Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/177995
PCT/US2020/041022
Electrode Applicators for Conjunctive Use in a Dental Implant Treatment System
CROSS REFERENCE TO PRIOR APPLICATIONS
100011 This application claims priority to United States Patent
Application No.
16/884,664, filed on May 27, 2020, as well as United States Patent Application
No. 62/984,332,
filed on March 3, 2020, each of the above documents being incorporated by
reference in their
entirety.
TECHNIC AL FIELD
100021 The application is directed generally to the field of
treatment systems used to
disrupt bacteria from surgically implanted devices, and more specifically to
supporting and
configuring electrodes used in systems for treating infected metal dental
implants.
BACKGROUND
100031 Metal implants are used in patients with many different
injuries or medical
problems. For example, various orthopedic devices such as knee, hip or
shoulder joint
replacements can be surgically implanted. Similarly, metal implants may be
used for any
individual that needs to replace a tooth in a dental procedure. Dental
implants are commonly used
to completely replace a tooth. More specifically, dental implants are made up
of three (3)
components; namely, a metallic post that is osseointegrated to the jaw bone of
the patient, an
abutment extending from the metallic post, and a prosthetic tooth (a dental
crown), in which the
latter can be made from an electrically non-conductive material, which is
disposed over the
abutment.
100041 One potential problem with metal implants in general is
that they tend to allow for
the growth of bacteria on the surface. This may increase the patient's risk
for an infection. This
issue is especially prevalent in the mouth due to a large bacterial presence.
As bacteria colonize
upon foreign surfaces such as metal, biofilms are formed. Biofilms are
protective extracellular
matrix materials that encapsulate bacterial colonies onto a surface and
protect them. Biofilms can
be 500-5000 times more resistant to antibiotics than common planktonic
bacteria because the
antibiotics cannot penetrate the biofilm. Statistically, a significant
percentage (greater than 14
1
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
percent) of dental implants acquire periimplantitis, or bacterial infection of
the implant that can
cause complications with implant loosening, gum and bone loss.
100051 To decrease the risk of infection, electrodes can provide
electrical stimulation to
disrupt the growth of bacteria. It has been shown in scientific literature
that the application of a
suitable cathodic current to metal samples create chemical reactions at the
surface of the implant
that can disrupt and kill bacterial biofilms that exist on the metal.
100061 For electrochemical processes to occur, there must be an
anode and a cathode
within an electrolyte solution. The anode is a metallic surface where
oxidative reactions occur, and
the cathode is another metallic surface where reduction reactions occur. A
reduction reaction is
essentially when the material of interest gains electrons and thereby
decreases the oxidation state
of the molecules. The electrolyte that the electrodes each reside in provides
the electrical
connection by facilitating the flow of electrons shuttled by ion carriers such
as sodium or potassium
ions. Electrons are driven from the anode to the cathode through the
electrical path via a
potentiostat. A potentiostat is an instrument used to drive current from a
counter electrode to a
working electrode in order to keep the voltage on the working electrode at a
constant value
compared to a stable reference electrode. A treatment technique based on
cathodic voltage
controlled electrical stimulation (CVCES) is described in U.S. Patent No.
9,616,142, herein
incorporated in its entirety by reference. In this treatment technique, the
anode represents the
counter electrode and the cathode represents the working electrode. Using a
potentiostat, a user
can dictate which electrochemical process is occurring on the working
electrode and at what rate
it occurs simply by adjusting the applied voltage parameters with respect to a
separate reference
electrode. The cathodic reactions occurring at the working electrode produce
hydroxide ions,
resulting in an alkaline pH at on the implant surface while also producing
different reactive
oxidative chemical species that are bactericidal for existing biofilms.
100071 In a research setting, the above treatment technique has
been shown as a way to
fight bacterial biofilm infections on metallic implants in the most minimally
invasive way possible.
In this setting, the patient's bodies can act as an electrochemical cell by
using the metal implant as
the cathode and the counter electrode as the anode. The treatment system uses
the electrochemical
2
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
properties of the two electrodes in a DC circuit to chemically kill the
biofilm, which means the
electrodes must be submersed or contacting an electrolyte that transports the
electrical energy
through chemical reactions to the other electrode. Human bone and soft tissue
provide this
electrolyte media for conduction, and thus the complete surface area of the
dental implant
embedded in the bone receives treatment. Full surface treatment optimizes
effectiveness against
biofilm infections.
[0008] For dental implants in particular, an electrical
connection to the implant can be
difficult due to the non-conductive crown or crown coating that sits on top of
the metal post and
abutment, and above the gum line. Various approaches to electrically connect
to the metal post for
treatment include removing the crown or using a needle to pierce through the
gum. Each of these
approaches are impractical and inconvenient, as well as uncomfortable for the
patient. Moreover,
the design of any dental apparatus or medical device must highly consider
patient safety and
comfort. The treatment device must be both efficient and non-toxic relative to
the patient.
BRIEF DESCRIPTION
100091 As described above, it has been demonstrated that applying
cathodic voltages to a
metallic material kills any form of bacterial biofilm that exists on the
metal. When applying this
therapy to an infected dental implant, it is preferable to keep the prosthetic
crown attached, as
opposed to alternative attachment mechanisms that need to connect to the
implant abutment
directly with no crown. The disclosed apparatus provides means of contact to
exposed portions of
the abutment, or specialized electrical contact points provide on a
specialized crown, such as that
described in U.S. Patent Application No. 16/884,664, herein incorporated by
reference or other
variants. The apparatus and related method of the present invention also
involves novel features
that optimize the cathodic voltage system for patient safety in the oral
cavity.
100101 The disclosed invention presents a novel apparatus to both
make electrical contact
with an exposed dental abutment or a specialized crown with exposed metal, as
well as novel
embodiments of the application of the counter electrode (anode) and the
reference electrode within
the mouth. Optimal application of the implant (working electrode) connector,
the counter
electrode, and the reference electrode allows for efficient and concise
connection to an external
3
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
voltage source. This system and related method allows the physician to treat
the fully bone-
embedded implant surface, while still maintaining patient safety parameters
and its minimally
invasive profile.
100111 The present invention relates to the use of voltage
controlled electrical treatment to
metallic surfaces as a method to prevent and eradicate microbial colonization
on the surface, such
as in the case of periimplantitis, common to dental implants. This invention
is implemented when
a DC electrical current is applied to a metallic implant. The system requires
at least two (2)
electrodes, but can also utilize three (3) or more electrodes. Specifically
and in the case of three
(3) electrodes, a counter electrode, a working electrode, and a reference
electrode are provided in
which the counter electrode delivers the current to the working electrode in
order to maintain a
steady DC potential with respect to the stable reference electrode. In the
case of a dental implant,
the metallic surface of the implant post and abutment act as the working
electrode.
100121 Novel mechanisms are disclosed to reliably attach
electrodes to the dental implant
and the tissue within the mouth to enable the chemical reaction for biofilm
treatment to proceed
safely and effectively. The herein disclosed apparatus provides a novel way of
incorporating all
elements necessary to provide an effective cathodic voltage electrical
stimulation to a dental
implant while maintaining patient safety and optimizing the treatment of the
biofilm infection.
These elements include the various electrodes of the treatment system, as well
as physical
applicator apparatus as described herein.
100131 Therefore and according to one aspect, there is provided
an apparatus for use with
a treatment system that disrupts bacteria from a metallic dental implant, the
treatment system
comprising a device capable of producing a stimulation voltage, a counter
electrode, and a working
electrode each coupled to the device capable of producing the stimulation
voltage. The apparatus
comprises a connective body configured for connection to the device capable of
producing a
stimulation voltage and having at least one feature configured for attachment
to the mouth of a
patient, the body including at least one metal contact configured for
electrical contact with an
exposed metal area of the metallic dental implant as the working electrode.
