Note: Descriptions are shown in the official language in which they were submitted.
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
PACKAGING SYSTEMS FOR DRUG DELIVERY DEVICES AND PACKAGING KITS
FIELD
[0001] Packaging systems and kits are disclosed for drug delivery devices,
particularly
intranasal drug delivery devices.
BACKGROUND
[0002] Pharmaceutical packaging protects the packaged product from damage and
contamination during shipping. Blister packaging is one type of pharmaceutical
packaging that
is commonly used for packaging pills. Recently, blister packaging has been
implemented in the
packaging of drug delivery devices. However, while being transported, the
packaged drug
delivery device(s) can rattle within the blister packs. This is because
typical blister packs fail to
sufficiently secure the drug delivery device(s) in a manner that prevents
rattle. This rattle can
have negative effects on the drug delivery devices, and ultimately, can cause
the drug delivery
devices to be defective for their intended use.
[0003] Further, some drug delivery devices that are packaged in blister packs
can include a
plunger that is used for dispensing a medicament disposed therein.
Unfortunately, movement of
these drug delivery devices within typical blister packs can result in
actuation of the plunger
before it reaches the end-user, e.g., during shipping, handling, storage,
sterilization, and the like.
As a result, medicament can be prematurely expelled from the drug delivery
device. This
premature expulsion of drug can render the drug delivery device ineffective,
and thus, can
require the disposal thereof.
[0004] Accordingly, there remains a need for improved packaging systems for
drug delivery
devices.
SUMMARY
[0005] Various packaging systems for drug delivery devices and packaging kits
are disclosed.
[0006] In one exemplary embodiment, a packaging system is provided and
includes a base
extending from a first end to a second end and configured to receive a drug
delivery device
1
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
having a device body having an upper segment and a lower segment, and an
actuator operatively
coupled to the lower segment, in which the base is configured to house the
received drug
delivery device therein at a non-zero angle relative to a longitudinal axis of
the base that extends
between the first and second ends to thereby substantially prevent movement of
the received
drug delivery device while within the base. The base includes a retaining
cavity and a gripping
cavity adjacent the retaining cavity. The retaining cavity is configured to
create an interference
fit with at least a portion of an upper segment of a drug delivery device
received within the base
to thereby releasably retain the received drug delivery device within the
base. The gripping
cavity is configured to receive a lower segment of a device body and a portion
of an actuator of a
drug delivery device received within the base.
[0007] The base can have a variety of configurations. For example, in some
embodiments, the
base can include an end cavity positioned between the first end of the base
and the retaining
cavity, in which the end cavity can be configured to receive at least a
portion of an upper
segment and an outer sleeve of a drug delivery device received within the
base. In other
embodiments, the base can include an end cavity positioned between the
gripping cavity and the
second end of the base, in which the end cavity can be configured to receive a
portion of an
actuator of a drug delivery device received within the base.
[0008] In some embodiments, the base can be substantially transparent such
that a drug
delivery device received within the base is viewable through the base.
[0009] In some embodiments, the non-zero angle can be from about 1 degree to 5
degrees. In
one embodiment, the non-zero angle can be about 4 degrees.
[0010] The retaining cavity can have a variety of configurations. For example,
in some
embodiments, the retaining cavity can have a substantially c-shaped
configuration.
[0011] In some embodiments, the packaging system includes a lid that can be
releasably sealed
to the base.
[0012] The drug delivery device can have a variety of configurations. For
example, in some
embodiments, the drug delivery device can include an outer sleeve about a
portion of the upper
segment of the device body. The outer sleeve can have a first set of opposing
flanges extending
2
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
from a first end thereof. In such embodiments, the gripping cavity can include
a support element
that can be configured to support a lower segment of a drug delivery device
received within the
base such that the first set of opposing flanges of the received drug delivery
device is positioned
between the retaining cavity and the support element to thereby substantially
prevent actuation of
an actuator of the received drug delivery while within the base. In one
embodiment, the support
element can have a substantially c-shaped configuration.
[0013] In some embodiments, the gripping cavity can be configured to allow a
device body of
a drug delivery device received within the base to be gripped to remove the
received drug
delivery device from the base.
[0014] In another exemplary embodiment, a packaging kit is provided and
includes a drug
delivery device and a packaging system having the drug delivery device
disposed therein. The
drug delivery device includes a device body having an upper segment and a
lower segment, and
an actuator operatively coupled to the lower segment. The packaging system
includes a base
extending from a first end to a second end and houses the drug delivery device
therein at a non-
zero angle relative to a longitudinal axis of the base that extends between
the first and second
ends to thereby substantially prevent movement of the drug delivery device
within the base. The
base includes a retaining cavity that creates an interference fit with at
least a portion of the upper
segment of the drug delivery device to thereby releasably retain the drug
delivery device within
the base, and a gripping cavity adjacent to the retaining cavity and houses
the lower segment of
the device body and a portion of the actuator of the drug delivery device.
