Note: Descriptions are shown in the official language in which they were submitted.
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
SYRINGE ADAPTER FOR MEDICATION
BACKGROUND
[0001] The present invention relates to improved apparatus for use with
medication, and
method(s) of using same, particularly for higher-viscosity medication.
[0002] Medication is needed for various purposes, including illness treatment
and illness
prevention.
BRIEF SUMMARY
[0003] The present invention is directed to improved apparatus for use with
medication, and
method(s) of using same, and is particularly useful for medication having a
relatively high
viscosity. In one aspect, a syringe adapter for withdrawing fluid medication
from a container
comprises a sidewall extending between a proximal end and a distal end, the
sidewall having
an interior surface defining a chamber, the proximal end configured to be
connected to a
syringe while withdrawing at least a portion of the fluid medication from the
container
through the chamber and into a barrel of the syringe and the distal end
configured for
inserting into the container for the withdrawal, wherein an opening at the
distal end is
relatively large in diameter to facilitate withdrawing fluid medication having
a relatively high
viscosity and the syringe adapter is configured to be removed from the syringe
and replaced
with a needle prior to subsequently injecting (for example, into an animal)
the fluid
medication (or at least some portion thereof) withdrawn into the barrel. The
relatively large
opening is directed toward improved syringeability of the fluid medication.
The viscosity of
the fluid medication is preferably greater than or equal to 50 centipoise
units when a
temperature of the fluid medication is at least 5 degrees Celsius.
[0004] The syringe is preferably configured as a pistol-grip syringe or a tab-
handled syringe,
1
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
and may therefore provide improved leverage for the subsequent injection. In
an
embodiment, the diameter of the opening at the distal end of the syringe
adapter is preferably
about 0.05 inches to about 0.1585 inches, and more preferably, this inner
diameter is about
0.078 inches to about 0.14 inches, and even more preferably, is about 0.09
inches to about
0.11 inches, and generally, is approximately 0.10 inches. In an embodiment,
the sidewall is
approximately 0.05 inches in thickness at the distal end. Optionally, the
syringe adapter
further comprises a flanged area that extends laterally from the proximal end.
Optionally, the
syringe adapter may further comprise a radial extension member that extends
perpendicularly
and radially outward from an exterior surface of the syringe adapter. In an
embodiment, an
outer shape of the syringe adapter is generally conical in a first portion and
generally
cylindrical in a second portion. In an embodiment, an inner shape of the
syringe adapter, for
at least a portion of the proximal end, is generally conical. In an
embodiment, the inner shape
of the syringe adapter tapers from the proximal end toward the distal end, for
at least a
portion of the proximal end, at approximately 6 percent. The syringe adapter
preferably
connects to the syringe using a Luer-type connection, the Luer-type connection
selected from
the group consisting of a Luer-type lock and a Luer-type slip.
[0005] In another aspect, a method of administering fluid medication (for
example, to an
animal) comprises: affixing a syringe adapter to a syringe, the syringe
adapter comprising a
sidewall extending between a proximal end and a distal end, the sidewall
having an interior
surface defining a chamber, the proximal end configured to be connected to a
distal end of the
syringe; inserting the distal end of the syringe adapter into a container of
fluid medication
having a relatively high viscosity; withdrawing, from the container, at least
a portion of the
fluid medication through the chamber and into a barrel of the syringe, wherein
an opening at
the distal end of the syringe adapter is relatively large in diameter to
facilitate withdrawing
2
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
the relatively-high-viscosity medication; removing the syringe adapter from
the syringe
subsequent to the withdrawing; affixing a needle to the distal end of the
syringe, subsequent
to the removing; and injecting (for example, into an animal) the fluid
medication (or at least
some portion thereof) previously withdrawn into the barrel. The distal end
opening is
preferably about 0.05 inches to about 0.1585 inches, and more preferably, is
about 0.078
inches to about 0.14 inches, and even more preferably, is about 0.09 inches to
about 0.11
inches, and generally, is approximately 0.10 inches.
[0006] In yet another aspect, the syringe adapter is configured for receiving
a needle at its
distal end, such that the needle is affixed to the distal end of the syringe
adapter subsequent to
withdrawing fluid medication into the barrel of the syringe, and the syringe
adapter is
configured to remain in place while injecting the fluid medication (or at
least some portion
thereof) into a recipient with the needle. In this aspect, administering the
fluid medication
may be repeated (for example, for another recipient) by removing the needle,
using the in-
place syringe adapter for withdrawing more fluid medication (from the same or
a different
container), re-affixing the needle to the syringe adapter, and then injecting
this medication (or
some portion thereof). In this aspect, the distal end of the syringe adapter
preferably provides
for a Luer-type connection with the needle, and the proximal end of the
syringe adapter is
preferably configured with a Luer-type locking member for connecting to the
syringe. The
syringe adapter may further comprise an extension member that extends
perpendicularly
outward from an exterior surface of the syringe adapter.
[0007] In still another aspect, a syringe adapter for withdrawing viscous
fluid medication
from a container comprises a sidewall extending between a proximal end and a
distal end
opposite the proximal end, the sidewall having an interior surface defining a
chamber, a first
opening at the distal end being defined by a first end of the sidewall and a
second opening at
3
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
the proximal end being defined by a second end of the sidewall. In this
aspect, the proximal
end is configured to be connected to a distal end of a syringe, the distal end
of the syringe
having a third opening; the distal end of the sidewall of the syringe adapter
is configured to be
inserted into a container of viscous fluid medication; the first opening
provides for fluid
communication of the viscous fluid medication from the container into the
chamber; the
second opening provides for fluid communication of the viscous fluid
medication between
the chamber and, through the third opening, the barrel of the syringe; and the
first opening has
an inner diameter sized to facilitate withdrawing at least a portion of the
viscous fluid
medication through the first opening and into the chamber and then into the
barrel. The inner
diameter of the first opening is preferably about 0.05 inches to about 0.1585
inches, and more
preferably, is about 0.078 inches to about 0.14 inches, and even more
preferably, is about
0.09 inches to about 0.11 inches, and generally, is approximately 0.10 inches.
Preferably, at
least a portion of the viscous fluid medication is caused to be withdrawn from
the container,
through the first opening and into the chamber, and then through the second
and third
openings into the barrel, upon activation of a drawing mechanism of the
syringe while the
proximal end of the syringe adapter is connected to the syringe and while the
distal end of the
syringe adapter is inserted into the container.
[0008] In a further aspect, a method of withdrawing viscous fluid medication
from a
container comprises: affixing a syringe adapter to a syringe, the syringe
adapter comprising a
sidewall extending between a proximal end and a distal end opposite the
proximal end, the
sidewall having an interior surface defining a chamber, a first opening at the
distal end being
defined by a first end of the sidewall and a second opening at the proximal
end being defined
by a second end of the sidewall, the proximal end configured to be connected
to a distal end
of the syringe; inserting the distal end of the sidewall of the syringe
adapter into a container of
4
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
viscous fluid medication; and withdrawing, from the container, at least a
portion of the
viscous fluid medication through the first opening and into the chamber, and
from the
chamber through the second opening and then through a third opening in the
distal end of the
syringe and into a barrel of the syringe, wherein the first opening has an
inner diameter sized
to facilitate the withdrawing. The inner diameter of the first opening is
preferably about 0.05
inches to about 0.1585 inches, and more preferably, is about 0.078 inches to
about 0.14
inches, and even more preferably, is about 0.09 inches to about 0.11 inches,
and generally, is
approximately 0.10 inches. In this aspect, the syringe adapter is preferably
configured to be
removed from the syringe subsequent to the withdrawing and replaced by
affixing a needle to
the distal end of the syringe to thereby enable injecting, into a recipient
with the needle, at
least a portion of the viscous fluid medication withdrawn into the barrel.
[0009] In another aspect, the needle is removably affixed to a needle holder
that, in turn, is
removably affixed to the in-place syringe adapter for the injection. In an
embodiment, the
syringe adapter is configured with a support hub member for removably
receiving the needle
holder, the support hub member radially / laterally surrounding at least a
portion of a length
of a sidewall of the syringe adapter, the sidewall extending between a
proximal end and a
distal end opposite the proximal end, the sidewall having an interior surface
defining a
chamber, a first opening at the distal end being defined by a first terminal
end of the sidewall
and a second opening at the proximal end being defined by a second terminal
end of the
sidewall, wherein: a proximal end of the support hub member is configured for
slipping over
an exterior of a syringe tip located at a distal end of a syringe, the syringe
tip having a third
opening in a protrusion extending distally therefrom; the proximal end of the
sidewall is
configured to be connected to an internal threaded portion of the syringe tip,
and to receive
the protrusion within the chamber, while the proximal end of the support hub
member is
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
slipped over the exterior; the distal end of the sidewall is configured for
inserting into a
container of viscous fluid medication; the first opening provides for fluid
communication of
the viscous fluid medication from the container into the chamber; the second
opening
provides for fluid communication of the viscous fluid medication between the
chamber and,
through the third opening, a barrel of the syringe; the first opening has an
inner diameter sized
to facilitate withdrawing at least a portion of the viscous fluid medication
through the first
opening and into the chamber and then into the barrel; and a distal end of the
support hub
member, located opposite the proximal end of the support hub member, is
configured for
removably receiving a proximal end of a needle holder, the needle holder
further comprising
a distal end opposite the proximal end of the needle holder, the distal end of
the needle holder
adapted for removably affixing thereto a needle. The inner diameter of the
opening at the
distal end of the syringe adapter is at least about 0.05 inches, and is
preferably about 0.05
inches to about 0.1585 inches, and more preferably, this inner diameter is
about 0.078 inches
to about 0.14 inches, and even more preferably, is about 0.09 inches to about
0.11 inches, and
generally, is approximately 0.10 inches. In another embodiment, a syringe
adapter for
withdrawing viscous fluid medication comprises a support hub member radially /
laterally
surrounding an inner chamber, the syringe adapter further comprising an
extension from a
proximal end of the support hub member and a sidewall extending from a distal
end of the
support hub member, the distal end of the support hub member being opposite
the proximal
end of the support hub member, the inner chamber extending through the
sidewall and the
extension, a terminal end of the sidewall defining a first opening into the
inner chamber and a
terminal end of the extension defining a second opening into the inner
chamber, wherein: a
proximal end of the syringe adapter is affixed to a distal end of a syringe by
inserting, into a
cavity at the distal end of the syringe, the extension from the proximal end
of the support hub
6
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
member, wherein the cavity defines a third opening into the syringe; the
terminal end of the
sidewall is configured for inserting into a container of viscous fluid
medication; the first
opening provides for fluid communication of the viscous fluid medication from
the container
into the inner chamber; the second opening provides for fluid communication of
the viscous
fluid medication between the inner chamber and, through the third opening, a
barrel of the
syringe; the first opening has an inner diameter sized to facilitate
withdrawing at least a
portion of the viscous fluid medication through the first opening and into the
inner chamber
and then into the barrel; the distal end of the support hub member is
configured for removably
receiving a proximal end of a needle holder, the needle holder further
comprising a distal end
opposite the proximal end of the needle holder, the distal end of the needle
holder adapted for
removably affixing thereto a needle. The inner diameter of the first opening
is at least about
0.05 inches, and is preferably about 0.05 inches to about 0.1585 inches, and
more preferably,
is about 0.078 inches to about 0.14 inches, and even more preferably, is about
0.09 inches to
about 0.11 inches, and generally, is approximately 0.10 inches. In this
aspect, the needle
preferably remains affixed to the needle holder following an injection,
whereby the needle
may be removed from the syringe adapter (for example, in preparation for using
the syringe
adapter for withdrawing additional fluid medication from a container) by
removing, as a
single unit, the needle holder and the needle affixed thereto, and the needle
holder preferably
affixes to the distal end of the support hub member of the syringe adapter
using a Luer-type
locking connection.
[0010] In an aspect, a method of administering fluid medication comprises:
inserting a distal
end of a syringe adapter into a container of viscous fluid medication, the
syringe adapter
being affixed to a syringe, the syringe adapter comprising a support hub
member radially /
laterally surrounding at least a portion of a length of a sidewall, the
sidewall extending
7
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
between a proximal end and the distal end (the distal end being opposite the
proximal end)
and having an interior surface defining a chamber, a first opening at the
distal end being
defined by a first terminal end of the sidewall and a second opening at the
proximal end being
defined by a second terminal end of the sidewall, the syringe adapter
configured to be affixed
to the syringe by slipping a proximal end of the support hub member over an
exterior of a
syringe tip located at a distal end of the syringe, the syringe tip having a
third opening in a
protrusion extending distally therefrom, and connecting the proximal end of
the sidewall to an
internal threaded portion of the syringe tip; withdrawing, from the container,
at least a portion
of the viscous fluid medication through the first opening and into the chamber
and then
through the second and third openings into a barrel of the syringe; affixing a
proximal end of
a needle holder to a distal end of the support hub member, subsequent to the
withdrawing
from the container, the needle holder adapted for removably affixing a needle
to a distal end
thereof; and injecting, into a recipient with the needle affixed to the needle
holder, at least a
portion of the viscous fluid medication previously withdrawn into the barrel.
An inner
diameter of the first opening is at least about 0.05 inches, and is preferably
about 0.05 inches
to about 0.1585 inches, and more preferably, is about 0.078 inches to about
0.14 inches, and
even more preferably, is about 0.09 inches to about 0.11 inches, and
generally, is
approximately 0.10 inches.
