Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/091583
PCT/US2020/016597
INTRA-ORAL ELECTROENCEPHALOGRAPHY DEVICE AND METHOD
CROSS-REFERENCE TO RELATED APPLICATIONS
100011 This application is a Continuation-in-Part of U.S.
Application No. 16/673,077 filed
November 4, 2019, which is a Continuation-in-Part of U.S. Application No.
16/202,204 filed
November 28, 2018, which is a Continuation-in-Part of U.S. Application No.
15/479,737 filed
April 5,2017, which claims the benefit of U.S. Provisional No. 62/319,443
filed April 7,2016.
Each of these applications are incorporated herein by reference in its
entirety.
BACKGROUND
[0002] Sleep apnea is a common medical condition during
which a person experiences one
or more pauses in breathing, and in some instances, experiences shallow
breaths during sleep.
While there are several types of sleep apnea, the most common type is
obstructive sleep apnea.
In this medical condition, one or more of the person's throat muscles relax
during sleep causing
surrounding tissues in the posterior portions of the mouth, nose and throat to
collapse, thereby
creating a pharyngeal obstruction that can block the upper airway. Persons
suffering from
obstructive sleep apnea have inadequate oxygen exchange during sleep, which
can lead to
daytime fatigue, lack of concentration and mood changes. Left untreated,
obstructive sleep apnea
can have a significant impact on a person's health, often leading to
cardiovascular, stroke and
metabolic disorders.
[0003] Known methods for treatment of obstructive sleep
apnea include both surgical and
nonsurgical devices. A popular surgical procedure is
uvulopalatopharyngoplasty, which may be
performed for patients who have anatomical abnormalities that cause their
obstructive sleep
apnea and/or make them less likely to tolerate nonsurgical devices.
Uvulopalatopharyngoplasty
may be a complicated surgery, during which a portion of the soft palate is
removed in an effort to
prevent closure of the airway by excess tissue during sleep. A disadvantage of
this procedure,
however, is that the operation is often expensive and may damage throat
muscles necessary for
swallowing and/or cause other undesirable disorders, such as, nasal
regurgitation and numbness
of the lower front teeth.
[0004] To reduce this risk, various nonsurgical approaches
have been employed. One such
nonsurgical approach includes using standardized oral appliances to
incrementally advance
and/or protrude the mandible (lower jaw) relative to the maxilla (upper jaw).
These standardized
oral appliances, commonly referred to as a mandibular advancement device,
("MAD"), typically
1
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
include upper and lower dental trays, whereby the lower dental tray is
designed to advance the
mandible, and hence, move the tongue forward to increase the space in the
posterior part of the
throat and the oropharynx, which in turn may serve to increase the flow of air
during sleep. The
distance (degree of advancement) required to protrude and/or reposition the
mandible may be, at
least in part, dependent on the severity of the individual's obstructive sleep
apnea, as well as
psychological variables among the users. A disadvantage of using these
standard oral appliances
is that they may not sufficiently provide for and/or address individualized
anatomical variances,
such as difference in dental arches, dentition alignment and/or jaw
flexibility. Another
disadvantage is that in instances where the degree of advancement is
excessive, the appliance
may lead to long-term temporomandibular joint ("TMJ") disorders, muscular
aggravation,
dentition discomfort and/or myofascial disorders. As a result, use of these
standard appliances
has an approximate compliance rate of 75% over a 2-year period. For a detailed
study of
compliance with use of MAD, see Non-CPAP therapies in obstructive sleep
apnoea: mandibular
advancement device therapy, see Eur Respir J 2012; 39: 1241-1247, which is
incorporated by
reference in its entirety. Thus, such oral appliances may not treat
obstructive sleep apnea in a
manner that prevents and/or limits impacts on a person's health.
[0005] FIG. 1 depicts a system 1 including an intraoral
stimulator device 2 used for
providing treatment of a sleep disorder. The intraoral stimulator device 2 is
powered by a
rechargeable battery and includes a housing of a hollow dental retainer
wireframe or mouthguard
(in the case of a bilateral configuration) or a molar teeth clip (in the case
of unilateral
configuration) for positioning on the lower teeth. The housing 4 includes a
single pair or two
pairs of bilateral electrodes 5a, 5b for positioning ventral-laterally and
sublingually at the
posterior to middle section under the tongue for recruiting a large section of
the genioglossus
muscle and base-of-tongue for stimulation to regain muscle tone during sleep.
The system 1
includes an external inductive recharger sub-system 6, configured to receive
electrical power
from a wall outlet 7 and use the electrical power to recharge a rechargeable
battery (not shown)
provided in the intraoral stimulator device 2 by transferring power through
electromagnetic
induction.
[0006] The oral appliance 1 further includes a non-
rechargeable battery-operated hand-held
appliance 3 that communicates instructions to the intraoral stimulator device
2. The non-
rechargeable battery-operated hand-held appliance 3 is used by the patient's
sleep medicine
2
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
physician to program the stimulation and to set system parameters in the
intraoral stimulator
device 2. The stimulation can be pre-programmed or can occur as a result of
change in the user's
breathing pattern, as tested by accelerometer, temperature, piezoelectric film
and EMG.
Alternatively, the stimulation therapy may be programmed and setup up by a
physician so that
the therapy begins as soon as the device is turned On and ceases when the
device turns Off,
without regard to changes in the user's breathing pattern. An issue with
continuous stimulation is
that over stimulation can lead to nerve and/or muscle fatigue/damage.
Moreover, while a
physician can set and/or send instructions to the intraoral stimulator, the
physician cannot store
and or assess the breathing and/or snoring pattern of a patient in a way that
allows the physician
to modify treatment as may be necessary. The lack of specialized treatment
measures in
individual patients with unique medical needs can be problematic, particularly
because they fail
to store patient behavior and/or medical data that can assist medical
providers in the design
and/or improvement of specialized treatment measures for individual patients.
Thus, such
intraoral stimulator devices may fail to treat obstructive sleep apnea in a
manner that prevents
and/or limits impacts on a person's health.
[0007] Other methods of treating obstructive sleep apnea
include the administration of
positive air pressure via a continuous positive airway pressure ("CPAP")
machine. The CPAP
machine is often assembled for use in combination with various face or nasal
masks and may
provide continuously pressurized and/or forced air during the person's sleep.
A disadvantage of
this assembly is that it may cause nasal and/or oral mucosa] dryness due to
the continuously
forced air and may also cause claustrophobia due to the presence of a mask on
the patient's face.
As a result, use of these assemblies has an approximate compliance rate of 50%
over a 5-year
period. For a detailed study of compliance with use of CPAP machines, see Long-
term
compliance with continuous positive airway pressure in patients with
obstructive sleep apnea,
Can Respir J. 2008 Oct; 15(7): 365-369, which is incorporated by reference in
its entirety.
Another disadvantage is that standard masks are not properly adapted for a
customized fit for
persons with unique and/or variable facial anatomies that may be natural or
created by loss of
muscle tone secondary to facial paralysis and/or stroke. Ill-fitting masks may
lead to leakage of
air and/or inadequate air intake. In addition, the masks used with CPAP
machines have been
found to be a breeding ground for bacteria and fungi. Despite routine washing
and cleaning
measures, the bacteria and fungi on these masks can grow exponentially, and
lead to infections,
3
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
such as pneumonia, in the airways of persons who use them. Moreover, such
assemblies may not
sufficiently treat obstructive sleep apnea and may fail to promote patient
compliance with the
treatment method.
[0008] The aforementioned treatment techniques may not
provide sufficient treatment of
obstructive sleep apnea, may cause and/or promote other negative health
situations for the user
and may not foster compliance with treatment methods.
[0009] In view of the disadvantages associated with
currently available methods and devices
for treating obstructive sleep apnea, there is a need for a device and method
that treats
obstructive sleep apnea while storing patient behavior and/or medical data
relating to a user's
breathing pattern, snoring pattern and/or clenching/grinding behaviors, that
can assist medical
providers in the design, improvement and/or modification of specialized
treatment measures for
individual patients. Further, there is a need for a device and method that
treats obstructive sleep
apnea in a single removable oral appliance and prevents and/or limits long-
term TMJ disorders,
muscular aggravation and/or myofascial disorders that may occur with continued
use of currently
available appliances.
[0010] Electroencephalography is a technique for recording
and interpreting electrical
activity occurring within the brain. The EEG technique is based on the nerve
cells of the brain
generating electrical impulses that fluctuate in particular patterns. The
pattern produced by an
electroencephalograph machine, which may be recorded, is called an
electroencephalogram
(EEG).
