Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 440
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 440
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
I
Inhibitors of Transglutaminases
Description
The invention relates to novel inhibitors of transglutaminases, in particular
transglutaminase 2, methods for their synthesis and to their use for the
prophylaxis and
treatment of diseases associated with transglutaminases, in particular
transglutaminase
2.
Background of the invention
Transglutaminases are part of the class of transferases and according to EC
nomenclature they are correctly designated as "protein-glutamine: amine y-
glutamyl
transferases" (EC 2.3.2.13). They link the E-amino group of the amino acid
lysine and
the y-glutamyl group of the amino acid glutamine forming an isopeptide bond
while
ammonia is released. In the absence of suitable amines and/or under certain
conditions,
deamidation of the glutamine may occur resulting in the corresponding glutamic
acid.
Additionally, transglutaminases play an important role in many therapeutic
areas such as
the cardiovascular diseases (thrombosis and atherosclerosis), autoimmune
diseases
(celiac disease, Duhring-Brocq-disease, gluten ataxia), neurodegenerative
diseases
(Alzheimer's disease, Parkinson's disease, Huntington's disease),
dermatological
diseases (ichthyosis, psoriasis, acne) as well as in wound healing and
inflammatory
diseases (e.g. tissue fibrosis) (J.M. Wodzinska, Mini-Reviews in medical
chemistry, 2005,
5, 279 - 292).
Celiac disease, a gluten intolerance, however, is one of the most important
indications.
Celiac disease is characterized by a chronic inflammation of the mucosa of the
small
intestine. In susceptible patients, the intestinal epithelium is successively
destroyed after
ingestion of gluten-containing food resulting in reduced absorption of
nutrients which
again has massive impact on the patients affected and is for example
associated with
symptoms such as loss of weight, anemia, diarrhea, nausea, vomiting, loss of
appetite
and fatigue. Due to these findings, there is a large demand for the
development of a
medicament for the treatment of celiac disease as well as of other diseases
associated
with tissue transglutaminase (transglutaminase 2, TG2, tTG).
The tissue
transglutaminase is a central element during pathogenesis. The endogenous
enzyme
catalyses the deamidation of gluten/gliadin in the small intestinal mucosa and
thus
triggers the inflammatory response. Therefore inhibitors of tissue
transglutaminase are
suitable to be used as active agents for medication.
Another very important group of indications for tissue transglutaminase
inhibitors are
fibrotic disorders. Fibrotic disorders are characterized by the accumulation
of cross-
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
2
linked extracellular matrix proteins. Diabetic nephropathy, cystic
fibrosis, idiopathic
pulmonary fibrosis, kidney fibrosis as well as liver fibrosis belong to the
most important
fibrotic disorders to be addressed with the compounds disclosed.
US 9,434,763 B2 discloses pyridinone derivatives having a warhead comprising
at least
one acceptor-substituted double bond, such as a Michael System, as
irreversible
transglutaminase inhibitors. Alkylacetamido and arylacetamido pyridinones
showed
inhibitory activity regarding tissue transglutaminase TG2 in nanomolar range
(IC50).
Tse et al. (J. Med. Chem. 2020, 63, 11585-11601) report on replacement of
phenyl
residues by non-classical bioisosteres, such as cubane and
bicyclo[1.1.1]pentane (BCP),
in anti-malarial triazolopyrazine compounds in order to alter compound
solubility and
metabolic stability. The authors further evaluated in vitro antiplasmodial
activity of
bioisosteric modified triazolopyrazines against the 3D7 strain of P.
falciparum.
Replacement of phenyl by bioisosteric saturated heterocyclic residues resulted
in
complete loss of activity. Adamantyl residues as well as other hydrocarbon-
caged
derivatives led to potency up to 2-9 times lower than the corresponding phenyl
triazolopyrazine compounds. In contrast, higher potencies were achieved by
replacing
phenyl with closo-1,2- and 1,7-carborane isomers. The authors concluded that
the effect
of non classical bioiostere replacement on biological properties cannot be
predicted
accurately and that a considerable range of possible bioisosteres has to be
tested first in
order to identify a suitable replacement leading to the desired properties of
a given
molecule.
Subbaiah et al. (J. Med. Chem. 2021, 64, 19, 14046-14128) report on
bioisosteres of the
phenyl ring in lead optimization and drug design. It is noted that
bioisosteric phenyl ring
replacement with heterocyclic and carbocyclic moieties can lead to enhanced
potency,
solubility, and metabolic stability while reducing lipophilicity, plasma
protein binding,
phospholipidosis potential, and inhibition of cytochrome P450 enzymes and the
hERG
channel. However, this effect depends strongly on the properties of the
compound itself
and the addressed target.
US 11,072,634 B2 discloses reversible transglutaminase inhibitors comprising
an
aldehyde, a ketone, an a-ketoaldehyde, an a-ketoketone, an a-ketoacid, an a-
ketoester,
an a-ketoamide or a halogenmethylketone as warhead. The inhibitors showed
inhibitory
activity regarding tissue transglutaminase TG2 in nanomolar and micromolar
range (IC50).
The objective of the present invention is to provide novel, most probably
reversible
inhibitors of transglutaminases, in particular transglutaminase 2 and methods
for the
synthesis of said inhibitors as well as several uses of these inhibitors.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
3
Said objective is solved by the technical teachings of the independent claims.
Further
advantageous embodiments, aspects and details of the invention are evident
from the
dependent claims, the description and the examples.
Surprisingly, it has been found that reversible inhibitors having a chemical
warhead as
disclosed herein inhibit effectively transglutaminases including tissue
transglutaminase
called transglutaminase 2 or TG2. Herein these terms are used synonymous.
Preferably, such chemical warhead moiety is particularly selected from
reversible
warheads such as a-ketoamides. The compounds of the present invention act as
selective inhibitors of transglutaminase 2.
In order to prove inventiveness of the compounds of the present application,
reference
compounds were synthesized and tested in comparison to the most similar
compounds
of the present application. A skilled person might notice compound A8 from our
patent
US 9,434,763 B2 which we introduce as Ref. 3 to highlight the inventive and
preferred
features of the claimed compounds. From US 9,434,763 B2, it is clear, that
aromatic
moieties (C-terminal) restrict the efficacy of those compounds (compare to Al,
A8, A37,
A44, A47). In sharp contrast, branched alkyl moieties are highly preferred as
indicated
by more potent compounds (A28, A29, A59, A61, A63, A67, A68, A79).
To illustrate the advantage of branched alkyl moieties over aromatic moieties,
we refer to
reference compounds Ref. 2 (ZED1227, US 9,434,763 B2) and Ref. 3 (A8,
ZED1047).
Inhibition data were determined using the classical fluorescent transamidation
assay
(dansylcadaverine incorporation into methylated casein, DCC-assay) as
described
[Bach Id, C.; HiIs, M.; Gerlach, U.; Weber, J.; Pelzer, C.; Heil, A.;
Aeschlimann, D.;
Pasternack, R. Features of ZED1227: The First-In-Class Tissue Transglutaminase
Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac
Disease. Cells 2022, //, 1667. https://doi.org/10.3390/cells11101667]. Casein
is one of
the best known high molecular weight (24 kDa) protein substrates for
transglutaminases.
Please note, the ICso value of Ref. 3 (A8) published in US 9,434,763 B2 cannot
be
compared to the present data, relying on a fluorogenic isopeptidase assay.
Measured
in the DCC-assay, Ref. 2 (ICso = 53 nM) is 80-fold more potent compared to
Ref. 3 (ICso
= 4,268 nM).
Accordingly, a person skilled in the art of medicinal chemistry would choose
branched
alkyl moieties as lead structures, such excluding aromatic moieties, e.g. the
phenyl group.
It is of common knowledge, that bridged cycloalkyl groups are non-classical
bioisosters
of the phenyl group. By replacement of the phenyl group in A8 by e.g. an
adamantane
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
4
group, a skilled person would expect similar physico-chemical or biochemical
properties
excluding to invest efforts. Since aromatic moieties are clearly not
preferred, bridged
cycloalkyl groups would not be considered improving the compounds.
This is further supported by additional reference compounds. ZED3641 (Ref. 1,
as
disclosed in US 11,072,634 B2; reversible acting a-ketomethylamide analogue to
Ref. 2,
ZED1227) is about 15-fold more potent compared to Ref. 4 (compare table 1).
Ref. 4 is
analogous to compound A8 disclosed in US 9,434,763 B2 with respect to the
backbone
proving again superiority of branched alkyl moieties compared to aromatic
derivatives in
combination with reversible acting warheads.
However, surprisingly, replacement of the preferred branched alkyl moieties by
bridged
cycloalkyl groups further significantly improve the potency of the compounds
as shown in
table 1. Therefore, we credit bridged cycloalkyl groups as disclosed with an
outstanding
inventive manner.
In summary, the inventive compounds rated "A" show efficacies of about 30-fold
higher
compared to Ref. 3 (A8, compare to table1).
Further, compounds with activities rated "B" or "C" are still preferred (lower
ICso values)
to Ref. 3 (A8). These compounds can also be considered inventive, since
peripheric
ligands affect physico-chemical or biochemical properties. Therefore, also
less potent
compounds might be of high value, depending on the application.
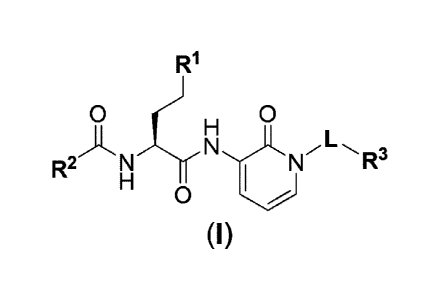
Thus, the present invention relates to compounds of the general formula (I):
R1
0 0
H J (I) CN LR3
R2 N 1 N
Ho
wherein
L represents ¨L1-L2¨;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨,
¨CH2C0¨, or
¨CH2CH2C0¨;
L2 represents a bond, _NRN1_, _NRI41CH2¨,
¨NRI41CH2CH2¨, or
¨NRN1CH(CH3)¨,
0
R1 represents
0 -
,
R2 represents
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
5
RN
- _. 0 Rs R11 _R8 -._ S Ra RzvS R8 ---
11 R8
\ / lr ,\ r
Rlo R9 ; / R9 , R10 R9 ; R9
RN R9 ;
R10 D9
;
RN RN RN RN RN RN
R11 N R8 R11
RN
N, R11 N, _.. N, R11 N
_ - ii
0.8
i ___________ r --14N I iN
i er.
N N
Ro Rlo s, / R9 Rlo R9 Rlo
, / ,
RN Rio Rio
s ii 0 - 0 pp8
_____.(, .)7_ _ Rti_.(õ\,0,R8 --1 __--..
)-\.-
\):IR8
R
0
N N ,\ __ N N N
Rio Rio , Rio R8 N '= , , ' , ,
RN RN
sR8 _..1SRa -,<N ,N
Rio R11 S R11 N,
R
I ,N
) __ N N N N
N-r-CR8 Rio , Rio Rio ,
, , , ,
RN RN
,N, ,N, io RN
-__ , R12 R12
-------------------------------------------------------------------------- )
vl
N N N , N, R N /') R13 ------------- R=-
\ _________ i( ir Iii,N --\- N----
u,,,,,_<,\,;j
,
, Rio ,,\¨N ; N-N ; N=N ; R14
N Rizt
, ,
R12
,
R12 R12
N R12 , R12
1 q
R.- r
.- -\ i
Rio_tsr>R13
N
\N \- R
R.4
C)---4 14 ;
N R14 N Ria N= R14 Rio
,
RN
Riz R12
Rii
R12 S-----/Y,) 13 \% R12
i\I /') 13
---\ TR ---
TR
R104------r/ - \R14 R10_trnR13
C)---i13 R10 S-----\44 R10
R14
, RN
,
R12
R12 R12
Rlo / N---/y,) '-/ 13
RN , R1."A R14 -------/".>7,,
N
R13
__
N----- \/\\I-R14
, ,
,
7R
R13 N,- Rii ,,
RN RN
R14
ICI R9
N,R9
Nli ----___Ri4 ..(cDp9
i" [ / ,I R10 N \J
Rio N s R1O - \ -7----R10 RN'
Rlo
N'S ,
,
,
RN R8 R8
,S --
R9 D.....:,9 ,S - - ),_ - N 1
1_1_ R o1R9 N\\ I N''' s: ----7-
o)/¨R10 cr...,NR10 N R8 \s_...N N-_-_-N Rs
1 0 ,
R8
a ,s - ,,,-- . N - - 0 S
R'-'-----\ il N6- R'a N ---- -7 Ru----
N---N ; b-N , N-0 , o-N , N N
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
a !`g
Fri 6
-0
X cD
, / ,' I I I I
I
,c) m , , , ' ,
c m i
,
CD 2
a .,-0- z \-0 2Z
r)No) (,(j) ,r-z CZ-Az z/iz ----- /Cr)--Z---- (Z----
o) H
/Z
rEP 2 \ / z--( Z zj z
z /( 0 --------// \ ------;"---cn µ___-_ z
i
CD = M \ Zo / Z, \ /Z Z / \ Z cZ vC) Z N
\
CI 0
CD 20 Z Z _ V 1/ _
CD
0- N,
N) *
0
0
N)
i
N) ,r1
C)
clo , , , ,
,
0 I 1 1 I
I z
_ \
z_.
2
z' -
07 2Z N cn N c/37 ,(, - zi ¨ \ C z
, , , /
¨ ¨
¨Kz
\ /
z
cz----- e-----z
--z
\ //z \ / \z
z-z z z-0
.. _________ .. ..
.. .. ..
..
..
I ,' ,
I I I I I
Cr)-Z
(z
________________________________________________________________ z \( z--(/
z z ,' 0 z
I
' / Z¨K Z
¨ _
0
-
-
/ \
// \ Z/ Z/ \Z ¨/ \ / ,
,
I '
1 1 ,
1 i
1
i
- ____________________________________ Z
0_ z --
/ Z \
0 N 1 ,('-zi 1 Iz, z
Z Z Zj Z z
Z "cn
i - C z----
cn
_ Z----Cn
_ Z
-
_
-
7
_
1 I
--. ---;---õ,----
N N N N
' , ,
-,
'-IN 'r1;1 --Ir
N, N NN,
,
-.fNI\I -, N N
1 f -
N N ,
N I\I NN
N N
'
-,,NN,N
1
N N
, ,
1
,
,
-,,NN -'NN -,,NN
N 1 II
1 N N N NN ,
, ,
1 II
NN N1,N N N
,
. N. -, -,,N,N
N'N,
N 1
1\1
, ,
N,
''' 'NI -I
N9 N1\1
, N
, ,
'r 1
NN , Ne , NN
,
''IrN
1 i\ii
N
N N , NN NN
, ,
NN NN-,N --N
, NN, ,
I
N,NN Th N,NN \1N ,
, ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
8
H
NH
HN NH ,
,
, ,
.,
N NH ,
HN , NH, H,
N=\ 0 N=N N=\ N-
- - /(1\1
- -N
---0
\ N
N/ \ " ,N
,
,
'
N¨ N¨ N¨ ¨N ¨N
- - \ - - \ - - \ /N - - -_
/ N \ ,N
N N ,
, ________ ,
/¨N ¨N ¨N/¨\ /¨\
( / N - - -\ sN
- -
________ - - / N \ /N N , ________________ ' , N N ,
, ,
i ¨ \
N N
N _________________________________________________________
N=\ N=\
- - -- /(N
\ / N\/ N / N N N
N N¨N , N // N
N=N N=N N=N N=N N=N
- ______________ - \ /N - - - - - - - - -,
\ N /
N N
,
, ,
N=\ N=\ N=\ N=\ N-
- - /(1\1 - - -,\__. / N - - __ /N __ - - /N -
-
\ /71 , \ \ N N \ ,N
N N ,
,
N¨ N¨ N¨ N¨ N¨
- 1 ) 1 1
N N N N\/ N /
N¨ //, N¨N N N
, , , ,
¨N N Ns ¨N ¨N
,
\/N , \ \ N \ ,N
N N N , , , ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
9
/-N
--- /
\ N
N/ N //N
N-N N N __ ,
,
, , _________ ,
-\/-\ -\ -\ -\
--- N -- --i --- --i
/ - ( /N ( /N (
\ SN N
N \ N1 \ , N N N
//
, '
- \
0 .
Ns /
Nss \ //N N N
// Ns N N-N
,-
N , N-N , N N-// ,or
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 - R14, and R" and preferably with 1 to 3 of the substituents
R11 -
R13;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl, diem
antyl,
or hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one or more C=C double bond and/or are optionally substituted one or more of
Ra,
Rb, Rc, Rd, and Re;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CON H2 ,
-CONHCH3, -CON(CH3)2, -CONHC2H5, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H5, -CH2CONH2, -CH2CONHCH3,
-CH2CON(CH3)2,
-CH2CONHC2H5, -NHCOCH3, -NHCOC2H5, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C2H5, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 represents -CH2CH3, and R7 represents -H;
R9, R9, R19, R11, R12, R13, and R14 represent independently of each other
-H, -F, -Cl, -Br, -1, -OH, -CN, -NO2, -CH3, -C2H5, -C3H7, -CH(CH3)2,
-C4H9, -CH2-CH(CH3)2, -CH(CH3)-C2H5, -C(CH3)3, -cyclo-C3H5,
-CH2-cyclo-C3H5, -CH2F, -CH F2, -CF3, -CH2CI,
-CH2Br, -CH21,
-CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21,
-CH2OH, -OCH3, -0C2H5, -0C3H7, -OCH(CH3)2, -0C(CH3)3, -0C4H9,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
10
-OCHF2, -0CF3, -OCH2CF3, -0C2F5, -OCH2OCH3, -O-CyCIO-C3H5,
-OCH2-cyclo-C3H5, -0-C2H4-cyclo-C3H5, -CHO, -COCH3, -COCF3,
-00C21-15, -00C3H7, -COCH(CH3)2, -00C(CH3)3, -COOK -COOCH3,
-CO0C2H5, -CO0C3H7, -COOCH (CH3)2, -COOC(CH3)3, -00C-CH3,
-00C-CF3, -00C-C2H5, -00C-C3H7, -00C-CH (CH3)2, -00C-C(CH3)3,
-NH2, -NHCH3, -NHC2H5, -NHC3H7, -NHCH(CH3)2, -NHC(CH3)3,
-N (CH3)2, -N(C2H5)2, -N(C3H7)2, -N[CH(CH3)2]2, -N[C(CH3)3]2, -NHCOCH3,
-NHCOCF3, -NHCOC2H5, -NHCOC3H7, -N HCOCH (CH3)2, -NHCOC(CH3)3,
-CONH2, -CONHCH3, -
CONHC2H5, -CONHC3H7,
-CON HCH (CH3)2, -CON H-cyclo-C3H5, -CON
HC(CH3)3, -CON(CH3)2,
-CON (C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2, -CON[C(CH3)3]2, -SO2NH2,
-SO2NHCH3, -SO2NHC2H5, -
SO2NHC3H7, -SO2NHCH(CH3)2,
-SO2NH-cyclo-C3H5, -SO2NHC(CH3)3, -
SO2N(CH3)2, -SO2N(C2H5)2,
-SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C2H5, -NHSO2C3H7, -NHSO2CH
(CH3)2,
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
N
r
--Nn rTh N/N rN1 r rN
r N r N
/ _______________________________________ \ / __ \ / \
N\ /NH N\ Or N\ \
or R8 and R9 or R9 and R1 can form together one of the following five-
membered
or six-membered rings:
õo
-- , N
-
or ---%N
or R12 and R13 or R13 and R14 can form together one of the following five-
membered or six-membered rings:
0
.0 ,0 ,0
''N N
'0 H or I
R" represents -H, -CH3, -C2H5, -C3H7, -CH(CH3)2,
-C4H9,
-CH2-CH (CH3)2, -CH(CH3)-C2H5, -C(CH3)3, -
cyclo-C3H5, -cyclo-C4H7,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
11
-cyclo-CsHo, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-05H9, -CH2F,
-CH F2; -CF3, -CH2CI, -CH2Br, -CH21, -
CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C2H5, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -CO-cyclo-C3H5, -CO-cyclo-C4H7, -CO-cyclo-CsHo, -COOCH3,
-CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -S02C2H5, -
S02C3H7, -S02CH(CH3)2,
-S02-cyclo-C3H5, or -S02C(CH3)3;
rc -N1
represent -H, -CH3, or -CH2CH3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomer, a racemate, a solvate, a hydrate, or a pharmaceutically acceptable
salt
thereof.
The inventors have found that the reversible inhibitors of formula (I)
disclosed herein
having a bridged bicyclic residue R3 exhibit increased potency over the
compounds of the
prior art. Particularly, it is demonstrated herein, that the inventive
compounds have an
improved inhibitory activity compared to the known compounds bearing aromatic
moieties
R3 instead of bridged bicyclic residues. In order to prove inventiveness of
the
compounds of the present application, known compounds from US 9,434,763 B2 and
US 11,072,634 B2 (Reference 1 (E16 from US 11,072,634 B2), Reference 3 (A8
from US
9,434,763 B2), and Reference 4) were synthesized and tested as reference
compounds
in comparison to the most similar compounds of the present application. To
this extent,
inhibition data were determined using the classical fluorescent transamidation
assay
(dansylcadaverine incorporation into methylated casein, DCC-assay) as
described in
Buchold et al. [Buchold, C.; Nils, M.; Gerlach, U.; Weber, J.; Pelzer, C.;
Heil, A.;
Aeschlimann, D.; Pasternack, R. Features of ZED1227: The First-In-Class Tissue
Transglutaminase Inhibitor Undergoing Clinical Evaluation for the Treatment of
Celiac
Disease. Cells 2022, 1/, 1667. https://doi.org/10.3390/cells11101667]. Casein
is one of
the best known high molecular weight (24 kDa) protein substrates for
transglutaminases.
Inhibition data of the inventive compounds was compared with inhibition of
compounds
disclosed in US 9,434,763 B2, particularly, compound A8, which is denoted
herein as
Reference 3. It is noteworthy that the ICso values of compounds A8 published
in US
9,434,763 B2 and E16 from US 11,072,634 B2 cannot be compared to the present
data,
relying on a fluorogenic isopeptidase assay.
Thus, the inventive compounds of formula (I) rated "A" showed efficacies of
about 100-
fold higher compared to Ref. 3 (A8). The same argumentation applies to Ref. 4.
Ref. 4
shows also that aromatic moiety of this reference comopund reduces the
inhbibitory
activity against TG2 strongly.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
12
Accordingly, a person skilled in the art would exclude aromatic moieties, e.g.
the phenyl
group. It is of common knowledge, that bridged cycloalkyl groups are non-
classical
bioisosters of the phenyl group. By replacement of the phenyl group in A8 by
e.g. an
adamantane group, a skilled person would expect similar physico-chemical or
biochemical properties excluding to invest efforts. Since aromatic moieties
are clearly not
preferred, bridged cycloalkyl groups would not be considered improving the
compounds.
Surprisingly, the inventive compounds demonstrate that bridged cycloalkyl
groups
improve the potency of the compounds by orders of magnitude compared to the
aromatic
reference compounds. Therefore, we credit bridged cycloalkyl groups as
disclosed with
an outstanding inventive manner.
In another set of preferred compounds
R2 represents
RN
Rii 0 Ra -__ S R8 Rii \ S R8 --
II R8
- -(
Rio R9 ;
' R9 Rio R9
RN RN RN RN
N 1
Rii 0 __ wIi ON_R8
Rii N R8 Doi N, Rii N, __ N, ( lµi /1\1 i /N
/ ( --1K \I ir
N
/ R9 R10 µ, , R9 Rio R9 R10 ,
, ,
,
R10 R10 µi
R10 Ril S _ R11 S R8
Rio
?!riR8 cl -
N N¨ ) N __ N R8
,
RN RN
RN RN
S 8 -, N ii ii ,N, ,N, _R RN
R N. < ,N wõ...),õ ,N N N N io N.
\¨N
Rio ' nio , __ N ' \Rio / N¨N
N=N
, , ' , = '
R12 R12 R12 R12
N R12
N r
-------------------- -Ri3 Ri3 Ri3 Ri3
L.1 xj \-) \N \-) \-)
------R14 N R14 N R14 N R14 N R14
,
,
R12 R12
' Rii
R12 R12
---TRL5
R10R13
R14 ¨ \-J R14
Rio 0---"\-114 0- 21-113 R10
; ; rk ; ;
RN
pp12 µ, RN
, Ri2
, R12 i\iy4'
/ 1\1---__/y
Rio_tr/R13 ---TR13
N --- 1 \ RN
s-----1414 Rio R1,1 Rio
R.,
, R13 '
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
13
R13
R12 Ri2 R11
s,
,,,------.õõ...---:-/,,,,,
- 1 jL _ R13 ), Nv----R14
Rioill-- R., 1 A
S
R14 R14 Rio N __
N S
RN
RN RN
0=e ii R9
9 rN-/IR9 r Y1 y I_ . _ .R 9
R10 m R9
r_R
Rio
R10 ,l' --/- R10
- \____/-----R10 L...,.,.,_....) ,--, rµNr"
N----
-
, ,
R8 R8 - - R8
- /s1 -
NI \ 1 N N p8
s)- S--7-1 - - Ni RsN ..
\N R8 \s-N \N----N Rs N-N b-N N---
' , ,
N 0
R8--- I- S - -
- -
O'N N N
_yN
- - -eNNH - - -eNNH N
- - A ¨_<N3
N _____ (
NN
- N N __ \(
S S 7
S S z c N
---eNN H - - -eNNH
IIN , NJN __ (
0 ONJ
,
'
_N _ N
- - -\ - -\13 N - - -eN0
N N __ \ (
0
IIN , NN3
0 7 , c N N
, ,
N
H S
-K7/ -II,
- N N Ns
H ' H
'
S-_-N _ _7,__--/ ___N S __ _---:--- \N
\S-N - - -____ -- N
N S' N
, ,
______n N 0 H
N __ / -----/ _ -__ri
- -CT-)
\ N -NI/ S'N'N N / N-0 , ,
,
__________ 0
_ _ _e---- - /0---N, ....._N
\ -N - 7
N-N ) N 0 - \--N-1 - - -<71---O-N
H H ,
, ,
0 -,.. N N
*4
,
1 I I
N
/ N
, N -, --
' N
, / N
-- - N
-. , -, ,N
1 II
N 1\r N
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
14
_
I -N
-I
NI,N / , N
, ,
-, N -, --
'N 'N
N N
N
,
-,,I\I N -,,NN
NA 1
,
- N
N I 1
N N
N
1 N I
N N N N
,
N
I
N N- NN ,
,
,N N
II -, N N
1 f -
1 i\il
N
Nõ," N N
,
-,,NN,N
N 1
N
,
1 I
,
,
-,,NN -''NN -,,NN
1
N 1
N II
N,,, ,
1 N f
N--1\1 NN ,
N
, ,
-,,N, -,,rN -, N
1 II
N1,N ,
-, N. -, -,,N,N
NA
1
N ,
N N
, ,
N2 N A Nr N,
N, ,
ir 1
NN , NN , NN
,
'-
'-IN
1 F\11
N,N
N-, N
N
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
15
I I I
N N , 1\1N NN
--1\11
I --1
N.N
, N1\1-N
, ,
I I
1
N.NN N N.NN N ,
H
NH
HN NH
, ,
.,
N NH ,
HN , NH H , ,
N=\ 9\ N=N N=\ N-
--- /(N
--N
)''---0
\ /N \ ,N
" ,
N¨ N¨ N¨ ¨N ¨N
/N -- \
--- / N ,N
N N _____________________________________
,
/ _________ N ' ¨N ¨N __\
/(N-- __________________________________________
/ N \ //
________ N 1\1
N , ________________________________________ ' , _____ N ,
, ,
-- \ /\N
\ ¨\
--i /N
I\1 , --
\ N --
\ N
N -- .
N N
N N
,
N=\ N=\
--- /(N 1 /N
\ / N\/ N / N N N
N N¨N N // N
, ,
,
N=N N=N N=N N=N N=N
--- ____________________________________________ ---_/ --,
\ ,N /
, _________ // , _____ N, N ,
,
N=\ N=\ N=\ N=\ N-
-- /(N ---. /N --- /N ---
\ /71, \ \ _______ N \ ,N
N N N ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
16
N-
--- ______________________________ --- _____ \ _______ -- ---
\ N
N N --- / N / N, /
N/ //
, N¨N N N __ ;
, ,
¨N ¨N ¨N ' cNs ¨N
N N /KN
1
\ /71, \ _______ 1\1 , \ ,N
¨N ¨N /¨N ¨N ¨N
--- --i
\ \
--- /
\ N N N N / N, /
N/ // N¨N N __ N ; ,
____________________________________ ,
¨\ ¨\
i¨\
,N N \ N
N , N=/ // N¨N N
,
__\ 0 =
- -- -i /N
-- --
N, \ N N N
// N, ,N N¨N ;
N , N¨N , N sINI/ , or
'
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14, and RN; and preferably with 1 to 3 of the substituents
R11 ¨
R13; and the substituents R9 ¨R14, and RN have the meanings as defined
informula
(I); and more preferably R2 represents
RN RN
0rR8 R11 0 R8 ____õ,s, _R8 R11 s R8 __ R8 Rii ,, R8
\ / (- \ , \ , \ ,
Ric) R9 R9 R10 R9 R9 Rio R9 ,' D9
r` ,
RN RN RN
Rii N,k. RN N, R11 0 __ R11 0 R8 -- -
)" R8
I /(1 4
--121(\1 --\__, I(\ I ----- \ //
) _____________________________________________ N N N
Rio ss R9 Rio R9 Rio Rio
, '
R10 Rio
,R10 Ril SN Rii S
O\ Rs -----SNR8
--
CaR8 )\ /7-
N 1 , //
N N N N
R8 N¨ µ R8 Rio Rio
' ' , , , ,
RN
N RN RN RN R12
___________ 'NI
N Rii N, ,N Rio
------ ,N 1\1\¨ri . N, N, D N
- ---'- N''`
/-)
LI.,.,.õ,\,:j R13
) ______________________ N
Rio N¨N ; N=N , D14
,
" ,
R12
R12 R12 R12 N R12 0---__//
R13 /`) R13 --------------------------- N/-)
R13 R13 ---),IRA 13
\N \-)
N R14 N R14 N R14 N R14 R10 R.,
, ,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
17
RN
R12 Ri2 RN
S---.../Y,) _ N-....//,
1 i D13 N,/Y,)
R12
R12
--- .,
R14 R14 N-----' ,A \jR
Rio Rio R., R14
R13 RN RN RN
R12
\
_______________________ N71-.õ).___Riit N, R9 _ Rio
,NI ,R9
II 1 R13 9 f_ '
4. 1 _____ S r-ii R
r /1 Rio
R1._ R1._0
, ,
- R9 R9 p S
,I\I /¨R1 0--/-m, 1
__`-,N----- 1-µ N N
,
'
Icl 0 s ---eNNH
\ \ \ __________________ N (
, S
,
---eNNH
-- A - - N -(3
N N \(
,
SNJ
S S y N
NH ---eNNH
IIN , NNN3 N __ (
0 ONJ
-- A - N N
\(
6 y 11 Ni
o N3 N , N , NN
,
H N,--S N __ ___(//-1-
N-N ___________________________________________
H H
S--...._-N ----r-N\ S ___(-1-'-\---N
---____ ---- N
N,...(/
,
H
S-N-N/
0 ___,----µ 0,..--N / N
- Cf--
r-N
H H , ,
,
0,N (----r-N\ 0
N 0-N-N/
,
i ..
1 I I
N /
N,
,
'N
, N
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
18
- - - N
-, , -.N
1 II
N N N /
.,
-, -,
I N'N N
-I
N,N / , N
, ,
-. N -,
'N 'N
N N
N
,
.,
-,,NN -,,N ,N
1 N I 1
N N ,
I
N --.N % --- N N----õ,..-- ¨
, , ,
, --
1rN 1(1\11
N , N NN ,
,
-- -,,N N -, N N
f -
N N,,, N NN ,
,
-,,NN
- ---- -:-.,--- -:,1
Il I 1
N
N,,," N
N , -N
, ,
-,,NN,N
1 1
N N
,
1 II -,,NN
1
N,,,
,
NN -'''NN -,,NN
I
N 1
N II
N ,
- N
I 1 f
NN ,
-,,N -,fN -, N
1 II
NN NN N,N
, , ,
N,N -. N,N
''I N'N
N,
N I
N N-"N NN
' , ,
Ir 1
NN , NN
N N
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
19
- -
-'1rN
N.N
I I
N N , 1\IN NN
I -'1
' ,
--,r1
I -,,NN
1
N.NN N,NN
NN
H
NH
HN KNH ,
,
.,
N NH ,
HN H , NH , '
N=\ 0 N=N N=\ N-
--N
---0
-- \ /
\ N
\ ' //N
,
,
'
N- N- N- -N -N
/N -- \
/ N
N _____________________ N , ,
/ _________ N ' -N -N _ __ \
-- -- \ -1\-/(\N ----\
/N
1 N \ /71
N, N N
,
,
-- \
\ /N
-
-\
--i /N
N\\ , --
*
N N
N _________________________________________________________
,
N=\ N=\
-- --
i /(N --- /N
\ / N / N / N N N
N
'
N-N \IV \\ // N
, ,
' ,
N=N N=N N=N N=N N=N
-- \ /N ---, -- -t _____ -- --,
\ ,N
N N
,
' ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
20
N=\ N=\ N=\ N=\ N-
-
(
\ //N, \ S \ ) N N /)', \ ,,N1 N
,
N¨ NI/Z/ N //N
N¨N N \ __
,
,
1 Z(
N\\ N N N ,
- - ______________ -- i
\ \
-- \\ // \
\ N NN \ / N / Ns /
N¨z/ N¨N N µ1\1 __ ,
¨\ ---7\NI - - -- -, --i \
z(N z(N z(N
\ __________ NN, N N
N/ * __ //N N
\
, , ,
_ __________ \
- - Ns /
N s \ ,,N1 N N
// Ns N \N¨N ;
_____________ , N¨N , ' N sINIZ ,or
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and RN; and preferably with 1 to 3 of the substituents
R11 ¨
R13; and the substituents R9 ¨R14 and RN have the meanings as defined herein.
Preferred; the unsubstituted bicyclic residues which can be substituted with 1
to 5 of
the substituents R9 ¨ R14 and RN; have the following structure and the
substituents
R9 ¨ R14 and RN have the meanings as defined herein:
R11
RN R11 R11 R11
i\I R12 0 R12 S R12 - - ..eN - RN
Rio \ Rio \ Rio \ N __ (
R13 R13 R13 S
Ria
R13
' ' Ria , Ria
, , , Riz
,
Rii Rii
N Rii
- - -NNI- RN - \
N N
N
S.NRii R13Y 7 R12 N
\(
rC----
Riz R13 R13-ez N
Riz , ; , R12,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
21
R11 R11 R11
R11
-----eS ----N-RN ---N-RN
---eS
N __________________________________________ (
N ,
N/N---\
N Riz R130 0 R12
R13 N R12
' R13 R12 ; R13
' ,
R11 R11
N R11 ____NN R11 R11
Z 0
R134,y, ,c) 6 7 Riz N \(
N _________________________________________________ \ N,13..._
N
R12 , N R13 Dp,13/N, ,L12
N Riz
" N '`
' R12 ; R13 ,
RN R11 R11 R11
N R12
0 w2
S Riz
--- --- N R13 N R13 --4 N
R13
R14 Ru R14
,
RN R11 R11 R11
N R12
0 Riz
S R12
R13 R13 R13
N S
Rio R14 R10
R14 R10
R114
, ,
RN R13 R11 R1 R12
,r¨ \
-r-S/> _________________________________________ R 12 -_ / N-N \
iq
R-
--¨N R NI-N1 -S
Rii R12 ; --S
R11 RN. R13 ; RN
, ,
R11
R13 R11 R12
S------N
_-N ,e R13 __ R N-... ---------1\1 12 ---$SX(N
S'
___--------:N
S-1\I ./( IN/(
wi w2 ;
R13 ; Rii R12 R13 ;
,
R13 RN R13
R11 12 N-0 \
R13 N
/ S-NN R13
-N R11 R12 ; 0
-
R11 ; R11
R11 R11 R12 R11
-- &:1 R12 R-- --------- 13 1--Ne R13
R R12
--
NO - 0-N />
R12
N.0 ii
RN1 R13 RN ; R13 ;
;
R11
0,N R13
R11 R12
- __________ - N ____e R13 _"----T_____N 0
i , __ R12 -- -0 __ R12
0-N / /
RI 1 R12 ; " N 0-1\LN
R13
R13 ; Rii
' ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
22
R14 R10 R11 R9 R14
-, N R13 . R12
I
,
1 I
R12 R9 N N R13 Ri2
R9
Rlo Rii R14 Rlo Rii
R14 R14 R9 R14 R9
R9 -, N
N IA
N
R10 R10
R13 R13 R13
R12 R11 R12 R11 R12 R11
' , ,
R12 R11 R14 R13
R10 - N R13 -, N R12
-.
1 il
R13 N R' Rlo N R12 N R11
R14 R11 R10
, , ,
R10 R11 R14 R14
Ri2 NN
. -, -, Nr R9
III
N.N
R13 a N
R-
R12 R12
R14 Rii Rlo Rii Rlo
' , ,
R14 R14 R9 R14 R9
-, -, -,
N-R9
N N
, N R
N N R.-
in
R12 12 R12
RU Rii Rlo R11
,
, ,
R11 Rlo R13
- N N R13
-, R9 ----, ---,-.-.--- -:-.---.---- -.,N
I 1 N
---- 1
N-,N R9 R12
---'" / 10
R.-
R13 Rlo Rii R9
R14 , Rlo iv
R13 R13 Rlo
- NR12 - NR12 -,N R11
1 1 _...., 1
R9rN R9------,r---V---R11 R9--'-N---R12
R10 R11 R10 R13
, , ,
R10 R11 R10 R11
R10 R11
R12
''--1 N , R12
I I
R9-----Nr-M-AR12 R9-1\1-N I
R9-N N R13
R13 R13 '
, ,
R9 R9 R11 R9 R11
N,R11
-'Nr1 N Ri2 ..
, --..- -,.., -,..,
I I
N R._ 1 9 N R._ 1 9 NN
Rlo R13 Rlo R13 Rlo R13
,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
23
R9 R13
R -,f NY NY R12 -,f NY NY R12
--.., --.., .=
I N N
N---,r-N--2----Rii
Rlo Rlo R11
R10 , ,
,
R12
-,,N N N N R12 -----, N-----.----Ny- R12
-- ---, --,--.-.-----,-.----
' I ,
II I -----õN:.---õ,ff-- N
N N
'rN R11 R1o7 NR11 R10
, R11
R10 ,
,
- N N, - N N R12 - _ , N ,N , R12
N-----, --,---.-.--- --:----- ----, --,--- --,---
R9
9r-r1 N I ,,, - --- R12 R R9------r-e---- R11
R10 R11 R10 R11 R10
,
R12
- N N R13
1 -,f NY NY R13
-,,NN
R10iNI R12 N R12 1 ,
7-õ-- N
Rii Rio Rii R9 y--.1
, , Rlo R11 ,
R12 R13 R13
-,,N)Ni -, ,I\IN ,N )N
1 1 1
R9 -N R" Rlo R12 Rlo y......õ1õ--
N y,.....õ1õ--
y N R12
R10 R11 R11
, , ,
R12 R13
R12
- N,,, Ril N R11 -. N R12
-
--- ,
I I f
R97 r-rN N--N Rlo N ------. -------õ,,- N
N
Rio , Rio Rii
R13 R13 R14
-,NN ..,_ R12 -,,N R13
- N R12
-_,-- õ,.. -,..õ. II II
1
R1Or NN,
NrN R ------7"--ii
NNR11 R , R10 R11 12
R13 R13 R12
-, NN _ N
1 1
N-----. --------1,'
R12 Ri2 N R1 Rii Ny<-.R9
R11 1-110 R11 R10
rk , , ,
R12 R13 R10
N. R10 ,,,,, NõR11
-. -, N -
I I
N
R12 N: N
R9-NrN
R9
R11 R10 R11 R12
, , ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
24
R10 R10
R10
R11 Nri\I R11
-, -,,N
N R11
_
I--,---'-c---, -=.---.-- I
NN I 12 N ----..T.7"-
N%----R12
R9-N N R
R9 R12 ; R9
R10 R11 R10 R11 R10
N R11 `. ,
'N '-'. N
I
,N 1 .
N-NR12 Ri2 N- R12---N----"-yN
R13 R13 R9 ,
, ,
R10 R11 R10 R11
R10 R11
I . I
1
NN N R'` NNR12
..-_,.:L ,
W N
R9 R12, ; R9 ,
R10 R11 R10 R11
R10 R11
, N R12 R12
-' r-
I - I
R13N NN-,N R-
1\1-"N
R13 ; R9
, ,
R10 R11
R10 R11 R11
R.,
p12 `.,N
¨ ,
¨ . 'N
N,NrN I 1
N,
N N R1¨, Rio N N R'`
R13
'
R14 R13 R12 R13
R14 RN R14 R9
R14 ,, N R9 -,
N_RN
RN¨N Rii R11 N¨RN Rio Rlo
R13 1,, R13
R ¨ Ri.i . R12 R11
R9 Rio ; Rio R9 ,
R14 R13 R12 R13
R12 R11 R12 R11
__ R12 _ - R14 . R10 . R10
R9 R11 R11 R9 RN
N R9 R13
/N
N R14 RN R14 R9
RN R10 R10 RN
R9 0 R9 R9
N_( ---(:) N=N
9 =( N¨i
-- \ /N--N R14 -- \ /R - - N
\ /N _
1 / I 0µ
( R11 R14
R11
\ N R11 Ri4 R11 "
R11 R13 \ //I
N _________ z( R12 R13 ; \
D12; R12 R12 R13 ; R12 R13
''
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
25
\ ________ 1\/1R9r, N NR9 R9 R9 R9
N_ N¨ tR10
N_ N_ ¨N
/ R --
___
___________________ -- ________ z(R13 __ Dii R13 __ \ / Rii N/ R11
/ R11 Rii \ N
R12 R12 R13 R12 R13 R12 R12
R.,i,
, , ,
Rlo R9 Rio Rlo R9 Rlo R9
¨N
--(
Dio __ R9 -___1\--/(N
--_ (R9 --$¨ ., -- \ /N
(
Rii \ / ____________ Ri3 R11 __________ \ / R13 N / _____ R13 Rii R11
ifl
R=-
N N , \ /N
( 1, N
R12 R12 RU R12 IR¨ D., R12
' , F` , ,
R11 R12
Rlo R9 Rlo R9 R11 R12 R11 R12
--( --( ---- R13 R13
-- /N --- /N
N N
z
R11 \ / R13 N / __ R13 Rio \ /NI R9 \ N z(
N NI N __ ( R9 Rlo
R12 R11 R12 R9 R10
; ; ,
;
R11 R12 R11 R12 R10 R10
R11 R12
N¨( N¨(
R13 -- R13 --
-- R13 -) __ /N
( --- /N
R9 \ / Rlo Ns /2¨R1 N \ / Rlo N N N R11
N sN ____________ z( N¨N
' R9 R9 R12 R11 N
R12
, ,
N=N N=N N=N N=N N=N
-- \ ;NI _____ /tRio it /(Rio _ x_ (Rio
fR0
Rii R14 R13 \ /NI R13 \ / R11 R13 \ /
¨11
rc N / ___________________________________________________________ R11
10 ( ii N N
R12 R13 IR¨ R.. R12 R12 ; R13 R12
,
, , ,
___
Rlo Rlo Rlo Rlo
N_( N_( N_( N¨( N¨T
-- /N -1 /N ) /N --- /N R10
(
R13 \ /N Ri \ S_Rii R13 \ / Rii N / ____ R11 o012
IA \ / N
NI
, ( ii N N
R12 D 1 1 R12 R12 R13 R12 R11
" ; ; ; ;
R9 R9 R9
N_ N_
_ _ _$N¨/R9 R9
R1 o _ _ ii¨/ R1
_Rio ___$ _Rlo ¨
\ N N N R N / ____ R11 Ns / __ R11
N ______ z R
( z( 12 \ / ii
N N
R12 R11 N¨N , R12 R12
,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
26
R10 R10 R10 R10
R9
¨N
-- \ /1\1 .__
( ¨N
-- \
\ /N ¨N
-- \ /N ¨N
R12
R13 \i, /(Nii R13 \ NRii R13 \N / Ril
N \ / __ R11
R. r,.. R12 Riz
R13 R12 R11
,
rc , ' , ,
R9 R9
R9 R9 R9
¨N
__i Rlo
¨N
i¨N
--_$ _____________________________________ (R11 R10 R10 __ \ / R10
N
R12 \ N N Z R12 N N / __ R11 Ns / __ R11
N sN
R11
; R12 R11 N¨N , Riz Riz
R9 Rlo R9 R10 R9 R10 R9 R10
--( --( --( R9 R10
--(
N --_ N --- z(N ¨(
-- \ z(
\ zZ( -- i /N
R12
R1
\ ,N R12 \ N
N N Riz \ _Rii __ Nµ\
S IA
R11 R11 R12 R11 ; R12
; ; ' ;
R9 R10 R11 R12 R11 R12 R11 R12
¨( R11 R12
N / I R11 Rio \ N
N¨I4
R12 , Rio Rlo
, , ,
Also preferred are the compounds of the general formual (I)
R.1
0 J 0 CINI
R2 N 1 N R-
H
0
(I)
wherein
L represents ¨L1¨L2¨;
LI represents ¨CH2C0¨;
L2 represents ¨NRI41¨; and R3 represents 1-adamantyl ; or
L2 represents ¨NRN1CH2¨; and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues are
optionally contain one or more C=C double bond and/or are optionally
substituted
one or more of Ra; Rb; Rc; Rd; and Re;
0
RI represents ''-N- R6
H
0 -
,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
27
RN RN RN
Ril Il pli IV 8 - - N8
R2 represents 1 ----- '' ---R or ---"R
N ) N
R1 , __ N R10 -
, ' , ,
R6 represents -C2H5;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CON H2,
-CON HCH3, -CON(CH3)2, -
CONN C2H5, -CH2CO2H , -CH2CO2CH3,
-CH2CO2C2H5, -CH2CON H2, -
CH2CON HCH3, -CH2CON (CH 3)2,
-CH2CON HC2H5, -NHCOCH3, -NHCOC2H5, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C2H5, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R6, R10, and R11 represent independently of each other
-H, -F, -Cl, -Br, -I, -OH, -CN, -NO2, -CH3, -C2H5, -C3H7,
-CH(CH3)2, -C4H9, -CH2-CH(CH3)2, -
CH (CH3)-C2H5, -C(CH3)3,
-cyclo-C3H5, -CH2-cyclo-C3H5, -CH2F, -CH F2, -CF3, -CH2CI, -CH2Br,
-CH21, -CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br,
-CH2-CH21, -OCH3, -0C2H5, -0C3H7, -OCH(CH3)2, -0C(CH3)3,
-0C4H9, -OCHF2, -0CF3, -OCH2CF3, -0C2F5, -OCH2OCH3,
-0-cyclo-C3H5, -OCH2-cyclo-C3H5, -0-C2H4-cyclo-C3H5, -CH 0, -COCH 3,
-COCF3, -00C2H5, -00C3H7, -
COCH (CH3)2, -00C(CH3)3,
-COOH, -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3,
-00C-CH3, -00C-CF3, -00C-C2H5, -00C-C3H7, -00C-CH (CH3)2,
-00C-C(CH3)3, -NH2, -NHCH3, -NHC2H5, -NHC3H7, -NHCH(CH3)2,
-N HC(CH3)3, -N(CH3)2, -N(C2H5)2, -N(C3H7)2, -N[CH(CH3)2]2, -N[C(CH3)3]2,
-NHCOCH3, -NHCOCF3, -NHCOC2H5, -NHCOC3H7, -NHCOCH(CH3)2,
-NHCOC(CH3)3, -CONH2, -CONHCH3, -CONHC2H5, -CONHC3H7,
-CON HCH (CH3)2, -CON H-cyclo-C3H5, -
CON HC(CH3)3, -CON(CH3)2,
-CON (C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2, -CON[C(CH3)3]2, -SO2NH2,
-SO2NHCH3, -SO2NHC2H5, -
SO2NHC3H7, -SO2NHCH(CH3)2,
-SO2NH-cyclo-C3H5, -SO2NHC(CH3)3, -
SO2N(CH3)2, -SO2N(C2H5)2,
-SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C2H5, -
NHSO2C3H7, -NHSO2CH (CH3)2,
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
28
/ H
rN I
N H
N H
r rN
--NO N
--N/N
0
I H I
rN rN
/ _______________________________________ \ / \ / \
o o 0 N\ /NH _________________ N\ /N-;
or N\ 71 \;
RN represents -H, -CH3, -C2E-13, -C3H7, -
CH(CH3)2, -C4H9,
-CH2-CH(CH3)2, -CH(CH3)-C2H5, -C(CH3)3, -cyclo-C3H3, -cyclo-C4H7,
-cyclo-C3I-19, -CH2-cyclo-C3H3, -CH2-cyclo-C4H7, -CH2-cyclo-C3H9, -CH2F,
-CH F2; -CF3, -CH2CI, -CH2Br, -
CH21, -CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-13, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -CO-cyclo-C3H3, -CO-cyclo-C4H7, -CO-cyclo-C31-19, -COOCH3,
-COOC2H3, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -
S02C21-13, -S02C3H7, -S02CH(CH3)2,
-S02-cyclo-C3H3, or -S02C(CH3)3;
RI" represent -H, -CH3, or -CH2CH3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomer, a racemate, a solvate, a hydrate, or a pharmaceutically acceptable
salt
thereof.
In a preferred set of compounds
RN RN RN
cr.- - 8
R2 represents Ri,) R8 or
N
R1 R10
, , ,
and R8, R10, R11, and RN have the meanings as defined herein.
The residues bicyclo[1.1.1]pentyl,
bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl, and
hexamethylenetetraminyl used herein, have the following parent structures
respectively:
bicyclo[1.1.1]pentyl bicyclo[2.1.1]hexyl bicyclo[2.2.1]heptyl
-2--L ...-- --
bicyclo[3.1.1]heptyl bicyclo[2.2.2]octyl bicyclo[3.2.1]octyl
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
29
- -
bicyclo[3.2.2]nonyl bicyclo[3.3.2]decyl bicyclo[3.3.3]undecyl
4-homoisotwistyl adamantyl diamantyl
hexamethylenetetraminyl
N,'
N N
and the afore-mentioned residues optionally contain one or more C=C double
bond
and/or are substituted optionally one or more of Ra, Rb, Rc, Rd, and R.
Preferred are the compounds of the formula (la):
0
ON,R6
0 0
3
R2 N ,N L 'R-
H 0
(1a),
and L, R2, R3, R6 have the same meanings as defined in the formula (I)
Preferably, the present invention relates to the comopund of the formula (I)
0 0
R2 N N
0I
(I)
wherein
L represents ¨L1¨L2¨ ;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, or ¨CH2C0¨,
L2 represents ¨NR141¨, or ¨NRNICH2;;
and preferably L represents ¨CH2¨, ¨CH2CH2¨,
¨CH2CH2CH2¨,
¨CH2CONH¨, ¨CH2CONH¨CH2¨,
¨CH2CON(CH3)¨CH2¨,
or ¨CH2CONH¨CH(CH3)¨,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
30
o
R1 represents -'')N-R6
H
0 -
,
R2 represents
RN RN RN
R11 1\1
i II
R11 N R8 --.R8 --( \_R8 0 .,
11 0 R8
N
N
R10 D10 D10 R9 / R9 R10
,-, N9
/
" , " ,
RN RN RN RN RN
Rii \ s R8 _ _ __õ, iir R8 R1:__LNõr R8 R1=1,
Rii ri, __ N,
-\\
1 iN
K /N
(
, R9 R10 R9 // 09 R10 sµ09 p .in ..,
0.9
" 5 5 " 5 " N
5
R10 RN
1
1 .
CD -
R11 0 R8 - _ ....,-ON _R8 Rtl),õSõ,
_õS,õ _R8 -___,,N,N
.,
? , -\
N¨ N , ' , __ N N N
R8 0, 1 0 R1 R1 0 R1 0
, , ,
RN RN RN
,N , R12 Ri 2
Rii ii
1 - ,1/4, N N N RN oi
_N, ' goN pe13
I R13
/ri ic
-------------------------------------------------------- . .,
N / Rio NEN , D14 , " N
D14
' , " ,
Ri2 ,
R12 R12 N R12 R12
R13 ----------------------- R13 -- r ) R13 - - -\-ji RA 13
Rio_----er/R13
\N \-
N Dia N= R14 N R14 Rio R.-, ()-
11.4
"
RN
R' R12 Ri
2
R12 S--_/y,) _ \ R12 N--__//
1 1 R13
Rio4-----r/ ---TIRli
Rio4-----r/ Ri3 ---$___ -
0--13 Rio R14
S14 , Rlo
R14
,
RN p12
R12 p12
R10 /
R13
N ,'
- - -(\ 1 tR13 '-1 -R13 -----.,---`
II 1 N-%\-
RN R1A .-, R14 R14
, , , ,
R13
RN
R11 I,
RN
\
. _____________________ ,A.Nr...õ14 , R9 0 R9
2/ N RioNI ---- __ R14 r_r R9 r /1
1 f) ___________ S _ _ 1_ R 0
N \J
R._ N
, 1\1-----S - "\___,----- Ri o
' N- ' ppl 0
' ' ,
, , r<
RN R8
ri R9 9
r Y,
o)
,r\ R9
N\ II N
L-R'10 IN õ, _R10
\N-
R8 \SI\I R8 ,S , ' R8
, N 1 S,-- N , N --
.. ----(,:\
R''a s=- II N R8
z -- R8--
N'N R8 N-N b-N N-C) , or 0-N -
, ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
31
R3 represents bicyclo[1.1.1]pentan-1-yl,
bicyclo[2.1.1]hexan-1-yl,
bicyclo[3.1.1]heptan-3-yl, bicyclo[2.2.1]heptan-2-yl, bicyclo[2.2.1]hept-5-en-
2-yl,
bicyclo[2.2.1]heptan-7-yl, bicyclo[2.2.1]heptan-2-yl, bicyclo[2.2.2]octan-2-
yl, 1-
adamantyl, 2-adamantyl , 4-homoisotwistyl, 1-diamantyl, or 4-diamantyl,
and the afore-mentioned bicyclo[1.1.1]pentan-1-yl, bicyclo[2.1.1]hexan-1-yl,
bicyclo[3.1.1]heptan-3-yl, bicyclo[2.2.1]heptan-2-yl, bicyclo[2.2.1]hept-5-en-
2-yl,
bicyclo[2.2.1]heptan-7-yl, bicyclo[2.2.1]heptan-2-yl, bicyclo[2.2.2]octan-2-
yl, 1-
adamantyl, 2-adamantyl , 4-homoisotwistyl, 1-diamantyl, or 4-diamantyl
residues
are optionally contain one or more C=C double bond and/or are optionally
substituted one or more of Ra, Rb, Rc, Rd, and Re;
R6 represents ¨C2H5;
and R6, R6, R10, R11, R12, R13, R14, Ra; R13, Rc; Rd; Re ; RN and rt "N1
have the same
meanings as defined in formula (I) or a diastereomer, an enantiomer, a mixture
of
diastereomers, a mixture of enantiomer, a racemate, a solvate, a hydrate, or a
pharmaceutically acceptable salt thereof.
In some embodiments, the present invention relates to the comopund of the
formula (I)
R1
0 J 0
H CN yL
R2 N 1 N R3
H
0I
(I)
wherein
L represents ¨I-1-1-2¨ ;
LI represents ¨CH2C0¨,
L2 represents ¨NR141¨, and R3 represents 1-adamantyl; or
I-2 represents ¨NR1ICH2¨, and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues are
optionally contain one or more C=C double bond and/or are optionally
substituted
one or more of Ra, Rb, Rc, Rd, and Re;
0
,,,A ,R6
Ri represents N
H
0 -
,
R2 represents
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
32
RN RN RN
R11 1\1
_ - R11 1\1
r R8 ---)--"IN---R8 ---s--0 Rs R11 0 Ra -_ S r, m8
1 ________________________________________________________________________ r r
i __ , ___
) ___________ N N
Rlo N Dio D10 R9 /'
R9 R10 .-, rC9
lµ , " ,
RN RN RN RN RN
R11 s R8 __ N r-s8 R11 ii R8 R11 1\1, R11 rj, __
N,
1 ___________ r K i _____ ( ,, i ,N
( n , ,N
1, ( n
. R9 R10 ___ R9 / n9 R10 sµ / , R._
nõo
R11 S - - --
rµ rµ ,
rµ ,
Rio RN
o, ,,_ ,- Rii 0 R8 -___sõ-ON __R8 ),-.,._
N¨ 1 ----
N \ ir
N --
) __ N S R8
1
N
N
R8 Dio Rio Rio RiO
, , ' , , , ,
RN RN RN
Rii ii ,1/4,
- N N "N i
N Ro - ,N, p¨
o R12
R1-
1
R12
------------------------------------------------------------------- I R13
1 '' \\ /c
\¨N ----\- N--
------------------------------------------------------ u,,,,,,õ\,)
N / o10 , N=1\1 , 0 '1,4 N
R14
' , = ,
R12
R12 R12
N R12 0 -/,) %% R12
R13
¨, TR13
---------- I R- R = -12 ¨ ¨ -\- R1
o_(%.--..-- o 1,4r/ R13
\N \-) .-, '\-4 5 N Ri4
, N Ri4
, N Ri4 , Rlo RiA
N
S-
R
R11 R12
pp12
R12 -/ 13 \ R12 i\i-
/Y s
1 '
R13
W 0 4.--------(> - - ,:j R
R 1 o _(---r> R13
0 ---\13 Rio Ria
S14 , Rio Ria
,µ RN
13p. 12
R12 p12
R10 / i\I ----_/' '
- -- -(\ 1 tR13 13 ,,--------,õõ----"N`
II 1 4R13
N N--%\-'1A \7R
RN R., Ria Ria
,
R13
RN
R11 1,
RN
Niv_____ R14
--=' /1
) --- N . R
R 1 _ r._" 9 rNiR9
__ i_ _Ro N \ j
Rio N __ g 10_1 __ R14 v - S \___,------010 ,
K,) Ri\I `Rio
' ,
'
RN R8
R9
9
r Yi
/1.r N\ X N
1
--/-_-, Rio - õ, _Rio
, N
\
- ,." R8 \O
,
R8 /S , ' , R8
N H
-- N - - N --
N R,RS ,.. ---ii N6- Ro___i ------
-i R8--- 1
N'N R8 , N-N , b-N , N-1:3 , or 0--N -
R6 represents ¨C2H5;
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
33
and R8; R9; R113, R11, R12, R13, R14, Ra; Ri3; Rc; Rd; Re RN and RNi have the
same
meanings as defined in formula (I) or a diastereomer; an enantiomer; a mixture
of
diastereomers; a mixture of enantiomer; a racemate; a solvate; a hydrate; or a
pharmaceutically acceptable salt thereof.
In some embodiments; the present invention relates to the comopund of the
formula (I)
0 J 0 CN L R2 N N R3
0I
(I)
wherein
L represents ¨L1¨L2¨;
LI represents ¨CH2C0¨;
L2 represents ¨NRI41¨; and R3 represents 1-adamantyl; or
L2 represents ¨NRN1CH2¨; and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues are
optionally contain one or more C=C double bond and/or are optionally
substituted
one or more of Ra; Rb; Rc; Rd; and Re;
0
RI represents
0
R2 represents
RN RN RN
R8
11 R8
) __ N
N
R10 R10 =
R6 represents ¨C2H5;
and R8; R113, R11, Ra; Ri3; Rc; Rd; Re RN and RN1 have the same meanings as
defined
in formula (I) or a diastereomer; an enantiomer; a mixture of diastereomers; a
mixture of enantiomer; a racemate; a solvate; a hydrate; or a pharmaceutically
acceptable salt thereof.
In some embodiments; the present invention relates to the compound of the
formula
(la):
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
34
0
0 IL
'1\1-R6
H
0 _i,_, 0
H,)-L , L ,
R2i N 1 N R3
H
0 (1a),
wherein
L represents ¨L1¨L2¨;
L1 represents ¨CH2C0¨,
L2 represents ¨NRI41¨, and R3 represents 1-adamantyl; or
L2 represents ¨NRN1CH2¨, and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues are
optionally contain one or more C=C double bond and/or are optionally
substituted
one or more of Ra, Rb, Rc, Rd, and Re;
R2 represents
RN RN RN
N
1
Ri N
_N _Ra ___ N ,_,8 _ _ 0 R8 R1 _1 0 Ra -
__ S ,-, m8
-\\ 'r ir 1 ______ r -\\ r r
R10 N Rio Di R9 , R9 R10
,-,9
I-C ,
RN RN RN RN RN
R11 S R8 N Rs R1N Rs Rii N, R11 II, -_
N,
-14N I iN
/ K ¶
/ R9 R10 R9 / ,--,9 R10 `, / ,-,,-.-,n
R10 ,--,9
N 1 o N 1 N
1
Rlo RN
0 8 R11 S -
S, Dp8 -_,..õ,,n.
x - R11 0 R8 J R =------ /7-----
1 --- \ 8 ________________________________
N N N ) __ N N N
R8 R10 R10 R10 R10
,
RN RN RN
,N, R12 R12
Rii N,m N N ,N1 Rio
N Nr--- ,N, DN 11
R=- 1 2
R =-
1 ,r1 1/ - - y N----
)'N ------------------- u,,,, \-)
N
NEN , r-114 N
R14
/ R10 /
, ,
' N
R12
R12 R12 N R12 0---__//, %, R12
------------ /') R13 N/-) Ri 3 R=-, r 1 ---,TR13
-\- 1, R10_tr>niR1
3
N R14 N R14 N et R10 R.-,
0"---R14 ,
RN
R12 R12
R11 R12
S ---__/ N----,
R12 13
-- TR13
R10 4--------r/ -\' R10_t---r> R13 ---,__---- M
(21-13 R10 R14
S"----414 , R10 R14
,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
35
RN
R12 R1 / i\l-A -_, R12 R12
---_-------7,
-- 4 1 R13 - I R13 - L _I 1
R13
N N'%\i4 ___\7
iA
, RN R14 R14 R-
R13
R
_____________ R11 N RN 2/ NIVR14 ii
R9 r
9 r '/1
inX S R10-N1 ____ 14 riIIR
R=- N R RN,N\J1.<,..10 S
,
,
RN R8
ri /R9
r / io m i --
N'\ I N6-
_Rf_Rio _. 1-------'
- R8
N II - ,S,- - ,- a N, -
- - ,-----N R'-'a ---\ 11 N li - R' ---- -/ -
R8 --N I- -
, or 0---N ;
R6 represents -C2H5;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CON H2 ,
-CONHCH3, -CON(CH3)2, -CONHC2F15, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H5, -CH2CONH2, -
CH2CONHCH3, -CH2CON(CH3)2,
-CH2CONHC2F15, -NHCOCH3, -NHCOC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R6, R6, R10, R11, R12, R13, and R14 represent independently of each other
-H, -F, -Cl, -Br, -I, -OH, -
CN, -NO2,
-CH3, -C2H5, -C3H7, -CH(CH3)2, -
C4H9, -CH2-CH(CH3)2,
-CH(CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5, -CH2F, -CH F2,
-CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2, -CH2-CF3,
-CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2OH, -OCH3, -0C21-15, -0C3H7,
-OCH(CH3)2, -0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH2CF3, -0C2 F5 ,
-OCH 20CH 3 , -0-CYCIO-C3H 5 , -
OCH 2-CYCIO-C3 H5 , -0-C2H4-CYCIO-C3 H5 ,
-CHO, -COCH3, -COCF3, -00C21-15, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -
COOC(CH3)3, -00C-CH3, -00C-CF3, -00C-C2H5, -
00C-C3H7,
-00C-CH(CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5, -NHC3H7,
-NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -
N(C2H5)2, -N(C3H7)2,
-N[CH(CH3)2]2, -N[C(CH3)3]2, -NHCOCH3, -NHCOCF3, -NHCOC2H5,
-NHCOC3H7, -NHCOCH(CH3)2, -NHCOC(CH3)3, -CON H2, -CONHCH3,
-CONHC2H5, -CONHC3H7, -
CONHCH(CH3)2, -CONH-cyclo-C3H5,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
36
-CON HC(CH3)3, -CON(CH3)2, -CON (C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2,
-CON[C(CH3)3]2, -SO2NH2, -SO2NHCH3, -SO2NHC21-15, -SO2NHC3H7,
-SO2NHCH(CH3)2, -SO2NH-cyclo-C31-15, -SO2NHC(CH3)3, -S02N(CH3)2,
-SO2N(C2H5)2, -SO2N(C3H7)2, -
SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2,
-NHSO2CH3, -NHSO2CF3, -NHSO2C21-15, -NHSO2C3H7, -N HSO2CH (CH3)2,
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
/ H 1 H H
N N N
,K - -Nn ---------------------- N/N
__J 0
1 H 1
r N r N
/ _______________________________________ \ / \ / \
N\ /NH N\ 71- ; or N\ /1\1 __ \;
RN represents -H, -CH3, -C2H5, -C3H7, -CH(CH3)2, -
C4H9,
-CH2-CH (CH3)2, -CH(CH3)-C2H5, -
C(CH3)3, -cyclo-C3H5, -cyclo-C4H7,
-cyclo-051-19, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-051-19, -CH2F,
-CH F2; -CF3, -CH2CI, -CH2Br, -
CH21, -CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-15, -00C3H7, -COCH (CH3)2,
-00C(CH3)3, -CO-cyclo-C3H5, -CO-cyclo-C4H7, -CO-cyclo-051-19, -COOCH3,
-CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -S02C21-15, -
S02C3H7, -S02CH (CH3)2,
-S02-cyclo-C3H5, or -S02C(CH3)3;
RNI represents -H, -CH3, or -C2F15;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomer, a racemate, a solvate, a hydrate, or a pharmaceutically acceptable
salt
thereof.
Preferably, the present invention relates to the compound of the formula (la):
0
0)-LN . R6
H
0 ( H 0
A N - L ,
R2 N 1 N R3
H
0 (la);
wherein
L represents -L1-L2- ;
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
37
L1 represents -CH2C0-,
L2 represents -NRN1-, and R3 represents 1-adamantyl; or
L2 represents -NRN1CH2-, and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues are
optionally contain one or more C=C double bond and/or are optionally
substituted
one or more of Ra, Rb, Rc, Rd, and Re;
R2 represents
RN RN RN
R11 N R8
IV
R1 N R0
R6 represents -C2H5;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CONH2,
-CON HCH3, -CON(CH3)2, -
CONN C2H5, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H5, -CH2CON H2, -
CH2CONHCH3, -CH2CON(CH3)2,
-CH2CONHC2F15, -NHCOCH3, -NHCOC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R8, R10, and
R11 represent independently of each other
-H, -F, -Cl, -Br, -OH, -
CN, -NO2,
-CH3, -C2H5, -C3H7, -
CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH (CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5, -CH2F, -CH F2,
-CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2, -CH2-CF3,
-CH2-CH2CI -CH2-CH213r, -CH2-CH21, -OC H3,
-0C2H5, -0C3H7,
-OCH (CH3)2, -0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH2CF3, -0C2F5,
-OCH20C H3, -0-CYCIO-C3H5, -
OCH2-CYCIO-C3H5, -0-C2H4-CYCIO-C3H5,
-CHO, -COCH3, -COCF3, -00C21-15, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -
COOC(CH3)3, -00C-CH3, -00C-CF3, -00C-C2H5, -
00C-C3H7,
-00C-CH (CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5, -NHC3H7,
-NHCH(CH3)2, -NHC(CH3)3, -
N(CH3)2, -N(C2H5)2, -N(C3H7)2,
-N [CH (CH3)2]2, -N[C(CH3)3]2, -
NHCOCH3, -NHCOCF3, -NHCOC2H5,
-NHCOC3H7, -NHCOCH(CH3)2, -NHCOC(CH3)3, -CONH2, -CONHCH3,
-CON HC2H5, -CON HC3H7, -
CONHCH (CH3)2, -CON H-cyclo-C3H5,
-CON HC(CH3)3, -CON(CH3)2, -CON (C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
38
-CON[C(CH3)3]2, -SO2NH2, -SO2NHCH3, -SO2NHC21-13, -SO2NHC3H7,
-SO2NHCH(CH3)2, -SO2NH-cyclo-C3H3, -SO2NHC(CH3)3, -SO2N(CH3)2,
-SO2N(C2H3)2, -SO2N(C3H7)2, -
SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2,
-NHSO2CH3, -NHSO2CF3, -NHSO2C21-13, -NHSO2C3H7, -N HSO2CH (CH3)2,
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
r1\1 r1\1 rk1
- -NOrN
r N r N r N
/ _______________________________________ \ / __ \ / __ \
K(:) N\ /NH N\ or N\ \
R" represents -H, -CH3, -C2E-13, -C3H7, -CH(CH3)2, -C4H9,
-CH2-CH (CH3)2, -CH(CH3)-C2H3, -
C(CH3)3, -cyclo-C3H3, -cyclo-C4H7,
-cyclo-C31-19, -CH2-cyclo-C3H3, -CH2-cyclo-C4H7, -CH2-cyclo-C3H9, -CH2F,
-CH F2; -CF3, -CH2CI, -CH2Br, -CH21, -
CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-13, -00C3H7, -COCH (CH3)2,
-00C(CH3)3, -CO-cyclo-C3H3, -CO-cyclo-C4H7, -CO-cyclo-C31-19, -COOCH3,
-COOC2H3, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -000CH2Ph,
-S02CH3, -S02CF3, -S02C21-13, -
S02C3H7, -S02CH (CH3)2,
-S02-cyclo-C3H3, or -S02C(CH3)3;
RI" represents -H, -CH3, or -C2F13;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomer, a racemate, a solvate, a hydrate, or a pharmaceutically acceptable
salt
thereof.
Preferalby, the compound of the invention has the formula (II):
0
ON
0 ( H 0 (II)
I\O-L
R2N
N
0 Li
wherein
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
39
L2 represents -NRI41-, and R3 represents 1-adamantyl; or
L2 represents -NRNICH2-, and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned adamantyl and bicyclo[3.1.1]heptyl residues are
optionally
contain one or more C=C double bond and/or are optionally substituted one or
more
of Ra, Rb, Rc, Rd, and Re;
R2 represents
RN RN RN
R11 II -
--- Ri __-R8 --IN-----R8
) __ N Y __ N
R1 RI -
, _____________________ N , , ,
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H3, -CON H2,
-CON HCH3, -CON(CH3)2, -
CONN C2F13, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H3, -CH2CON H2, -
CH2CONHCH3, .. -CH2CON(CH3)2,
-CH2CONHC2H3, -NHCOCH3, -NHCOC21-13, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-13, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R" represents -H, -CH3, -C2E-13, -C3H7, -
CH(CH3)2, -C4H9,
-CH2-CH (CH3)2, -CH(CH3)-C2H3, -
C(CH3)3, -cyclo-C3H3, -cyclo-C4H7,
-cyclo-C3I-19, -CH2-cyclo-C3H3, -CH2-cyclo-C4H7, -CH2-cyclo-C3H9, -CH2F,
-CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-13, -00C3H7, -COCH (CH3)2,
-00C(CH3)3, -CO-cyclo-C3H3, -CO-cyclo-C4H7, -CO-cyclo-C31-19, -COOCH3,
-COOC2H3, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -S02C21-13, -S02C3H7, -S02CH(CH3)2, or
-S02C(CH3)3;
RI" represent -H, -CH3, or -CH2CH3;
R8, R", and R" represent independently of each other
-H, -F, -Cl, -Br, -I, -OH, -
CN, -- -NO2,
-CH3, -C2E-13, -C3H7, -
CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH (CH3)-C2H3, -cyclo-C3H3, -CH2-cyclo-
C3H3, -CH2F, -CHF2,
-CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2, -CH2-CF3,
-CH2-CH2CI , -CH2-CH2Br, -CH2-CH21, -
OCH3, -0C2H5, -0C3H7,
-OCH (CH3)2, -0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH2CF3, -0C2F5,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
40
-OCH2OCH3, -0-cyclo-C3H3; -OCH2-cyclo-C3H3, -0-C2H4-cyclo-C3H3;
-CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -
CH=CH-CH3,
-CECH; -CEC-CH3, -CH2-CECH, or -Ph;
or a diastereomer; an enantiomer; a mixture of diastereomers; a mixture of
enantiomer; a racemate; a solvate; a hydrate; or a pharmaceutically acceptable
salt
thereof.
The term "1-adamantyl" and "2-bicyclo[3.1.1]heptyl" have the following
structures
respectively:
1-adamantyl
Rd IR' Re 2-
bicyclo[3.1.1]heptyl
Rb Ra Ra
Rb
and Ra; Rb; Rc; Rd and Re have the same meanings as defined herein.
Preferred; 2-bicyclo[3.1.1]heptyl have the following structure:
Ra
Rb ,or Rb
and Ra and Rb; have the same meanings as defined herein.
More preferred; the compound has any one of the formulae (II-a) - (II-I), (11-
b1)- (II-b2),
and (III-a) - (III-I):
0 Rd Rc Re 0
(DN Rb
0 0 0 0
Ra
R2
NrN1H N
N R2 N N
Rb
0 0
0 0
(II-a) (II-b)
0
0 1.4 0 0 0
a
Ra
R2 N N
Rb
Rb R2 1-1jC
0 0 0 0
(II-bl) (II-b2)
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
41
0
0
0-1-,N.-----,..
0
N
H
HH 0 Rb R2 N Rc
R2
0H 0
N N , L1
N L2 AN 1 N H
0
H
0 0
Ra Ra
(II-c) (II-d)
0 0
0-L ..----õ, 0)- -----õ,,
N N
H H N
Rb Ra
Rb Ra
0 ( H 0 0
A H 0 Rc
L Rc N L2
R2 A FIN %N )-LI N 0 Rd R2 N
H 1
0 0 Rd
(II-e) (II-f)
0 0 Rd
0, A
-1\1 Rb Ra 0, JL
H Rc H Rc
0H 0 0 ( H 0
R2A NN 1 N L2 R2 A N N
_ J1 NL2
H
0 0 H
0 0
(II-g) (II-h)
O 0
ON 0,-,,)-1,N.----,õ
H Rb Ra H
N kli )-L
R2A N
J1 NirL2
R2AN
1\i
_ J1 rL2
H Rd H
0 0 0 0
(II-i) (11-j)
0 0
0 0, A
N
' -1\1 rf_frj
H H
0 JCH 0 0 JCL, 0
R2A N 1 N R2 N 1 N
H
0 0 H
0 0
(II-k) (II-I)
wherein L1, L2, R2, RN, Ra, Rb, Rc, Rd and Re have the same meanings as
defined
in the formula (I), preferred in the formula (la), more preferred in the
formula (II).
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
42
o Rd Rc Re o
Rb Ra
H H
0 ti 0 0 i_i 0
Ra
Nr\lcriN)-L ,NH
1 N Tr N -y-LNcrN)-L NH
1 N-r Rb
__N H 0 0
____N H 0 0
'RN 'RN
(III-a) (III-b)
0
9
oN 0 1 N
H I H
0 0 o 0
o id H
N H H
N,ryr N
R8 N Re 1 hl 1 -(XliC N
\ 0 0 0 I oa
R. 0 R" t
(11I-c) (11I-d)
0
O 0
N
H (:))-LN
H
H 0 0
S Nj- N R8 H
N 1 N \ I H R8 N
0 0 0 0
Rio S R11
(11I-e) (III-f)
0 0
ON N
H H
0 H 0 0 0
H H
,H
R8-? N
___ZAN R8-- __Ai_l
N Rio N Rio 0 0
(11I-g) (11I-h)
0
(21)-
N11.-'1' 0
H 0
UN
'----1-11'=
0 0 H
H 0 ti 0
R13 0 1 hi NH
1 N N R13 s H
i i NljciNN-1N
0 0 I H 0 0
R10
Rl
R12 R12
(iii-i) (nil)
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
43
0
o
0)-N
OA
H N'
H
0 H 0
0 _rH 0
R13 0 N)-L NH H H
N r
I H 0 0 I H I I
0 0
Rio Rio
Ri2 R13 12
(III-k) (III-I)
wherein LI, L2, R8, R10; R11, R12, R13, R11, RN, Ra; Rb; Rc; Rd and K i-ie
have the
same meanings as defined in the formula (I), preferred in the formula (la).
In a preferred embodmiment, the invention refers to the compound of the
formulae (I),
(la) and (II), wherein
R3 represents
,
,
.,
d.--- ,
d--
-----K -----
' , i
' ' ' '
, , ,
,
,,, ..
.--'s
,
, , ,
, i i
, ,
,
F
CI CF3 OH CO2Me
i i ,
,
F
F
. . .,
. '
,
F Br,
, . . ., ., . .
.
CI OH
CO2H ;
CO2Me , CO2H
, ,
. 0
., .
CON H2 ; C0NMe2 ; HN) CF3
,
Br OH
'
,
'
_- ; ,- ; _-
' '
, or
Also preferred, R3 represents
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
44
F CI
. . . . .
F
CF3 jZOH ZCO2Me F 01
. . . . .
N
i i i . 1 ,or i = , ,,
, ,
More preferred, R3 represents
'
.,
,or .
In a preferred embodmiment, the invention refers to the compound of any one of
the
formulae (I), (la) and (II), (II-a) - (II-1), (II-b1)¨ (II-b2), wherein
R2 represents
RN RN
RN
R11 il ,,8 _ 0 D8 R 11 0 ,-,8
_ - R11 II R8 - - ,.---IIry - --s" r , ..,
rr, ___.,õ.s.r8
¨, , , \ , \ , , ,
i __ N ) N N
R10 R10 010 R9 R9 R10 .-,9
" I-C ,
RN RN RN RN RN
R11 s R8 _____NrR8 R1,N,(Rõ R1:N:, Rii õ N,
\ , -,\ , -\\ , , ,N __ i ,N
. ,
,N
.õ (
, R9 Rio R9 . R9 R10 sµ , c9 D 1 ..,
r-,9
1 o N 1 " N 1
R10 RN
Rt....5 N
Rii 0, D8 - _ ,ON____ R8 - _ S .-
,8 - _ _..,.., ,n ,
0,- 1 r-- ii -\ ----- 1 IN
N
N N R10 __ N
R10 N
R10 ________________________________________________________ N
Rio
R8 ' 1
RN RN RN
R12 R12
R11
N N ,N R10
N N, DN pp 1 3 R 1 3
N'''
-------------------------------------------------------- ,, .,
1
N / R10 , ' , ' N r` =N , o014 N R14
R12
R12 R12 N R12 0 ---_// %
% R12
R13 N r R 1 3 - - - $__ , j R 1 3
R13 1 A
R10_t----r> R13
N R14 N Ri4 N Ri4 Rio R.-, (:)----414 ,
,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
45
RN
R12 R11 R12
, N
R12 S ---./A 13 R12 -
R13
R1134/\1 - - R
R19-(/ 1 '1R13 -$%\
0----\2=-Ri3 R19 R14 S------Ri4 Rio
R14
, RN
R12 R12 R12
R19 /
RN R14 R14 IR-
R13
R11
RN RN . 0 R9
il Rl __ N S ' R19- R14 _ _r\_" RF9zio - -cr
''' Rio RN,
N S
rµ ,
RN
R8
0) I D
il R9 S - - -
r -/i 9 , .
, N6-
,''' --/_=-= R10 _R10 N,I
R8
. - . N,..õ, -
S: ---T - - ,----N R8-- N / ii R8-----N'-'-T--
Fz u--- I/ -
N'N , or O-N ;
wherein
R8, R9, R10, R11, R12, R13, and R14, represent independently of each other
-H, -F, -Cl, -Br, -I, -OH, -
CN, -NO2,
-CH3, -C2H5, -C3H7, -CH(CH3)2, -
C4H9, -CH2-CH(CH3)2,
-CH(CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5, -CH2F, -CH F2,
-CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2, -CH2-CF3,
-CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2OH, -OCH3, -0C21-15, -0C3H7,
-OCH(CH3)2, -0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH2CF3, -0C2F5,
-OCH2OCH3, -0-CYCIO-C3H5, -OCH2-CYCIO-C3H5, -0-C2H4-CYCIO-C3H5,
-CHO, -COCH3, -COCF3, -00C21-15, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -
COOC(CH3)3, -00C-CH3, -00C-CF3, -00C-C2H5, -
00C-C3H7,
-00C-CH(CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5, -NHC3H7,
-NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -
N(C2H5)2, -N(C3H7)2,
-N[CH(CH3)2]2, -N[C(CH3)3]2, -NHCOCH3, -NHCOCF3, -NHCOC2H5,
-NHCOC3H7, -NHCOCH(CH3)2, -NHCOC(CH3)3, -CON H2, -CONHCH3,
-CONHC2H5, -CONHC3H7, -
CONHCH(CH3)2, -CONH-cyclo-C3H5,
-CONHC(CH3)3, -CON(CH3)2, -CON(C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2,
-CON[C(CH3)3]2, -SO2NH2, -SO2NHCH3, -SO2NHC2H5, -SO2NHC3H7,
-SO2NHCH(CH3)2, -SO2NH-cyclo-C3H5, -SO2NHC(CH3)3, -SO2N(CH3)2,
-SO2N(C2H5)2, -SO2N(C3H7)2, -
SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2,
-NHSO2CH3, -NHSO2CF3, -NHSO2C2H5, -NHSO2C3H7, -NHSO2CH(CH3)2,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
46
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
/ H 1 H H
N
..K --No :1) -NrN1 __r rN N
_---1 r
0
,
1 H
NI
N N
r r r / __ \ / __ \ / __ \
N\ /NH N\ 71- , or N\
71 \;
Preferably, R8, R9, R", R11, R12, R13, and R14, represent independently of
each
other -H, -F, -Cl, -Br, -OH, -CN, -
NO2,
-CH3, -C2H5, -C3H7, -CH(CH3)2, -
C4H9, -CH2-CH(CH3)2,
-CH (CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5, -CH2F, -CH F2,
-CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2, -CH2-CF3,
-CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2OH, -OCH3, -0C2H5, -0C3H7,
-OCH (CH3)2, -0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH2CF3, -0C2F5,
- OCH20C H3, -0-CYCIO-C3H5, -
OCH2-CYCIO-C3H5, -O-C2H4-CYCIO-C3H5,
-CHO, -COCH3, -COCF3, -00C2H5, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -
COOC(CH3)3, -00C-CH3, -00C-CF3, -00C-C2H5, -
00C-C3H7,
-00C-CH (CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5, -NHC3H7,
-NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -
N(C2H5)2, -N(C3H7)2,
-N [CH (CH3)2]2, -N[C(CH3)3]2, -
NHCOCH3, -NHCOCF3, -NHCOC2H5,
-NHCOC3H7, -NHCOCH(CH 3)2, -NHCOC(CH3)3, -CON H2, -CONHCH 3 ,
-CON HC2H5 , -CON HC3H7, -
CONHCH (CH3)2, -CON H-cyclo-C3H5 ,
-CON HC(CH3)3, -CON(CH3)2, -CON (C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2,
-CON[C(CH3)3]2, -SO2NH2, -SO2NHCH3, -SO2NHC2H5, -SO2NHC3H7, -Ph, -
0-Ph, -0-CH2-Ph, or
More preferably, R8, R9, R", R11, R12, R13, and R14, represent independently
of each
other -H, -F, -Cl, -Br, -OH, -CN, -
NO2,
-CH3, -C2H5, -C3H7, -CH(CH3)2, -C(CH3)3, -cyclo-C3H5,
-CF3, -CH2OH, -OCH3, -0CF3, -CHO, -COCH3, -COOK -COOCH3,
-NH2, -N(CH3)2, -CONH2, -SO2NH2, -Ph, or --
R" represents -H, -CH3, -C2H5, -C3H7, -
CH(CH3)2, -C4H9,
-CH2-CH (CH3)2, -CH(CH3)-C2H5, -
C(CH3)3, -cyclo-C3H5, -cyclo-C4H7,
-cyclo-05H9, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-05H9, -CH2F,
-CH F2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-
CH2F, -CH2-CHF2,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
47
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-15, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -CO-cyclo-C3H5, -CO-cyclo-C4H7, -CO-cyclo-051-19, -COOCH3,
-CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -S02C2H5, -S02C3H7, -
S02CH(CH3)2,
-S02-cyclo-C3H5, or -S02C(CH3)3;
Preferably, R" represents -H, -CH3, -C2H5, -CH(CH3)2, -cyclo-C3H5,
-cyclo-C4H7, -cyclo-05H9, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-05H9,
or -COCH3, more preferably, R" represents -H, -CH3, -C2H5, -CH(CH3)2, -
cyclo-C4H7, -cyclo-05H9, or -COCH3.
In a preferred embodmiment, the invention refers to the compound of any one of
the
formulae (I), (la) and (II), (II-a) - (II-I), (II-b1)- (II-b2), R2 represents
H I H
N I
N '9 9 H
N N N H
N H
N
N N
- - - -
N ,N1 ,N1 ,N ,N ,N ,N
N N /
,
,
CI
I I I I
1 _ N ,
N___--- N H __-Br -----N--
I\I - N 171 .---1\1 71
N N N N )
--N ; \--N ; ,
CI I I
- -
HN 0 , - ---_ 7N --.. N ,
N ,
. -
NC ---- N N N N
-/ i\I ____
iii\I
/
I ---N , __
H2N HN \ ,,=/
' ' ,
'
I I
I N,
/õ_ , =
- _ N, N - 'N N N,N _ ,J\1 , - - 0_._-
Br icl__--
Cm N\-N -Y NH
1\1-"N N ; / ; / ; / ; N=N ;
,
---q-C1 --1S_z-Br ---q-S CI _ )SN Br'
o - 1
\ f
H2N/ \o-' , Br, Br ; Br ; CI ;
'
--p-S Br Cli___S ci BrS Br 0 _\
N -- Me0-- - -
)-----<\ - -
Br , ,
S s
0__-Br --1S-CF3
'----3 N __ 1 N Br Br
, , ,
S , - S , -
S - ,S - N
N 1
- N, - NI
S - - NI, µIs-i H
- - -= II 1 µ II - Sµ 1 N
0 NN S-N _--N N----N
0 ;
,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
48
s -- N's 0 H2N H
N 111
-- .----<\N--- ---- (-, -- (----
HO , O-N , N-0 0-N HN--
H -'
NO2,
,
HN- Nr-
o) N - - _.õ------,
-._õ-----NO2
I I
-1-- ¨N-1\1,-- CO2Me
-,CO2H .,, -, N,N '-. --.N N.---
0
I N
I N I
0 0
0
, 0 , CI Br , I , 0
, ,
F , H
-- 0 ,- S S
N
/ / - \
0 , \
, , S , ,
,
H
N
H
N H
F \
F
S 0
,-
________________________________ -----,,__.\_,--$
N S N NL S N S H .. , HN
, ,
OH ,
,
, 3%, ,...õ0 N - , ,
1
r ,
-
I ---
I
N-- or
N N N N N ,
,
,
Also preferred, R2 represents
H I H
N I 9 H
N N N H
N H
N N
__
N
\ ---- N---
N , ________________________________________ N N , ___ N , N N N
NN ,/ / , , , , / ,
CI
I I I I
H I )N N___¨ N__-Br
--N , ,
Still more preferred, R2 represents
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
49
CI
11\1 Br
)N I o
---q-CI
N / N / Br,
Br ,
,
S S
Br ClS
-- Br -- CI - S
r __-Br --p-S Br S Br
CI ij-
Br , CI , , Br , ,
., NO2
0 p,- Sc --1S-CF3 --.,__..---
,NO2
-5-F3
/----- j /----\ j \ #
N 1
N N , Br N CO2Me ,
N
0 0
0
-.0O2H 0 -- \ --
' \ S
1 \
N -- \ -- , CI Br -- \
' ,
H
F H N
N H
S -- \ -- \
F ___(\N
\
, , F , N C, or .
