Note: Descriptions are shown in the official language in which they were submitted.
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
mRNA TRANSFECTION OF IMMUNE CELLS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to and the benefit of U.S.
Provisional Patent
Application No. 63/044,855, filed June 26, 2020, the entirety of which is
incorporated herein by
reference.
BACKGROUND
[0002] Although immunotherapies have been investigated for many diseases
and
disorders, including cancer, Alzheimer's disease, Parkinson's disease,
amyotrophic lateral
sclerosis (ALS), systemic amyloidosis, prion disease, cardiovascular disease,
atherosclerosis,
fibrosis, functional limitations that have been encountered still need to be
addressed.
[0003] Therefore, a need exists for the development of therapeutic
modalities optimized
for enhanced expression, viability, and function.
SUMMARY OF THE INVENTION
[0004] The present disclosure encompasses, among other things, methods,
systems and
compositions for modifying immune cells including monocytes, macrophages
and/or dendritic
cells. In some embodiments, provided methods, systems, and/or compositions
provide for
enhanced production and/or enhanced properties of modified immune cells.
Surprisingly, the
present disclosure encompasses the recognition that use of modified mRNA
(e.g., a 5'-cap or
uridine modified mRNA encoding a transgene of interest such a chimeric antigen
receptor
(CAR)) can result in significantly increased levels of expression, persistence
of expression, and
viability of human monocytes, macrophages and/or dendritic cells.
Additionally, the present
disclosure encompasses the recognition that use of interferon beta can result
in significantly
increased levels of expression, persistence of expression, and function of CAR
monocytes,
macrophages, and/or dendritic cells that have been transfected with mRNA
encoding a CAR or
other transgene. The present disclosure also encompasses the recognition that
use of an RNAse
1
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
inhibitor (e.g., an RNAseL inhibitor) can result in significantly increased
levels of expression of
a desired polynucleotide and/or polypeptide (e.g., a transgene).
[0005] In one aspect, the present disclosure provides methods of
modifying an immune
cell, the method comprising the steps of: modifying a messenger RNA (mRNA)
encoding a
chimeric antigen receptor (CAR), purifying the mRNA, and delivering the mRNA
to the immune
cell, wherein the immune cell comprises a macrophage, a monocyte or a
dendritic cell, and
wherein the modified immune cell comprises a CAR.
[0006] In some embodiments, a step of modifying comprises causing mRNA to
include a
modified nucleotide, an alteration to the 5' or 3' untranslated region (UTR),
a cap structure,
and/or a poly(A) tail. In some embodiments, a cap structure comprises AGCapl,
m6AGCap1, or
Anti-Reverse Cap Analog (ARCA). In some embodiments, a modified nucleotide
comprises
pseudouridine (PsU), 5-methoxyuridine (5moU), 5-methylcytidine/pseudouridine
(5meC PsU),
Ni-methyl-pseudouridine (N1mPsU), or combinations thereof. In some
embodiments, a step of
purifying comprises silica membrane purification and/or high performance
liquid
chromatography (HPLC). In some embodiments, a step of delivering comprises
transfection.
[0007] In some embodiments, a step of modifying comprises causing mRNA to
include
AGCapl and 5moU, a step of purifying comprises silica membrane purification,
and a step of
delivering comprises electroporation. In some embodiments, a step of modifying
comprises
causing mRNA to include AGCapl and PsU, a step of purifying comprises HPLC,
and a step of
delivering comprises electroporation. In some embodiments, a step of modifying
comprises
causing mRNA to include AGCapl and N1mPsU, a step of purifying comprises HPLC,
and a
step of delivering comprises electroporation. In some embodiments, a step of
modifying
comprises causing mRNA to include m6-AGCap1 and N1mPsU, a step of purifying
comprises
HPLC, and a step of delivering comprises electroporation. In some embodiments,
a step of
modifying comprises causing mRNA to include m6-AGCap1 and PsU, a step of
purifying
comprises HPLC, and a step of delivering comprises electroporation. In some
embodiments, a
step of modifying comprises modifying mRNA to include AGCapl and PsU, a step
of purifying
comprises HPLC, and a step of delivering comprises transfection. In some
embodiments, a step
of modifying comprises causing mRNA to include m6-AGCap1 and PsU, a step of
purifying
comprises HPLC, and a step of delivering comprises transfection. In some
embodiments, a step
2
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
of modifying comprises causing mRNA to include m6-AGCap1 and N1mPsU, a step of
purifying comprises HPLC, and a step of delivering comprises transfection. In
some
embodiments, a step of modifying comprises causing mRNA to include AGCapl and
5moU. In
some embodiments, a step of modifying comprises causing mRNA to include
m6AGCap1 and
5moU.
