Note: Descriptions are shown in the official language in which they were submitted.
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
DEVICE ALLOWING LARGE BORE TRANSSEPTAL ACCESS
WITH SUBSEQUENT ATRIAL RE-ACCESS
AND METHOD THEREOF
RELATED DISCLOSURE
[0001] This disclosure claims priority to U.S. Provisional Application
Serial No.
63/036,435 filed June 8, 2020 titled Large Bore Septal Closure and U.S.
Provisional
Application Serial No. 62/873,383 filed July 12, 2019 titled Large Bore Atrial
Preclose,
both of which are hereby incorporated by reference in their entirety.
TECHNICAL FIELD
[0002] The present disclosure relates to medical devices and more
particularly, to
transcatheter-delivered interatrial septal crossing and closing techniques for
a large
bore instrument allowing access into an atrium (e.g., the left atrium) with
subsequent
atrial re-access.
BACKGROUND
[0003] An increasingly common approach for left heart catheter procedures
may
be to puncture and cross an interatrial septum using a mechanical or radio
frequency
(RF) powered needle. This procedure is generally straightforward for small
bore
catheters, which are typically less than 24 French. If larger catheters or
bores,
however, are to be sent across the atrial septum, a puncture site dilation is
typically
used in order to advance the catheter through the septum. Current
methodologies for
dilating the initial septal puncture site may involve the use of a dilator, or
by inflating a
balloon, to open the access site. This may use multiple tool exchanges by the
physician and may have undesirable consequences on the tissue due to the
uncontrolled nature of the dilation techniques.
[0004] In addition, minimally-invasive, catheter-based therapies are being
developed that allow physicians to provide treatments to patients whose
existing
comorbidities may preclude them from having a needed, but more invasive,
surgical
procedure. Over the last few years, catheter based procedures have developed
which
may involve implantation of repair or replacement mitral valves, which may use
large
- 1 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
bore transseptal access. The transseptal puncture may result in the formation
of an
iatrogenic atrial septal defect which may need to be subsequently closed by an
atrial
septal defect device. However, that atrial septal defect device may preclude,
or make
difficult, subsequent transseptal crossing.
[0005] The present disclosure provides for a device allowing large bore
transseptal
access with subsequent atrial re-access and method thereof that addresses the
above
identified concerns. A controlled and precise atrial septostomy that permits
passage
of the large bore device across the interatrial septum and then provides a
rapid and
permissive closure of the procedurally created atrial septal defect is
described herein.
The word permissive may be defined as a mechanism which the septal defect is
closed
and may allow future crossings of the interatrial septum by standard
transseptal
methods. Other benefits and advantages will become clear from the disclosure
provided herein and those advantages provided are for illustration. The
statements in
this section merely provide the background related to the present disclosure
and does
not constitute prior art.
SUMMARY
[0006] This summary is provided to introduce a selection of concepts in a
simplified
form that are further described below in the DESCRIPTION OF THE DISLCOSURE.
This summary is not intended to identify key features of the claimed subject
matter,
nor is it intended to be used as an aid in determining the scope of the
claimed subject
matter.
[0007] According to one aspect of the present disclosure, a vascular device
for
performing a transseptal puncture is provided. The device may include a body,
an
anchor extending from a distal end of the body through a shaft disposed within
the
body, at least one suture coupled to at least one needle within the anchor, at
least one
catch extending from the body to pull the at least one needle into the body
for placing
the at least one suture, and a cutting implement between the body and anchor
coupled
to an actuating shaft aligned with the at least one suture.
[0008] According to another aspect of the present disclosure, a septal
orifice
closure apparatus allowing re-access is provided. The apparatus may include a
body
- 2 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
on a first side of a septal orifice in a septum of a heart, an anchor on a
second side of
the septal orifice extending from a distal end of the body through a shaft
disposed
within the body, at least one suture coupled to at least one needle disposed
within the
anchor, at least one catch extending from the body to pull the at least one
needle into
the body for placing the at least one suture, and a cutting implement between
the body
and anchor coupled to an actuating shaft aligned with the at least one suture.
[0009] According to yet another aspect of the present disclosure, a
vascular closure
apparatus is provided. The apparatus may include an anchor positioned through
a
puncture in a vessel wall and operable between retracted and expanded
positions from
a body, at least one suture disposed within the anchor, at least one needle
coupled to
the at least one suture extending through the vessel wall adjacent to the
puncture to
connect the at least one suture when the anchor is in the expanded position,
at least
one catch extending from the body to pull the at least one needle into the
body for
placing the at least one suture, and a cutting implement between the body and
anchor
coupled to an actuating shaft aligned with the at least one suture.
[0010] According to another aspect of the present disclosure, a method of
performing a septal crossing in a vessel wall is provided. The method may
include
providing a delivery catheter having a body and an anchor, inserting the
anchor
through a puncture in the vessel wall, operating the anchor into an expanded
position
capturing the vessel wall between the body and the anchor to expose at least
one
needle, capturing the at least one needle through the vessel wall adjacent to
the
puncture and into engagement with at least one suture, and positioning the at
least
one suture in the vessel wall.
[0011] According to one aspect of the present disclosure, a vascular
apparatus is
provided. The apparatus may include a delivery system having at least one
anchor
penetrating a tissue plane, the at least one anchor having a suture, a cutting
implement
positioned into the tissue plane facilitating an incision, a therapeutic
instrument
advanced into the incision, and a fastener securing the suture with tissue of
the tissue
plane.
[0012] According to yet another aspect of the present disclosure, a septal
orifice
closure apparatus is provided. The apparatus may include a first pledget
introduced
- 3 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
into a tissue plane through a cannula, wherein the first pledget is coupled to
a control
line tensioning the first pledget after introduction into the tissue plane, a
second
pledget introduced into the tissue plane through the cannula, wherein the
second
pledget is coupled to a control line tensioning the second pledget after
introduction
into the tissue plane, a cutting implement making an incision between the
first pledget
and second pledget, a therapy device passed through the incision, and a knot
made
of the control line of the first pledget and the control line of second
pledget tensioning
the first pledget and second pledget with tissue from the tissue plane
therebetween.
[0013] According to another aspect of the present disclosure, a device for
puncturing an atrial septum of a patient is provided. The device may include a
body, a
tip extending from a distal end of the body, and a cutting member in a
collapsed state
disposed between the body and tip, wherein the tip followed by the cutting
member
penetrates into a tissue plane, the cutting member expanded after passing
through the
tissue plane.
[0014] According to one aspect of the present disclosure, a vascular
apparatus is
provided. The apparatus may include a delivery system, a tip extending from a
distal
end of the delivery system, and a cutting implement disposed between the
delivery
system and tip.
[0015] According to another aspect of the present disclosure, a method of
instrumenting the left atrium is provided. The method may include puncturing a
septum with a needle, placing at least one suture behind the septum, advancing
a
therapeutic instrument into the puncture, and cinching the at least one suture
closing
the puncture.
[0016] According to another aspect of the present disclosure, a method of
closing
a septal orifice is provided. The method may include creating a transseptal
access
through a wire, inserting a delivery catheter over the wire, enlarging the
transseptal
access through a cutting implement of the delivery catheter, inserting at
least one
suture coupled to a needle that passes around the transseptal access, cinching
the
transseptal access with the at least one suture, and removing the delivery
catheter.
- 4 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] The novel features believed to be characteristic of the disclosure
are set
forth in the appended claims. In the descriptions that follow, like parts are
marked
throughout the specification and drawings with the same numerals,
respectively. The
drawing FIGURES are not necessarily drawn to scale and certain FIGURES may be
shown in exaggerated or generalized form in the interest of clarity and
conciseness.
The disclosure itself, however, as well as a preferred mode of use, further
objectives
and advantages thereof, will be best understood by reference to the following
detailed
description of illustrative embodiments when read in conjunction with the
accompanying drawings, wherein:
[0018] FIG. 1 is a front view schematic representation of an illustrative
human
venous circulatory system of a patient with a guidewire routed from the
femoral vein
into the right atrium in accordance with one aspect of the present disclosure;
[0019] FIG. 2 is a front view schematic representation of the illustrative
human
venous circulatory system of the patient with an exemplary vascular device
advanced
into a right atrium in accordance with one aspect of the present disclosure;
[0020] FIG. 3 is a cross-sectional illustration of a heart with the
exemplary vascular
device positioned at an atrial septum and a septal penetrator advanced across
the
atrial septum into a left atrium in accordance with one aspect of the present
disclosure;
[0021] FIG. 4 is a cross-sectional illustration of the heart with the
exemplary
vascular device advanced into the left atrium across the atrial septum and the
septal
penetrator withdrawn in accordance with one aspect of the present disclosure;
[0022] FIG. 5 is a cross-sectional illustration of the heart showing
illustrative initial
incisions followed by lengthening them in accordance with one aspect of the
present
disclosure;
[0023] FIG. 6 is an illustrative side view of the exemplary vascular device
in
accordance with one aspect of the present disclosure;
- 5 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0024] FIG. 7 is an isometric view of a distal end of the exemplary
vascular device
in a low profile condition in accordance with one aspect of the present
disclosure;
[0025] FIG. 8 is an isometric view of the distal end of the exemplary
vascular device
viewed from a different perspective with a portion of the device advanced
revealing
components used to puncture tissue and pass sutures in accordance with one
aspect
of the present disclosure;
[0026] FIG. 9 is an isometric view of the distal end of the exemplary
vascular device
with the portion of the device advanced through the tissue of the atrial
septum in
accordance with one aspect of the present disclosure;
[0027] FIG. 10 is a cross sectional view of the exemplary vascular device
having
an illustrative lumen configuration in accordance with one aspect of the
present
disclosure;
[0028] FIG. 11 is an isometric, sectioned view of the distal end of the
exemplary
vascular device revealing the inner geometry of components therein in
accordance
with one aspect of the present disclosure;
[0029] FIG. 12 is an isometric view of the distal end of the exemplary
vascular
device revealing snares that have been advanced out of a body of the vascular
device
in accordance with one aspect of the present disclosure;
[0030] FIG. 13 is an isometric view of the distal end of the exemplary
vascular
device after four suture needles and suture ends have been passed through the
tissue,
snared and pulled through the length of the device in accordance with one
aspect of
the present disclosure;
[0031] FIG. 14 is an isometric view of the distal end of the exemplary
vascular
device after the suture has been pulled completely through the length of the
device
and is now pulled taught against the tissue in accordance with one aspect of
the
present disclosure;
- 6 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0032] FIG. 15 is an isometric view of the distal end of the vascular
device after a
cutting implement has been partially advanced revealing the cutting elements
in
accordance with one aspect of the present disclosure;
[0033] FIG. 16 is an isometric view of the distal end of the vascular
device with the
cutting implement expanded in accordance with one aspect of the present
disclosure;
[0034] FIG. 17. is an isometric view of the exemplary vascular device
positioned to
cut the tissue in accordance with one aspect of the present disclosure;
[0035] FIG. 18 is an isometric view of sutures that would be inserted into
the tissue
in accordance with one aspect of the present disclosure;
[0036] FIG. 19 is an isometric view of an incision after the tissue is
engaged with
the suture in accordance with one aspect of the present disclosure;
[0037] FIGS. 20A-L are various illustrative cut patterns that may be
created using
the vascular device in the atrial septum which may be made from at least one
cutting
implement that may be rotated and used multiple times in accordance with one
aspect
of the present disclosure;
[0038] FIG. 21 is an isometric view of an illustrative cut pattern for
incision to
promote tissue edge apposition in accordance with one aspect of the present
disclosure;
[0039] FIG. 22 is an isometric view of the illustrative cut pattern for
incision to
promote the tissue edge apposition, while under slight tension, demonstrating
tissue
edge control and overlap of edges with tension applied in accordance with one
aspect
of the present disclosure;
[0040] FIG. 23 is an isometric view of the cut patterns for incision to
promote the
tissue edge apposition and an illustrative helical anchor controlling tissue
edges in
accordance with one aspect of the present disclosure;
[0041] FIG. 24 is an isometric view of an illustrative expandable radio
frequency
(RF) cutting implement with four expandable members in accordance with one
aspect
of the present disclosure;