[0011] Various embodiments of these and other aspects of the present invention
may be
provided in view of the present disclosure. It should be noted that the
foregoing is a summary
and thus contains, by necessity, simplifications, generalizations, and
omissions of detail;
consequently, those of ordinary skill in the art will appreciate that the
summary is illustrative
only and is not intended to be in any way limiting. Other aspects, inventive
features, and
advantages of the present invention, as defined by the appended claims, will
become apparent
8
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
in the non-limiting detailed description set forth below.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0012] The present invention will be described with reference to the following
drawings, in
which like reference numbers denote the same element throughout.
[0013] FIGs. 1 - 3 depict examples of prior art syringes;
[0014] FIG. 4 depicts an example of a prior art needle;
[0015] FIGs. 4A and 4B illustrate bottom views showing how a proximal end of a
needle
may be configured for securable attachment to a syringe;
[0016] FIGs. 5 - 6 illustrate first and second preferred embodiments of the
syringe adapter
disclosed herein;
[0017] FIGs. 7 - 8 illustrate alternative embodiments of the syringe adapter
disclosed herein;
[0018] FIG. 9 illustrates a still further embodiment of the syringe adapter
disclosed herein;
[0019] FIG. 10 illustrates a syringe adapter placed upon a syringe, and FIG.
11 illustrates a
needle placed upon a syringe adapter;
[0020] FIGs. 12 and 13 illustrate yet other embodiments of the syringe adapter
disclosed
herein, and also illustrate placement thereof upon a syringe;
[0021] FIG. 14 (comprising FIGs. 14A through 14D) illustrates a further
embodiment of the
disclosed syringe adapter, showing exterior views as well as cross-sectional
views of
placement thereof upon a syringe and as exploded;
[0022] FIG. 15 (comprising FIGs. 15A through 15D) illustrates a still further
embodiment of
the disclosed syringe adapter that includes a needle holder to which a needle
may be
removably affixed, showing exterior views as well as cross-sectional views of
placement
thereof upon a syringe and as exploded;
[0023] FIGs. 16 (comprising FIGs. 16A through 16D) and 17 (comprising FIGs.
17A through
9
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
17D) illustrate yet other embodiments of the disclosed syringe adapter that
include a needle
holder to which a needle may be removably affixed, showing exterior views as
well as cross-
sectional views of placement thereof upon a syringe and as exploded, where
FIG. 17 shows a
syringe adapter having a sharp tip and both FIGs. 16 and 17 illustrate a
needle holder adapted
for a locking connection with the needle;
[0024] FIGs. 18 (comprising FIGs. 18A through 18D) and 19 (comprising FIGs.
19A through
19D) illustrate embodiments of the disclosed syringe adapter that replace a
conventional
syringe tip, showing exterior views as well as cross-sectional views of
placement thereof
upon a syringe and as exploded, and FIGs. 20 - 21 (comprising FIGs. 20A
through 20D and
21A through 21D, respectively) illustrate alternative approaches for a portion
of the syringe
adapters and needle holders shown in FIGs. 18 and 19; and
[0025] FIG. 22 presents tables containing measurements from tests conducted to
compare use
of a sample version of the disclosed syringe adapter to use of conventional
needles.
DETAILED DESCRIPTION
[0026] As noted earlier, medication is needed for various purposes, including
illness
treatment and illness prevention. Discussions are presented herein with
reference to
medication used for animals, primarily in terms of livestock animals; this is
by way of
illustration and not of limitation, however, and it should be noted that the
disclosed syringe
adapter may be beneficial with medication used for all types of animal life,
including humans.
[0027] Treatment of animals using medication may be desired whether the animal
is a family
pet, part of a livestock operation, is the subject of research, and so forth.
Examples of
medicating animals for illness treatment will be obvious, and may span a wide
variety of
illnesses. One example of medicating an animal for illness prevention is a
proactive
vaccination; another example is to proactively administer an antibiotic. In a
commercial
CA 03158155 2022-04-13
WO 2021/108257
PCT/US2020/061523
livestock operation, animals may be proactively medicated before they are
introduced into
another group of livestock, for example to guard against introducing an
illness that they may
carry or simply to ensure that all animals in the group have received an
identical medication
regimen. Medication might also be administered in anticipation of, or in
response to, a
change in weather conditions or a change in geographical location for an
animal (such as
moving from one climate to another). Hereinafter, animal medications are
discussed without
differentiation of the purpose for such medication.
[0028] Medication may be found in various forms, including solid and fluid.
Solid
substances may be ingestible, for example, while fluids may be injectable or
may be
administered orally or nasally. Embodiments of the present invention are
directed toward
improved apparatus for use with medication in fluid form, and the scope of the
present
invention also includes method(s) for using such apparatus.
[0029] Medications provided in fluid form may vary widely in their viscosity,
depending
upon their chemical formulation. Viscosity is sometimes defined as the
resistance of a
substance to flow. The viscosity of water is relatively low, for example,
while the viscosity
of honey is relatively high. The viscosity of some substances can be changed
by applying
heat; for example, melting butter increases its ability to flow. Some fluid
medications may
have a viscosity that is relatively low and is similar to that of water, for
example, and thus
will flow quite easily. Other fluid medications are known that have a
viscosity that is
markedly different from water.
[0030] Fluid medications intended for use with animals are commonly marketed
in multi-
dose packaging, such as bottles that hold enough fluid for administering
several doses. A
bottle of medication might hold 500 milliliters, for example (equivalently,
500 cubic
centimeters), which is roughly equivalent to 16.9 ounces. The bottle might be
made of glass
11
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
or plastic, and a container having a configuration other than a bottle might
be used.
Hereinafter, the term "bottle" is used for ease of reference, and by way of
illustration and not
of limitation, as a container type in which medication may be contained.
[0031] One reason for marketing animal medication in multi-dose bottles is
economic. The
cost of the medication may be reduced, for example, by selling a larger
quantity container and
thereby reducing the relative cost of the packaging. Another reason for
marketing animal
medication in multi-dose bottles is that the dosage of many (if not all)
medications is
prescribed with regard to the animal's body weight. Accordingly, the correct
amount of
medication to use on a particular animal can be calculated and then withdrawn
from the
multi-dose bottle, after which it may be injected into the animal, and the
remaining
medication is then available for subsequent use.
[0032] A multi-dose bottle of fluid medication is typically marketed with a
rubber membrane
covering at least a portion of an opening at the top of the bottle.
Conventionally, the fluid
medication is withdrawn from such bottle by placing a needle onto the tip of a
syringe,
inserting a tip of the needle into the rubber membrane, and withdrawing a
plunger of the
syringe until an appropriate amount of fluid is pulled into the syringe body
(referred to herein
as the syringe "barrel"); this same needle is then used for injecting the
medication from the
syringe into the animal. FIG. 1 shows an example of a prior art syringe 100,
and illustrates
how the hollow barrel 130 of syringe 100 is commonly marked with fill lines
110 that are
provided for measuring the amount of fluid contained therein. A needle is
placed over (or
inside) the tip 140, and fluid enters through an opening or eye of the needle
and into the
syringe barrel 130. The syringe includes a retractable plunger, a terminal end
of which is
shown at 120. (As will be obvious, as fluid medication is withdrawn from the
bottle into the
barrel 130, the plunger 120 movably extends outward from the proximal end of
the syringe
12
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
100, although this is not illustrated in FIG. 1.) Commonly, a syringe as
illustrated in FIG. 1 is
constructed of plastic, making it relatively cheap to purchase.
[0033] A tab-shaped member 150 is also provided on syringe 100. When
administering the
medication from the barrel 130, a person's index finger is placed on the tab-
shaped member
150 at one side of barrel 130 and the person's middle finger is placed on the
tab-shaped
member 150 at the opposing side of barrel 130, and the person's thumb is then
used to
depress the terminal end of plunger 120 into the barrel in order to expel the
medication from
the barrel.
[0034] As an alternative to the syringe 100 of FIG. 1, an example of a so-
called "pistol-grip"
syringe is illustrated in FIG. 2. Fluid medication is drawn into a syringe of
this type by
pulling plunger 220 outwardly from the barrel 230. A tab-shaped member is not
provided on
a syringe of this type, as compressing or squeezing the handles 210 serves to
expel
medication from the barrel of a syringe having a pistol-grip configuration.
[0035] FIG. 3 illustrates yet another prior art syringe 300, and is referred
to herein as a "tab-
handled" syringe. In this configuration, the syringe has a tabbed member 350
near the
proximal end of barrel 330, and includes a handle-style tabbed member 320
affixed to the
terminal end of the plunger. The tabbed member 350 is used in a similar manner
to tab-
shaped member 150 of FIG. 1, whereby a person places fingers on the tabbed
member 350 on
opposing sides of barrel 330; the person then presses down on tabbed member
320 using the
person's palm to depress the terminal end of the plunger into the barrel in
order to expel the
medication from the barrel. As will be readily understood, fluid medication is
drawn into a
syringe of this type by pulling handle 320 to thereby draw the attached
plunger outwardly
from the barrel 330. As compared to tab-shaped member 150 and plunger end 120
of FIG. 1,
the tabbed members 320, 350 of FIG. 3 typically provide improved comfort for
the person
13
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
using the tab-handled syringe.
[0036] The tips 240, 340 may be generally on the order of 3/8 to 7/16 inch in
diameter and
generally of similar height (and similarly, tip 140), and are generally
constructed of metal.
An interior area of this tip is intended for securably attaching a needle and
is generally
threaded for at least a portion thereof A height of this threaded area is
believed to be
generally on the order of 1/8 inch to 1/4 inch (and it is believed that a
height of 5.4
millimeters, or approximately 0.2125 inches, is used for syringe tip threads
that conform to
ISO 80369-7:2016, which is further discussed below). While not illustrated in
detail on tips
240, 340 of FIGs. 2 and 3, the syringe tip also typically includes a
protrusion (illustrated
herein in FIGs. 14D, 15D, 16D, and 17D; see reference number 341) that is
centered within
the exterior wall of the tip and that provides the opening through which a
substance enters
into the syringe barrel. (Notably, tips 140, 240, 340 are not designed for
inserting through the
rubber membrane of a medicine bottle.)
[0037] Syringes 200, 300 are often constructed, at least in part, of metal.
Glass or plastic
might be used for the syringe barrel. A metal commonly used for syringes, by
way of
example, is stainless steel; another example is aluminum.
[0038] FIG. 4 illustrates an example of a prior art needle 400, which may be
affixed to the
distal end of syringes 100, 200, or 300. Needles are typically sold in
standardized sizes, and
thus the distal syringe ends 140, 240, 340 typically conform to the standard
size of the
proximal end of a needle. FIGs. 4A and 4B illustrate bottom views showing
examples of how
a proximal end of needle 400 may be configured for securable attachment to the
distal end of
a syringe that has an internal threaded portion. In an approach 410 as shown
in FIG. 4A, a
flanged area 420 extends radially outward from the proximal end of the needle
(as is generally
illustrated in FIG. 4). Reference number 440 depicts the opening in the tip of
the needle, and
14
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
reference number 430 generally depicts the sidewall of needle 400. In another
approach 450
as shown in FIG. 4B, a flanged area 460 extends perpendicularly outward from
the proximal
end of the needle, but in this configuration, is fashioned as having tabs as
side edges that are
not generally round. (Note that it is believed that the flanged area 460 of a
needle extends
perpendicularly, although more generally, this will be understood as a
"lateral" extension.
Accordingly, references herein to a "perpendicular" extension for connecting
to a syringe
should be interpreted more generally as a "lateral" extension for making such
connection.)
Reference number 480 depicts the opening in the tip of the needle, and
reference number 470
generally depicts the sidewall of needle 400. In either case, a flanged area
420, 460 on the
proximal end of a needle is designed to securably attach to a corresponding
receiving area on
the distal end of a syringe. In yet another approach (not illustrated), the
securable attachment
of a needle to a syringe tip relies on friction instead of an exterior flanged
area, whereby the
proximal end of needle 400 is placed over an exterior of the distal end (e.g.,
tip 140 of FIG. 1)
of a syringe. These approaches are commonly referred to as a Luer-style lock
approach and a
Luer-style slip approach, respectively, as is discussed in further detail
below. (As is readily
understood, a Luer-style lock relies upon a threaded attachment of what are
commonly
denoted as "male" and "female" parts, which may be achieved by placing tabs as
lateral
extensions on one part, these tabs designed to rotatably descend within
corresponding threads
of the other part.) Note that if flanged area 420 is configured to extend
perpendicularly
outward as illustrated in FIG. 4A, it is preferably intended for use in a Luer-
type slip
connection rather than a Luer-type lock connection, due to the so-called
"double start" or
double helix configuration that is described for the internal threads of a
Luer-type lock hub
according to International Standards 594-1:1986 and ISO 594-2:1998(E) and
their
replacement ISO 80369-7:2016, which are discussed in further detail below. As
an
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
alternative, tabs may be added to the outer edge of flanged area 420, where
these tabs are
configured for engaging the internal threads of the Luer-type lock hub (and
specifications
pertaining to such tabs are provided in ISO 80369-7:2016).
[0039] For withdrawing fluid medication from a bottle into the barrel of
syringe 100, 200, or
300 using known techniques, the sharp tip at the distal end of the needle 400
is inserted
through the rubber membrane of the bottle. For subsequently administering the
fluid
medication from the barrel of the syringe, the sharp tip of that same needle
is inserted into an
animal's body, and the person holds tab-shaped member 150 while simultaneously
depressing
plunger 120 of syringe 100, squeezes the handles 210 of pistol-grip syringe
200, or holds
tabbed member 350 while simultaneously depressing handle-style tabbed member
320 of tab-
handled syringe 300.
