Note: Descriptions are shown in the official language in which they were submitted.
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
THERMALLY MODIFIED OXIDE BASED PRETREATMENTS FOR
METALS AND METHODS OF MAKING THE SAME
CROSS-REFERENCE TO RELATED APPLICATION
[0001] The present application claims priority to and filing benefit of
U.S. provisional patent
application Ser. No. 63/015,056, filed April 24, 2020, which is incorporated
herein by reference
in its entirety.
FIELD
[0002] The present disclosure generally relates to processing of metal
substrates, such as
aluminum alloys. More specifically, the present disclosure relates to thermal
modification of
pretreated metal substrates.
BACKGROUND
[0003] Certain metal products, such as aluminum alloys, can benefit from
pretreatment, e.g.,
the application or production of a pretreatment film on a surface of the metal
product. These
benefits include bond durability, color stability, ease of maintenance,
aesthetics, health and
safety, and low cost. However, it is difficult to produce aluminum alloy coils
having a
pretreatment film that meets flexibility, durability and/or surface
characteristics requirements for
downstream processing, including joining of aluminum alloy products.
Furthermore,
conventional methods require limiting the exposure of the pretreated metal to
high temperatures,
e.g., to avoid loss of the above-described benefits. This limits the types of
products, e.g., the
tempers of the aluminum alloys, that may be pretreated.
SUMMARY
[0004] Covered embodiments of the invention are defined by the claims, not
this summary.
This summary is a high-level overview of various aspects of the invention and
introduces some
of the concepts that are further described in the Detailed Description section
below. This
summary is not intended to identify key or essential features of the claimed
subject matter, nor is
it intended to be used in isolation to determine the scope of the claimed
subject matter. The
subject matter should be understood by reference to appropriate portions of
the entire
specification, any or all drawings, and each claim.
-1-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0005] In one aspect, the present disclosure describes a method of making a
corrosion
resistant substrate, the method comprising producing a pretreatment film on a
surface of a metal
substrate to provide a pretreated metal substrate; and heating the pretreated
metal substrate at a
first temperature to provide the corrosion resistant substrate, wherein the
first temperature is
greater than 300 C; and wherein the metal substrate and/or the pretreated
metal substrate is in an
F temper, a T4 temper, or a T6 temper. In some cases, the metal substrate
comprises an
aluminum alloy (e.g., a 5xxx series aluminum alloy, a 6xxx series aluminum
alloy, or a 7xxx
series aluminum alloy). In some cases, the corrosion resistant substrate is in
a T6 temper. In
some cases, the pretreatment film comprises an oxide layer. In some cases, the
oxide layer
comprises an aluminum oxide, a silicon oxide, a titanium oxide, a chromium
oxide, a manganese
oxide, a nickel oxide, a yttrium oxide, a zirconium oxide, a molybdenum oxide,
or combinations
thereof. In some cases, producing the pretreatment film comprises applying an
inorganic
pretreatment composition to the surface of the metal substrate. In some cases,
producing the
pretreatment film comprises anodizing the surface of the metal substrate. In
some cases,
producing the pretreatment film comprises flame hydrolyzing the surface of the
metal substrate.
In some cases, the first temperature is from 300 C to 550 C. In some cases,
the heating
comprises heating the pretreated metal substrate at the first temperature for
less than 30 minutes.
Optionally, the heating further comprises heating the pretreated metal
substrate at a second
temperature. In some cases, the second temperature is lower than the first
temperature. In some
cases, the second temperature is from 75 C to 250 C. In some cases, the
heating comprises
heating the pretreated metal substrate at the second temperature from 1 hour
to 48 hours. In some
cases, the metal substrate is a continuous coil.
[0006] In another aspect, the present disclosure describes a corrosion
resistant coil
comprising an aluminum alloy continuous coil, wherein a surface of the
aluminum alloy
continuous coil comprises an inorganic pretreatment film, and wherein the
aluminum alloy
continuous coil is in an F temper, a T4 temper, or a T6 temper. In some cases,
the aluminum
alloy continuous coil comprises a 5xxx series aluminum alloy, a 6xxx series
aluminum alloy, or
a 7xxx series aluminum alloy. In some cases, the inorganic pretreatment film
comprises an oxide
layer. In some cases, the oxide layer comprises an aluminum oxide, a silicon
oxide, a titanium
oxide, a chromium oxide, a manganese oxide, a nickel oxide, a yttrium oxide, a
zirconium oxide,
a molybdenum oxide, or combinations thereof.
-2-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0007] In another aspect, the present disclosure describes a method of
making a corrosion
resistant substrate, the method comprising producing a pretreatment film on a
surface of a metal
substrate to provide a pretreated metal substrate; and heating the pretreated
metal substrate at a
first temperature to provide the corrosion resistant substrate, wherein the
metal substrate and/or
the pretreated metal substrate is in an F temper, and wherein the corrosion
resistant substrate is in
a T5 temper, a T6 temper, a T61 temper, a T7 temper, T8x temper, or a T9
temper.
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] The disclosure is described in detail below with reference to the
appended drawings.
[0009] FIG. 1 illustrate results from glow discharge optical emission
spectrometry (GDOES)
analysis of corrosion resistant substrates according to certain aspects of the
present disclosure.
DETAILED DESCRIPTION
[0010] Described herein are methods for making a corrosion resistant metal
substrate, such
as a corrosion resistant aluminum alloy substrate. The corrosion resistant
substrates described
herein can be used, for example, to produce corrosion resistant products that
have superior
surface qualities and minimized surface defects as compared to products
prepared from metal
substrates that have not been processed according to the present disclosure.
[0011] Various pretreatments are often employed in conventional processing
of metal
substrates, such as aluminum alloys. Some conventional processes produce a
pretreatment film
on one or more surfaces of the metal substrate by chemical or electrolytic
modification. The
pretreatment film may alter properties of the metal substrate, such as bond
durability, adhesion,
or corrosion rate. In conventional methods, the metal substrates are not
subjected to thermal
modification after pretreatment. In particular, conventional methods avoid
exposing pretreatment
films on surfaces of metal substrates to high temperatures (e.g., temperatures
greater than 400
C). For example, conventional methods dry pretreated surfaces at temperatures
less than 100
C. This is due to the commonly held belief by those of ordinary skill in the
art that exposure to
high temperatures would degrade the pretreatment film, e.g., by burning the
pretreatment film, or
otherwise reducing the effectiveness of the pretreatment film. Furthermore,
because of the
commonly held belief that exposure to high temperatures provides no advantage,
thermal
modification of metal substrates after pretreatment was considered to be an
unnecessary
additional cost to be avoided.
-3-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0012] Despite the conventional belief urging otherwise, the methods
described herein
include intentionally exposing pretreatment films to high temperatures. The
present disclosure
provides methods of making a corrosion resistant metal substrate by producing
a pretreatment
film on a surface of a metal substrate and heating the pretreated metal
substrate to a temperature
greater than 400 C. The exposure of pretreatment films to high temperatures
(e.g., greater than
400 C) according to the methods described herein do not degrade or negatively
impact the
pretreatment film. On the contrary, the high temperatures improve (e.g.,
enhance) the properties
of the pretreatment film. Heating the pretreated metal substrate according to
the present
disclosure dries and/or densifies the pretreatment film, improving the bond
durability, adhesion,
and/or corrosion resistance imparted by the pretreatment film.
[0013] Thus, the corrosion resistant substrates produced by the methods
described herein
exhibit excellent physical properties, such as bond durability. Furthermore,
the processes
described herein are suitable for coil-to-coil lines as well as batch
processing.
Definitions and Descriptions
[0014] As used herein, the terms "invention," "the invention," "this
invention" and "the
present invention" are intended to refer broadly to all of the subject matter
of this patent
application and the claims below. Statements containing these terms should be
understood not to
limit the subject matter described herein or to limit the meaning or scope of
the patent claims
below.
[0015] In this description, reference is made to alloys identified by
aluminum industry
designations, such as "series" or "7xxx." For an understanding of the number
designation system
most commonly used in naming and identifying aluminum and its alloys, see
"International
Alloy Designations and Chemical Composition Limits for Wrought Aluminum and
Wrought
Aluminum Alloys" or "Registration Record of Aluminum Association Alloy
Designations and
Chemical Compositions Limits for Aluminum Alloys in the Form of Castings and
Ingot," both
published by The Aluminum Association.
[0016] As used herein, a plate generally has a thickness of greater than
about 15 mm. For
example, a plate may refer to an aluminum product having a thickness of
greater than 15 mm,
greater than 20 mm, greater than 25 mm, greater than 30 mm, greater than 35
mm, greater than
40 mm, greater than 45 mm, greater than 50 mm, or greater than 100 mm.
