Note: Descriptions are shown in the official language in which they were submitted.
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
- 1 ¨
REACTIVE HYDROGEL FORMING FORMULATIONS AND RELATED
METHODS
TECHNICAL FIELD
Compositions and methods related to hydrogel tissue sealants are generally
described.
BACKGROUND
Pneumothorax is a problematic complication of a lung biopsy procedure in which
air passes into the pleural space as a result of a puncture of the parietal
and visceral
pleura. Pneumothorax poses significant concerns for clinicians performing and
patients
undergoing percutaneous lung biopsies. The incidence of pneumothorax in
patients
undergoing percutaneous lung biopsy has been reported to be anywhere between
about
9% and about 54% of patients, with an average of about 15%. In addition, on
average,
about 7% of all percutaneous lung biopsies result in pneumothorax requiring a
chest tube
to be placed into the patient, which subsequently results in an average
hospital stay of
about 3 days. Factors increasing the risk of pneumothorax include increased
patient age,
obstructive lung disease, increased depth of lesion, multiple pleural passes,
increased
time of needle across the pleura, and traversal of a fissure. Pneumothorax can
occur
during or immediately after the lung biopsy procedure. Furthermore, other
complications of percutaneous lung biopsy include hemoptysis, hemothorax,
infection,
and air embolism. The development of a novel hydrogel tissue sealant with the
ability to
adhere to and/or seal tissues (e.g. the pleura) to address pneumothorax and
other surgical
applications, and related methods, would be beneficial.
SUMMARY
Compositions and methods for forming hydrogel tissue sealants are generally
described. The subject matter of the present invention involves, in some
cases,
interrelated products, alternative solutions to a particular problem, and/or a
plurality of
different uses of one or more systems and/or articles.
In some embodiments, a hydrogel forming composition for forming a hydrogel
tissue sealant is described. In certain embodiments, the hydrogel forming
composition
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 2 ¨
comprises a first component comprising a crosslinking agent, wherein the
crosslinking
agent is a difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the hydrogel forming
composition
comprises a second component comprising a protein that is capable of cros
slinking with
the crosslinking agent. In some embodiments, the hydrogel forming composition
comprises one or more solvents able to dissolve the first component and the
second
component, and a surfactant. In certain embodiments, when the first component,
the
second component, and the surfactant are all dissolved in the one or more
solvents,
crosslinking of the crosslinking agent and the protein occurs to form the
hydrogel tissue
sealant.
In some embodiments, a hydrogel forming composition for forming a hydrogel
tissue sealant comprises a crosslinking agent, wherein the crosslinking agent
is a
difunctionalized polyalkylene oxide-based component of the formula:
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 3 ¨
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)-
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the hydrogel forming
composition
comprises a protein that is capable of crosslinking with the crosslinking
agent, one or
more solvents able to dissolve the first component and the second component,
and a
surfactant, wherein when the crosslinking agent, the protein, and the
surfactant are all
dissolved in the one or more solvents, crosslinking of the crosslinking agent
and the
protein occurs to form the hydrogel tissue sealant.
According to certain embodiments, a hydrogel forming composition for forming
a hydrogel tissue sealant comprises a first component comprising a
crosslinking agent,
wherein the crosslinking agent is a difunctionalized polyalkylene oxide-based
component
of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 4 ¨
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In some embodiments, the hydrogel forming
composition
comprises a second component comprising a protein that is capable of cros
slinking with
the crosslinking agent and one or more solvents able to dissolve the first
component and
the second component, wherein when the first component and the second
component are
dissolved in the one or more solvents, upon mixing of the first component and
the second
component dissolved in the one or more solvents, crosslinking of the
crosslinking agent
and the protein occurs with a gel time less than or equal to 20 seconds to
form the
hydrogel tissue sealant.
According to some embodiments, a hydrogel forming composition for forming a
hydrogel tissue sealant comprises a first component comprising a crosslinking
agent,
wherein the crosslinking agent is a difunctionalized polyalkylene oxide-based
component
of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 5 ¨
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the hydrogel forming
composition
comprises a second component comprising a protein that is capable of cros
slinking with
the crosslinking agent, a first solvent able to dissolve the first component,
and a second
solvent able to dissolve the second component, wherein when the second
component is
dissolved in the second solvent the pH of the solution of the second component
in the
second solvent is greater than or equal to 10.2 and less than or equal to
10.6, and wherein
when the first component is dissolved in the first solvent and combined with
the solution
of the second component in the second solvent a cros slinking solution of the
first
component and the second component is formed.
In certain embodiments, a hydrogel forming composition for forming a hydrogel
tissue sealant comprises a first component comprising a crosslinking agent,
wherein the
cros slinking agent is a difunctionalized polyalkylene oxide-based component
of the
formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 6 ¨
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
.. aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
.. fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. According to certain embodiments, the hydrogel
forming
composition comprise a second component comprising a protein that is capable
of
.. crosslinking with the crosslinking agent, and one or more solvents able to
dissolve the
first component and the second component such that when the first component
and the
second component are separately mixed with the one or more solvents, at least
the
second component is able to have a dissolution time at 25 C of less than or
equal to 30
seconds.
In certain embodiments, a method of forming a hydrogel tissue sealant is
described. In some embodiments, the method comprises dissolving in a first
solvent a
first component, wherein the first component comprises a crosslinking agent,
wherein the
cros slinking agent is a difunctionalized polyalkylene oxide-based component
of the
formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 7 ¨
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
.. an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
.. fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the method comprises
dissolving in a
second solvent a second component, wherein the second component comprises a
protein
.. that is capable of crosslinking with the crosslinking agent, and combining
the dissolved
first component and the dissolved second component to form a hydrogel forming
composition comprising the crosslinking agent, the protein, and a surfactant,
to initiate
crosslinking of the crosslinking agent and the protein, thereby forming the
hydrogel
tissue sealant.
According to certain embodiments, a method of forming a hydrogel tissue
sealant
comprises dissolving in a first solvent a first component, wherein the first
component
comprises a crosslinking agent, wherein the crosslinking agent is a
difunctionalized
polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 8 ¨
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)-
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In some embodiments, the method comprises
dissolving in a
second solvent a second component, wherein the second component comprises a
protein
that is capable of crosslinking with the crosslinking agent, and combining the
dissolved
first component and the dissolved second component to form a hydrogel forming
composition comprising the crosslinking agent and the protein, thereby
initiating
crosslinking of the crosslinking agent and the protein to form the hydrogel
tissue sealant
such that crosslinking is characterized by a gel time less than or equal to 20
seconds.
In some embodiments, a method of forming a hydrogel tissue sealant comprises
dissolving in a first solvent a first component to form a solution of the
first component,
wherein the first component comprises a crosslinking agent, wherein the
crosslinking
agent is a difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 9 ¨
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the method comprises
dissolving in a
second solvent a second component to form a solution of the second component,
wherein
the second component comprises a protein that is capable of cros slinking with
the
cros slinking agent and wherein the solution of the second component has a pH
greater
than or equal to 10.2 and less than or equal to 10.6, and combining the
solution of the
first component and the solution of the second component to form a hydrogel
forming
composition comprising the crosslinking agent and the protein, thereby
initiating
crosslinking of the crosslinking agent and the protein to form the hydrogel
tissue sealant.
According to certain embodiments, a method of forming a hydrogel tissue
sealant
comprises dissolving in a first solvent a first component, wherein the first
component
comprises a crosslinking agent, wherein the crosslinking agent is a
difunctionalized
polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
- 10 ¨
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments. and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In some embodiments, the method comprises
dissolving in a
second solvent a second component, wherein the second component comprises a
protein
that is capable of crosslinking with the crosslinking agent, wherein the
dissolution time
of the second component in the second solvent at 25 C is less than or equal
to 30
seconds, and combining the dissolved first component and the dissolved second
component to form a hydrogel forming composition comprising the crosslinking
agent
and the protein, thereby initiating crosslinking of the crosslinking agent and
the protein
to form the hydrogel tissue sealant.
In some embodiments, a method of forming a hydrogel tissue sealant comprises
forming a hydrogel forming composition comprising a crosslinking agent that is
a
difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
- 11 ¨
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)-
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In some embodiments, the solution comprises a
protein that is
capable of crosslinking with the crosslinking agent, and a surfactant, wherein
the
hydrogel forming composition, upon formation, results in initiation of
crosslinking of the
crosslinking agent and the protein, thereby forming the hydrogel tissue
sealant.
In some embodiments, a method of sealing tissue is described. In certain
embodiments, the method comprises delivering a hydrogel forming composition to
a
tissue site, wherein the hydrogel forming composition comprises a reaction
product of:
a first component comprising a crosslinking agent, wherein the crosslinking
agent
is a difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 12 ¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl; and
a second component comprising a protein that is capable of crosslinking with
the
crosslinking agent. In certain embodiments, the hydrogel forming composition
further
comprises a surfactant.
According to certain embodiments, a method of sealing tissue comprises
delivering a hydrogel forming composition to a tissue site, wherein the
hydrogel forming
composition is a reaction product of:
a solution of a first component comprising a crosslinking agent, wherein the
cros slinking agent is a difunctionalized polyalkylene oxide-based component
of the
formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)-
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 13 ¨
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl; and
a solution of a second component comprising a protein that is capable of
cros slinking with the crosslinking agent, wherein the solution of the second
component
has a pH greater than or equal to 10.2 and less than or equal to 10.6.
According to some embodiments, a method of sealing tissue, comprises
delivering a hydrogel forming composition to a tissue site, wherein the
hydrogel
composition comprises a reaction product of:
a first component comprising a crosslinking agent, wherein the crosslinking
agent
is a difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
.. monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 14 ¨
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl; and
a second component comprising a protein that is capable of crosslinking with
the
crosslinking agent. In certain embodiments, the method comprises forming a
hydrogel
tissue sealant at the tissue site via a crosslinking reaction characterized by
a gel time less
than or equal to 20 seconds.
In certain embodiments, a kit for forming a hydrogel tissue sealant is
described,
wherein the kit comprises a first component contained within a first
container, wherein
the first component comprises a crosslinking agent, wherein the crosslinking
agent is a
difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)-
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the kit comprises a second
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 15 ¨
component contained with a second container, wherein the second component
comprises
a protein that is capable of crosslinking with the crosslinking agent, and a
surfactant.
According to some embodiments, a kit for forming a hydrogel tissue sealant
comprises a first component in powder form contained within a first container,
wherein
the first component comprises a crosslinking agent, wherein the crosslinking
agent is a
difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)-
.. (CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In some embodiments, the kit comprises a second
component
in powder form contained with a second container, wherein the second component
comprises a protein that is capable of crosslinking with the crosslinking
agent, a first
aqueous hydration solution contained within a third container, wherein the
first aqueous
hydration solution is able to dissolve the first component, and a second
aqueous
hydration solution contained with a fourth container, wherein the second
aqueous
hydration solution is able to dissolve the second component.
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 16 ¨
In certain embodiments, a kit for forming a hydrogel tissue sealant comprises
one
or more syringes collectively comprising at least three separate containers,
wherein a
first container comprises a first component in powder form, a second container
comprises a second component in powder form, and at least a third container
comprises
one or more solvents, wherein the one or more syringes are configured such
that the first
container and the second container are able to be placed in fluid
communication with the
at least a third container comprising the one or more solvents to facilitate
mixing of the
first component with the one or more solvents to form a solution of the first
component
and to facilitate mixing of the second component with the one or more solvents
to form a
solution of the second component, and wherein the one or more syringes are
further
configured to mix the solution of the first component and the solution of the
second
component to form a cros slinking solution of the first component and the
second
component able to form the hydrogel tissue sealant, wherein the first
component
comprises an electrophilic biodegradable polymer and the second component
comprises
a nucleophilic biodegradable polymer able to cros slink with the electrophilic
biodegradable polymer.
According to some embodiments, a hydrogel forming composition for forming a
hydrogel tissue sealant comprises a first component comprising a crosslinking
agent
which is a difunctionalized polyalkylene oxide-based component of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
.. formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical
of the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
.. an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 17 ¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl. In certain embodiments, the hydrogel forming
composition
comprises a second component comprising a protein that is capable of cros
slinking with
the crosslinking agent, and one or more solvents, wherein the first component
and the
second component are dissolved in the one or more solvents.
In certain embodiments of the hydrogel forming composition, the
difunctionalized polyalkylene oxide-based component has the formula G-LM-
(OCH2CH2),0-LM-G where n is an integer from 10 to 500, preferably 50 to 200.
In certain embodiments of the hydrogel forming composition the leaving group G
in the difunctionalized polyalkylene oxide-based component is N-
oxysuccinimidyl.
In certain embodiments of the hydrogel forming composition the difunctional
linking moiety LM in the difunctionalized polyalkylene oxide-based component
is
selected from ¨(CH2)b¨C(0)¨ and ¨C(0)¨(CH2),¨C(0)¨, wherein b and c are
both integers from 1 to 10.
In certain embodiments of the hydrogel forming composition the
difunctionalized
polyalkylene oxide-based component is selected from:
0
0
= 0"¨
H-4.) -='\, 'LOCH CH
2 21n
\\11 =
0
and
0
Q 0
N
N ¨0' = k 0
6
0
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 18 ¨
wherein in both formulae n is an integer from 10 to 500, preferably 50 to 200.
In certain embodiments of the hydrogel forming composition the protein is
selected from the group consisting of human serum albumin, recombinant human
serum
albumin, and animal sourced albumin.
In certain embodiments of the hydrogel forming composition the protein is
recombinant human serum albumin.
In certain embodiments of the hydrogel forming composition the composition
further comprises a surfactant dissolved in the one or more solvents.
In certain embodiments of the hydrogel forming composition the surfactant is
selected from a non-functionalized PEG preferably with a weight average
molecular
weight of 1000 g/mol to 40000 g/mol, dextran sulfate, a poloxamer, a
polysorbate, an oil,
a siloxane, a stearate, and/or a glycol.
In certain embodiments of the hydrogel forming composition the one or more
solvents include water in an amount of 50 wt.% to 100 wt.%, preferably 90 wt.%
to 100
wt.%, based on the total amount of solvent.
In certain embodiments of the hydrogel forming composition the
difunctionalized
polyalkylene oxide-based component is selected from:
0 0
, t
0
s\ri ,0 L.H2t=Ha)n---- =
0 , r
= .
0
a and
0
0 0
it ...."-
r
N ...................... 0 s--
õ,
.,,,....õ...,õ,
0
0
wherein in both formulae n is an integer from 10 to 500, preferably 50 to 200;
the protein is recombinant human serum albumin;
the surfactant is a non-functionalized PEG; and
water makes up 90 wt.% or more of the total amount of the one or more
solvents.
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 19 ¨
In certain embodiments of the hydrogel forming composition the composition
further comprises a crosslinking initiator, an antioxidant, and/or a
radiopaque agent.
In certain embodiments of the hydrogel forming composition the composition
comprises a base or basic buffer, preferably a carbonate and/or a bicarbonate.
In certain embodiments of the hydrogel forming composition the composition
comprises an antioxidant, preferably butylated hydroxyanisole, butylated
hydroxytoluene, propyl gallate d-alpha tocopheryl polyethylene glycol-1000
succinate,
or sodium metabisulfite, and/or mixtures thereof.
In certain embodiments of the hydrogel forming composition the composition
comprises a radiopaque agent, preferably gold, silver, iodine, potassium
chloride, barium
sulfate, iohexol, or diatrizoate, and/or mixtures thereof.
In certain embodiments of the hydrogel forming composition a first component
of
the compositionis dissolved in a first solvent.
In certain embodiments of the hydrogel forming composition a second
.. component of the composition is dissolved in a second solvent.
In certain embodiments of the hydrogel forming composition a second
component dissolved in the second solvent has a pH of from 10.2 to 10.6.
In certain embodiments kit for forming a hydrogel tissue sealant, comprising:
a first container containing a first component comprising the crosslinking
agent as
defined in this disclosure; a second container containing a second component
comprising
a protein, preferably a protein selected from the group consisting of human
serum
albumin, recombinant human serum albumin, and animal sourced albumin; and
optionally one or more additional containers containing one or more solvents,
preferably
water, for dissolving the first component and the second component.
