Note: Descriptions are shown in the official language in which they were submitted.
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
MODIFIED SEMAPHORIN 3A, COMPOSITIONS COMPRISING THE SAME AND
USES THEREOF
FIELD OF THE INVENTION
The present invention relates to modified forms of Semaphorin 3A (Sema3A)
polypeptide
having amino acid(s) substitution and/or deletion compared to a wild type
Sema3A protein. The
invention further relates to compositions including the modified Sema3A and
uses thereof for
treating various immune-related conditions.
BACKGROUND OF THE INVENTION
Semaphorins are a family of membrane bound and soluble proteins classified
into eight sub-
classes based on their structural domains. Semaphorins were found to regulate
axon guidance,
organogenesis, angiogenesis, lymphangiogenesis and immune responses and to
modulate tumor
progression. The Semaphorins are divided into several subfamilies.
The seven class-3 Semaphorins (Semaphorin 3s), designated by the letters A-G,
are the only
vertebrate secreted Semaphorins. Neuropilins (Nrps) and the type A/D family
Plexins (Plexin-Al ,
-A2, A3, A4 and Plexin-D1) act as receptors for class-3 Semaphorins. Each
Semaphorin 3 family
member shows distinct binding preference for Nrps. Each Sema3-Nrp complex
associates with
specific plexins to mediate downstream signaling, including transducing
signals that induce the
collapse of the actin cytoskeleton of target cells. Most membrane-bound
vertebrate Semaphorins
directly bind plexins, while the class-3 Semaphorins, with the exception of
sema3E and sema3C,
require Neuropilins as obligate co-receptors.
Semaphorin 3A (Sema3A), a class-3 secreted member of the Semaphorin family,
has been
established as an axonal guidance factor during development. Sema3A has also
been shown to be
expressed by activated T cells and inhibit T cell proliferation and cytokine
secretion. Additionally,
Neuropilin-1 expression on regulatory T cells has been shown to enhance
interactions with immature
dendritic cells (DCs) during antigen recognition, resulting in higher
sensitivity to limiting amounts
of antigen. In addition to its role as an axon guidance factor, Sema3A
functions as an inhibitor of
angiogenesis and as a blood vessels permeabilizing agent, functions mediated
through the
neuropilin-1 receptor. Sema3A also functions as an inhibitor of tumor
progression in a variety of
solid tumors as well as in hematological malignancies such as multiple
myeloma.
1
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
Sema3A was also characterized as a modulator of immune responses. It inhibits
primary T-
cell proliferation and pro-inflammatory cytokines production under anti-CD3
plus anti-CD28
stimulating conditions and inhibits the migration of thymocytes. Sema3A
production by bone
marrow derived mesenchymal stem cells seems to mediate at least part of their
immune suppressive
effects. In addition, sema3A was suggested to have beneficial effects in a
variety of auto-immune
diseases. For example, it was found that sema3A reduced kidney failure in
NZB/W mouse model
of lupus nephritis and reduced the severity of asthma in mouse models of
asthma and allergic rhinitis.
Such beneficiary effects were likely due in part to sema3A stimulation of
FoxP3 and IL-10
expression in Treg cells and the significant reduction in TLR-9 expression in
B cells. It was found
that the concentration of Sema3A is strongly reduced in the sera of patients
afflicted with immune-
mediated (e.g. Familial Mediterranean fever (FMF)) and auto-immune diseases
such as systemic
lupus erythematosus and systemic sclerosis. Furthermore, it was found that
systemic administration
of recombinant Sema3A inhibits the development of kidney failure in the NZB/W
mouse model of
lupus nephritis, and alleviates asthma in an asthma model, It was further
found that Sema3A
promotes the expression of immune suppressive cytokines such as IL-10 from
regulatory T cells
(Treg) and the expansion of a subpopulation of regulatory B cells (Breg) that
highly express IL10,
suggesting that Sema3A is a master regulator that inhibits immune responses,
at least in part, by the
regulation of the expression of inhibitory cytokines.
Thus, for example, US Patent No. 10,105,413 relates to Semaphorin 3A and use
thereof in
treatment and prognosis of Systemic Lupus Erythematosus (SLE). US Patent No.
10,568,932 is
related to Semaphorin 3A for treatment and assessment of severity of asthma.
International
publication No. 2016/128966 relates to Semaphorin 3A for treatment and
assessment of severity of
Inflammatory Bowel Disease (IBD).
International application WO 2016135130 relates to non-natural Semaphorins 3
and their
medical use and discloses various mutated Semaphorin 3 molecules and methods
of using them in
the treatment of disease, in particular in the medical intervention of
angiogenic diseases, tumors
and/or cancer.
Nevertheless, there is a need in the art for modified forms of Sema3A that
exhibit improved
properties, compared to unmodified Sema3A molecules, and which can be used for
safe, efficient
and cost effective treatment of various immune-related conditions.
2
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
SUMMARY OF THE INVENTION
According to some embodiments, there is provided an advantageous modified
Semaphorin 3A
polypeptide, which includes one or more point mutations and/or truncations,
compared to a wild-
type (non-modified) Semaphorin 3A. According to some embodiments, the novel,
non-naturally
occurring, modified Sema3A disclosed herein is advantageous, as it is stable,
easy to produce, and
exhibit a desired biological activity, as further detailed herein. Further
provided are nucleic acids
encoding for the modified Sema3A polypeptide, methods for the preparation of
the modified
Sema3A, compositions comprising the same and uses thereof in treating various
medical conditions,
in particular, immune-related conditions.
According to some embodiments, the advantageous modified/non-naturally
occurring/genetically modified/mutated Semaphorin 3A polypeptide includes at
least one point
mutation and/or deletion (truncation) of a stretch of amino acids, compared to
a WT, unmodified,
naturally occurring Sema3A.
According to some embodiments, the modified Sema3A (also referred to herein as
"T-
sema3A") includes one amino acid substitution and a C-terminal deletion (of at
least 100 amino
acids), as compared to a WT Sema3A.
In some embodiments, the modified Sema3A includes an amino acid substitution
in position
257 of the human amino acid sequence of wild type Sema3A (represented by amino
acid sequence
denoted by SEQ ID NO: 1), whereby the amino acid Serine (Ser) in the WT
sequence is replaced by
amino acid Cysteine (Cys). Thus, the modified Sema3A includes a 5257C sequence
substitution.
In some exemplary embodiments, the modified Sema3A further includes a
deletion/truncation of
254 amino acids from the C-terminus of the WT Sema3A. That is, the modified
Sema3A is truncated
at amino acid 516 of the WT Sema3A. In some exemplary embodiments, the
modified Sema3A
polypeptide comprises an amino acid sequence as denoted by SEQ ID NO: 3.
According to further embodiments, the modified Sema3A may further include one
or more
additional tag sequences at the N-terminal and/or C-terminal thereof. In some
embodiments, the
Tag sequence may be used for marking/identification and/or purification of the
modified Sema3A.
In some embodiments, the tag sequence may be selected from His tag (i.e.,
including a stretch of
Histidine amino acids, for example, 8 Histidine amino acids), FLAG-tag, Myc-
tag, and the like. The
tag sequences may be placed in-frame at the N-terminal of the modified
proteins and/or on the C-
terminal of the modified protein. In some exemplary embodiments, the modified
Sema3A protein
3
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
may include a stretch of 8 Histidine (8-His-Tag) at the C-terminus of the
polypeptide.
According to some embodiments, as mentioned above, WT Sema3A binds to the
neuropilin-
1 receptor (nrpl) which subsequently associates with type-A plexin receptors
that function as the
signal transducing elements in the functional sema3A receptor. Classically,
signaling via these
receptor complexes induces the collapse of the cytoskeleton in target cells.
Surprisingly, the
inventors of the present application have revealed that CD72 receptor also
functions as a sema3A
receptor (in addition to the known neuropilin-1 which was considered to be the
sole sema3A binding
receptor), and that CD72 mediated signal transduction can control anti-
inflammatory gene
expression in primary B-lymphoblastoid cells lacking neuropilin receptors.
Thus, as disclosed
herein, the anti-immune effects of sema3A may be mediated, at least in part,
by the CD72 receptor.
Accordingly, without wishing to be bound to any theory or mechanism, the
advantageous, non-
naturally occurring modified Sema3A exhibits a differential activation as
compared to a WT
Sema3A. In other words, the modified Sema3A protein, having a truncation at
the C-terminal region
of the protein, will not be able to activate neuropilin-1 mediated signal
transduction, but does retain
its ability to activate CD72 mediated signal transduction. Thus, the
advantageous modified Sema3A
protein disclosed herein may retain its anti-immune properties, mediated via
CD72 binding, yet be
devoid of undesired side effects which in the wild type sema3A are mediated
via the neuropilin-1
receptor. Further, since the Sema3A is active as a homodimer, in order to
allow the modified
Sema3A to retain dimerization capabilities (which are found in the WT protein
in the C-terminal
region), the 5257C point mutation mentioned above was introduced.
According to some embodiments, as further exemplified herein, the advantageous
modified
Sema3A retains the immune beneficiary properties of wild type Sema3A while and
because it
interacts with only a subset the sema3A receptors, displays fewer side
effects, as compared with
wild type sema3A. Further, as exemplified herein, the modified Sema3A was
found to be at least
as effective as wild type sema3A in increasing T regulatory cells function. In
further embodiments,
as disclosed herein, the modified sema3A is capable of reducing activity and
metabolism of
activated T-cells. According to some embodiments, T-Sema3A can affect
(decrease) the glycolytic
rate of activated T-cells, i.e., down regulate aerobic glycolysis in such
activated immune cells.
Accordingly, in some embodiments, the modified-sema3A can therefore be used
for the
successful treatment of various immune-mediated conditions, such as, auto-
immune diseases (such
as, for example, Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis,
inflammatory bowel
disease (IBD), Uveitis, Psoriasis), allergic conditions (such as, bronchial
asthma, allergic
4
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
conjunctivitis, allergic rhinitis and atopic dermatitis), conditions related
to over activation of the
immune system (such as, for example, sepsis, cytokine storm-due to infectious
diseases and/or
CAR-T treatment, graft-versus host disease (GVHD), inflammatory diseases (such
as, Chronic
Obstructive Pulmonary Disease (COPD), Familial Mediterranean fever (FMF)). In
some
exemplary embodiments, the immune-mediated condition may include, for example,
Systemic
Lupus Erythematosus (SLE), asthma, IBD, and the like.
According to some embodiments, there is thus provided a novel, non-naturally
occurring
modified sema3A (T-sema3A) that is unable to signal via neuropilins yet
capable of displaying
anti-inflammatory effects at least as good as, if not better, compared to wild
type sema3A in
various assays. The disclosed T-sema3A is advantageous as it is smaller in
size, compared to the
wild type sema3A, and may therefore be more diffusible and less difficult to
produce in large
quantities. In addition, it may be safer and more potent for use in treating
various immune-
mediated disorders. Wild type sema3A has beneficial effects in several
autoimmune diseases.
However, it also affects additional biological processes such as angiogenesis
and axon guidance
as a result of its binding to receptors of the neuropilins family. Thus,
treatment with wild type
sema3A may be accompanied by diverse side effects resulting from the
activation of neuropilins
mediated signaling in various body compartments. Thus, without wishing to be
bound to any
theory or mechanism, the disclosed T-sema3A, which retains the immune
beneficial effects of wild
type sema3A, but un-able to activate he undesired neuropilin mediated signal
transduction, may
consequently exhibit fewer side effects. Thus, according to some embodiments,
the herein
disclosed modified Sema3A surprisingly exhibit better in vivo and/or in vitro
properties as
compared to naturally occurring Sema3A (WT-Sema3A). In some embodiments, the
modified
Sema3A disclosed herein exhibit improved therapeutic activity of immune-
related conditions, as
compared to a WT Sema3A. In some embodiments, the modified Sema3A exhibit one
or more
improved properties as compared to a WT Sema3A, the properties may include:
pharmacologic
effects, pharmacokinetic, stability, half-life, delivery, efficiency, cellular
targets, side effects, and
the like, or any combination thereof.
According to some embodiments, in view of the 5257C substitution introduced in
the T-
Sema3A, the modified Sema3A can function as a dimer, whereby two monomeric T-
Sema3A can
form a dimer, via sulfide bonds, between the respective Cysteine residues
introduced into the
sequence. Therefore, the modified Sema3A proteins disclosed herein can
preferably and
advantageously be in the form of the dimer. In some embodiments, the thus
formed dimer is a homo-
5
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
dimer. The term "homo-dimer" indicates that two identical T-Sema3A monomers
are in the form of
a dimer.
In some embodiments, the two monomers of the dimer can be comprised in one
fusion
protein. In some embodiments, the two modified Sema3A monomers of the dimer
may be encoded
by a single nucleic acid molecule. In some embodiments, the two monomers of
the dimer can be
formed independently in a tube or a cell, and form a dimer in-vitro or in-
vivo, for example, after
being produced or placed under physiological conditions.