4
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100141 The connective body according to at least one embodiment
can comprise a mouth
guard, which is shaped and configured to fit over the teeth and gums of a
patient. In at least one
version, the at least one metal contact is integrated into a wall of the mouth
guard. In another
version, the at least one metal contact can be releasably attached to a wall
of the mouth guard
100151 The mouth guard can include a grounding plate imbedded in
the wall of the mouth
guard, wherein the at least one metal contact is formed on a clip member that
is releasably
attachable to the mouth guard. According to at least one version, the
apparatus can comprise two
or more clip members that can be releasably disposed on the mouth guard.
100161 In an embodiment, the at least one metal contact of the
mouth guard is biased into
contact with an exposed metal area of at least one dental implant when
attached to the mouth of a
patient. The biasing can occur in a number of ways. For example and according
to one version,
the at least one metal contact can comprise a section of a conductive sponge
or steel wool.
According to another version, the at least one metal contact comprises a
spring section of steel or
other conductive material.
100171 According to at least one other version, the connective
body comprises at least one
clip member configured for direct attachment to at least one tooth of a
patient. The at least one
clip member can include a torsional spring configured to bias the at least
metal contact into contact
with an exposed metal area of at least one dental implant when attached. The
at least one clip
member can further comprise a soft pad opposite the at least one metal contact
configured for
contacting the tooth of a patient when attached.
100181 According to another aspect of the invention, there is
described an apparatus for
use with a treatment system that disrupts bacteria from a metallic dental
implant, the treatment
system comprising a device capable of producing a stimulation voltage, a
counter electrode, and a
working electrode each coupled to the device capable of producing the
stimulation voltage, the
working electrode comprising the metallic dental implant, the apparatus
comprising the counter
electrode including a connective body adapted for attachment to the gum line
of the patient.
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100191 According to at least one version, the connective body
comprises a flexible member
configured to wrap about the teeth and gums of at least a portion of the mouth
of a patient, the
flexible member comprising a conductive anodic layer.
100201 According to another version, the apparatus comprises a
conductive member
disposed within a container external to the mouth of the patient, the
container containing a
conductive fluid that is fluidically connected to an applicator disposed
within the mouth of the
patient. The conductive fluid is preferably neutral to basic in pH, wherein
the container is
configured to deliver conductive fluid to the connective body. In at least one
version, the
connective body supports at least one cotton roll that is saturated by the
conductive fluid wherein
the conductive fluid is transferred using a hollow tube disposed between the
container and at least
one cotton roll.
100211 According to yet another aspect, there is provided a
treatment system to disrupt
bacteria from a metallic dental implant comprising a device capable of
producing a cathodic
stimulation voltage, a working electrode that comprises the metallic dental
implant and a counter
electrode. Each of the counter electrode and working electrode are connected
via a circuit to the
device capable of producing a stimulation voltage. The counter electrode
comprises a container
retaining a conductive fluid and an electrically conductive member, the
container being connected
to the device capable of producing the stimulation voltage. The system further
comprises a
connective body fluidically coupled to the container and the gum interface of
a patient in relation
to the metallic dental implant, the connective body being configured to
receive conductive fluid
and current created by the device capable of producing the stimulation
voltage.
100221 According to another aspect, there is provided an
apparatus for use with a treatment
system that disrupts bacteria from a metallic dental implant, the treatment
system comprising a
device capable of producing a stimulation voltage, a counter electrode, and a
working electrode
each coupled to the device capable of producing the stimulation voltage, the
apparatus comprising
a mouth guard configured for attachment to the mouth of a patient and having
at least one metal
contact configured for electrical contact with an exposed metal area of the
metallic dental implant
as the working electrode.
6
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100231 According to yet another aspect, there is provided an
apparatus for use with a
treatment system that disrupts bacteria from a metallic dental implant, the
treatment system
comprising a device capable of producing a stimulation voltage, a counter
electrode, and a working
electrode each coupled to the device capable of producing the stimulation
voltage, the apparatus
comprising at least one clip member configured for attachment to at least one
tooth of a patient.
[0024] According to yet another aspect, there is provided a
treatment system for disrupting
bacteria from a metallic dental implant, the system comprising a device
capable of providing a
cathodic stimulation voltage, a working electrode capable of making electrical
contact with at least
one metallic dental implant; and a counter electrode electrically coupled with
the gum line of a
patient in proximity to the at least one metallic dental implant, each of the
working and counter
electrode being coupled in a circuit.
[0025] In at least one embodiment, the treatment system further
comprises a reference
electrode coupled to the circuit, the reference electrode being configured for
monitoring treatment
of the at least metallic dental implant.
[0026] According to at least one embodiment, the working
electrode further comprises a
connective body configured for attachment to the mouth of a patient, the
connective body including
at least one metal contact for engaging an exposed metal area of the at least
one metallic dental
implant. In at least one version, the connective body comprises a mouth guard
shaped and
configured to fit over the teeth and gums of a patient in which the at least
one metal contact is
integrated into a wall of the mouth guard or alternatively the least one metal
contact is releasably
attachable to a wall of the mouth guard.
[0027] A grounding plate is imbedded in the wall of the mouth
guard wherein the at least
one metal contact can be formed on a clip member that is releasably attachable
to the mouth guard.
According to at least one version, two or more clip members are configured to
be releasably
disposed on the mouth guard.
7
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100281 In at least one embodiment, the connective body comprises
at least one clip member
configured for attachment to at least one tooth of a patient. Means are
provided for biasing the at
least one metal contact into contact with the exposed metal area of at least
one dental implant when
the connective body is attached to the mouth of a patient. In one version, the
at least one metal
contact can comprise a section of conductive sponge or steel wool. In another
version, the at least
one metal contact can comprise a spring section made from conductive material.
[0029] In at least one version, the clip member can comprise a
torsional spring configured
to bias the at least metal contact into contact with an exposed metal area of
at least one dental
implant when attached. The at least one clip member can further comprise a
soft pad opposite the
at least one metal contact configured for contacting the tooth of a patient
when attached.
[0030] The metallic dental implant comprises a crown disposed
over a post, wherein the
crown includes a metallic core having an exposed end in electrical contact
with a metallic post
fused to the jaw of the patient.
[0031] According to another embodiment, the connective body can
comprise a flexible
member configured to wrap about the teeth and gums of at least a portion of
the mouth of a patient,
the flexible member comprising a conductive anodic layer. The flexible member
can further
comprise a conductive mesh layer disposed between the conductive anodic layer
and an exterior
adhesive layer. In addition, a hydrogel layer having a buffered agent is
disposed between the
conductive anodic layer and exterior adhesive layer.
[0032] According to another embodiment, the counter electrode
comprises at least one
conductive member disposed within a container external to the mouth of the
patient, the container
containing a conductive fluid that is fluidically connected to the connective
body disposed within
the mouth of the patient. In some versions, the conductive fluid is neutral to
basic in pH.
8
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100331 The container is configured to deliver conductive fluid to
the connective body in
which conductive fluid can be delivered to the connective body by at least one
hollow tube and
wherein the connective body includes at least cotton roll configured to
receive conductive fluid
from the container.
100341 An advantage is that the herein described apparatus
provides alternative means for
dentists to treat infections that statistically affect roughly 14% of all
people who receive a dental
implant in a manner that is very minimally invasive.
100351 The novel embodiments of the herein described dental
implant treatment system
that include abutment and crown contact mechanisms, as well as novel counter
electrode
embodiments give the physician optimal ability to apply a cathodic voltage
system that can
effectively disrupt and eliminate biofilm from a dental implant without
removing the crown. A
distinct differentiator from alternative dental treatment techniques is that
this system promotes
conduction over the entire bone-embedded surface of the dental implant, not
just within the abscess
pocket. This is key, especially in regard to dental implant posts. The posts
are manufactured to
have a very rough, coarse microsurface to promote osseointegration. One issue
this surface can
create is that bacteria are able to "hide" within the crevices of the
microstructure, even when bone
matrix are apparently grown into the surface. The approach and design of this
novel system allows
for thorough treatment of all microstructures in the metal, even with bone
present, to eliminate all
bacteria from those location. It has been found in scientific literature that
at optimizes treatment
parameters, matrix embedded bone cells that are local to the reaction are not
affected to a high
degree.