[0011] Obtaining an EEG typically begins with the
attachment of a number of pairs of
electrodes to the subject's scalp. Each pair of electrodes sends a signal to
one of several
recording channels of the electroencephalograph; the signal is a measure of
the voltage
difference between the pair. This voltage difference can be rhythmic and shown
as waves on a
line graph by the recording channel. For a normal, fully conscious adult in a
relaxed state, the
EEG shows regularly oscillating waves known as alpha waves. Subjecting the
person to
excitement or startling the person results in the alpha waves being replaced
by rapid irregular
waves of low-voltage relative to the alpha waves. A sleeping adult's brain
waves become
extremely slow. This is also true for a person in a coma. Other abnormal
conditions have known
EEG patterns. For example, delta waves are irregular slow waves in the
vicinity of an area of
brain damage. Although certainly not useful in all circumstances,
electroencephalography has
4
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
been useful as a diagnostic aid in cases of serious head injuries, brain
tumors, sleep disorders,
cerebral infections, epilepsy, some degenerative diseases of the nervous
system and brain death.
[0012] In a sleep lab, delta waves may be utilized to
assess the depth of sleep. The stronger
the delta rhythm, the deeper the sleep. Increased delta power (an increased
quantity of delta wave
recordings) has also been found to be associated with increased concentration
on internal
working memory tasks.
[0013] As noted, collection of EEG data is performed with
electrodes attached to the
subject's scalp. One reason for this placement is to have the electrodes as
close as possible to the
brain with as little intervening structure as possible. Other than for bald
subjects, it is not
possible to really 'attach' electrodes to the scalp. This presents a problem
because movement of
electrodes can interfere with the quality of the received voltages. In
addition, since muscle cells
also generate an electrical potential, electroencephalograph machines
typically try to avoid
muscles intervening between the electrode and the brain.
[0014] In addition to above, there is a need for a device
and method capable of determining
when a user is having arousals or being awoken from deep sleep, entering or in
an obstructive
sleep apnea condition. Such a monitoring device and method may be coupled with
active
treatment regimens. That is, a determination made that the user is being
awoken from or having
arousals from sleep, entering or in an obstructive sleep apnea condition may
be used a trigger for
the active treatment regimens.
BRIEF DESCRIPTION
[0015] According to an aspect, the present embodiments are
associated with an oral
appliance for treatment of sleep apnea in a user. The present embodiments may
comprise a
mouthpiece configured to be retained in the mouth of the user, a plurality of
electrodes, a
microprocessor and at least one stimulator. The mouth of the user contains
oral tissue and the
mouthpiece has a lingual wall and a buccal wall. A plurality of electrodes are
attached to the
mouthpiece and electrically connected to the microprocessor; the electrodes
and microprocessor
are configured to determine electroencephalograph rhythms of the user's brain.
The
microprocessor is configured to assess the rhythms for an immediate presence
of sleep apnea in
the user. The at least one stimulator is also attached to the mouthpiece and
is configured to
respond to the immediate presence of sleep apnea in the user by stimulating
the user's
genioglossus. The oral appliance mouthpiece may have an upper portion and a
lower portion.
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
100161 Embodiments of the disclosure are further associated
with an oral appliance for
treatment of sleep apnea in a user comprising a mouthpiece, a plurality of
electrodes, at least one
electrical stimulator, a microprocessor. The mouthpiece is configured to be
retained in the mouth
of the user and may have an upper portion and a lower portion, each with a
lingual wall and a
buccal wall. The mouth of the user has oral tissue. The plurality of
electrodes are attached to the
mouthpiece and the microprocessor is configured to receive signals from the
electrodes such that
the electrodes and microprocessor cumulatively operate as an
electroencephalograph and detect
electrical rhythms from the user's brain. The electrical rhythms may be
indicative of an
immediate presence of arousals from deep sleep, or the presence of sleep apnea
in the user. The
at least one electrical stimulator is attached to the mouthpiece and
microprocessor; the stimulator
is configured for emitting an electrical current or field in response to the
immediate presence of
sleep apnea in the user based on the electrical rhythms.
100171 Embodiments of the disclosure are further associated
with a mouthpiece for being
worn on the upper dentition and jaw (or upper jaw, without teeth) of the user.
The mouthpiece
includes electrodes that detect electrical rhythms of the user's brain. The
mouthpiece may be
useful in a variety of applications, such as athletics, gaming, and personal
hobbies. It is
contemplated that the mouthpiece may be used to detect, in real time,
traumatic blows to the
head of a user during an athletic activity, to be detected in real time, while
the athlete wears the
upper mouthpiece. The user's EEG can also be monitored on the side lines by
trained operators.
This technique may detect early onset of acute brain trauma or concussion
prior to any clinical
presentations and help save the user from potential future damaging side
effects of the trauma.
BRIEF DESCRIPTION OF THE FIGURES
[0018] A more particular description will be rendered by
reference to specific embodiments
thereof that are illustrated in the appended drawings. Understanding that
these drawings depict
only typical embodiments thereof and are not therefore to be considered to be
limiting of its
scope, exemplary embodiments will be described and explained with additional
specificity and
detail through the use of the accompanying drawings in which:
[0019] FIG. 1 is a perspective view of a prior art oral
device;
[0020] FIG. 2 is a top view of an oral appliance, according
to an embodiment;
100211 FIG. 3 is a perspective view of an oral appliance,
according to an embodiment;
[0022] FIG. 4 is a perspective view of an oral appliance,
according to an embodiment;
6
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
100231 FIG. 5 is a perspective view of an oral appliance
kit, according to an embodiment;
[0024] FIG. 6 is a schematic of a method for providing
electrical genioglossus stimulation,
according to an embodiment;
[0025] FIG. 7 is a bottom view of a top mouthpiece oral
appliance, according to an
embodiment;
[0026] FIG. 8A is a bottom, left-side perspective view of
the mouthpiece oral appliance,
according to an embodiment;
[0027] FIG. 8B is a bottom, right-side perspective view of
the mouthpiece oral appliance
shown in FIG. 8A;
[0028] FIG. 9 is a bottom, anterior perspective view of the
mouthpiece oral appliance,
according to an embodiment; and
[0029] FIG. 10 is a schematic of a method for providing
electrical genioglossus stimulation,
according to an embodiment.
[0030] Various features, aspects, and advantages of the
embodiments will become more
apparent from the following detailed description, along with the accompanying
figures in which
like numerals represent like components throughout the figures and text. The
various described
features are not necessarily drawn to scale but are drawn to emphasize
specific features relevant
to some embodiments.
DETAILED DESCRIPTION
100311 Reference will now be made in detail to various
embodiments. Each example is
provided by way of explanation and is not meant as a limitation and does not
constitute a
definition of all possible embodiments.
[0032] Embodiments of the disclosure relate generally to
devices / appliances and methods
for treating obstructive sleep apnea, a device for providing electrical
stimulation to a user's
tongue to inhibit and/or limit snoring that may be caused by obstructive sleep
apnea as well as a
device including a pharmaceutical delivery reservoir for delivery of a drug
for treating
obstructive sleep apnea. Such devices provide particular utility in providing
electrical stimulation
to the user's tongue in such a manner that the stimulation does not awaken the
user during sleep.
Alternatively or supplemental to electrical stimulation, the device may
include a pharmaceutical
compound, such as an ionized medication, that treats obstructive sleep apnea.
The
pharmaceutical compound may be provided in a reservoir / pharmaceutical
reservoir, separate
7
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
from the device, or as part of the physical matrix of the device. Particularly
in the former option,
the reservoir may be refilled or replaced on a daily or less frequent
schedule.
[0033] The oral appliance contemplated includes a
mouthpiece that is configured to receive
at least temporary, permanent and/or artificial lower dentition of the user.
The mouthpiece may
include various electronic components including one or more of the following:
an oxygen sensor,
a pressure sensor, an airflow sensor, a noise detector, an actigraphy sensor,
a stimulator, data
recorder, battery and a microprocessor. The mouthpiece may also be comprised
of a material,
e.g., a polymer matrix, into which a pharmaceutical compound may be
incorporated for delivery
to the user. Alternatively, one or more reservoirs containing a pharmaceutical
compound may be
attached to the mouthpiece. Each reservoir is capable of delivering a drug
directly to one or
more oral cavity membrane surfaces of the user. The mouthpiece may include
customizable
materials that provide a comfortable fit for a user while retrieving data
related to the user's
oxygen saturation levels, clenching and/or grinding of dentition surfaces,
actual airflow levels
and noise levels associated with snoring, analyzing the data, and preparing a
set of instructions to
the stimulator.