According to some embodiments, as exemplified herein, it was surprisingly
found that the
replacement of the S257C and the truncation of the C-terminal region (which
includes the native
binding region of the Nrp 1 receptors), results in binding to CD72 receptor,
independently of Nrpl
binding. As further exemplified herein the modified Sema3A exhibit activation
of regulatory T-
cells. For example, the modified Sema3A can bind to cellular CD72 receptor.
For example, the
modified Sema3A can active CD4+ regulatory T-cells and induce IL-10 secretion.
For example,
the modified Sema3A does not induce cell-contraction.
According to some embodiments, provided are methods and compositions for
treatment of
immune-related condition, said methods comprising administration of a
pharmaceutical composition
comprising the modified Semaphorin 3A to a subject in need thereof. In some
embodiments, the
immune-related condition is selected from Asthma, IBD and systemic Lupus
Eryhtmus (SLE).
According to some embodiments, there is provided a modified Semaphorin 3A
polypeptide,
the modified Semaphorin 3A polypeptide includes an amino acid
substitution/replacement at a
position corresponding to position 257 in a wild type Semaphorin 3A protein
having an amino acid
sequence as denoted by SEQ ID NO: 1, wherein the replacement is with Cysteine
(C); and a deletion
of at least 100 amino acids of the C-terminal region of the corresponding wild
type Semaphorin 3A.
According to some embodiments, the amino acid substitution is 5257C and the C-
terminal
deletion is of amino acids 517-771 of the corresponding wild type Semaphorin
3A.
According to some embodiments, the modified Semaphorin 3A and the wild type
Semaphorin
3A are of human origin.
According to some embodiments, the polypeptide may further include a Tag
sequence at the
N-terminus and/or the C-terminus thereof.
According to some embodiments, tag sequence is positioned in frame at the C-
terminal region
of the polypeptide. According to some embodiments, the Tag sequence is
selected from: His-Tag,
6
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
Myc-Tag and FLAG-tag.
According to some embodiments, the Tag sequence may include a stretch of 6 or
more
consecutive Histidine residues.
According to some embodiments, the modified Semaphorin 3A polypeptide has an
amino acid
sequence as denoted by SEQ ID NO: 3. According to some embodiments, the
modified Semaphorin
3A polypeptide has an amino acid sequence as denoted by SEQ ID NO: 5.
According to some embodiments, the modified Semaphorin 3A polypeptide is
configured to
or is capable of forming a homo-dimer with a modified Semaphorin 3A
polypeptide via S-S bonds
formed between Cysteine 257 in each of the modified polypeptides.
According to some embodiments, the modified Semaphorin 3A polypeptide is
capable of
binding CD72 receptor.
According to some embodiments, the modified Semaphorin 3A polypeptide un-
capable of
binding to Nrpl.
According to some embodiments, the modified Semaphorin 3A polypeptide is
unable to
induce cell contraction.
According to some embodiments, the modified Semaphorin 3A polypeptide s
capable of
inducing/changing/affecting expression of one or more anti-inflammatory
cytokines.
According to some embodiments, the modified Semaphorin 3A polypeptide is
capable of
inducing expression of IL-10 in CD4+ regulatory T-cells.
According to some embodiments, there is provided a composition comprising the
modified
Semaphorin 3A polypeptide disclosed herein.
According to some embodiments, the modified Semaphorin 3A polypeptide
disclosed herein,
or the composition comprising the same may be used for treating an immune-
related condition in a
subject in need thereof.
According to some embodiments, the immune related condition is selected from
Asthma, SLE
and IBD.
According to some embodiments, there is provided a nucleic acid molecule
(polynucleotide)
encoding the modified Semaphorin 3A disclosed herein.
According to some embodiments, the nucleic acid molecule encoding the modified
7
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
Semaphorin 3A has a nucleotide sequence as denoted by any one of SEQ ID NO: 4
and SEQ ID NO:
6.
According to some embodiments, there is provided a vector including the
nucleic acid
molecule encoding for the modified Semaphorin 3A. In some embodiments, the
vector is an
expression vector, further including one or more regulatory sequences.
According to some embodiments, the nucleic acid molecule encoding the modified
Semaphorin 3A or the vector including the nucleic acid may be used for
treating an immune-related
condition in a subject in need thereof.
According to some embodiments, there is provided a method of treating an
immune related
.. condition in a subject in need thereof, the method includes administering
to the subject in need
thereof a therapeutically effective amount of the modified Sema3A polypeptide
disclosed herein, or
a composition including the same.
According to some embodiments, there is provided a method of treating an
immune related
disorder in a subject in need thereof, the method includes administering to
the subject in need thereof
a therapeutically amount of nucleic acid molecule encoding the modified
Semaphorin 3A or the
vector including the same.
According to some embodiments, there is provided a host cell harboring the
nucleic acid
encoding the modified Sema3A.
According to some embodiments, there is provided a host cell transformed or
transfected with
the vector including the nucleic acid molecule encoding the modified Sema3A.
According to further embodiments, there is provide a host cell which includes
or expresses the
modified Sema3A polypeptide disclosed herein.
According to some embodiments, there is provided a method of producing the
modified
Sema3A polypeptide, the method includes the steps of: (i) culturing the host
cells under conditions
.. such that the polypeptide comprising the modified Sema3A is expressed; and
(ii) optionally
recovering the modified Sema3A from the host cells or from the culture medium.
Further embodiments, features, advantages and the full scope of applicability
of the present
invention will become apparent from the detailed description and drawings
given hereinafter.
However, it should be understood that the detailed description, while
indicating preferred
embodiments of the invention, are given by way of illustration only, since
various changes and
8
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
modifications within the spirit and scope of the invention will become
apparent to those skilled in
the art from this detailed description.
BRIEF DESCRIPTION OF THE FIGURES
Figs. 1A-C - Presents amino acid and nucleotide sequences of human WT-Sema3A
and
modified Sema3A. Fig. lA Shows the 771 amino acid sequence of the human WT-
Sema3A (SEQ
ID NO: 1), with the C-terminal region (amino acids 517-771) marked (gray
background); Fig. 1B
shows the amino acid sequence of a modified Sema3A (T-sema3A), which includes
a 5257C
substitution (marked by Capital C), and a C-terminal truncation at amino acid
R516. The modified
Sema3A sequence presented in Fig. 1B further includes an in-frame 8X-His tag
(HHHHHHHH
(SEQ ID NO: 7 (marked)) at the C-terminal end of the modified protein. The
amino acid sequence
presented in Fig. 1B corresponds to SEQ ID NO: 5; Fig. 1C presents the nucleic
acid sequence of
the cDNA encoding for a modified His-tagged Sema3A. The cDNA sequences
presented in Fig. 1C
corresponds to SEQ ID NO: 6, and includes a codon modification (bases 769-
771), whereby the
codon encoding for a Serine residue (in the WT Sema3A protein, SEQ ID NO: 2),
was changed to a
tgt codon encoding cysteine at bases 769-771. In addition, a cDNA sequence
encoding 8 histidine
residues (caccatcaccatcaccatcaccatcaccat (SEQ ID NO: 8), highlighted) was
fused in frame at
nucleotide 1548, followed by a stop codon (tga);
Fig. 2¨ shows a vector map of the NSPI-CMV-MCS-myc-His lentiviral expression
vector
which harbors a coding sequence of the modified Sema3A, according to some
embodiments;
Figs. 3A-D ¨ Sema3A binds to the CD72 receptor: Fig. 3A ¨ shows pictogram of
Western
Blot analysis of cell extracts probed with antibodies directed against
neuropilin-1 and/or CD72. The
cells include Parental U87MG cells (par), cells in which the gene expressing
neuropilin-1 was
knocked out using CRISPR/Cas9 (U87MG-ANrp1), and cells in which the gene
expressing
.. neuropilin-1 was knocked out and that were further infected with empty
lentiviruses or lentiviruses
directing expression of CD72 (U87MG-ANrp1+CD72) to which a V5 epitope tag was
fused in frame
upstream of the stop codon; Fig. 3B- shows pictograms of the cells to which
Sema3A-AP was bound
to. The cells were consequently washed and bound sema3A-AP was detected using
BICP/NBT; Fig.
3C ¨ presents line graphs showing the effect of increasing concentrations of
purified Sema3A-AP
that were bound for 30 minutes at room temperature to the three cell types.
Following binding, the
cells were washed and the amount of bound Sema3A-AP per microscopic field was
assessed using
an alkaline phosphatase colorimetric assay; Fig. 3D ¨Sema3A-AP (5 g/m1) was
bound to the three
9
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
cell types in the presence of increasing concentrations of sema4D. The amount
of bound sema3A-
AP/microscopic field was then determined and presented in the line graphs of
Fig. 3D;
Figs. 4A-C - Sema3A transduces signals using CD72. Fig. 4A ¨ shows pictograms
of
Western Blot analysis in which a primary B-lymphoblastoid cell line (BLCL) was
infected with
lentiviruses directing expression of CD72. The BLCL cells and the BLCL cells
expressing CD72
were probed with antibodies directed against neuropilin-1 (Nrpl) and CD72.
Parental BLCL cell do
not express either of these receptors. Fig. 4B and Fig. 4C - BLCL cells and
BLCL cells expressing
CD72 (BLCL+CD72) were stimulated with Sema3A. The phosphorylation state (p) of
STAT-4 (Fig.
4B) and P38 (Fig. 4C) were than determined. Shown in the figures are Western
blots probed with
antibodies directed against the total (t) proteins and against specific
phosphorylation (p) sites in Stat-
4 protein (Fig. 4B) and P38 (Fig. 4C). Also shown are bar graphs representing
quantification of the
Western blot results (i.e., the ratio between phosphorylated (p) and total (t)
STAT-4 and P-38);
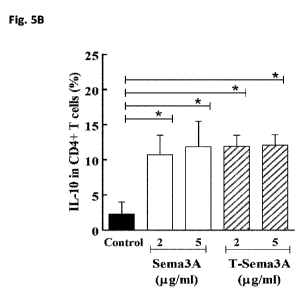
Fig. 5A-B -Modified Sema3A (T-Sema3A) transduces signals using the CD72
receptor but
is unable to induce endothelial cell contraction mediated by the neuropilin-1
receptor: Fig. 5A shows
pictograms of Human umbilical vein derived endothelial cells (HUVEC) that were
stimulated with
conditioned medium from control HEK293 cells (Control), or with conditioned
medium containing
similar concentrations of WT Sema3A or T-sema3A derived from HEK293 cells
expressing either
recombinant WT Sema3A or T-sema3A. Cells were photographed 30 minutes after
addition of the
conditioned media; Fig. 5B shows bar graphs of the percentage of T-cells
expressing IL-10 as
determined using FACS analysis. CD4+ T-cells were stimulated with the
indicated concentrations
of purified WT Sema3A or T-Sema3A. *=P<0.05;
Fig. 6 shows line graphs of glycolysis stress test of activated T-cells in the
presence or
absence of T-Sema3A. Purified CD4+ T cells were activated with anti-CD3 and
anti-CD28 for 24
hours at 37 C. The activated cells were treated with 5tig/m1 T-Sema3A or PBS
(as a control) and
incubated for 24 hours at 37 C. Cells were harvested and transferred to medium
without glucose
for 2 hours. Thereafter, Glucose, Oligomycin and 2-deoxy-glucose (2-DG), were
added to the
cells at the indicated time points. The real-time (in live) ECAR
(extracellular acidification rate)
measurements were performed using Agilent Seahorse XF Analyzers (Seahorse Reg-
Time Cell
Metabolic Analysis assay (Agilent)).
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
DETAILED DESCRIPTION OF THE INVENTION
The principles, uses, and implementations of the teachings herein may be
better understood
with reference to the accompanying description and figures. Upon perusal of
the description and
figures present herein, one skilled in the art will be able to implement the
teachings herein without
undue effort or experimentation. In the figures, same reference numerals refer
to same parts
throughout.
Definitions
To facilitate an understanding of the present invention, a number of terms and
phrases are
defined below. It is to be understood that these terms and phrases are for the
purpose of description
and not of limitation, such that the terminology or phraseology of the present
specification is to be
interpreted by the skilled artisan in light of the teachings and guidance
presented herein, in
combination with the knowledge of one of ordinary skill in the art.
As referred to herein, the terms "polynucleotide molecules",
"oligonucleotide",
,'polynucleotide", "nucleic acid" and "nucleotide" sequences may
interchangeably be used. The
terms are directed to polymers of deoxyribonucleotides (DNA), ribonucleotides
(RNA), and
modified forms thereof in the form of a separate fragment or as a component of
a larger construct,
linear or branched, single stranded (ss), double stranded (ds), triple
stranded (ts), or hybrids
thereof. The polynucleotides may be, for example, or polynucleotide sequences
of DNA or RNA.
