Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/254808 PCT/EP2021/065172
1
Process for making an electrode, and electrode active materials
The present invention is directed towards a process for making an electrode
wherein the pro-
cess comprises the following steps
(a) providing a particulate lithiated transition metal oxide according to
the formula Li1,TM1_x02
wherein xis in the range of from zero to 0.1 and TM contains nickel and at
least one of
Co, Mn and Al,
(b) mixing the lithiated transition metal oxide from step (a) with carbon
in electrically conduc-
tive form,
(c) exposing the mixture obtained in step (b) to a pressure in the range of
from 100 to 500
MPa over a period of time of from one second to one minute, thereby causing
cracks in at
least some of the particles of the electrode active material,
(d) mixing the mixture from step (c) with a binder polymer and, optionally,
with further carbon
in electrically conductive form and with a solvent,
(e) applying the mixture from step (d) to a metal foil.
Additionally, the present invention is directed to electrode active materials.
Lithiated transition metal oxides are currently being used as electrode active
materials for lithi-
um-ion batteries. Extensive research and developmental work have been
performed in the past
to improve properties like the reduced cycle life and capacity loss that may
adversely affect the
lifetime or applicability of a lithium-ion battery. Additional effort has been
made to improve man-
ufacturing methods.
In a typical process for making cathode materials for lithium-ion batteries,
first a so-called pre-
cursor is being formed by co-precipitating the transition metals as
carbonates, oxides or prefer-
ably as hydroxides that may or may not be basic. The precursor is then mixed
with a source of
lithium such as, but not limited to Li0H, Li2O or Li2CO3 and calcined (fired)
at high temperatures.
Lithium salt(s) can be employed as hydrate(s) or in dehydrated form. The
calcination ¨ or firing
¨ generally also referred to as thermal treatment or heat treatment of the
precursor ¨ is usually
carried out at temperatures in the range of from 600 to 1000 C. During the
thermal treatment a
solid-state reaction takes place, and the electrode active material is formed.
The thermal treat-
ment is performed in the heating zone of an oven or kiln.
An ongoing issue remains the problem of capacity fade. Various theories exist
about the reason
for the capacity fade, and ¨ among others ¨ the surface properties the cathode
active materials
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
2
have been modified, for example by coating with an inorganic oxide or with
polymers. All of the
suggested solutions leave room for improvement. Various theories have been
developed with
respect to the reason for such capacity fade upon repeated cycling. One of
those theories is
related to crack formation and attempts to prevent crack formation, for
example by coating
methods, US 2017/0104217, by tungsten oxide addition into the particle
interspace
EP 3 553 856, or by specific co-precipitation conditions, US 2018/0166687. All
said references
teach to avoid crack formation but leave room for improvement.
It was therefore an objective of the present invention to provide electrodes
with low capacity
fading and thus a high cycling stability. It was further an objective to
provide a process for mak-
ing such electrodes with both a low capacity fading and thus a high cycling
stability. It was fur-
ther an objective to provide electrode active materials with low capacity
fading and thus a high
cycling stability.
Accordingly, the process defined at the outset has been found, hereinafter
also referred to as
inventive process or process according to the present invention.
The inventive process comprises the following steps (a) to (e), hereinafter
also referred to as
step (a) or step (b) or step (c) or step (d) or step (e), or briefly as (a) or
(b) or (c) or (d) or (e),
respectively: Steps (a) to (e) will be described hereinafter in more detail
below.
Step (a) starts off from a lithiated transition metal oxide according to the
general formula
Li1-,,TM102 wherein xis in the range of from zero to 0.1, from zero to 0.1,
preferably 0.01 to
0.05, and TM contains nickel and at least one of Co, Mn and Al.
Said TM may contain traces of further metal ions, for example traces of
ubiquitous metals such
as sodium, calcium or zinc, as impurities but such traces will not be taken
into account in the
description of the present invention. Traces in this context will mean amounts
of 0.05 mol-(Yo or
less, referring to the total metal content of TM.
In one embodiment of the present invention lithiated transition metal oxide
according to general
formula Li1,TM102 has an average particle diameter (D50) in the range of from
3 to 20 pm,
preferably from 5 to 16 pm. The average particle diameter may be determined,
e. g., by light
scattering or LASER diffraction or electroacoustic spectroscopy. The particles
are usually com-
posed of agglomerates from primary particles, and the above particle diameter
refers to the
secondary particle diameter.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
3
In one embodiment of the present invention, the primary particles of lithiated
transition metal
oxide according to general formula Li1.E.TM1_.02 have an average diameter
(D50) in the range
from 1 to 2000 nm, preferably from 10 to 1000 nm, particularly preferably from
50 to 500 nm.
The average primary particle diameter can, for example, be determined by SEM
or TEM, or by
LASER scattering.