[0015] The base can have a variety of configurations. For example, in some
embodiments, the
base can include an end cavity positioned between the first end of the base
and the retaining
cavity and receives at least a portion of the upper segment and the outer
sleeve of the drug
delivery device. In other embodiments, the base can include an end cavity
positioned between
the gripping cavity and the second end of the base and receives a portion of
the actuator of the
drug delivery device.
[0016] In some embodiments, the packaging system can include a lid that can be
releasably
sealed to the base.
3
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
[0017] In some embodiments, the non-zero angle can be from about 1 degree to 5
degrees. In
other embodiments, the non-zero angle can be about 4 degrees.
[0018] The retaining cavity can have a variety of configurations. For
example, in some
embodiments, the retaining cavity can have a substantially c-shaped
configuration.
[0019] The drug delivery device can have a variety of configurations. For
example, in some
embodiments, the drug delivery device can include an outer sleeve positioned
about a portion of
the upper segment of the device body. The outer sleeve can have a first set of
opposing flanges
extending from a first end thereof. In such embodiments, the gripping cavity
can include a
support element that supports the lower segment of the drug delivery device
such that the first set
of opposing flanges of the drug delivery device is positioned between the
retaining cavity and the
support element to thereby substantially prevent actuation of the actuator
while the drug delivery
device is within the base. In one embodiment, the support element can have a
substantially c-
shaped configuration.
[0020] In some embodiments, the base can be substantially transparent such
that the drug
delivery device is viewable through the base.
[0021] The gripping cavity can have a variety of configurations. For
example, in some
embodiments, the gripping cavity can allow the device body of the drug
delivery device to be
gripped to remove the drug delivery device from the base.
BRIEF DESCRIPTION OF THE DRAWINGS
[0022] This invention will be more fully understood from the following
detailed description
taken in conjunction with the accompanying drawings, in which:
[0023] FIG. 1A is a front view of one embodiment of an intranasal drug
delivery device;
[0024] FIG. 1B is a side view of the intranasal drug delivery device of FIG.
1A;
[0025] FIG. 1C is an exploded view of the intranasal drug delivery device of
FIG. 1A;
[0026] FIG. 2 is a partially exploded view of one embodiment of a packaging
kit that includes
4
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
a packaging system having a base and a lid, and the intranasal drug delivery
device of FIG. 1A
housed within the packaging system;
[0027] FIG. 3 is a perspective view of the packaging kit of FIG. 2;
[0028] FIG. 4 is a front magnified view of the lid of FIG. 3;
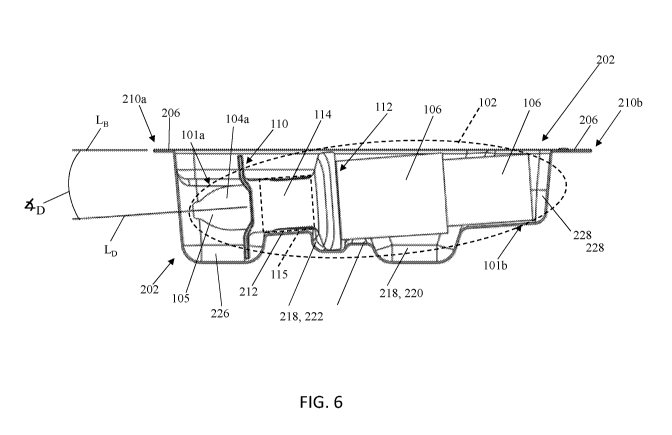
[0029] FIG. 5 is a top view of the base of the packaging system of FIG. 2,
showing the
intranasal drug delivery device disposed therein;
[0030] FIG. 6 is a cross-sectional side view of the base of the packaging
system of FIG. 2
taken at line 6-6, showing the intranasal drug delivery device disposed
therein;
[0031] FIG. 7 is a perspective view of the base of the packaging system of
FIG. 2 with the
intranasal drug delivery device removed;
[0032] FIG. 8 is a top view of the base of FIG. 7;
[0033] FIG. 9 is a cross-sectional view of the base of FIG. 8 taken at line 9-
9; and
[0034] FIG. 10 is a cross-sectional view of the base of FIG. 7 taken at line
10-10.
DETAILED DESCRIPTION
[0035] Certain exemplary embodiments will now be described to provide an
overall
understanding of the principles of the structure, function, manufacture, and
use of the packaging
systems and kits disclosed herein. One or more examples of these embodiments
are illustrated in
the accompanying drawings. Those skilled in the art will understand that the
devices, systems,
and methods specifically described herein and illustrated in the accompanying
drawings are non-
limiting exemplary embodiments and that the scope of the present invention is
defined solely by
the claims. The features illustrated or described in connection with one
exemplary embodiment
may be combined with the features of other embodiments. Such modifications and
variations are
intended to be included within the scope of the present invention.