- 7 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0042] FIG. 25 is an isometric view of an exemplary vascular device with
the
expandable cutting implement having a tip for piercing the tissue in
accordance with
one aspect of the present disclosure;
[0043] FIG. 26 is an isometric view of the exemplary vascular device with
the
illustrative expandable cutting implement placed beyond the tissue in
accordance with
one aspect of the present disclosure;
[0044] FIG. 27 is an isometric view of the exemplary vascular device with
the
illustrative expandable cutting implement making incisions into the tissue in
accordance with one aspect of the present disclosure;
[0045] FIG. 28 is an isometric view of the exemplary vascular device with
the
illustrative expandable cutting implement advancing anchor mechanisms in
accordance with one aspect of the present disclosure;
[0046] FIG. 29 is an isometric view of the exemplary vascular device with
the
illustrative expandable cutting implement with push anchors further inserted
to
advance the anchor mechanisms in accordance with one aspect of the present
disclosure;
[0047] FIG. 30 is an isometric view of the exemplary vascular device having
the
anchor mechanisms removed in accordance with one aspect of the present
disclosure;
[0048] FIG. 31 is an isometric view of the exemplary cutting implement
removed
from the tissue leaving tissue anchors against it in accordance with one
aspect of the
present disclosure;
[0049] FIG. 32 is an isometric view of an exemplary toggle within the
tissue in
accordance with one aspect of the present disclosure;
[0050] FIG. 33 is an isometric view of an illustrative cutting implement
having an
atraumatic tip deployed from an exemplary vascular device in accordance with
one
aspect of the present disclosure;
- 8 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0051] FIG. 34 is an isometric view of an illustrative cutting implement
having a slit
in a sheath deployed from an exemplary vascular device in accordance with one
aspect of the present disclosure;
[0052] FIG. 35 is a side view of the illustrative cutting implement having
a cutting
element extending from the slit in the sheath in accordance with one aspect of
the
present disclosure;
[0053] FIG. 36 is an isometric view of a distal end of the exemplary
cutting
implement in accordance with one aspect of the present disclosure;
[0054] FIG. 37 is a cross-sectional illustration of the cutting implement
in
accordance with one aspect of the present disclosure;
[0055] FIGS. 38A-E are schematics showing how the exemplary cutting
implements may be used to optimize cutting performance and minimize power
input
in accordance with one aspect of the present disclosure;
[0056] FIG. 39 is an isometric view of exemplary tissue being crossed using
an
illustrative guidewire in accordance with one aspect of the present
disclosure;
[0057] FIG. 40 is an isometric view of suture anchors in delivery sheaths
in
accordance with one aspect of the present disclosure;
[0058] FIG. 41 is an isometric view of illustrative suture anchors in
delivery sheaths
about to penetrate the tissue in accordance with one aspect of the present
disclosure;
[0059] FIG. 42 is an isometric view of the illustrative suture anchors in
delivery
sheaths engaging or penetrating the tissue in accordance with one aspect of
the
present disclosure;
[0060] FIG. 43 is an isometric view of the illustrative suture anchors in
delivery
sheaths engaging the tissue with suture control lines attached in accordance
with one
aspect of the present disclosure;
[0061] FIG. 44 is an isometric view of an illustrative cutting implement in
a sheathed
position in accordance with one aspect of the present disclosure;
- 9 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0062] FIG. 45 is an isometric view of the illustrative cutting implement
in an
unsheathed position in accordance with one aspect of the present disclosure;
[0063] FIG. 46 is an isometric view of the illustrative cutting implement
in an
unsheathed position and expanded in accordance with one aspect of the present
disclosure;
[0064] FIG. 47 is an isometric view of the illustrative cutting implement
making an
incision or cut into the tissue in accordance with one aspect of the present
disclosure;
[0065] FIG. 48 is an isometric view of an illustrative incision or cut in
the tissue with
a guidewire passing through it in accordance with one aspect of the present
disclosure;
[0066] FIG. 49 is an isometric view of an advancing of an exemplary
therapeutic
instrument passing through the cut in the tissue over the guidewire in
accordance with
one aspect of the present disclosure;
[0067] FIG. 50 is an isometric view of an illustrative tissue anchor lock
with helical
barbs in accordance with one aspect of the present disclosure;
[0068] FIG. 51 is an isometric view of the illustrative tissue anchor lock
with helical
barbs engaged in the tissue passed over the suture control lines in accordance
with
one aspect of the present disclosure;
[0069] FIG. 52 is an isometric view of the illustrative tissue anchor lock
with helical
barbs engaged in the tissue passed over the suture control lines and the
control lines
trimmed to the level of the anchor in accordance with one aspect of the
present
disclosure;
[0070] FIG. 53 is an isometric view of the illustrative tissue anchor lock
with helical
barbs engaged in an other side of the tissue in accordance with one aspect of
the
present disclosure;
[0071] FIG. 54 is an isometric view of an illustrative pledget made from
biocompatible or bio-absorbable material in accordance with one aspect of the
present
disclosure;
- 1 0 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0072] FIG. 55 is an isometric view of an exemplary cannula piecing heart
tissue in
accordance with one aspect of the present disclosure;
[0073] FIG. 56 is an isometric view of the exemplary cannula piecing heart
tissue
and the illustrative pledget made from biocompatible or bioabsorbable material
advanced through the cannula in accordance with one aspect of the present
disclosure;
[0074] FIG. 57 is an isometric view of the exemplary cannula piecing heart
tissue
and the illustrative pledget made from biocompatible or bioabsorbable material
advanced out of the cannula in accordance with one aspect of the present
disclosure;
[0075] FIG. 58 is an isometric view of the exemplary cannula piecing heart
tissue
and the illustrative pledget made from biocompatible or bioabsorbable material
advanced out of the cannula and a control line tensioned to shorten the
pledget in
accordance with one aspect of the present disclosure;
[0076] FIG. 59 is an isometric view of the illustrative pledget made from
biocompatible or bioabsorbable material tensioned to shorten the pledget with
the
exemplary cannula piecing heart tissue withdrawn and retained on a heart
tissue
surface in accordance with one aspect of the present disclosure;
[0077] FIG. 60 is an isometric view of an illustrative concentric pledget
made from
biocompatible or bioabsorbable material in accordance with one aspect of the
present
disclosure;
[0078] FIG. 61 is an isometric view of the exemplary cannula piecing heart
tissue
next to the pledget in accordance with one aspect of the present disclosure;
[0079] FIG. 62 is an isometric view of the exemplary cannula piecing heart
tissue
and the illustrative concentric pledget made from biocompatible or
bioabsorbable
material advanced out of the cannula in accordance with one aspect of the
present
disclosure;
[0080] FIG. 63 is an isometric view of the exemplary cannula piecing heart
tissue
and the illustrative concentric pledget made from biocompatible, or
bioabsorbable
-11-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
material advanced out of the cannula and a control tensioned to shorten the
pledget
in accordance with one aspect of the present disclosure;
[0081] FIG. 64 is an isometric view of the illustrative concentric pledget
made from
biocompatible or bioabsorbable material tensioned to shorten the pledget and
an
exemplary incision made between the pledgets in accordance with one aspect of
the
present disclosure;
[0082] FIG. 65 is an isometric view of an exemplary therapeutic instrument
placed
into the incision between the pledgets into the tissue in accordance with one
aspect of
the present disclosure;
[0083] FIG. 66 is an isometric view of an illustrative knot advanced up to
a heart
tissue with the two pledget control lines in accordance with one aspect of the
present
disclosure;
[0084] FIG. 67 is an isometric view of the illustrative knot advanced up to
the heart
tissue with the two pledget control lines and tightened from a knot side of
the tissue in
accordance with one aspect of the present disclosure;
[0085] FIG. 68 is an isometric view of the illustrative knot advanced up to
the tissue
with the two pledget control lines tightened from the pledget side in
accordance with
one aspect of the present disclosure;
[0086] FIG. 69 is an isometric view of the illustrative incision closed
between the
pledgets in accordance with one aspect of the present disclosure; and
[0087] FIG. 70 is an illustrative flow chart showing exemplary processes
for
allowing a large bore transseptal access with subsequent atrial re-access in
accordance with one aspect of the present disclosure.
DESCRIPTION OF THE DISCLOSURE
[0088] The description set forth below in connection with the appended
drawings
is intended as a description of exemplary embodiments of the disclosure and is
not
intended to represent the only forms in which the present disclosure may be
constructed and/or utilized. The description sets forth the functions and the
sequence
-12-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
of blocks for constructing and operating the disclosure in connection with the
illustrated
embodiments. It is to be understood, however, that the same or equivalent
functions
and sequences may be accomplished by different embodiments that are also
intended
to be encompassed within the spirit and scope of this disclosure.
[0089] The present disclosure relates to medical devices. More
particularly, this
disclosure describes a vascular device allowing large bore transseptal access
with
subsequent atrial re-access by preplacing closures/tissue approximating
sutures prior
to creating a septostomy. Generally, the device may include a delivery
catheter for
puncturing and cutting the interatrial septum. An anchor of the delivery
catheter may
secure the suture in an atrium to a septum wall, for example, the left atrium.
Incisions
may be made by an expandable cutting implement which may use mechanical or
radio
frequency (RF) energy without interfering with the suture. The suture may be
made
of a high temperature resistant material to prevent damage if it is in contact
with the
cutting implement. A therapeutic instrument may be advanced through the tissue
plane after the incisions are made by the cutting implement. Closure of the
incision
may be performed with the previously placed sutures.
[0090] Numerous other modifications or configurations to the vascular
device will
become apparent from the description provided below. For example, closing the
incision of the interatrial septum may involve needles that may puncture the
septum
and pass push anchors into the tissue. Control lines tied to two or more
pledgets may
be also used and placed through the tissue plane via a cannula to promote
tissue edge
overlap and apposition.
[0091] Advantageously, the initial puncture with suture management nearby
allows
for a rapid closure of the procedurally created iatrogenic atrial septal
defect (ASD)
while permissively allowing multiple instruments within the single delivery
catheter
access at the atrial septum. Incisions made therefrom are easily cinched
allowing re-
access through the anchors/sutures. The vascular device may be useful in
procedures
requiring large bore trans-venous access to a left atrium for transcatheter
mitral valve
replacement, where the delivery systems commonly create a large residual ASD.
Other benefits and advantages will become clear from the disclosure provided
herein
and those advantages provided are for illustration.
-13-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0092] FIGS. 1-5 represent a human venous circulatory system including a
heart
with an exemplary vascular device with guidewire defined therein while FIGS. 6-
23
describe a first embodiment of the device with illustrative incisions. FIGS.
24-38
describe a second embodiment of the vascular device with additional
illustrative
incisions. FIGS. 39-53 describe a third embodiment with delivery sheaths and a
helical
anchor for tying tissue together. FIGS. 54-69 provide a fourth embodiment
localizing
a plurality of pledgets and sutures made of biodegradable materials secured
together
with a knot to allow re-access. FIG. 70 provides for different techniques.
Components
described below within the embodiments may be interchanged, removed, or added
within or to one another to come up with derivatives of the device which are
within the
scope of the present disclosure.
[0093] Turning to FIG. 1, a front view schematic representation of an
illustrative
human venous circulatory system of a patient 100 with a guidewire 102 routed
from a
femoral vein 104 into a right atrium 106 in accordance with one aspect of the
present
disclosure is provided. The guidewire 102 may permit a continuous presence
whereby
multiple tools may be exchanged. For example, these tools may provide
placement
of pre-closure sutures, subsequent controlled atrial septostomy by a
retractable blade,
and delivery of a large bore catheter or other medical device to the left
atrium.
[0094] Initially, as shown, a vascular introduction sheath 112 may be
inserted into
the right femoral vein 104 via a percutaneous puncture or incision.
Alternatively, the
vascular introduction sheath 112 may be placed into a non-femoral site such as
a
jugular vein, subclavian artery, subclavian vein, or brachial artery and vein.
Other
approaches or access sites may include an approach of the opposite leg from
the
therapy catheter.
[0095] The guidewire 102 may be inserted through a vascular introduction
sheath
112 and routed cranially up the inferior vena cava 110 to the right atrium
106, one of
the chambers of the heart 108. In this illustration, the left anatomical side
of the
patient 100 is toward the right. The guidewire 102 may be placed so that it is
used to
direct therapeutic or diagnostic catheters into a region of the heart 108.
[0096] The venous circulation, through which the guidewire 102 has been
routed,
may generally be at a lower pressure between 0 and 20 mm Hg than is the
systemic
-14-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
circulation, of which the descending aorta is a part of. The
pressure within the
systemic circulation may range from 60 to over 300 mm Hg depending on the
level of
hypertension or hypotension existent in the patient 100. By accessing the
heart 108
through the venous circulation, at the femoral vein 104, the chance of
hemorrhage
from the catheter insertion site may be minimized.