In one embodiment of the present invention, TM is a combination of transition
metals according
to general formula (I)
(Ni 1 ,CobMne) M -d-1 d (I)
wherein
a is in the range of from 0.6 to 1.0, preferably 0.7 to 0.9, and more
preferably 0.75 to 0.85,
b being in the range of from zero to 0.2, preferably 0.05 to 0.2,
c being in the range of from zero to 0.2, preferably 0.01 to 0.1, and
d being in the range of from zero to 0.1, preferably 0.001 to 0.005,
M1 is selected from Al, Ti, Zr, W, Nb, Ta, Mo, Mg and combinations of at least
two of the afore-
mentioned, of which Al and Ti and Zr and combinations of at least two of the
aforementioned
are preferred,
a + b + c = 1, and
at least one of b, c and d is greater than zero.
In one embodiment of the present invention, lithiated transition metal oxide
provided in step (a)
have a specific surface (BET), hereinafter also referred to as "BET surface",
in the range of from
0.1 to 1.0 m2/g. The BET surface may be determined by nitrogen adsorption
after outgassing of
the sample at 200 C for 30 minutes and, beyond this, according to DIN-ISO
9277:2003-05.
In step (b), the above lithiated transition metal oxide is mixed with carbon
in electrically conduc-
tive form, hereinafter also referred to as carbon (B). Carbon (B) can be
selected from soot, ac-
tive carbon, carbon nanotubes, graphene, and graphite. Carbon (B) can be added
as such dur-
ing the inventive process. A preferred carbon (B) is graphite.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
4
In step (b), no binder (C) is added or present. Binders (C) ¨ also referred to
as binder polymers
(C) ¨ are described in more detail below.
In step (b), it is preferred to mix lithiated transition metal oxide with
carbon (B) wherein the
quantities of carbon (B) are lower than of the lithiated transition metal
oxide. Preferably, in step
(b), the weight ratio of lithiated transition metal oxide provided in step (a)
and carbon (B) is in
the range of from 100:1 to 20:1, preferably 60:1 to 25:1.
In one embodiment of the present invention, the average particle diameter of
carbon (B) in step
(b) is in the range of from 1 to 20 pm. Preferably, carbon (B) is selected
from synthetic graphite
with platelet-shaped secondary particles.
In one embodiment of the present invention, the mixing in step (b) is
performed in a high-shear
mixer, in a plough-share mixer, in a free-fall mixer, or in a ball mill. On
laboratory scale, shakers
and roller mixers are suitable as well. On laboratory scale, mortars with
pestles are useful as
well.
In step (c), the mixture obtained in step (b) is exposed to a pressure in the
range of from 100 to
500 MPa, preferably 120 to 350 MPa, thereby causing cracks in at least some of
the particles of
the electrode active material. The crack level is in the range of from 5 to
30, preferably at least
20 and refers to at least 25% of all particles of the respective electrode
active material.
Step (c) may be performed in various types of vessels. Suitable are, for
example, isostatic
pressing devices. Isostatic pressing devices may be selected from so-called
"cold" isostatic
pressing devices and "hot" isostatic pressing devices, and they are known from
the formation of
green bodies of ceramics, and from the casting industry. On laboratory scale,
tablet presses are
useful as well.
In one embodiment of the present invention, step (c) is performed at a
temperature in the range
of from 10 to 50 C. It is possible to perform external cooling during step
(c). Preferably, step (c)
is performed without external heating or cooling.
The duration of step (c) is in the range of from one second to one minute,
preferably 5 to 30
seconds.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
In the course of step (c), cracks are formed in the particles of lithiated
transition metal oxide.
The cracks may be detected by SEM (Scanning Electron Microscopy), and the
crack level is
determined as follows: the SEM picture is analyzed by an edge detection
algorithm, which com-
putes the gradient of image intensity and thus detects the cracks.
Such cracks may have any shape. They may follow primary particles but not
affect them. In an
alternative embodiment, cracks may affect primary particles as well.
Preferably, such cracks
affect secondary particles but not primary particles. Cracks may be in one
direction only or may
display turns, for example, they may be zig-zag shaped.
In the subsequent step (d), the mixture from step (c) is mixed with a binder
polymer (C) and,
optionally, with further carbon in electrically conductive form, carbon (B),
and with a solvent.
Cathodes according to the present invention contain carbon in electrically
conductive modifica-
tion, in brief also referred to as carbon (B). Carbon (B) can be selected from
soot, active carbon,
carbon nanotubes, graphene, and graphite. Carbon (B) can be added as such
during prepara-
tion of electrode materials according to the invention.
Electrodes according to the present invention can comprise further components.
They can
comprise a current collector (D), such as, but not limited to, an aluminum
foil. They further com-
prise a binder polymer (C), hereinafter also referred to as binder (C).
