Note: Descriptions are shown in the official language in which they were submitted.
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
LUBRICATING OIL COMPOSITIONS WITH IMPROVED OXIDATIVE PERFORMANCE COMPRISING
ALKYLATED
DIPHENYLAMINE ANTIOXIDANT AND SULFONATE DETERGENTS
CROSS REFERENCE TO RELATED APPLICATIONS
[001] This application is related to U.S. Provisional Application entitled
"IMPROVED OXIDATIVE PERFORMANCE WITH CARBOXYLATE DETERGENTS"
(ATTORNEY DOCKET: T-11173) filed on March 11, 2020, the contents of which are
herein incorporated by reference.
TECHNICAL FIELD
[002] This disclosure relates to lubricating oil additives that disrupt
oxidation
and increase the useful life of lubricating oils. More particularly, this
disclosure relates
to lubricating oil compositions that include alkylated diphenylamine
antioxidant and
sulfonate detergent.
BACKGROUND
[003] Oxidation is a concern for in-service lubricating oils as it can cause
thickening of the oil, sludge, varnish, acid number increase and corrosion.
These
outcomes are generally detrimental to proper operation of automotive engines
and
limit useful life of the lubricating oil. With continually evolving engine
designs,
operating conditions and oil performance expectations, oxidation continues to
be an
important ongoing technical challenge.
[004] One way to slow down oxidation in engines is to introduce antioxidants
to lubricating oils. Additionally, antioxidants can also extend drain
intervals, maintain
viscosity, reduce deposit, reduce foam formation, protect against corrosion as
well as
protect the lubricating oil against high temperature.
[005] There are many antioxidants that have varying degrees of effectiveness.
Commercial lubricants are usually formulated with one or more antioxidants to
protect
the fluid under a wide range of conditions (e.g., temperature, time, air
mixtures,
pressure, etc.).
1
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
[006] In particular, alkylated diphenylamines are used as antioxidants. Widely-
used alkylated diphenylamine antioxidants include nonylated (C9) diphenylamine
which can be added into organic fluids such as engine oils, gear oils,
hydraulic fluids,
compressor oils, turbine oils, and grease.
SUMMARY
[007] This disclosure relates to lubricating oil additives that disrupt
oxidation
and increase the useful life of lubricating oils. More particularly, this
disclosure relates
to compositions that include alkylated diphenylamines and sulfonate
detergents.
[008] In one aspect, there is provided a lubricating oil composition
comprising:
a base oil; a primary antioxidant comprising alkylated diphenylamines having
an alkyl
group derived from propylene tetramers; and a sulfonate detergent.
[009] In a further aspect, there is provided a method of improving oxidation
stability of a lubricating oil, the method comprising: supplying to an engine
a
lubricating oil composition comprising: a base oil; a primary antioxidant
comprising
alkylated diphenylamines having an alkyl group derived from propylene
tetramers; and
a sulfonate detergent.
BRIEF DESCRIPTION OF THE DRAWINGS
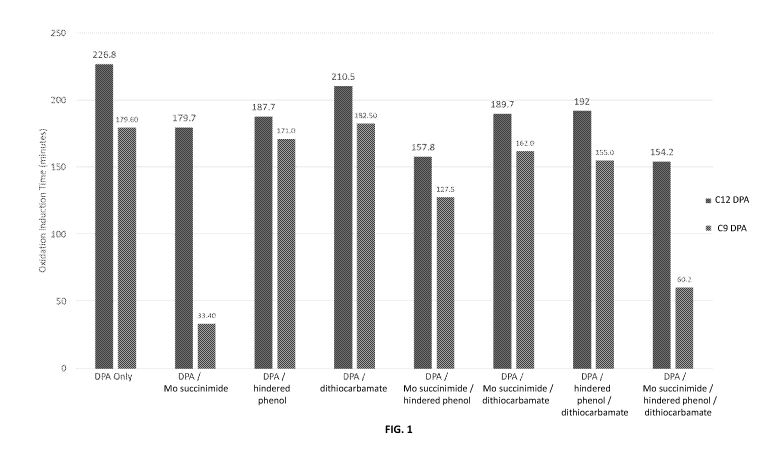
[010] FIG. 1 illustrates a comparison of oxidative induction time of
formulated
oil samples as described in the Example.