[0036] While the packaging systems, kits, and methods described herein can be
applicable to a
number of drug delivery devices, they are described herein in the context of
an intranasal drug
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
delivery device.
[0037] In general, the intranasal drug delivery devices can include a device
body and an
actuator that is operatively coupled to the device body and, upon actuation,
is configured to expel
one or more doses of medicament from the device. While the actuator can have a
variety of
configurations such as, for example, a plunger, a button, a lever, and the
like, the actuator
described herein is in the context of a plunger. Further, while the intranasal
drug delivery
devices can be configured to expel one or more doses of a medicament disposed
therein, they are
described herein in the context of a bi-dose intranasal drug delivery device.
[0038] For example, as shown in FIGS. 1A-1C, an exemplary intranasal drug
delivery device
100 includes a device body 102 having an upper segment 104a and a lower
segment 104b, and a
plunger 106 operatively coupled to the lower segment 104b. In use, a user
pushes the plunger
106 towards the upper segment 104a of the device body 102, thereby causing a
dose of
medicament (not shown) disposed within the device 100 to be expelled
therefrom. Further, after
the plunger 106 resets, the user can then subsequently push the plunger 106
again towards the
upper segment 104a of the device body 102, thereby causing an additional dose
of medicament
(not shown) disposed within the device 100 to be expelled therefrom.
[0039] A "medicament" as used herein refers to a therapeutic agent (a drug, a
biologic, a
biological material, etc.) that when administered to a subject will have the
intended prophylactic
effect, e.g., preventing or delaying the onset (or reoccurrence) of an injury,
disease, pathology or
condition, or reducing the likelihood of the onset (or reoccurrence) of an
injury, disease,
pathology, or condition, or their symptoms or the intended therapeutic effect,
e.g., treatment or
amelioration of an injury, disease, pathology or condition, or their symptoms
including any
objective or subjective parameter of treatment such as abatement; remission;
diminishing of
symptoms or making the injury, pathology or condition more tolerable to the
patient; slowing in
the rate of degeneration or decline; making the final point of degeneration
less debilitating; or
improving a patient's physical or mental well-being. Non-limiting examples of
suitable
medicaments include esketamine, ketamine, naloxone, and sumatriptan.
[0040] The upper segment 104a of the device body 102 includes first and second
indicator
windows 108a, 108b. The first indicator window 108a is configured to indicate
to a user or
6
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
healthcare provider (HCP) that a first dose of the medicament has been
expelled from the device
100, and the second indicator window 108b is configured to indicate to a user
or HCP that a
second dose of the medicament has been expelled from the device 100. For
example, prior to
actuation of the plunger 106, the indicator windows 108a, 108b can show a
color such as green.
After a first actuation of the plunger 106, and thus the expulsion of the
first dose of medicament
from the device 100, the indicator window 108a can show a different color such
as white.
Further, after the second actuation of the plunger 106, and thus the expulsion
of the second dose
of medicament from the device 100, the indicator window 108b can likewise show
the color
white. In other embodiments, the indicator windows 108a, 108b can each show a
different color
prior to and/or after corresponding actuation of the plunger 106.
[0041] The intranasal drug delivery device 100 can include other elements. For
example, in
this illustrated embodiment, the intranasal drug delivery device 100 includes
a tip 105 that
extends from an end of the upper segment 104a, which is configured to be
placed in a user's
nostril, a depth guide 110, and a finger rest 112. The depth guide 110 is
conjoined to the finger
rest 112 via an elongated tubular body 114, also referred to herein as an
"outer sleeve." The
depth guide 110 includes a first set of opposing flanges 116a, 116b extending
from a first end
114a of the elongated tubular body 114. The first set of opposing flanges
116a, 116b are
configured to limit the insertion depth of the upper segment 104a of the
device body 102 into a
user's nostril. The finger rest 112 includes a second set of two opposing
flanges 118a, 118b
extending from a second, opposing end 114b of the elongated tubular body 114.
The finger rest
112 acts as a positioning guide for fingers of a user, e.g., the index and
middle fingers of a user,
such that a user can grasp and hold the device 100 while depressing the
plunger 106 toward the
upper segment 104a of the device body 102 using their thumb. The elongated
tubular body 114
includes an oblong shaped hole 120 such that the first and second indicator
windows 108a, 108b
are visible therethrough.
[0042] In general, the packaging kits described herein include a packaging
system and a drug
delivery device, such as an intranasal drug delivery device, housed within the
packaging system.
