Note: Descriptions are shown in the official language in which they were submitted.
Title: Method of Breath Screening of Viral Infection
Field of the Invention
[001] This invention relates to the field of testing for COVID-19 infection in
subjects.
Background of Invention
[002] The Covid-19 pandemic has created a need for large scale testing for
cases of SARS-CoV-
2, the virus that causes Covid-19. People requiring tests include the public
at large, and also,
longitudinal testing of high-risk sub-populations, such as health care
workers.
[003] COVID-19 testing is currently based on quantitative reverse
transcription polymerase
chain reaction (RT-qPCR) assays detecting SARS-CoV-2 RNA in nasopharyngeal
(NP) and/or
oral swabs. These tests: require authorized laboratories with a minimum
Biological Safety Class
2 specification; take time to ship, process and report; and are prone to false
negative results (from
errors in swab sampling or laboratory processing, or because the virus is not
yet/no longer
present in the oropharynx). The false negative rate for one-time NP testing by
RT-qPCR is 30%
to 50% for COVID-19 samples acquired in community or clinical care settings,
while the area-
under-the-receiver operator characteristic (AUROC) for a single RT-qPCR test
is about 0.8.
Repeat RT-qPCR tests, combined with hematological variables and chest computed
tomography,
are advised for diagnosis, along with caution in the interpretation of
negative RT-qPCR tests.
[004] Exhaled breath analysis has attracted notable scientific and clinical
interest in recent years.
Volatile organic compounds (VOCs) have the potential to minor various
metabolic processes
both locally within the respiratory system and systemically, via blood
circulation. VOCs have
been utilized as diagnostic, prognostic, and treatment response biomarkers for
various respiratory
illnesses, including infections.
[005] US patents No. 9,170,232 and No. 9,541,525 describe an ion-mobility
spectrometer with
front fast GC separation of the sample analytes. US patent No. 9,329,156
describes a filter used
in the collection of sample breath and enrichment of the sample. US patent No.
5,395,589
discloses an apparatus for preconcentrating trace amounts of organic vapors in
a sample of air for
subsequent detection.
Summary of the Invention
[006] It is desirable to provide a point-of-care testing apparatus and method
because results
can be obtained relatively quickly (preferably less than 10 minutes). Also,
because there is
no sample that needs to be transported for analysis, there is much reduced
risk that it will be
lost, damaged, or contaminated during sample collection, transport, and
analysis.
[007] It is also desirable to provide a non-invasive or less invasive form of
COVID-19 testing.
There are test subjects who have an aversion to more invasive types of sample
collection
instruments, such as swabs. This aversion can range from moderate to severe.
Some test
Date Recue/Date Received 2022-05-25
subjects will avoid testing completely, or at least suffer anxiety and
discomfort from invasive
sample collection. A non-invasive mode of sample collection can ameliorate
these problems.
[008] COVID-19 is a multi-system condition. It was hypothesized that a
combination of
inflammatory and host-response VOCs would differentiate between the breath of
patients
with COVID-19 and those with other respiratory or cardiac problems. After the
start of the
COVID-19 pandemic, independent feasibility studies were rapidly carried out
with the
following objectives: (1) to trial point-of-care testing using self-contained
GC-IMS breath
analyzers, and (2) to evaluate the breath biochemistry for possible markers of
COVID-19.
[009] Several studies have shown that specific VOCs related to disease are
present as a result of
contraction of that disease, and that some diseases can be identified using
biomarkers(s) found in
the subject's breath. Since COVID-19 eventually reaches the stage where it
damages the lungs of
the subject, it was hypothesized that some volatile and non-volatile
biomarker(s), strongly related
to the COVID-19 disease would be present in the alveolar breath and breath
condensate as well as
in body odour of the infected population that could be distinguished from the
control (healthy)
population.