[0040] This known approach of withdrawing fluid medication from a bottle using
a needle
and then administering the medication using the same needle works well for
fluids having a
low viscosity. (Consider, by way of reference, the relative ease of drawing a
low-viscosity
fluid such as water through the tip / opening of a needle 400 affixed to a
syringe.) However,
animal medications are marketed that have a relatively high viscosity (that
is, they are
relatively thick in consistency), and this higher viscosity makes the
medications very difficult
to withdraw from a bottle using a needle, and also typically more difficult to
expel from the
syringe. Stated another way, such higher-viscosity medications are not readily
"syringeable".
[0041] When a medication is not readily syringeable, it may take a
considerable amount of
time for the person tasked with withdrawing the medication from the bottle to
withdraw even
a small amount of medication. When a large amount of such medication must be
administered, and/or when the higher-viscosity medication must be administered
to multiple
animals, the person may experience frustration or even fatigue due to this
long withdrawal
16
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
time. As a result, use of the higher-viscosity medication by animal care-
givers may be
diminished, which may lead to the medication failing to reach its potential
market share.
Thinning the medication is undesirable as an answer to improving the
syringeability problem,
as the effectiveness of the medication could be altered.
[0042] In addition to the above-described issues with withdrawing higher-
viscosity
medication into a syringe, the higher viscosity of the medication makes the
injection process
more time-consuming and physically more difficult for the person tasked with
medicating the
animal. In particular, the general configuration of a plastic syringe as
illustrated in FIG. 1
does not enable a person using the syringe to have sufficient leverage when
attempting to
inject the medication into an animal. Tab-shaped member 150 is known to
collapse or break
in some instances, due to the physical force that must be exerted while
depressing plunger
120. The plunger shaft is also known to break in some instances, for example
due to
misalignment as it moves within the barrel or due to age-related brittleness.
The needle may
also be forced off the syringe when attached thereto by a friction-based Luer-
type slip
connection, which may in turn lead to leakage and/or waste of the medication
through the
now-opened end of the syringe. Additionally, the syringe tips of plastic
syringes are known
to break off while medicating an animal (for example, due to the animal moving
or thrashing
about), which can lead to waste of medication in the syringe. These problems
are more likely
to occur with the increased physical force required for injecting higher-
viscosity medications.
[0043] In sharp contrast to use of a plastic syringe, the syringes 200, 300 of
FIGs. 2 - 3 are
better adapted for withstanding the physical force required for expelling a
higher-viscosity
medication from the syringe barrel and for allowing the person using the
syringe to have
better leverage during the injection process. (Because the leverage is
improved, the time
required to complete the injection may be shortened as compared to use of a
plastic syringe
17
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
configured as shown at 100 of FIG. 1, which benefits the person and the
animal.)
[0044] In view of the above-described issues, preferred embodiments of the
present invention
are directed toward improved syringeability of medications having a relatively
high viscosity.
(The disclosed syringe adapter may function suitably with lower-viscosity
medications as
well, and is therefore not deemed to be limited to use with particular
medications.)
[0045] A preferred embodiment of the present invention provides a new tip that
operates as a
syringe adapter for withdrawing medication from a bottle. This tip is
preferably affixed to a
pistol-grip syringe of the type illustrated in FIG. 2 or a tab-handled syringe
of the type
illustrated in FIG. 3. The pistol-grip or tab-handled syringe may be formed
from plastic,
metal, or other substance(s), as noted earlier. Accordingly, use of an
embodiment of the
present invention addresses the issue of drawing a higher-viscosity fluid from
a bottle as well
as the issue of providing sufficient leverage for subsequent injection. That
is, the larger
opening of the disclosed syringe adapter (as compared to a conventional
needle) addresses
syringeability issues by improving draw time of higher-viscosity medications
and, when this
adapter is affixed to a pistol-grip or tab-handled syringe, the medication
withdrawn into the
pistol-grip or tab-handled syringe can be more easily administered from the
syringe barrel
(noting that, in some embodiments, the syringe adapter will be replaced with a
needle prior to
injecting the medication).
[0046] While discussions herein refer to preferably using the disclosed
syringe adapter with a
pistol-grip or tab-handled syringe, it should be noted that the disclosed
syringe adapter may
also be used advantageously with a syringe of the type shown in FIG. 1 (and
such usage is
within the scope of the present invention).
[0047] FIG. 5 illustrates one embodiment of the syringe adapter disclosed
herein. The
syringe adapter has a sidewall extending between a proximal end (i.e., the end
on which
18
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
reference number 510 is located) and a distal end (denoted generally at 520),
and the interior
surface of the sidewall defines a chamber through which fluid medication
flows. Reference
number 530 denotes the location of the opening in the distal end of syringe
adapter 500. The
length and shape of the syringe adapter, as well as the thickness of portions
of the sidewall
and the width of its interior chamber, may vary from illustrations depicted
herein without
deviating from the scope of the present invention. In the embodiment
illustrated in FIG. 5,
the shape of the syringe adapter 500 is generally conical in an upper portion
and generally
cylindrical in a lower portion. While not illustrated in FIG. 5, an interior
of at least a portion
of the lower portion is preferably tapered, with a 6 percent taper extending
from the proximal
end toward the distal end. This tapered shape conforms the interior surface to
the above-
noted International Standards ISO 594-1:1986 and ISO 594-2:1998(E) and their
replacement
ISO 80369-7:2016, which are directed toward conical fittings for health-care
applications.
Preferably, the overall length of the syringe adapter is not shorter than 3/8
to 1/2 inch, by way
of illustration but not of limitation, as this length will enable the syringe
adapter to
sufficiently extend into a bottle of medication to be withdrawn. An upper
range of the overall
length, conversely, may be on the order of 1 to 2 inches, by way of
illustration but not of
limitation.
[0048] A preferred diameter of the hole in the distal end of the tip of the
syringe adapter is on
the order of 0.10 inches, although embodiments are not limited to this
diameter (and as
discussed in further detail hereinafter, the diameter used in embodiments of
the present
invention is preferably about 0.05 inches to about 0.1585 inches, and more
preferably, is
about 0.078 inches to about 0.14 inches, and even more preferably, is about
0.09 inches to
about 0.11 inches, and generally, is approximately 0.10 inches). Thickness of
the sidewall of
the syringe adapter is preferably on the order of 0.050 inches, although
embodiments are not
19
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
limited to this thickness. Using a sidewall thickness of 0.050 inches and an
opening of 0.10
inches results in a syringe adapter having an overall diameter of 0.20 inches
at the end to be
inserted into the bottle of medication, in this example configuration. The
above-cited
International Standards indicate that sidewall thickness for a Luer tip
(whether Luer-type lock
or Luer-type slip) that conforms to the International Standards may range
between 0.021
inches as a minimum and 0.039 inches as a maximum. Accordingly, in another
example
configuration, an embodiment of the syringe adapter may use a sidewall
thickness of about
0.03 inches and an opening sized at 0.09 inches, in which case the outer
diameter at the end to
be inserted into the bottle is 0.15 inches. Advantageously, it is noted that
the opening formed
by the sidewall at the distal end of the syringe adapter is depicted herein
for several
embodiments as generally blunt, as contrasted with a sharp metal point found
on the tip of a
needle, thereby improving safety for someone who may, for example, carry a
syringe adapter
on his or her person. (FIGs. 17 - 19, discussed below, depict embodiments
where the opening
is not generally blunt.)
[0049] Preferably, the proximal end of the disclosed syringe adapter attaches
to a syringe
using a Luer-type lock or a Luer-type slip (although it will be understood
that this is by way
of illustration and not of limitation). Luer-type locks and Luer-type slips
are known
approaches for making leak-free connections on fluid fittings, and are
described in the above-
cited International Standards. A Luer-type lock provides a threaded
attachment, whereby two
pieces of a configuration are held together by rotating a flanged area (such
as flanged area 460
of FIG. 4B) of one piece within threads of the other piece, whereas a Luer-
type slip is non-
threaded and provides attachment using friction. In one approach for securably
attaching
syringe adapter 500 using a Luer-type lock, the syringe adapter 500 as
illustrated in FIG. 5 has
an external flanged area 510 on the proximal end (shown without tabs extending
from the
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
outer edge, for drafting convenience), and a two-part connection is made by
inserting this
flanged end into corresponding internal threads on a distal end of a syringe
(as discussed
above with reference to the syringe tips illustrated at 240, 340). As noted
earlier, a
conventional height for this internal threaded portion of a pistol-grip or tab-
handled syringe
tip is believed to be approximately 1/8 inch to 1/4 inch in length and a
standardized height
thereof is believed to be 5.4 millimeters, and accordingly, a flanged area 510
on the proximal
end of syringe adapter 500 is preferably on the order of at least 1/16 to 1/8
inch in height.
The shape of flanged area 510 may correspond generally to flanged area 420 or
460 (for
example, by extending radially or perpendicularly from the proximal end of the
syringe
adapter, although a strictly circular shape is not required), although another
shape providing
for a securable attachment may be used without deviating from the scope of the
present
invention. (In view of this ability to use shapes other than strictly circular
for securable
attachment, it will be readily understood that references herein to "radial"
or "radially" may
be more generally understood as "lateral" or "laterally".) Notably, with
regard to the
securable attachments noted herein that are described as preferably using a
Luer-lock
connection, it will be readily understood that the shape and size of the
attachment area ¨ such
as the area denoted in FIG. 5 by reference number 510 ¨ preferably conforms to
the well-
known shape and size required for such connection.
[0050] In another approach, the proximal end of the syringe adapter 500 may
omit the flanged
area shown at 510 and is attached and held to the distal end of the syringe by
friction in a
Luer-type slip approach.
[0051] FIG. 6 illustrates another embodiment of the syringe adapter disclosed
herein. In this
embodiment, syringe adapter 600 includes a radial extension feature 610, which
is preferably
configured as extending perpendicularly and radially outward from the body of
the syringe
21
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
adapter and is shown in FIG. 6 as being located relatively near to the
proximal end of syringe
adapter 600 (where the proximal end of syringe adapter 600 is the end on which
reference
number 620 is located, and the distal end is denoted generally by reference
number 630;
reference number 640 denotes the location of the opening in this distal end).
Alternatively,
radial extension feature 610 may be placed at another location on the syringe
adapter, for
example being located closer to the conical portion thereof In addition to
enabling a person
to more easily grasp the syringe adapter 600, the radial extension feature 610
also serves to
prevent inserting the syringe into the medication bottle far enough that the
attachment point
(e.g., Luer-type slip or lock) between the syringe and the syringe adapter
would come into
contact with the medication. Stated another way, an extension feature (or
equivalently,
"extension member") on an embodiment of the disclosed syringe adapter is
preferably sized
so as to halt insertion of the syringe adapter into the container beyond a
location of the
extension feature. Accordingly, in a preferred embodiment, a diameter of
radial extension
feature 610 is sufficiently large as to meet or exceed the diameter of a
conventional rubber
membrane on a medicine bottle, although it will be understood that embodiments
are not
limited to this diameter. The diameter of radial extension feature 610 may be,
by way of
example, on the order of twice the diameter of the cylindrical portion of
syringe adapter 600.
(Syringe adapter 600 may omit the flanged area 620 when relying on a Luer-type
slip
attachment, and is depicted without tabs extending from the outer edge for
drafting
convenience, as was discussed above with reference to flanged area 510.)
[0052] An extension feature might alternatively be used that is not round,
although this has
not been illustrated in FIG. 6. (For example, a hexagonal shape might be used
for an
extension feature, and in view of this ability to use an extension feature
that is not round, it
will be readily understood that references herein to a "radial" extension
feature are by way of
22
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
illustration but not of limitation and are not to be construed as requiring
the extension feature
to have a round outer edge. More generally, the extension feature may be
termed a "lateral"
extension feature or simply an "extension feature") As noted above, a
preferred diameter for
an extension feature as disclosed herein (as illustrated by the example at
reference number
610) is selected to provide compatibility with a size of the rubber membrane
on a bottle from
which fluid will be withdrawn. It will be readily understood that a common
diameter of the
rubber membrane fitted in the top of bottles holding 250 milliliters ("m1") of
fluid and also
bottles holding 500 ml is 0.75 inches. (Most common multi-dose fluid
medication bottles are
sold in bottles having volumes of 100 ml, 250 ml, and 500 ml, while some less-
common
smaller-dose bottles hold 20 ml of fluid.) It is readily understood that a
common outer
diameter of the top of such bottles is about 1.3 inches. Accordingly, a
preferred diameter for
the extension feature when used with such bottles is in the range of about
0.50 inches to about
1.3 inches, and more preferably, the diameter is about 0.70 inches to about
0.80 inches, and
most preferably, is about 0.75 inches as noted herein. The range in size for
the extension
feature has been selected, through experimentation and testing, by noting that
an extension
feature diameter appreciably exceeding the outer width of the medicine bottle
may be
cumbersome for the person holding the bottle and the syringe with affixed
syringe adapter,
and that an extension feature diameter about the diameter of the rubber
membrane ¨ or
ranging between about the diameter of the rubber membrane and about the outer
diameter of
the top of the bottle ¨ provides an advantageous size that allows the person
to grip the outer
edges of the extension feature while also gripping the bottle, and at the same
time, also
ensuring that the extension feature is unlikely to push through the rubber
membrane. If used
with a bottle having other dimensions than those noted above, it will be
understood that the
diameter of the extension feature may be adapted to provide compatibility
therewith. In such
23
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
adaptation, it is preferred that the diameter of the extension feature is
preferably within about
the diameter of the rubber membrane and about the outer diameter of the top of
the bottle, and
more generally, ranges between about two-thirds the diameter of the rubber
membrane and
about the diameter of the top.
