Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/146724
PCT/US2021/014001
METHODS AND DEVICES FOR ENDO VASCULAR ABLATION OF A SPLANCHNIC
NERVE
INCORPORATION BY REFERENCE
[0001] This application claims priority to U.S. Provisional
Application No. 62/962,627,
filed January 17, 2020 and U.S. Provisional Application No. 63/086,516, filed
October 1, 2020,
the disclosures of which are incorporated by reference herein in their
entireties for all purposes.
[0002] All publications and patent applications mentioned in this
specification are herein
incorporated by reference to the same extent as if each individual publication
or patent
application was specifically and individually indicated to be incorporated by
reference.
[0003] This disclosure is related by subject matter to the
disclosure in U.S. Provisional
Application 62/864,093, filed June 20, 2019, U.S. Provisional Application
62/881,251, filed July
31, 2019, U.S. Provisional Application 62/962,627 filed January 17, 2020, U.S.
Pub. Nos.
US2019/0175912, US2019/0183569, Patents US 10,376,308, US 10,207,110, App.
Nos.
16/510,503, 62/836,720, 62/837,090, 62/864,093, PCT/US2019/15400,
PCT/US2020/038934,
and PCT Pub. Nos. W02018/023132, W02019/118976, and WO/2020/257763, all of
which are
incorporated herein by reference in their entirety for all purposes.
BACKGROUND
[0004] Heart failure (HF) is a medical condition that occurs when
the heart is unable to
pump sufficiently to sustain the organs of the body. Heart failure is a
serious condition and
affects millions of patients in the United States and around the world.
[0005] One common measure of heart health is left ventricular
ejection fraction (LVEF) or
ejection fraction. By definition, the volume of blood within a ventricle
immediately before a
contraction is known as the end-diastolic volume (EDV). Likewise, the volume
of blood left in a
ventricle at the end of contraction is end-systolic volume (ESV). The
difference between EDV
and ESV is stroke volume (SV). SV describes the volume of blood ejected from
the right and
left ventricles with each heartbeat. Ejection fraction (EF) is the fraction of
the EDV that is
ejected with each beat; that is, it is SV divided by EDV. Cardiac output (CO)
is defined as the
volume of blood pumped per minute by each ventricle of the heart. CO is equal
to SV times the
heart rate (HR).
- 1 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0006] Cardiomyopathy, in which the heart muscle becomes weakened,
stretched, or
exhibits other structural problems, can be further categorized into systolic
and diastolic
dysfunction based on ventricular ejection fraction.
[0007] While a number of drug therapies successfully target
systolic dysfunction and
HFrEF, for the large group of patients with diastolic dysfunction and HFpEF no
promising
therapies have yet been identified. The clinical course for patients with both
HFrEF and HFpEF
is significant for recurrent presentations of acute decompensated heart
failure (ADHF) with
symptoms of dyspnea, decreased exercise capacity, peripheral edema, etc.
Recurrent admissions
for ADHF utilize a large part of current health care resources and could
continue to generate
1 0 enormous costs.
[0008] While the pathophysiology of HF is becoming increasingly
better understood,
modern medicine has, thus far, failed to develop new therapies for chronic
management of HF or
recurrent ADHF episodes. Over the past few decades, strategies of ADHF
management and
prevention have and continue to focus on the classical paradigm that salt and
fluid retention is
the cause of intravascular fluid expansion and cardiac decompensation.
[0009] Thus, there remains a need for improved therapies for heart
failure patients that are
safe and effective, and devices and systems that are adapted and configured to
perform those
therapies.
SUMMARY OF THE DISCLOSURE
2 0 [0010] The disclosure is related to methods of, devices for, and
approaches for ablating a
thoracic splanchnic nerve or a thoracic splanchnic nerve root. The ablations
can be performed to
treat at least one of hypertension and heart failure, but the general methods
may also be used for
other treatments as well. For example, the methods herein can be used in the
treatment of pain, or
even to generally benefit the subject to reducing the amount of blood that is
expelled from the
splanchnic bed into the central thoracic veins.
[0011] The treatments herein may be accomplished by increasing
splanchnic capacitance.
The therapies generally include ablating a patient' s preganglionic thoracic
splanchnic nerve or
thoracic splanchnic nerve root to increase splanchnic capacitance, and thereby
treat at least one
of hypertension and heart failure.
[0012] Methods herein describe ablating thoracic splanchnic nerves, such as
a greater
splanchnic nerve or greater splanchnic nerve roots. While methods herein may
provide specific
examples of targeting greater splanchnic nerve or greater splanchnic nerve
roots, it may be
- 2 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
possible to alternatively, or in addition to, ablate other thoracic splanchnic
nerves (e.g., lesser,
least) to perform one or more treatments herein.
[0013] One aspect of the disclosure is a method of ablating tissue
by positioning a medical
device intravascularly in the vicinity of target tissue, and using the medical
device to ablate
tissue and create a lesion. One aspect of the disclosure a method of ablating
tissue by positioning
a medical device intravascularly into one or more target vessels, and using
the medical device to
ablate tissue and create a lesion. The methods herein can thus be described as
methods that
position a medical device near target tissue to be ablated and/or methods that
position a medical
device in one or more vessels, where the target tissue is relatively near to
the target regions
within the one or more vessels. Any of the method steps herein (including, for
example without
limitation, in the claims or the Description section) can be incorporated into
any other method of
use herein unless specifically indicated to the contrary herein.
[0014] One aspect of the disclosure is a method of ablating a
greater splanchnic nerve or a
greater splanchnic nerve root to increase splanchnic venous blood capacitance
and/or venous
compliance, the method including advancing a medical device into a first
vessel, advancing the
medical device at least partially into a second vessel, and delivering
ablation energy from the
medical device to create a lesion in tissue surrounding the first vessel.
[0015] In some embodiments the first vessel is an azygos vein and
the second vessel is an
intercostal vein. The intercostal vein may be one of the three lowest
intercostal veins. The
2 0 intercostal vein may be a T9, T10, or T11 intercostal vein.
[0016] The methods may include positioning a distal end of an
ablation element in the
second vessel and no more than 30 mm (e.g., 20 mm, 15 mm, 12 mm) from a
junction between
the first vessel and the second vessel when delivering the energy from the
ablation element.
[0017] The methods may include a proximal portion of an ablation
element being disposed
in the second vessel when delivering energy.
[0018] The methods may include aligning or positioning the
ablation element with respect
to a honey landmark, such as a costovertebral joint at the same vertebral
level at which the
second vessel (e.g., intercostal vein) resides.
[0019] In some embodiments aligning or positioning the ablation
element with respect to a
boney landmark, such as a costovertebral joint, includes viewing the boney
landmark with
medical imaging such as fluoroscopy.
[0020] In some embodiments viewing the boney landmark with medical
imaging such as
fluoroscopy includes orienting the medical imaging perspective at an anterior
oblique angle in a
range of 25 to 65 from AP (e.g., in a range of 30 to 60 , in a range of 35
to 55 ) toward the
side of the patient where the target nerve resides.
-3 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0021] In some embodiments viewing the boney landmark with medical
imaging such as
fluoroscopy includes orienting the medical imaging perspective approximately
perpendicular to a
line between the patient's first vessel (e.g., azygos vein) and the boney
landmark (e.g.,
costovertebral joint).
[0022] In some embodiments aligning the ablation element with respect to a
boney
landmark includes aligning a radiopaque marker positioned on the catheter
containing the
ablation element with the boney landmark.
[0023] The method may include creating a lesion at a distance of
5mm around the ablation
element. Creating a lesion may include ablating a portion of a thoracic
splanchnic nerve or a
thoracic splanchnic nerve root, e.g., a greater splanchnic nerve or GSN root.
A lesion may be a
continuous lesion. The lesion may have a length from 5 aim to 25 nana, such as
10 mm to 25 mm,
such as 15 mm to 20 mm. A lesion may be a circumferential lesion all the way
around the second
vessel. The lesion may, however, be less than circumferential all the way
around the second
vessel, such as 225 degrees or less, 180 degrees or less, 135 degrees or less,
90 degrees or less,
45 degrees or less.
[0024] The methods may include positioning an entire ablation
element in the second
vessel, while the method can also include positioning less than the entire
length of the ablation
element in the second vessel.
[0025] The methods may include performing an ablation process from
within more than
one target vessel, such as an intercostal vein or an azygos vein. The methods
of ablation herein
may also be performed in the second vessel.
[0026] The methods may include performing an ablation confirmation
test, such as any of
the tests herein. If desired or needed, an ablation element may be
repositioned into a second
target vessel, which may be an azygos vein or a different intercostal vein.
[0027] The methods can also include, prior to, during, and/or subsequent to
delivering the
ablation energy, delivering stimulation energy to first and second stimulation
electrodes carried
by the medical device. Delivering stimulation energy may help determine if the
ablation element
is in a target location within the intercostal vein, and /or if an ablation
procedure was effective.
[0028] One aspect of the disclosure is a method that includes
delivering an ablation
catheter comprising an energy delivery element (or member) through a venous
system of the
patient, positioning the energy delivery element at least partially
(optionally completely) inside a
vein selected from T9, T10 and T11 intercostal veins, delivering ablation
energy from the energy
delivery element to create a continuous lesion having a depth of at least 5 mm
and a length from
10 to 25 mm. The continuous lesion and its parameters can be formed by
selecting or choosing
certain energy delivery parameters that will create the lesion. In some
embodiments, the lesion
- 4 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
can extend from an ostium of an azygos vein to up to 20 mm along the
intercostal vein. Any of
the other method steps herein that are described in the context of other
methods can be
performed with this exemplary method.
[0029] in some alternative methods herein, a plurality of
ablations (i.e.., from ablation
energy on to energy ablation off) can be performed within a single target
vessel (e.g., an
intercostal vein) to create a total lesion made from two or more lesions made
from the plurality
of ablations. The total lesion made from the plurality of lesions can have any
of characteristics
of the other lesions herein. For example, the total lesion can be continuous
(made by the
connection of a plurality of lesions created during different ablations), may
be up to 20 mm long,
1 0 can be circumferential (or not), etc. After a first ablation, the
ablation device can be moved
within the same vessel and create a second lesion, which may or may not
overlap with a first
lesion. This can be repeated as many times as desired. Any of the stimulation
or testing steps
herein can be performed before, during, or after any ablation step, even if a
plurality of ablations
are performed in a single vessel.
[0030] One aspect of the disclosure is a method of positioning an ablation
catheter in a T9,
T10, or T11 intercostal vein in a position for ablating a greater splanchnic
nerve (GSN), the
method including imaging a portion of a subject, the portion including at
least one of a T9, T10,
or T11 intercostal vein and a portion of the subject's spine; positioning a
distal section of an
ablation catheter in the T9. T10, or T11 intercostal vein; and positioning an
ablation catheter
radiopaque marker at a location based on the position of the radiopaque marker
relative to an
anatomical landmark, such as one or more of a portion of the spine, a rib, a
costovertebral joint,
an azygous vein, or an ostium between the azygous vein and the T9, T10. or T11
intercostal vein.
The method may further include delivering energy from an ablation catheter
ablation element to
ablate tissue.
[0031] One aspect of the disclosure is a method that includes
characterizing a relative
position of a patient's azygos vein to determine if the azygos is centered or
substantially
centered, right-biased (to the patient's right of center), or left-biased (to
the patient's left of
center). The characterization step may occur while viewing a particular
portion of the patient's
anatomy, and from a particular viewpoint that allows the characterization to
accurately take
place. The method may further include positioning an ablation catheter based
on the
characterization step.
[0032] One aspect of this disclosure is a method of characterizing
the position of a human
patient's azygos vein relative to a portion of the patient's spine,
comprising: imaging at least a
portion of the patient' s spine and vasculature, in particular the azygos vein
and/or one or more
intercostal veins, using an imaging device, in particular using a radiographic
imaging device with
-5 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
a radiopaque contrast agent injected into the patient's vasculature, or
imaging at least one
radiopaque device, positioned in the azygos vein and/or in one or more
intercostal veins, relative
to a portion of the spine, using an imaging device, in particular using a
radiographic imaging
device, to thereby characterize the position of the patient's azygos vein
relative to a midline of
the spine, the radiopaque device optionally comprising a radiopaque portion of
a guidewire; and
determining if the azygos vein is centered, left-biased or right biased with
respect to the midline
of the vertebra based on one or more images generated by said imaging device.
This aspect may
further include a method of determining a proper position where a catheter
should be inserted in
a vasculature of a human patient, in particular in order to allow ablating a
greater splanchnic
nerve or greater splanchnic nerve roots, the method comprising determining
where to place an
ablation element of a catheter for transvascular ablation, in particular any
of the ablation
catheters herein, based on said determination of if the azygos vein is
centered, left-biased or right
biased with respect to the midline of the vertebra.
[0033] This aspect may further comprise determining where to place
a radiopaque marker
carried by the distal section of an ablation catheter, optionally a proximal
radiopaque marker
positioned proximal to any ablation element canied by the same distal section,
based on said
determination of if the azygos vein is centered, left-biased or right biased
with respect to the
midline of the vertebra.
[0034] One aspect of the disclosure is a method of determining
proper positioning of a
2 0 catheter inserted in a vasculature of a human patient, optionally of a
catheter according to any of
the claims or disclosure herein, wherein the catheter comprises an elongate
shaft with a distal
section carrying one or more ablation elements and a proximal radiopaque
marker, with the distal
section of the elongate shaft positioned in a T9, T10, or T11 intercostal
vein; wherein the method
comprises: determining if the azygos vein is centered, left-biased or right
biased with respect to
the midline of the vertebra, assessing the position of the proximal radiopaque
marker relative to
the midline of the vertebra, verifying if the catheter is properly positioned
relative to a patient's
anatomical landmark, wherein verifying comprises: considering that the
catheter is properly
positioned when one of the following circumstances takes place: if the azygos
vein is right-
biased, the proximal radiopaque marker is placed at the ostium of the
intercostal vein, to the right
of midline of the vertebra, if the azygos vein is centered or left-biased, the
proximal radiopaque
marker is aligned with the midline of the vertebra.
[0035] In any of the method aspects herein, the proximal
radiopaque marker may be
carried by the distal section and may be positioned proximal to all the
ablation element(s). The
proximal radiopaque marker may be positioned directly proximal to the ablation
element or
- 6 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
directly proximal to the most proximal of the ablation elements carried by the
distal section of
the catheter.
[0036] In any of the method aspects herein, the catheter may
comprise a distal radiopaque
marker positioned distal to all the ablation el ement(s) and wherein the step
of verifying also
includes: assessing the position of the distal radiopaque marker relative to
the patient's
costovertebral joint and/or rib, ascertaining that the distal radiopaque
marker is spaced from the
costovertebral joint and/or rib at least a prefixed threshold distance. The
distal radiopaque marker
may be positioned directly distal to the ablation element, or directly distal
to the most distal of
the ablation elements carried by the distal region of the catheter, and
wherein ascertaining
1 0 comprises ascertaining that the distal radiopaque marker is at least 3
mm, preferably at least 5
mm, far from the costovertebral joint.
[0037] In any of the method aspects herein, verifying may comprise
considering that the
catheter is not properly positioned when none of the following circumstances
takes place: if the
azygos vein is right-biased, the proximal radiopaque marker is placed at the
ostium of the
intercostal vein, to the right of midline of the vertebra, if the azygos vein
is centered or left-
biased, the proximal radiopaque marker is aligned with the midline of the
vertebra.
[0038] In any of the method aspects herein, if it has been
verified that the catheter is not
properly positioned, the method may further include adjusting the position of
the catheter by
aligning the proximal radiopaque marker on the ablation catheter with the
respective anatomical
2 0 landmark, and/or by further distancing the distal radiopaque marker
from the costovertebral joint.
[0039] In any of the method aspects herein, a step of determining
if the azygos vein is
centered, left-biased or right biased with respect to the midline of the
vertebra may comprise:
imaging at least a portion of the patient's spine and vasculature, in
particular the azygos vein
and/or one or more intercostal veins, using an imaging device, in particular
using a radiographic
imaging device with a radiopaque contrast agent injected into the patient's
vasculature, or
imaging at least one radiopaque device, positioned in the azygos vein and/or
in one or more
intercostal veins, relative to a portion of the spine, using an imaging
device, in particular using a
radiographic imaging device, to thereby characterize the position of the
patient's azygos vein
relative to a midline of the spine, the radiopaque device optionally
comprising a radiopaque
portion of a guidewire.
[0040] In any of the method aspects herein, a step of assessing
the position of the proximal
radiopaque marker relative to the midline of the vertebra may comprise
imaging, using an
imaging device, in particular using a radiographic imaging device, at least a
portion of the
catheter comprising the proximal radiopaque marker.
- 7 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0041] In any of the method aspects herein, a step of assessing
the position of the distal
radiopaque marker relative to the costovertebral joint may comprise imaging,
using an imaging
device, in particular using a radiographic imaging device, at least a portion
of the catheter
comprising the distal radiopaque marker.
[0042] One aspect of the disclosure is a method of determining proper
positioning of a
catheter inserted in a vasculature of a human patient, optionally of a
catheter according to any
one of the claims or disclosure herein, wherein the catheter comprises an
elongate shaft with a
distal section carrying one or more ablation elements and a distal radiopaque
marker, with the
distal section of the elongate shaft positioned in a T9, T10, or T11
intercostal vein; wherein the
method comprises: determining the position of the distal radiopaque marker
relative to the
patient's costovertebral joint, verifying if the catheter is properly
positioned relative to a patient's
anatomical landmark, wherein verifying comprises: considering that the
catheter is properly
positioned when the distal radiopaque marker is spaced from the costovertebral
joint at least a
prefixed threshold distance. The distal radiopaque marker may be positioned
directly distal to the
ablation element, or directly distal to the most distal of the ablation
elements carried by the distal
section of the catheter, and wherein the prefixed threshold distance is at
least 3 mm, preferably at
least 5 mm.
[0043] In this aspect, if it has been verified that the catheter
is not properly positioned, the
method may further comprise adjusting the position of the catheter by further
distancing the
2 0 distal radiopaque marker from the costovertebral joint.
[0044] In this aspect, a step of determining the position of the
distal radiopaque marker
relative to the patient' s costovertebral joint may comprises imaging at least
a portion of the
patient's spine and vasculature, in particular the azygos vein and/or one or
more intercostal
veins, using an imaging device, in particular using a radiographic imaging
device with a
radiopaque contrast agent injected into the patient's vasculature, or imaging
at least one
radiopaque device, positioned in the azygos vein and/or in one or more
intercostal veins, relative
to a portion of the spine, using an imaging device, in particular using a
radiographic imaging
device, to thereby characterize the position of the patient's azygos vein
relative to a midline of
the spine, the radiopaque device optionally comprising a radiopaque portion of
a guidewire; and
imaging, using an imaging device, in particular using a radiographic imaging
device, at least a
portion of the catheter comprising the distance radiopaque marker.
[0045] One aspect of the disclosure is an ablation catheter for
transvascular ablation of
thoracic splanchnic nerves, particularly for ablating a greater splanchnic
nerve or greater
splanchnic nerve roots, comprising: an elongate shaft having a length such
that a distal section of
the elongate shaft can be positioned in a T9. T10, or T11 intercostal vein,
proximal and distal
-8 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
electrically conductive flexible ablation elements carried by the elongate
shaft distal section, a
length from a distal end of the distal ablation element to a proximal end of
the proximal ablation
element being from 10 mm - 25 mm.
[0046] In this aspect the distal section of the elongate shaft may
have an outer diameter
from 1.5 mm to 3 mm.
[0047] In this aspect an axial spacing may exist between the
proximal and distal ablation
elements that is from .1 mm to 5 mm, such as .1 mm to 3 mm, such as .1 mm to 2
mm, such as 5
mm to 1-mm.
[0048] In this aspect the distal and proximal ablation elements
may be electrodes.
1 0 [0049] In this aspect the distal and proximal ablation elements may
each have a length,
wherein the lengths are the same.
[0050] In this aspect the distal and proximal ablation elements
may each have a length,
wherein the lengths are not the same.
[0051] In this aspect the distal and proximal ablation elements
may each have a length
from 5 mm to 12 mm, such as from 6 mm to 10 mm, such as from 7 mm to 9 mm.
such as any
length in any of these ranges.
[0052] In this aspect the distal ablation element may have a
helical configuration and
wherein the proximal ablation element may a helical configuration. A helical
configuration of the
distal and proximal ablation elements may the same. Helical configurations of
the distal and
2 0 proximal ablation elements have one or more different features, such as
one or more of coil
direction (e.g. left-handed vs right-handed), pitch, or thickness.
[0053] In this aspect the distal and proximal ablation elements
may each have curvilinear
cross-sectional configurations.
[0054] In this aspect the distal and proximal ablation elements
may each have rectilinear
cross-sectional configurations.
[0055] In this aspect the distal and proximal ablation elements
may be made from a
superelastic material such as Nitinol.
[0056] In this aspect distal and proximal ablation elements may be
sufficiently flexible and
sized to allow the distal section to be advanced from an azygos vein into one
of a T9, T10, or
T 1 1 intercostal vein.
[0057] In this aspect the distal and proximal ablation elements
may each be attached to the
shaft at distal and proximal end regions, but not in between the distal and
proximal end regions.
[0058] In this aspect the catheter may further comprise a
radiopaque marker. The
radiopaque marker may be disposed distal to a distal end of the distal
ablation element. The
radiopaque marker may be 0 mm to 5 mm distal to the distal end of the distal
ablation element,
- 9 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
optionally 0 mm to 3 mm, or 0 mm to 2 mm. The radiopaque marker may be
disposed proximal
to a proximal end of the proximal ablation element. The radiopaque marker may
be 0 mm to 5
mm proximal to the distal proximal of the distal ablation element, optionally
0 mm to 3 mm, or 0
mm to 2 mm.
[0059] In this aspect the distal and proximal ablation elements are each
not configured to
deploy to a deployed configuration.
[0060] In this aspect the distal and proximal ablation elements
each have an operational
configuration that is the same or substantially the same as a delivery
configuration.
[0061] In this aspect the distal and proximal ablation elements
each have an outer diameter
in an operational state that is the same or substantially the same as an outer
diameter in a delivery
state.
[0062] In this aspect the distal and proximal ablation elements
may each have expanded
configurations different than delivery configurations.
[0063] In this aspect the catheter may further comprise a
temperature sensor carried by the
shaft. The temperature sensor may be disposed at a distal end of the distal
ablation element. The
temperature sensor may be disposed at a proximal end of the proximal ablation
element. The
catheter may comprise a second temperature sensor, the temperature sensor
disposed at a distal
end of the distal ablation element, the second temperature sensor disposed at
a proximal end of
the proximal ablation element.
[0064] In this aspect, the catheter may further comprise one or more
irrigation ports in fluid
communication with an irrigation lumen that is connectable to a fluid source
at a proximal region
of the ablation catheter. One of the one or more irrigation ports may be
axially in between the
distal and proximal ablation electrodes. Optionally none of the one or more
irrigation ports may
be disposed radially under an ablation element structure. One or more
irrigation ports may be
disposed between helical windings of the distal and proximal ablation
electrodes. In a side view,
an irrigation port may be disposed between every adjacent pair of ablation
element helical
sections of the distal ablation element and the proximal ablation element.