2
CA 03173369 2022-08-26
WO 2021/181286
PCT/IB2021/051971
DETAILED DESCRIPTION
[011] In this specification, the following words and expressions, if and when
used, have the meanings ascribed below.
[012] The term "antioxidant" or equivalent term (e.g., "oxidation stabilizer"
or
"oxidation inhibitor") refers to a composition and its ability to resist
deleterious attacks
in an oxidizing environment. Antioxidants are often used in organic fluids
(e.g.,
lubricating oil, gear oil, compressor oil, mineral oil, hydraulic fluid, etc.)
to improve the
oxidation stability of the organic fluid.
[013] The term "alkyl" or related term refers to a saturated hydrocarbon
group,
that can be linear, branched, cyclic, or a combination of cyclic, linear
and/or branched.
[014] The term "olefin" refers to a hydrocarbon that has at least one carbon-
carbon double bond that is not part of an aromatic ring or ring system.
Olefins may
include aliphatic and aromatic, cyclic and acyclic, and/or linear and branched
compounds having at least one carbon-carbon double bond that is not part of an
aromatic ring or ring system, unless specifically stated otherwise. Olefins
having only
one, only two, only three, etc., carbon-carbon double bonds can be identified
by use
of the term "mono," "di," "tri," etc., within the name of the olefin. The
olefins can be
further identified by the position of the carbon-carbon double bond(s).
Depending on
the context, the term "olefin" may refer to an "olefin oligomer" or to an
"olefin
monomer" or both.
[015] An "olefin oligomer" is an oligomer made from oligomerization of
"olefin monomers." For example, a "propylene oligomer" is made from the
oligomerization of propylene monomers. Examples of propylene oligomers include
propylene tetramer and propylene pentamer. A "propylene tetramer" is an olefin
oligomer product resulting from the oligomerization of nominally 4 propylene
monomers. These terms also can be used generically to describe homo-oligomers,
co-oligomers, salts of oligomers, derivatives of oligomers, and the like.
3
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
[016] A "minor amount" or related term means less than 50 wt % of a
composition, expressed in respect of the stated additive and in respect of the
total
weight of the composition, reckoned as active ingredient of the additive.
[017] A "major amount" or related term means an amount greater than 50 wt
% based on the total weight of the composition.
Antioxidant Composition
[018] The present invention relates to antioxidant compositions that disrupt
oxidation and increase the useful life of lubricating oils. More particularly,
the present
invention describes antioxidant compositions comprising a plurality of
lubricant
additives. The lubricant additives include at least one antioxidant and at
least one
detergent working together to provide enhanced oxidative performance. The
enhanced performance is a result of a previously unknown synergy arising from
the
lubricant additive components of the present invention in lubricating oil
compositions.
Antioxidants and detergents compatible with the present invention will be
described
herein.
Primary Antioxidant
[019] The antioxidant composition comprises a primary antioxidant and one
or more secondary antioxidants. The primary antioxidant of the present
invention is
an allwlated diphenylamine having one or more relatively long alkyl groups.
Conventional allwlated diphenylamine antioxidants typically utilize relatively
short
alkyl groups. These include, for example, nonylated diphenylamine ("propylene
trimer") which nominally has 9 carbons and can be formed from the
oligomerization
of propylene.
[020] The alkylated diphenylamines of the present invention have been
allwlated by propylene tetramers (having nominally 12 carbons) or by a mixture
comprising propylene tetramers, wherein the propylene tetramer is the
predominant
olefin oligomer alkylating agent. Propylene tetramers can be obtained by the
oligomerization of 4 propylene monomers. There are several potential
advantages of
4
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
propylene tetramer over propylene trimer including, but not limited to,
increased oil
solubility, cheaper cost, and superior stability against oxidation.
[021] The alkylated diphenylamine of the present invention may be present at
about 0.4 wt % to about 20 wt % of the lubricating oil composition, such as
from about
0.5 wt % to about 15 wt %, 0.1 wt % to about 10 wt %, 0.5 wt % to about 8 wt
%, or 1
wt % to about 5 wt %.