[0008] In some embodiments, a method of the present invention further
comprises a step
of treating the immune cell with an RNaseL inhibitor. In some embodiments, an
RNaseL
inhibitor comprises sunitinib. In some embodiments, an RNaseL inhibitor
comprises ABCE1.
[0009] In some embodiments, a step of treating occurs before a step of
delivering.
[0010] In some embodiments, a method of the present invention further
comprises a step
of culturing an immune cell with a cytokine or immune stimulating recombinant
protein. In
some embodiments, a cytokine comprises IFN-a, IFN-f3, IFN-y, TNFa, IL-6,
STNGL, LPS, a
CD40 agonist, a 4-1BB ligand, a recombinant 4-1BB receptor, a TLR agonist,
beta-glucan, IL-4,
IL-13, IL-10, TGF-f3, a glucocorticoid, an immune complex, or a combination
thereof In some
embodiments, a cytokine comprises IFN-f3.
[0011] In some embodiments, a step of culturing occurs after the step of
delivering.
[0012] In some embodiments, a method of the present invention results in
a modified
immune cell that expresses a CAR. In some embodiments, CAR expression is
increased relative
to CAR expression in a modified immune cell of the same type wherein
unmodified mRNA
encoding a CAR was delivered. In some embodiments, a modified immune cell
exhibits
increased effector activity relative to effector activity in a modified immune
cell of the same type
wherein unmodified mRNA encoding the CAR was delivered.
[0013] In another aspect, the present disclosure provides a modified
immune cell made
by a method of the present invention. In some embodiments, a modified immune
cell exhibits
increased viability relative to a modified immune cell of the same type
comprising unmodified
mRNA encoding a CAR. In some embodiments, a modified immune cell exhibits
increased
expression of mRNA encoding a CAR relative to a modified immune cell of the
same type
comprising unmodified mRNA encoding a CAR. In some embodiments, a modified
immune
cell exhibits increased CAR expression relative to a modified immune cell of
the same type
3
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
comprising unmodified mRNA encoding a CAR. In some embodiments, a modified
immune
cell exhibits increased longevity of mRNA encoding a CAR relative to a
modified immune cell
of the same type comprising unmodified mRNA encoding a CAR. In some
embodiments, a
modified immune cell exhibits increased longevity of a CAR relative to a
modified immune cell
of the same type comprising unmodified mRNA encoding a CAR. In some
embodiments, a
modified immune cell exhibits increased effector activity relative to a
modified immune cell of
the same type comprising unmodified mRNA encoding a CAR. In some embodiments,
a
modified immune cell exhibits increased M1 polarization relative to a modified
immune cell of
the same type comprising unmodified mRNA encoding a CAR.
[0014] In another aspect, the present disclosure provides compositions
comprising one or
more modified mRNA, wherein the one or more modified mRNA comprise a modified
nucleotide, an alteration to the 5' or 3' untranslated region (UTR), a cap
structure, a poly A tail,
or a combination thereof, and one or more RNaseL inhibitors.
[0015] In some embodiments, a cap structure comprises AGCapl or m6AGCapl.
In
some embodiments, a modified nucleotide comprises pseudouridine (PsU), 5-
methoxyuridine
(5moU), 5-methylcytidine/pseudouridine (5meC PsU), or N1-methyl-pseudouridine
(N1mPsU).
In some embodiments, one or more RNaseL inhibitors comprise sunitinib. In some
embodiments, one or more RNaseL inhibitors comprise ABCE1.
BRIEF DESCRIPTION OF THE DRAWING
[0016] The drawings are for illustration purposes only, not for
limitation.
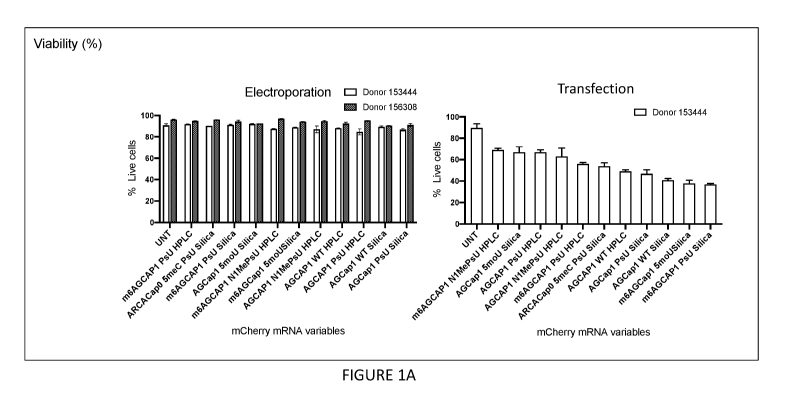
[0017] Figures 1A-1C show exemplary graphs illustrating macrophage
viability (Figure
1A), mean fluorescent intensity (Figure 1B) and persistence (Figure 1C) after
electroporation or
transfection with mCherry mRNA comprising a variety of modifications.