In a preferred embodmiment, the invention refers to the compound of any one of
the
formulae (I), (la) and (II), (II-a) - (II-1), (II-b1) ¨ (II-b2), wherein
L represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2CONH¨,
¨CH2CONH¨CH2¨, ¨CH2CON(CH3)¨CH2¨, ¨CH2CONH¨CH(CH3)¨,
Most preferred are the following compounds of formula (I):
Cpd Structure Name
1-1: 0 (S)-N1-ethyl-N6-(1-(2-(1-
ON
adamantylamino)-2-oxoethyl)-2-oxo-1,2-
H
0 1.4 0 dihydropyridin-3-y1)-5-(1-methy1-
1H-
k imidazole-5-carboxamido)-2-
NIN
. . a t.,,..,...) a oxohexanediamide
\
1-2: 0 (S)-N1-ethy1-5-(1 H-imidazole-4-
0N carboxamido)-N6-(1-(2-(1-
H
0 0 adamantylamino)-2-oxoethyI)-2-oxo-
1,2-
N dihydropyridin-3-yI)-2-
oxohexanediamide
HNI1-1[1 1 N-rNH
---=---N 0 0
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
50
1-3: 0 (S)-N1-ethyl-N6-(1-(2-(1-
ON adamantyl(methyl)amino)-2-oxoethyl)-2-
H
O oxo-1,2-dihydropyridin-3-y1)-5-(1-methyl-
1\iANN 1 H-imidazole-5-carboxamido)-2-
NNI H
0 0 oxohexanediamide
--NN
1-4: 0 (S)-N1-ethyl-N6-(1-(2-(3,5-
ON
dimethyladamantane-1-amino)-2-
H
O 0 oxoethy1)-2-oxo-1,2-
dihydropyridin-3-y1)-
NH 5-(1-methy1-1H-imidazole-5-
NHNjc N I N(
0 ,!,.,,,,,) 0 carboxamido)-2-oxohexanediamide
\
1-5: 0 (S)-N1-ethyl-N6-(1-(2-(3-
O11L.N.-----õ,
ethyladamantane-1-amino)-2-oxoethyl)-
H
O 0 2-oxo-1,2-dihydropyridin-3-y1)-5-
(1-
N
H 11
N)..r NH methyl-I H-imidazole-5-carboxamido)-2-
NHN
0 1,,õ...7,1 0 oxohexanediamide
N
1-6: o (S)-N1-ethyl-N6-(1-(2-(3-
oN cF3
trifluoromethyladamantane-1-amino)-2-
H
O 0 oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
H u
N'N NH 5-(1-methy1-1H-imidazole-5-
NN FIN (:c 8
carboxamido)-2-oxohexanediamide
\
1-7: 0 (S)-N1-ethyl-N6-(1-(2-(3-
ON N - OH
hydroxyadamantane-1-amino)-2-
H
O 0 oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
111 j- NH
1\l/N1 1 NM( 5-(1-methy1-1H-imidazole-5-
--N 0 0 carboxamido)-2-oxohexanediamide
\
1-8: 0 (S)-N1-ethyl-N6-(1-(2-(3-
F
O N fluoroadamantane-1-amino)-2-oxoethyly
H
O 0 2-oxo-1,2-dihydropyridin-3-y1)-5-
(1-
H 11
'N NH methyl-I H-imidazole-5-carboxamido)-2-
NHN N
_--N - 0 0 oxohexanediamide
\
0
1-9: (S)-N1-ethyl-N6-(1-(2-(3-
chloroadamantane-1-amino)-2-oxoethyly
H
O 0 2-oxo-1,2-dihydropyridin-3-y1)-5-
(1-
H u
N 'NNH methyl-I H-imidazole-5-carboxamido)-2-
NHN r() 8
oxohexanediamide
"N
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
51
0
1-10: (S)-N1-ethyl-N6-(1-(2-(3-
N )-
-
bromoadamantane-1-amino)-2-
OL Br
H
0 0 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
H I]
r\l/YN N 1 NThr NH 5-(1-methy1-1H-imidazole-5-
---N 0 0 carboxamido)-2-oxohexanediamide
\
o
I-11: (S)-N1-ethyl-N6-(1-(2-(3-methyl
ON CO2Me
H adamantane-3-carboxylate-1-amino)-2-
oxoethyl)-2-oxo-1 ,2-di hydropyridin-3-y1)-
1\1 ,NH
N -7.'=-=NI-1 1 N Tr 5-(1-methy1-1H-
imidazole-5-
N H 0 0
carboxamido)-2-oxohexanediamide
1-12: 0 F F (S)-N1-ethyl-N6-(1-(2-(4,4-
ON-----,, difluoroadamantane-1-amino)-2-
H
0 0 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
H It
N'NNH 5-(1-methy1-1H-imidazole-5-
NN jc
0 8 carboxamido)-2-oxohexanediamide
N
o
1-13: (S)-N1-(1-(2-(((1S,2R,5S)-6,6-
0,,,,,,AN------õ,
H dimethylbicyclo[3.1 .1]heptan-2-
o o yl)methylamino)-2-oxoethyl)-2-oxo-
1,2-
N 611
dihydropyridin-3-y1)-N6-ethy1-2-(1-
1\1_7--FIN µ1 H C) 0 methyl-I H-imidazole-5-
carboxamido)-5-
oxohexanediamide
o
1-14: (S)-N1-(1-(2-(((1R,2R,5R)-6,6-
OLN-----.,
H dimethylbicyclo[3.1 .1]heptan-2-
o 0
H it H ei yl)methylamino)-2-oxoethy1)-2-oxo-1,2-
NNN -NN'"
dihydropyridin-3-y1)-N6-ethyl-2-(1-
H 0 8
\ methyl-I H-imidazole-5-carboxamido)-5-
oxohexanediamide
1-15: 0
(S)-N1-ethyl-N6-(1-(2-(3-
H ethyladamantane-1-amino)-2-oxoethyl)-
o 0 2-oxo-1,2-dihydropyridin-3-y1)-5-
(1-
H it
,NH
H Ny
¨NN N methyl-I H-imidazole-4-carboxamido)-2-
0 8
oxohexanediamide
1-16: 0
OJL
(S)-N1-ethyl-N6-(1-(2-(3-
---õ,,
N--- H ethyladamantane-1-amino)-2-oxoethyl)-
O H 0 2-oxo-1,2-dihydropyridin-3-y1)-5-(1-
II
(N)N NH N'rN-r methyl-I H-imidazole-2-
carboxamido)-2-
-H 0 0 oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
52
1-17: 0 (S)-2-(1,4-dimethy1-1H-imidazole-5-
H carboxamido)-N6-ethyl-N1-(1-(2-(3-
, õ r H
0 ethyladamantane-1-amino)-2-oxoethyl)-
N
,Nj- -),(NH
N fl 1 N 2-oxo-1,2-dihydropyridin-3-y1)-5-
" .. 0 ,1,,,._...;_j 0
"N oxohexanediamide
o
1-18: o.õ)-N..---,,, (S)-N1-ethy1-5-(1-isobuty1-1H-
imidazole-
L.
H 4-carboxamido)-N6-(1-(2-(3-
o 0
H i ethyladamantane-1-amino)-2-oxoethyl)-
/---N:--N HNIN C)io NH
----\ \ 2-oxo-1,2-dihydropyridin-3-y1)-2-
oxohexanediamide
o
1-19: (S)-2-(1-cyclopenty1-1H-imidazole-4-
o..--...õ
N
H carboxamido)-N6-ethyl-N1-(1-(2-(3-
0 0 N H
N N-{N1H ethyladamantane-1-amino)-2-oxoethyl)-
---N
H 0jJ6 2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
o
1-20: o-jt.N..---,,, (S)-2-(1-cyclobuty1-1H-imidazole-4-
H carboxamido)-N6-ethyl-N1-(1-(2-(3-
ethyladamantane-1-amino)-2-oxoethyl)-
- 0N N N N NINH
H 2-oxo-1,2-dihydropyridin-3-y1)-5-
\_,--- 0 0
oxohexanediamide
1-21: 0 (S)-2-(1,4-dimethy1-1H-imidazole-5-
0N
H carboxamido)-N6-ethyl-N1-(1-(2-(3,5-
0 H j dimethyladamantane-1-amino)-2-
NH
1 NI
N1)\FIN 0 N 0 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
\ 5-oxohexanediamide
o
1-22: 0,)-1...N (S)-N1-ethyl-N6-(1-(2-(3,5-
-.õ,----.õ
H dimethyladamantane-1-amino)-2-
o l oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
NH
1
¨NN Fi N 5-(1-methyl-1H-imidazole-4-
H
carboxamido)-2-oxohexanediamide
o
1-23: OAN (S)-N1-ethyl-N6-(1-(2-(3,5-
H dimethyladamantane-1-amino)-2-
o 0
N
/\ N(iF11)-N'-iNH oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
5-(1-methy1-1H-imidazole-2-
\_N H 0 0
carboxamido)-2-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
53
1-24: 0 (S)-2-(1,2-dimethy1-1H-imidazole-5-
H carboxamido)-N6-ethyl-N1-(1-(2-(3,5-
O H 0 dimethyladamantane-1-amino)-2-
1\1)-
NH oxoethy1)-2-oxo-1,2-dihydropyridin-3-
y1)-
-N H 0 0
5-oxohexanediamide
1-25: 0
0,L.N (S)-N1-ethyl-N6-(1-(2-(3-
H .----
methyladamantane-1-amino)-2-
O 0 oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N[lji )-LI N rNH 5-(1-methy1-1H-imidazole-5-
---N \ 0 0 carboxamido)-2-oxohexanediamide
0
1-26: (S)-2-(2-chloro-1-methy1-1H-imidazole-5-
{..N.---,,
H carboxamido)-N6-ethyl-N1-(1-(2-(3,5-
O 0 dimethyladamantane-1-amino)-2-
N H
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
,--N \ 0 0
5-oxohexanediamide
CI
1-27: 0
(S)-2-(1,2-dimethy1-1H-imidazole-5-
0N
H carboxamido)-N6-ethyl-N1-(1-(2-(3-
O o methyladamantane-1-amino)-2-
N FIN {NH
1\l/Ncr oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-
-N \ H 0 8
5-oxohexanediamide
0
1-28: (S)-N1-ethyl-N6-(1-(2-(3,5,7-trimethy1-1-
oN
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
O H. o dihydropyridin-3-y1)-5-(1-
methy1-1H-
N NH
N
imidazole-5-carboxamido)-2-
i--INN
0 .,,,,0
\ oxohexanediamide
1-29: o
(S)-2-(2-chloro-1-methy1-1H-imidazole-5-
0N----õ,
H carboxamido)-N6-ethyl-N1-(1-(2-(3,5,7-
O trimethy1-1-adamantylamino)-2-
N Sil\ii--11 N c-INH
oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-
-N \
5-oxohexanediamide
CI
0
0..1.,N (S)-2-(benzofuran-2-carboxamido)-N6-
,....õ1.----...õ,,
H ethyl-N1-(1-(2-(2-adamantylamino)-2-
1-30 o k o
F oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
o NJcr 1\INNI
1 H 0 8 5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
54
o
o..LN (S)-N1-ethyl-N6-(1-(2-(2-
)
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
o o
1-31 H II H dihydropyridin-3-yI)-5-(3-
0 NrN-.Kil.rN
1 H 0 0 methylbenzofuran-2-carboxamido)-2-
oxohexanediamide
o
o)-N, (S)-2-(3-chlorobenzofuran-2-
H carboxamido)-N6-ethyl-N1 -(1 -(2-(2-
1-32 H H
0 0 NJcr
0 adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
Nj-NrN
I H 1 I 0 0 dihydropyridin-3-yI)-5-
oxohexanediamide
CI
0
(S)-2-(4-bromobenzofuran-2-
H carboxamido)-N6-ethyl-N1 -(1 -(2-(2-
1-33 0
o 0
H ii H adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
NiJc
0 N'N.rN
I H dihydropyridin-3-yI)-5-
oxohexanediamide
0
Br
0
0 (S)-2-(benzo[b]thiophene-2-
N`
H carboxamido)-N6-ethyl-N1 -(1 -(242-
1-34 0 H ? H
S N. adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N N-INI
I H 1
0 0 dihydropyridin-3-yI)-5-oxohexanediamide
o
0 N (S)-N1-ethyl-5-(7-
..---.,
H fluorobenzo[b]thiophene-2-
o 1.4 0
H
1-35 NA carboxamido)-N6-(1 -(242-
---- N i IIIINI
S H 0 0 adamantylamino)-2-oxoethyl)-2-oxo-1,2-
F dihydropyridin-3-y1)-2-oxohexanediamide
0
ON (S)-2-(4,5-difluoro-1H-indole-2-
H carboxamido)-N6-ethyl-N1 -(1 -(2-(2-
o Nr E., 0
1-36 H
N N,A adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N-_r H N
I H I
0 0 dihydropyridin-3-yI)-5-oxohexanediamide
F F
0
o-.N (S)-N1 -ethyl-N6-(1 -(242-
. .JL ,---,,,
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
o iFi o
1-37 H H
N Nj-L dihydropyridin-3-y1)-5-(3-methy1-1 H.
N-rrµj
I H U indole-2-carboxamido)-2-
o o
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
55
o
0)N (S)-2-(1H-benzo[d]imidazole-2-
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
FAo
H
adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N 1 It
o 'N
1-38 H
41 N H 0 _ (NN0 N dihydropyridin-3-yI)-5-oxohexanediamide
o
011-.N (S)-2-(2,3-dihydro-1H-indene-2-
--",õ
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-39 o H 0
l'iN H adamantylamino)-2-oxoethyl)-2-oxo-
N
0
_ Ji riµi H 1,2-dihydropyridin-3-yI)-5-
0
oxohexanediamide
o
0..,,,J-L.N (S)-N1-ethyl-N6-(1-(2-(2-
.õ---,,,
H adamantylamino)-2-oxoethyl)-2-oxo-
o o
1-40 s_.A NH H
N 1,2-dihydropyridin-3-yI)-5-(4-methyl-
F3c----- i Njcro 1
2-(trifluoromethyl)thiazole-5-
N
carboxamido)-2-oxohexanediamide
o
o....}..N
(S)-2-(4-bromo-2-
..----õ,õ
H (trifluoromethyl)thiazole-5-
ki
0 H
carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-41 F3c--e_ALIr 1 Nf
N ' ' 0 0 adamantylamino)-2-oxoethyl)-2-oxo-
Br
1,2-dihydropyridin-3-yI)-5-
oxohexanediamide
0
o N (S)-N1-ethyl-N6-(1-(2-(2-
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
o o
1-42 S_.)-L N IN1 I 1r\J I:1 dihydropyridin-3-y1)-5-(4-
methyl-2-
\ I H
N 0 0 phenylthiazole-5-carboxamido)-2-
oxohexanediamide
o
0.t.N.- (S)-2-(5-bromo-3-methylthiophene-2-
-...,
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
o
H
s o r[l
adamantylamino)-2-oxoethyl)-2-oxo-1,2-
1-43
\ i H 0 8 dihydropyridin-3-yI)-5-
oxohexanediamide
o
0 0
(S)-2-(3,5-dibromothiophene-2-
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
H N
Br
1-44 H adamantylamino)-2-oxoethyl)-2-oxo-
Br N
\ I H 0 8 N 1,2-dihydropyridin-3-yI)-5-
oxohexanediamide
o
oN (S)-2-(5-bromo-3-methylfuran-2-
...---.......
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-45 0
H H
0 N Br1 HN N adamantylamino)-2-oxoethyl)-2-oxo-
\ N
0 8
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
56
1,2-dihydropyridin-3-yI)-5-
oxohexanediamide
o
o)N (S)-2-(2,5-dichlorothiophene-3-
õ,,.. --,......
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
O r Fo
1-46 adamantylamino)-2-oxoethyl)-2-oxo-
a /
s 0 1 hif 1 rnf 1,2-dihydropyridin-3-yI)-5-
0
CI oxohexanediamide
o
0 N (S)-2-(2,5-dibromothiophene-3-
---..õ
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
O r on
1-47 adamantylamino)-2-oxoethyl)-2-oxo-
Thr rN, 0 1,2-dihydropyridin-3-yI)-5-
s 0
Br
oxohexanediamide
o
0 ,N,- (S)-2-(2,5-dichlorothiazole-4-
-,..
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
0
1-48 N,, Nj-LNI -1
adamantylamino)-2-oxoethyl)-2-oxo-
FI\1
CI----- I pi
s o o 1,2-dihydropyridin-3-yI)-5-
a oxohexanediamide
o
0 .N (S)-2-(2,5-dimethylfuran-3-
.--
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
o o
1-49 adamantylamino)-2-oxoethyI)-2-oxo-
/ 1 iljCr 1,2-dihydropyridin-3-yI)-5-
o 0 0
oxohexanediamide
o
0 .N (S)-2-(4-bromothiazole-2-
.---,õ
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-50 0 SN f)l 0
H adamantylamino)-2-oxoethyI)-2-oxo-
Nj-
1 N-11\1 1,2-dihydropyridin-3-yI)-5-
$ JN H 0 0
oxohexanediamide
Br
0
(S)-2-(4-bromothiophene-2-
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
o ti o
1-51
, s)tNJc(N H adamantylamino)-2-oxoethyl)-2-oxo-
1 N-rNj 1,2-dihydropyridin-3-yI)-5-
Br \ i H 0 0
oxohexanediamide
0
0-I-L.N (S)-2-(4-bromo-5-chlorothiophene-2-
,,,...----,,
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
O H 0
1-52 F adamantylamino)-2-oxoethyl)-2-oxo-
s N r\lANN-1
CI \ I H 0 8 1,2-dihydropyridin-3-yI)-5-
Br oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
57
o
oN (S)-2-(4,5-dibromothiophene-2-
---....õ
H carboxam ido)-N6-ethyl-N1 -(1-(2-(2-
0
H 0
1-53 Nj -1 kli adamantylamino)-2-oxoethyl)-2-oxo-
S
Br i N 1 N
\ i H 0 0 1,2-dihydropyridin-3-yI)-5-
Br oxohexanediamide
o
0y),N (S)-2-(4,5-dichlorothiophene-2-
.--,õ
H carboxam ido)-N6-ethyl-N1 -(1-(2-(2-
.(Fi 0
1-54 s i N Nj= kil adamantylamino)-2-oxoethyl)-2-oxo-
0
ci , N-r
\ i H 0 0 1,2-dihydropyridin-3-yI)-5-
ci oxohexanediamide
o
ON.-------õ, (S)-2-((S)-1-acetylpyrrolidine-2-
H carboxamido)-N6-ethyl-N1-(1-(2-(2-
o ,
,.., N_AN __.(fiy o
H adamantylamino)-2-oxoethy1)-2-oxo-
- NJ. N
N-r 1,2-dihydropyridin-3-yI)-5-
U H 0 0
oxohexanediamide
o
0)-1,..N (S)-N1-ethyl-N6-(1 -(242-
.----,,,..
H adamantylam ino)-2-oxoethyl)-2-oxo-
1-56 o jcH 0
H 1,2-dihydropyridin-3-yI)-5-(1-methyl-
Nj N
N/A1F1 1 N 1H-1 ,2,3-triazole-5-carboxam ido)-2-
`1\\,1-N \ 0 0
oxohexanediamide
o
0N
(S)-N1-ethyl-N6-(1 -(2-(2-
------..õ
H adamantylam ino)-2-oxoethyl)-2-oxo-
1-57 o o
, H 1,2-dihydropyridin-3-yI)-2-oxo-5-(2H-
HN 1 YiN
tetrazole-5-
µN----,N o 0
carboxamido)hexanediamide
o
(S)-N1-ethyl-N6-(1 -(245-
H hydroxyadamantane-2-am ino)-2-
0 H 0
1-58 1\lj Nj ill oxoethyl)-2-oxo-1 ,2-dihyd ropyridin-3-
1 N 1 N If
H
0 0 yI)-2-oxo-5-(pyrazine-2-
OH
carboxamido)hexanediamide
o
0_,.-1-1..N (S)-N1-ethyl-N6-(1 -(245-
...----..,
H fluoroadamantane-2-am ino)-2-
1-59 c ,jo. (rFi j H oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
N 1 NI ThrN
= H y1)-2-oxo-54(S)-pyrrolidine-3-
HN---- 0 0 F
carboxamido)hexanediamide
o
(S)-N1-ethyl-N6-(1 -(245-
H chloroadamantane-2-am ino)-2-
1-60 H 0 (rH 0 Fd oxoethyI)-2-oxo-1,2-dihydropyridin-3-
N Nj-Ni
- N
= H
----..,....--- 0 0 CI
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
58
y1)-2-oxo-54(S)-piperidine-2-
carboxamido)hexanediamide
o
(S)-N1-ethyl-N6-(1-(2-(5-
0,-,,AN,---,,
H bromoadamantane-2-amino)-2-
0 r N 0
1-61 H oxoethy1)-2-oxo-1,2-dihydropyridin-3-
HNNN , N-rN
H 0 0 Br y1)-2-oxo-5((R)-piperidine-3-
carboxamido)hexanediamide
o
(S)-N1-ethyl-N6-(1-(2-(5-
0,,,,:,A.N..^....õ
H methyladamantane-2-amino)-2-
1-62 H H 0
H oxoethy1)-2-oxo-1,2-dihydropyridin-3-
N)AN N)-L
Ji NN
y1)-5-((R)-morpholine-3-
H
0 0 0
carboxamido)-2-oxohexanediamide
o
o N (S)-N1-ethyl-N6-(1-(2-(2-
H carbonitrileadamantane-2-amino)-2-
x n
1-63 N- 0
H H CN oxoethyl)-2-oxo-1,2-dihydropyridin-3-
i
N
_ JNrN
H y1)-2-oxo-54(3S,4R)-quinuclidine-3-
o o
carboxamido)hexanediamide
o
0AN (S)-methyl 3-(6-(ethylamino)-1-(1-(2-
---õ,
H (methyl 2-aminoadamantane-2-
1-64 o2N N jcrN,)HNNI-1 C 21VIe ,2-
LJJ
0 0 LJi dihydropyridin-3-ylamino)-1,5,6-
CO2Me trioxohexan-2-ylcarbamoy1)-5-
nitrobenzoate
o
(S)-N1-(1-(2-(1-
o
H adamantylmethylamino)-2-oxoethyl)-
1\110 N
H
1-65 o2N I N ii 2-oxo-1,2-dihydropyridin-3-y1)-N6-
H
The 0 N 0 ethy1-2-(5-nitronicotinamido)-5-
oxohexanediamide
0
0 5-((2S)-1-(1-(2-1-
N
H adamantylmethylamino)-2-oxoethyl)-
0 õ 0
H
1-66 1 Ho2cN Nj- ,N 2-oxo-1,2-dihydropyridin-3-ylamino)-
H 1 N y
0 0
The 6-(ethylamino)-1,5,6-trioxohexan-2-
ylcarbamoyl)nicotinic acid
o
o N (S)-N1-ethyl-N6-(1-(2-(2-
H adamantyl(methyl)amino)-2-oxoethyl)-2-
1-67 -r-----\- Nj_, oxo-1,2-dihydropyridin-3-y1)-5-(6-
) ,N
hl N 1NTr
methylimidazo[2,1-b]thiazole-5-
N 0 0
carboxamido)-2-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
59
o
(S)-N1-(1-(2-((1S,2R,4R)-
,1
H bicyclo[2.2.1]heptan-2-ylamino)-2-
1-68 0
H 0 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
0 N NNThrNH
1 H y1)-N6-ethy1-2-(3-methylbenzofuran-
0 0
2-carboxamido)-5-oxohexanediamide
0 (S)-N1-ethy1-5-(3-methylbenzofuran-
ON---....õ
2-ca-2-oxo-N6-(2-oxo-1-
H
0 0 (2-oxo-24(1R,2S,4R)-1,7,7-
1-69 H II
0 N NN INH trimethylbicyclo[2.2.1]heptan-2-
I H 1 1
0 0 ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
o
0õ,.)L,N (S)-N1-(1-(2-((1R,2R,4S)-
,.,-,õ.
H c H bicyclo[2.2.1Theptan-2-ylamino)-2-
1-70 0 j 0
0 N oxoethyl)-2-oxo-1,2-dihydropyridin-3-
N 1 Ni
1 H
0 0 y1)-N6-ethy1-2-(3-methylbenzofuran-
2-carboxamido)-5-oxohexanediamide
0 (S)-N1-(1-(2-((1s,4R)-
0
bicyclo[2.2.1]heptan-1-ylamino)-2-
H
1-71 0
NNH y1)-N6-ethyl-2-(isonicotinamido)-5-
N)LI N 0 Nt 0
H oxohexanediamide
0 (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-
0.N.---- õTr
ylamino)-2-oxoethyl)-2-oxo-1,2-
H
1-72 0 H 0 dihydropyridin-3-y1)-N6-ethyl-5-oxo-2-
N N N
'A ,NH
1 I (pyridazine-4-
NI.N H 0 0 carboxamido)hexanediamide
0
(S)-N1-(1-(2-((1R,2R,4R)-
N
H bicyclo[2.2.1]hept-5-en-2-ylamino)-2-
1-73 0 iY jcH 0 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
NA ,NH iNi 1 N Ii y1)-N6-ethy1-5-oxo-2-(pyridazine-3-
0 0
N-'1\1 carboxamido)hexanediamide
o
0 N (2S)-N1-(1-(2-(bicyclo[2.2.2]octan-2-
H ylamino)-2-oxoethyl)-2-oxo-1,2-
1-74 0 JIIIIH ? H dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-
vy(N NI NQI
N (2H-1,2,3-triazole-4-
i-k-N H 0 0
carboxamido)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
60
o
0,.N (S)-N1-ethyl-5-(1-methyl-1 H-1,2,3-
..-----õ,
H triazole-4-carboxamido)-2-oxo-N6-(2-
1-75
o jc H 0
H oxo-1-(2-oxo-2-((1 R,2R,4R)-1,7,7-
/A N
_NJ ---- N Y-Ni trimethylbicyclo[2.2.1]heptan-2-
sr\I--N H 0 0
ylamino)ethyl)-1 ,2-d ihydropyrid in-3-
yl)hexanediamide
o
0
N (S)-N1-ethyl-5-(1-methyl-1 H-1 ,2,4-
H triazole-3-carboxamido)-2-oxo-N6-(2-
o o oxo-1- 2-oxo-2- 1R 2R 3R 5S -
( (( õ , )
1-76 1\1Y-NljcrEl
I\1 ,ENI
1 N If ''. 2,6,6-trimethylbicyclo[3.1.1]heptan-3-
N_N H 0 0
/ ylamino)ethyl)-1 ,2-d ihydropyrid in-3-
yl)hexanediam ide
o
0N (S)-2-(benzofuran-3-carboxam ido)-
.-----,,,
H N6-ethyl-5-oxo-N1 -(2-oxo-1-(2-oxo-2-
0 H 0
Nj-L L1 ((I S,2S,3S,5R)-2,6,6-
1-77 1 N N n
H 0 0 trimethylbicyclo[3.1.1]heptan-3-
o
s,-
ylamino)ethyl)-1 ,2-d ihydropyrid in-3-
yl)hexanediam ide
o
(S)-2-(benzo[b]thiophene-3-
0,-,...A...N...---..õ
H carboxam ido)-N6-ethyl-N1 -(1-(2-(4-
1-78 o jc H o
F homoisotwistane-3-amino)-2-
N N'AN-rN-1
1 H 0 0 oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-
S
y1)-5-oxohexanediamide
0 (S)-N1-ethyl-N6-(1-(2-(diamantane-1-
0-LN pg
am ino)-2-oxoethyl)-2-oxo-1 ,2-
H
0 di h dro rid in-3- 1 -5- 1-meth 1- H-
1-79Y PY Y ) ( y 1
N 1\i
/y N JC Filj-L NH pyrazole-4-carboxam ido)-2-
/
11 r
I 14
N - 0 0 oxohexanediamide
/
o
0.N (S)-N1-ethyl-N6-(1-(2-(diamantane-4-
.õ---,õ
H am ino)-2-oxoethyl)-2-oxo-1 ,2-
1-80 0
H ? di hydropyrid in-3-y1)-5-(1-methy1-1 H-
,
L, H pyrazole-3-carboxam ido)-2-
N-11 0 0
/ oxohexanediamide
0 0 (S)-N1-(1-(1-adamantylmethy1)-2-
.-----..õ.õ
N oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-
H
1-81 _ i:j: H 0 2- 1-meth 1-1H- razole-5-
( Y PY
N N
carboxamido)-5-oxohexanediamide
----- 1
T -H
N---"\ 0
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
61
0 O N (S)-N1-(1((3-hydroxy-1-
H
adamantyl)methyl)-2-oxo-1,2-
1-82 N
0 0 di hydropyrid in-3-y1)-2-(4-
cyclopropyl-
H II
,S_1 ,NJCNN 1,2,3-th iadiazole-5-carboxam ido)-N6-
H
N 0 ethyl-5-oxohexanediamide
OH
0 (S)-N1-(1((3-bromo-1-
0 A
H
N - adamantyl)methyl)-2-oxo-1,2-
0 di hydropyrid in-3-y1)-N6-ethyl-5-oxo-
2-
1-83 - 1 JCI-d N (1 ,2,5-thiadiazole-3-
N/ 1 r Y -N H
\S---N 0 carboxamido)hexanediamide
Br
0 0 (S)-N1-(1-(2-adamantylmethyl)-2-
N
oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-
H
0 0 2-(4-(hydroxymethyl)-1 ,2,3-
1-84 H N1 II
,,
,N thiadiazole-5-carboxam ido)-5-
1 7
N 0 oxohexanediamide
HO
0
OAN
(S)-2-(4-tert-butyl-1 H-pyrrole-3-
H carboxam ido)-N1 -(1 -(241 -
o N,cNH oll adamantylamino)ethyl)-2-
oxo-1 ,2-
1-85
N di hydropyrid in-3-y1)-N6-ethyl-5-
HN H
0 oxohexanediamide
o
N (S)-2-(4-cyano-1-methyl-1 H-pyrrole-
õ---.õ.,
H 2-carboxamido)-N1-(1-(1-
0 hi o
1-86 adamantylam ino)propy1)-2-oxo-1 ,2-
NC --- 1\lciNI N
\ N H 0 di hydropyrid in-3-y1)-N6-ethyl-5-
\
oxohexanediamide
0
(S)-N1-(1-(3-(2-adamantylamino)-3-
0y1LN...--,_
H oxopropy1)-2-oxo-1 ,2-dihydropyridi n-
1-87 0 rFi 0 0
3-y1)-N6-ethy1-2-(5-methoxyoxazole-
Me0_7)
-- H" 0 H
2-carboxamido)-5-oxohexanediamide
o
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
oN------,õ
H ylamino)-2-oxoethyl)-2-oxo-1 ,2-
1-88 0
H 0 di hydropyrid in-3-y1)-N6-ethyl-2-(3-
o NJcrr\O-N(NFI
I H I 1 methylbenzofuran-2-carboxam ido)-5-
0 0
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
62
o
(S)-2-(2-acetyloxazole-4-
0N-----õ,,
H carboxamido)-N1-(1-(2-
1-89 (bicyclo[1.1.1]pentan-1-ylamino)-2-
L'\\ NDAN NN_rN,IH
oxoethyl)-2-oxo-1,2-dihydropyridin-3-
o 0 o
y1)-N6-ethy1-5-oxohexanediamide
o
0 ,N (S)-N1-(1-(2-(bicyclo[2.1.1Thexan-1-
..--.,
H ylamino)-2-oxoethyl)-2-oxo-1,2-
1-90 0 ( Hr,__, 0
0_ J J H dihydropyridin-3-y1)-N6-ethyl-2-(2-
C)I
isopropyloxdamideaizole-5-carboxamido)-5-
N " 0
hexane oxo/ 0
0
(2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-
0N----,,
H ylamino)-2-oxoethyl)-2-oxo-1,2-
1-91 \ ? o dihydropyridin-3-yI)-2-(3,5-
j-
10,1)1c i ji 1\1 H 1\1 dimethylisoxazole-4-
carboxamido)-
N 0 0 N6-ethyl-5-oxohexanediamide
o
0N (S)-N1-ethyl-N6-(1-(2-(5-carboxy-2-
.---,õ...,
H aminoadamantane)-2-oxoethyl)-2-
o o
1-92 H Li oxo-1,2-dihydropyridin-3-yI)-5-(4-
r\r H 0 0 co2H methylpyrimidine-5-carboxamido)-2-
oxohexanediamide
0
0 (5S)-N1-ethyl-N6-(1-(2-(4-
N-----'
H
0 H H 0 aminoadamantane-N,N-dimethy1-1-
1-93 H
N NN-rN carboxamide)-2-oxoethyl)-2-oxo-1,2-
H 0 0
N--...
dihydropyridin-3-yI)-2-oxo-5-(1,2,3,4-
0
tetrahydronaphthalene-2-
carboxamido)hexanediamide
o
0,,A.N (S)-2-((S)-1,4-
...--...õ
H diazabicyclo[2.2.2]octane-2-
NN 0
HcrFi 0
.._-- '..A INIA carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-94
N N 1 NMI
H
0 0 adamantylamino)-2-oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
o
(S)-N1-ethy1-5-(1H-indole-3-
0,N----..õ
H carboxamido)-N6-(1-(2-(2-
1-95 o ciFi o
1- adamantylamino)-2-oxoethyl)-2-oxo-
N ININThr1\11
I H 0 0 1,2-dihydropyridin-3-yI)-2-
N
H oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
63
o
(S)-N1-ethyl-N6-(1 -(242-
0,=.,)--1-NN.---,,,,
H adamantylamino)-2-oxoethyl)-2-oxo-
F
o .rF., o
1-96 ------3)-L Nj-L N1 1,2-d ihydropyridin-3-yI)-5-(6-
N's methylimidazo[2,1-b]thiazole-3-
o 0
carboxamido)-2-oxohexanediamide
o
or\ ,o__ (S)-2-(benzo[d]thiazole-2-
H carboxam ido)-N1 -(1 -(2-((1 S,2R,4R)-
1-97 0 0 bicyclo[2.2.1]heptan-2-ylami no)-2-
S ,i)-.N Nj-Nl_r NH
NI H i I oxoethyl)-2-oxo-1 ,2-dihyd ropyridin-3-
0 0
y1)-N6-ethyl-5-oxohexanediamide
o
o N
(S)-N1-(1-(2-((1 S,2R,4R)-
H bicyclo[2.2.1Theptan-2-ylami no)-2-
1-98
o 0 '-'1"\-- oxoethyl)-2-oxo-1 ,2-dihyd
ropyridin-3-
NcrEN-111 NI ThrNH
y1)-N6-ethyl-2-(im idazo[2,1-
E>5-:-N H o 0 b]thiazole-6-carboxamido)-5-
s
oxohexanediamide
0
(S)-N1-(1-(2-((1 S,2R,4R)-
0 N.--....õ ,0
H bicyclo[2.2.1]heptan-2-ylami no)-2-
OHO , 0
oxoethyl)-2-oxo-1 2-dihyd ropyridin-3-
1_99 F3c- --- . N N N-Thf - = ' - ' -
I H 0 0
r\J y1)-N6-ethy1-2-(4-hydroxy-6-
(trifluoromethoxy)quinoline-3-
carboxamido)-5-oxohexanediamide
o
0 N-- (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
--õ, ,7L
H ylamino)-2-oxoethyl)-2-oxo-1 ,2-
1-100 0
H 0 dihydropyridin-3-yI)-2-(cinnoline-3-
Ncr\ij-.N(NI-1
I , H
0 0 carboxamido)-N6-ethyl-5-
NN oxohexanediamide
o
O N (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
H ylamino)-2-oxoethyl)-2-oxo-1 ,2-
o o
1-101 H II di hydropyrid in-3-y1)-N6-ethyl-2-(3-
0 N.iN,NNH
i H 1 1 ethylbenzofu ran-2-carboxam ido)-5-
0 0
oxohexanediamide
o
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
0.N.."..õ
H ylamino)-2-oxoethyl)-2-oxo-1 ,2-
1-102 di hydropyrid in-3-y1)-N6-ethy1-2-(1-
N N Nj-r\JrNH
1 H
0 0 ethyl-1H-indole-2-carboxamido)-5-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
64
o
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
..,____)\
H ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-103 o ri_11 o
di hydropyrid in-3-y1)-N6-ethy1-2-(2-
N 1 N If methy1-1,8-naphthyridine-3-
, H 0 I J 0
NN carboxamido)-5-oxohexanediamide
o
0
(S)-N1-(1-(2-(bicyclo[2.1.1Thexan-1-
N
H ylam ino)-2-oxoethyl)-2-oxo-1,2-
0
H 0
1-104 N,A NI di hydropyrid in-3-y1)-N6-ethy1-5-oxo-
2-
N N
H 0 8 19 (1,2,3,4-tetrahydroquinoline-6-
N
H carboxamido)hexanediamide
0
0 N...--., (S)-N1-ethyl-N6-(1-(2-(2-carboxy-2-
H
0 0 amino-5-
0 u CO2H
(trifluoromethyl)adamantane)-2-
H
0 0
1-105 HN CF3 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-oxo-5-(3-oxo-1,2,3,4-
tetrahydroisoquinoline-6-
carboxamido)hexanediamide
0
0 N..---,,, (S)-N1-ethyl-N6-(1-(2-(5-
H ethyladam antane-2-am ino)-2-
0 H 0 H
1-106 oxoethyl)-2-oxo-1,2-dihyd ropyridin-3-
1 H
N 0 u lor
y1)-5-(1,6-naphthyridine-2-
carboxamido)-2-oxohexanediamide
o
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-
oN
H ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-107 N 0 H 0
H di hydropyrid in-3-y1)-N6-ethy1-2-(2,6-
1 Nj-
naphthyridine-1 -carboxamido)-5-
N H
0 0
oxohexanediamide
0 0)-1,,N (S)-2-(4-ami no-1,2 ,5-oxad iazole-3-
..-----,.... .._.7L
H carboxam ido)-N1-(1-(2-
1-108 H2N 0 0 (bicyclo[1.1.1]pentan-1-ylamino)-2-
id
L
N IYN HjC 11 NTh(NH oxoethyl)-2-oxo-1,2-dihydropyridin-3-
6-N 0 0 y1)-N6-ethyl-5-oxohexanediamide
o
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
oN
0
H
1-109 N ylam ino)-2-oxoethyl)-2-oxo-1,2-
0 Jci-HC) di hydropyrid in-3-y1)-2-(6-
N _
N ir
NH
\ I H 0 0 (dimethylamino)benzofuran-2-
N
/ carboxamido)-N6-ethy1-5-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
65
o
(S)-2-(2-acetamidothiazole-5-
0.N.---,õ ..4,
H carboxam ido)-N1 -(1 -(2-
1-110 o ) H o
NH
(bicyclo[1.1.1]pentan-1-ylamino)-2-
Nõ,
oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-
----\( N 0 0
0 y1)-N6-ethy1-5-oxohexanediamide
o
oN (S)-N4-(1 -(1 -(2-(bicyclo[1
.1.1]pentan-
H 1-ylamino)-2-oxoethyl)-2-oxo-1,2-
o o
1-111 0 ri NThr NH dihydropyridin-3-ylamino)-6-
H2N HN
/ I iljCr I (ethylamino)-1,5,6-trioxohexan-2-y1)-
0 0
1H-pyrrole-2,4-dicarboxamide
0
(S)-N1-ethyl-N6-(1-(2-(1-acetylamino-
H
0 0 4-am inoadam antane)-2-oxoethyl)-2-
0
1-112 (:)N AN -rFNI 0 H2N oxo-1 ,2-d ihydropyrid in-3-
yI)-2-oxo-5-
0
N
0
I H 0
H (5-sulfamoylfuran-3-
carboxamido)hexanediamide
0
O N--,,,, (S)-2-(benzofuran-5-
carboxamido)-
H N6-ethyl-N1 -(1-(2-(1 -acetylam ino-4-
0 H Ij? H
1-113/ NN, am inoadamantane)-2-oxoethyl)-2-
N N
0
H
0
0 0
N
H oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
0
(S)-2-(benzofuran-6-carboxamido)-
0
H N6-ethyl-NI-(1-(2-(4-
0
1-114 0 N NirN am inoadamantane-1-carboxamide)-
\ H
0 <1 0 H NH2 2-oxoethyl)-2-oxo-1 ,2-d ihydropyridin-
0
3-yI)-5-oxohexanediamide
0
O N..----,, (S)-N1-ethyl-N6-(1-(2-(4-
H
0 0 am inoadamantane-1-carboxamide)-
H H t-i
____\7)-LN N,N_rN 2-oxoethyl)-2-oxo-1 ,2-d ihydropyridin-
1-115 \I \N-0 " o 0 NH 3-y1)-5-(3-(1-methylcyclopropyl)-
0
1,2,4-oxadiazole-5-carboxamido)-2-
oxohexanediamide
o
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
oN õ_...).
H ylam ino)-2-oxoethyl)-2-oxo-1 ,2-
1-116 rFl 0 di hydropyrid in-3-y1)-N6-ethy1-2-(5-
N NH
----Ni il _ 'I NThr methyl-1 ,2,4-oxadiazole-3-
o-N o o carboxamido)-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
66
ID
13.)(N S (1-r(2d-in(13-3ic_yycol-
oN[16.-1e.t1h]yple-5n_toaxn0-1-2-
H _
- ylamino)-2-oxoethyl)-2-oxo-1,2-
1-117 0
c 0 µ---)' d( i h) y- N 1 d r o- y
InilAN lNH (1,2,3-thiadiazole-4-
s
I\ N'
\F-N H 0 c)
carboxamido)hexanediamide
ID ID1 (S)-N1-(1-(2-
(bicyclo[1.1.1]pentan-1-
,
H
N ylamino)-2-oxoethyl)-2-oxo-1,2-
1-118 0 0 ---/L dihydropyridin-3-y1)-N6-ethy1-5-oxo-
2-
j-L N,sy -LriJci (1,2,4-thiadiazole-5-
.--N 0 0 carboxamido)hexanediamide
0 0 N (S)-N1-(1-(2-
(bicyclo[1.1.1]pentan-1-
H ____),
ylamino)-2-oxoethyl)-2-oxo-1,2-
1-119
ip 1.1 0 dihydropyridin-3-y1)-N6-ethy1-5-
oxo-2-
N
S Nj-L (NH (1,3,4-thiadiazole-2-
IN1
N-N 0 0 carboxamido)hexanediamide
o
(S)-N1-ethy1-5-(4-formy1-1,2,3-
0N.---,õ
H thiadiazole-5-carboxamido)-N6-(1-
(2-
, 0
H
1-120 NI,jcNriUN ii N (2-adamantylamino)-2-oxoethyl)-2-
I
NJ H N H I 1 oxo-1,2-dihydropyridin-3-yI)-2-
\ o o
oxohexanediamide
o
0 (S)-N1-ethyl-N6-(1-(2-(3,5-
0N
dimethyladamantane-1-amino)-2-
H
1-121 0 jcH 0 oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
AN N'ANNH yI)-5-(nicotinamido)-2-
I H
0 0 oxohexanediamide
N
Method for production of inventive compounds
In some embodiments, the present invention relates to a method for the
synthesis of a
compound of formula (I), especially any compound of the formula (la):
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
67
0
0 N ,R6
H
0 0
R2NJCH
N N R -L, 3
1
H
0I
(la)
As shown in Scheme 1,
a method for producing the compound of the formula (la) comprising:
Step 1A: providing a compound 4a
0
AcON,R6
H
PWNOH
H
0 4a;
Step 2A: performing coupling reaction of the compound 4a with a compound 5
0
H2N....... ....1_, ,
1 N Rs'
I
5
to obtain a compound 6a
0
AcON-R6
H
0
PG3,Nk-11j- ,L .,
1 N 'R-
H
0I
6a;
Step 3A: deprotecting an amino protecting group PG3to obtain a compound 7a
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
68
0
AcON,R6
H
0
H2N1-N
N R-
0
7a;
Step 4A: performing coupling reaction of the compound 7a with a carboxylic
acid
(R2-CO2H 8) to obtain a compound 9a
0
AcON,R6
H
0
---,.... ,...CH
0
R2 N
N 3
1 N R
H
0I
9a;
Step 5A: performing oxidation reaction of the compound 9a to produce the
compound of the formula (la)
0
ON,R6
H
0
JCH
R2 N 0
1 N R
H
0I
(la);
wherein L, R2, R3,and R6 have the same meanings as defined above in formula
(la),
and
PG3 is an amino protecting group.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
69
Scheme 1
(b) coupling ¨ ¨ (c) deprotection PG1 & PG2
reaction 0 Ac0)-L N" R6 0
CEN¨R6
reprotection with PG3
OH _ + Ac0 )-N, R6
2a H 1) TFA H
PG1,N PG2 AcOH PG1.N_i PG2 2) PG320
PGNJcrOH
DCM
PG1 0 PG1 0 DMF, DIPEA H 0
4a
1 3a
0
H2N N -LR3
, Step 2A:
1
coupling
reaction
(HATU, DIPEA )
0 0
Ac0)-N - R6 Ac0 N - R6
H Step 3A H
0 H2N deprotection
H 9
rE ,L. 2 PG3N N ,L.
1 N R- (TFA) 1 N R3
0 H
0
6a
7a
Step 4A
R2¨CO2H 8 coupling
reaction
(HATU, DIPEA )
Y
0 0
AcO)-N,R6 0)-NR6.
H Step 5A H
2 0 0 oxidation 0 0
reaction
R2 N N 'r Ni R3
H
0 1) K2CO3, Me0H H
0
2) Dess-Martin
9a periodinane la
DMF
5
Optionally, Step 1A" is carried out before the step 1A:
(a) providing a protected aldehyde 1
1:::, H
PG1N PG2
l'Gl 0 I;
(b) performing a coupling reaction of the aldehyde 1 with an isocyanide (CN-
R6) 2a to
obtain an intermediate compound 3a
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
70
0
AcON'R6
H
PG1N PG2
-
PG1 0 3a;
(c)deprotecting the protecting groups PG1 and PG2 of the compound 3a
preferably under
acidic condition and introducing an amino protecting group PG3 to obtain a
compound
4a
0
AcON-R6
H
PG3N Jc OH
-
H 0 4a;
wherein R2, R6 have the same meanings as defined in formula (la),
PG1 and PG3 are amino protecting groups,
PG2 is a carboxyl protecting group.
Therefore, the following method for the production of compounds of the formula
(la) is
preferred:
Step 1A":
(a) providing a protected aldehyde 1
OH
PG1.NPG2
PG1 0 I;
(b) performing a coupling reaction of the aldehyde 1 with an isocyanide (CN-
R6)
2a to obtain an intermediate compound 3a
0
Ac0)-LN-R6
H
pG1N pG2
-
PG1 0 3a;
(c) deprotecting the protecting groups PG1 and PG2 of the compound 3a
preferably under acidic condition and introducing an amino protecting group
PG3 to obtain a compound 4a
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
71
0
Ac0)-LN-R6
H
PGNJCOH
H 0 4a;
Step 1A: providing a compound 4a
0
Ac0)-LN-R6
H
PGNON
H 0 4a;
Step 2A: performing coupling reaction of the compound 4a with a compound 5
0
H2N )-L ,L,
, N R3
5
to obtain a compound 6a
0
Ac0)-LN-R6
H
, 0
, N R-
H 0
6a;
Step 3A: deprotecting the amino protecting group PG3 to obtain a compound 7a
0
Ac0)-LN-R6
H
0
H
H2NjcNN-1--R3
0
7a;
Step 4A: performing coupling reaction of the compound 7a with a carboxylic
acid
(R2-CO2H 8) to obtain a compound 9a
0
Ac0)-N-R6
H
0 iCH 0
R2J-LN 1\1 ,L,
H 0
9a;
Step 5A: performing oxidation reaction of the compound 9a to produce the
compound of the formula (la)
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
72
0
0
N-R6
0 0
)-L
R2 N H 1 N L- 'R-
H 0
(la);
wherein L, R2, R3,and R6 have the same meanings as defined above in the
formula (la), and PG1 and PG3 are amino protecting groups,
PG2 is a carboxyl protecting group.
In an alternative route first all protecting groups PG1 and PG2 are
simultaneously removed
and the protecting group PG3 is selectively introduced. Preferably, PG1 and
PG3 are
same.
The term "protecting groups" as used herein refers to commonly used protection
groups
in organic synthesis, preferably for amino and carboxyl groups.
PG1, PG3, and PG5
preferably are suitable protecting groups for amino groups. PG2 and PG4
preferably are
suitable protecting groups for carboxyl groups. Preferably, PG1, PG3, and PG5
may be
selected from the group consisting of or comprising: acetyl, benzoyl,
benzyloxycarbonyl
(Cbz), tert-butylcarbonyl, tert-butyloxycarbonyl (Boc), and
fluorenylmethylenoxy group
(Fmoc). PG2 and PG4 may be selected from the group consisting of or
comprising:
methoxy, ethoxy, isobutoxy, tert-butoxy, benzyloxy; preferably, tert-butoxy
group.
In Step 2A to promote the coupling reaction with amino group of intermediate
compound,
activating reagents are commonly used to activating carboxylic acid (õPeptide
Coupling
Reagents, More than a Letter Soup", Ayman El-Faham and Fernando Albericio,
Chemical
Reviews, 2011, ///(//), p.6557-6602). The activation may be introduced
separate
reaction or in situ reaction. Preferably, any of the following coupling
reagent can be used
to activate carobxylic acid group: BOP (Benzotriazole-1-yl-oxy-tris-
(dimethylamino)-
phosphonium hexafluorophosphate), PyBOP (Benzotriazole-1-yl-oxy-tris-
pyrrolidino-
phosphonium hexafl uorophosphate), AOP
(7-(Azabenzotriazol-1-yl)oxy
tris(dimethylamino)phosphonium hexafluorophosphate), PyAOP ((7-Azabenzotriazol-
1-
yloxy)tripyrrolidinophosphonium hexafluorophosphate), TBTU (2-(1H-
Benzotriazole-1-
y1)-1,1,3,3-tetramethylaminium tetrafluoroborate), EEDQ (N-Ethoxycarbony1-2-
ethoxy-
1,2-dihydroquinoline), Polyphosphoric Acid (PPA), DPPA (Diphenyl phosphoryl
azide),
HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-
oxide
hexafluorophosphate), H BTU
(0-Benzotriazol-1-yl-N,N ,N',N'-tetramethyluronium
hexafluorophosphate), HOBt (1-Hyd roxybenzotriazo le),
HOAt (1-Hydroxy-7-
azabenzotriazole), DCC (N,W-Dicyclohexylcarbodiimide), EDC (or EDAC or EDCI, 1-
Ethyl-3-(3-dimethylaminopropyl)carbodiim ide), BOP-C1
(Bis(2-oxo-3-
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
73
oxazolidinyl)phosphinic chloride), TFFH
(Tetramethylfluoroformamidinium
hexafluorophosphate), BroP (Bromo tris(dimethylamino)
phosphonium
hexafluorophosphate), PyBroP
(Bromo-tris-pyrrolidino-phosphonium
hexafluorophosphate) and CI P
(2-Chloro-1,3-dimethylimidazolidinium
hexafluorophosphate), or further, similar acting reagents, providing an
activated
intermediate,or a mixture thereof.
Pharmaceutical Composition & Medical Use
Therefore another aspect of the present invention relates to compounds
according to the
general formula (I) as medicine as well as their use in medicine. Especially
preferred is
the use as inhibitors of transglutaminases, in particular transglutaminsase 2
(TG2).
Thus the compounds of formula (I) described herein or according to the present
invention
may be administered themselves or in form of a pharmacologically acceptable
salt.
The compounds of the present invention may form of a pharmacologically
acceptable salt
with organic or inorganic acids or bases. Examples of suitable acids for such
acid
addition salt formation are hydrochloric acid, hydrobromic acid, sulfuric
acid, phosphoric
acid, acetic acid, citric acid, oxalic acid, malonic acid, salicylic acid, p-
aminosalicylic acid,
malic acid, fumaric acid, succinic acid, ascorbic acid, maleic acid, sulfonic
acid,
phosphonic acid, perchloric acid, nitric acid, formic acid, propionic acid,
gluconic acid,
lactic acid, tartaric acid, hydroxymaleic acid, pyruvic acid, phenylacetic
acid, benzoic acid,
p-aminobenzoic acid, p-hydroxybenzoic acid, methanesulfonic acid,
ethanesulfonic acid,
nitrous acid, hydroxyethanesulfonic acid, ethylenesulfonic acid, p-
toluenesulfonic acid,
naphthylsulfonic acid, sulfanilic acid, camphorsulfonic acid, china acid,
mandelic acid, o-
methylmandelic acid, hydrogen-benzenesulfonic acid, picric acid, adipic acid,
d-o-
tolyltartaric acid, tartronic acid, (o, m, p)-toluic acid, naphthylamine
sulfonic acid,
trifluoroacetic acid, and other mineral or carboxylic acids well known to
those skilled in
the art. The salts are prepared by contacting the free base form with a
sufficient amount
of the desired acid to produce a salt in the conventional manner. Preferred is
the
mesylate salt, hydrochloride salt and the trifluoroacetate salt and especially
preferred is
the trifluoroacetate salt and the hydrochloride salt.
In the case the inventive compounds bear acidic groups, salts could also be
formed with
inorganic or organic bases. Examples for suitable inorganic or organic bases
are, for
example, NaOH, KOH, NH4OH, tetraalkylammonium hydroxide, lysine or arginine
and the
like. Salts may be prepared in a conventional manner using methods well known
in the
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
74
art, for example by treatment of a solution of the compound of the general
formula (I) with
a solution of an acid, selected out of the group mentioned above.
Methods of Use
In a further aspect of the present invention, the novel compounds according to
the general
formula (I) are used as pharmaceutically active agent, i.e. the compound of
the formula
(I) is used in medicine.
Furthermore, the present invention relates to a pharmaceutical composition
comprising
at least one compound according to the general formula (I), as an active
ingredient or a
pharmacologically acceptable salts thereof as an active ingredient, together
with at least
one pharmacologically acceptable carrier, excipient and/or diluent.
The compounds according to general formula (I) described herein are especially
suitable
for the treatment and prophylaxis of diseases associated with and/or caused by
transglutaminase 2.
Celiac disease, a gluten intolerance is associated with tissue
transglutaminase (TG 2).
Another very important group of indications for tissue transglutaminase
inhibitors are
fibrotic disorders. Fibrotic disorders are characterized by the accumulation
of cross-linked
extracellular matrix proteins. Diabetic nephropathy, cystic fibrosis,
idiopathic pulmonary
fibrosis, kidney fibrosis as well as liver fibrosis belong to the most
important fibrotic
disorders to be addressed with the compounds disclosed.
In the biological example B-1, it is proven that the inventive compounds as
reversible
inhibitors effectively inhibit the activity of TGs, especially TG2.
As used herein the term "inhibiting" or "inhibition" refers to the ability of
a compound to
downregulate, decrease, reduce, suppress, inactivate, or inhibit at least
partially the activity
of an enzyme, or the expression of an enzyme or protein.
Therefore, another aspect of the present invention is the use of the inventive
compounds
of the general formula (I), or the pharmaceutical composition thereof as
described in the
treatment or prophylaxis of autoimmune and inflammatory diseases, vascular
diseases,
fibrotic diseases, liver diseases, cholestatic liver diseases, cancer,
neurodegenerative
diseases, ocular diseases, and skin disorders.
Further aspects of the present invention relate to the use of the compounds of
general
formula (I) for the preparation of a pharmaceutical composition useful for
prophylaxis
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
75
and/or treatment of autoimmune and inflammatory diseases, vascular diseases,
fibrotic
diseases, liver diseases, cholestatic liver diseases, cancer,
neurodegenerative diseases,
ocular diseases, and skin disorders.
In a further aspect of the present invention, a method for preventing and/or
treating
autoimmune and inflammatory diseases, vascular diseases, fibrotic diseases,
liver
diseases, cholestatic liver diseases, cancer, neurodegenerative diseases,
ocular
diseases, and skin disorders, which method comprises administering to a
subject, in
particular a human, a pharmaceutically effective amount of at least one
compound of the
general formula (I), to prevent and/or treat said autoimmune and inflammatory
diseases,
vascular diseases, fibrotic diseases, liver diseases, cholestatic liver
diseases, cancer,
neurodegenerative diseases, ocular diseases, and skin disorders.
Preferred, the autoimmune and inflammatory diseases comprises multiple
sclerosis,
celiac disease, Duhring-Brocq-disease (dermatitis herpetiformis), gluten
ataxia, gluten
neuropathy, diabetes, rheumatoid arthritis, Graves' disease, inflammatory
bowel disease,
systemic lupus erythematosus psoriasis, and gingivitis;
the vascular diseases comprise atherosclerosis, thrombosis, vascular
stiffness;
the fibrotic diseases affecting the lung, the kidney, the liver, the skin or
the gut like cystic
fibrosis, kidney fibrosis and diabetic nephropathy, intestinal fibrosis,
idiopathic lung
fibrosis, liver fibrosis;
the liver diseases like alcoholic hepatitis, alcoholic steatohepatitis,
nonalcoholic
steatohepatitis, non-alcoholic fatty liver disease, liver cirrhosis,
autoimmune hepatitis or
liver inflammation;
the cholestatic liver diseases comprise primary biliary cholangitis and
primary sclerosing
cholangitis;
the cancer comprises glioblastoma, melanoma, pancreatic cancer, renal cell
carcinoma,
meningioma, and breast cancer,
the neurodegenerative diseases comprise Parkinson's disease, Huntington's
disease, or
Alzheimer's disease,
the ocular diseases comprise glaucoma, cataracts, macular degeneration, or
uveitis;
the skin disorders comprise acne, psoriasis, scarring, and skin aging.
More preferred, the compound of the formula (I), or the pharmaceutical
composition
thereof is useful in the treatment or prophylaxis of celiac disease.
Furthermore, the compounds of the general formula (I), can be administered in
form of
their pharmaceutically active salts, optionally using essentially non-toxic
pharmaceutically
acceptable carriers, adjuvants or extenders. Medications are prepared in a
known
manner in a conventional solid or fluid carrier or in extenders and a
conventional
pharmaceutically acceptable adjuvant/expedient in a suitable dose. The
preferred
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
76
preparations are provided in an administrable form suitable for oral
application, such as
pills, tablets, film tablets, coated tablets, capsules and powders.
Tablets, film tablets, coated tablets, gelatin capsules and opaque capsules
are the
preferred pharmaceutical formulations. Any pharmaceutical compositions
contains at
least one compound of the general formula (I), and/or pharmaceutically
acceptable salts
thereof in an amount of 5 mg to 500 mg, preferably 10 mg to 250 mg and most
preferred
in an amount of 10 to 100 mg per formulation.
Besides, the object of the present invention also includes pharmaceutical
preparations
for oral, parenteral, dermal, intradermal, intragastric, intracutaneous,
intravascular,
intravenous, intramuscular, intraperitoneal, intranasal, intravaginal,
intrabuccal,
percutaneous, rectal, subcutaneous, sublingual, topic, transdermal or
inhalative
application, containing, in addition to typical vehicles and extenders, a
compound of the
general formula (I), and/or a pharmaceutically acceptable salt thereof as
active
component.
The pharmaceutical compositions of the present invention contain one of the
compounds
of the formula (I) disclosed herein as active component, typically mixed with
suitable
carrier materials, selected with respect to the intended form of
administration, i.e. tablets
to be administered orally, capsules (filled either with a solid, a semi-solid
or a liquid),
powders, orally administrable gels, elixirs, dispersible granulates, syrups,
suspensions
and the like in accordance with conventional pharmaceutical practices. For
example, the
compound of the formula (I) can as active agent component be combined with any
oral,
non-toxic, pharmaceutically acceptable, inert carrier, such as lactose,
starch, sucrose,
cellulose, magnesium stearate, dicalcium phosphate, calcium sulfate, talc,
mannitol, ethyl
alcohol (liquid forms) and the like for the oral administration in form of
tablets or capsules.
Moreover, suitable binders, lubricants, disintegrants and colorants can be
added to the
mixture if required. Powders and tablets can consist of said inert carriers to
an extent from
.. about 5% per weight to about 95% per weight of the inventive composition.
Suitable binders include starch, gelatin, natural sugars, sweeteners made of
corn, natural
and synthetic gums, such as acacia gum, sodium alginate,
carboxymethylcellulose,
polyethylene glycol and waxes. Possible lubricants for the use in said dosage
forms
include boric acid, sodium benzoate, sodium acetate, sodium chloride and the
like.
Disintegrants include starch, methylcellulose, cyclodextrins, guar gum and the
like. If
required, sweeteners and flavor additives and preservatives can also be
included. Some
of the terms used above, namely disintegrants, extenders, lubricants, binders
and the like
are discussed in greater detail below.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
77
Additionally, the compositions of the present invention can be formulated in a
form with
sustained release to provide a controlled release rate of any one or more
components or
active components, in order to optimize the therapeutic effect, i.e. the
inhibitory activity
and the like. Suitable dosage forms for sustained release include layered
tablets
containing layers with varying degradation rates or controlled release
polymeric matrices
impregnated with the active components and in the form of a tablet or capsule
containing
such impregnated or encapsulated porous polymeric matrices.
Preparations in fluid form include solutions, suspensions and emulsions.
Exemplarily
mentioned are water or water propylene glycol solutions for parenteral
injections or the
addition of sweeteners and opacifiers for oral solutions, suspensions and
emulsions.
Aerosol preparations suitable for inhalation may include solutions and solids
in the form
of powders which can be combined with a pharmaceutically acceptable carrier,
such as
a compressed inert gas, e.g. nitrogen.
For the preparation of suppositories a low melting wax, such as a mixture of
fatty acid
glycerides, e.g. cocoa butter, is melted firstly and the active component is
homogenously
dispersed therein by stirring or similar mixing operations. The melted
homogenous
mixture is then poured in fitting forms, cooled and thus hardened.
Further preparations in solid form which are to be converted into preparations
in fluid form
for either oral or parenteral administration shortly before use are included.
Such fluid
forms include solutions, suspensions and emulsions.
Furthermore, the compounds of the present invention may be administered via
transdermal application. The transdermal compositions can have the form of
crèmes,
lotions, aerosols and/or emulsions.
The term capsule refers to a special container or casing composed of
methylcellulose,
polyvinyl alcohols or denatured gelatins or starches, in which the active
agents can be
enclosed. Typically, hard shell capsules are prepared from mixtures of bones
and porcine
skin gelatins having comparatively high gel strength. The capsule itself can
contain small
amounts of colorants, opacifiers, softening agents and preservatives.
Tablet means a compressed or cast solid dosage form containing the active
components
with suitable extenders. The tablet can be produced by compressing mixtures or
granulates obtained by wet granulation, dry granulation or compaction, which
are known
to the one skilled in the art.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
78
Oral gels refer to the active components dispersed or solubilized in a
hydrophilic semi-
solid matrix.
Powders for compositions refer to powder mixtures containing the active
components and
suitable extenders which can be suspended in water or juices.
Suitable extenders are substances which usually form the largest part of the
composition
or dosage form. Suitable extenders include sugars such as lactose, sucrose,
mannitol
and sorbitol; starches derived from wheat, corn, rice and potatoes; and
celluloses such
as microcrystalline cellulose. The amount of extenders in the composition can
range from
about 5 to about 95% per weight of the total composition, preferably form
about 25 to
about 75% per weight and further preferred from about 30 to about 60% per
weight.
The term disintegrants refers to materials added to the composition in order
to support
disintegration and release of the medicinal substance. Suitable disintegrants
include
starches, modified starches which are soluble in cold water, such as sodium
carboxymethyl starch; natural and synthetic gums such as locust bean gum,
caraya, guar
gum, tragacanth and agar; cellulose derivatives such as methylcellulose and
sodium
carboxymethylcellulose, microcrystalline celluloses and crosslinked
microcrystalline
celluloses such as croscarmellose sodium; alginates such as alginic acid and
sodium
alginate; clays such as bentonites and foaming mixtures. The amount of
disintegrants
used in the composition can range from about 2 to 20% per weight of the
composition
and further preferred from about 5 to about 10% per weight.
Binders characterize substances binding or "gluing" powders to each other and
they
consequently serve as "glue" in the formulation. Binders add a cohesion starch
which is
already available in the extenders or the disintegrant. Suitable binders
include sugar, such
as sucrose; starches derived from wheat, corn, rice and potatoes; natural gums
such as
acacia gum, gelatin and tragacanth; derivatives of sea weed such as alginic
acid, sodium
alginate and ammonium calcium alginate, cellulose materials such as methyl
cellulose
and sodium carboxymethylcellulose and hydroxypropyl methylcellulose,
polyvinylpyrrolidone and inorganic compounds, such as magnesium aluminum
silicate.
The amount of binders in the composition can range from about 2 to about 20%
per weight
of the total composition, preferably form about 3 to about 10% per weight and
further
preferred from about 3 to about 6% per weight.
The term lubricant refers to a substance added to the dosage form in order to
allow for
the tablet, granulate, etc. to be released from the casting mold or pressing
mold, after
compression, by reducing the friction. Suitable lubricants include metallic
stearates such
as magnesium stearate, calcium stearate or potassium stearate; stearic acid;
waxes with
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
79
high melting points and water soluble lubricants such as sodium chloride,
sodium
benzoate, sodium acetate, sodium oleate, polyethylene glycols and D,L-leucine.
Due to
the fact that lubricants have to be present on the surface of the granulates
as well as
between the granulates and parts of the tablet press they are typically added
during the
last step prior to compression. The amount of lubricants in the composition
can range
from about 0.2 to about 5% per weight of the total composition, preferably
form about 0.5
to about 2% per weight and further preferred from about 0.3 to about 1.5 % per
weight.
Lubricants are materials preventing caking and improving the flow
characteristics of
granulates so that the flow is smooth and uniform. Suitable lubricants include
silicon
dioxide and talc. The amount of lubricants in the composition can range from
about 0.1
to about 5 % per weight of the total composition, preferably form about 0.5 to
about 2 %
per weight.
Colorants are adjuvants coloring the composition or dosage form. Such
adjuvants can
include colorants having food quality which are adsorbed on a suitable
adsorption means,
such as clay or aluminum oxide. The amount of the colorant used can vary from
about
0.1 to about 5% per weight of the composition and preferably from about 0.1 to
about 1%
per weight.
As used herein, a "pharmaceutically effective amount" of a transglutaminase
inhibitor is
the amount or activity effective for achieving the desired physiological
result, either in
cells treated in vitro or in a patient treated in vivo. Specifically, a
pharmaceutical effective
amount is such an amount which is sufficient for inhibiting, for a certain
period of time,
one or more of the clinically defined pathological processes associated with
transglutaminase 2. The effective amount can vary according to the specific
compound
of the formula (I) and additionally depends on a plurality of factors and
conditions related
to the subject to be treated and the severity of the disease. If, for example,
an inhibitor is
to be administered in vivo, factors such as age, weight and health of the
patients as well
as dose reaction curves and data regarding toxicity obtained from preclinical
animal
studies are amongst the data to be considered. If the inhibitor in form of the
compound of
the formula (I) described herein is to be brought in contact with the cells in
vivo, a plurality
of preclinical in vitro studies would be designed in order to determine
parameters such as
absorption, half-life, dose, toxicity, etc. Determining a pharmaceutically
effective amount
fora given pharmaceutically active ingredient is part of the ordinary skills
of the one skilled
in the art.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
80
Examples
Following abbreviations used in the examples have the following meaning.
Boc (tert-butoxycarbonyl), Boc0Su (N-tert-butoxycarbonyloxy-succinimide) DCM
(dichloromethane), DMAP (4-(Dimethylamino)-pyridine), TEA (triethylamine), DMF
(dimethylformamide), DMP (Dess-Martin periodiane), DIPEA (N-
Ethyldiisopropylamine),
Glu (glutamic acid), EDC (1-ethyl-3-(3'-dimethylaminopropyl)carbodiimide),
TFA
(trifluoroacetic acid), THF (tetrahydrofuran), Et0Ac (ethyl acetate), HATU (1-
[Bis(dimethylam ino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid
hexafluorophosphate), HOBt (hydroxybenzotriazole), MTBE (methyl tert-butyl
ether),
tBu (tert-butyl),
Chemical Examples
The following examples are intended to illustrate the invention with selected
compounds
without limiting the protecting scope of the present intellectual property
right on these
concrete examples. It is clear for a person skilled in the art that analogous
compounds
and compounds produced according to analogous synthetic ways fall under the
protecting
scope of the present intellectual property right.
Example I. Synthetic method 1
Scheme 1-1
OH chloroacetic acid
34 cl) 0
02NA, ___________ 02N ,OH
1 N 1
j\I NaP0 (a 0
ZED1 657
1
1-adamantylamine
EDC, DIPEA, DMF
10% Pd/C
0
H2 0
H2N N NH
j\I -01 Me0H JJ 0
ZED3913 ZED3912
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
81
Preparation of compound ZED1657
0
02N N OH
0
2-(3-n itro-2-oxopy rid in-1(2H)-yl)acetic acid
Chemical Formula: C7H6N205
Exact Mass: 198.03
Molecular Weight: 198.13
30.0 g (214 mmol) of 2-hydroxy-3-nitropyridine and 40.5 g (2 eq) of
chloroacetic acid were
suspended in 600 mL water. At 40 C, 245 g (3 eq) trisodium phosphate
dodecahydrate
were added, and the reaction was stirred at room temperature overnight. 250 mL
HCI
(32%) were added, and the suspension was stirred for another night at 4 C. The
precipitate was filtered and dried.
Yield: 41.2 g, 97%; ESI-MS: 199.3 [M+H]
Preparation of compound ZED3912
0
02N
NNH
0
N-(1-adamantyI)-2-(3-nitro-2-oxopyridin-1(2H)-yl)acetamide
Chemical Formula: 017H21N304
Exact Mass: 331.15
Molecular Weight: 331.37
7.0 g (35.3 mmol) of ZED1657, 6.63 g (1 eq) of 1-adamantanamine hydrochloride
and
4.77 g (1 eq) of HOBt were dissolved in 80 mL DMF and 7.38 mL (1.2 eq) DI PEA.
7.45 g
(1.1 eq) of 1-ethyl-3-(3'-dimethylaminopropyl)carbodiimide hydrochloride were
added and
the reaction was stirred at room temperature overnight. The solvent was
evaporated, and
the residue was dissolved in 200 mL DCM. The solution was washed with each 100
mL
citric acid solution (10%), NaHCO3 solution (10%) and brine. The organic phase
was dried
over Na2SO4, filtered and the solvent was evaporated.
Yield: 10.3 g, 88%; ESI-MS: 332.4 [M+H]
Preparation of compound ZED3913
0
2-(3-amino-2-oxopyridin-1(2H)-y1)-N-(1-adamantyl)acetamide
Chemical Formula: C17H23N302
Exact Mass: 301.18
Molecular Weight: 301.38
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
82
10.3 g (31.0 mmol) of ZED3912 were suspended in 200 mL Me0H before 1.0 g of
palladium (10%) on activated carbon (unreduced) were added. The suspension was
stirred overnight at room temperature under an atmosphere of hydrogen. The
catalyst
was filtered, and the solvent was evaporated.
Yield: 7.51 g, 78%
ESI-MS: 302.3 [M+H]+
Scheme. 1-2
0
C) OH 1. DMF, CH3I 0 H 1. Ethyl AcON
Cs2003 isocyanide
2. DMAP, ACN AcOH,
DCM H
Boc20 2. TEA
Boo, ______________________ ).- Boo,N ________________________________ ,-
Boc,N OH
N CO2tBu CO2tBu
H H
3. DIBAL-H Boo 3. Boc20
0
Et20, -78 C DIPEA, DMF
ZED3632
ZED3913 ci
0 HATU, DMF
DIPEA
H2N,)-L ,NH
N if
0
O 0
Ac(21)-N Ac0)-N
H H
? JCH 0 1) TEA, DCM 0
N _________________________________ NH ________ -4
I\1/ r_ii 1 N-r Boo,NJ(NFiNrNH
_--N 0 0 2) HATU, DIPEA, DMF H 0 0
N 1-methyl-1H-imidazole-
I-1 b 5-carboxylic acid
I-la
1 K2CO3, Me0H
O 0
HON
H H
0 0 Dess-Martin 0 0
)-L NH periodinane
____________________________________________ *- NYI\IjcNH1H N-r NH
NLY1,1 (10 N 1\1cc
____m H 0 0
-N DMF -N
1-1
1-1c
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
83
Preparation of compound ZED788
0 OMe
BooN
. JC0tBu
H 0
(S)-1-tert-butyl 5-methyl 2-(tert-butoxycarbonylamino)pentanedioate
Chemical Formula: C15H27N06
Exact Mass: 317,18
Molecular Weight: 317,38
12.0 g of Boc-L-Glu-OtBu (39.6 mmol) and 7.09 g of cesium carbonate (21.8
mmol, 0.55
eq) were suspended in 100 ml of DMF and stirred for 1 h at room temperature.
2.47 ml
iodomethane (39.6 mmol) we added, and the mixture was stirred at room
temperature
overnight. The solvent was evaporated, and the residue was dissolved in ethyl
acetate
and washed twice with each citric acid solution (10%), NaHCO3 solution (10%)
and brine.
The organic phase was dried over Na2SO4, filtered and the solvent was
evaporated. The
raw product was used without further purification.
Yield: 13.4 g, >100%
ESI-MS: 318.3 [M+H]
Preparation of compound ZED720
0 OMe
Boc,N OtBu
Boo 0
(S)-1-tert-butyl 5-methyl 2-(bis(tert-butoxycarbonyl)amino)pentanedioate
Chemical Formula: C20H35N08
Exact Mass: 417,24
Molecular Weight: 417,49
13.4 g of ZED788 (-39,6 mmol) and 986 mg of N,N-dimethy1-4-aminopyridine
(DMAP)
were dissolved in 30 ml of acetonitrile. 17.6 g of di-tert-butyl bicarbonate
(77.1 mmol) in
100 ml of acetonitrile was added and the solution was stirred at room
temperature
overnight. The solvent was evaporated, and the residue was dissolved in ethyl
acetate
and washed twice with each citric acid solution (10%), NaHCO3 solution (10%)
and brine.
The organic phase was dried over Na2SO4, filtered and the solvent was
evaporated. The
raw product was used without further purification.
Yield: 13.7 g, 83%
ESI-MS: 418.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
84
Preparation of compound ZED721
O H
Boc ,N J(OtBu
I3oc 0
(S)-tert-butyl 2-(bis(tert-butoxycarbonyhamino)-5-oxopentanoate
Chemical Formula: C19H33N07
Exact Mass: 387,23
Molecular Weight: 387,47
13.7 g of ZED720 (32.8 mmol) were dissolved in 200 ml of dry diethyl ether and
cooled
to -78 C under argon atmosphere. 36.1 ml of diisobutylaluminum hydride (1M in
hexane)
were added dropwise and the solution was stirred for 30 min at -78 C before
being
quenched with potassium sodium tartrate (Rochelle salt) solution. The organic
layer was
separated, dried over Na2SO4, filtered, and concentrated to dryness. The raw
product
was used without further purification.
Yield: 13.3 g, >100%
ESI-MS: 388.3 [M+H]
Preparation of compound ZED3221
AcOjL N
Boc,NOH
0
(2S)-5-acetoxy-2-(tert-butoxycarbonylamino)-6-(ethylamino)-6-oxohexanoic acid
Chemical Formula: C15H26N207
Exact Mass: 346.17
Molecular Weight: 346.38
7.0 g (18.1 mmol) of the aldehyde (S)-tert-butyl 2-(bis(tert-
butoxycarbonyl)amino)-5-
oxopentanoate (ZED721) were dissolved in 30 mL DCM. At 0 C 1.04 g (1.05 eq)
ethyl
isocyanide and 1.09 mL (1.05 eq) acetic acid were added, and the reaction was
stirred at
room temperature overnight. 35 mL TFA were added, and the reaction was stirred
for
another 3 h. The solvent was evaporated, and the residue was dissolved in 20
mL DMF.
6.29 mL (2 eq) DIPEA and 4.73 g (1.2 eq) di-tert-butyl dicarbonate in 5 mL DMF
were
added and the reaction was stirred at room temperature overnight. The solvent
was
evaporated, and the residue was dissolved in DCM. After extraction with NaHCO3
solution
(1.05 eq in water), 1.5 eq citric acid was added to the aqueous phase,
followed by re-
extraction with DCM. The organic phase was dried over Na2SO4, filtered and the
solvent
was evaporated. The residue was purified by flash chromatography.
Yield: 5.77 g, 92%
ESI-MS: 347.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
85
Preparation of compound I-la
0
Ac0j-L
H 0
Boc, A
NNH
0 Li
(5S)-5-(tert-butoxycarbonylamino)-1-(ethylamino)-6-(1-(2-(1-adamantylamino)-
2-oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-ylamino)-1 ,6-dioxohexan-2-y1 acetate
Chemical Formula: C32F147N508
Exact Mass: 629.34
Molecular Weight: 629.74
5.77 g (16.7 mmol) of ZED3221, 6.33 g (1 eq) HATU and 5.02 g (1 eq) ZED3913
were
dissolved in 100 mL DMF and 5.80 mL DIPEA (2 eq) and stirred at 45 C
overnight. The
solvent was evaporated; the residue was dissolved in 50 mL Et0Ac and washed
twice
with each 35 mL citric acid solution (10%), NaHCO3 solution (10%) and brine.
The organic
phase was dried over Na2SO4, filtered and the solvent was evaporated.
Yield: 7.41 g, 71%
ESI-MS: 630.5 [M+H]
Preparation of compound I-lb
0
AcOAN
0 ( H 0
7.'''-zi)LNNJ-L NH
N
N H 0 0
(5S)-1-(ethylamino)-6-(1-(2-(1-adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-d ihyd
ropyrid in-
3-ylamino)-5-(1 -methyl-1 H-imidazole-5-carboxamido)-1,6-dioxohexan-2-y1
acetate
Chemical Formula: C32H43N707
Exact Mass: 637.32
Molecular Weight: 637.73
600 mg (0.95 mmol) of I-la were dissolved in 5 ml DCM/TFA (1:1) and stirred at
room
temperature for 1 h. The solvent was evaporated, and the residue was dissolved
in 5 ml
DMF. 120 mg (1 eq) 1-methyl-1H-imidazole-5-carboxylic acid, 362 mg (1 eq) HATU
and
332 pl (2 eq) DIPEA were added, and the reaction was stirred at room
temperature
overnight. The solvent was evaporated; the residue was dissolved in 25 mL
Et0Ac and
washed with each 15 mL citric acid solution (10%), NaHCO3 solution (10%) and
brine.
The organic phase was dried over Na2SO4, filtered and the solvent was
evaporated.
Yield: 527 mg, 87%
ESI-MS: 638.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
86
Preparation of compound 1-1c
0
H0J-LN
0 ti 0
H 0 0
(56)-N 1-ethyl-2-hydroxy-N6-(1 -(2-(1-adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
dihydropyrid in-
3-y1)-5-(1-methyl-1H-imidazole-5-carboxamido)hexanediamide
Chemical Formula: C301-141N706
Exact Mass: 595.31
Molecular Weight: 595.69
527 mg (0.83 mmol) of 1-lb were dissolved in 7 ml Me0H. 171 mg (1.5 eq)
potassium
carbonate were added, and the reaction was stirred at room temperature for 1
h. The
solution was diluted with DCM and washed with water. The organic phase was
dried over
Na2SO4, filtered and the solvent was evaporated.
Yield: 469 mg, 95%
ESI-MS: 596.5 [M+H]
Preparation of compound 1-1
0
01
iCH 0
N
N NH
HN 0 0
(S)-N 1 -ethyl-N6-(1 -(2-(1-adamantylami no)-2-oxoethyl)-2-oxo-1 ,2-
dihydropyridin-3-y1)-
5-(1-methyl-1H-imidazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C30F139N706
Exact Mass: 593.30
Molecular Weight: 593.67
469 mg (0.60 mmol) of I-1c were dissolved in 5 ml DMF. 534 mg (1.6 eq) Dess-
Martin
periodinane (DMP) were added and the reaction was stirred at room temperature
over 2
h. The precipitate was filtered off and the filtrate was evaporated. The
residue was purified
by HPLC.
Yield: 362 mg, 77%
ESI-MS: 594.5 [M+H]
1H-NMR (DMSO-D6, 500 MHz, O [ppm]: 1.27 (t, 3H, ethyl-CH3), 1.74 (m, 6H,
adamantyl-
C4-H2), 1.97 // 2.15 (m // m, 1H // 1H, [3-CH2), 2.09 (m, 3H, adamantyl-C3-H),
2.17 (m,
6H, adamantyl-C2-H2), 3.23 (m, 2H, ethyl-CH2), 2.91 (t, 2H, y-CH2), 3.74 (s,
3H,
imidazole-N-CH3), 4.54 (ddd, 1H, a-CH2), 4.61 (s, 2H, N-CH2), 6.26 (t, 1H,
pyridinone-05-
H), 7.31 (d, 1H, pyridinone-C6-H), 7.69 (s, 1H, imidazole-CH), 7.77 (s, 1H,
imidazole-
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
87
CH), 8.04 (d, 1H, adamantyl-NH), 8.17 (d, 1H, pyridinone-C4-H), 8.44 (q, 1H,
ethylamide-
NH), 8.56 (d, 1H, a-NH), 9.16 (s, 1H, pyridinone-NH).
13C-NMR (DMSO-D6, 500 MHz, O [ppm]: 15.14 (ethyl-CH3), 24.54 (13-CH2), 29.19
(adamantyl-C3-H), 33.54 (imidazole-N-CH3), 33.71 (y-CH2), 34.41 (ethyl-CH2),
35.89
(adamantyl-C4-H2), 40.77 (adamantyl-C2-H2), 44.12 (adamantyl-C1), 51.74 (N-
CH2),
52.42 (a-CH2), 104.66 (pyridinone-05-H), 122.39 (pyridinone-C4-H), 125.22 (im
idazole-
Cq), 127.92 (pyridinone-N-Cq), 132.85 (imidazole-CH), 133.27 (pyridinone-C6-
H), 142.23
(imidazole-CH), 156.63 (pyridinone-C=0), 160.32 (imidazole-C=0), 161.10 (C=O-
NH-
CH2CH3), 165.75 (C=0-adamantylamide), 170.56 (C=O-NH-pyridinone), 198.43 (C=0-
ethylam ide).
Preparation of compound 1-2
0
0 ti 0
HNYL IAN "
1 NCNH
0 0
(S)-N1-ethy1-5-(1 H-imidazole-4-carboxamido)-N6-(1-(2-(1-adamantylamino)-2-
oxoethyl)-
2-oxo-1 ,2-dihydropyridin-3-y1)-2-oxohexanediamide
Chemical Formula: 029F137N706
Exact Mass: 579.28
Molecular Weight: 579.65
The synthesis of compound 1-2 was performed according to compound 1-1, using 1-
Boc-
imidazole-4-carboxylic acid instead of 1-methyl-1H-imidazole-5-carboxylic
acid. The final
product was obtained by deprotection (DCM/TFA) as described above and purified
by
HPLC.
Yield: 45 mg, 63% (last step)
ESI-MS: 580.4 [M+H]
Preparation of compound 1-3
0
? 0
N"-7N
HN 0 0
(S)-N1 -ethyl-N6-(1 -(241 -adamantyl(methyl)amino)-2-oxoethyl)-2-oxo-1 ,2-di
hydropyridin-3-yI)-
5-(1 -methyl-1 H-imidazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C311-141N706
Exact Mass: 607.31
Molecular Weight: 607.70
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
88
The synthesis of compound 1-3 was performed according to compound 1-1, using N-
methy1-1-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 26 mg, 52% (last step)
ESI-MS: 608.5 [M+H]
Preparation of compound 1-4
0
0)-L
0 0
I\1YLN NH
N
0 0
(S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-carboxamido)-2-
oxohexanediamide
Chemical Formula: C32H43N706
Exact Mass: 621.33
Molecular Weight: 621.73
To the a-hydroxyester precursor of compound 1-4 (382 mg, 0.61 mmol, prepared
by using
3,5-dimethy1-1-adamantanamine in step 2 according to compound ZED3912) in 10
mL of
acetonitrile, 1 mg of TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl, 1 mol%)
were added.
88 mg of calcium hypochlorite (1 eq) were added at 0 C and the reaction
mixture was
stirred at 25 C for 2 h. The suspension was filtered, diluted with ethyl
acetate and washed
with NaHCO3 solution (10%) and brine. The organic phase was dried over Na2SO4,
filtered and the solvent was evaporated. The residue was purified by HPLC.
Yield: 176 mg, 46%
ESI-MS: 622.5 [M+H]
Preparation of compound 1-5
0)LN
0 0
NYLNjcNA NH
H 0 0
(S)-N1-ethyl-N6-(1-(2-(3-ethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methyl-1H-imidazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C32H43N706
Exact Mass: 621.33
Molecular Weight: 621/3
To the a-hydroxyester precursor of compound 1-5 (162 mg, 0.26 mmol, prepared
by using
3-ethyl-1-adamantanamine in step 2 according to compound ZED3912) in 5 ml
DMSO,
145 mg of 2-iodoxybenzoic acid (IBX, 2 eq) were added and the reaction mixture
was
stirred at room temperature for 3 h. NaHCO3 solution (10%) was added and the
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
89
suspension was extracted with Et0Ac. The organic phase was dried over Na2SO4,
filtered
and the solvent was evaporated. The residue was purified by HPLC.
Yield: 59 mg, 37% (last step)
ESI-MS: 622.5 [M+H]
Preparation of compound 1-6
0
oJL CF3
0 0
N NH
, N
N '/Y-LFINjor 0
(S)-N1-ethyl-N6-(1-(2-(3-trifluoromethyladamantane-1-amino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-y1)-5-(1-methyl-1H-imidazole-5-carboxamido)-2-
oxohexanediamide
Chemical Formula: C31F138F3N706
Exact Mass: 661.28
Molecular Weight: 661.67
The synthesis of compound 1-6 was performed according to compound 1-1, using 3-
trifluoromethy1-1-adamantanamine instead of 1-adamantanamine in step 2
(according to
ZED3912).
Yield: 36 mg, 51% (last step)
ESI-MS: 662.4 [M+H]
Preparation of compound 1-7
OH
0 0
H j_LN
NH
N "-/YLN-rN
I
0 0
(S)-N1-ethyl-N6-(1-(2-(3-hydroxyadamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methy1-1H-imidazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C30H39N707
Exact Mass: 609.29
Molecular Weight: 609.67
The synthesis of compound 1-7 was performed according to compound 1-1, using 3-
hydroxy-1-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 21 mg, 45% (last step)
ESI-MS: 610.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
90
Preparation of compound 1-8
0
O 0
HN N
NYLN(r H)-L N-r
0 0
(S)-N1-ethyl-N6-(1-(2-(3-fluoroadamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methy1-1H-imidazole-5-carboxamido)-2-oxonexanediamide
Chemical Formula: C30H38FN706
Exact Mass: 611.29
Molecular Weight: 611.66
The synthesis of compound 1-8 was performed according to compound 1-1, using 3-
fluoro-
1-adamantanamine instead of 1-adamantanamine in step 2 (according to ZED3912).
Yield: 52 mg, 61% (last step) ESI-MS: 612.5 [M+H]
Preparation of compound 1-9
0
Cl
O 0
N/y-LNicN)-L NH
N
0 0
(S)-N1-ethyl-N6-(1-(2-(3-chloroadamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methyl-1H-imidazole-5-carboxamido)-2-oxonexanediamide
Chemical Formula: C301-138C1N706
Exact Mass: 627.26
Molecular Weight: 628.12
The synthesis of compound 1-9 was performed according to compound 1-1, using 3-
chloro-1-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 49 mg, 68% (last step) ESI-MS: 628.3 / 630.3 [M+H]
Preparation of compound 1-10
0
Br
O 0
NH
/Y-LN(1 N
N H 0 0
(S)-N1-ethyl-N6-(1-(2-(3-bromoadamantane-l-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methyl-1H-imidazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C30H38BrN706
Exact Mass: 671.21
Molecular Weight: 672.57
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
91
The synthesis of compound 1-10 was performed according to compound 1-1, using
3-
bromo-1-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 59 mg, 71% (last step)
ESI-MS: 672.3 / 674.3 [M+H]
Preparation of compound 1-11
0
CO2Me
0 0
HN N
NYLNjc
N-r
0 0
(S)-N1-ethyl-N6-(1-(2-(3-methyl adamantane-3-carboxylate-1-amino)-2-oxoethyl)-
2-oxo-1,2-
dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-carboxamido)-2-
oxohexanediamide
Chemical Formula: C32F141 N708
Exact Mass: 651.30
Molecular Weight: 651.71
The synthesis of compound 1-11 was performed according to compound 1-1, using
methyl
3-aminoadamantane-1-carboxylate instead of 1-adamantanamine in step 2
(according to
ZED3912).