Propylene Oligomers
[022] The propylene oligomers (i.e., propylene tetramers) of the present
invention can be prepared by any compatible method known in the art. By way of
an
example, a process for preparing the propylene oligomers employs a liquid
phosphoric
acid oligomerization catalyst. Descriptions of liquid phosphoric acid-
catalyzed
propylene oligomerization process can be found in U.S. Pat. Nos. 2,592,428;
2,814,655;
and 3,887,634, the relevant portions of which are hereby incorporated by
reference.
[023] An unrefined product of oligomerization process typically includes a
mixture of branched olefins having a distribution in number of carbons. In a
commercial setting, olefin oligomers are subject to extreme conditions during
the
oligomerization process which results in cracking, recombination,
isomerization and
the like. Refined or processed oligomerization products typically have higher
concentration of the desired product. Thus, the term "propylene tetramer" may
not
necessarily refer to a pure propylene tetramer product but a mixture of
olefins or olefin
oligomer products. Accordingly, the product of alkylation involving
diphenylamine
and propylene tetramer can have a distribution in number of carbons within the
allwlated alkyl groups.
[024] Propylene tetramers can be obtained from the oligomerization of 4
propylene monomers. The propylene tetramer is a cost effective olefin to
manufacture. As a product of oligomerization, it features a highly branched
chain of
to 15 carbons with high degree of methyl branching that imparts exceptional
oil
solubility and compatibility with other oil soluble lubricant additive
components. In
5
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
some embodiments, the average carbon number can range from about 10 to about
15.
[025] The product of oligomerization can vary in degree of branching. For
example, the propylene tetramer can exhibit a total branching (i.e., sum of
olefinic and
aliphatic branching) ranging from 1 to 15. In some embodiments, the average
total
branching can range from about 1 to about 15.
[026] The propylene tetramers of the present invention generally comprise at
least 50 wt % of Cio to C15 carbon atoms. In an embodiment, the propylene
tetramers
contain a distribution of carbon atoms which comprise at least 60 wt % of Cio
to C15
carbon atoms. In an embodiment, the propylene tetramers contain a distribution
of
carbon atoms which comprise at least 70 wt % of Cio to C15 carbon atoms. In an
embodiment, the propylene oligomers contain a distribution of carbon atoms
which
comprise at least 80 wt % of Cio to C15 carbon atoms. In an embodiment, the
propylene
oligomers contain a distribution of carbon atoms which comprise at least 90 wt
% of
Cio to C15 carbon atoms.
[027] As will be apparent to a person of ordinary skill in the art, the
propylene
oligomers employed herein may also contain a minor amount of lower molecular
weight propylene oligomer(s) such as propylene trimer, as well as higher
molecular
weight propylene oligomer(s) such as propylene pentamer. For example, the
propylene tetramer of the present invention may be a mixture of olefinic
hydrocarbons
containing 0-1 wt % C9H18, 0-5 wt % C10H20, 0-10 wt % C11H22, 50-90 wt %
C12H24, 10-
20 wt % C13H26, 5-15 wt % C14H28, and/or 1-10 wt % C15H30.
Alkylation
[028] The alkylated diphenylamine of the present invention can be obtained
by any allwlation process compatible with the present invention. For example,
US
6,355,839, hereby incorporated by reference, describes the preparation of
alkylated
diphenylamine wherein the diphenylamine is alkylated with polyisobutylene.
[029] Any suitable catalyst may be used. For example, the alkylation of
diphenylamine may proceed in the presence of a clay catalyst. Temperature of
this
6
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
reaction can range from 140 C to 200 C, more typically between 150 C to 190 C.
In
some embodiments, the temperature of the reaction ranges between 160 C to 180
C.
The reaction can be carried out at a single temperature, or sequentially, at
different
temperatures. The propylene oligomer can be charged at a charge mole ratio
(CMR)
between 2:1 to 8:1 in relation to the diphenylamine charge. In some
embodiments,
the CMR is between 3:1 to 7:1 or between 4:1 and 6:1. The reaction product can
be
filtered to remove the catalyst and then distilled to remove unreacted olefin
oligomers
and diphenylamines. The use of clay as catalyst is disclosed in U.S. Pat. No.