The DNA or RNA molecules may be, for example, but are not limited to:
complementary DNA
(cDNA), genomic DNA, synthesized DNA, recombinant DNA, or a hybrid thereof or
an RNA
molecule such as, for example, mRNA. Accordingly, as used herein, the terms
"polynucleotide
molecules", "oligonucleotide", "polynucleotide", "nucleic acid" and
"nucleotide" sequences are
meant to refer to both DNA and RNA molecules. The terms further include
oligonucleotides
composed of naturally occurring bases, sugars, and covalent inter nucleoside
linkages, as well as
oligonucleotides having non-naturally occurring portions, which function
similarly to respective
naturally occurring portions. As used herein, nucleotides (A, G, C or T) and
nucleotide sequences
are marked in lowercase letters (a, g, c or t)
The terms "polypeptide," "peptide" and "protein" are used interchangeably
herein to refer
to a polymer of amino acid residues. The terms also apply to amino acid
polymers in which one
or more amino acid residue is an artificial chemical analogue of a
corresponding naturally
occurring amino acid, as well as to naturally occurring amino acid polymers.
In some
embodiments, one or more of amino acid residue in the polypeptide, can contain
modification,
11
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
such as but be not limited only to, glycosylation, phosphorylation or
disulfide bond shape. Also
provided are conservative amino acid variants of the peptides and protein
molecules disclosed
herein. Variants according to the invention also may be made that conserve the
overall molecular
structure of the encoded proteins or peptides. Given the properties of the
individual amino acids
comprising the disclosed protein products, some rational substitutions will be
recognized by the
skilled worker. Amino acid substitutions, i.e. "conservative substitutions,"
may be made, for
instance, on the basis of similarity in polarity, charge, solubility,
hydrophobicity, hydrophilicity,
and/or the amphipathic nature of the residues involved. As used herein, Amino
acids and peptide
sequences are marked using conventional Amino Acid nomenclature (single letter
or 3-letters
code). For example, amino acid "Serine" may be marked as "Ser" or "S" and
amino acid
"Cysteine" may be marked as "Cys" or "C".
As referred to herein, the term "complementarity" is directed to base pairing
between
strands of nucleic acids. As known in the art, each strand of a nucleic acid
may be complementary
to another strand in that the base pairs between the strands are non-
covalently connected via two
or three hydrogen bonds. Two nucleotides on opposite complementary nucleic
acid strands that
are connected by hydrogen bonds are called a base pair. According to the
Watson-Crick DNA base
pairing, adenine (A or a) forms a base pair with thymine (T or t) and guanine
(G or g) with cytosine
(C or c). In RNA, thymine is replaced by uracil (U or u). The degree of
complementarity between
two strands of nucleic acid may vary, according to the number (or percentage)
of nucleotides that
form base pairs between the strands. For example, "100% complementarity"
indicates that all the
nucleotides in each strand form base pairs with the complement strand. For
example, "95%
complementarity" indicates that 95% of the nucleotides in each strand from
base pair with the
complement strand. The term sufficient complementarity may include any
percentage of
complementarity from about 30% to about 100%.
The term "construct", as used herein refers to an artificially assembled or
isolated nucleic
acid molecule which may be comprises of one or more nucleic acid sequences,
wherein the nucleic
acid sequences may be coding sequences (that is, sequence which encodes for an
end product),
regulatory sequences, non-coding sequences, or any combination thereof. The
term construct
includes, for example, vectors, plasmids but should not be seen as being
limited thereto. The term
"regulatory sequence" in some embodiments, refers to DNA sequences, which are
necessary to
affect the expression of coding sequences to which they are operably linked
(connected/ligated).
The nature of the regulatory sequences differs depending on the host cells.
For example, in
12
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
prokaryotes, regulatory/control sequences may include promoter, ribosomal
binding site, and/or
terminators. For example, in eukaryotes regulatory/control sequences may
include promoters (for
example, constitutive of inducible), terminators enhancers, transactivators
and/or transcription
factors. A regulatory sequence which is "operably linked" to a coding sequence
is ligated in such
.. a way that expression of the coding sequence is achieved under suitable
conditions. In some
embodiments, a "Construct" or a "DNA construct" refer to an artificially
assembled or isolated
nucleic acid molecule which comprises a coding region of interest and
optionally additional
regulatory or non-coding sequences.
As used herein, the term "vector" refers to any recombinant polynucleotide
construct (such
as a DNA construct) that may be used for the purpose of transformation, i.e.
the introduction of
heterologous DNA into a host cell. One exemplary type of vector is a "plasmid"
which refers to a
circular double stranded DNA loop into which additional DNA segments can be
ligated. Another
exemplary type of vector is a viral vector, wherein additional DNA segments
can be ligated into
the viral genome. Certain vectors are capable of autonomous replication in a
host cell into which
they are introduced. The term "Expression vector" refers to vectors that have
the ability to
incorporate and express heterologous nucleic acid fragments (such as DNA) in a
foreign cell. In
other words, an expression vector comprises nucleic acid sequences/fragments
(such as DNA,
mRNA), capable of being transcribed or expressed in a target cell. Many viral,
prokaryotic and
eukaryotic expression vectors are known and/or commercially available.
Selection of appropriate
.. expression vectors is within the knowledge of those having skill in the
art. The expression vectors
can include one or more regulatory sequences.
As used herein, a "primer" defines an oligonucleotide which is capable of
annealing to
(hybridizing with) a target nucleotide sequence, thereby creating a double
stranded region which
can serve as an initiation point for DNA synthesis under suitable conditions.
As used herein, the term "transformation" refers to the introduction of
foreign DNA into
cells. The terms "transformants" or "transformed cells" include the primary
transformed cell and
cultures derived from that cell regardless to the number of transfers. All
progeny may not be
precisely identical in DNA content, due to deliberate or inadvertent
mutations. Mutant progeny
that have the same functionality as screened for in the originally transformed
cell are included in
.. the definition of transformants.
As used herein, the terms "introducing" and "transfection" may interchangeably
be used and
refer to the transfer of molecules, such as, for example, nucleic acids,
polynucleotide molecules,
13
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
vectors, and the like into a target cell(s), and more specifically into the
interior of a membrane-
enclosed space of a target cell(s). The molecules can be "introduced" into the
target cell(s) by any
means known to those of skill in the art, for example as taught by Sambrook et
al. Molecular
Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, New York
(2001), the
contents of which are incorporated by reference herein. Means of "introducing"
molecules into a
cell include, for example, but are not limited to: heat shock, calcium
phosphate transfection, PEI
transfection, electroporation, lipofection, transfection reagent(s), viral-
mediated transfer,
injection, and the like, or combinations thereof. The transfection of the cell
may be performed on
any type of cell, of any origin, such as, for example, human cells, animal
cells, plant cells, and the
like. The cells may be isolated cells, tissue cultured cells, cell lines,
cells present within an
organism body, and the like.
The terms "upstream" and "downstream", as used herein refers to a relative
position in a
nucleotide sequence, such as, for example, a DNA sequence or an RNA sequence.
As well known,
a nucleotide sequence has a 5' end and a 3' end, so called for the carbons on
the sugar (deoxyribose
or ribose) ring of the nucleotide backbone. Hence, relative to the position on
the nucleotide
sequence, the term downstream relates to the region towards the 3' end of the
sequence. The term
upstream relates to the region towards the 5' end of the strand.
As used herein, the term "treating" includes, but is not limited to one or
more of the following:
abrogating, ameliorating, inhibiting, attenuating, blocking, suppressing,
reducing, delaying, halting,
alleviating or preventing symptoms associated with a condition. Each
possibility represents a
separate embodiment of the present invention. In some embodiments, the
condition is an immune
related condition. In some exemplary embodiments, the condition may be
selected from, Asthma,
Lupus, inflammatory bowel diseases, and the like.
The terms "Semaphorin 3A", "sema3A", "Sema3A" and "Sema 3A" may
interchangeably be
used. Further, it is to be understood that Semaphorin 3A is interchangeable
with any alternative
name or synonym of this protein known in the art. Typical Semaphorin 3A
synonyms include, but
are not limited to, collapsin 1, semaphorin III and Sema3A. The terms refer to
a protein or
polypeptide, primarily to a human protein. The terms further refer to a
nucleic acid encoding for
the corresponding polypeptide. The amino acid sequences and encoding
nucleotide sequences of
wild-type Semaphorin 3A are well known in the art. Nucleic acid sequences can
be retrieved in
public databases like NCBI. In some embodiments, the Homo sapiens Wild type
Sema3A
accession number gil 100913215IrefiNM_006080.21 corresponds to SEQ ID NO: 1.
14
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
The term "wild type Sema3A", "WT Sema3A", "naturally occurring Sema3A" and "un-
modified Sema3A" may interchangeably be used. The terms refer to the naturally
occurring form
of Sema3A (i.e., an endogenous, non-mutated Sema3A or full-length Sema3A). In
some
embodiments, the WT-Sema3A is from a mammalian origin. In some embodiments,
the WT-
Sema3A is of human origin. In some embodiments, the WT-Sema3A of human origin
has an amino
acid sequence as denoted by SEQ ID NO: 1. In some embodiments, WT-Semaphorin
3A as used
herein is a human Semaphorin 3A having an amino-acid sequence as set forth in
SEQ ID NO: 1.
The polynucleotide sequence as set forth in SEQ ID NO: 2 corresponds to the
cDNA encoding
human WT Semaphorin 3A as set forth in SEQ ID NO: 1.
As used herein the terms "modified Sema3A", "mutated Sema3A", "non-naturally
occurring
Sema3A", "short-Sema3A" and "T-Sema3A" may interchangeably be used. The terms
relate to a
mutated/modified form of the corresponding wild-type (WT) or natural form of
the Sema3A. In
some embodiments, the Sema3A is of human origin. In some embodiments, the
Sema3A is of
mammalian origin. In some embodiments, the modified Sema3A differs from the
corresponding
wild type Semaphorin 3A by at least one mutation selected from amino acid
substitution(s), and/or
deletions(s). In particular, the mutated form of the human Semaphorin 3A
includes a replacement
of the Serine (S) by a Cysteine (C) amino acid at the position that by
comparison of homology
corresponds to position 257 of the wild type Semaphorin 3A as shown in SEQ ID
NO: 1, as well
as a C-terminal truncation/deletion of a stretch of at least 50 amino acids,
at least 100 amino acids,
at least 150 amino acids, at least 200 amino acids, at least 250 amino acids,
or at least 254
consecutive amino acids of the WT Semaphorin 3A. Accordingly, in some
embodiments, the
modified human Sema3A includes an amino acid sequence as denoted by SEQ ID NO.
3. In some
embodiments, a modified Sema3A of an origin other than human may include a
corresponding point
mutation and/or deletion in the respective WT-Sema3A, which are equivalent or
homologous to the
mutations introduced in the human WT Sema3A.
According to some embodiments, Semaphorin 3A is an isolated Semaphorin 3A. In
some
embodiments, T-sema3A is an isolated T-sema3A. According to some embodiments,
WT-Sema3A
and/or the modified Sema3A is a recombinant protein, polypeptide or peptide.
As used herein, the
term "isolated" means either: 1) separated from at least some of the
components with which it is
usually associated in nature with respect of the Wild-Type Sema3A; 2) prepared
or purified by a
process that involves the hand of man (with respect to WT or modified Sema3A);
3) not occurring
in nature (with respect of the modified Sema3A).
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
In some embodiments, there is further provided a nucleic acid molecule
encoding a
polypeptide comprising an amino acid sequence of a modified Sema3A, wherein
the Serine
corresponding to position 257 of the wild type Semaphorin 3A (SEQ ID NO: 1) is
replaced by
Cysteine, and further includes a C-terminal truncation of 254 amino acids of
the wild type
Semaphorin 3A (SEQ ID NO: 1). In some embodiments, there is further provided a
nucleic acid
molecule having a nucleotide sequence as denoted by SEQ ID NO: 4, encoding a
polypeptide
having an amino acid sequence of the modified Sema3A (having an amino acid
sequence as
denoted by SEQ ID NO: 3).
In some embodiments, the nucleic acid molecule encoding for the modified
Sema3A
disclosed herein is preferably at least 50% homologous/identical to the
nucleic acid sequence as
shown in SEQ ID NO: 2. It is understood that such nucleic acid sequences can
also include
orthologous/homologous/identical (and thus related) sequences. More
preferably, the nucleic acid
sequence encoding the provided modified Sema3A is at least 52%, 53%, 55%, 60%,
65%, 70%,
75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
homologous/identical
to the nucleic acid sequence as shown in SEQ ID NO: 2, wherein the higher
values of sequence
identity are preferred.
According to some embodiments, the modified Sema3A may further include a
protein tag.
As used herein, the term "protein tag" refers to a peptide sequence bound to
the N-terminus or C-
terminus of the protein. According to some embodiments, the protein tag may
comprise a
glycoprotein. According to some embodiments, the protein tag may be used for
separation,
purification and/or identification/tracking of the tagged protein. Non-
limiting examples of protein
tags include: Myc-Tag, Human influenza hemagglutinin (HA), Flag-Tag, His-Tag,
Glutathione-S-
Transferase (GST) and a combination thereof. Each possibility represents a
separate embodiment of
the present invention. In some exemplary embodiments, the tag includes a
stretch of 6-8 Histidine
residues ("His-tag"). In some embodiments, the tag may be a 8X-His tag (SEQ ID
NO: 7), located
at the C-terminal end of the modified Sema3A. In some exemplary embodiments, a
modified
Sema3A with a C-terminal His-tag has an amino acid sequence as denoted by SEQ
ID NO: 5. In
some exemplary embodiments, the nucleic acid molecule encoding the modified
Seam 3A with a C-
terminal His -tag has a nucleotide sequence as denoted by SEQ ID NO: 6.). In
some embodiments,
there is provided a nucleic acid molecule having a nucleotide sequence as
denoted by SEQ ID NO:
6, encoding a polypeptide having an amino acid sequence of the modified His-
Tagged Sema3A
(having an amino acid sequence as denoted by SEQ ID NO: 5).