100361 These and other technical features and advantages will be
readily apparent from the
following Detailed Description, which should be read in conjunction with the
accompanying
drawings.
9
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
BRIEF DESCRIPTION OF THE DRAWINGS
[0037] FIG. 1 (a) is a side elevational view of a dental implant
that enables access by a
treatment system without removal of the crown;
[0038] FIG. 1(b) is a sectioned view of the dental implant of
FIG. 1(a) taken through
Section A-A;
[0039] FIG. 1 (c) is a partially exploded view of the dental
implant of FIGS. 1(a) and 1(b);
[0040] FIG. 2(a) is schematic diagram of an exemplary CVCES
treatment system;
[0041] FIG. 2(b) is a schematic diagram of another exemplary
CVCES treatment system
that includes working, counter and reference electrodes;
[0042] FIG. 2(c) is a schematic diagram of yet another CVCES
treatment system
incorporating a plurality of electrodes;
[0043] FIG. 3 (a) is a top perspective view of an apparatus for
use in a CVCES treatment
system, including but not limited to those shown in FIGS. 2(a) ¨ 2(c), which
is made in accordance
with aspects of the invention;
[0044] FIG. 3(b) is a top perspective view of the apparatus of
FIG.3 (a), including a contact
portion made in accordance with aspects of the invention;
[0045] FIGS. 3(c) and 3(d) are top perspective views of the
apparatus of FIG. 3(a),
including contact portions made in accordance with alternative aspects of the
invention;
[0046] FIG. 4(a) depicts a top perspective view of an apparatus
in accordance with an
exemplary embodiment, including one or more releasably attachable contacts,
according to one
configuration;
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100471 FIG. 4(b) depicts the top perspective view of the
apparatus of FIG. 4(a), including
the one or more releasable contacts as disposed in another configuration;
100481 FIG. 4(c) is a partial sectioned view depicting the
attachment of a releasable contact
to the apparatus of FIGS. 4(a) and 4(b);
[0049] FIG. 4(d) depicts an enlarged view of the releasable
contact attachment of FIG.
4(c);
[0050] FIG. 4(e) is a partial perspective view of the apparatus
of FIGS. 4(a) ¨ 4(d),
depicting another alternative placement of the releasable contacts;
[0051] FIG. 4(f) is a perspective view of the apparatus of FIGS.
4(a) ¨ 4(e), depicting the
releasable attachability of the contacts;
100521 FIG. 5(a) is a perspective view of an apparatus made in
accordance with other
aspects of the invention and as attached to a patient;
[0053] FIG. 5(b) is an elevational view of the apparatus of FIG.
5(a), showing a contact
portion made in accordance with an exemplary embodiment;
[0054] FIG. 5(c) is a top perspective view of the apparatus of
FIGS. 5(a) and 5(b);
[0055] FIG. 5(d) and 5(e) are perspective view of the apparatus
of FIGS. 5(a) ¨ (c),
showing alternative contact portions made in accordance with aspects of the
invention;
[0056] FIG. 6(a) is a perspective view of an apparatus made in
accordance with another
exemplary embodiment;
[0057] FIG. 6(b) is an exploded view of an electrode made for use
in the apparatus of FIG.
6(a);
11
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100581 FIGS. 7 and 8 are schematic views of an external
electrolytic delivery system in
accordance with aspects of the invention; and
[0059] FIGS. 9(a) ¨ 9(c) are views of the placement of cotton or
similar material into the
oral cavity of a patient for use with the external electrolytic delivery
system of FIGS. 7 and 8.
DETAILED DESCRIPTION
[0060] The present disclosure provides several novel embodiments
of apparatus used in
conjunction with treatment systems in order to disrupt and remove biofilms
from a metallic dental
implant. The treatment systems discussed utilize electrochemical stimulation
therapy through an
established electrical connection to the metallic dental implant and
application of a suitable
cathodic voltage. Connection is described in the following embodiments to a
specific form of
dental implant that enables treatment without requiring removal of the crown
portion. It will be
readily apparent, however, that this implant is an example and the herein
described apparatus can
be adapted for use with other types of dental implants. The novel apparatus
designs that are
discussed improve the overall ease and efficiency of treating metallic dental
implants with suitable
cathodic stimulation voltages.
[0061] As a matter of background and when a patient has a tooth
that needs to be removed,
the standard procedure is to replace that tooth with a dental implant. The
dental implant typically
is made up of three (3) main components that include a metallic post that is
osseointegated to the
jaw bone of a patient and a prosthetic tooth (crown) that is placed over an
abutment of the metallic
post. The tooth and roots are extracted and the bone is reamed to properly fit
the metal post. A
healing abutment is placed until the site is sufficiently healed. The healing
abutment is then
removed, and another metal abutment is screwed onto the post. The prosthetic
crown can then be
adjoined to the abutment. The crown typically has a hollowed core that allows
the abutment to be
press fit inside. It is also common to have an abutment that screws into the
crown itself. The screw
hole is typically at the top of the tooth and then is filled once the complete
implant is in place. In
many cases of this implantation, especially if there is an infection present
that causes recession of
tissue, the metal abutment is visibly exposed at the base. This situation
provides a means of
12
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
directly contacting with the abutment via a working electrode connection in
order to provide
cathodic stimulation for treatment of the infection and to disrupt biofilm
layers.
100621 The herein described system and method relies upon the
introduction of an
electrical current to an electrochemical cell. As a matter of background and
for electrochemical
(redox) processes to occur, there must be an anode and a cathode within an
electrolyte solution.
The anode is a metallic surface where oxidative reactions occur, and the
cathode is another metallic
surface where reduction reactions occur. A reduction reaction occurs when the
material of interest
gains electrons and thereby decreases the oxidation state of the molecules.
The electrolyte that the
electrodes each reside in provides the electrical connection by facilitating
the flow of electrons
shuttled by ion carriers, such as electrolytic sodium or potassium ions.
Electrons are driven from
the anode to the cathode through the electrical path via a potentiostat or
similar device. More
specifically, a potentiostat is an instrument used to drive current from a
counter electrode to a
working electrode in order to keep the voltage on the working electrode at a
constant value
compared to a stable reference electrode. One such procedure used for the
treatment of biofilms
on a metallic implant is described in U.S. Patent No. 9,616,142, the entire
contents of which are
herein incorporated by reference.
100631 According to this treatment procedure, the anode
represents the counter electrode
and the cathode represents the working electrode. Using a potentiostat, a user
can dictate which
electrochemical process is occurring on the working electrode and at what rate
the process occurs
simply by adjusting the applied voltage parameters with respect to a separate
reference electrode.
The cathodic reactions occurring at the working electrode produce hydroxide
ions, resulting in an
alkaline pH at on the implant surface, while also producing different reactive
oxidative chemical
species that are bactericidal for existing biofilms.
100641 In a research setting, the above-noted technique has been
shown as a way to fight
bacterial biofilm infections on metallic implants in the most minimally
invasive way possible. In
this setting, the patient's body can act as an electrochemical cell by using
the metal implant as the
cathode and the counter electrode as the anode. It has been shown that the
above techniques can
be used for the treatment of various orthopedic implants, including metallic
dental implants.
13
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100651 Referring to FIGS. 1(a) ¨ 1(c), an exemplary dental
implant 200 includes a metal
abutment 208 and a dental crown 220, the latter being made from a non-
conductive material such
as ceramic, porcelain or a suitable polymer having an open end and an enclosed
hollow cavity 224
that is suitably sized and shaped to be fitted over the metal abutment 208.
