Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/163448
PCT/US2021/017813
-1-
Vaccine and Methods for Detecting and
Preventing Filariasis
Introduction
[0001] This application claims the benefit of priority from
U.S. Application Serial Number 16/790,277, filed February
13, 2020, the contents of which are incorporated herein by
reference in their entireties.
[0002] This invention was made with government support
under contract numbers AI064745 and AI116441 awarded by the
National Institutes of Health. The government has certain
rights in the invention.
Background
[0003] Lymphatic filariasis caused by the filarial
nematodes Wuchereria bancrofti, Brugia malayi, and Brugia
timori, affects more than 120 million people worldwide (WHO
(1992) World Health Organ. Tech. Rep. Ser. 821:1-71). Mass
drug administration program by the World Health
Organization, is significantly reducing the incidence rate
of lymphatic filariasis in many parts of the world (Hotez
(2009) Clin. Pharmacol. Ther. 85(6):659-64). Nevertheless,
lack of effectiveness to the mass drug administration has
been reported from several endemic regions mainly due to
noncompliance (Babu & (2008) Trans. R. Soc. Trop. Med. Hyg.
102(12):1207-13; El-Setouhy, et al. (2007) Am. J. Trop.
Med. Hyg. 77(6):1069-73). In addition, drug resistance has
been reported to at least one of the drugs in the mass drug
combination (Horton (2009) Ann. Trop. Med. Parasitol.
103(1):S33-40; Schwab, et al. (2007) Parasitology 134(Pt
7):1025-40). Since yearly administration of the mass drugs
is required for effective control, there is an alarming
concern for selecting drug resistant parasites. Therefore,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-2-
there is an immediate need for a multipronged approach in
controlling this mosquito borne infection.
[0004] As with lymphatic filariasis, treatment of
dirofilariasis (heartworm disease) in canids and felids has
included the use of macrolide agents such as ivermectin,
milbemycin oxime, moxidectin and selamectin, which prevent
larval development during the first 2 months after
infection. However, these agents must be administered
monthly for effectiveness and can be very expensive to a
pet owner.
[0005] Vaccination is one strategy for controlling these
infections and several subunit candidate vaccine antigens
have been tested in laboratory animals with variable
results (Bottazzi, et al. (2006) Expert Rev. Vaccines
5(2):189-98; Chenthamarakshan, et al. (1995) Parasite
Immunol. 17(6):277-85; Dissanayakc, at al. (1995) Am. J.
Trop. Med. Hyg. 53(3):289-94; Li, et al. (1993) J. Immunol.
150(5):1881-5; Maizels, et al. (2001) Int. J. Parasitol.
31(9):889-98; Thirugnanam, et al. (2007) Exp. Parasitol.
116(4):483-91; Veerapathran, et al. (2009) PLoS Negl. Trop.
Dis. 3(6):e457). Lymphatic filariasis is a multicellular
organism with complex life cycle and produce large array of
host modulatory molecules. Thus, fighting against this
infection with a single antigen vaccine can be difficult.
By screening a phage display cDNA expression library of the
B. malayi parasite with sera from immune individuals,
several potential vaccine candidates were identified
(Gnanasekar, et al. (2004) Infect. Immun. 72(8):4707-15).
However, a varying degree of protection was achieved with
each of the candidate vaccine antigens when given as a DNA,
protein or prime boost vaccine (Veerapathran, et al. (2009)
supra).
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-3-
Summary of the Invention
[0006] The present invention is a multivalent immunogenic
composition composed of two or more antigens from
Dirofilaria immitis. In some embodiments, the antigens are
protein-based, DNA-based, or a combination thereof. In
other embodiments, the antigens include an Abundant Larval
Transcript (ALT), Small heat shock protein (HSP) 12.6,
Thioredoxin Peroxidase 2 (TXP2), or optionally Tetraspanin
(TSP). In some aspects, the antigens include an ALT antigen
having the amino acid sequence of SEQ ID NO:98 or SEQ ID
NO:99; an H5P12.6 antigen having the amino acid sequence of
SEQ ID NO:100 or SEQ ID NO:101; and/or a TXP2 antigen
having the amino acid sequence of SEQ ID NO:83 or SEQ ID
NO:101. In certain aspects, the ALT antigen has the amino
acid sequence of SEQ ID NO:93; the HSP12.6 antigen has the
amino acid sequence of SEQ ID NO:91; and the TXP2 antigen
has the amino acid sequence of SEQ ID NO:95. In certain
aspects, the ALT antigen has the amino acid sequence of SEQ
ID NO:121 or SEQ ID NO:122; the HSP 12.6 antigen has the
amino acid sequence of SEQ ID NO:81 or SEQ ID NO:123; the
TSP antigen has the amino acid sequence of SEQ ID NO:82;
and the TXP2 antigen has the amino acid sequence of SEQ ID
NO:83 or SEQ ID NO:124. In certain aspects, the antigens
are covalently attached. This invention also provides a
recombinant vector harboring nucleic acids encoding the
multivalent immunogenic composition, a recombinant host
cell harboring the recombinant vector, and the inclusion of
an adjuvant in the multivalent immunogenic composition.
Methods for inducing a protective immune response in a
subject and immunizing an animal against filariasis or
dirofilariasis are also provided. In some embodiments of
these methods, the multivalent immunogenic composition is
administered with an adjuvant, e.g., in one or more
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-4-
additional doses by subcutaneous or intramuscular
injection.
Brief Description of the Drawings
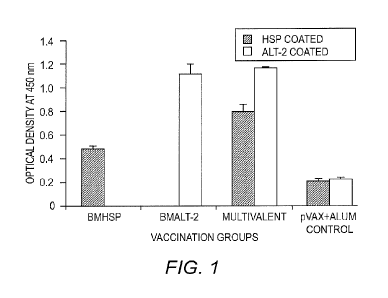
[0007] FIG. 1 shows the titer of anti-BmHSP and anti-BmALT2
IgG antibodies in the sera of vaccinated mice. 6-week-old
balb/c mice were immunized using a prime boost approach
with a monovalent immunogenic composition (Bmhsp prime and
rBmHSP boost or Bmalt2 prime and rBmALT2 boost) and
multivalent immunogenic composition (Rmhsp/Bma1t2 prime and
rBmHSP and rBmALT2 boost). Titer of IgG antibodies were
measured in the sera using an indirect ELISA. The data
presented is the antibody titer 2 weeks after the last
booster. Results show that both bivalent and multivalent
immunogenic compositions induce significant IgG antibodies
against each of the component antigens. The findings also
show that the antigens in the monovalent and multivalent
formulations act synergistically in boosting the immune
responses. N=5. Statistically significant ** p <0.001, * p
< 0.05. Values represented are mean SD.
[0008] FIGS. 2A-2B show the number of IL-4 (FIG. 2A) and
IFN-y (FIG. 2B) secreting cells in the spleen of mice
vaccinated with monovalent (BmHSP or BmALT2) or multivalent
immunogenic composition. An ELISPOT assay was performed
after stimulating the cells with rBmHSP or rBmALT (1
g/ml). Single cell preparations of spleen cells were
stimulated with respective antigens for 48 hours and spot
forming cells were counted. Results show that both
monovalent and multivalent immunogenic compositions
promoted IL-4 secreting cells. Multivalent vaccination
induced the higher number of IL-4 producing cells than
controls. IFN-y producing cells were comparatively low.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-5-
These findings further confirm that BmHSP and BmALT2
synergistically boost the immune responses in vaccinated
animals following a multivalent vaccination. N=5. Results
are expressed as mean number of spot forming units per 3 x
106 cells SD.
[0009] FIG. 3 shows the degree of protection conferred by a
multivalent immunogenic composition in a mouse model.
Balb/c strain of mice were immunized with HAT
(HSP/ALT2/TSP) hybrid DNA, with recombinant HAT protein or
a combination of both using a prime boost approach. HAT
hybrid DNA was used for priming. Two weeks following the
priming, mice were boosted with HAT hybrid protein. Another
group of mice were immunized with HAT hybrid DNA or with
HAT hybrid protein. Control groups of mice received only
blank vector or alum adjuvant. Two weeks after the last
immunization, mice were challenged with 20 infective larvae
of Brugia malayi by placing them in a micropore chamber in
the peritoneal cavity of the immunized mice. After 48
hours, larval death was measured to determine the success
of vaccination.
[0010] FIG. 4 shows multivalent immunogenic composition-
induced protection against Brugia malayi infection in
macaques. All animals (vaccinated and control) were
challenged with 130-180 L3s of Brugia malayi one month
after the last immunization. In weeks 5, 10, 15 and 18
post-challenge, 10 ml of blood was collected from each
macaque between 18:00 and 22:00 hours and screened for the
presence of microfilariae using a modified Knott technique
and analyzed by PCR for the Ma-1 repeats. Absence of
infection in microfilaria (Mf)-negative animals was further
confirmed by SXP-1 (B. malayi diagnostic antigen) ELISA.
Results show that rBmHAXT+AL019 (alum plus glucopyranosyl
lipid adjuvant-stable emulsion) is a better immunogenic
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-6-
composition formulation than the other formulations tested
(n = 10 per group). Chi-square test and Fisher's exact test
were used to compare the proportions across the groups.
[0011] FIG. 5 shows the results of an antibody-dependent
cell-mediated cytotoxicity (ADCC) assay. Approximately 10
Brugia malayi larvae were incubated for 72 hours at 37 C
with 2 x 105 peripheral blood mononuclear cells (PBMCs) and
50 pl of sera samples from each macaque. Larval death in
each well was monitored under a light microscope. Each data
point indicates the percent larval death using a serum
sample from one animal. '+' indicates the average percent
larvicidal activity for that group. n = 10 macaques per
group. *P 0.005 compared with the AL019 (alum plus
glucopyranosyl lipid adjuvant-stable emulsion) group.
Statistical analysis was performed by a Kruskal-Wallis test
followed by Bonferroni correction for multiple tests.
rBmHAXT, recombinant B. malayi HSP/ALT-2/TPX-2/TSPLEL.
[0012] FIG. 6 shows the results of an ADCC assay for
killing of drug-sensitive and drug-resistant Dirofilaria
immitis in dogs. Approximately 8-10 D. immitis larvae were
incubated for 96 hours at 37 C with 0.5 million PBMCs and
100 pl of sera samples from each dog. Larval death in each
well was monitored under a light microscope. Each data
point indicates the percent larval death using a serum
sample from one animal. rBmHAXT, recombinant B. malayi
HSP/ALT-2/TPX-2/TSPLEL.
Detailed Description of the Invention
[0013] A multivalent immunogenic composition for filariasis
has now been developed. Combinations of antigens, such as
Abundant Larval Transcript (ALT2), Tetraspanin (TSP), Small
heat shock protein (HSP) 12.6, Vespid Venom Allergen
homologue-Like protein (VAL-1), Glutathione S-Transferase
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-7-
(GST), and Thioredoxin Peroxidase 2 (TPX-2), and fragments
thereof, were tested in experimental animals (i.e., mouse,
jirds, mastomys, macaque, and dogs) and shown to provide
>80% protection against infection by filarial nematodes
such as Brugia malayd and Dirofilaria immitis. Accordingly,
the present invention features protein-based and DNA-based
compositions composed of filarial nematode antigens or
nucleic acids encoding the same and use of the immunogenic
compositions to prevent or control filariasis in humans and
animals, in particular canids and felids. In addition to
vaccination, the present invention also provides assays and
kits for detecting the presence of a filarial nematode.
[0014] For the purposes of the present invention, a
multivalent or polyvalent immunogenic composition refers to
an immunogenic composition or vaccine prepared from several
antigens. According to some embodiments, the antigen is a
nucleic acid molecule, which is referred to herein as a
"DNA-based" antigen. According to other embodiments, the
antigen is a protein or polypeptide, which is referred to
herein as "protein-based" antigen. A multivalent
immunogenic composition of the invention can be composed of
two, three, four, five, six or up to ten antigens or their
fragments in various permutation combinations. In
particular embodiments, the multivalent immunogenic
composition is composed of two, three or four antigens. In
some embodiments, the multivalent immunogenic composition
is composed of solely of protein antigens. In other
embodiments, the multivalent immunogenic composition is
composed solely of DNA-based antigens. In yet other
embodiments, the multivalent immunogenic composition is
composed of a mixture of protein- and DNA-based antigens.
[0015] Antigens of the instant invention can be provided or
expressed from a single nucleic acid molecule containing,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-8-
e.g., internal ribosome entry sites between the antigens.
Moreover, the antigens of the multivalent immunogenic
composition of this invention can be covalently attached to
form a hybrid or chimeric molecule or fusion protein,
wherein the antigens are immediately adjacent to one
another (e.g., an in-frame fusion with or without a short
spacer). Alternatively, antigens of the instant invention
can be provided as a mixture of individual antigens.
Moreover, it is contemplated that the instant immunogenic
composition can be composed of a hybrid molecule
containing, e.g., two antigens, in admixture with a third
non-covalently attached antigen. By way of illustration, a
multivalent immunogenic composition of the invention can be
composed of a chimeric TSP-HSP protein in admixture with a
nucleic acid molecule encoding ALT2.
[0016] In one embodiment, the antigens of the multivalent
immunogenic composition are different proteins from one
species of filarial nematode. As an example of this
embodiment, the multivalent immunogenic composition is
composed of ALT2, HSP, and TSP and/or TPX2 or GST antigens
isolated from one or more strains of B. malayi or D.
immitis. In another embodiment, the antigens are the same,
but from different species of filarial nematodes. As an
example of this embodiment, the multivalent immunogenic
composition is composed of the ALT2 antigen isolated from
W. bancrofti, B. malayi, B. timori, and D. immitis. In yet
a further embodiment, the multivalent immunogenic
composition is composed of a combination of different
antigens from different species of filarial nematodes. By
way of illustration, the multivalent immunogenic
composition can be composed of the ALT2 antigen isolated
from W. bancrofti, 0. volvn]us and L. loa and the HSP
antigen isolated from B. malayi and D. immitis.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-9-
[0017] For preparing multivalent DNA-based or multivalent
recombinant DNA-based immunogenic composition, the DNA
sequence of the gene of interest (also used interchangeably
as DNA molecule) need not contain the full length of DNA
encoding the corresponding protein. Likewise, when
preparing fusion protein-based or multivalent recombinant
protein immunogenic compositions, the protein sequence need
not contain the full-length protein. In most cases, a
fragment of the protein or gene which encodes an epitope
region is sufficient for immunization. The DNA/protein
sequence of an epitope region can be found by sequencing
the corresponding part of the gene from various strains or
species and comparing them. The major antigenic
determinants are likely to be those showing the greatest
heterology. Also, these regions are likely to lie
accessibly in the conformational structure of the proteins.
One or more such fragments of proteins or genes encoding
the antigenic determinants can be prepared by chemical
synthesis or by recombinant DNA technology. These fragments
of proteins or genes, if desired, can be linked together or
linked to other proteins or DNA molecules, respectively.
[0018] As described herein, the ALT2, TSP, VAL-1, GST and
HSP antigens were identified as providing protection
against infection by filaria larvae. Accordingly, in
particular embodiments, the instant immunogenic composition
includes the ALT2, TSP, VAL-1, TPX2, GST and/or HSP protein
antigens and/or nucleic acid molecules encoding the ALT2,
TSP, VAL-1, TPX2, GST and/or HSP protein, or fragments
thereof. Protein and nucleic acid sequences for these
antigens are available under the GENBANK accession numbers
and/or sequences listed in Table 1.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-10-
TABLE 1
SEQ ID
SEQ ID
Antigen Source Protein Nucleic Acid
NO: NO:
P90708 37 BM084723 38
B. malayi
XP 001896203 39 XM 001896168 40
ALT2 W. bancrofti AAC35355 41 AF084553
42
L. loa XP 003151340 43
XM 003151292 44
D. immitis AAC47031 93 - 92
TSP B. malayi ABN55911 45 EF397425
46
L. loa XP 003136177 47
XM 003136129 48
B. malayi AAU04396 49 AY692227 50 ,
0. vo1vulus CAA48633 51 X68669 52
HSP
L. boa XP 003139338 53
XM 003139290 54
D. immitis QHA79233 91 - 90
B. malayi AAB97283 55 AF042088 56
W. bancrofti AAD16985 57 AF109794 58
VAL-1 O. volvulus AAB69625 59 AF020586
60
L. boa XP 003146897 61
XM 003146849 62
_
TPX2 B. malayi Q17172 71 U47100
72
D. immitis AAC38831 95 - 94
W. bancrofti AA045827 85 AY195867 86
GST D. immitis P46426 103 -
102
B. malayi XP 001898233 120 - -
[0019] In addition, the nucleotide sequence encoding 0.
voivuius TSP can be found under GENBANK Accession No.
JN861043. The protein antigens and nucleic acid molecules
of the invention can be used as full length molecules or
less than full length molecules. In this respect, the
present invention further includes the use of fragments of
the above-referenced protein antigens and nucleic acid
molecules. Fragments are defined herein as 20, 30, 40, 50,
60, 70, 80, 90, 100, 150, or 200 amino acid residue
portions of full-length protein antigens (e.g., those
listed in Table 1) or 60, 90, 120, 150, 180, 210, 240, 270,
300, 350, or 600 nucleotide portion of full-length nucleic
acid molecules (e.g., those listed in Table 1). Exemplary
protein fragments include the large extracellular loop
(LEL) domain of TSP (see, e.g., the LEL domain of B. malayi
TSP of SEQ ID NO:63 or SEQ ID NO:77) and N-terminal
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-11-
deletion of HSP 12.6 (cHSP; see, e.g., the B. malayi HSP
fragment of SEQ ID NO:64), as well as the nucleic acid
molecules encoding the same (see, SEQ ID NO:65 and SEQ ID
NO:66, respectively). An exemplary fusion protein
containing ALT2, HSP and TSP protein sequences is set forth
in SEQ ID NO:70. An exemplary fusion protein containing
ALT2, HSP and TPX2 protein sequences is set forth in SEQ ID
NO:73 and SEQ ID NO:97. An exemplary fusion protein
containing ALT2, HSP, TSP and TPX2 protein sequences is set
forth in SEQ ID NO:74.
[0020] In particular embodiments, the protein or protein
fragments of this invention have one or more antigenic
sequences for eliciting an immune response in an animal. In
certain embodiments, the ALT2 protein of the invention is a
B. malayi ALT2 protein or fragment comprising or consisting
of the sequence VSESDEEFDDSAADDTDDSEAGGGSEGGDEYVT (SEQ ID
NO:78) and/or EFVETDGKKKECSSHEACYDQREPQ (SEQ ID NO:79) or
D. immitis ALT2 protein or fragment comprising or
consisting of the sequence ASESQEETVSFEESDEDYEDDSE (SEQ ID
NO:98) and/or FVESDGKMKHCKTHEACYDQREPQ (SEQ ID NO:99),
which, based upon the Bepipred Linear Epitope Prediction
method (Larsen, et al. (2006) Immunome Res. 2:2), are
predicted B-cell epitopes. In other embodiments, the HSP
protein of the invention is a B. malayi HSP protein or
fragment comprising or consisting of the sequence
WSAEQWDWPLQH (SEQ ID NO:80) and/or KLPSDVDTKTL (SEQ ID
NO:81) or D. immitis HSP protein or fragment comprising or
consisting of the sequence NWSADQWDWPLQHNDDVVKVTNTNDK (SEQ
ID NO:100) and/or KLPSDVDTKTL (SEQ ID NO:81), which are
predicted B-cell epitopes. In further embodiments, the TSP
protein of the invention is a B. malayi TSP protein or
fragment comprising or consisting of the sequence
KTGESEDEMQ (SEQ ID NO:82), which is a predicted B-cell
CA 03167346 2022- 8-8
WO 2021/163448 PCT/US2021/017813
-12-
epitope. In yet a further embodiment, the TPX2 protein of
the invention is a B. malayi TPX2 protein or fragment
comprising or consisting of the sequence FIGQPAPNFKT (SEQ
ID NO:83) and/or GEVCPANWHPGSETIKPGVKESKA (SEQ ID NO:84) or
D. immitis TPX2 protein or fragment comprising or
consisting of the sequence FIGQPAPNFKT (SEQ ID NO:83)
and/or GEVCPANWQPGSEAIKPGVKESKA (SEQ ID NO:101), which are
predicted B-cell epitopes.
[0021] In certain embodiments, ALT2 protein fragments of
this invention comprise or consist of the amino acid
sequences X1X2E5DEX3X4X5DX6 (SEQ ID NO:121), wherein
independently X1 is V or F, X2 is S or E, X3 is E or D, X4 is
F or Y, X5 is D or E, and X6 is S or D; or
FVEX1DGKX2KX3CX4X5HEACYDQREPQ (SEQ ID NO:122), wherein
independently X1 is S or T; X2 is M or K; X3 is E or H, X4 is
S or K, and X5 is S or T. In other embodiments, HSP protein
fragments of this invention comprise or consist of the
amino acid sequences WSAX1QWDWPLQH (SEQ ID NO:123), wherein
independently Xi is Glu or Asp; or KLPSDVDTKTL (SEQ ID
NO:81). In further embodiments, TPX2 protein fragments of
this invention comprise or consist of the amino acid
sequences FIGQPAPNFKT (SEQ ID .. NO:83); ..
or
GEVCPANWX1PGSEX2IKPGVKESKA (SEQ ID NO:124), wherein
independently X is H or Q, and X2 is T or A.
[0022] With respect to certain embodiments of the
invention, the multivalent immunogenic composition of the
invention includes other known antigens from filarial
nematodes. Examples of other suitable antigens include, but
are not limited to, glutathione peroxidase (see Cookson, et
al. (1992) Proc. Natl. Acad. Sci. USA 89:5837-5841;
Maizels, et al. (1983) Parasitology 87:249-263; Maizels, et
al. (1983) Clin. Exp. Immunol. 51:269-277); recombinant
antigen (BmR1; see Noordin, et al. (2004) Filaria J. 3:10);
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-13-
class II aminoacyl-tRNA synthetase (see Kron, et al. (1995)
FEBS Lett. 374:122-4); heat shock cognate 70 (hsc70)
protein (see Selkirk, et al. (1989) J. Immuno]. 143:299-
308); paramyosin (see Li, et al. (1991) Mbl. Biochem.
Parasitol. 49:315-23); tropomyosin (Hartmann, et al. (2006)
Vaccine 24(17):3581-90); chitinase (Adam, et al. (1996) J.
Biol. Chem. 271(3):1441-7); Abundant Larval Transcript
(ALT)-1 (Gregory, et al. (2000) Infect. Tmmun. 68(7):4174-
9); immunodominant hypodermal antigen SPX1 (Bradley, et al.
(1993) Exp. Parasitol. 77(4):414-424). In some embodiments,
the antigen is obtained from a filarial nematode selected
from the group of W. bancrofti, B. malayi, 0. volvulus, L.
loa, D. immitis and B. timori. In certain embodiments, the
antigen is B. malayi or Dirofilaria tropomyosin having an
amino acid sequence as set forth in SEQ ID NO:104 and SEQ
ID NO:105, respectively, or a fragment thereof; B. malayi
or Dirofilaria chitinase having an amino acid sequence as
set forth in SEQ ID NO:106 and SEQ TD NO:107, respectively,
or a fragment thereof; B. malayi or Dirofilaria ALT-1
having an amino acid sequence as set forth in SEQ ID NO:108
and SEQ ID NO:109, respectively, or a fragment thereof; B.
malayi or Dirofilaria SPX1 having an amino acid sequence as
set forth in SEQ ID NO:110 and SEQ ID NO:111, respectively,
or a fragment thereof; B. malayi or D. immitis venom
allergen antigen 5-like protein having an amino acid
sequence as set forth in SEQ ID NO:112 and SEQ ID NO:113,
respectively, or a fragment thereof; B. malayi or D.
immitis Macrophage migration Inhibitory Factor (MIF)-1
protein having an amino acid sequence as set forth in SEQ
ID NO:114 and SEQ ID NO:115, respectively, or a fragment
thereof; B. malayi or Dirofilaria MIF-2 protein having an
amino acid sequence as set forth in SEQ ID NO:116 and SEQ
ID NO:117, respectively, or a fragment thereof; or B.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-14-
malayi or Dirofilaria cystatin protein having an amino acid
sequence as set forth in SEQ ID NO:118 and SEQ ID NO:119,
respectively, or a fragment thereof.
[0023] According to the present invention, the antigens of
the fusion protein and immunogenic composition are isolated
from a filarial nematode. In this respect, an isolated
nucleic acid molecule or protein is a nucleic acid molecule
or protein that has been removed from its natural milieu
(i.e., that has been subjected to human manipulation). As
such, "isolated" does not reflect the extent to which the
nucleic acid molecule or protein has been purified. In
particular embodiments, the antigens are purified (e.g.,
purified to greater than 95% homogeneity). An isolated and
optionally purified nucleic acid molecule or protein of the
present invention can be obtained from its natural source
or produced using recombinant DNA technology (e.g.,
polymerase chain reaction (PCR) amplification or cloning)
or chemical synthesis. Isolated nucleic acid molecules and
proteins can also include, for example, natural allelic
variants or isomers that induce an immune response in the
host.
[0024] One embodiment of the present invention includes a
recombinant vector, which includes at least one isolated
nucleic acid molecule of the present invention, inserted
into a vector capable of delivering the nucleic acid
molecule into a host cell. Such a vector contains
heterologous nucleic acid sequences, that are nucleic acid
sequences that are not naturally found adjacent to nucleic
acid molecules of the present invention and that preferably
are derived from a species other than the species from
which the nucleic acid molecule(s) are derived. The vector
can be either prokaryotic or eukaryotic, and typically is a
virus or a plasmid. Recombinant vectors can be used in the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-15-
cloning, sequencing, and/or otherwise manipulating the
nucleic acid molecules of the present invention.
[0025] The present invention also includes an expression
vector, which includes a nucleic acid molecule of the
present invention in a recombinant vector that is capable
of expressing the nucleic acid molecule when transformed
into a host cell. Preferably, the expression vector is also
capable of replicating within the host cell. Expression
vectors can be either prokaryotic or eukaryotic, and are
typically viruses or plasmids. Expression vectors of the
present invention include any vectors that function (i.e.,
direct gene expression) in recombinant cells of the present
invention, including in bacterial, fungal, parasite,
insect, other animal, and plant cells. Preferred expression
vectors of the present invention can direct gene expression
in bacterial, yeast, helminth or other parasite, insect and
mammalian cells.
[0026] In particular, expression vectors of the present
invention contain regulatory sequences such as
transcription control sequences, translation control
sequences, origins of replication, and other regulatory
sequences that are compatible with the recombinant cell and
that control the expression of nucleic acid molecules of
the present invention. In particular, recombinant molecules
of the present invention include transcription control
sequences. Transcription control sequences are sequences
which control the initiation, elongation, and termination
of transcription. Particularly important transcription
control sequences are those which control transcription
initiation, such as promoter, enhancer, operator and
repressor sequences. Suitable transcription control
sequences include any transcription control sequence that
can function in at least one of the recombinant cells of
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-16-
the present invention. A variety of such transcription
control sequences are known to those skilled in the art.
Preferred transcription control sequences include those
which function in bacterial, yeast, helminth or other
endoparasite, or insect and mammalian cells, such as, but
not limited to, tac, lac, trp, tro, oxy-pro, omp/lpp, rrnB,
bacteriophage lambda (such as lambda pi, and lambda PR and
fusions that include such promoters), bacteriophage T7,
T7lac, bacteriophage T3, bacteriophage SP6, bacteriophage
SP01, metallothionein, alpha-mating factor, Pichia alcohol
oxidase, alphavirus subgenomic promoter, antibiotic
resistance gene, baculovirus, Heliothis zea insect virus,
vaccinia virus, herpesvirus, raccoon poxvirus, other
poxvirus, adenovirus, cytomegalovirus (such as immediate
early promoter), simian virus 40, retrovirus, actin,
retroviral long terminal repeat, Rous sarcoma virus, heat
shock, phosphate and nitrate transcription control
sequences as well as other sequences capable of controlling
gene expression in prokaryotic or eukaryotic cells.
Additional suitable transcription control sequences include
tissue-specific promoters and enhancers as well as
lymphokine-inducible promoters (e.g., promoters inducible
by interferons or interleukins). Transcription control
sequences of the present invention can also include
naturally occurring transcription control sequences
naturally associated with parasitic helminths, such as B.
malayi or D. immitis transcription control sequences.
[0027] Recombinant molecules of the present invention may
also contain (a) secretory signals (i.e., signal segment
nucleic acid sequences) to enable an expressed protein of
the present invention to be secreted from the cell that
produces the protein and/or (b) fusion sequences which lead
to the expression of nucleic acid molecules of the present
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-17-
invention as fusion proteins. Examples of suitable signal
segments include any signal segment capable of directing
the secretion of a protein of the present invention.
Preferred signal segments include, but are not limited to,
tissue plasminogen activator (t-PA),
interferon,
interleukin, growth hormone, histocompatibility and viral
envelope glycoprotein signal segments. In addition, a
nucleic acid molecule of the present invention can be
joined to a fusion segment that directs the encoded protein
to the proteosome, such as a ubiquitin fusion segment.
Eukaryotic recombinant molecules may also include
intervening and/or untranslated sequences surrounding
and/or within the nucleic acid sequences of nucleic acid
molecules of the present invention.
[0028] Another embodiment of the present invention includes
a recombinant host cell harboring one or more recombinant
molecules of the present invention. Transformation of a
nucleic acid molecule into a cell can be accomplished by
any method by which a nucleic acid molecule can be inserted
into the cell. Transformation techniques include, but are
not limited to, transfection,
electroporation,
microinjection, lipofection, adsorption, and protoplast
fusion. A recombinant cell may remain unicellular or may
grow into a tissue, organ or a multicellular organism.
Transformed nucleic acid molecules of the present invention
can remain extrachromosomal or can integrate into one or
more sites within a chromosome of the transformed (i.e.,
recombinant) cell in such a manner that their ability to be
expressed is retained.
[0029] Suitable host cells to transform include any cell
that can be transformed with a nucleic acid molecule of the
present invention. Host cells can be either untransformed
cells or cells that are already transformed with at least
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-18-
one nucleic acid molecule (e.g., nucleic acid molecules
encoding one or more proteins of the present invention
and/or other proteins useful in the production of
multivalent immunogenic compositions). Host cells of the
present invention either can be endogenously (i.e.,
naturally) capable of producing proteins of the present
invention or can be capable of producing such proteins
after being transformed with at least one nucleic acid
molecule of the present invention. Host cells of the
present invention can be any cell capable of producing at
least one protein of the present invention, and include
bacterial, fungal (including yeast), parasite (including
helminth, protozoa and ectoparasite), other insect, other
animal and plant cells. Preferred host cells include
bacterial, mycobacterial, yeast, helminth, insect and
mammalian cells. More preferred host cells include
Salmonella, Escherichia, Bacillus, Listeria, Saccharomyces,
Spodoptera, Mycobacteria, Trichnplusia, BHK (baby hamster
kidney) cells, MDCK cells (Madin-Darby canine kidney cell
line), CRFK cells (Crandell feline kidney cell line), CV-1
cells (African monkey kidney cell line used, for example,
to culture raccoon poxvirus), COS (e.g., COS-7) cells, and
Vero cells. Particularly preferred host cells are
Escherichia coli, including E. coli K-12 derivatives;
Salmonella typhi; Salmonella typhimurium; Spodoptera
frugiperda; Trichoplusia ni; BHK cells; MDCK cells; CRFK
cells; CV-1 cells; COS cells; Vero cells; and non-
tumorigenic mouse myoblast G8 cells (e.g., ATCC CRL 1246).