Current collector (D) is not
further described here.
Suitable binders (C) are preferably selected from organic (co)polymers.
Suitable (co)polymers,
i.e. homopolymers or copolymers, can be selected, for example, from
(co)polymers obtainable
by anionic, catalytic or free-radical (co)polymerization, especially from
polyethylene, polyacrylo-
nitrile, polybutadiene, polystyrene, and copolymers of at least two comonomers
selected from
ethylene, propylene, styrene, (meth)acrylonitrile and 1,3-butadiene.
Polypropylene is also suita-
ble. Polyisoprene and polyacrylates are additionally suitable. Particular
preference is given to
polyacrylonitrile.
In the context of the present invention, polyacrylonitrile is understood to
mean not only polyacry-
lonitrile homopolymers but also copolymers of acrylonitrile with 1,3-butadiene
or styrene. Pref-
erence is given to polyacrylonitrile homopolymers.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
6
In the context of the present invention, polyethylene is not only understood
to mean homopoly-
ethylene, but also copolymers of ethylene which comprise at least 50 mol% of
copolymerized
ethylene and up to 50 mol% of at least one further comonomer, for example a-
olefins such as
propylene, butylene (1-butene), 1-hexene, 1-octene, 1-decene, 1-dodecene, 1-
pentene, and
also isobutene, vinylaromatics, for example styrene, and also (meth)acrylic
acid, vinyl acetate,
vinyl propionate, C1-C10-alkyl esters of (meth)acrylic acid, especially methyl
acrylate, methyl
methacrylate, ethyl acrylate, ethyl methacrylate, n-butyl acrylate, 2-
ethylhexyl acrylate, n-butyl
methacrylate, 2-ethylhexyl methacrylate, and also nnaleic acid, nnaleic
anhydride and itaconic
anhydride. Polyethylene may be HDPE or LDPE.
In the context of the present invention, polypropylene is not only understood
to mean homopoly-
propylene, but also copolymers of propylene which comprise at least 50 mol% of
copolymerized
propylene and up to 50 mol% of at least one further comonomer, for example
ethylene and a-
olefins such as butylene, 1-hexene, 1-octene, 1-decene, 1-dodecene and 1-
pentene. Polypro-
pylene is preferably isotactic or essentially isotactic polypropylene.
In the context of the present invention, polystyrene is not only understood to
mean homopoly-
mers of styrene, but also copolymers with acrylonitrile, 1,3-butadiene,
(meth)acrylic acid, C1-
Cio-alkyl esters of (meth)acrylic acid, divinylbenzene, especially 1,3-
divinylbenzene, 1,2-
diphenylethylene and a-methylstyrene.
Another preferred binder (C) is polybutadiene.
Other suitable binders (C) are selected from polyethylene oxide (PEO),
cellulose, carboxy-
methylcellulose, polyimides and polyvinyl alcohol.
In one embodiment of the present invention, binder (C) is selected from those
(co)polymers
which have an average molecular weight NA,, in the range from 50,000 to
1,000,000 g/mol, pref-
erably to 500,000 g/mol.
Binder (C) may be cross-linked or non-cross-linked (co)polymers.
In a particularly preferred embodiment of the present invention, binder (C) is
selected from hal-
ogenated (co)polymers, especially from fluorinated (co)polymers. Halogenated
or fluorinated
(co)polymers are understood to mean those (co)polymers which comprise at least
one
(co)polymerized (co)monomer which has at least one halogen atom or at least
one fluorine at-
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
7
om per molecule, more preferably at least two halogen atoms or at least two
fluorine atoms per
molecule. Examples are polyvinyl chloride, polyvinylidene chloride,
polytetrafluoroethylene, pol-
yvinylidene fluoride (PVdF), tetrafluoroethylene-hexafluoropropylene
copolymers, vinylidene
fluoride-hexafluoropropylene copolymers (PVdF-HFP), vinylidene fluoride-
tetrafluoroethylene
copolymers, perfluoroalkyl vinyl ether copolymers, ethylene-
tetrafluoroethylene copolymers,
vinylidene fluoride-chlorotrifluoroethylene copolymers and ethylene-
chlorofluoroethylene copol-
ymers.
Suitable binders (C) are especially polyvinyl alcohol and halogenated
(co)polymers, for example
polyvinyl chloride or polyvinylidene chloride, especially fluorinated
(co)polymers such as polyvi-
nyl fluoride and especially polyvinylidene fluoride and
polytetrafluoroethylene.
Suitable solvents that may be added are organic non-protic solvents such as N-
methyl pyrroli-
done (NMP), N-ethyl pyrrolidone (NEP), dimethyl sulfoxide (DMSO), aromatic
hydrocarbons
such as, but not limited to toluene and ethylbenzene and xylene, for example m-
xylene and o-
xylene and mixtures of the isomers of xylene.
