Note: Descriptions are shown in the official language in which they were submitted.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
TISSUE RESECTION CONTROL SYSTEMS AND METHODS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to and the benefit of United States
(US) Patent
Application Number 63/035,913 filed June 8, 2020 and United States (US) Patent
Application
Number 63/042,124 filed June 22, 2020, each of which is hereby incorporated by
reference in
their entirety.
BACKGROUND
[0002] In certain instances, tissue may need to be removed from the body. As
an
example, cancerous or infected tissue may be removed from the body as part of
a treatment.
Cancer is not a single disease, but rather a collection of related diseases
that may start essentially
anywhere in the body. Common amongst all types of cancer is that the body's
cells begin to
divide without stopping, proliferating and potentially spreading into
surrounding tissues. In the
normal course of events, cells grow and divide to form new cells as required
by the body and
when they become damaged or old, they die, and new cells replace the damaged
or old cells;
however, cancer interrupts this process. With cancer, the cells become
abnormal, and cells that
should die do not and new cells form when they are not needed. These new cells
may reproduce
or proliferate without stopping and may form growths called tumors.
[0003] Cancerous tumors are malignant, which means they may spread into or
invade
surrounding healthy tissue. In addition, cancer cells may break off and travel
to remote areas in
the body through blood or in the lymph system. Benign tumors, unlike malignant
tumors, do not
spread or invade surrounding tissue; however, they may grow large and cause
damage. Both
malignant and benign tumors may be removed or treated. Malignant tumors tend
to grow back,
whereas benign tumors may grow back but are much less likely to do so.
[0004] Cancer is a genetic disease in that it is caused by changes in the
genes that
control the ways that cells function, especially in how they grow and divide.
Genetic changes
that cause cancer may be inherited or they may arise over an individual's
lifetime as a result of
errors that occur as cells divide or because of damage to DNA caused by
certain environmental
exposure, for example, industrial/commercial chemicals and ultraviolet light.
The genetic
changes that may cause cancer tend to affect three types of genes; namely
proto-oncogenes
1
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
which are involved in normal cell growth and division, tumor suppressor genes
which are also
involved in controlling cell growth and division, and DNA repair genes which,
as the name
implies, are involved in repairing damaged DNA.
[0005] More than one-hundred distinct types of cancer have been identified.
The type
of cancer may be named for the organ or tissue where the cancers arise, for
example, lung
cancer, or the type of cell that formed them, for example squamous cell
cancer. Cancer,
unfortunately, is a leading cause of death both in the United States and world-
wide. According to
the World Health Organization, the number of new cancer cases will rise to
twenty-five (25)
million per year over the next two decades.
[0006] Lung cancer is one of the most common cancers today. According to the
World
Cancer Report 2014 from the World Health Organization, lung cancer occurred in
14 million
people and resulted in 8.8 million deaths world-wide, making it the most
common cause of
cancer-related death in men and the second most common cause of cancer-related
death in
women. Lung cancer or lung carcinoma is a malignant lung tumor that if left
untreated may
metastasize into neighboring tissues and organs. The majority of lung cancer
is caused by long-
term tobacco smoking; however, about 10 to 15 percent of lung cancer cases are
not tobacco
related. These non-tobacco cases are most often caused by a combination of
genetic factors and
exposure to certain environmental conditions, including radon gas, asbestos,
second-hand
tobacco smoke, other forms of air pollution, and other agents. The chance of
surviving lung
cancer as well as other forms of cancer depends on early detection and
treatment.
[0007] Improvements in removing tissue are needed.
SUMMARY
[0008] It may be desirable to remove a core of tissue from other target tissue
sites
including, but not limited to, the lungs, the liver, pancreas, or
gastrointestinal (GI) tract, for
which managing post-coring bleeding may be desired. A core of tissue may have
a prescribed
(e.g., pre-defined) shape (e.g., columnar) and dimension based on a coring
apparatus. Such
coring apparatus may be used to core the same or substantially the same shaped
tissue core in a
repeatable manner. Such coring may be distinguished from other tissue removal,
for example
using scissors or scalpel, where the cut tissue will not have a pre-defined
shape or dimensions.
2
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0009] Methods may comprise removing a core of tissue from a tissue site. Such
coring
may further comprise introducing a tissue resection device to a tissue site,
using the tissue
resection device to create a core of tissue, removing the core of tissue from
the body to create a
tissue cavity, and sealing the tissue cavity.
[0010] In certain aspect, removing a core of tissue from a tissue site may
further
comprise one or more of: determining the location of a tissue lesion using one
or more imaging
modalities, navigating an instrument to the tissue site such as the tissue
lesion (with and without
image guidance), coupling (e.g., anchoring) the instrument to the tissue
lesion, obtaining access
to the tissue site (making an incision, introduction through a port/trocar, or
direct access via an
open procedure), introducing a tissue resection device to the tissue site
(with and without using
the anchor as a guide), using the tissue resection device to create a core of
tissue or amputating
the core of tissue from the tissue site, removing the core of tissue from the
body (with and
without leaving a cavity "access sleeve"), analyzing the tissue core sample
(tissue histology,
ROSE, DNA sequencing, etc.), sealing the tissue cavity, removing some or all
instrumentation,
or closing tissue access points.
[0011] In certain aspects, removing a core of tissue from a tissue site and
subsequent
diagnosis may further comprise one or more of: determining a location of a
tissue lesion using
one or more imaging modalities, navigating an instrument to a tissue site such
as the tissue lesion
(with and without image guidance), coupling (e.g., anchoring) the instrument
to the tissue lesion,
obtaining access to the tissue site (making an incision, introduction through
a port/trocar, or
direct access via an open procedure), introducing a tissue resection device to
the tissue site (with
and without using the anchor as a guide), using the tissue resection device to
create a core of
tissue or amputating the core of tissue from the tissue site, removing the
core of tissue from the
body (with and without leaving a cavity "access sleeve"), analyzing the tissue
core sample
(tissue histology, ROSE, DNA sequencing, etc.), sealing the tissue cavity,
removing some or all
instrumentation, or closing tissue access points.
[0012] In certain aspects, removing a core of tissue from a tissue site,
subsequent
diagnosis, and therapeutic management of confirmed malignancy may further
comprise one or
more of: determining the location of a tissue lesion using one or more imaging
modalities,
navigating an instrument to the tissue lesion (with and without image
guidance), coupling (e.g.,
anchoring) the instrument to the tissue lesion, obtaining access to the tissue
site (making an
3
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
incision, introduction through a port/trocar, or direct access via an open
procedure), introducing a
tissue resection device to the tissue site (with and without using the anchor
as a guide), using the
tissue resection device to create a core of tissue or amputating the core of
tissue from the tissue
site, removing the core of tissue from the body (with and without leaving a
cavity "access
sleeve"), analyzing the tissue core sample (tissue histology, ROSE, DNA
sequencing, etc.),
performing therapeutic management of tissue such as benign or malignant
tissue, sealing the
tissue cavity, removing some or all instrumentation, closing tissue access
points.
[0013] Methods for coring tissue may comprise disposing a tissue resection
device at a
target tissue site, causing the tissue resection device to resect a core of
tissue from the target
tissue site, and removing the core of tissue from the body, wherein the
removing the core of
tissue from the body creates a core cavity at the target tissue site. The core
of tissue comprises at
least a portion of a tissue lesion. The resecting the core of tissue from the
target tissue site may
comprise mechanical transection. The resecting the core of tissue from the
target tissue site may
comprise the delivery of radiofrequency energy. The resecting the core of
tissue from the target
tissue site may comprise mechanical compression and the delivery of
radiofrequency energy.
The resecting the core of tissue from the target tissue site may comprise
transection with an
energized wire. The resecting the core of tissue from the target tissue site
may comprise one of
more of mechanical compression, the delivery of radiofrequency energy, the
delivery of
microwave energy, the delivery of ultrasonic energy, or transection with an
energized wire.
Other resection devices and procedures may be used. The resection device may
be configured for
one or more of mechanical compression, the delivery of radiofrequency energy,
the delivery of
microwave energy, the delivery of ultrasonic energy, or transection with an
energized wire.
[0014] Methods for coring tissue may further comprise inserting a sleeve into
the core
cavity to support a wall of the core cavity. Methods for coring tissue may
further comprise
delivering radiofrequency energy to at least a portion of a wall defining the
core cavity. Methods
for coring tissue may further comprise delivering chemotherapy to at least a
portion of a wall
defining the core cavity. Methods for coring tissue may further comprise
delivering microwave
energy to at least a portion of a wall defining the core cavity. Methods for
coring tissue may
further comprise delivering thermal energy to at least a portion of a wall
defining the core cavity.
Methods for coring tissue may further comprise delivering ultrasonic energy to
at least a portion
of a wall defining the core cavity.
4
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0015] Methods for coring tissue may further comprise sealing biological fluid
vessels.
The sealing biological fluid vessels may minimize flow of biological fluids
into the cavity core.
The sealing may be effected using at least mechanical compression. The sealing
may be effected
using at least radiofrequency energy. The sealing may be effected using at
least microwave
energy. The sealing may be effected using at least ultrasonic energy. The
sealing may be effected
using one or more of compression or delivery of energy such as radiofrequency,
microwave,
ultrasonic, or thermal energy.
[0016] The present disclosure relates to a system, device and method for
performing
lung lesion removal. A lung needle biopsy is typically performed when an
abnormality is found
on an imaging test, for example, an X-ray or CAT scan. In a lung needle
biopsy, a fine needle is
used to remove sample of lung tissue for examining under a microscope to
determine the
presence of abnormal cells. Tissue diagnosis is challenging in small (<6mm)
and intermediate (6-
12mm) nodules. CT guided biopsy of peripheral lesions, either through the
chest wall (80%) or
by means of a bronchoscope (20%) yields only a .001-.002 cm2 of diagnostic
tissue, and as such,
cancer, when present, is only successfully identified in 60% of small and
intermediate nodules.
Although bronchoscopic techniques and technology continue to evolve, biopsy
accuracy,
specificity, and sensitivity will always be limited when dealing with small
and intermediate
nodules in the periphery of the lung.
[0017] If it is determined that the lesion is cancerous, a second procedure
may be
performed to remove the lesion and then followed up with chemotherapy and/or
radiation. The
second procedure most likely involves lung surgery. These procedures are
typically done through
an incision between the ribs. There are a number of possible procedures
depending on the state
of the cancer. Video-assisted thoracic surgery is a less invasive procedure
for certain types of
lung cancer. It is performed through small incisions utilizing an endoscopic
approach and is
typically utilized for performing wedge resections of smaller lesions close to
the surface of a
lung. In a wedge resection, a portion of the lobe is removed. In a sleeve
resection, a portion of a
large airway is removed thereby preserving more lung function.
[0018] Nodules deeper than 2-3 cm from the lung surface, once identified as
suspicious
for cancer, are difficult to localize and excise using laparoscopic or robotic
lung sparing
technique despite pre-procedure image guided biopsy and localization. Thus,
surgeons perform
an open thoracotomy or lobectomy to remove lung nodules that are 2-3 cm from
the lung
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
surface. A thoracotomy is an open approach surgery in which a portion of a
lobe, a full lobe or
an entire lung is removed. In a pneumonectomy, an entire lung is removed. This
type of surgery
is obviously the most aggressive. In a lobectomy, an entire section or lobe of
a lung is removed
and represents a less aggressive approach than removing the entire lung. All
thoracoscopic lung
surgeries require trained and experienced thoracic surgeons and the
favorability of surgical
outcomes track with operative experience.
[0019] Any of these types of lung surgery is a major operation with possible
complications which depend on the extent of the surgery as well as the
patient's overall health.
In addition to the reduction in lung function associated with any of these
procedures, the
recovery may take from weeks to months. With a thoracotomy, spreading of the
ribs is required,
thereby increasing postoperative pain. Although video-assisted thoracic
surgery is less invasive,
there may still be a substantial recovery period. In addition, once the
surgery is complete, full
treatment may require a system chemotherapy and/or radiation treatment.
[0020] As set forth above, a fine needle biopsy may not prove to be totally
diagnostic.
The fine needle biopsy procedure involves guiding a needle in three-
dimensional space under
two-dimensional imaging. Accordingly, the doctor may miss the lesion, or even
if he or she hits
the correct target, the section of the lesion that is removed through the
needle may not contain
the cancerous cells or the cells necessary to assess the aggressiveness of the
tumor. A needle
biopsy removes enough tissue to create a smear on a slide. The device of the
present disclosure is
designed to remove the entire lesion, or a substantial portion of it, while
minimizing the amount
of healthy lung tissue removal. This offers a number of advantages. Firstly,
the entire lesion may
be examined for a more accurate diagnosis without confounding sampling error,
loss of cell
packing or gross architecture. Secondly, since the entire lesion is removed, a
secondary
procedure as described above may not be required. Thirdly, localized
chemotherapy and/or
energy-based tumor extirpation, such as radiation, may be introduced via the
cavity created by
the lesion removal.
[0021] In at least one embodiment, the disclosure defines a method for
removing a
tissue lesion including coupling (e.g., anchoring) to the tissue lesion;
creating a channel in the
tissue leading to the tissue lesion; creating a tissue core including the
tissue lesion; ligating the
tissue core at a ligation point downstream from the tissue lesion; amputating
the tissue core form
6
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
the tissue between the ligation point and the tissue lesion; and removing the
tissue core from the
channel.
[0022] In keeping with aspects of the disclosure, the sleeve may be inserted
in the
channel prior to or after removing the tissue core. The sleeve may also be
anchored to the tissue.
In keeping with another aspect of the disclosure, a localized treatment may be
delivered through
the sleeve.
[0023] In some embodiments, creating a tissue core includes cauterizing and
cutting
tissue. Ligating tissue may include tissue may include cauterizing tissue at a
specific location
known as the ligation point. Amputation of the tissue core may be performed
with a snare, an
energized wire or any other device capable of slicing tissue.
[0024] In some embodiments, the tissue core is created by first sealing blood
vessels
then slicing tissue to form the core.
BRIEF DESCRIPTION OF THE DRAWINGS
[0025] The following drawings show generally, by way of example, but not by
way of
limitation, various examples discussed in the present disclosure. In the
drawings:
[0026] FIG. 1 shows an example method in accordance with the present
disclosure.
[0027] FIG. 2 shows an example method in accordance with the present
disclosure.
[0028] FIG. 3 shows an example method in accordance with the present
disclosure.
[0029] FIG. 4 shows an example method in accordance with the present
disclosure.
[0030] FIG. 5 illustrates a blade with an open channel.
[0031] FIG. 6 illustrates a distal tip of the blade of FIG. 5.
[0032] FIG. 7 illustrates a distal end of air channel connected to a flexible
or rigid tube.
[0033] FIGS. 8A-8B illustrate an example trocar.
[0034] FIGS. 9A-9B illustrate an example trocar.
[0035] FIG. 10 illustrates an example trocar.
[0036] FIG. 11 depicts a tissue resection device in accordance with an
embodiment of
the present disclosure.
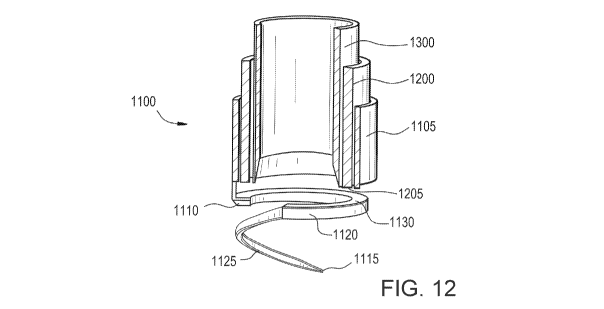
[0037] FIG. 12 illustrates a sectional view of the tissue resection device of
FIG. 11.
[0038] FIG. 13 shows a sectional view of a tissue resection device in
accordance with
an embodiment of the present disclosure.
7
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0039] FIG. 14 depicts a sectional view of a tissue resection device in
accordance with
an embodiment of the present disclosure.
[0040] FIG. 15 illustrates an exemplary anchor that may be employed in a
lesion
removal method in accordance with an embodiment of the present disclosure.
[0041] FIG. 16 shows a series of incision blades for use in a lesion removal
method in
accordance with an embodiment of the present disclosure.
[0042] FIG. 17 displays tissue dilators suitable for use in a lesion removal
method in
accordance with an embodiment of the present disclosure.
[0043] FIG. 18 shows an example workflow of tissue sample analysis.
[0044] FIG. 19 shows an application of an example system for sealing tissue.
[0045] FIG. 20 shows an application of an example system for sealing tissue.
[0046] FIGS. 21A, 21B, and 21C show an application of an example system for
sealing
tissue.
[0047] FIGS. 22A and 22B show an application of an example system for sealing
tissue.
[0048] FIGS. 23A, 23B, and 23C show an application of an example system for
sealing
tissue.
[0049] FIG. 24 illustrates an example therapy system and method in accordance
with
the present disclosure.
[0050] FIG. 25 illustrates an example therapy system and method in accordance
with
the present disclosure.
[0051] FIG. 26 illustrates an example therapy system and method in accordance
with
the present disclosure.
[0052] FIG. 27 illustrates an example therapy system and method in accordance
with
the present disclosure.
[0053] FIG. 28 illustrates an example therapy system and method in accordance
with
the present disclosure.
[0054] FIG. 29 illustrates an example therapy system and method in accordance
with
the present disclosure.
[0055] FIG. 30 illustrates an example therapy system and method in accordance
with
the present disclosure.
8
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0056] FIG. 31 illustrates a flow diagram of an example method in accordance
with the
present disclosure.
[0057] FIG. 32 illustrates an examples handle design in accordance with the
present
disclosure.
[0058] FIG. 33 illustrates an example rotation control assembly (e.g.,
planetary gear
assembly) in accordance with the present disclosure.
[0059] FIG. 34 illustrates a linear translation control assembly in accordance
with the
present disclosure.
[0060] FIG. 35 illustrates an anchor position monitor in accordance with the
present
disclosure.
[0061] FIG. 36 illustrates an example ligation and amputation system in
accordance
with the present disclosure.
[0062] FIGS. 37A-37B illustrate example methods of the present disclosure.
[0063] FIG. 38 illustrates an example handle design of the present disclosure.
[0064] FIG. 39 illustrates a rotation control assembly in accordance with the
present
disclosure.
[0065] FIG. 40 illustrates an example clamp in accordance with the present
disclosure.
[0066] FIG. 41 illustrates an example ligation and amputation system in
accordance
with the present disclosure.
[0067] FIG. 42 illustrates an examples schematic and flow diagram in
accordance with
the present disclosure.
DETAILED DESCRIPTION
[0068] The present disclosure relates to systems and methods for coring
tissue. Various
tissue and sites may benefit from the disclosed systems and methods.
[0069] A core of tissue may have a prescribed (e.g., pre-defined) shape (e.g.,
columnar)
and dimension based on a coring apparatus. Such coring apparatus may be used
to core the same
or substantially the same shaped tissue core in a repeatable manner. Such
coring may be
distinguished from other tissue removal, for example using scissors or
scalpel, where the cut
tissue will not have a pre-defined shape or dimensions.
9
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0070] FIG. 1 shows an example method, which may comprise removing a core of
tissue from a tissue site. Such coring may further comprise introducing a
tissue resection device
to a tissue site (102), amputating a core of tissue such as using the tissue
resection device to
create a core of tissue (104), removing the core of tissue from the body to
create a tissue cavity
(106), and sealing the tissue cavity (108).
[0071] As illustrated in FIG. 2, removing a core of tissue from a tissue site
may further
comprise one or more of: determining the location of a tissue lesion using one
or more imaging
modalities (202), navigating an instrument to a site such as the tissue lesion
(with and without
image guidance) (204), coupling (e.g., anchoring) the instrument to the tissue
lesion (206),
obtaining access to the tissue site (making an incision, introduction through
a port/trocar, or
direct access via an open procedure) (208), introducing a tissue resection
device to the tissue site
(with and without using the anchor as a guide) (210), using the tissue
resection device to create a
core of tissue (212) or amputating the core of tissue from the tissue site
(214), removing the core
of tissue from the body (with and without leaving a cavity "access sleeve")
(216), analyzing the
tissue core sample (tissue histology, ROSE, DNA sequencing, etc.) (218),
sealing the tissue
cavity (220), removing some or all instrumentation (222), or closing tissue
access points (224).
[0072] As illustrated in FIG. 3, removing a core of tissue from a tissue site
and
subsequent diagnosis may further comprise one or more of: determining a
location of a tissue
lesion using one or more imaging modalities (302), navigating an instrument to
a site such as the
tissue lesion (with and without image guidance) (304), coupling (e.g.,
anchoring) the instrument
to the tissue lesion (306), obtaining access to the tissue site (making an
incision, introduction
through a port/trocar, or direct access via an open procedure) (308),
introducing a tissue resection
device to the tissue site (with and without using the anchor as a guide)
(310), using the tissue
resection device to create a core of tissue (312) or amputating the core of
tissue from the tissue
site, removing the core of tissue from the body (with and without leaving a
cavity "access
sleeve") (314), analyzing the tissue core sample (tissue histology, ROSE, DNA
sequencing, etc.)
(316), diagnosing based on at least the tissue core sample (318), sealing the
tissue cavity (320),
removing some or all instrumentation (322), or closing tissue access points
(324).