-4-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0017] As used herein, a shate (also referred to as a sheet plate)
generally has a thickness of
from about 4 mm to about 15 mm. For example, a shate may have a thickness of 4
mm, 5 mm, 6
mm, 7 mm, 8 mm, 9 mm, 10 mm, 11 mm, 12 mm, 13 mm, 14 mm, or 15 mm.
[0018] As used herein, a sheet generally refers to an aluminum product
having a thickness of
less than about 4 mm. For example, a sheet may have a thickness of less than 4
mm, less than 3
mm, less than 2 mm, less than 1 mm, less than 0.5 mm, less than 0.3 mm, or
less than 0.1 mm.
[0019] As used herein, "bond durability" refers to an ability of a bonding
agent bonding two
products together to withstand cycled mechanical stress after exposure to
environmental
conditions that initiate failure of the bonding agent. Bond durability is
characterized in terms of
the number of mechanical stress cycles applied to the bonded products, while
the bonded
products are exposed to the environmental conditions, until the bond fails.
[0020] As used herein, terms such as "cast metal product," "cast product,"
"cast aluminum
alloy product," and the like are interchangeable and refer to a product
produced by direct chill
casting (including direct chill co-casting) or semi-continuous casting,
continuous casting
(including, for example, by use of a twin belt caster, a twin roll caster, a
twin block caster, or any
other continuous caster), electromagnetic casting, hot top casting, or any
other casting method.
[0021] As used herein, a "coil-to-coil" line or "coil-to-coil processing"
refers to a continuous
processing method on a continuous line whereby the alloy, e.g., aluminum
alloy, processed in the
method is fed into the processing from a coil, uncoiled during the processing,
and re-coiled after
completing the processing. An alloy processed is such a processing method is
referred to herein
as a "continuous coil" or an "aluminum alloy continuous coil."
[0022] Reference is made in this application to alloy condition or temper.
For an
understanding of the alloy temper descriptions most commonly used, see
"American National
Standards (ANSI) H35 on Alloy and Temper Designation Systems." An F condition
or temper
refers to an aluminum alloy as fabricated. An 0 condition or temper refers to
an aluminum alloy
after annealing. A Ti condition or temper refers to an aluminum alloy cooled
from hot working
and naturally aged (e.g., at room temperature). A T2 condition or temper
refers to an aluminum
alloy cooled from hot working, cold worked, and naturally aged. A T3 condition
or temper refers
to an aluminum alloy solution heat treated, cold worked, and naturally aged. A
T4 condition or
temper refers to an aluminum alloy solution heat treated and naturally aged. A
T5 condition or
temper refers to an aluminum alloy cooled from hot working and artificially
aged (at elevated
-5-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
temperatures). A T6 condition or temper refers to an aluminum alloy solution
heat treated and
artificially aged. A T7 condition or temper refers to an aluminum alloy
solution heat treated and
artificially overaged. A T8x condition or temper refers to an aluminum alloy
solution heat
treated, cold worked, and artificially aged. A T9 condition or temper refers
to an aluminum alloy
solution heat treated, artificially aged, and cold worked.
[0023] As used herein, the meaning of "a," "an," or "the" includes singular
and plural
references unless the context clearly dictates otherwise.
[0024] As used herein, the modifier "about" is intended to include the
described term without
the word "about" (e.g., "about 10" is intended to include "10").
[0025] As used herein, the meaning of "room temperature" can include a
temperature of
from about 15 C to about 30 C, for example about 15 C, about 16 C, about
17 C, about 18
C, about 19 C, about 20 C, about 21 C, about 22 C, about 23 C, about 24
C, about 25 C,
about 26 C, about 27 C, about 28 C, about 29 C, or about 30 C.
[0026] All ranges disclosed herein are to be understood to encompass any
and all subranges
subsumed therein. For example, a stated range of "1 to 10" should be
considered to include any
and all subranges between (and inclusive of) the minimum value of 1 and the
maximum value of
10; that is, all subranges beginning with a minimum value of 1 or more, e.g. 1
to 6.1, and ending
with a maximum value of 10 or less, e.g., 5.5 to 10.
Metal Substrate
[0027] As noted, the present disclosure provides methods for making a
corrosion resistant
metal substrate. More specifically, the methods described herein produce a
pretreatment film on
the surface of a metal substrate. The composition of the metal substrate on
which the
pretreatment film is formed is not particularly limited. The pretreatment film
can be applied, for
example, to any suitable aluminum alloy, such as a continuous coil of an
aluminum alloy.
Suitable aluminum alloys include, for example, 1 xxx series aluminum alloys,
2xxx series
aluminum alloys, 3xxx series aluminum alloys, 4xxx series aluminum alloys,
5xxx series
aluminum alloys, 6xxx series aluminum alloys, 7xxx series aluminum alloys, and
8xxx series
aluminum alloys.
[0028] By way of non-limiting example, exemplary lxxx series aluminum
alloys for use as
the metal substrate can include AA1100, AA1100A, AA1200, AA1200A, AA1300,
AA1110,
AA1120, AA1230, AA1230A, AA1235, AA1435, AA1145, AA1345, AA1445, AA1150,
-6-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
AA1350, AA1350A, AA1450, AA1370, AA1275, AA1185, AA1285, AA1385, AA1188,
AA1190, AA1290, AA1193, AA1198, or AA1199. In some cases, the aluminum alloy
is at least
99.9 % pure aluminum (e.g., at least 99.91 %, at least 99.92 %, at least 99.93
%, at least 99.94 %,
at least 99.95 %, at least 99.96 %, at least 99.97 %, at least 99.98 %, or at
least 99.99 % pure
aluminum).
[0029] Non-
limiting exemplary 2xxx series aluminum alloys for use as the metal substrate
can include AA2001, AA2002, AA2004, AA2005, AA2006, AA2007, AA2007A, AA2007B,
AA2008, AA2009, AA2010, AA2011, AA2011A, AA2111, AA2111A, AA2111B, AA2012,
AA2013, AA2014, AA2014A, AA2214, AA2015, AA2016, AA2017, AA2017A, AA2117,
AA2018, AA2218, AA2618, AA2618A, AA2219, AA2319, AA2419, AA2519, AA2021,
AA2022, AA2023, AA2024, AA2024A, AA2124, AA2224, AA2224A, AA2324, AA2424,
AA2524, AA2624, AA2724, AA2824, AA2025, AA2026, AA2027, AA2028, AA2028A,
AA2028B, AA2028C, AA2029, AA2030, AA2031, AA2032, AA2034, AA2036, AA2037,
AA2038, AA2039, AA2139, AA2040, AA2041, AA2044, AA2045, AA2050, AA2055,
AA2056, AA2060, AA2065, AA2070, AA2076, AA2090, AA2091, AA2094, AA2095,
AA2195, AA2295, AA2196, AA2296, AA2097, AA2197, AA2297, AA2397, AA2098,
AA2198, AA2099, or AA2199.
[0030] Non-
limiting exemplary 3xxx series aluminum alloys for use as the metal substrate
can include AA3002, AA3102, AA3003, AA3103, AA3103A, AA3103B, AA3203, AA3403,
AA3004, AA3004A, AA3104, AA3204, AA3304, AA3005, AA3005A, AA3105, AA3105A,
AA3105B, AA3007, AA3107, AA3207, AA3207A, AA3307, AA3009, AA3010, AA3110,
AA3011, AA3012, AA3012A, AA3013, AA3014, AA3015, AA3016, AA3017, AA3019,
AA3020, AA3021, AA3025, AA3026, AA3030, AA3130, or AA3065.
[0031] Non-
limiting exemplary 4xxx series aluminum alloys for use as the metal substrate
can include AA4004, AA4104, AA4006, AA4007, AA4008, AA4009, AA4010, AA4013,
AA4014, AA4015, AA4015A, AA4115, AA4016, AA4017, AA4018, AA4019, AA4020,
AA4021, AA4026, AA4032, AA4043, AA4043A, AA4143, AA4343, AA4643, AA4943,
AA4044, AA4045, AA4145, AA4145A, AA4046, AA4047, AA4047A, or AA4147.