In certain embodiments a kit comprises a first container containing a first
component; a second container containing a second component; and a third
container
containing a solvent, preferably water, for dissolving the first component and
the second
component.
In certain embodiments a kit comprises two syringes, wherein a first syringe
.. comprises a first container and a second container; and wherein a second
syringe
comprises a third container; wherein a first component and a second component
contained in the syringe are in powder form; wherein the first syringe and the
second
syringe are configured to be fluidically connectable to each other such that
the first
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 20 ¨
container and the second container are able to be placed in fluid
communication with the
third container to facilitate mixing of the first component and the second
component with
a solvent to form a solution of the first component in the first container and
a solution of
the second component in the second container, and wherein the first syringe is
further
configured to mix the solution of the first component and the solution of the
second
component to form a hydrogel forming composition for forming a hydrogel tissue
sealant. In certain embodiments such kit comprises a first container
containing the first
component; a second container containing the second component; a third
container
containing a solvent, preferably water, for dissolving the first component;
and a fourth
container containing a solvent, preferably water, for dissolving the second
component,
and may further comprises two syringes, wherein a first syringe comprises the
first
container and the second container; and wherein a second syringe comprises the
third
container and the fourth container; wherein the first component and the second
component are in powder form; wherein the first syringe and the second syringe
are
configured to be fluidically connectable to each other such that the first
container and the
second container are able to be placed in fluid communication with the third
container
and the fourth container, respectively, to facilitate mixing of the first
component with the
solvent in the third container to form a solution of the first component in
the first
container and to facilitate mixing of the second component with the solvent in
the fourth
container to form a solution of the second component in the second container,
wherein
the first syringe is further configured to mix the solution of the first
component and the
solution of the second component to form a hydrogel forming composition for
forming a
hydrogel tissue sealant.
In certain embodiments, any hydrogel forming composition described herein,
and/or prepared using any kit described herein is suitable for use in a method
of
treatment by surgery. In certain embodiments, such method of treatment by
surgery
includes delivering the hydrogel forming composition to a tissue site and
forming a
hydrogel tissue sealant at that tissue site. In certain embodiments the
treatment by
surgery is a lung biopsy procedure, and wherein the composition is used to
prevent or
.. reduce the risk of pneumothorax during or after the lung biopsy procedure,
which can be
a procedure wherein any hydrogel forming composition described herein is
delivered to
the pleural space of the patient to form a hydrogel tissue sealant through
which a biopsy
sample is taken.
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 21 ¨
In certain embodiments, a kit for forming a hydrogel tissue sealant comprises
a
first container containing a first component comprising a crosslinking agent,
a second
container containing a second component comprising a protein, preferably a
protein
selected from the group consisting of human serum albumin, recombinant human
serum
albumin, and animal sourced albumin, and optionally one or more additional
containers
containing one or more solvents, preferably water, for dissolving the first
component and
the second component.
According to certain embodiments, the hydrogel forming composition as
described above or as prepared using the kit as described above may be used in
a method
of treatment by surgery.
Other advantages and novel features of the present invention will become
apparent from the following detailed description of various non-limiting
embodiments of
the invention when considered in conjunction with the accompanying figures. In
cases
where the present specification and a document incorporated by reference
include
conflicting and/or inconsistent disclosure, the present specification shall
control.
BRIEF DESCRIPTION OF THE DRAWINGS
Non-limiting embodiments of the present invention will be described by way of
example with reference to the accompanying figures, which are schematic and
are not
intended to be drawn to scale. In the figures, each identical or nearly
identical
component illustrated is typically represented by a single numeral. For
purposes of
clarity, not every component is labeled in every figure, nor is every
component of each
embodiment of the invention shown where illustration is not necessary to allow
those of
ordinary skill in the art to understand the invention. In the figures:
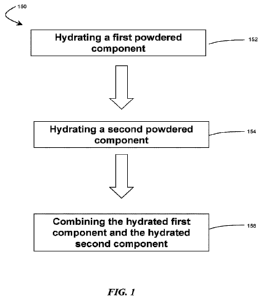
FIG. 1 shows, in accordance with certain embodiments, steps in an exemplary
method for forming a hydrogel tissue sealant;
FIG. 2A shows, in accordance with certain embodiments, a schematic diagram of
a syringe device that is configured for storing and/or mixing one or more
components of
the hydrogel forming composition;
FIG. 2B shows, in accordance with certain embodiments, a schematic diagram of
a syringe device that is configured for delivering a hydrogel forming
composition to a
tissue site;
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 22 ¨
FIG. 3A shows, in accordance with certain embodiments, a cross-sectional
schematic diagram of the syringe device shown in FIG. 2A;
FIG. 3B shows, in accordance with certain embodiments, a cross-sectional
schematic diagram of the syringe device shown in FIG. 2B;
FIG. 4 shows, in accordance with certain embodiments, steps in an exemplary
method for hydrating and delivering a hydrogel forming composition;
FIG. 5A shows, in accordance with certain embodiments, a X-ray image of a
swine lung model;
FIG. 5B shows, in accordance with certain embodiments, a X-ray image of a post
biopsy swine lung model;
FIG. 6A shows, in accordance with certain embodiments, a X-ray image of the
coaxial insertion of a syringe needle to deliver a hydrogel tissue sealant to
a swine lung
model;
FIG. 6B shows, in accordance with certain embodiments, a X-ray image of swine
lung model with a hydrogel tissue sealant;
FIG. 7A shows, in accordance with certain embodiments, an image of a hydrogel
tissue sealant adhered to the parietal pleura of a swine lung model;
FIG. 7B shows, in accordance with certain embodiments, an image of a hydrogel
tissue sealant adhered to the parietal and visceral pleura of swine lung
model;
FIG. 8A shows, in accordance with certain embodiments, a schematic diagram of
the syringe device depicted in FIG. 2B having its coaxial cannula inserted
into the
pleural space of a subject being treated prior to deployment of the hydrogel
forming
composition contained within the syringe;
FIG. 8B shows, in accordance with certain embodiments, a schematic diagram of
the syringe device of FIG. 8A with the plunger depressed for deploying the
hydrogel
forming composition to deliver and form the hydrogel lung sealant in the
pleural space of
the subject being treated;
FIG. 8C shows, in accordance with certain embodiments, a schematic diagram of
a biopsy needle inserted through the coaxial cannula of the syringe device of
FIG. 8B;
FIG. 9A shows, in accordance with certain embodiments, a CT scan of a test
subject (Test Subject 5) three days after deployment of the hydrogel and
subsequent lung
biopsy procedure;
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 23 ¨
FIG. 9B shows, in accordance with certain embodiments, a CT scan of a control
subject (Control Subject 9) immediately after a lung biopsy procedure, showing
an air
embolism;
FIG. 9C shows, in accordance with certain embodiments, a CT scan of a control
subject (Control Subject 10) immediately after a lung biopsy procedure,
showing
pneumothorax; and
FIG. 9D shows, in accordance with certain embodiments, a CT scan of a control
subject (Control Subject 6) two days after a lung biopsy procedure, showing
pneumothorax.
DETAILED DESCRIPTION
Compositions and methods related to hydrogel tissue sealants are generally
described. In certain embodiments, a hydrogel forming composition is provided
in dry
form (e.g. as one or more powder mixtures) and comprises at least a
crosslinking agent
and a protein that is capable of cros slinking with the cros slinking agent. A
solvent (i.e.,
one or more solvents) able to dissolve the crosslinking agent and the protein
can be
provided and used to dissolve the hydrogel forming composition to facilitate
crosslinking. A surfactant that is capable of stabilizing the hydrogel forming
composition, increasing the rate of dissolving the protein in the one or more
solvents,
and/or preventing aggregation of the protein can also be added, either to one
or more
components of the powder mixture or the one or more solvents. While in certain
embodiments all of the ingredients of the composition may be part of a single
dry
mixture (e.g., a powder mixture), in other embodiments that may result in
added stability
and improved shelf life, the composition may be segregated into two or more
reactive
components (e.g. two or more dry powdered mixtures), with at least a first and
a second
component comprising an ingredient that reacts with one or more ingredients of
another
of the components. Preferably, in such embodiments, the ingredients in each of
the
components are not substantially reactive with other ingredients in such
component, so
that reaction can be prevented until the components are dissolved in one or
more suitable
solvents (e.g. hydrated) and combined prior to or during use, enabling them to
react to
form the hydrogel. In instances wherein the ingredients are segregated into
components
that are, with respect to the other ingredients in such component, not
substantially
reactive, the components may be formulated and stored in a hydrated, flowable
form as
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 24 ¨
opposed to a dry, non-hydrated form. In much of the discussion and examples
below,
the composition is provided as two dry powder components prior to hydration
and
mixing of the components to form a crosslinked hydrogel tissue sealant, but as
indicated
above, other dry and flowable formulations are possible.
As one example, crosslinking to form the hydrogel tissue sealant may be
initiated
by combining a first component comprising the crosslinking agent with a second
component comprising the protein. In certain embodiments, the surfactant may
be part
of the second component. The first component may further include, for example,
an
antioxidant (e.g., a first antioxidant), which can be selected to increase the
stability of the
crosslinking agent. The second component may further comprise a second
antioxidant,
which can be selected to increase the stability of the protein. As a result,
the hydrogel
forming composition can have both an increased shelf-life and enhanced storage
capabilities as compared to other conventional hydrogel forming compositions.
For
example, in some embodiments, the hydrogel forming compositions described
herein
may be stored at room temperature for long periods of time (e.g., three years
or more)
without requiring refrigeration. Other advantages of the hydrogel forming
compositions
may include a shorter, tunable gel time, and an increased pot life, both of
which are
further described below in greater detail.
In certain embodiments, a multicomponent (e.g., two component, three
component, four component) composition formulation may be used. In some
embodiments, a first component comprises a difunctionalized polyalkylene oxide
crosslinking agent, and a second component comprises a protein (e.g.,
lyophilized
albumin) that is capable of crosslinking with the difunctionalized
polyalkylene oxide. In
certain embodiments, the second component may also comprise a crosslinking
initiator
(e.g., a base or basic buffer, such as sodium carbonate) that initiates cros
slinking of the
crosslinking agent with the protein. In certain embodiments, as indicated
above, the first
component and the second component may both be provided and stored as powdered
mixtures. The powdered mixtures may be separately or concurrently hydrated
(e.g., with
a solvent, such as water, a biocompatible organic solvent, or an aqueous
solution), then
combined (if separately hydrated), to form the hydrogel tissue sealant. In
certain
embodiments, the hydrating solution that hydrates the first component (and/or
second
component) may further comprise a radiopaque agent that permits the hydrogel
tissue
sealant to be, for example, spectroscopically visible. The hydrating solution
that
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 25 ¨
hydrates the second component (and/or first component) may comprise an anti-
foaming
additive, such as a poloxamer. In certain embodiments, the anti-foaming
additive may
assist in refolding of the protein upon hydration.
The hydrogel forming composition may be used to bond or seal tissue in vivo.
In
certain non-limiting embodiments, for example, it may be particularly useful
to use the
hydrogel forming composition as a pleural lung sealant to seal off air or
fluid from
entering the pleural space. In some such embodiments, the hydrogel lung
sealant may
advantageously decrease the occurrence of complications during and/or
following the
lung biopsy procedure, such as, for example, pneumothorax. In certain
embodiments, in
addition to, or instead of, use as a hydrogel tissue sealant, compositions and
methods
described herein may be useful for a variety of other medical applications,
such as a
postsurgical adhesion barrier or a wound dressing material.
As used herein, the term "crosslink" refers to a chemical reaction between two
or
more similar or dissimilar polymers, copolymers, oligomers, and/or macromers
that links
the two or more similar or dissimilar polymers, copolymers, oligomers, or
macromers via
formation of at least one covalent bond and/or ionic bond, or a chain
extension between
one or more polymers, copolymers, oligomers, and/or macromers to provide a
longer
chain of the one or more polymers, copolymers, oligomers, and/or macromers via
formation of at least one covalent bond and/or ionic bond.
Electrophilic Crosslinking Agents
According to certain embodiments, the hydrogel forming composition comprises
an electrophilic biodegradable polymer. In certain embodiments, the
electrophilic
biodegradable polymer may be a synthetic or naturally occurring polymer that
contains
or is functionalized to contain one or more, and preferably two or more,
reactive
electrophilic groups. Many suitable electrophilic biodegradable polymers are
known to
those of ordinary skill in the art. In some embodiments, for example, a
particularly
advantageous and preferred crosslinking agent of the hydrogel forming
composition
comprises a difunctionalized polyalkylene oxide. In certain embodiments, the
difunctionalized polyalkylene oxide has a composition described by the
formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 26 ¨
each LM is a difunctional linking moiety independently selected from the group
consisting of a carbonate diradical of the formula ¨C(0)¨, a monoester
diradical of the
formula ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 10, a diester radical of
the
formula ¨C(0)¨(CH2),¨C(0)¨ where c is an integer from 1 to 10 and where the
aliphatic portion of the radical may be saturated or unsaturated, a
dicarbonate diradical of
the formula ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is an integer from 1 to 10, an
amide containing diradical of the formula ¨N(H)¨C(0)¨(CH2)d¨C(0)¨ where d is
an integer from 1 to 10, an amide containing diradical of the formula
¨(CH2),¨C(0) ¨
N(H)¨(CH2)d¨ where c is an integer from 1 to 10 and d is an integer from 1 to
10, and
an oligomeric diradical represented by the formulas ¨R¨C(0)¨, ¨R¨C(0)¨
(CH2),¨C(0)¨, ¨R¨C(0)-0¨(CH2)d¨O¨C(0)¨, ¨R¨N(H)¨C(0)¨
(CH2)d¨C(0)¨, or ¨R¨(CH2),¨C(0) ¨N(H)¨(CH2)d¨ where c is an integer from
1 to 10, d is an integer from 1 to 10, and R is a polymer or copolymer having
1 to 10
monomeric lactide, glycolide, trimethylene carbonate, caprolactone or p-
dioxanone
fragments; and
each G is a leaving group independently selected from the group consisting of
N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl.
According to certain embodiments, the crosslinking agent is a difunctionalized
polyalkylene oxide of the formula:
G-LM-PEG-LM-G;
wherein:
PEG is polyethylene glycol;
each LM is the same and is a difunctional linking moiety represented by the
formulas ¨C(0)¨, ¨(CH2)b¨C(0)¨ where b is an integer from 1 to 5, ¨C(0)¨
(CH2),¨C(0)¨ where c is an integer from 2 to 10 and where the aliphatic
portion of the
radical may be saturated or unsaturated, ¨C(0)-0¨(CH2)d¨O¨C(0)¨ where d is
an integer from 2 to 10, and an oligomeric diradical represented by the
formulas ¨R¨
C(0)¨, ¨R¨C(0)¨(CH2),¨C(0)¨, or ¨R¨C(0)-0¨(CH2)d¨O¨C(0)-
where c is an integer from 2 to 10, d is an integer from 2 to 10, and R is a
polymer or
copolymer having 1 to 10 monomeric lactide, glycolide, trimethylene carbonate,
caprolactone or p-dioxanone fragments; and
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨27 ¨
each G is the same and is a leaving group selected from the group of N-
oxysuccinimidyl, N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-
oxyimidazolyl, and tresyl.
According to some embodiments, the hydrogel forming composition comprises
.. any of a variety of suitable crosslinking agents (e.g., difunctionalized
polyalkylene
oxides). In some embodiments, the crosslinking agent is or includes:
a 2-arm PEG disuccinimidyl succinate (PEG(SS)2) of the form:
a
0
1,..4'"--Thrks.,-11,40C142042)R--0
0 0 0
In certain embodiments, the crosslinking agent is or includes:
a 2-arm PEG carboxymethyl ester of the form:
0
0
0
N-0
0
0
0
According to certain embodiments, the crosslinking agent (e.g., a
difunctionalized polyalkylene oxide) of the formula G-LM-PEG-LM-G may have any
of
a variety of suitable weight average molecular weights. For example, in
certain
embodiments, the degree of ethoxylation in PEG (and the value for n in the
formulae
above) is such that the crosslinking agent may have a weight average molecular
weight
of greater than or equal to 1 kDa, greater than or equal to 2 kDa, greater
than or equal to
3 kDa, greater than or equal to 4 kDa, greater than or equal to 5 kDa, greater
than or
equal to 10 kDa, or greater than or equal to 15 kDa. In certain embodiments,
the
crosslinking agent may have a weight average molecular weight of less than or
equal to
20 kDa, less than or equal to 15 kDa, less than or equal to 10 kDa, less than
or equal to 5
kDa, less than or equal to 4 kDa, less than or equal to 3 kDa, or less than or
equal to 2
kDa. Combinations of the above recited ranges are also possible (e.g., the
crosslinking
agent may have a weight average molecular weight of greater than or equal to 1
kDa and
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 28 ¨
less than or equal to 20 kDa, the crosslinking agent may have a weight average
molecular weight of greater than or equal to 3 kDa and less than or equal to 5
kDa).