[0065] In this aspect the distal and proximal ablation elements
may be electrically
configured to be independently energized in monopolar mode.
[0066] In this aspect the distal and proximal ablation elements may be
electrically
configured to be energized in bipolar mode.
[0067] In this aspect the distal section may be no more than 7 cm
from a distal tip of the
ablation catheter.
- 10 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0068] In this aspect the distal and proximal ablation elements
may be sized and adapted to
create a continuous ablation having a length in a range of 5 nun to 25 =a,
such as 10 to 25 mm,
such as 15 mm to 20 mm.
[0069] In this aspect the distal section may be adapted for
flexibly traversing a bend from
an azygos vein to a T9, T10 or T11 intercostal vein.
[0070] In this aspect the catheter may further comprise a
guidewire lumen within the
elongate shaft and having a distal port at a distal tip of the catheter.
[0071] In this aspect the distal and proximal ablation elements
may each comprise one or
more of an RF ablation electrode, a coiled wire electrode, a laser cut RF
electrode, a RF
electrode printed with conductive ink, a RF electrode on an expandable balloon
(e.g., conductive
ink, flexible circuits,), a conductive membrane RF electrode, a RF electrodes
on an expandable
cage or mesh, an ultrasound ablation transducer, an electroporation
electrodes, an cryoablation
element, or a virtual RF electrode.
[0072] In this aspect the distal and proximal ablation elements
may each be adapted and
configured to deliver ablation energy circumferentially to create a
circumferential lesion.
[0073] One aspect of the disclosure is an ablation catheter for
transvascular ablation of
thoracic splanchnic nerves, particularly for ablating a greater splanchnic
nerve or greater
splanchnic nerve roots, comprising: an elongate shaft having a length such
that a distal section of
the elongate shaft can be positioned in a T9. T10, or T11 intercostal vein,
and an electrically
conductive flexible ablation element carried by the elongate shaft distal
section, the ablation
element having a length from 10 mm - 25 nana, and a radiopaque marker carried
by the elongate
shaft.
[0074] In this aspect the distal section of the elongate shaft may
have an outer diameter
from 1.5 mm to 3 mm.
[0075] In this aspect the radiopaque marker carried by the elongate shaft
may be disposed
from 0 mm to 5 mm from an end of the ablation element, such as from 0 to 4 mm,
or from 0 to 3
mm. or 0 to 2 mm. The end may be a distal end of the ablation element. The end
may be a distal
end of a distal ablation electrode, and the ablation element may further
comprising a proximal
ablation electrode axially spaced from the distal ablation electrode.
[0076] In this aspect the end may be a proximal end of the ablation
element.
[0077] In this aspect the catheter may further comprise a second
radiopaque marker carried
by the elongate shaft and disposed from 0 mm to 5 mm (e.g., 0 to 4 mm, 0 to 3
mm, or 0-2 mm
from a second end of the ablation element).
- 11 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0078] In this aspect the ablation element may comprise distal and
proximal ablation
electrodes. The radiopaque marker may be distal to the distal ablation
electrode, wherein catheter
may include a second marker proximal to the proximal ablation electrode.
[0079] In this aspect, the radiopaque marker may be disposed from
0 mm to 3 mm from the
end of the ablation element, optionally 1 mm.
[0080] In this aspect, the ablation element may comprise a distal
ablation electrode axially
spaced from a proximal ablation electrode. The distal and proximal ablation
electrodes may each
have a length, wherein the lengths are the same or wherein the lengths that
are not the same. The
distal and proximal ablation electrodes may each have a length from 5 mm to 12
mm. The distal
and proximal ablation electrodes may be axially spaced from .1 mm to 5 mm
apart, such as from
.1 mm to 3 mm apart, optionally from .5 mm to 1 mm apart. Distal and proximal
ablation
elements in this aspect may be any of the distal and proximal ablation
elements herein, such as
coiled elements. In this aspect a cross-sectional outer profile of a distal
ablation electrode may be
different than a cross-sectional outer profile of a proximal ablation
electrode. Distal and proximal
ablation electrodes may be made from a superelastic material such as nitinol.
Distal and proximal
ablation electrodes may be sufficiently flexible to allow the distal region to
be advanced from an
azygos vein into one of a T9, T10, or T11 intercostal vein.
[0081] In this aspect, the ablation element may not be configured
to deploy to a deployed
configuration.
2 0 [0082] In this aspect, the ablation element may have an operational
configuration that is the
same or substantially the same as a delivery configuration.
[0083] In this aspect, the distal section may have a linear at-
rest configuration.
[0084] In this aspect, the ablation element may have an outer
diameter in an operational
state that is the same or substantially the same as an outer diameter in a
delivery state.
[0085] In this aspect the catheter may further comprise one or more
temperature sensors
carried by the shaft. A temperature sensor may be disposed at a distal end of
the ablation
element. A temperature sensor may be disposed at a proximal end of the
ablation element. The
catheter may further comprise a second temperature sensor, the temperature
sensor may be
disposed at or near a distal end of the ablation element, the second
temperature sensor may be
disposed at or near a proximal end of the ablation element.
[0086] In this aspect the catheter may comprise one or more
irrigation ports in fluid
communication with an irrigation lumen that is connectable to a fluid source
at a proximal region
of the ablation catheter, including any of the one more irrigation ports
herein. One of the one or
more irrigation ports may be axially in between the distal and proximal
ablation electrodes.
Optionally none of the one or more irrigation ports may be disposed radially
under an ablation
- 12 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
element structure. The one or more irrigation ports may be disposed between
windings of the
distal and proximal ablation electrodes, and wherein none of the one or more
irrigation ports may
be disposed radially under an ablation element structure. In a side view, an
irrigation port may be
disposed between every adjacent pair of ablation element helical sections.
[0087] In this aspect the ablation element may comprise first and second
ablation elements,
each of which may be electrically configured to be independently energized in
monopolar mode.
[0088] In this aspect the ablation element may comprise first and
second ablation elements
that are electrically configured to be energized in bipolar mode.
[0089] In this aspect the distal section may be no more than 7 cm
from a distal tip of the
1 0 ablation catheter.
[0090] In this aspect the ablation element may be adapted to
create an ablation having a
length in a range of 10 to 25 mm, such as 15 mm to 20 mm.
[0091] In this aspect the distal section may be adapted for
flexibly traversing a bend from
an azygos vein to a T9, T10, or Ti 1 intercostal vein.
[0092] In this aspect the catheter may further comprise a guidewire lumen
within the
elongate shaft and having a distal port at a distal tip of the catheter.
[0093] In this aspect the ablation element may comprise one or
more of an RF ablation
electrode, a coiled wire electrode, a laser cut RF electrode, a RF electrode
printed with
conductive ink, a RF electrode on an expandable balloon (e.g., conductive ink,
flexible circuits,),
a conductive membrane RF electrode, a RF electrodes on an expandable cage or
mesh, an
ultrasound ablation transducer, an electroporation electrodes, an cryoablation
element, or a
virtual RF electrode.
[0094] In this aspect the ablation element may be adapted and
configured to deliver
ablation energy circumferentially to create a circumferential lesion.
[0095] One aspect of the disclosure is an ablation catheter for ablating a
greater splanchnic
nerve, comprising: an elongate shaft, an electrically conductive flexible
ablation element
(optionally distal and proximal coiled elements) carried by a distal section
or region of the
elongate shaft, and a plurality of irrigation ports in the distal section of
the elongate shaft. The
electrically conductive flexible ablation element may have an axial length
(e.g., from a proximal
end to a distal end) from 5 mm ¨ 25 mm.
[0096] In this aspect, an elongate shaft may have a length such
that at least a portion of a
distal section of the elongate shaft can be positioned in a T9, T10, or T11
intercostal vein. In this
aspect, an electrically conductive flexible ablation element may comprise
distal and proximal
electrically conductive flexible ablation elements (optionally coiled) carried
by the elongate shaft
distal section.
- 13 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0097] In this aspect, a first subset of the plurality of
irrigation ports may be disposed
between windings of an electrically conductive flexible ablation element, such
as an RF
electrode, which may be a first electrode or a second electrode. A subset of
the plurality of
irrigation ports may be distal to the electrically conductive flexible
ablation element. A subset of
the plurality of irrigation ports may be disposed axially between distal and
proximal ablation
elements.
[0098] In this aspect, the elongate shaft may be void of or free
of irrigation ports between
at least one winding at distal and/or proximal ends of an electrically
conductive flexible ablation,
optionally void of or free of irrigation ports between at least one winding at
distal and/or
proximal ends of first and second coiled electrodes.
[0099] In this aspect, an electrically conductive flexible
ablation element may include
distal and proximal coiled electrodes. Distal and proximal ends of each of
distal and proximal
electrodes may comprise a coil with a varying pitch.
[0100] In this aspect, distal irrigation ports may be within 2 mm
of a distal end of an
electrically conductive flexible ablation, which may be a distal end of a
distal ablation element.
In some instances, the number of distal irrigation ports may be from two to
four, or more. Distal
irrigation ports herein may be axially aligned, such as shown in exemplary
figure 8E.
[0101] In this aspect, distal and proximal electrically conductive
flexible ablation elements
may be are axially spaced no more than 2 mat apart, optionally no more than
1.5 mm apart.
2 0 [0102] In this aspect, central irrigation ports between distal and
proximal ablation may
include from two to four ports, or more, and may be axially aligned, such as
shown in exemplary
figure 8E.
[0103] In this aspect, the plurality of irrigation ports may have
a combined and total area in
a range of 1.51e-4 to 1.08c-3 in2.
[0104] In this aspect, a diameter of all of the plurality of irrigation
ports may be in a range
of 0.002" to 0.009".
[0105] In this aspect, the quantity of the plurality of irrigation
ports may be in a range of
17 to 344.
[0106] In this aspect, the plurality of irrigation ports may have
a size and quantity such that
a Weber number is in a range of 0.4 ¨ 53 when irrigation fluid is delivered
from the plurality of
irrigation ports, optionally at a rate of 15 ml/min to 50 ml/min, and
optionally with saline.
[0107] In this aspect, the distal section may have a distal length
from 60 him to 70 mm and
may be sufficiently flexible to be advanced from an azygous vein into an
intercostal vein. In this
aspect, the elongate shaft may also have a central transition section proximal
to the distal section,
the central section optionally having a central length from 15 mm to 25 mm and
optionally
- 14 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
having a central stiffness and that is greater than a distal stiffness of the
distal section. In this
aspect, the elongate shaft may have a proximal section proximal to a central
section, the
proximal section optionally having a length that is greater than the distal
length and greater than
the central length, the proximal section optionally having a proximal
stiffness that is greater than
the central stiffness and greater than the distal stiffness. A central section
in this aspect may be
immediately axially adjacent and proximal to the distal section. A proximal
section in this aspect
may be immediately axially adjacent and proximal to a central section. In this
aspect, a distal
section may have a durometer from 50 D to 60 D, optionally 55D. In this
aspect, a central section
may have a durometer from 60 D to 70 D, optionally from 60 D to 65 D. In this
aspect, a
proximal section may have a distal end not closer than 50 mm from a distal end
of the catheter. A
proximal section in this aspect may have a distal end that is from 75 mm to
100 mm away from a
distal end of the catheter, and may optionally extend to a proximal end of the
elongate shaft. In
this aspect, a proximal section may include a braided reinforcing structure
therein, and distal and
central sections may optionally be free of a braided reinforcing structure. In
this aspect, a
proximal section may as a durometer from 70 D to 80 D, optionally from 70 D to
75 D.
[0108] In this aspect, a distal section of an elongate shaft may
have a linear or straight
configuration (such as shown in exemplary figure 8E) and may have an outer
diameter from 1.5
mm to 3 mm when the distal section is outside of a sheath.
[0109] Any first and second ablation elements in this aspect may
have coiled
configurations, such as those shown in exemplary figure 8E.
[0110] In this aspect, the distal section may include a plurality
of irrigation ports having a
helical configuration. There may be multiple sets of ports, each of which has
a separate helical
configuration, such as shown in the multiple sets of irrigation ports shown in
exemplary figure
8E. The multiple sets may be between distal and proximal end of any particular
electrode, such
as shown in exemplary figure 8E.
[0111] In this aspect, a distal section of the shaft may have a
distal diameter, a central
section may have a central diameter, and a proximal section may have a
proximal diameter, the
distal diameter optionally less than the central diameter and the central
diameter optionally less
than the proximal diameter. In this aspect, a distal diameter may be from 1.5
nun to 2.5 mm,
optionally 2 turn. A central diameter may be from 2.0 mm to 3.0 rum,
optionally 2.5 mm. A
proximal diameter may be from 2.5 mm to 3.5 mm, optionally 3 mm.
[0112] One aspect of this disclosure is related to tracking or
calculating how much volume
of liquid has been delivered through a catheter and into a patient. This
aspect may include a
computer executable method that is adapted to calculate an accumulated volume
of liquid that
has been delivered through a catheter to a patient while excluding (or not
including) from the
- 15 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
accumulated volume liquid that may be delivered through the catheter but not
into the patient's
vasculaturc. The method may include initiating a method that calculates an
accumulated volume
of liquid that has been delivered from outside of a catheter, through the
catheter, and into a
patient, and in response to an exclusion event that is indicative of the
catheter not being inside
the patient, stopping the method that calculates the accumulated volume of
liquid to avoid
including a volume of fluid that is not delivered into the patient's
vasculature from being
included in the accumulated volume.
[0113] In this aspect, an exclusion event may comprise an operator
action that causes the
method to be stopped.
[0114] In this aspect, an exclusion event may comprise an automatic action
that causes the
method to be stopped.
[0115] In this aspect, a method that calculates an accumulated
volume of liquid may
comprise calculating an accumulated volume by multiplying a flow rate by an
elapsed time. A
flow rate may be determined by multiplying a volume per pulse by pulses per
second.
[0116] This aspect may also include calculating or tracking an accumulated
volume of the
liquid that is not delivered into the patient's vasculature when it is
determined that the catheter is
not in the patient's vasculature.
[0117] In this aspect, an exclusion event may optionally comprise
an impedance
measurement or calculation that is outside of a range, or higher than a high
threshold, for
2 0 example. An exclusion event may comprise an impedance measurement or
calculation that is
above 700 to 900 Ohms in a monopolar mode, for example. An exclusion event may
comprise an
impedance measurement or calculation that is above 300 to 600 Ohms in bipolar
mode, for
example. An exclusion event may comprise an impedance measurement or
calculation that is
outside of 60 to 80 Ohms, for example.
[0118] In this aspect, an exclusion event may comprise an impedance
measurement or
calculation that determines if the catheter is out of the body. Initially, the
catheter is out of the
body and when a low threshold is crossed the algorithm may be adapted to
determine that the
catheter has passed into the body, wherein pumped saline is included in the
accumulation
calculation. When the catheter is determined to be in the body and a high
threshold is passed, the
algorithm may be adapted to determine that the catheter has passed from in the
body to out of the
body, wherein pumped saline is excluded. An exclusion event may comprise a
determination that
the catheter is out of the body. If the catheter is out of the body an
exclusion event may comprise
an impedance measurement that is higher than a low threshold. If the catheter
is in the body an
exclusion event may comprise an impedance measurement that is higher than a
high threshold.
- 16 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0119] In this aspect, a method that calculates an accumulated
volume of liquid may
continue uninterrupted until an exclusion event occurs.
[0120] In this aspect, the liquid may be saline.
[0121] Any method in this aspect may stored on an external energy
delivery console of an
ablation system, which may be any of the external systems described herein
that are adapted to
be placed in operable communication with any of the ablation catheters herein.
[0122] One aspect of the disclosure is related to methods of
delivering ablative energy to
tissue, such as tissue surrounding an intercostal vein. The methods may
include delivering
waveforms from any of the external systems herein to any of the suitable
ablation catheters
herein, and may include the external system receiving information from any of
the suitable
ablation catheters herein.
[0123] In this aspect, a method may include delivering from a
power module (e.g. part of
an external system) to a first electrode a first waveform of ablative RF
energy with an initial
power from 15-50, delivering from the power module to a second electrode a
second waveform
of ablative RF energy with an initial power from 15-50 W, receiving
information indicative of at
least one of sensed temperature or measured impedance, determining if at least
one of the sensed
temperature or the measured impedance is at or above a limit, if at least one
of the sensed
temperature or the measured impedance is at or above a threshold limit,
decreasing the power of
at least one of the first waveform and the second waveform.
[0124] The methods in this aspect may be used with any suitable catheter
herein. For
example, a first waveform may be delivered to a first electrode (optionally
coiled), and a second
waveform may be delivered to a second electrode (optionally coiled).
[0125] In this aspect, if at least one of the sensed temperature
or the measured impedance
is at or above a threshold limit, and a minimum therapy time has not yet
passed, the reducing
step may comprise reducing the power of at least one of the first waveform and
second
waveform to a secondary power less than the initial power. In this aspect, a
secondary power
may be from 5-10 W less than any initial power.
[0126] In this aspect, if at least one of the sensed temperature
or the measured impedance
is at or above a threshold limit, and a minimum therapy time has passed, the
reducing step may
comprise reducing the power of at least one of the first waveform and second
waveform to a
secondary power that is from 0 W to 1 W.
[0127] In this aspect, the first and second waveforms may be
multiplexed.
[0128] In this aspect, the first and second waveforms may be
asynchronous.
[0129] In this aspect, delivering from a power module to a first
electrode may comprise
delivering from a power module to a first electrode a first waveform of
ablative RF energy with
- 17 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
an initial power of 25 W. Delivering from a power module to a second electrode
may comprise
delivering from a power module to the second electrode a second waveform of
ablative RF
energy with an initial power of 25 W.
[0130] In this aspect, the first and second waveforms may be
alternating waveforms that
alternate between an ablative power amplitude and a non-ablative power
amplitude. A non-
ablative power amplitude in this aspect may be in a range of 0 W to 1 W.
[0131] In this aspect, the determining step may comprise
determining if the sensed
temperature is at or above 40 C to 95 C, optionally at or above 90 C.
[0132] In this aspect, the receiving step may comprise receiving
information from a
1 0 temperature sensor associated with a first electrode, such as any of
the coiled electrodes herein.
In this aspect, the receiving step may comprise receiving information from a
second temperature
sensor associated with a second electrode, such as any of the coiled
electrodes herein.
[0133] In this aspect, the determining step may comprise
determining if a measured
impedance is at or above 200 to 500 ohms, optionally at or above 500 ohms.
[0134] In this aspect. reducing the power of at least one of the first
waveform and the
second waveform may comprise reducing the power of at least one of the first
waveform and the
second waveform to a power from 10 W to 30 W, optionally 20 W.
[0135] In this aspect, reducing the power of at least one of the
first waveform and the
second waveform may comprise reducing the power of at least one of the first
waveform and the
second waveform by a power decrement from 1 W to 30 W, optionally from 5-10W.
[0136] In this aspect, at least one of the first and second
waveforms may have a pulse
width in a range of .5 seconds to 4 seconds.
[0137] In this aspect, the power of the first waveform may be
decreased if a sensed
temperature corresponding to a first electrode is at or above the limit, and
wherein the power of
the second waveform may be decreased if the sensed temperature corresponding
to a second
electrode is at or above the limit.
[0138] In this aspect, the delivering step may occur for at least
60 seconds.
[0139] In this aspect, the delivering step may occur with a
default setting to occur from 30
seconds to 180 seconds.
[0140] Any method in this aspect may further comprise delivering irrigation
fluid to an
ablation catheter at a flow rate in a range of 10 to 30 ml/min. Delivering
irrigation fluid to an
ablation catheter may include delivering fluid to, through, and out of any of
the ablation catheters
herein, including any and all of the description of the irrigation ports from
which irrigation fluid
may be delivered into the subject.
- 18 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0141] One aspect of the disclosure is related to external devices
(which may include one
or more separate components) that are adapted for use with any of the ablation
catheters herein.
External devices as used herein generally refers to one or more components of
a system that
remain outside of a subject, such as a power module, energy generator, etc.
The external devices
herein may be adapted to be coupled to or with any of the ablation catheters
herein to create
operable communication therebetween. External devices herein may be referred
to as external
systems, and it is understood that this refers to the external nature of the
one or more
components. An ablation catheter and one or more external components may
together be referred
to herein as a system. Any feature of this aspect may be incorporated with the
previous aspect
described herein that is related to delivering ablative energy, and vice
versa. For example, any of
the methods set forth in the previous aspect may be stored in one or more
memories on any of
the external devices in this aspect of the disclosure, and may be used with
any of the ablation
catheters in this aspect.
[0142] This aspect may include an external device or system that
is adapted for use with an
ablation catheter that includes first and second ablation electrodes. The
external device may
comprise a power output module that adapted to deliver a first waveform of
ablative RF energy
with an initial power from 15-50 W and a second waveform of ablative RF energy
with an initial
power from 15-50 W. The external device may also include a module adapted to
receive
information indicative of at least one of sensed temperature or measured
impedance and
2 0 determine if at least one of the sensed temperature or the measured
impedance is at or above a
limit, and if at least one of the sensed temperature or the measured impedance
is at or above a
threshold limit, causing the power output module to decrease the power of at
least one of the first
waveform and the second waveform.
[0143] In this aspect, the module may comprise at least one of a
temperature limit module
or an impedance limit module.
[0144] One aspect of this disclosure is related to delivering
irrigation fluid to an ablation
catheter. This aspect may include a method of delivering ablation energy and
irrigation fluid to a
catheter for ablating a greater splanchnic nerve, wherein the method includes
positioning an
ablation catheter in an intercostal vein, delivering ablative energy to one or
more ablation
elements carried by a distal region of an ablation catheter, ablating a
greater splanchnic nerve
outside of the intercostal vein, and delivering irrigation fluid out of a
plurality of irrigation ports
in the distal region or section of the ablation catheter at a rate from 15
ml/min ¨ 50 ml/min.
[0145] Any feature of this aspect of the disclosure may be
included or incorporated with
any step or steps of any other aspect, including aspects related to delivering
ablative energy using
any of the ablation catheters herein.
- 19 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0146] In this aspect, delivering the irrigation fluid may
comprise delivering irrigation fluid
out of a plurality of irrigation ports in the distal region of the ablation
catheter at a rate of 30
ml/min.
[0147] In this aspect, delivering the irrigation fluid may
comprise delivering the irrigation
fluid out of 17 to 344 irrigation ports in the distal region or section of the
catheter.
[0148] In this aspect, delivering the irrigation fluid comprises
delivering the irrigation fluid
out of a plurality of distal irrigation ports, the distal irrigation ports
being disposed distal to the
one or more ablation elements.
[0149] In this aspect, delivering the irrigation fluid may
comprise delivering the irrigation
fluid out of a plurality of central irrigation ports, the central irrigation
ports disposed between a
proximal ablation element and a distal ablation element.