16
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
According to some embodiments, the T-Sema3A may include a protein tag upon
production,
which may be consequently cleaved and/or removed from T-Sema3A prior to
incorporation into a
composition or prior to being introduced to cells/ administered. Cleavage
and/or removal of a tag
may be performed by any method known in the art, such as, but not limited to,
enzymatic and/or
chemical cleaving.
Reference is now made to Figs. 1A-C, which presents amino acid and/or
nucleotide
sequences of human WT-Sema3A and modified Sema3A, while highlighting the
modifications/differences between the sequences of the WT and modified forms.
Fig. 1A presents
the 771 amino acid sequence of the human WT-Sema3A (SEQ ID NO: 1), with the C-
terminal
region (amino acids 517-771, that includes, inter alia, Nrpl binding domain
and dimerization
domain and which is deleted from the corresponding T-Sema3A) marked (gray
background). Fig.
1B presents the amino acid sequence of the modified Sema3A, which includes a
5257C
substitution (marked by Capital C), and a C-terminal truncation at amino acid
R516. The modified
Sema3A sequence presented in Fig. 1B further includes an in-frame 8X-His tag
(HHHHHHHH
(SEQ ID NO: 7 (marked)) at the C-terminal end of the modified protein. The
amino acid sequence
presented in Fig. 1B corresponds to SEQ ID NO: 5. Fig. 1C presents the nucleic
acid sequence
(cDNA) encoding for the modified tagged Sema3A (SEQ ID NO: 5). The cDNA
sequences
presented in Fig. 1C corresponds to SEQ ID NO: 6, and includes a codon
modification (bases 769-
771), whereby the codon encoding for a Serine residue (in the WT Sema3A
protein, SEQ ID NO:
2), was changed to a TGT codon encoding cysteine at bases 769-771. In
addition, a cDNA
sequence encoding 8 histidine residues (caccatcaccatcaccatcaccatcaccat (SEQ ID
NO: 8),
highlighted) was fused in frame at nucleotide 1548, followed by a stop codon
(tga).
According to some embodiments, the modified Sema-3A as disclosed herein may be
produced by recombinant or chemical synthetic methods. According to some
embodiments, T-
Sema3A as disclosed herein may be produced by recombinant methods from
genetically-modified
host cells. Any host cell known in the art for the production of recombinant
proteins may be used
for the present invention. According to some embodiments, the host cell is a
prokaryotic cell.
Representative, non-limiting examples of appropriate prokaryotic hosts include
bacterial cells,
such as cells of Escherichia coli and Bacillus subtilis. According to other
embodiments, the host
cell is a eukaryotic cell. According to some exemplary embodiments, the host
cell is a fungal cell,
such as yeast.
17
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
According to some exemplary embodiments, a coding region of interest is a
coding region
encoding WT-Semaphorin 3A. According to some exemplary embodiments, a coding
region of
interest is a coding region encoding modified Sema3A. According to some
exemplary
embodiments, a coding region of interest is a coding region encoding for human
modified Sema3A
as set forth in SEQ ID NOs: 4 or 6.
In some embodiments, the modified Sema3A may be synthesized by expressing a
polynucleotide molecule encoding the modified Sema3A in a host cell, for
example, a
microorganism cell transformed with the nucleic acid molecule.
In some embodiments, DNA sequences encoding wild type polypeptides, such as
Wild-
type Semaphorin 3A, may be isolated from any cell producing them, using
various methods well
known in the art. For example, a DNA encoding the wild-type polypeptide may be
amplified from
genomic DNA by polymerase chain reaction (PCR) using specific primers,
constructed on the
basis of the nucleotide sequence of the known wild type sequence. The genomic
DNA may be
extracted from the cell prior to the amplification using various methods known
in the art.
According to some embodiments, the polynucleotide encoding the T-Sema
polypeptide
may be cloned into any vector known in the art.
According to some embodiments, upon isolation and/or cloning of the
polynucleotide
encoding the wild type polypeptide, desired mutation(s) may be introduced by
modification at one
or more base pairs, using methods known in the art, such as for example, site-
specific mutagenesis,
cassette mutagenesis, recursive ensemble mutagenesis and gene site saturation
mutagenesis.
Methods are also well known for introducing multiple mutations into a
polynucleotide. For
example, introduction of two and/or three mutations can be performed using
commercially
available kits, such as the QuickChange site-directed mutagenesis kit
(Stratagene). In some
embodiments, as exemplified herein, point mutation is introduced into the
sequence encoding for
the WT-Semaphorin 3A (represented by SEQ ID NO: 2), whereby nucleotide c -at
position 770
(of SEQ ID NO: 2) is replaced/changed to nucleotide g. Such a point mutation
results in codon
modification (from tct (in the WT) to tgt (in the modified Sema3A) that will
translate to a Serine
(S or Ser) to Cysteine (C or Cys) amino acid substitution in the peptide
expressed therefrom. In
addition, the modified Sema3A coding sequence ends at nucleotide 1548 (g) of
the corresponding
WT-Sema3A (SEQ ID NO: 2). In some embodiments, a stop codon (any Stop codon
known in
the art, such as, tga, may be placed immediately after (downstream) nucleotide
1548. In some
embodiments, a tag may be placed after nucleotide 1548. In some embodiments, a
nucleotide
18
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
sequence encoding for a tag may be placed after nucleotide 1548. In some
embodiments, the
nucleotide sequence encoding tag may be a His-tag, Myc- tag, FLAG-tag, and the
like. In some
embodiments, a Stop codon may be placed after the tag-encoding sequence. In
some exemplary
embodiments, a nucleotide sequence encoding for modified Sema3A, having a stop
codon after
nucleotide 1548 is represented by SEQ ID NO: 4. For example, a nucleotide
sequence encoding
for modified Sema3A, having a nucleotide encoding tag (His-tag in this
example) followed by a
stop codon is represented by SEQ ID NO: 6.
According to some embodiments, an alternative method to producing a
polynucleotide with
a desired sequence is the use of a synthetic gene. A polynucleotide encoding a
desired polypeptide
may be prepared synthetically, for example using the phosphoroamidite.
According to some embodiments, the polynucleotide thus produced may then be
subjected
to further manipulations, including one or more of purification, annealing,
ligation, amplification,
digestion by restriction endonucleases and cloning into appropriate vectors.
The polynucleotide
may be ligated either initially into a cloning vector, or directly into an
expression vector that is
appropriate for its expression in a particular host cell type.
In some embodiments, in case of a fusion protein, or a protein fused with a
protein tag,
different polynucleotides may be ligated to form one polynucleotide. In some
embodiments, the
polynucleotide encoding the WT or modified Sema3A polypeptide, may be
incorporated into a
wide variety of expression vectors, which may be transformed into in a wide
variety of host cells.
According to some embodiments, introduction of a polynucleotide into the host
cell can be
effected by well-known methods, such as chemical transformation (e.g. calcium
chloride
treatment), electroporation, conjugation, transduction, calcium phosphate
transfection, DEAE-
dextran mediated transfection, transvection, microinjection, cationic lipid-
mediated transfection,
scrape loading, ballistic introduction and infection. Representative, non-
limiting examples of
appropriate hosts include bacterial cells, such as cells of E. coli and
Bacillus subtilis.
In some embodiments, the polypeptides may be expressed in any vector suitable
for
expression. The appropriate vector is determined according to the selected
host cell. Vectors for
expressing proteins in E. coli, for example, include, but are not limited to,
pET, pK233, pT7 and
lambda pSKF. Other expression vector systems are based on betagalactosidase
(pEX); maltose
binding protein (pMAL); and glutathione S-transferase (pGST).
19
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
According to some embodiments, as detailed above, the polypeptides may be
designed to
include a protein tag, for example, a His-Tag (6-8 consecutive histidine
residues), which can be
isolated and purified by conventional methods.
According to some embodiments, selection of a host cell transformed with the
desired
vector may be accomplished using standard selection protocols involving growth
in a selection
medium which is toxic to non-transformed cells. For example, in the case of E.
coli, it may be
grown in a medium containing an antibiotic selection agent; cells transformed
with the expression
vector which further provides an antibiotic resistance gene, will grow in the
selection medium. In
some embodiments, upon transformation of a suitable host cell, and propagation
under conditions
appropriate for protein expression, the polypeptide may be identified in cell
extracts of the
transformed cells. Transformed hosts expressing the polypeptide may be
identified by analyzing
the proteins expressed by the host, for example, using SDS-PAGE and comparing
the gel to an
SDS-PAGE gel obtained from the host which was transformed with the same vector
but not
containing a nucleic acid sequence encoding the desired polypeptide.
According to some embodiments, the desired polypeptides which have been
identified in
cell extracts may be isolated and purified by conventional methods, including
ammonium sulfate
or ethanol precipitation, acid extraction, salt fractionation, ion exchange
chromatography,
hydrophobic interaction chromatography, gel permeation chromatography,
affinity
chromatography, and combinations thereof. The polypeptides of the invention
may be produced
as fusion proteins, attached to an affinity purification protein tag, such as
a His-tag, in order to
facilitate their rapid purification.
According to some embodiments, the isolated polypeptide may be analyzed for
its various
properties, for example, specific activity, using methods known in the art. In
a non-limiting
example, isolated modified Semaphorin 3A may be analyzed for its ability to
bind CD72, lack of
binding to Neuropilin 1 receptor, lack of ability to mediate of cell
contraction, activation of CD72
signaling (as determined, for example, by increasing phosphorylation of
regulatory molecules,
such as, STAT-4), inducing/affecting/increasing IL-10 secretion in immune
cells (for example, T-
cells and/or B-cells), affecting aerobic glycolysis in immune cells (such as,
activated T-cells and/or
B-cells), and the like, or any combination thereof.
According to some embodiments, a modified Sema3A according to the present
invention
may also be produced by synthetic means using well known techniques, such as
solid phase
synthesis. Synthetic polypeptides may be produced using commercially available
laboratory
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
peptide design and synthesis kits. In addition, a number of available FMOC
peptide synthesis
systems are available. Assembly of a polypeptide or fragment can be carried
out on a solid support
using for example, an Applied Biosystems, Inc. Model 431A automated peptide
synthesizer. The
polypeptides may be made by either direct synthesis or by synthesis of a
series of fragments that
can be coupled using other known techniques.
According to some embodiments, there is provided a process for the production
of a
modified Sema3A polypeptide the process includes culturing/raising a suitable
host cells under
conditions allowing the expression of the modified Sema3A polypeptide and
optionally
recovering/isolating the produced polypeptide from the cell culture.
According to some embodiments, there is provided a nucleic acid encoding for
the
modified Sema3A polypeptide. In some embodiments, there is provide a DNA
construct/vector
(such as, an expression vector) harboring or comprising a nucleic acid
encoding for the modified
Sema3A polypeptide (optionally in addition to one or more regulatory
sequences, non-coding
sequences, and the like).
In some embodiments, various suitable vectors are known to those skilled in
art, and the
choice of which depends on the function desired. Such vectors include, for
example, plasmids,
cosmids, viruses, bacteriophages and other vectors. In some embodiments, the
polynucleotides
and/or vectors harboring the same can be reconstituted into vehicles, such as,
for example,
liposomes for delivery to target cells. Any cloning vector and/or expression
vector known in the
art may be used, depending on the purpose, the host cell, and the like. Such
vectors may be used
for in-vitro and/or in-vivo introduction/expression.
According to some embodiments, the encoding nucleic acid molecules and/or the
vectors
disclosed herein may be designed for direct introduction or for introduction
via carrier, such as,
liposomes, viral vectors (adenoviral, retroviral) into target cells.
According to some embodiments, there is provided a host cell harboring or
expressing the
modified Sema3A. In some embodiments, the host cell may be
transformed/transfected with the
vector of the present invention or with the nucleic acid encoding for the
modified Sema3A. In
some embodiments, there is provided a host cell harboring or comprising the
nucleic acid molecule
of the invention. In some embodiments, the presence of at least one vector or
at least one nucleic
acid molecule in the host may mediate the expression of the modified Sema3A in
the cell. In some
embodiments, the nucleic acid molecule or vector comprising the same, may
either integrate into
the genome of the host cell, or it may be maintained extrachromosomally. In
some embodiments,
21
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
the host cell may be any prokaryotic or eukaryotic cell. In some embodiments,
the host cell is a
mammalian cell.
According to some embodiments the nucleic acid molecules can be used alone or
as part
of a vector to express the modified Sema3A polypeptide of the invention in
cells, for purification
and/or for therapy.