[0053] A preferred material for the disclosed syringe adapter is plastic,
which will allow it to
be economically produced as a disposable item, although another material may
be used
without deviating from the scope of the present invention. As one alternative
to use of
plastic, the syringe adapter or portion(s) thereof may be constructed from
stainless steel,
aluminum, or another metal (or combinations thereof), noting that metal
generally provides
increased strength and durability as compared to plastic. It will be readily
understood that
constructing the syringe adapter from a material such as plastic
advantageously allows it to be
recyclable, in addition to being easily disposable (as contrasted to disposal
of a sharp needle,
for which care must be taken upon disposal to ensure that, inter al/a, persons
or animals are
protected from injury therefrom). Notably, the disclosed syringe adapter does
not need to
come into physical contact with a particular animal (i.e., because the
physical contact occurs
at the needle used to inject the medication), and thus re-use of the syringe
adapter for
medicating multiple animals need not introduce cross-contamination concerns.
[0054] While FIGs. 5 and 6 illustrate a syringe adapter shape that is
generally conical in an
upper portion and generally cylindrical in a lower portion, this is by way of
illustration and
not of limitation. As one alternative embodiment, an outer shape of the
syringe adapter may
be generally cylindrical while still preferably having a tapered interior
shape for at least a
portion of the proximal end as in the embodiments illustrated in previously-
discussed FIGs. 5
and 6, noting that such interior taper enables the syringe adapter to comply
with the above-
cited International Standards. This alternative embodiment is illustrated in
FIG. 7, where
24
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
dotted lines are used to illustrate a general shape of the interior. FIG. 8
provides another
alternative embodiment, where an outer shape of the syringe adapter may be
generally conical
in an upper portion and generally cylindrical in a lower portion, and in this
alternative, the
relative length of the upper and lower portions varies from the embodiments
illustrated in
FIGs. 5 and 6 (and again, at least a portion of such configuration preferably
has a tapered
interior shape at the proximal end, as shown by the dotted lines, to thereby
conform to the
above-cited International Standards). Notably, as compared to the cylindrical
exterior shape
as illustrated in FIG. 7, the exterior taper of the upper portion as
illustrated in FIGs. 5 - 6 and
8 may tend to provide a better seal, and thus be less likely to leak, during
such time as the
syringe adapter is inserted through the rubber membrane of a bottle. While not
illustrated in
FIGs. 7 or 8, a radial extension member (such as that shown at reference
number 610 of FIG.
6) may be added to these configurations if desired.
[0055] FIG. 9 illustrates yet another embodiment of the disclosed syringe
adapter. In this
embodiment, syringe adapter 900 includes a radial extension feature 910,
similar to the
previously-discussed radial extension feature 610 of FIG. 6. FIG. 9 depicts
radial extension
feature 910 as being located approximately midway along the length of the
syringe adapter,
by way of illustration but not of limitation. Whereas the radial extension
feature illustrated at
610 of FIG. 6 is illustrated as having a disk-like shape with generally flat
upper and lower
surfaces, FIG. 9 illustrates an alternative shape where an upper surface of
the radial extension
feature 910 has a somewhat domed or tapered shape. This tapered or domed
portion is shown
at reference number 920 and sits atop a disk-like portion 930. Optionally, the
lower surface
of the radial extension feature may taper in addition to, or instead of, the
upper surface
thereof, although this has not been illustrated. (Note that the particular
shape and dimensions
of portions 920, 930 may vary, and thus FIG. 9 provides one example by way of
illustration
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
but not of limitation.)
[0056] FIG. 9 also illustrates the upper portion 940, at the distal end, of
the syringe adapter
900 as having a generally conical shape which is somewhat less tapered than
the upper
portion as illustrated for the syringe adapters 500, 600 of FIGs. 5 and 6, and
having a
generally cylindrical shape for the lower portion 950, located at the proximal
end of syringe
adapter 900. Reference number 960 denotes the location of the opening in the
distal end of
syringe adapter 900. (As noted below with regard to FIG. 10, the proximal end
of the syringe
adapter preferably makes a Luer-type lock connection with the tip of a
syringe, and it will
therefore be readily understood that the shape illustrated at 970 is merely an
example and is
not to be construed as limiting.)
[0057] By way of illustration but not of limitation, a length of the conical
portion 940 may be
.32 inches; a length of the cylindrical portion 950 may be .48 inches; a
height or thickness of
portion 930 may be .07 inches; a diameter of radial extension feature 910 may
be .75 inches;
a diameter of the distal and proximal ends of conical portion 940 may be .156
inches and .174
inches, respectively; and a diameter of cylindrical portion 950 may be .24
inches. (When
using the above-noted example sidewall thickness of 0.05 inches, and if the
thickness is
uniform throughout, it will be noted that these illustrative measurements
indicate an example
where the inner diameter of the cylindrical portion 950 is 0.14 inches and the
inner diameter
of the distal and proximal ends of conical portion 940 are .056 inches and
.074 inches,
respectively.)
[0058] FIG. 10 illustrates placement of a syringe adapter on a syringe. By way
of illustration
but not of limitation, the syringe in FIG. 10 corresponds to the tab-handled
syringe 300 of
FIG. 3 and the syringe adapter corresponds to the embodiment shown at 900 of
FIG. 9.
Syringe tip 340' provides a point of attachment for the syringe adapter 900,
and syringe tip
26
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
340' is shown as being generally cylindrical; as contrasted with syringe tip
340 as earlier
illustrated, syringe tip 340' is shown with a ribbed exterior mid-section 345
that may provide
for a person to securely grip the syringe tip 340' while the syringe adapter
900 is being
inserted therein (or removed therefrom). The connection between syringe tip
340' and
syringe adapter 900 is preferably a Luer-type lock, but a Luer-type slip may
be used
alternatively without deviating from the scope of the present invention. (It
will be understood
that in FIG. 10, a portion of the proximal end of syringe adapter 900 is
located inside the
distal end of tip 340', following the connection.)
[0059] FIG. 11 illustrates one example of placement of a needle on a syringe
adapter, for an
embodiment in which the syringe adapter remains in place while medication is
administered
through an attached needle. By way of illustration but not of limitation, the
syringe adapter in
FIG. 11 corresponds to the embodiment shown at 900 of FIG. 9, the attachment
between the
syringe and syringe adapter 900 corresponds to the attachment illustrated in
FIG. 10, and the
needle corresponds to the needle 400 of FIG. 4. In FIG. 11, needle 400 is
shown as having its
proximal end placed over the distal end of the syringe adapter. In this
example, needle 400
includes a small flanged area 420 that enables it to securably attach to the
interior of a Luer-
type lock, although the illustrated attachment in FIG. 11 is a Luer-type slip
connection.
Accordingly, the needle 400 may be attached to, and removed from, the syringe
adapter with
relative ease (e.g., by pushing the proximal end of the needle onto the distal
end of the syringe
adapter and pulling it therefrom, respectively). Note that while flanged area
420 is shown as
directly abutting the portion of the syringe adapter shown as having a domed
shape, this is by
way of illustration: alternatively, there may be a gap beneath flanged area
420.
[0060] FIG. 12 illustrates still another embodiment of the disclosed syringe
adapter, and its
placement on a syringe. In this embodiment, syringe adapter 1200 includes a
radial extension
27
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
feature 1210 with a tapered or domed upper surface, similar to the previously-
discussed radial
extension feature 910 of FIG. 9. As contrasted to syringe adapter 900, the
conical portion of
the syringe adapter 1200 is somewhat longer (and it should be noted that
embodiments of the
present invention are not limited to a specific dimension, as has been
discussed).
[0061] FIG. 12 also illustrates a Luer-type connecting member 1220 affixed to
the proximal
end of syringe adapter 1200 (where 2 horizontally-oriented "ribs" are
illustrated on an upper
portion of the surface of member 1220, by way of illustration but not of
limitation, and may
serve as a grasping member that assists a person using the syringe adapter in
firmly grasping).
This connecting member 1220 is shown in FIG. 12 as connecting syringe adapter
1200 to a
syringe, which may be syringe 300 of FIG. 3 (by way of illustration but not of
limitation).
The point of connection on syringe 300 is shown in FIG. 12 as comprising a
syringe tip 340',
similar to that which was discussed above with reference to FIG. 10 by way of
illustration,
into which the proximal end of connecting member 1220 of syringe adapter 1200
is
removably inserted. In one approach, connecting member 1220 is made from metal
while
remaining portions of syringe adapter 1200 are made from plastic, and a bond
is made
between the metal and plastic during manufacturing. Preferably, connecting
member 1220
attaches to syringe tip 340' with a Luer-type lock connection (rather than a
Luer-type slip
connection). While not shown in FIG. 12, connecting member 1220 preferably
includes a
flanged area at its proximal end (such as flanged area 460 of FIG. 4B), and
the Luer-type lock
connection is made by inserting this flanged area of connecting member 1220
into syringe tip
340' and then twisting the syringe adapter 1200 until the flanged area locks
into place in the
internal threaded portion of the syringe tip 340'. This type of connection is
deemed beneficial
for providing a more secure attachment between the syringe and the syringe
adapter.
Reference number 1220 generally denotes the proximal end of syringe adapter
1200, although
28
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
as stated above, a portion of the proximal end is located within syringe tip
340' and is
therefore not visible. Reference number 1230 generally denotes the distal end
of syringe
adapter 1200, while reference number 1240 denotes the location of the opening
therein.
[0062] FIG. 13 illustrates yet another embodiment of the disclosed syringe
adapter and its
placement on a syringe. In this embodiment, syringe adapter 1300 differs from
syringe
adapter 1200 of FIG. 12 in that a Luer-type connecting member 1320 affixed to
the proximal
end of syringe adapter 1300 uses a different configuration. As shown in FIG.
13, an upper
portion 1322 of connecting member 1320 has a multi-sided exterior shape, shown
by way of
illustration as being hexagonal in at least a portion thereof, which may serve
as a grasping
member that assists a person using the syringe adapter in firmly grasping.
More particularly,
in the example shown in FIG. 13, connecting member 1320 has a hexagonal upper
portion
1322 and a cylindrical lower portion 1321. In one approach, connecting member
1320 is
made from metal while remaining portions of syringe adapter 1300 are made from
plastic,
and a bond is made between the metal and plastic during manufacturing.
Preferably,
connecting member 1320 attaches to syringe tip 340' with a Luer-type lock
connection (rather
than a Luer-type slip connection). While not shown in FIG. 13, connecting
member 1320
preferably includes a flanged area at its proximal end (such as flanged area
460 of FIG. 4B),
and the Luer-type lock connection is made by inserting this flanged area of
connecting
member 1320 into syringe tip 340' and then twisting the syringe adapter 1300
until the
flanged area locks into place in the internal threaded portion of the syringe
tip 340'. As noted
above, the Luer-type lock connection is deemed beneficial for providing a more
secure
attachment between the syringe and the syringe adapter. Reference number 1320
generally
denotes the proximal end of syringe adapter 1300, although as stated above, a
portion of the
proximal end is located within syringe tip 340' and is therefore not visible.
Reference
29
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
number 1330 generally denotes the distal end of syringe adapter 1300, while
reference
number 1340 denotes the location of the opening therein.
[0063] FIG. 14 (comprising FIGs. 14A through 14D) illustrates a further
embodiment of the
disclosed syringe adapter, showing exterior views as well as cross-sectional
views of
placement thereof upon a syringe and as exploded. By way of illustration but
not of
limitation, the syringe in FIG. 14 corresponds to the tab-handled syringe 300
of FIG. 3 with
its syringe tip 340. Syringe adapter 1400, in this embodiment, includes a
radial extension
member 1410 and the proximal end as denoted by reference number 1420 includes
a Luer-
type connecting member, where proximal end 1420 in turn includes a flanged
area 1421 at its
proximal end (such as flanged area 460 of FIG. 4B) for removably attaching
syringe adapter
1400 to the distal end of syringe 300 and an upper portion 1422. The Luer-type
lock
connection is made by inserting flanged area 1421 of proximal end 1420 into
syringe tip 340
and then twisting the syringe adapter 1400 until the flanged area 1421 locks
into place in the
internal threaded portion of the syringe tip 340 (as illustrated in the non-
exploded cross-
sectional view of FIG. 14C) to thereby provide a secure attachment between the
syringe and
the syringe adapter. It is noted that the above-cited International Standard
ISO 80369-7 states
acceptable measurements for inner and outer diameters of male and female
portions of
components intended for a Luer-type connection. For example, inside diameter
for a male
connector is stated as a range between 2.100 millimeters and 2.900 millimeters
(i.e., 0.082677
inches and 0.114173 inches), and outside diameter for a male connector is
stated as ranging
between 3.970 millimeters and 4.072 millimeters (i.e., 0.156299 inches and
0.160315 inches),
with a nominal outside diameter of 4.021 millimeters (i.e., 0.158307 inches);
dimensions for
corresponding female connectors are stated as roughly 0.20 millimeters
smaller. In an
embodiment, inner and outer diameters of flanged area 1421 are preferably
designed for
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
compatibility with such measurements to enable syringe adapter 1400 to make a
secure
attachment to the internal threaded portion of a syringe tip 340 that is
manufactured in
conformance with such measurements. As noted above with reference to the ribs
at 1220 and
upper portion 1322, upper portion 1422 may serve as a grasping member that
assists a person
using the syringe adapter with firmly grasping (for example, while inserting
and twisting the
flanged area 1421 into the syringe tip 340). Reference number 1430 generally
denotes the
distal end of syringe adapter 1400, while reference number 1440 denotes the
location of the
opening therein. (As can be seen with reference to FIGs. 14C and 14D,
embodiments are not
limited to using a uniform sidewall thickness throughout.)