[0032] Non-
limiting exemplary 5xxx series aluminum alloys for use as the metal substrate
can include AA5182, AA5183, AA5005, AA5005A, AA5205, AA5305, AA5505, AA5605,
AA5006, AA5106, AA5010, AA5110, AA5110A, AA5210, AA5310, AA5016, AA5017,
-7-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
AA5018, AA5018A, AA5019, AA5019A, AA5119, AA5119A, AA5021, AA5022, AA5023,
AA5024, AA5026, AA5027, AA5028, AA5040, AA5140, AA5041, AA5042, AA5043,
AA5049, AA5149, AA5249, AA5349, AA5449, AA5449A, AA5050, AA5050A, AA5050C,
AA5150, AA5051, AA5051A, AA5151, AA5251, AA5251A, AA5351, AA5451, AA5052,
AA5252, AA5352, AA5154, AA5154A, AA5154B, AA5154C, AA5254, AA5354, AA5454,
AA5554, AA5654, AA5654A, AA5754, AA5854, AA5954, AA5056, AA5356, AA5356A,
AA5456, AA5456A, AA5456B, AA5556, AA5556A, AA5556B, AA5556C, AA5257, AA5457,
AA5557, AA5657, AA5058, AA5059, AA5070, AA5180, AA5180A, AA5082, AA5182,
AA5083, AA5183, AA5183A, AA5283, AA5283A, AA5283B, AA5383, AA5483, AA5086,
AA5186, AA5087, AA5187, or AA5088.
[0033] Non-
limiting exemplary 6xxx series aluminum alloys for use as the metal substrate
can include AA6101, AA6101A, AA6101B, AA6201, AA6201A, AA6401, AA6501, AA6002,
AA6003, AA6103, AA6005, AA6005A, AA6005B, AA6005C, AA6105, AA6205, AA6305,
AA6006, AA6106, AA6206, AA6306, AA6008, AA6009, AA6010, AA6110, AA6110A,
AA6011, AA6111, AA6012, AA6012A, AA6013, AA6113, AA6014, AA6015, AA6016,
AA6016A, AA6116, AA6018, AA6019, AA6020, AA6021, AA6022, AA6023, AA6024,
AA6025, AA6026, AA6027, AA6028, AA6031, AA6032, AA6033, AA6040, AA6041,
AA6042, AA6043, AA6151, AA6351, AA6351A, AA6451, AA6951, AA6053, AA6055,
AA6056, AA6156, AA6060, AA6160, AA6260, AA6360, AA6460, AA6460B, AA6560,
AA6660, AA6061, AA6061A, AA6261, AA6361, AA6162, AA6262, AA6262A, AA6063,
AA6063A, AA6463, AA6463A, AA6763, A6963, AA6064, AA6064A, AA6065, AA6066,
AA6068, AA6069, AA6070, AA6081, AA6181, AA6181A, AA6082, AA6082A, AA6182,
AA6091, or AA6092.
[0034] Non-
limiting exemplary 7xxx series aluminum alloys for use as the metal substrate
can include AA7011, AA7019, AA7020, AA7021, AA7039, AA7072, AA7075, AA7085,
AA7108, AA7108A, AA7015, AA7017, AA7018, AA7019A, AA7024, AA7025, AA7028,
AA7030, AA7031, AA7033, AA7035, AA7035A, AA7046, AA7046A, AA7003, AA7004,
AA7005, AA7009, AA7010, AA7011, AA7012, AA7014, AA7016, AA7116, AA7122,
AA7023, AA7026, AA7029, AA7129, AA7229, AA7032, AA7033, AA7034, AA7036,
AA7136, AA7037, AA7040, AA7140, AA7041, AA7049, AA7049A, AA7149, AA7204,
AA7249, AA7349, AA7449, AA7050, AA7050A, AA7150, AA7250, AA7055, AA7155,
-8-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
AA7255, AA7056, AA7060, AA7064, AA7065, AA7068, AA7168, AA7175, AA7475,
AA7076, AA7178, AA7278, AA7278A, AA7081, AA7181, AA7185, AA7090, AA7093,
AA7095, or AA7099.
[0035] Non-limiting exemplary 8xxx series aluminum alloys for use as the
metal substrate
cane include AA8005, AA8006, AA8007, AA8008, AA8010, AA8011, AA8011A, AA8111,
AA8211, AA8112, AA8014, AA8015, AA8016, AA8017, AA8018, AA8019, AA8021,
AA8021A, AA8021B, AA8022, AA8023, AA8024, AA8025, AA8026, AA8030, AA8130,
AA8040, AA8050, AA8150, AA8076, AA8076A, AA8176, AA8077, AA8177, AA8079,
AA8090, AA8091, or AA8093.
[0036] While aluminum alloy products are described throughout the
disclosure, the methods
and products apply to any metal substrate. In some embodiments, the metal
substrate is
aluminum, an aluminum alloy, magnesium, a magnesium-based material, titanium,
a titanium-
based material, copper, a copper-based material, steel, a steel-based
material, bronze, a bronze-
based material, brass, a brass-based material, a composite, a sheet used in
composites, or any
other suitable metal or combination of materials. The product may include
monolithic materials,
as well as non-monolithic materials such as roll-bonded materials, clad
materials, composite
materials, or various other materials. In some examples, the metal substrate
is a metal coil, a
metal strip, a metal plate, a metal shate, a metal sheet, a metal billet, a
metal ingot, or other metal
article.
[0037] The alloys can be produced by direct chill casting (including direct
chill co-casting)
or semi-continuous casting, continuous casting (including, for example, by use
of a twin belt
caster, a twin roll caster, a block caster, or any other continuous caster),
electromagnetic casting,
hot top casting, or any other casting method.
[0038] The metal substrate can be prepared from an alloy of any temper. In
some
embodiments, the metal substrate is an alloy in an F temper, a T4 temper, or a
T6 temper. As
discussed below, the temper of the metal substrate may be altered by the
thermal modification
described herein. In one embodiment of the method, for example, a metal
substrate is provided in
an F temper, a pretreatment film is produced on a surface of the metal
substrate, and the
pretreated metal substrate is heated such that the final corrosion resistant
substrate is in a T6
temper without compromising the pretreatment film.
-9-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
Pretreatment
[0039] The methods described herein include producing a pretreatment film
on a surface of
the metal substrate to provide a pretreated metal substrate. The pretreatment
films described
herein improve properties, such as adhesion and/or corrosion resistance, of
the metal substrates
on the surfaces of which the pretreatment films are produced.
[0040] The method of producing the pretreatment film on a surface of the
metal substrate is
not particularly limited, and any suitable method known in the art may be
used. In some
embodiments, producing the pretreatment film may comprise applying a
pretreatment
composition (e.g., an inorganic pretreatment composition) to the surface of
the metal substrate.
In some cases, for example, the pretreatment composition (e.g., an inorganic
pretreatment
composition) may be sprayed on a surface of the metal substrate. In some
cases, the metal
substrate may be submerged in a pretreatment composition (e.g., an inorganic
pretreatment
composition). The pretreatment composition (e.g., an inorganic pretreatment
composition) may
be specially formulated to produce a pretreatment film on the surface of the
metal substrate. For
example, the pretreatment composition may include chromium, molybdenum,
titanium,
zirconium, manganese, or combinations thereof.
[0041] In some embodiments, producing the pretreatment film may comprise
anodizing a
surface of the metal substrate. Anodizing may comprise, for example,
contacting the surface of
the metal substrate with an electrolyte solution and applying an electric
current (e.g., alternating
current (AC) power and/or direct current (DC)) to the metal substrate. In some
cases, anodizing
the metal substrates produces a pretreated metal substrate having a thin
pretreatment film, which
may comprise an oxide layer. Suitable methods for anodizing are described in
U.S. Pub. No.
2020/0082972, which is incorporated herein by reference.
[0042] In some embodiments, producing the pretreatment film may comprise a
pyrogenic
process. For example, the pretreatment film may be produced by flame pyrolysis
deposition.
Flame pyrolysis deposition may comprise burning (e.g., combusting) a metallic
product to
produce a deposit on the surface of the metal substrate. The composition of
the deposit will vary
with the gas mixture and/or metallic compound, which may be specially
formulated for the flame
pyrolysis deposition. In some cases, the deposit, which may comprise an oxide,
forms a
pretreatment film.
-10-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0043] The composition or structure of the pretreatment film on the
pretreated metal
substrate is not particularly limited, and any pretreatment film known in the
art may be produced
or used. Pretreatment films known in the art may be classified as organic
pretreatment films,
inorganic pretreatment films, and combination pretreatment films. Organic
pretreatment films
comprise an organic compound (i.e., a carbon-containing compound), such as
organic polymers.
Inorganic pretreatment films comprise an inorganic compound (i.e., a non-
carbon containing
compound), such as metal ion analogues and metallic coordination complexes.
Combination
pretreatment films comprise both an organic compound and an inorganic compound
or an
organic-inorganic compound that includes both organic and inorganic moieties.
[0044] In some embodiments, the pretreatment film produced in the disclosed
methods is an
organic pretreatment film. Preferably, however, the pretreatment film is an
inorganic
pretreatment film or a combination pretreatment film. As described herein,
thermal modification
(discussed below) of metal substrates that have been pretreated with inorganic
and/or
combination pretreatment films surprisingly improves properties (e.g.,
adhesion, corrosion
resistance) of the metal substrates. On the contrary, the present inventors
have found that thermal
modification of metal substrates that have been pretreated with organic
pretreatment films may
not improve properties (e.g., may not improve properties to the same degree).