[0034] When utilized in combination with a pharmaceutical
compound, the stimulator
components may be utilized to effect transfer of the drug from the device to
the oral mucosa of
the user. This drug delivery function may be in addition to the electrical
stimulation of a user's
oral musculature or may be alternative thereto, i.e., the electrical
stimulation may only function
as a drug release/delivery mechanism. The stimulators may operate to rupture
or pierce the
pharmaceutical reservoir(s) attached to or otherwise associated with the
mouthpiece upon
receiving and instruction to do so. Alternatively, the stimulators may be
utilized in combination
with pharmaceuticals bearing an electrically charged surface, as will be
further explained. In the
event that the microprocessor sends a signal resulting in the rupture of
pharmaceutical
reservoir(s), notification of the user that the reservoir is in need of
replacement can be conveyed
by the microprocessor to the user. Such notification may take the form of a
smartphone
notification of the user or visual notification, e.g., activation of LED light
when user is next able
to see said light.
[0035] According to an aspect, the mouthpiece is customized
to be receivably positioned
and/or secured on the mandible of the user. According to an aspect, the
mouthpiece is
customized to receive the lower dentition of the user. In any event the
mouthpiece may be
8
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
customized such that it provides a comfortable fit that enhances the user's
comfort and reinforces
the user's likelihood of repeated wear of the mouthpiece, i.e., the user's
compliance rate.
[0036] For purposes of illustrating features of the
embodiments, embodiments will now be
introduced and referenced throughout the disclosure. Those skilled in the art
will recognize that
this example is illustrative and not limiting and is provided purely for
explanatory purposes.
[0037] In an embodiment, and with particular reference to
FIGS. 2-4, an oral appliance 10
for treatment of sleep apnea in a user is provided. The oral appliance 10 is
illustrated as having a
mouthpiece 20 and several components. In an embodiment, the mouthpiece 20 is
"customizable", that is, customized to the individual user's mouth in such a
manner that it
provides for a comfortable fit over and around surfaces of the user's hard
(teeth/dentition) and/or
soft tissues (general mouth structure, including gums). When customized, the
mouthpiece 20
may fit over temporary, permanent, primary natural and/or artificial lower
dentition of adult
and/or child users. The mouthpiece 20 may be configured to receive a removable
denture of the
user. According to an aspect, the mouthpiece 20 is fabricated over the lower
jaw, that is, the
mandible, with partial or complete absence of dentition. When customized, the
mouthpiece 20
can be formed of any self-conforming material that may be adaptable to
variances and/or
changes in mouth structure, or through use of a dental impression of the
individual user's
dentition, as would be understood by a person having ordinary skill in the art
In other words, a
mandibular impression and/or a dental impression can be taken, whereby a
negative imprint of
the user's hard and/or soft tissues are used to create a positive reproduction
(or cast) customized
for the user.
[0038] The types of materials selected to form the
mouthpiece 20 would be known to one of
ordinary skill in the art and includes polymers, thermoplastics, acrylics,
silicone, rubber, metal
wires or any other material that can be used to form the mouthpiece 20
conformed to the user's
dentition. In an embodiment, the materials are medical-grade, latex-free, BPA-
free and any other
material known to minimize patient health risks. According to an aspect, the
mouthpiece 20 may
be formed from the impression made in a thin, resilient material. The
mouthpiece material may
also be selected, particularly from polymers, for its ability to have a
pharmaceutical compound
incorporated within the structural matrix.
[0039] In an embodiment and as illustrated in FIGS. 2 and
3, the mouthpiece 20 includes a
central channel 29 bounded by a lingual portion 24 and a buccal portion 23.
The central channel
9
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
29 may be configured to be receivably positioned over and/or receive one or
more of the user's
dentition such that the mouthpiece 20 is secured thereon. When the mouthpiece
20 is in use, the
central channel 29 may receive the user's dentition and may extend over and/or
cover occlusal or
bite surfaces of the user's teeth. The lingual portion 24 of the mouthpiece 20
extends between the
user's teeth and the user's tongue. In an embodiment and as illustrated in
FIGS. 2 and 3, the
buccal portion 23 of the mouthpiece 20 extends between the user's teeth and
the user's cheek.
[0040] According to an aspect, the mouthpiece 20 is
configured to be secured to the user's
dentition. In an embodiment and as illustrated in FIG. 4, the mouthpiece 20
includes the lingual
portion 24 and dentition attachment members 28 coupled to the lingual portion
24. The dentition
attachment members 28, as well as the lingual portion 24, may be customizable,
such that the
dentition attachment members 28 have a shape and size that substantially
conforms to the
dentition of the user, thereby providing the user with the mouthpiece 20
having a secured and
customized fit. Typically, the dentition attachment members 28 are provided in
a wire-frame
form, in a way that extends from the lingual portion 24 to wrap over or around
the individual
user's dentition and anchor the lingual portion 24 between the lingual surface
of the teeth and the
tongue. According to an aspect, at least a portion of the dentition attachment
members 28 is
shaped to form a retention loop around one or more teeth of the user.
100411 Similar to the dentition receiving cavities 25
described for the mouthpiece 20 of
FIGS. 2 and 3, the lingual portion 24 depicted in FIG. 4 may also be
customized to have a shape
that is substantially the same as the shape of the individual user's dentition
for which it has been
molded and/or shaped to fit, thereby assisting the retention function of the
dentition attachment
members 28. In any event, the mouthpiece 20 is capable of being at least
temporarily fixed in
place by virtue of having been molded and conformed to the dentition of the
user and/or being
provided with the dentition attachment members 28, thus providing the
customized fit. As such,
the mouthpiece 20 may provide a retention function thereby allowing the oral
appliance 10 to
remain in place during the user's sleep, particularly in situations where the
user may make slight
to moderate movements during sleep and/or when the user may be awake. Thus,
the mouthpiece
20 may be substantially immovable unless positive effort is applied to remove
the mouthpiece
20. In other words, the user may remove the mouthpiece 20 at any time, if
desired, by exerting a
little pressure to remove the mouthpiece 20. Since the mouthpiece 20 is not
permanently affixed
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
to the dentition, it can be worn and/or subsequently removed by the user at
any time. Therefore,
the oral appliance 10 may be used for varying lengths of time.
[0042] According to an aspect and as illustrated in FIGS. 2-
4, the components positioned on
and/or embedded within the mouthpiece 20 include one or more of the following
components: an
oxygen sensor 30, a pressure sensor 32, an airflow sensor 34, a noise detector
35, an actigraphy
sensor 36, a stimulator 40, a pharmaceutical reservoir 42, a microprocessor
50, a data recorder 60
and a battery 70. According to an aspect, the mouthpiece 20 includes dry
protective areas or
covering to these electronic components that substantially inhibit and/or
limit water and/or tissue
damage to the components (not shown). Such dry/protected zones may be formed
by virtue of
the components being embedded within the mouthpiece 20 itself
[0043] As illustrated in FIGS. 2-4, the oxygen sensor(s) 30
may be provided near an anterior
portion 21 of the mouthpiece 20, i.e., towards the user's lips and away from
the user's pharynx.
According to an aspect, the oxygen sensor 30 is configured to monitor and/or
determine actual
oxygen saturation levels of the user's hemoglobin. The oxygen sensor 30 may be
adapted to
monitor and/determine the pulse and/or heart rate of the user. The oxygen
sensor 30 may be
positioned on or in the lingual portion 24 of the mouthpiece 20. In an
embodiment, the oxygen
sensor 30 is positioned primarily towards lateral portions of the tongue,
which are generally
understood to be the most vascular areas of the tongue, i.e., having numerous
blood vessels, as
well as the buccal regions of the upper jaw. According to an aspect, the
oxygen sensor 30 is a
transceiver such as a pulse oximeter configured to monitor/sense the oxygen
saturation level of a
user by analyzing the change in color of the user's blood. The pulse oximeter
may measure the
pulse rate of the user, typically in beats per minute, based on variations
and/or deviations in the
user's oxygen saturation level. An exemplary pulse oximeter, for example, may
include light
emitting diodes configured to transmit red and infrared lights to vascular
surfaces of the user's
tongue and sense changes in oxygen level in the user's tongue. According to an
aspect, two
oxygen sensors 30 are provided on the lingual portion 24 of the mouthpiece 20.
It is
contemplated that oxygen sensors 30 may be placed in other locations of the
oral cavity, such as
the buccal bone, such that the oxygen sensors gather oxygen saturation data
from the gum
surface overlaying the buccal bone. The two oxygen sensors 30 may be
bilaterally positioned on
the mouthpiece 20. While FIGS. 2-4 illustrate two oxygen sensors 30 being
positioned on the
11
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
mouthpiece 20, it is to be understood that the number of oxygen sensors
provided may be 3, 4, 5,
6 or more.