[0073] As illustrated in FIG. 4, removing a core of tissue from a tissue site,
subsequent
diagnosis, and therapeutic management of confirmed malignancy may further
comprise one or
more of: determining the location of a tissue lesion using one or more imaging
modalities (402),
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
navigating an instrument to a site such as the tissue lesion (with and without
image guidance)
(404), coupling (e.g., anchoring) the instrument to the tissue lesion (406),
obtaining access to the
tissue site (making an incision, introduction through a port/trocar, or direct
access via an open
procedure) (408), introducing a tissue resection device to the tissue site
(with and without using
the anchor as a guide) (410), using the tissue resection device to create a
core of tissue or
amputating the core of tissue from the tissue site (412), removing the core of
tissue from the
body (with and without leaving a cavity "access sleeve") (416), analyzing the
tissue core sample
(tissue histology, ROSE, DNA sequencing, etc.) (418), performing therapeutic
management of
tissue such as benign or malignant tissue (418), sealing the tissue cavity
(420), removing some or
all instrumentation (422), and closing tissue access points (424).
[0074] The present disclosure relates to methods and systems for coring
tissue.
Methods for coring tissue may comprise disposing a tissue resection device at
a target tissue site,
causing the tissue resection device to resect a core of tissue from the target
tissue site, and
removing the core of tissue from the body. The removing the core of tissue
from the body may
create a core cavity at the target tissue site. The core of tissue may
comprise at least a portion of
a tissue lesion. The resecting the core of tissue from the target tissue site
may comprise
mechanical transection. The resecting the core of tissue from the target
tissue site may comprise
the delivery of radiofrequency energy. The resecting the core of tissue from
the target tissue site
may comprise mechanical compression and the delivery of radiofrequency energy.
The resecting
the core of tissue from the target tissue site may comprise transection with
an energized wire.
The resecting the core of tissue from the target tissue site may comprise one
of more of
mechanical compression, the delivery of radiofrequency energy, the delivery of
microwave
energy, the delivery of ultrasonic energy, or transection with an energized
wire. Other resection
devices and procedures may be used. The resection device may be configured for
one or more of
mechanical compression, the delivery of radiofrequency energy, the delivery of
microwave
energy, the delivery of ultrasonic energy, or transection with an energized
wire.
[0075] The present disclosure relates to methods and systems for coring tissue
and
sealing the core cavity created by removing the tissue core. Such methods may
comprise
disposing a fill material in the core cavity. Methods may comprise applying
pressure to a portion
of the core cavity such as to a wall defining the core cavity. Methods may
comprise ablating a
portion of the core cavity such as a wall defining the core cavity. Methods
may comprise causing
11
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
a cavity closure device, such as suture thread, a stapling device, an
ultrasonic tissue sealing
device, a bipolar radiofrequency sealing device, or any combination thereof to
close the tissue
cavity. Methods may comprise disposing a cavity sealing material, such as a
tissue graft, a
hemostatic patch, a hemostatic agent such as fibrin or thrombin, a biological
adhesive material
such as Dermabond , or any combination thereof to close the tissue cavity.
[0076] Methods may comprise any combination or permutation of: 1) disposing an
anchoring device into a tissue cavity, 2) disposing a tissue access port into
the tissue cavity, 3)
disposing a tissue sealing device into the tissue cavity (with or without a
tissue access port, with
or without guidance from an anchoring device), 4) causing the tissue sealing
device to seal at
least a portion of the tissue cavity, 5) introducing a fill material into the
tissue cavity (with or
without a fill material delivery device, with or without being proceeded by
disposing a tissue
sealing device into the tissue cavity, with or without removing the tissue
sealing device after
sealing at least a portion of the tissue cavity, with or without a tissue
access port), 6) disposing a
cavity sealing material adjacent to the tissue cavity (with or without being
proceeded by
disposing a tissue sealing device into the tissue cavity, with or without
removing the tissue
sealing device after sealing at least a portion of the tissue cavity, with or
without being
proceeded by introducing a fill material into the tissue cavity), 7) disposing
a cavity closure
device adjacent to the tissue, and 8) causing a cavity closure device to close
the tissue cavity
(with or without being proceeded by any combination or permutation of the
above steps). As
described herein, methods may be used to core and/or seal tissue at various
target sites. Although
a lung is used as an illustrative example, it should not be so limiting, as
other target sites may be
punctured or actively cored and may benefit from the disclosed sealing
methods.
Ima2in2 systems
[0077] Various systems, devices, and apparatus may be used to locate a target
site such
as a target tissue site in a human body. For example, imaging systems may be
used such as
computed tomography (CT), ultrasound, magnetic resonance imaging (MRI),
endoscope, visual,
electromagnetic, and/or X-ray.
CT
12
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0078] In conventional X-ray systems, a beam of X-rays is directed through an
object
such as the human body onto a flat X-ray photographic film. The beam of X-rays
is selectively
absorbed by structures within the object, such as bones within the human body.
Since the
exposure of the X-ray film varies directly with the transmission of X-rays
through the body (and
varies inversely with the absorption of X-rays), the image that is produced
provides an accurate
indication of any structures within the object that absorbed the X-rays. As a
result, X-rays have
been widely used for non-invasive examination of the interior of objects and
have been
especially useful in the practice of medicine.
[0079] The image that is formed from the X-ray is basically the shadow of the
structures within the object that absorb the X-rays. As a result, the image
formed on the X-ray is
only two-dimensional, and if multiple X-ray absorbing structures lie in the
same shadow,
information about some of these structures is likely to be obscured. Moreover,
in the case of
medical applications, it is often quite difficult to use conventional X-ray
systems to examine
portions of the body such as the lungs that consist mostly of air when
inflated and do not absorb
X-rays significantly.
[0080] Many of the limitations of conventional X-ray systems may be avoided by
X-
ray computer tomography, which is often referred to as CT. In particular, CT
provides three-
dimensional views and the imaging of structures and features that are unlikely
to be seen very
well in a conventional X-ray.
[0081] A CT scanning equipment typically includes a computer, a large toroidal
structure and a platform that is movable along a longitudinal axis through the
center of the
toroidal structure. Mounted within the toroidal structure are an X-ray source
(not shown) and an
array of X-ray detectors (not shown). The X-ray source is aimed substantially
at the longitudinal
axis and is movable around the interior of the toroidal structure in a plane
that is substantially
perpendicular to the longitudinal axis. The X-ray detectors are mounted all
around the toroidal
structure in substantially the same plane as the X-ray source and are aimed at
the longitudinal
axis. To obtain a CT X-ray image, a patient is placed on the platform and the
platform is inserted
into the center of the toroidal structure. The X-ray source then rotates
around the patient
continuously emitting X-rays and the detectors sense the X-ray radiation that
passes through the
patient. Since the detectors are in the same plane as the X-ray source, the
signals they receive
relate essentially to a slice through the patient's body where the plane of
the X-ray source and
13
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
detectors intersect the body. The signals from the X-ray detectors are then
processed by the
computer to generate an image of this slice known in the art as an axial
section.
[0082] As an example, X-rays may be emitted continuously for the full 360
around the
patient and numerous features are observed but the overall approach is
generally the same.
[0083] While the patient remains motionless, the platform is moved along the
longitudinal axis through the toroidal structure. In the course of this
movement, X-ray exposures
are continuously made of the portion of the patient on which CT is to be
performed. Since the
table is moving during this process, the different X-ray exposures are
exposures of different
slices of the portion of the patient being examined and the images generated
by the computer are
a series of axial sections depicting in three dimensions the portion of the
patient's body that is
being examined. The spacing between adjacent CT sections depends on the
minimum size of the
features to be detected. For detection at the highest resolution, center-to-
center spacing between
adjacent sections should be on the order of less than 2 mm.
[0084] Because of the superior imaging capabilities of CT, the use of CT in
medical
imaging has grown rapidly in the last several years due to the emergence of
multi-slice CT. One
application of medical CT is detection and confirmation of cancer. The
diagnostically superior
information now available in CT axial sections, especially that provided by
multidetector CT
(multiple slices acquired per single rotation of the gantry) where acquisition
speed and
volumetric resolution provide exquisite diagnostic value, however, enables the
detection of
potential cancers at the earliest and most treatable stage. For example, the
minimum detectable
size of a potentially cancerous nodule in an axial section of the lung is
about 2 mm (1/10 of
inch), a size that is potentially treatable and curable if detected.
[0085] Recently, medical professionals have been able to diagnose lung cancer
with the
aid of computed tomography (CT) imaging systems. Radiologists are able to
examine these
series of cross sectional images to diagnose pulmonary nodules. The
radiologists' examinations
also diagnose whether these pulmonary nodules are malignant or benign. If a
radiologist
confirms confidently that a pulmonary nodule is benign, further medical
examination may be
avoided.
[0086] To enable accurate diagnosis of pulmonary nodules that have the size
around the
resolution of the CT scanner, it may be advantageous to combine the CT scan
with a computer-
aided diagnostic (CAD) scheme to assist radiologists.
14
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[0087] A procedure in accordance with the present disclosure may be performed
with
CT guidance. CT is particularly well suited for solid organ interventions.
With CT fluoroscopy,
which shows the motion of organs and devices in real time, the trajectory of a
needle may be
tracked in real time, which allows the physician to make adjustments as
appropriate. This
advantage has made procedures shorter with equivalent or better success rates
than those with
standard intermittent CT imaging.
[0088] A CT scan be used to locate target sites for the anchor. CT scans may
be used to
reconstruct the 3D positioning of the target site with respect to fiducial
markers on the body of
the patient. This reconstructed 3D image of CT slices may be loaded to a
system that helps the
physician navigate the devices of the present disclosure through the patient's
body and/or help
determine the best route for access.
[0089] The devices of the present disclosure may be fitted with an
accelerometer and/or
gyroscope that helps determine the position of the instrument tip in 3D space
at all times. By
enabling communication between such devices of the present disclosure (fitted
with 3D tracking)
and the CT software, the tip of the devices of the present disclosure may be
determined with
respect to the desired target spot. The software may help keep the device on
the planned
trajectory and help achieve optimal outcomes.
[0090] Additionally or alternatively, CT scans may be combined with other
imaging
modalities, such as ultrasound or electromagnetic tracking of the tip, to
facilitate navigation of
the devices of the present disclosure.
[0091] In an aspect of the present disclosure, a patient may be placed in a CT
scanner
and the nodule may be imaged. Using standard CT guided interventional
techniques commonly
used in CT guided biopsy of the lung, an anchor needle may be advanced through
the skin, chest
wall, pleural space and lung and through to the target tissue to be sampled.
Once the distal end of
the anchor needle has passed through the nodule or interstitial abnormality,
anchoring members
comprised of shape memory metal such as Nitinol, are advanced out of the
distal end of the
needle.
Ultrasound
[0092] An ultrasound probe may be used to facilitate detection and/or location
of target
tissue sites. An ultrasound probe consists of a piezoelectric transducer that
generates ultrasonic
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
waves. These ultrasonic waves are reflected differently from various tissues
based on their
mechanical and constitutional properties. The reflected waves are then
acquired through the
receiver and interpreted to translate the properties and location of the
tissue. By tracking the
location of the ultrasound in 3D space, it is possible to generate a 3D map of
the tissue imaged
using ultrasound.
[0093] Alternatively or additionally to providing the location of the specific
target
tissue sites, ultrasound is also capable of distinguishing tissue stiffness.
This is of critical
importance as tumors are known for different mechanical and elastic properties
than their
surrounding tissue. Hence, ultrasound may enable rapid detection and imaging
of the tumor site,
in addition to providing details on its location, size and other physical
properties.
[0094] The ultrasound may also be in a probe format that may be inserted into
the
pleural space, or navigated through the bronchial space. The probe may be in
the form of a
catheter configured to facilitate visualization. Such a catheter may be
rotated continuously to get
a complete 360 ultrasound map as the catheter navigates through the space
(iVUS).
[0095] The tip has a lubricious covering that allows the operator to run the
ultrasound
probe over the surface of the lung until the nodule is localized. Once the
nodule is localized, a
suction apparatus around the perimeter of the ultrasound probe may be actuated
so that the lung
is sucked into the scope/probe, thus securing the area and locking the probe
into place. A needle
may be advanced through the lung (e.g., by an operatory) under ultrasound
guidance to access
the nodule.
MRI/ Magnetic Detection
[0096] MRI or magnetic resonance imaging relies on the use of high flux
electromagnets to oscillate polar molecules and thereby image the localization
of those polar
molecules. The most ubiquitous polar molecule is water present in human
tissue. The water
content of normal tissue is different from tumor tissues. For example, tumors
usually have
elaborate blood supply and drainage, compared to normal tissue. This may be
used to visualize a
target tissue site. Depending on the target tissue properties, a contrast
agent may be added to
enhance the resolution of the imaging technique. The contrast agent may
comprise components
that have a high dipole moment or respond, through motion, emission or
vibration, to changes in
surrounding magnetic fields.
16
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Endoscope
[0097] An endoscope may be used to facilitate visualization of a target tissue
site.
Specifically for the lung, endoscopy may be used within the chest, thereby
precluding the need
for a large thoracotomy incision. Thoracoscopy is the use of a specialized
viewing instrument,
usually a rigid endoscope, introduced through a thoracostomy, or a small hole
placed in between
the ribs. Once the endoscope is placed in the space that surrounds the lung,
known as the pleural
space, additional thoracostomy holes may be made to introduce additional
instruments.
Additional instruments include grasping instruments, cutting instruments,
and/or a cutting
stapler, such as the Ethicon Endosurgery Endo GIA 45 mm stapler. Using the
endoscope and the
other instruments, a "triangulation" technique is utilized where, for example,
the endoscope is
used to view as the grasping instrument is brought in from one direction, and
the stapler is
brought in from another, and tissue is cut with the stapler and removed
through one of the ports.
Visual
[0098] Visual imaging may be done using the following modalities: Laser
Doppler
perfusion imaging (LDPI), Laser speckle contrast imaging (LSCI), Tissue
viability imaging
(TiVi), Photoacoustic Imaging (PAT), Optical coherence tomography (OCT),
Infrared based
imaging, optical camera
[0099] A wide range of visualization techniques may be used for detection and
imaging
of the target tissue site. These techniques employ a certain wavelength range
or combination of
multiple wavelengths to yield deterministic results. Depending on the
wavelength range used by
the source, the penetration depth may vary and therefore, it is possible to
image the target tissue
site non-invasively. The light (radiation source) could be a hand held probe
that is used scan the
patient's body from exterior, similar to an ultrasound probe, for
visualization or detection of the
target tissue site. Alternatively, the light source could be mounted on a
probe and navigated
through the patient's body up to a point close enough to visualize the target
tissue site. Such a
probe could be advanced through the pleural cavity along the trachea and used
to detect or
visualize the target tissue in the lungs.
[00100] These imaging techniques could be combined with other imaging
modalities,
such as ultrasound, electrical detection, etc., to enhance the resolution.
17
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00101] Additionally, external agents may be administered, such as contrast,
nanoparticles, fluorescing agents, etc., to enhance the resolution or
detection capabilities of
visual imaging techniques.
Electromagnetic/Electrical Potential/Impedance
[00102] An electromagnetic probe may be used to visualize the target tissue
site.
[00103] An electromagnetic guided probe may also be used to remotely control
the
navigation to the target tissue site.
[00104] A probe capable of detecting differences in zeta potential changes as
it is
navigated through the tissue may be used for detection and visualization of
the target tissue site.
[00105] Bioimpedance analysis relates to the measurement that an organ or
tissue
responds additional applied current. The bio-impedance parameter that may
record is as
resistance, reactance, phase angle, and it is to determine for the purpose of
blood flow and body
composition (such as, water and fat content).However, there is the physical
evidence of
accumulation, at least the phase angular dimensions of bioimpedance analysis
measures at body
composition, as general health situation index and forecasting tool likely
exceeded its stage
generally used. Phase angle it has been generally acknowledged that, such as,
be cell membrane
integrity and the fluid index in the intra or extracellular spatial
distribution of cellular level.
Ongoing research shows, phase angle also may reflect other biological
attribute.
[00106] Based on Cole-Cole model and Hanai method, a kind of method of bio-
impedance frequency spectrum (BIS) of utilizing has been proposed to be used
in measurement
extracellular liquid volume (ECV) and intracellular fluid body volume
(ICV).Now, multi-
frequency bioimpedance analysis method may provide some information about
extracellular
fluid and intracellular fluid volume in health compartment total or sections.
[00107] The ability of recognizing cancer cells using bioimpedance is well
established
in the biomedical literature. The usual method for measuring bioimpedance is
by introducing a
sample into a special chamber and applying an AC current through it while
recording the voltage
across the sample at each frequency. More modern methods rely on multiple
electrode matrices
which are connected with the human body and measure physiological and
pathological changes.
Some of the methods aim to localize tumor cells inside the human body and to
form an image.
Another technique, based on magnetic' bioimpedance, measures the bioimpedance
by magnetic
18
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
induction. This technique consists of a single coil acting as both an
electromagnetic source and a
receiver operating typically in the frequency range 1-10 MHz. When the coil is
placed in a fixed-
geometric relationship to a conducting body, the alternating electric field in
the coil generates
electrical eddy current. A change in the bioimpedance induces changes in the
eddy current, and
as a result, a change in the magnetic field of those eddy currents. The coil
acts as a receiver to
detect such changes. Experiments with this technique achieved sensitivity of
95%, and
specificity of 69%, distinguishing between 1% metastasis tumor and 20%
metastasis tumor.
Distinguishing between tumor and normal tissue is even better.
X-ray
[00108] X-rays are electromagnetic radiation with high penetration
capabilities.
Differences in elemental properties of tissues will pose differences in
resistance to X-ray
radiation. This property of the target tissue may be used to detect and
visualize the target tissue
site.
[00109] Fiducial markers, comprised of material opaque to X-rays, for example,
lead,
may be placed on the patient's body to aid navigation to the target tissue and
for trajectory
planning.
Nayi2ation systems
[00110] Various systems, devices, and apparatus may be used to navigate
instruments
and/or devices to a target site such as a target tissue site in a human body.
For example,
navigation systems may be used such as Auris, robotic, CT/ultrasound fusion,
electromagnetic
navigation, fluoroscopic, etc.
[00111] A tissue coring system may comprise a tissue resection apparatus
comprising a
helical coil electrode; and a tracking apparatus configured to determine a
position of the helical
coil electrode in three dimensional space. The helical coil electrode may be
configured to deliver
energy to tissue. The helical coil electrode may be configured to determine
electrical properties
of tissue. The tissue resection device may further comprise a first clamping
element comprising
the helical coil electrode, a second clamping element comprising a second
electrode, the second
clamping element being positioned to oppose at least a portion of the first
clamping element, and
a cutting element configured for the transection of tissue. The tracking
apparatus may comprise
19
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
one or more of an X-ray device, a computed tomography device, or a fluoroscopy
device. Other
devices and technologies may be used. The system may further comprise an
anchor configured to
guide movement of the helical coil electrode. The system may further comprise
a non-invasive
anchor configured to guide movement of the helical coil electrode. The system
may further
comprise computing logic configured to control movement of the helical coil
electrode. The
system may further comprise computing logic configured to determine a target
trajectory of the
helical coil electrode. The system may further comprise computing logic
configured to determine
energy dosage provided by the helical coil electrode. The system may further
comprise
computing logic configured to determine energy dosage provided to the helical
coil electrode.
The system may further comprise computing logic configured to receive position
information
indicative of the position of the helical coil electrode and to determine,
based on at least the
position information, deviation from a target route or target trajectory. The
system may further
comprise computing logic configured to receive position information indicative
of the position of
the helical coil electrode and to determine, based on at least the position
information, modulate
an energy supplied to the helical coil electrode. The system may further
comprise computing
logic configured to receive position information indicative of the position of
the helical coil
electrode and to determine, based on at least the position information, a stop
point at which tissue
resection is intended to be implemented.
[00112] A method for navigating a tissue resection apparatus may comprise
disposing a
tissue resection apparatus into the body of a patient, the tissue resection
apparatus comprising a
helical coil electrode; and determining a position of the helical coil
electrode in three
dimensional space. Methods may further comprise controlling movement of the
helical coil
electrode based at least on the determined position of the helical coil
electrode. Methods may
further comprise determining a target trajectory of the helical coil
electrode; and determining
deviation from the target trajectory based at least on the determined position
of the helical coil
electrode. Methods may further comprise determining energy dosage provided by
the helical
coil electrode based at least on the determined position of the helical coil
electrode. Methods
may further comprise determining energy dosage provided to the helical coil
electrode based at
least on the determined position of the helical coil electrode. Methods may
further comprise
comprising determining, based on at least on the determined position of the
helical coil electrode,
a stop point at which tissue resection is intended to be implemented.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00113] The navigation methods and systems could follow the logic as described
in
FIG. 42. As shown, a processing unit 4202 may communicate with a graphical
user interface
(GUI) 4204 to display a current device path 4206 and/or a desired trajectory
4208. The desired
trajectory 4208 may be calculated based on the target location 4210 and an
entry point. Other
inputs may be used. The entry point may be selected based on the patient scan
data 4212 or
selected based on real time device position feedback 4214. Additionally, the
real time device
position feedback 4214 may be used to determine the current path 4206 for
device navigation.