In some cases, the
thermal modification of metal substrates having an organic pretreatment film
may even degrade
properties of the substrate. It is theorized that organic compounds present in
conventional
organic pretreatment films (e.g., organic polymers) may undergo undesirable
chemical reactions
(e.g., combustion) when subjected to the thermal modification described
herein. Thus, in some
embodiments of the methods, the pretreatment film is not an organic
pretreatment film (i.e., a
pretreatment film including an organic compound only).
[0045] In some cases, producing the pretreatment film on a surface of the
metal substrate
comprises creating an oxide layer on the surface. Said another way, the
pretreatment film may
comprise an oxide layer. For example, the pretreatment film may comprise an
inorganic oxide
layer. The oxide layer comprises one or more oxides, such as metallic oxides.
[0046] The composition of the oxide layer is not particularly limited, and
any suitable oxide
layer known in the art may be used. The oxide layer may comprise, for example,
an aluminum
oxide (e.g., A120, A10, and/or A1203), a silicon oxide (e.g., 5i02 and/or
Si0), a titanium oxide
(e.g., Ti20, TiO, Ti203, and/or TiO2), a chromium oxide (e.g., CrO, Cr203,
Cr02, and/or Cr05), a
-11-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
manganese oxide (e.g., MnO, Mn304, M-1203, Mn02, Mn03, and/or Mn207), a nickel
oxide (e.g.,
NiO and/or Ni203), a yttrium oxide (e.g., Y203), a zirconium oxide (e.g.,
ZrO2), a molybdenum
oxide (e.g., Mo02 and/or Mo03), or combinations thereof.
[0047] In some embodiments, the pretreatment film comprises an oxide layer
of aluminum
oxide. In some embodiments, the pretreatment film comprises an oxide layer of
silicon oxide. In
some embodiments, the pretreatment film comprises a combination of oxides. For
example, the
pretreatment film may comprise on oxide layer of titanium oxide and zirconium
oxide.
[0048] Generally, the pretreatment film comprises a thin layer on a portion
(e.g., at least a
portion) of a surface of the metal substrate. In some cases, the pretreatment
film may be
produced on one surface of the metal substrate. In some cases, the
pretreatment film may be
produced on one or more surfaces of the metal substrate, e.g., two surfaces.
In some cases, the
pretreatment film is produced on all surfaces of the metal substrate.
[0049] The thickness of the pretreatment film may vary. As noted, the
pretreatment film is
generally a thin layer. The thickness of the pretreatment film can range from
about 1 nm to about
1000 nm. In some cases, the pretreatment film is less than about 1000 nm in
thickness, e.g., less
than about 900 nm, less than about 800 nm, less than about 700 nm, less than
about 600 nm, less
than about 500 nm, less than about 400 nm, less than about 300 nm, less than
about 200 nm, or
less than about 100 nm. For example, the pretreatment film can be from about 5
nm to about
1000 nm, from about 10 nm to about 900 nm, from about 20 nm to about 800 nm,
or from about
30 nm to about 700 nm in thickness. In some examples, the pretreatment film
can be about 1 nm,
about 5 nm, about 10 nm, about 15 nm, about 20 nm, about 25 nm, about 30 nm,
about 35 nm,
about 40 nm, about 45 nm, about 50 nm, about 55 nm, about 60 nm, about 65 nm,
about 70 nm,
about 75 nm, about 80 nm, about 85 nm, about 90 nm, about 95 nm, about 100 nm,
about 150
nm, about 200 nm, about 250 nm, about 300 nm, about 400 nm, about 500 nm,
about 600, about
700 nm, about 750 nm, about 800 nm, about 850 nm, about 900 nm, about 950 nm,
or about
1000 nm in thickness, or anywhere in between.
[0050] In some cases, the pretreatment film on the pretreated metal
substrate may be
composed of multiple layers. In particular, certain methods of producing the
pretreatment film
may produce distinct layers within the pretreatment film. For example,
anodizing the metal
substrate may produce a pretreatment film including a barrier layer (e.g.,
composed of aluminum
oxide, such as nonporous aluminum oxide) and a filament layer (e.g., composed
of aluminum
-12-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
oxide, such porous aluminum oxide). The characteristics of either layer may be
controlled by the
method of producing the pretreatment film (e.g., the anodizing parameters or
conditions).
[0051] The temper of the substrate is generally not affected (e.g.,
altered) by producing the
pretreatment film. That is, the pretreated metal substrate generally is in the
same temper as the
metal substrate before pretreatment. In some embodiments, the pretreated metal
substrate is an
alloy in an F temper, a T4 temper, or a T6 temper. As discussed below, the
temper of the metal
substrate may be altered by the thermal modification described herein. In one
embodiment, for
example, the pretreated metal substrate is in F temper, and the thermal
modification of the
pretreated metal substrate produces a substrate in a T6 temper.
Thermal Modification
[0052] The methods described herein include heating the pretreated metal
substrate to
provide the corrosion resistant substrate. As noted above, conventional
methods of pretreating
metal substrates avoid exposing the metal substrate to high temperatures
(e.g., temperatures
greater than 400 C) after a pretreatment film has been produced. It was
believed by those of
ordinary skill in the art that exposure to high temperatures would degrade the
pretreatment film
or otherwise reduce the effectiveness of the pretreatment film. On the
contrary, heating a
pretreated metal substrate according to the methods described herein enhances
the pretreatment
film.
[0053] The thermal modification of the present disclosure includes heating
the pretreated
metal substrate at a first temperature, which is generally a high temperature
relative to
conventional methods. In some embodiments, the first temperature is from 300
C to 550 C,
e.g., from 300 C to 540 C, from 300 C to 530 C, from 300 C to 520 C,
from 300 C to
510 C, from 300 C to 500 C, from 325 C to 550 C, from 325 C to 540 C,
from 325 C to
530 C, from 325 C to 520 C, from 325 C to 510 C, from 325 C to 500 C,
from 350 C to
550 C, from 350 C to 540 C, from 350 C to 530 C, from 350 C to 520 C,
from 350 C to
510 C, from 350 C to 500 C, from 375 C to 550 C, from 375 C to 540 C,
from 375 C to
530 C, from 375 C to 520 C, from 375 C to 510 C, from 375 C to 500 C,
from 400 C to
550 C, from 400 C to 540 C, from 400 C to 530 C, from 400 C to 520 C,
from 400 C to
510 C, from 400 C to 500 C, from 425 C to 550 C, from 425 C to 540 C,
from 425 C to
530 C, from 425 C to 520 C, from 425 C to 510 C, from 425 C to 500 C,
from 450 C to
-13-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
550 C, from 450 C to 540 C, from 450 C to 530 C, from 450 C to 520 C,
from 450 C to
510 C, or from 450 C to 500 C.
[0054] In terms of upper limits, the first temperature may be less than 550
C, e.g., less than
540 C, less than 530 C, less than 520 C, less than 510 C, or less than 500
C. In terms of
lower limits, the first temperature may be greater than 300 C, e.g., greater
than 325 C, greater
than 350 C, greater than 375 C, greater than 400 C, greater than 425 C, or
greater than
450 C.
[0055] In some cases, the first temperature may be about 375 C, about 385
C, about
395 C, about 400 C, about 405 C, about 410 C, about 415 C, about 420 C,
about 425 C,
about 430 C, about 435 C, about 440 C, about 445 C, about 450 C, about
455 C, about
460 C, about 465 C, about 466 C, about 467 C, about 468 C, about 469 C,
about 470 C,
about 471 C, about 472 C, about 473 C, about 474 C, about 475 C, about
476 C, about
477 C, about 478 C, about 479 C, about 480 C, about 481 C, about 482 C,
about 483 C,
about 484 C, about 485 C, about 486 C, about 487 C, about 488 C, about
489 C, about
490 C, about 491 C, about 492 C, about 493 C, about 494 C, about 495 C,
about 496 C,
about 497 C, about 498 C, about 499 C, about 500 C, about 510 C, about
520 C, about
525 C, about 530 C, about 540 C, or about 550 C, or any temperature
therebetween.
[0056] The thermal modification of the described methods may include
prolonged exposure
to high temperatures, e.g., the first temperature. Prolonged exposure to high
temperatures may
enhance the pretreatment film, e.g., by (further) drying and/or densifying the
pretreatment film.
Thus, in some embodiments, the pretreated metal substrate may be heated at the
first temperature
for a period of time.