The packaging systems described herein include a base that is designed to
house an intranasal
drug delivery device in a manner that substantially prevents movement of the
device within the
packaging system during transportation. In particular, the packaging system
can house the
7
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
device therein at a non-zero-angle with respect to the longitudinal axis of
the base. As such,
rattling of the device during transport, which commonly occurs when using
conventional
packaging systems, can therefore be substantially prevented. Further, the
present packaging
systems can be configured to releasably retain the intranasal drug delivery
device therein so as to
prevent the device from being prematurely released from the packaging system,
e.g., during
opening of the packaging system. The intranasal drug delivery device can
therefore be securely
retained within the packaging system until the user or HCP decides to remove
the device
therefrom, e.g., at a point of use.
[0043] In some embodiments, the packaging systems can be designed to orient
the intranasal
drug delivery devices such that a first end of the devices, like first end
101a, is angled down and
an opposing, second end of the devices, like second end 101b, is angled up. In
other
embodiments, the packaging systems can be designed in which the first end of
the devices is
angled up and the second end of the devices is angled down.
[0044] An exemplary packaging system can include a variety of features to
facilitate secure
positioning and orientation of an intranasal drug delivery device therein, as
described herein and
illustrated in the drawings. However, a person skilled in the art will
appreciate that the
packaging systems can include only some of these features and/or can include a
variety of other
features known in the art. Thus, the packaging systems described herein are
merely intended to
represent certain exemplary embodiments.
[0045] FIGS. 2 and 3 illustrate an exemplary embodiment of a packaging kit 200
that includes
a base 202 and a lid 204, which are collectively referred to herein as a
packaging system, and the
intranasal drug delivery device 100 as shown in FIGS. 1A-1C. FIG. 2
illustrates the packaging
kit 200 in a partially exploded view, and FIG. 3 illustrates the packaging kit
200 in an assembled
view in which the intranasal drug delivery device 100 (obscured) is housed
within the packaging
system.
[0046] The packaging system is configured to house and seal the intranasal
drug delivery
device 100 disposed therein. In this illustrated embodiment, the packaging
system is in a form of
a blister pack, in which the lid 204 is releasably sealed to the base 202. The
lid 204 can be
sealed to at least a portion of the base 202 using any suitable sealing
method, e.g., lamination,
8
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
heat sealing, adhesive, heat and pressure sealing, and the like. Further, in
this illustrated
embodiment, the packaging system is designed such that the first end 101a of
the device 100 is
angled down and the second end 101b of the device 100 is angled up (see FIG.
6).
[0047] The base 202 can be created via thermoforming, vacuum forming, or any
other suitable
process. In the case of thermoforming, a plastic film or sheet is unwound from
a reel and guided
through a pre-heating station. The temperature of the pre-heating plates is
such that the plastic
will soften and become pliable. The warm plastic then arrives in a forming
station where a large
pressure forms a base into a negative mold. The mold is cooled such that the
plastic becomes
firm again and maintains its shape when removed from the mold. In instances of
vacuum
forming, a plastic film or sheet is heated to a forming temperature, stretched
onto a single-
surface mold, and forced against the mold by a vacuum.
[0048] The base 202 can be formed of a variety of one or more base materials.
The one or
more base materials can be moldable material(s), e.g., thermoform material(s),
and configured to
retain a desired shape and to be crush resistant so prevent a device disposed
within the base 202
from being damaged, e.g., during transport. In some embodiments, the one or
more base
materials can impart specific characteristics to the base 202, e.g., a desired
water vapor
transmission rate, a desired oxygen transmission rate, a desired melting
point, and the like. The
one or more base materials can be opaque, transparent, or translucent.
Further, compatibility of
the one or more base materials with a device to be contained therein can also
be a factor in the
selection of the one or more base materials. Non-limiting examples of suitable
base materials
include a variety of polymeric materials such as polyvinyl chloride,
polyethylene terephthalate
(PET), a modified version of PET, e.g., a glycol modified version of PET
(PETG), and the like.
[0049] In embodiments where the base 202 is transparent, the indicator windows
108a and
108b can be visible to the user or HCP. As discussed above, the first
indicator window 108a is
configured to indicate to a user or HCP that a first dose of the medicament
has been expelled
from the device 100, and the second indicator window 108b is configured to
indicate to a user or
HCP that a second dose of the medicament has been expelled from the device
100. As such, the
user or HCP can verify that the device 100 has not been prematurely actuated
by simply looking
through the base 202 at the first and second indicator windows 108a, 108b of
the assembled
9
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
packaging kit 200 rather than having to open the packaging kit 200.
[0050] The lid 204 can have a variety of configurations. For example, in some
embodiments,
the lid 204 can be formed of a single layer, whereas in other embodiments, the
lid 204 can be
formed of multiple layers. In this illustrated embodiment, as shown in more
detail in FIG. 4, the
lid 204 includes an adhesive layer 207, a printing layer 208, and a sealing
layer 205 that is sealed
to and interposed therebetween. When the lid 204 is sealed to at least a
portion of the top surface
206 of the base 202, the adhesive layer 207 is positioned between the sealing
layer 205 and the
base 202. Further, the lid 204, as illustrated in FIGS. 2-3, is in the form of
a rounded rectangle
and defines a planar upper surface of the packaging system, and thus the
packaging kit 200. In
other embodiments, the lid 204 can have other suitable shapes and sizes. A
person skilled in the
art will appreciate that the size and shape of the lid depends at least upon
the shape of the top
surface 206 of the base 202.