[0097]
FIG. 2 is a front view schematic representation of the illustrative human
venous circulatory system of the patient 100 with an exemplary vascular device
200
advanced into a right atrium 106 in accordance with one aspect of the present
disclosure. The view is a frontal illustration, looking posteriorly from the
anterior side
of the patient 100. The vascular introduction sheath 112 of FIG. 1 has been
removed
from the right femoral vein 104 and a vascular device 200 has been inserted
into the
venous circulation over the guidewire 102. The device 200 may be routed
through the
inferior vena cava 110 into the right atrium 106 of the heart 108 through the
same
guidewire 102 used by the introduction sheath.
[0098]
With reference to FIG. 3, a cross-sectional illustration of the heart 108 with
the exemplary vascular device 200 positioned at an atrial septum 300 and a
septal
penetrator 304 advanced across the atrial septum 300 into a left atrium 302 in
accordance with one aspect of the present disclosure is shown. An ascending
aorta,
aortic valve, pulmonary artery, and pulmonary valve have been removed from
this
illustration for clarity and to show the atrial septum 300. The body of the
vascular
device 200, substantially located within the right atrium 106, is shown with
its long axis
perpendicular to the atrial septum 300. The proximal end of the vascular
device 200 is
shown resident within the inferior vena cava 110. A septal penetrator 304 is
shown
extended through a puncture 306 in the atrial septum 300 and is routed into
the left
atrium 302 on a distal end of the vascular device 200.
[0099] The
septal penetrator 304 may be a needle or axially elongate structure with
a sharp, pointed distal end. The septal penetrator 304 may be resident within
the
guidewire 102, with the penetrator 304 being removable. The
septal
penetrator 304 may be actuated at the proximal end of the vascular device 200
through a control mechanism such as a button, lever, handle, or trigger which
may be
-15-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
affixed, permanently or removably by way of a linkage, pusher rod, electrical
bus, or
the like that runs the length of the device 200.
[0100] In operation, the septal penetrator 304 through a wall of the left
atrium 302 opposite the atrial septum 300 may be guided and advanced using
fluoroscopy, magnetic resonance imaging (MRI), ultrasound, or the like. Care
may be
taken not to inadvertently pierce the aorta through the penetrator 304 in the
region
upstream or anatomically proximal to an aortic arch of the patient 100. The
distal
portion of the vascular device 200 may be bent, deflected, or articulated
through an
angle of between 30 and 120 degrees to achieve approximate perpendicularity
with
the atrial septum 300.
[0101] The septal penetrator 304 may be solid, it may be hollow like a
hypodermic
needle, or it may have a "U" or "C"-shaped cross-section. The center or core
of a
hollow "C" or "U"-shaped septal penetrator 304 may be filled with a guidewire
or other
core element to prevent incorrect tissue penetration. The septal penetrator
304 may
be rigid or it may be flexible but retain column strength. Such flexible
configurations
may include cutouts in the wall of the penetrator 304 or guidewire-like
construction.
The septal penetrator 304 may be initially straight or it may be initially
curved. The
septal penetrator 304 may be fabricated from shape memory material such as
nitinol and heat treated to cause curving once the material is heated from
martensitic
to austenitic temperatures. Such heating may be performed using electrical
heating,
hot water injection, or the like. The septal penetrator may utilize energy to
facilitate
puncture of the septal tissue such as RF (Radio Frequency).
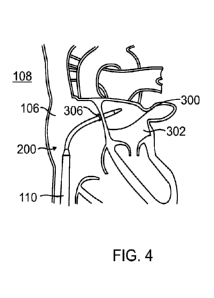
[0102] Referring to FIG. 4, a cross-sectional illustration of the heart 108
with the
exemplary vascular device 200 advanced into the left atrium 302 across the
atrial
septum 300 and the septal penetrator withdrawn in accordance with one aspect
of the
present disclosure is shown. The vascular device 200 having expanded at a
distal
portion has advanced across the atrial septum 300 from the right atrium 106
and into
the left atrium 302 through the puncture 306. Through this, the distal portion
of the
vascular device 200 may provide placement of pre-closure sutures or other
apparatuses.
-16-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0103] The proximal region, or body, of the vascular device 200 has
advanced so
that the proximal region is located not only in the inferior vena cava 110 but
also within
the right atrium 106. This may be guided by fluoroscopy, magnetic resonance
imaging
(MRI), ultrasound, or the like, which was described earlier.
[0104] FIG. 5 is a cross-sectional illustration of the heart 108 showing
illustrative
initial incisions 502 or 506 and followed by lengthening them to a user
selected/controlled length in accordance with one aspect of the present
disclosure. A
cross-sectional view of the heart 108 is shown viewed from a right atrial
side. The
atrial septum 300 may be surrounded by the superior vena cava rim 510 of a
superior
vena cava 512, posterior rim 514, inferior vena cava rim 516 of the inferior
vena
cava 110, atrioventricular valve rim 518, aortic rim 520, and superior rim
522. The
vascular device 200 may create a user defined adjustably controlled atrial
septostomy
by a retractable cutting implement, in conjunction with suture-mediated
closure
created by the atrial septostomy.
[0105] After the puncture is made into the atrial septum 300 by the septal
penetrator at an ideal location, the distal end of the vascular device 200 may
be
inserted through the tissue plane. An initial incision 502 or 506 on the
atrial septal wall
may be made and then subsequently lengthened to a specified desired amount 504
or 508 appropriate to allow for a therapeutic instrument. Additionally,
positional control
and visualization may enable the user to avoid areas 524 of the anatomy that
are not
desirable to disturb such as the aortic rim 520 or superior rim 522 or
puncturing outside
the atrium, or cutting through myocardium
[0106] The target of the tissue incision 502 or 506 may be on the atrial
septum 300
in such a location to allow access to the desired target of therapy such as
the mitral
valve. In one example, the incision length or size may be set to accommodate
the
procedural instrumentation or device without further damaging the tissue
plane. The
tissue when cut to a specific length that is large enough may facilitate no
ripping of the
tissue beyond the desired incision length. This may be accomplished by having
the
perimeter of the incision 502 or 506 along with the desired amount 504 or 508
match
the circumference of the therapeutic instrument. In one embodiment, creating a
cut
that is slightly larger than the subsequent therapy catheter may enable more
flexibility
-17-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
to the user. In a typical septal puncture, if the initial puncture site is not
adequate, the
user may need to retract the catheter, re-puncture, and re-dilate with the
risk of tearing
the tissue.
[0107] The length may also be adjusted to account for stretching of the
tissue
plane. The tissue rim surrounding the atrial fossa may be used or referenced
to begin
or limit the incision 502 or 506 of the tissue. Extending the incision 502 or
506 beyond
or outside the rim of the fossa may require more force or energy to create and
therefor
utilized as a feedback loop to determine the location of the incision 502 or
506.
[0108] In one example, which will be shown below, the vascular device 200
may
have one cutting arm and a centering puncture member. This configuration may
have
the operator rotate the tool to create a slit in a desired direction. The edge
of the fossa
of the heart 108 may be used as the starting point as this point may be easier
to
puncture through first and then rotate the cutting member to cut along a
direction of
choice. Alternatively, two symmetrical cutting arms extending from center
(with or
without a centering puncture face) may be used. This configuration may allow
the
physician to puncture a known height or position which may allow them to be
sure that
the center of the cut may be at the position as they intend, as the cut is
symmetric.
[0109] Turning to FIG. 6, which discloses a first embodiment, an
illustrative side
view of the exemplary vascular device 200 in accordance with one aspect of the
present disclosure is provided. The vascular device 200 may have a distal
region,
which may be the tip or anchor, expandable from a body of the device 200. A
length
of sheath tubing 606 may extend the body to a sheath hub 608. The tubing 606
may
be substantially curved near its distal end to provide deflection of catheters
in a
direction approximately 180 degrees from the exit path of the guidewire 102.
As
illustrated, the sheath hub 608 may be a tri port hub 608. The hub 608 may be
configured, in other embodiments, with less or more ports. Multiple
instruments may
be inserted and retracted from the hub 608, which will be shown below.
[0110] Broadly described, the vascular device 200 may be used to place
sutures
into the atrial septum, either before or after creating a controlled atrial
septostomy
through instruments placed into the hub 608. Other medical devices may gain
entry
and location to the left atrium, with the ability to subsequently close that
ASD.
-18-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
Advantageously, the way the ASD is closed may permit future tissue crossings
in the
event that is necessary for a subsequent catheter based procedure.
[0111] Turning to FIG. 7, an isometric view of the distal end of the
exemplary
vascular device 200 in a low profile condition in accordance with one aspect
of the
present disclosure is provided. The device 200 may permit placement of the
atrial
septostomy closure sutures prior to introduction of the cutting implement,
that is, a
blade septostomy. The entire device 200 may run over the guidewire, which was
disposed to the atrial septum through the right atrium.
[0112] The vascular device 200 described herein may facilitate creation of
a
controlled and or adjustable size and position an access incision in a tissue
plane such
as an atrial septum allowing for a therapeutic instrument or catheter to
easily perform
a controlled closure of the incision after the procedure is complete. The
closure may
be tailored to be sealed hemodynamically, or conversely allow for certain
amounts of
flow. The therapeutic instrument may perform diagnosis and therapeutic
intervention
to correct atrial fibrillation, perform mitral valve repair, correct septal
defects, or
perform implantation of a cardiac prosthesis, for example.
[0113] The vascular device 200 at a distal end may include a catheter shaft
702 or
body, anchor 704, and guidewire lumen 706. These components may be made of,
for
example, a polymeric material. Elastomeric materials may be used to construct
the
catheter shaft 702 to maximize flexibility. These materials may be used to
construct
an inner and outer wall of the shaft 702. Reinforcing structures within the
device 200
may be made from metals, such as stainless steel, titanium, or the like. In
this
embodiment, the reinforcement structure is malleable but retains sufficient
force to
overcome any forces imparted on it.
[0114] The catheter shaft 702, or delivery catheter, may have a body that
is tubular
in structure. The shaft 702 may, in one embodiment, have a circular cross-
section for
housing components. These components may extend towards a proximal end of the
vascular device 200. Left atrial appendage implants described below may be
radially
collapsible during delivery through the shaft 702. In one embodiment, the
implants
may be delivered through 14 French or larger catheters with a radially
expandable
delivery sheath.
-19-
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0115] Continuing with FIG. 7, the anchor 704 of the vascular device 200
may be
delivered to the atrial septum and extendable from the catheter shaft 702. The
anchor
704 and catheter shaft 702 may incorporate components which perform specific
functions that enable the delivery of sutures across tissue from a remote
location. The
anchor 704, for purposes of this embodiment, may be in a cone-shape.
Alternatively,
the anchor 704 may have a shape that is, but is not limited to, funneled,
rounded,
tipped, or peaked. The anchor 704 may have a beveled edge which may conform to
the atrial septum.
[0116] Traveling or within the catheter shaft 702 and anchor 704 of the
vascular
device 200 may be the guidewire lumen 706. The lumen 706 may permit
advancement
and delivery of the vascular device 200 over the previously placed guidewire.
The
guidewire, which was described earlier, may enter into the patient through a
femoral
vein up into the inferior vena cava and into the right atrium. The septal
penetrator for
placing the initial puncture may be resident within the guidewire, which may
be
removable therefrom.
[0117] FIG. 8 is an isometric view of the distal end of the exemplary
vascular device
200 viewed from a different perspective with a portion of the device 200
advanced
revealing components used to puncture tissue and pass sutures in accordance
with
one aspect of the present disclosure. The anchor 704, having a cone-shape, may
be
advanced within the heart of the patient from a tip of the catheter shaft 702.
The
amount of distance that the anchor 704 advances may depend on the thickness of
the
tissue it is crossing and the size of the chamber that it is entering.
[0118] The vascular device 200 may be designed to permit or restrict a
varying
amount of the anchor 704 to travel. The travel may be as small as a few
millimeters
to upwards of several centimeters. For purposes of this disclosure, it is
assumed that
the anchor 704 may cross the interatrial septum from the right atrium into the
left atrium
and the catheter shaft 702 may remain on the right side of the heart within
the right
atrium. The anchor 704 may be extended such that needles 804 of the anchor 704
are allowed to be hooked or grabbed within the left atrium 304.
[0119] Once the anchor 704 has been advanced, the needles 804 may be exposed.