Yield: 85 mg, 74% (last step)
ESI-MS: 652.5 [M+H]
Preparation of compound 1-12
0 FF
N -
H
0 0
NAyLNjcrN)-L NH
N-r
0 0
(S)-N1-ethyl-N6-(1-(2-(4,4-difluoroadamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methy1-1H-imidazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C301-137F2N706
Exact Mass: 629.28
Molecular Weight: 629.65
The synthesis of compound 1-12 was performed according to compound 1-1, using
4,4-
difluoro-1-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 23 mg, 47% (last step)
ESI-MS: 630.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
92
Preparation of compound 1-13
0
0
O Li 0
NYL N
Nr
H 0 0
(S)-N1-(1-(2-(((1S,2R,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-Amethylamino)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-N6-ethyl-2-(1-methyl-1H-imidazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C30H41N706
Exact Mass: 595.31
Molecular Weight: 595.69
The synthesis of compound 1-13 was performed according to compound 1-1, using
(-)-
cis-myrtanylamine instead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 74 mg, 68% (last step) ESI-MS: 596.5 [M+H]
Preparation of compound 1-14
0
OAN
O ti 0
NYLHN N Nr
¨ 0 0
(S)-N1-(1-(2-(((1R,2R,5R)-6,6-dimethylbicyclo[3.1.1]heptan-2-yhmethylamino)-2-
oxoethyl)-2-oxo-
1 ,2-dihydropyridin-3-y1)-N6-ethyl-2-(1-methyl-1 H-imidazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C301-141N706
Exact Mass: 595.31
Molecular Weight: 595.69
The synthesis of compound 1-14 was performed according to compound 1-1, using
(-)-
cis-(pinan-2-ylmethyl)amine instead of 1-adamantanamine in step 2 (according
to
ZED3912).
Yield: 52 mg, 63% (last step) ESI-MS: 596.5 [M+H]
Preparation of compound 1-15
0
0
O ti 0
j-L NH
¨1\l/AN(r N I
H 0 0
(S)-N1-ethyl-N6-(1-(2-(3-ethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methy1-1H-imidazole-4-carboxamido)-2-oxohexanediamide
Chemical Formula: C32H43N706
Exact Mass: 621.33
Molecular Weight: 621.73
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
93
The synthesis of compound 1-15 was performed according to compound 1-5, using
1-
methy1-1H-imidazole-4-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 26 mg, 67% (last step)
ESI-MS: 622.5 [M+H]
Preparation of compound 1-16
0
0)-L N
0 0
N N NHN NH
\ H
\
(S)-N1-ethyl-N6-(1-(2-(3-ethyladamantane-l-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1 -methyl-1 H-imidazole-2-carboxamido)-2-oxohexanediamide
Chemical Formula: C32F143N706
Exact Mass: 621.33
Molecular Weight: 621.73
The synthesis of compound 1-16 was performed according to compound 1-5, using
1-
methyl-1H-imidazole-2-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 46 mg, 71% (last step)
ESI-MS: 622.5 [M+H]
.. Preparation of compound 1-17
0
0)-LN
0H 0
N NH
N N
H 0 0
(S)-2-(1,4-dimethy1-1H-imidazole-5-carboxamido)-N6-ethyl-N1-(1-(2-(3-
ethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C33F145N706
Exact Mass: 635.34
Molecular Weight: 635.75
The synthesis of compound 1-17 was performed according to compound 1-5, using
1,4-
dimethy1-1H-imidazole-5-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
.. Yield: 69 mg, 75% (last step)
ESI-MS: 636.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
94
Preparation of compound 1-18
0
O 0
H j_L
NNH
0 0
(S)-N1-ethy1-5-(1-isobuty1-1H-imidazole-4-carboxamido)-N6-(1-(2-(3-
ethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxohexanediamide
Chemical Formula: C35H49N706
Exact Mass: 663.37
Molecular Weight: 663.81
The synthesis of compound 1-18 was performed according to compound 1-5, using
1-
isobuty1-1H-imidazole-4-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 38 mg, 62% (last step) ESI-MS: 664.5 [M+H]
Preparation of compound 1-19
0
O 0
Nj-I NH
FiNjcor H NiTho
(S)-2-(1-cyclopenty1-1H-imidazole-4-carboxamido)-N6-ethyl-N1-(1-(2-(3-
ethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C36F149N706
Exact Mass: 675.37
Molecular Weight: 675.82
The synthesis of compound 1-19 was performed according to compound 1-5, using
1-
cyclopenty1-1H-im idazole-4-carboxylic acid instead of 1-methy1-1H-imidazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 24 mg, 57% (last step) ESI-MS: 676.5 [M+H]
Preparation of compound 1-20
0
O 0
Nj-L NH
N-r
(S)-2-(1-cyclobuty1-1H-imidazole-4-carboxamido)-N6-ethyl-N1-(1-(2-(3-
ethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C35H47N706
Exact Mass: 661.36
Molecular Weight: 661.79
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
95
The synthesis of compound 1-20 was performed according to compound 1-5, using
1-
cyclobuty1-1H-im idazole-4-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 32 mg, 66% (last step)
ESI-MS: 662.5 [M+H]
Preparation of compound 1-21
0
0 ( H 0
NH
NNN,
0 0
Chemical Formula: C33F145N706
Exact Mass: 635.34
Molecular Weight: 635.75
(S)-2-(1,4-dimethy1-1H-imidazole-5-carboxamido)-N6-ethyl-N1-(1-(2-(3,5-
climethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 1-21 was performed according to compound 1-4, using
1,4-
dimethy1-1H-imidazole-5-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 25 mg, 56% (last step)
ESI-MS: 636.5 [M+H]
Preparation of compound 1-22
0
0 0
JCH
N)-L NH
¨NYLN N
0 0
Chemical Formula: C32F143N706
Exact Mass: 621.33
Molecular Weight: 621.73
(S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(1-methyl-1H-imidazole-4-carboxamido)-2-
oxohexanediamide
The synthesis of compound 1-22 was performed according to compound 1-4, using
1-
methy1-1H-im idazole-4-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 45 mg, 77% (last step)
ESI-MS: 622.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
96
Preparation of compound 1-23
0
0
0 0
N CNF1)- NH
H 0 0
Chemical Formula: C32F-143N706
Exact Mass: 621.33
Molecular Weight: 621.73
(S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(1-methyl-1 H-imidazole-2-carboxamido)-2-
oxohexanediamide
The synthesis of compound 1-23 was performed according to compound 1-4, using
1-
methy1-1H-im idazole-2-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
.. acid in step 6 (according to compound 1-1b).
Yield: 18 mg, 50% (last step)
ESI-MS: 622.5 [M+H]
Preparation of compound 1-24
0 0
NA NH
1\1Th
0 0
Chemical Formula: C33H4.5N706
Exact Mass: 635.34
Molecular Weight: 635/5
(S)-2-(1,2-dimethy1-1 H-imidazole-5-carboxamido)-N6-ethyl-N 1-(1-(2-(3,5-d
imethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 1-24 was performed according to compound 1-4, using
1,2-
dimethy1-1H-imidazole-5-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 44 mg, 72% (last step)
ESI-MS: 636.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
97
Preparation of compound 1-25
0
)LN
0 0
Nj-LN NH
N/YN
0 0
Chemical Formula: C311-141N706
Exact Mass: 607.31
Molecular Weight: 607.70
(S)-N1-ethyl-N6-(1-(2-(3-methyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methy1-1H-imidazole-5-carboxamido)-2-oxohexanediamide
The synthesis of compound 1-25 was performed according to compound 1-1 , using
3-
methy1-1-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912). Yield: 37 mg, 59% (last step) ESI-MS: 608.5
[M+H]
Preparation of compound 1-26
0
0)-LN
0 0
N NH
N'r
0 0
CI
Chemical Formula: C32H42C1N706
Exact Mass: 655.29
Molecular Weight: 656.17
(S)-2-(2-chloro-1-methy1-1H-imidazole-5-carboxamido)-N6-ethyl-N1-(1-(2-(3,5-
dimethyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 1-26 was performed according to compound 1-4, using
2-
chloro-1-methyl-1H-imidazole-5-carboxylic acid instead of 1-methyl-1H-im
idazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 57 mg, 71% (last step) ESI-MS: 656.5 / 658.5 [M+H]
Preparation of compound 1-27
0
0 0
0 0
Chemical Formula: C32F-143N706
Exact Mass: 621.33
Molecular Weight: 621.73
(S)-2-(1,2-dimethy1-1H-imidazole-5-carboxamido)-N6-ethyl-N1-(1-(2-(3-
methyladamantane-
1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
98
The synthesis of compound 1-27 was performed according to compound 1-25, using
1,2-
dimethy1-1H-im idazole-5-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 29 mg, 52% (last step)
ESI-MS: 622.5 [M+H]
Preparation of compound 1-28
0
0
0
Nj-L NH
'JCL NFI N
N 0 0
"
Chemical Formula: C33F-145N706
Exact Mass: 635.34
Molecular Weight: 635.75
(S)-N1-ethyl-N6-(1-(2-(3,5,7-trimethy1-1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1 -methyl-1 H-imidazole-5-carboxamido)-2-oxohexanediamide
The synthesis of compound 1-28 was performed according to compound 1-1, using
3,5,7-
trimethy1-1-adamantanamineinstead of 1-adamantanamine in step 2 (according to
ZED3912).
Yield: 48 mg, 63% (last step)
ESI-MS: 636.5 [M+H]
Preparation of compound 1-29
0
0 0
NH
N-r
0 0
CI
Chemical Formula: C33H44C1N706
Exact Mass: 669.30
Molecular Weight: 670.20
(S)-2-(2-ch loro-1 -methyl-1 H-imidazole-5-carboxamido)-N6-ethyl-N1 -(1 -(2-
(3,5,7-trimethyl-
1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
The synthesis of compound 1-29 was performed according to compound 1-28, using
2-
chloro-1-methy1-1H-im idazole-5-carboxylic acid instead of 1-methyl-1H-im
idazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 23 mg, 52% (last step)
ESI-MS: 670.5 / 672.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
99
Preparation of compound 1-30
0
)LN
0 0
0 )-L
N-r
I I
I-1 0 0
(S)-2-(benzofuran-2-carboxamido)-N6-ethyl-N1 -(1 -(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1 ,2-idihydropyridin-3-yI)-5-oxohexanediamide
Chemical Formula: C34H39N507
Exact Mass: 629.28
Molecular Weight: 629.70
The synthesis of compound 1-30 was performed according to compound 1-1, using
1-
adamantanamine instead of 1-adamantanamine in step 2 (according to ZED3912)
and
benzofuran-2-carboxylic acid instead of 1-methyl-1H-imidazole-5-carboxylic
acid in step
6 (according to compound I-1b).
Yield: 73 mg, 68% (last step)
ESI-MS: 630.4 [M+H]
Preparation of compound 1-31
0
o
oLN
0
0 N
r-rC
0 0
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-clihydropyridin-
3-y1)-
5-(3-methylbenzofuran-2-carboxamido)-2-oxohexanediamide
Chemical Formula: C35H41N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 1-31 was performed according to compound 1-30, using
3-
methylbenzo[b]furan-2-carboxylic acid instead of benzofuran-2-carboxylic acid
in step 6
(according to compound I-1b).
Yield: 90 mg, 77% (last step)
ESI-MS: 644.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
100
Preparation of compound 1-32
0
0 0
0 FNINFI).L
NN
I I
0 0
CI
(S)-2-(3-chlorobenzofuran-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C34.H38CIN507
Exact Mass: 663.25
Molecular Weight: 664.15
The synthesis of compound 1-32 was performed according to compound 1-30, using
3-
chlorobenzofuran-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 56 mg, 72% (last step)
ESI-MS: 664.4 / 666.4 [M+H]
Preparation of compound 1-33
0
0 0
0
NN)-LNN
I H I
0 0
Br
(S)-2-(4-bromobenzofuran-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C34H38BrN507
Exact Mass: 707.20
Molecular Weight: 708.60
The synthesis of compound 1-33 was performed according to compound 1-30, using
4-
bromo-1-benzofuran-2-carboxylic acid instead of benzofuran-2-carboxylic acid
in step 6
(according to compound I-1b).
Yield: 64 mg, 69% (last step)
ESI-MS: 708.3 / 710.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
101
Preparation of compound 1-34
0
0,-õ)-1,N------õ,
H
0
S 1 NNI-1)-LI NNEI
i H 0 0
(S)-2-(benzo[b]thiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C34.H39N506S
Exact Mass: 645.26
Molecular Weight: 645.77
The synthesis of compound 1-34 was performed according to compound 1-30, using
benzo[b]thiophene-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 51 mg, 67% (last step)
ESI-MS: 646.4 [M+H]
Preparation of compound 1-35
0
ON
H
0
N-11\iNMN
S H 0 0
F
(S)-N1-ethyl-5-(7-fluorobenzo[b]thiophene-2-carboxamido)-N6-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-clihydropyridin-3-y1)-2-oxohexanediamide
Chemical Formula: C34H38FN506S
Exact Mass: 663.25
Molecular Weight: 663.76
The synthesis of compound 1-35 was performed according to compound 1-30, using
7-
fluorobenzo[b]thiophene-2-carboxylic acid instead of benzofuran-2-carboxylic
acid in step
6 (according to compound I-1b).
Yield: 36 mg, 64% (last step)
.. ESI-MS: 664.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
102
Preparation of compound 1-36
0
0
0 0
N N
I H 0
F F
(S)-2-(4,5-difluoro-1H-indole-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C34F138F2N606
Exact Mass: 664.28
Molecular Weight: 664.70
The synthesis of compound 1-36 was performed according to compound 1-30, using
4,5-
difluoro-1H-indole-2-carboxylic acid instead of benzofuran-2-carboxylic acid
in step 6
(according to compound I-1b).
Yield: 48 mg, 67% (last step)
ESI-MS: 665.4 [M+H]
Preparation of compound 1-37
0
0)L
N
0 (rH 0
N
H 0 0
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-(3-methyl-1H-indole-2-carboxamido)-2-oxohexanediamide
Chemical Formula: C35H42N606
Exact Mass: 642.32
Molecular Weight: 642.74
The synthesis of compound 1-37 was performed according to compound 1-30, using
3-
methyl-1H-indole-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 42 mg, 64% (last step)
ESI-MS: 643.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
103
Preparation of compound 1-38
0
0
0 0
N
0 0
(S)-2-(1H-benzo[d]imidazole-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C33H35N706
Exact Mass: 629.30
Molecular Weight: 629.71
The synthesis of compound 1-38 was performed according to compound 1-30, using
1H-
benzo[d]imidazole-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 26 mg, 55% (last step)
ESI-MS: 630.5 [M+H]
Preparation of compound 1-39
0
0 H 0
N)^ N
0 0
(S)-2-(2,3-dihydro-1H-indene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C35H43N506
Exact Mass: 629.32
Molecular Weight: 629.75
The synthesis of compound 1-39 was performed according to compound 1-30, using
2,3-
dihydro-1H-indene-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 19 mg, 46% (last step)
ESI-MS: 630.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
104
Preparation of compound 1-40
0
0)L
N -
H
N
N
_ J1 r\irN
F3C----
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-clihydropyridin-
3-y1)-
5-(4-methy1-2-(trifluoromethypthiazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C31F137F3N606S
Exact Mass: 678.24
Molecular Weight: 678.72
The synthesis of compound 1-40 was performed according to compound 1-30, using
4-
methy1-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 34 mg, 61% (last step)
ESI-MS: 679.4 [M+H]
Preparation of compound 1-41
0
H
0 1.1 0 H
F3C.--K\ _IAN
H 0 :01
N
Br
(S)-2-(4-bromo-2-(trifluoromethypthiazole-5-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H34BrF3N606S
Exact Mass: 742.14
Molecular Weight: 743.59
The synthesis of compound 1-41 was performed according to compound 1-30, using
4-
bromo-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 39 mg, 56% (last step)
ESI-MS: 743.3 / 745.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
105
Preparation of compound 1-42
0
0 0
1\1¨
H 0 0
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-(4-methy1-2-phenylthiazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C36H4.2N606S
Exact Mass: 686.29
Molecular Weight: 686.82
The synthesis of compound 1-42 was performed according to compound 1-30, using
4-
methy1-2-phenylthiazole-5-carboxylic acid instead of benzofuran-2-carboxylic
acid in step
6 (according to compound I-1b).
Yield: 43 mg, 69% (last step)
ESI-MS: 687.5 [M+H]
Preparation of compound 1-43
0
0
0 H 0
NN
Br \ H 0 I
0
(S)-2-(5-bromo-3-methylthiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C31 H38BrN506S
Exact Mass: 687.17
Molecular Weight: 688.63
The synthesis of compound 1-43 was performed according to compound 1-30, using
5-
bromo-3-methylthiophene-2-carboxylic acid instead of benzofuran-2-carboxylic
acid in
step 6 (according to compound 1-1b).
Yield: 55 mg, 67% (last step)
ESI-MS: 688.3 /690.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
106
Preparation of compound 1-44
0
N
0 0
N
Br , N N
\I Ho I I
0
Br
(S)-2-(3,5-dibromothiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H35Br2N506S
Exact Mass: 751.07
Molecular Weight: 753.50
The synthesis of compound 1-44 was performed according to compound 1-30, using
3,5-
dibromothiophene-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 43 mg, 60% (last step)
ESI-MS: 752.2 / 754.2 / 756.2 [M+H]
Preparation of compound 1-45
0
0
0 0
0 Nj-L N
N
Br , N
\I Ho I
0
(S)-2-(5-bromo-3-methylfuran-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C31H38BrN507
Exact Mass: 671.20
Molecular Weight: 672.57
The synthesis of compound 1-45 was performed according to compound 1-30, using
5-
bromo-3-methylfuran-2-carboxylic acid instead of benzofuran-2-carboxylic acid
in step 6
(according to compound I-1b).
Yield: 52 mg, 71% (last step)
ESI-MS: 672.3 /674.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
107
Preparation of compound 1-46
0
0liN
0 0
H u
CI ,
0 0
CI
(S)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H35Cl2N506S
Exact Mass: 663.17
Molecular Weight: 664.60
The synthesis of compound 1-46 was performed according to compound 1-30, using
2,5-
dichlorothiophene-3-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 85 mg, 72% (last step)
ESI-MS: 664.3 / 666.3 [M+H]
Preparation of compound 1-47
0
0 A
N
0 jCH 0
N
Br
0 0
Br
(S)-2-(2,5-dibromothiophene-3-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H35Br2N506S
Exact Mass: 751.07
Molecular Weight: 753.50
The synthesis of compound 1-47 was performed according to compound 1-30, using
2,5-
dibromothiophene-3-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 64 mg, 68% (last step)
ESI-MS: 752.2 / 754.2 / 756.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
108
Preparation of compound 1-48
0
0)-L
0 ICH 0
N I NN
o o
CI
(S)-2-(2,5-dichlorothiazole-4-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C29H34.Cl2N606S
Exact Mass: 664.16
Molecular Weight: 665.59
The synthesis of compound 1-48 was performed according to compound 1-30, using
2,5-
dichlorothiazole-4-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 26 mg, 48% (last step)
ESI-MS: 665.3 / 667.3 [M+H]
Preparation of compound 1-49
0
ON
0 ( H 0
NN
H
0 0 0
(S)-2-(2,5-dimethylfuran-3-carboxamido)-N6-ethyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C32H41N507
Exact Mass: 607.30
Molecular Weight: 607.70
The synthesis of compound 1-49 was performed according to compound 1-30, using
2,5-
dimethylfuran-3-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 53 mg, 74% (last step)
ESI-MS: 608.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
109
Preparation of compound 1-50
0
0 A
N
H
0 0 H
SANJcr NH )-L N
1 N
Br
(S)-2-(4-bromothiazole-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C29H35BrN606S
Exact Mass: 674.15
Molecular Weight: 675.59
The synthesis of compound 1-50 was performed according to compound 1-30, using
4-
bromothiazole-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 37 mg, 59% (last step)
ESI-MS: 675.3 / 677.3 [M+H]
Preparation of compound 1-51
0
0)-LN
H
0 )C H 0
H
N N N
\ I H 0 1 1
0
Br
(S)-2-(4-bromothiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H36BrN506S
Exact Mass: 673.16
Molecular Weight: 674.61
The synthesis of compound 1-51 was performed according to compound 1-30, using
4-
bromothiophene-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 52 mg, 66% (last step)
ESI-MS: 674.3 /676.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
110
Preparation of compound 1-52
0
0
0 r H 0
NN
CI\I Ho II
0
Br
(S)-2-(4-bromo-5-chlorothiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H35BrCIN506S
Exact Mass: 707.12
Molecular Weight: 709.05
The synthesis of compound 1-52 was performed according to compound 1-30, using
4-
bromo-5-chlorothiophene-2-carboxylic acid instead of benzofuran-2-carboxylic
acid in
step 6 (according to compound 1-1b).
Yield: 48 mg, 65% (last step)
ESI-MS: 708.2 / 710.2 [M+H]
Preparation of compound 1-53
0
0
0 JCH 0
Br\i Ho II
0
Br
(S)-2-(4,5-dibromothiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C30H356r2N506S
Exact Mass: 751.07
Molecular Weight: 753.50
The synthesis of compound 1-53 was performed according to compound 1-30, using
4,5-
dibromothiophene-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 52 mg, 63% (last step)
ESI-MS: 752.2 / 754.2 / 756.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
111
Preparation of compound 1-54
0
0,
N
0 0
N
\ I Ho I I
0
CI
(S)-2-(4,5-dichlorothiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C301-135Cl2N506S
Exact Mass: 663.17
Molecular Weight: 664.60
The synthesis of compound 1-54 was performed according to compound 1-30, using
4,5-
dichlorothiophene-2-carboxylic acid instead of benzofuran-2-carboxylic acid in
step 6
(according to compound I-1b).
Yield: 63 mg, 68% (last step)
ESI-MS: 664.2 / 666.2 [M+H]
Preparation of compound 1-55
0
n 7NJ-L JGH 0
Nj-L
- N
cõ) H 0 _ r\irN
0
(S)-2-((S)-1-acetylpyrrolidine-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C32H4.4.N607
Exact Mass: 624.33
Molecular Weight: 624.73
The synthesis of compound 1-55 was performed according to compound 1-30, using
(S)-
1-acetylpyrrolidine-2-carboxylic acid instead of benzofuran-2-carboxylic acid
in step 6
(according to compound I-1b).
Yield: 76 mg, 73% (last step)
ESI-MS: 625.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
112
Preparation of compound 1-56
0
oLN
H
I N
-N 0 0
N
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-(1-methy1-1H-1,2,3-triazole-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C29F138N806
Exact Mass: 594.29
Molecular Weight: 594.66
The synthesis of compound 1-56 was performed according to compound 1-30, using
1-
methyl-1H-1,2,3-triazole-5-carboxylic acid instead of benzofuran-2-carboxylic
acid in step
6 (according to compound I-1b).
Yield: 50 mg, 61% (last step)
ESI-MS: 595.5 [M+H]
Preparation of compound 1-57
0
0 H0
N
HN H-
0 0
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethy1)-2-oxo-1,2-clihydropyridin-
3-y1)-2-oxo-5-(2H-tetrazole-5-carboxamido)hexanediamide
Chemical Formula: C271-135N906
Exact Mass: 581.27
Molecular Weight: 581.62
The synthesis of compound 1-57 was performed according to compound 1-30, using
2H-
tetrazole-5-carboxylic acid instead of benzofuran-2-carboxylic acid in step 6
(according
to compound I-1b).
Yield: 32 mg, 48% (last step)
ESI-MS: 582.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
113
Preparation of compound 1-58
ON
0 0
, N N
0 0
OH
(S)-N1-ethyl-N6-(1-(2-(5-hydroxyadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-oxo-5-(pyrazine-2-carboxamido)hexanediamide
Chemical Formula: C30H37N707
Exact Mass: 607.28
Molecular Weight: 607.66
The synthesis of compound 1-58 was performed according to compound 1-1 , using
5-
hydroxy-2-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and pyrazine-2-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 26 mg, 53% (last step)
ESI-MS: 608.5 [M+H]
.. Preparation of compound 1-59
0
0llN
0 0
LNi(rH
N-rN
I I
HN¨ 0 0
(S)-N1-ethyl-N6-(1-(2-(5-fluoroadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-oxo-5-((S)-pyrrolidine-3-carboxamido)hexanediamide
Chemical Formula: C30H41FN606
Exact Mass: 600.31
Molecular Weight: 600.68
The synthesis of compound 1-59 was performed according to compound 1-1 , using
5-
fluoro-2-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and (S)-1-Boc-pyrrolidine-3-carboxylic acid instead of 1-methy1-1H-
imidazole-
.. 5-carboxylic acid in step 6 (according to compound I-1b). The final product
was obtained
by deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 35 mg, 83% (last step)
ESI-MS: 601.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
114
Preparation of compound 1-60
o
0)-L
H H 0
NA
- N
_ NThrN
= H
0 0 1ci
(S)-N1-ethyl-N6-(1-(2-(5-chloroadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-oxo-5-((S)-piperidine-2-carboxamido)hexanediamide
Chemical Formula: C31F143CIN606
Exact Mass: 630.29
Molecular Weight: 631.16
The synthesis of compound 1-60 was performed according to compound 1-1, using
5-
chloro-2-adamantanamine instead of 1-adamantanamine in step 2 (according to
.. ZED3912) and (S)-1-Boc-piperidine-2-carboxylic acid instead of 1-methy1-1H-
imidazole-
5-carboxylic acid in step 6 (according to compound 1-1b). The final product
was obtained
by deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 37 mg, 78% (last step)
ESI-MS: 631.4 / 633.4 [M+H]
Preparation of compound 1-61
0
0 0
NJC-iN)-L
, N
H 0 0
Br
(S)-N1-ethyl-N6-(1-(2-(5-bromoadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-oxo-5-((R)-piperidine-3-carboxamido)hexanediamide
Chemical Formula: C31H43BrN606
Exact Mass: 674.24
Molecular Weight: 675.61
The synthesis of compound 1-61 was performed according to compound 1-1, using
5-
bromo-2-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and (R)-1-Boc-piperidine-3-carboxylic acid instead of 1-methy1-1H-
imidazole-
5-carboxylic acid in step 6 (according to compound 1-1b). The final product
was obtained
by deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 35 mg, 72% (last step)
ESI-MS: 675.3 / 677.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
115
Preparation of compound 1-62
0
H H 0
0 Nj-1 r\ir0N
(S)-N1-ethyl-N6-(1-(2-(5-methyladamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-((R)-morpholine-3-carboxamido)-2-oxohexanediamide
Chemical Formula: C31H44N607
Exact Mass: 612.33
Molecular Weight: 612.72
The synthesis of compound 1-62 was performed according to compound 1-1 , using
5-
methy1-2-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and (R)-4-Boc-morpholine-3-carboxylic acid instead of 1-methy1-1H-
imidazole-5-carboxylic acid in step 6 (according to compound 1-1b). The final
product was
obtained by deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 31 mg, 80% (last step)
ESI-MS: 613.5 [M+H]
Preparation of compound 1-63
0
0llN
N- H CN
0 NON
(S)-N1-ethyl-N6-(1-(2-(2-carbonitrileadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-oxo-5-((3S,4R)-quinuclidine-3-carboxamido)hexanediamide
Chemical Formula: C34H45N706
Exact Mass: 647.34
Molecular Weight: 647.76
The synthesis of compound 1-63 was performed according to compound 1-1 , using
2-
aminoadamantane-2-carbonitrile instead of 1-adamantanamine in step 2
(according to
ZED3912) and quinuclidine-3-carboxylic acid instead of 1-methy1-1H-imidazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 24 mg, 56% (last step)
ESI-MS: 648.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
116
Preparation of compound 1-64
0
0
0 0
H CO2Me
02N
Njc N
0 0
CO2Me
(S)-methyl 3-(6-(ethylamino)-1-(1-(2-(methyl 2-aminoadamantane-2-carboxylate)-
2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-ylamino)-1,5,6-trioxohexan-2-ylcarbamoy1)-5-
nitrobenzoate
Chemical Formula: C36H4.2N6012
Exact Mass: 750.29
Molecular Weight: 750.75
The synthesis of compound 1-64 was performed according to compound 1-1 , using
2-
methyl 2-aminoadamantane-2-carboxylate instead of 1-adamantanamine in step 2
(according to ZED3912) and mono-methyl 5-nitroisophthalate instead of 1-methyl-
1H-
imidazole-5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 46 mg, 71% (last step)
ESI-MS: 751.5 [M+H]
Preparation of compound 1-65
ON
0 0
02N N H
NrN
0 0
(S)-N1-(1-(2-(1-adamantylmethylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-ethy1-2-(5-nitronicotinamido)-5-oxohexanediamide
Chemical Formula: C32H39N708
Exact Mass: 649.29
Molecular Weight: 649.69
The synthesis of compound 1-65 was performed according to compound 1-1 , using
1-
adamantanemethylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 5-nitronicotinic acid instead of 1-methyl-1H-imidazole-5-
carboxylic acid in
step 6 (according to compound I-1b).
Yield: 61 mg, 74% (last step)
ESI-MS: 650.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
117
Preparation of compound 1-66
0
0,
N
0 0
H
HO2CNJc N
J IN N
0 0
5-((2S)-1-(1-(2-1-adamantylmethylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-ylamino)-6-(ethylamino)-1,5,6-trioxohexan-2-ylcarbamoyl)nicotinic acid
Chemical Formula: C34F142N608
Exact Mass: 662.31
Molecular Weight: 662.73
The synthesis of compound 1-66 was performed according to compound 1-1, using
1-
rimantadine instead of 1-adamantanamine in step 2 (according to ZED3912) and
3,5-
pyridinedicarboxylic acid instead of 1-methyl-1H-imidazole-5-carboxylic acid
in step 6
(according to compound I-1b).
Yield: 31 mg, 56% (last step)
ESI-MS: 663.5 [M+H]
Preparation of compound 1-67
0
0
0
N GE1
S, N
0 0
(S)-N1-ethyl-N6-(1-(2-(2-adamantyl(methyl)amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(6-methylimidazo[2,1-1D]thiazole-5-carboxamido)-2-
oxohexanediamide
Chemical Formula: C33H41N706S
Exact Mass: 663.28
Molecular Weight: 663.79
The synthesis of compound 1-67 was performed according to compound 1-1, using
N-methyl-2-adamantanamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 6-methylimidazo[2,1-b]thiazole-5-carboxylic acid instead of 1-
methyl-1H-
imidazole-5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 23 mg, 52% (last step)
ESI-MS: 664.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
118
Preparation of compound 1-68
0
0
0 0
o
hiN NH jC
0 0
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-
1 ,2-dihydropyridin-3-y1)-N6-ethy1-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
Chemical Formula: C32H37N507
Exact Mass: 603.27
Molecular Weight: 603.67
The synthesis of compound 1-68 was performed according to compound 1-1, using
( )-
endo-2-norbornylamine instead of 1-adamantanamine in step 2 (according to
ZED3912)
and 3-methylbenzo[b]furan-2-carboxylic acid instead of 1-methyl-1H-im idazole-
5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 106 mg, 78% (last step)
ESI-MS: 604.4 [M+H]
Preparation of compound 1-69
0
0 H
N)-L N
o Nr N H
1-1 0 0
(S)-N1-ethy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
((1R,2S,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
Chemical Formula: C35H43N507
Exact Mass: 645.32
Molecular Weight: 645.75
The synthesis of compound 1-69 was performed according to compound 1-1, using
(R)-
(+)-bornylamine instead of 1-adamantanamine in step 2 (according to ZED3912)
and 3-
methylbenzo[b]furan-2-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound I-1b).
Yield: 86 mg, 72% (last step)
ESI-MS: 646.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
119
Preparation of compound 1-70
0
0 H
0 , Njc N
H 0 0
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C32H37N507
Exact Mass: 603.27
Molecular Weight: 603.67
The synthesis of compound 1-70 was performed according to compound 1-1, using
exo-
2-aminonorbornane instead of 1-adamantanamine in step 2 (according to ZED3912)
and
3-methylbenzo[b]furan-2-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 94 mg, 79% (last step)
ESI-MS: 604.4 [M+H]
Preparation of compound 1-71
0
0
NH
N
0 0
(S)-N1-(1-(2-((ls,4R)-bicyclo[2.2.1]heptan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-ethyl-2-(isonicotinamido)-5-oxohexanediamide
Chemical Formula: C28F-134N606
Exact Mass: 550.25
Molecular Weight: 550.61
The synthesis of compound 1-71 was performed according to compound 1-1, using
bicyclo[2.2.1]heptan-1-ylamine instead of 1-adamantanamine in step 2
(according to
ZED3912) and isonicotinic acid instead of 1-methyl-1H-imidazole-5-carboxylic
acid in
step 6 (according to compound I-1b).
Yield: 77 mg, 72% (last step)
ESI-MS: 551.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
120
Preparation of compound 1-72
0
N 0 17
0 jcI N N NH
0 0
(S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-5-oxo-2-(pyridazine-4-carboxamido)hexanediamide
Chemical Formula: C271-133N706
Exact Mass: 551.25
Molecular Weight: 551.59
The synthesis of compound 1-72 was performed according to compound 1-1, using
bicyclo[2.2.1]heptan-7-ylamine instead of 1-adamantanamine in step 2
(according to
ZED3912) and pyridazine-4-carboxylic acid instead of 1-methy1-1H-imidazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 44 mg, 61% (last step)
ESI-MS: 552.4 [M+H]
Preparation of compound 1-73
0
NN H
0 0
N NH
NjC1-1 .. N
0 0
(S)-N1-(1-(2-((1R,2R,4R)-bicyclo[2.2.1]hept-5-en-2-ylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-y1)-N6-ethyl-5-oxo-2-(pyridazine-3-carboxamido)hexanediamide
Chemical Formula: C27F131 N706
Exact Mass: 549.23
Molecular Weight: 549.58
The synthesis of compound 1-73 was performed according to compound 1-1, using
bicyclo[2.2.1]hept-5-en-2-amine instead of 1-adamantanamine in step 2
(according to
ZED3912) and pyridazine-3-carboxylic acid instead of 1-methy1-1H-imidazole-5-
carboxylic acid in step 6 (according to compound I-1b).
Yield: 31 mg, 52% (last step)
ESI-MS: 550.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
121
Preparation of compound 1-74
0
0 0
N
N1 I
HµN-N 0 0
(2S)-N1-(1 -(2-(bicyclo[2.2.2]octan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-5-oxo-2-(2H-1,2,3-triazole-4-carboxamido)hexanediamide
Chemical Formula: C26H341\1806
Exact Mass: 554.26
Molecular Weight: 554.60
The synthesis of compound 1-74 was performed according to compound 1-1, using
bicyclo[2.2.2]oct-2-ylamine instead of 1-adamantanamine in step 2 (according
to
ZED3912) and 2H-1,2,3-triazole-4-carboxylic acid instead of 1-methy1-1H-
imidazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 35 mg, 57% (last step)
ESI-MS: 555.4 [M+H]
Preparation of compound 1-75
0
0 0 õ
,s
¨NYLNjEr H
N
0 0
(S)-N1-ethyl-5-(1-methyl-1 H-1 ,2,3-triazole-4-carboxamido)-2-oxo-N6-(2-oxo-1-
(2-oxo-2-((1 R,2R,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yOhexanediamide
Chemical Formula: C29H40N806
Exact Mass: 596.31
Molecular Weight: 596.68
The synthesis of compound 1-75 was performed according to compound 1-1, using
(R)-
(-)-isobornylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and
1-methyl-1H-1,2,3-triazole-4-carboxylic acid instead of 1-methyl-1H-im idazole-
5-
carboxylic acid in step 6 (according to compound I-1b).
Yield: 44 mg, 68% (last step)
ESI-MS: 597.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
122
Preparation of compound 1-76
0
0)-LN
0 0
YLN NH )'LN
(S)-N1-ethyl-5-(1-methyl-1H-1,2,4-triazole-3-carboxamido)-2-oxo-N6-(2-oxo-1-(2-
oxo-24(1R,2R,3R,5S)-
2,6,6-trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1 ,2-dihydropyridin-3-
yhhexanediamide
Chemical Formula: C29H40N306
Exact Mass: 596.31
Molecular Weight: 596.68
The synthesis of compound 1-76 was performed according to compound 1-1, using
(1R,2R,3R,5S)-(-)-isopinocampheylamine instead of 1-adamantanamine in step 2
(according to ZED3912) and 1-methyl-1H-1,2,4-triazole-3-carboxylic acid
instead of 1-
methyl-1H-imidazole-5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 51 mg, 72% (last step)
ESI-MS: 597.5 [M+H]
Preparation of compound 1-77
0
0
0 0
, N
r\ir N
H
0 0 0
(S)-2-(benzofuran-3-carboxamido)-N6-ethyl-5-oxo-N1-(2-oxo-1-(2-oxo-2-
((1S,2S,3S,5R)-2,6,6-
trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
Chemical Formula: C34H41N507
Exact Mass: 631.30
Molecular Weight: 631.72
The synthesis of compound 1-77 was performed according to compound 1-1, using
(1S,2S,3S,5R)-(+)-isopinocampheylamine instead of 1-adamantanamine in step 2
(according to ZED3912) and benzofuran-3-carboxylic acid instead of 1-methyl-1H-
imidazole-5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 66 mg, 75% (last step)
ESI-MS: 632.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
123
Preparation of compound 1-78
0
0
0 0
N
, N N
I H I
0 0
(S)-2-(benzo[b]thiophene-3-carboxamido)-N6-ethyl-N1-(1-(2-(4-homoisotwistane-
3-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C35H41 N506S
Exact Mass: 659.28
Molecular Weight: 659.79
The synthesis of compound 1-78 was performed according to compound 1-1 , using
3-
amino-4-homoisotwistane instead of 1-adamantanamine in step 2 (according to
ZED3912) and benzo[b]thiophene-3-carboxylic acid instead of 1-methy1-1H-
imidazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 42 mg, 59% (last step)
ESI-MS: 660.4 [M+H]
Preparation of compound 1-79
0
0 N
0
N NH
"
N 0 0
(S)-N1-ethyl-N6-(1-(2-(diamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methyl-1H-pyrazole-4-carboxamido)-2-oxohexanediamide
Chemical Formula: C34.H4.3N706
Exact Mass: 645.33
Molecular Weight: 645.75
The synthesis of compound 1-79 was performed according to compound 1-1 , using
1-
aminodiamantane instead of 1-adamantanamine in step 2 (according to ZED3912)
and
1-methyl-1H-pyrazole-4-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound I-1b).
Yield: 28 mg, 53% (last step)
ESI-MS: 646.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
124
Preparation of compound 1-80
0
0 )-L
N
H
e_LO f H Oil H
-rHN N /
(S)-N1-ethyl-N6-(1-(2-(diamantane-4-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1-methyl-1H-pyrazole-3-carboxamido)-2-oxohexanediamide
Chemical Formula: C34F143N706
Exact Mass: 645.33
Molecular Weight: 645.75
The synthesis of compound 1-80 was performed according to compound 1-1 , using
4-
aminodiamantane instead of 1-adamantanamine in step 2 (according to ZED3912)
and
1-methyl-1H-pyrazole-3-carboxylic acid instead of 1-methyl-1H-imidazole-5-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 36 mg, 59% (last step)
ESI-MS: 646.5 [M+H]
Scheme 1-3 New Building Block
OH 1-(Bromomethyl)- 0
adamantane
02N ..õ,j\I --L, 02N j-
, N
DMF, DIPEA
ZED4893
1
10% Pd/C
Me0H
0
H2Nj-L
j\I
ZED4894
1 Preparation of compound ZED4893
0
02N j-
U\I
1-(1-adamantylmethyl)-3-nitropyridin-2(1H)-one
Chemical Formula: C16H20N203
Exact Mass: 288.15
Molecular Weight: 288.34
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
125
500 mg (3.57 mmol) of 2-hydroxy-3-nitropyridine and 818 mg (1 eq) of 1-
(bromomethyl)adamantane were dissolved in 10 mL DMF and 1.24 mL DIPEA (2 eq)
and
stirred at room temperature overnight. The solvent was evaporated; the residue
was
dissolved in 30 mL Et0Ac and washed twice with each 10 mL citric acid solution
(10%),
NaHCO3 solution (10%) and brine. The organic phase was dried over Na2SO4,
filtered
and the solvent was evaporated. The residue was purified by HPLC.
Yield: 484 mg, 47%
ESI-MS: 289.3 [M+H]
2 Preparation of compound ZED4894
0
H2Nj-N
3-amino-1-(1-adamantylmethyl)pyridin-2(1 H)-one
Chemical Formula: C16H22N20
Exact Mass: 258.17
Molecular Weight: 258.36
484 mg (1.68 mmol) of ZED4893 were suspended in 30 mL Me0H before 50 mg of
palladium (10%) on activated carbon (unreduced) were added. The suspension was
stirred for 3 h at room temperature under an atmosphere of hydrogen. The
catalyst was
filtered, and the solvent was evaporated.
Yield: 339 mg, 78%
ESI-MS: 259.4 [M+H]
Preparation of compound 1-81
0
0 ( H 0
(,
N
H
0
N-NN
(S)-N1-(1-(1-adamantylmethyl)-2-oxo-1 ,2-di hydropyridin-3-y1)-N6-ethyl-
2-(1 -methyl-1 H-pyrazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C29H38N605
Exact Mass: 550.29
Molecular Weight: 550.65
The synthesis of compound 1-81 was performed according to compound 1-1, using
ZED4894 instead of ZED3913 in step 5 (according to I-1a) and 1-methyl-1H-
pyrazole-5-
carboxylic acid instead of 1-methyl-1H-imidazole-5-carboxylic acid in step 6
(according to
compound I-1b).
Yield: 43 mg, 65% (last step)
ESI-MS: 551.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
126
Preparation of compound 1-82
0
,SN
0
0 H 0
)-L
N
No IA
0
OH
(S)-N1-(14(3-hydroxy-1-adamantyl)methyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-(4-
cyclopropyl-1,2,3-thiadiazole-5-carboxamido)-N6-ethyl-5-oxohexanediamide
Chemical Formula: C30H38N606S
Exact Mass: 610.26
Molecular Weight: 610.72
The synthesis of compound 1-82 was performed according to compound 1-81, using
3-
(bromomethyl)-1-adamantanol instead of 1-(bromomethyl)adamantane (according to
ZED4893) and 4-cyclopropyl-[I,2,3]thiadiazole-5-carboxylic acid instead of 1-
methyl-1H-
pyrazole-5-carboxylic acid.
Yield: 26 mg, 46% (last step)
ESI-MS: 611.4 [M+H]
Preparation of compound 1-83
0
0
N
0
H
N
N I Hr I ¨I
0
Br
(S)-N1-(1-((3-bromo-1-adamantyl)methyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-ethy1-5-oxo-2-(1,2,5-thiadiazole-3-carboxamido)hexanediamide
Chemical Formula: C27H33BrN605S
Exact Mass: 632.14
Molecular Weight: 633.56
The synthesis of compound 1-83 was performed according to compound 1-81, using
1-
bromo-3-(bromomethyl)adamantane instead of 1-(bromomethyl)adamantane
(according
to ZED4893) and 1,2,5-thiadiazole-3-carboxylic acid instead of 1-methyl-1H-
pyrazole-5-
carboxylic acid.
Yield: 42 mg, 61% (last step)
ESI-MS: 633.3 / 635.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
127
Preparation of compound 1-84
o
0
0
N)-L
N
N, H
0
1\1
HO
(S)-N1-(1-(2-adamantylmethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-2-(4-
(hydroxymethyl)-1,2,3-thiadiazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C28F-136N606S
Exact Mass: 584.24
Molecular Weight: 584.69
The synthesis of compound 1-84 was performed according to compound 1-81, using
2-
(bromomethyl)adamantane instead of 1-(bromomethyl)adamantane (according to
ZED4893) and 4-((tetrahydro-2H-pyran-2-yloxy)methyl)-1,2,3-thiadiazole-5-
carboxylic
acid instead of 1-methyl-1H-pyrazole-5-carboxylic acid. The tetrahydropyranyl
(Thp)
protecting group was cleaved by TFA in the final step.
Yield: 15 mg, 68% (last step)
ESI-MS: 585.4 [M+H]
Preparation of compound 1-85
0
0 N
0 ( H 0
N N
HN
0
(S)-2-(4-tert-buty1-1H-pyrrole-3-carboxamido)-N1-(1-(2-(1-
adamantylamino)ethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-5-oxohexanediamide
Chemical Formula: C34.H4.7N505
Exact Mass: 605.36
Molecular Weight: 605.77
The synthesis of compound 1-85 was performed according to compound 1-81, using
1-(2-
bromoethyl)adamantane instead of 1-(bromomethyl)adamantane (according to
ZED4893) and 5-tert-butyl-1H-pyrrole-3-carboxylic acid instead of 1-methyl-1H-
pyrazole-
5-carboxylic acid.
Yield: 32 mg, 58% (last step)
ESI-MS: 606.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
128
Preparation of compound 1-86
0
N
0 0
NCNNjc NH
N \ 0
(S)-2-(4-cyano-1 -methyl-1 H-pyrrole-2-carboxamido)-N1-(1-(1-
adamantylamino)propy1)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide
Chemical Formula: 033H42N605
Exact Mass: 602.32
Molecular Weight: 602.72
The synthesis of compound 1-86 was performed according to compound 1-81, using
1-(3-
bromopropyl)adamantane instead of 1-(bromomethyl)adamantane (according to
ZED4893) and 4-cyano-1-methyl-1H-pyrrole-2-carboxylic acid instead of 1-methyl-
1H-
pyrazole-5-carboxylic acid.
Yield: 28 mg, 51% (last step)
ESI-MS: 603.5 [M+H]
Preparation of compound 1-87
0
0)-LN
0 H 0 0
0?LNJciNj-LN
H 0
(S)-N1-(1-(3-(2-adamantylamino)-3-oxopropy1)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-ethy1-2-(5-methoxyoxazole-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31H4oN608
Exact Mass: 624.29
Molecular Weight: 624.68
The synthesis of compound 1-87 was performed according to compound 1-1, using
3-
chloropropionic acid instead of chloroacetic acid (according to ZED1657) and 5-
methoxyoxazole-2-carboxylic acid instead of 1-methyl-1H-pyrazole-5-carboxylic
acid in
step 6 (according to compound I-1b).
Yield: 39 mg, 64% (last step)
ESI-MS: 625.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
129
Preparation of compound 1-88
0
N
0 JCH 0
0 N N N NH
H 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C30H33N507
Exact Mass: 575.24
Molecular Weight: 575.61
The synthesis of compound 1-88 was performed according to compound 1-1 , using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 3-methylbenzo[b]furan-2-carboxylic acid instead of 1-methyl-1H-
imidazole-5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 107 mg, 81% (last step)
ESI-MS: 576.4 [M+H]
Preparation of compound 1-89
0-(N
0 0
0
Nj-L NH
N
0 0 0
(S)-2-(2-acetyloxazole-4-carboxamido)-N1-(1-(2-(bicyclo[1A A ]pentan-1-
ylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide
Chemical Formula: C26H30N608
Exact Mass: 554.21
Molecular Weight: 554.55
The synthesis of compound 1-89 was performed according to compound 1-1 , using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 2-acetyloxazole-4-carboxylic acid instead of 1-methyl-1H-
imidazole-5-
carboxylic acid in step 6 (according to compound I-1b).
Yield: 68 mg, 75% (last step)
ESI-MS: 555.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
130
Preparation of compound 1-90
0)LN
0 0
U\IN
H 0 0
(S)-N1-(1-(2-(bicyclo[2.1A]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(2-isopropyloxazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C28H36N607
Exact Mass: 568.26
Molecular Weight: 568.62
The synthesis of compound 1-90 was performed according to compound 1-1, using
1-
bicyclo[2.1.1]hexan-1-amine instead of 1-adamantanamine in step 2 (according
to
ZED3912) and 2-isopropyloxazole-5-carboxylic acid instead of 1-methy1-1H-
imidazole-5-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 46 mg, 60% (last step)
ESI-MS: 569.4 [M+H]
Preparation of compound 1-91
0
0)-LN
0
NN
I I
\ 0 0
(2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-(3,5-dimethylisoxazole-4-carboxamido)-N6-ethyl-5-oxohexanediamide
Chemical Formula: C29H38N607
Exact Mass: 582.28
Molecular Weight: 582.65
The synthesis of compound 1-91 was performed according to compound 1-1, using
1-
bicyclo[3.2.1]octan-8-amine instead of 1-adamantanamine in step 2 (according
to
ZED3912) and 3,5-dimethylisoxazole-4-carboxylic acid instead of 1-methyl-1H-
imidazole-5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 32 mg, 54% (last step)
ESI-MS: 583.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
131
Preparation of compound 1-92
0
0)-L N
0 0
NNCN N N
H 0 0
CO2H
(S)-N1-ethyl-N6-(1-(2-(5-carboxy-2-aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(4-methylpyrimidine-5-carboxamido)-2-oxohexanediamide
Chemical Formula: C32H39N708
Exact Mass: 649.29
Molecular Weight: 649.69
The synthesis of compound 1-92 was performed according to compound 1-1 , using
4-
aminoadamantane-1-carboxylic acid instead of 1-adamantanamine in step 2
(according
to ZED3912) and 4-methylpyrimidine-5-carboxylic acid instead of 1-methyl-1H-
imidazole-
5-carboxylic acid in step 6 (according to compound I-1b).
Yield: 35 mg, 46% (last step)
ESI-MS: 650.5 [M+H]
Preparation of compound 1-93
0 N
0 H 0
N N N N
0 0
N
0
(5S)-N1-ethyl-N6-(1-(2-(4-aminoadamantane-N,N-dimethy1-1-carboxamide)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-2-oxo-5-(1,2,3,4-tetrahydronaphthalene-2-
carboxamido)hexanediamide
Chemical Formula: C37H45N507
Exact Mass: 67133
Molecular Weight: 671.78
The synthesis of compound 1-93 was performed according to compound 1-1 , using
4-
aminoadamantane-N,N-dimethy1-1-carboxamide instead of 1-adamantanamine in step
2
(according to ZED3912) and 1,2,3,4-tetrahydronaphthalene-2-carboxylic acid
instead of
1-methyl-1H-imidazole-5-carboxylic acid in step 6 (according to compound I-
1b).
Yield: 31 mg, 55% (last step)
ESI-MS: 672.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
132
Preparation of compound 1-94
oLN
0 0
N
N
0 0
(S)-2-((S)-1,4-diazabicyclo[2.2.2]octane-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C32F145N706
Exact Mass: 623.34
Molecular Weight: 623.74
The synthesis of compound 1-94 was performed according to compound 1-30, using
1,4-
diazabicyclo[2.2.2]octane-2-carboxylic acid instead of benzofuran-2-carboxylic
acid in
step 6 (according to compound 1-1b).
Yield: 39 mg, 58% (last step)
ESI-MS: 624.5 [M+H]
Preparation of compound 1-95
0
0
N
0 H
N
0 0
(S)-N1-ethy1-5-(1H-indole-3-carboxamido)-N6-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-clihydropyridin-3-y1)-2-oxohexanediamide
Chemical Formula: C34H40N606
Exact Mass: 628.30
Molecular Weight: 628.72
The synthesis of compound 1-95 was performed according to compound 1-30, using
1H-
indole-3-carboxylic acid instead of benzofuran-2-carboxylic acid in step 6
(according to
compound I-1b).
Yield: 56 mg, 74% (last step)
ESI-MS: 629.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
133
Preparation of compound 1-96
0
OAN
0 0
N Hjc
0 0
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-(6-methylimidazo[2,1-b]thiazole-3-carboxamido)-2-oxohexanediamide
Chemical Formula: C32H39N706S
Exact Mass: 649.27
Molecular Weight: 649.76
The synthesis of compound 1-96 was performed according to compound 1-30, using
6-
methylimidazo[2,1-b][1,3]thiazole-3-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 33 mg, 56% (last step)
ESI-MS: 650.5 [M+H]
Preparation of compound 1-97
0
o
jcH 0
Nj-L NH
Ni
0 0
(S)-2-(benzo[d]thiazole-2-carboxamido)-N1-(1-(2-(( IS,2R,4R)-
bicyclo[2.2.1]heptan-2-
ylamino)-2-oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-5-
oxohexanediamide
Chemical Formula: C30H34N606S
Exact Mass: 606.23
Molecular Weight: 606.69
The synthesis of compound 1-97 was performed according to compound 1-1, using
( )-
endo-2-norbornylamine instead of 1-adamantanamine in step 2 (according to
ZED3912)
and 1,3-benzothiazole-2-carboxylic acid instead of benzofuran-2-carboxylic
acid in step
6 (according to compound I-1b).
Yield: 45 mg, 73% (last step)
ESI-MS: 607.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
134
Preparation of compound 1-98
0
0
0 0
NNjcN)-L NH
N
C 0 0
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-ethyl-2-(imidazo[2,1-b]thiazole-6-carboxamido)-5-
oxohexanediamide
Chemical Formula: C28H33N706S
Exact Mass: 595.22
Molecular Weight: 595.67
The synthesis of compound 1-98 was performed according to compound 1-1, using
( )-
endo-2-norbornylamine instead of 1-adamantanamine in step 2 (according to
ZED3912)
and imidazo[2,1-b][1,3]thiazole-6-carboxylic acid instead of benzofuran-2-
carboxylic acid
in step 6 (according to compound 1-1b).
Yield: 26 mg, 50% (last step)
ESI-MS: 596.4 [M+H]
Preparation of compound 1-99
ON
OH 0 0
(õ0 N N)-L
NH
I H
0 0
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-ethyl-2-(4-hydroxy-6-(trifluoromethoxy)quinoline-3-carboxamido)-5-
oxohexanediamide
Chemical Formula: C33F135F3N608
Exact Mass: 700.25
Molecular Weight: 700.66
The synthesis of compound 1-99 was performed according to compound 1-1, using
( )-
endo-2-norbornylamine instead of 1-adamantanamine in step 2 (according to
ZED3912)
and 4-hydroxy-6-(trifluoromethoxy)quinoline-3-carboxylic acid instead of
benzofuran-2-
carboxylic acid in step 6 (according to compound I-1b).
Yield: 15 mg, 42% (last step)
ESI-MS: 701.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
135
Preparation of compound 1-100
0
0
o)LN
H 0
NH II
N NH
I ,N H- Y
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-(cinnoline-3-carboxamido)-N6-ethyl-5-oxohexanediamide
Chemical Formula: C29F131N706
Exact Mass: 573.23
Molecular Weight: 573.60
The synthesis of compound 1-100 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 3-cinnolinecarboxylic acid instead of benzofuran-2-carboxylic
acid in step
6 (according to compound I-1b).
Yield: 37 mg, 65% (last step)
ESI-MS: 574.4 [M+H]
Preparation of compound 1-101
0
N
0 H 0
0 N N NH
I H 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(3-ethylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31H35N507
Exact Mass: 589.25
Molecular Weight: 589.64
The synthesis of compound 1-101 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 3-ethylbenzofuran-2-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound I-1b).
Yield: 55 mg, 73% (last step)
ESI-MS: 590.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
136
Preparation of compound 1-102
0
M 0 0
N INHCr H NNH N
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-l-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(1-ethyl-1H-indole-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31H36N606
Exact Mass: 588.27
Molecular Weight: 588.65
The synthesis of compound 1-102 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 1-ethyl-1H-indole-2-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 43 mg, 68% (last step)
ESI-MS: 589.4 [M+H]
Preparation of compound 1-103
0
N
0 0
NH
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(2-methyl-1,8-naphthyridine-3-carboxamido)-5-oxohexanediamide
Chemical Formula: C301-133N706
Exact Mass: 587.25
Molecular Weight: 587.63
The synthesis of compound 1-103 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 2-methyl-1,8-naphthyridine-3-carboxylic acid instead of
benzofuran-2-
carboxylic acid in step 6 (according to compound I-1b).
Yield: 40 mg, 64% (last step)
ESI-MS: 588.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
137
Preparation of compound 1-104
0
0
)LN
0 0
U\IN
0 0
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-5-oxo-2-(1,2,3,4-tetrahydroquinoline-6-
carboxamido)hexanediamide
Chemical Formula: C31 H38 N1606
Exact Mass: 590.29
Molecular Weight: 590.67
The synthesis of compound 1-104 was performed according to compound 1-1, using
bicyclo[2.1.1]hexan-1-amine instead of 1-adamantanamine in step 2 (according
to
ZED3912) and N-Boc-1,2,3,4-tetrahydroquinoline-6-carboxylic acid instead of
benzofuran-2-carboxylic acid in step 6 (according to compound I-1b). The final
product
was obtained by deprotection (DCM/TFA) as described above and purified by
HPLC.
Yield: 27 mg, 73% (last step)
ESI-MS: 591.4 [M+H]
Preparation of compound 1-105
0
0
0 H 0 H CO2H
ON N N
HN 0 0
CF3
(S)-N1-ethyl-N6-(1-(2-(2-carboxy-2-amino-5-(trifluoromethyl)adamantane)-2-
oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-oxo-5-(3-oxo-1,2,3,4-tetrahydroisoquinoline-6-
carboxamido)hexanediamide
Chemical Formula: C37F141F3N609
Exact Mass: 770.29
Molecular Weight: 770.75
The synthesis of compound 1-105 was performed according to compound 1-1, using
2-
amino-5-(trifluoromethyl)adamantane-2-carboxylic acid instead of 1-
adamantanamine in
step 2 (according to ZED3912) and 3-oxo-1,2,3,4-tetrahydroisoquinoline-6-
carboxylic
acid instead of benzofuran-2-carboxylic acid in step 6 (according to compound
1-1b).
Yield: 19 mg, 44% (last step)
ESI-MS: 771.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
138
Preparation of compound 1-106
otLN
0
N N
I I
N 0 0
(S)-N1-ethyl-N6-(1-(2-(5-ethyladamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(1,6-naphthyridine-2-carboxamido)-2-oxohexanediamide
Chemical Formula: C36F143N706
Exact Mass: 669.33
Molecular Weight: 669.77
The synthesis of compound 1-106 was performed according to compound 1-1, using
5-
ethyladamantane-2-amine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 1,6-naphthyridine-2-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 25 mg, 54% (last step)
ESI-MS: 670.5 [M+H]
Preparation of compound 1-107
oN
N N
0 0
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethy1-2-(2,6-naphthyridine-1-carboxamido)-5-oxohexanediamide
Chemical Formula: C30H33N706
Exact Mass: 587.25
Molecular Weight: 587.63
The synthesis of compound 1-107 was performed according to compound 1-1, using
bicyclo[2.1.1]hexan-1-amine instead of 1-adamantanamine in step 2 (according
to
ZED3912) and 2,6-naphthyridine-1-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound I-1b).
Yield: 58 mg, 74% (last step)
ESI-MS: 588.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
139
Preparation of compound 1-108
0-(N
H2N 0 0 -.4\
N I H
b-N 0 0
(S)-2-(4-amino-1,2,5-oxadiazole-3-carboxamido)-N1-(1-(2-(bicyclo[1A A ]pentan-
1-
ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-5-
oxohexanediamide
Chemical Formula: C23H28N807
Exact Mass: 528.21
Molecular Weight: 528.52
The synthesis of compound 1-108 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 4-Boc-amino-1,2,5-oxadiazole-3-carboxylic acid instead of
benzofuran-2-
carboxylic acid in step 6 (according to compound I-1b). The final product was
obtained
by deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 27 mg, 77% (last step)
ESI-MS: 529.4 [M+H]
Preparation of compound 1-109
0
)-LN
0 0
0
Pi 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
2-(6-(dimethylamino)benzofuran-2-carboxamido)-N6-ethyl-5-oxohexanediamide
Chemical Formula: C3iH36N607
Exact Mass: 604.26
Molecular Weight: 604.65
The synthesis of compound 1-109 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 6-(dimethylamino)benzofuran-2-carboxylic acid instead of
benzofuran-2-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 35 mg, 57% (last step)
ESI-MS: 605.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
140
Preparation of compound 1-110
0
0)LN
0 jc H 0
e3A\IN JN NH
1F
HN
N 0 0
0
(S)-2-(2-acetamidothiazole-5-carboxamido)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
ylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide
Chemical Formula: C26H31 N1707S
Exact Mass: 585.20
Molecular Weight: 585.63
The synthesis of compound 1-110 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 2-acetylamino-5-thiazolecarboxylic acid instead of benzofuran-2-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 25 mg, 49% (last step)
ESI-MS: 586.4 [M+H]
Preparation of compound 1-111
ON
0 0
H
0 Nj-L NH
jC
H2N HN 0 0
(S)-N4-(1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-ylamino)-6-(ethylamino)-1,5,6-trioxohexan-2-y1)-1H-pyrrole-2,4-dicarboxamide
Chemical Formula: C26H31N707
Exact Mass: 553.23
Molecular Weight: 553.57
The synthesis of compound 1-111 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 5-carbamoy1-1H-pyrrole-3-carboxylic acid instead of benzofuran-2-
carboxylic acid in step 6 (according to compound I-1b).
Yield: 19 mg, 42% (last step)
ESI-MS: 554.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
141
Preparation of compound 1-112
0
oLN
0 0
0
0,11 Nj-L
N 0
H2N 0 0 0
(S)-N1-ethyl-N6-(1-(2-(1-acetylamino-4-aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-oxo-5-(5-sulfamoylfuran-3-carboxamido)hexanediamide
Chemical Formula: C32H41 N7010S
Exact Mass: 715.26
Molecular Weight: 715.77
The synthesis of compound 1-112 was performed according to compound 1-1, using
1-
acetylamino-4-aminoadamantane instead of 1-adamantanamine in step 2 (according
to
ZED3912) and 5-sulfamoylfuran-3-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 24 mg, 53% (last step)
ESI-MS: 716.4 [M+H]
Preparation of compound 1-113
0
0 0
Njc NH 0
0 0 0
(S)-2-(benzofuran-5-carboxamido)-N6-ethyl-N1-(1-(2-(1-acetylamino-4-
aminoadamantane)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C36F142N608
Exact Mass: 686.31
Molecular Weight: 686.75
The synthesis of compound 1-113 was performed according to compound 1-1, using
1-
acetylamino-4-aminoadamantane instead of 1-adamantanamine in step 2 (according
to
ZED3912) and benzofuran-5-carboxylic acid instead of benzofuran-2-carboxylic
acid in
step 6 (according to compound I-1b).
Yield: 30 mg, 58% (last step)
ESI-MS: 687.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
142
Preparation of compound 1-114
0
0 ON
0 Njc NH )-LNN
0 0 NH2
0
(S)-2-(benzofuran-6-carboxamido)-N6-ethyl-N1-(1-(2-(4-aminoadamantane-1-
carboxamide)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C35H4.0N608
Exact Mass: 672.29
Molecular Weight: 672.73
The synthesis of compound 1-114 was performed according to compound 1-1, using
4-
aminoadamantane-1-carboxamide instead of 1-adamantanamine in step 2 (according
to
ZED3912) and benzofuran-6-carboxylic acid instead of benzofuran-2-carboxylic
acid in
step 6 (according to compound 1-1b).
Yield: 37 mg, 65% (last step)
ESI-MS: 673.5 [M+H]
Preparation of compound 1-115
0
0)-(N
JCH 0
N
0 0 NH2
0
(S)-N1-ethyl-N6-(1-(2-(4-aminoadamantane-1-carboxamide)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(3-(1-methylcyclopropy1)-1,2,4-oxadiazole-5-carboxamido)-2-
oxohexanediamide
Chemical Formula: C33H4.2N808
Exact Mass: 678.31
Molecular Weight: 678.74
The synthesis of compound 1-115 was performed according to compound 1-1, using
4-
aminoadamantane-1-carboxamide instead of 1-adamantanamine in step 2 (according
to
ZED3912) and 3-(1-methylcyclopropyI)-1,2,4-oxadiazole-5-carboxylic acid
instead of
benzofuran-2-carboxylic acid in step 6 (according to compound I-1b).
Yield: 23 mg, 47% (last step)
ESI-MS: 679.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
143
Preparation of compound 1-116
0
0
N
0 H
yLrilJc N NH
0-N 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-2-(5-methyl-1,2,4-oxadiazole-3-carboxamido)-5-oxohexanediamide
Chemical Formula: C24H29N707
Exact Mass: 527.21
Molecular Weight: 527.53
The synthesis of compound 1-116 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 5-methyl-1,2,4-oxadiazole-3-carboxylic acid instead of benzofuran-
2-
carboxylic acid in step 6 (according to compound 1-1b).
Yield: 36 mg, 62% (last step)
ESI-MS: 528.4 [M+H]
Preparation of compound 1-117
0
/)?LN
N NH
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-5-oxo-2-(1,2,3-thiadiazole-4-carboxamido)hexanediamide
Chemical Formula: C23H27N706S
Exact Mass: 529.17
Molecular Weight: 529.57
The synthesis of compound 1-117 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 1,2,3-thiadiazole-4-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound I-1b).
Yield: 31 mg, 57% (last step)
ESI-MS: 530.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
144
Preparation of compound 1-118
o
0
S- N Jcri\lj- NH
ri y
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-5-oxo-2-(1,2,4-thiadiazole-5-carboxamido)hexanediamide
Chemical Formula: 023H27N706S
Exact Mass: 529.17
Molecular Weight: 529.57
The synthesis of compound 1-118 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 1,2,4-thiadiazole-4-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 39 mg, 63% (last step)
ESI-MS: 530.3 [M+H]
Preparation of compound 1-119
0
0)-LN
0 0
N.r NH N NH
N-N H 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-ethyl-5-oxo-2-(1,3,4-thiadiazole-2-carboxamido)hexanediamide
Chemical Formula: C23H27N706S
Exact Mass: 529.17
Molecular Weight: 529.57
The synthesis of compound 1-119 was performed according to compound 1-1, using
1-
bicyclo[1.1.1]pentylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and 1,3,4-thiadiazole-5-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound I-1b).
Yield: 28 mg, 52% (last step)
ESI-MS: 530.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
145
Preparation of compound 1-120
o
m's Nc7 N
H I I
0 0
0
(S)-N1-ethy1-5-(4-formy1-1,2,3-thiadiazole-5-carboxamido)-N6-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxohexanediamide
Chemical Formula: C29H35N707S
Exact Mass: 625.23
Molecular Weight: 625/0
The synthesis of compound 1-120 was performed according to compound 1-30,
using 4-
(hydroxymethyl)-1,2,3-thiadiazole-5-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to compound 1-1b).
Yield: 17 mg, 41% (last step)
ESI-MS: 626.4 [M+H]
Preparation of compound 1-121
0
0)-(N
0 0
NH
N(-1H N
0 0
(S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(nicotinamido)-2-oxohexanediamide
Chemical Formula: C31F138N606
Exact Mass: 590.29
Molecular Weight: 590.67
The synthesis of compound 1-120 was performed according to compound 1-1, using
nicotinic acid instead of benzofuran-2-carboxylic acid in step 6 (according to
compound
I-1b).
Yield: 56 mg, 60% (last step)
ESI-MS: 591.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
146
Preparation of reference compound Ref. 4
0
OA
N
0 0
N
)LN N
0 0
(S)-N1 -ethy1-5-(nicotinamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
(phenethylamino)ethyl)-1 ,2-dihydropyridin-3-yl)hexanediamide
Chemical Formula: C29H32N606
Exact Mass: 560.24
Molecular Weight: 560.60
The synthesis of reference compound Ref. 4 was performed according to compound
1-1,
using 2-phenylethylamine instead of 1-adamantanamine in step 2 (according to
ZED3912) and nicotinic acid instead of benzofuran-2-carboxylic acid in step 6
(according
to compound l-lb).
Yield: 68 mg, 77% (last step)
ESI-MS: 561.4 [M+H]
Biological Examples
Example B-1. Inhibitory effect of the compounds according to the invention
Transglutaminase assay
For the determination of potency of inhibitors against tissue
transglutaminase, the
incorporation of dansylcadaverine into dimethylcasein (Zedira product T036,
Lorand et
a/., Anal Biochem, 1971, 44:221-31) was measured using recombinant human
transglutaminase 2 (Zedira Product T022).
The tissue transglutaminase is diluted in buffer (50 mM Tris-HCI, 7.5 mM
CaCl2, 150 mM
NaCI, pH = 7.4). The final concentration of TG2 in the assay is 10 nM.
A 10 mM inhibitor stock solution is prepared in DMSO, and from this a serial
1:2-fold
dilution series is prepared also in DMSO. Each of the initial dilutions is
subsequently
diluted 1:50-fold with buffer (50 mM Tris-HCI, 7.5 mM CaCl2, 150 mM NaCI, pH =
7.4) to
yield the final working dilutions containing 2% (v/v) DMSO.
15 pl of inhibitor working dilution are added per well of a 96 well microtiter
plate. As
control, 15 pl of a 2% (v/v) DMSO solution prepared using the buffer mentioned
above
are added per well.
Immediately before starting the assay, 600 pl transglutaminase working
solution are
added to 11.4 ml assay buffer (50 mM Tris-HCI, 10 mM CaCl2, 10 mM glutathione,
2.5%
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
147
glycerol, 16.7 pM dansylcadaverine, 4 pM N,N-dimethylcasein, 200 mM NaCI, pH =
8.0).
285 pl of this reaction mix are added per well containing the inhibitor.
Increase in fluorescence is measured using Aex = 330 nm and Aem = 500 nm at 37
C for
30 min. A slope of the increase in fluorescence between 20 and 30 min is
calculated for
determination of the ICso value (inhibitor concentration at which 50% of the
initial activity
is blocked).
Analysis of enzymatic activity is performed by calculation of the slope of an
increase in
fluorescence intensity. ICso values are calculated by plotting the enzymatic
activity (as
percentage from control containing 2% DMSO instead of inhibitor) against the
inhibitor
concentration. ICso is defined as the inhibitor concentration blocking 50% of
initial enzyme
activity.
The inhibitory activity of the inventive compounds in regard to tissue
transglutaminase
(TG2) is shown in the following table 1 using ICso-values.
Table 1. efficacy of reversible TG2 inhibitors
A: ICso < 150 nM, B: 150 nM ICso <600 nM, C: 600 nM ICso < 3,500 nM,
D: 3,500 nM ICso < 10,000 nM
ICso I C50
Compound Compound
TG2 [nM] TG2 [nM]
1-1 C 1-61 B
1-2 C 1-62 B
1-3 C 1-63 B
1-4 A 1-64 A
1-5 B 1-65 A
1-6 B 1-66 B
1-7 C 1-67 C
1-8 B 1-68 A
1-9 B 1-69 B
1-10 B 1-70 A
1-11 B 1-71 B
1-12 B 1-72 B
1-13 C 1-73 B
1-14 B 1-74 B
1-15 C 1-75 B
1-16 C 1-76 B
1-17 C 1-77 B
1-18 C 1-78 B
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
148
1-19 C 1-79 B
1-20 C 1-80 B
1-21 C 1-81 B
1-22 C 1-82 B
1-23 C 1-83 B
1-24 B 1-84 B
1-25 B 1-85 C
1-26 B 1-86 C
1-27 C 1-87 C
1-28 A 1-88 A
1-29 A 1-89 A
1-30 A 1-90 A
1-31 A 1-91 B
1-32 A 1-92 B
1-33 A 1-93 B
1-34 A 1-94 C
1-35 A 1-95 B
1-36 A 1-96 C
1-37 A 1-97 B
1-38 A 1-98 C
1-39 A 1-99 C
1-40 A 1-100 C
1-41 A 1-101 B
1-42 B 1-102 B
1-43 A 1-103 C
1-44 A 1-104 C
1-45 A 1-105 C
1-46 A 1-106 C
1-47 A 1-107 C
1-48 B 1-108 C
1-49 A 1-109 B
1-50 A 1-110 C
1-51 A 1-111 C
1-52 A 1-112 C
1-53 A 1-113 B
1-54 A 1-114 B
1-55 B 1-115 C
1-56 A 1-116 C
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
149
1-57 C 1-117 B
1-58 B 1-118 B
1-59 B 1-119 B
1-60 B 1-120 B
Ref. 1 C 1-121 A
Ref. 2 A
Ref. 3 D Ref. 4 D
Ref. 1 (ZED3641) Ref. 2 (ZED1227)
00Me
0
o)-LN
H
H H
1\1 N .----,,,,,N.õ..---,,_,--
NN -c NH')N-M-IN'----'"-----
/YNcN
H 0 8
.--N 0 0
N \
Ref. 3 (A8, ZED1047) Ref. 4
o,o
o
0)*.N.-----,....,
H
0 u 0 0 0
H H
Nljc-rINI
II NN
0 1\ljcrNH')
_ JI NN
I H I ,, H
0 0
N 0
l\r
Example B-2. logD values of the inventive compounds
10 In order to classify the inventive compounds according to their
lipophilicity, LogD values
(distribution coefficient) were determined by means of the well-established
shake flask
method, measuring the partition of a compound between an octanol and phosphate-
buffered saline (PBS, pH 7.4) by HPLC.
Compounds with a moderate lipophilicity (LogD values from 0 to 3) are usually
15 advantaged for oral absorption, being in balance between solubility and
permeability.
However, sophisticated formulation of a compound might improve oral
bioavailability for
highly lipophilic compounds.
Table 2. logD values of reversible TG2 inhibitors
20 A: logD < 1, B: 1 logD < 3, C: 3 logD < 5
Compound logD Compound logD
1-1 A 1-61 B
1-2 A 1-62 B
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
150
1-3 B 1-63 A
1-4 B 1-64 B
1-5 B 1-65 B
1-6 A 1-66 C
1-7 A 1-67 C
1-8 A 1-68 B
1-9 A 1-69 C
1-10 A 1-70 B
1-11 A 1-71 A
1-12 A 1-72 A
1-13 B 1-73 A
1-14 B 1-74 A
1-15 B 1-75 A
1-16 B 1-76 B
1-17 B 1-77 C
1-18 C 1-78 C
1-19 C 1-79 B
1-20 B 1-80 B
1-21 B 1-81 B
1-22 B 1-82 A
1-23 B 1-83 C
1-24 B 1-84 B
1-25 B 1-85 C
1-26 B 1-86 C
1-27 B 1-87 B
1-28 B 1-88 A
1-29 B 1-89 A
1-30 C 1-90 A
1-31 C 1-91 A
1-32 C 1-92 A
1-33 C 1-93 A
1-34 C 1-94 B
1-35 C 1-95 B
1-36 C 1-96 C
1-37 C 1-97 B
1-38 C 1-98 B
1-39 C 1-99 C
1-40 B 1-100 A
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
151
1-41 B 1-101 A
1-42 C 1-102 B
1-43 C 1-103 A
1-44 C 1-104 A
1-45 C 1-105 A
1-46 C 1-106 C
1-47 C 1-107 A
1-48 B 1-108 A
1-49 B 1-109 A
1-50 C 1-110 A
1-51 C 1-111 A
1-52 C 1-112 A
1-53 C 1-113 A
1-54 C 1-114 A
1_55 B 1-115 A
1-56 A 1-116 A
1-57 A 1-117 A
1-58 A 1-118 A
1-59 A 1-119 A
1-60 B 1-120 A
Ref. 1 A 1-121 A
Ref. 2 B
Ref. 3 B Ref. 4 A
Example B-3. Caco-2 permeability assay of the inventive compounds
Permeability coefficients (Papp values) were obtained from Caco-2 barrier
studies
predicting oral/intestinal bioavailability of the tested compounds. The assays
were
performed by using CacoReadyTM ready-to-use kits from ReadyCell according to
the
manufacturers protocol.
It is considered that compounds bearing Papp values above 1x10-6 cm/s are
classified as
permeable whereas compounds bearing Papp values below 1x10-6 cm/s are
classified as
not permeable.
Table 3. Caco2 permeability assay of reversible TG2 inhibitors
A: Papp < 1x10-6 cm/s, B: Papp 1x10-6 cm/s Papp < 10x10-6 cm/s
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
152
Papp [CM/S] Papp [CM/S]
Compound Compound
x10-6 x10-6
1-1 A 1-61 A
1-2 A 1-62 A
1-3 A 1-63 A
1-4 A 1-64 B
1-5 A 1-65 B
1-6 A 1-66 A
1-7 A 1-67 A
1-8 A 1-68 B
1-9 A 1-69 B
1-10 A 1-70 B
1-11 A 1-71 A
1-12 A 1-72 A
1-13 A 1-73 A
1-14 A 1-74 A
1-15 A 1-75 B
1-16 A 1-76 A
1-17 A 1-77 B
1-18 A 1-78 B
1-19 A 1-79 A
1-20 A 1-80 A
1-21 A 1-81 A
1-22 A 1-82 A
1-23 A 1-83 A
1-24 A 1-84 A
1-25 A 1-85 B
1-26 A 1-86 B
1-27 A 1-87 B
1-28 A 1-88 B
1-29 A 1-89 A
1-30 B 1-90 B
1-31 B 1-91 B
1-32 B 1-92 A
1-33 B 1-93 A
1-34 B 1-94 A
1-35 B 1-95 B
1-36 B 1-96 A
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
153
1-37 B 1-97 B
1-38 A 1-98 A
1-39 B 1-99 A
1-40 B 1-100 A
1-41 B 1-101 B
1-42 B 1-102 B
1-43 B 1-103 A
1-44 B 1-104 A
1-45 B 1-105 A
1-46 B 1-106 A
1-47 B 1-107 A
1-48 B 1-108 A
1-49 B 1-109 A
1-50 B 1-110 A
1-51 B 1-111 A
1-52 B 1-112 A
1-53 B 1-113 A
1-54 B 1-114 A
1-55 A 1-115 A
1-56 B 1-116 A
1-57 A 1-117 A
1-58 A 1-118 A
1-59 A 1-119 A
1-60 A 1-120 A
Ref. 1 A 1-121 A
Ref. 2 A
Ref. 3 A Ref. 4 A
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
154
CLAIMS
1. A compound of the general formula (I):
R1
0 (0
H (I)
R2 N N
7,.N yL R3
1
H 1
0
wherein
L represents ¨1_1-L2¨;
LI represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
L2 represents a bond, ¨NRI41¨, ¨NRN1CH2¨, ¨NRN1CH2CH2¨, or
¨NRNICH(CH3)¨;
0
R1 represents
0 =
'
R2 represents
RN
- _ 0 Rs Rii 0 Ra - _ S Rs R11 S Rs --
11 R8
Rlo R9 ;
' R9 Rlo R9 ;
' R9
Rlo R9 ;
, ,
RN RN RN RN RN
RN
R11 N R8 R11 N, Rii N, R11 N _ _
--14N 1 iN
.,), K n ----
R1,...1 11R8
) _________________________________________________________ N
N
/ R9 Rlo µ, / Ro R.- IR Rlo
, / ,
RN Rlo Rlo
R1-1.(:)_...- Ril 0R8 --_0 R8 os)--..-- R8
13)
Y __________ N ) __ N N Y __ N
R10
; R10
; ,
/ R10
; R8
' N -
\ ,
RN RN
\
os?..R10 R1, 5._._ RiR8 -- S R8 .---11N Rt,l,c11-N
N.--1\ R8 N
N \ n';
, N
R1)8 N R10 N ,
R10
; ;
'
RN RN
,N, ,Nx_Rlo RN R12 R12
N N N N /-1 R13
- - ---N'N'IRN
R13
i(
/ Rlo ,,
\-1\1 LI xj \-)
; N¨N ; N=N ; ------- R14 N R14
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
155
R12
R12 R12 N R12 R12
R13 N R13 R13 ---,,\TR
\N \-) Ria RioR13
N Ria N=Ria N Ria Rio
(:)----\44 ,
RN
R12 R12
R11 , N---/y
R12 S---/y,) , R12
- --TR13 ---,
R10_<)---r/ -\ R1013 7R13
0"---13 R10 R14 S"---14 R10 R14
%
% RN
R12 R12 R12
Rlo / N---/y,) '-1 /') 13
-- 4 1 71 R13 II 1 +R13
N N----' c7R
1
RN , R14 , R14 R._A
R13
R11 ,,
RN RN
. R9
. __________ Niv...._R14 (1 N, R9 0,1
Rio_---N1 14 \1R9 r
Rio N __ S , R NS - \____,------R10 RN Rio
, ,
RN R8 R8
IR9
R...ki 9 R9 ,S -- ),,,,__ -
-
_ _Rio 4/, in N \ X N./ II --
o) ". --/--, io
¨R N----R'" \N R8 \s_N N-_-_N
-
' ,
,S -- Fi8
N ' 0 ,S.õ,,,-- / ,- 0 N,, - 0
.---N Ru----\ il N6- Ru---- -I - R8---NI-- ---
R8 N-N b-N WO
, ,
S
---
N,
N _yN
N __________ (
Nj N( i - N N N __ \(
S S z S S z N
,
----eNS ---eNS ----eNH ---eNNH
IIN, ITij N __ (
0 01\ij
..N _ N
-- \ --( ----eN0 ----eN0 ---eN0
N N __ \(
Z
6 7N
o N3 , N
, ,
,
H
N,_-S
--N- /N S
H,
S---___-N ____N
__C:1; --1/ S--_- ___C-r\-
---/___N--(/N
S-NN
,
, , ,
H
N,.-0 N
--
A-NI
S-N
-1\1/
--"1-.),
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
156
0--__-N _NI
-N
H H
p-...__N
/
,
i ..
1 I I
N
/ N ,
,
_ N -- --
'N
, N
-- -, N -N
1 II
N
,
.,
I -I
,
'
-, N -, --
'N 'N
N 1\11
N
,
_
-_,1\1 I\1 -_,I\IN
- N
N N le 1
,
.,
N
1
NN I
1\1 1\1 N
-, -,
1rN 'rNii '-'r
N, N NN,
,
'-- -, N N -, N. N
N,s, NN NN,
-,fl\IN
--%
N
Nk," NN N
,
-_,NN,N
N 1
--..._õ----__
1
Th\l N,
,
,
-,,, ---!NN I\I N -,1\IN
1 1\1 I II
I 1 , f
N N--N --, ------õ,--,N NN,
, ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
157
-,,N
1 II
Nle , Nie , N.N
,
I\IN -,,N,N
'N
1
IV ,
N, ,
N. -, I\1 -,,I\I
'Nil
N,2 N N N--N
, , ,
N N , 1\1-N1-, NN
,
-'
'-lr"N
N,N
N-, N I
1\1-
N N , N N N
I I I
NN
, NN--N NN-,N
-,,N,N
I I
1
N. NN N N
el\I
,
H
NH
HN NH ,
,
.,
N NH ,
HN , NH H , ,
N=\ 0 N=N N=\ N-
--N
---0
\ N
N- N- N- -N -N
--- ;IV - -
--- / N \ JV
N' N
-- __
N \
\ //IV
N, /
___________________________________ , _______ '
N ______________________ ,
--\ /,\N
-\
I\1 , --
\ N --
\ N -- N =
N
N IV N/ //
,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
158
N=\ N=\
--- /(1\1
\ / N\/ N / N
N
N N¨N 'IV N //N
N=N N=N N=N N=N N=N
' N --,
\ /N / N
, ________ , __________________________________ ,
N=\ N=\ N=\ N=\ N-
--
(
\ /71; \ S \ N
/ N N N ,
\
N/ N //N
N¨N N N/
,
---\ N ,
\ /71; \ S N N , ____ ¨\ ,N N N
,
¨N ¨N _¨N
-- --i
)
\ )
\
/
N / N¨N N N
,
______________________________________________ ,
¨\ ___\
-- N ---
\ N \ S N S
N ; N¨//
N¨N N
* *
Ns /
Ns \ /IV N N
" N, /N N¨N -
N N¨N ; N si\l/ , or
______________________ , ,
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and R" ; and preferably with 1 to 3 of the substituents
R11 ¨
R13;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl;
diamantyl;
or hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one or more C=C double bond(s) and/or are optionally substituted by one or
more
of Ra; Rb; Rc; Rd; and Re;
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
159
Ra, Rb, Rc, Rd, and Re represent independently of each other -H, -F, -Cl, -Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CONH2,
-CON HCH3, -CON(CH3)2, -
CON HC2F15, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C21-15, -CH2CONH2, -
CH2CONHCH3, -CH2CON(CH3)2,
-CH2CONHC2F15, -NHCOCH3, -NHCOC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R4 represents -N R6R7;
R6 represents -CH2CH3; and R7 represents -H;
R6, R6, R10, R11, R12, R13, and R14 represent independently of each other
-H, -F, -Cl, -Br, -I, -OH, -CN, -NO2, -CH3, -C2H5, -C3H7,
-CH(CH3)2, -C4H9, -CH2-CH (CH3)2, -CH(CH3)-C2H5, -C(CH3)3,
-cyclo-C3H5, -CH2-cyclo-C3H5, -
CH2F, -CH F2, -CF3, -CH2CI,
-CH2Br, -CH21, -CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI,
-CH2-CH2Br, -CH2-CH21, -CH2OH, -OCH3, -0C2H5, -0C3H7, -OCH(CH3)2,
-0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH 2C F3 , -0C2F5, -OCH2OCH3,
-0-cyclo-C3H5, -OCH2-cyclo-C3H5, -0-C2H4-cyclo-C3H5, -CHO,
-COCH3, -COCF3, -00C2H5, -00C3H7, -COCH(CH3)2, -00C(CH3)3,
-COOH, -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3,
-00C-CH3, -00C-CF3, -00C-C2H5, -00C-C3H7, -00C-CH (CH3)2,
-00C-C(CH3)3, -NH2, -NHCH3, -NHC2H5, -NHC3H7, -NHCH(CH3)2,
-N HC(CH3)3, -N(CH3)2, -N(C2H5)2, -N(C3H7)2, -N[CH(CH3)2]2, -N[C(CH3)3]2,
-NHCOCH3, -NHCOCF3, -NHCOC2H5, -NHCOC3H7, -NHCOCH(CH3)2,
-NHCOC(CH3)3, -CONH2, -CONHCH3, -CONHC2H5, -CONHC3H7,
-CON HCH (CH3)2, -CON H-cyclo-C3H5, -
CON HC(CH3)3, -CON(CH3)2,
-CON (C2H5)2, -CON(C3H7)2, -CON[CH(CH3)2]2, -CON[C(CH3)3]2, -SO2NH2,
-SO2NHCH3, -SO2NHC2H5, -
SO2NHC3H7, -SO2NHCH(CH3)2,
-SO2NH-cyclo-C3H5, -SO2NHC(CH3)3, -
SO2N(CH3)2, -SO2N(C2H5)2,
-SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C2H5, -
NHSO2C3H7, -NHSO2CH (CH3)2,
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
160
/ H 1
I\1 H
I\1 H
N
--
N
,
,
, ,
1 H 1
1\1 1\1
r N r r / __ \ / __ \ / __ \ __
-N\ /NH, --N\ N-
/ , or - -N N
\ ________________________________________________________________ / \ =
, ,
or R8 and R9 or R9 and R1 can form together one of the following five-
membered
or six-membered rings:
ci _-1-d --- -- --- --I\1 ---N --- ---
--) -- -- 1 --_1 '-./ ''0 --,% --.% '-,
'-.N 'le,
__1\1 õ-N - ,N
-I
-
'1\1 'N or ---1\1-
or R12 and R13 or R13 and R14 can form together one of the following five-
membered or six-membered rings:
,o
,ici ,,ici ,-C) ,-0__-
_.0
-0 --0 ,,.--...,
, --./ '--O ''I:Y H or I - ,
Ri4 represents -H, -CH3, -C2F13, -C3H7, -CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH(CH3)-C2H3, -C(CH3)3, -cyclo-C3H3, -cyclo-C4H7, -cyclo-C31-19,
-CH2-cyclo-C3H3, -CH2-cyclo-C4H7, -CH2-cyclo-C3H9, -CH2F, -CHF2, -CF3,
-CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2; -CF12-CF3, -CH2-CH2CI,
-CH2-CH2Br, -CH2-CH21, -
CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-13, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -CO-cyclo-C3H3, -CO-cyclo-C4H7, -CO-cyclo-C31-19, -COOCH 3;
-COOC2H3, -CO0C3H7, -COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -S02C21-13, -S02C3H7, -
S02CH(CH3)2,
-S02-cyclo-C3H3, or -S02C(CH3)3;
RNi represents -H, -CH3, or -CH2CH3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomer, a racemate, a solvate, a hydrate, or a pharmaceutically acceptable
salt
thereof.