[010] In the biomarker discovery phase, a broad scope, untargeted
investigation for volatile organic
compounds (VOCs) found in breath and body scent of patients affected by COVID-
19 was
performed. Sample collection involved use of vapor enrichment cartridges
(e.g., Tenax GC) with
aspiration sample pump that can be used to screen a person's breath for
several minutes. This
allowed trapping and enrichment of VOCs exhaled from the patients. The
intention was to screen
and compare the sample of people who had tested positive and negative for
COVID-19. The
trapping cartridges were analysed at an accredited analytical laboratory using
GCxGC separation
and Time of Light Mass spectrometry (TOFMS) detection system. Chemometrics
software with
was used with the capability to extract maximum analytical information and
identification of key
components in samples of infected people compared to non-infected people. This
facilitated
discovery of differences between sample classes.
[011] The disclosed invention relates in one aspect to collection media to
trap exhaled breath from
a person. Preferably, the subject being tested exhales five or more times and
allowing enrichment
of the exhaled air onto the collector media. The enriched breath sample is
introduced into a short
gas chromatography-ion mobility spectrometer for thermal desorption and
analysis in a span of 20
seconds. The GC-plasmagram profile is analyzed by four-layer detection
algorithm and an AT
decision making process. The method was validated using COVID-19 infected
subjects and
healthy subjects. The invented method can be deployed at airports, shopping
malls, office
entrances, railway stations, universities, caregiver homes, etc. for rapid
screening of the mass
population. A non-invasive sampling approach of the method makes it a
promising and fast
technique for monitoring the health status of patients or volunteers during
the vaccine trial and
after vaccination.
Date Recue/Date Received 2022-05-25
Brief Description of the Drawings
[012] Reference will now be made, by way of example only, to preferred
embodiments of the
invention and in which:
[013] FIG. 1 is a representation of a breath sample being given;
[014] FIG. 2 is a schematic representation of the preferred analyser system;
[015] FIG. 3 is a typical 3D ion mobility spectrum or plasmagram 100 of a non-
infected person;
[016] FIG. 4 is a 3D ion mobility spectrum or plasmagram 200 of the breath of
a COVID-19
infected person;
[017] FIG. 5 is a schematic representation of the detector circuitry; and
[018] FIG. 6 is a schematic representation of alternative detector circuitry.
Detailed Description
[019] In an aspect of the invention, a GC is used as a tool to separate out
predetermined analytes,
specifically volatile organic compounds (VOCs) whose presence has been found
to be associated
with Covid-19 infection.
[020] A sampling card 38 is illustrated in FIG. 1. The sampling card 38
comprises a substrate 50
coated with a combination of adsorbent/absorbent materials. The
adsorbent/absorbent materials
function as a chemical filter, concentrating vapors and entrapping fine
airborne particles when air
is directed over the sampling card 38. A handle 52 is formed at one end of the
substrate 50. The
handle 52 facilitates handling of the sampling card 38, allowing the sampling
card to be readily
inserted into and removed from a suitable sampler.
[021] The substrate 50 may be formed of a stainless-steel mesh. Other possible
substrate
materials include nickel, copper, aluminum, fiberglass, porous Teflon, cotton,
Nomex and other
man-made fibers. Still other materials may be used. What is important is that
materials be used
that will retain the relevant VOCs for analysis.
[022] The combination of adsorbent/absorbent materials may comprise two or
more of
diphenylene oxide polymer(s) prepared in chloroform, carbon composite
materials such as
graphite, fullerenes, polymeric carbons from soot produced from nitro
substituted alkylbenzenes,
divinyl benzene, mono-alkyl substituted benzenes, di-alkyl substituted
benzene, toluene, xylenes,
ethylbenzene, silicone oils with high thermal stability and boiling points and
adsorption
properties for wide range of organic compounds or other suitable materials,
although silicone oils
are not preferred.