In one embodiment, as shown, four needles 804 may be removably coupled into
the
- 20 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
anchor 704. The needles 804 may be rearward facing. For example, the needles
804
may extend towards the catheter shaft 702, or body, of the vascular device 200
when
the anchor 704 has extended into the left atrium. The face of the catheter
shaft 702
and anchor 704 may be angled a prescribed amount to permit a more orthogonal
contact with the tissue which should promote a more stable interface between
the
tissue and catheter shaft 702.
[0120] An advancement shaft 802 for the anchor 704 of the vascular device 200
may be rectangular in shape and travel through a corresponding rectangular-
shaped
lumen within the catheter shaft 702 in order to maintain a precise rotational
alignment
with the catheter shaft 702. Typically, the alignment between the anchor 704
and
catheter shaft 702 is maintained as it permits device functionality. The
advancement
shaft 802 may be extended and retracted at a proximal end of the vascular
device 200,
which as shown above may be located at the percutaneous puncture or incision
point.
[0121] Referring to FIG. 9, an isometric view of the distal end of the
exemplary
vascular device 200 with the portion of the device 200 advanced through the
tissue
900 of the atrial septum in accordance with one aspect of the present
disclosure is
provided. The vascular device 200 has been placed through a representative
section
of tissue 900. The anchor 704, having a cone-shape, has been advanced across
the
puncture site of the tissue 900 which was performed earlier by the septal
penetrator.
The catheter shaft 702 may be larger than the puncture created by the septal
penetrator, thus precluding the catheter shaft 702 from entering into the
tissue 900.
This may allow for the tissue to be tight or secured around the narrow aspect
of the
apparatus.
[0122] FIG. 10 is a cross sectional view of the exemplary vascular device
200
having an illustrative lumen configuration in accordance with one aspect of
the present
disclosure. The catheter shaft 702 may include several channels or lumens that
permit
certain components to pass therethrough. As an example, four lumens 1002
having
equal diameters may permit snares to grab, hook or loop the needles removably
coupled to the anchor with tethered sutures to pass through them. The central
rectangular-shaped channel 1004 may permit the rectangular-shaped advancement
shaft of the anchor to travel through it. The configuration may maintain an
alignment
-21 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
of the four needles and cutting implements, which will be shown later, within
the
catheter shaft 702.
[0123] Different configuration to the cross section of the vascular device
200 may
be implemented depending on suture placements. For example, more than four
lumens 1002 may be channeled through the catheter shaft 702 with each being
equidistant from the center. Advantageously, the lumens 1002 may provide a
proper
spacing to the initial puncture such that sutures management may be had.
Punctures
used to capture the needles into the lumens 1002 may be placed such that no
unnecessary tearing of tissue is made yet still proper suture placement is
performed.
The shown configuration may allow for two sutures through four needles, but
other
configurations may exist and are within the scope of the present disclosure.
In one
embodiment, apertures may radially surround the central lumen where the user
may
selectively advance any number of needles/sutures through. The sutures may be
loaded from the proximal end in any configuration the user has chosen.
[0124] FIG. 11 is an isometric, sectioned view of the distal end of the
exemplary
vascular device 200 revealing the inner geometry of components therein in
accordance with one aspect of the present disclosure. For illustrative
purposes, a
section of the anchor 704 has been removed permitting a view of a bundled
suture
1102 that is stowed in a recess channel 1104 of the cone-shaped anchor 704.
[0125] Ends of the suture bundles 1102 may be coupled to two needles 804 on
opposite ends. Two suture bundles 1102, as shown, may have four needles 804.
The
suture bundles 1104 may be placed on opposite sides of one another delineated,
or
separated, by the advancement shaft. The needles 804 on both sides may be
engaged simultaneously with the recess channel 1104 unspooling both suture
bundles
1102. The recess channel 1104 may rotate for two different bundled sutures
1102
while the needles 804 are being drawn into the catheter shaft 702.
[0126] The recess channel 1104 may be shaped to permit the unspooling and
release of the suture bundles 1102. In one example, sutures of the suture
bundles
1102 may be spooled into the recess channel 1104 which may be held taught
within
the anchor 704. When the needles tethered to the suture bundles 1102 are
pulled,
the suture bundles 1102 may be unspooled. The sutures of the suture bundles
1102
- 22 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
may be released from the anchor 704 after the suture bundles 1102 are pulled a
predetermined amount by the needles 804 which are drawn into the catheter
shaft
702. This amount may be, for example, a couple of centimeters.
[0127] With reference to FIG. 12, an isometric view of the distal end of
the
exemplary vascular device 200 revealing snares 1202 that have been advanced
out
of a body of the vascular device 200 in accordance with one aspect of the
present
disclosure is provided. Four snares 1202 may be used to grab, hook or loop the
suture
bundles with each suture bundle having on opposite ends needles 804 for
snaring.
Small punctures may be made for the snares 1202 to pass through the tissue, or
the
snares 1202 themselves may have a tip capable of puncturing the tissue. The
snares
1202 may be placed through the catheter shaft 702 at a proximal end and out of
the
previously described lumens. Once the needles 804 have been captured, the
snares
1202 may be retracted through the catheter shaft 702.
[0128] In one example, recesses 1204 placed within the needles 804 may be
used
by the snares 1202. These recesses 1204 may be slanted and directed towards
the
anchor 704 of the vascular device 200. When the snares 1202 are pushed through
the catheter shaft 702, a hook of the snare 1202 may be pulled and tethered
against
the recess 1204.
[0129] A mechanism may be used at a proximal end of the catheter shaft 702 to
push multiple snares 1202 therethrough simultaneously. The snares 1202 may
grab
and then pull the needles 804 through the tissue at the same time. In an
alternative
embodiment, the snares 1202 may be individually pushed into the shaft 702 to
capture
or hook a single needle 804 at one time through its recess 1204. In one
embodiment,
two snares 1202 may work in tandem to pull two corresponding needles 804
through
the shaft 702. The two needles 804 may be connected to opposite sides of the
suture
bundle. The needles 804 may be trimmed from the suture after being pulled into
the
shaft 702.
[0130] Snares 1202 may be made of a variety of materials. For example, the
snares 1202 may be made of a radiopaque platinum coil and tip for enhanced
visibility.
The snare 1202 may include a helical loop design for a smaller profile yet a
longer
reach than right-angle loops. A durable cobalt chromium loop may add strength
and
- 23 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
retain its shape. The snare 1202 may have a loop that varies in size: 1 mm, 2
mm
and 3 mm loop diameters for clinical versatility.
[0131] FIG. 13 is an isometric view of the distal end of the exemplary
vascular
device 200 after four suture needles 804 and suture ends have been passed
through
the tissue 900, snared and pulled through the length of the device 200 in
accordance
with one aspect of the present disclosure. The catheter shaft 702 of the
vascular
device 200 may receive the needles 804 after being pulled by the snares. From
the
bundled sutures with the pull of the needles 804 coupled on opposite ends,
sutures
1302 may be unspooled from the recess channel and tensioned.
[0132] The suture 1302, in one embodiment, may be made of finely woven
nylon
material. Other materials may be used such as, but not limited to,
polypropylene, silk
or polyester. The sutures 1302 may be made of a sturdy, but bendable material.
The
sutures 1302 may be in a "U" or "C" shape. The sutures 1302 may be soaked in a
sterile mineral oil immediately prior to its use. The edges of the suture 1302
may be
sutured easily to margins of an incision in the atrial septum. The suture may
be made
of a high temperature resistant material to prevent damage if it is in contact
with the
cutting implement.
[0133] FIG. 14 is an isometric view of the distal end of the exemplary
vascular
device 200 after the suture 1302 has been pulled completely through the length
of the
device 200 and is now pulled taught against the tissue in accordance with one
aspect
of the present disclosure. The sutures 1302 may be fully unspooled from the
recess
of the anchor 704. The ends of the suture 1302 may be available to the user
through
the proximal end of the catheter shaft 702 of the vascular device 200. In
operation,
the suture lines may be slackened or tightened according to a user's need at a
particular time.
[0134] With reference now to FIG. 15, an isometric view of the distal end
of the
vascular device 200 after a cutting implement 1500 has been partially advanced
revealing the cutting elements 1506 in accordance with one aspect of the
present
disclosure is provided. Once the sutures 1302 are pulled against the tissue,
such that
they may not be cut, the anchor 704, having the cone-shape, may be further
advanced
into the left atrium through the advancement shaft 802. The shaft 802 may be
extend
- 24 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
through the catheter shaft 702 of the vascular device 200 towards and through
the
puncture in the atrial septum. The advancement shaft 802 may be extended
through
the proximal end of the vascular device 200.
[0135] By advancing the shaft 802 through the tissue of the atrial septum,
a second,
rectangular-shaped telescoping cutting implement 1500 may be sent through the
rectangular-shaped channel of the catheter shaft 702. The cutting implement
1500
may include an expansion actuating shaft 1502 that may telescope over the
advancement shaft 802 for the anchor 704. That is, the expansion actuating
shaft
1502 may slide over the advancement shaft 802 of the anchor 704.
[0136] The expansion actuating shaft 1502 may be advanced through the
puncture
and be located within the left atrium extending the cutting implement 1500.
When the
expansion actuating shaft 1502 is pushed forward, the cutting implement 1500
with a
linkage system 1504 is exposed. A distal end of the cutting implement 1500 may
be
temporarily locked to a top portion of the advancement shaft 802 of the anchor
704
while a proximal end of the cutting implement 1500 may be connected to a lower
portion of the expansion actuating shaft 1502.
[0137] The linkage system 1504 may bow outward and expand the cutting
elements 1506 to a length much greater than the diameter of the vascular
device 200
when the anchor 704 is retracted and the expansion actuating shaft 1502 is
held in
place. The linkage system 1504 may bend at symmetrical points 1508 when the
advancement shaft 802 is retracted. The cutting elements 1506, as shown, are
positioned behind the anchor 704 facing towards the catheter shaft 702. In
operation,
the linkage system 1504 may remove any potential to cut the sutures 1302,
which are
parallel thereto, as the cutting elements 1506 are expanded.
[0138] FIG. 16 is an isometric view of the distal end of the vascular
device 200 with
the cutting implement 1500 expanded in accordance with one aspect of the
present
disclosure. The anchor 704, in one configuration, has been retracted covering
the
advancement shaft. When this is performed, the top of the cutting implement
1500 is
held and lowered with the anchor 704 and the expansion actuating shaft 1502
holds a
bottom portion of the cutting implement 1500 in place. This may cause a bend
in the
cutting implement 1500 within the linkage system 1504 and expose the cutting
- 25 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
elements 1506. The cutting elements 1506 may be perpendicular to the shafts,
such
that the cutting elements 1506 expand past a diameter of the catheter shaft
702.
[0139] The parallel alignment of the cutting elements 1506 relative to the
sutures
1302 may be made so that the cutting elements 1506 do not inadvertently cut
the
sutures 1302. Multiple cuts or incisions may be made by cutting elements 1506
by
slicing through the tissue and pulling through the cutting elements 1506 back
to the
left atrium. The processes may be repeated depending on the number of
incisions
needed. Accordingly, the number and location of sutures/needles may vary
depending
on the size and number of incisions made.
[0140] When completed, the cutting elements 1506 may be retracted by
advancing
the anchor 704. The linkage system 1504 may be collapsed through this
advancement
and the system 1504 may condense into a narrow channel without the cutting
elements 1506 exposed. The lock coupling the top of the advancement shaft and
the
cutting implement 1500 may be removed. The cutting implement 1500 may then be
pulled through the catheter shaft 702 without moving the anchor 704. After
removing
the cutting implement 1500, the vascular device may be removed leaving the
sutures
1302 in place.
[0141] Other technique or devices may be used to expand and collapse the
cutting
elements 1506 such that no tissue is inadvertently cut when the anchor 704 of
the
vascular device 200 is being used in the left atrium. The linkage system 1504,
allowing
the cutting elements 1506 to be used, may come in a variety of forms and is
not
necessarily limited to that shown in this embodiment. For example, the linkage
system
1504 may entirely reside on the expansion actuating shaft 1502 whereby
mechanisms
on the proximal end may be used to expand and collapse the cutting elements
1506
without the need to retract the anchor 704. This may use a separate knob, pull-
wire,
or the like to expand and collapse the cutting elements 1506. Other variations
may
exist and are within the scope of the present disclosure.