2. The compound according to Claim 1, wherein
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
161
R2 represents
RN
- 0 Ra R11 0 Ra _ _ S ,-, - 8 Rt1 S
Ro - - 11 R8
r -\\ r (
R1 R9 ; ' R9 R10 .-,9 ; ' R9 R10
R9
; K
RN RN RN RN
R11 N R8 R11 N, R11 ri, _ _ ,N,N R11 ON
_ _ R11 0 pp 8
N
/ ____________________________________ l(
Y /K ir 1 -----
N N
R9 R10 s, / R9 R10 R9 R10 /
R10 R10
- _2:)____ R8 ,_ R10 Ril SN Rii S R8
. -
0? 10) R8 C/./ ir 1
N \ N
¨7 R N
Rlo N R8 N
N \ R- Rio
, ,
RN RN RN
RN
S Ra - - N -õ,
õ
1
Rii ri N
, ,N, _Rlo
-\___ 1 N
N I ir
N ________ N N ,N, N, '"
DN
--- N
R1 , R10, , , ,/ , N-N ; N=N ;
R12 R12 R12 R12 N R12
R13 /-1
I R13 (R13 N/ R13 r
1
Ri3
\N
-----Ria N-Ria N R14 N-Ria N Ria
,
RN
R12 R12 R12 RN
i\i ---- R12
0___-y 13 s
13 i\i -,) 3
I
R1
-\- -- 'p -
R14 R14 R14 iA
Rlo Rlo R1 o R -
,
R13
R12 R12
RN
RN
sµ
N R9
N)---- R14 ii R9 r >1
iX ____________________________________________________ _ )...i _
R1.4 \-i)R14 ' .o. -n N S cõ,
' , , ,
RN R8
ri R9
r\
\____ i___ 9 R9
_ ir_ -/* R 1 0 --/ __ , Ri n N \ 1\
\ I l/
IT
<:., m _ -
N R8
\S-N
R8 ,S, - - R8
S)- - - N II
__-N R' . S - -
--- il
N./ Rµ-'
IT - . N, - -
--- -I N
R8--
\N=N R8 , N-N ; b-N
, N-0
0 S
- - - -
N N
, ,
Icl 0 s ---eNNH
\ \ \ N __ (
, i S
,
, ; , ,
,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
162
N _ N
---eNNH ---( --(N ---eNS
N N __ \(
SNjz
S , N
---eNNH ---eNNH
IIN , i\il\IJ N __ (
0 ONjz
-- A -
N N N __ \(
, NiNj
o ciN, N
H s
N--...._-S
Nr-1,
-
- --- N -) --.1------) N N-N-S
,
S----N
-k-N, ------S-N ---c_rN
N0 H
N
S-N-N/ ----N, --C----)
, N-0
0----N
-----N-N No ------N,
H H ,
,
-C-----Q
-, I\1 .,
1 I I
N /
N,
,
-, N -,
' N
-- - N
-. , -N
I II
N 1\r N /
-, N,
I 'N
-I
N.N / , N
, '
-, N -. --
'N 'N
N N
N
,
-,,I\I I\1 -,,I\IN
-,N I
N
- N
I N I
IN 1\1-
N
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
_
I
- - - -
- - z
Z /z¨Z /z¨Z 7/ // Z Z Z \ / Z - Z /¨Z
Z¨ - Z Z¨
) Z\ , Z\ , Z\ Z ) Z' ZZ ) Z
) Z-
Z K Z
/ ) Z)/ Z2/ Z)/ \ Z2/ \ ZZ ZZ \ Z \ Z 0 /
/ / 2/ µ Z Z
¨Z Z ¨/ ¨ ¨ Z , ¨/ , ¨/ , ¨Z ¨/ ¨Z ¨Z
¨/
-
2Z
-
Z - - z ______________________ - z - - z __ - z -
2 z \ z' 'z z/z 'z z"¨ z\ z' \ 0 'z z' ¨'z z"¨ \ z z/z 'z T
z" z¨z / z / _______ z
)
/\ zz z)z __ z)z \ z)z __ \ z/z z/z z4
¨z z ¨/ ¨ z ¨/ ¨/ z c
z
z __ z
, _/ , _/
/
_
I
z
e5
cõ
i 51
9
- .
N
i
c
?
z7/ / __ ; Z ¨z \ z7/ __ V , ¨z r% ,- z\- /_ rz\ ,
z\ , __ z\ . ._
0 / z)/ z)/ __ \ z)/ µz z)/ z)/ z)/ µz
0 ((z
¨/ ¨ ¨ ¨/ , ¨z , ¨z , ¨z
, ¨z - :wc' ' ca!
z
0
0
59- --
Qo
/ I 1 Lucn (9,
, ,
I 9 0
0
ct
a
q
0
a,
,
,r
a
'
r v
164
.,
HN , NH H , ;
N=\ 0 N=N N=\ N-
-
¨ ¨N
--13
¨ ¨ \ / -- \ /N ¨ ¨ ¨_
\ N
N/ \ ' ,,N
,
N¨ N¨ N¨ ¨N ¨N
¨ ¨
¨ -- / N
N N
,
¨ ¨ _______________________ -- \ ¨ --\ /(N
¨ / N \ /IV \ /
N N , ________________________ ' , ______ N ,
¨ ¨ \ /\N
¨
\
1\1 ¨\
, \ N \ N
//
N/
N N
N N
, ,
N=\ N=\
¨ ¨ ¨, /(N
N s / N
N¨N
N N N //N
N
N=N N=N N=N N=N N=N
¨ ¨ \ -- ______________ -- ¨.__ -- ¨,
\ ,N
N N
, ______ // , ,
, ,
N=\ N=\ N=\ N=\ N-
- ¨ /N ¨ ¨ ¨_ /N ) /N --- 51 __ --
(
\/N , \ N, \ N \ ,N
/ N N ,
N¨ N¨ N¨ N¨ N-
- ¨ __________ ¨ ¨ \ i 1
N
\ N -- / N / N / / N N //'
N¨N, N, N __
, ,
¨N ¨N ¨N ¨N ¨N
,
/N ¨ ¨ /N N
\/N , \ \ N \ ,N
N N N , , , ,
¨N ¨N _¨N ¨N ¨N
¨ ¨ __________ ¨ ¨i
\ \
--
N N /
\ N , N / N /
N/ //
N¨N N N
, ' , ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
165
¨\
---, N -- N --i /N
\ N \ S N S
N , N¨// //N N¨N N
, ,
-- . -- =
--i /N --
N, \ ,N N N
N N N¨N ;
N _____________ , N¨N , N sis\l¨//
, ,
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and RN; and preferably with 1 to 3 of the substituents
R11 ¨
R13;
and the substituents R9 ¨ R14 and RN have the meanings as defined in claim 1.
3. The compound according to Claim 1, wherein
RN RN RN
R11 il
-
R11 N R8 ---Ii\_¨R8
R2 represents 1 ---
1 - - --
N
N
R10 R10
RN
- _ 0 R8 R11 0 R8 - __ S R8 R11 S R8 11
R8
R10 R9 ;
/ R9 R10 R9 ;
/ R9 R10 R9 ;
, ,
RN RN RN RN R10
R11 N R8 R11 N, R11 N, N ,
---cji - - --L i\i - - R11 0 R8
, , R9 R10 ,R9 Rlo R9 N¨ 8 N
1 1 1 1 N , 1
RN RN RN
¨8 R11 S _._ S
N ) __ N N - 11 N I ,N,
1
--, ____.R8 -_. -NI R11.õcN,N
)--- \ .'
, N N N
, ______________________________________________________________________
i(
Rio Rio Rio Rio
, , R
, , , , , io ,
,
RN
R12 R12 R12 R
N iz
,N Rlo /-R13
1 /-) R13 N/
"Nr---, ¨
R13
)¨N LI _ \ -)R13 -- \N
,
, N=N , ------R14 N R14 N R14 N
R14
,
R12 R11
N R12 0---/y,) ,
% R12 _()
R12
R13 ---, TR"
-\' 1 A
Q14 Rio_tr>R13
õ, Rlo
N R14 Rlo R.-, R14
(:)---13 ;
,
RN ,
R12 R12 %
%
s-r/,
-- p 13 % R12 1\11 /') p13 R10 /
Rio_Ri3 - - ¨
R14 R14 fl
Rio S------4 14 R10
, , , RN
'
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
166
RN R13
R12 R12 R12
13
N \ s= v_____ R14
\R13 - -c\7R N)
----"- n 7 __ S
R1.A , R14 R14 Ri., N
,
R11 i,
RN RN RN
0,11:e
1
ril /IR9
_ _R10
R1ONII ----- R14 (N
rNR9 R9 R1 N \J o)
N-:"----S - "\___,------- Ri 0 ,N' R10
, , , rµ
R8
\)LRm 9 R9 ,S - - - -
- -1_--, R10 ,IL R1 N\ Z
N I
, - µ.1`i \N R8 \S-N
R8 R8
N 1 )' . S, - - . Nõ,---,-7- - - ,-- N6- "
R8
S N R ----- ----- --I R8---
1
R8 NO , \O-N , N-0 or O-N
, , ,
and R8, R9, R10, R11, R12, R13, KI-114,
and R" have the meanings as defined in claim
1.
4. The compound according to Claim 1, wherein
R1
0 (,..N..õ0
H (I)
R2 N 1 NR3
Ho
wherein
L represents ¨L1¨L2¨;
L1 represents ¨CH2C0¨,
L2 represents ¨NRNI¨, and R3 represents 1-adamantyl; or
L2 represents ¨NRNICH2¨, and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues
optionally
contain one or more C=C double bond(s) and/or are optionally substituted by
one
or more of Ra, Rb, Rc, Rd, and Re;
0
R1 represents
=
,
H
0
RN RN RN
ii _ _ _ N
R2 represents
--- R 1 1 R8
1 ----- ¨R8 ,
N N N
R10 R10
,
, ,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
167
RN
-__ 0 Ra R11 rRa R8 S Ra 0 R1
-- 11 R8
\ / 1 __Isr
-\\ r (
Rlo R9 ;
' R9 R10 R9 ;
' R9 R10 -
I-C9
' ; ;
R,,, 11
ON _R8
RN RN RN RN Rio
R11 N R8 R11 N, R11 N \ N,
1 ___________ ( ----¶ I N
/ /K ---LIK\I
\ / C)\) I ir-
,
Ro R10 s, , R9 Rlo R9 N¨K ._...8 ___ N
;
,
1 1 1 1 1
RN RN RN
0 N
8 R11 S S li 8 -_
' -- __-R ----- - - --___ 'N R1:1 ,I1,N
,, N N
)--N ) __ N Y __ N N )\ N i(
Rlo ' Rio ' Rio , Rio , , ,/
R10,
RN
,
RN
R12 R12 R12 Riz
,N wo /-1 --------------------- N/
N ---
N, Dp N
N''` ¨R13 -- R13 ------------------
R13
\¨N Q A \N R13
,
, N=N , ------- D14 I\1 R14 N
Ri4 N R14
, '` ,
R12 _F;v11
,
N Ri2 0---/Y,) 13 %
R12 R12
RioRi3 wo
R14
N Ri4 R10 0---\44 0----\43
RN ,
R12 Riz ,
,
S--_./y _ , R12 i\l----r/ R13 R10R13 wo /
- -$___---) -
ffl
Rio
R14 N s-----414 ; Rio
R14 , RN
, ,
RN R13
R12 R12 p12
-1 _________________ N
i\i---/A
--- 1 \,tR13 ` s= V.___ 14 N R
I 4R13
N----.%\- c__\R13
1 S
R14 R14 R.-A
w_n N
,
R11 ,,
RN RN RN
0 R9
---.. ,--1
rN /IR9
R10-11--- ________ R14 r_.IIR9 rN!rZ9
__ R10
N \J _
_Rio
N S - "\____/------ Ri 0 ; rµ mr\l' R10 o)
R8
1_79
N \ X N
I ..
N- --/,
_R10 õ,R10 N
\
.' ,.1`1 R8 \S-N
R8 S-,-- R8
). -NT N
----<\
S: 7-- , N R8 ---e- - - N R.-- 8 -
R8---- 1
Nr-N , R8 N-N , 0-N , N- , or
,
R6 represents ¨C2H5;
and R6, R6, R113, R11, R12, R13, R14, Ra; R13, Rc; Rd; Re ; RN and rt"Isll
have the same
meanings as defined in claim 1 or a diastereomer, an enantiomer, a mixture of
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
168
diastereomers, a mixture of enantiomer, a racemate, a solvate, a hydrate, or a
pharmaceutically acceptable salt thereof.
5. The compound according to Claim 1 0r4 represented by formula (II):
0
oN
H
o N H 0 (II)
R2 N 1 N R3
H
0I 0
wherein
L2 represents -NIRN1-, and R3 represents 1-adamantyl; or
L2 represents -NR111CH2-, and R3 represents 2-bicyclo[3.1.1]heptyl,
and the afore-mentioned 1-adamantyl and 2-bicyclo[3.1.1]heptyl residues
optionally
contain one or more C=C double bond(s) and/or are optionally substituted by
one
or more of Ra, Rb, Rc, Rd, and Re;
RN RN RN
Rli"Ncr- Rii 11R8 -- il R8 .
R2 represents or
N 1
N
N
R1 , / R10
,
Ra, Rb, Rc, Rd, and Re represent independently of each other -H, -F, -Cl, -Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -0O2H, -CO2CH3, -0O2C2H5, -CONH2,
-CON HCH3, -CON(CH3)2, -
CONN C2F15, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H5, -CH2CON H2, -
CH2CONHCH3, -CH2CON(CH3)2,
-CH2CONHC21-15, -NHCOCH3, -NHCOC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
RN represents -H, -CH3, -C2F-15, -C3H7, -CH(CH3)2, -
C4H9,
-CH2-CH(CH3)2, -CH(CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -cyclo-C4H7,
-cyclo-051-19, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-051-19, -CH2F,
-CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-15, -00C3H7, -COCH(CH3)2,
-00C(CH3)3, -CO-cyclo-C3H5, -CO-cyclo-C4H7, -CO-cyclo-051-19, -COOCH3,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
169
-COOC2H3, -CO0C3H7, -COOCH (CH3)2, -COOC(CH3)3, -COOCH2Ph,
-S02CH3, -S02CF3, -S02C2H3, -S02C3H7, -S02CH(CH3)2, or -S02C(CH3)3;
RI" represents -H, -CH3, or -CH2CH3;
R8, R", and R" represent independently of each other
-H, -F, -Cl, -Br, -1, -OH, -CN, -NO2, -CH3, -C2F13, -C3H7, -CH(CH3)2,
-C4H9, -CH2-CH (CH3)2, -CH(CH3)-C2H3, -cyclo-C3H3, -CH2-cyclo-C3H3,
-CH2F, -CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CHF2,
-CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -OCH3, -0C21-13,
-0C3H7, -OCH(CH3)2, -0C(CH3)3, -0C4H9, -OCHF2, -0CF3, -OCH2CF3,
-0C2F5, -OCH2OCH3, -
0-cyclo-C3H3, -OCH2-cyclo-C3H3,
-0-C2H4-cyclo-C3H3, -CH=CH2, -CH2-CH=CH2,
-C(CH3)=CH2,
-CH=CH-CH3, -CECH, -CEC-CH3, -CH2-CECH, or -Ph,
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomer, a racemate, a solvate, a hydrate, or a pharmaceutically acceptable
salt
thereof.
6. The compound according to any one of claims 1õ wherein the compound has any
one of the formula (II-a) - (II-1), (II-b1)- (II-b2), and (III-a) - (I11-1):
0 Rd Rc Re 0
0)LN Rb Ra
0 0 0
0 H (rH
0- H
N NH
Ra
R2 N 1\ N R2 N N
Rb
0 0 HjC
0 0
(II-a) (II-b)
0
ON N
0 H 0 0 0
NH Ra 2)-rµi NH N H
R2 N Rb R -
Rb
0 0 0 0
(II-b1) (II-b2)
0
H 0
R2 N N
0
Ra
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
170
0 (II-d)
0-L
N
H
0 H 0 Rb
A N
R2 N I N L2
H
0 0
Ra
(II-C)
0 0
(31AN 0
N
H H Rb Ra
Rb Ra
0H 0 0 JCH 0 RC
1 N Rc A N L2
R2 N R2 N 1 N
H H 0 0 Rd 0 0 Rd
(II-e) (II-f)
0 0 Rd
Rb Ra
0)-LN,---.õ
H RC H Rc
0 H 0 0 ( H 0
A N L2 A N
1 N
H
R2 N 1 N R2 N
0 0 H
0 0
(II-g) (II-h)
0 0
OA ()N
N
H Rb Ra H
0H 0 Rc 0 A JCH 0 ----k
N L2 A N ..-----,.. L2
H
R2 N 0 0 Rd 1 N R2 N 1 N
H 0 0
(Iki) (11-j)
0 0
(31)-
H H
0 JCH 0 0 0
N L2
R2A N 1 N N -------õ,..- L2
H
0 0 R2AN NH
H
0 0
(II-k) (II-I)
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
171
o Rd Rc Re 0
OAN Rb Ra 0N
H H
0 H 0 0 0 Ra
N N cFNIA NH
N r\i 1 N Rb
0 0 _____N H 0 ,i,,.,,,J, .. 0
'RN 'RN
(III-a) (III-b)
O 0
0N 0
H H
0 0
0 0 H
N H 0,
H
N H
R8 \ 1 rii
Re-eil'14 0 b ,p; N A
0 0 0
Rio i- R11
C) (11I-d)
0
0 )-L 0
'-- N-...''
H 0-LN------õ,,
H
0H 0
H 0 0
S N N IR11 H
N
R8 \ 1 ril 1 N R8 NI If
0 0 o o
R10 s R11
(11I-e) (III-f)
0 0
0)-N 0N
H H
0 0 0 (,H 0
H H
S Elm N N N õ,___J-1-...
,----..,,õ N
8 0
R8-- _e[ijc
N 0 R -- _e H
_ il "
'19
N 0 N
R10 R10 0 0
(11I-g) (11I-h)
0
01N 0
H ON
0 H 0 H
0 0
R13 0 N ...õ.õ,-11-.. N H
..----..,..õ-N H
i 1 R13 S
N
I H
0 0 1 INACI _ il '''fN
R10
R12 R12
(Ill-i) (III-D
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
172
0
0)-L o
N
0.A
H N
H
0 H 0
0 jcH 0
N N )-LNH
N
I H
0 0 I H I I
0 0
Rio Rio
Riz R13 12
(11I-k) (111-I)
wherein L1; L2, R2; R8, R10; R11, R12, R13, R14, RN, Ra; Rb; Rc; Rd and K i-ie
have the
same meanings as defined in claim 1.
7. The compound according to any one of the claims 1 ¨ 5, wherein R3
represents
----- ----
.--/j[-
,
,
,
.d1--
______________________________ , ,
_.
-- , ,s , ,
, ,
,
fiZF Zci jZCF3 jZOH jZCO2Me
F
F
., .,
., .,
Br ; CI OH
., CO2Me
', ', ', 0 '=
CO2H CONH2 ; CONMe2;
H
Br OH
CO2H ,
'
CF3 -- , -- , -" , ,
, or
-
8. The compound according to any one of the claims 1, - 7, wherein R2
represents
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
173
N
H I H
N I
N '9 4
N N H
N H H
N
N
t ---
N --
,N ,N ,N ,N ,N
N ,N ,N
N , ,
I I I
I I I
N,õ-Br _________ .--, _____ p N Y N ) N N
N N , , / ,
CI CI
I
--
)N N,
vt 0 ,
'N NN HN - ----NINN ----N NN N
- NC--0. _i \\
I N
H2N HN \ ,
I H I I
N, I N N, ,N
/\1 C-_-----_ -...,1\1,N Ni, ;I\I (\ ,N r\cLii
, NN N \\ ii , 2 __ / , ! N
N=I\1
S
---q,-Br -- , s CI --
__--Br
0-- -= \ / \ / \ / \ //
H2N 0 Br, Br , Br , CI ,
' ,
Br N_S Br 0 \ __
--, yS Br CI SCI
IV Me0
-11--
Br
S rsc S
Br - - - -)O¨Br --)S__--CF3
N
,
,S -- S --
H , N' N
Ni\iFi ,
5N S.,,,-/--
ll Nr/ IT 1\1/ - s .. N
--____Il
\ N 0 N-N S-N _--N 0
,
,
,S --
H2N 0
H
1\lis\v. )--,-/ -- ci\___<\N,T,- ' (111:_
N I (--- --
HO 0-N N-0 0-N HN-2- \/
, , ,
NO2
H -
N -----", ',NO2
HN
.--- 1\1)-- N_
I I
"O N- - ¨N,-- CO2Me
-_,CO2H , -N,N ---/ ---N N'.--- 0
I
I N I N I
-IV
,-, -- \
NI
, ,
0 0 ,
,
/
0 _
-- \ / -- _ \ \
Br , I , 0
, ,
2489-CIPO-DESCRIPTION-ZED-P04402W027.docx
Date Recue/Date Received 2022-06-30
174
F
0
\
0 , S
\
-<\
0
N¨L-S H HN
OH
F3C-0
-
N
N N NN or
9. The compound according to claim 1 selected from the group consisting
of:
Compound Name
1-1: (S)-N1-ethyl-N6-(1-(2-(1-adamantylamino)-2-oxoethyl)-2-
oxo-1,2-
dihydropyridin-3-y1)-5-(1-methy1-1H-im idazole-5-carboxamido)-2-
oxohexanediamide,
1-2: (S)-N1-ethy1-5-(1H-im idazole-4-carboxam ido)-N6-(1-(2-(1-
adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
oxohexanediamide,
1-3: (S)-N1-ethyl-N6-(1-(2-(1-adamantyl(methyl)amino)-2-
oxoethyl)-2-
oxo-1,2-dihyd ropyridin-3-y1)-5-(1-methy1-1H-im idazole-5-
carboxam ido)-2-oxohexanediam ide,
1-4: (S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-5: (S)-N1-ethyl-N6-(1-(2-(3-ethyladamantane-1-amino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-im idazole-5-
carboxam ido)-2-oxohexanediam ide,
1-6: (S)-N1-ethyl-N6-(1-(2-(3-trifluoromethyladamantane-1-
amino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-7: (S)-N1-ethyl-N6-(1-(2-(3-hydroxyadamantane-1-amino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-8: (S)-N1-ethyl-N6-(1-(2-(3-fluoroadamantane-1-amino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-im idazole-5-
carboxam ido)-2-oxohexanediam ide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
175
1-9: (S)-N1-ethyl-N6-(1-(2-(3-chloroadamantane-1-amino)-2-oxoethyl)-
2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-10: (S)-N1-ethyl-N6-(1-(2-(3-bromoadamantane-1-amino)-2-oxoethyl)-
2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-11: (S)-N1-ethyl-N6-(1-(2-(3-methyl
adamantane-3-ca rboxylate-1-
am ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-
imidazole-5-carboxam ido)-2-oxohexanediam ide,
1-12: (S)-N1-ethyl-N6-(1-(2-(4,4-difluoroadamantane-1-amino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-13: (S)-N1-(1-(2-(((1S,2R,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-
yl)methylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-
2-(1-methyl-1H-imidazole-5-carboxamido)-5-oxohexanediamide,
1-14: (S)-N1-(1-(2-(((1R,2R,5R)-6,6-dimethylbicyclo[3.1.1]heptan-2-
yl)methylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethyl-
2-(1-methyl-1H-imidazole-5-carboxamido)-5-oxohexanediamide,
1-15: (S)-N1-ethyl-N6-(1-(2-(3-ethyladamantane-1-amino)-2-oxoethyl)-
2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-4-
carboxamido)-2-oxohexanediamide,
1-16: (S)-N1-ethyl-N6-(1-(2-(3-ethyladamantane-1-amino)-2-oxoethyl)-
2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-2-
carboxamido)-2-oxohexanediamide,
1-17: (S)-2-(1,4-dimethy1-1H-imidazole-5-carboxamido)-N6-ethyl-N1-(1-
(2-
(3-ethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-oxohexanediamide,
1-18: (S)-N1-ethy1-5-(1-isobuty1-1H-imidazole-4-carboxamido)-N6-(1-
(2-(3-
ethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-oxohexanediamide,
1-19: (S)-2-(1-cyclopenty1-1H-imidazole-4-carboxamido)-N6-ethyl-N1-
(1-
(2-(3-ethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
1-20: (S)-2-(1-cyclobuty1-1H-imidazole-4-carboxamido)-N6-ethyl-N1-(1-
(2-
(3-ethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-oxohexanediamide,
1-21: (S)-2-(1,4-d imethy1-1H-im idazole-5-carboxamido)-N6-ethyl-N1-
(1-(2-
(3,5-d imethyladam antane-1-am i no)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
176
1-22: (S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-4-
carboxamido)-2-oxohexanediamide,
1-23: (S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-2-
carboxamido)-2-oxohexanediamide,
1-24: (S)-2-(1,2-d imethy1-1H-im idazole-5-carboxamido)-N6-ethyl-N1-
(1-(2-
(3,5-d imethyladam antane-1-am i no)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
1-25: (S)-N1-ethyl-N6-(1-(2-(3-methyladamantane-1-amino)-2-oxoethyl)-
2-
oxo-1,2-dihydropyridin-3-y1)-5-(1-methy1-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-26: (S)-2-(2-chloro-1-methy1-1H-imidazole-5-carboxamido)-N6-ethyl-
N1-
(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
1-27: (S)-2-(1,2-dimethy1-1H-imidazole-5-carboxamido)-N6-ethyl-N1-(1-
(2-
(3-methyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
1-28: (S)-N1-ethyl-N6-(1-(2-(3,5,7-trimethy1-1-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(1-methyl-1H-imidazole-5-
carboxamido)-2-oxohexanediamide,
1-29: (S)-2-(2-chloro-1-methy1-1H-imidazole-5-carboxamido)-N6-ethyl-
N1-
(1-(2-(3,5,7-trimethy1-1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
(S)-2-(benzofuran-2-carboxam ido)-N6-ethyl-N1-(1-(2-(2-
1-30 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
1-31 dihydropyridin-3-y1)-5-(3-methylbenzofuran-2-carboxamido)-2-
oxohexanediamide,
(S)-2-(3-chlorobenzofuran-2-carboxam ido)-N6-ethyl-N1-(1-(2-(2-
1-32 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(4-bromobenzofuran-2-carboxam ido)-N6-ethyl-N1-(1-(2-(2-
1-33 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(benzo[b]thiophene-2-carboxam ido)-N6-ethyl-N1-(1-(2-(2-
1-34 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
177
(S)-N1-ethy1-5-(7-fluorobenzo[b]thiophene-2-carboxamido)-N6-(1-(2-
1-35 (2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
2-
oxohexanediamide,
(S)-2-(4,5-difluoro-1H-indole-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-36 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
1-37 dihydropyridin-3-y1)-5-(3-methy1-1H-indole-2-carboxamido)-2-
oxohexanediamide,
(S)-2-(1H-benzo[d]imidazole-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-38 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(2,3-dihydro-1H-indene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-39 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
1-40 dihydropyridin-3-y1)-5-(4-methy1-2-(trifluoromethyl)thiazole-5-
carboxamido)-2-oxohexanediamide,
(S)-2-(4-bromo-2-(trifluoromethyl)thiazole-5-carboxamido)-N6-ethyl-
1-41 N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
1-42 dihydropyridin-3-y1)-5-(4-methy1-2-phenylthiazole-5-
carboxamido)-2-
oxohexanediamide,
(S)-2-(5-bromo-3-methylthiophene-2-carboxamido)-N6-ethyl-N1-(1-
1-43 (2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-5-
oxohexanediamide,
(S)-2-(3,5-dibromothiophene-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-44 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(5-bromo-3-methylfuran-2-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-45 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-46 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(2,5-dibromothiophene-3-carboxamido)-N6-ethyl-N1-(1-(2-(2-
1-47 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
178
(S)-2-(2, 5-dich lorothiazole-4-carboxam ido)-N6-ethyl-N 1-(1-(2-(2-
1-48 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(2, 5-dimethylfuran-3-carboxam ido)-N6-ethyl-N 1-(1-(2-(2-
1-49 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(4-bromothiazole-2-carboxam ido)-N6-ethyl-N 1-(1-(2-(2-
1-50 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(4-bromothiophene-2-carboxam ido)-N6-ethyl-N1-(1-(2-(2-
1-51 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(4-bromo-5-chlorothiophene-2-carboxam ido)-N6-ethyl-N 1-(1-
1-52 (2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-5-
oxohexanediamide,
(S)-2-(4,5-dibromothiophene-2-carboxam ido)-N6-ethyl-N 1-(1-(2-(2-
1-53 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-(4,5-dichlorothiophene-2-carboxam ido)-N6-ethyl-N 1-(1-(2-(2-
1-54 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-2-((S)-1-acetylpyrrolidine-2-carboxam ido)-N6-ethyl-N 1-(1-(2-(2-
1-55 adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
1-56 dihydropyridin-3-y1)-5-(1-methy1-1H-1,2,3-triazole-5-carboxam
ido)-2-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
1-57 di hyd ropyridin-3-y1)-2-oxo-5-(2 H-tetrazole-5-
carboxam ido)hexanediamide,
(S)-N1-ethyl-N6-(1-(2-(5-hydroxyadamantane-2-am ino)-2-oxoethyl)-
1-58 2-oxo-1,2-dihydropyridin-3-y1)-2-oxo-5-(pyrazine-2-
carboxam ido)hexanediamide,
(S)-N1-ethyl-N6-(1-(2-(5-fluoroadamantane-2-am ino)-2-oxoethyl)-2-
1-59 oxo-1,2-dihydropyridin-3-y1)-2-oxo-5-((S)-pyrrolidine-3-
carboxam ido)hexanediamide,
(S)-N1-ethyl-N6-(1-(2-(5-chloroadamantane-2-am ino)-2-oxoethyl)-2-
1-60 oxo-1,2-dihydropyridin-3-y1)-2-oxo-5-((S)-piperidine-2-
carboxam ido)hexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
179
(S)-N1-ethyl-N6-(1-(2-(5-bromoadamantane-2-amino)-2-oxoethyl)-2-
1-61 oxo-1,2-dihydropyridin-3-y1)-2-oxo-5-((R)-piperidine-3-
carboxamido)hexanediamide,
(S)-N1-ethyl-N6-(1-(2-(5-methyladamantane-2-amino)-2-oxoethyl)-2-
1-62 oxo-1,2-dihydropyridin-3-y1)-5-((R)-morpholine-3-carboxamido)-
2-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-carbonitrileadamantane-2-amino)-2-
1-63 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxo-54(3S,4R)-
quinuclidine-3-carboxamido)hexanediamide,
(S)-methyl 3-(6-(ethylamino)-1-(1-(2-(methyl 2-aminoadamantane-2-
1-64 carboxylate)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-ylamino)-
1,5,6-
trioxohexan-2-ylcarbamoy1)-5-nitrobenzoate,
(S)-N1-(1-(2-(1-adamantylmethylamino)-2-oxoethyl)-2-oxo-1,2-
1-65 dihydropyridin-3-y1)-N6-ethy1-2-(5-nitronicotinamido)-5-
oxohexanediamide,
54(2S)-1-(1-(2-1-adamantylmethylamino)-2-oxoethyl)-2-oxo-1,2-
1-66 dihydropyridin-3-ylamino)-6-(ethylamino)-1,5,6-trioxohexan-2-
ylcarbamoyl)nicotinic acid,
(S)-N1-ethyl-N6-(1-(2-(2-adamantyl(methyl)amino)-2-oxoethyl)-2-
1-67 oxo-1,2-dihydropyridin-3-y1)-5-(6-methylimidazo[2,1-b]thiazole-
5-
carboxamido)-2-oxohexanediamide,
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-
1-68 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediamide,
(S)-N1-ethy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-
1-69 oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-
2-
ylamino)ethyl)-1,2-dihydropyridin-3-yl)hexanediamide,
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
1-70 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediamide,
(S)-N1-(1-(2-((1s,4R)-bicyclo[2.2.1]heptan-1-ylamino)-2-oxoethyl)-2-
1-71 oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-2-(isonicotinamido)-5-
oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-ylamino)-2-oxoethyl)-2-oxo-1,2-
1-72 dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-(pyridazine-4-
carboxamido)hexanediamide,
(S)-N1-(1-(2-((1R,2R,4R)-bicyclo[2.2.1]hept-5-en-2-ylamino)-2-
1-73 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-
(pyridazine-3-carboxamido)hexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
180
(2S)-N1-(1-(2-(bicyclo[2.2.2]octan-2-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-74 dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-(2H-1,2,3-triazole-4-
carboxamido)hexanediamide,
(S)-N1-ethy1-5-(1-methyl-1H-1,2,3-triazole-4-carboxam ido)-2-oxo-
1 75 N6-(2-oxo-1-(2-oxo-2-((1R,2R,4R)-1,7 ,7-
-
trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
y1)hexanediamide,
(S)-N1-ethy1-5-(1-methyl-1H-1,2,4-triazole-3-carboxam ido)-2-oxo-
1 76 N6-(2-oxo-1-(2-oxo-2-((1R,2R,3R,5S)-2,6,6-
-
trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1,2-dihydropyridin-3-
y1)hexanediamide,
(S)-2-(benzofuran-3-carboxam ido)-N6-ethy1-5-oxo-N1-(2-oxo-1-(2-
1-77 oxo-2-((1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-
ylamino)ethyl)-1,2-dihydropyridin-3-y1)hexanediamide,
(S)-2-(benzo[b]thiophene-3-carboxam ido)-N6-ethyl-N 1-(1-(2-(4-
1-78 homoisotwistane-3-am ino)-2-oxoethyl)-2-oxo-1,2-d ihydropyrid
in-3-
y1)-5-oxohexanediam ide,
(S)-N1-ethyl-N6-(1-(2-(diamantane-1-am ino)-2-oxoethyl)-2-oxo-1,2-
1-79 dihydropyridin-3-y1)-5-(1-methy1-1H-pyrazole-4-carboxamido)-2-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(diamantane-4-am ino)-2-oxoethyl)-2-oxo-1,2-
1-80 dihydropyridin-3-y1)-5-(1-methy1-1H-pyrazole-3-carboxamido)-2-
oxohexanediamide,
1 81 (S)-N1-(1-(1-adamantylmethyl)-2-oxo-1,2-d ihydropyrid in-3-y1)-
N6-
-
ethyl-2-(1-methy1-1H-pyrazole-5-carboxamido)-5-oxohexanediamide,
(S)-N1-(14(3-hydroxy-1-adamantyl)methyl)-2-oxo-1 ,2-
1-82 dihydropyridin-3-y1)-2-(4-cyclopropy1-1,2,3-thiadiazole-5-
carboxam ido)-N6-ethyl-5-oxohexanediam ide,
(S)-N1 -(14(3-bromo-1 -adamantyl)methyl)-2-oxo-1 ,2-d ihydropyrid in-
1-83 3-y1)-N6-ethy1-5-oxo-2-(1,2,5-thiadiazole-3-
carboxamido)hexanediamide,
(S)-N1-(1-(2-adamantylmethyl)-2-oxo-1,2-d ihydropyrid in-3-y1)-N6-
1-84 ethy1-2-(4-(hydroxymethyl)-1,2,3-thiadiazole-5-carboxamido)-5-
oxohexanediamide,
(S)-2-(4-tert-butyl-1H-pyrrole-3-carboxam ido)-N1-(1-(2-(1-
1-85 adamantylamino)ethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-
5-
oxohexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
181
(S)-2-(4-cyano-1-methy1-1H-pyrrole-2-carboxam ido)-N1-(1-(1-
1-86 adamantylamino)propy1)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-
5-
oxohexanediamide,
(S)-N1-(1-(3-(2-adamantylam ino)-3-oxopropy1)-2-oxo-1,2-
1-87 dihydropyridin-3-y1)-N6-ethy1-2-(5-methoxyoxazole-2-
carboxamido)-
5-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-88 dihydropyridin-3-y1)-N6-ethy1-2-(3-methylbenzofuran-2-
carboxamido)-5-oxohexanediamide,
(S)-2-(2-acetyloxazole-4-carboxam ido)-N1-(1-(2-
1-89 (bicyclo[1.1.11pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-90 dihydropyridin-3-y1)-N6-ethy1-2-(2-isopropyloxazole-5-
carboxamido)-
5-oxohexanediamide,
(2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-91 dihydropyridin-3-y1)-2-(3,5-dimethylisoxazole-4-carboxamido)-
N6-
ethy1-5-oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(5-carboxy-2-am inoadamantane)-2-oxoethyl)-
1-92 2-oxo-1,2-dihydropyridin-3-y1)-5-(4-methylpyrimidine-5-
carboxamido)-2-oxohexanediamide,
(5S)-N1-ethyl-N6-(1-(2-(4-aminoadamantane-N,N-dimethy1-1-
1-93 carboxamide)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxo-
5-
(1,2,3,4-tetrahydronaphthalene-2-carboxamido)hexanediamide,
(S)-2-((S)-1,4-diazabicyclo[2.2.2]octane-2-carboxam ido)-N6-ethyl-
1-94 N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-oxohexanediamide,
(S)-N1-ethy1-5-(1H-indole-3-carboxam ido)-N6-(1-(2-(2-
1-95 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
1-96 dihydropyridin-3-y1)-5-(6-methylimidazo[2,1-b]thiazole-3-
carboxamido)-2-oxohexanediamide,
(S)-2-(benzo[d]thiazole-2-carboxam ido)-N1-(1-(2-((1S,2R,4R)-
1-97 bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide,
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
1-98 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-ethy1-2-
(imidazo[2,1-
b]thiazole-6-carboxamido)-5-oxohexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
182
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
1-99 oxoethyl)-2-oxo-1,2-di hydro pyridi n-3-y1)-N6-ethy1-2-(4-
hydroxy-6-
(trifluoromethoxy)quinoli ne-3-carboxam ido)-5-oxohexanediam ide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-100 dihydropyridin-3-y1)-2-(cinnoline-3-carboxamido)-N6-ethy1-5-
oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-101 dihydropyridin-3-y1)-N6-ethy1-2-(3-ethylbenzofuran-2-
carboxamido)-
5-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-102 dihydropyridin-3-y1)-N6-ethy1-2-(1-ethy1-1H-indole-2-
carboxamido)-5-
oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-103 dihydropyridin-3-y1)-N6-ethy1-2-(2-methy1-1,8-naphthyridine-3-
carboxamido)-5-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-104 dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-(1,2,3,4-
tetrahydroquinoline-6-
carboxamido)hexanediamide,
(S)-N1-ethyl-N6-(1-(2-(2-carboxy-2-am ino-5-
1105 (trifluoromethyl)adamantane)-2-oxoethyl)-2-oxo-1,2-d
ihydropyrid in-
-
3-y1)-2-oxo-5-(3-oxo-1,2,3,4-tetrahydroisoquinoline-6-
carboxamido)hexanediamide,
(S)-N1-ethyl-N6-(1-(2-(5-ethyladamantane-2-am ino)-2-oxoethyl)-2-
1-106 oxo-1,2-dihydropyridin-3-y1)-5-(1,6-naphthyridine-2-
carboxamido)-2-
oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-107 dihydropyridin-3-y1)-N6-ethy1-2-(2,6-naphthyridine-1-
carboxamido)-5-
oxohexanediamide,
(S)-2-(4-am ino-1,2, 5-oxadiazole-3-carboxamido)-N 1-(1-(2-
1-108 (bicyclo[1.1.11pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
1-109 dihyd ropyridin-3-y1)-2-(6-(d imethylami no)benzofu ran-2-
carboxam ido)-N6-ethyl-5-oxohexanediam ide,
(S)-2-(2-acetam idoth iazole-5-carboxam ido)-N 1-(1-(2-
1-110 (bicyclo[1.1.11pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-ethy1-5-oxohexanediamide,
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
183
(S)-N4-(1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-
1-111 1,2-dihydropyridin-3-ylamino)-6-(ethylamino)-1,5,6-
trioxohexan-2-yI)-
1H-pyrrole-2,4-dicarboxamide,
(S)-N1-ethyl-N6-(1-(2-(1-acetylamino-4-aminoadamantane)-2-
1-112 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxo-5-(5-
sulfamoylfuran-
3-carboxamido)hexanediamide,
(S)-2-(benzofuran-5-carboxamido)-N6-ethyl-N1-(1-(2-(1-
1-113 acetylamino-4-aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
(S)-2-(benzofuran-6-carboxamido)-N6-ethyl-N1-(1-(2-(4-
1-114 aminoadamantane-1-carboxamide)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(4-aminoadamantane-1-carboxamide)-2-
1-115 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(3-(1-
methylcyclopropy1)-
1,2,4-oxadiazole-5-carboxamido)-2-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
1-116 dihydropyridin-3-y1)-N6-ethy1-2-(5-methy1-1,2,4-
oxadiazole-3-
carboxamido)-5-oxohexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
1-117 dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-(1,2,3-thiadiazole-
4-
carboxamido)hexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
1-118 dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-(1,2,4-thiadiazole-
5-
carboxamido)hexanediamide,
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
1-119 dihydropyridin-3-y1)-N6-ethy1-5-oxo-2-(1,3,4-thiadiazole-
2-
carboxamido)hexanediamide,
(S)-N1-ethy1-5-(4-formy1-1,2,3-thiadiazole-5-carboxamido)-N6-(1-(2-
1-120 (2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-
oxohexanediamide,
(S)-N1-ethyl-N6-(1-(2-(3,5-dimethyladamantane-1-amino)-2-
1-121 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-(nicotinamido)-
2-
oxohexanediamide
or a pharmaceutically acceptable salt thereof.
10. A pharmaceutical composition comprising a compound of any one of the
claims
1 ¨ 9 as an active ingredient, together with at least one pharmaceutically
acceptable
carrier, excipient and/or diluent.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
184
11. Compound according to any one of the claims 1 ¨ 9 for use in medicine.
12. Compound according to any one of the claims 1 ¨ 9 or the pharmaceutical
composition according to claim 8 for use in the treatment or prophylaxis of
autoimmune and inflammatory diseases, vascular diseases, fibrotic diseases,
liver
diseases, cholestatic liver diseases, cancer, neurodegenerative diseases,
ocular
diseases, and skin disorders.
13. Compound for use, or the pharmaceutical composition for use according to
claim
12, wherein
the autoimmune and inflammatory diseases comprises multiple sclerosis, celiac
disease, Duhring-Brocq-disease (dermatitis herpetiformis), gluten ataxia,
gluten
neuropathy, diabetes, rheumatoid arthritis, Graves' disease, inflammatory
bowel
disease, systemic lupus erythematosus psoriasis, and gingivitis;
the vascular diseases comprise atherosclerosis, thrombosis, vascular
stiffness;
the fibrotic diseases affecting the lung, the kidney, the liver, the skin or
the gut like
cystic fibrosis, kidney fibrosis and diabetic nephropathy, intestinal
fibrosis, idiopathic
lung fibrosis, liver fibrosis;
the liver diseases like alcoholic hepatitis, alcoholic steatohepatitis,
nonalcoholic
steatohepatitis, non-alcoholic fatty liver disease, liver cirrhosis,
autoimmune
hepatitis or liver inflammation;
the cholestatic liver diseases comprise primary biliary cholangitis and
primary
sclerosing cholangitis;
the cancer comprises glioblastoma, melanoma, pancreatic cancer, renal cell
carcinoma, meningioma, and breast cancer,
the neurodegenerative diseases comprise Parkinson's disease, Huntington's
disease, or Alzheimer's disease;
the ocular diseases comprise glaucoma, cataracts, macular degeneration, or
uveitis;
and
the skin disorders comprise acne, psoriasis, scarring, and skin aging.
14. Compound for use, or the pharmaceutical composition for use according to
any one
of the claims 12 and 13 in the treatment or prophylaxis of celiac disease.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
185
15. A method for producing the compound of formula (la) according to claim 1
comprising:
Step 1A: providing a compound 4a
0
AcON-R6
H
PGNJ((OH
H 0 4a;
Step 2A: performing coupling reaction of the compound 4a with a compound 5
0
H2N)-L _I__
, N R3
5
to obtain a compound 6a
0
Ac0-LN-R6
H
, N R-
H 0
6a;
Step 3A: deprotecting an amino protecting group PG3to obtain a compound 7a
0
Ac0)-LN-R6
H
0
H
H2Nj(iNN-1¨R3
0
7a;
Step 4A: performing coupling reaction of the compound 7a with a carboxylic
acid
(R2-CO2H 8) to obtain a compound 9a
0
AcO)-N-R6
H
0 iCH 0
R2N J-L 1\1 N ,L
, 'R3
H 0
9a;
Step 5A: performing oxidation reaction of the compound 9a to produce the
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
186
compound of the formula (la)
0
ON.R6
H
0 H 0
A N)-L ,L, ,
R2 N , N R-
H 0
(1a),
wherein L, R2, R3, and R6 have the same meanings as defined in claim 1, and
PG3 is an amino protecting group.
2489-CIPO-DESCRIPTION-ZED-P04402W027 docx
Date Recue/Date Received 2022-06-30
187
Inhibitors of Transglutaminases
Description
The invention relates to novel inhibitors of transglutaminases, in particular
transglutaminase 2, methods for their synthesis and to their use for the
prophylaxis and
treatment of diseases associated with transglutaminases, in particular
transglutaminase
2.
Background of the invention
Transglutaminases are part of the class of transferases and according to EC
nomenclature they are correctly designated as "protein-glutamine: amine y-
glutamyl
transferases" (EC 2.3.2.13). They link the E-amino group of the amino acid
lysine and
the y-glutamyl group of the amino acid glutamine forming an isopeptide bond
while
ammonia is released. In the absence of suitable amines and/or under certain
conditions,
deamidation of the glutamine may occur resulting in the corresponding glutamic
acid.
Additionally, transglutaminases play an important role in many therapeutic
areas such as
the cardiovascular diseases (thrombosis and atherosclerosis), autoimmune
diseases
(celiac disease, Duhring-Brocq-disease, gluten ataxia), neurodegenerative
diseases
(Alzheimer's disease, Parkinson's disease, Huntington's disease),
dermatological
diseases (ichthyosis, psoriasis, acne) as well as in wound healing and
inflammatory
diseases (e.g. tissue fibrosis) (J.M. Wodzinska, Mini-Reviews in medical
chemistry, 2005,
5, 279 - 292).
Celiac disease, a gluten intolerance, however, is one of the most important
indications.
Celiac disease is characterized by a chronic inflammation of the mucosa of the
small
intestine. In susceptible patients, the intestinal epithelium is successively
destroyed after
ingestion of gluten-containing food resulting in reduced absorption of
nutrients which
again has massive impact on the patients affected and is for example
associated with
symptoms such as loss of weight, anemia, diarrhea, nausea, vomiting, loss of
appetite
and fatigue. Due to these findings, there is a large demand for the
development of a
medicament for the treatment of celiac disease as well as of other diseases
associated
with tissue transglutaminase (transglutaminase 2, TG2, tTG).
The tissue
transglutaminase is a central element during pathogenesis. The endogenous
enzyme
catalyses the deamidation of gluten/gliadin in the small intestinal mucosa and
thus
triggers the inflammatory response. Therefore inhibitors of tissue
transglutaminase are
suitable to be used as active agents for medication.
Another very important group of indications for tissue transglutaminase
inhibitors are
fibrotic disorders. Fibrotic disorders are characterized by the accumulation
of cross-
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
188
linked extracellular matrix proteins. Diabetic nephropathy, cystic
fibrosis, idiopathic
pulmonary fibrosis, kidney fibrosis as well as liver fibrosis belong to the
most important
fibrotic disorders to be addressed with the compounds disclosed.
.. US 9,434,763 B2 discloses pyridinone derivatives having a warhead
comprising at least
one acceptor-substituted double bond, such as a Michael System, as
irreversible
transglutaminase inhibitors. Alkylacetamido and arylacetamido pyridinones
showed
inhibitory activity regarding tissue transglutaminase TG2 in nanomolar range
(IC50).
Tse et al. (J. Med. Chem. 2020, 63, 11585-11601) report on replacement of
phenyl
residues by non-classical bioisosteres, such as cubane and
bicyclo[1.1.1]pentane (BCP),
in anti-malarial triazolopyrazine compounds in order to alter compound
solubility and
metabolic stability. The authors further evaluated in vitro antiplasmodial
activity of
bioisosteric modified triazolopyrazines against the 3D7 strain of P.
falciparum.
Replacement of phenyl by bioisosteric saturated heterocyclic residues resulted
in
complete loss of activity. Adamantyl residues as well as other hydrocarbon-
caged
derivatives led to potency up to 2-9 times lower than the corresponding phenyl
triazolopyrazine compounds. In contrast, higher potencies were achieved by
replacing
phenyl with closo-1,2- and 1,7-carborane isomers. The authors concluded that
the effect
__ of non classical bioiostere replacement on biological properties cannot be
predicted
accurately and that a considerable range of possible bioisosteres has to be
tested first in
order to identify a suitable replacement leading to the desired properties of
a given
molecule.
Subbaiah et al. (J. Med. Chem. 2021, 64, 19, 14046-14128) report on
bioisosteres of the
phenyl ring in lead optimization and drug design. It is noted that
bioisosteric phenyl ring
replacement with heterocyclic and carbocyclic moieties can lead to enhanced
potency,
solubility, and metabolic stability while reducing lipophilicity, plasma
protein binding,
phospholipidosis potential, and inhibition of cytochrome P450 enzymes and the
hERG
channel. However, this effect depends strongly on the properties of the
compound itself
and the addressed target.
US 11,072,634 B2 discloses reversible transglutaminase inhibitors comprising
an
aldehyde, a ketone, an a-ketoaldehyde, an a-ketoketone, an a-ketoacid, an a-
ketoester,
an a-ketoamide or a halogenmethylketone as warhead. The inhibitors showed
inhibitory
activity regarding tissue transglutaminase TG2 in nanomolar and micromolar
range (IC50).
The objective of the present invention is to provide novel, most probably
reversible
inhibitors of transglutaminases, in particular transglutaminase 2 and methods
for the
synthesis of said inhibitors as well as several uses of these inhibitors.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
189
Said objective is solved by the technical teachings of the independent claims.
Further
advantageous embodiments, aspects and details of the invention are evident
from the
dependent claims, the description and the examples.
Surprisingly, it has been found that reversible inhibitors having a chemical
warhead as
disclosed herein inhibit effectively transglutaminases including tissue
transglutaminase
called transglutaminase 2 or TG2. Herein these terms are used synonymous.
Preferably, such chemical warhead moiety is particularly selected from
reversible
warheads such as a-ketoamides. The compounds of the present invention act as
selective inhibitors of transglutaminase 2.
In order to prove inventiveness of the compounds of the present application,
reference
compounds were synthesized and tested in comparison to the most similar
compounds
of the present application. A skilled person might notice compound A8 from our
patent
US 9,434,763 B2 which we introduce as Ref. 3 to highlight the inventive and
preferred
features of the claimed compounds. From US 9,434,763 B2, it is clear, that
aromatic
moieties (C-terminal) restrict the efficacy of those compounds (compare to Al,
A8, A37,
A44, A47). In sharp contrast, branched alkyl moieties are highly preferred as
indicated
by more potent compounds (A28, A29, A59, A61, A63, A67, A68, A79).
To illustrate the advantage of branched alkyl moieties over aromatic moieties,
we refer to
reference compounds Ref. 2 (ZED1227, US 9,434,763 B2) and Ref. 3 (A8,
ZED1047).
Inhibition data were determined using the classical fluorescent transamidation
assay
(dansylcadaverine incorporation into methylated casein, DCC-assay) as
described
[Bach Id, C.; HiIs, M.; Gerlach, U.; Weber, J.; Pelzer, C.; Heil, A.;
Aeschlimann, D.;
Pasternack, R. Features of ZED1227: The First-In-Class Tissue Transglutaminase
Inhibitor Undergoing Clinical Evaluation for the Treatment of Celiac
Disease. Cells 2022, //, 1667. https://doi.org/10.3390/cells11101667]. Casein
is one of
the best known high molecular weight (24 kDa) protein substrates for
transglutaminases.
Please note, the ICso value of Ref. 3 (A8) published in US 9,434,763 B2 cannot
be
compared to the present data, relying on a fluorogenic isopeptidase assay.
Measured
in the DCC-assay, Ref. 2 (ICso = 53 nM) is 80-fold more potent compared to
Ref. 3 (ICso
= 4,268 nM).
Accordingly, a person skilled in the art of medicinal chemistry would choose
branched
alkyl moieties as lead structures, such excluding aromatic moieties, e.g. the
phenyl group.
It is of common knowledge, that bridged cycloalkyl groups are non-classical
bioisosters
of the phenyl group. By replacement of the phenyl group in A8 by e.g. an
adamantane
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
190
group, a skilled person would expect similar physico-chemical or biochemical
properties
excluding to invest efforts. Since aromatic moieties are clearly not
preferred, bridged
cycloalkyl groups would not be considered improving the compounds.
This is further supported by additional reference compounds. ZED3641 (Ref. 1,
as
disclosed in US 11,072,634 B2; reversible acting a-ketomethylamide analogue to
Ref. 2,
ZED1227) is about 10-fold more potent compared to Ref. 6 (compare table 1).
Ref. 6 is
analogous to compound A8 disclosed in US 9,434,763 B2 with respect to the
backbone
proving again superiority of branched alkyl moieties compared to aromatic
derivatives in
combination with reversible acting warheads.
However, surprisingly, replacement of the preferred branched alkyl moieties by
bridged
cycloalkyl groups further significantly improve the potency of the compounds
as shown in
table 1. Therefore, we credit bridged cycloalkyl groups as disclosed with an
outstanding
inventive manner.
In summary, the inventive compounds rated "A" show efficacies of about 100-
fold higher
compared to Ref. 3 (A8, compare to table1).
Further, compounds with activities rated "B" or "C" are still preferred (lower
ICso values)
to Ref. 3 (A8). These compounds can also be considered inventive, since
peripheric
ligands affect physico-chemical or biochemical properties. Therefore, also
less potent
compounds might be of high value, depending on the application.
Thus, the present invention relates to compounds of the general formula (I):
R1
0 0
H J (I) CN L R3
R2 N 1 N
Ho
wherein
L represents ¨L1¨ or _L1-L2¨; preferably ¨1_1-L2¨;;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
L2 represents a bond, ¨NRN1¨, ¨NRN1CH2¨, ¨NRN1CH2CH2¨, or ¨NRN1CH(C1-13)¨,
0
R1 represents
0 -
,
R2 represents
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
191
RN RN
___ 0 R8 R1.0 R8 _ _ S .-,8 R1S R8 - _ li
Ra R1'11 Ra
Irm , __________________________________________ r , __ (
Rlo R9 , R9 R10 R9 , R9 Rlo R9 , I-
C,_,9
,
RN RN RN RN RN
11
RN N, R N \ __ N, R11 il RN
_ _ 4 4 K il K---- R8
-----\_4N
1
R, 1 IN R8
N
N
N
Rlo s, , R9 R10 R9 Rlo
, Rio
' ,
Rio , ,
R1 ,
Rtl,c(0õ, __ Rzc,0õ,_ R8 - - 15 r ¨ R8 0 ---- ' - _8
_A,..R1C) R1:J.,6_ _ _
\ /7--- o y
N N N 1\A
R10 , ' R10 N - R8 ? N.¨ \ R8
n10
N
/
, I-µ ,
RN RN RN
1 RN
1
Rii s R8 -_).,,SN Ra --,._1\i, RNN N,
, ,N Rlo Ri o,N, N _RN
, ______ N N
,
R10 N-N
,
, K
R12 R12 R12 R12 N R12
- _NI, DN ---------- /') ppl3 /`) R13 ------- iq
R.- N/ 1,
R.- 1,1
R .-
- '' N''` ft j .- \) \N
, N=14 , ---- 1-µo14
, N R14
, 1\1-R14
, N Ria
' N Ria
,
RN
R12 R12 Riz RN
0----//, S-----, N-----' R2
R12
---U tR13 ii--.//µ .../-----.....
Rlo
R14 R10 R14 Rlo ,--, - / R14 N-----\--
N1A ,\-)
R14
, ,
R13 RN
RN
p RN
`s 14 Ni yR9
,õ------..,---`
H 12 I 1 R13 n 71\1)7¨NsR
(ri R9 r N 1R9
R14
\-'
n14 , I-C n1,, N , IA - \___,--------
mio, R10
, ,
R8 R8,
0Rlo
,S,,
(--_ , ,
'
R9 ,S N I/
_R10 ,-- " _Rio
N--/ N _., N \ 1 --
N I S)'-- ---N
\
,-s( IN R8' \S-N N'N R8
,
,
R8
õ,--,-- 0
R8----S il NhT IR----- Ru---- il ---
N-N b-N , N- ,
0-N N ,
,
H
N 0 S S
\ \ \ --
N ,
, , ,
, , '
,
_
¨ -eNNH -- _ -(N _<N -- NH --eNS
N N __ \(
S S z S S 7 N
----eNS ----eNS ---eNNH ---eNNH
N N __ (
Ni Nj
, Nj
0 0 7
,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
192
/
-N ----(N3
N( 11
0 Nj \N , N ,
,
0 z N
, ,
H S
- --N& - ----N----$ -\ --1,
N-
H N -N
'S
---S H ,
S --____--N _ y/Th_-N S ___C-1-N
----:-\--
----N-,.. \S-I -- N -,õ(/N S-1\1-//
,
N-,õ_-0 H
N
-- ------N---N&o NJ'N-0
H H
/
,
-, I\1 .,
1 I I
N
N
-, N ., .,
' N
, N ,
,
.,
-.,1\1 -, ,N
1 II
N Th\r N /
. . N,N
I -I
NN , N
,
-. N -.
' N 'N
N N
N
,
.,
-, ,1\1 I\1 - N,
N
1 N 1
N
N
1\1 NN N le ,
N , N N N ,
,
N -, N N , f -
N , NN N N ,
N NNI IVN
"
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
193
-õNN,N
-,! N N
I
1\1- N ,
,
,
-,,I\IN -,,I\IN -,,NN
1
N 1
1 1 f
N--N NN N N ,
-,,1\1 -, N -, ,I\I
1\i-Ni-, NN N1,N
, ,
1\1-
N leN N"
'
N -,,1\1 -,,1\1
N N
'N
N,N
N:N I
I I
NN, NN NN
, ,
1 -r
N,N NN-.N NN:1\1
' , ,
- - ,
-1r N '-,-
I --'1 'N
I
N,NN N,NN
1\IN
'
H -,
NH
HN KNH ,
,
N NH,
HN , NH, H'
N=\ CR\ N=N N=\ N-
--N
\ N
N/ \ //N
__________________________________________________________ // ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
194
N¨ N¨ N¨ ¨N ¨N
/N
¨ _______________________ ¨ ¨ N
N' N",
/¨N ¨N ¨N ¨ \
¨ ¨ ¨ ¨\ // ¨ ¨ \
N / N
(
/71 , -- \ /N
I\
N N , ____________________ ¨-N\ ,
, ,
I\1 , ¨ ¨
\ '/N ¨ ¨
\ N ¨ =
N N
N N N¨// // ,
,
N=\ N=\
¨ ¨ /(1\1 ¨ ¨ ¨. /N
\ / N\/ N / N N N S
N N¨N sIV // N
N=N N=N N=N N=N N=N
\ /jv N /
N, N /
,
,
N=\ N=\ N=\ N=\ N-
- ¨ /(N ¨ ¨ /N ¨ ¨ /N ¨ ¨ 5
N1 ¨ ¨ __
\//N , \ S \ _______ 1\1 / , N
N N
, , ,
N¨ N¨ N¨ N¨ N-
- ¨ ________ ¨ ¨ _________ \ ¨ ¨ _____ ¨ ¨ __
\ N N N ¨ ¨ / N / N /
N¨// // N¨N N sIV __
, ' , , ,
¨N ¨N ¨Ns ¨N ¨N
,
¨ ¨ --, /s(N ¨ ¨ ;IV ¨ ¨ /N ¨ ¨ i / N ¨ ¨
\//N , \ S \ _______ 1\1 , N
N N N
, , ,
¨N ¨N /¨N ¨N ¨N
¨ ¨ /
\ N N
N N
// N¨N \\_N
,
_______ ' , ' N ___ ,
¨ \
/ ¨ ¨ N ¨7 / /
N ¨ ¨ i N ¨ ¨ / N -- i /N
( ( (
N N N \ S N S
N N // , N¨N N
__________ , ,
¨ ¨ = ¨ ¨ * ¨ ¨
Ns, \ ,N N /N N N N /
N , / ss
or 1V , N¨N N ¨N ;
, N ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
195
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 - R14 and R" ; and preferably with 1 to 3 of the substituents
R11 - R13;
R3 represents bicyclo[1.1.1]pentyl,
bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl,
hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one or
more C=C double bond(s) and/or are optionally substituted by one or more of
Ra, Rb, Rc,
Rd, and Re;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3, -CH2CF3, -
COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C21-13, -CON H2, -CONHCH3,
-CON(CH3)2, -CONHC2H3, -CH2CO2H, -CH2CO2CH3, -CH2CO2C2H3, -CH2CON H2,
-CH2CONHCH3, -CH2CON(CH3)2, -CH2CONHC2H3, -NHCOCH3, -NHCOC21-13,
-NHCOCF3, -NHCOCH2CF3, -NHSO2CH3, -NHSO2C21-13, -NHSO2CHF2,
-NHSO2CF3, -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -CH3, -CH2CH2CH3,
-CH(CH3)2, -CH2CH2CH2CH3, -CH2CH2CH2CH2CH3, -CH2CH(CH3)2, -C(CH3)3,
-CH2CH=CH2, -CH2CH=CH(CH3), -CH2CH=C(CH3)2, -CH2CH=CHCH2CH3,
-cyclo-C3H3, -cyclo-C4H7, -cyclo-C31-19, -
cyclo-C6H11, -CH2-cyclo-C3H3,
-CH2-cyclo-C4H7, -CH2-cyclo-C31-19, -CH2-cyclo-C6H11, -Ph, -CH2-Ph,
-CH2OCH3, -CH2OCH2CH3, -
CH2CH2OCH3, -CH2CH2OCH2CH3,
-CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR6R7 is -N(C2F13)2, - -N - -N - -NO
or N/ \
\ ___________________________________________________________________ / =
R6, R9, R10, R11, R12, R13, and R14 represent independently of each other -H, -
F, -Cl,
-Br, -I, -OH, -CN, -NO2, -CH3, -C2F13, -C3H7, -CH(CH3)2, -C4H9,
-CH2-CH(CH3)2, -CH(CH3)-C2H3, -C(CH3)3, -cyclo-C3H3, -CH2-cyclo-C3H3,
-CH2OH, -CH2F, -CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F,
-CH2-CH F2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -OCH3,
-0C21-13, -0C3H7, -OCH(CH3)2, -0C(CH3)3, -0C4H9, -OCHF2, -0CF3,
-OCH2CF3, -0C2F3, -OCH2OCH3, -0-cyclo-C3H3, -OCH2-cyclo-C3H3,
-0-C2H4-cyclo-C3H3, -
CHO, -COC H3, -COCF3, -00C2H5, -00C3H7,
-COCH(CH3)2, -00C(CH3)3, -COOK -COOCH3, -COOC2H3, -CO0C3H7,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
196
-COOCH (CH3)2, -COOC(CH3)3, -
00C-CH3, -00C-CF3, -00C-C2H5,
-00C-C3H7, -00C-CH(CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5,
-NHC3H7, -NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -N(C2H5)2, -N(C3H7)2,
-N[CH(CH3)2]2, -N[C(CH3)3]2, -NHCOCH3, -NHCOCF3, -NHCOC2H5,
-NHCOC3H7, -NHCOCH(CH3)2, -NHCOC(CH3)3, -CONH2, -CONHCH3,
-CONHC2H5, -CONHC3H7, -
CONHCH(CH3)2, -CON H-cyclo-C3H5,
-CONHC(CH3)3, -CON(CH3)2, -CON (C2H5)2, -CON (C3H7)2, -CON [CH(CH3)2]2,
-CON[C(CH3)3]2, -SO2N H2; -SO2N H CH3; -
SO2NH C2H5, -SO2NHC3H7,
-SO2NHCH (CH3)2, -SO2NH-cyclo-
C3H5, -SO2NHC(CH3)3, -SO2N(CH3)2,
-SO2N(C2H5)2, -SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C2H5, -NHSO2C3H7, -NHSO2CH (CH3)2, -NHSO2C(CH3)3,
-CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3, -CECH, -CEC-CH3,
-CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
= ----------------------------- -NO _1\1/N
1\1 1\1
r r r r
1\1
r r r
--N NH --N N- --N N
KO- , or \ ____ ;
or R8 and R9 or R9 and R19 can form together one of the following five-
membered or
six-membered rings:
0
--1
I
'N , or -'--1\1;
or R12 and R13 or R13 and R14 can form together one of the following five-
membered or
six-membered rings:
.0 o-0 .0
- -0 - -0 H or I
R" represents -H, -CH3, -C2H5, -C3H7, -CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH(CH3)-C2H5, -C(CH3)3, -
cyclo-C3H5, -cyclo-C4H7, -cyclo-05H9,
-CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-05H9, -CH2F, -CHF2, -CF3,
-CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2; -CH2-CF3, -CH2-CH2CI,
-CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2, -CH2-CECH, -CHO, -COCH3,
-00C2H5, -00C3H7, -
COCH (CH3)2, -00C(CH3)3, -CO-cyclo-C3H5,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
197
¨CO¨cyclo-C4H7, ¨CO¨cyclo-CsHo, ¨COOCI-13, ¨CO0C2H5, ¨CO0C31-17,
¨COOCH(CH3)2, ¨COOC(C1-13)3, ¨COOCH2Ph, ¨SO2CH3, ¨SO2CF3, ¨S02C2H5,
¨S02C31-17, ¨S02CH(CH3)2, ¨S02¨cyclo¨C31-15, or ¨S02C(CH3)3;
RI" represent ¨H, ¨CF13, or ¨CH2CH3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers,
a racemate, a solvate, a hydrate, or a pharmaceutically acceptable salt
thereof.
The inventors have found that the reversible inhibitors of formula (1)
disclosed herein
having a bridged bicyclic residue R3 exhibit increased potency over the
compounds of the
prior art. Particularly, it is demonstrated herein, that the inventive
compounds have an
improved inhibitory activity compared to the known compounds bearing aromatic
moieties
R3 instead of bridged bicyclic residues.
In order to prove inventiveness of the
compounds of the present application, known compounds from US 9,434,763 B2 and
US 11,072,634 B2 (Reference 1 (E16 from US 11,072,634 B2), Reference 3 (A8
from US
9,434,763 B2), and Reference 6) were synthesized and tested as reference
compounds
in comparison to the most similar compounds of the present application. To
this extent,
inhibition data were determined using the classical fluorescent transamidation
assay
(dansylcadaverine incorporation into methylated casein, DCC-assay) as
described in
Buchold et al. [Buchold, C.; Nils, M.; Gerlach, U.; Weber, J.; Pelzer, C.;
Heil, A.;
Aeschlimann, D.; Pastemack, R. Features
of ZED1227:
The First-In-Class Tissue Transglutaminase Inhibitor Undergoing Clinical
Evaluation for
the Treatment of Celiac Disease.
Cells 2022, 1/, 1667.
https://doi.org/10.3390/cells11101667]. Casein is one of the best known high
molecular
weight (24 kDa) protein substrates for transglutaminases. Inhibition data of
the inventive
compounds was compared with inhibition of compounds disclosed in US 9,434,763
B2,
particularly, compound A8, which is denoted herein as Reference 3. It is
noteworthy that
the ICso values of compounds A8 published in US 9,434,763 B2 and E16 from
US 11,072,634 B2 cannot be compared to the present data, relying on a
fluorogenic
isopeptidase assay.
Thus, the inventive compounds of formula (I) rated "A" showed efficacies of
about
100-fold higher compared to Ref. 3 (A8). The same argumentation applies to
Ref. 6 which
is except of the phenylethyl group identical to compound 11-111. Ref. 6 is
more than 25-
times less potent in comparison to compound 11-111 as evident from Table 1.
This finding was particularly surprising as a skilled person would not have
expected an
improved inhibitory activity of the inventive compounds bearing a bridged
bicyclic residue
over the compounds of the prior art bearing aromatic residues since it is
common
knowledge that bridged bicyclic groups or bridged cycloalkyl groups are non-
classical
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
198
bioisosters of the phenyl group, such that a skilled person would only expect
to obtain a
compound having similar physico-chemical and biological properties, including
inhibitory
activity, when replacing a phenyl group with a bridged bicyclic group. As
aromatic
moieties showed lower potency, bridged cycloalkyl groups would not be
considered
improving the physico-chemical and biological properties of the compounds.
The residues bicyclo[1.1.1]pentyl,
bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl, and
hexamethylenetetraminyl used herein, have the following parent structures
respectively:
bicyclo[1.1.1]pentyl bicyclo[2.1.1]hexyl bicyclo[2.2.1]heptyl
-
- -
bicyclo[3.1.1]heptyl bicyclo[2.2.2]octyl bicyclo[3.2.1]octyl
- -
- -
bicyclo[3.2.2]nonyl bicyclo[3.3.2]decyl bicyclo[3.3.3]undecyl
4-homoisotwistyl adamantyl diamantyl
hexamethylenetetraminyl
N ¨N
and the afore-mentioned residues optionally contain one or more C=C double
bond(s)
such as bicyclo[2.2.1]hept-5-enyl (s. 11-97) and/or are optionally substituted
by one or more
.. of Ra; Rb; ¨c,
rc Rd, and R.
The unsubstituted bicyclic residues which can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and RN ; have the following structure and the
substituents R9 ¨
and RN have the meanings as defined herein:
R11
RN R11 R11 R11
R12
0 R12 R12 -RN
Ri
Ri o Ri 0 \ N __ (
R13 R13 R13
Ria ' Ria
R12
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
199
R11 R11
_ -( _rN Rii ___ N R11
Z N-R - \N __ Z--- N -----NS
N R13.7S R12 N __ \(
S-----Rii -
R12 R13 R13N
R12 ; ; R12,
;
R11 R11 R11
R11
N N
----eS - - -eN-R -- --NN-R
----NS
N _________ \ R13 N R._
12 N, N Ri2 RR1300 v z Ri2
; R13 R12 ; R13
; ;
R11 R11
R11 ___ _NI, R11 R11
-(N __ r N ---e0
-4NO -4NO
Ri3-4kr,õo 6 7 R12 N __ \(
N ______________________________________________ \
NNN \ Ri2
R12 N R13 R13
N Ri2
, ' R12 ; ' R13 ;
RN R11 R11 R11
N
R12 R12 R12
--- --- 0 S ---
N R13 N R13 N R13
R14 R14 R14
; ;
RN R11 R11 R11
N R12
0 R12
S R12
R13 R13 R13
R10 A
R4,.,. R10
R14 R10 RI ,
A
1
,
R.12
RN\ R13 R11
R.1
N,S
-$ N N "---1.-S -,e R13 __ , ,
_<õ,-,--L-___Ri2 _ _ N,e __ R12 R13
R11 R12 ; N" NI-1'1-s
Rii R12
R11 RN R13 ; RN
, ,
Rii
R13
S N
)11,e R13 -- -'/Ni
R12
, N
S-1\le S-1\1/ Rii R12 INI---</ I-C NI.)
' R13 ; R.. .-,IG R13 ;
;
R13 R11 R12 N 0
RN\ R13 ,..-
-$ N -N,e R13 --
__ / -- _________________________________________________________
/ R13 12
N R11 R ; " 0
S-N-N
R11 , R11
, ;
R11 R11 R12 R11
ON
_ _(-----rO
-$
R12 __ R13 / \ --
_N ,e R13 _ _<,------r-N
, __ R12
N-1\ie N-N-0 R11 R12
RN R13 ; RN ' ; R13 ; 2489-
CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
200
R11
0 R13
,N
R11 R12
- -
-$¨N ,e R13 _ _ _-----i___-__-N R 0
p12 ¨ /
0-"N
Rii R12 1--IN ¨ 0-1\1-N/ R13
' R13 , R11
Rtet R10 R11 R9 R14
-, N R13 -, R12 -, R13
1
1 1 1
----- N
R12 R9 N R13 R12
R9
R10 R11 R14 R10 R11
R14 R14 R9 R14 R9
-, IA N R9
R13 o10
N
--- --- N
Rio R10
R13 R13
R12 R11 R12 R11 R12 R11
, , ,
R12 R11 R14 R13
Fµ
os10 N R13 R12
-. - -,
,
I 11
N
R13 N R"n Rlo N R12 R11
R14 R11 R10
'
R10 R11 R14 R14
NN
-. R' -, -_ N-R9
I
N,N ---- N
R13 R12
R12 .
R12
R14 R11 R10 R11 R10
' , ,
R14 R14 R9 R14 R9
NR9
N N
N
N Rin '' N R10
R12 R12 R12
R11 R11 IA n10 , R11
, ,
R11 R10 R13
- N N R13
-, R9
1 1 N
--- ---
N-,N R9 R12 I
----- .--' io
R13 R10 R11 R9 R¨
R14 , R10 R11
R13 R13 R10
- NR12 - NR12 N R11
I 1 ..õ._ I
R9-N Ro --y-----N!"---Rii Ro-----N--- R12
R10 R11 R10 R13
, , ,
R10 R11 R10 R11
R10 R11
R12
.,
R12
N -,.., -,.., ,
I I
R9-----NR12 R9-1\1-N I
R9-N N R13
R13 R13 '
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
201
R9 R9 R11 R9 R11
TIL -, N R11 ,,
', -,..õ. -,..õ,
NrAl N I Ri2
N R1 N 1 9 N N
.9._
Rlo R13 Rlo R13 Rlo R13
,
R9 R13
R2 -.Nr NY NY R12 -,f NY NY R12
.µ
1 N r-rN
N NRii
N NRii
Rlo Rlo R11
Rlo , ,
'
R12
-------, NY Ny R12
-- -2, N---,-.-.-2N---,-.-.-2 R12
N I
II I ,¨õN-------.õ1.7.--
N
N !Rii Rlo
N R11 R10 NN
7
R11
,
Rlo ,
,
- N NN R12 N N R12, -
- - -,--- N--=:,.----Ny-
1 I 1 R9
R1 ,,,
---y--...T-- -,.- N .
-,
R9 R9----y--- N-2.-- R11
R10 R11 R10 R11 R10
,
- N N R13 N N R13 R12
1 'N
N I 1
R10iNI R12 R12
Rii Rio Rii R9 1 --,.,r---
, , R10 R11 ,
R12 R13 R13
-,,NN N --,
1 'N -,,N
1 ' N
I 1 1
õ,...-- ....,-...I.õ,. R" ,,,, Rlo R¨ ..----. 2.---,y---1-,. 1,
Rlo R ..----. 2.----y2..-1-,. 1,
N N ¨
Rlo Rii Rii
' , ,
R12 R13
R12
- N R11 -,,N R12
- N R11 --. --,.
II
N:N I 10 N N N
2,--------- N
R
Rio , Rio Rii
R13 R13 R14
-. NR12 -,NN R13
- N Ri2
, .....,. 11
1 NN,
N N Rii
RlorNNRii R12
, R10 R11
, ,
R13 R13 R12
NN
N I
----. 2-----y--I-2 ,. R9 R12 Ri2 N Rm Ri 1 N
Rii Rlo Rii Rlo
, , ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
202
R12 Rm Rlo
.,
N. N R10 N R11
'
-, ------L-, -----:,..---
I I
N
R9 N-,N
R12
R9-NrN
R11 R10 R11 R12
' , ;
R10 R10
R10
-. I\I Ru NR11 R11
-, , -.,N
I I
NN 1 N ---õri"---N%---
R12
R9-NN R12
R9 R12 ' R9
R10 R11 Rm R11 R10
-, NrNI R11 -.
N----,,--k-....,,--j-=-:
I 1 1 11
,N
N-N\IR12 Ri2 N- R12---N--------y-%N
R13 R13 R9 ,
, ,
Rm Ru Rm Ru
R10 R11
I 1 I
Nr-rN
IR
, N N R
_----N-=R12
-1--'`
R9 R12, ; R9 ,
Rlo R11 R10 R11
-,
Nr"1 -' R10 R11
N
- - R12 .
- --õ,,. --õ,,.
1 R12
R13-NN NN-,N-"N
Rm ; R9
, ,
R10 R11
R10 R11 R11
p12
.,
--õ, --õ, ¨ p12 -.,N
- 1 1\1
N.INI-IN I I
N.
N N R1-,/ Rio N N R'`
Rm
,
R14 R13 R12 R13
R14 RN R14 R9
I
R12 __ R14 R1 ., N NR
R9 .
.
RN-N R11 R11 N-RN R10 R13 R10
3
R4IL,, R4I 4 I R12 R11
R9 R10 ; R10 R9 ;
R14 R13 R12 R13
R12 R11 R12 R11
R10 R10
R9 R11 R11 R9 N R9 Rm N. m
R-
N N R14 RN R14 R9
RN R10 R10 'RN
, ;
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
203
R9 o R9 R9
N¨( --10 N=N
N=(
--) /(1\1 --N R14 -- \ / R9
_11=_Rio
Rii Ri4
R11 \ N R11 R14 R11 N
N /( Rii R13
R12 R13 ; \ __ //
õ, \ õ.,
012 R12 R12 R13 1R, R ¨
, ;
" ;
R9 R9 R9 R9 R9
N N¨ N¨ N¨ _______________ ¨N
/ R1 1 _ _ \ / R10 _ _ _. / R10 _ _ __ / R10
- \
R13 \ / __ R1 R13 __ \ / Rh1 N \ / Rh1 _________ N\\ / Rh1
R11 \ /N
N N 2 (
R12 R12 R13 R12 R13 R12 R õ, ¨
R.,õ,
, , , ,
R10 R9 R10 R10 R9 R10 R9
--( --(
/
--.__ ¨R9 ___ R10 _ - i R9 - - \
/ N - - \ N
Rii \ / _________ Ri3 R11 ______________ \ z R13 N / R13 R11 \
/(N R11 \ _R13
R12 R12 R11 R12 R12õ¨ D 1õ., .., R12
; ; " ; ;
R10 R9 R10 R9 R11 R12 R11 R12 R11 R12
¨ ( ¨ (
- - --
--_ / N --i /N
Rii \ / R13 N\ / _____ R13 R10 R13 R13
\ N R9 \ N N N
N ) N N( /(
R12 R11 R12 R9 R10 R9 R10
; ; ; ;
R11 R12 R11 R12 R10 R10
R11 R12
N¨( N¨(
R13
R13 - - R13 -- -- / N --- .. /N
-- - -
(
N / R19 N \ / R19 N N __ N -S R11
\ /
N N /(
Ro Rio N
N¨N
' R9 R9 R12 .-, I-C11 R12
; , , ;
N=N N=N
___\ tRi 0 ___t / R10
N=N N=N N=N
-- \ ;NI ___ \_ /Rio Rio
R11 11 R14 R13 n 1
\ //IN
1, \ 1 R13 \ / R11 __ R13 \ / R11 N /
Rii
N N
R12 R13 ; IR¨ R.1 R12 ; R12 ; Ri3 R12
; ;
_ :¨
R10 R10 R10 R10 R9
N¨( N¨( N¨( N¨(
N -- \ /N ) /N
\
\
R13-- N 13- R
// \ _Rii R13 \ / Rii -- --,-
//N1 R10
Rii R \ ,N
õ, /( , N N N
Ric ID 11 R12 R12 R13 R12 R11
'N
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
204
R9 R9
R9
N¨ N¨
_ _ _ . c ¨ z R9 R10 :9 R10 ,/, _R10 _ __. _R10 ¨
\ z Rio
\ N N N N / ____ R11 N, / __ R11
N _______ /( /( R12 \ / R11
N N
R11 R12 R11 N¨N , R12 R12 ,
, ,
R10 R10 R10 R10 R9
¨N
-- \ /N
( ¨N
-- \ /N _iR
/
_N
--__.$1:(µN ¨N
--i ;1\1 ¨N
__ \ io
\ Nz/
R13 _________ , /(N R13 \ 1\/1 R11 R13 \N z R11
1\1 / R11 R12 N
R,¨ 0,..ii . R12 R12 , R13 R12 R11
Fµ , , , ,
R9 R9 R9
R9
--1\1/ R10 --R9\--Nz
¨N ¨N
¨N Rio
two tRio
Rio
--_ _________________________________
R124 4 /( R12 R11
N N N
N N / __ R11 N, / __ Rii
\ /
N N
R11
, R12 R11 N¨N , Riz R12
R9 R10 R9 R10 R9 R10 R9 R10
R12 ¨( R9 R10
\ /1\1 --_ N --i /(1\1 --(
-- ( \ /( --_$ /N --
,N R12
D
\ , _ \ N N N R12 11
\ R11 N \ -S IA
N N __ /( /( N¨N N
R11 R11 R12 0411 , R12
Fµ , ,
R9 R10 R11 R12 R11 R12
¨( R11 R12 R11 R12
--i /N
-- R13 - R13 -- R13 -- R13
N, / __________ R11 Rio \ N N N N N
N, / R10
12
N 14 sN __ /(
N¨N,/ , R10 , R10 , or N¨N
R =
,
One embodiment is directed to compounds of the general formula (I):
R1
0 0
(NH yl-R3 (I)
R2 N 1 N
H
0I
wherein
L represents ¨L1¨ or ¨L1-L2¨; preferably ¨L1-L2¨;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
0
R1 represents -'--H-R4
0 -
,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
205
R2 represents
RN RN
__ ,0 R8 Rii 0 R8 _____S ,R8 R11 s,R8
___...,,,,,R8 R1,, ,,,R8
Ri0 R9 / R9 R10 R9 / R9 R10 R9 . N,-
,9
,
RN RN R10
R10
R11 11µ R11 II, R11 0 -
-1 /1\1 1 /N ---- Ri---1 C:1__- R8 - - -(:)---- R8 0?--Z
R8
, K a, ) N N __ Y N N¨
cl\)
Rio ____ ss / R. Rio Rio R8 N¨ '
,
, , ,
RN RN RN
,,
Rio Ri_i S, _ Rii S D8 -__S___R8 - -
_N-I\I Rii N, ,N, _Rio
----",N N ir
N¨
RIR - Rlo , Rlo Rio ,
' ,
RN R12 R12 R12
Rio_...N,N_RN
_ N. _ N, DN --- R13 R13 R1-3
------------------------------------------------------------------------- ) -
N''` LI xj = - N-N , N=N , -"---- R14
, N R14
, N
Ria
,
RN
R12 R12 R12
R12 N R12 0-/,) 13 S----
/Y,) 13 N----__A
I '1 D13
N/-) Ri3
-\-) -\-)
\- \- R.,1, R1, .,
N Ria N Ria R10 R10 R10 R14
,
RN R13
R12
R12 R12
, RN
s)/ N___Rizt N R9
N-----\
¨R13
R14 L j 7R13
- iA x)
---& ____________________________________________________ S (
.2....i _
ni. - R14 \-R14 0 '._in N
----\ -7---- Ri 0
, , , , ,
RN RN R8
m
,N, R9 ril /1R9
m 9 R9 ,s,,-
r /1 _ R10
¨R R10 \
"- ---/--= 10 "--/--= N \ N
N -
\ I
R8 S'N
' ,
R8 ,S,-' R8
. N II - N6-
S - .- . N -- . N,-- 0
- ---N R8--- 1 Ru--- Ru----
- -
S)-
\N-=-"N R8 N-N b-N N-0
,
H
N 0 S S
\
, , N
, , , ,
, ,
N
NH NH - N
---eN ---eN - ---eNS
--_,N3
N N __ \(
S S 7 S S 7 N
---eNNH ---eNNH
N
, NNJ\ N __ (
0 Nj
0 z , 2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
206
/
-N ----(N3
N( 11
0 Nj \N , N ,
,
0 z N
, ,
H S
- --N& - ----N----$ -\ --1,
N-
H N -N
'S
-S H ,
S --___-- N _ y/Th_-N S ___C-1---N
----:-\--
----N-,.. \S-I -- N -,õ(/N S-1\1-//
,
N-,õ_-0 H
N
-- ------N---N&o NJ'N-0
H H
0---r-N N
/
,
-, I\1 .,
1 I I
N
N
-, N ., .,
' N
, N ,
,
.,
-.,1\1 -, ,N
1 II
N Th\r N /
. . N,N
I -I
NN , N
,
-. N -.
' N 'N
N N
N
,
.,
-, ,1\1 I\1 - N,
N
1 N 1
N
N
1\1 NN N le ,
N , N N N ,
,
N -, N N , f -
N , NN N N ,
N N le leN
"
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
207
-õNN,N
-,! N N
I
1\1- N ,
,
,
-,,I\IN -,,I\IN -,,NN
1
N 1
1 1 f
N--N NN N N ,
-,,1\1 -, N -, ,I\I
1\i-Ni-, NN N1,N
, ,
1\1-
N leN N"
'
N -,,1\1 -,,1\1
N N
'N
N,N
N:N I
I I
NN, NN NN
, ,
1 -r
N,N NN-.N NN:1\1
' , ,
- - ,
-1r N '-,-
I --'1 'N
I
N,NN N,NN
1\IN
'
H -,
NH
HN KNH ,
,
N NH,
HN , NH, H'
N=\ CR\ N=N N=\ N-
--N
\ N
N/ \ //N
__________________________________________________________ // ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
208
N¨ N¨ N¨ ¨N ¨N
/N
¨ _______________________ ¨ ¨ N
N' N",
/¨N ¨N ¨N ¨ \
¨ ¨ ¨ ¨\ // ¨ ¨ \
N / N
(
/71 , -- \ /N
I\
N N , ____________________ ¨-N\ ,
, ,
I\1 , ¨ ¨
\ '/N ¨ ¨
\ N ¨ =
N N
N N N¨// // ,
,
N=\ N=\
¨ ¨ /(1\1 ¨ ¨ ¨. /N
\ / N\/ N / N N N S
N N¨N sIV // N
N=N N=N N=N N=N N=N
\ /jv N /
N, N /
,
,
N=\ N=\ N=\ N=\ N-
- ¨ /(N ¨ ¨ /N ¨ ¨ /N ¨ ¨ 5
N1 ¨ ¨ __
\//N , \ S \ _______ 1\1 / , N
N N
, , ,
N¨ N¨ N¨ N¨ N-
- ¨ ________ ¨ ¨ _________ \ ¨ ¨ _____ ¨ ¨ __
\ N N N ¨ ¨ / N / N /
N¨// // N¨N N sIV __
, ' , , ,
¨N ¨N ¨Ns ¨N ¨N
,
¨ ¨ --, /s(N ¨ ¨ ;IV ¨ ¨ /N ¨ ¨ i / N ¨ ¨
\//N , \ S \ _______ 1\1 , N
N N N
, , ,
¨N ¨N /¨N ¨N ¨N
¨ ¨ /
\ N N
N N
// N¨N \\_N
,
_______ ' , ' N ___ ,
¨ \
/ ¨ ¨ N ¨7 / /
N ¨ ¨ i N ¨ ¨ / N -- i /N
( ( (
N N N \ S N S
N N // , N¨N N
__________ , ,
¨ ¨ = ¨ ¨ * ¨ ¨
Ns, \ ,N N /N N N N /
N , / ss
or 1V , N¨N N ¨N ;
, N ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
209
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9- R14 and R" ; and preferably with 1 to 3 of the substituents
R11- R13;
wherein
i) L2 represents a bond, -NRN1CH2-, -NRN1CH2CH2-, or -NRN1CH(CH3)-; and
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
1-bicyclo[3.1.1]heptyl, 3-bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl, bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-
homoisotwistyl,
diamantyl, hexamethylenetetraminyl and the afore-mentioned residues optionally
contain one or more C=C double bond(s) and/or are optionally substituted by
one or
more of Ra, Rb, Rc, Rd, and Re; or
ii) L2 represents a bond, -NRI41-, -NRN1CH2CH2-, or -NRN1CH(CH3)-; and
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl,
bicyclo[3.3.3]undecyl, 4-homoisotwistyl, 2-adamantyl,
diamantyl,
hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one or
more C=C double bond(s) and/or are optionally substituted by one or more of
Ra, Rb,
Rc, Rd, and Re;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3, -CH2CF3, -
COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C21-15, -CON H2, -CONHCH3,
-CON(CH3)2, -CONHC2H5, -CH2CO2H, -CH2CO2CH3, -CH2CO2C2H5, -CH2CON H2,
-CH2CONHCH3, -CH2CON(CH3)2, -CH2CONHC2H5, -NHCOCH3, -NHCOC21-15,
-NHCOCF3, -NHCOCH2CF3, -NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2,
-NHSO2CF3, -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -CH3, -CH2CH2CH3,
-CH(CH3)2, -CH2CH2CH2CH3, -CH2CH2CH2CH2CH3, -CH2CH(CH3)2, -C(CH3)3,
-CH2CH=CH2, -CH2CH=CH(CH3), -CH2CH=C(CH3)2, -CH2CH=CHCH2CH3,
-cyclo-C3H5, -cyclo-C4H7, -cyclo-051-19, -
cyclo-C6H11, -CH2-cyclo-C3H5,
-CH2-cyclo-C4H7, -CH2-cyclo-051-19, -CH2-cyclo-
C6H11, -Ph, -CH2-Ph,
-CH2OCH3, -CH2OCH2CH3, -
CH2CH2OCH3, -CH2CH2OCH2CH3,
-CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR6R7 is -N(C2F15)2, - -N -10 - -NO or N/ \\ /
=
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
210
R8, R9, R10, R11, R12, R13, and R14 represent independently of each other -H, -
F, -Cl,
-Br, -I, -OH, -CN, -NO2, -CH3, -C2F15, -C3H7, -CH (CH3)2, -C4H9,
-CH2-CH (CH3)2, -CH (CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5,
-CH2OH, -CH2F, -CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F,
-CH2-CH F2; -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -OCH3,
-0C21-15, -0C3H7, -OCH (CH 3)2, -0C(CH3)3, -0C4H9, -OCHF2, -0CF3,
-OCH2CF3, -0C2F5, -OCH2OCH3, -0-cyclo-C3H5, -OCH2-cyclo-C3H5,
-0-C2H4-cyclo-C3H5, -
CHO, -COC H3; -COCF3, -00C2H5, -00C3H7,
-COCH(CH3)2, -00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7,
-COOCH (CH 3)2, -COOC(CH3)3, -00C-CH3, -00C-
CF3, -00C-C2H5,
-00C-C3H7, -00C-CH(CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2F15,
-NHC3H7, -NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -N(C2F15)2, -N(C3H7)2,
-N[CH(CH3)2]2, -N[C(CH3)3]2, -NHCOCH3, -NHCOCF3, -NHCOC21-15,
-NHCOC3H7, -NHCOCH(CH3)2, -NHCOC(CH3)3, -CONH2, -CONHCH3,
-CONHC2F15, -CONHC3H7, -CONHCH(CH3)2, -
CONH-cyclo-C3F15,
-CONHC(CH3)3, -CON(CH 3)2, -CON (C2F15)2, -CON (C3H7)2, -CON [CH(CH3)2]2,
-CON[C(CH3)3]2, -SO2N H2; -SO2N H CH3; -
SO2NH C2H5, -SO2NHC3H7,
-SO2NHCH (CH3)2, -SO2NH-cyclo-C31-
15, -SO2NHC(CH3)3, -SO2N(CH3)2,
-SO2N(C2H5)2, -SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C21-15, -NHSO2C3H7, -NHSO2CH (CH3)2, -NHSO2C(CH3)3,
-CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3, -CECH, -CEC-CH3,
-CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
= ----------------------- -NO
r
rN r r
N\ /NH N\ N\
or
or R8 and R9 or R9 and R1 can form together one of the following five-
membered or
six-membered rings:
õ-0 ,-N
- -
'I\1 or '-%1\I
'
or R12 and R13 or R13 and R14 can form together one of the following five-
membered or
six-membered rings:
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
211
,0
,O\
_ --O --O -N
H or I
R" represents -H, -CH3, -C2F13, -C3H7, -CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH(CH3)-C2H3, -C(CH3)3, -cyclo-C3H3, -
cyclo-C4H7, -cyclo-C31-19,
-CH2-cyclo-C3H3, -CH2-cyclo-C4H7, -CH2-cyclo-C31-19, -CH2F, -CHF2, -CF3,
-CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2; -CH2-CF3, -CH2-CH2CI,
-CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2, -CH2-CECH, -CHO, -COCH3,
-00C21-13, -00C3H7, -COCH(CH3)2, -
00C(CH3)3, -CO-cyclo-C3H3,
-CO-cyclo-C4H7, -CO-cyclo-C31-19, -COOCH3, -COOC2H3, -CO0C3H7,
-COOCH (CH3)2, -COOC(CH3)3, -COOCH2Ph, -S02CH3, -S02CF3, -S02C21-13,
-S02C3H7, -S02CH(CH3)2, -S02-cyclo-C3H3, or -S02C(CH3)3;
RNi represent -H, -CH3, or -CH2CH3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers,
a racemate, a solvate, a hydrate, or a pharmaceutically acceptable salt
thereof.