[023] VOCs on people's breath are usually present in the low parts per billion
concentration and
therefore, may well require some enrichment to bring the levels to the
detection limit of the
Date Recue/Date Received 2022-05-25
detector. The filter 50 is used to capture the volatile components on the
infected person and
excluding water and other light weight gases and preferably trap the target
volatiles that indicate
COVID-19 infection.
[024] The subject being tested will exhale, preferably ten times or more, onto
the substrate 50, so
that sample 12 comprising any relevant VOCs are absorbed into / adsorbed to
the substrate
material 50. Then the card 38, and the substrate 50, are inserted into heated
desorber 14.
[025] Referring now to FIG. 2, a schematic representation of the preferred
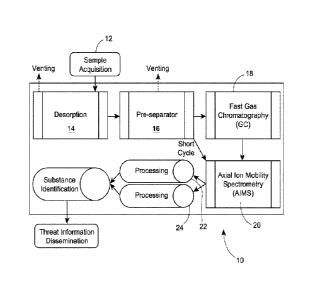
analyser system 10,
according to an aspect of the invention, is shown. The sample 12 is acquired
by the analyser
through interfacing with desorber 14. Desorber 14 communicates with pre-
separator 16, which
communicates both with GC 18, and AIMS 20. Processing means 22 and 24 are in
communication with AIMS 20, and the outputs of means 22, 24 are used to
identify substances
of interest, after which identification information is disseminated. In the
preferred embodiment, a
carrier gas (discussed below) carries the sample from the desorber 14, to the
pre-separator 16, the
GC 18 and the AIMS 20.
[026] Preferably, the desorber 14 includes means for ramping up temperature
upon receipt of a
sample to evaporate volatile compounds not of interest, thus cleaning the
sample. These volatile
contaminants are preferably vented. As the temperature continues to rise, the
cleaned sample is
then evaporated and travels to the pre-separator 16.
[027] The desorber is heated to 200 C which is sufficient to destroy any
biological sample that is
collected on the filter. Another aspect of the invention is trapping the
effluent after internal
sterilization on internal charcoal filter(s). This aspect of the invention
makes the filter reusable
for the next person.
[028] Preferably, the desorber 14 communicates with the pre-separator 16 via a
six-port heated
valve, which functions to keep the sample evaporated until it condenses in the
pre-separator 16.
The pre-separator 16 is kept cool while the sample is transferred from the
desorber 14, so that the
sample will condense and thus be trapped.
[029] The pre-separator 16 preferably operates as follows. It is heated in a
ramping fashion with
power pulses ranging from 100-500 sec to assist in the thermal separation of
different
compounds based on their physical and chemical properties. Each compound will
be released at
a different temperature, and thus at a different time, creating a temporal
separation between the
individual predetermined analytes present. The pre-separator 16 also functions
to release other
volatile compounds not of interest that were not removed by the desorber 14,
while separating in
time the release of potential analytes of interest as the pulsed increase in
temperature proceeds.
[030] Thus, the desorber 14 and pre-separator 16 function to eliminate
unwanted compounds
and/or contaminants (such as volatile compounds), and thus to preselect for
analysis compounds
likely to be of interest.
[031] Preferably, the pre-separated sample emerging from the pre-separator 16
is split into main
and bypass samples. The bypass sample is carried directly to AIMS 20,
permitting a faster
Date Recue/Date Received 2022-05-25
analysis because of the GC step being skipped for the bypass sample. This
faster analysis can, in
the preferred embodiment, take about 20-30 seconds, providing a quick
detection of threat
substances followed by confirmation after GC analysis of the main sample is
completed is
completed. This offers flagging of the sample for further investigation.
[032] On the other hand, if the short cycle shows no detection, there is a
strong likelihood that
the sample is clean. Preparations can begin to test the next sample. In the
unlikely event that the
long cycle shows detection when the short cycle did not, the relevant object
(e.g., shipping
containers, luggage, etc.) can be extracted and dealt with accordingly.
