Note: Descriptions are shown in the official language in which they were submitted.
CA 03168650 2022-07-20
METHOD AND APPARATUS FOR REMOVABLE CATHETER VISUAL LIGHT
THERAPEUTIC SYSTEM
RELATED APPLICATION
[0001] This is an international PCT application entitled METHODS AND APPARATUS
FOR REMOVABLE CATHETER VISUAL LIGHT THERAPEUTIC SYSTEM and claims
the benefit of United States Patent Application, Serial No. 16/747,315 that
was filed on January
20, 2020.
BACKGROUND OF THE INVENTION
Field of the Invention
[0002] The present invention is a method and apparatus to provide therapeutic
doses of non-
ultraviolet light to inactivate infectious agents residing on, within, or
generally around a
catheter while the catheter is residing within a body cavity and/or to
stimulate healthy cell
growth causing a healing effect. In particular, the disclosure is a medical
device assembly that
utilizes non-ultraviolet visual therapeutic electromagnetic radiation (EMR) at
a high enough
intensity to stimulate healthy cell growth causing a healing effect and/or to
reduce or eliminate
infectious agents in, on, and around a catheter while it resides inside a body
cavity.
[0003] Various exemplary embodiments of the present invention are described
below. Use
of the term "exemplary" means illustrative or by way of example only, and any
reference herein
to "the invention" is not intended to restrict or limit the invention to exact
features or steps of
any one or more of the exemplary embodiments disclosed in the present
specification.
References to "exemplary embodiment," "one embodiment," "an embodiment," "some
embodiments," "various embodiments," and the like, may indicate that the
embodiment(s) of
the invention so described may include a particular feature, structure, or
characteristic, but not
every embodiment necessarily includes the particular feature, structure, or
characteristic.
Further, repeated use of the phrase "in one embodiment," or "in an exemplary
embodiment,"
do not necessarily refer to the same embodiment, although they may.
The Relevant Technology
[0004] Catheters are used commonly as channels to inject medications into
or retrieve
fluid samples from a patient. Each catheter comprises a tube, usually made
from plastic or
other polymers, such as silicone, polyurethane, and the like, that is inserted
into an area of the
body and may contain one or more separate lumens through which fluids may be
delivered or
1
Date Recue/Date Received 2022-07-20
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
retrieved. A "lumen" designates an enclosed pathway within the catheter that
goes from outside
the body to inside the body. Catheters are used in various applications,
including
intravascularly, urologically, gastrointestinally, ophthalmically, within the
respiratory tract,
within the cranium, and the like. In all cases, the catheter may be placed
inside of a space in
the body where the catheter resides, herein referred to as a "body cavity".
These devices
frequently give rise to infections caused by growth of infectious agents in,
on, and around the
catheter. Infectious agents can include bacteria, fungi, viruses, or the like
that enter the body
and lead to illness of the patient. Depending on the location of the catheter
placement, these
infections can arise in the form of urinary tract infections, blood stream
infections, soft tissue
infection, and the like.
[0005] Catheter related infections (CRIs) are a large problem in medicine,
leading to high
morbidity and mortality rates. Current methods for reducing or eliminating the
number of
infectious agents in and on a catheter are of low efficacy. Typically,
catheters may be removed
if they are suspected to be harboring infectious agents, increasing both the
cost associated with
treatment and patient discomfort. Various methods to deter or eliminate growth
of infectious
agents in catheters have been attempted such as using sterile handling
techniques, antibiotics,
and replacing the catheter when an infection is suspected. Despite these
techniques, infections
resulting from catheters remain a major problem. According to the Centers for
Disease Control
and Prevention, over 31,000 people died specifically from catheter-related
bloodstream
infections in 2010. These infections, along with urinary tract infections,
gastrointestinal
infections, and other infections from catheters, increase both medical costs
and patient
discomfort.
[0006] Catheters come in various sizes. Those that are smaller in diameter,
such as many PICC
lines (peripherally inserted central catheters), have small diameter lumens.
Such smaller
diameter catheters may be suitable for prolonged insertion. Consequently, with
smaller
diameter catheters, there may be inadequate thickness to the catheter wall to
carry a sterilization
delivery system.
[0007] Accordingly, there exists a need for a method and apparatus designed to
deliver non-
antibiotic, bactericidal therapeutics in-vivo. Such a method and apparatus,
using novel
technology, may provide removable delivery of safe, effective, and
reproducible disinfection.
2
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
SUMMARY OF THE INVENTION
[0008] The exemplary embodiments of the present disclosure comprise methods
and
apparatuses for inactivating infectious agents and/or stimulating healthy cell
growth causing a
healing effect on, around, and in connection with catheters. In particular,
the methods utilize
removably insertable apparatuses for this inactivation and/or healing to occur
while the catheter
is residing within a patient's body cavity. Generally, this disclosure
addresses a medical device
assembly for removable insertion into a lumen within the catheter. The medical
device
assembly comprises an electromagnetic radiation (EMR) source, a removable EMR
conduction
system, and at least one coupling to connect the radiation source to the EMR
conduction
system. The EMR source provides non-ultraviolet, therapeutic EMR having
intensity
sufficient to inactivate one or more infectious agents and/or to stimulate
healthy cell growth
causing a healing effect. The removable EMR conduction system is at least
partially insertable
into and removable from the lumen of the catheter.
[0009] The EMR source can be from a single or group of EMR sources including,
but not
limited to, a light emitting diode, a semiconductor laser, a diode laser, an
incandescent (filtered
or unfiltered) and a fluorescent (filtered or unfiltered) light source. This
EMR source provides
non-ultraviolet, therapeutic EMR providing one or more wavelengths in the
range of above 380
nm to about 900 nm. In order to provide sufficient inactivation of infectious
species and/or
stimulation of healthy cell growth, each EMR wavelength should be of a narrow
spectrum and
centered around one wavelength from the group. The intensity should be
sufficient to inactivate
one or more infectious agents and/or to stimulate healthy cell growth causing
a healing effect.
This group includes several wavelengths: 400 nm, 405 nm, 415 nm, 430 nm, 440
nm, 445 nm,
455 nm, 470 nm, 475 nm, 632 nm, 632.8 nm, 640 nm, 650 nm, 660 nm, 670 nm, 680
nm, 780
nm, 808 nm, 830 nm, and 904 nm.
[0010] The EMR source may require drivers and electronic support for full
functionality.
Consideration should be given to accommodating the support hardware and/or
software, which
may encompass a significant portion of the EMR source's functionality and
efficacy. It is
possible that the EMR source may generate heat, which could be detrimental to
the EMR source
and may need to be limited.
[0011] One exemplary embodiment of the EMR source and support components is
simplified
to contain only the EMR source and necessary components. In another exemplary
embodiment
of the EMR conduction system, a passive heat sink is required to diffuse the
heat generated
into the surrounding environment. In yet another exemplary embodiment of the
EMR source,
3
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
a heat sink may be couple to at least one fan to actively dissipate heat
generated by the EMR
source.