[0142] With reference now to FIG. 17, an isometric view of the exemplary
vascular
device 200 positioned to cut the tissue 900 in accordance with one aspect of
the
present disclosure is provided. The vascular device 200 may be angled at a
- 26 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
prescribed amount to permit a more orthogonal contact with the tissue 900.
This may
promote a more stable interface between the tissue 900 and catheter shaft 702.
[0143] The distal end of the catheter shaft 702 may be angled to conform to
the
tissue 900. The shaft 702 typically does not go through the initial puncture
or the slit
1702 created by the cutting elements 1506. The anchor 704 of the vascular
device
200, however, may extend through the puncture and into the left atrium. The
linkage
system 1504 may be expanded and the cutting elements 1506, which may be in the
form blades, may be used to cut a slit 1702 through the tissue 900. The length
of the
slit 1702 may be controlled by how much the linkage system 1504 is expanded.
The
vascular device 200 may be rotated to produce other slits 1702 or combinations
of slits
1702.
[0144] In operation, the anchor 704 of the vascular device 200 may advance
through the initial puncture. Needles may extend towards the catheter shaft
702. The
sutures may then be brought towards the tissue 900 from a backend before any
incision 1702 is made. The sutures may then be managed after the incision 1702
and
the therapeutic instrument has been used.
[0145] FIG. 18 is an isometric view of sutures 1302 that would be inserted
into the
tissue 900 in accordance with one aspect of the present disclosure. The two
sutures
1302 may correspond to those that were unspooled from the recess of the
anchor.
After removing the vascular device, two lengths of sutures 1302 may remain in
place
behind the atrial wall that extends from the left atrium.
[0146] In addition, the guidewire previously used by the vascular device
may also
be left in place. The guidewire may be used by a larger bore device, such as a
therapeutic instrument, that may now travel over the guidewire and gain easy
access
into the left atrium through the slit and run adjacent to the previously
placed sutures.
Once the large bore device has been removed, the free ends of the suture 1302
may
be knotted and pushed towards the tissue to create a closing force. This may
reduce
the size of the cut or hole within the tissue, which the large bore device
used.
Advantageously, this may prevent or minimize the amount of hemodynamic
communication between chambers and allow the user to leave behind a simple
knot
on the interatrial septum. Typically, the hole may close in the short term. In
the long
- 27 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
term, this may be used to permit a subsequent access into the left atrium if
another
catheter-based procedure or intervention is needed for the patient.
[0147] FIG. 19 is an isometric view of an incision after the tissue 900 is
engaged
with the suture 1302 in accordance with one aspect of the present disclosure.
A close-
up provides a view of the tissue 900 after a knot 1902 has been tied. A slit,
which was
created by the cutting implement has been cinched down in the middle by the
knot
1902, which may reduce or eliminate an amount of hemodynamic flow and
communication between chambers. For illustrative purposes the knot 1902 may be
represented by a simple "X" configuration. Alternatively, the knot 1902 may be
one of
several different types of surgical knots that may be tied and pushed down the
vascular
device via a remote location.
[0148] In one embodiment, the knot 1902 may be formed and advanced with
a knot pusher having a pusher rod fitted with a distal side port and severing
member in
the form of a sharpened outer sheath. The knot 1902 may hold an associated
patch in
place where an excess line may be trimmed by the shearing action of the pusher
rod distal side port and the distal sharpened portion of the severing member.
The
excess line and other elements may be removed from the catheter.
[00100] Knot pushers that are known in the art include Edwards ThruPort knot
pusher; Medline Endoscopic Pushers; Laparoscopic Knot Pushers by Cooper
Surgical.
[00101] Arthrex offers several options: The Single-Hole Knot Pusher provides a
simple method to advance sliding knots and half-hitches. This closed end knot
pusher
has a modified handle that provides an ergonomic feel. The distal tip has also
been
modified for easier advancement of slipknots and half-hitches. The 6th finger
was
designed to tie the surgeon's knots and allows the surgeon to apply and
maintain
tension to the first throw while advancing subsequent throws. The CrabClaw
incorporates an opening jaw to allow intraarticular capture of suture.
[0149] A simple incision with closure was described beforehand. Turning to
FIGS.
20A-L, various illustrative cut patterns that may be created using the
vascular device
in the atrial septum which may be made from at least one cutting implement
that may
- 28 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
be rotated and used a single or multiple times in accordance with one aspect
of the
present disclosure are provided. The embodiments may create the various
cutting
shapes in the atrial septum. These shapes may be created with multiple cutting
arms,
or using one cutting arm that is rotated and used multiple times, such as the
cutting
implement shown above. The length of the incision may be controlled by the
user
through activation of the adjustable cutting implement. The incision may take
a
number of different geometries commonly used to facilitate both passage of the
therapeutic device and closure of the tissue plane post therapy.
[0150] With reference to FIGS. 20A-20C, apposition of the tissue edges may
be
controlled by the nature of the location of the anchors or sutures 2002 and
2004. To
increase overlap or tissue apposition, a first distance 2006 between the
sutures 2002
and 2004 may be increased to a second distance 2010. Another method or
technique
may be to add additional suture locations or sutures 2012 at various and
intersecting
paths. The incision 2008 may be in the middle of the sutures 2002, 2004 and
2012.
[0151] The incision 2008 may take the form of many patterns such as a
straight
cut, v cut 2020, zig zag 2022, or crescent arc 2024, to name a few, and as
shown in
FIGS. 200-F. These cuts may then be coupled together with other shapes
yielding
multiple flaps of tissue 2030, 2032, or 2034 shown in FIGS. 20J-L. The various
configurations may be advantageous depending on the shape and size of the
device
needed to pass through and or the type or amount of closure that is desired
post
procedure. Some incision shapes such as FIGS. 200-F may facilitate a tissue
plane
overlap 2026 upon closure due to the shape of the cut and the tension of the
tissue
before and during healing. This overlap may greatly aid the healing of the
tissue edges
together. That is, in FIGS. 20G-I what is shown is the tissue plane overlap
2026 from
the different incision shapes 2020, 2022, and 2024.
[0152] The embodiments described herein manage the variables to control the
closure of the incision 2008. The tissue edges may be managed by having
control
members in place before the incision 2008 is made. For example, and as shown
above, having sutures 2002, 2004 and 2012 in place before incisions 2008 are
made
by the cutting implement may secure the tissue. In one embodiment, control
features
may be applied after the incision 2008 is made.
- 29 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0153] The tissue edge position and apposition of those edges relative to
each
other may be controlled to manage an amount of tissue overlap, apposition
pressure,
and amount of residual flow after a closer is applied. This control may be
done by
controlling where the suture lines are positioned relative to the incision. An
example
of this, is the distance of the suture lines farther away from the incision
edge may
cause more tissue bunching and/or tissue overlap. Increasing the number of
tissue
anchors and/or suture passing locations may increase the amount of tissue
apposition
along the length of the incision. The amount of tension or pressure applied to
the
suture tension lines may further affect the amount of closure on the incision
2008.
These mechanisms may be managed in real-time and monitored under echo flow
monitoring and/or fluoroscopy visualization.
[0154] In one embodiment, a mechanism may be used that would leave no long-
term implant left in the patient. The mechanism may be designed to either seal
the
tissue, have the tissue heal in a sealed state, or have the tissue heal in a
partially
sealed state. The percentage of the hemostasis may be adjusted by varying the
application of the mechanism. The mechanism may be designed to secure the
tissue
through various time points. These time points may correlate to various tissue
healing
cascade points such as time for tissue to coagulate, adhere, endothelialize,
and
scaring.
[0155] An absorbable body may be left that would facilitate the closure and
be
absorbed by the body over time. In one embodiment, the absorbable body may be
removed from the body at a later point in time. The closure may be fully or
partially
engaged into the tissue plane near the incision 2008 before the incision 2008
is made.
In another embodiment, applying the closure fully or partially engaged into
the tissue
plane may be near the incision 2008 after the incision is made. In one
embodiment,
the closure mechanism may be applied fully or partially engaged into the
tissue plane
near the incision 2008 after the incision 2008 and therapeutic instrument is
removed
from the incision 2008.
[0156] The cutting implements described herein may be used to control the
condition of the tissue edges based on the method of creating the incision in
the tissue.
The methods of creating the incision may include, but are not limited to, a
sharp blade
- 30 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
made from durable material such as metal or ceramic, electrocautery
techniques, RF
energy, plasmajet vaporization, ultra-sonics, high voltage vaporization,
controlled
dilation, heat, cold, and others. A state of the cells on the edge of the cut
surface may
be controlled to optimize the desired healing cascade utilizing these various
methods
of incision creation.
[0157] FIG. 21 is an isometric view of an illustrative cut pattern 2106 for
incision to
promote tissue edge apposition in accordance with one aspect of the present
disclosure. A cut pattern 2106 may be defined by a first end 2102 and second
end
2104. A cut pattern 2106 may be made into the tissue 900. The inside edge 2108
is
separated from the outside edge 2110. This may be a cut pattern 2106 of a
straight
and arc combination for incision to promote tissue edge overlap and
apposition.
[0158] FIG. 22 is an isometric view of the illustrative cut pattern for
incision to
promote tissue edge apposition, while under slight tension, demonstrating
tissue edge
control and overlap of edges with tension applied in accordance with one
aspect of the
present disclosure. This demonstrates overlap of the inside edge 2108 and
outside
edge 2110 with yield overlap 2202 in the tissue 900 caused by the cut pattern
between
the first end 2102 and second end 2104.
[0159] FIG. 23 is an isometric view of the cut patterns for incision to
promote tissue
edge apposition and a helical anchor 2300 controlling tissue edges in
accordance with
one aspect of the present disclosure. Tension may be applied in the direction
of the
ends 2102 and 2104 of the cut. The inside edge 2108 and outside edge 2110 with
yield overlap 2202 in the tissue 900 may be secured with a helical anchor
2300, or the
like.
[0160] Previously, a first embodiment of a vascular device was described.
FIGS.
24-38 describe a second embodiment of a vascular device 2400 with additional
illustrative incisions. It will be understood from the present disclosure that
components
with these embodiments may be interchanged, added or deleted based on a
reasonable configuration. New embodiments using these modifications are within
the
scope of this disclosure.
- 31 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0161] Turning to FIG. 24, an isometric view of an illustrative expandable
RF cutting
implement 2410 with four expandable members 2416 in accordance with one aspect
of the present disclosure is provided. The cutting implement 2410 may be
between a
catheter shaft and a distal anchor, as previously described. While four
cutting
members 2414 are shown, fewer or more may be used with each cutting member
2412
equidistant from one another.
[0162] The cutting implement 2410 may be expandable and collapsible through
similar linkage systems described above. The cutting implement 2410 may be
collapsed when advanced through the initial puncture and expanded after
passing
through the tissue. The cutting implement 2410 may have four expandable
members
2414 connected to four cutting members 2412. The cutting members 2412 may be
provided on a proximal end of the cutting implement 2410 such that the cutting
implement is pulled back towards the tissue to make cuts.
[0163] The cutting members 2412 may extend radially from the center of the
cutting
implement 2410. The width of the cutting members may vary in width to change
the
incision length based on a French size of the delivery catheter. The cutting
implement
2410 may also include a tip piercing device 2416 at a distal end of the
cutting
implement 2410. This may be used to puncture the tissue. The cutting members
2412
and tip piercing device 2416 may use mechanical or electrical energy. The
mechanical
or electrical energy may come from at least one of a blade, ceramic,
electrocautery
technique, RF, plasmajet vaporization, ultra-sonic, high voltage vaporization,
controlled dilation, heat and cold. In one example, the cutting members 2412
and tip
piercing device 2416 may both use mechanical energy. Alternatively, they may
use
both electrical energy. In yet another variation, the cutting members 2412 and
tip
piercing device 2416 may use different types of energy. The cutting implement
2410
along with its members 2412 and arms may radially expand in a controlled
manner or
plane such that they minimize or prevent the likelihood that the cutting
implement 2410
inadvertently cuts or negatively impacts the previously placed closing
sutures.
[0164] FIG. 25 is an isometric view of the exemplary vascular device 2400
with the
expandable cutting implement 2410 having a tip for piercing the tissue 900 in
accordance with one aspect of the present disclosure. A tip piercing device
2416 may
- 32 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
be coupled to the cutting implement 2410 and may be pushed through the tissue
900
to create a puncture within the tissue 900. The puncture may be made through a
mechanism on a proximal end allowing a physician to control the vascular
device 2400.
The cutting implement 2410 may be in a retracted state before advancing
through the
tissue 900.