[033] Preferably, the main sample is carried to the GC, and the preferred GC
operates to
evaporate the main sample by upward ramping of temperature. The main sample
molecules are
preferably trapped by adsorption, condensation, surface interaction on a
cooled trapping material
consisting of an inert coated metal surface like GC liquid phase and other
means of trapping
molecules. The trap is resistively heated by applying power across its
terminals to release
trapped materials into the carrier gas and transfer the evaporated main sample
into the analytical
GC column. The preferred GC column can contain polar, semi-polar or non-polar
bonded liquid
phase for effective separation of molecules of interest.
[034] Temperature ramping of the preferred GC column under an internal carrier
gas is
accomplished by resistive heating of the column from 40 to 220 degrees
Celsius, which allows
separation of volatile and non-volatile (higher boiling point) compounds,
typically in a span of 1-
3 minutes. Positive and negative ions are formed for each analyte of interest,
as well as dimer
peaks because of the internal ion-molecular ionization processes. The initial
temperature of the
GC before heating is preferably maintained by an electrically driven cooling
fan.
[035] Those skilled in the art will appreciate that the analysis using the IMS
20 involves
ionization, typically both positive and negative, of the sample entering the
IMS. IMS devices, in
general terms, identify analytes of interest by measuring mobility of
associated ions using a drift
tube and detector. Chemical ionization reagents (CIRs) are deployed in the
IMS' ionization
chamber to facilitate ionization of the substances in the sample for
detection.
[036] The preferred embodiment of the system is configured to time the
deployment of CIRs to
be concurrent with the GC peaks of analytes of interest. In the preferred
embodiment, then, CIRs
are conserved, and wastage reduced, since CIRs are deployed only when needed
for ionization.
In the preferred embodiment, the microprocessor controlling the system 10 is
programmed to as
to release CIRs to the IMS only concurrently with GC peaks, that is, when
potential analytes of
interest are arriving for analysis. CIRs are preferably withheld during the
absence of GC peaks.
[037] Referring now to FIG. 5, the IMS assembly preferably comprises a
microprocessor or CPU
57 which is configured to switch on and off high voltage power supply 58
(HVPS). HVPS 58
and CPU 56 are operatively connected to switching and monitoring circuit 60,
which is used by
CPU 56 to monitor the voltage from the HVPS and to switch the voltage.
[038] The AIMS 20 receives the switching voltage and provides the raw output
used to calculate
ion mobility and identify, if appropriate, analytes of interest. The output is
amplified by a pre-
Date Recue/Date Received 2022-05-25
amplifier 62 prior to delivery to a data grabber circuit 64. It will be
appreciated that the pre-
amplifier is vulnerable to damage from sudden large changes in electric field
resulting from
changes in polarity and ionization of the sample. Specifically, damage may
result from sudden
change of voltages and voltage surge on the guard electrode located in front
of the IMS' Faraday
collector plate. The system 10 is thus configured to provide a protective
blanking pulse signal to
the pre-amplifier timed to coincide with the changes in the electric field,
thus preventing the
damage.
[039] Circuit 60 preferably provides the high voltage polarity needed to
operate the axial ion
mobility spectrometer (AIMS) in one polarity and the appropriate gating pulse
to introduce
single polarity ions into the single glass or ceramic tube drift tube. The
process is under CPU
control. The signal generated at the preamplifier 62 is fed to the data
grabber board 64 which
controls the blanking pulse and feedback to the switching and monitoring
circuit and to the CPU
56.
[040] In the preferred embodiment, the circuit 60 comprises a half H instead
of four H bridge,
which offers a simpler and faster switching circuit capability over other
configurations.
[041] Alternation between ion polarities is preferably governed by a timing
circuit of duration
varying from 100-500 msec, depending on the eluting GC peak from the
chromatography
column. In this mode, several positive ion scans are collected in one polarity
and several
negative ion scans are collected in the opposite polarity mode. This is
possible because the GC
peak is wide enough, and the switching frequency high enough, to provide
enough data points
associated with a single GC peak, for both positive and negative polarities.
