Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/158831
PCT/US2021/016681
CHARACTERISTICS OF MEAT PRODUCTS
CROSS REFERENCE TO RELATED APPLICATIONS
100011 This application claims priority to U.S. non-provisional patent
application number
17/033,635, filed on September 25, 2020, and U.S. provisional patent
application number
62/970,109, filed on February 4, 2020, the contents of which are incorporated
herein by
reference in their entireties.
BACKGROUND
100021 Animal meat is a first-choice source of protein for many people all
over the world. The
total estimated consumption of meat (chicken, turkey, veal, lamb, beef, pork)
in 2018 in the
USA was 219 pounds per capita. It is expected that the traditional
(conventional) method of
harvesting meat from slaughtered animals, such as livestock, poultry, and
fish, usually involving
slaughter, may not be sufficient to meet the future demand for meat.
Additionally, meat
production from these sources is associated with several drawbacks, although
legally acceptable,
such as increased levels of microbial contamination, and exposure to the
hormones and
antibiotics traditionally found in conventional meat products. Conventional
meat production is
also associated with environmental drawbacks such as poor conversion of
caloric inputs,
greenhouse gas emissions, land usage, water usage, and local pollution. One
alternative to
conventional meat production involving slaughter is the production of meat by
culturing
metazoan cells in the laboratory (also referred to herein interchangeably as
cell-based meat, cell-
based meat, cell culture-based meat, cell-based meat, cell-based meat, or
cultured meat).
100031 Adoption of cell-based meat products into the food supply chain will
depend on a
variety of factors such as the quantifiable features of the meat itself,
including, but not limited to,
the macro and micro nutrient profiles, levels of hormones, and antibiotics,
and shelf life.
Comparisons to conventional products will need to be made to ensure comparable
nutrient
profiles, while seeking out distinguishable factors, such as low/no hormonal
or antibiotic content
and microbial counts. Cell-based meat products are not yet commercially
available but
ultimately regulatory bodies will also require quantification and
accountability of the meat
products intended for the market, food safety establishment prior to sale as
well as post-market
compliance of the meat products intended for the market.
1
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0004] Production of cell-based meat and customizations to flavor and texture
still remain
limited by several factors, consistent and methodical production being a few.
Provided herein
are compositions and methods that address this and other related needs.
SUMMARY
100051 Provided herein are methods and compositions related to the production
of slaughter-
free meat products generated from cells grown in culture; these meat products
are
interchangeably referred to herein as cell-based meat products.
[0006] In one aspect, provided herein is a slaughter-free meat product for
dietary consumption
exhibiting an extended shelf life, wherein the shelf life is extended for
varying durations
following harvest, when compared to conventional meat obtained by slaughter.
In a related
aspect, provided herein is a slaughter-free meat product for dietary
consumption exhibiting a
lower microbial contamination count as compared to conventional meat obtained
by slaughter,
wherein the lower microbial contamination count is exhibited for varying
durations following
harvest. The slaughter-free meat product may be of any species as disclosed
herein.
[0007] Also provided herein are methods of generating the slaughter-free meat
products of the
disclosure, comprising culturing cells such as fibroblasts, myoblasts,
adipocytes, endothelial
cells, cells of a mesodermal lineage, and combinations thereof, in either
suspension culture, or in
adherent formats.
BRIEF DESCRIPTION OF THE DRAWINGS
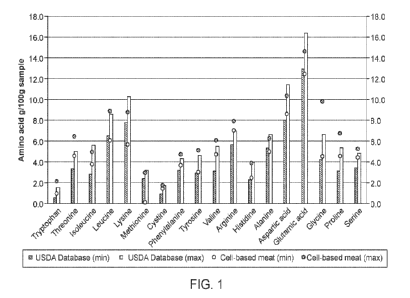
[0008] FIG. 1 shows the amino acid profiles found in exemplary slaughter-free,
cell-based
meat samples, as compared to conventional meat. The USDA database includes
amino acid data
from: bluefin tuna, tilapia, yellowfin tuna, turkey, lamb, chicken, beef,
cornish game hens,
guinea fowl, pheasant, quail, squab, goose, duck, ostrich ,beef top sirloin,
beef short ribs, beef
shank, chicken breast, chicken thigh, pork shoulder, grass fed bison, chicken
wing, chicken
neck, turkey breast, turkey wing, turkey thigh, and lamb shank.
[0009] FIG. 2 shows the mean concentration range of hydroxyproline in
exemplary cell-
based meat samples (expressed as grams of hydroxyproline per 100g of wet mass
of cell-based
meat).
2
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0010] FIG. 3 shows representative plates with bacteria colonies, indicative
of microbial
contamination, showing results for exemplary samples of slaughter-free cell-
based duck, store-
bought (conventional) beef, and store-bought chicken.
[0011] FIG. 4 shows total fatty acid composition for saturated,
monounsaturated and
polyunsaturated fatty acids across exemplary of slaughter-free cell-based meat
samples.
[0012] FIG. 5 shows the principal component analysis (PCA) of fatty acid data,
gathered from
the USDA database for conventional meat products.
[0013] FIG. 6 shows the ratio of Omega 6 to 3 fatty acids in an exemplary
sample of
slaughter-free cell-based chicken meat.
[0014] FIG. 7 shows that the presence of serum in the media used to generate
cell-based meat
(CBM) from cells in culture can affect fatty acid profiles; the figure shows
the fatty acid
percentages in exemplary slaughter-free cell-based meat samples, produced in
serum free media
vs. media containing serum. W3 =omega 3 FA; W=6= omega 6 FA; W=9 = omega 9 FA.
[0015] FIG. 8 shows that using serum from different sources imparts different
fatty acid
profiles in slaughter-free cell-based meat samples. The data are from Method
10 from Table 1.
Key - BS: Bovine serum; CS: Chicken serum; FBS: Fetal bovine serum; Hy: soy-
based plant
hydrolysate; Media contained 8-10% of particular serum; DMEM-F12 was used as
the base
media.
[0016] FIG. 9 shows that isolated clones of myoblast cells from a polyclonal
population of
myoblast cells can impact the fatty acid profile of slaughter-free cell-based
meat samples.
[0017] FIG. 10 shows that fatty acid profiles of slaughter-free cell-based
meat are affected by
media composition, and the addition of an agonist that targets the Liver X
Receptor 13. (um).
[0018] FIG. 11 shows that fatty acid profiles of slaughter-free cell-based
meat are affected by
media composition, and the addition of riboflavin.
[0019] FIG. 12 shows that the titration of fatty acids into the media used for
culturing cell-
based meat can change the fatty acid profile of slaughter-free cell-based
meat.
3
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0020] FIG. 13 shows the cooked hardness of slaughter-free cell-based meat
generated from
fibroblast/myoblast co-cultures, and monocultures, as compared to conventional
chicken and
beef.
DETAILED DESCRIPTION
100211 Provided herein are methods and compositions related to the slaughter-
free production
of meat products, and rely on the use of cell culture based methods for the
growth, harvesting,
and formulation of cells into meat products. There are several differences in
the slaughter-free
meat products of the disclosure when compared to conventional meat obtained by
slaughter, and
these differences are described throughout.
[0022] Before describing particular embodiments in detail, it is to be
understood that the
disclosure is not limited to the particular embodiments described herein,
which can vary. It is
also to be understood that the terminology used herein is for the purpose of
describing particular
illustrative embodiments only, and is not intended to be limiting unless
otherwise defined. The
terms used in this specification generally have their ordinary meaning in the
art, within the
context of this disclosure and in the specific context where each term is
used. Certain terms are
discussed below or elsewhere in the specification to provide additional
guidance to the
practitioner in describing the compositions and methods of the invention and
how to make and
use them. The scope and meaning of any use of a term will be apparent from the
specific
context in which the term is used. As such, the definitions set forth herein
are intended to
provide illustrative guidance in ascertaining particular embodiments of the
invention, without
limitation to particular compositions or biological systems.
[0023] As used in the present disclosure and the appended claims, the singular
forms "a," "an"
and "the" include plural references unless the content clearly dictates
otherwise.
[0024] Throughout the present disclosure and the appended claims, unless the
context requires
otherwise, the word "comprise", or variations such as "comprises" or
"comprising", will be
understood to imply the inclusion of a stated element or group of elements but
not the exclusion
of any other element or group of elements.
100251 The term "edible" in the context of the cell-based meat as used herein
encompasses
raw or uncooked meat as well as partially or fully cooked meat.
4
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0026] Unless specific definitions are provided, the nomenclature utilized in
connection with,
and the laboratory procedures and techniques of, molecular biology, cell
biology, analytical
chemistry, and synthetic organic chemistry described herein are those well-
known and
commonly used in the art. Standard techniques may be used for recombinant
technology,
molecular biological, microbiological, chemical syntheses, and chemical
analyses.
[0027] The term "slaughter" as applied to the manner in which conventional
meat is obtained
covers all methods traditionally used to kill an animal, with the purpose of
directly harvesting its
meat for dietary consumption.
[0028] The term "slaughter-free" as applied to the cell based meat products of
the disclosure
refer to the process by which the meat is generated, starting with cells in
culture, and that
method which does not involve the slaughter of an animal in order to directly
obtain meat from
that animal for dietary consumption. It is understood that in some
embodiments, it is possible
that the starting cells for use in the cell culture methods may have been
obtained following the
slaughter of an animal, or a biopsy - although the starting cells for use in
culture may have been
obtained in this manner, the meat resulting from the culturing of cells, by
harvest and a possible
subsequent formulation are still considered to be meat obtained in a slaughter-
free manner. It is
noted that as a general matter, as used herein, harvesting of the slaughter-
free cell-based meat
product may involve using a buffered solution of water (or other aqueous
solution) to remove the
meat where it is grown (e.g. the surface of a bioreactor, or in a container
comprising a the cells
cultured in suspension), and the meat may then captured in a collection device
(e.g. net, sieve,
colander). In some embodiments the meat may be harvested by physical methods
(such as
scraping), enzymatic methods, and/or chemical methods. In some embodiments the
meat may be
harvested by any of the above mentioned methods and subsequently rinsed with
buffered
solutions (or other aqueous solutions).
[0029] The phrases "cell-based meat", "slaughter-free cell-based meat", "in
vitro produced
meat-, "in vitro cell-based meat-, "cultured meat-, "slaughter-free cultured
meat-, "in vitro
produced cultured meat", "in vitro meat", "in vitro cultured meat" and other
similar such
phrases are interchangeably used herein, and refer to the meat that is
generated in vitro, starting
with cells in culture, and that method which does not involve the slaughter of
an animal in order
to directly obtain meat from that animal for dietary consumption.
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
I. Generation of Slaughter-Free Cell-Based Meat
100301 Provided herein are methods to produce cell-based meat products in a
slaughter-free
manner.
A. Cells
100311 The slaughter-free cell-based meat products of the disclosure are
compositions
produced by the culturing of naturally occurring, transgenic, or modified
cells in culture.
100321 The cells used in the methods of the present disclosure can be primary
cells, or cell
lines. The methods provided herein are applicable to any metazoan cell in
culture. Generally,
the cells can be from any metazoan species whose tissues are suitable for
dietary consumption.
In some embodiments, the cells demonstrate the capacity for skeletal muscle
tissue specification
(e.g. myoblasts). In other embodiments, the cells do not demonstrate the
capacity for skeletal
muscle tissue specification.
100331 In some embodiments, the cells are derived from any non-human animal
species
intended for human or non-human dietary consumption. In some embodiments the
cells may be
of avian, ovine, caprine, porcine, bovine, or piscine origin. In some
embodiments the cells may
be of livestock, poultry, avian, game, or aquatic species.
100341 In some embodiments, the cells are from livestock such as domestic
cattle, pigs, sheep,
goats, camels, water buffalo, rabbits and the like. In some embodiments, the
cells are from
poultry such as domestic chicken, turkeys, ducks, geese, pigeons and the like.
In some
embodiments, the cells are from game species such as wild deer, gallinaceous
fowl, waterfowl,
hare and the like. In some embodiments, the cells are from aquatic species or
semi-aquatic
species harvested commercially from wild fisheries or aquaculture operations,
or for sport,
including certain fish, crustaceans, mollusks, cephalopods, cetaceans,
crocodilians, turtles, frogs
and the like
100351 In some embodiments, the cells are from exotic, conserved or extinct
animal species. In
some embodiments, the cells are from Gallus gal/its, Gallus domesticus, Bos
taurusõcous
scrofa, Meleagris gallopavo, Anas platytynchos, Salmo salar, Thunnus thymus,
Ovis aries,
Coturnix, Capra aegagrus hircus, or Homarus americanus. Accordingly, exemplary
slaughter-
6
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
free cell-based meat products of the disclosure include avian meat products,
chicken meat
products, duck meat products, and bovine meat products.
100361 In some embodiments, the cells are primary stem cells, self-renewing
stem cells,
embryonic stem cells, pluripotent stem cells, induced pluripotent stem cells,
or
transdifferentiated pluripotent stem cells.
100371 In some embodiments, the cells are modifiable by a genetic switch to
induce rapid and
efficient conversion of the cells to skeletal muscle for cultured meat
production.
100381 In some embodiments, the cells are myogenic cells, destined to become
muscle, or
muscle-like cells. In some embodiments, the myogenic cells are natively
myogenic, e.g.
myoblasts. Natively myogenic cells include, but are not limited to, myoblasts,
myocytes,
satellite cells, side population cells, muscle derived stem cells, mesenchymal
stem cells,
myogenic pericytes, or mesoangioblasts.
100391 In some embodiments, cells are of the skeletal muscle lineage. Cells of
the skeletal
muscle lineage include myoblasts, myocytes, and skeletal muscle progenitor
cells, also called
myogenic progenitors that include satellite cells, side population cells,
muscle derived stem
cells, mesenchymal stem cells, myogenic pericytes, and mesoangioblasts.
100401 In some embodiments, the cells are non-myogenic, and such non-myogenic
cells can
be programmed to be myogenic, for example the cells may comprise fibroblasts
modified to
express one or more myogenic transcription factors. In exemplary embodiments,
the myogenic
transcription factors include MY0D1, MYOG, MYF5, MYF6, PAX3, PAX7, paralogs,
orthologs, and genetic variants thereof. In some embodiments, the cells are
modified to express
one or more myogenic transcription factors as described in a PCT publication,
WO/2015/066377, incorporated by reference herein in its entirety.
100411 Tn some embodiments, the cells comprise a mixture of cell populations
described
herein, e.g. a mixture of fibrogenic cells and myogenic cells in co-culture,
e.g. a mixture of
fibroblasts and myoblasts in co-culture. In some embodiments the cells used
for the production
of cell-based meat are a mixture of fibroblasts and myoblasts in a suspension
co-culture. In
some embodiments the cells used for the production of cell-based meat are a
mixture of
fibroblasts and myoblasts in an adherent co-culture. In some embodiments cells
in co-culture
7
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
comprise additional cell types such as adipocytes, endothelial cells, and
generally cells from the
mesoderm lineage.
100421 In some co-culture embodiments, the cells are in a suspension co-
culture, in some
embodiments, the cells are in anadherent co-culture, and in some embodiments,
the culturing
processing makes use of both techniques. The co-cultures provide herein
comprise a mixture of
at least fibroblasts and myoblasts. In some embodiments, the ratio of the
fibroblasts to
myoblasts (designated as F and M) ranges from about 5F:95M to about 95F:5M. In
exemplary
embodiments, the ratio of the fibroblasts to myoblasts is about 5F:95M,
10F:90M, 15F:85M,
20F:80M, 25F:75M, 30F:70M, 35F:65M, 40F:60M, 45F:55M, 50F:50M, 55F:45M,
60F:40M,
65F:35M, 70F:30M, 75F:25M, 80F:20M, 85F:15M, 90F:10M, or even about 95F:5M.
[0043] In some embodiments, the cells are genetically modified to inhibit a
pathway, e.g. the
HIPPO signaling pathway. Exemplary methods to inhibit the HIPPO signaling
pathway as
described in a PCT Application No. PCT/US2018/031276, incorporated by
reference herein in
its entirety.
[0044] In some embodiments, the cells are modified to express telomerase
reverse
transcriptase (TERT) and/or inhibit cyclin-dependent kinase inhibitors (CKI).
In some
embodiments, the cells are modified to express TERT and/or inhibit cyclin-
dependent kinase
inhibitors as described in a PCT publication, WO 2017/124100, incorporated by
reference herein
in its entirety.
100451 In some embodiments, the cells are modified to express glutamine
synthetase (GS),
insulin-like growth factor (IGF), and/or albumin. Exemplary methods of
modifying cells to
express GS, IGF, and/or albumin are described in a PCT Application No.
PCT/US2018/042187
which is incorporated by reference herein in its entirety.
[0046] In some embodiments, the cells may comprise any combinations of the
modifications
described herein.
B. Cultivation Infrastructure
[0047] As referred to herein, a cultivation infrastructure refers to the
environment in which the
cells are cultured or cultivated to provide a two-dimensional or three-
dimensional meat product.
8
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0048] A cultivation infrastructure includes but is not limited to a roller
bottle, a tube, a
cylinder, a flask, a petri-dish, a multi-well plate, a dish, a vat, an
incubator, a bioreactor, and an
industrial fermenter.