[0018] Figures 2A-2C show exemplary graphs illustrating macrophage
viability (Figure
2A) and CAR expression (Figures 2B and 2C) after electroporation or
transfection with CAR
mRNA comprising a variety of modifications.
[0019] Figures 3A-3D show exemplary graphs illustrating an effect of CAR
mRNA
modifications on macrophage function. Figure 3A shows a tumor growth curve two
days after
4
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
macrophages were electroporated with CAR mRNA and Figures 3B-3D show tumor
growth
curves when cancer cells were co-cultured with CAR macrophages at an Effector
(CAR
macrophage) to Target (cancer cell) ratio of 4:1, 2:1 and 1:1, respectively.
[0020] Figures 4A-4F show exemplary graphs illustrating CAR macrophage
viability
(Figure 4A and Figure 4C), indicated surface marker mean fluorescent intensity
(Figure 4B,
Figure 4D, Figure 4E, and Figure 4F) after treatment with cytokines.
[0021] Figures 5A-5C show exemplary graphs illustrating macrophage
viability (Figure
5A), CAR expression (Figure 5B), and mean fluorescent intensity (Figure 5C)
after transfection
with CAR mRNA comprising a variety of modifications and treatment with an
interferon
cytokine.
[0022] Figure 6 shows an exemplary graph illustrating persistence of CAR
expression in
macrophages treated with an interferon cytokine.
[0023] Figures 7A and 7B show exemplary graphs illustrating CAR
macrophage
viability, CAR expression and mean fluorescent intensity (Figure 7A) and
induction of M1
markers (Figure 7B) after transfection with CAR mRNA and treatment with a
variety of IFN-f3
concentrations.
[0024] Figures 8A and 8B show exemplary graphs illustrating CAR
macrophage M2 and
M1 marker mean fluorescent intensity two days (Figure 8A) or seven days
(Figure 8B) after
electroporation with CAR mRNA and treatment with IFN-f3.
[0025] Figures 9A-9C show exemplary graphs illustrating anti-tumor
function of CAR
macrophages. Figure 9A shows results from macrophages transfected with CAR
mRNA with or
without priming with various concentrations of IFN-f3. Figure 9B shows results
from
macrophages transfected with CAR mRNA comprising a variety of modifications
and treated
with IFN-0. Figure 9C shows results from macrophages transfected with CAR mRNA
and
treated with interferon cytokines.
[0026] Figure 10 shows an exemplary graph illustrating an effect of
treating CAR
macrophages with interferons on cytokine secretion.
[0027] Figures 11A-11C show exemplary graphs illustrating an effect of
treatment with
interferons on CAR mRNA persistence in macrophages and duration of CAR
macrophage
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
functionality. Figure 11A shows results from tests of viability and CAR
expression in CAR
macrophages that had been treated with interferon cytokines. Figure 11B and
Figure 11C show
tumor growth results from cancer cells cultured with macrophages
electroporated with CAR
mRNA and treated with interferon cytokines.
[0028] Figures 12A-12C show exemplary graphs illustrating an effect of
treatment with
interferons on macrophage viability, CAR expression, M1 marker expression, and
CAR
macrophage functionality. Figure 12A shows viability, CAR expression and M1
marker
expression of macrophages transfected with CAR mRNA and treated with
interferons. Figure
12B and Figure 12C show tumor killing results from cancer cells cultured with
macrophages
electroporated with CAR mRNA and treated with interferon cytokines.
[0029] Figure 13 shows an exemplary graph illustrating an effect of IFN-y
on transfected
macrophages.
[0030] Figures 14A-14C show exemplary graphs illustrating an effect of
RNaseL
inhibitors on CAR macrophages. Figure 14A shows mCherry expression in
transfected
macrophages after treatment with IFN-y and the RNaseL inhibitor sunitinib.
Figure 14B shows
a tumor growth curve for cancer cells cultured with CAR macrophages treated
with sunitinib.
Figure 14C shows tumor killing activity of CAR macrophages treated with
sunitinib.
[0031] Figure 15 shows exemplary graphs illustrating macrophage viability
and
mCherry expression of macrophages co-transfected with mRNA encoding mCherry
and with
mRNA encoding the RNaseL inhibitor ABCE1.