The target location 4210 may be selected through the GUI 4204, or directly
inputted to the
processing unit 4202 and may be based on the patient scan data 4212.
[00114] An example of planned trajectory may be based on an anchor path as
described
herein. An example of real time device position feedback may comprise use of
navigation
systems described herein.
[00115] A surgical instrument system for coring tissue from a target tissue
site may
comprise: a tissue resection device configured for coring tissue, wherein the
device comprises: a
helical coil electrode, and a cutting element configured to cooperate with the
helical coil
electrode for the transection of tissue; a handle assembly configured to
facilitate interaction
between tissue the tissue resection device; and a tracking apparatus
configured to determine a
position of the helical coil electrode in three dimensional space.
Auris
[00116] Auris is a system and tools for endolumenal robotic procedures that
provide
improved ergonomics, usability, and navigation. Endoscopy is a widely-used,
minimally invasive
technique for both imaging and delivering therapeutics to anatomical locations
within the human
body. Typically a flexible endoscope is used to deliver tools to an operative
site inside the
body¨e.g., through small incisions or a natural orifice in the body (nasal,
anal, vaginal, urinary,
throat, etc.)¨where a procedure is performed. Endoscopes may have imaging,
lighting and
steering capabilities at the distal end of a flexible shaft enabling
navigation of non-linear lumens
or pathways.
[00117] Auris typically uses a sheath with a lumen, having a controllable and
articulable distal end, which is mounted to a first robotic arm having at
least 3 DOF, but
preferably 6 or more DOF. This embodiment also includes a flexible endoscope
having a
21
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
controllable and articulable distal end, a light source and video capture unit
at the distal end
thereof, and at least one working channel extending. The flexible endoscope is
slidingly disposed
in the lumen of the sheath, and is mounted to a second robotic arm having at
least 3 DOF, but
preferably 6 or more DOF. Further included are first and second modules,
operatively coupled,
respectfully, to the proximal ends of the sheath and flexible endoscope. The
modules are
mounted to the first and second robotic arms, thereby mounting the sheath and
flexible
endoscope to first and second robotic arms, respectively. The modules provide
the mechanics to
steer and operate the sheath and flexible endoscope, and receive power and
other utilities from
the robotic arms. The robotic arms are positioned such that the first module
is distal to the second
module and the proximal end of the sheath is distal to the proximal end of the
flexible
endoscope. Movement of the first and second robotic arms relative to each
other and relative to
the patient causes movement of the sheath relative to the flexible endoscope
and movement of
either relative to the patient.
Robotic/Electromagnetic navigation
[00118] Robotically-enabled medical systems may be used to perform a variety
of
medical procedures, including both minimally invasive procedures, such as
laparoscopic
procedures, percutaneous and non-invasive procedures, such as endoscopic
procedures.
[00119] Among endoscopic procedures, robotically-enabled medical systems may
be
used to perform bronchoscopy, ureteroscopy, gastroenterology, etc. During such
procedures, a
physician and/or computer system may navigate a medical instrument through a
luminal network
of a patient. The luminal network may include a plurality of branched lumens
(such as in
bronchial or renal networks), or a single lumen (such as a gastrointestinal
tract). The robotically-
enabled medical systems may include navigation systems for guiding (or
assisting with the
guidance of) the medical instrument through the luminal network. This
navigation may be guided
using mechanical means, such as that of Auris, or use of electromagnets.
[00120] Among percutaneous procedures, robotically-enabled medical systems may
be
used to perform minimally invasive surgeries. The methods include advancing a
first alignment
sensor into the cavity through a patient lumen. The first alignment sensor
provides its position
and orientation in free space in real time. The alignment sensor is
manipulated until it is located
in proximity to the object. A percutaneous opening is made in the patient with
a surgical tool,
22
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
where the surgical tool includes a second alignment sensor that provides the
position and
orientation of the surgical tool in free space in real time. The surgical tool
is directed towards the
object using data provided by both the first and the second alignment sensors.
[00121] The alignment sensor may, for example, be an anchor coupled with an EM
sensor which works in conjunction with EM field generators placed around the
patient and an
associated CT (or other) scan to provide position and orientation information
for EM sensor in
the patient's body. The alignment sensor is placed via a cavity, such as the
devices of the present
disclosure, and together with a camera is used to identify the location of the
target tissue site.
The alignment sensor provides a guidance mechanism for directing the
percutaneous cut for
accessing the target tissue site within lungs. Further, as at this point in
the procedure, a scope is
already present, a working channel of the scope may be used to advance other
tools to assist in
the removal of the target tissue through a port created by the access devices
of the present
disclosure.
CT/fluoroscopy and/or combining with ultrasound
[00122] In conventional X-ray systems, a beam of X-rays is directed through an
object
such as the human body onto a flat X-ray photographic film. The beam of X-rays
is selectively
absorbed by structures within the object, such as bones within the human body.
Since the
exposure of the X-ray film varies directly with the transmission of X-rays
through the body (and
varies inversely with the absorption of X-rays), the image that is produced
provides an accurate
indication of any structures within the object that absorbed the X-rays. As a
result, X-rays have
been widely used for non-invasive examination of the interior of objects and
have been
especially useful in the practice of medicine.
[00123] As an illustrative example, the image that is formed from the X-ray is
effectively the shadow of the structures within the object that absorb the X-
rays. As a result, the
image formed on an X-ray is only two-dimensional, and if multiple X-ray
absorbing structures
lie in the same shadow, information about some of these structures is likely
to be obscured.
Moreover, in the case of medical applications, it is often quite difficult to
use conventional X-ray
systems to examine portions of the body such as the lungs that consist mostly
of air when
inflated and do not absorb X-rays significantly.
23
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00124] Many of the limitations of conventional X-ray systems are avoided by X-
ray
computer tomography, which is often referred to as CT. In particular, CT
provides three-
dimensional views and the imaging of structures and features that are unlikely
to be seen very
well in a conventional X-ray.
[00125] Tracking and/or navigation of a resection device of the present
disclosure may
be performed with CT guidance. CT is particularly well suited for solid organ
interventions.
With CT fluoroscopy, which shows the motion of organs and devices in real
time, the trajectory
of a needle may be tracked in real time, which allows the physician to make
adjustments as
appropriate. This advantage has made procedures shorter with equivalent or
better success rates
than those with standard intermittent CT imaging.
[00126] A CT scan, for example, may be used to locate target sites for the
anchor. CT
scans may be used to reconstruct the 3D positioning of the target site with
respect to fiducial
markers on the body of the patient. This reconstructed 3D image of CT slices
may be loaded to a
system that helps the physician navigate a resection device through the
patient's body and/or help
determine the best route for access.
[00127] A resection device may be fitted with an accelerometer and/or
gyroscope that
helps determine the position of the instrument tip in 3D space at all times.
By enabling
communication between such a resection device (fitted with 3D tracking) and
the CT software, a
tip (e.g., coil electrode) of the resection device may be determined with
respect to the desired
target spot. Computing logic such as software (e.g., CT software) may be used
keep the device
on the planned trajectory and help achieve optimal outcomes.
[00128] Additionally or alternatively, CT scans may be combined with other
imaging
modalities, such as ultrasound or electromagnetic tracking of the tip, to
facilitate navigation of
the resection device. Other technologies may be used alone or in combination.
[00129] As a further example, an ultrasound probe may be used to facilitate
detection
and/or location of target tissue sites. An ultrasound probe may comprise a
piezoelectric
transducer that generates ultrasonic waves. These ultrasonic waves are
reflected differently from
various tissues based on their mechanical and constitutional properties. The
reflected waves are
then acquired through the receiver and interpreted to translate the properties
and location of the
tissue. By tracking the location of the ultrasound in 3D space, it is possible
to generate a 3D map
of the tissue imaged using ultrasound.
24
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00130] Alternatively, or in addition to providing the location of the
specific target
tissue sites, ultrasound is also capable of distinguishing tissue stiffness.
This may be important,
as tumors are known for different mechanical and elastic properties than their
surrounding tissue.
Hence, ultrasound may enable rapid detection and imaging of the tumor site, in
addition to
providing details on its location, size and other physical properties.
[00131] Systems and methods are described for navigating a probe to a location
within
a body of a patient. The probe may comprise a needle, introducer, catheter,
stylet, or sheath.
Other probes may be used. Methods may comprise visualizing a three-dimensional
image of a
region of a body of a patient. As an example, the three-dimensional image of a
region of a body
of a patient may be based on one or more of magnetic resonance imaging (MRI),
computer
tomography (CT), or ultrasound. Other imaging techniques may be used. Methods
may comprise
receiving a selection of a target location within said three-dimensional image
of a region of a
patient's body. As an example, the receiving a selection of a target location
may be via
interaction with a display device configured to output one or more of the
visualizing steps. Other
inputs may be used to effect selection. Methods may comprise determining and
visualizing a
preferred pathway for the probe to follow from an external entry point on the
patient's body to
the target location. The preferred pathway may be determined by transforming a
selected point in
a two-dimensional view of the three-dimensional image of a region of a body of
a patient into a
line (e.g., line of sight) through the three-dimensional image of a region of
a body of a patient.
Methods may further comprise calibrating the preferred pathway to compensate
for shift of
anatomical structures pre-operatively. Alternatively or additionally, methods
may further
comprise calibrating the preferred pathway to compensate for shift of
anatomical structures intra-
operatively. Methods may comprise registering the three-dimensional image to
the current actual
position of the corresponding region of the patient's body. Methods may
comprise registering the
current actual position of the probe to the three-dimensional image and the
current actual
position of the patient's body. Methods may further comprise updating the
registration of the
three-dimensional image to the patient to compensate for shift of anatomical
structures. Methods
may comprise visualizing the preferred pathway for the probe simultaneously
with an indication
of the current actual position of the probe in real time such that the
simultaneous visualizations
enables a user to align the current actual position of the probe with the
preferred pathway. As an
example, the indication of the current actual position of the probe may
comprise the position of
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
the probe in three-dimensional space. As a further example, the indication of
the current actual
position of the probe may comprise the projected extension of the probe in
three-dimensional
space. Methods may comprise updating and visualizing an indication of the
current actual
position of the probe in real time as the probe is advanced to the target
location. Additionally,
output of an auditory or visual feedback may be used to warn the user about
information
regarding proximity to the target location and/or to warn the user about
information regarding
proximity to critical anatomical structures.
[00132] The procedures of the present disclosure may be performed with CT
guidance.
CT is particularly well suited for solid organ interventions. With CT
fluoroscopy, which shows
the motion of organs and devices in real time, the trajectory of a needle may
be tracked in real-
time, which allows the physician to make adjustments as appropriate. This
advantage has made
procedures shorter with equivalent or better success rates than those with
standard intermittent
CT imaging.
[00133] A CT scan be used to locate target sites for the anchor. CT scans may
be used
to reconstruct the 3D positioning of the target site with respect to fiducial
markers on the body of
the patient. This reconstructed 3D image of CT slices may be loaded to a
system that helps the
physician navigate devices of the present disclosure through the patient's
body and/or help
determine the best route for access.
[00134] The devices of the present disclosure may be fitted with an
accelerometer
and/or gyroscope that helps determine the position of the instrument tip in 3D
space at all times.
By enabling communication between such as devices of the present disclosure
(fitted with 3D
tracking) and the CT software, the tip of the devices of the present
disclosure may be determined
with respect to the desired target spot. The software may help keep the device
on the planned
trajectory and help achieve optimal outcomes.
[00135] Additionally, CT scans may be combined with other imaging modalities,
such
as ultrasound or electromagnetic tracking of the tip, to facilitate navigation
of the devices of the
present disclosure.
[00136] In an embodiment of the present invention, a patient may be placed in
a CT
scanner and the nodule may be imaged. Using standard CT guided interventional
techniques
commonly used in CT guided biopsy of the lung, an anchor needle may be
advanced through the
skin, chest wall, pleural space and lung and through to the target tissue to
be sampled. Once the
26
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
distal end of the anchor needle has passed through the nodule or interstitial
abnormality,
anchoring members comprised of shape memory metal such as Nitinol, may be
advanced out of
the distal end of the needle.
Fluoroscopic
[00137] Fluoroscopy uses lower doses of radiation, similar to a CT scanner, to
minimize negative effects to the patient.
Magnetic resonance imaging or Radiofrequency based navigation
[00138] Magnetic resonance imaging (MRI) methods may utilize the interaction
between magnetic fields and nuclear spins in order to form two-dimensional or
three-
dimensional images are widely used, notably in the field of medical
diagnostics, because for the
imaging of soft tissue they are superior to other imaging methods in many
respects, do not
require ionizing radiation and are usually not invasive.
[00139] For example, during MRI, the body of the patient to be examined is
arranged
in a strong, uniform magnetic field BO whose direction at the same time
defines an axis
(normally the z-axis) of the co-ordinate system to which the measurement is
related. The
magnetic field BO causes different energy levels for the individual nuclear
spins in dependence
on the magnetic field strength which may be excited (spin resonance) by
application of an
electromagnetic alternating field (RF field) of defined frequency (so-called
Larmor frequency, or
MR frequency). From a macroscopic point of view the distribution of the
individual nuclear
spins produces an overall magnetization which may be deflected out of the
state of equilibrium
by application of an electromagnetic pulse of appropriate frequency (RF pulse)
while the
corresponding magnetic field B1 of this RF pulse extends perpendicular to the
z-axis, so that the
magnetization performs a precession motion about the z-axis. The precession
motion describes a
surface of a cone whose angle of aperture is referred to as flip angle. The
magnitude of the flip
angle is dependent on the strength and the duration of the applied
electromagnetic pulse. In the
example of a so-called 90 pulse, the magnetization is deflected from the z
axis to the transverse
plane (flip angle 90 ).
[00140] After termination of the RF pulse, the magnetization relaxes back to
the
original state of equilibrium, in which the magnetization in the z direction
is built up again with a
27
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
first time constant Ti (spin lattice or longitudinal relaxation time), and the
magnetization in the
direction perpendicular to the z-direction relaxes with a second and shorter
time constant T2
(spin-spin or transverse relaxation time). The transverse magnetization and
its variation may be
detected by means of receiving RF antennae (coil arrays) which are arranged
and orientated
within an examination volume of the magnetic resonance examination system in
such a manner
that the variation of the magnetization is measured in the direction
perpendicular to the z-axis.
The decay of the transverse magnetization is accompanied by dephasing taking
place after RF
excitation caused by local magnetic field inhomogeneities facilitating a
transition from an
ordered state with the same signal phase to a state in which all phase angles
are uniformly
distributed. The dephasing may be compensated by means of a refocusing RF
pulse (for example
a 180 pulse). This produces an echo signal (spin echo) in the receiving
coils.
[00141] In order to realize spatial resolution in the subject being imaged,
such as a
patient to be examined, constant magnetic field gradients extending along the
three main axes are
superposed on the uniform magnetic field BO, leading to a linear spatial
dependency of the spin
resonance frequency. The signal picked up in the receiving antennae (coil
arrays) then contains
components of different frequencies which may be associated with different
locations in the
body. The signal data obtained via the receiving coils correspond to the
spatial frequency domain
of the wave-vectors of the magnetic resonance signal and are called k-space
data. The k-space
data usually include multiple lines acquired of different phase encoding. Each
line is digitized by
collecting a number of samples. A set of k-space data is converted to an MR
image by means of
Fourier transformation.
[00142] The transverse magnetization dephases also in presence of constant
magnetic
field gradients. This process may be reversed, similar to the formation of RF
induced (spin)
echoes, by appropriate gradient reversal forming a so-called gradient echo.
However, in case of a
gradient echo, effects of main field inhomogeneities, chemical shift and other
off-resonances
effects are not refocused, in contrast to the RF refocused (spin) echo.
[00143] In order to increase spatial resolution, certain elements (nuclei) may
be used
that provide higher contrast when excited by the magnetic field gradients.
Real time visualization
of the anchor or resection device may be achieved if the body of the
respective device is
comprised of such materials.
28
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Visual
[00144] Visual imaging may be implemented using at least the following example
modalities: Laser Doppler perfusion imaging (LDPI), Laser speckle contrast
imaging (LSCI),
Tissue viability imaging (TiVi), Photoacoustic Imaging (PAD, Optical coherence
tomography
(OCT), Infrared based imaging, and/or optical camera.
[00145] A wide range of visualization techniques may be used for detection and
imaging of the target tissue site. These techniques employ a certain
wavelength range or
combination of multiple wavelengths to yield deterministic results. Depending
on the wavelength
range used by the source, the penetration depth may vary and therefore, it is
possible to image
the target tissue site non-invasively. The light (radiation source) could be a
hand held probe that
is used scan the patient's body from exterior, similar to an ultrasound probe,
for visualization or
detection of the target tissue site. Alternatively, the light source could be
mounted on a probe and
navigated through the patient's body up to a point close enough to visualize
the target tissue site.
Such a probe could be advanced through the pleural cavity along the trachea
and used to detect
or visualize the target tissue in the lungs.
[00146] These imaging techniques could be combined with other imaging
modalities,
such as ultrasound, electrical detection, etc., to enhance the resolution.
[00147] Additionally or alternatively, external agents may be administered,
such as
contrast, nanoparticles, fluorescing agents, etc., to enhance the resolution
or detection
capabilities of visual imaging techniques.
[00148] An example of use of an optical camera would be use of an endoscope.
An
endoscope may be used to facilitate visualization of a target tissue site.
Specifically for the lung,
endoscopy may be used within the chest, thereby precluding the need for a
large thoracotomy
incision. Thoracoscopy is the use of a specialized viewing instrument, usually
a rigid endoscope,
introduced through a thoracostomy, or a small hole placed in between the ribs.
Once the
endoscope is placed in the space that surrounds the lung, known as the pleural
space, additional
thoracostomy holes may be made to introduce additional instruments. Additional
instruments
include grasping instruments, cutting instruments, and/or a cutting stapler,
such as the Ethicon
Endosurgery Endo GIA 45 mm stapler. Using the endoscope and the other
instruments, a
"triangulation" technique is utilized where, for example, the endoscope is
used to view as the
29
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
grasping instrument is brought in from one direction, and the stapler is
brought in from another,
and tissue is cut with the stapler and removed through one of the ports.
Anchorin2
[00149] Various anchor devices may be used. A needle may be anchored to guide
the
coring device. Non-invasive anchoring may be used. For example, a needle may
be advanced to
the desired target site via the use of a real time or virtual image guided
procedure. The advancing
process may be carried out by a person's hands directly, by a person manually
using a robotic
arm, or autonomously robotically guided per a digital 2D or 3D image. Once the
desired position
has been achieved, Nitinol fingers may be engaged into the target tissue.
Anchored needle to guide one or more devices
[00150] Many medical procedures are undertaken through small tracts formed
within a
patient's tissue. These procedures are minimally invasive. In order to form
the tract running from
outside of the patient to a target within the patient, an anchor typically is
inserted in the initial
stages of a procedure. Such an example anchor may run from the surface of the
patient's skin to
the target. As a further example, later in the procedure, this initial
insertion may be enlarged to
accommodate other medical devices necessary for the procedure.
[00151] Additionally, localized applications, such as biopsies, thermoablation
or
localized injection of therapeutic substances are currently performed in
combination with
imaging means, such as ultrasound, X-ray, fluoroscopy, CT scan, MIZI, visual,
optical, etc. As an
example, in the case of use of an X-ray emitting device, X-ray energy passes
through the
patient's body and differentially impinges on a fluoroscope screen, exciting
fluorescent material,
such as calcium tungstate, to create a screen display of the body and anchor.
The anchor is
visualized on the fluoroscope as it enters the patient on the display of the
medical device. This
anchor may appear on the screen because it does not allow the energy to pass
through it (i.e., it
may be opaque).
[00152] These imaging modalities allow visualization of the anchor and the
target
region to safely orient and move the target to the target point. Furthermore,
these visualization
modalities also allow proper positioning and/or repositioning of the anchor.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Non-invasive anchoring
[00153] In certain cases, there may be no need for coring the tissue to access
the target
site. Examples of such cases may include growing cancer where the access has
been established
through a prior biopsy procedure, or a superficial target location. In such
cases, it may be
possible to have a non-invasive anchor that helps guide the device along the
desired trajectory.
[00154] A non-invasive anchor may sit on the patient's body surface, such as
skin, and
could provide guidance for a device. The device could be inserted through the
non-invasive
anchor or from a position adjacent to the anchor. Guidance for the device
being navigated to the
target site could be provided either through the means of a mechanical guide
(cannula), above
listed imaging and navigation technologies, sensors mounted on the device or
the anchor itself,
or a combination of these.
[00155] In an aspect, an anchor may comprise a ring placed on the patients'
body
surface, embedded with sensors. As the resection device is inserted through
the ring, the sensors
on the anchor tracks the resection device position in 3D space. As an example,
the sensors on
anchor may interact with sensors on the resection device to improve accuracy
or resolution.
Some examples of sensors include, but are not limited to, electromagnetic,
photodiode, optical,
IR, magnetic, FET, eddy current sensors.