[0049] While the cultivation infrastructure itself may have a three-
dimensional structure or
shape, the cells cultured in the cultivation infrastructure may form a
monolayer of cells.
Compositions and methods of the present disclosure can promote a three-
dimensional growth of
metazoan cells in the cultivation infrastructure to provide a scaffold-less
self-assembly of a
three-dimensional cellular biomass.
100501 A three-dimensional cultivation infrastructure may be sculpted into
different sizes,
shapes, and forms, as desired, to provide the shape and form for the muscle
cells to grow and
resemble different types of muscle tissues such as steak, tenderloin, shank,
chicken breast,
drumstick, lamb chops, fish fillet, lobster tail, etc. The three-dimensional
cultivation
infrastructure may be made from natural or synthetic biomaterials that are non-
toxic so that they
may not be harmful if ingested. Natural biomaterials may include, for example,
collagen,
fibronectin, laminin, or other extracellular matrices. Synthetic biomaterials
may include, for
example, hydroxyapatite, alginate, polyglycolic acid, polylactic acid, or
their copolymers. The
three-dimensional cultivation infrastructure may be formed as a solid or
semisolid support.
100511 A cultivation infrastructure can be of any scale, and support any
volume of cellular
biomass and culturing reagents. In some embodiments, the cultivation
infrastn.icture ranges
from about 10 p.L to about 100,000 L. In exemplary embodiments, the
cultivation infrastructure
is about 10 III-, about 100 III-, about 1 mL, about 10 mL, about 100 mL, about
1 L, about 10 L,
about 100 L, about 1000 L, about 10,000 L, or even about 100,000L.
[0052] In some embodiments, the cultivation infrastructure comprises a
substrate. A
cultivation infrastructure may comprise a permeable substrate (e.g. permeable
to physiological
solutions) or an impermeable substrate (e.g. impermeable to physiological
solutions). The
substrate can be flat, concave, or convex. The substrate may be textured so as
to promote cell
growth and cell sheet attachment.
100531 In some embodiments, the culturing of cells in the cultivation
infrastructure can induce
the production of extracellular matrix (ECM) that may act as an autologous
scaffold to direct
9
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
three-dimensional cellular growth, e.g. to direct attachment, proliferation
and hypertrophy of
cells on a plane perpendicular to the substrate.
100541 In some embodiments, the cultivation infrastructure does not comprise
an exogenously
added scaffold to promote self-assembly of a three-dimensional cellular
biomass. In some
embodiments, the cultivation infrastructure does not comprise exogenous
scaffolds such as a
hydrogel or soft agar.
C. Culturing Conditions
100551 The culturing conditions for the generation of cell-based meat are
generally aseptic,
and sterile.
100561 Cells can be grown in an adherent culture format to form a cell sheet
or can be grown
in a suspension culture format to form a cell pellet. Table 1 provides
exemplary culture methods
for the various meat products that can be produced.
100571 In some embodiments, the media is substantially free of serum or other
components
derived from an animal.
100581 Accordingly, in some embodiments, provided herein is a method of
producing
slaughter-free cell-based meat comprising: (a) providing cells from a non-
human organism; (b)
culturing the cells in media under conditions under which the cells grow in
either suspension
culture or adherent culture, wherein the media is substantially free of serum
and other
components derived from an animal; and optionally (c) isolating the cells and
producing the
slaughter-free meat product. In some embodiments the cells in culture comprise
fibroblasts,
myoblasts, or a co-culture of fibroblasts and myoblasts; in some embodiments
cells in co-culture
comprise additional cell types such as adipocytes, endothelial cells, and
generally cells from the
mesoderm lineage.
100591 In some embodiments, provided herein is a method of producing a
slaughter-free meat
product exhibiting an extended shelf life and/or lower microbial content
compared to
conventional meat obtained by slaughter, the method comprising: (a) providing
cells from a non-
human organism; (b) culturing the cells in media under suspension culture
conditions, or
adherent culture conditions, wherein the media is substantially free of serum
and other
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
components derived from an animal; and optionally (c) isolating the cells and
producing the
slaughter-free meat product. In some embodiments the cells in culture comprise
fibroblasts,
myoblasts, or a co-culture of fibroblasts and myoblasts; in some embodiments
cells in co-culture
comprise additional cell types such as adipocytes, endothelial cells, and
generally cells from the
mesoderm lineage.
100601 In some embodiments, the cells are grown in a suspension culture, e.g.
in a shake flask,
and the product of the culture yields a cell pellet. In some embodiments the
product may be
obtained by physical methods (e.g. centrifugation, gravity-assisted settling),
chemical methods,
enzymatic methods, sedimentation, concentration, flocculation, and the like.
In other
embodiments, the cells are grown in adherent culture, and the product of the
culture is a cell
sheet.
D. Harvesting and Formulation
100611 In some embodiments, the slaughter-free cell-based meat of the
disclosure is harvested
from a bioreactor (or other cell growth apparatus) and assessed for its
properties prior to
formulation. In some embodiments, the harvesting is carried out under aseptic
conditions (e.g.
using sterile gloves and working conditions, in a laminar flow hood).
100621 The slaughter-free cell-based meat of the disclosure may be formulated
post-harvest
(e.g. manipulated, processed) into a specific edible food type, any variety of
products, including,
but not limited to, a meatball, patty, surimi, cutlet, sausage, loaf, tender,
filet-style products, hot
dog, nugget, etc. Slaughter-free cell-based meat formulated products of the
disclosure may also
include meat that has been seasoned or dried such as jerky or snack-stick type
products, e.g. in
order to further extend the shelf life. Slaughter-free cell-based meat
formulated products of the
disclosure may also comprise additional ingredients (additives) such as
binders, spices,
stabilizers, preservatives, and the like. In exemplary embodiments,
formulation includes adding
one or more of the following ingredients to the harvested cell-based meat:
vital wheat gluten,
calcium chloride, iota carrageenan, flavor precursor mix, transglutaminase
enzyme powder
(maltodextrin, enzyme), protein concentrates, protein isolates,
polysaccharides, carrageenans,
flavorings, yeast extracts, enzymes, fibers, texturized proteins, pectins,
starches.
11
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
II. Characteristics of Cell-Based Meat
100631 Provided herein are slaughter-free cell-based meat products comprising
a number of
unique features that allow them to be distinguished from conventional meat
(which involves the
slaughter or otherwise demise of a live animal). The methods can also be
tailored to achieve
desired traits such as health benefits and sensory benefits. The points of
distinction include at
least the extended shelf life, levels of hormones, antibiotics, and microbial
contamination in the
cell-based meat, but can also include further customization such as altered
levels of fat content,
amino acid profiles, texture, and the like. These are considered in turn
below.
A. Hormones
100641 As compared to conventional meat, the slaughter-free cell-based meat of
the disclosure
comprises significantly lower amounts of steroid hormones. For example, using
the culturing
methods described, there need not be any exogenous hormones added into culture
thus resulting
in lower or non-existent hormonal levels in the resulting meat. Accordingly,
in some
embodiments, the slaughter-free cell-based meat product is substantially free
of steroid
hormones (i.e. contains little or no steroid hormones). This is in contrast to
animals raised for
conventional meat production, where they are often fed or otherwise
administered exogenous
hormones. It is noted that even animals (e.g. chicken, livestock) raised for
conventional meat
production that are not fed or administered any exogenous hormones, still have
testosterone,
estradiol, progesterone, among an array of others hormones, simply due to the
basal production
levels by the animals' glandular systems. Estradiol, progesterone, and
testosterone are natural
hormones found in conventional meat at some low level depending on animal
gender. In
contrast, the cell-based meat of the disclosure comprises lower levels of
steroid hormones or is
even substantially free of steroid hormones. For example, ELISA results for
1713-estradiol
indicated that slaughter-free chicken meat samples yielded a lower
concentration compared to
conventional chicken. 1713-estradiol levels were on average 35 ng estradiol/kg
wet mass for
slaughter-free chicken meat using the ELISA kit whereas conventional chicken,
procured from
the local grocery, was 90 ng/kg estradiol/kg wet mass.
100651 Accordingly, in some embodiments, the cell-based meat of the disclosure
comprises no
more than about lug, 0.5ug, 0.1ug, 0.05ug, 0.01ug, 0.005ug, or even about
0.001ug steroid
hormone/kg dry mass of cell-based meat. In some embodiments, the cell-based
meat comprises
12
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
no more than about lug, 0.5ug, 0.1ug, 0.05ug, 0.0 lug, 0.005ug, or even about
0.00lug
progesterone/kg dry mass of cell-based meat. In some embodiments, the cell-
based meat
comprises no more than about lug, 0.5ug, 0.1ug, 0.05ug, 0.0lug, 0.005ug, or
even about
0.00 lug testosterone/kg dry mass of cell-based meat. In some embodiments, the
cell-based meat
comprises no more than about 0.05ug, 0.0 lug, 0.005ug, or even about 0.00 lug
estradiol/kg dry
mass of cell-based meat. In exemplary embodiments, the cell-based meat
comprises no more
than about 35 ng estradiol/kg dry mass of cell-based meat.
B. Microbial Contamination
100661 Using the culturing methods described, the slaughter-free cell-based
meat product is
substantially free of microbial contaminants. "Substantially free" means that
the concentration of
microbes or parasites is below a clinically significant level of
contamination, e.g., below a level
wherein ingestion would lead to disease or adverse health conditions. Such low
levels of
contamination leads to an increased shelf life. This is in contrast to animals
raised for
conventional meat production destined for slaughter. As used herein, microbial
contamination
includes, but is not limited to, bacteria, fungi, viruses, protozoa, and
combinations thereof.
Harmful microbes may include coliforms (fecal bacteria), E. coli, yeast, mold,
Campylobacter,
Salmonella, Listeria, Enterobacteriaceae, Enterobacter cloacae complex,
Influenza Type A,
Influenza Type B, and Staph. A skilled artisan would understand that any
contaminant can be
measured.
100671 It is noted that the lower microbial contamination associated with the
slaughter-free
cell-based meat product of the disclosure as compared to conventional meat
obtained by
slaughter is exhibited at all temperatures: e.g. from about 0 C to about 30 C,
e.g. both at
standard domestic refrigerator temperatures (e.g. about 2 C to about 6 C) and
at room
temperature(e.g. about 22 C to about 25 C). It is also noted that the lower
microbial
contamination associated with the slaughter-free cell-based meat product of
the disclosure as
compared to conventional meat obtained by slaughter is exhibited for at least
3 days, 7 days, 14
days, 30 days, or 148 days following harvest, is exhibited for at least about
1, 2, 3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, and more weeks following
harvest. It is also noted
that the lower contamination is observed both under aseptic conditions as well
as under non-
aseptic conditions, and the lower contamination is observed when the meat is
measured post-
13
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
harvest with no subsequent formulation, as well as when the meat is measured
post-harvest, and
post-formulation.
100681 In addition, cells grown in culture may be substantially free from
parasites such as
tapeworms that infect cells of whole animals and that are transferred to
humans through
consumption of insufficiently cooked meat.
100691 Aseptic techniques may also be employed in packaging the meat products
as they come
off the biological production line. Such quality assurance may be monitored by
standard assays
for microorganisms or chemicals that are already known in the art.
100701 As compared to conventional meat, the slaughter-free cell-based meat of
the disclosure
comprises a significantly lower amount of microbial contamination. Example 3,
Example 9,
Tables 12 and 13 provides a comparison of contaminants in the slaughter-free
cell-based meat
versus conventional grocery store meat obtained by slaughter. Conventional
duck meat, and
especially conventional beef, had significantly higher amounts of microbial
contamination.
FIG. 3 shows representative plates indicating bacteria colonies, specifically
showing results for
cell-based duck, conventional beef, and conventional chicken.
100711 Accordingly, in some embodiments, provided herein is a slaughter-free
meat product
for dietary consumption exhibiting a lower microbial contamination count as
compared to
conventional meat obtained by slaughter, wherein the lower microbial
contamination count is
exhibited for at least 3 days following harvest.
100721 In some embodiments, the lower microbial contamination count is
exhibited at least 2,
3,4, 5, 6, 7, 10, 14, 15, 20, 21, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110,
120, 130, 140, 148, 150,
160, 170, 180, 190, 200, 210, 220, 230, 240, or at least 250 days following
harvest.
100731 In some embodiments, the microbial contamination count is determined
following
harvest, and prior to formulation In other embodiments, the microbial
contamination count is
determined following formulation.
100741 In some embodiments, the slaughter-free meat product is maintained
under non-aseptic
conditions and still exhibits the lower microbial contamination counts.
14
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0075] In some embodiments the microbial contamination count is determined by
measuring
the total microbial count (TC), E. coli/coliform count (EC), E. coli microbial
count, the
coliforms count, or the E. coli/coliforms count. In some embodiments the
microbial
contamination count is determined by measuring the count for other microbe
including, but not
limited to mold, yeast, salmonella, listeria, and staph.
[0076] In some embodiments the slaughter-free meat product comprises:
(a) no more than 100 cfus microbial contamination/g wet mass determined as per
the FDA
bacteriological analytical manual;
(b) no more than 10 cfus coliform contamination/g wet mass determined as per
the FDA
bacteriological analytical manual;
(c) no more than 10 cfus E. coli contamination/g wet mass determined as per
the FDA
bacteriological analytical manual;
(d) no more than 10 cfus yeast contamination/g wet mass determined as per the
FDA
bacteriological analytical manual;
(e) no more than 10 cfus mold contamination/g wet mass determined as per the
FDA
bacteriological analytical manual;
(f) no detectable level of salmonella contamination/25g wet mass determined as
per the
FDA bacteriological analytical manual;
(g) no detectable level of listeria contamination/25g wet mass determined as
per the AOAC
2004.06 method;
(h) no more than 10 cfus Staph contamination/g wet mass determined as per the
AOAC
2003.07 method; and/or
(i) no more than 55 cfus total aerobic count contamination/g wet mass
determined as per the
CompactDry protocol.
[0077] In some embodiments, the slaughter-free meat product comprises
slaughter-free
chicken, duck, or bovine meat, and the conventional meat comprises chicken,
duck or bovine
meat obtained by slaughter.
100781 Examples 3 and 9 provide a variety of exemplary protocols under which
the shelf life
and microbial contamination is observed. A skilled artisan would understand
that there are a
number of methods by which to measure microbial contamination. These are
provided at least by
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
the following texts: (1) FDA Bacteriological Analytical Manual (BAM) (Edition
8, Revision A
/1998) and (2) USDA Food Safety and Inspection Service Microbiology Laboratory
Guidebook.
The AOAC also provides at least the following tests for determination of
microbial
contamination:
a. Enterobacteriaceae, AOAC 2003.01
b. E. coli and coliforms, AOAC 998.08
c. Yeast and mold, FDA BAM Ch. 18
d. Listeria, AOAC 2004.06
e. Salmonella (25 g), AOAC 2011.03
f. Campylobacter, AOAC RI 051201
g. AOAC 2003.07 - Staph
h. Aerobic plate counts, AOAC 990.12
i. Salmonella, AOAC 2013.02, RI PTM 081201
j. Li steria species, AOAC-RI PTM #081401
k. Aerobic Count, AOAC 990.12
1. Coliforms & E. coli, AOAC 99L14
m. Y&M Count, AOAC 2014.05
C. Antibiotics
100791 As compared to conventional meat, the slaughter-free cell-based meat of
the disclosure
comprises significantly lower amounts of antibiotics, or is substantially free
of antibiotics, or is
free of antibiotics entirely. For example using the culturing methods
described, the use of
antibiotics in culture can be controlled or eliminated, thus resulting in
lower or non-existent
antibiotic levels in the resulting cell-based meat. Accordingly, in some
embodiments, the
slaughter-free cell-based meat product is substantially free of antibiotics
(i.e. contains little or no
antibiotics). This is in contrast to animals raised for conventional meat
production, where they
are often fed or otherwise administered exogenous antibiotics.
100801 Accordingly, in some embodiments, the cell-based meat of the disclosure
comprises no
more than about 100 ug antibiotics/kg dry mass of cell-based meat, 90 ug
antibiotics/kg dry
mass of cell-based meat, 80 ug antibiotics/kg dry mass of cell-based meat, 70
ug antibiotics/kg
dry mass of cell-based meat, 60 ug antibiotics/kg dry mass of cell-based meat,
50 ug
16
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
antibiotics/kg dry mass of cell-based meat, 40 ug antibiotics/kg dry mass of
cell-based meat, 30
ug antibiotics/kg dry mass of cell-based meat, 20 ug antibiotics/kg dry mass
of cell-based meat,
ug antibiotics/kg dry mass of cell-based meat, 5 ug antibiotics/kg dry mass of
cell-based
meat, 1 ug antibiotics/kg dry mass of cell-based meat, 0.5 ug antibiotics/kg
dry mass of cell-
based meat, 0.1 ug antibiotics/kg dry mass of cell-based meat, 0.05 ug
antibiotics/kg dry mass
of cell-based meat, or even about 0.01 ug/kg of antibiotics/kg dry mass of
cell-based meat.
D. Lipids
100811 As compared to conventional meat, the slaughter-free cell-based meat of
the disclosure
comprises a lower average total lipid (fat) content. Cell-based meat generally
has an average
total fat content between about 0.5% to about 5.0%, whereas the fatty acid
content in
conventional meat varies widely and can range from about 3% to about 18%,
depending on the
cut of meat.