Preferably, a time gap
is afforded between each polarity to allow stabilization of reagent ions and
baseline.
[042] In an alternate embodiment shown in FIG. 6, there are instead two HVPSs,
58a and 58b,
one set to output positive voltage, and the other negative. In this
embodiment, supplies 58a and
58b may both draw power from a 24 VDC power supply 66. The power supplies 58a
and 58b
themselves do not switch polarity. Rather, the circuit 60 switches between one
HVPS and the
other. Preferably, the data grabber rate is 100 k samples/sec or down to 10
microseconds/sample
for improved peak resolution. The advantage of two separate high voltage power
supply is ability
to adjust the polarity independently for each HVPS. Also switch time is
reduced, because
polarity does not switch¨preferably, switch time is reduced as low as 500
microseconds.
[043] It has been discovered that the presence of C1-C3 alcohols, C2-C8
aldehydes, C3-C4
ketones and/or C4-C6 alkyl esters on a subject's breath are indicative of
COVID-19 infections.
In an embodiment of the invention, if three or more of these compounds are
present then a
COVID-19 positive result is returned.
[044] The following were found to be strongly indicative of COVID-19
infection: Ethyl butyrate
(an ester); Propionaldehyde (aldehyde); 2-butanone (a ketone); Heptaldehyde
(aldehyde);
Octanal (aldehyde); Butyraldehyde (aldehyde). Each chemical is detected as
protonated ion
(MW) and its dimer ion (M2H+). The said protonated molecular ion can cluster
with an internal
CIR to form an additional ionic signature of the analyte of interest. Some of
the substances form
negative ions by clustering with internal CIRs. In an embodiment of the
invention, a positive
Date Recue/Date Received 2022-05-25
result is returned if three or more of these strongly indicative compounds are
sensed. In another
embodiment, the sensing of four of these strongly indicative compounds returns
a positive result.
[045] Each of these VOCs has a number of peaks in both positive and negative
IMS modes,
associated with different reduced mobilities, as per table 1 below.
Preferably, COVID-19
positive subjects are identified by means of a combined result of four or more
channels as
programmed.
I Chemical compounir
I type/ functional
S19 Charnel Name VOC _____ 9fouP
COTO Baty rote -
C-4 ',IOC Ciwirttl __ EsteT
Prno. n'l,c1d0 pie
c ,
ZhLt nalf.
_c+ 01,44L4VA 4t9114
Heietaktetryde
P.2 VOC .1 chow* Arc.lt=,,y
m Imo*
C+3 vOC 4,1 _________________________ 4*i
wiileohvoir
Cf.4 VOC 1%1' -1
r),:tarhi -1
Lt',,, t,,Arote +
044 VOC Ester
. r ' yde AltkJ 'iy it
0.1.µ4% õ +.e ,,r
Table 1: COVID-19 substance VOC IMS channels as programmed
[046] In an aspect of the invention, there is provided a novel Retention-Time
Separation-
Analysis (RTCA) test-system casting substance-quantifiers as distinguishable
nest-peaks. A
Drift-Time-Peak-Separation (DTPS) technique was used for time-clustered
structures. Another
aspect of the invention is use of combined Derivative-Based-Retention-Time-
Separation-
Approach (DBRTA), which allowed identification of low signal-noise peaks over
background
baseline.
[047] Complex-cluster benchmarks with 3-4 nested peaks residing in the analyte
of interest
identification area with relatively high peak intensity were addressed by this
novel-architecture
of multi-Shard detection designed to reduce misdetection working in dual-
single polarity
schemes. This advancement increased resolution of nested structures
characteristics of sensed
complex chemical compositions.
[048] Figure 3 shows a typical 3D ion mobility spectrum or plasmagram 100 of a
non-infected
person.