[0012] Of particular interest to this disclosure is the use of light between
380 nm and about
900 nm wavelengths. Additionally, the intensity and power of the light emitted
bear
significantly on the inactivation of infectious agents, thus a range of
radiant exposures covering
0.1 J/cm2 to 1 kJ/cm2 and a range of powers from 0.005 mW to 1 W, and power
density range
covering 1 mW/cm2 and 1 W/cm2 are of interest for these exemplary device
assemblies and
methods. These ranges of wavelengths, power densities, and radiant exposures
have been
shown to have either antimicrobial effects or positive biological effects on
healing tissue. These
positive biological effects include reduction of inflammatory cells, increased
proliferation of
fibroblasts, stimulation of collagen synthesis, angiogenesis inducement and
granulation tissue
formation.
[0013] For each exemplary embodiment described herein, the EMR conduction
system and
method for disinfection/healing could be utilized in an adjustable or
predetermined duty cycle.
If treatments began immediately after sterile procedure was initiated, device
related infections
may be inhibited. This includes device related biofilm growth.
[0014] For the purposes of this disclosure the use of the term "duty cycle"
should be
understood to mean of or relating to the delivery of non-ultraviolet visual,
therapeutic EMR by
at least one of a single, multiple, variable, continuous, indefinite,
increasing-intensity lead-in,
decreasing-intensity phase-out, or any combination thereof on-and-off period
of EMR dosing
via the EMR conduction system of this disclosure.
[0015] A treatment may include at least one wavelength of therapeutic EMR
that acts as a
predominant wavelength selected to sterilize one or more target organisms and
selected from
the group of wavelengths centered about 400 nm, 405 nm, 415 nm, 430 nm, 440
nm, 445 nm,
455 nm, 470 nm, 475 nm, 660 nm, and 808 nm. Or, a predominant wavelength
selected to
promote healing and healthy cell growth may be selected from the group of
wavelengths
centered about 632 nm, 632.8 nm, 640 nm, 650 nm, 660 nm, 670 nm, 680 nm, 780
nm, 808
nm, 830 nm, and 904 nm. Another treatment may include alternating the
predominant
wavelength between a first predominant wavelength and a second predominant
wavelength
(differing from the first predominant wavelength) in a selected treatment
pattern. Further,
sterilizing EMR and EMR that stimulates healthy cell growth may be transmitted
simultaneously in tandem or alternatively.
4
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
[0016] The removably insertable EMR conduction system may comprise at least
one
optical element having an elongate body conducive to the axial propagation of
the therapeutic
EMR through the elongate body. This elongate body may have an exterior surface
between a
coupling end and a distal end tip. The exterior surface may have at least one
modified portion
wherein the modified portion permits the radial emission of therapeutic EMR
from the elongate
body proximate the modified portion.
[0017] The at least one coupling to connect the radiation source to the EMR
conduction system
may comprise at least one feature that allows for the coupling to be readily
removable from the
removable EMR conduction system. This coupling may be achieved by utilizing a
uniquely
designed connection, a pre-manufactured coupling system, or any combination
thereof that
optimizes the coupling efficiency and utility. Further, the coupling that
couples the removably
insertable EMR conduction system to the EMR source may comprise more than one
coupling
with an intermediate section optimized to further the propagation of the EMR.
In one
exemplary embodiment, the EMR source is coupled to a patch cable or EMR
conduction
extending segment, which is then coupled to the formal removably insertable
EMR conduction
system.
[0018] For the purposes of this disclosure the use of the term
"therapeutic" should be
understood to mean of or relating to the treatment of disease, including
reducing or eliminating
infectious agents, as well as serving or performed to maintain health,
including enhancing
healthy cell growth.
[0019] The optical element further comprises at least one optical feature
selected from a group
of optical features such as a reflective surface, an optically transmissible
material, a lens, a
fiber optic filament, and any combination thereof. The optical element also
may be capable of
transmitting more than one wavelength or intensity EMR. Multiple wavelengths
may be
transmitted simultaneously, one after another or in tandem, or a combination
thereof (for
example, one constantly on and the other wavelength pulsed). Multiple
intensities may be
transmitted through the same element simultaneously. Alternating patterns of
light treatments
may also be transmitted.
[0020] The EMR conduction system may be configured to insert, at least
partially, into one of
any number of catheters, such as by way of example only and not to be
limiting: a central
venous catheter, a peripheral insertion catheter, a peripheral insertion
central catheter, a midline
catheter, a jugular catheter, a subclavian catheter, a femoral catheter, a
cardiac catheter, a
cardiovascular catheter, a urinary Foley catheter, an intermittent urinary
catheter, an
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
endotracheal tube, a gastrointestinal catheter, a nasogastric tube, a wound
drainage catheter, or
any similar accessing medical catheter or tube that has been inserted into a
patient for the
purpose of delivering or retrieving fluids or samples.
[0021] One exemplary embodiment of the EMR conduction system has an optical
element
comprising a single, insertable optical fiber. With a single optical fiber,
the single fiber may
allow light to transmit radially or axially at various sections along its
length. For sections where
light will transmit radially, the exterior surface of the optical element may
be modified by
chemical etching, physical etching, or electromagnetic ablation through plasma
or lasers to
modify various sections along the length of the optical fiber. The modified
portions pellnit
light to emit radially.
[0022] For purposes of this disclosure, light emitted radially means that the
light has a radial
component. Hence, the light emitted radially may emit perpendicularly and/or
obliquely to the
central axis of the optical fiber at the axial point of emission.
[0023] For embodiments having modified sections, the material comprising the
optical fiber
may be selected from a group of materials comprising optical fibers including
plastic, silica,
fluoride glass, phosphate glass, chalcogenide glass, plastic, and any other
suitable material that
is capable of axial light propogation and surface modification to achieve
radial emission. In
addition, the optical fibers may be single mode, multi-mode, or plastic
optical fibers that may
have been optimized for modification using a chemical, physical, or
electromagnetic
manufacturing modification process. The optical fibers may also be optimized
for modification
post-production.
[0024] Yet another exemplary embodiment employs a physical abrasion method of
alteration
to modify the EMR conduction system comprised of at least one optical fiber.
This fiber would
be utilized based on its optimal optical response to the physical abrasion
process. This process
may include, but is not limited to, sanding, media blasting, grinding,
buffing, or media blasting
at least one section of the optical fiber. The physical abrasion process would
also necessarily
be optimized in terms of the extent of physical abrasion to optimize the
appropriate radial EMR
emission or lack thereof This may be accomplished by adjusting at least one of
velocity,
acceleration, pressure, modification time, or abrasion material utilized in
modifying the optical
fiber.
[0025] Yet another exemplary embodiment employs microscopic porous structures
in the
optical fiber to achieve radial transmission of light. These microscopic
structures may be
positioned within the core and/or core-cladding boundary of the optical fiber.