Additional appropriate mammalian cell hosts include other
kidney cell lines, other fibroblast cell lines (e.g.,
human, murine or chicken embryo fibroblast cell lines),
myeloma cell lines, Chinese hamster ovary cells, mouse
NIH/3T3 cells, LMTK31 cells and/or HeLa cells. In one
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-19-
embodiment, the proteins may be expressed as heterologous
proteins in myeloma cell lines employing immunoglobulin
promoters.
[0030] A recombinant cell is preferably produced by
transforming a host cell with one or more recombinant
molecules, each comprising one or more nucleic acid
molecules of the present invention and one or more
transcription control sequences, examples of which are
disclosed herein.
[0031] Recombinant DNA technologies can be used to improve
expression of transformed nucleic acid molecules by
manipulating, for example, the number of copies of the
nucleic acid molecules within a host cell, the efficiency
with which those nucleic acid molecules are transcribed,
the efficiency with which the resultant transcripts are
translated, and the efficiency of post-translational
modifications. Recombinant techniques useful for increasing
the expression of nucleic acid molecules of the present
invention include, but are not limited to, operatively
linking nucleic acid molecules to high-copy number
plasmids, integration of the nucleic acid molecules into
one or more host cell chromosomes, addition of vector
stability sequences to plasmids, substitutions or
modifications of transcription control signals (e.g.,
promoters, operators, enhancers), substitutions
or
modifications of translational control signals (e.g.,
ribosome binding sites, Shine-Dalgarno sequences),
modification of nucleic acid molecules of the present
invention to correspond to the codon usage of the host
cell, deletion of sequences that destabilize transcripts,
and use of control signals that temporally separate
recombinant cell growth from recombinant enzyme production
during fermentation. The activity of an expressed
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-20-
recombinant protein of the present invention may be
improved by fragmenting, modifying, or derivatizing nucleic
acid molecules encoding such a protein. Moreover, while
non-codon-optimized sequences may be used to express fusion
proteins in host cells such as E. coil (see Table 1), in
embodiments pertaining to DNA vaccines, the nucleic acid
molecule may be codon-optimized to facilitate expression in
mammalian cells. In this respect, codon-optimized sequences
for BmALT2, N-terminal deleted HSP 12.6 (cHSP) of B.
malayd, and LEL domain of B. malayi Tetraspanin are set
forth in SEQ ID NO:67, SEQ ID NO:68, and SEQ ID NO:69,
respectively. Moreover, to facilitate expression of one or
more of the recombinant proteins in a recombinant host
cell, the protein sequence can be manipulated. By way of
illustration, the insertion of a glycine residue after the
N-terminal methionine residue of the B. malayi ALT2 protein
was found to improve expression of this protein in E. coil.
[0032] Isolated protein-based antigens of the present
invention can be produced in a variety of ways, including
production and recovery of natural proteins, production and
recovery of recombinant proteins, and chemical synthesis of
the proteins. In one embodiment, an isolated protein of the
present invention is produced by culturing a cell capable
of expressing the protein under conditions effective to
produce the protein, and recovering the protein. A
preferred cell to culture is a recombinant cell of the
present invention. Effective culture conditions include,
but are not limited to, effective media, bioreactor,
temperature, pH and oxygen conditions that permit protein
production. An effective, medium refers to any medium in
which a cell is cultured to produce a protein of the
present invention. Such medium typically includes an
aqueous medium having assimilable carbon, nitrogen and
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-21-
phosphate sources, and appropriate salts, minerals, metals
and other nutrients, such as vitamins. Cells of the present
invention can be cultured in conventional fermentation
bioreactors, shake flasks, test tubes, microtiter dishes,
and petri plates. Culturing can be carried out at a
temperature, pH and oxygen content appropriate for a
recombinant cell. Such culturing conditions are within the
expertise of one of ordinary skill in the art.
[0033] Depending on the vector and host system used for
production, resultant proteins of the present invention may
either remain within the recombinant cell; be secreted into
the fermentation medium; be secreted into a space between
two cellular membranes, such as the periplasmic space in E.
co/i; or be retained on the outer surface of a cell or
viral membrane.
[0034] Recovery of proteins of invention can include
collecting the whole fermentation medium containing the
protein and need not imply additional steps of separation
or purification. Proteins of the present invention can be
purified using a variety of standard protein purification
techniques, such as, but not limited to, affinity
chromatography, ion exchange chromatography, filtration,
electrophoresis, hydrophobic interaction chromatography,
gel filtration chromatography, reverse
phase
chromatography, concanavalin A
chromatography,
chromatofocusing and differential solubilization. Proteins
of the present invention are preferably retrieved in
substantially pure form thereby allowing for the effective
use of the protein as a therapeutic composition. A
therapeutic composition for animals, for example, should
exhibit no substantial toxicity and preferably should be
capable of stimulating the production of antibodies in a
treated animal.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-22-
[0035] One embodiment of the present invention is an
immunogenic composition or vaccine that, when administered
to an animal in an effective manner, is capable of
protecting that animal from filariasis or dirofilariasis
caused by a filarial nematode such as a Dirofilaria
nematode. In some embodiments, the invention provides a
method for treating or protecting an animal from a disease
caused by a filarial nematode. In other embodiments, the
invention provides a method for treating or protecting an
animal, e.g., a dog or cat, from dirofilariasis (heartworm
disease). Immunogenic compositions include protective
molecules such as an isolated antigenic protein of the
present invention, an isolated nucleic acid molecule of the
present invention, and hybrids and mixtures thereof. As
used herein, the multivalent immunogenic composition of the
invention induces a proLecLive immune response when
administered in an effective manner to an animal such as a
human, cat or dog thereby treating, ameliorating, and/or
preventing disease caused by a filarial or dirofilarial
nematode including, but not limited to, W. bancrofti, B.
malayi, 0. volvulus, L. loa, D. immitis, Mansonella
streptocerca, Dracunculus medinensis, M. perstans, M.
ozzardi, and/or B. timori. Immunogenic composition of the
present invention can be administered to any animal
susceptible to such therapy, preferably to mammals, and
more preferably to humans, pets such as dogs and cats, and
economic food animals and/or zoo animals.
[0036] In one embodiment, a multivalent immunogenic
composition of the present invention when administered to
the host can develop antibodies that can kill the parasites
in the vector in which the filarial nematode develops, such
as in a mosquito when they feed the host.
CA 03167346 2022- 8-8
WO 2021/163448 PCT/US2021/017813
-23-
[0037] In order to protect an animal from disease caused by
a filarial nematode, an immunogenic composition of the
present invention is administered to the animal in an
effective manner such that the composition is capable of
protecting that animal from a disease caused by the
filarial nematode. Compositions of the present invention
can be administered to animals prior to infection in order
to prevent infection (i.e., as a preventative vaccine)
and/or can be administered to animals after infection in
order to treat disease caused by the filarial nematode
(i.e., as a therapeutic vaccine).
[0038] Compositions of the present invention can be
formulated in an excipient that the animal to be treated
can tolerate. Examples of such excipients include water,
saline, Ringer's solution, dextrose solution, Hank's
solution, and other aqueous physiologically balanced salt
solutions. Nonaqueous vehicles, such as fixed oils, sesame
oil, ethyl oleate, or triglycerides may also be used. Other
useful formulations include suspensions containing
viscosity enhancing agents, such as sodium
carboxymethylcellulose, sorbitol, or dextran. Excipients
can also contain minor amounts of additives, such as
substances that enhance isotonicity and chemical stability.
Examples of buffers include phosphate buffer, bicarbonate
buffer and Tris buffer, while examples of preservatives
include thimerosal, m- or o-cresol, formalin and benzyl
alcohol. Standard formulations can either be liquid
injectables or solids which can be taken up in a suitable
liquid as a suspension or solution for injection. Thus, in
a non-liquid formulation, the excipient can comprise
dextrose, human serum albumin, preservatives, etc., to
which sterile water or saline can be added prior to
administration.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-24-
[0039] In one embodiment of the present invention, the
immunogenic composition can include an adjuvant. An
"adjuvant," as defined herein, is a substance that serves
to enhance the immunogenicity of an immunogenic composition
of the invention. An immune adjuvant may enhance an immune
response to an antigen that is weakly immunogenic when
administered alone, e.g., inducing no or weak antibody
titers or cell-mediated immune response, increase antibody
titers to the antigen, and/or lowers the dose of the
antigen effective to achieve an immune response in the
individual. Thus, adjuvants are often given to boost the
immune response and are well known to the skilled artisan.
[0040] Suitable adjuvants to enhance effectiveness of the
immunogenic composition include, but are not limited to:
(1) aluminum salts (alum), such as aluminum hydroxide,
aluminum phosphate, aluminum sulfate, etc.;
(2) calcium-based salts;
(3) silica;
(4) oil-in-water emulsion formulations (with or without
other specific immunostimulating agents such as muramyl
peptides (defined below) or bacterial cell wall
components), such as, for example,
(a) ME59 (WO 90/14837), containing 5% squalene, 0.5%
polysorbate 80, and 0.5% sorbitan trioleate (optionally
containing various amounts of muramyl tripeptide
phosphatidylethanolamine) formulated into
submicron
particles using a microfluidizer such as Model 110Y
microfluidizer (Microfluidics, Newton, MA),
(b) SAF, containing 10% squalene, 0.4% polysorbate
80, 5% pluronic-blocked polymer L121, and thr-MDP either
microfluidized into a submicron emulsion or vortexed to
generate a larger particle size emulsion,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-25-
(c) Ribi' adjuvant system (RAS), (Corixa, Hamilton,
MT) containing 2% squalene, 0.2% polysorbate 80, and one or
more bacterial cell wall components from the group
consisting of 3-0-deacylated monophosphorylipid A (MPL')
described in US 4,912,094, trehalose dimycolate (TDM), and
cell wall skeleton (CWS), preferably MPL+CWS (Detox"); and
(d) a Montanide ISA;
(5) saponin adjuvants, such as those sold under the
tradenames QUIL-AO or QS-21 STIMULONO (Antigenics,
Framingham, MA) (see, e.g., US 5,057,540), may be used or
particles generated therefrom such as
ISCOM
(immunostimulating complexes formed by the combination of
cholesterol, saponin, phospholipid, and amphipathic
proteins) and IscomatrixTM (having essentially the same
structure as an ISCOM but without the protein);
(6) bacterial components (e.g., endotoxins, in
particular superantigens, exotoxins and cell wall
components) and lipopolysaccharides, synthetic lipid A
analogs such as aminoalkyl glucosamine phosphate compounds
(AGP), or derivatives or analogs thereof, which are
available from Corixa, and described in US 6,113,918; one
such AGP is 2-[(R)-3-tetradecanoyloxytetradecanoylamino]
ethyl
2-Deoxy-4-0-phosphono-3-0-[(R)-3-tetradecanoyloxy-
tetradecanoy1]-2-[(R)-3-tetradecanoyloxytetradecanoylamino]
-b-D-glucopyranoside, which is also known as 529 (formerly
known as RC529), which is formulated as an aqueous form or
as a stable emulsion;
(7) synthetic polynueleotides such as oligonucleotides
containing CpG motif(s) (US 6,207,646);
(8) cytokines and chemokines (e.g., granulocyte
macrophage colony stimulating factor (GM-CSF), granulocyte
colony stimulating factor (G-CSF), macrophage colony
stimulating factor (M-CSF), colony stimulating factor
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-26-
(CSF), erythropoietin (EPO), interleukin 2 (IL-2), IL-3,
IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IL-18,
interferon gamma, interferon gamma inducing factor I
(IGIF), transforming growth factor beta, RANTES (regulated
upon activation, normal T-cell expressed and presumably
secreted), macrophage inflammatory proteins (e.g., NIP-1
alpha and NIP-1 beta), tumor necrosis factor (TNF),
costimulatory molecules B7-1 and B7-2, and Leishmania
elongation initiating factor (LEIF));
(9) complement, such as a trimer of complement
component C3d;
(10) toll-like receptor agonists, e.g., TLR4 agonists
such as glucopyranosyl lipid adjuvant (GLA);
(11) serum proteins, e.g., transferrin;
(12) viral coat proteins, e.g., rotavirus capsid VP6
protein; and
(13) block copolymer adjuvants, e.g., Hunter's
TITERMAX0 adjuvant (VAXCEL, Inc. Norcross, GA).
[0041] Muramyl peptides include, but are not limited to, N-
acetyl-muramyl-L-threonyl-D-isoglutamine (thr-MDP),
N-
acetyl-normuramyl-L-alanine-2-(1'-21dipalmitoyl-sn-glycero-
3-hydroxyphosphoryloxy)-ethylamine (MTP-PE), etc.
[0042] Protein adjuvants of the present invention can be
delivered in the form of the protein themselves or of
nucleic acid molecules encoding such proteins using the
techniques described herein.
[0043] In certain embodiments, the adjuvant includes an
aluminum salt. The aluminum salt adjuvant may be an alum-
precipitated vaccine or an alum-adsorbed vaccine. Aluminum-
salt adjuvants are well-known in the art and are described,
for example, in Harlow & Lane ((1988) Antibodies: A
Laboratory Manua], Cold Spring Harbor Laboratory) and
Nicklas ((1992) Res. Immunol. 143:489-493). The aluminum
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-27-
salt includes, but is not limited to, hydrated alumina,
alumina hydrate, alumina trihydrate (ATH), aluminum
hydrate, aluminum trihydrate, aluminum (III) hydroxide,
aluminum hydroxyphosphate sulfate, Aluminum Phosphate
Adjuvant (APA), amorphous alumina, trihydrated alumina, or
trihydroxyaluminum.
[0044] APA is an aqueous suspension of aluminum
hydroxyphosphate. APA is manufactured by blending aluminum
chloride and sodium phosphate in a 1:1 volumetric ratio to
precipitate aluminum hydroxyphosphate. After the blending
process, the material is size-reduced with a high-shear
mixer to achieve a monodisperse particle size distribution.
The product is then diafiltered against physiological
saline and steam sterilized.
[0045] In certain embodiments, a commercially available
Al(OH)3 (e.g., aluminum hydroxide gel sold under the
tradename Alhydrogele) is used to adsorb proteins in a
ratio of 50-200 ug protein/mg aluminum hydroxide.
Adsorption of protein is dependent, in another embodiment,
on the pI (Isoelectric pH) of the protein and the pH of the
medium. A protein with a lower pI adsorbs to the positively
charged aluminum ion more strongly than a protein with a
higher pI. Aluminum salts may establish a depot of antigen
that is released slowly over a period of 2-3 weeks, be
involved in nonspecific activation of macrophages and
complement activation, and/or stimulate innate immune
mechanism (possibly through stimulation of uric acid). See,
e.g., Lambrecht, et al. (2009) Curr. Opin. Immunol. 21:23.
[0046] In some embodiments, the adjuvant is a mixture of 2,
3, or more of the above adjuvants, e.g., SBAS2 (an oil-in-
water emulsion also containing 3-deacylated monophosphoryl
lipid A and QS-21); or alum in combination with GLA
(AL019).
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-28-
[0047] The multivalent immunogenic composition of the
invention can be formulated as single dose vials, multi-
dose vials or as pre-filled glass or plastic syringes.
[0048] In one embodiment, multivalent immunogenic
compositions of the present invention are administered
orally, and are thus formulated in a form suitable for oral
administration, i.e., as a solid or a liquid preparation.
Solid oral formulations include tablets, capsules, pills,
granules, pellets and the like. Liquid oral formulations
include solutions, suspensions, dispersions, emulsions,
oils and the like.
[0049] Pharmaceutically acceptable carriers for liquid
formulations are aqueous or non-aqueous solutions,
suspensions, emulsions or oils. Examples of nonaqueous
solvents are propylene glycol, polyethylene glycol, and
injectable organic esters such as ethyl oleate. Aqueous
carriers include water, alcoholic/aqueous solutions,
emulsions or suspensions, including saline and buffered
media. Examples of oils are those of animal, vegetable, or
synthetic origin, for example, peanut oil, soybean oil,
olive oil, sunflower oil, fish-liver oil, another marine
oil, or a lipid from milk or eggs.
[0050] The pharmaceutical composition may be isotonic,
hypotonic or hypertonic. However, it is often preferred
that a composition for infusion or injection is essentially
isotonic, when it is administrated. Hence, storage of the
composition may preferably be isotonic or hypertonic. If
the composition is hypertonic for storage, it may be
diluted to become an isotonic solution prior to
administration.
[0051] The isotonic agent may be an ionic isotonic agent
such as a salt or a non-ionic isotonic agent such as a
carbohydrate. Examples of ionic isotonic agents include but
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-29-
are not limited to NaCl, CaCl2, KC1 and MgCl2. Examples of
non-ionic isotonic agents include but are not limited to
mannitol, sorbitol and glycerol.
[0052] It is also preferred that at least one
pharmaceutically acceptable additive is a buffer. For some
purposes, for example, when the composition is meant for
infusion or injection, it is often desirable that the
composition includes a buffer, which is capable of
buffering a solution to a pH in the range of 4 to 10, such
as 5 to 9, for example 6 to 8.
[0053] The buffer may, for example, be selected from Tris,
acetate, glutamate, lactate, maleate, tartrate, phosphate,
citrate, carbonate, glycinate, histidine,
glycine,
succinate and triethanolamine buffer. The buffer may be
selected from USP compatible buffers for parenteral use, in
particular, when the formulation is for parenteral use. For
example the buffer may be selected from the group of,
monobasic acids such as acetic, benzoic, gluconic, glyceric
and lactic; dibasic acids such as aconitic, adipic,
ascorbic, carbonic, glutamic, malic, succinic and tartaric,
polybasic acids such as citric and phosphoric; and bases
such as ammonia, diethanolamine, glycine, triethanolamine,
and Tris.
[0054] Parenteral vehicles (for subcutaneous, intravenous,
intraarterial, or intramuscular injection) include sodium
chloride solution, Ringer's dextrose, dextrose and sodium
chloride, lactated Ringer's and fixed oils. Intravenous
vehicles include fluid and nutrient replenishers,
electrolyte replenishers such as those based on Ringer's
dextrose, and the like. Examples are sterile liquids such
as water and oils, with or without the addition of a
surfactant and other pharmaceutically acceptable adjuvants.
In general, water, saline, aqueous dextrose and related
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-30-
sugar solutions, glycols such as propylene glycols or
polyethylene glycol, Polysorbate 80 (PS-80), Polysorbate 20
(PS-20), and Poloxamer 188 (P188) are preferred liquid
carriers, particularly for injectable solutions. Examples
of oils are those of animal, vegetable, or synthetic
origin, for example, peanut oil, soybean oil, olive oil,
sunflower oil, fish-liver oil, another marine oil, or a
lipid from milk or eggs.
[0055] The formulations of the invention may also contain a
surfactant. Preferred surfactants include, but are not
limited to, the polyoxyethylene sorbitan esters
surfactants, especially PS-20 and PS-80; copolymers of
ethylene oxide (EO), propylene oxide (PO), and/or butylene
oxide (BO), sold under the tradename DOWFAXTM, such as
linear BO/PO block copolymers; octoxynols, which can vary
in the number of repeating ethoxy (oxy-1,2-ethanediy1)
groups, with octoxynol-9 (Triton X-100, or t-
octylphenoxypolyethoxyethanol) being of
particular
interest; (octylphenoxy)polyethoxyethanol (IGEPAL CA-
630/NP-40); phospholipids such as phosphatidylcholine
(lecithin); nonylphenol ethoxylates, such as the Tergitolm
NP series; polyoxyethylene fatty ethers derived from
lauryl, cetyl, stearyl and oleyl alcohols (known as Brij
surfactants), such as triethyleneglycol monolauryl ether
(Brij 30); and sorbitan esters, such as sorbitan trioleate
and sorbitan monolaurate. A preferred surfactant for
including in the emulsion is PS-80.
[0056] Mixtures of surfactants can be used. A combination
of a polyoxyethylene sorbitan ester such as polyoxyethylene
sorbitan monooleate (PS-80) and an octoxynol such as t-
octylphenoxypolyethoxyethanol is also suitable. Another
useful combination comprises laureth 9 plus a
polyoxyethylene sorbitan ester and/or an octoxynol.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-31-
[0057] Poloxamer may also be used in the compositions of
the invention. A poloxamer is a nonionic triblock copolymer
composed of a central hydrophobic chain of polyoxypropylene
(poly(propylene oxide)) flanked by two hydrophilic chains
of polyoxyethyiene (poly(ethylene oxide)). Poloxamers are
also known by the tradename Pluronice. Because the lengths
of the polymer blocks can be customized, many different
poloxamers exist that have slightly different properties.
For the generic term "poloxamer", these copolymers are
commonly named with the letter "P" (for poloxamer) followed
by three digits, the first two digits x 100 give the
approximate molecular mass of the polyoxypropylene core,
and the last digit x10 gives the percentage polyoxyethylene
content (e.g., P407=Poloxamer with a polyoxypropylene
molecular mass of 4,000 g/mol and a 70% polyoxyethylene
content). For the Pluronice tradename, coding of these
copolymers starts with a letter to define its physical form
at room temperature (L=liquid, P=paste, F=flake (solid))
followed by two or three digits. The first digit (two
digits in a three-digit number) in the numerical
designation, multiplied by 300, indicates the approximate
molecular weight of the hydrophobe; and the last digit x10
gives the percentage polyoxyethylene content (e.g., L6lis a
Pluronice with a polyoxypropylene molecular mass of 1,800
g/mol and a 10% polyoxyethylene content). See US 3,740,421.
[0058] Preferably, the poloxamer generally has a molecular
weight in the range from 1100 to 17,400 Da, from 7,500 to
15,000 Da, or from 7,500 to 10,000 Da. The poloxamer can be
selected from poloxamer 188 or poloxamer 407. The final
concentration of the poloxamer in the formulations is from
0.001% to 5% weight/volume, or 0.025% to 1% weight/volume.
In certain aspects, the polyol is propylene glycol and is
at final concentration from 1% to 20% weight/volume. In
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-32-
certain aspects, the polyol is polyethylene glycol 400 and
is at final concentration from 1% to 20% weight/volume.
[0059] Suitable polyols for the formulations of the
invention are polymeric polyols, particularly polyether
diols including, but are not limited to, propylene glycol
and polyethylene glycol, Polyethylene glycol monomethyl
ethers. Propylene glycol is available in a range of
molecular weights of the monomer from about 425 to about
2700. Polyethylene glycol and Polyethylene glycol
monomethyl ether is also available in a range of molecular
weights ranging from about 200 to about 35000 including but
not limited to PEG200, PEG300, PEG400, PEG1000, PEG MME
550, PEG MME 600, PEG MME 2000, PEG MME 3350 and PEG MME
4000. A preferred polyethylene glycol is polyethylene
glycol 400. The final concentration of the polyol in the
formulations of the invention may be 1% to 20%
weight/volume or 6% to 20% weight/volume.
[0060] The formulation may also contain a pH-buffered
saline solution. The buffer may, for example, be selected
from the group consisting of Tris, acetate, glutamate,
lactate, maleate, tartrate, phosphate, citrate, carbonate,
glycinate, histidine, glycine, succinate, HEPES (4-(2-
hydroxyethyl)-1-piperazineethanesulfonic acid), MOPS (3-(N-
morpholino)propanesulfonic acid). MES
(2-(N-
morpholino)ethanesulfonic acid) and triethanolamine buffer.
The buffer is capable of buffering a solution to a pH in
the range of 4 to 10, 5.2 to 7.5, or 5.8 to 7Ø In certain
aspects of the invention, the buffer is selected from the
group of phosphate, succinate, histidine, MES, MOPS, HEPES,
acetate or citrate. The buffer may furthermore, for
example, be selected from USP compatible buffers for
parenteral use, in particular, when the pharmaceutical
formulation is for parenteral use. The concentrations of
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-33-
buffer will range from 1 mM to 100 mM. The concentrations
of buffer will range from 10 mM to 80 mM. The
concentrations of buffer will range from 1 mM to 50 mM or 5
mM to 50 mM.
[0061] While the saline solution (i.e., a solution
containing NaCl) is preferred, other salts suitable for
formulation include but are not limited to, CaCl2, KC1 and
MgCl2 and combinations thereof. Non-ionic isotonic agents
including but not limited to sucrose, trehalose, mannitol,
sorbitol and glycerol may be used in lieu of a salt.
Suitable salt ranges include, but are not limited to 25 mM
to 500 mM or 40 mM to 170 mM. In one aspect, the saline is
NaCl, optionally present at a concentration from 20 mM to
170 mM.
[0062] In some aspects, the composition of the invention is
administered to a subject by one or more methods known to a
person skilled in the art, such as parenterally,
transmucosally, transdermally,
intramuscularly,
intravenously, intra-dermally,
intra-nasally,
subcutaneously, intra-peritonealy, and
formulated
accordingly. In one embodiment, a composition of the
present invention is administered via epidermal injection,
intramuscular injection, intravenous, intra-arterial,
subcutaneous injection, or intra-respiratory mucosal
injection of a liquid preparation. Liquid formulations for
injection include solutions and the like.
[0063] One embodiment of the present invention is a
controlled release formulation that is capable of slowly
releasing a composition of the present invention into an
animal. As used herein, a controlled release formulation
includes a composition of the present invention in a
controlled release vehicle. Suitable controlled release
vehicles include, but are not limited to, biocompatible
CA 03167346 2022- 8-8
WO 2021/163448 PCT/US2021/017813
-34-
polymers, other polymeric matrices,
capsules,
microcapsules, microparticles, bolus preparations, osmotic
pumps, diffusion devices, liposomes, lipospheres, and
transdermal delivery systems. Other controlled release
formulations of the present invention include liquids that,
upon administration to an animal, form a solid or a gel in
situ. Preferred controlled release formulations are
biodegradable (i.e., bioerodible).
[0064] A preferred controlled release formulation is
capable of releasing an immunogenic composition of the
present invention into the blood of the treated animal at a
constant rate sufficient to attain therapeutic dose levels
of the composition to protect an animal from disease caused
by a filarial nematode. For example, the immunogenic
composition can be administered using intravenous infusion,
a transdermal patch, liposomes, or other modes of
administration. In another embodiment, polymeric materials
are used, e.g., in microspheres in or an implant. The
immunogenic composition is preferably released over a
period of time ranging from about 1 to about 12 months. A
controlled release formulation of the present invention is
capable of effecting a treatment preferably for at least
about 1 month, more preferably for at least about 3 months,
even more preferably for at least about 6 months, even more
preferably for at least about 9 months, and even more
preferably for at least about 12 months.
[0065] Immunogenic compositions or vaccines of the present
invention can be administered to animals prior to infection
in order to prevent infection and/or can be administered to
animals after infection in order to treat disease caused by
a filarial nematode. For example, proteins, nucleic acids
and mixtures thereof can be used as immunotherapeutic
agents. Acceptable protocols to administer compositions in
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-35-
an effective manner include individual dose size, number of
doses, frequency of dose administration, and mode of
administration. Determination of such protocols can be
accomplished by those skilled in the art. A suitable single
dose is a dose that is capable of protecting an animal from
disease when administered one or more times over a suitable
time period. For example, a preferred single dose of a
protein-based vaccine is from about 1 microgram (pg) to
about 10 milligrams (mg) of protein-based vaccine per
kilogram body weight of the animal. Booster vaccinations
can be administered from about 2 weeks to several years
after the original administration. Booster administrations
preferably are administered when the immune response of the
animal becomes insufficient to protect the animal from
disease. A preferred administration schedule is one in
which from about 10 pg to about 1 mg of the vaccine per kg
body weight of the animal is administered from about one to
about two times over a time period of from about 2 weeks to
about 12 months. Modes of administration can include, but
are not limited to, subcutaneous, intradermal, intravenous,
intranasal, oral, transdermal and intramuscular routes.
[0066] Wherein the immunogenic composition includes a
nucleic acid molecule, the immunogenic composition can be
administered to an animal in a fashion to enable expression
of that nucleic acid molecule into a protective protein in
the animal. Nucleic acid molecules can be delivered to an
animal in a variety of methods including, but not limited
to, administering a naked (i.e., not packaged in a viral
coat or cellular membrane) nucleic acid as a genetic
vaccine (e.g., as naked DNA molecules, such as is taught,
for example in Wolff, et al. (1990) Science 247:1465-1468);
or administering a nucleic acid molecule packaged as a
recombinant virus vaccine or as a recombinant cell vaccine
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-36-
(i.e., the nucleic acid molecule is delivered by a viral or
cellular vehicle).
[0067] A genetic (i.e., naked nucleic acid) vaccine of the
present invention includes a nucleic acid molecule of the
present invention and preferably includes a recombinant
molecule of the present invention that preferably is
replication, or otherwise amplification, competent. A
genetic vaccine of the present invention can include one or
more nucleic acid molecules of the present invention in the
form of, for example, a dicistronic recombinant molecule.
Preferred genetic vaccines include at least a portion of a
viral genome (i.e., a viral vector). Preferred viral
vectors include those based on alphaviruses, poxviruses,
adenoviruses, herpesviruses, picornaviruses,
and
retroviruses, with those based on alphaviruses (such as
sindbis or Semliki forest virus), species-specific
herpesviruses and poxviruses being particularly preferred.
Any suitable transcription control sequence can be used,
including those disclosed as suitable for protein
production. Particularly preferred transcription control
sequences include cytomegalovirus immediate early
(preferably in conjunction with Intron-A), Rous sarcoma
virus long terminal repeat, and tissue-specific
transcription control sequences, as well as transcription
control sequences endogenous to viral vectors if viral
vectors are used. The incorporation of a "strong"
polyadenylation signal is also preferred.
[0068] Genetic vaccines of the present invention can be
administered in a variety of ways, including intramuscular,
subcutaneous, intradermal, transdermal, intranasal and oral
routes of administration. Moreover, it is contemplated that
the vaccine can be delivered by gene gun, skin patch,
electroporation, or nano-based delivery. In this respect,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-37-
DNA-based and protein-based vaccines can be administered at
the same time. A preferred single dose of a genetic vaccine
ranges from about 1 nanogram (ng) to about 600 rig,
depending on the route of administration and/or method of
delivery, as can be determined by those skilled in the art.
Suitable delivery methods include, for example, by
injection, as drops, aerosolized and/or topically. Genetic
vaccines of the present invention can be contained in an
aqueous excipient (e.g., phosphate-buffered saline) alone
or in a carrier (e.g., lipid-based vehicles).
[0069] A recombinant virus vaccine of the present invention
includes a recombinant molecule of the present invention
that is packaged in a viral coat and that can be expressed
in an animal after administration. Preferably, the
recombinant molecule is packaging- or replication-deficient
and/or encodes an attenuated virus. A number of recombinant
viruses can be used, including, but not limited to, those
based on alphaviruses, poxviruses,
adcnoviruses,
herpesviruses, picornaviruses, and retroviruses. Preferred
recombinant virus vaccines are those based on alphaviruses
(such as Sindbis virus), raccoon poxviruses, species-
specific herpesviruses and species-specific poxviruses.