3,452,056,
which is hereby incorporated by reference.
[030] As would be expected to a person of ordinary skill in the art, the
reaction
conditions may vary significantly depending on the catalyst used. For example,
reactions involving homogeneous acid catalysts may only require temperatures
ranging between 75 C to 100 C.
[031] Depending on the reaction conditions, the alkylated diphenylamine
product can have various relative amounts of mono-alkylated, di-alkylated,
and/or tri-
allwlated diphenylamine products. It should be apparent that for a given di-
or tri-
allwlated diphenylamine molecule, the two or more alkylated alkyl groups may
be
identical or different in accordance with this disclosure.
Secondary Antioxidants
[032] The present invention employs one or more secondary antioxidants in
combination with the primary antioxidant. The secondary antioxidant may be
present
at about 0.01 wt % to about 20 wt % of the lubricating oil composition, such
as from
about 0.05 wt % to about 15 wt %, 0.1 wt % to about 10 wt %, 0.5 wt % to about
8 wt
%, or 1 wt % to about 5 wt %.
[033] A number of secondary antioxidants are compatible with the present
invention. Examples of secondary antioxidants include molybdenum succinimides,
dithiocarbamates and hindered phenols. These oil-soluble components are
generally
known.
7
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
[034] For example, the mono and polysuccinimides that can be used to
prepare the molybdenum complexes described herein are disclosed in numerous
references and are well known in the art. Certain fundamental types of
succinimides
and the related materials encompassed by the term of art "succinimide" are
taught in
U.S. Pat. No's. 3,219,666; 3,172,892; and 3,272,746, the disclosures of which
are hereby
incorporated by reference. The term "succinimide" is understood in the art to
include
many of the amide, imide, and amidine species which may also be formed. The
predominant product however is a succinimide and this term has been generally
accepted as meaning the product of a reaction of an alkenyl substituted
succinic acid
or anhydride with a nitrogen-containing compound.
[035] Preferred succinimides, because of their commercial availability, are
those succinimides prepared from a hydrocarbyl succinic anhydride, wherein the
hydrocarbyl group contains from about 24 to about 350 carbon atoms, and an
ethylene amine, said ethylene amines being especially characterized by
ethylene
diamine, diethylene triamine, triethylene tetramine, and tetraethylene
pentamine.
Particularly preferred are those succinimides prepared from polyisobutenyl
succinic
anhydride of 70 to 128 carbon atoms and tetraethylene pentamine or triethylene
tetramine or mixtures thereof.
[036] Also included within the term "succinimide" are the cooligomers of a
hydrocarbyl succinic acid or anhydride and a poly secondary amine containing
at least
one tertiary amino nitrogen in addition to two or more secondary amino groups.
Ordinarily this composition has between 1,500 and 50,000 average molecular
weight.
A typical compound would be that prepared by reacting polyisobutenyl succinic
anhydride and ethylene dipiperazine.
[037] Succinimides having an average molecular weight of 1000 or 1300 or
2300 and mixtures thereof are most preferred. Such succinimides can be post
treated
with boron or ethylene carbonate as known in the art.
[038] Suitable dithioca rba mates include, but are not limited to,
dithiocarbamates wherein the metal is zinc, copper or molybdenum, ashless
8
CA 03173369 2022-08-26
WO 2021/181286
PCT/IB2021/051971
thiocarbamates or dithioca rba mates (i.e., essentially metal free) such as
methylenebis(dia lkyldithioca rba mate),
ethylenebis(dialkyldithiocarbamate), and
isobutyl disulfide-2,2'-bis(dialkyldithiocarbamate) where the alkyl groups of
the
diallyldithiocarbamate can preferably have from 1 to 6 carbon atoms. Examples
of
preferred ashless dithiocarbamates are methylenebis(dibutyldithiocarbamate),
ethylenebis(dibutylthiocarbamate) and isobutyl disulfide-
2,2'-
bis(dibutyldithiocarbamate).
[039] The secondary antioxidant employed in the lubricating oil of the present
invention may be a sterically hindered phenol. The hindered phenol antioxidant
often
contains a secondary butyl and/or a tertiary butyl group as a sterically
hindering group.