The microscopic
6
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
structures having a refractive index lower than the region free of microscopic
structures. The
microscopic structures may be a material added to the optical fiber core or
the core-cladding
boundary, such as a metal, rubber, or plastic. The microscopic structures may
also be the lack
of material creating an aberration within the optical fiber core or the core-
cladding boundary.
For example, the presence of microscopic bubbles in the optical fiber core
would create an
aberration or imperfection that would alter the materials refractive index,
resulting in EMR
being radially emitted from the optical fiber.
[0026] Another exemplary embodiment may comprise at least one optical fiber
with cladding
modified to optimize the radial or axial propagation of EMR. For example, the
cladding may
be modified to at least partially remove or thin the cladding in order to
achieve partial radial
transmission of EMR. Another example could include an optical fiber with only
certain
portions containing cladding, the EMR transmitting axially in the clad
portions and at least
partially axially and radially in the non-clad portions.
[0027] Yet another exemplary embodiment achieves radial transmission
equivalency wherein
the radially emitting portion of the optical fiber has substantially
equivalent intensity over the
length of the emitting portion along the optical fiber. This may be done
through chemical
etching, physical etching, plasma ablation, or laser ablation in a gradient
pattern. By altering at
least one of velocity, acceleration, pressure gradients, flow, modification
time, or modification
material or process, it is possible to achieve radial transmission equivalency
throughout each
portion or the entire length of the modified optical fiber. During
manufacturing, a gradient-
provided equivalency also may be achieved through addition of microscopic
structures
positioned within the core and/or core-cladding boundary in a gradient
pattern. Also, radial
transmission equivalency achieved through gradient cladding or core features
are contemplated
for achieving desired radial emission, whether substantially equivalent over a
portion length or
varying as desired.
[0028] Still another exemplary embodiment achieves a gradient radial
transmission wherein at
least one portion of the optical fiber emits EMR radially in a gradient
distribution. The gradient
distribution may also be accomplished through chemical etching, physical
etching, plasma or
laser ablation in a uniform or gradient pattern. By altering at least one of
velocity, acceleration,
pressure gradients, flow, modification time, or modification material or
process, it is possible
to achieve a gradient radial transmission throughout a portion of modified
optical fiber. This
may also be achieved through addition of microscopic structures positioned
within the core
and/or core-cladding boundary.
7
CA 03168650 2022-07-20
[0029] A further exemplary embodiment of the removable EMR conduction system
comprises an optical element such as at least one LED, its associated wiring
components, and
a scaffold. The LED(s) may emit EMR based on the LED's inherent distribution,
or may utilize
another optical element, such as a lens or minor, to focus or diffuse the EMR
in the direction
of interest. In addition, more than one LED could be arranged in an array to
appropriately emit
EMR for maximal therapeutic benefit. The LED(s), together with associated
wiring
components may be permanently or removably attached to the scaffold, which
allows for
removable insertion of the EMR conduction system into a catheter. The scaffold
may be rigid,
semi-rigid, malleable, elastic, or flexible, or any combination thereof.
[0030] Another exemplary embodiment comprises a plurality of optical elements
where the
lumen is sufficiently large to receive a plurality of optical elements. The
elongate body of each
optical element has a length and at least two of the elongate bodies may have
differing lengths
such that elongate bodies of differing lengths terminate at varying distances
from the coupling
end of the elongate body. Additionally, where in-lumen space permitting,
multiple optical
elements of various lengths may be arranged to achieve a gradient distribution
pattern and/or
multiple emitting portions.
[0030a] In another embodiment of the present invention there is provided a
medical device
assembly for delivering electromagnetic radiation (EMR) into, onto, and around
a catheter
indwelling within a patient's body and having at least one lumen, the medical
device assembly
comprising: an EMR source for providing non-ultraviolet, therapeutic EMR
having an intensity
comprising at least one of a radiant exposure of at least 0.5 J/cm2 and up to
1 kJ/cui2, power of
at least 0.005 mW and up to 1 W, and power density of at least 0.1 mW/cm2 and
up to 1 W/cm2,
whereby such intensity causes a therapeutic effect of at least one of
inactivating one or more
infectious agents and promoting healing; an EMR conduction system comprising
an optical
element connector and an optical element having an elongate body and a distal
end tip, at least
a portion of the optical element being insertable into a disposition within
and removable from
the lumen of the catheter while the catheter resides within the patient's
body, the elongate body
being conducive to the axial propagation of the non-ultraviolet, therapeutic
EMR through the
elongate body, the elongate body having an exterior surface between a proximal
coupling end
and the distal end tip, the exterior surface having at least one radial
emission portion between
the proximal coupling end and the distal end tip allowing the emission of non-
ultraviolet,
therapeutic EMR radially from the elongate body into the lumen of the catheter
and through
the catheter, thereby delivering a duty cycle of the non-ultraviolet,
therapeutic EMR into, onto,
8
Date Recue/Date Received 2022-07-20
CA 03168650 2022-07-20
and around the catheter while the optical element resides in the disposition
and the catheter
resides within the patient's body, the duty cycle comprising at least one of a
single, multiple,
variable, continuous, increasing-intensity lead-in, decreasing-intensity phase-
out, and any
combination thereof on-and-off periods; and a coupling to connect the EMR
source to the
optical element connector of the EMR conduction system and to deliver the non-
ultraviolet,
therapeutic EMR from the EMR source to the optical element for axial
propagation of the non-
ultraviolet, therapeutic EMR through the elongate body.
[0030113] In a further embodiment of the present invention there is provided a
medical system
for delivering electromagnetic radiation (EMR) into a patient's body, medical
system
comprising: a catheter having at least one lumen; a medical device assembly
for delivering
(EMR) into, onto, and around the catheter: an EMR source for providing non-
ultraviolet,
therapeutic EMR having a wavelength in a range of above 380 nm to 904 nm and
having an
intensity comprising at least one of a radiant exposure of at least 0.5 J/cm2
and up to 1 kJ/cm2,
power of at least 0.005 mW and up to 1 W, and power density of at least 0.1
mW/cm2 and up
to 1 W/cm2, whereby such intensity causes a therapeutic effect of at least one
of inactivating
infectious agents and promoting healing; an EMR conduction system comprising
at least one
optical element having an elongate body conducive to the axial propagation of
the non-
ultraviolet, therapeutic EMR along the elongate body, at least one of the
optical elements being
at least partially insertable into and removable from at least one of the
lumen of the catheter
while the catheter resides within the patient's body, the non-ultraviolet,
therapeutic EMR
emitting radially from the elongate body into the lumen and through the
catheter, thereby
delivering a duty cycle of the non-ultraviolet, therapeutic EMR into, onto,
and around the
catheter while the catheter resides within the patient's body, the duty cycle
comprising at least
one of a single, multiple, variable, continuous, increasing-intensity lead-in,
decreasing-
intensity phase-out, and any combination thereof on-and-off periods; and at
least one coupling
to connect the EMR source to the EMR conduction system and to deliver the non-
ultraviolet,
therapeutic EMR from the EMR source to the optical element for axial
propagation of the non-
ultraviolet, therapeutic EMR through the elongate body.