In a preferred embodiment of the present invention, the amounts of carbon (B)
and binder pol-
ymer (C) are selected as follows:
(A) 80 to 99 % by weight cathode active material obtained from step (c),
(B) 0.5 to 19.5 % by weight of carbon,
(C) 0.5 to 9.5 % by weight of binder polymer,
percentages referring to the sum of (A), (B) and (C).
The amount of solvent ¨ if applicable ¨ is selected that a slurry or paste is
formed, for example
25 (:)/0 by weight up to the 10-fold of the sum of cathode active material
obtained from step (c)
and carbon (B) and binder polymer (C).
Mixing may be performed in any suitable vessel. Mixing is preferably performed
until a lump-
free slurry or paste is obtained.
In step (e), the mixture obtained from step (e) is applied to a metal foil,
for example an alumini-
um foil. Such metal foil then serves as a current collector. Said applying
includes placing slurry
or paste from step (d) on said metal foil, for example by spraying or by
dipping or by a doctor
blade or with a squeegee, securing the layer of slurry or paste from step (d)
to have a homoge-
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
8
neous thickness, and removing the solvent ¨ if present ¨ for example by drying
under reduced
pressure.
Step (e) may further include a calendaring step.
Cathodes made according to the inventive process are excellent components for
electrochemi-
cal cells and especially for lithium ion batteries.
Another aspect of the present invention is related to electrode active
materials, hereinafter also
referred to as inventive electrode active materials or inventive cathode
active materials. In-
ventive electrode materials may be manufactured in accordance with the
inventive process.
Inventive electrode materials are described in more detail below.
Inventive electrode materials are characterized in that they are particulate
electrode active ma-
terial according to the formula Li1-,,TM102 wherein x is in the range of from
zero to 0.1, prefer-
ably 0.01 to 0.05, and TM contains nickel and at least one of Co, Mn and Al,
for example Ni, Co
and Al or Ni, Co and Mn, or Ni, Mn and Al, and wherein at least 25 % of all
particles having a
crack level of at least 5, for example is in the range of from 5 to 30, and
wherein such cracks
contain carbon in electrically conductive form.
In one embodiment of the present invention inventive electrode active
materials have an aver-
age particle diameter (D50) in the range of from 3 to 20 pm, preferably from 5
to 16 pm. The
average particle diameter may be determined, e. g., by light scattering or
LASER diffraction or
electroacoustic spectroscopy. The particles are usually composed of
agglomerates from primary
particles, and the above particle diameter refers to the secondary particle
diameter.
In one embodiment of the present invention, the primary particles of inventive
electrode active
materials have an average diameter (D50) in the range from Ito 2000 nm,
preferably from 10 to
1000 nm, particularly preferably from 50 to 500 nm. The average primary
particle diameter can,
for example, be determined by SEM or TEM, or by LASER scattering.
In one embodiment of the present invention, TM in the formula Li1-,,TM102 is a
combination of
elements according to general formula (I)
(NiaCobMnG)i-dMid (I)
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
9
wherein
a is in the range of from 0.6 to 1.0, preferably 0.7 to 0.9, and more
preferably 0/5 to 0.85,
b being in the range of from zero to 0.2, preferably 0.05 to 0.2,
c being in the range of from zero to 0.2, preferably 0.01 to 0.1, and
d being in the range of from zero to 0.1, preferably 0.001 to 0.005,
M1 is selected from Al, Ti, Zr, W, Nb, Ta, Mo, Mg and combinations of at least
two of the afore-
mentioned, of which Al and Ti and Zr and combinations of at least two of the
aforementioned
are preferred,
and
a + b + c = 1.
Inventive electrode active materials are further characterized in that at
least 25 % of the parti-
cles have a crack level of at least 5, for example in the range of from 5 to
30, preferably of at
least 20, and such cracks contain carbon in electrically conductive form,
preferably graphite.
The cracks may be detected by SEM (Scanning Electron Microscopy), and the
crack level is
determined as follows: the respective SEM picture is analyzed by an edge
detection algorithm,
which computes the gradient of image intensity and thus detects the cracks.
In one embodiment of the present invention, inventive electrode active
materials have a specific
surface (BET) in the range of from 0.1 to 1.0 m2/g. The BET surface may be
determined by ni-
trogen adsorption after outgassing of the sample at 200 C for 30 minutes and,
beyond this, ac-
cording to DIN-ISO 9277:2003-05.
In one embodiment of the present invention, inventive electrode active
materials the weight ratio
of lithiated transition metal oxide provided and carbon (B) is in the range of
from 100:1 to 20:1,
preferably 60:1 to 25:1.
The cracks usually do not contain binder polymer (C).