[0150] In this aspect, delivering the irrigation fluid may
comprise avoiding delivering the
irrigation fluid out of any part of the distal region or section of the shaft
that is proximal to the
one or more ablation elements, optionally due to the absence of any irrigation
ports proximal to
the one or more ablation elements.
[0151] In this aspect, delivering ablative energy may comprises
delivering energy at a
power from 15 W-50 W, optionally at 35 W.
[0152] In this aspect, delivering the irrigation fluid may
comprise delivering irrigation fluid
distal to the one or more ablation elements and not delivering irrigation
fluid proximal to the one
2 0 or more ablation elements.
[0153] In this aspect, delivering the irrigation fluid may
comprise delivering irrigation fluid
out of a plurality of irrigation ports, wherein the plurality of irrigation
ports optionally having a
combined area in a range of 1.51e-4 to 1.08e-3 in2.
[0154] In this aspect, a diameter of the plurality of irrigation
ports may be in a range of
0.002" to 0.009".
[0155] In this aspect, delivering the irrigation fluid out of the
plurality of irrigation ports at
a rate from 15 ml/min ¨ 50 ml/min may create a Weber number in a range of 0.4
¨ 53.
[0156] In this aspect, delivering the ablative energy may comprise
delivering the ablation
energy to first and second coiled ablation elements that are axially spaced
apart on the shaft.
[0157] In this aspect, delivering irrigation fluid may comprise delivering
the irrigation fluid
out of at least some of the plurality of ports that are disposed between
windings of first and
second coiled ablation elements.
- 20 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
BRIEF DESCRIPTION OF THE DRAWINGS
[0158] The drawings included herewith are for illustrating various
examples of articles,
methods, and apparatuses of the present specification and are not intended to
limit the scope of
what is taught in any way. In the drawings:
[0159] Figure 1 is an isometric view schematic illustration of an ablation
catheter
positioned in an intercostal vein for ablation of a thoracic splanchnic nerve.
[0160] Figure 2 is a transverse view schematic illustration of an
ablation catheter
positioned in an intercostal vein and a centered azygos vein.
[0161] Figures 3 is a transverse view schematic illustration of
anatomy showing a right-
1 0 biased azygos vein.
[0162] Figure 4 is a transverse view schematic illustration of
anatomy showing a left-
biased azygos vein.
[0163] Figure 5 is a transverse view schematic illustration of
anatomy showing a range of
position of azygos veins and a range of position of a right GSN.
[0164] Figure 6 is an AP fluoroscopic image of a patient's T8 to T12
thoracic region.
[0165] Figure 7 is an RA030 fluoroscopic image of a patient's T8
to T12 thoracic region.
[0166] Figure 8A is a schematic illustration of an ablation
catheter with two coiled RF
electrodes.
[0167] Figure 8B is a schematic illustration of an ablation
catheter with two coiled RF
2 0 electrodes and a distal deployable element.
[0168] Figure 8C is a schematic illustration of a first, second
and third section of a catheter
shaft.
[0169] Figure 8D is a schematic illustration of a distal portion
or section of an ablation
catheter having irrigation holes arranged in a helical pattern between
windings of a helical
electrode and an irrigation hole distal to the distal electrode.
[0170] Figure 8E is a schematic illustration of a distal portion
of an ablation catheter
having irrigation holes arranged in a helical pattern between at least some
windings of a helical
electrode and a plurality of in-igation holes distal to a distal electrode and
between proximal and
distal electrodes.
[0171] Figure 9 is a schematic illustration of an ablation catheter with
two coiled RF
electrodes, a distal deployable element, and a proximal deployable element.
[0172] Figure 10 is a schematic illustration of an ablation
catheter with two coiled RF
electrodes, a distal deployable element, a proximal deployable element, and a
middle deployable
element.
- 21 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0173] Figure 11 is a schematic illustration of an ablation
catheter with an RF electrode
comprising expandable wire struts.
[0174] Figure 12 is a schematic illustration of an ablation
catheter with an RF electrode
comprising an expandable balloon with an RF electrode on its surface.
[0175] Figure 13A and 13B are schematic illustrations of an ablation
catheter with an RF
electrode comprising an expandable balloon with an RF electrode made from
conductive ink on
its surface.
[0176] Figure 14 is a schematic illustration of an ablation
catheter with an RF electrode
comprising an expandable balloon with an RF electrode on its surface in a zig-
zag pattern.
1 0 [0177] Figure 15 is a schematic illustration of an ablation catheter
with an RF electrode in
a cavity defined by a membrane.
[0178] Figure 16 is a schematic illustration of an ablation
catheter with a plurality of RF
electrode sections on a tapered shaft
[0179] Figure 17A and 17B are schematic illustrations of an
ablation catheter with RF
electrode pads on an expandable balloon.
[0180] Figure 18 is a schematic illustration of an ablation
catheter with ultrasound
transducers.
[0181] Figure 19 includes plots of RF Power delivered to a first
electrode and a second
electrode as well as temperature monitored by sensors associated with the
first and second
2 0 electrodes and bioelectric impedance monitored from the first and
second electrodes on the same
time axis.
[0182] Figure 20 is an exemplary machine state diagram of an
exemplary saline tracking
algorithm.
[0183] Figure 21A is a schematic illustration of an ablation
catheter with flat helical
electrodes.
[0184] Figure 21B is a schematic illustration of an ablation
catheter with flat helical
electrodes.
DETAILED DESCRIPTION
[0185] The disclosure herein is generally related to methods of
treating at least one of heart
failure and hypertension by increasing splanchnic capacitance. Some approaches
include
systems, devices, and methods for transvascular (e.g., transvenous) ablation
of target tissue to
increase splanchnic venous capacitance or venous compliance. The devices and
methods may, in
some examples, be used for ablating a splanchnic nerve to increase splanchnic
capacitance. For
- 22 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
example, the devices disclosed herein may be advanced endovascularly to a
target vessel or
plurality of vessels in the region of a thoracic splanchnic nerve (-TSN"),
such as a preganglionic
greater splanchnic nerve ("GSN"), lesser splanchnic nerve, or least splanchnic
nerve or one of
their roots (a TSN nerve root). The target vessel may be, for example, an
intercostal vein or an
azygos vein (or both) or a vein of the azygos vein system, preferably, one or
more of the lowest
(i.e., most caudal) three intercostal veins (which may be T9, T10, or T11).
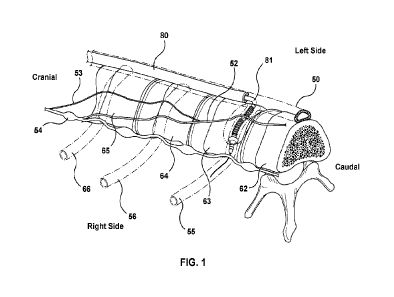
[0186] Figure 1 shows a patient's thoracic spine, including T12
(62), T11 (63), T10 (64),
and T9 (65) vertebrae, intervertebral discs, a sympathetic trunk 54, an azygos
vein 50, a right
T11 intercostal vein 55, a right T10 intercostal vein 56, a right T9
intercostal vein 66, GSN roots
1 0 53, and a fully-formed GSN 52. The lesser and least splanchnic nerves
and their roots are
omitted for simplicity. A primary objective of the proposed procedure is to
ablate the GSN or its
roots as will be discussed in detail herein. It is noted that ablation of the
lesser or least splanchnic
nerves or their roots may also have therapeutic effects and may be a
procedural objective. A
delivery sheath 80 is shown positioned in the azygos vein and an ablation
catheter 81 is shown
delivered through the sheath and passing from the azygos vein into the T11
intercostal vein. The
sympathetic trunk runs substantially parallel to the spine, consistently
passing close to each
costovertebral joint 61 (see Figure 2). On the right side of the body the GSN
roots branch from
the sympathetic trunk, typically cranial to the T9 vertebra, and converge to
form the GSN, which
travels at an angle from the sympathetic trunk toward the anterior-center of
the spine and is
2 0 positioned anterior to the intercostal veins between the intercostal
veins and parietal pleura 60
(see Figure 2). The azygos vein 50 travels along the anterior of the spine and
may be somewhat
straight and parallel to the axis of the spine as shown in Figure 1. However,
the precise position
of the azygos vein relative to the spine is variable from patient to patient
and at different
vertebral levels. At the T9, T10. and T11 vertebral levels the azygos vein 50
may be centered
with respect to the midline of the vertebra 69 as shown in Figure 2, may be a
right-biased azygos
vein 50R with respect to the midline of the vertebra 69 as shown in Figure 3,
or be a left-biased
azygos vein 50L with respect to the midline of the vertebra 69 as shown in
Figure 4. Cadaver
studies conducted by the authors indicate that the range of azygos position
relative to the center
of the spine at the T9, T10, and T11 levels is within 10 mm to the left or
right of center for a
large majority of people. Figure 5 shows a left-biased azygos vein 50L, a
right-biased azygos
vein 50R, and a centered azygos vein 50C along with the range 67 of the azygos
vein relative to
the center of the spine 69. Furthermore, the precise position of the right GSN
from patient to
patient is somewhat variable including where it originates from the
sympathetic trunk, the angle
at which it travels, and its destination relative to the spine. Thus, the
position of the GSN
relative to the vertebra at T9, T10 and T11 can vary. Cadaver studies
conducted by the authors
- 23 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
indicate that the range of right side GSN position relative to the center of
the vertebra at the T9,
T10 and T11 levels is from 0 mm to 25 mm to the right of center 69 as shown by
the range box
68 in Figure 5.
[0187] An endovascular approach to transvascularly ablate a TSN,
particularly a GSN may
involve one or more of the following steps: accessing venous vasculature at
the patient's jugular
vein or femoral vein with an access introducer sheath (e.g. 12F); delivering a
delivery sheath
(e.g., 9F sheath) to an azygos vein (e.g., to one or two thoracic levels above
the target intercostal
vein); optionally, delivering contrast agent through the sheath to show
location of veins on
fluoroscopy; optionally, delivering a guidewire (e.g., 0.014" guidewire)
through the delivery
sheath and into a targeted T9, T10, or T11 intercostal vein; and delivering an
ablation catheter
through the delivery sheath to the azygos vein, optionally over the guidewire,
positioning an
ablation element in an intercostal vein, azygos vein or both; and aligning a
radiopaque marker on
the ablation catheter with an anatomical landmark (or positioning it relative
thereto) to position
an ablation element in a region that maximizes efficacy of ablating a target
TSN/GSN while
minimizing risk of injuring one or more non-target structures.
[0188] Some important anatomical structures in the vicinity of
this region that should not
be injured include the sympathetic trunk 54, vagus nerve, thoracic duct, and
esophagus.
Therefore, to ensure safety an ablation zone should be contained within a safe
region that does
not injure such structures. Due to the variability of position of the azygos
vein and GSN relative
to the T9. T10 and T11 vertebrae, the relative position of the GSN with
respect to the intercostal
vein or azygos vein in which an ablation element is positioned is also
variable.
[0189] Bones, blood vessels if injected with radiopaque contrast
medium, and medical
devices if made from radiopaque material, are visible on fluoroscopy but
nerves are not. An
ablation device designed for transvascular (e.g., transvenous) ablation of a
TSN (e.g., GSN) from
an intercostal vein, azygos vein, or both along with procedural steps may be
provided to ensure
efficacious ablation of the TSN (e.g., GSN) while ensuring safety. The
procedural steps may
include fluoroscopic imaging to position the ablation element(s) of the
ablation catheter with
respect to honey or vascular structures.
[0190] In a first embodiment of a method of ablating a right GSN
an ablation catheter
having a proximal radiopaque marker 136, a distal radiopaque marker 130, an
ablation element
131 or plurality of ablation elements 132, 133, and an optional gap 135
between the ablation
element and the distal radiopaque marker is advanced from an azygos vein 50
into an intercostal
vein 55 at one of the lower three thoracic levels (e.g., T9, T10, T11). The C-
Arm is placed in
Anterior-Posterior (AP) orientation. The proximal radiopaque marker 136 is
aligned with the
midline of the vertebra 69, which is possible if the azygos vein 50 is
centered or left-biased. If
- 24 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
the azygos vein 50 is left-biased the proximal radiopaque marker will need to
be advanced into
the intercostal vein to align it with the midline of the vertebra 69. If the
azygos vein is right-
biased the proximal radiopaque marker 136 will not be able to be placed at the
midline of the
vertebra 69. In this case the proximal radiopaque marker 136 may be placed at
the ostium of the
intercostal vein, which will be to the right of midline 69. Optionally, the
position of a distal
radiopaque marker 130 relative to the costovertebral joint may be assessed
(e.g., with the C-Arm
in a RAO orientation) to ensure the sympathetic trunk is not at risk of
injury, for example with
patients who are very small and have an extreme right-biased azygos vein. The
C-Arm may be
obliquely angled to the right (RAO orientation) to maximize the 2D projection
of the section of
1 0 intercostal vein between the costovertebral joint 61 and anterior
midline of the vertebra 69
(Figure 7). For example. the C-arm may be positioned with a Right Anterior
Oblique (RAO)
angle in a range of 20 to 70 from AP (e.g., in a range of 300 to 60 , in a
range of 35 to 55 ,
about 30 , at an angle that maximizes projected distance between the proximal
and distal RO
markers). With this view the user may check to make sure the distal radiopaque
marker is not
too close to the costovertebral joint 61. For example, if the distal
radiopaque marker is positioned
directly distal to the ablation element a distance of at least 3 mm (e.g., at
least 5 mm) may be
chosen to ensure the sympathetic trunk is not injured. In another example, if
the distal
radiopaque marker is positioned distal to the ablation element with a known
space between them
the distal radiopaque marker may be aligned with the costovertebral joint or
proximal to it to
2 0 ensure safety of the sympathetic joint. If the distal radiopaque marker
is too close to or beyond
the costovertebral joint the catheter may be pulled back until an acceptable
distance between the
distal radiopaque marker and the costovertebral joint is seen, which may place
the proximal
radiopaque marker in the azygos vein especially if the azygos vein is right
biased. If the ablation
element is comprised of a plurality of ablation elements (e.g., two) an
ablation may first be
performed from the more proximal ablation element prior to pulling the
catheter back to
appropriately place the distal radiopaque marker relative to the
costovertebral joint. Then a
subsequent ablation may be made from the more distal ablation element.
[0191] In a second embodiment of a method of ablating a right GSN
an ablation catheter
having a proximal radiopaque marker 136, a distal radiopaque marker 130, an
ablation element
131 or plurality of ablation elements 132, 133, and an optional gap 135
between the ablation
element and the distal radiopaque marker is advanced from an azygos vein 50
into an intercostal
vein 55 at one of the lower three thoracic levels (e.g., T9, T10, T11). The C-
Arm is placed in
Anterior-Posterior (AP) orientation. The proximal radiopaque marker 136 is
aligned with the
intercostal vein ostium 59. The ostium can be found for example by injecting
contrast agent and
viewing the vasculature on fluoroscopy or if a guidewire was previously
positioned in a target
- 25 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
intercostal vein a bend in the guidewire or ablation catheter may indicate the
location of the
ostium. If the azygos vein is left-biased the catheter is advanced distal to
the ostium to align the
proximal radiopaque marker 136 with the midline of the vertebra 69. In this
placement strategy
the proximal radiopaque marker 136 will be aligned with the midline of the
vertebra 69 if the
azygos vein is left-biased or centered, and to the right of the midline of the
vertebra if the azygos
vein is right-biased. Concurrently, the proximal radiopaque marker 136 will be
aligned with the
ostium if the azygos vein is right-biased or centered, and at the midline of
the vertebra 69 if the
azygos vein is left-biased. Optionally, the position of a distal radiopaque
marker 130 relative to
the costovertebral joint may be assessed (e.g., with the C-Arm in a RAO
orientation) to ensure
the sympathetic trunk is not at risk of injury, for example with patients who
are very small and
have an extreme right-biased azygos vein. The C-Arm may be obliquely angled to
the right
(RAO orientation) to maximize the 2D projection of the section of intercostal
vein between the
costovertebral joint 61 and anterior midline of the vertebra 69 (Figure 7).
For example, the C-
arm may be positioned with a Right Anterior Oblique (RAO) angle in a range of
20 to 70 from
AP (e.g., in a range of 30 to 60 , in a range of 35 to 55 , about 30 , at an
angle that maximizes
projected distance between the proximal and distal RO markers). With this view
the user may
check to make sure the distal radiopaque marker is not too close to the
costovertebral joint 61.
For example, if the distal radiopaque marker is positioned directly distal to
the ablation element a
distance of at least 3 mm (e.g., at least 5 mm) may he chosen to ensure the
sympathetic trunk is
2 0 not injured. In another example, if the distal radiopaque marker is
positioned distal to the
ablation element with a known space between them the distal radiopaque marker
may be aligned
with the costovertebral joint or proximal to it to ensure safety of the
sympathetic joint. If the
distal radiopaque marker is too close to or beyond the costovertebral joint
the catheter may be
pulled back until an acceptable distance between the distal radiopaque marker
and the
costovertebral joint is seen, which may place the proximal radiopaque marker
in the azygos vein
especially if the azygos vein is right biased.
1_0192]
In a third embodiment of a method of ablating a right GSN an ablation
catheter
having a distal radiopaque marker 130, an ablation element 131 or plurality of
ablation elements
132, 133, and a gap 135 between the ablation element and the distal radiopaque
marker is
advanced from an azygos vein 50 into an intercostal vein 55 at one of the
lower three thoracic
levels (e.g., T9, T10, T11). The C-Arm is obliquely angled to the right to
maximize the 2D
projection of the section of intercostal vein between the costuvertebral joint
61 and anterior
midline of the vertebra 69 (Figure 2). For example, the C-arm may be
positioned with a Right
Anterior Oblique (RAO) angle in a range of 20 to 70 from AP (e.g., in a
range of 30 to 60 , in
a range of 35 to 55 , about 30 , at an angle that maximizes projected
distance between the
- 26 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
proximal and distal RO markers). A fluoroscopy image in an anterior-posterior
(AP) view is
shown in Figure 6. In comparison a fluoroscopy image in a RAO 300 is shown in
Figure 7. The
catheter is advanced to align the distal radiopaque marker 130 with the
costovertebral joint 61.
Since the sympathetic trunk 54 is next to the costovertebral joint 61 the gap
between the distal
radiopaque marker and the ablation element may ensure the sympathetic trunk is
not injured.
The gap may be for example a length in a range of 0 to 25 mm (e.g., a range of
3 to 25 mm, a
range of 5 to 25 mm, a range of 5 to 20 mm). Optionally, an inflatable balloon
134 may be
positioned on the catheter shaft within the gap, which may help to anchor the
catheter or contain
ablation energy proximal to the balloon. Optionally, the catheter shaft 138
distal to the ablation
1 0 element may be narrower or more flexible than the remainder of the
shaft to facilitate delivery
through the narrower distal portion of the intercostal vein. Optionally, the
ablation element(s)
has a length capable of ablating to the anterior midline of the vertebra 69
when the distal
radiopaque marker is aligned with the costovertebral joint. For example, the
ablation element(s)
may have a total length in a range of 5 to 25 mm (e.g., in a range of 10 to 25
nun, in a range of
15 to 20 mm). The ablation catheter may have a proximal radiopaque marker
located just
proximal to the ablation element(s). Optionally, prior to delivering ablation
energy a user may
image the proximal radiopaque marker to ensure it is at the anterior midline
of the vertebra 69. If
the proximal radiopaque marker is to the left of the midline 69, for example
if the patient is
extremely small, there may be a risk of injuring a non-target tissue such as
the thoracic duct or
esophagus. To mitigate this risk a catheter with a smaller sized ablation
element may be used or
if the ablation element is made of a plurality of ablation elements only the
elements between the
midline 69 and distal radiopaque marker may be activated for ablation.
Conversely, if the
proximal radiopaque marker is to the right of the midline 69, for example if
the patient is
extremely large, there may be a risk of missing the GSN. To mitigate this risk
another ablation
may be performed at another intercostal level or within the same intercoastal
vein with the
position of the ablation element retracted until the proximal radiopaque
marker is aligned with
the midline 69.
[0193] In a fourth embodiment of a method of ablating a right GSN
an ablation catheter
having an ablation element 131, which may include a plurality of ablation
elements, a distal
radiopaque marker located at a distal end of the ablation element(s), and a
proximal radiopaque
marker located at a proximal end of the ablation element(s) is advanced from
an azygos vein into
an intercostal vein at one of the lower three thoracic levels (e.g., T9, T10,
T11). The C-Arm is
obliquely angled to the right to maximize the 2D projection of the section of
intercostal vein
between the costovertebral joint 61 and anterior midline of the vertebra 69
(Figure 5). For
example, the C-arm may be positioned with a Right Anterior Oblique (RAO) angle
in a range of
- 27 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
25 to 65 from AP (e.g., in a range of 30 to 600, in a range of 350 to 55 ,
about 30 ). The
catheter is advanced to align the distal radiopaque marker with a position
relative to the
costovertebral joint and the opposing edge of the vertebral body in the
oblique view. For
example, the distal radiopaque marker may be aligned with a point that is
midway between the
costovertebral joint and the opposing edge of the vertebral body in the
oblique view. The
ablation element(s) may have a total length expected to cover the GSN position
range 68 in most
patients. Similar to the previously described methods, the proximal end of the
ablation
element(s) may be at the anterior midline of the vertebra 69 or to the left in
centered or left-
biased azygos situations and may be in the azygos vein in right-biased azygos
situations.
Ablation energy may be delivered from the ablation element(s) to ablate the
range without
moving the catheter. Optionally, the catheter may be moved to another
intercostal level and a
second ablation may be made using the same method steps.
[0194] Performing any of the exemplary embodiments of placement
strategy disclosed
above, when the ablation element 131 has a total length less than 30 mm (e.g.,
less than 25 mm,
less than 20 mm, about 15 mm) it is expected that in a large majority of
patients the sympathetic
trunk will be spared from injury even if the azygos vein is right-biased.
Additionally, when
performing the methods herein, when the ablation element 131 has a total
length greater than or
equal to 15 mm it is expected that in a large majority of patients the GSN
will be ablated.
Therefore, the ablation clement 131 may have a total length in a range of 15
mm to 30 mm to be
2 0 effective and safe for a large majority of patients using these
placement strategies. However,
smaller ablation element total length may be suitable for exceptional
patients. For example, the
ablation element may have a total length in a range of 5 to 25 mm (e.g., in a
range of 10 to 20
mm, or in a range of 10 to 15 mm).
[0195] As used herein, ablation element may refer to a single
structure or a plurality of
structures. For example, as used herein, ablation element may include a
plurality of ablation
electrodes that are axially spaced apart, and each of which may be adapted to
facilitate the
delivery of ablation energy.
[0196] Once acceptable ablation element placement is achieved, for
example using one of
the exemplary embodiments of placement strategy herein, ablation energy may be
delivered from
the ablation element or plurality of ablation elements without having to move
the catheter.