100821 Table 14 shows the total fatty acid analysis for several exemplary
slaughter-free cell-
based meat samples. FIG. 4 shows total fatty acid composition for saturated,
monounsaturated
and polyunsaturated fatty acid across exemplary slaughter-free cell-based meat
samples.
100831 Accordingly, in some embodiments, the cell-based meat of the disclosure
comprises an
average total fat content of about 0.5%, about 0.6%, about 0.7%, about 0.8%,
about 0.9%, about
1.0%, about 1.1%, about 1.2%, about 1.3%, about 1.4%, about 1.5%, about 1.6%,
about 1.7%,
about 1.8%, about 1.9%, about 2.0%, about 2.1%, about 2.2%, about 2.3%, about
2.4%, about
2.5%, about 2.6%, about 2.7%, about 2.8%, about 2.9%, about 3.0%, about 3.1%,
about 3.2%,
about 3.3%, about 3.4%, about 3.5%, about 3.6%, about 3.7%, about 3.8%, about
3.9%, about
4.0%, about 4.1%, about 4.2%, about 4.3%, about 4.4%, about 4.5%, about 4.6%,
about 4.7%,
about 4.8%, about 4.9%, or about 5.0%, when measured as a % of total wet mass
of the cell-
based meat.
100841 In some exemplary embodiments, the cell-based meat comprises one or
more of the
following fatty acids classes in the amounts indicated, expressed as % of that
class over total
fatty acids:
17
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
a. saturated fatty acids content between about 10% to about 60%, e.g. about
20% to about
50%, about 30% to about 40%, about 10% to about 50%, about 10% to about 40%,
about
10% to about 30%, and/or about 10% to about 20%.
b. monounsaturated fatty acids content between about 10% to about 60%, e.g.
about 20% to
about 50%, about 30% to about 40%, about 10% to about 50%, about 10% to about
40%,
about 10% to about 30%, and/or about 10% to about 20%.
c. polyunsaturated fatty acids content between about 1% to about 50%, e.g.
about 10% to
about 40%, about 20% to about 30%, about 30% to about 20%, and/or about 40% to
about 10%.
100851 In some embodiments, the cell-based meat of the disclosure comprises a
ratio of about
2:1 to about 18:1 of omega 6:3 fatty acids classes. (ct-linolenic acid (ALA),
eicosapentaenoic
acid (EPA) and docosahexaenoic acid (DHA) are major omega 3 fatty acids).
Accordingly, in
some embodiments, the cell-based meat of the disclosure comprises a ratio of
about 2:1, 3:1, 4:1,
5:1, 6:1, 7:1, 8:1, 9:1, 10:1, 11:1, 12:1, 13:1, 14:1, 15:1, 16:1, 17:1, or
about 18:1 of omega 6:3
fatty acids classes. On the other hand, conventional meat, e.g. conventional
chicken comprises a
ratio of >18:1 omega 6:3 fatty acids classes.
100861 The lower fat content of cell-based meat provides a lower caloric
content, lower omega
6:3 ratios, as well as other related health benefits, when compared to
conventional meat.
100871 The slaughter-free cell-based meat production can further be customized
to achieve
desired profiles. Post-harvest desiccation can further increase the fat
content and/or other solid
components. Increasing lipid content in the growth medium can increase the fat
content as well.
100881 The flavor and aroma of the cell-based meat of the disclosure can be
altered during the
production. Generally, the higher proportion of unsaturated fatty acids in the
meat gives more
unsaturated volatile aldehydes and such compounds may be important in
determining the
specific aromas of these species.
100891 Accordingly, the methods provided herein can alter specific fatty acid
profiles to
achieve desired flavor characteristics or fatty acid profiles such as Omega
3:6 ratio through at
least the following mechanisms:
18
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
a. In some embodiments, the presence of serum in the media can affect fatty
acid profiles.
FIG. 7 shows the fatty acid percentages in serum free media vs. media
containing serum.
b. In some embodiments, serum of different sources can be used in culture to
achieve
different fatty acid profiles in the slaughter-free cell-based meat product.
(FIG. 8)
c. In some embodiments, the use of isolated clones from a polyclonal
population can be
used to alter fatty acid profiles as well. (FIG. 9).
d. In some embodiments, the fatty acids profiles are modulated by altering the
media's fatty
acid composition or by the addition of media components including compounds
added to
change fatty acid composition such as, but not limited to an agonist (e.g. an
agonist of
LXR13), or riboflavin. Such adjustments to media can impact fat profiles.
(FIG. 10, FIG.
11).
e. In some embodiments, the fatty acid profiles are modulated by the
composition of the
cells in culture. Accordingly, in some embodiments, the fibroblasts in
culture, the
myoblasts in culture, or the fibroblasts/myoblasts in co-culture can be
further modified to
include adipocytes.
100901 In FIG. 9, tissue (cell sheets) were formed using a co-culture method
(described in the
Method 15 in Table 1) using culture media with enhanced levels of various
compounds to
modulate specific biochemical pathways. Riboflavin, a vitamin and common co-
factor, was
titrated into cell culture media. The global effect on fatty acid
concentrations are shown.
100911 The lower levels of fatty acids in the cell-based meat of the
disclosure also promote an
extended shelf life of the meat, for example by leading to lower levels of
fatty acid oxidation
products in the meat.
E. Amino Acids
100921 It is also desirable that the slaughter-free cell-based meat product of
the disclosure
shares similarities with conventional meat products. The slaughter-free cell-
based meat product
of the disclosure generally comprises about 50 g to about 95 g by weight of
amino acids per 100
g dry mass. For example, the cell-based meat of the disclosure comprises one
or more of the
following amino acids in the indicated amounts (the amounts expressed as g of
amino acid/100g
total amino acid): Tryptophan about 1 to about 2.2, Threonine about 4.6 and
6.5, Isoleucine
about 3.8 to about 5, Leucine about 6.1 to about 8.9, Lysine about 5.7 to
about 8.8 , Methionine
19
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
about 0.14 to about 3.0, Cysteine about L5 to about L8, Phenylalanine about
3.7 to about 4.8,
Tyrosine about 3.0 to about 5.2, Valine about 4.8 to about 6.1, Arginine about
7.0 to about 8.0,
Histidine about 2.5 to about 4, Alanine about 5.0 to about 6.3, Aspartic acid
about 8.6 to about
10.4, Glutamic acid about 12.5 to about 14.6, Glycine about 4.6 to about 9.8,
Proline about 4.6
to about 6.8, Serine 4.4 to 5.3 , and/or Hydroxyproline about 0.0 to 4Ø
100931 In some embodiments, hydroxyproline levels are elevated in the cell-
based meat
generated from fibroblast monocultures, as compared to the conventional
counterpart. Without
being held to any theory or mechanism, such an increase in the hydroxyproline
levels may be
due higher levels of collagen formation resulting from the secretion of
extracellular matrix
components by fibroblast cells. In some embodiments, when myoblasts (MB) are
added to the
culture system either as a polyclonal cell mixture (mixed population of
myoblasts) or
monoclonal myoblast cell mixture (single-cell isolated from a mixed population
and expanded),
the hydroxyproline concentration can be reduced to close to that of
conventional meat. It is
noted that in the embodiments provided herein the hydroxyproline concentration
may also be
modulated to alter the texture of the meat product.
F. Vitamin E Content
100941 As compared to conventional meat, the slaughter-free cell-based meat of
the disclosure
comprises a higher Vitamin E (a-Tocopherol) content. In some embodiments, the
slaughter-free
cell-based meat product of the disclosure comprises at least about 0.5mg, at
least about 0.6mg, at
least about 0.7mg, at least about 0.8mg, at least about 0.9mg, or at least
about 1.0mg/Vitamin
E/100g wet mass of cell-based meat.
G. Moisture Content
[0095] The slaughter-free cell-based meat product the disclosure generally has
a moisture
content of about 65% to about 95%. In some embodiments, the moisture content
is measured
after harvesting, but before formulation. In other embodiments, the moisture
content is
measured after formulation.
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
H. Architecture of Cell-Based Meat
100961 There are additional points of distinction between slaughter-free cell-
based meat and
conventional meat. White it is not required for cells grown purely for meat
purposes (as
described herein) to possess functional characteristics that would allow for
transplantation and
function, under some circumstances they may be further engineered to include
components that
would confer functionality.
100971 Cell-based meat, unless otherwise manipulated to include, does not
include vascular
tissues, such as veins and arteries, whereas conventional meat does contain
such vasculature, and
contains the blood found in the vasculature. Accordingly, in some embodiments,
the cell-based
meat is substantially free of any vasculature. As generally contemplated
herein, vasculature
includes blood and/or lymphatic fluid.
[0098] Likewise, cell-based meat, although composed of muscle or muscle-like
tissues, unless
otherwise manipulated to include, does not comprise functioning muscle tissue.
Accordingly, in
some embodiments, the cell-based meat does not comprise functioning muscle
tissue.
[0099] It is noted that features such as vasculature (that may contain blood,
lymph, etc.) and
functional muscle tissue can be further engineered into the cell-based meat,
should there be a
desire to do so. For example, protocols that lead to sprouting of vessels may
be utilized to
introduce vasculature into the slaughter-free meat products of the disclosure,
and allow for
increased perfusion to the meat product.
I. Flavor
[0100] The fatty acid composition of meat generally impacts the overall meat
quality and
influences flavor, juiciness, and tenderness of the meat (Wood et al.,
Manipulating meat quality
and composition. Proceedings of the Nutrition Society. 1999;58:363-370. DOT:
org/10.1017/S009665199000488). Specific fatty acids, such 1VETIF'A oleic acid
(18:1 oi9) and
MUFA palmitoleic acid (16:1 w9) are fatty acids that are often primarily
associated with good
flavor.
[0101] Fattier meat is generally tastier, but with greater fat content comes
greater risk of
adverse Fat profiles in meat drive key organoleptic profiles that not only
drive consumer
21
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
preferences, but also establish unique species identification. However,
certain fat types are
associated with a greater risk of adverse health consequences such as heart
disease. Thus, overall
cell culture media composition, fatty acid supplementation into the culture,
and/or the ratio of
myoblasts/fibroblasts/adipocytes/endothelial cells/other cells mesodermal
lineage may be
regulated in culture to produce the slaughter-free cell-based meat products
with optimal flavor
and health effects. In exemplary embodiments, the ratio of adipocytcs is
altered in tch co-culture.
Regulation may be achieved by selecting specific clones of myogenic cells,
controlling the ratio
of the cells that are initially seeded in culture, and/or by varying, as
desired, the concentrations
and ratio of growth factors or other media components that act upon the cells.
Specific fatty
acids, like MUFA oleic acid (18:1 (09), can be enriched through media
composition and
nutritional design.
J. Supplementation
101021 In other embodiments, other nutrients such as vitamins may be added to
increase the
nutritional value of the meat. For example, this may be achieved through the
exogenous addition
of the nutrients to the growth medium or through genetic engineering
techniques.
K. Cooked Bite Force and Hardness
101031 The slaughter-free slaughter-free cell-based meat product of the
disclosure can be
modified to achieve certain textual features, such as a desired cooked bite
force or cooked
hardness. Table 17 shows the cooked texture of exemplary cell-based meat
samples. FIG. 13
shows the cooked hardness of exemplary meat generated from cells in either
myoblast:fibroblast
co-culture or fibroblast monoculture.
101041 The cooked bite force of a cell-based meat of the disclosure can range
from about 100g
to 5000g. In some exemplary embodiments, the cooked bite force of a cell-based
meat of the
disclosure, as harvested from adherent cells in culture, ranges from about
450g to about 3000g.
In some embodiments, the cooked hardness of a cell-based meat of the
disclosure as harvested
from adherent cells in culture, ranges from about 2500g to about 2000g. In
some embodiments
the cooked bite force and/or cooked hardness of a cell-based meat of the
disclosure is at or
below the detection limit, e.g. in some embodiments where the meat is
harvested from cells
grown in a suspension culture.
22
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
L. Shelf Life
101051 A significant portion of meat and meat products are spoiled every year.
It is estimated
that approximately 3.5 billion kg of poultry and meat are wasted at the
consumer, retailer, and
foodservice levels which have a substantial economic and environmental impact
(Kantor et al.
(1997)). A significant portion of this loss is due to microbial spoilage.
101061 Conventional meat is perishable and has a relatively short
shelf life stability
(interchangeably referred to as simply "shelf life" herein). The composition
of conventional
meat and the conditions used to slaughter and harvest the meat generally
create favorable growth
conditions for various microorganisms including fecal bacteria (e.g. coliform
bacteria);
conventional meat is also very susceptible to spoilage due to chemical,
oxidative, and enzymatic
activities. Accordingly, as used herein, in some embodiments, the shelf life
is the amount of time
a food remains fit for dietary consumption while still being palatable, e.g.
not causing any
disease or adverse health effects, such as vomiting, diarrhea, nausea, and the
like upon ingestion,
and not producing an aroma that would suggest that the process of decay (e.g.
microbially-
induced, molecular decay, physical decay) has begun.
101071 Without being bound by theory or mechanism, it is generally regarded
that microbial
growth, oxidation, and enzymatic autolysis are three mechanisms responsible
for the spoilage of
meat, thereby reducing the shelf life. The breakdown of fat, protein, and
carbohydrates of meat
results in the development of off-odors and off-flavor and these the off-odors
and off-flavors
make the meat objectionable for human consumption. Depending on the species
and method of
harvest, conventional meat products are not safe to consume after a relatively
short period of
storage time. For example, chicken should be cooked within a few days of
purchasing. Cooked
poultry can be safely stored in the refrigerator for about only 3-5 days and
the freezer for up to
about 3-5 months. It is, therefore, necessary to control meat spoilage in
order to increase its
shelf life and maintain its nutritional value, texture and flavor.
101081 The shelf life of conventional meat is often increased by various
processes including
adding preservatives, pickling, salting, dehydrating, canning, fermenting, or
storing in darkness.
The cell-based meat of the disclosure exhibits extended shelf life, without
the use of any of these
methods, but it is noted that such methods could be added to even further
enhance the shelf life.
Accordingly in some embodiments, the cell-based meat of the disclosure
exhibits a measurement
23
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
indicative of an increase in shelf life, as compared to conventional meat
obtained by slaughter,
where the conventional meat is unprocessed (e.g. no process has been further
applied, such as
those listed above).
101091 Slaughter-free cell-based meat, through its method of production and
composition,
produces a meat product that has extended shelf life compared to conventional
meat products
and does not require the addition of preservative agents to obtain the shelf
life stability. The
composition of cell-based meat is such that fewer off-odors and off-flavors
are detected. In
addition, the manufacturing methods used to produce cell-based meat require
clean and aseptic
conditions. These conditions ensure that microbial cell counts in both
harvested products and
subsequent food processing are low. These multiple factors contribute to
extended shelf life
stability of cell-based meat
101101 The shelf life due to spoilage of the cell-based meat of the disclosure
is enhanced
relative to conventional meat. This is the case both at all temperatures, e.g.
at room temperature
(about 22 C to about 26 C) and at colder temperatures akin to domestic
refrigerator temperatures
(e.g. at about 2 C to about 4 C). The extended shelf life is associated with
reduced
contamination, composition of the cell-based meat, reduced degradation of the
cell-based meat
and slower rates of change in color, spoilage, smell and flavor of the cell-
based meat, and allows
the meat to be maintained for dietary consumption.
101111 Without being bound to theory or mechanism, there is a decrease in
total fatty acid
content in the cell-based meat, as compared to conventional meat, resulting in
lower levels of
fatty acid oxidation products, leading to slower rates of change in the color,
smell, or flavor of
the meat. Oxidative rancidity is associated with the degradation by oxygen in
the air. The double
bonds of an unsaturated fatty acid can be cleaved by free-radical reactions
involving molecular
oxygen. This reaction causes the release of malodorous and highly volatile
aldehydes and
ketones. Oxidation primarily occurs with unsaturated fats. For example, even
though meat is
held under refrigeration or in a frozen state, the polyunsaturated fat can
continue to oxidize and
slowly become rancid. The fat oxidation process begins immediately after the
animal is
slaughtered and the muscle, intra-muscular, inter-muscular, and surface fat
becomes exposed to
oxygen of the air.
24
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0112] Without being bound to theory or mechanism, there is a decrease the
number of lipases
in the cell-based meat, as compared to conventional meat, resulting in lower
levels of fatty acid
breakdown, leading to slower rates of change in the color, smell, or flavor of
the meat.
[0113] Without being bound to theory or mechanism, due to the absence of
vasculature in the
cell-based meat, when compared to conventional meat, there is less oxygen
present, resulting in
lower levels of fatty acid oxidation and growth of aerobic bacteria, leading
to reduced microbial
contamination levels, and leading to slower rates of change in the color,
smell, aroma or flavor
of the meat.
[0114] Without being bound to theory or mechanism, due to the absence of
functional muscle
tissue (e.g. myoglobin) in the cell-based meat when compared to conventional
meat, there is less
oxygen present, resulting in lower levels fatty acid oxidation and the growth
of aerobic bacteria,
leading to reduced microbial contamination levels, and leading to slower rates
of change in the
color, smell, or flavor of the meat.