Examples of methods to produce and use alphavirus
recombinant virus vaccines are disclosed in PCT Publication
No. NO 94/17013.
[0070] When administered to an animal, a recombinant virus
vaccine of the present invention infects cells within the
immunized animal and directs the production of a protective
protein that is capable of protecting the animal from
filariasis caused by filarial nematodes. By way of
illustration, a single dose of a recombinant virus vaccine
of the present invention can be from about 1X104 to about
1X108 virus plague forming units (pfu) per kilogram body
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-38-
weight of the animal. Administration protocols are similar
to those described herein for protein-based vaccines, with
subcutaneous, intramuscular, intranasal and oral as routes
of administration.
[0071] A recombinant cell vaccine of the present invention
includes recombinant cells of the present invention that
express a protein of the present invention. Preferred
recombinant cells for this embodiment include Salmonella,
E. coli, Listeria, Mycobacterium, S. frugiperda, yeast,
(including Saccharomyces cerevisiae and Pichia pastoris),
BHK, CV-1, myoblast G8, COS (e.g., COS-7), Vero, MDCK and
CRFK recombinant cells. Recombinant cell vaccines of the
present invention can be administered in a variety of ways
but have the advantage that they can be administered
orally, preferably at doses ranging from about 108 to about
1012 cells per kilogram body weight. Administration
protocols are similar to those described herein for
protein-based vaccines. Recombinant cell vaccines can
include whole cells, cells stripped of cell walls or cell
lysates.
[0072] In some embodiments of the composition of the
invention, all of the antigens are present in the
composition in the same amount. In further embodiments, the
antigens are present in the composition in different
amounts (i.e., at least one antigen is present in an amount
that is different than one or more of the other antigens of
the composition).
[0073] Optimal amounts of components for a particular
immunogenic composition can be ascertained by standard
studies involving observation of appropriate immune
responses in subjects. For example, in another embodiment,
the dosage for human vaccination is determined by
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-39-
extrapolation from animal studies to human data. In another
embodiment, the dosage is determined empirically.
[0074] As is known in the art, there are three groups of
filarial nematodes, classified according to the niche
within the body that they occupy: lymphatic filariasis,
subcutaneous filariasis, and serous cavity filariasis.
Lymphatic filariasis is caused by the worms W. banclufli,
B. malayi and B. timori. These worms occupy the lymphatic
system, including the lymph nodes, and cause fever,
lymphadenitis (swelling of the lymph nodes), lymphangitis
(inflammation of the lymphatic vessels in response to
infection), and lymphedema (elephantiasis). Subcutaneous
filariasis may be caused by Loa loa (the African eye worm),
Mansonella stretocerca, 0. volvulus,
Diaouhculus
medinensis, or Dirofilaria immitis. Many of these worms
occupy the subcutaneous layer of the skin, in the fat
layer, and present with skin rashes, urticarial papules,
and arthritis, as well as hyper- and hypopigmentation
macules. Onchocerca volvulus manifests itself in the eyes,
causing "river blindness." Adult Dirofilaria immitis reside
in pulmonary arteries and are the causal agent of heartworm
disease. Serous cavity filariasis is caused by the worms M.
perstans and M. ozzardi, which occupy the serous cavity of
the abdomen. Serous cavity filariasis presents with
symptoms similar to subcutaneous filariasis, in addition to
abdominal pain, because these worms are also deep tissue
dwellers.
[0075] Dogs infected with Brugia malayi develop clinical
lymphedema, scrotal enlargement, conjunctivitis and
lymphagitis similar to the human lymphatic filariasis;
however, the pathology is not as severe as in the human.
Since dogs carry the infection in the nature, humans can
get the Brugia malayi infections from dogs. Thus, zoonotic
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-40-
infections are common in the endemic areas, where dogs and
cats carry the infection in the nature and they transmit
the infection to the humans. Dogs and cats can also be
infected with Brugia malayi under laboratory conditions.
Thus, an immunogenic composition developed against
lymphatic filariasis in dogs are also important in blocking
transmission of the disease in the human.
[0076] The efficacy of a multivalent immunogenic
composition of the present invention to protect an animal
from filariasis or dirofilariasis caused by filarial
nematodes can be tested in a variety of ways including, but
not limited to, detection of protective antibodies (using,
for example, proteins of the present invention), detection
of cellular immunity within the treated animal, and/or
challenge of the treated animal with the a filarial
nematode Lo determine whether the treated animal is
resistant to disease and fails to exhibit one or more signs
of disease. Challenge studies can include implantation of
chambers including filarial nematode larvae into the
treated animal and/or direct administration of larvae to
the treated animal. In one embodiment, therapeutic
compositions can be tested in animal models such as mice,
jirds (Meriones unguiculatus), mastomys (e.g., Mastamys
natalensis) and/or dogs. Such techniques are known to those
skilled in the art.
[0077] To detect the presence/amount of anti-filarial
nematode antibodies, e.g., protective or neutralizing
antibodies resulting from the vaccination of an animal,
this invention also provides a method and kit for efficacy
evaluation, as well as for detecting prior exposure to
filarial proteins and/or infection with a filarial
nematode. In accordance with such a method, one or more
antigenic proteins/epitopes is contacted with a biological
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-41-
sample from an animal and binding between the antigenic
proteins/epitopes and antibodies in the biological sample
is quantitively or qualitatively determined as described
herein, wherein the presence and/or amount of antibodies to
the antigenic proteins/epitopes is indicative of vaccine
efficacy, as well as prior exposure to filarial proteins or
an existing infection with a filarial nematode. In certain
embodiments, the method and kit use an array-based format
in which serial dilutions of one or more antigens or
epitopes are printed. In some embodiments, the one or more
of the filarial nematode proteins are present on one or
more solid surfaces or particles. In other embodiments, the
one or more of the filarial nematode proteins are in an
array so that the presence of multiple antibodies can be
assessed in a single assay due to the multiplexing
capability of an array-based approach. In this respect, the
array can contain one or more of ALT2, TSP, VAL-1, TPX2,
GST or HSP protein or an epitope thereof. In other
embodiments, the array at least contains each of the
proteins used in the multivalent immunogenic composition.
For example, to assay for protective or neutralizing
antibodies against a multivalent immunogenic composition
containing HSP, ALT2 and TSP, the array would contain HSP,
ALT2 and TSP, or a fusion protein thereof.
[0078] For testing for the presence of a filarial nematode,
this invention also provides a method and kit for detecting
a filarial nematode. The assay method generally includes
the steps of contacting, in vitro, a biological sample with
one or more binding agents against filarial nematode
proteins selected from the group of ALT2, TSP, VAL-1, TPX2,
GST and HSP or fragments thereof. The bound binding agents
are then detected. The bound binding agents can be detected
using automated detection of binding such as an image
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-42-
reader of an ELISA assay, and if a bound binding agent is
detected, the data indicating that a bound binding agent
has been detected can be transferred, e.g., to a computer
display or on a paper print out. Detection of a filarial
nematode protein indicates that the sample or subject from
which the sample was obtained has filariasis. Therefore,
detection allows selection of treatment options for the
subject. Thus, in one embodiment, if one or more of ALT2,
TSP, VAL-1, TPX2, GST and HSP is detected, the patient will
be given a treatment suitable for filariasis, including but
not limited to treatment with diethylcarbamazine,
mebendazole, flubendazole, albendazole, ivermectin or a
combination thereof.
[0079] A biological sample is any material to be tested for
the presence or amount of a protein of interest (e.g., an
antibody or antigen/epitope). The sample can be a fluid
sample, preferably a liquid sample. Examples of liquid
samples that may be tested in accordance with this
invention include bodily fluids including blood, serum,
plasma, saliva, urine, ocular fluid, semen, and spinal
fluid. Viscous liquid, semi-solid, or solid specimens
(e.g., human tissue, or mosquito or fly tissue) may be used
to create liquid solutions, eluates, suspensions, or
extracts that can be samples. In some embodiments, the
biological sample is undiluted. In other embodiments, the
sample is diluted or concentrated depending on the
detection application.
[0080] In certain embodiments, one can concentrate the
proteins in the sample by using a solid surface coated with
a monoclonal antibody to capture the protein. The recovered
captured proteins can then be analyzed using any suitable
method described herein. The solid surface can be, e.g.,
beads, such as magnetic beads, polystyrene beads, or gold
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-43-
beads, or in an array or a microarray format using a glass,
a plastic or a silicon chip. Such protein capture can be
also a part of a channel in a microfluidic device.
[0081] Binding agents of use in this invention include an
antibody, an antibody fragment, or an antibody derivative
(e.g., an aptamer) which specifically binds to a cognate
filarial nematode protein. Specific binding between two
entities generally refers to an affinity of at least 106,
107, 108, 109, or 1010 M-1. Affinities greater than 108 M-1 are
desired to achieve specific binding.
[0082] When the binding agent is an antibody, the antibody
can be produced by natural (i.e., immunization) or partial
or wholly synthetic means. Antibodies can be monoclonal or
polyclonal and include commercially available antibodies.
An antibody can be a member of any immunoglobulin class,
including any of the human classes: IgG, IgM, IgA, IgD, and
IgE. Bispecific and chimeric antibodies are also
encompassed within the scope of the present invention.
Derivatives of the IgG class, however, are desirable.
Further, an antibody can be of human, mouse, rat, goat,
sheep, rabbit, chicken, camel, or donkey origin or other
species which may be used to produce native or human
antibodies (i.e., recombinant bacteria, baculovirus or
plants).
[0083] For example, naturally-produced
monoclonal
antibodies can be generated using classical cloning and
cell fusion techniques or techniques wherein B-cells are
captured and nucleic acids encoding a specific antibody are
amplified (see, e.g., US 20060051348). In such methods, a
collection of proteins or an individual protein (e.g., a
peptide or polypeptide) can be used for the initial
immunization and in the context of antibody production is
referred to herein as the antigen. The antigen of interest
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-44-
is typically administered (e.g., intraperitoneal injection)
to wild-type or inbred mice (e.g., BALB/c) or rats,
rabbits, chickens, sheep, goats, or other animal species
which can produce native or human antibodies. The antigen
can be administered alone, or mixed with an adjuvant. After
the animal is boosted, for example, two or more times, the
spleen or large lymph node, such as the popliteal in rat,
is removed and splenocytes or lymphocytes are isolated and
fused with myeloma cells using well-known processes, for
example, see Kohler & Milstein ((1975) Nature 256:495-497)
or Harlow & Lane (Antibodies: A Laboratory Manual (Cold
Spring Harbor Laboratory, New York (1988)). The resulting
hybrid cells are then cloned in the conventional manner,
e.g., using limiting dilution, and the resulting clones,
which produce the desired monoclonal antibodies, are
cultured (see Stewart (2001) Monoclonal Antibody
Production. In: Basic Methods in Antibody Production and
Characterization, Howard and Bothell (eds.), CRC Press,
Boca Raton, FL, pp.51-67).
[0084] Alternatively, antibodies can be derived by a phage
display method. Methods of producing phage display
antibodies are known in the art, e.g., see Huse, et al.
((1989) Science 246(4935):1275-81). Selection of antibodies
is based on binding affinity to a protein or proteins of
interest.
[0085] An antibody fragment encompasses at least a
significant portion of the full-length antibody's specific
binding ability. Examples of antibody fragments include,
but are not limited to, Fab, Fab', F(ab')2, scFv, Fv, dsFv,
diabody, Fd fragments or microbodies. An antibody fragment
can contain multiple chains which are linked together, for
instance, by disulfide linkages. A fragment can also
optionally be a multi-molecular complex. A functional
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-45-
antibody fragment will typically include at least about 50
amino acid residues and more typically will include at
least about 200 amino acid residues. The antibody fragment
can be produced by any means. For instance, the antibody
fragment can be enzymatically or chemically produced by
fragmentation of an intact antibody or it can be
recombinantly-produced from a gene encoding the partial
antibody sequence. Alternatively, the antibody fragment can
be wholly or partially synthetically-produced.
[0086] Peptide aptamers which specifically bind to a
protein are, in general, rationally designed or screened
for in a library of aptamers (e.g., provided by Aptanomics
SA, Lyon, France). In general, peptide aptamers are
synthetic recognition molecules whose design is based on
the structure of antibodies. Peptide aptamers are composed
of a variable peptide loop attached at both ends to a
protein scaffold. This double structural constraint greatly
increases the binding affinity of the peptide aptamer to
levels comparable to that of an antibody (nanomolar range).
[0087] Recombinant production of binding agents of this
invention can be achieved using conventional molecular
biology techniques and commercially available expression
systems. Furthermore, binding agents can be produced using
solid-phase techniques (see, e.g., Merrifield (1963) J. Am.
Chem. Soc. 85:2149-2154; Seeberger (2003) Chem. Commun.
(Comb) (10):1115-21). Protein synthesis can be performed
using manual techniques or by automation. Automated
synthesis can be achieved, for example, using Applied
Biosystems 431A Peptide Synthesizer (Perkin Elmer, Boston,
MA). Various fragments of a binding agent can be
chemically-synthesized separately and combined using
chemical methods to produce a full-length molecule.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-46-
[0088] Moreover, combinatorial chemistry approaches can be
used to produce binding agents (see, e.g., Lenssen, et al.
(2002) Chembiochem. 3(9):852-8; Khersonsky, et al. (2003)
Curr. Top. Med. Chem. 3(6):617-43; Anthony-Cahill &
Magliery (2002) Curr. Pharm. Biotechnol. 3(4):299-315).
[0089] The binding agents described herein can be labeled.
In some embodiments, the binding agent is an antibody
labeled by covalently linking the antibody to a direct or
indirect label. A direct label can be defined as an entity,
which in its natural state, is visible either to the naked
eye or with the aid of an optical filter and/or applied
stimulation, e.g., ultraviolet light, to promote
fluorescence. Examples of colored labels which can be used
include metallic sal particles, gold sal particles, dye sal
particles, dyed latex particles or dyes encapsulated in
liposomes. Other direct labels include radionuclides and
fluorescent or luminescent moieties.
[0090] Indirect labels such as enzymes can also be used
according to the invention. Various enzymes are known for
use as labels such as, for example, alkaline phosphatase,
horseradish peroxidase, lysozyme, glucose-6-phosphate
dehydrogenase, lactate dehydrogenase and urease. For a
detailed discussion of enzymes in immunoassays see Engvall
(1980) Methods of Enzymology 70:419-439.
[0091] The proteins described herein (i.e., antibodies or
antigens/epitopes) can be attached to a surface. Examples
of useful surfaces on which the protein can be attached for
diagnostic purposes include nitrocellulose,
PVDF,
polystyrene, nylon or other suitable plastic. The surface
or support may also be a porous support (see, e.g., US
7,939,342).
[0092] Further, the proteins of the invention can be
attached to a particle or bead. For example, antibodies to
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-47-
the filarial nematode proteins or the filarial nematode
proteins themselves can be conjugated to superparamagnetic
microparticles, e.g., as used in LUMINEX-based multiplex
assays.
[0093] The filarial nematode proteins of this invention may
be isolated and/or purified or produced synthetically or
using recombinant nucleic acid technology. The purification
may be partial or substantial. With reference to filarial
nematode protein fragments, the term "fragment" refers to a
protein having an amino acid sequence shorter than that of
the proteins described herein. Preferably, such fragments
are at least 5 consecutive amino acids long or up to 35
amino acids long. In certain embodiments, the protein
fragment includes at least one epitope. An "epitope" is a
feature of a molecule, such as primary, secondary and/or
tertiary peptide structure, and/or charge, that forms a
site recognized by an immunoglobulin, T cell receptor or
HLA molecule. Alternatively, an epitope can be defined as a
set of amino acid residues which is involved in recognition
by a particular immunoglobulin, or in the context of T
cells, those residues necessary for recognition by T cell
receptor proteins and/or Major Histocompatibility Complex
(MHC) receptors.
[0094] In some embodiments, the protein fragment of the
invention is a fragment of ALT2 comprising or consisting of
the epitope of SEQ ID NO:121, in particular epitopes of SEQ
ID NO:78 or SEQ ID NO:98. In other embodiments, the protein
fragment of the invention is a fragment of ALT2 comprising
or consisting of the epitope of SEQ ID NO:122, in
particular SEQ ID NO:79 or SEQ ID NO:99. In further
embodiments, the protein fragment of the invention is a
fragment of HSP comprising or consisting of the epitope of
SEQ ID NO:81 or SEQ ID NO:123, in particular SEQ ID NO:80
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-48-
or SEQ ID NO:100. In certain embodiments, the protein
fragment of the invention is a fragment of TSP comprising
or consisting of the epitope of SEQ ID NO:82. In other
embodiments, the protein fragment of the invention is a
fragment of TPX2 comprising or consisting of the epitope of
SEQ ID NO:83 or SEQ TD NO:124, in particular SEQ ID NO:84
or SEQ ID NO:101.
[0095] The fragments of the invention can be isolated,
purified or otherwise prepared/derived by human or non-
human means. For example, epitopes can be prepared by
isolating the filarial nematode protein fragment from a
bacterial culture, or they can be synthesized in accordance
with standard protocols in the art. Synthetic epitopes can
also be prepared from amino acid mimetics, such as D
isomers of natural occurring L amino acids or non-natural
amino acids such as cyclohexylalanine.
[0096] In some embodiments, the filarial nematode protein
or protein fragment is conjugated or fused to a high
molecular weight protein carrier to facilitate antibody
production. In some embodiments, the high molecular weight
protein is bovine serum albumin, thyroglobulin, ovalbumin,
fibrinogen, or keyhole limpet hemocyanin. A particularly
preferred carrier is keyhole limpet hemocyanin.
[0097] Any suitable immunoassay method may be used,
including those which are commercially available, to
determine the level of at least one of the specific
filarial nematode proteins, protein fragments or
protective/neutralizing antibodies according to the
invention. Extensive discussion of the known immunoassay
techniques is not required here since these are known to
those of skill in the art. Typical suitable immunoassay
techniques include sandwich enzyme-linked immunoassays
(ELISA), radioimmunoassays (RIA), competitive binding
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-49-
assays, homogeneous assays, heterogeneous assays, etc.
Various of the known immunoassay methods are reviewed,
e.g., in Methods in Enzymology (1980) 70:30-70 and 166-198.
[0098] In some embodiments, the immunoassay method or assay
includes a double antibody technique for measuring the
level of the filarial nematode proteins or protein
fragments in the biological sample. According to this
method one of the antibodies is a "capture" antibody and
the other is a "detector" antibody. The capture antibody is
immobilized on a solid support which may be any of various
types which are known in the art such as, for example,
microtiter plate wells, beads, tubes and porous materials
such as nylon, glass fibers and other polymeric materials.
In this method, a solid support, e.g., microtiter plate
wells, coated with a capture antibody, preferably
monoclonal, raised against the particular protein of
interest, constitutes the solid phase. The biological
sample, which may be diluted or not, typically at least 1,
2, 3, 4, 5, 10, or more standards and controls are added to
separate solid supports and incubated. When the protein of
interest is present in the sample it is captured by the
immobilized antibody which is specific for the protein in
question. After incubation and washing, a detector
antibody, e.g., a polyclonal rabbit anti-marker protein
antibody, is added to the solid support. The detector
antibody binds to the protein bound to the capture antibody
to form a sandwich structure. After incubation and washing
an anti-IgG antibody, e.g., a polyclonal goat anti-rabbit
IgG antibody, labeled with an enzyme such as horseradish
peroxidase (HRP) is added to the solid support. After
incubation and washing a substrate for the enzyme is added
to the solid support followed by incubation and the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-50-
addition of an acid solution to stop the enzymatic
reaction.
[0099] The degree of enzymatic activity of immobilized
enzyme is determined by measuring the optical density of
the oxidized enzymatic product on the solid support at, the -
appropriate wavelength, e.g., 450 nm for HRP. The
absorbance at the wavelength is proportional to the amount
of protein of interest in the sample. A set of marker
protein standards is used to prepare a standard curve of
absorbance vs. filarial nematode protein concentration.
This method is useful because test results can be provided
in 45 to 50 minutes and the method is both sensitive over
the concentration range of interest for each filarial
nematode protein and is highly specific.
[00100] The standards may be positive samples containing
various concentrations of the protein to be detected to
ensure that the reagents and conditions work properly for
each assay. The standards also typically include a negative
control, e.g., for detection of contaminants. In some
aspects of the embodiments of the invention, the positive
controls may be titrated to different concentrations,
including non-detectable amounts and clearly detectable
amounts, and in some aspects, also including a sample that
shows a signal at the threshold level of detection in the
biological sample.
[00101] The method of the invention can be carried out in
various assay device formats including those described in
US 6,426,050, US 5,910,287, US 6,229,603, and US 6,232,114
to Aurora Biosciences Corporation. The assay devices used
according to the invention can be arranged to provide a
quantitative or a qualitative (present/not present) result.
In some embodiments, the method includes the use of a
microtiter plate or a microfluidic device format. The
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-51-
assays may also be carried out in automated immunoassay
analyzers which are known in the art and which can carry
out assays on a number of different samples. These
automated analyzers include continuous/random access types.
Examples of such systems are described in US 5,207,987, US
5,518,688, US 6,448,089, and US 6,814,933. Various
automated analyzers that are commercially available include
the OPUS and OPUS MAGNUM analyzers.
[00102]Another assay format which can be used according to
the invention is a rapid manual test which can be
administered at the point-of-care at any location.
Typically, such point-of-care assay devices will provide a
result which is either "positive," i.e., showing the
protein is present, or "negative" showing that the protein
is absent. Typically, a control showing that the reagents
worked in general is included with such point-of-care
system. Point-of-care systems, assays and devices have been
well described for other purposes, such as pregnancy
detection (see, e.g., US 7,569,397 and US 7,959,875).
Accordingly, the invention also provides devices, such as
point-of-care test strips and microfluidic devices to
perform the in vitro assays of the present invention.
[00103] It should be recognized also that the assay devices
used according to the invention can be provided to carry
out one single assay for a particular protein or to carry
out a plurality of assays, from a single volume of body
fluid, for a corresponding number of different filarial
nematode proteins or antibodies thereto. In some
embodiments, an assay device of the latter type is one
which can provide a semi-quantitative result for the
filarial nematode protein or antibodies measured according
to the invention, i.e., one or more of ALT2, TSP, VAL-1,
TPX2, GST and HSP, or antibodies thereto. These devices
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-52-
typically are adapted to provide a distinct visually
detectable colored band at the location where the
particular protein of interest is located when the
concentration of the protein is above the threshold level.
For additional detailed discussion of assay types which can
be utilized according to the invention as well as various
assay formats and automated analyzer apparatus see, e.g.,
US 5,747,274. Filarial nematode protein detection can
further be performed using multiplex technologies.
[00104] In other embodiments, the assays or immunoassays of
the invention include beads coated with a binding agent
against a filarial nematode protein or a fragment thereof,
or antibody. Commonly used are polystyrene beads that can
be labeled to establish a unique identity. Detection is
performed by flow cytometry. Other types of bead-based
immunoassays are known in the art, e.g., laser bead
immunoassays and related magnetic bead assays (see, e.g.,
Fritzler, et al. (2009) Expert Opinion on Medical
Diagnostics 3:81-89).
[00105] The methods of the invention can be automated using
robotics and computer directed systems. The biological
sample can be injected into a system, such as a
microfluidic devise entirely run by a robotic station from
sample input to output of the result. The step of
displaying the result can also be automated and connected
to the same system or in a remote system. Thus, the sample
analysis can be performed in one location and the result
analysis in another location, the only connection being,
e.g., an internet connection, wherein the analysis is
subsequently displayed in a format suitable for either
reading by a health professional or by a patient.
[00106] In certain embodiments, the presence of any one or
any combination of protective/neutralizing antibodies
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-53-
described herein identifies a subject as having been
immunized with a multivalent immunogenic composition
against a filarial nematode. Thus, depending on antibody
titer, the subject may or may not receive additional
booster vaccinations.
[00107] In some embodiments, the presence of any one or any
combination of the filarial nematode proteins described
herein identifies a subject as having a filarial nematode
infection. Thus, the subject is diagnosed as having
filariasis and, in certain embodiments of this invention,
treated with diethylcarbamazine, mebendazole, flubendazole,
albendazole, ivermectin or a combination thereof. In one
embodiment, the diagnosis can be made if the presence of
any one of the filarial nematode proteins is detected in
the subject's sample. In another embodiment, treatment is
prescribed or administered if at least two of the filarial
nematode proteins are identified positively in the
biological sample.
[00108] Kits provided according to this invention include
one or more binding agents, e.g., antibodies or antibody
fragments, or filarial nematode proteins, and optionally a
device with a solid surface. In some embodiments, the solid
surface is a bead, slide, assay plate (e.g., a mulLiwell
plate) or a lateral flow device, to which the binding
agents/proteins are bound. In some embodiments, the kit
further includes one or more standards or controls.
[00109] In some embodiments, the invention provides a
microplate-based array for multiplex immunoassays. In
accordance with some embodiments, each well can contain a
single antibody against at least one of the listed filarial
nematode proteins. In other embodiments, each well contains
an array of antibodies against at least two or more of the
listed filarial nematode proteins. In certain embodiments,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-54-
each well of the plate includes an antibody to two, three,
four, or five of the following proteins: ALT2, TSP, VAL-1,
TPX2, GST and HSP. In particular embodiments, each well of
the plate includes an antibody to each of ALT2, TSP, VAL-1,
TPX2, GST and HSP.
[00110] In other embodiments, each well contains an array of
at least two or more of the filarial nematode proteins of
this invention. In certain embodiments, each well of the
plate includes two, three, four, or five of the following
proteins: ALT2, TSP, VAL-1, TPX2, GST and HSP. In
particular embodiments, each well of the plate includes
each of ALT2, TSP, VAL-1, TPX2, GST and HSP.
[00111] In other embodiments, the invention provides simple
to use point-of-care diagnostic test strips akin to
pregnancy detection strips, wherein the strip includes at
least one antibody against at least one of the listed
filarial nematode proteins. In alternative embodiments, the
invention provides simple to use point-of-care diagnostic
test strips, wherein the strip includes at least one of the
instant filarial nematode proteins.
[00112] The test strip may include a positive and negative
control to show the user that the reagents work properly
and/or that the sample has been added to the strip
properly. The strips may be provided with or without a
casing and with or without additional reagents. Diagnostic
test strips for lateral flow assays, such as the test strip
assay described herein, may be constructed as described in
the art, see, e.g., US 2010/0196200; US 2010/0129935; US
2009/0253119; and US 2009/0111171. Suitable materials for
test strips include, but are not limited to, materials
derived from cellulose, such as filter paper,
chromatographic paper, nitrocellulose, and cellulose
acetate, as well as materials made of glass fibers, nylon,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-55-
dacron, PVC, polyacrylamide, cross-linked dextran, agarose,
polyacrylate, ceramic materials, and the like. The material
or materials of the test strip may optionally be treated to
modify their capillary flow characteristics or the
characteristics of the applied sample. For example, the
sample application region of the test strip may be treated
with buffers to correct the pH or specific gravity of an
applied sample, to ensure optimal test conditions.
[00113] The invention is described in greater detail by the
following non-limiting examples.
Example 1: Small Heat Shock Protein Vaccine
[00114] Parasites. B. malayi L3s were obtained from the
NIAID/NIH Filariasis Research Reagent Resource Center (FR3)
at the University of Georgia, Athens, GA.
[00115] Human Sera Samples. About 10 ml of blood samples
were collected from the following clinical groups of
subjects (1) Endemic normal (EN) subjects, these were
individuals who were asymptomatic and non-microfilaraemie;
(2) asymptomatic microfilaraemic subjects (Mf) who had
circulating microfilaria in their blood and were identified
by microscopic examination of their night blood smears; (3)
Chronic Pathology (CP) patients include those subjects who
exhibited lymph edema and other chronic clinical symptoms
of filariasis and (4) Non-endemic normal subjects (NEN) who
lived in non-endemic areas and had no circulating parasites
or antibodies and showed no evidence of any filarial
disease. Sera were separated from these blood samples and
were stored at -80 C until use.
[00116] Expression and Purification of Recombinant B. malayi
Heat Shock Protein. To produce recombinant B. malayi small
heat shock protein 12.6 (rBmHSP), the full-length gene
sequence was cloned into pRSET-A (with an N-terminal
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-56-
hexahistidine tag) and was transformed into BL21(DE3)
containing pLysS (Invitrogen, Carlsbad, CA) to minimize
toxicity due to the protein. When absorbance of the
cultures reached 0.6 OD value, 1 mM of IPTG (isopropyl
thio-d-galactopyranoside) was added to the cultures and
incubated for an additional 3 hours to induce gene
expression. After lysing the cells, total proteins were
separated in a 15% SDS-PAGE to confirm the expression of
his-tagged protein. Subsequently, the histidine-tagged
recombinant protein was purified using an immobilized
cobalt metal affinity column chromatography (Clontech,
Mountain View, CA) as per the manufacturer's
recommendations. Recombinant protein was then separated in
a 15% SDS-PAGE and stained with COOMASSIE brilliant blue
R250. A single band was obtained after column purification.
[00117] Three-Dimensional Model of BmHSP. A
three-
dimensional model of BmHSP protein was constructed by
homology modeling. BLAST sequence homology searches were
performed to identify template proteins in the PDB
database. Human alpha-crystallin A, a recently crystallized
protein, showed significant sequence identity and was
therefore chosen as the template for modeling BmHSP. Model
building was performed using MODELLER 9v6 (Sali & Blundell
(1993) J. Mol. Biol. 234:779-815). The 3-0 structure
obtained was subsequently validated using PROCHECK program
(Laskowski, et al. (1993) J. Appl. Cryst. 26:283-29). The
best model predicted by PROCHECK had a score of -0.46 and
was chosen for further modeling and for generating the 3-D
structure using Rasmol program.
[00118] Analysis of the Structure of BmHSP. The secondary
structure and protein-protein interaction site of BmHSP was
predicted at PDBsum and the Predict Protein E-mail server
at the European Molecular Biology Laboratory, Heidelberg
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-57-
(Roos, et al. (1995) Parasitol. Today 11:148-150). Motif
scanning was carried out via PROSITE pattern analysis to
identify the functional motifs in BmHSP. B-cell, T-cell and
CTL epitopes in BmHSP sequences were predicted using Immune
Epitope Database and Analysis Resource (1EDB).
[00119] Phylogenetic Analysis of BmHSP. Amino acid sequences
of BmHSP were compared with members of other small heat:
shock family of proteins from different organisms. The
following sequences were analyzed. Accession numbers are
given in parenthesis. Aconthocheilonema vitae (CAA48631);
Archaeoglobus fulgidus (028308); Artibeus jamaicensis
(P02482); Aspergillus fumigatus (Q4WV00); Arabidopsis
thaliana (081822); Artemia persimilis
(DQ310578);
Azotobacter vinelandii (P96193); Brugia pahangi (CAA61152),
Brugia malayi (AAU04396); Buchnera aphidicola (P57640);
Bombyx mori (AF315318 1); Bradyrhizobium
japonicum
(P70918); Caenorhabditis elegans (Q7JP52); Coccidioides
immitis (Q1E6R4); Carica papaya (Q69BI7); Caenorhabditis
remanei (AAZ42349); Dictyostelium discoideum (Q54191);
Escherichia coil (ibpA; P00054); Escherichia coli (ibpB;
P00058); Homo sapiens (P02489); Raemonchus contortus
(AAN05752); Lygodactylus pdcturatus (Q6EWI0); Onchocercara
volvulus (0AA48633), Ostertagia ostergi (0AG25499); Macaca
mulatta (P02488); Mycobacterium tuberculosis (P0A5B7); Mus
musculus (AAA37861); Nippostrongy1 us
brasiliensis
(BAI81970); Plasmodium falciparum (Q8IB02);
Rattus
(CAA42910); Saccharomyces cerevisiae (P15992); Solanum
lycopersicum (082545); Streptococcus thermophilus (P80485);
Trichinella spiralis (ABJ55914); Trypanosoma bruced
(Q57V53); Toxoplasma gondii (Q6DUA8). The alpha-crystallin
domain from all sHSP sequences were aligned using ClustalW
algorithm and the data set were used to build a
phylogenetic tree with the PHYLIP software. The trees were
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-58-
made using the neighbor joining method, with Poisson-
corrected amino acid distances.