[0051] While not illustrated, when the packaging kit 200 is assembled, a
portion of the lid 204
is unsealed to a portion of the top surface 206 of the base 202 such that the
unsealed portion of
the lid 204 can be grasped and used to open the packaging kit 200 to access
the device 100
disposed within the base 202. For example, in use, the sealing layer 205 can
be configured to be
removed from the base 202 via a user or HCP grasping the unsealed position of
the lid 204 and
peeling the lid 204 in a direction away from the base 202.
[0052] The sealing layer 205 can be sealed to at least a portion of the top
surface 206 of the
base 202 such that the resulting seal is air-tight, and the device 100 housed
within the base 202
can be protected from the outside environment. Further, the resulting seal can
function as a form
of tamper evidence when compromised, e.g., by the resulting seal being at
least partially
removed from the base 202 prior to the device 100 reaching a desired point of
use.
[0053] The sealing layer 205 can formed of any one or more materials that are
configured to
suppress permeation of oxygen and/or water from the outside environment into
the base 202.
For example, the sealing layer 205 can be a metal foil such as an aluminum
foil, a silver film or a
gold foil, a film on which a metal such as aluminum, silver or gold has been
deposited, or a film
on which an inorganic substance such as SiOx has been deposited. In some
embodiments, the
sealing layer 205 is an aluminum foil.
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
[0054] The sealing layer 205 can be sealed to at least a portion of the top
surface 206 of the
base 202 using any suitable processes. In this illustrated embodiment, the
sealing layer 205 is
sealed to at least a portion of the top surface 206 of the base 202 via the
adhesive layer 207. The
adhesive layer 207 can be applied to the entire outer surface 205a of the
sealing layer 205, as
shown in FIG. 4, or alternatively, to one or more portions of the outer
surface 205a of the sealing
layer 205. The adhesive layer 207 can be formed of one or more adhesives, such
as hot melt
laminate adhesive(s), or any other suitable adhesive(s) that can sufficiently
seal the sealing layer
205 to at least a portion of the top surface 206 of the base 202. In some
embodiments, the
adhesive layer 207 can be heat sealed to a portion of the top surface 206,
whereas in other
embodiments, the adhesive layer 207 can be sealed to the entire top surface
206 of the base 202.
In other embodiments, the sealing layer 206 can be sealed directly to at least
a portion of the top
surface 206 of the base 202.
[0055] The printing layer 208 can be formed of any one or more materials that
allows printing
of indicia thereon, such as the name of a medicament filled in the device to
be housed inside the
base, a fill content of the medicament, a skew number, a lot number
corresponding to the device
itself and/or the medicament, an expiration date of the medicament, the
company name
associated with the device and/or medicament, instructions indicating where to
open the
packaging system to access the device disposed therein. In some embodiments,
the printing
layer 208 can be formed of white paper that can allow for quick ink drying
time and provide a
visible contrast to a colored ink. In other embodiments, the printing layer
208 can be omitted,
and any desired indicia can be printed directly on the sealing layer 205.
[0056] The base 202 can have a variety of configurations. The base 202 extends
from a first
end 210a to an opposing second end 210b. As will be discussed in more detail
below, the base
202 is configured to house and position the intranasal drug delivery device
100 therein (see FIG.
5) such that the longitudinal axis (LD) of the device 100 extends at a non-
zero angle (4D) relative
to the longitudinal axis (LB) of the base 202 (see FIG. 6). The non-zero angle
(4D) depends at
least upon the height (HF) of the finger rest 112 and the end of the plunger
106 that would
otherwise contact the lid 204 if the longitudinal axis (LD) of the device 100
extended at a zero
angle relative to the longitudinal axis (LB) of the base 202. In some
embodiments, the non-zero
angle (4D) can be from about 1 degree to 5 degrees. In one embodiment, the non-
zero angle
11
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
(4D) can be about 4 degrees. Further, the base 202 is configured to
substantially secure the
device 100 thereto in a manner that substantially prevents movement of the
device 100 within the
base 202, e.g., during transport of the packaging kit 200. As a result, this
resistance to
movement can substantially hinder the device 100 from rattling within the base
202, which
would otherwise occur within conventional packaging systems.
[0057] As shown in FIGS. 5-10, the base 202 includes a retaining cavity 212
that is defined by
two conjoined shoulder surfaces 214a, 214b. The retaining cavity 212 creates
an interference fit
with at least a portion of the upper segment 104a of the device body 102 (see
FIG. 5). More
specifically, as shown in FIG. 5 and partially shown in FIG. 6, a portion 115
of the outer sleeve
114, and thus the portion of the upper segment 104a of the device body 102
that extends through
this portion 115 of the outer sleeve 114, is positioned within the retaining
cavity 212. As a
result, the interference fit is formed between the outer surface of this
portion 115 of the outer
sleeve 114 and the two conjoined shoulder surfaces 214a, 214b. As such, the
width (WR) of the
retaining cavity 212 can be equal to or less than the outer diameter (Do) of
the outer sleeve 114,
excepting for dimensional tolerances in manufacturing. As used herein, the
width (WR) of the
retaining cavity 212 refers to the largest distance between the two shoulder
surfaces 214a, 214b
(see e.g., FIG. 9). A person skilled in the art will appreciate that the width
(WR) and depth (DR)
of the retaining cavity 212 can vary and is dependent at least upon the
structural configuration of
the outer sleeve 114.
[0058] The interference fit that is created between the outer sleeve 114 and
the shoulder
surfaces 214a, 214b can prevent movement, e.g., orthogonal movement, of the
device 100 within
the base 202, e.g., during transport of the packaging kit 200. This
interference fit can releasably
retain the device 100 within the base 202 such that the device 100 is secured
to the base 202
during transport and opening of the packaging kit 200, while also allowing a
user or HCP to
remove the device 100 from the base 202 when desired. For example, during the
opening the
packaging kit 200, e.g., when a user or HCP separates the lid 204 from the
base 202 (e.g., by
pulling the lid 204 away from the base 202), the device 100 can remain secured
within the base
202. Further, by having the interference fit between the outer sleeve 114 and
the shoulder
surfaces 214a, 214b, the device 100 can be secured to the base 202 without the
need to grip onto
the plunger 106. This can reduce the chance of a user or HCP accidentally
actuating the device
12
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
100 when removing it from the base 202.
[0059] The retaining cavity 212 can have a variety of configurations, For
example, in this
illustrated embodiment, as shown in more detail in FIGS. 7 and 9, each of the
two conjoined
shoulder surfaces 214a, 214b have a curved configuration, and as a result, the
retaining cavity
212 has a substantially c-shaped configuration. A person skilled in the art
will appreciate that
the configuration of each of the two shoulder surfaces 214a, 214b, and thus
the retaining cavity
212 defined thereby, depends at least upon the structural configuration of the
outer sleeve 114.
As such, in other embodiments, each of the two shoulder surfaces 214a, 214b,
and thus the
retaining cavity 212, can have any other shaped configuration that can create
an interference fit
with the portion of a device that extends through the retaining cavity 212.
For example, in other
embodiments, the retaining cavity 212 can have a U-shaped configuration, a V-
shaped
configuration, a polygonal-shaped configuration (e.g., a square-shaped
configuration, an
octagon-shaped configuration, etc.), and the like.
[0060] As mentioned above, the base 202 is configured to house the device 100
therein at a
non-zero angle. That is, the base 202 is configured to house and position the
intranasal drug
delivery device 100 therein (see FIG. 5) in which the longitudinal axis (Lb)
of the device 100
extends at a non-zero angle (4b) relative to the longitudinal axis (Ls) of the
base 202 (see FIG.
6). This can be achieved for example, as shown in more detail in FIG. 10, by
having at least the
bottom surface 212a of the retaining cavity 212 (i.e., the point at which the
two shoulder surfaces
214a, 214b conjoin) extend at a non-zero angle (4R) relative to the
longitudinal axis (Ls) of the
base 202. As result, when the device 100 is inserted into and secured to the
base 202 via the
interference fit created between the outer sleeve 114 and the two shoulder
surfaces 214a, 214b
that define the retaining cavity 212, the longitudinal axis (Lb) of the device
100 extends at a non-
zero angle (4b) relative to the longitudinal axis (LB) of the base 202 (see
FIG. 6). In some
embodiments, the non-zero angle (4R) can be equal to the non-zero angle (4b),
whereas in other
embodiments, the non-zero angle (4R) can be less than or greater than the non-
zero angle (4-b).
[0061] As shown in FIGS. 5-8 and 10, the base 202 includes a gripping cavity
218 that is
adjacent to the retaining cavity 212. The gripping cavity 218 receives and
houses the finger rest
112, the lower segment 104b, and a portion of the plunger 106 of the device
body 102. The
13
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
gripping cavity 218 includes a first portion 220, a second portion 222, and a
support element 224
that separates the first portion 220 from the second portion 222. The first
portion 220 can be
configured to enable gripping, via digits of a hand of a user or HCP (e.g., a
user's or HCP's
finger and thumb), of a particular portion of the device 100 housed therein.
In some
embodiments, the portion of the device 100 enabled for gripping may be
isolated within the first
portion 220 of the gripping cavity 218, such that the device 100 cannot be
gripped, or would
otherwise be difficult to grip, elsewhere. In the illustrated embodiment, the
first portion 220 is
configured for insertion of the aforedescribed digits of a hand to engage with
opposed sides of
the lower segment 104b of the device body 102 housed within the gripping
cavity 218.
[0062] The first portion 220 can therefore be structurally dimensioned, i.e.,
having a length
(LGO, a width (Wu), and a depth (Du) as shown in FIGS. 8-10, so as to provide
sufficient space
for insertion of at least a single digit on either side of the lower segment
104b of the device body
102 in an opposed manner, whereby the lower segment 104b can be gripped by
said digits (e.g.,
a user's or HCP's finger and thumb) with a pincer type action. In this way,
the user or HCP can
grip and pull the lower segment 104b away from the base 202 with a sufficient
amount of force
that can overcome the frictional force of the interference fit between the
outer sleeve 114 and the
shoulder surfaces 214a, 214b, and thus, remove the device 100 from the base
202. Thus, the
structural configuration of the first portion 220 can encourage a user or HCP
to remove the
device 100 from the base 202 via the lower segment 104b of the device body
102, which can
keep their fingers away from the device tip 105, e.g., for hygiene purposes,
and/or the plunger
106, e.g., to prevent accidental actuation of the device 100. Further, in
instances where the HCP
removes the device 100 from the base 202, the removal thereof via the lower
segment 104b of
the device body 102 can allow for a smooth transition or hand-over of the
device 100 from the
HCP to the user. In some embodiments, the first portion 220 can be
structurally dimensioned so
as to provide a clearance from about 1 mm to 5 mm.
[0063] The support element 224 is configured to support the lower segment 104b
of the device
body 102, and thus the device 100, such that the finger rest 112 is positioned
between the
retaining cavity 212 and the support element 224, and thus the finger rest 112
is positioned
within the second portion 222 of the gripping cavity 218. That is, when the
device 100 is
inserted into and secured to the base 202, the finger rest 112 is confined
between the retaining
14
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
cavity 212 and the support element 224. Since the finger rest 112 is fixedly
coupled to the
device body 102 and the plunger 106 is operatively coupled to the device body
102, the position
of the finger rest 112 within the second portion 222 of the gripping cavity
218, and the
confinement of the finger rest 112 between the retaining cavity 212 and the
support element 224,
substantially prevents movement of the device 100 in a longitudinal direction
(i.e., a direction
that runs along the longitudinal axis (LB) of the base 202). As a result, the
plunger 106 can be
maintained in its unactuated position, and thus premature actuation of the
plunger 106 can be
avoided, while the device 100 is housed within and secured to the base 202.
[0064] Further, the positioning and confinement of the finger rest 112 within
the second
portion 222 of the gripping cavity 218 can control the rotational orientation
of device 100, and
thus the orientation of the indicator windows 108a, 108b relative to the base
202. As a result, in
embodiments where the base is transparent, the interaction of the finger rest
112 with the second
portion 222 of the gripping cavity 218 can position the indicators windows
108a, 108b at an
orientation that is visible to a user or HCP through the base 202.
[0065] The support element 224 can have a variety of configurations. For
example, the
support element 224, as shown in FIGS. 5 and 7-9, has a substantially c-shaped
configuration.
Further, the width (Ws) of the support element 224 can be configured to allow
the support
element 224 to receive and make contact with a portion of the lower segment
104b of the device
body 102 (see FIGS. 5-6 and 9). In other embodiments, the support element 224
can have any
other suitable shape that can support the lower segment 104b of the device
body 102 and can
cooperate within the structural configuration of the retaining cavity 212 so
as to confine the
finger rest 112 therebetween. A person skilled in the art will appreciate that
the structural
configuration of the support element 224 can depend at least upon the
structural configuration of
the lower segment 104b of the device body 102 and the structural configuration
of the retaining
and gripping cavities 212, 218.
[0066] In some embodiments, the base 202 can include one or more additional
cavities for
housing other portions of the device 100. For example, as shown in FIGS. 5-8
and 10, the base
202 includes a first end cavity 226 and a second, opposing end cavity 228. The
first end cavity
226 is configured to receive and house at least a portion of the upper segment
104a and of the
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
outer sleeve 114 (i.e., portions thereof that are not housed in any other
cavity of the base 202).
Further, the first end cavity 226 can be configured to house the distal tip
105 and the depth guide
110 of the device 100 (see FIGS. 5-6). As shown, the first end cavity 226 is
positioned between
the retaining cavity 212 and the first end 210a of the base 202, and as a
result, the retaining
cavity 212 is positioned between the first end cavity 226 and the gripping
cavity 218. In this
illustrated embodiment, the retaining cavity 212 extends from the first end
cavity 226 to the
gripping cavity 218.