[00156] In an aspect, an anchor may comprise hard stops that limit the freedom
of
motion for the resection device. Once the anchor has been deployed, the anchor
may act as a
guide for insertion and advancement of the resection device. The advancement
of the resection
device may not require any imaging or monitoring and may rely on the hard
stops of the anchor
to advance and position the device at the target location and position. As a
further example, the
anchor may have sensors disposed along the body of the anchor. Once the anchor
has been
deployed, the sensors may monitor and provide feedback on the advancement and
position of the
resection device. Although reference is made to the resection device, other
devices such as
sealing devices and fluid delivery devices may be used in the same or similar
manner.
[00157] Various processes and mechanism may be used to navigate an anchor to a
target location (such as a tissue location where coring may be desired). As an
example, an anchor
may be disposed at a target lesion using CT technology similar to a guided
needle biopsy. Other
systems such as imaging system may be used, for example ultrasound, X-ray or
the like.
31
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00158] As a further example, a larger sheath needle may be disposed at a
target
location using CT technology similar to a guided needle biopsy until a distil
tip of the sheath
needle touches or is adjacent a target lesion. An anchor may then be inserted
through the sheath
needle to be placed at the target location (e.g., lesion). The sheath needle
may then be removed.
Other systems such as imaging system may be used, for example ultrasound, X-
ray or the like.
[00159] As a further example, a position sensor may be disposed at or adjacent
the tip
of the anchor and configured to scan the target location (e.g., lung) to
generate a 3D position of
the target location (e.g., lesion). The anchor may be guided to the target
lesion based on the
sensor position.
[00160] As a further example, a position sensor may be disposed on a larger
sheath
needle and configured to scan the target location (e.g., lung) to generate a
3D position of the
target location (e.g., lesion). The sheath needle may be guided until the
distal tip touches the
target location (e.g., lesion). The anchor may then be inserted through the
sheath needle to be
placed at the target lesion. The sheath needle may then be removed.
[00161] As a further example, a position sensor may be disposed at an end of
an anchor
and use of Auris or other comparable system to place a position sensor at a
target lesion through
the airway. The anchor may be guided to the lesion based on locations of the
two sensors.
[00162] As a further example, a position sensor may be disposed at the tip of
a larger
sheath needle and use of Auris or other comparable system to place a position
sensor at a target
lesion through the airway. The sheath needle may be guided until the distal
tip touches the lesion
based on the locations of the two sensors. The anchor may then be inserted
through the sheath
needle to be placed at the target lesion. The sheath needle may then be
removed.
Tissue site access
[00163] Various systems, devices, and apparatus may be used to provide or
support
access to a target site such as a target tissue site in a human body. For
example, chest wall
incision blades, deployable access ports, tissue dilation, trocar, and/or open
incisions may be
used.
Chest wall incision blades
32
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00164] Once the anchor is placed and deployed at the target location, to
access the
chest cavity through the chest wall without causing puncture to the lung,
there is a need to break
the vacuum of the intrapleural space. The chest wall incision blade may be
designed with an
open channel next to the center hole, which allows the blade to be advanced
and cut through
chest wall tissue along the anchor. The open channel may be used to allow air
to be introduced
into the pleural space when the first layer of the pleural space is
penetrated. The intrapleural
vacuum may be lost, and thus the lung may be dropped away to minimize the
potential of
damaging to the lung pleura.
[00165] FIG. 5 illustrates a blade 500 with an open channel 502. The open
channel 502
may be an air channel and may be connected to the sharp distal tip 504 of the
blade at a distal
end 506 to allow air to continuously flow to the distal tip 504 of the blade
500 (see, e.g., FIG. 6).
FIG. 6 illustrates a distal tip 504 of the blade of FIG. 5. The proximal end
508 of the open
channel 502 may be connected to a rigid or flexible tube 510. Air may enter
the open channel
502 by ambient pressure or by a higher pressurized air (see, e.g., FIG. 7).
FIG. 7 illustrates a
proximal end 508 of an open channel 502 connected to a flexible or rigid tube
510.
Cavity access sleeve
[00166] Post coring and amputation of the target tissue, prior to removing the
coring
device with the target tissue inside, a cavity access sleeve may be placed on
the outside diameter
of the coring device shaft to maintain access to the location where the target
tissue was removed
from. Re-access to the location may be desirable for post coring treatment,
such as adding a
marking device of the tissue location for subsequent surgery, cavity seal,
cavity ablation,
delivery of drug or local chemotherapy. Without placing a cavity access sleeve
prior to removing
the coring device, re-access to the removed target tissue location could be
difficult in an organ
that has large movement, such as the lung.
Tissue dilation
[00167] After the anchor is deployed at a target tissue location of an organ,
such as a
target lesion in a human lung, to spare the healthy tissue between the organ
surface and the target
tissue from being removed, the tissue may be dilated to allow subsequent
insertion of the coring
device to remove the target tissue only. The dilation may be achieved as
follows:
33
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00168] Rigid rods with center holes may be advanced over the anchor until the
distal
ends of the rods reach the target tissue. The rigid rods may have a diameter
increasing from
small to larger diameters.
[00169] An expandable rod may be advanced over the anchor until the distal end
of the
expendable rod reaches the target tissue. At this point, the distal end of the
rod may be expanded
to a desired diameter.
[00170] A balloon catheter in its collapsed state may be advanced over the
anchor.
Once the distal end of the balloon catheter reaches the target site, the
balloon may be expanded
to dilate the tissue. The balloon may have a similar shape as an angioplasty
balloon, or it may be
configured to have square corners at the distal end. Also, the body of the
balloon may have
features, such as a corrugated balloon, to minimize tissue slippage along the
balloon as the
balloon is inflated.
Trocar
[00171] Access to a target tissue site may be achieved via a trocar. Example
trocars
800, 900, 1000 are shown in FIGS. 8-10. Trocars may comprise a trocar channel
(e.g., trocar
channel 802 of FIG. 8B and/or trocar channel 902 of FIG. 9B). Trocar channel
may be used to
allow air to be introduced into the pleural space when the first layer of the
pleural space is
penetrated. The intrapleural vacuum may be lost, and thus the lung may be
dropped away to
minimize the potential of damaging to the lung pleura. Once a lesion has been
successfully
located, an anchoring device may be used to stabilize the target tissue
lesion. The tissue coring
device may also be introduced directly to the location of the target lesion
using a trocar or under
direct visualization with or without a guide anchor and perform the tissue
resection.
Open incision
[00172] Access to a target tissue site may be achieved via an open incision.
Specifically
for the lung, a thoracotomy may be performed and consists of creating a 300 to
450mm (12 to 18
inches) incision on the chest wall followed by division or dissection of the
major back muscles to
move them out of the way, partial removal of the rib, and the placement of a
rib spreader to
provide intra thoracic access to the operating surgeon. The advantage of a
thoracotomy is that the
surgeon has excellent access to the intrathoracic structures, and may see and
manually feel the
34
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
lung and other structures directly. Once a lesion has been successfully
located, an anchoring
device (such as the above) may be used to stabilize the target tissue lesion.
The tissue coring
device may also be introduced directly to the location of the target lesion
using an endoscope or
under direct visualization with or without a guide anchor and perform the
tissue resection.
Tissue coring
[00173] Various methods, devices, and systems may be used to core or remove
tissue.
[00174] A method for removing a tissue lesion may comprise introducing a
tissue
resection device to a target tissue site, causing the tissue resection device
to resect a core of
tissue from the target tissue site, and removing the core of tissue from the
body. The core of
tissue may comprise at least a portion of a tissue lesion. A method may
further comprise creating
a core cavity at the target tissue site. A method may further comprise
inserting a sleeve into the
core cavity. A method may further comprise delivering radiofrequency energy
through the core
cavity. A method may further comprise delivering chemotherapy through the core
cavity. A
method may further comprise delivering microwave radiation through the core
cavity. A method
may further comprise delivering thermal energy through the core cavity. A
method may further
comprise delivering ultrasonic energy through the core cavity. The tissue
resection device may
be configured for the delivery of radiofrequency energy. The tissue resection
device may be
configured for mechanical transection. The tissue resection device may
comprise mechanical
compression and the delivery of radiofrequency energy. A method may further
comprise
amputating the core of tissue from the target tissue site. As an example, the
means for
amputation of the core of tissue may comprise mechanical transection. As a
further example, the
means for amputation of the core of tissue may comprise the delivery of
radiofrequency energy.
The means for amputation of the core of tissue may comprise mechanical
compression and the
delivery of radiofrequency energy. The means for amputation of the core of
tissue may comprise
transection with an energized wire. Other devices may be used.
[00175] A method for removing a core of tissue may comprise introducing a
tissue
resection device to a target tissue site, causing the tissue resection device
to resect a core of
tissue from the target tissue site, and removing the core of tissue from the
body. A method may
further comprise creating a core cavity at the target tissue site. A method
may further comprise
inserting a sleeve into the core cavity. A method may further comprise
delivering radiofrequency
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
energy through the core cavity. A method may further comprise delivering
chemotherapy
through the core cavity. A method may further comprise delivering microwave
radiation through
the core cavity. A method may further comprise delivering thermal energy
through the core
cavity. A method may further comprise delivering ultrasonic energy through the
core cavity. The
tissue resection device may be configured for the delivery of radiofrequency
energy. The tissue
resection device may be configured for mechanical transection. The tissue
resection device may
be configured for mechanical compression and the delivery of radiofrequency
energy. A method
may further comprise amputating the core of tissue from the target tissue
site. The means for
amputation of the core of tissue may comprise mechanical transection. The
means for amputation
of the core of tissue may comprise the delivery of radiofrequency energy. The
means for
amputation of the core of tissue may comprise mechanical compression and the
delivery of
radiofrequency energy. The means for amputation of the core of tissue may
comprise transection
with an energized wire.
[00176] A method for removing a core of tissue may comprise introducing a
tissue
resection device to a target tissue site. The tissue resection device may
comprise one or more of:
a first clamping element comprising a helical coil and a first electrode, or a
second clamping
element comprising a second electrode. Where a second clamping element is
included, the
second clamping element may be positioned to oppose at least a portion of the
first clamping
element. The method may further comprise causing the tissue resection device
to resect a core of
tissue from the target tissue site and removing the core of tissue from the
body. A method may
further comprise creating a core cavity at the target tissue site. A method
may further comprise
inserting a sleeve into the core cavity. A method may further comprise
delivering radiofrequency
energy through the core cavity. A method may further comprise delivering
chemotherapy
through the core cavity. A method may further comprise delivering microwave
radiation through
the core cavity. A method may further comprise delivering thermal energy
through the core
cavity. A method may further comprise delivering ultrasonic energy through the
core cavity. The
tissue resection device may be configured for resecting the core of tissue
comprises the delivery
of radiofrequency energy. The tissue resection device may be configured for
resecting the core of
tissue comprises mechanical transection. The tissue resection device may be
configured for
resecting the core of tissue comprises mechanical compression and the delivery
of
radiofrequency energy. A method may further comprise amputating the core of
tissue from the
36
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
target tissue site. The means for amputation of the core of tissue may
comprise mechanical
transection. The means for amputation of the core of tissue may comprise the
delivery of
radiofrequency energy. The means for amputation of the core of tissue may
comprise mechanical
compression and the delivery of radiofrequency energy. The means for
amputation of the core of
tissue may comprise transection with an energized wire.
[00177] A method for sealing biological fluid vessels may comprise piercing a
target
tissue site containing a least a portion of at least one target biological
fluid vessel with a helical
tissue sealing mechanism. The helical tissue sealing mechanism may comprise a
helical piercing
element and a clamping element. The method may comprise causing the helical
tissue sealing
mechanism to apply mechanical compression to at least one target biological
fluid vessel and
delivering energy to seal at least one target biological fluid vessel. The
helical piercing element
may comprise the clamping element. The mechanical compression may be applied
between the
helical piercing element and the clamping element. A method may further
comprise a second
clamping element. The mechanical compression may be applied between the first
and second
clamping elements. The delivered energy may comprise monopolar radiofrequency
energy. The
delivered energy may comprise bipolar radiofrequency energy. The delivered
energy may
comprise thermal energy. The delivered energy may comprise ultrasonic energy.
[00178] A method for sealing biological fluid vessels may comprise piercing a
target
tissue site with a helical piercing element, adjusting the pitch of the
helical piercing element to
apply mechanical compression to the target tissue, and delivering energy to
seal at least one
biological fluid vessel in the target tissue. The helical piercing element may
comprise a plurality
of tissue sealing electrodes. The delivered energy may comprise monopolar
radiofrequency
energy. The delivered energy may comprise bipolar radiofrequency energy. The
delivered energy
may comprise thermal energy. The delivered energy may comprise ultrasonic
energy.
[00179] A tissue resection apparatus may comprise a first clamping element
comprising
a helical coil, a second clamping element, the second clamping element being
positioned to
oppose at least a portion of the first clamping element, a first and second
electrode configured for
the delivery of radiofrequency energy for sealing tissue, and a cutting
element configured for the
transection of at least a portion of the sealed tissue. A tissue resection
device may further
comprise: a first actuator operable to actuate the first or second clamping
element to apply
mechanical compression to tissue and a second actuator operable to actuate the
cutting element
37
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
to transect tissue. The helical coil may include first and second contiguous
coil segments. The
first coil segment may comprise a generally planar open ring. The first coil
segment may be
helical and may have a pitch of zero. The second coil segment may be helical
and may have a
non-zero pitch. The second coil segment may have a variable pitch. The first
coil segment may
be helical and may have a first pitch and the second coil segment may be
helical and may have a
second pitch, and at least one of the first and second pitches may be
variable. The first electrode
may be comprised of at least a portion of the first clamping element. The
second electrode may
be comprised of at least a portion of the second clamping element. The helical
coil may comprise
a blunt tip. The first and second electrodes may comprise surface profiles
that are matching or
substantially matching. At least a portion of the cutting element may comprise
a sharpened edge.
The cutting element may comprise at least one electrode configured for the
delivery of
radiofrequency energy. The cutting element may comprise an ultrasonic blade.
The tissue
resection device may further comprise a second cutting element configured for
the amputation
the core of tissue from the target tissue site. At least a portion of the
second cutting element may
comprise a sharpened edge. The second cutting element may comprise at least
one electrode
configured for the delivery of radiofrequency energy. The second cutting
element may comprise
an energized wire. The second cutting element may comprises a suture. The
tissue resection
device may further comprise an actuator operable to actuate the second cutting
element to
transect tissue.
[00180] A tissue resection apparatus may comprise a first clamping element
having a
helical coil disposed on a distal end, a second clamping element, the second
clamping element
being positioned to oppose at least a portion of the first clamping element, a
first and second
electrode configured for the delivery of radiofrequency energy for sealing
tissue, and a cutting
element configured for the transection of at least a portion of the sealed
tissue. The tissue
resection device may further comprise a first actuator operable to actuate the
first or second
clamping element to apply mechanical compression to tissue and a second
actuator operable to
actuate the cutting element to transect tissue. The helical coil may comprise
first and second
contiguous coil segments. The first coil segment may comprise a generally
planar open ring. The
first coil segment may be helical and may have a pitch of zero. The second
coil segment may be
helical and may have a non-zero pitch. The second coil segment may have a
variable pitch. The
first coil segment may be helical and may have a first pitch and the second
coil segment may be
38
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
helical and may have a second pitch, and at least one of the first and second
pitches may be
variable. The first electrode may be comprised of at least a portion of the
helical coil. The first
electrode may be comprised of at least a portion of the first clamping
element. The second
electrode may be comprised of at least a portion of the second clamping
element. The helical coil
may comprise a blunt tip. The first and second electrodes may comprise surface
profiles that are
matching or substantially matching. At least a portion of the cutting element
may comprise a
sharpened edge. The cutting element may comprise at least one electrode
configured for the
delivery of radiofrequency energy. The cutting element may comprise an
ultrasonic blade. The
tissue resection device may further comprise a second cutting element
configured for the
amputation the core of tissue from the target tissue site. At least a portion
of the second cutting
element may comprise a sharpened edge. The second cutting element may comprise
at least one
electrode configured for the delivery of radiofrequency energy. The second
cutting element may
comprise an energized wire. The second cutting element may comprise a suture.
The tissue
resection device may further comprise an actuator operable to actuate the
second cutting element
to transect tissue.
[00181] A tissue resection apparatus may comprise a first clamping element
comprising
a helical coil and a first electrode, and a second clamping element comprising
a second electrode,
the second clamping element being positioned to oppose at least a portion of
the first clamping
element. The first and second clamping elements may be configured for: (a) the
delivery of
radiofrequency energy for sealing tissue, and (b) the application of
mechanical compression for
the transection of tissue. The tissue resection device may further comprise a
first actuator
operable to actuate the first or second clamping element to apply mechanical
compression to
tissue and a second actuator operable to actuate the cutting element to
transect tissue. The helical
coil may comprise first and second contiguous coil segments. The first coil
segment may
comprise a generally planar open ring. The first coil segment may be helical
and may have a
pitch of zero. The second coil segment may be helical and may have a non-zero
pitch. The
second coil segment may have a variable pitch. The first coil segment may be
helical and may
have a first pitch and the second coil segment may be helical and may have a
second pitch, and at
least one of the first and second pitches may be variable. The first electrode
may be comprised
by at least a portion of the helical coil. The first electrode may be
comprised of at least a portion
of the first clamping element. The second electrode may be comprised of at
least a portion of the
39
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
second clamping element. The helical coil may comprise a blunt tip. The first
and second
electrodes may comprise surface profiles that are matching or substantially
matching. At least a
portion of the cutting element may comprise a sharpened edge. The cutting
element may
comprise at least one electrode configured for the delivery of radiofrequency
energy. The cutting
element may comprise an ultrasonic blade. The tissue resection device may
further comprise a
second cutting element configured for the amputation the core of tissue from
the target tissue
site. At least a portion of the second cutting element may comprise a
sharpened edge. The second
cutting element may comprise at least one electrode configured for the
delivery of
radiofrequency energy. The second cutting element may comprise an energized
wire. The second
cutting element may comprise a suture. The tissue resection device may further
comprise an
actuator operable to actuate the second cutting element to transect tissue.
[00182] A surgical instrument system for the resection of tissue may comprise
an end
effector operable to cut and seal tissue, wherein the end effector and a
generator configured to
provide power to the end effector having the first and second electrodes for
sealing tissue. The
end effector may comprise a first clamping element comprising a helical coil,
a second clamping
element, the second clamping element being positioned to oppose at least a
portion of the first
clamping element, a first and second electrode configured for the delivery of
radiofrequency
energy for sealing tissue, and a cutting element configured for the
transection of at least a portion
of the sealed tissue. The surgical instrument system may further comprise a
controller in
communication with the generator, wherein the controller is configured to
control the generator
to provide radiofrequency energy sufficient to seal tissue to the first and
second electrodes of the
end effector, based on at least one sensed operating condition of the end
effector. The controller
may be configured to sense the presence of tissue at the end effector. The
controller may be
configured to sense the presence of tissue at the end effector based on a
measured impedance
level associated with the first and second electrodes. The controller may be
configured to sense
an amount of force applied to at least one of the first or second clamping
elements to detect the
presence of tissue at the end effector. The controller may be configured to
sense the position of
the cutting element relative to at least one of the first or second clamping
elements. The
controller may be configured to control the generator to provide
radiofrequency energy at the end
effector when the second actuator is actuated and no tissue is sensed at the
end effector. The
controller may be configured to control the generator to provide a continuous
amount of
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
radiofrequency energy. The controller may be configured to control the
generator to
automatically provide an increase or decrease in the amount of radiofrequency
energy. The
system may further comprise a first actuator operable to actuate the first or
second clamping
element to apply mechanical compression to tissue, and a second actuator
operable to actuate the
cutting element to transect tissue. The helical coil may comprise first and
second contiguous coil
segments, the first coil segment including the first electrode. The first coil
segment may
comprise a generally planar open ring. The first coil segment may be helical
and may have a
pitch of zero. The second coil segment may be helical and may have a non-zero
pitch. The
second coil segment may have a variable pitch. The first coil segment may be
helical and may
have a first pitch and the second coil segment may be helical and may have a
second pitch, and at
least one of the first and second pitches may be variable. The first electrode
may be comprised of
at least a portion of the helical coil. The first electrode may be comprised
of at least a portion of
the first clamping element. The second electrode may be comprised of at least
a portion of the
second clamping element. The helical coil may comprise a blunt tip. The first
and second
electrodes may comprise surface profiles that are matching or substantially
matching. At least a
portion of the cutting element may comprise a sharpened edge. The cutting
element may
comprise at least one electrode configured for the delivery of radiofrequency
energy. The cutting
element may comprise an ultrasonic blade. The tissue resection device may
further comprise a
second cutting element configured for the amputation the core of tissue from
the target tissue
site. At least a portion of the second cutting element may comprise a
sharpened edge. The second
cutting element may comprise at least one electrode configured for the
delivery of
radiofrequency energy. The second cutting element may comprise an energized
wire. The second
cutting element may comprise a suture. The tissue resection device may further
comprise an
actuator operable to actuate the second cutting element to transect tissue.