[0115] Without being bound to theory or mechanism, due to higher amounts of
Vitamin E in
the cell-based meat when compared to conventional meat, there are higher
levels of antioxidant
activity, resulting in protection against fatty acid oxidation, and leading to
slower rates of change
in the color, smell, or flavor of the meat. Oxidation of lipids in meat
depends on several factors
including fatty acid composition, the level of the antioxidant vitamin E (a-
tocopherol), and
prooxidants such as the free iron presence in muscles.
[0116] Accordingly, in some embodiments, as compared to conventional meat, the
shelf life of
slaughter-free cell-based meat product is extended by at least about 1.5x, at
least about 2x, at
least about 2.5x, at least about 3x, at least about 3.5x, at least about 4x,
at least about 4.5x, at
least about 5x, at least about 5.5x, at least about 6x, at least about 6.5x,
at least about 7x, at least
about 7.5x, at least about 8x, at least about 8.5x, at least about 9x, at
least about 9.5x, or even at
least about 10x. The shelf life increases are observed both at about 2 C, and
about 26 C, and all
temperatures in between, inclusive of the endpoints.
101171 In some embodiments, provided herein is a slaughter-free cell-based
meat product for
dietary consumption exhibiting an extended shelf life, wherein the shelf life
is extended
compared to conventional meat obtained by slaughter, and wherein the shelf
life is extended at
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
all temperatures. In some embodiments the extended shelf life is maintained
for at least 2, 3, 4,
5, 6, 7, 10, 14, 15, 20, 21, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120,
130, 140, 148, 150, 160,
170, 180, 190, 200, 210, 220, 230, 240, or at least 250 days following
harvest. In some
embodiments the extended shelf life is maintained for at least 3, 7, 14, 30,
or 148 days following
harvest at about 22 C - 26 C. In some embodiments the shelf life is determined
after harvest,
and prior to formulation. In some embodiments the shelf life is determined
after formulation. In
some embodiments the shelf life is extended under non-aseptic conditions, both
at about 2 C to
about 6 C, and at about 22 C- 26 C. In some embodiments the shelf life is
determined by
measuring the total microbial count (TC), for E. coli/coliforms (EC), E. coli
microbial count, or
the coliforms count.
[0118] In some embodiments the TC measurement of conventional meat obtained by
slaughter
is at least 1.5x, 2x, 2.5x, 3x, 3.5x, 4x, 4.5x, 5x, 10x, 15x, 20x, or even 25x
higher than that of the
TC measurement of slaughter-free meat product, and leads to its lower shelf
life.
101191 In some exemplary embodiments the slaughter-free cell-based meat
product comprises
one or more of the following features, which in part contributes to the
exhibited extended shelf
life:
(a) no detectable level or no more than 1, 10, 50, or 100 cfus microbial
contamination/g wet
mass determined as per the FDA bacteriological analytical manual;
(b) no detectable level or no more than 1, 10, 50, or 100 cfus coliform
contamination/g wet
mass determined as per the FDA bacteriological analytical manual;
(c) no detectable level or no more than 1, 10, 50, or 100 cfus E. coli
contamination/g wet
mass determined as per the FDA bacteriological analytical manual;
(d) no detectable level or no more than 1, 10, 50, or 100 cfus yeast
contamination/g wet mass
determined as per the FDA bacteriological analytical manual;
(e) no detectable level or no more than 1, 10, 50, or 100 cfus mold
contamination/g wet mass
determined as per the FDA bacteriological analytical manual;
(f) no detectable level or no more than 1, 10, 50, or 100 cfus of salmonella
contamination/g
wet mass determined as per the FDA bacteriological analytical manual;
(g) no detectable level of or no more than 1, 10, 50, or 100 cfus listeria
contamination/g wet
mass determined as per the AOAC 2004.06 method;
26
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
(h) no detectable level of or no more than 1, 10, 50, or 100 cfus Staph
contamination/g wet
mass determined as per the AOAC 2003.07 method; and/or
(i) no detectable level of or no more than 1, 10, 50, or 100 cfus no more than
55 cfus total
aerobic count contamination/g wet mass determined as per the CompactDry
protocol.
101.201 In some embodiments the slaughter-free meat product exhibiting the
extended shelf life
comprises slaughter-free chicken, duck, or bovine meat, and the conventional
meat comprises
chicken, duck or bovine meat obtained by slaughter.
101211 Example 9 discusses a variety of exemplary protocols under which the
shelf life and
microbial contamination is observed. A skilled artisan would understand that
there are a number
of methods by which to measure shelf life, and parameters associated with
shelf life, such as
microbial contamination. These are provided at least by the following texts:
(1) FDA
Bacteriological Analytical Manual (BAM) (Edition 8, Revision A /1998) and (2)
USDA Food
Safety and Inspection Service Microbiology Laboratory Guidebook. The AOAC also
provides at
least the following tests for determination of microbial contamination:
a) Enterobacteriaceae, AOAC 2003.01
b) E. coli and coliforms, AOAC 998.08
c) Yeast and mold, FDA BAM Ch. 18
d) Listeria, AOAC 2004.06
e) Salmonella (25 g), AOAC 2011.03
f) Campylobacter, AOAC RI 051201
g) AOAC 2003.07 - Staph
h) Aerobic plate counts, AOAC 990.12
i) Salmonella, AOAC 2013.02, RI PTM 081201
j) Li steri a species, AOAC-RI PTM #081401
k) Aerobic Count, AOAC 990.12
1) Coliforms & E. coli, AOAC 991.14
m) Y&M Count, AOAC 2014.05
101221 Additional endpoints may be tested as indicative of shelf life. These
include but are not
limited to: rancidity testing; accelerated shelf life study; moisture, pH and
water activity
27
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
variability; product stability under varying storage conditions;
microbiological, chemical and
physical testing; sensory evaluation; and probiotic stability.
III. Enumerated Embodiments
101231 The invention may be defined by reference to the following sets of
enumerated,
illustrative sets of embodiments:
Set 1
101241 Embodiment 1. A cell-based meat product, wherein the cell-
based meat product
comprises at least two of the following features:
a. no more than about 1 ug steroid hormone/kg dry mass cell-based meat
product;
b. no more than about 100 ug antibiotics/kg dry mass cell-based meat
product;
c. no more than about 100 cfus microbial contamination/g wet mass of cell-
based
meat product;
d. an average total fat content between about 0.5% to about 5.0% when
measured as
% of wet mass of cell-based meat product;
e. is substantially free of vasculature; and
f. has at least a 2x increase in shelf life as compared to conventional
meat.
101251 Embodiment 2. The cell-based meat product of embodiment 1,
wherein the cell-
based meat product comprises at least, three, four, five, or six of the
features (a) to (f).
101261 Embodiment 3. The cell-based meat product of embodiments 1
to 2, wherein the
cell-based meat product comprises no more than about 1 ug progesterone /kg dry
mass of cell-
based meat.
101271 Embodiment 4. The cell-based meat product of embodiments 1
to 3, wherein the
cell-based meat product comprises no more than about 1 ug testosterone /kg dry
mass of cell-
based meat.
28
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0128] Embodiment 5. The cell-based meat product of any one of
embodiments 1 to 4,
wherein the cell-based meat product comprises no more than about 35 ng
estradiol/kg dry mass
of cell-based meat.
[0129] Embodiment 6. The cell-based meat product of any one of
embodiments 1 to 5,
wherein the cell-based meat product comprises about 50 g to about 90 g by
weight of amino
acids per 100 g dry mass.
[0130] Embodiment 7. The cell-based meat product of any one of
embodiments 1 to 6,
wherein the cell-based meat product comprises one or more of the following
amino acids in the
indicated amounts (expressed as g of amino acid/100g total amino acid):
Tryptophan about Ito
about 2.2, Threonine about 4.6 and 6.5, Isoleucine about 3.8 to about 5,
Leucine about 6.1 to
about 8.9, Lysine about 5.7 to about 8.8 , Methionine about 0.14 to about 3.0,
Cysteine about
1.5 to about 1.8, Phenylalanine about 3.7 to about 4.8, Tyrosine about 3.0 to
about 5.2, Valine
about 4.8 to about 6.1, Arginine about 7.0 to about 8.0, Histidine about 2.5
to about 4, Alanine
about 5.0 to about 6.3, Aspartic acid about 8.6 to about 10.4, Glutamic acid
about 12.5 to about
14.6, Glycine about 4.6 to about 9.8, Proline about 4.6 to about 6.8, Serine
about 4.4 to about 5.3
, and/or Hydroxyproline about 0.0 to 4Ø
[0131] Embodiment 8. The cell-based meat product of any one of
embodiments Ito 7,
wherein the cell-based meat product has a moisture content of about 65% to
about 95%.
[0132] Embodiment 9. The cell-based meat product of embodiment 8,
wherein the
moisture content is measured after harvesting, but before formulation.
[0133] Embodiment 10. The cell-based meat product of embodiment 8,
wherein the
moisture content is measured after formulation and a dehydration process, but
before the
addition of ingredients.
[0134] Embodiment 11. The cell-based meat product of any one of
embodiments 1 to 10,
wherein the cell-based meat product is a cell-based avian meat product.
101351 Embodiment 12. The cell-based meat product of embodiment 11,
wherein the cell-
based avian meat product is a chicken cell-based meat product.
29
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0136] Embodiment 13. The cell-based meat product of embodiment 11,
wherein the cell-
based avian meat product is a duck cell-based meat product.
101371 Embodiment 14. The cell-based meat product of any one of
embodiments 1 to 10,
wherein the cell-based meat product is a cell-based bovine meat product.
101381 Embodiment 15. The cell-based meat product of any one of
embodiments 1 to 14,
wherein the cell-based meat product is a cell pellet.
[0139] Embodiment 16. The cell-based meat product of any one of
embodiments 1 to 14,
wherein the cell-based meat product is a cell sheet.
[0140] Embodiment 17. The cell-based meat product of any one of
embodiments 1 to 16,
wherein the cell-based meat product is generated from fibroblasts in culture.
[0141] Embodiment 18. The cell-based meat product of any one of
embodiments 1 to 16,
wherein the cell-based meat product is generated from myoblasts in culture.
[0142] Embodiment 19. The cell-based meat product of any one of
embodiments 1 to 16,
wherein the cell-based meat product is generated from a co-culture comprising
fibroblasts and
myoblasts in culture.
[0143] Embodiment 20. The cell-based meat product of any one of
embodiments 16 to 19,
wherein the culture further comprises adipocytes, endothelial cells, and/or
cells of a mesodermal
lineage.
[0144] Embodiment 21. The cell-based meat product of embodiment 19,
wherein the ratio
of fibroblasts to myoblasts in co-culture are at a ratio of about 95F:5M to
about 5F:95M.
[0145] Embodiment 22. The cell-based meat product of any one of
embodiments 1 to 21,
wherein the cell-based meat product comprises at least about 0.5 mg Vitamin
E/100 g wet mass
of cell-based meat.
[0146] Embodiment 23. The cell-based meat product of any one of
embodiments 1 to 22,
wherein the cell-based meat product comprises one or more of the following
fatty acids classes
in the amounts indicated, expressed as % of that class over total fatty acids:
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
a. saturated fatty acids content between about 29% to about 42%;
b. monounsaturated fatty acids content between about 19% to about 54%; and
c. polyunsaturated fatty acids content between about 5% to about 36%.
101471 Embodiment 24. The cell-based meat product of any one of
embodiments 1 to 23,
wherein the cell-based meat product comprises a ratio of about 2:1 to 18:1
omega 6:3 fatty acids
classes.
101481 Embodiment 25. The cell-based meat product of embodiments 23
or 24, wherein
the cell-based meat product is produced from chicken cells, duck cells, or
bovine cells.
101491 Embodiment 26. The cell-based meat product of embodiments 23
or 24, wherein
the cell-based meat is generated in a medium in which the fatty acid content
has been
manipulated.
101501 Embodiment 27. The cell-based meat product of any one of
embodiments 1 to 25,
wherein the cell-based meat product comprises at least one of the following
textural features:
a. cooked bite force from 450g to about 2970g; and
b. cooked hardness from about 280g to about 1900g.
101511 Embodiment 28. The cell-based meat product of any one of
embodiments 1 to 27,
the cell-based meat product has at least a 10x increase in stability and shelf
life as compared to
conventional meat.
101521 Embodiment 29. The cell-based meat product of embodiment 28,
wherein the
increase in shelf life is measured at about 4 C.
101531 Embodiment 30. The cell-based meat product of embodiment 28,
wherein the
increase in shelf life is measured at about 25 C.
101541 Embodiment 31. A method of producing cell-based meat
comprising:
a. providing fibroblasts and/or myoblasts from a non-human
organism;
31
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
b. culturing the fibroblasts and/or myoblasts in media under suspension
culture
conditions, or adherent culture conditions, wherein the media is substantially
free of
serum and other components derived from an animal; and
c. isolating the cells and producing the cell-based meat.
101551 Embodiment 32. The method of embodiment 31, wherein the
fibroblasts and/or
myoblasts are provided at a ratio of about 95F:5M to about 5F:95M.
101561 Embodiment 33. The method of any one of embodiments 31 to
32, wherein the cell-
based meat product has at least a 2x increase in shelf life, as compared to
conventional meat.
101571 Embodiment 34. The method of any one of embodiments 31 to
33, wherein the
method comprises adjusting the fatty acid content of the media, wherein the
resulting cell-based
meat product has a ratio of about 2:1 to about 18:1 of the omega 6:3 fatty
acids classes.
Set 2
101581 Embodiment 1. A slaughter-free meat product for dietary
consumption exhibiting
an extended shelf life, wherein the shelf life is extended compared to
conventional meat obtained
by slaughter, and wherein the shelf life is extended for at least 3 days
following harvest.
101591 Embodiment 2. The slaughter-free meat product of embodiment
1, wherein the
extended shelf life is maintained for at least 3 days following harvest at
about 0 C to about
30 C.
101601 Embodiment 3. The slaughter-free meat product of any one of
embodiments 1 to 2,
wherein the shelf life is determined after harvest, and prior to formulation.
101611 Embodiment 4. The slaughter-free meat product of any one of
embodiments 1 to 3,
wherein the shelf life is determined after formulation.
101621 Embodiment 5. The slaughter-free meat product of embodiment
3, wherein the
shelf life is extended when the meat is harvested under non-aseptic
conditions.
32
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0163] Embodiment 6. The slaughter-free meat product of any one of
embodiments 1 to 5,
wherein the shelf life is determined by measuring the total microbial count
(TC), E.
coli/coliforms count (EC), E. coli microbial count, or the coliforms count.
[0164] Embodiment 7. The slaughter-free meat product of embodiment
6, wherein the TC
measurement of conventional meat obtained by slaughter is at least 1.5x higher
than that of the
TC measurement of slaughter-free meat product.
[0165] Embodiment 8. The slaughter-free meat product of any one of
embodiments 1 to 7,
comprising no more than 1 cfus microbial contamination per g/wet mass.
[0166] Embodiment 9. The slaughter-free meat product of any one of
embodiments 1 to 8,
wherein the slaughter-free meat product comprises no more than about lug
steroid hormone.
[0167] Embodiment 10. The slaughter-free meat product of any one of
embodiments 1 to
9, wherein the slaughter-free meat product comprises about 50g to about 90 g
by weight of
amino acids per 100 g dry mass.
[0168] Embodiment 11. The slaughter-free meat product of any one of
embodiments 1 to
10, wherein the slaughter-free meat product comprises one or more of the
following amino acids
in the indicated amounts (expressed as g of amino acid/100g total amino acid):
Tryptophan
about 1 to about 2.2, Threonine about 4.6 and 6.5, Isoleucine about 3.8 to
about 5, Leucine
about 6.1 to about 8.9, Lysine about 5.7 to about 8.8 , Methionine about 0.14
to about 3.0,
Cysteine about 1.5 to about 1.8, Phenylalanine about 3.7 to about 4.8,
Tyrosine about 3.0 to
about 5.2, Valine about 4.8 to about 6.1, Arginine about 7.0 to about 8.0,
Histidine about 2.5 to
about 4, Alanine about 5.0 to about 6.3, Aspartic acid about 8.6 to about
10.4, Glutamic acid
about 12.5 to about 14.6, Glycine about 4.6 to about 9.8, Proline about 4.6 to
about 6.8, Serine
about 4.4 to about 5.3 , and/or Hydroxyproline about 0.0 to 4Ø
[0169] Embodiment 12. The slaughter-free meat product of any one of
embodiments 1 to
11, wherein the slaughter-free meat product has a moisture content of about
65% to about 95%,
wherein the moisture content is measured after harvest, but before
formulation.
33
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0170] Embodiment 13. The slaughter-free meat product of any one of
embodiments 1 to
12, wherein the slaughter-free meat product comprises at least about 0.5mg
Vitamin E/100g wet
mass of the slaughter-free meat product.
[0171] Embodiment 14. The slaughter-free meat product of any one of
embodiments 1 to
13, wherein the slaughter-free meat product comprises one or more of the
following fatty acids
classes in the amounts indicated, expressed as % of that class over total
fatty acids:
a. saturated fatty acids content between about 10% to about 60%;
b. monounsaturated fatty acids content between about 10% to about 60%; and
c. polyunsaturated fatty acids content between about 1% to about 50%.