Ablation energy may be delivered from the ablation element to ablate tissue
circumferentially
around the intercostal vein a depth in a range of 2 mm to 10 mm (e.g., a range
of 2 mm to 8 mm,
a ranee of 3 mm to 8 mm, about 5 mm). Optionally, the procedure may be
repeated at another
thoracic level (e.g., a more cranial level, a more caudal level, another of
T9, T10, T11 intercostal
veins on the same side of the patient) especially if the azygos is right
biased. Alternatively or in
- 28 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
addition to having distal and proximal radiopaque markers at both ends of an
ablation element or
plurality of ablation elements, the ablation element(s) itself may be
radiopaque and the same
methods herein may be used to position the distal or proximal end of the
ablation element(s)
relative to anatomical landmarks (e.g., midline of the spine, costovertebral
joint, etc.). The
phrase radiopaque marker as used herein may thus describe an ablation element
if the ablation
element is radiopaque. In some alternative embodiments, a radiopaque markers
may comprise a
relatively longer radiopaque marker positioned under or next to one or more
ablation elements
wherein the proximal end of the long radiopaque marker is at least aligned
with the proximal end
of the ablation element or extending proximal of the ablation element by up to
3 mm and the
distal end of the long radiopaque marker is at least aligned with the distal
end of the ablation
element or extending distal to the ablation element by up to 3 mm.
[0197] With any of the exemplary embodiments of placement strategy
disclosed above,
there may be situations when a portion of the ablation element(s) is in the
azygos vein while the
remainder is in the intercostal vein, in particular when the ablation catheter
has an ablation
element or plurality of elements having a total length in a range of 10 to 25
mm. The azygos
vein is larger than the intercostal vein and has greater blood flow, which may
impact the ability
to create an effective ablation around the azygos vein or even in the
intercostal vein and may
require different energy delivery parameters than an ablation made completely
in an intercostal
vein. To resolve this, the ablation catheter may have a plurality of ablation
elements wherein at
2 0 least one is fully positioned in an intercostal vein and the remainder
may be in the intercostal
vein or in the azygos vein or both. Different ablation energy delivery
parameters may be used
for the different scenarios, for example higher power or energy may be
delivered to the ablation
element in the azygos vein or ablation energy may only be delivered to the
element(s) that are
fully or partially in the intercostal vein. The location of the plurality of
ablation elements may be
determined with fluoroscopic imaging or by monitoring electrical impedance
between each
ablation element (e.g., RF electrode) and a dispersive electrode.
[0198] Optionally, two or even three levels may be ablated,
particularly if the azygos is
right-biased hut even if the azygos is centered or left-biased, which may
further increase
efficacy.
[0199] Alternative devices and methods of use may include a shorter
ablation element that
is used to create a relatively shorter ablation and repositioned a plurality
of times to create
multiple ablations within the GSN position range 68. If the azygos is centered
or left-biased all
ablations may be made in the intercostal vein 55 and cover the range 68. If
the azygos is right-
biased, ablations may be made in the intercostal vein to cover a portion of
the range 68, and then
ablations may be made at another intercostal level to improve the probability
of ablating the
- 29 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
GSN. Optionally, ablations may be made from the azygos vein, which may use
different energy
delivery parameters for example, higher energy or power.
[0200] An ablation catheter adapted to ablate a TSN (e.g., GSN)
from an intercostal vein
and or an azygos vein, for example using one or more of the embodiments of
placement
strategies disclosed herein, may have features that allow it to be delivered
transvascularly to a
desired location in a T9, T10, or T11 intercostal vein, be positioned relative
to anatomical
features to effectively ablate a target TSN while safely avoiding important
non-target structures
in a large majority of patients, and to deliver ablative energy capable of
ablating the target TSN.
The ablation catheter and system features may allow a user to ablate a TSN
with relative ease
and efficiency without sacrificing efficacy or safety. For example, once the
ablation element(s)
of the catheter are positioned (e.g., using methods disclosed herein),
ablation energy may be
delivered from a computerized ablation console with the press of a button or
at least with
minimal adjustments, repositioning, dragging, torqueing of the catheter or
minimal user
decisions regarding energy delivery. Even considering the variability of
location of the GSN 68
and azygos vein 67 (see Figure 5), features of ablation catheters and systems
disclosed herein
may allow a TSN/GSN to be ablated from one placement and energy delivery
procedure or in
some cases from an additional placement (e.g., in another of a T9, T10, or T11
intercostal vein)
and energy delivery with a high probability of success in a large majority of
patients.
[0201] An ablation catheter for transvascular ablation of a GSN
may have a proximal end,
a distal end, an elongate shaft therebetween, a distal section (e.g.,
comprising the distal-most
7cm), and an ablation element on, at or carried by the distal section. The
ablation element may be
adapted (including sized and/or configured) to create an ablation having a
length in a range of 5
mm to 25 mm, preferably 10 to 25 mm (such as 15 mm to 20 mm) and a radial
depth of at least 5
mm from the vessel surface. A handle may be located on the proximal end of the
catheter to
contain electrical or fluid connections or facilitate handling of the
catheter. The elongate shaft
from a strain relief region to the distal tip may have a length of 100 cm to
140 cm (such as from
110 cm to 130 cm, such as about 120 cm) allowing the distal section to be
delivered from an
arteriotomy such as a femoral vein access (or other access location such as
jugular vein, brachial
vein, radial vein, hepatic vein or subclavian vein) to a T11 intercostal vein
in a large majority of
human patients, or a length of 50 cm to 140 cm allowing the distal section to
be delivered from a
jugular vein access to a T11 intercostal vein in most patients. To be
deliverable through a 9F
delivery sheath the catheter may have a maximum outer diameter of 3 mm (e.g.,
2.5 mm, 2 mm,
1.5 mm) at least in its delivery state. The catheter may optionally have a
deployable structure that
expands beyond this dimension once advanced from the delivery sheath and
positioned in a
target vessel in some embodiments. An ablation catheter for delivering an
ablation element to an
- 30 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
intercostal vein, in particular a T9, TIO or T11 intercostal vein, from an
endovascular approach
including approaching the intercostal vein from an azygos vein may have a
shaft with features
that facilitate easy tracking over a guidewire, pushability, transfer of
translation forces from the
handle of the catheter, and passing over a tight bend from the azygos vein to
the intercostal vein
without kinking. As shown in Figure 8C, the catheter shaft may comprise a
first section 340, a
second section 34 land a third section 342. The first section 340 may be more
flexible than the
second and third sections and may carry the ablation element such as two
coiled electrodes 133
and 132 as shown. This first section may have a flexibility capable of passing
over the tight
bend from the azygos vein to intercostal vein (e.g., having a radius of
curvature >, 5 mm, and
angle up to 120 degrees). The first section may have a length in a range of 60
nana to 100 mm
(e.g., about 65 mm) and may be made from a single lumen Pebax tube having a
durometer
from 50 to 60 D, such as 55D.
[0202] The second section 341 may have a flexibility between that
of the first and third
sections and function as a transition region and strain relief to resist
kinking. For example, the
second section may have a length in a range of 15 mm to 25 mm (e.g., about 20
mm) and may be
made from a single lumen Pebax 0 tube having a durometer from 60D ¨ 70D, such
as from 60D
- 65D, such as 63D.
[0203] The third section 342 may be at least a portion of the
proximal region of the
elongate shaft and may be adapted for pushability, kink resistance, torque
transmission, and
flexibility. For example, the third section of the elongate shaft may span
from the proximal end
of the catheter to about 85 mm (e.g., in a range of 75 mm to 100 nun) from the
distal end and
may optionally have a metal wire braid embedded into an outer layer of the
shaft. An example
material for the third section of the elongate shaft may be extruded Pebax
having a durometer
from 70D to 75D, such as 72D, for example. For example, the first section 340
may be more
flexible than the second section 341 section, which may be more flexible than
the third section
342 and flexibility may be increased by using a lower durometer material or
more flexible
braided outer layer or no braided outer layer. The maximum outer diameter of
the elongate shaft,
at least in a delivery state, may be in a range of 1.5 to 3 mm. Optionally, as
shown in Figure 8C,
the first section 340 of the shaft may be made from a tube having a smaller
diameter than the
second section 341, which in turn may have a smaller diameter than the third
section 342 of the
shaft. For example, the first section may be made of a tube having an outer
diameter of 2 mm;
the second section may be made of a tube having an outer diameter of 2.5 mm;
and the third
section may be made of a tube having an outer diameter of 3 'mi. Optionally,
the elongate shaft
may have a tapered. soft distal tip 345, which may have a length in a range of
5 mm to 30 mm
(e.g., about 8 mm), and which may be softer than the first section.
Optionally, the first, second,
- 31 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
or third sections of the shaft may have a lubricious coating on the exterior
surface to further
improve delivery through vasculaturc. A guidewire lumen may pass through the
elongate shaft
with an exit port 82 at the distal tip of the shaft. The guidewire lumen may
be made from, for
example, a 0.014" ID polyimide tube located in a lumen of the shaft.
[0204] The ablation catheters may have an ablation element adapted to
deliver ablative
energy to a target nerve up to 5 mm from the vessel surface for a total length
in a range of 10 mm
to 25 mm, such as 10 mm to 20 mm, such as 15 mm to 20 mm. The ablation element
may be
made of a plurality of ablation elements (e.g., two) positioned within a
region of the shaft having
a total length in a range of 10 mm to 25 mm, such as 10 to 20 mm, such as 15
mm to 20 mm
even if the ablation elements are axially spaced apart. The ablation
element(s) may include one
or more of an RF ablation electrode, a coiled wire electrode, a laser cut RF
electrode, an RF
electrode printed with conductive ink, an RF electrode on an expandable
balloon (e.g., made
from conductive ink or flexible circuits), a conductive membrane RF electrode,
an RF electrode
on an expandable cage or mesh, an ultrasound ablation transducer,
electroporation electrodes, a
cryoablation element, or a virtual RF electrode.
[0205] The ablation element may be adapted to deliver ablation
energy circumferentially,
that is radially symmetric around the ablation element and around the vessel
in which the
ablation element is positioned. Although the GSN always passes anterior to the
intercostal vein
and azygos, it is safe and acceptable to ablate tissue around the intercostal
or azygos veins, and
2 0 ablating circumferentially may allow for a simpler and faster procedure
that is also less prone to
user error because aiming the energy delivery is not necessary. Features that
may allow for
circumferential ablation may include, without limitation, ablation electrodes
that expand to
contact the vessel wall evenly around the circumference of the vessel,
ablation electrodes that are
used with an electrically conductive fluid, electrically insulative balloons
or deployable
structures that contain ablative energy in a segment of a target vessel
allowing it to be directed
radially, ablation elements that direct ablation energy circumferentially such
as cylindrical
ultrasound transducers.
[0206] In sonic embodiments, the ablation element is an RF
electrode and saline may be
delivered to the vessel in fluid communication with the RF electrode. An
irrigation lumen in
communication with irrigation ports may located distal to the ablation
element, under the
ablation element (in some designs where irrigated saline can pass through the
ablation element),
or in a deployable structure in some embodiments). An irrigation lumen may be
for example a
lumen in the elongate shaft in fluid communication with a tube on the
catheter's proximal end
that is connectable to a fluid source and pump.
- 32 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0207] Optionally, at least one deployable occlusive structure
(e.g., balloon, bellows, wire
mesh, wire braid, coated wire mesh, or coated wire braid) may be positioned on
the shaft distal to
the ablation element. The deployable structure may function to anchor the
catheter in place
during energy deli very and possibly to improve safety by avoiding ablation of
the sympathetic
trunk by providing an electrical insulator or containing saline proximal to
the deployable
structure. Optionally, a deployable occlusive structure may be located just
proximal to the
proximal end of the ablation element(s) which may function to divert blood
flowing in the
azygos vein away from the ablation zone. For example, a deployable occlusive
structure may be
a balloon such as a urethane balloon having a length (along the axis of the
shaft) of about 2.5 mm
and an inflated diameter of about 2.5 mm to 7 mm (e.g., 3 mm to 6 mm, 4 mm to
5 mm). The
balloon may be in fluid communication with an inflation port connecting the
balloon with an
inflation lumen connectable to an inflation source on the proximal end of the
catheter.
Optionally, the inflation lumen may be in fluid communication with an
irrigation lumen
connectable to an irrigation source and pump. Optionally such a catheter may
have a balloon
with holes that allow irrigation fluid to exit the inflated balloon and flow
toward the ablation
element(s).
[0208] Ablation catheters may have a proximal radiopaque marker
positioned on the shaft
at or proximal to the proximal end of the ablation element(s). Optionally,
ablation catheters may
include a distal radiopaque marker which may be positioned on the shaft at or
distal to the distal
end of the ablation element. Optionally, there may be a space between a distal
radiopaque marker
and the distal end of the ablation element, the space having a length in a
range of .1 mm to 25
mm, such as .1 mm to 5 mm, such as .1 mm to 3 mm, such as .5 nana, 1 mm, or
1.5 mm. For
example, as shown in Figure 2 a distal radiopaque marker 130 may be aligned
with or positioned
relative to an anatomical landmark such as the costovertebral joint 61 and a
space 135 (e.g., .1
mm to 25 mm) is between the distal radiopaque marker 130 and the distal end of
the ablation
element 132 ensuring the ablation element is safely distant from the
sympathetic trunk 54.
Optionally, a deployable structure 134 may be positioned in the space
transitionable between a
contracted state (OD similar to the shaft OD e.g., in a range of 1.5 mm to 3
mm) and deployed
state (OD increases to a range of 3 to 7 mm). The deployable structure may be
a balloon,
bellows, wire mesh, wire braid, coated wire mesh, or coated wire braid.
[0209] An example of an ablation catheter that is sized and
adapted for GSN ablation is
shown in Figure 2. Ablation catheter 81 has an elongated shaft sized and
adapted to reach a T11
intercostal vein from an introduction site at a femoral vein or jugular vein.
The distal section of
catheter 81, shown positioned in an intercostal vein 55, includes a distal
radiopaque marker 130
that is aligned with or positioned relative to a costovertebral joint 61, an
ablation element 131
- 33 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
comprising or consisting of a distal conductive coiled RF electrode 132 and a
proximal
conductive coiled RF electrode 133, an optional inflatable balloon 134
disposed between the
ablation element 131 and the distal radiopaque electrode 130. The distal
radiopaque marker 130
is optionally spaced distally apart from the distal end of the ablation
element 132 by a distance
135 for example in a range of 0 to 25 mm (e.g., such as a range of .1 mm to 20
mm, such as a
range of.1 mm to 15 mm, a range of .1 mm to 3 nun, such as .5 nun. 1 mm, or
1.5 mm). Catheter
81 also includes a proximal radiopaque marker 136 that is located at or near a
proximal edge of
the ablation element 131. In some embodiments proximal radiopaque marker 136
is axially
spaced between 0 mm and 25 mm from a proximal end of ablation element 31
(which may be
1 0 from a proximal end of ablation element 133).
[0210] The exemplary axial distances between markers and
electrodes described herein
(e.g., 0 mm to 25 mm, or 0 mm to 15 mm) may be integrated into any other
ablation catheter
herein unless indicated herein to the contrary.
[0211] Ablation electrodes 132 and 133 (or any other ablation
electrode herein) may be
made from, for example, Nitinol wire coiled around the catheter shaft, which
may allow the
electrodes to be flexible so they can traverse a tight bend from the azygos
vein to the intercostal
vein and also create a long ablation (e.g., 5 to 25 mm). Nitinol is an example
of a superelastic
material that allows the ablation element(s) to bend when traversing
anatomical bends, and then
elastically return to a linear or straight configuration once the electrode is
past the bend.
2 0 [0212] Any of the distal sections herein may thus be described as a
distal section that has
an at-rest (as manufactured) linear or straight configuration. This would be
in contrast to distal
sections that may revert or assume non-linear at-rest configurations (e.g., a
distal section with
electrodes thereon that returns to a coiled configuration).
[0213] Optionally, the ablation catheter 81 includes at least one
irrigation port 137 (as
shown in figure 2) in fluid communication with an irrigation lumen that is
near the coil
electrodes for delivering a fluid such as saline. Saline delivery may
facilitate delivery or removal
of the device, or can be used during energy delivery to improve ablation
formation and prevent
overheating, for example. Optionally, catheter 81 may include a guidewire
lumen 82 for delivery
over a guidewire 79.
[0214] Figure 8A illustrates a portion of an exemplary ablation catheter,
including at least a
portion of a distal section thereof. The ablation catheter in figure 8A
includes an ablation element
that includes a distal ablation element and a proximal ablation element. The
ablation element
(and other ablation elements herein) includes or consists of a distal
conductive coiled RF
electrode 132 and a proximal conductive coiled RF electrode 133, as shown in
Figure 8A. Both
distal and proximal coiled electrodes may be helical coils positioned around
and at least partially
- 34 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
on the outer surface of the shaft, optionally in a groove in the shaft. The
coiled electrodes may be
helical, and may have varying directions, pitches. or wire thickness, and may
be made from a
round wire or ribbon wire of electrically conductive material such as
stainless steel or
superelastic Nitinol, optionally electropolished, optionally including a
radiopaque material such
as platinum iridium. Alternatively, one or more coiled electrodes may be made
from a laser cut
tube such as a Nitinol tube forming a coiled pattern or other flexible
pattern. Alternatively, the
ablation element (e.g., ablation element 131) may be made from a distal and a
proximal flexible
electrode in the form of wire mesh or braid. Alternatively, the flexible
ablation element may
comprise a plurality of ring electrodes each having a length no more than 5
mm, such as 3 mm.
Optionally, the flexible ablation element may have an expandable diameter
transitionable from a
contracted delivery state to an expanded deployed state (e.g., having an outer
diameter up to
about 5 mm) so it can expand to contact the vessel wall.
[0215] Electrodes herein, such as the proximal and distal
electrodes herein (e.g., distal
electrode 132 and proximal electrode 133) may have a length that is in a range
of 4 mm to 12
mm, such as 5 mm to 11 mm, and in some embodiments they are or about 5 mm,
5.5. mm, 6 mm,
6.5 mm, 7.0 mm, 7.5 mm, 8 mm, 8.5 mm, 9 mm, 9.5. mm, 10 mm, 10.5 mm, or 11 mm.
Proximal and distal electrodes may have the same or substantially the same
lengths, including
lengths that are in the ranges provided herein (e.g., 5 mm to 11 mm). In some
embodiments
electrodes may have different lengths. For example, in some examples distal
electrode 132 may
2 0 be longer than proximal electrode 133, but the electrodes individually
may have any of the
lengths herein. In some examples distal electrode 132 may be shorter than
proximal electrode
133, but the electrodes individually may have any of the lengths herein.
[0216] For catheters that have a plurality of electrodes, each
electrode may be connected to
an independent conductor passing through the elongate shaft to the proximal
region of the
catheter where it is connectable to an extension cable or ablation energy
source. This can allow
each electrode to be independently energized in monopolar mode or bipolar
mode.
[0217] For some catheters with distal and proximal electrodes, the
catheters may include a
gap between a distal end of the proximal electrode and a proximal end of the
distal electrode. In
some embodiments the gap may be in a range of 0 to 5 mm, such as 0 mm 4 mm,
such as .1 mm
to 1.25 mm, such as .25 mm, .5 mm, .75 mm, 1 mm, or 1.25 mm. Preferably the
proximal and
distal electrodes are not in electrical communication with one another.
Alternatively, the
proximal and distal electrodes may at least partially overlap one another
along their lengths, as
long as they are not in electrical communication with one another.
[0218] A gap between proximal and distal electrodes may be such
that it is not so large that
it prevents a continuous ablation lesion to be formed. Gaps described herein
(e.g., 0 mm to 5
- 35 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
mm. such as .1 mm to 1.25 mm, such as .25 mm, .5 mm, .75 mm, 1 mm, or 1.25 mm)
can
provide the exemplary benefit of providing for continuous lesion formation.
[0219] Ablation catheters herein may include one or more
temperature sensors. Figure 8A
illustrates an exemplary ablation catheter that comprises at least one
temperature sensor. The
ablation catheter shown includes, for example, a proximal temperature sensor
139 that may be
positioned in contact with proximal electrode 133, and optionally on the
proximal end of
proximal electrode 133. The ablation catheter shown also includes a distal
temperature sensor
140 that may be positioned in contact with distal electrode 132, and
optionally on the distal end
of the distal electrode. Any of the ablation catheters herein may optionally
include another
temperature sensor that may be positioned between proximal and distal
electrodes, or between a
plurality of electrodes. For catheters that include one or more temperature
sensors, the
temperature sensor(s) may be thermocouples (e.g.. T-type) or thermistors.
Optionally, at least
one temperature sensor may radially extend or be radially extendable from the
catheter shaft to
contact tissue up to 3 mm away from the catheter surface. The temperature
sensor(s) may be
connectable at the proximal region of the catheter to a computerized energy
delivery console
where signals from the sensors may be input and used in an energy delivery
control algorithm.
[0220] Any of the ablation catheters herein may include one or
more irrigation ports
(which may be referred to herein as holes or apertures) in fluid communication
with an irrigation
lumen that is connectable to a fluid source at the proximal region of the
catheter for delivering a
2 0 fluid such as saline (e.g., normal or hypertonic saline) to the vessel.
The ports may be formed in
one or more layers of the elongate shaft to create the fluid communication
between the port and
the irrigation lumen. The fluid may function to cool or remove heat from the
electrode(s) and/or
vessel wall, to flush blood from the vessel to reduce risk of clot formation
or improve ablation
consistency, to conduct electrical energy from the ablation electrodes, to
control pressure in the
vessel, to facilitate delivery of the distal section of the ablation catheter
to a target vessel (e.g.,
intercostal vein), or to facilitate removal of the distal section of the
ablation catheter from the
target vessel. Optionally, one or more irrigation ports may be distal to the
ablation element(s), or
distal to each of the plurality of flexible ablation elements. In sonic
embodiments, any of the
irrigation port(s) may be positioned radially under the flexible ablation
element(s). In some
embodiments, one or all irrigation ports may be disposed between windings of
coiled ablation
element, such that the port is not radially under the winding of the ablation
element. Optionally,
an irrigation port may be positioned in an axial gap or space between adjacent
ablation
electrodes. Optionally, one or more irrigation ports may be in a cavity of a
deployable occlusive
structure (e.g., balloon) and may function to inflate the balloon, wherein the
balloon may have a
- 36 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
perforation on its proximal side that allows the fluid to escape the balloon
into the target region
of the vessel.
[0221] Figures 8A-10 illustrate distal sections of ablation
catheters that include a plurality
of irrigation ports between windings of coiled ablation elements (although
only one port 137 is
labeled, the others can be seen in the figures). In the side views shown in
figures 8A, 8B, 9 and
10, the exemplary ports are linearly aligned, parallel to a long axis of the
distal section.
Additionally shown in the side views of figures 8A, 8B, 9 and 10, there is an
irrigation port
between every adjacent pair of winding material (even though coiled elements
132 and 133 are
each formed by a continuous winding along their lengths). The central port 137
axially between
the ablation elements may or may not be included. In any of the embodiments,
every port in the
distal section may be between a winding (in the side view). Alternatively
stated, in any of the
embodiments, none of the ports may be radially under a winding structure of
the ablation
element.