[0044] According to an aspect and as illustrated in FIGS. 2
and 3, the oral appliance 10 may
include one or more pressure sensors 32. According to an aspect, the one or
more pressure
sensors 32 are configured to detect signs of clenching and/or grinding by the
user that occur, for
example, while the user is asleep. The pressure sensors 32 may be positioned
in or on the central
channel 29. In an embodiment, the pressure sensors 32 are positioned in the
dentition receiving
cavities 25, such that the pressure sensors 32 are positioned substantially
adjacent to the user's
mandibular occlusal and/or bite surfaces. According to an aspect, the pressure
sensors 32 are on
an exterior surface of the central channel 29, where the central channel 29
has an interior surface
configured for receiving the dentition receiving cavities 25 and the exterior
surface is positioned
opposite of the interior surface, such that the pressure sensors 32 are
positioned substantially
adjacent to the user's maxillary occlusal and/or bite surfaces. In some
embodiments (not shown),
the pressure sensors may be provided on the dentition attachment members 28,
such as those
manufactured by Tekscan under the brand FlexiForceTM Force Sensors. Such signs
of clenching
may include force sensors configured to measure the force that is being
applied to occlusal
and/or bite surfaces of the user's teeth. According to an aspect, the pressure
sensors 32 are a thin
resilient material. The one or more pressure sensors 32 may be electrically
sealed and/or
impervious to liquids, saliva and/or oral tissue. The number of pressure
sensors 32 provided on
the mouthpiece 20 may be selected based on the user's proclivity to grinding
and/or clenching.
According to an aspect, the number of pressure sensors 32 provided is 2, 3, 4,
5, 6 or more.
[0045] In an embodiment, the mouthpiece 20 includes one or
more airflow sensors 34
configured to measure the actual airflow and/or breathing rate of the user,
i.e., the rate of air that
is inhaled and/or exhaled through the mouthpiece 20 by the user. According to
an aspect, the
airflow sensor 34 is configured to detect any reduction and/or cessation of
airflow during sleep.
The airflow sensor 34 may be arranged at any position on the mouthpiece 20
that is in a general
flow path of air inhaled and/or exhaled by the user. As illustrated in FIG. 2,
the airflow sensor 34
may be positioned near a posterior portion 22 of the mouthpiece 20. According
to an aspect, the
airflow sensor 34 is bilaterally positioned on the mouthpiece 20. As
illustrated in FIGS. 2-3, one
airflow sensor 34 may be positioned to the left of the lingual portion 24,
while another airflow
sensor 34' may be positioned to the right of the lingual portion 24. In any
event, both airflow
12
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
sensors 34, 34' may work in tandem to measure the user's airflow rate. Airflow
sensors 34 may
be arranged in/on at least one of the lingual portion 24 and the buccal
portion 23 of the
mouthpiece 20. The number of airflow sensors 34 provided on the mouthpiece may
be selected
based on the needs of the user. According to an aspect, the number of airflow
sensors provided is
2, 3, 4, 5 or more.
[0046] According to an aspect and as illustrated in FIGS. 2-
4, the mouthpiece 20 may
include an actigraphy sensor 36 configured to monitor and capture data related
to sleep activity,
including sleep position and movement of the user during sleep. The actigraphy
sensor 36 may
embedded in or otherwise connected to the mouthpiece 20, at any desired
position. According to
an aspect and as illustrated in FIG. 2-3, the actigraphy sensor 36 is position
at the buccal portion
23 of the mouthpiece 20. In an alternate embodiment and as illustrated in FIG.
4, the actigraphy
sensor 36 may be positioned at the lingual portion 24 of the mouthpiece 20.
The actigraphy
sensor 36 may determine the user's sleep positions, such as, for example, a
supine position
during which the user is positioned on his/her back, a prone position during
which the user is
lying face down and/or lateral recumbent positions during which the user is
lying on their left or
right sides. The actigraphy sensor 36 may measure the time the user sleeps in
each identified
position and/or the frequency of the user changing from one sleep position to
another sleep
position.
[0047] The oral appliance 10 may include a noise detector
35 configured to detect actual
noise and/or vibrations caused by the user's snoring. According to an aspect,
the noise detector
35 is internally hard-wired to one or more components coupled to or otherwise
embedded in the
mouthpiece 20, such as, for example, the stimulator 40, the microprocessor 50
and the data
recorder 60, such that the noise detector 40 can communicate with the
components. The noise
detector 35 may be configured to wirelessly communicate with at least one of
the stimulator 40,
the microprocessor 50 and the data recorder 60. The noise detector 35 may be
positioned on or
otherwise embedded in the mouthpiece 20 at any desired location. According to
an aspect, the
noise detector 35 is positioned at the posterior portion 22 of the mouthpiece
20, such that
relevant snoring information may be detected close to a sound source, i.e.,
the user's pharynx. In
an embodiment, the noise detector 34 is positioned at the anterior portion 21
of the mouthpiece
20. As illustrated in FIG. 3, the noise sensor 35 may be positioned at the
buccal portion 23 of the
mouthpiece 20. In an embodiment and as illustrated in FIG. 4, the noise sensor
35 is positioned
13
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
at the lingual portion 24 of the mouthpiece 20. While FIGS. 3-4 illustrate a
single noise detector
35 being provided on the mouthpiece 20, it is to be understood that 2, 3,4 or
more noise
detectors 35 may be provided.
[0048] According to an aspect and as illustrated in FIGS. 2-
4, the at least one stimulator 40 is
provided near the posterior portion 22 of the mouthpiece 20, that is generally
near the back of the
user's mouth. The stimulator 40 is configured to provide a gentle stimulation
to the tongue of the
user, as will be described in more detail hereinbelow. In an embodiment, the
stimulator 40 is
positioned on the lingual portion 24 of the mouthpiece 20, adjacent to the
tongue. The stimulator
40 may be bilaterally positioned on the mouthpiece 20, such that bilateral
stimulation may be
provided to both sides of the user's tongue. The stimulator 40 may be
positioned substantially
adjacent to a base of the user's tongue, for example, adjacent to the user's
genioglossus muscle.
Thus, the stimulator 40 may be configured for providing stimulation to the
genioglossus muscle
of the user's tongue in a manner that allows the muscle tone of the
genioglossus muscle to be
regained. Such stimulation may be electrical impulses that cause the
genioglossus muscle to
contract and/or cause the user to reduce the amount of force being applied to
occlusal and/or bite
surfaces of the user's teeth. In some embodiments, contraction of the
genioglossus muscle may
cause the user's tongue to protrude, thereby creating more space in the user's
pharynx and
helping the user breathe more easily in a manner that increases the oxygen
saturation levels of
the user's hemoglobin. The stimulation may be in response to the actual
saturation level of
hemoglobin of the user, as measured by the at least one oxygen sensor 30.
[0049] According to an aspect, the stimulator 40 is
activated based on measurements
received from the oxygen sensors 30, the pressure sensors 32, the airflow
sensors 34 and/or the
noise detector 35. The stimulator 40 may be activated if the oxygen sensor 30
determines that the
actual oxygen saturation level of hemoglobin of the user is at a predetermined
oxygen level, that
is, that a certain oxygen level has been pre-determined to be insufficient.
The stimulator 40 may
provide at least intermittent stimulation to the genioglossus muscle of the
user's tongue until the
oxygen saturation level of hemoglobin rises above the predetermined oxygen
level. In an
embodiment, the stimulator 40 is activated if the oxygen sensor 30 determines
that the actual
oxygen saturation level of hemoglobin of the user is below about 95% oxygen
saturation.
Stimulation of the user's genioglossus muscle may facilitate an increase in
respiratory flow to the
user, thereby increasing the availability of oxygen to the user and the
increase of oxygen
14
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
saturation levels of hemoglobin. According to an aspect, when the oxygen
sensor 30 determines
that the oxygen saturation level of hemoglobin of the user is above about 95%
oxygen saturation,
the stimulator 40 is not activated. In an embodiment, the stimulator 40 is
activated if the pressure
sensors 32 detect grinding and/or clenching by the user. According to an
aspect, the stimulator
40 provides stimulation until the force applied to occlusal and/or bite
surfaces of the user's teeth
are below a predetermined force level. The stimulator 40 may stop stimulation
once the pressure
sensors 32 detect that grinding and/or clenching has substantially decreased
and/or ceased, as
evidenced by the detected force level. According to an aspect, the stimulator
40 is activated
when the airflow sensor 34 determines that the frequency of air inhaled and/or
exhaled by the
user is below a predetermined airflow level. In an embodiment, the stimulator
40 is activated
when the airflow sensor 34 determines that airflow is at or below 30% of the
user's natural
airflow or breathing rate, i.e., air inhaled and/or air exhaled by the user
while the user is awake
(natural airflow), has been reduced by 30%. The stimulator 40 may provide
stimulation to the
genioglossus muscle until the predetermined airflow level is achieved and/or
airflow to the user
is at least about 30% of the user's natural airflow rate. In an embodiment,
the stimulator 40 is
activated if the noise detector 35 detects that the actual noise and/or
vibrations are above a
predetermined noise level. In this embodiment, the stimulator 40 provides
gentle electrical
stimulation to the genioglossus muscle of the user's tongue until the actual
noise and/or
vibrations are below the predetermined noise level.