The abutment 208 is
tied to a metallic post 210, the latter component being shown more clearly in
FIGS. 2(a) 2(c),
wherein the metallic post 210 is implanted directly into the jawbone 240 of
the patient. According
to this embodiment, the metal abutment 208 is press fit into the crown 220,
the latter having an
integrated metallic core 230 made in accordance with a specialized embodiment.
The metallic
core 230 is made from a suitable metallic material that enables and
facilitates electrical conduction.
In a preferred embodiment, the metallic core 230 is composed of a
biocompatible metal commonly
used in dentistry, such as but not limited to titanium, stainless steel and/or
their alloys. When the
metal abutment 208 is surgically implanted, the abutment 208 is placed into
direct physical contact
with a distal end of the metallic core 230, thereby creating an electrical
connection. The metallic
core 230 can alternatively be made integral with the abutment 208 (or can be
formed as part of the
crown 230), according to this embodiment.
100661 More specifically and according to this specific
embodiment, the metallic core 230
extends upwardly, as shown more specifically in FIG. 1(b), through the hollow
cavity 224 of the
dental crown 220, including a transverse portion of the core 230 that further
extends to an opening
formed in a side wall of the dental crown 220. A proximal end of the metallic
core 230 is preferably
flush with the side wall of the dental crown 220, thereby exposing a small
metallic surface zone
234 on the dental crown 220, as shown. The shape of the exposed metallic
surface zone 234 is
circular according to this specific embodiment, but it will be understood that
the shape of the
defined zone 234 and the metallic core 230 can be suitably varied. Making the
proximal end of
the core 230 flush to the surface of the crown 220 is preferable so not to
produce an overhang or
create sharp edges. According to one version, the exposed surface area 234 of
the metallic core
230 may be dimpled to allow for better mating with the crown attachment
mechanism. In a
preferred embodiment, the exposed metallic surface zone 234 resides on the
inner facing wall of
the crown 220 relative to the patient, such that the exposed metallic surface
zone 234 is not visible.
14
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100671 The overall shape and configuration of the metallic core
230 can be suitably varied
provided that the dental crown 220 can adequately and structurally function
primarily as a
prosthetic tooth. For example and in lieu of extending transversely as shown,
the proximal end of
the metallic core 230 can extend vertically through the hollow cavity 224
until exposed at a top
surface of the crown 220. Other similarly based versions of implants that
enable access to the
metallic abutment and post, but without requiring removal of the crown are
described in U.S.
Patent Application No. 16/884,664, which is incorporated herein by reference.
As noted, this
implant design provides a great advantage, when compared to other
technologies, because the
crown does not have to be removed in order to perform treatment.
100681 Various systems are shown schematically in FIGS 2(a) ¨
2(c) that can be utilized
for treatment of an infected dental implant, such as implant 200, based on the
application of a
suitable cathodic stimulation voltage to the exposed metal surface area 234
without having to first
remove the crown 220 to disrupt biofilm layers on the implant. The overall
principles of this form
(CVCES) of treatment are described in U.S. Patent No. 9,616,142, previously
incorporated herein
in its entirety. As shown in FIGS. 2(a) ¨ 2(c), the herein described dental
implant 200 or other
dental implants can be treated in conjunction with various CVCES treatment
configurations or
systems, herein labeled 400, 500, and 600, respectively. For purposes of this
discussion, the dental
implant 200 is shown schematically in use with each treatment system 400, 500,
and 600. It will
be understood that other metallic dental implants can be similarly treated
using any of these
exemplary treatment systems.
100691 Each CVCES treatment system 400, 500 and 600, as shown
diagrammatically in
FIGS. 2(a) ¨ 2(c), respectively, commonly includes a potentiostat 404 or
similar device that is
capable of generating an electrical potential as well as a number of
electrodes, minimally including
a working electrode and a counter electrode. The minimal configuration is
shown with reference
to FIG. 2(a), for a first exemplary CVCES treatment system 400, employing a
pair of electrodes;
namely, a working electrode and a counter electrode 420. The working electrode
is the dental
implant 200 based on the availability of the exposed metallic surface area 234
of the crown 230,
while the counter electrode 420 is preferably made up of carbon, although
other materials can be
used. The counter electrode 420 is attached to the gum/jawbone 240 area of the
patient via an
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
electrical lead or wire 412 coupled to the potentiostat 404. An electrical
lead 408 is further
provided extending to a conductive member that is placed into contact with the
exposed metallic
area 234 of the crown 230. Electrochemical current is caused to flow based on
voltages applied
by the potentiostat 404 via an electrochemical circuit being formed between
the working electrode
200 and the counter electrode 420. Due to the exposure area 234, the
potentiostat 404 is
electrically connected to the metal abutment 208, and thus the entire post-
abutment-core system is
electrically connected. However, the only metallic materials that are in
contact with a conductive
electrolyte (bone and soft tissue) are the metal abutment 208 and the post
210. Because the metallic
core 230 is encapsulated by the non-conductive crown material, the metal core
230 essentially acts
as an electrical wire or lead capable of transferring electrical energy (i.e.,
current) from the
potentiostat lead to the dental implant 200. This system 400 provides a
significant advantage,
when compared to other comparable potentiometric treatment systems or
techniques, because the
dental crown 220 does not have to be first removed in order to perform
treatment.
100701 FIG. 2(b) diagrammatically illustrates a three (3)
electrode system 500 that includes
the working electrode (the implant 200), as well as a counter electrode 520
functioning in the same
manner as that of the prior system 400. Each electrode 200, 520 is coupled to
the potentiostat 404
via electrical leads 508 and 512, respectively. A third (reference) electrode
524 is applied along
with the counter electrode 520 to the gums/jawbone area 240 of the subject and
is electrically
coupled to the potentiostat 404 via a corresponding lead 516. The reference
electrode 524 permits
greater electrochemical control for the treatment system 500. In a preferred
embodiment, the
reference electrode 524 is made from silver/silver chloride, thus producing a
stable electrochemical
biopotential for the working electrode (implant 200). Further details relating
to the functioning of
the potentiostat and counter and reference electrodes of this system 500 are
described in greater
detail in U.S. Patent No. 9,616,142, previously incorporated by reference in
its entirety.
100711 FIG. 2(c) diagrammatically illustrates yet another version
of a CVCES treatment
configuration 600 that employs four (4) electrodes. As in the preceding, the
dental implant 200
due to the exposed metallic area 234 acts a working electrode as electrically
coupled to the
potentiostat 404 by electrical lead 608. A counter electrode 620 and reference
electrode 624 are
attached to the gum/jawbone area 240 of the patient as connected to the
potentiostat 404 by leads
16
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
612 and 616, respectively. Each of the electrodes 620 and 624 operate in the
same manner as those
previously described. In addition, this treatment system 600 is further
equipped with a working
sense electrode 632, similarly attached to the gum/jawbone 240 area of the
patient and coupled to
the potentiostat 404 by electrical lead 618. The working sense electrode 632
enables further
control and data feedback of the working electrode (implant 200). Other
suitable cathodic voltage
treatment configurations or systems can also be utilized. In addition and
though the counter,
reference and working sense electrodes are shown according to this embodiment
being attached to
the jawbone/gum area 240, FIGS. 2(a) ¨ 2(c), of the patient, other suitable
positioning of these
electrodes is permitted. For example, any or all of these electrodes could
also be located outside
the mouth on the face or completely external to the body and connected via a
salt bridge.