In one embodiment the herein disclosed inventive compounds, the moiety -L-R3
is not
0
or
0
0
In one embodiment of the herein disclosed inventive compounds, L represents -
1_1-L2-;
L1 represents -CH2-, or -CH2C0-; and
L2 represents a bond, -NH-, -NHCH2-, -NHCH2CH2-, or -NHCH(CH3)-.
In one embodiment of the herein disclosed inventive compounds, L represents -
CH2-,
-CH2CO-NH-, -CH2CO-NH-CH2-, or -CH2CO-NH-CH(CH3)-.
In preferred embodiments of the inventive compound of formula (I), R2
represents
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
0C-90-ZZOZ peAieoef alea/an5a aleCI
xoop-vzomeovvod-a3z-Noadiens3a-odo-satz
r ' N
rp 0, ,N
N N ) __ N N
HNN-__ HN-__
,
Ny s,
, , s,
)\ NI N N N ) __ N
r
1-11\1-_,..
N -- N --
,
, , , ,
' N , ,
\ \ \
S S 0 N
H
, , ,
N N--R 0-N
4 /2- gei =L -9'el
--
NQ N-N 81 N---:-N\ N-S\ 0 N ---\ -
'
:V---N
-Q-9e1 N --i , , -L,- t S -1/-,e N \ \
- ---( -' ...,
Ii N
- ' S 6'u
, 0:1 -' S' oH 0:1
,
H o [H ---C-\ õ N 0
OiT .:1
I----N ,.., r
0,7/ ) 0, ,, -)--
H,,,-)
6 IN s __ /
' _______________________________________________________________
6H
NH NH NH
, C[H
171.H 171.H VI.H OM
1-\
E N
[H¨ 1 C[el, 1 _ _
H Z
/----- cM,,, 1
>-
z[U z0:1 Z[H
N [ - - -
NH
9
v[eir___0_1 KH 0[H .fr[:I.N t[H .N t[H .N
N\ -------------------------------------------------------------------
--
/:/"--S /-----0 zM N zM zM
zM z[el
N M , ,
Kel 17 N,N N-N ,'
,i_ni Ni' NI_ ,_,,N-\
---------------------- N- 'N --- 'N --
zM Z[H NH NU-N'Ny oM 00:I N'N
NH
' ' , ' 9H
/ /
OM ' ____
N-(-()M N50M N __ (/ N (
NIIN\ _--( el-- --
N z;_____N N
o k,__?
sH
0:1 N.N -_ 0:1 s-- 9 S [[el - - s õ?:1
NH
NH OM
, , , , ,
sH o[H '
0[H 6H 0H 6H\ / µs OM
N N N __ (/
N ____________________________________ (
0 9ei-- _ 771
"µ, u - - 0 [[el N 'N -- N N
'N [___M N [0=1
oH
NH NH NH
, , , ,
6H / 6H 0H 6H = 6H OM 6H / 6H OM
) / \
2e1)N [[H 9e1 N ' --S [[H 9H 5 - - 8H)0 H 9H 0 - -
NH NH
Z I=Z
213
/
-N - - --(N3
N( 11
Nj \N , Ni ,
,
0 0 z N
, ,
H S
- --N& - ----N----$ -\ --1,
1\r"
H N -N
'S
-S H ,
S --____--N _ y/Th_-N S __ _C-1----N
----:-\--
- ---N,..._ \S-I - - N -...(/N S-1\1-//
,
N-,..._-0 H
N
- - ------N1-"N&o NI'N-0
H H
0---r-N N
/
,
-, I\1 .,
i -
1 I I
N
/ N
-, N ., .,
'N
,
.,
-.,1\1 - , ,N
1 II
N Th\r N /
. . N,N
I -I
NN
,
-. N -. -,
'N 'N
N N
N
,
.,
-,,1\1 I\1 - N,
N
1 N 1
N
N '---,
1\1 NN Nle ,
N , N N N ,
,
N -, N N , f -
NNi , NN N N ,
N NNI leN
"
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
214
-õNN,N
-,! N N
I
1\1- N ,
,
,
-,,I\IN -,,I\IN -,,NN
1
N 1
1 1 f
N--N NN N N ,
-,,1\1 -, N -, ,I\I
1\i-Ni-, NN N1,N
, ,
1\1-
N leN N"
'
N -,,1\1 -,,1\1
N N
'N
N,N
N:N I
I I
NN, NN NN
, ,
1 -r
N,N NN-.N NN:1\1
' , ,
- - ,
-1r N '-,-
I --'1 'N
I
N,NN N,NN
1\IN
'
H -,
NH
HN KNH ,
,
N NH,
HN , NH, H'
N=\ CR\ N=N N=\ N-
--N
\ N
N/ \ //N
__________________________________________________________ // ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
215
N¨ N¨ N¨ ¨N ¨N
/N
¨ _______________________ ¨ ¨ N
N' N",
/¨N ¨N ¨N ¨ \
¨ ¨ ¨ ¨\ // ¨ ¨ \
N / N
(
/71 , -- \ /N
I\
N N , ____________________ ¨-N\ ,
, ,
I\1 , ¨ ¨
\ '/N ¨ ¨
\ N ¨ =
N N
N N N¨// // ,
,
N=\ N=\
¨ ¨ /(1\1 ¨ ¨ ¨. /N
\ / N\/ N / N N N S
N N¨N sIV // N
N=N N=N N=N N=N N=N
\ /jv N /
N, N /
,
,
N=\ N=\ N=\ N=\ N-
- ¨ /(N ¨ ¨ /N ¨ ¨ /N ¨ ¨ 5
N1 ¨ ¨ __
\//N , \ S \ _______ 1\1 / , N
N N
, , ,
N¨ N¨ N¨ N¨ N-
- ¨ ________ ¨ ¨ _________ \ ¨ ¨ _____ ¨ ¨ __
\ N N N ¨ ¨ / N / N /
N¨// // N¨N N sIV __
, ' , , ,
¨N ¨N ¨Ns ¨N ¨N
,
¨ ¨ --, /s(N ¨ ¨ ;IV ¨ ¨ /N ¨ ¨ i / N ¨ ¨
\//N , \ S \ _______ 1\1 , N
N N N
, , ,
¨N ¨N /¨N ¨N ¨N
¨ ¨ /
\ N N
N N
// N¨N \\_N
,
_______ ' , ' N ___ ,
¨ \
/ ¨ ¨ N ¨7 / /
N ¨ ¨ i N ¨ ¨ / N -- i /N
( ( (
N N N \ S N S
N N // , N¨N N
__________ , ,
¨ ¨ = ¨ ¨ * ¨ ¨
Ns, \ ,N N /N N N N /
N , / ss
or 1V , N¨N N ¨N ;
, N ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
216
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and R" ; and preferably with 1 to 3 of the substituents
R11 ¨ R13 and
the substituents R9 ¨ R14 and RN3 have the meanings as defined herein.
In even more preferred embodiments, R2 represents:
RN RN
__ 0 R8 R11 0 R8 ___ S R8 R1SN _R8 __, N
R8 R11 N R8
1 , __ (-- \ , \ ,
. .
Rlo _____ R9 , R9 R10 R9 , R9 R10 R9 / R9
,
RN RN RN RN RN
Ri 1 ri, RN __ N,N R11 ii _ R
_ RN
N R8 - - il
R8
I /i\i
---tii(\i 1 /
( 1
N N
-\\ _____________________________________________________ I--
R10 ss , R9 R1- R9 R10 R10
; ' R10 , , ;
R10 ,
, R1_R10 R11 SN _
0,/,/._ R0õ. _R8 --<0R8 0 ----... --
R8 ,c,µ ir-
\ /7--- o?'
N N )\ N N-2-r-R8
Rlo , Ri 0 __ N R8 N¨ R = lo
N
,
, ' RN
RN R
. N RN
R1- S R8 - - _S R8 - - _\I , N RiNN , N,I\rL _Rio
RioN_RN
, \ i
N N N N N
Rlo Rlo , N-N
, , ,
,
R12 R12 R12 R12
N R12
/') ) 13 ------------------------------------- 3 --- N/ 11 r 3
- ,N, DoN ------------ pp13
- -c N-F\
U,,,,.,,,;::\,, J . s \_) R - \IIR1 \_) R.- R1
, N=N , mr-,14 N Rizt N R1,4 N Ria N Ria ,
,
R-
'
R12 R12 Riz RN
s---/Y j\i-/
, R12
i\L..._õ, 12
- -TR13
R R-----%\- x)
Rlo 14 Rlo 14 ol, ¨ Rlo 14 N
R1."A
R14
,
'
R13 RN
RN
R12
12 RN
s \ ,õ---------../,',` 014 , N, R9
r /I
-1 j R13 il Nv--F\ 9 r /1 -- Rio
R1 S r_.IIR
_Rlo
)
\i-R14 .--,._ _________ N - \ ----7--- Rio
R4' , N,S 1 -
- '
1-(
R8
R9 ,S -- ,
R10
,--- . , _R , u N\\ 1 N
N I - - ---N
,-s(N R8 \S-N s\N'N R8
-
' ' ,
R8
N,- - N --
R---õ \ n N- R---- I R8--- 1
N-N b-N N-0 0-N
' ,
'
H
\ \ \ N __ \(
, N
,
,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
217
S N,--S
-¨N
ONj N , ,
,
I I I
N
N
., .,
-_,1\1 N
I
N,N I N N ,
H - -
NH .,
N NH ,
, H
' '
N_
or
N,
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 - R14 and RN ; and preferably with 1 to 3 of the substituents
R11 - R13 and
the substituents R9 - R14 and RN have the meanings as defined herein.
Thus, the present invention relates to compounds of the general formula (I):
R1
0 0
H (I)
N ,LR3
R2 N 1 N
Ho
wherein
L represents -L1- or -L1-L2-; preferably -L1-L2-;
L1 represents -CH2-, or -CH2C0-;
L2 represents a bond, -NH-, -NHCH2-, -NHCH2CH2-, or -NHCH(CH3)-,
0
R1 represents -'--H-R4
0 -
'
R2 represents
RN RN
_ 0 R rx 8 mil o -<8 ______srR8 Rsr,õ __ R8
Rii\i, R8
r I r
/
R10 R9 / R9 R10 1,9 / R9 R10 1-,9 ' R9
RN RN RN RN
-1
Rii , 1\1 R -
R111 N R8 - -),,,IIN Rs Rtt\sõON __ Rii ON _R8 / \ /7-
--1 __________________________________________________________________ r
N N N , N N
R10 ss Rto R10 R10
/
'
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
218
R10
Ri (1 ni
0 R8 R8 \ R8 , R10 RS. _ _ RSN _ R8 - - IS R8
- - _._-- 0, --),----,-
- 02 Cl ZT \ ir
Y ______ N N \ N p= t N N N
Ri 0
RN RN
RN Ri2 Ri
2
Rii lj N 1\1=--
Rio Rio(rN , N _ RN
õ ,N, /') pp 13 --------------------------------------------------- /')
LI \_) R13
,) ________ N N-N ------ R14 N R
i 4
;
R12 R12
R12 R12
N R12 0 ---__/y S---../y
N /-) Ri 3 -\ -$____, 1 A
R 1-r R14
N R14 N R14 N R14 R10 R10
RN
Ri2 RN
N--__//,) Ri2
R12 R12 RN
- - -R 13 _ _ 4i \ I ----r/õ Ri3 ' - < - - - - - - --/') 13
1 1 7R13 N
R9
R10 ; ni. -
R14 N ----%\IA 1 A ; R
\/\\-
; R14 R14 ' '
RN RN
N R9 ri , R9
0)
_ N II
R8 R.a i u n \ N \ I R8 N-. s(
:
N
,
S --N,.,
R8---- 1 N II R8----- --/ R''p,
N-N , b---N , N-C) , O'N N , Or N
,
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl,
bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl,
10 bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl,
hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one or
more C=C double bond(s) and/or are optionally substituted by one or more of
Ra, Rb, Rc,
Rd, and Re;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3, -CH2CF3, -
COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C21-15, -CON H2, -CONHCH3,
-CON(CH3)2, -CONHC2H5, -CH2CO2H, -CH2CO2CH3, -CH2CO2C2H5, -CH2CONH2,
-CH2CONHCH3, -CH2CON(CH3)2, -CH2CONHC2F15, -NHCOCH3, -NHCOC21-15,
-NHCOCF3, -NHCOCH2CF3, -NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2,
-NHSO2CF3, -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -CH3, -CH2CH2CH3,
-CH(CH3)2, -CH2CH2CH2CH3, -CH2CH2CH2CH2CH3, -CH2CH(CH3)2,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
219
-CH2CH=CH2, -CH2CH=CH(CH3), -CH2CH=C(CH3)2, -CH2CH=CHCH2CH3,
-cyclo-C3H5, -cyclo-C4H7, -cyclo-05H9, -
cyclo-C6H11, -CH2-cyclo-C3H5,
-CH2-cyclo-C4H7, -CH2-cyclo-05H9,
-CH2-cyclo-C6H1 1, -CH2OCH3,
-CH2OCH2CH3, -
CH2CH2OCH3, -CH2CH2OCH2CH3,
-CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR8R7 is -N(C2F15)2, --I\1 --I0 --NO
or N/ \
\ __ / =
R8, R9, R10, R11, R12, R13, and R14 represent independently of each other -H, -
F, -CI,
-Br, -OH, -CN, -NO2, -CH3, -C2H5,
-C3H7, -CH (CH3)2, -C4H9,
-CH2-CH (CH3)2, -CH
(CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5,
-CH2OH, -CH2F, -CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F,
-CH2-CH F2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -OCH3,
-0C2H5, -0C3H7, -OCH (CH3)2, -0C(CH3)3, -0C4H9, -OCHF2, -0CF3,
-OCH2CF3, -0C2F5, -OCH2OCH3, -0-cyclo-C3H5, -OCH2-cyclo-C3H5,
-0-C2H4-cyclo-C3H5, -CHO, -COC H3, -COCF3, -00C2H5, -
00C3H7,
-COCH(CH3)2, -00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7,
-COOCH (CH3)2, -COOC(CH3)3, -
00C-CH3, -00C-CF3, -00C-C2H5,
-00C-C3H7, -00C-CH(CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5,
-NHC3H7, -NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -N(C2H5)2, -N(C3H7)2,
-N[CH(CH3)2]2, -N[C(CH3)3]2, -NHCOCH3, -NHCOCF3, -NHCOC2H5,
-NHCOC3H7, -NHCOCH(CH3)2, -NHCOC(CH3)3, -CONH2, -CONHCH3,
-CONHC2H5, -CONHC3H7, -
CONHCH(CH3)2, -CON H-cyclo-C3H5,
-CONHC(CH3)3, -CON(CH3)2, -CON (C2H5)2, -CON (C3H7)2, -CON [CH(CH3)2]2,
-CON[C(CH3)3]2, -SO2N H2, -SO2N H CH3; -
SO2NH C2H5, -SO2NHC3H7,
-SO2NHCH (CH3)2, -SO2NH-cyclo-C3H5, -SO2NHC(CH3)3, -
SO2N(CH3)2,
-SO2N(C2H5)2, -SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C2H5, -NHSO2C3H7, -NHSO2CH (CH3)2, -NHSO2C(CH3)3,
-CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3, -CECH, -CEC-CH3,
-CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
H I H H
=-
-. -NO ------------- t-N/
Li
N____
r
0
, ,
I H I
N N N
r r r / __ \ / __ \ / __ \
0 (:) (:) --N \ / / NH --N\ /N- --N\ /N \ .
, or \
or R8 and R9 or R9 and R1 can form together one of the following five-
membered or
six-membered rings:
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
220
H _,ID ,N
_ - ) --- --- - ---N - -
- -1 -- -1 '-,/ ''0 '-,% '-.% '- --,N
---N ,N
or ----%N . ,
or R12 and R13 or R13 and R14 can form together one of the following five-
membered or
six-membered rings:
,0
,o ,,0 ,-C) ,-() --- _.13
- ) - -0 --0 , C) 0
'-,/ '', -' H or I -
R" represents -H, -CH3, -C2F13, -C3H7, -CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH(CH3)-C2H3, -C(CH3)3, -cyclo-C3H3, -
cyclo-C4H7, -cyclo-C31-19,
-CH2-cyclo-C3H3, -CH2-cyclo-C4H7, -CH2-cyclo-C31-19, -CH2F, -CHF2, -CF3,
-CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2; -CF12-CF3, -CH2-CH2CI,
-CH2-CH2Br, -CH2-CH21, -CH2-CH=CH2, -CH2-CECH, -CHO, -COCH3,
-00C21-13, -00C3H7, -COCH(CH3)2, -
00C(CH3)3, -CO-cyclo-C3H3,
-CO-cyclo-C4H7, -CO-cyclo-C31-19, -COOCH3, -COOC2H3, -CO0C3H7,
-COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph, -S02CH3, -S02CF3, -S02C21-13,
-S02C3H7, -S02CH(CH3)2, -S02-cyclo-C3H3, or -S02C(CH3)3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers,
a racemate, a solvate, a hydrate, or a pharmaceutically acceptable salt
thereof.
Preferred are the compounds of the formula (lb):
0
0,,,
R6
ii
0 H R70
A N , L ,
R2 N 1 N R3
H
0 (Ib);
and L, R2, R3, R6, R7 have the same meanings as defined in the formula (I)
Preferred are the compounds of the formula (lb):
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
221
0
ON,R6
0
H L
R2AN ')-NIõR3
H
0
(lb)
wherein
L represents ¨L1¨ or ¨L1-L2¨; preferably ¨L1-L2¨;;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
L2 represents a bond, ¨NRI41¨, ¨NRN1CH2¨, ¨NRN1CH2CH2¨, or ¨NRN1CH(CH3)¨;
R2 represents
RN RN
0 R8 R11 0 R8 -__ S Rs R11 S Rs -_ N Rs R11N Rs
Rlo R9 / R9 Rlo R9 / R9 Rio R9 ,
mg
R ,
RN RN RN wo
R11 rj, R11 rj,
/NI
r.,,i), ( n R1:JON __ RON _R8 - - __õ-ON____.R8
\ ir
, __ N \
N N¨
Ri 0 `s / R9 i-K - R. R10
R10 R8
,
, , , ,
'
R1 RN RN
)1 ,, 1
R8 2xRio Rzc.,\õ_ RSRs ---1SR8 --<1\1:N Rttc.,
N ,N
N N )\ N
, __ N
N¨ ', R8 Rio , /' N Rio , Rio ,
, ,
RN RN R12 R12
Rio.,..N ,N_RN N .,,Nr, _Rio
\¨N
N - N, ,-,N R13
NI'm -- LI, \')
= N-N , N IA=N ,
------ n, 14 N et
, ,
R12 R12
R12 R12 N R12 0 S--
---/Y,) 13
R13 r
-\'
\N
N Rizt N Rizt N Rizt Rio R14 Rio R14
,
RN
R12 RN
N---__A RN
Ny,
R12
S R12
---TR13 9 __ - - -(\ 1 tR13
\7R
R14 r_.IR
N
Rio - \ -7---Rio R.,iA
N R14, , , ,
_r_ /R910'
R13
r\zi:
R12
N
Ri
- H
7R13 , NI.-S
S
.A Ri_- n _______________________ N _
R
c-N----)
, ,
RN
H
ii R9 N ,
, ,
,
D____R:k, R9
r -/i i
n
_ R10 \ / /TiiiiIJi el-
--/_-= R1 :, k , _R . u
0) , II , 0 , S , ' , ,µ.11
,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
222
N_
'===õ,, ,S -'
-_,N,
\N
I - N\ \ I
N N R8
R8 R8 ' R8
S
)
N6-- -- ---N R8---- ii NhT- Ru---- -1
\S-N N R8
or
,
R`-'a
-- ii
0-N ;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl,
hexamethylenetetraminyl, and the afore-mentioned residues optionally contain
one or
more C=C double bond(s) and/or are optionally substituted with one or more of
Ra, Rb,
Rc, Rd, and Re;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H3, -CON H2,
-CONHCH3, -CON(CH3)2, -CONHC2F13, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H3, -CH2CONH2, -
CH2CONHCH3, -CH2CON(CH3)2,
-CH2CON HC2F13, -NHCOCH3, -NH COC21-13, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-13, -NHSO2CHF2, -NHSO2CF3, -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -CH3, -CH2CH2CH3,
-CH(CH3)2, -CH2CH2CH2CH3, -CH2CH2CH2CH2CH3, -CH2CH(CH3)2, -C(CH3)3,
-CH2CH=CH2, -CH2CH=CH(CH3), -CH2CH=C(CH3)2, -CH2CH=CHCH2CH3,
-cyclo-C3H3, -cyclo-C4H7, -cyclo-C31-19, -cyclo-C6H11, -CH2-cyclo-C3H3,
-CH2-cyclo-C4H7, -CH2-cyclo-C31-19, -CH2-cyclo-
C6H11, -Ph, -CH2-Ph,
-CH2OCH3, -CH2OCH2CH3, -
CH2CH2OCH3, -CH2CH2OCH2CH3,
-CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR6R7 is -N(C2F13)2, - -N N/-- --NO or __ N/ \\
/ =
RN represents -H, -CH3, -C2E-13, -C3H7, -
CH(CH3)2, -C4H9,
-CH2-CH(CH3)2, -CH(CH3)-C2H3, -
C(CH3)3, -cyclo-C3H3, -cyclo-C4H7,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
223
-cyclo-05H9, -CH2-cyclo-C3H5, -CH2F, -CH F2; -
CF3, -CH2CI, -CH2Br,
-CH21, -CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br,
-CH2-CH21, -CH2-CH=CH2, -CH2-CECH, -CHO, -COCH3, -00C21-15,
-00C3H7, -COCH(CH3)2, -00C(CH3)3, -COOCH3, -CO0C2H5, -CO0C3H7,
-COOCH(CH3)2, -COOC(CH3)3, -
COOCH2Ph, -S02CH3, -S02CF3,
-S02C21-15, -S02C3H7, -S02CH(CH3)2, or -S02C(CH3)3;
RNi represent -H, -CH3, or -CH2CH3;
and R8- R14 have the meanings as defined above for formula (I);
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers,
a racemate, a solvate, a hydrate, or a pharmaceutically acceptable salt
thereof.
Preferably, R2 represents
RN RN
__ _ 0 8 R11 0 R8 - _ R8 R1,srR8 ___,,c,,, R8
R1,_,,,,,,,,,
R
Rlo _____ R9 / R9 Rlo R9 , R9 Rlo R9 /
,,,,,9
'`
,
RN RN RN Rio
Rii N, R11 N,
-_)(11.N R1:1),,,O.N __ Ro, _R8 - _ )õ-O.N___ Rs
I /1\1 1 N
/ l(
/K \ ir
N \ Ir-
N \ // 0,
N N- NR:
Rlo ss / Ro R10 Ro Rio , Rlo
N.
Rii RN ;
,
, ,
Rio ',
R8 ______ R10 Rti,Sr _ R1_1SR8 - -
IS \r-R8 - - 1 : --_c m
(:)?zy
N N N N \ ..
N
R8 , Rio , / Rio Rio /,
N
RN ;
RN R12 R12
, N N Rio Rio N_RN
13 12
" ,N, DN R - R=-
----V LI -- _\õ.) \-)
, N-N ; N=N ; -------R14 N
R14
,
R12 R12
R12 R12 N R12 0---./Y,) s
N'/,) r ---__)R13 ---
$____) R13
R13 R13 Ri3 - \ 44
\ N \' \'
N R14
, N Ri4 ; N Ri4 , Rlo RI4
, Rlo R14
,
RN
Dp. 12 RN
i\l-/y4' R12 R12 p12
N---__/Y,
-- -\TR13 \,tR13 ''l
/') 13 ,õ------`
I 1 R13
R14 N----%\ 1 A __\7 R
Rio R., Rizt Rizt
,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
224
R13 N RN
14 R
R9
ss R
RN
17\)1R9R9
R N o 0)
Di 0 N\\ S\)- N R8
¨R N R8 \s-N R8
R8
'
Nhi R8I 8
b¨N N¨C) , or O¨N
Preferably, -NR6R7 of the formula (lb) represents -NH2, -NHCH3, -N(CH3)2,
-NHCH(CH3)2, -NHCH2CH2CH3, -NH-CH2CH=CH2, -NHCH2CH2CH2CH3,
-NHCH2CH(CH3)2, -NHC(CH3)3, -
NHCH2CH2CH2CH2CH3, -NH-cyclo-C3F15,
-NH-cyclo-C4H7, -NH-cyclo-051-19, -NH-cyclo-C6Hii, -NHCH2-cyclo-C3F15,
-NHCH2-cyclo-C4H7, -NHCH2-cyclo-051-19, -NHCH2-cyclo-C6H11, -NHCH2-Ph,
-NHCH2OCH3, -NHCH2OCH2CH3, -NHCH2CH2OCH3, -NHCH2CH2NHCH3,
-NHCH2CH2N(CH3)2,
- ¨N ¨10 --NO
--N
or
In some embodiments, the present invention relates to the compound of the
formula (I),
IRI
0 H 0
JC
R2 N N R3 (I)
0I
wherein
L represents -L1- or -L1-L2-; preferably -L1-L2-;;
L1 represents -CH2-, -CH2CH2-, -CH2CH2CH2-, -CH2C0-, or -CH2CH2C0-;
L2 represents a bond, -NRNI-, -NRNICH2-, -NRN1CH2CH2-, or -NRN1CH(CH3)-;
0
R1 represents ''N-R6
0
R2 represents
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
225
RN RN
0 Rs R10 Ra -_ S Rs R1: .!õsR8 ___,,R8
i__rR8
\ , r 1 __ r ,\ , ,\ , ,\ ,
R10 R9 / __ , R10 R9 , R9 R10 R9 R1, /
,Ii
N ,
RN RN RN Rlo
R11 ii, Rii
N N;, N __ , Ril 0 R(
ii 0 R 8 - - 0 R8
R10 sµ R K = - Rn R10 I\1 R
,
---\_4 I /N
/ ( ---, ----/ I
1---- \)-------R8
/ :, P.
, / , , ,
RN
R10
ii
\
R8 /_______. R10 Rtl.õ.(\õSNit _ _ R1_SR8 - -
1S)r-R8 - R8
, Rio R10 R10 __ N
N N N -'
/
RN RN RN
N. RN R12
-- õK.- N Rij1i ,N,\1 _ R1o.N,N_RN .. N,N, _Rio
_ ,N, ,N, DN
13
R -
\ " .1
N N =r\i \ r
)=N --\\ ,N ---- N''`
------------------------------------------------------------------------
LI,.,.,.,,,:\,,,j
Rio , , N-N N=N
R14
,
R12
R12 R12 R12 0 ,
r N 1 ,1211
N/-)
R13 R1 -3 R1'-',
R- -\-
iA
\-) , N Ria
, N Ria
, N Ria
, N Ria
' R10 R."
,
RN
R12 p12 RN
S--/y,) 13 i\i--/' s RN N2 RN
S
)
- - R - - - ¨R13
9
r_.R ¨4 1 R13
N
R14 R14 _ N'%\-iA
R10 R10 \____ -R10 ni..-, ,
, , I-µ ,
R13
R12 R12
H
R13 N .
L j ¨A - ii __ N77----R14
NSõ-S
- c S
R14 R14 Do._ in N -N--.,_?
Fµ
RN RN H ,,
,N, R9 r 1 i
R10 -/ 1 R9 N
m 9
r /1 _ Rio \ / / ' N- --/--\
¨R 1 o
_ 1::1) ,
, -
, 0 s
, , ,
i
i õ.
R9 N
_Rto N
,;(IN N N N N)
,
, R8 R8 ,S - - R8
=, N,S NI' II
\\ I
ff St\ 7 N ,--N R . S,--
---- il N-.-
NN N Ra \s-N NN Ra N-N b-N
, ,
R8 N,--
IR'--- -/ R8--- 1
N-0 or 0-N -
R3 represents bicyclo[1.1.1]pentyl,
bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
226
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl,
hexamethylenetetraminyl, and the afore-mentioned residues optionally contain
one or
more C=C double bond(s) and/or are substituted with one or more of Ra, Rb, Rc,
Rd, and
Re;
R6 represents -H, -CH3, -CH2CH=CH2, -cyclo-C3H5, -CH2CH2CH2CH2CH3;
and R8 - R14, Ra; Rt.; Rc; Rd; Re; RN; and rc -N1
have the meanings and preferred meanings
as defined herein.
Also preferred are compounds of the general formula (I),
R1
J
0 0
(I) CINI 71- R3
R2 N 1 N
Ho
wherein
L represents -L1- or
L1 represents -CH2-, -CH2CH2-, -CH2CH2CH2-, -CH2C0-, -CH2CH2C0-;
L2 is a bond, -NR'-, -NRN1CH2-, -NRN1CH2CH2-, or -NRN1CH(CH3)-;
0
R1 represents
,
0
R2 represents
RN RN
____,I;IrR8 Rii
R8
Ri______i 0 R8 ____.s R8 w______.1 s R8
-\ r r ,\ r ,\ __ r )\ , \ ,
Ri0 R9 / __ R9 Ri0 R9 / R9 Ri0 R9 / R9
;
RN RN RN
R11 1;1, R11 1;1, __ 11, Rii 0 __
Ri:).,0R8 -__(:)___Rs
N
Rio `, ; R9 Rio R9 Rio , , rµ
mio
,
,
R N
1 RN
RN
Rti)rs, __ RS, _R8 S, Rs --).-N-N Ri_i N,
\ rk __ 1 ,N li - - -1\IRN
N N N IN N
Rio , rµ mio Rlo , N¨N N=N
, ,
R12 R12 R12 Ri2 Ri2
N ,. , --:-.7
pp 13 r N4)
1R 3 1R 3 1R 3
R¨ 13
\')
, R14 N R14 N R14 N R14 , N R14
,
,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
227
RN
R12 W2 R12
N - - - / .,.__
R12
Rl W
-R13 - - -S_____\T R13 - - iis__j\T R13 N
R1313
0 41 ,õ R14
Wu N-----\%\
R14
R14
R13 RN RN
R12 RN
µµ), ________________________ N-- R14 N R9 ri R9
iR13
S r_IiR9 _r_ % R10 _Tr- '/R10
R14i- W N - \-->R1 _
R10
- m R10
or -- " , ,
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl,
hexamethylenetetraminyl, and the afore-mentioned residues optionally contain
one or
more C=C double bond and/or are substituted one or more of Ra, Rb, Rc, Rd, and
Re;
Ra, Rb, Rc, Rd, and Re represents independently of each other -H, -F, -Cl, -
Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CONH2,
-CONHCH3, -CON(CH3)2, -CONHC2H5, -CH2CO2H, -CH2CO2CH3,
15 -CH2CO2C2H5, -CH2CONH2, -CH2CONHCH3, -CH2CON(CH3)2,
-CH2CONHC2H5, -NHCOCH3, -NHCOC2H5, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C2H5, -NHSO2CHF2, -NHSO2CF3, -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -
CH3,
-CH2CH2CH3, -CH(CH3)2, -
CH2CH2CH2CH3, -CH2CH2CH2CH2CH3,
-CH2CH(CH3)2, -C(CH3)3, -
CH2CH=CH2, -CH2CH=CH(CH3),
-CH2CH=C(CH3)2, -CH2CH=CHCH2CH3,
-cyclo-C3H5, -cyclo-C4H7,
-cyclo-05H9, -cyclo-C6H11, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-05H9,
-CH2-cyclo-C6H11, -CH2-Ph, -
CH2OCH3, -CH2OCH2CH3,
-CH2CH2OCH3, -CH2CH2OCH2CH3, -CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR6R7 represents or --N \ / =
R" represents -H, -CH3, -C2H5, -C3H7, -CH(CH3)2, -
C4H9,
-CH2-CH(CH3)2, -CH(CH3)-C2H5, -
C(CH3)3, -cyclo-C3H5, -cyclo-C4H7,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
228
-cyclo-05H9, -CH2-cyclo-C3H5, -CH2F, -CHF2, -CF3, -
CH2CI, -CH2Br,
-CH21, -CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br,
-CH2-CH21, -CH2-CH=CH2, -CH2-CECH, -CHO, -COCH3, -00C21-15,
-00C3H7, -COCH(CH3)2, -00C(CH3)3, -COOCH3, -CO0C2H5, -CO0C3H7,
-COOCH(CH3)2, -COOC(CH3)3, -
COOCH2Ph, -S02CH3, -S02CF3,
-S02C21-15, -S02C3H7, -S02CH(CH3)2, or -S02C(CH3)3;
RNi represent -H, -CH3, or -CH2CH3;
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers,
a racemate, a solvate, a hydrate, or a pharmaceutically acceptable salt
thereof.
Also preferred are compounds of the formula (I) or (lb), wherein
L1 represents -CH2-, or -CH2C0-;
L2 represents a bond, -NR'-, -NRN1CH2-, or -NRN1CH(CH3)-;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl,
bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, 4-homoisotwistyl, adamantyl, or
diamantyl, and
the afore-mentioned residues optionally contain one or more C=C double bond(s)
and/or
are substituted by one or more of Ra, Rb, Rc, Rd; and Re; and
Ra, Rb, Rc, Rd; Re and RI" have the same meanings as defined herein.
Preferably, R2 of the formula (I) or (lb) represents
R10
Rio R9 R11 0 RR R8 -__ S R8 R11 R8 R8 S
R8 ,
, N
,-,9
' R9
N- .= ,
RN RN RN
nn Rio Rii SN Ril Sõ8 -- SR8 -- ri ___
R8 -- N-
Rc NN
0, /7--- --i /7---- -,r,,, ,
N
R8 Rio , Rio Rio Rio
RN
Ri2 Ri2 Ri2
,N R Rio_(rN,N _ RN
---------------------------------------------------------- /-1
N io ---
\-N
=r\j N, p N 1R 3
R13 -R=-
13
, N=N R14 N R14 N
R14
'
R'"
Ri2 Ri2 Ri2 RN
0--/y,) S --/y,) iN --/y,., R12
R12
---,\ TI R13 , i \ 1 - - - - - - ---' '/----i - , . - - - = - - - "
'----/,
-\R14 - - -\ 1 \ _T R13 R13
R 1-r R14 N-----\--1A
Rio Rio Rio ni.."
R14
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
229
R13 RN RN RN
N v ri /R9
\ __Ri 4 N R9 rN r' r 1 1
1 n S (- )..õ. _ 9
_ _ _ _R10 - 1 - TR 0 /
D.- /
N - -\____/"--1---Ri 0 ,
, " , ,
R8 R8 ,S -. R8
,\ S - ' N II ,,,-
! N i N/ - - S)- - - -.--N
a, R',S ----- il NhT
N R8 \s-N \Ns-- N R8 N - N b-N
, ,
n N, - ' n N,
R ---- -] R --- 1/
N-0 or
,
and R8¨ R14 and RN have the meanings as defined in formula (I) or (lb).
Preferred are compounds having any one of the formulae (IV-a) ¨ (IV-o) and
(V-a) ¨ (V-d):
0 0
A ,R6
0)-LN , R6
NH
H
0 H 0 Rb 0 c 0 RC
R2 N N
A N L Ra R2. NH NH N
1 .L2 Rb
1 2
1-. `= '
I-
U
H
0 0 0
Ra
(IV-a)
(IV-b)
o 0
oANR6 0 N-R6
"
H H Rb Ra
0H 0 0 NN 1_ H 0
A ,,..,..-1-1.., 2 CNN L2
Rc
A N 1
R2 H 0 0 R R2
a H
0 0 Rd
IR' Rb
(IV-d)
(IV-c)
0 0
a
OAN , R6 ON, R Rb R
6
H Rb Ra H RC
o( 0
0 Rc 0 H 0
N N ,L2
R2A N
, il NL2
R2A N 1 N
H Rd H
0 0 0 0
(IV-e) (IV-f)
0 Rd 0
-
H Rc H Rb
Ra
0NR6 0N-R6
0 0 0 0 RC
A H II
N L2 A H
N L2
R2 N 0 1 N R2 N 1 N
H
0 H
0 0 Rd
(IV-g) (IV-h)
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
230
0 0
0 . 6
N R oA6 Ra NR -
H
Rb A
H
0 JCH 0 IR' 0 0
R2 N R2 N
I N L Rd H H
0 0 0 N 0
(IV-i ) (IV+
0 0
OA N , R6 i. .: OA N .R6 [7:p
H H
0 0 0 0
N 2
H H L2
R2 A N 1 N R2)- N crN
_ J1 NL
H
0 0 H
0 0
(IV-k) (IV-I)
o o
Q_ANFIR6 cl__H HR6
--- N
L2
R2NH NH N L2 I I R2-NH H NH N H
0 0 0 0
(IV-m) (IV-n)
0 0
0 06
0 .R6
N
H
0 ,C 0 0 H 0
R2,/NH NH 1 , 2
N/\1- 0 N 1 2
O
0 0 0
Rio
R9
(IV-o)
(V-a)
0 o
o R6 o )-L R6
N - N -
H H
0 H 0 H 0
S N .-
R8 hi N 1 2
..-----,,,_,... -,R3 R- 8 S N N N L2
H
R3
\ I 1 -- I 1
0 0 N 0 0
Ri 0 R10
R9
(V-C)
(V-b)
0
0 N .R6
H
0 H 0
Rii 0 N ki L2,, R3
R12 /7 \ \ I hi 1 I
(/ \ R10 o 0
R13 -
(V-d)
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
231
and R2, R3, R6, R8; Rs; R10, R11, R12, R13, Ra; R13, Rc; Rd L and - . 2
have the same meanings
as defined herein, preferably as defined in formula (I) or (lb).
Also preferred are the compounds of any of the formula (IVa-1),
0
0)-R6
H
0 H 0
H Rb
N
R2 N
, JI N N
H
0 0 Ra
(IVa-1)
wherein
R6 represents ¨H, ¨CF13, ¨CH(CH3)2, ¨CH2CH2C1-13,
¨CH2CH=CH2,
¨CH2CH2CH2CH3, ¨CH2CH(CH3)2, ¨C(CH3)3, ¨CH2CH2CH2CH2CH3, ¨cyclo-C3H5,
¨cyclo-05H8, ¨cyclo-C8H11 or ¨CH2¨cyclo-C3H5.
and R2, Ra, and Rb have the same meanings as defined above.
Preferably, in the compounds of formula (I) or (lb), Ra and Rb represent
independently of
each other ¨H, ¨F, ¨Cl, ¨Br, ¨OH, ¨CN, ¨CF13, ¨C2H5, or ¨0O2Me.
Preferably, in any of the formula (I), (lb), (IV-a) ¨ (IV-o), or (IVa-1):
R2 represents
o
r0 Br , -- Br 0 Br 0 \ /
s Br
,
S _ _
S
- S CI \\/ p , -B-rY-1-Br
\ /
-- - -- -- 1
S Br
Br Br Br,
,
-ySz-Br -- - S CI -- r- S B y
S
Br
CI Br , Br , CI Me() Br
, ,
---- 0
.--
\ I
S _ \ /
-CI CI B
CI r-õD____S
\ / Br HN
NC -0 --- H2N N
0 S,
\ .-- Nõ-
Me0 ---)\1--- H N3
0 N-NBr, N-
N
, ,
\
\s Br s C I ------s Br ------s_.-- CI BrS
Br CI-----cS-C1
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
0C-90-ZZOZ peAieoef alea/an5a aleCI
xoop-vzonnottod-a3z-Noadiens3a-odo-68tz
pue N-0 JO ' N-
C)
k ----
LN
-- N --'
, NZH
,
El-----N\\N \\ N I \\ N _õ- 1 \=\ N 1 \ \N 1
\ N " - S/
N-N NN.
N-S N N \NN
õ0 ----s'1\1 õ- S JUN I
-- S .--- / N 1 1
, - -----N 'W ,
:
N H 0 [
WN
1 1 NH N
__, \ _,, / ,-' 1 N 1 ---=
co , HO s\>- , , ---
, ,
N N J
--- N
- - J
\
H N
N H
H
'c'- , , , \ ,
_le s
--\ -- \ __ __ \ __ , /
N N S S S
, , ,
0 0 ' ' J9 10
1 0 0
0
\ __ N------9 /9---r-N ,--7N---- ...--\-------"A
\
0 \
,¨NH J9
,
--N
NH - , N
- N H -- -NN) / __, N
H 0
H
' N
N ' .,1\1 N
-N '-- '-- -N '-- - elf\lz00-- Hz00-- zON --
,
91/\1z00
1 HN --__ iii¨\\ iii¨\\ N ____ N Ii
Ni'
N ,N N ,N N-N N, NN
zON ., 1 I N N 1
, N-N , '
-----(5--
NN \--N\
NH N \ // N N N
\-----IN-- -- 10-5¨<-- -
_ -
S ---
I,
J9 10
N N N N N N
Lld'-s-- elAlOs- - cdo _ _OS 9 J -- c --s-
s 5
ZCZ
233
R6 represents ¨H, ¨CH3, ¨CH(CH3)2, ¨CH2CH2CH3, ¨CH2CH=CH2,
¨CH2CH2CH2CH3, ¨CH2CH(CH3)2, ¨C(CH3)3, ¨CH2CH2CH2CH2CH3, ¨cyclo-C3H3,
¨cyclo-C31-19, ¨cyclo-C6H11 or ¨CH2¨cyclo-C3H3.
Due to the specially selected substituents R2 on the N-terminal side and
substituents R3
on the C-terminal side and of the inventive compound according to the
invention the steric
dimension can be adjusted very precisely, so that a binding pocket of a
desired target
molecule may be addressed with highly matching measurements.
Preferred, are the compound of any of the formulae (I), (lb), (IV-a) ¨ (IV-o),
and
(V-a) ¨ (V-d), wherein
R3 represents
,
,
,
,
,
.,,
,
'
,
, , ,
., .
'
F Br ; CI OH
CO2H CONH2 CONMe2 Nj
H,
CO2Me ,, CO2H Br OH
CF3,
F
F
F jZci ZcF3 ZOH jZco2Me
i , , , , or .
Surprisingly, it was found that the inventive compounds bound to the
transglutaminase 2
reversibly and inhibit the transglutaminase effectively. The electrophilic
warheads in
combination with the preferred embodiment specifically react with highly
nucleophilic
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
234
thiols in the active site of the transglutaminase 2. Accordingly, it was found
that potential
unspecific reactions with off-targets are reduced.
In one embodiment, the present invention refers to the compound selected from
the group
consisting of:
11-2: oANI (S)-2-(benzofuran-2-carboxamido)-
N1 -(1 -
(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
0
H 1 ,2-dihydropyridin-3-y1)-N6-
methy1-5-
N
I H I oxohexanediamide
o o
11-3: (S)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o 0
H N6-methy1-2-(3-methylbenzofuran-2-
0 N N
I H carboxamido)-5-oxohexanediamide
o o
11-4: (S)-2-(3-ch lorobenzofuran-2-
0 1\1
carboxamido)-N1 -(1 -(2-(2-
0
H
0 N N adamantylamino)-2-oxoethyl)-2-oxo-
1 ,2-
I H
0 0 dihydropyridin-3-y1)-N6-methy1-5-
cI
oxohexanediamide
11-5: (S)-2-(4-bromobenzofuran-2-
0)-LNJ
carboxamido)-N1 -(1 -(2-(2-
0
H adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
o
I H 0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
Br
0
11-6: ON (S)-2-(4-bromobenzofuran-2-
carboxamido)-N1 -(1 -(2-(2-
0
H adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
S N
I 0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-7: OLN (S)-2-(benzo[b]thiophene-2-
carboxamido)-
N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
0
HH 2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-
s
I H 0 0 5-oxohexanediamide
Br
0
11-8: 0)-L (S)-2-(5-bromobenzo[b]thiophene-2-
NJ
carboxamido)-N1 -(1 -(2-(2-
oH 0 N adamantylamino)-2-oxoethyl)-2-oxo-1
HN ThiNj
0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
235
o
11-9: (S)-2-(1H-indole-2-carboxamido)-N1-(1-
(2-
o,A --
N
H (2-adamantylamino)-2-oxoethyl)-2-oxo-
FN1 i o UN Ed
1,2-dihydropyridin-3-y1)-N6-methy1-5-
, NThr
H
0 0 oxohexanediamide
o
11-10: ON (S)-2-(4,5-difluoro-1H-indole-2-
H carboxamido)-N1-(1-(2-(2-
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
H N r\jAN-IN
I H 0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
F F
0
11-1 1: 0)-1\1 (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o H 0
H H N6-methy1-2-(3-methy1-1H-indole-2-
N N,A
N NN carboxamido)-5-oxohexanediamide
1 H 0 8
0
11-12:
o,)-LN (S)-2-(1H-benzo[d]imidazole-2-
H carboxamido)-N1-(1-(2-(2-
0 N rEi 0
I, " NThr IR1 adamantylamino)-2-oxoethyl)-2-oxo-
1,2-
iii,j H 0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
o
11-13: (S)-2-(2,3-dihydro-1H-indene-2-
o
N
H carboxamido)-N1-(1-(2-(2-
O 0 adamantylamino)-2-oxoethy1)-2-oxo-1,2-
N N
H , Ji dihydropyridin-3-y1)-N6-methy1-5-
o o
oxohexanediamide
o
11-14: OAN (S)--(2-bromo-4-methylthiazole-5-
H carboxamido)-N1-(1-(2-(2-
o H o
s -
FNI1 adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N
Br--- k)-L'Fir i NI
0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
o
11-15: (S)-N1-(1-(2-(2-adamantylamino)-2-
0)-LN
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o H 0
_N' N6-methyl-2-(4-methyl-2-
F3c___ekHN_A 1 N ff
E
N 0 0 (trifluoromethyl)thiazole-5-
carboxamido)-5-
oxohexanediamide
o
11-16: 0)-N (S)-2-(4-bromo-2-
(trifluoromethyl)thiazole-
H 5-carboxamido)-N1-(1-(2-(2-
o F
F3c-- H o
S_Aõ, NJLI\jr NI adamantylamino)-2-oxoethyl)-2-oxo-1,2-
- 1 [sil
N 0 0 dihydropyridin-3-y1)-N6-methy1-5-
Br
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
236
o
11-17: OAN (S)-2-(2,4-dichlorothiazole-5-
H carboxamido)-N1 -(1 -(2-(2-
o , o
j-L adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
ci----
N s
__I\Icki
H 0 0 dihydropyridin-3-y1)-N6-methyl-5-
CI
oxohexanediamide
o
11-18: 0 (S)-N1-(1-(2-(2-adamantylamino)-2-
r\J
H oxoethyl)-2-oxo-1 ,2-d i hydropyrid
in-3-y1)-2-
0 o
s-L N r11J-Nil (2-methoxy-4-methylthiazole-5-
Me0--- 1 H
N 0 0 carboxamido)-N6-methyl-5-
oxohexanediamide
o
11-19: (S)-N1-(1-(2-(2-adamantylamino)-2-
0 N
H oxoethyl)-2-oxo-1 ,2-d i hydropyrid
in-3-y1)-
0 H H
= \s 1 N N,CN N,Q1, N6-methy1-2-(4-methy1-2-
phenylthiazole-5-
H 0 8
N carboxamido)-5-oxohexanediamide
o
11-20:
oN (S)-2-(2,4-dimethylthiazole-5-
H carboxamido)-N1 -(1 -(2-(2-
o o
s__A fl- H adamantylamino)-2-oxoethyl)-2-oxo-1
,2-
----N1 il 1 NifN
dihydropyridin-3-y1)-N6-methy1-5-
0 o
oxohexanediamide
o
11-21: o N (S)-2-(5-bromo-3-methylthiophene-2-
)-L
H carboxamido)-N1 -(1 -(2-(2-
Br1
o
H ? H adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
S N N
\ 1 N N
dihydropyridin-3-y1)-N6-methyl-5-
H o o
oxohexanediamide
o
11-22: 0)-N (S)-2-(3,5-dibromothiophene-2-
H carboxamido)-N1 -(1 -(2-(2-
Br
o H N H adamantylamino)-2-oxoethyl)-2-
oxo-1 ,2-
s
Br N N N
Tr
\ I H 0 0 dihydropyridin-3-y1)-N6-methyl-5-
oxohexanediamide
o
11-23: oN (S)-2-(5-bromothiophene-2-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o
s o ci.1
H
Nj-LNN 2-oxo-1 ,2-d i hydropyrid in-3-y1)-N6-methyl-
5-oxohexanediamide
o
11-24: oN (S)-2-(5-chlorothiophene-2-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
CI\ (
o H H 2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-
s Nj -
HN 1 Nr -iNj
5-oxohexanediamide
o o
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
237
o
11-25: oN (S)-2-(5-bromo-3-methylfuran-2-
H carboxamido)-N1 -(1 -(2-(2-
Br
o
o 0 ( )-L
El H
NNN adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
,
\ I HN 0 8 dihydropyridin-3-y1)-N6-methyl-5-
oxohexanediamide
o
11-26: oN (S)-2-(5-chlorofuran-2-carboxamido)-
N1-
H (1-(2-(2-adamantylamino)-2-oxoethyl)-2-
H oxo-1 ,2-dihydropyridin-3-y1)-N6-methy1-5-
0 N2-
N , N
CI \I H I I N oxohexanediamide
o o
o
11-27: Ol-L.N (S)-2-(5-chlorothiophene-3-
carboxamido)-
y.-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
CI
o 0
H H 2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-
0
N )-LNThiN
I 5-oxohexanediamide
s 0
0
11-28: oAN (S)-2-(2,5-dichlorothiophene-3-
H carboxamido)-N1 -(1 -(2-(2-
o 0
H
N2- N adamantylamino)-2-oxoethyl)-2-
oxo-1 ,2-
a /1 N 1 N'Ir
s 0 0 dihydropyridin-3-y1)-N6-methy1-5-
ci
oxohexanediamide
o
11-29: o)LN (S)-2-(2,5-dibromothiophene-3-
H carboxamido)-N1 -(1 -(2-(2-
o (H 0
N)-L kil
Br / 1 N'i 1 N,'.r adamantylamino)-2-oxoethyl)-2-
oxo-1 ,2-
S 0 0 dihydropyridin-3-y1)-N6-methyl-5-
Br
oxohexanediamide
o
11-30: oN (S)-2-(5-bromothiophene-3-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o o
H 2-oxo-1 ,2-d i hydropyrid in-3-y1)-N6-methyl-
0
Br / 1 hic i NI -rNi 5-oxohexanediamide
s 0
0
11-31: o)N (S)-2-(2-chloro-5-methylthiazole-4-
,t. ..---
H carboxamido)-N1 -(1 -(2-(2-
o 0
H)L
I\11 adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
CI N
i N,
0 0 dihydropyridin-3-y1)-N6-methyl-5-
s
oxohexanediamide
o
11-32: 0)-I\J (S)-2-(2,5-dichlorothiazole-4-
L
H carboxamido)-N1 -(1 -(2-(2-
o 0
H H adamantylamino)-2-oxoethyl)-2-oxo-1
,2-
ci---1
0 N 1 NI N dihydropyridin-3-y1)-N6-methyl-5-
s 0
CI oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
238
o
11-33: 0N (S)-2-(2,5-dibromothiazole-4-
H carboxamido)-N1 -(1 -(2-(2-
Br o H o
adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
0 i
j-LN
, i EN11 dihydropyridin-3-y1)-N6-methyl-5-
/s - 0
Br oxohexanediamide
o
11-34: oN (S)-2-(2-bromo-5-methylthiazole-4-
H carboxamido)-N1 -(1 -(2-(2-
Br-- 0 rFi 0
adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
N__)-LN NThili
-- 1 H
H
S 0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
o
11-35: O N (S)-2-(2-bromothiazole-4-carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o H o
H 2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-
o
Br----(NjAHN fiNI
/ 5-oxohexanediamide
s - - il N N
0
11-36: ON (S)-2-(2-chlorothiazole-4-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o 0
H)L 2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-
0
a-----N N 1 NI LI 5-oxohexanediamide
s 0
0
11-37: N (S)-2-(2,5-dimethylfuran-3-
carboxamido)-
0A
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o ti 0
H 2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-
/ N-11\1-ANN 5-oxohexanediamide
I H 0 8
0
0
11-38: o N (S)-2-(4,5-dimethylthiazole-2-
H carboxamido)-N1 -(1 -(2-(2-
o H 0
H adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
s,IAN N,AN N
H 0 0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-39: 0
O N (S)-2-(4-bromothiazole-2-carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o N S H 0
H 2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-
N N 5-oxohexanediamide
5AH 0 ,-,0,,N
Br
0
11-40: O N (S)-2-(4-bromothiophene-2-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
0
H 2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-
s 0 Nr NH
5-oxohexanediamide
\ 1 H 0
Br 0
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
239
0
11-41:
0-N (S)-2-(4-bromo-3-methylthiophene-2-
H carboxamido)-N1-(1-(2-(2-
s
0 0
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
NN.rN
\I N H I 1 dihydropyridin-3-y1)-N6-methyl-5-
0 0
oxohexanediamide
Br
11-42: 0 (S)-2-(3-bromothiophene-2-
carboxamido)-
ON
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
0 0
s N H 2-oxo-1,2-dihydropyridin-3-yI)-N6-
methyl-
H 0 .iNNfN
5-oxohexanediamide
\I 1 1
0
Br
0
11-43: (S)-2-(3-chloro-4-methylthiophene-2-
ON
H carboxamido)-N1-(1-(2-(2-
0 0
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
s NNN),iN
\ I H i I dihydropyridin-3-y1)-N6-methyl-5-
0
a 0 oxohexanediamide
o
11-44: o,,,)-I (S)-2-(4-bromo-5-chlorothiophene-2-
.,-.. --
HN carboxamido)-N1-(1-(2-(2-
CI
o
s o -
-.IRI adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N II
\ I N H 0 0 dihydropyridin-3-y1)-N6-methyl-5-
Br oxohexanediamide
o
11-45: o)LN (S)-2-(4,5-dibromothiophene-2-
H carboxamido)-N1-(1-(2-(2-
Br -_7('N
o .. jo
s
1 -y --k1 adamantylamino)-2-oxoethyl)-2-oxo-1,2-
1 N II
\ i H 0 0 dihydropyridin-3-y1)-N6-methyl-5-
Br oxohexanediamide
o
11-46: oN (S)-2-(4,5-dibromo-3-methoxythiophene-
2-
H carboxamido)-N1-(1-(2-(2-
Br
o
s o -
ciRilj-L NI adamantylamino)-2-oxoethyl)-2-oxo-1,2-
OMe NThr
0 H N 0 0 dihydropyridin-3-y1)-N6-methyl-5-
Br oxohexanediamide
o
11-47: 0 (S)-2-(4-bromofuran-2-carboxamido)-N1-
N
H (1-(2-(2-adamantylamino)-2-oxoethyl)-2-
o o
H oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
0 r\jciNNrN
oxohexanediamide
\ I H 0
Br 0
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
240
o
11-48: ON (S)-2-(4,5-dibromofuran-2-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
o
o o NciRlij-L _NI 2-oxo-1,2-d i
hydropyrid in-3-y1)-N6-methyl-
Br N if
\I H 0 0 5-oxohexanediamide
Br
0
11-49: o..,JL.N...-
(S)-2-(4,5-dichlorothiophene-2-
H carboxamido)-N1-(1-(2-(2-
o
s o ci.,
adamantylamino)-2-oxoethy1)-2-oxo-1,2-
ci 1 N 1 N Thr
Ed
\ , H 0 0 dihydropyridin-3-y1)-N6-methy1-5-
CI oxohexanediamide
o
11-50: 0)-N (S)-2-((S)-1-acetylpyrrolidine-2-
H carboxamido)-N1-(1-(2-(2-
0
----- 0 0
N¨) N cFNI)"N ld adamantylamino)-2-oxoethy1)-2-oxo-1,2-
,
\ _ j H , JIi
0 0 dihydropyridin-3-y1)-N6-methyl-5-
oxohexanediamide
11-51: o
OAN
(S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-
H
0 0 N6-methyl-2-(1-methy1-1H-1,2,3-
triazole-5-
NilJ FRI 1 NrN
carboxamido)-5-oxohexanediamide
0 0
0
11-52: 0)-I\J (S)-N1-(1-(2-(2-adamantylamino)-2-
L
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-
0 o N6-methyl-5-oxo-2-(2H-tetrazole-5-
N
HNs'NN:IAN FIN H jiN'io FN1 carboxamido)hexanediamide
0
11-53: 0)-LI\J (S)-N1-(1-(2-(2-adamantylamino)-2-
H
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
0 N6-methyl-5-oxo-2-(pyrazine-2-
N)-LI\J
0 rEl
H
N
carboxamido)hexanediamide
I H _ JI NTh(N
N 0 0
11-54: 0 (S)-N1-(1-(2-(2-adamantylamino)-2-
H ---
N oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
\ 0 iF1 0
H N6-methyl-24(S)-1-methylpyrrol id ine-2-
N )-LNf N carboxamido)-5-oxohexanediamide
0 0
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
241
0
11-55
O r\I (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
0 0 H N6-methyl-5-oxo-2((S)-pyrrolidine-3-
H
_-AN-IN'Al N-rN carboxamido)hexanediamide
HN¨ H 0 0
11-56: o O N (S)-24(2S,4S)-4-bromopyrrolidine-2-
H carboxamido)-N1-(1-(2-(2-
E
H
0 adamantylamino)-2-oxoethyl)-2-oxo-1,2-
d J EN_I
0
dihydropyridin-3-y1)-N6-methyl-5-
oxohexanediamide
131
11-58: 0
0It. (S)-N1-(1-(2-(2-adamantylamino)-2-
,....,-
H--
N oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
H N6-methyl-5-oxo-2-((S)-piperidine-2-
NA N)-L carboxamido)hexanediamide
Nnf
---õ,--- 0 0
0
11-59: Or\j (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
0 0 H N6-methyl-5-oxo-2-((R)-piperidine-3-
HNNFdj-L
N
carboxamido)hexanediamide
H 0 INo
11-60: o
OAN (S)-N1-(1-(2-(2-adamantylamino)-2-
H
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
I- N6-methyl-24(R)-((R)-3-
N))-NciNNIVI carboxamido)-5-oxohexanediamide
H
0 0
0
0
11-61: oAN (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
N
N- 0 r H ? H N6-methy1-5-oxo-2-(quinuclidine-3-
NnN
Ii NN carboxamido)hexanediamide
H
0 0
0
11-62: okN (S)-methyl 3-(1-(1-(2-(2-
adamantylamino)-
y.-
H 2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
0 H 0
ylamino)-6-(methylamino)-1,5,6-
N-1 N 'AI N Fd
H
0 0 trioxohexan-2-ylcarbamoy1)-5-
02N
nitrobenzoate
CO2Me
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
242
o
11-63: 0AN (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o 0
H H
02N N. i-ir1µ12-N.rN N6-methy1-2-(5-
nitronicotinamido)-5-
1 H
0 0 oxohexanediamide
N
0
11-64: o-LI\j (S)-5-(1-(1-(2-(2-adamantylamino)-2-
)
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
HO2C 0 LN AN E
rEl 0
ylamino)-6-(methylamino)-1,5,6-
i N, rNI H
0 0 trioxohexan-2-ylcarbamoyl)nicotinic
acid
N
0
11-65: (S)-methyl 5-(1-(1-(2-(2-adamantylamino)-
0.N..-
H 2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-
0(rFi 0
ylamino)-6-(methylamino)-1,5,6-
Me02CN N AN
i H 0 8 a trioxohexan-2-ylcarbamoyl)nicotinate
N
0
11-66: oN (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
S 0 o
f-'-'N N'II H it H N6-methyl-2-(6-methylimidazo[2,1 -
N
__A
\\ I H b]thiazole-5-carboxamido)-5-
N 0 0 -10 N
oxohexanediamide
o
11-67: o -N (S)-N1-(1-(2-(2-
adamantyl(methyl)amino)-
...)L. --
H 2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o 0
I N6-methyl-2-(3-methylbenzofuran-2-
0 N .iN12-N N
I H I 1 carboxamido)-5-oxohexanediamide
0 o
o
11-68: (S)-N1-(1-(2-(5-hydroxyadamantane-2-
0 N
H amino)-2-oxoethyl)-2-oxo-1,2-
o o
O N id NI
N Thr dihydropyridin-3-y1)-N6-methyl-2-(3-
1 H 0 0 methylbenzofuran-2-carboxamido)-5-
OH
oxohexanediamide
o
11-69: 0 N (S)-N1-(1-(2-(5-fluoroadamantane-2-
H amino)-2-oxoethyl)-2-oxo-1,2-
o o
O N IR11)L H dihydropyridin-3-y1)-N6-methy1-
2-(3-
N N
methylbenzofuran-2-carboxamido)-5-
o 0 F oxohexanediamide
o
11-70: o,AN (S)-N1-(1-(2-(5-chloroadamantane-2-
H amino)-2-oxoethyl)-2-oxo-1,2-
o
o -
,N1 dihydropyridin-3-y1)-N6-methy1-2-(3-
N ri
I H 0 0 methylbenzofuran-2-carboxamido)-5-
ci
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
243
o
11-71: OAN (S)-N1-(1-(2-(5-bromoadamantane-2-
H amino)-2-oxoethyl)-2-oxo-1,2-
0 H 0 dihydropyridin-3-y1)-N6-methyl-2-(3-
o N.r1\1AN,r1
I H 0 8 methylbenzofuran-2-carboxamido)-5-
Br
oxohexanediamide
o
11-72: 0)NI (S)-N1-(1-(2-(5-methyladamantane-2-
L
H amino)-2-oxoethyl)-2-oxo-1,2-
o 0
H II H dihydropyridin-3-y1)-N6-methy1-2-(3-
ol N.iNNrN
methylbenzofuran-2-carboxamido)-5-
1 H 0 0
oxohexanediamide
o
11-73: (S)-N1-(1-(2-(2-carbonitrileadamantane-2-
OANI
H amino)-2-oxoethyl)-2-oxo-1,2-
o , 0 H CN dihydropyridin-3-y1)-N6-
methyl-2-(3-
1
O 4
0 f N N
methylbenzofuran-2-carboxamido)-5-
H 0
oxohexanediamide
o
11-74: o-LN (S)-N1-(1-(2-(2-methyl adamantane-
2-
H carboxylate-2-amino)-2-oxoethyl)-2-
oxo-
o 0
H II H CO2Me 1,2-dihydropyridin-3-y1)-N6-methy1-2-(3-
O I
0 NNN.irµi
methylbenzofuran-2-carboxamido)-5-
H 0
oxohexanediamide
o
11-87: 0 (S)-N1-(1-(2-(1-adamantylmethylamino)-
2-
N
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
O 0
H ii h N N6-methyl-2-(3-methylbenzofuran-2-
0 N ,NrN
i H
0 0 carboxamido)-5-oxohexanediamide
o
11-88: 0N (S)-N1-(1-(2-(1-(1-
H adamantyl)ethanamino)-2-oxoethyl)-2-
oxo-
O H ?
O N, rl 1,2-dihydropyridin-3-y1)-N6-
methy1-2-(3-
, N 1 NThr
i H
0 0 methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
o
11-90: O N (S)-N1-(1-(2-((1R,2S,4S)-
H bicyclo[2.2.1]heptan-2-ylamino)-2-
o 0
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o NiNNThiNFI
I H I 1 N6-methyl-2-(3-methylbenzofuran-2-
0 0
carboxamido)-5-oxohexanediamide
11-92: o (S)-N1-methy1-5-(3-methylbenzofuran-2-
oN carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-
2-
H
0 0 ((1S,2S,4R)-1,7,7-
0 N NNNH 1 H trimethylbicyclo[2.2.1Theptan-2-
1 I 11
0 0 ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
244
o
11-94: oN (S)-N1-(1-(2-((1R,2R,4S)-
H bicyclo[2.2.1]heptan-2-ylamino)-2-
F
O rFi 0 oxoethyl)-2-oxo-1,2-d i
hydropyrid in-3-y1)-
O N NO-LN,N11=/_L
N6-methy1-2-(3-methylbenzofuran-2-
1 H 0 8
carboxamido)-5-oxohexanediamide
0
11-95: (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-1-
0 N ylamino)-2-oxoethyl)-2-oxo-1,2-
H
0 H 0 d\_..
di hydropyridi n-3-y1)-N6-methy1-2-(3-
ii
0 N N 2-NH methylbenzofuran-2-carboxamido)-5-
1 H
0 0 oxohexanediamide
11-96: 0 (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-
0 -1N
-....)-.. .-- T
ylami no)-2-oxoethyl)-2-oxo-1,2-
0 0 di hydropyridi n-3-y1)-N6-methy1-2-(3-
0 NNNThrNH methylbenzofuran-2-carboxamido)-5-
1 H I 1
0 0 oxohexanediamide
o
11-97: (S)-N1-(1-(2-(bicyclo[2.2.1]hept-5-en-2-
oN
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o 0
di hydropyridi n-3-y1)-N6-methy1-2-(3-
O NJciN 2-Nr NH
I H I 1 methylbenzofuran-2-carboxamido)-5-
o o
oxohexanediamide
o
11-98: 0N (2S)-N1-(1-(2-(bicyclo[2.2.2]octan-2-
H ylami no)-2-oxoethyl)-2-oxo-1,2-
0
N o
H di hydropyridi n-3-y1)-N6-methy1-2-(3-
O iN N II N
H o methylbenzofuran-2-carboxamido)-5-
1 1TIII o
oxohexanediamide
o
11-99: 0)N (S)-N1-methy1-5-(3-methylbenzofuran-2-
H carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
O o ((1R,2R,4R)-1,7,7-
H ii H
O N NN_,1\1 ssµ
tri methyl bicyclo[2.2.1]heptan-2-
1 H I 1 II
0 0 ylamino)ethyl)-1,2-dihydropyridi n-3-
yl)hexanediamide
o
11-100: oN (S)-N1-methy1-5-(3-methylbenzofuran-2-
H carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
O o
O INic illNMIft, 1 ((1R,2R,3R,5S)-
2,6,6-
0 0
tri methyl bicyclo[3.1.1]heptan-3-
H
ylamino)ethyl)-1,2-dihydropyridi n-3-
yl)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
245
o
11-101:
oN (S)-N1-methy1-5-(3-methylbenzofuran-2-
H carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-
2-
o o ((1S,2S,3S,5R)-2,6,6-
o NINII
I H 0 0 trimethylbicyclo[3.1.1]heptan-3-
ylamino)ethyl)-1,2-dihydropyridin-3-
yhhexanediamide
o
11-103: (S)-N1-(1-(2-(4-homoisotwistane-3-
o, )-L
N
H amino)-2-oxoethyl)-2-oxo-1,2-
o 0
H dihydropyridin-3-y1)-N6-methyl-2-(3-
O NiNNThiN
I H I 1 methylbenzofuran-2-carboxamido)-5-
0 0 oxohexanediamide
o
11-104: (S)-N1-(1-(2-(diamantane-1-amino)-2-
Or\i pj
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
o 0
N6-methy1-2-(3-methylbenzofuran-2-
o Nini 2-N( NH
I H I 1 carboxamido)-5-oxohexanediamide
0 0
o
11-105: (S)-N1-(1-(2-(diamantane-4-amino)-2-
0 N
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
0
H 9
NN HN6-methy1-2-(3-methylbenzofuran-2-
0 N
1 N-1
I H
0 0 carboxamido)-5-oxohexanediamide
11-107: 0 0 (S)-N1-(1-(1-adamantylmethyl)-2-oxo-
1,2-
-,....)-L.
H .--
N dihydropyridin-3-y1)-N6-methyl-2-(3-
O 0 methylbenzofuran-2-carboxamido)-
5-
H II
O NJcr N 2-cN oxohexanediamide
I H 1 1
0
11-108: 0 (2S)-N1-(1-((3-hydroxy-1-
0 .--
N adamantypmethyl)-2-oxo-1,2-
H
O 0 dihydropyridin-3-y1)-N6-methy1-2-
(3-
H II
O N N N methylbenzofuran-2-carboxamido)-
5-
I H 1 1
0 oxohexanediamide
OH
11-109: 0 (2S)-N1-(1-((3-bromo-1-
0 .--
N adamantypmethyl)-2-oxo-1,2-
H
O 0 dihydropyridin-3-y1)-N6-methy1-2-
(3-
H II
O 1\iciN2-N methylbenzofuran-2-
carboxamido)-5-
I H 1 1
0 oxohexanediamide
Br
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
246
0
11-110: (S)-N1-(1-(2-adamantylmethyl)-2-oxo-1,2-
0...,)-1-.. ---
N dihydropyridin-3-y1)-N6-methy1-2-(3-
H
0 H 0 methylbenzofuran-2-carboxamido)-5-
0 NNN oxohexanediamide
I H 0 1 1
11-111: 0 0)-L (S)-N1-(1-(2-(2-adamantylamino)-2-
H N oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
N H 011 H N6-methyl-2-(nicotinamido)-5-
I N INThiN
H
0 0 oxohexanediamide
N
0
11-112: 0)N (S)-2-(isonicotinamido)-N1-(1-(2-(2-
-
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
O rH 011 H dihydropyridin-3-y1)-N6-methyl-5-
H
rAN N 'N N oxohexanediamide
I -
N 0 0
0
11-113: OAr\j (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
O r1-1 OH N6-methyl-5-oxo-2-
(pyridazine-4-
N
H
r)-LNI
H- yN N N
carboxamido)hexanediamide
I
.N 0 0
0
11-114: 0)-L (S)-N1-(1-(2-(2-adamantylamino)-2-
H
N oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
O jcH 011 * H N6-methyl-5-oxo-2-
(pyridazine-3-
NN carboxamido)hexanediamide
I H 0 0
-
0
11-115: 0)-LN (S)-N1-cyclopropyl-N6-(1-(2-(2-
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
o 0
H i i H dihydropyridin-3-y1)-5-(3-
O N.iNINfl,rN
I 1-1 0 I I methylbenzofuran-2-carboxamido)-2-
o
oxohexanediamide
o
11-116: (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
o H 0u H (3-methylbenzofuran-2-
carboxamido)-5-
o N N N
H 8
N oxo-N6-pentylhexanediamide
I 0
0
11-117: (S)-N1-allyl-N6-(1-(2-(2-adamantylamino)-
0)-LN
H 2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o o
N
HA H 5-(3-methylbenzofuran-2-carboxamido)-
2-
o N N -1N
oxohexanediamide
I H U
0 0
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
247
o
11-118: (S)-N1-(1-(2-(2-adamantylamino)-2-
0-LNH2 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
o H 0
H (3-methylbenzofuran-2-carboxamido)-5-
0 N 1\1)-LNN
I H I 1 0 oxohexanediamide
0
0
11-119: 0)-1\1 (S)-N1-ally1-5-(benzofuran-2-
L
H carboxamido)-N6-(1-(2-(2-
O H ? H adamantylamino)-2-oxoethy1)-2-
oxo-1,2-
N
0 1 NN
dihydropyridin-3-y1)-2-oxohexanediamide
O 0
0
11-120: OAN (S)-2-(benzofuran-2-carboxamido)-N6-
H isopropyl-N1-(1-(2-(2-adamantylamino)-2-
0
O H ? H oxoethy1)-2-oxo-1,2-
dihydropyridin-3-y1)-5-
o 0 N, 1 NN
oxohexanediamide
0
11-121: o)N L (S)-2-(benzofuran-2-carboxamido)-N6-
H cyclopropyl-N1-(1-(2-(2-adamantylamino)-
NH
0
H 2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
O 0 )L(:) 0 1 NN
5-oxohexanediamide
11-122: 9 140 o (S)-2-(benzofuran-2-carboxamido)-N1-
(1-
H
N (2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
H
O 0 1,2-dihydropyridin-3-y1)-5-oxo-
N6-
0 0 H II
0 NiJciNNrN phenylhexanediamide
I H I 1
0
11-123: 0 (S)-2-(benzofuran-2-carboxamido)-N6-
'Ail 10 benzyl-N1-(1-(2-(2-adamantylamino)-2-
o 0
H oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-5-
o o NL 0 1 H NN
oxohexanediamide
o
11-124: (S)-2-(benzofuran-2-carboxamido)-N1-
(1-
)-LN H2 (2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
H 1,2-dihydropyridin-3-y1)-5-
0
NH )L(:) oxohexanediamide
o o
o
11-125: (S)-2-(2,5-dichlorothiophene-3-
0)-LN H2 carboxamido)-N1-(1-(2-(2-
o 0
H H adamantylamino)-2-oxoethy1)-2-oxo-1,2-
NNN
dihydropyridin-3-y1)-5-oxohexanediamide
S 0 0
CI
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
248
o
11-126: (S)-N1-(1-(2-(2-adamantylamino)-2-
o)-NH2 oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-
o , o
s -
õ, N,)- (4-methyl-2-(trifluoromethyl)thiazole-5-
F3c--- k) pi j)' carboxamido)-5-oxohexanediamide
N 0 0
11-127: o (S)-N1-(1-(2-(2-adamantylamino)-2-
0
NH2 oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-2-
O 0 (1 -methyl-1 H-1 ,2,3-triazole-5-
H H
N, l\iN _ Ji NMI N carboxamido)-5-oxohexanediamide
0 0
0
11-128: (2S)-N1-(1-(2-((1R,2R,4S)-
0)-LN
H bicyclo[2.2.1]heptan-2-ylamino)-2-
o 0
H H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-
NN
CI / 1 hi N )- 1 1 1 "IL (2,5-dichlorothiophene-3-
carboxamido)-
s 0 o
CI N6-methyl-5-oxohexanediamide
o
11-129: oN (2S)-N1-(1-(2-((1R,2R,4S)-
H bicyclo[2.2.1]heptan-2-ylamino)-2-
o Ei 0 H,,d oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
k, N)-LNrN
F3C¨<77 r
Ari 1 N6-methyl-2-(4-methyl-2-
N 0 0
(trifluoromethyl)thiazole-5-carboxamido)-5-
oxohexanediamide
0
11-130: (2S)-N1-(1-(2-((1 R,2R,4S)-
0 )-L
H
N bicyclo[2.2.1]heptan-2-ylamino)-2-
O 11 0 oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
j N H
N-rN-3_ N6-methyl-2-(1 -methyl-1 H-1 ,2,3-
triazole-5-
N
c, 0 FiNci
carboxamido)-5-oxohexanediamide
11-131: 0
(S)-N1-(1-(2-(2-adamantylamino)-2-
0)-Lr\i
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-
3-y1)-
O id H 0 N6-methyl-5-oxo-2-(2H-1,2,3-
tri azole-4-
N
'-lAN
N I H I NI carboxamido)hexanediamide
\N-N 0 0
H
11-132: 0 (S)-N1-(1-(2-(2-adamantylamino)-2-
N
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-
0 0 J H N6-methyl-5-oxo-2-(1H-1,2,3-triazole-
4-
HNEll 1 NTh(N carboxamido)hexanediamide
fel 0 0
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
249
0
11-133: Or\j (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-
3-y1)-
0
H N6-methyl-2-(1 -methyl-1 H-1 ,2,3-
triazole-4-
¨No HN IrICI-O(cIN carboxamido)-5-oxohexanediamide
\W-N '-----
11-134: 0 0 1\1 (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-
0 0
N?L H H N6-methy1-5-oxo-2-(1H-1,2,4-triazole-
3-
I INI ii N(N
carboxamido)hexanediamide
NN 0 0
H
0
11-135: O N (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o 0
id 11 N6-methyl-2-(1 -methyl-1 H-1 ,2,4-
triazole-3-
NAINI 1 N(
carboxamido)-5-oxohexanediamide
NN 0 0
/
0
11-136: O (S)-2-(benzofuran-3-carboxamido)-N1-
(1-
N
H (2-(2-adamantylamino)-2-oxoethyl)-2-
oxo-
0 0
IcLA 11 1,2-dihydropyridin-3-y1)-N6-methy1-5-
o1 INICI 1 Yi oxohexanediamide
0 0
o
11-137: ON (S)-2-(benzo[b]thiophene-3-
carboxamido)-
H N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
o o
H 2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-
s1 NC I NniN
0 0 5-oxohexanediamide
o
11-138 0 -.N (S)-N1-(1-(2-(2-adamantylamino)-2-
)
H oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-
W
N6-methyl-2-(1 -methyl-1 H-pyrazole-3-
NjrH A N
carboxamido)-5-oxohexanediamide
N-N H 0 NH
/
0
11-139 o)-. (S)-N1-(1-(2-(2-adamantylamino)-2-
N
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
H N6-methyl-2-(1 -methyl-1 H-pyrazole-4-
NV NicH-A N.,,N
,NYH 0 8 carboxamido)-5-oxohexanediamide
/
o
11-140 0)N (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
H N6-methyl-2-(1 -methyl-1 H-pyrazole-5-
<.- (j) NicH Ac)NN carboxamido)-5-oxohexanediamide
NIII:H1 0 8
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
250
0
11-141 ON (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
0 0
H ii H N6-methyl-2-(4-methyl-1,2,3-thiadiazole-5-
,sNJc N,N..rN
N, I H carboxamido)-5-oxohexanediamide
\N 0 0
0
11-142 OAN (S)-N1-(1-(2-(2-adamantylamino)-2-
H oxoethy1)-2-oxo-1,2-dihydropyridin-3-
y1)-
/ 0 N_r FNI 0 H N6-methyl-5-oxo-2-(1,2,5-
thiadiazole-3-
NN
NfIrF1 carboxamido)hexanediamide
0
11-143 O N (S)-2-(4-iodo-1 -methyl-1 H-pyrazole-
5-
H carboxamido)-N1 -(1 -(242-
I 0 jcH 0
H adamantylamino)-2-oxoethy1)-2-oxo-1,2-
N N)'
il Nrr\j dihydropyridin-3-y1)-N6-methy1-5-
\ oxohexanediamide
0
11-144 (S)-N1-(1-(2-(2-adamantylamino)-2-
A NH2
oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-2-
0 ,CLN f FNI II
H (1 -methyl-1 H-pyrazole-5-carboxamido)-5-
NN oxohexanediamide
0
11-145 (S)-N1-(1-(2-(2-adamantylamino)-2-
A NH2
oxoethyl)-2-oxo-1,2-d i hydropyrid in-3-y1)-2-
0
H 9 H (4-methyl-1,2,3-thiadiazole-5-
ci i) I(carboxamido)-5-oxohexanediamide
iv 0 0
o
11-146 (S)-2-(benzofuran-2-carboxamido)-N1-(1-
o
NH2
(2-((1 R,2R,4S)-bicyclo[2.2.1]heptan-2-
0
NH9NThry. ylamino)-2-oxoethyl)-2-oxo-1 ,2-
0 N
I I-I 0 0 dihydropyridin-3-y1)-5-
oxohexanediamide
o
11-147 (S)-N1-(1-(2-((1R,2R,4S)-
o
NH2
bicyclo[2.2.1]heptan-2-ylamino)-2-
0
H 9 oxoethyl)-2-oxo-1,2-d i hydropyrid in-
3-y1)-2-
0 N NNThr
I H 0 0 ----1-.- (3-methyl benzofuran-2-carboxa
mido)-5-
oxohexanediamide
o
11-148 (S)-2-(benzofuran-2-carboxamido)-N1-
(1 -
NH2 -i
(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-
o kilj NH ylamino)-2-oxoethyl)-2-oxo-1 ,2-
I FNI 1 Nr
0 0 dihydropyridin-3-y1)-5-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
251
11-149 N H2 (S)-N1-(1-(24(1S,2R,4R)-
0 0
bicyclo[2.2.1]heptan-2-ylamino)-2-
0 o
o ri_i
Nj-LN NH oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-
N
I H 0 0 (3-methyl benzofuran-2-carboxa mido)-5-
oxohexanediamide
o
11-150 NH2 (S)-2-(benzofuran-2-carboxamido)-5-
oxo-
o 0
o
Ni -(2-oxo-1 -(2-oxo-2-((1 R,2S,4R)-1 ,7, 7-
tri methyl bicyclo[2.2.1]heptan-2-
0 N NNThrN1H
I H 0 0 ylamino)ethyl)-1 ,2-dihydropyridi n-3-
yl)hexanediamide
0
11-151 (S)-2-(3-methylbenzofuran-2-
NH2 carboxamido)-5-oxo-N1-(2-oxo-1 -(2-oxo-2-
0 0
((1R,2S,4R)-1 ,7,7-
0 N.r1\12-.N_r NH
I H I I tri methyl bicyclo[2.2.1Theptan-2-
0 0
ylamino)ethyl)-1 ,2-dihydropyridi n-3-
yl)hexanediamide
0
11-152 0N (S)-N1-(1-(2-((1 R,2R,4S)-
H bicyclo[2.2.1]heptan-2-ylamino)-2-
0 jc H 0 H A oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
,s,
N i p 1 ", ri 'c..___1.. N6-methyl-2-(4-
methyl-1 ,2,3-th lad iazole-5-
'N 0 0
carboxamido)-5-oxohexanediamide
0
11-153 ON (S)-N1-(1-(2-((1 R,2R,4S)-
H bicyclo[2.2.1]heptan-2-ylamino)-2-
0 (it N jcErci H H _.. oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-
NN N6-methyl-2-(1 -methyl-1 H-pyrazole-5-
-N H 0 0
N--- \ carboxamido)-5-oxohexanediamide
0
11-154 0 (S)-N1-(1-(2-((1S,2R,4R)-
A N
H bicyclo[2.2.1]heptan-2-ylamino)-2-
0 j(i N El ,NH 0 oxoethy1)-2-oxo-1,2-
dihydropyridin-3-y1)-
jci
F3C¨S_KIII 1 y h N6-methyl-2-(4-methyl-2-
N 0 0
(trifluoromethyl)thiazole-5-carboxamido)-5-
oxohexanediamide
0
11-155 (S)-N1-(1-(24(1S,2R,4R)-
ON
H bicyclo[2.2.1]heptan-2-ylamino)-2-
0( 0
NH oxoethyl)-2-oxo-1,2-d i hydropyrid in-
3-y1)-2-
(2,5-dichlorothiophene-3-carboxamido)-
s 0 0
CI N6-methyl-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
252
0
11-156 0A (S)-N1-(1-(2-((1S,2R,4R)-
N
H obxiColOy20.-22.:10xhoe_it,a2n:d2ih-
yldarmopinyorid-2in--3_yo-
Y [ i P Y )
O 0 '--1
N, 1 1 NI NH
ciRliJ-
N6-methy1-2-(4-methy1-1,2,3-th lad iazole-5-
N 0 0
carboxamido)-5-oxohexanediamide
0
11-157 (S)-N1-(1-(2-((1S,2R,4R)-
0AN
H bicyclo[2.2.1]heptan-2-ylamino)-2-
0 0 oxoethyl)-2-oxo-1,2-d i hydropyrid in-
3-y1)-
id jN NH
Nr'-'-'--7)-Njcr N6-methyl-2-(1-methy1-1H-1,2,3-
triazole-5-
H 0 8
carboxamido)-5-oxohexanediamide
0
11-158 (S)-N1-(1-(24(1S,2R,4R)-
0)-LN
H bicyclo[2.2.1]heptan-2-ylamino)-2-
O 0 T1 H oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
<
H
N ,NH
N if -----)ANcr 1 N6-methyl-2-(1-methy1-1H-pyrazole-5-
N-N 0 0
\ carboxamido)-5-oxohexanediamide
0
11-159 (S)-Ni-methyl-5-(4-methyl-2-
0AN
H (trifluoromethypth iazole-5-carboxamido)-2-
0 0 oxo-N6-(2-oxo-1-(2-oxo-2-((1R,2S,4R)-
S idN if
,NH
F3C--- F\ici
1,7,7-trimethylbicyclo[2.2.1Theptan-2-
N i0 0
ylamino)ethyl)-1,2-dihydropyridi n-3-
yl)hexanediamide
0
11-160 OA N (S)-2-(2,5-dichlorothiophene-3-
H carboxamido)-N6-methy1-5-oxo-N1-(2-oxo-
Lf(0 0 1-(2-oxo-24(1R,2S,4R)-1,7,7-
11
N 1 N'-r NH trimethylbicyclo[2.2.1Th
Cl eptan-2-
S 0 0 ylamino)ethyl)-1,2-dihydropyridi n-3-
yl)hexanediamide
0
11-161 (S)-N1-methy1-5-(4-methy1-1,2,3-
0N
H th lad iazole-5-carboxamido)-2-oxo-N6-(2-
0 0 oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
H II
N
N/S_kANjcr 1 yThr NH tri methyl bicyclo[2.2.1Theptan-2-
N 0 0 ylamino)ethyl)-1,2-dihydropyridi n-3-
yhhexanediamide
0
11-162 (S)-N1-methy1-5-(1-methy1-1H-1,2,3-
Oli
N
H triazole-5-carboxamido)-2-oxo-N6-(2-oxo-
I? H 0 1-(2-oxo-2-((1R,2S,4R)-1,7,7-
N NH tri methyl bicyclo[2.2.1]heptan-2-
NN
H 0 N 0
i\i-N \ ylamino)ethyl)-1,2-dihydropyridi n-3-
yl)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
253
0
11-163 (S)-N1-methy1-5-(1-methy1-1H-pyrazole-5-
0N
H carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-
2-
,it N 0 ((1R,2S,4R)-1,7,7-
ill )-LNr NH trimethylbicyclo[2.2.1]heptan-2-
'El 0 0
N-N \ ylamino)ethyl)-1,2-dihydropyridi n-3-
yl)hexanediamide
o
11-164 N (2S)-2-(4-tert-butyl-1H-pyrrole-3-
o
H carboxamido)-N6-methyl-N1-(1-(2-(1-
o o
adamantylamino)ethyl)-2-oxo-1,2-
N
HN --- FiNjcorill N dihydropyridin-3-yI)-5-
oxohexanediamide
¨
o
11-165 0)-.LNI (2S)-2-(4-cyano-1-methy1-1H-pyrrole-2-
H carboxamido)-N6-methyl-N1-(1-(3-(1-
H o
adamantylamino)propyI)-2-oxo-1,2-
o
NN
\ N H 0 dihydropyridin-3-yI)-5-oxohexanediamide
\
o
11-166 (S)-N1-(1-(3-(2-adamantylamino)-3-
H oxopropy1)-2-oxo-1,2-dihydropyridin-3-y1)-
0 0 0
0?LN N,)-NN 2-(5-methoxyoxazole-2-carboxamido)-N6-
MeON H 0 H
methyl-5-oxohexanediamide
o
11-167 0 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
1\1
H ylamino)-2-oxoethyl)-2-oxo-1,2-
O H o
dihydropyridin-3-y1)-N6-methy1-2-(3-
0 N_iNNNH
I H 0 8 methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
o
11-168 0 ,1 (S)-2-(2-acetyloxazole-4-carboxamido)-
H N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-
o o
j( FdN.rN,IH 2-oxoethy1)-2-oxo-1,2-dihydropyridin-
3-y1)-
J h' N6-methy1-5-oxohexanediamide
o 0
o
o
11-169 0)-.N (S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o o
H II H dihydropyridin-3-y1)-N6-methy1-2-(3-
o NrN,IN,N)
I H 0 8 methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
o
11-170 0 (S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-
1\1
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o 0
H li H dihydropyridin-3-y1)-2-(2-
isopropyloxazole-
oiNNN
5-carboxamido)-N6-methy1-5-
N 0 0
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
254
o
11-171 ON (2S)-2-(benzofuran-2-carboxamido)-N1-
(1-
H 0 (2-(bicyclo[3.2.1]octan-8-ylamino)-2-
o
H
0 N NF1 II
-2N(INI oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
I H 0 0 N6-methyl-5-oxohexanediamide
0
11-172 (2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-
0AN
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 0
H dihydropyridin-3-y1)-2-(3,5-
--- N NH
N N dimethylisoxazole-4-carboxamido)-N6-
L, H
'NJ- 0 0
methyl-5-oxohexanediamide
o
11-173 O (S)-N1-(1-(2-(5-carboxy-2-
NI
H aminoadamantane)-2-oxoethyl)-2-oxo-
1,2-
0
dihydropyridin-3-y1)-N6-methy1-2-(4-
LI H n 0 CO2H methylpyrimidine-5-carboxamido)-5-
1 ''
oxohexanediamide
o
11-174 0 N (2S)-N1-(1-(2-(4-aminoadamantane-N,N-
o
H dimethy1-1-carboxamide)-2-oxoethyl)-2-
o
N 1 N Tr I oxo-1,2-dihydropyridin-3-y1)-N6-methy1-
5-
H
0 0 N-..._ oxo-2-(1,2,3,4-
tetrahydronaphthalene-2-
o
carboxamido)hexanediamide
o
11-175 (S)-24(S)-1,4-diazabicyclo[2.2.2]octane-2-
0N--v
IN 0
H ? carboxamido)-N6-tert-butyl-N1-(1-(2-
(2-
H I H
_1._'_\_j r\j---ANJc,NNThrN adamantylamino)-2-oxoethyl)-2-oxo-1,2-
H
0 0 dihydropyridin-3-y1)-5-
oxohexanediamide
o
11-176 (S)-N1-tert-buty1-5-(1H-indole-3-
H carboxamido)-N6-(1-(2-(2-
0
H
N kil adamantylamino)-2-oxoethyl)-2-oxo-1,2-
H
i
N 0 0 U dihydropyridin-3-y1)-2-oxohexanediamide
H
0
11-177 (S)-N1-tert-butyl-N6-(1-(2-(2-
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
A) jc( Fii 9 dihydropyridin-3-y1)-5-(6-
N 0 rjmor
methylimidazo[2,1-b]thiazole-3-
s
carboxamido)-2-oxohexanediamide
11-178 0 ji)
(S)-2-(benzo[d]thiazole-2-carboxamido)-
ON
H N1-(1-(24(1S,2R,4R)-
bicyclo[2.2.1]heptan-
S):)1111j- 0 N_r NH 2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-cyclopenty1-5-
N 0 0
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
255
11-179 ojNiL) (S)-N1-(1-(24(16,2R,4R)-
H bicyclo[2.2.1]heptan-2-ylamino)-2-
0 EIJ o oxoethy1)-2-oxo-1,2-dihydropyridin-3-
y1)-
N NNH
NAYFINjc N6-cyclopenty1-2-(imidazo[2,1-b]thiazole-
E --N 0 0
6-carboxamido)-5-oxohexanediamide
s
11-180 o 0 n
(S)-N1-(1-(24(16,2R,4R)-
N
H OH 0 bicyclo[2.2.1Theptan-2-ylamino)-2-
0
, 0 N NNThrNH oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-
1-3U
1 H
0 0
N N6-cyclopenty1-2-(4-hydroxy-6-
(trifluoromethoxy)quinoline-3-
carboxamido)-5-oxohexanediamide
11-181 o AJO (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
oN
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o dihydropyridin-3-y1)-2-(cinnoline-3-
o JEI
N,A NH
N 1 N n carboxamido)-N6-cyclohexy1-5-
0 0
N oxohexanediamide
11-182 o n (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
0A1\1
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o di hydropyridin-3-y1)-N6-cyclohexy1-2-(3-
o NiCri 1\1Hj-N,r1 NH
I H 0 0 ethylbenzofuran-2-carboxamido)-5-
oxohexanediamide
11-183
ojN,0 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
H ylamino)-2-oxoethyl)-2-oxo-1,2-
Th o o di hydropyridin-3-y1)-N6-cyclohexy1-2-
(1-
N
N 1 ri_rH NThrNH
j-
ethy1-1H-indole-2-carboxamido)-5-
o o
o( sx )o_ hNeix_ aon-e(2d _i a( bmi ci dy e
11-184 c 1 0
o n [1.1.1]pentan-1-
0
N
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o di hydropyridin-3-y1)-N6-cyclohexy1-2-(2-
0 JEI
Nj. ,NH
methyl-1,8-naphthyridine-3-carboxamido)-
H 0 U 0
NN 5-oxohexanediamide
0
11-185 O (S)-N1-(1-(2-(bicyclo[2.1.1Thexan-1-
N
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 H 0
H di hydropyridin-3-y1)-N6-methy1-5-oxo-
2-
N 'iNj1 N, N (1,2,3,4-tetrahydroquinoline-6-
0
H 0
N carboxamido)hexanediamide
H
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
256
o
11-186 0 N_, (S)-N1-(1-(2-(2-carboxy-2-amino-5-
H (trifluoromethypadamantane)-2-oxoethyly
o o
o NNANJ(N c02H
2-oxo-1,2-dihydropyridin-3-yI)-N6-methyl-
H 1
HN 0 0 CF3 5-oxo-2-(3-oxo-1,2,3,4-
tetrahydroisoquinoline-6-
carboxamido)hexanediamide
o
11-187 0 N (S)-N1-(1-(2-(5-ethyladamantane-2-
o
H amino)-2-oxoethyl)-2-oxo-1,2-
o H
1\1N kll,ANN dihydropyridin-3-y1)-N6-methy1-2-(1,6-
r\il H 0 0
naphthyridine-2-carboxamido)-5-
oxohexanediamide
0
11-188 (S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 F1 0
H dihydropyridin-3-y1)-N6-methy1-2-(2,6-
Njt.,
1 rirN naphthyridine-1-carboxamido)-5-
1 H
N 0 0
oxohexanediamide
0
11-189 (S)-2-(4-amino-1,2,5-oxadiazole-3-
ajt.