The phenol group is often further substituted with a hydrocarbyl group and/or
a
bridging group linking to a second aromatic group. Suitable hindered phenols
include,
but are not limited to, 2,6-di-tert-butylphenol, 4-methyl-2,6-di-tert-
butylphenol, 4-
ethyl-2,6-di-tert-butylphenol, 4-propy1-2,6-di-tert-butylphenol or 4-butyl-2,6-
di-tert-
butylphenol, or 4-dodecy1-2,6-di-tert-butylphenol.
Detergents
[040] The antioxidant composition of the present invention includes one or
more detergents. Detergents may be present at about 0.01 wt % to about 10 wt %
of
the lubricating oil composition, such as from about 0.05 wt % to about 8 wt %,
0.1 wt
% to about 5 wt %, 0.5 wt % to about 4 wt %, or 1 wt % to about 3 wt %.
[041] Detergents are normally salts (e.g., overbased salts) and are single
phase,
homogeneous Newtonian systems characterized by a metal content in excess of
that
which would be present according to the stoichiometry of the metal and the
particular
acidic organic compound reacted with the metal.
[042] The detergents of the present invention include sulfonate detergents.
Metallic detergents such as sulfonate detergents typically contain a polar
head group
and a hydrocarbon tail or an oleophilic group. In general, the hydrocarbon
tail can
range from about 3 carbons to 50 carbons in length.
9
CA 03173369 2022-08-26
WO 2021/181286
PCT/IB2021/051971
[043] Sulfonate detergents may be natural or synthesized. The detergent may
be neutral or overbased. Overbased detergents may range in degree of
overbasing
(as measured by ASTM D2896). Compatible overbased sulfonates include low
overbased, medium overbased, high overbased, and high high overbased sulfonate
detergents. In some embodiments, the detergent may be borated.
[044] Examples of sulfonate detergents include alkyl aryl sulfonates and the
like. Specific examples include magnesium allwItoluene sulfonates which are
described in U520110136711. Other examples include calcium alkyl aryl
sulfonates,
calcium alkyltoluene sulfonates, and magnesium alkylbenzene sulfonates.
[045] Metals of detergents can also include alkali or alkaline earth metals,
e.g.,
barium, sodium, potassium, lithium, calcium, and magnesium. The most commonly
used metals are calcium and magnesium, which both may be present in detergents
used in lubricants, and mixtures of calcium and/or magnesium with sodium.
[046] In some embodiments, additional detergents may be used. The
additional detergents include phenates, salicylates, phenolates, phosphonates,
thiophosphonates, ionic surfactants, and the like. In some embodiments,
additional
detergents include hybrid and/or complex detergents.
Lubricating Oil Compositions
[047] The antioxidant compositions of present disclosure may be used in
lubricating oil to impart oxidation stability to the lubricating oil. The
primary
antioxidant, secondary antioxidant, and one or more detergents may be present
in any
ratio provided that their concentrations fall within the guidelines provided
herein.
[048] In general, the antioxidant compositions are oil soluble meaning that
they are, for instance, soluble or stably dispersible in oil to an extent
sufficient to exert
their intended effect in the environment in which the oil is employed.
Moreover, the
additional incorporation of other additives may also permit incorporation of
higher
levels of a particular additive, if desired. The term oil-soluble does not
necessarily
indicate that the compounds or additives are soluble, dissolvable, miscible,
or capable
of being suspended in the oil in all proportions. If other antioxidants are
present in
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
the lubricating oil composition, a lesser amount of the antioxidant of the
present
invention may be used.
[049] Oils used as the base oil will be selected or blended depending on the
desired end use and the additives in the finished oil to give the desired
grade of engine
oil, e.g. a lubricating oil composition having an Society of Automotive
Engineers (SAE)
Viscosity Grade of OW, OW-8, OW-16, OW-20, OW-30, OW-40, OW-50, OW-60, 5W, 5W-
20, 5W-30, 5W-40, 5W-50, 5W-60, 10W, 10W-20, 10W-30, 10W-40, 10W-50, 15W,
15W-20, 15W-30, or 15W-40. Straight grade based oils such as SAE 30, 40, 50,
and 60
may also be used.