In some embodiments, the nucleic acid molecules (or vectors harboring the
same) and/or
the modified Sema3A polypeptide, can be used as a medicament (as is, or in the
form of a
composition, such as a pharmaceutical composition), for treating various
conditions, in particular,
immune related conditions.
According to some embodiments, there is provided a composition (also referred
to herein
as pharmaceutical composition) which includes the modified Sema3A polypeptide,
the nucleic
acid encoding therefor, or vectors harboring the nucleic acids. Each
possibility is a separate
embodiment. In some embodiments, the composition may include one or more
suitable excipients,
according to the purpose, type and/or use of the composition. In some
embodiments, excipient is
a pharmaceutical excipient which may include or a pharmaceutical carrier,
vehicle, buffer and/or
diluent.
In some embodiments, the composition disclosed herein may be used as a
medicament for
treating various immune related conditions.
Thus, according to some embodiments, the modified-sema3A (polypeptide or
nucleic acid
encoding the same) can be used for the successful treatment of various immune-
mediated
conditions, such as, auto-immune diseases, allergic conditions, conditions
related to over
activation of the immune system, inflammatory diseases, and the like.
In some embodiments, auto-immune diseases may include such conditions as, but
not
limited to: Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis,
inflammatory bowel
disease (IBD), Uveitis, Psoriasis and the like.
In some embodiments, allergic conditions may include such conditions as, but
not limited
to: bronchial asthma, allergic conjunctivitis, allergic rhinitis and atopic
dermatitis.
In some embodiments, conditions related to over activation of the immune
system may
include such conditions as, but not limited to: sepsis, cytokine storm-due to
infectious diseases and
/or inducement by CAR-T, graft-versus host disease (GVHD), and the like.
22
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
In some embodiments, inflammatory diseases may include such diseases as, but
not limited
to: Chronic Obstructive Pulmonary Disease (COPD), Familial Mediterranean fever
(FMF), and
the like.
According to some embodiments, any suitable route of administration to a
subject may be
used for the nucleic acid, polypeptide or the composition of the present
invention, including but
not limited to, local and systemic routes. Exemplary suitable routes of
administration include, but
are not limited to: orally, intra-nasally, parenterally, intravenously,
topically, enema or by
inhalation. According to another embodiment, systemic administration of the
composition is via
an injection. For administration via injection, the composition may be
formulated in an aqueous
solution, for example in a physiologically compatible buffer including, but
not limited, to Hank's
solution, Ringer's solution, or physiological salt buffer. Formulations for
injection may be
presented in unit dosage forms, for example, in ampoules, or in multi-dose
containers with,
optionally, an added preservative.
According to another embodiment, administration systemically is through a
parenteral
route. According to some embodiments, parenteral administration is
administration intravenously,
intra-arterially, intramuscularly, intraperitoneally, intradermally,
intravitreally, or subcutaneously.
Each of the abovementioned administration routes represents a separate
embodiment of the present
invention. According to another embodiment, parenteral administration is
performed by bolus
injection. According to another embodiment, parenteral administration is
performed by continuous
infusion. According to some embodiments, preparations of the composition of
the invention for
parenteral administration include sterile aqueous or non-aqueous solutions,
suspensions, or
emulsions, each representing a separate embodiment of the present invention.
Non-limiting
examples of non-aqueous solvents or vehicles are propylene glycol,
polyethylene glycol, vegetable
oils such as olive oil and corn oil, gelatin, and injectable organic esters
such as ethyl oleate.
According to another embodiment, parenteral administration is transmucosal
administration. According to another embodiment, transmucosal administration
is transnasal
administration. For transmucosal administration, penetrants appropriate to the
barrier to be
permeated are used in the formulation. Such penetrants are generally known in
the art. The
preferred mode of administration will depend upon the particular indication
being treated and will
be apparent to one of skill in the art.
Aqueous injection suspensions may contain substances that increase the
viscosity of the
suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran.
Optionally, the
23
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
suspension may also contain suitable stabilizers or agents that increase the
solubility of the active
ingredients, to allow for the preparation of highly concentrated solutions.
According to another embodiment, compositions formulated for injection may be
in the
form of solutions, suspensions, dispersions or emulsions in oily or aqueous
vehicles, and may
contain formulatory agents such as suspending, stabilizing, and/or dispersing
agents. Non-limiting
examples of suitable lipophilic solvents or vehicles include fatty oils such
as sesame oil, or
synthetic fatty acid esters such as ethyl oleate or triglycerides.
According to another embodiment, the composition is administered
intravenously, and is
thus formulated in a form suitable for intravenous administration. According
to another
embodiment, the composition is administered intra-arterially, and is thus
formulated in a form
suitable for intra-arterial administration. According to another embodiment,
the composition is
administered intramuscularly, and is thus formulated in a form suitable for
intramuscular
administration.
According to another embodiment, administration systemically is through an
enteral route.
According to another embodiment, administration through an enteral route is
buccal
administration. According to another embodiment, administration through an
enteral route is oral
administration. According to some embodiments, the composition is formulated
for oral
administration.
According to some embodiments, oral administration is in the form of hard or
soft gelatin
capsules, pills, capsules, tablets, including coated tablets, dragees,
elixirs, suspensions, liquids,
gels, slurries, syrups or inhalations and controlled release forms thereof.
According to some embodiments, suitable carriers for oral administration are
well known
in the art. Compositions for oral use can be made using a solid excipient,
optionally grinding the
resulting mixture, and processing the mixture of granules, after adding
suitable auxiliaries as
desired, to obtain tablets or dragee cores. Non-limiting examples of suitable
excipients include
fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol,
cellulose preparations such
as, maize starch, wheat starch, rice starch, potato starch, gelatin, gum
tragacanth, methyl cellulose,
hydroxypropylmethyl-cellulose, and sodium carbomethylcellulose, and/or
physiologically
acceptable polymers such as polyvinylpyrrolidone (PVP).
In some embodiments, if desired, disintegrating agents, such as cross-linked
polyvinyl
pyrrolidone, agar, or alginic acid or a salt thereof, such as sodium alginate,
may be added. Capsules
24
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
and cartridges of, for example, gelatin, for use in a dispenser may be
formulated containing a
powder mix of the composition of the invention and a suitable powder base,
such as lactose or
starch.
According to some embodiments, solid dosage forms for oral administration
include
capsules, tablets, pill, powders, and granules. In such solid dosage forms,
the composition of the
invention is admixed with at least one inert pharmaceutically acceptable
carrier such as sucrose,
lactose, or starch. Such dosage forms can also comprise, as it normal
practice, additional
substances other than inert diluents, e.g., lubricating, agents such as
magnesium stearate. In the
case of capsules, tablets and pills, the dosage forms may also comprise
buffering, agents. Tablets
and pills can additionally be prepared with enteric coatings.
In some embodiments, liquid dosage forms for oral administration may further
contain
adjuvants, such as wetting agents, emulsifying and suspending agents, and
sweetening, flavoring
and perfuming agents. According to some embodiments, enteral coating of the
composition is
further used for oral or buccal administration. The term "enteral coating", as
used herein, refers to
a coating which controls the location of composition absorption within the
digestive system. Non-
limiting examples for materials used for enteral coating are fatty acids,
waxes, plant fibers or
plastics.
According to some embodiments, administering is administering topically.
According to
some embodiments, the composition is formulated for topical administration.
The term "topical
administration", as used herein, refers to administration to body surfaces.
Non-limiting examples
of formulations for topical use include cream, ointment, lotion, gel, foam,
suspension, aqueous or
cosolvent solutions, salve and sprayable liquid form. Other suitable topical
product forms for the
compositions of the present invention include, for example, emulsion, mousse,
lotion, solution and
serum.
According to some embodiments, the administration may include any suitable
administration regime, depending, inter alia, on the medical condition,
patient characteristics,
administration route, and the like. In some embodiments, administration may
include
administration twice daily, every day, every other day, every third day, every
fourth day, every
fifth day, once a week, once every second week, once every third week, once
every month, and
the like.
According to some embodiments, the T-Sema3A polypeptide, the nucleic acid
encoding
the same, and/or the composition comprising the polypeptide or the nucleic
acid molecules, when
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
used for used for treating an immune-related may be used in combination with
other therapeutic
agents. The
components of such combinations may be administered sequentially or
simultaneously/concomitantly in separate or combined pharmaceutical
formulations by any
suitable administration route.
According to some embodiments, there is provided a method of treating an
immune related
condition, the method includes administration to a subject in need thereof a
therapeutically
effective amount of modified Sema3A. In some embodiments, the modified Sema3A
may be
administered as a polypeptide as is, or in a suitable pharmaceutical
composition. In some
embodiments, the modified Sema3A may be administered as a polynucleotide
encoding for the
polypeptide as is, or in a suitable pharmaceutical composition.
According to some embodiments, a therapeutically effective amount refers to an
amount
sufficient to ameliorate and/or prevent at least one of the symptoms
associated with an immune-
related disorder.
According to some exemplary embodiments, there is provided a method for
treating
Asthma, the method comprising administering to a subject in need thereof a
pharmaceutical
composition comprising a therapeutically effective amount of modified Sema3A.
According to some exemplary embodiments, there is provided a method for
treating
Inflammatory bowel disease, the method comprising administering to a subject
in need thereof a
pharmaceutical composition comprising a therapeutically effective amount of
modified Sema3A.
According to some exemplary embodiments, there is provided a method for
treating Lupus,
the method comprising administering to a subject in need thereof a
pharmaceutical composition
comprising a therapeutically effective amount of modified Sema3A.
According to some embodiments, there are provided kits comprising the modified
Sema3A
peptide and/or the nucleic acid molecule encoding the same and/or the
composition as disclosed
herein. Such a kit can be used, for example, in the treatment of various
immune-related conditions,
such as, for example, Asthma, Lupus, and IBD.
In the description and claims of the application, the words "include" and
"have", and forms
thereof, are not limited to members in a list with which the words may be
associated. As used
herein, the term comprising includes the term consisting of.
As used herein, the term "about" may be used to specify a value of a quantity
or parameter
(e.g. the length of an element) to within a continuous range of values in the
neighborhood of (and
26
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
including) a given (stated) value. According to some embodiments, "about" may
specify the value
of a parameter to be between 80 % and 120 % of the given value. According to
some embodiments,
"about" may specify the value of a parameter to be between 90 % and 110 % of
the given value.
According to some embodiments, "about" may specify the value of a parameter to
be between 95
% and 105 % of the given value.
As used herein, according to some embodiments, the terms "substantially" and
"about"
may be interchangeable.
While a number of exemplary aspects and embodiments have been discussed above,
those
of skill in the art will recognize certain modifications, permutations,
additions and sub-
combinations thereof. It is therefore intended that the following appended
claims and claims
hereafter introduced be interpreted to include all such modifications,
permutations, additions and
sub-combinations as are within their true spirit and scope.
The following examples are presented in order to more fully illustrate some
embodiments
of the invention. They should, in no way be construed, however, as limiting
the broad scope of the
invention. One skilled in the art can readily devise many variations and
modifications of the
principles disclosed herein without departing from the scope of the invention.
EXAMPLES
Example 1: Construction of a modified Sema3A protein
A modified (truncated and mutated) human Sema3A, which retains the signal
sequence
and the Sema-domain of the WT protein was created. The modified Sema3A (T-
Sema3A) was
derived from wild type human Semaphorin 3A, using standard genetic engineering
techniques.
The Sema3A includes a stretch of amino acids 1-516 (compared to the WT
Sema3A)) with one
point mutation in amino acid 257 (S257C). To this aim, the corresponding
region of the Sema3A
gene was amplified by PCR using 3 sets of primers (detailed below). The PCR
reaction was used
to introduce a point mutation at base 770 (from c to g), to result in
consequent substitution of
amino acid 257 by replacing Serine (in the WT sequence) to Cysteine in the
modified Sema3A),
in order to allow s-s bonds and the formation of a dimer in the truncated,
modified molecule.
Additionally, a C-terminal truncation of the sequence was included at
nucleotide 1548, to from a
truncated modified Sema3A. In some instances, at the 3' end of the molecule, a
nucleotide
sequence that is translated to a stretch of 8 Histidine amino acids in
included in-frame. The His-
27
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
tag is followed by a stop codon, thus resulting in the generation of a cDNA
encoding the modified
Sema3A. The amino acid sequence of such His tagged modified Sema3A is shown in
Fig. 1B and
is represented by SEQ ID NO: 5. The nucleic acid sequence encoding for such
modified Sema3A
is shown in Fig. 1C and is represented by SEQ ID NO: 6. In other instances, if
a tag sequence is
not introduced, an appropriate stop codon is inserted. The amino acid sequence
of such modified
Sema3A is represented by SEQ ID NO: 3. The nucleic acid sequence encoding for
such modified
Sema3A is represented by SEQ ID NO: 4. The amplification products were then
assembled and
ligated into the NSPI-CMV-MCS-myc-His lentiviral expression vector (Shown in
Fig. 2), by
recombination. This procedure was preformed using NEBuilder HiFi DNA Assembly
Master Mix,
according to the instructions of the manufacturer (New England Biolabs).