Other ranges are also possible. In certain embodiments, for the formulae of
the 2-arm
PEG disuccinimidyl succinate and the 2-arm PEG carboxymethyl ester shown
above, n is
in the range of 10 to 500, more preferably 50 to 200. In some embodiments, the
weight
average molecular weight of the crosslinking agent is determined using size
exclusion
chromatography-multi-angle laser light scattering (SEC-MALLS).
According to certain embodiments, difunctionalized polyalkylene oxide
crosslinking agents describable by the formula G-LM-PEG-LM-G, such as but not
limited to the examples noted above, may be prepared by any of a variety
suitable
synthetic methods known to those skilled in the art. See, for example U.S.
Patent
6,576,263, U.S. Patent RE38,827, and U.S. Patent RE38,158, each of which are
incorporated herein by reference in its entirety.
In some embodiments, difunctionalized polyalkylene oxides describable by the
formula G-LM-PEG-LM-G may be prepared using known processes, procedures, or
synthetic methods such as the procedures reported in U.S. Patent 4,101,380 or
U.S.
Patent 4,839,345, the procedure reported in International Application Ser. No.
PCT/U590/02133 filed Apr. 19, 1990, or the procedure reported by Abuchowski et
al.,
Cancer Biochem. Biophys., 7:175-186 (1984), each of which are incorporated
herein by
reference in its entirety. Briefly, in certain embodiments, a polyalkylene
oxide-based
compound (e.g., polyethylene glycol discussed below as exemplary) and a
suitable acid
anhydride are dissolved in a suitable polar organic solvent in the presence of
base and
refluxed for a period of time sufficient to form a polyethylene glycol diester
diacid. The
diester diacid is then reacted with a leaving group, such as a N-hydroxy imide
compound, in a suitable polar organic solvent in the presence of
dicyclohexylcarbodiimide or another condensing agent, and stirred at room
temperature
to form the desired difunctional crosslinking agent.
All or some of the difunctionalized polyalkylene oxide-based compounds
describable by the formula G-LM-PEG-LM-G may be purchased from commercial
sources, including, but not limited to, NOF America Corporation, Laysan Bio,
Inc,
Sigma-Aldrich, and/or JenKem Technology USA. The difunctionalized polyalkylene
oxide-based compounds may also be readily synthetized by persons of ordinary
skill in
the chemical synthesis art in view of the teaching and exemplary methods
described
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 29 ¨
herein for exemplary compositions, published literature, and the level of
ordinary skill
and knowledge of the skilled artisan.
In certain non-limiting embodiments, PEG(SS)2 can be synthesized by obtaining
a linear PEG with an average weight average molecular weight of 3,350 Da,
representing
75.7 oxyethylene repeat units. The linear PEG can be obtained, for example,
from Dow
Chemical Company. In some embodiments, the linear PEG may be converted to
PEG(SS)2 via a two-step synthesis. For instance, in some examples, the first
step may
comprise reacting the linear PEG with two equivalents of succinic anhydride to
form an
ester. The second step may comprise reacting the ester with two equivalents of
the N-
.. hydroxysuccinimide (NHS) to produce the crosslinking agent PEG(SS)2,
resulting in a
white solid of a 2-arm crosslinking agent that possesses two succinimidyl
groups per
molecule.
In certain embodiments, difunctionalized polyalkylene oxide-based compounds
of the formula G-LM-PEG-LM-G comprise a leaving group G (e.g., N-
oxysuccinimidyl,
N-oxymaleimidyl, N-oxyphthalimidyl, nitrophenoxyl, N-oxyimidazolyl, and
tresyl). In
such embodiments, leaving group G is an electrophilic leaving group that is
capable of
reacting with a nucleophilic group, for example an amine group of a protein.
According
to certain embodiments, the leaving group G reacts with an amine group of the
nucleophile (e.g., protein) to produce a crosslinked composition by formation
of amide
bonds upon release of the leaving group G. Such reactivity is further
described in U.S.
Patent Number 6,458,147, which is incorporated herein by reference in its
entirety.
According to certain embodiments, the purity of the difunctionalized
polyalkylene oxide crosslinking agent may be determined by its percent
difunctionality.
A high percentage of difunctionality may advantageously result in a higher
degree and/or
rate of crosslinking to provide the a hydrogel formed from the hydrogel
forming
composition with enhanced performance characteristics, such as a fast gel
time, a longer
pot life, and/or a longer shelf life, and/or improved mechanical properties or
resorption
time, each of which are explained below in greater detail. In some
embodiments, the
difunctionalized polyalkylene oxide crosslinking agent has a percent
difunctionality
greater than or equal to 75 wt.%, greater than or equal to 80 wt.%, greater
than or equal
to 85 wt.%, greater than or equal to 90 wt.%, greater than or equal to 95
wt.%, or greater
than or equal to 99 wt.%. In certain embodiments, the difunctionalized
polyalkylene
oxide crosslinking agent has a percent difunctionality between 70 wt.% and
99.9 wt.%,
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 30 ¨
or between 90 wt.% and 95%. Other ranges are also possible. The percent
difunctionality of the difunctionalized polyalkylene oxide crosslinking agent
as used
herein is determined by high-performance liquid chromatography (HPLC).
In a powdered form, the difunctionalized polyalkylene oxide crosslinking agent
may have a relatively low weight percent moisture content. A low weight
percent
moisture content for the powdered difunctionalized polyalkylene oxide
crosslinking
agent can advantageously provide a hydrogel forming composition with an
improved
shelf life, as hydrolysis of the difunctionalized polyalkylene oxide is
reduced, therefore
preserving reactivity of the crosslinking agent over storage time. In certain
embodiments, for example, the weight percent moisture content of the powdered
difunctionalized polyalkylene oxide crosslinking agent may be less than or
equal to 10
wt.%, less than or equal to 9 wt.%, less than or equal to 8 wt.%, less than or
equal to 7
wt.%, less than or equal to 6 wt.%, less than or equal to 5 wt.%, less than or
equal to 4
wt.%, less than or equal to 3 wt.%, or less than or equal to 2 wt.% based on
the total
weight of the powdered difunctionalized polyalkylene oxide cros slinking
agent. In
certain embodiments, the weight percent moisture content of the powdered
crosslinking
agent may be between 1 wt.% and 10 wt.% based on the total weight of the
powdered
difunctionalized polyalkylene oxide crosslinking agent, or between 4 wt.% and
6 wt.%
based on the total weight of the powdered difunctionalized polyalkylene oxide
crosslinking agent. Other ranges are also possible. The weight percent
moisture content
as stated herein is determined using a moisture analyzer and/or a Karl-Fischer
titration.
According to certain embodiments, the hydrogel forming composition comprises
the powdered crosslinking agent in any of a variety of suitable amounts in
weight percent
(wt.%) by mass versus the total weight of the powdered hydrogel forming
composition.
For example, in some embodiments, the hydrogel forming composition comprises
the
crosslinking agent in an amount, on a powdered basis, greater than or equal to
10 wt.%,
greater than or equal to 15 wt.%, greater than or equal to 20 wt.%, greater
than or equal
to 25 wt.%, greater than or equal to 30 wt.%, greater than or equal to 35
wt.%, greater
than or equal to 40 wt.%, greater than or equal to 45 wt.%, greater than or
equal to 50
wt.%, or greater than or equal to 55 wt.% of the total weight of the powdered
hydrogel
forming composition. In certain embodiments, the hydrogel forming composition
comprises the crosslinking agent, on a powdered basis, in an amount less than
or equal to
60 wt.%, less than or equal to 55 wt.%, less than or equal to 50 wt.%, less
than or equal
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 31 ¨
to 45 wt.%, less than or equal to 40 wt.%, less than or equal to 35 wt.%, less
than or
equal to 30 wt.%, less than or equal to 25 wt.%, less than or equal to 20
wt.%, or less
than or equal to 15 wt.% of the total weight of the powdered hydrogel forming
composition. Combinations of the above recited ranges are also possible (e.g.,
the
hydrogel forming composition comprises the crosslinking agent in an amount, on
a
powdered basis, greater than or equal to 10 wt.% and less than or equal to 60
wt.% of the
total weight of the powdered hydrogel forming composition, the hydrogel
forming
composition comprises the crosslinking agent in an amount, on a powdered
basis, greater
than or equal to 25 wt.% and less than or equal to 30 wt.% of the total weight
of the
powdered hydrogel forming composition). Other ranges are also possible.
Nucleophilic Biodegradable Polymers, such as Proteins
In certain embodiments, the hydrogel forming composition comprises a
nucleophilic biodegradable polymer that is able to crosslink with the
electrophilic
biodegradable polymer. In certain embodiments, the nucleophilic biodegradable
polymer may be a synthetic or naturally occurring polymer that contains or is
functionalized to contain one or more, and preferably two or more, reactive
nucleophilic
groups. Many suitable nucleophilic biodegradable polymers are known to those
of
ordinary skill in the art. In some embodiments, for example, a particularly
advantageous
and preferred nucleophilic biodegradable polymer is a protein. According to
some
.. embodiments, for example, the hydrogel forming composition comprises a
protein that is
capable of crosslinking with the above described electrophilic crosslinking
agents (e.g.,
PEG(SS)2). In certain embodiments, the protein comprises serum albumin (SA).
The
serum albumin may be, in some embodiments, human serum albumin (HSA) derived
from donor blood, recombinant human serum albumin (rHSA) expressed in yeast
and/or
rice, and/or animal sourced albumin, such as, for example, bovine serum
albumin (BSA).
According to certain embodiments, the protein (e.g., rHSA) may be lyophilized.
Lyophilization of the protein may advantageously impede the degradation of the
protein
and improve shelf life and/or dissolution time when dissolved in an aqueous
solvent, as
described below, according to some embodiments.
In certain non-limiting embodiments, the protein may be Cohn analog culture
grade BSA obtained from Proliant Biologicals. In some embodiments, the
recombinant
human serum albumin may be Cellastim recombinant human serum albumin,
Healthgen
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 32 ¨
recombinant human serum albumin, Optibumin recombinant human serum albumin,
InVitria human serum albumin, or Albumedix human serum albumin.
In certain embodiments, the protein may be or include collagen or gelatin.
Other
proteins are also possible.
According to certain embodiments, the purity and/or amount of protein
aggregation may be determined by the percent of the amount of monomer of the
protein
in the protein source. In certain embodiments, for example, the protein may
comprise
greater than or equal to 60 wt.% protein monomer, greater than or equal to 65
wt.%
protein monomer, greater than or equal to 70 wt.% protein monomer, greater
than or
equal to 75 wt.% protein monomer, greater than or equal to 80 wt.% protein
monomer,
greater than or equal to 85 wt.% protein monomer, greater than or equal to 90
wt.%
protein monomer, or greater than or equal to 95 wt.% protein monomer. In
certain
embodiments, the protein comprises less than or equal to 99 wt.% protein
monomer, less
than or equal to 95 wt.% protein monomer, less than or equal to 90 wt.%
protein
monomer, less than or equal to 85 wt.% protein monomer, less than or equal to
80 wt.%
protein monomer, less than or equal to 75 wt.% protein monomer, less than or
equal to
70% protein monomer, less than or equal to 70 wt.% protein monomer, or less
than or
equal to 65 wt.% protein monomer. Combinations of the above recited ranges are
also
possible (e.g., the protein comprises greater than or equal to 60 wt.% protein
monomer
and less than or equal to 99 wt.% protein monomer, the protein comprises
greater than or
equal to 90 wt.% protein monomer and less than or equal to 95 wt.% protein
monomer).
Other ranges are also possible. As is explained in further detail below,
certain
components of the hydrogel forming composition (e.g., the surfactant and/or
anti-
foaming agent) may act to prevent aggregation and/or the formation of protein
dimers or
higher order multimeric structures.
The powdered protein may have a relatively low weight percent moisture
content.
In certain embodiments, for example, the weight percent moisture content of
the
powdered protein component may be greater than or equal to 1 wt.%, greater
than or
equal to 2 wt.%, greater than or equal to 3 wt.%, greater than or equal to 4
wt.%, greater
than or equal to 5 wt.%, greater than or equal to 6 wt.%, greater than or 7
wt.%, greater
than or equal to 8 wt.%, or greater than or equal to 9 wt.% versus the total
weight of the
powdered protein component. In some embodiments, the weight percent of
moisture
content of the powdered protein may be less than or equal to 10 wt.%, less
than or equal
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 33 ¨
to 9 wt.%, less than or equal to 8 wt.%, less than or equal to 7 wt.%, less
than or equal to
6 wt.%, less than or equal to 5 wt.%, less than or equal to 4 wt.%, less than
or equal to 3
wt.%, or less than or equal to 2 wt.% versus the total weight of the powdered
protein
component. Combinations of the above recited ranges are also possible (e.g.,
the percent
moisture content of the powdered protein may be between greater than or equal
to 1
wt.% and less than or equal to 10 wt.% versus the total weight of the powdered
protein
component, the percent moisture content of the powdered protein may be between
greater than or equal to 4 wt.% and less than or equal to 6 wt.% versus the
total weight of
the powdered protein component). Other ranges are also possible. As explained
herein,
the weight percent of moisture content is determined using a moisture analyzer
and/or a
Karl-Fischer titration.
According to certain embodiments, an overall powdered hydrogel forming
composition comprises the protein (e.g., albumin) in any of a variety of
suitable amounts
in weight percent (wt.%) by mass versus the total weight of the powdered
hydrogel
forming composition (i.e., based on the combined weight of both the protein
containing
powdered component and the electrophilic polymer containing crosslinking agent
powdered component). For example, in certain embodiments, the hydrogel forming
composition comprises the protein in an amount, on a powdered basis, greater
than or
equal to 40 wt.%, greater than or equal to 45 wt.%, greater than or equal to
50 wt.%,
greater than or equal to 55 wt.%, greater than or equal to 60 wt.%, greater
than or equal
to 65 wt.%, greater than or equal to 70 wt.%, or greater than or equal to 75
wt.% of the
total weight of the powdered hydrogel forming composition. In certain
embodiments,
the hydrogel forming composition comprises the protein, on a powdered basis,
in an
amount of less than or equal to 80 wt.%, less than or equal to 75 wt.%, less
than or equal
to 70 wt.%, less than or equal to 65 wt.%, less than or equal to 60 wt.%, less
than or
equal to 55 wt.%, less than or equal to 50 wt.%, or less than or equal to 45
wt.% of the
total weight of the powdered hydrogel forming composition. Combinations of the
above
recited ranges are also possible (e.g., the hydrogel forming composition
comprises the
protein in an amount, on a powdered basis, greater than or equal to 40 wt.%
and less than
or equal to 80 wt.% of the total weight of the powdered hydrogel forming
composition,
the hydrogel forming composition comprises the protein in an amount, on a
powdered
basis, greater than or equal to 55 wt.% and less than or equal to 65 wt.% of
the total
weight of the powdered hydrogel forming composition). Other ranges are also
possible.
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 34 ¨
The ratio of the leaving group G (e.g., NHS) in the difunctionalized
polyalkylene
oxide-based compound of the formula G-LM-PEG-LM-G to the number of amine
groups
(e.g., of the protein) can be any of variety of suitable ratios. In certain
embodiments, for
example, the ratio of the leaving group G to the amine groups is greater than
or equal to
0.5:1, greater than or equal to 1:1, greater than or equal to 1.5:1, greater
than or equal to
2:1, or greater than or equal to 2.5:1. In some embodiments, the ratio of the
leaving
group G to the amine groups is less than or equal to 3:1, less than or equal
to 2.5:1, less
than or equal to 2:1, less than or equal to 1.5:1, or less than or equal to
1:1.