[00183] A tissue resection apparatus may comprise a first clamping element
comprising
a helical coil, a second clamping element, the second clamping element being
positioned to
oppose at least a portion of the first clamping element, a first and second
electrode configured for
the delivery of radiofrequency energy for sealing tissue, a first cutting
element configured for the
transection of at least a portion of the sealed tissue, a first and second
ligating element, and a
second cutting element positioned between said first and second ligating
elements. The tissue
resection device may further comprise a first actuator operable to actuate the
first or second
41
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
clamping element to apply mechanical compression to tissue, and a second
actuator operable to
actuate the cutting element to transect tissue. The helical coil may comprise
first and second
contiguous coil segments. The first coil segment may comprise a generally
planar open ring. The
first coil segment may be helical and may have a pitch of zero. The second
coil segment may be
helical and may have a non-zero pitch. The second coil segment may have a
variable pitch. The
first coil segment may be helical and may have a first pitch and the second
coil segment may be
helical and may have a second pitch, and at least one of the first and second
pitches may be
variable. The first electrode may be comprised of at least a portion of the
helical coil. The first
electrode may be comprised of at least a portion of the first clamping
element. The second
electrode may be comprised of at least a portion of the second clamping
element. The helical coil
may comprise a blunt tip. The first and second electrodes may comprise surface
profiles that are
matching or substantially matching. At least a portion of the cutting element
may comprise a
sharpened edge. The cutting element may comprise at least one electrode
configured for the
delivery of radiofrequency energy. The cutting element may comprise an
ultrasonic blade. The
tissue resection device may further comprise a second cutting element
configured for the
amputation the core of tissue from the target tissue site. At least a portion
of the second cutting
element may comprise a sharpened edge. The second cutting element may comprise
at least one
electrode configured for the delivery of radiofrequency energy. The second
cutting element may
comprise an energized wire. The second cutting element may comprise a suture.
The tissue
resection device may further comprise an actuator operable to actuate the
second cutting element
to transect tissue.
[00184] A tissue sealing mechanism may comprise a helical coil with a
generally
obround cross section and a tapered point disposed at a distal end, a first
and second helical
tissue sealing surface, wherein the first and second helical tissue sealing
surfaces are provided by
the parallel planar surfaces of the helical coil, a first electrode disposed
on the first helical tissue
sealing surface, and a second electrode disposed on the second helical tissue
sealing surface,
wherein the first and second electrodes are configured to apply bipolar
radiofrequency energy for
sealing tissue. The helical coil may comprise first and second contiguous coil
segments. The
helical coil may comprise a blunt tip. The first and second electrodes may
have surface profiles
that are substantially matching. The first and second helical tissue sealing
surfaces may further
comprise a plurality of electrodes configured for the delivery of bipolar
radiofrequency energy.
42
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00185] FIGS. 11-17 shown examples devices that may be used to effect a coring
process, as described herein. For example, a resection device of the present
invention may
comprise an energy-based arrangement capable of penetrating tissue towards a
target lesion. In
one embodiment depicted in FIG. 11, tissue resection device 1100 includes an
outer tube 1105
may be provided having a distal edge profile and having an inner diameter
IDouter. A coil 1110
may be attached to an outer tube 1105 where the coil turns are spaced from and
opposed to a
distal end of the outer tube 1105. The coil 1110 preferably has a slightly
blunted tip 1115 to
minimize the possibility that it will penetrate through a blood vessel while
being sufficiently
sharp to penetrate tissue such as pleura and parenchyma. In some embodiments,
the coil 1110
may take the form of a helix having a constant or variable pitch. The coil
1110 may also have a
variable cross-sectional geometry. An electrode 1130 may be disposed on a
surface or embedded
within the coil 1110.
[00186] In some embodiments, as illustrated in FIG. 11, the coil 1110 may
include a
plurality of contiguous coil segments, e.g., coil segments 1120 and 1125. The
coil segment 1120
may comprises a helical member having a pitch of zero, e.g., a generally
planar open ring
structure, having an inner diameter IDcoil and an outer diameter 0Dcoil. The
coil segment 1125
may comprise a helical structure of constant or variable pitch and constant or
variable cross-
sectional geometry. In this embodiment, the electrode 1130 may be disposed on
a surface of or
embedded in the coil segment 1120.
[00187] A central tube 1200 may be provided having a distal end with an edge
profile
comprising one or more surface segments and having an outer diameter 0Dcentral
and an inner
diameter IDcentral. As illustrated in FIG. 12, an electrode 1205 may be
disposed on or
embedded within at least one of the surface segments. The central tube 1200
may be slidably
disposed within the outer tube 1105 and positioned such that the electrode
1205 opposes and
overlaps at least a portion of electrode 1130. The space between electrode
1205 and electrode
1130 may be referred to as the tissue clamping zone. In keeping with an aspect
of the present
disclosure, 0Dcentral > IDcoil and 0Dcoil > IDcentral. In some embodiments,
0Dcentral may
be about equal to 0Dcoil. Accordingly, the central tube 1200 may be advanced
through the
tissue clamping zone towards coil 1110 such that electrode 1205 abuts
electrode 1130.
43
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00188] A cutting tube 1300 may be slidably disposed within the central tube
1200.
The distal end of the cutting tube 1300 may be provided with a knife edge to
facilitate tissue
cutting.
[00189] To enable tissue resection, the resection device 1100 may be inserted
into
tissue and the outer tube 1105 may be advanced a predetermined distance
towards a target. The
coil segment 1125 may allow the device to penetrate the tissue in a manner
similar to a cork
screw. As the coil segment 1125 penetrates tissue, any vessel in its path may
either be moved to
planar coil segment 1120 or pushed away from the coil 1100 for subsequent
turns. A coil tip
1115 may be made blunt enough to minimize chances that it will penetrate
through a blood
vessel, while still sharp enough to penetrate certain tissue, such as the lung
pleura and
parenchyma. The central tube 1200 may then be advanced a predetermined
distance towards the
target. Any vessels that are disposed in the tissue clamping zone will be
clamped between
electrode 1130 and electrode 1205. The vessels may then be sealed by the
application of bipolar
energy to electrode 1130 and electrode 1205. Once blood vessels are sealed,
the cutting tube
1300 may be advanced to core the tissue to the depth that the outer tube 1105
has reached. The
sealing and cutting process may be repeated to create a core of desired size.
[00190] In keeping with an aspect of the present disclosure, the resection
device may
be further configured to dissect a target lesion and seal tissue proximate the
dissection point. To
facilitate dissection and sealing, as illustrated in FIG. 13, the central tube
1200 may be provided
with a ligation snare 1230, first and second ligation electrodes 1215 and
1220, and an amputation
snare 1225. As used herein, the word "snare" refers to a flexible line, e.g.,
a string or a wire.
The inner wall surface of the central tube 1200 may include upper and lower
circumferential
grooved pathways 1212 and 1214 disposed proximate the distal end. The first
and second
ligation electrodes 1215 and 1220 may be disposed on the inner wall of central
tube 1200 such
that lower circumferential groove 1214 may be between them. The upper grooved
pathway 1212
may be disposed axially above the ligation electrodes 1215 and 1220.
[00191] The ligation snare 1230 may be disposed in the lower circumferential
groove
1214 and extends through the central tube 1200 and axially along the outer
wall surface to a
snare activation mechanism (not shown). The amputation snare 1225 may be
disposed in the
upper circumferential groove 1212 and extends through the central tube 1200
and axially along
the outer wall surface to a snare activation mechanism (not shown). The outer
surface of the
44
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
central tube 1200 may be provided with a plurality of axially extending
grooved pathways which
receive the amputation snare 1225 and the ligation snare 1230 and are in
communication with the
upper and lower circumferential grooved pathways 1212 and 1214. In addition,
electrode leads
for the ligation electrodes 1215 and 1220 may extend to an energy source via
the axially
extending grooved pathways.
[00192] In operation, the resection device of this embodiment may detach and
seal the
tissue core. The cutting tube 1300 may be retracted to expose the ligation
snare 1230 which may
be preferably made of flexible line, e.g., suture. The ligation snare 1230 may
be engaged to snag
tissue and pull tissue against the inner wall surface between the first and
second ligation
electrodes 1215 and 1220. Bipolar energy may then be applied to the first and
second electrodes
1215 and 1220 to seal, i.e., cauterize, the tissue. Once sealed, the cutting
tube 1300 may be
further retracted to expose the amputation snare 1225 which may then be
activated to sever the
tissue core upstream from the point where the tissue was sealed (ligation
point). In some
embodiments, the amputation snare 1225 has a smaller diameter than that of
ligation snare 1230.
The smaller diameter facilitates tissue slicing. Accordingly, the resection
device 1100 according
to this embodiment may both create a tissue core and disengage the core from
surrounding tissue.
[00193] In an alternative embodiment, the resection device of the present
disclosure
may be provided with a single snare disposed between ligation electrodes which
both ligates and
cuts tissue. In this embodiment, the single snare may first pull tissue
against the inner wall
surface of the central tube 1200 between the ligation electrodes 1215 and
1220. Bipolar energy
may then applied to the first and second electrodes 1215 and 1220 to seal,
i.e., cauterize, the
tissue. Once sealed, the snare may further pulled to sever the tissue core.
[00194] In yet another embodiment, cutting and sealing may be performed
without
employing electrodes. In this embodiment, the ligation snare 1230 may include
a set of knots
1235 and 1240 which tighten under load, shown, for example, in FIG. 14.
Ligation may be
performed by retracting the cutting tube 1300 to expose the ligation snare
1230 and activating
the ligation snare 1230, which lassos tissue as ligation knot tightens. Once
the tissue is lassoed,
the cutting tube 1300 may be further retracted to expose the amputation snare
1225 which may
then be activated to sever the tissue core upstream from the point where the
point where the
tissue was lassoed.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00195] The present disclosure also contemplates a method and system for using
the
resection device to remove tissue lesions, for example, lung lesions. The
method generally
comprises anchoring the lesion targeted for removal, creating a channel in the
tissue leading to
the target lesion, creating a tissue core which includes the anchored lesion,
ligating the tissue
core and sealing the surrounding tissue, and removing the tissue core
including the target lesion
from the channel.
[00196] Anchoring may be performed by, any suitable structure for securing the
device
to the lung. Once the lesion is anchored, a channel may be created to
facilitate insertion of the
resection device 1100. The channel may be created by making an incision in the
lung area and
inserting a tissue dilator and port into the incision. A tissue core which
includes the anchored
lesion may be created. In keeping with the present disclosure, the resection
device 1100 may be
used to create the tissue core, to ligate the tissue core and to seal the
tissue core and sever it from
the surrounding tissue as described hereinabove. The tissue core may then be
removed from the
channel. As an example, a cavity port may be inserted in the channel to
facilitate subsequent
treatment of the target lesion site through chemotherapy and/or energy-based
tumor extirpation
such as radiation. As a further example, a cavity port may be disposed on the
perimeter of the
tissue resection apparatus. When the apparatus is removed from the tissue
site, the cavity port
may remain in place or may be removed.
[00197] The anchor depicted in FIG. 15 may be suitable for use in performing
the
method for removing tissue lesions described herein. The anchor may comprise
an outer tube
1422 having a sufficiently sharp edge to pierce the chest cavity tissue and
lung without causing
excess damage and an inner tube 1424 disposed within the outer tube 1422. One
or more tines or
fingers 1426 formed or preformed from shape memory material, e.g., Nitinol,
may be attached to
the end of inner tube 1424. The outer tube 1422 may be retractably disposed
over the inner tube
1424 such that when the outer tube 1422 may be retracted, the tines 1426
assume their preform
shape as shown. In keeping with the present disclosure, the outer tube 1422
may be retracted
after it has pierced the lung lesion thereby causing the tines 1426 to engage
the lung lesion.
Other suitable anchors may include coils and suction-based structures.
[00198] The incision blades depicted in FIG. 16 are suitable for use in
performing the
method for removing tissue lesions described herein. Once the anchor 1400 is
set, it may be
preferable to create a small cut or incision to facilitate insertion of chest
wall tissue dilator.
46
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Incision blades 1605 may be used to make a wider cut. The incision blades 1605
may successive.
The incision blades 1605 may include a central aperture which may allow them
to be coaxially
advanced along the anchor needle 1405 to create a wider cut in the chest wall,
with each
successive blade being larger than the previous blade, thereby increasing the
width of the
incision.
[00199] The tissue dilator depicted in FIG. 17 may be suitable for use in
performing the
method for removing tissue lesions described herein. The tissue dilator may
comprise any
suitable device for creating a channel in organic tissue. In one exemplary
embodiment, the tissue
dilator assembly includes a single cylindrical rod with a rounded end 1510 or
a cylindrical rod
with rounded end and a rigid sleeve arrangement 1515. Successive tissue
dilators may be
coaxially advanced along the anchor needle to create tissue tract or channel
in the chest wall,
with each successive dilator being larger than the previous dilator, thereby
increasing the
diameter of the channel. Once a final dilator with rigid sleeve is deployed,
the inner rod 1505
may be removed, leaving the rigid sleeve in the intercostal space between ribs
to create direct
passage to the lung pleura.
[00200] Any tissue resection device capable of penetrating lung tissue and
creating a
tissue core including a target lesion may be suitable for use in performing
the method for
removing tissue lesions described herein. The tissue resection device 1100
described
hereinbefore is preferred.
[00201] Once the tissue resection device 1100 is removed, a small channel in
the lung
may exist where the target lesion was removed. This channel may be utilized to
introduce an
energy-based ablation device and/or localized chemotherapy depending on the
results of the
tissue diagnosis. Accordingly, the method and system of the present disclosure
may not only be
utilized to ensure an effective biopsy is performed but also complete removal
of the lesion with
minimal healthy lung tissue removal is accomplished.
Generator
[00202] Electrical energy applied by the devices of the present disclosure may
be
transmitted to the devices by a generator. The electrical energy may be in the
form of radio
frequency ("RF") energy. In application, an electrosurgical instrument may
transmit RF energy
through tissue, which causes ionic agitation, or friction, in effect resistive
heating, thereby
47
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
increasing the temperature of the tissue. Because a sharp boundary is created
between the
affected tissue and the surrounding tissue, surgeons may operate with a high
level of precision
and control, without sacrificing un-targeted adjacent tissue. The low
operating temperatures of
RF energy is useful for removing, shrinking, or sculpting soft tissue while
simultaneously sealing
blood vessels.
[00203] The devices of the present disclosure is designed to work with any
commercially available bipolar energy generator, such as an Enseal generator
or a Bovie
generator. The devices of the present disclosure may interface with a "brand-
agnostic" generator
adapter that enables device operation regardless of the proprietary brand of
generator used to
delivery radiofrequency energy. In an exemplary embodiment, the adapter may
automatically or,
with the assistance of a user, manually identify the specific generator
product that is connect to
any of the devices of the present disclosure. The generator adapter may
modify, modulate, or
change the output of the generator (which may have subtle characteristic
differences depending
on the specific generator used) to ensure optimal tissue sealing using the
tissue coring devices of
the present disclosure. The generator provides radiofrequency power to drive
the devices of the
present disclosure such as an electrosurgical coring instrument that is used
during open or
laparoscopic general surgery to cut and seal vessels and to cut, grasp, and
dissect tissues. The
generator has an Adaptive Tissue Technology, which delivers intelligent energy
for greater
precision and efficiency.
Sample analysis
[00204] Various systems, devices, processes, and apparatus may be used to
analyze a
sample such as a cored tissue sample. For example, tissue histology, DNA
sequencing, rapid on-
site evaluation (ROSE), or a combination of the same may be used. The coring
method described
provides a large tissue sample. Following the removal of a core of tissue from
a site of interest,
the specimen may be analyzed for diagnostic purposes using any of the methods
described
below, independently or in combination.
[00205] FIG. 18 shows an example workflow 1800 of tissue sample analysis. As
illustrated in FIG. 18, tissue sample analysis may further comprise one or
more of: removing
core tissue (1802) and determining if the removed core tissue is adequate
(1804), or
inadequate/non-diagnostic (1806). If adequate, the removed tissue core may be
analyzed using a
48
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
designated analysis technique (1808). If inadequate, the workflow may perform
an additional
pass (1810), and the cycle may continue, starting with step 1802.
Rapid on-site evaluation (ROSE)
[00206] Rapid on-site examination (ROSE) is a rapid, real-time examination
method of
the specimen at hand. Use of ROSE during lung lesion biopsy sampling has been
suggested to
improve diagnostic yield. Reported advantages of ROSE include reduced number
of biopsies
performed, a lower procedural risk, and an improved accuracy yield. The core
of tissue isolated
may be analyzed using ROSE techniques. Using ROSE, one may check the sample
adequacy and
establish a preliminary diagnosis by performing a rapid stain in the
bronchoscopy suite or
operating room, with evaluation by a cytopathologist or a trained
cytotechnologist.
Histology
[00207] Morphologic assessment of the core tissue sample may be performed by
routine hematoxylin-eosin (H&E) staining, thereby allowing for interpretation
of the biopsy.
Immunohistochemistry
[00208] A vast majority of neoplasms arising from lung or pleura are initially
diagnosed based on the histologic evaluation of tissue biopsies. Although most
diagnoses may be
determined by morphology alone, immunohistochemistry may be a valuable
diagnostic tool in
the workup of problematic cases. The core tissue sample may also be analyzed
using
immunohistochemistry. This may help differentiate between lung adenocarcinoma
and squamous
cell carcinoma (SqCC), lung adeno-carcinoma and malignant mesothelioma (MM),
primary and
metastatic carcinomas, and small cell lung carcinoma (SCLC) and carcinoid
tumor.
Electron microscopy
[00209] The cored tissue sample may be evaluated using electron microscopy.
Electron
microscopy may be used to visualize details of a cancer cell's structure that
provide clues to the
exact type of the cancer.
Flow cytometry
49
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00210] Flow cytometry is used to detect the presence of tumor markers, such
as
antigens, on the surface of the cells. It may be used to help in the diagnosis
of cancer. The core
of tissue isolated may be analyzed using flow cytometry.
Image cytometry
[00211] DNA image cytometry (DNA-ICM) has gained attention for its diagnostic
advantages, including objectivity, convenience and a high positive rate, in
diagnosing various
malignant cancer types. Thus technique has been successfully used for lung
biopsies. The core
of tissue isolated may be analyzed using image cytometry.
Polymerase Chain Reaction (PCR)
[00212] The core of tissue isolated may be analyzed using PCR. PCR may be used
to
look for certain changes in a gene or chromosome, which may help find and
diagnose a genetic
condition or a disease, such as cancer.
Gene expression microarrays
[00213] The core of tissue isolated may be analyzed using gene expression
microarrays. Microarray-based technology is an ideal way in which to study the
effects and
interactions of multiple genes in cancer.
Fluorescent in situ hybridization (FISH)
[00214] The core of tissue isolated may be analyzed using FISH technology.
FISH may
be used to identify where a specific gene is located on a chromosome, how many
copies of the
gene are present, and any chromosomal abnormalities. It is used to help
diagnose diseases, such
as cancer.
Genetic sequencing
[00215] Next-generation sequencing (NGS) helps to characterize cancer and is
rapidly
being implemented to guide therapy. It has been previously demonstrated that
small lung biopsy
samples yield adequate quality DNA and RNA, enabling high-quality NGS
analysis. The core of
tissue isolated may be analyzed using NGS techniques.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Atomic force microscopy
[00216] The core of tissue isolated may be analyzed using atomic force
microscopy.
Atomic force microscopy (AFM) allows for nanometer-scale investigation of
cells and
molecules. The physicochemical properties of live cells undergo changes when
their
physiological conditions are altered. These physicochemical properties may
therefore reflect
complex physiological processes occurring in cells. When cells are in the
process of
carcinogenesis and stimulated by external stimuli, their morphology,
elasticity, and adhesion
properties may change. AFM may perform surface imaging and ultrastructural
observation of
live cells with atomic resolution under near-physiological conditions,
collecting force
spectroscopy information which allows for the study of the mechanical
properties of cells. For
this reason, AFM has potential to be used as a tool for the analysis and
diagnosis of lung biopsy
samples.
Surface enhanced Ramen spectroscopy
[00217] The core of tissue isolated may be analyzed using surface enhanced
Ramen
spectroscopy. Ramen spectroscopy may characterize biomolecules, because each
macromolecule
(lipid, protein, DNA, etc.) has unique finger-printing information about the
modes of vibration
and rotation. Therefore, Raman spectroscopy may be a promising tool for cancer
diagnostics in
the future. Nevertheless, Raman spectroscopy has the deficiency of low
sensitivity in practical
application. Compared with conventional Raman spectroscopy, Raman scattering
signals may be
strengthened by 4-15 orders of magnitude utilizing surface-enhanced Raman
spectroscopy
(SERS) technology. Studies have shown that the Raman enhancement effect may be
obtained by
utilizing silver nanospheres, gold nanospheres, and similar particulates. In
clinical detection,
label-free SERS detection of tissue provides a rapid and facile way to
differentiate tumors from
normal tissues. The differences in SERS spectra between lung cancer and normal
tissue may be
used to potentially diagnose lung cancer.
SEALING
[00218] The present disclosure relates to a method to deliver a fill material
such as
autologous blood to the core site that may be used to seal and provide
pneumostasis. As an
51
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
example, once the tissue specimen is cored and removed from the lung, there
may be a need to
seal the core site to provide pneumostasis. As a further example, pneumostasis
may be achieved
in the same surgery session as the tissue removal.