[0172] Embodiment 15. The slaughter-free meat product of any one of
embodiments 1 to
14, wherein the slaughter-free meat product comprises a ratio of about 2:1 to
18:1 omega 6:3
fatty acids classes.
[0173] Embodiment 16. The slaughter-free meat product of any one of
embodiments 1 to
15, wherein the slaughter-free meat product comprises slaughter-free chicken,
duck, or bovine
meat, and the conventional meat comprises chicken, duck or bovine meat
obtained by slaughter.
[0174] Embodiment 17. The slaughter-free meat product of any one of
embodiments 1 to
16, wherein the slaughter-free meat product is substantially free of
vasculature.
[0175] Embodiment 18. The slaughter-free meat product of any one of
embodiments 1 to
17, wherein the conventional meat is not processed.
[0176] Embodiment 19. A slaughter-free meat product for dietary
consumption exhibiting
a lower microbial contamination count as compared to conventional meat
obtained by slaughter,
wherein the lower microbial contamination count is exhibited for at least 3
days following
harvest.
[0177] Embodiment 20. The slaughter-free meat product of embodiment
19, wherein the
lower microbial contamination count is maintained for at least 3 days
following harvest at about
0 C to about 30 C.
34
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0178] Embodiment 21. The slaughter-free meat product of any one of
embodiments 19 to
20, wherein the microbial contamination count is determined following harvest,
and prior to
formulation.
101791 Embodiment 22. The slaughter-free meat product of any one of
embodiments 19 to
21, wherein the slaughter-free meat product is maintained under non-aseptic
conditions.
[0180] Embodiment 23. The slaughter-free meat product of any one of
embodiments 19 to
22, wherein the microbial contamination count is determined by measuring the
total microbial
count (TC), E. coli/coliforms count (EC), E. coli microbial count, or the
coliforms count.
[0181] Embodiment 24. The slaughter-free meat product of any one of
embodiments 19 to
23, comprising no more than 1 cfus microbial contamination per g/wet mass.
[0182] Embodiment 25. The slaughter-free meat product of any one of
embodiments 19 to
24, wherein the TC measurement of conventional meat obtained by slaughter is
at least 1.5x
higher than that of the TC measurement of slaughter-free meat product.
[0183] Embodiment 26. The slaughter-free meat product of any one of
embodiments 19 to
25, wherein the slaughter-free meat product comprises no more than about lug
steroid hormone.
[0184] Embodiment 27. The slaughter-free meat product of any one of
embodiments 19 to
26, wherein the slaughter-free meat product comprises one or more of the
following amino acids
in the indicated amounts (expressed as g of amino acid/100g total amino acid):
Tryptophan
about 1 to about 2.2, Threonine about 4.6 and 6.5, Isoleucine about 3.8 to
about 5, Leucine
about 6.1 to about 8.9, Lysine about 5.7 to about 8.8 , Methionine about 0.14
to about 3.0,
Cysteine about 1.5 to about 1.8, Ph enylalanine about 3.7 to about 4.8,
Tyrosine about 3.0 to
about 5.2, Valine about 4.8 to about 6.1, Arginine about 7.0 to about 8.0, Hi
stidine about 2.5 to
about 4, Alanine about 5.0 to about 6.3, Aspartic acid about 8.6 to about
10.4, Glutamic acid
about 12.5 to about 14.6, Glycine about 4.6 to about 9.8, Proline about 4.6 to
about 6.8, Serine
about 4.4 to about 5.3 , and/or Hydroxyproline about 0.0 to 4Ø
101851 Embodiment 28. The slaughter-free meat product of any one of
embodiments 19 to
27, wherein the slaughter-free meat product comprises one or more of the
following fatty acids
classes in the amounts indicated, expressed as % of that class over total
fatty acids:
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
a. saturated fatty acids content between about 10% to about 50%;
b. monounsaturated fatty acids content between about 10% to about 54%; and
c. polyunsaturated fatty acids content between about 1% to about 50%.
101861 Embodiment 29. The slaughter-free meat product of
embodiments 19 to 28, wherein
the slaughter-free meat product comprises a ratio of about 2:1 to 18:1 omega
6:3 fatty acids
classes.
101871 Embodiment 30. The slaughter-free meat product of
embodiments 19 to 29, wherein
the slaughter-free meat product comprises slaughter-free chicken, duck, or
bovine meat, and the
conventional meat comprises chicken, duck or bovine meat obtained by
slaughter.
101881 Embodiment 31. A method of producing a slaughter-free meat
product exhibiting an
increase in shelf life compared to unprocessed conventional meat obtained by
slaughter, the
method comprising:
a. providing cells from a non-human organism;
b. culturing the cells in media under suspension culture conditions or
adherent
culture conditions, wherein the media is substantially free of serum and other
components derived from an animal; and
c. isolating the cells and producing the slaughter-free meat product.
101891 Embodiment 32. The method of embodiment 31, wherein the
cells comprise
myoblasts, fibroblasts, adipocytes, endothelial cells, cells of a mesoderm
lineage, and
combinations thereof.
101901 Embodiment 33. The method of any one of embodiments 31-32,
wherein the cells
comprise at least fibroblasts and myoblasts, and the fibroblasts and myoblasts
are provided at a
ratio of about 95F:5M to about 5F:95M.
101911 Embodiment 34. The method of any one of embodiments 31-33
the method
comprises adjusting the fatty acid content of the media, wherein the resulting
slaughter-free meat
product has a ratio of about 2:1 to about 18:1 of the omega 6:3 fatty acids
classes.
36
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
Set 3
[0192] Embodiment 1. A slaughter-free meat product for dietary
consumption exhibiting
an extended shelf life, wherein the shelf life is extended compared to
conventional meat obtained
by slaughter, and wherein the shelf life is extended for at least 3 days
following harvest.
101931 Embodiment 2. The slaughter-free meat product of embodiment
1, wherein the
extended shelf life is maintained for at least 3 days following harvest at
about 0 C to about 30 C.
[0194] Embodiment 3. The slaughter-free meat product of embodiment
1, wherein the
shelf life is determined after harvest, and prior to formulation.
[0195] Embodiment 4. The slaughter-free meat product of embodiment
1, wherein the
shelf life is determined after formulation.
[0196] Embodiment 5. The slaughter-free meat product of embodiment
3, wherein the
shelf life is extended when the meat is harvested under non-aseptic
conditions.
101971 Embodiment 6. The slaughter-free meat product of embodiment
1, wherein the
shelf life is determined by measuring the total microbial count (TC), E.
coli/coliforms count
(EC), E. coli microbial count, or the coliforms count.
101981 Embodiment 7. The slaughter-free meat product of embodiment
6, wherein the TC
measurement of conventional meat obtained by slaughter is at least 1.5x higher
than that of the
TC measurement of slaughter-free meat product.
[0199] Embodiment 8. The slaughter-free meat product of embodiment
1, comprising no
more than 1 cfus microbial contamination per g/wet mass.
[0200] Embodiment 9. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises no more than about lug steroid hormone.
[0201] Embodiment 10. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises about 50g to about 90 g by weight of
amino acids per 100
g dry mass.
37
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0202] Embodiment 11. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises one or more of the following amino acids
in the indicated
amounts (expressed as g of amino acid/100g total amino acid): Tryptophan about
1 to about 2.2,
Threonine about 4.6 and 6.5, Isoleucine about 3.8 to about 5, Leucine about
6.1 to about 8.9,
Lysine about 5.7 to about 8.8, Methionine about 0.14 to about 3.0, Cysteine
about 1.5 to about
1.8, Phcnylalaninc about 3.7 to about 4.8, Tyrosine about 3.0 to about 5.2,
Valinc about 4.8 to
about 6.1, Arginine about 7.0 to about 8.0, Histidine about 2.5 to about 4,
Alanine about 5.0 to
about 6.3, Aspartic acid about 8.6 to about 10.4, Glutamic acid about 12.5 to
about 14.6, Glycine
about 4.6 to about 9.8, Proline about 4.6 to about 6.8, Serine about 4.4 to
about 5.3 , and/or
Hydroxyproline about 0.0 to 4Ø
[0203] Embodiment 12. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product has a moisture content of about 65% to about 95%,
wherein the
moisture content is measured after harvest, but before formulation.
[0204] Embodiment 13. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises at least about 0.5mg Vitamin E/100g wet
mass of the
slaughter-free meat product.
[0205] Embodiment 14. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises one or more of the following fatty acids
classes in the
amounts indicated, expressed as % of that class over total fatty acids:
a. saturated fatty acids content between about 10% to about 60%;
b. monounsaturated fatty acids content between about 10% to about 60%; and
c. polyunsaturated fatty acids content between about 1% to about 50%.
[0206] Embodiment 15. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises a ratio of about 2:1 to 18:1 omega 6:3
fatty acids classes.
[0207] Embodiment 16. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product comprises slaughter-free chicken, duck, or bovine
meat, and the
conventional meat comprises chicken, duck or bovine meat obtained by
slaughter.
38
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0208] Embodiment 17. The slaughter-free meat product of embodiment
1, wherein the
slaughter-free meat product is substantially free of vasculature.
102091 Embodiment 18. The slaughter-free meat product of embodiment
1, wherein the
conventional meat is not processed.
102101 Embodiment 19. A slaughter-free meat product for dietary
consumption exhibiting
a lower microbial contamination count as compared to conventional meat
obtained by slaughter,
wherein the lower microbial contamination count is exhibited for at least 3
days following
harvest.
[0211] Embodiment 20. The slaughter-free meat product of embodiment
19, wherein the
lower microbial contamination count is maintained for at least 3 days
following harvest at about
0 C to about 30 C.
[0212] Embodiment 21. The slaughter-free meat product of embodiment
19, wherein the
microbial contamination count is determined following harvest, and prior to
formulation.
[0213] Embodiment 22. The slaughter-free meat product of embodiment
19, wherein the
slaughter-free meat product is maintained under non-aseptic conditions.
[0214] Embodiment 23. The slaughter-free meat product of embodiment
19, wherein the
microbial contamination count is determined by measuring the total microbial
count (TC), E.
coli/coliforms count (EC), E. coli microbial count, or the coliforms count.
[0215] Embodiment 24. The slaughter-free meat product of embodiment
19, comprising no
more than 1 cfus microbial contamination per g/wet mass.
[0216] Embodiment 25. The slaughter-free meat product of embodiment
19, wherein the
TC measurement of conventional meat obtained by slaughter is at least 1.5x
higher than that of
the TC measurement of slaughter-free meat product.
[0217] Embodiment 26. The slaughter-free meat product of embodiment
19, wherein the
slaughter-free meat product comprises no more than about lug steroid hormone.
39
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0218] Embodiment 27. The slaughter-free meat product of embodiment
19, wherein the
slaughter-free meat product comprises one or more of the following amino acids
in the indicated
amounts (expressed as g of amino acid/100g total amino acid): Tryptophan about
1 to about 2.2,
Threonine about 4.6 and 6.5, Isoleucine about 3.8 to about 5, Leucine about
6.1 to about 8.9,
Lysine about 5.7 to about 8.8, Methionine about 0.14 to about 3.0, Cysteine
about 1.5 to about
1.8, Phenylalanine about 3.7 to about 4.8, Tyrosine about 3.0 to about 5.2,
Valinc about 4.8 to
about 6.1, Arginine about 7.0 to about 8.0, Histidine about 2.5 to about 4,
Alanine about 5.0 to
about 6.3, Aspartic acid about 8.6 to about 10.4, Glutamic acid about 12.5 to
about 14.6, Glycine
about 4.6 to about 9.8, Proline about 4.6 to about 6.8, Serine about 4.4 to
about 5.3 , and/or
Hydroxyproline about 0.0 to 4Ø
[0219] Embodiment 28. The slaughter-free meat product of embodiment
19, wherein the
slaughter-free meat product comprises one or more of the following fatty acids
classes in the
amounts indicated, expressed as % of that class over total fatty acids:
a. saturated fatty acids content between about 29% to about 42%;
b. monounsaturated fatty acids content between about 19% to about 54%; and
c. polyunsaturated fatty acids content between about 5% to about 36%.
[0220] Embodiment 29. The slaughter-free meat product of embodiment
19, wherein the
slaughter-free meat product comprises a ratio of about 2:1 to 18:1 omega 6:3
fatty acids classes.
[0221] Embodiment 30. The slaughter-free meat product of embodiment
19, wherein the
slaughter-free meat product comprises slaughter-free chicken, duck, or bovine
meat, and the
conventional meat comprises chicken, duck or bovine meat obtained by
slaughter.
102221 Embodiment 31. A method of producing a slaughter-free meat
product exhibiting an
increase in shelf life compared to unprocessed conventional meat obtained by
slaughter, the
method comprising:
a providing cells from a non-human organism;
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
b. culturing the cells in media under suspension culture conditions or
adherent
culture conditions, wherein the media is substantially free of serum and other
components derived from an animal; and
c. isolating the cells and producing the slaughter-free meat product.
102231 Embodiment 32. The method of embodiment 31, wherein the cells comprise
myoblasts, fibroblasts, adipocytes, endothelial cells, cells of a mesoderm
lineage, and
combinations thereof.
102241 Embodiment 33. The method of embodiment 31, wherein the
cells comprise at least
fibroblasts and myoblasts, and the fibroblasts and myoblasts are provided at a
ratio of about
95F:5M to about 5F:95M.
102251 Embodiment 34. The method of embodiment 31, wherein the
method comprises
adjusting the fatty acid content of the media, wherein the resulting slaughter-
free meat product
has a ratio of about 2:1 to about 18:1 of the omega 6:3 fatty acids classes.
102261 The inventions disclosed herein are further illustrated by the
following additional
examples that should not be construed as limiting. Those of skill in the art
should, in light of the
present disclosure, appreciate that many changes can be made to the specific
embodiments
which are disclosed and still obtain a like or similar result without
departing from the spirit and
scope of the invention.
102271 All patent and non-patent documents referenced throughout this
disclosure are
incorporated by reference herein in their entirety.
EXAMPLES
Example 1: Generation of Cell Culture-Based Meat Products
102281 By way of example, meat from Gallus (chicken), Anas platyrhynchos
(duck), and Bos
taunts (bovine, beef) was generated in culture as described below, and in
Table 1.
41
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0229] Culture conditions: The cell sheets (tissues) and cells analyzed were
generated by a
monoculture or co-culture of fibroblasts (F) and myoblasts (M). All
populations are polyclonal
unless specified as monoclonal (e.g. Method 14).
[0230] Adherent culture format: Cells were thawed into vessels and grown in
adherent culture
to near confluence (70-90%). The cells were expanded 6 to 10-fold in adherent
culture until
reaching numbers appropriate to seed for tissue formation. Tissues were
generated by seeding
cells adherently at a target density (target range: 10,000-20,000 cells per
cm2) and, if the tissue
contained two or more cell types, at a specific ratio. The cells were cultured
for an amount of
time (10-20 days) in a media containing a specific amount of animal serum. The
meat tissue was
physically harvested at the end of culture and washed in a buffer to remove
media components.
[0231] Suspension culture format: Cells were thawed into media containing
specific amounts
of animal serum and grown in suspension culture. Fresh media was added to
maintain a density
of 50,000 to 1,000,000 cells per mL to expand cell population.
[0232] Table 1 provides exemplary culture methods for the various meat
products produced
iherein. All populations are polyclonal unless specified as monoclonal (e.g.
Methods 14, 15,
monoclonal myoblast population).
[0233] Key for the table ¨ Fibroblast: F; Myoblast: M; Bovine serum: BS;
Chicken serum: CS;
Fetal bovine serum: FBS; Horse serum: HS; (High): media contained 8-10% of
particular serum;
(Low): media contained 1-2.5% of particular serum. DMEM-F12 with 10% FBS was
used
unless otherwise described in the seed train or tissue formation section
Table 1: Cell culture methods used to generate cell-based meat
Culture
Condition
Method
Sample ID Culture format Base
media
Cell Type(s)
(ratio)
A. platyrhynchos (duck) -cu DM EM-F12
with FBS
Colture
1 fibroblast/myoblast F/M (50/50) Adherent
(High), BS (High), CS
tissue 1
(Low), HS (Low)
DM EM-F12 with FBS
A. Platyrhynchos (duck) Monoculture
2 Adherent (High),
BS (High), CS
fibroblast tissue 1
(Low), HS (Low)
42
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
DM EM-F12 with FBS
Bos (Cow) fibroblast Monoculture
3 Adherent (High),
BS (High), CS
tissue 1 F
(Low), HS (Low)
Gallus (chicken) Monoculture DM EM-F12
with FBS
4 Adherent
fibroblast tissue 1 F
(High), CS (Low)
Gallus (chicken) Monoculture DMEM-F12
with CS
Adherent
fibroblast tissue 2 F
(High), BS (Low)
Gallus (chicken) Monoculture DMEM-F12
with CS
6 Adherent
fibroblast tissue 3 F
(High), BS (High)
Gallus (chicken) Monoculture DMEM-F12
with BS
7 Adherent
fibroblast tissue 4 F
(High), CS (Low)
Gallus (chicken) Monoculture DMEM-F12
with 10%
8 Adherent
fibroblast cells 1 F FBS
Gallus (chicken)
Co-culture DM EM-F12
with FBS
9 fibroblast/myoblast Adherent
F/M (30/70)
(High), CS (Low)
tissue 1
Gallus (chicken) Monoculture DMEM-F12
with BS
Adherent
fibroblasttissue 5 F
(High), CS (Low)
Gallus (chicken) Monoculture DMEM-F12
with BS
11 Suspension
myoblast cells 1 M
(High), CS (Low)
Gallus (chicken)
Co-culture DMEM-F12
with BS
12 fibroblast/myoblast Adherent
F/M (30/70)
(High), CS (Low
tissue 2
Gallus (chicken)
Co-culture DMEM-F12
with BS
13 fibroblast/myoblast Adherent
F/M (50/50)
(High), CS (Low)
tissue 3
Gallus (chicken) Co-culture
DMEM-F12 with BS
14 fibroblast/myoblast F/Monoclonal
Adherent
(High), CS (Low
tissue 4 M (50/50)
Gallus (chicken) Co-culture
Chemically-defined
fibroblast/myoblast F/Monoclonal Adherent
media with BS (low)
tissue 5 M (70/30)
Chemically defined
Gallus (chicken) Monoculture
16 Suspension media
formula. No
myoblast cells 2 M
serum
Gallus (chicken) Monoculture SMEM-F12
with BS
17 Suspension
myoblast cells 3 M
(high), CS (low)
Example 2: Amino Acid Profiles of Cell Culture-Based Meat Products
102341 Meat tissues were generated in culture, as described in Example 1 using
Methods
described and Table 1.