[0057] In some embodiments, the pretreated metal substrate is heated at the
first temperature
for a period of time from 10 seconds to 30 minutes, e.g., from 10 seconds to
25 minutes, from 10
seconds to 20 minutes, from 10 seconds to 15 minutes, from 10 seconds to 10
minutes, from 15
seconds to 30 minutes, from 15 seconds to 25 minutes, from 15 seconds to 20
minutes, from 15
seconds to 15 minutes, from 15 seconds to 10 minutes, from 30 seconds to 30
minutes, from 30
seconds to 25 minutes, from 30 seconds to 20 minutes, from 30 seconds to 15
minutes, from 30
seconds to 10 minutes, from 60 seconds to 30 minutes, from 60 seconds to 25
minutes, from 60
seconds to 20 minutes, from 60 seconds to 15 minutes, from 60 seconds to 10
minutes, from 75
seconds to 30 minutes, from 75 seconds to 25 minutes, from 75 seconds to 20
minutes, from 75
-14-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
seconds to 15 minutes, from 75 seconds to 10 minutes, from 90 seconds to 30
minutes, from 90
seconds to 25 minutes, from 90 seconds to 20 minutes, from 90 seconds to 15
minutes, or from
90 seconds to 10 minutes.
[0058] In terms of upper limits, the pretreated metal substrate may be
heated at the first
temperature for less than 30 minutes, e.g., less than 25 minutes, less than 20
minutes, less than
15 minutes, or less than 10 minutes. In terms of lower limits, the pretreated
metal substrate may
be heated at the first temperature for at least 10 seconds, e.g., at least 15
seconds, at least 30
seconds, at least 60 seconds, at least 75 seconds, or at least 90 seconds.
[0059] In some cases, for example, the pretreated metal substrate is heated
at the first
temperature for about 1 minute, about 2 minutes, about 3 minutes, about 4
minutes, about 5
minutes, about 6 minutes, about 7 minutes, about 8 minutes, about 9 minutes,
or about 10
minutes, or any length of time therebetween.
[0060] In some embodiments, the first temperature may be maintained during
the period of
time by an appropriate heating process. In some cases, for example, heat may
be continuously
and/or continually applied to the pretreated metal substrate for the period of
time.
[0061] In some embodiments, the first temperature may not be maintained
during the period
of time. In some cases, for example, the pretreated metal substrate may be
exposed to the first
temperature, and no additional heat may be applied during the period of time,
such that the
temperature at which the pretreated metal substrate is heated during the
period of time may
diminish slightly, e.g., by less than 25 C, less than 20 C, less than 15 C,
less than 10 C, less
than 5 C, less than 3 C, less than 2 C, or less than 1 C.
[0062] In some embodiments, the thermal modification includes additional
heating steps. For
example, the pretreated metal substrate may be heated at a second temperature
(e.g., before
and/or after having been heated at the first temperature). In some cases,
heating at the second
temperature, according to the described methods, further enhances the
pretreatment film, e.g., by
drying and/or densifying the pretreatment film. As a result, the corrosion
resistant substrate may
demonstrate improved adhesion, bond durability, and/or corrosion resistance.
[0063] The second temperature is generally a higher temperature relative to
conventional
methods. The second temperature may or may not be related to the first
temperature. In some
embodiments, for example, the second temperature is less than the first
temperature. In some
embodiments, the first temperature and the second temperature are about the
same.
-15-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0064] In some embodiments, the second temperature is from 75 C to 250 C,
e.g., from
75 C to 240 C, from 75 C to 230 C, from 75 C to 220 C, from 75 C to 210
C, from 75 C
to 200 C, from 80 C to 250 C, from 80 C to 240 C, from 80 C to 230 C,
from 80 C to
220 C, from 80 C to 210 C, from 80 C to 200 C, from 85 C to 250 C, from
85 C to
240 C, from 85 C to 230 C, from 85 C to 220 C, from 85 C to 210 C, from
85 C to
200 C, from 90 C to 250 C, from 90 C to 240 C, from 90 C to 230 C, from
90 C to
220 C, from 90 C to 210 C, from 90 C to 200 C, from 95 C to 250 C, from
95 C to
240 C, from 95 C to 230 C, from 95 C to 220 C, from 95 C to 210 C, from
95 C to
200 C, from 100 C to 250 C, from 100 C to 240 C, from 100 C to 230 C,
from 100 C to
220 C, from 100 C to 210 C, or from 100 C to 200 C. In some embodiments,
the second
temperature is from 150 C to 250 C, e.g., from 150 C to 240 C, from 150 C
to 230 C, from
150 C to 220 C, from 150 C to 210 C, from 150 C to 200 C, from 155 C to
250 C, from
155 C to 240 C, from 155 C to 230 C, from 155 C to 220 C, from 155 C to
210 C, from
155 C to 200 C, from 160 C to 250 C, from 160 C to 240 C, from 160 C to
230 C, from
160 C to 220 C, from 160 C to 210 C, from 160 C to 200 C, from 165 C to
250 C, from
165 C to 240 C, from 165 C to 230 C, from 165 C to 220 C, from 165 C to
210 C, from
165 C to 200 C, from 170 C to 250 C, from 170 C to 240 C, from 170 C to
230 C, from
170 C to 220 C, from 170 C to 210 C, from 170 C to 200 C, from 175 C to
250 C, from
175 C to 240 C, from 175 C to 230 C, from 175 C to 220 C, from 175 C to
210 C, from
175 C to 200 C, from 180 C to 250 C, from 180 C to 240 C, from 180 C to
230 C, from
180 C to 220 C, from 180 C to 210 C, or from 180 C to 200 C.
[0065] In terms of upper limits, the second temperature may be less than
250 C, e.g., less
than 240 C, less than 230 C, less than 220 C, less than 210 C, or less
than 200 C. In terms of
lower limits, the second temperature may be greater than 75 C, e.g., greater
than 80 C, greater
than 85 C, greater than 90 C, greater than 95 C, or greater than 100 C.
[0066] In some cases, for example, the second temperature may be about 90
C, about 91 C,
about 92 C, about 93 C, about 94 C, about 95 C, about 96 C, about 97 C,
about 98 C,
about 99 C, about 100 C, about 101 C, about 102 C, about 103 C, about 104
C, about
105 C, about 106 C, about 107 C, about 108 C, about 109 C, about 110 C,
about 111 C,
about 112 C, about 113 C, about 114 C, about 115 C, about 116 C, about
117 C, about
118 C, about 119 C, about 120 C, about 121 C, about 122 C, about 123 C,
about 124 C,
-16-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
about 125 C, about 126 C, about 127 C, about 128 C, about 129 C, about
130 C, about
131 C, about 132 C, about 133 C, about 134 C, about 135 C, about 136 C,
about 137 C,
about 138 C, about 139 C, about 140 C, about 141 C, about 142 C, about
143 C, about
144 C, about 145 C, about 146 C, about 147 C, about 148 C, about 149 C,
or about 150 C,
or any temperature therebetween.
[0067] As with the first temperature, in some cases, the pretreated metal
substrate may be
heated at the second temperature for a prolonged time. In some embodiments,
the pretreated
metal substrate is heated at the second temperature for a period of time
greater than the time it is
exposed to the first temperature. In some embodiments, the preheated metal
substrate is heated at
the second temperature for a period of time less than the time it is exposed
to the first
temperature. In some embodiments, the pretreated metal substrate is exposed to
the first
temperature and the second temperature for about the same amounts of time.
[0068] In some embodiments, the pretreated metal substrate is heated at the
second
temperature for a period of time from 1 hour to 48 hours, e.g., from 1 hour to
42 hours, from 1
hour to 38 hours, from 1 hour to 34 hours, from 1 hour to 30 hours, from 2
hours to 48 hours,
from 2 hours to 42 hours, from 2 hours to 38 hours, from 2 hours to 34 hours,
from 2 hours to 30
hours, from 4 hours to 48 hours, from 4 hours to 42 hours, from 4 hours to 38
hours, from 4
hours to 34 hours, from 4 hours to 30 hours, from 8 hours to 48 hours, from 8
hours to 42 hours,
from 8 hours to 38 hours, from 8 hours to 34 hours, from 8 hours to 30 hours,
from 12 hours to
48 hours, from 12 hours to 42 hours, from 12 hours to 38 hours, from 12 hours
to 34 hours, from
12 hours to 30 hours, from 18 hours to 48 hours, from 18 hours to 42 hours,
from 18 hours to 38
hours, from 18 hours to 34 hours, from 18 hours to 30 hours, from 22 hours to
48 hours, from 22
hours to 42 hours, from 22 hours to 38 hours, from 22 hours to 34 hours, or
from 22 hours to 30
hours.