[00501 In an embodiment, the stimulator 40 is configured to
provide constant stimulation to
the genioglossus muscle of the user's tongue. Alternatively, the stimulator 40
may provide
variant stimulation to the genioglossus muscle of the user's tongue. The
variant stimulation may
increasingly stimulate the genioglossus muscle of the tongue until the oxygen
saturation level is
at the predetermined oxygen level, such as, for example, at or above 95%. In
an embodiment, the
variant stimulation increasingly stimulates the genioglossus muscle until the
force applied to the
occlusal and/or bite surfaces is below the predetermined force level. The
variant stimulation
provided by the stimulator 40 to may increasingly stimulate the genioglossus
muscle until the
predetermined airflow level is achieved and/or until the actual noise and/or
vibrations are below
the predetermined noise level. According to an aspect, the strength and
frequency of the
electrical impulses in variant mode will depend on how quickly the oxygen
saturation of
hemoglobin and/or the predetermined force level is achieved. The constant or
variant stimulation
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
may be a gentle stimulation that does not disturb and/or awaken the user
during sleep. According
to an aspect, the constant or variant stimulation is gentle enough so that the
user does not
recognize it when wearing it when the user is at least slightly awake. The
stimulator 40 may
alternate between a constant stimulation mode and a variant stimulation mode.
In an
embodiment, the at least one stimulator 40 is an electrode configured to
provide gentle electrical
impulses. The gentle electrical impulses may be provided to the genioglossus
muscle of the
user's tongue in a non-invasive manner and in such a manner that stimulation
does not awaken
the user during sleep.
[0051] In an embodiment, the mouthpiece 20 or structures
associated with the mouthpiece 20
allow delivery of a pharmaceutical compound to foster retention or
reacquisition of muscle tone
of the genioglossus muscle. Such a pharmaceutical compound may cause the
genioglossus
muscle to contract. Activation of the genioglossus muscle may be achieved
utilizing cholinergic
drugs such as neostigmine. Other stimulants and/or drugs that activate and/or
increase calcium
ion release/activation affecting muscle contraction may also be used to
activate the genioglossus
muscle, such compounds include norepinephrine and caffeine.
[0052] In another embodiment, genetically engineered light
stimulation of the nerves and
muscles, specific to the desired site, may be utilized. This concept is called
optogenetics
Optogenetics makes it possible to stimulate neurons with light by inserting
the gene for a protein
called channelrhodopsin-2, from green algae. When a modified neuron is exposed
to blue light,
the protein initiates electrical activity inside the cell that then spreads
from neuron to neuron. The
optical control method provides advantages over electrical stimulation for
muscle and the
biomechanics of human movement. That is, photons are released by the
mouthpiece 20 instead of
electrical charge/current.
[0053] In an embodiment, the pharmaceutical compound may be
incorporated into the
material of mouthpiece 20 for active or passive release. Passive release may
be triggered by
environmental factors in the users mouth such as change in temperature, pH or
similar variables.
Active release may involve electrical stimulation controlled by the
microprocessor 50 responsive
to inputs from one or more of the sensors associated with mouthpiece 50.
Electrical stimulation
resulting in drug release is discussed further below.
[0054] Iontophoresis is a drug delivery process utilizing a
voltage gradient. Molecules are
transported through a semipermeable material or barrier by electrophoresis
and/or
16
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
electroosmosis. Electrophoresis is the motion of charged particles, ions or
anions, in the presence
of an electric field. Particles bearing a surface charge present in a liquid
or gel, i.e., capable of
substantial movement relative to the medium in which they are contained, are
most amenable to
electrophoresis, though movement through other materials is possible.
Electroosmosis is the
motion of liquid induced by an applied electrical potential across a porous
material, capillary
tube, membrane, microchannel, or any other fluid conduit. Iontophoresis is an
active transport of
matter resulting from an applied electric current. Such transport is measured
in units of chemical
flux, commonly pmol/(cmmhour).
[0055] The material chosen for mouthpiece 20 may be, for
example, a polymer acting as a
semipermeable retainer of a selected pharmaceutical compound. That is, the
material of
mouthpiece 20 will retain the pharmaceutical compound under storage and other
conditions
while releasing the pharmaceutical compound under certain passive or active
conditions. In the
case of active release, an electric charge or electric field may be applied to
some portion of
mouthpiece 20, causing the pharmaceutical compound to flow out of the
mouthpiece 20 and be
made available for absorption through the user's oral mucosa precisely to the
tissues to which it
is designed to treat. Whether released actively or passively, once a reservoir
42 is empty,
notification of the user that a reservoir 42 is in need of replacement can be
conveyed by the
microprocessor 50 to the user. Such notification may take the form of a
smartphone notification
of the user or visual notification, e.g., activation of LED light when user is
next able to see said
light. Replacement reservoir(s) 42 may be provided to user and have means,
e.g., friction or
adhesive (e.g., pressure sensitive adhesives / PSA), for attachment to
mouthpiece 20 upon
notification of the user regarding the need for replacement.
[0056] In an embodiment, a reservoir 42 containing a
liquid, gel or similar state of matter
may be associated with the mouthpiece 20. For example, the reservoir 42 may
comprise a pouch
attached to a surface of mouthpiece 20 and containing a pharmaceutical
compound. In an
embodiment, the pouch is formed from a material that will rupture when
subjected to an electric
charge or field by activation of stimulator 40. This activation may be the
result of microprocessor
31 responding to input from one or more sensors, as described previously. The
reservoir 42
pouch will typically be attached to mouthpiece 20 at a surface unlikely to
bear much force
associated with the user's teeth biting or rubbing against one another or the
mouthpiece 10. Thus,
the lingual wall 24 or buccal wall 23 are ideal for placement of reservoir(s)
42. The reservoir 42
17
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
may be removed after use or simply dissolve during use; either way, placement
of a new
reservoir 42 immediately prior to insertion of the mouthpiece 20 by the user
can be done when
needed.
[00571 In an embodiment, the material of the pouch walls 44
forming reservoir(s) 42 may be
a semipermeable polymer through which the pharmaceutical compound may pass
under
specified passive conditions or one through which the pharmaceutical may pass
when an
electrical stimulus or field is applied to the pouch reservoir 42. When
electrical stimulus is
required for iontophoresis, besides considerations of reservoir 42 placement
discussed above, it
is also important to consider placement relative to electrical stimulator(s)
40. A feature of the
stimulating reservoir(s) 42 to dispense the pharmaceutical compound is that
delivery of the
compound may be initiated, halted and reinitiated according to readings
sensors 30, 32, 34 and/or
36 convey to microprocessor 50. Thus, instead of having the pharmaceutical
compound delivered
as a bollus, it may be delivered closer to the profile of user's need.
[0058j Another semi-permeable bather through which
molecules of the pharmaceutical
compound may be transported is the outermost layer of human skin, i.e., the
stratum corneum
and other oral mucosa layers. Thus, however released from mouthpiece 20, the
pharmaceutical
compound is absorbed by the oral mucosa of the user. In some embodiments,
pharmaceutically
induced contraction of the genioglossus muscle may cause the user's tongue to
protrude, thereby
creating more space in the user's pharynx and helping the user breathe more
easily in a manner
that increases the oxygen saturation levels of the user's hemoglobin. The
stimulation may be in
response to the actual saturation level of hemoglobin of the user, as measured
by the at least one
oxygen sensor 30. Release of the pharmaceutical compound resulting in
stimulation to the
genioglossus muscle of the user's tongue may continue until the oxygen
saturation level of
hemoglobin rises above the predetermined oxygen level. In an embodiment, the
stimulator 40 is
activated if the oxygen sensor 30 determines that the actual oxygen saturation
level of
hemoglobin of the user is below about 95% oxygen saturation. Stimulation of
the user's
genioglossus muscle may facilitate an increase in respiratory flow to the
user, thereby increasing
the availability of oxygen to the user and the increase of oxygen saturation
levels of hemoglobin.