[0030c1 In yet another embodiment of the present invention there is provided a
method for
effectively delivering non-ultraviolet, therapeutic EMR into a catheter while
the catheter is
indwelling within a patient's body, the catheter having a lumen with an
interior surface
dimension, comprising the steps of: inserting an optical element of an EMR
conduction system
into a disposition within the lumen of the catheter, the optical element
having an exterior
surface dimension which is less than the interior surface dimension of the
lumen such that the
8a
Date Recue/Date Received 2022-07-20
CA 03168650 2022-07-20
optical element is removably insertable into the lumen of the catheter;
transmitting a duty cycle
of non-ultraviolet, therapeutic EMR from an EMR source into the optical
element of the EMR
conduction system for an amount of time and at an intensity comprising at
least one of a radiant
exposure of at least 0.5 J/cm2 and up to 1 kJ/cm2, power of at least 0.005 mW
and up to 1 W,
and power density of at least 0.1 mW/cui2 and up to 1 W/cm2, whereby such
intensity causes a
therapeutic effect of at least one of inactivating one or more infectious
agents and promoting
healing; emitting the duty cycle of non-ultraviolet, therapeutic EMR radially
into the lumen of
the catheter and through the catheter, the duty cycle comprising at least one
of a single,
multiple, variable, continuous, increasing-intensity lead-in, decreasing-
intensity phase-out, and
any combination thereof on-and-off periods; delivering the duty cycle of the
non-ultraviolet,
therapeutic EMR into, onto, and around the catheter while the optical element
resides in the
disposition and the catheter resides within the patient's body; and removing
the optical element
of the EMR conduction system from the lumen of the catheter, the catheter
remaining
indwelling within the patient's body.
[0031] For each exemplary embodiment, the assembly and method for disinfection
may be
utilized in an adjustable or predetermined duty cycle or duty cycles. If
treatments begin
immediately after sterile procedure has been initiated, device-related
infections may be
inhibited. Also, if treatments begin after device-related infection(s) have
been detected, the
treatment may cause the inactivation of one or more infectious agents. It
should be understood
that inactivating infectious agents includes inhibiting infectious agents and
infectious agents
includes device-related biofilm growth.
BRIEF DESCRIPTION OF THE DRAWINGS
[0032] In order that the manner in which the above-recited and other features
and
advantages of the present disclosure are obtained will be readily understood,
reference is made
to exemplary embodiments thereof which are illustrated in the appended
figures.
Understanding that these figures depict only typical exemplary embodiments and
are not
therefore to be considered limiting of the scope of the present disclosure,
the exemplary
embodiments will be described and explained through the use of the
accompanying figures in
which:
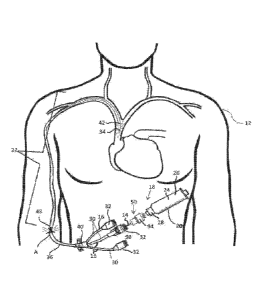
[0033] FIG. 1 is
a schematic view of a triple lumen catheter, an insertable optical element,
and an EMR component;
8b
Date Recue/Date Received 2022-07-20
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
[0034] FIG. 2 is a perspective view of a dual lumen catheter with the
insertable component
outside the catheter;
[0035] FIG. 3 is a perspective view of a dual lumen catheter with the
insertable component
disposed partially inside the catheter;
[0036] FIG. 4 is a perspective, partially exploded view of a dual lumen
catheter with the
insertable component disposed partially inside the catheter and showing an
intermediate
coupling;
[0037] FIG. 5 is a series of elevation views of several exemplary embodiments
of an insertable
optical element with varying locations, lengths, and degrees of modification,
and with the
optical element connector shown as transparent;
[0038] FIG. 6 shows cross-sectional views of multiple portions of an
insertable optical element
with various EMR radial, gradient dispersion levels;
[0039] FIG. 7 shows the cross-sectional views of various gradient dispersion
levels of FIG. 6
showing the sections with EMR ray diagrams of internal reflection, and
relative radial
emission;
[0040] FIG. 8 shows cross-sectional views of various exemplary dispersals of
microscopic
structures (such as flecks or bubbles) within a fiber optic's core, cladding,
and the core/cladding
boundary;
[0041] FIG. 9 is a schematic view of a treatment being applied to the
insertable optical
element;
[0042] FIG. 10 is a perspective, transparent view of an optical element
connector showing an
exemplary optical collimating element; and
[0043] FIG. 11 shows plan views of an optical element assembly and EMR power
source,
detached and attached, which does not require a collimating lens.
REFERENCE NUMERAL S
catheter 10 patient's body 12
insertable optical element 14 line tubing 16
EMR conduction system 18 electromagnetic radiation component 20
insertable catheter component 22 elongate body 24
electromagnetic radiation power source 26 coupling element 28
internal lumen 30 proximal catheter hub assembly 32
distal end tip 34 elongate catheter body 36
9
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
catheter of varying lengths 38 convergence chamber 40
termination of the optical element 42 flexible protection tubing 44
line clamp 46 transdermal area 48
optical assembly 50 intermediate coupling 52
patch cable 54 EMR conduction extending segment 56
forward connector 58 rearward connector 60
exterior surface 62 tip 64
connecting element 88 EMR hub connector 90
collimating lens 92 optical element connector 94
alignment shaft 98 an aligning bore 99
non-modified optical span 100 segment-modified optical span 102
single modified portion 103 fully-modified optical span 104
single elongated modified portion 105 multi-modified optical span 106
modified tip portion 107 first section 108
microscopic structures free area 109 second section 110
minimal concentration 111 third section 112
moderate concentration 113 fourth section 114
maximal concentration 115 core 116
microscopic structures 117 optical element cladding 118
cladding boundary 120 first dispersal 121
control device 122 second dispersal 123
wand 124 third dispersal 125
acid spray 126 outer region 127
inner region 129 boundary region 131
insertion site A
DETAILED DESCRIPTION OF THE INVENTION
[0044] The exemplary embodiments of the present disclosure will be best
understood by
reference to the drawings, wherein like parts are designated by like numerals
throughout. It
should be understood that the components of the exemplary embodiments, as
generally
described and illustrated in the figures herein, could be arranged and
designed in a wide variety
of different configurations. Thus, the following more detailed description of
the exemplary
embodiments of the apparatus, system, and method of the present disclosure, as
represented in
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
FIGS. 1 through 11, is not intended to limit the scope of the invention, as
claimed, but is merely
representative of exemplary embodiments.