100721 Advantageously, each of the above systems/configurations
permit reliable
treatment of the dental implant 200, but without requiring removal of the
crown. The exposed
metallic surface area 234 of the crown permits electrical conduction to the
remainder of the dental
implant. Exposed metal surfaces are both safe and cosmetically acceptable when
applying these
designs and embodiments. A distinct differentiator from alternative dental
treatment techniques
is that the herein described implant promotes conduction over the entire bone-
embedded surface
of the dental implant, and not just conduction, for example, within the
abscess pocket. This
differentiator is a significant advance, especially in regard to dental
implant posts. Implant posts
are typically manufactured with a very rough, coarse microsurface to promote
osseointegration.
One issue this microsurface can create is that bacteria are able to "hide"
within the crevices of the
microstructure, even when bone matrix are apparently grown into the surface.
The approach and
design of herein described apparatus allows for thorough treatment of all
microstructures in the
metal, even with bone present, to eliminate all bacteria from those locations.
It has been found
and substantiated in scientific literature that at optimized treatment
parameters, matrix embedded
bone cells that are local to the reaction are not affected to a high degree.
17
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100731 Apparatus are now described in accordance with a number of
embodiments
according to various aspects of the present invention for use in a treatment
system. More
specifically, the following described apparatus can be used in connection with
treatment systems
for enabling electrical contact with the working electrode (metallic dental
implant) and/or retaining
the counter and/or reference electrode.
100741 With reference to FIGS. 3(a) ¨ 3(d), there is shown a
first embodiment of an
apparatus 700 that can used in conjunction with the CVCES treatment systems
400, 500, 600,
FIGS. 2(a) ¨ 2(c), as well as other systems designed to remove biofilms from
metallic surfaces.
The herein described apparatus 700 is described by way of example for use with
the dental implant
200, FIGS. 1(a) ¨ 1(c), having the exposed metallic surface area 234, FIGS.
1(a) ¨ 1(c), on the
crown 220 to enable direct electrical connection to the metal abutment/post of
the dental implant
200. It will be understood, however, that the specialized dental implant 200
is merely discussed
as an example wherein the described apparatus 700 is suitable for use with
other dental implant
designs.
[0075] As previously described and in a CVCES treatment system
such as system 500,
FIG. 2(b), the dental implant 200 is employed as the working electrode and the
cathode of the
formed electrochemical cell. The herein described apparatus 700 is a
connective body that is
configured to provide electrical engagement between an external electrical
source such as a
potentiostat 404, FIG. 2(b), and the dental implant 200. More specifically and
according to this
embodiment, the herein described apparatus 700 is defined by a semi-circularly
shaped mouth
guard 720 The mouth guard 720 is made from a moldable biofriendly
thermoplastic or other
suitable structural material that is sized and configured to fit within the
mouth of a patient. More
specifically and according to this embodiment, the mouth guard 720 includes an
outer side or
surface 722 and an opposing inner side or surface 723. A front or outer
circumferential section
728 and a rear or inner circumferential section 732 each project from the
inner surface 723, each
of the front and rear circumferential sections 728, 732 being separated by an
open-ended
circumferential recess 736. The recess 736 is defined by a height and width
dimension enabling
the mouth guard 720 to be placed over the teeth (not shown) of a patient in
either the entire upper
or lower portion of the mouth. Alternative mouth guard designs are
contemplated for purposes of
18
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
this invention. For example, a single mouth guard can be sized and configured
to cover both the
upper and lower sets of teeth of a patient at the same time.
100761 According to this embodiment and as shown in FIG. 3(b),
the mouth guard 720 is
custom formfitted to the patient and includes at least one metal contact 752
integrated in a wall of
at least one of the front and rear circumferential sections 728, 732. The
location of the at least one
metal contact can be determined during the manufacturing process of the mouth
guard 720 and is
patient specific, depending on the location of the implant(s). Since the
apparatus 700 is patient
specific, a single metal contact can be provided or the apparatus 700 can
include two (2) or more
metal contacts for use with patients having multiple infected dental implants.
100771 In terms of electrical connection and still referring to
FIG. 3(b), the at least one
metal contact 752 is coupled to a grounding plate 744 imbedded within the wall
of the mouth guard
720, the latter grounding plate 744 being coupled by to an imbedded wire 748
that extends to an
extending electrical lead 740 at one end of the mouth guard 720. The extending
electrical lead 740
is preferably coated with a polymer or other insulating material and
configured for attachment to
an external electrical supply, such as a potentiostat 404, FIGS. 2(a) ¨ 2(c).
100781 According to at least one version, the mouth guard 720 may
be reusable or
alternatively could be designed as a single patient or single use apparatus.
According to a preferred
embodiment, the at least one metal contact 752 is biasedly positioned on the
interior surface (i.e.,
the surface facing the circumferential recess 736) of either the outer or
inner circumferential
sections 728, 732 of the mouth guard 720 to promote electrical contact with
the exposed metal
area 234, FIG. 1(b), of the dental implant 200.
100791 Various means for biasing the at least one metal contact
752 of the mouth guard
720 can be employed. For example, the at least one metal contact 752 can be
spring loaded relative
to one of the circumferential sections 728, 732 of the mouth guard 720.
Alternatively, biasing can
be provided by manufacturing the at least one metal contact 752 from a section
of a spring steel,
as shown in FIG. 3(b).
19
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100801 Alternatively and as shown in FIGS. 3(c) and 3(d), the at
least one metal contact
can be made from a conductive sponge 756 or a section of steel wool 760, each
of the latter
materials inherently providing a spring-like quality, albeit a weaker one than
those provided by
either spring loading or manufacturing the at least one metal contact from a
section of spring steel.
Each of the foregoing contacts 752, 756, 760 are electrically coupled to the
grounding plate 744,
FIG. 3(b), and imbedded wire 748, FIG. 3(b), disposed within the mouth guard
720 wherein each
or any of the foregoing techniques can be used for insuring that the at least
one spring contact 752,
756, 760 is biased into contact with the exposed metal area 234, FIG. 1(b), of
the dental implant
200, FIG. 1(a) ¨ 1(c). As noted, the grounding plate 744 and imbedded wire 748
provides a suitable
electrical connection to the coated electrical lead 740, partially shown,
leading to an external
voltage supply of a treatment system, for example CVCES treatment systems 400,
500, 600, FIGS.
2(a) ¨ 2(c), each having a potentiostat 404 or other suitable device that is
capable of providing a
cathodic stimulation voltage.
100811 When using a conductive sponge or steel wool 756, 760 as a
metal contact as shown
in FIGS. 3(c) and (d), respectively, the contact components should be made as
condensed as much
as possible within the defined recess 736 without sacrificing contactability
to the abutment or
crown of the dental implant in order to reduce extra metallic surface area
involved in the working
electrode reaction. In addition, all of the contact components described
according to this
embodiment may be coated with an insulating polymer that exposes only the
points of contact
needed for electrical connection in order to reduce extraneous metal surface
area.
100821 The at least one electrical contact, as described
according to this embodiment and
having embedded contact points, allows for treatment of a dental implant
without having to remove
the crown. As previously discussed, removing the crown is an option that many
dentists prefer
not to perform because the dental crown may break or cause extra trauma to the
afflicted tissue.
In the case where a biofilm exists on the post and abutment, the flow of
electrons into the bulk
metal, out the metal surface, and into the electrolytic environment, will
create bactericidal
chemical species that attack the biofilm from the metal surface outwards. pH
is also a large factor
in the bactericidal effect as laboratory testing has shown that
microenvironment pH levels microns
away from the surface can reach an alkaline level of 12 within minutes of
electrical stimulation.
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
[0083] Alternatively and for dental implants in which the
abutment of the dental implant
is not exposed or the crown does not provide an exposed electrical contact
point, the contact
mechanism embedded in the mouth guard 720 may alternatively include a needle
(not shown), the
latter being appropriately sized and configured to pierce the tissue and
contact the abutment of a
metal dental implant directly.
[0084] With reference to FIGS. 4(a) ¨ 4(f), another exemplary
apparatus 800 is described.