102351 The amino acid profile of the cell-culture based meat products was
assayed as follows.
Total amino acid profiles include the summation of free and bound amino acids,
whereas free
amino acid profiles include amino acids that are not protein bound
43
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0236] Sample preparation: About 100 mg of wet sample was obtained in a 1.7 mL
microtube.
The sample was freeze dried in the tube for 48 hours until completely dried.
102371 Total Hydrolysis: About 4 mg of lyophilized tissue was placed into a
hydrolysis tube
(noting the actual mass of the sample). The samples were soaked in 5001A, of
formic acid
overnight, and then dried. Liquid phase hydrolysis on samples was performed
(2001.tL 6N
hydrochloric acid/1% phenol @ 110 C for 24hrs), and then samples were dried.
Samples were
then vortexed, spun down and 50[EL was used for analysis. Thus, total
hydrolysis was
accomplished via hydrochloric acid digestion.
[0238] Free Hydrolysis: About 18 mg of lyophilized tissue was placed into a
hydrolysis tube
(noting the actual mass of the sample). The samples were dissolved in lmL 0.1N
hydrochloric
acid, and then sonicated for 15 min with glass beads (BioEruptor). The entire
sample was then
precipitated with 250uL 10% 5-sulfosalicylic acid, and then allowed to stand
for 15 minutes at
room temperature, following which the samples were frozen at -20C overnight.
The samples
were then centrifuged, and the supernatant was taken and AE-cys (or NorLeu)
dilutions were
prepared to final dilution as indicated. Samples were then vortexed, spun down
and 50 L was
used for analysis. This method was employed for all amino acids except MET,
CYS, and TRP,
which require different hydrolysis conditions as hydrochloric acid destroys
them. For MET and
CYS, a separate hydrolysis was employed using performic acid, and TRP requires
a basic
hydrolysis employing sodium hydroxide was used.
[0239] Analysis: Acid-stable amino acids were assayed via Hitachi L-8900 and L-
8800a
analyzers that utilize a lithium citrate buffer system, which is optimized for
physiological
samples. The analyzers used ion-exchange chromatography to separate amino
acids followed by
a "post-column" ninhydrin reaction detection system. Each amino acid was
identified by peak
retention time (RT) and quantified by the peak area; representative RT values
are listed below in
Table 2 for the amino acids of interest.
Table 2: Peak Retention Times for Amino Acids
Amino acid Abbreviation RT (min)
Aspartic acid ASX 8.5
Threonine THR 12.2
Serine SER 13.4
44
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/US2021/016681
Glutamic acid GLX 16.8
Proline PRO 25.7
Glycine GLY 26.9
Alanine ALA 28.4
Valine VAL 33.9
Isoleucine ILE 53.4
Leucine LEU 57.5
Tyrosine TYR 61.4
Phenylalanine PHE 66.4
Lysine LYS 100.5
Histidine HIS 103.2
Arginine ARG 116
102401 Tables 3-5 below summarize the amino acid (AA) profile data for several
of the
samples listed in Table 1 - in some cases measurements were taken in duplicate
or triplicate.
Values are expressed as g/100g of total amino acids. The column marked "Total"
represents the
total grams of amino acids, excluding TRP, CYS, MET, HYP, HYL, and was used to
normalize
the presented amino acid values. TRP, CYS, MET, HYP, and HYL were excluded as
the
measurements were inconsistent across all samples. NT = Not tested.
Table 3: Amino Acid Profiles
Tryptophan Threonine Isoleucine Leucine
Lysine Methionine
Method
Total* TRP THR ILE LEU LYS
MET
ID
1 96.13 NT 5.76 4.57 7.55 6.6
NT
1 96.50 NT 5.83 4.7 7.63 6.69
NT
1 96.25 NT 5.85 4.69 7.66 6.66
NT
3 96.00 NT 5.82 4.71 7.67 6.73
NT
3 96.19 NT 5.93 4.71 7.64 6.56
NT
4 96.70 NT 5.72 4.74 7.94 7.06
NT
4 97.44 NT 5.42 4.94 8.6 8.37
NT
91.50 2.22 5.3 4.35 7.55 6.94 2.95
6 92.12 2.12 5.22 4.39 7.71 7.17
2.95
7 92.26 1.98 5.32 4.37 7.63 7.21
2.96
7 99.98 NT 6.47 4.95 8 7.02
NT
8 95.15 NT 5.1 4.65 8.93 8.6
2.55
9 95.00 NT 5.09 4.74 8.61 8.8
2.7
9 93.47 NT 4.89 4.25 7.68 7.23
2.28
9 93.27 NT 4.97 4.25 7.56 7.06
2.24
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
10 97.24 NT 5.72 4.78 8.43 7.96 0.14
11 94.30 NT 5.65 4.68 8.05 7.57 2.46
11 93.72 NT 5.41 4.32 7.54 6.87 2.2
Table 4: Amino Acid Profiles
Cysteine Phenylalanine Tyrosine Valine Arginine Histidine Alanine
Method
CYS PHE TYR VAL ARG HIS
ALA
ID
1 NT 4.14 4.09 5.78 7.63 3.46 5.3
1 NT 4.18 4.13 5.86 7.52 3.47 5.25
1 NT 4.15 4.08 5.83 7.54 3.37 5.27
3 NT 4.2 4.04 5.81 7.47 3.36 5.29
3 NT 4.17 4.17 5.86 7.57 3.37 5.2
4 NT 4.29 4.05 5.92 7.59 3.56 5.42
4 NT 4.62 3.96 5.86 7.45 3.94 5.52
5 1.72 4.27 3.93 5.33 7.07 3.51 5.02
6 1.6 4.38 3.92 5.35 7.17 3.52 5.08
7 1.54 4.09 3.77 5.5 7.21 3.45 5.12
7 NT 4.23 5.15 5.88 7.95 2.51 5.54
8 1.8 4.76 3.94 6.1 7.6 3.85 5.63
9 1.7 4.65 3.89 5.95 7.37 3.9 5.58
9 1.72 4.39 3.69 5.32 7.13 3.38 5.95
9 1.69 4.42 3.72 5.37 7.12 3.46 5.91
10 1.7 4.52 3.98 6.07 7.56 3.79 5.6
11 1.82 4.34 4.01 5.89 7.33 3.83 5.37
11 1.67 4.13 3.73 5.53 7.41 3.41 5.71
Table 5: Amino Acid Profiles
Aspartic Glutamic Hydroxy-
Alanine Glycine Proline
Serine
acid acid
proline
Method
ALA ASX GLX GLY PRO SER
HYP
ID
1 5.3 9.91 13.52 7.03 5.99 4.8 1.58
1 5.25 9.98 13.57 6.8 6.04 4.85 1.32
1 5.27 9.87 13.51 6.89 6.07 4.81 1.26
3 5.29 9.82 13.47 6.73 6.09 4.79 1.37
3 5.2 9.91 13.49 6.82 5.95 4.84 1.28
4 5.42 9.71 13.73 6.39 5.75 4.83 1.07
4 5.52 9.97 13.92 5 4.91 4.96 NT
5 5.02 9.19 13.55 5.63 5.16 4.7 1.02
6 5.08 9.31 13.64 5.39 5.16 4.71 0.64
7 5.12 9.41 13.8 5.56 5.03 4.79 0.86
7 5.54 10.39 14.64 6.18 5.78 5.29 0
8 5.63 9.48 12.63 4.56 4.59 4.73 0
9 5.58 9.57 12.47 4.94 4.78 4.66 0
46
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
9 5.95 9.15 13.29 7.06 5.49 4.57
1.51
9 5.91 9.05 13.15 7.12 5.6 4.51
1.71
5.6 9.89 13.24 5.33 5.3 5.07 0.36
11 5.37 9.57 12.67 5.44 5.05 4.85
0.8
11 5.71 9.38 12.65 6.95 6.01 4.67
1.64
102411 Tables 6-8 show the composite average amino acid values (represented as
% total
amino acids) for chicken, duck, and bovine separately. The % of total amino
acids are
represented as the g/100g value for each individual amino acid normalized by
the summation
across all measured analytes, excluding tryptophan (TRP), cysteine (CYS),
methionine (MET),
hydroxyproline (HYP), and hydroxylysine (HYL) as those parameters were not
measured
consistently across all samples. Thus, the % of total values allow for direct
comparison of all
samples, regardless of whether TRP, CYS, MET, HYP, and HYL were specifically
measured.
102421 Table 9 shows the data of Tables 6-8 combined - all cell-based meat
samples
(composite of methods in Table 1) amino acid composition data
Table 6: Chicken cell-based meat amino acid composition
Table 6: Chicken - % Total AA
THR WE LEU LYS MET
CYS
AVG 5.53 4.60 7.91 7.28 2.34
1.70
STDEV 0.40 0.22 0.44 0.70 0.83
0.08
N 18 18 18 18 10
10
95% CI 0.19 0.10 0.20 0.32 0.51
0.05
MIN 4.89 4.25 7.54 6.56 0.14
1.54
MAX 6.47 4.95 8.93 8.80 2.96
1.82
PH E TYR VAL ARG HIS
ALA
AVG 4.33 4.01 5.73 7.43 3.51
5.43
STDEV 0.20 0.32 0.26 0.23 0.32
0.27
N 18 18 18 18 18
18
95% CI 0.09 0.15 0.12 0.10 0.15
0.12
MIN 4.09 3.69 5.32 7.07 2.51
5.02
MAX 4.76 5.15 6.10 7.95 3.94
5.95
ASX GLX GLY PRO SER
HYP
AVG 9.64 13.39 6.10 5.49 4.80
0.97
47
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/US2021/016681
STDEV 0.35 0.54 0.86 0.50 0.18
0.59
N 18 18 18 18 18
17
95% CI 0.16 0.25 0.40 0.23 0.08
0.28
MIN 9.05 12.47 4.56 4.59 4.51
0.00
MAX 10.39 14.64 7.12 6.09 5.29
1.71
Table 7: Duck cell-based meat amino acid composition
Table 7: Duck - % Total AA
THR ILE LEU LYS MET
CYS
AVG 5.10 3.99 6.42 6.35 2.09 1.61
STDEV 0.09 0.15 0.43 0.33 0.06 0.09
N 3 3 3 3
3 3
95% CI 0.10 0.17 0.48 0.38 0.07 0.10
MIN 5.01 3.82 6.10 6.09 2.02 1.51
MAX 5.18 4.12 6.90 6.73 2.13 1.68
PH E TYR VAL ARG HIS
ALA
AVG 4.08 3.26 4.99 7.36 2.76 6.15
STDEV 0.19 0.18 0.23 0.25 0.15 0.17
N 3 3 3 3
3 3
95% CI 0.21 0.21 0.26 0.28 0.17 0.19
MIN 3.87 3.05 4.78 7.12 2.65 5.96
MAX 4.23 3.40 5.24 7.62 2.93 6.28
ASX GLX GLY PRO SER
HYP
AVG 8.78 13.16 8.76 6.56 4.56 3.48
STDEV 0.24 0.31 0.73 0.34 0.08 0.54
N 3 3 3 3
3 3
95% CI 0.28 0.35 0.83 0.39 0.09 0.61
MIN 8.61 12.80 7.95 6.17 4.49 3.01
MAX 9.06 13.36 9.38 6.79 4.65 4.07
48
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
Table 8: Beef cell-based meat amino acid composition
Table 8: Beef - % Total AA
THR ILE LEU LYS MET
CYS
AVG 4.60 3.96 7.03 5.96 2.01 1.53
STDEV 0.02 0.09 0.24 0.39 0.01 0.06
N 2 2 2 2
2 2
95% CI 0.03 0.13 0.33 0.54 0.01 0.09
MIN 4.58 3.89 6.86 5.68 2.00 1.48
MAX 4.61 4.02 7.20 6.23 2.01 1.57
PHE TYR VAL ARG HIS
ALA
AVG 3.82 3.37 4.89 7.25 2.74 6.07
STDEV 0.14 0.02 0.13 0.28 0.07 0.10
N 2 2 2 2
2 2
95% CI 0.20 0.03 0.18 0.39 0.10 0.14
MIN 3.72 3.35 4.80 7.05 2.69 6.00
MAX 3.92 3.38 4.98 7.45 2.79 6.14
ASX GLX GLY PRO SER
HYP
AVG 8.77 13.03 9.27 6.26 4.50 3.21
STDEV 0.22 0.46 0.79 0.40 0.06 0.42
N 2 2 2 2
2 2
95% CI 0.30 0.64 1.10 0.56 0.08 0.58
MIN 8.61 12.70 8.71 5.97 4.46 2.91
MAX 8.92 13.35 9.83 6.54 4.54 3.50
Table 9: All cell-based meat samples (composite of methods in Table 1) amino
acid
composition (g amino acid per 100g sample)
AVG STDEV N MIN MAX
Tryptophan TRP 1.67 0.60 5 1 2.22
Threonine THR 5.39 0.46 23 4.58 6.47
Isoleucine ILE 4.46 0.33 23 3.82 4.95
Leucine LEU 7.64 0.68 23 6.1 8.93
Lysine LYS 7.05 0.78 23 5.68 8.80
Methionine MET 2.25 0.68 15 0.14 2.96
Cysteine CYS 1.66 0.10 15 1.48 1.82
Phenylalanine PHE 4.25 0.25 23 3.72 4.76
Tyrosine TYR 3.86 0.41 23 3.05 5.15
49
CA 03166478 2022- 7- 28
WO 2021/158831 PCT/ITS2021/016681
Valine VAL 5.56 0.41 23
4.78 6.10
Arginine ARG 7.40 0.23 23
7.05 7.95
Histidine HIS 3.34 0.43 23
2.51 3.94
Alanine ALA 5.58 0.38 23
5.02 6.28
Aspartic acid ASX 9.45 0.49 23 8.61 10.39
Glutamic acid GLX 13.32 0.50 23 12.47 14.64
Glycine GLY 6.72 1.46 23
4.56 9.83
Proline PRO 5.69 0.61 23
4.59 6.79
Serine SER 4.74 0.20 23
4.46 5.29
102431 Collectively, the amino acid profiles of the meat are comparable to
conventional meat
(FIG. 1), however there are critical differences. For example, hydroxyproline
concentrations in
the cell-based meat is higher than in conventional meat (USDA database Food
Central Database
[https://fdc.nal.usda.gov/]). Comparison to cell-based meat is shown in Table
10 (units shown in
grams of hydroxyproline per 100g of total protein). Concentrations are shown
for conventional
and cell-based meat beef, duck and chicken. The cell-based meat was generated
using Methods
2, 3 and 7 from Table 1. Hydroxyproline concentrations were elevated in the
cell-based meat in
three species used as comparators.
Table 10: Hydroxyproline Concentrations (g of Hydroxyproline/100g total
protein)
USDA/Literature Experimental Analysis
Conventional Cell-based meat
Beef Duck Chicken Chicken Beef Duck Chicken
Mean 0.839 0.852 0.371 0.223 3.205
3.477 1.424
SD 0.489 0.167 0.160 0.146 0.417
1.521 0.288
8 40 8 4 2 3 10
102441 FIG. 2 and Table 11 show the hydroxyproline concentrations for chicken
cell-based
meat for cell-based meat generated from Methods 7, 13, and 14, with further
treatments. FIG. 2
shows the hydroxyproline concentrations in grams per 100g of wet mass of cell-
based meat.
Hydroxyproline mean concentration across the cell-based meat ranges between
0.15 and 0.17
g/100g wet mass of cell-based meat (FIG. 2). Table 11 shows the hydroxyproline
concentrations
in grams per 100g total protein. The control conditions (Ctrl) were generated
using Methods 7,
13, 14 from Table 1 for FB, FB/MB(poly), and FB/MB(mono), respectively.
