Note: Descriptions are shown in the official language in which they were submitted.
CARTRIDGE FOR RESPIRATION DEVICE
[0001] Deliberately left blank.
TECHNICAL FIELD
[0002] The following relates to respirator devices, and more particularly
to cartridges for
elastomeric respirator devices.
BACKGROUND
[0003] Humans have been affected by respiratory illnesses for many
centuries. Common
respiratory illnesses include tuberculosis, seasonal flu, pertussis, swine
flu, SARS (severe acute
respiratory syndrome), MERS (middle east respiratory syndrome) and COVID-19.
These
respiratory illnesses have been reported in all parts of the world including
Asia, North America,
and Europe. Many respiratory illnesses spread primarily by close person-to-
person contact with
symptomatic individuals (e.g., persons with fever, cough, sneezing or other
respiratory
symptoms). The respiratory illnesses can be spread by touching the skin of
other individuals or
objects contaminated with infectious droplets and then touching one's own
eyes, nose, or
mouth. Contamination occurs when someone with the illness coughs or sneezes
droplets onto
themselves, other individuals, or nearby surfaces. It also is possible that
the respiratory illness
can be spread further through the air by very small particles, referred to as
airborne
transmission.
[0004] One method to slow the spread of illness is disinfection. Surfaces
which commonly
harbor bacteria and/or viruses may be disinfected. In disinfection, the
bacteria and/or viruses
are killed such that they are not able to reproduce and cause illness.
Chlorination, ozone and
ultraviolet (UV) light are commonly known methods of disinfection. Chemical
disinfectants such
as alcohol, iodine, chlorine may also be used for disinfecting. However, these
methods of
disinfections are largely used for disinfecting surfaces which commonly harbor
bacteria and/or
viruses. These methods are generally not suitable for disinfecting humans
which carry the
contagion. It may also be challenging to disinfect the air used for breathing.
[0005] There are 3 main types of UV light: UVA (long wave, low energy, 315-
400 nm
wavelength); UVB (medium wave, medium energy, 280-315 nm wavelength); and UVC
(short
wave, high energy, 100-280 nm wavelength). UV-C light has been used in the
past to disinfect
1
23881838.1
Date Recue/Date Received 2022-06-21
objects. The UV-C light will effectively kill viruses, bacteria and other
airborne contagion. In
particular, the wavelength of 264 nm is effective at killing viruses and
bacteria.
[0006] Another method to stop or slow the spread of the illnesses is
through the use of
respirators or face masks. Respirators provide a physical barrier which
prohibits the bacteria
and viruses from entering into a host's body. Since the bacteria or viruses
are prohibited from
entering a host's body, they are unable to reproduce and cause disease or
illness. The most
critical places where bacteria and viruses enter the body include: the nose,
eyes, ears and
mouth. Therefore, it is critical to provide a physical barrier to cover these
orifices. Respirators
may have half-face forms that cover only the bottom half of the face including
the nose and
mouth, and full-face forms that cover the entire face.
[0007] Respirators typically have a facepiece held to the wearer's head
with straps, a cloth
harness, or some other method. Facepieces come in many different styles and
sizes, to
accommodate all types of face shapes. The differences in respirator design
impact the
respirator assigned protection factors, i.e. the resulting degree of
protection.
[0008] Particulate filtering facepiece respirators are a subset of
respirators which are
discarded when they become unsuitable for further use due to considerations of
hygiene,
excessive resistance, or physical damage. An example of a particulate
filtering facepiece is
shown in FIG. 1A. Powered air-purifying respirators are another subset of
respirators which
have a battery-powered blower that moves the airflow through filters. An
example of a powered
air-purifying respirator is shown in FIG. 1B.
[0009] Elastomeric respirators are yet another subset of respirators which
are reusable.
Elastomeric respirators comprise a facepiece as well as at least one filter
cartridge. An example
of an elastomeric respirator is shown in FIG. 2. The facepiece can be cleaned
and reused, but
the filter cartridges must be discarded and replaced when they become
unsuitable for further
use. Filter cartridges generally comprise layers of various chemical filters
such as activated
carbon layer, charcoal layer, aerosol filter layer, absorbent layer, etc. An
example of the
replaceable filter cartridge is shown in FIG. 3.