[00219] Although autologous blood is described herein as an example, other
fill
materials and additives may be used. For example, a hemostatic adjunct such as
an absorbable
gelatin foam (e.g., SURGIFOAMO), biologic, oxidized regenerated cellulose
(ORC),
fibrin/thrombin spray, etc. As a further example, a patient may have a rare
disorder of
hemophilia in which their blood does not clot normally. Other patients may be
on blood thinning
medicines which could inhibit blood clotting formation. For such patients, to
seal the cored
cavity, thrombin and/or fibrinogen may be added to the autologous blood sample
to aid in clot
formation. Reactive polyethylene glycol (PEG), ammonium sulfate, ethanol,
calcium chloride,
or magnesium chloride may also be added to the blood sample to aid in clot
formation. Another
source for the blood to be used to seal the cored cavity is donated blood from
other people or
blood bank. Donated blood may be used with or without clotting agents as
mentioned above.
[00220] Systems and/or methods for sealing tissue are described herein. An
example
method may comprise disposing a port to provide access to a target site. The
target site may
comprise biological tissue. The target site may comprise tissue of a lung. The
target site may
comprise a cored tissue. The target site may comprise a punctured tissue.
Other sites may benefit
from the disclosed methods.
[00221] Example methods may comprise anchoring an anchor device (e.g., via the
port) to a surface at the target site. Anchoring may be performed by any
suitable structure for
securing the device to the lung. Example methods may comprise disposing (e.g.,
via the port) a
sealing device adjacent the target site. Example methods may comprise
disposing a sealing
device adjacent the target site using the anchoring device as a guide. The
sealing device may
comprise an inflatable balloon. The sealing device may comprise an inflatable
balloon with an
array of radio frequency (RF) electrodes configured to ablate and seal tissue.
The sealing device
may comprise an inflatable balloon configured to seal tissue using a thermal
fluid. The sealing
device may comprise an inflatable balloon catheter. The sealing device may
comprise an access
port with an array of RF electrodes configured to ablate and seal tissue. The
sealing device may
comprise at least one microwave ablation probe.
52
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00222] Example methods may comprise causing the sealing device to seal the
target
site. The causing the sealing device to seal the target site may comprise
causing at least a portion
of the sealing device to abut a portion of the target site. Example methods
may comprise
disposing a fill material adjacent the target site. Example methods may
comprise disposing a fill
material adjacent the target site via a fill material delivery device such as
a catheter. The fill
material may comprise autologous blood, donated blood, recirculated blood,
hemostatic adjuncts
such as fibrin and/or thrombin, biological tissue adhesives such as Dermabond
, ORC,
absorbable gelatin, or any combination thereof. The fill material may promote
pneumostasis. The
fill material may additionally promote hemostasis. Other materials may be
used. The sealing
device may minimize escape of the fill material from the target site.
[00223] As an illustrative example, the target site may comprise at least a
portion of a
lung. The lung may be caused to collapse prior to disposing the sealing device
adjacent the
target site. The lung may be allowed to ventilate while the sealing device is
sealing the target
site. The sealing device may be spaced (e.g., removed, separated, etc.) from
the target site after
the fill material is disposed.
[00224] Systems and/or methods for sealing are described herein. An example
method
may comprise disposing a sealing device adjacent a target site of a lung. The
sealing device may
be disposed adjacent the target site while the lung is collapsed. However, the
lung may be
ventilated. Example methods may comprise causing the sealing device to seal
the target site.
Example methods may comprise disposing a sealing device adjacent the target
site using the
anchoring device as a guide. The sealing device may comprise an inflatable
balloon. The sealing
device may comprise an inflatable balloon with an array of RF electrodes
configured to ablate
and seal tissue. The sealing device may comprise an inflatable balloon
configured to seal tissue
using a thermal fluid. The sealing device may comprise an inflatable balloon
catheter. The
sealing device may comprise an access port with an array of RF electrodes
configured to ablate
and seal tissue. The sealing device may comprise at least one microwave
ablation probe.
Example methods may comprise disposing a fill material adjacent the target
site. Example
methods may comprise disposing a fill material adjacent the target site via a
fill material delivery
device such as a catheter. The fill material may comprise autologous blood,
donated blood,
recirculated blood, hemostatic adjuncts such as fibrin, thrombin, biological
tissue adhesives such
as Dermabond , ORC, absorbable gelatin, or any combination thereof. The fill
material may
53
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
promote pneumostasis. The fill material may additionally promote hemostasis.
Other materials
may be used. The sealing device may minimize escape of the fill material from
the target site.
[00225] Systems and/or methods for sealing are described herein. An example
method
may comprise disposing a fluid delivery device into a target site of a lung.
The sealing device
may be disposed adjacent the target site while the lung is collapsed. However,
the sealing device
may be disposed adjacent the target site when the lung is ventilated. Example
methods may
comprise disposing a fill material into the target site. Example methods may
comprise spacing
(e.g., removing, separating, etc.) the sealing device from the target site.
[00226] The sealing device may comprise an inflatable balloon. The sealing
device
may comprise an inflatable balloon with an array of RF electrodes configured
to ablate and seal
tissue. The sealing device may comprise an inflatable balloon configured to
seal tissue using a
thermal fluid. The sealing device may comprise an inflatable balloon catheter.
The sealing device
may comprise an access port with an array of RF electrodes configured to
ablate and seal tissue.
The sealing device may comprise at least one microwave ablation probe. The
systems and/or
methods described herein may allow clotted blood to provide a seal to achieve
pneumostasis.
Example methods may comprise disposing a fill material adjacent the target
site. Example
methods may comprise disposing a fill material adjacent the target site via a
fill material delivery
device such as a catheter. The fill material may comprise autologous blood,
donated blood,
recirculated blood, hemostatic adjuncts such as fibrin, thrombin, biological
tissue adhesives such
as Dermabond , ORC, absorbable gelatin, or any combination thereof. The fill
material may
promote pneumostasis. The fill material may additionally promote hemostasis.
Other materials
may be used. The sealing device may minimize escape of the fill material from
the target site.
[00227] The target site may comprise a cavity. The cavity may be closed, for
example,
after sealing. Closing the cavity may comprise using biological tissue
adhesive such as
Dermabond , tissue grafts, hemostatic sealing patches, staple closure,
sutures, or the like.
[00228] FIG. 19 shows an example system 1900. The system 1900 may comprise a
port such as chest port 1902 configured to provide access, such as via a
channel to a portion of a
body. It should be understood that various channels or ports may be used
throughout the body
and the chest port 1902 is shown as a non-limiting example. As an illustrative
example, the chest
port 1902 is shown disposed adjacent ribs 1906 to provide access to lungs 1910
of a patient.
However, other sites may be used and a chest port 1902 (or other port) may not
be necessary. An
54
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
anchor device 1904 may be anchored to tissue, such as the lung 1910. An
example anchor device
is shown in FIG. 6 for illustration. However, any suitable device for
anchoring to the target site
1912 may be used. As show, the anchor device 1904 extends via the chest port
1902, through the
pleura 1908, and anchors to tissue in the lung 1910. The anchor device 1904
may be anchored
(e.g., releasably coupled) to a tissue at a target site 1912. The target site
1912 may comprise a
core site where a portion of lung tissue has been cored, punctured, or
removed. The anchor
device 1904 may be placed at the target site 1912 while the lung is inflated.
However, other
processes may be implemented while the lung is collapsed.
[00229] FIG. 20 shows an application of an example sealing device 2000. The
sealing
device 2000 may comprise an inflatable balloon 2002. Other sealing mechanisms
may be used.
The sealing device 2000 may comprise and/or be in contact with a balloon
catheter. The balloon
catheter may be a single lumen balloon catheter. The balloon catheter may be
multi-lumen
balloon catheter. The sealing device 2000 may be disposed adjacent the target
site 2012. As
such, the sealing device 2000 may seal the target site 2012 to minimize exit
of a fluid or material
from the target site 2012. As an example, a fill material 2004 may be disposed
at the target site
2012 and may be sealed in the target site 2012 by the sealing device 2000. As
an illustrative
example, the inflatable balloon 2002 may provide sealing while the lung 110
moves (e.g.,
inflates and deflates). The sealing device 2000 may be implemented when the
lung 2010 is
inflated or collapsed.
[00230] Example sealing procedures are described herein and include fill
materials,
ablation, mechanical pressure, energy emission (e.g., RF energy), and others,
for example.
Causing the sealing device to seal at least a portion of the core cavity at
the target site may
comprise causing at least a portion of the sealing device to abut a wall
defining the core cavity.
Causing the sealing device to seal at least a portion of the core cavity at
the target site may
comprise ablating a wall defining the core cavity. Causing the sealing device
to seal at least a
portion of the core cavity at the target site may comprise applying pressure
to a wall defining the
core cavity. Methods may further comprise disposing a fill material in the
core cavity, wherein
the sealing device minimizes escape of the fill material from the core cavity.
The fill material
may comprise autologous blood. As an example, the target site may comprise at
least a portion of
a lung and the method may further comprise causing the lung to collapse prior
to disposing the
sealing device adjacent the target site. As a further example, the target site
may comprise at least
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
a portion of a lung and methods may further comprise allowing the lung to
ventilate while the
sealing device is sealing the target site.
[00231] An example system for implementing one or more of the methods of the
present disclosure may comprise a guided anchor. The example system may
comprise a single
lumen balloon catheter. The example system may comprise a multi-lumen balloon
catheter. The
example system may comprise a coring device. Post coring by the coring device,
an anchor may
be introduced into the tissue cavity to ensure access to a cored site. The
chest port may be
removed, and the lung may be collapsed. The balloon catheter may be inserted
over the anchor.
Once the balloon catheter is in the chest cavity, the balloon catheter may be
inflated. The
inflated balloon catheter may be moved forward and pushed slightly against
lung tissue.
Autologous blood may be injected into a core site through the inflated balloon
catheter. The
inflated balloon catheter and autologous blood may be held in place for a
predetermined time
period (e.g., one (1) minute, etc.) to allow the blood to clot at the core
site. The lung may be
allowed to resume ventilation. The inflated balloon catheter may be allowed to
go up and down
with the lung while maintaining contact with the lung to keep the blood at the
core site to
facilitate further clotting. The balloon catheter may be deflated. The balloon
catheter and
anchor may be removed after a predetermined time period (e.g., three (3)
minutes, etc.). The
autologous blood may be clotted at the core site to provide pneumostasis.
[00232] In an embodiment, the anchor and/or the balloon catheter may be used
to
deposit autologous blood at the core site with the lung collapsed. The anchor
and/or the balloon
catheter may be removed right after the autologous blood is delivered. The
blood may be
allowed to clot in place with a predetermined time period (e.g., five (5)
minutes, etc.) before the
lung is allowed to resume ventilation.
[00233] The example system may cause autologous blood to be delivered to the
core
site. Other fill materials may be used.
[00234] The example system may allow clotted blood to provide a seal to
achieve
pneumostasis.
[00235] In an embodiment, a method and apparatus are provided whereby a plug
or
series of stitches are on a wire within the chest in a compressed
configuration. When it is desired
to seal the pleural space, the wire may be pulled back towards the operator,
bringing the plug or
stitches in opposition to the internal opening of the body space. The device
may then be actuated
56
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
to insert the plug or Stitches into the internal body space opening, and the
wire breaks away,
thereby closing the hole and preventing fluid from leaking out or air from
getting sucked back in.
[00236] Polypeptide / protein-based adhesives, fibrin-based adhesives, gelatin-
based
adhesives, collagen-based adhesives, albumin based adhesives, polysaccharide-
based adhesives,
chitosan-based adhesives, human blood-based adhesives, and animal-based
adhesives, and
synthetic and semi-synthetic adhesives (such as cyanoacrylates, polyethylene
glycol hydrogels,
urethane-based adhesives, and other synthetic adhesives). The fluid may fill
the volume of the
tract and may be heated with RF energy or laser beyond the temperature of the
surrounding
tissue, to a temperature sufficient to cauterize and seal the surrounding
tissue. The combination
of the fluid and the RF seals the surrounding tissues
[00237] Various methods, devices, and systems may be used to core or remove
tissue.
Therapy
[00238] Various therapies may be implemented.
[00239] FIGS. 21-22 show illustrative examples, but other methods of ablation
or
energy emission may be used for sealing tissue. For example, a shaped mesh
catheter may be
used. As such, a catheter with collapsed meshed shape may be inserted into the
cavity and the
cavity sheath may be removed. The mesh may be then expanded, and suction may
be applied to
pull tissue to contact with the mesh. Energy, e.g. RF, may then be applied to
ablate the cavity
tissue wall.
Margin ablation
[00240] Introducing an energy delivery device into a tissue cavity and
delivering
energy to eradicate cancerous tissue. Once the target tissue has been cored
out and removed, the
tissue wall of the cavity may be ablated. For example, any of the following
ablation methods
could be used:
Rotating Ablation Probe
[00241] FIGS. 21A-21C show an example application. As shown, once a target
site has
been cored out and the tissue core removed, there may be a need to ablate the
tissue wall of the
cavity. As such, the following ablation methods could be used. For example, a
rotating ablation
57
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
probe may be used. FIG. 21A shows a cored-out cavity 2112 in tissue 2110 with
the cavity
sheath 2102 in place to keep the cavity open. A rotating probe 2100 may then
be inserted into
the cavity sheath 2102, as shown in FIG. 21B. The probe 2100 may be equipped
with an energy
source such as an array of energy heads or a continuous energy strip. The
energy may be
microwave, RF, other output form. Once the probe 2100 is in place, the cavity
sheath 2102 may
remain in place or be removed. The energy may then be applied while the
probe/energy heads
are rotated to give a radially continuous ablation on the wall and bottom
tissue 2110 of the
cavity, as shown in FIG. 21C.
Hot Balloon Catheter
[00242] FIGS. 22A-22B show an example application. As shown, a hot balloon
catheter may be used. For example, a balloon catheter 2200 may be placed into
a cavity 2212
formed in tissue 2210 and a cavity sheath may be removed to expose the cavity
2212 needed to
be ablated, as shown in FIG. 22A. The balloon 2200 may then be inflated with
hot fluid or hot
air/gas to ablate the cavity wall tissue 2210, as shown in FIG. 22B.
[00243] FIGS. 23A-23C show an example application. As shown, once a target
site has
been cored out and the tissue core removed, there may be a need to seal the
cut tissue wall of the
cavity. As such, the following example procedure may be used. A device 2300
may comprise a
fluid conduit 2301 and an inflatable absorbable balloon 2302. The balloon 2302
may be coated
on the exterior with absorbable bio adhesive that will seal against the tissue
of the cored cavity
post coring, as shown in FIGS. 23A-23B. Once the deflated balloon 2302 may be
placed in the
desired location, the balloon 2302 may be inflated with CO2 (or other fluid),
for example via
fluid conduit 2301, so that the bio adhesive is pressed against the tissue
wall of the cored cavity
to achieve sealing to prevent air leak. The CO2 filled balloon 2302 may be
pressurized to an
appropriate pressure and may be left behind inside the cored cavity.
Shaped Mesh Catheter
[00244] A catheter with a collapsed meshed shape may be inserted into the
cavity and
the cavity sheath may be removed. The mesh may then be expanded, and suction
may be applied
to pull tissue to contact the mesh. Energy, e.g. RF, may then be applied to
ablate the cavity
tissue wall.
58
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Microwave ablation
[00245] FIG. 24 illustrates an example therapy system. A catheter probe 2402
containing an antenna 2404 which emits microwaves may be inserted into a
tissue cavity 2412
cored out of tissue 2410, such as illustrated in FIG. 24. The probe produces
intense heat that
ablates (e.g., destroys) the target tissue.
Cryoablation
[00246] FIG. 25 illustrates an example therapy system. A cryoablation probe
2502
may be inserted into a tissue cavity 2512 cored out of target tissue 2510,
such as shown in FIG.
25. The probe produces extremely cold temperatures to ablate the target tissue
2510 within a
cryoablation zone 2504.
Chemical ablation (chemoablation)
[00247] Hypertonic saline gel, solid salt, and/or acetic acid gel may be
implanted into
the cavity to promote damage of the target cells.
Laser ablation (photoablation)
[00248] A probe that emits a laser beam at a specific wavelength and pulse
length may
be inserted into the cavity. The emitted laser beam may be used to kill the
target tissue in the
cavity.
Ethanol ablation
[00249] In this procedure, concentrated alcohol in liquid or gel form may be
injected
directly into the target cavity to damage the cells.
Chemotherapy drugs
[00250] At the cored site, administration of chemotherapy drugs such as
doxorubicin,
fluorouracil, and/or cisplatin may be done via direct injection of the agent
into the cored tissue
site.
59
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00251] FIG. 26 illustrates an example therapy system. The method of
drug/therapy
delivery may be achieved by placing a cavity sheath 2602 into a tissue cavity
2612 at the cored
site of cored tissue 2610. Then, a delivery probe 2604 containing one or more
lumens 2606 at the
distal end may be inserted into the cavity sheath 2602. Said delivery probe
2604 may extend out
of the distal opening of said cavity sheath into the cored tissue cavity. The
desired therapeutic
and/or diagnostic agent may then be delivered through the delivery lumen 2606
to the tissue via
the distal end of the delivery probe via direct injection using a drug/therapy
injection port with
plunger 2608, such as shown in FIG. 26.
[00252] FIG. 27 illustrates an example therapy system. In some scenarios, a
biodegradable plug 2702 may be placed over the cored site of cored tissue 2710
following the
addition of the drug/therapy to the cavity, such as shown in FIG. 27. Namely,
drug 2704 may be
delivered into a tissue cavity 2712 at the cored site of cored tissue 2710.
The plug 2702 may be
secured in place using a biocompatible glue.
Chemotherapy drug-eluting particles
[00253] Chemotherapy drug-eluting particles may be delivered to the cored
tissue site,
thereby promoting controlled and sustained locoregional release of therapeutic
agents in high
concentration with prolonged administration. For example, doxorubicin may be
encapsulated
into nanoparticles to form micelles for targeted drug delivery. Additionally,
anti-cancer drugs
may be vectorized using porous particles, such as mesoporous silica
nanoparticles, and delivered
to the cored tissue site.
Co-delivery of siRNA and chemotherapy drugs
[00254] Chemotherapy drugs and short interfering RNA (siRNA) may be co-
delivered
to the cored tissue site through direct injection to promote cancer cell
death. Multidrug
resistance in cancer cells may be suppressed using siRNA-based formulations to
induce specific
silencing of a broad range of genetic targets. Delivering siRNAs in
combination with
chemotherapy drugs may enhance the efficacy of the chemotherapy through
conquering the
resistance mechanism of the cancer cells. For example, siRNA encapsulated in
mesoporous silica
nanoparticles may be co-delivered with doxorubicin to the target core site.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Biodegradable hydrogel-based controlled drug delivery
[00255] FIGS. 28-29 illustrate an example therapy system and method. The
method of
hydrogel/plug delivery may be achieved by placing a cavity sheath 2802 into a
tissue cavity 2812
at the cored site 2810. Then, a delivery plunger 2804, further comprising a
delivery plunger
sheath 2806, and containing the hydrogel 2806 at the distal end 2814 may be
inserted through the
cavity sheath 2802 into the cored site 2812. The hydrogel 2806 may then be
delivered into the
cored site 2812 through the plunging mechanism of the delivery plunger 2802,
such as is shown
in FIGS. 28-29.
Photodynamic therapy (PDT)
[00256] A combination of chemotherapy drug(s) and photodynamic therapy (PDT)
may
be directly delivered to the cored tissue site. PDT is a treatment modality
which relies on a
photosensitizer and light to generate reactive oxygen species (ROS) to kill
cancer cells.
Degradable polymer/scaffold system
[00257] FIG. 30 illustrates an example therapy system. Polymer systems
containing
chemotherapy drugs may be delivered to the cored tissue site 3012 of cored
tissue 3010 via direct
implantation. Porous biodegradable polymers, such as sponges or scaffolds
3002, may be
designed to carry chemotherapy drugs, such as cisplatin. These polymers
degrade overtime,
thereby releasing the chemotherapy drug at a controlled rate within the
targeted site. The
excellent biodegradability of the scaffolds, such as porous scaffolds,
overcome the limitations of
non-biodegradable systems which support the sustained release of the
chemotherapy drugs and
degrade after a specific time period. The scaffold 3002 may be manufactured in
manner that is
convenient for surgical delivery, such as shown in FIG. 30.
Hyperthermia of cored tissue site
[00258] Hyperthermia may be used to treat the desired cored tissue site. Using
this
approach, the cored tissue site may be exposed to higher than normal
temperatures to promote
selective destruction of abnormal cells, which minimizes the size effects on
healthy cells. For
example, light-absorbing metal particles, such as gold nanoparticles or iron
oxide microparticles,
61
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
may be delivered to the cored tissue site. Then, by applying a short-pulsed
laser, cancer cells
targeted with the metal particles may be killed.
Control System
[00259] The present disclosure generally relates to electrosurgical systems
configured
for the resection a core of tissue from a tissue site. The present disclosure
generally relates to
electrosurgical methods for optimizing tissue coring, for example, based on
the type of tissue
being treated, employing multiple energy modalities based on tissue
parameters, based on tissue
impedance, and employing simultaneous energy modalities based on tissue
parameters.