'------ N
H carboxamido)-N1-(1-(2-
H2N 0 0 (bicyclo[1.1.1]pentan-1-ylamino)-2-
INI
Y(N Nr.----,irNH
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N I H
b-N 0 0
N6-methyl-5-oxohexanediamide
o
11-190 0}1..N (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
,,,....--
H ylamino)-2-oxoethyl)-2-oxo-1,2-
o o
0 N NH dihydropyridin-3-yI)-2-(6-
\
N 1 H 0 8 (dimethylamino)benzofuran-2-
/ carboxamido)-N6-methy1-5-
oxohexanediamide
o
11-191 ojN--
(S)-2-(2-acetamidothiazole-5-
.,
H carboxamido)-N1-(1-(2-
NH
(bicyclo[1.1.1]pentan-1-ylamino)-2-
s ,,,Jcki
FINI--- DA" N if
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
o N6-methyl-5-oxohexanediamide
o
11-192 0IL.N (S)-N4-(1-(1-(2-(bicyclo[1.1.1]pentan-
1-
.,-
H j ylamino)-2-.oxoethyl)-2-oxo-1,2-
o o
N
0 HN , dihydropyndm-3-ylamino)-6-
H2N HN
/ NrF-IN if
I H 0 0 (methylamino)-1,5,6-trioxohexan-2-yI)-
1 H-
pyrrole-2,4-dicarboxamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
257
0 ________________________________________________________________________
11-193 0 N (S)-N1-(1-(2-(1-acetylamino-4-
0
H
aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
H 0 H
0,t(JAN N,ANThrN
0 dihydropyridin-3-y1)-N6-methyl-5-oxo-
2-(5-
NJ
H sulfamoylfuran-3-
carboxamido)hexanediamide
o
11-194 0 N (S)-2-(benzofuran-5-carboxamido)-N1-
(1-
H
0 H 0
(2-(1-acetylamino-4-aminoadamantane)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
H
0 Nj
H N6-methyl-5-oxohexanediamide
o
11-195 0 N,-- (S)-2-(benzofuran-6-carboxamido)-N1-
(1-
H
0
H (2-(4-aminoadamantane-1-carboxamide)-
NJ,) ENI
0 N 1 N-r 2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-
\ H
0 0 NH2 N6-methyl-5-oxohexanediamide
o
11-196 o- o (S)-N1-(1-(2-(4-aminoadamantane-1-
0 9
H carboxamide)-2-oxoethyl)-2-oxo-1,2-
r[i H
A e N r,j,---,õ,,,N dihydropyridin-3-y1)-N6-methy1-
2-(3-(1-
N-0 H 0 8
NH2 methylcyclopropyI)-1,2,4-oxadiazole-5-
o
carboxamido)-5-oxohexanediamide
0
11-197 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
OAN
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 (.rr\I i 0
H j,. dihydropyridin-3-y1)-N6-methy1-2-(5-
NINI N,r NH
methyl-1,2,4-oxadiazole-3-carboxamido)-
0 0
5-oxohexanediamide
0
11-198 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
0)-LN
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 0 dihydropyridin-3-y1)-N6-methyl-5-oxo-
2-
S/
NNH ANcrEA (1,2,3-thiadiazole-4-
0 8
carboxamido)hexanediamide
0
11-199 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
N
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 H 0 ----)' dihydropyridin-3-y1)-N6-methyl-5-oxo-
2-
N,3,7AN N NH
..----N/ _r H I I (1,2,4-thiadiazole-5-
0 0
carboxamido)hexanediamide
0
11-200 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
OAN
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0 ( id 0 dihydropyridin-3-y1)-N6-methyl-5-oxo-
2-
e-,AN-NThrNH
(1,3,4-thiadiazole-2-
N---N H 0 0
carboxamido)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
258
0
11-201 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
0
N
H ylamino)-2-oxoethyl)-2-oxo-1,2-
0
H ii? dihydropyridin-3-y1)-2-(4-cyclopropy1-
1,2,3-
N, I h' 1 N, MCNH
thiadiazole-5-carboxamido)-N6-methy1-5-
N 0 0
oxohexanediamide
o
11-202 0).N (S)-2-(4-cyclopropy1-1,2,3-
thiadiazole-5-
H carboxamido)-N1-(1-(2-(2-
0 H 0
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N
N',SHI\jjCi N NI dihydropyridin-3-y1)-N6-methy1-5-
N 0 o
oxohexanediamide
o
11-203 0).N (S)-2-(4-isopropyl-1,2,3-thiadiazole-
5-
H carboxamido)-N1-(1-(2-(2-
o 0 r H
H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
r,,,s NfN')N-rN
N I H dihydropyridin-3-y1)-N6-methy1-5-
N 0 0
oxohexanediamide
o
11-204 0)N (S)-2-(4-ethy1-1,2,3-thiadiazole-5-
-.
H carboxamido)-N1-(1-(2-(2-
o 0 H
N'S N( H
N)- ,N adamantylamino)-2-oxoethyl)-2-oxo-1,2-
N IT
dihydropyridin-3-y1)-N6-methyl-5-
N 0 0
oxohexanediamide
o
11-205 0 (S)-2-(4-formy1-1,2,3-thiadiazole-5-
N
H carboxamido)-N1-(1-(2-(2-
0
H
FN11 adamantylamino)-2-oxoethyl)-2-oxo-1,2-
,S
dihydropyridin-3-y1)-N6-methy1-5-
\N H 0
oxohexanediamide
o
o
11-206 (S)-2-(4-(hydroxymethyl)-1,2,3-thiadiazole-
N
H 5-carboxamido)-N1-(1-(2-(2-
N 0 0
H il N H adamantylamino)-2-oxoethyl)-2-oxo-1,2-
NJrN
kr,\ I 1 H 0 c N2-.0 dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
HO
0
11-207 OJt (S)-N1-(1-(2-(1-adamantylamino)-2-
N
H oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
0 H 0 N6-methyl-2-(1-methyl-1H-imidazole-5-
ii
1\l/YN N'l N'r NH
carboxamido)-5-oxohexanediamide
\\ Ki ¨ 0 ...,..,0
'-------IIN
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
259
11-208 0 (S)-N1-(1-(2-(((1 S,2R,5S)-6,6-
dimethylbicyclo[3.1.1]heptan-2-
0 H
21,1AN k-11 yl)methylamino)-2-oxoethyl)-2-oxo-1,2-
N
H 0 0 dihydropyridin-3-y1)-N6-methy1-2-(1-
methyl-1 H-imidazole-2-carboxamido)-5-
oxohexanediamide
11-209 0 (S)-N1-(1-(2-(((1 R,2R,5R)-6,6-
dimethylbicyclo[3.1.1]heptan-2-
o o
HeN FIN NOThr yl)methylamino)-2-oxoethyl)-2-oxo-1,2-
0 o
dihydropyridin-3-y1)-2-(1H-imidazole-4-
carboxamido)-N6-methyl-5-
oxohexanediamide
0
(S)-N1-(1-(2-(3,5-dimethyladamantane-1-
N
amino)-2-oxoethyl)-2-oxo-1 ,2-
11-210 o jcr H 0 dihydropyridin-3-y1)-N6-methyl-2-(1-
NH
Thr methyl-1 H-imidazole-5-carboxamido)-5-
-N
oxohexanediamide
0
(S)-N1-(1-(2-(3,5,7-trimethy1-1-
adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-
11-211 0
dihydropyridin-3-y1)-N6-methy1-2-(1-
N N NH f'''=----/A-r NThr methyl-1 H-imidazole-5-
carboxamido)-5-
--N H 0 0
oxohexanediamide
or a pharmaceutically acceptable salt thereof.
Especially preferred are the following compounds:
11-2: (S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-3: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-4: (S)-2-(3-chlorobenzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-5: (S)-2-(4-bromobenzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-6: (S)-2-(4-bromobenzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-7: (S)-2-(benzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-8: (S)-2-(5-bromobenzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
260
11-9: (S)-2-(1 H-indole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-10: (S)-2-(4,5-difluoro-1 H-indole-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-11 : (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-2-(3-methy1-1H-indole-2-carboxamido)-5-oxohexanediamide
11-12: (S)-2-(1 H-benzo[d]imidazole-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-13: (S)-2-(2,3-dihydro-1 H-indene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-14: (S)--(2-bromo-4-methylthiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-15: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(4-methy1-2-(trifluoromethypthiazole-5-carboxamido)-5-
oxohexanediamide
11-16: (S)-2-(4-bromo-2-(trifluoromethyl)thiazole-5-carboxamido)-N1-(1 -
(242-
adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-17: (S)-2-(2,4-dichlorothiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-18: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-2-(2-
methoxy-4-methylthiazole-5-carboxamido)-N6-methy1-5-oxohexanediamide
11-19: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(4-methy1-2-phenylthiazole-5-carboxamido)-5-oxohexanediamide
11-21: (S)-2-(5-bromo-3-methylthiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-22: (S)-2-(3,5-dibromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-23: (S)-2-(5-bromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-24: (S)-2-(5-chlorothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-25: (S)-2-(5-bromo-3-methylfuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-26: (S)-2-(5-chlorofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-27: (S)-2-(5-chlorothiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-28: (S)-2-(2,5-dichlorothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
261
11-29: (S)-2-(2,5-dibromothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-30: (S)-2-(5-bromothiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-31: (S)-2-(2-chloro-5-methylthiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-32: (S)-2-(2,5-dichlorothiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-33: (S)-2-(2,5-dibromothiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-34: (S)-2-(2-bromo-5-methylthiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-35: (S)-2-(2-bromothiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-36: (S)-2-(2-chlorothiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-37: (S)-2-(2,5-dimethylfuran-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-39: (S)-2-(4-bromothiazole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-40: (S)-2-(4-bromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-41: (S)-2-(4-bromo-3-methylthiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-42: (S)-2-(3-bromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-44: (S)-2-(4-bromo-5-chlorothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-45: (S)-2-(4,5-dibromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-46: (S)-2-(4,5-dibromo-3-methoxythiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-47: (S)-2-(4-bromofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-48: (S)-2-(4,5-dibromofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-49: (S)-2-(4,5-dichlorothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
262
11-50: (S)-24(S)-1-acetylpyrrolidine-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
11-51: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(1-methy1-1H-1,2,3-triazole-5-carboxamido)-5-oxohexanediamide
11-51 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-5-oxo-2-(pyrazine-2-carboxamido)hexanediamide
11-54: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-24(S)-1-methylpyrrolidine-2-carboxamido)-5-oxohexanediamide
11-55: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-5-oxo-24(S)-pyrrolidine-3-carboxamido)hexanediamide
11-56: (S)-24(2S,4S)-4-bromopyrrolidine-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-58: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-5-oxo-24(S)-piperidine-2-carboxamido)hexanediamide
11-59: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-5-oxo-24(R)-piperidine-3-carboxamido)hexanediamide
11-60: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-24(R)-morpholine-3-carboxamido)-5-oxohexanediamide
11-61: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-5-oxo-2-(quinuclidine-3-carboxamido)hexanediamide
11-62: (S)-methyl 3-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-ylcarbamoy1)-5-nitrobenzoate
11-61 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(5-nitronicotinamido)-5-oxohexanediamide
11-64: (S)-5-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-ylamino)-
6-(methylamino)-1,5,6-trioxohexan-2-ylcarbamoyl)nicotinic acid
11-65: (S)-methyl 5-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-ylcarbamoyl)nicotinate
11-68: (S)-N1-(1-(2-(5-hydroxyadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-69: (S)-N1-(1-(2-(5-fluoroadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-70: (S)-N1-(1-(2-(5-chloroadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-71: (S)-N1-(1-(2-(5-bromoadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-72: (S)-N1-(1-(2-(5-methyladamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-71 (S)-N1-(1-(2-(2-carbonitrileadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
di hydropyrid in-3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
263
11-87: (S)-N1-(1-(2-(1-adamantylmethylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-88: (S)-N1-(1-(2-(1-(1-adamantypethanamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-90: (S)-N1-(1-(24(1R,2S,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-
2-oxo-1,2-
dihydropyridin-3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
11-92: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-
(2-oxo-2-
((1S,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-
3-yl)hexanediamide
11-94: (S)-N1-(1-(24(1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-
2-oxo-1,2-
dihydropyridin-3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
11-95: (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-96: (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-97: (S)-N1-(1-(2-(bicyclo[2.2.1]hept-5-en-2-ylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
11-98: (2S)-N1-(1-(2-(bicyclo[2.2.2]octan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-99: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-
(2-oxo-2-
((1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-
3-yl)hexanediamide
11-100: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-
1-(2-oxo-2-
((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1,2-
dihydropyridin-3-yl)hexanediamide
11-101: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-
1-(2-oxo-2-
((1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1,2-
dihydropyridin-3-yl)hexanediamide
11-103: (S)-N1-(1-(2-(4-homoisotwistane-3-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-104: (S)-N1-(1-(2-(diamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-105: (S)-N1-(1-(2-(diamantane-4-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-107: (S)-N1-(1-(1-adamantylmethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-108: (2S)-N1-(14(3-hydroxy-1-adamantypmethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-N6-
methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
264
11-109: (2S)-N1-(14(3-bromo-1-adamantypmethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-N6-methyl-
2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-110: (S)-N1-(1-(2-adamantylmethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-111: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-2-(nicotinamido)-5-oxohexanediamide
11-112: (S)-2-(isonicotinamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-
oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-113: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-5-oxo-2-(pyridazine-4-carboxamido)hexanediamide
11-114: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-5-oxo-2-(pyridazine-3-carboxamido)hexanediamide
11-116: (S)-N1-cyclopropyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-0-5-(3-methylbenzofuran-2-carboxamido)-2-oxohexanediamide
11-116: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(3-
methylbenzofuran-2-carboxamido)-5-oxo-N6-pentylhexanediamide
11-117: (S)-N1-allyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
5-(3-methylbenzofuran-2-carboxamido)-2-oxohexanediamide
11-119: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-119: (S)-N1-ally1-5-(benzofuran-2-carboxamido)-N6-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxohexanediamide
11-120: (S)-2-(benzofuran-2-carboxamido)-N6-isopropyl-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
11-121: (S)-2-(benzofuran-2-carboxamido)-N6-cyclopropyl-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
11-124: (S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-0-5-oxohexanediamide
11-126: (S)-2-(2,5-dichlorothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
11-126: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(4-
methy1-2-(trifluoromethypthiazole-5-carboxamido)-5-oxohexanediamide
11-127: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(1-
methy1-1H-1,2,3-triazole-5-carboxamido)-5-oxohexanediamide
11-129: (2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-methyl-5-
oxohexanediamide
11-129: (2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methyl-2-(4-methyl-2-(trifluoromethypthiazole-5-
carboxamido)-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
265
11-130: (2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1,2-
di hydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-1,2,3-triazole-5-carboxamido)-5-
oxohexanediamide
11-131: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-5-oxo-2-(2H-1,2,3-triazole-4-carboxamido)hexanediamide
11-135: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methy1-2-(1-methy1-1H-1,2,4-triazole-3-carboxamido)-5-oxohexanediamide
11-136: (S)-2-(benzofuran-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-137: (S)-2-(benzo[b]thiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-138 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(1-methy1-1H-pyrazole-3-carboxamido)-5-oxohexanediamide
11-139 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(1-methy1-1H-pyrazole-4-carboxamido)-5-oxohexanediamide
11-140 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(1-methy1-1H-pyrazole-5-carboxamido)-5-oxohexanediamide
11-141 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-2-(4-methy1-1,2,3-thiadiazole-5-carboxamido)-5-oxohexanediamide
11-142 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-
methy1-5-oxo-2-(1,2,5-thiadiazole-3-carboxamido)hexanediamide
11-145 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-2-(4-
methy1-1,2,3-thiadiazole-5-carboxamido)-5-oxohexanediamide
11-146 (S)-2-(benzofuran-2-carboxamido)-N1-(1-(24(1R,2R,4S)-
bicyclo[2.2.1]heptan-2-
ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
11-147 (S)-N1-(1-(24(1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-
2-oxo-1,2-
di hydropyridin-3-y1)-2-(3-methylbenzofuran-2-carboxamido)-5-oxo hexanediamide
11-148 (S)-2-(benzofuran-2-carboxamido)-N1-(1-(24(1S,2R,4R)-
bicyclo[2.2.1]heptan-2-
ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
11-149 (S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-
2-oxo-1,2-
di hydropyridin-3-y1)-2-(3-methylbenzofuran-2-carboxamido)-5-oxo hexanediamide
11-150 (S)-2-(benzofuran-2-carboxamido)-5-oxo-N1-(2-oxo-1-(2-oxo-
24(1R,2S,4R)-1,7,7-
trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yhhexanediamide
11-151 (S)-2-(3-methylbenzofuran-2-carboxamido)-5-oxo-N1-(2-oxo-1-(2-oxo-2-
((1R,2S,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
11-152 (S)-N1-(1-(24(1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-
2-oxo-1,2-
di hydropyridin-3-y1)-N6-methy1-2-(4-methy1-1,2,3-th iadiazole-5-carboxamido)-
5-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
266
11-154 (S)-N1-(1-(2-((1 S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1 ,2-
di hydropyrid in-3-y1)-N6-methy1-2-(4-methy1-2-(trifluoromethyl)th iazole-5-
carboxamido)-5-oxohexanediamide
11-155 (S)-N1-(1-(2-((1 S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1 ,2-
dihydropyridin-3-y1)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-methy1-5-
oxohexanediamide
11-156 (S)-N1-(1-(2-((1 S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1 ,2-
di hydropyrid in-3-y1)-N6-methy1-2-(4-methy1-1,2, 3-th iadiazole-5-
carboxamido)-5-
oxohexanediamide
11-157 (S)-N1-(1-(2-((1 S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1 ,2-
di hydropyrid in-3-y1)-N6-methy1-2-(1 -methyl-1 H-1 ,2,3-triazole-5-
carboxamido)-5-
oxohexanediamide
11-158 (S)-N1-(1-(2-((1 S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1 ,2-
di hydropyrid in-3-y1)-N6-methy1-2-(1 -methyl-1 H-pyrazole-5-carboxamido)-5-
oxohexanediamide
11-159 (S)-N1-methy1-5-(4-methy1-2-(trifluoromethypthiazole-5-carboxamido)-
2-oxo-N6-(2-
oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-
ylamino)ethyl)-1,2-
dihydropyridin-3-yphexanediamide
11-160 (S)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-methy1-5-oxo-N1-(2-
oxo-1-(2-oxo-2-
((1 R,2S,4R)-1 ,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-
3-yl)hexanediamide
11-161 (S)-N1-methy1-5-(4-methy1-1 ,2,3-thiad iazole-5-carboxamido)-2-oxo-
N6-(2-oxo-1 -(2-
oxo-24(1 R,2S,4R)-1 ,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1 ,2-
dihydropyridin-3-yl)hexanediamide
11-162 (S)-N1-methy1-5-(1-methy1-1 H-1 ,2,3-triazole-5-carboxamido)-2-oxo-
N6-(2-oxo-1 -(2-
oxo-24(1 R,2S,4R)-1 ,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1 ,2-
dihydropyridin-3-yl)hexanediamide
11-163 (S)-N1-methy1-5-(1-methy1-1 H-pyrazole-5-carboxamido)-2-oxo-N6-(2-
oxo-1-(2-oxo-2-
((1 R,2S,4R)-1 ,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-
3-yl)hexanediamide
11-167 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-168 (S)-2-(2-acetyloxazole-4-carboxamido)-N1-(1-(2-(bicyclo[1 .1
.1]pentan-1-ylamino)-2-
oxoethyl)-2-oxo-1,2-di hydropyrid in-3-y1)-N6-methy1-5-oxohexaned iamide
11-169 (S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-170 (S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-2-(2-isopropyloxazole-5-carboxamido)-N6-methyl-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
267
11-171 (2S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(bicyclo[3.2.1]octan-8-
ylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-172 (2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-2-(3,5-dimethylisoxazole-4-carboxamido)-N6-methy1-5-oxohexanediamide
11-173 (S)-N1-(1-(2-(5-carboxy-2-aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(4-methylpyrimidine-5-carboxamido)-5-oxohexanediamide
11-174 (2S)-N1 -(1-(2-(4-aminoadamantane-N,N-d imethyl-1 -carboxamide)-2-
oxoethyl)-2-oxo-
1,2-d ihydropyrid in-3-y1)-N6-methyl-5-oxo-2-(1 ,2,3,4-tetrahydronaphthalene-2-
carboxamido)hexanediamide
11-178 (S)-2-(benzo[d]thiazole-2-carboxamido)-N1-(1-(24(1S,2R,4R)-
bicyclo[2.2.1]heptan-2-
ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-cyclopentyl-5-
oxohexanediamide
11-182 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-cyclohexy1-2-(3-ethylbenzofuran-2-carboxamido)-5-oxohexanediamide
11-183 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-cyclohexy1-2-(1-ethy1-1H-indole-2-carboxamido)-5-oxohexanediamide
11-194 (S)-2-(benzofuran-5-carboxamido)-N1-(1-(2-(1-acetylamino-4-
aminoadamantane)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-195 (S)-2-(benzofuran-6-carboxamido)-N1-(1-(2-(4-aminoadamantane-1-
carboxamide)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
11-198 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-5-oxo-2-(1,2,3-thiadiazole-4-carboxamido)hexanediamide
11-199 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-5-oxo-2-(1,2,4-thiadiazole-5-carboxamido)hexanediamide
11-200 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-N6-methy1-5-oxo-2-(1,3,4-thiadiazole-2-carboxamido)hexanediamide
11-201 (S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-
y1)-2-(4-cyclopropy1-1,2,3-thiadiazole-5-carboxamido)-N6-methyl-5-
oxohexanediamide
11-202 (S)-2-(4-cyclopropy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
11-203 (S)-2-(4-isopropy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
11-204 (S)-2-(4-ethy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
11-205 (S)-2-(4-formy1-1 ,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
268
11-206 (S)-244-(hydroxymethyl)-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-
(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-
oxohexanediamide
11-207 (S)-N1-(1-(2-(1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-
methyl-2-(1-methyl-1H-imidazole-5-carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
11-210 dihydropyridin-3-y1)-N6-methy1-241-methyl-1H-imidazole-5-
carboxamido)-5-
oxohexanediamide
(S)-N1 -(1-(2-(3,5,7-trimethyl-1 -adamantylam ino)-2-oxoethyl)-2-oxo-1 ,2-
11-211 dihydropyridin-3-y1)-N6-methy1-241-methyl-1H-imidazole-5-
carboxamido)-5-
oxohexanediamide
or a pharmaceutically acceptable salt thereof.
Method for production of inventive compounds
In some embodiments, the present invention relates to a method for the
synthesis of a
compound of formula (I), especially any compound of the formula (lb):
0
OA N .R6
---.
R7
Ao N H o
)-L ,,
R2 N 1 N L R-
,
H
0 (lb)
The compound of the formula (lb) can be produced and thus, the present
invention relates
to a method for producing the compound of formula (lb) comprising the
following steps in
the following order:
Step 1B: providing a compound 4b
0
Ac0)-L
N - R6
R7
PGNKOH
H 0 4b;
Step 2B: performing coupling reaction of the compound 4b with a compound 5
0
H2N N)-L , L ,
IR'
,
5
to obtain a compound 6b
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
269
0
Ac0 N , R6
i_i R70
PG3Ni\O- ,L.
1 N R3
H 0
6b;
Step 3B: deprotecting an amino protecting group PG3to obtain a compound 7b
0
Ac0)-N , R6
R70
HN rl N , L
1 'R3
0 7b;
Step 4B: performing coupling reaction of the compound 7b with a carboxylic
acid
(R2-CO2H 8) to obtain a compound 9b
0
Ac0)-N L , R6
0 H R70
R2 L
1 N" 'R3
H 0
9b;
Step 5B: performing oxidation reaction of the compound 9b to produce the
compound of the formula (lb)
0
OA .R6
--.. N
0 H R70
R2AN N , N L .
1 R3
H 0
(1b);
wherein L, R2, R3, R6 and R7 have the same meanings as defined above in the
formula (lb), and PG3 is an amino protecting group.
0
AcO N
-L , R6
In the step 5B the chemical warhead precursor i H
i
may be firstly converted to
0 0
HO , R6
N HO N
, R6
' H ; H
i under a basic condition such as treating with K2CO3, and then
0
0)-L N .R6
is converted to the corresponding chemical warhead H
by an oxidation
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
270
method, preferably by using Dess-Martin periodinane (DMP), iodoxybenzoic acid
(IBX),
or hypochlorite/TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) in a polar
solvent, as
described in the chemical examples.
In an alternative route first all protecting groups PG1 and PG2 are
simultaneously removed
and the protecting group PG3 is selectively introduced. Preferably, PG1 and
PG3 are
same.
The term "protecting groups" as used herein refers to commonly used protection
groups
in organic synthesis, preferably for amino and carboxyl groups. PG1, PG3,
and PG5
preferably are suitable protecting groups for amino groups. PG2 and PG4
preferably are
suitable protecting groups for carboxyl groups. Preferably, PG1, PG3, and PG5
may be
selected from the group consisting of or comprising: acetyl, benzoyl,
benzyloxycarbonyl
(Cbz), tert-butylcarbonyl, tert-butyloxycarbonyl (Boc), and
fluorenylmethylenoxy group
(Fmoc). PG2 and PG4 may be selected from the group consisting of or
comprising:
methoxy, ethoxy, isobutoxy, tert-butoxy, benzyloxy; preferably, tert-butoxy
group.
In Step 2B, to promote the coupling reaction with amino group of intermediate
compound, activating reagents are commonly used to activating carboxylic acid
(õPeptide
Coupling Reagents, More than a Letter Soup", Ayman El-Faham and Fernando
Albericio,
Chemical Reviews, 2011, 111(11), p.6557-6602). The activation may be
introduced
separate reaction or in situ reaction. Preferably, any of the following
coupling reagent
can be used to activate carobxylic acid group: BOP (Benzotriazole-1-yl-oxy-
tris-
(dimethylamino)-phosphonium hexafluorophosphate), PyBOP (Benzotriazole-1-yl-
oxy-
tris-pyrrolidino-phosphonium hexafluorophosphate), AOP (7-(Azabenzotriazol-1-
yl)oxy
tris(dimethylamino)phosphonium hexafluorophosphate), PyAOP ((7-Azabenzotriazol-
1-
yloxy)tripyrrolidinophosphonium hexafluorophosphate), TBTU (2-(1H-
Benzotriazole-1-
y1)-1,1,3,3-tetramethylaminium tetrafluoroborate), EEDQ (N-Ethoxycarbony1-2-
ethoxy-
1,2-dihydroquinoline), Polyphosphoric Acid (PPA), DPPA (Diphenyl phosphoryl
azide),
HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-
oxide
hexafluorophosphate), H BTU
(0-Benzotriazol-1-yl-N,N ,N',N'-tetramethyluronium
hexafluorophosphate), HOBt (1-Hyd roxybenzotriazo le),
HOAt (1-Hydroxy-7-
azabenzotriazole), DCC (N,W-Dicyclohexylcarbodiimide), EDC (or EDAC or EDCI, 1-
Ethy1-3-(3-dimethylam inopropyl)carbodi im ide), BOP-C1
(Bis(2-oxo-3-
oxazolidinyl)phosphinic chloride), TFFH
(Tetram ethylfl uo roformam id i n i um
hexafluorophosphate), BroP (Bromo tris(d imethylam ino)
phosphonium
hexafluorophosphate), PyBroP
(Bromo-tris-pyrrolidino-phosphonium
hexafluorophosphate) and CI P
(2-Chloro-1,3-dimethylimidazolidinium
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
271
hexafluorophosphate), or further, similar acting reagents, providing an
activated
intermediate,or a mixture thereof.
Pharmaceutical Composition & Medical Use
Therefore another aspect of the present invention relates to compounds
according to the
general formula (I) as medicine as well as their use in medicine. Especially
preferred is
the use as inhibitors of transglutaminases, in particular transglutaminase 2
(TG2).
Thus the compounds of formula (I) described herein or according to the present
invention
may be administered themselves or in form of a pharmacologically acceptable
salt.
The compounds of the present invention may form of a pharmacologically
acceptable salt
with organic or inorganic acids or bases. Examples of suitable acids for such
acid
addition salt formation are hydrochloric acid, hydrobromic acid, sulfuric
acid, phosphoric
acid, acetic acid, citric acid, oxalic acid, malonic acid, salicylic acid, p-
aminosalicylic acid,
malic acid, fumaric acid, succinic acid, ascorbic acid, maleic acid, sulfonic
acid,
phosphonic acid, perchloric acid, nitric acid, formic acid, propionic acid,
gluconic acid,
lactic acid, tartaric acid, hydroxymaleic acid, pyruvic acid, phenylacetic
acid, benzoic acid,
p-aminobenzoic acid, p-hydroxybenzoic acid, methanesulfonic acid,
ethanesulfonic acid,
nitrous acid, hydroxyethanesulfonic acid, ethylenesulfonic acid, p-
toluenesulfonic acid,
naphthylsulfonic acid, sulfanilic acid, camphorsulfonic acid, china acid,
mandelic acid, o-
methylmandelic acid, hydrogen-benzenesulfonic acid, picric acid, adipic acid,
d-o-
tolyltartaric acid, tartronic acid, (o, m, p)-toluic acid, naphthylamine
sulfonic acid,
trifluoroacetic acid, and other mineral or carboxylic acids well known to
those skilled in
the art. The salts are prepared by contacting the free base form with a
sufficient amount
of the desired acid to produce a salt in the conventional manner. Preferred is
the
mesylate salt, hydrochloride salt and the trifluoroacetate salt and especially
preferred is
the trifluoroacetate salt and the hydrochloride salt.
In the case the inventive compounds bear acidic groups, salts could also be
formed with
inorganic or organic bases. Examples for suitable inorganic or organic bases
are, for
example, NaOH, KOH, NH4OH, tetraalkylammonium hydroxide, lysine or arginine
and the
like. Salts may be prepared in a conventional manner using methods well known
in the
art, for example by treatment of a solution of the compound of the general
formula (I) with
a solution of an acid, selected out of the group mentioned above.
Methods of Use
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
272
In a further aspect of the present invention, the novel compounds according to
the general
formula (I) are used as pharmaceutically active agent, i.e. the compound of
the formula
(I) is used in medicine.
Furthermore, the present invention relates to a pharmaceutical composition
comprising
at least one compound according to the general formula (I), as an active
ingredient or a
pharmacologically acceptable salts thereof as an active ingredient, together
with at least
one pharmacologically acceptable carrier, excipient and/or diluent.
The compounds according to general formula (I) described herein are especially
suitable
for the treatment and prophylaxis of diseases associated with and/or caused by
transglutaminase 2.
Celiac disease, a gluten intolerance is associated with tissue
transglutaminase (TG 2).
Another very important group of indications for tissue transglutaminase
inhibitors are
fibrotic disorders. Fibrotic disorders are characterized by the accumulation
of cross-linked
extracellular matrix proteins. Diabetic nephropathy, cystic fibrosis,
idiopathic pulmonary
fibrosis, kidney fibrosis as well as liver fibrosis belong to the most
important fibrotic
disorders to be addressed with the compounds disclosed.
In the biological example B-I, it is proven that the inventive compounds as
reversible and
irreversible TG inhibitors effectively inhibit the activity of TGs, especially
TG2.
As used herein the term "inhibiting" or "inhibition" refers to the ability of
a compound to
downregulate, decrease, reduce, suppress, inactivate, or inhibit at least
partially the activity
of an enzyme, or the expression of an enzyme or protein.
Therefore, another aspect of the present invention is the use of the inventive
compounds
of the general formula (I), or the pharmaceutical composition thereof as
described in the
treatment or prophylaxis of autoimmune and inflammatory diseases, vascular
diseases,
fibrotic diseases, liver diseases, cholestatic liver diseases, cancer,
neurodegenerative
diseases, ocular diseases, and skin disorders.
Further aspects of the present invention relate to the use of the compounds of
general
formula (I) for the preparation of a pharmaceutical composition useful for
prophylaxis
and/or treatment of autoimmune and inflammatory diseases, vascular diseases,
fibrotic
diseases, liver diseases, cholestatic liver diseases, cancer,
neurodegenerative diseases,
ocular diseases, and skin disorders.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
273
In a further aspect of the present invention, a method for preventing and/or
treating
autoimmune and inflammatory diseases, vascular diseases, fibrotic diseases,
liver
diseases, cholestatic liver diseases, cancer, neurodegenerative diseases,
ocular
diseases, and skin disorders, which method comprises administering to a
subject, in
particular a human, a pharmaceutically effective amount of at least one
compound of the
general formula (I), to prevent and/or treat said autoimmune and inflammatory
diseases,
vascular diseases, fibrotic diseases, liver diseases, cholestatic liver
diseases, cancer,
neurodegenerative diseases, ocular diseases, and skin disorders.
Preferred, the autoimmune and inflammatory diseases comprises multiple
sclerosis,
celiac disease, Duhring-Brocq-disease (dermatitis herpetiformis), gluten
ataxia, gluten
neuropathy, diabetes, rheumatoid arthritis, Graves' disease, inflammatory
bowel disease,
systemic lupus erythematosus psoriasis, and gingivitis;
the vascular diseases comprise atherosclerosis, thrombosis, vascular
stiffness;
the fibrotic diseases affecting the lung, the kidney, the liver, the skin or
the gut like cystic
fibrosis, kidney fibrosis and diabetic nephropathy, intestinal fibrosis,
idiopathic lung
fibrosis, liver fibrosis;
the liver diseases like alcoholic hepatitis, alcoholic steatohepatitis,
nonalcoholic
steatohepatitis, non-alcoholic fatty liver disease, liver cirrhosis,
autoimmune hepatitis or
liver inflammation;
.. the cholestatic liver diseases comprise primary biliary cholangitis and
primary sclerosing
cholangitis;
the cancer comprises glioblastoma, melanoma, pancreatic cancer, renal cell
carcinoma,
meningioma, and breast cancer,
the neurodegenerative diseases comprise Parkinson's disease, Huntington's
disease, or
Alzheimer's disease,
the ocular diseases comprise glaucoma, cataracts, macular degeneration, or
uveitis;
the skin disorders comprise acne, psoriasis, scarring, and skin aging.
More preferred, the compound of the formula (I), or the pharmaceutical
composition
thereof is useful in the treatment or prophylaxis of celiac disease.
Furthermore, the compounds of the general formula (I), can be administered in
form of
their pharmaceutically active salts, optionally using essentially non-toxic
pharmaceutically
acceptable carriers, adjuvants or extenders. Medications are prepared in a
known
manner in a conventional solid or fluid carrier or in extenders and a
conventional
pharmaceutically acceptable adjuvant/expedient in a suitable dose. The
preferred
preparations are provided in an administrable form suitable for oral
application, such as
pills, tablets, film tablets, coated tablets, capsules and powders.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
274
Tablets, film tablets, coated tablets, gelatine capsules and opaque capsules
are the
preferred pharmaceutical formulations. Any pharmaceutical compositions
contains at
least one compound of the general formula (I), and/or pharmaceutically
acceptable salts
thereof in an amount of 5 mg to 500 mg, preferably 10 mg to 250 mg and most
preferred
in an amount of 10 to 100 mg per formulation.
Besides, the object of the present invention also includes pharmaceutical
preparations
for oral, parenteral, dermal, intradermal, intragastric, intracutaneous,
intravascular,
intravenous, intramuscular, intraperitoneal, intranasal, intravaginal,
intrabuccal,
percutaneous, rectal, subcutaneous, sublingual, topic, transdermal or
inhalative
application, containing, in addition to typical vehicles and extenders, a
compound of the
general formula (I), and/or a pharmaceutically acceptable salt thereof as
active
component.
The pharmaceutical compositions of the present invention contain one of the
compounds
of the formula (I) disclosed herein as active component, typically mixed with
suitable
carrier materials, selected with respect to the intended form of
administration, i.e. tablets
to be administered orally, capsules (filled either with a solid, a semi-solid
or a liquid),
powders, orally administrable gels, elixirs, dispersible granulates, syrups,
suspensions
.. and the like in accordance with conventional pharmaceutical practices. For
example, the
compound of the formula (I) can as active agent component be combined with any
oral,
non-toxic, pharmaceutically acceptable, inert carrier, such as lactose,
starch, sucrose,
cellulose, magnesium stearate, dicalcium phosphate, calcium sulfate, talc,
mannitol, ethyl
alcohol (liquid forms) and the like for the oral administration in form of
tablets or capsules.
.. Moreover, suitable binders, lubricants, disintegrants and colorants can be
added to the
mixture if required. Powders and tablets can consist of said inert carriers to
an extent from
about 5% per weight to about 95% per weight of the inventive composition.
Suitable binders include starch, gelatine, natural sugars, sweeteners made of
corn,
natural and synthetic gums, such as acacia gum, sodium alginate,
carboxymethylcellulose, polyethylene glycol and waxes. Possible lubricants for
the use in
said dosage forms include boric acid, sodium benzoate, sodium acetate, sodium
chloride
and the like. Disintegrants include starch, methylcellulose, cyclodextrins,
guar gum and
the like. If required, sweeteners and flavor additives and preservatives can
also be
.. included. Some of the terms used above, namely disintegrants, extenders,
lubricants,
binders and the like are discussed in greater detail below.
Additionally, the compositions of the present invention can be formulated in a
form with
sustained release to provide a controlled release rate of any one or more
components or
active components, in order to optimize the therapeutic effect, i.e. the
inhibitory activity
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
275
and the like. Suitable dosage forms for sustained release include layered
tablets
containing layers with varying degradation rates or controlled release
polymeric matrices
impregnated with the active components and in the form of a tablet or capsule
containing
such impregnated or encapsulated porous polymeric matrices.
Preparations in fluid form include solutions, suspensions and emulsions.
Exemplarily
mentioned are water or water propylene glycol solutions for parenteral
injections or the
addition of sweeteners and opacifiers for oral solutions, suspensions, and
emulsions.
Aerosol preparations suitable for inhalation may include solutions and solids
in the form
of powders which can be combined with a pharmaceutically acceptable carrier,
such as
a compressed inert gas, e.g. nitrogen.
For the preparation of suppositories a low melting wax, such as a mixture of
fatty acid
glycerides, e.g. cocoa butter, is melted firstly and the active component is
homogenously
dispersed therein by stirring or similar mixing operations. The melted
homogenous
mixture is then poured in fitting forms, cooled and thus hardened.
Further preparations in solid form which are to be converted into preparations
in fluid form
for either oral or parenteral administration shortly before use are included.
Such fluid
forms include solutions, suspensions and emulsions.
Furthermore, the compounds of the present invention may be administered via
transdermal application. The transdermal compositions can have the form of
crèmes,
lotions, aerosols and/or emulsions.
The term capsule refers to a special container or casing composed of
methylcellulose,
polyvinyl alcohols or denatured gelatins or starches, in which the active
agents can be
enclosed. Typically, hard shell capsules are prepared from mixtures of bones
and porcine
skin gelatins having comparatively high gel strength. The capsule itself can
contain small
amounts of colorants, opacifiers, softening agents and preservatives.
Tablet means a compressed or cast solid dosage form containing the active
components
with suitable extenders. The tablet can be produced by compressing mixtures or
granulates obtained by wet granulation, dry granulation or compaction, which
are known
to the one skilled in the art.
Oral gels refer to the active components dispersed or solubilized in a
hydrophilic semi-
solid matrix.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
276
Powders for compositions refer to powder mixtures containing the active
components and
suitable extenders which can be suspended in water or juices.
Suitable extenders are substances which usually form the largest part of the
composition
or dosage form. Suitable extenders include sugars such as lactose, sucrose,
mannitol
and sorbitol; starches derived from wheat, corn, rice and potatoes; and
celluloses such
as microcrystalline cellulose. The amount of extenders in the composition can
range from
about 5 to about 95% per weight of the total composition, preferably form
about 25 to
about 75% per weight and further preferred from about 30 to about 60% per
weight.
The term disintegrants refers to materials added to the composition in order
to support
disintegration and release of the medicinal substance. Suitable disintegrants
include
starches, modified starches which are soluble in cold water, such as sodium
carboxymethyl starch; natural and synthetic gums such as locust bean gum,
caraya, guar
gum, tragacanth and agar; cellulose derivatives such as methylcellulose and
sodium
carboxymethylcellulose, microcrystalline celluloses and crosslinked
microcrystalline
celluloses such as croscarmellose sodium; alginates such as alginic acid and
sodium
alginate; clays such as bentonites and foaming mixtures. The amount of
disintegrants
used in the composition can range from about 2 to 20% per weight of the
composition
and further preferred from about 5 to about 10% per weight.
Binders characterize substances binding or "gluing" powders to each other and
they
consequently serve as "glue" in the formulation. Binders add a cohesion starch
which is
already available in the extenders or the disintegrant. Suitable binders
include sugar, such
as sucrose; starches derived from wheat, corn, rice and potatoes; natural gums
such as
acacia gum, gelatine and tragacanth; derivatives of sea weed such as alginic
acid, sodium
alginate and ammonium calcium alginate, cellulose materials such as methyl
cellulose
and sodium carboxymethylcellulose and hydroxypropyl methylcellulose,
polyvinylpyrrolidone and inorganic compounds, such as magnesium aluminium
silicate.
The amount of binders in the composition can range from about 2 to about 20%
per weight
of the total composition, preferably form about 3 to about 10% per weight and
further
preferred from about 3 to about 6% per weight.
The term lubricant refers to a substance added to the dosage form in order to
allow for
the tablet, granulate, etc. to be released from the casting mold or pressing
mold, after
compression, by reducing the friction. Suitable lubricants include metallic
stearates such
as magnesium stearate, calcium stearate or potassium stearate; stearic acid;
waxes with
high melting points and water soluble lubricants such as sodium chloride,
sodium
benzoate, sodium acetate, sodium oleate, polyethylene glycols and D,L-leucine.
Due to
the fact that lubricants have to be present on the surface of the granulates
as well as
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
277
between the granulates and parts of the tablet press they are typically added
during the
last step prior to compression. The amount of lubricants in the composition
can range
from about 0.2 to about 5% per weight of the total composition, preferably
form about 0.5
to about 2% per weight and further preferred from about 0.3 to about 1.5 % per
weight.
Lubricants are materials preventing caking and improving the flow
characteristics of
granulates so that the flow is smooth and uniform. Suitable lubricants include
silicon
dioxide and talc. The amount of lubricants in the composition can range from
about 0.1
to about 5 % per weight of the total composition, preferably form about 0.5 to
about 2 %
per weight.
Colorants are adjuvants coloring the composition or dosage form. Such
adjuvants can
include colorants having food quality which are adsorbed on a suitable
adsorption means,
such as clay or aluminium oxide. The amount of the colorant used can vary from
about
0.1 to about 5% per weight of the composition and preferably from about 0.1 to
about 1%
per weight.
As used herein, a "pharmaceutically effective amount" of a transglutaminase
inhibitor is
the amount or activity effective for achieving the desired physiological
result, either in
cells treated in vitro or in a patient treated in vivo. Specifically, a
pharmaceutical effective
amount is such an amount which is sufficient for inhibiting, for a certain
period of time,
one or more of the clinically defined pathological processes associated with
transglutaminase 2. The effective amount can vary according to the specific
compound
of the formula (I) and additionally depends on a plurality of factors and
conditions related
to the subject to be treated and the severity of the disease. If, for example,
an inhibitor is
to be administered in vivo, factors such as age, weight and health of the
patients as well
as dose reaction curves and data regarding toxicity obtained from preclinical
animal
studies are amongst the data to be considered. If the inhibitor in form of the
compound of
the formula (I) described herein is to be brought in contact with the cells in
vivo, a plurality
of preclinical in vitro studies would be designed in order to determine
parameters such as
absorption, half-life, dose, toxicity, etc. Determining a pharmaceutically
effective amount
fora given pharmaceutically active ingredient is part of the ordinary skills
of the one skilled
in the art.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
278
Examples
Following abbreviations used in the examples have the following meaning.
Boc (tert-butoxycarbonyl), Boc0Su (N-
tert-butoxycarbonyloxy-succinim ide)
DCM (dichloromethane), DMAP (4-(Dimethylamino)-pyridine), TEA (triethylamine),
DMF (dimethylformamide), DMP (Dess-Martin
periodiane), DIPEA
(N-Ethyldiisopropylamine), Glu (glutamic acid), EDC
(1-ethyl-3-(3'-
dimethylaminopropyl)carbodiimide), TFA (trifluoroacetic acid), THF
(tetrahydrofuran),
Et0Ac (ethyl acetate), HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-
triazolo[4,5-
b]pyridinium 3-oxid hexafluorophosphate), HOBt (hydroxybenzotriazole), MTBE
(methyl
tert-butyl ether), tBu (tert-butyl),
Chemical Examples
The following examples are intended to illustrate the invention with selected
compounds
without limiting the protecting scope of the present intellectual property
right on these
concrete examples. It is clear for a person skilled in the art that analogous
compounds
and compounds produced according to analogous synthetic ways fall under the
protecting
scope of the present intellectual property right.
Example II. Synthetic method 11
Scheme 11-1
OH Chloroacetic acid 0
02N Na3PO4 (acl) ...õ..AN 02N N OH
1
0
ZED1657
I2-Adamantylamine
EDC, DIPEA, DMF
10% Pd/C
0
H Me0H 0 H
H2NAN N -. ________ 02N ).L N
1 N
0
0
ZE03906 ZE03905
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
279
1 Preparation of compound ZED1657
0
02N)-L NThrOH
0
2-(3-nitro-2-oxopyridin-1(2H)-yl)acetic acid
Chemical Formula: C7H6N205
Exact Mass: 198.03
Molecular Weight: 198.13
30.0 g (214 mmol) of 2-hydroxy-3-nitropyridine and 40.5 g (2 eq) of
chloroacetic acid were
suspended in 600 mL water. At 40 C, 245 g (3 eq) trisodium phosphate
dodecahydrate
were added, and the reaction was stirred at room temperature overnight. 250 mL
HCI
(32%) were added, and the suspension was stirred for another night at 4 C. The
precipitate was filtered and dried. Yield: 41.2 g, 97% ESI-MS: 199.3 [M+H]
2 Preparation of compound ZED3905
0
02N
Tof
N-(2-adamantyI)-2-(3-nitro-2-oxopyridin-1(2H)-yl)acetamide
Chemical Formula: 017H21N304
Exact Mass: 331.15
Molecular Weight: 331.37
17.0 g (85.8 mmol) of ZED1657, 16.1 g (1 eq) of 2-adamantanamine hydrochloride
and
11.6 g (1 eq) of HOBt were dissolved in 200 mL DMF and 17.9 mL (1.2 eq) DIPEA.
18.1
g (1.1 eq) of 1-ethyl-3-(3'-dimethylaminopropyl)carbodiimide hydrochloride
were added
and the reaction was stirred at room temperature overnight. The solvent was
evaporated,
and the residue was dissolved in 500 mL DCM. The solution was washed with each
200
mL citric acid solution (10%), NaHCO3 solution (10%) and brine. The organic
phase was
dried over Na2SO4, filtered and the solvent was evaporated.
Yield: 24.1 g, 85% ESI-MS: 332.4 [M+H]
3 Preparation of compound ZED3906
0
H2Nj-(
N-IN
0
2-(3-amino-2-oxopyridin-1(2H)-y1)-N-(2-adamantyl)acetamide
Chemical Formula: C17H23N302
Exact Mass: 301.18
Molecular Weight: 301.38
24.2 g (73.0 mmol) of ZED3905 were suspended in 600 mL Me0H before 2.42 g of
palladium (10%) on activated carbon (unreduced) were added. The suspension was
stirred overnight at room temperature under an atmosphere of hydrogen. The
catalyst
was filtered, and the solvent was evaporated. Yield: 15.7 g, 71% ESI-MS: 302.4
[M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
280
Scheme 11-2
0
OH 1. DMF, CH3I OH 1. Methyl AcOAN
Cs2003 isocyanide
2. DMAP, ACN AcOH, DCM
Boc20 2. TFA
Boc, Boc,
Boc,N OH
N CO2tBu N CO2tBu
3. DIBAL-H Boc 3. Boc.20 0
Et20, -78 C DIPEA, DMF
ZED721 ZED3632
N HATU, DMF
o DIPEA
ZED3906
0 0
Ac0j-N Ac0j-LN
0 0
H
0 IRLA
1) TFA, DCM
__________________________________________________________ Boc,NcrN
1NrCr
0 0 2) 3-Methylbenzo[b]- 0 0
furan-2-carboxylic acid
ZED3264 ZED3907
HATU, DIPEA, DMF
K2CO3, Me0H
0 0
H011N
OAN
0 0 Dess-Martin 0 0
0 IRLA periodinane H
N
1-1Cr U\Irr\I oI DMF 1NrCr U\IThrN
0 0 0 0
ZED3266 11-3
Preparation of compound ZED788
0 OMe
Boc,NOtBu
0
(S)-1-tert-butyl 5-methyl 2-(tert-butoxycarbonylamino)pentanedioate
Chemical Formula: C15H27N06
Exact Mass: 317,18
Molecular Weight: 317,38
12.0 g of Boc-L-Glu-OtBu (39.6 mmol) and 7.09 g of cesium carbonate (21.8
mmol, 0.55
eq) were suspended in 100 ml of DMF and stirred for 1 h at room temperature.
2.47 ml
iodomethane (39.6 mmol) we added, and the mixture was stirred at room
temperature
overnight. The solvent was evaporated, and the residue was dissolved in ethyl
acetate
and washed twice with each citric acid solution (10%), NaHCO3 solution (10%)
and brine.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
281
The organic phase was dried over Na2SO4, filtered and the solvent was
evaporated. The
raw product was used without further purification.
Yield: 13.4 g, >100%
ESI-MS: 318.3 [M+H]
Preparation of compound ZED720
00Me
Boo, OtBu
60c 0
(S)-1-tert-butyl 5-methyl 2-(bis(tert-butoxycarbonyl)amino)pentanedioate
Chemical Formula: C201-135N08
Exact Mass: 417,24
Molecular Weight: 417,49
13.4 g of ZED788 (-39,6 mmol) and 986 mg of N,N-dimethy1-4-aminopyridine
(DMAP)
were dissolved in 30 ml of acetonitrile. 17.6 g of di-tert-butyl bicarbonate
(77.1 mmol) in
100 ml of acetonitrile was added and the solution was stirred at room
temperature
overnight. The solvent was evaporated, and the residue was dissolved in ethyl
acetate
and washed twice with each citric acid solution (10%), NaHCO3 solution (10%)
and brine.
The organic phase was dried over Na2SO4, filtered and the solvent was
evaporated. The
raw product was used without further purification.
Yield: 13.7 g, 83%
ESI-MS: 418.3 [M+H]
Preparation of compound ZED721
0 H
Boc,NJC0tBu
Boo 0
(S)-tert-butyl 2-(bis(tert-butoxycarbonyl)amino)-5-oxopentanoate
Chemical Formula: C19H33N07
Exact Mass: 387,23
Molecular Weight: 387,47
13.7 g of ZED720 (32.8 mmol) were dissolved in 200 ml of dry diethyl ether and
cooled
to -78 C under argon atmosphere. 36.1 ml of diisobutylaluminum hydride (1M in
hexane)
were added dropwise and the solution was stirred for 30 min at -78 C before
being
quenched with potassium sodium tartrate (Rochelle salt) solution. The organic
layer was
separated, dried over Na2SO4, filtered, and concentrated to dryness. The raw
product
was used without further purification.
Yield: 13.3 g, >100%
ESI-MS: 388.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
282
4 Preparation of compound ZED3632
0
AcON
Boc ,N (rOH
0
(2S)-5-acetoxy-2-(tert-butoxycarbonylamino)-6-(methylamino)-6-oxohexanoic acid
Chemical Formula: C14H24N207
Exact Mass: 332,16
Molecular Weight: 332,35
.. 15.0 g (38.7 mmol) of the aldehyde (S)-tert-butyl 2-(bis(tert-
butoxycarbonyl)amino)-5-
oxopentanoate (ZED721) were dissolved in 60 mL DCM. At 0 C 2.42 mL (1.05 eq)
methyl
isocyanide and 2.33 mL (1.05 eq) acetic acid were added, and the reaction was
stirred at
room temperature overnight. 75 mL TFA were added, and the reaction was stirred
for
another 3 h. The solvent was evaporated, and the residue was dissolved in 40
mL DMF.
13.2 mL (2 eq) DIPEA and 10.4 g (46.6 mmol) di-tert-butyl dicarbonate in 10 mL
DMF
were added and the reaction was stirred at room temperature overnight. The
solvent was
evaporated, and the residue was dissolved in DCM. After extraction with NaHCO3
solution
(1.05 eq in water), 1.5 eq citric acid was added to the aqueous phase,
followed by re-
extraction with DCM. The organic phase was dried over Na2SO4, filtered and the
solvent
.. was evaporated. The residue was purified by flash chromatography.
Yield: 12.5 g, 95%
ESI-MS: 333.5 [M+H]
5 Preparation of compound ZED3907
0
Ac0j-BocNLN
0
_ JI N
0 0
(5S)-5-(tert-butoxycarbonylamino)-6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-ylamino)-1-(methylamino)-1,6-dioxohexan-2-ylacetate
Chemical Formula: C31H45N508
Exact Mass: 615.33
Molecular Weight: 615.72
19.8 g (59.5 mmol) of ZED3632, 22.6 g (1 eq) HATU and 17.9 g (1 eq) ZED3906
were
dissolved in 400 mL DMF and 20.8 mL DIPEA (2 eq) and stirred at 45 C
overnight. The
solvent was evaporated; the residue was dissolved in 200 mL Et0Ac and washed
twice
with each 150 mL citric acid solution (10%), NaHCO3 solution (10%) and brine.
The
organic phase was dried over Na2SO4, filtered and the solvent was evaporated.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
283
Yield: 27.4 g, 75%
ESI-MS: 616.4 [M+H]
6 Preparation of compound ZED3264
0
Ac0j-N
0 0
0
I NThr NH
I H
0 0
(5S)-6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
ylamino)-1-
(methylamino)-5-(3-methylbenzofuran-2-carboxamido)-1,6-dioxohexan-2-ylacetate
Chemical Formula: C36F-143N508
Exact Mass: 673.31
Molecular Weight: 673.76
480 mg (0.78 mmol) of ZED3907 were dissolved in 4 ml DCM/TFA (1:1) and stirred
at
room temperature for 1 h. The solvent was evaporated, and the residue was
dissolved in
4 ml DMF. 137 mg (1 eq) 3-methylbenzo[b]furan-2-carboxylic acid, 296 mg (1 eq)
HATU
and 272 pl (2 eq) DIPEA were added, and the reaction was stirred at room
temperature
overnight. The solvent was evaporated; the residue was dissolved in 20 mL
Et0Ac and
washed with each 10 mL citric acid solution (10%), NaHCO3 solution (10%) and
brine.
The organic phase was dried over Na2SO4, filtered and the solvent was
evaporated.
Yield: 409 mg, 78%
ESI-MS: 674.4 [M+H]
7 Preparation of compound ZED3266
0
H0)-LN
0
0 Njcr NH N
I H 0 0
(2S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
hydroxy-A6-methyl-2-(3-methylbenzofuran-2-carboxamido)hexanediamide
Chemical Formula: C34H41N507
Exact Mass: 631.30
Molecular Weight: 631.72
409 mg (0.61 mmol) of ZED3264 were dissolved in 5 ml Me0H. 126 mg (1.5 eq)
potassium carbonate were added, and the reaction was stirred at room
temperature for 1
h. The solution was diluted with DCM and washed with water. The organic phase
was
dried over Na2SO4, filtered and the solvent was evaporated.
Yield: 377 mg, 98%
ESI-MS: 632.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
284
8 Preparation of compound 11-3
0
0)-
N
H
0 0
H II H
0 NJCNNThr N
I H 0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C34H39N507
Exact Mass: 629.28
Molecular Weight: 629.70
377 mg (0.60 mmol) of ZED3266 were dissolved in 2 ml DMF. 405 mg (1.6 eq) Dess-
Martin periodinane (DMP) were added and the reaction was stirred at room
temperature
over 2 h. The precipitate was filtered off and the filtrate was evaporated.
The residue was
purified by HPLC.
Yield: 314 mg, 67%
ESI-MS: 630.4 [M+H]
1H-NMR (DMSO-D6, 500 MHz, 8 [ppm]: 1.46 // 1.98 (d lid, 2H //2H, adamantyl-C4-
H2),
1.68 // 1.78 (m, 4H, adamantyl-C4-H2), 1.71 (m, 2H, adamantyl-C1-H), 1.75 (m,
2H,
adamantyl-C6-H2), 1.78 (m, 2H, adamantyl-05-H), 2.05 II 2.16 (m //m, 1H // 1H,
13-CH2),
2.53 (s, 3H, benzofuran-CH3), 2.64 (d, 3H, amide-N-CH3), 2.96 (t, 2H, y-CH2),
3.82 (m,
1H, adamantyl-C2-H), 4.64 (s, 2H, N-CH2), 4.70 (ddd, 1H, a-CH2), 6.25 (t, 1H,
pyridinone-
C5-H), 7.33 (d, 1H, pyridinone-C6-H), 7.36 (t, 1H, benzofuran-CH), 7.51 (t,
1H,
benzofuran-CH), 7.63 (d, 1H, benzofuran-CH), 7.76 (d, 1H, benzofuran-CH), 8.06
(d, 1H,
adamantyl-NH), 8.21 (d, 1H, pyridinone-C4-H), 8.54 (q, 1H, methylamide-NH),
8.87 (d,
1H, a-NH), 9.36 (s, 1H, pyridinone-NH).
13C-NMR (DMSO-D6, 500 MHz, 8 [ppm]: 8.62 (benzofuran-CH3), 24.50 (13-CH2),
25.37
(amide-N-CH3), 26.57 // 26.62 (adamantyl-05-H), 30.83 (adamantyl-C4-H2), 31.35
(adamantyl-C1-H), 33.61 (y-CH2), 36.66 (adamantyl-C4'-H2), 37.01 (adamantyl-C6-
H2),
51.64 (N-CH2), 52.80 (a-CH2), 53.24 (adamantyl-C2-H), 104.51 (pyridinone-05-
H),
111.55 (benzofuran-CH), 121.09 (benzofuran-CH), 121.72 (benzofuran-Cq), 122.53
(pyridinone-C4-H), 123.19 (benzofuran-CH), 127.28 (pyridinone-N-Cq), 127.89
(benzofuran-CH), 129.02 (benzofuran-Cq), 133.27 (pyridinone-C6-H), 142.31
(benzofuran-Cq), 152.68 (benzofuran-Cq), 156.55 (pyridinone-C=0), 159.59
(benzofuran-C=0), 161.32 (C=O-NH-CH3), 165.65 (C=0-adamantylamide), 170.42
(C=O-NH-pyridinone), 198.06 (C=0-methylamide).
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
285
9 Preparation of compound 11-2
0
0 0-L
N
H
0 H
0 1 õ,J(rNFIL
1 N-rN
1 I
il 0 0
(S)-2-( benzofu ran-2-carboxamido)-N 1-(1-(2-(2-adamantylamin o)-2-oxoethyl)-2-
oxo-
1,2-d i hyd ropyrid in-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: 033H37N507
Exact Mass: 615.27
Molecular Weight: 615.68
To the a-hydroxyester precursor of compound 11-2 (242 mg, 0.39 mmol, prepared
by using
benzofuran-2-carboxylic acid in step 6 according to compound ZED3264) in 8 mL
of
.. acetonitrile, 1 mg of TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl, 1 mol%)
were added.
56 mg of calcium hypochlorite (1 eq) were added at 0 C and the reaction
mixture was
stirred at 25 C for 2 h. The suspension was filtered, diluted with ethyl
acetate and washed
with NaHCO3 solution (10%) and brine. The organic phase was dried over Na2SO4,
filtered and the solvent was evaporated. The residue was purified by HPLC.
Yield: 102 mg, 42%
ESI-MS: 616.3 [M+H]
10 Preparation of compound 11-4
0
0 N....,),. ..,--
H
0 0
H
0 1 rrc NH )-L
1 N -rN
1 I
I 0 0
C
(S)-2-(3-chlorobenzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C33H36CIN507
Exact Mass: 649.23
Molecular Weight: 650.12
To the a-hydroxyester precursor of compound 11-4 (124 mg, 0.19 mmol, prepared
by using
3-chlorobenzofuran-2-carboxylic acid in step 6 according to compound ZED3264)
in 4 ml
DMSO, 106 mg of 2-iodoxybenzoic acid (IBX, 2 eq) were added and the reaction
mixture
was stirred at room temperature for 3 h. NaHCO3 solution (10%) was added and
the
suspension was extracted with Et0Ac. The organic phase was dried over Na2SO4,
filtered
and the solvent was evaporated. The residue was purified by HPLC.
Yield: 37 mg, 30% (last step)
ESI-MS: 650.3 / 652.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
286
11 Preparation of compound 11-5
0
0)-LN
0 0
N
o hijCr
0 0
Br
(S)-2-(4-bromobenzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C33H36BrN507
Exact Mass: 693.18
Molecular Weight: 694.57
The synthesis of compound 11-5 was performed according to compound 11-3, using
4-
bromo-1-benzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 69 mg, 72% (last step)
ESI-MS: 694.3 / 696.3 [M+H]
12 Preparation of compound 11-6
0
N
0 H
N
I i'dr
0 0
(S)-2-(benzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: 033H37N506S
Exact Mass: 631.25
Molecular Weight: 631.74
The synthesis of compound 11-6 was performed according to compound 11-3, using
benzo[b]thiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 287 mg, 76% (last step)
ESI-MS: 632.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
287
13 Preparation of compound 11-7
0
N
0 0
/SN
N
N
I H
0 0
Br
(S)-2-(5-bromobenzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C33H36BrN506S
Exact Mass: 709.16
Molecular Weight: 710.64
The synthesis of compound 11-7 was performed according to compound 11-3, using
5-
bromobenzo[b]thiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 145 mg, 59% (last step)
ESI-MS: 710.2 / 712.2 [M+H]
14 Preparation of compound 11-8
0
OAN
0 0
N NH N N
S 0 0
(S)-2-(7-fluorobenzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C33H36FN506S
Exact Mass: 649.24
Molecular Weight: 649.73
The synthesis of compound 11-8 was performed according to compound 11-3, using
7-
fluorobenzo[b]thiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 78 mg, 71% (last step)
ESI-MS: 650.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
288
15 Preparation of compound 11-9
o
o
).N
H
0 õ 0
H H
N kl1)-L
I H I I
0 0
(S)-2-(1H-indole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C33H38N606
Exact Mass: 614.29
Molecular Weight: 614.69
The synthesis of compound 11-9 was performed according to compound 11-3, using
1H-
indole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid in
step 6
(according to ZED3264).
Yield: 57 mg, 69% (last step)
ESI-MS: 615.4 [M+H]
16 Preparation of compound 11-10
0
0......õ..k. ---
N
H
0 (rH 0
H H
N N Nj-LNN
,
I H 0 0
F F
(S)-2-(4,5-difluoro-1H-indole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C33F136F2N606
Exact Mass: 650.27
Molecular Weight: 650.67
The synthesis of compound 11-10 was performed according to compound 11-3,
using 4,5-
difluoro-1H-indole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 47 mg, 65% (last step)
ESI-MS: 651.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
289
17 Preparation of compound 11-11
0 0
I H I I
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-2-(3-methyl-1H-indole-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C34H40N606
Exact Mass: 628.30
Molecular Weight: 628.72
The synthesis of compound 11-11 was performed according to compound 11-3,
using
3-methyl-1H-indole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 58 mg, 72% (last step)
ESI-MS: 629.4 [M+H]
18 Preparation of compound 11-12
0
=)LN
0
NTh'N
41 INT -H I I
0 0
(S)-2-(1H-benzo[d]imidazole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C32F-137N706
Exact Mass: 615.28
Molecular Weight: 615.68
The synthesis of compound 11-12 was performed according to compound 11-3,
using
1H-benzo[d]imidazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 27 mg, 48% (last step)
ESI-MS: 616.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
290
19 Preparation of compound 11-13
0
N
0 0
N N
r\iN
0 0
(S)-2-(2,3-dihydro-1H-indene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C34F141N506
Exact Mass: 615.31
Molecular Weight: 615.72
The synthesis of compound 11-13 was performed according to compound 11-3,
using
2,3-dihydro-1H-indene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 38 mg, 61% (last step)
ESI-MS: 616.4 [M+H]
20 Preparation of compound 11-14
0
0 0
[\11N N
N
Br
0 0
(S)-2-(2-bromo-4-methylthiazole-5-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H35BrN606S
Exact Mass: 674.15
Molecular Weight: 675.59
The synthesis of compound 11-14 was performed according to compound 11-3,
using
2-bromo-4-methylthiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 98 mg, 70% (last step)
ESI-MS: 675.2 /677.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
291
21 Preparation of compound 11-15
0
ON
N
0 0
S Nr N
.1-
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-2-(4-methyl-2-(trifluoromethyl)thiazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C30H36F3N606S
Exact Mass: 664.23
Molecular Weight: 664.70
The synthesis of compound 11-15 was performed according to compound 11-3,
using
4-methyl-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-
2-carboxylic acid in step 6 (according to ZED3264).
Yield: 67 mg, 51% (last step)
ESI-MS: 665.4 [M+H]
22 Preparation of compound 11-16
0
0 0
N
F3C---e_fL N NTh
o o
Br
(S)-2-(4-bromo-2-(trifluoromethyl)thiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H32BrF3N606S
Exact Mass: 728.12
Molecular Weight: 729.57
The synthesis of compound 11-16 was performed according to compound 11-3,
using
4-bromo-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 136 mg, 63% (last step)
ESI-MS: 729.3 / 731.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
292
23 Preparation of compound 11-17
0
OAN
0 r H 0
N
H 0
CI
(S)-2-(2,4-dichlorothiazole-5-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C28F132C12N606S
Exact Mass: 650.15
Molecular Weight: 651.56
The synthesis of compound 11-17 was performed according to compound 11-3,
using
2,4-dichlorothiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
.. in step 6 (according to ZED3264).
Yield: 102 mg, 71% (last step)
ESI-MS: 651.2 / 653.2 [M+H]
24 Preparation of compound 11-18
0
0
0 0
,S NN N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
(2-
methoxy-4-methylthiazole-5-carboxamido)-N6-methy1-5-oxohexanediamide
Chemical Formula: C30H38N607S
Exact Mass: 626.25
Molecular Weight: 626.72
The synthesis of compound 11-18 was performed according to compound 11-3,
using
2-methoxy-4-methylthiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-
2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 56 mg, 63% (last step)
.. ESI-MS: 627.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
293
25 Preparation of compound 11-19
0
OA N
0 õ 0
S N m
H ¨I
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(4-methy1-2-phenylthiazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C35H4.0N606S
Exact Mass: 672.27
Molecular Weight: 672.79
The synthesis of compound 11-19 was performed according to compound 11-3,
using
4-methyl-2-phenylthiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 46 mg, 67% (last step)
ESI-MS: 673.4 [M+H]
26 Preparation of compound 11-20
0
oR
0 0
/S H
N N N
N
H 0
(S)-2-(2,4-dimethylthiazole-5-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-clihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: 030H38N606S
Exact Mass: 610.26
Molecular Weight: 610.72
The synthesis of compound 11-20 was performed according to compound 11-3,
using
2,4-dimethylthiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 216 mg, 77% (last step)
ESI-MS: 611.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
294
27 Preparation of compound 11-21
0
0 jc 0
Br
\I H 0 0
(S)-2-(5-bromo-3-methylthiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C301-1366rN506S
Exact Mass: 673.16
Molecular Weight: 674.61
The synthesis of compound 11-21 was performed according to compound 11-3,
using
5-bromo-3-methylthiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
.. carboxylic acid in step 6 (according to ZED3264).
Yield: 178 mg, 79% (last step)
ESI-MS: 674.2 / 676.2.4 [M+H]
28 Preparation of compound 11-22
0
0)-L
N
0 0
Br N(rN')-LNN
\ I H 0
Br
(S)-2-(3,5-dibromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-clihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H33Br2N506S
Exact Mass: 737.05
Molecular Weight: 739.48
The synthesis of compound 11-22 was performed according to compound 11-3,
using
3,5-dibromothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 89 mg, 67% (last step)
ESI-MS: 738.2 / 740.2 / 742.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
295
29 Preparation of compound 11-23
0
N
0 0
Nj-LNN
Br
\I H 0 0
(S)-2-(5-bromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34BrN506S
Exact Mass: 659.14
Molecular Weight: 660.58
The synthesis of compound 11-23 was performed according to compound 11-3,
using
5-bromothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 141 mg, 72% (last step)
ESI-MS: 660.2 / 662.2 [M+H]
30 Preparation of compound 11-24
0
0
0 0 r H
(S)-2-(5-chlorothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34CIN506S
Exact Mass: 615.19
Molecular Weight: 616.13
The synthesis of compound 11-24 was performed according to compound 11-3,
using
5-chlorothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 117 mg, 78% (last step)
.. ESI-MS: 616.3 /618.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
296
31 Preparation of compound 11-25
0
N
0 0 0
Nj-LN
Br \I Ho I I
0
(S)-2-(5-bromo-3-methylfuran-2-carboxamido)-N1 -(1 -(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-311)-N6-methy1-5-oxohexanediamide
Chemical Formula: C30H36BrN507
Exact Mass: 657.18
Molecular Weight: 658.54
The synthesis of compound 11-25 was performed according to compound 11-3,
using
5-bromo-3-methylfuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 173 mg, 72% (last step)
ESI-MS: 658.2 / 660.2 [M+H]
32 Preparation of compound 11-26
0
0 0 0
CI \I H 0 I I
0
(S)-2-(5-chlorofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34.CIN507
Exact Mass: 599.21
Molecular Weight: 600.06
The synthesis of compound 11-26 was performed according to compound 11-3,
using
5-chlorofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in
step 6 (according to ZED3264).
Yield: 127 mg, 56% (last step)
ESI-MS: 600.3 /602.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
297
33 Preparation of compound 11-27
0
N
0 0
N N
CI / ric
0 0
(S)-2-(5-chlorothiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34.CIN506S
Exact Mass: 615.19
Molecular Weight: 616.13
The synthesis of compound 11-27 was performed according to compound 11-3,
using
5-chlorothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 112 mg, 65% (last step)
ESI-MS: 616.3 /618.3 [M+H]
34 Preparation of compound 11-28
0
OAN
0 0
H u
CI / FNicNNI
0 0
CI
(S)-2-(2,5-dichlorothiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H33Cl2N506S
Exact Mass: 649.15
Molecular Weight: 650.57
The synthesis of compound 11-28 was performed according to compound 11-3,
using
2,5-dichlorothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 319 mg, 77% (last step)
ESI-MS: 650.3 /652.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
298
35 Preparation of compound 11-29
o ON
0
N
Br /
0 0
Br
(S)-2-(2,5-dibromothiophene-3-carboxamido)-N141-(242-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H33Br2N506S
Exact Mass: 737.05
Molecular Weight: 739.48
The synthesis of compound 11-29 was performed according to compound 11-3,
using
2,5-dibromothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 98 mg, 52% (last step)
ESI-MS: 738.2 / 740.2 / 742.2 [M+H]
36 Preparation of compound 11-30
0
0
ll
0 jCH 0
Br
0 0
(S)-2-(5-bromothiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34BrN506S
Exact Mass: 659.14
Molecular Weight: 660.58
The synthesis of compound 11-30 was performed according to compound 11-3,
using
5-bromothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 171 mg, 73% (last step)
ESI-MS: 660.2 /662.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
299
37 Preparation of compound 11-31
0
00
0 0
(S)-2-(2-chloro-5-methylthiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H38C1N606S
Exact Mass: 630.20
Molecular Weight: 631.14
The synthesis of compound 11-31 was performed according to compound 11-3,
using
2-chloro-5-methylthiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 32 mg, 41% (last step)
ESI-MS: 631.3 / 633.3 [M+H]
38 Preparation of compound 11-32
0
ou
0 0
H NH
C1 N--
0 0
CI
(S)-2-(2,5-dichlorothiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C28H32Cl2N606S
Exact Mass: 650.15
Molecular Weight: 651.56
The synthesis of compound 11-32 was performed according to compound 11-3,
using
2,5-dichlorothiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 41 mg, 35% (last step)
ESI-MS: 651.2 /653.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
300
39 Preparation of compound 11-33
0
0
N
0 0
N
H 0 0
Br
(S)-2-(2,5-dibromothiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C28H32Br2N606S
Exact Mass: 738.05
Molecular Weight: 740.46
The synthesis of compound 11-33 was performed according to compound 11-3,
using
2,5-dibromothiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 21 mg, 32% (last step)
ESI-MS: 739.2 / 741.2 / 743.2 [M+H]
40 Preparation of compound 11-34
0
0
ll
0 0
N
N
H 0 0
(S)-2-(2-bromo-5-methylthiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H35BrN606S
Exact Mass: 674.15
Molecular Weight: 675.59
The synthesis of compound 11-34 was performed according to compound 11-3,
using
2-bromo-5-methylthiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 42 mg, 57% (last step)
ESI-MS: 675.2 /677.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
301
41 Preparation of compound 11-35
0
N
0 H 0
N
H 0 0
(S)-2-(2-bromothiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C28H33BrN606S
Exact Mass: 660.14
Molecular Weight: 661.57
The synthesis of compound 11-35 was performed according to compound 11-3,
using
2-bromothiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 66 mg, 46% (last step)
ESI-MS: 661.2 / 663.2 [M+H]
42 Preparation of compound 11-36
0
0
0H 0
C1--</N3A N N
H 0 0
(S)-2-(2-chlorothiazole-4-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C28H33CIN606S
Exact Mass: 616.19
Molecular Weight: 617.12
The synthesis of compound 11-36 was performed according to compound 11-3,
using
2-chlorothiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 74 mg, 58% (last step)
ESI-MS: 617.3 /619.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
302
43 Preparation of compound 11-37
0
N
0 ( H 0
0 0 0
(S)-2-(2,5-dimethylfuran-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C31H39N507
Exact Mass: 593.28
Molecular Weight: 593.67
The synthesis of compound 11-37 was performed according to compound 11-3,
using
2,5-dimethylfuran-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 152 mg, 73% (last step)
ESI-MS: 594.4 [M+H]
44 Preparation of compound 11-38
0
N
0 H
N
1_SeNJ(or u\IN
0
(S)-2-(4,5-dimethylthiazole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C30H38N606S
Exact Mass: 610.26
Molecular Weight: 610.72
The synthesis of compound 11-38 was performed according to compound 11-3,
using
4,5-dimethylthiazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 46 mg, 38% (last step)
ESI-MS: 611.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
303
45 Preparation of compound 11-39
0
0)-LN
0 0
SAN
5_1N H o 0
Br
(S)-2-(4,5-dimethylthiazole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-clihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C28H33-RrN606S
Exact Mass: 660.14
Molecular Weight: 661.57
The synthesis of compound 11-39 was performed according to compound 11-3,
using
4-bromothiazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 84 mg, 68% (last step)
ESI-MS: 661.2 / 663.2 [M+H]
46 Preparation of compound 11-40
0
0)-LN
0 H 0
Nci\iINN
\ H 0
Br
(S)-2-(4-bromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34BrN506S
Exact Mass: 659.14
Molecular Weight: 660.58
The synthesis of compound 11-40 was performed according to compound 11-3,
using
4-bromothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 163 mg, 73% (last step)
ESI-MS: 660.2 /662.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
304
47 Preparation of compound 11-41
0
0)L
N
H
0 ti 0
H
S
, NjcNNN
\I H 0 0
Br
(S)-2-(4-bromo-3-methylthiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C301-136BrN506S
Exact Mass: 673.16
Molecular Weight: 674.61
The synthesis of compound 11-41 was performed according to compound 11-3,
using
4-bromo-3-methylthiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 114 mg, 68% (last step)
ESI-MS: 674.2 / 676.2 [M+H]
48 Preparation of compound 11-42
0
0 N
.A
H
0 0
H
N jcr N ).L N
\I H 0 0
Br
(S)-2-(3-bromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34.BrN506S
Exact Mass: 659.14
Molecular Weight: 660.58
The synthesis of compound 11-42 was performed according to compound 11-3,
using
3-bromothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 149 mg, 76% (last step)
ESI-MS: 660.2 /662.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
305
49 Preparation of compound 11-43
ON
0 0
N
\ hIjorH
0
CI
(S)-2-(3-chloro-4-methylthiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C301-136CIN506S
Exact Mass: 629.21
Molecular Weight: 630.15
The synthesis of compound 11-43 was performed according to compound 11-3,
using
3-chloro-4-methylthiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-
2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 110 mg, 64% (last step)
ESI-MS: 630.3 / 632.3 [M+H]
50 Preparation of compound 11-44
0
0 0
N
0 jcH
N
N
\ I H 0 0
Br
(S)-2-(4-bromo-5-chlorothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H33BrCIN506S
Exact Mass: 693.10
Molecular Weight: 695.02
The synthesis of compound 11-44 was performed according to compound 11-3,
using
4-bromo-5-chlorothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 126 mg, 59% (last step)
ESI-MS: 694.2 / 696.2 / 698.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
306
51 Preparation of compound 11-45
o
H 0
Br \I H 0 0
Br
(S)-2-(4,5-dibromothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H33Br2N506S
Exact Mass: 737.05
Molecular Weight: 739.48
The synthesis of compound 11-45 was performed according to compound 11-3,
using
4,5-dibromothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 89 mg, 51% (last step)
ESI-MS: 738.2 / 740.2 / 742.2 [M+H]
52 Preparation of compound 11-46
o
0
N
NjcH 0
l\i)LI N
Br \I H 0 0
OMe
Br
(S)-2-(4,5-dibromo-3-methoxythiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C301-135Br2N507S
Exact Mass: 767.06
Molecular Weight: 769.50
The synthesis of compound 11-46 was performed according to compound 11-3,
using
4,5-dibromo-3-methoxythiophene-2-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 59 mg, 40% (last step)
ESI-MS: 768.2 / 770.2 / 772.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
307
53 Preparation of compound 11-47
0
0 Li 0
0
\ I H 0 0
Br
(S)-2-(4-bromofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H34BrN507
Exact Mass: 643.16
Molecular Weight: 644.51
The synthesis of compound 11-47 was performed according to compound 11-3,
using
4-bromofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in
.. step 6 (according to ZED3264).
Yield: 90 mg, 69% (last step)
ESI-MS: 644.3 / 646.3 [M+H]
54 Preparation of compound 11-48
0
0 hi 0
0
N(-r
Br N
\ H 0
Br
(S)-2-(4,5-dibromofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C29H33Br2N507
Exact Mass: 721.07
Molecular Weight: 723.41
The synthesis of compound 11-48 was performed according to compound 11-3,
using
4,5-dibromofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 72 mg, 56% (last step)
ESI-MS: 722.2 / 724.2 / 726.2 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
308
55 Preparation of compound 11-49
0
N
0 0
N )LNN
CI
\I H I
0 0
CI
(S)-2-(4,5-dichlorothiophene-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethy1)-2-oxo-1,2-clihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H33Cl2N506S
Exact Mass: 649.15
Molecular Weight: 650.57
The synthesis of compound 11-49 was performed according to compound 11-3,
using
4,5-dichlorothiophene-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
__ acid in step 6 (according to ZED3264).
Yield: 55 mg, 48% (last step)
ESI-MS: 650.3 / 652.3 [M+H]
56 Preparation of compound 11-50
)'(N
0
0
Nj_ N NH J-(i NN
H 0 0
(S)-2-((S)-1-acetylpyrrolidine-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C31H42N607
Exact Mass: 610.31
Molecular Weight: 610/0
The synthesis of compound 11-50 was performed according to compound 11-3,
using
(S)-1-acetylpyrrolidine-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 190 mg, 78% (last step)
ESI-MS: 611.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
309
57 Preparation of compound 11-51
0
C)AN
0 0
NH
NThr N
N 0 0
N".
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-2-(1-methyl-1 H-1,2,3-triazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C281--136N806
Exact Mass: 580.28
Molecular Weight: 580.64
The synthesis of compound 11-51 was performed according to compound 11-3,
using
1-methyl-1H-1,2,3-triazole-5-carboxylic acid instead of 3-methylbenzo[b]fu ran-
2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 28 mg, 35% (last step)
ESI-MS: 581.4 [M+H]
58 Preparation of compound 11-52
0
0)-LN
0 N H
N
HN I
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-5-oxo-2-(2H-tetrazole-5-carboxamido)hexanediamide
Chemical Formula: C26H33N906
Exact Mass: 567.26
Molecular Weight: 567.60
The synthesis of compound 11-52 was performed according to compound 11-3,
using
2H-tetrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 23 mg, 31% (last step)
ESI-MS: 568.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
310
59 Preparation of compound 11-53
0
OAN
0 H 0
_
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methyl-5-oxo-2-(pyrazine-2-carboxamido)hexanediamide
Chemical Formula: C29F135N706
Exact Mass: 577.26
Molecular Weight: 577.63
The synthesis of compound 11-53 was performed according to compound 11-3,
using
pyrazine-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid
in step 6
(according to ZED3264).