[0010] Respirators in this family are rated as N, R, or P for protection
against oils. This
rating is important in industry because some industrial oils can drastically
alter or degrade the
filter performance. Respirators are rated "N," if they are Not resistant to
oil, "R" if somewhat
Resistant to oil, and "P" if strongly resistant to oil (oil-Proof).
Furthermore, each of the N, R and
2
23881838.1
Date Recue/Date Received 2022-06-21
P ratings have 3 varieties of filtration limits. Respirators can additionally
be rather either 95, 99
or 100. Respirators rated 95 filters at least 95% of airborne particles;
respirators rated 99 filters
at least 99% of airborne particles; and respirators rated 100 filter at least
99.97% of airborne
particles. Thus, there are nine types of disposable particulate respirators: N-
95, N-99, and N-
100; R-95, R-99, and R-100; and P-95, P-99, and P-100.
[0011] National Institute for Occupational Safety and Health ("NIOSH") uses
very high
standards to test and approve respirators for occupational uses. NIOSH-
approved disposable
respirators are marked with the manufacturer's name, the part number (P/N),
the filter rating
(e.g., N-95), and "NIOSH." This information is printed on the facepiece,
exhalation valve cover,
or head straps. While masks like N95 and others are effective, they are mostly
one time use and
are expensive to maintain. Providing respirators that are reusable, effective,
and can be used by
the general public and the healthcare industry is a challenge.
[0012] Another problem with respirators is that they do not kill the
viruses and/or bacteria
which cause illness. They simply provide a physical barrier which keeps the
viruses and
bacteria out of a host's body. However, it is still possible for some of the
viruses and bacteria to
make it past the physical layer and into the host's body. Therefore, there is
a need for a
respirator which exterminates as well as provides a physical barrier against
airborne contagions.
SUMMARY
[0013] In one aspect, a reusable respiration device is provided which is
configured to kill
infectious virus or other air borne contagion (examples of such viruses and
bacteria include
SARS, MERS, M RSA, Ebola, norovirus and C-DIFF) before it enters the user's
airways. The
respiration device comprises a wearable mask portion, an evacuation filter in
fluid connection to
the wearable mask portion; and at least one UV (ultraviolet) cartridge in
fluid connection to the
wearable mask portion. The UV cartridge comprises a housing having an inlet at
a first end and
an outlet at a second end opposite the first end; a series of UV bulbs located
within the housing;
and a powering means. Ambient air enters the respirator device through the
inlet and is subject
to UV radiation emitted from the series of UV bulbs such that the ambient air
becomes sterilized
air. Sterilized air exits the UV cartridge and enters the wearable mask
portion via the outlet. A
user is able to inhale the sterilized air entering the mask portion and exhale
the used air through
the evacuation filter.
3
23881838.1
Date Recue/Date Received 2022-06-21
BRIEF DESCRIPTION OF THE DRAWINGS
[0014] Embodiments will now be described with reference to the appended
drawings
wherein:
[0015] FIG. 1A is a perspective view of a particulate filtering facepiece
respirator which
forms part of the prior art.
[0016] FIG. 1B is a perspective view of a powered air-purifying respirator
which forms part
of the prior art.
[0017] FIG. 2 is a front view of an elastomeric respirator which forms part
of the prior art.
[0018] FIG. 3 is a perspective view of replaceable filter cartridges to be
used with
elastomeric respirators which are prior art.
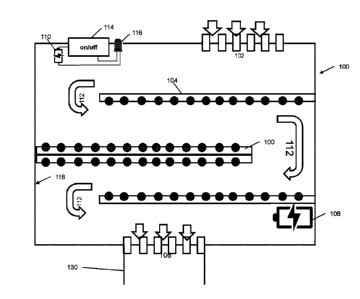
[0019] FIG. 4 is a schematic cross-sectional view of the UV cartridge.
DETAILED DESCRIPTION
[0020] Elastomeric respirators 200, as known in the prior art, typically
comprise a mask
portion 120 as well as at least one filter cartridge 122, as shown in FIG. 2.
The elastomeric
respirator 200 may also comprise strapping means 126 from which straps can be
connected to
such that the respirator 200 and mask 120 can be properly fitted on a user.