[00120] Chaperone Assay. One of the typical characteristics
of chaperone is that they can bind to and protect cellular
proteins from heat damage. When proteins are exposed to
heat damage, they aggregate (thermal aggregation).
Chaperones prevent this aggregation. To determine whether
BmHSP could prevent thermal aggregation, a citruline
synthase (CS) (Sigma, St. Louis, MO) thermal aggregation
assay was used. CS was selected because this protein is
highly sensitive to heat denaturation. An established
method was used (Gnanasekar, et al. (2009) Biochem.
Biophys. Res. Commun. 386:333-337). Briefly, 1 uM of CS was
exposed to 45 C in the presence or absence of BmHSP (2 uM)
suspended in 50 mM of sodium phosphate pH 7.4 buffer
containing 100 mM NaCl. BSA was used as a control. CS was
incubated with BmHSP at a molar ratio of (1:2) for various
time intervals from 0 to 40 minutes. Thermal denaturation
(aggregation) was monitored spectrophotometrically at 360
nm.
[00121] In Vitro Peptide Binding Assay for Chaperone
Activity. Another characteristic of heat shock proteins is
that they can bind to a variety of proteins. To determine
whether BmHSP also possesses this function, CS and another
protein, luciferase, were chemically denatured with 6M
guanidine hydrochloride according to known methods
(Gnanasekar, et al. (2009) supra). Native and chemically
denatured proteins were then coated onto 96-well plates
overnight at 4 C. After washing with PBS, wells were
blocked with 3% BSA at room temperature. Following further
washing, wells were incubated with his-tagged rBmHSP for 1
hour at 37 C. After washing again with PBS, optimally
diluted anti-his-tagged HRP conjugate was added and
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-59-
incubated at 37 C for 1 hour. After final washing, color
was developed with ABTS
[2,2'-azinobis(3-
ethylbenzothiazoline-6-sulfonic acid)] and OD was measured
at 405 nm.
[00122] Anti-BmHSP Antibody Levels in Human Sera. A total of
20 sera samples belonging to different clinical groups such
as Mf, CP, EN and NEN were analyzed for the presence and
titer of anti-BmHSP IgG antibodies using an indirect ELISA
(Cheirmaraj, et al. (1992) J. Trop. Med. Hyg. 95:47-51).
Briefly, wells of a 96-well microtiter plate were coated
with rBmHSP (1 ug/m1) in carbonate buffer, pH 9.6,
overnight at 4 C and blocked with 3% BSA for 1 hour at
37 C. Sera samples were added to the wells and the plates
were incubated overnight at 4 C. After washing the wells,
HRP-labeled mouse anti-human IgG was added (1:5000) and
incubated further for 1 hour at 37 C. Color was developed
using ABTS substrate. Absorbance was measured at 405 nm in
a microplate reader (BIO-RAD, Hercules, CA). The isotype of
anti-BmHSP IgG antibodies in the sera of subjects was also
determined using an isotype-specific ELISA. Biotinylated
mouse monoclonal antihuman IgGl, TgG2, IgG3 and IgG4 were
used as the secondary antibodies and color was developed
with avidin-HRP conjugate (Sigma, St. Louis, MO) as the
secondary antibodies.
[00123] Cloning of Codon-Optimized BmHSP into pVAX Vector
for DNA Vaccine. Codon-optimized Bmhsp genes were cloned
into eukaryotic expression vector pVAX (Invitrogen) using
insert-specific primers (forward primer, 51-CGC GGA TCC ATG
GAA GAG AAG GTG GTG-3' (SEQ ID NO:1) containing BamHT site
and reverse primer, 5'-CCG GAA TTC TCA CTT GTC GTT GGT G-3'
(SEQ ID NO:2) containing EcoRI site). PCR parameters were
as follows: 94 C of denaturation for 30 seconds, 50 C of
primer annealing for 30 seconds, 72 C of primer extension
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-60-
for 30 seconds for 30 cycles; and a final extension of 5
minutes was performed at 72 C. Insert DNA was sequenced to
ensure authenticity of the cloned nucleotide sequence on
both strands. Plasmids were maintained and propagated in E.
coli TOP1OF' cells. Subsequently, plasmids were purified
using endotoxin-free plasmid extraction kit (Qiagen,
Hilden, Germany). DNA was analyzed by agarose gel
electrophoresis and quantified by spectrophotometry (OD
260/280, ratio> 1.8).
[00124] Immunization of Mice. Six-week-old male Balb/c mice
purchased from Charles River Laboratories were used in
these experiments. Humane use of animals in this study and
the protocol was approved by the IACUC committee at the
College of Medicine, University of Illinois Rockford. Each
group was composed of five (5) mice and all mice were
immunized intraperitoneally using three different
immunization regimens. Group A mice were immunized using a
prime-boost regimen. Mice were primed twice at two-week
intervals with 100 pg of endotoxin-free, codon-optimized
pVAX Bmhsp DNA suspended in 50 pl volume. Following
priming, all mice received two booster doses of 15 pg of
rBmHSP protein (50 pl each) suspended in alum at two weeks
interval. Group B mice were immunized with rBmHSP protein
alone. These mice received four doses of 15 pg of rBmHSP
protein suspended in alum given at two-week intervals.
Group C mice were immunized with DNA alone. These mice
received four doses of 100 pg of pVAX Bmhsp DNA given at
two-week intervals. Group D animals received 100 pg of pVAX
vector control and adjuvant at the same interval and
remained as negative controls. Blood samples were collected
from each mouse before immunization and one month after the
last booster dose. After separating the sera, titer of
circulating anti-BmHSP IgG antibodies and the respective
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-61-
isotypes were determined. Sera that showed high titer of
antibodies against BmHSP were used in the Antibody
Dependent Cellular Cytotoxicity (ADCC) assay described
herein.
[00125] Anti-BmHSP Antibody Levels in the Sera of Mace.
Anti-BmHSP IgG antibody levels in the sera of immunized and
control groups of mice were determined using an indirect
ELISA (Veerapathran, et al. (2009) PLoS Negl. Trop. Dis.
3:e457). IgGl, IgG2a, IgG2b and IgG3 anti-BmHSP antibody
levels were also determined using a mouse antibody
isotyping ELISA kit (ThermoFisher Scientific, Rockford,
IL). Color was developed with ABTS (2,2'-azinobis (3-ethyl
benzothiazoline-6-sulfonic acid) chromogen substrate and
the absorbance was measured at 405 nm in an ELISA reader
(BIO-RAD).
[00126] Depletion of Anti-BmHSP Antibodies from Human and
Mice Sera. Anti-BmHSP antibodies were depleted from pooled
sera of EN subjects and immunized mice by incubating the
pooled sera with cobalt IMAC resin coupled with his-tagged
rBmHSP according to established methods (Veerapathran, et
al. (2009) supra). Briefly, 1 mg of his-tagged rBmHSP was
coupled to 2 ml bed volume of IMAC resin for 2 hours at
37 C. After washing the resin once with 10 ml of PBS
(pH.8), 200 pl of pooled sera was added and incubated
overnight at 4 C. After incubation, the resin mixture was
centrifuged for 2 minutes at 750 rpm and the supernatant
was collected. Depletion of anti-BmHSP antibodies in the
supernatant was confirmed by ELISA as described herein.
[00127]Anti-BmHSP IgGl, anti-BmHSP IgG2a, anti-BmHSP IgG2b,
anti-BmHSP IgG3 and anti-BmHSP IgG4 antibodies from pooled
sera of EN subjects and pooled sera of immunized mice were
depleted using NHS (N-hydroxysuccinimidyl) resin (Thermo
fisher scientific). Briefly, 1 pg of respective monoclonal
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-62-
antibodies were coupled to NHS resin column. After washing
the resin twice with PBS (pH.8), 100 pl of sera were passed
through the column. The flow through was collected as the
antibody depleted sera. Depletion of the specific isotype
of antibody was confirmed by an isotype-specific ELISA as
described herein. After washing the column three times with
PBS (pH 7.4), bound antibodies were eluted using Glycine-
HCI buffer (pH 2.7) from the resin and the pH was adjusted
to 7.4 with 1 M Tris buffer (pH 8). The recovered elute
contained the specific antibody as confirmed again by an
ELISA. The antibody depleted sera was also reconstituted
with the eluted antibodies. An aliquot of depleted sera was
reconstituted with the eluted antibodies to its original
concentration using values determined by an earlier ELISA
on the neat serum samples. Antibody depleted sera, eluted
antibodies and reconstituted sera samples were then used in
an ADCC assay.
[00128] Antibody-Dependent Cellular Cytotoxicity
(ADCC)
Assay. In vitro ADCC assay was performed according to known
methods (Chandrasekhar, et al. (1985) Parasite Immunol.
7:633-641). Briefly, ten (10) L3 of B. malayi were
incubated with 2 x 105 peritoneal cells (PEC) collected from
normal mice, 50 pl of pooled mouse sera samples and 50 pl
of RPMI 1640 media in a 96-well culture plate (Thermo
Fisher Scientific). After 48 hours of incubation at 37 C
and 5% CO2, larval viability was determined at 400X using a
light microscope. Larvae that were limpid and damaged were
counted as dead. In addition, dead larvae also had clumps
of cells adhered to it and were more transparent than live.
Larvae that were active, coiled and translucent were
counted as live. ADCC was estimated as the percent larval
death calculated using the formula:
Number of Dead larvae Total Number of Larvae x 100.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-63-
[00129]ADCC assay was also performed with pooled human sera
samples as described herein except that the human sera
samples were incubated with 2 x 105 PBMCs collected from
normal health subjects and 6-12 B. malayi L3 for 48 hours
at 37 C and 5% CO2. Larval viability and death were
determined as described above.
[00130] Protection Studies in Mice. Vaccine potential of
BmHSP was evaluated in a mouse model of challenge
infection. Mice were immunized as described above using
prime-boost, DNA alone or protein alone approach. Vector
and alum group served as negative controls. Immunized and
control animals were challenged using a micropore chamber
method as known in the art (Abraham, et al. (1986)
Immunology 57:165-169). Briefly, micropore chambers were
assembled using 14 x 2 mm PLEXIGLASS (acrylic) rings
(Millipore Corporations, Bedford, MA) and 5.0 pm NUCLEOPORE
polycarbonate membranes (Millipore Corporations). The
membranes were attached to the PLEXIGLASS rings with
cyanoacrylic adhesive and dental cement. The chambers were
immersed overnight at 37 C in sterile RPMI medium
containing gentamycin and antimycotic solution. Before
challenge experiments, 20 live, infective L3s suspended in
RPMI 1640 medium supplemented with 15% heat-inactivated
fetal calf serum (FCS) were introduced into the micropore
chambers and the opening was sealed with dental cement.
Micropore chamber containing the L3s were then surgically
implanted into the peritoneal cavity of each mice under
anesthesia. Aseptic conditions were followed for the
surgical procedures. After 48 hours of implantation,
animals were sacrificed and the chambers were recovered
from peritoneal cavity. Contents of each chamber were
emptied and larvae were examined microscopically for
adherence of cells and for larval death. Dead and live
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-64-
larvae were identified as described above under ADCC. The
percentage of protection was expressed as the number of
dead parasites number of total parasites recovered x 100.
[00131] Splenocyte Proliferation Assay. Spleens
were
collected from all mice from the above experiment and
single-cell suspension of spleen cells was prepared.
Approximately 2 x 105 cells/well suspended in complete RPMT
1640 medium supplemented with 10% heat-inactivated FCS were
incubated at 37 C and 5% CO2 for 72 hours with rBmHSP (1
ug/m1), ConA (1 pg/m1) or with medium alone. After
incubation, cell proliferation was determined using cell
counting kit (CCK-8) purchased from Dojindo Molecular
Technologies, Inc. (Gaithersburg, MD). Stimulation index of
spleen cell proliferation was calculated using the formula:
Absorbance of stimulated cells
Absorbance of unstimulated
cells.
[00132] Cytokine Analysis. Spleen cells from immunized and
control mice were cultured at 37 C and 5% CO2 for 72 hours
with rBmHSP (1 lag/m1), ConA (1 pg/m1) or with medium alone
as described above. After 72 hours, culture supernatants
and cell pellets were collected separately for cytokines
analysis. For measuring cytokine mRNA, cell pellets were
suspended in TRIZOL (phenol, guanidinium and thiocyanate)
reagent (GIBCO-BRL, Life technologies, Carlsbad, CA) and
total RNA was extracted as per the manufacturer's
instructions. After ethanol washes, RNA pellets were
dissolved in RNAse-free water (Sigma) and treated with
DNase I before determining total RNA concentration using a
Beckman spectrophotometer at 260 nm. Reverse transcription
of total RNA was performed using first strand cDNA
synthesis kit (SABiosciences, Frederick, MD) as per
manufacturer's recommendations. Relative quantification of
the expression of genes of interest was measured in an
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-65-
Applied BioSystems 7300 real-time PCR machine (Applied
BioSystems, Foster City, CA). PCR amplifications were
performed with the LIGHTCYCLER-DNA SYBR Green (cyanine dye)
mix (SAbiosciences). The reaction was performed using the
following PCR conditions: 15 minutes activation step at
95 C for one cycle, 15 seconds denaturation step at 95 C,
annealing of primers for 20 seconds at 50 C and elongation
step for 15 seconds at 72 C. DNA was amplified for 50
cycles. The fluorescent DNA binding dye SYBR Green (cyanine
dye) was monitored. RT-PCR data array set was generated and
analyzed using SABiosciences web-based data analysis
system.
[00133] Culture supernatants were then collected from
splenocyte cultures 72 hours after incubation with rBmHSP
(1 pg/m1), ConA (1 pg/m1) or with medium alone. Secreted
levels of IL-2, IL-4, IFN-y and IL-10 protein in the
culture supernatants were determined using a sandwich ELISA
kit purchased from ThermoFisher Scientific. Concentration
of each cytokine was determined from a standard curve
plotted using recombinant mouse IL-2, IL-4, IFN-y or IL-10.
[00134] Statistical Analysis.
Statistical analysis was
performed using XL STAT software v.7.5.2 (Kovach Computing
Services, Anglesey, UK). Statistical significance between
comparable groups was estimated using appropriate non-
parametric tests, with the level of significance set at
p<0.05.
[00135] Expression of Recombinant BmHS512.6
BmHSP
was cloned in pRSET A vector and was expressed as a
histidine-tagged (his-tagged) fusion protein in E. coli
BL21 (DE3)PLysS. Recombinant BmHSP protein was subsequently
purified using IMAC column. The molecular mass of the
purified recombinant his-tag fusion protein was found to be
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-66-
approximately 18 kDa. The column-purified recombinant
protein appeared as a single band in SDS-PAGE.
[00136] Predicted Three-Dimensional Structure of BmHSP.
Amino acid sequences of the human alpha crystalline A chain
share 42% similarity with BmHSP. Since crystal structure of
the human alpha crystalline A chain is already available,
this was used that as a template to model the putative
structure of BmHSP using the Modeller 9v6 program. PROCHECK
analysis was used to select the best model that showed a
score of -0.41 compared to the template (Laskowski, et al.
(1993) J. Appl. Cryst. 26:283-29). A Ramachandran plot
analysis was also performed on the BmHSP sequence. These
analyses showed that 92% of residues were in the most
favorable region with no steno hindrance. About 6.7%
residues were found in the additional allowed region.
Models that showed over 90% residues in the most favored
regions were predicted as the most ideal three-dimensional
model as predicted by the Ramachandran plot (Balazs, et al.
(2001) Protein Eng. Des. Sci. 14:875-880). Secondary
structure prediction analysis was also performed on BmHSP
protein using PDBsum server at EMBL. This analysis showed
that each alpha-crystalline domain of BmHSP monomer had an
immunoglobulin core composed of seven 3-strands arranged in
two anti-parallel sheets. The secondary structure
prediction of BmHSP showed two sheets, four beta hairpins,
one beta bulge, seven strands, two helices, seven beta
turns and one gamma turn in the structure of BmHSP.
[00137] Previous studies showed that BmHSP binds to human
IL-10 receptor I a chain (Gnanasekar, et al. (2008) Mbl.
Biochem. Parasit. 159:98-103). To identify the IL-10
receptor binding site on BmHSP, a predictive protein-
protein interaction analysis was performed (Ofran & Rost
(2007) Bioinformatics 23:e13-e16). Results from the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-67-
prediction analysis showed that the N-terminal fragment of
BmHSP (amino acids from Metl to Asn26) had a strong
protein-protein interaction region. Further sequence
analysis of this region showed that the amino acid
sequences from Val5 to Glu42 had significant sequence
identity to human IL-10R binding region of human IL-10.
These findings confirm that the N-terminal region of BmHSP
may be involved in the binding of BmHSP to human IL-10
receptor I a chain.
[001381 Motif and Phylogenetic Analysis on BmHSP. Motif
analysis performed at PROSITE showed several putative post-
translation modification sites such as N-glycosylation
sites (residues 11 to 14 and 98 to 101), protein kinase-c
phosphorylation sites (residues 83 to 85 and 100 to 102),
casein kinase II phosphorylation sites (residues 68 to 71
and 88 to 91) and N-myristylation sites (residues 40 to 45)
on BmHSP. Similar motifs were also observed in human IL-10
further indicating that BmHSP may mimic human IL-10
function (Gnansekar, et al. (2008) supra). Epitope mapping
on BmHSP revealed the presence of B-cell, T-cell and CTL
epitope regions, indicating that BmHSP is potentially a
highly immunogenic protein (Table 2).
TABLE 2
Position of Epitope in
Epitope Peptide SEQ ID
the Amino Acid
Predicted Sequence NO:
Sequence
12-23 WSAEQWDWPLQH
3
B-Cell 26-35
EVIKTNTNDK 4
Epitopes 67-74 SRAEHYGE
5
84-94
KLPSDVDTKTL 6
45-53
FTPKEIEVK 7
50-57 IEVKVAGD
8
T-C 38-45
VGLDASFF 9
ell
39-47
GLDASFFTP 10
Epitopes
52-60
VKVAGDNLV 11
84-92
KLPSDVDTK 12
92-100
KTLTSNLTK 13
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-68-
27-35 VIKTNTNDK 14
90-98 DTKTLTSNL 15
44-52 FFTPKEIEV 16
38-46 VGLDASFFT 17
100-108 KRGHLVIAA
18
73-81 GEIKREISR 19
43-51 SFFTPKEIE 20
78-86 REISRTYKL 21
CTL
74-82 GEIKRETSR 22
Epitopes
70-78 AEHYGEIKR 23
[00139] A phylogenetic analysis
performed using
representative sHSP sequences from different groups of
organisms showed that BmHSP, C. eiegans HSP and C. remani
HSP form a monophyletic group, separated from the other
groups of organisms.
[00140] BmHSP is a Chaperone. Most of the heat shock
proteins reported to date have chaperone function. To
determine whether BmHSP also has similar chaperone
function, a thermal aggregation reaction was performed
using a model substrate, Citrulline synthase (CS).
Incubation of CS at 42 C resulted in unfolding of the
protein and subsequent aggregation within 10 minutes.
Addition of BmHSP to CS protein (at a molar ratio of 1:2),
before the heat treatment, significantly (P<0.01) inhibited
the thermal aggregation of CS protein. A non-chaperone
control protein, BSA, had no effect on the heat-induced
aggregation of CS protein.
[00141] Another function of chaperone proteins is that they
can specifically bind to denatured proteins. To determine
whether BmHSP can specifically bind to denatured proteins,
rBmHSP was incubated with native and denatured CS or native
and denatured luciferase substrates. These studies showed
that rBmHSP preferentially bound to denatured protein
substrates compared to native or control protein. These
findings thus confirmed that BmHSP can act as a molecular
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-69-
chaperone potentially protecting the parasite cellular
proteins from the damaging effects of the host.
[00142] Antibody Responses in Human. The results presented
herein indicate that BmHSP has several T-cell and B-cell
epitopes. Therefore, it was evaluated whether filariasis-
infected individuals carry antibodies to BmHSP.
Accordingly, the titer of anti-BmHSP IgG antibodies in the
sera of EN, CP, Mf and NEN subjects was measured. The
results showed that the EN subjects had the highest levels
of anti-BmHSP antibodies (p<0.001). Subsequent isotype
analysis of the IgG antibodies showed that compared to the
infected groups (Mf and CP) of individuals, sera from EN
subjects had high titers of IgG1 and IgG3 anti-BmHSP
antibodies. Mf carriers had only significant levels of
anti-BmHSP IgG2 antibodies in their sera. Similarly, CP
individuals had only significant levels of anti-BmHSP IgG4
antibodies in their sera. Anti-BmHSP IgG1 and IgG3 levels
were very low in the sera of these Mf and CP individuals.
Anti-BmHSP antibodies were not detectable in the sera of
NEN subjects.
[00143] Results of ADCC Assay. Since antibodies to BmHSP
were present in all infected groups of individuals (Mf and
CP) and EN subjects, it was determined whether these
antibodies were functional. Using an antibody-dependent
cell cytotoxicity assay, it was tested if anti-BmHSP12.6
IgG antibodies had any protective function against B.
malayi. These studies showed that pooled EN sera promoted
adherence of PBMC's to L3 and induced significant (77.37%)
death of B. malayi L3s in vitro (Table 3), whereas, pooled
sera from Mf and CP failed to participate in the ADCC
function. These findings indicated that EN sera have anti-
parasitic activities. To determine if this function is
associated with antibodies, antibody depletion studies were
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-70-
performed. Depletion of anti-BmHSP antibodies from EN sera
resulted in significant reduction (21.42%) in larval death
(Table 3) confirming that anti-BmHSP antibodies in the sera
of EN subjects, but not Mf or CP subjects, participate in
larval killing.
TABLE 3
% Larval
Dead Live Total
Groups
Death
L3 L3 L3
(Mean SD*)
1 6
Endemic Normal (EN) sera
77.37 8.41
5 2 7
EN sera depleted of anti- 2 5 7
21.42 10.12
rBmHSP antibodies 1 6 7
Non-Endemic Normal (NEN) 1 5 6
19.44 3.92
sera 2 7 9
*Values represent mean SD of three wells.
[00144] Further depletion studies showed that the anti-
parasitic effect of anti-BmHSP antibodies was associated
with IgG1 isotype of antibodies. Depletion of IgG1
antibodies from EN sera significantly (40%) inhibited the
ADCC function (Table 4). Reconstitution of anti-BmHSP
antibody depleted EN sera with eluted anti-BmHSP IgG1
antibodies regained the ADCC function (Table 3). These
findings thus indicated that anti-BmHSP IgG1 antibodies are
critical for ADCC function.
TABLE 4
EN Sera j % Larval Death
Neat Sera 72
IgG1 40
IgG2 Depleted of: 71.43
IgG3 60
IgG4 62.5
IgG1 70
Reconstituted IgG2 j 69.23
with: IgG3 I 66.67
IgG4 54.55
Values represent mean of three wells.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-71-
[00145] Antibody Responses in Mice. Mice immunized with
rBmHSP developed significant levels of anti-BmHSP IgG
antibodies. More specifically, prime-boost vaccine regimen
induced significantly higher titer of IgG antibodies
compared to DNA vaccine alone group (p < 0.05). However,
rBmHSP protein vaccine induced the highest IgG antibody
titer. Analysis of the isotype of anti-BmHSP IgG antibodies
showed that predominantly IgGl, IgG2a and IgG2b anti-BmHSP
antibodies were present in the sera of vaccinated animals.
The ADCC assay was also performed with mouse sera. These
studies showed that sera from BmHSP-vaccinated mice
promoted adherence of peritoneal exudate cells to L3 and
participated in ADCC function (83.02% larval killing)
compared to control sera (13%) (p<0.002) (Table 5).
TABLE 5
Immunization Regimen % Larval
Death
Bmhsp DNA prime and rBmHSP protein boost
83.02 3.62
Bmhsp DNA 43.7
8.12
rBmHSP protein
55.08 1.15
pVAX & alum control 13
2.35
Values represent mean SD of three wells.
[00146] Similar to human sera, individual isotypes of IgG
antibodies were depleted from the sera of vaccinated mice
to determine the isotype of anti-BmHSP antibodies that
participate in the ADCC function. Results from these
studies showed that, similar to that observed with EN sera,
anti-BmHSP IgG1 antibodies were involved in ADCC-mediated
killing of L3 in mice as well (Table 6).
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-72-
TABLE 6
Immunized Mice Sera % Larval Death
Neat Sera of BmHSP prime-boost 80.16
IgG1 37
IgG2a 72
Depleted of:
IgG2b 71
IgG3 80
IgG1 00
IgG2a 63
Reconstituted with:
IgG2b 87
IgG3 71
Values represent mean of three wells.
[00147] Vaccine Potential of BmHSP in Mace. Vaccine
potential of BmHSP was assessed in Balb/c mice using a
micropore chamber method. Results showed that mice
immunized using the prime-boost vaccination regimen and
protein vaccine of BmHSP exhibited nearly 72% and 58%
mortality, respectively, of L3s implanted into the
peritoneal cavity of the immunized mice (Table 7). While
chambers implanted in the control groups of animals showed
only 7% mortality of the parasite, the difference between
the protection of control group of mice and vaccinated mice
was significant (3<0.001). On the other hand, mice
immunized by DNA vaccine alone induced only 31% protection.
Thus, the prime-boost vaccination regimen appeared to be
highly efficient in conferring vaccine-induced protection
against a challenge infection compared to DNA alone or
protein alone immunization protocols.
TABLE 7
Immunization Regimen % Larval
Death
Bmhsp DNA prime and rBmHSP protein boost 72 10.22
Bmhsp DNA
31 5.23
rBmHSP12.6 protein
58 7.76
pVAX & alum control 7
5.2
Values represent mean SD. N=5. Data is from one of two
similar experiments showing comparable results.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-73-
[00148] Immune Responses in BmHSP Vaccinated Mice. To
determine cellular immune responses to BmHSP in the
vaccinated mice, spleen cells collected from vaccinated and
control mice were cultured in the presence of rBmHSP
protein and their proliferative responses and cytokine
profiles were evaluated. Proliferative response of spleen
cells from animals immunized with the prime-boost vaccine
regimen was significantly (P>0.05) higher (stimulation
index of 3.35 0.176) compared to rBmHSP protein alone
vaccination group (stimulation index of 2.22 0.018) or
Bmhsp DNA vaccination alone group (stimulation index of
3.53 0.102). Spleen cells from the control group of
animals failed to proliferate in response to rBmHSP
(stimulation index of 0.98 0.013) and was similar to
media alone controls. Since the spleen cells from
vaccinated animals were proliferating significantly to
recall response to rBmHSP, levels of cytokines in the
culture supernatants were measured. These results showed
that IFN-y was the predominant cytokine secreted by spleen
cells from vaccinated animals at 72 hours after stimulation
with rBmHSP. A real time-PCR cytokine gene array was
performed on mRNA collected from the spleen cells
stimulated with rBmHSP. These results showed that both Thl
(IFN-y, CD-28, IL-12, IL-2) and Th2 (IL-4, IL-5, IL-1R)
cytokine genes were significantly increased in vaccinated
animals.
Example 2: rEimALT2+rBmHSP Multivalent immunogenic
composition
[00149] Parasite. Brugia malayi L3s were obtained from the
NIAID/NIH Filariasis Research Reagent Resource Center (FR3)
at the University of Georgia, Athens, GA.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-74-
[00150] Construction of Monovalent and Multivalent DNA
Vaccines. Monovalent DNA vaccine was composed of Bmhsp or
Bmalt2 in pVAX1 vector. To prepare the monovalent vaccine,
codon optimized Bmhsp or BmALT2 genes were cloned into the
eukaryotic expression vector pVAX1 (Invitrogen, Carlsbad,
CA) using insert-specific primers (Gnanasekar, et al.
(2004) supra). The multivalent immunogenic composition was
composed of Bmhsp and Bmalt2 genes in the same pVAX1
vector. Codon optimized Bmhsp gene was first cloned into
pVAX1 vector with no stop codon in the reverse primer (5'-
CCG GAA TTC TCA CTT GTC GTT GGT G-3'; SEQ ID NO:24) but
contained a PstI site. Codon optimized Bmalt2 gene was then
inserted into this clone using gene specific primers
(Gnanasekar, et al. (2004) supra). PCR parameters for all
the three constructs were: 94 C denaturation for 30
seconds, 50 C primer annealing for 30 seconds, 72 C primer
extension for 30 seconds for 30 cycles; a final extension
of 5 minutes was performed at 72 C. Insert DNA was finally
sequenced to ensure authenticity of the cloned nucleotide
sequence on both strands. Plasmids were maintained and
propagated in E. coli TOP1OF' cells. Plasmids were purified
using endotoxin-free plasmid extraction kit (Qiagen,
Valencia, CA). DNA was analyzed by agarose gel
electrophoresis and quantified in a spectrophotometer (OD
260/280, ratio >1.8).
[00151] Expression and Purification of Recombinant Proteins.
All the genes were cloned in pRSET-A vector (with an N-
terminal hexahistidine tag) to produce recombinant
proteins. Bmhsp and Bmalt2 constructs were transformed into
BL21(DE3) containing pLysS E. coli host (Invitrogen) to
minimize toxicity due to the protein. When absorbance of
the cultures reached 0.6 OD value, 1 mM of IPTG (isopropyl
thio-d-galacto pyranoside) was added to the cultures and
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-75-
incubated for an additional 3 hours to induce the gene
expression. After lysing the cells, total proteins were
separated in 15% and 12% SDS-PAGE to confirm the expression
of his-tag recombinant BmHSP (rBmHSP) and rBmALT2 proteins.
The recombinant proteins were then purified using an
immobilized cobalt metal affinity column chromatography
(Clontech, Mountain View, CA) as per the manufacturer's
recommendations. Recombinant proteins were then separated
in SDS-PAGE and stained with COOMASSIE brilliant blue R250
and silver stain. These studies showed that a single band
was obtained after column purification. Endotoxins if any
in the recombinant preparations were removed by passing the
recombinant proteins through polymyxin B affinity columns
(Thermo Fisher Scientific, Rockford, IL) and the levels of
endotoxin in the final preparations were determined using
an E-TOXATE kit (Sigma, St Louis, MO) as per manufacturer's
instructions. Endotoxin levels were below detection limits
in these recombinant protein preparations.
[00152] Immunization of Mice. Six-weeks old male Balb/o mice
purchased from Charles River Laboratories were used in
these experiments. Humane use of animals in this study and
the protocol was approved by the IACUC committee at the
College of Medicine, University of Illinois Rockford. Mice
were divided into four (4) groups of five (5) animals each.