[0222] Optionally, as shown in Figure 8D, irrigation holes (which
may be referred to
herein as apertures or ports)137 may be positioned between windings of the
coil electrodes and
be circumferentially distributed to deposit saline along the length of the
ablation electrodes as
well as circumferentially around the electrodes. In Figure 8D the irrigation
holes 137 follow a
helical path, optionally of the same pitch as the coil electrode with equal
spacing between holes
as shown in figure 8D. As shown, even though the example in figure 8D does not
include a
2 0 central port between electrodes (as do the examples in figures 8A and
8B), the irrigation holes
may still be considered to have or follow a helical path. That is, there may
be a greater spacing
between sections of helical ports than between adjacent ports in the sections.
The example shown
in figures 8D may, however, also include a central irrigation port between
electrodes (as in the
examples of figures 8A and 8B).
[0223] The irrigation holes may be created (e.g., laser drilled) in the
tube (or tubular
member) prior to or after positioning or connecting the electrode coil(s) to
the tube. Optionally,
size and quantity of irrigation holes are chosen along with an irrigation flow
rate range to
maintain a back pressure in the irrigation lumen so that irrigated saline jets
from the irrigation
holes, which may evenly, consistently and predictably fill the vessel (e.g.,
intercostal vein) with
saline. For example, an ablation catheter may be adapted to accept a saline
flow rate in a range
of 30 to 50 mL/min during ablation and may have irrigation holes with a
diameter of 0.003" and
a quantity of 34 holes or alternatively holes with a diameter of 0.009" and a
quantity of 17 holes.
[0224] Optionally, there may be more holes associated with the
distal electrode than the
proximal electrode, or vice versa. Optionally, one or more irrigation holes
may be positioned
distal to the distal electrode, for example within 3 mm distal of the distal
electrode. This may
- 37 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
improve cooling of the distal electrode including a temperature sensor in
communication with
the distal electrode, in particular if the temperature sensor is located at
the distal end of the distal
electrode. For example, as shown in Figure 8E, a schematic illustration of a
distal portion of an
ablation catheter, irrigation holes 137 may be arranged in a helical pattern
between at least some
windings of a proximal helical electrode 133 and likewise irrigation holes 137
may be arranged
in a helical pattern between at least some windings of a distal helical
electrode 132, and a
plurality of irrigation holes 461 may be arranged distal to the distal
electrode and a plurality of
irrigation holes 460 between the proximal and distal electrodes. In this
example, the irrigation
holes 137 that are between windings of the coiled proximal 133 and distal 132
electrodes follow
1 0 a helical path around the shaft 340 having the same pitch as the
helical coil electrodes or at least
sufficiently the same pitch such that the holes 137 remain between the
windings of the coiled
electrodes. Furthermore. the holes 137 may be spaced apart from one another
along the helical
pathway in regular intervals, for example every 96 degrees (or a regular
interval in a range of 4
to 110 degrees), which may provide an even circumferential distribution of
irrigation. The pitch
of the coiled electrodes may get tighter at one or both ends of each coil. For
example, each end
of each coil may wrap around the shaft 340 and contact the adjacent turn of
the coil making a
closed loop at each end of the coil, where the connections are soldered
together. This may help
to hold the coil securely to the shaft and contain the ends to avoid a risk of
a loose wire end
getting caught on tissue or the delivery sheath. The same solder joint may
include an RF
conductor and optionally thermocouple wires forming a thermocouple junction as
shown as
distal thermocouple junction 140, which is shown at the distal end of the
distal electrode 132,
and proximal thermocouple junction 139, which is shown at the proximal end of
the proximal
electrode 133. Due to the decreased coil pitch at the ends of the coils. there
is less room to place
irrigation holes. Optionally, irrigation holes may be positioned only in
central regions of the
coiled electrodes and not where the pitch decreases at the ends, for example
within the beginning
or ending 3 turns. To compensate for reduced irrigation at the ends of the
coiled electrodes when
holes are not placed in the last few turns, irrigation boles 461 may be
positioned distal to the
distal electrode 132, and irrigation holes 460 may be positioned between the
proximal 133 and
distal 132 electrodes. For example, a quantity of distal holes 461 in a range
of 1 to 5 (e.g., 3)
may be circumferentially evenly spaced (e.g., radially symmetric) and within a
distance 462
(e.g., in a range of 0.1 to 3 mm, in a range of 0.1 to lmm) of the distal end
of the distal electrode
132. Similarly, for example, a quantity of holes 460 between the electrodes in
a range of 1 to 5
(e.g., 3) may be circumferentially evenly spaced (e.g., radially symmetric)
and within a space
463 (e.g., in a range of 0.5 to 1.0 mm) between the distal electrode 132 and
proximal electrode.
Since blood flows from distal to proximal in the intercostal vein, irrigated
saline flowing out of
- 38 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
distal holes 461 would sufficiently bathe and cool the distal few turns of the
electrode 132; the
proximal few turns of the distal electrode would be bathed and cooled by
saline flowing from
holes between windings as well as from the distal holes 461; likewise the
distal few turns of the
proximal electrode 133 would be cooled and bathed by the holes 460 between the
electrodes; and
the proximal few turns of the proximal electrode would be bathed and cooled by
saline flowing
from holes between windings as well as from the boles 460 and from other holes
137 and 462
associated with the distal electrode. In one exemplary embodiment as shown in
Figure 8E the
catheter has three circumferentially evenly spaced distal irrigation holes
461, three
circumferentially evenly spaced irrigation holes 460 between the proximal and
distal electrodes,
15 helically evenly spaced irrigation holes 137 between windings in the distal
electrode, and 15
helically evenly spaced irrigation holes 137 between windings in the proximal
electrode, totaling
36 irrigation holes, each having a diameter of 0.003".
[0225] Alternatively, in any of the examples herein, irrigation
holes may be positioned
under the coil electrode windings as well as between the windings.
[0226] Alternatively, any of the devices herein may include a section of
tube that the
electrodes are positioned over that may be a porous tube made from a material
that is inherently
porous, for example a mesh or woven tube.
[0227] Alternatively, any of the coiled electrodes herein may have
a flat profile such as a
ribbon of conductive material wrapped helically around a tube. A flat profile
compared to a
2 0 round wire profile may in some situations be easier to deliver or
remove from a tight vessel.
Figure 21A shows an exemplary distal portion of an ablation catheter having an
ablation element
385 having a plurality of coiled electrodes (a first electrode 386 and a
second coiled electrode
387 in this example) made from flat ribbon wrapped helically around a tubular
shaft 388. The
flat ribbon may be a conductive material, optionally a superelastic Nitinol
ribbon shape set into a
helical coil having, for example only, an inner diameter of 0.069" +/- 0.004"
and a pitch of about
0.047". Superclastic Nitinol may have a benefit of kink resistance and
elastically returning to a
preset shape during or after being deformed when delivering the device to a
target vessel.
However, alternative materials such as stainless steel or a conductive alloy
could be used.
Optionally, at least a portion of the ribbon electrode may be made with a
radiopaque material
such as platinum iridium. Optionally, the surface of the ribbon electrode may
be etched and
passivated. The flat ribbon may have a thickness in a range of, for example
only, 0.002" to
0.003" and a width 389 in a range of 0.010" to 0.020". The length 390 of each
coil may be about
8 mm +/- 0.5 mm. The flat ribbon electrodes may be applied to be flush with
the surface of the
tubular shaft. For example, the tubular shaft may be indented where the flat
ribbon connects
with the shaft or the tubular shaft may re-molded or softened during
application of the flat ribbon
- 39 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
to allow it to sink into the shaft. Alternatively, the flat ribbon electrodes
may extend beyond the
surface of the shaft, for example by the thickness of the ribbon, which may be
in a range of
0.002" to 0.003". Optionally, the edges 391 of the ribbon electrode, for
example on the outer
diameter, may be rounded, chamfered or tapered, which may further facilitate
delivery or
removal of the catheter into the target vessel or may reduce high current
density during delivery
of RF energy. Irrigation ports 137 may be positioned between the windings of
the flat ribbon
electrodes as shown in Figure 21A, or in other configurations disclosed
herein. Another
alternative form of a flat helical electrode, as shown in Figure 21B, may
include an assembly
made from a conductive material 396 such as superelastic Nitinol, stainless
steel or an alloy, on a
non-conductive substrate 397 (e.g., a flex circuit), which may facilitate
manufacturing. The non-
conductive substrate 397 may be for example, polyimide, and the conductive
trace 396 may be
connected to the substrate with adhesive. The assembly may have a wire strain
relief 398 in the
substrate, through which conductors may pass from a lumen in the catheter
shaft to a wire solder
pad 399 that is in electrical communication with the conductive material 396.
Optionally,
temperature sensors 139, 140, such as thermocouples, may be positioned on the
wire solder pad
along with conductors supplying RF to the electrodes. The assembly may have a
thickness in a
range of 0.002" to 0.003", with a conductor thickness in a range of 0.0015" to
0.0025". The
width of each trace may be in a range of 0.010" to 0.020".
[0228] Optionally, the ablation catheter may have a deployable element
transitionable from
a contracted delivery state (e.g., having an OD in a range of 1.5 mm to 3 mm)
to an expanded
deployed state (e.g., having an OD in a range of 2.5 mm to 6 mm) that
functions to one or more
of anchor the distal section of the catheter in the target region of the
vessel, to occlude blood
flow, to contain delivered fluid such as saline, to maintain vessel patency,
or to act as an
electrical insulator. For example, as shown in Figure 8B, any catheter herein
may also include a
distal deployable element 134 coupled with optimized irrigation flow that may
create a virtual
electrode that provides an effective ablation without the need for wall
contact. Distal deployable
element 134 may be a balloon (e.g., compliant balloon) as shown in Figure 8B,
or alternatively a
bellows or coated stent or mesh. Distal deployable element 134 is distal to
the ablation element,
which may include proximal and distal electrodes as shown in Figure 8B.
[0229] Optionally, any of the ablation catheters herein may have a
proximal deployable
element. Figure 9 illustrates an exemplary ablation catheter that includes
proximal deployable
element 141 that can be contracted to have an OD in a range of 1.5 to 3 mm in
a delivery state,
and be deployed to have an OD in a range of 4 to 10 mm in a deployed state as
shown in Figure
9. The proximal deployable element 141 may function to one or more of anchor
the distal section
- 40 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
of the catheter in the target region of the vessel, to occlude blood flow, to
contain delivered fluid
such as saline, to act as an electrical insulator, to maintain vessel patency.
to act as a depth
stopper (e.g., having a deployed OD larger than the targeted intercostal vein)
to prevent the distal
section from being advanced too far into the intercostal vein, or to direct
blood flow in the
azygos vein away from the ostium to facilitate ablation near the ostium. A
proximal deployable
element and a distal deployable element coupled with optimized irrigation flow
may create a
virtual electrode that provides an effective ablation without the need for
wall contact. A proximal
deployable element may be a balloon (e.g., compliant balloon) as shown in
Figure 9, or
alternatively a bellows or coated stent or mesh. Any of the catheters herein
may include a
proximal deployable element and a distal deployable element.
[0230] Optionally, any of the ablation catheters herein may
include a middle or central
deployable element. Figure 10 illustrates an exemplary ablation catheter that
includes a middle
deployable element 142 that can be contracted to have an OD in a range of 1.5
to 3 mm in a
delivery state, and be deployed to an expanded state (e.g., having an OD in a
range of 2.5 mm to
6 mm) as shown in Figure 10. The middle deployable element may function to one
or more of
anchor the distal section in the target region of the vessel, to occlude blood
flow, to contain
delivered fluid such as saline, to maintain vessel patency, or to act as an
electrical insulator. A
middle deployable element may be used to isolate the vessel between a distal
deployable element
and the middle deployable element and around the distal ablation element to
create a virtual
2 0 electrode that provides an effective ablation without the need for wall
contact. Likewise, the
section of vessel between the middle deployable element and a proximal
deployable element
may be isolated. The middle deployable element may be a balloon (e.g.,
compliant balloon) as
shown in Figure 10. or alternatively a bellows or coated stent or mesh. In an
embodiment
wherein the ablation energy is electroporation, the middle deployable element
may function as an
electrical insulator to direct electrical current out of the vessel in through
tissue around the vessel
to more effectively ablate the target nerve. In alternative embodiments, an
ablation catheter may
have a middle deployable element and only a distal deployable element (i.e.,
no proximal
deployable element) or only a proximal deployable element (i.e., no distal
deployable element).
[0231] The disclosure above described exemplary methods of
positioning an ablation
catheter within an intercostal vein to ablate a GSN while minimizing or
avoiding damage to non-
target structures. The ablation catheters above, including those shown in
figures 8A, 8B, 9, and
10, included one or more radiopaque markers (e.g., distal marker 130 and
proximal marker 136)
that can be used as part of those methods of positioning. While the ablation
catheters in figures
8A, 8B, 9 and 10 are examples of ablation catheters that can be used when
performing the
methods herein, it is understood that the methods can be performed with a
variety of ablation
- 41 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
catheters. It is thus understood that the methods herein are not limited by
the particular ablation
catheters herein. It is also understood that the ablation catheters herein
need not be used with the
positioning methods herein.
[0232] Alternative embodiments of TSN/GSN ablation catheters may
have one or more the
features that are described herein, such as proximal and distal radiopaque
markers spaced as
described, irrigation lumens( s), temperature sensor(s), guide wire lumens,
flexible shaft section,
and may also include alternative ablation elements. For example, ablation
elements may be RF
electrodes having different configurations or ablation elements that deliver a
different type of
ablation energy such as ultrasound, electroporation, cryoablation, laser,
chemical or other
ablation modality. Ablation catheter features that are described with respect
to one embodiment
or example herein may be incorporated into other suitable embodiments unless
the disclosure
indicates otherwise. Features with the same or similar reference numbers are
understood to be
optionally included and can be the same component.
[0233] For example, Figure 11 illustrates a distal section of an
ablation catheter. The
ablation catheter includes an ablation element that may be an RF electrode
that includes a
plurality of wire struts 143 running the length of the ablation element and
arranged around the
circumference of the shaft. The wire struts are electrically conductive, for
example made from
stainless steel, Nitinol or the like, and transitionable from a contracted
delivery state (e.g., having
an OD in a range of 1.5 to 3 mm) to an expanded deployed state (e.g., having
an OD in a range
2 0 of 2.5 mm to 6 mm) to contact the vessel wall, in particular an
intercostal vein. The wire struts
may be deployed by applying tension to a pull wire that moves a collar holding
or otherwise
secured to one end of the wire struts, shortening the distance between the two
ends, which causes
the wire struts to bend outward. The struts may be heat set in a biased
configuration, such as
those shown in figure 11. Optionally, an RF electrode may have multiple (e.g.,
two) RF
electrodes made of wire struts, wherein the multiple electrodes are positioned
next to one another
similar to the coiled electrodes shown in figures 8 to 10. Optionally, the
wire struts may be made
from a laser cut tube. Optionally the distal end, proximal end or both ends of
the expandable
wire electrode may have a membrane that functions to occlude the vessel when
expanded and
function similar to the deployable structures (e.g., balloons) shown in
Figures 8A to 10.
[0234] Figure 12 illustrates an exemplary ablation catheter with ablation
element(s) carried
by an expandable balloon. Figure 12 illustrates a distal section of an
ablation catheter with an RF
ablation element, wherein the ablation element includes one or more
electrically conductive
element(s) positioned on expandable balloon 144. The conductive elements may
be a film or
conductive ink or flexible circuits. Sensors (e.g., temperature sensors) may
be positioned on the
balloon as well. Optionally the balloon may be inflated by delivering fluid
such as saline or air
- 42 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
into the balloon. Optionally, the conductive element(s) or the balloon may
have perforations
allowing fluid to pass through to cool the electrode or conduct energy. The
pattern of the
conductive element(s) may be cylindrical 148 (Figure 12), helical 149 (Figure
13A), a plurality
of electrodes each having a helical configuration 150 (Figure 13B), electrodes
with a wavy (e.g.,
sine wave) or zig-zag pattern 151 (Figure 14), or other pattern adapted to
circumferentially ablate
around a vessel. The examples shown in figures 12 to 14 include optional
distal and proximal
radiopaque markers that can be used with any of the methods of positioning
described above.
102351 Figure 15 illustrates an additional exemplary distal
section of an ablation catheter
that includes an electrically conductive element within a membrane. The
catheter in figure 15
1 0 includes an RF ablation element that is an electrically conductive wire
145 (e.g., wire coil) on or
around the catheter shaft within a cavity defined by a membrane 185. The
membrane may be an
ionomer, a conductive membrane, or a weeping membrane. The optional distal and
proximal
markers are shown distal and proximal to the balloon, respectively.
[0236] Figure 16 illustrates an example of a distal section of an
ablation catheter, which
can may be used with the methods of positioning herein. Another embodiment of
an RF ablation
element is shown in Figure 16 wherein the ablation elements are a plurality of
shorter RF
electrodes 146 on a tapered shaft 147. This embodiment is different in that
the total length of the
shaft carrying ablation elements may be longer than previously described as 5
mm to 25 mm
(preferably 10 mm to 15 mm). Instead, the catheter includes multiple sections
(e.g., two or three)
2 0 that each have a length in this range, but are selectively chosen to
deliver ablation energy
depending on how they fit in the intercostal vein. The tapered shaft may
function to fit a range of
intercostal veins (e.g., in a range of 2.5 mm to 5 mm). The distal end is
narrower than the
proximal end and the electrodes may be independently and selectively
energized. If the distal
section of the catheter is delivered to a relatively narrow intercostal vein,
for example having an
inner diameter of about 2.5 mm, the distal narrow portion may be advanced into
the vein and
selected for energy delivery, while the proximal larger portion may remain in
the azygos vein
and not used to delivery ablation energy. If the intercostal vein is larger,
for example 5 mm inner
diameter, the distal section may be advanced further into the intercostal vein
until the larger
electrodes are wedged into the vessel contacting the wall. The larger proximal
electrodes may be
selected for energy delivery while the distal electrodes are inactive to avoid
injury to the
sympathetic trunk. Optionally and intermediate section of electrodes may be
sized to fit an
intercostal vein having an inner diameter of about 3.5 mm. The plurality of
electrodes may be
coiled wire, laser cut tube, or solid electrodes. The electrodes may be
radiopaque or have
radiopaque markers associated with them so the user can image where the
electrodes are
positioned in the intercostal vein and choose which section of electrodes to
activate.
- 43 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0237] Another embodiment of a transvascular ablation catheter 241
for ablating a TSN or
GSN from within an intercostal nerve is shown in Figure 17A. The catheter 241
may extend
along a longitudinal axis. An expandable member, for example in the form of a
balloon 242
having an unexpanded state and an expanded state, may be coupled to a distal
section 243 of the
catheter. The expandable member (e.g., balloon) may have a circumferential
treatment zone 248
(e.g., having a length in a range of 5 to 25 mm, in a range of 10 to 15 mm)
extending along the
longitudinal axis in the expanded state and surrounding the vessel 55. The
catheter includes an
electrode assembly 252, which comprises a plurality of electrode pads 244, may
be mounted or
otherwise secured to the balloon 242. Each electrode pad assembly may include
a substrate
supporting first and second electrode pads with each electrode pad having a
pair of elongate
bipolar electrodes and connected with an electrical trace 249. The electrode
pads of each
electrode pad assembly may be longitudinally and circumferentially offset from
one another.
The method may also include expanding the balloon in the intercostal vein so
as to electrically
couple the electrodes with a wall of the intercostal vein and driving bipolar
energy between the
electrodes of each bipolar pair so as to therapeutically alter the TSN or GSN
within 5 mm of the
intercostal vein such that the blood volume of the patient is redistributed
for treatment of
diseases such as pulmonary hypertension, or heart failure (e.g., HFpEF).
[0238] Each electrode pad may include a temperature sensor
disposed between the
electrodes of the pair. The expanding of the balloon may couple the
temperature sensors with
2 0 the wall of the intercostal vein. In some embodiments, the method may
further include directing
the energy to the bipolar pairs in response to a temperature signal from the
temperature sensor so
as to heat the wall approximately evenly.
[0239] To create an ablation having a depth of 5 mm to target a
GSN from an intercostal
vein the electrode pads may be cooled to allow greater power to be delivered
without desiccating
tissue of the vein wall, which impedes ablation depth. The electrodes may be
cooled for
example, by circulating coolant in the balloon 242. In one embodiment coolant
may be injected
into the balloon 242 from a coolant injection port 246 at one end of the
balloon chamber and the
coolant may exit the chamber through an exit port 247 at the opposing end of
the chamber and
allowed to return through the catheter through an exit lumen.
[0240] In another embodiment coolant may be deposited into the blood stream
instead of
returning through a lumen in the catheter. This embodiment may allow a
thinner, more flexible
catheter shaft or a larger coolant delivery lumen to increase flow rate of the
coolant. A coolant
exit port may be smaller than the coolant injection port to allow pressure to
increase in the
balloon to inflate it. The coolant exit port may be in communication with a
lumen that does not
pass through the full catheter shaft to the proximal end but instead passes to
the distal end of the
- 44 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
catheter to deposit the coolant (e.g., normal saline) into the intercostal
vein. Optionally the
coolant exit lumen may be the same lumen as a guidewire delivery lumen.
[0241] Electrode pads may be positioned around the balloon to make
a circumferential
ablation pattern that is as long as the target ablation zone 58 (e.g., up to
20 min, about 15 mm,
between 12 and 18 mm). For example, as shown in Figure 17B, a balloon with
electrode pads
mounted to an elongate shaft 253 may have an undeployed state having a
diameter of about 1
mm to 2.5 mm and a circumference of about 3.14 mm to 7.85 mm and be expandable
to a
deployed state having a diameter in a range of about 3 mm to 5 mm and a
circumference in a
range of about 9.4 mm to 15.7 mm. Electrode pads 244 may be separated or
spaced by a distance
250 of less than 5 mm (e.g., less than 2.5 mm) and width or arc length 251 in
a range of 3 mm to
3.5 mm. Electrode pads 244 may have a length of about 3 to 5 mm each. As shown
in Figure
17A, an electrode pad assembly 252 may comprise multiple electrode pads 244
arranged on four
separate rows connected together by electrical traces 249, the rows evenly
spaced around the
circumference of the balloon 242 (e.g., four rows at each 90 degree quadrant).
Longitudinally.
the pads 244 on one row may be offset from pads of adjacent rows. When the
balloon is in its
unexpanded state the space between the electrode pads is decreased (e.g., to
about 0 to 1 mm)
and the adjacent rows interlock with one another. In its expanded state the
space 250 between the
pads expands due to the expandable balloon 242 to about 2 narn to 5 rum. The
balloon 242 may
be a compliant material such as latex or a non-compliant material that
flexibly folds to contract.