Upon sub-cloning of the modified Sema3A into the NSPI lentiviral expression
vector, it
was used to infect HEK293 cells (as detailed below). T-sema3A was then
purified from the
conditioned medium using nickel affinity chromatography.
Primers used in the PCR reaction for the formation of the modified Sema3A,
using WT-
Sema3A as a template:
shs3A 5' taagcttggtaccgagctcgatgggctggttaactaggattg (SEQ ID NO: 9)
shs3a s257c 5' caatagatggagaacactgtggaaaagctactcacgctag (SEQ ID NO:
10)
shs3a s257c 3' ctagcgtgagtagcttttccacagtgttctccatctattg (SEQ ID NO:
11)
shs3a 8hi5+stop 3' tcaatggtgatggtgatggtgatggtgccggtgtaaagggagctggg (SEQ ID
NO: 12)
shs3A 3' caccacactggactagtgtcaatggtgatggtgatggt (SEQ ID NO: 13)
Example 2 ¨ Expression and purification of Modified Sema3A polypeptide
The cDNA encoding the modified-sema3A was subcloned into the NSPI lentiviral
expression vector, as detailed above. Lentiviruses directing expression of the
T-sema3A were
generated in HEK293-T cells as previously described (Varshaysky, A., et.
al.,(2008) Cancer Res.
68, 6922-6931) and used to infect HEK293 cells. Serum free conditioned medium
was collected
48 hours after infection and purified on a Nickel-agarose column as per the
instructions of the
vendor ("Ni-NTA-QUIAGEN").
The transfected HEK293 cells were grown to 70% confluence and incubated for
48h in
serum free medium. Conditioned medium was collected and then loaded on 1.5cm
diameter
column containing 2m1 Ni-NTA agarose at 4 C (QIAGEN). The beads were washed
twice with
28
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
10m1 wash buffer (50mM phosphate buffer pH-8 containing 100mM NaC1). Then, the
beads were
eluted five times using 2m1 elution buffer (50mM phosphate buffer pH-8
containing 100mM NaC1
and 150mM imidazole). The peptide concentration was determined using Coomassie
blue staining
by comparison to known concentration of bovine serum albumin fraction V
protein (MP
B iomedicalsTm).
The eluate was subsequently dialyzed against PBS and the purified T-sema3A was
kept
frozen at -80 C.
Example 3- binding of WT-Sema3A to Nrpl and CD72 receptors
Materials and methods:
Cells:
-Parental U87MG: Human glioblastoma cell line (ATCC) originally/endogenously
expressing Nrpl.
-U87MG-ANrp1 : U87MG knockout for Nrpl (achieved by CRISPR-Cas9 method).
-U87MG-ANrp1+CD72: U87MG knockout for Nrpl and stably express CD72. These
cells
were generated by introducing full-length CD72 cDNA (Human CD72 9432bp
sequence, clone
5226648, DharmaconTM) into pLenti6.3/V5-DEST lentiviral expression vector in
frame with a
C-terminal V5 tag (Gateway, Thermo Fisher Scientific). By infection, this
vector was introduced
to U87MG ANrpl , followed by Blasticidin selection.
Sema3A-alkaline phosphatase concentration
HEK293-Sema3A-AP cells (HEK-293 transfected with WT-Sema3A in frame with
alkaline phosphatase) were grown to 70% confluence and incubated for 48h in
serum free medium.
Conditioned medium was concentrated using 30KDa Amicon Ultra centrifugal
filter devices for
50-fold concentration.
The Sema3A-AP concentration was determined using Coomassie blue staining by
comparison to known concentration of BSA.
Alkaline phosphatase colorimetric assay
Cells were incubated with concentrated Sema3A-AP for 1.5h at 4 C, followed by
PBS
wash and 20min fixation with 4% paraformaldehyde, and lh incubation at 65 C.
Next, 5-bromo-
4-chloro-3-indoly1 phosphate and nitro blue tetrazolium (BCIP /NBT) liquid
substrates for AP-
29
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
enzyme (SIGMA) were added for over-night incubation at 4 C. At the next day, a
visible yellow-
brown precipitate was microscopely demonstrated in case of AP-Sema3A binding
and the mean
color intensity per field (11.1m/tim2) was analyzed using Image-Pro software.
Alkaline phosphatase colorimetric assay: competitive inhibition assay
To analyze the kinetics of sema3A binding to CD72 receptor, Sema4D, which is
the known
ligand of CD72 was used as competitive inhibitor. The phosphatase colorimetric
assay was
performed with increasing concentration of recombinant human Sema4D protein (0-
50 g/m1)
(abcam) with constant concentration of Sema3A-AP (51.1,g/m1). The Graph Pad
Prism software was
used to draw the kinetics graphs and calculate the binding parameters.
Results:
Reference is made to Fig. 3A which shows a pictogram of a Western Blot
analysis of
modified or non-modified U87MG cells extract, probed with antibodies directed
against
neuropilin-1 and/or CD72. As can be seen in Fig. 3A, parental U87MG cells
(par) express Nrpl,
but do not express CD72. U87MG cells in which the gene expressing neuropilin-1
was knocked
out using CRISPR/Cas9 ("U87MG-ANrp1 ") were further infected with empty
lentiviruses or
lentiviruses directing expression of CD72 ("U87MG-ANrp1+CD72") to which a V5
epitope tag
was fused in frame upstream of the stop codon. The results demonstrate that
U87MG-ANrp1
indeed do not express Nrpl nor CD72, whereas the U87MG-ANrp1+CD72 cells
express CD72,
and do not express Nrpl.
Next, Sema3A-AP was bound to these cells for 60 minutes at 37 C. The cells
were then
washed and bound Sema3A-AP was detected using BICP/NBT. The results are
presented in the
pictogram shown in Fig. 3B.
Next, increasing concentrations of purified Sema3A-AP were incubated for 30
minutes at
room temperature with the three cell types. Following incubation (binding),
the cells were washed
and the amount of bound Sema3A-AP bound per microscopic field assessed using
an alkaline
phosphatase colorimetric assay. The results are presented in the line graphs
of Fig. 3C, which
clearly show that WT-Sema3A can bind CD72.
Next, Sema3A-AP (5 [tg/m1) was bound/incubated to the three cell types in the
presence
of increasing concentrations of sema4D, which is an authentic known ligand of
CD72. The amount
of bound sema3A-AP/microscopic field was then determined. The results
presented in Fig. 3D,
strengthen the finding that indeed WT-Sema3A can bind CD72 and that the
binding affinity of
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
sema3A to CD72 is very similar to its binding affinity to neuropilin-1.
Thus, the results presented in Figs. 3A-D demonstrate that wild type Sema3A is
able to
bind the CD72 receptor and that this binding is with similar binding affinity
as to Nrpl.
Example 4- Signal transduction of Sema3A using CD72 receptor
Materials and methods:
Cells:
- BLCL (donor#213, healthy female donor): B-Lymphoblastoid Cell Lines, an
Epstein-Barr
Virus transformed primary B-lymphoblastoid cells (ASTARTE BIOLOGICS, INC.).
- BLCL-CD72: BLCL stably express CD72. These cells were generated by
introducing full-
length CD72 cDNA into pBABE-EGFP lentiviral expression vector (Gateway, Thermo
Fisher Scientific). By infection, this vector was introduced to BLCL, followed
by EGFP
sorting.
Sema3A purification
HEK293-Sema3A cells (HEK-293 transfected with Sema3A in frame with a C-
terminal
His tag) were grown to 70% confluence and incubated for 48h in serum free
medium. Conditioned
medium was collected and then loaded on 1.5cm diameter column containing 2m1Ni-
NTA agarose
at 4 C (QIAGEN). The beads were washed twice with 10m1 wash buffer (50mM
phosphate buffer
pH-8 containing 100mM NaCl). Then, the beads were eluted five times using 2m1
elution buffer
(50mM phosphate buffer pH-8 containing 100mM NaCl and 150mM imidazole). The
Sema3A
concentration was determined using Coomassie blue staining by comparison to
known
concentration of bovine serum albumin fraction V protein (MP BiomedicalsTm).
Phosphorylation assay
Cells were serum starved for 16h. At the day of the experiment, cells were
activated with
51.1,g/m1 anti IgM for 5min at 37 C and then 101.1g/m1 of Sema3A or elution
buffer as a control were
added for extra 10min at 37 C. The experiment was terminated by a wash with
ice cold PBS and
lysed with phosphorylation lysis buffer (50mM Tris-HC1 pH-7.5, 150mM NaCl, 2mM
EDTA,
2mM EGTA, 5mM NaF, 2mM Na3VO4, 10mM Na4P207, 1% Triton X-100). 801.1,g of
proteins
were subjected to SDS-PAGE and immunoblotted with an antibody directed against
phosphorylated target protein, the blot was then stripped and re-probed with
an antibody directed
against total protein. Western blots were probed with the following
antibodies: anti STAT4 (C-4)
31
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
(Santa Cruz Biotechnology), anti phospho-STAT4 (Tyr693) (Santa Cruz
Biotechnology), p38
MAPK Antibody (Cell Signaling Technology, Inc), Phospho-p38 MAPK
(Thr180/Tyr182)
Antibody (Cell Signaling Technology, Inc). Quantification of band intensity
was performed using
ImageQuant LAS 4000 program.
Results:
To test whether Sema3A signal transduction is mediated by CD72, Furthermore,
CD72
was expressed in primary B-lymphoblastoid cells that lack neuropilin-1. The
results presented
herein in Figs. 4A-C demonstrate that Sema3A can signal via CD72 to increase
or inhibit the
phosphorylation state of several secondary signal transducers in these cells.
As shown in Fig. 4A,
a primary B-lymphoblastoid cell line (BLCL) was infected with lentiviruses
directing expression
of CD72. The BLCL cells and the BLCL cells expressing CD72 were probed with
antibodies
directed against neuropilin-1 (Nrp 1) and CD72. parental BLCL cell do not
express either of these
receptors. Next, BLCL cells and BLCL cells expressing CD72 (BLCL+CD72) were
stimulated
with WT-Sema3A peptide. The phosphorylation state of Stat-4 and P38 was than
determined. The
results are shown in Fig 4B and Fig. 4C which show pictograms of Western Blots
of cells extracts
probed with antibodies directed against the total proteins and against
specific phosphorylation sites
in Stat-4 (Fig. 4B) and P38 (Fig. 4C).
Thus, the results demonstrate that Sema3A can bind CD72 and further exert
cellular effects
via this receptor.
Example 5- Characterization of the biological properties of the modified-
Sema3A
1. Endothelial cells contraction assay
HUVECs (Human umbilical vein derived endothelial cells) plated on gelatin
plates were
incubated with conditioned medium from control HEK2963 cells or with
conditioned medium
containing similar concentration of wild-type Sema3A or T-Sema3A for 30
minutes (min) in a
humidified incubator, at 37 C. After the incubation the cells were
photographed using phase-
contrast inverted microscope (Ziess).
2. CD4+T cell purification and culturing
Peripheral blood samples from healthy controls were drown to heparin-washed
tubes, and
then loaded on Lymphoprep- a ficoll gradient to collect PBMCs. CD4+T cells
were positively
isolated from PBMCs using anti-human CD4 microbeads (Miltenyi-Biotec)
according to the
32
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
manufacturer's instructions. The purified CD4+T cells were cultured in plates
pre-coated with
g/m1 of anti-CD3 for 4 hours at 37 C, then were stimulated with 1 tig/m1 of
anti-CD28 and
1iug/m1 of IL-2, in addition to purified wild-type Sema3A or T-Sema3A (2-
51.1,g/m1) for 48 hours
at 37 C.
5 3. Flow Cytometry (FACS) staining
To determine the percentage of T-cells expressing IL-10 after 48h stimulation
with wild-
type Sema3A or T-sema3A, CD4+T cells were stained with FITC-anti-CD4 antibody
for 30min
at room temperature, then they were fixed with Fix and Perm medium A for
10min, afterward they
were permeabilized with Fix and Perm medium B, and APC-anti-IL-10 antibody was
added for
10 extra 30 min at room temperature. The CD4+ T cells expressing IL-10 were
evaluated using
Navios EX flow cytometer followed by Kaluza analysis software (Beckman Coulter
Life
Sciences).