All mice were immunized subcutaneously using a DNA prime -
protein boost vaccine regimen. All experimental groups of
mice were primed with two injections of endotoxin-free
codon optimized DNA given in 50 pl volume and boosted with
two doses of recombinant proteins suspended in alum (50 pl
each) given at two weeks interval.
[00153] Group A mice were primed with 100 pg of pVAXBmhsp
and boosted with 15 pg of rBmHSP; Group B mice were primed
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-76-
with 100 pg of pVAX Bma1t2 and boosted with 15 pg of
rBmALT2; Group C mice were primed with 100 pg of
pVAXBmhsp/Bmart2 DNA and boosted with 15 pg of rBmHSP and
15 g of rBmALT2. Group D mice received 100 pg of pVAX1
vector plus 50 pl of alum and served as controls. Blood
samples were collected from each mouse before immunization
and one month after the last booster dose. Sera were
separated and stored at -80 C.
[00154] Evaluation of Antibody Responses in Mice. Levels of
anti-BmHSP and anti-BmALT2 antibodies were measured in the
sera of immunized and control groups of mice using an
indirect ELTSA according to established methods
(Veerapathran, et al. (2009) supra; Gnanasekar, et al.
(2004) supra). Briefly, wells of 96-well microtiter plates
were coated with rBmHSP, rBmALT2 or rBmHSP (1 pg/ml) in
carbonate buffer (pH 9.6) overnight at 4 C. After washing
the wells, unbound sites were blocked with 3% BSA for 1
hour at 37 C. Diluted sera samples were then added to the
wells and incubated further overnight at 4 C. After washing
the wells, HRP-labelled rabbit anti-mouse IgG was added
(1:5000) and incubated further for 1 hour at 37 C. Color
was developed using ABTS (2, 2"-azinobis (3-
ethylbenzothiazoline-6-sultonic acid))
substrate.
Absorbance was measured at 405 nm in a microplate reader
(BIO-RAD, Hercules, CA).
[00155] Protection Studies in Mice. Vaccine potential of the
monovalent and multivalent immunogenic composition
formulations were then evaluated in a mice model. Mice were
immunized as described above using the prime boost
approach. Vector plus alum group served as negative
controls. Immunized and control animals were challenged
using a micropore chamber method known in the art (Abraham,
et al. (1989) Am. J. Trop. Med. Hyg. 40(6):598-604).
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-77-
Briefly, micropore chambers were assembled using 14 x 2 mm
PLEXIGLASS (acrylic) rings (Millipore Corporations,
Bedford, MA) and 5.0 pm NUCLEOPORE polycarbonate membranes
(Millipore Corporations) that were attached to the
PLEXIGLASS (acrylic) rings with cyanoacrylic adhesive and
dental cement. The chambers were immersed overnight at 37 C
in sterile RPMI medium containing gentamycin and
antimycotic solution. Before challenge experiments, 20 live
infective L3s suspended in RPMI1640 medium supplemented
with 15% heat inactivated fetal calf serum (FCS), were
introduced into the micropore chambers and the opening was
sealed with dental cement. Micropore chamber containing the
L3s were then surgically implanted into the peritoneal
cavity of each mice under anaesthesia. Aseptic conditions
were followed for the surgical procedures. After 48 hours
of implantation, animals were sacrificed and the chambers
were recovered from peritoneal cavity. Contents of each
chamber were emptied and larvae were examined
microscopically for adherence of cells and for larval
death. Larval viability was determined microscopically at
100 x. The percentage of protection was expressed as the
number of dead parasites 4- number of total parasites
recovered x 100.
[00156] Cytokine Analysis in Mice. The percent of rBmHSP and
rBmALT2 specific interferon-y (IFN-y) and interleukin-4
(IL-4) secreting cells were determined in the spleen of
control and vaccinated mice using an ELISPOT assay.
Briefly, MILLIPORE MULTISCREEN HTS Filter plates were
coated with monoclonal rat anti mouse IFN-y or monoclonal
rat anti-mouse IL-4 antibodies (BD Pharmigen, San Diego,
CA) at a concentration of 10 ug/m1 in PBS buffer. After
washing the plates, non-specific sites were blocked by
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-78-
incubating the wells in complete RPMI with 10% fetal calf
serum for one hour at room temperature. Approximately 3 x
106 spleen cells suspended in complete RPMI1640 medium
supplemented with 10% heat inactivated FBS were then added
to each well. Cells were stimulated with rBmHSP or rBmALT2
(1 pg/ml). Unstimulated cells served as controls. Forty-
eight hours after incubation at 37 C in humidified 5% CO2,
plates were washed and further incubated for 1 hour at room
temperature with 2 pg of biotinylated rat anti-mouse IFN-y
or biotinylated rat anti-mouse IL-4 antibody (BD
Pharmigen). After washing the plates, streptavidin-
conjugated horseradish peroxidase (Thermo
Fisher
Scientific) was added (1:800) to each well and incubated at
room temperature for one hour. Plates were washed and color
developed using DAB substrate (Thermo Fisher Scientific).
Total numbers of spots were counted under a dissection
microscope.
[00157] Statistical Analysis.
Statistical analysis was
performed using XL STAT software v.7.5.2 (Kovach Computing
Services, Anglesey, UK). Statistical significance between
comparable groups was estimated using appropriate non-
parametric tests, with the level of significance set at
p<0.05.
[00158] Antibody Responses in Mice. It was first determined
whether the multivalent immunogenic composition could
elicit significant antibodies against each of the antigenic
components. Previous studies have shown that mice similarly
vaccinated with B. malayi antigens elicited significant
host protective IgG antibodies (Veerapathran, et al. (2009)
supra). Therefore, IgG antibody titers were analyzed. The
results of this analysis indicated that the monovalent
immunization with Bmhsp + rBmHSP and Bmalt2 + rBmALT2
elicited significant (p< 0.005) titers of anti-BmHSP and
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-79-
anti-BmALT2 IgG antibodies (FIG. 1). The multivalent
immunogenic composition also elicited significant IgG
antibody titers. Following multivalent immunogenic
composition, the mice produced IgG antibodies against both
BmHSP and BmALT2 equally, suggesting that the antigens do
not interfere or compete for dominance. An interesting
finding was that the multivalent immunogenic composition
elicited 1.5- to 1.75-fold higher (p< 0.005) titers of IgG
antibodies compared to the monovalent vaccine (FIG. 1).
These finding indicated that the two antigens in the
multivalent formulation can act synergistically by
increasing the vaccine-induced antibody responses against
each antigen in the vaccinated mice. The findings also
indicated that combining these two antigens in the vaccine
formulation has a great advantage. Given the robust IgG
antibody responses induced following vaccination, it is
also possible that the concentration of the component
antigens in the multivalent preparation can be reduced.
[00159] Multivalent immunogenic composition
Induces
Significant Protection in Mice. The results herein showed
that significant IgG antibodies were elicited following
vaccination with monovalent and multivalent immunogenic
composition preparations. To test if the immune responses
elicited following vaccination were protective, vaccinated
animals were challenged with live third stage infective
larvae (L3) of B. malayd. Since the parasites do not reach
maturity in these animals, a better recovery of worms is
obtained if the parasites are surgically implanted into the
animals. A standard micropore chamber challenge method
(Abraham, et al. (1989) supra). These studies showed that
close to 61% protection could be achieved in mice immunized
with a monovalent vaccine (Table 8). This was highly
significant (p <0.001) compared to negative controls. This
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-80-
finding also showed that rBmHSP and rBmALT2 are of use in
vaccines for lymphatic filariasis. Challenge experiments in
mice immunized with multivalent immunogenic composition
showed that significantly (p <0.005) higher protection
could be achieved compared to monovalent vaccination (Table
8). These findings also clearly correlated with the higher
IgG antibody titer in these animals and support the above
finding that rBmALT2 and rBmHSP can synergistically enhance
the protective immune responses in vaccinated animals when
given as a prime boost regimen (Table 8).
TABLE 8
Percent
Vaccination regimen
Groups
Larval Deatha ________________________________________________________
Bmhsp DNA prime and rBmHSP
61 4.24 Monovalent
protein boost
Bma1t2 DNA prime and rBmALT2
76 8.21 Monovalent
protein boost
Bmhsp+Bmalt2 prime and rBmHSP
90 7.53 Multivalent
and rBmALT2 protein boost
pVAX plus alum control 22 10.41
Control
aValues are mean + SD. N=5. Data is from one of two similar
experiments showing comparable results.
[00160] To further demonstrate efficacy, mice were immunized
with various prime-boost combinations. As shown in FIG. 3,
100% protection can be achieved in mice following
immunization with HAT hybrid protein or after prime boost
immunization with HAT hybrid DNA and HAT hybrid protein.
[00161] CyLuAine Responses. The
immunological
characteristics of the protective responses in vaccinated
mice were determined by evaluating the secreted cytokine
responses of spleen cells in response to the vaccine
antigens. When spleen cells were stimulated with rBmHSP or
rBmALT there was significant antigen-specific proliferation
of spleen cells suggesting a strong recall cellular
response to the antigens. To identify the cytokine profile
of these antigen-responding cells, the IFN-y and IL-4
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-81-
secreting cells were counted using an ELISPOT assay.
Results from these studies showed that spleen cells from
mice vaccinated with multivalent immunogenic composition
were predominantly secreting IL-4 (FIG. 2A). The numbers of
IFN-y secreting cells were very low (FIG. 2B). Overall,
these findings indicated that vaccine-induced protection
was largely mediated by Th2 type responses.
Example 3: BmVal-1+BmALT2 Multivalent immunogenic
composition
[00162] Sera. Sera samples used in this study were from
archived samples stored at the Mahatma Gandhi Institute of
Medical Sciences, Sevagram, India. These samples were
collected as part of epidemiological surveys in and around
Wardha, an area endemic for lymphatic filariasis.
[00163] No demographic data was available to this study
except that the sera samples were classified into
microfilaremic (MF), chronic pathology (CP) or Endemic
normals (EN) based on the detection of circulating
parasites, parasite antigens or by evaluating clinical
symptoms of lymphatic filariasis. Circulating microfilariae
were detected in the blood of subjects according to known
methods (Haslbeck, et al. (2005) Nat. Struct. Mbl. Biol.
12:842-846; Yoo, et al. (2005) Biotechnol. Lett. 27:443-
448). The presence of circulating antigen was detected
using an 0g4C3 kit and a WbSXP-based enzyme-linked
immunosorbent assay (ELISA). Subjects with no circulating
antigen or microfilariae were classified as EN, whereas
subjects with circulating microfilariae and/or circulating
antigen, as detected by ELISA, were considered as MF.
Subjects showing lymphedema and other visible clinical
symptoms of filariasis were grouped into CP. Control non-
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-82-
endemic normal (NEN) sera were collected at the University
of Illinois Clinic at Rockford, IL.
[00164] Parasites. Brugia malayd L3s were obtained from the
NIAID/NIH Filariasis Research Reagent Resource Center (FR3)
at the University of Georgia, Athens, GA.
[00165] Construction of Monovalent and Multivalent DNA
Vaccines. To prepare monovalent vaccine, codon optimized
Bmval-1 or Bmalt-2 genes were cloned into the eukaryotic
expression vector pVAX1 (Invitrogen, Carlsbad, CA) using
insert-specific primers (Yoo, et al. (2005) supra; Huang,
et al. (2005) Immunol. Lett. 101:71-80). To prepare
multivalent immunogenic composition, codon-optimized Bmval-
1 gene was first cloned into pVAX1 vector with no stop
codon using already published primer sequences with a PstI
site. Codon-optimized Bmalt-2 gene was then inserted into
this clone using gene-specific primers. FOR parameters for
all the constructs were: 94 C denaturation for 30 seconds,
50 C primer annealing for 30 seconds, 72 C primer extension
for 30 seconds for 30 cycles; and a final extension of 5
minutes was performed at 72 C. Insert DNA was sequenced to
ensure authenticity of the cloned nucleotide sequence on
both strands. Plasmids were maintained and propagated in E.
coil TOP1OF' cells. Plasmids were purified using endotoxin-
free plasmid extraction kit (Qiagen, Valencia, CA). DNA was
analyzed by agarosc gel electrophoresis and quantified in a
spectrophotometer (OD 260/280, ratio > 1.8).
[00166] Expression and Purification of Recombinant Proteins.
Recombinant BmVAL-1 and rBmALT2 were expressed in pRSET-A
vector and purified using an immobilized cobalt metal
affinity column chromatography according to published
methods (Norimine, et al. (2004) Infect. Immun. 72:1096-
1106; Shinnick, et al. (1988) Infect. Immun. 56:446-451).
Endotoxin in the recombinant preparations were removed by
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-83-
passing the recombinant proteins through polymyxin B
affinity columns (Thermo Fisher Scientific, Rockford, IL)
and the levels of endotoxin in the final preparations were
determined using an E-TOXATE kit (Sigma, St Louis, MO) as
per manufacturer's instructions. Endotoxin levels in the
final preparations (0.005 EU/ml) were below detection
limits in these recombinant protein preparations.
[00167] Inmunoreactivity of Human Sera. To determine if the
human sera samples carried antibodies against BmVAL-1 or
BmALT2, an ELISA was performed (Haslbeck, et al. (2005)
supra; Yoo, et al. (2005) supra). For isotype-specific
ELISA, alkaline phosphatase-conjugated goat anti-human
IgGl, anti-human IgG2, anti-human IgG3, and anti-human IgG4
antibodies (Sigma) were used as the secondary antibodies.
[00168] Immunization Protocol for Mice and Jirds. Six-week
old male Balb/c mice and 35-40 gm outbred male mongolian
gerbils (jirds) purchased from Charles River Laboratories
(Wilmington, MA) were used in these experiments. Animals
were treated as per the guidelines in the Guide for the
Care and Use of Laboratory Animals. Two different animal
models were used because B. malayi parasite does not mature
into adults in mouse, so vaccine-induced protection against
the L3 stages can be evaluated in the mouse model. In
addition, significant immunological parameters can be
measured in mice. Conversely, B. malayi parasite develops
into mature adult worms in jirds. Therefore, vaccine-
induced protection can be evaluated against adult worm
establishment in jirds.
[00169] Three sets of experiments were performed:
(1)
monovalent BmVAL-1 vaccination, (2) monovalent BmALT2
vaccination and (3) multivalent mVAL-1/BmALT2 vaccination.
Each experimental set had four groups (a) DNA prime plus
DNA boost (homologous), (b) protein prime plus protein
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-84-
boost (homologous), (c) DNA prime plus protein boost
(heterologous) and pVAX plus alum controls. Each group
included ten (10) animals each. All animals were immunized
subcutaneously with codon-optimized DNA (100 jig) in 50 pl
volume or with recombinant protein (150 jig) plus alum in 50
pl volume. Control group received 100 pg of pVAX1 blank
vector or 50 pl of alum. Blood samples were collected at
frequent intervals, sera separated and stored at -80 C. The
protocol used for immunizing mice and jirds was as follows.
Animals were prebled and given a first dose on day 0. A
second dose was administered on day 14 and subsequently
bled. Third and fourth doses were administered on days 28
and 42, respectively, and the animals were subsequently
bled. Mice were challenged on day 56 and protection was
determined on day 58. Jirds were challenged on day 60 and
protection was determined on day 155.
[00170] Protection Studies in Mice. Challenge studies were
conducted in mice by surgically implanting twenty live,
infective B. malayi L3s into the peritoneal cavity in a
micropore chamber (Veerapathran, et al. (2009) supra;
Abraham, et al. (1988) supra). Aseptic conditions were
followed for the surgical procedures. Forty-eight hours
after implantation, chambers were recovered from the
peritoneal cavity and viability of the larvae was
determined under a light microscope. The percentage of
protection was expressed as the number of dead parasites +
number of total parasites recovered x 100.
[00171] Splenocyte Proliferation and cytokine Assays.
Single-cell suspension of spleen cells (0.5 x 106 cells per
well suspended in 200 pl media) were prepared from each
mouse and cultured in triplicate wells with either (1) 1
pg/ml rBmVAL-1, (2) 1 pg/ml rBmALT2, (3) 1 pAg/m1 rBmVAL-
1+Bm7\LT2, (4) a nonspecific recombinant protein (1 pg/ml of
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-85-
Schistosoma mansoni G-binding protein) or (5) were left
unstimulated in the media. All cells were incubated for 3
days at 37 C with 5% CO2. After 3 days, 3H-Thymidine (0.5
pCi per well, Amersham Biosciences) was added to each well
and further incubated. Cells were harvested 16 hours later
and 3H-thymidine uptake was measured in a liquid
scintillation counter and expressed as stimulation index
(SI) = (counts per minute of stimulated cultures counts per
minute of unstimulated cultures). Cell culture supernatants
collected from the spleen cultures were assayed for IFN-y,
IL-4, IL-5 and IL-10 using an ELISA kit purchased from
eBioscience Inc. (San Diego, CA).
[00172] BmVAL-1 and BmALT2 Specific IgG Antibodies in the
Sera of Immunized Mace. Titer of anti-BmVAL-1- and anti-
BmALT2-specific antibodies was determined in the sera of
immunized mice using an ELISA (Veerapathran, et al. (2009)
supra; Gnanasekar, et al. (2004) Infect. Immun. 72:4707-
15). Pre-immune sera served as controls. HRP-conjugated
goat anti-mouse IgG was used as the secondary antibody
(Thermo Fisher Scientific) for mouse assays. OPD (Sigma)
was used as the substrate and optical density (OD) was
measured at 405 nm.
[00173] Anti-BmVAL-1- and anti-BmALT2-specific IgGl, IgG2a,
IgG2b, IgG3 and IgG4 antibodies were determined in the sera
of mouse using a mouse antibody isotyping kit purchased
from Thermo Fisher Scientific. All ELISAs were performed as
per the manufacturer's recommendation and absorbance was
read at 405 nm. Respective HRP-labeled goat anti-IgG
isotype antibody was used as the secondary antibodies and
color was developed using OPD substrate.
[00174] Challenge Studies in Jirds. Jirds were challenged
with 100 B. malayi L3s and worm establishment was
determined on day 95 after challenge according to
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-86-
established methods (Weil, et al. (1992) supra). Jirds are
permissive hosts for B. malayi and the worms mature into
adult males and females in about 75 days. Presence of
mature worms in the control group of jirds was confirmed by
demonstrating microfilariae in their blood on day 80 after
challenge. Percent reduction in the worm establishment was
calculated using the formula: average number of worms
recovered from control worms - average number of worms
recovered from vaccinated animals / average number of worms
recovered from control animals x 100.
[00175] Statistical Analysis.
Statistical analysis was
performed using SIGMASTAT program (Jandel Scientific, San
Rafel, California) and STATVIEW (SAS Institute, Cary, NC)
software. Wilcoxon signed rank test was used to compare
paired data; comparison between the groups was performed
using the Mann-Whitney U test. p value of p<0.05 was
considered statistically significant.
[00176] EN individuals Carry High Titer of Antibodies
Against BmVAL-1 and BmALT2. Significant anti-BmVAL-1 and
anti-BmALT2 IgG antibodies were present in the sera of EN
subjects compared to MF subjects (p < 0.01) and CP subjects
(p < 0.005). NEN subjects did not carry IgG antibodies
against either of the antigens. Subsequent analysis of the
IgG isotype of antibodies in the sera of EN subjects showed
that anti-BmVAL-1 and anti-BmALT2 antibodies were
predominantly of IgG1 and IgG3 isotypes.
[00177] High Titer of Antibody Responses in the Sera of
Immunized Mice. It has been shown that mice vaccinated with
B. malayi antigens elicit significant host protective IgG
antibodies. Therefore, IgG antibody titers in the sera of
immunized mice were determined. Monovalent immunization
with BmVal-1 and monovalent immunization with BmAlt2 both
elicited significant (p< 0.005) titers of anti-BmVAL-1 and
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-87-
anti-BmALT2 IgG antibodies in the sera of mice. Compared to
controls, the prime boost immunized group gave the maximum
titer of antibodies followed by protein immunized and DNA
immunized groups. Immunization with the multivalent
immunogenic formulation (BmVAL-1 + BmALT2) also elicited
significant IgG antibody titers against both rBmVAL-1 and
rBmALT2 and the titers were comparable, indicating that the
antigens do not interfere with each other or compete for
dominance. An interesting finding was that the multivalent
immunogenic elicited significantly higher (p< 0.001) titer
of IgG antibodies in mice compared to any of the monovalent
vaccines. These finding indicated that the two antigens in
the multivalent formulation synergistically increased the
vaccine-induced antibody responses.
[00178] Overall, protein vaccination elicited higher titer
of IgG antibodies compared to DNA vaccines, indicating that
protein vaccinations were highly immunogenic. Another
observation was that a heterologous prime boost approach
gave a higher seroconversion than homologous prime boost
approach. Thus, overall heterologous prime boost approach
appeared to stimulate the highest titer of antibodies.
[00179] IgG antibody subset analysis showed that BmVAL-1
vaccination elicited primarily IgG1 and IgG2a isotype of
antibodies, whereas, BmALT2 vaccination induced IgGl, IgG2a
and IgG3 isotype of antigen-specific antibody responses.
Antigen-specific IgG4 antibody responses were not evident.
The prime boost approach significantly amplified the IgG
isotype responses. Following multivalent vaccination
regimen IgGl, IgG2a and IgG3 subset of antigen specific
antibodies were present in the sera of mouse.
[00180] Antigen-Specific Responses in the Spleen of Mace.
Spleen cells from immunized mice stimulated with either
rBmVAL-1 or rBmALT2 proliferated significantly (SI 10.8
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-88-
1.1 and SI 14.6 1.2, respectively) compared to the media
control (SI 2.1 0.9). Spleen cells from mice immunized
with the multivalent construct responded to both rBmVAL-1
(SI 18.9 2.6) and rBmALT2 (SI 23.5 3.1), indicating
that a strong recall cellular response was generated to
both BmVAL-1 and BmALT2 following vaccination with the
multivalent construct.
[00181] Cytokine Analysis from Proliferated
Culture
Supernatants. To identify the cytokine profile of the
antigen-responding cells, the culture supernatant of mouse
spleen cells stimulated with respective antigen (rBmVAL-1
or rBmALT2) was collected and the level of IFN-y, IL-4, IL-
and IL-10 was measured. These results showed that
significant levels of IL-5 and IFN-y were secreted by the
spleen cells in response to rBmVAL-1. Spleen cells
stimulated with rBmALT2 predominantly secreted IL-4 and IL-
5.
[00182]Multivalent immunogenic composition
Induces
Significant Protection in Mice and Jirds. The results
herein indicated that significant IgG antibodies were
elicited following vaccination with monovalent and
multivalent immunogenic preparations. To test if the immune
responses elicited following vaccination were protective,
vaccinated animals were challenged with live, third stage
infective larvae (L3) of B. malayi. Since the parasites do
not reach to maturity in mice, a standard micropore chamber
challenge method was used (Gnanasekar, et al. (2004),
supra). These studies showed that 39% to 74% protection was
achieved in mice following immunization with monovalent
vaccine (Table 9).
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-89-
TABLE 9
Mean SD Percent
Vaccination Group
Live L3s Protection
pVAXBmVAL-1 DNA monovalent
12.2 4.5 39.0 1.7%**
homologous
rBmVAL-1 protein monovalent
10.4 3.1 48.0 2.1%*
homologous
pVAXBmVAL-1 DNA plus rBmVAL-1
9.2 2.2 54.0 3.1%*
monovalent heterologous
pVAXBmALT2 DNA monovalent
9.8 2.1 51.0 2.5%*
homologous
rBmALT2 protein monovalent
7.0 1.1 65.0 4.2%*
homologous
pVAXBmALT2 DNA plus rBmALT2
5.1 0.5 74.5 3.1%*
monovalent heterologous
pVAXBmVAL-1/ALT2 DNA multivalent
8.6 0.1 57.0 2.2%*
homologous
rBmVAL-1/rBmALT2 protein
5.2 1.1 74.0-19.9%*
multivalent homologous
pVAX13mVAL-1/BmALT2 DNA plus
rBmVAL-1/rBmALT2 multivalent 4.4 0.4
82.0 2.2%*
heterologous
pVAX + Alum control 20 0 0%
Significance, * p<0.01, **p<0.05 compared to control.
[00183] Protein vaccination gave better results than DNA
vaccination. The prime boost regimen gave the best results
overall. Vaccination with BmALT2 gave higher percent of
protection compared to BmVAL-1. Similarly, multivalent
vaccination regimen gave the 57% to 82% protection compared
to the monovalent vaccination regimen. These finding
indicated that BmVAL-1 and BmALT2 synergistically enhance
the protective immune responses in vaccinated animals when
given as a multivalent immunogenic composition .
[00184]Analysis of the thick blood smear prepared from the
control group of jirds on day 80 after challenge showed
that all five jirds were positive for microfilaria,
whereas, microfilaria were not detected in the peripheral
blood of vaccinated jirds. Fifteen (15) days later the
animals were sacrificed and the male and female worms in
CA 03167346 2022- 8-8
W02021/163448
PCT/US2021/017813
-90-
the peritoneal, pelvic and pleural cavities were counted
and the results between controls and vaccinated groups were
compared (Table 10). Findings from vaccination of jirds
also confirmed that the multivalent prime boost regimen
gave the highest rate of protection. No female worms were
recovered from the multivalent vaccinated animals.
TABLE 10
Vaccination Group Percent Production
pVAXBmVAL-1 DNA monovalent 50 3.7%
homologous _______________
rBmVAL-1 protein monovalent 40.0
3.1%
homologous
pVAXBmVAL-1 DNA plus rBmVAL-1 52.4
2.5%
monovalent heterologous
pVAXBmALT2 DNA monovalent 58.3
2.1%
homologous
rBmALT2 protein monovalent 72.0
5.5%
homologous
pVAXBmALT2 DNA plus rBmALT2 78.5
3.2%
monovalent heterologous
pVAXBmVAL-1/ALT2 DNA 77.1
2.0%
multivalent homologous
rBmVAL-1/rBmALT2 protein 79.9
3.5%
multivalent homologous
pVAXBmVAL-1/BmALT2 DNA plus 85.0
1.4%
rBmVAL-1/rBmALT2 multivalent
heterologous
pVAX + Alum control 0
Significance, p<0.01 compared to control.
Example 4: BmHSP+BmALT2+BmTSP Multivalent Immunogenic
Composition
[00185] Parasites. Brugia malayi L3s were obtained from the
NIAID/NIH Filariasis Research Reagent Resource Center (FR3)
at the University of Georgia, Athens, GA.
[00186] Construction of pVAX Bmhsp+Bmalt2+Bmtsp DNA vaccine.
The codon-optimized DNA sequence coding for Bmhsp was
amplified with the forward primer 5'-CGC GGA TCC ACC GTG
ATC CAT TGT CG-3' (SEQ ID NO:25) containing BamHI
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-91-
restriction site and the reverse primer 5'-AAC TGC AGO TGT
TTT CCA TTT CCA TTC-3' (SEQ ID NO:26) containing PstI
restriction site without the stop codon and cloned into
pVAX vector. The resulting plasmid was designated as pVAX
Bmhsp*. Codon-optimized Bmalt2 gene was amplified with the
forward primer 5'-AAC TGC AGA TGG GTA ACA AGO TCC TCA TOG-
3' (SEQ ID NO:27) and the reverse primer without the stop
codon 5'-CGC GAA TTC GGC GCA CTG CCA ACC TGC-3' (SEQ ID
NO:28). Underlined sequences indicate PstI and EcoRI
restriction sites in the forward and reverse primers,
respectively. The amplified Bmalt2 DNA insert was then
subcloned into pVAX Bmhsp* plasmid at the PstI and EcoRI
restriction sites, resulting in pVAX Bmhsp+Bmalt2* plasmid.
To clone the final product of pVAXBmhsp+Bmalt2+Bmtsp
plasmid, the gene sequence encoding Bmtsp ECL domain alone
was amplified with the forward primer 5'-CGC GAA TTC ACC
ATG GTC CTG GAG-3' (SEQ ID NO:29) containing EcoRI
restriction site and the reverse primer with stop codon 5'-
GOT CTA GAT CAG TCC TTC TGG CTA G-3' (SEQ ID NO:30)
containing XbaI restriction site and cloned into pVAX
Bmhsp+Bmalt2* plasmid. Bivalent constructs of HSP+TSP,
TSP+ALT and HSP+ALT were also constructed with their
respective primers.
[00187] Construction of pRSETA Bmhsp+Bmalt24-Bmtsp,
a
Multivalent Fusion Protein. The Bmhsp+Bmalt2+Bmtsp fusion
protein was constructed in the same manner as above. The
primer sequences of HSP, ALT2 and TSP were as follows.
Bmhsp, forward primer, Y-CGG GAT CCA TGG AAG AAA AGG TAG
TG-3' (SEG, ID NO:31) containing BamHI and reverse primer, 5'-
CCC TOG AGT GOT TTC TTT TTG GCA GC-3' (SEQ ID NO:32)
containing XhoI. Bmalt2, forward primer, 5'-CCC TOG AG A TGA
ATA AAC TTT TAA TAG OAT-3' (SEQ ID NO:33) containing XhcI or
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-92-
5'-AAC TGC AGA TGG GTA ACA AGO TCC TCA TCG-3' (SEQ ID
NO:27) and reverse primer, 5'-GGG TAO COG CGC ATT GCC AAC
00-3' (SEQ ID NO:34) containing KpnI. Bmtsp, forward primer,
5'-GGG GTA CCC CGG CAA GGA TCA ATT TAA AA-3' (SEQ ID NO:35)
containing KionI and reverse primer, 5'-CGG AAT TOT CAA TCT
TTT TGA GAT GAA T-3' (SEQ ID NO:36) containing EcoRI were
used to amplify the Bmtsp fragment of SEQ ID NO:77. Primers
were also designed to amplify a Tetraspanin Large
Extracellular Loop (LEL) fragment of SEQ ID NO:63. Bivalent
constructs (HA, HT and TA) were also cloned individually
into a pRSETA vector.
[00188] Immunization of Animals. Six-week-old Balb/C mice
were immunized with 100 pg of DNA intradermally (i.d.) as
DNA vaccine or with 15 pg of recombinant protein
subcutaneously (s.c.) as protein vaccine or with two doses
of DNA and two doses of protein as prime-boost vaccine.
Mice were randomly divided into 15 groups with 5 mice per
group. Animals from groups 1-3 were immunized with HSP+ALT2
(HA). Groups 4-6 were immunized with HSP+TSP (HT), and 7-9
were immunized with TSP+ALT2 (TA). Mice from groups 10-12
were immunized with the multivalent immunogenic composition
HSP+ALT2+TSP (HAT). Control group of animals received pVAX
vector and/or alum (Infectious Disease Research Institute
(IDRI)). This experiment was repeated twice with all the
groups.
[00189] Analysis of Antibody Response in Immunized Animals.
IgG antibody levels in the sera of immunized and control
groups of animals against all the three proteins were
determined using an indirect ELISA (Anandharaman, et al.