[0069] In terms of upper limits, the pretreated metal substrate may be
heated at the second
temperature for less than 48 hours, e.g., less than 42 hours, less than 38
hours, less than 34 hours,
or less than 30 hours. In terms of lower limits, the pretreated metal
substrate may be heated at the
second temperature for at least 1 hour, e.g., at least 2 hours, at least 4
hours, at least 8 hours, at
least 12 hours, at least 18 hours, or at least 22 hours.
[0070] In some cases, for example, the pretreated metal substrate is heated
at the second
temperature for about 18 hours, about 19 hours, about 20 hours, about 21
hours, about 22 hours,
-17-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
about 23 hours, about 24 hours, about 25 hours, about 26 hours, about 27
hours, about 28 hours,
about 29 hours, about 30 hours, about 31 hours, or about 32 hours, or any
length of time
therebetween.
[0071] As with the first temperature, in some embodiments, the second
temperature may be
maintained during the period of time by an appropriate heating process. In
some cases, for
example, heat may be continuously and/or continually applied to the pretreated
metal substrate
for a period of time. In some embodiments, the second temperature may not be
maintained
during the period of time. In some cases, for example, the pretreated metal
substrate may be
exposed to the second temperature, and no additional heat may be applied
during the period of
time, such that the temperature at which the pretreated metal substrate is
heated during the period
of time may diminish slightly, e.g., by less than 25 C, less than 20 C, less
than 15 C, less than
C, less than 5 C, less than 3 C, less than 2 C, or less than 1 C.
[0072] The thermal modification of the described methods may artificially
age the pretreated
metal substrate. That is, the corrosion resistant substrate produced by the
methods described
herein may be an artificially aged alloy. Artificial aging may be
accomplished, for example, by
heating the pretreated metal substrate at the first temperature alone and/or
by heating the
pretreated metal substrate at both the first temperature and the second
temperature.
[0073] By artificially aging the pretreated metal substrate, the thermal
modification
described herein produces a corrosion resistant substrate in a different
temper from that of the
metal substrate and/or the pretreated metal substrate. In some embodiments,
for example, the
metal substrate is in an F temper or a T4 temper, and the thermal modification
produces a
substrate in a T6 temper. Further discussion of the temper of the corrosion
resistant substrate is
provided below.
Corrosion Resistant Substrate
[0074] The methods described herein produce a corrosion resistant
substrate. In particular,
the methods produce a corrosion resistant substrate having a pretreatment film
(e.g., an enhanced
pretreatment film, such as a dried pretreatment film or a densified
pretreatment film). The
pretreatment film imparts desirable characteristics, such as corrosion
resistance and/or increased
adhesion, on the corrosion resistant substrate. As a result of the described
methods, the corrosion
resistant substrate demonstrates excellent bond durability, adhesion, and/or
corrosion resistance.
-18-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0075] In some embodiments, the thermal modification does not alter (e.g.,
chemically alter)
the pretreatment film. In some cases, for example, the chemical composition of
the pretreatment
film is substantially unchanged by the thermal modification. In some cases,
the thermal
modification dries the pretreatment film (e.g., removes adsorbed and/or
absorbed water). The
pretreatment film of the corrosion resistant substrate may comprise an oxide
layer. For example,
the pretreatment film of the corrosion resistant substrate may comprise an
inorganic oxide layer.
The oxide layer comprises one or more oxides, such as metallic oxides. In
particular, the
pretreatment film of the corrosion resistant substrate may comprise any of the
oxides discussed
above or combinations thereof.
[0076] In some cases, exposing an aluminum alloy to high temperatures
causes surface
enrichment of certain alloying elements. For example, high temperatures
typically cause surface
enrichment of magnesium and/or silicon, which can contribute to corrosion.
Surface enrichment
of these and other elements is not an issue for the thermal modification of
the present disclosure,
because the pretreatment film (e.g., the oxide layer) may act as a barrier.
[0077] In some cases, the physical structure of the pretreatment film after
thermal
modification is unchanged as compared to the physical structure of the
pretreatment film before
the thermal modification as described herein. In some cases, the thermal
modification forms
metal-oxide bridges, producing a dense, anhydrous oxide film. As noted above,
the pretreatment
film on the pretreated metal substrate may be composed of multiple layers.
These layers may
remain intact after the thermal modification. For example, the corrosion
resistant substrate may
include a pretreatment film including a barrier layer (e.g., composed of
aluminum oxide, such as
nonporous aluminum oxide) and a filament layer (e.g., composed of aluminum
oxide, such
porous aluminum oxide).
[0078] As noted above, thermal modification may artificially age the
pretreated metal
substrate. Thus, the corrosion resistant substrate may be in a temper
corresponding to an
artificially aged alloy. In some embodiments, the corrosion resistant
substrate is in a T5 temper, a
T6 temper, a T61 temper, a T7 temper, T8x temper, or a T9 temper. In some
cases, in particular,
the corrosion resistant substrate may be in a T6 temper.
Use of Corrosion Resistant Substrate
[0079] The corrosion resistant substrates made according to the methods
described herein
can be used in producing products, including products for use in, among
others, automotive,
-19-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
electronics, and transportation applications, such as commercial vehicle,
aircraft, or railway
applications. The continuous coils and methods described herein provide
products with surface
properties desired in various applications. The products described herein can
have high strength,
high deformability (elongation, stamping, shaping, formability, bendability,
or hot formability),
and/or high resistance to corrosion. Preparing the corrosion resistant
substrate as a continuous
coil provides a product that is deformable without damaging the pretreatment.
[0080] In certain aspects, the corrosion resistant substrates can be
coated, e.g., Zn-
phosphated and electrocoated (E-coated). The corrosion resistant substrates
display an improved
adhesion of coatings as compared to continuous coils that do not contain a
pretreatment film.
[0081] In some further aspects, the corrosion resistant substrates display
a high level of
adhesion of laminates or lacquer films onto the surface of the continuous
coils. Additionally,
laminates and lacquers can be cured after application at temperatures of up to
about 230 C. The
corrosion resistant substrates are not damaged by elevated temperatures used
in certain
downstream processing of aluminum alloy products, providing a thermally
resistant pretreatment
for aluminum alloy products.
[0082] In some further aspects, the corrosion resistant substrates display
excellent bond
durability.
[0083] In some examples, the corrosion resistant substrates can be used for
chassis, cross-
member, and intra-chassis components (encompassing, but not limited to, all
components
between the two C channels in a commercial vehicle chassis) to gain strength,
serving as a full or
partial replacement of high-strength steels. In certain aspects, the corrosion
resistant substrates
can be used to prepare motor vehicle body part products, e.g., automobile body
parts, such as
bumpers, side beams, roof beams, cross beams, pillar reinforcements (e.g., A-
pillars, B-pillars,
and C-pillars), inner panels, side panels, floor panels, tunnels, structure
panels, reinforcement
panels, inner hoods, or trunk lid panels. The disclosed corrosion resistant
substrates can also be
used in aircraft or railway vehicle applications, to prepare, for example,
external and internal
panels.
[0084] In some examples, the corrosion resistant substrates can also be
used to prepare
housings for electronic devices, including mobile phones and tablet computers.
For example, the
corrosion resistant substrates can be used to prepare housings for the outer
casing of mobile
phones (e.g., smart phones) and tablet bottom chassis. Exemplary consumer
electronic products
-20-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
include mobile phones, audio devices, video devices, cameras, laptop
computers, desktop
computers, tablet computers, televisions, displays, household appliances,
video playback and
recording devices, and the like. Exemplary consumer electronic product parts
include outer
housings (e.g., facades) and inner pieces for the consumer electronic
products.
[0085] The corrosion resistant substrates can be used in any other desired
application.
Illustrations
[0086] Illustration 1 is a method of making a corrosion resistant
substrate, the method
comprising: producing a pretreatment film on a surface of a metal substrate to
provide a
pretreated metal substrate; and heating the pretreated metal substrate at a
first temperature to
provide the corrosion resistant substrate, wherein the first temperature is
greater than 300 C; and
wherein the metal substrate and/or the pretreated metal substrate is in an F
temper, a T4 temper,
or a T6 temper.
[0087] Illustration 2 is the method of any preceding or subsequent
illustration, wherein the
metal substrate comprises an aluminum alloy.
[0088] Illustration 3 is the method of any preceding or subsequent
illustration, wherein the
metal substrate comprises a 5xxx series aluminum alloy, a 6xxx series aluminum
alloy, or a 7xxx
series aluminum alloy.
[0089] Illustration 4 is the method of any preceding or subsequent
illustration, wherein the
corrosion resistant substrate is in a T6 temper.
[0090] Illustration 5 is the method of any preceding or subsequent
illustration, wherein the
pretreatment film comprises an oxide layer.
