Note: Descriptions are shown in the official language in which they were submitted.
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
ADAMTS INHIBITORS, PREPARATION METHODS AND
MEDICINAL USES THEREOF
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority under 35 U.S.C. 119(e) to United States
Provisional
Patent Application No. 62/969,992, filed on February 4, 2020; No. 63/046,267,
filed on June
30, 2020; No. 63/066,148, filed on August 14, 2020; and No. 63/087,656, filed
on October 5,
2020, the disclosures of all of which are incorporated herein by reference in
their entireties.
FIELD OF THE DISCLOSURE
The present disclosure relates to compounds and methods in inhibiting the
function of
ADAMTS-5 and/or ADAMTS-4 and their application in the treatment of diseases
involving
degradation of cartilage or disruption of cartilage homeostasis, such as
osteoarthritis and/or
rheumatoid arthritis.
BACKGROUND OF THE DISCLOSURE
Cartilage is the highly specialized connective tissue of diarthrodial joints.
Its principal
function is to provide the joints the capability of load bearing and
compression resistance.
Chondrocyte is the cellular component of articular cartilage, taking about
only 5% of the tissue
volume. The main component of cartilage is extracellular matrix comprising
aggrecan and
collagen. Under physiological conditions, cartilage homeostasis is maintained
by a balance
between production (anabolism) and degradation (catabolism) of aggrecan and
collagen.
However, the balance is shifted to catabolism in diseases such as
osteoarthritis.
Osteoarthritis is the most common chronic joint disease and a leading cause of
pain and
disability in developed countries. It can happen to the joints of the hips,
knees, spines, hands
and others. It was estimated that worldwide 250 million people are currently
being affected by
osteoarthritis, and the prevalence is progressively rising (Hunter et al.,
Lancet. 2019, 393:
1745-1759). Pain and loss of functional capacity are accompanied by an
increased risk of
additional disease conditions such as diabetes, cancer or cardiovascular
disease (Valdes AM
and Stocks J Osteoarthritis and ageing. Eur Med J. 2018, 3:116-123).
Osteoarthritis is a whole
joint disease, the structural changes of which are found to be degradation of
articular cartilage,
synovitis and alterations in subchondral bone and other periarticular tissues
(Goldring MB and
Otero M Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011, 23: 471-
478). The
pathogenesis of osteoarthritis is not very clear, with mechanical damage,
inflammation, aging,
and metabolism factors being involved. Osteoarthritis is not a passive
degenerative disease, but
1
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
an active dynamic alteration arising from an imbalance between the repair and
destruction of
joint tissues (Hunter et al., Lancet. 2019, 393: 1745-1759). Currently, the
pharmacological
treatments available for osteoarthritis are limited to symptomatic relief of
pain and
inflammation. Disease-modifying drugs that arrest or slow down disease
progression are not
available.
Progressive loss of articular cartilage is currently viewed as an early event
in
osteoarthritis. Aggrecan may have a role protecting loss of collagen (Pratta
et al., J Biol Chem.
2003, 278: 45539-45545). These studies suggest the critical role of aggrecan
in osteoarthritis
and other joint diseases. Aggrecan is a proteoglycan, possessing a core
protein with covalently
attached sulfated glycosaminoglycan (GAG) chains. Its core protein has three
globular domains,
G1 and G2 domains in the N-terminus, and G3 in the C-terminus. The extensive
region between
the G2 and G3 domains is heavily modified by GAG keratan sulfate (KS) and
chondroitin
sulfate (CS). Based on the difference in the amino acid sequence, the CS
domain is further
divided into two subdomains, CS 1 and C52. The GAG chains provide aggrecan
with its high
anionic charge. Multiple aggrecan monomers bind to hyaluronan (HA) through G1
domains,
which is stabilized by a link protein, forming large supramolecular
aggregates. The large
aggrecan aggregates absorb water and provide the resilient properties for the
cartilage
(Roughley et al., The Journal of Experimental Orthopaedics. 2014, 1: 8). A
high concentration
of aggrecan, a high degree of sulfation and the ability to form large
aggregation is required for
the normal function of cartilage.
The extended structure of aggrecan can be cleaved by proteolytic enzymes,
leading to
impaired normal function of cartilage. ADAMTS (a disintegrin and
metalloproteinase with
thrombospondin motifs) is a family of zinc ion-dependent metalloproteases.
ADAMTS-4 and
-5, also termed "aggrecanases", degrade aggrecan at several specific locations
in the IGD and
the C52 domain. It was demonstrated that ADAMTS-5 deficiency protects against
aggrecan
loss and cartilage damage in mouse osteoarthritis disease model induced by
surgeries (Glasson
et al., Nature. 2005, 434: 644-648; Stanton et al., Nature. 2005, 434:648-
652), implicating
ADAMTS-5 in driving cartilage loss and osteoarthritis disease severity.
However, some studies
in human cartilage explant culture suggested that not only ADAMTS-5, but also
ADAMTS-4
are important for human osteoarthritis (Verma et al., Journal of Cellular
Biochemistry. 2011,
112: 3507-3514). These studies strongly suggest that inhibiting the enzymatic
function of
ADAMTS-5 and ADAMT-4 might provide a protecting role in osteoarthritis.
2
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
In sum, the role of ADAMTS-5 and/or ADAMTS-4 in cartilage degradation has been
well-established. Therefore, compounds that can inhibit ADAMTS-5 and/or ADAMTS-
4 may
be of therapeutic value in the treatement of arthritis.
SUMMARY OF THE DISCLOSURE
The compounds of this disclosure inhibit the function of ADAMTS-5 and/or
ADAMTS-4 and accordingly may serve as therapeutic agents for the treatment of
diseases
involving degradation of cartilage or disruption of cartilage homeostasis, in
particular,
osteoarthritis and/or rheumatoid arthritis.
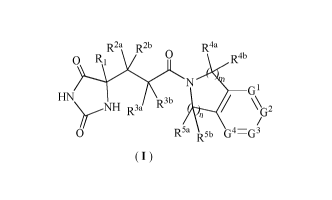
The present disclosure, in one aspect, provides a compound of formula (I), or
a
tautomer, mesomer, racemate, enantiomer, diastereomer thereof, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof:
R2a R2b R4a
0 R1
0 R4b
G1
HN R3b R3a \\G2
0 R5a R5b G4G3
(I)
wherein:
G1, G2, G3 and G4 are each independently N or CR6, provided that no more than
two of
them are N;
R1 is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl,
cycloalkyl,
heterocyclyl and heteroaryl, wherein the alkyl, cycloalkyl, heterocyclyl and
heteroaryl are each
optionally substituted with one or more, preferably one to five, and sometimes
more preferably
one to three, groups independently selected from the group consisting of
halogen, hydroxy,
cyano, alkyl, alkoxy, hydroxyalkyl, SO2R11a, NRilaR1113, C(=0)0R1 la,
C(=0)NRilaR1113, NH
c(=o)Riia, NH C(=0)0R11a, cycloalkyl, heterocyclyl, aryl and heteroaryl;
R2a, R213, R3a and
are each independently selected from the group consisting of
hydrogen, halogen, alkyl, alkoxy, hydroxy, haloalkyl, haloalkoxy,
hydroxyalkyl, cyano, amino,
nitro, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein the alkyl,
cycloalkyl, heterocyclyl,
aryl and heteroaryl are each optionally substituted with one or more,
preferably one to five, and
sometimes more preferably one to three, groups independently selected from the
group
consisting of halogen, alkyl, alkoxy, hydroxy, hydroxyalkyl, cyano, amino,
nitro, NR12aR1213,
3
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
C(=0)0R12a, C(=0)NR12aR1213, NHC(=o)R12a, NHC(=0)0R12a, oR12a, cycloalkyl,
heterocyclyl, aryl and heteroaryl;
or alternatively, two of R2a, R21), R3a and R31 together with the carbon atom
to which
they are attached form cycloalkyl or heterocyclyl;
R4a, R4b, R5a and x -.-s5b
are each independently selected from the group consisting of
hydrogen, deuterium, halogen, alkyl, alkoxy, hydroxy, haloalkyl, haloalkoxy,
hydroxyalkyl,
cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein
the alkyl, cycloalkyl,
heterocyclyl, aryl and heteroaryl are each optionally substituted with one or
more, preferably
one to five, and sometimes more preferably one to three, groups independently
selected from
the group consisting of halogen, alkyl, alkoxy, hydroxy, hydroxyalkyl, cyano,
amino, nitro,
cycloalkyl, heterocyclyl, aryl and heteroaryl;
or alternatively, two of R4a, R41, R5a and R5b together with the carbon atom
they are
attached form cycloalkyl or heterocyclyl;
R6 at each occurrence is independently selected from the group consisting of
hydrogen,
halogen, alkyl, alkoxy, haloalkyl, haloalkoxy, hydroxy, hydroxyalkyl, cyano,
amino, nitro,
SO2R13a, SO2NR13aRl3b, NR13aR1313, C(=0)0R13a, C(=0)NR13aR1313, NHC(=0)R13a,
NHC(=0)0R13a, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein the
alkyl, cycloalkyl,
heterocyclyl, aryl and heteroaryl are each optionally substituted with one or
more, preferably
one to five, and sometimes more preferably one to three, groups independently
selected from
the group consisting of halogen, alkyl, alkoxy, hydroxy, hydroxyalkyl, cyano,
amino, nitro,
SO2R14a, SO2NR14aRl4b, NR14aRl4b, C(=0)0R14a, C(=0)NR14aR1413, NHC(=o)R14a,
NHC(=0)0R14a, cycloalkyl, heterocyclyl, aryl and heteroaryl;
each of R1 la, R12a, R13a and K-14a
is independently selected from the group consisting of
hydrogen, alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein the
alkyl, cycloalkyl,
heterocyclyl, aryl and heteroaryl are each optionally substituted with
halogen, hydroxy, alkoxy,
alkyl, aryl and cycloalkyl;
each of Rlib, R121), R131, and R1413 is independently selected from the group
consisting of
hydrogen and alkyl, wherein alkyl is optionally substituted with halogen,
hydroxyl and alkoxy;
n is 1 or 2; and
m is 1 or 2.
In another aspect, this disclosure provides a preparation process of a
compound of
formula (I), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, the
preparation process
comprising the steps of:
4
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R4a
R2a R2b R4b R2a R2b
-pp 4a
0 0 " R4b
Ri Ri
OH HN
Gi ___________________________________________________________________ Gi
HN
R3b R3b
R3a
R3a
R5a R5b G4=G3
0 0 R5a
R5b G4 ¨G3
( IA ) ( IB ) ( I )
reacting a compound of formula (IA) with a compound of formula (IB), or a
pharmaceutically acceptable salt thereof, to obtain the compound of formula
(I), wherein:
the pharmaceutically acceptable salt of the compound (IB) preferably is
hydrochloride;
and
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (I).
In another aspect, the present disclosure provides a pharmaceutical
composition
comprising a compound of formula (I), or an isomer, pharmaceutically
acceptable salt, solvate
or prodrug thereof thereof, and a pharmaceutically acceptable carrier.In
another aspect, the
present disclosure provides a method of preventing and/or treating
inflammatory conditions,
and/or diseases involving degradation of cartilage, and/or disruption of
cartilage homeostasis,
comprising a step of administering to a subject in need thereof a
therapeutically effective
amount of a compound of formula (I), or an isomer, pharmaceutically acceptable
salt, solvate,
or prodrug, or a pharmaceutical composition containing the compound.
In another aspect, the present disclosure also relates to use of a compound of
formula
(I), or an isomer, pharmaceutically acceptable salt, solvate or prodrug
thereof, or a
pharmaceutical composition containing the compound, in the manufacture of a
medicament for
treatment of an inflammatory condition, and/or a disease involving degradation
of cartilage,
and/or disruption of cartilage homeostasis.
The disease or condition includes arthritis, preferably, rheumatoid arthritis,
psoriatic
arthritis, osteoarthrosis and hypertropic arthritis, which are further
preferably related to the
activity of ADAMTS-5 and/or ADAMTS-4.
Other aspects or advantages of the disclosure will be better appreciated in
view of the
following detailed description, examples, and claims.
DETAILED DESCRIPTION OF THE DISCLOSURE
In one aspect, the present disclosure provides a compound of formula (I), or
an isomer,
pharmaceutically acceptable salt, solvate or prodrug thereof:
5
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R2a R2b R4a
0 R4b
0 R1
G1
HN R3b
R3a G2
0 R5a R5b G4¨G3
(I)
wherein:
G1, G2, G3 and G4 are each identical or different, and each is N or CR6,
provided that
no more than two of them are N;
IV is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl,
cycloalkyl,
heterocyclyl and heteroaryl, wherein the alkyl, cycloalkyl, heterocyclyl or
heteroaryl is
optionally substituted with one or more groups independently selected from the
group
consisting of halogen, hydroxy, cyano, alkyl, alkoxy, hydroxyalkyl, SO2R11a,
NRi laR1 lb,
C(=0)0R1 la, C(=0)NR1 laR1 lb, mic(=o)Rila NHC(=0)0R1la, cycloalkyl,
heterocyclyl, aryl
and heteroaryl;
R2a, R213, R3a and R31
are each identical or different, and each is independently selected
from the group consisting of hydrogen, halogen, alkyl, alkoxy, hydroxy,
haloalkyl, haloalkoxy,
hydroxyalkyl, cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl and
heteroaryl, wherein the
alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl is optionally substituted
with one or more
.. groups selected from the group consisting of halogen, alkyl, alkoxy,
hydroxy, hydroxyalkyl,
cyano, amino, nitro, NR12aR1213, C(=0)0R12a, C(=0)NR12aR1213, NHC(=o)R12a,
NHC(=0)0R12a, oR12a, cycloalkyl, heterocyclyl, aryl and heteroaryl;
or two of R2a, R213, R3a and R31 together with the carbon atom to which they
are attached
form cycloalkyl or heterocyclyl;
R4a, R413, R5a and x -.-s5b
are each identical or different, and each is independently selected
from the group consisting of hydrogen, deuterium, halogen, alkyl, alkoxy,
hydroxy, haloalkyl,
haloalkoxy, hydroxyalkyl, cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl
and heteroaryl,
wherein the alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl is optionally
substituted with one
or more groups selected from the group consisting of halogen, alkyl, alkoxy,
hydroxy,
hydroxyalkyl, cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl and
heteroaryl;
or two of R4a, R41, R5a and R5b together with the carbon atom to which they
are attached
form cycloalkyl or heterocyclyl;
6
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
each R6 is identical or different, and each is independently selected from the
group
consisting of hydrogen, halogen, alkyl, alkoxy, haloalkyl, haloalkoxy,
hydroxy, hydroxyalkyl,
cyano, amino, nitro, SO2R13a, SO2NR13aR1313, NR13aR1313, C(=0)0R13a,
C(=0)NR13aR1313,
NHC(=0)R13a, NHC(=0)0R13a, cycloalkyl, heterocyclyl, aryl and heteroaryl,
wherein the
alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl is optionally substituted
with one or more
groups selected from the group consisting of halogen, alkyl, alkoxy, hydroxy,
hydroxyalkyl,
cyano, amino, nitro, SO2R14a, SO2NR14aR1413, NR14aR1413, C(=0)0R14a,
C(=0)NR14aR1413,
NHC(=0)R14a, NHC(=0)0R14a, cycloalkyl, heterocyclyl, aryl and heteroaryl;
R11a, R12a, Rt3a, and R14 are each independently selected from the group
consisting of
hydrogen, alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein the
alkyl, cycloalkyl,
heterocyclyl, aryl or heteroaryl is optionally substituted with one or more
groups selected from
the group consisting of halogen, hydroxy, alkoxy, alkyl, aryl and cycloalkyl;
Rub, R1213, R131, R1413 are each independently selected from the group
consisting of
hydrogen and alkyl, wherein alkyl is optionally substituted with one or more
groups selected
.. from the group consisting of halogen, hydroxyl and alkoxy;
n is 1 or 2; and
m is 1 or 2.
In some embodiments of the disclosure, in the compound of formula (I), or a
tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt, solvate or prodrug thereof, G1, G2, G3 and G4 are each
identical or different,
and each is N or CR6, provided that no more than two of them are N;
R1 is selected from the group consisting of alkyl, haloalkyl, hydroxyalkyl,
cycloalkyl,
heterocyclyl and heteroaryl, wherein the alkyl, cycloalkyl, heterocyclyl or
heteroaryl is
optionally substituted with one or more groups independently selected from the
group
consisting of halogen, hydroxy, cyano, alkyl, alkoxy, hydroxyalkyl, SO2R11a,
NRi laR1 lb,
C(=0)0R1 la, C(=0)NRilaR1113, mic(=o)Rila NHC(=0)0R1 la, cycloalkyl,
heterocyclyl, aryl
and heteroaryl;
R2a, R213, R3a and R31
are each identical or different, and each is independently selected
from the group consisting of hydrogen, halogen, alkyl, alkoxy, hydroxy,
haloalkyl, haloalkoxy,
hydroxyalkyl, cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl and
heteroaryl, wherein the
alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl is optionally substituted
with one or more
groups selected from the group consisting of halogen, alkyl, alkoxy, hydroxy,
hydroxyalkyl,
cyano, amino, nitro, NR12aR1213, C(=0)0R12a, C(=0)NR12aR1213, NHC(=o)R12a,
NHC(=0)0R12a, cycloalkyl, heterocyclyl, aryl and heteroaryl;
7
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
or two of R2a, R21), R3a and R36 together with the carbon atom to which they
are attached
form cycloalkyl or heterocyclyl;
R4a, R413, R5a and x -.-s5b
are each identical or different, and each is independently selected
from the group consisting of hydrogen, halogen, alkyl, alkoxy, hydroxy,
haloalkyl, haloalkoxy,
hydroxyalkyl, cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl and
heteroaryl, wherein the
alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl is optionally substituted
with one or more
groups selected from the group consisting of halogen, alkyl, alkoxy, hydroxy,
hydroxyalkyl,
cyano, amino, nitro, cycloalkyl, heterocyclyl, aryl and heteroaryl;
or two of R4a, R413, R5a and R5b together with the carbon atom to which they
are attached
form cycloalkyl or heterocyclyl;
each R6 is identical or different, and each is independently selected from the
group
consisting of hydrogen, halogen, alkyl, alkoxy, haloalkyl, haloalkoxy,
hydroxy, hydroxyalkyl,
cyano, amino, nitro, SO2R13a, SO2NR13aR1313, NR13aR1313, C(=0)0R13a,
C(=0)NR13aR1313,
NHC(=0)R13a, NHC(=0)0R13a, cycloalkyl, heterocyclyl, aryl and heteroaryl,
wherein the
alkyl, cycloalkyl, heterocyclyl, aryl or heteroaryl is optionally substituted
with one or more
groups selected from the group consisting of halogen, alkyl, alkoxy, hydroxy,
hydroxyalkyl,
cyano, amino, nitro, SO2R14a, SO2NR14aR1413, NR14aR1413, C(=0)0R14a,
C(=0)NR14aR1413,
NHC(=0)R14a, NHC(=0)0R14a, cycloalkyl, heterocyclyl, aryl and heteroaryl;
Rua, R12a, Rt3a, and R14 are each independently selected from the group
consisting of
hydrogen, alkyl, cycloalkyl, heterocyclyl, aryl and heteroaryl, wherein the
alkyl, cycloalkyl,
heterocyclyl, aryl or heteroaryl is optionally substituted with one or more
groups selected from
the group consisting of halogen, hydroxy, alkoxy, alkyl and cycloalkyl;
Rub, R1213, R131, R1413 are each independently selected from the group
consisting of
hydrogen and alkyl, wherein alkyl is optionally substituted with one or more
groups selected
from the group consisting of halogen, hydroxyl and alkoxy;
n is 1 or 2; and
m is 1 or 2.
In some embodiments of the disclosure, the compound of formula (I), or a
tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt, solvate or prodrug thereof, is a compound of formula (II), or
a tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt, solvate or prodrug thereof:
8
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R2a R2b R4a
0 R4b
0 Ri
G1
HN R3b \\G2
R3a
0 R5a R5b G4--k_Tr'3
( II )
wherein Gl, G2, G3, G4, R1, R2a to R5a, R21 to R5b, n and m are each as
defined in formula
(I).
In some embodiments of the disclosure, in the compound of formula (I) or
formula (II),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof, Gl and G2 are
each independently
N or CR6; G3 and G4 are each CR6; and R6 is as defined in formula (I) .
In some embodiments of the disclosure, the compound of formula (I), or a
tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt, solvate or prodrug thereof, is a compound of formula (III) or
(Ma), or a
tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or
a
pharmaceutically acceptable salt, solvate or prodrug thereof:
R2a R2b R4a
0 R4b R2a R2b R4a
0 R1 0
0 R1 R4b
HN eio 6\
R3b ) )
HN , ,6r,NH R3a \
17 R3b (R.1sr,NH
R3a \
0 R5a R5b
0 R5a R5b
( III) ( )
wherein:
S iS 0, I or 2; and
R2a to R5a, R21 to R5b, R6, n and m are each as defined in formula (I) above.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III) or formula (Ma), or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, Rl is
selected from the group consisting of alkyl, cycloalkyl and heteroaryl,
wherein the alkyl,
cycloalkyl and heteroaryl are optionally substituted with one or more groups
selected from the
group consisting of alkyl and alkoxy; preferably Rl is selected from the group
consisting of
C1-6 alkyl, 3 to 8-member cycloalkyl and 5 to 10-member heteroaryl, wherein
the C1_6 alkyl, 3
9
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
to 8-member cycloalkyl and 5 to 10-member heteroaryl are optionally
substituted with one or
more, some times preferably one to three, groups independently selected from
the group
consisting of C1_6 alkyl and C1_6 alkoxy; more preferably, R1 is selected from
the group
_/
consisting of ¨17< N¨R
, -CH2OCH3,
Rlw R1w
N,0
N¨R1w N N I I Ns= \
and ;
and Rlw is C1-6
alkyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III) or formula (Ma), or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, R1 is alkyl,
cycloalkyl or heteroaryl, each optionally substituted by an alkyl or alkoxy;
preferably R1 is
cycloalkyl, sometimes more preferably cyclopropyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III) or formula (Ma), or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, R1 is
cycloalkyl or heteroaryl, each optionally substituted by alkyl; preferably R1
is cycloalkyl,
sometimes more preferably cyclopropyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(I),
formula (II), formula (III) or formula (Ina), or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof, R1 is heteroaryl, optionally substituted by alkyl; preferably R1 is
pyrazolyl, thiazolyl,
imidazolyl, pyridyl or pyrimidyl, each optionally substituted by C1_6 alkyl;
more preferably R1
is imidazolyl, optionally substituted by C1_6 alkyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(I),
formula (II), formula (III) or formula (Ina), or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof, R2a and R21 are each identical or different, and each is
independently selected from the
group consisting of hydrogen, halogen, alkyl, alkoxy, hydroxy, haloalkyl,
haloalkoxy,
hydroxyalkyl and cyano; preferably R2a and R21 are each hydrogen.
In some embodiments of the disclosure, the compound of formula (I) or formula
(III),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
pharmaceutically acceptable salt, solvate or prodrug thereof, is a compound of
formula (IV) or
(IVa), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof:
Rt.a R4a
0 R4b 0 R4b
0 0
HN R5a HN
)r-NH R31' R6 )rNH R3b R6
0 R5b 0 R5a R5b
R6 R6
(IV) 1Va )
wherein:
R4a, R5a, R31 to R513,
n and m are each as defined in formula (I).
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III) or formula (Ma), or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, R3a and
R31 are each independently selected from the group consisting of hydrogen,
halogen, alkyl,
alkoxy and hydroxyalkyl, wherein the alkyl is optionally substituted with one
or more,
sometimes preferably one to three, groups independently selected from the
group consisting of
halogen, OR12a and alkoxy.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III) or formula (Ma), or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, R3a and
R31 are each independently selected from the group consisting of hydrogen,
halogen, alkyl,
alkoxy and hydroxyalkyl, wherein the alkyl is optionally substituted with one
or more,
sometimes preferably one to three, groups independently selected from the
group consisting of
halogen and alkoxy.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III) or formula (Ma), or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, R3a and
R31 are identical or different, and each is selected from the group consisting
of hydrogen,
halogen, C1_6 alkyl, C1_6 alkoxy and C1_6 hydroxyalkyl, wherein the C1_6 alkyl
is optionally
substituted with one or more, sometimes preferably one to three, groups
independently selected
from the group consisting of halogen and C1_6 alkoxy.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV) or formula (IVa), or a tautomer,
mesomer, racemate,
11
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof, R4a, R4b, R5a and R5b are each dentical or different, and
each is independently
selected from the group consisting of hydrogen, deuterium, halogen and C1_6
alkyl; or R5 and
R5b together with the carbon atom to which they are attached form 3 to 8-
member cycloalkyl,
R4a. and R41 are each dentical or different, and each is independently
selected from the group
consisting of hydrogen, deuterium, halogen and C1_6 alkyl; or R4a and R41
together with the
carbon atom to which they are attached form 3 to 8-member cycloalkyl, R5a and
R5b are dentical
or different, and each is independently selected from the group consisting of
hydrogen,
deuterium, halogen and C1_6 alkyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV) or formula (IVa), or a tautomer,
mesomer, racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof, R4a, R4b, R5a and R5b are each identical or different, and
each is independently
selected from the group consisting of hydrogen, deuterium, halogen, alkyl,
alkoxy, hydroxy,
haloalkyl, haloalkoxy, hydroxyalkyl and cyano; preferably R4a, R41, R5a and
R5b are each
independently selected from the group consisting of hydrogen, deuterium,
halogen and alkyl;
and sometimes more preferably R4a, 1('-µ413, R5" and R5b are each
independently selected from the
group consisting of hydrogen, halogen and alkyl.
In some embodiments of the disclosure, in the compound of formula (I) or
formula (II),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
R4a
Rab
).1\T
GI
/1
/
'
pharmaceutically acceptable salt, solvate or prodrug thereof, R5 R5b G4 ¨G3 is
selected from
4_
RR45a b R4b
RI
R4b
"555'4'N µ \ (R6)s
µ (R6) 4
R6a
the group consisting of R6Rb4a¨/ , 9 ,
12
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R4a
R4a Raa
(R6),
:a / __ (/ R 6)s and :a4/3 (R6)s ; sometimes preferably :a N or
R4a
*(N
(R6)s
=
s is 0,1 or 2; and
R4a, R413, R5a, R513, R6
and s are as defined in formula (I).
In some embodiments of the disclosure, in the compound of formula (I) or
formula (II),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
R4a
Rab
G1
\\G2
11
pharmaceutically acceptable salt, solvate or prodrug thereof, R5a R5b G4¨G3 is
selected from
R4a R4a
)(N
the group consisting of R5a R5a
9 9
R4a
R4a R4a
N (R6)s
N
and R5a , and preferably
R5a or
R4a
(R6)s
R5a =
s is 0,1 or 2; and
K-4a,
R5a and R6 are as defined in formula (I) above.
In some embodiments of the disclosure, in the compound of formula (I) or
formula (II),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
13
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R4a
R4b
kO
N
at6)s
pharmaceutically acceptable salt, solvate or prodrug thereof, R5a R5b is
selected
Rita R4a
N N
(R6)s (R6)s
(R6),
from the group consisting of R5a R5a and =
9
S is 0,1 or 2; and
¨4a,
R5a and R6 are as defined in formula (I) , above.
In some embodiments of the disclosure, in the compound of formula (I) or
formula (II),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
R46
ittb
,55s.-1\T
R6
R5a R5b
pharmaceutically acceptable salt, solvate or prodrug thereof, R6 is
selected
R4a R4a
.cs!
R6 R6 V**-N R6
R5a R5a
from the group consisting of R6 R6 , and R6 ;
s is 0,1 or 2; and
K-4a,
R5a and R6 are as defined in formula (I) above.
In some embodiments of the disclosure, the compound of formula (I), formula
(III),
formula (IV), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, is a
compound of formula
(V), (Va) or (Vb), or a tautomer, mesomer, racemate, enantiomer, diastereomer,
or mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof:
R4a
0 Ri 0 R4a
Ri 0
HN
)rNH R3b 40 R6 FIN)r-NH R3b R6
R5a R5a
0 0
R6 R6
( V ) ( Va )
14
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R4a
0 R1 0
HN
R3b R6
II
R5a
0
R6
( Vb )
wherein:
Rl is selected from the group consisting of cycloalkyl and heteroaryl, wherein
the
cycloalkyl or heteroaryl is optionally substituted with one or more groups
independently
selected from the group consisting of halogen, hydroxy, cyano, alkyl, alkoxy
and hydroxyalkyl;
and
R31), K-rs4a,
R5a and R6 are each as defined in formula ( I) above.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV), formula (IVa), formula (V),
formula (Va) or formula
(Vb), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt thereof, solvate or prodrug thereof, R6 is
identical or different,
and at each occurrence is independently selected from the group consisting of
hydrogen,
halogen, alkyl, alkoxy, haloalkyl, haloalkoxy, cyano and C(=0)0R13a; R13a is
as defined in
formula (I); preferably, R6 is identical or different, and at each occurrence
is independently
selected from the group consisting of hydrogen, halogen, C1_6 alkyl, C1_6
alkoxy, C16 haloalkyl,
C1_6 haloalkoxy, cyano and C(=0)0R13a; and R13 is hydrogen or C1_6 alkyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV), formula (IVa), formula (V),
formula (Va) or formula
(Vb), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt thereof, solvate or prodrug thereof, R6 at
each occurrence is
independently selected from the group consisting of hydrogen, halogen, alkyl,
alkoxy,
haloalkyl, hydroxyalkyl, amino, nitro, C(=0)oR13a, NR14aR1413, haloalkoxy and
cyano;
sometimes preferably R6 at each occurrence is independently selected from the
group
consisting of hydrogen, halogen, alkyl, alkoxy, haloalkyl, haloalkoxy and
cyano.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV), formula (IVa), formula (V),
formula (Va) or formula
(Vb), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt thereof, solvate or prodrug thereof, R11a,
R12a, R13, and R14a
are each independently selected from the group consisting of hydrogen and
alkyl, wherein the
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
alkyl is optionally substituted with one or more, sometimes preferably one to
three, groups
independently selected from the group consisting of halogen, hydroxy, alkoxy
and aryl.
In some embodiments of the disclosure, in the compound of formula (I), or a
tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt thereof, solvate or prodrug thereof, R 11b, R1213, R131, R14b
are each independently
selected from the group consisting of hydrogen and alkyl.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV) or formula (IVa), or a tautomer,
mesomer, racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt thereof,
solvate or prodrug thereof, n is 1.
In some embodiments of the disclosure, in the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV) or formula (IVa), or a tautomer,
mesomer, racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt thereof,
solvate or prodrug thereof, m is 1.
Exemplified compounds of the disclosure include, but are not limited to:
Example
Compound structure and name
No.
(:) V 0
HN
101 1 1 CF
0
(S)-5 -c ycloprop yl- 5 -(3 -oxo-3 -(5 -(trifluoromethyl) iso indolin-2 -
yl)prop yl) imidazolidine-2 ,4-dione
0 0
IHNNEd
2
0 2 C I
(S)-5 -(3 -(5 -chloroiso indolin-2 - y1)- 3 -oxoprop y1)-5 -cycloprop
ylimidazolidine-
2,4-dione
0 0
3
0 3 411 CN
16
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-2-(3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-yl)propanoyl)isoindoline-5-
carbonitrile
0 o
HN NH N
4 )T 4 AP
0 00F3
(S)-5 -cyclopropy1-5-(3-oxo-3-(5-(trifluoromethoxy)isoindolin-2-
yl)propyflimidazolidine-2,4-dione
0 V
eNH
5
0
(S)-5 -cyclopropy1-5-(3-(7-methoxy-4,5-dihydro-1H-benzo [di azepin-3(2H)-
y1)-3-oxopropyflimidazolidine-2,4-dione
0
HN)LT441
CF3
6 NH 6
0
(S)-5 -cyclopropy1-5-(3-oxo-3-(6-(trifluoromethyl)-3,4-dihydroisoquinolin-
2(1H)-yl)propyl)imidazolidine-2,4-dione
0
)4fr)LN
101 CF3
HN
NH
7 0 7
(S)-5 -cyclopropy1-5-(3-oxo-3-(7-(trifluoromethyl)-3,4-dihydroisoquinolin-
2(111)-yl)propyflimidazolidine-2,4-dione
0
HN )LT)LN
=
8 0 8
CI
(5 S)- 5 -(3-(8-chloro-l-methy1-4,5-dihydro-1H-benzo [di azepin-3(2H)-y1)-3-
oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
17
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
V 0
OL
9
0 9
(S)-5 -cyclopropy1-5-(3-(4,5-dihydro-1H-benzo [d] azepin-3(2H)-y1)-3-
oxopropyl)imidazolidine-2,4-dione
0 V
H N
)NH C H F2
0 10
(S)-5 -cyclopropy1-5-(3-(5-(difluoromethoxy)isoindolin-2-y1)-3-
oxopropyflimidazolidine-2,4-dione
0 V
H N
11 NH
=
0 1 1
(S)-5 -cyclopropy1-5-(3-(isoindolin-2-y1)-3-oxopropyl)imidazolidine-2,4-dione
%M
HN11---r
NH \
12 0 12
(S)-5 -cycloprop y1-5-(3-oxo-3-(5H-pyrrolo13,4-blpyridin-6(7H)-
yl)propyl)imidazolidine-2,4-dione
HNI Ng,
13 .NH N
¨/
0 13
(S)-5 -cyclopropy1-5-(3-oxo-3-(1H-pyrrolo13,4-clpyridin-2(3 H) -
yl)propyl)imidazolidine-2,4-dione
0 V
f " 14 HN; N
NH
0 14
18
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -cyclopropy1-5 -(3 -((R)- 1-methylisoindolin-2 -y1)-3 -
oxopropyl)imidazolidine-2,4-dione
0 V
15 y NH
0 15
(S)-5 -cycloprop y1-5 -(3 -((S)- 1-methylisoindolin-2 -y1)-3 -
oxopropyflimidazolidine-2,4-dione
0 V
=)7,-= NH
16 0 16
(S)-5 -cycloprop y1-5 -(3 -(4-fluoroisoindolin-2-y1)-3 -
oxopropyflimidazolidine-
2,4-dione
O V
HNN
= )r-NH F
17 0 17
(S)-5 -cycloprop y1-5 -(3 -(5 -fluoroisoindolin-2-y1)-3 -
oxopropyflimidazolidine-
2,4-dione
V 0
HN),r-NH
18 0 18
(5S)-5-cyclopropy1-5 -(3 -oxo-3 -(1,2,3,4 -tetrahydro-1,4 -epiminonaphthalen-9-
yl)propyl)imidazolidine-2 ,4-dione
O V
HN()LN
)r, NH F
19 0 19
(S)-5 -cyclopropy1-5 -(3-(5 ,6-difluoroisoindolin-2-y1)-3 -
oxopropyflimidazolidine-2,4-dione
19
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
V 0
HNC"--21"
NH 41 CI
20 0 20
CI
(S)-5 -cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione
(:) V
H NH
CI
21 0 21
(S)-5 -(3-((R)-8-chloro-1-methy1-4,5-dihydro-1H-benzokllazepin-3(2H)-y1)-3-
oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
)r-NH
22 HF
0 22
(5 S)-5 -cyclopropy1-5-(3-(8-fluoro-l-methy1-4,5-dihydro-1H-benzo [d] azepin-
3(2H)-y1)-3-oxopropyl)imidazolidine-2,4-dione (Mixture of two
diastereomers)
CI
)r-
23
23 NH0
(S)-5 -(3-(7-chloro-4,5-dihydro-1H-benzokllazepin-3(2H)-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
(:) VHN
0
OMe
24 0 24
(S)-5 -cyclopropy1-5-(3-(5-methoxyisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione
OMe
25 HN)rNH
0 25
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -cycloprop y1-5 -(3 -(6-methoxy-4,5 -dihydro-1H-benzo [d] azepin-3 (211)-
y1)-3 -o xoprop yflimidazolidine-2 ,4 -dione
0
HHN
26 P 26
(S)-5 -c ycloprop y1-5 -(3 -(1,1 -dimethy1-4 ,5 -dihydro- 1H-benzoldlazepin-3
(211)-
y1)-3 -o xoprop yflimidazolidine-2 ,4 -dione
0
HN)r-NH
27 27
0
(S)-5-c ycloprop y1-5 -(3 -((R)-8-fluoro- 1 -methyl-1,2,4,5 -tetrahydro-3 H-
benzo [di azepin-3 - y1)-3 -o xoprop yflimidazolidine-2 ,4 -dione
0 V
HN"
)r-NH
0 28
28
(S)-5-c ycloprop y1-5 -(3 -((S)-8 -fluoro-1 -methyl-1,2 ,4,5 -tetrahydro-3 H-
benzo [di azepin-3 - y1)-3 -o xoprop yflimidazolidine-2 ,4 -dione
0 V 0
29 )(NH C F3
0 29
(5S)-5 -c ycloprop y1-5 -(2-methyl-3-oxo-3-(5 -(trifluoromethyl)isoindolin-2-
yl)propyllimidazolidine-2,4-dione
0
o
29-1
HNI \NH
CF3
0
29-1
(S)-5 -cyclopropy1-54(S)-2-methyl-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
y1)propyl)imidazolidine-2,4-dione
21
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
V 0
N
HNNH
29-2 Nz CF
0
29-2
(S)-5 -cycloprop y1-5 -((R)-2-methyl-3 -oxo-3 -(5-(trifluoromethyl)isoindolin-
2-
yl)propyl)imidazolidine-2 ,4-dione
HNOn)CL)
)i-NH =CF3
30 30
0
(5 R)-5-cyclopropy1-5-(2-methy1-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione
0
z N
HN rNH
4C F3
30-1 0
30-1
(R)-5 -cyclopropy1-5 -((R)-2-methyl-3 -oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2 ,4-dione
0)
HN yNH
30-2 0 CF3
30-2
(R)-5-cyclopropy1-54(S)-2-methyl-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione
HN)i-NHri CF3
31 0
5-cyclopropy1-5-(2-(5-(trifluoromethyflisoindoline-2-
carbonyl)butyl)imidazolidine-2,4-dione
22
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Or*L)
HN)(NH = cF3
31-1 0
31-1
(5R)-5 -cyclopropy1-5-(2-(5-(trifluoromethyl)isoindoline-2-
carbonyl)butyl)imidazolidine-2,4-dione
V
HN
yeNH cF3
31-2 0
31-2
(5 S)-5-cyclopropy1-5-(2-(5-(trifluoromethyl)isoindoline-2-
carbonyl)butyl)imidazolidine-2,4-dione
0 V 0
)r-NH
CF3
32 0
32
(S)-5-cyclopropy1-54(S)-2-(5-(trifluoromethyl)isoindoline-2-
carbonyl)butyl)imidazolidine-2,4-dione
0 VH~I 0
NH 110,
CF3
33 0
33
(S)-5-cyclopropy1-54(R)-2-(5-(trifluoromethyflisoindoline-2-
carbonyl)butyl)imidazolidine-2,4-dione
OH L
HN)rNH = CI
34 0
CI
5-cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1)-2-methyl-3-
oxopropyl)imidazolidine-2,4-dione
23
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
040 JL
(R)
HN),rNH
CI
34-1 0
34-1 CI
(5R)-5 -cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1)-2-methyl-3-
oxopropyl)imidazolidine-2,4-dione
HNs)
yNH
CI
34-2 0
34-2 CI
(SS)-5 -cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1)-2-methyl-3-
oxopropyl)imidazolidine-2,4-dione
0 V 0
)r-NH CI
34-2-A 0
34-2-A CI
(SS)-5 -cyclopropy1-5-((S)-(3-(5,6-dichloroisoindolin-2-y1)-2-methy1-3-
oxopropy1))imidazolidine-2,4-dione
H N
34-2-B 0
34-2-B CI
(SS)-5 -cyclopropy1-5-((R)-(3-(5,6-dichloroisoindolin-2-y1)-2-methy1-3-
oxopropy1))imidazolidine-2,4-dione
CI elHrTs)
NH OMe
35 0
(S)-5-(3-(5-chloro-6-methoxyisoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
24
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
36 HN
0,W
yNH OMe
0
CI
-(3 -(5-chloro-6-methoxyisoindolin-2- y1)-2-methy1-3 -o xoprop y1)-5 -
c ycloprop ylimidazolidine-2 ,4-dione
0 0
si r CI \)(N
(s)
HN)i-NH OM e
36-1 0 36-1
(5 S)-5 -(3 -(5-chloro-6-methoxyisoindolin-2- y1)-2-methy1-3 -o xoprop y1)-5 -
c ycloprop ylimidazolidine-2 ,4-dione
HN)7,-NH CI
37 0 37 CI
(S)-5 -c ycloprop y1-5 -(3 -(7,8-dichloro-1 -methyl-1,2,4,5 -tetrahydro-3H-
benzo [di azepin-3 - y1)-3 -o xoprop yl)imidazolidine-2 ,4-dione
HN)r-NH CI
37-1 CI
37-1
(S)-5 -cycloprop y1-5 -(3 -((S)-7,8-dichloro-1 -methyl- 1,2 ,4,5 -tetrahydro-3
H -
benzo [di azepin-3 - y1)-3 -o xoprop yl)imidazolidine-2 ,4-dione
0 V 9
(\2N
HN CI
37-2 CI
37-2
(S)-5 -c ycloprop y1-5 -(3 -((R)-7,8 -dichloro-l-methyl-1,2,4,5 -tetrahydro-3
H-
benzo [di azepin-3 - y1)-3 -o xoprop yl)imidazolidine-2 ,4-dione
38 HNN CI
CI
0 38
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -c ycloprop y1-5 -(3 -(8,9-dichloro-1 -methyl-1,2,4,5 -tetrahydro-3H-
benzo [di azepin-3 - y1)-3 -o xoprop yflimidazolidine-2 ,4-dione
CI
HNNH CI
38-1 0 38-1
(S)-5 -cycloprop y1-5 -(3 -((S)-8,9-dichloro-1 -methyl- 1,2 ,4,5 -tetrahydro-3
H -
benzo [di azepin-3 - y1)-3 -o xoprop yflimidazolidine-2 ,4-dione
HN),i-NH CI
38-2 0 38-2
(S)-5 -c ycloprop y1-5 -(3 -((R)-8,9-dichloro-l-methy1-1,2,4,5 -tetrahydro-3 H-
benzo [di azepin-3 - y1)-3 -o xoprop yflimidazolidine-2 ,4-dione
(:) V
HN)r-NH
CF3
39 0 39
(S)-5 -c ycloprop y1-5 -(3 -(5-fluoro-6-(trifluoromethyflisoindolin-2- y1)-3 -
oxoprop yl)imidazolidine-2,4-dione
HN)r-NH CF3
40 0 40
CI
(S)-5 -(3 -(5 -chloro-6-(trifluoromethyflisoindolin-2-y1)-3 -oxoprop y1)-5 -
c ycloprop ylimidazolidine-2 ,4-dione
r)Lo
(:)
HNNH CF3
41 0 41
CI
(SS)-5 -(3 -(5 -chloro-l-methy1-6-(trifluoromethyflisoindolin-2- y1)-3 -
o xoprop y1)-5 -cycloprop ylimidazolidine-2,4-dione
26
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
V
HN
CI
0
41-1 C F3
41-1
(S)-5 -(3 - ((S)- 5 -chloro-1 -methyl-6-(trifluoromethyl)isoindolin-2- y1)-3 -
o xoprop y1)-5 -cycloprop ylimidazolidine-2,4-dione
V
HN
y NH CI
41-2 0
CF3
41-2
(S)-5 -(3 -((R)-5 -chloro-1 -methyl-6-(trifluoromethyl)isoindolin-2- y1)-3 -
o xoprop y1)-5 -cycloprop ylimidazolidine-2,4-dione
HN' 4.el\JH
OH CI
0
42 42 CI
-cycloprop y1-5 -(3 -(5 ,6-dichloroisoindolin-2- y1)-2-(hydroxymethyl)-3 -
oxopropyl)imidazolidine-2,4-dione
NH 0
HN
0 /s
N
43
43
CF3
5 -(3 -o xo-3-(5 -(trifluoromethyl)isoindolin-2- yl)prop y1)-5 -(thiazol-2 -
yl)imidazolidine-2,4-dione
0 0
44
HNLN
0
44
27
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -cyclopropy1-5-(3-(7-fluoro-4,5-dihydro-1H-benzo [di azepin-3(2H)-y1)-3-
oxopropyl)imidazolidine-2,4-dione
V 0
CF3
NH
45 0 45
(S)-5 -cyclopropy1-5-(3-oxo-3-(7-(trifluoromethyl)-4,5-dihydro-1 H-
benzokllazepin-3(2H)-yl)propyl)imidazolidine-2,4-dione
D D
HNIThs) N CI
?r-NH
46 CF3
0
46
(S)-5-(3-(5-chloro-6-(trifluoromethypisoindolin-2-y1-1,1,3,3-d4)-3-
oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
N CI
0
47 DI
47
(S)-5-cyclopropy1-5-(3-(5',6'-dichlorospiro1cyclopropane-1,1'-isoindolin1-2'-
y1)-3-oxopropyl)imidazolidine-2,4-dione
JN
0
HNNH CI
48 48
CF3
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(pyridin-3-
yl)imidazolidine-2,4-dione
0
0
49 HNLN )r-NH CF3
0
28
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
5-cyclopropy1-5-(3-methy1-2-(5-(trifluoromethyflisoindoline-2-
carbonyflbutyflimidazolidine-2,4-dione
HN1
yNH CF3
49-1 0
49-1
(5R)-5-cyclopropy1-5-(3-methyl-2-(5-(trifluoromethyflisoindoline-2-
carbonyflbutyflimidazolidine-2,4-dione
0 V
HN(33L
yNH CF3
49-2 0
49-2
(5S)-5-cyclopropy1-5-(3-methy1-2-(5-(trifluoromethyflisoindoline-2-
carbonyflbutyflimidazolidine-2,4-dione
7 0
HN NH =
= CF
49-2a 0
49-2a
(5S)-5-cyclopropy1-5-R2R)-3-methy1-2-[5-(trifluoromethyl)-1, 3, 3a, 7a-
tetrahydroisoindole-2-carbonyl] butyl] imidazolidine-2, 4-dione
o, 7
HN NXH
441 CF3
0
49-2b
49-2b
(5R)-5-cyclopropy1-5-R2S)-3-methy1-2-[5-(trifluoromethyl)-1, 3, 3a, 7a-
tetrahydroisoindole-2-carbonyl] butyl] imidazolidine-2, 4-dione
0
(s)
50 HN)r_NH 40 50 NO2
0
CI
29
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5-(3-(5-chloro-6-nitroisoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
V
51
HN1 HN OH
)r-N51
0
(5 S)- 5 -cyclopropy1-5-(3-(5-(1-hydroxyethyDisoindolin-2-y1)-3-
oxopropyflimidazolidine-2,4-dione
(:) V
HN
)r-NH cHF2
52 0 52
(S)-5-cyclopropy1-5-(3-(5-(difluoromethyflisoindolin-2-y1)-3-
oxopropyflimidazolidine-2,4-dione
0 V 0
HNyNH 41 I
53 0
53 CI
(S)-5-(3 -(5-chloro-6-iodoisoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
HNnP)
NH CI
54 0
54 NH2
(S)-5-(3-(5-amino-6-chloroisoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
JV
0\\
HNRs)
y NH = NH
55 0
55 CI
(S)-N-(6-chloro-2-(3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-
yl)propanoyl)isoindolin-5-yl)acetamide
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 V
56
HN1 HN 0
y. N OH
0 56
(S)-2-(3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-yl)propanoyl)isoindoline-5-
carboxylic acid
V 0
HN L(s) 1010I
)r NH CH3
57 0
57
(S)-5 -(3-(5-chloro-6-methylisoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
0 V
)(NH CI
58 0 58 CI
(SS)-5 -cyclopropy1-5-(3-(5,6-dichloro-1-ethylisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione
0 V
)r, NH
CF3
59 0 59
Br
(S)-5 -(3-(5-bromo-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
0
HN--1NH 0
0
60 /
60 40
L.,E3
5-(3-oxo-3-(5-(trifluoromethyflisoindolin-2-yflpropy1)-5-(pyridin-2-
y1)imidazolidine-2,4-dione
31
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
o
61
HNy NH
CI
0 61
CI
-(3 -(5 ,6-dichloroisoindolin-2- y1)-3 -o xoprop y1)-5 -(pyridin-2-
yl)imidazolidine-2,4-dione
0
HN'ANH 0
0
N
-
62
62 C F3
5 -(1-methyl- 1H-imidazol-2- y1)-5-(3 -oxo-3 -(5-(trifluoromethyflisoindolin-2
-
yflpropyflimidazolidine-2,4-dione
0
HN-1(0
0.1 \NI
N
62-1
C F3
62-1
(S)-5 -(1 -methyl- 1H-imidazol-2- y1)-5 -(3-o xo-3 -(5 -
(trifluoromethyl)isoindolin-
2 - yl)prop yl)imidazolidine-2 ,4-dione
0
HN-1( 0
0 .4
N%\
62-2 __Ls2 ¨
C F3
62-2
(R)-5-(1 -methyl- 1H-imidazol-2 - y1)-5 -(3 -o xo-3-(5 -
(trifluoromethyl)isoindolin-
2 - yl)prop yl)imidazolidine-2 ,4-dione
32
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 0
HN 's
63 yNH CF3
0 63
-(methoxymethyl)-5 -(3 -oxo-3 -(5 - (triflu oromethyl)iso indolin-2 -
yl)propyl)imidazolidine-2 ,4-dione
0 0
0 /
H
63-1 N)rNH afr CF
0
63-1
(S)-5 -(methoxymethyl)-5 -(3 -oxo-3 -(5 -(trifluoromethyl) isoindolin-2-
yl)prop yl) imidazolidine-2 ,4-dione
C) 0 0
63-2
HN)r. NH afr C F3
0
63-2
(R)-5 - (metho xymethyl)-5 -(3 -oxo-3 -(5- (trifluoromethyl) isoindo lin-2-
yl)prop yl) imidazolidine-2 ,4-dione
jCL
H Ny NH CF3
64 0 64
5 -(1 -methylc yc loprop y1)-5 -(3 -oxo-3 - (5- (trifluoromethyl) isoindolin-2-
yl)prop yl) imidazolidine-2 ,4-dione
Od%
HN/
y NH = 64-1 0 C F3
64-1
(R)-5-(1-methylcycloprop y1)-5 - (3-o xo -3 -(5 -(triflu oro methyl) isoindo
lin-2-
yl)prop yl) imidazolidine-2 ,4-dione
33
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0
HN
CF3
64-2 'NH0
64-2
(S)-5-(1-methylcyclopropy1)-5-(3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione
OZ)Ct
HNIN ' 141 )r 441 0 CF3
0
65
5-(2-((benzyloxy)methyl)-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-yl)propy1)-
5-cyclopropylimidazolidine-2,4-dione
O
HNLN
),r NH CI
66 0
66 CI
(SS)-5 -cyclopropy1-5-(3-(5,6-dichloro-1-methylisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione
O V
HNL
yNH CI
0
66-1 CI
66-1
(S)-5 -cyclopropy1-5-(3 - ((S) -5 ,6 - dichlo ro -1-methylisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione
O V
CI
0
66-2 CI
66-2
(S)-5 -cyclopropy1-5-(3 - ((R) -5 ,6- dichlo r o-l-methylisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione
34
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0
N
CI
67 0 67
(5S)-5 -(3 -(8-chloro-9-fluoro-1 -methyl- 1 ,2,4,5-tetrahydro-3H-benzo
kflazepin-
3 - y1)-3 -oxoprop y1)-5 -c ycloprop ylimidazolidine-2 ,4-dione
V 0
=s- F
HN),rNH CI
67-i 67-1
0
(S)-5-(3 -((R)-8-chloro-9-fluoro-1 -methyl- 1 ,2,4,5 -tetrahydro-3H-
benzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -c ycloprop ylimidazolidine-2 ,4-
dione
O V
HNH") CI
yNH
67 -2 0 67-2
(S)-5 -(3 -((S)-8-chloro-9-fluoro-1 -methyl- 1 ,2,4,5-tetrahydro-3H-
benzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -c ycloprop ylimidazolidine-2 ,4-
dione
HN)r_NH CI
68 0 68
(5S)-5 -(3 -(8-chloro-7 -fluoro-1 -methyl- 1 ,2,4,5-tetrahydro-3H-
benzoldlazepin-
3 - y1)-3 -oxoprop y1)-5 -c ycloprop ylimidazolidine-2 ,4-dione
O
68-1
HNHHLN
CI
y-N0 68-1
(S)-5 -(3 -((S)-8-chloro-7 -fluoro-1 -methyl- 1 ,2,4,5-tetrahydro-3H-
benzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -c ycloprop ylimidazolidine-2 ,4-
dione
O V
68-2 HNHHLN
CI
y-N
0 68-2
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5-(3 -((R)-8-chloro-7-fluoro-1-methy1-1 ,2,4,5 -tetrahydro-3H-
benzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -c ycloprop ylimidazolidine-2 ,4-
dione
O 0
ci
69
HN (s) N
CI
D D
0
69
(5S)-5 -cycloprop y1-5 -(3 -(5,6-dichloro-l-methylisoindolin-2-y1-3 ,3 -d2)-3-
oxoprop yflimidazolidine-2,4-dione
O V 0 D D
HN (s) N
)i-NH
69-1 0
CI
69-1
(S)-5 -cycloprop y1-5 -(3 -((S)-5 ,6-dichloro-1 -methylisoindolin-2- y1-3 ,3 -
d2)-3 -
oxoprop yflimidazolidine-2,4-dione
D D
(s)
HNy-NH
(R) CI
69-2 0
CI
69-2
(S)-5 -c ycloprop y1-5 -(3 -((R)-5 ,6-dichloro-l-methylisoindolin-2- y1-3 ,3 -
d2)-3 -
oxoprop yflimidazolidine-2,4-dione
O V 0
HN's---r# )L
),rNH CI
70 0
70 CI
(5S)-5 -cycloprop y1-5 -(3 -(5 ,6-dichloro-l-methylisoindolin-2- y1)-2-methy1-
3 -
oxoprop yl)imidazolidine-2,4-dione
0 0
cF3
71
(s) N
HN),r-NH CI
D D
0
71
(SS)-5 -(3 -(5 -chloro-l-methy1-6-(trifluoromethyflisoindolin-2- y1-3 ,3 -d2)-
3 -
o xoprop y1)-5 -cycloprop ylimidazolidine-2,4-dione
36
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
HN/
irNH CI
H3C
71-1 0
CF3
71-1
(S)-5-(3 -((R)-5-chloro-l-methy1-6-(trifluoromethyflisoindolin-2- y1-3 ,3-d2)-
3 -
o xoprop y1)-5 -cycloprop ylimidazolidine-2,4-dione
00
)LV
HN/
NH H3C" CI
71-2 0
CF3
71-2
(S)-5 -(3 -((S)-5 -chloro-l-methy1-6-(trifluoromethypisoindolin-2- y1-3 ,3-d2)-
3 -
o xoprop y1)-5 -cycloprop ylimidazolidine-2,4-dione
HN()L
72 0 72 CF3
(S)-5 -cycloprop y1-5 -(3 -o xo-3-(5 -(2,2,2-trifluoroethyl)isoindolin-2-
yl)prop yl)imidazolidine-2 ,4-dione
/=\
N N 0
HN)rNH CF3
73 0
73 CI
-(3 -(5-chloro-6-(trifluoromethyl)isoindolin-2- y1)-3 -oxoprop y1)-5 -(1-
methyl-
1H-imidazol-2- yflimidazolidine-2 ,4-dione
N 0
0 N,
HN),r-NH CF3
73 -1
0 73-1 CI
(S)-5 -(3 -(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3 -oxoprop y1)-5 -(1-
methy1-1H-imidazol-2- yflimidazolidine-2,4-dione
37
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
/=\
1\1 0
0
H
73-2 N)7,-NH CF3
0 73-2 CI
(R)-5 -(3 -(5 -chloro-6-(trifluoromethyl)isoindolin-2- y1)-3 -o xoprop y1)-5 -
(1-
methy1-1H-imidazol-2- yl)imidazolidine-2,4-dione
(:) V
HNrjL
)(NH CHF2
74 0 74
(S)-5 -cycloprop y1-5 -(3 -(5 -(difluoromethyl)-6-fluoroisoindolin-2- y1)-3 -
oxoprop yl)imidazolidine-2,4-dione
tr)
/
,N 0
HN),rNH
75 ci
0
CF3
5 -(3 -(5 -chloro-6-(trifluoromethyl)isoindolin-2- y1)-3 -oxoprop y1)-5 -(5 -
methyliso xazol-3 - yl)imidazolidine-2,4-dione
13 V 0
HN)r NH
CF3
76 0
76 NH
(S)-5-c ycloprop y1-5 -(3 -(5 -(methylamino)-6-(trifluoromethyl)isoindolin-2-
y1)-
3 -o xoprop yl)imidazolidine-2,4-dione
HN
NH
CF3
77 0
77 CI
5 -(3 -(5-chloro-6-(trifluoromethyl)isoindolin-2- y1)-3 -oxoprop y1)-5 -(1-
methyl-
1H-p yrazol-3 - yl)imidazolidine-2 ,4-dione
38
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Hd 0
HN 110 CF3
78
78 CI
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(5-methyl-
1H-pyrazol-3-yl)imidazolidine-2,4-dione
N 1\1 0
HN)r-NH CF3
79
0 79 CI
5-(3-(5-chloro-6-(trifluoromethypisoindolin-2-y1)-3-oxopropy1)-5-(pyrimidin-
2-yflimidazolidine-2,4-dione
HNyNH
0
CI
80 CI
(S)-5-cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1-1,1,3,3-d4)-3-
oxopropyl)imidazolidine-2,4-dione
II
HNyNH CF3
81
81 CI
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(2-
methyloxazol-4-yflimidazolidine-2,4-dione
82 NH
CF3
0 82
CI
39
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(1-ethyl-
1H-imidazol-2-yl)imidazolidine-2,4-dione
N S 0
0
1-1Ny NH CF3
83
CI
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(thiazol-2-
yl)imidazolidine-2,4-dione
N S 0
0,1,µõ),
N
HN)r_ NH CF3
83-1 0
83-1 CI
(S)-5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
(thiazol-2-yl)imidazolidine-2,4-dione
N S 0
0 Nr
R
HN)r_ NH CF3
83-2
83-2 CI
(R)-5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
(thiazol-2-yl)imidazolidine-2,4-dione
0 0
HN
84 )7,-NH CF
84 CI
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(pyridin-2-
yl)imidazolidine-2,4-dione
I
4N o
o
HN)rNH = CF3
84-1
84-1 CI
(R)-5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
(pyridin-2-yl)imidazolidine-2,4-dione
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 0
HN>rNH cF,
84-2 84-2 CI
(S)-5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
(pyridin-2-yflimidazolidine-2,4-dione
0
H
85 N),[-NH 40 a
cF,
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(pyrazin-2-
yl)imidazolidine-2,4-dione
4N 0
0
==µµ\)N
HN)(NH =CI
85-1
85-1 cF,
(R)-5-(3-(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-3-oxopropy1)-5-
(pyrazin-2-yl)imidazolidine-2,4-dione
0 0
HNN
y-NH
85-2 CI
85-2 CF3
(S)-5-(3-(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-3-oxopropy1)-5-
(pyrazin-2-yl)imidazolidine-2,4-dione
%._\/
HNI-T N
NH ClOH
0
86-1 cF,
86-1
(S)-54(S)-3-(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-2-(hydroxymethyl)-
3-oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
41
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 V 0
N
NH
OH CI
86-2 HN
86-2 CF3
(S)-5-((R)-3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-2-(hydroxymethyl)-
3-oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
HN = y NH
OH CI
86-3 CF3
86-3
(R)-54(S)-3-(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-2-
(hydroxymethyl)-3-oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
s
HN1 1
)r¨NH N
OH CI
86-4 86-4 CF3
(R)-5 -((R)-3 -(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-2-(hydroxymethyl)-
3-oxopropy1)-5-cyclopropylimidazolidine-2,4-dione
HN)i_NH CF3
5-cyclopropy1-5-(2-methy1-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione
HN)r¨NH
OMe CF3
5-cyclopropy1-5-(2-(methoxymethyl)-3-oxo-3-(5-(trifluoromethyl)isoindolin-
2-yl)propyl)imidazolidine-2,4-dione
o
HNN
)r¨NH CF3
0
42
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(5 S)-5 -cyclopropy1-5-(3-(1-methyl-8-(trifluoromethyl)-4,5-dihydro-1H-
benzoldlazepin-3(2H)-y1)-3-oxopropyflimidazolidine-2,4-dione
0
H N
)r_ NH
0
(5S)-5 -cyclopropy1-5-(3-(1-fluoro-4,5-dihydro-1H-benzoldlazepin-3(2H)-y1)-
3-oxopropyflimidazolidine-2,4-dione
HN)rNH
0
(S)-5 -cyclopropy1-5-(3-(6-fluoro-4,5-dihydro-1H-benzoldlazepin-3(2H)-y1)-3-
oxopropyflimidazolidine-2,4-dione
V o
HN)r-NH CHF2
0
CI
(S)-5 -(3-(5-chloro-6-(difluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-dione
N-N
V 0
HN)r NH CF3
0
CI
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(1-methyl-
1H-pyrazol-4-yl)imidazolidine-2,4-dione
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (I), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, the
preparation process
comprising the steps of:
43
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
R4a
R2a R2b R4b R2a R2b
0 0 R4a
0 Ri 0 Ri R4b
OH HN
+
R3b G2 R3b / %2 )r..-NH R3a
)r--NH 3a n
/ n
R3a R5b G4=G3
/
0 0 R3a R5b
G4¨G3
( IA ) ( IB ) ( I )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (IB), or a salt
thereof, to obtain the compound of formula (I) or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof,
wherein:
the salt of the compound (IB) preferably is hydrochloride; and
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (I).
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (II), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, the
preparation process
comprising the steps of:
R4a
R2a R2b R2a R2b D 4a
0 R4h 0 -1' R4b
0 R1 0 Ri
HN
+
R3b
)r,NH 3a G2
/ 17
R5a R5b G4G3 /
0 0 R5a
R5b G4G3
( IIA ) ( IB ) ( II )
reacting a compound of formula (IIA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (IB), or a salt
thereof, to obtain the compound of formula (II) or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof;
wherein:
the salt of the compound (IB) preferably is hydrochloride; and
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (II).
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (III), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
44
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
or a pharmaceutically acceptable salt, solvate or prodrug thereof, the
preparation process
comprising the steps of:
R2a R2b R4a
R4a R4b
R2a R2 b 0b R1
0
0 R1
HN
OH +
R3b
0 R5a R5a R5b
R5b
0
( IA ) ( IIIB ) ( III )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (IIIB), or a salt
thereof, to obtain the compound of formula (III) or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof;
wherein:
the salt of the compound (IIIB) preferably is hydrochloride; and
Rl, R2a to R5a, R21 to R5b, R6, n, m and s are each as defined in formula
(III).
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (Ma), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
the preparation
process comprising the steps of:
R2a R
R4a 4b 2a R2b R4a R2b R
0 R4b
0 Ri
Ri
OH HN
6s
(R
HN
R3b R3b
R3a R3a
p 5a
0 R5b
0 R5a R5b
( IIA ) ( IIIB ) ( )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (IIIB), or a salt
thereof, to obtain the compound of formula (Ma) or a tautomer, mesomer,
racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof;
wherein:
the salt of the compound (IIIB) preferably is hydrochloride; and
Rl, R2a to R5a, R21 to R5b, R6, n, m and s are each as defined in formula
(Ma).
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (IV), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, the
preparation process
comprising the steps of:
R4a
0 R4a
R4b
R4b 0
0
0
HN
OH
HN
HN R3b
R6
R6
)rNH R3b
D 5a 0 n 5a
0 R5b R5b
R6 R6
( WA ) ( IVB ) ( W)
reacting a compound of formula (IVA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (IVB), or a salt
thereof, to obtain the compound of formula (IV) or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof;
wherein:
the salt of the compound (IV) preferably is hydrochloride; and
R4a, R5a. R31 to R5b K-rs
n and m are each as defined in formula (IV).
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (IVa), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
the preparation
process comprising the steps of:
R4a R4a
R4b
R4b
0
HN
OH H R3b
HN
HN R3b
)
R6 R6 r,N
n 5a n 5a
R5b R5b
R6 R6
( IVaA ) ( IVB ) ( IVa )
reacting a compound of formula (IVaA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (IVB), or a salt
thereof, to obtain the compound of formula (IVa) or a tautomer, mesomer,
racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof;
wherein:
the salt of the compound (IVB) preferably is hydrochloride; and
46
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R4a, R5a, R31 to R5b,
K n and m are each as defined in formula (IVa).
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (V), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, the
preparation process
comprising the steps of:
R4a 0 R4a
0 Ri
R1 0
0
FIN
OH
HN
HN )r¨NH R3b
R6 R6
)r,NH R3b
R5a 0 R5a
0
R6 R6
( VA ) ( VB ) (V)
reacting a compound of formula (VA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (VB), or a salt
thereof, to obtain the compound of formula (V) or a tautomer, mesomer,
racemate, enantiomer,
diastereomer, or mixture thereof, or a pharmaceutically acceptable salt,
solvate or prodrug
thereof;
wherein:
the salt of the compound (VB) preferably is hydrochloride; and
R4a, R5a, R31, 1
K and R6 are each as defined in formula (V).
In another aspect, this disclosure provides a process for preparation of a
compound of
formula (Va) or (Vb), or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
the preparation
process comprising the steps of:
R4a R4a
Ri
Ri
HN
OH +
HN
HN ) R6 )r¨NH R3b
R6 R6 r,NH
R3b
R5a 0 R5a
0
R6
( VaA ) ( VB ) ( Va )
R4a R4a
Ri
_________________________________________ . HN
HN R3b
R3b R6 R6
R5a 0 R5a
0
R6 R6
( VbA ) ( VB ) ( Vb )
47
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
reacting a compound of formula (VaA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (VB), or a salt
thereof, to obtain the compound of formula (Va) or a tautomer, mesomer,
racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof;
reacting a compound of formula (VbA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof, with a compound of
formula (VB), or a salt
thereof, to obtain the compound of formula (Vb) or a tautomer, mesomer,
racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof;
wherein:
the salt of the compound (VB) preferably is hydrochloride; and
R4a, R5a, R31
,
tc and R6 are each as defined in formula (V).
In another aspect, the present disclosure also provides a pharmaceutical
composition,
comprising a compound of formula (I), formula (II), formula (III), formula
(Ma), formula (IV),
formula (IVa), formula (V), formula (Va) or formula (Vb), or a tautomer,
mesomer, racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof, and one or more pharmaceutically acceptable carriers,
diluents and/or other
excipients.
In another aspect, the present disclosure provides a method of inhibiting
ADAMTS-5
and/or ADAMTS-4, comprising a step of administering to a subject in need
thereof a
therapeutically effective amount of a compound of formula (I), formula (II),
formula (III),
formula (Ina), formula (IV), formula (IVa), formula (V), formula (Va) or
formula (Vb), or a
tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or
a
pharmaceutically acceptable salt, solvate or prodrug thereof, or a
pharmaceutical composition
containing the compound.
In another aspect, the present disclosure provides a method of preventing
and/or
treating inflammatory conditions, and/or diseases involving degradation of
cartilage, and/or
disruption of cartilage homeostasis, comprising a step of administering to a
subject in need
thereof a therapeutically effective amount of a compound of formula (I),
formula (II), formula
(III), formula (Ina), formula (IV), formula (IVa), formula (V), formula (Va)
or formula (Vb),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof, or a
pharmaceutical composition
containing the compound.
48
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
In another aspect, the present disclosure provides a method of preventing
and/or
treating arthritis, comprising a step of administering to a subject in need
thereof a
therapeutically effective amount of the compound of formula (I), formula (II),
formula (III),
formula (Ina), formula (IV), formula (IVa), formula (V), formula (Va) or
formula (Vb), or a
tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or
a
pharmaceutically acceptable salt, solvate or prodrug thereof, or a
pharmaceutical composition
containing the compound; preferably, wherein arthritis is selected from the
group consisting of
rheumatoid arthritis, psoriatic arthritis, osteoarthrosis and hypertropic
arthritis.
In another aspect, the present disclosure also relates to use of a compound of
formula
(I), formula (II), formula (III), formula (Ina), formula (IV), formula (IVa),
formula (V),
formula (Va) or formula (Vb), or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, or a
pharmaceutical composition containing the compound, in the manufacture of a
medicament for
the inhibition of ADAMTS-5 and/or ADAMTS-4.
In another aspect, the present disclosure also relates to use of a compound of
formula
(I), formula (II), formula (III), formula (Ina), formula (IV), formula (IVa),
formula (V),
formula (Va) or formula (Vb), or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, or a
pharmaceutical composition containing the compound, in the manufacture of a
medicament for
preventing and/or treating inflammatory conditions, and/or diseases involving
degradation of
cartilage, and/or disruption of cartilage homeostasis.
In another aspect, the present disclosure also relates to use of a compound of
formula
(I), formula (II), formula (III), formula (Ina), formula (IV), formula (IVa),
formula (V),
formula (Va) or formula (Vb), or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof, or a
pharmaceutical composition containing the compound, in the manufacture of a
medicament for
preventing and/or treating arthritis; preferably, rheumatoid arthritis,
psoriatic arthritis,
osteoarthrosis and hypertropic arthritis.
The present disclosure further relates to the compound of formula (I), formula
(II),
formula (III), formula (Ina), formula (IV), formula (IVa), formula (V),
formula (Va) or formula
(Vb), or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof, or a
pharmaceutical composition
containing the same, for use as a medicament.
49
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
The present disclosure also relates to the compound of formula (I), formula
(II), formula
(III), formula (Ina), formula (IV), formula (IVa), formula (V), formula (Va)
or formula (Vb),
or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof, or a
pharmaceutical composition
.. containing the compound, for use in inhibiting ADAMTS-5 and/or ADAMTS-4 .
The present disclosure also relates to the combination of the compound of
formula (I),
formula (II), formula (III), formula (Ina), formula (IV), formula (IVa),
formula (V), formula
(Va) or formula (Vb), or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof, or
a pharmaceutical
composition containing the compound, for use in preventing and/or treating
inflammatory
conditions, and/or diseases involving degradation of cartilage, and/or
disruption of cartilage
homeostasis.
The present disclosure also relates to the combination of the compound of
formula (I),
formula (II), formula (III), formula (Ina), formula (IV), formula (IVa),
formula (V), formula
(Va) or formula (Vb), or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof, or
a pharmaceutical
composition containing the compound, for use in preventing and/or treating
arthritis;
preferably, rheumatoid arthritis, psoriatic arthritis, osteoarthrosis and
hypertropic arthritis.
Further, the present disclosure encompasses all compounds that can exist based
on any
chemically plausible combinations of the embodiments disclosed or substituents
on the
exemplified compounds, as would be understood by a person of skill in the art.
The term "inflammatory conditions" refers to the group of conditions including
rheumatoid arthritis, osteoarthritis, juvenile idiopathic arthritis,
psoriasis, psoriatic arthritis,
allergic airway disease (e.g. asthma, rhinitis), chronic obstructive pulmonary
disease (COPD),
inflammatory bowel diseases (e.g. Crohn's disease, ulcerative colitis),
endotwdn-driven disease
states (e.g.complications after bypass surgery or chronic endotwdn states
contributing to e.g.
chronic cardiacfailure), and related diseases involving cartilage, such as
that of the joints.
Particularly refers to rheumatoid arthritis, osteoarthritis, allergic airway
disease (e.g. asthma),
chronic obstructive pulmonary disease (COPD) and inflammatory bowel diseases.
More
particularly refers to rheumatoid arthritis, and osteoarthritis (OA). Most
particularly refers to
osteoarthritis (OA).
The term "diseases involving degradation of cartilage and/or disruption of
cartilage
homeostasis" includes conditions such as osteoarthritis, psoriatic arthritis,
juvenile rheumatoid
arthritis, gouty arthritis, septic or infectious arthritis, reactive
arthritis, reflex sympathetic
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
dystrophy, algodystrophy, achondroplasia, Paget's disease, Tietze syndrome or
costal
chondritis, fibromyalgia, osteochondritis, neurogenic or neuropathic
arthritis, arthropathy,
sarcoidosis, amylosis, hydarthrosis, periodical disease, rheumatoid
spondylitis, endemic forms
of arthritis like osteoarthritis deformans endemic, Mseleni disease and
Handigodu disease;
degeneration resulting from fibromyalgia, systemic lupus erythematosus,
scleroderma and
ankylosing spondylitis; and particularly, refers to osteoarthritis (OA).
The compositions of this disclosure can be formulated by conventional methods
using
one or more pharmaceutically acceptable carriers. Thus, the active compounds
of this
disclosure can be formulated as various dosage forms for oral, buccal,
intranasal, parenteral
(e.g., intravenous, intramuscular or subcutaneous), rectal administration,
inhalation or
insufflation administration. The compounds of this disclosure can also be
formulated as
sustained release dosage forms.
Suitable dosage forms include, but are not limited to, a tablet, troche,
lozenge, aqueous
or oily suspension, dispersible powder or granule, emulsion, hard or soft
capsule, or syrup or
elixir. Oral compositions can be prepared according to any known method in the
art for the
preparation of pharmaceutical compositions. Such compositions can contain one
or more
additives selected from the group consisting of sweeteners, flavoring agents,
colorants and
preservatives, in order to provide a pleasing and palatable pharmaceutical
preparation. Tablets
contain the active ingredient and nontoxic pharmaceutically acceptable
excipients suitable for
the manufacture of tablets. These excipients can be inert excipients,
granulating agents,
disintegrating agents, and lubricants. The tablet can be uncoated or coated by
means of a known
technique to mask the taste of the drug or delay the disintegration and
absorption of the drug
in the gastrointestinal tract, thereby providing sustained release over an
extended period. For
example, water soluble taste masking materials can be used.
Oral formulations can also be provided as soft gelatin capsules in which the
active
ingredient is mixed with an inert solid diluent, or the active ingredient is
mixed with a water
soluble carrier.
An aqueous suspension contains the active ingredient in admixture with
excipients
suitable for the manufacture of an aqueous suspension. Such excipients are
suspending agents,
dispersants or humectants, and can be naturally occurring phospholipids. The
aqueous
suspension can also contain one or more preservatives, one or more colorants,
one or more
flavoring agents, and one or more sweeteners.
An oil suspension can be formulated by suspending the active ingredient in a
vegetable
oil, or in a mineral oil. The oil suspension can contain a thickener. The
aforementioned
51
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
sweeteners and flavoring agents can be added to provide a palatable
preparation. These
compositions can be preserved by adding an antioxidant.
The present pharmaceutical composition can also be in the form of an oil-in-
water
emulsion. The oil phase can be a vegetable oil, or a mineral oil, or mixture
thereof. Suitable
emulsifying agents can be naturally occurring phospholipids. Sweeteners can be
used. Such
formulations can also contain moderators, preservatives, colorants and
antioxidants.
The pharmaceutical composition can be in the form of a sterile injectable
aqueous
solution. The acceptable vehicles and solvents that can be employed are water,
Ringer's
solution and isotonic sodium chloride solution. The sterile injectable
preparation can also be a
sterile injectable oil-in-water microemulsion in which the active ingredient
is dissolved in the
oil phase. The injectable solution or microemulsion can be introduced into an
individual's
bloodstream by local bolus injection. Alternatively, it can be advantageous to
administer the
solution or microemulsion in such a way as to maintain a constant circulating
concentration of
the present compound. In order to maintain such a constant concentration, a
continuous
intravenous delivery device can be utilized. An example of such a device is
Deltec CADD-
PLUS . TM. 5400 intravenous injection pump.
The pharmaceutical composition can be in the form of a sterile injectable
aqueous or
oily suspension for intramuscular and subcutaneous administration. Such a
suspension can be
formulated with suitable dispersants or wetting agents and suspending agents
as described
above according to known techniques. The sterile injectable preparation can
also be a sterile
injectable solution or suspension prepared in a nontoxic parenterally
acceptable diluent or
solvent. Moreover, sterile fixed oils can easily be used as a solvent or
suspending medium, and
fatty acids can also be used to prepare injections.
The present compound can be administered in the form of a suppository for
rectal
administration. These pharmaceutical compositions can be prepared by mixing
the drug with a
suitable non-irritating excipient that is solid at ordinary temperatures, but
liquid in the rectum,
thereby melting in the rectum to release the drug.
For buccal administration, the compositions can be formulated as tablets or
lozenges
by conventional means.
For intranasal administration or administration by inhalation, the active
compounds of
the present disclosure are conveniently delivered in the form of a solution or
suspension
released from a pump spray container that is squeezed or pumped by the
patient, or as an aerosol
spray released from a pressurized container or nebulizer, with the use of a
suitable propellant,
e.g., dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, carbon
52
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
dioxide or other suitable gas. In the case of a pressurized aerosol, the
dosage unit can be
determined by providing a valve to deliver a metered amount. The pressurized
container or
nebulizer can contain a solution or suspension of the active compound.
Capsules or cartridges
(for example, made from gelatin) for use in an inhaler or insufflator can be
formulated
containing a powder mix of the present disclosure and a suitable powder base
such as lactose
or starch.
It is well known to those skilled in the art that the dosage of a drug depends
on a variety
of factors, including but not limited to, the following factors: activity of
the specific compound,
age, weight, general health, behavior, diet of the patient, administration
time, administration
route, excretion rate, drug combination and the like. In addition, the best
treatment, such as
treatment mode, daily dose of the compound of formula (I) or the type of
pharmaceutically
acceptable salt thereof can be verified by traditional therapeutic regimens.
Unless otherwise stated, the terms used in the specification and claims take
ordinary
meanings as understood by those in the ordinary skill in the relevant art.
Certain terms have
the meanings described below.
"Alkyl" refers to a saturated aliphatic hydrocarbon group including C1-C12
(for example,
including 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 carbons) straight chain and
branched chain
groups. Preferably an alkyl group is an alkyl having 1 to 8 carbon atoms,
sometimes more
preferably 1 to 6 carbon atoms, and sometime more preferably 1 to 4 carbon
atoms.
Representative examples include, but are not limited to methyl, ethyl, n-
propyl, isopropyl, n-
butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl, 1,1-dimethyl propyl, 1,2-
dimethyl propyl, 2,2-
dimethyl propyl, 1-ethyl propyl, 2-methylbutyl, 3 -methylbutyl, n-hexyl, 1-
ethy1-2-
methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl,
1,3-dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3 -methylpentyl, 4-
methylpentyl, 2,3-
dimethylbutyl, n-heptyl, 2-methylhexyl, 3 -methylhexyl, 4-methylhexyl, 5 -
methylhexyl, 2,3-
dimethylpentyl, 2,4-dimethylpentyl, 2,2-dimethylpentyl, 3,3 -dimethylpentyl, 2-
ethylpentyl, 3-
ethylpentyl, n-octyl, 2,3-dimethylhexyl, 2,4-dimethylhexyl, 2,5-dimethylhexyl,
2,2-
dimethylhexyl, 3,3-dimethylhexyl, 4,4-dimethylhexyl, 2-ethylhexyl, 3-
ethylhexyl, 4-
ethylhexyl, 2-methyl-2-ethylpentyl, 2-methyl-3-ethylpentyl, n-nonyl, 2-methyl-
2-ethylhexyl,
2-methyl-3-ethylhexyl, 2,2-diethylpentyl, n-decyl, 3,3-diethylhexyl, 2,2-
diethylhexyl, and the
isomers of branched chain thereof. The alkyl group can be substituted or
unsubstituted. When
substituted, the substituent group(s) can be substituted at any available
connection point,
preferably the substituent group(s) is one or more, preferably one to five,
and more preferably
one to three, groups independently selected from the group consisting of
alkyl, halogen, alkoxy,
53
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
alkenyl, alkynyl, alkylsulfo, alkylamino, thiol, hydroxy, nitro, cyano, amino,
cycloalkyl,
heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic,
cycloalkylthio, heterocylic
alkylthio and oxo group.
"Alkenyl" refers to an alkyl defined as above that has at least two carbon
atoms and at
least one carbon-carbon double bond, for example, vinyl, 1-propenyl, 2-
propenyl, 1-, 2-, or 3-
butenyl, etc. preferably C2_12 (for example, including 2, 3, 4, 5, 6, 7, 8, 9,
10, 11 and 12 carbons)
alkenyl, more preferably C2_8 alkenyl, sometimes more preferably C2_6 alkenyl,
and sometimes
more preferable C2_4 alkenyl. The alkenyl group can be substituted or
unsubstituted. When
substituted, the substituent group(s) is preferably one or more, preferably
one to five, and more
preferably one to three, group(s) independently selected from the group
consisting of alkyl,
halogen, alkoxy, alkenyl, alkynyl, alkylsulfo, alkylamino, thiol, hydroxy,
nitro, cyano, amino,
cycloalkyl, heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic,
cycloalkylthio,
heterocylic alkylthio and oxo group.
"Alkynyl" refers to an alkyl defined as above that has at least two carbon
atoms and at
least one carbon-carbon triple bond, for example, ethynyl, 1-propynyl, 2-
propynyl, 1-, 2-, or 3-
butynyl etc., preferably C2-12 (for example, including 2, 3, 4, 5, 6, 7, 8, 9,
10, 11 and 12 carbons)
alkynyl, more preferably C2_6 alkynyl, some times more preferably C2_6
alkynyl, and sometimes
more preferable C2_4 alkynyl. The alkynyl group can be substituted or
unsubstituted. When
substituted, the substituent group(s) is preferably one or more, preferably
one to five, and more
preferably one to three, group(s) independently selected from the group
consisting of alkyl,
alkenyl, alkynyl, alkoxy, alkylsulfo, alkylamino, halogen, thiol, hydroxy,
nitro, cyano,
cycloalkyl, heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic
alkoxyl,
cycloalkylthio and heterocylic alkylthio.
"Alkylene" refers to a saturated linear or branched divalent aliphatic
hydrocarbon
group, derived by removing two hydrogen atoms from the same carbon atom or two
different
carbon atoms of the parent alkane. The straight or branched chain group
containing 1 to 12 (for
example, including 1,2, 3,4, 5, 6,7, 8, 9, 10, 11 and 12 carbons) carbon
atoms, preferably has
1 to 8 carbon atoms, more preferably 1 to 6 carbon atoms, and sometimes more
preferably 1 to
4 carbon atoms. Non-limiting examples of alkylene groups include, but are not
limited to,
methylene (-CH2-), 1,1-ethylene (-CH(CH3)-), 1,2-ethylene (-CH2CH2)-, 1,1-
propylene (-
CH(CH2CH3)-), 1,2-propylene (-CH2CH(CH3)-), 1,3-propylene (-CH2CH2CH2-), 1 ,4-
butylidene (-CH2CH2CH2CH2-) etc. The alkylene group can be substituted or
unsubstituted.
When substituted, the substituent group(s) is preferably one or more,
sometimes preferably 1
to 5, and sometimes more preferably 1 to 3, group(s) independently selected
from the group
54
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
consisting of selected from alkyl, alkenyl, alkynyl, alkoxy, alkylsulfo,
alkylamino, halogen,
thiol, hydroxy, nitro, cyano, cycloalkyl, heterocyclic alkyl, aryl,
heteroaryl, cycloalkoxyl,
heterocylic alkoxyl, cycloalkylthio and heterocylic alkylthio.
"Alkenylene" refers to an alkylene defined as above that has at least two
carbon atoms
and at least one carbon-carbon double bond, preferably C2-12 (for example,
including 2, 3, 4,
5, 6, 7, 8, 9, 10, 11 and 12 carbons) alkenylene, more preferably C2_8
alkenylene, sometimes
more preferably C2_6 alkenylene, and sometimes even more prefereably C2_4
alkenylene. Non-
limiting examples of alkenylene groups include, but are not limited to, -CH=CH-
, -
CH=CHCH2-, -CH=CHCH2CH2-, -CH2CH=CHCH2- etc. The alkenylene group can be
substituted or unsubstituted. When substituted, the substituent group(s) is
preferably one or
more, sometimes preferably 1 to 5, and sometimes more preferably 1 to 3,
group(s)
independently selected from the group consisting of selected from alkyl,
alkenyl, alkynyl,
alkoxy, alkylsulfo, alkylamino, halogen, thiol, hydroxy, nitro, cyano,
cycloalkyl, heterocyclic
alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic alkoxyl, cycloalkylthio and
heterocylic
.. alkylthio.
"Cycloalkyl" refers to a saturated and/or partially unsaturated monocyclic or
polycyclic hydrocarbon group having 3 to 20 carbon atoms, preferably 3 to 12
(for example,
including 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 carbons) carbon atoms, more
preferably 3 to 10
carbon atoms, sometimes more preferably 3 to 8 carbon atoms, and sometimes
even more
.. preferably 3 to 6 carbon atoms. Representative examples of monocyclic
cycloalkyls include,
but are not limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl,
cyclohexyl,
cyclohexenyl, cyclohexadienyl, cycloheptyl, cycloheptatrienyl, cyclooctyl,
etc. Polycyclic
cycloalkyl includes a cycloalkyl having a spiro ring, fused ring or bridged
ring.
"Spiro Cycloalkyl" refers to a 5 to 20 membered polycyclic group with rings
connected
through one common carbon atom (called a spiro atom), wherein one or more
rings can contain
one or more double bonds. Preferably a spiro cycloalkyl is 6 to 14 membered
(for example,
including 6, 7, 8, 9, 10, 11, 12, 13 and 14 carbons), and more preferably 7 to
10 membered.
According to the number of common spiro atoms, a spiro cycloalkyl is divided
into mono-spiro
cycloalkyl, di-spiro cycloalkyl, or poly-spiro cycloalkyl, and preferably
refers to a mono-spiro
cycloalkyl or di-spiro cycloalkyl, more preferably 4-membered/4-membered, 4-
membered/5-
membered, 4-membered/6-membered, 5 -membered/5 -membered, or 5 -membered/6-
membered mono-spiro cycloalkyl. Representative examples of spiro cycloalkyl
include, but
are not limited to the following groups:
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
g414 Era7nd .
"Fused Cycloalkyl" refers to a 5 to 20 membered polycyclic hydrocarbon group,
wherein each ring in the system shares an adjacent pair of carbon atoms with
another ring,
wherein one or more rings can contain one or more double bonds. Preferably, a
fused cycloalkyl
group is 6 to 14 membered (for example, including 6, 7, 8, 9, 10, 11, 12, 13
and 14 carbons),
more preferably 7 to 10 membered. According to the number of membered rings,
fused
cycloalkyl is divided into bicyclic, tricyclic, tetracyclic or polycyclic
fused cycloalkyl, and
preferably refers to a bicyclic or tricyclic fused cycloalkyl, more preferably
5-membered/5-
membered, or 5-membered/6-membered bicyclic fused cycloalkyl. Representative
examples of
fused cycloalkyls include, but are not limited to, the following groups:
and '
"Bridged Cycloalkyl" refers to a 5 to 20 membered polycyclic hydrocarbon
group,
wherein every two rings in the system share two disconnected carbon atoms. The
rings can
have one or more double bonds. Preferably, a bridged cycloalkyl is 6 to 14
membered (for
example, including 6, 7, 8, 9, 10, 11, 12, 13 and 14 carbons), and more
preferably 7 to 10
membered. According to the number of membered rings, bridged cycloalkyl is
divided into
bicyclic, tricyclic, tetracyclic or polycyclic bridged cycloalkyl, and
preferably refers to a
bicyclic, tricyclic or tetracyclic bridged cycloalkyl, more preferably a
bicyclic or tricyclic
bridged cycloalkyl. Representative examples of bridged cycloalkyls include,
but are not limited
to, the following groups:
and 1;1.-- =
The cycloalkyl includes the cycloalkyl said above fused to the ring of an
aryl, heteroaryl
or heterocyclic alkyl, wherein the ring bound to the parent structure is
cycloalkyl.
56
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Representative examples include, but are not limited to indanylacetic,
tetrahydronaphthalene,
benzocycloheptyl and so on. The cycloalkyl is optionally substituted or
unsubstituted. When
substituted, the substituent group(s) is preferably one or more, sometimes
preferably 1 to 5,
and sometimes more preferably 1 to 3, groups independently selected from the
group consisting
of alkyl, halogen, alkoxy, alkenyl, alkynyl, alkylsulfo, alkylamino, thiol,
hydroxy, nitro, cyano,
amino, cycloalkyl, heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl,
heterocylic,
cycloalkylthio, heterocylic alkylthio and oxo group.
"Heterocycly1" refers to a 3 to 20 membered saturated and/or partially
unsaturated
monocyclic or polycyclic hydrocarbon group having one or more heteroatoms
selected from
the group consisting of N, 0, S, S(0) and S(0)2 as ring atoms, but excluding -
0-0-, -0-S- or -
S-S- in the ring, the remaining ring atoms being C. Preferably, heterocyclyl
is a 3 to 12
membered having 1 to 4 heteroatoms(for example, including 1, 2, 3 or 4
heteroatoms.); more
preferably a 3 to 10 membered (for example, including 3, 4, 5, 6, 7, 8, 9 or
10 ring atoms)
having 1 to 3 heteroatoms (for example, including 1, 2 or 3 heteroatoms.);
most preferably a 5
to 6 membered having 1 to 2 heteroatoms. Representative examples of monocyclic
heterocyclyls include, but are not limited to, pyrrolidyl, piperidyl,
piperazinyl, morpholinyl,
sulfo-morpholinyl, homopiperazinyl, and so on. Polycyclic heterocyclyl
includes the
heterocyclyl having a spiro ring, fused ring or bridged ring.
"Spiro heterocyclyl" refers to a 5 to 20 membered polycyclic heterocyclyl with
rings
connected through one common carbon atom (called a spiro atom), wherein said
rings have
one or more heteroatoms selected from the group consisting of N, 0, S, 5(0)
and S(0)2 as ring
atoms, the remaining ring atoms being C, wherein one or more rings can contain
one or more
double bonds. Preferably a spiro heterocyclyl is 6 to 14 membered (for
example, including 6,
7, 8, 9, 10, 11, 12, 13 or 14 ring atoms), and more preferably 7 to 10
membered. According to
the number of common spiro atoms, spiro heterocyclyl is divided into mono-
spiro heterocyclyl,
di-spiro heterocyclyl, or poly-spiro heterocyclyl, and preferably refers to
mono-spiro
heterocyclyl or di-spiro heterocyclyl, more preferably 4-membered/4-membered,
4-
membered/5 -membered, 4-membered/6-membered, 5 -membered/5 -membered, or 5-
membered/6-membered mono-spiro heterocyclyl. Representative examples of spiro
heterocyclyl include, but are not limited to the following groups:
57
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
-LA
N
¨07-7
x
o 0 Q 0_ _________ and N
"Fused heterocyclyl" refers to a 5 to 20 membered polycyclic heterocyclyl
group,
wherein each ring in the system shares an adjacent pair of carbon atoms with
the other ring,
wherein one or more rings can contain one or more double bonds, and wherein
said rings have
one or more heteroatoms selected from the group consisting of N, 0, S, S(0)
and S(0)2 as ring
atoms, the remaining ring atoms being C. Preferably a fused heterocyclyl is 6
to 14 membered
(for example, including 6, 7, 8, 9, 10, 11, 12, 13 or 14 ring atoms), and more
preferably 7 to
membered. According to the number of membered rings, fused heterocyclyl is
divided into
bicyclic, tricyclic, tetracyclic or polycyclic fused heterocyclyl, preferably
refers to bicyclic or
10 tricyclic fused heterocyclyl, more preferably 5-membered/5-membered, or 5-
membered/6-
membered bicyclic fused heterocyclyl. Representative examples of fused
heterocyclyl include,
but are not limited to, the following groups:
c 1) 7
,The N
CINTt
Nst.õ 0j ak)
cD,
and
"Bridged heterocyclyl" refers to a 5 to 14 membered polycyclic heterocyclic
alkyl
group, wherein every two rings in the system share two disconnected atoms, the
rings can have
one or more double bonds, and the rings have one or more heteroatoms selected
from the group
consisting of N, 0, S, S(0) and S(0)2 as ring atoms, the remaining ring atoms
being C.
Preferably a bridged heterocyclyl is 6 to 14 membered (for example, including
6, 7, 8, 9, 10,
11, 12, 13 or 14 ring atoms), and more preferably 7 to 10 membered. According
to the number
of membered rings, bridged heterocyclyl is divided into bicyclic, tricyclic,
tetracyclic or
polycyclic bridged heterocyclyl, and preferably refers to bicyclic, tricyclic
or tetracyclic
bridged heterocyclyl, more preferably bicyclic or tricyclic bridged
heterocyclyl. Representative
examples of bridged heterocyclyl include, but are not limited to, the
following groups:
58
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
kNA ___________________________
N
-AA
and1N-----)
The ring of said heterocyclyl include the heterocyclyl said above which fused
to the
ring of an aryl, heteroaryl or cycloalkyl, wherein the ring bound to the
parent structure is
heterocyclyl. Representative examples include, but are not limited to the
following groups:
AT N . $'-xj
a
. s . , N) ' - $N
/
_ -/
H H H
0 N N
1.I lel : N 110
0 00 and s , etc.
The heterocyclyl is optionally substituted or unsubstituted. When substituted,
the
substituent group(s) is preferably one or more, sometimes preferably 1 to 5,
and sometimes
more preferably 1 to 3, group(s) independently selected from the group
consisting of alkyl,
alkenyl, alkynyl, alkoxy, alkylsulfo, alkylamino, halogen, thiol, hydroxy,
nitro, cyano,
cycloalkyl, heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic
alkoxyl,
cycloalkylthio and heterocylic alkylthio.
"Aryl" refers to a 6 to 14 membered all-carbon monocyclic ring or a polycyclic
fused
ring (a "fused" ring system means that each ring in the system shares an
adjacent pair of carbon
atoms with another ring in the system) group, and has a completely conjugated
pi-electron
system. Preferably aryl is 6 to 10 membered, such as phenyl and naphthyl, most
preferably
phenyl. The aryl include the aryl said above which fused to the ring of
heteroaryl, heterocyclyl
or cycloalkyl, wherein the ring bound to parent structure is aryl.
Representative examples
include, but are not limited to, the following groups:
IL N
0 H H
N
wi 0 / 0
0 0 0 0 (00
H H H
N s N N N
N'
\ . 1 0 /
N S e N 0 0 and .
59
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
The aryl group can be substituted or unsubstituted. When substituted, the
substituent
group(s) is preferably one or more, sometimes preferably 1 to 5, and sometimes
more
preferably 1 to 3, groups independently selected from the group consisting of
alkyl, alkenyl,
alkynyl, alkoxy, alkylsulfo, alkylamino, halogen, thiol, hydroxy, nitro,
cyano, cycloalkyl,
heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic alkoxyl,
cycloalkylthio and
heterocylic alkylthio.
"Heteroaryl" refers to an aryl system having 1 to 4 heteroatoms (for example,
including
1, 2, 3 or 4 heteroatoms) selected from the group consisting of 0, S and N as
ring atoms and
having 5 to 14 annular atoms (for example, including 5, 6,7, 8, 9, 10, 11, 12,
13 or 14 annular
atoms). Preferably a heteroaryl is 5- to 10- membered, more preferably 5- or 6-
membered, for
example, thiadiazolyl, pyrazolyl, oxazolyl, oxadiazolyl, imidazolyl,
triazolyl, thiazolyl, furyl,
thienyl, pyridyl, pyrrolyl, N-alkyl pyrrolyl, pyrimidinyl, pyrazinyl,
imidazolyl, tetrazolyl, and
the like. The heteroaryl include the heteroaryl said above which fused with
the ring of an aryl,
heterocyclyl or cycloalkyl, wherein the ring bound to parent structure is
heteroaryl.
Representative examples include, but are not limited to, the following groups:
N-N N N-N
N
efT N I
.rNH NH HN z HN V N 0 N
N
5C0 0 and
The heteroaryl group can be substituted or unsubstituted. When substituted,
the
substituent group(s) is preferably one or more , sometimes preferably 1 to 5,
and sometimes
more preferably 1 to 3, groups independently selected from the group
consisting of alkyl,
alkenyl, alkynyl, alkoxy, alkylsulfo, alkylamino, halogen, thiol, hydroxy,
nitro, cyano,
cycloalkyl, heterocyclic alkyl, aryl, heteroaryl, cycloalkoxyl, heterocylic
alkoxyl,
cycloalkylthio and heterocylic alkylthio.
"Alkoxy" refers to an -0-(alkyl) group, wherein the alkyl is defined as above.
Representative examples include, but are not limited to, methoxy, ethoxy,
propoxy, butoxy,
and the like. The alkoxyl can be substituted or unsubstituted. When
substituted, the substituent
is preferably one or more, sometimes preferably 1 to 5, and sometimes more
preferably 1 to 3,
groups independently selected from the group consisting of alkenyl, alkynyl,
alkoxy,
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
alkylsulfo, alkylamino, halogen, thiol, hydroxy, nitro, cyano, cycloalkyl,
heterocyclic alkyl,
aryl, heteroaryl, cycloalkoxyl, heterocylic alkoxyl, cycloalkylthio and
heterocylic alkylthio.
"Bond" refers to a covalent bond using a sign of "¨".
"Hydroxyalkyl" refers to an alkyl group substituted by one or more hydroxy
group(s),
wherein alkyl is as defined above.
"Hydroxy" refers to an -OH group.
"Halogen" refers to fluoro, chloro, bromo or iodo atoms.
"Amino" refers to a -NH2 group.
"Cyano" refers to a -CN group.
"Nitro" refers to a -NO2 group.
"Oxo group" refers to a =0 group.
"Carboxyl" refers to a -C(=0)0H group.
"Alkoxycarbonyl" refers to a -C(=0)0(alkyl) or -C(=0)0 (cycloalkyl) group,
wherein
the alkyl and cycloalkyl are defined as above.
"Optional" or "optionally" means that the event or circumstance described
subsequently can, but need not, occur, and the description includes the
instances in which the
event or circumstance may or may not occur. For example, "the heterocyclic
group optionally
substituted by an alkyl" means that an alkyl group can be, but need not be,
present, and the
description includes the case of the heterocyclic group being substituted with
an alkyl and the
heterocyclic group being not substituted with an alkyl.
"Substituted" refers to one or more hydrogen atoms in the group, preferably up
to 5,
more preferably 1 to 3 hydrogen atoms, independently substituted with a
corresponding number
of substituents. The person skilled in the art is able to determine if the
substitution is possible
or impossible without paying excessive efforts by experiment or theory. For
example, the
combination of amino or hydroxyl group having free hydrogen and carbon atoms
having
unsaturated bonds (such as olefinic) may be unstable.
A "pharmaceutical composition" refers to a mixture of one or more of the
compounds
described in the present disclosure or physiologically/pharmaceutically
acceptable salts or
prodrugs thereof and other chemical components such as
physiologically/pharmaceutically
acceptable carriers and excipients. The purpose of a pharmaceutical
composition is to facilitate
administration of a compound to an organism, which is conducive to the
absorption of the
active ingredient and thus displaying biological activity.
"Pharmaceutically acceptable salts" refer to salts of the compounds of the
disclosure,
such salts being safe and effective when used in a mammal and have
corresponding biological
61
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
activity. The salts can be prepared during the final isolation and
purification of the compounds
or separately by reacting a suitable nitrogen atom with a suitable acid. Acids
commonly
employed to form pharmaceutically acceptable salts include inorganic acids
such as
hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid,
phosphoric acid, hydrogen
bisulfide as well as organic acids, such as para-toluenesulfonic acid,
salicylic acid, tartaric acid,
bitartaric acid, ascorbic acid, maleic acid, besylic acid, fumaric acid,
gluconic acid, glucuronic
acid, formic acid, glutamic acid, methanesulfonic acid, ethanesulfonic acid,
benzenesulfonic
acid, lactic acid, oxalic acid, para-bromophenylsulfonic acid, carbonic acid,
succinic acid, citric
acid, benzoic acid, acetic acid acid, and related inorganic and organic acids.
Basic addition salts can be prepared during the final isolation and
purification of the
compounds by reacting a carboxy group with a suitable base such as the
hydroxide, carbonate,
or bicarbonate of a metal cation or with ammonia or an organic primary,
secondary, or tertiary
amine. The cations of pharmaceutically acceptable salts include, but are not
limited to, lithium,
sodium, potassium, calcium, magnesium, and aluminum, as well as nontoxic
quaternary amine
cations such as ammonium, tetramethylammonium, tetraethylammonium,
methylamine,
dimethylamine, trimethylamine, triethylamine, diethylamine, ethylamine,
tributylamine,
pyridine, N,N-dimethylaniline, N-methylpiperidine, and N-methylmorpholine.
As a person skilled in the art would understand, the compounds of formula (I)
or
pharmaceutically acceptable salts thereof disclosed herein may exist in
prodrug or solvate
forms, which are all encompassed by the present disclosure.
The term "solvate," as used herein, means a physical association of a compound
of this
disclosure with one or more, preferably one to three, solvent molecules,
whether organic or
inorganic. This physical association includes hydrogen bonding. In certain
instances the
solvate will be capable of isolation, for example, when one or more,
preferably one to three,
solvent molecules are incorporated in the crystal lattice of the crystalline
solid. Exemplary
solvates include, but are not limited to, hydrates, ethanolates, methanolates,
and
isopropanolates. Methods of solvation are generally known in the art.
"Prodrug" refers to compounds that can be transformed in vivo to yield the
active parent
compound under physiological conditions, such as through hydrolysis in blood.
Common
.. examples include, but are not limited to, ester and amide forms of a
compound having an active
form bearing a carboxylic acid moiety. Amides and esters of the compounds of
the present
disclosure may be prepared according to conventional methods. In particular,
in the present
disclosure, a prodrug may also be formed by acylation of an amino group or a
nitrogen atom
in a heterocyclyl ring structure, which acyl group can be hydrolyzed in vivo.
Such acyl group
62
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
includes, but is not limited to, a Ci-C6 acyl, preferably Ci-C4 acyl, and more
preferably Ci-C2
(formyl or acetyl) group, or benzoyl.
The term "pharmaceutically acceptable," as used herein, refers to those
compounds,
materials, compositions, and/or dosage forms which are, within the scope of
sound medical
judgment, suitable for use in contact with the tissues of patients without
excessive toxicity,
irritation, allergic response, or other problem or complication commensurate
with a reasonable
benefit/risk ratio, and are effective for their intended use.
The term "therapeutically effective amount," as used herein, refers to the
total amount
of each active component that is sufficient to show a meaningful patient
benefit, e.g., a
sustained reduction in viral load. When applied to an individual active
ingredient, administered
alone, the term refers to that ingredient alone. When applied to a
combination, the term refers
to combined amounts of the active ingredients that result in the therapeutic
effect, whether
administered in combination, serially, or simultaneously.
The term "treat", "treating", "treatment", or the like, refers to: (i)
inhibiting the disease,
disorder, or condition, i.e., arresting its development; and (ii) relieving
the disease, disorder, or
condition, i.e., causing regression of the disease, disorder, and/or
condition. In addition, the
compounds of present disclosure may be used for their prophylactic effects in
preventing a
disease, disorder or condition from occurring in a subject that may be
predisposed to the
disease, disorder, and/or condition but has not yet been diagnosed as having
it.
As used herein, the singular forms "a", "an", and "the" include plural
reference, and
vice versa, unless the context clearly dictates otherwise.
When the term "about" is applied to a parameter, such as pH, concentration,
temperature, or the like, it indicates that the parameter can vary by 10%,
and some times more
preferably within 5%. As would be understood by a person skilled in the art,
when a parameter
is not critical, a number is often given only for illustration purpose,
instead of being limiting.
The compound of the present disclosure, any atom not specifically designated
as a
specific isotope means any stable isotope of that atom. Unless otherwise
stated, when a position
is specifically designated as "H" or "hydrogen", the position should be
understood as having
hydrogen according to its natural abundance isotopic composition. Likewise,
unless otherwise
specified, when a position is specifically designated as "D" or "deuterium",
the position should
be understood as deuterium having an abundance of at least 3000 times greater
than the natural
abundance of deuterium (which is 0.015%) (That is, at least 45% of deuterium
is incorporated).
63
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
SYNTHESIS METHODS
In order to complete the purpose of the disclosure, the present disclosure
applies, but is
not limited to, the following technical solution:
Scheme 1
A preparation process of a compound of formula (I), or a tautomer, mesomer,
racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof, comprising a step of:
R2a R2b R4b R2a R2b R4a
0 0 R4b
0 Ri 0 Ri
OH FIN
+ Gi N 1
FIN / \\G2 FIN G
R3b R3b / \\G2 )r.,-NH R3a
)r,NH R3a n / n
R5a R5b GziG3 /
0 0 R3a
R5b G4 G3
( IA ) ( IB ) ( I )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(IB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (I) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (IB) preferably is hydrochloride salt; and
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (I).
Scheme 2
A preparation process of a compound of formula (II), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, comprising a step of:
R4a
R2a R2b R4b R2a R2b pp 4a
0 0
0 Ri 0 Ri ¨ R4b
FIN / \\G2 FIN
R3b )r
3 R3b / \\G2 ¨NH Ra
)r-NH R3a n
/ n
/
R5a R5b G4 G3
R5a R5b G4 ¨G3
0 0
( ILA ) ( IB ) ( II )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(IB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
64
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
compound of formula (II) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (IB) preferably is hydrochloride salt; and
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (II).
Scheme 3
A preparation process of a compound of formula (III), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, comprising a step of:
R2a R2b R4a
R4a 0
R2a R2b R4b 0 Ri R4b
0
0 Ri
OH
FIN
(R6),
\ 7 (R6), R3b
D 5a 0 R5a R5b
0 R5b
( IA ) (IIIB ) ( III )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(IIIB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (III) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (IIIB) preferably is hydrochloride salt; and
Rl, R2a to R5a, R26 to R5b, R6, n, m and s are each as defined in formula
(III).
Scheme 4
A preparation process of a compound of formula (Ina), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, comprising a step of:
Ra R2b R4a
R2a R2b 4a R4b R2 0 R4b
0 0 R1
0 Ri
OH FIN
(R6)
(R6),
FIN ><, s FIN
R3b
R3a R3
n 5a
0 1\- R5b ____________ 0 R5a R5b
( IIA ) ( IIIB ) ( IIIa )
reacting a compound of formula (IA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(IIIB) or a salt
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (Ma) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (IIIB) preferably is hydrochloride salt; and
Rl, R2a to R5a, R21 to R5b, R6, n, m and s are each as defined in formula
(Ma).
Scheme 5
A preparation process of a compound of formula (IV), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, the preparation process comprising the steps of:
R4a
R4a
R4b
R4b
0
HN
OH
HN
HN R3b
R6 R6
).rNH R3b
p 5a 5a
R5b R R5b
R6 R6
( IVA ) ( IVB ) ( IV)
reacting a compound of formula (IVA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(IVB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (IV) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (IVB) preferably is hydrochloride salt; and
R4a, R5a, R31 to R5b,
K n and m are each as defined in formula (IV).
Scheme 6
A preparation process of a compound of formula (IVa), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, the preparation process comprising the steps of:
R4a R4a
R4b
R4b
-7;
OH HN
HN
HN )r )r,NH R3b
R6 R6 ¨NH
R3b
D 5a D 5a
R5b R5b
R6 R6
( IVaA ) ( IVB ) ( IVa )
66
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
reacting a compound of formula (IVaA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(IVB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (IVa) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (IVB) preferably is hydrochloride salt; and
R4a, R5a, R31 to R5b,
K n and m are each as defined in formula (IVa).
Scheme 7
A preparation process of a compound of formula (V), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, the preparation process comprising the steps of:
R4a R4a
0 Ri 0
0 R1 0
HN
OH
HN
HN R3b
R6 R6
)rNH R3b
R5a 0 R5a
0
( VA ) ( VB ) R6 (V) R6
reacting a compound of formula (VA) or a tautomer, mesomer, racemate,
enantiomer,
.. diastereomer, or mixture thereof, or a salt thereof with a compound of
formula (VB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (V) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (VB) preferably is hydrochloride salt; and
R4a, R5a, R3b,
K and R6 are each as defined in formula (V).
Scheme 8
A preparation process of a compound of formula (Va) or (Vb), or a tautomer,
mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, the preparation process comprising the steps of:
67
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
R4a o R4a
o R1
o R1 0
HN N
OH HN
-1.-
HN + R6 )r,NH R3b
R6
R5a 0 R5a
0
6
( VaA ) ( VB ) R ( Va ) R6
R4a
0 R4a
OH + HN N
HN _,,.
)R3b R5a
)r..-NH R3b
R6 R6 r,NH 0 R5a
0
( VbA ) ( VB ) R6 ( Vb ) R6
reacting a compound of formula (VaA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(VB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (Va) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
reacting a compound of formula (VbA) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with a compound of formula
(VB) or a salt
thereof under alkaline conditions and in the presence of a condensing agent to
obtain the
compound of formula (Vb) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or
mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
the salt of the compound (VB) preferably is hydrochloride salt; and
R4a, R5a, R313, R1 and R6 are each as defined in formula (V).
Scheme 9
A preparation process of a compound of formula (II) or (Ilb), or a tautomer,
mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, the preparation process comprising the steps of:
2R a R2b 0 R4a 2R a R2b 0 R4a R2a R2b 0 R4a
0 RI R4b 0 RI R4b 0 RI R4b
N N
+
HN
n n
0 R3a R5b 04-03 0 R3a R5b 04-03 0
R3a R5b 04-03
( I ) ( II ) ( IIb )
Formula (I) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture thereof,
or a pharmaceutically acceptable salt, solvate or prodrug thereof, was chiral
separated to give
68
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Formula (II) and (Ilb) or a tautomer, mesomer, racemate, enantiomer,
diastereomer, or mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof;
wherein:
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (II).
Scheme 10
A preparation process of a compound of formula (Ma) or (11Th), or a tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt, solvate or prodrug thereof, the preparation process
comprising the steps of:
R2a R2b R2a R2b R46 R26 R2b
0 R46 R4b 0 R4b \& );IL R46 R4b
0 RI 0 RI 0 RI
N N 3a) \'R3b N
)7,----N1-1 R36 R3b )7,----NH R36 R3b a 1 R
0 R56 R5b 0 R56 R5b 0 R56 R5b
(III) ( Ma ) ( Mb )
Formula (III) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
was chiral separated
to give Formula (Ma) and (IIIb) or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
IV, R2a to R5a, R21 to R5b, R6, s, n and m are each as defined in formula
(III).
Scheme 11
A preparation process of a compound of formula (IVa) or (IVb), or a tautomer,
mesomer, racemate, enantiomer, diastereomer, or mixture thereof, or a
pharmaceutically
acceptable salt, solvate or prodrug thereof, the preparation process
comprising the steps of:
0 R4a < 0 R4a Rab 0 R4a R4b
HN HN
R6 HN)r,NH R3b ( ,
R6 )r...NH R3b , R6
,
0 R5a R5b 0 R56 R5b 0 R56 R5b
R6 R6 R6
(W) (IVa) (IVb)
Formula (IV) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
was chiral separated
to give Formula (IVa) and (IVb) or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
R' to R5a, R36 to R5b, R6, n and m are each as defined in formula (IV).
69
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Scheme 12
A preparation process of a compound of formula (Va) or (Vb), or a tautomer,
mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, the preparation process comprising the steps of:
0 R4a 0 R4a
HN 0 R4a
0 Ri 0 Ri 0 R HN
R3bR3b 1
R6 )r-NH R3b R6
0 R" 0 R5a 0 R5a
R6 R6 R6
(Va) (Vb)
Formula (V) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or
mixture
thereof, or a pharmaceutically acceptable salt, solvate or prodrug thereof,
was chiral separated
to give Formula (Va) and (Vb) or a tautomer, mesomer, racemate, enantiomer,
diastereomer,
or mixture thereof, or a pharmaceutically acceptable salt, solvate or prodrug
thereof;
wherein:
Rl, R4a to R5a, R36, and R6 are each as defined in formula (V).
Scheme 13
A preparation process of a compound of formula (I), or a tautomer, mesomer,
racemate,
enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable
salt, solvate or
prodrug thereof, comprising a step of:
R2a R2b R Rza R2b 4a R4a
0 0 R4b R4b
Ri 0 R1
Gi Gi
0 FIN
R3b R3b \\G2
R'aG2)r,NH R3 a
R5a R5b G3 0 R5a R5b G4 G3
( IC ) ( I )
reacting a compound of formula (IC) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with ammonium carbonate
(or ammonium
bicarbonate) and sodium cyanide (or potassium cyanide) to obtain the compound
of formula
(I) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof;
wherein:
Gl, G2, G3, G4, Rl, R2a to R5a, R21 to R5b, n and m are each as defined in
formula (I).
70
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Scheme 14
A preparation process of a compound of formula (III), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, comprising a step of:
R2a R2 2b
R2a R2b R4a
0 R4b 0 R4b
Ri 0 R1
0 (R6) \(R6)s
R3b HN R3b
R3a R3a
R5a R5b 0 R5a R5b
( IIIC ) ( III )
reacting a compound of formula (IIIC) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with ammonium carbonate
(or ammonium
bicarbonate) and sodium cyanide (or potassium cyanide) to obtain the compound
of formula
(III) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof;
wherein:
Rl, R2a to R5a, R21 to R5b, R6, n, m and s are each as defined in formula
(III).
Scheme 15
A preparation process of a compound of formula (IV), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, comprising a step of:
R4a R4a
R4b
0 0 R4b
FIN
R3b R3b
R6 R6
a a
R5a R5b 0 R5a R5b
R6 R6
( WC) (1V)
reacting a compound of formula (IVC) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with ammonium carbonate
(or ammonium
bicarbonate) and sodium cyanide (or potassium cyanide) to obtain the compound
of formula
(IV) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof;
wherein:
71
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
R4a, R5a, R31 to R5b,
n and m are each as defined in formula (IV).
Scheme 16
A preparation process of a compound of formula (V), or a tautomer, mesomer,
racemate, enantiomer, diastereomer, or mixture thereof, or a pharmaceutically
acceptable salt,
solvate or prodrug thereof, comprising a step of:
R4a R4a
R1 0 0 Ri 0
Ok>CN HN
R3b R3b
R6 R6
R5a 0 R5a
R6 R6
(VC) (V)
reacting a compound of formula (VC) or a tautomer, mesomer, racemate,
enantiomer,
diastereomer, or mixture thereof, or a salt thereof with ammonium carbonate
(or ammonium
bicarbonate) and sodium cyanide (or potassium cyanide) to obtain the compound
of formula
(V) or a tautomer, mesomer, racemate, enantiomer, diastereomer, or mixture
thereof, or a
pharmaceutically acceptable salt, solvate or prodrug thereof;
wherein:
R4a, R5a, R31
,
tc and R6 are each as defined in formula (V).
The agent which provides the alkaline condition includes organic bases and
inorganic
bases, wherein the organic base includes, but is not limited to,
triethylamine, N,N-
disopropylethylamine, n-butyllithium, lithium diisopropylamide, potassium
acetate, sodium
tert-butwdde and potassium tert-butoxide, and wherein the inorganic base
includes, but is not
limited to, magnesium chloride, sodium hydride, potassium phosphate, sodium
carbonate,
potassium carbonate, cesium carbonate and
N-(3 -Dimethylaminoprop y1)-N'-
ethylcarbodiimide hydrochloride (EDCI).
The condensing agent includes, but is not limited to, 1-(3-
dimethylaminopropy1)-3-
ethylcarbodiimide hydrochloride, N,N'-dicyclohexylcarbodiimide,
diisopropylcarbodiimide, 0-benzotriazole-N,N,N',N'-tetramethyluronium
tetrafluoroborate, 1-
hydroxyb enzo triazole, 1 -hydro xy-7 - azobenzo triazo le, 0-
benzo triazo le-N,N,N',N'-
tetramethyluronium hexafluoro phosphate,
2 - (7-o xobenzo triazo le)-N,N,N',N'-
tetramethyluroniu m hexafluorophosphate, 2 -
(7- azobenzotriazole)-N,N,N',N'-
tetramethyluronium hexafluorophosphate,
benzotriazol- 1 -
ylo xytri s(dimethylamino)pho sphonium hexafluorophosphate
and benzo triazol- 1 -yl-
oxytripyrrolidinylphosphonium phosphate.
72
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
The reaction is preferably conducted in a solvent, wherein solvent used herein
includes,
but is not limited to, acetic acid, methanol, ethanol, toluene,
tetrahydrofuran, dichloromethane,
dimethylsulfoxide, 1,4-dioxane, water, N, N-dimethylformamide, and the
mixtures thereof.
The following examples serve to illustrate the disclosure, but the examples
should not be
considered as limiting the scope of the disclosure. If specific conditions for
the experimental
method are not specified in the examples of the present disclosure, they are
generally in
accordance with conventional conditions or recommended conditions of the raw
materials and
the product manufacturer. The reagents without a specific source indicated are
commercially available, conventional reagents.
The structures of the compounds were identified by nuclear magnetic resonance
(NMR)
and/or mass spectrometry (MS). NMR was determined by a Bruker AVANCE II (or
III)-
400MHz. The solvents are deuterated-dimethyl sulfoxide (DMSO-d6), deuterated-
chloroform
(CDC13) and deuterated-methanol (CD30D) with tetramethylsilane (TMS) as an
internal
standard. NMR chemical shifts (5) are given in 10-6 (ppm).
LC/MS (ESI) analyses were performed on a Shimadzu LCMS2020 equipped with a
Sunfire C18 (Sum 50x4.6mm) column, Waters UPLC-QDa equipped with an ACQUITY
UPLC 0 BEH (2.1*50mm 1.7um) column, Agilent Agilent6120 equipped with a
Xbridge C18
(Sum 50x4.6mm) column.
HPLC analyses were performed on an Agilent 1200DAD equipped with a Sunfire C18
(Sum 150x4.6mm) column and Shimadzu UFLC equipped with an Xbridge C18 (Sum
150x4.6mm) column.
Chiral HPLC analyses were performed on a Waters-UPC2 instrument.
The known raw materials of the present disclosure were prepared by the
conventional
synthesis methods in the art, or purchased from Aldrich Chemical Company,
Fisher Scientific
or Combi-Blocks, etc.
Unless otherwise stated, the reactions were carried out under nitrogen
atmosphere.
Unless otherwise stated, the reaction temperature in the reactions refers to
room
temperature, and the range of the temperature was 20 C to 30 C.
The reaction process was monitored by LC-MS or thin layer chromatography
(TLC), and
the developing solvent system includes: A: dichloromethane and methanol, B:
hexane and ethyl
acetate. The ratio of the volume of the solvent was adjusted according to the
polarity of the
compounds. The elution system for purification of the compounds by column
chromatography,
thin layer chromatography and CombiF/ash flash rapid preparation instrument
includes: A:
73
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
dichloromethane and methanol, B: hexane and ethyl acetate. The ratio of the
volume of the
solvent was adjusted according to the polarity of the compounds, and sometimes
a small
amount of basic reagent such as ammonia or acidic reagent such as acetic acid
was added.
Prep-HPLC was performed on Shimadzu (LC-20AD, SPD20A) Preparative HPLC
(Phenomenex Gemini-NX 5uM C18 21.2x100mm column), Waters 2767 equipped with a
Sunfire Pre C18 (10um 19x250mm) column and Waters 2767-QDa equipped with an
Xbridge
Pre C18 (10um 19x250mm) column instrument.
Pre-SFC was performed on a Waters-SFC80 equipped with Daciel AD/OD/al/IC/IA/ID
(10um 20x250mm) column instrument.
CombiFlash was performed on systems from Teledyne ISCO or Agela Technologies.
The following abbreviations are used:
AIBN is 2,2 -Azobis(2-methylpropionitrile),
DAST is (Diethylamino)sulfur trifluoride,
DIPEA (or DIEA) is N,N-diisopropylethylamine,
EDCI is N-(3-Dimethylaminopropy1)-N' -ethylcarbodiimide hydrochloride,
HOBt is 1-Hydroxybenzotriazole hydrate,
HATU is 0-
(7-Azabenzotriazol-1-y1)-N,N,N',N'-te-tramethyluronium
hexafluorophosphate,
HBTU is 0-(Benzotriazol-1-y1)-N,N,N ,N -tetramethyluronium
hexafluorophosphate,
HC1 is hydrogen chloride,
LDA is Lithium diisopropylamide,
LiHMDS is Lithium bis(trimethylsilyl)amide,
n-BuLi is n-Butyllithium,
NBS is N-Bromosuccinimide,
NCS is N-Chlorosuccinimide,
Pd(dppf)C12 is 111,1 -Bis(diphenylphosphino)ferrocene[dichloropalladium(II),
TEA is triethylamine,
DCE is 1,2-dichloroethane,
DCM is dichloromathane,
DMF is N,N-dimethylformamide,
Et0Ac (or EA) is ethyl acetate,
Et0H is ethanol,
MeCN or ACN is acetonitrile,
74
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Me0H is methanol,
n-BuOH is n-Butanol,
PE is petroleum ether,
THF is Tetrahydrofuran,
NMR is nuclear magnetic resonance,
MS is mass spectroscopy with (+) referring to the positive mode which
generally gives a
M+1 (or M+H) absorption where M = the molecular mass.
Prep HPLC is Prepative High performance liquid chromatography.
SFC is Supercritical fluid chromatography.
Intermediate 1 (Int-1)
(S)-3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-yl)propanoic acid Intermediate 1
(Int-1)
0 V 0
HN (S) OH
y-NH
It-1
0
0
I-1 r\._vNI-j( 0
0
Step 1 Step 2 Step 3
___________________________________ ,v)Hr0 ___
v7L0 0
0
It-1-1 It-1-2 It-1-3
0 0
H N 0 Step 4 H N "AN0
0V\).... 0 H 0 0 H
It-1-4 It-1
Step 1
Tert-butyl 4-cyclopropy1-4-oxobutanoate Int-1-2
The solution of LDA (15.28 g, 142.66 mmol, 71.43 mL) in THF (50 mL) was cooled
to -78 C before the solution of cyclopropyl methyl ketone Int-1-1 (10 g,
118.88 mmol) in THF
(10 mL) was added dropwise. The resulting solution was warmed to 20 C and
stirred for 30
min. The reaction mixture was then re-cooled to -78 C and tert-butyl 2-
bromoacetate (23.19 g,
118.88 mmol) in THF (10 mL) was added slowly. The reaction was stirred at room
temperature
overnight. After the reaction completed, the reaction was quenched with
saturated NH4C1 (50
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
mL, aq.), the mixture was extracted with Et0Ac (50 mL X 3), the organic phase
was washed
with brine (100 mL), dried over Na2SO4 and concentrated to give the crude
tittle compound
Int-1-2 (22 g, 110.97 mmol, 93.34% yield).
1H NMR (400 MHz, CDC13): 6 2.83 (t, 2H), 2.50 (t, 2H), 1.97-1.92 (m, 1H), 1.45
(s,
9H), 1.06-1.01 (m, 2H), 0.91-0.86 (m, 2H).
Step 2
Tert-butyl 3 -(4 -cyc loprop y1-2,5 -dio xo imidazolidin-4- yl)prop ano ate
Int-1-3
The mixture of Int-1-2 (8.2 g, 41.36 mmol), ammonium carbonate (33.78 g,
351.56
mmol), sodium cyanide (5.07 g, 103.40 mmol), Et0H (50 mL) and water (50 mL)
was sealed
and heated to 80 C for 18h. The reaction mixture was cooled and poured into a
mixture of
Et0Ac (100 mL) and water (100 mL), the layers were separated, and the aq.
layer was extracted
with Et0Ac (100 mL X 3). The organic solution was combined and washed with
brine, dried
over Na2SO4 and concentrated. The residue was purified by silica gel
chromatagraphy
(Et0Ac/hexane =1/2) to give the tittle compound Int-1-3 (5.7 g, 21.24 mmol,
51.36% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.61 (s, 1H), 7.66 (s, 1H), 2.29-2.08 (m, 2H),
1.93-1.88 (m, 2H), 1.29 (s, 9H), 1.09-1.02 (m, 1H), 0.47-0.26 (m, 3H), 0.11-
0.04 (m, 1H).
Steps 3 and 4
(S)-3 -(4-c yc loprop y1-2,5 -dioxoimid azolidin-4- yl)prop anoic acid It-1
The solution of Int-1-3 (7.2 g, 26.83 mmol) in HO/Dioxane (4M, 50 mL) was
stirred
at room temperature for 4h and concentrated. The resulting solid was
triturated in MeCN
(30mL) for lh and filtrated to give pure racemic target as a white solid. The
solid was chiral
separated by SFC (using a chiral column CHIRALPAK AD-H 10um 2.5 *25 cm; Flow
Rate/detection: 70 g/min; Detector Wavelength: 214 nm; Mobile phase A:
Supercritical CO2,
Mobile phase B: methanol) to give the tittle compound It-1 (2 g, 9.42 mmol,
35.12% yield).
1H NMR (400 MHz, DMSO-d6): 6 12.20 (s, 1H), 10.63 (s, 1H), 7.71 (s, 1H), 2.32-
2.09 (m, 2H), 1.99-1.87 (m, 2H), 1.11-1.03 (m, 1H), 0.48-0.27 (m, 3H), 0.12-
0.05 (m, 1H).
Chiral HPLC: 98.04% cc, Rt: 2.918 mm.
LCMS: MS m/z (ES!): 213.1 1M+11
Intermediate 2 (Int-2)
3 -(4-c ycloprop y1-2 ,5 -dioxoimidazo lidin-4 - y1)-2-methylprop ano ic acid
Int-2
76
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
OH
HN NH
Int-2
0
0 0 0
Step 1 0 Step 2
0<
0
0
Int-2-1 Int-2-2 Int-2-3
O
Step 3 0_Y(*-Lo Step 4 OH
HN NH HN NH
0 0
Int-2-4 Int-2
Step 1
1-(tert-butyl) 4-ethyl 3-(cyclopropanecarbony1)-2-methylsuccinate Int-2-2
To a mixture of ethyl 3-cyclopropy1-3-oxopropanoate Int-2-1 (32 g, 204.89
mmol) in butan-2-
one (400 mL) was added tert-butyl 2-bromopropanoate (44.54 g, 213.04 mmol),
K2CO3 (57 g,
409.79 mmol) and NaI (3.07 g, 20.49 mmol). The reaction mixture was heated at
100 C for
16h and cooled to RT. Water was added and the reaction mixture acidified to pH
8. The the
mixture was extracted with Et0Ac. The combined organic layers were washed with
water and
brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuum. The
residue was
purified by silica gel chromatagraphy (hexane) to give Int-2-2 (11.7 g, 41.15
mmol, 20.08%
yield).
11INMR (400 MHz, CDC13): 6 4.25-4.21 (m, 2H), 3.92 (dd, 1H), 3.19-3.09 (m,
1H), 2.17-2.07
(m, 1H), 1.43 (d, 9H), 1.30-1.24 (m, 3H), 1.19-1.13 (m, 3H), 1.11-1.06 (m,
2H), 0.98-0.91 (m,
2H).
Step 2
tert-butyl 4-cyclopropy1-2-methyl-4-oxo-butanoate Int-2-3
To a solution of the Int-2-2 (4 g, 14.07 mmol) in THF (50 mL) was added LiOH
(1.68 g, 70.34
mmol) and H20 (50 mL). The reaction mixture was stirred at room temperature
overnight. To
the mixture was added aq. Citric acid solution to adjust to pH<5 and then
stirred at 40-50 C for
10 min. After the reaction was completed, water was added and the reaction
mixture was
extracted with Et0Ac. The combined organic layers were washed with water and
brine, dried
77
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was
purified by silica
gel chromatagraphy to give Int-2-3 (1.81 g, 8.55 mmol, 60.74% yield).
11INMR (400 MHz, CDC13): 6 2.97 (dd, 1H), 2.89-2.79 (m, 1H), 2.56 (dd, 1H),
1.96-1.88 (m,
1H), 1.43 (s, 9H), 1.15 (d, 3H), 1.05-1.00 (m, 2H), 0.90-0.84 (m, 2H).
Step 3
Tert-butyl 3- 4 -cyc loprop y1-2 ,5 -dio xo -imidazolidin-4- y11-2-methyl-prop
ano ate Int-2-4
To a solution of the Int-2-3 (1.89 g, 8.91 mmol) in Et0H (60 mL) was added
(NH4)2CO3 (6.86
g, 71.36 mmol), H20 (60 mL) and NaCN (1.09 g, 22.30 mmol). The reaction
mixture was
stirred at 80 C in a seal tube overnight and cooled to rt. Water was added and
reaction mixture
extracted with Et0Ac. The combined organic layers were washed with water and
brine, dried
over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was
triturated with
Et0Ac/hexane (1/5), filtered and dried to give Int-2-4 (1.23 g, 4.34 mmol,
48.71% yield).
11INMR (400 MHz, DMSO-d6): 6 10.54 (brs, 0.5H) 10.52(brs, 0.5H), 7.64 (brs,
0.5H), 7.52
(brs, 0.5H), 2.41-2.06 (m, 2H), 1.70 (dd, 0.5H), 1.56 (dd, 0.5H), 1.37 (d,
9H), 1.07 (d, 1.5H),
1.08-1.01 (m, 1H), 1.02 (d, 1.5H), 0.48-0.29 (m, 3H), 0.14-0.05 (m, 1H).
Step 4
3 -(4-c ycloprop y1-2 ,5 -dioxoimidazo lidin-4 - y1)-2-methylprop ano ic acid
Int-2
The solution of Int-2-4 (100 mg, 354.19 umol) in HC1/Dioxane (2N, 4 mL) was
stirred at room
temperature overnight. The mixture was concentrated in vacuum to give Int-2
(100 mg). The
product was used directly in the next step without further purification.
Intermediate 2A (Int-2A) and Intermediate 2B (Int-2B)
3 -((S)-4-c yc loprop y1-2,5-dioxoimid azo lidin-4 -y1)-2 -methylprop ano ic
acid Int-2A
3 -((R)-4-c ycloprop y1-2,5 -dioxoimidazolidin-4- y1)-2-methylprop anoic acid
Int-2B
V 0
OL 0
OH OH
HN 1,(NH HN NH
0 Int-2A I nt-2B
0
Int-2-4 (1.22g) was separated by silica gel chromagraphy (hexane:Et0Ac=20:1)
to give
diastereoisomers 1 (360 mg) and diastereoisomers 2 (350 mg). Diastereoisomers
1 were
converted to Int-2A using similar procdure as Step 4 for Int-2.
Diastereoisomers 2 were
converted to Int-2B using similar procdure as Step 4 for Int-2.
Int-2A
78
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
11INMR (400 MHz, DMSO-d6): 6 12.15 (brs, 1H), 10.62 (s, 1H), 7.64 (s, 1H),
2.33-2.27 (m,
1H), 2.19-2.13 (m, 1H), 1.77-1.71 (m, 1H), 1.05 (d, 4H), 0.45-0.26 (m, 3H),
0.09-0.04 (m, 1H).
LCMS: MS nth (ES!): 227.2 1M+Hr.
Int-2B
1HNMR (400 MHz, DMSO-d6): 6 12.16 (brs, 1H), 10.57 (s, 1H), 7.68 (s, 1H), 2.40-
2.35 (m,
1H), 2.30-2.24 (m, 1H), 1.60 (dd, 1H), 1.12- 1.05(m, 1H), 1.11 (d, 3H), 0.43-
0.30 (m, 3H),
0.13-0.07 (m, 1H).
LCMS: MS nth (ES!): 227.2 1M+Hr.
EXAMPLES
Example 1
(S)-5 -c ycloprop y1-5 -(3 -o xo -3-(5 -(triflu oromethyl) iso indolin-2 -
yl)prop yl) imidazo lidine-2 ,4-
dione 1
0 V
HNN
)r-NH 1 CF3
0
HN
OH
CF HN.niNH oA HN)r.NH
C F3
0 0
1-1 It-1 1
To a mixture of 5-(trifluoromethyl)isoindoline 1-1 (150 mg, 801.45 umol) in
DMF (10
mL) was added triethylamine (324 mg, 3.21 mmol), Int-1 (170mg, 801.45 umol)
and HATU
(365 mg, 961.74 umol). The reaction was stirred at r.t for 18h. Water (20 mL)
was added and
the mixture was extracted with Et0Ac (20 mL X 2). The combined layer was
washed with
brine, dried over Na2SO4 and concentrated. The residue was purified with Prep-
HPLC to give
title compound example 1 (80 mg, 209.78 umol, 26.18% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.77-7.74 (m, 2H), 7.66 (d, 1H),
7.58 (d, 1H), 4.89-4.69 (m, 4H), 2.48-2.38 (m, 1H), 2.33-2.24 (m, 1H), 2.04-
1.99 (m, 2H),
1.16-1.08 (m, 1H), 0.50-0.46 (m, 1H), 0.43-0.29 (m, 2H), 0.15-0.08 (m, 1H).
LCMS: MS nth (ES!): 382.1 1M+Hr.
Example 2
(S)-5 -(3 -(5 -chloro isoindolin-2- y1)-3 -oxoprop y1)-5 -cyc loprop ylimidazo
lidine-2,4-dione 2
79
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0oN
HN NH
)T
0 2 II CI
NH HCI 0)L
OH
HN),r-NH
CI HN/I\IH
11 .410. ci
0 0
2-1 It-1 2
To a solution of 5-chloroisoindoline hydrochloride 2-1 (100 mg, 526.12 umol)
in DMF
(3 mL) was added TEA (0.22 mL), It-1 (134 mg, 631.35 umol) and HATU (240 mg,
631.35
umol). The mixture was stirred at room temperature overnight. LCMS showed
starting material
was completely reacted. Water (20 mL) was added and the mixture extracted with
Et0Ac (15
mL X 2). The combined organic layers are washed with water (30 mL) and brine
(30 mL X 2),
dried over anhydrous Na2SO4, filtered and concentrated in vacuum. The crude
was purified by
prep-HPLC to give title compound 2 (70 mg, 38.03%).
11INMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.73 (s, 1H), 7.45(s, 1H), 7.39-
7.33
(m, 2H), 4.77(d, 2H), 4.61 (d, 2H), 2.43-2.33 (m, 1H), 2.31-2.22 (m, 1H), 2.00
(t, 2H), 1.15-
1.07 (m, 1H), 0.49-0.45 (m, 1H), 0.40-0.29 (m, 2H), 0.13-0.08 (m, 1H).
LCMS: MS nth (ES!): 348.1 1M+Hr.
Example 3
(S)-2-(3 - (4-cycloprop y1-2,5 -dioxoimidazolidin-4- yl)prop ano yl) isoindo
line-5 -carbonitrile 3
0 \7 0
0 3 ON
V NH + 0 V 0
1
0(
OH ___
(s) 10 hilpTANH HN
NC NH
= ON
0 0
3-1 It-1 3
To a mixture of isoindoline-5-carbonitrile 3-1 (50 mg, 346.81 umol) in DMF (10
mL)
was added Triethylamine (140 mg, 1.39 mmol), It-1 (88 mg, 416.17 umol) and
HATU (158
mg, 416.17 umol). The reaction was stirred at r.t for 18h. Water (20 mL) was
added and the
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
mixture was extracted with Et0Ac (20 mL X 2). The combined layer was washed
with brine,
dried over Na2SO4 and concentrated. The residue was purified with prep-HPLC to
give title
compound 3 (25 mg, 73.89 umol, 21.30% yield).
111 NMR (400 MHz, CDC13): 6 7.97-7.92 (m, 1H), 7.65-7.57 (m, 2H), 7.45-7.38
(m,
1H), 6.15 (brs, 1H), 4.84 (brs, 4H), 2.58-2.30 (m, 4H), 1.25-1.18 (m, 1H),
0.64-0.57 (m, 1H),
0.50-0.44 (m, 1H), 0.42-0.32 (m, 2H).
LCMS: MS raiz (ES!): 339.1 [M+Hr.
Example 4
(S)-5 -c yc loprop y1-5 -(3 -oxo-3 -(5 -(trifluorometho xy)isoindo lin-2-
yl)propyl)imidazolidine-
2,4-dione 4
0
HN)rNH
0 4 OCF3
0 OH 0 OS
0)-N= 11
step 1
4-1 4-2
0
,=-"\AOH
0
.NH 0 V
Int-1 o N HN ,* 0
F
________________ HN )7-NH
step 2 step 3 0
4-3 4
Step 1
tert-butyl 5 - (((methylthio)c arbonothio yl)o xy) isoindoline-2-c arboxyl ate
4-2
To a solution of tert-butyl 5-hydroxyisoindoline-2-carboxylate 4-1 (1.5 g,
6.38 mmol)
in DMF (20 ml) was added sodium hydride (0.4 g, 9.57 mmol), at 0 C. After
stirring for 30
minutes, carbon disulfide (0.5 ml) was added. It was stirred for one hour, and
methyl iodide
(0.6 ml, 8.30 mmol) was added. The reaction mixture was stirred at ambient
temperature for
14 hours. It was quenched with ice water and extracted with ethyl acetate. The
organic layer
was washed with water, dried, and concentrated to get 4-2 as a pale solid
(1.95 g, 93.8 % yield).
81
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
1H NMR (400 MHz, CDC13): 7.24 (d, 1 H), 7.18 (s, 1 H), 6.95 9d, 1 H), 4.61
(dd, 4
H), 2.60 (s, 3 H), 1.44 (s, 9 H).
LCMS: MS mh (ES!): 326 [M+Hr.
Step 2
5 -(trifluorometho xy) isoindoline 4-3
To a solution of 4-2 in pyridine-hydrogen fluoride complex (15 ml) was added
1,3-
dibromo-5,5-dimethylimidazolidine-2,4-dione (5.5 g, 19.2 mmol), at 0 C. The
reaction
mixture was stirred at ambient temperature for 14 hours. It was quenched with
ice water and
extracted with ethyl acetate. The organic layer was washed with water, dried,
and concentrated.
.. The residue was purified on a silica gel column, eluting with ethyl acetate
in hexanes, to get 4-
3, as a white solid (910 mg).
LCMS: MS mh (ES!): 204 [M+Hr.
Step 3
(S )-5-c yc loprop y1-5 -(3 -oxo-3 - (5- (trifluorometho xy) isoindo lin-2-
yl)prop yl) imid azolidine-
2,4-dione 4
A solution of Int-1 (10 mg. 0.047 mmol), EDCI (14 mg, 0.071 mmol) and HATU (27
mg, 0.071 mmol) in DMF (1.5 ml) was stirred at ambient temperature for 20
minutes, before
4-3 (10 mg, 0.047 mmol) was added. The reaction mixture was stirred at ambient
temperature
for 2 hours. It was directly loaded onto and purified on a reverse phase HPLC.
The appropriate
fraction was lyophilized to afford example 4.
1H NMR (400 MHz, CD30D): 8.00-7.78 (m, 3 H) 4.87 (m, 2 H), 4.68 (m, 2 H), 3.70
(m, 1 H), 3.10 (m, 1 H), 2.26 (m, 2 H), 1.14 (m, 1 H), 0.49 (m, 1 H), 0.32 (m,
2 H), 0.25 (m, 1
H).
LCMS: MS mh (ES!): 398 [M+Hr.
Example 5
(S)-5 -c yc loprop y1-5 - (3 -(7-methoxy-4,5-dihydro-1H-benzo [d] azep in-3
(2H)- y1)-3 -
oxoprop yllimidazolidine-2 ,4-dione 5
0 V 0
HN)4fr)L
e NH 400 5 0/
0
82
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0
HN (:) 0
HN)H)LN
HCI )1.- NH 41 01
OH 0
5-1 It-1 5
A solution of It-1 (10 mg. 0.047 mmol), EDCI (14 mg, 0.071 mmol) and HATU (27
mg, 0.071 mmol) in DMF (1.5 ml) was stirred at ambient temperature for 20
minutes, before
7 -metho xy-2 ,3,4,5-tetrahydro-1H-benzo kllazepine hydrochloride 5-1 (10 mg,
0.047 mmol)
was added. The reaction mixture was stirred at ambient temperature for 2
hours. It was directly
loaded onto and purified on a reverse phase HPLC. The appropriate fraction was
lyophilized
to afford 5.
1H NMR (400 MHz, CD30D): 7.06 (dd, 1 H), 6.74 (m, 1 H), 6.70 (dt, 1 H), 3.77
(d, 3
H), 3.72-3.59 (m, 4 H), 2.96-2.84 (m, 4 H), 2.54 (m, 1 H), 2.42 (m, 1 H), 2.11
(m, 2 H), 1.21
(m, 1 H), 0.56 (m, 1 H), 0.44-0.30 (m, 3 H).
LCMS: MS rniz (ES!): 372 [M+Hr.
Example 6
(S)-5 -c ycloprop y1-5 -(3 -oxo-3 - (6-(triflu oromethyl)- 3 ,4-dihydroiso qu
ino lin-2 (1 H)-
yl)propyl)imidazolidine-2,4-dione 6
NH
CF3
0 6
0 V 0
0 t>, 1101
HN = HN"..:,,&\..4 e NH
CF3 + HN N CF
0
OH 0
6-1 It-1 6
A solution of It-1 (10 mg. 0.047 mmol), EDCI (14mg, 0.071 mmol) and HATU (27
mg, 0.071 mmol) in DMF (1.5 ml) was stirred at ambient temperature for 20
minutes, before
6-(trifluoromethyl)-1,2,3,4-tetrahydroisoquinoline 6-1 (10 mg, 0.047 mmol) was
added. The
reaction mixture was stirred at ambient temperature for 2 hours. It was
directly loaded onto and
purified on a reverse phase HPLC. The appropriate fraction was lyophilized to
afford 6.
1H NMR (400 MHz, CD30D): 8.15-7.50 (m, 3 H), 4.10 (m, 1 H), 3.55-3.85 (m, 4
H), 3.00 (m,
2 H), 2.50-2.10 (m, 3 H), 1.21 (m, 1 H), 0.60 (m, 1 H), 0.49-0.30 (m, 3 H).
LCMS: MS rniz (ES!): 396 [M+Hr.
83
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Example 7
(S)-5 -c ycloprop y1-5 -(3 -oxo-3 - (7 -(triflu oromethyl)- 3 ,4-dihydroiso qu
ino lin-2 (1 H)-
yl)pr op yl)imid azolidine -2 ,4 -dione 7
0
)
HN )LN
C F3 LT
NH
0 7
0
0 N,
HN cF3 HN CF
eNH
OH 0
7-1 It-1 7
A solution of It-1 (10 mg. 0.047 mmol), EDCI (14 mg, 0.071 mmol) and HATU (27
mg, 0.071 mmol) in DMF (1.5 ml) was stirred at ambient temperature for 20
minutes, before
7-(trifluoromethyl)-1,2,3,4-tetrahydroisoquinoline 7-1 (10 mg, 0.047 mmol) was
added. The
reaction mixture was stirred at ambient temperature for 2 hours. It was
directly loaded onto and
purified on a reverse phase HPLC. The appropriate fraction was lyophilized to
afford 7, as a
white solid.
11-1 NMR (400 MHz, CD30D): 8.10-7.60 (m, 3 H), 4.12 (m, 1 H), 3.55-3.85 (m, 4
H), 3.00 (m,
2 H), 2.50-2.10 (m, 3 H), 1.21 (m, 1 H), 0.60 (m, 1 H), 0.49-0.30 (m, 3 H).
LCMS: MS mh (ES!): 396 [M+Hr.
Example 8
(5 S)-5 -(3 -(8-chloro-1-methy1-4,5-dihydro-1H-benzo [di azep in-3 (2H)- y1)-3
-oxoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 8
0 o
HN N
eNH
0 8
CI
0 V 0
ip 01 0
NH =HN
o."-N1H 0
OH
CI
8-1 It-1 8
84
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
A solution of It-1 (10 mg. 0.047 mmol), EDCI (14 mg, 0.071 mmol) and HATU (27
mg, 0.071 mmol) in DMF (1.5 ml) was stirred at ambient temperature for 20
minutes, before
8-chloro-1-methy1-2,3,4,5-tetrahydro-1H-benzoldlazepine 8-1 (12 mg, 0.047
mmol) was
added. The reaction mixture was stirred at ambient temperature for 2 hours. It
was directly
loaded onto and purified on a reverse phase HPLC. The appropriate fraction was
lyophilized
to afford 8 (mixture of two diastereomers).
LCMS: MS nth (ES!): 390 [M+Hr.
Example 9
(S)- 5 -c yc loprop y1-5 - (3 - (4,5 -dihydro -1H-benzo [d] azep in-3 (21/)-
y1)-3 -
oxoprop yl)imidazolidine-2 ,4-dione 9
V
O
HN NH
0 9
0 0 0
HN HN
(s)
+ (s) OH ________
HN zNH
9-1 Int-1 0 9
To a solution of 2,3,4,5-tetrahydro-1H-benzoldlazepine 9-1 (100 mg, 679.27
umol) in
DMF (4 mL) was added Triethylamine (275 mg, 2.72 mmol, 377.67 uL), It-1 (173
mg, 815.13
umol) and HATU (310 mg, 815.13 umol). The mixture was stirred at room
temperature
overnight. LCMS showed starting material was completely reacted. Water (25 mL)
was added
and the reaction mixture extracted with Et0Ac (20 mL X2). The combined organic
layers are
washed with water (40 mL) and brine (40 mL X 2), dried over anhydrous Na2SO4,
filtered and
concentrated in vacuum. The crude was purified by prep-HPLC to give title
compound 9 (11
mg, 4.74%).
1H NMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.72 (s, 1H), 7.15-7.13 (m, 4H),
3.58-3.51 (m, 4H), 2.91-2.90 (m, 2H), 2.82-2.81 (m, 2H), 2.45-2.35 (m, 1H),
2.30-2.25 (m,
1H), M.94 (m, 2H), 1.12-1.08 (m, 1H), 0.48-0.46 (m, 1H), 0.40-0.29 (m, 2H),
0.10-0.08 (m,
1H).
LCMS: MS nth (ES!): 342.2 1M+Hr.
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Example 20
(S)-5 -c ycloprop y1-5 - (3- (5 ,6-dichloroiso indo lin-2 -y1)-3 -o xoprop yl)
imidazolidine-2 ,4-dione
(:) V 0
H 1\1
y NH CI
0
20 CI
Step 1 Step 2 CI Int-1
0 NH ___________ NH Ste HNN
CI CI CI )r-NH CI
p 3
0 0 20
0
20a 20b 20c
5 ci
Step 1
5,6 -dichloro isoindoline- 1 ,3 -dione 20b
The mixture of 5,6-dichloroisobenzofuran-1,3-dione 20a (5 g, 23.04 mmol) in
formamide
(1.04 g, 23.04 mmol) was stirred at 200 C for 2 h. The mixture was poured into
ice water
10 (100 mL), the mixture was filtered and the solid was dried to afford the
tittle compound 20b
(4.5 g, 20.83 mmol, 90.41% yield).
111 NMR (400 MHz, DMSO-d6): 6 11.62 (s, 1H), 8.12 (s, 2H).
Step 2
5 ,6-dichloroisoindoline 20c
15 To a solution of 20b (1 g, 4.63 mmol) in THF (5 mL) was added BH3 (1 M
in THF, 46.29 mL).
The resulting mixture was stirred at 80 C for 16 h. Con. HC1 (10 mL) was added
to quench the
reaction. Then aq NaOH was added to adjust the mixture to pH-13, the aqueous
phase was
extracted with Et0Ac (80 mL X 3), the combined organic phases were washed with
brine (80
mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The
residue was
20 purified by silica gel chromatography (eluting with DCM/Me0H=10/1) to
afford the tittle
compound 20c (150 mg, 797.64 umol, 17.23% yield).
111 NMR (400 MHz, DMSO-d6): 6 7.55 (s, 2H), 4.06 (s, 4H).
Step 3
(S)-5-cycloprop y1-5 - (3 -(5 ,6 -dichloro isoindo lin-2- y1)-3 -o xoprop yl)
imid azolidine-2 ,4 -dio ne 20
To a solution of It-1 (140 mg, 659.75 umol) in DMF (7 mL) was added DIEA (111
mg,
857.67 umol). The resulting mixture was followed by the addition of 20c (149
mg, 791.70 umol)
and EDCI (116 mg, 857.67 umol). The resulting mixture was stirred at room
temperature for 2
86
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
h. The mixture was purified by prep-HPLC to afford the tittle compound 20 (109
mg, 283.66
umol, 43.00% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.74 (s, 1H), 7.67 (s, 2H), 4.77
(brs, 2H),
4.60 (brs, 2H), 2.50-2.37 (m, 1H), 2.29-2.24 (m, 1H), 2.02-1.97 (m, 2H), 1.12-
1.10 (m, 1H),
0.46-0.33 (m, 3H), 0.13-0.11 (m, 1H).
LCMS: MS miz (ES!): 382.011M+Hr.
Examples 29 and 30
(SS)-5 -cycloprop y1-5 -(2-methyl-3 - o xo -3- (5 -(trifluoro methyl) isoindo
lin-2-
yl)propyl)imidazolidine-2 ,4-dione 29
(5R)-5 -c ycloprop y1-5 -(2-methyl-3 - o xo-3 -(5 - (triflu oro methyl)
isoindo lin-2-
yl)prop yl) imidazolidine-2 ,4-dione 30
0 7 0 0HL0
HN CF3 HN),T,NH
C F3
0 29 0 30
To a solution of 5-(trifluoromethyl)isoindoline 1-1 (1.0 g, 4.47 mmol, HC1
salt) in DMF (30
mL) was added TEA (3.5 mL), Int-2 (1.21 g, 5.37 mmol) and HATU (2.04 g, 5.37
mmol). The
mixture was stirred at room temperature overnight. LCMS monitoring, the
starting material
was completely reacted. Water was added and the mixture was extracted with
Et0Ac. The
combined organic layer was washed with water and brine, dried over anhydrous
Na2SO4,
filtered and concentrated. The residue was purified by prep-HPLC to give two
sets of
diastereomers 29 (960 mg, 2.43 mmol, 56.56% yield) and 30 (300 mg, 0.76 mmol,
17.68%
yield).
Examples 29-1 and 29-2
(S)-5 -c ycloprop y1-5 -((S)-2-methyl-3 -oxo-3 -(5 - (trifluoromethyl) isoindo
lin-2-
yl)propyl)imidazolidine-2,4-dione 29-1
(S)-5 -cycloprop y1-5 -((R)-2-methyl-3 -oxo-3 -(5 - (trifluoromethyl) iso
indolin-2-
yl)propyl)imidazolidine-2,4-dione 29-2
87
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0
0 0 7
HNIN:NH
CF3 HN1rNH E
0 CF3
0
29-1 29-2
29 (200 mg) was separated by SFC (Daicel CHIRALPAK AS) to give two single
enantiomers
(67 mg and 75 mg).
Enantiomer (shorter retention time):
1HNMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.75 (d, 1H), 7.69-7.65 (m, 2H),
7.58 (t, 1H),
4.92 (d, 2H), 4.75-4.64 (m, 2H), 2.66 (br, 1H), 2.39-2.32 (m, 1H), 1.74-1.70
(m, 1H), 1.06 (d,
3H), 1.06-1.00 (m, 1H), 0.43-0.38 (m, 1H), 0.31-0.21 (m, 2H), 0.03-0.01 (m,
1H).
LCMS: MS m/z (ES!): 396.1 1M+Hr.
Chiral HPLC (CO2/Me0H/DEA 5%-40% 1.5m1/min AS,3um,3*100(Daicel)): cc: 100%,
Rt:
1.370 min.
Enantiomer (longer retention time):
11INMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.75 (d, 1H), 7.69-7.66 (m, 2H),
7.59 (t, 1H),
4.93 (d, 2H), 4.75-4.64 (m, 2H), 2.65 (br, 1H), 2.39-2.32 (m, 1H), 1.74-1.70
(m, 1H), 1.07 (d,
3H), 1.07-1.05 (m, 1H), 0.38 (br, 1H), 0.29-0.25 (m, 2H), 0.03-0.00 (m, 1H).
LCMS: MS m/z (ES!): 396.1 1M+Hr.
Chiral HPLC (CO2/Me0H/DEA 5%-40% 1.5m1/min AS,3um,3*100(Daicel)): cc: 96.62%,
Rt: 1.972 min.
Examples 30-1 and 30-2
(R)-5 -cyclopropy1-5-((R)-2-methy1-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione 30-1
(R)-5 -cyclopropy1-54(S)-2-methyl-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione 30-2
007 0
iõ),0
: N j N
HNNzNH HNN,NH
C F3 n cF3
0 0
30-1 30-2
30 (300 mg) was separated by SFC (Daicel CHIRALPAK IA) to give two single
enantiomers (70.6 mg and 71.6 mg).
88
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Enantiomer (shorter retention time):
11INMR (400 MHz, DMSO-d6): 6 10.50 (s, 1H), 7.75 (d, 1H), 7.70-7.65 (m, 2H),
7.59 (t, 1H),
4.98-4.86 (m, 2H), 4.72-4.59 (m, 2H), 2.81 (br, 1H), 2.41-2.34 (m, 1H), 1.69-
1.64 (m, 1H),
1.11 (d, 3H), 1.11-1.06 (m, 1H), 0.46-0.40 (m, 1H), 0.35-0.26 (m, 2H), 0.11-
0.06 (m, 1H).
19FNMR (376.5 MHz, DMSO-d6): 6 -60.72
LCMS: MS miz (ES!): 396.0 1M+Hr.
Chiral HPLC (CO2/Me0H/DEA 5%_40% 1.5m1/min IA, 3um 3.0*100(Daicel)): ee:
99.48%,
Rt: 3.580 min.
Enantiomer (longer retention time):
11INMR (400 MHz, DMSO-d6): 6 10.50 (s, 1H), 7.75 (d, 1H), 7.70-7.65 (m, 2H),
7.60-7.56
(m, 1H), 4.98-4.86 (m, 2H), 4.72-4.59 (m, 2H), 2.84-2.78 (m, 1H), 2.41-2.34
(m, 1H), 1.69-
1.64 (m, 1H), 1.11 (d, 3H), 1.11-1.04 (m, 1H), 0.45-0.40 (m, 1H), 0.36-0.27
(m, 2H), 0.12-0.09
(m, 1H).
19FNMR (376.5 MHz, DMSO-d6): 6 -60.57
LCMS: MS m/z (ES!): 396.1 1M+Hr.
Chiral HPLC (CO2/Me0H/DEA 5%_40% 1.5m1/min IA, 3um 3.0*100(Daicel)): ee:
96.26%,
Rt: 4.368 min.
Examples 31-1 and 31-2
(5R)-5 -c ycloprop y1-5 -(2 -(5-(trifluoromethyl) iso indoline-2-c arbo nyl)bu
tyl)imidazolidine-2 ,4-
dione 31-1
(5S)-5 -c ycloprop y1-5 -(2-(5 -(triflu oro methyl) i so indoline-2-c arbo
nyl)b utyl)imidazolidine-2 ,4 -
dione 31-2
OL(:) N _________
N =HN2rNH = 0F3 0F3
31-1 31-2
0
0 0
Step 1 A Step 2 cy< Step 3
0 0 0
'11
Int-2-1 31b 31c
0
t=-0.< Step 4 "LOH Step 5 N H N
HN)./..- NH HN)./..- NH HN)r, NH ____ 110 CF3
CF3
31d 31e 31-1 31-2
Step 1
89
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
1-(tert-butyl) 4-ethyl 3-(cyclopropanecarbony1)-2-ethylsuccinate 31b
To a mixture of Int-2-1 (30 g, 192.09 mmol) and tert-butyl 2-bromobutanoate
(45.00 g, 201.69
mmol) in 2-butanone (400 mL) was added potassium carbonate (53.10 g, 384.18
mmol) and Sodium iodide (2.88 g, 19.21 mmol). The reaction was stirred at 100
C for 72h.
The mixture was filtered and the filtrate was diluted with water (500 mL); the
mixture was
extracted with Et0Ac (200 mL x 3), the combined organic solution was washed
with brine,
dried over Na2SO4 and concentrated. The residue was purified by silica gel
chromatography to
give 31b (55.6 g, 186.34 mmol, 97.01% yield).
Step 2
Tert-butyl 4-cyclopropy1-2-ethyl-4-oxobutanoate 31c
To a mixture of 31b (7.5 g, 25.14 mmol) in THF (80 mL) was added a solution of
Li0H.H20
(3.17 g, 75.41 mmol) in water (80 mL). The reaction was stirred at r.t for
18h. The reaction
mixture was diluted with Citric acid to pH =6 and then stirred at 50 C for
10min. The mixture
was diluted with water (100 mL) and the mixture was extracted with Et0Ac (100
mL x 3). The
combined organic layers were washed with brine, dried over Na2SO4 and
concentrated. The
residue was purified by silica gel chromatography (hexane:Et0Ac=20:1) to give
31c (5.1 g,
22.54 mmol, 89.65% yield).
Step 3
Tert-butyl 2-((4-cyclopropy1-2,5-dioxoimidazolidin-4-yl)methyl)butanoate 31d
To a mixture of 31c (5.1 g, 22.54 mmol) in Et0H (80 mL) was added to a
solution
of ammonium carbonate (17.32 g, 180.28 mmol) in water (80 mL). Then sodium
cyanide (2.76
g, 56.34 mmol) was added. The reaction was stirred at 80 C for 18h in the
sealed tube. The
reaction was cooled to r.t and the mixture was poured into a mixture of water
and Et0Ac, the
obtained mixture was extracted with Et0Ac (100 mL x 2). The organic layers
were washed
with brine, dried over Na2SO4 and concentrated. The residue was triturated
with hexane (50
mL) for lh and filtered; the solid was dried to give 31d (mixture, 1.5 g, 5.06
mmol, 22.46%
yield).
Step 4
24(4 -cyc loprop y1-2,5 -dioxoimidazolidin-4-y0methyl)butanoic acid 31e
The solution of 31d (1.5 g, 5.06 mmol) in HCl/Dioxane (4N, 20 mL) was stirred
at r.t for 18h.
The mixture was concentrated, the residue was triturated with hexane (50 mL)
and filtered, the
solid was dried to give 31e (1.4 g, 5.06 mmol, 99.96% yield).
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
11INMR (400 MHz, DMSO-d6): 6 12.13 (brs, 1H), 10.58, 10.53 (s, 1H), 7.64, 7.60
(s, 1H),
2.27-2.06 (m, 2H), 1.81-1.77 (m, 1H), 1.64-1.36 (m, 2H), 1.21-1.00 (m, 1H),
0.84-0.79 (m,
3H), 0.47-0.25 (m, 3H), 0.14-0.02 (m, 1H).
Step 5
(SR)-5 -cyc loprop y1-5 -(2-(5 -(triflu oro methyl)isoindo line-2 -carbonyl)b
utyl)imid azolidine-2,4 -
dione (diastereoisomers 1, Rt: 6.567 min) 31-1
(SS)-5 -cyc loprop y1-5 -(245 -(trifluoromethyl) isoindo line-2 -carbonyl)b
utyflimid azolidine-2,4-
dione (diastereoisomers 2, Rt: 7.063 min) 31-2
To a
solution of 31e (400 mg, 1.66 mmol) in DMF (15 mL) was added 5-
(trifluoromethyl)isoindoline 1-1 (372.31 mg, 1.66 mmol, HC1 salt) and
Triethylamine (673.88
mg, 6.66 mmol), then HATU (696.35 mg, 1.83 mmol) was added. The reaction was
stirred at
r.t for 4h. Water (100 mL) was added and the mixture was extracted with Et0Ac
(50 mL x 2),
the combined organic layers were washed with brine, dried over Na2SO4 and
concentrated. The
residue was purified by prep-HPLC to give 31-1 (12.5 mg) and 31-2 (55 mg).
31-1:
11INMR (400 MHz, DMSO-d6): 6 10.48 (s, 1H), 7.78-7.56 (m, 4H), 4.93-4.90 (m,
1H), 4.81-
4.50 (m, 3H), 2.69-2.67 (m, 1H), 2.40-2.32 (m, 1H), 1.78-1.65 (m, 1H), 1.59-
1.39 (m, 3H),
1.08-1.02 (m, 1H), 0.89-0.79 (m, 3H), 0.49-0.25 (m, 3H), 0.12-0.05 (m, 1H).
HPLC (SunFire C18 Sum 4.6*150mm 1.0ml/min 16min, 0.03% CH3CN/H20): Rt: 6.567
min.
LCMS: MS miz (ES!): 410.2 [M+Hr.
31-2:
11INMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.78-7.55(m, 4H), 4.92-4.90 (m,
1H), 4.78-
4.53 (m, 3H), 2.48-2.42 (m, 1H), 2.33-2.24 (m, 1H), 1.84-1.76 (m, 1H), 1.58-
1.37 (m, 2H),
1.08-1.00 (m, 1H), 0.89-0.83 (m, 3H), 0.45-0.22 (m, 3H), 0.05-0.00 (m, 1H).
HPLC (SunFire C18 Sum 4.6*150mm 1.0ml/min 16min, 0.03% CH3CN/H20): Rt: 7.063
min.
LCMS: MS miz (ES!): 410.1 [M+Hr.
Examples 32 and 33
(S)-5 -c ycloprop y1-5 -((S)-2 -(5-(trifluoromethyl) isoindoline-2-c
arbonyebutyflimid azo lidine-
2,4-dione 32
(S)-5 -cyc loprop y1-5 -((R)-2-(5 -(trifluoro methyl) isoindo line-2 -
carbonyflb utyflimid azolidine-
2,4-dione 33
91
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
_ = HN'N
)i-NH
CF3 yNNH N
CF3
0 0
32 33
31-2 (350 mg) was separated by SFC (Daicel CHIRALPAK IG, 250*25mm 10p,m) to
give
single enantiomers (120 mg and 100 mg).
Enantiomer (shorter retention time):
1HNMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.75 (d, 1H), 7.68 (d, 1H), 7.63-
7.55 (m,
2H), 4.96-4.85 (m, 2H), 4.79-4.66 (m, 2H), 2.48-2.43 (m, 1H), 2.32-2.25 (m,
1H), 1.83-1.78
(m, 1H), 1.56-1.37 (m, 2H), 1.08-1.00 (m, 1H), 0.86 (t, 3H), 0.44-0.40 (m,
1H), 0.32-0.22 (m,
2H), 0.03-0.00 (m, 1H).
LCMS: MS m/z (ES!): 410.1 [1\4+Hr
Chiral HPLC (CO2/Et0H/DEA 5%-40% 1.5ml/min IG,3um,3*100(Daicel)): cc: 100%,
Rt:
3.222 min.
Enantiomer (longer retention time):
11INMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.75 (d, 1H), 7.68 (d, 1H), 7.63-
7.55 (m, 2H),
4.96-4.85 (m, 2H), 4.79-4.66 (m, 2H), 2.48-2.43 (m, 1H), 2.33-2.25 (m, 1H),
1.84-1.78 (m,
1H), 1.57-1.38 (m, 2H), 1.08-1.00 (m, 1H), 0.86 (t, 3H), 0.44-0.39 (m, 1H),
0.29-0.22 (m, 1H),
0.04-0.00 (m, 1H).
LCMS: MS m/z (ES!): 410.1 [1\4+Hr
Chiral HPLC (CO2/Et0H/DEA 5%-40% 1.5ml/min IG,3um,3*100(Daicel)): cc: 99.40%,
Rt:
3.854 min.
Example 34-1
(5 R)-5 -c ycloprop y1-5 -(3 -(5 ,6-dichloroiso indolin-2 -y1)-2 -methy1-3 -
oxoprop yl) imidazolidine-
2,4 -dio ne
o
JL
(R)
HNrNH
CI
0
34-1 CI
92
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0
A 0
HN HN AR) OH 10
NH
(R)
C I
It-2B
HN)(NH
0
CI
0
CI
CI
20c 34-1
To a mixture of 5,6-dichloroisoindoline 20c (9 mg, 0.047 mmol) in DMF (2 mL)
was added
Triethylamine (17.22 mg, 0.132 mmol), Int-2B (10 mg, 0.044 mmol) and HATU
(21.8 mg,
0.0574 mmol). The reaction was stirred at r.t for 18h. Water (3 mL) added and
the mixture was
extracted with Et0Ac (20 mL X 2). The combined layer was washed with brine,
dried over
Na2SO4 and concentrated. The residue was purified with prep-HPLC to give title
compound
34-1 (5 mg, 0.0125 mmol, 26 % yield).
111 NMR (400 MHz, Methanol-d4): 6 7.53 (d, 2H), 4.70 (d, 4H), 2.58 (dd, 1H),
1.86 (dd, 2H),
1.25-1.14 (m, 3H), 0.62-0.50 (m, 1H), 0.48-0.36 (m, 2H), 0.40-0.25 (m, 2H).
LCMS: MS nth (ES!): 397 1M+H1 .
Example 34-2
(5S)-5 -c ycloprop y1-5 - (3 - (5 ,6-dichloroiso indolin-2 - y1)-2-methy1-3 -o
xoprop yl) imidazolidine-
2,4 -dione
(:)V icj
HN (s) N
= CI
0
34-2 CI
0 V 0
HO 0 V 0
HN HN).(NH
104 CI 0 Int-2A
CI
0
CI
20c 34-2 CI
To a mixture of 5,6-dichloroisoindoline 20c (72 mg, 0.38 mmol) in DMF (4 mL)
was added
Triethylamine (144 mg, 1.11 mmol), Int-2A (80 mg, 0.37mmo1) and HATU (171.8
mg, 0.45
mmol). The reaction was stirred at r.t for 18h. Water (5 mL) was added and the
mixture was
extracted with Et0Ac (40 mL X 3). The combined organic layer was washed with
brine, dried
93
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
over Na2SO4 and concentrated. The residue was purified with prep-HPLC to give
title
compound 34-2 (55 mg, 0.138 mmol, 37 % yield).
111 NMR (400 MHz, Methanol-d4): 6 7.53 (d, 2H), 4.73 (s, 4H), 2.72 (m, 1H),
2.08-1.94 (m,
2H), 1.31-1.16 (m, 3H), 0.53 (td, 1H), 0.49-0.34 (m, 2H), 0.34-0.24 (m, 2H).
LCMS: MS m/z (ES!): 396 [M+Hr.
Example 34-2-A and Example 34-2-B
(SS)-5 -cyclopropy1-5-((S)-(3-(5,6-dichloroisoindolin-2-y0-2-methyl-3-
oxopropy1))imidazolidine-2,4-dione 34-2-A
(SS)-5 -cyclopropy1-54(R)-(3-(5,6-dichloroisoindolin-2-y0-2-methyl-3-
oxopropy0)imidazolidine-2,4-dione 34-2-B
HN HNLN
yNH = CI ),r-NH z CI
0 0
34-2-A CI 34-2-B CI
34-2 (27 mg) was separated by chiral HPLC (column: CHIRALPAK IG-3) to give
title
compounds (9 mg and 10 mg).
Enantiomer (shorter retention time):
Chiral HPLC: (CHERALPAK IG-3, 100% Me0H): Rt= 4.119 mm.
LCMS: m/z (ES!): 394.2 [M-H1-.
Enantiomer (longer retention time):
Chiral HPLC: (CHERALPAK IG-3, 100% Me0H): Rt= 5.239 mm.
LCMS: m/z (ES!): 394.1 [M-H1-.
Example 35
(S)-5 -(3-(5-chloro-6-methoxyisoindolin-2-y1)-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-
dione
N is CI
HNyNH OMe
0
25
94
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
jtV
Me0JOJ Me0 Int-1 CI
NH i"Fsr"--N
Step 1 101 NH _____
CI CI step 2 HNr)7.¨NH OMe
0
35a 35b
Step 1
5 -chloro -6-metho xyisoindoline 35b
To a solution of 5-chloro-6-methoxyisoindolin- 1-one 35a (1 g, 5 mmol) in THF
(10 mL) was
5 added borane¨tetrahydrofuran (1M, 30 mL) dropwise under N2. The resulting
mixture was
stirred at 60 C for 24h. The reaction mixture was cooled to ambient
temperature and quenched
with Me0H (5 mL) until the bubbling ceased. Then 4N HC1 in water (5 mL) was
added and
the mixture was heated at 80 C for 3 h. After cooled down to RT, 5N KOH was
added to adjust
pH to 7. The mixture was concentrated under reduced pressure and the residue
was purified by
10 silica-gel column (DCM: Me0H(2%NH4OH) = 10: 1) to afford 5-chloro-6-
methoxyisoindoline 35b (300 mg, 60% yield).
Step 2
(S)-5-(3 - (5-chloro -6-metho xyisoindo lin-2- y1)-3 -o xoprop y1)-5 -c
ycloprop ylimid azolidine-2 ,4-
dione 35
15 To a mixture of 5-chloro-6-methoxyisoindoline 35b (10 mg, 0.0514 mmol)
in DMF (2 mL)
was added triethylamine (18 mg, 0.141 mmol), It-1 (10 mg, 0.047 mmol) and HATU
(22 mg,
0.0567 mmol). The reaction was stirred at r.t for 18h. Water (4 mL) added and
the mixture was
extracted with Et0Ac (20 mL X 2). The combined organic layers were washed with
brine,
dried over Na2SO4 and concentrated. The residue was purified with prep-HPLC to
give title
20 compound 35 (5 mg, 132.2 umol, 28 % yield).
111 NMR (400 MHz, Methanol-d4): 6 7.52 (s, 2H), 4.66 (t, 4H), 3.69 (d, 3H),
1.25 (m, 4H),
0.60 (m, 1H), 0.46 (m, 2H), 0.42-0.30 (m, 2H).
LCMS: MS nth (ES!): 378 [M+Hr.
25 Example 36-1
(5S)-5 -(3 - (5-chloro -6-metho xyisoindo lin-2- y1)-2-methy1-3 -o xoprop y1)-
5 -
c ycloprop ylimidazolidine-2 ,4-dione
0 0
ci
HN)r-NH OMe
0 36-1
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
To a mixture of 5-chloro-6-methoxyisoindoline 35b (10 mg, 546.44umo1) in DMF
(2 mL) was
added triethylamine (18 mg, 0.141 mmol), Int-2A (10 mg, 442.4 umol) and HATU
(21 mg,
564 umol). The reaction was stirred at r.t for 18h. Water (4 mL) was added and
the mixture
was extracted with Et0Ac (20 mL X 2). The combined organic layers were washed
with brine,
dried over Na2SO4 and concentrated. The residue was purified with prep-HPLC to
give the title
compound 36-1 (5 mg, 127 umol, 28 % yield).
111 NMR (400 MHz, Methanol-d4): 6 7.52 (s, 2H), 4.66 (t, 4H), 3.69 (d, 3H),
2.25 (m, 1H),
1.95 (m, 2H), 1.25 (m, 1H), 0.60 (m, 4H), 0.46 (m, 2H), 0.42-0.30 (m, 2H).
LCMS: MS nth (ES!): 392 [M+Hr.
Example 37
(S)-5 -cyc loprop y1-5 -(3 -(7,8 -dichloro -1 -methyl- 1 ,2,4,5 -tetrahydro-3H-
benzo [di azep in-3 - y1)-
3 -o xoprop yl) imid azolidine-2,4-dione 37
0 V 0
H CI
N
0 37 CI
ci
CI Step 1 CI CF3 Step 2 CI CF3
CI CF3
0 CI 0 0
8-1 37b 37c1 37c2
0 V
CI CF3 Step 3 CI Step 4
(s)
N¨µ NH
Int-1 HN
)i-NH CI
CI 0 CI 0
37c1 37d 37 CI
Step 1
1 -(8-chloro-1 -methyl- 1,2,4 ,5 -tetrahydro-3H-benzo [di azepin-3 -y1)-2 ,2,2-
trifluoroethan- 1 -o ne
37b
The solution of 8-chloro-l-methyl-2,3,4,5-tetrahydro-1H-benzo [di azep ine 8-1
(5 g, 21.54
mmol, HC1 salt) in DCM (100 mL) was cooled to 0 C before pyridine (3.41 g,
43.08
mmol) was added, then trifluoroacetic anhydride (5.43 g, 25.85 mmol) was
added. The reaction
was stirred at r.t for lh. The reaction solution was washed with HC1 (2N, aq.
150 mL), NaHCO3
(aq., 150 mL) and brine, dried over Na2SO4 and concentrated to give crude 37b
(6.2 g, 21.25
mmol, 98.69% yield).
96
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
11INMR (400 MHz, CDC13): 6 7.18-7.12 (m, 2H), 7.08-7.02 (m, 1H), 4.09-3.39 (m,
2H), 3.70-
3.25 (m, 2H), 3.20-2.89 (m, 3H), 1.38-1.31 (m, 3H).
Step 2
1-(7 ,8-dichloro-l-methy1-1 ,2 ,4,5 -tetrahydro -3H-b enzo ldl azep in-3 - y1)-
2,2,2 -triflu oro ethan-1 -
one 37c1
1 -(8,9-dichloro-1 -methyl-1 ,2 ,4,5 -tetrahydro -3H-b enzo ldl azep in-3 -
y1)-2,2,2 -triflu oro ethan-1 -
one 37c2
To a solution of 37b (1 g, 3.43 mmol) in DCE (20 mL) was added NCS (595.10 mg,
4.46
mmol) and trifluoromethanesulfonic Acid (5.15 g, 34.28 mmol). The reaction was
stirred at 90 C
for 72h. Then the mixture was diluted with DCM (100 mL) and washed with brine,
the organic
solution was dried over Na2SO4 and concentrated. The residue was purified with
prep-HPLC
to give 37c1 (270 mg) and 37c2 (210 mg).
37c1
11INMR (400 MHz, DMSO-d6): 6 7.48-7.42 (m, 2H), 3.81-3.74 (m, 2H), 3.59-3.41
(m, 2H),
3.33-3.30 (m, 1H), 3.12-3.01 (m, 2H), 1.27-1.20 (m, 3H).
37c2
11INMR (400 MHz, DMSO-d6): 6 7.46 (d, 1H), 7.22-7.19 (m, 1H), 4.05-3.47 (m,
5H), 3.30-
3.21 (m, 1H), 3.03-2.95 (m, 1H), 1.21-1.17 (m, 3H).
Step 3
7,8 -dichloro-1 -methyl-2 ,3 ,4,5 -tetrahydro -1H-benzo [di azep ine 37d
To a solution of 37c1 (100.00 mg, 306.62 umol) in Me0H (5 mL) was added a
solution
of NaOH (61.32 mg, 1.53 mmol) in water (5 mL). The reaction was stirred at r.t
for 2h. Water
(20 mL) was added and the mixture was extracted with EA (20 mL X 3), the
combined organic
layers were washed with brine, dried over Na2SO4 and concentrated to give
crude 37d (70 mg,
304.17 umol, 99.20% yield).
LCMS: MS m/z (ES!): 230.1 [M+Hr.
Step 4
(S)-5 -cyc loprop y1-5 -(3 -(7,8 -dichloro -1 -methyl-1 ,2,4,5 -tetrahydro-3H-
benzo [di azep in-3 - y1)-
3 -o xoprop yl) imid azolidine-2,4-dione 37
To a solution of 37d (70.00 mg, 304.17 umol) and It-1 (71.00 mg, 334.59 umol)
in DMF (10
mL) was added triethylamine (92.34 mg, 912.52 umol) and HATU (127.22 mg,
334.59 umol).
The reaction was stirred at r.t for 2h. The reaction mixture was diluted with
water (50 mL) and
the mixture was extracted with EA (50 mL X 2). The combined layers were washed
with brine,
97
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
dried over Na2SO4 and concentrated. The residue was purified by prep-HPLC to
give 37 (40
mg, 94.27 umol, 30.99% yield).
11INMR (400 MHz, DMSO-d6): 6 10.62, 10.61 (s, 1H), 7.76-7.70 (m, 1H), 7.46-
7.39 (m, 2H),
3.66-3.32 (m, 4H), 3.24-2.81 (m, 3H), 2.45-2.19 (m, 2H), 1.91-1.76 (m, 2H),
1.24-1.15 (m,
3H), 1.09-1.01 (m, 1H), 0.46-0.27 (m, 3H), 0.13-0.05 (m, 1H).
LCMS: MS m/z (ES!): 424.1 [M+Hr.
Examples 37-1 and 37-2
(S)-5 -cycloprop y1-5 -(3-((S)-7 ,8-dichloro-1 -methyl- 1,2,4,5 -tetrahydro-3H-
benzo [d] azep in-3 -
y1)-3-oxopropyl)imidazolidine-2,4-dione 37-1
(S)-5 -cyc loprop y1-5 -(3-((R)-7,8 -dichloro -1 -methyl- 1,2,4,5 -tetrahydro-
3H-benzo [d] azep in-3 -
y1)-3-oxopropyl)imidazolidine-2,4-dione 37-2
0 V
H
CI HNH CI
y N y
0 CI 0 CI
37-1 37-2
37 (55 mg) was chiral separated by SFC to give title compounds (15mg and
17mg).
Enantiomer (shorter retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.60 (s, 1H), 7.71 (d, 1H), 7.44 (d, 1H), 7.40
(d, 1H),
3.65-3.38 (m, 4H), 3.23-2.81 (m, 3H), 2.41-2.21 (m, 2H), 1.93-1.77 (m, 2H),
1.20 (dd, 3H),
1.10-1.01 (m, 1H), 0.47-0.26 (m, 3H), 0.13-0.04 (m, 1H).
LCMS: m/z (ES!): 424.1 [M+Hr.
ChirHPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:1.446
min,
ee:100%.
Enantiomer (longer retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.61 (br, 1H), 7.72 (d, 1H), 7.44 (d, 1H), 7.40
(d, 1H),
3.66-3.36 (m, 4H), 3.26-2.80 (m, 3H), 2.44-2.17 (m, 2H), 1.93-1.77 (m, 2H),
1.19 (dd, 3H),
1.09-1.02 (m, 1H), 0.48-0.27 (m, 3H), 0.12-0.05 (m, 1H).
LCMS: m/z (ES!): 424.1 [M+Hr
ChirHPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:2.547
min,
ee:99.38%.
Example 38
98
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -cyc loprop y1-5 -(3 -(8,9 -dichloro -1-methy1-1 ,2,4,5 -tetrahydro-3H-
benzo [di azep in-3 - y1)-
3-oxopropyl)imidazolidine-2,4-dione 38
CI
HN)r...NH CI
0 38
CI CI 0
CI
CF3 Step 1 CI Step 2 CI
(s)
_____________________________________________________ HN CI
0
0
3702 38b 38
Step 1
8,9 -dichloro-1 -methyl-2 ,3 ,4,5 -tetrahydro -1H-benzo [di azep ine 38b
To a solution of 37c2 (100 mg, 306.62 umol) in Me0H (5 mL) was added a
solution of NaOH
(61.32 mg, 1.53 mmol) in water (5 mL). The reaction was stirred at r.t for 2h.
Water (20 mL)
was added and the mixture was extracted with Et0Ac (20 mL X 3), the combined
organic
layers were washed with brine, dried over Na2SO4 and concentrated to give
crude 38b (70 mg,
304.17 umol, 99.20% yield).
LCMS: MS in/z (ES!): 230.1 [M+Hr.
Step 2
(S)-5 -cyc loprop y1-5 -(3 -(8,9 -dichloro -1-methy1-1 ,2,4,5 -tetrahydro-3H-
benzo [di azep in-3 - y1)-
3-oxopropyl)imidazolidine-2,4-dione 38
To a solution of 38b (70 mg, 304.17 umol) and It-1 (71.00 mg, 334.59 umol) in
DMF (10
mL) was added triethylamine (92.34 mg, 912.52 umol) and HATU (127.22 mg,
334.59 umol).
The reaction was stirred at r.t for 2h. The reaction was diluted with water
(50 mL) and the
mixture was extracted with Et0Ac (50 mL X 2). The combined organic layers were
washed
with brine, dried over Na2SO4 and concentrated. The residue was purified by
prep-HPLC to
give 38 (35 mg, 82.48 umol, 27.12% yield).
11INMR (400 MHz, DMSO-d6): 6 10.61, 10.58 (s, 1H), 7.74, 7.65 (s, 1H), 7.42
(d, 1H), 7.16
(t, 1H), 3.90-3.35 (m, 5H), 3.25-3.10 (m, 1H), 2.92-2.81 (m, 1H), 2.45-2.07
(m, 2H), 1.85-1.68
(m, 2H), 1.19-1.13 (m, 3H), 1.07-0.95 (m, 1H), 0.45-0.25 (m, 3H), 0.11-0.02
(m, 1H).
LCMS: MS nth (ES!): 424.1 [M+Hr.
Examples 38-1 and 38-2
99
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
(S)-5 -cycloprop y1-5 -(3 -((S)-8,9-dichloro-1 -methyl- 1,2 ,4,5 -tetrahydro-
3H-b enzo [di azepin-3 -
y1)-3-oxopropyflimidazolidine-2,4-dione 38-1
(S)-5 -cyc loprop y1-5 -(3-((R)-8,9 -dichloro -1 -methyl- 1,2,4,5 -tetrahydro-
3H-benzo lcll azep in-3 -
y1)-3-oxopropyl)imidazolidine-2,4-dione 38-2
0
0 =ss C
CI
N
HN)r-NH CI )r-NH CI
0 38-1 0 38-2
38 (53 mg) was chiral separated by SFC to give title compounds (14mg and
13mg).
Enantiomer (shorter retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.59 (d, 1H), 7.68 (d, 1H), 7.41 (d, 1H),7.17
(t, 1H), 3.90-
3.47 (m, 5H), 3.30-3.10 (m, 1H), 2.92-2.81 (m, 1H), 2.38-2.09 (m, 2H), 1.91-
1.66 (m, 2H),
1.16 (dd, 3H), 1.09-0.95 (m, 1H), 0.45-0.24 (m, 3H), 0.11-0.03 (m, 1H).
LCMS: m/z (ES!): 424.1 [M+Hr.
ChirHPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:1.076
mm,
ee:100%.
Enantiomer (longer retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.58 (brs, 1H), 7.68 (d, 1H), 7.41 (d, 1H),7.16
(t, 1H),
3.90-3.37 (m, 5H), 3.27-3.10 (m, 1H), 2.91-2.80 (m, 1H), 2.42-2.07 (m, 2H),
1.99-1.69 (m,
2H), 1.19-1.13 (dd, 3H), 1.09-0.98 (m, 1H), 0.46-0.24 (m, 3H), 0.12-0.02 (m,
1H).
LCMS: m/z (ES!): 424.1 [M+Hr.
ChirHPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:2.237
mm,
ee:99.70%.
Examlpe 40
(S)-5 -(3-(5 -chloro -6-(trifluoromethyl) iso indolin-2 - y1)-3 -oxoprop y1)-5
-
cyclopropylimidazolidine-2,4-dione 40
0 V
N
HNL
)r-NH 4100 CF3
0 40
CI
100
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0 0 0
H2N Step 1 H2N Step 2 H2N Step 3
OH __________________________________ OH __________________ c) ______
CF3 CF3 Br CF3 Br
40a 40b 40c
O 0 0
H2N Step 4 CI Step 5 CI Step 6
0 __________________________________ 0 __________________ 0 _______
Br
CF3 CF3 CF3
40d 40e 40f
0 V 0
0
CI
CI Step 7 Step 8
NH _____________________________________________________ HN'-"-Ted
NH CF3
CF3
CF3 0
40g 40h 40 CI
Step 1
5-amino-2-bromo-4-(trifluoromethyl)benzoic acid 40b
To a solution of 3-amino-4-(trifluoromethyl)benzoic acid 40a (1 g, 4.87 mmol)
in DMF (20
mL) was added NBS (870 mg, 4.89 mmol). The mixture was stirred at room
temperature for 2
hours, the resulting mixture was poured into ice water (20 mL) and the mixture
was extracted
with Et0Ac (20 mL X 2). The combined organic phase was washed with water (20
mL), brine
(20 mL), dried over Na2SO4(s) and filtered. The filtrate was concentrated to
afford crude 40b
(1 g, 3.52 mmol, 72.22% yield).
Step 2
Methyl 5-amino-2-bromo-4-(trifluoromethyl)benzoate 40c
To a solution of 40b (1 g, 3.52 mmol) in Me0H (10 mL) was added H2504 (18 M,
0.7 mL)
dropwise. After the mixture was stirred at 75 C overnight, the mixture was
cooled down to
room temperature and poured into ice water (20 mL), the mixture was extracted
with Et0Ac
(50 mL). The organic fraction was dried over Na2SO4(s) and filtered. The
filtrate was
concentrated to afford crude 40c (1 g, 3.36 mmol, 95.29% yield).
111 NMR (400 MHz, DMSO-d6): 6 7.57 (s, 1H), 7.21 (s, 1H), 6.11 (brs, 2H), 3.85
(s, 3H).
Step 3
Methyl 5 - amino -2-methy1-4- (trifluoromethyl)b enzo ate 40d
To a solution of 40c (1 g, 3.36 mmol) in DMF (10 mL) was added Pd(PPh3)4 (430
mg, 372.11
umol), K3PO4 (2.2 g, 10.36 mmol) and methylboronic acid (1 g, 16.71 mmol).
After the
mixture was stirred at 130 C under N2 atmosphere overnight, the mixture was
cooled down to
101
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
room temperature and filtered. The filtrate was concentrated, and the residue
was purified by
silica gel chromatography column to afford 40d (500 mg, 2.14 mmol, 63.91%
yield).
LCMS: MS m/z (ES!): 234.1 [M+Hr.
Step 4
Methyl 5 -chloro-2-methy1-4-(trifluoromethyl)benzo ate 40e
Concentrated HC1 (2 mL) was added to a solution of 40d (2.0 g, 8.58 mmol) in
acetone (20
mL), and the mixture was stirred at room temperature for 20 min. The mixture
was cooled to -
5-0 C, a solution of NaNO2 (600 mg, 8.70 mmol) in H20 (2.5 mL) was added
dropwise, and
the mixture was stirred at an ambient temperature for 30 min. CuCl (849.11 mg,
8.58 mmol)
was added portion-wise at 0 C, and the mixture was stirred at room temperature
for 2h. After
completion of the reaction, the mixture was poured into 1N HC1 (50 mL) and the
mixture was
extracted with Et0Ac. The combined organic layer was washed with water and
brine, dried
over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was
purified by
column chromatography to afford 40e (1.3g, 5.15 mmol, 60.00% yield).
Step 5
Methyl 2-bromo-5-chloro-4-(trifluoromethyl)benzoate 40f
To a solution of 40e (1.3 g, 5.15 mmol) in CC14 (20 mL) was added NBS (1.10 g,
6.18 mmol)
and AIBN (25.35 mg, 154.38 umol), the mixture was heated to 70 C and stirred
overnight. The
mixture was coldecl to room temperature and filtered, the cake was washed with
CC14, the
filtrate was concentrated in vacuo to give crude 40f (1.9 g, 5.73 mmol,
111.37% yield).
Step 6
6-chloro-5 -(trifluoro methyl) isoindo lin- 1 -o ne 40g
To a solution of 40f (1.9 g, 5.73 mmol) in Me0H (10 mL) was added NH3/Me0H (20
mL) and
the mixture was stirred at room temperature overnight. The reaction mixture
was concentrated
in vacuo. The residue was purified by column chromatography (hexane:
Et0Ac=1:1) to afford
40g (920 mg, 3.91 mmol, 68.14% yield).
LCMS: MS m/z (ES!): 236.0 [M+Hr.
Step 7
5 -chloro-6 -(triflu oro methyl) isoindo line 40h
To a solution of 40g (570 mg, 2.42 mmol) in THF (5 mL) was added BH3/THF
(167.36 mg,
12.10 mmol, 15 mL) and the mixture was stirred at 60 C overnight. The reaction
was colded
to room temperature and quenched with methanol. The mixture was adjusted to
pH1-2 with
1M HC1. Then the mixture was heated to 45 C and stirred for 30 min. After
cooled to rt, the
102
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
mixture was adjusted to pH 7-8 with 1M NaOH. Water was added and the mixture
was
extracted with Et0Ac. The combined organic layer was washed with water and
brine, dried
over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was
purified prep-
TLC (DCM: Me0H=10:1) to give 40h (10 mg, 45.13 umol, 1.87% yield).
1HNMR (400 MHz, DMSO-d6): 6 7.78 (s, 1H), 7.65 (s, 1H), 4.16 (br, 2H), 4.14
(br, 2H).
LCMS: MS nth (ES!): 222.1 1M+H1 .
Step 8
(S)-5 -(3 -(5 -chloro-6-(triflu oro methyl)isoindo lin-2 -y1)-3 -o xoprop y1)-
5 -
cyclopropylimidazolidine-2 ,4-dione 40
To a solution of 40h (10 mg, 45.12 umol) in DMF (2 mL) was added TEA (50 uL),
It-1 (10
mg, 47.12 umol), and HATU (17.16 mg, 45.12 umol). The reaction mixture was
stirred at room
temperature for 3h. Water was added, the mixture extracted with Et0Ac. The
combined organic
layers were washed with water and brine, dried over anhydrous Na2SO4, filtered
and
concentrated in vacuo. The crude was purified by prep-HPLC to give 40 (5 mg,
12.03 umol,
.. 26.65% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.90 (s, 1H), 7.76 (s, 1H), 7.75
(s, 1H), 4.85
(d, 2H), 4.67 (d, 2H), 2.46-2.22 (m, 2H), 2.03-1.98 (m, 2H), 1.15-1.08 (m,
1H), 0.49-0.31 (m,
3H), 0.15-0.08 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.86.
LCMS: MS nth (ES!): 416.4 1M+H1 .
Example 41
(SS)-5 -(3 -(5-chloro-1 -methyl-6-(trifluoromethyl) is oindo lin-2- y1)-3 -o
xoprop y1)-5 -
c yc loprop ylimidazolidine-2 ,4-dio ne 41
0
HN>r-NH
41
CF3
0
CI
103
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
H2N 401
Step 1 H2N Step 2 H2N Step 3 CI
CF Br CF3 CF3 CF3
40c 41b 41c 41d
0 0
CI is
Step 4 CI
C NH _____
Step 5 CI Step 6 Step 7
NH _____________________________________________________________________
Br CF3 Int-1
F3 CF3
41e 41f 41g
HN)r-NH = CF3
0 41
CI
Step 1
Methyl 5 - amino-4 -(triflu oro methyl)-2 - vinyl-benzo ate 41b
To a solution of 40c (5.45 g, 18.29 mmol) and potassium vinyltrifluoroborate
(2.45 g, 18.29
mmol) in dioxane (50 mL) and water (10 mL) was added Pd(dppf)C12 (1.34 g, 1.83
mmol) and
K2CO3 (6.35 g, 45.71 mmol). The resulting mixture was evacuated and refilled
with N2 for 3
times. The resulting mixture was stirred at 80 C for 16 h. The mixture was
diluted with Et0Ac
(100 mL), the combined organic phase was washed with brine (100 mL), dried
over Na2SO4,
filtered and concentrated under reduced pressure. The residue was purified by
silica gel column
chromatography to afford the tittle compound 41b (3.56 g, 14.52 mmol, 79.40%
yield).
LCMS: MS m/z (ES!): 246.1 [M+Hr.
Step 2
Methyl 5-amino-2-ethy1-4-(trifluoromethyl) benzoate 41c
To a solution of 41b (3.56 g, 14.52 mmol) in Me0H (20 mL) was added Pd/C (1.55
g, 1.45
mmol, 285.48 uL, 10% purity). The resulting mixture was evacuated and refilled
with H2. The
resulting mixture was stirred at room temperature for 16 h and the LCMS
indicated the reaction
was finished. The mixture was filtered and the cake was washed with Me0H, the
filtrate was
concentrated under reduced pressure to afford the tittle compound 41c (3.45 g,
13.96 mmol,
96.12% yield).
LCMS: MS m/z (ES!): 248.1 [M+Hr.
Step 3
Methyl 5-chloro-2-ethy1-4-(trifluoromethyl) benzoate 41d
104
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of 41c (3.36 g, 13.59 mmol) in acetone (34 mL) was added HC1
(3.36 mL). The
resulting mixture was stirred at room temperature for 20 min. After the
mixture was cooled to
0 C, a solution of NaNO2 (1.88 g, 27.18 mmol) in water (5 mL) was added. Then
CuCl (1.48
g, 14.95 mmol) was added in small portions at 0 C. The resulting mixture was
stirred at room
temperature for 1 h. The mixture was poured into 1M HC1 (60 mL), the aqueous
phase was
extracted with Et0Ac (100 mL X 3), the combined organic phases were washed
with brine
(100 mL), dried over Na2SO4, filtered and concentrated under reduced pressure.
The residue
was purified by silica gel column chromatography (eluting with hexane /
Et0Ac=50/1) to
afford the title compound 41d (2.23 g, 8.36 mmol, 61.53% yield).
111 NMR (400 MHz, DMSO-d6): 6 7.99 (s, 1H), 7.87 (s, 1H), 3.88 (s, 3H), 2.92
(q, 2H), 1.17
(t, 3H).
Step 4
Methyl 2-(1-bromoethyl)-5-chloro-4-(trifluoromethyl) benzoate 41e
To a solution of 41d (2.23 g, 8.36 mmol) in CC14 (35 mL) was added AIBN
(412.00 mg, 2.51
.. mmol) and NBS (1.64 g, 9.20 mmol). The resulting mixture was stirred at 80
C for 16 h. The
mixture was filtered. The solid was washed with DCM and the filtrate was
concentrated in
vacuo to afford the crude tittle compound 41e (2.5 g, 7.24 mmol, 86.51%
yield).
1H NMR (400 MHz, DMSO-d6): 6 8.17 (s, 1H), 8.04 (s, 1H), 6.08 (q, 1H), 3.92
(s, 3H), 2.05
(d, 3H).
Step 5
6 -chloro-3 -methyl-5 - (triflu oro methyl) isoindo lin- 1 -o ne 41f
To a solution of 41e (2.5 g, 7.24 mmol) in Me0H (10 mL) was added NH3/Me0H (7
M, 30
mL). The resulting mixture was stirred at room temperature for 16 h. The
mixture was purified
by prep-HPLC to afford the tittle compound 41f (1.18 g, 4.73 mmol, 65.34%
yield).
1H NMR (400 MHz, DMSO-d6): 6 9.11 (brs, 1H), 8.20 (s, 1H), 7.91 (s, 1H), 4.71
(q, 1H),
1.42 (d, 3H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.99.
LCMS: MS m/z (ES!): 250.0 [M+Hr.
Step 6
5 -chloro-1 -methyl-6 - (triflu oro methyl) isoindo line 41g
To a solution of 41f (730 mg, 2.92 mmol) in THF (5 mL) was added BH3/THF (2 M,
30.93
mL). The resulting mixture was stirred at 60 C for 16 h. The mixture was
quenched with Me0H
(5 mL) and HC1 (4 M, 5 mL), the mixture was stirred at 60 C for 3 h. The
mixture was basified
105
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
with aq. NaOH and extracted with Et0Ac (50 mL X 3), the combined organic phase
was
washed with brine (50 mL), dried over Na2SO4, filtered and concentrated under
reduced
pressure. The residue was purified by silica gel column chromatography
(eluting with
DCM/Me0H=20/1) to afford the tittle compound 41g (300 mg, 1.27 mmol, 43.53%
yield).
LCMS: MS rn/z (ES!): 236.1 [M+Hr.
Step 7
(SS)-5 -(3 - (5-chloro-1 -methyl-6- (trifluoromethyl) is oindo lin-2- y1)-3 -o
xoprop y1)-5 -
cyclopropylimidazolidine-2 ,4-dione 41
To a solution of It-1 (54.03 mg, 254.63 umol) in DMF (4 mL) was added TEA
(53.68 mg,
530.49 umol), and then 41g (50 mg, 212.19 umol) and HATU (96.82 mg, 254.63
umol). The
resulting mixture was stirred at room temperature for 2 h. The mixture was
purified by prep-
HPLC to afford the tittle compound 41 (25 mg, 57.89 umol, 27.28% yield).
111 NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.88 (s, 1H), 7.79-7.70 (m, 2H),
5.22-5.18
(m, 1H), 4.91-4.49 (m, 2H), 2.44-2.19 (m, 2H), 2.02-1.97 (m, 2H), 1.42 (d,
3H), 1.14-1.08 (m,
1H), 0.46-0.31 (m, 3H), 0.14-0.08 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.74.
LCMS: MS rn/z (ES!): 430.0 [M+Hr.
Examples 41 -1 and 41-2
(S)-5 -(3 -((S)-5 -chloro-l-methy1-6-(trifluoromethyl)isoindolin-2- y1)-3 -
oxoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 41-1
(S)-5 -(3 -((R)-5 -chloro -1 -methyl-6- (triflu oromethyl)iso indolin-2 - y1)-
3 -oxoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 41-2
0 V 00 V 110
HNN
HNN
N H sõ,= CI )7,-NH CI
0 0
CF3 CF3
41-1 41-2
The two enantiomers of 41 (60mg) was separated by chiral SFC (DAICELCHIRALCEL
IG,
250*25 mm 10 pm) to give title compound as single enantiomers (20mg and 18
mg).
Enantiomer (shorter retentsion time):
106
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
11INMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.92-7.88 (m, 1H), 7.80-7.71 (m,
2H), 5.25-
5.18 (m, 1H), 4.90-4.54 (m, 2H), 2.37-2.28 (m, 2H), 2.04-1.97 (m, 2H), 1.44
(dd, 3H), 1.15-
1.08 (m, 1H), 0.46-0.32 (m, 3H), 0.14-0.09 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.75.
LCMS: MS m/z (ES!): 430.1 1M+Hr.
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 1.8m1/min IG,3um,3*100(Daicel)): Rt:
1.171
min, ee: 100%.
Enantiomer (longer retentsion time):
11INMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.92-7.88 (m, 1H), 7.80-7.73 (m,
2H), 5.21-
5.19 (m, 1H), 4.89-4.80 (m, 2H), 2.46-2.24 (m, 2H), 2.01-1.97 (m, 2H), 1.44
(dd, 3H), 1.13-
1.08 (m, 1H), 0.45-0.32 (m, 3H), 0.13-0.11 (m, 1H).
19FNMR (376.5 MHz, DMSO-d6): 6 -60.74.
LCMS: MS m/z (ES!): 430.1 1M+Hr.
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 1.8m1/min IG,3um,3*100(Daicel)): Rt:
1.982
min, ee: 100%.
Example 42
5-cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1)-2-(hydroxymethyl)-3-
oxopropyl)imidazolidine-2,4-dione 42
HN)r-- NH
OH CI
0
42 CI
0 7) Br OBn
Step 1 0 Ly 0 Step 2 0 Step 3
42a 42b 42c
0
OBn Step 4 Step 5 A
v)wo 0 OH
0 YN
OBn 0 41 CI
110 CI
42d 42e 42f
107
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0
Step 6 y N =
Step 7 NH 0 40 CI _____________________________ HN)rNH
OH 40 CI
0
CI 0
CI
42g 42
Step 1
Diethyl (2-cyclopropy1-2-oxoethyl)phosphonate 42b
To a mixture of triethyl phosphite (5 g, 30.09 mmol) in acetonitrile (150 mL)
was
5 added sodium iodide (460 mg, 3.07 mmol). Then 2-bromo-1-
cyclopropylethanone 42a (25 g,
153.36 mmol) was added. The reaction was atirred at r.t for 18h. Water (100
mL) was added
and the mixture was extracted with Et0Ac (100mL X 2), the organic solution was
washed
with brine, dried over Na2SO4 and concentrted. The residue was purified with
silica gel
chromatography (hexane: Et0Ac=1:1) to give 42b (5.4 g, 79.95% yield).
10 iHNIMR (400 MHz, CDC13): 6 4.18-4.13 (m, 4H), 3.24 (s, 1H), 3.18 (s,
1H), 2.23-2.16 (m,
1H), 1.34 (t, 6H), 1.13-1.10 (m, 2H), 0.99-0.96 (m, 2H).
Step 2
(E)-4 -(benzylo xy)-1 -cyc loprop ylb ut-2-en-1 -one 42c
To a mixture of 42b (5.4 g, 24.52 mmol) in acetonitrile (90 mL) was added LiC1
(987.55 mg,
23.30 mmol). Then 2-benzyloxyacetaldehyde (3.13 g, 20.84 mmol) was added. The
reaction
was atirred at r.t for 18h. Water (100 mL) was added and the mixture was
extracted with Et0Ac
(100 mL X 2), the organic solution was washed with brine, dried over Na2S 04
and concentrted.
The residue was purified with silica gel chromatography (hexane:Et0Ac =1:1) to
give 42c (3.4
g, 64.11% yield).
iHNIMR (400 MHz, CDC13): 6 7.37-7.28 (m, 5H), 6.92-6.86 (dt, 1H), 6.53-6.48
(dt, 1H), 4.58
(s, 2H), 4.22 (dd, 2H), 2.17-2.10 (m, 1H), 1.12-1.07 (m, 2H), 0.95-0.90 (m,
2H).
Step 3
4 -(benzylo xy)-1 -cyc loprop y1-3 -(5 -methylfuran-2-yl)butan-1 -one 42d
To a solution of 42c (3.4 g, 15.72 mmol) in Me0H (75 mL) was added 2-
methylfuran (2.58 g,
31.44 mmol) and palladium chloride (279 mg, 1.57 mmol). The reaction was
stirred at r.t for
18h. The solution was filtered, the filtrate was concentrated. The residue was
purified by silica
gel chromatography (hexane: Et0Ac=10:1) to give 42d (2.6 g, 55.43% yield).
108
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
11INMR (400 MHz, CDC13): 6 7.33-7.25 (m, 5H), 5.93 (d, 1H), 5.85-5.83 (m, 1H),
4.49 (s,
2H), 3.69-3.55 (m, 3H), 3.02-2.85 (m, 2H), 2.23 (d, 3H), 1.94-1.87 (m 1H),
1.00-0.95 (m, 2H),
0.84-0.79 (m, 2H).
Step 4
2-((b enzylo xy)methyl)-4-c yc loprop y1-4-o xobu tanoic acid 42e
To a solution of 42d (1 g, 3.35 mmol) in hexane (6 mL), ethyl acetate (18 mL)
and water (24
mL) was added, followed by sodium periodate (5.02 g, 23.46 mmol). The reaction
was stirred
at room temperature for 10 min, ruthenium(III) chloride (695 mg, 3.35 mmol)
was added. The
reaction was stirred at room temperature for lh. The mixture was filtered, the
filtrate was
concentrated. The residue was purified by silica gel chromatography
(DCM:Me0H=20:1) to
give 42e (810 mg, 92.14% yield).
LCMS: m/z (ES!): 261.1 1M-11-.
Step 5
2-((b enzylo xy)methyl)-4-c yc loprop yl- 1-(5 ,6-dichloroiso indo lin-2 -
yl)bu tane- 1,4-dione 42f
To a solution of 42e (200 mg, 762.48 umol), 20c (174 mg, 914.98 umol) and HATU
(435 mg,
1.14 mmol) in DMF (3 mL) was added DIEA (295 mg, 2.29 mmol), the mixture was
stirred at
room temperature for 2h. The mixture was diluted with water and extracted with
Et0Ac, the
organic solution was concentrated and the residue was purified by silica gel
chromatagraphy
(hexane:Et0Ac=1:1) to give the target 42f (180 mg, 414.41 umol, 54.35% yield).
LCMS: MS m/z (ES!): 432.4 1M+H1 .
Step 6
5 -(2-((b enz ylo xy)methyl)-3 -(5,6-dichloroiso indolin-2 - y1)-3 -oxoprop
y1)-5 -
cycloprop ylimidazolidine-2 ,4-dione 42g
The mixture of 42f (120 mg, 276.27 umol), (NH4)2CO3 (27 mg, 276.27 umol) and
NaCN (15
mg, 276.27 umol) in Me0H (5 mL) and H20 (1 mL) was sealed and stirred at 90 C
under Ar
for 16h. The mixture was concentrated and the residue was purified by prep-
HPLC to give the
target 42g (20 mg, 39.65 umol, 14.35% yield).
111 NMR (400 MHz, Methanol-d4): 6 7.51 (s, 1H), 7.44 (s, 1H), 7.20 (brs, 5H),
5.00-4.96 (m,
1H), 4.82-4.73 (m, 2H), 4.64-4.59 (m, 1H), 4.50-4.42 (m, 2H), 3.65-3.59 (m,
2H), 3.30-3.20
(m, 1H), 2.47 (dd, 1H), 1.83 (dd, 1H), 1.18-1.14 (m, 1H), 0.54-0.51 (m, 1H),
0.42-0.38 (m,
1H), 0.33-0.29 (m, 2H).
109
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: MS m/z (ES!): 502.5 1M+H1 .
Step 7
5-cyclopropy1-5-(3-(5,6-dichloroisoindolin-2-y1)-2-(hydroxymethyl)-3-
oxopropyl)imidazolidine-2,4-dione 42
To a solution of 42g (15 mg, 29.74 umol) in Et0Ac (3 mL) was added Pd/C (2 mg,
11.30 umol),
then the mixture was stirred at room temperature for 15 mm under H2. The
mixture was filtered,
and the filtrate was concentrated, the residue was purified by prep-HPLC to
give the target 42
(8.0 mg, 19.31 umol, 64.94% yield).
111 NMR (400 MHz, Methanol-d4): 6 7.51 (s, 1H), 7.48 (s, 1H), 5.04-4.99 (m,
1H), 4.86-4.71
(m, 2H), 4.67-4.57 (m, 1H), 3.67-3.57 (m, 2H), 2.87-2.83 (m, 1H), 2.30 (dd,
1H), 2.03-1.97 (m,
1H), 1.19-1.16 (m, 1H), 0.52-0.50 (m, 1H), 0.42-0.35 (m, 2H), 0.29-0.26 (m,
1H).
LCMS: MS m/z (ES!): 412.0 1M+H1 .
Example 43
5-(3-oxo-3-(5-(trifluoromethyl)isoindolin-2-yl)propy1)-5-(thiazol-2-
y1)imidazolidine-2,4-
dione 43
0
)LNH 0
HN
0 )2"--5
N
43
=
CF3
0
0 0 HN ANN
Step 1 m Step 2 Step 3
S I N
N5 0
N S
43a 43b 43c 0
HN
0
II
0
411 CF3HN)LNH
HNANH
co 1-1 y N
N S 01-I Step 4
410
43d 43 CF3
110
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Step 1
Tert-butyl 4-oxo-4-(thiazol-2-yl)butanoate 43b
To a solution of 1-(thiazol-2-yl)ethanone 43a (21.88 g, 172.06 mmol) in THF
(175 mL) was
added LiHMDS (34.55 g, 206.48 mmol) and 1,3-dimethyltetrahydropyrimidin-2(1H)-
one (44
mL) at -78 C, then tert-butyl 2-bromoacetate (40.27 g, 206.47 mmol) was added
at -78 C.
The resulting mixture was stirred at-78 C for 16 h, and then the mixture was
diluted with
Et0Ac (200 mL) and H20 (100 mL), the organic phase was washed with water (100
mL X 2),
brine (100 mL), dried over Na2SO4, filtered and concentrated under reduced
pressure. The
residue was purified by silica gel column chromatography to afford 43b (16 g,
66.31 mmol,
38.54% yield).
LCMS: MS rah (ES!): 242.1 1M+H1 .
Step 2
tert-butyl 3 -(2,5 -dioxo-4 - (thiazol-2 - yl)imidazolidin-4- yl)prop ano ate
43c
A mixture of 43b (5.0 g, 20.72 mmol), (NH4)2CO3 (16.91 g, 176.12 mmol), NaCN
(2.75 g,
51.80 mmol), Et0H (25 mL) and H20 (25 mL) was heated in an autoclave at 85 C
for 18 hours.
The resulting mixture was diluted with water (60 mL), and extracted with Et0Ac
(200 mL X
5).The organic layers were combined, washed with brine (200 mL), dried over
Na2SO4 and
filtered. The filtrate was concentrated under reduced pressure. The residue
was purified by
column chromatography to afford 43c (650 mg, 2.09 mmol, 10.08% yield).
LCMS: MS rah (ES!): 312.11M+Hr.
Step 3
3-(2,5-dioxo-4-(thiazol-2-y0imidazolidin-4-y0propanoic acid 43d
To a solution of 43c (650 mg, 2.09 mmol) in DCM (5 mL) was added HC1/dioxane
(10 mL)
drop-wise. The reaction mixture was stirred at room temperature for 18 hours.
Then the
reaction mixture was concentrated under reduced pressure to afford crude 43d
(600 mg, 2.35
mmol, 112.60% yield).
LCMS: MS rah (ES!): 256.01M+Hr.
Step 4
5 -(3 -o xo -3- (5 -(triflu oro methyl) isoindo lin-2- yl)prop y1)-5 -(thiazol-
2 - yl)imid azo lidine-2 ,4-
dione 43
A mixture of 43d (100 mg, 391.77 umol), 1-1 hydrochloride (87.61 mg, 391.77
umol), Et3N
(118.71 mg, 1.18 mmol), HATU (148.96 mg, 391.77 umol) and DMF (10 mL) was
stirred at
111
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
room temperature for 18 hours. Then the reaction mixture was purified by prep-
HPLC to afford
43 (20 mg, 47.13 umol, 12.03% yield).
1H NMR (400 MHz, DMSO-d6): 6 11.10 (br, 1H), 8.47-8.44 (m, 1H), 7.82 (d, 1H),
7.76-7.73
(m, 2H), 7.66 (d, 1H), 7.59-7.55 (m, 1H), 4.83 (d, 1H), 4.69 (brs, 2H), 2.46-
2.38 (m, 4H).
LCMS: MS nth (ES!): 425.2 1M+Hr.
Example 44
(S)-5 -cyc loprop y1-5 -(3 -(7-flu oro -1,2,4 ,5-tetrahydro -3H-b enzo [di
azepin-3 - y1)-3 -
oxoprop yl)imidazo lidine-2,4-dione
0 0
N
HN),i-NH
0
44
0 0 0 0
_________________________________________________ HN
+ HN
HN HATU TEA
DMF 0
0 44a 44
It-1
To a mixture of 7-fluoro-2,3,4,5-tetrahydro-1H-benzoldlazepine 44a (23 mg,
0.139 mmol,
purchased from Acme Bioscience) in DMF (5 mL) was added Triethylamine (70 mg,
0.51
mmol), It-1 (30 mg, 0.141 mmol) and HATU (57 mg, 0.151 mmol). The reaction was
stirred
at room temperature for 18h. Water (3 mL) was added and the mixture was
extracted with
Et0Ac (20 mL X 2). The combined layer was washed with brine, dried over Na2SO4
and
concentrated. The residue was purified with prep-HPLC to give title compound
44 (25 mg,
69.5 umol, 48 % yield).
1H NMR (400 MHz, Methanol-d4): 6 7.16 (dt, 1H), 6.98-6.81 (m, 2H), 3.80 - 3.55
(m, 4H),
3.37 (s, 1H), 3.03-2.90 (m, 4H), 2.19-2.03 (m, 4H), 1.22 (m, 1H), 0.59 (m,
1H), 0.40 (m, 3H).
LCMS: MS nth (ES!): 360 1M+Hr.
Example 45
(S)-5 -cyc loprop y1-5 -(3 -o xo-3 -(7-(triflu oromethyl)- 1,2,4,5 -tetrahydro-
3H-b enzoldl azepin-3 -
yl)propyl)imidazolidine-2,4-dione
112
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
V 0
HN Y---teL(s) CF3
OH
0
0 V 0
0
HATU/ TEA
+ HN CF3 DMF HN)r-NH CF3
HNy-NH
0
0 45
It-1
To a mixture of 7-(trifluoromethyl)-2,3,4,5-tetrahydro-1H-benzoldlazepine (23
mg, 0.139
mmol) in DMF (5 mL) was added Triethylamine (70 mg, 0.51 mmol), It-1 (30 mg,
0.141
5 mmol)
and HATU (57 mg, 0.151 mmol). The reaction was stirred at r.t for 18h. Water
(3 mL)
added and the mixture was extracted with Et0Ac (20 mL X 2). The combined
organic layers
were washed with brine, dried over Na2SO4 and concentrated. The residue was
purified with
prep-HPLC to give the title compound (18 mg, 31 % yield).
111 NMR (400 MHz, CDC13): 6 7.31 (q, 2H), 7.15 (t, 1H), 3.65 (m, 2H), 3.59 ¨
3.45 (m, 2H),
10 2.94
¨2.79 (m, 4H), 2.45 (hept, 2H), 2.23 (dt, 1H), 2.14 (dt, 7.4 Hz, 1H), 1.12
(td, 1H), 0.40 ¨
0.26 (m, 4H).
LCMS: MS nth (ES!): 410 [M+Hr.
Example 48
15 5 -(3 -(5 -chloro-6- (triflu oro methyl) iso indolin-2 - y1)-3 -oxoprop
y1)-5 - (p yridin-3-
yl)imidazolidine-2,4-dione 48
N
0
HN 40 CI
0 48
CF3
113
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
N 0
0
Step 1 Step 2
Oro __________________________________________
HN)r.- NH
C)<
0
48a 48b 48c
0
0
Step 3 Step 4
OH ______________________________________
H N H HN)r-NH 41 CI
0
0
48 CF3
48d
Step 1
Tert-butyl 4-oxo-4-(pyridin-3-yl)butanoate 48b
To a solution of 1-(pyridin-3-y0ethan-1-one 48a (4.97 g, 41.01 mmol) in THF
(50 mL) was
added NaHMDS (7.52 g, 41.01 mmol) dropwise at -70 C. The resulting mxiture was
stirred at
this temperature for 30 min before tert-butyl 2-bromoacetate (8.0 g, 41.01
mmol) was added
dropwsie. After addition, the reaction mixture was stirred at -20 C for 1.0
hour, then warmed
to room temperature and stirred for 18 hours. The resulting mixture was cooled
to 0 C,
quenched with aq. NaHCO3 (20 mL). The whole solution was extracted with Et0Ac
(30 mL
X 4). The organic layers were combined, dried over Na2SO4 and filtered. The
filtrate was
concentrated under reduced pressure. The residue was purified by silica gel
chromatography
(Et0Ac/hexane= 1/20 to 1/4) to afford 48b (800 mg, 3.40 mmol, 8.29% yield).
LCMS: MS rah (ES!): 236.1 [M+Hr.
Step 2
Tert-butyl 3 -(2,5 -dio xo -4-(p yridin-3- yl)imidazolidin-4- yl)prop ano ate
48c
To the solution of 48b (500 mg, 2.13 mmol) in H20 (8 mL) and Et0H (10 mL) was
added
NaCN (282 mg, 5.31 mmol), (NH4)2CO3 (1.63 g, 17.00 mmol). The reaction was
stirred at 85 C
overnight. LCMS showed product produced. Then the reaction mixture was diluted
with water
and extracted with Et0Ac (10 mL X 2). The organic solution was concentrated,
and the residue
was purified by silica gel chromatography (Et0Ac/hexane= 1/2 to 1/1) to give
48c (250 mg,
818.79 umol, 38.53% yield).
114
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
111 NMR (400 MHz, DMSO-d6): 6 11.21 (s, 1H), 9.07 (s, 1H), 8.92 (s, 1H), 8.90
(s, 1H), 8.45
(d, 1H), 7.90 (s, 1H), 2.57-2.30 (m, 3H), 2.19-2.15 (m, 1H), 1.41 (s, 9H).
LCMS: MS m/z (ES!): 306.1 1M+H1 .
Step 3
3 -(2,5 -dio xo -4-(p yridin-3 - yl)imidazolidin-4- yl)prop ano ic acid 48d
The mixture of 48c (250 mg, 818.79 umol) in HC1/1,4-dioxane (2 mL, 2N) was
stirred at room
temperature for lh. LCMS showed that the product produced. The mixture was
concentrated
to give 48d (220 mg, 882.75 umol, 107.81% yield).
LCMS: MS m/z (ES!): 250.1 1M+H1 .
Step 4
5 -(3 -(5 -chloro-6-(triflu oro methyl) iso indolin-2 - y1)-3 -oxoprop y1)-5 -
(p yridin-3 -
yl)imidazolidine-2,4-dione 48
To the solution of 48d (100 mg, 401 umol) in THF (5 mL) was added HATU (232
mg, 609.19
umol), DIEA (63 mg, 487.35 umol) and 40h (90 mg, 406.13 umol). The reaction
was stirred at
room temperature overnight. LCMS showed that the product produced. The mixture
was
purified by prep-HPLC to give title compound 48 (5.55 mg, 12.26 umol, 3.02%
yield).
111 NMR (400 MHz, DMSO-d6): 6 11.01 (brs, 1H), 8.87 (d, 1.6 Hz, 1H), 8.72 (d,
1H), 8.56
(dd, 1H), 7.94-7.88 (m, 2H), 7.55 (d, 1H), 7.46 (dd, 1H), 4.81-4.77 (m, 2H),
4.67-4.63 (m, 2H),
2.42-2.23 (m, 4H).
LCMS: MS m/z (ES!): 453.4 1M+H1 .
Examples 49-1 and 49-2
(SR)-5 -cyc loprop y1-5 -(3 -methy1-2 -(5-(trifluoromethyl) isoindo line-2 -
carbonyl)butyl)imidazolidine-2,4-dione 49-1
(SS)-5 -c ycloprop y1-5 -(3 -methyl-2-(5 -(triflu oro methyfliso indoline-2-
carbonyflbutyflimidazolidine-2,4-dione 49-2
115
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0
V 0
H>X y HN
NH yNhl
CF3 CF3
0 0
49-1 49-2
00
0 0
V')
Step1 C) Step 2
0
Int2-1 49a
49b 49c
0 0 y 0 N
Step 3
Step 4
________________________________ HN)r-NH
CF3 CF3 HN)T-NH CF3
0 0
49d 49-1 49-2
Step 1
Diethyl 2- (c yc loprop anec arbony1)-3 -isopropyl-bu tanedio ate 49b
To a solution of Int2-1 (3.59 g, 22.99 mmol) and ethyl 2-bromo-3-
methylbutanoate 49a (5 g,
23.91 mmol) in methyl ethyl ketone (60 mL) were added K2CO3 (6.36 g, 45.99
mmol) and NaI
(3.45 g, 22.99 mmol). The resulting mixture was stirred at 110 C for 48 h. The
mixture was
poured into water (100 mL), the aqueous phase was extracted with Et0Ac (80 mL
X 3), the
combined organic phase was washed with brine (100 mL), dried over Na2SO4,
filtered and
concentrated under reduced pressure. The residue was purified by silica gel
column
chromatography to afford the tittle compound 49b (4.29 g, 15.09 mmol, 65.61%
yield).
Step 2
4-cyclopropy1-2-isopropyl-4-oxo-butanoic acid 49c
To a solution of 49b (2.29 g, 8.05 mmol) in THF (8 mL) and water (8 mL) was
added LiOH
(482.21 mg, 20.13 mmol). The resulting mixture was stirred at room temperature
for 16 h. To
the mixture was added HC1 (1N, 10 mL), the resulting mixture was stirred at 60
C for 2h. Then
the mixture was adjusted to pH=13 with the addition of NaOH (aq) and the
layers were
separated. The combined aq. phase was adjusted to pH-7 with 1 M HC1 (10 mL),
the mixture
was then extracted with DCM (50 mL X 3), the organic solution was dried over
Na2S0),
filtered and concentrated under reduced pressure to afford the crude tittle
compound 49c (300
mg, 1.63 mmol, 20.22% yield).
Step 3
4-c ycloprop y1-2 -isoprop y1-1- [5- (trifluoromethyl)- 1, 3, 3a, 7 a-
tetrahydroisoindo1-2- yll butane-
1, 4-dione 49d
116
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of 49c (270 mg, 1.52 mmol) in DMF (8 mL) was added TEA (753.42
mg, 7.45
mmol), 5-(trifluoromethyl)isoindoline 1-1 (333 mg, 1.49 mmol) and HATU (679.45
mg, 1.79
mmol). The resulting mixture was stirred at room temperature for 2 h. The
mixture was poured
into water (80 mL), the aqueous phase was extracted with Et0Ac (80 mL X 3),
the combined
organic phase was washed with brine (100 mL), dried over Na2SO4, filtered and
concentrated
under reduced pressure. The residue was purified by silica gel column
chromatography (eluting
with hexane/Et0Ac=3/1) to afford the tittle compound 49d (340 mg, 956.68 umol,
64.25%
yield).
LCMS: MS nilz (ES!): 354.1 [M+Hr.
Step 4
(51?)-5 -c yc loprop y1-5 -(3 -methyl-2-(5 -(trifluoromethyl)iso indoline-2-
carbo nyl)b utyl) imidazolidine-2 ,4 -dio ne 49-1
(5S)-5 -c ycloprop y1-5 -(3 -methyl-2-(5 -(triflu oro methyl)iso indoline-2-
carbo nyl)b utyl) imidazolidine-2 ,4 -dio ne 49-2
To a solution of 49d (340 mg, 956.69 umol) in Me0H (5 mL) was added (NH4)2CO3
(735.39
mg, 7.65 mmol), water (5 mL) and NaCN (126.86 mg, 2.39 mmol). The resulting
mixture was
stirred at 80 C for 16 h. The reaction mixture was poured into water (100 mL),
the mixture was
extracted with Et0Ac (80 mL X 3), the combined organic phases were washed with
brine (100
mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The
residue was
.. purified with prep-HPLC to afford the tittle compound 49-1 (40 mg, 94.02
umol, 9.83% yield)
and 49-2 (250 mg, 587.62 umol, 61.42% yield).
49-1:
111 NMR (400 MHz, DMSO-d6): 6 10.46 (s, 1H), 7.77-7.72 (m, 2H), 7.66 (d, 1H),
7.60-7.56
(m, 1H), 4.91-4.88 (m, 2H), 4.72-4.69 (m, 1H), 4.63-4.58 (m, 1H), 2.58-2.50
(m, 1H), 2.42-
2.35 (m, 1H), 1.78-1.73 (m, 2H), 1.07-1.03 (m, 1H), 0.92 (dd, 6H), 0.41-0.27
(m, 3H), 0.10-
0.07 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.57.
HPLC: SunFire C18 Sum 4.6*150mm, 0.03%TFA CH3CN/H20, Rt 7.478 min.
LCMS: MS nilz (ES!): 424.1 [M+Hr.
49-2:
111 NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.74 (d, 1H), 7.68-7.65 (m, 1H),
7.60-7.54
(m, 2H), 4.90-4.85 (m, 2H), 4.74-4.71 (m, 2H), 2.33-2.28 (m, 2H), 1.88-1.75
(m, 2H), 1.07-
1.03 (m, 1H), 0.92-0.89 (m, 6H), 0.42-0.39 (s, 1H), 0.29-0.25 (m, 2H), 0.01-
0.00 (m, 1H).
117
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
19F NMR (376.5 MHz, DMSO-d6): 6 -60.57.
HPLC: SunFire C18 Sum 4.6*150mm, 0.03%TFA CH3CN/H20, Rt 8.141 min.
LCMS: MS m/z (ES!): 424.3 [M+Hr.
Examples 49-2a and 49-2b
(5S)-5 -c ycloprop y1-5 - [(2R)-3-methy1-2- [5-(trifluoromethyl)- 1, 3, 3a, 7
a-tetrahydro isoindo le-
2-carbonyl] butyl] imidazolidine-2, 4-dione 49-2a
(51?)-5 -c yc loprop y1-5 - [(2S)-3-methy1-2- [5-(trifluoromethyl)- 1, 3, 3a,
7 a-tetrahydro isoindo le-
2-carbonyl] butyl] imidazolidine-2, 4-dione 49-2b
f, V 0 0 o
_=
\- X1(N
HN NH
HN NH =
itS CF3 CF3
0 0
49-2a 49-2b
49-2 (140 mg) was seperated by chiral SFC to afford the title compounds (50 mg
and 50mg).
Enantiomer (shorter retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.74 (d, 1H), 7.68-7.65 (m, 1H),
7.61-7.54
(m, 2H), 4.90-4.86 (m, 2H), 4.74-4.71 (m, 2H), 2.33-2.28 (m, 2H), 1.87-1.75
(m, 2H), 1.05-
1.03 (m, 1H), 0.92-0.89 (m, 6H), 0.40-0.38 (m, 1H), 0.28-0.25 (m, 2H), 0.01-
0.00 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.57.
LCMS: MS m/z (ES!): 424.5 [M+Hr.
Chiral HPLC (CO2/Et0H/DEA 5%-40% 1.5ml/min IG,3um,3*100(Daicel)): Rt:3.003
min,
cc: 100%.
Enantiomer (longer retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.75 (d, 1H), 7.68-7.65 (m, 1H),
7.60-7.54
(m, 2H), 4.90-4.86 (m, 2H), 4.73-4.69 (m, 2H), 2.32-2.28 (m, 2H), 1.88-1.74
(m, 2H), 1.05-
1.02 (m, 1H), 0.92-0.86 (m, 6H), 0.42-0.40 (m, 1H), 0.28-0.25 (m, 2H), 0.01-
0.00 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.57.
LCMS: MS m/z (ES!): 424.2 [M+Hr.
Chiral HPLC (CO2/Et0H/DEA 5%-40% 1.5ml/min IG,3um,3*100(Daicel)): Rt:3.494
min,
cc: 100%.
Example 50
118
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -(3 - (5 -chloro-6-nitroiso indolin-2 - y1)-3 -oxoprop y1)-5 -c ye
loprop ylimidazolidine-2 ,4-
dione 50
(s)
HNNH 44I 50 NO2
0
CI
CI
NH BH3 in T.HF CI Int-1 (s)
= NH HN),rNH
02N step1
02N step 2 410, NO2
0 0
50a 50b 50 CI
Step 1
5-chloro-6-nitroisoindoline (50b)
To a solution of 5-chloro-6-nitroisoindoline-1,3-dione 50a (1 g, 4.42 mmol) in
THF (15 mL)
was added borane¨tetrahydrofuran (1M, 35 mL) dropwise under N2. The resulting
mixture was
stirred at 60 C for 24h. The reaction mixture was cooled to ambient
temperature and quenched
with Me0H (5 mL) until the bubbling ceased. Then 4N HC1 in water (4 mL) was
added and
the mixture was heated at 80 C for 3 h. After cooled down to RT, 5N KOH was
added to adjust
pH to 7. The mixture was concentrated under reduced pressure and the residue
was purified by
silica-gel column (DCM: Me0H(2%NH4OH) = 10: 1) to afford 5-chloro-6-
nitroisoindoline
50b (300 mg, 33% yield).
Step 2
(S)-5 -(3 - (5 -chloro-6-nitroiso indolin-2 - y1)-3 -oxoprop y1)-5 -c ye
loprop ylimidazolidine-2 ,4-
dione 50
To a mixture of 5-chloro-6-nitroisoindoline 50b (10 mg, 0.05m01) in DMF (2 mL)
was added
triethylamine (18 mg, 0.138 mmol), It-1 (10 mg, 0.047 mmol) and HATU (21mg,
0.055
mmol). The reaction was stirred at r.t for 18h. Water (4 mL) was added and the
mixture was
extracted with Et0Ac (20 mL X 2). The combined organic layers were washed with
brine,
dried over Na2SO4 and concentrated. The residue was purified with prep-HPLC to
give the title
compound 50 (4 mg, 0.01 mmol, 21% yield).
1H NMR (400 MHz, Methanol-d4): .94 (d, 1H), 7.67 (d, 1H), 4.95 (d, 2H), 4.81
(d, 2H), 2.57
(m, 1H), 2.45 (m, 1H), 2.33 ¨2.14 (m, 2H), 1.34¨ 1.20 (m, 1H), 0.61 (m, 1H),
0.53 ¨0.30 (m,
3H).
LCMS: MS nth (ES!): 393 1M+Hr.
119
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Example 51
(5S)-5 -c yc loprop y1-5 -(3 -(5-(1 -hydro xyethyl) isoindo lin-2- y1)-3 -o
xoprop yl) imid azolidine-2,4 -
dione 51
0 V 0
HNlH
)r-NH
51
0
Boc, Boc, HN
Step 1 Step 2
0
0
Br
51a 51b 51c
0 V 0 0 V 0
Step 3 Ysi")L
____________ HN)rNH 0N Step 4
______________________________________________ HNY--1")L OH
I nt-1 ),r-NH
0 51d 0 51
Step 1
Tert-butyl 5-acetylisoindoline-2-carboxylate Sib
To a mixture of tert-butyl 5-bromoisoindoline-2-carboxylate 51a (500 mg, 1.68
mmol),
tributy1(1-ethoxyvinyl)stannane (726.72 mg, 2.01 mmol) in dioxane (5 mL) was
added
Pd(PPh3)4 (193.78 mg, 167.69 umol). The reaction was stirred under N2 at 100 C
for 6h. The
reaction was cooled to rt, the mixture was diluted with water (50 mL), the
mixture was extracted
with ethylacetate (20 mL X 3). The organic solution was washed with brine,
dried over Na2SO4
and concentrated. The residue was purified by prep-TLC (hexane:Et0Ac=10:1) to
give 51b
(105 mg, 401.81 umol, 23.96% yield).
111 NMR (400 MHz, DMSO-d6): 6 7.90-7.84 (m, 2H), 7.35-7.32 (m, 1H), 4.73 (br,
2H), 4.70
(br, 2H), 2.61 (s, 3H), 1.45 (s, 9H).
Step 2
1 -(isoindolin-5 -yl)ethan- 1 -one 51c
To a mixture of 51b (50 mg, 191.34 umol) in DCM (3 mL) was added dioxane/HC1
(1N, lmL).
The reaction was stirred at room temperature for 16h. The mixture was
concentrated to give
crude 51c, the crude product was used to the next step directly without
purification.
Step 3
(S)-5 -(3 -(5- acetylisoindolin-2- y1)-3 -oxoprop y1)-5 -cyc loprop ylimidazo
lidine-2,4-dione 51d
120
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of 51c (56 mg, 347.39 umol) in DMF (10 mL) was added It-1 (73.72
mg, 347.39
umol) and HATU (198.13 mg, 521.09 umol). The mixture was stirred at room
temperature 2h.
Water (30 mL) was added and the mixture was extracted with Et0Ac (20 mL X 2).
The
combined organic layers were washed with water (40 mL) and brine (40 mL X 2),
dried and
concentrated. The crude was purified by prep-HPLC to give 51d (52 mg, 146.32
umol, 42.12%
yield).
1H NMR (400 MHz, CDC13): 6 7.94-7.87 (m, 2H), 7.57 (brs, 1H), 7.42-7.34 (m,
1H), 6.09 (d,
1H), 4.83 (brs, 4H), 2.62 (s, 3H), 2.66-2.62 (m, 1H), 2.47-2.27 (m, 3H), 1.26-
1.20 (m, 1H),
0.62-0.59 (m, 1H), 0.48-0.36 (m, 3H).
Step 4
(SS)-5 -c yc loprop y1-5 -(3 -(5-(1 -hydro xyethyl) isoindo lin-2- y1)-3 -o
xoprop yl) imid azolidine-2,4 -
dione 51
To a mixture of 51d (22 mg, 61.90 umol) in Me0H (3 mL), NaBH4 (11.70 mg,
309.52 umol)
was added. The reaction was stirred at room temperature for 4h. The reaction
was quenched
with water (30 mL), and the mixture was extracted with Et0Ac (20 mL X 3), The
organic
solution was washed with brine, dried over Na2SO4, filtered and concentrated.
The crude was
purified by prep-HPLC to give 51 (7 mg, 19.59 umol, 31.64% yield).
1H NMR (400 MHz, DMSO-d6): 610.51 (brs, 1H), 7.63 (brs, 1H), 7.21-7.12 (m,
3H), 5.06-
5.05 (m, 1H), 4.66-4.59 (m, 3H), 4.48 (d, 2H), 2.33-2.26 (m, 1H), 2.18-2.13
(m, 1H), 1.89 (t,
2H), 1.19 (d, 3H), 1.02-0.98 (m, 1H), 0.35-0.19 (m, 3H), 0.01-0.00 (m, 1H).
LCMS: MS nth (ES!): 358.2 [M+Hr.
Example 52
(S)-5 -c ycloprop y1-5 -(3 -(5 -(difluoromethyl) isoindolin-2- y1)-3 -o xoprop
yl) imid azolidine-2,4 -
dione 52
0 V
N
NH CHF2
0 52
121
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Boc, B,N Boc
oc ,N
Step 1 Step 2 Step 3
4
\ 11 Br 0
51a 52b 52c
Boc, HN 0 V
Step 4 Step 5
CHF2 _________________________ * _____________________ HN)r-NH
CHF2 It-1
CHF2
0
52d 52e 52
Step 1
Tert-butyl 5-vinylisoindoline-2-carboxylate 52b
To a mixture of 51a (1 g, 3.35 mmol), K2CO3 (925.63 mg, 6.71 mmol) and
potassium
vinyltrifluoroborate (538.88 mg, 4.02 mmol) in dioxane (10 mL) and water (2
mL) was added
Pd(dppf)C12 (197.54 mg, 335.37 umol). The reaction was stirred under N2 at 80
C for 3h. The
reaction mixture was cooled to room temperature and diluted with water (50
mL), extracted
with Et0Ac (30 mL X 3). The combined organic layers were washed with brine,
dried over
Na2SO4 and concentrated. The residue was purified by SGC (hexane:Et0Ac=10:1)
to give 52b
(786 mg, 3.20 mmol, 95.54% yield).
1H NMR (400 MHz, CDC13): 6 7.32-7.15 (m, 3H), 6.71 (dd, 1H), 5.73 (dd, 1H),
5.23 (d, 1H),
4.67 (br, 2H), 5.22 (br, 2H), 1.52 (d, 9H).
Step 2
Tert-butyl 5-formylisoindoline-2-carboxylate 52c
To a mixture of 52b (400 mg, 1.63 mmol), NaI04 (697.88 mg, 3.26 mmol) in
dioxane (7 mL)
and water (4 mL) was added osmium tetroxide (41.45 mg, 163.05 umol). The
reaction was
stirred at room temperature for 0.5 h and then diluted with water (40 mL). The
mixture was
extracted with ethylacetate (20 mL X 3), the organic solution was washed with
brine, dried
over Na2SO4 and concentrated. The residue was purified by silica gel
chromatography
(hexane:Et0Ac=5:1) to give 52c (98 mg, 396.30 umol, 24.30% yield).
1H NMR (400 MHz, CDC13): 6 10.01 (s, 1H), 7.82-7.76 (m, 2H), 7.44-7.40 (m,
1H), 4.76-
7.73 (m, 4H), 1.53 (s, 9H).
Step 3
Tert-butyl 5-(difluoromethyl)isoindoline-2-carboxylate 52d
To a mixture of 52c (50 mg, 202.19 umol) and Et0H (930.09 ug, 20.22 umol) in
DCM (3 mL)
was added DAST (162.96 mg, 1.01 mmol). The reaction was stirred at room
temperature for
16h. The reaction was quenched with water (30 mL), and the mixture was
extracted with Et0Ac
122
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(20 mL X 3). The organic solution was washed with brine, dried over Na2SO4 and
concentrated
to give crude 52d (52 mg, 193.10 umol, 95.50% yield).
1H NMR (400 MHz, CDC13): 6 7.36-7.19 (m, 3H), 6.57 (t, 1H), 4.65 (br, 2H),
4.61 (br, 2H),
1.45 (s, 9H).
19F NMR (400 MHz, CDC13): 6 -109.86.
Step 4
5 -(difluoromethyl)isoindoline 52e
To the mixture of 52d (30 mg, 111.41 umol) in DCM (3 mL) was add HO/dioxane
(1N, 1 mL).
The reaction was stirred at room temperature for 16 hours. The mixture was
concentrated to
give the crude 52e.
Step 5
(S)-5 -c ycloprop y1-5 -(3 -(5 -(difluoromethyl) isoindolin-2- y1)-3 -o xoprop
yl) imid azolidine-2,4 -
dione 52
To a solution of 52e (35 mg, 206.89 umol) in DMF (3 mL) was added It-1 (43.90
mg, 206.89
umol), TEA (62.81 mg, 620.67 umol) and HATU (94.40 mg, 248.27 umol). The
mixture was
stirred at room temperature for 2h. Water (30 mL) was added and the mixture
was extracted
with EA (20 mL X 2). The combined organic layers were washed with water (30
mL) and
brine (30 mL), dried and concentrated. The crude was purified by prep-HPLC to
give 52 (5 mg,
13.76 umol, 6.65% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.64 (brs, 0.5H), 7.73 (brs, 0.5H), 7.73-7.70
(m, 1H),
7.58-7.49 (m, 2H), 7.05 (t, 1H), 4.88-4.84 (m, 2H), 4.67-4.65 (m, 2H), 2.50-
2.28 (m, 2H), 2.07-
1.97 (m, 2H), 1.12-0.85 (m, 1H), 0.46-0.27 (m, 2H), 0.20-0.05 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -108.63.
LCMS: MS nth (ES!): 364.0 1M+Hr.
Example 53
(S)-5 -(3 -(5 -chloro -6-iodo isoindo lin-2- y1)-3 -oxoprop y1)-5 -cyc loprop
ylimid azo lidine-2,4-
dione 53
0 7 0
H Ny NH 41 I
0
53 CI
123
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
CI step 1 TI:IfIIIIICI step 2 CI
step 3 CI
NH _____________________________ NH -1' NH
02N H2N NH
0 0 0
50a 53b 3c 53d
0 V 0
step 4
(s)
_______________ HN)r-NH
Int-1
0
53 CI
Step 1
5- amino -6- chloro isoindo line- 1 ,3-dione 53b
To a mixture of 50a (1 g, 4.4 mmol) in Me0H (20 mL) was added ammonia solution
(3 mL),
5 H20 (5m1) and Na2S204 (7.6 g, 44 mmol). The reaction was stirred at r.t
for 24h. Water (4 mL)
was added and the mixture was extracted with Et0Ac (30 mL X 4). The combined
organic
layers were washed with brine, dried over Na2SO4 and concentrated to afford
53b (0.5 g, 2.5
mmol, yield 56%). The residue was used in next step without further
purification.
Step 2
5 - chloro-6 - iodoisoindoline- 1 ,3-dione 53c
To a stirred suspension of 53b (0.5 g 2.5 mmol) in 15 mL water was added
dropwise at 10 C.
A solution of 0.4 mL concentrated sulfuric acid in 5 mL water. After the
mixture was cooled
to 5 C, a solution of sodium nitrite (276 mg, 4 mmol) in 5 mL water was added
dropwise and
the stirring continued at 0 C for 90 mins. A solution of potassium iodide
(1.4 g, 8.8 mmol) in
8 mL water was then added dropwise over 40 mins while maintaining the reaction
temperature
between 0 C and 5 C. The reaction mixture was then warmed to room
temperature and
subsequently heated at 35 C for 45 mins and then 60 C for 30 mm. Then the
mixture was
cooled down to room temperature and was extracted with Et0Ac (30 mL X 4). The
combined
organic layers were dried, filtered and concentrated. The residue was
resuspended in 30 mL
DCM, stirred for 10 mins at room temperature and the resulting crystals
collected by filtration
to yield compound 53c (240 mg, 0.78 mmol, 31% yield).
Step 3
5-chloro-6-iodoisoindoline 53d
To a solution of 53c (120mg, 0.38 mmol) in THF (5 mL) was added
borane¨tetrahydrofuran
(1M, 60 mL) dropwise under N2. The resulting mixture was stirred at 60 C for
24h. The
reaction mixture was cooled to ambient temperature and quenched with Me0H (6
mL) until
124
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
the bubbling ceased. Then 4N HC1 in water (2 mL) was added and the mixture was
heated at
80 C for 3 h. Then the mixture was cooled down to room temperature and 5N KOH
was added
to adjust pH to 7. The mixture was concentrated under reduced pressure and the
residue was
purified by silica-gel column (DCM: Me0H(2%NH4OH) = 10: 1) to afford 53d (51
mg, 0.18
mmol 47 % yield).
Step 4
(S)-5 -(3 - (5 -chloro-6-iodoiso indolin-2 - y1)-3 -oxoprop y1)-5 -c yc loprop
ylimidazolidine-2 ,4-
dione 53
To a mixture of 53d (10 mg, 0.035 mmol) in DMF (2 mL) was added triethylamine
(12mg, 0.1
mmol), It-1 (9 mg, 0.042 mmol) and HATU (21 mg, 0.055 mmol). The reaction was
stirred
at r.t for 18h. Water (3 mL) was added and the mixture was extracted with
Et0Ac (20 mL X
2). The combined organic layers were washed with brine, dried over Na2SO4 and
concentrated.
The residue was purified with prep-HPLC to give title compound 53 (4 mg, 0.008
mmol, 22 %
yield).
1H NMR (400 MHz, Methanol-d4): 6 7.92 (d, 1H), 7.53 (d, 1H), 4.84 (s,2H), 4.71
(s, 2H),
2.61-2.33 (m, 2H), 2.30-2.15 (m, 2H), 1.20-1.30 (m, 1H), 0.68-0.32 (m, 4H).
LCMS: nth (ES!): 474 [M+1-11+
Example 54
(S)-5 -(3 - (5 - amino-6-chloroiso indolin-2 - y1)-3 -oxoprop y1)-5-
cyclopropylimidazolidine-2 ,4-
dione 54
HN NH S)
CI
0
54 NH2
To a mixture of 50 (10 mg, 0.025 mmol) in Me0H (2 mL) was added ammonia
solution (0.3m1),
H20 (1m1) and Na2S204 (65mg, 0.375 mmol). The reaction was stirred at r.t for
18h. Water (6
mL) was added and the mixture was extracted with Et0Ac (20 mL X 2). The
combined organic
layers were washed with brine, dried over Na2SO4 and concentrated. The residue
was purified
with prep-HPLC to give title compound 54 (6 mg, 0.016 mmol, 64% yield).
1H NMR (400 MHz, Methanol-d4): 6 7.64 (d, 1H), 7.32 (d, 1H), 6.98 (d, 2H),
4.79 (d, 2H),
4.67 (s, 2H), 2.55 (m, 1H), 2.42 (m, 1H), 2.31 ¨2.13 (m, 2H), 1.34 ¨ 1.19 (m,
1H), 0.60 (td,
1H), 0.53 ¨0.30 (m, 3H).
125
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: MS raiz (ES!): 363 [IVI+Hr.
Example 55
(S)-N-(6-chloro-2-(3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-
y0propanoy0isoindolin-5-
yl)acetamide 55
JV
0\\
HI\nP)
r NH 411. 55 NH
0
CI
To a mixture of 54 (3 mg, 0.08 mmol) in THE (2 mL) was added acetic anhydride
(8mg 0.075
mmol) and triethylamine (18 mg 0.14 mmol). The reaction was stirred at 30 C
for 18h. The
mixture was concentrated. The residue was purified with prep-HPLC to give
title compound
55 (2.1 mg, 0.005 mmol, 62% yield).
1H NMR (400 MHz, Methanol-d4): 6 7.74 (s, 1H), 7.47 (d, 1H), 4.84 (s, 2H),
4.74 (s, 2H),
2.68-2.33 (m, 2H), 2.30-2.12 (m, 5H), 1.30 ¨ 1.21 (m, 1H), 0.68-0.29 (m, 4H).
LCMS: MS raiz (ES!): 405 [IVI+Hr.
Example 56
(S)-2-(3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-y0propanoy0isoindoline-5-
carboxylic acid
56
0 V
0
)r_ N H
0 56 OH
The title compound was prepared with similar method as Example 1.
LCMS: MS raiz (ES!): 358 [IVI+Hr.
Example 57
(S)-5-(3-(5-chloro-6-methylisoindolin-2-y0-3-oxopropy1)-5-
cyclopropylimidazolidine-2,4-
dione 57
126
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 0
CI
H N (s)
)r-NH CH3
0
57
CI NH step 1 CI s N¨Boc step 2 CI
N¨Boc
53d 57a 57b
0
step 3 CI step 4 N ei CI
NH HNyNH CH3
57c 0
57
Step 1
tert-butyl 5-chloro-6-iodoisoindoline-2-carboxylate 57a
To a mixture of 53d (40 mg, 0.143 mmol) in 5N KOH solution (2 mL) was added di-
tert-butyl
dicarbonate (100 mg, 0.45 mmol), the reaction was stirred at r.t for 18h. The
mixture was
cooled to 0 C, then filtered to give 57a (28 mg, 0.09 mmol, 63% yield).
Step 2
tert-butyl 5-chloro-6-methylisoindoline-2-carboxylate 57b
To a solution of 57a (20 mg, 0.052 mmol) in DME (3 mL) was added water (2 mL),
CH3B(OH)2 (20mg 0.33 mmol), K2CO3 (15 mg 0.1 mmol) and Pd (PPh3)2C12 (3 mg,
0.0052
mmol). The reaction mixture was stirred at 80 C for 18h under N2 atmosphere.
After the
reaction was completed, the reaction mixture was quenched and extracted with
Et0Ac (20 mL
X 2). The combined organic layers were washed with brine, dried over Na2SO4
and
concentrated to afford 57b. The crude product was used without purification
for next step.
Step 3
5 -chloro -6-methylisoindo line 57c
To a solution of 57b (20 mg, 0.052 mmol) in DCM (3 mL) was added 4N HC1 in 1,4
dioxane(2
mL). After the reaction was complete. The mixture was concentrated under
reduced pressure
and the residue was purified by silica-gel column (DCM: Me0H(2%NH4OH) = 10: 1)
to afford
57c (5 mg, 0. 029 mmol 60 % yield).
Step 4
127
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -(3 - (5-chloro-6-methylisoindo lin-2- y1)-3 -o xoprop y1)-5-c ycloprop
ylimid azolidine-2 ,4 -
dione 57
To a mixture of 57c (5 mg, 0.029 mmol) in DMF (2 mL) was added Triethylamine
(13 mg,
0.1mmol), It-1 (5 mg, 0.022 mmol) and HATU (12 mg, 0.031 mmol). The reaction
was stirred
at r.t for 18h. Water (3 mL) added and the mixture was extracted with Et0Ac
(20 mL X 2).
The combined layer was washed with brine, dried over Na2SO4 and concentrated.
The residue
was purified with prep-HPLC to give title compound 57 (3 mg, 0.008 mmol, 36 %
yield).
1H NMR (400 MHz, Methanol-d4): 6 7.36 (d, 1H), 7.28 (d, 1H), 4.83 (s, 2H),
4.71 (d, 2H),
2.63-2.41 (m, 2H), 2.39 (s, 3H), 2.23 (m, 2H), 0.60 (td, 2H), 0.53-0.38 (m,
2H), 0.35 (dt, 1H).
LCMS: nth (ES!): 3621M+Hr.
Example 58
(5S)-5 -c yc loprop y1-5 - (3 - (5,6-dichloro-1 -ethylisoindo lin-2- y1)-3 -o
xoprop yl) imid azolidine-
2 ,4-dio ne 58
V..r)L0
HN)r-NH CI
0
58
CI
0 0
0 CI CI CI
Step 1 Step 2 N Step 3
o NH
0 CI OH
CI CI CI
CI 0 0 0
20a 58b 58c 58d
0
Step 4 CI Step 5 )""tfrAN
HN)r_NH
NH-3" CI
CI 0
58e 58 ci
Step 1
4,5-dichloro-2-propionylbenzoic acid 58b
The mixture of 20a (1.0 g, 4.61 mmol), 2-methylpropanedioic acid (761.85 mg,
6.45 mmol)
and pyridine (1 mL) was stirred at 75 C for 3 h. The mixture was diluted with
water (2 mL)
and concentrated HC1 (2 mL), and the mixture was heated to 140 C and stirred
for 2 h. After
the reaction mixture was cooled to RT, the mixture was filtered and the cake
was dried to give
58b (640 mg, 2.59 mmol, 56.21% yield).
128
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: MS in/z (ES!): 249.3 [M+Hr.
Step 2
6,7-dichloro-4-ethyl- 1H-b enzo [d] [1,2]oxazin- 1 -o ne 58c
58b (200 mg, 809.47 umol) was added to the solution of KOH (129.45 mg, 2.31
mmol) in H20
.. (1.2 mL), and then hydroxylamine hydrochloride (120.38 mg, 1.73 mmol) was
added portion-
wise. The reaction was stirred at 30 C overnight. Then the mixture was
filtered and the filter
cake was dried to give 58c (80 mg, 327.77 umol, 40.49% yield).
11INMR (400 MHz, DMSO-d6): 6 8.40 (s, 1H), 8.31 (s, 1H), 3.01 (q, 2H), 1.24
(t, 3H).
LCMS: MS m/z (ES!): 244.3 [M+Hr.
Step 3
5 ,6-dichloro-3 -ethyliso indolin- 1 -one 58d
To a solution of 58c (350 mg, 1.43 mmol) in acetic acid (5 mL) was added Zn
(937.69 mg,
14.34 mmol). The mixture was heated to 118 C and stirred overnight. The
mixture was filtered
and the cake was washed with DCM, the filtrate was concentrated, and the
residue was
triturated with ether and filtered to give 58d (180 mg, 782.30 umol, 54.55%
yield).
11INMR (400 MHz, DMSO-d6): 6 8.96 (brs, 1H), 7.95 (s, 1H), 7.82 (s, 1H), 4.59-
4.56 (m,
1H), 2.01-1.90 (m, 1H), 1.65-1.54 (m, 1H), 0.79 (t, 3H).
LCMS: MS m/z (ES!): 230.4 [M+Hr.
Step 4
5,6-dichloro-1 -ethylisoindo line 58e
To a solution of 58d (1.32 g, 5.74 mmol) in THF (10 mL) was added BH3/THF
(793.68 mg,
57.37 mmol, 55 mL). The mixture was heated to 60 C and stirred overnight. The
reaction was
quenched with Me0H (5 mL) and then 6 M HC1 (adjusted pH to 1-2). The mixture
was heated
to 80 C for lh and then cooled to RT. The mixture was adjusted pH to 7-8 with
aq. NaOH (6N).
Then the mixture was dried over anhydrous Na2SO4 and concentrated in vacuo.
The residue
was purified by column chromatography (Et0Ac/hexane=1:20-1:1) to afford 58e
(630 mg,
2.92 mmol, 50.82% yield).
11INMR (400 MHz, DMSO-d6): 6 7.50 (s, 1H), 7.47 (s, 1H), 4.19-4.16 (m, 1H),
4.03 (s, 2H),
1.80-1.71 (m, 1H), 1.47-1.43 (m, 1H), 0.90 (t, 3H).
.. LCMS: MS m/z (ES!): 216.4 [M+Hr.
Step 5
(5S)-5 -c yc loprop y1-5 - (3 - (5,6-dichloro-1 -ethylisoindo lin-2- y1)-3 -o
xoprop yl) imid azolidine-
2,4-dione 58
129
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
To a solution of 58e (50 mg, 231.37 umol) in DMF (2 mL) was added TEA (0.3
mL), It-1
(53 mg, 249.76 umol) and HATU (100 mg, 263.00 umol). The mixture was stirred
at room
temperature for 3 h. Water was added, and then the mixture was extracted with
Et0Ac. The
combined organic layers were washed with water and brine, dried over anhydrous
Na2SO4,
filtered and concentrated in vacuo. The residue was purified by prep-HPLC to
give 58 (30 mg,
73.12 umol, 31.60% yield).
11INMR (400 MHz, DMSO-d6): 6 10.58 (br, 1H), 7.79-7.63(m, 3H), 5.27-5.21 (m,
1H), 4.83-
4.66 (m, 2H), 2.42-1.72 (m, 6H), 1.16-1.05 (m, 1H), 0.61-0.51 (m, 3H), 0.47-
0.29 (m, 3H),
0.16-0.07 (m, 1H).
LCMS: MS nth (ES!): 410.1 [M+Hr.
Example 59
(S)-5 -(3 -(5-bromo-6 - (triflu oro methyl) iso indolin-2 -y1)-3 -o xoprop y1)-
5 -
c ycloprop ylimidazolidine-2 ,4-dione 59
0 V 0
HN 15 NH CH
0 59
Br
0 0 0
H2N Step 1 Br Step 2 Br Step 3
CFI CFI CF3 Br
40d 59b 590
Oy..*=V
0
Br Step 4 Br Step 5
CF3
NH OIC __ HN NH )r- NH CF3
CF3 0 59
59d 59e Br
Step 1
Methyl 5 -bromo -2-methy1-4- (trifluoromethyl)b enzo ate 59b
To a suspension of 40d (500 mg, 2.14 mmol) in CH3CN (20 mL) was added isoamyl
nitrite
(377 mg, 3.22 mmol) and CuBr2 (960 mg, 4.30 mmol). After the mixture was
stirred at 70 C
overnight, the mixture was cooled down to room temperature and poured into ice
water (20
mL). Then the mixture was extracted with Et0Ac (50 mL). The organic phase was
dried
over Na2SO4(,), filtered. The filtrate was concentrated to afford crude 59b
(600 mg, 2.02 mmol,
94.20% yield).
130
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
111 NMR (400 MHz, CDC13): 6 8.20 (s, 1H), 7.56 (s, 1H), 3.93 (s, 3H), 2.59 (s,
3H).
Step 2
Methyl 5 -bromo-2- (bro momethyl)-4- (trifluoromethyl)benzo ate 59c
To a solution of 59b (100 mg, 336.62 umol) in CC14 (3 mL) was added AIBN (1.66
mg, 10.10
umol) and NBS (71.89 mg, 403.95 umol), and the mixture was stirred at 70 C
overnight. The
mixture was cooled to room temperature and filtered, the cake was washed with
DCM, the
filtrate was concentrated in vacuo to give crude 59c (150 mg, 398.97 umol,
118.52% yield).
Step 3
6 -bro mo -5- (triflu oromethyl) iso indolin- 1 -one 59d
To a solution of 59c (150 mg, 398.97 umol) in Me0H (1 mL) was added NH3/Me0H
(4 mL),
the mixture was stirred at room temperature overnight. The reaction mixture
was concentrated
in vacuo, and the residue was purified by silica gel chromatography
(Et0Ac/hexane=1/5) to
give 59d (60 mg, 214.25 umol, 53.70% yield).
11INMR (400 MHz, DMSO-d6): 6 9.03 (brs, 1H), 8.16 (s, 1H), 8.08 (s, 1H), 4.44
(s, 2H).
LCMS: MS m/z (ES!): 280.3 1M+Hr.
Step 4
5 -bromo -6- (trifluoromethyl) isoindo line 59e
To a solution of 59d (60 mg, 214.25 umol) in THF (2 mL) was added BH3/THF
(29.64 mg,
2.14 mmol, 5 mL), and the mixture was heated to 60 C and stirred overnight.
The reaction was
quenched with Me0H (5 mL) and the mixture was adjusted to pH 1-2 with 6M HC1.
The
mixture was heated to 80 C and stirred for lh. The reaction was cooled to room
temperature
and adjusted to pH 7-8 with 6 M NaOH. The mixture was extracted with
ethylacetate and the
organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. The
residue was
purified by silica gel chromatography (Me0H/DCM=1/20) to afford 59e (20 mg,
75.17 umol,
35.09% yield).
LCMS: MS m/z (ES!): 268.2 1M+H1 .
Step 5
(S)-5 -(3 -(5-bromo-6 - (triflu oro methyl) iso indolin-2 -y1)-3 -o xoprop y1)-
5 -
c ycloprop ylimidazolidine-2 ,4-dione 59
To a solution of 59e (20 mg, 75.17 umol) in DMF (1.5 mL) was added TEA (0.2
mL), Int-1
(16 mg, 75.40 umol) and HATU (30 mg, 78.90 umol). The mixture was stirred at
room
temperature for 2 h. Water was added and the mixture was extracted with EA.
The combined
organic layers were washed with water and brine, dried over anhydrous Na2SO4,
filtered and
131
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
concentrated in vacuo. The residue was purified by prep-HPLC to give 59 (10
mg, 21.73 umol,
28.90% yield).
11INMR (400 MHz, DMSO-d6): 6 10.62 (brs, 1H), 7.92 (s, 1H), 7.89 (s, 1H), 7.75
(s, 1H),
4.86-4.81 (m, 2H), 4.68-4.63 (m, 2H), 2.41-2.22 (m, 2H), 2.03-1.98 (m, 2H),
1.15-1.07 (m,
1H), 0.50-0.29 (m, 3H), 0.16-0.07 (m, 1H).
19F NMR (400 MHz, CDC13): 6 -60.79.
LCMS: MS raiz (ES!): 462.3 11\4+Hr.
Example 60
5-(3-oxo-3-(5-(trifluoromethyflisoindolin-2-yflpropy1)-5-(pyridin-2-
yflimidazolidine-2,4-
dione 60
0
HN-ANH 0
0
\ /
60 410
CF3
0
0 0 HNANH
Step 1 Step 2 Step 3
I I
0
a 0
60a
0 c 60b 0
0
HN-1(NH 0
HN-ANH 0
Step 4 0
0 OH
\ /
\ /
490
60d 60 CF3
Step 1
15 Tert-butyl 4-oxo-4-
(pyridin-2-yl)butanoate 60b
To a solution of 1-(pyridin-2-yl)ethanone 60a (24.2 g, 199 mmol) in THF (300
mL) at -70 C
was added LDA (120 mL, 240 mmol) dropwise. After the addition, the reaction
mixture was
stirred at this temperature for 30 min before tert-butyl 2-bromoacetate (39 g,
199 mmol) was
added dropwise. Then the resulting mixture was stirred atroom temperature for
18 hours. The
132
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
reaction mixture was diluted with aq. NH4C1 (100 mL). The mixture was
extracted with Et0Ac
(400 mL X 3). The organic layers were combined, washed with brine (400 mL),
dried over
Na2SO4, and filtered. The filtrate was concentrated under reduced pressure.
The residue was
purified by column chromatography (Et0Ac/hexane = 1/10 to 1/2) to afford 60b
(11.0 g, 46.7
mmol, yield: 23.4%).
LCMS: MS m/z (ES!): 236.1[1\4+Hr.
Step 2
Tert-butyl 3 -(2,5 -dio xo -4-(p yridin-2- yl)imidazolidin-4- yl)prop ano ate
60c
A mixture of 60b (4.7 g, 20.0 mmol), (NH4)2CO3 (16.3 g, 170 mmol), NaCN (2.45
g, 50.00
mmol), Et0H (25 mL) and H20 (25 mL) was heated in an autoclave at 85 C for 18
hours. The
resulting mixture was diluted with water (60 mL). The mixture was extracted
with Et0Ac (200
mL X 5). The organic layers were combined, washed with brine (200 mL), dried
over Na2SO4
and filtered. The filtrate was concentrated under reduced pressure. The
residue was purified by
column chromatography (Et0Ac/hexane = 1/10 to 1/1) to afford 60c (3.0 g, 9.83
mmol, yield:
49.2%).
LCMS: MS m/z (ES!): 306.1[1\4+Hr.
Step 3
3 -(2,5 -dio xo -4-(p yridin-2- yl)imidazolidin-4- yl)propanoic acid 60d
To a solution of 60c (1.0 g, 3.28 mmol) in DCM (10 mL) was added HC1/1,4-
dioxane (30 mL,
3.0 M). The resulting mixture was stirred at room temperature for 18 hours.
Then the reaction
mixture was concentrated under reduced pressure. The residue was washed with
Et20 (10 mL),
dried in vacuum to afford 60d (800 mg, 3.21 mmol, 98.01% yield).
LCMS: MS m/z (ES!): 248.2 [M-11-.
Step 4
5 -(3 -oxo-3- (5 -(trifluoro methyl) isoindo lin-2- yflprop y1)-5 - (p yridin-
2- yl) imid azolidine-2 ,4-
dione 60
A mixture of 60d (100 mg, 401.25 umol), 5-(trifluoromethyl)isoindoline 1-1
(89.7 mg,
401um01), Et3N (140 mg, 1.39 mmol), HATU (153 mg, 401um01) and DMF (10 mL) was
stirred at room temperature for 18 hours. Then the reaction mixture was
purified by prep-HPLC
to afford 60 (30 mg, 71.71 umol, 17.9% yield).
111 NMR (400 MHz, DMSO-d6): 6 10.92 (brs, 1H), 8.59 (d, 1H), 8.16-8.10 (m,
1H), 7.84 (t,
1H), 7.75 (d, 1H), 7.66 (d, 1H), 7.59-7.51 (m, 2H), 7.37-7.34 (m, 1H), 4.83
(brs, 2H), 4.69 (brs,
2H), 4.43-4.28 (m, 4H).
133
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: MS raiz (ES!): 419.0 [M+Hr.
Example 61
5-(3 -(5,6-dichloro isoindolin-2- y1)-3 -oxoprop y1)-5 -(p yridin-2 -y1) imid
azo lidine-2,4-dione 61
O
HN)i-NH CI
0 61
CI
To a solution of 60d (66.87 mg, 268.31 umol) in DMF (6 mL) was added DIEA
(173.06 mg,
1.34 mmol), followed by the addition of 20c (60 mg, 268.31 umol), EDCI (128.59
mg, 670.77
umol) and HOBt (47.13 mg, 348.80 umol). The mixture was stirred for 2 hours
and then the
mixture was purified by prep-HPLC to afford the tittle compound 61 (8.8 mg,
20.93 umol, 7.80%
yield).
111 NMR (400 MHz, DMSO-d6): 6 10.87 (s, 1H), 8.61 (s, 1H), 8.52 (s, 1H), 7.86
(t, 1H), 7.66
(s, 1H), 7.65 (s, 1H), 7.54 (d, 1H), 7.39-7.37 (m, 1H), 4.74 (brs, 2H), 4.60
(brs, 2H), 2.50-2.39
(m, 2H), 2.33-2.30 (m, 2H).
LCMS: MS raiz (ES!): 419.0 1M-PH1 .
Example 62
5-(1-methy1-1H-imidazol-2-y1)-5-(3-oxo-3-(5-(trifluoromethyflisoindolin-2-
yflpropyflimidazolidine-2,4-dione 62
0
HN-ANH 0
ON
N
62 CF3
134
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0 0
Step 1 N-yc Step 2 N Step 3
_____________________ /
0
62a 62b 62c
0
0 0
HNI-ANH 0
HWANH HN-1.NH 0
Step 4 Step 5 0
0 0 0 OH N-'
N¨
N
62d 62e 62 C F3
Step 1
1 -(1 -methyl- 1H-imid azol-2- yl)ethan- 1 -one 62b
To a solution of 1-methyl-1H-imidazole 62a (39 g, 475.01 mmol) in THF (350 mL)
at -70 C
was added n-BuLi (356 mL, 1.6N, 570.01 mmol) dropwise. After the addition, the
resulting
mixture was allowed to warm to 0 C and stirred at this temperature for 30 mm,
and then the
reaction was re-cooled to -70 C. Ethyl acetate (104.63 g, 1.19 mol) was added
dropwise at -
70 C. The reaction mixture was stirred at room temperature for 18 hours. The
reaction mixture
was diluted with aq. NH4C1 (100 mL). The whole mixture was extracted with
Et0Ac (400 mL
X 3). The organic layers were combined, washed with brine (400 mL), dried over
Na2SO4 and
filtered. The filtrate was concentrated under reduced pressure. The residue
was purified by
column chromatography (Et0Ac/hexane = 1/10 to 1/2) to afford 62b (26 g, 209.44
mmol, 44.09%
yield).
111 NMR (400 MHz, CDC13): 6 7.14 (s, 1H), 7.04 (s, 1H), 4.00 (d, 3H), 2.66 (d,
3H).
Step 2
Tert-butyl 4 -(1 -methyl- 1H-imid azol-2- y1)-4-o xobutano ate 62c
A solution of LDA (77.3 mL, 2N, 154.66 mmol) in THF (200 mL) was cooled to -78
C. A
solution of 62b (16.0 g, 128.89 mmol) was added dropwise, then the resulting
mixture was
warmed to 0 C and stirred for 30 mm. The reaction mixture was re-cooled to -78
C and tert-
butyl 2-bromoacetate (25.14 g, 128.89 mmol) was added slowly. The reaction was
stirred at
room temperature overnight. The reaction was quenched with saturated NH4C1
aq.(150 mL),
the whole mixture was extracted with Et0Ac (150 mL X 3). The combined organic
layer was
washed with brine (100 mL), dried over Na2SO4 and concentrated under reduced
pressure. The
residue was purified by column chromatography (Et0Ac/hexane = 1/8 to 1/1) to
afford 62c (13
g, 54.56 mmol, 42.33% yield).
135
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
111 NMR (400 MHz, CDC13): 6 7.15-7.13 (m, 1H), 7.06 (s, 1H), 4.01-3.99 (m,
3H), 3.44-3.38
(m, 2H), 2.67-2.61 (m, 2H), 1.46-1.43 (m, 9H).
Step 3
Tert-butyl 3 -(4-(1 -methyl-1H-imidazol-2 - y1)-2 ,5 -dio xo imidazolidin-4-
yflprop ano ate 62d
A mixture of 62c (4.0 g, 16.79 mmol), (NH4)2CO3 (13.70 g, 142.69 mmol), NaCN
(2.23 g,
41.97 mmol), Et0H (25 mL) and H20 (25 mL) was added to sealed vessel and
heated to 85 C
reacted for 18 hours. The reaction mixture was diluted with water (100 mL).
The whole mixture
was extracted with n-BuOH (100 mL X 3). The combined organic layers were
washed with
brine (50 mL), concentrated under reduced pressure. The residue was purified
by column
chromatography (DCM/Me0H= 100/1 to 10/1) to afford 62d (700 mg, 2.27 mmol,
13.52%
yield).
LCMS: miz (ES!): 309.4 1M+H1 .
Step 4
3 -(4 -(1-methy1-1H-imid azol-2- y1)-2,5 -dioxoimid azo lidin-4 - yflprop ano
ic acid 62e
To a solution 62d (700 mg, 2.27 mmol) in dioxane (20 mL) was added HO/dioxane
(20 mL,
6N, 120 mmol). The resulting mixture was stirred at room temperature for 18
hours. The
reaction mixture was concentrated under reduced pressure to afford crude 62e
(700 mg, 2.78
mmol).
LCMS: miz (ES!): 253.1 1M+H1 .
Step 5
5 -(1 -methy1-1H-imidazol-2- y1)-5 -(3 -o xo -3 -(5 -(trifluoro methyl) iso
indolin-2 -
yl)propyl) imidazolidine-2 ,4-dione 62
The mixture of 62e (100 mg, 396.47 umol) and 5-(trifluoromethyl)isoindoline 1-
1 (89 mg,
396.47 umol) in DMF (5 mL) was added HATU (150mg, 396.47 umol) and Et3N (160
mg,
1.59 mmol), the reaction was stirred at room temperature for 18 hours. The
reaction mixture
was purified by prep-HPLC to afford 62 (30 mg, 71.20 umol, 17.96% yield).
111 NMR (400 MHz, DMSO-d6): 6 8.59-8.57 (m, 1H), 7.77 (s, 1H), 7.68-7.65 (m,
1H), 7.60-
7.58 (m, 1H), 7.19 (s, 1H), 6.85 (s, 1H), 4.86-4.85 (m, 2H), 4.71 (br, 2H),
3.55 (s, 3H), 2.61-
2.54 (m, 2H), 2.50-2.38 (m, 2H).
LCMS: MS miz (ES!): 422.4 1M+H1 .
Examples 62-1 and 62-2
136
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5-(1-methy1-1H-imidazol-2-y1)-5-(3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
y1)propyl)imidazolidine-2,4-dione 62-1
(R)-5-(1-methy1-1H-imidazol-2-y1)-5-(3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
y1)propyl)imidazolidine-2,4-dione 62-2
0 0
HN-ANH40 HNA - 0
01"'l7_ 0
N N -="-\
CF3 CF3
62-1 62-2
62 (200 mg) was separated by SFC to give two single enantiomers (62 mg and 63
mg).
Enantiomer (shorter retention time):
111 NMR (400 MHz, DMSO-d6) 6 11.22 (s, 1H), 8.58 (d, 1H), 7.76 (s, 1H), 7.66
(d, 1H), 7.58
(dd, 1H), 7.19 (s, 1H), 6.85 (t, 1H), 4.86-4.84 (m, 2H), 4.71 (brs, 2H), 3.54
(s, 3H), 2.62-2.53
(m, 2H), 2.49-2.39 (m, 2H).
LCMS: m/z (ES!): 422.1 11\4+Hr.
ChirHPLC (CO2/Et0H/DEA 60/40/0.04 2.8m1/min OZ,5um,4.6*250(Daicel)): Rt:3.326
mm,
ee:94.58%.
Enantiomer (longer retention time):
111 NMR (400 MHz, DMSO-d6) 6 11.23 (s, 1H), 8.59 (d, 1H), 7.76 (s, 1H), 7.66
(d, 1H), 7.59
(dd, 1H), 7.20 (s, 1H), 6.86 (s, 1H), 4.86-4.84 (d, 2H), 4.71 (brs, 2H), 3.55
(s, 3H), 2.61-2.53
(m, 2H), 2.49-2.42 (m, 2H).
LCMS: m/z (ES!): 422.1 11\4+Hr.
ChirHPLC (CO2/Et0H/DEA 60/40/0.04 2.8m1/min OZ,5um,4.6*250(Daicel)): Rt:4.538
mm,
ee:100%.
Example 63
5-(methoxymethyl)-5-(3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yflpropyflimidazolidine-2,4-
dione 63
0 0
0
HN)r-NH CF3
0 63
137
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 0 0
_____________________________________________________ )
HO)
Step 1 Step 2 Step 3 r
HO 0
0 0
63a 63b 63c
0 0 0
Step 4
0
0 CF3 HN)i-NH CF3
63d 0 63
Step 1
Methyl 5-methoxy-4-oxopentanoate 63b
To a solution of iodosylbenzene (16.82 g, 76.45 mmol) in DCM (700 mL) at 0 C
was added
pent-4-ynoic acid 63a (5 g, 50.97 mmol), boron trifluoride etherate (14.47 g,
101.94 mmol)
was added dropwise. The reaction was stirred at r.t for lh. The resulting
precipitate was
separated by filtration and the solid was dried. Me0H (20 mL) was added, the
reaction was
stirred at r.t for 18h. The solution was concentrated and the residue was
purified by silica gel
chromatography (hexane:Et0Ac=10:1) to give 63b (3.6 g, 44.10% yield).
1H NMR (400 MHz, CDC13): 6 4.07 (s, 2H), 3.68 (s, 3H), 3.43 (s, 3H), 2.77-2.75
(m, 2H),
2.67-2.64 (m, 2H).
Step 2
5-methoxy-4-oxopentanoic acid 63c
To a solution of 63b (500 mg, 3.12 mmol) in Water (10 mL) and THF (10 mL) was
added
LiOH (149 mg, 6.24 mmol). The reaction was stirred at r.t for 2h. Water (20
mL) was added
and the mixture was extracted with Et0Ac (20 mL X 2). The organic solution was
washed
with brine, dried over Na2SO4 and concentrated to give crude 63c (260 mg,
56.99% yield). The
crude product was used directly in next step.
Step 3
5 -metho xy- 1 - (5 - (triflu oro methyl) isoindo lin-2- yl)pentane- 1 ,4-dio
ne 63d
To a solution of 63c (260 mg, 1.78 mmol) and 5-(trifluoromethyl)isoindoline 1-
1 (333 mg, 1.49
mmol, HC1) in DMF (20 mL) was added triethylamine (720 mg, 7.12 mmol) and HATU
(812mg, 2.13 mmol). The reaction was stirred at r.t for 2h. Water (40 mL) was
added and the
mixture was extracted with Et0Ac (30 mL X 2), the organic solution was washed
with brine,
dried over Na2SO4 and concentrated. The residue was purified by silica gel
chromatography
(hexane: Et0Ac=2:1) to give 63d (320 mg, 96.10% yield).
LCMS: raiz (ES!): 316.1 [M+Hr.
138
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Step 4
-(metho xymethyl)-5 -(3-o xo -3 -(5 -(triflu oro methyl)isoindo lin-2- yl)prop
yl) imidazolidine-2 ,4-
dione 63
To a solution of 63d (200 mg, 634.34 umol) in Et0H (5 mL) was added to a
solution of
5 Ammonium carbonate (488 mg, 5.07 mmol) in water (5 mL). Then Sodium
cyanide (78 mg,
1.59 mmol) was added. The reaction was stirred at 80 C for 18h in the sealed
vessel. The
solution was cooled to r.t. Water (50 mL) was added and the mixture was
extracted with Et0Ac
(40 mL X 2). The organic solution was washed with brine, dried over Na2SO4 and
concentrated.
The residue was purified by prep-HPLC to give 63 (140 mg, 57.27% yield).
111 NMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.89 (s, 1H), 7.75 (s, 1H), 7.67
(d, 1H), 7.59
(d, 1H), 4.88-4.85 (m, 2H), 4.68 (br, 2H), 3.51 (d, 1H), 3.35 (d, 1H), 3.25
(s, 3H), 2.43-2.34
(m, 1H), 2.27-2.18 (m, 1H), 1.89-1.75 (m, 2H).
LCMS: m/z (ES!): 386.1 lIVI+Hr.
Examples 63-1 and 63-2
(S)-5 -(methoxymethyl)-5 -(3 -oxo-3 -(5 -(trifluoromethyl) isoindolin-2-
yl)prop yl)imid azo lidine-
2,4-dione 63-1
(R)-5 -(metho xymethyl)-5 -(3 -oxo-3 -(5-(trifluoromethyl) isoindo lin-2-
yl)prop yl)imid azolidine-
2,4 -dione 63-2
0 0
/0 0
HNn
y
H N"--)L NH CF3 NH CF3
0
0
63-1 63-2
63 was seperated by SFC to give the title single enantiomer compounds (42 mg
and 55 mg).
Enantiomer (shorter retention time):
111 NMR (400 MHz, DMSO-d6): 6 10.59 (br, 1H), 7.89 (d, 1H), 7.75 (s, 1H), 7.67
(d, 1H),
7.58 (d, 1H), 4.88-4.85 (m, 2H), 4.68 (br, 2H), 3.50 (d, J= 9.6 Hz, 1H), 3.35
(d, 1H), 3.25 (s,
3H), 2.42-2.32 (m, 1H), 2.26-2.17 (m, 1H), 1.89-1.76 (m, 2H).
LCMS: m/z (ES!): 386.1 [1\4+Hr
ChirHPLC (Et0H/DEA 5%_40% 1.5m1/min IC, 3um 3.0*100(Daicel)): Rt:4.084 mm,
cc: 100%.
Enantiomer (longer retention time):
139
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
111 NMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.89 (s, 1H), 7.75 (s, 1H), 7.67
(d, 1H), 7.58
(d, 1H), 4.88-4.85 (m, 2H), 4.68 (br, 2H), 3.50 (d, 1H), 3.35 (d, 1H), 3.25
(s, 3H), 2.43-2.32
(m, 1H), 2.27-2.17 (m, 1H), 1.88-1.76 (m, 2H).
LCMS: raiz (ES!): 386.11M+Hr.
ChirHPLC (Et0H/DEA 5%_40% 1.5m1/min IC, 3um 3.0*100(Daicel)): Rt:4.766 min,
ee:100%.
Example 64
5-(1 -methylc ycloprop y1)-5 -(3 -oxo-3 -(5-(triflu oromethyl) iso indolin-2 -
yl)propyl)imidazolidine-2,4-dione 64
HN C F3
0 64
j3
0 L
0
)c Step 1 Step 2 0 Step 3
>i
__________________________________ >1)(0 ___________ HNy.NH
0 64a 64b 064c
HN = CF3
0 j)L
OH
HN Step 4 NH HNyNH 4100 CF3
64d 0
64
Step 1
Tert-butyl 4-(1 -methylc yc loprop y1)-4-o xobu tano ate 64b
The solution of LDA (1.31 g, 12.23 mmol) in THF (5 mL) was colded to -78 C and
stirred for
min under N2 atmosphere. Then the solution of 1-(1-methylcyclopropyl)ethanone
64a (1.0
g, 10.19 mmol) in THF (3 mL) was added dropwise before the reaction mixture
was warmed
to 20 C for 30 min. Then the mixture was re-cooded to -78 C before the
solution of tert-butyl
2-bromoacetate (1.99 g, 10.19 mmol) in THF (2 mL) was added dropwise. The
mixture was
20 stirred at room temperature overnight. The reaction was quenched by
saturated NH4C1 and then
extracted with ethylacetate. The combined organic layers were washed with
water and brine,
140
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
dried over anhydrous Na2SO4, filtered and concentrated. The residue was
purified by silica gel
chromatography (Et0Ac/hexane=1/10) to afford 64b (1.6 g, 7.54 mmol, 73.97%
yield).
Step 2
Tert-butyl 3 -(4- (1 -methylc ycloprop y1)-2 ,5-dioxoimidazolidin-4- yl)prop
ano ate 64c
To a solution of 64b (1.7 g, 8.01 mmol) in Me0H (25 mL) was added (NH4)2CO3
(4.16 g,
43.24 mmol), H20 (25 mL) and NaCN (1.06 g, 20.02 mmol). The reaction mixture
was stirred
at 80 C in a seal tube overnight and then cooled to RT. Water was added, the
mixture was
extracted with ethylacetate. The combined organic layers were washed with
water and brine,
dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue
was triturated
with Et0Ac/hexane=1/5, filtered and dried to give 64c (880 mg, 3.28 mmol,
40.96% yield).
11INMR (400 MHz, CDC13): 6 8.31 (brs, 1H), 6.01 (brs, 1H), 2.32-2.24 (m, 2H),
2.18-2.07
(m, 2H), 1.44 (s, 9H), 1.21 (s, 3H), 0.76-0.70 (m, 1H), 0.61-0.55 (m, 1H),
0.41-0.28 (m, 2H).
Step 3
3 -(4- (1 -methylc yclopropy1)-2,5-dioxoimidazolidin-4- yl)propanoic acid 64d
To a solution of 64c (880 mg, 3.28 mmol) in Me0H (10 mL) was added HC1/Dioxane
(16 mL,
6N), and the mixture was stirred at room temperature for 2 h. The reaction
mixture
was concentrated in vacuo and the residue was triturated with ether, filtered
and dried to give
64d (630 mg, 2.97 mmol, 90.52% yield). The product was directly used for the
next reaction.
Step 4
5 -(1 -methylc ycloprop y1)-5 -(3 -oxo-3 - (5- (trifluoromethyl) iso indolin-2
-
yl)prop yl) imidazolidine-2 ,4-dione 64
To a solution of 5-(trifluoromethyl)isoindoline 1-1 (90 mg, 480.87 umol) in
DMF (10 mL)
was added TEA (0.3 mL), 64d (100 mg, 471.25 umol) and HATU (197.10 mg, 518.37
umol).
The mixture was stirred at room temperature for 2h. Water was added, and the
mixture was
extracted with Et0Ac. The combined organic layers were washed with water and
brine, dried
over anhydrous Na2SO4, filtered and concentrated. The residue was purified by
prep-HPLC
to give 64 (130 mg, 340.90 umol, 72.34% yield).
11INMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.90 (s, 1H), 7.78 (s, 1H), 7.66
(d, 1H), 7.58
(d, 1H), 4.86 (brs, 2H), 4.70 (brs, 2H), 2.42-2.20 (m, 2H), 2.07-1.94 (m, 2H),
1.15 (s, 3H),
0.65-0.58 (m, 1H), 0.50-0.44 (m, 1H), 0.29-0.18 (m, 2H).
19FNMR (376.5 MHz, DMSO-d6): 6 -60.55.
LCMS: MS m/z (ES!): 396.4 [M+Hr.
141
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Examples 64 -1 and 64-2
(R)-5-(1-methylcyclopropy1)-5-(3-oxo-3-(5-(trifluoromethyflisoindolin-2-
yl)propyflimidazolidine-2,4-dione 64-1
(S)-5 -(1-methylcyclopropy1)-5-(3-oxo-3-(5-(trifluoromethyl)isoindolin-2-
yl)propyl)imidazolidine-2,4-dione 64-2
Od; 0
HN1 HN
)r-NH CF 3 )i-NH = CF3
0 0
64-1 64-2
64 (70 mg) was separated by SFC (DAICELCHIRALCELOIG) to give two enantiomers
(22
mg and 18 mg).
Enantiomer (shorter retention time):
11INMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.90 (d, 1H), 7.77 (s, 1H), 7.66
(d, 1H), 7.58
(d, 1H), 4.87-4.85 (m, 2H), 4.70 (brs, 2H), 2.40-2.19 (m, 2H), 2.08-1.94 (m,
2H), 1.15 (s, 3H),
0.65-0.58 (m, 1H), 0.50-0.44 (m, 1H), 0.30-0.17 (m, 2H).
19FNMR (376.5 MHz, DMSO-d6): 6 -60.56.
LCMS: MS m/z (ES!): 396.4 1M+H1 .
Chiral HPLC (CO2/Me0H/DEA 5%-40% 1.5m1/min IG,3um,3*100(Daicel)): Rt: 5.027
min,
cc: 100%.
Enantiomer (longer retention time):
11INMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.90 (s, 1H), 7.77 (s, 1H), 7.66
(d, 1H), 7.58
(d, 1H), 4.86 (brs, 2H), 4.70 (brs, 2H), 2.41-2.20 (m, 2H), 2.04-1.93 (m, 2H),
1.15 (s, 3H),
0.63-0.60 (m, 1H), 0.47 (br, 1H), 0.29-0.17 (m, 2H).
19FNMR (376.5 MHz, DMSO-d6): 6 -60.56.
LCMS: MS m/z (ES!): 396.4 1M+H1 .
Chiral HPLC (CO2/Me0H/DEA 5%-40% 1.5m1/min IG,3um,3*100(Daicel)): Rt: 5.892
min,
cc: 100%.
Example 65
5-(2-((benzyloxy)methyl)-3-oxo-3-(5-(trifluoromethyl)isoindolin-2-yflpropy1)-5-
cyclopropylimidazolidine-2,4-dione 65
142
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0
0
HNyNhl
0 CF3
0
0
v)X-I
0 Step 1 AT Step 2 HN
OBn 0 CF3 ________________________ CF3
0
42e 65a SI 65
Step 1
2 -((benzylo xy)methyl)-4 -cyc loprop yl- 1 - (5 - (trifluoromethyl) iso
indolin-2- yl)b utane- 1,4-dio ne
5 65a
To a solution of 42e (200 mg, 762.48 umol) and 5-(trifluoromethyl)isoindoline
1-1 (120 mg,
638.66 umol) in DMF (10 mL), triethylamine (232 mg, 2.29 mmol) and HATU
(347.90 mg,
914.98 umol) were added. The reaction was stirred at room temperature for 2h.
Water (20 mL)
was added and the mixture was extracted with Et0Ac (20 mL X 2), the organic
solution was
10 washed with brine, dried over Na2SO4 and concentrated. The residue was
purified by prep-TLC
to give 65a (200 mg, 60.80% yield).
LCMS: m/z (ES!): 432.1 [M+Hr.
Step 2
5 -(2- ((b enz ylo xy)methyl)-3 -oxo-3 - (5- (triflu oromethyl)iso indolin-2 -
yflprop y1)-5 -
15 cyclopropylimidazolidine-2,4-dione 65
To a solution of 65a (200 mg, 463.56 umol) in Et0H (2 mL), ammonium carbonate
(356.35
mg, 3.71 mmol), NaCN (61.47 mg, 1.16 mmol) and water (2 mL) were added. The
reaction
was stirred at 80 C for 18h. Water (20 mL) was added and the mixture was
extracted with
Et0Ac (20 mL X 2), the organic solution was washed with brine, dried over
Na2SO4 and
20 concentrated. The residue was purified by prep-HPLC to give 65 (85 mg,
36.56% yield).
111 NMR (400 MHz, DMSO-d6): 6 10.68 (brs, 1H), 7.78-7.66 (m, 3H), 7.61-7.53
(m, 1H),
7.23 (br, 5H), 5.03-4.64 (m, 4H), 4.45 (s, 2H), 3.55-3.42 (m, 2H), 2.99-2.91
(m, 1H), 2.33-2.23
(m, 1H), 1.86-1.82 (m, 1H), 1.08-0.99 (m, 1H), 0.44-0.24 (m, 3H), 0.04-0.01
(m, 1H).
LCMS: m/z (ES!): 502.2 [M+Hr.
Example 66
143
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(5 S)-5 -c ycloprop y1-5 - (3 - (5 ,6-dichloro-1 -methylisoindo lin-2- y1)-3 -
o xoprop yl) imidazolidine-
2 ,4-dione 66
0 V 0
N H CI
0
66 CI
0 0
0 CI CI
Step 1 Step 2 CI Step 3
oI _____________________________________________________________________ NH
0 CI OH
CI CI CI
CI 0 0 0
20a 66b 66c 66d
0 V 0
Step 4 CI Step 5
NH ____ . HNH 40 CI
CI 0
66e 66 CI
Step 1
2-acetyl-4,5-dichlorobenzoic acid 66b
To a mixture of 5,6-dichloroisobenzofuran-1,3-dione 20a (5g, 23.04 mmol) and
3,3-
dihydroxypropanoic acid (3.67 g, 34.56 mmol) in pyridine (5 mL) was stirred at
75 C for 2h.
Water (16 mL) and conc. HC1 (16 mL) were added, the reaction was stirred at
130 C for 30min.
The mixture was cooled to r.t, then filtered to give 66b (2.1 g, 39.11%
yield).
LCMS: MS rah (ES!): 230.9 1M-H1-.
Step 2
6,7 -dichloro-4-methyl- 1H-b enzo [d][1,21oxazin- 1 -o ne 66c
To a solution of 66b (2.1 g, 9.01 mmol) in water (12 mL) was added KOH (1.52
g, 27.03 mmol).
Then hydroxylamine hydrochloride (1.25 g, 18.02 mmol) was slowly added to the
solution.
The reaction was stirred at room temperature for 18h. The solution was cooled
to 0 C, the
resulting precipitate was filtered. The solid was dried to give 66c (1.2 g,
57.89% yield).
LCMS: MS rah (ES!): 230.1 1M+Hr.
Step 3
5 ,6 -dichloro-3 -methyliso indolin- 1 -o ne 66d
To a solution of 66c (3 g, 13.04 mmol) in acetic acid (20 mL) was added Zn (10
g, 153.85
mmol). The reaction was stirred at 115 C for 24h. The mixture was cooled to
room temperature
144
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
and filtered, the cake was washed with DCM, and the filtrate was concentrated.
The residue
was purified by trituration (hexane: Et0Ac=4:1) to give 66d (2 g, 70.98%
yield).
LCMS: MS nth (ES!): 216.1 [M+Hr.
Step 4
5 ,6-dichloro-1 -methylisoindo line 66e
To a solution of 66d (2 g, 9.26 mmol) in THF (10 mL) was added borane-
tetrahydrofuran
complex (629.44 mg, 37.03 mmol, 20 mL). The reaction was stirred at 60 C for
18h. Me0H
(2 mL) was added dropwise and HC1 (6M, 2mL) was added, the reaction was
stirred at 80 C
for 2h. Then NaOH (5M) was added to adjust the mixture to pH=7, the solution
was dried and
concentrated. The residue was purified by was added (DCM:Me0H=20:1) to give
66e (900
mg, 4.45 mmol, 48.11% yield).
111 NMR (400 MHz, DMSO-d6): 6 7.62 (s, 2H), 4.63-4.58 (m, 1H), 4.31-4.19 (m,
2H), 1.46
(d, J= 6.8 Hz, 3H).
LCMS: nth (ES!): 202.1 [M+Hr.
Step 5
(5 S)-5 -c ycloprop y1-5 - (3 - (5 ,6-dichloro-1 -methylisoindo lin-2- y1)-3 -
o xoprop yl) imidazolidine-
2,4-dione 66
To a solution of 66e (1 g, 4.95 mmol) and Int-1 (1.16 g, 5.44 mmol) in DMF (50
mL),
triethylamine (1.50 g, 14.85 mmol) and HATU (2.26 g, 5.94 mmol) were added.
The reaction
was stirred at room temperature for 2h. Water (100 mL) was added and the
mixture was
extracted with Et0Ac (100 mL X 2). The combined organic phases were washed
with brine,
dried over Na2SO4 and concentrated. The residue was purified by silica gel
chromatagraphy
(DCM: Me0H=50:1) to give 66 (900 mg, 46% yield).
LCMS: MS nth (ES!): 396 [M+Hr
111 NMR (400 MHz, Methanol-d4) 6 7.40 (d, 2H), 5.14 (q, 1H), 4.71 (d, 2H),
2.56-2.23 (m,
2H), 2.22-2.01 (m, 2H), 1.39 (t, 3H), 1.13 (m, 1H), 0.48 ¨0.17 (m, 4H).
Examples 66-1 and 66-2
(S)-5 -c yc loprop y1-5 -(3 - ((S) - 5 ,6 -dichloro -1 -methylisoindo lin-2-
y1)-3 -
oxoprop yl)imidazolidine-2,4-dione 66-1
(S)-5 -c yc loprop y1-5 -(3 - ((R) - 5 ,6-dichlor o-1 -methylisoindo lin-2 -
y1)-3 -
oxoprop yl)imidazolidine-2,4-dione 66-2
145
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
FdNI),r NH HNN
CI yNH * I CI
0 0
CI C
66-1 66-2
Racemate 66 (900 mg) was seperated by SFC to give to give two isomers (340 mg
and 320 mg
separately).
Isomer with longer retention time:
111 NMR (400 MHz, DMSO-d6): 6 10.62 (s, 1H), 7.78-7.65 (m, 3H), 5.20 -5.10 (m,
1H), 4.81-
4.71 (m, 2H), 2.49-2.18 (m, 2H), 2.03-1.97(m, 2H), 1.44-1.39 (m, 3H), 1.15-
1.07 (m, 1H),
0.49-0.29 (m, 3H), 0.15-0.08 (m, 1H).
LCMS: nth (ES!): 396.0 1M+H1 .
ChirHPLC(CO2/Me0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:3.001
min,
ee 97.10%.
Isomer with shorter retention time:
111 NMR (400 MHz, DMSO-d6): 6 10.64 (br, 1H), 7.80-7.65 (m, 3H), 5.25-5.10 (m,
1H), 4.81-
4.71 (m, 2H), 2.51-2.23 (m, 2H), 2.10-1.93 (m, 2H), 1.43-1.38 (m, 3H), 1.15-
1.05 (m, 1H),
0.49-0.29 (m, 3H), 0.14-0.06 (m, 1H).
LCMS: nth (ES!): 396.0 1M+H1 .
ChirHPLC (CO2/Me0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:1.689
min,
ee:100%.
Examples 67-1, 67-2, 68-1, 68-2
(S)-5 -(3 -((R)-8 -chloro-9-fluoro-1 -methyl-1,2 ,4,5 -tetrahydro-3H-b enzo
[di azepin-3 - y1)-3 -
oxoprop y1)-5-cyclopropylimidazolidine-2,4-dione 67-1
(S)- 5 -(3 -((S)-8-chloro-9-fluoro-l-methy1-1,2,4 ,5-tetrahydro-3H-benzo [di
azepin-3 -y1)-3 -
oxoprop y1)-5-cyclopropylimidazolidine-2,4-dione 67-2
(S)-5 -(3 -((S)-8-chloro-7 -fluoro-l-methyl-1,2,4 ,5-tetrahydro-3H-benzo [di
azepin-3 -y1)-3 -
oxopropy1)-5-cyclopropylimidazolidine-2,4-dione 68-1
(S)-5 -(3 -((R)-8 -chloro-7 -fluoro-1 -methyl-1,2 ,4,5 -tetrahydro-3H-b enzo
[di azepin-3 - y1)-3 -
oxoprop y1)-5-cyclopropylimidazolidine-2,4-dione 68-2
146
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
N N
HN CI HN-------r CI
yNH )r...NH
0 67-1 0 67-2
0 V 0
0 V 0
H H
NN CI HN)r...NH CI
)r-N
0 68-1 F 0 68-2 F
F F
CI CF3 Step 1 CI CF3 CI CF3 Step 2
CI CI
0 F 0 0 F
37b 68b 67b 67c 68c
N N
HN V. HN F N
CI
)r-NH )7...NH CI HN NH . CI
)7--
Step 3 0 67 SFC 0 67-2 F 0 67-1
)r-NH HN)rNH CI Fdr-r----
-N CI
0 68 F )7...NH
68-1 0 68-2 F
Step 1
Mixture of 1-(8-chloro-9-fluoro-1-methy1-1,2,4,5-tetrahydro-3H-benzo[d]azepin-
3-y1)-2,2,2-
trifluoro ethan-1 -one 67b & 1-(8-chloro-7-fluoro-l-methy1-1,2,4,5-tetrahydro-
3H-
benzo [d] azepin-3 - y1)-2,2,2-trifluoro ethan-1 -one 68b
To a solution of 37b (2 g, 6.86 mmol) in 1,2-dichloroethane (12 mL),
trifluoromethanesulfonic
acid (10.29 g, 68.56 mmol) and Selectfluor (4.86 g, 13.71 mmol) were added.
The reaction was
stirred at 75 C for 18h. Water (50 mL) was added and the mixture was extracted
with DCM
(20 mL X 2). The combined organic phases were washed with brine, dried over
Na2SO4 and
concentrated. The residue was purified by silica gel chromatagraphy (hexane:
Et0Ac = 20: 1)
to give the mixture of 67b & 68b (800 mg, 37.68% yield).
LCMS: MS mh (ES!): 310.0 [M+Hr.
Step 2
Mixture of 8-chloro-9-fluoro-l-methy1-2,3,4,5-tetrahydro-1H-benzo[d]azepine
67c & 1-(8-
chloro-9-fluoro-l-methy1-1,2,4,5-tetrahydro-3H-benzo [d]azepin-3 - y1)-2,2,2-
trifluoro ethan-1 -
one 68c
147
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of 67b & 68b (800 mg, 2.58 mmol) in Me0H (18 mL) was added a
solution of
NaOH (207 mg, 5.17 mmol) in water (5 mL). The reaction was stirred at room
temperature for
2h. Water (20 mL) was added and the mixture was extracted with Et0Ac (20 mL X
3), the
combined layers was washed with brine, dried over Na2SO4 and concentrated to
give the
mixture of 67c & 68c (550 mg, 2.57 mmol, 99.64% yield). The crude solid was
used to next
step directly.
Step 3
Mixture of (5S)-5-(3 -(8-chloro-9-fluoro-1-methyl- 1,2,4,5 -tetrahydro-3H-
benzo [di azep in-3 -
y1)-3 -o xoprop y1)-5 -c ycloprop ylimid azolidine-2,4 -dio ne 67 & (SS)-5 -(3
-(8-chloro-7-flu oro -1-
methyl-1 ,2,4,5 -tetrahydro -3H-b enzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 68
To a solution of 67c & 68c (500 mg, 2.34 mmol) and 34(45)-4-cyclopropy1-2,5-
dioxo-
imidazolidin-4-yllpropanoic acid It-1 (601 mg, 2.83 mmol) in DMF (20 mL),
triethylamine
(710.34 mg, 7.02 mmol) and HATU (1.07 g, 2.81 mmol) were added. The reaction
was stirred
at room temperature for 2h. The reaction was diluted with water (50 mL) and
the mixture was
extracted with Et0Ac (50 mL X 2). The combined organic layers were washed with
brine,
dried over Na2SO4 and concentrated. The residue was purified by prep-HPLC to
give the
mixture of 67 & 68 (700 mg, 73.35% yield).
(S)-5 -(3 -((R)-8 -chloro-9 -fluoro-1 -methyl-1,2 ,4,5 -tetrahydro-3H-b enzo
[di azepin-3 - y1)-3 -
oxopropy1)-5-cyclopropylimidazolidine-2,4-dione 67-1 & (S)-5 -(3-((S)-8-chloro
-9-fluoro-1 -
methyl-1 ,2,4,5 -tetrahydro -3H-b enzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 67-2
(S)- 5 -(3 -((S)-8-chloro-7 -flu oro -1 -methyl- 1,2,4 ,5-tetrahydro-3H-benzo
[di azepin-3 -y1)-3 -
o xoprop y1)-5 -cyc loprop ylimid azo lidine-2,4-dione 68-1 & (S)-5 -(3 -((R)-
8 -chloro-7 -fluoro-1-
methyl-1 ,2,4,5 -tetrahydro -3H-b enzo [di azepin-3 - y1)-3 -o xoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 68-2
Mixture of 67 & 68 was separated by SFC (Me0H/DEA 5%_40% 1.5ml/min IA, 3um
3.0*100(Daicel)) to give four isomers.
Isomer (peak 1, Rt: 4.729 min):
111 NMR (400 MHz, DMSO-d6): 6 10.59 (d, 1H), 7.70 (d, 1H), 7.33 (t, 1H), 7.02
(t, 1H),
3.91-3.48 (m, 4H), 3.33-3.30 (m, 1H), 3.28-3.07 (m, 1H), 2.94-2.82 (m, 1H),
2.40-2.15 (m,
2H), 1.97-1.73 (m, 2H), 1.15 (dd, 3H), 1.08-0.99 (m, 1H), 0.43-0.25 (m, 3H),
0.10-0.03 (m,
1H).
148
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: m/z (ES!): 408.1[1\4+Hr.
ChirHPLC 1 (Me0H/DEA 5%_40% 1.5m1/min IA, 3um 3.0*100(Daicel)): peak 1, Rt:
4.729
min.
ChirHPLC 2 (CO2/Et0H/DEA 60/40/0.04 2.8m1/min AD,5um,4.6*250(Daicel)):
Rt:2.736
min, ee:100%.
Isomer (peak 2, Rt: 5.284 min):
1H NMR (400 MHz, DMSO-d6): 6 10.59 (d, 1H), 7.71 (d, 1H), 7.33 (t, 1H),7.02
(t, 1H), 3.89-
3.47 (m, 4H), 3.31-3.06 (m, 2H), 2.91-2.82 (m, 1H), 2.36-2.00 (m, 2H), 1.95-
1.78 (m, 2H),
1.15 (dd, 3H), 1.08-1.00 (m, 1H), 0.46-0.25 (m, 3H), 0.11-0.04 (m, 1H).
LCMS: m/z (ES!): 408.1[1\4+Hr.
ChirHPLC 1 (Me0H/DEA 5%_40% 1.5m1/min IA, 3um 3.0*100(Daicel)): peak 2, Rt:
5.284
min.
ChirHPLC 2 (CO2/Et0H/DEA 60/40/0.04 2.8m1/min AD,5um,4.6*250(Daicel)):
Rt:5.410
min, ee:99.42%.
Isomer (peak 3, Rt: 5.970 min):
1H NMR (400 MHz, DMSO-d6): 6 10.60 (d, 1H), 7.70 (d, 1H), 7.34 (dd, 1H), 7.23
(t, 1H),
3.65-3.38 (m, 4H), 3.22-2.80 (m, 3H), 2.41-2.21 (m, 2H), 1.93-1.75 (m, 2H),
1.20 (dd, 3H),
1.10-1.01 (m, 1H), 0.46-0.26 (m, 3H), 0.11-0.05 (m, 1H).
LCMS: m/z (ES!): 408.1[1\4+Hr.
Chiral HPLC 1 (Me0H/DEA 5%_40% 1.5m1/min IA, 3um 3.0*100(Daicel)): peak 3, Rt:
5.970 min.
Chiral HPLC 2 (CO2/Et0H/DEA 60/40/0.04 2.8m1/min AD,5um,4.6*250(Daicel)): Rt:
3.801
min, ee:100%.
Isomer (peak 4, Rt: 6.442 min):
1H NMR (400 MHz, DMSO-d6): 6 10.60 (s, 1H), 7.72 (d, 1H), 7.34 (t, 1H), 7.23
(dd, 1H),
3.66-3.38 (m, 4H), 3.23-2.80 (m, 3H), 2.45-2.16 (m, 2H), 1.93-1.78 (m, 2H),
1.20 (dd, 3H),
1.09-1.00 (m, 1H), 0.48-0.26 (m, 3H), 0.12-0.05 (m, 1H).
LCMS: m/z (ES!): 408.1[1\4+Hr.
ChirHPLC 1 (Me0H/DEA 5%_40% 1.5m1/min IA, 3um 3.0*100(Daicel)): peak 4, Rt:
6.442
min.
ChirHPLC 2 (CO2/Et0H/DEA 60/40/0.04 2.8m1/min AD,5um,4.6*250(Daicel)): Rt:
8.117
min, ee:100%.
149
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Example 69
(5S)-5 -c yc loprop y1-5 - (3 -(5,6-dichloro-1-methylisoindolin-2- y1-3 ,3 -
d2)-3-
oxopropyl)imidazolidine-2,4-dione 69
0
CI
(s)
HN)r-NH
CI
D D
0
69
CI CI CI
NH Step 1 NH Step 2
(s)
________________________________________________ HN),r-NH
CI CI CI
0 D D
0
66d 69e 69
Step 1
5 ,6-dichloro-1 -methylisoindo line-3 ,3 -d2 69e
To a solution of 66d (500 mg, 2.314 mmol) in THF (4 mL), borane-d3-THF complex
solution
(1M, 15 mL) was added. The reaction was stirred at 60 C for 18h. Me0H (2 mL)
was added
dropwise followed by HC1 (6M, 2 mL), the reaction was stirred at 80 C for 2h.
Then NaOH
(5M) was added to adjust the mixture to pH=7, the solution was dried and
concentrated. The
residue was purified by silica gel chromatagraphy (DCM:Me0H=20:1) to give 69e
(320 mg,
1.5 mmol, 65% yield).
LCMS: nth (ES!): 204 [M+Hr.
Step 2
(5S)-5 -c yc loprop y1-5 - (3 -(5,6-dichloro-1-methylisoindolin-2- y1-3 ,3 -
d2)-3-
oxopropyl)imidazolidine-2,4-dione 69
To a mixture of 69e (20 mg, 0.094 mmol) in DMF (2 mL), triethylamine (40 mg,
0.28 mmol),
It-1 (23 mg, 0.108 mmol) and HATU (57 mg, 0.13 mmol) were added. The reaction
was
stirred at room temperature for 18h. Water (3 mL) was added and the mixture
was extracted
with Et0Ac (20 mL X 3). The combined organic layers were washed with brine,
dried over
Na2SO4 and concentrated. The residue was purified with prep-HPLC to give title
compound 69
(22 mg, 0.055mo1, 50 % yield).
111 NMR (400 MHz, Methanol-d4): 7.39 (s, 2H), 5.14 (q, 1H), 2.58 ¨2.22 (m,
2H), 2.20-2.02
(m, 3H), 0.49 (tt, 1H), 0.41-0.18 (m, 4H).
LCMS: nth (ES!): 39811M+Hr.
150
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Examples 69-1 and 69-2
(S)-5-cyclopropy1-5-(3-((S)-5,6-dichloro-1-methylisoindolin-2-y1-3,3-d2)-3-
oxopropyl)imidazolidine-2,4-dione 69-1:
(S)-5-cyclopropy1-5-(3-((R)-5,6-dichloro-1-methylisoindolin-2-y1-3,3- d2)-3 -
oxopropyl)imidazolidine-2,4-dione 69-2:
(S) N
(s)
HNTh
N H H N N H
4s) C I (R) CI
0 0
CI CI
69-1 69-2
69 (85mg) was separartecl by SFC to give two diastereomers (15mg, 16mg).
Diastereomer (shorter retention time):
1HNMR (400 MHz, DMSO-d6): 6 10.66-10.63 (m, 1H), 7.80-7.65 (m, 3H), 5.25-5.10
(m, 1H),
2.40-2.22 (m, 2H), 2.09-1.93 (m, 2H), 1.40 (dd, 3H), 1.15-1.06 (m, 1H), 0.49-
0.29 (m, 3H),
0.14-0.07 (m, 1H).
LCMS: MS miz (ES!): 398.0 1M+H1 .
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt:
1.581
min, de: 100%.
Diastereomer (longer retention time):
1H NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.79-7.65 (m, 3H), 5.22-5.10 (m,
1H), 2.44-
2.36 (m, 1H), 2.26-2.17 (m, 1H), 2.03-1.96 (m, 2H), 1.40 (dd, 3H), 1.15-1.07
(m, 1H), 0.48-
0.29 (m, 3H), 0.14-0.08 (m, 1H).
LCMS: miz (ES!): 398.0 1M+H1 .
ChirHPLC (CO2/Me0H/DEA 60/40/0.04 1.8m1/min IA, 3um 3*100(Daicel)): Rt: 3.053
mm,
de:99.38%.
Example 70
(SS)-5 -cyclopropy1-5-(3-(5,6-dichloro-1-methylisoindolin-2-y1)-2-methyl-3 -
oxopropyl)imidazolidine-2,4-dione
0
()L
HNHN C I
0
70 CI
151
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a mixture of 66e (10 mg, 0.049 mmol) in DMF (2 mL), triethylamine (20 mg,
0.15 mmol),
Int-2A (9 mg, 0.042 mmol) and HATU (22.8 mg, 0.06 mmol) were added. The
reaction was
stirred at room temperature for 18h. Water (3 mL) added and the mixture was
extracted with
Et0Ac (20 mL X 2). The combined layer was washed with brine, dried over Na2SO4
and
concentrated. The residue was purified with prep-HPLC to give title compound
70 (6 mg, 0.014
mmol, 33 % yield).
111 NMR (400 MHz, Methanol-d4): 6 7.26 (t, J = 3.3 Hz, 2H), 4.99 (q, 1H), 4.52
(d, 2H), 2.21
(m, 1H), 1.76 (m, 2H), 1.29 (dd, 3H), 0.97 (dd, 4H), 0.37 ¨0.05 (m, 4H).
LCMS: nth (ES!): 411 [M+Hr.
Example 71
(5S)-5 -(3 - (5-chloro -1-methy1-6- (trifluoromethyl) is oindolin-2- y1-3 ,3 -
d2)-3-o xoprop y1)-5 -
c yc loprop ylimidazolidine-2 ,4-dione 71
0 V 0
CF3
HN (s) N
)i¨NH CI
D D
0
71
0 V 0
CF3 CF3 CF3
NH NH
step 1 CI
CI CI step 2 D D
0 71b 0
41f 71
Step 1
5 -chloro -1 -methyl-6 - (triflu oromethyl)iso indoline-3 ,3 -d2 71b
Small scale
To a solution of 41f (50 mg, 0.2 mmol) in THF (2 mL) was added Borane-d3-THF
complex
solution (6 mmol, 6 mL). The reaction was stirred at 60 C for 18h. Me0H (2 mL)
was added
dropwise followed by HC1 (6M, 2mL). The reaction was stirred at 80 C for 2h.
Then NaOH
(5M) was added to adjust the mixture to pH=7, the solution was dried and
concentrated. The
residue was purified by silica gel chromatagraphy (DCM:Me0H=20:1) to give 71b
(39 mg,
0.147 mmol, 70% yield).
Large scale
To a solution 6-chloro-3-methy1-5-(trifluoromethyl)isoindolin-1-one 41f (800
mg, 3.20 mmol)
in THF (10 ml) was added BD3 (1M in THF, 64 ml, 64 mmol). After addition, the
reaction
152
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
was stirred at 60 C (in a sealed tube) for 10 hours. It was quenched with
Me0H (10 ml),
followed by HC1 (6 M, 20 ml). It then was stirred at 80 C for 8 hours. 2 N
NaOH was added
to adjust pH to 7 and extracted with Et0Ac, the combined organic phases were
washed with
brine (50 mL), dried over Na2SO4, filtered and concentrated under reduced
pressure. The
residue was purified by silica gel column chromatography eluting with 5 % Me0H
in DCM,
to get the desired product 71b which is used for next step.
LCMS: MS miz (ES!): 238.1 [M+Hr.
Step 2
(5 S)-5 -(3 - (5-chloro -1-methy1-6- (trifluoromethyl) is oindolin-2- y1-3 ,3 -
d2)-3-o xoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 71
Small scale
To a mixture of 71b (20 mg, 0.084 mmol) in DMF (2 mL) was added triethylamine
(40 mg,
0.28 mmol), It-1 (23 mg, 0.108 mmol) and HATU (57 mg, 0.13 mmol). The reaction
was
stirred at r.t for 18h. Water (3 mL) was added and the mixture was extracted
with Et0Ac (20
mL X 3). The combined organic layers were washed with brine, dried over Na2SO4
and
concentrated. The residue was purified with prep-HPLC to give title compound
71 (24 mg,
0.055 mol, 51 % yield).
Large scale
To a solution of (S)-3-(4-cyclopropy1-2,5-dioxoimidazolidin-4-yl)propanoic
acid It-1 (680
.. mg, 3.2 mmol) in DMF (10 ml) was added EDCI (920 mg, 4.8 mmol) and HATU
(1.83 g, 4.8
mmol). After stirring for 10 minutes, the isoindoline 71b collected from the
previous step was
added. The reaction was stirred at ambient temperature for 3 hours. LCMS
showed that the
reaction was completed. It was directly purified on a reverse phase HPLC to
get the desired
product 71 (1.10 g, 79.6% yield over two steps).
111 NMR (400 MHz, CD30D,): 7.76 (s, 1 H), 7.63-7.60 (m, 1 H), 5.57-5.53 (m, 1
H), 2.59-
2.40 (m, 2 H), 2.28-2.19 (m, 2 H), 1.56-1.50 (m, 3 H), 1.28-1.21 (m, 1 H),
0.62-0.58 (m, 1 H),
0.49-0.41 (m, 3 H).
LCMS: MS miz (ES!): 432 [M+Hr.
Examples 71-1 and 71-2
(S)-5-(3 -((R)-5 -chloro -1 -methyl-6 - (triflu oro methypiso indolin-2 - y1-3
,3 -d2)-3-oxoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 71-1
153
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
(S)-5-(3 -((S)-5 -chloro-1 -methyl-6-(triflu oromethyl)isoindo lin-2 - y1-3 ,3
-d2)-3 -oxoprop y1)-5 -
cyclopropylimidazolidine-2,4-dione 71-2
()LN
HN HN
CI CI
H3C H3C
0 0
CF3 CF3
71-1 71-2
71 (1.10 g) was separarted by SFC to give two diastereomers (325mg and 415mg
separately).
Enantiomers (shorter retention time):
1H NMR (500 MHz, DMSO-d6) 6 10.63 (s, 1H), 7.89 (d, 1H), 7.75 (t, 2H), 5.30 ¨
5.16 (m,
1H),4.23 (d, 1H), 2.37 ¨ 2.27 (m, 2H), 1.99 (dq, 2H), 1.53 (s, 1H), 1.43 (dd,
3H), 1.11 (td, 1H),
0.49 ¨0.30 (m, 3H), 0.11 (dt, 1H).
LCMS: MS m/z (ES!): 432.3 [1\4+Hr
ChirHPLC (1% DEA in Et0H/hexane 60/40, 1.0 mL/min, 35 C, CHIRALPAK 1G,
150*4.6mm, Sum): Rt: 4.594 min, de:100%.
Enantiomers (longer retention time):
1H NMR (500 MHz, DMSO-d6) 6 10.53 (s, 1H), 7.89 (d, 1H), 7.80 ¨ 7.66 (m, 2H),
5.30 ¨
5.12 (m, 1H), 4.23 (d, 1H), 2.44 ¨ 2.36 (m, 1H), 2.31 ¨2.20 (m, 1H), 2.05 ¨
1.95 (m, 2H), 1.44
(dd, 3H), 1.11 (td, 1H), 0.50 ¨ 0.29 (m, 3H), 0.16 ¨ 0.08 (m, 1H).
LCMS: MS m/z (ES!): 432.3 lIVI+Hl+
ChirHPLC (1% DEA in Et0H/hexane 60/40, 1.0 mL/min, 35 C, CHIRALPAK 1G,
150*4.6mm, Sum): Rt: 10.931 min, de:100%.
Example 72
(S)-5 -cycloprop y1-5 -(3 -o xo -3-(5 -(2,2,2 -triflu oro ethyl)isoindo lin-2-
yl)prop yl)imid azolidine-
2,4-dione 72
0 V
(\)N
HN)r-NH
0 CF3
72
154
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Boo, Boc, HN
N r.
Step 1 Step 2
HCI
Br 3
CF3
51a 72b 72c
Step 3
HN),r 72 r.NH
It-1
0 ¨.3
Step 1
Tert-butyl 5-(2,2,2-trifluoroethyl)isoindoline-2-carboxylate 72b
To a mixture of 51a (500.00 mg, 1.68 mmol) and 1,1,1-trifluoro-2-iodoethane
(1.76 g, 8.38
mmol) in DMSO (3 mL) was added copper (1.07 g, 16.77 mmol). The reaction was
stirred at
120 C for 40h. The reaction was cooled to rt, and water (100 mL) was added.
The mixture was
extracted with Et0Ac (30 mL X 2), and the combined organic layers were washed
with brine,
dried over Na2SO4 and concentrated. The crude was purified by prep-HPLC to
give 72b (33
mg, 109.52 umol, 6.53% yield).
1H NMR (400 MHz, CDC13): 6 7.21-7.15 (m, 3H), 4.69-4.65 (m, 4H), 3.37 (q, 2H),
1.52 (s,
9H).
19FNMR (400 MHz, CDC13): 6 -66.03.
Step 2
5-(2,2,2-trifluoroethyflisoindoline hydrochloride 72c
The mixture of 72b (23 mg, 76.41 umol) in DCM (3 mL) was add HO/dioxane (1N, 1
mL).
The reaction was stirred at room temperature for 16 hours. The mixture was
concentrated to
give 72c as crude which was used to the next step.
Step 3
(S)-5 -c yc loprop y1-5 -(3 - (5- (diflu oro methyl) isoindolin-2- y1)-3 -o
xoprop yl) imid azo lidine-2,4-
dione 72
To a solution of 72c (23 mg, 206.89 umol) in DMF (3 mL) was added It-1 (23mg,
109.35
umol), TEA (30 mg, 298.23 umol) and HATU (45 mg, 119.29 umol). The mixture was
stirred
at room temperature 2h. Water (30 mL) was added and the mixture was extracted
with Et0Ac
(20 mL X 2). The combined organic layers were washed with water (30 mL) and
brine (30
mL), dried and concentrated. The crude was purified by prep-HPLC to give 72
(13 mg, 32.88
umol, 33.08% yield).
155
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
1H NMR (400 MHz, CDC13): 6 10.62 (brs, 1H), 7.74 (s, 1H), 7.37-7.27 (m, 3H),
4.80 (br,
2H), 4.62 (br, 2H), 3.67 (q, 2H), 2.47-2.40 (m, 1H), 2.30-2.26 (m, 1H), 2.01
(t, 2H), 1.13-
1.09 (m, 1H), 0.47-0.31 (m, 3H), 0.13-0.10 (m, 1H).
19F NMR (400 MHz, CDC13): 6 -64.44.
LCMS: MS nth (ES!): 396.1 11\4+Hr.
Example 73
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(1-methyl-1H-
imidazol-2-
yflimidazolidine-2,4-dione 73
1\1 N.-- 0
0
HNNH
CF3
0
73 CI
The title compound was prepared with similar method as Examples 40 and 62.
1H NMR (400 MHz, DMSO-d6): 6 8.57 (s, 1H), 7.91 (s, 1H), 7.77 (s, 1H), 7.19
(d, 1H), 6.84
(d, 1H), 4.85-4.81 (m, 2H), 4.71-4.67 (m, 2H), 3.54 (s, 3H), 2.59-2.54 (m,
2H), 2.45-2.38 (m,
2H).
LCMS: MS nth (ES!): 455.9 11\4+Hr.
Examples 73-1 and 73-2
(S)-5 -(3 -(5 -chloro-6-(trifluoromethyl)isoindolin-2-y1)-3 -oxoprop y1)-5 -(1
-methyl-1H-
imidazol-2-yl)imidazolidine-2,4-dione 73-1
& (R)-5-(3-(5-chloro-6-(trifluoromethypisoindolin-2-y1)-3-oxopropy1)-5-(1-
methyl-1H-
imidazol-2-y1)imidazolidine-2,4-dione 73-2
0
0 Ny 0
HN),r-NH = HN)r-NH CF3 = CF3
0 73_1
CI 0 73_2
CI
73 (24 mg) was chirally separated by SFC to afford two enantiomers (5.0 mg,
5.0 mg).
Enantiomer (shorter retention time):
1H NMR (400 MHz, DMSO-d6): 6 11.16 (br, 1H), 8.57 (s, 1H), 7.91 (s, 1H), 7.77
(s, 1H),
7.19 (s, 1H), 6.84 (s, 1H), 4.85-4.81 (m, 2H), 4.71-4.67 (m, 2H), 3.54 (s,
3H), 2.59-2.54 (m,
2H), 2.45-2.38 (m, 2H).
156
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
LCMS: MS mh (ES!): 456.1 [M+Hr.
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 2.8ml/min OD,5um,4.6*250(Daicel)): Rt:
2.823
min, ee: 100%.
Enantiomer (longer retention time):
111 NMR (400 MHz, DMSO-d6): 6 11.18 (br, 1H), 8.59 (s, 1H), 7.91 (s, 1H), 7.77
(s, 1H),
7.20 (s, 1H), 6.85 (s, 1H), 4.85-4.81 (m, 2H), 4.71-4.67 (m, 2H), 3.54 (s,
3H), 2.59-2.54 (m,
2H), 2.45-2.38 (m, 2H).
LCMS: MS nth (ES!): 456.0 [M+Hr.
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 2.8ml/min OD,5um,4.6*250(Daicel)): Rt:
3.878
min, ee: 96.48%.
Example 74
(S)-5-cyclopropy1-5-(3-(5-(difluoromethyl)-6-fluoroisoindolin-2-y1)-3-
oxopropyl)imidazolidine-2,4-dione 74
o V
Hr\()(N
)õr NH 40 CHF2
o
74 F
0 0 0 0
0
F OH _____
Step 1 Br OH Step 2 Br 0 Step 3 Br
o
Br
F F F
74a 74b 74c 74d
0
Step 4 ,... Br / Step 5 Br i& NH ____ Step 6 Br = N
Boc Step 7
3.- ..-
NH
F F
F
74e 74f 74g
/ Step 8 (:) Step 9 F2HC Step 10
N¨Boc __________________________________ N¨Boc __________ N Boc __ ..-
F F F
74h 74i 74j
0 V
F2HC i& Step 11
NH ______________________ .- HNC)N
F CHF2
F
0
74k 74
157
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Step 1
5-Bromo-4-fluoro-2-methylbenzoic acid 74b
To a solution of 4-fluoro-2-methylbenzoic acid 74a (10 g, 64.88 mmol) in H2504
(50 mL) was
added NBS (11.6 g, 65.18 mmol) in portions at 0 C. The reaction mixture was
stirred at 0-5 C
for 2 his. The resulting mixture was poured into ice-water. The solid was
collected by filtration
and dried in vacuum to give 74b (14 g, 60.08 mmol, 92.60% yield).
111 NMR (400 MHz, DMSO-d6): 6 8.07 (d, 1H), 7.39 (d, 1H), 2.51 (s, 3H).
Step 2
Methyl 5-bromo-4-fluoro-2-methylbenzoate 74c
To a solution of 74b (15 g, 64.37 mmol) in Me0H (150 mL) at 0 C was added
SOC12 (22.97
g, 193.10 mmol, 14 mL) slowly. The reaction mixture was heated to 80 C for 2
hr. Then the
mixture was cooled to room temperature and concentrated in vacuum. The residue
was diluted
with aq NH4C1 and extracted with Et0Ac. The combined organic layers were
washed with
brine, dried over Na2SO4, filtered and concentrated in vacuum to afford crude
74c (15.2 g,
61.52 mmol, 95.58% yield).
111 NMR (400 MHz, DMSO-d6): 6 8.02 (d, 1H), 7.37 (d, 1H), 3.78 (s, 3H), 2.45
(s, 3H).
Step 3
Methyl 5-bromo-2-(bromomethyl)-4-fluorobenzoate 74d
To a solution of 74c (15.2 g, 61.52 mmol) in CC14 (250 mL) was added NBS
(13.14 g, 73.83
mmol) and AIBN (1.01 g, 6.15 mmol). The reaction mixture was stirred at 80 C
overnight.
Then the mixture was cooled down to room temperature and filtered. The cake
was washed
with CC14, the filtrate was concentrated in vacuum. The residue was purified
by silica gel
chromatagraphy (Et0Ac: hexane=1:20) to afford 74d (19 g, 58.29 mmol, 94.74%
yield).
111 NMR (400 MHz, DMSO-d6): 6 8.16 (d, 1H), 7.71 (d, 1H), 4.98 (s, 2H), 3.88
(s, 3H).
Step 4
6-bromo-5 -fluoroiso indo lin- 1 -one 74e
The solution of 74d (4.6 g, 14.11 mmol) in NH3/Me0H (40 mL, 7N) was stirred at
room
temperature overnight. The reaction mixture was concentrated in vacuum, the
residue
158
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
was purified by silica gel chromatagraphy (MeOH: DCM=1:50) to give 74e (3.0 g,
13.04 mmol,
92.41% yield).
LCMS: MS m/z (ES!): 230.3 1M+Hr.
Step 5
5-bromo-6-fluoroisoindoline 74f
To a solution of 74e (3.0 g, 13.04 mmol) in THF (20 mL) was added BH3/THF (1N
in THF,
90 mmol, 90 mL), and the mixture was heated to 65 C overnight. The reaction
was quenched
with methanol (5 mL) and 6M HC1 to adjust pH to 2. The mixture was heated to
80 C for 2 h,
and cooled to RT. The mixture was adjusted to pH 7-8 with 6M NaOH, and was
extracted with
ethyl acetate (3x). The combined organic phases was dried over anhydrous
Na2SO4,
and concentrated in vacuum. The crude mixture was purified by silica gel
chromatagraphy
(MeOH: DCM=1:20) to afford 74f (350 mg, 1.62 mmol, 12.42% yield).
1H NMR (400 MHz, DMSO-d6): 6 7.62 (d, 1H), 7.33 (d, 1H), 4.14-4.10 (m, 4H).
LCMS: MS m/z (ES!): 216.2 1M+Hr.
Step 6
Tert-butyl 5-bromo-6-fluoroisoindoline-2-carboxylate 74g
To a solution of 74f (350 mg, 1.62 mmol) in DCM (5 mL) was added TEA (492 mg,
4.86
mmol) and Boc20 (425 mg, 1.94 mmol), the mixture was stirred at room
temperature for 4 h.
The reaction mixture was concentrated in vacuum. The crude mixture was
purified by silica
gel chromatagraphy (Et0Ac:hexane=1:20) to afford 74g (580 mg, 1.83 mmol,
113.24% yield).
1H NMR (400 MHz, DMSO-d6): 6 7.71-7.67 (m, 1H), 7.40-7.35 (m, 1H), 4.55-4.52
(m, 4H),
1.47-1.44 (m, 9H).
LCMS: MS m/z (ES!): 316.2 1M+Hr.
Step 7
Tert-butyl 5-fluoro-6-vinylisoindoline-2-carboxylate 74h
To a solution of 74g (580 mg, 1.83 mmol) in 1,4-dioxane (20 mL) and H20 (3 mL)
was
added potassium trifluoro(vinyl)borate (270 mg, 2.02 mmol), Pd(dppf)C12 (150
mg, 183.45
umol) and K2CO3 (760 mg, 5.50 mmol), the reaction was replaced with N2 for
three times. The
159
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
mixture was stirred at 100 C overnight. Water was added and the reaction
mixture was
extracted with Et0Ac. The combined organic layers were washed with water and
brine, dried
over anhydrous Na2SO4, filtered and concentrated in vacuum. The crude mixture
was
purified by silica gel chromatagraphy (Et0Ac:hexane=1:20) to afford 74h (400
mg, 1.52 mmol,
82.81% yield).
1H NMR (400 MHz, DMSO-d6): 6 7.63-7.58 (m, 1H), 7.22-7.17 (m, 1H), 6.82 (dd,
1H), 5.89
(dd, 1H), 5.41 (d, 1H), 4.58-4.52 (m, 4H), 1.45 (s, 9H).
LCMS: MS m/z (ES!): 208.0 [M-tBu+Hr.
Step 8
Tert-butyl 5-fluoro-6-formylisoindoline-2-carboxylate 74i
To solution of 74h (400 mg, 1.52 mmol) in 1,4-dioxane (8 mL) was added NaI04
(650 mg,
3.04 mmol) and H20 (2 mL), and the mixture was stirred at RT. Then 0s04 (39
mg, 151.91
umol) was added. The reaction mixture was stirred at room temperature for 3 h.
Saturated
sodium bicarbonate was added, and then the reaction mixture was extracted with
Et0Ac. The
combined organic layers were washed with water and brine, dried over anhydrous
Na2SO4,
filtered and concentrated in vacuum. The crude mixture was purified by silica
gel
chromatagraphy (Et0Ac:hexane=1:20) to give 74i (180 mg, 678.53 umol, 44.67%
yield).
1H NMR (400 MHz, DMSO-d6): 6 10.20 (s, 1H), 7.81-7.76 (m, 1H), 7.44-7.39 (m,
1H), 4.67-
4.58 (m, 4H), 1.45 (s, 9H).
LCMS: MS m/z (ES!): 210.4 [M-tBu+Hr.
Step 9
Tert-butyl 5-(difluoromethyl)-6-fluoroisoindoline-2-carboxylate 74j
To a solution of 74i (180 mg, 678.53 umol) in DCM (5 mL) was added Et0H (3.1
mg, 67.85
umol), then DAST (547 mg, 3.39 mmol) was added dropwise at RT. The mixture was
stirred
at room temperature for 3 h. Water was added and the reaction mixture was
extracted with
DCM. The combined organic layers were washed with water and brine, dried over
anhydrous
Na2SO4, filtered and concentrated in vacuum. The crude mixture was purified by
silica gel
chromatagraphy (Et0Ac:hexane=1:20) to afford 74j (180 mg, 626.57 umol, 92.34%
yield).
160
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
1H NMR (400 MHz, DMSO-d6): 6 7.62-7.58 (m, 1H), 7.40-7.33 (m, 1H), 7.20 (t,
1H), 4.64-
4.57 (m, 4H), 1.45 (s, 9H).
LCMS: MS in/z (ES!): 232.4 [M-tBu+Hr.
Step 10
5 -(difluoromethyl)-6-fluoroiso indoline 74k
To a solution of 74j (180 mg, 626.57 umol) in the flask was added HC1 in1,4-
dioxane (4N, 10
mL). The mixture was stirred at room temperature for 2 h. The reaction mixture
was
concentrated in vacuum to give 74k (110 mg, 587.73 umol, 93.80% yield).
LCMS: MS nth (ES!): 188.1 [M+Hr.
Step 11
(S)-5 -c yc loprop y1-5 -(3 -(5-(diflu oro methyl)-6-fluoroiso indo lin-2- y1)-
3 -
oxopropylnmidazolidine-2,4-dione 74
To a solution of 74k (110 mg, 587.73 umol) in DMF (5 mL) was added TEA (0.4
mL), (S)-3-
(4-c ycloprop y1-2 ,5 -dio xo imidazo lidin-4- yl)prop ano ic acid Int-1 (125
mg, 587.73
umol) and HATU (246 mg, 646.50 umol). The mixture was stirred at room
temperature for 2
h. Water was added and reaction mixture was extracted with Et0Ac. The combined
organic
layers were washed with water and brine, dried over anhydrous Na2SO4, filtered
and
concentrated in vacuum. The residue was purified by prep-HPLC to give 74 (24
mg, 62.93
umol, 10.71% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.51 (brs, 1H), 7.62 (s, 1H), 7.51 (d, 1H), 7.28
(dd, 1H),
7.09 (t, 1H), 4.73-4.68 (m, 2H), 4.55-4.50 (m, 2H), 2.33-2.25 (m, 1H), 2.20-
2.10 (m, 1H), 1.91-
1.86 (m, 2H), 1.08-0.96 (m, 1H), 0.38-0.17 (m, 3H), 0.03-0.00 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -112.85, --120.80.
LCMS: MS nth (ES!): 382.4 [M+Hr.
Exampe 75
5 -(3 -(5 -chloro-6-(triflu oro methyl) iso indolin-2 - y1)-3 -o xoprop y1)-5 -
(5 -methyli so xazol-3 -
yl)imid azo lidine-2,4-dione 75
161
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
bN 0
0.......)L
N
HN),rNH 40 CI
0
CF3
0 0
0
N Step 1
0/
)..3)
__________________________ "'
0 0 _______
Ol< Step 2 0
02
HNCNNH I
0
75a 75b 75c
ti 0N 0
0
, N 0
Step 3 0 Step 4 0,.......)(
OH N
HN),r..-N1H
HI\l,NFI
0 441 CI
0 CF3
75d 75
Step 1
Tert-butyl 445 -methyliso xazol-3 - y1)-4 -oxobutano ate 75b
5 To a solution of 1-(5-methylisoxazol-3-y0ethan-1-one 75a (3.00 g, 23.99
mmol) in THF (10
mL) was added NaHMDS (4.40 g, 23.99 mmol) dropwise at -70 C. The resulting
mxiture was
stirred at this temperature for 30 mm before tert-butyl 2-bromoacetate (4.68
g, 23.99 mmol)
was added dropwsie. After addition, the reaction mixture was stirred at -20 C
for 1.0 hour, then
warmed to room temperature for 18 hours. The resulting mixture was cooled to 0
C, quenched
10 with aq. NaHCO3 (20 mL). The whole mixture was extracted with Et0Ac (30
mL x 4). The
organic layers were combined, dried over anhydrous Na2SO4 and filtered. The
filtrate was
concentrated under reduced pressure. The residue was purified by silica gel
chromatography
(Et0Ac/hexane= 1/20 to 1/4) to afford 75b (400 mg, 1.67 mmol, 6.97% yield).
LCMS: MS rah (ES!): 240.5 [M+Hr.
15 Step 2
Tert-butyl 3 -(4- (5-methyliso xazol-3 - y1)-2 ,5 -dio xo imidazolidin-4-
yl)prop ano ate 75c
162
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To the solution of 75b (350 mg, 1.46 mmol) in H20 (5 mL) and Me0H (5 mL) was
added
(NH4)2CO3 (1.12 g, 11.70 mmol) and NaCN (176 mg, 3.66 mmol). The reaction was
stirred at
85 C overnight. The mixture was concentrated and the residue was extracted
with Et0Ac. The
organic solution was dried and concentrated. The residue was slurred with Et20
to give 75c
(180 mg, 581.93 umol, 39.78% yield).
LCMS: MS raiz (ES!): 332.1 1M+Nar.
Step 3
3 -(4 - (5-methyliso xazol-3 - y1)-2 ,5-dio xo imidazo lidin-4 - yl)propanoic
acid 75d
The mixture of 75c (30 mg, 96.99 umol) in HC1/1,4-dixoane (2 mL, 4N) was
stirred at room
temperature for lh. The mixture was oncentrated to give crude 75d (20 mg,
78.99 umol, 81.44%
yield).
LCMS: MS raiz (ES!): 254.0 1M+H1 .
Step 4
5 -(3 -(5 -chloro-6- (triflu oro methyl) iso indolin-2 - y1)-3 -o xoprop y1)-5
-(5 -methyli so xazol-3 -
yl)imidazolidine-2,4-dione 75
To the solution of 5-chloro-6-(trifluoromethyl)isoindoline 40h (17.50 mg,
78.99 umol) in DMF
(2 mL) was added HATU (33 mg, 86.88 umol), Et3N (24 mg, 236.96 umol) and 75d
(20 mg,
78.99 umol). The mixture was stirred at room temperature for lh. The reaction
mixture was
purified by prep-HPLC to give 75 (2.5 mg, 5.47 umol, 6.93% yield).
1H NMR (400 MHz, CDC13): 6 8.45 (br, 1H), 7.62 (br, 1H), 7.48 (br, 1H), 7.00
(br, 1H), 6.20
(brs, 1H), 4.80 (br, 4H), 2.60-2.45 (m, 4H), 2.42 (s, 3H).
LCMS: MS raiz (ES!): 457.0 1M+H1 .
Example 76
(S)-5 -c ycloprop y1-5 -(3 -(5 - (methylamino)-6-(trifluoromethyl) iso indolin-
2 - y1)-3 -
oxopropyl)imidazolidine-2,4-dione 76
163
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 V
N
HN'
)r-NH 41fr CF3
0
76 NHMe
Br Br
Step 1 Step 2 1\1
NH __________________________ . N¨Boc _____ CF3= N¨Boc
CF3 CF3
59e 76a 76b
0 V
H Ii
Step 3 1\1 Step 4
NH ____ HN)i-NH CF3
CF3 0
76c 76 NHMe
Step 1
Tert-butyl 5 -bromo-6-(trifluoromethyl)isoindoline-2-carboxylate 76a
To a solution of 59e (920 mg, 3.46 mmol) in THF (20 mL) was added TEA (1.8 mL)
and Boc20
(906 mg, 4.15 mmol). The mixture was stirred at room temperature for 4h. The
reaction mixture
was concentrated in vacuo and the residue was purified by silica gel
chromatography (ethyl
acetate/hexane=1/20) to afford 76a (810 mg, 2.21 mmol, 63.97% yield).
LCMS: MS raiz (ES!): 311.9 lIVI+H--tBur.
Step 2
Tert-butyl 5 -(methylamino)-6-(trifluoromethyl)isoindoline-2-carboxylate 76b
To a solution of 76a (100 mg, 273.09 umol) in 1,4-dioxane (5 mL) was added
MeNH2/THF
(1N in THF, 0.5 mL, 0.5 mmol), Pd2(dba)3 (25 mg, 27.31 umol), Cs2CO3 (267 mg,
819.28
umol) and XantPhos (32 mg, 54.62 umol). The reaction was stirred at 90 C in a
sealed tube
under N2 overnight. Water was added and the mixture was extracted with Et0Ac.
The
combined organic layers were washed with water and brine, dried over anhydrous
Na2SO4,
filtered and concentrated in vacuo. The residue was purified by prep-HPLC to
give 76b (45
mg, 142.26 umol, 52.09% yield).
11INMR (400 MHz, DMSO-d6): 6 7.38 (d, 1H), 6.69 (s, 1H), 5.59 (d, 1H), 4.56-
4.52 (m, 2H),
4.48-4.45 (m, 2H), 2.75 (t, 3H), 1.45 (s, 9H).
LCMS: MS raiz (ES!): 358.1 lIVI+H+CH3C1\11 .
164
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Step 3
N-methyl-6-(trifluoromethyl) iso indolin-5 -amine 76c
The solution of 76b (45 mg, 142.26 umol) of HC1/1,4-dioxane (5 mL, 1N) was
stirred at room
temperature for 3h. The reaction mixture was concentrated in vacuo to give 76c
(30 mg, 138.76
umol, 97.54% yield).
LCMS: MS nth (ES!): 217.1 1M+1-11 .
Step 4
(S)-5 -c ycloprop y1-5 -(3 -(5 -(methylamino)-6-(trifluoromethyl) iso indolin-
2 -y1)-3 -
oxopropyl)imidazolidine-2,4-dione 76
To a solution of 76c (30 mg, 138.76 umol) in DMF (2 mL) was successively added
TEA (0.1
mL), It-1 (30 mg, 138.76 umol) and HATU (58 mg, 152.63 umol). The mixture was
stirred
at room temperature for 2h. Water was added and the mixture was extracted with
Et0Ac. The
combined organic layers were washed with water and brine, dried over anhydrous
Na2SO4,
filtered and concentrated in vacuo. The crude mixture was purified by prep-
HPLC to give 76
(10 mg, 24.37 umol, 17.56% yield).
1H NMR (400 MHz, DMSO-d6): 6 10.64 (s, 1H), 7.74 (s, 1H), 7.41 (s, 1H), 6.72
(s, 1H), 5.61
(d, 1H), 4.76 (brs, 1H), 4.67 (brs, 1H), 4.59 (brs, 1H), 4.51 (brs, 1H), 2.77
(d, 3H), 2.44-2.21
(m, 2H), 2.05-1.96 (m, 2H), 1.14-1.09 (m, 1H), 0.50-0.29 (m, 3H), 0.16-0.12
(m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -61.20.
LCMS: MS nth (ES!): 411.2 1M+H1 .
Example 77
5 -(3 -(5-chloro-6-(trifluoromethyl) isoindo lin-2- y1)-3 -o xoprop y1)-5 -(1-
methyl- 1H-p yrazol-3 -
yl)imid azo lidine-2,4-dione 77
0
HN)r-NH CF3
0 77 CI
165
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
isL) 0
CStep 1 / N Step 2 NN Ste C)(y<
0 _________________________________________
0 FIN)r- NH
0 0
77a 77b 77c
0
0 N)
Step 3 Step 4
OH NH FIN)rNH
CF3
0
0
77d 77 CI
Step 1
Tert-butyl 4-(1 -methyl- 1H-p yrazol-3 - y1)-4-o xobu tano ate 77b
To the solution of 1-methyl-1H-pyrazole-3-carbaldehyde 77a(3.87 g, 35.11 mmol)
in THF (10
mL) was added P(Bu)3 (5.4 g, 42.13 mmol), and the reaction mixture was heated
at 50 C for
5min, tert-butyl prop-2-enoate (4.5 g, 35.11 mmol) was added and the mixture
is stirred at 80 C
for 3h. More tert-butyl prop-2-enoate (4.5 g, 35.11 mmol) was added and this
process was
repeated until no elution is observerd by TLC. The mixture was purified by
silica gel
chromatagraphy (hexane/Et0Ac=10/1) to give 77b (1.2 g, 5.04 mmol, 14.34%
yield).
LCMS: MS raiz (ES!): 183.1 1M+1-tBur.
Step 2
Tert-butyl 3 -(4- (1 -methyl- 1H-p yrazol-3 - y1)-2,5-dioxoimid azo lidin-4 -
yl)prop ano ate 77c
To the solution of 77b (300 mg, 1.26 mmol) in H20 (2 mL) and Me0H (2 mL) was
added
NaCN (155 mg, 3.15 mmol) and (NH4)2CO3 (967 mg, 10.07 mmol). The reaction was
stirred
at 85 C overnight. LCMS showed the product produced. Water was added and the
mixture was
extracted with Et0Ac (10 mL x 2), the combined organic layers were
concentrated to give
crude 77c (100 mg, 324.33 umol, 25.76% yield).
111 NMR (400 MHz, DMSO-d6): 6 10.74 (s, 1H), 8.35 (s, 1H), 7.65 (d, 1H), 6.18
(d, 1H), 3.81
(s, 3H), 2.23-2.19 (m, 4H), 1.39 (s, 9H).
LCMS: MS raiz (ES!): 309.1 1M+Hr.
Step 3
3 -(4- (1 -methyl- 1H-p yrazol-3 - y1)-2,5 -dioxoimid azo lidin-4 -yl)prop
anoic acid 77d
166
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
The mixture of 77c (110 mg, 356.76 umol) in H0/1,4-dioxane (2 mL, 2N) was
stirred at room
temperature for lh. LCMS showed that the product formed. The mixture was
concentrated to
give 77d (100 mg, 396.47 umol, 111.13% yield).
LCMS: MS raiz (ES!): 253.1 [M+Hr.
Step 4
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y0-3-oxopropy1)-5-(1-methyl-1H-
pyrazol-3-
y1)imidazolidine-2,4-dione 77
To the solution of 40h (20 mg, 79.29 umol) in DMF (2 mL) was added HOBT (12.85
mg,
95.15 umol), EDCI (19 mg, 95.15 umol) and 77d (17.57 mg, 79.29 umol). The
reaction was
stirred atroom temperature overnight. LCMS showed that the product formed.
The mixture was purified by prep-HPLC to give 77 (0.88 mg, 1.93 umol, 2.43%
yield).
111 NMR (400 MHz, CDC13): 6 7.63-7.58 (m, 2H), 7.46-7.40 (m, 1H), 7.33 (d,
1H), 6.35-6.31
(m, 2H), 4.80-4.77 (m, 4H), 3.86 (s, 3H), 2.61-2.44 (m, 4H).
19F NMR (376.5 MHz, CDC13): 6 -62.45.
LCMS: MS raiz (ES!): 456.4 [M+Hr.
Example 78
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y0-3-oxopropy1)-5-(5-methyl-1H-
pyrazol-3-
y1)imidazolidine-2,4-dione 78
HNIN j 0
HNNH CF3
0
HN
78 CI
Step 1 THPN \ Step 2 THPN I Step 3 THPY \ Step
4 THPN 0 (
N \ N
/ 0
COOH COOH 0 \ 0 0
78a 78b 78c 78d 78e
THPN 0 E iNj 0 HNNJ 0
Step 5 Step 6 Step 7
HN)r-NIH HN2r- 78g NH HN2r- 78 NH CF3
0 78f CI
167
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Step 1
-methyl- 1 - (tetrahydro-2H-p yran-2- y1)- 1H-p yrazole-3 -carboxylic acid 78b
To a solution of 5-methyl-1H-pyrazole-3-carboxylic acid 78a (1 g, 7.94 mmol)
and 3,4-
dihydro-2H-pyran (1.33 g, 15.88 mmol) in THF (20 mL) was added PTSA (71 mg,
0.4 mmol).
5 The resulting mixture was stirred at r.t for 18 h. The solution was
concentrated, the residue was
purified by silica gel column chromatography (eluting with DCM/Me0H = 50/1) to
afford the
tittle compound 78b (907 mg, 4.32 mmol, 54.41% yield).
111 NMR (400 MHz, CDC13): 6 6.63 (s, 1H), 5.37 (d, 1H), 4.04-4.01 (m, 1H),
3.69-3.63 (m,
1H), 2.50-2.41 (m, 1H), 2.38 (s, 3H), 2.14-2.11 (m, 1H), 2.01-1.98 (m, 1H),
1.75-1.55 (m, 4H).
Step 2
N-methoxy-N,5 -dimethyl- 1 - (tetrahydro -2H-p yran- 2 - y1)- 1H-p yrazo le-3 -
carboxamide 78c
To a solution of 78b (907 mg, 4.32 mmol) in DCM (15 mL) was added TEA (2.1 mL,
15.12
mmol), the solution was cooled to 0 C. Then HOBt (642 mg, 4.75 mmol) and EDCI
(1 g, 5.18
mmol) were added, the reaction mixture was stirred at 0 C for 30min. N,0-
dimethylhydroxylamine hydrochloride (505 mg, 5.18 mmol) was added. The
reaction was
stirred at r.t for 18h. Water (50 mL) was added and the mixture was extracted
with DCM (50
mL x 2), the organic solution was washed with brine, dried over anhydrous
Na2SO4 and
concentrated. The residue was purified by silica gel chromatography (eluting
with
hexane/Et0Ac = 1/1) to afford the tittle compound 78c (810 mg, 3.2 mmol,
74.11% yield).
Step 3
1-(5 -methyl- 1 - (tetrahydro-2H-p yran-2- y1)- 1H-p yrazol-3 - yl)ethan- 1 -
one 78d
To a solution of 78c (810 mg, 3.2 mmol) in THF (15 mL) was added MeMgC1 (3N,
3.2 mL,
9.6 mmmol). The reaction mixture was stirred at 0 C for 1 h. The reaction was
quenched with
aqueous NH4C1 (20 mL), extracted with Et0Ac (20 mL x 2), the organic solution
was washed
with brine, dried over anhydrous Na2SO4 and concentrated, the residue was
purified by silica
gel chromatography (eluting with hexane/Et0Ac = 4/1) to afford the tittle
compound 78d (450
mg, 2.16 mmol, 67.5% yield).
168
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
111 NMR (400 MHz, CDC13): 6 6.54 (d, 1H), 5.34 (dd, 1H), 4.04-4.00 (m, 1H),
3.70-3.64 (m,
1H), 2.56 (s, 3H), 2.52-1.45 (m, 1H), 2.36 (s, 3H), 2.18-2.12 (m, 1H), 2.01-
1.96 (m, 1H), 1.75-
1.61 (m, 3H).
Step 4
Tert-butyl 4-(5 -methyl- 1 - (tetrahydro-2H-p yran-2-y1)- 1H-p yrazol-3 - y1)-
4-o xobu tano ate 78e
To a solution of 78d (200 mg, 0.96 mmol) in THF (3 mL) was added NaHMDS (2 N,
0.55 mL,
1.1 mmoL) dropwise at -78 C. After lh, tert-butyl bromoacetate (0.146 mL, 1
mmoL) was
added to the solution dropwise. The reaction was stirred at r.t for 18h. Water
(20 mL) was
added and the mixture was extracted with Et0Ac (20 mL x 2). The combined
organic layeres
were washed with brine, dried over anhydrous Na2SO4 and concentrated. The
residue was
purified by silica gel column chromatography (eluting with hexane/Et0Ac =
10/1) to afford
the tittle compound 78e (180 mg, 0.87 mmol, 90.58% yield).
111 NMR (400 MHz, CDC13): 6 6.55 (s, 1H), 5.34 (dd, 1H), 4.02-3.99 (m, 1H),
3.69-3.63 (m,
1H), 3.38-3.22 (m, 2H), 2.67-2.56 (m, 2H), 2.51-2.44 (m, 1H), 2.35 (s, 3H),
2.19-2.14 (m, 1H),
1.99-1.95 (m, 1H), 1.73-1.65 (m, 3H), 1.43 (s, 9H).
Step 5
Tert-butyl 3 -(4 - (5-methyl- 1 - (tetrahydro-2H-p yran-2- y1)- 1H-p yrazol-3 -
y1)-2,5 -
dioxoimid azo lidin-4 -yl)prop ano ate 78f
To a solution of 78e (2 g, 6.21 mmol) in Et0H (30 mL) and water (30 mL) was
added
(NH4)2CO3 (4.77 g, 49.69 mmol) and NaCN (760.73 mg, 15.53 mmol). The reaction
was done
in sealed tube and stirred at 90 C for 18h. The mixture was poured to water
(100 mL) and
extracted with Et0Ac (50 mL x 3), the combined organic layers were washed with
brine, dried
over anhydrous Na2SO4 and concentrated. The residue was triturated with hexane
to afford the
tittle compound 78f (2.2 g, 5.61 mmol, 90.37% yield).
LCMS: MS m/z (ES!): 393.2 1M+Hr.
Step 6
3 -(4-(5 -methyl-1H-p yrazol-3 - y1)-2,5 -dioxoimid azolidin-4 -yl)prop anoic
acid 78g
169
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
A solution of 78f (500 mg, 1.28 mmol) in H0/1,4-dioxane (4N, 10 mL) was
stirred at r.t for
18h. The solution was concentrated to afford the tittle compound 78g (350 g,
1.21 mmol, 94.53%
yield).
111 NMR (400 MHz, DMSO-d6): 6 10.82 (s, 1H), 8.42 (s, 1H), 6.04 (s, 1H), 2.25-
2.18 (m,
7H).
Step 7
5 -(3 -(5-chloro-6-(trifluoromethyl) isoindo lin-2- y1)-3 -o xoprop y1)-5 -(5-
methyl- 1H-p yrazol-3 -
yl)imid azo lidine-2,4-dione 78
To a solution of 78g (50 mg, 0.17 mmol) in DMF (5 mL) was added TEA (0.12 mL,
0.85 mmol)
and 5-chloro-6-(trifluoromethyl)isoindoline 40h (44 mg, 0.17 mmol), then HATU
(65 mg, 0.17
mmol) was added. The reaction was stirred at r.t for 2h. Water (20 mL) was
added and the
mixture was extracted with Et0Ac (20 mL x 2). The combined organic layers were
washed
with brine, dried over anhydrous Na2SO4 and concentrated. The residue was
purified by prep-
HPLC to afford the tittle compound 78 (18 mg, 0.04 mmol, 23.53% yield).
111 NMR (400 MHz, DMSO-d6): 6 12.50 (brs, 1H), 10.71 (brs, 1H), 8.35 (s, 1H),
7.89 (d, 1H),
7.75 (d, 1H), 5.96 (s, 1H), 4.81 (d, 2H), 4.67 (d, 2H), 2.50-2.29 (m, 4H),
2.20 (s, 3H).
LCMS: MS raiz (ES!): 456.1 [M+Hr.
Example 79
5 -(3 -(5-chloro -6-(trifluoromethyl) isoindolin-2- yl) -3 -oxoprop y1)-5 -(p
yrimidin-2-
yl)imid azo lidine-2,4-dione 79
NN o
HN)rNH CI
0 79 C F3
170
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
0 NN N A\I 0
Step 1 Step 2 0
N 0
HN/NH
(:)<
0
79a 79b 79c
(m
N A\I 0 0
Step 3 0 Step 4
OH ___
HN )r-NH
HN/NH CI
0
0
CF3
79d 79
Step 1
Tert-butyl 4-oxo-4-(pyrimidin-2-yl)butanoate 79b
To the solution of 1-(pyrimidin-2-yl)ethan- 1-one 79a (3 g, 24.57 mmol) in THF
(100 mL) was
added DMPU (4.15 g, 32.43 mmol) and LiHMDS (5.39 g, 29.48 mmol) at 0 C, the
reaction
was stirred at this temperature for 30 min. Then tert-butyl 2-bromoacetate
(4.79 g, 24.57 mmol)
was added at 0 C, and the reaction was stirred for 3h. The reaction mixture
was quenched by
water and concentrated. The residue was purified by silica gel chromatography
(Et0Ac/hexane
= 1/20) to give 79b (500 mg, 8.61% yield).
LCMS: MS rah (ES!): 237.1 [M+Hr.
Step 2
Tert-butyl 3 -(2,5 -dioxo-4 -(p yrimidin-2 - yl)imid azolidin-4 - yl)prop ano
ate 79c
To the solution of 79b (100 mg, 423.25 umol) in H20 (1 mL) and Me0H (1 mL) was
added
NaCN (56.12 mg, 1.06 mmol) and (NH4)2CO3 (325.06 mg, 3.39 mmol). The reaction
was
stirred at 85 C overnight. The mixture was concentrated and extracted with
Et0Ac. The organic
solution was dried and concentrated; the residue was slurred with Et20 to give
crude 79c (25
mg, 19.28% yield) for next step as is.
LCMS: MS rah (ES!): 305.0 [M-Ht.
Step 3
3 -(2,5-dioxo-4-(pyrimidin-2- yl)imidazolidin-4- yl)propanoic acid 79d
171
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
The solution of 79c (25 mg, 81.62 umol) in 4N HO/dixoane (2 mL) was stirred at
room
temperature for lh. LCMS showed that the product was produced and the mixture
was
concentrated to give 79d (16 mg, 78.35% yield) for next step as it is.
LCMS: MS raiz (ES!): 251.0 [M+Hr.
Step 4
5 -(3 - (5-chloro -6- (trifluoromethyl) isoindolin-2- y1)-3 -oxoprop y1)-5 -
(p yrimidin-2-
yl)imidazolidine-2,4-dione 79
To the solution of 79d (16 mg, 0.0634 mmol) in THF (5 mL) was added HATU
(231.63 mg,
609.19 umol), DIEA (62.38 mg, 487.35 umol) and 5-chloro-6-
(trifluoromethyl)isoindoline 40h
(14 mg, 0.063 mmol). The reaction was stirred at room temperature overnight.
The mixture
was concentrated and the residue was purified by prep-HPLC to give 79 (2.13
mg, yield 7.34%).
1H NMR (400 MHz, CDC13): 6 8.80 (brs, 1H), 8.79 (brs, 1H), 8.15 (brs, 1H),
7.60 (d, 1H),
7.60 (d, 1H), 7.32 (br, 1H), 6.46 (brs, 1H), 4.84-4.75 (m, 4H), 2.85-2.78 (m,
2H), 2.56 (br, 2H).
LCMS: MS raiz (ES!): 454.1 [M+Hr.
Example 80
(S)-5 -cyc loprop y1-5 - (3 -(5 ,6-dichloro isoindo lin-2- yl- 1,1,3,3 -d4)-3 -
o xoprop yl) imidazolidine-
2,4-dione 80
0 \7'
D D
HN (s)NH
0
CI
80 CI
V
0).L
OH
0 (S)NH
D D HN D D
CI CI
HN Bal/THF, 60 jc
HNJC
0 epInt-1
HNNH
CI then HCI, 80C CI 0
0 D D st 2 CI
Step1
20b 80a 80 CI
Step 1
172
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
,6 -dichloro isoindoline- 1,1 ,3 ,3 -c/4 80a
To a solution of 4,5-dichlorophthalimide 20b (500 mg, 2.3 mmol) in THF (15 mL)
was added
BD3¨tetrahydrofuran (1M, 20 mL) dropwise under N2. The resulting mixture was
stirred at
60 C for 24h. The reaction mixture was cooled to ambient temperature and
quenched with
5 Me0H (6 ml) until the bubbling ceased. Then 4N HC1 in water (20 ml) was
added and the
mixture was heated at 80 C for 1 h. After cooled down to room temperature and
5N KOH was
added to adjust pH to 7. The mixture was concentrated under reduced pressure
and the residue
was purified by silica-gel column (DCM: Me0H(2%NH4OH) = 10: 1) to afford 5,6-
dichloroisoindoline-1,1,3,3-d480a (300 mg, 70% yield).
Step 2
(S)-5 -cycloprop y1-5 - (3 - (5 ,6 -dichloro isoindo lin-2- yl- 1,1,3 ,3 -d4)-
3-oxoprop yl)imid azolidine-
2,4-dione 80
To a mixture of 5,6-dichloroisoindoline-1,1,3,3-d4 80a (35 mg, 0.19 mmol) in
DMF (4 mL)
was added triethylamine (72 mg, 0.56 mmol), It-1 (40 mg, 0.16mmol) and HATU
(98.8 mg,
0.23 mmol). The reaction was stirred at r.t for 18h. Water (5 mL) was added
and the mixture
was extracted with Et0Ac (40 mL x 3). The combined layer was washed with
brine, dried over
Na2SO4 and concentrated. The residue was purified with prep-HPLC to give the
title compound
(30 mg, 40 % yield).
111 NMR (400 MHz, Methanol-d4): 6 7.54 (d, 2H), 2.56 (ddd, 2H), 2.32-2.13 (m,
2H), 1.34-
1.19 (m, 1H), 0.60 (td, 2H), 0.53-0.30 (m, 2H).
LCMS: MS m/z (ES!): 386 [M+Hr.
Example 81
5 -(3 -(5 -chloro-6- (triflu oro methyl) iso indolin-2 - y1)-3 -oxoprop y1)-5 -
(2-methylo xazol-4 -
yl)imidazolidine-2,4-dione 81
ro
No
0
HNyNH CF3
0
81 CI
173
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0 COOH Step \ /
1 Nr...N) µN-0 Step
0
Step 3
0 0
81a 81b 81c 81d
0 0 0
Step 4 0 (:),< Step 5, Step 6
HN HN)r-
HN N H NH 1,(NH CF3
0 0 0 81
81e 81f CI
Step 1
N-methoxy-N,2-dimethylo xazole-4-c arbo xamide 81b
To a solution of 2-methyloxazole-4-carboxylic acid 81a (500 mg, 3.94 mmol) in
DCM (15 mL)
was added TEA (1.9 mL, 13.79 mmol), the solution was cooled to 0 C. Then HOBt
(585 mg,
4.33 mmol) and EDCI (906 mg, 4.73 mmol) were added. The reaction mixture was
stirred at
0 C for 30min. N,O-Dimethylhydroxylamine hydrochloride (460 mg, 4.73 mmol) was
added.
The reaction was stirred at r.t for 18h. Water (50 mL) was added and the
mixture was extracted
with DCM (50 mL x 2). The combined organic layers were washed with brine,
dried over
anhydrous Na2SO4 and concentrated. The residue was purified by silica gel
chromatography
(eluting with hexane/Et0Ac = 1/1) to afford the tittle compound 81b (600 mg,
3.53 mmol,
89.58% yield).
111 NMR (400 MHz, CDC13): 6 8.07 (s, 1H), 3.74 (s, 3H), 3.37 (s, 3H), 2.51 (s,
3H).
Step 2
1 -(2 -methylo xazol-4- yl)ethan- 1 -one 81c
To a solution of 81b (200 mg, 1.18 mmol) in THF (20 mL) was added MeMgC1 (3N,
1.18 mL,
3.52 mmmol). The reaction mixture was stirred at 0 C for 1 h. The reaction was
diluted with
aqueue NH4C1 (30 mL) and extracted with EA (20 mL x 2). The combined organic
layers were
washed with brine, dried over anhydrous Na2SO4 and concentrated. The residue
was purified
by silica gel chromatography (eluting with hexane/Et0Ac = 4/1) to afford the
tittle compound
81c (100 mg, 0.59 mmol, 49.86% yield).
111 NMR (400 MHz, CDC13): 6 8.10 (s, 1H), 2.51 (s, 6H).
Step 3
174
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Tert-butyl 4-(2-methyloxazol-4-y1)-4-oxobutanoate 81d
To a solution of 81c (1.25 g, 10 mmol) in THF (100 mL) was added dropwise
NaHMDS (2 N,
5.5 mL,11 mmoL) at -78 C. After lh, tert-Butyl bromoacetate (1.46 mL, 10 mmoL)
was added
to the solution dropwise. The reaction was stirred at r.t for 18h. Water (200
mL) was added and
the mixture was extracted with Et0Ac (100 mL x 2). The combined organic phases
were
washed with brine, dried over anhydrous Na2SO4 and concentrated. The residue
was purified
by silica gel chromatography (eluting with hexane/Et0Ac = 10/1) to afford the
tittle compound
81d (680 mg, 2.85 mmol, 28.45% yield).
Step 4
Tert-butyl 3 - (4- (2-methylo xazol-4- y1)-2,5 -dioxoimidazolidin-4- yl)prop
ano ate 81e
To a solution of 81d (700 mg, 2.93 mmol) in Et0H (10 mL) and water (10 mL) was
added
(NH4)2CO3 (2.25 g, 23.43 mmol) and NaCN (359 mg, 7.33 mmol). The reaction was
sealed in
a vessel and stirred at 90 C for 18h. The mixture was poured to Water (100 mL)
and extracted
with Et0Ac (50 mL x 3). The combined organic phases were washed with brine,
dried over
anhydrous Na2SO4 and concentrated. The residue was purified by reversed phase
column
chromatography to afford the tittle compound 81e (180 mg, 0.58 mmol, 19.88%
yield).
LCMS: MS mh (ES!): 254.5 [M-tBu+Hr.
Step 5
3 -(4- (2-methylo xazol-4- y1)-2 ,5 -dioxoimid azo lidin-4 - yflprop anoic
acid 81f
A solution of 81e (60 mg, 0.19 mmol) in H0/1,4-dioxane (4N, 4 mL) was stirred
at r.t for 4h.
The solution was concentrated to afford the tittle compound 81f (55 mg, 0.19
mmol, 100%
yield).
Step 6
5 -(3 -(5 -chloro-6- (triflu oro methyl) iso indolin-2 - y1)-3 -oxoprop y1)-5 -
(2-methylo xazol-4 -
yl)imidazolidine-2,4-dione 81
To a solution of 81f (55 mg, 0.19 mmol) in DMF (5 mL) was added TEA (0.12 mL,
0.85 mmol)
and 5-chloro-6-(trifluoromethyl)isoindoline 40h (44 mg, 0.17 mmol), then HATU
(65 mg, 0.17
mmol) was added. The reaction was stirred at r.t for 2h. Water (20 mL) was
added and the
mixture was extracted with Et0Ac (20 mL x 2). The combined organic phases were
washed
175
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
with brine, dried over anhydrous Na2SO4 and concentrated. The residue was
purified by prep-
HPLC to afford the tittle compound 81 (20 mg, 0.044 mmol, 23.08% yield).
111 NMR (400 MHz, DMSO-d6): M0.88 (s, 1H), 8.35 (s, 1H), 8.06 (s, 1H), 7.90
(d, 3.2 Hz,
1H), 7.76 (d, 1H), 4.84 (d, 2H), 4.68 (d, 2H), 2.39 (s, 3H), 2.36-2.23 (m,
4H).
LCMS: MS raiz (ES!): 457.1 [1\4+Hr
Example 82
5-(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-(1-ethy1-1H-
imidazol-2-
yl)imidazolidine-2,4-dione 82
0
HN)r-NH CF3
0 82
CI
/=\
0
Step 1 N(yjHro Step 2
CHO HNyNH
0
82a 82b 0 82c
/=\ /=\
N N
n NN,/ o 0
Step 3 OH __ Step 4
HNy-NH
HN)rNH 44I CF3
0
0
82d 82 CI
Step 1
Tert-butyl 4-(1-ethy1-1H-imidazol-2-y1)-4-oxobutanoate 82b
To a solution of 1-ethyl-1H-imidazole-2-carbaldehyde 82a (500 mg, 4.03 mmol)
in THE (6
mL) was added tributylphosphine (0.82 mL, 3.843 mmol), the solution was
stirred at 50 C for
5min. Then tert-butyl acrylate (468 mg, 3.66 mmol) was added. The reaction was
stirred at 80 C
for 18h. The solution was concentrated. The residue was purified by silica gel
chromatography
(eluting with hexane/Et0Ac = 10/1) to afford the tittle compound 82b (84 mg,
0.33 mmol, 8.27%
yield).
176
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
1H NMR (400 MHz, CDC13): V.14 (d, 1H), 7.08 (d, 1H), 4.43 (q, 2H), 3.41 (t,
2H), 2.63 (t,
2H), 1.43 (s, 9H), 1.41 (t, 3H).
Step 2
Tert-butyl 3 -(4 -(1-ethy1-1H-imidazol-2 -y1)-2 ,5 -dioxo imidazolidin-4-
yflprop ano ate 82c
To a solution of 82b (1.7 g, 6.66 mmol) in Et0H (30 mL) and water (30 mL) was
added
(NH4)2CO3 (5.12 g, 53.3 mmol) and NaCN (816 mg, 16.66 mmol). The reaction was
sealed in
a vessel and stirred at 90 C for 18h. The mixture was poured to water (100 mL)
and extracted
with 1-butanol (50 mL x 8). The combined organic phases were dried over
anhydrous Na2SO4
and concentrated. The residue was purified by flash reversed phase column
chromatography to
.. afford the tittle compound 82c (220 mg, 0.68 mmol, 10.26% yield).
LCMS: MS m/z (ES!): 323.5 [M+Hr.
Step 3
3 -(4 -(1-ethyl- 1H-imidazol-2 -y1)-2,5 -dio xo imidazolidin-4- yflprop ano ic
acid 82d
A solution of 82c (50 mg, 0.16 mmol) in H0/1,4-dioxane (4N, 4 mL) was stirred
at r.t for 4h.
.. The solution was concentrated to afford the tittle compound 82d (45 mg,
0.16 mmol, 100%
yield), which was used directly for next step reaction.
Step 4
5 -(3 -(5 -chloro-6-(triflu oro methyl) iso indolin-2 - y1)-3 -oxoprop y1)-5 -
(1 -ethyl- 1H-imid azol-2-
yl)imidazolidine-2,4-dione 82
To a solution of 82d (45 mg, 0.16 mmol) in DMF (5 mL) was added TEA (0.09 mL,
0.85 mmol)
and 5-chloro-6-(trifluoromethyl)isoindoline 40h (32 mg, 0.12 mmol), then HOBt
(22 mg, 0.16
mmol) and EDCI (31 mg, 0.16 mmol) were added. The reaction was stirred at r.t
for 18h. The
mixture was purified by prep-HPLC to afford the tittle compound 82 (2.8 mg,
0.006 mmol,
3.75% yield).
.. 1H NMR (400 MHz, Methanol-d4): 6 7.78 (d, 1H), 7.67 (s, 1H), 7.62 (d, 1H),
7.46 (s, 1H),
4.93-4.90 (m, 2H), 4.79-4.77 (m, 4H), 4.43 (q, 2H), 2.81-2.60 (m, 4H), 1.53
(t, 7.2 Hz, 3H).
LCMS: MS m/z (ES!): 470.1 [M+Hr.
177
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Examples 83-1 & 83-2
(S)-5 -(3 -(5-chloro -6-(trifluoromethyl) isoindo lin-2-y1)-3 -o xoprop y1)-5 -
(thiazol-2-
yl)imidazolidine-2,4-dione 83-1 &
(R)-5 -(3 -(5 -chloro-6-(trifluoromethyl) isoindo lin-2-y1)-3 -o xoprop y1)-5 -
(thiazol-2 -
yl)imidazolidine-2,4-dione 83-2
/-=\
Nr¨\, N S 0
00r? ssS;jL N
MI 1 um (R)
2.rNH 11, CF3 NH CF3
83-1 CI 83-2 CI
0 S 0 S 0
0S 0 NYS
J1,0H Step 1 SFC
*
HN HN4tNH (s) (R) N
HN, _NH
CF3 )r-NH c3 CF3
43d 83 CI 83-1 CI 83-2 CI
Step 1
(S)-5 -(3 -(5-chloro -6-(trifluoromethyl) isoindo lin-2-y1)-3 -o xoprop y1)-5 -
(thiazol-2-
yl)imidazolidine-2,4-dione 83-1 & (R)-5-(3 -(5 -chloro-6-(trifluoromethyl)
isoindo lin-2- y1)-3 -
oxoprop y1)-5 -(thiazol-2- yl) imidazolidine-2 ,4 -dio ne 83-2
A mixture of 43d (102 mg, 400 umol), 40h (103 mg, 400 umol), HATU (152 mg, 400
umol)
and Et3N (161 mg, 1.60 mmol) in DMF (4 mL) was stirred at room temperature for
18 hours.
Then the reaction mixture was purified by prep-HPLC (Waters 2767/2545/2489,
Waters
Xbridge C18 10 um OBD 19*250 mm, Mobile Phase A: 0.1% NH4OH in water, Mobile
Phase
B: CH3CN, Flow: 20 mL/min, Column temp: RT) to afford racemic mixture 83 (60
mg), which
was separated by SFC to afford two enantiomers (18 mg, 39.23 umol, 9.82%
yield, and 20 mg,
43.59 umol, 10.9% yield, separately).
enantiomer with shorter retention time:
1H NMR (400 MHz, DMSO-d6): 6 11.10 (br, 1H), 8.83 (br, 1H), 7.91-7.85 (m, 2H),
7.81-7.74
(m, 2H), 4.83-4.78 (m, 2H), 4.69-4.66 (m, 2H), 2.44-2.33 (m, 4H).
HPLC: 99.985% @ 254 nm, 99.985% @ 214 nm.
Chiral HPLC ((CO2/Et0H/DEA 60/40/0.04 1.8m1/min IG,3um,3*100(Daicel)): Rt:
2.119
min, cc: 100%.
178
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: MS m/z (ES!): 459.0 [IVI+HIt
enantiomer with longer retention time:
111 NMR (400 MHz, DMSO-d6): 6 11.10 (br, 1H), 8.83 (br, 1H), 7.91-7.85 (m,
2H), 7.81-7.74
(m, 2H), 4.83-4.78 (m, 2H), 4.69-4.66 (m, 2H), 2.44-2.33 (m, 4H).
HPLC: 99.078% @ 254 nm, 99.403% @ 214 nm.
Chiral HPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IG,3um,3*100(Daicel)): Rt:
5.782 min;
ee: 100%.
LCMS: MS m/z (ES!): 459.0 [M+Hr.
Examples 84-1 and 84-2
(R)-5 -(3-(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-3-oxopropy1)-5-
(pyridin-2-
yl)imidazolidine-2,4-dione 84-1
(S)-5 -(3-(5-chloro-6-(trifluoromethyl)isoindolin-2-y1)-3-oxopropy1)-5-
(pyridin-2-
yl)imidazolidine-2,4-dione 84-2
HN
m
rNH CF3 y NH CF3
84-1 CI 84-2 CI
04:1N1 0 0 CNC' 0
N 0
0
HN
HN -NH ,IrNH afr CF3- HN)r-NI-1
CF3 HN)r-NI-1 CF3
0
60d 84 CI 84-1 CI 84-2 CI
Step 1
(R)-5 -(3-(5-chloro-6-(trifluoromethyflisoindolin-2-y1)-3-oxopropy1)-5-
(pyridin-2-
yl)imidazolidine-2,4-dione 84-1 & (S)-5 -(3-(5-chloro-6-
(trifluoromethyl)isoindolin-2-y1)-3 -
oxopropy1)-5-(pyridin-2-yl)imidazolidine-2,4-dione 84-2
A mixture of 60d (114 mg, 399 umol), 40h (78 mg, 302 umol), HATU (152 mg, 399
umol),
TEA (162 mg, 1.60 mmol) and DMF (4.0 mL) was stirred at room temperature for
18 hours.
The reaction mixture was purified by Prep-HPLC (Waters 2767/2545/2489, Waters
Xbridge
179
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
C18 10 urn OBD 19*250 mm, Mobile Phase A: 0.1% NH4OH in water, Mobile Phase B:
CH3CN, Flow: 20 mL/min, Column temp: RT) to afford racemic mixture 84 (60 mg),
which
was separated by SFC to afford two enantiomers (15 mg, 33.13 umol, 8.30%
yield, and 15 mg,
33.13 umol, 8.30% yield).
enantiomer with shorter retention time:
111 NMR (400 MHz, DMSO-d6): 6 10.87 (brs, 1H), 8.62 (dd, 1H), 8.51 (s, 1H),
7.91-7.84 (m,
2H), 7.76 (d, 1H), 7.54 (d, 1H), 7.41-7.37 (m, 1H), 4.83-4.80 (m, 2H), 4.69-
4.66 (m, 2H), 2.51-
2.41 (m, 2H), 2.36-2.30 (m, 2H).
LCMS: MS m/z (ES!): 453.0 1M+H1 .
Chrial HPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IG,3um,3*100(Daicel)): RT,
1.852;
Purity: 100%.
enantiomer with longer retention time:
111 NMR (400 MHz, DMSO-d6): 6 10.88 (brs, 1H), 8.62 (dd, 1H), 8.53 (s, 1H),
7.91-7.85 (m,
2H), 7.76 (d, 1H), 7.54 (d, 1H), 7.41-7.37 (m, 1H), 4.83-4.80 (m, 2H), 4.69-
4.66 (m, 2H), 2.51-
2.44 (m, 2H), 2.36-2.30 (m, 2H).
LCMS: MS m/z (ES!): 453.1 1M+H1 .
Chrial HPLC (CO2/Et0H/DEA 60/40/0.04 1.8m1/min IG,3um,3*100(Daicel)): RT,
2.713;
Purity: 99.32%.
Examples 85-1 and 85-2
(R)-5 -(3-(5 -chloro -6-(trifluoromethyl) iso indolin-2 -y1)-3 -oxoprop y1)-5 -
(p yrazin-2-
yl)imidazolidine-2 ,4-dione 85-1
& (S)-5 -(3 -(5 -chloro-6-(trifluoro methyl) isoindo lin-2- y1)-3 -o xoprop
y1)-5 -(p yrazin-2-
yl)imidazolidine-2,4-dione 85-2
0 0 0
(R) N
HN HN
410 CI 2(-NH 410 CI
85-1 CF3 85-2 CF3
180
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
0 HNANH HNANH 0
Step 1 Step 2 Step 3
k 0 0 OH
N
N
N N
0
85a 85b 85c 85d
0
0 04N 0
Step 4
410
HN SFC )r- HN)r HN
NH )r.-NH 0 CI -NH CI 1100 CI
0 0 0
85 CF3 85-1 CF3 85-2 CF3
Step 1
tert-butyl 4-oxo-4-(thiazol-2-yffbutanoate 85b
To a solution of 1-(pyrazin-2-yl)ethan-1-one 85a (12.0 g, 98.3 mmol) in THF
(200 mL) at -70 C
was added NaHMDs (49.2 mL, 98.3 mmol, 2.0 M in THF) dropwise. The resulting
mxiture
was stirred at this temperature for 30 mm before tert-butyl 2-bromoacetate
(19.2 g, 98.3 mmol)
was added drop-wsie. After addition, the reaction mixture was stirred at -20 C
for 1.0 hour,
then warmed to room temperature and stirred for 18 hours. The resulting
mixture was cooled
to 0 C, then quenched with aq. NaHCO3 (200 mL). The mixture was extracted with
Et0Ac
(300 mL x 4). The organic layers were combined, dried over anhydrous Na2SO4
and filtered.
The filtrate was concentrated under reduced pressure. The residue was purified
by silica gel
chromatography (Et0Ac/hexane = 1/20 to 1/4) to afford 85b (13.5 g, 57.14 mmol,
58.2% yield).
LCMS: MS rah (ES!): 237.1[1\4+Hr.
Step 2
tert-butyl 3 - (2 ,5 -dioxo-4- (p yrazin-2- yl)imidazolidin-4-yl)propanoate
85c
A mixture of 85b (13.0 g, 55.0 mmol), (NH4)2CO3 (44.9 g, 468 mmol) and NaCN
(11.7 g, 220
mmol) in Et0H (60 mL) and H20 (60 mL) was heated to 120 C in an autocalve and
stirred for
18 hours. The resulting mixture was diluted with water (100 mL). The mixture
was extracted
with Et0Ac (200 mL x 3) and n-BuOH (200 mL x 3). The combined organic layers
were
washed with brine (200 mL) and concentrated under reduced pressure. The
residue was purified
by silica gel chromatography (Me0H/DCM = 1/100 to 1/30) to afford 85c (3.0 g,
9.79 mmol,
17.80% yield).
LCMS: MS rah (ES!): 307.1[1\4+Hr.
181
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
Step 3
3 -(2,5 -dio xo -4-(p yrazin-2 - yl)imidazo lidin-4 - yl)prop ano ic acid 85d
To a solution of 85c (900 mg, 2.94 mmol) in DCM (30 mL) was added HC1/1,4-
dioxane (30
mL, 4.0 M) drop wise. The reaction mixture was stirred room temperature for 18
hours. The
resulting mixture was filtered in vacuum. The filter cake was collected, dried
in vacuum to
afford 85d (600 mg, 2.40 mmol, 81.62% yield).
LCMS: MS nth (ES!): 249.01M-H1-.
Step 4
(R)-5 -(3-(5 -chloro -6-(trifluoromethyl) iso indolin-2 -y1)-3 -oxoprop y1)-5 -
(p yrazin-2-
yl)imidazolidine-2,4-dione 85-1
& (S)-5 -(3 -(5 -chloro-6-(trifluoro methyl) isoindo lin-2- y1)-3 -o xoprop
y1)-5 -(p yrazin-2-
yl)imidazolidine-2,4-dione 85-2
A mixture of 85d (130 mg, 519.56 umol), 40h (134 mg, 519.56 umol), TEA (157.72
mg, 1.56
mmol) and HATU (198 mg, 519.56 umol) in DMF (5 mL) was stirred at room
temperature for
18 hours. The resulting mixture was purified by prep-HPLC (Waters
2767/2545/2489, Waters
Xbridge C18 10 um OBD 19*250 mm, Mobile Phase A: 0.1% NH4OH in water, Mobile
Phase
B: CH3CN, Flow: 20 mL/min, Column temp: RT) to afford racemic mixture 85
(about 50 mg),
which was further separated by SFC to afford two enantiomers (16 mg, 35.26
umol, 6.79%
yield, and 15 mg, 33.05 umol, 6.36% yield).
enantiomer with shorter retention time:
111 NMR (400 MHz, DMSO-d6): 6 11.05 (brs, 1H), 8.83 (d, 1H), 8.73-8.71 (m,
1H), 8.67 (d,
1H), 8.65 (br, 1H), 7.90 (d, 1H), 7.75 (d, 1H), 4.84-4.80 (m, 2H), 4.69-4.65
(m, 2H), 2.49-2.46
(m, 2H), 2.37-2.33 (m, 2H).
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 2.8m1/min AY,5um,4.6*250(Daicel)): Rt:
2.652min, cc: 100%.
LCMS: MS nth (ES!): 454.1 1M+H1t
enantiomer with longer retention time:
182
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
111 NMR (400 MHz, DMSO-d6): 6 11.01 (br, 1H), 8.82 (d, 1H), 8.72-8.70 (m, 1H),
8.67 (d,
1H), 8.57 (brs, 1H), 7.90 (d, 1H), 7.75 (d, 1H), 4.84-4.80 (m, 2H), 4.69-4.65
(m, 2H), 2.49-
2.46 (m, 2H), 2.37-2.33 (m, 2H).
Chiral HPLC (CO2/Me0H/DEA 60/40/0.04 2.8m1/min AY,5um,4.6*250(Daicel)): Rt:
4.485;
ee: 99.43%.
LCMS: MS raiz (ES!): 454.1 [M+Hr.
Examples 86-1, 86-2, 86-3 and 86-4
(S)-5 -((S)-3 -(5 -chloro-6-(trifluoromethyl) isoindo lin-2-34)-2-
(hydroxymethyl)-3 -oxoprop y1)-
5 -cyc loprop ylimid azo lidine-2,4-dione 86-1 &
(S)-54(R)-3 -(5-chloro-6-(triflu oromethyl) isoindo lin-2-34)-2-
(hydroxymethyl)-3 -o xoprop y1)-
-cyc loprop ylimid azo lidine-2,4-dione 86-2 &
(R)-5 -((S)-3 -(5 -chloro-6-(trifluoromethyl) isoindo lin-2- y1)-2-
(hydroxymethyl)-3 -oxoprop y1)-
5 -cyc loprop ylimid azo lidine-2,4-dione 86-3 &
(R)-5 -((R)-3 -(5-chloro-6-(triflu oro methyl) isoindo lin-2-34)-2-
(hydroxymethyl)-3 -o xoprop y1)-
-c yc loprop ylimidazolidine-2 ,4 -dio ne 86-4
0 7 0V
o0
rNH 411 CI y-NH
OH 411 CI
0 0
86-1 CF3 86-2 CF3
0
0
)7OH
HN NH CI HN)r,NH ,-
OH 40 CI
0 0
86-3 CF3 86-4 CF3
183
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
o V. o 7.
HNL
so NH - N HNILL
. CI rNH 0 I/ CI
.Ayyt 0 0
OH N
Step 1 Step 2 r cõ CF3
86b-1 86b-2
40 0 cõ
_ N 04, 0
t65e 86a HNI 1 HN2r NH NH -
,,o II CI 0 . CI
1110 CF3 IN CF3
86b-3 86h-4
ck.2:.....,_ .J.LV.
HN/ 7 7 " =
,---(-)LN
step 3 HI\li.....XILN Step 4 N
2rNH --,0 . CI HN - r,..NH __ . CI
HN'..H.........tit'
2r-NH -.,0H = CI 0 27.-NH
OH * CI
so cõ=so CF3
CF3 CF3
86b-1 86-1 86b-2 86-2
cD__. C1/4 k
y so r
'N
HN1 1 Step 5 HN .' Step 6 N NH 7,0 ii cõ
HNIH CI _,.. 1 )r-NH _,.. HNI iNH's. OH t
crN ,...OH * CI 0 e0 ii CI * 1
CF3 CF3
86h-4 86-3 86h-4 86-4
Step 1
2-((benzyloxy)methyl)-1-(5-chloro-6-(trifluoromethyl)isoindolin-2-34)-4-
cyclopropylbutane-
1,4-dione 86a
To a solution of 2-((benzyloxy)methyl)-4-cyclopropy1-4-oxobutanoic acid 65e
(350 mg, 1.33
mmol) and 5-chloro-6-(trifluoromethyl)isoindoline 40h (310 mg, 1.20 mmol) in
DMF (10
mL) was added TEA (675 mg, 6.67 mmol) and HATU (609 mg, 1.60 mmol). The
reaction was
stirred at r.t for 2h. Water (20 mL) was added and the mixture was extracted
with Et0Ac (20
mL X 2), The combined organic phases were washed with brine, dried over
anhydrous Na2SO4
and concentrated. The residue was purified by prep-TLC to give 86a (400 mg,
858.57 umol,
64.34% yield).
LCMS: rah (ES!): 466.1 [M+Hr.
Step 2
Four isomers of 5-(2-((benzyloxy)methyl)-3-(5-chloro-6-
(trifluoromethyl)isoindolin-2-34)-3 -
oxopropy1)-5-cyclopropylimidazolidine-2,4-dione 86b
184
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of 86a (600 mg, 1.29 mmol) in Et0H (20 mL) was added ammonium
carbonate
(991 mg, 10.32 mmol), NaCN (158 mg, 3.23 mmol) and water (20 mL). The reaction
was
stirred at 90 C for 18h. Water (20 mL) was added and the mixture was extracted
with Et0Ac
(20 mL x 3). The combined organic phases were washed with brine, dried over
anhydrous
Na2SO4 and concentrated. The residue was purified by flash reversed phase
column
chromatography to give 86b (370 mg), which was further seperated by SFC to
give four
isomers (127 mg, 118 mg, 55 mg and 48 mg).
isomers 1 (1st peak from SFC):
1H NMR (400 MHz, DMSO-d6): 6 10.66 (s, 1H), 7.90 (d, 1H), 7.76 (d, 1H), 7.68
(d, 1H),
7.25-7.22 (m, 5H), 5.01-4.62 (m, 4H), 4.44 (s, 2H), 3.54-3.43 (m, 2H), 2.96-
2.90 (m, 1H), 2.28-
2.21 (m, 1H), 1.84-1.81 (m, 1H), 1.06-1.00 (m, 1H), 0.42-0.23 (m, 3H), 0.02-
0.01 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.88.
Chiral HPLC: Rt: 3.12 min, cc: 100%.
LCMS: miz (ES!): 536.1 lIVI+Hr.
isomers 2 (2nd peak from SFC):
1H NMR (400 MHz, DMSO-d6): 6 10.66 (s, 1H), 7.91 (d, 1H), 7.77 (d, 1H), 7.71
(d, 1H),
7.25-7.22 (m, 5H), 5.01-4.62 (m, 4H), 4.44 (s, 2H), 3.54-3.43 (m, 2H), 2.96-
2.90 (m, 1H), 2.29-
2.21 (m, 1H), 1.85-1.81 (m, 1H), 1.06-1.00 (m, 1H), 0.42-0.23 (m, 3H), 0.03-
0.01 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.87.
Chiral HPLC: Rt: 3.34 min, cc: 100%.
LCMS: miz (ES!): 536.1 lIVI+Hr.
isomers 3 (3th peak from SFC):
1H NMR (400 MHz, DMSO-d6): 6 10.52 (s, 1H), 7.91 (s, 1H), 7.77-7.75 (s, 2H),
7.25-7.20
(m, 5H), 5.00-4.54 (m, 4H), 4.44 (s, 2H), 3.55-3.47 (m, 2H), 3.16-3.09 (m,
1H), 2.31-2.25 (m,
1H), 1.73-1.70 (m, 1H), 1.06-1.01 (m, 1H), 0.43-0.26 (m, 3H), 0.13-0.06 (m,
1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.85.
Chiral HPLC: Rt: 4.25 min, cc: 100%.
185
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
LCMS: rniz (ES!): 536.1 [1\4+Hr.
isomers 4 (4th peak from SFC):
1H NMR (400 MHz, DMSO-d6): 6 10.52 (s, 1H), 7.91 (s, 1H), 7.77-7.75 (m, 2H),
7.25-7.20
(m, 5H), 5.00-4.54 (m, 4H), 4.44 (s, 2H), 3.55-3.47 (m, 2H), 3.16-3.09 (m,
1H), 2.31-2.25 (m,
1H), 1.73-1.70 (m, 1H), 1.06-1.01 (m, 1H), 0.43-0.26 (m, 3H), 0.13-0.06 (m,
1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.85 nm.
Chiral HPLC: Rt: 4.60 min, ee: 98.76%.
LCMS: rniz (ES!): 536.1 11\4+Hr.
Step 3
(S)-5 -((S)-3 -(5 -chloro-6-(trifluoromethyl) isoindo lin-2- y1)-2-
(hydroxymethyl)-3 -oxoprop y1)-
5 -c yc loprop ylimidazolidine-2 ,4 -dio ne 86-1
To a solution of isomers 1 from step 2 (120 mg, 0.22 mmol) in Et0Ac (60 mL)
was added
PdC12 (60 mg). Then the reaction was stirred at room temperature under H2
atmosphere for 2h.
The mixture was filtered, and the filtrate was concentrated. The residue was
purified by flash
reversed phase column chromatography to give the compound 86-1(74 mg, 74.75%).
1H NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.90 (d, 1H), 7.77 (s, 1H), 7.66
(d, 1H),
5.04-4.97 (m, 2H), 4.89-4.83 (m, 1H), 4.77-4.60 (m, 2H), 3.51-3.36 (m, 2H),
2.77-2.70 (m,
1H), 2.20-2.13 (m, 1H), 1.80-1.74 (m, 1H), 1.05-0.98 (m, 1H), 0.42-0.22 (m,
3H), 0.04--0.029
(m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.84 nm.
Chiral HPLC: Rt: 3.29 min, cc: 100%.
LCMS: rn/z (ES!): 446.1 11\4+Hr.
Step 4
(S)-54(R)-3 -(5-chloro-6-(triflu oromethyl) isoindo lin-2- y1)-2-
(hydroxymethyl)-3 -o xoprop y1)-
5 -c yc loprop ylimidazolidine-2 ,4 -dio ne 86-2
186
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of isomers 2 from step 2 (110 mg, 0.21 mmol) in Et0Ac (50 mL)
was added
PdC12 (50 mg). Then the reaction was stirred at room temperature under H2
atmosphere for 2h.
The mixture was filtered and the filtrate was concentrated. The residue was
purified by flash
reversed phase column chromatography to give the compound 86-2(65 mg, 71.04%).
1H NMR (400 MHz, DMSO-d6): 6 10.63 (s, 1H), 7.90(d, 1H), 7.77 (s, 1H), 7.66 d,
1H), 5.04-
4.97 (m, 2H), 4.89-4.82 (m, 1H), 4.77-4.60 (m, 2H), 3.51-3.36 (m, 2H), 2.76-
2.70 (m, 1H),
2.20-2.13 (m, 1H), 1.80-1.75 (m, 1H), 1.05-0.98 (m, 1H), 0.43-0.23 (m, 3H),
0.04--0.031 (m,
1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.84 nm.
Chiral HPLC: Rt: 4.66 min, cc: 100%.
LCMS: raiz (ES!): 446.1 11\4+Hr.
Step 5
(R)-5 -((S)-3 -(5 -chloro-6-(trifluoromethyl) isoindo lin-2- y1)-2-
(hydroxymethyl)-3 -oxoprop y1)-
5 -c yc loprop ylimidazolidine-2 ,4 -dio ne 86-3
To a solution of isomers 3 from step 2 (50 mg, 0.093 mmol) in Et0Ac (25 mL)
was added
PdC12 (25 mg). Then the reaction was stirred at room temperature under H2
atmosphere for 2h,
the mixture was filtered and the filtrate was concentrated. The residue was
purified by flash
reversed phase column chromatography to give the compound 86-3(37 mg, 89.16%).
1H NMR (400 MHz, DMSO-d6): 6 10.50 (s, 1H), 7.91 (s, 1H), 7.78 (d, 1H), 7.74
(d, 1H),
5.04-4.97 (m, 2H), 4.87-4.80 (m, 1H), 4.74-4.67 (m, 1H), 4.60-4.53 (m, 1H),
3.51-3.34 (m,
2H), 2.96-2.89 (m, 1H), 2.25-2.19 (m, 1H), 1.67-1.63 (m, 1H), 1.07-1.00 (m,
1H), 0.43-0.25
(m, 3H), 0.11-0.05 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.84.
Chiral HPLC: Rt: 5.73 min, cc: 97.83%.
LCMS: raiz (ES!): 446.1 11\4+Hr.
Step 5
(R)-5 -((R)-3 -(5-chloro-6-(triflu oro methyl) isoindo lin-2- y1)-2-
(hydroxymethyl)-3 -o xoprop y1)-
5 -c yc loprop ylimidazolidine-2 ,4 -dio ne 86-4
187
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
To a solution of isomers 4 from step 2 (45 mg, 0.084 mmol) in Et0Ac (25 mL)
was added
PdC12 (25 mg). Then the reaction was stirred atroom temperature under H2
atmosphere for 2h,
the mixture was filtered and the filtrate was concentrated. The residue was
purified by flash
reversed phase column chromatography to give the compound 86-4(37 mg, 89.16%).
1H NMR (400 MHz, DMSO-d6): 6 10.50 (s, 1H), 7.91 (s, 1H), 7.78 (d, 1H), 7.74
(d, 1H),
5.05-4.97 (m, 2H), 4.87-4.81 (m, 1H), 4.75-4.68 (m, 1H), 4.60-4.53 (m, 1H),
3.51-3.40 (m,
2H), 2.96-2.89 (m, 1H), 2.25-2.20 (m, 1H), 1.67-1.63 (m, 1H), 1.07-1.00 (m,
1H), 0.43-0.24
(m, 3H), 0.11-0.05 (m, 1H).
19F NMR (376.5 MHz, DMSO-d6): 6 -60.83.
Chiral HPLC: Rt: 7.07 mm, cc: 97.16%.
LCMS: raiz (ESI): 446.1 1M+1-11 .
The following compounds were prepared using the similar methods as Examples 1-
86.
Ex# Structure and chemical name 1H NMR and mass spectra
10 oV 1H NMR (400 MHz, CD30D): 6 7.25-7.23
HN/ N = 0HCF2 (m, 1H), 7.04-7.01 (m, 1H), 7.02-6.98
(m,
N H
0 10
1H), 4.77-4.73 (m, 2H), 4.65-4.62 (m, 2 H),
(S)-5 -c ycloprop y1-5 -(3-(5 - 2.46-2.41 (m, 2H), 2.37-2.33 (m, 1H),
2.14-
(difluoromethoxy)isoindolin-2- 2.10 (m, 2H), 1.17-1.15 (m, 1H), 0.50-
0.47
y1)-3 -o xoprop yl) imidazolidine- (m, 1H), 0.34-0.30 (m, 2H), 0.27-0.22
(m,
2,4-dione 1H).
LC-MS: MS m/z (ESI): 1M+Hr. 380.2
11 0 V 0 1H NMR (400 MHz, CD30D): 6 7.22-1.16
HNL
(m, 4H), 4.78-4.73 (m, 2H), 4.67-4.62 (m, 2
NH
0 11 H), 2.46-2.44 (m, 1H), 2.37-2.34 (m, 1
H),
(S)-5 -c yc loprop y1-5 -(3 - 2.14-2.10 (m, 2H), 1.16-1.12 (m, 1H),
0.51-
(isoindolin-2-y1)-3- 0.47 (m, 1H), 0.34-0.30 (m, 2H), 0.27-
0.23
oxopropyl)imidazolidine-2,4- (m, 1H).
dione LC-MS: MS m/z (ESI): 1M+H1 . 314.2
188
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
12 1H NMR (400 MHz, CD30D): 6 8.41-8.37
(m, 1H), 7.81-7.78 (m, 1H), 7.34-7.31 (m,
\
o 12 1H), 4.72-4.68 (m, 2H), 3.57-3.55
(m, 2 H),
(S)-5-cyclopropy1-5-(3 -(5,7- 2.46-2.42 (m, 1H), 2.36-2.34 (m, 1H), 2.14-
dihydro-6H-pyrro1o[3,4- 2.12 (m, 2H), 1.16-1.14 (m, 1H), 0.50-0.47
b]pyridin-6-y1)-3- (m, 1H), 0.33-0.30 (m, 2H), 0.27-0.23 (m,
oxopropyl)imidazolidine-2,4- 1H).
dione LC-MS: MS m/z (ESI): [M+Hr. 315.2
13 a V (D.L 1H NMR (400 MHz, CD30D): 6 8.74-8.72
HN/ q/
2r-NH 13 / (m, 1H), 8.67-8.63 (m, 1H), 7.90-7.87 (m,
1H), 5.04-5.01 (m, 2H), 4.88-4.85 (m, 2 H),
(S)-5-cyclopropy1-5-(3 -(1,3- 2.47-2.45 (m, 1H), 2.39-2.35 (m, 1 H),
2.18-
dihydro-2H-pyrro1o[3,4- 2.11 (m, 2H), 1.15-1.13 (m, 1H), 0.50-0.48
c]pyridin-2-y1)-3- (m, 1H), 0.33-0.30 (m, 2H), 0.27-0.23 (m,
oxopropyl)imidazolidine-2,4- 1H).
dione LC-MS: MS m/z (ESI): [M+Hr. 315.2
14 o V 1H NMR (400 MHz, CD30D): 6 7.36-7.28
HN H LN
)7,-N
= (m, 4H), 5.28 (q, 1H), 4.89-4.84 (m, 2H),
o 14
2.57-2.43 (m, 2H), 2.25-2.22 (m, 2H), 1.50
(S)-5-cyclopropy1-5-(3 -((R)-1- (d, 3H), 1.40-1.37 (m, 2H), 1.28-1.26 (m,
methylisoindolin-2-y1)-3- 1H), 0.50-0.48 (m, 1H), 0.34-0.32 (m, 2
H),
oxopropyl)imidazolidine-2,4- 0.27-0.23 (m, 1H).
dione LC-MS: MS m/z (ESI): [M+Hr. 328.2
15 0 V 0 1H NMR (400 MHz, CD30D): 6 7.35-7.28
=-=-:-.(0=\)LN
HN
)r-NH 410k (m, 4H), 5.28 (q, 1H), 4.89-4.85 (m, 2H),
o 15 2.57-2.43 (m, 2H), 2.25-2.21 (m,
2H), 1.50
(S)-5-cyclopropy1-5-(3 -((S)-1- (d, 3H), 1.39-1.34 (m, 2H), 1.28-1.22 (m,
methylisoindolin-2-y1)-3- 1H), 0.50-0.44 (m, 1H), 0.34-0.30 (m, 2
H),
oxopropyl)imidazolidine-2,4- 0.27-0.23 (m, 1H).
dione LC-MS: MS m/z (ESI): [M+Hr. 328.2
16 o y 0 1H NMR (400 MHz, CD30D): 6 7.25-7.22
HNN
NH (m, 1H), 7.08-7.06 (m, 1H), 6.96-6.92 (m,
y.
O 16 1H), 4.85-4.81 (m, 2H), 4.69-4.66
(m, 2 H),
189
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(S)-5 -c yc loprop y1-5 -(3 -(4- 2.47-2.42 (m, 1H), 2.36-2.34 (m, 1H), 2.12-
fluoroisoindolin-2-y1)-3- 2.09 (m, 2H), 1.17-1.14 (m, 1H), 0.50-
00.47
oxopropyllimidazolidine-2,4- (m, 1H), 0.33-0.31 (m, 2H), 0.27-0.23 (m,
dione 1H).
LC-MS: MS m/z (ESI): 1M+Hr. 332.2
17 o V 1H NMR (400 MHz, CD30D): 6 7.38 ¨7.27
FdNN
)r-NH = F (m, 1H), 7.16 ¨ 6.96 (m, 2H), 4.72 (d,
2H),
0 17
3.37 (s, 2H), 2.60 ¨2.54 (m, 1H), 2.48 ¨ 2.42
(S)-5-cyclopropy1-543 (m, 1H), 2.33 ¨ 2.11 (m, 2H), 1.25 (tt,
1H),
fluoroisoindolin-2-y1)-3- 0.69 ¨ 0.53 (m, 1H), 0.53 ¨ 0.37 (m, 2H),
oxopropyllimidazolidine-2,4- 0.37 ¨ 0.23 (m, 1H).
dione LC-MS: MS m/z (ESI): 1M+Hr. 332.2
18 0 V 0 1H NMR(400 MHz, CD30D): 7.34-7.30 (m,
HN
2H), 7.20-7.17 (m, 2H), 5.50 (d, 1 H), 5.32
L
),r-NH (d, 1H), 2.46-2.02 (m, 6H), 1.42-1.30 (m,
0 18 2H), 1.19-1.16 (m, 1H), 0.54-0.51 (m, 1H),
(SS)-5 -cycloprop y1-5 -(3 -o xo -3- 0.44-0.27 (m, 3H).
(1,2,3,4 -tetrahydro- 1,4 - LC-MS: MS m/z (ESI): 1M+Hr. 340.2;
epiminonaphthalen-9-
yl)propyllimidazolidine-2,4-
dione
19 0 V 1H NMR (400 MHz, CD30D) 6 7.27 (dt,
)r-NH F 2H), 4.85 (s, 2H), 4.72 (s, 2H), 2.58-2.53
(m,
0 19 1H), 2.45 ¨ 2.41 (m, 1H), 2.29 ¨ 2.18 (m,
(S)-5-cyclopropy1-543 2H), 1.32 ¨ 1.18 (m, 1H), 0.64 ¨0.58 (m,
difluoroisoindolin-2-y1)-3- 1H), 0.52 ¨ 0.23 (m, 3H).
oxopropyllimidazolidine-2,4- LC-MS: MS m/z (ESI): 1M+H1t 350.2
dione
21 0 V 1H NMR (500 MHz, DMSO-d6) 6 10.07 (br,
HNtNH 21 CI 1H), 7.74 (d, 1H), 7.14-7.06 (m, 3H), 3.56-
3.33 (m, 4H), 3.12-3.03 (m, 1H), 2.98-2.70
(S)-5-(3 -((R)-8-chloro -1 -methyl-
(m, 2H), 2.41-2.12 (m, 2H), 1.83-1.72 (m,
1,2,4,5 -tetrahydro-3H-
b enzold] azep y1)-3
190
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
oxopropy1)-5- 2H), 1.11 (m, 3H), 0.98-0.96 (m, 1H), 0.40-
cyclopropylimidazolidine-2,4- 0.20 (m, 3H), 0.05--0.06 (m, 1H).
dione LCMS: MS m/z (ESI): 390.1 [M+Hr.
22 o Mixture of two diastereomers
1H NMR (400 MHz, CD30D): 7.08-6.98 (m,
HNNH 22
0 1H), 6.90-6.70 (m, 2H), 3.66-3.58 (m, 2H),
Mixture of two diastereomers 3.48-3.35 (m, 2H), 3.18-3.11 (m, 1 H),
3.04-
(5 S)-5 -cycloprop y1-5 -(3 -(8- 2.94 (m, 1H), 2.87-2.73 (m, 1H), 2.41-2.18
flu oro -1-methyl- 1,2,4,5 - (m, 2H), 1.98-1.85 (m, 2H), 1.37-1.28 (m,
tetrahydro-3H-benzo [d] azep in-3 - 3H), 1.11-1.02 (m, 1H), 0.48-0.42 (m, 1H),
y1)-3-oxopropyflimidazolidine-
0.38-0.15 (m, 3H).
2,4-dione MS m/z (ESI): [M+Hr. 374.2
23 o V 1H NMR (400 MHz, CD30D): 7.18-7.16 (m,
HN CI 1H), 7.01-6.99 (m, 2H), 3.63-3.58 (m, 2H),
o 23 3.16-3.14 (m, 1H), 3.10-3.07 (m, 1 H),
2.98-
(S)-5 -(3 -(7-chloro-1,2 ,4,5 - 2.94 (m, 1H), 2.91-2.87 (m, 1H), 2.39-2.36
tetrahydro-3H-benzo [d] azep in-3 - (m, 1H), 2.29-2.26 (m, 1H), 1.97-1.90 (m,
y1)-3-oxopropy1)-5- 2H), 1.10-1.06 (m, 2H), 0.46-0.44 (m, 1H),
cyclopropylimidazolidine-2,4- 0.33-0.18 (m, 4H).
dione LC-MS: MS m/z (ESI): [M+Hr. 376.2
24 0 V
MS m/z (ESI): [M+Hr. 344.2
HN2r- 24NH OMe
(S)-5-cyclopropy1-5-(3 -(5-
methoxyisoindolin-2-y1)-3-
oxopropyflimidazolidine-2,4-
dione
25 0 V
OMe 1H NMR (400 MHz, CD30D): 6 7.29-7.25
HNrNH 25 irk (m, 1H), 7.0-6.89 (m, 2H), 3.62-3.58 (m,
2H), 3.67 (s, 3H), 3.16-3.14 (m, 1H), 3.10-
(S)-5-cyclopropy1-5-(3 -(6-
3.08 (m, 1H), 2.98-2.96 (m, 1H), 2.90-2.88
metho xy- 1,2 ,4,5 -tetrahydro-3 H -
(m, 1H), 2.38-2.35 (m, 1H), 2.28-2.25 (m,
benzo[d]azepin-3-y1)-3-
191
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
oxopropyl)imidazolidine-2,4- 1H), 1.95-1.91 (m, 2H), 1.10-1.05 (m, 2H),
dione 0.45-0.42 (m, 1H), 0.33-0.18 (m, 4H).
LC-MS: MS m/z (ESI): [M+Hr. 372.2
26 0., V lic) 1H NMR (400 MHz, CD30D) 6 7.40 (t, 1H),
7.21 (dt, 1H), 7.15 ¨ 7.02 (m, 2H), 3.81 -
H
\_- 26
0 3.70 (m, 3H), 3.15 (dt, 2H), 2.60 ¨ 2.49
(m,
(S)-5-cyclopropy1-5-(3-(1,1- 1H), 2.49 ¨ 2.35 (m, 1H), 2.24 ¨ 2.03 (m,
dimethy1-1,2,4,5-tetrahydro-3H- 3H), 1.44 ¨ 1.27 (m, 6H), 1.27 ¨ 1.12 (m,
benzo[d1azepin-3-y1)-3- 1H), 0.65 ¨ 0.57 (m, 1H), 0.49 ¨ 0.21 (m,
oxopropyl)imidazolidine-2,4- 3H).
dione LC-MS: MS m/z (ESI): [M+Hr. 370.2
27&28 o V 3 1H NMR (500 MHz, DMSO-d6) 6 10.61 (d,
HN NH F 1H), 7.72 (d, 1H), 7.16 (td, 1H), 6.98 ¨
6.92
r 27
(m, 2H), 3.68 ¨ 3.49 (m, 2H), 3.23 ¨2.74
(S)- 5 -cyclopropy1-5-(3-((R)-8- (m, 4H), 2.41-2.36 (m, 1H), 2.27-2.20 (m,
fluoro-1-methyl-1,2,4,5- 1H), 1.95-1.90 (m, 2H), 1.21 (dd, 4H),
1.09
tetrahydro-3H-benzo[d]azepin-3- _ 1.01 (m, 1H), 0.94 ¨0.80 (m, 1H), 0.47 ¨
y1)-3-oxopropyl)imidazolidine- 0.28 (m, 2H), 0.09 (dd, 1H).
2,4-dione
Enantiomer 1 with shorter retention
o _IV time: HPLC: 99.560% @ 214 nm
N
yNH F
Chiral HPLC: Rt: 13.049 min, ee: 92.3%.
0 28
LC-MS: MS m/z (ESI): [M+Hr. 374.2
(S)-5 -cyclopropy1-5-(3-((S)-8-
Enantiomer 2 with longer retention time
fluoro-l-methyl-1,2,4,5-
HPLC: 100.000% @ 214 nm
tetrahydro-3H-benzo[d]azepin-3-
Chiral HPLC: Rt: 13.070 min, cc: 89.4%.
y1)-3-oxopropyl)imidazolidine-
LC-MS: MS m/z (ESI): [M+Hr. 374.2
2,4-dione
39 o y 0 1H NMR (400 MHz, CD30D) 6 7.78 ¨ 7.64
HNLN
OM 1H), 7.36 (t, 1H), 4.91 (s, 2H), 4.80 (d,
rNH CF3
39 2H), 2.57 (m, 1H), 2.45 (m, 1H), 2.24 (m,
(S)-5 -cyclopropy1-5-(3-(5-fluoro- 2H), 1.26 (m, 1H), 0.68 ¨0.52 (m, 1H),
6-(trifluoromethyl)isoindolin-2- 0.54 ¨ 0.28 (m, 3H).
19F NMR (376.5 MHz, CD30D) 6 -77.44, -
62.82
192
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
y1)-3-oxopropyflimidazolidine- LCMS: MS m/z (ESI): 400.0 1M+Hl+
2,4-dione
46 o 1H NMR (400 MHz, CD30D): 6 7.67 (s,
NH N 4. CI 1H), 7.51 (s, 1H), 2.49-2.42 (m, 1H), 2.36-
D D
46 CF3
2.30 (m, 1H), 2.18-2.06 (m, 2H), 1.30-1.10
(S)-5-(3-(5-chloro-6- (m, 1H), 0.52-0.47 (m, 1H), 0.39-0.24 (m,
(trifluoromethyl)isoindolin-2-yl- 3H).
1,1,3,3-d4)-3-oxopropy1)-5-
LC-MS: MS m/z (ESI): 1M+Hr. 420.2
cyclopropylimidazolidine-2,4-
dione
47 o V._ 1 1H NMR (400 MHz, CD30D): 6 7.36 (s,
Hkr-13) N CI 1H), 6.89 (s, 1H), 4.83 (s, 2H), 2.41-2.33 (m,
)r-NH
0 47 CI
4H), 2.29-2.21 (m, 1H), 2.09-1.99 (m, 2H),
(S)-5-cyclopropy1-5-(3-(5',6'-
1.13-1.08 (m, 1H), 0.81-0.78 (m, 2H), 0.50-
dich1orospirolcyc1opropane-1,1'-
0.44 (m, 1H), 0.37-0.31 (m, 3H).
isoindolin1-2'-y1)-3-
LC-MS: MS m/z (ESI): 1M+Hl+ 408.1.
oxopropyl)imidazolidine-2,4-
dione
The following compounds can be prepared using the similar methods as Examples
1-86.
Structure
O
HN)r-NH CF3
0
Dv
HNNH
OMe CF3
t
0
HNN
CF3
2r-NH
0 V. o F
N
rNH
193
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
IL F
HN
)r..NH
=
0
0 V 0
HN.L
rNH = CHF2
0
CI
N-N
0
HNrNH CF3
0
CI
BIOLOGICAL ASSAYS
The present disclosure will be further described with reference to the
following test
examples, but the examples should not be considered as limiting the scope of
the disclosure.
Test Example 1. In vitro fluorescence assay of ADAMTS-4 or ADAMTS-5 activity
A FRET (fluorescence resonance energy transfer) peptide was cleaved by
recombinant
ADAMTS-4 or ADAMTS-5 proteins into two separate fragments resulting in an
increase of
fluorescence signal which was quantified. The peptide was 5-FAM-
TEGEARGSVILLK(5-
TAMRA)K-NH2, customized from ANASPEC. ADAMTS-4 recombinant protein (catalog #
4307-AD) and ADAMTS-5 recombinant protein (catalog # 2198-AD) were purchased
from
R&D Systems.
An assay buffer containing 50 mM HEPES pH 7.5, 100 mM NaCl, 5 mM CaCl2, 0.1%
CHAPS and 5 % Glycerol was prepared. A volume of 2.5 jai of compound in the
assay buffer
was dispensed to a 384-well plate, and 2.5 jai of ADAMTS-4 or ADAMTS-5 protein
(final
concentration in the reaction was 10 nM) was added. The compounds and proteins
were pre-
incubated at room temperature for 15 minutes. Then, 5 jai of substrate was
added to each well.
The final substrate concentrations for ADAMTS-4 and ADAMTS-5 were 15 iaM and 8
iaM,
respectively. The fluorescence signal in each well was determined, after
incubation at 37 C for
3 hours, on a TECAN plate reader (Excitation, 490 nm; Emission, 520 nm).
Data Analysis:
The data was inputted into GraphPad Prism, and the IC5() values were
calculated using
function "log (inhibitor) vs. response -- Variable slope (four parameters)".
(See Table 1)
194
CA 03169225 2022-07-26
WO 2021/158626 PCT/US2021/016364
Table 1. The IC50 values of the exemplified compounds in FRET-peptide
enzymatic
assays.
Example No. ADAMTS-4 (IC50, ADAMTS-5
nM) (IC50, nM)
1 60 88
2 91 190
4 170 480
5 46 73
6 56 240
8 66 53
9 42 37
10 41 260
11 110 410
12 2300 1500
13 1400 4000
14 290 1000
16 1700 6200
17 310 370
19 260 330
20 22 37
21 20 16
22 51 61
24 120 360
25 5800 3600
26 3800 5400
27 170 270
28 47 48
29 14 22
compound with shorter retention time 8 9
in 29-1 and 29-2
compound with longer retention time 12 9
in 30-1 and 30-2
195
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
compound with shorter retention time 12 7.1
in 32 and 33
34-2 7 9
compound with longer retention time 3 7
in 34-2-A and 34-2-B
35 230 110
36-1 21 15
37 5 13
compound with longer retention time 52 98
in 37-1 and 37-2
compound with shorter retention time 8 12
in 37-1 and 37-2
38 62 31
compound with longer retention time 330 110
in 38-1 and 38-2
compound with shorter retention time 120 42
in 38-1 and 38-2
39 110 62
40 42 25
41 36 21
compound with shorter retention time 50 27
in 41-1 and 41-2
42 11 14
43 150 250
44 15 37
45 19 12
49-2 15 20
compound with shorter retention time 10 11
in 49-2a and 49-2b
50 120 220
52 30 66
53 240 170
54 11 110
196
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
57 43 130
59 82 46
60 170 220
61 40 42
62 100 89
compound with longer retention time 44 38
in 62-1 and 62-2
compound with longer retention time 280 280
in 64-1 and 64-2
65 16 21
66 43 52
66-2 12 10
compound with a retention time of 21 37
4.729 mm in 67-1, 67-2, 68-1 and 68-
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
compound with a retention time of 170 250
5.284 mm in 67-1, 67-2, 68-1 and 68-
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
compound with a retention time of 93 104
6.442 mm in 67-1, 67-2, 68-1 and 68-
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
compound with a retention time of 7 5
5.970 mm in 67-1, 67-2, 68-1 and 68-
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
69 68 40
compound with shorter retention time 24 18
in 69-1 and 69-2
70 20 26
71 54 26
197
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
compound with shorter retention time 18 31
in 71-1 and 71-2
compound with longer retention time 4300 4900
in
71-1 and 71-2
72 43 130
73 61 27
compound with shorter retention time 54 25
in 73-1 and 73-2
74 56 100
75 120 75
77 400 160
78 160 57
79 250 110
80 28 32
81 260 77
82 80 25
compound with longer retention time 61 34
in 83-1 and 83-2
compound with longer retention time 74 32
in 84-1 and 84-2
compound with longer retention time 100 39
in 85-1 and 85-2
compound with shortest retention time 16 15
in 86-1, 86-2, 86-3 and 86-4
Conclusion: The compounds of the present disclosure have a significant
inhibition
effect on the enzymatic activity of ADAMTS-4 and ADAMTS-5.
Test Example 2. In vitro ELISA (enzyme-linked immunosorbent assay) of ADAMTS-5
activity
In this assay, the enzymatic activity of recombinant ADAMTS-5 protein (catalog
#
2198-AD, R&D Systems) was assayed with a protein substrate, the aggrecan IGD
protein. The
aggrecan IGD protein is a polypeptide connecting human aggrecan globular
domains 1 and 2
198
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
(T331 ¨ G458) expressed in E. Coli with a C-terminal His-tag (catalog#
30411000, BIOTEZ).
The enzymatic product ARGSVIL-peptide was detected using an ELISA kit from
BioTEZ
(catalog# 30510111).
An assay buffer containing 50 mM HEPES pH 7.5, 100 mM NaCl, 5 mM CaCl2, 0.1%
CHAPS and 5 % Glycerol was prepared. Recombinant ADAMTS-5 protein was diluted
to 0.3
nM in the assay buffer. Ten jai of buffer and 10 jai of compound solution was
transferred to
each well of a 96-well plate and incubated at room temperature for 15 minutes.
Substrate
aggrecan-IGD was diluted to 100 nM with the assay buffer and 20 jai was added
to each well.
The plate was incubated at 37 C for 45 minutes. After incubation, the newly
generated epitope
ARGSVIL-peptides were measured using the Aggrecanase Activity ELISA Assay Kit
following the manufacturer's instructions. Then, 100 jai of stop solution was
added and the
absorbance of each well was read at 450 nM, using 620 nM as reference on a
TECAN plate
reader.
Data Analysis:
A standard curve of the ELISA assay was generated in GraphPad Prism using
Sigmoidal 4PL function and the corresponding peptide concentrations were
calculataed based
on the standard curve. The IC50 values were calculated using function "log
(inhibitor) vs.
response -- Variable slope (four parameters)". (See Table 2).
Table 2. The IC50 values of the exemplified compounds from the Aggrecan-IGD
enzymatic assay.
Example No. ADAMTS-5 (IC50, nM)
1 140
2 860
4 730
5 240
6 650
8 130
9 56
11 560
22 140
24 840
29 56
199
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
compound with shorter retention time
19
in 29-1 and 29-2
31-2 33
compound with shorter retention time
18
in 32 and 33
34-2 17
compound with longer retention time
18
in 34-2-A and 34-2-B
35 170
36-1 36
37 16
38 29
compound with longer retention time
220
in 38-1 and 38-2
compound with shorter retention time
52
in 38-1 and 38-2
39 65
40 92
41 18
compound with shorter retention time
17
in 41-1 and 41-2
42 17
44 51
45 25
49-2 55
compound with shorter retention time
39
in 49-2a and 49-2b
52 48
54 200
57 190
59 46
61 72
62 290
200
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
compound with longer retention time
in 62-1 and 62-2
65 31
66 83
66-2 19
compound with a retention time of
4.729 mm in 67-1, 67-2, 68-1 and 68-
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
compound with a retention time of
6.442 mm in 67-1, 67-2, 68-1 and 68-
290
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
compound with a retention time of
5.970 mm in 67-1, 67-2, 68-1 and 68-
2 (Me0H/DEA 5%_40% 1.5ml/min
IA, 3um 3.0*100(Daicel)
69 47
compound with shorter retention time
in 69-1 and 69-2
70 83
71 29
compound with shorter retention time
13
in 71-1 and 71-2
73 47
compound with shorter retention time
26
in 73-1 and 73-2
75 110
78 76
79 210
81 120
compound with longer retention time
in 84-1 and 84-2
201
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
compound with longer retention time
in 85-1 and 85-2
Conclusion: The compounds of the present disclosure have a significant
inhibition
effect on the enzymatic activity of ADAMTS-5.
5
Test Example 3. Mouse Explant Assay
In this assay, fresh mouse femoral head cartilage was treated with IL-la
protein (Sigma-
Aldrich, catalog# 12778) in culture media, which induces the cartilage
catabolism. Then, the
GAGs attached to the cleaved aggrecan fragments (released in the media) and
the GAGs
10 attached to the intact aggrecan were measured by dimethylmethylene blue dye
in the
Glycosaminoglycans Assay Kit (Chondrex, catalog# 6022).
Femoral head cartilage samples were isolated from mice (25 days old, male,
C57BL/6,
from Charles River Lab) and put into 2.0 ml tubes filled-up with media (DMEM,
10% FBS, 4
mM Glutamine, penicillin-streptomycin, 20 mM HEPES). Two hundred pl of media
without
15 FBS was added to each well of a 48-well plate, and one piece of
cartilage was transferred to a
well in the plate. Then the media was aspirated, and compounds and IL-la
protein were added
to the plate in a total volume of 400 pl of fresh media without FBS. The final
concentration of
IL-la was 1 ng/ml. The plate was incubated at 37 C for 72 hours in a
humidified incubator
with 5% CO2 supply.
20 The
supernatant was transferred to a 1.5 ml tube and kept at -20 C. Each cartilage
sample was tranferred to another 1.5 ml tube containing 400 plof freshly made
papain solution.
The papain solution contained 125 pg/ml papain (Sigma-Aldrich, catalog#
P3125), 0.1 M
sodium acetate (Sigma-Aldrich, catalog# S7899), pH 5.5 and 5 mM EDTA and 5 mM
L-
cysteine-HC1 (Sigma-Aldrich, catalog# C7880). The cartilage samples were kept
rocking in a
25 60 C water bath for 24 hours.
The lysates were vortexed for 10 seconds and spinned at 10,000 rpm for 2
minutes.
Both the supernatant and the lysate samples were diluted with PBS and mixed
with 100 pl of
dye from the Glycosaminoglycans Assay Kit. The optical density from each well
was
determined with a TECAN plate reader set to a wavelength of 525 nm.
30 Data Analysis:
The concentrations of GAGs in the supernatant and lysates were determined
based on
the standard curve with a dose range of chondroitin sulfate provided in the
kit. The percentage
202
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
[GAG]supernatant
of GAG release was calculated as the following: GAG% =(
[GAG]supernatant + [GAG]lysate) x
100%.
The test compound effect was expressed as the percent of inhibition using the
following
formula:
Inhibition ____________________ % =(1h GAG%(Compound+ILla)¨ GAG%(Vehicle))
x 100%
GAG%(Vehicle+IL1c)¨ GAG%(Vehicle)
The inhibition data of selected exemplified compounds at 2 iaM and 20 iaM
concentrations were listed in Table 3.
Table 3. The IC50 values of the exemplified compounds from the mouse explant
assay.
Example No. Inhibition % at 2 It M Inhibition % at 20
P. M
1 27 61
5 19 51
8 22 63
9 22 55
20 67 100
21 39 100
compound with shorter retention
46 99
time in 29-1 and 29-2
compound with shorter retention
65 97
time in 32 and 33
compound with longer retention
71 100
time in 34-2-A and 34-2-B
40 2 52
42 51 92
compound with shorter retention
50 74
time in 41-1 and 41-2
44 13 70
45 45 96
52 10 48
59 16 66
compound with longer retention
27 60
time in 62-1 and 62-2
66-2 23 87
203
CA 03169225 2022-07-26
WO 2021/158626
PCT/US2021/016364
compound with shorter retention
31 98
time in 69-1 and 69-2
compound with shorter retention
48 89
time in 71-1 and 71-2
compound with shorter retention
31 70
time in 73-1 and 73-2
80 30 73
The foregoing embodiments and examples are provided for illustration only and
are not
intended to limit the scope of the disclosure. Various changes and
modifications to the
disclosed embodiments will be apparent to those skilled in the art based on
the present
disclosure, and such changes and modifications may be made without departure
from the spirit
and scope of the present disclosure. All literature cited are incorporated
herein by reference in
their entireties without admission of them as prior art.
204