Results:
Effects on the cytoskeleton of endothelial cells:
Sema3A binds to the neuropilin-1 receptor which is expressed on endothelial
cells. This
induces the association of neuropilin-1 with the plexin-Al and plexin-A4 of
the endothelial cells
which then transduce a sema3A signal that induces the localized disassembly of
the actin
cytoskeleton resulting in cell contraction. Thus, Cell contraction in these
cells is mediated by the
neuropilin-1 receptor. In order to identify whether T-Sema3A has lost its
ability to signal using
neuropilin-1, cell contraction in human umbilical vein derived endothelial
cells (HUVEC) was
induced by incubation/stimulation with wild-type Sema3A or T-Sema3A. The
results presented
in Fig. SA clearly demonstrate that in contrast to WT Sema3A, T-sema3A failed
to induce the
contraction of endothelial cells, indicating that it is not able to transduce
signals via the neuropilin-
1 receptor. Specifically, addition of T-sema3A (0.5-10 lig/m1) to the cells,
surprisingly failed to
induce the contraction of either human umbilical vein derived endothelial
cells, or U87MG
glioblastoma cells, whereas wild type Sema3A induced their contraction. The
results presented in
Fig. 5A clearly show the differential effect of WT-Sema3A and T-Sema3A on the
cells
contraction. Human umbilical vein derived endothelial cells (HUVEC) were
stimulated with
conditioned medium from control HEK293 cells (Control), or with conditioned
medium
containing similar concentrations of Sema3A or T-sema3A derived from HEK293
cells expressing
either recombinant Sema3A or T-sema3A. Cells were photographed 30 minutes
after addition of
the conditioned media.
33
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
The results thus implicate that un-like wild-type sema3A, the modified sema3A
is unable to induce
signal transduction via the neuropilin-1 receptor.
Effects on CD4+ T cells:
Next, it was sought to identify whether the modified Sema3A can transduce
signals via the
CD72 receptor. To this aim, increasing concentrations of modified-sema3A or
wild type sema3A
were added to CD4+ T cells that were activated using anti-CD3 and anti-CD28
for 48 hours. It
was found that both short-sema3A and wild type sema3A induced effectively
secretion of IL-10,
which is the most important anti-inflammatory cytokine secreted by activated
CD4+ T cells and T
regulatory cells. A concentration of 2 lig\ml was the most effective dose for
both wild type sema3A
and short-sema3A (Fig. 5B). As shown in Fig. 5B, CD4+ T-cells were stimulated
with the indicated
concentrations of purified Sema3A or T-Sema3A. The percentage of T-cells
expressing IL-10 was
than determined using FACS analysis.
The results suggest that the modified Sema3A can indeed successfully transduce
signals
via CD72. The results further suggest that the anti-inflammatory effect of
modified-sema3A is at
least similar to that of wild type sema3A.
Thus, it can be concluded that modified-sema3A can be used for treatment of
immune-
related disease, such as, autoimmune diseases, including lupus nephritis or
asthma as it would have
to be free of side effects associated with the activation of neuropilin-1
mediated signal
transduction.
Example 6: Effect of modified Sema3A on metabolic activity of activated T-
cells¨
To determine the effect of modified Sema3A on cellular metabolism (glycolysis)
of activated
T-cells, the effect on extracellular acidification rate (ECAR) was determined
using seahorse
technology (Agilent). The aim of the study was to test the ability of T-Sema3A
to down regulate
aerobic glycolysis in activated immune cells.
As known, the bioenergetic needs of quiescent T cells are met mainly by
mitochondrial
oxidative phosphorylation (OXPHOS), as a way to generate ATP from a glucose
substrate. However,
once activated, these cells rapidly proliferate and produce cytokines,
therefore, they undergo a
metabolic switch, where they utilize aerobic glycolysis as a main source of
energy production.
Generally, purified T cells were activated with anti-CD3 and anti-CD28 for 24
hours at 37 C
in the presence or absence of 51.1,g of T-Sema3A. At the day of the
experiment, cells were harvested
and transferred to medium without glucose for 2 hours. The ECAR rate
(extracellular acidification
34
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
rate) was measured based on glycolysis test using the seahorse technology, in
accordance with the
manufacturer protocol (Seahorse XF technology, Agilent). Briefly, after
glucose starvation, glucose
is added to the medium. Thereafter, oligomycin is added. Oligomycin, which is
an ATP synthase
inhibitor, inhibits mitochondrial ATP production, and shifts the energy
production to glycolysis, with
the subsequent increase in ECAR revealing the cellular maximum glycolytic
capacity. Next, 2-
deoxy-glucose (2-DG) is added. 2-DG is a glucose analog, which inhibits
glycolysis through
competitive binding to glucose hexokinase. The resulting decrease in ECAR
confirms that the
ECAR produced in the experiment is due to glycolysis. The glycolysis phase is
measured during
the time period between the addition of glucose and the addition of
oligomycin. The glycolytic
capacity is determined during the time period between the addition of
oligomycin and the addition
of 2-DG.
More specifically, CD4+ T cells were purified from peripheral blood of healthy
controls,
according to the manufacturer's instructions (#130-045-101, Miltenyi Biotec)
and cultured in plates
pre-coated with 10 g/m1 anti-CD3 (#16-0038-85, eBioscienceTM) for 4 hours at
37 C and 1 tig/m1
anti-CD28 (#16-0289-85, eBioscienceTM) as activators. In addition, cells were
treated with 5 g/m1
T-Sema3A or PBS (as a control) and incubated for 24 hours at 37 C.
On the day of the experiment, cells were harvested and seeded in a 96 well-
plate (#102416-
100, Seahorse XFe96 FluxPak, Agilent) pre-coated with 22.4 g/mL cell-tak
(#FAL354240, Lapidot
Pharma), and incubated in glucose free DMEM basic media supplied with 2mM
Glutamine (ph=7.4)
.. for 2 hours at 37 C.
The sensors cartridge (#102416-100, Seahorse XFe96FluxPak, Agilent) which was
hydrated
a day earlier was calibrated one hour prior to the experiment and the A, B and
C ports were loaded
to the final concentrations of 10mM Glucose, 2 M Oligomycin and 50mM 2-DG,
respectively. The
in live ECAR (extracellular acidification rate) measurements were performed
using Agilent Seahorse
XF Analyzers. The Glycolysis rate was calculated as (Maximum rate measurement
before
Oligomycin injection) - (Last rate measurement before Glucose injection).
Whereas the Glycolytic
Capacity was calculated as (Maximum rate measurement after Oligomycin
injection) - (Last rate
measurement before Glucose injection) which reflects the maximal rate in which
glucose is
converted to pyruvate.
The results are presented in Fig. 6, which clearly shows the effect of T-sema3
on the
metabolism of activated T-cells. As can be seen in Fig. 6, the T-sema3A
significantly reduces
glycolysis and glycolytic capacity in the activated T-cells. The results
demonstrate that naive cells
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
perform minimal glycolysis, whereas activated T cells undergo metabolic switch
to aerobic
glycolysis. The addition of T-Sema3A to activated T cells decreased the
glycolytic rate of these cells,
further substantiating the ability of T-Sema3A to down regulate aerobic
glycolysis.
Collectively, the results indicate that T-sema3A can reduce metabolism and
activity of
activated T-cells, further substantiating its effect on the immune system as
an immuno-regulator.
Example 7: In vivo studies for determining modified Sema3A effect in treating
Asthma
In order to assess the effect of administration of modified Semaphorin 3A on
asthma, the
Ovalbumin (OVA)-induced asthma mouse model is utilized. This mouse model is
widely used to
reproduce the airway eosinophilia, pulmonary inflammation and elevated IgE
levels found during
asthma. Balb/c female mice are induced for OVA sensitization and airway
challenge by
intraperitoneal injection with 50 g ovalbumin (OVA; grade V; Sigma-Aldrich)
plus 1 mg Alum
hydroxide (Sigma-Aldrich) in 200 [d 0.9% sodium chloride (saline; Hospira)
every week and until
the end of the experiment. Control group is treated identically except that
OVA is absent in the
solutions. Modified Sema3A is administered to mice with aerosolized 50 [tg
recombinant modified
Sema3A in 50 [L1 saline 12 hours prior to each administration of OVA by nasal
administration or
intraperitoneal administration. Mice are euthanized on day 24 and efficiency
of sensitization is
assessed as changes in airway function after challenge with aerosolized
methacholine (Sigma-
Aldrich). The effect of modified Sema3A on airway hyper-responsiveness is
compared to the effect
of administration of dexamethasone (3mg\kg), a synthetic member of the
glucocorticoid. Mice are
anesthetized, tracheostomized, mechanically ventilated, and lung function is
assessed starting from
24 h after the final OVA challenge. The lungs are challenged with increasing
doses of aerosolized
methacholine using flexiVentTM (Scireq -Scientific Respiratory Equipment).
Lung resistance is
continuously analyzed and compared between the different treatment groups. In
addition, serum total
IgE levels and assessment of eosinophilia and total inflammatory cells count
is assesses on serum
samples. The total IgE and OVA-specific IgE levels is measured in serum
samples collected from
mice on 16 day is determined using enzyme-linked immunosorbent assay (ELISA)
kits (Serotec,
Oxford, UK) according to the manufacturer's instructions. The absorbance is
measured at 450 nm
by a micro plate ELISA reader.
Bronchoalveolar lavage fluid (BALF) is taken from the mice and analyzed. BALF
is
centrifuged, the supernatant is analyzed for inflammatory cell count including
eosinophil,
lymphocyte, neutrophil, macrophage and total cells, by using direct
microscopic counting with a
hemocytometer after exclusion of dead cells by trypan blue staining. Th2
cytokines including IL-4
36
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
and IL-5 are analyzed in the BALF using an enzyme-linked immunosorbent assay
(ELISA) kits
(BioSource International, Camarillo, CA) according to the manufacturer's
protocol.
Example 8: Examining the Effect of Administration of modified Sema3A on
systemic lupus
erythematosus (SLE)
In order to assess how modified Semaphorin 3A affects SLE disease progression
in NZB/NZW
Fl mice (serving as a model system for SLE), mice are divided into 4 groups:
Prevention Group: In this group, 5 mice are injected with recombinant modified
Sema3A on
a daily basis and 5 mice are injected with PBS, as a control group. Mice are
injected from the age of
6 weeks for 90 days. During this period, both groups are assessed for the
development of auto-
antibodies (e.g. anti-dsDNA and anti-cardiolipin), kidney function tests
(creatinine and BUN),
complete blood count on weekly basis and detection of early proteinuria. In
addition clinical status
of the mice is evaluated by assessing their weight. After this period, the
mice are sacrificed and a
histological evaluation of their kidneys is performed.
Treatment Group: In this group, 5 mice are injected with recombinant modified
Sema3A on
a daily basis and 5 mice are injected with PBS, as a control group. Mice are
injected from the onset
of clinical and laboratory signs of SLE (at four months of age with early
proteinuria) and continue
for 90 days. During this period, both groups are assessed for the development
of auto-antibodies
(e.g. anti-dsDNA and anti-cardiolipin), kidney function tests (creatinine and
BUN), complete blood
count on weekly basis and detection of early proteinuria. In addition clinical
status of the mice is
evaluated by assessing their weight. After this period, the mice are
sacrificed and a histological
evaluation of their kidneys is performed.
Example 9: Examining the effect of administration of modified Sema3A in
Inflammatory
bowel disease (IBD) model
In the following study, the beneficial effect of T-sema3A in improving the
outcome of
inflammatory bowel disease (IBD) is tested. I
BD mice model was generated as follow: thirty three 8 week old (WNO) BALB \c
female
mice were feed with DSS in their water for eight days. Three (3) mice were not
treated with DSS
and served as naive group. From the 9th day- the mice were divided into 3
groups: 13 mice were
injected intraperitoneal every other day with 50 micrograms of T-Sema3A for 10
days, 13 mice-
.. were injected intraperitoneal every other day with 50 micrograms of control
solution and 4 mice-
37
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
served as disease control, without any treatment. Following 10 days of
treatment, mice were
sacrificed, their spleen and intestine were removed. T regulatory cells were
purified from the
spleens and the intestine was subjected to hematoxylin-eosin staining. Serum
was also evaluated
for pro- and anti- inflammatory cytokines. Consequently, Function of T-
regulatory cells is tested,
in addition to histopathological results and changes in cytokine status.
The foregoing description of the specific embodiments will so fully reveal the
general nature
of the invention that others can, by applying current knowledge, readily
modify and/or adapt for
various applications such specific embodiments without undue experimentation
and without
departing from the generic concept, and, therefore, such adaptations and
modifications should and
are intended to be comprehended within the meaning and range of equivalents of
the disclosed
embodiments. It is to be understood that the phraseology or terminology
employed herein is for the
purpose of description and not of limitation. The means, materials, and steps
for carrying out various
disclosed functions may take a variety of alternative forms without departing
from the invention. It
is to be understood that further trials are being conducted to establish
clinical effects.
Listed below are the Amino acid sequences and nucleic acid sequences of wild
type or
modified Sema3A forms, as disclosed herein.