(2009) supra). Briefly, wells of a 96-well microtiter plate
were coated with recombinant proteins (rHSP, rALT2 or rTSP;
1 pg/ml) in carbonate buffer, pH 9.6, overnight at 4 C and
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-93-
blocked with 3% BSA for 1 hour at 37 C. Sera samples were
added to the wells and the plates were incubated overnight
at 4 C. After washing, HRP-labeled mouse anti-human IgG was
added (1:5000) and incubated further for 1 hour at 37 C.
The color was developed with OPD (o-phenylene diamine)
substrate (Sigma Aldrich, USA). Absorbance was measured at
450 nm in a microplate reader (BIO-RAD, Hercules, CA).
[00190] Immunoblot analysis was also performed with the
immunized mice sera. Sera samples from the mice immunized
with the recombinant proteins (rHAT, rALT2, rTSP or rBmHAT)
were used for the immunoblot study. The color of the blot
was developed with the diaminobenzidine (DAB) substrate.
[00191] ADCC Assay. To evaluate the protection efficacy of
the antigen combinations, in vitro ADCC was performed with
the sera from the mice immunized with bivalent and
trivalent vaccine constructs. The in vitro ADCC assay was
performed according to known methods (Chandrasekhar, et al.
(1990) supra). Briefly, Peritoneal Exudates Cells (PEC)
were collected from normal Balb/c mice by washing the
peritoneal cavity with sterile RPMI 1640 media. The cells
were washed and suspended in RPMI 1640 medium supplemented
with 10% Fetal Calf Serum (FCS). Ten L3 of B. malayi were
added to 2 x 105 peritoneal exudates cells (PEC)/well in 96-
well culture plates (Thermo Fisher Scientifics, USA.), 50
pl of immunized mice sera and 50 pl of RPMI 1640 media were
added to the wells in triplicates and incubated for 48
hours in 5% CO2 at 37'C. Larval viability was determined
microscopically after 48 hours of incubation. Larvae that
were limpid, damaged and with the clumps of cells adhered
to it were counted as dead. ADCC was estimated as the
percent larval death calculated using the formula: Number
of Dead larvae Total number of larvae x 100.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-94-
[00192] Depletion of IgG Antibodies from the Sera Samples.
Sera from mice immunized with multivalent immunogenic
composition was depleted of recombinant antigen specific
IgG antibodies using cobalt IMAC resin coupled with his-
tagged recombinant antigens (Anandharaman, et al (2009)
supra). Briefly, 1 mg of his-tagged recombinant protein
(rHSP) was coupled to 2 ml bed volume of IMAC resin for 2
hours at 37 C. The cobalt column was washed with ten bed
volumes of PBS (pH.8) and incubated overnight at 4 C with
200 ul of pooled sera from the mice immunized with
multivalent immunogenic composition. Supernatant containing
the depleted sera was collected by centrifugation. Anti-
HSP-depleted serum was incubated overnight at 4 C in rALT2-
coupled column. The supernatant containing anti-HSP- and
anti-ALT2-depleted serum was collected and incubated in
rTSP-coupled column. Anti-HSP-, anti-ALT2-, and anti-TSP-
depleted serum was collected and used. Depletion of IgG
antibodies against_ specific antigens was confirmed by ELISA
as described above. Antibody-depleted sera were then used
in an ADCC assay.
[00193] Analysis of in situ Cytotoxicity Against L3 Larvae
in Immunized Mice (Micropore Chamber Technique). The
protective efficacy of vaccination was analyzed by
challenging the immunized animals with infective L3 using
micropore chamber method (Abraham, et al. (1989) supra).
Micropore chambers were assembled using 14 x 2 mm
PLEXIGLASS (acrylic) rings and 5.0 pm NUCLEOPORE
polyearbonate membranes (Millipore Corporations, Bedford,
MA). After 48 hours of implantation, animals were
sacrificed and the chambers were recovered from peritoneal
cavity. Contents of each chamber were examined
microscopically for cell adherence and death of infective
L3. The parasite was considered dead if it was not motile
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-95-
and limpid, and had several adherent cells on the surface.
The percentage protection was calculated using the formula:
number of dead parasites
number of recovered parasites x
100. This experiment was repeated twice with five animals
in each group.
[00194] Splenocyte Proliferation. Vaccinated and control
mice were sacrificed on day 60 and the spleens were removed
aseptically. Single-cell suspensions were prepared in RPMI
1640 medium supplemented with 10% heat-inactivated FCS,
passed through a NYLON (aliphatic polyamide) mesh (BD
Biosciences, Bedford, USA). After determining the viability
of cells using trypan blue dye exclusion, approximately 2 x
106 cells per well in triplicates were plated in 96-well
culture plates (ThermoFisher, USA). The splenocytes were
stimulated with 1pg/100pl/well of recombinant proteins
(rHSP, rALT2 or rTSP) or ConA or with medium alone
(Unstimulated) for 72 hours at 37 C in the atmosphere of 5%
CO2. Cell proliferation was determined using cell counting
kit (CCK-8) purchased from Dojindo Molecular Technologies,
Inc. (Gaithersburg, MD). Stimulation index of spleen cell
proliferation was calculated using the formula: Absorbance
of stimulated cells
Absorbance of unstimulated cells. All
cultures were taken in triplicates and the results
expressed as mean S.I. SEM.
[00195] Real Time-PCR (RT-PCR). Cytokine levels in the mRNA
of the spleen cell pellets were analyzed by real time-PCR.
The spleen cells of vaccinated and control group mice were
cultured as above at a concentration of 2x106
cells/100pl/well in 96-well plates and stimulated with
recombinant antigens (1 pg/ml). After 72 hours, cells were
centrifuged (1000 rpm for 5 minutes) and total RNA was
extracted from the cell pellets using TRIZOL (phenol,
guanidinium and thiocyanate) reagent (Invitrogen) as per
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-96-
description of the manufacturer. Followed by RNA
extraction, first-strand cDNA was synthesized by RT2 First
Strand Kit (SuperArray Bioscience Corporation, Frederick,
MD). FOR array analysis was performed according to the
manufacturer protocol with the RT2 Real-Time TM SYBR Green
(cyanine dye) PCR Master Mix. Aliquots from this mix were
added to a 96-well plate, where each well contained
predispensed gene-specific primer sets.
Relative
quantification of the genes of interest that expressed was
measured in an Applied BioSystem 7300 real-time PCR machine
(Applied BioSystems, Foster City, CA). Cycling parameters
were as follows: 95 C for 10 minutes for activation of
HOTSTART DNA polymerase, followed by 40 cycles of
denaturation at 95 C for 15 seconds and primer extension at
60 C for 1 minute. RT-PCR data array set was generated and
analyzed using SABiosciences web-based data analysis
system. Results were expressed in terms of fold change of
immunized mice compared to control mice by normalizing the
expression of housekeeping genes.
[00196] gytokine Assay. Splenocyte cell culture supernatants
were collected after 72 hours incubation stimulated with
recombinant antigens (1 pg/m1) or with medium alone.
Secreted levels of IL-4 and IFN-y cytokines in the culture
supernatants were determined using a sandwich ELISA kit
purchased from Thermo Scientifics, USA. All concentrations
were derived from standard curves and data expressed in
pg/ml.
[00197] Construction of cHAT Plasmid and Expression of
Fusion Proteins. Since the N-terminal region of HSP is
involved in IL-10 binding, this region was deleted and cHAT
recombinant protein was prepared as 37 KDa His-tagged
protein.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-97-
[00198] Construction of Recombinant Plasmids and Expression
of Fusion Proteins. The full-length hsp, a1t2 and tsp genes
of B. malayi L3 stage were constructed with the expected
size (850 bp). These fragments were further directionally
cloned into the expression vectors pVAX1 and pRSETA with
the specified restriction enzyme cutting sites. Results of
the DNA sequence analysis confirmed gene insertion
direction. rBmHAT was expressed as a 45 KDa His-tagged
fusion protein, which was purified and analyzed in SDS-
PAGE. The results indicated that the fusion protein was
pure without any contaminating proteins. The presence of
antibodies against all the three antigens was confirmed by
immunoblot analysis.
[00199] Antibody Titer in the Immunized Mice Sera. The mean
peak antibody titer of the sera samples from the mice
immunized with prime-boost or protein vaccine was
significantly higher (p<0.001) compared to the DNA group.
Sera collected from rBmHAT-immunized animals showed the
maximum titer of 30,000 against rALT2 antigen, while the
antibody titer against rHSP or rTSP antigen was in the
range of 18,000-20,000. Similarly, the mice immunized with
the bivalent vaccine showed the maximum titer of 30,000
against ALT2 antigen while anti-HSP and anti-TSP antibodies
were in the range of 8,000-15,000.
[00200]Antibody-Dependent Cell-Mediated
cytotoxicity.
Antibody-mediated adherence and cytotoxicity of immune
cells to R. ma7ayi L3 larvae was observed after 48 hours of
incubation of parasites, with the sera and normal immune
cells. ADCC showed maximum cytotoxicity of approximately
90% (p<0.001) in the sera of mice immunized with rBmHAT or
rWbHA vaccine constructs (Table 11). Bivalent vaccine
constructs of rWbHT and rWbTA also gave better protection
of 82% and 87%, respectively, which was significant
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-98-
compared to monovalent-vaccinated and control animals
(p<0.001). To evaluate the protection mediated by the
antibodies generated against HSP, ALT and TSP antigens, IgG
antibodies were depleted from the immunized sera and used
in ADCC. Depleted antibodies showed only 6% protection
against L3.
TABLE 11
Groups % Cytotoxicity
H+A 90 2.4*
H+T 82.30 12.9*
_T+A 87.06 9.8*
H+A+T 88.69 7.5*
anti-HSP + anti-ALT + anti-
TSP antibodies depleted from 5.55+1.5
HAT immunized sera
Values represent mean+SD of three wells. *Significant
larval death (P<0.001) compared to other mice groups.
[00201] In situ Protection Study. Two weeks after the final
immunization, the ability of the vaccine candidates, to
kill the filarial parasites in the immunized animals was
evaluated by in situ micropore chamber studies. The data
was combined from the two similar experiments and
represented as mean count SEM. The analysis of percentage
reduction in worm burden compared with control showed that
multivalent immunogenic composition (HAT) conferred the
maximum protection of 100% and 94% for protein and prime-
boost vaccine, which was very significant protection (Table
12) (P<0.0001) compared to control groups (5%).
Interestingly, the percentage worm reduction of bivalent
vaccines HA, TA and HT were 90%, 80% and 82%, respectively,
which was also significantly high compared to the control.
In the entire bivalent vaccine group, prime-boost
vaccination was more protective compared to DNA and protein
vaccination.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-99-
TABLE 12
Trial 1 Trial 2 Trial 3
DNA Vaccine Protein Vaccine
Prime-Boost Vaccine
Group Group Group
Cytotoxicity Cytotoxicity
Cytotoxicity
pVAX 5 4.23 Alum 3 I 4.23
pVAX+Alum 5.9 4.23
H+A 81 11.23* H+A 78 11.23*
H+A 90 11.23*
H+T 72 12.03* 69 12.03*
H+T 80 12.03*
T+A 74 11.21* T+A 66 11.21*
T+A 82 11.21*
H+A+T 91 11.92* H+A+T 100 0*
H+A+T 94 11.92*
Values are mean SD. N=5. Data is from one of two similar
experiments showing comparable results. *Significant larval
death (P<0.001) compared to other mice groups.
[00202] Splenocyte Proliferation. Spleen cells isolated from
vaccinated and control animals were stimulated in vitro
individually with rHSP, rALT2 or rTSP to analyze the
protein-specific T-cell proliferation in vaccinated
animals. Mice immunized with the prime-boost regimen in all
the vaccine combinations and HAT as protein vaccine gave
the highest protection. Hence the splenocytes were
collected only from these animals analyzed for the immune
response. Splenocytes from bivalent- and trivalent-
vaccinated animals stimulated with respective recombinant
proteins showed significantly high (9<0.001) proliferation
(mean S.I. = 4.25-5.8) when compared to monovalent and
unstimulated controls. The proliferation index of spleen
cells immunized with the monovalent construct showed
significant proliferation. The stimulation of cells was
comparable to the positive controls.
[00203] RT-PCR Array. To determine the cellular immune
responses to multivalent constructs in the vaccinated mice,
spleen cells collected from vaccinated and control mice
were cultured in the presence of respective recombinant
proteins and their proliferative responses and cytokine
profiles were evaluated. Since the spleen cells from
vaccinated animals were proliferating significantly to
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-100-
recall response, levels of cytokine mRNA were measured. An
RT-PCR cytokine gene array was performed on mRNA collected
from the spleen cells stimulated with recombinant proteins.
These results showed that both Thl (IFN-y, IL-2) and Th2
(IL-4) cytokine genes were significantly increased in
vaccinated animals.
[00204] Cytokine Levels. After identifying the presence of
IFN-y and IL-4 cytokine expression in the mRNA isolated
from the vaccinated spleen cells, the secretion of same
cytokines in the supernatant was investigated. The data
were normalized with the unstimulated controls.
Interestingly, the cytokine profiles observed in the
supernatant exhibited significantly higher levels of IFN-y
showing a Thl-biased immune response. These results
demonstrated that recombinant proteins stimulated the
production of IFN-y and induced a Thl-mediated protective
response.
Example 5: Analysis of cHAT Vaccine in Various Adjuvant
Formulations
[00205] Preparation of cHAT. Previous studies showed that
the N-terminal sequence of BmHSP12.6 can bind to human IL-
receptor and trigger IL-10-mediated responses
(Gnanasekar, et al. (2008) Mol. Biochem. Parasitol.
159(2):98-103). Since IL-10 is an immunosuppressive agent,
the IL-10 receptor binding sequences were deleted from HSP.
The truncated sequence was referred to as cHSP. The cHSP
was then used to replace the HSP gene and HSP protein in
the multivalent HAT hybrid vaccine. Thus, the resulting new
vaccine was called cHAT.
[00206] Protection Studies Using cHAT-Fusion Protein Vaccine
in Mice. Mice were immunized with four doses of cHAT fusion
protein at two-week intervals. One month after the final
immunization, the ability of the vaccine candidates to kill
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-101-
the filarial parasites was evaluated by in situ micropore
chamber studies. Results showed that when mice were
immunized with cHAT fusion protein with alum as the
adjuvant, the vaccine conferred 81% protection (Table 13)
(P < 0.0001) compared to control groups (2%) that received
only phosphate-buffered saline (PBS) and alum. Different
adjuvants were then tested to see if changing the adjuvant
would improve the protection ability of cHAT. Two
additional adjuvants were tested: alum containing a TLR4
agonist (purchased from Infectious Disease Research
Institute, Seattle, Washington) and ALHYDROGEL (purchased
from Sigma, St. Louis, MO). cHAT with no adjuvants remained
as a control. Results from these studies (Table 13) showed
that 78% protection was achieved with alum plus TLR4
agonist and cHAT given in ALHYDROGEL adjuvant gave 70%
protection. An interesting finding in these studies was
that cHAT without any adjuvant also gave 72% protection
indicating that the cHAT fusion protein vaccine could be
administered without any adjuvant and still obtain
significant protection.
TABLE 13
Group % Larval Death (Mean SD)
cHAT + Alum 81 7.8
PBS + Alum Control 1.7 1.3
cHAT + Alum with TLR4 agonist 78 8.4
cHAT + ALHYDROGEL 70 13
cHAT With No adjuvant 72 12
Values are mean SD. N=5. Data is from one of two similar
experiments showing comparable results. *Significant larval
death (P<0.001) compared to other mice groups.
Example 6: Homologues of HSP, ALT2 and TSP
[00207] Homologues of the vaccine antigens, HSP, ALT2 and
Tetraspanin are present in 0. volvulus and L. loa.
Comparison of the nucleotide sequence of HSP, ALT2 and
Tetraspanin from 0. volvulus and L. loa show that there is
CA 03167346 2022- 8-8
WO 2021/163448 PCT/US2021/017813
-102-
significant sequence homology (>90%) between the proteins
from all filarial parasites. These findings indicate that
the cHAT fusion protein vaccine developed in Example 5 can
be used as a vaccine against 0. volvulus and L. loa.
[00208] As an example, 0. volvulus tetraspanin was cloned
from 0. volvulus L3 cDNA library and recombinant proteins
were prepared. Sera sample from mice vaccinated with cHAT
vaccine that gave the 81% protection in Table 13 was used
to probe the recombinant 0. volvulus tetraspanin after
separating the protein in a 12% SDS-PAGE gel. B. malayi
tetraspanin was used as a positive control. Results showed
that the sera sample significantly reacted with 0. volvulus
tetraspanin thereby indicating that the cHAT vaccine
developed in Example 5 is of use as a vaccine against 0.
volvulus.
Example 7: Multivalent Immunogenic Composition Against
Lymphatic Filariasis in Rhesus macaque Model
[00209] Parasites. B. malayi infective third stage larvae
(L3) were obtained from the NIAID/NIH Filariasis Research
Reagent Resource Center (University of Georgia, Athens,
GA).
[00210] Multivalent Fusion Protein rBmHAT. The multivalent
fusion protein rBmHAT expressed in Escherichia coli BL21
(pLysS), was purified and endotoxin removed by Pierce High
Capacity Endotoxin removal resin column (Thermo Fisher
Scientific, Rockford, IL) as described herein.
[00211] Immunizations of rBmHAT. Five macaques each received
200 pg of rBmHAT vaccine mixed with 100 pg of AL007 alum
(IDRI, Seattle, WA) under ABSL-2 conditions. Five (5)
macaques that received alum (AL007) only remained as
controls. Each animal was anesthetized
with
ketamine/xylazine and the vaccine was administered
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-103-
intramuscularly in each thigh (one injection site per thigh
per vaccination). Animals were immunized at 4-week
intervals on days 0, 28 and 56. Intramuscular route is
commonly used for clinical vaccine trials and hence the
same procedure was followed for macaques. The injection
sites were monitored daily for signs of fever, any adverse
reactions (redness, swelling, etc.) for up to 7 days post
immunization.
[00212] B. malayi L3 Challenge. On day 84, one month after
the final dose of vaccine, macaques were anesthetized with
ketamine HC1 and challenged subcutaneously with 400-500 B.
malayi L3. To facilitate the production of the relatively
large number of L3 (500 L3/animal) required for challenging
immunized macaques, the animals were divided into 2
subgroups within each group. The subgroups were challenged
one week apart. Before challenge, B. malayi L3 were counted
and examined for viability under a microscope. Only viable
parasites were used for challenge.
[00213] Monitoring of Each Animal After Challenge. All
animals were monitored daily for clinical signs after the
challenge. Behavioral observations were similarly conducted
during the entire post-challenge period. Clinical
monitoring included serum chemistry, hematology, complete
blood count (CBC) analysis (IDEXX) and CD4+/CD8+ T cell
flow cytometry analysis. Body weights, body condition,
lymphoedema and lymph node measurements were also recorded
each time the animal was sedated for procedures (like
immunizations, challenge, and blood collections).
[00214] Sample Collection. Blood samples and peripheral
blood mononuclear cells (PBMC) were collected. Whole blood
was collected into BD VACUTAINER SST tubes according to
manufacturer's instructions. Heparinized blood (1 ml) was
collected from the femoral vein of each animal during the
CA 03167346 2022- 8-8
WO 2021/163448 PCT/US2021/017813
-104-
immunization period and from the saphenous vein during the
challenge period. The shift in blood collection site was to
eliminate any potential interference with the inguinal
lymph node measurements or assessments of edema. Blood
samples were obtained at multiple time points during the
entire follow-up period.
[00215] Isolation of PBMC. The blood pellets after plasma
separation was diluted in phosphate-buffered saline (PBS;
1:2) and subjected to gradient density centrifugation for
30 minutes at 2200 rpm using a 90% HISTOPAQUE separation
solution (Sigma, St. Louis, MO). The opaque interface
containing mononuclear cells was collected, washed three
times in PBS by centrifugation at 800 rpm. PBMC were
enumerated using Trypan blue dye exclusion method and
resuspended in RPMI 1640 medium containing 10% FBS (100
U/ml Penicillin/Streptomycin, and 2 mM L-glutamine). PBMC
collected before the challenge was analyzed for T cell
proliferation and TFN-y secretions. PBMC collected after
the challenge experiments were tested for T cell
proliferation and ELTSPOT assays. Proliferation assay was
performed with PBMC isolated on the same day of blood
collection. PBMC suspended in RPMI media with 10% PBS were
used for ADCC assay and for cytokines analysis.
[00216] T Cell Proliferation and Flow
Cytometry.
Carhoxyfluorescein diacetate succinimidyl ester (CFSE)-
based assay was used for assessment of antigen-specific
proliferation within the T cell population (Parish, et al.
(2009) Curr. Protoc. Immunol. Chapter 4: Unit 49). A 5 mM
CFSE stock solution (Invitrogen, Grand Island, NY) was
prepared according to manufacturer's instructions. PBMC
collected four weeks after the final immunization were
gently resuspended at 10/ celis/mi in 5 pM CFSE and
incubated in the dark at 37 C for 15 minutes. Cells were
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-105-
centrifuged and washed with RPMI containing 10% FBS (100
U/ml Penicillin/Streptomycin, and 2 mM L-glutamine) and
incubated for an additional 30 minutes at 37 C. Cells were
then washed, resuspended in RPMI containing FBS, plated in
a 24-well plate at 2x106 cells/ml per well and incubated
overnight at 37 C. The medium (-500 pl) was removed the
following day and cells were stimulated with 1 pg/mL of
rBmHAT. Samples incubated only with RPMI medium served as
negative controls. As a positive control for each animal,
cells were stimulated with phytohemagglutinin (PHA). Cells
were cultured and harvested after 5 days of stimulation.
Following a washing step with PBS/0.2% FBS, cells were
surface stained with an antibody cocktail of CD3-APC-Cy,
CD4-PE and CD8-PerCP and incubated for 20 minutes at room
temperature. After an additional washing step with PBS/0.2%
FBS the cells were acquired on BD FACS CANTO II flow
cytometer (BD, San Jose, CA) and analyzed on a BD FACS DIVA
Software v6.1.2. At least 50,000 events within the live
lymphocyte gate were acquired.
[00217] Cell Counts, Serum Chemistry and Complete Blood
Count (CBC) Analysis. CBC, serum chemistries and eosinophil
counts were analyzed using commercial automated hematology
and serum chemistry analyzers by IDEXX. Samples collected
prior to the initiation of the study served as a normal
reference baseline for each animal.
[00218] Measurement of Secreted Levels of IFN-y. PBMC (1x106
cells) were stimulated in vitro with 1 pg/ml of rBmHAT for
days at 37 C. Following stimulation, the supernatants
were harvested and assayed for secreted levels of TFN-y
using an ELISA kit (Mabtech AS, Ashburn, VA) according to
manufacturer's instructions.
[00219] ELISPOT Assay. An ELTSPOT assay was performed to
determine the antigen-specific IFN-y and IL-10 secreting
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-106-
cells in the PBMC of vaccinated and control macaques. A
monkey ELISPOT kit purchased from U-Cytech biosciences
(Yalelaan, The Netherlands) was used to determine the spot
forming units as per the manufacturer's instruction. PBMC
collected 20 weeks post challenge were plated in 96-well
plates at 1x106 cells/ml and were stimulated with 100
ng/well of B. malayi adult soluble antigen (BmA) for 24
hours at 37 C and 5% CO2. Wells of ELISPOT plates were
coated with 100 p1/well of capture antibodies (anti-IL-10
or anti-IFN-y) diluted in sterile coating buffer and
incubated overnight at 4 C. Plates were washed 2 times with
sterile coating buffer. After blocking the plates with 200
pl/well of blocking buffer for 1 hour at room temperature,
PBMC that were already stimulated with BmA antigens or only
media (negative control) were added to the wells of the
ELISPOT plates at 100 p1/well and incubated for 24 hours at
37 C and 5% CO2. All the cells were removed from the plates
and the membrane was washed 3 times with sterile PBS.
Following wash, 100 pl of detection antibodies were added
to each well and incubated at room temperature for 2 hours.
After washing the plate 4 times with wash buffer, avidin-
HRP reagent was added (100 p1/well) and incubated for 45
minutes at room temperature. After a final wash with PBS,
freshly prepared 3-amino-9-ethylcarbazole (AEC) substrate
solution was added (100 p1/well) and monitored for the
development of spots at room temperature for 10-60 minutes.
The substrate reaction was stopped by washing wells 3 times
with 200 p1/well ultrapure water. The plates were air
dried. Spots were counted using a dissecting microscope.
The plates were stored in the dark prior to reading.
Antigen-specific responses were determined by subtracting
the number of spots in the negative control wells from the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-107-
wells containing antigens. Results are shown as the mean
value of spots obtained from triplicate wells.
[00220] Analysis of Serum Antibody Titers in Macaques.
Levels of IgG, IgGl, IgG2, IgG3, IgA and IgE antibodies
against rBmHSP, rBmALT2, rBmTSP or rBmHAT were determined
in the sera (collected one month after the final dose of
vaccine) of each rhesus macaque using an indirect ELISA as
described herein. Briefly, wells of a 96-well microtiter
ELISA plates were coated with 100 ng/well of antigens
(rBmHSP, rBmALT2, rBmTSP or rBmHAT) in 0.05 M carbonate-
bicarbonate buffer, pH 9.6. The wells were blocked with 3%
BSA in 0.05% PBS-TWEEN 20 (PBS-T), and 100 pl of sera
samples (diluted in the range of 1:100-1:50,000 in PBS-T)
from each macaque were added to each well. Goat anti-monkey
IgG antibodies conjugated to peroxidase (Rockland
lmmunochemicals, Gilbertsville, PA) was used as secondary
antibodies to determine IgG titer antibodies. The color was
developed using OPD substrate and absorbance was read at
492 nm in the ELISA reader (BioRad, Hercules, CA). To
determine the levels of isotype antibodies, biotinylated
anti-monkey IgG1 (1:2000), IgG2 (1:200), IgG3 (1:2000), IgA
(1:2000) and IgE (1:1000) antibodies (NHP Reagent
Resources, Boston, MA) were used as secondary antibodies.
After washing the plates, optimally diluted streptavidin
conjugated horse radish peroxidase (HRP) was added and
further incubated for 60 minutes at room temperature and
the color was developed.
[00221]ADCC Assay. PBMC were prepared from heparinized
whole blood from a naive healthy animal as described above.
Briefly, ten B. malayi L3 (suspended in 50 pl RPMI 1640
medium containing 10% FBS) were incubated with 2x105 PBMC
(in 50 pl RPMI 1640) and 50 pl of serum from each animal
(collected one month after the final dose of vaccine) in a
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-108-
96-well round bottom tissue culture plate. Five replicates
were performed for each serum sample. Control wells
contained B. malayi L3 incubated in media, with sera alone
or cells alone. The plates were incubated at 37 C with 5%
CO2 for 48 hours. Following incubation, B. malayi L3 were
examined under a microscope at 24 and 48 hours to determine
larval viability. Dead L3 were defined as those having a
limpid or straight appearance with no movements for an
additional observation period of 8 hours at 37 C. Live
larvae were active, coiled and motile. The percentage
larval death was expressed as the ratio of the number of
dead L3 to that of the total number recovered within the
experimental period multiplied by 100. Average larval death
in 5 wells were calculated and expressed as percent
protection in each animal.
[00222] Knott Test to Determine Microfilaremia OM in
Macaques. The presence of Mf in the blood of macaques was
detcctcd using the Knott technique as described previously
(Liu, et al. (1989) J. Trop. Med. Hyg. 92:93-96).
Peripheral blood of macaques was screened weekly for Mf
starting from 5 weeks to 20 weeks post challenge. Briefly,
whole blood was mixed with 9 ml of a 2% formalin solution
(prepared in PBS) in a 15 ml conical centrifuge tube. The
tubes were gently rocked for 2 minutes at room temperature
and centrifuged at 1,500 rpm for 5 minutes. The supernatant
was then thoroughly decanted by turning the tube completely
upside down to remove all the liquid. Following this 5 ml
of ACK lysis buffer (Quality Biologicals, Gaithersburg, MD)
was added to the pellet and the tube was vortexed. Two to
three drops of methylene blue solution (Fisher Scientific,
Hannover Park, IL) was then added to the tubes, gently
mixed, and smeared onto five glass slides. The samples were
allowed to dry and read under a microscope using 40X lens
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-109-
objective. A comparison of Mf counts in blood collected
from the saphenous and femoral veins showed similar
results.
[00223] Detection of Mf in the Peripheral Blood by PCR. PCR-
based assays are more sensitive in detecting the presence
of Mf in the blood samples (Mishra, et al. (2005) Acta
Trop. 93:233-7; Tao, et al. (2006) J. Clin. Microbic].
44:3887-93). Therefore, the PCR based assay was also used
to confirm the presence of Mf in the blood samples of all
macaques 20 weeks after challenge. Whole blood samples were
centrifuged at 10,000 rpm for 5 minutes and the supernatant
containing serum was stored at -20 C. DNA was isolated from
the pellet using DNEASY Blood & Tissue Kit (Qiagen,
Valencia, CA) according to the manufacturer's instruction.
Primers were synthesized at Integrated DNA Technologies
Inc., (Coralville, IA) for HhaI tandem repeats. Primer
sequences for HhaI tandem repeats were: Forward 5'-GCG CAT
AAA TTC ATC AGC-3' (SEQ ID NO:75) and Reverse 5'-GCG CAA
AAC TTA ATT ACA AAA GC-3' (SEQ ID NO:76). PCR parameters
were initial denaturation of 94 C for 5 minutes, followed
by 40 cycles of 1 minute at 94 C, 1 minute at 56 C, 1
minute at 72 C and a final extension of 10 minutes at 72 C.
Following PCR reaction, 10 pl of each PCR product was
analyzed on a 1% agarose gel.
[00224] PBMC Proliferations Assay. PBMC collected 10 weeks
post-challenge were cultured in 96-well tissue culture
plates at a concentration of 1x106 cells/well in RPMI 1640
supplemented with 10% FCS. Cells were stimulated either
with rBmHAT antigen (1 mg/ml) or Concanavalin A (1 mg/ml)
or with medium alone (unstimulated) in triplicate wells.
PBMC were stimulated in triplicate wells and the plates
were incubated at 37 C in 5% CO2. After 72 hours, cell
proliferation was measured using cell counting kit (CCK-8)
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-110-
(Dojindo Molecular Technologies, Inc., Gaithersburg, MD).
Stimulation index of PBMC proliferation was calculated
using the formula: Absorbance of
stimulated
cells/Absorbance of unstimulated cells.
[00225] Statistical Analysis. Data are represented as the
mean standard error. One-way ANOVA tests (Kruskal-Wallis)
was performed for the antibody titer and T cell
proliferation using GraphPad Prism software. Student T test
was performed for protection studies. A probability (P)
value of Ø001 was considered statistically significant.
[00226] rBmHAT Vaccination Does Not Induce Any Adverse
Reactions in Macaques. The injection sites were monitored
closely for signs of any adverse reactions (redness,
swelling, etc.) for 7 days post-immunization. There were no
adverse reactions in any of the vaccinated or control
animals. Clinical monitoring showed no dramatic loss of
body weight (>10% of the original weight), changes in
eating habits or any other behavioral changes. Temperature
measurements obtained daily following immunizations did not
show any significant variations. Temperature measurements
were also performed at regular intervals using implanted
transponders. There were no significant variations in the
body temperature in vaccinated and control animals.