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
A further aspect of the present invention is an electrochemical cell,
containing
(1) a cathode comprising inventive electrode active material (A), carbon
(B), and binder (C),
(2) an anode, and
(3) at least one electrolyte.
Embodiments of cathode (1) have been described above in detail.
Anode (2) may contain at least one anode active material, such as carbon
(graphite), TiO2, lithi-
um titanium oxide, silicon or tin. Anode (2) may additionally contain a
current collector, for ex-
ample a metal foil such as a copper foil.
Electrolyte (3) may comprise at least one non-aqueous solvent, at least one
electrolyte salt and,
optionally, additives.
Non-aqueous solvents for electrolyte (3) can be liquid or solid at room
temperature and is pref-
erably selected from among polymers, cyclic or acyclic ethers, cyclic and
acyclic acetals and
cyclic or acyclic organic carbonates.
Examples of suitable polymers are, in particular, polyalkylene glycols,
preferably poly-C1-C4-
alkylene glycols and in particular polyethylene glycols. Polyethylene glycols
can here comprise
up to 20 mol% of one or more Ci-C4-alkylene glycols. Polyalkylene glycols are
preferably poly-
alkylene glycols having two methyl or ethyl end caps.
The molecular weight M of suitable polyalkylene glycols and in particular
suitable polyethylene
glycols can be at least 400 g/mol.
The molecular weight M of suitable polyalkylene glycols and in particular
suitable polyethylene
glycols can be up to 5,000,000 g/mol, preferably up to 2,000,000 g/mol.
Examples of suitable acyclic ethers are, for example, diisopropyl ether, di-n-
butyl ether,
1,2-dimethoxyethane, 1,2-diethoxyethane, with preference being given to 1,2-
dimethoxyethane.
Examples of suitable cyclic ethers are tetrahydrofuran and 1,4-dioxane.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
11
Examples of suitable acyclic acetals are, for example, dimethoxymethane,
diethoxymethane,
1,1-dimethoxyethane and 1,1-diethoxyethane.
Examples of suitable cyclic acetals are 1,3-dioxane and, in particular, 1,3-
dioxolane.
Examples of suitable acyclic organic carbonates are dimethyl carbonate, ethyl
methyl carbonate
and diethyl carbonate.
Examples of suitable cyclic organic carbonates are compounds of the general
formulae (II) and
(III)
0 0
3 R3
R1)R2R
(II) (III)
where R1, R2 and R3 can be identical or different and are selected from among
hydrogen and
Ci-C4-alkyl, for example methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl, sec-butyl and tert-
butyl, with R2 and R3 preferably not both being tert-butyl.
In particularly preferred embodiments, R1 is methyl and R2 and R3 are each
hydrogen, or R1, R2
and R3 are each hydrogen.
Another preferred cyclic organic carbonate is vinylene carbonate, formula
(IV).
0
o7-No
\_/ (IV)
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
12
The solvent or solvents is/are preferably used in the water-free state, i.e.
with a water content in
the range from 1 ppm to 0.1% by weight, which can be determined, for example,
by Karl-Fischer
titration.
Electrolyte (3) further comprises at least one electrolyte salt. Suitable
electrolyte salts are, in
particular, lithium salts. Examples of suitable lithium salts are LiPF6,
LiBF4, LiC104, LiAsF6,
LiCF3S03, LiC(CnF2n-EiS02)3, lithium imides such as LiN(CnF2n-EiS02)2, where n
is an integer in
the range from 1 to 20, LiN(SO2F)2, Li2SiF6, LiSbF6, LiAIC14 and salts of the
general formula
(CnF2n+1S02)tYLi, where m is defined as follows:
t = 1, when Y is selected from among oxygen and sulfur,
t = 2, when Y is selected from among nitrogen and phosphorus, and
t = 3, when Y is selected from among carbon and silicon.
Preferred electrolyte salts are selected from among LiC(CF3S02)3,
LiN(CF3S02)2, LiPF6, LiBF4,
LiC104, with particular preference being given to LiPF6 and LiN(CF3S02)2.
In a preferred embodiment of the present invention, electrolyte (3) contains
at least one flame
retardant. Useful flame retardants may be selected from trialkyl phosphates,
said alkyl being
different or identical, Maryl phosphates, alkyl dialkyl phosphonates, and
halogenated trialkyl
phosphates. Preferred are tri-Ci-C4-alkyl phosphates, said Ci-C4-alkyls being
different or identi-
cal, tribenzyl phosphate, triphenyl phosphate, C1-C4-alkyl di- C1-C4-alkyl
phosphonates, and
fluorinated tri-C1-C4-alkyl phosphates,
In a preferred embodiment, electrolyte (3) comprises at least one flame
retardant selected from
trimethyl phosphate, CH3-P(0)(OCH3)2, triphenylphosphate, and tris-(2,2,2-
trifluoroethyl)-
phosphate.