The apparatus 800 according to this embodiment is defined by another
connective body and more
specifically by a generic mouth guard 824, which like the preceding described
custom or formfitted
version 700, FIGS. 3(a) ¨ 3(d), has a semicircular shape or configuration. The
mouth guard 820
includes an outer facing side or surface 821, an opposing inner side or
surface 823, as well as an
outer or front circumferential section 826, an inner or rear circumferential
section 830, and an
open-ended circumferential recess 834, each extending from the inner surface
823. The recess 834
has a width and height dimension that enables the mouth guard 820 to be placed
over the teeth and
gums of the patient. Preferably, the mouth guard 820 is made from a moldable
thermoplastic
material. According to one version, the apparatus 800 can be cleaned or
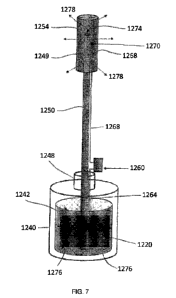
recycled for reuse. In
another version, the apparatus 800 is designed for single patient or single
use.
[0085] As shown in FIG. 4(a), 4(c) and 4(d), the herein described
apparatus 800 further
contains a metal grounding plate 844, preferably made from sheet metal, which
is embedded within
the mouth guard 820, as well as an embedded wire 848 further extending to an
electrical lead 840,
the latter extending from the mouth guard 820 to an external voltage supply
404, FIGS. 2(a) ¨ (c),
of a CVCES implant treatment system 400, 500, 600, FIGS. 2(a) ¨ (c).
Preferably, the extending
electrical lead 840 is coated with a protective polymeric or other suitable
insulating layer.
[0086] According to this specific embodiment and rather than
specifically integrating at
least one electrical contact, at least one releasably attachable contact 850
is configured for
placement over one of the inner and outer circumferential sections 826, 830 of
the mouth guard
820. As shown in FIGS. 4(c) and 4(d), the at least one releasably attachable
contact 850 is
fabricated in the form of a clip-like member defined by a base portion 854, as
well as pair of arm
portions 860, 864 extending in parallel relation from the base portion 854
with a spacing 858 being
21
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
defined between the arm portions 860, 864 that is sized to seat the releasable
attached contact 850
over the outer or inner circumferential portion 826, 830 of the mouth guard
820. More
specifically, the arm portion 864 is longer than the other arm portion 860 and
further configured,
when attached, to extend into the recess 834 of the mouth guard 820. The
exterior side of the arm
portion 864 is further configured with a contact portion, the latter being
configured for engaging
the dental implant.
[0087]
As further shown in FIG. 4(d), the releasably attachable contact 850
according to
this embodiment has embedded metal on the interior surfaces of each of the arm
portions 860, 864
to "bite" into the sheet metal of the grounding plate 844 imbedded in the
mouth guard 820.
[0088]
Advantageously and according to this embodiment, the number and
location of
releasably attachable contacts 850 can be varied as needed in order to create
alignment with an
implant, irrespective of the implant's location in the mouth of the patient.
Again referring to FIGS.
3(a) ¨ (c), the contact mechanism according to this embodiment may be spring
loaded, spring
steel/leaf-spring, or comprise a section of a conductive sponge or steel wool
to create biasing, each
enabling electrical contact with the exposed metal area 234, FIGS. 1(a) ¨
1(c), of the dental implant
200. Alternatively and in lieu of a contact, the leg portion 864 can be
separately provided with a
needle (not shown) extending therefrom and configured and positioned to
directly engage the
tissue below the gum line for contact with the abutment/post of the implant.
[0089]
The ability to attach the herein releasable attachable contact(s) 850
to one of the
wall sections 826, 830 of the mouth guard 820 provides considerable
versatility, enabling
placement of the contact(s) in literally any portion of the mouth of a patient
as shown in FIGS.
4(a), (b) and (e), without the need for pre-molding a custom guard. However,
in the case in which
the dentist wishes to have no mouth guard in order to use conjunctive
irrigational therapies to
disrupt the biofilm, alternative embodiments of the working electrode contact
are described that
attach directly to the crown and without the need for a fitted mouth guard.
22
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100901 FIGS. 5(a) ¨ 5(e) illustrates an apparatus 900 made in
accordance with aspects of
the invention to a connective body that can be used without an intermediate
mouth guard. More
specifically, the apparatus 900 is a spring loaded clip 920 having first and
second half portions
924, 928. Each of the half portions 924, 928 commonly include a leg portion
932, 936,
respectively, vertically extending from a distal end of each half portion 924,
928. An arm portion
940, 944 outwardly and transversely extends from an upper end of each leg
portion 932, 936 in a
curved configuration wherein each of the arm portions 940, 944 inwardly extend
and then cross
with one of the arm portions 944 being configured beneath the other arm
portion 940. A torsional
spring 950 provided on the upper arm portion 940 is coupled to the remaining
lower arm portion
944 at the junction between the curved arm portions 940, 944. The torsional
spring 950 according
to this embodiment biases the leg portions 932, 936 of the apparatus 900 and
more specifically
defines a spacing 964 between the leg portions 932, 936 that can be further
opened by inwardly
squeezing the proximal ends of the arm portions 940, 944 to enable the
apparatus 900 to be
releasably secured over the teeth and gums of a patient, the latter being
schematically shown as
904 in FIG. 5(a).
100911 Referring to FIGS. 5(a) and 5(c), the legs 932, 936
include inwardly faced surfaces
at each side of the defined spacing 964. The inwardly facing surface of leg
portion 932 is provided
with a metal contact 968 with the inwardly facing surface of the remaining leg
portion 936 being
provided with a soft pad 972, that is adhesively or otherwise attached. The
metal contact 968 is
directly wired through an imbedded wire (not shown) extending through the half
section 924 to a
proximal end of the arm portion 940, and further extending as an electrical
lead 976, shown only
in FIGS. 5(a) and 5(c), which is configured to make an electrical connection
to an external voltage
supply, such as potentiostat 404, FIG. 2(b), of the treatment system 500, FIG.
2(b).
100921 According to this exemplary embodiment, the metal contact
968 is configured to
make electrical contact with the exposed metal area 234 of the dental implant
200, FIGS. 1(a) ¨
1(c), while the soft pad 972 on the remaining leg portion 936 of the apparatus
900 is preferably
made from silicon, to help grip the tooth. As shown in FIGS. 5(b) and 5(d),
the metal contact could
be formed as a cantilevered section of spring steel 968A, as shown in FIG.
5(b), or a spring loaded
pin 968B, as shown in FIG. 5(d). Alternatively, the metal contact 968 could
also be made from a
23
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
conductive sponge material or steel wool such as those described in prior
embodiments, each of
which is preferably biased to make an electrical connection with a dental
implant, such as 200,
FIGS. 1(a) ¨ 1(c), or otherwise the clip member 900 could be configured with a
needle (not shown)
positioned to engage the dental implant below the gum line. According to
another alternative
version shown in FIG. 5(e), a half circular clip 968C on the lower end of the
leg portion 932 may
also be appropriate to clip on and contact the base of an abutment of an
implant (not shown) that
is very exposed. As in the preceding versions described, the apparatus 900 is
preferably configured
to provide connection to the working electrode of a CVCES treatment system,
such as those shown
in FIGS. 2(a) ¨ 2(c), with the dental implant serving as the working
electrode.
100931 As previously discussed and in order to complete a
circuit, there must at least exist
one other electrode in a CVCES or other electrochemically based treatment
system other than the
metal dental implant, the latter acting as a working electrode, as previously
shown in FIGS. 2(a),
2(b) and 2(c). In the case of a dental implant, a counter electrode of a CVCES
treatment system
ideally interfaces with the gums of the patient and more specifically, the gum
in which the implant
is implanted as opposed to a gum on the opposite side of the jaw or the other
jaw entirely (upper
or lower). Electrochemical current will flow between the implant and the
counter electrode due to
the conductiveness of the tissue. Though a two (2) electrode treatment system
400, such as shown
in FIG. 2(a) will function, a two-electrode treatment system only allows for
minimal control of the
electrochemical processes on the working electrode because the potentials of
the metal(s) of the
implant can drift into thermodynamic regions that can cause corrosion or metal
immunity.