[050] The oil of lubricating viscosity (sometimes referred to as "base stock"
or
"base oil") is the primary liquid constituent of a lubricant, into which
additives and
possibly other oils are blended, for example to produce a final lubricant (or
lubricant
composition). A base oil, which is useful for making concentrates as well as
for making
lubricating oil compositions therefrom, may be selected from natural
(vegetable,
animal or mineral) and synthetic lubricating oils and mixtures thereof.
[051] Definitions for the base stocks and base oils in this disclosure are the
same as those found in American Petroleum Institute (API) Publication 1509
Annex E
("API Base Oil Interchangeability Guidelines for Passenger Car Motor Oils and
Diesel
Engine Oils," December 2016). Group I base stocks contain less than 90%
saturates
and/or greater than 0.03% sulfur and have a viscosity index greater than or
equal to
80 and less than 120 using the test methods specified in Table E-1. Group II
base
stocks contain greater than or equal to 90% saturates and less than or equal
to 0.03%
sulfur and have a viscosity index greater than or equal to 80 and less than
120 using
the test methods specified in Table E-1. Group III base stocks contain greater
than or
equal to 90% saturates and less than or equal to 0.03% sulfur and have a
viscosity
index greater than or equal to 120 using the test methods specified in Table E-
1. Group
IV base stocks are polyalphaolefins (PAO). Group V base stocks include all
other base
stocks not included in Group I, II, Ill, or IV.
11
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
[052] Natural oils include animal oils, vegetable oils (e.g., castor oil and
lard
oil), and mineral oils. Animal and vegetable oils possessing favorable thermal
oxidative
stability can be used. Of the natural oils, mineral oils are preferred.
Mineral oils vary
widely as to their crude source, for example, as to whether they are
paraffinic,
naphthenic, or mixed paraffinic-naphthenic. Oils derived from coal or shale
are also
useful. Natural oils vary also as to the method used for their production and
purification, for example, their distillation range and whether they are
straight run or
cracked, hydrorefined, or solvent extracted.
[053] Synthetic oils include hydrocarbon oil. Hydrocarbon oils include oils
such as polymerized and interpolymerized olefins (e.g., polybutylenes,
polypropylenes,
propylene isobutylene copolymers, ethylene-olefin copolymers, and ethylene-
alphaolefin copolymers). Polyalphaolefin (PAO) oil base stocks are commonly
used
synthetic hydrocarbon oil. By way of example, PAOs derived from C8 to
C14olefins, e.g.,
C8, C10, C12, C14 olefins or mixtures thereof, may be utilized.
[054] Other useful fluids for use as base oils include non-conventional or
unconventional base stocks that have been processed, preferably catalytically,
or
synthesized to provide high performance characteristics.
[055] Non-conventional or unconventional base stocks/base oils include one
or more of a mixture of base stock(s) derived from one or more Gas-to-Liquids
(GTL)
materials, as well as isomerate/isodewaxate base stock(s) derived from natural
wax or
waxy feeds, mineral and or non-mineral oil waxy feed stocks such as slack
waxes,
natural waxes, and waxy stocks such as gas oils, waxy fuels hydrocracker
bottoms, waxy
raffinate, hydrocrackate, thermal crackates, or other mineral, mineral oil, or
even non-
petroleum oil derived waxy materials such as waxy materials received from coal
liquefaction or shale oil, and mixtures of such base stocks.
[056] Base oils for use in the lubricating oil compositions of present
disclosure
are any of the variety of oils corresponding to API Group I, Group II, Group
III, Group
IV, and Group V oils, and mixtures thereof, preferably API Group II, Group
III, Group IV,
12
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
and Group V oils, and mixtures thereof, more preferably the Group III to Group
V base
oils due to their exceptional volatility, stability, viscometric and
cleanliness features.
[057] Typically, the base oil will have a kinematic viscosity at 100 C (ASTM
D445) in a range of 2.5 to 20 mm2/s (e.g., 3 to 12 mm2/s, 4 to 10 mm2/s, or
4.5 to 8
mm2/s).