Electrolyte (3) may contain 1 to 10% by weight of flame retardant, based on
the total amount of
electrolyte.
In one embodiment of the present invention, electrolyte (3) is solid at
ambient temperature and
contains sulfur and phosphorus, hereinafter also referred to as solid
electrolyte (3).
In this context, the term "solid" refers to the state of matter at ambient
temperature.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
13
In one embodiment of the present invention, solid electrolyte (3) has a
lithium-ion conductivity at
25 C of 0.1 mS/cm, preferably in the range of from 0.1 to 30 mS/cm, measurable
by, e.g.,
impedance spectroscopy.
In one embodiment of the present invention, solid electrolyte (3) comprises
Li3PS4, yet more
preferably orthorhombicp-Li3PS4.
In one embodiment of the present invention, solid electrolyte (3) is selected
from the group con-
sisting of Li2S-P2S5, Li2S-P2S5-Lil, Li2S-P2S5-Li2O, Li2S-P2S5-Li2O-Lil, L12S-
SiS2-P2S5-Lil, L12S-
P2S5-ZmSn wherein m and n are positive numbers and Z is a member selected from
the group
consisting of germanium, gallium and zinc, Li2S-SiS2-Li3PO4, Li2S-SiS2-LiyPOz,
wherein y and z
are positive numbers, Li7P3Sii, Li3PS4., LiiiS2PS12, Li7P2S8I, and Li
.74-2s PS6-r-sXir wherein X1 is
chlorine, bromine or iodine, and the variables are defined as follows:
0.8 r 1.7
0 s (-0.25 r) + 0.5.
A particularly preferred example of solid electrolytes (3) is Li6PS5CI, thus,
r = 1.0 and s = zero.
In one embodiment of the present invention, solid electrolyte (3) is doped
with at least one of Si,
Sb, Sn. Si is preferably provided as element. Sb and Sn are preferably
provided as sulfides.
In an embodiment of the present invention, batteries according to the
invention comprise one or
more separators (4) by means of which the electrodes are mechanically
separated. Suitable
separators (4) are polymer films, in particular porous polymer films, which
are unreactive toward
metallic lithium. Particularly suitable materials for separators (4) are
polyolefins, in particular
film-forming porous polyethylene and film-forming porous polypropylene.
Separators (4) composed of polyolefin, in particular polyethylene or
polypropylene, can have a
porosity in the range from 35 to 50%. Suitable pore diameters are, for
example, in the range
from 30 to 500 nm.
In another embodiment of the present invention, separators (4) can be selected
from among
PET nonwovens filled with inorganic particles. Such separators can have a
porosity in the range
from 40 to 55%. Suitable pore diameters are, for example, in the range from 80
to 750 nm.
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
14
Batteries according to the invention can further comprise a housing which can
have any shape,
for example cuboidal or the shape of a cylindrical disk. In one variant, a
metal foil configured as
a pouch is used as housing.
Batteries according to the invention provide a very good discharge and cycling
behavior, in par-
ticular at high temperatures (45 C or higher, for example up to 60 C) in
particular with respect
to the capacity loss.
Batteries according to the invention can comprise two or more electrochemical
cells that com-
bined with one another, for example can be connected in series or connected in
parallel. Con-
nection in series is preferred. In batteries according to the present
invention, at least one of the
electrochemical cells contains at least one electrode according to the
invention. Preferably, in
electrochemical cells according to the present invention, the majority of the
electrochemical
cells contain an electrode according to the present invention. Even more
preferably, in batteries
according to the present invention all the electrochemical cells contain
electrodes according to
the present invention.
The present invention further provides for the use of batteries according to
the invention in ap-
pliances, in particular in mobile appliances. Examples of mobile appliances
are vehicles, for
example automobiles, bicycles, aircraft or water vehicles such as boats or
ships. Other exam-
ples of mobile appliances are those which move manually, for example
computers, especially
laptops, telephones or electric hand tools, for example in the building
sector, especially drills,
battery-powered screwdrivers or battery-powered staplers.
The present invention is further illustrated by working examples.