Accordingly, a treatment system having additional electrodes as shown in FIGS.
2(b) and 2(c) is
preferable.
100941 Though any number of electrodes can be used, a three-
electrode system that
includes an additional stable reference electrode is more favorable due to its
balance of sufficient
electrochemical control and number of electrodes that need to be in the mouth
of the patient. In a
preferred embodiment, the reference electrode is made from Ag/AgC1, thus
providing a stable
electrochemical biopotential for the working electrodes voltage to be
referenced to. This function
keeps the working electrode in safe electrochemical regions of thermodynamics.
Although the
counter electrode needs to interface with the gums to promote electrochemical
current through the
24
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
tissue to the dental implant, the electrode's metal surface can exist either
internal or external to the
mouth as described herein.
100951 According to one version, shown in FIG. 6(a) and 6(b), an
exemplary apparatus
1000 is defined by a highly flexible connective body 1006 retaining a flexible
electrode 1008
fabricated from a plurality of stacked layers that create an electrical
connection. The connective
body 1006 according to this embodiment is shaped and configured to be placed
within the mouth
of a patient and more specifically placed or wrapped over the teeth, with
opposing sides of the
flexible electrode 1008 being in contact with the gums of the patient.
100961 According to this embodiment and as shown in FIG. 6(b),
the flexible electrode
1008 is shown in an exploded form and includes a hydrogel layer 1012, which is
preferably carbon
backed with at least one buffering agent, an anodic conductive film layer
1016, a conductive mesh
layer 1020, and an exterior layer 1024. The exterior layer 1024 is preferably
being made from a
flexible material, such as a fabric, that is sized and configured to surround
the assembly 1000 and
further includes an exterior adhesive that permits flexible attachment over
the teeth and gums 1004
of the patient. An electrical lead 1030 extends from a stimulation device such
as a potentiostat
404, FIG. 2(b), and attaches through the back of the exterior layer 1024. The
conductive mesh
layer 1020 behind the conductive anodic (preferably carbon) layer 1016 spreads
the point of
contact over a considerably larger area, wherein the mesh layer 1020 is
preferably made from
copper or platinum. The carbon film layer 1016 behind the buffered hydrogel
layer 1012 acts as
the conductive electrode surface for the reaction. Layer 1016 can
alternatively be made from
platinum, or other suitable metal that is chemically stable under anodic
reactions. Additional
details relating to this assembly are described in copending U.S. Patent
Application Serial No.
62/984,332, the entire contents of which are incorporated by reference.
100971 In a preferred embodiment, the flexible connective body
1006 of the apparatus 1000
interfaces with the gums on both the inner and outer sides of the jaw around
the implant, though
in a less preferred embodiment there may exist only one electrode on only one
side of the implant.
Preferably, the electrode 1008 of the herein described apparatus 1000 is
sufficiently flexible and
may wrap over the teeth to adhere to both sides of the gum, or alternatively
exist as two (2) separate
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
electrodes that adhere to both sides of the gum, but are electrically
connected to one another.
Having the flexible electrode 1008 on both sides of the gums creates a more
evenly distributed
treatment on the dental implant itself. The electrode 1008 may incorporate
flexible segments that
contour more effectively to the jaw. The anodic reaction will build up an
acidic pH within the
hydrogel, and thus the starting pH of the hydrogel will be neutral or basic,
preferably with a pH
between 6 and IL Preferably, the surface area of the electrode 1008 should be
at least the same
as the surface area of the dental implant to promote a more optimized
treatment. This embodiment
is directed to the counter electrode of a CVCES treatment system, such as
treatment system 500,
FIG. 2(b), but may also contain the reference electrode, herein shown as 1011
that can be
incorporated into the flexible connective body 1006. The reference electrode
1011 may
alternatively be provided as a separate electrode adhered to the gums, as
opposed to being built
into the herein described counter electrode while maintaining electrical
isolation between the
carbon and the Ag/Ag/C1 metal surfaces of the counter and reference
electrodes, respectively.
100981 The foregoing described an apparatus used in connection
with a CVCES or other
suitable treatment system, such as treatment systems 400, 500, 600, FIGS. 2(a)
¨ 2(c), with the
counter electrode being disposed within the mouth of the patient proximate the
dental implant. As
noted above, the counter electrode of the treatment system can alternatively
be disposed external
to the mouth of a patient. As shown in FIGS. 7 and 8, an external electrolytic
system 1200 is
represented, the system 1200 including a metal counter electrode surface 1220
supported within a
container 1240 or cartridge that is external to the mouth of the patient. The
container 1240 is
shaped and configured to retain a suitable quantity of a conductive fluid
1242, such as a salt
solution, for enabling electrochemical current transport to the gum interface.
In a preferred
embodiment, the salt solution would be composed of sodium chloride and water;
however, the salt
solution may contain any suitable electrolytic salt compound that can be
safely maintained within
the oral cavity. According to one version, the container 1240 retains
approximately 100 mL of
conductive fluid. In other embodiments, for example, the container 1240 can be
configured and
sized to retain between 20 mL and 10 liters of conductive fluid, though it
will be apparent that the
amount of a retained conductive fluid 1242 can be easily varied.
26
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
100991 According to this embodiment and in order to create an
electrolytic bridge, the
conductive fluid 1242 exits the container 1240 through a lid 1248 via a hollow
tube 1250 having
one proximal end extending through the lid 1248 and into the interior of the
container 1240. The
electrode 1220 according to this embodiment is defined by a carbon sheet or a
platinum mesh that
is fully or substantially immersed within the conductive fluid 1242 retained
in the container 1240.
The conductive fluid 1242 according to this embodiment can possess any pH and
can be mildly
acidic (pH of about 5.0), but preferably is neutral to basic and even more
preferably is basic in pH
in order to counteract the acid generation during treatment.
101001 According to this embodiment, at least one cotton roll
1254 is disposed at a distal
end 1249 of the hollow tube 1250. The at least one cotton roll 1254 can
preferably be made from
traditionally used dental cotton that often lines the gums for dental
procedures. Alternatively, the
roll 1254 can be made from synthetic cotton or other similar material. In a
preferred embodiment,
the distal end 1249 of the tube 1250 inserts into an end of the cotton roll
1254, as opposed to the
cotton roll 1254 being inserted into the opening of the hollow tube 1250. A
single cotton roll 1254
is shown for illustrative purposes, but it will be understood that one or more
cotton rolls can be
used. According to a preferred embodiment, the tube 1250 can be bifurcated
with tube portions
being separately attached to two cotton rolls that are disposed within the
oral cavity of a patient.
101011 The conductive fluid 1242 can be caused to flow from the
container 1240 to the
cotton roll(s) 1254 via the hollow tube 1250 by various means. For example,
the container 1240
can be made from a flexible material that can be squeezed. According to
another version, the
container 1240 can be pressurized during its manufacture and provided with a
seal (not shown)
that can be broken by the dentist or physician/caregiver prior to use. In
another version, the
container 1240 can be provided with a one-way valve 1262, FIG 8, permitting
the user to push air
into the container 1240 in order to displace conductive fluid 1242 through the
attached tube 1250
to the cotton roll(s) 1254. According to yet another version, the container
1240 can be suspended
or hung above the head of the patient wherein gravity can be used in order to
feed the conductive
fluid 1242 to the cotton roll(s) 1254. The foregoing are merely examples, as
it will be readily
understood that other suitable means configured for moving conductive fluid
1242 from the
container 1240 to the cotton roll(s) 1254 can be employed.