[0064] FIG. 15 illustrates a still further embodiment of the disclosed syringe
adapter,
showing exterior views as well as cross-sectional views of placement thereof
upon a syringe
and as exploded. The embodiment illustrated in FIG. 15 includes a needle
holder to which a
needle may be removably affixed. In this embodiment, a needle 400'
(illustrated as having an
outer shape somewhat different from needle 400, by way of illustration but not
of limitation)
is removably affixed to a needle holder 1510 that, in turn, is removably
affixed to a syringe
adapter 1500 that is removably affixed to a syringe 300 for the injection of
medication into a
recipient. When the injection is completed, the needle 400' preferably remains
affixed to the
needle holder 1510, enabling needle 400' and needle holder 1510 to be removed
from syringe
adapter 1500 as a single unit (for example, in preparation for using syringe
adapter 1500 for
withdrawing additional fluid medication from a container). This enables the
combination of
needle holder and needle to be quickly and easily re-installed on the syringe
adapter, after the
syringe adapter is used for withdrawing a next dosage of medication, for
administering that
next dosage if the needle is deemed to be reusable for that next dosage. While
discussions
herein of embodiments using a needle holder as depicted in FIGs. 15 - 21 refer
to the
31
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
possibility of reusing a needle that has been attached to the needle holder,
it is recognized that
needle reuse is not advisable in some situations, such as when there is a risk
of cross-
contamination. In such situations, the used needle is preferably removed from
the needle
holder of FIGs. 15 - 21 and replaced with an unused needle. (It should be
noted that the
cross-sectional views in FIG. 15 illustrate a preferred interior and exterior
shape of
components 1500, 1510, but embodiments are not limited to the specific shapes
and/or
relative dimensions as shown except as otherwise noted herein. It should also
be noted that
the syringe adapters as described herein with reference to FIGs. 15 - 21 have
separate utility
without use of the corresponding needle holder; that is, the syringe adapters
illustrated in
FIGs. 15 - 21 provide benefits analogous to those discussed herein with regard
to the
embodiments illustrated in FIGs. 5 - 14.)
[0065] Preferably, syringe adapter 1500 is configured with a support hub
member 1501 that
radially surrounds at least a portion of the length of the syringe adapter
sidewall 1502.
Preferably, the connection of syringe adapter 1500 to syringe tip 340 is made
as a Luer-type
lock by inserting a flanged proximal end 1504 of syringe adapter sidewall 1502
into an
internal threaded portion of syringe tip 340 and then twisting the syringe
adapter 1500 until
the flanged area locks into place in the internal threaded portion of the
syringe tip 340. A
proximal end of support hub member 1501 (i.e., the end where reference number
1501 is
generally located) is preferably sized so as to slip over the exterior of
syringe tip 340, while
the proximal end of syringe adapter sidewall 1502 (i.e., the end where
reference number 1504
is generally located) is sized so as to fit within the threaded interior of
syringe tip 340 and to
receive protrusion 341 of the syringe tip 340 within the chamber defined by
the interior
surface of sidewall 1502. This is illustrated in the non-exploded cross-
sectional view of FIG.
15C. As discussed above with reference to FIG. 14, it is noted that the above-
cited
32
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
International Standard ISO 80369-7 states acceptable measurements for inner
and outer
diameters of male and female portions of components intended for a Luer-type
lock
connection. In an embodiment, inner and outer diameters of support hub member
1501 and
flanged proximal end 1504 are preferably designed for compatibility with such
measurements
to enable syringe adapter 1500 to make a secure attachment to the internal
threaded portion of
a syringe tip 340 that is manufactured in conformance with such measurements,
and similarly
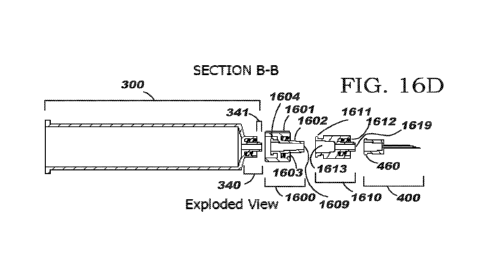
for support hub members 1601, 1701 and flanged proximal ends 1604, 1704 of
syringe
adapters 1600, 1700.
[0066] In an embodiment, the opening 1509 at the distal end of sidewall 1502
(noting that the
distal end of sidewall 1502 is the end at which reference number 1502 is
generally located) is
approximately .10 inches in inside diameter (and as discussed in further
detail hereinafter, the
diameter is preferably about 0.05 inches to about 0.1585 inches, and more
preferably, is about
0.078 inches to about 0.14 inches, and even more preferably, is about 0.09
inches to about
0.11 inches, and generally, is approximately 0.10 inches), and this distal end
of sidewall 1502
extends beyond the distal end of support hub member 1501 (i.e., the end of
support hub
member where reference number 1503 is generally located) to allow the distal
end of sidewall
1502 to penetrate the rubber membrane on the medication bottle. In an
embodiment, an outer
wall of support hub member 1501 is shaped as a cylinder. In another
embodiment, by way of
illustration but not of limitation, the outer wall may be configured to have a
multi-sided
exterior shape (such as, for example, a hexagonal shape, and in view of this
ability to use
shapes other than strictly cylindrical for the exterior shape, it will be
readily understood that
references herein to a support hub member that "radially surrounds" or is
"radially
surrounding" may be more generally understood as "laterally surrounds" or is
"laterally
surrounding").
33
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
[0067] A distal end of support hub member 1501 is preferably configured with
an internal
threaded portion to facilitate removably attaching needle holder 1510. The
threads are
illustrated in FIG. 15 generally by small extensions 1503 on the interior wall
of support hub
member 1501, and this internal threaded portion receives a flanged area 1511
(similar to the
above-described flanged area 460 of FIG. 4B, which may alternatively be
referred to as a
tabbed area) that is located at the proximal end of needle holder 1510. In an
embodiment, the
threads illustrated by extensions 1503 correspond to a double-helix
configuration as described
in the above-cited International Standards. Needle holder 1510 thereby makes a
Luer-type
lock connection with syringe adapter 1500. In a configuration as illustrated
in FIG. 15,
support hub member 1501 also provides additional support for needle holder
1510. See the
non-exploded cross-sectional view in FIG. 15C.
[0068] Needle holder 1510 preferably includes a radial extension feature 1512,
which may be
located (by way of example) near the proximal end of the needle holder (noting
that the
proximal end of needle holder 1510 is the end where reference number 1511 is
generally
located, and the distal end of the needle holder is opposite thereto). Radial
extension feature
1512 may enable a person to more easily grasp the needle holder 1510. In a
configuration as
illustrated in FIG. 15, radial extension feature 1512 also serves to provide
additional strength
for the needle holder 1510, and thus may reduce the likelihood of a physical
failure during
use.
[0069] Needle holder 1510 is configured for removably attaching a needle 400',
and is
illustrated in FIG. 15 as using a Luer-type slip connection that makes the
attachment by
placing the proximal end of needle 400' over the distal end of needle holder
1510 (as
illustrated by FIGs. 15C and 15D). Needle holder 1510 may alternatively be
configured to
support a Luer-type lock connection with needle 400' without deviating from
the scope of the
34
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
present invention, although this is not illustrated in FIG. 15. Such
alternative configuration
for a needle holder may be used with syringe adapter 1500, in place of needle
holder 1510,
within the scope of the present invention. (FIGs. 16 - 21, discussed below,
illustrate
embodiments that use Luer-type lock connections between a needle and needle
holder.)
[0070] Preferably, syringe adapter 1500 and needle holder 1510 are constructed
from a plastic
or a composite. Syringe adapter 1500 and/or needle holder 1510 may
alternatively be
constructed from another material, such as stainless steel, aluminum, or
another metal (or a
combination thereof), without deviating from the scope of the present
invention ¨ although as
discussed earlier, using a material such as plastic for the syringe adapter
and needle holder
advantageously allows these components to be recyclable.
[0071] Syringe adapter 1500 may optionally be configured as a permanent
attachment to (or
replacement for) syringe tip 340, rather than being removably affixed thereto.
(See the
discussion of FIGs. 18 - 21, below, which depict embodiments that replace a
conventional
syringe tip.) A distal end of the sidewall 1502 of syringe adapter 1500 may be
relatively
sharp, for ease of inserting this distal end into a bottle of medication (for
example), as
contrasted to the generally blunt opening that is illustrated for this
embodiment in FIG. 15D.
(See the discussion of FIGs. 17 - 19, below, which depict embodiments having a
relatively
sharp tip ¨ i.e., a tip that is not generally blunt ¨ on the distal end of a
syringe adapter.)
[0072] In an embodiment, the opening 1519 at the distal end of needle holder
1510 is
approximately .08 inches in inside diameter (see, for example, the above-noted
reference to
male connectors ranging between 2.100 and 2.900 millimeters), and the outside
diameter of
the distal end of needle holder 1510 preferably conforms to suggested
dimensions for a Luer-
type tip in the above-cited International Standards to thereby facilitate
connection to standard
needles. Accordingly, in an embodiment, while the inner diameter for opening
1519 is
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
approximately .08 inches as noted above, outer diameter is preferably about
0.16 inches to
accommodate a Luer-type slip connection to a needle 400' that is manufactured
in
conformance with such measurements. In an embodiment as illustrated in FIG.
15, an outer
wall of needle holder 1510 has a conical shape at the distal end and a similar
shape at the
proximal end, where both the proximal end and the distal end are generally
conical in shape.
Preferably, a length of an inner chamber 1513 of needle holder 1510 extends
from the
attachment point in support hub member 1501 to cover the distal end of syringe
adapter 1500
(as illustrated in the non-exploded cross-sectional view in FIG. 15C). Note
that while the
needle holder 1510 and needle 400' may be removed as a unit from the syringe
adapter 1500,
this is by way of example; alternatively the needle may be removed from the
needle holder
and the needle holder may then be removed from the syringe adapter, without
deviating from
the scope of the present invention.
[0073] FIG. 16 illustrates yet another embodiment of the disclosed syringe
adapter, showing
exterior views as well as cross-sectional views of placement thereof upon a
syringe and as
exploded. The embodiment illustrated in FIG. 16 includes a needle holder to
which a needle
may be removably affixed. In this embodiment, a needle 400 is removably
affixed to a needle
holder 1610 that, in turn, is removably affixed to a syringe adapter 1600 that
is removably
affixed to a syringe 300 for the injection of medication into a recipient.
When the injection is
completed, the needle 400 preferably remains affixed to the needle holder
1610, enabling
needle 400 and needle holder 1610 to be removed from syringe adapter 1600 as a
single unit
(for example, in preparation for using syringe adapter 1600 for withdrawing
additional fluid
medication from a container). This enables the combination of needle holder
and needle to be
quickly and easily re-installed on the syringe adapter, after the syringe
adapter is used for
withdrawing a next dosage of medication, for administering that next dosage.
(It should be
36
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
noted that the cross-sectional views in FIG. 16 illustrate a preferred
interior and exterior
shape of components 1600, 1610, but embodiments are not limited to the
specific shapes
and/or relative dimensions as shown except as otherwise noted herein.)
[0074] Preferably, syringe adapter 1600 is configured with a support hub
member 1601 that
radially surrounds at least a portion of the length of the syringe adapter
sidewall 1602. In an
embodiment as illustrated in FIG. 16, the exterior of the outer wall of
support hub member
1601 has a hexagonal shape. This hexagonal shape may enable a person to have a
better
grasp when connecting or disconnecting the syringe adapter 1600 to syringe 300
and/or
needle holder 1610. (Note that while FIG. 16 illustrates the exterior of
support hub member
1601 as being hexagonal in shape, this is by way of illustration but not of
limitation, and the
exterior may be configured to have a different shape without deviating from
the scope of the
present invention.)
[0075] A Luer-type lock connection between syringe adapter 1600 and syringe
tip 340 is
preferably made in the manner discussed above with reference to FIG. 15, and
accordingly,
details of the connection are not repeated here. A result of the connection is
illustrated in the
non-exploded cross-sectional view in FIG. 16C.
[0076] In an embodiment, the opening 1609 at the distal end of sidewall 1602
(noting that the
distal end of sidewall 1602 is the end at which reference number 1602 is
generally located,
and reference number 1604 denotes a flanged proximal end of sidewall 1602) is
approximately .10 inches in inside diameter (and as discussed in further
detail hereinafter, the
diameter is preferably about 0.05 inches to about 0.1585 inches, and more
preferably, is about
0.078 inches to about 0.14 inches, and even more preferably, is about 0.09
inches to about
0.11 inches, and generally, is approximately 0.10 inches), and this distal end
of sidewall 1602
extends beyond the distal end of support hub member 1601 (i.e., the end where
reference
37
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
number 1603 is generally located, the proximal end of support hub member 1601
being
opposite thereto) to allow the distal end of sidewall 1602 to penetrate the
rubber membrane
on the medication bottle.