Combinations of the above recited ranges are also possible (e.g., the ratio of
the leaving
group G to the amine groups is greater than or equal to 0.5:1 and less than or
equal to
3:1, the ratio of the leaving group G to the amine groups is greater than or
equal to 2:1
and less than or equal to 2.5:1). Other ranges are also possible.
In certain non-limiting embodiments, the crosslinking agent is PEG(SS)2 and
the
protein is rHSA, and the ratio of NHS groups of PEG(SS)2 to amine groups of
rHSA is
2.21:1. In other non-limiting embodiments, the cros slinking agent is PEG(SS)2
and the
protein is rHSA, and the ratio of NHS groups of PEG(SS)2 to amine groups of
rHSA is
2.65:1.
Crosslinking Initiators
In some embodiments, the crosslinking reactions that occur between the
electrophilic crosslinking agent and the nucleophile (e.g., protein) are pH
sensitive. In
certain such embodiments, for example, the crosslinking reactions are
inhibited at acidic
pH and can be initiated and sustained by increasing the pH to neutral or basic
values. In
some embodiments, the hydrogel forming composition comprises a crosslinking
initiator
that initiates crosslinking of the crosslinking agent with the nucleophile
(e.g., protein).
In certain embodiments, the crosslinking initiator may be combined as a powder
mixture
with the protein. In some such embodiments, the crosslinking initiator may be
lyophilized with the protein.
In certain embodiments, the crosslinking initiator may be, in some
embodiments,
a base or a basic buffer. In certain embodiments, for example, the
crosslinking initiator
comprises a base and/or basic buffer that facilitates the reaction between the
leaving
group G in a difunctionalized polyalkylene oxide-based compound of the formula
G-
LM-PEG-LM-G and the amine group of a protein. Any of a variety of suitable
bases or
basic buffers may be utilized. In certain embodiments in which the
nucleophilic
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 35 ¨
compound comprises amine groups that react with the crosslinking agent, the
basic
crosslinking initiator is a base and/or basic buffer that does not include
amine
functionalities. In some embodiments, the base comprises a carbonate and/or a
bicarbonate (e.g., a carbonate and/or a bicarbonate salt). For example, in
certain
embodiments, the base or basic buffer comprises sodium carbonate. In some
embodiments, the base or basic buffer comprises sodium bicarbonate. Other
bases or
basic buffers are possible.
The crosslinking reaction between the leaving group G and the amine group of
the nucleophile (e.g., protein) may occur at any of a variety of suitable pH
values. In
some embodiments, the crosslinking reaction is favored at high pH values. In
certain
embodiments, for example, the crosslinking reaction between the leaving group
G and
the amine group of the nucleophile (e.g., protein) is initiated and occurs at
a pH greater
than or equal to7, greater than or equal to 8, greater than or equal to 9,
greater than or
equal to 10, or greater than or equal to 11. In certain embodiments, the cros
slinking
reaction between the leaving group G and the amine group of the nucleophile is
initiated
and occurs at a pH less than or equal to 12, less than or equal to 11, less
than or equal to
10, or less than or equal to 9. Combinations of the above recited ranges are
also possible
(e.g., the crosslinking reaction between the leaving group G and the amine
group of the
nucleophile is initiated and occurs at a pH between greater than or equal to 7
and less
than or equal to 11, the crosslinking reaction between the leaving group G and
the amine
group of the nucleophile is initiated and occurs at a pH between greater than
or equal to 8
and less than or equal to 11, the crosslinking reaction between the leaving
group G and
the amine group of the nucleophile is initiated and occurs at a pH between
greater than or
equal to 9 and less than or equal to 11, or the crosslinking reaction between
the leaving
group G and the amine group of the nucleophile is initiated and occurs at a pH
between
greater than or equal to 10 and less than or equal to 11. Other ranges are
also possible.
In certain non-limiting embodiments, the crosslinking reaction between the
leaving group G and the amine group of the nucleophile (e.g. protein) can be
initiated to
occur at a pH suitable for facilitating reaction by combining a solution of
the
cros slinking agent with a solution of the nucleophile, wherein the pH of the
solution of
the nucleophile is between greater than or equal to 10.2 and less than or
equal to 10.6.
The hydrogel forming composition may comprise the powdered crosslinking
initiator (e.g., base or basic buffer) in any of a variety of suitable amounts
in weight
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 36 ¨
percent (wt.%) by mass based on the total weight of the powdered hydrogel
forming
composition. The amount of the base or basic buffer may affect the reactivity
of the
hydrogel forming composition, such as the gel time (described below), or other
measure
of the time it takes for the crosslinking agent to crosslink with the
nucleophile (e.g.,
protein). Accordingly, in certain embodiments, it may be advantageous to
select the type
and/or amount of base or basic buffer in order to facilitate a crosslinking
rate and/or
degree enabling the hydrogel to crosslink and form prior to or upon delivery
of the
hydrogel forming composition to a tissue site so as to effectively seal
tissue.
In certain embodiments, the hydrogel forming composition comprises the
crosslinking initiator in an amount, on a powdered basis, greater than or
equal to 0.1
wt.%, greater than or equal to 0.2 wt.%, greater than or equal to 0.5 wt.%,
greater than or
equal to 1 wt.%, greater than or equal to 1.5 wt.%, greater than or equal to 2
wt.%,
greater than or equal to 2.5 wt.%, greater than or equal to 3 wt.%, greater
than or equal to
4 wt.%, or greater than or equal to 5 wt.%, greater than or equal to 6 wt.%,
greater than
or equal to 7 wt.%, greater than or equal to 8 wt.%, or greater than or equal
to 9 wt.% of
the total weight of the powdered hydrogel forming composition. In certain
embodiments, the hydrogel forming composition comprises the crosslinking
initiator in
an amount, on a powdered basis, less than or equal to 10 wt.%, less than or
equal to 9
wt.%, less than or equal to 8 wt.%, less than or equal to 6 wt.%, less than or
equal to 5
wt.%, less than or equal to 4 wt.%, less than or equal to 3 wt.%, less than or
equal to 2
wt.%, less than or equal to 1.5 wt.%, less than or equal to 1 wt.%, less than
or equal to
0.5 wt.%, or less than or equal to 0.2 wt.%, or less than or equal to 5 wt.%
of the total
weight of the powdered hydrogel forming composition. Combinations of the above
recited ranges are also possible (e.g., the hydrogel forming composition
comprises the
crosslinking initiator in an amount, on a powdered basis, greater than or
equal to 0.1
wt.% and less than or equal to 10 wt.% of the total weight of the powdered
hydrogel
forming composition or greater than or equal to 0.1 wt.% and less than or
equal to 5
wt.% of the total weight of the powdered hydrogel forming composition, the
hydrogel
forming composition comprises the crosslinking initiator in an amount, on a
powdered
basis, greater than or equal to 4 wt.% and less than or equal to 8 wt.% of the
total weight
of the composition). Other ranges are also possible.
According to certain embodiments, the crosslinking initiator may be
compartmentalized to be part of the second component. For example, in some
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 37 ¨
embodiments, the crosslinking initiator may be combined with the protein
(e.g., albumin)
as a powder mixture. In some such embodiments, the crosslinking initiator and
the
protein may be lyophilized. In some alternative embodiments, the crosslinking
initiator
may be dissolved in a solvent (e.g., water) and used as the hydration solution
to hydrate
the second component (e.g., the protein).
Surfactants
In certain embodiments, the hydrogel forming composition comprises a
surfactant. In some embodiments, the surfactant is capable of stabilizing one
or more
components (e.g., the first component, the second component) of the hydrogel
forming
composition. In certain embodiments, the surfactant is capable of increasing
the rate of
dissolution of the protein (e.g., albumin) in one or more solvents used to
dissolve the
protein. In some embodiments, the surfactant may be selected to prevent
aggregation
(e.g., clumping) of the protein (e.g., albumin).
Any of a variety of suitable surfactants may be utilized. In some embodiments,
for example, the surfactant comprises a non-functionalized polyethylene glycol
(PEG).
Any of a variety of non-functionalized PEGs may be utilized. In certain
embodiments,
the non-functionalized PEG will be a solid at room temperature. In certain
embodiments, the non-functionalized PEG will have a weight average molecular
weight,
for example, greater than or equal to 100 g/mol and less than or equal to
40,000 g/mol.
In certain embodiments, the non-functionalized PEG is PEG 8000 (e.g., a PEG
with a
molecular weight of 8000 g/mol). In certain embodiments, the surfactant
comprises
dextran sulfate. In some embodiments, the surfactant may comprise a poloxamer,
a
polysorbate (e.g., TWEENC)), or encompass a purely lipophilic material such as
an oil
(e.g., mineral oil, vegetable oil), a siloxane, a stearate, a glycol, and/or
mixtures thereof.
In certain embodiments, for example, the poloxamer is Pluronic L61. Other
Pluronic
poloxamers may be also utilized.
In some embodiments, the surfactant may additionally function as an anti-
foaming additive (although not all anti-foaming additives need to be
surfactants). The
anti-foaming additive may advantageously prevent foaming and/or air bubbles
from
forming when the hydrogel forming composition is hydrated to facilitate
crosslinking. In
some embodiments, for example, the anti-foaming additive prevents the
formation of air
bubbles that would otherwise be present in the hydrated hydrogel forming
composition if
an anti-foaming additive were not present. Such air bubbles may disrupt
crosslinking
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 38 ¨
and weaken the resulting hydrogel network of the tissue sealant. In certain
non-limiting
embodiments, a poloxamer (e.g., Pluronic L61) is an anti-foaming additive.
According to certain embodiments, the hydrogel forming composition comprises
the powdered or liquid surfactant in any of a variety of amounts in weight
percent (wt.%)
by mass versus the total weight of the powdered or aqueous solution of the
powdered
hydrogel forming composition. In some embodiments, for example, the hydrogel
forming composition comprises the surfactant in an amount, on a powdered or
liquid
basis, greater than or equal to 0.01 wt.%, greater than or equal to 0.1 wt.%,
greater than
or equal to 0.5 wt.%, greater than or equal to 1 wt.%, greater than or equal
to 5 wt.%,
greater than or equal to 10 wt.%, or greater than or equal to 15 wt.% of the
total weight
of the powdered or aqueous hydrogel forming composition. In certain
embodiments, the
hydrogel forming composition comprises the surfactant, on a powdered or liquid
basis, in
an amount less than or equal to 20 wt.%, less than or equal to 15 wt.%, less
than or equal
to 10 wt.%, less than or equal to 5 wt.%, less than or equal to 1 wt.%, less
than or equal
to 0.5 wt.%, less than or equal to 0.1 wt.%, less than or equal to 0.01 wt.%
of the total
weight of the powdered or aqueous hydrogel forming composition. Combinations
of the
above recited ranges are also possible (e.g., the hydrogel forming composition
comprises
the surfactant in an amount, on a powdered or liquid basis, greater than or
equal to 1
wt.% and less than or equal to 30 wt.% of the total weight of the powdered or
aqueous
hydrogel forming composition, the hydrogel forming composition comprises the
surfactant in an amount, on a powdered or liquid basis, greater than or equal
to 10 wt.%
and less than or equal to 20 wt.% of the total weight of the powdered or
aqueous solution
of the powdered hydrogel forming composition). Other ranges are also possible.
In some embodiments, the surfactant may be a part of the second component.
For example, in certain embodiments, the surfactant may be combined with the
protein
(and the crosslinking initiator, in some embodiments) as a powder mixture. In
some
such embodiments, the protein, crosslinking initiator, and surfactant may be
lyophilized
(e.g., prior to dissolution in the solvent). Without wishing to be bound by
theory, in
some embodiments wherein a liquid surfactant is utilized (e.g., Pluronic
L61), the
.. liquid surfactant may hydrogen bond with one or more powder components of
the second
component (e.g., the protein). In some embodiments, the surfactant may be
dissolved in
and/or mixed with a solvent (e.g., water) that is used as a hydration solution
to hydrate
the second component to form a hydrated solution that is capable of
crosslinking with the
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 39 ¨
first component when mixed with a solution of the first component. In certain
embodiments, for example, the surfactant may be dispersed and/or suspended in
a
solvent used to hydrate the second component.
According to certain embodiments, the hydrogel forming composition may
comprise more than one surfactant (e.g., two surfactants, three surfactants,
etc.), with
each individual surfactant, or the cumulative amount of all the surfactants
together
falling in any of the weight percent ranges listed above.
Antioxidants
According to certain embodiments, the hydrogel forming composition may
comprise at least one antioxidant. An antioxidant may advantageously increase
the
storage stability of one or more components of the hydrogel forming
composition. For
example, the use of one or more antioxidants may increase the shelf-life
and/or storage
capabilities of the hydrogel forming composition. Because the one or more
antioxidants
are more susceptible to oxidation than the crosslinking reagents for forming
the hydrogel
(e.g., due to a lower oxidation potential), the antioxidants become oxidized
during
storage prior to the crosslinking reagents, resulting in a hydrogel forming
composition
that has a longer shelf-life than a hydrogel forming composition that is
otherwise
comparable but does not have the one or more antioxidants.
Any of a variety of suitable antioxidants may be utilized. In certain
embodiments, for example, the composition comprises butylated hydroxytoluene
(BHT).
In some such embodiments, BHT prevents free radical-mediated oxidation. In
certain
embodiments, BHT may be utilized to prevent oxidation of the difunctionalized
polyalkylene oxide-based crosslinking agent. In some embodiments, the
composition
comprises N-acetyl-DL-tryptophan. In some such embodiments, N-acetyl-DL-
tryptophan prevents oxidation of one or more amino acids and/or other residues
of the
protein. In certain embodiments, the antioxidant is or comprises butylated
hydroxyanisole, butylated hydroxytoluene, propyl gallate d-alpha tocopheryl
polyethylene glycol-1000 succinate, sodium metabisulfite, and/or mixtures
thereof.
In some embodiments, the hydrogel forming composition comprises at least two
antioxidants. For example, in certain embodiments, the hydrogel forming
composition
may comprise a first antioxidant (e.g., BHT) to prevent oxidation of the
difunctionalized
polyalkylene oxide-based crosslinking agent and a second antioxidant (e.g., N-
acetyl-
DL-tryptophan) to prevent oxidation of the protein.
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 40 ¨
In addition to preventing oxidation of one or more ingredients of the hydrogel
forming composition, the one or more antioxidants can in some cases stabilize
one or
more ingredients of the hydrogel forming composition (e.g., the crosslinking
agent, the
protein, etc.) to allow sterilization with a lethal dose of radiation (e.g.,
electron beam or
gamma radiation). In certain embodiments, for example, one or more components
of the
hydrogel forming composition (e.g., the first component and/or the second
component)
may be sterilized using electron beam radiation. In some such embodiments, the
one or
more components of the hydrogel may be exposed to one or more doses of
electron beam
radiation with a cumulative dose between greater than or equal to 25 kGy and
less than
or equal to 30 kGy.
According to certain embodiments, the hydrogel forming composition comprises
each antioxidant (e.g., a first antioxidant, a second antioxidant) in any of a
variety of
suitable amounts in weight percent (wt.%) by mass versus the total weight of
the
powdered hydrogel forming composition. In some embodiments, for example, the
hydrogel forming composition comprises each antioxidant in an amount, on a
powdered
basis, greater than or equal to 0.1 wt%, greater than or equal to 1 wt.%,
greater than or
equal to 2 wt.%, greater than or equal to 5 wt.%, greater than or equal to 10
wt.%, or
greater than or equal to 15 wt.% of the total weight of the powdered hydrogel
forming
composition. In certain embodiments, the hydrogel forming composition
comprises each
antioxidant in an amount, on a powdered basis, less than or equal to 20 wt.%,
less than or
equal to 15 wt.%, less than or equal to 10 wt.%, less than or equal to 5 wt.%,
less than or
equal to 2 wt.%, less than or equal to 1 wt.%, or less than or equal to 0.1
wt.% of the
total weight of the powdered hydrogel forming composition. Combinations of the
above
recited ranges are also possible (e.g., the hydrogel forming composition
comprises each
antioxidant in an amount, on a powdered basis, greater than or equal to 0.1
wt.% and less
than or equal to 20 wt.% of the total weight of the powdered hydrogel forming
composition, the hydrogel forming composition comprises each antioxidant in an
amount, on a powdered basis, greater than or equal to 1 wt.% and less than or
equal to 5
wt.% of the total weight of the powdered hydrogel forming composition). Other
ranges
.. are also possible.