[058] The present lubricating oil compositions may also contain conventional
lubricant additives for imparting auxiliary functions to give a finished
lubricating oil
composition in which these additives are dispersed or dissolved. For example,
the
lubricating oil compositions can be blended with antioxidants, ashless
dispersants,
anti-wear agents, detergents such as metal detergents, rust inhibitors,
dehazing
agents, demulsifying agents, friction modifiers, metal deactivating agents,
pour point
depressants, viscosity modifiers, a ntifoaming agents, co-solvents, package
compatibilizers, corrosion-inhibitors, dyes, extreme pressure agents and the
like and
mixtures thereof. A variety of the additives are known and commercially
available.
These additives, or their analogous compounds, can be employed for the
preparation
of the lubricating oil compositions of the invention by the usual blending
procedures.
[059] Each of the foregoing additives, when used, is used at a functionally
effective amount to impart the desired properties to the lubricant. Thus, for
example,
if an additive is an ashless dispersant, a functionally effective amount of
this ashless
dispersant would be an amount sufficient to impart the desired dispersancy
characteristics to the lubricant. Generally, the concentration of each of
these additives,
when used, may range, unless otherwise specified, from about 0.001 to about 20
wt %,
such as about 0.01 to about 10 wt %.
[060] The following illustrative examples are intended to be non-limiting.
EXAMPLES
[061] As shown in FIG. 1, oxidation induction times of fully formulated engine
oils were tested. The fully formulated engine oils include one or more
antioxidants
13
CA 03173369 2022-08-26
WO 2021/181286
PCT/IB2021/051971
and a sulfonate detergent as well as common lubricant additives such as
dispersant,
and corrosion inhibitor.
[062] The first engine oil samples ("DPA Only") includes a sulfonate detergent
and an alkylated diphenylamine. The alkylated diphenylamine is a nonylated
diphenylamine or a diphenylamine alkylated with a propylene tetramer. Gas
chromatography analysis of the diphenylamine alkylated with propylene tetramer
is
summarized in Table 1 below. The analysis shows that roughly half of the
sample is
mono alkylated diphenylamine. Roughly another half of the sample is di
alkylated
diphenylamine. There is a very small amount of diphenylamine with C3-C8 alkyl
group.
TABLE 1
DPA Dimer C3-C8 DPA Total Mono DPA Total Di DPA
0.0% 0.0% 0.6% 50.8% 48.6%
[063] Other engine oil samples include one or more additional antioxidants
(i.e., molybdenum succinimide, hindered phenol, dithiocarbamate). In a mixed
engine
oil sample with multiple antioxidants, each antioxidant present is present in
equal treat
levels / weight percent.
[064] Test engine oil samples featuring two antioxidants include an alkylated
diphenylamine with molybdenum succinimide ("DPA/Mo succinimide"), hindered
phenol ("DPA/hindered phenol") or dithiocarbamate ("DPA/dithiocarbamate").
Test
engine oil samples featuring three antioxidants include an alkylated
diphenylamine
with molybdenum succinimide and hindered phenol ("DPA/Mo succinimide/hindered
phenol"), molybdenum succinimide and dithiocarbamate ("DPA/Mo
succinimide/dithiocarbamate"), or hindered phenol and dithiocarbamate
("DPA/hindered phenol/dithiocarbamate"). Test engine oil samples feature four
antioxidants include an alkylated diphenylamine with molybdenum succinimide,
hindered phenol, and dithiocarbamate ("DPA/Mo succinimide/hindered
phenol/dithiocarbamate").
14
CA 03173369 2022-08-26
WO 2021/181286 PCT/IB2021/051971
[065] For each test sample, sulfonate detergent (low and medium overbased
sulfonates) is present in 78 mM while the total concentration of the
antioxidant(s) is
1.5 wt %.
[066] The data shows consistently higher oxidation induction times in samples
with diphenylamine alkylated with propylene tetramers as compared to samples
with
nonylated diphenylamine.
[067] Oxidation induction times were evaluated using Pressurized Differential
Scanning Calorimetry (PDSC) according to ASTM D6186 test protocol. Greater
oxidation induction times indicated greater oxidation stability.