[0077] A distal end of support hub member 1601 is preferably configured with
an internal
threaded portion, similar to the threaded portion discussed above with
reference to support
hub member 1501 of FIG. 15, and a Luer-type lock connection between syringe
adapter 1600
and needle holder 1610 is preferably made using the threads (illustrated in
FIG. 16 generally
by small extensions 1603) and the flanged area 1611 (which may alternatively
be referred to
as a tabbed area) at the proximal end of needle holder 1610 in the manner
discussed above
with reference to FIG. 15. See the non-exploded cross-sectional view in FIG.
16C.
[0078] Needle holder 1610 is configured for removably attaching a needle 400.
Needle
holder 1610 includes an internal threaded portion (see reference number 1612)
on its distal
end (i.e., the end where reference number 1612 is generally located), where
these threads are
configured to provide a Luer-type lock connection with the flange 460 at the
proximal end of
needle 400, thus providing a relatively secure connection with the needle.
More particularly,
the connection between needle holder 1610 and needle 400 is preferably made by
inserting
the flanged proximal end 460 into internal threaded portion 1612 and then
twisting the needle
until the flanged area locks into place in the internal threaded portion of
the needle holder.
[0079] In an embodiment as depicted in FIG. 16, an exterior wall of needle
holder 1610 is
preferably hexagonal in shape in a lower portion and cylindrical in shape in
an upper portion,
as shown in the isometric view of FIG. 16A, with exception of the proximal end
where flange
1611 is located (and the protrusion at the distal end). As shown in FIG. 16,
the proximal end
immediately above the flange 1611 has a conical shape, by way of illustration
but not of
limitation (see, for example, the illustration in FIG. 16D, which depicts the
exterior shape
38
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
thereof as being somewhat tapered); as one alternative, the shape may be
cylindrical and still
engage the threads on 1600 properly. Needle holder 1610 may serve to provide
additional
strength for the assembly, and thus may reduce the likelihood of a physical
failure during use.
[0080] Preferably, syringe adapter 1600 and needle holder 1610 are constructed
from a plastic
or a composite. Syringe adapter 1600 and/or needle holder 1610 may
alternatively be
constructed from another material, such as stainless steel, aluminum, or
another metal (or a
combination thereof), without deviating from the scope of the present
invention ¨ although as
discussed earlier, using a material such as plastic for the syringe adapter
and needle holder
advantageously allows these components to be recyclable.
[0081] Syringe adapter 1600 may optionally be configured as a permanent
attachment to (or
replacement for) syringe tip 340, rather than being removably affixed thereto.
(See the
discussion of FIGs. 18 - 21, below, which depict embodiments that replace a
conventional
syringe tip.) While not illustrated in FIG. 16, the distal end of the sidewall
1602 of syringe
adapter 1600 may be relatively sharp, for ease of inserting this distal end
into a bottle of
medication (for example), as contrasted to the generally blunt opening that is
illustrated for
this embodiment in FIG. 16D. (See the discussion of FIGs. 17 - 19, below,
which depict
embodiments having a relatively sharp tip ¨ i.e., a tip that is not generally
blunt ¨ on the distal
end of a syringe adapter.)
[0082] Reference number 1619 denotes the opening in the distal end of needle
holder 1610.
As discussed above with reference to needle holder 1500, the inside diameter
of opening
1619 is preferably approximately .08 inches, and in an embodiment, the outside
diameter
conforms to measurements for Luer-type lock connections as stated in
International Standard
ISO 80369-7:2016 to accommodate a Luer-type lock connection to a needle 400
that is
manufactured in conformance with such measurements. Preferably, a length of an
inner
39
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
chamber 1613 of needle holder 1610 extends from the attachment point in
support hub
member 1601 to cover the distal end of syringe adapter 1600 (as illustrated in
the non-
exploded cross-sectional view in FIG. 16C). Note that while the needle holder
1610 and
needle 400 may be removed as a unit from the syringe adapter 1600, this is by
way of
example; alternatively the needle may be removed from the needle holder and
the needle
holder may then be removed from the syringe adapter, without deviating from
the scope of
the present invention.
[0083] FIG. 17 illustrates still another embodiment of the disclosed syringe
adapter, showing
exterior views as well as cross-sectional views of placement thereof upon a
syringe and as
exploded. The embodiment illustrated in FIG. 17 includes a needle holder to
which a needle
may be removably affixed. In this embodiment, a needle 400 is removably
affixed to a needle
holder 1710 that, in turn, is removably affixed to a syringe adapter 1700 that
is removably
affixed to a syringe 300 for the injection of medication into a recipient.
When the injection is
completed, the needle 400 preferably remains affixed to the needle holder
1710, enabling
needle 400 and needle holder 1710 to be removed from syringe adapter 1700 as a
single unit
(for example, in preparation for using syringe adapter 1700 for withdrawing
additional fluid
medication from a container). This enables the combination of needle holder
and needle to be
quickly and easily re-installed on the syringe adapter, after the syringe
adapter is used for
withdrawing a next dosage of medication, for administering that next dosage.
(It should be
noted that the cross-sectional views in FIG. 17 illustrate a preferred
interior and exterior
shape of components 1700, 1710, but embodiments are not limited to the
specific shapes
and/or relative dimensions as shown except as otherwise noted herein.)
[0084] Preferably, syringe adapter 1700 is configured with a support hub
member 1701 that
radially surrounds at least a portion of the length of the syringe adapter
sidewall 1702. In an
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
embodiment as illustrated in FIG. 17, the exterior of the outer wall of
support hub member
1701 has a hexagonal shape. This hexagonal shape may enable a person to have a
better
grasp when connecting or disconnecting the syringe adapter 1700 to syringe 300
and/or
needle holder 1710. (Note that while FIG. 17 illustrates the exterior of
support hub member
1701 as being hexagonal in shape, this is by way of illustration but not of
limitation, and the
exterior may be configured to have a different shape without deviating from
the scope of the
present invention.)
[0085] Notably, a tip at the distal end of sidewall 1702 (noting that the
distal end of sidewall
1702 is the end at which reference number 1702 is generally located, and
reference number
1704 denotes a flanged proximal end of sidewall 1702) is shown in FIG. 17 as
having a
relatively sharp point. The sharp point, or tip, is designed to assist in
inserting the syringe
adapter 1700 into the rubber membrane on a medicine bottle. The particular
taper illustrated
in FIG. 17 for this sharp point may be adjusted, thereby altering the degree
of sharpness,
without deviating from the scope of the present invention.
[0086] A Luer-type lock connection between syringe adapter 1700 and syringe
tip 340 is
preferably made in the manner discussed above with reference to FIG. 15, and
accordingly,
details of the connection are not repeated here. A result of the connection is
illustrated in the
non-exploded cross-sectional view in FIG. 17C.
[0087] In an embodiment, the opening 1709 at the distal end of sidewall 1702
is
approximately .10 inches in inside diameter (and as discussed in further
detail hereinafter, the
diameter is preferably about 0.05 inches to about 0.1585 inches, and more
preferably, is about
0.078 inches to about 0.14 inches, and even more preferably, is about 0.09
inches to about
0.11 inches, and generally, is approximately 0.10 inches). In the illustration
as shown, the
distal end of sidewall 1702 extends beyond the distal end of support hub
member 1701 (i.e.,
41
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
the end where reference number 1703 is generally located, the proximal end of
support hub
member 1701 being opposite thereto) to allow the distal end of sidewall 1702
to penetrate the
rubber membrane on the medication bottle.
[0088] A distal end of support hub member 1701 is preferably configured with
an internal
threaded portion, similar to the threaded portion discussed above with
reference to support
hub member 1501 of FIG. 15, and a Luer-type lock connection between syringe
adapter 1700
and needle holder 1710 is preferably made using the threads (illustrated in
FIG. 17 generally
by small extensions 1703) and the flanged area 1711 (which may alternatively
be referred to
as a tabbed area) at the proximal end of needle holder 1710 in the manner
discussed above
with reference to FIG. 15. See the non-exploded cross-sectional view in FIG.
17C.
[0089] Needle holder 1710 is configured for removably attaching a needle 400.
Needle
holder 1710 includes an internal threaded portion (see reference number 1712)
on its distal
end (i.e., the end where reference number 1712 is generally located), where
these threads are
configured to provide a Luer-type lock connection with the flange 460 at the
proximal end of
needle 400, thus providing a relatively secure connection with the needle.
More particularly,
the connection between needle holder 1710 and needle 400 is preferably made by
inserting
the flanged proximal end 460 into internal threaded portion 1712 and then
twisting the needle
until the flanged area locks into place in the internal threaded portion of
the needle holder.
[0090] In an embodiment as depicted in FIG. 17, an exterior wall of needle
holder 1710 is
preferably hexagonal in shape in a lower portion and cylindrical in shape in
an upper portion,
as shown in the isometric view in FIG. 17A, with exception of the proximal end
where flange
1711 is located (and the protrusion at the distal end). As shown in FIG. 17,
the proximal end
immediately above the flange 1711 has a conical shape, by way of illustration
but not of
limitation (see, for example, the illustration in FIG. 17D, which depicts the
exterior shape
42
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
thereof as being somewhat tapered); as one alternative, the shape may be
cylindrical and still
engage the threads on 1700 properly. Needle holder 1710 may serve to provide
additional
strength for the assembly, and thus may reduce the likelihood of a physical
failure during use.
[0091] Reference number 1719 denotes the opening in the distal end of needle
holder 1710.
As discussed above with reference to needle holder 1500, the inside diameter
of opening
1719 is preferably approximately .08 inches, and in an embodiment, the outside
diameter
conforms to measurements for Luer-type lock connections as stated in
International Standard
ISO 80369-7:2016 to accommodate a Luer-type lock connection to a needle 400
that is
manufactured in conformance with such measurements. As contrasted to the inner
chamber
1613 of needle holder 1610, it will be noted that the inner chamber 1713 of
needle holder
1710 is approximately twice as long as chamber 1613. This added length serves
to accept the
full length of the elongated tip 1702 of syringe adapter 1700, as can be seen
in the non-
exploded cross-sectional view in FIG. 17C, and thus the length of inner
chamber 1713
extends from the attachment point in support hub member 1701 to cover the
distal end of
syringe adapter 1700.
[0092] Preferably, syringe adapter 1700 and needle holder 1710 are constructed
from a plastic
or a composite. Syringe adapter 1700 and/or needle holder 1710 may
alternatively be
constructed from another material, such as stainless steel, aluminum, or
another metal (or a
combination thereof), without deviating from the scope of the present
invention ¨ although as
discussed earlier, using a material such as plastic for the syringe adapter
and needle holder
advantageously allows these components to be recyclable.
[0093] Syringe adapter 1700 may optionally be configured as a permanent
attachment to (or
replacement for) syringe tip 340, rather than being removably affixed thereto.
(See the
discussion of FIGs. 18 - 21, below, which depict embodiments that replace a
conventional
43
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
syringe tip.)
[0094] Note that while the needle holder 1710 and needle 400 may be removed as
a unit from
the syringe adapter 1700, this is by way of example; alternatively the needle
may be removed
from the needle holder and the needle holder may then be removed from the
syringe adapter,
without deviating from the scope of the present invention.
[0095] FIGs. 18 and 19 illustrate additional embodiments of the disclosed
syringe adapter,
showing exterior views as well as cross-sectional views of placement thereof
upon a syringe
and as exploded. FIGs. 18 and 19 are similar, and thus will be described
together. The
embodiments illustrated in FIGs. 18 and 19 include a needle holder to which a
needle may be
removably affixed. In these embodiments, a needle 400 is removably affixed to
a needle
holder 1810 or 1910 that, in turn, is removably affixed to a syringe adapter
1800 or 1900 that
is removably affixed to a syringe 300' for the injection of medication into a
recipient. When
the injection is completed, the needle 400 preferably remains affixed to the
needle holder
1810 or 1910, enabling needle 400 and needle holder 1810 or 1910 to be removed
from
syringe adapter 1800 or 1900 as a single unit (for example, in preparation for
using syringe
adapter 1800, 1900 for withdrawing additional fluid medication from a
container). This
enables the combination of needle holder and needle to be quickly and easily
re-installed on
the syringe adapter, after the syringe adapter is used for withdrawing a next
dosage of
medication, for administering that next dosage. (It should be noted that the
cross-sectional
views in FIGs. 18 and 19 illustrate a preferred interior and exterior shape of
components
1800, 1810, 1900, 1910, but embodiments are not limited to the specific shapes
and/or
relative dimensions as shown except as otherwise noted herein.)
[0096] As shown in FIGs. 18 and 19, syringe adapters 1800, 1900 are preferably
configured
with a support hub member 1801, 1901 that is a solid piece surrounding an
inner chamber, in
44
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
contrast to the approach of the support hub members shown in FIGs. 15 - 17,
with an
extension from the proximal end (see 1804, 1904) and an extension from the
distal end (see
1802, 1902) of this support hub member, where this distal end extension is
analogous to
previously-discussed distal end of a sidewall. (The proximal end of support
hub members
1801, 1901 is the end where reference numbers 1806, 1906 are generally
located, and the
distal end of the support hub members 1801, 1901 is the end where reference
numbers 1803,
1903 are generally located.) In embodiments as illustrated in FIGs. 18 and 19,
the exterior of
the support hub members 1801, 1901 have a generally hexagonal shape, although
another
shape (such as cylindrical) may be used without deviating from the scope of
the present
invention.