[00260] Depending upon specific instrument configurations and operational
parameters, electrosurgical instruments may provide substantially simultaneous
cutting of tissue
and hemostasis through the application of radiofrequency energy, desirably
minimizing patient
trauma. For resection devices of the present disclosure, the tissue sealing
action may be realized
by clamping tissue between a helical coil and a corresponding circular ring
(referred to as first
and second clamping elements), delivering radiofrequency energy to two RF
electrodes on the
surface of the helical coil and circular ring (referred to as first and second
electrode elements),
and finally the cutting action is typically realized by a blade tip (or
cutting element). Elements
may be located at the distal end of the tissue coring instrument. The devices
of the present
disclosure may be configured for open surgical use, laparoscopic, or
endoscopic surgical
procedures including robotic-assisted procedures.
[00261] Electrosurgical devices for applying electrical energy to tissue in
order to treat
and/or destroy the tissue are also finding increasingly widespread
applications in surgical
procedures. An electrosurgical device typically includes a hand piece, an
instrument having a
distally-mounted end effector (e.g., one or more electrodes). The end effector
may be positioned
against the tissue such that electrical current may be introduced into the
tissue. Electrosurgical
devices may be configured for bipolar or monopolar operation. During bipolar
operation, current
may be introduced into and returned from the tissue by active and return
electrodes, respectively,
of the end effector. During monopolar operation, current may be introduced
into the tissue by an
active electrode of the end effector and returned through a return electrode
(e.g., a grounding
pad) separately located on a patient's body. Heat generated by the current
flowing through the
tissue may form hemostatic seals within the tissue and/or between tissues and
thus may be
62
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
particularly useful for sealing blood vessels, for example. The end effector
of an electrosurgical
device also may include a cutting member that may be movable relative to the
tissue and the
electrodes to transect the tissue.
[00262] Electrical energy applied by an electrosurgical device may be
transmitted to
the instrument by a generator in communication with the hand piece. The
electrical energy may
be in the form of radio frequency ("RF") energy. RF energy is a form of
electrical energy that
may be in the frequency range of 200 kilohertz (kHz) to 1 megahertz (MHz). In
application, an
electrosurgical device may transmit low frequency RF energy through tissue,
which causes ionic
agitation, or friction, in effect resistive heating, thereby increasing the
temperature of the tissue.
Because a sharp boundary may be created between the affected tissue and the
surrounding tissue,
surgeons may operate with a high level of precision and control, without
sacrificing un-targeted
adjacent tissue. The low operating temperatures of RF energy may be useful for
removing,
shrinking, or sculpting soft tissue while simultaneously sealing blood
vessels. RF energy works
particularly well on connective tissue, which is primarily comprised of
collagen and shrinks
when contacted by heat.
[00263] The RF energy may be in a frequency range described in EN 60601-2-
2:2009+A11:2011, Definition 201.3.218-HIGH FREQUENCY. For example, the
frequency in
monopolar RF applications may be typically restricted to less than 5 MHz.
However, in bipolar
RF applications, the frequency may be almost anything. Frequencies above 200
kHz may be
typically used for monopolar applications in order to avoid the unwanted
stimulation of nerves
and muscles that would result from the use of low frequency current. Lower
frequencies may be
used for bipolar applications if the risk analysis shows the possibility of
neuromuscular
stimulation has been mitigated to an acceptable level. Normally, frequencies
above 5 MHz are
not used in order to minimize the problems associated with high frequency
leakage currents.
Higher frequencies may, however, be used in the case of bipolar applications.
It is generally
recognized that 10 mA is the lower threshold of thermal effects on tissue.
[00264] One challenge of using conventional medical devices is the inability
to control
and customize the power output depending on the type of tissue being treated
by the devices. It
would be desirable to provide a surgical instrument configured for the coring
of tissue that
overcomes some of the deficiencies of current instruments.
63
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00265] In an aspect, a surgical instrument for resecting a core of tissue may
be
provided, the surgical instrument comprising a processor; an end effector at a
distal end of the
surgical instrument, the end effector configured to interact with tissue, the
end effector
comprising: first and second clamping elements; first and second electrode
elements; a force
sensor in communication with the processor and configured to measure a force
applied to tissue
located between the first and second clamping elements; and a temperature
sensor in
communication with the processor; first and second electrode elements
configured to receive
radiofrequency energy from a generator and deliver RF energy to tissue
interposed between the
first and second clamping elements to seal tissue; wherein the processor is
configured to:
determine a type of tissue interacting with the end effector based on a tissue
coefficient of
friction, wherein the tissue coefficient of friction is determined based on
the force applied to the
tissue by the end effector and a rate of heat generated by the end effector;
and dynamically
control the energy delivered to the first and second electrode elements based
on the type of tissue
interacting with the end effector. Specifically, the output power of a
surgical instrument may be
modulated as a function of a desired impedance trajectory where the impedance
trajectory results
in a desired tissue effect or outcome. In one aspect, the RF output may be
therapeutic, e.g. tissue
treating, or sub-therapeutic, e.g. sensing only. The RF output may be applied
to the tissue and the
voltage and current, or representations of the voltage and current, are
measured or estimated. The
impedance may be calculated by determining the ratio of the voltage to the
current.
[00266] In an aspect, a tissue coring instrument may comprise a controller and
processing unit configured to optimize the delivery of radiofrequency energy
for coring tissue
by: a) identifying the brand and/or model of electrosurgical generator
supplying power to the
device, b) directly adjusting the dynamics of RF energy delivery to tissue to
optimize tissue
sealing, and/or c) communicating with the electrosurgical generator to affect
generator operation.
Identification of the device-connected generator may be achieved automatically
be the controller,
or be manually determined, identified, and/or selected by a user.
[00267] The controller may be integrated within the device, or comprise an
external
unit placed in-line between the device and the corresponding electrosurgical
generator. The
controller may additionally comprise an assortment of proprietary connectors
to enable "brand
agnostic" use of the device (i.e. to assure that the device may perform
resection of a core of
64
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
tissue regardless of the type of generator used, the controller may comprise a
modular connector
system).
[00268] The controller may affect energy delivery to the device via two-way
communication between a corresponding electrosurgical generator and the
device. Various
operating settings for energy delivery and tissue sealing parameters may be
transferred from the
generator to the controller. Real-time tissue sensing parameters may be
additionally transferred
from the device to the generator.
[00269] Additionally, the controller and processing unit may serve other
purposes,
including providing an interface for the connection of other devices or
accessories such as a
display to visualize and communicate useful information to a user (such as
tissue sealing
progress; device positioning relative to a target tissue site; proper device
positioning and
orientation; relevant device diagnostic data and error reports; device misuse
warnings; navigation
systems to determine the position of the device in 3D space and/or relative to
anatomic
landmarks obtained via various medical imaging modalities such as CT, MRI, and
ultrasound).
[00270] In another embodiment, a custom generator configured for the coring of
tissue
may comprise a controller and processing unit directly integrated into the
generator. This
exemplary tissue coring electrosurgical generator may comprise an interface
for the connection
of other devices or accessories such as a display to visualize and communicate
useful
information to a user (such as tissue sealing progress; device positioning
relative to a target
tissue site; proper device positioning and orientation; relevant device
diagnostic data and error
reports; device misuse warnings; navigation systems to determine the position
of the device in
3D space and/or relative to anatomic landmarks obtained via various medical
imaging modalities
such as CT, MRI, and ultrasound).
[00271] For any of the exemplary surgical instruments configured for the
resection of a
core of tissue from a target tissue site as described above (i.e. a tissue
coring electrosurgical
generator specifically configured for the coring of tissue, an integrated
controller within the
tissue core resection device configured to affect RF energy delivery from an
electrosurgical
generator to optimize tissue sealing, or an external controller configured to
affect RF energy
delivery to the tissue core resection device from an electrosurgical
generator), RF energy
delivery to tissue may be modulated, controlled, affected, or otherwise
changed to achieve a
desired tissue effect or outcome.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00272] RF impedance is known to change during the heating and coagulation of
tissue.
RF impedance may be used as an indicator of the state of the tissue and
therefore may be used to
indicate progress in a coagulation cycle, vessel sealing cycle, cutting, etc.
An extension of this
change in RF impedance may be used to form a desired treatment cycle if the
output is
modulated such that the RF impedance follows a particular, desired course of
change in
impedance. The desired course of impedance may be pre-determined based on the
instrument's
operating parameters or determined by selection of the surgeon or measurement
of tissue
parameters to set this course of treatment. The course of impedance may
determine one or more
of the output power, output waveform or wave shape, selection of energy mode
or modality or a
point to terminate the application of energy to tissue.
[00273] During tissue treatment, a parameterized tissue model may be fitted to
the
tissue. The parameters that are found in the model may be used to generate an
optimal controller
in real-time and could also be correlated to specific tissue characteristics.
The present application
provides real-time optimization on a generator control system based on RF
impedance and real-
time tissue evaluation.
[00274] These techniques may be used to model the tissue in real-time and
develop
controllers in real-time, specific to a particular tissue type to maximize
sealing, minimize
sticking of tissue, and cycle times. Furthermore, control of the output of a
surgical instrument
based on tissue characteristics and changes in tissue during sealing and
cutting cycles is
provided.
[00275] RF output may be configured to supply electrosurgical energy to the
tissue via
at least one electrode configured to apply electrosurgical energy to the
tissue, sensing circuitry
configured to measure impedance of the tissue, and a controller programmed to
determine
whether a tissue reaction has occurred as a function of impedance values and a
predetermined
rise in impedance, where the tissue reaction corresponds to a boiling point of
tissue fluid, to
generate a target impedance trajectory as a function of measured impedance and
a predetermined
desired rate of change of impedance based on the tissue reaction
determination, where the target
impedance trajectory includes a plurality of target impedance values for each
of a plurality of
time steps, and to drive tissue impedance along the target impedance
trajectory by adjusting the
output level of the ultrasonic output stage to substantially match tissue
impedance to a
corresponding target impedance value for at least a predetermined minimum time
period.
66
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00276] FIG. 31 shows a schematic diagram and flowchart of a device 3100 that
would
be able to advance to the target site, identify the tumorous tissue from
normal tissue and
successfully treat or resect the tumorous tissue. The device 3100 may comprise
a processing unit
3102 and an external display 3104- (e.g., a graphical user interface (GUI)).
The processing unit
3102 may be configured to receive one or more inputs, including, for example,
3-dimensional
(3D) data 3106 from a patient scan and/or energy from an energy source 3108.
The processing
unit 3102 may be further configured to receive device positioning information
3110 and confirm
3112 a current location of the tissue. The processing unit 3102 may be further
configured to
confirm 3114 that the device 3100 is at a target location and send such a
confirmation to a tissue
sensing unit 3116. The processing unit 3102 may further be configured to
activate 3118 a
delivery of energy to a tissue site. Once energy delivery is activated, the
processing unit 3102
may be configured to provide automated advancement 3120 of the device 3100 and
provide new
location information to the tissue sensing unit 3116. The processing unit 3102
may configured to
receive information from the tissue sensing unit 3116 and adjust 3122 energy
delivery
accordingly. The processing unit 3102 may be further configured to provide
instructions 3124 to
a device component for ablating and/or resecting the tissue.
[00277] In an aspect, a surgical instrument system for coring tissue from a
target tissue
site may comprise: a tissue resection device configured for coring tissue,
wherein the device
comprises: a helical coil electrode, and a cutting element configured to
cooperate with the helical
cold electrode for the transection of tissue; and a handle assembly configured
to facilitate
interaction between tissue the tissue resection device.
[00278] In an aspect, a surgical instrument system for coring tissue from a
target tissue
site may comprise a tissue resection device configured for coring tissue,
wherein the device
comprises: a first clamping element comprising a helical coil and a first
electrode, a second
clamping element comprising a second electrode, the second clamping element
being positioned
to oppose at least a portion of the first clamping element, and a cutting
element configured for
the transection of tissue; and a handle assembly configured to facilitate
interaction between
tissue and at least one of the first clamping element, the second clamping
element, or the cutting
element.
[00279] The handle assembly may facilitate connection of at least one
electrode such as
at least one of the first electrode and the second electrode to a generator.
The handle assembly
67
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
may facilitate connection of at least one electrode such as at least one of
the first electrode and
the second electrode to a computing device. The handle assembly may facilitate
connection of at
least one electrode such as at least one of the first electrode and the second
electrode to a robotic
system. The handle assembly may be configured to automate advancement of at
least one
electrode such as at least one of the first electrode and the second
electrode. The handle assembly
may be configured to automate delivery of energy to at least one electrode
such as at least one of
the first electrode and the second electrode.
[00280] In an aspect, a surgical instrument system for coring tissue from a
target tissue
site may comprise a tissue resection device configured for coring tissue,
wherein the device
comprises: a helical coil electrode, and a cutting element configured to
cooperate with the helical
cold electrode for the transection of tissue; and computing logic configured
to automate use one
or more functions of the tissue resection device.
[00281] In an aspect, a surgical instrument system for coring tissue from a
target tissue
site may comprise a tissue resection device configured for coring tissue,
wherein the device
comprises: a first clamping element comprising a helical coil and a first
electrode, a second
clamping element comprising a second electrode, the second clamping element
being positioned
to oppose at least a portion of the first clamping element, and a cutting
element configured for
the transection of tissue; and computing logic configured to automate use one
or more functions
of the tissue resection device.
[00282] The computing logic may be configured to automate advancement of at
least
one electrode such as at least one of the first electrode and the second
electrode. The computing
logic may be configured to automate delivery of energy to at least one
electrode such as one of
the first electrode and the second electrode. The computing logic may be
configured to determine
an energy distribution provided via the tissue resection device. The computing
logic may be
configured to receive one or more inputs relating to the resection device such
as one or more of
the first clamping element, the second clamping element, or the cutting
element. The computing
logic may be disposed in a handle assembly associated with the tissue
resection device. The
computing logic may be disposed in a generator in communication with the
tissue resection
device.
Handle Desi2n Sequence
68
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00283] A tissue coring device with helix coil and anvil electrodes is
provided to track
along the anchor to remove a target tissue. Once the coring device is placed
over the anchor and
has made contact with the tissue surface with the distal tip of the helix
coil, the following
sequence of steps may be performed to core the target tissue while sealing
fluid vessels
simultaneously. An example method 3700 comprising an operational sequence is
shown for
illustration in FIGS. 37A-37B. Method may comprise a coring procedure start
step 3702. For
instance, a coring device may be placed over an anchor. The method 3700 may
further comprise
one or more of the following steps.
[00284] At step 3704, the anchor may be placed at a depth of 3-5 cm (e.g.,
using an
insertion stopper), based on the targeted tissue depth from organ surface.
[00285] At step 3706, the anchor may be deployed and the insertion stopper may
be
removed.
[00286] At step 3708, a center aid may be placed over the anchor.
[00287] At step 3710, the anchor may be passed through the coring device until
a
coring device coil is above the pleura and centered around the anchor.
[00288] At step 3712, an advancement button may be activated (e.g., pressed).
[00289] At step 3714, a coil electrode may be rotated a 5/4 turn through an
organ
surface (e.g., the pleura of a lung). Initial rotation of the coil electrode
may engage coil electrode
into tissue. If using a helix coil electrode, fluid vessels that may be caught
in the helix section of
the helix coil may be moved to a flat portion of the helix coil.
[00290] At step 3716, an anvil electrode may be clamped against the coil
electrode. In
some embodiments, tissue may be clamped between the helix coil and one or more
anvil
electrodes to a predetermined gap that is suitable for vessel sealing.
[00291] At step 3718, a controller may apply RF energy to one or more
electrodes to
cauterize the tissue and seal any fluid vessels clamped between the
electrodes. RF energy may be
applied between the helix coil and anvil electrodes to perform vessel sealings
between the
electrodes.
[00292] At step 3720, a hold or wait period of about ten (10) seconds may be
initiated.
[00293] At step 3722, a determination may be made as to whether a generator
warning
and/or instruction was received during cauterization.
[00294] If yes, steps 3724 ¨ 3730 may be initiated.
69
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00295] At step 3724, a warning button may be activated (e.g., pressed or
selected) to
clear the warning.
[00296] At step 3726, one or more electrodes may be deactivated.
[00297] At step 3728, one or more anvil electrodes may be unclamped from one
or
more coil electrodes.
[00298] At step 3730, a coil electrode may be reversed a 1/36 counterclockwise
turn.
[00299] After completing steps 3724-3730, the method 3700 may cycle back to
step
3716.
[00300] If no, the process may advance to step 3716 and proceed as described
herein.
[00301] At step 3732, an advancement button may be activated (e.g., pressed).
[00302] At step 3734, one or more electrodes may be deactivated and
disconnected.
[00303] At step 3738, a blade tube may be turned a 1/2 turn to dissect cored
tissue from
surrounding tissue. For instance, the tissue core may be dissected via a
mechanical blade tube.
[00304] At step 3740, the blade tube may be retracted and the coil electrode
may be
disconnected.
[00305] Continuing to FIG. 37B from FIG. 37A as indicated, at step 3742, a
determination may be made as to whether the anchor is locked into the coring
device (i.e.,
indicating targeted tissue at at least 3 cm depth has been reached).
[00306] If no, at step 3744, the coil electrode may be turned a 3/4 turn and
the method
3700 may cycle back to step 3716.
[00307] If yes, the method 3700 may advance to step 3746, where an advancement
button may be activated (e.g., pressed).
[00308] At step 3748, a cutting tool may be fully retracted.
[00309] At step 3750, a ligation line may be pulled and held in tension.
[00310] At step 3752, one or more electrodes may be activated. RF energy may
be
applied between a second set of electrodes to seal any fluid vessels within
the ligation line loop
and between the electrodes.
[00311] At step 3754, a hold or wait period of about ten (10) seconds may be
initiated.
[00312] At step 3756, one or more electrodes may be deactivated. The anvil
electrode
may be separated from the helix electrode.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00313] The cycle of rotating the helix coil, clamping tissue between
electrodes,
applying RF energy to seal vessel, dissecting the tissue core and separating
the anvil and helix
electrodes maybe repeated as needed. Once the target tissue is cored and is
within the blade tube,
a ligation line may be deployed to squeeze the distal end of the target tissue
between a second set
of electrodes.
[00314] At step 3758, ligation line tension may be maintained.
[00315] At step 3760, an amputation line may be activated to amputate cored
tissue
from surrounding tissue. For instance, a machinal line may be deployed to
amputate the target
tissue at a proximal position to the ligation line.
[00316] At step 3762, a cavity port may be spun clockwise and down until
resistance is
felt.
[00317] At step 3764, a cavity port may be spun counter-clockwise and down
until
resistance is felt.
[00318] At step 3766, the coil electrode may be turned a 3/4 turn counter-
clockwise.
For instance, a helix coil may be rotated to disengage the helix coil from the
surrounding tissue.
[00319] At step 3768, the coring device may be removed with the cored tissue
(e.g., the
target tissue sample).
[00320] At step 3770, the anchor may be unlocked.
[00321] At step 3772, the anchor may be undeployed and removed from the coring
device.
[00322] At step 3774, the cored tissue may be removed from the anchor for
subsequent
tissue lab work.
[00323] At step 3776, the coring procedure may be complete.
[00324] To reduce the number of manual steps that a user needs to perform the
tissue
coring device, there is a need to incorporate a handle mechanism to drive the
sequence of steps
described above. The mechanism is controlled through electrical hardware
enclosed in the
device handle with the coring device gear mechanism and firmware sequence
installed in a
generator (also refers a controller). The sequence may have different modes of
input, e.g.
operator action, advancement, warning, etc. Following is a concept of using
said modes of input
to automate the post tissue coring procedure.
71
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00325] Following are the design description of handle mechanism and the step
sequence to be controlled by the controller. The sequence shows a target
tissue to be cored out
at 3cm below the organ surface as an example.
[00326] A handle design 3200 is shown, for example, in FIG. 32. As shown, the
handle
3200 may be configured for a tissue coring device with the helix coil and
anvil electrodes, and
may comprise dependent mechanisms (e.g., four) for automated rotation, tissue
clamp, device
position control relative to the anchor, and ligation/amputation of the target
tissue. As described
above, once the coring device is placed over the anchor and in contact with
the tissue surface
with the distal tip of the helix coil, the said sequence of steps is performed
with a planetary gear
system with three rotational states for coil and mechanical blade rotation, a
clamping cam shaft
3202 for vessel sealing, an optic anchor position monitoring mechanism to
identify when the
cored target tissue is within a mechanical blade tube, and an integrated bi-
directional pulley
system 3204 for ligation/amputation of the cored target tissue. The handle
3200 may further
comprise an automated housing cap 3206, a solenoid 3208, manual clutch wings
3210, an
automation handle housing 3212, a coil cam pin 3214, and a mechanical blade
cam pin 3216 The
following mechanisms are described in greater detail below.