[0242] Alternatively, electrode pads may be positioned only on one side
(e.g., 50%, 40%,
30%, 25% of the balloon's circumference) to generate a directional ablation
pattern that is all
toward the same side and of a length of the target ablation zone 58. For a
directional ablation
catheter, a radiopaque marker may be positioned on the distal section of the
catheter to indicate
radial direction. For example, a radiopaque marker may be asymmetric and
positioned on the
same side or opposing side as the directional electrode pads to indicate and
in use a physician
may torque the catheter to aim the radiopaque marker and thus the electrode
pads away from the
vertebra, which is always toward the GSN. Figure 17A shows several small
electrode pads.
Alternatively, the device may have larger and fewer electrode pads, for
example two or three
directional electrode pads (e.g., 3 to 5 mm long) on the same side of the
balloon that span the
target ablation zone 58. A gap (e.g., 1 to 3 mm) between electrode pads may
facilitate bending
of the device to traverse from the azygos vein to the intercostal vein. The
ablation catheter in
figures 17A and 17B can include proximal and/or distal radiopaque markers, and
may be used
with methods of positioning described herein.
[0243] Just proximal to the balloon the catheter shaft may
comprise a flexible neck 245
that allows the ablation balloon to sit in the intercostal vein's natural
orientation. Given the
- 45 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
small bend radius at this location a stiff shaft could apply force to the
ablation balloon causing it
to distort the intercostal vein and reduce predictability of ablation zone. A
flexible neck may be
made of a softer durometer polymer (e.g., Pebaxe) and may have a wire coil
embedded in the
material, which may allow flexible bending while providing pushability. This
type of flexible
neck may be incorporated into other ablation catheters herein.
[0244] The electrode(s) that are most proximal may be placed just
in the intercostal vein
near the ostium. Blood flow through the azygos vein may metabolically cool
tissue near it
impeding ablation creation. A larger amount of ablation power (e.g., RF) or
longer duration may
be delivered to this proximal electrode(s) than the rest of the electrode(s)
to compensate for the
blood flow cooling.
[0245] The catheter 241 may have a distal radiopaque marker 255
positioned distal to the
ablation elements, for example distal to the balloon 242, and/or a proximal
radiopaque marker
254 positioned proximal to the ablation elements 244, for example proximal to
the balloon 242.
The distal and proximal radiopaque markers 255, 254 may be separated along the
longitudinal
axis of the shaft by a distance in a range of 5 mm to 25 mm (e.g., 10 mm to 15
mm). Any other
features or description of radiopaque markers herein may apply to markers 255
and/or 254.
[0246] Figure 18A illustrates an exemplary ultrasound ablation
catheter. Catheter 220
includes an elongate shaft 225 with a proximal region and a distal section and
an ablation
assembly 232 mounted to or at the distal section. The ultrasound ablation
catheter 220 has an
inflatable balloon 221 which may have a geometry suitable for expansion in an
intercostal vein
(e.g., outer diameter 222 in a range of 2.5 to 5 mm in its inflated state) and
a length 223 in a
range of 8 to 30 mm. Within the balloon 221, multiple ultrasound transducers
224 are positioned
on a shaft 233 centered in the balloon 221. The transducers 224 may be placed
serially spanning
a length 226 that is in a range of 5 to 25 mm to generate an ablation of a
similar length capable of
creating an ablation the length of the target ablation zone 58. Due to the
small diameter of the
intercostal vein the reduced balloon size may risk contacting the transducer
or getting over
heated by the transducer, which may rupture the balloon or reduce efficacy of
the ablation. To
remedy this risk struts or protrusions 227 may be positioned between the
transducer and balloon.
The struts 227 may be for example polymer strands elastically pre-shaped to
radially expand
away from the transducers 224. To make a longer ablation to span the targeted
ablation zone,
multiple transducers may be incorporated (e.g., three 4 mm long transducers)
and spaced apart
with flexible gaps 228 between them to facilitate traversing the small bend
radius from the
azygos vein to intercostal vein. For example, shaft 225 may be a braid
reinforced polyimide tube
with an optional guidewire lumen 229 for delivery over a guidewire 79 and
carry electrical
conductors that energize the transducers 224. The ultrasound transducers 224
may be cylindrical
- 46 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
for producing circumferential ablation around the target vein. Alternatively,
the ultrasound
transducers may be flat or hemicylindrical to produce an ablation that is a
partial segment of the
circumference of the vein and a radially identifiable radiopaque marker 230
may be positioned
on the distal section allowing a user to orient the direction of ablation
toward the patient's
anterior where the GSN passes over the vein 55. Optionally, the ultrasound
transducer may be
configured to image as well as ablate and the imaging function may be used to
assess nearby
structures such as the lung, vertebra, ribs. Imaging ultrasound may be used to
confirm the
transducer is aiming toward the lung, which is the direction of the target
GSN. Optionally, the
shaft may have a flexible neck 231 within 10 mm proximal of the balloon 221 to
allow the distal
1 0 section to sit well in the intercostal vein.
[0247] In an alternative embodiment of an ultrasound ablation
catheter, the catheter can be
composed of an active ultrasound transducer and an inflatable reflector
balloon, which may be
on the same catheter or alternatively be on separate catheters. The reflector
balloon may have an
inflated diameter in a range of 2.5 to 4 mm and on its proximal surface have a
shape such as a
concave curvature that focuses reflected waves on to the target ablation zone.
The reflector
balloon is located distal to the transducer and is inserted in the narrower
intercostal vein, while
the ultrasound transducer remains in the larger azygos vein. The ultrasound
transducer may be
exposed to blood flow in the azygos vein or alternatively may be contained in
a chamber in an
inflatable balloon filled with coolant (e.g., circulating coolant such as
sterile water or saline). The
ultrasound energy is directed toward the distal reflector balloon and
reflected and focused into
tissue surrounding the splanchnic nerve. The advantage of this approach is
that an active
ultrasound transducer can be made larger and is not required to go through the
sharp turn from
azygos to intercostal vein. A second advantage is that several intercostal
veins can be used to
target ablation with the same catheter.
[0248] The catheter 220 may have a distal radiopaque marker 230 positioned
distal to the
ablation elements, for example distal to the balloon 221 and a proximal
radiopaque marker
positioned proximal to the ablation elements, for example proximal to the
balloon. The distal and
proximal radiopaque markers may be separated along the longitudinal axis of
the shaft by a
distance in a range of 5 mm to 25 mm (e.g., 10 mm to 15 mm).
[0249] Figures 8A to 10 illustrate exemplary ablation catheters. The
ablation catheters in
these examples includes an ablation element that includes first and second
flexible coiled
ablation electrodes that are axially spaced. It may be beneficial to have
first and second
electrodes rather than a single longer electrode to avoid a tendency of the
single longer electrode
to heat tissue mostly towards one end of the electrode. Having more than one
electrode thus can
help to create a long and consistent ablation in tissue. Figures 8A to 10 are
thus examples of
- 47 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
ablation catheters that can more consistently create a continuous ablation of
the desired length,
such as 10 mm to 25 nana. such as 15 mm to 25 nana. such as 15 mm to 20 mm.
[0250] An additional exemplary benefit of having first and second
electrodes versus a
single longer electrode is that only a single relatively shorter electrode may
be energized rather
than a single longer electrodes. This can be advantageous when the patient's
anatomy requires or
may benefit from making shorter ablations, such as if the azygos is right
centered. In these cases,
a longer single electrode may make it difficult or dangerous to safely ablate
tissue while avoiding
non-target structures. This is described in more detail elsewhere herein.
[0251] Additionally, Figures 8A to 10 illustrate ablation
catheters that have first and
second ablation elements axially separated by a gap or spacing. This gap is
small enough (i.e.,
not too large) such that a continuous lesion is formed when energizing the
first and second
ablation elements, yet is large enough to avoid short circuiting.
[0252] Design features of distal sections of ablation catheters
herein (e.g., figures 8A to 10)
thus provide exemplary benefits that allow the distal section to be advanced
into position in an
intercostal vein and reliably create a continuous ablation of at least 10 mm
to 25 mm in length,
while allowing shorter ablation sections if needed based on the patient's
anatomy.
[0253] In some methods of use, the ablation energy is RF, and an
energy delivery
controller is adapted to deliver RF power in a range of 15W to 50W. In some
embodiments, the
controller is adapted to deliver RF power in a range of 15W to 40W, in a range
of 15W to 35W.
or in a range of 20W to 35W, such as about 25W, about 30W or about 35W.
[0254] In some methods of use, energy is delivered over a period
of time between 25
seconds and 120 seconds. For example, energy may be delivered for 90 seconds,
for 100
seconds, for 110 second, or for 120 seconds, wherein for a portion (e.g.,
half) of the period of
time energy, may be delivered to a first electrode and for the remainder
(e.g., half) of the period
energy may be delivered to a second electrode.
[0255] In some methods of use, an irrigation flow rate is from 10
mL/min to 50 mL/min,
(e.g., 10 mL/min, 15 mL/min, 20 mL/min) during ablation. Optionally, flow rate
may be
changed automatically by the control algorithm in response to changes in
measured temperature,
impedance or phase. With devices and methods disclosed herein, the TSN may be
ablated in a
relatively safe manner, with minimal or reduced adverse effects (such as
damage to the lungs or
other nerves). Some method of use embodiments herein may temporarily occlude
blood flow
and reduce an effect of vein collapse, thus advantageously avoiding challenges
of a changing
thermal and electrical environment during the heating process. Some method of
use
embodiments herein may ablate a nerve up to 5 mm from the target vessel. Some
of the devices
- 48 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
herein are dimensioned and configured for delivery and positioning in
vasculature specified for
ablating a target nerve (e.g., TSN. GSN).
[0256] Some of the devices herein may have one or more features
that provides for a safe
delivery to the target vessel.
[0257] Some of the devices and methods of use herein may safely deliver
energy with
temperature monitored energy delivery.
[0258] Some of the methods of use herein may generate a lesion
capable of targeting a
nerve up to 5 mm away from the target vessel and within a target region having
a continuous
lesion length from 5 mm to 25 mm, such as 10 mm to 25 mm, such as 15 mm to 20
ulna, (e.g., 15
mm. 16 mm, 17 mm, 18 mm, 19 mm, 20 mm), with a single positioning and delivery
of energy.
[0259] Some of the devices and methods herein are adapted to avoid
risks of boiling, hot
spots, or erratic energy delivery that could decrease ablation efficacy.
Furthermore, some
embodiments may include nerve stimulation to identify a target nerve or non-
target nerve to
confirm positioning prior to ablation, or to confirm technical success during
or following
ablation.
[0260] It may be preferred, but not required, that the methods of
ablation create a
continuous ablation zone (i.e., not having separate, discrete regions of
ablated tissue that are not
connected to each other). This ensures that the region of tissue where the
target GSN nerve or
GSN nerve root is likely to be located is most likely to be effectively
ablated by the ablation
2 0 energy. The continuous ablation zone may be circumferential, Or less
than circumferential.
[0261] Optionally, an ablation confirmation test can then be
performed, for example, by
delivering a nerve stimulation signal. Monitoring can be performed for a
physiological response
(e.g., splanchnic vasoconstriction, increased heart rate, increased blood
pressure) to the ablation
confirmation test. If the physiological response demonstrates that the first
lesion did not provide
a clinically significant amount of GSN blocking (e.g., by observing a lack of
physiological
response) then ablation energy can be delivered from the ablation catheter to
create a second
lesion in tissue up to 5 mm from the second intercostal vein. The distal
section of the ablation
catheter can he moved to a third intercostal vein that is superior to (e.g.,
superior and adjacent to)
the second intercostal vein. The same or different ablation confirmation test
can be performed,
followed by another monitoring test. If the physiological response
demonstrates that the first
lesion and second lesion did not provide a clinically significant amount of
GSN blocking (e.g.,
by observing a lack of physiological response) then ablation energy can be
delivered from the
ablation catheter to create a third lesion in tissue up to 5 mm from the third
intercostal vein. Any
of the the ablation confirmation tests may comprise delivering a nerve
stimulation signal from a
stimulation electrode positioned on the distal section of the ablation
catheter configured to
- 49 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
generate an action potential in the thoracic splanchnic nerve. Alternatively
or in addition to, the
ablation confirmation test may comprise a leg raise test. Alternatively or in
addition to, the
ablation confirmation test may comprise adding fluid volume to the venous
system. Alternatively
or in addition to, the ablation confirmation test may comprise a hand-grip
test. Alternatively or in
addition to, the ablation confirmation test may comprise measuring venous
compliance or
capacitance.
[0262] In exemplary methods in which an ablation confirmation test
includes a leg raise
test, the method may comprise any of the following steps. Prior to ablation in
the lowest
intercostal vein, a baseline measurement may be obtained by raising the legs
and measuring the
1 0 change in central venous pressure and waiting for equilibration, that
is a measure of the total
venous compliance including the central veins and splanchnic bed. The legs can
then be lowered,
to allow equilibration so blood redistributes back to the legs. An ablation in
the lowest intercostal
vein (e.g. T11) can then be performed as set forth herein. The legs can then
be raised, followed
by waiting for equilibration and re-measure central venous pressure. A
measurement can then be
made to determine if there was an appropriate reduction in total venous
compliance. If yes, then
the GSN has successfully been ablated. If no, then an ablation in the next
higher intercostal vein
(e.g., T10) can be performed, as set forth herein. The measurement can be
repeated. A
determination can then be made to see if there was an appropriate reduction in
total venous
compliance. If yes, then the GSN has successfully been ablated. If no, then an
ablation in the
next higher intercostal vein (e.g., T9) can be performed.
[0263] In exemplary methods in which an ablation confirmation test
comprises a hand-grip
or other activity that increases sympathetic nervous system (SNS) outflow to
the splanchnic bed
may comprise the following steps. An ablation can be performed in a lowest
intercostal vein
(e.g., T11). Venous compliance can then be measured. A hand-grip can then be
performed for a
predetermined amount of time (e.g., 60 seconds). Venous compliance can then be
remeasured. If
there is no change in venous compliance, the initial ablation was sufficient
to achieve a clinically
significant outcome. If there still is a decrease in compliance, some of the
SNS activity caused by
the hand-grip is getting through. The ablation in the lowest intercostal vein
was thus insufficient
to achieve a clinically significant effect. An ablation in the next higher
intercostal vein (e.g.,
T10) can then be performed. A hand grip test for a predetermined amount of
time (e.g., 60
seconds) can be performed. Venous compliance can then be remeasured. If there
is no change in
compliance, the second ablation was sufficient. If there is a decrease in
compliance, some of the
SNS activity caused by the hand-grip is getting through, and the ablation in
the next higher
intercostal vein was thus insufficient to achieve a clinically significant
effect. Ablation is the
- 50 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
next higher intercostal vein (T9) can then be performed. The procedure is done
at this point as
ablation at a level higher than the 3rd lowest intercostal vein is not
anticipated.
[0264] Energy delivery algorithms
[0265] One aspect of the disclosure herein is related to energy
delivery algorithms that are
adapted to be particularly suited for ablating tissue circumferentially around
a narrow blood
vessel such as an intercostal vein or other similar vessel to a depth of at
least 5 mm and up to 10
mm and from an ablation catheter. The ablation catheter may be any of the
catheter embodiments
shown in Figures 1, 2, 8A, 8B, 8C, 8D, 8E, 9, 10, 21A and 21B, wherein the
ablation catheter
comprises first and second electrodes (e.g., two coiled electrodes each having
a length in a range
of 2.5 to 10 mm, preferably 5 to 8 mm, and an outer diameter in a range of
about 1.5 to 3 mm,
and a distance between the electrodes in a range of 0 to 5 mm).
[0266] A first embodiment of an energy delivery algorithm is
referred to as "Multiplexed
Monopolar RF", wherein pulses of RF arc delivered to the plurality (e.g., two)
electrodes in
monopolar configuration with asynchronous waveforms. Each electrode receives a
pulsed
waveform of RF energy alternating on and off at a steady frequency. The
waveforms may be for
example square wave, sinusoidal, or other form of alternating waveform. The on
period delivers
an ablative level of RF power while the off period delivers a non-ablative
level of RF power
(e.g., in a range of OW to 1 W, about 0.1 W). The waveforms for each electrode
are
asynchronous, that is to say the waveforms are aligned in time so that an on
period for one
2 0 electrode is aligned with off periods of the remaining electrode(s) and
vice versa. The algorithm
has an ablation mode initiated by user activation for example by depressing a
button or foot
pedal. The Ablation Mode Algorithm, as shown in Figure 19, may include
parameters that are
optionally user defined or may be set by default until a user changes them or
may be
automatically defined. Note that Figure 19 is not to scale and the total time,
t TOTAL is shortened
for a simplified illustration of the parameters and concepts. For example, if
total time is 180 s
and both the first and second electrode pulse widths arc each 2 s, a true plot
would show 45
cycles, however fewer cycles are shown for simplicity. The parameters may
include Initial
Power, Põ First Electrode Pulse Width, PW1, Second Electrode Pulse Width, PW2,
Total
Therapy Time, t TOTAL, Minimum Therapy Time, Lockout Period I L0, Secondary
Power P?, and
optionally further lower power levels. Initial Power, Pi, refers to the
amplitude of radiofrequency
power in Watts that is initially delivered to each of the ablation electrodes
(e.g., the proximal 133
and distal electrodes 132 shown in Figures 8A, 8B, 8C, 8D, 8E, 9 and 10) at
the beginning of the
energy delivery protocol. Initial Power may be selectable in a range of 15W to
50W, preferably
in a range of 20W to 50W, and may a have default setting of 35W (e.g., when
the flow rate is in
a range of 10 to 50 ml/min). First Electrode Pulse Width, PW1, is the duration
of each pulse of
- 51 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
RF energy (i.e., ablative portion of waveform) delivered to the distal
electrode 132 and may be
selectable in a range of 0.5-4s (e.g., 1-3s, preferably 2s) and have a default
setting of preferably
2s. Second Electrode Pulse Width, PW2, is the duration of each pulse of RF
energy (i.e.,
ablative portion of waveform) delivered to the proximal electrode 133 and may
be selectable in a
range of 0.5-4s (e.g., 1-3s) and have a default setting of preferably 2s. Some
embodiments may
have more than two electrodes and accordingly may have parameters of pulse
width associated
with each of them. The off period of an electrodes waveform may equal the
duration of the on
period(s) of the remaining electrode(s). In an embodiment having four
electrodes, alternating
electrodes (e.g., the first and third) may be synchronized together and
asynchronous with the
1 0 remaining electrodes (e.g., the second and fourth). The Total Therapy
Time, t TOTAL, is the
duration of time from the beginning of delivery of ablative energy to the end
and may be
selectable in a range of 60s to 400s (e.g. 120 to 200 s), preferably 180s.
Minimum Therapy Time,
is an optional portion of Total Therapy Time (e.g., less than or equal to)
beginning at the start of
delivery of ablative energy; if a temperature or impedance limit is reached
before Minimum
Therapy Time is complete then power may be decreased to the Secondary Power
level or
subsequent lower power level; if a temperature or impedance limit is reached
after Minimum
Therapy Time is complete then power may be decreased to zero (e.g., ablative
energy delivery
may be terminated). Lockout Period, t in, is a period of time following an
event that triggers a
reaction (e.g., a Temperature or Impedance Limit is passed, and the algorithm
reacts by
decreasing power) to allow tissue temperature to respond to the reaction
(e.g., decrease in
temperature). During a Lockout Period the algorithm may ignore the temperature
or impedance
measurements to either the electrode associated with the trigger, or all
electrodes unless they are
indicative of a critical error such as a critical upper temperature limit, T
Cu, (e.g., 105 C or
higher), critical lower temperature limit, T CL. (e.g., 20 C or lower),
critical upper impedance
limit, Z CU (e.g., 800 or more Ohms, 900 or more Ohms, 1000 or more Ohms, a
user selectable
value between 800 and 2000 Ohms), or critical lower impedance limit (e.g., 50
Ohms or less)
which may be indicative of damaged equipment. The Lockout Period may be
selectable in a
range of 2 s to 7 s, or alternatively the length of one pulse width up to the
length of 4 pulse
widths, and may have a default setting of 5 s. Secondary Power, P2, refers to
the amplitude of
radiofrequency power in Watts that is less than the Initial Power, for example
5 to 10 W less than
the Initial Power. The power level is changed to Secondary Power if a
Temperature, TL, or
Impedance Limit, ZL (e.g., in a range of 200 to 500 Ohms), is reached or
passed, for the
electrode (e.g., distal 132 or proximal 133 electrode) associated with the
temperature sensor
(e.g., distal 140 or proximal 139 temperature sensor) that sensed the
Temperature Limit or with
the electrode through which the Impedance Limit was measured. Alternatively,
if one of the
- 52 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
temperature sensors measures temperature above the Temperature Limit, TL,
power may be
decreased to all of the electrodes. Optionally, algorithm parameters may
include further power
levels that are less than the Secondary Power, such as a Tertiary Power Level,
Quaternary Power
Level, and so on. Alternatively, a user defined parameter may be a Power
Decrement, Pd, instead
of Secondary Power. Power Decrement, Pd, is an amount of decrease in power
amplitude
triggered by an over temperature or over impedance limit and may be selectable
in a range of 1
W to 30 W, with a default of 5 W. Optionally, the Power Decrement may be
variable or be
calculated as a percentage of the previous power level (e.g., a percentage in
a range of 1% to
30%). In the event that Power is decreased, either to absolute levels such as
Secondary Power or
by Power Decrements, and a Minimum Power, Põõõ, (e.g., in a range of 1 to 10
W, e.g., 5 W) is
reached and temperature is still above the Temperature Limit or impedance is
still above the
Impedance Limit, then the algorithm may react by a) terminating ablative power
to the electrode
associated with the trigger and continue to deliver ablative power to the
remaining electrode(s)
either using the current alternating waveform or in continuous RF, b)
terminating ablative power
to all electrodes, c) increase flow rate of the irrigation fluid, or d) adjust
the Temperature Limit.
If a treatment is terminated due to inability to maintain temperature below
the Temperature Limit
or impedance below the Impedance Limit or due to any other error, the user may
be instructed by
the algorithm to reposition the device, remove it for inspection, or inspect
the equipment setup.
[0267] Saline may be pumped from an irrigation source through the
catheter and out of
irrigation ports 137 upon activation by a user. This may be done before the
device is put in the
patient to prime the irrigation lumen or test functionality or while the
device is being advanced
into position or during removal of the device and may facilitate delivery or
removal, during
which flow rate or pump speed may be selected by the user within a range of 0
to 50 mL/nain.
Optionally ablation will not start unless flow is on within a range of 15 to
30 ml/min.