[0045] In
this application, the phrases "connected to", "coupled to", and "in
communication
with" refer to any form of interaction between two or more entities, including
mechanical,
capillary, electrical, magnetic, electromagnetic, pneumatic, hydraulic,
fluidic, and thermal
interactions.
[0046]
The phrases "attached to", "secured to", and "mounted to" refer to a form of
mechanical coupling that restricts relative translation or rotation between
the attached, secured,
or mounted objects, respectively. The phrase "slidably attached to" refer to a
form of
mechanical coupling that permits relative translation, respectively, while
restricting other
relative motions. The phrase "attached directly to" refers to a form of
securement in which the
secured items are in direct contact and retained in that state of securement.
[0047]
The term "abutting" refers to items that are in direct physical contact with
each
other, although the items may not be attached together. The term "grip" refers
to items that are
in direct physical contact with one of the items firmly holding the other. The
term "integrally
formed" refers to a body that is manufactured as a single piece, without
requiring the assembly
of constituent elements. Multiple elements may be formed integral with each
other, when
attached directly to each other to form a single work piece. Thus, elements
that are "coupled
to" each other may be formed together as a single piece.
[0048] FIG. 1 of the present disclosure depicts a schematic view of an
exemplary triple lumen
catheter 10 with the catheter 10 shown disposed within a patient's body 12. An
insertable
optical element 14 is inserted partially into the catheter 10, and an EMR
component 20 is
connected to the insertable optical element 14.
[0049] The catheters 10 depicted in FIGS. 1-4 are exemplary multiple lumen
catheters 10 each
also comprises line tubing 16, one or more (in FIG. 1, three are shown, in
FIGS. 2-4, two are
shown) proximal catheter hub assemblies 32, an elongate catheter body 36, a
distal end tip 34,
and a convergence chamber 40. Internal lumen 30 has an inner diameter (i.e.,
an interior
surface dimension) and runs the length of the catheter 10, from the proximal
catheter hub
assembly 32, through the line tubing 16, the convergence chamber 40, and the
elongate catheter
body 36, to the distal end tip 34. The insertable optical element 14 is
elongate with an outer
diameter (i.e., an exterior surface dimension) sufficiently small to be
insertable within at least
one of the internal lumens 30 and may extend at least as far into the catheter
10 as a termination
of the optical element 42, although the insertion may be less than that length
if desired.
11
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
100501 Catheters 10 suitable for use with the insertable optical element 14
may be of several
different makes, sizes, and functions. For example, catheters that are
translucent may be
particularly suited to permit the passage of radially emitted EMR therethrough
to the tissue
surrounding the catheter 10. Catheters 10 that have an interior surface
dimension (inside
diameter) sufficiently larger than the exterior surface dimension (outer
diameter) of the
insertable optical element 14 may permit the injection or withdrawal of fluid
(liquid or gas)
simultaneously through the catheter while that insertable optical element 14
resides within the
catheter 10.
[0051] Also, some catheters 10 have radiopacifiers embedded within the walls
of the catheter
so that an image of where the catheter 10 is located within the patient's body
12 may be
determined. However, some catheters have no such radiopacifiers. In either
case, it is
contemplated by this disclosure that radiopacifiers may be contained in or on
the insertable
optical element 14 to provide detection of the location of the catheter 10
within the patient's
body 12 when the catheter 10 does not have radiopacifiers, and to provide
detection of the
location of the insertable optical element 14 disposed within the catheter 10
whether or not the
catheter 10 has radiopacifiers (this may require differing radiopacifiers in
some instances so
that the catheter 10 and the insertable optical element 14 may be
distinguished).
[0052] With some exemplary embodiments, at least one of the proximal catheter
hub
assemblies 32 may have an optical fiber element alignment shaft 98 that aligns
an optical
element connector 94 and the insertable optical element 14.
[0053] FIG. 1 shows the catheter 10, in a schematic view, inserted at an
insertion site A in an
arm of the patient's body 12. The depiction shows how non-ultraviolet,
therapeutic EMR may
be delivered at the insertion site A and to other sites within the patient's
body 12. At the
insertion site A, the therapeutic EMR may be delivered to a transdermal area
48 to inactivate
infectious agents in that area and to enhance healing of the insert site A.
Similarly, proximate
the distal end tip 34, in this case within the vena cava, therapeutic EMR may
be delivered to
inactivate infectious agents and/or to enhance healing in that proximate
vicinity.
[0054] The EMR component 20 comprises the EMR power source 26 (FIGS. 2-4), a
light
source (not shown, such as a laser or the like), electrical circuitry (not
shown), and optics (not
shown, but dependent upon the light source) all housed within an elongate body
24. A coupling
element 28 connects the EMR component 20 to an optical assembly 50. The
optical assembly
50 comprises the insertable optical element 14 and the optical element
connector 94. The
combination of the EMR component 20, the coupling element 28, and the optical
assembly 50,
12
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
comprising the insertable optical element connector 94 and the insertable
optical element 14,
will be referred to herein as a removable EMR conduction system 18.
[0055] Of particular interest to each of the embodiments is the use of light
having wavelengths
ranging from above 380 nm and about 900 nm. Additionally, the intensity and
power of the
light emitted server to inactivate of infectious agents and/or to promote
healing. A range of
radiant exposures covering 0.1 J/cm2 to 1 kJ/cm2 and a range of powers from
0.005 mW to 1
W, and power density range covering 1 mW/cm2 and 1 W/cm2 are of interest for
these
exemplary device assemblies and methods. These ranges of wavelengths, power
densities, and
radiant exposures have been shown to have either antimicrobial effects or
positive biological
effects on healing tissue. These positive biological effects include reduction
of inflammatory
cells, increased proliferation of fibroblasts, stimulation of collagen
synthesis, angiogenesis
inducement and granulation tissue formation.
[0056] For each exemplary embodiment described herein, the EMR conduction
system 18
and method for disinfecting/healing could be utilized in an adjustable or
predetermined duty
cycle or duty cycles. If treatments began immediately after sterile procedure
was initiated,
device-related infections may be inhibited. Also, if treatments begin after
device-related
infection(s) have been detected, the treatment may cause the inactivation of
one or more
infectious agents. It should be understood that inactivating infectious agents
includes inhibiting
infectious agents and infectious agents includes device-related biofilm
growth.
[0057] An exemplary adjustable, predetermined duty cycle may effectively
utilize at least
one of a power ranging from 0.005 mW to 1 W, a power density from a range
covering 1
mW/cm2 and 1 W/cm2 and radiant exposure from a range covering 0.1 J/cm2 to 1
kJ/cm2 and
at least one of a single, multiple, variable, continuous, indefinite,
increasing-intensity lead-in,
decreasing-intensity phase-out, or any combination thereof on-and-off periods.
EMR dosing
uses the EMR conduction system of this disclosure and operated in duty cycles.