[00227] The lymph nodes in the left and right leg of all
animals were monitored weekly starting approximately 2
weeks prior to challenge (to establish a baseline) and
throughout the challenge period. The lymph nodes were
measured with a caliper and observed for edema. The
measurements showed an overall increase in the mean size of
the inguinal lymph nodes in both legs during the 5-8 week
post-challenge period in all groups. Compared to the
baseline (14.5 mm) the lymph node size in control animals
were 22 1 mm and rBmHAT group were 26.2 1 mm. Following
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-111-
this period, the sizes of the lymph nodes decreased to near
pre-challenge levels in all macaques.
[00228] Challenge with B. malayi L3 did not alter the body
temperature in macaques. Analyses of the serum chemistry
and hematology (CBC) values showed that they were all in
the normal range for all cell types except for a slight
increase in the eosinophil counts following L3 challenge in
infected animals.
[00229] All Three Antigens in the Multivalent Immunogenic
Construct Were Immunogenic in Macaques. Analysis of the IgG
antibody titer in vaccinated macaques showed that all the
macaques developed high titers (1:40,000) of IgG antibodies
after third immunization against rBmHAT. The titer of
antibodies against each of the three component antigens in
the vaccine construct was then analyzed. All macaques
developed high titers of IgG antibodies against_ rBmHSP12.6
(1:16,000), rBmALT2 (1:24,000) and rBmTSP-LEL (1:16,000).
There were slight individual variations in the titer of
antibodies between each vaccinated macaque. On a
comparative basis, macaque #5242, #5258 and #5259 showed
the highest titer of IgG antibodies against the component
antigens (except anti-rBmHSP12.6 antibodies in macaque
#5258 and anti-rBmTSP antibodies in macaque #5259). Macaque
#4996 and 5254 developed only low titers of antibodies to
rBmALT2 and rBmTSP (Table 14).
TABLE 14
Antibody Titer
Animal ID
rBmHSP12.6 rBmALT2 rBmTSP rBmHAT
4996 6400 3200 16000*
40000
5242 16000* 24000** 16000*
40000
5254 6400 800 12800*
40000
5258 6400 24000** 16000*
40000
5259 16000* 24000** 6400
40000
Macaques were immunized with 200 pg of rBmHAT with alum
adjuvant. Anti-rBmHAT antibodies against rBmHSP12.6,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-112-
rBmALT2, rBmTSP LEL or rBmHAT were evaluated. Each animal
differed in the antibody titer against each antigen.
*P<0.05 and **P<0.001 statistically significant antibody
IgG antibody titer compared to other animals.
[00230] Isotype analysis showed that nearly all of the
antibodies were of IgG1 isotype against all the four
antigens tested (rBmHSP, rBmALT2, rBmTSP and rBmHAT).
Levels of IgG2, IgG3, IgA and IgE did not show any
significant difference from the background values.
[00231] rBmHAT Responding Cells Were Present in the PBMC of
Immunized Rhesus Macaques. To determine the antigen
specific proliferative responses, PBMC was collected four
weeks after the final vaccination. Cell proliferation was
determined after stimulating CFSE labeled PBMC with rBmHAT
proteins for 5 days and counting the labeled cells in a
flow cytometer. These results showed that the proliferation
frequency of antigen-responding cells in the immunized
animals were 3-fold higher (stimulation index 6.1 0.86)
compared to the control animals (stimulation index
2.2+1.42). As expected, PBMC from all the animals showed
robust proliferative responses (stimulation index 87.4 0)
upon stimulation with pan-T mitogen, PHA. PBMC cultured in
control medium had only low-level proliferation following
5-day incubation. The proliferation frequency value for
each sample was obtained by subtracting the medium alone
control value.
[00232] Frequency of CFSE-labeled CD3+, CD4+ and CD8+ PBMC
proliferating in response to antigen stimulation were
determined by flow cytometry. These studies showed that
there was an increase in the proliferation of antigen-
responding T cells in all immunized macaques compared to
control macaques. Subset analysis showed that in immunized
animals approximately 12.7% of the antigen responding T
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-113-
cells were CD4+ cells and 7.9% of T cells were CD8+
subsets. Background proliferation in the presence of rBmHAT
antigen in the PBMC of control animals were 1.4% for CD4+
cells and 2.3% for CD8+ cells.
[00233] Antigen Responding Cells in the PBMC of Immunized
Monkeys Secrete IFN-y. Antigen responding cells in the
spleen of rBmHAT immunized mice and gerbils predominantly
secreted high levels of IFN-y. Therefore, it was determined
whether macaques also show a similar response after
immunization but before challenge. These studies showed
that PBMC from three immunized macaques (#5242, #5258 and
#5259) all secreted significant amounts of IFN-y when
stimulated with rBmHAT antigen (Table 15). Culture
supernatants of PBMC from macaque #4996 and #5254 only had
background levels of 1FN-y similar to that of the PBMC from
control macaques.
TABLE 15
1FN-y Secretion (pg/ml)
Animal ID Control (alum only) Animal ID rBmHAT + alum
4995 0 4996 0
5240 0 5242
62.5
5249 0 5254 0
5252 0 5258
62.5
5253 0 5259
62.5
[00234] Anti-rBmHAT Antibodies in the Sera of Immunized
Macaques can Participate in the Killing of B. malayi L3. To
determine the protective ability of anti-rBmHAT antibodies
in the sera of immunized macaques, an in vitro ADCC assay
was performed. Results showed that the PBMC from vaccinated
macaque were able to participate in the killing of 35% of
B. malayi L3 (Table 16). When sera from individual macaques
were evaluated maximum killing potential in the ADCC was
45% in the sera of macaque #5258. Sera from macaque #5242
and #5259 also showed significant killing potential with
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-114-
38% and 35% killing respectively. Sera from macaque #4996
and #5259 had the least ADCC property with 25% and 31%
killing respectively. No larval death occurred when sera
from control macaques were used in these assays.
TABLE 16
% Larval Mean
%
Animal ID Live L3a Dead L3a
Deatha
Larval Death
4995 10 0 0 0
(control)
5240 10 0 0
5249 10 0 0
5252 10 0 0
5253 10 0 0
35% 6.1*
4996 7.5 0.6 1.5 0.6 25
5.2*
(immunized)
5242 6.5 0.6 4 0.6 38 6.9*
5254 6.5 1 3 0.6 31 7.4*
5258 6.5 1.5 5 0.6 45 6.3*
5259 7 1.2 3.5 1.2 35 11.5*
a Results are presented as Mean SD of five wells.
Significant larval death *(P<0.05) compared to other
macaques. Control wells were L3 incubated with media, cells
alone or sera alone.
[00235] Immunization with rBmHAT Conferred
Partial
Protection in Macaques. One month after the final
vaccination, all 10 monkeys were challenged with 500 B.
malayd L3 and screened for the appearance of Mf in the
peripheral blood circulation. A Knott test and PCR analysis
were used to detect Mf. The Knott test was performed weekly
from week 5 post-challenge until the animals became
positive. In these studies, challenged macaques became
positive for Mf starting from week 10 post-challenge.
During weeks 11-20 post challenge, three of the control
macaques became positive for Mf. Unfortunately, the
remaining two control macaques remained negative through
the end of the study. In the vaccinated group, three of the
macaques (#5242, #5254 and #5259) remained negative
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-115-
throughout the study. However, two of the vaccinated
macaques (#4996 and #5258) became positive for Mf. To
further confirm the infection, a PCR analysis was
performed, where Hhal antigen-specific primers were used to
amplify for the presence of Mf-specific DNA in the blood of
infected monkeys. PCR analysis confirmed infections in
macaque #5249 and #4996. The other three positive animals
identified by Knott technique were negative by PCR.
(00236] rBmHAT Responding Cells were Present in the PBMC of
Immunized Rhesus Macaques After Challenge. PBMC collected
weeks post challenge was stimulated with rBmHAT to
determine the antigen-specific T cell response. PBMC of
three animals #5242 (S.I. - 0.928 0.01), #5258 (S.I. -
1.091 0.16) and #5256 (S.I. - 1.0181 0.13) from the
vaccinated group that were negative for Mf showed
significant proliferation upon rBmHAT stimulation. Whereas,
two of the vaccinated animals #4996 (S.I. - 0.258 0.12) and
#5254 (S.I. - 0.379 0.03) positive for Mf did not show
significant proliferation upon rBmHAT stimulation. No
significant proliferation was observed in any of the
control animals #4995 (S.I. - 0.280 0.03), 5240 (S.I. -
0.415 0.09), 5249 (S.I. - 0.300 0.26), 5252 (S.I. -
0.507 0.03) or 5253 (S.I. - 0.475 0.25). S.I of PBMC
stimulated with Concanavalin was in the range of 2.0-3.8.
[00237] Eosinophil Numbers were High in Infected Macaques
Showing Mf. Microfilaremic individuals show high eosinophil
counts in their blood (Pearlman, et al. (1993) Exp.
Parasitol. 76:200-8; Pearlman, et al. (1993) J. Immunol.
151:4857-64). A similar finding was observed in rhesus
macaques as well. Absolute counts of eosinophils were
determined on weeks 13, 9, and 5 prior to challenge, on the
day of challenge and on weeks 1, 5, 10, and 14 post-
challenge. The results showed that there was an increase in
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-116-
the frequency of eosinophil numbers in the peripheral blood
of microfilaremic macaques around l0 weeks post-challenges.
One macaque (#5259) that was negative for Mf also showed
some eosinophilla. Eosinophil counts were 10-fold higher in
control macaques that had microfilariae in their peripheral
blood.
[00238] High Titer of Antigen-Specific IgG Antibodies and
Elevated Antigen-Specific Secretion of IFN-y from PBMC
Correlated with Protection in the Immunized Macaques. Since
two of the macaques in the immunized group showed presence
of infection following challenge, vaccine-induced immune
responses were compared in the two infected macaques with
similar responses in the three uninfected macaques within
the immunized group. Values before and after challenge were
compared. Values before challenge eliminated any bias due
to the challenge of parasites. Comparative immunological
values are presented in Table 17.
TABLE 17
PBMC Proliferation, Mean
S.I. S.D. (n=3)
Macaque Group Animal ID
Stimulated
Stimulated
with ConA
with rBmHAT
4995 3.260 0.01
0.280+0.03
Control 5240 3.090+0.58
0.41510.09
(immunized 5249 2.982 0.24
0.300 0.26
with alum) 5252 3.674 0.83
0.507 0.03
5253 2.58210.72
0.475 0.25
4996 3.874 0.47
0.258 0.12
rBmHAT 5242 2.170 0.43
0.928 0.001**
(immunized
with rBmHAT + 5254 2.068 0.18
0.379 0.03
alum) 5258 3.304 0.64
1.091 0.16**
5259 2.883 0.27 1.0181
0.13**
**Significant proliferation of PBMC **(P<0.001) compared to
PBMC from other macaques.
[00239] Results showed that the titer of IgG antibodies was
significantly high in the three immunized macaques that did
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-117-
not develop the infection after the challenge. Similarly,
PBMC from the same three macaques secreted higher levels of
IFN-y when stimulated with the rBmHAT antigen. PBMC from
the two immunized macaques that developed the infection
after challenge were unable to secrete similar levels of
IFN-y in response to rBmHAT stimulation. An ELISPOT assay
was performed using PBMC from vaccinated and control
macaques. Results showed that in all the infected macaques
there was a significant increase in the number of antigen-
specific IL-10 secreting cells compared to IFN-y secreting
cells. When the ratios of IFN-y to IL-10 secreting cells in
the PBMC of immunized macaques were compared, there was a
significant increase in the IL-10 secreting cells in the
two vaccinated macaques that showed infection (Table 18).
These findings suggest a clear correlation between the type
immune responses elicited and the failure to establish
infection in the vaccinated macaques.
TABLE 18
Immunological
Immunological values before L3
values after L3
challenge
challenge
Animal Antibody titer of Ratio
of
ID >12,000 ____________ IFN-y Mf IFN-y:IL-
10
rBmTSP
secreting
rBmHSP rBmALT2
LEL
cells
4995a - - - - + 1:3
5240a - - - - - 1:1
5249a - - - - + 1:11
5252a - - - - -
1:0.01
5253a - - - - +
1:13
4996b _ _ + - + 1:4
__
5242b + + + + -
1:0.003
5254b - - + - + 1:2
5258b + + + + -
1:0.001
5259b + + - + -
1:0.02
a Control, immunized with alum. b rBmHAT, immunized with
rBmHAT + alum.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-118-
Example 8: Valency Comparisons
[00240] Monovalent, bivalent and trivalent vaccination
trials of recombinant heat shock protein 12.6 (rHSP12.6),
abundant larval transcript-2 (rALT-2) and tetraspanin large
extracellular loop (rTSP-LEL) proteins were compared.
Recombinant proteins were prepared as described herein. The
bivalent immunogenic compositions and multivalent
immunogenic compositions (SEQ ID NO:70) were produced as
fusion proteins. Mice (N=5) were immunized subcutaneously
using a protein prime-boost vaccine regimen. Immunized and
control animals were challenged with live third stage
infective larvae (L3) of B. malayi using a micropore
chamber method. After 48 hours of implantation, animals
were sacrificed and the chambers were recovered from
peritoneal cavity. Contents of each chamber were emptied
and larvae were examined microscopically at 100X to assess
larval death. The results of this analysis are presented in
Table 19.
TABLE 19
Percent Larval Death
Group Protein Vaccine
(protection)
Control Alum 9 3.4
rHSP12.6 (rH) 58 7.8
Monovalent rALT-2 (rA) 78 3.7
rTSP LEL (rT) 49 2.2
rHA 81 6.5
Bivalent rAT 72 1.1
rHT 68 4.4
__
Multivalent rHAT 95 3.1
[00241] The results indicate that the multivalent
immunogenic composition synergistically enhanced the
protective immune responses in vaccinated animals compared
to monovalent and bivalent compositions.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-119-
[00242] B. malayi parasite does not mature into adults in
mice. However, vaccine-induced protection against adult
worm establishment can be determined in jirds. Therefore,
monovalent, bivalent and trivalent vaccines were evaluated
in jirds. Animals (N-10) were immunized subcutaneously with
recombinant proteins. Jirds were challenged with 100 B.
malayi L3s and worm establishment was determined on day 95
after challenge. Percent protection values were calculated
as the percent reduction in worm establishment compared
with control jirds. The results of this analysis are
presented in Table 20.
TABLE 20
Group Protein Vaccine
Percent Protection
Control Alum 15.2 3.3
rHSP12.6 (H) 70.0 12.6
Monovalent rALT-2 (A) 72.7 8.8
rTSP LEL (T) 68.1 2.4
rHA 83.3 3.3
Bivalent rAT 77.1 12.3
rHT 70.2 11.8
Multivalent rHAT 90.2 9.1
[00243] The results indicate that
the multivalent
immunogenic composition synergistically enhanced the
protective immune responses in vaccinated animals compared
to monovalent and bivalent vaccines.
Example 9: Vaccine Comparisons
[00244]Monovalent, bivalent and multivalent immunogenic
compositions of this disclosure were compared in mice,
jirds and mastomys. Animals were immunized as described,
challenged with B. malayi L3 and worm establishment was
determined. The results of these analyses are presented in
Table 21. Of note, rBmHAX immunization gave 98% protection
in mice and 97% protection in jirds. These findings show
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-120-
that both rBmHAT and rBmHAX are excellent vaccine
candidates for lymphatic filariasis.
TABLE 21
G Mice* Jirds Mastomys
roup
Test Control Test Control Test Control
;
rWbALT2a 73+3.7% 2+0% 73+1%
0+1% 71.66+8.8% 4.2+1.3%
rBmHSPa 58+7.8% 0+0% 61+0%
4+0% 69.97+12.6% 2.1+0.2%
rWbTSPa 49+2.2% 3+1% 33+2% 1+1% 68.13+2.4% 1.1+1.1%
rBmTPXa 48+2.1% 0+0% 52+2.5% 0+0% ND
ND
rWbGSTa 49+3.1% 2+1% 61+ 1% 0+0% ND
ND
rWbHA' 81+6.5% 3+3.2% ND ND
83.25+3.3% 7.2+1.1%
rWbATb 72+1.1% 1+2.1% ND ND
77.13+12.3% 5.4+2.3%
rWbHTb 68+4.4% 6+3.8% ND ND
70.23+11.8% 7.1+3.3%
rBmAX' 74+3.3% 0+0% 80+3.5 0+0% ND
ND
rWbGAb 68+2.5% 2+4.1% 72+3.3% 0+0% ND
ND
rBmHATc 98+2.1% 4+3.3% 95+3.5% 2+1% 95.23+9.1% 4.4+1.2%
rBmHAXc** 98+1.2% 3+1.0% 97+2.1% 0+0% ND
ND
aMonovalent vaccine. Wb, W. bancrofit. Bm,. B. malayi.
hBivalent vaccine. H, HSP. A, ALT2. T, TSP. X, TPX. G, GST.
cTrivalent vaccine.
*Animals were immunized s/c with four injections of 15 ug
of the vaccine antigen plus 15 pg of alum at 2-week
intervals. Test animals were challenged with 100 L3 and
worm establishment was determined on day 90 post-challenge.
The micropore chamber challenge method was used in mice. In
this method, 20 L3 were placed in a micropore chamber,
which was implanted into the peritoneal cavity. After 48
hours the chambers were removed to determine live and dead
larvae. Data mean + SD. N=10.
**Mice and jirds were immunized with 15 pg of rBmHAX plus
15 pg of alum with a total of four immunizations at 2 weeks
interval. Blood was collected on day 0, 14, 28, 42, 49 and
70 to monitor the titer of antibodies against each of the
component antigens. The following titers were observed on
day 49 (ALT-2 1:60,000; HSP 1:40,000, TPX 1:40,000). All
the animals were challenged on day 49 with 20 B. malayi L3
for mice and 100 B. malayi L3 for jirds. Worm establishment
or worm death in immunized animals was observed at 48 hours
after surgical implantation of L3 in mice or 90 days after
infection in jirds. Percent protection was calculated as
described herein.
Example 10: Tetravalent Fusion Protein (rEimHAXT) Vaccine
Antigen Against Lymphatic Filariasis in a Mouse Mode
[00245] Cloning, Expression and Purification of rBmRAXT
Recombinant Protein. GenScript (Piscataway, NJ) supplied
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-121-
the sequences of bmhsp12.6 (GENBNAK Accession No.
AY692227.1), bmalt-2 (GENBNAK Accession No. JF795950.1),
bmtpx-2 (GENBNAK Accession No. AF319997.1) and bmtsp
(GENBNAK Accession No. JF795955.1) in the pUC57 vector. The
genes were amplified using forward 5'-CGG GAT CCA TGG AAG
AAA AGG TAG TG-3' (SEQ ID NO:31) and reverse 5'-CCC GAA TTC
TTA ATG TTT CTC AAA ATA TGC TTT-3' (SEQ ID NO:89) with
restriction sites for BamHI and EcoRI. The PCR-amplified
products were cloned into the pRSETA expression vector,
transformed into competent BL21 (DE3) Escherichia coli
cells for expression of the recombinant proteins with 6X
histidine tag. Recombinant fusion proteins were purified
using immobilized metal affinity Nit-charged agarose
chromatography column sold under the tradename SEPHAROSE8
(GE Healthcare Life Sciences, Pittsburg, PA) and eluted
with 300 mM imidazole. Endotoxin in the final purified
protein preparation was removed using an endotoxin removal
column (Thermo Fisher Scientific, Rockford, IL). The
expression and purity of recombinant proteins was confirmed
in 12% SDS-PAGE gel and western blot using anti-His
antibodies (Qiagen, Valencia, CA). Protein concentration
was determined using a Bradford reagent (Thermo Fisher
Scientific).
[00246] Adjuvants. Three different adjuvant formulations
were used with recombinant BmHAXT. Alum (AL007) and Alum
plus GLA, a synthetic TLR4 agonist (AL019) was purchased
from the Infectious Disease Research Institute, Seattle, WA
and Mannosylated Chitosan (MCA) was a gift from Pacific
GeneTech, Hong Kong.
[00247] Animals and Parasite. Six to eight weeks old Balb/c
mice purchased from Taconic biosciences (Hudson, NY) were
used in these experiments. Use of animal in this study was
approved by the animal care committee of the University of
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-122-
Illinois, Rockford following the National Institutes of
Health guidelines for the care and use of laboratory
animals. The infective larval stage (L3) of B. malayi was
obtained from the NIAID/NIH Filariasis Research Reagent
Resource Center (University of Georgia, Athens, GA).
[00248] Immunization of Balb/c Mice. For the immunization,
mice were randomly divided into seven groups of five mice
per group: (1) rBmHAXT + AL007 given s/c, (2) rBmHAXT +
AL019 given s/c, (3) rBmHAXT + MCA (first dose s/c and
booster doses given orally), (4) AL007 control given s/c,
(5) AL019 control given s/c, (6) MCA control (first dose
s/c and booster doses given orally), (7) rBmHAXT + MCA
control (all doses were given orally). Each mouse received
three doses of 15 pg of rBmHAXT and 15 pg of respective
adjuvant formulation at 15 days interval.
[00249] Collection of Serum, Peritoneal Fluid and Spleen.
Blood samples were collected from the submandibular vein of
each mouse on day 0 (pre-immune), and then two weeks after
each immunization and kept at room temperature for 1 hour
to clot. Serum was separated, and aliquots were kept frozen
at -80 C for further use. Peritoneal cavity was washed with
500 pl of sterile saline solution and the fluid was
collected from each mouse and processed. Spleen was then
collected from each animal, washed three times with
complete RPMI-1640 medium supplemented with 10% FBS and 1X
antibiotic/mycotic solution (Sigma, St. Louis, MO).
[00250] Titer of IgG Antibodies in the Serum and Peritoneal
Fluids. The titers of rBmHAXT-specific IgG antibodies in
the sera samples and in the peritoneal fluids were
evaluated using an indirect ELISA. Wells were coated with 1
pg/ml of rBmHAXT overnight at 4 C. After washing and
blocking of the plates, diluted (1:100, 1:1,000, 1:5,000,
1:10,000, 1:20,000 and 1:40,000) sera or peritoneal fluid
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-123-
samples were added and incubated for 1 hour at room
temperature. HRP-conjugated chicken anti-mouse IgG
antibodies (Thermo Fisher scientific) were used as the
secondary antibodies and color was developed using the 1-
step Ultra TMB-ELISA substrate (Thermo Fisher Scientific).
The reaction was stopped using 0.16 M H2SO4, and optical
density was determined at 450 nm in a BioTek Synergy 2
ELISA reader.
[00251] Levels of Antigen Specific Antibody Isotypes in the
Serum and Peritoneal Fluids. Levels of rBmHAXT-specific
antibody isotypes (IgGl, IgG2a, IgG2b, IgG3, IgE, IgM) were
determined in the sera and peritoneal fluid samples using
an indirect ELISA. Respective isotype-specific biotinylated
goat anti-mouse antibodies (Sigma) and streptavidin-HRP
(1:20,000) were used as the secondary antibodies. Color was
developed with 1-step Ultra-TMB. The reaction was stopped
using 0.16 M H2504, and optical density was determined at
450 nm in a BioTek Synergy 2 ELISA reader.
[00252] Challenge Studies. To determine vaccine-induced
\
protection, a micropore chamber challenge method was used
as described previously (Dakshinamoorthy, et al. (2013)
Vaccine 31(12):1616-22). Briefly, 20 L3s of B. malayi were
placed in a micropore chamber and surgically implanted into
the peritoneum of each mouse. Seventy-two hours after
implantation, the micropore chambers were recovered.
Contents of each chamber were emptied and larvae were
counted and examined microscopically for adherence of cells
and for larval death. Larvae that were clear, straight and
with no movement were counted as dead. Larvae that were
active, coiled and translucent were counted as live. The
percentage of protection was expressed as the number of
dead parasites/number of total parasites recovered x 100.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-124-
[00253] Levels of Secreted Cytokines in Culture Supernatant
of Splenocytes. A single cell suspension of spleen cells
was prepared and stimulated with 1 pg/ml of rBmHAXT or
ConA. Unstimulated spleen cells were kept as negative
control for the assay. After a 72-hour incubation, culture
supernatants were collected and levels of IL-2, IL-4, IL-6,
IFNy, TNFa, IL-10, and IL-17A were determined using a
cytokine bead array kit (BD Bio Sciences, San Jose, CA).
[00254] Analysis of T Cell Subsets by Flow Cytometer. Spleen
cells from the above cultures were then washed and labeled
with fluorescent labeled anti-mouse CD3 (ABC), CD4 (PE) and
CD8 (PE/cya7) and the percent population of each cell type
was determined in a flow cytometer. Briefly, cells were
incubated with FeyII receptor blocker in staining buffer
(2% FBS + 0.1% sodium azide) for 30 minutes at 4 C with
subsequent wash in staining buffer. All three fluorescent-
labeled antibodies were added to the cells and incubated
for 1 hour at 4 C in the dark. After washing with staining
buffer, cells were fixed in 4% paraformaldehyde and
analyzed in a BD FACSCaliburTM (BD Biosciences) flow
cytometer.
[00255]Another set of cells from the above experiment was
stained with CD3 (ABC) and within the CD3-gated population,
the CD62L (PE/Cya7) and CCR7 (PE) positive T cells were
identified as T-central memory cells. The cell population
was also stained for intracellular IFN-y (FITC) to
determine the percent of 1FN-y positive T-central memory
cells.
[00256] Statistical Analysis. Data presented are mean
standard deviation (SD). Statistical significance of mean
differences among different sample groups was analyzed
using non-parametric Kruskal-Wallis test followed by
Bonferroni correction for multiple tests using SPSS
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-125-
software (v24.0, IBM, NY). The significance level was
defined as P<0.05.
[00257] Titer of rBmHAXT-Specific IgG Antibody. Recombinant
BmHAXT protein was prepared and expressed. On the SDS-PAGE
gel, the molecular mass of rBmHAXT was approximately 60 kDa
and appeared as a single band. Endotoxin levels in the
final purified preparations was <3 EU/0.1 mg of protein.
The titer of rBmHAXT-specific IgG antibody was high
(1:20000) in the sera of the rBmHAXT + AL007 group and in
the rBmHAXT + AL019 group (p<0.05). However, the titer was
less (1:10000) in rBmHAXT + MCA group. In rBmHAXT + MCA
group where all the doses were given orally, there was very
little titer of antigen-specific antibodies, nearly same as
AL007, AL019 and MCA adjuvant control groups. Similarly,
when the peritoneal fluids were analyzed, high titer of
antigen-specific IgG antibody in rBmHAXT + AL019 and
rBmHAXT + AL007 vaccinated animals were observed compared
to rBmHAXT + MCA group. The titer of IgG antibodies was
less in the peritoneal fluids when compared to the
respective sera samples from the same animals.
[00258] Antibody Isotypes in Serum and Peritonea] Fluid. To
determine the type of humoral immune response generated
against rBmHAXT, the antibody isotypes IgGl, IgG2a, IgG2b,
IgG3, IgE, IgM and IgA were determined in serum and
peritoneal fluid samples. The results showed that IgG1 was
the predominant isotype of antibodies in all vaccinated
groups (p=0.0001) except rBmHAXT + MCA (all oral dose)
group, which was similar to the controls. Titer of IgG2a
and IgG2b antibodies were also significantly high in all
vaccinated animals (p<0.05) compared to controls except in
rBmHAXT + oral MCA vaccinated group. Titer of IgG3, IgE,
IgM, and IgA antibodies in the vaccinated animals did not
show any significant changes compared to the controls.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-126-
Significantly, high titers of IgG1 antibodies were present
in the peritoneal fluids of rBmHAXT + AL007 and rBmHAXT +
AL019 vaccinated animals (p=0.0001) compared to rBmHAXT +
MCA subcutaneous group and controls. Titer of IgG2a and
IgG2b antibodies in the peritoneal fluid of all vaccinated
animals were not significantly different from the controls.
[00259] Vaccination with rBmHAXT+AL019 Conferred Maximum
Protection. Vaccine-induced protection was determined using
a micropore chamber challenge method. The results showed
that maximum protection was observed in animals vaccinated
with rBmHAXT+AL019 (88.05 3.9%; p=0.0001) followed by
rBmHAXT+AL007 (79.47 2.6%; p=0.0001) and rBmHAXT+MCA
(78.67 5.47%; p=0.0001). Several cells were found
attached to the surface of the dead larvae. These results
indicate that AL019 may be a better adjuvant for rBmHAXT
compared to AL007 (p=0.0037) and MCA (p-0.02). Vaccination
with rBmHAXT + oral MCA conferred only 17.97 5.75%, which
was similar to the adjuvant control groups (AL007, 20.55%;
AL019, 24.19%; and MCA, 15.742%).
[00260] Spleen Cells from
rBmHAXT-Vaccinated Animals
Secreted Both Thl and Th2 Cytokines. Cytokines level in the
culture supernatants of spleen cells were determined using
a cytokine bead array. The results showed that secreted
levels of Thl (IFN-y, IL-2, IL-6, IL-17A) and Th2 (IL-4 and
IL-10) cyLokines were significantly (p<0.05) increased in
the culture supernatants of spleen cells from rBmHAXT+AL019
and rBmHAXT+AL007 vaccinated animals compared to the
respective adjuvant control animals. Spleen culture
supernatants from rBmHAXT+MCA vaccinated animals had
significantly high levels of IFN-y (p=0.01) and IL-6
(p=0.0001) compared to the respective adjuvant control.
However, there was no significant difference in the levels
of the other cytokines measured. Cytokine levels in the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-127-
culture supernatants from rBmHAXT + oral MCA group were
similar to the adjuvant controls.
[00261] Tcm Cells were Generated in the Spleen of rBmHAXT-
Vaccinated Animals. Spleen cells were cultured at 37 C for
72 hours, stimulated with 1 pg/ml of rBmHAXT protein. After
72 hours, cells were harvested and stained with CD3/CD4/CD8
antibodies and evaluated via flow cytometer. There was a
slight but significant increase in the CD8+ cell population
in the rBmHAXT+AL019-treated group (p<0.05) compared to the
other groups. To determine the percent of Tcm cells in the
spleen, splenocytes were stained with CD62L/CCR7 antibodies
and analyzed in a flow cytometer. Cells that were dual
positive for CD62L/CCR7 were considered to be Tcm cells. The
results showed that rBmHAXT-treated animals showed high
percentage of Tcm cells irrespective of the adjuvant used
(p<0.001).
[00262] Tcm Cells were Predominantly IFNy+. IFN-y secreting
Tcm cells are believed to play a major role in vaccine-
induced protection in parasitic infections (Maggioli, et
al. (2016) Front. Immunol. 7:421). Therefore, the
percentage of CD62L+ CCR7+ Tcm cells that expressed
intracellular IFN-y was measured. The results showed that
cells from rBmHAXT+AL019-vaccinated animals had a
significantly (p<0.01) high percentage of IFNy+ Tcm cells
compared to rBmHAXT+AL007- and rBmHAXT+MCA-vaccinated
groups.
Example 11: Prophylactic Vaccine Against Human Lymphatic
Filariasis in Non-Human Primates
[00263] Non-Human Primates. Forty male or female disease-
free rhesus macaques (3 to 5 years old) were purchased from
PrimGen (Hines, IL) and housed at the Bioqual's facility at
Rockville, MD. All animals were screened for the absence of
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-128-
filarial infections prior to enrolling them in the study by
analyzing the blood for the presence of microfilarial Hha-1
by PCR (Hoti, et al. (2003) Acta Trop. 88:77-81; Rao, et
al. (2006) J. Clin. Microbiol. 44:3887-3893) and serum for
the presence of antibodies against rBmSXP-1 (Vasuki, et al.