[0268] Saline tracking is a feature that has an algorithm that calculates a
volume of saline
that has been delivered to the patient, for example, by multiplying flow rate
and elapsed time or
calculating the area under a plot of flowrate vs time, that saline has been
delivered to the
patient's vasculature using said flow rate and displaying the volume on a user
interface (e.g., on
the computerized console). Furtheilitore, the algorithm may determine if the
portion of the
catheter that delivers irrigation fluid is out of the body or in the body,
either with a manual input
or with an automatic detection algorithm using one or more input signals such
as temperature
sensed by temperature sensors on the catheter (e.g., sensor 139 or 140 in
Figure 8A), or
monopolar impedance, or bipolar impedance. When the algorithm determines the
catheter is in
the patient's body (or in a delivery sheath that is inserted into the patient)
any saline pumped by a
pump connected to the computerized console will be accounted for in the
calculation of saline
- 53 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
volume delivered to the patient. When the algorithm determines the catheter is
not in the
patient's body, any saline pumped by the pump connected to the computerized
console will not
be accounted for in the calculation of saline volume delivered to the patient.
This feature helps a
user determine how much saline has been introduced to the patient's fluid
system, which may be
a concern for some patients. Optionally, a warning may be triggered if a
predetermined saline
volume has been reached or is approached. Saline irrigation flow rate may be
turned on when
the device is out of the body, for example to prime the irrigation lumen or to
test the catheter and
irrigation system function. To determine how much volume is delivered to the
body the saline
tracking algorithm may distinguish if the catheter is in or out of the body
with manual input.
This may be done by having the user press an actuator when the catheter is
entered into the body
that signals the algorithm to begin calculating volume when the pump is
activated. If the catheter
is removed from the body the user may press an actuator to signal the
algorithm that the catheter
is not in the body wherein calculation of accumulating saline volume is
paused. Any volume
delivered outside of the body is not included in the calculation of saline
volume delivered to this
patient. If the catheter or other catheter is put back in the patient for
subsequent treatments any
saline delivered to the patient is added to the volume calculation by the user
restarting tracking
by pressing an actuator. Alternatively, the saline tracking algorithm may
automatically identify
if the irrigated ablation catheter is in the body or not by monitoring
monopolar impedance
measured between one or more ablation electrodes and the grounding pad, or
alternatively
monitoring bipolar impedance measured between two ablation electrodes.
Monopolar
impedance has an advantage over bipolar impedance for detecting in vivo vs ex
vivo because
monopolar impedance completes an electrical circuit from at least one of the
electrodes on the
catheter through the body to a dispersive grounding pad placed on the patient'
s skin, whereas
bipolar impedance completes a circuit from a first electrode on the catheter
through a conductive
medium to a second electrode on the catheter. The conductive medium may be
within the patient
such as blood or tissue but it also could include saline or a conductive
medium outside the body,
for example if the electrodes are immersed in a saline bath or if saline is
irrigated through the
catheter and wets the electrodes closing the circuit. However, bipolar
impedance could still be
used to detect a change in environment and be useful in a saline tracking
algorithm. A very low
(non-ablative, e.g., 0.1 W) power may be delivered when an ablation treatment
is not running so
impedance can be measured. For example in monopolar mode, if the catheter is
in the body and
connected to the console and a grounding pad in electrical communication with
the console is
connected to the patient's skin, monopolar impedance may be within a certain
range that is
discernable from a catheter out of the body. For example, as determined
experientially, a
monopolar impedance measurement within a range of 700-900 Ohms in monopolar
mode may
- 54 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
indicate the distal region of the catheter having the electrodes and
irrigation holes is in a sheath
in the patient's vasculature; an impedance measurement that is a significant
drop from the
sheathed impedance, for example in a range of 80 to 130 Ohms, may indicate the
distal region is
in the vasculature and out of the sheath; above a high impedance threshold
(e.g., a high
impedance threshold or 900 Ohms, higher than 2000 Ohms, higher than 3000 Ohms)
in
monopolar mode may indicate the electrodes are out of the body, or that a
grounding pad is
incorrectly connected. Alternatively, bipolar impedance (e.g., measured by
passing current
through conductive medium between two ablation electrodes on the distal region
of the catheter)
measured in a range of about 300 to 600 Ohms (e.g., about 500 Ohms) may
indicate the distal
region is in a sheath and in the body; or a bipolar impedance in a range of 60
to 80 Ohms may
indicate the distal region is in the vasculature out of the sheath; a high
impedance threshold (e.g.,
higher than 600 Ohms, higher than 900 Ohms, higher than 2000 Ohms, higher than
3000 Ohms)
may indicate the electrodes are out of the body, or that the catheter' s
electrical circuit has been
broken. The algorithm may determine that the distal region of the ablation
catheter, where saline
is released, is in the body if measured impedance is below the high impedance
threshold,
wherein accumulating saline volume is accounted for; and that the distal
region is out of the body
if measured impedance is above the high impedance threshold, wherein saline
pumped during
this scenario is not accounted for in the accumulated volume. Optionally, when
a change of in
vivo / ex vivo state has been detected the algorithm may display a message
asking the user to
acknowledge the change. Optionally, a user may input a known volume of saline
that has been
injected by other means such as with a contrast solution injected from a
syringe into the delivery
sheath and the known volume may be added to the accumulated volume
calculation.
[0269] Figure 20 shows a machine state diagram of an automatic
saline tracking algorithm.
Beginning at a Main Therapy Screen 321 with the ablation catheter connected to
the console and
out of the body and a grounding pad connected to the patient and console a
user may press a
button 325 to enable saline tracking 326. This may begin automatic calculation
of saline volume
pumped wherein the calculation determines how much of the volume of saline is
deposited in the
body and optionally how much is pumped while the catheter is out of the body.
If the ablation
catheter is inserted into the body the bioelectric impedance should drop 322
to within the range
indicating tissue contact and a message is displayed suggesting that the user
start saline tracking
323. The user may press a button to acknowledge 324 the message which tells
the algorithm to
include the accumulating pumped saline to the total accumulated volume of
saline deposited in
the body. If the catheter is removed from the body an impedance rise to a
level out of the range
associated with body contact is measured 327 and a message is automatically
displayed to
suggest the user Pause saline tracking 328. The user may press a button to
acknowledge 329 the
- 55 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
message which tells the algorithm to exclude the accumulating pumped saline to
the total
accumulated volume of saline deposited in the body. Furthermore, a user may
press a button 330
at any time to pause saline tracking, or to pause including the pumped saline
volume in the
calculation of saline deposited into the body 331. Alternatively, instead of
automatically defining
that the catheter is in the body based on impedance, an algorithm may alert
the user that it thinks
the catheter is out of the body and the user may manually select that flow
shall be excluded from
the saline tracking total. A reset actuator may be pressed by a user to reset
the total volume to
zero.
[0270] An alternative saline tracking algorithm may ignore a quick
increase in impedance
within a predetermined amount that may be caused by the injection of contrast
solution or saline
in the vicinity of the ablation electrode(s) while the catheter is in the body
to avoid a false
determination of removal. To distinguish the difference between injecting
contrast solution or
saline and removing or inserting the distal region of the catheter from the
patient, when a large
change in impedance is detected, the algorithm may have two impedance
thresholds that are used
depending on whether the system is in in vivo or ex vivo mode. A first
impedance threshold
(e.g., in a range of 400 Ohms to 600 Ohms, about 500 Ohms) may be used if the
catheter is not
in the patient's body (i.e., ex vivo) to automatically indicate that the
catheter has been inserted
into the body when impedance drops below this first threshold. A second
impedance threshold
(e.g., in a range of 800 Ohms to 3000 Ohms, about 900 Ohms) may be used if the
catheter is in
the patient's body (i.e., in vivo) to automatically indicate that the catheter
has been removed from
the body when impedance rises above this second impedance threshold. For
example, the
ablation catheter may be out of the patient and impedance may be above the
second threshold,
say 900 Ohms; if the saline pump is running the algorithm determines that the
catheter is not in
the body and no saline volume is included in an accumulation calculation; the
catheter may be
inserted into the patient and impedance may drop below the first threshold,
say 500 Ohms,
wherein the algorithm determines the catheter is in the body and any pump
movement is
accounted for in the accumulation calculation; injection of saline or contrast
may raise
impedance above the first threshold but since the catheter is in the body the
algorithm determines
the rise does not indicate removal so any pump movement continues to be
accounted for in the
accumulation calculation; if the catheter is removed from the body impedance
will rise above the
second threshold, say 900 Ohms, and the tracking algorithm will determine the
catheter has been
removed and any pump movement will not be accounted for in the accumulation
calculation.
Optionally, the first and second thresholds may be adjusted or selected in
user settings. The
catheter may be indicated for use with a consistent concentration of saline,
for example 0.9%
Normal Saline, for the algorithm to function properly.
- 56 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0271] In addition to calculating accumulated saline injected, the
algorithm may optionally
change other feature behaviors depending on whether the system is in the in
vivo or ex vivo
mode, for example as described in Table 1.
Table 1
Feature In vivo behavior Ex vivo behavior
Saline volume tracking active paused
Require confirmation to stop enabled disabled
pump
Remind user to turn on pump Triggered upon entry to in n/a
if not running vivo mode
Remind user Pump Prime not Triggered upon entry to in n/a
completed since last power vivo mode
cycle
Require confirmation to run In vivo Warning Message
Prime
Notify user that pump is still n/a Triggered upon
entry to ex
running vivo mode
RF energy delivery Allowed Restricted
Prime Mode (bypass pump Restricted Allowed
bubble detector)
[0272] Another use of bipolar impedance monitoring by an algorithm
may be used to
display a message to the user to check if the dispersive grounding pad is not
correctly connected
if bipolar impedance is low (e.g., less than 500 Ohms) and monopolar impedance
is high (e.g.,
above 900 Ohms).
[0273] Another use of bipolar impedance monitoring by an algorithm
may be used to
display a message to the user to check if there is an open circuit on one or
both electrodes, if
bipolar impedance is high (e.g., above 900 Ohms), and the irrigation pump is
running, and the
system is in in vivo mode.
[0274] During the Ablation Mode Algorithm, the pump may be activated so
saline is
irrigated from irrigation ports 137 with a flowrate in a range of 15 to 30
ml/min before ablation
energy begins to be delivered, for example for a time of 5s. Then
radiofrequency electrical
energy (RF), for example having a frequency in a range of 350 to 500 kIIz, is
delivered from the
computerized energy console to a first of the plurality of electrodes (e.g.,
the distal electrode
- 57 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
132) in monopolar mode (i.e., returned through a grounding pad) with the
Initial Power for a
duration of a pulse width (e.g., the First Electrode Pulse Width). Then the
first electrode (e.g.,
distal electrode) enters its off period of the waveform (e.g., having a power
of OW or a low
power less than 1W) while RF is delivered to a second electrode (e.g., the
proximal electrode)
starting at the Initial Power for a duration of the Second Electrode Pulse
Width. Optionally, if the
ablation device has more than two electrodes power may be then delivered to
the subsequent
electrode(s) for an according pulse width before repeating power delivery to
the first electrode.
Alternatively, power may be delivered to the electrodes in other orders or
combinations without
deviating from the spirit of the disclosure herein. RF power continues to be
multiplexed through
1 0 each electrode for the Total Therapy Time unless an event is triggered
that titrates or stops
delivery of ablative RF.
[0275] Throughout the Ablation Mode Algorithm and optionally
before or after,
temperature may be measured by the temperature sensors (140 and 139 in Figures
8A, 8B, 9, and
10) and displayed on the console. During the Ablation Mode Algorithm these
temperatures may
be compared to a predefined Temperature Limit, TL, which may be in a range of
40 C to 95 C,
preferably 90 C. Since the space in the vessel around the electrodes and
temperature sensors is
irrigated the measured temperatures can be expected to be less than the
hottest tissue
temperature. Due to the variability of vessel size and shape the relationship
between measured
temperature and the hottest tissue temperature or ablation volume may vary.
However. a
2 0 measured temperature that is higher than the Temperature Limit may be
an indication of too
much power. The Temperature Limit may be considered as a safety control where
measured
temperature above the limit needs to be reduced. However, if measured
temperature is below the
limit it is not necessarily an indication of low tissue temperature. If the
measured temperatures
are below the Temperature Limit throughout the Total Therapy Time then RF
Power remains at
the Initial Power. If one of the measured temperatures is above or optionally
equal to the
Temperature Limit, TL, during treatment (total therapy time) t TOTAL, or
optionally before
Minimum Therapy Time is complete, then power may be decreased to the Secondary
Power, P2,
preferably for the active electrode as shown in Figure 19, or alternatively
for the electrode
associated with said measured temperature or all electrodes. The measured
temperature is
expected to drop below the Temperature Limit within about 5 seconds (or about
2 to 3 pulse
widths) of the power decrease, however if it does not or if it does but then
raises to or above the
Temperature Limit again prior to completion of the Total Therapy Time (or
optionally the
minimum therapy time) then power may be decreased to OW or a low level less
than 1W
preferably to all electrodes, or alternatively to the active electrode or the
electrode associated
with the measured temperature. Alternatively, power may be decreased to the
Tertiary Power
- 58 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
and so on. Following completion of the Minimum Therapy Time if any of the
measured
temperatures reaches or exceeds the Temperature Limit then power may be
decreased to OW or a
low level less than 1W instead of titrating power to a lower ablative level.
[0276] Optionally, the Ablation Mode Algorithm may further have an
Impedance Limit,
ZL, which may be in a range of 200 to 500 ohms, preferably 500, which may be
an indication of
tissue desiccation. If monopolar impedance measured from one of the plurality
of electrodes in
electrical communication with the grounding pad, rises above the Impedance
Limit delivery of
ablative energy to the associated electrode may terminated to avoid steam
formation or injury.
Optionally or additionally, if an Impedance Limit is passed before minimal
therapy time is
complete then power of the ablative RF energy may be reduced to the Secondary
Power or
optionally other lower power levels if there are subsequent occurrences. As
shown in Figure 19,
if impedance for all electrodes remains below the Impedance Limit, ZL, and
above a Critical Low
Impedance Limit, ZcL, then there are no resulting changes to power for each
electrode.
[0277] Optionally, if temperature or impedance for a particular
electrode goes above the
Temperature Limit or Impedance Limit when the secondary power is being
delivered then
ablative RF power may drop to OW preferably for the active electrode, or
alternatively for the
electrode associated with the sensor or for all electrodes.
[0278] Optionally, the Total Therapy Time or Minimum Therapy Time
(if included) may
be extended if power has been decreased to the Secondary Power, or optional
subsequent lower
power levels, for example match the amount of energy being delivered if power
were not
decreased.
[0279] In addition to the Temperature and Impedance Limits the
algorithm may have an
Upper Critical Temperature Limit, Tcu, Lower Critical Temperature Limit. TcL,
Upper Critical
Impedance Limit, Zcu and Lower Critical Impedance Limit, ZCL. An Upper
Critical Temperature
Limit, Tcu, may be used to identify a damaged temperature sensor or an
ultimately high tissue
temperature above which is not desirable, and may be equal to or above 105 C.
A Lower Critical
Temperature Limit, TCL, may indicate something is incorrect about placement or
device damage
and may be equal to or below body temperature (e.g., 35 C). An Upper Critical
Impedance
Limit, Zcu, may be used to identify damage to the catheter such as broken
wires or improperly
applied ground pad and may be in a range of 800 to 2000 Ohms. A Lower Critical
Impedance
Limit, ZcL, may be used to identify damage to the catheter such as short
circuit or a damaged
electrode and may be equal to or below 20 Ohms.
[0280] Optionally, an energy delivery algorithm may have a bipolar
RF component where
RF electrical current passes from the first electrode to the second electrode
(bipolar mode).
Bipolar RF concentrates current density between the two electrodes which may
result in an
- 59 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
ablation pattern that heats tissue between the electrodes greater than the two
electrodes
delivering monopolar RF independently from one another. A bipolar RF component
may be
added to the beginning or end of a Multiplexed Monopolar RF period. For
example, a bipolar
RF component may have a duration in a range of 30 s to 120 s, preferably about
60 s, and deliver
power at an initial level in a range of 10 to 50 W (e.g., 20 to 35 W,
preferably about 30 W) and
be delivered either before or after a multiplexed monopolar RF treatment.
[0281] Alternatively and optionally, an ablation waveform may be
similar to the
Multiplexed Monopolar RF algorithm but have an additional pulse width wherein
the electrodes
deliver Bipolar RF. For example, a bipolar pulse width may be in a range of .5
to 5 s (e.g., 2 s).
The waveform may have an alternating cycle of monopolar RF from a first
electrode for a first
pulse width, monopolar RF from a second electrode for a second pulse width,
and bipolar RF
between the first and second electrodes for a bipolar pulse width that
repeats. If the ablation
catheter has more than two electrodes the waveform may include a repeating
cycle of monopolar
RF to each electrode for respective pulse widths and bipolar RF between each
adjacent pair of
electrodes for bipolar pulse widths.
[0282] An alternative embodiment of an Ablation Energy Delivery
Algorithm used to
create a desired lesion for GSN ablation, is referred to as "Sequential
Monopolar with Bipolar
Fill", wherein ablative RF energy is delivered in monopolar mode to a first
ablation electrode
(e.g., the distal electrode 132 shown in Figures 1, 2, 8A, 8B, 9, and 10) for
a First Electrode
Monopolor Duration, then to a second ablation electrode (e.g., the proximal
electrode 133) for a
Second Electrode Monopolar Duration, then ablative RF energy is delivered in
bipolar mode
between the first and second electrodes for a Bipolar Duration and with an
Initial Bipolar Power.
If temperature measured by a temperature sensor associated with the electrode
receiving ablation
energy raises above an Upper Monopolar Temperature Limit the Initial Monopolar
Power of RF
energy may be decreased to a Secondary Monopolar Power or alternatively be
decreased by a
Power Decrement. If the temperature rises above the upper Temperature Limit
again while the
lower power is being delivered then the power may be decreased again, either
to a Tertiary
Power or by the Power Decrement. Optionally, a user may define parameters such
as Initial
Power to each ablation electrode, First and Second Electrode Monopolor
Durations, Power
Decrement or Secondary, Tertiary etc Monopolar Power. Likewise, during the
Bipolar phase the
Initial Bipolar Power may be decreased to a Secondary Bipolar Power or by a
Power Decrement
if measured temperature from either of the temperature sensors associated with
the activated
electrodes rises above an Upper Bipolar Temperature Limit.
- 60 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0283] Initial Monopolar Power is the amplitude of RF power that
is initially delivered to
either ablation electrode during the monopolar phases and may be selectable in
a range of 20 W
to 50 W, with a default setting of 25 W.
[0284] First Electrode Monopolor Duration is the amount of time
that ablative RF energy is
delivered to the first electrode in Monopolar mode and may be selectable in a
range of 30 s to
180 s, with a default setting of 60 s.
[0285] Second Electrode Monopolor Duration is the amount of time
that ablative RF
energy is delivered to the second electrode in Monopolar mode and may be
selectable in a range
of 30 s to 180 s, with a default setting of 60 s.
[0286] Secondary Monopolar Power is the amplitude of RF power that is lower
than the
Initial Monopolar Power, triggered by measured temperature rising above the
Upper
Temperature Limit. It may be selectable in a range of 10 W to 50 W. as long as
it is below the
Initial Monopolar Power, with a default setting of 20 W.
[0287] Monopolar Power Decrement, an alternative to Secondary
Monopolar Power (and
optionally Tertiary etc.), is the amount of decrease in Power triggered by
measured temperature
rising above the Upper Monopolar Temperature Limit and may be selectable in a
range of 1 to
W, with a default setting of 5 W.
[0288] Initial Bipolar Power is the amplitude of RF power that is
initially delivered to two
ablation electrodes (e.g., the two electrodes that were previously activated
with monopolar RF)
20 during the bipolar phase and may be selectable in a range of 10 W to 50
W, with a default setting
of 20 W.
[0289] Bipolar Duration is the amount of time that ablative RF
energy is delivered to the
two electrodes in Bipolar mode and may be selectable in a range of 10 s to 180
s, with a default
setting of 20 s.
[0290] Secondary Bipolar Power is the amplitude of RF power that is lower
than the Initial
Bipolar Power, triggered by measured temperature rising above the Upper
Bipolar Temperature
Limit. It may be selectable in a range of 5 W to 50 W, as long as it is below
the Initial Bipolar
Power, with a default setting of 15 W.
[0291] Bipolar Power Decrement, an alternative to Secondary
Bipolar Power (and
optionally Tertiary etc.), is the amount of decrease in Power triggered by
measured temperature
rising above the Upper Bipolar Temperature Limit and may be selectable in a
range of 1 to 20
W, with a default setting of 5 W.
[0292] Upper Monopolar Temperature Limit is a threshold
temperature that measured
monopolar temperature is compared to during a monopolar phase. It may be
selectable within a
range of 60 to 90 C, with a default setting of 90 C.
- 61 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0293] Upper Bipolar Temperature Limit is a threshold temperature
that measured bipolar
temperature is compared to. It may be selectable within a range of 60 to 90
C, with a default
setting of 90 C.
[0294] Optionally, if an Upper Temperature Limit is passed during
either a monopolar or
bipolar phase Initial Power may be decreased to the Secondary Power or by the
Power
Decrement and the Duration may be repeated, optionally with the electrodes in
the same
position. If the Upper Temperature Limit is passed a subsequent time the
therapy may be
terminated with an error message. The user may attempt an ablation procedure
with the
electrodes repositioned or with a new catheter.
[0295] Optionally, the algorithm may have an Upper Monopolar Impedance
Limit, which
is a threshold impedance that measured monopolar impedance is compared to
during a
monopolar phase. It may be selectable within a range of 150 to 300 Ohms, with
a default setting
of 200 Ohms.
[0296] Optionally, the algorithm may have an Upper Bipolar
Impedance Limit, which is a
threshold impedance that measured bipolar impedance is compared to during the
bipolar phase.
It may be selectable within a range of 100 to 300 Ohms, with a default setting
of 150 Ohms.
[0297] The disclosure that follows provides some exemplary methods
of use and steps
thereof. Some embodiments of a method of use may include one or more of the
following steps,
2 0 the order of which may in some instances be varied, and not all steps
of which need necessarily
be performed. Methods herein may include interventional access, which may
include one or
more of the following treat the patient with an anti-coagulation regimen that
is appropriate for
venous interventional procedures; place a return electrode on the patient's
right chest; follow
standard techniques for femoral, subclavian, or jugular vein puncture, guide
wire insertion, and
sheath placement using heparinized saline where appropriate; place 0.035
exchange length guide
wire (e.g., Cordis Amplatz Super Stiff 260 cm or equivalent); advance a 6F
general purpose
catheter (e.g. JR4 or equivalent) over the guide wire to the azygous vein
ostium; using the 6F
general purpose catheter, inject a bolus of radiopaque contrast to identify
the azygos vein ostium
using fluoroscopy; engage the azygos vein ostium with the guide wire and 6F
general purpose
catheter and advance the guide wire through the valve (if applicable) into the
azygos vein;
exchange the 6F general purpose catheter for an azygos access sheath, wherein
the azygos access
sheath may be 9F and at least 100 cm long (e.g., Arrow 9F Super Arrow Flex
Introducer Sheath
or equivalent); position the azygos access sheath approximately to the T9
level; adjust the C-arm
off the vertical axis to obtain the optimal view of the azygos vein tree via
shooting contrast prior
to introduction of the Ablation Catheter; load a 0.014 exchange length guide
wire (e.g. ChoICE
- 62 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
Pt LS Floppy or equivalent) into the azygos access sheath; and advance the
0.014 guide wire and
deep seat into first target intercostal vein (e.g., T11 intercostal vein).