Wild type Sema3A polypeptide (Amino Acids)- SEQ ID NO: 1
MGWLTRIVCLFWGVLLTARANYQNGKNNVPRLKLS YKEMLESNNVITFNGLANS SSYH
TFLLDEERSRLYVGAKDHIFSFDLVNIKDFQKIVWPVSYTRRDECKWAGKDILKECANFI
KVLKAYNQTHLYACGTGAFHPICTYIEIGHHPEDNIFKLENSHFENGRGKSPYDPKLLTA
SLLIDGELYSGTAADFMGRDFAIFRTLGHHHPIRTEQHDSRWLNDPKFISAHLISESDNPE
DDKVYFFFRENAIDGEHSGKATHARIGQICKNDFGGHRSLVNKWTTFLKARLICSVPGP
NGIDTHFDELQDVFLMNFKDPKNPVVYGVFTTSSNIFKGSAVCMYSMSDVRRVFLGPY
AHRDGPNYQWVPYQGRVPYPRPGTCPSKTFGGFDSTKDLPDDVITFARSHPAMYNPVFP
MNNRPIVIKTDVNYQFTQIVVDRVDAEDGQYDVMFIGTDVGTVLKVVSIPKETWYDLE
EVLLEEMTVFREPTAISAMELSTKQQQLYIGS TAGVAQLPLHRCD IYGKACAECCLARD
PYCAWDGSACSRYFPTAKRRTRRQDIRNGDPLTHCSDLHHDNHHGHSPEERIIYGVENS
STFLECSPKS QRALVYWQFQRRNEERKEEIRVDDHIIRTDQGLLLRSL QQKD S GNYLCH
AVEHGFIQTLLKVTLEVIDTEHLEELLHKDDDGDGS KT KEMSNS MTPS QKVWYRDFMQ
LINHPNLNTMDEFCE QVWKRDRKQRRQRPGHTPGNS NKWKHLQENKKGRNRRTHEFE
RAPRSV
38
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
Wild type Sema3A nucleotide sequence (nucleic acids of coding sequence)- SEQ
ID NO: 2
atgggctggt taactaggat tgtctgtctt ttctggggag tattacttac
agcaagagca aactatcaga atgggaagaa caatgtgcca aggctgaaat
tatcctacaa agaaatgttg gaatccaaca atgtgatcac tttcaatggc
ttggccaaca gctccagtta tcataccttc cttttggatg aggaacggag
taggctgtat gttggagcaa aggatcacat attttcattc gacctggtta
atatcaagga ttttcaaaag attgtgtggc cagtatctta caccagaaga
gatgaatgca agtgggctgg aaaagacatc ctgaaagaat gtgctaattt
catcaaggta cttaaggcat ataatcagac tcacttgtac gcctgtggaa
cgggggcttt tcatccaatt tgcacctaca ttgaaattgg acatcatcct
gaggacaata tttttaagct ggagaactca cattttgaaa acggccgtgg
gaagagtcca tatgacccta agctgctgac agcatccctt ttaatagatg
gagaattata ctctggaact gcagctgatt ttatggggcg agactttgct
atcttccgaa ctcttgggca ccaccaccca atcaggacag agcagcatga
ttccaggtgg ctcaatgatc caaagttcat tagtgcccac ctcatctcag
agagtgacaa tcctgaagat gacaaagtat actttttctt ccgtgaaaat
gcaatagatg gagaacactc tggaaaagct actcacgcta gaataggtca
gatatgcaag aatgactttg gagggcacag aagtctggtg aataaatgga
caacattcct caaagctcgt ctgatttgct cagtgccagg tccaaatggc
attgacactc attttgatga actgcaggat gtattcctaa tgaactttaa
agatcctaaa aatccagttg tatatggagt gtttacgact tccagtaaca
ttttcaaggg atcagccgtg tgtatgtata gcatgagtga tgtgagaagg
gtgttccttg gtccatatgc ccacagggat ggacccaact atcaatgggt
gccttatcaa ggaagagtcc cctatccacg gccaggaact tgtcccagca
aaacatttgg tggttttgac tctacaaagg accttcctga tgatgttata
acctttgcaa gaagtcatcc agccatgtac aatccagtgt ttcctatgaa
caatcgccca atagtgatca aaacggatgt aaattatcaa tttacacaaa
ttgtcgtaga ccgagtggat gcagaagatg gacagtatga tgttatgttt
atcggaacag atgttgggac cgttcttaaa gtagtttcaa ttcctaagga
gacttggtat gatttagaag aggttctgct ggaagaaatg acagtttttc
gggaaccgac tgctatttca gcaatggagc tttccactaa gcagcaacaa
ctatatattg gttcaacggc tggggttgcc cagctccctt tacaccggtg
tgatatttac gggaaagcgt gtgctgagtg ttgcctcgcc cgagaccctt
actgtgcttg ggatggttct gcatgttctc gctattttcc cactgcaaag
agacgcacaa gacgacaaga tataagaaat ggagacccac tgactcactg
ttcagactta caccatgata atcaccatgg ccacagccct gaagagagaa
tcatctatgg tgtagagaat agtagcacat ttttggaatg cagtccgaag
tcgcagagag cgctggtcta ttggcaattc cagaggcgaa atgaagagcg
aaaagaagag atcagagtgg atgatcatat catcaggaca gatcaaggcc
ttctgctacg tagtctacaa cagaaggatt caggcaatta cctctgccat
gcggtggaac atgggttcat acaaactctt cttaaggtaa ccctggaagt
cattgacaca gagcatttgg aagaacttct tcataaagat gatgatggag
atggctctaa gaccaaagaa atgtccaata gcatgacacc tagccagaag
gtctggtaca gagacttcat gcagctcatc aaccacccca atctcaacac
39
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
aatggatgag ttctgtgaac aagtttggaa aagggaccga aaacaacgtc
ggcaaaggcc aggacatacc ccagggaaca gtaacaaatg gaagcactta
caagaaaata agaaaggtag aaacaggagg acccacgaat ttgagagggc
acccaggagt gtctga
Modified Sema 3A polypeptide (Amino Acids)¨ Seq ID NO: 3
MGWLTRIVCLFWGVLLTARANYQNGKNNVPRLKLS YKEMLESNNVITFNGLANS SSYH
TFLLDEERSRLYVGAKDHIFSFDLVNIKDFQKIVWPVSYTRRDECKWAGKDILKECANFI
KVLKAYNQTHLYACGTGAFHPICTYIEIGHHPEDNIFKLENSHFENGRGKSPYDPKLLTA
SLLIDGELYSGTAADFMGRDFAIFRTLGHHHPIRTEQHDSRWLNDPKFISAHLISESDNPE
DDKVYFFFRENAIDGEHCGKATHARIGQICKNDFGGHRSLVNKWTTFLKARLICSVPGP
NGIDTHFDELQDVFLMNFKDPKNPVVYGVFTTSSNIFKGSAVCMYSMSDVRRVFLGPY
AHRDGPNYQWVPYQGRVPYPRPGTCPSKTFGGFDSTKDLPDDVITFARSHPAMYNPVFP
MNNRPIVIKTDVNYQFTQIVVDRVDAEDGQYDVMFIGTDVGTVLKVVSIPKETWYDLE
EVLLEEMTVFREPTAISAMELSTKQQQLYIGSTAGVAQLPLHR*
Modified Sema3A nucleotide sequence- SEQ ID NO: 4
atgggctggttaactaggattgtctgtcttttctggggagtattacttacagcaagagcaaact
atcagaatgggaagaacaatgtgccaaggctgaaattatcctacaaagaaatgttggaatccaa
caatgtgatcactttcaatggcttggccaacagctccagttatcataccttccttttggatgag
gaacggagtaggctgtatgttggagcaaaggatcacatattttcattcgacctggttaatatca
aggattttcaaaagattgtgtggccagtatcttacaccagaagagatgaatgcaagtgggctgg
aaaagacatcctgaaagaatgtgctaatttcatcaaggtacttaaggcatataatcagactcac
ttgtacgcctgtggaacgggggcttttcatccaatttgcacctacattgaaattggacatcatc
ctgaggacaatatttttaagctggagaactcacattttgaaaacggccgtgggaagagtccata
tgaccctaagctgctgacagcatccottttaatagatggagaattatactctggaactgcagct
gattttatggggcgagactttgctatcttccgaactcttgggcaccaccacccaatcaggacag
agcagcatgattccaggtggctcaatgatccaaagttcattagtgcccacctcatctcagagag
tgacaatcctgaagatgacaaagtatactttttcttccgtgaaaatgcaatagatggagaacac
tGtggaaaagctactcacgctagaataggtcagatatgcaagaatgactttggagggcacagaa
gtctggtgaataaatggacaacattcctcaaagctcgtctgatttgctcagtgccaggtccaaa
tggcattgacactcattttgatgaactgcaggatgtattcctaatgaactttaaagatcctaaa
aatccagttgtatatggagtgtttacgacttccagtaacattttcaagggatcagccgtgtgta
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
tgtatagcatgagtgatgtgagaagggtgttccttggtccatatgcccacagggatggacccaa
ctatcaatgggtgccttatcaaggaagagtcccctatccacggccaggaacttgtcccagcaaa
acatttggtggttttgactctacaaaggaccttcctgatgatgttataacctttgcaagaagtc
atccagccatgtacaatccagtgtttcctatgaacaatcgcccaatagtgatcaaaacggatgt
aaattatcaatttacacaaattgtcgtagaccgagtggatgcagaagatggacagtatgatgtt
atgtttatcggaacagatgttgggaccgttcttaaagtagtttcaattcctaaggagacttggt
atgatttagaagaggttctgctggaagaaatgacagtttttcgggaaccgactgctatttcagc
aatggagctttccactaagcagcaacaactatatattggttcaacggctggggttgcccagctc
cctttacaccggTGA
Modified Sema3A polypeptide with C-terminal His-Tag (Amino Acids)¨ Seq ID NO:
5
MGWLTRIVCLFWGVLLTARANYQNGKNNVPRLKLSYKEMLESNNVITFNGLANSSSYH
TFLLDEERSRLYVGAKDHIFSFDLVNIKDFQKIVWPVSYTRRDECKWAGKDILKECANFI
KVLKAYNQTHLYACGTGAFHPICTYIEIGHHPEDNIFKLENSHFENGRGKSPYDPKLLTA
SLLIDGELYSGTAADFMGRDFAIFRTLGHHHPIRTEQHDSRWLNDPKFISAHLISESDNPE
DDKVYFFFRENAIDGEHCGKATHARIGQICKNDFGGHRSLVNKWTTFLKARLICSVPGP
NGIDTHFDELQDVFLMNFKDPKNPVVYGVFTTSSNIFKGSAVCMYSMSDVRRVFLGPY
AHRDGPNYQWVPYQGRVPYPRPGTCPSKTFGGFDSTKDLPDDVITFARSHPAMYNPVFP
MNNRPIVIKTDVNYQFTQIVVDRVDAEDGQYDVMFIGTDVGTVLKVVSIPKETWYDLE
EVLLEEMTVFREPTAISAMELSTKQQQLYIGSTAGVAQLPLHRHHHHHHHH
Modified Sema3A with C-terminal His-Tag nucleotide sequence- SEQ ID NO: 6
atgggctggttaactaggattgtctgtcttttctggggagtattacttacagcaagagcaaact
atcagaatgggaagaacaatgtgccaaggctgaaattatcctacaaagaaatgttggaatccaa
caatgtgatcactttcaatggcttggccaacagctccagttatcataccttccttttggatgag
gaacggagtaggctgtatgttggagcaaaggatcacatattttcattcgacctggttaatatca
aggattttcaaaagattgtgtggccagtatcttacaccagaagagatgaatgcaagtgggctgg
aaaagacatcctgaaagaatgtgctaatttcatcaaggtacttaaggcatataatcagactcac
ttgtacgcctgtggaacgggggcttttcatccaatttgcacctacattgaaattggacatcatc
ctgaggacaatatttttaagctggagaactcacattttgaaaacggccgtgggaagagtccata
tgaccctaagctgctgacagcatccottttaatagatggagaattatactctggaactgcagct
gattttatggggcgagactttgctatcttccgaactcttgggcaccaccacccaatcaggacag
41
CA 03185618 2022-11-29
WO 2021/245670
PCT/IL2021/050660
agcagcatgattccaggtggctcaatgatccaaagttcattagtgcccacctcatctcagagag
tgacaatcctgaagatgacaaagtatactttttcttccgtgaaaatgcaatagatggagaacac
tGtggaaaagctactcacgctagaataggtcagatatgcaagaatgactttggagggcacagaa
gtctggtgaataaatggacaacattcctcaaagctcgtctgatttgctcagtgccaggtccaaa
tggcattgacactcattttgatgaactgcaggatgtattcctaatgaactttaaagatcctaaa
aatccagttgtatatggagtgtttacgacttccagtaacattttcaagggatcagccgtgtgta
tgtatagcatgagtgatgtgagaagggtgttccttggtccatatgcccacagggatggacccaa
ctatcaatgggtgccttatcaaggaagagtcccctatccacggccaggaacttgtcccagcaaa
acatttggtggttttgactctacaaaggaccttcctgatgatgttataacctttgcaagaagtc
atccagccatgtacaatccagtgtttcctatgaacaatcgcccaatagtgatcaaaacggatgt
aaattatcaatttacacaaattgtcgtagaccgagtggatgcagaagatggacagtatgatgtt
atgtttatcggaacagatgttgggaccgttcttaaagtagtttcaattcctaaggagacttggt
atgatttagaagaggttctgctggaagaaatgacagtttttcgggaaccgactgctatttcagc
aatggagctttccactaagcagcaacaactatatattggttcaacggctggggttgcccagctc
cctttacaccggcaccatcaccatcaccatcaccatcaccatTGA
42