[0165] A catheter shaft 2504, or delivery catheter, may house, but is not
limited to,
the cutting implement 2410 and anchor mechanisms 2502. The anchor mechanisms
2502 may be equidistant from the center of the catheter shaft 2504. While four
anchor
mechanisms 2502 are shown, fewer or more exist depending on a closure strategy
of
incisions made into the tissue 900.
[0166] The vascular device 2400, while not shown, may include visualization
tools
for determining a location of the device 2400 within the patient. In one
embodiment,
a sensor may be affixed to the device 2400 to determine a location and
orientation of
the catheter. Alternatively and/or additionally, an independent tracking
system may
be based on ultrasound, impedance or fluoroscopy tracking. In the case of
impedance,
electrical potential generated by electric field generators may be detected by
the
existing electrodes. In the case of fluoroscopy, electrode location may be
detected by
an image processing scheme that identifies and tracks the electrodes and/or
opaque
markers located on the device 2400.
[0167] FIG. 26 is an isometric view of the exemplary vascular device 2400
with the
illustrative expandable cutting implement 2410 placed beyond the tissue 900 in
accordance with one aspect of the present disclosure. The cutting implement
2410,
in its retracted state, may be pushed through after the puncture is made to
the tissue
900. The cutting implement 2410 may be sent through by an advancement shaft
which
may be controlled at a proximal end whereas the catheter shaft 2504 is not
distributed
therethrough.
[0168] After the cutting implement 2410 has been distributed into the left
atrium,
the cutting implement 2410 may be expanded. The expandable members 2414 may
be radially extended from its center which spreads to a larger diameter than a
diameter
of the device 2400 itself.
- 33 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0169] An anchor mechanism 2502 within the catheter shaft 2504 may be used to
push delivery mechanisms, which will be described below. The anchor mechanisms
2502 may be distributed within the catheter shaft 2504 and enclosed within
lumens.
The anchor mechanisms 2502 may surround the centralized cutting implement 2410
and be equidistant from one another.
[0170] Referring to FIG. 27, an isometric view of the exemplary vascular
device
2400 with the illustrative expandable cutting implement 2410 making incisions
into the
tissue 900 in accordance with one aspect of the present disclosure is
provided. After
the expandable members 2414 are extended, the advancement shaft may be pulled
back, along with the tip piercing device 2416, toward the catheter shaft 2504
to make
incisions in the tissue 900. These incisions may be made in a backplane of the
left
atrium. The cutting implement 2410 may be extended, rotated, and then
retracted to
make additional incisions into the tissue 900. During this time, the anchor
mechanisms
2502 may be held stationary.
[0171] FIG. 28 is an isometric view of the exemplary vascular device 2400
with the
illustrative expandable cutting implement 2410 advancing anchor delivery
mechanisms 2502 in accordance with one aspect of the present disclosure. When
the
anchor mechanisms 2502 are pushed through the catheter shaft 2504, it may
extend
through the tissue 900 to the other side, that is, the left atrium. The anchor
mechanisms 2502 may be coupled to a delivery mechanism 2802 that may be
transferred through the tissue 900. The delivery mechanisms 2802 may have
tissue
piercing points.
[0172] Four delivery mechanisms 2802, coupled to four anchor mechanisms
2502,
may pierce the tissue 900. Fewer or more combined structures may be present
within
the vascular device 2400. The delivery mechanisms 2802 may use similar
energies
to the expandable members 2414 and tip piercing device 2416. That is,
combinations
of mechanical and/or electrical energies may be used.
[0173] FIG. 29 is an isometric view of the exemplary vascular device 2400
with the
illustrative expandable cutting implement 2410 with push anchors 2902 further
inserted to advance the anchor mechanisms 2502 in accordance with one aspect
of
the present disclosure. The anchor mechanisms 2502 may advance the delivery
- 34 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
mechanisms 2802 within the catheter shaft 2504 into and beyond the tissue 900,
that
is, within the left atrium. The push anchors 2902 may thereafter be deployed
by the
delivery mechanisms 2802. The push anchors 2902 may be used to secure the
tissue
900, and its surrounding area. The anchors 2902 may be made from PLGA, PLLA,
nylon, polyester, PEEK, or other biocompatible material. It should be noted
that cutting
implement 2410 may be advanced into the tissue 900 after the push anchors 2902
are
set in place.
[0174] Other anchors may be used for securing the tissue 900. For example,
tissue
anchor lines may be utilized. These may include, but are not limited to
sutures,
toggles, helical structures, grabbing devices, inverting clips, expanding
structures,
mesh structures, stent-like structures, patch structures, clips, expandable
valves, and
suture knot configurations.
[0175] FIG. 30 is an isometric view of the exemplary vascular device 2400
having
the delivery mechanisms 2802 removed in accordance with one aspect of the
present
disclosure. The anchor mechanism 2502 coupled to the delivery mechanisms 2802
may be pulled at a proximal end of the catheter shaft 2504. This may retract
the
delivery mechanism 2802 from the left atrium 302 into the right atrium 106.
The cutting
implement 2410 with its expandable members 2414 and tip piercing device 2416
may
still be inserted into the left atrium of the patient. The anchors 2902 may be
left against
the tissue 900. These may be embedded therein.
[0176] FIG. 31 is an isometric view of the exemplary cutting implement 2410
removed from the tissue 900 leaving tissue anchors 2902 against it in
accordance with
one aspect of the present disclosure. The cutting implement 2410 may be
removed
through the catheter shaft 2504 of the vascular device 2400. The catheter
shaft 2504
may still be placed in the right atrium of the patient at this time.
[0177] Referring to FIG. 32, an isometric view of an exemplary toggle 3202
within
the tissue 900 in accordance with one aspect of the present disclosure is
provided.
The toggle 3202 may be pushed or advanced through the tissue longitudinally
from
the right atrium into the left atrium. The toggle 3202 may be shifted
horizontally and
secured on the septal wall to hold the tissue in place. The toggle 3202 may be
made
of similar materials to the anchor, which may be biodegradable. The presence
of this
- 35 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
closure apparatus or of others herein disclosed are of a size and location
that they will
not preclude another access procedure in the future.
[0178] Multiple cutting implements were described beforehand.
These
implements, as well as those described below, may use mechanical or RF energy.
When using electrical energy, an amount of exposed metal may be minimized
through
insulation such that the exposed metal may only exist in the desired tissue
cutting
region of the tool. The smaller the amount of exposed metal, a better cutting
effect on
the tissue may be realized. Advantageously, this may use less power. To
achieve the
best cutting effect, the operator may ensure the cutting region is in good
mechanical
contact with the target tissue.
[0179] The
initial septal puncture site and the septal cut may be performed using
separate applications of energy. For example, using electrical energy, a first
puncture
may be performed using a first circuit and a larger cut may be achieved using
a second
circuit. If the first puncture is done separately from the cut, the operator
may then
rotate the cutting implement to align a cutting arm with a direction they
intend to cut.
If performing the second cut after the initial puncture by advancing the
cutting tool from
the right atrium to the left atrium, it may be beneficial to have the cutting
regions of the
initial puncture and the second larger cut overlap so there is no chance of a
piece of
tissue not being cut.
[0180] If
the tool has symmetric cutting arms on either side of the center puncture
element, the center of the overall cut may be at the intended puncture site,
and not
shifted in one direction. This is important for the success of a subsequent
procedural
step that may require being a certain distance above the target structures.
[0181] The
following cutting implements may create a continuous cut form a center
puncture site to the edge of the cut. The intent of these implements is to cut
all the
way from the center to the edge. The adjustability or expandability of the
cutting
implement may be achieved using a pull-wire/ring mechanism, such that the
cutting
implement may be compressed and bowed outward as the wire is pulled, which was
described above. It may also be performed using a spring, or by using a shaped
tool
made of shape memory alloy.
- 36 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0182] Turning now to FIG. 33, an isometric view of an illustrative cutting
implement
3300 having an atraumatic tip 3304 deployed from an exemplary vascular device
in
accordance with one aspect of the present disclosure is provided. The cutting
implement 3300 may be deployed from a distal end of a tubular member 3302 with
electrical insulation selectively removed. This cutting implement 3300 may be
fixed in
size or adjustable through mechanism design.
[0183] The design may incorporate an atraumatic tip 3304 that may be used
for
finding the fossa ovalis or other desired target location. It also has cutting
surfaces
3306 that may extend to both sides of the atraumatic tip 3304, symmetrically.
In these
types of embodiments, the initial septal puncture and slit creation may be
performed
in one motion with the same continuous cutting surface 3306, that is, they may
be part
of the same circuit for delivering energy, and they may also be on individual
circuits.
If the puncture/cutting energy is to be RF, microwave, or other electrical
energy,
insulation may be strategically removed from the metal structure. The amount
of
insulation removal, or alternatively metal exposure, may be varied to optimize
performance. It may wrap entirely around the cutting arm, for example, or may
be
present in a narrow line along the length of the cutting surface arm. The goal
may be
to ensure the cutting surface being energize and in good contact with the
tissue, with
minimal direct communication to a blood pool.
[0184] FIG. 34 is an isometric view of an illustrative cutting implement
3400 having
a slit 3404 in a sheath 3402 deployed from an exemplary vascular device in
accordance with one aspect of the present disclosure. A cutting tool that
incorporates
one cutting arm may expand radially through the slit 3404 in the needle type
sheath
3402, which will be shown below. In this embodiment, with only one cutting
arm, the
size of the arm may be fixed or adjustable through a mechanism, for example,
using
a pull-wire, spring, or the like. A distal portion 3406 of the cutting
implement 3400 may
be used for the initial puncture. Unlike the embodiments described above, the
initial
septal puncture site and slit creation are a part of separate parallel
circuits, if electrical
energy is used for cutting the tissue.
[0185] FIG. 35 is a side view of the illustrative cutting implement 3400
having a
cutting element 3502 extending from the slit 3404 in the sheath 3402 in
accordance
- 37 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
with one aspect of the present disclosure. The expandable cutting arm 3504 is
a part
of a pull-wire that may run the length of the catheter and the puncture needle
face is
a part of another.
[0186] In one embodiment, the initial puncture needle may be entirely
insulated,
with only the distal region having exposed metal for energy delivery to the
tissue. The
expandable cutting element 3502, in the form of a radially extending arm, may
have
exposed metal circumferentially, or be mostly insulated with a thin line of
exposed
metal running along the cutting surface (or any number of patterns for
exposing
minimal surface area of the metal). It may be possible and more desirable for
these
two different cutting surfaces to be energized at the same time with one
switch or
through different switches on the handle end to allow for energy application
at different
times during the procedure. The benefit of only puncturing with the needle
first is that
it may create an anchor point in the tissue. Once this initial puncture is
made, the
operator may rotate the cutting implement 3400 until the expandable cutting
element
3502 aligns with where they want the length of the cut to go.
[0187] FIG. 36 is an isometric view of a distal end of the exemplary
cutting
implement 3400 in accordance with one aspect of the present disclosure. The
expandable cutting arm 3504 for the cutting element 3502 may be a wire that
runs the
length of the catheter. A puncture needle 3602 may be part of another
mechanism to
be actuated. The cutting element 3502 may have exposed metal
circumferentially, or
be mostly insulated with a thin line of exposed metal running along the
cutting surface.
In operation, the expandable cutting arm 3504 may be pushed up and down to
change
the shape of the cutting element 3502.
[0188] The initial puncture needle 3602 may be entirely insulated, with
only the
distal region having exposed metal for energy delivery to the tissue. In one
embodiment, the cutting implement 3400 may have two different cutting surfaces
to
be energized at the same time with one switch or through different switches on
the
handle end to allow for energy application at different times during the
procedure. The
benefit of puncturing with the needle 3602 first is that it may create an
anchor point in
the tissue. Once the initial puncture is made, the operator may rotate the
tool until the
expandable cutting arm aligns with where they want the length of the cut to
go.
- 38 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0189]
Furthermore, the cutting element 3502 may be retracted inward when the
expandable cutting arm 3504 is pulled in a proximal direction. The cutting
element
3502 may be retracted towards the center of the cutting implement 3400. The
energy
applied to the cutting element 3502 may be removed to prevent inadvertent
cutting.
[0190]
FIG. 37 is a cross-sectional illustration of the cutting implement 3400 in
accordance with one aspect of the present disclosure. A schematic showing how
the
cutting arms of the embodiment may be made to allow exposed metal 3702 in
specific
areas to optimize the cutting performance of the tool and minimize the
required power
input is shown, which may use a thinnest possible insulation in order to
ensure better
tissue contact with the exposed metal surface. The sheath 3402 with no exposed
metal using insulation 3704 is also provided. In this embodiment, the cutting
element,
or the cutting arm, is not used. Rather, it may use RF power for cutting.