[0091] Illustration 6 is the method of any preceding or subsequent
illustration, wherein the
oxide layer comprises an aluminum oxide, a silicon oxide, a titanium oxide, a
chromium oxide, a
manganese oxide, a nickel oxide, a yttrium oxide, a zirconium oxide, a
molybdenum oxide, or
combinations thereof.
[0092] Illustration 7 is the method of any preceding or subsequent
illustration, wherein
producing the pretreatment film comprises applying an inorganic pretreatment
composition to
the surface of the metal substrate.
[0093] Illustration 8 is the method of any preceding or subsequent
illustration, wherein
producing the pretreatment film comprises anodizing the surface of the metal
substrate.
-21-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0094] Illustration 9 is the method of any preceding or subsequent
illustration, wherein
producing the pretreatment film comprises flame hydrolyzing the surface of the
metal substrate.
[0095] Illustration 10 is the method of any preceding or subsequent
illustration, wherein the
first temperature is from 300 C to 550 C.
[0096] Illustration 11 is the method of any preceding or subsequent
illustration, wherein the
heating comprises heating the pretreated metal substrate at the first
temperature for less than 30
minutes.
[0097] Illustration 12 is the method of any preceding or subsequent
illustration, wherein the
heating further comprises heating the pretreated metal substrate at a second
temperature.
[0098] Illustration 13 is the method of any preceding or subsequent
illustration, wherein the
second temperature is lower than the first temperature.
[0099] Illustration 14 is the method of any preceding or subsequent
illustration, wherein the
second temperature is from 75 C to 250 C.
[0100] Illustration 15 is the method of any preceding or subsequent
illustration, wherein the
heating comprises heating the pretreated metal substrate at the second
temperature from 1 hour to
48 hours.
[0101] Illustration 16 is the method of any preceding or subsequent
illustration, wherein the
metal substrate is a continuous coil.
[0102] Illustration 17 is a corrosion resistant coil comprising: an
aluminum alloy continuous
coil, wherein a surface of the aluminum alloy continuous coil comprises an
inorganic
pretreatment film, and wherein the aluminum alloy continuous coil is in an F
temper, a T4
temper, or a T6 temper.
[0103] Illustration 18 is the method of any preceding or subsequent
illustration, wherein the
aluminum alloy continuous coil comprises a 5xxx series aluminum alloy, a 6xxx
series
aluminum alloy, or a 7xxx series aluminum alloy.
[0104] Illustration 19 is the corrosion resistant coil of any preceding or
subsequent
illustration, wherein the inorganic pretreatment film comprises an oxide
layer.
[0105] Illustration 20 is the corrosion resistant coil of any preceding or
subsequent
illustration, wherein the oxide layer comprises an aluminum oxide, a silicon
oxide, a titanium
oxide, a chromium oxide, a manganese oxide, a nickel oxide, a yttrium oxide, a
zirconium oxide,
a molybdenum oxide, or combinations thereof.
-22-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
[0106] Illustration 21 is a method of making a corrosion resistant
substrate, the method
comprising producing a pretreatment film on a surface of a metal substrate to
provide a
pretreated metal substrate; and heating the pretreated metal substrate at a
first temperature to
provide the corrosion resistant substrate, wherein the metal substrate and/or
the pretreated metal
substrate is in an F temper, and wherein the corrosion resistant substrate is
in a T5 temper, a T6
temper, a T61 temper, a T7 temper, T8x temper, or a T9 temper.
EXAMPLES
[0107] The following examples will serve to further illustrate the present
invention without,
however, constituting any limitation thereof. On the contrary, it is to be
clearly understood that
resort may be had to various embodiments, modifications, and equivalents
thereof which, after
reading the description herein, may suggest themselves to those of ordinary
skill in the art
without departing from the spirit of the invention.
Example 1: Bond Durability Testing
[0108] As noted above, the thermal modification method described herein
produces
corrosion resistant substrates that demonstrate excellent bond durability.
This example serves to
illustrate the improvement of the bond durability of corrosion resistant
substrates produced
according to the methods described herein relative to metallic substrates
pretreated according to
conventional methods and not thermally modified.
[0109] Several samples of a corrosion resistant substrate were prepared
according to the
disclosed methods using an AA7075 aluminum alloy to test the properties of the
corrosion
resistant substrate. Each of the samples tested is shown in Table 1. Samples
were prepared with
varying pretreatment methods and comprising varying components, as indicated
in Table 1.
Furthermore, samples were prepared from varying tempers, as indicated in Table
1. Each sample
was subjected to thermal modification as follows: each sample was heated at
485 C for 5
minutes, and subsequently heated at 125 C for 24 hours. Following this
thermal modification,
each sample was in a T6 temper.
[0110] As shown in Table 1, several comparative samples (Comp. 1 ¨ 7) were
also prepared
and tests. The comparative samples were prepared by producing a pretreatment
film. These
samples did not undergo thermal modification.
-23-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
Table 1
Intl. Final
Sample ID Pretreatment Film Thermal Modification
Temper Temper
Sample 1 = Anodized, 485 C for 5 minutes; T6
Al Oxide 125 C for 24 hours
Sample 2 F Anodized, = 485 C for 5 minutes; T6
Al Oxide 125 C for 24 hours
Sample 3 F Anodized, 485 C for 5 minutes; T6
Al Oxide 125 C for 24 hours
Sample 4 T6 = Anodized, 485 C for 5 minutes; T6
Al Oxide 125 C for 24 hours
Sample 5 T6 Anodized, 485 C for 5 minutes; T6
Al Oxide 125 C for 24 hours
Sample 6 T6 Anodized, 485 C for 5 minutes; T6
Al Oxide 125 C for 24 hours
Sample 7 F Flame hydrolyzed, 485 C for 5 minutes; T6
Si Oxide 125 C for 24 hours
Sample 8 F Flame hydrolyzed, = 485 C for 5 minutes; T6
Si Oxide 125 C for 24 hours
Sample 9 F Flame hydrolyzed, 485 C for 5 minutes; T6
Si Oxide 125 C for 24 hours
Sample 10 F Flame hydrolyzed, 485 C for 5 mins; T6
Si Oxide 125 C for 24 hours
Sample 11 F Inorg. composition, = 485 C for
5 mins; T6
Ti Oxide / Zr Oxide 125 C for 24 hours
Sample 12 F Combination 485 C for 5 mins; T6
(org. & inorg.) 125 C for 24 hours
pretreatment
Sample 13 F Combination 485 C for 5 mins; T6
(org. & inorg.) 125 C for 24 hours
pretreatment
Comp. 1 T6 Anodized, N/A T6
Al Oxide
Comp. 2 T6 Anodized, N/A T6
Al Oxide
Comp. 3 T6 = Anodized, N/A T6
Al Oxide
Comp. 4 T6 Anodized, N/A T6
Al Oxide
-24-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
Intl. Final
Sample ID Pretreatment Film Thermal Modification
Temker Temper
Comp. 5 T6 Anodized, N/A T6
Al Oxide
Comp. 6 T6 Anodized, N/A T6
Al Oxide
Comp. 7 T6 Flame hydrolyzed, N/A T6
Si Oxide
[0111] The
above samples and comparatives were subjected to bond durability testing. In
this testing, a set of six lap joints/bonds of each sample were connected in
sequence by bolts and
positioned vertically in a 90% relative humidity (RH) humidity cabinet. The
temperature was
maintained at 50 C. A force load of 2.4 kN was applied to the bond sequence.
The bond
durability test is a cyclic exposure test that is conducted for up to 60
cycles. Each cycle lasts for
24 hours. In each cycle, the bonds are exposed in the humidity cabinet for 22
hours, then
immersed in 5% NaCl for 15 minutes, and finally air-dried for 105 minutes.
Upon the breaking
of four joints, the test is discontinued for the particular set of joints and
is indicated as a bond
failure. For this disclosure, the completion of 45 cycles without a bond
failure indicates that the
set of joints passed the bond durability test. Upon the completion of 60
cycles, the test is
discontinued.