[0058] Infectious agents may include but are not limited to bacteria,
fungi, viruses, and
protozoa that invade the body and lead to or cause illness of the patient.
Depending on the
application, infectious agents vary by type and sensitivity to EMR
inactivation. Effective duty
cycles and EMR dosing may be standardly optimized by those skilled in the art
through
empirical derivation. Through such empirical derivation, effective duty cycles
may be
identified whereby duty cycle intensity causes a therapeutic effect of at
least one of inactivating
one or more infectious agents and promoting healing.
13
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
[0059] One exemplary application of interest pertains to catheters utilized
in environments
subjected to continuous contamination from infectious agents. In this
exemplary application
one effective duty cycle (on-and-off period) is repeated continuously for the
effective life of
the catheter. Treatments composed of multiple effective duty cycles each with
intensity
sufficient to inactivate one or more infectious agents may inhibitor eradicate
device-related
infections and/or device-related biofilm growth.
[0060] Another exemplary duty cycle may include the EMR conduction system
delivering
EMR with an intensity sufficient to inactivate one or more infectious agents
and/or to stimulate
healthy cell growth causing a healing effect by utilizing an "on" period of
120 minutes followed
by an "off" period of 10 minutes. This on-and-off pattern may be repeated
continuously for 30
days providing sterilizing and/or healing EMR for the effective life of the
catheter.
[0061] The foregoing being only representative examples, it should be
understood that a
duty cycle(s) is/are adjustable and may be comprised of an unlimited number of
possible
combinations of at least one of a power ranging from 0.005 mW to 1 W, a power
density from
a range covering 1 mW/cm2 and 1 W/cm2 and a radiant exposure from a range
covering 0.1
J/cm2 to 1 kJ/cm2 and at least one of a single, multiple, variable,
continuous, indefinite,
increasing-intensity lead-in, decreasing-intensity phase-out, or any
combination of on-and-off
period(s). On-and-off periods may comprise of at least one of an infinite
combination of
nanoseconds, milliseconds, seconds, minutes, hours, or days.
[0062] A treatment may include at least one wavelength of therapeutic EN/IR
that acts as a
predominant wavelength selected to sterilize one or more target organisms and
selected from
the group of wavelengths centered about 400 nm, 405 nm, 415 nm, 430 nm, 440
nm, 455 nm,
470 nm, 475 nm, 660 nm, and 808 nm. Another treatment may include alternating
the
predominant wavelength between a first predominant wavelength and a second
predominant
wavelength (differing from the first predominant wavelength) in a selected
treatment pattern.
Further, sterilizing EMR and EMR that stimulates healthy cell growth may be
transmitted
simultaneously in tandem or alternatively.
[0063] Another embodiment of the present disclosure is depicted in FIG. 2,
showing a
perspective view of a dual lumen catheter 10 with the removable EMR conduction
system 18
outside the catheter 10. The catheter 10 portion of the depiction shows
flexible protection
tubing 44 that protects the coupling of the proximal catheter hub assembly 32
with the line
tubing 16 and also protects line tubing 16 from wear imposed by line clamps
46.
14
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
[0064] FIG. 3 shows the dual lumen catheter 10 of FIG. 2 with the removably
insertable EMR
conduction system 18 partially inserted into one of the lumens 30 of the
catheter 10.
[0065] FIG. 4 shows an exploded perspective view of an exemplary EMR
conduction system
18 as partially inserted into the proximal catheter hub assembly 32 and an
internal lumen 30.
With this exemplary embodiment, an intermediate coupling 52 is shown. Such
intermediate
coupling 52 may comprise a patch cable 54 or an EMR conduction extending
segment 56 used
to extend the distance between the EMR power source 26 and the optical element
connector 94
of the insertable optical element 14, without appreciable loss of light
intensity. Each of the
patch cable 54 or EMR conduction extending segment 56 may have a forward
connector 58 to
securely engage coupling element 28, and a rearward connector 60 to securely
engage the
optical element connector 94. Hence, by using a patch cable 54 or an EMR
conduction
extending segment 56, the EMR power source 26 may be operated some desired
distance from
the patient to reduce noise or heat concerns and/or to position the EMR power
source 26 closer
to a power source (not shown) such as an electrical outlet or battery pack.
[0066] FIG. 5 is a series of elevation views of several exemplary embodiments
of an optical
assembly 50 showing various locations with gradient degrees of modification on
the exterior
surface 62 of the insertable optical element 14. Each view of the series of
views shows an
optical assembly 50 with an insertable optical element 14 connected to the
optical element
connector 94. The optical element connector 94 (see also FIG. 10) has a
connecting element
88, an EMR hub connection 90, a collimating lens 92, and an alignment shaft
98.
[0067] The first view (uppermost) of the series of views shows a non-modified
optical span
100 of the insertable optical element 14 that is without any radial dispersion
(i.e., the insertable
optical element 14 has not been modified to provide radial emission of light
from the body of
the insertable optical element 14). With this embodiment, therapeutic, non-
ultra-violet EMR
may be provided to a tip 64 with no radial emission from the non-modified
optical span 100
other than at the tip 64.
[0068] The second view (next view down) of the series of views shows an
exemplary radial
transmission equivalency over a single modified portion 103 (i.e., modified
portion 103 has a
gradient modification such that the emitted light has substantially the same
intensity and power
over the length of the modified portion 103) that provides radially dispersed
light from a
segment-modified optical span 102. The location of the single modified portion
103, in this
instance, corresponds to where the catheter 10 enters the insertion site A
when the insertable
optical element 14 is inserted fully into the catheter 10. With this
embodiment, radially emitted
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
visual light may sterilize the insertion site A and the transdermal area 48 or
any other
predetermined site within the patient's body 12.
[0069] The third view of the series of views shows an example of a single
elongated modified
portion 105 that provides radially dispersed light from optical element 14
extending along most
of a fully-modified optical span 104. The location of the single elongated
modified portion
105 corresponds generally to the length of the insertable catheter component
22 of the catheter
10. With this embodiment, therapeutic light may be provided for substantially
the entire length
that the catheter 10 would be inserted within the patient's body 12.
[0070] The fourth view of the series of views shows an example of radial
transmission
equivalency at multiple locations. A single modified portion 103 and an
additional radial
transmission equivalency at a modified tip portion 107 are spaced along a
multi-modified
optical span 106. The locations of the modified portion 103 and the modified
tip portion 107
correspond to areas of the body, including for example the insertion site A,
where the delivery
of non-ultraviolet, therapeutic EMR may be desired for sterilization and/or
healing. It should
be understood that there may be more than one modified portion 103 disposed
along the length
of the multi-modified optical span 106 and/or each modified portion 103 may
have various
lengths.
[0071] Also, it should be understood that in each of these views the modified
portions depicted
may be of modifications other than modification of the exterior surface 62 of
the insertable
optical element 14, such as for example, modifications including microscopic
structures
embedded within the insertable optical element 14 that allow radial
transmission of light from
the insertable optical element 14. Further, such modified portions 103, 105,
107 may have
gradient patterns that allow for an overall substantially-uniform distribution
of light over the
length of the modified portion 103, 105, 107.