[0191]
FIGS. 38A-E are schematics showing how the cutting implements may be
used to optimize cutting performance and minimize power input in accordance
with
one aspect of the present disclosure. Various cutting shapes are shown that
may be
created by the embodiments through the interatrial septum. These shapes may be
created with multiple cutting arms or using a single cutting arm that may be
rotated
and used multiple times.
[0192]
FIGS. 39-53 describe a third embodiment with delivery sheaths and a helical
anchor for tying tissue together. Components described below may be placed
into a
catheter shaft. The shaft internally may have a number of different lumens and
channels for these components. In one embodiment, separate tools may be used
for
each of the components. These tools may follow along the guidewire that is in
place.
Techniques and/or devices are shown below to provide large bore transseptal
access
with subsequent atrial re-access.
[0193]
Turning to FIG. 39, an isometric view of exemplary tissue 900 being crossed
using an illustrative guidewire 102 in accordance with one aspect of the
present
disclosure is provided. The
technique may initially begin with the guidewire 102
piercing or crossing into the tissue 900. The guidewire 102 may be brought in
through
the vascular introduction sheath which may be routed cranially up the inferior
vena
- 39 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
cava to the right atrium. The guidewire 102 may be placed to direct
therapeutic or
diagnostic catheters into a region of the heart.
[0194] FIG. 40 is an isometric view of suture anchors 4002 in delivery
sheaths 4004
in accordance with one aspect of the present disclosure. The delivery sheaths
4004
may house other components in addition to the tissue anchors 4002. Using the
guidewire, the delivery sheaths 4004 may be directed towards the tissue 900 of
the
atrial septum.
[0195] FIG. 41 is an isometric view of illustrative suture anchors 4002 in
delivery
sheaths 4004 about to penetrate the tissue 900 in accordance with one aspect
of the
present disclosure. The suture anchors 4002 may be placed at a first puncture
point
4102 and a second puncture point 4104 which may be directed through a
guidewire
102. Thereafter, they may be inserted into the tissue 900.
[0196] Referring to FIG. 42, an isometric view of the illustrative suture
anchors
4002 in delivery sheaths 4004 engaging or penetrating the tissue 900 in
accordance
with one aspect of the present disclosure is provided. The suture anchors 4002
in
delivery sheaths 4004 may engage or penetrate the tissue 900 near the
guidewire
102.
[0197] FIG. 43 is an isometric view of the illustrative suture anchors 4002
in delivery
sheaths 4004 engaging the tissue 900 with suture control lines 4302 attached
in
accordance with one aspect of the present disclosure. The suture control lines
4302
may be used for holding sutures in place within the tissue 900 near the
guidewire 102
while operations are being performed. The delivery sheaths have been removed
to
expose the suture control lines 4302.
[0198] FIG. 44 is an isometric view of an illustrative cutting implement
4400 in a
sheathed position in accordance with one aspect of the present disclosure. The
cutting
implement 4400 may be tucked away into the sheath 4402. The cutting implement
4400 may be circuitous with a guidewire, that is, its guidance may be based on
the
guidewire to the tissue.
- 40 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0199]
Turning to FIG. 45, an isometric view of the illustrative cutting implement
4400 in an unsheathed position in accordance with one aspect of the present
disclosure is provided. The cutting implement 4400 may be extended outside the
sheath 4402. This may be performed at a proximal end by an advancing
mechanisms
through the sheath 4402.
[0200]
FIG. 46 is an isometric view of the illustrative cutting implement 4400 in an
unsheathed position and expanded in accordance with one aspect of the present
disclosure. The cutting implement 4400 may be spring loaded such that after
extending outside the sheath 4402, it may be expand symmetrically. The cutting
implement 4400 may also be expanded through a pull-wire or other mechanism.
Expanding the cutting implement 4400 may facilitate tissue incision.
[0201]
FIG. 47 is an isometric view of the illustrative cutting implement 4400 making
an incision or cut into the tissue 900 in accordance with one aspect of the
present
disclosure. The suture anchors 4002 in the delivery sheaths may be used to
hold the
tissue 900 in place while allowing guidance of the cutting implement 4400. The
cutting
implement 4400 may make incisions between the two suture anchors 4002 in the
delivery sheaths.
[0202]
Referring to FIG. 48, an isometric view of an illustrative incision 4800 in
the
tissue 900 with a guidewire 102 passing through it in accordance with one
aspect of
the present disclosure is provided. With the cutting implement removed, the
two suture
anchors 4002 coupled to the suture control lines 4302 along with the guidewire
102
remain.
[0203]
FIG. 49 is an isometric view of an advancing of an exemplary therapeutic
instrument 4900 passing through the incision in the tissue 900 over the
guidewire in
accordance with one aspect of the present disclosure. The
therapeutic
instrumentation 4900 may be advanced through the guidewire and through the
incision
in the tissue 900. The therapeutic instrumentation 4900 may be disposed
between
the two suture anchors 4002 coupled to the suture control lines 4302.
[0204]
FIG. 50 is an isometric view of an illustrative tissue anchor lock 5000 with
helical barbs 5002 in accordance with one aspect of the present disclosure.
The lock
-41 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
5000 with helical barbs 5002 may be implanted into the tissue, which will be
shown
below, to close an incision.
[0205]
Turning to FIG. 51, an isometric view of the illustrative tissue anchor lock
5000 with helical barbs engaged in the tissue 900 passed over the suture
control lines
4302 in accordance with one aspect of the present disclosure is provided. In
this
technique, the tissue 900, having the incision 4800, may be twisted with the
tissue
anchor lock 5000 using the helical barbs. This may secure the sutures, anchors
and
tissue 900 to one another.
[0206]
FIG. 52 is an isometric view of the illustrative tissue anchor lock 5000 with
helical barbs engaged in the tissue passed over the suture control lines 4302
trimmed
to the level of the anchor in accordance with one aspect of the present
disclosure. The
excess suture control lines 4302 may be trimmed through a separate cutting
implement that is guided to the tissue using the guidewire. The incision 4800
may be
closed with the lock 5000 which has minimal control lines 4302 attached
thereto.
[0207]
FIG. 53 is an isometric view of the illustrative tissue anchor lock 5000 with
helical barbs 5002 engaged in the tissue 900 from the other side of the tissue
900 in
accordance with one aspect of the present disclosure. The lock 5000 may be
interspersed between the tissue 900 to either heal with the tissue 900 and
endothelize,
or be absorbed or dissolve. The view is on an opposite side of atrial wall
that was
shown in FIG. 52. The lock 5000 may grab or hook tissue 900 and then be
twisted to
seal the incision.
[0208]
Multiple techniques were described above for closing an incision and
allowing re-access. In addition, FIGS. 54-69 provide a fourth embodiment
localizing a
plurality of pledgets made of biodegradable materials secured together with a
knot to
close the incision while allowing re-access at a later time. In the following,
two
pledgets may be used, however, fewer or more may be passed into the tissue for
securing the area. The pledgets may be constructed from a bioabsorbable,
biodegradable material including but not limited to; sugars, salts, collagen,
PLGA,
PLLA, other absorbable polymers, magnesium, and or other materials. The
pledgets
may be constructed from a combination of materials to facilitate different
structural
properties. Some of these materials may be traditional implant materials such
as
- 42 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
metals or polymers including, but not limited to, stainless steel, nitinol,
cobalt chrome,
PEEK, HDPE, and others.
[0209] Turning to FIG. 54, an isometric view of an illustrative pledget
5402 made
from biocompatible or bio-absorbable material in accordance with one aspect of
the
present disclosure is provided. The pledget 5402, made from biocompatible or
bioabsorbable material, may have a control line 5404 interlaced throughout.
The
pledget 5402 may be a single piece of material that is elongated with the
control lines
5404 dispersed between the pledget 5402 and tied or fastened at the end of the
pledget 5402. The control lines 5404 may be interspersed between apertures
within
the pledget 5402.
[0210] FIG. 55 is an isometric view of an exemplary cannula 5500 piecing
heart
tissue 900 in accordance with one aspect of the present disclosure. The
cannula 5500
may have a sharp distal end, but preferably uses RF energy to pierce the
tissue 900.
The cannula 5500 may use RF at a distal end with insulation covering the
tubular
structure. The tubular structure may allow a pledget, or other mechanism, to
be
distributed therethrough. The cannula 5500 may be inserted into a vein, such
as the
femoral vein, to reach the patient's heart. The cannula 5500 may be set firmly
in place.
Different variations of cannulas 500 exist and the technique described herein
is not
limited to the cannula 500 shown.
[0211] FIG. 56 is an isometric view of the exemplary cannula 5500 piecing
heart
tissue 900 and the illustrative pledget 5402 made from biocompatible or
bioabsorbable
material advanced through the cannula 5500 in accordance with one aspect of
the
present disclosure. The cannula 5500 may pierce the tissue 900 with a circular
cut.
Typically, the cut may be small such that the pledget when compacted may plug
or fill
the cut, providing sufficient apposition to then tension the tissue for later
closure. Other
shapes for the cut may be used depending on the cross-section of the cannula
5500.
[0212] Referring to FIG. 57, an isometric view of the exemplary cannula
5500
piecing heart tissue 900 and the illustrative pledget 5402 made from
biocompatible or
bioabsorbable material advanced out of the cannula 5500 in accordance with one
aspect of the present disclosure is provided. An advancing member 5702 may be
placed into the cannula 5500 from a proximal end to force the pledget 5402
with control
- 43 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
lines 5404 into the left atrium. In one embodiment, a proximal end of the
pledget 5402
is coupled to the distal end of the advancing member 5702 through the control
lines
5404 for activation of the pledget by shortening the distance and expanding
the cross
sectional area of the pledget.
[0213] FIG. 58 is an isometric view of the exemplary cannula 5500 piecing
heart
tissue 900 and the illustrative pledget 5402 made from biocompatible or
bioabsorbable
material advanced out of the cannula 550 and a pull member tensioned to
shorten the
pledget 5402 in accordance with one aspect of the present disclosure. The
control
line 5404 may be pulled through the advancing member 5702. The control line
5404,
which was interspersed between apertures within the pledget 5402, may then
cause
the pledget 5402 to be retracted or shortened. This may cause the pledget 5402
to
be bunched up.
[0214] FIG. 59 is an isometric view of the illustrative pledget 5402 made
from
biocompatible or bioabsorbable material tensioned to shorten the pledget 5402
with
the exemplary cannula piecing heart tissue 900 withdrawn and retained on a
heart
tissue surface in accordance with one aspect of the present disclosure. With
the
cannula withdrawn and the pledget 5402 shortened and compacted, the initial
cut
made by the cannula has been plugged or secured.
[0215] Referring to FIG. 60, an isometric view of an illustrative
concentric pledget
6002 made from biocompatible or bioabsorbable material in accordance with one
aspect of the present disclosure is provided. The concentric pledget 6002,
made from
biocompatible or bioabsorbable material, may be introduced in a similar manner
as
the other pledget. The concentric pledget 6002 may be made of the same or
similar
materials as the other pledget. A control line 6004 may be coupled to the
concentric
pledget 6002 allowing the pledget 6002 to be pulled back such that the
concentric
pledget 6002 may become contracted or bundled expanding the cross sectional
area
to provide for greater apposition force to the tissue plane.
[0216] FIG. 61 is an isometric view of the exemplary cannula 5500 piecing
heart
tissue 900 next to the pledget 5402 in accordance with one aspect of the
present
disclosure. The cannula 5500 may be placed into the tissue 900 near the other
pledget
5402. Typically, and as described earlier, the pledgets may be inserted by one
another
- 44 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
to maintain the integrity of the surrounding tissue as well as allow re-
access. The
placement may provide for a cutting implement to be disposed between the
pledgets
to make an incision as well as for a therapeutic or diagnostic instrument to
be inserted
into the incision.
[0217] FIG. 62 is an isometric view of the exemplary cannula 5500 piecing
heart
tissue 900 and the illustrative concentric pledget 6002 made from
biocompatible or
bioabsorbable material advanced out of the cannula 5500 in accordance with one
aspect of the present disclosure. The advancing member 5702 may be used to
push
or advance the pledget 6002 through the cannula 5500. The pledget 6002
connected
to the control line 6004 may be pushed through the tissue 900 via the cannula
5500
next to the other pledget 5402.