According to certain embodiments, the antioxidant may be a part of the first
and/or second component. In some embodiments, for example, at least one
antioxidant
may be combined with the crosslinking agent and/or protein as a powder
mixture. In
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 41 ¨
some such embodiments, the antioxidant may by lyophilized with the protein
(and/or the
cros slinking agent, in some cases). In certain alternative embodiments, the
antioxidant
may be dissolved (e.g., in a solvent such as water) that is used as the
hydration solution
to hydrate the first and/or second component.
Radiopaque Agents
In some embodiments, the hydrogel forming composition comprises a radiopaque
agent. The use of a radiopaque agent can provide the resulting hydrogel with
the ability
to be spectroscopically imaged, for example, by X-ray or computed tomography
(CT)
imaging. Any of a variety of suitable radiopaque agents may be added. In some
embodiments, the radiopaque agent comprises gold (e.g., gold nanoparticles),
silver (e.g.,
silver nanoparticles), or iodine. In certain embodiments, the radiopaque agent
is
potassium chloride (KC1), barium sulfate, iohexol, or diatrizoate.
According to certain embodiments, the hydrogel forming composition comprises
the radiopaque agent in any of a variety of amounts in weight percent (wt.%)
by mass
versus the total weight of the powdered hydrogel forming composition. In some
embodiments, for example, the hydrogel forming composition comprises the
radiopaque
agent in an amount, on a powdered basis, greater than or equal to 0.1 wt.%,
greater than
or equal to 0.5 wt.%, greater than or equal to 1 wt.%, greater than or equal
to 5 wt.%,
greater than or equal to 10 wt.%, or greater than or equal to 15 wt.% of the
total weight
of the powdered hydrogel forming composition. In certain embodiments, the
hydrogel
forming composition comprises the radiopaque agent, on a powdered basis, in an
amount
less than or equal to 20 wt.%, less than or equal to 15 wt.%, less than or
equal to 10
wt.%, less than or equal to 5 wt.%, less than or equal to 1 wt.%, or less than
or equal to
0.5 wt.% of the total weight of the powdered hydrogel forming composition.
Combinations of the above recited ranges are also possible (e.g., the hydrogel
forming
composition comprises the radiopaque agent in an amount, on a powdered basis,
greater
than or equal to 0.1 wt.% and less than or equal to 20 wt.% of the total
weight of the
powdered hydrogel forming composition, the hydrogel forming composition
comprises
the radiopaque agent in an amount, on a powdered basis, greater than or equal
to 1 wt.%
and less than or equal to 10 wt.% of the total weight of the powdered hydrogel
forming
composition). Other ranges are also possible.
Other Agents
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 42 ¨
In any of the above described embodiments, the hydrogel forming composition
may comprise other active agents or ingredients for various purposes, for
example any of
a variety of suitable active agents, such as antimicrobials, anti-
inflammatories,
hemostatic agents, etc.
According to some embodiments, the hydrogel forming composition is in the
form of one or more powders (e.g., during storage of the hydrogel forming
composition).
In certain embodiments, the one or more powders of the hydrogel forming
composition
may be hydrated with water or one or more aqueous solutions in order to form
an
aqueous solution of the hydrogel forming composition comprising the
crosslinking agent,
the protein, the optional surfactant(s), the optional antioxidant(s), and/or
the optional
cros slinking initiator(s). In certain embodiments, forming the aqueous
solution form of
the hydrogel forming composition comprising the crosslinking agent and the
protein
initiates crosslinking of the crosslinking agent and the protein, thereby
forming a
hydrogel tissue sealant.
As mentioned above, in certain embodiments, the hydrogel forming composition
may be stored and/or provided as a multicomponent formulation where certain of
the
ingredients are segregated from others in different powdered or hydrated
components. In
some embodiments, for example, the hydrogel forming composition comprises at
least a
first component, a second component, a solvent able to dissolve the first
component and
the second component, and, optionally, a surfactant. In some such embodiments,
the
first component comprises the crosslinking agent and an optional antioxidant,
and the
second component comprises the protein. The surfactant, in certain
embodiments, may
be a part of the second component (e.g., a powder mixture with the protein),
or may be
dissolved in and/or otherwise mixed with a solvent that is used to hydrate the
first and/or
second components. In some embodiments, the hydrogel forming composition
comprises a crosslinking initiator, which may be a part of the second
component (e.g., a
powder mixture with the protein), or may be dissolved in a solvent that is
used to hydrate
one or the components (e.g., the second component). In certain embodiments,
for
example, the second component is a mixture (e.g., a lyophilized powder
mixture)
including the protein, the crosslinking initiator, and the surfactant. The
hydrogel forming
composition may also comprise at least one antioxidant, in some embodiments,
which
may be a part of the first and/or second component, or may be dissolved in a
solvent that
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 43 ¨
is used to hydrate the first and/or second component. In certain embodiments,
the
hydrogel forming composition may also comprise a radiopaque agent, in some
embodiments, which may be a part of the first and/or second component, or may
be
dissolved in a solvent that is used to hydrate the first and/or second
component.
It may be advantageous, in certain embodiments, to separately store the first
component (e.g., comprising the crosslinking agent (and optional antioxidant))
and the
second component (e.g., comprising the protein (and optionally a crosslinking
initiator
and/or surfactant)) in order to avoid any crosslinking between the
crosslinking agent and
the protein during storage and/or to delay crosslinking until the hydrogel
forming
.. composition is delivered to the tissue site. In certain embodiments, at
least the second
component comprising the protein, the crosslinking initiator, and the
surfactant may be
lyophilized, or at least the protein of such component is lyophilized.
According to some embodiments, the first component may be in the form of a
first powder mixture and the second component may be in the form of a second
powder
mixture (e.g., during storage of the first component and/or the second
component). In
certain embodiments, the first component and/or the second component that are
powder
mixtures may be separately solvated or hydrated (in the description below,
"hydrated" is
used for brevity, but it should be understood that for embodiments where a non-
aqueous
solvent is used, "solvated" should be substituted for "hydrated") with water
or one or
.. more solvents (e.g., water, biocompatible organic solvent such as DMSO) or
aqueous
solutions, thereby providing a first component that is in the form of a first
solution (e.g.,
first aqueous solution) and a second component that is in the form of a second
solution
(e.g., second aqueous solution). In certain embodiments, it may be
advantageous to
dissolve the first component (e.g., the crosslinking agent) in a biocompatible
organic
solvent, such as DMSO, in order to extend the pot life of the hydrogel forming
composition. In some embodiments, the hydrated first component and the
hydrated
second component may be combined in order to initiate crosslinking of the
crosslinking
agent and the protein, thereby forming the hydrogel tissue sealant.
In certain embodiments, the first solution (e.g., that hydrates the first
component
comprising the crosslinking agent) may comprise the radiopaque agent and/or
the first
antioxidant. In some embodiments, the second solution (e.g., that hydrates the
second
component comprising the protein) may comprise the surfactant, the
crosslinking
initiator, and/or the second antioxidant.
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 44 ¨
The second component, when a protein is included as the nucleophilic polymer,
may be hydrated such that the concentration of the protein in the resulting
hydrated
solution is any of a variety of suitable amounts. In some embodiments, for
example, the
concentration of the protein in the hydrated second component is greater than
or equal to
.. 10% mass by volume, greater than or equal to 15% mass by volume, greater
than or
equal to 20% mass by volume, greater than or equal to 25% mass by volume, or
greater
than or equal to 30% mass by volume. In certain embodiments, the concentration
of the
protein in the hydrated second component is less than or equal to 35% mass by
volume,
less than or equal to 30% mass by volume, less than or equal to 25% mass by
volume,
less than or equal to 20% mass by volume, or less than or equal to 15% mass by
volume.
Combinations of the above recited ranges are also possible (e.g., the
concentration of the
protein in the hydrated second component is greater than or equal to 10% mass
by
volume and less than or equal to 35% mass by volume, the concentration of the
protein
in the hydrated second component is greater than or equal to 20% mass by
volume and
less than or equal to 25% mass by volume). Other ranges are also possible.
According to certain embodiments, the lyophilized second component comprising
the protein may have a relatively fast dissolution time. As used herein, the
term
"dissolution time" is given its ordinary meaning in the art and generally
refers to the time
it takes for the second component comprising the protein to completely
dissolve when
.. hydrated (or solvated) with mixing or agitation. A relatively fast
dissolution time may
advantageously reduce the time it takes to form the hydrogel tissue sealant.
The
dissolution time is calculated by starting a timer, hydrating the second
component by
mixing the second component and the hydration solution, and stopping the timer
when
the second component is completely dissolved. In some embodiments, the
dissolution
time of the second component at 25 C may be greater than or equal to 10
seconds,
greater than or equal to 15 seconds, greater than or equal to 20 seconds,
greater than or
equal to 25 seconds, greater than or equal to 30 seconds, or greater than or
equal to 35
seconds. In certain embodiments, the dissolution time of the second component
at 25 C
is less than or equal to 40 seconds, less than or equal to 35 seconds, less
than or equal to
30 seconds, less than or equal to 25 seconds, less than or equal to 20
seconds, or less than
or equal to 15 seconds. Combinations of the recited ranges are also possible
(e.g., the
dissolution time of the second component at 25 C is between greater than or
equal to 10
seconds and less than or equal to 40 seconds, the dissolution time of the
second
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 45 ¨
component at 25 C is between greater than or equal to 20 seconds and less
than or equal
to 30 seconds). Other ranges are also possible.
In certain embodiments, the dissolution time of the second component may
depend on the amount of protein in the second component upon hydration, i.e.
its mass
by volume of the solution. For example, in some embodiments, the dissolution
time of
the second component is directly proportional to the amount of protein in the
second
component. In some non-limiting embodiments, for example, a second component
comprising a relatively low amount of protein (e.g., 10% mass by volume in the
resulting
hydration solution) will have a shorter dissolution time as compared to a
second
component that is otherwise equivalent but has a relatively high amount of
protein (e.g.,
30% mass by volume in the resulting hydration solution), assuming that the
final volume
is the same between the second component comprising the lower amount of
protein and
the second component comprising the higher amount of protein.
The solution of the lyophilized second component (e.g., comprising the protein
.. and the cros slinking initiator) may have a relatively high pH upon
dissolution. In certain
embodiments, for example, the solution of the lyophilized second component,
upon
dissolution, has a pH greater than or equal to 9, greater than or equal to
9.5, greater than
or equal to 10, or greater than or equal to 10.5. In some embodiments, the
solution of the
lyophilized second component, upon dissolution, has a pH less than or equal to
11, less
.. than or equal to 10.5, less than or equal to 10, or less than or equal to
9.5. Combinations
of the above recited ranges are also possible (e.g., the solution of the
lyophilized second
component, upon dissolution, has a pH between greater than or equal to 9 and
less than
or equal to 11, the solution of the lyophilized second component, upon
dissolution, has a
pH between greater than or equal to 10 and less than or equal to 10.5). Other
ranges are
.. also possible.
It should be understood that, when specifying a second component, when
hydrated as explained herein, produces a pH of a resulting solution in the
above ranges,
the amount of protein added need not be specified, so long as there exists an
amount of
the protein that can be hydrated to produce such a pH. That said, in some
embodiments,
dissolution of even a relatively small amount of the second component (e.g.,
10% mass
by volume) can produce a solution with a pH in the above ranges. For example,
in some
embodiments, the second component, when hydrated to form a resulting solution
of the
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 46 ¨
protein, produces a solution having a pH of greater than or equal to 9,
greater than or
equal to 9.5, greater than or equal to 10, or greater than or equal to 10.5.
In certain embodiments, as explained herein, the first component and the
second
component may be dissolved in one or more solvents then combined/mixed
together to
form a cros slinking hydrogel forming composition comprising a solution of the
first
component and the second component. In some embodiments, for example, the
first
component is dissolved in a first solvent and the second component is
dissolved in a
second solvent, which are then combined to form the hydrogel forming
composition
solution. The hydrogel forming composition solution of the first component and
the
second component may have a pH that is less than or substantially similar to
the pH of
the solution of the lyophilized second component. In certain embodiments, for
example,
the crosslinking solution of the first component and the second component has
a pH
greater than or equal to 7, greater than or equal to 8, greater than or equal
to 9, greater
than or equal to 9.5, greater than or equal to 10, or greater than or equal to
10.5. In some
embodiments, the cros slinking solution of the first component and the second
component
has a pH less than or equal to 11, less than or equal to 10.5, less than or
equal to 10, or
less than or equal to 9.5. Combinations of the above recited ranges are also
possible
(e.g., the crosslinking solution of the first component and the second
component has a
pH greater than or equal to 9 and less than or equal to 11, the crosslinking
solution of the
first component and the second component has a pH greater than or equal to 10
and less
than or equal to 10.5). Other ranges are also possible.
In certain non-limiting but advantageous embodiments, the hydrogel forming
composition solution of the first component and the second component is formed
by
combining an unbuffered solution of the first component with a solution of the
second
component that has a pH greater than or equal to 10.2 and less than or equal
to 10.6.
The time it takes for the hydrated hydrogel forming composition to crosslink
and
form a gel may determine how fast the composition can act as a tissue sealant
when the
hydrogel/hydrogel forming composition is delivered to a tissue site. It may be
beneficial
for the hydrogel forming composition to crosslink within a sufficiently short
time frame
to permit the applied composition to quickly seal a tissue puncture site or
other wound
surface when the hydrogel forming composition is applied to the tissue site.
The
"measured gel time" as used herein is determined by dispensing the hydrated
first
component and the hydrated second component to a vial containing a stir bar on
a stir
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨47 ¨
plate adjusted to 300 RPM and recording the initial time (To) upon dispensing
the
components of the composition, followed by recording the end time (TF) when
gelation
causes the stir bar to stop spinning. The measured crosslink time is the time
when the
timer is stopped minus the initial time. In certain embodiments, rheometry may
be used
to determine the measured gel time.
The hydrogel forming composition may have any of a variety of suitable
measured gel times for particular uses and application methods. In some
embodiments,
for example, the hydrogel forming composition may have a measured gel time of
greater
than or equal to 0.1 seconds, greater than or equal to 0.5 seconds, greater
than or equal to
1 second, greater than or equal to 2 second, greater than or equal to 3
seconds, greater
than or equal to 4 seconds, greater than or equal to 5 seconds, greater than
or equal to 10
seconds, or greater than or equal to 15 seconds. In certain embodiments, the
hydrogel
forming composition has a measured gel time of less than or equal to 20
seconds, less
than or equal to 15 seconds, less than or equal to 10 seconds, less than or
equal to 5
seconds, less than or equal to 4 seconds, less than or equal to 3 seconds,
less than or
equal to 2 seconds, less than or equal to 1 second, or less than or equal to
0.5 seconds.
Combinations of the above recited ranges are also possible (e.g., the hydrogel
forming
composition may have a measured gel time of greater than or equal to 0.1
seconds and
less than or equal to 20 seconds, the hydrogel forming composition may have a
measured
cros slink time of greater than or equal to 1 second and less than or equal to
3 seconds).
Other ranges are also possible.
According to certain embodiments, the measured gel time may be rendered
advantageously short by adjusting the amount of the crosslinking initiator. In
some
embodiments, for example, sufficient crosslinking initiator is added to
provide a suitable
pH value, as explained herein, to initiate the crosslinking reaction between
the
crosslinking agent and the protein in a given surgical environment. In certain
embodiments, the cros slinking initiator provides a pH value of greater than
or equal to 10
(e.g., between 10.2-10.6, between 10.3-10.4), which facilitates a faster
crosslinking
reaction since the reaction is generally favored at higher pH values. In
certain
embodiments, the gel time is tunable, depending on the amount of the base or
basic
buffer in the hydrogel forming composition.