Hydroxyproline
levels are elevated in cell-based meat generated from fibroblast cultures
alone, as compared to
the conventional counterpart. When myoblasts (MB) are added to the culture
system either as a
CA 03166478 2022- 7- 28
WO 2021/158831 PCT/ITS2021/016681
polyclonal cell mixture (mixed population of myoblasts) (e.g. Method 13 from
Table 1) or
monoclonal myoblast cell mixture (Method 14 from Table 1) (single-cell
isolated from a mixed
population and expanded), the hydroxyproline concentration is reduced to close
to that of
conventional meat. Hydroxyproline concentrations further decrease using
modified culture
conditions (Treatment 1 Ursoloic acid, 20mM; or Treatment 2 Leucine, 20mM).
These
treatments were applied to Methods 13 and 14 from Table 1.
Table 11: Hydroxyproline Concentrations (g/100g total protein)
FB FB/MB(poly) FB/MB(mono)
Cnt Cnt Tx-1 Tx-2 Cnt Tx-1 Tx-2
Mean 1.424 0.898 0.649 0.308 0.814 0.510
0.162
SD 0.288 0.449 0.044 0.112 0.438 0.014
0.090
10 8 3 3 6 3 3
Example 3: Assay for microbial contamination of the cell-based meat product
102451 The cell-based meat tissues were assessed for microbial contamination,
for example for
coliform bacteria, yeast, mold, salmonella and listeria.
102461 These studies were performed by a third-party lab, Anresco
Laboratories. Standard
plate counts (SPC), E. coli/coliforms, and yeast/mold were determined by
standard FDA
Biological Analytical Method protocols. AOAC methods were used for Salmonella
(AOAC
2011.03), Listeria (AOAC 2004.06), and Staphylococcus (AOAC 2003.07).
102471 Briefly, SPC were accomplished by preparing decimal dilutions of a cell-
based meat
homogenate and pipetting 1 mL aliquots per dilution into separate, duplicate,
approximately
marked petri dishes, to which 12-15 mL of plate count agar was added. Sample
dilutions and
agar medium were mixed thoroughly and the agar allowed to solidify. The
solidified petri dishes
were inverted and incubated at 35 C for 48 2 hours, after which time plate
counts were read.
(https://www.fda.gov/food/laboratory-methods-food/bam-aerobic-plate-
count#conventional)
102481 E. coli/coliform measurements were determined by preparing 50 g of cell-
based meat
homogenized sample to 450 mL of Butterfield's phosphate buffer and mixed;
decimal dilutions
were prepared and 1 mL volume aliquoted into each of 3 lauryl tryptose (LST)
broth for a 3 tube
51
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
most probable number (MPN). LST tubes were incubated at 35 C and examined
after 24 2
hours to observe for gas displacement or effervescence; any gas-negative tubes
were incubated
for an additional 24 hours to confirm a negative. From each gassing LST tube,
to confirm
coliforms, a loopful of sample was transferred to a tube of brilliant green
lactose bile (BGLB)
broth and incubated at 35 C for 48 3 hours. MPN was calculated based on the
proportion of
confirmed gassing LST tubes for 3 consecutive dilutions. From each gassing LST
tube, to
confirm for E. coli, a loopful of sample was transferred to a tube of EC broth
and incubated at
44.5 C for 24 2 hours. Any negative results were re-incubated and examined
again at 48 hours.
Further, each gassing EC tube was sampled by removing a loopful of broth and
streak-isolating
on a L-EMB agar plate at 35 C for 18-24 hours; any E. coli colonies were
transferred to PCA
slants and further incubated at 35 C for 18-24 hours. MPN was calculated
based on the
proportion of EC tubes in 3 successive dilutions that contain E. coli.
(https://www.fda.gov/food/laboratory-methods-food/bam-4-enumeration-
escherichia-coli-and-
coliform-bacteria#conventional)
102491 Yeast/mold counts were obtained by analyzing 25-50 g cell-based meat
sample
digested in 0.1% peptone water to achieve a 10' dilution and homogenized in a
stomacher for 2
min or blending for 30-60 s. Spread-plate or pour-plate plating was performed,
and incubated in
the dark at 25 C. Plates were counted after 5 days; negative plates were
incubated for an
additional 48 hours. (https://www.fda.gov/food/laboratory-methods-food/bam-
yeasts-molds-and-
mycotoxins)
102501 Table 12 provides a comparison of contaminants in the cell-based meat
versus
conventional grocery store meat. Conventional duck meat, and especially
conventional beef had
significantly higher amounts of microbial contamination.
102511 The conventional duck meat was purchased at a local grocery store
(Berkeley, CA).
The meat was separated from skin and fat and was finely chopped with a
sterilized knife and
cutting board. Meat was packed into 50mL falcon tubes & sealed. The closed
tube was sprayed
with 70% ethanol and frozen at -80 C. Samples were frozen at -80 C for, then
held at 4 C prior
to testing.
102521 The cell-based duck meat was a combination of tissues using Methods 1
and 2
described in Table 1. Tissues were removed from frozen storage, mixed, and
chopped using a
52
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/US2021/016681
sterilized cutting board and knife. Meat was packed into 50mL falcon tubes &
sealed. The closed
tubes were sprayed with 70% ethanol and frozen at -80 C. Samples were frozen
at -80 C for,
then held at 4 C prior to testing.
102531 Extra lean (97% lean) ground beef was purchased from a local grocery
store (Berkeley,
CA). Beef was packed into 50mL falcon tubes directly. The tubes were sealed
and sprayed with
70% ethanol. Tubes were frozen at -80 C. Samples were frozen at -80 C for,
then held at 4 C
prior to testing.
102541 The cell-based beef meat was a combination of tissues from using Method
3 in Table
1. Tissues were removed from frozen storage, mixed, and chopped using a
sterilized cutting
board and knife. Meat was packed into 50 mL falcon tubes & sealed. The closed
tubes were
sprayed with 70% ethanol and frozen at -80 C. Samples were frozen at -80 C
for, then held at
4 C prior to testing.
Table 12: Contaminant Comparison in Conventional and Cell-Based Meat
Standard
Plate Confirmed
Listeria CP
Count Coliforms E. coil Yeast Mold
Salmonella (per Staph
Sample (cfu/g) (cfu/g) (cfu/g) (cfu/g)
(cfu/g) (per 25g) 25g) (cfu/g)
Conventional
Duck 100 <10 <10 <10 <10 Negative
Negative <10
Cell-based
Duck <100 <10 <10 <10 <10 Negative
Negative <10
Positive -
L.
monocto
Conventional genes
Beef 6000000 1300 <10 2300 <10 Negative
detected <10
Cell-based
Beef <100 <10 <10 <10 <10 Negative
Negative <10
[0255] In further experiments, CompactDry plates were used to assess total
aerobic counts and
E. coli/coliform counts for cell-based meat samples and conventional meat
samples; all
evaluated samples were uncooked and raw.
[0256] A CompactDry protocol was deployed, which involved collecting a 1 g
sample size
with ethanol-sterilized supplies and transferring to a sterile tube; sterile
Butterfield's phosphate
53
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
buffer was added to maintain a ratio of 25 g sample to 225 mL of buffer. Tubes
were vortexed,
sat at room temperature for 10 min to transfer any bacteria to solution, then
centrifuged at 300xg
for 5 min to pull solids to the bottom; the supernatant was collected into a
sterile tube and
decimal dilutions prepared. 1 mL aliquots per dilution were plated onto the
Total Count
CompactDryTM TC and CompactDryTM EC plates for total aerobic counts (TC) and
E.
coli/coliforms (EC), respectively. Plates were incubated according to
manufacturer
specifications, and colonies counted as cfu/mL and converted to cfu/g based on
the 25 g per 225
mL sample digestion ratio.
[0257] In general, the cell-based meat recoveries were low, usually less than
the limit of
detection (-10 cfu/g) for the assay and were thus not detected (ND), Table 13.
More
specifically, different batches of cell-based duck meat yielded from ND (<9
cfu/g) to 54 cfu/g
for TC, and ND (<9 cfu/g) for total EC. For TC, cell-based beef samples
yielded ND (<9 cfu/g)
and cell-based chicken samples yielded from ND (<9 cfu/g) to 18 cfu/g. When
cell-based duck
and beef samples (with low total aerobic counts) were intentionally
contaminated with
conventional raw chicken (in Table 13 represented as cell-based sample
"contaminated with
chicken"), the IC spiked to >900 cfu/g, indicating utility of the assay with
the sample matrix
and confirming that no signal suppression was occurring due to matrix effects.
Conventional raw
beef and chicken samples both exhibited >900 cfu/g TC, and 315 cfu/g and >900
cfu/g EC,
respectively. Compared to conventional raw meat samples, the cell-based meat
samples all
exhibited not detectable-to-low (<100 cfu/g) TC and EC counts indicating
significantly less
bioburden. FIG. 3 shows representative CompactDry plates indicating bacteria
colonies,
specifically showing EC and TC results for cell-based duck, conventional beef,
and conventional
chicken. This is quantified in Table 13.
Table 13: Bioburden of Conventional and Cell-Based Meat Samples
Sample Total aerobic counts, cfu/g E. coil /
coliforms, cfu/g
Conventional beef >1000 350
Conventional chicken >1000 >1000
Cell-Based duck 60 <10
Cell-Based duck, contaminated
>1000 Not
measured
with chicken
Cell-Based beef <10 Not
measured
Cell-Based beef, contaminated
>1000 Not
measured
with chicken
Cell-Based chicken <10 to 20 Not
measured
54
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
Cell-Based chicken,
>1000 Not
measured
contaminated with chicken
Example 4: Assay for fatty acid content of cell-based meat product
102581 The majority of data in this section involved assaying fatty acid
profiles via a GC-
FAME method (Method FA1), whereby samples underwent direct hydrolysis (using
hydrochloric acid under heat with the sample dissolved in chloroform and
ethanol) to break
down the sample matrix, liberate the triglycerides (TG), and convert TG to
fatty acids. Fatty
acids were recovered via ether extraction and collected as the non-polar
layer. Fatty acids were
derivatized to their fatty acid methyl ester (FAME) counterparts via
methanolic sulfuric acid,
and the final FAME compounds recovered via ether extraction. The non-polar
FAME-containing
layer was injected for analysis via GC-FID. Individual FAME compounds were
identified based
on retention time, and quantified by area under the peak as compared to a
standard curve.
Recovery was evaluated based on a triglyceride internal standard. The data
presented in FIG. 8
were assayed via a technically different (although similar) method (Method
FA2), according to
AOCS CE 1F-96, whereby gas chromatography was employed to separate fatty acid
derivatives
(FAME) by their chain length, degree of saturation/unsaturation, and position
of unsaturation.
102591 In both methods, the raw values recovered from the analysis are
reported as each fatty
acid's percentage of the total fatty acid. In Method FA1, the values reported
from the assay were
ug fatty acid per g of wet mass. The % of total fatty acids are represented as
the g/100g value for
each individual fatty acid normalized by the summation across all measured
fatty acid analytes.
Method FA2 reported fatty acid as percent of total fat.
102601 Table 14 shows the total fatty acid analysis for cell-based meat. Total
FA was
determined in the cell-based meat prepared, using the methods of Table 1, as
indicated. The data
in table is measured as % of wet mass. When the cell-based meat is adjusted to
mimic the
moisture content of conventional meat (on cell-based meat which had undergone
a forced air
dehydration process to adjust moisture content to between 65% to 85%), as is
typically measured
and recorded by the USDA, the % FA went up to a maximum of about 5%.
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/US2021/016681
Table 14: Total Fatty Acid Analysis
Method name Percent Total FA (%) of wet mass
(Table 1)
7 0.97
14 0.88
14 0.77
17 1.11
17 2.17
13 1.12
13 1.15
14 1.45
14 1.25
14 0.96
17 0.57
17 1.15
17 1.01
17 0.86
16 0.96
14 1.01
14 1.2
16 0.68
16 0.64
16 0.66
7 1.12
7 1.07
7 1.4
14 1.38
Avg 1.06
Min 0.57
Max 2.17
Store-bought chicken 3.96
Store-bought chicken 3.98
Store-bought chicken 4.13
Avg 4.02
56
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0261] FIG. 4 shows total fatty acid composition for saturated,
monounsaturated and
polyunsaturated fatty acids across the samples (N=24) including representative
samples using
each method in Table 1. The data are presented as percent of FA class/total
FA. All samples
(Table 1, 1-15) were in serum-containing media except suspension monoculture M
(Method 16,
Table 1) which was in chemically-defined (CD) cell culture media. The figure
shows
distribution of fatty acids derived from all species cell-based meat
prototypes across four major
fatty acid class designations (saturated fatty acids, monounsaturated fatty
acids, polyunsaturated
fatty acids and highly unsaturated fatty acids (HUFA)). The ends of the box
are the upper and
lower quartiles, so the box spans the interquartile range, the median is
marked by a vertical line
inside the box, the whiskers or lines that bracket the box are the two lines
outside the box that
extend to the highest and lowest observations.
102621 FIG. 5 shows the PCA of USDA species FA. It shows that FA are different
across
species but similar in like species (e.g. poultry). Principal component
analysis (PCA) of fatty
acid data were gathered from the USDA database meat products, FIG. 5. The
graph on the left
shows the principal components of the analysis. Triangles represent poultry
products, circles
represent fish products, squares are beef, and diamonds are pork. This
analysis accounts for
66.3% of the variation found in the fatty acid data. The graph on the right
shows the directional
and magnitude effects that each fatty acid has on the analysis of the
components. From this
analysis it is clear that the different types of meat (chicken, pork, beef,
and fish) cluster around
each other in separate and distinct groups based on the fatty acid profile of
each meat. In
conventional meat, diet significantly affects the fatty acids profile.
Likewise, fatty acid profiles
are distinct for different species.
102631 FIG. 6 shows the ratio of Omega 6 to 3 fatty acids in cell-based
chicken meat N=23.
The outlier data points were all derived from chemically-defined (CD) cell
culture media per
Method 16 from Table 1. Conventional chicken (locally procured from grocery
store) meat ratio
of Omega 6:3 fatty acid were > 18:1 in a sample size of n=3.
102641 The methods provided herein can alter specific lipid profiles to
achieve desired flavor
characteristics or fatty acid profiles such as Omega 3/6 ratio through several
mechanisms:
a. The presence of serum in the media can affect fatty acid profiles. FIG. 7
shows the fatty
acid percentages in serum free media vs. media containing serum.
57
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
b. Serum of different sources imparts different fatty acid profiles in
cultured tissues. (FIG. 8)
c. Isolated clones from a polyclonal population affects FA profile as well.
Myoblast Clone 7
vs 8 (FIG. 9).
d. Fatty acids profiles are affected by media composition and by the addition
of media
components including compounds added to change FA composition like an agonist,
or
riboflavin (for example). Adjustments to media can impact fat profiles. (FIG.
10, FIG.
11).
[0265] FIG 10. Tissues were formed using a co-culture method (described in the
Method 15 in
Table 1) using culture media with enhanced levels of various compounds to
modulate specific
biochemical pathways In FIG. 10, Agonist TO901317 was titrated into cell
culture media. The
global effect on fatty acid concentrations are shown. Agonist TO901317 targets
the Liver X
Receptor 13 (LXRf3). Inhibition of LXR13, up-regulates stearoyl-CoA Desaturase
(SCD)
synthesis, resulting in the further conversion of saturated fatty acids to
unsaturated fatty acid
(e.g. steric acid-18:0 to oleic acid-18:1 and linoleic acid-18:2.
[0266] FIG. 11 Tissue were formed using a co-culture method (described in
Method 15 in
Table 1) using culture media with enhanced levels of various compounds to
modulate specific
biochemical pathways. In the figure, Riboflavin, a vitamin and common co-
factor, was titrated
into cell culture media. The global effects on fatty acid concentrations are
shown.
[0267] FIG. 12 shows the titration of FA into media to change the profile of
specific FAs.
Based on their prevalence in conventional chicken, four specific fatty acids,
palmitoleic (C16:1),
palmitic (C16:0), linoleic (C18:2), and oleic acid (C18:1), were chosen for
targeted increase
through supplementation into the cell culture media. Tissue were formed using
a co-culture
method (described Method 14 in Table I) using culture media with enhanced
levels of each fatty
acid as depicted in FIG. 12 (10 mg/L total FA: 2.8 mg/L C16:0, 0.5 mg/L C16:1,
4.2 mg/L
C18:1, and 2.5 mg/L C18:2; 20 mg/L total FA: 5.6 mg/L C16:0, 1.0 mg/L C16:1,
8.4 mg/L
C18:1, and 5.0 mg/L C18:2).
Example 5: Analysis of Macronutrients in Cell-Based Meat Products
[0268] The meat tissues were assessed for macronutrient content including
moisture, protein,
and fat.
58
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0269] Total moisture was assayed by one of two methods, either Moisture-1 or
Moisture-2. In
Moisture 1, AOAC method 950.46 was employed. In short, this method employs a 2
g sample
that is weighed in an aluminum dish and dried for 16-18 hours at 100-102 C in
a mechanical
convection air oven. The dried sample is cooled in a desiccator and reweighed;
the moisture is
reported at the loss in sample weight. In Moisture-2, a >100 mg sample is
added to a pre-
weighed aluminum dish and dried at least overnight at 70 C to constant mass.