[0112] The
bond durability test results are shown below in Table 2. In Table 2, each of
the
joints are numbered 1 through 6, where joint 1 is the top joint and joint 6 is
the bottom joint
when oriented vertically. Unless otherwise noted, the number in the cells
indicates the number of
successful cycles before a break. An asterisk ("*") next to a number indicates
that the joint was
unbroken but that the test was discontinued. The results are summarized in
Table 2 below:
Table 2
Trial Sample Bond Durability Test Coupon Arrangement
1 2 3 4 5 6
Top Bottom
1 Sample 1 60* 60* 60* 60* 60* 60*
2 Sample 2 60* 60* 60* 60* 60* 60*
3 Sample 3 60* .. 60* 60* 60* 60* 60*
4 Sample 4 53* 46 53* 45 53 50
-25-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
Trial Sample Bond Durability Test Coupon Arrangement
1 2 3 4 5 6
Top Bottom
Sample 5 40* 40* 39 40 34 29
6 Sample 6 47* 24 47* 27 47 26
7 Sample 7 60* 60 59 60* 59 60*
8 Sample 8 52* 52* 49 48 52 50
9 Sample 9 51* 44 50 49 51 51*
Sample 10 57* 52 57 50 57* 56
11 Sample 11 60* 60* 60* 60* 60* 60*
12 Sample 12 7* 7* 6 7 7 5
13 Sample 13 10* 10* 10 8 8 9
14 Comp. 1 7* 7* 5 5 7 6
Comp. 2 21* 21* 16 13 15 21
16 Comp. 3 10* 10* 10 7 7 5
17 Comp. 4 10* 8 9 10* 10 6
18 Comp. 5 11* 11* 11 9 5 10
19 Comp. 6 25* 25* 24 25 23 15
Comp. 7 2 4* 4* 4 4 4
[0113] The exemplary corrosion resistant substrates which were subjected to
thermal
modification according to the present disclosure demonstrated excellent bond
durability, with all
but three samples (Samples, Sample 12, and Sample 13) passing the test.
Notably, two of the
three samples that did not pass the durability test included organic
pretreatment films. The
comparative substrates demonstrated comparatively poorer bond durability, with
each
comparative substrate failing the durability test.
Example 2: Bond Durability Testing
[0114] This example further illustrates the improvement of the bond
durability of corrosion
resistant substrates produced according to the methods described herein
relative to metallic
substrates pretreated according to conventional methods and not thermally
modified.
-26-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
[0115] Several samples of a corrosion resistant substrate were prepared
according to the
disclosed methods using an AA7075 aluminum alloy to test the properties of the
corrosion
resistant substrate. Each of the samples tested is shown in Table 3. Samples
were prepared by
etching with an acid and applying a varying titanium/zirconium pretreatment
(Gardobond 4591
from Chemetall GmbH (Frankfurt, Germany)), as detailed in Table 3. Each sample
was prepared
at an initial F temper. Each sample was thermally modified to adjust the
temper, as indicated in
Table 3.
Table 3
Intl. Final
Sample ID Acid Etch Pretreatment Film Thermal Modification
Temper
Temper
Sample 14 F 0.2 g/m2, Ti: 22 mg/m2, 125 C for 24 hours T6
65 C, Zr: 20 mg/m2,
seconds 5 seconds
25 C
Sample 15 F 0.2 g/m2, Ti: 22 mg/m2, 125 C for 24 hours T6
65 C, Zr: 20 mg/m2,
5 seconds 5 seconds
25 C
Sample 16 F 0.2 g/m2, Ti: 17 mg/m2, 125 C for 24 hours T6
50 C, Zr: 11 mg/m2,
1 second 10 seconds
25 C
Sample 17 F 0.2 g/m2, Ti: 17 mg/m2, 125 C for 24 hours T6
50 C, Zr: 11 mg/m2,
1 second 10 seconds
25 C
Sample 18 F 0.8 g/m2, Ti: 13 mg/m2, 125 C for 24 hours T6
50 C, Zr: 8 mg/m2,
5 seconds 10 seconds
25 C
Sample 19 F 0.8 g/m2, Ti: 13 mg/m2, 125 C for 24 hours T6
50 C, Zr: 8 mg/m2,
5 seconds 10 seconds
25 C
Sample 20 F 0.2 g/m2, Ti: 42 mg/m2, 125 C for 24 hours T6
50 C, Zr: 20 mg/m2,
1 second 10 seconds
45 C
-27-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
Intl. Final
Sample ID Acid Etch Pretreatment Film Thermal Modification
Temker
Tem,per
Sample 21 F 0.2 g/0, Ti: 42 mg/ml, 125 C for 24 hours T6
50 C, Zr: 20 mg/m2,
1 second 10 seconds
45 C
Sample 22 F 0.2 g/m2, Ti: 16 mg/m2, 125 C for 24 hours T6
50 C, Zr: 12 mg/m2,
1 second 10 seconds
25 C
Sample 23 F 0.2 g/m2, Ti: 16 mg/m2, 125 C for 24 hours T6
50 C, Zr: 12 mg/m2,
1 second 10 seconds
25 C
[0116] The
above samples were subjected to bond durability testing. In this testing, a
set of
six lap joints/bonds of each sample were connected in sequence by bolts and
positioned
vertically in a 90% relative humidity (RH) humidity cabinet. The temperature
was maintained at
50 C. A force load of 2.4 kN was applied to the bond sequence. The bond
durability test is a
cyclic exposure test that is conducted for up to 60 cycles. Each cycle lasts
for 24 hours. In each
cycle, the bonds are exposed in the humidity cabinet for 22 hours, then
immersed in 5% NaCl for
15 minutes, and finally air-dried for 105 minutes. Upon the breaking of four
joints, the test is
discontinued for the particular set of joints and is indicated as a bond
failure. For this disclosure,
the completion of 45 cycles without a bond failure indicates that the set of
joints passed the bond
durability test. Upon the completion of 60 cycles, the test is discontinued.
[0117] The
bond durability test results are shown below in Table 4. In Table 4, each of
the
joints are numbered 1 through 6, where joint 1 is the top joint and joint 6 is
the bottom joint
when oriented vertically. Unless otherwise noted, the number in the cells
indicates the number of
successful cycles before a break. An asterisk ("*") next to a number indicates
that the joint was
unbroken but that the test was discontinued. The results are summarized in
Table 4 below:
-28-
CA 03167652 2022-07-11
WO 2021/216950 PCT/US2021/028766
Table 4
Sample Bond Durability Test Coupon Arrangement
1 2 3 4 5 6
Top Bottom
Sample 14 59* 59* 59 59 56 47
Sample 15 67* 67* 66 67 63 59
Sample 16 64* 59 62 64 64* 63
Sample 17 49* 49* 42* 40 41 49
Sample 18 61* 60 59 61 58 61*
Sample 19 63* 63 63* 59 55 56
Sample 20 63 67* 59 67 67* 62
Sample 21 63 63* 49 54 63* 57
Sample 22 71* 59 71* 57 58 71
Sample 23 69* 65 69* 66 64 69
[0118] The exemplary corrosion resistant substrates which were subjected to
thermal
modification according to the present disclosure demonstrated excellent bond
durability, with all
but one sample (Sample 17) passing the test.
Example 3: GDOES Depth Profiling
[0119] Several samples of a corrosion resistant substrate were prepared
according to the
disclosed methods using an AA7075 aluminum alloy to test the properties of the
corrosion
resistant substrate. Each of the samples tested is shown in Table 5. Samples
were prepared by
etching with an acid and applying a varying titanium/zirconium pretreatment
(Gardobond 4591
from Chemetall GmbH (Frankfurt, Germany)), as detailed in Table 5. Each sample
was prepared
at an initial F temper. Each sample was thermally modified to adjust the
temper, as indicated in
Table 5.
-29-
CA 03167652 2022-07-11
WO 2021/216950
PCT/US2021/028766
Table 3
Intl. Final
Sample ID Acid Etch Pretreatment Film Thermal Modification
Temper
Temper
Sample 25 F 0.2 g/m2, Ti: 22 mg/m2, 125 C for 24 hours T6
65 C, Zr: 20 mg/m2,
seconds 10 seconds
65 C
Sample 26 F 0.2 g/m2, Ti: 17 mg/m2, 125 C for 24 hours T6
50 C, Zr: 11 mg/m2,
1 second 10 seconds
25 C
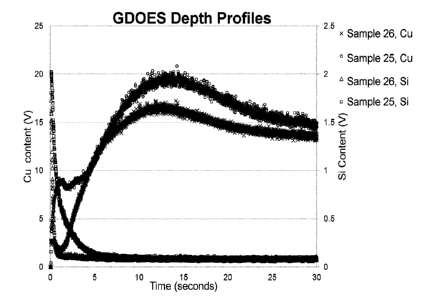
[0120] To analyze the surface and depth profile, each sample was subjected
to glow
discharge optical emission spectrometry (GDOES). GDOES gives the quantitative
depth
distribution of elements in the thin surface film of each sample. The results
of the GDOES depth
profiling are illustrated FIG. 1, which shows the enrichment of copper and
silicon on the surface
of the samples. With regard to copper, Samples 25 and 26 exhibited similar
profiles with the
presence of copper enrichment after 12 seconds of sputtering. With regard to
silicon, Sample 25
had higher yet thinner enrichment of silicon than Sample 26, which may be due
to the difference
in the acid etching between the two samples.
-30-