In some cases, it may be advantageous for the hydrogel forming composition to
have a sufficiently long measured pot life. As used herein, the term "measured
pot life"
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 48 ¨
refers to the duration of time that the hydrated first and second components
of the
hydrogel forming composition remain usable after hydration of one or more of
the
powdered reactive components (e.g., the first component and/or the second
component)
but prior to combining the solutions of the first and second components to
form the
crosslinking hydrogel forming composition solution. A sufficiently long pot
life, in
some embodiments, may advantageously allow the hydrated first and second
components
of the hydrogel forming composition to remain usable after a user (e.g., a
physician) has
hydrated the first and second components until the user is ready to deliver
the one or
more components to the site of administration to form the hydrogel tissue
sealant. The
"measured pot life" as used herein is determined by measuring certain
performance
metrics, such as, for example, the gel time and/or the burst strength (both of
which are
explained herein in greater detail) of a crosslinked hydrogel forming
composition or
formed hydrogel and comparing to an equivalent hydrogel forming composition or
formed hydrogel formed from freshly hydrated first and second components of
the
hydrogel forming composition that have not been subject to storage or delayed
use after
hydration. The measured pot life is the time it takes for the performance
metric of the
crosslinked hydrogel composition resulting from the one or more hydrated
components
that have been stored for a period of time to differ from the performance
metric of the
crosslinked hydrogel composition resulting from the one or more freshly
hydrated
components by a defined percentage (e.g., +/- 10%) based on a clinically based
minimum value for each performance metric to ensure that the hydrogel tissue
sealant
can safely perform its function (e.g., sealing tissue).
The hydrogel forming components of the composition may have any of a variety
of suitable pot life times (defined as performance metric differing by no more
than +/-
10%). In some embodiments, for example, the hydrogel forming composition has a
pot
life of greater than or equal to 10 minutes, greater than or equal to 20
minutes, greater
than or equal to 30 minutes, greater than or equal to 1 hour, greater than or
equal to 2
hours, greater than or equal to 5 hours, or greater than or equal to 10 hours.
In certain
embodiments, the hydrogel forming composition has a pot life less than or
equal to 24
hours, less than or equal to 10 hours, less than or equal to 5 hours, less
than or equal to 2
hours, less than or equal to 1 hour, less than or equal to 30 minutes, or less
than or equal
to 20 minutes. Combinations of the above recited ranges are also possible
(e.g., the
hydrogel forming composition has a pot life between greater than or equal to
10 minutes
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 49 ¨
and less than or equal to 24 hours, the hydrogel forming composition has a pot
life
greater than or equal to 1 hour and less than or equal to 2 hours). Other
ranges are also
possible.
According to certain non-limiting embodiments, the pot life of either or both
reagent components of the hydrogel forming composition may be increased,
particularly
for embodiments where a component is provided in solvated form, by dissolving
such
component(s) in a biocompatible non-polar organic solvent, such as DMSO, to
form the
solvated component(s) of the hydrogel forming composition. Use of such a
solvent may
in certain instances substantially increase the pot life, to be, for example,
greater than or
equal to 1 week, greater than or equal to 1 month, greater than or equal to 6
months,
greater than or equal to 1 year, or greater than or equal to 2 years.
According to some embodiments, it may be advantageous for the hydrogel
forming composition to have a sufficiently long measured shelf life. As used
herein, the
term "measured shelf life" refers to the duration of time that one or more of
the
powdered components of the hydrogel forming composition remains suitably
usable after
storage of the one or more powdered components. A sufficiently long shelf
life, in some
embodiments, may advantageously allow the hydrogel forming composition to
remain
usable after long-term storage of the hydrogel forming composition. The
"measured
shelf life" as used herein is determined by measuring certain performance
metrics, such
as, for example, the gel time, dissolution time, and/or the burst strength
(which are
explained herein in greater detail) of a crosslinking hydrogel forming
composition
solution and/or a crosslinked hydrogel composition formed therefrom prepared
that has
been subject to a storage period as compared to the corresponding measured
metrics
resulting from such one or more powdered components of the hydrogel forming
composition (e.g., the first component and/or the second component) that have
not been
subject to storage prior to hydration. The measured shelf life is the time it
takes for the
performance metric of the crosslinked hydrogel composition resulting from the
one or
more powdered components that have been subject to storage for a period of
time prior
to hydration to differ from the same metric measured for fresh ingredients by
a specified
percentage (e.g., +/- 10%) based on a clinically based minimum value for each
performance metric to ensure that the hydrogel tissue sealant can safely
perform its
function (e.g., sealing tissue).
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 50 ¨
The first and second components of the hydrogel forming composition may have
any of a variety of suitable shelf life times. In some embodiments, for
example, the
hydrogel forming composition has a shelf life (defined as resulting is a
difference
compared to fresh ingredients of +/- 10%) of greater than or equal to 1 week,
greater than
or equal to 1 month, greater than or equal to 6 months, greater than or equal
to 1 year,
greater than or equal to 2 years, greater than or equal to 3 years, or greater
than or equal
to 4 years. In certain embodiments, the hydrogel forming composition has a
shelf life
less than or equal to 5 years, less than or equal to 4 years, less than or
equal to 3 years,
less than or equal to 2 years, less than or equal to 1 year, less than or
equal to 6 months,
or less than or equal to 1 month. Combinations of the above recited ranges are
also
possible (e.g., the hydrogel forming composition has a shelf life between
greater than or
equal to 1 week and less than or equal to 5 years, the hydrogel forming
composition has
a shelf life greater than or equal to 1 year and less than or equal to 2
years). Other ranges
are also possible.
Methods of forming a hydrogel tissue sealant are provided. In some
embodiments, the method comprises forming a crosslinking solution comprising
at least
a crosslinking agent and a nucleophilic biodegradable polymer (e.g., a
protein), wherein
forming the crosslinking solution initiates crosslinking of the crosslinking
agent and the
nucleophilic biodegradable polymer (e.g., a protein), thereby forming the
hydrogel tissue
sealant. In certain embodiments, as explained herein, the crosslinking
solution
comprises a crosslinking initiator, a surfactant, and/or an antioxidant.
According to certain embodiments, the method of forming the hydrogel tissue
sealant comprises dissolving a first powdered component comprising a
crosslinking
agent (e.g., an electrophilic biodegradable polymer) and a second powdered
component
comprising a nucleophilic biodegradable polymer (e.g., a protein) in one or
more
solvents. In some embodiments, for example, the first component is dissolved
in a first
solvent (e.g., water or an aqueous solution) and the second component is
dissolved in a
second solvent (e.g., water or an aqueous solution). In some such embodiments,
the
dissolved fist component and the dissolved second component are combined to
form a
crosslinking hydrogel forming composition in the form of a solution comprising
the
crosslinking agent and the protein, thereby initiating crosslinking of the
crosslinking
agent and the nucleophilic biodegradable polymer (e.g., a protein) to from the
hydrogel
tissue sealant.
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 51 ¨
In certain embodiments, the method of forming a hydrogel tissue sealant
comprises hydrating a first powdered component including a crosslinking agent
and at
least one antioxidant, hydrating a second powdered component including a
protein, a
crosslinking initiator, and a surfactant, and combining the hydrated first
component and
the hydrated second component to initiate crosslinking of the crosslinking
agent and the
protein. FIG. 1, shows steps in such an exemplary method for forming a
hydrogel tissue
sealant. Method 150, in step 152 comprises hydrating a first powdered
component,
comprising, for example, a crosslinking agent. In some embodiments, the first
powdered
component optionally comprises an antioxidant. In certain embodiments, the
first
powdered component is hydrated with a first solvent comprising water or a
first aqueous
solution. The first solvent may include, in some embodiments, a radiopaque
agent. Step
154 comprises hydrating a second powdered component comprising, for example, a
protein. In certain embodiments, the second powdered component comprises a
crosslinking initiator and/or a surfactant. In some embodiments, the second
powdered
component is hydrated with a second solvent comprising water or a second
aqueous
solution. In certain embodiments, the second solvent comprises an anti-foaming
agent.
According to certain embodiments, step 152 and step 154 may occur
simultaneously (but
in separate containers). Step 156 comprises combining the hydrated first
component and
the hydrated second component to initiate crosslinking of the crosslinking
agent and the
protein to form the hydrogel tissue sealant.
Methods are also disclosed herein related to sealing tissue with the formed
hydrogel compositions. In some embodiments, for example, such a method
comprises
delivering the hydrogel forming composition comprising the first component and
the
second component to a tissue site, or delivering a partially or fully
crosslinked hydrogel
composition to the tissue site, wherein the hydrogel composition comprises the
reaction
product of the above described first component and second component reagents.
The hydrogel composition may be delivered to the tissue site in any of a
variety
of suitable ways. In some embodiments, the first component of the hydrogel
forming
composition and the second component of the hydrogel forming composition may
be at
least partially combined to initiate crosslinking prior to delivering the
hydrogel
composition to the tissue site. In certain embodiments, the first component of
the
hydrogel forming composition and the second component of the hydrogel forming
composition are fully combined prior to delivering the hydrogel composition to
the tissue
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 52 ¨
site. According to certain embodiments, the hydrated first component of the
hydrogel
forming composition and the hydrated second component of the hydrogel forming
composition may crosslink as the hydrogel composition is being delivered to
the tissue
site. In some embodiments, for example, the hydrogel tissue sealant is at
least partially
formed prior to or upon delivery to the tissue site. In certain embodiments,
the tissue site
is a pleural site, such as the parietal pleura and/or the visceral pleura.
In certain embodiments, the hydrogel forming composition may be delivered to
the tissue site using one or more syringes, sprayers, or other applicators. In
certain
embodiments, for example, the applicator that can be used to deliver the
hydrogel
forming compositions is described in U.S. Patent Application Serial No.
62/822,490,
titled "LUNG BIOPSY FLOWABLE SEALANT DELIVERY SYSTEM," or
PCT/U52020/023772, titled "SEALANT DELIVERY APPARATUS, AND SYSTEM
AND METHOD FOR PREPARING SAME, FOR USE IN A LUNG PROCEDURE,"
both of which are incorporated herein by reference in their entireties. The
following
application, filed on even date herewith, is also incorporated by reference in
its entirety:
International Application No. PCT/US21/23171, filed on March 19, 2021, titled
"MULTI-COMPONENT SEALANT DELIVERY SYSTEMS INCORPORATING
QUARTER TURN CONNECTORS." Further details regarding the hydrogel forming
composition delivery device are described below.
According to some embodiments, the hydrogel tissue sealant can be formulated
so that it adheres to the tissue site. In certain embodiments, the adherence
of the
hydrogel tissue sealant at the tissue site may be determined by a liquid burst
pressure
strength model based on ASTM F2392-04 (the Standard Test Method for Burst
Strength
of Surgical Sealants). According to some embodiments, the test is designed to
determine
the pressure needed to rupture the sealant patch covering a simulated liquid
leak and
indirectly measure the adhesion property of the sealant to simulated tissue.
In certain
embodiments, the hydrogel tissue sealant may have any of a variety of suitable
burst
pressure strengths (e.g., liquid burst pressure strengths). For example, in
some
embodiments, the burst pressure strength of the hydrogel tissue sealant
measured by such
test is greater than or equal to 10 mm Hg, greater than or equal to 50 mm Hg,
greater
than or equal to 100 mm Hg, greater than or equal to 150 mm Hg, greater than
or equal
to 200 mm Hg, or greater than or equal to 250 mm Hg. In certain embodiments,
the
burst pressure strength of the hydrogel tissue sealant measured by such test
is less than or
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 53 ¨
equal to 300 mm Hg, less than or equal to 250 mm Hg, less than or equal to 200
mm Hg,
less than or equal to 150 mm Hg, less than or equal to 100 mm Hg, less than or
equal to
50 mm Hg. Combinations of these ranges are also possible (e.g., the burst
pressure
strength of the hydrogel tissue sealant is greater than or equal to 10 mm Hg
and less than
or equal to 300 mm Hg, the burst pressure strength of the hydrogel tissue
sealant is
greater than or equal 100 mm Hg and less than or equal to 150 mm Hg). Other
ranges
are also possible.
In some embodiments, the crosslinked hydrogel tissue sealant may swell (e.g.,
with water) after delivery to the tissue site. In some embodiments,
advantageously, the
hydrogel tissue sealant may have a relatively high swelling rate and/or extent
(characterized by mass gain after a defined swelling period). A hydrogel
tissue sealant
with a relatively high swelling rate may be advantageous, as the hydrogel
tissue sealant
may swell and conform to the tissue delivery site to improve sealing
properties. In
certain embodiments, and as explained below in greater detail, the hydrogel
composition
may be delivered to the tissue site via a coaxial cannula. In certain
embodiments the
coaxial cannula becomes surrounded by the hydrogel composition during and/or
after
delivery of the hydrogel composition in order to perform a biopsy procedure
(e.g., a lung
biopsy). The coaxial cannula may, in some embodiments, be removed through the
bulk
of the hydrogel after the biopsy procedure, resulting in a puncture, void, or
hole in the
hydrogel tissue sealant. In some such embodiments, the hydrogel tissue sealant
may
swell (e.g., with water) after removal of the coaxial cannula, therefore
substantially
closing and/or filling the puncture, void, and/or hole caused by the coaxial
cannula. The
swelling rate of the hydrogel tissue sealant may be determined by forming the
crosslinked hydrogel sealant as explained herein, recording the weight of the
hydrogel
composition, incubating the hydrogel composition in a phosphate-buffered
saline (PBS)
solution at 37 C, removing the hydrogel composition from the PBS solution
after two
hours, and recording the weight of the hydrogel composition, wherein the
percent is
calculated by the percent weight gain.
According to certain embodiments, the hydrogel tissue sealant has a swelling
mass gain greater than or equal to 30%, greater than or equal to 35%, greater
than or
equal to 40%, greater than or equal to 45%, greater than or equal to 50%,
greater than or
equal to 55%, greater than or equal to 60%, or greater than or equal to 65%.
In some
embodiments, the hydrogel tissue sealant has a swelling mass gain less than or
equal to
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 54 ¨
70%, less than or equal to 65%, less than or equal to 60%, less than or equal
to 55%, less
than or equal to 50%, less than or equal to 45%, less than or equal to 40%, or
less than or
equal to 35%. Combinations of the above recited ranges are also possible
(e.g., the
hydrogel tissue sealant has a swelling mass gain between greater than or equal
to 30%
and less than or equal to 70%, the hydrogel tissue sealant has a swelling rate
between
greater than or equal to 40% and less than or equal to 50%). Other ranges are
also
possible.
According to certain embodiments, the hydrogel tissue sealant may be utilized
as
a pleural lung sealant to seal off air and/or fluid from entering the pleural
space. In some
embodiments, the hydrogel tissue sealant may be utilized to seal the pleura
together.
The hydrogel tissue sealant may degrade in the subject over time. In certain
embodiments, the degradation time of the hydrogel may be determined by a
degradation
model based on ASTM F1635 (the Standard Test Method for in Vitro Degradation).
In
some embodiments, the test is designed to determine the degradation rate (that
is, the
mass loss rate) and change in material or structural properties, or both, of
materials used
in surgical implants. The hydrogel tissue sealant may have any of a variety of
suitable
degradation times. In some embodiments, for example, the hydrogel tissue
sealant has a
degradation time greater than or equal to 1 day, greater than or equal to 5
days, greater
than or equal to 7 days, greater than or equal to 10 days, or greater than or
equal to 15
.. days. In certain embodiments, the hydrogel tissue sealant has a degradation
time less
than or equal to 20 days, less than or equal to 15 days, less than or equal to
10 days, less
than or equal to 7 days, or less than or equal to 5 days. Combinations of the
above
recited ranges are also possible (e.g., the hydrogel tissue sealant has a
degradation time
between greater than or equal to 1 day and less than or equal to 20 days, the
hydrogel
tissue sealant has a degradation time between greater than or equal to 10 days
and less
than or equal to 15 days). Other ranges are also possible.