27
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
101021 FIG. 7 schematically depicts the flow of current through
the external electrolytic
system 1200. Lines 1264 represent current flow that transports electrically
from the external
voltage supply, such as potentiostat 404, FIG. 5(b) of a treatment system, to
the metal electrode
surface 1222 (counter electrode) disposed in the fluid container 1240 via a
connector, shown
diagrammatically as 1260. The current then is converted to electrochemical
current through
faradaic and non-faradaic reactions and enters the conductive fluid 1242,
shown as arrows 1276.
The current can then shuttle via the electrolyte through the hollow tube 1250,
into the at least one
saturated cotton roll(s) 1254, and into the gum interface as shown by arrows
1278. The cotton
roll(s) 1254 are retained at the gum interface of a patient, as shown in FIGS.
9(a) ¨ 9(c) using an
connective body disposed within the oral cavity of a patient in order to
permit the conductive fluid
1242 to be directed to the gum interface. An example of a suitable connective
body is described
in U.S. Patent No. 5,203,699, herein incorporated by reference in its
entirety. The connective body
according to the above patent is described for removing saliva from a patient
in a dental procedure
wherein the present apparatus is configured to support one and preferably two
(or more) cotton
rolls in a frame that is configured to positively engage and receive the
conductive fluid (and
current) to the gum interface for purposes of implant treatment.
101031 As noted, it is preferable that a three-electrode
treatment system or configuration
such as shown in FIG. 2(b) be utilized. Regarding the herein described
external electrolytic system
embodiment, it is highly preferred that a stable reference electrode 1270 such
as Ag/Ag-Cl stays
internal to the mouth to be as close to the working electrode (e.g., the
dental implant) as possible.
High resistance between the working electrode and the reference electrode 1270
can cause marked
drops in treatment current. Therefore, the reference electrode 1270 can be
disposed as a typical
adhesive hydrogel electrode to the gum line or alternatively be incorporated
into the body of the
cotton roll 1254 as shown schematically in FIGS. 7 and 8. In each of the
foregoing arrangements,
the reference electrode 1270 is separately coupled via lead 1268 to the
external voltage supplying
device (potentiostat 404, FIG. 2(b)) of the treatment system 500, FIG. 2(b)
through the connector
1260.
28
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
101041 The fluidic configuration of the external system 1200 is
shown in FIG. 8. An
electrode applicator is configured to retain cotton roll(s) 1254 on opposing
sides of a frame (not
shown) and receive conductive fluid 1242 from the container 1240, saturating
the cotton roll(s)
1254 and creating an electrolytic bridge with the gums of the patient and
forming an
electrochemical cell with the working electrode (metal dental implant). Once
an amount of
conductive fluid 1242 is moved from the container 1240 and saturates the at
least one cotton roll
1254, the cotton roll(s) 1254 is mechanically stabilized to the gum interface
of the patient in a
manner similar to that described with regard to the internally disposed
electrode 1008, previously
shown in FIGS. 6(a) and 6(b), including both the inner and outer sides of the
gum. The continuous
electrolyte bridge created and extending from the dental implant, through the
gums, the at least
one cotton roll(s) 1254, the conductive fluid 1242, and the metal surface 1222
enables this external
electrolytic system 1200. As such, the herein disclosed embodiment is
configured to supply the
cotton roll(s) 1254 with a fluid in order to promote a conductive pathway to
the metallic dental
implant.
101051 A main advantage of the foregoing external electrolytic
system 1200, as compared
to the version of FIGS. 6(a) and 6(b), is that acid build-up from the anodic
reaction on the counter
electrode can be mitigated to a much higher degree with an external metal
surface 1220. The
externally disposed metal surface 1220 can be provided with a much higher
surface area than an
internally disposed counter electrode because there is no size restriction,
such as found in the
mouth of the patient. The external metal surface 1220 may exist as a planar
sheet, a conductive
mesh, or alternatively as folded sheets in order to increase the surface area.
With an increase in
surface area, fewer faradaic chemical reactions are needed to take place to
support the reaction at
the working electrode and thus acid build-up is reduced. Also, the container
1240 of conductive
fluid 1242 provides a much larger volume of electrolyte for the acid to
diffuse into, and thus
concentrations of acid per volume can be reduced. As noted, the conductive
fluid 1242 also
preferably exists as a neutral to basic pH to assist in neutralizing any acid
build-up. However, one
disadvantage of the described external system 1200 is that there now exists
more electrochemical
resistance between the electrodes due to distance and volume of fluid between
the electrodes. This
increase in resistance may cause losses in current and thus therapy strength
to the biofilm layer of
the implant(s) being treated. This challenge can be overcome by increasing the
conductivity of
29
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
the conductive fluid 1242, optimizing the overall distance of the hollow tube
1250 (thus reducing
volume of fluid), maximizing the surface area size of the counter electrode
sheet or mesh 1220,
and ensuring that the electronics of the external power supply of the
stimulation device of the
treatment system, such as system 500, FIG. 2(b), contains suitable voltage
limitations, such that
the voltages required by the treatment reaction can be accommodated by the
external power supply
(potentiostat 404, FIG. 2(b)).
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
PARTS LIST FOR FIGS. 1 ¨ 9(c)
200 dental implant
208 abutment
210 post
220 crown
224 hollow cavity, crown
230 metallic core
234 exposed surface area
240 jawbone/gums
400 treatment system
404 potentiostat
408 electrical lead
412 electrical lead
420 counter electrode
500 treatment system
508 electrical lead
512 electrical lead
516 electrical lead
520 working electrode
524 reference electrode
600 treatment system
608 electrical lead
612 electrical lead
616 electrical lead
620 working electrode
624 reference electrode
632 sense electrode
700 apparatus
720 custom mouth guard
721 outer side or surface
723 inner side or surface
31
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
728 front or outer circumferential section
732 rear or inner circumferential section
736 circumferential recess
740 electrical lead
744 grounding plate
748 imbedded wire
752 contact
756 conductive sponge
760 steel wool
800 apparatus
820 working mouth guard
821 outer facing side or surface
823 inner surface
826 front or outer circumferential section
830 rear or inner circumferential section
834 recess
840 electrical lead
844 grounding plate
848 imbedded wire
850 releasably attachable contact
854 base portion, contact
858 spacing, contact
860 leg portion, contact
864 leg portion, contact
900 apparatus
904 jaw line/ gums (patient)
920 torsional clip
924 half section, clip
928 half section, clip
932 leg portion
936 leg portion
32
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
940 arm portion
944 arm portion
950 torsional spring
964 spacing
968 contact, metal
968A cantilevered piece of metal
968B spring-loaded pin
968C half-circular clip
972 pad
976 electrical lead
1000 apparatus
1006 connective body
1008 electrode
1012 buffered hydrogel layer
1016 conductive layer
1020 conductive mesh layer
1024 exterior layer
1030 electrical lead
1200 external electrolytic system
1220 metal electrode surface
1240 container
1242 conductive fluid
1248 lid, container
1249 distal end, tube
1250 tube
1254 cotton roll(s)
1260 connector
1262 one way valve
1264 line
1268 line
1270 reference electrode
33
CA 03168137 2022- 8- 16
WO 2021/177995
PCT/US2020/041022
1276 arrows
1278 arrows
1280 arrows
101061 The preceding embodiments are examples and it will be
understood to the reader
that a number modifications and variations can be made in accordance with the
present invention
including the following claims. For example and though the embodiments have
been described
for use with specific electrodes, the various apparatus could be used in
conjunction with other
electrodes. For example, the internal mouth disposed counter electrode could
also be optimal to
use in conjunction with the torsional clip working electrode contacting
mechanism.
34
CA 03168137 2022- 8- 16