[0298] Methods herein may include device, generator, and accessory
preparation, which
may include one or more of the following steps: inspect the catheter package
prior to use; open
the Ablation Catheter package using sterile technique; while maintaining
sterility, remove the
Catheter from its package and place in a sterile field; visually inspect the
electrodes and ablation
catheter carefully for integrity and overall condition; fill a lOcc or larger
syringe with saline and
connect the syringe to the guidewire lumen hub on the handle of the ablation
catheter. Flush the
guidewire lumen with the saline to remove all air; prepare the ablation
catheter by connecting the
ablation catheter irrigation line to a 3-way stopcock, connecting the tube set
to the 3-way
stopcock and connecting the saline spike on a hanging sterile saline bag, and
ensuring the
stopcocks on the saline inlet and saline outlet lines are in the open
position; place the irrigation
pump tubing into the pump, through the bubble detectors and close the pump
door; power ON
the Generator (also referred to as a computerize console) and initialize the
pump; flush the
irrigation lumen of the ablation catheter using the pump to pump the saline
through the irrigation
lumen; confirm that the irrigation ports are patent; purge the tubing and
ablation catheter of air
bubbles; watch the saline tubing and Catheter tip for bubbles and continue to
de-bubble until
there is no air in the ablation catheter irrigation lumen and tube set; to
avoid occlusion of the
irrigation conduits and prevent ingress of air into the ablation catheter, the
ablation catheter may
2 0 be continuously irrigated when within the vasculature, for example at a
rate 2 mL/min; irrigation
may only be stopped after removal of the ablation catheter from the body;
confirm user
selectable ablation parameters on the Generator; plug the ablation catheter
with a cable into the
RF Generator; observe connector polarity;
[0299] Methods herein may include Ablation Catheter Insertion and
Ablation Energy
Delivery, which may include one or more of the following steps: with the 0.014
guide wire deep
seated in the first target intercostal vein, advance the ablation catheter
over the guide wire into
the intercostal vein; initiate saline tracking (examples of which are set
forth herein) from the
Generator once the ablation catheter is inserted into the patient; the
ablation catheter may be
passed from a peripheral vessel to the desired position with the aid of
fluoroscopy; the ablation
catheter saline infusion rate may be increased to a maximum of 50 mL/min to
assist with device
entry to the target intercostal vein; place the proximal marker at the
anterior midline of the
vertebrae in the AP view (if possible); if the azygos to intercostal vein
ostium is to the patient's
right of midline, advance the device so the proximal radiopaque marker is in
the azygos vein
proximal to the ostium to the intercostal vein and approximately at the
patient's midline; rotate
the C-arm to RA030 (or an appropriate angle that maximizes the projected
length between the
- 63 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
proximal and distal radiopaque markers) and confirm that the distal marker is
not past the
costovertebral joint, and adjust as appropriate; confirm that a valid
impedance reading (e.g..
within 80 to 150 Ohms in monopolar mode, or within 60 to 80 Ohms in bipolar
mode) is
displayed for both electrodes on the Generator; activate a saline infusion
rate of 15m1/min to
30m1/min before initiating ablative energy delivery; a recommended saline
infusion rate during
ablation may be 15mllmin; The saline infusion rate can be adjusted after
initiation of RF delivery
to within 15 ml/min to 30 ml/min; initiate the RF ablation mode algorithm from
the Generator;
monitor the impedance display on the RF Generator, before, during, and after
RF power
delivery; if a sudden rise in impedance is noted during RF delivery that does
not exceed the
1 0 preset limit, manually discontinue the power delivery; clinically
assess the situation; if necessary,
remove the ablation catheter and inspect it for damage; in case of a steam pop
or automatic shut
off, discontinue RF and remove the ablation catheter, terminate saline
tracking from the RF
Generator and perform a visual inspection, checking for coagulum, charring, or
other catheter
defects; confirm saline infusion rate and flush the ports prior to reinsertion
in the patient,
resuming saline tracking once inserted; if the ablation catheter has defects,
exchange it for a new
one; re-position the ablation catheter and attempt another RF application;
optionally, no more
than two 180s RF applications should be completed at a single target site; if
the pump alarms and
stops the irrigation, immediately remove the Catheter from the patient and
inspect and re-flush
the ablation catheter; when the ablation in the first target intercostal vein
(e.g., T11) is finished,
2 0 remove the guide wire and ablation catheter from the first target
intercostal vein and keep in the
azygos access sheath in place; the ablation catheter saline infusion rate may
be increased to a
maximum of 50cc/min to assist with device removal from the target intercostal
vein; the ablation
catheter may be removed for inspection; deliver contrast agent to visualize a
second target
intercostal vein (e.g., T10) from the azygos access sheath; repeat Ablation
Catheter Insertion and
Ablation Energy Delivery steps to advance the ablation catheter over the guide
wire into the
second target intercostal vein and ablate; when the ablation in the second
target intercostal vein
is finished, withdraw the ablation catheter into the 9F azygos access sheath
and deliver contrast
from the azygos access sheath to obtain a fluoroscopic image of the azygos
tree.
[0300] Methods herein include device withdrawal, which may include
one or more of the
following steps: withdraw the ablation catheter into the 9F azygos access
sheath and out of the
patient; terminate saline tracking; it may be helpful to disconnect the
connector cable; inspect the
ablation catheter; withdraw the azygos sheath from the patient and close the
venous puncture;
after use, dispose of the devices in accordance with hospital, administrative,
and/or local
governmental policy.
- 64 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0301] In any of the methods herein, including ablation
confirmation tests herein, not all of
the steps need necessarily to be performed. And some of the steps may occur in
different orders.
It is of note that the procedures herein are intending to target particular
nerves or nerve roots, and
are doing so from particular target veins, and even within those veins are
placing ablation
elements or members within certain regions. The anatomical regions that are
being accessed and
targeted necessitate certain design requirements. In other treatments that are
targeting different
anatomical locations for placement, and targeting different target nerves, the
device design
constraints for those approaches are very different, and thus the devices that
can be used in those
treatments may be very different. The disclosure herein thus provides specific
reasons for
designing particular devices, and those reasons include being able to
effectively carry out the
treatments specifically set forth herein.
[0302] While the above description provides examples of one or
more processes or
apparatuses, it will be appreciated that other processes or apparatuses may be
within the scope of
the accompanying claims.
[0303] To the extent any amendments, characterizations, or other assertions
previously
made (in this or in any related patent applications or patents, including any
parent, sibling, or
child) with respect to any art, prior or otherwise, could be construed as a
disclaimer of any
subject matter supported by the present disclosure of this application,
Applicant hereby rescinds
and retracts such disclaimer. Applicant also respectfully submits that any
prior art previously
considered in any related patent applications or patents, including any
parent, sibling, or child,
may need to be re-visited.
[0304] Specific embodiments described herein are not intended to
limit any claim and any
claim may cover processes or apparatuses that differ from those described
below, unless
specifically indicated otherwise. The claims are not limited to apparatuses or
processes having all
of the features of any one apparatus or process described below or to features
common to
multiple or all of the apparatuses described below, unless specifically
indicated otherwise. It is
possible that an apparatus or process described below is not an embodiment of
any exclusive
right granted by issuance of this patent application. Any subject matter
described below and for
which an exclusive right is not granted by issuance of this patent application
may be the subject
matter of another protective instrument, for example, a continuing patent
application, and the
applicants, inventors or owners do not intend to abandon, disclaim or dedicate
to the public any
such subject matter by its disclosure in this document.
[0305] Additional Examples
[0306] A first additional example is a method of characterizing
the position of a patient's
azygos vein relative to a portion of the patient's spine, comprising: while
imaging at least a
- 65 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
portion of the patient's spine; intravascularly delivering a device into a
patient's azygos vein;
performing at least one of: injecting a radiopaque contrast agent (e.g., dye)
from the device into
the patient's vasculature (e.g., into the azygos vein and/or one or more
intercostal veins) to
visualize the vasculature relative to a position of the spine, or identifying
the position of at least a
portion of the device relative to a portion of the spine, to thereby
characterize (e.g., qualify
and/or quantify) the position of the patient's azygos vein relative to a
portion of the spine (e.g.
relative to a midline of the spine).
[0307] In this first additional example, imaging may comprise
imaging in an anterior-to-
posterior view.
1 0 [0308] This first additional example may further comprise
deteimining a lateral position of
a patient's azygos vein, where it meets an intercostal vein, relative to the
patient's spine.
Determining a lateral position of the patient' s azygos vein may be performed
while imaging the
patient's azygos vein. Imaging may comprise radiographic imaging (e.g.
fluoroscopy) after
injecting a radiopaque contrast agent (e.g., dye) from the device into the
patient's vasculature.
Determining a lateral position may be used to determine where to place an
ablation catheter
relative to the intercostal vein, as part of an ablation procedure (optionally
to ablate a GSN).
[0309] A second additional example is a method that includes
assessing a position of a
patient's azygos vein to determine if it is centered, right-biased (to the
patient's right of center),
or left-biased (to the patient's left of center). Assessing a position of the
patient's azygos vein
may be performed while imaging the patient's azygos vein. Imaging may comprise
radiographic
imaging (e.g., fluoroscopy). Imaging may comprise imaging in an anterior-to-
posterior view.
Assessing the position may be used to determine where to place an ablation
catheter as part of an
ablation procedure (optionally intended to ablate a GSN).
[0310] In this second additional example, an assessing step can be
used to determine where
to place a radiopaque marker of an ablation catheter (optionally a proximal
radiopaque marker),
wherein the ablation catheter includes an ablation clement distal to the
radiopaque marker.
[0311] In this second additional example, the assessing step is
used to determine whether
to place the radiopaque marker at an ostium where the azygos vein meets an
intercostal vein, or
at (including substantially at) a midline of the spine.
[0312] In this second additional example, if an assessing step indicates
that the azygos vein
is right-biased or centered (including substantially centered), the method may
include positioning
the radiopaque marker at an ostium where the azygos vein meets the intercostal
vein.
[0313] In this second additional example, if the assessing step
indicates that the azygos
vein is left-biased, the method may include positioning the radiopaque marker
at or substantially
at a midline of the spine (for example, as determined in an anterior-to-
posterior imaging view).
- 66 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0314] In this second additional example, the assessing step may
be used to determine
where to place an ablation element (e.g., one or more electrodes) that is part
of the ablation
catheter.
[0315] In this second additional example, the method may further
comprise assessing a
position of a distal radiopaque marker relative to at least one or more of a
portion of the spine, a
rib, or a costovertebral joint. The method may further comprise retracting the
ablation catheter
proximally if the assessment indicates that the distal radiopaque marker is
positioned too far
distally, which thereby indicates the ablation element is positioned too far
distally. The method
may further ensure that the distal radiopaque marker is not further distally
than the costovertebral
joint.
[0316] A third additional example is a method of intravascularly
positioning an ablation
catheter for GSN ablation, comprising: positioning an ablation catheter in one
or more of an
intercostal vein (e.g. T9, T10, or T11) and an azygos vein, wherein the
position of the ablation
catheter is selected based on a characterized relative position of a portion
of the spine and a
location of the azygos vein where it meets the intercostal vein.
[0317] A fourth additional example is a method of characterizing a
position of a distal
section of an ablation catheter to facilitate placement of at least a portion
of the ablation catheter
in an intercostal vein, comprising: positioning an ablation catheter in a
patient's intercostal vein
(e.g. a T9, T10, or T11 intercostal vein); while imaging a portion of the
patient that includes the
intercostal vein and a portion of the spine, determining a location of one or
more components of
the ablation catheter relative to one or more of a portion of the spine, a
rib, or a costovertebral
joint.
[0318] A fifth additional example is a method of any claim herein,
comprising accessing
venous vasculature at the patient's jugular vein or femoral vein with an
access introducer sheath
(e.g. 12F).
[0319] A sixth additional example is a method of any claim herein,
comprising delivering a
delivery sheath (e.g., 9F sheath) to an azygos vein (e.g., to one or two
thoracic levels above the
target intercostal).
[0320] A seventh additional example is a method of any claim
herein, comprising
delivering contrast agent to show a location of an azygos vein and one or more
intercostal veins
while imaging the azygos vein and one or more intercostal vein.
[0321] Any of additional examples may include an imaging step that
comprises imaging in
an anterior-to-posterior direction (e.g., with a C-arm in an AP position).
[0322] Any of additional examples may include positioning a C-arm
in a Right Anterior
Oblique angle.
- 67 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0323] Any of additional examples may include positioning a C-arm
in a range of 20
degrees to 70 degrees. such as 30 to 60 degrees.
[0324] Any of additional examples may include positioning a C-arm
at an angle that
maximizes a projected distance between first and second axially spaced
locations on the ablation
catheter (e.g., locations of proximal and distal radiopaque markers).
[0325] Any of additional examples may include assessing if a RU
marker (e.g., a distal RU
marker) is at or proximal to a particular anatomical location (e.g. a
costovertebral joint).
[0326] Any of additional examples may include, if the marker is at
or proximal to the
particular anatomical location, continuing with an ablation procedure (e.g.
ablating tissue). If the
marker is not at or proximal to the particular anatomical location, the method
may include
moving the ablation catheter within the intercostal vein. If the marker is not
at or proximal to the
particular anatomical location, the method may include generating ablative
energy within a
proximal ablation clement (e.g. coiled electrode) but not with a distal
ablation element (e.g.
coiled electrode).
[0327] An eighth additional example is an ablation catheter sized and
configured such that
a distal section of the ablation catheter can be advanced into a T9, T10, or
T11 intercostal vein
from an azygos vein, and adapted to deliver ablative energy, comprising: an
elongate shaft with a
length such that a distal section of the catheter can be positioned in a T9,
T10, or T11 intercostal
vein; and the distal section comprising an electrically conductive flexible
ablation element
carried by the elongate shaft, the electrically conductive flexible ablation
element (which may
comprise more than one ablation element) having a length from 5 mm ¨ 20 mm,
and the distal
section having an OD (at least in a delivery configuration) from 1.5 mm ¨ 3
mm.
[0328] A ninth additional example is an ablation catheter sized
and configured such that a
distal section of the ablation catheter can be advanced into a T9, T10, or T11
intercostal vein
from an azygos vein, and adapted to deliver ablative energy, comprising: an
elongate shaft with a
length such that a distal section of the catheter can be positioned in a T9,
T10, or T11 intercostal
vein; and the distal section comprising an electrically conductive flexible
ablation element
carried by the elongate shaft.
[0329] In this ninth additional example, the ablation element may
comprise a first ablation
element axially spaced from a second ablation element, the first and second
ablation elements
carried by the shaft. The first ablation element may have a coiled
configuration, and wherein the
second ablation element may have a coiled configuration. A coiled
configuration of the first
ablation element may be the same in all regards as a coiled configuration of
the second ablation
element. A coiled configuration of the first ablation element may be different
than a coiled
configuration of the second ablation element in at least one way.
- 68 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0330] In this ninth additional example, the first ablation
element may have a different
length than the second ablation element.
[0331] In this ninth additional example, the first ablation
element may have a different coil
direction (e.g. left handed vs right handed) than the second ablation element.
[0332] In this ninth additional example, the first ablation element may
have a different
pitch than the second ablation element.
[0333] In this ninth additional example, the first ablation
element may have a different wire
thickness than the second ablation element.
[0334] In this ninth additional example, an OD of the distal
section at the location of the
1 0 first ablation element may be different than an OD of the distal
section at the location of the
second ablation element.
[0335] In this ninth additional example, a first ablation element
and a second ablation
element may each have either a curvilinear (e.g. circular) or rectilinear
(e.g., rectangular) cross
sectional outer profile.
[0336] In this ninth additional example, a first ablation element and a
second ablation
element may be a superelastic material such as nitinol.
[0337] In this ninth additional example, a first ablation element
and a second ablation
element may be sufficiently flexible to allow the distal section to be
advanced from an azygos
vein into one of a T9, T10, or T11 intercostal vein.
[0338] In this ninth additional example, at least one of a first and second
ablation elements
may be made from a laser cut tubular element (e.g., a nitinol tube).
[0339] In this ninth additional example, at least one of a first
and second ablation elements
may comprise a wire mesh or braid.
[0340] In this ninth additional example, at least one of a first
and second ablation elements
may be a ring electrode having a length not more than 5 mm, optionally around
3mm.
[0341] In this ninth additional example, each of a first and
second ablation elements may
have a length from 1 mm ¨ 12 mm, optionally from 2 mm ¨ 12 m, optionally from
5 mm ¨ 12
rum, optionally from 6 mm ¨ 11 mm, optionally from 7 mm ¨ 10 mm, such as
around 8 mm.
[0342] In this ninth additional example, an axial spacing between
a first and second
ablation elements may be from 0 mm ¨ 8 mm, such as from 0 mm - 5 nun, such as
from .5 mm -
5 mm, such as from 1 mm ¨4 mm.
[0343] In this ninth additional example, an ablation element total
axial length may be from
1 mm - 25 mm, optionally from 2 mm - 22 mm, optionally from 5 narn - 20 mm,
optionally 8 mm
¨ 20 mm, optionally 10 mm ¨20 mm, optionally 10 mm ¨ 18 mm, optionally,
preferably 10 mm
¨ 15 mm.
- 69 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
[0344] In this ninth additional example, the ablation element, and
optionally both of a first
and second ablation elements, may have an expandable diameter.
[0345] In this ninth additional example, the ablation element may
comprise a plurality of
ablation elements, of which first and second ablation elements may he part of
and may define the
entirety of the plurality of ablation elements.
[0346] In this ninth additional example a plurality of ablation
elements may be configured
to be independently energized in monopolar mode (with a ground pad).
[0347] In this ninth additional example, any two of a plurality of
ablation elements may be
configured to be energized in bipolar mode.
1 0 [0348] In this ninth additional example, the catheter may include a
temperature sensor
disposed between the first and second ablation elements and carried by the
shaft.
[0349] In this ninth additional example, the catheter may further
comprise one or more of a
temperature sensor distal to a distal ablation element, or a temperature
sensor proximal to a
proximal ablation element.
[0350] In this ninth additional example, the catheter may include at least
one irrigation port
in fluid communication with an irrigation lumen that is connectable to a fluid
source at a
proximal region of the ablation catheter. The ablation catheter may further
comprise a second
irrigation port distal to the proximal ablation element.
[0351] In this ninth additional example, the catheter may include
one or more irrigation
ports between a distal end and a proximal end of a distal ablation member,
optionally between
the windings of a coiled distal ablation member.
[0352] In this ninth additional example, the catheter may comprise
one or more irrigation
ports between a distal end and a proximal end of a proximal ablation member,
optionally
between the windings of a coiled proximal ablation member.
[0353] In this ninth additional example, the catheter may include one or
more irrigation
ports under any of the flexible ablation elements, such as a distal ablation
element and/or a
proximal ablation member.
[0354] In this ninth additional example, the catheter may further
comprise a deployable
element carried by the shaft (optionally expandable). A deployable element may
be distal to the
ablation element, optionally distal to a distal ablation element. A deployable
element may be
inflatable, and wherein the shaft may include an inflation port within the
inflatable deployable
element. A deployable element may have a delivery configuration and a deployed
configuration
with an OD greater than the delivery configuration. A deployable element may
have an OD from
3-6 mm in the deployed configuration, such as 4 mm ¨ 6 mm. A deployable
element may have
an OD that is equal to or greater than the OD of the shaft in the distal
section by no more than
- 70 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
0.2 mm. A deployable element may comprise at least one of the following: a
balloon, a bellowed
member, or a coated stent or coated stent-like device (e.g., a reinforcing
member coated with a
one or more layers of material).
[0355] In this ninth example, the ablation catheter may further
comprise a proximal
deployable element carried by the shaft proximal to the ablation element,
which may be proximal
to a proximal ablation element. A proximal deployable element may be
inflatable, and wherein
the shaft may include an inflation port within the proximal deployable
element. A proximal
deployable element may have a delivery configuration and a deployed
configuration with an OD
greater than the delivery configuration. A deployable element may have an OD
from 4-10 mm in
the deployed configuration, and optionally larger than an OD of a distal
deployable member. A
proximal deployable element may have an OD that is equal to or greater than
the OD of the shaft
in the distal section by no more than 0.2 nana. A proximal deployable element
may comprise at
least one of the following: a balloon, a bellowed member, or a coated stent or
coated stent-like
device (e.g., a reinforcing member coated with a one or more layers of
material).
[0356] In this ninth additional example, the catheter may include a central
deployable
element. A central deployable element may include any of the features,
including any
combination thereof, of a distal or proximal deployable member herein.
[0357] In this ninth additional example, the catheter is
configured for transvascular
ablation of a GSN. The ablation catheter may include a distal section that
includes the distal-
2 0 most 7 cm of the ablation catheter. The ablation element may be adapted
to create an ablation
having a length in a range of 5 mm to 25 mm.
[0358] In this ninth additional example, a distal section may be
adapted for flexibly
traversing a bend from an azygos vein to a T9-T11 intercostal vein (e.g.,
having a radius of
curvature >= 5 mm, angle as much as 120 degrees.
[0359] In this ninth additional example, an outer diameter of the distal
section (at least in a
delivery state) is in a range of 1.5 to 3 mm.
[0360] In this ninth additional example, the ablation catheter may
further comprise a
guidewire lumen within the elongate shaft.
[0361] In this ninth additional example, a total length of the
ablation element (which may
comprise a plurality of individual ablation elements) may be from 5 mm to 20
mm, such as 10 to
15 mm.
[0362] In this ninth additional example, any of the ablation
elements may comprise one or
more of an RF ablation electrode, a coiled wire electrode, a laser cut RF
electrode, a RF
electrode printed with conductive ink, a RF electrode on an expandable balloon
(e.g., conductive
ink, flexible circuits,), a conductive membrane RF electrode, a RF electrodes
on an expandable
- 71 -
CA 03162954 2022- 6- 23
WO 2021/146724
PCT/US2021/014001
cage or mesh, an ultrasound ablation transducer, an electroporation
electrodes, an cryoablation
element, or a virtual RF electrode.
[0363] In this ninth additional example, the ablation element may
be adapted to deliver
ablation energy circumferentially (radially symmetric around the ablation
element / around the
vessel).
[0364] In this ninth additional example, the catheter may further
include a proximal
radiopaque marker positioned on the shaft at or proximal to a proximal end of
the ablation
element.
[0365] In this ninth additional example, the catheter may further
a distal radiopaque marker
positioned on the shaft distal to a distal end of the ablation element(s).
[0366] In this ninth additional example, the catheter may include
an axial space between a
distal radiopaque marker and a distal end of the ablation element.
[0367] Any of the methods in any of the additional methods may be
used with any of
catheters in the additional examples. Any of the catheters in the additional
examples may be used
with methods herein or used in ways that are not described herein.
- 72 -
CA 03162954 2022- 6- 23