Date Recue/Date Received 2022-05-25
[049] Figure 4 shows a 3D ion mobility spectrum or plasmagram 200 of the
breath of a COVID-
19 infected person.
[050] Embodiments of the invention include one or more of the following items.
1. Volatile organic compounds found on breath of infected subjects were
identified and
comprise of Cl-C3 alcohols, C2-C8 aldehydes, C3-C4 ketones and C4-C6 alkyl
esters.
2. A system as in item 1, wherein the system detects the ionic profile
produced by the viral
infection compared to breath samples from healthy people.
3. A system in item 2, wherein the VOCs are collected on a chemical treated
filter. Five or more
exhalation onto the filter to enrich the trapped VOCs.
4. A system as in item 1, wherein the temporal separation means comprises a
pre-separator of
the predetermined analytes and transfer into the chemical ionization source of
the IMS.
5. A method of detecting the presence of plurality of predetermined analytes
in the collected
breath sample.
6. A method as in item 5, wherein the detected ionic species are protonated
ions, dimers ions,
analyte-cluster with chemical ionization reagent and negative ions clustering
with oxygen
and reagent ion.
7. A detector for detecting the presence of analytes profile using nested
peaks within a non-
Gaussian signal-pattern structure. Advanced compaiimentalized Multi-Stacked
Sharding with
dynamic background-corrected noise identification algorithm.
8. A detector as in item 6, wherein the identification process uses multi-
layer pattern
recognition algorithm to identify the target analytes in the presence of
complex chemical
matrix.
9. An apparatus for detecting Covid-19 infection in a subject, the apparatus
comprising:
a. A sampling apparatus for collecting a breath sample from a subject;
b. An analyzer, comprising an ion mobility spectrometer (IMS), for receiving
the
sample from the sampling apparatus and for determining the presence in the
sample
of Volatile Organic Compounds (VOCs) indicative of Covid-19, the VOCs
comprising at least three compounds selected from the group consisting of Cl-
C3
alcohols, C2-C8 aldehydes, C3-C4 ketones and C4-C6 alkyl esters.
References:
1. Russkiewicz et al, EClinical Medicine 100609, 2020. Diagnosis of COVID-19
by analysis of
breath with gas chromatography-ion mobility spectrometry- a feasibility study.
2. Benjie Shan et all, ACS Nano. August 18, 2020 and related references.
Multiplexed
nanomaterial-based sensor array for detection of COVID-19 exhaled breath.
3. M.Sohrab et al, Clin.Microbial, 3 :3, 2014. Volatile organic compounds as
novel markers for
the detection of bacterial infections.
4. J.R.Belinato et al, J.Chrom. B, Volume 1110, March 15, 2019. Rapid
discrimination of fungal
strains isolated from human skin based on microbial volatile organic profiles.
5. R.M.S.Thorn and J.Greenman, J.Breath Res. 6, 2012. Microbial volatile
compounds in health
and disease conditions.
Date Recue/Date Received 2022-05-25
6. A.A.E1 Qader et al, Biomed.Chromatogr. 29, 1783-1790, 2015. Volatile
organic compounds
generated by cultures of bacteria and viruses associated with respiratory
infections.
7. B.Buszewski and T. L.igor, Anal.Bioanal.Chem, 404, 141-146, 2012.
Identification of
volatile lung cancer markers by GC-MS: comparison with discrimination by
canines.
8. R.Jiang et al, Analytical Chimica Acta 804, 111-119, 2013. A non-invasive
method for in
vivo skin volatile compounds sampling.
9. P.Mochalski et al, J.Chrom.B, 1076, 29-34, 2018. Monitoring of selected
skin and breath
borne volatile organic compounds emitted from the human body using GC-IMS.
10. Maosheng Yao et al, https;//doi.org/10.1101/2020.06.21.20136523. This
version posted on
June 24, 2020. Breath-borne VOC Biomarkers for COVID-19.
Date Recue/Date Received 2022-05-25