General:
NMP: N-methyl pyrrolidone
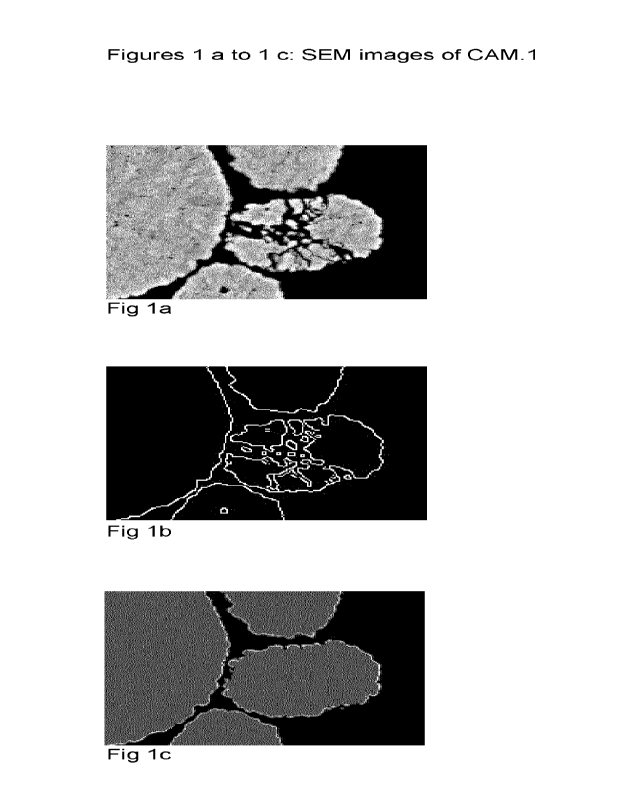
The crack level was determined as follows: the SEM pictures of arbitrarily
selected particles was
analyzed by an edge detection algorithm named "canny" which computed the
gradient of image
intensity. The algorithm applies double thresholding to determine potential
edges, and track
edge by hysteresis to detect final edge of object. It detects cracks inside of
secondary particles
and outlines of particles as edge, as, e.g., shown in figure 1 b. The outlines
of particles are re-
moved by eroding particle area, and remaining edges are defined as crack. Said
crack pixels
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
are normalized by particle area, which are shaded in grey in figure 1 c. The
detected crack pix-
els are normalized by particle are pixels, which are shown in figure 1C,
shaded area in gray.
Percentages are % by weight unless specifically indicated otherwise.
(B.1): synthetic graphite, platelet shape, 6 pm particle diameter
I. Manufacture of inventive electrode active materials
1.1 Synthesis of a precursor TM-OH.1
A stirred tank reactor was filled with deionized water and 49 g of ammonium
sulfate per kg of
water. The solution was tempered to 55 C and a pH value of 12 was adjusted by
adding an
aqueous sodium hydroxide solution.
The co-precipitation reaction was started by simultaneously feeding an aqueous
transition metal
sulfate solution and aqueous sodium hydroxide solution at a flow rate ratio of
1.8, and a total
flow rate resulting in a residence time of 8 hours. The transition metal
solution contained Ni, Co
and Mn at a molar ratio of 8.3:1.2:0.5 and a total transition metal
concentration of 1.65 mol/kg.
The aqueous sodium hydroxide solution was a 25 wt.% sodium hydroxide solution
and 25 wt.%
ammonia solution in a weight ratio of 6. The pH value was kept at 12 by the
separate feed of an
aqueous sodium hydroxide solution. Beginning with the start-up of all feeds,
mother liquor was
removed continuously. After 33 hours all feed flows were stopped. The mixed
transition metal
(TM) hydroxide precursor TM-OH.1, average particle diameter (D50): 10.6 pm,
was obtained by
filtration of the resulting suspension, washing with distilled water, drying
at 120 C in air and
sieving.
1.2 Conversion of TM-OH.1 into cathode active materials
1.2.1 Manufacture of a base cathode active material, B-CAM.1, step (a.1)
B-CAM.1 (base): The mixed transition metal hydroxide precursor TM-OH.1 was
mixed with Li-
OH monohydrate in a Li/(TM) molar ratio of 1.03, and with TiO2 and ZrO2. The
amounts of Ti
and Zr were adjusted to obtain 0.17% Ti as well as 0.17% Zr by mole referred
to the total
amount of TM (TM = Ni + Co + Mn + Ti + Zr). The resultant mixture was heated
to 780 C and
kept for 10 hours in a forced flow of a mixture of 60% oxygen and 40% nitrogen
(by volume).
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
16
After cooling to ambient temperature, the resultant powder was deagglomerated
and sieved
through a 32 pm mesh to obtain the base cathode active material B-CAM 1.
D50 = 10.6 pm determined using the technique of laser diffraction in a
Mastersize 3000 instru-
ment from Malvern Instruments. Residual moisture at 250 C was determined to
be 300 ppm.
Specific surface (BET): 0.17 m2/g.
1.2.2 Mixing with carbon (B), step (b.1)
B-CAM.1 and (B.1) were mixed in a weight ratio of 100:3 at ambient temperature
in a bottle on a
roller mixer for one hour. A mixture was obtained.
1.2.3 Exposure to pressure, step (c.1)
An amount of 5 g of a mixture obtained from step (b.1) was filled into a
pellet press, inner diam-
eter 16.07 mm, and pressed at 150 MPa for 10 seconds. The pressure was then
released and
the tablet-shaped body formed under the pressure was removed from the tablet
press, gently
delumped and sieved through a 32 pm vibrational screen. A free-flowing powder
of CAM.1 was
obtained. By image analysis, a crack level of 5.9 was determined.