[0072] FIG. 6 is a schematic view of an optical assembly 50 with an insertable
optical element
14 coupled to an optical element connector 94. The insertable optical element
14 has a fully-
modified optical span 104. Multiple locations along the insertable optical
element 14 are shown
in enlarged cross-sectional views. These locations are axially spaced along
the insertable
optical element 14 to assist in describing the nature of an exemplary
insertable optical element
14 at each location. As depicted, there are four section locations, a first
section 108, a second
section 110, a third section 112, and a fourth section 114. For brevity, the
modifications on
and in the insertable optical element 14 at each of the four sections are
combined in the
depictions of FIG. 6. Of course, the modified portion of the insertable
optical element 14 may
16
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
be singular or multiple, may be any length or gradient, and may be coincident,
overlapping or
not.
[0073] The first section 108 represents an internally reflected region of the
insertable optical
element 14. As shown at the first section 108, there is no ablation (or other
modification) and
no microscopic structure within the core 116 of the insertable optical element
14. No
therapeutic non-ultra-violet EMR will emit radially from the insertable
optical element 14 at
the first section 108.
[0074] The second section 110 represents a minimally emissive region of the
insertable optical
element 14. As shown at the second section 110, there is minimal ablation (or
other
modification) to the exterior surface 62 of the insertable optical element 14
and a minimal
dispersal of microscopic structures 117 within the core 116 of the insertable
optical element
14. From the second section 110, minimal therapeutic, non-ultra-violet EMR
will emit radially
from the insertable optical element 14. However, the amount of EMR emitted
should have
sufficient intensity and power to inactivate infectious agents and/or promote
healing proximate
the second section 110.
[0075] The third section 112 represents a moderately emissive region of the
insertable optical
element 14. As shown at the third section 112, there is moderate ablation (or
other
modification) to the exterior surface 62 of the insertable optical element 14
and moderate
dispersal of microscopic structures 117 within the core 116 of the insertable
optical element
14. From the third section 112, a moderate amount of therapeutic, non-ultra-
violet EMR will
emit radially from the insertable optical element 14 proximate the third
section 112. However,
prior to reaching the third section 112, the amount of light traveling down
the insertable optical
element 14 diminishes due to the radial emission of some of the light such as
at second section
110. Consequently, the degree of the gradient of modification is selected so
that the amount
of EMR emitted radially at third section 112 should be substantially
equivalent to the radial
emission at the second section 110. Hence, the intensity and power of the EMR
emitted may
be substantially equivalent to the intensity and power emitted at second
section 110 and is of
sufficient intensity and power to inactivate infectious agents and/or promote
healing.
[0076] The fourth section 114 represents a maximally emissive region of the
insertable optical
element 14. As shown at the fourth section 114, there is maximal ablation (or
other
modification) to the exterior surface 62 of the insertable optical element 14
and maximal
dispersal of microscopic structures 117 within the core 116 of the insertable
optical element
14. From the fourth section 114, a maximum amount of therapeutic, non-ultra-
violet EMR will
17
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
emit radially from the insertable optical element 14 proximate the fourth
section 114. Again,
prior to reaching the fourth section 114, the amount of light continuing to
travel down the
insertable optical element 14 diminishes due to the radial emission of some of
the light such as
at second section 110 and at third section 112. Consequently, the degree of
the gradient of
modification is selected so that the amount of EMR emitted radially at fourth
section 114
should be substantially equivalent to the emissions at second section 110 and
third section 112.
The intensity and power of the EMR emitted may be substantially equivalent to
the intensity
and power emitted at second section 110 and third section 112 and is of
sufficient intensity and
power to inactivate infectious agents and/or promote healing.
[0077] The modified portions may be modified by chemical, physical or other
cladding
modification (e.g., ablation) to alter the critical angle enough to allow
light to emit radially.
Additionally or alternatively, the modified portions may be modified by
dispersing microscopic
structures 117 of varying gradient concentration inside the core 116 of the
insertable element
14. The gradient concentration of microscopic structures 117 within the core
116 shown in
FIG. 6 range from a microscopic structures free area 109, to a minimal
concentration 111 of
microscopic structures 117, to a moderate concentration 113 of microscopic
structures 117, to
a maximal concentration 115 of microscopic structures 117.
[0078] The concentration of microscopic structures 117 within the core 116
affects the
refractive index of the core 116 and the core-cladding boundary 118. The
microscopic
structures 117 (which may be voids, such as bubbles) create changes in the
incident angle of
the light as it passes through the insertable optical element 14. At certain
incident angles, light
leaves the optical element cladding 119 and emits radially from the cladding
boundary 120.
[0079] FIG. 7 is a schematic view of the cross-sectional views of FIG. 6
depicting light rays
as arrows. The same cross-sectional views of the insertable optical element 14
are shown:
namely, the first section 108 (internally reflected), the second section 110
(minimally radially
emissive), the third section 112 (moderately radially emissive), and the
fourth section 114
(maximally radially emissive). These views also show light rays traveling down
the core 116,
that collide with microscopic structures 117 at an incident angle causing the
light ray to pass
through the optical element cladding 119. An increasing pixilated gradient is
depicted on the
cladding boundary 120 from the first section 108 (no pixilation), to the
second section 110
(minimal pixilation), to the third section 112 (moderate pixilation), to the
fourth section 114
(maximal pixilation) represents the chemical, physical or other cladding
modification (e.g.,
ablation) at the cladding boundary 120. Such modification of the insertable
optical element 14
18
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
alters critical angles enough to allow light to emit radially. As
schematically depicted, the
amount of rays leaving the optical element cladding 119 are substantially
equivalent at each
site although the amount of rays the core 116 diminishes as the light travels
from proximal to
distal. The microscopic structures 117 of varying gradient concentration are
also shown inside
the core 116, from the microscopic structure free area 109, to a minimal
concentration 111, to
a moderate concentration 113, to a maximal concentration 115. Each of the
microscopic
structures 117 has a refractive index that differs from that of the core 116
and the optical
element cladding 119. The microscopic structures 117 (which may be voids, such
as bubbles)
create changes in the incident angle of the light as it passes through the
insertable optical
element 14. At certain incident angles, light leaves the optical element
cladding 119 and emits
radially.