According to an aspect, if the oxygen sensor 30 determines that the oxygen
saturation level of
hemoglobin of the user is above about 95% oxygen saturation, stimulator 40 is
not activated and
reservoir 42 is not caused to dispense the pharmaceutical compound through
iontophoresis or
18
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
otherwise. In an embodiment, the stimulator 40 is activated if the pressure
sensors 32 detect
grinding and/or clenching by the user. According to an aspect, the stimulator
40 provides
electrical stimulus or an electrical field to reservoir(s) 42 as instructed by
microprocessor 50
acting in response to inputs from one or more of sensors 30, 32, 34 and 36.
[00591 As illustrated in FIGS. 2-4, a microprocessor 50 may
be provided on and/or
embedded within the mouthpiece 20. As illustrated in FIGS. 2 and 3, the
microprocessor 50 may
be positioned on or in the buccal portion 23. Alternatively, and as
illustrated in FIG. 4, the
microprocessor 50 may be positioned on or in the lingual portion 24 of the
mouthpiece 20. In
other words, it is possible to place the microprocessor 50 on the mouthpiece
20 wherever
available real estate may be found. Thus, when more than one component, such
as, for example,
the oxygen sensor 30 and the stimulator 40, are positioned at the lingual
portion 24 of the
mouthpiece 20, the microprocessor 50 may be positioned away from these regions
on the buccal
portion 23. In some embodiments and as illustrated in FIG. 4, the
microprocessor 50 is
positioned at the lingual portion 24 of the mouthpiece 20 and may be embedded
therein. It is to
be understood that the microprocessor 50 may be positioned at any location
that enables it to
communicate with the components included in the oral appliance 10, such as,
for example, the
oxygen sensor 30, the pressure sensor 32, the airflow sensor 34, the noise
detector 35, the
actigraphy sensor 36, the stimulator 40, the data recorder 60, andJor a
battery 70, while ensuring
that the location of the microprocessor 50 helps maintain a comfortable fit
and/or maintain
wearability of the mouthpiece 20 by the user. The microprocessor 50 may be
attached to and/or
positioned at any desired location on the mouthpiece 20, such as, anteriorly,
posteriorly and any
location therebetween. According to an aspect, the microprocessor 50 is sized
and/or positioned
to provide for a comfortable fit for the user. To be sure, the microprocessor
50 may be positioned
at any location that does not interfere with the comfortable fit of the
mouthpiece 20 for the user.
The microprocessor 50 may be configured to receive data corresponding to the
actual oxygen
saturation levels of hemoglobin from the at least one oxygen sensor 30, and
data relating to the
user's grinding and/or clenching behavior, actual airflow levels, actual noise
and/or snoring
levels. In an embodiment, the microprocessor 50 is configured to activate the
stimulator 40 if the
oxygen sensor 30 determines that the actual oxygen saturation level of
hemoglobin of the user is
at a predetermined level. According to an aspect, the microprocessor 50
activates the stimulator
40 if the pressure sensor 32 determines that the user is clenching and/or
grinding his/her
19
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
dentition at unacceptable levels. The microprocessor 50 may activate the
stimulator 40 if the
airflow sensor 34 determines that the user's airflow rate is below the
predetermined airflow
level. According to an aspect, the microprocessor 50 activates the stimulator
if the noise detector
35 determines that the user's actual noise and/or vibrations during sleep are
above the
predetermined noise level.
[0060] As illustrated in FIGS. 2-4 and in an embodiment,
the oral appliance 10 includes a
data recorder 60. The data recorder 60 may be positioned at, for instance, the
buccal portion 23
of the mouthpiece 20, (see, for instance, FIG. 2). According to an aspect and
as illustrated in
FIG.3, the data recorder 60 is positioned at the lingual portion 24 of the
mouthpiece 20. In an
embodiment, the data recorder 60 is configured to receive and/or store
information provided
from the microprocessor 50. According to an aspect, the data recorder 60
receives and/or stores
the actual oxygen saturation level of hemoglobin, the predetermined force
level of the user
applied to the occlusal and/or bite surfaces and/or the predetermined airflow
level, as provided
by the oxygen sensor 30, the pressure sensors 32 and the airflow sensor 34,
respectively. The
data recorder 60 may also receive and/or store stimulation information
regarding the quantity
and/or frequency of stimulations provided by the stimulator 40. The data
recorder 60 may also
store pharmaceutical compound dispensing information such as the volume/dosage
of
pharmaceutical dispensed from reservoir(s) 42 at each dispensing event and the
total volume
dispensed and, thus, remaining in reservoir(s) 42. This remaining
pharmaceutical compound data
may be used in signaling user as to replacement of reservoir(s) 42.
[0061] According to an aspect, the appliance 10 includes a
transceiver (not shown). The
transceiver may be configured to remotely monitor any additional components
provided on
and/or within the mouthpiece 20. In an embodiment, the transceiver may be
configured for use
with a customized web-based application for a handheld wireless communication
device. The
customized web-based application may include features such as, a graph of the
user's sleep
position and chart and/or graphical data related to oxygen saturation levels
of hemoglobin and
the pressure applied to occlusal surfaces of the user's dentition. According
to an aspect, the
customized web-based application may include data related to the user's heart
rate. In an
embodiment, the transceiver communicates with handheld wireless communication
devices
having Bluetooth capabilities. The transceiver may be communicable with
handheld wireless
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
communication devices, such as, for example, computers, smart watches, smart
phones, and the
like.
[0062] The oral appliance 10 may include a battery 70.
While it is contemplated that the
battery 70 is rechargeable, it may be disposable. The battery 70 may be
configured to provide
power to at least one of the oxygen sensor 30, the pressure sensor 32, the
airflow sensor 34, the
noise detector 35, the actigraphy sensor 36, the stimulator 40, the
microprocessor 50, the data
recorder 60 and the transceiver. According to an aspect, the battery 70
includes an energy store
and a contact element sealably arranged on the mouthpiece 20 (not shown). In
an embodiment,
the battery 70 is embedded within the mouthpiece 20, such that the battery 70
is not exposed to
liquids, saliva and/or oral tissue. The battery 70 may be positioned near the
buccal portion 23
(see, for instance, FIG. 2). According to an aspect, the battery 70 is
positioned near the lingual
portion 24 (see, for instance FIG. 4) of the mouthpiece 20.
[0063] As illustrated in FIG. 5, the oral appliance may
include a data transfer pod 80. The
data transfer pod 80 may be configured to charge and/or provide power to the
rechargeable
battery 70. According to an aspect the data transfer pod 80 is configured to
retrieve and/or store
information collection by the data recorder 60, such that the user and or
medical provider can
track and/or assess the collected information. According to an aspect, the
transceiver may
include power amplifiers (not shown) configured to reduce power requirements
of the oral
appliance 10, thereby helping to conserve life of the rechargeable battery 70.
The data transfer
pod 80 may be provided with an electrical contact component accessible to a
plug of a power
supply unit (not shown).
[0064] As illustrate in FIG. 5 and in an embodiment, an
oral appliance kit 100 for treatment
of sleep apnea in a user is provided. In an embodiment, the oral appliance kit
100 includes the
oral appliance 10, including the various electronic components, as
substantially described above
and illustrated in FIGS. 2-4, and the data transfer pod 80.
[0065] FIG. 6 is a flowchart illustrating an exemplary
operation 200 of the oral appliance 10.
Optionally, a customized mouthpiece is created 201 and various electronic
components are
assembled to form the oral appliance. The mouthpiece of the oral appliance is
positioned 210 in
the user's oral cavity. Oxygen sensors measure 220 oxygen saturation levels of
the users
hemoglobin, pressure sensors measure 222 the pressure applied to occlusal
surfaces of the
customized mandibular mouthpiece, airflow sensors measure 224 the actual
airflow and/or
21
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
breathing rate of the user, actigraphy sensors measure 226 data related to
sleep activity, including
sleep position and movement of the user during sleep and/or noise detectors
measure 228 the
actual noise and/or vibrations created by the user during sleep. The
microprocessor collects,
records and analyzes data 230 relating to oxygen saturation, pressure,
airflow, sleep activity and
actual noise levels. In the event that actual oxygen saturation levels of
hemoglobin are below a
predetermined level or in the event that the actual pressure applied to the
occlusal portion of the
mouthpiece is above the predetermined pressure level, the stimulator sends
impulses 240 to
stimulate the genioglossus muscle of the user's tongue. The oxygen sensors re-
measure 250 the
oxygen saturation level of hemoglobin, the pressure sensor re-measures 252 the
pressure applied
to occlusal surfaces of the customized mandibular mouthpiece, the airflow
sensors re-measure
254 actual airflow of the user, the actigraphy sensors re-measure 256 the
user's sleep activity,
and the noise detector re-measures 258 the actual noise and/or vibrations
created by the user
during sleep. Stimulation is stopped if the predetermined levels are achieved.