The mask portion
120 on an elastomeric respirator 200 can be cleaned and reused, but typically,
the filter
cartridges 122 have to be discarded and replaced when they become unsuitable
for further use.
Typical filter cartridges 122 for respirators comprise layers of various
chemical filters such as an
activated carbon layer, a charcoal layer, an aerosol filter layer or an
absorbent layer. Ambient
air enters the elastomeric respirator 200 through inlets 128 located on the
filter cartridges 122
and is subject to layers of various chemical filters located within the filter
cartridge 122 to
produce sterilized air. Sterilized air exits the filter cartridge 122 and
enters the wearable mask
portion 120 where a user is able to inhale the sterilized air entering the
mask portion 120. Once
the user breathes the sterilized air, they are able to exhale the used air
through the evacuation
filter 124.
[0021] FIG. 3 provides a perspective view of replaceable filter cartridges
122 to be used
with elastomeric respirators 200 which constitute prior art. The filter
cartridges 122 comprise a
4
23881838.1
Date Recue/Date Received 2022-06-21
first surface on which a plurality of inlets 128 are located. The inlets face
the ambient air and
allow the ambient air to enter the filter cartridge 122. On an opposite
surface, the filter cartridge
122 comprises a coupling means 130 to couple to the mask portion 120. The
filter cartridge 122
removably couples to the mask portion 120 such that the filter cartridges 122
can be easily
removed and replaced once it is unsuitable for further use.
[0022] A UV
cartridge 100 is herein provided which is capable of exterminating air-borne
viruses and bacteria before they enter a user's airways. It can be appreciated
that the UV
cartridge 100 can replace a filter cartridge 122 of a respiration device such
as an elastomeric
respirator device 200. The UV cartridge 100 also comprises a couplings means
130 to couple to
the mask portion of an existing elastomeric respirator 200. The UV cartridge
100 is reusable yet
completely sanitary and effective whilst being easy to operate and maintain.
[0023] A
schematic view of the UV cartridge 100 is provided in FIG. 4. The UV cartridge
100 can be used with an existing respiration device such as an elastomeric
respirator device
200. The UV cartridge 100 taught herein comprises a series of UV light bulbs
104 which can be
used to exterminate any air borne contagion such as viruses and/or bacteria.
The UV cartridge
100 comprises at least one UV lightbulb 104, preferably a series of UV light
bulbs 104. In one
embodiment, the UV lightbulbs 104 are of the LED type, however any suitable UV
light may be
used. LED light bulbs are preferable because they are lightweight and have
reported higher
operating efficiencies. The LED UV lightbulbs are preferably battery-operated;
however, they
may be powered by any suitable method including photovoltaic, capacitive, or
kinetic methods
of charging. The LED UV lightbulbs are preferably light-weight such that a
user may wear the
respiration device without discomfort. Additionally, the UV cartridge 100
comprises UV-C light,
preferably having a wavelength of 264 nm. This wavelength of light is the most
effective at
breaking down bacterial cell walls and viruses.
[0024]
Ambient air is absorbed from an inlet 102. The ambient air is then subject to
the
series of LED UV bulbs 104. In this embodiment, the ambient air is subject to
multiple layers of
UV bulbs 104. The UV bulbs 104 are charged through a first battery 108. The
first battery 108
may be rechargeable or replaceable. The longer exposure to ultraviolet light
will cause a higher
number of the airborne contagions to be exterminated. At an opposite end, the
cleaned air exits
the UV cartridge 100 and enters the user's nose, mouth and lungs through the
passage 106.
The flow rate of the air 112 passing through the UV cartridge 100 is primarily
dependent on the
user's breath. For instance, the air flow through the mask for a user taking
slow breaths may be
23881838.1
Date Recue/Date Received 2022-06-21
slower than the air flow through the mask for a user taking faster breaths.
The user's breath will
therefore ensure that the incoming ambient air passes through the various
layers of UV bulbs
104 before exiting through the passage 106. Once the user breathes the air, it
may be exhaled
through the UV cartridge 100 or through an evacuation filter 124. It can be
appreciated that the
UV cartridge 100 should contain at least the volume of space that would allow
ample air to enter
the space and exit. For instance, the tidal volume (volume of air breathed in
or out per breath) of
an average user is approximately 0.5 litres.