[00327] FIG. 33 illustrates an example rotation control assembly 3300 (e.g.,
planetary
gear assembly). The rotation control assembly 3300 shown in FIG. 33 may be
configured to
control bi-directional rotation for the tissue coring coil and single
direction rotation for a
mechanical blade tube 3310 with one motor control, requiring the system to
maintain two
degrees of freedom. To initialize the rotation of the coil to engage coil into
tissue, a motor 3302
may be rotated counter-clockwise and a coil one-way bearing 3316 in-line with
the motor shaft
3304 may be engaged. Subsequently, a planetary gear mechanism may be activated
and a ring
gear 3306 may be rotated clockwise, engaging a jack shaft 3322. The jack shaft
3322 may then
be rotated counter-clockwise and engages a coil tube gear 3312, allowing the
coil to rotate a
predetermined rotational distance. To initialize the rotation of a mechanical
blade tube 3310 to
dissect the tissue core, the motor 3302 may be rotated clockwise, a mechanical
blade one-way
bearing 3308 in-line with a motor shaft 3304 may be engaged, and the planetary
gear mechanism
may be inactive, allowing the mechanical blade tube 3310 to rotate a
predetermined rotational
distance. To initialize the counterclockwise rotation of the coil to disengage
coil into tissue, a
manual clutch 3320 may be shifted to the up position and a cone clutch 3318
may be moved into
72
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
the contact with the planetary gear system. Subsequently, the motor 3302 may
be rotated
counter-clockwise and a coil one-way bearing 3316 in-line with the motor shaft
3304 may be
engaged. The planetary gear mechanism may be active and a ring gear 3306 may
be rotated
counter-clockwise, engaging a jack shaft 3322. The jack shaft 3322 may then be
rotated
clockwise and engages a coil tube gear 3312, allowing the coil to rotate a
predetermined
rotational distance.
[00328] FIG. 34 illustrates a linear translation control assembly 3400 in the
form of a
clamping cam shaft and pin mechanism. The clamping cam shaft and pin mechanism
shown in
FIG. 34 controls linear translation of a mechanical blade tube (not shown) and
a coil tube 3408
for tissue engagement, vessel sealing, and dissection. After a helix coil is
rotated a predetermined
distance to engage target tissue, a cam shaft 3402 with machined slots housed
on a servo motor
3420 may be rotated clockwise. Subsequently, a coil cam pin 3404 follows a cam
path 3406 and
a coil tube 3408 may be linearly translated to clamp tissue between the helix
coil and anvil
electrodes to a predetermined gap that may be suitable for vessel sealing.
Following, the servo
motor 3420 may be rotated clockwise, a mechanical blade cam pin 3410 may
follow the
mechanical blade path 3412, and a mechanical blade tube (not shown) may be
linearly translated
to a dissection position. At the end of the cycle, the servo motor 3420 may be
rotated counter-
clockwise to separate the anvil electrode from the helix electrode and
translate the mechanical
blade tube out of the dissection position.
[00329] FIG. 35 illustrates an anchor position monitor 3500. The anchor
position
monitor 3500 may be an optic anchor position monitoring mechanism and may
actively monitor
the target core tissue location, on an anchor, relative to a tissue coring
device through an optical
sensor 3502 in-line with the anchor. When target tissue is cored and within a
mechanical blade
tube, a pre-set marking on the anchor will be in-line with the optical sensor
3502 or optical
sensing unit, alerting the system that the tissue coring device is in the
optimal position.
Subsequently, a solenoid 3504 may be fired and engage a spring-loaded anchor
lock 3506, and
lock the anchor position relative to the tissue coring device.
[00330] FIG. 36 illustrates an example ligation and amputation system 3600.
The
ligation and amputation system 3600 may be a bi-direction pulley system and
may control
deployment of the ligation and amputation machinal lines. Once a target tissue
is within a blade
tube as described relative to FIGS. 34-35, a servo motor 3602 may be rotated
clockwise.
73
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
Subsequently, a one-way bearing 3604 housed within the ligation pulley 3606
may be engaged, a
ligation machinal line may be deployed, and a ligation pawl 3608 may hold the
line in tension to
squeeze a distal end of the target tissue between a second set of electrodes.
After RF energy
between the second set of electrodes to seal fluid vessels within the ligation
loop is completed,
the servo motor 3602 may be rotated counter-clockwise and a one-way bearing
3610 housed
within an amputation pulley 3612 is engaged, deploying an amputation machinal
line to
amputate the target tissue at a proximal position to the ligation line.
[00331] FIG. 38 illustrates a handle design 3800 for the tissue coring device
comprising
a helix coil and one or more anvil electrodes and may comprise multiple
attachments to a tissue
coring device for automated rotation, tissue clamp, and ligation/amputation of
the target tissue.
As described above, once a coring device is placed over an anchor and in
contact with a tissue
surface with a distal tip of the helix coil, the above described sequence of
steps may be
performed with two independent gear systems for coil and mechanical blade
rotation, clamping
actuator plate, and two independent spring-loaded systems for ligation and
amputation. The
handle design 3800 may comprise a mechanical blade gear set 3802, a mechanical
blade stepper
motor 3804, a coil stepper motor 3806, a spring loaded amputation knob 3808, a
spring loaded
ligation knob 3810, a clamp plate housing 3812 and a clamp linear actuator
3814. The following
mechanisms are described in greater detail below.
[00332] FIG. 39 illustrates a rotation control assembly 3900. The rotation
control
assembly 3900 may be an independent gear system configured to control bi-
directional rotation
for a tissue coring coil and a mechanical blade tube with two motor control.
To initialize
clockwise rotation of the coil (i.e., to engage the coil into tissue), a coil
stepper motor (e.g., the
coil stepper motor 3806 of FIG. 38) may be rotated counter-clockwise and the
coil gear system
may be active. Subsequently, the coil may be allowed to rotate a predetermined
rotational
distance and fluid vessels that are caught in the helix section of the helix
coil are moved to the
flat portion of the helix coil. To initialize the rotation of the mechanical
blade tube to dissect the
target tissue core, the blade stepper motor (e.g., the mechanical blade
stepper motor 3804 of FIG.
38) may be rotated and the blade tube gear system may be active, allowing the
mechanical blade
tube to rotate a predetermined rotational distance. To initialize the
counterclockwise rotation of
the coil to disengage coil into tissue, the coil stepper motor may be rotated
clockwise, the coil
gear system 3902 may be active, and the coil tube may be allowed to rotate a
predetermined
74
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
rotational distance. The rotation control assembly 3900 may further comprise a
cutting tool
motor bracket 3904, a motor housing/handle 3906, a linear actuator clamp plate
3908, and a
linear actuator frame 3910
[00333] FIG. 40 illustrates an example clamp 4000. A clamping solenoid plate
controls
linear translation of the coil tube for tissue engagement and vessel sealing.
After the helix coil
may be rotated a predetermined distance clockwise to engage target tissue, the
linear actuators
4002 housed on the carrier plate 4004 may be activated. Subsequently, the
actuator arms may be
linearly translated to clamp tissue between the helix coil and anvil
electrodes to a predetermined
gap suitable for vessel sealing. At the end of the RF energy cycle, the linear
actuators may be de-
activated and the coil tube may be allowed to un-clamp under gravity. The
clamp 4000 may
further comprise a coil gear set 4006, a clamp plate housing 4008, a linear
actuator frame 4010.
[00334] FIG. 41 illustrates an example ligation and amputation system 4100.
Independent spring-loaded mechanisms for ligation and amputation may control
deployment of
the ligation and amputation machinal lines. Once the target tissue is within a
blade tube, a
ligation spring 4102 may be engaged (e.g., pushed down by, for instance, an
operator) and a pin
may be rotated clockwise. The ligation and amputation system may further
comprise a ligation
knob 4104, a spring base 4106, and a locking pathway 4108. Subsequently, the
spring-loaded pin
may be activated and the ligation machinal line may be deployed and held in
tension to squeeze
the distal end of the target tissue between the second set of electrodes.
After the application of
RF energy between the second set of electrodes to seal fluid vessels with the
ligation loop is
completed, the amputation spring may be engaged (e.g., by an operator) and the
pin may be
rotated clockwise, thereby activating the spring-loaded pin and deploying the
amputation
machinal line to amputate the target tissues at a proximal position to the
ligation line.
[00335] The present disclosure comprises at least the following aspects:
[00336] Aspect 1. A method for coring tissue from a target tissue site, the
method
comprising: delivering a first energy modality to an end effector of a
surgical instrument
interacting with tissue, wherein the end effector is configured for coring
tissue and comprises: a
first clamping element comprising a helical coil and a first electrode, a
second clamping element
comprising a second electrode, the second clamping element being positioned to
oppose at least
a portion of the first clamping element, and a cutting element configured for
the transection of
tissue.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00337] Aspect 2. The method of aspect 1, further comprising: determining a
tissue
parameter of the tissue interacting with the end effector; and delivering a
second energy modality
to the end effector based on the determined tissue parameter, wherein the
first energy modality is
different from the second energy modality.
[00338] Aspect 3. The method of aspect 2, wherein the tissue parameter
comprises
tissue impedance.
[00339] Aspect 4. The method of aspect 3, further comprising calculating the
tissue
impedance based on electrical parameters associated with the first energy
modality.
[00340] Aspect 5. The method of any one of aspects 2-4, further comprising:
controlling the first energy modality delivered to the end effector based on
the tissue parameter
as an input; and selecting the second energy modality to deliver to interact
with the tissue by the
end effector, wherein properties of the first energy modality and the second
energy modality
correspond to a type of interaction between the end effector and the tissue.
[00341] Aspect 6. A method for coring tissue from a target tissue site, the
method
comprising: delivering a first drive signal to an end effector of a surgical
instrument interacting
with tissue, wherein the end effector is configured for coring tissue and
comprises: a first
clamping element comprising a helical coil and a first electrode, a second
clamping element
comprising a second electrode, the second clamping element being positioned to
oppose at least
a portion of the first clamping element; and a cutting element configured for
the transection of
tissue; measuring a tissue parameter of tissue interacting with the end
effector; modulating
delivery of the first drive signal based on the measured tissue parameter; and
ceasing delivery of
the first drive signal when a termination parameter is met.
[00342] Aspect 7. The method of aspect 6, wherein the first drive signal is a
radio
frequency (RF) energy signal.
[00343] Aspect 8. The method of any one of aspects 6-7, wherein the first
drive signal
is an ultrasonic energy signal.
[00344] Aspect 9. The method of any one of aspects 6-8, further comprising
determining that the tissue is sealed based on at least one of initial tissue
impedance, initial
aperture defined by jaws of an end effector, current tissue impedance, rate of
change of tissue
impedance, ultrasonic energy driven into the tissue, radio frequency (RF)
energy driven into the
tissue, or transaction time.
76
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00345] Aspect 10. The method of any one of aspects 6-9, wherein the first
drive signal
comprises a first energy signal, and wherein modulating delivery of the first
energy signal based
on a measured tissue impedance comprises modifying an output power of a
generator, modifying
an output waveform of the generator, selecting a second energy signal to
deliver to the surgical
instrument, or modifying the termination parameter.
[00346] Aspect 11. The method of any one of aspects 6-10, further comprising
ceasing
delivery of the first drive signal upon the tissue parameter meeting or
exceeding a threshold
value of the tissue parameter.
[00347] Aspect 12. The method of any one of aspects 6-11, wherein the first
drive
signal comprises a first energy signal, wherein an amplitude of the first
energy signal is a first
amplitude, the method further comprising delivering a second drive signal to
tissue at a second
amplitude different from the first amplitude.
[00348] Aspect 13. The method of aspect 12, wherein the first drive signal is
a radio
frequency (RF) energy signal and the second drive signal is an ultrasonic
energy signal.
[00349] Aspect 14. The method of any one of aspects 6-13, wherein measuring
the
tissue parameter comprises measuring a rate of change of the tissue parameter.
[00350] Aspect 15. The method of any one of aspects 6-14, further comprising
determining a state of the tissue based on the measured tissue parameter.
[00351] Aspect 16. The method of aspect 15, wherein the state of the tissue
comprises
coagulated, sealed, or cut.
[00352] Aspect 17. The method of any one of aspects 6-16, wherein modulating
delivery of the first drive signal to the end effector to cause the tissue
parameter to change
according to a predetermined technique.
[00353] Aspect 18. The method of aspect 17, wherein the predetermined
technique
comprises adjusting the tissue parameter according to a threshold value of the
tissue parameter or
a threshold rate of change of the tissue parameter.
[00354] Aspect 19. The method of any one of aspects 6-18, wherein the tissue
parameter is based on dividing a voltage measurement of radio frequency (RF)
energy by a
current measurement of the RF energy.
77
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00355] Aspect 20. The method of aspect 19, wherein a threshold value of the
tissue
parameter corresponds to a termination impedance at which a seal is complete
during
coagulation of the tissue utilizing RF energy.
[00356] Aspect 21. A surgical instrument system for coring tissue from a
target tissue
site, the system comprising: a tissue resection device configured for coring
tissue, wherein the
device comprises: a first clamping element comprising a helical coil and a
first electrode, a
second clamping element comprising a second electrode, the second clamping
element being
positioned to oppose at least a portion of the first clamping element, and a
cutting element
configured for the transection of tissue; a handle assembly comprising a
trigger system, wherein
the trigger system is configured to facilitate interaction between tissue and
at least one of the first
clamping element, the second clamping element, or the cutting element; and a
generator
configured to deliver energy to the tissue resection device.
[00357] Aspect 22. The system of aspect 21, further comprising a controller in
communication with the generator, wherein the controller is configured to
control the generator
to provide radiofrequency energy sufficient to seal tissue to the first and
second electrodes of the
tissue resection device, based on at least one sensed operating condition of
the tissue resection
device.
[00358] Aspect 23. The system of aspect 22, wherein the controller is
configured to
sense an interaction of tissue with the tissue resection device.
[00359] Aspect 24. The system of aspect 23, wherein the controller is
configured to
sense the interaction of tissue with the tissue resection device based on a
measured impedance
level associated with the first and second electrodes.
[00360] Aspect 25. The system of aspects 23-24, wherein the controller is
configured to
sense an amount of force applied to at least one of the first or second
clamping elements to detect
the interaction of tissue with the tissue resection device.
[00361] Aspect 26. The system of any one of aspects 22-25, wherein the
controller is
configured to sense the position of the cutting element relative to at least
one of the first or
second clamping elements of the tissue resection device.
[00362] Aspect 27. The system of any one of aspects 22-26, wherein the
controller is
configured to control the generator to provide a continuous amount of
radiofrequency energy.
78
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00363] Aspect 28. The system of any one of aspects 22-27, wherein the
controller is
configured to control the generator to automatically provide an increase or
decrease in an amount
of radiofrequency energy.
[00364] Aspect 29. The system of any one of aspects 22-28, wherein the
controller is
configured to control the position of the first clamping element relative to
the second clamping
element.
[00365] Aspect 30. The system of any one of aspects 22-29, wherein the
controller is
configured to control the position of the cutting element relative to at least
one of the first or
second clamping elements of the tissue resection device.
[00366] Aspect 31. The system of any one of aspects 22-30, wherein the tissue
resection device and the generator are at least on of mechanically or
electrically connected to the
handle assembly.
[00367] Aspect 32. A method comprising: disposing a coring device over an
anchor,
the coring device comprising a helix coil having a coil section and a flat
portion; rotating the
helix coil of the coring device to engage the helix coil into tissue such that
fluid vessels that are
caught between the coil section are moved to the flat portion of the helix
coil; clamping tissue
between the helix coil and at least two anvil electrodes of the coring device;
applying radio
frequency (RF) energy between the helix coil and anvil electrodes to perform
vessel sealing
between the electrodes; dissecting a tissue core via a mechanical blade tube
of the coring device;
separating the electrodes from each other; and repeating a cycle of rotating
the helix coil,
clamping tissue between electrodes, applying RF to seal vessel, dissecting
tissue core, and
separating the electrodes.
[00368] Aspect 33. The method of aspect 32, further comprising: once a target
tissue is
cored and is within the blade tube, deploying a ligation line to squeeze a
distal end of the target
tissue between a second set of electrodes; applying RF energy between a second
set of electrodes
to seal any fluid vessels within a ligation line loop and between the
electrodes; deploying a
machinal line to amputate the target tissues at a proximal position to the
ligation line; rotating the
helix coil to disengage it from surrounding tissue; and remove the coring
device with target
tissue sample.
[00369] Aspect 34. A surgical instrument system for coring tissue from a
target tissue
site, the system comprising: a tissue resection device configured for coring
tissue, wherein the
79
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
device comprises: a first clamping element comprising a helical coil and a
first electrode, a
second clamping element comprising a second electrode, the second clamping
element being
positioned to oppose at least a portion of the first clamping element, and a
cutting element
configured for the transection of tissue; and a handle assembly configured to
facilitate interaction
between tissue and at least one of the first clamping element, the second
clamping element, or
the cutting element.
[00370] Aspect 35. The system of aspect 34, wherein the handle assembly
facilitates
connection of at least one of the first electrode and the second electrode to
a generator.
[00371] Aspect 36. The system of any one of aspects 34-35, wherein the handle
assembly facilitates connection of at least one of the first electrode and the
second electrode to a
computing device.
[00372] Aspect 37. The system of any one of aspects 34-36, wherein the handle
assembly facilitates connection of at least one of the first electrode and the
second electrode to a
robotic system.
[00373] Aspect 38. The system of any one of aspects 34-37, wherein the handle
assembly is configured to automate advancement of at least one of the first
electrode and the
second electrode.
[00374] Aspect 39. The system of any one of aspects 34-38, wherein the handle
assembly is configured to automate delivery of energy to at least one of the
first electrode and
the second electrode.
[00375] Aspect 40. A surgical instrument system for coring tissue from a
target tissue
site, the system comprising: a tissue resection device configured for coring
tissue, wherein the
device comprises: a first clamping element comprising a helical coil and a
first electrode, a
second clamping element comprising a second electrode, the second clamping
element being
positioned to oppose at least a portion of the first clamping element, and a
cutting element
configured for the transection of tissue; and computing logic configured to
automate use one or
more functions of the tissue resection device.
[00376] Aspect 41. The system of aspect 40, wherein the computing logic is
configured
to automate advancement of at least one of the first electrode and the second
electrode.
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
[00377] Aspect 42. The system of any one of aspects 40-41, wherein the
computing
logic is configured to automate delivery of energy to at least one of the
first electrode and the
second electrode.
[00378] Aspect 43. The system of any one of aspects 40-42, wherein the
computing
logic is configured to determine an energy distribution provided via the
tissue resection device.
[00379] Aspect 44. The system of any one of aspects 40-43, wherein the
computing
logic is configured to receive one or more inputs relating to one or more of
the first clamping
element, the second clamping element, or the cutting element.
[00380] Aspect 45. The system of any one of aspects 40-44, wherein the
computing
logic is disposed in a handle assembly associated with the tissue resection
device.
[00381] Aspect 46. The system of any one of aspects 40-45, wherein the
computing
logic is disposed in a generator in communication with the tissue resection
device.
[00382] Aspect 47. A surgical instrument system for coring tissue from a
target tissue
site, the system comprising: a tissue resection device configured for coring
tissue, wherein the
device comprises: a helical coil electrode, and a cutting element configured
to cooperate with the
helical coil electrode for the transection of tissue; and a handle assembly
configured to facilitate
interaction between tissue the tissue resection device.
[00383] Aspect 48. The system of aspect 47, wherein the handle assembly
facilitates
connection of the electrode to a generator.
[00384] Aspect 49. The system of any one of aspects 47-48, wherein the handle
assembly facilitates connection of the electrode to a computing device.
[00385] Aspect 50. The system of any one of aspects 47-49, wherein the handle
assembly facilitates connection of the electrode to a robotic system.
[00386] Aspect 51. The system of any one of aspects 47-50, wherein the handle
assembly is configured to automate advancement of the electrode.
[00387] Aspect 52. The system of any one of aspects 47-51, wherein the handle
assembly is configured to automate delivery of energy to the electrode.
[00388] Aspect 53. A surgical instrument system for coring tissue from a
target tissue
site, the system comprising: a tissue resection device configured for coring
tissue, wherein the
device comprises: a helical coil electrode, and a cutting element configured
to cooperate with the
81
CA 03186596 2022-12-07
WO 2021/250526 PCT/IB2021/054934
helical coil electrode for the transection of tissue; and computing logic
configured to automate
use one or more functions of the tissue resection device.
[00389] Aspect 54. The system of aspect 53, wherein the computing logic is
configured
to automate advancement of the electrode.
[00390] Aspect 55. The system of any one of aspects 53-54, wherein the
computing
logic is configured to automate delivery of energy to the electrode.
[00391] Aspect 56. The system of any one of aspects 53-55, wherein the
computing
logic is configured to determine an energy distribution provided via the
tissue resection device.
[00392] Aspect 57. The system of any one of aspects 53-56, wherein the
computing
logic is configured to receive one or more inputs relating to the tissue
resection device.
[00393] Aspect 58. The system of any one of aspects 53-57, wherein the
computing
logic is disposed in a handle assembly associated with the tissue resection
device.
[00394] Aspect 59. The system of any one of aspects 53-58, wherein the
computing
logic is disposed in a generator in communication with the tissue resection
device.
[00395] Although shown and described is what is believed to be the most
practical and
preferred embodiments, it is apparent that departures from specific designs
and methods
described and shown will suggest themselves to those skilled in the art and
may be used without
departing from the spirit and scope of the invention. For example, the
systems, devices and
methods described herein for removal of lesions from the lung. It will be
appreciated by the
skilled artisan that the devices and methods described herein may are not
limited to the lung and
could be used for tissue resection and lesion removal in other areas of the
body. The present
invention is not restricted to the particular constructions described and
illustrated, but should be
constructed to cohere with all modifications that may fall within the scope of
the appended
claims.
82