(2003) Acta Trop. 86:109-114; Abdul Rahman, et al. (2007)
Filaria J. 6:10) and rBmHAXT proteins were analyzed using
enzyme-linked immunosorbent assay (ELISA). Animals that
were positive for any of the proteins were not enrolled in
the study.
[00264] Parasites. Brugia malayi infective third stage
larvae (L3) were obtained from the NIATD/NIH Filariasis
Research Reagent Resource Center (University of Georgia,
Athens, GA).
[00265] Adjuvants. Two different adjuvants were compared in
this study. Alum (AL007) and Alum plus a synthetic TLR4
agonist GLA (AL019) purchased from Infectious Disease
Research Institute, Seattle, WA.
[00266] Cloning and Expression of Multivalent Recombinant
Proteins. rBmHAT protein was expressed in Escherichia coli
BL21 (DE3), purified and analyzed as described herein. The
coding sequence (CDS) of multivalent fusion protein rRmHAT
(composed of bmhsp 12.6, bmalt-2 and bmtsp) and rBmHAXT
(composed of bmhsp 12.6, bmalt-2, bmtpx2 and bmtsp) were
synthesized at GenScript (Piscataway, NJ). The sequences
were provided in pUC51 vector. Both CDS were PCR amplified
using the same gene specific primers (Forward primer: 5'-
CGG GAT CCA TGG AAG AAA AGG TAG TG-3', SEQ ID NO:31 &
Reverse primer: 5f-CGG AAT TCT CAA TCT TTT TGA GAT GAA T-
3', SEQ DI NO:36) with restriction sites for BamHI and
EcoRI and cloned into the expression vector pRSETA
(Invitrogen, Carlsbad, CA) with the 6X Histidine tag. The
ligated constructs for both bmhat and bmhaxt were further
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-129-
transformed into the expression strain of E. coli BL21
(DE3). Expression of recombinant proteins was induced with
1 mM IPTG. The recombinant proteins were purified using
nickel affinity column chromatography (GE Healthcare Life
Sciences, Pittsburg, PA) and the purity of the recombinant
proteins was confirmed in 12% SDS PAGE gel and by western
blot using anti-penta His antibodies (Qiagen, Velencia,
CA). Endotoxin in the final prep was removed using an
endotoxin removal column (Thermo Fisher Scientific,
Rockford, IL). Final concentration of rBmHAT and rBmHAXT
proteins was determined by Bradford assay (Qiagen).
[00267] Immunization or Macaques. This was a double-blinded
vaccination trial. A total of 40 macaques were randomly
divided into three treatment groups and one control group
with 10 macaques per group. All the treated animals
received four doses of 150 pg of the vaccine antigen and 2
mg of the adjuvant on days 0, 28, 56 and 84. Treatment
group 1 received rBmHAT + alum, treatment group 2 received
rBmHAT + AL019 and treatment group 3 received rBmHAXT +
AL019. Control animals received AL019 adjuvant only. The
injection sites were monitored daily for any adverse
reactions (redness, swelling, etc.) for up to 7 days post-
immunization. Blood samples were collected prior to each
immunization and before challenge.
[00268] Cell Counts, Serum Chemistry and Complete Blood
Count (CDC) Analysis. CBC and serum chemistries were
analyzed using commercial automated hematology and serum
chemistry analyzers by IDEXX. Samples collected prior to
the initiation of the study served as a normal reference
baseline for each animal.
[00269]Antigen-Specific Antibody Levels in Macaque Sera.
Levels of rBmHAT-, rBmHAXT-, rBmHSP-, rBmALT-2-, rBmTPX-,
or rBmTSP-specific total IgG, IgGl, TgG2, IgG3, IgM and IgE
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-130-
antibodies were determined in the sera of each rhesus
macaque using an indirect ELISA as described elsewhere
herein.
[00270] Antibody-Dependent Cell-mediated Cytotoxicity (ADCC)
Assay. ADCC assay was performed as described elsewhere
herein. Approximately ten live B. malayi L3 each were
incubated at 37 C with 5% CO2 in triplicate wells along with
2x105 PBMC and 50 pl of sera samples. Seventy-two hours
after incubation, viability of B. malayi L3 was determined.
The percentage larval death was expressed as the ratio of
the number of dead L3 to the total number recovered from
each well multiplied by 100.
[00271] The culture supernatant from the ADCC assay was also
collected to determine the level of myeloperoxidase (MPO)
activity using a kit purchased from Biovision (Milpitas,
CA) and the values are expressed as mil/minute in per ml of
culture supernatant.
[00272] Antigen-Specific Proliferation of Peripheral Blood
Mononuclear Cells (PBMC). PBMC (1x107 cells/well in 1 ml)
were incubated at 37 C in the dark for 15 minutes with 5 mM
of carboxyfluorescein diacetate succinimidyl ester (CFSE).
Following incubation, cells were washed, incubated for an
additional 30 minutes at 37 C and plated at 2x106 cells/well
in 1 ml in a 24-well tissue culture plate. Five hundred pl
of the medium was removed the next day and rRmHAXT (1 pg in
500 pl) was added to the wells. Cells incubated with RPMI
medium alone served as negative controls and concanavalin A
(1 mg/well)-stimulated cells served as positive controls.
Cells were harvested on day five after culture, labeled
with anti-CD3-APC antibody, fixed in 4% paraformaldehyde
for 10 minutes at room temperature and data acquired on a
BD FACSCaliburTM flow cytometer and analyzed using ModFit LT
software (Verity Software House, Topsham, ME).
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-131-
[00273] Parallel cultures of cells incubated for 3 days were
harvested, paraformaldehyde fixed and labeled with
combinations of anti-CD3-APC, anti-CD4-FITC, anti-CD8-PE,
anti-CD28-PE, or anti-CCR7-FITC. Intracellular IFN-y and
IL-4 were determined after fixing and permeabilization.
Data was acquired on BD FACSCalibur' flow cytometer and
analyzed using Flow Jo v10.1 (FlowJo, LLC, Ashland, OR).
[00274] Secreted levels of cytokines (GM-CSF, IFN-y, IL-
12p70, IL-113, IL-4, IL-5, IL-6, IL-15, IL-16 and TNF-a) in
the cell culture supernatants were measured using an
antibody-based Rhesus Cytokine Quantibody Array GS1
(RayBiotech, Inc., Norcross, GA) according
to
manufacturer's protocol. The intensity of the fluorescence
signals from the slide arrays was scanned and data analyzed
after subtracting the background signals and normalization
to positive controls.
[00275] B. malayi Infective Larval (L3) Challenge. One month
after the last immunization, all macaques were challenged
subcutaneously with 130-180 B. malayi L3. All the larvae
were examined microscopically for their viability, counted,
and only the viable larvae were used for challenge.
Following challenge, all the macaques were monitored
routinely for any possible alterations in the clinical
biochemistry panel (serum chemistry, hematology, complete
blood count analysis and CD4+/CD84- T-cell counts), physical
parameters (signs of adverse reactions at the site of
challenge, body weights, body temperature, body condition,
lymphoedema and lymph node measurements) and behavioral
patterns.
[00276] Confirming the Establishment of Challenge Infection
in Macaques. It is practically difficult to count the
number of adult worms established in each macaque. However,
several pieces of evidences were used to confirm the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-132-
presence of active infection in macaques including
microscopic and hha-I PCR analyses. For microscopic
analysis, on week 5, 10, 15 and 18 post-challenge, 10 ml of
blood was collected from each macaque between 6-10 pm and
screened for the presence of microfilariae using a modified
Knott technique. For Hha-I PCR analysis, DNA isolated from
200 pl of blood samples using the Gen Elute blood genomic
DNA kit (Sigma-Aldrich) were PCR amplified for Hha-I tandem
repeat genes as described previously (Hoti, et al. (2001)
Bull. Entomol. Res. 91:87-92) and the amplified PCR
products were sequenced to confirm the Hha-I sequence.
[00277] Titer of Anti-rBmSXP-1 Antibodies in Sera. BmSXP-1
is a highly sensitive and specific diagnostic antigen for
B. malayi infections. Typically, a single sex infection
will not have any Mf in the peripheral circulation. Thus,
microscopy and PCR approach may not detect these dormant
infections. However, presence of active worm in infected
subjects or animals can be confirmed by determining the
titer of IgG4 antibodies against BmSXP-1 antigen. An ELISA
was used to determine the titer of these antibodies in the
sera of macaques. Since no reliable commercial anti-macaque
IgG4 antibodies were obtainable, titer of anti-rBmSX5-1 IgG
antibodies was used as a marker to detect dormant
infections.
[00278] Lymphoscintigraphy Analysis. The lymphoscinLigraphic
analysis was carried out as described elsewhere herein.
[00279] Statistical Analysis. Data presented are mean
standard deviation (SD). Statistical significance of mean
differences among different sample groups was analyzed
using non-parametric Kruskal-Wallis test followed by
Bonferroni correction for multiple tests using SPSS
software (v24.0, IBM, NY). The significance level was
defined as P<0.05. To analyze the vaccine-induced
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-133-
protection, Chi-square test was used to compare the
proportions across the groups and Fisher's exact test was
used where appropriate. Odds ratios (OD) were calculated to
determine the differences between groups.
[00280] Immunization with the Vaccine Candidates Generated
High Titer of Antigen-Specific IgG Antibodies and Their
Isotypes. Molecular mass of rBmHAT is approximately 39 kDa
and rBmHAXT is approximately 60 kDa. Both proteins were
prepared to over 95% purity with endotoxin levels <10 EU/pg
of the protein. Immunization of macaques with the purified
proteins generated high titer of antigen-specific IgG
antibodies. The IgG antibodies were specific to each
component of the fusion protein. Maximum IgG antibody titer
was achieved after the second dose of immunization (Table
22) indicating that two doses of vaccine is sufficient to
achieve maximum antibody titer.
TABLE 22
Macaque Groups Immunizations
(n=10) First Second Third
Fourth
rBmHAT+AL007 1:1250 1:20000 1:20000
1:20000
rBmHAT+AL019 1:10000 1:20000 1:20000
1:20000
rBmHAXT+AL019 1:1250 ____ 1:20000 1:20000 1:20000
AL019 1:125 1:125 1:125
1:125
rBmHAT, recombinant Brugia malayd HSP12.6+ALT-2+TSPLEL;
rBmHAXT, recombinant B. malayi HSP12.6+ALT-2+TPX-2+TSPLEL.
[00281] IgG1 and IgG2 were the most predominant isotype of
IgG antibodies in the sera of all immunized animals. Levels
of IgG3 antibodies were significantly elevated in the sera
of rBmHAXT plus AL019 immunized group compared to the
control animals. IgM and IgE antibodies were not
significantly different from controls in the sera of
vaccinated animals.
[00282] Antigen Responding Memory Cells were Present in the
Blood of Vaccinated Macaques. Proliferation index of PBMCs
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
¨134¨
from vaccinated animals was high in response to the
respective antigens compared to controls. Cells from
control group replicated only once during the five days in
culture compared to the vaccinated group where the cells
divided up to eight generations. Evaluation of the memory
cell population within the dividing antigen-responding CD4+
and CD8+ T cells showed that both CD28+CCR7- effector memory
T cells (TEm) and CD28+ CCR7+ central memory T cells (Tcm)
were selectively expanded in rBmHAXT plus AL019 and rBmHAT
plus AL007 immunized macaques. The TENT cells predominantly
were positive for intracellular IFN-y and Tcm cells
predominantly positive for intracellular IL-4. Analysis of
the culture supernatants of the PBMCs showed a marked
increase in the secreted levels of cytokines (GM-CSF, IFN-
y, IL-12p70, IL-1p, IL-4, IL-5, IL-6, IL-15, IL-16 and TNF-
a) compared to AL019 controls. PBMCs from rBmHAXT+AL019
vaccinated animals secreted nearly 10-fold higher levels of
IFN-y compared to AL019 controls.
[00283] Immunization with rBmHAXT Conferred
Maximum
Protection. Ten weeks after challenge with B. malayi L3,
seven out of 10 control animals (70%) showed Mf in their
peripheral blood and they continued to be positive for Mf
until completion of the experiment (18 weeks post-
challenge). However, only 3 out of 10 macaques (30%) in the
rBmHAXT plus AL019 group showed Mf in their blood (FIG. 4).
These findings were further confirmed by PCR analyses of
the blood samples for the presence of B. ma/ayi-specific
Hha-1 and by an ELISA for IgG antibodies against SXP-1
antigen. Repeat examination of the blood did not show any
evidence challenge of infection in any of the negative
animals until completion of the experiment (18 weeks post)
indicating that immunization with rBmHAXT plus AL019
conferred 57.14% protection after adjusting the 30% of
CA 03167346 2022- 8- 8
WO 2021/163448
PCT/US2021/017813
-135-
amicrofilaremic macaques in the control group (p=0.073,
odds ratio=0.18). Five out of 10 animals in the
rBmHAT+AL019 group (p=0.649, odds ratio=0.42) and 7 out of
animals in the rBmHAT+AL007 group (p=1.0, odds
ratio=1.0) were positive for Mf. Statistical correlation
between different groups showed that rBmHAXT+AL019
conferred better protection than other vaccinated groups
and AL019 control (rBmHAXT+AL019 vs AL019 control -
p=0.073; rBmHAXT+AL019 VS rBmHAT+AL019
p=0.649;
rBmHAXT+AL019 vs rBmHAT+AL007 - p=0.073 and rBmHAT+AL019 vs
rBmHAT+AL007 - p=0.649). Thus, based on the odds-ratio, the
odds that the AL019 control group would be positive for Mf
were 2.33 times higher than the odds that rBmHAT+AL019
group would be Mf positive. The odds that the AL019 control
group would be positive for Mf were 5.43 times higher than
the odds that the rBmHAXT+AL019 group would be Mf positive.
The odds that the rBmHAT+AL019 group would be positive for
Mf were 1.95 times higher than the odds that the
rBmHAXT+AL019 group would be Mf positive. These statistical
analyses indicate that protection conferred by
rBmHAXT+AL019 immunization was significantly better than
rBmHAT+AL007 and rBmHAT+AL019 immunizations.
[00284]An antibody dependent cellular cytotoxicity (ADCC)
assay was also performed as an in vitro surrogate for
determining vaccine-induced protection. These assays also
confirmed that sera samples from macaque vaccinated with
rBmHAXT+AL019 were more efficient in killing the B. malayd
L3 in vitro compared to the sera samples from the other two
vaccinated groups (FIG. 5). Several cells were found
attached to the dead larvae. In fact, many of the dead
larvae were totally covered by cells within 24 hours after
incubation. In this study the morphology of the cells was
not identified. However, previous studies suggest that the
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-136-
bound cells are predominantly monocytes. To determine the
activation status of these cells, levels
of
myeloperoxidases (MPO) in the culture supernatants from
these ADCC assays were measured. The results showed an
increase in the secreted levels of MPO (2.08 to 2.78
mmol/min/ml) in the wells with sera from vaccinated animals
and PBMCs compared to the wells with sera from control
animals (1.99 mmol/min/ml), indicating activation of MPO
producing cells.
[00285] Mf-Negative Vaccinated Animals did not Develop
Lymphatic Pathology. Adult filarial parasites living within
the lymphatic vessels cause severe inflammation leading to
lymphedema and blockage of lymph flow. The presence of
lymphatic blockage in this study was determined by
lymphoscintigraphy. Lymph flow was compared between the
right and left leg in the same animal. Challenge infections
were given on the right leg. The results showed that on
week 16 post-challenge, there was a significant reduction
in the lymph flow in the right leg of all Mf positive
animals compared to their left leg indicating lymph
blockage. Lymph flow did not show any significant
differences between the right and left leg in all Mf
negative animals in the vaccinated group. These findings
thus demonstrate that lymphatic pathology was minimal or
was absent in vaccinated and challenged macaques that did
not show Mf in their peripheral blood.
Example 12: Prophylactic Vaccine Against Dirofilaria
immitis Infection in Dogs
[00286] Immunization Protocol. Six dogs were divided into
two groups of three animals per group. Each animal of the
first group received three rounds of 100 pg dose of rBmHAXT
vaccine plus 400 pl of AL019 adjuvant (40 pg TLR4 agonist +
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-137-
800 pg alum) s/c. Each animal of the second group was used
as a control and received three rounds of 400 pl of AL019
adjuvant only s/c. Injections were performed on days 0, 30,
and 60. In addition, at days 0, 30, 60, and 90, 20 mL of
blood was collected in EDTA tubes from the saphenous vein
of each dog prior to immunization. All animals were
monitored for adverse reactions including injection site
reactions, fever, loss of appetite, allergy, hair loss and
weight loss.
[00287]Antigen-Specific Antibody Levels in Dog Sera. Levels
of rBmHAXT-specific total IgG, Ig01, IgG2, IgG3, IgM and
IgE antibodies were determined in the sera of each dog
using an indirect ELISA as described elsewhere herein.
[00288] Antibody-Dependent Cell-mediated Cytotoxicity (ADCC)
Assay. ADCC assay was performed as described elsewhere
herein. Eight to ten live D. immitis L3 each were incubated
at 37 C with 5% CO2 in duplicate wells along with 0.5
million PBMC isolated from normal dog blood, 200 pl RPMI
1640 medium and 100 pl of sera samples. Plates were
monitored under light microscope every 24 hours for
viability of D. immitis L3. Larvae that were limpid, non-
motile or slowly motile were considered dead. The
percentage larval death was expressed as the ratio of the
number of dead L3 to the total number recovered from each
well multiplied by 100.
[00289] Immunization with rBmHAXT Vaccine Generated High
Titer of Antigen-Specific IgG Antibodies and Their
Isotypes. Immunization of dogs with the purified rBmHAXT
protein generated high titer of antigen-specific IgG
antibodies (over 1:20000). There was also a significant
increase in IgGl, IgG2 and IgA antibodies in the serum of
dogs after three immunizations compared to the control
group, with IgG1 and IgG2 being the most predominant
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-138-
isotype of IgG antibodies. Analysis of the sera samples for
the presence of protective antibodies against D. immitis
infective larvae showed that significant levels of
protective memory antibodies were present in the sera of
vaccinated animals. These protective
antibodies
proliferated in response to rBmHAXT and were able to kill
both drug-sensitive (-60%) and drug-resistant (-85%) of
infective larvae after two to three immunizations (FIG. 6).
Several cells were found attached to the dead larvae.
Further, culture supernatants of spleen cells from the
vaccinated dogs showed elevated levels of TNF-ot and IL-10.
These findings suggest that rBmHAXT is an excellent vaccine
candidate against heartworm infections in animals such as
dogs and cats.
[00290]All dogs were challenged s/c with 50 drug-sensitive
D. immitis infective larvae. Six months after the
challenge, all animals were euthanized to recover the
established adult worms from each dog. The results showed
that the mean worm load in rBmHAXT vaccinated animals was 7
1.73 compared to the AL019 control group, which had a
mean worm load of 29.3 3.51. This study demonstrated that
vaccination with rBmHAXT in dogs, conferred 76.11%
protection against a challenge infection with drug-
sensitive D. immitis. Following challenge infection, fewer
male and female worms were established in the rBmHAXT
vaccinated dogs.
Example 13: Comparison of rEMHAXT and rDIMAX Vaccines
Against Dirofilaria immitis Infection in Mice
[00291] Cloning, Expression and Purification of rBmHAXT
Recombinant Protein. GenScript (Piscataway, NJ) supplied
the sequences of bmhsp12.6 (GENBNAK Accession No.
AY692227.1), bmalt-2 (GENBNAK Accession No. JF795950.1),
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-139-
bmtpx-2 (GENBNAK Accession No. AF319997.1) and bmtsp
(GENBNAK Accession No. JF795955.1) in the pUC57 vector. The
genes were amplified using forward 5'-CGG GAT CCA TGG AAG
AAA AGG TAG TG-3' (SEQ ID NO:31) and reverse 5'-CCC GAA TTC
TTA ATG TTT CTC AAA ATA TGC TTT-3' (SEQ ID NO:89) with
restriction sites for BamHI and EcoRI. The PCR-amplified
products were cloned into the pRSETA expression vector,
transformed into competent BL21 (DE3) E. coil cells for
expression of the recombinant proteins with 6X histidine
tag. Recombinant fusion proteins were purified using
immobilized metal affinity Nit-charged
agarose
chromatography column sold under the tradename SEPHAROSE0
(GE Healthcare Life Sciences, Pittsburg, PA) and eluted
with 300 mM imidazole. Endotoxin in the final purified
protein preparation was removed using an endotoxin removal
column (Thermo Fisher Scientific, Rockford, IL). The
expression and purity of recombinant proteins was confirmed
in 12% SDS-PAGE gel and western blot using anti-His
antibodies (Qiagen, Valencia, CA). Protein concentration
was determined using a Bradford reagent (Thermo Fisher
Scientific).
[00292] Cloning, Expression and Purification of rDiHAX
Recombinant Protein. The contig nucleotide sequence of
DiHSP 12.6 (gene=nDi.2.2.2.g00663),
DiALT-2
(gene=nDi.2.2.2.g08197) and DiTPX-2 (gene=nDi.2.2.2.g06574)
were obtained from Wormbase ParaSite by blasting BmHSP
12.6, BmALT-2 and BmTPX-2 sequences. BmHSP 12.6, BmALT-2
and BmTPX-2 proteins were found to share 97%, 63% and 96%
sequence similarity with DiHSP 12.6, DiALT-2 and DiTPX-2
proteins, respectively. The nucleotide and amino acid
sequence of DiHSP are provided in SEQ ID NO:90 and SEQ ID
NO:91, respectively. The nucleotide and amino acid sequence
of DiALT-2 are provided in SEQ ID NO:92 and SEQ ID NO:93,
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-140-
respectively. The nucleotide and amino acid sequence of
DiTPX are provided in SEQ ID NO:94 and SEQ ID NO:95,
respectively. The nucleotide sequences of DiHSP 12.6,
DiALT-2 and D1TPX-2 were linearly combined and the
resulting dihax gene was synthesized by Invitrogen Life
Technologies'. The chimeric gene (SEQ ID NO:96) was
provided in pET100/D-TOPOO vector (ThermoFisher Scientific,
Rockford, IL), transformed into competent BL21*DE3 E. coil
and the rDiHAX fusion protein (SEQ ID NO:97) was expressed.
Briefly, an overnight seed culture was inoculated into 500
mL sterile LB broth and allowed to grow under the optimized
conditions and selection pressure until the 0D600 was
reached. The bacterial cells were then induced with 1 mM
IPTG (Research Products International, Mt. Prospect, IL)
and allowed to grow for an additional 4 hours. The cells
were then pelleted down by centrifuging at 12,000 rpm for
30 minutes at 4 C. For protein purification, the pellet was
re-suspended in 20 mL Tris-Buffered Saline (TBS) and 150 pl
of lysozyme was then added to the solution and incubated
for 30 minutes in a shaker platform at room temperature.
Following incubation, the pellet was sonicated for 4 cycles
at 1 minute each with a pause of 30 seconds in between.
Following sonication, the lysates were centrifuged at
12,000 rpm for 30 minutes at 4 C. The supernatant was
discarded, the pellet was washed and 15 mL of 8M Urea was
then added to the pellet and incubated overnight at 4 C
with constant mixing. After incubation, the lysate was
centrifuged at 12,000 rpm for 30 minutes at 4 C and the
supernatant was collected into a fresh 50 mL conical
centrifuge tube over ice. The recombinant protein was
expressed with an N-terminal six histidine residue tag.
This allowed for purification of the rDiHAX protein by
Immobilized Metal Affinity Chromatography (IMAC). In
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-141-
particular, the extracted protein was incubated with 2 mL
cobalt resin for 30 minutes in a shaker at room temperature
and packed into a 10 ml column. After washing the column
with 10 ml TBS, the column was washed with 30 mL of 10 mM
Imidazole prepared in TBS. The bound protein was then
eluted with 300 mM Imidazole containing 10% glycerol in
TBS. Purity and molecular size of the rDiHAX protein was
assessed on a 14% SDS-PAGE gel. The molecular weight of the
recombinant protein was approximately 60 kDa. After
desalting, endotoxin from the purified protein was removed
by passing the protein solution through a High Capacity
Endotoxin removal resin column (ThermoFisher Scientific).
The level of endotoxin in the concentrated protein sample
was analyzed using a Piercelm LAL Chromogenic Endotoxin
Quantitation Kit. The final amount of endotoxin in the
purified rDiHAX preparation was 3 EU per mg of protein.
[00293] Experimental Design. Balb/c mice (male 4-6 weeks of
age) were grouped into 10 mice per group and immunized
subcutaneously with 15 pg of rBmHAXT or rDiHAX antigen
along with 10 pg of AL019 (Alum plus GLA, a synthetic TLR4
agonist) as adjuvant. Four immunizations were given at 2-
week intervals. Control animals received AL019 adjuvant
only. Blood samples were collected from each mouse prior to
each immunization and 2 weeks after the last immunization
to analyze the serum levels of antigen-specific TgG, IgGl,
IgG2a, IgG2b and IgG3. An ADCC assay was performed by
incubating 10-15 D. immitis L3 with 50 ul of sera from
immunized mice and 1x105 peritoneal cells from control mice.
A challenge experiment was also performed by placing a
micropore chamber containing 15-20 D. immitis L3 in the
peritoneal cavity of all mice. Larval viability was
determined 72 hours post-challenge and spleen cells and
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-142-
peritoneal fluid/cells were analyzed for immunological
correlates of vaccine-induced protection.
[00294] Immunization with rBmHAXT and rDiHAX Vaccines
Generated High Titer of Antigen-Specific IgG Antibodies and
Their Isotypes. Immunization of mice with the purified
rBmHAXT and rDiHAX proteins generated high titer of
antigen-specific IgG antibodies. In particular, the results
showed that immunized animals developed high titers
(1:10000 titer) of IgGl, IgG2a, IgG2b and IgG3 antibodies
against rBmHAXT or rDiHAX compared to controls (p<0.05).
[00295] Results from the ADCC experiments showed that sera
samples from rBmHAXT immunized mice killed 93 8.83% larvae
and sera samples from rDiHAX immunized mice killed 76 5.69%
larvae compared to the sera samples from the AL019 group
that gave (20 5.93%) larval death. In the challenge
experiment, larval death was 83 4.14% and 71 8.99% for
rBmHAXT and rDiHAX immunized mice, respectively, compared
to the control (7.3 2.42%). Notably, there was significant
(p<0.05) cross-reactivity of antibodies in the sera samples
from rBmHAXT and rDiHAX vaccinated animals with rBmHAXT and
rDiHAX proteins as determined by ELISA and western blot
analysis.
[00296]Analysis of the cellular immune response showed that
there was an increase in the antigen-specific
CD3+CD62L+CCR7+ memory T cells in the spleen of vaccinated
animals compared to AL019 controls. The culture
supernatants of spleen cells from both rBmHAXT and rDiHAX
groups showed elevated levels of IL-17A, IL-6, IFN-y and
IL-10.
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-143-
Example 14: Intramuscular Injection of rBmHAXT Provides
Protection Against Dirofilaria immitis Infection in Dogs
[00297] Immunization Protocol. Six dogs were divided into
two groups of three animals per group. Each animal of the
first group received three rounds of 100 pg dose of rBmHAXT
vaccine plus 40 pg of alum adsorbed GLA-SE (AL019; TLR4
ligand GLA formulated as an oil-in-water emulsion) on days
0, 28 and 56 given i/m on the left flank region. Each
animal of the second group was used as a control and
received three rounds of adjuvant only on days 0, 28 and 56
given i/m on the left flank region. In addition, at days -
1, 0, 28, 56, and 84, blood samples were collected in EDTA
tubes from the saphenous vein of each dog prior to
immunization. Serum samples were analyzed for antibody
titer (IgG, IgGl, IgG2, IgA, IgM and IgE). Peripheral blood
mononuclear cells were analyzed for vaccine-induced memory
cells and for their cytokine production. Protective
antibodies were determined by performing an ADCC assay. All
animals were challenged with 50 drug-sensitive D. immitis
larvae to determine protection. Vital signs and clinical
laboratory parameters (CBC, urinalysis, liver function)
were monitored as was injection site reaction (swelling,
redness, pain).
[00298] Antigen-Specific Antibody Levels in Dog Sera. Levels
of rBmHAXT-specific total TgGl, TgG2, TgGA, IgM and IgE
antibodies were determined in the sera of each dog using an
indirect ELISA as described elsewhere herein.
[00299] Antibody-Dependent Cell-mediated Cytotoxicity (ADCC)
Assay. ADCC assay was performed as described elsewhere
herein. Eight to ten live D. immitis L3 each were incubated
at 37 C with 5% CO2 in duplicate wells along with 0.5
million PBMC isolated from normal dog blood, 200 pl RPMI
1640 medium and 100 pl of sera samples. Plates were
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-144-
monitored under light microscope every 24 hours for
viability of D. immitis L3. Larvae that were limpid, non-
motile or slowly motile were considered dead. The
percentage larval death was expressed as the ratio of the
number of dead L3 to the total number recovered from each
well multiplied by 100.
[00300] Immunization with rBmHAXT Vaccine Generated High
Titer of Antigen-Specific IgG Isotype Antibodies. Twenty-
eight days after the third vaccination, dogs vaccinated
with rBmHAXT antigen showed significant increases (p<0.01)
in IgG antibody titers (1:10,000) compared to the adjuvant
control group. There was also a significant increase in
IgGl, IgG2 and IgA antibodies in the serum of dogs after
three immunizations compared to the control group, with
IgG1 and IgG2 being the most predominant isotype of IgG
antibodies. While no change was observed in the levels of
IgE and IgM antibodies, this analysis demonstrated a
balanced Th1/Th2 immune response generated against the
vaccine antigens in the vaccinated animals. ADCC showed
that sera samples from rBmHAXT vaccinated animals killed
69 16.82% drug-sensitive larvae (p<0.05) compared to the
AL019 control group (20 12.17%). Similarly, ADCC showed
that sera samples from rBmHAXT vaccinated animals killed
86 7.50% drug-resistant larvae (p<0.05) compared to the
AL019 control group (38.2 22.80%). Tn rBmHAXT vaccinated
animals, drug-sensitive worm load was 7 1.73 (P=0.0025)
compared to the AL019 control group (29.3 3.51). This
represents a 76.11% reduction in worm establishment in
vaccinated animals. Indeed, worm recovery after challenge
=infection with drug-sensitive D. immitis L3s indicated a
significant reduction in rBmHAXT vaccinated animals
compared to the AL019 control group (6.67 vs. 29.33). The
results of these analysis indicate that after three doses
CA 03167346 2022- 8-8
WO 2021/163448
PCT/US2021/017813
-145-
of vaccinations with rBmHAXT, protective antibodies were
generated, and these antibodies were effective in killing
both drug-resistant and drug-sensitive D. immitis infective
larvae in vitro. In addition, challenge studies showed that
vaccination significantly reduced worm establishment. Thus,
the rBmHAXT vaccine is safe for use in canines.
CA 03167346 2022- 8-8