1.2.4 Exposure to pressure, step (c.2)
An amount of 5 g of a mixture obtained from step (b.1) was filled into a
pellet press, inner diam-
eter 16.07 mm, and pressed at 300 MPa for 10 seconds. The pressure was then
released and
the tablet-shaped body formed under the pressure was removed from the tablet
press, gently
delumped and sieved through a 32 pm vibrational screen. A free-flowing powder
of CAM.2 was
obtained. By image analysis, a crack level of 21.1 was determined.
II. Manufacture of electrodes
11.1 Cathode manufacture, steps (d) and (e)
Step (d.1) and (d.2) and C-(d.3): PVDF binder (Solef0 5130) was dissolved in
NMP (Merck) to
produce an 8.0 wt.% solution. For electrode preparation, binder solution (4
wt.%), and carbon
black (Li250, 3.5 wt.-%) were suspended in NMP. After mixing using a planetary
centrifugal
mixer (ARE-250, Thinky Corp.; Japan), either any of inventive CAM.1 (step
(d.1) or CAM.2 (step
(d.2) or base cathode active material B-CAM.1, step C-(d.3), (92.5 wt.%) was
added and the
CA 03181076 2022- 12- 1
WO 2021/254808
PCT/EP2021/065172
17
suspension was mixed again to obtain a lump-free slurry. The solid content of
the slurry was
adjusted to 65%.
Step (e.1): The slurry from (d.1) was coated onto Al foil using a KTF-S roll-
to-roll coater (Mathis
AG).
Step (e.2): The slurry from (d.2) was coated onto Al foil using a KTF-S roll-
to-roll coater (Mathis
AG).
Step C-(e.3): The slurry from C-(d.1) was coated onto Al foil using a KTF-S
roll-to-roll coater
(Mathis AG).
Electrode coating corresponded to 15 mg/cm2. All electrodes were dried at 120
C for 7 hours
before battery assembly.
11.2 Electrolyte Manufacture
A base electrolyte composition was prepared containing 12.7 wt% of LiPF6, 26.2
wt% of eth-
ylene carbonate (EC), and 61.1 wt% of ethyl methyl carbonate (EMC) (EL base
1), based on the
total weight of EL base 1. To this base electrolyte formulation 2wt.% of
vinylene carbonate (VC)
was added (EL base 2).
11.3 Test cell Manufacture
Coin-type half cells (20 mm in diameter and 3.2 mm in thickness) comprising a
cathode pre-
pared as described under 111.1.1 and lithium metal as working and counter
electrode, respective-
ly, were assembled and sealed in an Ar-filled glove box. In addition, the
cathode and anode and
a separator were superposed in order of cathode 1/ separator // Li foil to
produce a half coin cell.
Thereafter, 0.15 mL of the EL base 1 which is described above (111.2) were
introduced into the
coin cell.
III. Evaluation of coin half-cell performance
Cell performance were evaluated using the produced coin type battery. For the
battery perfor-
mances, initial capacity and reaction resistance of cell were measured. The
initial performance
and rate performance were measured as follows: Coin half cells according to
11.3.1 were tested
in a voltage range between 4.3 V to 3.0 V at 25 C. For the initial cycles, the
initial lithiation was
conducted in the CC-CV mode, i.e., a constant current (CC) of 0.1 C was
applied until reaching
0.01 C. Reductive lithiation was carried out at constant current of 0.1 C up
to 3.0V. After for-
CA 03181076 2022- 12- 1
WO 2021/254808 PCT/EP2021/065172
18
mation cycle, rate property was measured with 3C discharge. Relative rate
performance[%] is
based on discharge capacity at 0.1C. The results are summarized in Table 1.
Cycle performance was tested as follows; After the evaluation of initial
performance, the coin
cells were cycled with 0.50-CC-CV charge and 1C CC discharge at 25 deg. Direct
current inter-
nal resistance ("DCIR") was measured at before and after cycle, by applying
0.5C discharge for
30 seconds at state of charge 50%. Cycle retention and DCIR increase are based
on those of
before cycle as 100%. The results are summarized in Tables 1 and 2.
Table.1 Cell initial performance
Sample Crack level 1' discharge capacity [mA=h/g]
Rate performance [c/o]
CAM.1 5.9 195.8 87.0
CAM.2 21.1 197.5 87.2
B-CAM.1 1.7 193.2 85.9
Table.2 Cell cycle performance
Sample Cycle retention DCIR
increase
CAM.1 92.0
179.3
CAM.2 92.0
171.5
B-CAM. 1 92.1
187.6
CA 03181076 2022- 12- 1