[0025] The UV cartridge 100 may also comprise a second battery 110 and may
have a
coloured LED light 116 to show the status of the batteries 108, 110. For
instance, the coloured
LED light may show green for high charge, red for low charge, etc.
Furthermore, the respiration
device may also comprise an on/off switch 114 to conserve battery life of the
device. The on/off
switch 114 may also be a depressible button or any other suitable switch known
to a person
skilled in the art. It can be appreciated that the on/off switch 114 should
not accidentally turn off
during use and therefore should be placed accordingly. For instance, the
on/off switch 114 may
be placed on the side of the UV cartridge 100 where the user is not likely to
accidentally engage
the on/off switch 114. Other placements of the on/off switch may be possible
as well. The on/off
switch 114 may also include a protective member to decrease the likelihood of
it being
accidentally engaged. The on/off switch 114 may also be waterproof.
[0026] Additionally, the UV cartridge may be used to replace a filter
cartridge of an existing
elastomeric respirator. In this embodiment, the UV cartridge can be sized
according to a filter
cartridge such that it can easily replace the filter cartridge. For
construction masks, the UV
cartridge may also be equipped with a dust filter to prevent dust and
particulates from entering
the UVC cartridge. The dust filter may be in the form of an adapter at the
outside of the UV
cartridge 100.
[0027] It can be appreciated that the user should not be subject to the
emitted UVC
radiation from the UVC lightbulbs. As such, the UV cartridge 100 can be in
fluid connection to
the mask, however light from the UV bulbs will not reach the user. The
internal walls 118 of the
UV cartridge 100 may also include an anti-reflective (AR) coating in order to
maximize photon
emission from the LED UVC light bulbs. This can help increase the efficiency
of the respiration
device as the emitted photons of light will be reflected within the UV
cartridge 100, increasing
their contact with the airborne contagion and killing them faster. The UV
cartridge 100 can be
6
23881838.1
Date Recue/Date Received 2022-06-21
constructed from sapphire or fused silica. The anti-reflective coating may be
applied to one or
both faces of the UV cartridge 100.
[0028] Some materials including some plastics are susceptible to breakdown
by UV light.
Therefore, a UV stable material is preferable for the construction of the UV
cartridge 100.
Certain plastics such as acrylic, Ulteme, PVDF, and PTFE are inherently UV
stable. Therefore,
in a preferred embodiment the UV cartridge 100 is constructed from a UV-stable
plastic such as
acrylic, Ultem , PVDF, or PTFE.
[0029] The mask portion of the respiration device may be half-face mask,
without eye
protection; or a full-face mask including eye protection. The respiration
device may be
particularly useful for people in health care, for example, health care
workers, front line health
care workers, doctors, nurses, and patients. However, it can be appreciated
that other
populations, such as the general public could also use the mask.
[0030] In one embodiment, a full face mask can be constructed which
comprises a panel of
UVC bulbs. The UVC bulbs face away from the user such that the emitted
radiation cannot be
felt by the user.
[0031] For simplicity and clarity of illustration, where considered
appropriate, reference
numerals may be repeated among the figures to indicate corresponding or
analogous elements.
In addition, numerous specific details are set forth in order to provide a
thorough understanding
of the examples described herein. However, it will be understood by those of
ordinary skill in the
art that the examples described herein may be practiced without these specific
details. In other
instances, well-known methods, procedures and components have not been
described in detail
so as not to obscure the examples described herein. Also, the description is
not to be
considered as limiting the scope of the examples described herein.
[0032] It will be appreciated that the examples and corresponding diagrams
used herein are
for illustrative purposes only. Different configurations and terminology can
be used without
departing from the principles expressed herein. For instance, components and
modules can be
added, deleted, modified, or arranged with differing connections without
departing from these
principles.
7
23881838.1
Date Recue/Date Received 2022-06-21
[0033]
Although the above principles have been described with reference to certain
specific
examples, various modifications thereof will be apparent to those skilled in
the art as outlined in
the appended claims.
8
23881838.1
Date Recue/Date Received 2022-06-21