Other
temperatures, such as 50 C may also be used. In both Moisture-1 and Moisture-
2, the mass loss
of the sample post-drying is attributed to the percent of total moisture in
the sample.
[0270] Total protein was assayed by one of two methods, either Protein-1 or
Protein-2. In
Protein-1 the AOAC method 977.14 was employed. This method is a Kjeldahl
method where
nitrogen in the sample is reduced to ammonia in acid under heat with a
catalyst. The ammonia is
then distilled with water vapor and titrated with acid. A nitrogen factor of
6.25 is used to convert
the nitrogen content to crude protein In Protein-2, a modification of the
Pierce BCA assay was
used. A 100 mg sample was digested in 1 M sodium hydroxide at a ratio of 0.1
g/mL under
sonication for 3 hours to dissolve the sample matrix. The sample digestate was
then diluted and
assayed via the colorimetric Pierce BCA assay. The final jig/mL values are
converted to g
protein per g of wet mass using the 0.1 g/mL digestion ratio.
[0271] Total fat was assayed by one of two methods, either Fat-1 or Fat-2. In
Fat-1, petroleum
ether was used as a solvent to extract soluble fat from the sample matrix via
AOAC method
991.36. In this method, the sample was weighed in a thimble and inserted into
an extraction unit,
which adds the solvent to perform the extraction via a solvent recovery
system. The extraction
cups were dried and weighed. In a similar fashion, Fat-2 involves a modified
Folch extraction
where a >250 mg sample was weighed in a pre-weighed 16 mL vial and dried
overnight at 70
C; the mass loss post-drying was attributed to moisture. The dried sample
remained in the vial
and underwent an extraction using a Hydranal LipoSolverCM solvent (10 mL). The
vial was
capped with a PTFE-lined cap and shaken at 200 rpm at room temperature for 24
hours. Post-
extraction, the samples were filtered through a pre-weighed PTFE filter (0.2
[im pore size) and
dried at 50 C for 48 hours, venting to a chemical fume hood. In both Fat-1
and Fat-2, the mass
loss of the sample post-extraction was attributed to the percent of total fat
in the sample.
59
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
Example 6: Analysis of Hormones in the Cell Culture-Based Meat Product
[0272] Meat samples were assayed for hormone levels by a 3rd party analytical
laboratory
(Eurofins Central Analytical Laboratories) using a LC-MS/MS method with
internal reference.
Hormone ELISA assay results indicated that the conventional chicken meat
sample yielded
higher hormone concentration compared to the cell-based meat chicken samples
(grown as
adherent or suspension cultures). Additional data will be collected to confirm
the validity of this
assay for the sample matrices of interest. Briefly, the 1713-estradiol assay
was performed using
the R1DASCREEN 17p-Ostradiol kit from R-biopharm. In a glass vial with a PTFE-
lined cap,
1-1.5 g of wet meat sample was measured out and homogenized in 67 mM phosphate
buffered
saline (PBS) at a ratio of 1 mL buffer to 1 g of wet sample mass using a
handheld homogenizer
with a sawtooth accessory. Post-homogenization, 5 mL of methyl tert-butyl
ether (MTBE) were
added and then shaken for 30 min. The sample tube was centrifuged, and the
MTBE supernatant
layer was collected and an additional 5 mL extraction of MTBE was performed on
the sample;
both MTBE layers were combined for subsequent use. The MTBE solvent was
evaporated
overnight at 40 C and then 1 mL of 80% methanol was added to the "dried"
vial, vortexed to
mix, and then 2 mL of 20 mM PBS was added and vortexed. The resultant solution
was passed
through a RIDA C18 column that was pre-rinsed with methanol and conditioned
with 20 mM
PBS. After the sample fraction was passed through the column, the column was
cleaned with a
40% methanol solution (layer discarded), then dried under a nitrogen gas
stream, and the final
sample was collected by passing 80% methanol through the column and collecting
the layer. The
sample was dried using a vacuum concentrator and then resuspended in 50 L of
buffer just
prior to analysis. The kit was used according to manufacturer specifications,
using a calibration
range of 0 to 12.8 ng/L. Absorbance of the plate was finally measured using a
plate reader and
the sample values were determined according to the derived calibration curve.
The assay output
was in units of ng/L which were converted to ng/kg using the initial sample
preparation ratio (1
g sample to 1 mL extraction buffer).
[0273] Mass spectrometry methods did not have suitable limits of detection
(LOD) for the
hormone analytes of interest to yield quantitative recovery for samples the
levels in the cell-
based meat were very low or non-existent. For instance, the hormones
testosterone,
progesterone, and 170-estradiol have LOD values of 1, 1, and 20 ng/kg,
respectively. MS
chromatographs did not show any bands corresponding to these hormones in the
cell-based meat
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
samples. Table 15 shows a summary of LC-MS/MS results. All hormones returned
as not
detected (ND) or less than the limit of detection
102741 ELISA results for 17f3-estradiol indicated that cell-based chicken
samples yielded a
lower concentration compared to conventional chicken and beef samples. 1713-
estradiol levels
were on average 35 ng estradiol/kg wet mass for cell-based meat using the
ELISA kit whereas
conventional chicken, procured from the local grocery, was 90 ng/kg
estradiol/kg wet mass.
Negative controls in subsequent studies were in the 30 ng/kg estradiol/kg wet
mass range
indicating that cell-based meat samples also had levels that were near or
below the limit of
detection for both assays. Table 16 shows 1713-estradiol levels, using an
ELISA-based method to
assay conventional and cell-based chicken meat.
Table 15: Hormonal Levels in Cell-Based Meat Samples
Parameter Concentration (
g/kg)
Testosterone <1
Epitcstostcronc <10
Clostebol NR
Methyltestosterone <10
Testosterone propionate NR
Boldenone <10
17a-Boldenone <10
Di anabol <10
17a-Trenbolone <10
Trenbolone <10
16-Hydroxystanozolole NR
Stanozolol NR
Nandrolone <10
Trenbolone-acetate <10
17a-Ethinylestradiol NR
17a-Estradi ol <20
1713-Estradi ol <20
Dienestrol <10
Diethylstilbestrol <10
61
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
Parameter Concentration
(ug/kg)
Estriol <10
Estrone <5.0
Hexestrol <5
a-Zearalanol <10
13-Zearalanol <10
Progesterone <1.0
Medroxyprogesterone NR
Melengestrole acetate <10
Chlormadinone acetate <10
17a-Hydroxyprogesterone <10
Cortisone <10
Hydrocortisone <10
Fludrocorti sone acetate <10
Table 16. 17p-estradiol Levels
Sample ng/kg 1713-
estradiol
Chicken 89
Gallus (chicken) fibroblast/myoblast tissue 4
34
(Table 1)
Gallus (chicken) myoblast cells 2 (Table 1) 36
Example 7: Analysis of Cooked Texture in the Cell Culture-Based Meat Product
[0275] The cell-based meat samples were assessed for physical properties of
significance to
the human perception of texture, including "cooked bite force" and "cooked
hardness".
[0276] Analysis was conducted on meat products generated from cells grown in
adherent
culture which had undergone a forced air dehydration process to adjust
moisture content to
between 65% to 85%. Dehydration occurs at temperatures below 100 F so as not
to "cook" or
otherwise denature components within the meat products.
62
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
[0277] Analysis of all samples was performed by the company using a TA.XTplus
Texture
Analyzer equipped with a 5kg load cell. Samples for analysis have a mass of
400mg +/- 40mg
and were packed in a cylindrical container 10.4mm in diameter and 17mm in
height. Cooked
samples were held at 150 F in a water bath inside their individual sealed
containers for 90
minutes and chilled prior to analysis.
102781 "Cooked Bite Force" was measured using a stainless steel TA-45 incisor
probe under
the test settings:
(a) Test Mode Compression
(b) Pre-Test Speed 3.00 mm/sec
(c) Test Speed 3.00 mm/sec
(d) Post-Test Speed 10.00 mm/sec
(e) Target Mode Strain
(f) Force 100.0g
(g) Distance 5.000 mm
(h) Strain 98.0%
(i) Trigger Type Auto (Force)
(j) Trigger Force 1.0 g
(k) Trigger Distance 2.000 mm
(1) Break Mode Off
(m) Break Sensitivity 10.0 g
(n) Break Detect Stop
(0) Stop Plot At Start Position
(p) Tare Mode Auto
(q) Temperature Set Point 40.0 C
(r) Advanced Options Off
102791 "Cooked Hardness- was measured using a stainless steel TA-24
cylindrical probe
under the test settings:
(a) Pre-Test Speed 2.00 mm/sec
(b) Test Speed 1.00 mm/sec
(c) Post-Test Speed 5.00 mm/sec
63
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
(d) Target Mode Strain
(e) Force Threshold 0
(f) Distance 10.000 mm
(g) Strain 40.0%
(h) Time 5.00 sec
(1) Trigger Type Auto (Force)
Trigger Force 1.0 g
(k) Trigger Distance 2.000 mm
(1) Break Mode Off
(m) Break Sensitivity 10.0 g
(n) Tare Mode Auto
(o) Temperature 4.0 C
(p) Advanced Options On
(q) Control Oven Disabled
(r) Wait For Temperature Yes
(s) Temperature Zone 0.0 C
(t) Frame Deflection Correction Off (XT2 compatibility)
102801 Table 17 shows the cooked texture of the cell-based meat samples. FIG.
13 shows the
cooked hardness of co-culture and fibroblast monoculture tissues.
Table 17: Cooked Texture
Cooked Texture of Cell-Based Meat Samples
Cooked Hardness (g) Cooked Bite Force
(g)
Mmn 284 449
Max 1903 2966
Mean 947 1343
Median 779 1198
33 66
Example 8: Vitamin E Levels in Cell-Based Meat
102811 Cell-based meat has higher enrichment of CL-tocopherol (Vitamin E).
Table 18 shows
the amount of Vitamin E in an exemplary cell-based meat sample, has about 0.90
mg Vitamin
E/100g wet mass of cell-based meat compared to conventional meat (Table 18).
Vitamin E was
64
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
determined using an outside laboratory, Certified Labs, according to UPLC
method AOAC
2001.13.
Table 18: Vitamin E Levels
Sample n Mean value Std Dev
Cell-based meat 3 0.90 mg/100g 0.08mg/100g
Conventionally produced chicken 27 0.28 mg/100g 0.12 mg/100g
Example 9: Determination of Shelf Life
[0282] Methodology I: 0.5-1.5g of conventional or cell-based meat was
aliquoted into clean,
new 15mL falcon tubes. Samples were all frozen at -80 C in tubes. Frozen
sample tubes were
then removed from the -80 C and allowed to sit at room temperature for 0, 1,
2, 7, 14, and 28
days.
[0283] After the designated number of days had passed, sample tubes were
opened and serial
dilutions were made using Butterfields formulation. Serial dilutions were
plated on agar plates
(40g/L of Miller LB Agar powder) - 100uL of solution and spread with
ethanol/flame sterilized
spreader. Dilutions were 10-1- e.g. 1 g into 9m1. Two plates were prepared for
each sample
dilution - all plates were incubated at 37 C for 48 hours and photographed at
0, 24, and 48 hours.
Table 19 shows the results.
Table 19: Shelf Life at Room Temperature
0 days 1 day 2 day 7 day 14 day 28 days
Cell-based meat ND ND ND ND ND
ND
Duck ND TNTC TNTC TNTC TNTC TNTC
Chicken ND TNTC TNTC TNTC TNTC TNTC
TNTC = Too numerous to count
ND = Non detect
[0284] Methodology II: 1.0g of conventional or in-vitro cell-based meat was
aliquoted into
clean, new 15mL falcon tubes. Samples were stored at 4 C or 25 C (room
temperature) for 3
days.
[0285] After the 3 days had passed, sample tubes were opened and serial
dilutions were made
using Butterfields formulation. Serial dilutions were plated on agar plates
for Total microbial
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/US2021/016681
count (TC) or E. coli/coliform count (EC) ¨ lml of solution was added to
compact Dry plates as
above. Dilutions were 10.1 e.g. 1 g into 9 ml were prepared. Three plates were
prepared for each
sample dilution ¨ all plates incubated at 37 C for 48 hours and photographed
at 0, 24, and 48
hours. Table 20 shows the results.
Table 20: Shelf Life at Room Temperature, 3 days, EC and TC Counts
Method Storage temperature EC counts
TC counts
Sample ID
ID [C] (cfu/mL)
(cfu/mL)
Conventional chicken 4 ND 2
10 Cell-based meat (harvest) 4 ND ND
Cell-based meat
10 4 ND ND
(formulated)
10 Control (blank tube) 4 ND ND
10 Conventional chicken 25 5.80E+06
7.40E+07
10 Cell-based meat (harvest) 25 ND ND
Cell-based meat
10 25 ND 8.70E+06
(formulated)
10 Control (blank tube) 25 ND ND
[0286] Additional assays were carried out to assess shelf life for longer
periods of time, using
the methods provided above. After the designated number of days had passed at
4 C, sample
tubes were opened and serial dilutions were made using Butterfields
formulation. Serial dilutions
were plated on agar plates and Total aerobic bacterial count was assessed.
Table 21 shows the
results.
Table 21: Shelf Life at 4 C, 0-148 days, Total Aerobic Bacteria Count (cfu/g)
Day
0 2 3 14 30
148
Conventional
chicken 0 16 17 73
6854 1492063
Normal cell-based
chicken harvest 0 0 0 0
442 0
Aseptic cell-based
chicken harvest 0 0 0 0 49
0
102871 Additional assays were carried out to assess shelf life, in particular
to determine E. coli
and coliforms count, at 4 C and 23 C, out to 148 days. Tables 22-24 show the
results. Table 22
66
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/US2021/016681
show the E. coli and coliform counts as cfu/g, at 4 C, at Day 148. Tables 23
and 24 shows the
E. coli (Table 23) and coliform (Table 24) counts at 23 C at Day 0, 1, 2, 3,
7, 30, and 148.
TMTC designates too many colonies to count.
Table 22: Shelf Life at 4 C, 148 Days, E. Coil and Coliforms Count
4 C
Sample DAY 148
E. coli (cfu/g) Coliforms
(cfu/g)
Conventional chicken 0 990476
Cell-based meat harvest 0 0
Aseptic cell-based meat harvest 0 0
Cell-based meat formulated 0 0
Aseptic cell-based meat 0 0
to
Table 23: Shelf Life, 23 C, 0-148 Days, E. Coll Count
23 C, E. Coil
DAY 0 DAY 1 DAY 2 DAY 3 DAY 7 DAY 30
DAY 148
Conventional chicken 3.23E+00 1.33E+06 4.07E+06 3.84E+07 9.75E-F06
Terminated
Cell-based meat
harvest 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
0.00E+00 0.00E+00
Aseptic Cell-based
meat harvest 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
0.00E+00 0.00E+00
Cell-based meat
formulated 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
0.00E+00 0.00E+00
Aseptic Cell-based
meat formulated 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
0.00E+00 0.00E+00
Table 24: Shelf Life, 23 C, 0-148 Days, Coliforms Count
23 C, Coliforms
(cfu/g)
DAY 0 DAY 1 DAY 2 DAY 3 DAY 14 DAY 30
DAY 148
Conventional chicken 0.00E+00 0.00E+00 6.11E+06 5.77E+06 7.47E+05
Terminated
Cell-based meat
harvest 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
0.00E+00 0.00E+00
Aseptic Cell-based
meat harvest 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
0.00E+00 0.00E+00
67
CA 03166478 2022- 7- 28
WO 2021/158831
PCT/ITS2021/016681
Cell-based meat
formulated
0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
Aseptic Cell-based
meat formulated
0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00 0.00E+00
Example 10: Determination of moisture in cell-based poultry meat
1028R1 Moisture was assayed according to AOAC 950.46. Results shown in Table
25 include
three non-consecutive lots of cell-based poultry meat that were produced using
serum and three
non-consecutive lots of cell-based poultry meat that are serum-free. When
comparing cell-based
poultry meat results to USDA's Agricultural Research Service FoodData Central
database, two
categories were selected for conventional chicken: (1) All USDA chicken (no
organs) which
included data from 27 different published samples ranging across light meat
with and without
skin, dark meat with and without skin, ground raw, and other chicken samples
for roasting or
stewing, but did not include data for skin only or chicken organs; and (2)
USDA chicken white
meat (no skin) which included data from 4 published samples specified as light
meat, meat only.
102891 Table 25 shows the moisture data, reported as average (avg.) and
standard deviation
(std. dev.), for the triplicate serum-containing production lots, the
triplicate serum-free
production lots, as well as chicken data from USDA's Agricultural Research
Service FoodData
Central database (both "all USDA chicken (no organs)- and "USDA chicken white
meat (no
skin)").
Table 25. Moisture analysis
Parameter Units Serum- Serum-free All USDA chicken USDA
chicken
containing cell-based (no organs) white
meat
cell-based poultry meat (no
skin)
poultry meat
Avg. Std. Avg. Std. Avg. Std. N Avg. Std. N
Dev. Dev Dev.
Dev.
Moisture g per 80.68 0.45 75.10 2.67 71.00 5.20
27 74.08 0.68 4
100 g
68
CA 03166478 2022- 7- 28