Yield: 79 mg, 74% (last step)
ESI-MS: 578.3 [M+H]
60 Preparation of compound 11-54
o
0
N
H
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-2-((S)-1-methylpyrrolidine-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C30F142N606
Exact Mass: 582.32
Molecular Weight: 582.69
The synthesis of compound 11-54 was performed according to compound 11-3,
using
(S)-1-methylpyrrolidine-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 68 mg, 82% (last step)
ESI-MS: 583.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
311
61 Preparation of compound 11-55
0
N
0 0
H
NN
I I
HN¨ 0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-5-oxo-24(S)-pyrrolidine-3-carboxamido)hexanediamide
Chemical Formula: C29F140N606
Exact Mass: 568.30
Molecular Weight: 568.66
The synthesis of compound 11-55 was performed according to compound 11-3,
using
(S)-1-Boc-pyrrolidine-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264). The final product was obtained by
deprotection
(DCM/TFA) as described above and purified by HPLC.
Yield: 43 mg, 79% (last step)
ESI-MS: 569.4 [M+H]
62 Preparation of compound 11-56
oN
0 0
JC
0 N
I I
0
(S)-2-((2S,4S)-4-bromopyrrolidine-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C291-139BrN606
Exact Mass: 646.21
Molecular Weight: 647.56
The synthesis of compound 11-56 was performed according to compound 11-3,
using
(2S,4S)-1-Boc-4-bromopyrrolidine-2-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264). The final product was
obtained by
deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 45 mg, 73% (last step)
ESI-MS: 647.3 / 649.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
312
63 Preparation of compound 11-58
0
o*
H 9
N
- N N
= H
0 0
(S)-N1-(1-(242-adamantylamino)-2-oxoethyl)-2-oxo-1,2-clihydropyridin-
3-y1)-N6-methyl-5-oxo-2-((S)-piperidine-2-carboxamido)hexanediamide
Chemical Formula: C30F142N606
Exact Mass: 582.32
Molecular Weight: 582.69
The synthesis of compound 11-58 was performed according to compound 11-3,
using (S)-
1-Boc-piperidine-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264). The final product was obtained by deprotection
(DCM/TFA) as described above and purified by HPLC.
Yield: 53 mg, 86% (last step)
ESI-MS: 583.4 [M+H]
64 Preparation of compound 11-59
0
o õA.
N
0 H 0
H 0 N
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-5-oxo-2-((R)-piperidine-3-carboxamido)hexanediamide
Chemical Formula: C30F142N606
Exact Mass: 582.32
Molecular Weight: 582.69
The synthesis of compound 11-59 was performed according to compound 11-3,
using (R)-
1-Boc-piperidine-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264). The final product was obtained by deprotection
(DCM/TFA) as described above and purified by HPLC.
Yield: 43 mg, 77% (last step)
ESI-MS: 583.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
313
65 Preparation of compound 11-60
0
N
H 0
N
N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-2-((R)-morpholine-3-carboxamido)-5-oxohexanediamide
Chemical Formula: C29H4.0N607
Exact Mass: 584.30
Molecular Weight: 584.66
The synthesis of compound 11-60 was performed according to compound 11-3,
using (R)-
4-Boc-morpholine-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264). The final product was obtained by deprotection
(DCM/TFA) as described above and purified by HPLC.
Yield: 67 mg, 85% (last step)
ESI-MS: 585.4 [M+H]
66 Preparation of compound 11-61
0
N
N- H
N
JI N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methyl-5-oxo-2-(quinuclidine-3-carboxamido)hexanediamide
Chemical Formula: C321-144N606
Exact Mass: 608.33
Molecular Weight: 608.73
The synthesis of compound 11-61 was performed according to compound 11-3,
using
quinuclidine-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 24 mg, 54% (last step)
ESI-MS: 609.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
314
67 Preparation of compound 11-62
0 0
02N N
NN
I I
0 0
CO2Me
(S)-methyl 3-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-ylcarbamoy1)-5-nitrobenzoate
Chemical Formula: C331-138N6010
Exact Mass: 678.26
Molecular Weight: 678.69
The synthesis of compound 11-62 was performed according to compound 11-3,
using
mono-methyl 5-nitroisophthalate instead of 3-methylbenzo[b]furan-2-carboxylic
acid in
step 6 (according to ZED3264).
Yield: 57 mg, 66% (last step)
ESI-MS: 679.3 [M+H]
68 Preparation of compound 11-63
0
0
N
0 0
H
N)-L
N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-2-(5-nitronicotinamido)-5-oxohexanediamide
Chemical Formula: 030F-135N708
Exact Mass: 621.25
Molecular Weight: 621.64
The synthesis of compound 11-63 was performed according to compound 11-3,
using 5-
nitronicotinic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid in step
6 (according
to ZED3264).
Yield: 76 mg, 65% (last step)
ESI-MS: 622.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
315
69 Preparation of compound 11-64
0
N
0 0
H
HO2CLNJ(rN
N
0 0
(S)-5-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
ylamino)-
6-(methylamino)-1,5,6-trioxohexan-2-ylcarbamoyl)nicotinic acid
Chemical Formula: C31F136N608
Exact Mass: 620.26
Molecular Weight: 620.65
The synthesis of compound 11-64 was performed according to compound 11-3,
using 3,5-
pyridinedicarboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid
in step 6
(according to ZED3264).
Yield: 16 mg, 52% (last step)
ESI-MS: 621.3 [M+H]
70 Preparation of compound 11-65
0
0
0 0
H JL
Me02CNJc N
0 0
(S)-methyl 5-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-ylcarbamoyl)nicotinate
Chemical Formula: C32F138N608
Exact Mass: 634.28
Molecular Weight: 634.68
The synthesis of compound 11-65 was performed according to compound 11-3,
using 5-
(methoxycarbonyl)nicotinic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in
step 6 (according to ZED3264).
Yield: 34 mg, 62% (last step)
.. ESI-MS: 635.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
316
71 Preparation of compound 11-66
0
0 =)0
I N Nj-L
N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-2-(6-methylimidazo[2,1-1D]thiazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C31 H37N706S
Exact Mass: 635.25
Molecular Weight: 635.73
The synthesis of compound 11-66 was performed according to compound 11-3,
using 6-
methylimidazo[2,1-b]thiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 45 mg, 37% (last step)
ESI-MS: 636.4 [M+H]
72 Preparation of compound 11-67
0
OA
N
OHO I
0 NN
I H
0 0
(S)-N1-(1-(2-(2-adamantyl(methyl)amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-yI)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35 H41 N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 11-67 was performed according to compound 11-3,
using
N-methyl-2-adamantanamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 66 mg, 45% (last step)
ESI-MS: 644.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
317
73 Preparation of compound 11-68
0
0,)1,
N
0 JCH 0
0 N Nj-L
N ,
I H
0 0 OH
(S)-N1-(1-(2-(5-hydroxyadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C341-130\1508
Exact Mass: 645.28
Molecular Weight: 645.70
The synthesis of compound 11-68 was performed according to compound 11-3,
using 5-
hydroxy-2-adamantanamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 21 mg, 34% (last step)
ESI-MS: 646.4 [M+H]
74 Preparation of compound 11-69
0
N
0 0
0 N
N N I H
0 0
(S)-N1-(1-(2-(5-fluoroadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C34H38FN507
Exact Mass: 647.28
Molecular Weight: 647.69
The synthesis of compound 11-69 was performed according to compound 11-3,
using 5-
fluoro-2-adamantanamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 48 mg, 57% (last step)
ESI-MS: 648.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
318
75 Preparation of compound 11-70
0
OAN
0 0
o il(r
0 0 CI
(S)-N1-(1-(2-(5-chloroadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: 034.H380IN507
Exact Mass: 663.25
Molecular Weight: 664.15
The synthesis of compound 11-70 was performed according to compound 11-3,
using 5-
chloro-2-adamantanamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 45 mg, 35% (last step)
ESI-MS: 664.3 / 666.3 [M+H]
76 Preparation of compound 11-71
0
)-L
0 0
0 Nj-L
I H
0 0 Br
(S)-N1-(1-(2-(5-bromoadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C34.H38BrN507
Exact Mass: 707.20
Molecular Weight: 708.60
The synthesis of compound 11-71 was performed according to compound 11-3,
using 5-
bromo-2-adamantanamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 31 mg, 34% (last step)
ESI-MS: 708.3 / 710.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
319
77 Preparation of compound 11-72
0
oU
0 0
0 N
N NN
1 H I
0 0
(S)-N1-(1-(2-(5-methyladamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35H41N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 11-72 was performed according to compound 11-3,
using
5-methyl-2-adamantanamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 68 mg, 54% (last step)
ESI-MS: 644.4 [M+H]
78 Preparation of compound 11-73
0
o-JN
0 0 H CN
0
, N NrN
I H I
0 0
(S)-N1-(1-(2-(2-carbonitrileadamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35H38N607
Exact Mass: 654.28
Molecular Weight: 654.71
The synthesis of compound 11-73 was performed according to compound 11-3,
using
2-aminoadamantane-2-carbonitrile instead of 2-adamantanamine in step 2
(according to
ZED3905).
Yield: 26 mg, 46% (last step)
ESI-MS: 655.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
320
79 Preparation of compound 11-74
0
0 JCH 0
H CO2Me
0 N N
N ,
I H 0 0
(S)-N1-(1-(2-(2-methyl adamantane-2-carboxylate-2-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C36H41N509
Exact Mass: 687.29
Molecular Weight: 687.74
The synthesis of compound 11-74 was performed according to compound 11-3,
using 2-
methyl 2-aminoadamantane-2-carboxylate instead of 2-adamantanamine in step 2
(according to ZED3905).
Yield: 38 mg, 61% (last step)
ESI-MS: 688.4 [M+H]
80 Preparation of compound 11-87
0
0 A
N
0 0
0 N
N
NrN
I H 0 0
(S)-N1-(1-(2-(1-adamantylmethylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35H41N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 11-87 was performed according to compound 11-3,
using 1-
adamantanemethylamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 43 mg, 53% (last step)
ESI-MS: 644.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
321
81 Preparation of compound 11-88
0
N
0 0
N
o i'dC
0 0
(S)-N1-(1-(2-(1-(1-adamantyl)ethanamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35 H41 N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 11-88 was performed according to compound 11-2,
using 1-
rimantadine instead of 2-adamantanamine in step 2 (according to ZED3905).
Yield: 31 mg, 41% (last step)
ESI-MS: 644.4 [M+H]
82 Preparation of compound 11-90
0
0)-L N
0 0
N NH
oI i'dr
0 0
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31 H35N 507
Exact Mass: 589.25
Molecular Weight: 589.64
The synthesis of compound 11-90 was performed according to compound 11-3,
using ( )-
endo-2-norbornylamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 67 mg, 65% (last step)
ESI-MS: 590.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
322
83 Preparation of compound 11-92
0
0)LN
0 0
o
N iljCr NnrN
0 0
(S)-N1-methyl-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
((1R,2S,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yOhexanediamide
Chemical Formula: 034 H41 N507
Exact Mass: 631.30
Molecular Weight: 631.72
The synthesis of compound 11-92 was performed according to compound 11-3,
using (R)-
(+)-bornylamine instead of 2-adamantanamine in step 2 (according to ZED3905).
Yield: 52 mg, 66% (last step)
ESI-MS: 632.5 [M+H]
84 Preparation of compound 11-94
0
oUN
0 iCH 0
0 N
N
I H 0 0
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: 031 H35N507
Exact Mass: 589.25
Molecular Weight: 589.64
The synthesis of compound 11-94 was performed according to compound 11-3,
using exo-
2-aminonorbornane instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 62 mg, 68% (last step)
ESI-MS: 590.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
323
85 Preparation of compound 11-95
0
0)LN
0 rHH0
0 N NNNH
,
I H 0 0
(S)-N1-(1-(2-(bicyclo[2.2.Theptan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31H35N507
Exact Mass: 589.25
Molecular Weight: 589.64
The synthesis of compound 11-95 was performed according to compound 11-3,
using
bicyclo[2.2.1]heptan-1-ylamine instead of 2-adamantanamine in step 2
(according to
ZED3905).
Yield: 14 mg, 32% (last step)
ESI-MS: 590.4 [M+H]
86 Preparation of compound 11-96
0
CDN
0 JCH 0
0 NANr NH
, N
I H 0 0
(S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31H35N507
Exact Mass: 589.25
Molecular Weight: 589.64
The synthesis of compound 11-96 was performed according to compound 11-3,
using
bicyclo[2.2.1]heptan-7-ylamine instead of 2-adamantanamine in step 2
(according to
ZED3905).
Yield: 36 mg, 53% (last step)
ESI-MS: 590.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
324
87 Preparation of compound 11-97
0
N
0 0
0 N NH
r\ir
I H
0 0
(S)-N1-(1-(2-(bicyclo[2.2.1]hept-5-en-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C31H33N507
Exact Mass: 587.24
Molecular Weight: 587.62
The synthesis of compound 11-97 was performed according to compound 11-3,
using
bicyclo[2.2.1]hept-5-en-2-amine instead of 2-adamantanamine in step 2
(according to
ZED3905).
Yield: 21 mg, 44% (last step)
ESI-MS: 588.4 [M+H]
88 Preparation of compound 11-98
0 0
0
N
N MN
0 0 11
(2S)-N1-(1-(2-(bicyclo[2.2.2]octan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C32H37N507
Exact Mass: 603.27
Molecular Weight: 603.67
The synthesis of compound 11-98 was performed according to compound 11-3,
using
bicyclo[2.2.2]oct-2-ylamine instead of 2-adamantanamine in step 2 (according
to
ZED3905).
Yield: 25 mg, 41% (last step)
ESI-MS: 604.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
325
89 Preparation of compound 11-99
0
N
0 0
0
N
I H
0 0
(S)-N1-methyl-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
((1R,2R,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
y1)hexanediamide
Chemical Formula: C34H41N507
Exact Mass: 631.30
Molecular Weight: 631.72
The synthesis of compound 11-99 was performed according to compound 11-3,
using (R)-
(-)-isobornylamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 27 mg, 48% (last step)
ESI-MS: 632.5 [M+H]
90 Preparation of compound 11-100
0
ou
0 0
N
0 0
(S)-N1-methyl-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
((1R,2R,3R,5S)-
2,6,6-trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1,2-dihydropyridin-3-
y1)hexanediamide
Chemical Formula: C34H41N507
Exact Mass: 631.30
Molecular Weight: 631.72
The synthesis of compound 11-100 was performed according to compound 11-3,
using
(1R,2R,3R,5S)-(-)-isopinocampheylamine instead of 2-adamantanamine in step 2
(according to ZED3905).
Yield: 17 mg, 39% (last step)
ESI-MS: 632.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
326
91 Preparation of compound 11-101
0
0
0
0 Njc NHJN NH
(S)-N1-methyl-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-
((1S,2S,3S,5R)-
2,6,6-trimethylbicyclo[3.1.1]heptan-3-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
Chemical Formula: C34H41N507
Exact Mass: 631.30
Molecular Weight: 631.72
The synthesis of compound 11-101 was performed according to compound 11-3,
using
(1S,2S,3S,5R)-(+)-isopinocampheylamine instead of 2-adamantanamine in step 2
(according to ZED3905).
Yield: 25 mg, 41% (last step)
ESI-MS: 632.5 [M+H]
92 Preparation of compound 11-103
0
0
N
0
H
N
oI hijC
0 0
(S)-N1-(1-(2-(4-homoisotwistane-3-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: 035H41N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 11-103 was performed according to compound 11-3,
using 3-
amino-4-homoisotwistane instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 12 mg, 28% (last step)
ESI-MS: 644.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
327
93 Preparation of compound 11-104
0
0)-L
N
0 H 9
o
0 0
(S)-N1-(1-(2-(diamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C38H4.3N507
Exact Mass: 681.32
Molecular Weight: 681.78
The synthesis of compound 11-104 was performed according to compound 11-3,
using 1-
aminodiamantane instead of 2-adamantanamine in step 2 (according to ZED3905).
Yield: 17 mg, 35% (last step)
ESI-MS: 682.5 [M+H]
94 Preparation of compound 11-105
0
0
N
0 H LC)
0 N N
I H 0 0
(S)-N1-(1-(2-(diamantane-4-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C38H4.3N507
Exact Mass: 681.32
Molecular Weight: 681.78
The synthesis of compound 11-105 was performed according to compound 11-3,
using 4-
aminodiamantane instead of 2-adamantanamine in step 2 (according to ZED3905).
Yield: 8 mg, 26% (last step)
ESI-MS: 682.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
328
Scheme 11-3 New Building Block
OH 1-(Bromomethyl)- 0
adamantane
02Nj\I --1*-. 02N j-
______________________________________ . , N
DMF, DIPEA
ZED4893
I10% Pd/C
Me0H
0
H2Nj-LN
ZED4894
95 Preparation of compound ZED4893
0
02N j-
Ul
1-(1-adamantylmethyl)-3-nitropyridin-2(1H)-one
Chemical Formula: C16H20N203
Exact Mass: 288.15
Molecular Weight: 288.34
500 mg (3.57 mmol) of 2-hydroxy-3-nitropyridine and 818 mg (1 eq) of 1-
(bromomethyl)adamantane were dissolved in 10 mL DMF and 1.24 mL DIPEA (2 eq)
and
stirred at room temperature overnight. The solvent was evaporated; the residue
was
dissolved in 30 mL Et0Ac and washed twice with each 10 mL citric acid solution
(10%),
NaHCO3 solution (10%) and brine. The organic phase was dried over Na2SO4,
filtered
and the solvent was evaporated. The residue was purified by HPLC.
Yield: 484 mg, 47% ESI-MS: 289.3 [M+H]
96 Preparation of compound ZED4894
0
H2Nj-
j\I
3-amino-1-(1-adamantylmethyl)pyridin-2(1H)-one
Chemical Formula: C16H22N20
Exact Mass: 258.17
Molecular Weight: 258.36
484 mg (1.68 mmol) of ZED4893 were suspended in 30 mL Me0H before 50 mg of
palladium (10%) on activated carbon (unreduced) were added. The suspension was
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
329
stirred for 3 h at room temperature under an atmosphere of hydrogen. The
catalyst was
filtered, and the solvent was evaporated.
Yield: 339 mg, 78%
ESI-MS: 259.4 [M+H]
97 Preparation of compound 11-107
0
0)-LN
0 )C,, H
0 N
, N N
I H
0
(S)-N1-(1-(1-adamantylmethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: 033F138N406
Exact Mass: 586.28
Molecular Weight: 586.68
The synthesis of compound 11-107 was performed according to compound 11-3,
using
ZED4894 instead of ZED3906 in step 5 (according to ZED3907).
Yield: 41 mg, 49% (last step)
ESI-MS: 587.4 [M+H]
98 Preparation of compound 11-108
0
0)L
N
0 0
0 NJL
N
I H
0
OH
(2S)-N1-(1-((3-hydroxy-1-adamantypmethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: 033H38N407
Exact Mass: 602.27
Molecular Weight: 602.68
The synthesis of compound 11-108 was performed according to compound 11-107,
using
3-(bromomethyl)-1-adamantanol instead of 1-(bromomethyl)adamantane (according
to
ZED4893).
Yield: 16 mg, 36% (last step)
ESI-MS: 603.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
330
99 Preparation of compound 11-109
0
N
0 0
0 N
, N N
I H
0
Br
(2S)-N1-(1-((3-bromo-1-adamantyl)methyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C33H3713rN4.06
Exact Mass: 664.19
Molecular Weight: 665.57
The synthesis of compound 11-109 was performed according to compound 11-107,
using
1-bromo-3-(bromomethyl)adamantane instead of 1-(bromomethyl)adamantane
(according to ZED4893).
Yield: 24 mg, 41% (last step)
ESI-MS: 665.3 / 667.3 [M+H]
100 Preparation of compound 11-110
0
0 0
0 N
N
I H
0
(S)-N1-(1-(2-adamantylmethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-
2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C33F1381\1406
Exact Mass: 586.28
Molecular Weight: 586.68
The synthesis of compound 11-110 was performed according to compound 11-107,
using
2-(bromomethyl)adamantane instead of 1-(bromomethyl)adamantane (according to
ZED4893).
Yield: 46 mg, 62% (last step)
ESI-MS: 587.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
331
101 Preparation of compound 11-111
0
OAN
0 0
Njci NH
N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-2-(nicotinamido)-5-oxohexanediamide
Chemical Formula: C301-136N606
Exact Mass: 576.27
Molecular Weight: 576.64
The synthesis of compound 11-111 was performed according to compound 11-3,
using
nicotinic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid in step 6
(according to
ZED3264).
Yield: 65 mg, 46% (last step)
ESI-MS: 577.4 [M+H]
102 Preparation of compound 11-112
0
N
J.LN J3,1L
1 1\11N1
N 0 0
(S)-2-(isonicotinamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C30F136N606
Exact Mass: 576.27
Molecular Weight: 576.64
The synthesis of compound 11-112 was performed according to compound 11-3,
using
isonicotinic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid in step 6
(according
to ZED3264).
Yield: 47 mg, 52% (last step)
ESI-MS: 577.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
332
103 Preparation of compound 11-113
0
N
0 0
N
H 0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-5-oxo-2-(pyridazine-4-carboxamido)hexanediamide
Chemical Formula: C29F135N706
Exact Mass: 577.26
Molecular Weight: 577.63
The synthesis of compound 11-113 was performed according to compound 11-3,
using
pyridazine-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step 6
(according to ZED3264).
Yield: 34 mg, 46% (last step)
ESI-MS: 578.4 [M+H]
104 Preparation of compound 11-114
0
NN
0 0
N N
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-5-oxo-2-(pyridazine-3-carboxamido)hexanediamide
Chemical Formula: C29F135N706
Exact Mass: 577.26
Molecular Weight: 577.63
The synthesis of compound 11-114 was performed according to compound 11-3,
using
pyridazine-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step 6
(according to ZED3264).
Yield: 43 mg, 56% (last step)
ESI-MS: 578.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
333
105 Preparation of compound 11-115
0
0
0 0
N
o
0 0
(S)-N1-cyclopropyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(3-methylbenzofuran-2-carboxamido)-2-oxohexanediamide
Chemical Formula: C36H41N507
Exact Mass: 655.30
Molecular Weight: 655.74
The synthesis of compound 11-115 was performed according to compound 11-3,
using
cyclopropyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 47 mg, 64% (last step)
ESI-MS: 656.5 [M+H]
106 Preparation of compound 11-116
0
0
0
H 1:1:j
o hijC
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
2-(3-methylbenzofuran-2-carboxamido)-5-oxo-N6-pentylhexanediamide
Chemical Formula: C38H47N507
Exact Mass: 685.35
Molecular Weight: 685.81
The synthesis of compound 11-116 was performed according to compound 11-3,
using
pentyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 87 mg, 71% (last step)
ESI-MS: 686.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
334
107 Preparation of compound 11-117
0
0 0
0 N)-L
N
QJNH
0 0
(S)-N1-allyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-5-(3-methylbenzofuran-2-carboxamido)-2-oxohexanediamide
Chemical Formula: C36H41N507
Exact Mass: 655.30
Molecular Weight: 655.74
The synthesis of compound 11-117 was performed according to compound 11-3,
using
allyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 42 mg, 63% (last step)
ESI-MS: 656.5 [M+H]
Scheme 11-4
0
OH HO CN HO CN HO-NH
H202, LiOH
Boc.N(OtBu
Et3N, CH2C'12 Boc.N OtBu Me0H Boc.N OtBu
lioc 0 Lc 0 Lc 0 11
1) Ac20, NEt3 2) TFA/DCM
DMAP, DCM 3) Boc20
DIPEA, DMF
0
(t) H 0
AcOANH2 H2N
I :1'1N a
AcONH2
k 0
HATU, DMF BocN OtBu
r\iN
0 0 DIPEA 0
1) TFA/DCM 16
2) 3-Methylbenzo[b]-
furan-2-carboxylic acid
y HATU, DIPEA, DMF
0 0
AcOAN H2NH2
1) K2CO3, Me0H
0 0 0
H
0 0
Nj-N-rNj 2) Dess-Martin Iljcr
I H I
0 0 periodinane 0 0
DMF
11-118
Preparation of compound 10
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
335
HO CN
Boc ,N ,,OtBu
I3oc 0
(2 S)-ter t-butyl 2-(bis(tert-butoxycarbonyl)amino)-5-cyano-5-
hydroxypentanoate
Chemical Formula: C20H34N207
Exact Mass: 414,24
Molecular Weight: 414,49
15.0 g (38.7 mmol) of the aldehyde (S)-tert-butyl 2-(bis(tert-
butoxycarbonyl)amino)-5-
oxopentanoate (ZED721) were dissolved in 150 ml DCM. 6.42 ml (46.3 mmol)
trimethylamine and 7.37 ml (79.9 mmol) acetone cyanohydrin were added, and the
reaction was stirred at room temperature overnight. The solution was washed
twice with
each citric acid solution (10%) and brine. The organic phase was dried over
Na2SO4,
filtered and the solvent was evaporated. The residue was purified by flash
chromatography.
Yield: 16.2 g, >100%
ESI-MS: 437.6 [M+Na]
Preparation of compound 11
0
H0)-L NH2
Boc,N OtBu
Boo 0
(2S)-tert-butyl 6-amino-2-(bis(tert-butoxycarbonyhamino)-5-hydroxy-6-
oxohexanoate
Chemical Formula: 020H36N208
Exact Mass: 432,25
Molecular Weight: 432,51
16.2 g (-38.6 mmol) of cyanohydrin 10 were dissolved in 95 ml Me0H at 4 C and
1.91
g (45.5 mmol) lithium hydroxide monohydrate were added. 18.6 ml hydrogen
peroxide
(35%) were added dropwise, and the reaction was stirred at room temperature
for 1.5 h
before quenching with sodium thiosulfate solution (5%). The aqueous phase was
extracted with DCM. The combined organic phases were dried over Na2SO4,
filtered and
the solvent was evaporated. The residue was purified by flash chromatography.
Yield: 8.61 g, 52%
ESI-MS: 455.2 [M+Na]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
336
Preparation of compound 15
0
Ac0)-LNH2
Boc,NJc OtBu
Boo 0
(2S)-tert-butyl 5-acetoxy-6-amino-2-(bis(tert-butoxycarbonyl)amino)-6-
oxohexanoate
Chemical Formula: C22H38N209
Exact Mass: 474,26
Molecular Weight: 474,55
8.61 g (19.9 mmol) of hydroxyamide 10 were dissolved in 55 ml DCM. 3.45 ml
(24.9
mmol) 1.91 g (45.5 mmol) trimethylamine, 2.12 ml acetic anhydride and 62 mg
(0.50
mmol) DMAP were added, and the reaction was stirred at room temperature for 3
h. After
washing with water and brine, the organic phase was dried over Na2SO4,
filtered and the
solvent was evaporated. The product precipitates from MTBE solution by
addition of
hexane.
Yield: 8.08 g, 86%
ESI-MS: 475.5 [M+H]
Preparation of compound 16
0
AcONH2
Boc,NJCOH
0
(2S)-5-acetoxy-6-amino-2-(tert-butoxycarbonylamino)-6-oxohexanoic acid
Chemical Formula: C13H22N207
Exact Mass: 318,14
Molecular Weight: 318,32
8.08 g (17.0 mmol) of 15 were dissolved in 140 ml DCM/TFA (1:1) and stirred at
room
temperature for 3 h. The solvent was evaporated, and the residue was dissolved
in 40 ml
DMF. 5.80 ml (2 eq) DIPEA and 4.55 g (20.4 mmol)di-tert-butyl dicarbonate in
20 ml DMF
were added and the reaction was stirred at room temperature overnight. The
solvent was
evaporated, and the residue was dissolved in 80 ml Et0Ac. After extraction
with NaHCO3
.. solution (1.05 eq in water), the product precipitates from the aqueous
phase by addition
of 1.5 eq citric acid.
Yield: 1.64 g,30%
ESI-MS: 319.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
337
108 Preparation of compound 11-118
0 j
NH2
0
0 NNHiNNH
I H 0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C33H37N507
Exact Mass: 615.27
Molecular Weight: 615.68
The synthesis of compound 11-118 was performed according to compound 11-3,
using
compound 16 instead of ZED3632 in step 5 (according to ZED3907).
Yield: 158 mg, 56% (last step)
ESI-MS: 616.4 [M+H]
109 Preparation of compound 11-119
0 jN
0
H 11
0 NcNNN
I H 0 0
Chemical Formula: 035H39N507
Exact Mass: 641.28
Molecular Weight: 641.71
(S)-N1-ally1-5-(benzofuran-2-carboxamido)-N6-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxohexanediamide
The synthesis of compound 11-119 was performed according to compound 11-2,
using
allyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 56 mg, 71% (last step)
ESI-MS: 642.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
338
110 Preparation of compound 11-120
oJ
0 H
N
o 11jCr
0 0
Chemical Formula: C35H41N507
Exact Mass: 643.30
Molecular Weight: 643.73
(S)-2-(benzofuran-2-carboxamido)-N6-isopropyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 11-120 was performed according to compound 11-2,
using
isopropyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 62 mg, 65% (last step)
ESI-MS: 644.5 [M+H]
111 Preparation of compound 11-121
0
0
0 NjcNjN
H 0 0
Chemical Formula: C35H39N507
Exact Mass: 641.28
Molecular Weight: 641.71
(S)-2-(benzofuran-2-carboxamido)-N6-cyclopropyl-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 11-121 was performed according to compound 11-2,
using
cyclopropyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 44 mg, 51% (last step)
ESI-MS: 642.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
339
112 Preparation of compound 11-122
0
0)-L
N
0 0
0 NH
0 0
Chemical Formula: C35H39N507
Exact Mass: 677.28
Molecular Weight: 677.75
(S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-5-oxo-N6-phenylhexanediamide
The synthesis of compound 11-122 was performed according to compound 11-2,
using
phenyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 37 mg, 56% (last step)
ESI-MS: 678.4 [M+H]
113 Preparation of compound 11-123
(21A
N
0 0
0
LNCNNN
H 0 0
Chemical Formula: C39H41 N507
Exact Mass: 691,30
Molecular Weight: 691,77
(S)-2-(benzofuran-2-carboxamido)-N6-benzyl-N1-(1-(242-adamantylaminoy
2-oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 11-123 was performed according to compound 11-2,
using
benzyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632).
Yield: 46 mg, 52% (last step)
ESI-MS: 692.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
340
114 Preparation of compound 11-124
0
0)-L
NH2
0 0
0 Nj-L
N'IN
I H 0 0
Chemical Formula: 032H35N507
Exact Mass: 601.25
Molecular Weight: 601.65
(S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-
2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 11-124 was performed according to compound 11-118,
using
benzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 96 mg, 81% (last step)
ESI-MS: 602.4 [M+H]
115 Preparation of compound 11-125
0
OliNH2
0 ( H 0
CI /
0 0
CI
Chemical Formula: C25F131C12N506S
Exact Mass: 635.14
Molecular Weight: 636.55
(S)-2-(2,5-dichlorothiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
The synthesis of compound 11-125 was performed according to compound 11-124,
using
2,5-dichlorothiophene-3-carboxylic acid instead of benzofuran-2-carboxylic
acid in step 6
(according to ZED3264).
Yield: 78 mg, 71% (last step)
ESI-MS: 636.3 /638.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
341
116 Preparation of compound 11-126
0
0)-L
NH2
0 0
S JCH
N
NN
0 0
Chemical Formula: C29H33F3N606S
Exact Mass: 650.21
Molecular Weight: 650.67
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
(4-methyl-2-(trifluoromethyl)thiazole-5-carboxamido)-5-oxohexanediamide
The synthesis of compound 11-126 was performed according to compound 11-124,
using
4-methyl-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of benzofuran-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 53 mg, 67% (last step)
ESI-MS: 651.3 [M+H]
117 Preparation of compound 11-127
0
OA
NH2
0 0
H
NN
NN 0 0
Chemical Formula: C27H34N806
Exact Mass: 566.26
Molecular Weight: 566.61
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
2-(1 -methyl-1 H-1 ,2,3-triazole-5-carboxamido)-5-oxohexanediamide
The synthesis of compound 11-127 was performed according to compound 11-124,
using
1-methyl-1H-1,2,3-triazole-5-carboxylic acid instead of benzofuran-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 26 mg, 49% (last step)
ESI-MS: 567.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
342
118 Preparation of compound 11-128
o
o
)^LN
H
0 0
H H
Nj-L Nõ,,c3i.
CI
'''r
s 0 0
CI
(2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-y1)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-methyl-5-
oxohexanediamide
Chemical Formula: C26H29Cl2N506S
Exact Mass: 609.12
Molecular Weight: 610.51
The synthesis of compound 11-128 was performed according to compound 11-97,
using
2,5-dichlorothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 53 mg, 73% (last step)
ESI-MS: 610.3 /612.3 [M+H]
119 Preparation of compound 11-129
o
ON
H
0 jc H 0
H
S Nj-N N...õ.cL
F3C N-----/ i
i I-1 1 I
N 0 0
(2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-
1,2-dihydropyridin-
3-y1)-N6-methyl-2-(4-methyl-2-(trifluoromethyl)thiazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: 027H31F3N606S
Exact Mass: 624.20
Molecular Weight: 624.63
The synthesis of compound 11-129 was performed according to compound 11-97,
using 4-
methyl-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 42 mg, 60% (last step)
ESI-MS: 625.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
343
120 Preparation of compound 11-130
NN
0)-L
N
0 0
H H
1\1--NN 0 0
(2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-
1,2-dihydropyridin-
3-y1)-N6-methyl-2-(1-methyl-1H-1,2,3-triazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C25H32N806
Exact Mass: 540.24
Molecular Weight: 540.57
The synthesis of compound 11-130 was performed according to compound 11-97,
using 1-
methyl-1H-1,2,3-triazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 15 mg, 39% (last step)
ESI-MS: 541.3 [M+H]
121 Preparation of compound 11-131
0
0)-L
0 0
H
N
Y-LN
\N-N 0 0
Chemical Formula: C27H341\1806
Exact Mass: 566.26
Molecular Weight: 566.61 (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-yI)-N6-methyl-5-oxo-2-(2H-1,2,3-triazole-4-
carboxamido)hexanediamide
The synthesis of compound 11-131 was performed according to compound 11-3,
using 2H-
1,2,3-triazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 28 mg, 56% (last step)
ESI-MS: 567.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
344
122 Preparation of compound 11-132
0
0)-L
N
0 ( H0
HN Ay-LN N
N 0 0
Chemical Formula: C271-134N806
Exact Mass: 566.26
Molecular Weight: 566.61
(S)-N1-(1 -(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methy1-5-oxo-2-(1 H-1 ,2,3-triazole-4-carboxamido)hexanediamide
The synthesis of compound 11-132 was performed according to compound 11-3,
using 1H-
1,2,3-triazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 14 mg, 45% (last step)
ESI-MS: 567.4 [M+H]
123 Preparation of compound 11-133
0
0 011
N
N-r-N 0 0
Chemical Formula: C28H36N806
Exact Mass: 580.28
Molecular Weight: 580.64
(S)-N1 -(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-2-(1 -methyl-1 H-1,2,3-triazole-4-carboxamido)-5-oxohexanediamide
The synthesis of compound 11-133 was performed according to compound 11-3,
using 1-
methyl-1H-1,2,3-triazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 36 mg, 61% (last step)
ESI-MS: 581.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
345
124 Preparation of compound 11-134
0
011
0 0
NN
H 0
Chemical Formula: C27H34N806
Exact Mass: 566.26
Molecular Weight: 566.61
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methy1-5-oxo-2-(1H-1,2,4-triazole-3-carboxamido)hexanediamide
The synthesis of compound 11-134 was performed according to compound 11-3,
using 1H-
1,2,4-triazole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 21 mg, 43% (last step)
ESI-MS: 567.4 [M+H]
125 Preparation of compound 11-135
0
0)-(
N
111 H 0
N
JI NN
NN 0 0
Chemical Formula: C28H36N806
Exact Mass: 580.28
Molecular Weight: 580.64
(S)-N1 -(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(1-methy1-1 H-1 ,2,4-triazole-3-carboxamido)-5-oxohexanediamide
The synthesis of compound 11-135 was performed according to compound 11-3,
using 1-
methyl-1H-1,2,4-triazole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 39 mg, 67% (last step)
ESI-MS: 581.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
346
126 Preparation of compound 11-136
0
N
0
N
o I HjjCr II NrN
0 0
Chemical Formula: C33H37N507
Exact Mass: 615.27
Molecular Weight: 615.68
(S)-2-(benzofuran-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
The synthesis of compound 11-136 was performed according to compound 11-3,
using
benzofuran-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 56 mg, 71% (last step)
ESI-MS: 616.4 [M+H]
127 Preparation of compound 11-137
0
0 JCH 0
N)-L
H NrNEI
0 0
Chemical Formula: C33H37N506S
Exact Mass: 631.25
Molecular Weight: 631.74
(S)-2-(benzo[b]thiophene-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
The synthesis of compound 11-137 was performed according to compound 11-3,
using
benzo[b]thiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 49 mg, 64% (last step)
ESI-MS: 632.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
347
128 Preparation of compound 11-138
0
N
0
NEIHNThrN
/ 11
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-2-(1-methyl-1H-pyrazole-3-carboxamido)-5-oxohexanediamide
Chemical Formula: C29F-137N706
Exact Mass: 579.28
Molecular Weight: 579.65
The synthesis of compound 11-138 was performed according to compound 11-3,
using 1-
methyl-1H-pyrazole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 43 mg, 59% (last step)
ESI-MS: 580.4 [M+H]
129 Preparation of compound 11-139
0
N
0
H
N m
N, I H 1 41 I
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(1-methy1-1H-pyrazole-4-carboxamido)-5-oxohexanediamide
Chemical Formula: C29F-137N706
Exact Mass: 579.28
Molecular Weight: 579.65
The synthesis of compound 11-139 was performed according to compound 11-3,
using 1-
methyl-1H-pyrazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 53 mg, 62% (last step)
ESI-MS: 580.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
348
130 Preparation of compound 11-140
0
N
OHO H
*LN N N
, H
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methyl-2-(1-methyl-1H-pyrazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C29F-137N706
Exact Mass: 579.28
Molecular Weight: 579.65
The synthesis of compound 11-140 was performed according to compound 11-3,
using 1-
methyl-1H-pyrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 39 mg, 48% (last step)
ESI-MS: 580.4 [M+H]
131 Preparation of compound 11-141
0
N
0
N
No I H
, yThvN
0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(4-methy1-1,2,3-thiadiazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C28H35N706S
Exact Mass: 597.24
Molecular Weight: 597.69
The synthesis of compound 11-141 was performed according to compound 11-3,
using 4-
methyl-1,2,3-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 54 mg, 58% (last step)
ESI-MS: 598.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
349
132 Preparation of compound 11-142
0
0 ( H0
N NThv N
H
0 0
\S-N
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-
N6-methyl-5-oxo-2-(1,2,5-thiadiazole-3-carboxamido)hexanediamide
Chemical Formula: C27H33N706S
Exact Mass: 583.22
Molecular Weight: 583.66
The synthesis of compound 11-142 was performed according to compound 11-3,
using
1,2,5-thiadiazole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 36 mg, 43% (last step)
ESI-MS: 584.4 [M+H]
133 Preparation of compound 11-143
0
ON
I 0 0
N N
0 0
N-NN
(S)-2-(4-iodo-1-methyl-1H-pyrazole-5-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H36IN706
Exact Mass: 705.18
Molecular Weight: 705.54
The synthesis of compound 11-143 was performed according to compound 11-3,
using 4-
iodo-1-methyl-1H-pyrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 24 mg, 49% (last step)
ESI-MS: 706.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
350
134 Preparation of compound 11-144
0
0)L
NH2
jOt NH 0
N
N
Hs, I I
(S)-N1 -(1 -(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-dihydropyridin-
3-y1)-2-(1-methy1-1 H-pyrazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C28H35N706
Exact Mass: 565.26
Molecular Weight: 565.62
The synthesis of compound 11-144 was performed according to compound 11-118,
using
1-methyl-1H-pyrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 26 mg, 68% (last step)
ESI-MS: 566.4 [M+H]
135 Preparation of compound 11-145
0
OA
NH2
0 0
N
µ1\1 0 0
(S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-y1)-
244-methyl-I ,2,3-thiadiazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: 027H33N706S
Exact Mass: 583.22
Molecular Weight: 583.66
The synthesis of compound 11-145 was performed according to compound 11-118,
using
4-methyl-1,2,3-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-
2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 35 mg, 62% (last step)
ESI-MS: 584.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
351
136 Preparation of compound 11-146
0
NH2
0 H
N
o IF\11 N
0 0
(S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-
2-ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C29F131N507
Exact Mass: 561.22
Molecular Weight: 561.59
The synthesis of compound 11-146 was performed according to compound 11-118,
using
benzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264) and using exo-2-aminonorbornane instead of 2-
adamantan-
amine in step 2 (according to ZED3905).
Yield: 43 mg, 60% (last step)
ESI-MS: 562.4 [M+H]
137 Preparation of compound 11-147
0
0)L
NH2
0 H H
N
0 0
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C30H33N507
Exact Mass: 575.24
Molecular Weight: 575.61
The synthesis of compound 11-147 was performed according to compound 11-118,
using
using exo-2-aminonorbornane instead of 2-adamantanamine in step 2 (according
to
ZED3905).
Yield: 56 mg, 67% (last step)
ESI-MS: 576.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
352
138 Preparation of compound 11-148
0
0 9
oI 'NI
0 0
(S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-
2-ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C29H31N507
Exact Mass: 561.22
Molecular Weight: 561.59
The synthesis of compound 11-148 was performed according to compound 11-118,
using
benzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264) and using ( )-endo-2-aminonorbornane instead of 2-
adamantanamine in step 2 (according to ZED3905).
Yield: 49 mg, 66% (last step)
ESI-MS: 562.4 [M+H]
139 Preparation of compound 11-149
OI'0
NH2
0 H
0
N.r NiNH
H 0 0
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C30H33N507
Exact Mass: 575.24
Molecular Weight: 575.61
The synthesis of compound 11-149 was performed according to compound 11-118,
using
using ( )-endo-2-aminonorbornane instead of 2-adamantanamine in step 2
(according to
ZED3905).
Yield: 64 mg, 75% (last step)
ESI-MS: 576.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
353
140 Preparation of compound 11-150
0
NHfNN2
0 H 0
NjLr NH
o
0 0
(S)-2-(benzofuran-2-carboxamido)-5-oxo-N1-(2-oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
Chemical Formula: C32H37N507
Exact Mass: 603.27
Molecular Weight: 603.67
The synthesis of compound 11-150 was performed according to compound 11-118,
using
benzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in step
6 (according to ZED3264) and using (R)-(+)-bornylamine instead of 2-
adamantanamine
in step 2 (according to ZED3905).
Yield: 38 mg, 53% (last step)
ESI-MS: 604.4 [M+H]
141 Preparation of compound 11-151
0
0)-L
NH2
0 H
0 Nr N2-KN.r NH
H 0 0
(S)-2-(3-methylbenzofuran-2-carboxamido)-5-oxo-N1-(2-oxo-1-(2-oxo-2-
((1R,2S,4R)-1,7,7-
trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
Chemical Formula: C33H39N507
Exact Mass: 617.28
Molecular Weight: 617.69
The synthesis of compound 11-151 was performed according to compound 11-118,
using
using (R)-(+)-bornylamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
Yield: 31 mg, 59% (last step)
ESI-MS: 618.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
354
142 Preparation of compound 11-152
0
COL'
N
N, H jJ
1\1 0 0
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(4-methy1-1,2,3-thiadiazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C25H31 N706S
Exact Mass: 557.21
Molecular Weight: 557.62
The synthesis of compound 11-152 was performed according to compound 11-94,
using 4-
methyl-1,2,3-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 53 mg, 68% (last step)
ESI-MS: 558.4 [M+H]
143 Preparation of compound 11-153
0
N
0 ( H 0
Y-LNNJ-L
N N
\ H
0 0
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(1-methy1-1H-pyrazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C26H33N706
Exact Mass: 539.25
Molecular Weight: 539.58
The synthesis of compound 11-153 was performed according to compound 11-94,
using 1-
methyl-1H-pyrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 60 mg, 67% (last step)
ESI-MS: 540.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
355
144 Preparation of compound 11-154
0
(DAN
0 jcr H 0
)-LTh
F3C ________________________ SNNN(NH
N ,,r
0 0
(S)-N1-(1-(2-((16,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(4-methyl-2-(trifluoromethypthiazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C27H31F3N606S
Exact Mass: 624.20
Molecular Weight: 624.63
The synthesis of compound 11-154 was performed according to compound 11-90,
using 4-
methyl-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 68 mg, 74% (last step)
ESI-MS: 625.3 [M+H]
145 Preparation of compound 11-155
0
N
0
H ?
NH
0 0
CI
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-methyl-5-oxohexanediamide
Chemical Formula: C26H29Cl2N506S
Exact Mass: 609.12
Molecular Weight: 610.51
The synthesis of compound 11-155 was performed according to compound 11-90,
using
2,5-dichlorothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 74 mg, 68% (last step)
ESI-MS: 610.3 /612.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
356
146 Preparation of compound 11-156
0
0 jc H 0
:S N NH
0 0
(S)-N1-(1-(2-((16,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(4-methyl-1,2,3-thiadiazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C25H31N706S
Exact Mass: 557.21
Molecular Weight: 557.62
The synthesis of compound 11-156 was performed according to compound 11-90,
using 4-
methyl-1,2,3-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 56 mg, 61% (last step)
ESI-MS: 558.4 [M+H]
147 Preparation of compound 11-157
0
0)-(N
0
(I? NH NH
FIN 0 0
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(1-methyl-1H-1,2,3-triazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C25F132N806
Exact Mass: 540.24
Molecular Weight: 540.57
The synthesis of compound 11-157 was performed according to compound 11-90,
using 1-
methyl-1H-1,2,3-triazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 34 mg, 52% (last step)
ESI-MS: 541.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
357
148 Preparation of compound 11-158
0
jOt N 0
N
-H- 0
N-NN
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(1-methyl-1H-pyrazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C26H33N706
Exact Mass: 539.25
Molecular Weight: 539.58
The synthesis of compound 11-158 was performed according to compound 11-90,
using 1-
methyl-1H-pyrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 55 mg, 64% (last step)
ESI-MS: 540.4 [M+H]
149 Preparation of compound 11-159
0
0 H 9
NH
0 0
(S)-N1-methyl-5-(4-methyl-2-(trifluoromethyl)thiazole-5-carboxamido)-2-oxo-N6-
(2-oxo-1-(2-oxo-2-
((1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-3-yl)hexanediamide
Chemical Formula: C30H37F3N606S
Exact Mass: 666.24
Molecular Weight: 666.71
The synthesis of compound 11-159 was performed according to compound 11-92,
using 4-
methyl-2-(trifluoromethyl)thiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 52 mg, 57% (last step)
ESI-MS: 667.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
358
150 Preparation of compound 11-160
0
N
0 0
H
_NH
CI / r
0 0
CI
(S)-2-(2,5-dichlorothiophene-3-carboxamido)-N6-methyl-5-oxo-N1-(2-oxo-1-(2-oxo-
2-((1R,2S,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
Chemical Formula: C29H35Cl2N506S
Exact Mass: 651.17
Molecular Weight: 652.59
The synthesis of compound 11-160 was performed according to compound 11-92,
using
2,5-dichlorothiophene-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 66 mg, 61% (last step)
ESI-MS: 652.3 / 654.3 [M+H]
151 Preparation of compound 11-161
0
N
0 0
N NH
I
µ1\1 0 0
(S)-N1-methyl-5-(4-methyl-1,2,3-thiadiazole-5-carboxamido)-2-oxo-N6-(2-oxo-1-
(2-oxo-2-((1R,2S,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yOhexanediamide
Chemical Formula: C28H37N706S
Exact Mass: 599.25
Molecular Weight: 599.70
The synthesis of compound 11-161 was performed according to compound 11-92,
using 4-
methyl-1,2,3-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 41 mg, 51% (last step)
ESI-MS: 600.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
359
152 Preparation of compound 11-162
0
N
J(0
rN NH
N
NY 111
1
0 0 \1-1\1
(S)-N1 -methyl-5-(1 -methyl-1 H-1,2,3-triazole-5-carboxamido)-2-oxo-N6-(2-oxo-
1 -(2-oxo-2-((1 R,2S,4R)-
1 ,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yOhexanediamide
Chemical Formula: C28H38N806
Exact Mass: 582.29
Molecular Weight: 582.65
The synthesis of compound 11-162 was performed according to compound 11-92,
using 1-
methyl-1H-1,2,3-triazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 28 mg, 46% (last step)
ESI-MS: 583.5 [M+H]
153 Preparation of compound 11-163
0
N
0 0
a)L
N
N N NH
H 1 I
N--"\ 0 0
(S)-N1-methy1-5-(1 -methyl-1 H-pyrazole-5-carboxamido)-2-oxo-N6-(2-oxo-1-(2-
oxo-2-((1R,2S,4R)-
1,7,7-trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yOhexanediamide
Chemical Formula: C29H39N706
Exact Mass: 581.30
Molecular Weight: 581.66
The synthesis of compound 11-163 was performed according to compound 11-92,
using 1-
methyl-1H-pyrazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 44 mg, 58% (last step)
ESI-MS: 582.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
360
154 Preparation of compound 11-164
0
0)-LN
0 0
N N
HN 0
(2S)-2-(4-tert-buty1-1H-pyrrole-3-carboxamido)-N6-methyl-N1-(1-(2-(2-
adamantylamino)ethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C33H45N505
Exact Mass: 591.34
Molecular Weight: 591.74
The synthesis of compound 11-164 was performed according to compound 11-107,
using
1-(2-bromoethyl)adamantane instead of 1-(bromomethyl)adamantane (according to
ZED4893) and 5-tert-butyl-1H-pyrrole-3-carboxylic acid instead of 3-methyl-
benzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 32 mg, 56% (last step)
ESI-MS: 592.5 [M+H]
155 Preparation of compound 11-165
0
N
0 0
NC N c NH N
N H 0
(2S)-2-(4-cyano-1-methy1-1H-pyrrole-2-carboxamido)-N6-methyl-N 1-(1-(3-(1-
adamantylami no)propy1)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexaned iamide
Chemical Formula: 032H40N605
Exact Mass: 588.31
Molecular Weight: 588.70
The synthesis of compound 11-165 was performed according to compound 11-107,
using
1-(3-bromopropyl)adamantane instead of 1-(bromomethyl)adamantane (according to
ZED4893) and 4-cyano-1-methyl-1H-pyrrole-2-carboxylic acid instead of 3-methyl-
benzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 27 mg, 48% (last step)
ESI-MS: 589.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
361
156 Preparation of compound 11-166
0
0)-L
N
0 0 0
0?LNjcFr1J-LN
Me
H 0
(S)-N1-(1-(3-(2-adamantylamino)-3-oxopropy1)-2-oxo-1,2-dihydropyridin-3-y1)-
2-(5-methoxyoxazole-2-carboxamido)-N6-methy1-5-oxohexanediamide
Chemical Formula: C301-138N608
Exact Mass: 610.28
Molecular Weight: 610.66
The synthesis of compound 11-166 was performed according to compound 11-3,
using 3-
chloropropionic acid instead of chloroacetic acid (according to ZED1657) and 5-
methoxyoxazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in
step 6 (according to ZED3264).
Yield: 31 mg, 59% (last step)
ESI-MS: 611.4 [M+H]
157 Preparation of compound 11-167
0
N
0 JCH 0
0 N NH
Nr
I H 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C29F131N507
Exact Mass: 561.22
Molecular Weight: 561.59
The synthesis of compound 11-167 was performed according to compound 11-3,
using 1-
bicyclo[1.1.1]pentylamine instead of 2-adamantanamine in step 2 (according to
ZED3905).
.. Yield: 45 mg, 67% (last step)
ESI-MS: 562.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
362
158 Preparation of compound 11-168
o
N
0
0 Na,õ11,,N N NH
H I H N-r
0 0
0
(S)-2-(2-acetyloxazole-4-carboxamido)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
ylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-311)-N6-methyl-5-oxohexanediamide
Chemical Formula: C25F128N608
Exact Mass: 540.20
Molecular Weight: 540.53
The synthesis of compound 11-168 was performed according to compound 11-167,
using
2-acetyloxazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 26 mg, 48% (last step)
ESI-MS: 541.4 [M+H]
159 Preparation of compound 11-169
0
ON
0 0
0
N
I H 0 0
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
311)-N6-methyl-2-(3-methylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C30H33N507
Exact Mass: 575.24
Molecular Weight: 575.61
The synthesis of compound 11-169 was performed according to compound 11-3,
using
bicyclo[2.1.1]hexan-1-amine instead of 2-adamantanamine in step 2 (according
to
ZED3905).
Yield: 33 mg, 61% (last step)
ESI-MS: 576.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
363
160 Preparation of compound 11-170
0
OA
N
0 H 0
o o
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-(2-isopropyloxazole-5-carboxamido)-N6-methyl-5-oxohexanediamide
Chemical Formula: C27H34.N607
Exact Mass: 554.25
Molecular Weight: 554.59
The synthesis of compound 11-170 was performed according to compound 11-169,
using
2-isopropyloxazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 32 mg, 54% (last step)
ESI-MS: 555.4 [M+H]
161 Preparation of compound 11-171
0
N
0 H ?
N
o jC 1'
0 0
(2S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(bicyclo[3.2.1]octan-8-ylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-yh-N6-methyl-5-oxohexanediamide
Chemical Formula: C31H35N507
Exact Mass: 589.25
Molecular Weight: 589.64
The synthesis of compound 11-171 was performed according to compound 11-2,
using
bicyclo[3.2.1]octan-8-amine instead of 2-adamantanamine in step 2 (according
to
ZED3905).
Yield: 42 mg, 60% (last step)
ESI-MS: 590.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
364
162 Preparation of compound 11-172
ON
0 0
NjcH N
H
(2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-(3,5-dimethylisoxazole-4-carboxamido)-N6-methyl-5-oxohexanediamide
Chemical Formula: C28H36N607
Exact Mass: 568.26
Molecular Weight: 568.62
The synthesis of compound 11-172 was performed according to compound 11-171,
using
3,5-dimethylisoxazole-4-carboxylic acid instead of benzofuran-2-carboxylic
acid in step
6 (according to ZED3264).
Yield: 35 mg, 58% (last step)
ESI-MS: 569.4 [M+H]
163 Preparation of compound 11-173
0
0)-L
N
0 ti 0
N-LNjC-r N
H 0 NTh0r
CO2H
(S)-N1-(1-(2-(5-carboxy-2-aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(4-methylpyrimidine-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C31F-137N708
Exact Mass: 635.27
Molecular Weight: 635.67
The synthesis of compound 11-173 was performed according to compound 11-3,
using 4-
aminoadamantane-1-carboxylic acid instead of 2-adamantanamine in step 2
(according
to ZED3905) and 4-methylpyrimidine-5-carboxylic acid instead of 3-methyl-
benzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 25 mg, 49% (last step)
ESI-MS: 636.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
365
164 Preparation of compound 11-174
0
N
0 H 0
NN
0 0
N,
0
(2S)-N1-(1-(2-(4-aminoadamantane-N,N-dimethy1-1-carboxamide)-2-oxoethyl)-2-oxo-
1,2-dihydropyridin-
3-y1)-N6-methyl-5-oxo-241,2,3,4-tetrahydronaphthalene-2-
carboxamido)hexanediamide
Chemical Formula: C38H48N607
Exact Mass: 700.36
Molecular Weight: 700.82
The synthesis of compound 11-174 was performed according to compound 11-3,
using 4-
aminoadamantane-N,N-dimethy1-1-carboxamide instead of 2-adamantanamine in step
2
(according to ZED3905) and 1,2,3,4-tetrahydronaphthalene-2-carboxylic acid
instead of
3-methylbenzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 33 mg, 56% (last step)
ESI-MS: 701.5 [M+H]
165 Preparation of compound 11-175
0
0 0
N
1\jr N
0 0
(S)-24(S)-1,4-diazabicyclo[2.2.2]octane-2-carboxamido)-N6-tert-butyl-N141-(242-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
Chemical Formula: C34H49N706
Exact Mass: 651.37
Molecular Weight: 651.80
The synthesis of compound 11-175 was performed according to compound 11-3,
using
tert-butyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632) and
1,4-diazabicyclo[2.2.2]octane-2-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
.. carboxylic acid in step 6 (according to ZED3264).
Yield: 39 mg, 53% (last step)
ESI-MS: 652.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
366
166 Preparation of compound 11-176
0
0 0
0 0
(S)-N1-tert-buty1-5-(1H-indole-3-carboxamido)-N6-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-oxohexanediamide
Chemical Formula: C36F144N606
Exact Mass: 656.33
Molecular Weight: 656.77
The synthesis of compound 11-176 was performed according to compound 11-3,
using
tert-butyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632) and
1H-indole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid
in step 6
(according to ZED3264).
Yield: 52 mg, 62% (last step)
ESI-MS: 657.5 [M+H]
167 Preparation of compound 11-177
0
0)-(
N -
H
0 0
N,--/N3AN
N )=-L
N
-Ns I H 0 0
(S)-N1-tert-butyl-N6-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-5-(6-methylimidazo[2,1-b]thiazole-3-carboxamido)-2-oxohexanediamide
Chemical Formula: C34H43N706S
Exact Mass: 677.30
Molecular Weight: 677.81
The synthesis of compound 11-177 was performed according to compound 11-3,
using
tert-butyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632) and
6-methylimidazo[2,1-b][1,3]thiazole-3-carboxylic acid instead of 3-
methylbenzo[b]furan-
2-carboxylic acid in step 6 (according to ZED3264).
Yield: 42 mg, 53% (last step)
ESI-MS: 678.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
367
168 Preparation of compound 11-178
0
JN
1? t\li 9
s
0 0
(S)-2-(benzo[d]thiazole-2-carboxamido)-N1-(1-(24(1S,2R,4R)-
bicyclo[2.2.1]heptan-2-ylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-cyclopenty1-5-oxohexanediamide
Chemical Formula: 033H38N606S
Exact Mass: 646.26
Molecular Weight: 646.76
The synthesis of compound 11-178 was performed according to compound 11-90,
using
cyclopentyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
and 1,3-benzothiazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 54 mg, 61% (last step)
ESI-MS: 647.4 [M+H]
169 Preparation of compound 11-179
0 N
0 0
NH
N
N7-2)LFI
0 0
S/
(S)-N1-(1-(2-((1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-cyclopenty1-2-(imidazo[2,1-b]thiazole-6-carboxamido)-5-
oxohexanediamide
Chemical Formula: C31H37N706S
Exact Mass: 635.25
Molecular Weight: 635.73
The synthesis of compound 11-179 was performed according to compound 11-90,
using
cyclopentyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
and imidazo[2,1-b][1,3]thiazole-6-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 36 mg, 51% (last step)
ESI-MS: 636.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
368
170 Preparation of compound 11-180
0 9 L)
OH
F3C-0
IN HC N NH
0 0
(S)-N1 -(1 -(2-((1 S,2R,4R)-bicyclo[2.2.1 ]heptan-2-ylamino)-2-oxoethyl)-2-oxo-
1 ,2-dihydropyridin-3-y1)-
N6-cyclopenty1-2-(4-hydroxy-6-(trifluoromethoxy)quinoline-3-carboxamido)-5-
oxohexanediamide
Chemical Formula: 036H39F3N608
Exact Mass: 740.28
Molecular Weight: 740.73
The synthesis of compound 11-180 was performed according to compound 11-90,
using
cyclopentyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
__ and 4-hydroxy-6-(trifluoromethoxy)quinoline-3-carboxylic acid instead of 3-
methyl-
benzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 19 mg, 41% (last step)
ESI-MS: 741.5 [M+H]
171 Preparation of compound 11-181
0
0 0
NN N
)- NH
-r
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]9entan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-2-(cinnoline-3-carboxamido)-N6-cyclohexyl-5-oxohexanediamide
Chemical Formula: C33F-137N706
Exact Mass: 627.28
Molecular Weight: 627.69
The synthesis of compound 11-181 was performed according to compound 11-167,
using
cyclohexyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
and 3-cinnolinecarboxylic acid instead of 3-methylbenzo[b]furan-2-carboxylic
acid in
step 6 (according to ZED3264).
Yield: 37 mg, 56% (last step)
ESI-MS: 628.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
369
172 Preparation of compound 11-182
0
0 JCH 0
0 N N
j-L NH
I H
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-cyclohexy1-2-(3-ethylbenzofuran-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35H41N507
Exact Mass: 643.30
Molecular Weight: 643.73
The synthesis of compound 11-182 was performed according to compound 11-167,
using
cyclohexyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
and 3-ethylbenzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 53 mg, 68% (last step)
ESI-MS: 644.5 [M+H]
173 Preparation of compound 11-183
0
/NN
M 0 0
Nj-LNNH
I H
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethy1)-2-oxo-1,2-
clihydropyridin-
3-y1)-N6-cyclohexy1-2-(1 H-indole-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35F142N606
Exact Mass: 642.32
Molecular Weight: 642.74
The synthesis of compound 11-183 was performed according to compound 11-167,
using
cyclohexyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
and 1-ethyl-1H-indole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 41 mg, 57% (last step)
ESI-MS: 643.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
370
174 Preparation of compound 11-184
ou
NC)
0 C H 0
NN)L NH
N
H
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N6-cyclohexyl-2-(2-methyl-1,8-naphthyridine-3-carboxamido)-5-oxohexanediamide
Chemical Formula: C34H39N706
Exact Mass: 641.30
Molecular Weight: 641.72
The synthesis of compound 11-184 was performed according to compound 11-167,
using
cyclohexyl isocyanide instead of methyl isocyanide in step 4 (according to
ZED3632)
and 2-methyl-1,8-naphthyridine-3-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 26 mg, 54% (last step)
ESI-MS: 642.5 [M+H]
175 Preparation of compound 11-185
0
).LN
0H
NTh'N
0 0
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N6-methyl-5-oxo-2-(1,2,3,4-tetrahydroquinoline-6-carboxamido)hexanediamide
Chemical Formula: C301-136N606
Exact Mass: 576.27
Molecular Weight: 576.64
The synthesis of compound 11-185 was performed according to compound 11-169,
using
N-Boc-1,2,3,4-tetrahydroquinoline-6-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264). The final product was
obtained by
deprotection (DCM/TFA) as described above and purified by HPLC.
Yield: 39 mg, 61% (last step)
ESI-MS: 577.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
371
176 Preparation of compound 11-186
0
0
)LN
0 ( H 0
H CO2H
0
NN
N-vN
HJ!JN 0 0 CF3
(S)-N1-(1-(2-(2-carboxy-2-amino-5-(trifluoromethyl)adamantane)-2-oxoethyl)-2-
oxo-1,2-dihydropyridin-
3-y1)-N6-methy1-5-oxo-2-(3-oxo-1,2,3,4-tetrahydroisoquinoline-6-
carboxamido)hexanediamide
Chemical Formula: C36H39F3N609
Exact Mass: 756.27
Molecular Weight: 756.72
The synthesis of compound 11-186 was performed according to compound 11-3,
using 2-
amino-5-(trifluoromethyl)adamantane-2-carboxylic acid instead of 2-
adamantanamine in
step 2 (according to ZED3905) and 3-oxo-1,2,3,4-tetrahydroisoquinoline-6-
carboxylic
acid instead of 3-methylbenzo[b]furan-2-carboxylic acid in step 6 (according
to
ZED3264).
Yield: 12 mg, 36% (last step)
ESI-MS: 757.4 [M+H]
177 Preparation of compound 11-187
0
0)-L
N
0 0
N HJ-L
N 1\jrN
N 0 0
(S)-N1-(1-(2-(5-ethyladamantane-2-amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-
3-y1)-
N6-methy1-2-(1,6-naphthyridine-2-carboxamido)-5-oxohexanediamide
Chemical Formula: C35H41N706
Exact Mass: 655.31
Molecular Weight: 655.74
The synthesis of compound 11-187 was performed according to compound 11-3,
using 5-
ethyladamantane-2-amine instead of 2-adamantanamine in step 2 (according to
ZED3905) and 1,6-naphthyridine-2-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 26 mg, 51% (last step)
ESI-MS: 656.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
372
178 Preparation of compound 11-188
0
0)-L
N
N 0 0
*LN
NN
H 0 'le,
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(2,6-naphthyridine-1-carboxamido)-5-oxohexanediamide
Chemical Formula: C29F131N706
Exact Mass: 573.23
Molecular Weight: 573.60
The synthesis of compound 11-188 was performed according to compound 11-169,
using
2,6-naphthyridine-1-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid
in step 6 (according to ZED3264).
Yield: 33 mg, 56% (last step)
ESI-MS: 574.4 [M+H]
179 Preparation of compound 11-189
0
0)-L N
H2N 0 0
N)-L rNH
YLN
N HjC N
0 0
(S)-2-(4-amino-1,2,5-oxadiazole-3-carboxamido)-N1-(1-(2-(bicyclo[1.1.1]pentan-
1-
ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-
oxohexanediamide
Chemical Formula: C22H26N807
Exact Mass: 514.19
Molecular Weight: 514.49
The synthesis of compound 11-189 was performed according to compound 11-167,
using
4-Boc-amino-1,2,5-oxadiazole-3-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264). The amine was deprotected by
TFA in
the final step.
Yield: 16 mg, 69% (last step)
ESI-MS: 515.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
373
180 Preparation of compound 11-190
0
0)-LN
0 iCH 0
0 N Nj-Nr NH
,
\N I H 0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
2-(6-(dimethylamino)benzofuran-2-carboxamido)-N6-methyl-5-oxohexanediamide
Chemical Formula: C30H34N607
Exact Mass: 590.25
Molecular Weight: 590.63
The synthesis of compound 11-190 was performed according to compound 11-167,
using
6-(dimethylamino)benzofuran-2-carboxylic acid instead of 3-methylbenzo[b]furan-
2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 38 mg, 55% (last step)
ESI-MS: 591.4 [M+H]
181 Preparation of compound 11-191
0
N
0 )(vH 0
N)-LNNH
N 0 0
0
(S)-2-(2-acetamidothiazole-5-carboxamido)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-
ylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C25H29N707S
Exact Mass: 571.18
Molecular Weight: 571.61
The synthesis of compound 11-191 was performed according to compound 11-167,
using
2-acetylamino-5-thiazolecarboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 21 mg, 46% (last step)
ESI-MS: 572.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
374
182 Preparation of compound 11-192
0
N
0 0
0 NH
H2N HN 0 0
(S)-N4-(1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-y1)-1H-pyrrole-2,4-
dicarboxamide
Chemical Formula: C25H29N707
Exact Mass: 539.21
Molecular Weight: 539.54
The synthesis of compound 11-192 was performed according to compound 11-167,
using
5-carbamoy1-1H-pyrrole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 13 mg, 38% (last step)
ESI-MS: 540.4 [M+H]
183 Preparation of compound 11-193
0
o
N
0 H 0
0
011 Nj-L
FN11 I NI rNEI 0
H214 0 0 0
(S)-N1-(1-(2-(1-acetylamino-4-aminoadamantane)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-5-oxo-2-(5-sulfamoylfuran-3-carboxamido)hexanediamide
Chemical Formula: C31H39N7010S
Exact Mass: 701.25
Molecular Weight: 701.75
The synthesis of compound 11-193 was performed according to compound 11-3,
using 1-
acetylamino-4-aminoadamantane instead of 2-adamantanamine in step 2 (according
to
ZED3905) and 5-sulfamoylfuran-3-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 10 mg, 27% (last step)
ESI-MS: 702.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
375
184 Preparation of compound 11-194
0)-L
0
N
0 0
Njc H')-L
N NTh'N
0
0 0
NJL
0
(S)-2-(benzofuran-5-carboxamido)-N1-(1-(2-(1-acetylamino-4-aminoadamantane)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C35H4.0N608
Exact Mass: 672.29
Molecular Weight: 672.73
The synthesis of compound 11-194 was performed according to compound 11-3,
using 1-
acetylamino-4-aminoadamantane instead of 2-adamantanamine in step 2 (according
to
ZED3905) and benzofuran-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 23 mg, 52% (last step)
ESI-MS: 673.5 [M+H]
185 Preparation of compound 11-195
0
0)-L
N
0 H 0
0 N N N N
0 0 NH2
0
(S)-2-(benzofuran-6-carboxamido)-N1-(1-(2-(4-aminoadamantane-1-carboxamide)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
Chemical Formula: C34.H38N608
Exact Mass: 658.28
Molecular Weight: 658.70
The synthesis of compound 11-195 was performed according to compound 11-3,
using 4-
aminoadamantane-1-carboxamide instead of 2-adamantanamine in step 2 (according
to
ZED3905) and benzofuran-6-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 26 mg, 57% (last step)
ESI-MS: 659.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
376
186 Preparation of compound 11-196
0
N
0 0
IR11)-LNN
H 0
NH2
0
(S)-N1-(1-(2-(4-aminoadamantane-1-carboxamide)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N6-methy1-2-(3-(1-methylcyclopropy1)-1,2,4-oxadiazole-5-carboxamido)-5-
oxohexanediamide
Chemical Formula: C32H40N308
Exact Mass: 664.30
Molecular Weight: 664.71
The synthesis of compound 11-196 was performed according to compound 11-3,
using 4-
aminoadamantane-1-carboxamide instead of 2-adamantanamine in step 2 (according
to
ZED3905) and 3-(1-methylcyclopropyI)-1,2,4-oxadiazole-5-carboxylic acid
instead of 3-
methylbenzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 15 mg, 38% (last step)
ESI-MS: 665.5 [M+H]
187 Preparation of compound 11-197
0
0
N)-L
0-NH o0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
N6-methyl-2-(5-methyl-1,2,4-oxadiazole-3-carboxamido)-5-oxohexanediamide
Chemical Formula: C23H27N707
Exact Mass: 513.20
Molecular Weight: 513.50
The synthesis of compound 11-197 was performed according to compound 11-167,
using
5-methyl-1,2,4-oxadiazole-3-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 36 mg, 58% (last step)
ESI-MS: 514.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
377
188 Preparation of compound 11-198
oliN
0 0
N NH
N
S
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-5-oxo-2-(1,2,3-thiadiazole-4-carboxamido)hexanediamide
Chemical Formula: C22H25N706S
Exact Mass: 515.16
Molecular Weight: 515.54
The synthesis of compound 11-198 was performed according to compound 11-167,
using
1,2,3-thiadiazole-4-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 29 mg, 48% (last step)
ESI-MS: 516.3 [M+H]
189 Preparation of compound 11-199
0
0
N
)0L H 0
N NH
/ 'Y
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-5-oxo-2-(1,2,4-thiadiazole-5-carboxamido)hexanediamide
Chemical Formula: C22H25N706S
Exact Mass: 515.16
Molecular Weight: 515.54
The synthesis of compound 11-199 was performed according to compound 11-167,
using
1,2,4-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 35 mg, 56% (last step)
ESI-MS: 516.3 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
378
190 Preparation of compound 11-200
o
N
0
S ( H
Nj-L NH
N-r
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-5-oxo-2-(1,3,4-thiadiazole-2-carboxamido)hexanediamide
Chemical Formula: C22H25N706S
Exact Mass: 515.16
Molecular Weight: 515.54
The synthesis of compound 11-200 was performed according to compound 11-167,
using
1,3,4-thiadiazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in
step 6 (according to ZED3264).
Yield: 24 mg, 41% (last step)
ESI-MS: 516.3 [M+H]
191 Preparation of compound 11-201
0 0
N NH
N I 1_1
0 0
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-
2-(4-cyclopropy1-1,2,3-thiadiazole-5-carboxamido)-N6-methyl-5-oxohexanediamide
Chemical Formula: C25H29N706S
Exact Mass: 555.19
Molecular Weight: 555.61
The synthesis of compound 11-201 was performed according to compound 11-167,
using
4-cyclopropyl-[1,2,3]thiadiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 43 mg, 67% (last step)
ESI-MS: 556.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
379
192 Preparation of compound 11-202
0
ON
0 ( H 0
_ JI NrN
N I H
\ 0 0
(S)-2-(4-cyclopropy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: 0301-137N706S
Exact Mass: 623.25
Molecular Weight: 623.72
The synthesis of compound 11-202 was performed according to compound 11-3,
using 4-
cyclopropyl-[1,2,3]thiadiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 42 mg, 56% (last step)
ESI-MS: 624.4 [M+H]
193 Preparation of compound 11-203
0
0 H 0
,S Nr N
N H NrN
\N 0 0
(S)-2-(4-isopropy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C30H39N706S
Exact Mass: 625.27
Molecular Weight: 625.74
The synthesis of compound 11-203 was performed according to compound 11-3,
using 4-
(propan-2-yI)-1,2,3-thiadiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 36 mg, 52% (last step)
ESI-MS: 626.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
380
194 Preparation of compound 11-204
0
li
0 JCH 0
N
NN
N I H
0 0
(S)-2-(4-ethy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C29H37N706S
Exact Mass: 611.25
Molecular Weight: 611.71
The synthesis of compound 11-204 was performed according to compound 11-3,
using 4-
ethyl-1,2,3-thiadiazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
acid in step 6 (according to ZED3264).
Yield: 33 mg, 47% (last step)
ESI-MS: 612.4 [M+H]
195 Preparation of compound 11-205
0
N
0 0
SNCNNN
N
0 0
0
(S)-2-(4-formy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxonexanediamide
Chemical Formula: C28H33N707S
Exact Mass: 611.22
Molecular Weight: 611.67
The synthesis of compound 11-205 was performed according to compound 11-3,
using 4-
(hydroxymethyl)-1,2,3-thiadiazole-5-carboxylic acid instead of 3-
methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 13 mg, 32% (last step)
ESI-MS: 612.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
381
196 Preparation of compound 11-206
0
OA
o
N
H 0
,S NNYLNN
No I H
0 0
HO
(S)-2-(4-(hydroxymethyl)-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-5-oxohexanediamide
Chemical Formula: C28H35N707S
Exact Mass: 613.23
Molecular Weight: 613.69
The synthesis of compound 11-206 was performed according to compound 11-3,
using 4-
((tetrahydro-2H-pyran-2-yloxy)methyl)-1,2,3-thiadiazole-5-carboxylic acid
instead of 3-
methylbenzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264). The
tetrahydropyranyl (Thp) protecting group was cleaved by TFA in the final step.
Yield: 9 mg, 46% (last step)
ESI-MS: 614.4 [M+H]
197 Preparation of compound 11-207
0
0 ( H 011
N ,/y-LNNNNH
0 0
(S)-N1-(1-(2-(1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-2-(1-methy1-1H-imidazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C29F-137N706
Exact Mass: 579.28
Molecular Weight: 579.65
The synthesis of compound 11-207 was performed according to compound 11-3,
using 1-
adamantanamine instead of 2-adamantanamine in step 2 (according to ZED3905)
and
1-methyl-1H-imidazole-5-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic
.. acid in step 6 (according to ZED3264).
Yield: 64 mg, 71% (last step)
ESI-MS: 580.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
382
198 Preparation of compound 11-208
0
0)-LN
0 0
1j11
NH N
H 0 0
(S)-N1-(1-(2-(((1S,2R,5S)-6,6-dimethylbicyclo[3.1.1]heptan-2-yl)methylamino)-2-
oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methyl-2-(1-methyl-1H-imidazole-2-carboxamido)-5-
oxohexanediamide
Chemical Formula: C29H39N706
Exact Mass: 581.30
Molecular Weight: 581.66
The synthesis of compound 11-208 was performed according to compound 11-3,
using
(-)-cis-myrtanylamine instead of 2-adamantanamine in step 2 (according to
ZED3905)
and 1-methyl-1H-imidazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 43 mg, 59% (last step)
ESI-MS: 582.5 [M+H]
199 Preparation of compound 11-209
0
0)-L
N
0
HNYNN"..
0 0
(S)-N1-(1-(2-(((1R,2R,5R)-6,6-dimethylbicyclo[3.1.1]heptan-2-yl)methylamino)-2-
oxoethyl)-2-oxo-
1,2-dihydropyridin-3-y1)-2-(1H-imidazole-4-carboxamido)-N6-methyl-5-
oxohexanediamide
Chemical Formula: 028F137N706
Exact Mass: 567.28
Molecular Weight: 567.64
The synthesis of compound 11-209 was performed according to compound 11-3,
using
(-)-cis-myrtanylamine instead of 2-adamantanamine in step 2 (according to
ZED3905)
and 1-methyl-1H-imidazole-2-carboxylic acid instead of 3-methylbenzo[b]furan-2-
carboxylic acid in step 6 (according to ZED3264).
Yield: 31 mg, 55% (last step)
ESI-MS: 568.4 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
383
200 Preparation of compound 11-210
0
ON
0 LI 0
N/ANf;J-LNThNH
H 0 0
(S)-N1-(1-(2-(3,5-dimethyladamantane-1-amino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methy1-2-(1-methy1-1H-imidazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C311-141N706
Exact Mass: 607.31
Molecular Weight: 607.70
The synthesis of compound 11-210 was performed according to compound 11-3,
using
3,5-dimethy1-1-adamantanamine instead of 2-adamantanamine in step 2 (according
to
ZED3905) and 1-methyl-1H-imidazole-5-carboxylic acid instead of 3-methyl-
benzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 46 mg, 66% (last step)
ESI-MS: 608.5 [M+H]
201 Preparation of compound 11-211
0
)*LN
0 0
1 NC
H 0 0
'IN
(S)-N1-(1-(2-(3,5,7-trimethy1-1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-
3-y1)-N6-methyl-2-(1-methyl-1H-imidazole-5-carboxamido)-5-oxohexanediamide
Chemical Formula: C32F-143N706
Exact Mass: 621.33
Molecular Weight: 621.73
The synthesis of compound 11-211 was performed according to compound 11-3,
using
3,5,7-trimethy1-1-adamantanamine instead of 2-adamantanamine in step 2
(according to
ZED3905) and 1-methyl-1H-imidazole-5-carboxylic acid instead of 3-methyl-
benzo[b]furan-2-carboxylic acid in step 6 (according to ZED3264).
Yield: 53 mg, 70% (last step)
ESI-MS: 622.5 [M+H]
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
384
202 Preparation of Reference Compound 6
0
OA
0 0
NjEr N)'L N-r
I I
0 0
(S)-N 1 -methy1-5-(nicotinamido)-2-oxo-N6-(2-oxo-1 -(2-oxo-2-
(phenethylamino)ethyl)-1 ,2-dihydropyridin-3-yl)hexanediamide
Chemical Formula: C28H30N606
Exact Mass: 546.22
Molecular Weight: 546.57
The synthesis of reference compound 6 was performed according to compound 11-
3, using
2-phenylethylamine instead of 2-adamantanamine in step 2 (according to
ZED3905) and
nicotinic acid instead of 3-methylbenzo[b]furan-2-carboxylic acid in step 6
(according to
ZED3264).
Yield: 89 mg, 79% (last step)
ESI-MS: 547.4 [M+H]
Biological Examples
Example B-1. Inhibitory effect of the compounds according to the invention
Transglutaminase assay
For the determination of potency of inhibitors against tissue
transglutaminase, the
incorporation of dansylcadaverine into dimethylcasein (Zedira product T036,
Lorand et
a/., Anal Biochem, 1971, 44:221-31) was measured using recombinant human
transglutaminase 2 (Zedira Product T022).
The tissue transglutaminase is diluted in buffer (50 mM Tris-HCI, 7.5 mM
CaCl2, 150 mM
NaCI, pH = 7.4). The final concentration of TG2 in the assay is 10 nM.
A 10 mM inhibitor stock solution is prepared in DMSO, and from this a serial
1:2-fold
dilution series is prepared also in DMSO. Each of the initial dilutions is
subsequently
diluted 1:50-fold with buffer (50 mM Tris-HCI, 7.5 mM CaCl2, 150 mM NaCI, pH =
7.4) to
yield the final working dilutions containing 2% (v/v) DMSO.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
385
15 pl of inhibitor working dilution are added per well of a 96 well microtiter
plate. As
control, 15 pl of a 2% (v/v) DMSO solution prepared using the buffer mentioned
above
are added per well.
Immediately before starting the assay, 600 pl transglutaminase working
solution are
added to 11.4 ml assay buffer (50 mM Tris-HCI, 10 mM CaCl2, 10 mM glutathione,
2.5%
glycerol, 16.7 pM dansylcadaverine, 4 pM N,N-dimethylcasein, 200 mM NaCI, pH =
8.0).
285 pl of this reaction mix are added per well containing the inhibitor.
Increase in fluorescence is measured using Aex = 330 nm and Aem = 500 nm at 37
C for
30 min. A slope of the increase in fluorescence between 20 and 30 min is
calculated for
determination of the ICso value (inhibitor concentration at which 50% of the
initial activity
is blocked).
Analysis of enzymatic activity is performed by calculation of the slope of an
increase in
fluorescence intensity. ICso values are calculated by plotting the enzymatic
activity (as
percentage from control containing 2% DMSO instead of inhibitor) against the
inhibitor
concentration. ICso is defined as the inhibitor concentration blocking 50% of
initial enzyme
activity.
The inhibitory activity of the inventive compounds in regard to tissue
transglutaminase
(TG2) is shown in the following table 1 using ICso-values.
Table 1. efficacy of reversible TG2 inhibitors
A: ICso <40 nM, B: 40 nM ICso < 400 nM, C: 400 nM ICso < 2,500 nM,
D: 2,500 nM ICso < 10,000 nM
ICso IC50
Compound Compound
TG2 [nM] TG2 [nM]
11-2 A 11-117 B
11-3 A 11-118 A
11-4 A 11-119 B
11-5 A 11-120 B
11-6 A 11-121 B
11-7 A 11-122 C
11-8 A 11-123 C
11-9 A 11-124 B
II-10 A 11-125 B
11-11 A 11-126 B
11-12 B 11-127 B
11-13 A 11-128 B
11-14 A 11-129 B
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
386
11-15 A 11-130 B
11-16 A 11-131 B
11-17 B 11-132 C
11-18 B 11-133 C
11-19 B 11-134 C
11-20 C 11-135 B
11-21 A 11-136 B
11-22 A 11-137 B
11-23 B 11-138 C
11-24 B 11-139 C
11-25 B 11-140 B
11-26 B 11-141 B
11-27 B 11-142 B
11-28 A 11-143 C
11-29 A 11-144 C
11-30 A 11-145 B
11-31 B 11-146 A
11-32 B 11-147 A
11-33 B 11-148 A
11-34 B 11-149 A
11-35 B 11-150 B
11-36 B 11-151 B
11-37 B 11-152 B
11-38 C 11-153 C
11-39 B 11-154 B
11-40 A 11-155 A
11-41 B 11-156 B
11-42 B 11-157 B
11-43 C 11-158 B
11-44 A 11-159 B
11-45 A 11-160 B
11-46 B 11-161 B
11-47 A 11-162 B
11-48 A 11-163 B
11-49 A 11-164 C
11-50 A 11-165 C
11-51 B 11-166 C
11-52 C 11-167 A
2489-ClPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
387
11-53 B 11-168 B
11-54 B 11-169 A
11-55 B 11-170 B
11-56 B 11-171 A
11-58 B 11-172 B
11-59 B 11-173 B
11-60 B 11-174 B
11-61 B 11-175 C
11-62 A 11-176 C
11-63 A 11-177 C
11-64 B 11-178 B
11-65 B 11-179 C
11-66 C 11-180 C
11-67 C 11-181 C
11-68 B 11-182 B
11-69 A 11-183 B
11-70 A 11-184 C
11-71 A 11-185 C
11-72 A 11-186 C
11-73 B 11-187 C
11-74 C 11-188 C
11-87 B 11-189 C
11-88 B 11-190 C
11-90 B 11-191 C
11-92 B 11-192 C
11-94 A 11-193 C
11-95 B 11-194 B
11-96 B 11-195 B
11-97 B 11-196 C
11-98 B 11-197 C
11-99 B 11-198 B
11-100 B 11-199 B
11-101 B 11-200 B
11-103 B 11-201 B
11-104 B 11-202 B
11-105 B 11-203 B
11-107 B 11-204 B
11-108 B 11-205 B
2489-ClPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
388
11-109 B 11-206 B
11-110 B 11-207 B
11-111 A 11-208 C
11-112 A 11-209 C
11-113 B 11-210 B
11-114 B 11-211 B
11-115 B Ref. 1 C
11-116 B Ref. 2 B
Ref. 6 D Ref. 3 D
Ref. 1 (ZED3641) Ref. 2 (ZED1227)
00Me
0
ON %
H
H 11
N H H
NNN N
ThiN
/Y1\1 NN..-----yNõ,,,_õ.---õ,
/Y c H
----N 0 0 --N 0 0
Ref. 3 (A8, ZED1047) Ref. 6
o,,o o
o).N
H
OHOH
ll 0 fH Oil
H
1 HN NN-rN
0 0HN- yNN-rN
I
0 0
1\1 1\1
Example B-2. logD values of the inventive compounds
In order to classify the inventive compounds according to their lipophilicity,
LogD values
(distribution coefficient) were determined by means of the well-established
shake flask
method, measuring the partition of a compound between an octanol and phosphate-
buffered saline (PBS, pH 7.4) by HPLC.
The LogD is pH dependent and is a "predictor" for in-vivo properties. LogD
combines
lipophilicity (intrinsic structural property of the molecule, logP) and
ionizability (pKa).
Compounds with a moderate lipophilicity (LogD values from 0 to 3) are usually
advantaged for oral absorption, being in balance between solubility and
permeability.
However, sophisticated formulation of a compound might improve oral
bioavailability for
highly lipophilic compounds.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
389
Table 2. logD values of reversible TG2 inhibitors
A: logD <1, B: 1 logD <3, C: 3 logD <5
Compound logD Compound logD
11-2 B 11-117 C
11-3 B 11-118 B
11-4 B 11-119 C
11-5 C 11-120 C
11-6 C 11-121 C
11-7 C 11-122 C
11-8 C 11-123 C
11-9 B 11-124 B
11-10 C 11-125 B
11-11 B 11-126 A
11-12 C 11-127 A
11-13 B 11-128 B
11-14 B 11-129 B
11-15 B 11-130 A
11-16 B 11-131 A
11-17 B 11-132 A
11-18 B 11-133 A
11-19 C 11-134 A
11-20 B 11-135 A
11-21 C 11-136 B
11-22 C 11-137 C
11-23 C 11-138 A
11-24 B 11-139 A
11-25 B 11-140 A
11-26 B 11-141 A
11-27 B 11-142 B
11-28 B 11-143 A
11-29 C 11-144 A
11-30 C 11-145 A
11-31 B 11-146 A
11-32 B 11-147 A
11-33 B 11-148 A
11-34 B 11-149 A
11-35 B 11-150 B
2489-ClPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
390
11-36 B 11-151 B
11-37 B 11-152 A
11-38 C 11-153 A
11-39 B 11-154 B
11-40 C 11-155 B
11-41 C 11-156 A
11-42 B 11-157 A
11-43 B 11-158 A
11-44 C 11-159 B
11-45 C 11-160 B
11-46 C 11-161 A
11-47 B 11-162 A
11-48 C 11-163 A
11-49 C 11-164 C
11-50 A 11-165 C
11-51 A 11-166 B
11-52 A 11-167 A
11-53 B 11-168 A
11-54 B 11-169 A
11-55 A 11-170 A
11-56 B 11-171 A
11-58 B 11-172 A
11-59 B 11-173 A
11-60 A 11-174 A
11-61 B 11-175 B
11-62 B 11-176 C
11-63 B 11-177 C
11-64 B 11-178 C
11-65 B 11-179 B
11-66 B 11-180 C
11-67 C 11-181 B
11-68 A 11-182 B
11-69 B 11-183 B
11-70 B 11-184 B
11-71 B 11-185 A
11-72 C 11-186 A
11-73 B 11-187 C
11-74 B 11-188 A
2489-ClPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
391
11-87 C 11-189 A
11-88 C 11-190 A
11-90 B 11-191 A
11-92 C 11-192 A
11-94 B 11-193 A
11-95 B 11-194 A
11-96 B 11-195 A
11-97 B 11-196 A
11-98 B 11-197 A
11-99 C 11-198 A
11-100 C 11-199 A
11-101 C 11-200 A
11-103 B 11-201 A
11-104 B 11-202 A
11-105 C 11-203 B
11-107 C 11-204 B
11-108 B 11-205 A
11-109 C 11-206 A
11-110 C 11-207 A
11-111 B 11-208 B
11-112 B 11-209 B
11-113 A 11-210 B
11-114 A 11-211 B
11-115 C Ref. 1 A
11-116 C Ref. 2 B
Ref. 6 A Ref. 3 B
Example B-3. Caco-2 permeability assay of the inventive compounds
Permeability coefficients (Papp values) were obtained from Caco-2 barrier
studies
predicting oral/intestinal bioavailability of the tested compounds. The assays
were
performed by using CacoReadyTM ready-to-use kits from ReadyCell according to
the
manufacturers protocol.