[0218] FIG. 63 is an isometric view of the exemplary cannula 5500 piecing
heart
tissue 900 and the illustrative concentric pledget 6002 made from
biocompatible or
bioabsorbable material advanced out of the cannula 5500 and a pull member
tensioned to shorten the pledget in accordance with one aspect of the present
disclosure. The control line of the concentric pledget 6002 may be pulled
through the
advancing member 5702. By doing so, the concentric pledget 6002 may be shorted
or bundled.
[0219] Referring to FIG. 64, an isometric view of the illustrative
concentric pledget
6002 made from biocompatible or bioabsorbable material tensioned to shorten
the
pledget 6002 and an exemplary incision 6402 made between the pledgets 5402 and
6002 in accordance with one aspect of the present disclosure is provided. The
pledgets 5402 and 6002 may provide anchor points where the tissue 900 may be
tied
together.
[0220] A cutting implement, as previously described, may be used to make
the
incision 6402 between them. While a straight cut is shown, other types of
incisions
6402 may be made. These may include, but are not limited to, a straight cut, v
cut,
zig zag, or crescent arc. With both tissue securing pledgets 5402 and 6002 in
place,
the incision 6402 may then be made.
- 45 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
[0221] FIG. 65 is an isometric view of the exemplary therapeutic instrument
4900
placed into the incision 6402 between the pledgets 5402 and 6002 into the
tissue 900
in accordance with one aspect of the present disclosure. Widening of the
incision
6402 may occur when the therapeutic instrument 4900 is placed therein. The
therapeutic instrument 4900 may follow a guidewire that was inserted for the
other
mechanisms brought to the right atrium. A diagnostic instrument may also be
used.
[0222] FIG. 66 is an isometric view of an illustrative knot 6602 advanced
up to the
heart tissue 900 with the two pledget control lines 5404 and 6004 in
accordance with
one aspect of the present disclosure. Upon use and removal of the therapeutic
instrument 4900, the securing knot 6602 may be advanced through tightening of
the
control lines 5404 and 6004 of the pledgets.
[0223] Referring to FIG. 67, an isometric view of the illustrative knot
6602 advanced
up to the heart tissue 900 with the two pledget control lines 5404 and 6004
and
tensioned tight from the knot side of the tissue 900 in accordance with one
aspect of
the present disclosure is provided. When pulled, the control lines 5404 and
6004 may
advance the knot 6002 further to the tissue 900. The control lines 5404 and
6004 may
be cut to shorten them, which will be shown below.
[0224] FIG. 68 is an isometric view of the illustrative knot advanced up to
the heart
tissue 900 with the two pledget tension lines tightened from the pledget side
of the
tissue 900 in accordance with one aspect of the present disclosure. When
tightened,
the pledgets 5402 and 6002 may collapse towards one another with tissue 900
folded
therebetween.
[0225] FIG. 69 is an isometric view of the illustrative incision 6402
closed between
the pledgets in accordance with one aspect of the present disclosure. Control
lines
used to make the knot 6602 may be removed or cut. This may remove any
interference that they have within the patient.
[0226] In addition to suture and mechanical apparatuses to join the tissue
edges
together, adhesive materials may be used to seal or join the tissue either as
a primary
or supplementary or adjunct mechanism. These material may include, but are not
limited to, adhesive cyanoacrylates, methoxypropyl cyanoacrylates, alkyl
- 46 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
cyanoacrylates such as n-butyl, isobutyl or n-octyl cyanoacrylates,
octylcyanoacrylate,
butylcyanoacrylate, BioGlue Surgical Adhesive (BioGlue), bovine serum albumin
(BSA), glutaraldehyde of purified (BSA), extracellular matrix ECM human
connective
tissue, autologous and homologous fibrin sealants, fibrin glue, Polyethylene
glycol
(PEG)-Based Hydrogel Sealants, hydrogel, methacryloyl-substituted tropoelastin
(MeTro), and many others. These sealants may be biocompatible and resorbable.
[0227] In
one embodiment, a bipolar catheter type mechanism may be used to
seal the tissue back together. For example, bipolar coagulating forceps used
to stop
a bleeding vessel may be used. There may be procedural order options to
accomplish
this procedure.
[0228]
FIG. 70 is an illustrative flow chart showing exemplary processes for
allowing a large bore transseptal access with subsequent atrial re-access in
accordance with one aspect of the present disclosure. These processes are for
illustrative purposes and may be modified according to those techniques
described
herein. The processes may begin at block 7000.
[0229] At
block 7002, a guidewire may be inserted into the heart chamber and
distributed at the atrial septum. The guidewire may be inserted into the
venous
circulatory system through the vascular introduction sheath. The initial
percutaneous
puncture or incision, by way of example, may be at the patient's femoral vein.
Other
areas where the guidewire may enter into the patient may include, but is not
limited to,
a jugular vein, subclavian artery, subclavian vein, or brachial artery and
vein.
[0230] The
guidewire may be routed cranially up the inferior vena cava to the right
atrium of the heart. The guidewire may be placed at the atrial septum so that
it is used
to direct therapeutic or diagnostic catheters into a region of the heart. In
turn, the
guidewire may be temporarily or removably fixed at the atrial septum.
[0231] At
block 7004, a puncture may be made by a needle through the atrial
septum into the left atrium. The septal penetrator may be a needle or axially
elongate
structure with a sharp, pointed distal end. In
one embodiment, the septal
penetrator may be resident within the guidewire. The septal penetrator may be
actuated at the proximal end of the vascular device through a control
mechanism such
- 47 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
as a button, lever, handle, or trigger which may be affixed, permanently or
removably
by way of a linkage, pusher rod, electrical bus, or the like that runs the
length of the
device.
[0232] The guidewire may be used as the puncture device. The guidewire may
have a tip that facilitates the crossing of the septum such as, but not
limited to, a sharp
end, a helical end, a RF energy electrode tip, other energy tip, or other
device to aid
the tissue penetration.
[0233] Sutures may be pulled through the punctures at block 7006 through an
anchor. The sutures may be bundled within a suture bundle and stored in the
recess
of the anchor. The catheter shaft may be positioned in the right atrium with
the anchor,
having the suture bundle, advanced into the left atrium through the puncture.
In turn,
the suture bundle may be unspooled by snares which capture needles coupled to
the
end of the sutures. The snares with the needles may be pulled into the
catheter shaft.
The sutures from the suture bundles may be managed through the snares.
[0234] Sutures may be placed from the right to left atrium or from the left
to right
atrium depending on the apparatus. The suture may be placed repeatedly across
the
septum for multiple points of engagement. The sutures may be another type of
apparatus such as a helical anchor or barb device. The placement of the
sutures may
also be provided after block 7012, when the therapy is complete.
[0235] At block 7008, and after the sutures are in place, a cut may be made
at the
interatrial septum using the cutting implement proximal to the needle passing.
The
sutures may be deployed through holes made near the initial puncture. Cuts or
incisions that are made may be parallel such that the sutures are not cut by
the cutting
implement. The cutting implement may be coupled to the catheter to make sure
that
the cutter does not accidentally cut the sutures.
[0236] Various cutting implements were described herein. Mechanical or RF
energy may be used. When using electrical energy, the amount of exposed metal
may
be minimized through insulation such that the exposed metal may only exist in
the
desired tissue cutting region of the tool. Mechanical energy may use blades
that may
be deployed in the singular direction or may be symmetrical. The cutting may
be
- 48 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
performed from the right to left atrium or from the left to right depending on
the
apparatus. The cutting may be post anchor placement. Alternatively, the
cutting may
precede the suture anchor placement, described at block 7006. The cutting may
be
integrated into blocks 7002 and 7004 above with a device that punctures and
cuts the
tissue.
[0237] At
block 7010, the sutures may be managed. That is, the sutures may be
pulled towards the vessel wall and be shifted or manipulated such that they do
not
interfere or entangle with the therapy instrumentation catheters. The tissue
suture
management lines may be managed in a lumen of an access sheath or in a
separate
catheter.
[0238]
Therapy may be performed using the therapeutic instrument at block 7012.
The therapeutic instrument may perform diagnosis and therapeutic intervention
to
correct atrial fibrillation, perform mitral valve repair, correct septal
defects, perform
implantation of a cardiac prosthesis, or the like. The therapy or diagnosis
may be
performed in the left atrium. In turn, the therapeutic instrument may be
removed.
[0239] At
block 7014, the control sutures may be used to close the incision. The
amount of this closure may be adjusted to meet the desired therapeutic goals
of the
procedure. It may be desirable to completely seal the incision, or leave a
passage for
relief of excess pressure from one side to the other. In one example, the
control
sutures may be pressed against the tissue which was described above. The
processes may end at block 7016.
[0240]
Other techniques may be used for allowing large bore transseptal access
with subsequent atrial re-access. For example, and in this embodiment, the
method
may include puncturing the septum for needle passing, leaving sutures or some
other
anchor behind, and then performing a procedure. The anchors or sutures left
behind
may be cinched together to close the septum. Excess sutures may be then be
cut.
[0241] In
another technique for atrial re-access, the septum may be cut first. The
septum may be stabilized through a needle puncture radius so that the needles
may
pass through the previous cut septum. The needle passing device may be
introduced.
Needles on the left atrium side may then be snared/grabbed and pulled through
the
- 49 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
catheter. In turn, pre-shaped nitinol wire may be used to loop through the
septum.
Control structures may be used to close the incision with the excess sutures
cut.
[0242] In yet another technique, the transseptal access may be obtained via
a
standard transseptal approach. The guidewire may be left across the
transseptal
access site in the left atrium. The vascular device for slicing, enlarging and
placing
sutures is advanced over the guidewire. Four cutting members radially spaced
may
slice the septum and enlarge the transseptal access point in a controlled and
consistent manner. Needles may then puncture the septum and pass a suture
through
the interatrial tissue in a location between the slices such that when cinched
together
they most optimally close the iatrogenic ASD. The vascular device may then be
removed, leaving the four sutures in place across the septum. The sutures may
be
pulled out of the vein and remain temporarily in place in the inferior vena
cava vein
until later in the procedure.
[0243] In yet another technique the mechanism to facilitate the delivery of
the
suture, anchors, cutting implements, and closing features may be integrated
into the
therapeutic instrument to minimize the exchange of devices in the patient.
[0244] In yet another technique, no foreign body may be left behind. The
technique, with associated device or devices, may come into the atrium after
the
procedure and cinch the tissue to be apposed for a time to promote healing
while
sealing them. In turn, the technique may include removing the structure or
apparatus.
This technique may be the combination of a controlled incision and then later
cinching
with an apparatus described herein, or that is known in the art. The apparatus
may
include, but is not limited to, grabbers, forceps, helical anchors, pinchers,
knots,
suction devices, barbs, or the like. This technique may then promote the
tissue to heal
by itself due to cut morphology. The time of this temporary apposition may
range from
minutes to days, or weeks, depending on the amount of tissue healing desired.
[0245] In some embodiments, the anchors described above may subsequently be
removed from the tissue, or alternatively may be left in place. The techniques
or
procedures for removing the anchors may use grabbers, snares, cutting
elements, or
engagement features specific to the mechanism left in place. A mechanical
feature
may be added on the right atrial side of the anchoring device such that it may
be
- 50 -
CA 03185473 2022-11-30
WO 2021/011502 PCT/US2020/041853
subsequently grabbed and unscrewed. This mechanical feature may be in the
shape
of a hook, an oval, or the like. This feature may protrude off of the right
atrial septum
such that it may be grasped with a snare and rotated out of the tissue, for
example.
[0246] The foregoing description is provided to enable any person skilled
in the
relevant art to practice the various embodiments described herein. Various
modifications to these embodiments will be readily apparent to those skilled
in the
relevant art and generic principles defined herein may be applied to other
embodiments. Thus, the claims are not intended to be limited to the
embodiments
shown and described herein, but are to be accorded the full scope consistent
with the
language of the claims, wherein reference to an element in the singular is not
intended
to mean one and only one" unless specifically stated, but rather one or more."
All
structural and functional equivalents to the elements of the various
embodiments
described throughout this disclosure that are known or later come to be known
to those
of ordinary skill in the relevant art are expressly incorporated herein by
reference and
intended to be encompassed by the claims. Moreover, nothing disclosed herein
is
intended to be dedicated to the public regardless of whether such disclosure
is
explicitly recited in the claims.
- 51 -