According to an
aspect If the predetermined levels are not achieved, stimulation continues,
increases, decreases or
otherwise varies according to the measured values.
[0066] According to an aspect, an upper mouthpiece 120 is
configured to be secured to /
worn on the user's upper dentition. As illustrated in FIG. 7, the mouthpiece
120 includes
dentition attachment members 128 (or collectively, a dentition attachment
portion 128 which has
a generally arch shape of an upper dentition). The dentition attachment
portion 128 has a buccal
surface / wall 130a facing the user's lips and/or cheeks, and a lingual
surface / wall 130b
opposite the buccal surface 130a facing the user's tongue. The mouthpiece 120
further includes
a palate portion 122 adjacent to and integrally connected with the lingual
surface 130b of the
dentition attachment portion 128. The mouthpiece 120 also includes a gum
portion 132 (shown
in FIGS. 8A-9) integral with and extending upwardly from the buccal surface
130a of the
dentition attachment portion 128, such that the gum portion 132 lies along the
user's upper gum
adjacent to the user's maxillary bone. The dentition attachment portion 128,
palate portion 122,
and gum portion 132 are integral parts of a unitary body.
[0067] The gum portion 132, dentition attachment portion
128, and mouthpiece 120 overall
can each be described (when viewed in top plan view) as having a left side /
portion / wing 134a
(i.e., generally positioned on the user's left dentition), a right side /
portion / wing 134b (i.e.,
generally positioned on the user's right dentition), an anterior portion or
end 136a (i.e., generally
22
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
positioned on the user's front / anterior dentition), and a posterior portion
or end 136b (i.e.,
generally positioned on the user's back / posterior dentition). The palate
portion 122 thus
extends between and is partially surrounded by the left side of the dentition
attachment portion
and the right side of the dentition attachment portion.
[0068] As shown in FIG. 7, the dentition attachment members
128 (each and collectively)
have a shape and size that substantially conforms to the upper dentition of
the user. The palate
portion 122 of the upper mouthpiece 120 substantially conforms to the palate
of the user. Thus,
the user is presented with a mouthpiece 120 having a secured and customized
fit. The dentition
attachment members 128 may be provided with a wire-frame form for support or,
as shown in
FIG. 7, support may stem from the presence of the palate portion 122 and the
material chosen for
forming the upper mouthpiece 120. According to an aspect, a portion of the
dentition attachment
members 128 may be shaped to form a retention loop around one or more teeth of
the user. The
dentition attachment members 128 and palate portion 122 render the upper
mouthpiece 120
capable of being at least temporarily fixed in place by virtue of the
customized fit. As such, the
mouthpiece 120 may provide a retention function thereby allowing it to remain
in place during
the user's sleep, particularly in situations where the user may make slight to
moderate
movements during sleep and/or when the user may be awake.
[0069] Thus, the mouthpiece 120 may be substantially
immovable unless positive effort is
applied to remove the mouthpiece 120. In other words, the user may remove the
mouthpiece 120
at any time, if desired, by exerting a little pressure to remove the
mouthpiece 120. Since the
mouthpiece 120 is not permanently affixed to the dentition, it can be worn
and/or subsequently
removed by the user at any time.
[0070] As with mouthpiece 20, components may be positioned
on and/or embedded within
the upper mouthpiece 120. Components in or on the upper mouthpiece 120 may
include any of
the components associated with the mouthpiece 20. The components illustrated
in FIG. 7 include
a first pair of electrodes 112a, 112b, a second pair of electrodes 114a, 114b,
a microprocessor
118 and electrical leads 116 connecting each electrode to the microprocessor
118. The
microprocessor 118 may have a data recorder 60 and/or a battery 70 associated
and integral
therewith that can be embedded within the upper mouthpiece 120, such that any
component on
any surface of mouthpiece 120 is covered to eliminate tissue damage and damage
to the
components. Placement of the components should not interfere with the fit of
the upper
23
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
mouthpiece 120 in the user's mouth or irritate user's gums or palate.
Electrical leads 116 are also
either embedded in the material of the mouthpiece 120 or, if placed on the
surface of the
mouthpiece, covered to eliminate a number of readily apparent issues with
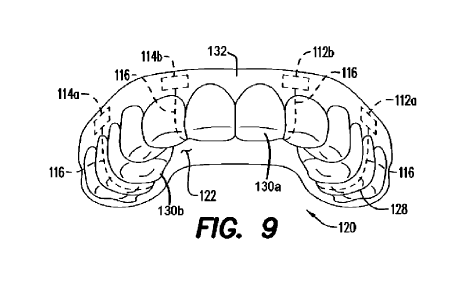
loose leads. FIG. 9
illustrates how the electrical leads 116 follow the shape of the upper
mouthpiece 120, either
within or on top of the mouthpiece material, from each electrode to the
microprocessor 118.
[0071] The microprocessor 118 of the upper mouthpiece 120
may also be provided with a
wireless transceiver that enables it, like the oral appliance 10, to
communicate with external
wireless communication devices, such as, for example, computers, smart
watches, smart phones,
and the like. In addition, the wireless transceivers of the upper mouthpiece
120 and oral
appliance 10 may communicate with one another. Thus, microprocessor 118 of the
upper
mouthpiece 120 may supply information to the oral appliance 10 is much the
same way that the
oral appliance receives information from external sources as well as its
constituent components.
Alternatively, it is contemplated that the upper mouthpiece 120 and oral
appliance 10 may be
wired together in a manner that would not be inconvenient or uncomfortable to
the user.
[0072] FIG. 7 shows an arrangement where the first
electrode pair 112a, 112b, the second
electrode pair 114a, 114b and microprocessor 118 are all located in or on the
palate portion 122
of the upper mouthpiece 120. Electrical leads 116 convey the electrical
signals from each of the
electrodes 112; 112b, 114; 114b to the microprocessor 118. Although there is
ample room
toward the posterior portion of the upper mouthpiece 120 palate portion 122,
it may be difficult
to obtain a strong electroencephalograph signal when electrodes are positioned
solely underneath
the palate portion.
[0073] FIGS. 8A and 8B show an arrangement where the first
electrode pair 112a, 112b and
the second electrode pair 114a, 114b are located on the gum portion 132 of the
upper mouthpiece
120 adjacent the buccal side of the maxillary bone of the user. That is, the
first electrode pair
112; 112b and the second electrode pair 114; 114b are located between the
upper gums and
inner lip/cheek of the user. The upper jaw, which includes the maxillary bone
of the user, is a
continuous extension of the skull bone, i.e., on the underside of the brain.
Thus, electrodes in
intimate contact with the upper jawbone (i.e., the bone in contact with the
inside of the cheeks
and upper lip of the user), on the buccal side, of the user will receive brain
waves in the same
manner as the scalp. Electrodes 112a and 114a (e.g., the left side electrodes)
are positioned
along the left side of the gum portion 132 / mouthpiece 120 (i.e., along the
left buccal side of the
24
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
maxillary bone of the user) and their paired electrodes 1126 and 1146 (e.g.,
the right side
electrodes) are positioned along the right side of the gum portion 132 /
mouthpiece 120 (i.e.,
along the right buccal side of the maxillary bone of the user). The locations
of the electrodes in
FIGS. 8A and 8B permit a good EEG signal, which can be clearly read and
interpreted, to be
achieved by the electrodes and microprocessor 118. The microprocessor 118,
though it cannot
be seen in FIGS. 8A and 8B, may be located in or on the palate portion 122 of
the upper
mouthpiece 120, i.e., in approximately the same location on the palate portion
122 as in FIG. 7.
Electrical leads 116 convey the electrical signals from each of the electrodes
112a, 112b, 114a,
114b to the microprocessor 118 by the most direct route along or within the
material of upper
mouthpiece 120. For example, the electrical leads 116 may follow the contour
of the dentition
attachment member 128 down, over and then up to the palate portion 122, where
the electrical
leads 116 then proceed to the microprocessor 118. The path of the electrical
leads in FIGS. 8A
and 8B are similar to those in FIG. 9, and FIG. 9 illustrates these paths more
clearly.
[0074] FIG. 9 shows an arrangement where the first
electrode pair 112a, 112b and the second
electrode pair 114a, 114b are, like in FIGS. 8A and 8B, located on the gum
portion 132 of upper
mouthpiece 120 adjacent the buccal side of the maxillary bone of the user.