It is considered that compounds bearing Papp values above 1x10-6 cm/s are
classified as
permeable whereas compounds bearing Papp values below 1x10-6 cm/s are
classified as
not permeable.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
392
Table 3. Caco2 permeability assay of reversible TG2 inhibitors
A: Papp < 1X10-6 CM/S, B: Papp 1X10-6 CM/S Papp < 10X10-6 CM/S
C: Papp 10X10-6 CM/S
Papp [CM/S] Papp [CM/S]
Compound Compound
x10-6 x10-6
11-2 B 11-117 B
11-3 B 11-118 B
11-4 B 11-119 B
11-5 B 11-120 B
11-6 B 11-121 B
11-7 B 11-122 B
11-8 B 11-123 B
11-9 B 11-124 B
11-10 A 11-125 B
11-11 B 11-126 B
11-12 A 11-127 A
11-13 B 11-128 B
11-14 B 11-129 A
11-15 B 11-130 A
11-16 B 11-131 A
11-17 B 11-132 A
11-18 A 11-133 B
11-19 A 11-134 A
11-20 B 11-135 B
11-21 B 11-136 B
11-22 B 11-137 B
11-23 B 11-138 A
11-24 B 11-139 A
11-25 B 11-140 A
11-26 B 11-141 A
11-27 B 11-142 A
11-28 B 11-143 A
11-29 B 11-144 A
11-30 B 11-145 A
11-31 B 11-146 B
11-32 B 11-147 B
11-33 C 11-148 B
11-34 C 11-149 B
2489-ClPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
393
11-35 B 11-150 B
11-36 B 11-151 B
11-37 B 11-152 A
11-38 B 11-153 A
11-39 B 11-154 B
11-40 A 11-155 B
11-41 B 11-156 A
11-42 B 11-157 A
11-43 B 11-158 A
11-44 B 11-159 B
11-45 B 11-160 B
11-46 A 11-161 A
11-47 B 11-162 A
11-48 B 11-163 A
11-49 B 11-164 B
11-50 A 11-165 B
11-51 B 11-166 B
11-52 A 11-167 B
11-53 A 11-168 A
11-54 A 11-169 B
11-55 A 11-170 B
11-56 A 11-171 B
11-58 A 11-172 B
11-59 A 11-173 A
11-60 A 11-174 A
11-61 A 11-175 A
11-62 B 11-176 B
11-63 A 11-177 B
11-64 A 11-178 B
11-65 A 11-179 B
11-66 A 11-180 A
11-67 B 11-181 A
11-68 A 11-182 B
11-69 B 11-183 B
11-70 B 11-184 A
11-71 B 11-185 A
11-72 A 11-186 A
11-73 A 11-187 B
2489-ClPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
394
11-74 A 11-188 A
11-87 B 11-189 A
11-88 A 11-190 A
11-90 A 11-191 A
11-92 B 11-192 A
11-94 B 11-193 A
11-95 B 11-194 A
11-96 B 11-195 A
11-97 B 11-196 A
11-98 B 11-197 A
11-99 B 11-198 A
11-100 B 11-199 A
11-101 B 11-200 A
11-103 B 11-201 A
11-104 B 11-202 A
11-105 B 11-203 A
11-107 B 11-204 A
11-108 A 11-205 A
11-109 B 11-206 A
11-110 B 11-207 A
11-111 A 11-208 A
11-112 A 11-209 A
11-113 A 11-210 A
11-114 A 11-211 A
11-115 B Ref. 1 A
11-116 B Ref. 2 A
Ref. 6 A Ref. 3 A
Bioavailability studies
Presumed from promising Papp values (permeability coefficients, see below),
the inventors
had proven the oral bioavailability of the inhibitors of the present
application by the
representative compounds 11-3, 11-15, and 11-28. For this selected set of
representative
compounds, pharmacokinetic profiles were determined in male C57BL/6 mice (N=3,
each). Briefly, the compounds were administered as single-dose soluble, oral
formulation
[20 mg/ml in PBS / (2-HydroxypropyI)-R-cyclodextrin formulation] at 200 mg/kg.
Plasma
samples were taken at (0, 0.25, 0.5, 1, 2, 4, and 6 hours) and analyzed by LC-
MS to
determine the concentration of the representative compounds.
The calculated pharmacokinetic parameters are summarized in the table.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
395
Cmax Cmax AUCt Kei MRT t112 CL/F Vd/F
R2
[ng/m L] [nM] [ng/m L*h] [l/h] [h] [h] [L/h/kg]
[L/h/kg]
11-3 13,594 21,588 12,023 1.0 1.0 0.7 16.6 16.3 0.87
11-28 15,143 23,277 14,468 0.6 1.6 1.1 14.8 23.8 0.98
11-15 32,322 48,626 36,878 0.9 1.1 0.9 6.1 7.5 0.93
Cmax: maximum plasma concentration.
AUCt: area under the plasma concentration time curve from administration to
last observed
concentration at time t measured by trapezoidal rules.
Kel: is estimated by the linear regression of the logarithm of the terminal
concentration as a
function of time. Point used to calculate the Kel are selected the 'Best Fit'
option of Winnonlin.
MRT: mean residence time.
t112: is calculated by application of the equation In2 /
CL/F: apparent plasma clearance calculated as follow: Dose/AUCinf.
Vd/F (L/kg): apparent volume of distribution following administration. The
parameter are calculated
as Vd/F = (CL/F) / Kei
R2: correlation coefficient.
Papp [CM/Si
IC50 [nM] ICoo [nM]
*10-6
11-3 23 180 8.4
11-28 24 240 7.4
11-15 35 375 5.9
The corresponding PK profiles reveal that the plasma levels for all
representative
compounds exceed the ICoo for 3.5 hours and the ICso during the whole study (6
hours).
In summary, the high cmax values observed in the PK-studies exceed the ICoo
levels >100-
times. We therefore expect to occupy all active TG2 accessible.
Additionally, in a multiple-dosing PK study, 11-3 was orally administered to 3
mice with 200
mg/kg doses (dose volume 10 mL/kg) twice daily (12 h intervals). After the
animals were
sacrificed, the liver and lung were removed. The homogenates of the respective
tissues
were analyzed by LC-MS to determine the concentration of the compound. The
tissue
concentration in lung and liver after the eighth dose (four days) was 6,800
and 10,400
ng/g, respectively, showing that the compound reaches the tissue at
pharmacological
active concentration.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
396
CLAIMS
1. A compound of the general formula (I):
R1
0 0
H (I)
R2 N 1 Ny R3
H 1
0
wherein
L represents ¨L1¨ or _L1-L2¨; preferably ¨L1-L2¨;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
L2 represents a bond, ¨NRNI¨, ¨NRNICH2¨, ¨NRNICH2CH2¨, or
¨NRNICH(CH3)¨;
0
R1 represents
0
R2 represents
RN
-, 0 r-t8
1 __________ rm R11 1 0 R8 r __ s R8
Rii , s ,R8 __ \,,,/ R8
R10 R9
/ R9 R10 R9 ; R9 Rlo R9 ;
' , ,
RN RN RN RN RN RN
R11 N R8 R11 N, Rii \I N, 011 1\1 __
\ / --I4N -1_1K 1 /NI
i)n ( '------ R1.1 11 R8
) ________________________________________________________ N
/ R9 R10 `s / R9 R.- R9 Rlo N
, ' ,
RN R19
R1
ri 8 Doll 0 _ R1_1 0R 8 -.._ 0 .-,i-c8
- _-- ?'''- -
/ R8
0 Os)
Y ____________ N ) __ N N )\ __ N N-
R10
; R10
; ; R10
; R8 N------
'
RN
RN
R10 R11 S __ 11 -_ SN_R8 -_ 1\1,
N 11 IJ,
0µ ---22-Z 1 RS R8 ii-- __ --.-Qi----- 1 ____ ri ii RI
4\1
N R8 Ri0 R10 R10
, , , ,
'
RN RN ,,,,,, R12
R12
,N R10 Rio_."1,N_RN
R13
N
//
=Nj II, N, N rl '/'''l D13
''''''' NN LI ".\,,,j .µ
' N¨N ; N=N ; -."------- R14
N. R14
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
397
R12 R12
R12 R12 N R12 0 - = - ./ y, S - = = _ / y,
------------------- R13 N2 R13 ri, , ___L IR13
___<\)\____L IR13
----------------------------------------- ¨R13 -\
\ii \- R14 Ria
N R14 N-R14 NyR14 Rlo Rio
,
RN
R12 RN
NI - = - ./ y,
R12
R12 p12
- - -TR13l 13 õ..----"`
II 1 4R13
R14 N-----%\-1A c____\7R
14.
Rlo R." R14 R._
,
R13
RN RN RN
9 ,ii, R9 rri /IR9
N- -/,R9
S rR
R10 Rio
0) _
R10 N _______ - Rio _ \ -7----Dio -
R8 R8 ,S-,
" , -
R9 ,S -- ., N II
1\--7_, N , 1 NV T- -- ,---N
N
N _R10 I s)'''''T
\
. R8 \S-N \NI:=N ,
R8
,
R8
. S, - - 1\1,-- . N..õõ--
R ---- 1/ d--- R8--- -1 Ru---- 0 ji
-
N-N b-N , NI -- -- 0-N N
H
N 0 S S
\ \ \ ---
, N
, , ,
, ,
N ---eNNH ---eNNH ---( --( N
- N N __ \(
S S z S S z N
---eNNH ---eNNH
11 NINN3 N __ (
0 ()NJ
N,
_rN N ----eN0 ---eN0
,3
NI
- N ___eN0
N
, \( N
N Nj
, \
0 0 7 N ,
H S
NI_ s N
--CNT-1-- -N-
___
, --1"D5 H H ,
S _NI N
s
_---------\N
S-1\i/2
,
NO H
N
, , 0
0 ___"'-µ 0,N _NI
-1-/
-----N--N \NI-N-07
H , H
,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
_
\ F
z
N O _
_
z - z -
__ - z-z- z - z
z z
-
i / \ 7, / r z r z7, z
c
i z
/ \ z/ \
z' \ / `z / C / z)/ z)/
`z z)/ z' \ z' \ z' z' `z
, ¨z , z ¨ ¨/ ¨/ ¨z z --/ ¨ ¨
z z v=i
,
,
, '
'
) ____ z
(y,
,
,
_
_
co z z z_z , z z ,
/ \ \ / \ Z/ / \ / 3Z//- Z Z/ / Z Z/ Z '
1 ,
C r 9
- / \ Z / \
Z Z Z) / \ Z/ I \ Z I \ Z) /- Z) /
µZ Z) / \ Z)/ \ Z? / \ Z Z/ Z Z/
Zy ¨ ¨ __ / ¨ ¨ ¨/ ¨Z -=Z --/ ¨
¨/ ¨/ -=Z
tT7Z i j j j
N O
0
,
e?
,
cc
:s< 9
"0 N
.4 N
0,1 0
0
0 ,
,r
- _________________________________ \\ - Z Z Z
0r a)
ZVk' Z/ \ / \ Z Z/ \\Z / µµZ c /
Z/ / Z---Z / \ Z/ r Z c µµZ
0 0
w a.)
r`i Ce
z
i---\\ Z 0 a)
/ \
iz" ca
Cy Z / \ Z Z/ \ / \Z ( \ / Z2/
Z)/ \ Z)/ \ Z Zi/ Z/ Z/ (Z 1- a
ce --
, ¨Z , ¨ ¨/ ¨Z ¨Z Z ¨
¨/ ¨ ¨
(,) =
1 , , ,
Lu 0,
1 '
9 a)
o ct
,r a
õ
399
I\IN -.N,N
N
N , 1
1\r'
N-,N TheN
' ,
N - , ,1\1
''IrN
NN
,
--
-''rN
N.N
N-,N I
,
NN 1\INI NN
, ,
'--ry
N.N NN--N ,
,
--
N.
NN N.NN 1
1\IN
H -,
HN NH ,
,
, ,
.,
N NH ,
HN , NH, H'
N=\ 0 N=N N=\ N-
--N
\ N
,
N- N- N- -N -N
--- \ --/N __ ---
---
N N/
,
N ' -N -N -\ -\
1 (N
- /
N
\ //1\1 ,
\ /
N, N , N ,
,
\ \ 1
-- \ iN -
-- zN
1\1 --
\ /1\1
, 2 -- 1
N N
,
N , ______________________________ N N \\ , ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
400
N=\ N=\
/N
\ / N\/ N / N¨N N //N
N S
N \IV
N
N=N N=N N=N N=N N=N
\ N ,,N, N / N /
, _______ z/ ,
N=\ N=\ N=\ N=\ N-
--
\
\ //N, \ S \ _______ I\1 / , N
N N N
,
\ --
N /
/ // N¨N \\_N N
, , , ,
'
,
\ /71, \ S \ _______ I\1 , N
N N N
'
¨N ¨N --? /¨N
-- ________________ --i
--i --i
/
N¨ N //N
V/ N¨N N __ I
, ,
,
¨\ ___\
N ; N¨ // N¨N N
--
Ns, \ /1\1 N N N N N /
_______________ , N¨N N N ; /
sis¨/ N / or ¨N - \l
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and R" ; and preferably with 1 to 3 of the substituents
R11 ¨
R13;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl;
diamantyl;
hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one
or more C=C double bond(s) and/or are optionally substituted by one or more of
Ra; Ri3; Rc; Rd; and Re;
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
401
Ra, Rb, Rc, Rd, and Re represent independently of each other -H, -F, -Cl, -Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CONH2,
-CONHCH3, -CON(CH3)2, -CONHC2F15, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C21-15, -CH2CONH2, -CH2CONHCH3, -
CH2CON(CH3)2,
-CH2CONHC2F15, -NHCOCH3, -NHCOC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -
CH3,
-CH2CH2CH3, -CH(CH3)2, -
CH2CH2CH2CH3, -CH2CH2CH2CH2CH3,
-CH2CH(CH3)2, -C(CH3)3, -
CH2CH=CH2, -CH2CH=CH(CH3),
-CH2CH=C(CH3)2, -CH2CH=CHCH2CH3, -
cyclo-C3H5, -cyclo-C4H7,
-cyclo-05H9, -cyclo-C6H11, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-
05H9, -CH2-cyclo-C6H11, -Ph, -CH2-Ph , -CH2OCH3, -CH2OCH2CH3,
-CH2CH2OCH3, -CH2CH2OCH2CH3, -CH2CH2NHCH3, or -CH2CH2N(CH3)2,
or -NR6R7 is -N(C2H5)2, - -N - -N - -NO
or N/ \
\ ___________________________________________________________________________
/ =
,
R8, R9, R10, R11, R12, R13, and R14 represent independently of each other
-H, -F, -Cl, -Br, -I, -OH, -CN, -NO2, -CH3, -C2H5, -C3H7,
-CH(CH3)2, -C4H9, -
CH2-CH (CH3)2, -CH(CH3)-C2H5, -C(CH3)3,
-cyclo-C3H5, -CH2-cyclo-C3H5, -CH2OH, -CH2F, -CH F2, -CF3, -CH2CI,
-CH2Br, -CH21, -CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI,
-CH2-CH2Br, -CH2-CH21, -OCH3, -0C2H5, -0C3H7, -OCH(CH3)2,
-0C(CH3)3, -0C4H9, -OCH F2, -0CF3, -OCH2CF3, -0C2F5, -OCH2OCH3,
-0-CYCIO-C3H5, -OCH2-CYCIO-C3H5, -
0-C2H4-CYCIO-C3H5, -CH 0,
-COCH3, -COCF3, -00C2H5, -00C3H7, -COCH(CH3)2, -00C(CH3)3,
-COOH, -COOCH3, -CO0C2H5, -CO0C3H7, -COOCH (CH3)2, -COOC(CH3)3,
-00C-CH3, -00C-CF3, -00C-C2H5, -00C-C3H7, -00C-CH(CH3)2,
-00C-C(CH3)3, -NH2, -NHCH3, -NHC2H5, -NHC3H7, -NHCH(CH3)2,
-N HC(CH3)3, -N(CH3)2, -N(C2H5)2, -N(C3H7)2, -N[CH(CH3)2]2, -N[C(CH3)3]2,
-NHCOCH3, -NHCOCF3, -NHCOC2H5, -NHCOC3H7, -NHCOCH (CH3)2,
-NHCOC(CH3)3, -CONH2, -CONHCH3, -CONHC2H5, -CONHC3H7,
-CON HCH (CH3)2, -CON H-cyclo-C3H5, -CON HC(CH3)3, -
CON(CH3)2,
-CON (C2H5)2, -CON(C3H7)2, -CON [CH(CH3)2]2, -CON [C(CH3)3]2, -SO2N H2,
-SO2NHCH3, -SO2NHC2H5, -
SO2NHC3H7, -SO2NHCH(CH3)2,
-SO2NH-cyclo-C3H5, -SO2NHC(CH3)3, -
SO2N(CH3)2, -SO2N(C2H5)2,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
402
-SO2N(C3H7)2, -SO2N[CH(CH3)2]2, -
SO2N[C(CH3)3]2, -NHSO2CH3,
-NHSO2CF3, -NHSO2C2H5, -
NHSO2C3H7, -NHSO2CH (CH3)2,
-NHSO2C(CH3)3, -CH=CH2, -CH2-CH=CH2, -C(CH3)=CH2, -CH=CH-CH3,
-CECH, -CEC-CH3, -CH2-CECH, -Ph, -0-Ph, -0-CH2-Ph,
r1\1
* -NO IL:12)1 -Nr-N -- r
r1\1 r1\1
r / __ \ / __ \ / __ \
N\ /NH N\ or N\ /N
or R8 and R9 or R9 and R19 can form together one of the following five-
membered
or six-membered rings:
__o
_1\1
- I
or ---%N
or R12 and R13 or R13 and R14 can form together one of the following five-
membered or six-membered rings:
0
.0 .0 .-
H or I =
RN represents -H, -CH3, -C2F15, -C3H7, -CH(CH3)2, -C4H9, -CH2-CH(CH3)2,
-CH (CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -
cyclo-C4H7, -cyclo-051-19,
-CH2-cyclo-C3F15, -CH2-cyclo-C4H7, -CH2-cyclo-051-19, -CH2F, -CHF2, -CF3,
-CH2CI, -CH2Br, -CH21, -CH2-CH2F, -CH2-CH F2; -CF12-CF3, -CH2-CH2CI,
-CH2-CH2Br, -
CH2-CH21, -CH2-CH=CH2,
-CH2-CECH, -CHO, -COCH3, -00C21-15, -00C3H7, -COCH (CH3)2,
-00C(CH3)3, -CO-cyclo-C3H5, -CO-cyclo-C4H7, -CO-cyclo-051-19, -COOCH 3;
-COOC2H5, -COOC3H7 -COOCH (C H3)2, -COOC(CH3)3, -000CH2Ph,
-S02CH3, -S02CF3, -S02C21-15, -S02C3H7,
-S02CH (CH3)2,
-S02-cyclo-C3H5, or -S02C(CH3)3;
RN1 represents -H, -CH3, or -CH2CH3;
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
403
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers, a racemate, a solvate, a hydrate, or a pharmaceutically
acceptable
salt thereof.
2. The compound according to claim 1, wherein R2 represents
R1:LS R8 RN
-... 0 r,8
1 _______________ rm R11 0 R8
\ , __ S Rs
1 __ r , ___ r __ ,,
\ , R8
R10 R9 / R9 R10 D9 ' R9
Rio R9 ;
' , rµ , ,
RN RN RN RN
Rii N Rs Rii N, RN N, __ N,
1(\1 1 iN RI-1.0x __ Rii 0
R8
ii -1 ---
, i) __ ( N N
, Ro Rio ss , R9 R.,,r, 09 R10 /
; F\ ;
R10
R10 nµ
pp10 R11 5
' --0,---R8 i_s)--,'' R8 A- _,- ...... --1 N
R1S,,s _R8
0s) Os ir \ ir-
)--N `-', _ N
N N¨ N
Rio R8 N¨ - R8 Rio
' ,,, ' , ,
R" RN RN RN
\ 1
-._ S R8 -__1,,,, Rii N, ,N Itt Rio ----- ,N
Rio ,N,N_RN
N
) __________________________________ N
Rio Rio N-N
, ,
,
R12 R12 R12 R12
N R12
,N, pp L1,,.,. -)R1=3 13
N ---- R13 ------- -
R=- r
=rl
¨R=-
----V N--"
..õ.j = - \
R - -------------------------------------------------------------------
N=N , Dia N Ria N Ria N Ria
"
RN
Riz Riz Riz RN
R12
0----/Y,) S-----_,) i\i----/Y ,)
i\i----/Y,)
---R13 -- ---, ¨R13
--- 1 \TR13
Ria Ria Ria N----%\- iA
Rio Rio Rio R.-
,
R13 RN RN
Riz
,µ
R r_
14
N, R9
Riz
_I _] ii 7--- 9 r R13
N ."R /1
_
--. __ S
Ria R..14 R._in N --\___,-------Rio
, , , ,
RN R8 R8
S..õ1,--
R9
_ R10
pi0 N/r---"` DA 10 N, µ _,K N I
s
-- )-
--
¨IA \ N R8 \ `
, S NO 'N ,
S,-- R8
1\1/, II S,,,,' 1\1,-- . N
R8---- ,-- 0
)\--N -
ii N./ IT R8---- -/ Ru--
---
R8 , N-N b-N WC' , 0-N N
, , ,
H
N 0 S S
\ \ \ ---
, N
, '
, , ,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
404
---eNNH ---eNNH
(i \ IN
N ______ (
1,\ij N N __ \(
S S 7 S S y , N
---eNNH ---eNNH
ITIN3 N __ (
0 ONJ
,
-- A - - N N --(N3 N( Z
-_,7,0
i ________________________________ \N , N
NJ
,
0 0 7 N
, , H S
----7Nr-S H N1 .-- -- -e------N&\ -N
'S
' -- H
'
, ,
S------N __N S ___ /(--'-\--- N
---:-\-- N
-k-N,õ// \S-I N,// S'INI
, ,
H
N 0,_-N
-------N-No N-N-0 -----N, ------0-N&\
H H
j0---r-N -w 0
/
-"Q
-, I\1 .,
i --
I I I
N /
/ N
-, N -- -,
' N
,
.. 1\1 -.,N
I II
N Th\r N /
-, NõN
I -I
NN
,
-. N -. -,
'N 'N
N N
N
.. 1\1 I\1 -.,z1\1N
1 N 1
N 1\rz
'--1 N
1\1 Nle ,
, ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
405
'-'N '---N
I
N , N NN,
,
N N
I II -.fNI\I
Nõ, " NN NN,
, ,
---- -:-,-,--- :-..1
II 1 N
N
NK," N N
,
1
-..,...,.----.._
" ,
1
N N , N,
,
- N,
'N -,,1\1N -,,N ,N
1N 1 II
1\1 N ,
1 N f
-1\1-"Ni
1 II II
NN NN N.N
N'N' , ,
N,N -.N,N
N,
N 1
1\1
N
N -,,1\1 -, 1\1 '
NN, NN N N
-, --
IN 'N
NN NN I
N
,
NN N NN
, , '
.,
1
N.N NN-,N :N
, NN , ,
I
N.
NN N,NN NN
'
_, Icl NH
HN NH ,
,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
406
HN H , NH , '
N=\ 0 N=N N=\ N-
--- \ /NI --
\ N
N/ \ /IV
___________________________________________________________ ' ,
'
N¨ N¨ N¨ ¨N ¨N
¨ ¨ _____________ \ ¨ ¨ \ ¨ ¨ \ ;IV ¨ ¨
¨ ¨ ¨¨ ________________ / N\
N' , N,
_¨N ¨N ¨N \ /¨\
N
-- ¨\ /N z(
¨ ¨ ¨ ¨ / N ¨-\1\1 , N
N, N
______________________ , ,
,
\ ¨ \
N , \ / N/ __ \ N 1\1
N ¨ ¨ =
N N
N \\ // ,
,
N=\ N=\
¨ ¨ ¨ ¨
¨ ¨ /(1\1
\ / N / Ns / N
N¨N S
N
N sN N //N
, , ,
N=N N=N N=N N=N N=N
¨ ¨ \ ;NI ¨ ¨ ¨ ¨ ¨ ¨ ___ 1
N
\ ,,N N /
N _________________________________________________________
N=\ N=\ N=\ N,\ N-
- ¨ z(N ¨ ¨ ¨\ z N ¨ ¨ /N ¨ ¨ ____ /N¨ ¨
\//N , \ S \ N\/ N
N, N / N
,
______________________ , ,
N¨ N¨ N¨ N¨ N-
- ¨ __________ ¨ ¨ _________ \
N N
\ ¨ ¨ __
\ N ¨ ¨ / N\ ¨N, V / N /
N/ //, N¨N, \ I __
, ,
¨N ¨N Ns ¨N ¨N
-- zs(N ¨ ¨ \ /N¨ ¨ i
¨ ¨N ,
i / N ___ ¨ ¨
N
\//N , \ S I\1 ,
N N N
, , ,
¨N ¨N /¨N _ _ _¨N ¨N
¨ ¨ __________ ¨ ¨ i
¨ ¨ /
\ N N\/ N /
N //N
N¨N _N 'IV
' ,
, , ,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
407
__\ __\
- - m --- m --i N --- /N -- --i /N
\ '\ /(
\ ,N ( N N N \ S N S
N ; N¨// // N-N N
,
,
¨\
1/N
Nss ___________ \ ,N N N
/ N N N, /
N N-N ; N Ni or \N-N ;
, , ,
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and RN; and preferably with 1 to 3 of the substituents
R11 ¨
R13 and the substituents R9 ¨ R14 and RN have the meanings as defined in claim
1.
3. The compound according to claim 1, wherein R2 represents
RN
0 m
- ,O r,8
1 __ r R11 0 R8
\ , __ 1 S Rs r Rii S R8
\ / -_ ii
\ / R8
R10 R9 ; / R9 R10 R9 ; / R9
R10 R9 ;
;
;
RN RN RN RN RN RN
R11 N R8 R11 N, R11 N, _ _ N, N I
R11
11
R=___(-r__R 8
K _ -
\
N \ i
N
/ R9 Rlo ss / R.n R10 R.,,,, Rlo
RN Rio
R1
N 8 Ril 0 R
_ 11 0 IR 8 - - 0 R8
- - 1---R - , ?-
0 - 0? R8
Y __ N ) N ________ N Y N R1 R Rio Rio
R8 N¨ '
,
RN RN
,
,
R10 RZyS, _ Rii s ,rµ8 -_,SR8 --õ,1`1,N Rii N,
C)\2 ir" 'N
R
N R8 __ N N N N io Rio Rio
,
RN RN R12 R12
,N Nir--
R10 R10.,,N ,N e. RN
\
, N , õN, oN ------------ r1
R13
-
R = -
12
N-F\
\
, N-N ; N=N ; I-C.--,14 N
R14
;
R12 R12
R12 R12
N R12 0---__/' s
I , p13 S/,) 13
R13 N/ Ri3 r
\
,,, 1 A
N R14 N R14 N R14 R10 R." R10 R.,
,
RN
D. 12 RN
N' ,712
N--/y,' s R12
,õ------.../,:',2
p12
- - -\TR13 - - 1 \ R." TR13 ''/ 13 -ji 1
R13
R14 N--%\- 1 A R14
R
\/\\--
Rio Ri 4
, , , ,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
408
R13 RN RN
RN \ R:
v____ R14 N R9 rii-/,R9
2, ___________ N rNiR9in Ri0
inX S ri
¨ __ _IR ,.,
R._ N - \___,------- N .-,1 o 0 ,
, ,
,S, - -
R9 ,S - - R8 R8
- S NI , I
N\ N6- \ I . )---
)\--N
õ, R10
, \" N R8 \S-N i\l'N , R8
Rd
N,
R ---- 1/ N' - R8--- -I R'a ----- 1/
N-N \O-N N-C)
,
H
N 0
\ \ \ N __ \(
, , , N
, ,
,
- -- -eN NH ¨ --eN0 - - S
N TI
N
- -c _________________________________________________________ \ N
IJ
' I
I I
N
,
- N N '' ---
NN I N N
,
NH .,
. ., .,
. NH
N NH ,
, H
N¨\
- - -_
or
N,
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 ¨ R14 and RN; and preferably with 1 to 3 of the substituents
R11 ¨
R13 and the substituents R9 ¨ R14 and RN have the meanings as defined in claim
I.
4. The compound according to claim 1, wherein the compound has the formula
(lb)
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
409
0
--- -N
i
R7
0 0
H
N ,L, 3
R2 N 1 N R
H
0
(lb)
wherein
L represents ¨L1¨ or _L1-L2¨; preferably ¨1_1-L2¨;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
L2 represents a bond, ¨NRNI¨, ¨NRNICH2¨,
¨NRNICH2CH2¨, or
¨NRNICH(CH3)¨;
R2 represents
RN
R11 0 Rs - _ S R8 R11 S R8 -- 8
\-11( R
R10 R9 ;
' R9 R10 R9 ;
' R9 R10
D9
, ; "
,
RN RN RN RN
R11 N N
R8 R11 , Rii N , R11 0 N _ _
Rii 0 R 8
/ ( n --1(\1 ---\ -----
__--
) ________________________________________________________ N N
R9 R10 `, / Ro R10 R9 R10
, = ,
; Rlo
R10
1 R
N Os
N¨ 0? R10 Rii S
R8 0?,----x ------- - - R1-_.1 S R8
)
N
R19 R8 N¨ ' R8 Rio __ N N /
, ,
RN
RN RN RN
-_ S Rs - _ ,s,N,,s,
1
N Rii N,
rill 1 __ /N RioN,N....RN Nio
N ¨1\¨N ,N
R10 Rio , '
N¨N
,
; ' , ' ,
,
R12 R12 R12 R12
R13 , D N
13 13
--------------------------- ¨ R R ----------------------------- N/
- N'
\ NR13
N=N R14 N R14 N R14 N
R14
,
RN
R12 R12 Riz
R12 0---__ _ S----_.// _ N--__// RN
N
r --R,, ---R ' - - R13
9
R14 R14 R14 _ (NR
N R14 Rlo Rlo R10
;
RN R12 R12 R12
N---__/y, - S H
--- 1 \R13
N .%\
N 13
R14 13 N -
-
7 \ 7R ----" i A
R
r-,.., , , R14 R14
,
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
410
R13 RN
RN H
, ri R9 N ,
,
NR14 Nk.õ..-S rN,R910 _r /I
R1 ON) ___________ S -
R -
, ; 0
,
õ
1
D___I_Im 9 R9
/
R10 ,1\1\1-1./=-= R10
N NN
; S ,µ.
, ' ,
,
N N , ,Sx
- N , I Nks_NI
N N NN/ \N R8
,
,S - IR
- ;t3
N7T ,S,-- ,- n N,-' n N--
-----N IR`-'n ---\ II NT R --- -! R ----- 1/
R8 N-N \O-N IN- Or
, , ,
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl,
diamantyl,
hexamethylenetetraminyl, and the afore-mentioned residues optionally contain
one
or more C=C double bond(s) and/or are substituted by one or more of Ra, Rb,
Rc,
Rd, and Re;
Ra, Rb, Rc, Rd, and Re represent independently of each other -H, -F, -Cl, -Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CONH2,
-CONHCH3, -CON(CH3)2, -CONHC2F15, -CH2CO2H, -CH2CO2CH3,
-CH2CO2C2H5, -CH2CONH2, -
CH2CONHCH3, -CH2CON(CH3)2,
-CH2CONHC2F15, -NHCOCH3, -NHCOC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R4 represents -NR6R7;
R6 and R7 represent independently of each other -H, -
CH3,
-CH2CH2CH3, -CH(CH3)2, -
CH2CH2CH2CH3, -CH2CH2CH2CH2CH3,
-CH2CH(CH3)2, -C(CH3)3, -CH2CH=CH2, -
CH2CH=CH(CH3),
-CH2CH=C(CH3)2, -CH2CH=CHCH2CH3, -
cyclo-C3H5, -cyclo-C4H7,
-cyclo-05H9, -cyclo-C6H11, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-
05H9, -CH2-cyclo-C6H11, -Ph, -CH2-Ph, -CH2OCH3, -CH2OCH2CH3,
-CH2CH2OCH3, -CH2CH2OCH2CH3, -CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR6R7 is -N(C2H5)2, - -1\I -10 - NO
or N/\ ).
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
411
RI4 represents -H, -CH3, -C2E-13, -C3H7, -CH(CH3)2, -
C4H9,
-CH2-CH(CH3)2, -CH(CH3)-C2H3, -C(CH3)3, -cyclo-C3H5, -cyclo-C4H7,
-cyclo-051-19, -CH2-cyclo-C3F15, -CH2F, -CH F2, -CF3, -CH2CI, -CH2Br,
-CH21, -CH2-CH2F, -CH2-CHF2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br,
-CH2-CH21, -CH2-CH=CH2, -CH2-CECH, -CHO, -COCH3, -00C21-15,
-00C3H7, -COCH(CH3)2, -00C(CH3)3, -COOCH3, -CO0C2H5, -CO0C3H7,
-COOCH(CH3)2, -COOC(CH3)3, -COOCH2Ph, -S02CH3, -S02CF3,
-S02C21-15, -S02C3H7, -S02CH(CH3)2, or -S02C(CH3)3;
RNi represent -H, -CH3, or -CH2CH3;
and R8 - R14 have the meanings as defined in formula (I);
or a diastereomer, an enantiomer, a mixture of diastereomers, a mixture of
enantiomers, a racemate, a solvate, a hydrate, or a pharmaceutically
acceptable
salt thereof.
5. The compound according to any one of claims 1 - 4, wherein
L1 represents -CH2-, or -CH2C0-;
L2 represents a bond, -NR'-, -NR1ICH2-, or -NRNICH(CH3)-;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl, 4-homoisotwistyl, adamantyl, or
diamantyl,
and the afore-mentioned residues optionally contain one or more C=C double
bond(s) and/or are substituted by one or more of Ra, Rb, Rc, Rd, and Re;
and Ra, Rb, Rc, Rd, Re and RI" have the same meanings as defined in claim I.
6. The compound according to any one of the claims 1 - 5, wherein
R2 represents
Rio
0 R8
0 R8 _ S R8 RSrR8
8
C)\ C)\) R
Rii3 R9 R9 Rio R9 R8 N-
N
RN RN
µ` R10 R11 S R11 S 8 S R8 - R8
C)\
) __________________________________ N
R- Rio Rio Rio
RN RN
Riz Riz
Rii N\ Rio
N. RN pe, 13
---------------------------------------------------------------------- -R13
N N--- -- u .=
N=N N
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
412
RN
R12 R12 Ri 2
R12 0 S /Y,) N
----------- /') R13 - - j-R13 - - T R13 \ ---_
¨R13
-\R14 i,
N R14 R10 R ., R10 R10 R14
, , , ,
RN R13
_r /TR%)
111=Z:
R12 R12 RN
N ----/Y,) . . . . / - - - - - - -----/, µs I
KR13 R14
N\ -%-R14 A-)
1 (1 _________________________________________ S r_. R9
R14 p . _ N
, ' ' , ' ' ,
,
RN - R8
ri R9 ,
N
D._.1, 9
1 -
(D) i 4. --/R1 0 N6'
-
\N
, 0 , S R8 \S-N
, , ,
R8 R8
N 1 , S,-- , N, - - N
R - - - -
,---N ,
S)- N6- R'a --i -1 R`-'Q ---
11
R8 N-N \O-N N "C) Or
, , ,
and R8 - R14 and RN have the meanings as defined in claim 1 or 2.
7. The compound according to any one of the claims 1 -6, wherein the
compound has
any one of the formulae (IV-a) - (IV-o) and (V-a) - (V-d):
0
0
0 )16
)-L NH
0
NR
"6
H 0 c 0 RC
N
R2 N N L2
0 ( H 0 Rb
R2j NH 1 NI-1 1- ,1 N-1¨L2
A )-L Rb
1
0
HO 0 Ra Ra
(IV-a) (IV-b)
o o
o .
NR6 o)L N. R6
H H Rb Ra
0 A 0 0 ( H 0 H
N L2 A N
Rc
R2 N 1 N R2 N 1 N L2
H 0 0 RRb Ra H
0 0 Rd
b
(IV-d)
(IV-c)
o o
o AN, R6 0 )-L N- R6
Rb Ra
H Rb Ra H Rc
0 H 0 Rc 0 0
A j(H
A N L2
R2 N N N L2
0 0
R2 N 1 N
H
0 0 Rd H
(IV-a) (IV-f)
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
413
O Rd 0
(DA R6 0 R6
N - N -
H Rc H Rb Ra
0 0 0 0 Rc
A H II
N N L2 AN JC
1111N ...-----õ,õ. L2
H
R2 N 1 R2
AN
0 H
0Ji 0 Rd
(IV-g) (IV-h)
0 0
0)- R6
N - Ra oN - R6
H
Rb j H
0 H 0 Rc 0 H 0
A N L2
R2 N 1 N
H
0 0 Rd H
0 U 0
(IV-i ) (IV-j)
0 0
0AN R6 if :!:.;:C c:1)- N , R6 7p
-
H H
0 iCH 0 0 0
..----õ,_õ L2 A N
J
R2 N 1 N R2 N 1 NL2
H 0 0 H
0 0
(IV-k) (IV-I)
o o
o NI- .R6
0
-NH L2
R2 NH H N L2 H R2NHIII
N
0 0 0 ,d 0
(IV-m) (IV-n)
0 0
0, NH . R6 0 N R6
- -
H
0 0 0 H 0
0 I\1)-L 12
...--,...õ_õ. -__
R2 NH ).Ntr " - N L2
1 R8 \ I hi 1 N ._ R3
0 0 0 0
Ri 0
R9
(IV-o)
(V-a)
0 o
o)-N ,R6
(DN - R6
H H
0 H 0 0
S 1 2
,----,_.___R3 SN /CH
N
R8 \ 1 N N 1 N R8 1 N L2-- R3
R10 Rio
O 0 N 0 0
R9
(V-c)
(V-b)
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
414
0
0 )-L N , R6
H
0 iFi 0
R1i 0 N L2
N
R12 d I
\ 0 0
Rio
R13 ¨
(V-d)
and R2; R3, R6, R8, R9, R1 0 ; R1 1 ; R1 2 ; R1 3 ; Ra ; RI3 ; Rc ; Rd L
and - . 2
have the same
meanings as defined in claim 1.
8. The compound
according to any one of the claims 1 ¨ 7, wherein
R2 represents
o
0 -- -(:)¨ Br -._ q
____O______ 0- " 0
-S -*
(:)-Br
H214
Br, ; Br ;
s B r \_-
S --- Br - -
\ // \ / y __ ,
_.-- -----u-C1
S S
Br
Br, ; Br) ; Br ,
S ---1Sr ci __-CSBr -----Sz-C1 --
- S Br
'Br, CI , Br ; Br ; CI ; Me Br ;
--"'
I CI
S S S Br
__- Br ci C --1 S Br HN
NC ___ 0---
, \
,
,
0
--- 0
------0
S
N ,--
H2N N meo - - - <72 1N1 - - - ,)-----(/ ) 0
I
H ; 0 0--J N - N-NBr
, ,
S,/ sr 5
__-C1 -------s__-Br -------
s_-C1 Br----s Br
- - I N N ) __ N N N
N --N , ,
CI __---C1 -jS--CF3 -- --)S __ Br - - -S__-C1 - -
-S-CF3
N N N ) __ N ) N
CI ; Br
-js,_-0Me -js-Ph :-.-)s-- ISN-CI ' C_ //\---NH
N N N N N // <=
N-N
0 N
I ftIcl NI, ,H 1
,N
,N,,..,.--- 1
-,,N,N NN ',N ,N
N\ N\
1 HN/"'----1 \\ , __ > / >\ __ N )\ N N N
N
N N ,
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
415
NO2
N,,CO2H -,._õ..--.--.., ,CO2Me
N=N , CO2Me 1\1" N , The , The
H
N
N ,N'-,1\1 N C)r--- / c
I ---
I 1\1 N
0-- \ __________ -::
-- Br''' -
,
H H
1\1- \ \
1\1õ.
r,_ HN"-
HN---r: \/ 0 ._-------1N- -- ___ ___-N
N S ;
0
0 0
-N---- --------------- 0 -- \ -- \ -- \
--
S-- __________ N \
; CI Br
, IJO1,
I ,
S
-- \
, o ; o----- s ,
, , ,
F H
S H \ N
S N N
-- \
Br
H
N
H S
F ___ ----<\ --
F N N
OH ;
.- -------------------------------- u
,,
F3
\ --
H - --
-
N HN 1 I I
N N-'N NN
:
, I\L -
S -- ,S --
fel"! ----
N N) \s-i\I s-1--- N's-'-' <\ 11--
N \ jrN
'N,--N *- N N---,,1,1 \N
;
S ;
õ--
,S - ,S -- H2N
\\ \\ I
N N \ N N I N
.\\ _..
\N \N N N H N
ci\?,),-- N ---
0 ; HO, N-0 O'N , or
,
N --
O-N and
R6 represents -H, -CH3, -CH(CH3)2, -CH2CH2CH3, -CH2CH=CH2,
-CH2CH2CH2CH3, -CH2CH(CH3)2, -C(CH3)3, -CH2CH2CH2CH2CH3,
-cyclo-C3H3, -cyclo-C3119, -cyclo-C6H11 or -CH2-cyclo-C3H3.
2489-CIPO-DESCRIPTION-ZED-P04403W024.docx
Date Recue/Date Received 2022-06-30
416
9. The compound according to claim 1 selected from the group consisting of:
Compound Name
11-2: (S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-3: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(3-methylbenzofuran-2-
carboxamido)-5-oxohexanediamide
11-4: (S)-2-(3-chlorobenzofuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-5: (S)-2-(4-bromobenzofuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-6: (S)-2-(4-bromobenzofuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-7: (S)-2-(benzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-8: (S)-2-(5-bromobenzo[b]thiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-9: (S)-2-(1H-indole-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-10: (S)-2-(4,5-difluoro-1H-indole-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-11: (S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(3-methy1-1H-indole-2-
carboxamido)-5-oxohexanediamide
11-12: (S)-2-(1H-benzo[d]imidazole-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-13: (S)-2-(2,3-dihydro-1H-indene-2-carboxam ido)-N 1414242-
adamantylam ino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediam ide
11-14: (S)--(2-bromo-4-methylthiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
417
methyl-5-oxohexanediamide
11-15: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(4-methy1-2-
(trifluoromethyl)thiazole-5-carboxamido)-5-oxohexanediamide
11-16: (S)-2-(4-bromo-2-(trifluoromethyl)thiazole-5-carboxamido)-N1-
(1-(2-
(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-17: (S)-2-(2,4-dichlorothiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-18: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(2-methoxy-4-methylthiazole-5-carboxamido)-
N6-methy1-5-oxohexanediamide
11-19: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(4-methy1-2-phenylthiazole-5-
carboxamido)-5-oxohexanediamide
11-20: (S)-2-(2,4-dimethylthiazole-5-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-21: (S)-2-(5-bromo-3-methylthiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-22: (S)-2-(3,5-dibromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-23: (S)-2-(5-bromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-24: (S)-2-(5-chlorothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-25: (S)-2-(5-bromo-3-methylfuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-26: (S)-2-(5-chlorofuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-27: (S)-2-(5-chlorothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
418
methyl-5-oxohexanediamide
11-28: (S)-2-(2,5-dichlorothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-29: (S)-2-(2,5-dibromothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-30: (S)-2-(5-bromothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-31: (S)-2-(2-chloro-5-methylthiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-32: (S)-2-(2,5-dichlorothiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-33: (S)-2-(2,5-dibromothiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-34: (S)-2-(2-bromo-5-methylthiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-35: (S)-2-(2-bromothiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-36: (S)-2-(2-chlorothiazole-4-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-37: (S)-2-(2,5-dimethylfuran-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-38: (S)-2-(4,5-dimethylthiazole-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-39: (S)-2-(4-bromothiazole-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-40: (S)-2-(4-bromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
419
methyl-5-oxohexanediamide
11-41: (S)-2-(4-bromo-3-methylthiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-42: (S)-2-(3-bromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-43: (S)-2-(3-chloro-4-methylthiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-44: (S)-2-(4-bromo-5-chlorothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-45: (S)-2-(4,5-dibromothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-46: (S)-2-(4,5-dibromo-3-methoxythiophene-2-carboxamido)-N1-(1-(2-
(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-47: (S)-2-(4-bromofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-
2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
11-48: (S)-2-(4,5-dibromofuran-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-49: (S)-2-(4,5-dichlorothiophene-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-50: (S)-2-((S)-1-acetylpyrrolidine-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-51: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-1,2,3-triazole-5-
carboxamido)-5-oxohexanediamide
11-52: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(2H-tetrazole-5-
carboxamido)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
420
11-53: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(pyrazine-2-
carboxamido)hexanediamide
11-54: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-24(S)-1-methylpyrrolidine-2-
carboxamido)-5-oxohexanediamide
11-55: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-24(S)-pyrrolidine-3-
carboxamido)hexanediamide
11-56: (S)-2-((2S,4S)-4-bromopyrrolidine-2-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
11-58: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-24(S)-piperidine-2-
carboxamido)hexanediamide
11-59: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-24(R)-piperidine-3-
carboxamido)hexanediamide
11-60: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-24(R)-morpholine-3-carboxamido)-5-
oxohexanediamide
11-61: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(quinuclidine-3-
carboxamido)hexanediamide
11-62: (S)-methyl 3-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-
ylcarbamoy1)-5-nitrobenzoate
11-63: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(5-nitronicotinamido)-5-
oxohexanediamide
11-64: (S)-5-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-
ylcarbamoyl)nicotinic acid
11-65: (S)-methyl 5-(1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-
1,2-
dihydropyridin-3-ylamino)-6-(methylamino)-1,5,6-trioxohexan-2-
ylcarbamoyl)nicotinate
11-66: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(6-methylimidazo[2,1-b]thiazole-5-
carboxamido)-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
421
11-67: (S)-N1-(1-(2-(2-adamantyl(methyl)am ino)-2-oxoethyl)-2-oxo-1,2-
dihyd ropyridin-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-68: (S)-N1-(1-(2-(5-hyd roxyadam antane-2-am i no)-2-oxoethyl)-2-
oxo-
1,2-di hyd ropyrid in-3-y1)-N6-methy1-2-(3-methylbenzofuran-2-
carboxam ido)-5-oxohexanediam ide
11-69: (S)-N1-(1-(2-(5-fluoroadamantane-2-am ino)-2-oxoethyl)-2-oxo-
1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-70: (S)-N1-(1-(2-(5-chloroadamantane-2-am ino)-2-oxoethyl)-2-oxo-
1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-71: (S)-N1-(1-(2-(5-bromoadamantane-2-am ino)-2-oxoethyl)-2-oxo-
1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-72: (S)-N1-(1-(2-(5-methyladamantane-2-am ino)-2-oxoethyl)-2-oxo-
1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-73: (S)-N1-(1-(2-(2-carbonitrileadamantane-2-am ino)-2-oxoethyl)-2-
oxo-
1,2-di hyd ropyrid in-3-y1)-N6-methy1-2-(3-methylbenzofuran-2-
carboxam ido)-5-oxohexanediam ide
11-74: (S)-N1-(1-(2-(2-methyl ad am
antane-2-carboxylate-2-am ino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediam ide
11-87: (S)-N1-(1-(2-(1-adamantylmethylam ino)-2-oxoethyl)-2-oxo-1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-88: (S)-N1-(1-(2-(1-(1-adamantyl)ethanamino)-2-oxoethyl )-2-oxo-
1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-90: (S)-N1-(1-(2-((1R,2S,4S)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediam ide
11-92: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxam ido)-2-oxo-N6-
(2-
oxo-1-(2-oxo-2-((1S ,2S,4R)-1,7,7-trimethyl bicyclo[2 .2.11 heptan-2-
ylam ino)ethyl)-1,2-di hydropyrid in-3-yl)hexanediam ide
11-94: (S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(3-
methylbenzofuran-2-carboxamido)-5-oxohexanediam ide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
422
11-95: (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-1-ylam ino)-2-oxoethyl)-2-
oxo-1,2-
dihyd ropyridin-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-96: (S)-N1-(1-(2-(bicyclo[2.2.1]heptan-7-ylam ino)-2-oxoethyl)-2-
oxo-1,2-
dihyd ropyridin-3-y1)-N6-methy1-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-97: (S)-N1-(1-(2-(bicyclo[2.2.1]hept-5-en-2-ylam ino)-2-oxoethyl)-
2-oxo-
1,2-di hyd ropyrid in-3-y1)-N6-methy1-2-(3-methylbenzofuran-2-
carboxam ido)-5-oxohexanediam ide
11-98: (2S)-N1-(1-(2-(bicyclo[2.2.2]octan-2-ylam ino)-2-oxoethyl)-2-
oxo-1,2-
dihyd ropyrid in-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-99: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-
oxo-1-(2-oxo-2-((1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-
ylamino)ethyl)-1,2-dihydropyridin-3-y1)hexanediamide
11-100: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-
oxo-1-(2-oxo-2-((1R,2R,3R,5S)-2,6,6-trimethylbicyclo[3.1.1]heptan-
3-ylamino)ethyl)-1,2-dihydropyridin-3-yl)hexanediamide
11-101: (S)-N1-methy1-5-(3-methylbenzofuran-2-carboxamido)-2-oxo-N6-(2-
oxo-1-(2-oxo-2-((1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptan-
3-ylamino)ethyl)-1,2-dihydropyridin-3-yl)hexanediamide
11-103: (S)-N1-(1-(2-(4-homoisotwistane-3-am ino)-2-oxoethyl )-2-oxo-
1,2-
dihyd ropyrid in-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediamide
11-104: (S)-N1-(1-(2-(d iamantane-1-am i no)-2-oxoethyl)-2-oxo-1,2-
dihyd ropyrid in-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-105: (S)-N1-(1-(2-(d iamantane-4-am i no)-2-oxoethyl)-2-oxo-1,2-
dihyd ropyrid in-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-107: (S)-N1-(1-(1-adamantylmethyl)-2-oxo-1,2-d ihydropyrid in-3-y1)-
N6-
methyl-2-(3-methyl benzofu ran-2-carboxam ido)-5-oxohexaned jam ide
11-108: (2S)-N1-(14(3-hydroxy-1-adamantyl)methyl)-2-oxo-1 ,2-
dihyd ropyrid in-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
11-109: (2S)-N1-(14(3-bromo-1-adamantyl)methyl)-2-oxo-1 ,2-
dihydropyridin-
3-y1)-N6-methy1-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
11-110: (S)-N1-(1-(2-adamantylmethyl)-2-oxo-1,2-d ihydropyrid in-3-y1)-
N6-
methyl-2-(3-methyl benzofu ran-2-carboxam ido)-5-oxohexaned jam ide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
423
11-111: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(nicotinamido)-5-
oxohexanediamide
11-112: (S)-2-(isonicotinamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-
oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
0-113: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(pyridazine-4-
carboxamido)hexanediamide
11-114: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(pyridazine-3-
carboxamido)hexanediamide
11-115: (S)-N1-cyclopropyl-N6-(1-(2-(2-adamantylamino)-2-oxoethy1)-2-
oxo-
1,2-dihydropyridin-3-y1)-5-(3-methylbenzofuran-2-carboxamido)-2-
oxohexanediamide
11-116: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethy1)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(3-methylbenzofuran-2-carboxamido)-5-oxo-
N6-pentylhexanediamide
11-117: (S)-N1-allyl-N6-(1-(2-(2-adamantylamino)-2-oxoethy1)-2-oxo-1,2-
dihydropyridin-3-y1)-5-(3-methylbenzofuran-2-carboxamido)-2-
oxohexanediamide
11-115: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethy1)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(3-methylbenzofuran-2-carboxamido)-5-
oxohexanediamide
11-119: (S)-N1-ally1-5-(benzofuran-2-carboxamido)-N6-(1-(2-(2-
adamantylamino)-2-oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-2-
oxohexanediamide
11-120: (S)-2-(benzofuran-2-carboxamido)-N6-isopropyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
11-121: (S)-2-(benzofuran-2-carboxamido)-N6-cyclopropyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
11-122: (S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxo-N6-
phenylhexanediamide
11-123: (S)-2-(benzofuran-2-carboxamido)-N6-benzyl-N1-(1-(2-(2-
adamantylamino)-2-oxoethy1)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
11-124: (S)-2-(benzofuran-2-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
424
8-125: (S)-2-(2,5-dichlorothiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
11-126: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(4-methy1-2-(trifluoromethyl)thiazole-5-
carboxamido)-5-oxohexanediamide
8-127: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-2-(1-methy1-1H-1,2,3-triazole-5-carboxamido)-5-
oxohexanediamide
8-128: (2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1 )-2-(2,5-d ich broth iophene-3-
carboxam ido)-N6-methyl-5-oxohexanediamide
11-129: (2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methyl-2-(4-methyl-2-
(trifluoromethyl)thiazole-5-carboxamido)-5-oxohexanediamide
8-130: (2S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1)-N6-methy1-2-(1-methy1-1H-
1,2 ,3-triazole-5-carboxam ido)-5-oxohexanediam ide
8-131: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(2H-1,2,3-triazole-4-
carboxamido)hexanediamide
11-132: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1H-1,2,3-triazole-4-
carboxamido)hexanediamide
8-133: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-1,2,3-triazole-4-
carboxamido)-5-oxohexanediamide
8-134: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1H-1,2,4-triazole-3-
carboxamido)hexanediamide
8-135: (S)-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-1,2,4-triazole-3-
carboxamido)-5-oxohexanediamide
8-136: (S)-2-(benzofuran-3-carboxamido)-N1-(1-(2-(2-adamantylamino)-2-
oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-
oxohexanediamide
8-137: (S)-2-(benzo[b]thiophene-3-carboxamido)-N1-(1-(2-(2-
adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
425
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
11-138 dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-pyrazole-3-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
11-139 dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-pyrazole-4-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
11-140 dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-1H-pyrazole-5-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
11-141 dihydropyridin-3-y1)-N6-methy1-2-(4-methy1-1,2,3-thiadiazole-5-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
11-142 dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1,2,5-thiadiazole-3-
carboxamido)hexanediamide
(S)-2-(4-iodo-1-methy1-1H-pyrazole-5-carboxam ido)-N1-(1-(2-(2-
11-143 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
11-144 dihydropyridin-3-y1)-2-(1-methy1-1H-pyrazole-5-carboxamido)-5-
oxohexanediamide
(S)-N1-(1-(2-(2-adamantylam ino)-2-oxoethyl)-2-oxo-1,2-
II-145 dihyd ropyridin-3-y1)-2-(4-methy1-1,2,3-thi ad iazole-5-
carboxam ido)-5-
oxohexanediam ide
(S)-2-(benzofuran-2-carboxam ido)-N1-(1-(2-((1R,2R,4S)-
11-146 bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-147 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-(3-methylbenzofuran-
2-
carboxamido)-5-oxohexanediamide
(S)-2-(benzofuran-2-carboxam ido)-N1-(1-(2-((1S ,2R,4R)-
11-148 bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide
(S)-N1-(1-(2-((1S,2R,4R)-b icyclo[2.2.1]heptan-2-ylam ino)-2-
II-149 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-(3-methylbenzofuran-
2-
carboxamido)-5-oxohexanediamide
(S)-2-(benzofuran-2-carboxam ido)-5-oxo-N1-(2-oxo-1-(2-oxo-2-
11-150 ((1R,25,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-
ylamino)ethyl)-1,2-
dihydropyridin-3-yl)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
426
(S)-2-(3-methylbenzofuran-2-carboxam ido)-5-oxo-N1-(2-oxo-1-(2-
11-151 oxo-2-((1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-
ylamino)ethyl)-1,2-dihydropyridin-3-y1)hexanediamide
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-152 oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1)-N6-methyl-2-(4-
methyl-
1,2 ,3-thiadiazole-5-carboxam ido)-5-oxohexaned iam ide
(S)-N1-(1-(2-((1R,2R,4S)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-153 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-
1H-
pyrazole-5-carboxamido)-5-oxohexanediamide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-154 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(4-methy1-
2-
(trifluoromethyl)thiazole-5-carboxamido)-5-oxohexanediamide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-155 oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1 )-2-(2,5-d ich broth
iophene-3-
carboxam ido)-N6-methyl-5-oxohexanediam ide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-156 oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1)-N6-methyl-2-(4-
methyl-
1,2 ,3-thiadiazole-5-carboxam ido)-5-oxohexaned iam ide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-157 oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1)-N6-methy1-2-(1-
methy1-1H-
1,2 ,3-triazole-5-carboxam ido)-5-oxohexaned iam ide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-158 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(1-methy1-
1H-
pyrazole-5-carboxamido)-5-oxohexanediamide
(S)-N1-methy1-5-(4-methy1-2-(trifl uoromethyl)thiazole-5-
11159 carboxam ido)-2-oxo-N6-(2-oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
-
trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-dihydropyridin-3-
yl)hexanediamide
(S)-2-(2,5-dich lorothiophene-3-carboxam ido)-N6-methy1-5-oxo-N1-
11-160 (2-oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
trimethylbicyclo[2.2.1]heptan-2-
ylamino)ethyl)-1,2-dihydropyridin-3-yl)hexanediamide
(S)-N1-methy1-5-(4-methy1-1,2 ,3-th iadiazole-5-carboxam ido)-2-oxo-
N6-(2-oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
11-161 trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-3-
yl)hexanediamide
(S)-N1-methy1-5-(1-methy1-1H-1,2,3-triazole-5-carboxam ido)-2-oxo-
N6-(2-oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
11-162 trimethylbicyclo[2.2.1]heptan-2-ylamino)ethyl)-1,2-
dihydropyridin-3-
yl)hexanediamide
(S)-N1-methy1-5-(1-methy1-1H-pyrazole-5-carboxam ido)-2-oxo-N6-
11-163 (2-oxo-1-(2-oxo-2-((1R,2S,4R)-1,7,7-
trimethylbicyclo[2.2.1]heptan-2-
ylamino)ethyl)-1,2-dihydropyridin-3-yl)hexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
427
(2S)-2-(4-tert-butyl-1H-pyrrole-3-carboxam ido)-N6-methyl-N1-(1-(2-
11-164 (1-adamantylamino)ethyl)-2-oxo-1,2-dihydropyridin-3-y1)-5-
oxohexanediamide
(2S)-2-(4-cyano-1-methyl-1H-pyrrole-2-carboxam ido)-N6-methyl-N1-
11-165 (1-(3-(1-adamantylamino)propy1)-2-oxo-1,2-dihydropyridin-3-y1)-
5-
oxohexanediamide
(S)-N1-(1-(3-(2-adamantylam ino)-3-oxopropy1)-2-oxo-1,2-
11-166 dihydropyridin-3-y1)-2-(5-methoxyoxazole-2-carboxamido)-N6-
methy1-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-167 dihyd ropyridin-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
(S)-2-(2-acetyloxazole-4-carboxam ido)-N1-(1-(2-
11-168 (bicyclo[1.1.11pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-169 dihyd ropyridin-3-y1)-N6-methyl-2-(3-methylbenzofu ran-2-
carboxam ido)-5-oxohexanediam ide
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-170 dihydropyridin-3-y1)-2-(2-isopropyloxazole-5-carboxamido)-N6-
methy1-5-oxohexanediamide
(2S)-2-(benzofuran-2-carboxam ido)-N1-(1-(2-(bicyclo[3.2.1]octan-8-
11-171 ylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-
5-
oxohexanediamide
(2S)-N1-(1-(2-(bicyclo[3.2.1]octan-8-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-172 dihydropyridin-3-y1)-2-(3,5-dimethylisoxazole-4-carboxamido)-
N6-
methy1-5-oxohexanediamide
(S)-N1-(1-(2-(5-carboxy-2-am inoadam anta ne)-2-oxoethyl)-2-oxo-
11-173 1,2-di hyd ropyrid in-3-y1)-N6-methyl-2-(4-methylpyrim idi ne-
5-
carboxam ido)-5-oxohexanediam ide
(2S)-N1-(1-(2-(4-am i noadamantane-N ,N-dimethy1-1-carboxam ide)-2-
11-174 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxo-2-
(1,2,3,4-
tetrahydronaphthalene-2-carboxamido)hexanediamide
(S)-2-((S)-1,4-diazabicyclo[2.2.2]octane-2-carboxam ido)-N6-tert-
11-175 butyl-N1-(1-(2-(2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-5-oxohexanediamide
(S)-N1-tert-buty1-5-(1H-indole-3-carboxam ido)-N6-(1-(2-(2-
11-176 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-2-
oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
428
(S)-N1-tert-butyl-N6-(1-(2-(2-adam antylam i no)-2-oxoethyl)-2-oxo-
11-177 1,2-di hyd ropyrid in-3-y1)-5-(6-methylim idazo[2,1-b]th
iazole-3-
carboxam ido)-2-oxohexanediam ide
(S)-2-(benzo[d]thiazole-2-carboxam ido)-N1-(1-(2-((1S,2R,4R)-
11-178 bicyclo[2.2.1]heptan-2-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-cyclopenty1-5-oxohexanediamide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11-179 oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-cyclopenty1-2-
(imidazo[2,1-13]thiazole-6-carboxamido)-5-oxohexanediamide
(S)-N1-(1-(24(1S,2R,4R)-bicyclo[2.2.1]heptan-2-ylam ino)-2-
11180 oxoethyl)-2-oxo-1,2-di hydro pyridi n-3-y1)-N6-cyclo penty1-2-
(4-
-
hydroxy-6-(trifluoromethoxy)quinoline-3-carboxamido)-5-
oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-181 dihydropyridin-3-y1)-2-(cinnoline-3-carboxamido)-N6-
cyclohexy1-5-
oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-182 dihydropyridin-3-y1)-N6-cyclohexy1-2-(3-ethylbenzofuran-2-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-183 dihydropyridin-3-y1)-N6-cyclohexy1-2-(1-ethy1-1H-indole-2-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-194 dihydropyridin-3-y1)-N6-cyclohexy1-2-(2-methy1-1,8-
naphthyridine-3-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-185 dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1,2,3,4-
tetrahydroquinoline-
6-carboxamido)hexanediamide
(S)-N1-(1-(2-(2-carboxy-2-am ino-5-(trifluoromethyl)adamantane)-2-
11-186 oxoethyl)-2-oxo-1,2-dihydropyridi n-3-y1)-N6-methy1-5-oxo-2-
(3-oxo-
1,2 ,3,4-tetrahyd roisoqui noline-6-carboxam ido)hexanediamide
(S)-N1-(1-(2-(5-ethyladamantane-2-am ino)-2-oxoethyl)-2-oxo-1,2-
11-187 dihydropyridin-3-y1)-N6-methy1-2-(1,6-naphthyridine-2-
carboxamido)-
5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[2.1.1]hexan-1-ylam ino)-2-oxoethyl)-2-oxo-1,2-
11-188 dihydropyridin-3-y1)-N6-methy1-2-(2,6-naphthyridine-1-
carboxamido)-
5-oxohexanediamide
(S)-2-(4-am ino-1,2,5-oxadiazole-3-carboxamido)-N1-(1-(2-
11-189 (bicyclo[1.1.11pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
429
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
11-190 dihydropyridin-3-y1)-2-(6-(dimethylamino)benzofuran-2-
carboxamido)-N6-methy1-5-oxohexanediamide
(S)-2-(2-acetamidothiazole-5-carboxamido)-N1-(1-(2-
11-191 (bicyclo[1.1.11pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
dihydropyridin-3-y1)-N6-methy1-5-oxohexanediamide
(S)-N4-(1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-
11-192 1,2-dihydropyridin-3-ylamino)-6-(methylamino)-1,5,6-
trioxohexan-2-
y1)-1H-pyrrole-2,4-dicarboxamide
(S)-N1-(1-(2-(1-acetylamino-4-aminoadamantane)-2-oxoethyl)-2-
11-193 oxo-1,2-dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(5-
sulfamoylfuran-3-
carboxamido)hexanediamide
(S)-2-(benzofuran-5-carboxamido)-N1-(1-(2-(1-acetylamino-4-
11-194 aminoadamantane)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
(S)-2-(benzofuran-6-carboxamido)-N1-(1-(2-(4-aminoadamantane-1-
11-195 carboxamide)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-
5-oxohexanediamide
(S)-N1-(1-(2-(4-aminoadamantane-1-carboxamide)-2-oxoethyl)-2-
11-196 oxo-1,2-dihydropyridin-3-y1)-N6-methy1-2-(3-(1-
methylcyclopropy1)-
1,2,4-oxadiazole-5-carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
11-197 dihydropyridin-3-y1)-N6-methy1-2-(5-methy1-1,2,4-oxadiazole-3-
carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
11-198 dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1,2,3-thiadiazole-4-
carboxamido)hexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
11-199 dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1,2,4-thiadiazole-5-
carboxamido)hexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
11-200 dihydropyridin-3-y1)-N6-methy1-5-oxo-2-(1,3,4-thiadiazole-2-
carboxamido)hexanediamide
(S)-N1-(1-(2-(bicyclo[1.1.1]pentan-1-ylamino)-2-oxoethyl)-2-oxo-1,2-
11-201 dihydropyridin-3-y1)-2-(4-cyclopropy1-1,2,3-thiadiazole-5-
carboxamido)-N6-methy1-5-oxohexanediamide
(S)-2-(4-cyclopropy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
11-202 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-y1)-N6-
methy1-5-oxohexanediamide
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
430
(S)-2-(4-isopropyl-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
11-203 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-N6-
methyl-5-oxohexanediamide
(S)-2-(4-ethyl-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
11-204 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-N6-
methyl-5-oxohexanediamide
(S)-2-(4-formy1-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-(2-
11-205 adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-N6-
methyl-5-oxohexanediamide
(S)-2-(4-(hydroxymethyl)-1,2,3-thiadiazole-5-carboxamido)-N1-(1-(2-
11-206 (2-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-N6-
methyl-5-oxohexanediamide
(S)-N1-(1-(2-(1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
11-207 y1)-N6-methy1-2-(1-methyl-1H-imidazole-5-carboxamido)-5-
oxohexanediamide
(S)-N1-(1-(2-(((1 6,2R,56)-6,6-dimethylbicyclo[3.1.1]heptan-2-
11-208 yl)methylamino)-2-oxoethyl)-2-oxo-1 ,2-dihydropyridin-3-
y1)-N6-methy1-2-(1-
methyl-1 H-imidazole-2-carboxamido)-5-oxohexanediamide
(S)-N1-(1-(2-(((1 R,2R,5R)-6,6-dimethylbicyclo[3.1.1]heptan-2-
11-209 yl)methylamino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-
y1)-2-(1H-
imidazole-4-carboxamido)-N6-methyl-5-oxohexanediamide
(S)-N1-(1-(2-(3,5-dimethyladamantane-l-amino)-2-oxoethyl)-2-oxo-1,2-
11-210 dihydropyridin-3-y1)-N6-methy1-2-(1-methyl-1H-imidazole-5-
carboxamido)-
5-oxohexanediamide
(S)-N1-(1-(2-(3,5,7-trimethy1-1-adamantylamino)-2-oxoethyl)-2-oxo-1,2-
11-211 dihydropyridin-3-y1)-N6-methy1-2-(1-methyl-1H-imidazole-5-
carboxamido)-
5-oxohexanediamide
or a pharmaceutically acceptable salt thereof.
10. A pharmaceutical composition comprising a compound of any one of the
claims
1 ¨ 9 as an active ingredient, together with at least one pharmaceutically
acceptable
carrier, excipient and/or diluent.
11. Compound according to any one of the claims 1 ¨ 9 for use in medicine.
12. Compound according to any one of the claims 1 ¨ 9 or the pharmaceutical
composition according to claim 9 for use in the treatment or prophylaxis of
autoimmune and inflammatory diseases, vascular diseases, fibrotic diseases,
liver
diseases, cholestatic liver diseases, cancer, neurodegenerative diseases,
ocular
diseases, and skin disorders.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
431
13. Compound for use, or the pharmaceutical composition for use according to
claim
12, wherein
the autoimmune and inflammatory diseases comprise multiple sclerosis, celiac
disease, Duhring-Brocq-disease (dermatitis herpetiformis), gluten ataxia,
gluten
neuropathy, diabetes, rheumatoid arthritis, Graves' disease, inflammatory
bowel
disease, systemic lupus erythematosus psoriasis, and gingivitis; wherein the
vascular diseases comprise atherosclerosis, thrombosis, vascular stiffness;
wherein
the fibrotic diseases affect the lung, the kidney, the liver, the skin or the
gut like
cystic fibrosis, kidney fibrosis and diabetic nephropathy, intestinal
fibrosis, idiopathic
lung fibrosis, liver fibrosis; wherein the liver diseases comprise alcoholic
hepatitis,
alcoholic steatohepatitis, nonalcoholic steatohepatitis, non-alcoholic fatty
liver
disease, liver cirrhosis, autoimmune hepatitis or liver inflammation; wherein
the
cholestatic liver diseases comprise primary biliary cholangitis and primary
sclerosing
cholangitis; wherein the cancer comprises glioblastoma, melanoma, pancreatic
cancer, renal cell carcinoma, meningioma, and breast cancer, wherein the
neurodegenerative diseases comprise Parkinson's disease, Huntington's disease,
or Alzheimer's disease, wherein the ocular diseases comprise glaucoma,
cataracts,
macular degeneration, or uveitis; and wherein the skin disorders comprise
acne,
psoriasis, scarring, and skin aging.
14. Compound for use, or the pharmaceutical composition for use according
to any one
of the claims 12 and 13 in the treatment or prophylaxis of celiac disease.
15. A method for producing the compound of formula (lb) according to claim 1
or 3
comprising:
Step 1B: providing a compound 4b
0
Ac0)-LN -R6
147
PG3N COH
'
H 0 4b;
Step 2B: performing coupling reaction of the compound 4b with a compound 5
0
H2N)-L L
, N- 'R3
5
to obtain a compound 6b
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
432
0
Ac0 , R6
R70
PG3Ni\O- _L.
N R-
H 0
6b;
Step 3B: deprotecting an amino protecting group PG3to obtain a compound 7b
0
Ac0)-L , R6
R70
H2N L
N 'R-
0
7b;
Step 4B: performing coupling reaction of the compound 7b with a carboxylic
acid
(R2-CO2H 8) to obtain a compound 9b
0
Ac0 , R6
0 R70
R2A N ,L.
N R-
H 0
9b;
Step 5B: performing oxidation reaction of the compound 9b to produce the
compound of the formula (lb)
0
0N R6
0H R70
N
R2 N NL R3
0
(1b);
wherein L, R2, R3, R6 and R7 have the same meanings as defined in claim 1, and
PG3 is an amino protecting group.
2489-CIPO-DESCRIPTION-ZED-P04403W024 docx
Date Recue/Date Received 2022-06-30
433
Inhibitors of Transglutaminases
Description
The invention relates to novel inhibitors of transglutaminases, in particular
transglutaminase 2, methods for their synthesis and to their use for the
prophylaxis and
treatment of diseases associated with transglutaminases, in particular
transglutaminase
2.
Background of the invention
Transglutaminases are part of the class of transferases and according to EC
nomenclature they are correctly designated as "protein-glutamine: amine y-
glutamyl
transferases" (EC 2.3.2.13). They link the E-amino group of the amino acid
lysine and
the y-glutamyl group of the amino acid glutamine forming an isopeptide bond
while
ammonia is released. In the absence of suitable amines and/or under certain
conditions,
deamidation of the glutamine may occur resulting in the corresponding glutamic
acid.
Additionally, transglutaminases play an important role in many therapeutic
areas such as
the cardiovascular diseases (thrombosis and atherosclerosis), autoimmune
diseases
(celiac disease, Duhring-Brocq-disease, gluten ataxia), neurodegenerative
diseases
(Alzheimer's disease, Parkinson's disease, Huntington's disease),
dermatological
diseases (ichthyosis, psoriasis, acne) as well as in wound healing and
inflammatory
diseases (e.g. tissue fibrosis) (J.M. Wodzinska, Mini-Reviews in medical
chemistry, 2005,
5, 279 - 292).
Celiac disease, a gluten intolerance, however, is one of the most important
indications.
Celiac disease is characterized by a chronic inflammation of the mucosa of the
small
intestine. In susceptible patients, the intestinal epithelium is successively
destroyed after
ingestion of gluten-containing food resulting in reduced absorption of
nutrients which
again has massive impact on the patients affected and is for example
associated with
symptoms such as loss of weight, anemia, diarrhea, nausea, vomiting, loss of
appetite
and fatigue. Due to these findings, there is a large demand for the
development of a
medicament for the treatment of celiac disease as well as of other diseases
associated
with tissue transglutaminase (transglutaminase 2, TG2, tTG).
The tissue
transglutaminase is a central element during pathogenesis. The endogenous
enzyme
catalyses the deamidation of gluten/gliadin in the small intestinal mucosa and
thus
triggers the inflammatory response. Therefore inhibitors of tissue
transglutaminase are
suitable to be used as active agents for medication.
Another very important group of indications for tissue transglutaminase
inhibitors are
fibrotic disorders. Fibrotic disorders are characterized by the accumulation
of cross-
2489-CIPO-DESCRIPTION-ZED-P04404W026 docx
Date Recue/Date Received 2022-06-30
434
linked extracellular matrix proteins. Diabetic nephropathy, cystic
fibrosis, idiopathic
pulmonary fibrosis, kidney fibrosis as well as liver fibrosis belong to the
most important
fibrotic disorders to be addressed with the compounds disclosed.
US 9,434,763 B2 discloses pyridinone derivatives having a warhead comprising
at least
one acceptor-substituted double bond, such as a Michael System, as
irreversible
transglutaminase inhibitors. Alkylacetamido and arylacetamido pyridinones
showed
inhibitory activity regarding tissue transglutaminase TG2 in nanomolar range
(IC50).
Tse et al. (J. Med. Chem. 2020, 63, 11585-11601) report on replacement of
phenyl
residues by non-classical bioisosteres, such as cubane and
bicyclo[1.1.1]pentane (BCP),
in anti-malarial triazolopyrazine compounds in order to alter compound
solubility and
metabolic stability. The authors further evaluated in vitro antiplasmodial
activity of
bioisosteric modified triazolopyrazines against the 3D7 strain of P.
falciparum.
Replacement of phenyl by bioisosteric saturated heterocyclic residues resulted
in
complete loss of activity. Adamantyl residues as well as other hydrocarbon-
caged
derivatives led to potency up to 2-9 times lower than the corresponding phenyl
triazolopyrazine compounds. In contrast, higher potencies were achieved by
replacing
phenyl with closo-1,2- and 1,7-carborane isomers. The authors concluded that
the effect
of non classical bioiostere replacement on biological properties cannot be
predicted
accurately and that a considerable range of possible bioisosteres has to be
tested first in
order to identify a suitable replacement leading to the desired properties of
a given
molecule.
Subbaiah et al. (J. Med. Chem. 2021, 64, 19, 14046-14128) report on
bioisosteres of the
phenyl ring in lead optimization and drug design. It is noted that
bioisosteric phenyl ring
replacement with heterocyclic and carbocyclic moieties can lead to enhanced
potency,
solubility, and metabolic stability while reducing lipophilicity, plasma
protein binding,
phospholipidosis potential, and inhibition of cytochrome P450 enzymes and the
hERG
channel. However, this effect depends strongly on the properties of the
compound itself
and the addressed target.
US 11,072,634 B2 discloses reversible transglutaminase inhibitors comprising
an
aldehyde, a ketone, an a-ketoaldehyde, an a-ketoketone, an a-ketoacid, an a-
ketoester,
an a-ketoamide or a halogenmethylketone as warhead. The inhibitors showed
inhibitory
activity regarding tissue transglutaminase TG2 in nanomolar and micromolar
range (IC50).
The objective of the present invention is to provide novel, most probably
irreversible
inhibitors of transglutaminases, in particular transglutaminase 2 and methods
for the
synthesis of said inhibitors as well as several uses of these inhibitors.
2489-CIPO-DESCRIPTION-ZED-P04404W026 docx
Date Recue/Date Received 2022-06-30
435
Said objective is solved by the technical teachings of the independent claims.
Further
advantageous embodiments, aspects and details of the invention are evident
from the
dependent claims, the description, and the examples.
Surprisingly, it has been found that irreversible inhibitors having a chemical
warhead as
disclosed herein inhibit effectively transglutaminases including tissue
transglutaminase
called transglutaminase 2 or TG2. Herein these terms are used synonymous.
Preferably, such chemical warhead moiety is particularly selected from
irreversible
warheads such as a,R-unsaturated-ketoester, a,R-unsaturated ketoamide and a,R-
unsaturated-sulfone. The compounds of the present invention act as selective
inhibitors
of transglutaminase 2.
Thus, the present invention relates to compounds of the general formula (I):
R1
0 0
C
(I) NH 7I-R3
R2 N 1 N
Ho
wherein
L represents ¨L1¨ or ¨L1-L2¨; preferably ¨L1-L2¨;
L1 represents ¨CH2¨, ¨CH2CH2¨, ¨CH2CH2CH2¨, ¨CH2C0¨, or ¨CH2CH2C0¨;
L2 represents a bond, ¨NRI41¨, ¨NRN1CH2¨, ¨NRN1CH2CH2¨, or ¨NRN1CH(CH3)¨,
0 0õ0
R1 represents or ,,-)Sl,R4 .
--R4
R2 represents
RN RN
_O R8 Ril 0 R8 _ _ _S R8 __ IR11 S R8 - _ _.$-Ii.,(R8 Rii
\i, r , r ,\ r r , ,R8
,
RN RN RN RN RN
N 1
R11 N , R1i 1 \ N -, N, RN RN
Rii lj R8 I R
- 8
---14
/ l(
- I --- - -¨
N
N
Rio µ, ; ' R9 Rio R9 ; Rio R10
;
w0 R1,1 S
R1 R10
R ,
i
0, iv 0 R8 -___,,ON R8 _--
\ ir --1--- \ ir ? 9
R8 1' 13 Rio
N N N N 'N Rio R8 N¨ ' R8 Rio
2489-CIPO-DESCRIPTION-ZED-P04404W026 docx
Date Recue/Date Received 2022-06-30
436
RN RN RN
N
R11,S, _R8 --1S\¨R8 ----- ',
N N N R11 __ N D10 R10 N_RN
--\\ /7--- ____
, N ,N NQ--1` TC=N
,)
'
RiO , , R10
, , ' , ,
RN RN R12 R12 R12
N r", 0,, (11R9 LI
N R13
----------------------------------------------------------------- ¨R=-
N-N NN - \____,-----Rio ------ R14 N R14 N R14 ,
RN
R12 R12 R12
R12 N R12 0- 13 S/ 13 i\i
I ,
N/-) Ri3 r Ri 3 - TR - - -R - - Dp13
-.,
N R14 N R14 Rlo R14 R14 Rio Rlo R14
,
'
RN R13
R12
pp12 pp12 RN
R13 I 7rN_
), N N R14 , R9
¨ I 1 R13 r /1
N---\-R14R14 R14 00._ in
Fµ N __ S __
_R10
RN R8 R8 ,S,--
N R9
R9 ,S,-' II
NI S)--- r\i"---N
r /I
R1 0 &/--, R1 0 No j(
N Ro \s--N \N-_---N Ro
R8
. N , - . N, - 0
R8--\- <SM-Ii -- N-. Ru-- '/ . R ---- il - - --
N-N , b-N , N-0 , 0-N N
' , ,
H
N 0 S s
, N ,
, , ,
, , ,
,
_N _,
---eNNH ---eNNH N
---\ -13 ---eNS
N( NN - N N __ \(
S S z S S y N
---eNNH
N(
N, NNJ\ N __ (
0 ONJ
N _ N ----eN0 ---eN0 ---
-(1\1 --(,N3 ----eN0
N __ \(
II
6 y N , Ni
vlo , N NN
, , ,
H
N,S N
,
,
S,--N
s
N
H
2489-CIPO-DESCRIPTION-ZED-P04404W026.docx
Date Recue/Date Received 2022-06-30
O W
Fri 6
-0
X o
o ,
, ,, , , , , 1
o ,
' , 1 , , , '
c c,
Z_
CD 2 /_ ___________________________ Z= Z_ Z) /_
n) H
/z \ /z z z(\ z $ /z $ / \ / Z/z
rp' 2 \ __ /K \ /(
CD m Z Z S Z \ , Z Z S Z Z
Z / Z Z Z
O 0 0
_ Z / Z \ // \ i Z / \ ___________ // \ Z Z /
__ µZµ / _ // -
C) . .'10
, -
-
CD
o_ R
N) c)
N) p ,
N) ..
a)
0
i 1
07C 2z3
, , , / , Z z
' '
z/ z z/ Z Z=Z ¨Z z/¨Z z=z Z_ z/
/¨
,
________________ 0 \ ____ /( \ /( /K \ _____ /( \ / / \
\ z z z
/K \ / \
.4,.
Z Z Z Z Z
// Z-Z
_
-
1 1
e0 e(:)
6 z(
N Z
, , , , ,
' ' , ,' -
Z=z Z=z _ ________________________________________ /_ Z= Z /
_____________________ /_ Z= Z_
Z \ / \ / \ /
\ .Z Z. .Z \ .Z \ / Z\ / Z\
\
\_ \ S \ S Z Z \ Z \ Z \ / Z / \ Z \ S Z / \ Z
\ /
Z / // Z¨// Z¨// Z ' ' \ __ // Z
Z Z¨// Z 1 1
_ ________________________________________________________
0 N 07
Z Z
\ cZN
-
438
-,,N
1 II
Nle , Nie , N.N
,
I\IN -,,N,N
'N
1
IV ,
N, ,
N. -, I\1 -,,I\I
'Nil
N,2 N N N--N
, , ,
N N , 1\1-N1-, NN
,
-'
'-lr"N
N,N
N-, N I
1\1-
N N , N N N
I I I
NN
, NN--N NN-,N
-,,N,N
I I
1
N. NN N N
el\I
,
H
NH
HN NH ,
,
.,
N NH ,
HN , NH H , ,
N=\ 0 N=N N=\ N-
--N
---0
\ N
N- N- N- -N -N
--- ;IV - -
--- / N \ JV
N' N
-- __
N \
\ //IV
N, /
___________________________________ , _______ '
N ______________________ ,
--\ /,\N
-\
I\1 , --
\ N --
\ N -- N =
N
N IV N/ //
,
2489-CIPO-DESCRIPTION-ZED-P04404W026.docx
Date Recue/Date Received 2022-06-30
439
N=\ N=\
--- /(1\1
\ / N\/ N / N
N
N N¨N 'IV N //N
N=N N=N N=N N=N N=N
' N --,
\ ,,N, _________________________________________________________ / N
, ,
N=\ N=\ N=\ N=\ N-
--
(
\ /71; \ S \ N
/ N N N ,
'
\ _______________________________________________________________
N IV// N //N
N¨N N \
,
---\ N ,
\ /71; \ S N N , ____ ¨\ ,N N N
'
¨N ¨N _¨N
-- --i
)
\ )
\
/
N / N¨N N __ \IV
,
_________________________________________________ ,
¨\ ___\
-- N ---
\ /IV \ N \ S N S
N ; N¨//
N¨N N
* *
N N
Ns\
N / s \ /IV N N
" N \ N N-N
or ; ¨N ; N N¨//
, , ,
wherein the unsubstituted bicyclic residues can be substituted with 1 to 5 of
the
substituents R9 -R14; and RN; and preferably with 1 to 3 of the substituents
R11 - R13;
R3 represents bicyclo[1.1.1]pentyl, bicyclo[2.1.1]hexyl, bicyclo[2.2.1]heptyl,
bicyclo[3.1.1]heptyl, bicyclo[2.2.2]octyl,
bicyclo[3.2.1]octyl, bicyclo[3.2.2]nonyl,
bicyclo[3.3.2]decyl, bicyclo[3.3.3]undecyl, 4-homoisotwistyl, adamantyl;
diamantyl; or
hexamethylenetetraminyl and the afore-mentioned residues optionally contain
one or
more C=C double bond(s) and/or are optionally substituted by one or more of
Ra; Ri3; Rc;
Rd; and Re;
2489-CIPO-DESCRIPTION-ZED-P04404W026 docx
Date Recue/Date Received 2022-06-30
440
Ra, Rb, Rc, Rd, and Re represent independently of each other -H, -F, -Cl, -Br,
-CN, -OH, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CHF2, -CF3,
-CH2CF3, -COCH3, -COCH2CH3, -CO2H, -CO2CH3, -0O2C2H5, -CON H2,
-CONN CH3, -CON(CH3)2, -
CONHC2F15, -CH2CO2H, -CH2CO2CH3,
5 -CH2CO2C2H5, -CH2CONH2, -CH2CONHCH3, -
CH2CON(CH3)2,
-CH2CONHC2F15, -NHCOCH3, -NH COC21-15, -NHCOCF3, -NHCOCH2CF3,
-NHSO2CH3, -NHSO2C21-15, -NHSO2CHF2, -NHSO2CF3, or -NHSO2CH2CF3;
R4 represents -R5, -0R5 or -NR6R7;
R5 represents -H, -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2,
-CH2CH2CH2CH3, -CH2CH (CH3)2, -
C(CH3)3, -CH2CH2CH2CH2CH3,
-CH2CH=CH2, -CH2CH=CH(CH3), -CH2CH=C(CH3)2, -CH2CH=CHCH2CH3,
-cyclo-C3H5, -cyclo-C4H7, -cyclo-051-19, -
cyclo-C6H11, -CH2-cyclo-C3H5,
-CH2-cyclo-C4H7, -CH2-cyclo-051-19, -CH2-cyclo-C6H11, -Ph, -CH2-Ph,
-CH2OCH3, -CH2OCH2CH3, -CH2CH2OCH3, or -CH2CH2OCH2CH3;
R6 and R7 represent independently of each other -H, -CH3, -
CH2CH3,
-CH2CH2CH3, -CH(CH3)2, -
CH2CH2CH2CH3, -CH2CH2CH2CH2CH3,
20 -CH2CH(CH3)2, -C(CH3)3, -CH2CH=CH2, -
CH2CH=CH(CH3),
-CH2CH=C(CH3)2, -CH2CH=CHCH2CH3, -
cyclo-C3H5, -cyclo-C4H7,
-cyclo-051-19, -cyclo-C6H11, -CH2-cyclo-C3H5, -CH2-cyclo-C4H7, -CH2-cyclo-051-
19,
-CH2-cyclo-C6H11, -Ph, -CH2-Ph, -
CH2OCH3, -CH2OCH2CH3,
-CH2CH2OCH3, -CH2CH2OCH2CH3, -CH2CH2NHCH3, -CH2CH2N(CH3)2,
or -NR6R7 represents - -N - -N - NO
or i\i/ \
\ ______________________________________________________________________ / -
,
R6, R5, R10, R11, R12, R13, and R14 represent independently of each other -H, -
F, -Cl,
-Br, -I, -OH, -CN, -NO2, -CH3, -C2F15, -C3H7, -CH(CH3)2, -C4H9,
-CH2-CH (CH3)2, -CH (CH3)-C2H5, -C(CH3)3, -cyclo-C3H5, -CH2-cyclo-C3H5,
-CH2OH, -CH2F, -CHF2, -CF3, -CH2CI, -CH2Br, -CH21, -CH2-CH2F,
-CH2-CH F2, -CH2-CF3, -CH2-CH2CI, -CH2-CH2Br, -CH2-CH21, -OCH3,
-0C21-15, -0C3H7, -OCH (CH3)2, -0C(CH3)3, -0C4H9, -OCHF2, -0CF3,
-OCH2CF3, -0C2F5, -OCH2OCH3, -0-cyclo-C3H5, -OCH2-cyclo-C3H5,
-0-C2H4-cyclo-C3H5, -
CHO, -COC H3, -COC F3, -00C2H5, -00C3H7,
-COCH(CH3)2, -00C(CH3)3, -COOK -COOCH3, -CO0C2H5, -CO0C3H7,
-COOCH (CH3)2, -COOC(CH3)3, -
00C-CH3, -00C-CF3, -00C-C2H5,
-00C-C3H7, -00C-CH(CH3)2, -00C-C(CH3)3, -N H2, -NHCH3, -NHC2H5,
-NHC3H7, -NHCH(CH3)2, -NHC(CH3)3, -N(CH3)2, -N(C2H5)2, -N(C3H7)2,
2489-CIPO-DESCRIPTION-ZED-P04404W026.docx
Date Recue/Date Received 2022-06-30
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 440
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 440
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: