Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/008899
PCT/GB2021/051717
1
AEROSOL PROVISION SYSTEM
TECHNICAL FIELD
The present invention relates to an aerosol provision system.
BACKGROUND
Electronic aerosol provision systems such as electronic cigarettes (e-
cigarettes)
generally contain an aerosol-generating material, such as a reservoir of a
source liquid
containing a formulation, typically including nicotine, or a solid material
such as a tobacco-
based product, from which an aerosol is generated for inhalation by a user,
for example
through heat vaporisation. Thus, an aerosol provision system will typically
comprise an
aerosol generator, e.g. a heating element, arranged to aerosolise a portion of
aerosol-
generating material to generate an aerosol in an aerosol generation region of
an air channel
through the aerosol provision system. As a user inhales on the device and
electrical power
is supplied to the aerosol generator, air is drawn into the device through one
or more inlet
holes and along the air channel to the aerosol generation region, where the
air mixes with
the vaporised aerosol generator and forms a condensation aerosol. The air
drawn through
the aerosol generation region continues along the air channel to a mouthpiece,
carrying
some of the aerosol with it, and out through the mouthpiece for inhalation by
the user.
It is common for aerosol provision systems to comprise a modular assembly,
often
having two main functional parts, namely an aerosol provision device and
disposable /
replaceable consumable part Typically the consumable will comprise the
consumable
aerosol-generating material and the aerosol generator (heating element), while
the aerosol
provision device part will comprise longer-life items, such as a rechargeable
battery, device
control circuitry and user interface features. The aerosol provision device
may also be
referred to as a reusable part or battery section and the consumable may also
be referred to
as a disposable part, cartridge or cartomiser.
The aerosol provision device and consumable are mechanically coupled together
at
an interface for use, for example using a screw thread, bayonet, latched or
friction fit fixing.
VVhen the aerosol-generating material in a consumable has been exhausted, or
the user
wishes to switch to a different consumable having a different aerosol-
generating material,
the consumable may be removed from the aerosol provision device and a
replacement
consumable may be attached to the device in its place.
A potential drawback for aerosol provision systems is that there is no means
to
monitor the usage of the aerosol provision system by the user. This may lead
to excessive
use of the system by the user. Equally, the factory settings of the system may
not resemble
the desired operation of the system by the user, thereby reducing the user
satisfaction.
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
2
Various approaches are described herein which seek to help address or mitigate
some of the issues discussed above.
SUMMARY
The disclosure is defined in the appended claims.
In accordance with some embodiments described herein, there is provided an
aerosol provision system comprising control circuitry for determining an
operational
parameter of the aerosol provision system, an aerosol generator configured to
aerosolize an
aerosol-generating material and a sensor configured to detect an inhalation on
the aerosol
provision system by a user of the aerosol provision system, and output
corresponding
inhalation detection signals to the control circuitry. The control circuitry
is configured to
determine a duration of the inhalation based on the inhalation detection
signals received
from the sensor, and determine an indication of an amount of an ingredient
delivered from
the aerosol-generating material to the user during the inhalation based on the
duration of the
inhalation and an indication of the operational parameter during the
inhalation.
The control circuitry may be configured to determine a duration of a session
based
on the duration a plurality of inhalations, where a time between each of the
plurality of
inhalations is less than a predetermined time. The control circuitry can then
be configured to
determine an indication of an amount of the ingredient delivered from the
aerosol-generating
material to the user during the session based on the duration of the session
and an
indication of the operational parameter during the session.
The control circuitry may be configured to determine an indication of an
amount of
the ingredient delivered from the aerosol-generating material to the user
during a rolling
predetermined period based on the duration of each inhalation during the
rolling
predetermined period and an indication of the operational parameter during
each inhalation
during the rolling predetermined period. The control circuitry can then be
configured to
determine a time between each inhalation based on the inhalation detection
signals, and
wherein the determination of the indication of the amount of the ingredient
delivered from the
aerosol-generating material to the user during the rolling predetermined
period is also based
on the time between each inhalation during the rolling predetermined period.
The determination of the indication of the amount of the ingredient delivered
from the
aerosol-generating material to the user may further be based on a
concentration of the
ingredient in the aerosol-generating material. In some embodiments the
ingredient is
nicotine, caffeine, taurine, theine, a vitamin, melatonin, or a cannabinoid.
In some embodiments the aerosol provision system also comprises a power source
configured to supply electrical power to the aerosol generator, and the
operational parameter
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
3
of the system is an amount of electrical power supplied to the aerosol
generator by the
power source.
The control circuitry may be configured to determine default user behaviour
based on
the indication of the amount of the ingredient delivered from the aerosol-
generating material
to the user for a plurality of inhalations. For example, the control circuitry
may be configured
to determine a time between each of the plurality of inhalations based on the
inhalation
detection signals, and wherein determining default user behaviour is also
based on the time
between each of the plurality of inhalations.
The control circuitry may be configured to alter a mode of operation of the
aerosol
provision system based on the default user behaviour.
The control circuitry may be configured to provide a notification to the user
based on
the indication of the amount of the ingredient delivered from the aerosol-
generating material
to the user during the inhalation and the default user behaviour.
The control circuitry may be configured to provide a notification to the user
when the
indication of an amount of an ingredient delivered from the aerosol-generating
material to the
user during the inhalation exceeds a puff threshold.
The control circuitry may be configured to provide a notification to the user
when the
indication of the amount of an ingredient delivered from the aerosol-
generating material to
the user during the session exceeds a session threshold.
The control circuitry may be configured to provide a notification to the user
when the
indication of the amount of an ingredient delivered from the aerosol-
generating material to
the user during the rolling predetermined period exceeds a period threshold.
The notification may be provided on the aerosol provision system and/or an
application on a remote device. The notification may be a haptic notification.
A parameter of
the haptic notification may be adjustable by the user.
In accordance with some embodiments described herein, there is provided a
system
comprising an aerosol provision system configured to generate aerosol from an
aerosol-
generating material and a computer. The computer is configured to receive
inhalation
detection signals from a sensor configured to detect the inhalation on the
aerosol provision
system by a user of the aerosol provision system, determine a duration of the
inhalation
based on the inhalation detection signals received from the sensor, and
determine an
indication of an amount of an ingredient delivered from the aerosol-generating
material to the
user during the inhalation based on the duration of the inhalation and an
indication of an
operational parameter of the aerosol provision system during the inhalation.
In accordance with some embodiments described herein, there is provided a
method
of determining an amount of an ingredient delivered to a user of an aerosol
provision
system. The method comprises receiving inhalation detection signals from a
sensor
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
4
configured to detect the inhalation on the aerosol provision system by a user
of the aerosol
provision system, determining a duration of the inhalation based on the
inhalation detection
signals received from the sensor, and determining an indication of an amount
of an
ingredient delivered from an aerosol-generating material to the user during
the inhalation
based on the duration of the inhalation and an indication of an operational
parameter of the
aerosol provision system during the inhalation, wherein an aerosol generator
is configured to
aerosolize the aerosol-generating material. There is also provided a computer
readable
storage medium comprising instructions which, when executed by a processor,
performs the
above method.
These aspects and other aspects will be apparent from the following detailed
description. In this regard, particular sections of the description are not to
be read in
isolation from other sections.
BRIEF DESCRIPTION OF DRAWINGS
Embodiments of the invention will now be described, by way of example only,
with
reference to accompanying drawings, in which:
Figures 1 and 2 are schematic diagrams of an aerosol provision system;
Figures 3A to 3C illustrate graphs of inhalation detection signal output by
the sensor
against time;
Figure 4 illustrates a system comprising an aerosol provision system and a
computer;
Figure 5 is a flow chart of a method of determining an amount of an ingredient
delivered to a user of an aerosol provision system.
DETAILED DESCRIPTION
Aspects and features of certain examples and embodiments are discussed /
described herein. Some aspects and features of certain examples and
embodiments may
be implemented conventionally and these are not discussed / described in
detail in the
interests of brevity. It will thus be appreciated that aspects and features of
articles and
systems discussed herein which are not described in detail may be implemented
in
accordance with any conventional techniques for implementing such aspects and
features.
The present disclosure relates to aerosol provision systems, which may also be
referred to as aerosol provision systems, such as e-cigarettes. Throughout the
following
description the term "e-cigarette" or "electronic cigarette" may sometimes be
used, but it will
be appreciated this term may be used interchangeably with aerosol provision
system and
electronic aerosol provision system.
As noted above, aerosol provision systems (e-cigarettes) often comprise a
modular
assembly including both a reusable part (aerosol provision device) and a
replaceable
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
(disposable) cartridge part, referred to as a consumable. Systems conforming
to this type of
two-part modular configuration may generally be referred to as two-part
systems or devices.
It is also common for electronic cigarettes to have a generally elongate
shape. For the sake
of providing a concrete example, certain embodiments of the disclosure
described herein
5 comprise this kind of generally elongate two-part system employing
disposable cartridges.
However, it will be appreciated the underlying principles described herein may
equally be
adopted for other electronic cigarette configurations, for example modular
systems
comprising more than two parts, as devices conforming to other overall shapes,
for example
based on so-called box-mod high performance devices that typically have a more
boxy
shape.
As described above, the present disclosure relates to (but it not limited to)
aerosol
provision devices and corresponding aerosol provision systems, such as e-
cigarettes and
electronic cigarettes.
Figure 1 is a highly schematic diagram (not to scale) of an example aerosol
provision
system 10, such as an e-cigarette, to which embodiments are applicable. The
aerosol
provision system has a generally cylindrical shape, extending along a
longitudinal or y axis
as indicated by the axes (although aspects of the invention are applicable to
e-cigarettes
configured in other shapes and arrangements), and comprises two main
components,
namely an aerosol provision device 20 and a consumable 30.
The consumable 30 is an article comprising or consisting of aerosol-generating
material 38, part or all of which is intended to be consumed during use by a
user. A
consumable 30 may comprise one or more other components, such as an aerosol-
generating material storage area, an aerosol-generating material transfer
component 37, an
aerosol generation area, a housing, a wrapper, a mouthpiece 35, a filter
and/or an aerosol-
modifying agent.
A consumable 30 may also comprise an aerosol generator 36, such as a heating
element, that emits heat to cause the aerosol-generating material 38 to
generate aerosol in
use. The aerosol generator 36 may, for example, comprise combustible material,
a material
heatable by electrical conduction, or a susceptor. It should be noted that it
is possible for the
aerosol generator 36 to be part of the aerosol provision device 20 and the
consumable 30
then may comprise the aerosol-generating material storage area for the aerosol-
generating
material 38 such that, when the consumable 30 is coupled with the aerosol
provision device
20, the aerosol-generating material 38 can be transferred to the aerosol
generator 36.
The aerosol-generating material 38 is a material that is capable of generating
aerosol, for example when heated, irradiated or energized in any other way.
The aerosol-
generating material 38 may, for example, be in the form of a solid, liquid or
gel which may or
may not contain an active substance and/or flavourants. In some embodiments,
the aerosol-
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
6
generating material 38 may comprise an "amorphous solid", which may
alternatively be
referred to as a "monolithic solid" (i.e. non-fibrous). In some embodiments,
the amorphous
solid may be a dried gel. The amorphous solid is a solid material that may
retain some fluid,
such as liquid, within it. In some embodiments, the aerosol-generating
material may for
example comprise from about 50wt%, 60wV/0 or 70wV/0 of amorphous solid, to
about 90wt%,
95wt% or 100wt /0 of amorphous solid.
The aerosol-generating material 38 comprises one or more ingredients, such as
one
or more active substances and/or flavourants, one or more aerosol-former
materials, and
optionally one or more other functional materials such as pH regulators,
colouring agents,
preservatives, binders, fillers, stabilizers, and/or antioxidants.
The active substance as used herein may be a physiologically active material,
which
is a material intended to achieve or enhance a physiological response. The
active
substance may for example be selected from nutraceuticals, nootropics,
psychoactives. The
active substance may be naturally occurring or synthetically obtained. The
active substance
may comprise for example nicotine, caffeine, taurine, theine, vitamins such as
B6 or B12 or
C, melatonin, cannabinoids, or constituents, derivatives, or combinations
thereof. The active
substance may comprise one or more constituents, derivatives or extracts of
tobacco,
cannabis or another botanical.
In some embodiments, the active substance comprises nicotine.
In some
embodiments, the active substance comprises caffeine, melatonin or vitamin
B12.
The aerosol provision device 20 includes a power source 14, such as a battery,
configured to supply electrical power to the aerosol generator 36. The power
source 14 in
this example is rechargeable and may be of a conventional type, for example of
the kind
normally used in electronic cigarettes and other applications requiring
provision of relatively
high currents over relatively short periods. The battery 14 may be recharged
through the
charging port (not illustrated), which may, for example, comprise a USB
connector.
The aerosol provision device 20 includes control circuitry 28 configured to
determine
one or more operational parameters of the aerosol provision system 10. The
control circuitry
also controls the operation of the aerosol provision system 10 based on the
determining and
provides conventional operating functions in line with the established
techniques for
controlling aerosol provision systems such as electronic cigarettes. The
control circuitry
(processor circuitry) 28 may be considered to logically comprise various sub-
units / circuitry
elements associated with different aspects of the electronic cigarette's
operation. For
example, depending on the functionality provided in different implementations,
the control
circuitry 28 may comprises power source control circuitry for controlling the
supply of
electrical power from the power source 14 to the aerosol generator 36, user
programming
circuitry for establishing configuration settings (e.g. user-defined power
settings) in response
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
7
to user input, as well as other functional units / circuitry associated
functionality in
accordance with the principles described herein and conventional operating
aspects of
electronic cigarettes. It will be appreciated the functionality of the control
circuitry 28 can be
provided in various different ways, for example using one or more suitably
programmed
programmable computer(s) and / or one or more suitably configured application-
specific
integrated circuit(s) / circuitry / chip(s) / chipset(s) configured to provide
the desired
functionality.
The aerosol provision device 20 illustrated in Figure 1 includes one or more
air inlets
21. In use, as a user inhales on the mouthpiece 35, air is drawn into the
aerosol provision
device 20 through the air inlets 21 and along an air channel 23 to the aerosol
generator 36,
where the air mixes with the vaporised aerosol-generating material 38 and
forms a
condensation aerosol. The air drawn through the aerosol generator 36 continues
along the
air channel 23 to a mouthpiece 35, carrying some of the aerosol with it, and
out through the
mouthpiece 35 for inhalation by the user. It will be appreciated that the one
or more air inlets
may be provided on the consumable 30 such that the air channel 23 is entirely
contained
within the consumable 30, or the aerosol provision device 20 and the
consumable 30 may
each comprise at least one air inlet 21 and a portion of the air channel 23.
By way of a concrete example, the consumable 30 comprises a housing (formed,
e.g., from a plastics material), a reservoir formed within the housing for
containing the
aerosol-generating material 38 (which in this example may be a liquid which
may or may not
contain nicotine), an aerosol-generating material transfer component 37 (which
in this
example is a wick formed of e.g., glass or cotton fibres, or a ceramic
material configured to
transport the liquid from the reservoir using capillary action), an aerosol
generating area, and
a mouthpiece 35. Although not shown, a filter and/or aerosol modifying agent
(such as a
flavour imparting material) may be located in, or in proximity to, the
mouthpiece 35. The
consumable of this example comprises a heater element formed from an
electrically resistive
material (such as NiCr8020) spirally wrapped around the aerosol-generating
material
transfer component 37, and located in the air channel 23. The area around the
heating
element and wick combination is the aerosol generating area of the consumable
30. The
consumable comprises suitable electrical contacts for coupling to electrical
contacts
provided on the aerosol provision device 20, such that electrical power may be
supplied
directly to the heater element.
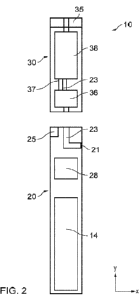
Figure 2 is a schematic diagram of a further example of an aerosol provision
system
10, where the same reference signs have been used for like elements between
the aerosol
provision system 10 illustrated in Figure 1 and the aerosol provision system
10 illustrated in
Figure 2.
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
8
The aerosol provision system 10 in Figure 2 comprises a sensor 25 configured
to
detect an inhalation on the aerosol provision system 10 by a user of the
aerosol provision
system 10. For example, the sensor 25 may be a flow sensor, a microphone, a
pressure
sensor, light sensor, touch sensor, accelerometer, gyroscope, or any other
type of sensor
suitable for directly or indirectly detecting or inferring an inhalation on
the aerosol provision
system 10 by a user of the aerosol provision system 10. Although the sensor 25
illustrated
in Figure 2 is part of the aerosol provision device 20, this is not essential.
In other
embodiments the sensor 25 may be part of the consumable 30.
The sensor 25 may be configured to detect an inhalation based on the flow of
air into
one or more of the air inlets 21, or in the air channel 23 through the aerosol
provision system
10. Alternative, the sensor may include a pressure sensor or light sensor on
the mouthpiece
35 configured to detect when the user's lips are placed around the mouthpiece
35, or a
pressure sensor or light sensor located on the aerosol provision device 20 to
detect when
the user places their hand around the aerosol provision device 20.
In some embodiments there is more than one sensor 25. For example, there may
be
a sensor 25 located proximate to an air inlet and a sensor 25 proximate to a
portion of the air
channel 23, the aerosol generator 36 and/or the mouthpiece 35 as described
above.
Accordingly, each sensor is configured to detect an inhalation on the aerosol
provision
system 10. Where there is more than one sensor 25, this can comprise more than
one type
of sensor, and/or multiple sensors of the same type.
In response to detecting an inhalation on the aerosol provision system 10 by a
user
of the system, the sensor 25 is configured to output corresponding inhalation
detection
signals to the control circuitry 28. In some embodiments, the sensor 25 is
configured to
continuously output inhalation detection signals, or to output inhalation
detection signals
periodically, such as every 0.01 seconds, every 0.1 seconds, or every 1
second. If the
sensor 25 outputs inhalation detection signals periodically, then in some
implementations the
period between the output of subsequent inhalation detection signals may be
set equal to or
less than the average, or a typical length of an user inhalation (e.g. between
2 to 5 seconds)
so as to ensure that an inhalation is not missed. In each case, the inhalation
detection
signals change when an inhalation on the system is detected by the sensor 25.
For
example, the inhalation detection signals could be a binary indication of
whether an
inhalation on the system is detected or not, for example a "1" to indicate an
inhalation has
been detected and a "0" to indicate that an inhalation has not been detected.
Alternatively,
the inhalation detection signals could correspond to an inhalation level or
strength detected
by the sensor 25. In other words, the inhalation detection signals could
provide an indication
of the draw strength detected by the sensor 25. For example, if the sensor 25
is a
microphone or a flow sensor, the inhalation detection signals could provide an
indication of
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
9
the air speed or mass flow through the aerosol provision system 10, thereby
providing an
indication of the magnitude or strength of the inhalation taken by the user.
In some
embodiments, the inhalation detection signals correspond to the signals
detected by the
sensor 25. In other words, the inhalation detection signals represent the raw
output from the
sensor 25 without any filtering or processing applied by the sensor 25.
The inhalation detection signals could be set to 0 when an inhalation has not
been
detected by the sensor 25 and correspond to the inhalation level or strength
detected by the
sensor 25 when an inhalation has been detected. In some embodiments, the
sensor 25 is
configured to only output inhalation detection signals when an inhalation has
been detected.
In other words, the sensor 25 is configured output inhalation detection in
response to
detecting an inhalation on the system and the sensor 25 is configured to stop
outputting
inhalation detection signals when the inhalation is no longer detected by the
sensor 25.
Figures 3A to 3C illustrate graphs of inhalation detection signals output by
the sensor
25 against time. In the example illustrated in Figure 3A, the sensor 25
outputs inhalation
detection signals continuously, and an inhalation being detected by the sensor
25
corresponds to the period when the inhalation detection signals are greater
than a detection
threshold 301. In the example illustrated in Figure 3B, until time
point 302, which
corresponds to the time point at which the sensor 25 detects an inhalation by
the user on the
aerosol provision system 10, the inhalation detection signal 305A output by
the sensor 25 is
"0". This may either represent no inhalation detection signal being output, or
an inhalation
detection signal being output with a value equal to "0". Between time point
301 and 302,
which corresponds to the times during which the sensor 25 detects an
inhalation by the user
on the aerosol provision system 10, the inhalation detection signal 305B
output by the
sensor 25 is "1". In other words, the sensor 25 outputs an inhalation
detection signal to
indicate that an inhalation is detected. After time point 303, the inhalation
detection signal
305A output by the sensor 25 is "0", indicating that the sensor 25 no longer
detects an
inhalation. As set out above, this may either represent no inhalation
detection signal being
output, or an inhalation detection signal being output with a value equal to
"0". In the
example illustrated in Figure 30 the inhalation detection signals 305A are set
to "0" when an
inhalation is not detected by the sensor 25, and the inhalation detection
signal 3050
corresponds to the signal recorded by the sensor 25 when the sensor 25 detects
an
inhalation.
As described above, the sensor 25 is configured to output the inhalation
detection
signals to the control circuitry 28. In response to receiving the inhalation
detection signals,
the control circuitry 28 is configured to determine a duration of the
inhalation based on the
inhalation detection signals received from the sensor 25. In other words, the
control circuitry
28 is configured to determine the elapsed time for an inhalation based on the
inhalation
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
detection signals received from the sensor 25. As set out above, the sensor 25
may be
configured to continuously or periodically output inhalation detection signals
to the control
circuitry, and the control circuitry is configured to use the change in these
signals described
above to determine a duration of an inhalation, for example by starting an
inhalation timer
5 when the inhalation detection signals change a first time and stop the
inhalation timer when
the inhalation detection signals change a second time. The control circuitry
28 may be
configured to start the inhalation timer when the first non-zero inhalation
detection signal is
received, or when the first inhalation detection signal indicative of an
inhalation being
detected by the sensor 25 is received, such as at time 302 in Figures 3B and
30
10 respectively. The control circuitry 28 may then be configured to stop
the inhalation timer
when the next zero value inhalation detection signal is received, or when the
next inhalation
detection signal indicative of the sensor 25 no longer detecting an inhalation
is received,
such as at time 303 in Figures 3A-3C. Using Figures 3A-3C as an example, the
duration of
inhalation determined by the control circuitry 28 is the elapsed time between
the time points
302 and 303.
As set out above, the sensor 25 may be configured to only output inhalation
detection
signals when an inhalation has been detected. In this case, the control
circuitry 28 can be
configured to determine the duration of the inhalation by activating the
inhalation timer when
an inhalation detection signal is received, and stopping the inhalation timer
when inhalation
detection signals are no longer received.
Alternatively, the duration of an inhalation may be determined based on
information
contained within the inhalation detection signals, such as a time stamp
associated with each
inhalation detection signal. For example, in the case illustrated in Figure 3B
or 3C, the
control circuitry 28 is configured to use the timestamp of a first non-zero
inhalation detection
signal (at time 302) received and the timestamp of the next zero value
inhalation detection
signal received (at lime 303) to determine the duration of the inhalation.
Alternatively, in the
example illustrated in Figure 3A, the control circuitry 28 is configured to
use the timestamp of
the first inhalation detection signal exceeding the detection threshold 301
received from the
sensor 25, corresponding to time point 302 in the Figure 3A, and the timestamp
of the next
inhalation detection signal indicative not exceeding the detection threshold
received from the
sensor, corresponding to time point 303 in Figure 3A, to determine the
duration of the
inhalation.
In the example where the inhalation detection signals are output periodically
by the
sensor 25, the control circuitry 28 may be configured to determine the
duration of an
inhalation by counting the number of consecutive, non-zero inhalation
detection signals
received, or the number of consecutive inhalation detection signals receive
from the sensor
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
11
25 indicative of an inhalation being detected by the sensor 25. The period of
output of the
airflow detection signals can then be used to determine the duration of the
inhalation.
In the example where there are two or more sensors 25, each sensor 25 is
configured to output inhalation detection signals in accordance with the
principles described
above. The control circuitry 28 is then configured to determine the duration
of an inhalation
based on the inhalation detection signals received from one or more of the
sensors 25. For
example, the control circuitry 28 may be configured to determine the duration
of an
inhalation in response to receiving inhalation detection signals indicating an
inhalation has
been detected from any one of the sensors 25. Alternatively, the control
circuitry 28 may be
configured to determine the duration of an inhalation in response to receiving
inhalation
detection signals indicating an inhalation has been detected from more than a
given
percentage of the total number of sensors 25, such as 25%, 50%, 80% or 100%.
In some embodiments, the control circuitry 28 is configured to determine a
time
between inhalations based on the inhalation detection signals. In other words,
the control
circuitry 28 is configured to determine the elapsed time between an inhalation
and the next
inhalation. This can be achieved using the same techniques as described above
with
respect to determining the duration of an inhalation, such as using a timer,
information
contained within the inhalation detection signals or the period of output of
the inhalation
detection signals. For example, the control circuitry 28 may be configured to
start an interval
timer in response to receiving the first zero value inhalation detection
signal after a non-zero
inhalation detection signal. The control circuitry 28 is then configured to
stop the interval
timer in response to receiving the next non-zero value inhalation detection
signal.
In the embodiment described above where the sensor 25 is configured to stop
outputting inhalation detection signals when an inhalation has not been
detected, the control
circuitry 28 can be configured to determine a time between inhalations by
activating the
interval timer when the sensor 25 stops outputting the inhalation detection
signals. In other
words, the control circuitry 28 is configured to start the interval timer in
response to the
sensor 25 stopping the output of inhalation detection signals following an
inhalation having
been detected by the sensor 25. The control circuitry 28 can then be
configured to stop the
interval timer when the next inhalation detection signal is outputted by the
sensor 25, thereby
allowing the control circuitry 28 to determine the time between inhalations.
The duration of each inhalation can be used to determine the duration of
multiple
inhalations by the user during a given predetermined time period, such as a
minute, an hour
or a day. For example, the control circuitry 28 can be configured to determine
the duration
of each inhalation during a rolling 24 hour period, which can then be summed
to determine
the total duration of inhalations in the rolling 24 hour time period. As will
be appreciated, a
rolling 24 hour period is intended to mean the 24 hours immediately prior to
any point in
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
12
time, such that the rolling 24 hour period represents the most recent 24 hours
in time from a
given time point. Accordingly, the rolling predetermined period represents a
period of time
immediately prior to any point in time, and the period of time is
predetermined. As set out
above, the rolling predetermined period may be a rolling minute, a rolling
hour, a rolling day
(24 hours) or longer such as a rolling week or other period of time.
As will be appreciated, many users of aerosol provision systems 10 do not take
single inhalations on the aerosol provision system 10, but rather perform a
session on the
aerosol provision system 10, where a session is a plurality of inhalations
within a time period
such as 1 to 2 minutes, sometimes longer such as 5 or 10 minutes. The control
circuitry 28
can therefore be configured to determine the duration of a session based on
the duration of
a plurality of inhalations using the inhalation detection signals received
from the sensor 25,
where the time between each of the plurality of inhalations is less than a
predetermined time.
The predetermined time may be set and altered by the user or the control
circuitry 28, or
may be a fixed value, for example based on empirical data. The predetermined
time could
be less than 1 minute, 1, 2, 5, 10 minutes or longer. The predetermined time
can be defined
as a rolling time period as described above such that each inhalation within
the most recent
predetermined time period is considered by the control circuitry 28 to be part
of the session.
Alternatively, the predetermined time may set such that the time between each
inhalation
must be less than the predetermined time in order for the inhalation to be
considered by the
control circuitry 28 as part of the same session. In this case, a session
timer could be
implemented to determine the duration of the session, where the session timer
is started
when the inhalation detection signals indicate that an inhalation has been
detected by the
sensor, and the session timer is stopped when the duration between an
inhalation exceeds
the predetermined time. Alternatively, as described above, a time stamp
associated with
each inhalation detection signal could be used to determine the duration of
the session
based on the duration of each inhalation and the time between each inhalation.
The control circuitry 28 is configured to determine an indication of an amount
of an
ingredient delivered from the aerosol-generating material 38 to the user
during the inhalation
based on the duration of the inhalation and an indication of an operational
parameter during
the inhalation. As it will be appreciated, the amount of aerosol, and by
extension the amount
of aerosol-generating material 38, delivered to the user during an inhalation
will vary based
on the duration of the inhalation, such that the longer the inhalation, the
more aerosol-
generating material 38 that will be delivered to the user during the
inhalation.
As described above, the aerosol-generating material 38 comprises one or more
ingredients. Accordingly, the amount of each of the one or more ingredients
delivered from
the aerosol-generating material 38 to the user during the inhalation will also
vary based on
the duration of the inhalation, and therefore the control circuitry 28 is
configured to use the
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
13
duration of the inhalation in the determination of the amount of an ingredient
delivered from
the aerosol-generating material 38 to the user during the inhalation.
As described above, the control circuitry 28 determines an operational
parameter of
the aerosol provision system 10. The amount of aerosol-generating material 38
delivered to
the user during the inhalation will varying depending on operational
parameters (settings) of
the aerosol provision system 10. Accordingly, an indication of the operational
parameter
during the inhalation is used along with the duration of the inhalation to
determine the
indication of the amount of the ingredient delivered from the aerosol-
generating material 38
to the user during the inhalation. The indication of the operational parameter
may be the
actual value of the operational parameter itself, or a numerical number
corresponding to a
setting, such as "0" for "off' and "1" for "on", or "1" for low, "2" for
medium and "3" for high.
As such, the indication is any suitable means of conveying the nature or state
of a
component of the aerosol provision system 10 for use in determining an
indication of the
amount of the ingredient delivered from the aerosol-generating material 38 to
the user during
the inhalation.
The operational parameter may be an amount of electrical power supplied to the
aerosol generator 36 by the power source 14. The control circuitry 28 is then
configured to
determine the indication of the amount of the ingredient delivered from the
aerosol-
generating material 38 to the user during the inhalation based on an
indication of the amount
of electrical power supplied to the aerosol generator 36 by the power source
14 during the
inhalation. For example, the indication of the amount of electrical power
supplied could be
the amount of power delivered or a voltage and/or current supplied to the
aerosol generator
36 during the inhalation, or could be a power setting for the aerosol
generator 36 during the
inhalation, such as an integer between 1 and 10 or "1" for low, "2" for medium
and "3" for
high. The amount of aerosol generated by the aerosol generator 36 during an
inhalation will
vary depending on the amount of electrical power supplied to the aerosol
generator 36, and
therefore a more accurate determination of the indication of the amount of the
ingredient
delivered from the aerosol-generating material 38 to the user during the
inhalation can be
achieved by considered the amount of electrical power in the calculation.
Alternatively or in addition, control circuitry 28 may determine one or more
other
operational parameters of the aerosol provision system, such as an amount of
charge in the
power source 14, a temperature of the aerosol generator 36 or a temperature
proximate to
the aerosol generator 36, an amount and/or speed of airflow through the
aerosol provision
system 10, indications of which are then used to determine the indication of
the amount of
the ingredient delivered from the aerosol-generating material 38 to the user
during the
inhalation. The operational parameter may change or vary during an inhalation,
for example
a decrease in the amount of charge in the power source 14 or an increase in
the
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
14
temperature of the aerosol generator 36. The determination of the operational
parameter by
the control circuitry 28 may therefore correspond to a maximum value, a
minimum value or
an average, model or median value of the operational parameter during the
inhalation.
Equally, the indication of the operational parameter may represent one or more
of a value for
the operational parameter at the start of the inhalation, a value for the
operational parameter
at the end of the inhalation, a maximum value of the operation parameter
during the
inhalation, a minimum value of the operation parameter during the inhalation
and an
average, model and/or median value for the operational parameter during the
inhalation.
As described above, the control circuitry 28 is configured to determine an
indication
of the amount of the ingredient delivered. The indication may represent the
actual amount of
the ingredient delivered, for example a mass or volume of the ingredient
delivered from the
aerosol-generating material 38 to the user during the inhalation. For example,
control
circuitry 28 may be configured to use an algorithm or look-up table to
determine the amount
of the ingredient delivered during the inhalation based on the duration of the
inhalation and
the operational parameter. The algorithm or look-up table may be based on
empirical data
related to the aerosol provision system 10, such as the maximum or average
mass flow of
air through the air channel 23, or an amount of the ingredient delivered for a
standard
inhalation profile, such as 55m1 of air in a 3 second inhalation every thirty
seconds (referred
to as an 55/3/30 profile). If the amount of an ingredient delivered for a
standard inhalation
profile is known, then this can be scaled using a look-up table or an
algorithm in order to
determine the amount of the ingredient that is delivered for an inhalation
with a different
duration and/or volume of aerosol delivered, and hence an indication of this
amount of
ingredient can be determined.
Alternatively, the indication of the amount of the ingredient delivered during
the
inhalation may relate to the amount of the ingredient delivered compared to a
capacity of the
aerosol-generating material storage area, such that the indication of the
amount of the
ingredient delivered indicates the amount of the ingredient and/or the aerosol-
generating
material remaining in the aerosol-generating material storage area. For
example, the
indication could be a percentage of the total amount of the aerosol-generating
material
present in the aerosol-generating material storage area when the aerosol-
generating
material storage area is full.
In some embodiments, the indication of the amount of the ingredient delivered
during
the inhalation is a rating on a fixed scale, for example an integer or real
number between 0
and 10, where 0 is the lowest value and 10 is the highest value, although
different forms and
granulations of scales can also be used. In this case, an indication of 2
represents that a
small amount of ingredient was delivered during the inhalation, whilst an
indication of 10
represents that a maximum amount of the ingredient was delivered. This rating
may be
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
calculated by multiplying the duration of the inhalation by the indication of
the operational
parameter during the inhalation and applying one or more scaling factors, or
by any other
suitable calculation technique. Using such a rating on a scale allows for
comparison
between indications from different inhalations without requiring as exact or
detailed a
5 calculation as when the indication corresponds to the actual amount of
the ingredient
delivered.
The determination of the indication of the amount of the ingredient delivered
from the
aerosol-generating material to the user may occur during the inhalation
itself. In other
words, the control circuitry 28 is configured to determine the indication of
the amount of the
10 ingredient that has been delivered as the inhalation takes place, such
that the determination
is ongoing during the inhalation. The determination of the indication of the
amount of the
ingredient delivered therefore occurs concurrently with the determination of
the duration of
the inhalation. For example, as described above, the control circuitry 28 may
be configured
to start a timer or otherwise begin the determination of the duration of the
inhalation in
15 response to receiving inhalation detection signals from the sensor 25,
or in response to a
change in the inhalation detection signals received from the sensor 25. The
determination of
the indication of the amount of the ingredient delivered would also begin at
the same time.
Both the determination of the duration of the inhalation and the determination
of the
indication of the amount of the ingredient delivered would therefore continue
until the
inhalation detection signals were no longer received from the sensor 25, or
the inhalation
detection signals received from the sensor 25 changed for a second time.
Alternatively, the determination of the duration of the inhalation may occur
during the
inhalation whilst the determination of the indication of the amount of the
ingredient delivered
occurs after the inhalation has concluded, or both determinations could be
performed after
the inhalation has concluded.
As described above, the control circuitry 28 may be configured to determine a
duration of a session based on the duration a plurality of inhalations. In
response the control
circuitry 28 may be configured to determine an indication of an amount of the
ingredient
delivered from the aerosol-generating material 38 to the user during the
session based on
the duration of the session and an indication of the operational parameter
during the
session. The determining of the indication of the amount of the ingredient
delivered from the
aerosol-generating material to the user during the session may also be based
on the
duration of each inhalation during the session and the time in between each
inhalation
during the session. As described above, this determination may be performed
for each
inhalation in the session, for example a separate determination performed
during each
inhalation or after each inhalation has concluded in the session.
Alternatively, the
determination may be performed once, either during the session or after the
entire session
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
16
has concluded. As described above, the indication of the operational parameter
during the
session may represent one or more of a value for the operational parameter at
the start of
the session, a value for the operational parameter at the end of the session,
a maximum
value of the operation parameter during the session, a minimum value of the
operation
parameter during the session and an average value for the operational
parameter during the
session. Alternatively, the indication of the operational parameter during the
session may
correspond to an indication of the operational parameter for each inhalation
in session.
In the embodiment described above where the control circuitry 28 is configured
to
determine the duration of each inhalation during the rolling predetermined
period, the control
circuitry 28 can also be configured to determine an indication of an amount of
the ingredient
delivered from the aerosol-generating material 38 to the user during the
rolling
predetermined period based on the duration of each inhalation during the
rolling
predetermined period and an indication of the operational parameter during
each inhalation
during the rolling predetermined period. As described above, the control
circuitry 28
determines an operational parameter of the aerosol provision system, and
therefore the
control circuitry 28 can be configured to determine the operational parameter
during each
inhalation during a rolling predetermined period in order to determine the an
indication of an
amount of the ingredient delivered from the aerosol-generating material 38 to
the user during
the rolling predetermined period.
Additionally, the determination of the indication of the amount of the
ingredient
delivered from the aerosol-generating material 38 to the user during the
rolling
predetermined period may also be based on the time between each inhalation
during the
rolling predetermined period. For some ingredients, the amount of residual
ingredient in the
user's body system will decrease over time as the ingredient is absorbed,
broken down,
expelled or otherwise depleted from the user's body system. By considering the
time
between each inhalation and the duration of each inhalation in a predetermined
period, the
indication of an amount of the ingredient delivered from the aerosol-
generating material 38 to
the user during the rolling predetermined period can indicate the amount of
residual
ingredient in the user's body system rather than the amount of the ingredient
delivered to the
user in the predetermined period.
The determination of the indication of the amount of the ingredient delivered
from the
aerosol-generating material 38 may also be based on other factors, such as a
concentration
of the ingredient in the aerosol-generating material 38. It will be
appreciated that, for a given
amount of aerosol generated by the aerosol generator 36 from the aerosol-
generating
material 38, the amount of the ingredient in the resulting aerosol will vary
depending on the
concentration, in other words the amount, of the ingredient in the aerosol-
generating material
38. As described above, the ingredient may be an active substance, such as
nicotine,
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
17
caffeine, taurine, theine, vitamins such as B6 or B12 or C, melatonin,
cannabinoids, or
constituents, derivatives, or combinations thereof. The ingredient may be a
flavourant, an
aerosol-former material or a functional material such as a pH regulator,
colouring agent,
preservative, binder, filler, stabilizer or antioxidant. Accordingly, the
concentration of the
ingredient in the aerosol-generating material 38 can be considered when
determining the
indication of the amount of the ingredient delivered from the aerosol-
generating material 38
in order to improve the accuracy of the determination. The concentration of
the ingredient in
the aerosol-generating material 38 may be provided to the control circuitry 38
by the user, for
example by inputting the concentration on a user input device associated with
the aerosol
provision system 10, or the control circuitry 28 may be configured to
determine the
concentration of the ingredient in the aerosol-generating material 38, for
example in
response to the consumable 30 being attached to the aerosol provision system
10. The
consumable 30 may comprise an electronic chip or tag, such as an RFIG tag,
which the
control circuitry 28 as able to read in order to determine the concentration
of the ingredient in
the aerosol-generating material 38, as well as other properties of the
consumable 30, such
as the identify of the manufacturer or the consumable, one or more flavourants
or other
ingredients contained within the aerosol-generating material 38 and the volume
or mass of
the aerosol-generating material 38 in the consumable 30.
In some embodiments, the control circuitry 28 is configured to determine
default user
behaviour based on the indication of the amount of the ingredient delivered
from the aerosol-
generating material 38 to the user for a plurality of inhalations. In other
words, the control
circuitry 28 is configured to detect patterns in the inhalations by the user
based on data
determined for the inhalations, such as the duration of an inhalation, the
duration of a
session, a time between inhalations, the amount of electrical power delivered
to the aerosol
generator 36 during the inhalation, a power level or setting for the aerosol
generator 36 for
the inhalation and the type and/or concentration of one or more of the
ingredients in the
aerosol-generating material 38. These patterns are then used to default user
behaviour with
respect to the amount of the ingredient delivered from the aerosol-generating
material 38 to
the user during an inhalation. The data determined for the inhalations can
also be used to
determine default user behaviour over another period, such as a session or
rolling
predetermined period as described above, a week, a month and/or a year.
Additionally, the
control circuitry 28 can continually update the determined default user
behaviour based on
changes in the indication of the amount of the ingredient delivered from the
aerosol-
generating material 38 to the user for inhalations over time.
For example, where the control circuitry 28 is configured to determine a time
between
each of the plurality of inhalations based on the inhalation detection
signals, the default user
behaviour can also be determined based on the time between each of the
plurality of
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
18
inhalations. This allows patterns of behaviour to be detected for the user,
such as if the user
takes a series of puffs, such as a session described above, then has an
extended period
between sessions, such as 30 minutes, 1 hour or longer, or whether the user
takes a small
number of inhalations, such as 1 or 2, but spaced more regularly, such as
every 10 of 20
minutes. Equally, the data collected may allows the control circuitry 28 to
determine
particular times of the day when the user takes more inhalations, such as in
the mornings or
the evenings, or if the number and duration of inhalations in a session change
during a day.
For example, the user may have a session comprising a plurality of long
inhalations with a
high power setting in the morning, but sessions in the evening comprise fewer,
short
inhalations with a lower power setting. The user may perform more inhalations
on
weekdays, whilst over the course of a month or a year the data may indicate
that the user is
performing fewer inhalations, for example due to the user trying to cut down
their usage of
the aerosol provision system 10. Such default behaviour can be determined
based on the
indication of the amount of the ingredient delivered from the aerosol-
generating material 38
to the user for a plurality of inhalations.
The control circuitry 28 may also be configured to alter a mode of operation
of the
aerosol generation system 10 based on the default user behaviour, such as an
amount of
electrical power supplied to the aerosol generator 36 by the power source 14,
the
temperature of the aerosol generator 36, a sensitivity or detection threshold
on the sensor
25, the colour and/or number of light indicators illuminated and/or the
volume, pitch and/or
duration of a sound emitted on the aerosol provision device 20 for an
inhalation.
For example, if it is determined that the user takes longs inhalations, such
as greater
than 10 seconds, the control circuitry 28 can be configured to alter the
amount of electrical
power supplied to the aerosol generator 36 by the power source 14 during the
inhalation in
order to prevent dry out or overheating of the aerosol generator 36. The power
supplied to
the aerosol generator 36 may be set to an initial value or power setting, and
then reduced as
the inhalation continues. Alternatively, if is determined that the user takes
very small or
gentle inhalations, for example with a low air speed or mass flow, the control
circuitry 28 can
be configured to change a sensitivity or detection threshold on the sensor 25
to ensure that
an inhalation is properly detected for the user.
In some embodiments, the control circuitry 28 is configured to provide a
notification
to the user based on the indication of the amount of the ingredient delivered
from the
aerosol-generating material 38 to the user during the inhalation and the
default user
behaviour. For example, a notification may be provided on the aerosol
provision system 10,
such as by activating an indicator light, emitting a sound from a speaker or
displaying a
message on a display screen on the aerosol provision device 20 and/or the
consumable 30.
The notification may also be a haptic notification on the aerosol provision
system 10, such as
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
19
a vibration or force feedback. For example, a vibration may be generated by an
eccentric
rotating mass (ERM) or piezoelectric actuator within the aerosol provision
device 20 and/or
the consumable 30, or a force may be generated by a motor within the aerosol
provision
device 20 and/or the consumable 30. The notification could also be a change in
a mode of
operation of the aerosol provision system 10 which the user would detect, such
switching off,
disabling or otherwise preventing electrical power from being supplied to the
aerosol
generator 36. For example, the aerosol generator 36 could be disabled for a
period of time,
such as 5 seconds, 10 seconds, a minute or longer.
Alternatively, or in addition, the notification may be provided on an
application on a
remote device. For example, the user of the aerosol provision system 10 may
have a device
associated with, but separate from, the aerosol provision system 10, and the
control circuitry
28 is configured to communicate with the remote device, for example by
Bluetooth,
Bluetooth Low Energy (BLE), ANT+, VVi-Fi or any other suitable wireless
communication
method. The control circuitry 28 can be configured to communicate with the
remote device
such that the notification is provided to the user on the remote device, such
as on an
application installed on the remote device. For example, a message may be
displayed on a
display screen on the remote device, an indicator light activated, a sound
emitted from a
speaker or a haptic notification means on the remote device as described
above. The
remote device may include any suitable electronic device that can be
communicatively
coupled to the aerosol provision system 10. For example, the remote device may
include a
mobile device (such as a smartphone), a PDA, a personal computer, laptop,
tablet,
smartwatch, etc.
Further, one or more parameters associated with the notification may be
adjustable
by the user. For example, the user may be able to adjust the number,
brightness and/or
colour of the indictor light that is activated, the volume, pitch and or
duration of the sound
emitted and/or the message that is displayed. The user may also be able to
adjust one or
more parameters of the haptic notification. For example the user may be able
to adjust the
duration, magnitude and/or pattern of the vibrations or forces provided by the
actuator and
motor respectively.
The user may be able to adjust the one or more parameters associated with the
notification on the aerosol provision system 10 and/or the remote device
regardless of
whether the notification is provided on the aerosol provision system 10 or the
remote device.
For example, the user may be able to use the application on the remote device
to adjust one
or more of the parameters associated with the notification even though the
notification itself
is provided on the aerosol provision system 10. For example, the user may
disable
notifications during an inhalation such that notifications are only received
when an inhalation
is not detected by the sensor 25.
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
In some embodiments, the control circuitry 28 is configured to provide a
notification
to the user when the indication of an amount of an ingredient delivered from
the aerosol-
generating material to the user during the inhalation exceeds a puff
threshold. The puff
threshold may correspond to a safe usage limit of the ingredient and/or
aerosol-generating
5
material 38, or a safe usage limit of the aerosol provision system 10 for an
inhalation, for
example to prevent overheating or drying out of one or more of the components
of the
aerosol provision system 10. The notification could be in any of the forms
described above.
The indication of the amount of the ingredient delivered from the aerosol-
generating
material 38 to the user during the inhalation might represent a proportion or
percentage of
10
the amount of the ingredient delivered from the aerosol-generating material 38
to the user
during the inhalation compared to the puff threshold. For example, the
indication might be a
percentage of the puff threshold, such as 10%, 20%, 50%, 80% or 110%, and the
notification
is provided to the user when the percentage is greater that 100%.
As described above, the determination of the indication of an amount of an
ingredient
15
delivered may occur during the inhalation. In this case, a comparison between
the indication
of an amount of an ingredient delivered and the puff threshold may be
performed during the
inhalation, either continuously or periodically (such as every second or every
5 seconds). In
other words, the indication of an amount of an ingredient delivered is
constantly determined
during the inhalation, and the value for the indication of an amount of an
ingredient delivered
20
at the current moment in time is compared to the puff threshold, such a
notification can be
provided to the user during the inhalation as soon as the amount of the
ingredient delivered
from the aerosol-generating material to the user exceeds the puff threshold.
In some embodiments the control circuitry 28 is configured to provide a
notification to
the user when the indication of the amount of an ingredient delivered from the
aerosol-
generating material 38 to the user during the session exceeds a session
threshold. In
addition or alternatively, the control circuitry 28 may be configured to
provide a notification to
the user when the indication of the amount of an ingredient delivered from the
aerosol-
generating material 38 to the user during the rolling predetermined period
exceeds a period
threshold. In a similar fashion to the puff threshold, the session threshold
and the period
threshold may correspond to a safe usage limit of the ingredient and/or
aerosol-generating
material 38, or a safe usage limit of the aerosol provision system 10 for a
session and a
predetermined period respectively. The notification could be in any of the
forms described
above. As described above, the determination of the indication of an amount of
an
ingredient delivered may occur during the session and/or the rolling
predetermined period.
The control circuitry 28 may be configured to alter one or more thresholds
described
above based on the default user behaviour, such as the puff threshold, session
threshold or
period threshold, thereby personalising or otherwise tailoring the operation
of the aerosol
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
21
provision system 10 to the user. Alternatively or in addition, the user may be
able to alter
one or more of the thresholds, for example by providing an input on an input
device on the
aerosol provision device 20 or consumable 30, or through an application on an
associated
remote device, thereby giving the user additional control over the operation
of the aerosol
provision system 10.
Figure 4 illustrates a system 400 comprising an aerosol provision system 10
configured to generate aerosol from an aerosol-generating material 38, such as
described
above. The system 400 also comprises a computer 40 configured to receive
inhalation
detection signals from a sensor 25 configured to detect the inhalation on the
aerosol
provision system 10 by a user of the aerosol provision system 10. The computer
40 is also
configured to determine a duration of the inhalation based on the inhalation
detection signals
received from the sensor 25 and determine an indication of an amount of an
ingredient
delivered from the aerosol-generating material 38 to the user during the
inhalation based on
the duration of the inhalation and an indication of an operational parameter
of the aerosol
provision system 10 during the inhalation.
As described above and illustrated in Figure 4, the computer 40 may be a
remote
device associated with the user and in communication with the aerosol
provision system 10.
Accordingly, it will be appreciated that the functions of the control
circuitry described herein,
such determining a duration of an inhalation, determining an indication of an
amount of an
ingredient delivered, determining default user behaviour and providing a
notification to the
user may be performed by a computer 40 separate from the aerosol provision
system 10,
such as a remote device.
Figure 5 is a flow chart of a method 500 of determining an amount of an
ingredient
delivered to a user of an aerosol provision system 10. The method begins at
step 501,
where inhalation detection signals are received from a sensor 25 configured to
detect the
inhalation on the aerosol provision system 10 by a user of the aerosol
provision system 10.
Next, at step 502, a duration of the inhalation is determined based on the
inhalation
detection signals received from the sensor 25. At step 503, an indication of
an amount of an
ingredient delivered from an aerosol-generating material 38 to the user during
the inhalation
is determined based on the duration of the inhalation and an indication of an
operational
parameter of the aerosol provision system 10 during the inhalation. As
described above, an
aerosol generator 36 is configured to aerosolize the aerosol-generating
material 38.
The method 500 illustrated in Figure 5 may be stored as instructions on a
computer
readable storage medium, such that when the instructions are executed by a
processor, the
method 500 described above is performed. The computer readable storage medium
may be
non-transitory.
CA 03172032 2022- 9- 15
WO 2022/008899
PCT/GB2021/051717
22
As described above, the present disclosure relates to (but it not limited to)
aerosol
provision system comprises control circuitry for determining an operational
parameter of the
aerosol provision system, an aerosol generator configured to aerosolize an
aerosol-
generating material and a sensor configured to detect an inhalation on the
aerosol provision
system by a user of the aerosol provision system, and output corresponding
inhalation
detection signals to the control circuitry. The control circuitry is
configured to determine a
duration of the inhalation based on the inhalation detection signals received
from the sensor,
and determine an indication of an amount of an ingredient delivered from the
aerosol-
generating material to the user during the inhalation based on the duration of
the inhalation
and an indication of the operational parameter during the inhalation.
Thus, there has been described an aerosol provision system, a system
comprising
an aerosol provision system and a computer, a method of determining an amount
of an
ingredient delivered to a user of an aerosol provision system, and computer
readable
storage medium.
The various embodiments described herein are presented only to assist in
understanding and teaching the claimed features. These embodiments are
provided as a
representative sample of embodiments only, and are not exhaustive and/or
exclusive. It is to
be understood that advantages, embodiments, examples, functions, features,
structures,
and/or other aspects described herein are not to be considered limitations on
the scope of
the invention as defined by the claims or limitations on equivalents to the
claims, and that
other embodiments may be utilised and modifications may be made without
departing from
the scope of the claimed invention. Various embodiments of the invention may
suitably
comprise, consist of, or consist essentially of, appropriate combinations of
the disclosed
elements, components, features, parts, steps, means, etc., other than those
specifically
described herein. In addition, this disclosure may include other inventions
not presently
claimed, but which may be claimed in future.
CA 03172032 2022- 9- 15