According to certain embodiments, a kit is provided. The kit may comprise one
or more devices, such as containers or syringes comprising containers (e.g.
barrels of the
syringes) that are capable of storing one or more components of the hydrogel
forming
.. composition, mixing one or more components of the hydrogel forming
composition,
and/or delivering the hydrogel forming composition (or one or more components
thereof)
to a tissue site. For example, FIG. 2A shows, according to certain
embodiments, a
schematic diagram of a dual-syringe, 4 compartment/container device 200 that
is capable
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 55 ¨
of storing and/or mixing one or more components of the hydrogel forming
composition
with one or more solvents, and FIG. 3A shows a cross-section thereof. FIG. 2B
shows,
according to some embodiments, a schematic diagram of a the device of FIG. 2A,
where
the second syringe component containing the hydrated/dissolved first and
second
components of the hydrogel forming composition is coupled with a coaxial
cannula to be
capable of delivering the hydrogel forming composition to a tissue site, and
FIG. 3B
shows a cross-section thereof.
The kit may comprise any of the above described first components contained
within a first container, which may, as illustrated in FIGs 2A-3B be
conveniently a barrel
of a syringe device. Referring to FIG. 3A, for example, syringe device 200
(e.g., with
two interconnected syringe components of the device configured for mixing the
stored
components with one or more solvents) can comprise the first component
comprising the
crosslinking agent contained within first barrel/container 102, e.g. during
storage. In
certain embodiments, the first component may further comprise an antioxidant
(e.g.,
BHT), a surfactant, and/or a radiopaque agent. The first component may be in
the form
of a first dry powder.
The kit may further comprise a second container containing any of the second
components described above. Referring to FIG. 3A, for example, syringe device
200 can
contain the second component within second barrel/container 104, e.g. during
storage.
In some embodiments, the second component may include a protein that is
capable of
crosslinking with the crosslinking agent. The second component may further
include a
crosslinking initiator that initiates crosslinking of the crosslinking agent
with the protein,
and/or a surfactant (e.g., PEG 8000). In some embodiments, the second
component may
further comprise a radiopaque agent. The second component may be in the form
of a
second dry powder.
In some embodiments, the kit further comprises a third, e.g., solvent,
component
contained within a third container. Referring, for example, to FIG. 3A, device
200 can
contain such third component within third barrel/container 106. According to
certain
embodiments, the third component comprises a first solvent (e.g., water, DMSO)
or
solution (e.g., aqueous solution) for dissolving and/or hydrating the first
powdered
component. In some embodiments, the first solvent or solution comprises an
antioxidant
(e.g., BHT), a radiopaque agent (e.g., KC1), and/or a surfactant.
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 56 ¨
In certain embodiments, the kit further comprises a fourth, e.g., solvent,
component contained within a fourth container. Referring, for example, to FIG.
3A,
device 200 can contain such fourth component within fourth barrel container
108.
According to some embodiments, the fourth compartment comprises a second
solvent
(e.g., water, DMSO) or solution (e.g., aqueous solution) for dissolving and/or
hydrating
the second powder component. In certain embodiments, the second solvent or
solution
comprises a crosslinking initiator (e.g., a base or basic buffer, such as
sodium carbonate).
In some embodiments, the second solvent or solution comprises an antioxidant
(e.g., N-
acetly-DL-tryptophan), a radiopaque agent, or a surfactant (e.g., Pluronic
L61).
According to some embodiments, and as illustrated, one or more of the first,
second, third, and fourth containers are compartments (barrels) of a syringe
or applicator.
Referring, for example, to FIGs. 2A and 3A, first barrel/container 102 and
second
barrel/container 104 may be double barrels of first a first syringe 100 (e.g.,
also used in
the disclosed embodiment as the applicator syringe¨See FIG. 2B), while third
barrel/container 106 and fourth barrel/container 108 are double barrels of a
second
syringe 120 (e.g., containing mixing or hydration solvents).
According to certain embodiments, the kit for forming a hydrogel tissue
sealant
comprises one or more syringes collectively providing at least three separate
containers.
In some embodiments, for example, a kit comprises a first container that
contains a first
component (e.g., an electrophilic biodegradable polymer) in powder form (e.g.,
first
container 102 in first syringe 100), a second container that contains a second
component
(e.g., a nucleophilic biodegradable polymer) in powder form (e.g., second
container 104
in first syringe 100), and at least a third container that contains one or
more solvents
(e.g., third container 106 and fourth container 108 in second syringe 120).
The kit may comprise one or more syringes (e.g., one syringe, two syringes,
three
syringes, four syringes). The one or more syringes may have any of a variety
of suitable
configurations. In certain embodiments, for example, the kit comprises two
syringes
(e.g., first syringe 100 and second syringe 120). Each syringe of the one or
more
syringes may, in certain embodiments, be a double-barrel syringe. First
syringe 100
(e.g., applicator syringe) may, in some embodiments, comprise first container
102
comprising the first component in powder form and second container 104
comprising the
second component in powder form. According to certain embodiments, second
syringe
120 (e.g., mixing or hydration syringe) comprises third container 106
comprising a first
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 57 ¨
solvent able to dissolve the first component, and fourth container 108
comprising a
second solvent able to dissolve the second component.
In some embodiments, the one or more syringes (e.g., first syringe 100 and
second syringe 120) are configured such that first container 102 and second
container
104 are able to be placed in fluid communication with at least third container
106
comprising the one or more solvents. Configuring the kit in this way
facilitates the
mixing of the first component with the one or more solvents to form a solution
of the
first component, and facilitates mixing of the second component with the one
or more
solvents to form a solution of the second component. For example, in certain
embodiments, first syringe 100 (e.g., applicator syringe) and second syringe
120 (e.g.,
mixing or hydration syringe) are configured to be fluidically connectable to
each other
such that first container 102 and second container 104 are able to be placed
in separate
fluid communication with third container 106 and fourth container 108,
respectively, to
facilitate mixing of the first component with the first solvent to form a
solution of the
first component in first container 102, and to facilitate mixing of the second
component
with the second solvent able to form a solution of the second component in
second
container 104.
The kit may comprise one or more devices that are capable of delivering the
hydrogel forming composition (or one or more components thereof) to a tissue
site. For
example, FIG. 2B shows, according to some embodiments, a schematic diagram of
a
device that is capable of delivering the hydrogel forming composition to a
tissue site, and
FIG. 3B shows a cross-section thereof. As shown in FIG. 2B, device 250 (e.g.,
delivery
device) comprises first syringe 100 and a needle assembly. The needle assembly
comprises coaxial cannula 130 and needle 132, in some embodiments. In some
embodiments, first syringe 100 is configured to mix and contain the dissolved
first
component (e.g., the solution of the first component) and the dissolved second
component (e.g., the solution of the second component) to form a cros slinking
solution
of the first component and the second component able to form the hydrogel
tissue sealant
upon delivery to the tissue site via the needle assembly.
FIG. 4 shows, in accordance with certain embodiments, a summary of the steps
in
an exemplary method for hydrating and delivering a hydrogel forming
composition using
the device depicted in FIGs. 2A-3B. Method 400 of hydrating and delivering a
hydrogel
forming composition comprises, in some embodiments, step 402 comprising
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 58 ¨
mechanically and fluidically interconnecting an applicator syringe (e.g.,
first syringe 100
in FIGs. 2A and 3A) and a mixing syringe (e.g., second syringe 120 in FIGs. 2A
and
3A). In some such embodiments, the applicator syringe (e.g., first syringe 100
in FIGs.
2A and 3A) comprises a first component contained with a first container (e.g.,
first
container 102 in FIGs. 2A and 3A) and a second component contained within a
second
container (e.g., second container 104 in FIGs. 2A and 3A), the mixing syringe
(e.g.,
second syringe 120 in FIGs. 2A and 3A) comprises a third component contained
with a
third container (e.g., third container 106 in FIGs. 2A and 3A) and a fourth
component
contained within a fourth container (e.g., fourth container 108 in FIGs. 2A
and 3A).
In certain embodiments, method 400 comprises step 404 comprising sequentially
depressing the plungers of the applicator syringe (e.g., first syringe 100 in
FIGs. 2A and
3A) and the mixing syringe (e.g., second syringe 120 in FIGs. 2A and 3A) to
hydrate the
first component contained within the first container (e.g., first container
102 in FIGs. 2A
and 3A) and the second component contained within the second container (e.g.,
second
container 104 in FIGs. 2A and 3A). Step 406 of method 400 comprises evaluating
whether the first component and/or the second component are fully hydrated
(e.g.,
completely dissolved). If the first component and/or the second component are
not fully
hydrated, then step 404 is repeated. If the first component and the second
component are
fully hydrated, then the user can proceed to step 408.
According to some embodiments, step 408 of method 400 comprises
disconnecting the mixing syringe (e.g., second syringe 120 in FIGs. 2A and 3A)
from the
applicator syringe (e.g., first syringe 100 in FIGs. 2A and 3A), when the
hydrated first
component and the hydrated second component are contained in the applicator
syringe.
Step 410 comprises connecting a needle assembly (e.g., coaxial cannula 130 and
needle
132 in FIG. 2B) to the applicator syringe (e.g., first syringe 100 in FIGs. 2B
and 3B).
According to some embodiments, method 400 comprises step 420 comprising
deploying the material (e.g., the solution of the first component and the
solution of the
second component) from the applicator syringe (e.g., first syringe 100 in
FIGs. 2B and
3B) to the tissue site. In certain embodiments, deploying the material
comprises mixing
the solution of the first component and the solution of the second component
to form a
cros slinking solution of the first component and the second component as the
material is
being delivered to the tissue site (e.g., at one or more mixing points within
the needle
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 59 ¨
assembly, proximal to the needle assembly and/or at the distal tip of the
needle
assembly).
According to certain embodiments, a needle assembly comprising a coaxial
cannula is used to deliver the hydrogel composition and to perform the biopsy
procedure
(e.g., the lung biopsy). In some embodiments, for example, device 250 (e.g.,
delivery
device) used to insert the coaxial cannula into the pleural space of a subject
such that the
hydrogel forming composition can be deployed at the tissue site. See, for
example, FIG.
8A, which shows, in accordance with certain embodiments, a schematic diagram
of a
hydrogel delivery device having a coaxial cannula inserted into the pleural
space of a
subject. The hydrated hydrogel forming composition is deployed to the tissue
site. See,
for example, FIG. 8B, which shows, in accordance with certain embodiments, a
schematic diagram of the hydrogel delivery device deploying the hydrogel
forming
composition via the coaxial cannula to provide a hydrogel tissue sealant at
the tissue site.
In some embodiments, the hydrogel forming composition is deployed during, or
subsequent to the user advancing the cannula through the tissue site.
Following delivery of the hydrogel composition, a biopsy is performed, in some
embodiments. For example, according to certain embodiments, syringe component
100
of device 250 is removed from the needle assembly comprising the coaxial
cannula, and
a biopsy device comprising a standard biopsy needle is inserted through the
coaxial
cannula. The biopsy (e.g., lung biopsy) procedure is then performed. See, for
example
FIG. 8C, which shows, in accordance with certain embodiments, a schematic
diagram of
a biopsy needle inserted through the coaxial cannula to perform a biopsy
procedure.
In certain embodiments, after deploying the hydrogel composition at the tissue
site to provide the hydrogel tissue sealant and performing the biopsy
procedure, the
biopsy device and the needle assembly comprising the coaxial cannula are
removed from
the administration site. According to some embodiments, the hydrogel tissue
sealant
may swell (e.g., with water), as described herein, sealing any punctures,
voids, and/or
holes in the hydrogel tissue sealant caused by removal of the needle assembly.
During storage, to prevent increases in moisture or oxygen uptake by the
powdered ingredients, one or more of containers/syringes containing one or
more of the
powdered components (particularly the powdered first component (e.g.,
comprising the
crosslinking agent)) may be placed in a sealed pouch (e.g., a sealed foil
pouch),
optionally flushed with and under an atmosphere of an inert gas such as
nitrogen, and
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 60 ¨
further optionally containing a desiccant material within the pouch (e.g., a
desiccant or
molecular sieve material, such as a desiccant packet containing either
PharmaKeep
(Mitsubishi Gas Chemical America, Inc.) or 4A molecular sieves (Multisorb
Filtration
Group).
EXAMPLE 1
The following example describes the use of a hydrogel tissue sealant in a
swine
lung model. A X-ray image of a swine lung model was obtained, as shown in FIG.
5A.
A biopsy was performed on the swine lung model. FIG. 5B shows the normal
airway
pressure of a post biopsy image of a swine lung model. The hydrogel forming
composition was prepared, comprising the reaction product of PEG(SS)2 and
albumin
with iohexol as the radiopaque material. As shown in FIG. 6A, the hydrogel
forming
composition was delivered upon initial puncture of the lung, via needle
(circled), to the
swine lung model prior to the biopsy. The hydrogel tissue sealant is visible
through the
soft tissue and pleural space. The coaxial component of the applicator was
left in place,
and the sealant application needle was removed. The biopsy needle was inserted
through
the coaxial component to the target tissue, the biopsy samples were acquired,
and the
coaxial component as well as the biopsy needle were removed. As shown in FIG.
6B,
the hydrogel tissue sealant (circled) remains in place and is easily
visualized post
procedure, where it closes off the puncture site. The hydrogel tissue sealant
permitted
normal ventilation. FIG. 5B shows a X-ray image of a swine lung model post
biopsy.
As shown in FIG. 7A, the hydrogel tissue sealant (circled) is adhered to the
parietal
pleura of the swine lung model. Furthermore, as shown in FIG. 7B, the hydrogel
tissue
sealant (circled) is protruding from the inside lung and is adhered to the
parietal and
visceral pleura of the swine lung model, therefore sealing the biopsy tract.
EXAMPLE 2
The following example describes the evaluation of the stability of a hydrogel
forming composition in an accelerated aging study.
Samples of hydrogel forming compositions including a PEG(SS)2-containing
component and a recombinant human serum albumin (rHSA)-containing component
were generated according to Table 1. PEG(SS)2 was obtained from Sigma (Samples
1, 3,
and 4) or Laysan Bio (Sample 2) and handled under a nitrogen environment. The
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 61 ¨
PEG(SS)2-containing component comprised either PEG(SS)2 or PEG(SS)2 with added
BHT. In certain cases, a desiccant or molecular sieve material was also
enclosed in the
pouch¨the desiccant packet containing either PharmaKeep (Mitsubishi Gas
Chemical
America, Inc.) or 4A molecular sieves (Multisorb Filtration Group). The
PEG(SS)2-
containing component was aliquoted into a first syringe and stored in a sealed
foil pouch
under an atmosphere of nitrogen gas. The rHSA-containing component solution
used to
prepare, via lyophilization, the rHSA-containing component used for forming
the
hydrogel included rHSA obtained from InVitria (Junction City, KS), with added
sodium
carbonate, and PEG 8000, combined with reverse osmosis (RO) water so that the
concentration of the rHSA was 30% mass by volume. The rHSA-containing
component
solution in the RO water was lyophilized, ground into a powder, aliquoted into
a second
syringe, and sealed in a foil pouch under a nitrogen environment.
A hydration kit was created using deionized (DI) water in a third syringe (for
hydrating the PEG(SS)2-containing component) and DI water containing Pluronic
L61
in a fourth syringe (for hydrating the lyophilized rHSA-containing component).
The
hydration kit was sealed in a foil pouch with two Leur-Lock connectors to be
connected to each syringe so that the powders mixtures could be hydrated at
the point of
use. The samples received two doses of electron beam sterilization. All
samples were
stored and conditioned at 40 C to simulate advanced aging compared to room-
temperature storage.
Sample PEG(SS)2-Containing rHSA-Containing
Number Component Component
1 PEG(SS)2 rHSA mixture
2 PEG(SS)2, BHT, and Desiccant rHSA mixture
3 PEG(SS)2 and PharmaKeep rHSA mixture
4 PEG(SS)2 and Desiccant rHSA mixture
Table 1: Components of tested hydrogel forming compositions.
The samples were aged according to Table 2, pulled at the indicated time
point,
and evaluated, as explained in further detail below.
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 62 ¨
Stability Time Points Simulated Advanced Age
Time zero 0
113 days 1 year
225 days 2 years
338 days 3 years
Table 2: Stability time points for the hydrogel forming composition and the
corresponding simulated advanced age.
The gel times of the four hydrogel forming composition samples denoted in
Table 1 were evaluated at each stability time point by: (i) hydrating the
PEG(SS)2-
containing component and the rHSA-containing component, (ii) waiting for a
period of
two minutes, thirty minutes, or sixty minutes after hydration, (iii)
dispensing the
hydrated components into a vial containing a stir bar on a stir plate adjusted
to 300 RPM,
(iv) recording the initial time upon dispensing the components, and (v)
recording the end
time when gelation caused the stir bar to stop spinning. Results are shown in
Table 3.