[0097] FIGs. 18 and 19 depict a tip 1802, 1902 extending as a sidewall from
the distal end of
support hub members 1801, 1901, where these tips are shown as having a
relatively sharp
point. Reference numbers 1809, 1909 indicate the opening formed by the
sidewall at the
distal end of syringe adapter 1800, 1900, respectively. In an embodiment,
openings 1809,
1909 are each approximately .10 inches in inside diameter (and as discussed in
further detail
hereinafter, the diameter is preferably about 0.05 inches to about 0.1585
inches, and more
preferably, is about 0.078 inches to about 0.14 inches, and even more
preferably, is about
0.09 inches to about 0.11 inches, and generally, is approximately 0.10
inches). As discussed
above, the sharp point is designed to assist in inserting the syringe adapter
1800, 1900 into the
rubber membrane on a medicine bottle, and the particular taper may be adjusted
from the
angle shown in the figures, thereby altering the degree of sharpness, without
deviating from
the scope of the present invention. (It should also be noted that the sharp
point may be
eliminated, using instead a tip generally similar to the shape illustrated in
FIGs. 15 - 16, and
such alternative shape is deemed to be within the scope of the present
invention. See FIGs.
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
20 and 21, which correspond to FIGs. 18 and 19, respectively, but show an
alternative
approach where the sharp point is not used. The descriptions of FIGs. 18 and
19 apply
equally to the embodiments depicted in FIGs. 20 and 21, except as noted
herein.)
[0098] FIGs. 18 and 19 also depict a change to how the syringe adapter 1800,
1900 attaches
to a syringe. Rather than forming a Luer-type lock connection between syringe
adapter 1800,
1900 and a syringe tip such as tip 340, the approach shown in FIGs. 18 and 19
is to remove
the syringe tip from the syringe (or equivalently, to use a syringe which has
not been fitted
with a syringe tip). In a preferred approach, connection between the syringe
adapter 1800,
1900 and a syringe is made by inserting an extension 1804, 1904 at the
proximal end of the
syringe adapter into a cavity 342 where the syringe tip would have been
located. Notably,
conventional syringe tips are made to be removable in some syringes, allowing
for cleaning
(for example). Accordingly, threads into which such removable syringe tips are
connected
may be leveraged for connecting the extensions 1804, 1904. The internal cavity
342 of a
typical syringe is configured to provide a female threaded portion, and the
proximal end of
the syringe adapter 1800, 1900 is thus preferably configured to provide a male
connector
(such as the previously-discussed flange or tabs) at extensions 1804, 1904. In
an
embodiment, a Luer-type locking connection is made between cavity 342 and
extensions
1804, 1904 by sizing extensions 1804, 1904 to conform to measurements for Luer-
type lock
connections as stated in International Standard ISO 80369-7:2016, thereby
enabling a secure
attachment to a cavity 342 that is manufactured in conformance with such
measurements.
The syringe adapters 1800, 1900 may therefore serve as a permanent attachment
to a syringe,
or as a replacement for a syringe tip. The syringe adapters 1800, 1900 may
alternatively be
viewed and/or configured as a semi-permanent attachment or replacement, in
that it can be
removed if desired. Preferably, syringe adapters 1800, 1900 include a rubber
gasket (or
46
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
similar fitting) on an underside of the support hub member 1801, 1901, as
shown by reference
numbers 1806, 1906. A result of the connection is illustrated in the non-
exploded cross-
sectional views in FIGs. 18C and 19C.
[0099] Syringe adapters 1800, 1900 differ in the width of extensions 1804,
1904. A
corresponding width is used for cavity 342. FIGs. 18 and 19 both illustrate
the exterior of
support hub members 1801, 1901 as being generally hexagonal in shape, although
this is by
way of illustration but not of limitation and the exterior may be configured
to have a different
shape without deviating from the scope of the present invention.
[0100] A distal end of support hub members 1801, 1901 is preferably configured
with an
internal threaded portion, similar to the threaded portion discussed above
with reference to
support hub member 1501 of FIG. 15, and a Luer-type lock connection between
syringe
adapter 1800, 1900 and needle holder 1810, 1910 is made using the threads
(illustrated in
FIGs. 18 and 19 generally by small extensions 1803, 1903) and flanged area
1811, 1911
(which may alternatively be referred to as a tabbed area) at the proximal end
of needle holder
1810, 1910 in the manner discussed above with reference to FIG. 15. See the
non-exploded
cross-sectional views in FIGs. 18C and 19C.
[0101] Needle holders 1810, 1910 are configured for removably attaching a
needle 400 using
a Luer-type lock connection between an internal threaded portion 1812, 1912 on
the distal
end of the needle holder (i.e., the end where reference numbers 1812, 1912 are
generally
located) and a flanged area 460 of needle 400 in the manner discussed above
with reference to
FIGs. 16 and 17, and accordingly, details of the connection are not repeated
here. A result of
the connection is illustrated in the non-exploded cross-sectional views in
FIGs. 18C and 19C.
[0102] In embodiments as depicted in FIGs. 18 and 19, an exterior wall of
needle holders
1810, 1910 is preferably hexagonal in shape in a lower portion and cylindrical
in shape in an
47
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
upper portion, as shown in the isometric views of FIGs. 18A and 19A, with
exception of the
proximal end where flange 1811, 1911 is located (and the protrusion at the
distal end). As
shown in FIGs. 18 and 19, the proximal end immediately above the flange 1811,
1911 has a
conical shape, by way of illustration but not of limitation (see, for example,
the illustrations in
FIGs. 18D and 19D, which depict the exterior shape thereof as being somewhat
tapered); as
one alternative, the shape may be cylindrical and still engage the threads on
1800, 1900
properly. Needle holder 1810, 1910 may serve to provide additional strength
for the
assembly, and thus may reduce the likelihood of a physical failure during use.
[0103] Reference numbers 1819, 1919 denote the opening in the distal end of
needle holders
1810, 1910. As discussed above with reference to needle holder 1500, the
inside diameter of
openings 1819, 1919 is preferably approximately .08 inches, and in an
embodiment, the
outside diameter conforms to measurements for Luer-type lock connections as
stated in
International Standard ISO 80369-7:2016 to accommodate a Luer-type lock
connection to a
needle 400 that is manufactured in conformance with such measurements. The
length of
inner chambers 1813, 1913 of needle holders 1810, 1910 preferably extends from
the
attachment point in support hub member 1801, 1901 to cover the distal end of
syringe adapter
1800, 1900, thus accepting the full length of the elongated tips 1802, 1902 of
syringe adapters
1800, 1900, as can be seen in the non-exploded cross-sectional views in FIGs.
18C and 19C.
The embodiments depicted in FIGs 20 and 21 differ from those of FIGs. 18 and
19 primarily
because the blunt tips 2002 and 2102 of syringe adapters 2000 and 2100,
respectively, are
somewhat shorter in length than sharp tips 1802, 1902; accordingly, the inner
chambers
2013, 2113 of needle holders 2010, 2110 may be shorter in length than chambers
1813, 1913
while still accepting the full length of tips 2002, 2102 as can be seen in the
non-exploded
cross-sectional view in FIGs. 20C and 21C.
48
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
[0104] Preferably, syringe adapters 1800, 1900 and needle holders 1810, 1910
are
constructed from a plastic or a composite. Syringe adapters 1800, 1900 and/or
needle holders
1810, 1910 may alternatively be constructed from another material, such as
stainless steel,
aluminum, or another metal (or a combination thereof), without deviating from
the scope of
the present invention ¨ although as discussed earlier, using a material such
as plastic for the
syringe adapters and needle holders advantageously allows these components to
be
recyclable.
[0105] Note that while the needle holders 1810, 1910 and needle 400 may be
removed as a
unit from the syringe adapters 1800, 1900, this is by way of example;
alternatively the needle
may be removed from the needle holder and the needle holder may then be
removed from the
syringe adapter, without deviating from the scope of the present invention.
[0106] It should be noted that while discussions herein refer primarily to
making a locking
connection by twisting a first feature within a second feature, it will be
obvious that the
second feature may be twisted within the first feature or that both features
may be twisted,
without deviating from the scope of the present invention.
[0107] Use of the disclosed syringe adapter while medicating an animal
operates, in some
embodiments, as follows: the syringe adapter is affixed to a syringe (which,
as noted earlier,
is preferably a pistol-grip or tab-handled syringe); the syringe adapter is
inserted into a bottle
of medication; the plunger of the syringe is pulled back to withdraw the
desired dosage of
medication from the bottle into the syringe barrel; the syringe adapter (which
remains
attached to the syringe) is removed from the bottle, while the plunger remains
stationary; the
syringe adapter is replaced with a needle; and the medication (or some portion
thereof) is then
injected by pushing the plunger forward (for example, by squeezing the pistol-
grip handles or
pressing down on the tabbed handle) to expel medication from the syringe
barrel. If it is
49
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
desired to reuse the syringe adapter, then the needle is removed from the
syringe, after which
the above process is repeated. (As noted earlier, the disclosed syringe
adapter is not limited
to use with medication intended for any particular type of animal life, and
therefore the
medication may be injected more generally into a "target" or a "recipient")
[0108] Use of the disclosed syringe adapter operates, in some other
embodiments, as follows:
the syringe adapter is affixed to a syringe (preferably a pistol-grip or tab-
handled syringe); the
syringe adapter is inserted into a bottle of medication; the plunger of the
syringe is pulled
back to withdraw the desired dosage of medication from the bottle into the
syringe barrel; the
syringe adapter (which remains attached to the syringe) is removed from the
bottle, while the
plunger remains stationary; a needle is affixed to the syringe adapter (and
note that the
syringe adapter remains affixed to the syringe); and the medication (or some
portion thereof)
is then injected by pushing the plunger forward (for example, by squeezing the
pistol-grip
handles or pressing down on the tabbed handle) to expel medication from the
syringe barrel.
If it is desired to reuse the syringe adapter (for example, for withdrawing
additional fluid for
medicating another animal), then the needle is removed from the syringe
adapter, after which
the above process of withdrawing medication using the syringe adapter,
affixing a needle
thereto, and then injecting the medication (or some portion thereof) is
repeated.
[0109] Use of the disclosed syringe adapter operates, in still other
embodiments, as follows:
the syringe adapter is affixed to a syringe (preferably a pistol-grip or tab-
handled syringe),
and this attachment may be temporary (i.e., removable), permanent, or semi-
permanent (that
is, intended as a permanent replacement for a conventional syringe tip,
although being
configured to be removable, such as for cleaning); the syringe adapter is
inserted into a bottle
of medication; the plunger of the syringe is pulled back to withdraw the
desired dosage of
medication from the bottle into the syringe barrel; the syringe adapter (which
remains
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
attached to the syringe) is removed from the bottle, while the plunger remains
stationary; a
needle holder, to which a needle is affixed (either before or after connecting
the needle holder
and the syringe adapter), is affixed to the syringe adapter (and note that the
syringe adapter
remains affixed to the syringe); and the medication (or some portion thereof)
is then injected
by pushing the plunger forward (for example, by squeezing the pistol-grip
handles or pressing
down on the tabbed handle) to expel medication from the syringe barrel. If it
is desired to
reuse the syringe adapter (for example, for withdrawing additional fluid for
medicating
another animal), then the needle holder and its affixed needle are removed
from the syringe
adapter (preferably as a single unit), after which the above process of
withdrawing medication
using the syringe adapter, affixing a needle holder with needle to the syringe
adapter, and then
injecting the medication (or some portion thereof) is repeated.
[0110] It should be noted that while embodiments are described herein as
conforming to the
above-cited International Standards and/or as using Luer-type connections to a
syringe, this is
by way of illustration but not of limitation. It should also be noted that the
figures are
directed toward illustrating aspects of the present invention, in combination
with descriptions
herein, and aspects shown therein (for example, length, width, and/or taper)
are not
necessarily drawn to scale.
[0111] While medications have been discussed herein as commonly being sold in
a multi-
dose bottle, this is by way of illustration and not of limitation. The
disclosed syringe adapter
may be used beneficially for medication that is sold in a single-use dosage.
Also, it should be
noted that while some discussions herein refer to expelling "the withdrawn
medication" or
"emptying" the syringe, this is by way of illustration and not of limitation:
the scope of the
present invention does not require withdrawn medication to be expelled in full
nor does it
require a syringe to be fully emptied.
51
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
[0112] Advantageously, the disclosed syringe adapter may be included with
purchase (e.g.,
within the packaging) of a higher-viscosity medication. As one alternative, a
multi-pack of
the disclosed syringe adapter may be included with such purchase, particularly
when the
medication is sold in a multi-dose bottle. The disclosed syringe adapter may
also be sold
separately from medication. Advantageously, a supplier of packaged syringe
adapters may
ensure that they are sterilized and/or sanitized by distributing them in
sealed packaging.
[0113] Examples of higher-viscosity animal medications with which the
disclosed syringe
adapter may be used beneficially include Nuflor , Nuflor Gold , and Resflor
Gold .
("Nuflor", "Nuflor Gold", and "Resflor Gold" are registered trademarks of
Intervet Inc. in the
United States, other countries, or both. Intervet is now known as "Merck
Animal Health".)