[0067] The first end cavity 226 can have a variety of configurations. For
example, in FIGS. 5-
7, the first end cavity 226 has a substantially rectangle-shaped
configuration. A person skilled in
the art will appreciate that the structural configuration of the first end
cavity 226 depends at least
upon the structural configuration of the portions of a device that is to be
housed therein, e.g., as
shown in FIGS. 5-6, a portion of the upper segment 104a and of the outer
sleeve 114, the distal
tip 105, and/or the depth guide 110 of the device 100. Further, as shown in
FIGS. 5 and 6, the
structural configuration of the first end cavity 226 also provides a clearance
between the distal
tip 105 and the base 202, which can help prevent accidental activation and/or
breakage of the
device 100. In other embodiments, the first end cavity 226 can have any other
suitable
configuration that can house one or more portions of the device 100.
[0068] The second end cavity 228 is configured to receive and house a portion
of the plunger
106 (i.e., the portion of the plunger 106 that is not housed within the
gripping cavity 218). As
shown, the second end cavity 228 is positioned between the gripping cavity 218
and the second
end 210b of the base 202, and as a result, the gripping cavity 218 is
positioned between the
retaining cavity 212 and the second end cavity 228.
[0069] The second end cavity 228 can have a variety of configurations. For
example, in FIGS.
5-7, the second end cavity 228 has a substantially rectangle-shaped
configuration. A person
skilled in the art will appreciate that the structural configuration of the
second end cavity 228
depends at least upon the structural configuration of the portions of a device
that is to be housed
therein, e.g., as shown in FIGS. 5-6, a portion of the plunger 106 of the
device 100. Further, as
shown in FIGS. 5 and 6, the structural configuration of the second end cavity
228 also provides a
clearance between the plunger 106 and the base 202 which can help prevent
accidental activation
16
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
and/or breakage of the device 100. In other embodiments, the second end cavity
228 can have
any other suitable configuration that can house one or more portions of the
device 100.
[0070] Further, similar to the bottom surface 212a of the retaining cavity
212, the bottom
surface 228a of the second end cavity 228 also extends at a non-zero angle
(4E2) relative to the
longitudinal axis (LB) of the base 202 (see FIG. 10). As a result, the
extension of the
longitudinal axis (Lb) of the device 100 at the non-zero angle (4b) can be
maintained (see FIG.
6). In some embodiments, the non-zero angle (4E2) can be equal to the non-zero
angle (4b)
and/or the non-zero angle (4R), whereas in other embodiments, the non-zero
angle (4E2) can be
less than or greater than the non-zero angle (4b) and/or the non-zero angle
(4R).
[0071] In other embodiments, the bottom surface 212a of the retaining cavity
212 and the
bottom surface 228a of the second end cavity 228 can each extend at a zero
angle relative to the
longitudinal axis (LB) of the base 202. In such instances, for example, the
non-zero angle (4b)
can be achieved and maintained by the relative heights between the bottom
surface 212a of the
retaining cavity 212 and the bottom surface 228a of the second end cavity 228
with respect to the
top surface 206 of the base 202. For example, the bottom surface 212a of the
retaining cavity
212 can be positioned at a first height and the bottom surface 238 of the
second end cavity 228
can be positioned at a second height that is greater than the first height.
[0072] Values or ranges may be expressed herein as "about" and/or from/of
"about" one
particular value to another particular value. When such values or ranges are
expressed, other
embodiments disclosed include the specific value recited and/or from/of the
one particular value
to another particular value. Similarly, when values are expressed as
approximations, by the use
of antecedent "about," it will be understood that here are a number of values
disclosed therein,
and that the particular value forms another embodiment. It will be further
understood that there
are a number of values disclosed therein, and that each value is also herein
disclosed as "about"
that particular value in addition to the value itself. In embodiments, "about"
can be used to
mean, for example, within 10% of the recited value, within 5% of the recited
value or within 2%
of the recited value.
[0073] For purposes of describing and defining the present teachings, it is
noted that unless
indicated otherwise, the term "substantially" is utilized herein to represent
the inherent degree of
17
CA 03164799 2022-06-15
WO 2021/122991 PCT/EP2020/086766
uncertainty that may be attributed to any quantitative comparison, value,
measurement, or other
representation. The term "substantially" is also utilized herein to represent
the degree by which a
quantitative representation may vary from a stated reference without resulting
in a change in the
basic function of the subject matter at issue.
[0074] One skilled in the art will appreciate further features and advantages
of the invention
based on the above-described embodiments. Accordingly, the invention is not to
be limited by
what has been particularly shown and described, except as indicated by the
appended claims. All
publications and references cited herein are expressly incorporated herein by
reference in their
entirety. Any patent, publication, or information, in whole or in part, that
is said to be
incorporated by reference herein is incorporated herein only to the extent
that the incorporated
material does not conflict with existing definitions, statements, or other
disclosure material set
forth in this document. As such, the disclosure as explicitly set forth herein
supersedes any
conflicting material incorporated herein by reference.
18