Time Point Time After Sample 1 Sample 2 Sample 3 Sample 4
Hydration Gel Time Gel Time Gel Time Gel Time
(sec) (sec) (sec) (sec)
Time zero 2 to 60 1 to 4 1 to 2 1 to 2 1 to 2.3
minutes
113 days 2 to 60 * 1 to 1.3 1.3 to 2 1 to 1.7
minutes
225 days 2 to 60 Not Tested 2 to 2.67 5.3
to 13 2
minutes
338 days 2 to 60 Not Tested 1 to 1.7 9 to 14 1 to 1.7
minutes
Table 3: Gel times for tested hydrogel forming compositions for samples aged
for the
indicated Stability Time Point (average of three replicate measurements). * =
two out of
the three samples did not gel.
The dissolution times of the four rHSA-containing component samples denoted
in Table 1 were evaluated at each stability time point by: (i) connecting the
syringe
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 63 ¨
containing the rHSA-containing component with the syringe containing DI water
and
Pluronic L61, (ii) starting a timer, (iii) pushing the fluid back and forth
into the powder
syringe, and (iv) stopping the timer when the rHSA-containing component
completely
dissolved. Results are shown in Table 4.
Time Point Sample 1 rHSA Sample 2 rHSA Sample 3 rHSA Sample 4 rHSA
Dissolution Time Dissolution Time Dissolution Time Dissolution Time
(sec) (sec) (sec) (sec)
Time zero 29 22 25 26
113 days 14 39 21 25
225 days Not Tested 49.67 18 64.67
338 days Not Tested 59.3 135 72.7
Table 4: Dissolution times of rHSA-containing components for samples aged for
the
indicated Stability Time Point (average of three replicate measurements).
The pHs of the four rHSA-containing component samples denoted in Table 1
were evaluated at each stability time point by: (i) dissolving the powder
mixture with the
syringe containing the DI water and Pluronic L61 as described above for the
dissolution time measurement, and (ii) measuring the pH of the solution using
a
calibrated Mettler Toledo FiveEasy pH Meter. Results are shown in Table 5.
Time Point Sample 1 Sample 2 Sample 3 Group 4A
rHSA pH rHSA pH rHSA pH rHSA pH
Time zero 10.34 10.43 10.37 10.35
113 days 10.33 10.64 10.44 10.52
225 days Not Tested 10.34 10.65 10.60
338 days Not Tested 10.38 10.22 10.35
Table 5: pHs of rHSA-containing components for samples aged for the indicated
Stability Time Point (average of three replicate measurements).
The swelling rates of the hydrogel compositions formed from the four samples
denoted in Table 1 were evaluated at each stability time point by: (i) forming
the
hydrogel composition by hydrating the PEG(SS)2-containing component and the
rHSA-
containing component, dispensing the components through a mixing tip, and
allowing
them to gel; (ii) recording the weight of the hydrogel composition at time
zero, (iii)
incubating the hydrogel composition in a phosphate-buffered saline (PBS)
solution at 37
C, (iv) removing the hydrogel composition from the PBS solution after two
hours; and
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 64 ¨
(v) recording the weight of the hydrogel composition. The percent swelling was
calculated by percentage weight gain. Results are shown in Table 6.
Time Point Sample 1 Sample 2 Sample 3 Sample 4
Swelling Rate Swelling Rate Swelling Rate Swelling Rate
Time zero 54.49% 44.63% 39.19% 45.08%
113 days Did not gel 45.77% 65.68% 66.75%
225 days Not tested 30.31% 78.77% 45.90%
338 days Not tested 30.01% Not Tested 40.02%
Table 6: Swelling rates of tested hydrogel compositions at two hours for
samples aged
for the indicated Stability Time Point (average of three replicate
measurements).
EXAMPLE 3
The following example describes the liquid burst pressure strength of hydrogel
compositions according to certain embodiments.
Hydrogel compositions including PEG(SS)2 and rHSA were generated according
to Table 7. The rHSA-containing component included rHSA lyophilized with
Pluronic
L61 and an antioxidant. Forty-five samples of three different hydrogel forming
compositions were investigated. The percent mass by volume of rHSA varied from
10-
30% between compositions, and the amount of PEG(SS)2 also varied so that the
NHS
ester: amine ratio was kept constant for all three compositions at 2.21.
Composition PEG(SS)2-Containing rHSA-Containing % Mass by Volume
Component Component of rHSA
1 PEG(SS)2 rHSA mixture 10%
2 PEG(SS)2 rHSA mixture 15%
3 PEG(SS)2 rHSA mixture 30%
Table 7: Components of tested hydrogel forming compositions.
The powdered PEG(SS)2 and the rHSA-containing component were aliquoted
into their own syringe and each was hydrated with a separate syringe
containing 1 mL of
water. The components were then dispensed through a mixing tip and allowed to
gel.
The adherence of the hydrogel composition was determined by a liquid burst
pressure
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 65 ¨
model based on ASTM F2392-04 (the Standard Test Method for Surgical Sealants).
Results are shown in Table 8.
Composition Average Liquid Burst Pressure
Strength (mm Hg)
1 25.92
2 111.83
3 208.97
Table 8: Average liquid burst pressure strength of tested hydrogel
compositions (average
of forty-five replicate measurements).
EXAMPLE 4
The following example describes the evaluation of inventive hydrogel
compositions as a sealant for use during lung biopsy procedures in swine
models to
prevent pneumothorax complications.
Five hydrogel compositions were generated. PEG(SS)2 was handled under a
nitrogen environment. PEG(SS)2 was aliquoted into a first syringe and stored
in a sealed
pouch under an atmosphere of nitrogen gas. The rHSA-containing component
solution
used to prepare, via lyophilization, the rHSA-containing component used for
forming the
hydrogel included rHSA with added sodium carbonate, PEG 8000, and Pluronic
L61,
combined with RO water. The rHSA-containing component solution in the RO water
was lyophilized, ground into a powder, aliquoted into a second syringe, and
sealed in a
foil pouch under a nitrogen environment.
A hydration kit (e.g., a double barrel syringe) was created using DI water in
a
first compartment of the double barrel syringe (for hydrating the PEG(SS)2-
containing
component) and DI water in a second compartment of the double barrel syringe
(for
hydrating the lyophilized rHSA-containing component). The hydration kit was
sealed in
a foil pouch with connections to each syringe containing PEG(SS)2 and the rHSA-
containing component so that the powder mixtures could be hydrated with their
respective solution at the point of use.
A total of ten swine subjects were evaluated. Five of the ten swine were
designated as test subjects and were implanted with a hydrogel composition in
the left
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 66 ¨
lower lung lobe. To deliver the hydrogel composition, a coaxial technique was
utilized
with computed tomography (CT) guidance. The delivery device was inserted
through
the soft tissue until adjacent to the lung and pleural space (see FIG. 8A).
The hydrogel
composition was hydrated and deployed through the ported needle system of the
delivery
device into the subcutaneous tissue, pleural space, and the immediately
adjacent area of
the lung parenchyma (see FIG. 8B). CT imaging was used to confirm placement of
the
hydrogel.
Following successful implantation of the hydrogel composition, a lung biopsy
was taken through use of a coaxial cannula within five minutes of implantation
of the
hydrogel composition. Briefly, the needle was adjusted as needed and advanced
to the
site of the biopsy and the ported needle delivery system was removed. A
standard
biopsy needle was inserted through the coaxial system, and a standard lung
biopsy
procedure was performed continuing to use CT guidance, utilizing 16G Bard
Mission
Biopsy Needles (see FIG. 8C).
A follow-up evaluation for two of the five test subjects was performed at 72 (
8)
hours post-implantation of the hydrogel, and evaluation of the other three
swine was
performed at 144 ( 8) hours post-implantation. During the follow-up
evaluation, a CT
scan was completed to assess for the presence of the hydrogel composition and
the
presence (or absence) of pneumothorax. After completing the CT scan, the
animals were
euthanized and a comprehensive necropsy was performed with target organs
(i.e., the
lung) removed for gross pathologic observation. The inner chest wall (e.g.,
the parietal
pleura) was also examined.
The five control swine received lung biopsy procedures as described above but
without implantation of the hydrogel composition. Follow-up evaluations
(including a
.. CT scan to assess for the presence (or absence) of pneumothorax) were
performed at 48
( 8) hours post-lung biopsy. The animals were then euthanized after their CT
scan
(unless otherwise noted) and a comprehensive necropsy was performed with
target
organs removed for gross pathologic observation.
A summary of the study design is shown in Table 9.
Sealant Lung Follow Up
Cohort Subject #
Location Period
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 67 ¨
Test subject 1 Left lower lobe 6 days
Test subject 2 Left lower lobe 3 days
Test subject 3 Left lower lobe 6 days
Test subject 4 Left lower lobe 6 days
Test subject 5 Left lower lobe 3 days
Control subject 6 Left lower lobe 2 days
Control subject 7 Left lower lobe 2 days
Control subject 8 Left lower lobe 2 days
Control subject 9 Left lower lobe 2 days
Control subject 10 Left lower lobe 2 days
Table 9: Summary of swine model study design.
Each of the five test subjects were successfully implanted with the hydrogel
composition prior to the lung biopsy procedure. Deployment of the hydrogel
composition was successful and did not cause any immediate issues or concerns
to the
physician performing the procedure. No pneumothorax complications developed
during
the biopsy procedures or during the 20-30-minute monitoring period after the
procedure.
All five test subjects survived until their slated follow-up date at either
day 3 or day 6.
Furthermore, all five test subjects showed no signs of post-operative or
delayed
pneumothorax on their post CT scans (see FIGs. 9A for Subject 5, as
representative
wherein the arrow indicates the site of the hydrogel). Necropsy revealed
retained
hydrogel material, as expected at day 3 or 6. The hydrogel compositions in the
day 6 test
subjects (i.e., samples 1, 3, and 4) demonstrated a decrease in hydrogel
firmness,
indicating resorption. All five test subjects showed minor irritation on the
parietal pleura
surrounding the needle insertion site, but nothing warranting major concern.
The five control subjects received a lung biopsy without application of the
hydrogel composition. Two of the five control subjects (i.e., Subjects 9 and
10)
developed intraprocedural pneumothorax and a subsequent air embolism, as
revealed by
CT. FIG. 9B shows an example of an air embolism (circled) for Subject 9, and
FIG. 9C
shows an example of pneumothorax (circled) for Subject 10. Due to the severe
nature of
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 68 ¨
these complications, the two test subjects were terminated after completing
the biopsy.
The three remaining control subjects tolerated the lung biopsy, and CT scans
at the time
of follow up showed one swine with a large pneumothorax present (i.e., Subject
6, see
FIG. 9D, wherein the circles indicate pneumothorax). The other two swine
(i.e., Subjects
7 and 8) were clear and free of complications. Necropsy revealed nothing of
note for any
of the control subjects.
A summary of the test results are shown in Table 10. The results showed an
improvement in the outcome of the lung biopsy procedure for the test subjects
(pneumothorax rate of 0%) as compared to the control subjects (pneumothorax
rate of
60%).
Subject # Post Biopsy Follow Up Necropsy Notes
Pneumothorax Pneumothorax
1 Negative Negative
Minor irritation of parietal pleura
2 Negative Negative
Minor irritation of parietal pleura
3 Negative Negative
Very minor irritation of parietal pleura
4 Negative Negative
Minor irritation of parietal pleura
5 Negative Negative Irritation of parietal
pleura
6 Negative Positive Nothing of note
7 Negative Negative Nothing of note
8 Negative Negative Nothing of note
9 Positive N/A Nothing of note
10 Positive N/A Nothing of note
Table 10: Summary of test results for the evaluation of pneumothorax in test
and control
subjects.
While several embodiments of the present invention have been described and
illustrated herein, those of ordinary skill in the art will readily envision a
variety of other
means and/or structures for performing the functions and/or obtaining the
results and/or
one or more of the advantages described herein, and each of such variations
and/or
modifications is deemed to be within the scope of the present invention. More
generally,
those skilled in the art will readily appreciate that all parameters,
dimensions, materials,
and configurations described herein are meant to be exemplary and that the
actual
parameters, dimensions, materials, and/or configurations will depend upon the
specific
application or applications for which the teachings of the present invention
is/are used.
Those skilled in the art will recognize, or be able to ascertain using no more
than routine
CA 03175344 2022-09-13
WO 2021/189024
PCT/US2021/023359
¨ 69 ¨
experimentation, many equivalents to the specific embodiments of the invention
described herein. It is, therefore, to be understood that the foregoing
embodiments are
presented by way of example only and that, within the scope of the appended
claims and
equivalents thereto, the invention may be practiced otherwise than as
specifically
described and claimed. The present invention is directed to each individual
feature,
system, article, material, and/or method described herein. In addition, any
combination
of two or more such features, systems, articles, materials, and/or methods, if
such
features, systems, articles, materials, and/or methods are not mutually
inconsistent, is
included within the scope of the present invention.
The indefinite articles "a" and "an," as used herein in the specification and
in the
claims, unless clearly indicated to the contrary, should be understood to mean
"at least
one."
The phrase "and/or," as used herein in the specification and in the claims,
should
be understood to mean "either or both" of the elements so conjoined, i.e.,
elements that
are conjunctively present in some cases and disjunctively present in other
cases. Other
elements may optionally be present other than the elements specifically
identified by the
"and/or" clause, whether related or unrelated to those elements specifically
identified
unless clearly indicated to the contrary. Thus, as a non-limiting example, a
reference to
"A and/or B," when used in conjunction with open-ended language such as
"comprising"
can refer, in one embodiment, to A without B (optionally including elements
other than
B); in another embodiment, to B without A (optionally including elements other
than A);
in yet another embodiment, to both A and B (optionally including other
elements); etc.
As used herein in the specification and in the claims, "or" should be
understood to have
the same meaning as "and/or" as defined above. For example, when separating
items in
a list, "or" or "and/or" shall be interpreted as being inclusive, i.e., the
inclusion of at least
one, but also including more than one, of a number or list of elements, and,
optionally,
additional unlisted items. Only terms clearly indicated to the contrary, such
as "only one
of' or "exactly one of," or, when used in the claims, "consisting of," will
refer to the
inclusion of exactly one element of a number or list of elements. In general,
the term
"or" as used herein shall only be interpreted as indicating exclusive
alternatives (i.e. "one
or the other but not both") when preceded by terms of exclusivity, such as
"either," "one
of," "only one of," or "exactly one of." "Consisting essentially of," when
used in the
claims, shall have its ordinary meaning as used in the field of patent law.
CA 03175344 2022-09-13
WO 2021/189024 PCT/US2021/023359
¨ 70 ¨
As used herein in the specification and in the claims, the phrase "at least
one," in
reference to a list of one or more elements, should be understood to mean at
least one
element selected from any one or more of the elements in the list of elements,
but not
necessarily including at least one of each and every element specifically
listed within the
list of elements and not excluding any combinations of elements in the list of
elements.
This definition also allows that elements may optionally be present other than
the
elements specifically identified within the list of elements to which the
phrase "at least
one" refers, whether related or unrelated to those elements specifically
identified. Thus,
as a non-limiting example, "at least one of A and B" (or, equivalently, "at
least one of A
.. or B," or, equivalently "at least one of A and/or B") can refer, in one
embodiment, to at
least one, optionally including more than one, A, with no B present (and
optionally
including elements other than B); in another embodiment, to at least one,
optionally
including more than one, B, with no A present (and optionally including
elements other
than A); in yet another embodiment, to at least one, optionally including more
than one,
A, and at least one, optionally including more than one, B (and optionally
including other
elements); etc.
In the claims, as well as in the specification above, all transitional phrases
such as
"comprising," "including," "carrying," "having," "containing," "involving,"
"holding,"
and the like are to be understood to be open-ended, i.e., to mean including
but not limited
.. to. Only the transitional phrases "consisting of' and "consisting
essentially of' shall be
closed or semi-closed transitional phrases, respectively, as set forth in the
United States
Patent Office Manual of Patent Examining Procedures, Section 2111.03.