These medications are commonly sold in 500-milliliter multi-dose bottles and
may be
administered, by way of example, in dosages of 36 to 60 milliliters per
animal. Accordingly,
a single multi-dose bottle may be used to treat generally 8 to 14 animals at
this dosage range.
[0114] As noted earlier, viscosity of a substance may vary with temperature.
Viscosity is
commonly measured in units termed "centipoise", which may be abbreviated as
"cP" or
"cps". Water, at 70 degrees Fahrenheit, has a viscosity of approximately 1
cps, and by way of
comparison, blood generally has a viscosity of about 10 cps. By convention, a
temperature of
70 degrees Fahrenheit is used as a reference point for measuring cps, and thus
when a
temperature is not mentioned for a particular cps measurement, it should be
assumed that the
temperature associated with the stated measurement is 70 degrees Fahrenheit.
[0115] According to a study documented in "Syringeability and Viscosity
Comparative of
Different Florfenicol Formulations" by S. Colomer, et at., date unknown, the
viscosity of
Nuflor at 5 degrees Celsius (which is approximately 41 degrees Fahrenheit)
was 321 cps.
U. S. Patent 8,034,845, titled "Compositions and Method for Treating Infection
in Cattle and
52
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
Swine", discusses a formulation believed to correspond to Nuflor Gold and
states that
formulations of the invention disclosed therein preferably "have a viscosity
of less than about
125 cps".
[0116] An embodiment of the present invention is believed to be advantageous
for fluid
medications having a viscosity of at least 50 to 100 cps at a temperature of
at least 5 degrees
Celsius, as well as for fluid medications having a higher cps at this
temperature (noting, as
stated above, that viscosity varies with temperature).
[0117] FIG. 22 presents tables containing measurements from tests conducted to
compare use
of a sample version of the disclosed syringe adapter to use of conventional
needles. The
tested medication was Resflor Gold , and a withdrawn quantity thereof was 30
cc (computed
as a desired volume for treating an animal with a body weight of 500 pounds).
Results of
these tests will now be discussed.
[0118] In a first test (denoted "Test #1" in FIG. 22), the medication was at
room temperature.
A withdrawal rate was measured using an 18-gauge needle having a 1-inch
length, a 16-gauge
needle having a 5/8-inch length, and the syringe adapter. A 16-gauge needle
has a larger tip
opening (i.e., a larger inside) than an 18-gauge needle, and will therefore
withdraw a solution
faster than the 18-gauge, although the 16-gauge needle is thought to be
disfavored for at least
some situations because it may allow the (relatively expensive) medication to
leak out of the
animal during the medicating process. In addition, a 16-gauge needle is
thought to be too
large to use on smaller animals. In the sample version, the diameter of the
opening in the
distal end of the syringe adapter was approximately 0.094 inches.
[0119] In this first test, the bottle of medication was placed upon a table
and the syringe
adapter was already mounted upon a syringe held by the tester, and the elapsed
withdrawal
times include picking up the bottle and inserting the syringe adapter into the
bottle. As
53
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
shown in FIG. 22, withdrawing 30 cc of Resflor Gold in this test environment
required 3
minutes 50.30 seconds using the 18-gauge needle and 35 seconds using the 16-
gauge needle,
as compared to 8 seconds using the syringe adapter.
[0120] Time to expel the 30 cc of medication was also tested in this first
test. Expelling the
medication in this test environment required 35 seconds using the 18-gauge
needle and 11
seconds using the 16-gauge needle. (Time to expel the medication was not
measured using
the syringe adapter, because the expel time depends on the needle used for
injecting the
medication.)
[0121] In a second test (denoted "Test #2" in FIG. 22), the medication was
again at room
temperature, but the bottle of medication was now held by the tester to
eliminate time
required to pick up the bottle. Accordingly, the elapsed withdrawal times in
this test begin
with inserting the syringe adapter into the bottle. As shown in FIG. 22,
withdrawing 30 cc of
Resflor Gold in this test environment required an average of 5.54 seconds
when using the
syringe adapter, where this average was computed by taking measurements 6
times and
discarding a time that appeared to be an outlier. (Because slightly more than
2 seconds were
gained by omitting the "pick up" time of the bottle, this test was not
performed using the
needles: it may be assumed that the withdrawal times using needles in the
first test would be
approximately 2 seconds less under the environment of this second test.)
[0122] In a third test (denoted "Test #3" in FIG. 22), the medication was now
at a
temperature of approximately 38 to 40 degrees Fahrenheit, having just been
removed from
refrigeration (noting that this temperature was intended to simulate a cold
weather
environment in which the tested medication might be used). The bottle of
medication and
syringe with affixed syringe adapter were again held by the tester (and the
elapsed times
began with inserting the syringe adapter into the bottle), as in the second
test. As shown in
54
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
FIG. 22, withdrawing 30 cc of the now-cooler-temperature Resflor Gold in this
test
environment required 3 minutes 42.27 seconds using the 16-gauge needle, as
compared to 1
minute 1 second using the syringe adapter. Note that this third test was not
conducted using
the smaller 18-gauge needle, but by comparison to the results of the first
test, it can be seen
that the withdrawal time using the smaller (i.e., 18-gauge) needle may be
expected to greatly
exceed the nearly 4-minute withdrawal time for the 16-gauge needle.
[0123] While a preferred diameter for the hole, or opening, in the distal end
of the syringe
adapter is about 0.10 inches, embodiments disclosed herein are not limited to
this preferred
diameter, as noted earlier. Instead, a syringe adapter as disclosed herein may
have an
opening, or distal end hole, within any suitable range. In addition to the
tests discussed above
with reference to FIG. 22, which were conducted using a sample version of the
syringe
adapter in which the distal end opening measured approximately .094 inches,
additional
experiments and testing have been performed using syringe adapter prototypes
with a range of
sizes for the distal end opening of the syringe adapter, in view of both draw
time and impact
upon the rubber membrane, and those results will now be discussed.
[0124] When considering an upper bound on the size of the opening in the
distal end of the
syringe adapter, it will be readily understood that throughput time of a fluid
drawn into a
syringe is necessarily limited by the size of the opening into the syringe
itself (that is, the
opening at the distal end of the syringe). It is believed that commercially-
available syringes
adhere to a standardized maximum outer diameter for the tip of a syringe,
where this
standardized maximum outer diameter is stated in a version of the above-cited
International
Standards as about 0.1585 inches. The version of the International Standards
did not appear
to state a standardized inner diameter for the tip of a syringe, and
accordingly, the
measurement of 0.1585 inches has been adopted for preferred embodiments
because it is
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
deemed to necessarily provide an upper bound on the inner diameter of the
syringe tip and
therefore on the width of the opening through which fluid will enter the
syringe. It is noted
that commercially-available syringes have distal end openings in different
inner diameters.
Several commercially-available syringes were measured, and were found to have
distal end
openings that range from an inner diameter of 0.0625 inches to 0.1094 inches,
for example.
In view of this information, it is noted that the draw time when using a
syringe adapter will
not be appreciably improved by increasing the syringe adapter distal end
opening size beyond
the limiting size of the opening into the syringe itself. Accordingly, for a
syringe having an
opening with a maximum inner diameter of about 0.1585 inches, a measurement of
about
0.1585 inches forms a de facto upper bound on the size of the distal end
opening of the
disclosed syringe adapter. In the more general case where the distal end
opening of a syringe
is smaller or larger than 0.1585 inches, an inner diameter of the distal end
opening in the
syringe adapter preferably provides compatibility with the size of an inner
diameter of the
distal end opening into such syringe. Advantageously, using a syringe adapter
distal end
opening with an inner diameter on the order of the 0.1 inch measurement
previously noted
herein provides compatibility with a plurality of different syringe opening
sizes.
[0125] It is noted that a standard opening size for the 16-gauge needle used
in the testing of
FIG. 22 is about 0.047 inches, and that the above discussion of FIG. 9
referred to an example
where the diameter of the distal end of conical portion 940 is approximately
.156 inches (and
when using a sidewall thickness of 0.05 inches, an inner diameter of
approximately 0.056
inches). Additional experiments and testing used prototypes with additional
opening sizes at
the distal end of the syringe adapter to validate earlier conclusions. Draw
times using
conditions analogous to Test #1 of FIG. 22 were measured for syringe adapter
prototypes
having distal end inner diameters in increments of 1/64 inch, from decimal
measurements of
56
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
0.0625 inches through 0.171875 inches (corresponding to 4/64 inches through
11/64 inches),
as well as a prototype having a 0.09 inch distal end inner diameter. The
average draw time
for the prototype with a 0.0625 inch inner diameter was 19.67 seconds. While
this is notably
improved over the results of the 18-gauge and 16-gauge needles of Test #1, it
is significantly
less attractive than the average draw time noted for the prototype having the
0.09 inch inner
diameter, which had an average draw time of 5.88 seconds. The prototype having
a distal end
inner diameter of 0.078125 inches yielded an average draw time of 8.45
seconds, showing
very significant improvement over the next-smaller 0.0625 inch opening. All
remaining
prototype sizes in this testing (ranging from 0.09375 inches to 0.17185
inches) showed an
average draw time within 0.5 seconds of the prototype with the 0.09 inch inner
diameter ¨
notably leveling off in draw time improvement, in spite of increasing the
distal end inner
diameter, to a variance that will not be detectable by a user. (For example,
the average draw
times for the prototypes with distal end inner diameters of 0.14 inches and
0.15625 inches
were 5.82 seconds and 5.81 seconds, respectively, while the average time for
the inner
diameter of 0.109 inches was 6.11 seconds.) In view of this information, a
preferred inner
diameter of the distal end opening in the syringe adapter is in the range of
about 0.05 inches
to about the maximum size of the distal end opening into the syringe (believed
to be about
0.1585 inches, as noted above), and more preferably, this inner diameter is
about 0.078 inches
to about 0.14 inches, and even more preferably, is about 0.09 inches to about
0.11 inches, and
generally, is about 0.10 inches.
[0126] A consideration for the outer diameter of the distal end of the syringe
adapter is the
impact of inserting the distal end of the syringe adapter into the rubber
membrane affixed to
the top of the bottle. It is noted that some vendors state (for example, in a
label affixed to a
medicine bottle or perhaps in its accompanying literature) an upper limit on
the number of
57
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
times the rubber membrane can safely be punctured. An example is commercially
known, for
example, in which the label states that the rubber membrane should not be
punctured more
than 20 times. This limit is believed to be due to a potentially compromising
effect that the
puncturing tip of a sharp needle may have on the rubber membrane (where, for
example,
compromising the rubber membrane may enable the fluid medication to leak out).
In view of
this consideration, experimentation and testing was performed using prototypes
with different
sizes for the outer diameter of the distal end of the syringe adapter to
determine what outer
diameter size might compromise the rubber membrane. In this testing, the
distal end of each
tested prototype syringe adapter was cylindrical in shape.
[0127] For the testing of rubber membrane compromise, it was noted above that
a common
diameter of the rubber membrane fitted in the top of 250 ml bottles and 500 ml
bottles is 0.75
inches. The typical rubber membrane has a dimple in the center where it is
intended for the
rubber membrane to be penetrated. A common size of this dimple is about 0.1730
inches to
about 0.1760 inches. In view of this 0.75 inch membrane size and approximately
0.1750 inch
dimple size, an initial test was conducted with a syringe adapter prototype
having an outer
diameter of approximately 0.26 inches on the distal end. Inserting this
prototype through the
rubber membrane was extremely difficult, and appeared to compromise the rubber
membrane.
Accordingly, no further testing above this 0.26 inch outer diameter was deemed
beneficial.
Another prototype syringe adapter was tested, this time having an outer
diameter of 0.1815
inches on the distal end. Inserting this prototype into the rubber membrane
was possible with
less effort, and did not appear to compromise the rubber membrane. In view of
these results,
an upper bound on the outer diameter of the distal end of the syringe adapter
is preferably
about 0.1815 inches for a rubber membrane sized at about 0.75 inches and a
corresponding
dimple of about 0.1750 inches. In a more general case, an upper bound on the
outer diameter
58
CA 03158155 2022-04-13
WO 2021/108257 PCT/US2020/061523
of the distal end of the syringe adapter is approximately about the size of
the dimple of the
rubber membrane.
[0128] As has been demonstrated, an embodiment of the present invention
improves
syringeability of higher-viscosity medications, allowing such medication to be
withdrawn
from a bottle in much less time as compared to the known approach of
withdrawal using a
needle. More animals may therefore be medicated in a given period of time,
leading to
improved productivity of persons caring for the animals as well as enabling
overall improved
health for the animals. No longer will higher viscosity be a barrier to the
market, and because
medication of this type will be more readily administered when using a syringe
adapter as
disclosed herein, improvement may be expected in animal health, and market
share and/or
market presence for the medication may improve as well.
[0129] It should be noted that various features discussed herein with
reference to "an
embodiment", "one embodiment", "a preferred embodiment", and so forth should
not be
construed as suggesting that each such feature is present in a single
embodiment, or in every
embodiment, of the present invention. Instead, it should be understood that
there may be
various combinations of the disclosed features present in any particular
embodiment.
[0130] While embodiments of the present invention have been described,
additional
variations and modifications in those embodiments may occur to those of
ordinary skill in the
art once they learn of the basic inventive concepts. Therefore, it is intended
that the appended
claims shall be construed to include the described embodiments and all such
variations and
modifications as fall within the spirit and scope of the invention.
59