Electrodes 112a and
114a (e.g., the posterior electrodes) are positioned along the posterior
portion of the gum portion
132 (and the mouthpiece 120) and their paired electrodes 112b and 114b (e.g.,
the anterior
electrodes) are positioned along the anterior portion of the gum portion 132
(and the mouthpiece
120). The locations of the electrodes in FIG. 9 permit a good EEG signal to be
achieved by the
electrodes and microprocessor 118. The microprocessor 118, though it cannot be
seen in FIG. 9,
is located in or on the palate portion 122 of the upper mouthpiece 120, i.e.,
in approximately the
same location on the palate portion 122 as in FIG. 7. Electrical leads 116
convey the electrical
signals from each of the electrodes 112a, 112b, 114a, 114b to the
microprocessor 118 by the
most direct route along or within the material of upper mouthpiece 120. That
is, the electrical
leads 116 will follow the contour of the dentition attachment members 128
down, over and then
up to the palate portion 122, where the electrical leads 116 then proceed to
the microprocessor
118.
[0075] With any of the electrode arrangements of electrodes
shown in FIGS. 7, 8A, 8B and
9, each of first electrode pair 112a, 112b and second electrode pair 114a,
114b may, along with
the microprocessor 118, comprise an electroencephalograph of sufficient
sensitivity to detect
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
rhythm changes in electrical signals in the brain. The electrodes 112a, 1126
and 114a, 1146
register voltage differences between the paired "a" electrode and "b"
electrode generated by
electrical signals in the brain. The signals detected by the electrodes are
provided, via the
electrical leads 116, to a signal processor that is part of the microprocessor
118.
[0076] As with any electroencephalograph, some
mental/consciousness states result in the
voltage differences detected between first electrode pair 112a, 112b and the
second electrode pair
114a, 114b being rhythmic, shown as waves on a graph by the recording channel.
The EEG
signal for conscious adult in a relaxed state will typically be an oscillating
wave known as an
alpha wave. A sleeping adult's brain waves become extremely slow. These slow
waves are
referred to as delta waves and may be utilized not only to identify sleep but
also to assess the
depth of sleep. That is, varying strength of the delta waves can be indicative
of a deeper sleep
state. The signal processor and other microprocessor 118 components are able
to differentiate
various EEG rhythms and to determine, based on these detected rhythms, the
various
consciousness and sleep states of the user. It is contemplated that the
mouthpiece 120 may be
able diagnose epilepsy by way of recording the user's brain activity ¨ such
brain activity may be
collected by the data recorder 60.
[0077] FIG. 10 is a flowchart illustrating an exemplary
operation 300 of the upper
mouthpiece 120 in cooperative communication with the oral appliance 10.
Optionally, a
customized upper mouthpiece 120 and oral appliance 10 mouthpiece are created
201 and various
electronic components are assembled to form the full cooperative appliance.
The upper
mouthpiece 120 and mouthpiece portion of oral appliance 10 are positioned 210
in the user's oral
cavity. The first electrode pair 112a, 112b and the second electrode pair
114a, 114b collect
voltage data and the microprocessor assesses an EEG rhythm 212 from this data.
The upper
mouthpiece microprocessor 118 may utilize the EEG rhythm to assess the sleep
state 214 of the
user and a determination of sleep state is communicated to the oral appliance
10. Alternatively,
the raw EEG rhythm may be communicated to the oral appliance 10 for assessment
at step 230.
In the event that the EEG data indicates that the user may be having an
arousal from sleep or has
exited a sleep state in an untimely manner, the microprocessor of the oral
appliance may cause
the stimulator to send impulses 240 to stimulate the genioglossus muscle of
the user's tongue.
The upper mouthpiece 120 is constantly assessing the EEG rhythms 262 and sleep
state 264 of
the user and providing data to the oral appliance. Stimulation is stopped if
the appropriate sleep
26
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
states are achieved. According to an aspect, if the predetermined EEG rhythms
are not regained,
stimulation continues, increases, decreases or otherwise varies according to
the measured values.
[0078] The flowchart shown in FIG. 10 may be utilized
independently of or in combination
with the flowchart shown in FIG. 6. Thus, the EEG data may be used in
combination with or
supplemental to oxygen sensors measure 220, pressure sensors measure 222,
airflow sensors
measure 224, actigraphy sensors measure 226 and/or noise detectors measure
228.
100791 The various upper mouthpieces described above (e.g.,
upper mouthpiece 120
described in connection with FIGS. 7, 8A, 8B, and 9) may find use in a variety
of other
applications. For example, the mouthpiece may be a mouthguard suitable for use
in athletic
activities. In such an application, the mouthpiece may be used to assess
potential medical
conditions or injuries, such as concussions or other head trauma. The data
and/or results can be
delivered via Bluetooth to a smart device or can be delivered to a remote
application via the
intermit (e.g., a cloud application). Other possibilities are contemplated, as
will be understood by
those of skill in the art. Such data may also be useful in generally studying
head trauma that
occurs in athletics.
[0080] As another example, the various upper mouthpieces
may find use in hobbyist or
gaming applications, such as personal meditation devices, virtual reality
games, video games,
learning / educational devices, or other personal activities that center
around brain activity.
[0081] The upper mouthpieces may be used with or without a
lower mouthpiece, such as
mouthpiece 10.
[0082] The components of the apparatus illustrated are not
limited to the specific
embodiments described herein, but rather, features illustrated or described as
part of one
embodiment can be used on or in conjunction with other embodiments to yield
yet a further
embodiment. It is intended that the apparatus include such modifications and
variations. Further,
steps described in the method may be utilized independently and separately
from other steps
described herein.
[0083] While the apparatus and method have been described
with reference to specific
embodiments, it will be understood by those skilled in the art that various
changes may be made
and equivalents may be substituted for elements thereof without departing from
the scope
contemplated. In addition, many modifications may be made to adapt a
particular situation or
material to the teachings found herein without departing from the essential
scope thereof.
27
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
100841 In this specification and the claims that follow,
reference will be made to a number of
terms that have the following meanings. The singular forms "a," "an" and "the"
include plural
referents unless the context clearly dictates otherwise. Furthermore,
references to "one
embodiment", "some embodiments", "an embodiment" and the like are not intended
to be
interpreted as excluding the existence of additional embodiments that also
incorporate the recited
features. Approximating language, as used herein throughout the specification
and claims, may
be applied to modify any quantitative representation that could permissibly
vary without
resulting in a change in the basic function to which it is related.
Accordingly, a value modified
by a term such as "about" is not to be limited to the precise value specified.
In some instances,
the approximating language may correspond to the precision of an instrument
for measuring the
value. Terms such as "first," "second: "upper," "lower" etc. are used to
identify one element
from another, and unless otherwise specified are not meant to refer to a
particular order or
number of elements.
[0085] As used herein, the terms "may" and "may be"
indicate a possibility of an occurrence
within a set of circumstances; a possession of a specified property,
characteristic or function;
and/or qualify another verb by expressing one or more of an ability,
capability, or possibility
associated with the qualified verb. Accordingly, usage of "may" and "may be"
indicates that a
modified term is apparently appropriate, capable, or suitable for an indicated
capacity, function,
or usage, while taking into account that in some circumstances the modified
term may sometimes
not be appropriate, capable, or suitable. For example, in some circumstances
an event or capacity
can be expected, while in other circumstances the event or capacity cannot
occur - this
distinction is captured by the terms "may" and "may be."
[0086] As used in the claims, the word "comprises" and its
grammatical variants logically
also subtend and include phrases of varying and differing extent such as for
example, but not
limited thereto, "consisting essentially of' and "consisting of" Where
necessary, ranges have
been supplied, and those ranges are inclusive of all sub-ranges therebetween.
It is to be expected
that variations in these ranges will suggest themselves to a practitioner
having ordinary skill in
the art and, where not already dedicated to the public, the appended claims
should cover those
variations.
[0087] Advances in science and technology may make
equivalents and substitutions possible
that are not now contemplated by reason of the imprecision of language; these
variations should
28
CA 03156309 2022-4-27
WO 2021/091583
PCT/US2020/016597
be covered by the appended claims This written description uses examples to
disclose the
method, machine and computer-readable medium, including the best mode, and
also to enable
any person of ordinary skill in the art to practice these, including making
and using any devices
or systems and performing any incorporated methods. The patentable scope
thereof is defined by
the claims, and may include other examples that occur to those of ordinary
skill in the art. Such
other examples are intended to be within the scope of the claims if they have
structural elements
that do not differ from the literal language of the claims, or if they include
equivalent structural
elements with insubstantial differences from the literal language of the
claims.
29
CA 03156309 2022-4-27