[0080] FIG. 8 shows cross-sectional views of various exemplary dispersals of
microscopic
structures 117 (such as flecks or bubbles) within a fiber optic's core 116,
cladding 119, and the
core/cladding boundary 118. With each of the exemplary embodiments depicted
microscopic
structures 117 are dispersed within the insertable optical element 14 (in this
case an optical
fiber) to achieve radial transmission of light. These microscopic structures
117 may be
positioned within the core 116 and/or at the core-cladding boundary 118 and/or
within the
cladding 119 of the optical fiber 14. The microscopic structures 117 having a
refractive index
lower than the region free of microscopic structures 117. The microscopic
structures 117 may
be a material added to the optical fiber core 116 or the core-cladding
boundary 118, such as a
metal, rubber, or plastic. The microscopic structures 117 may also be the lack
of material
creating an aberration within the optical fiber core 116 and/or the core-
cladding boundary 118
and/or within the cladding. For example, the presence of microscopic
structures 117 (such as
bubbles) in the optical fiber core 116 creates an aberration or imperfection
that would alter the
materials refractive index, resulting in EMR being emitted radially from the
optical fiber
(insertable optical element 14).
[0081] In FIG. 8, three exemplary dispersals, a first dispersal 121, a second
dispersal 123, and
a third dispersal 125, are depicted. The first dispersal 121 has microscopic
structures 117 (such
as flecks or bubbles) dispersed within and outer region 127 of the core 116
only. The second
dispersal 123 has microscopic structures 117 dispersed within an inner region
129 of the
cladding 119 as well as within the outer region 127 of the core 116. The third
dispersal 125
has microscopic structures 117 dispersed proximate to the core/cladding
boundary 118 and are
depicted as identifying a boundary region 131 that is thinner than the outer
region 127 of the
19
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
core 116 and the inner region 129 of the cladding 119. With each of these
exemplary dispersals,
at least some of the light traveling the length of the insertable optical
element 14 (fiber optic)
will not encounter any microscopic structures 117 while the remainder of the
light may
encounter at least one microscopic structure 117 and be deflected to emit
radially from the
insertable optical element 14.
[0082] FIG. 9 is a schematic view of an exemplary optical element modification
method for
creating gradient modification on the exterior surface 62 of the insertable
optical element 14.
Such modification of the core 116 or optical element cladding 118 alters the
incident angle of
light rays so that they differ from the critical angle needed to remain
internally reflected.
Depicted in FIG. 9 is a control device 122 with a wand 124 delivering an acid
spray 126 for
etching the insertable optical element 14.
[0083] There are several methods for achieving this gradient modification.
Chemically, the
insertable optical element 14 may be etched using a strong acid such as
hydrofluoric acid or
sulfuric acid and hydrogen-peroxide. Also, quartz powder, calcium fluoride, or
an etching
cream, usually carrying a fluorinated compound, may be used. Physically,
heating the
insertable optical element 14 or physical modification such as ablation by
sanding, media
blasting, grinding, or laser ablation modifications are also methods for
creating gradient
modification. Additionally, plasma ablation by laser modification causes the
ionization of
molecules and alteration of the exterior surface 62 of the insertable optical
element 14. Other
known methods for creating gradient ablation are contemplated by this
disclosure. Regardless
of the modification or manufacturing process, whether presently known or not,
the insertable
optical element 14 may be modified to have substantially equivalent radially
emitted light along
desired lengths. This uniformity in radially emitted light allows for a more
accurate treatment
dose for inactivating infectious agents and/or promoting healing.
[0084] In FIG. 10 of the present disclosure, a transparent view of the optical
element connector
94 is depicted, comprising a connecting element 88, an EMR hub connection 90,
a collimating
lens 92, an alignment shaft 98, and an aligning bore 99. The insertable
optical element 14 may
be inserted into the aligning bore 99 of the optical element connector 94 to
collimate the light
into a small diameter core 116 or one or more optical fibers.
[0085] The exemplary disclosure depicts an optical diversion element as a
single collimating
lens 92, but other types of optical diversion elements such as multiple lenses
or different types
of lenses may be used to collimate the light. Depending on the optical element
14 diameter,
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
numerical aperture, and refractive index, specific lenses will be needed as an
optical diversion
element to reduce light loss.
[0086] Referring now to FIG. 11 of the present disclosure, depicted are a pair
of EMR
conduction systems 18, one in exploded view and one in assembled view, each
EMR
conduction system has an EMR power source 26 that is attachable to an optical
assembly 50
having an optical element connector 94 without a collimating lens 92. In
instances where the
numerical aperture, diameter, and material can be matched with that of the
optical element 14,
a collimating lens 92 may not be required. In such instances the EMR hub
connector 90 may
connect directly to the EMR power source 26 and the optical element connector
94, as depicted.
[0087]
This disclosure anticipates that the system and methods of this disclosure may
be
embodied in other specific forms without departing from its structures,
methods, or other
essential characteristics as broadly described herein and claimed hereinafter.
The described
embodiments are to be considered in all respects only as illustrative, and not
restrictive. The
scope of the disclosure is, therefore, indicated by the appended claims,
rather than by the
foregoing description. All changes that come within the meaning and range of
equivalency of
the claims are to be embraced within their scope.
[0088]
For exemplary methods or processes of the invention, the sequence and/or
arrangement of steps described herein are illustrative and not restrictive.
Accordingly, it should
be understood that, although steps of various processes or methods may be
shown and
described as being in a sequence or temporal arrangement, the steps of any
such processes or
methods are not limited to being carried out in any particular sequence or
arrangement, absent
an indication otherwise. Indeed, the steps in such processes or methods
generally may be
carried out in various different sequences and arrangements while still
falling within the scope
of the present invention.
[0089]
Additionally, any references to advantages, benefits, unexpected results, or
operability of the present invention are not intended as an affirmation that
the invention has
been previously reduced to practice or that any testing has been performed.
Likewise, unless
stated otherwise, use of verbs in the past tense (present perfect or preterit)
is not intended to
indicate or imply that the invention has been previously reduced to practice
or that any testing
has been performed.
[0090]
Exemplary embodiments of the present invention are described above. No
element,
act, or instruction used in this description should be construed as important,
necessary, critical,
or essential to the invention unless explicitly described as such. Although
several exemplary
21
CA 03168650 2022-07-20
WO 2021/150279 PCT/US2020/056219
embodiments have been described in detail herein, those skilled in the art
will readily
appreciate that many modifications are possible in these exemplary embodiments
without
materially departing from the novel teachings and advantages of this
invention. Accordingly,
all such modifications are intended to be included within the scope of this
invention as defined
in the appended claims.
[0091] In the claims, any means-plus-function clauses are intended to cover
the structures
described herein as performing the recited function and not only structural
equivalents, but also
equivalent structures. Thus, although a nail and a screw may not be structural
equivalents in
that a nail employs a cylindrical surface to secure wooden parts together,
whereas a screw
employs a helical surface, in the environment of fastening wooden parts, a
nail and a screw
may be equivalent structures. Unless the exact language "means for"
(performing a particular
function or step) is recited in the claims, a construction under Section 112,
6th paragraph is not
intended. Additionally, it is not intended that the scope of patent protection
afforded the present
invention be defined by reading into any claim a limitation found herein that
does not explicitly
appear in the claim itself.
22