[0032] Figure 16 shows an exemplary graph illustrating CAR expression in
macrophages
co-transfected with mRNA encoding a CAR and mRNA encoding the RNaseL inhibitor
NS1.
[0033] Figure 17 shows an exemplary graph illustrating CAR mRNA stability
in
macrophages co-transfected with mRNA encoding a CAR and mRNA encoding either
the
RNaseL inhibitor ABCE1 or the RNaseL inhibitor NS1.
[0034] Figures 18A and 18B are graphs showing tumor killing ability
(Figure 18A) and
induction of expression of M1 and M2 markers (Figure 18B) after incubation of
macrophages
and CAR macrophages with CD40 ligand (CD4OL).
6
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0035] Figures 19A and 19B are graphs showing tumor killing ability
(Figure 19A) and
induction of expression of M1 and M2 markers (Figure 19B) after incubation of
macrophages
and CAR macrophages with 4-1BB.
[0036] Figures 20A and 20B are graphs showing killing tumor killing
ability (Figure
20A) and induction of expression of M1 and M2 markers (Figure 20B) after
incubation of
macrophages and CAR macrophages with 4-1BB ligand (4-1BBL).
[0037] Figure 21 shows exemplary graphs illustrating CAR expression in
human
monocytes electroporated with CAR mRNA.
[0038] Figure 22 shows an exemplary graph illustrating the efficacy of
CAR-
macrophages generated via mRNA electroporation, with or without IFN-f3
priming, in a
xenograft solid tumor mouse model.
[0039] Figure 23 shows an exemplary graph illustrating the efficacy of
CAR-
macrophages generated via mRNA electroporation, with or without IFN-f3
priming, in a
syngeneic solid tumor mouse model.
DEFINITIONS
[0040] In order for the present invention to be more readily understood,
certain terms are
first defined below. Additional definitions for the following terms and other
terms are set forth
throughout the specification. The publications and other reference materials
referenced herein to
describe the background of the invention and to provide additional detail
regarding its practice
are hereby incorporated by reference.
[0041] The articles "a" and "an" are used herein to refer to one or to
more than one (i.e.,
to at least one) of the grammatical object of the article. By way of example,
"an element" means
one element or more than one element.
[0042] Approximately or about: As used herein, the term "approximately"
or "about," as
applied to one or more values of interest, refers to a value that is similar
to a stated reference
value. In certain embodiments, the term "approximately" or "about" refers to a
range of values
that fall within 25%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%, 12%, 11%, 10%,
9%, 8%,
7%, 6%, 5%, 4%, 3%, 2%, 1 %, or less in either direction (greater than or less
than) of the stated
7
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
reference value unless otherwise stated or otherwise evident from the context
(except where such
number would exceed 100% of a possible value).
[0043] Activation: As used herein, the term "activation" refers to the
state of a cell, for
example a monocyte, macrophage, or dendritic cell that has been sufficiently
stimulated to
induce detectable cellular proliferation or has been stimulated to exert its
effector function.
Activation can also be associated with induced cytokine production,
phagocytosis, cell signaling,
target cell killing, and/or antigen processing and presentation.
[0044] Activated monocytes/macrophages/dendritic cells: As used herein,
the term
"activated monocytes/macrophages/dendritic cells" refers to, among other
things,
monocyte/macrophage/dendritic cells that are undergoing cell division or
exerting effector
function. The term "activated monocytes/macrophages/dendritic cells" refers
to, among others
thing, cells that are performing an effector function or exerting any activity
not seen in the
resting state, including phagocytosis, cytokine secretion, proliferation, gene
expression changes,
metabolic changes, and other functions.
[0045] Agent: As used herein, the term "agent" (or "biological agent" or
"therapeutic
agent"), refers to a molecule that may be expressed, released, secreted or
delivered to a target by
a modified cell described herein. An agent includes, but is not limited to, a
nucleic acid, an
antibiotic, an anti-inflammatory agent, an antibody or fragments thereof, an
antibody agent or
fragments thereof, a growth factor, a cytokine, an enzyme, a protein (e.g., an
RNAse inhibitor), a
peptide, a fusion protein, a synthetic molecule, an organic molecule (e.g., a
small molecule), a
carbohydrate, a lipid, a hormone, a microsome, a derivative or a variation
thereof, and any
combinations thereof An agent may bind any cell moiety, such as a receptor, an
antigenic
determinant, or other binding site present on a target or target cell. An
agent may diffuse or be
transported into a cell, where it may act intracellularly.
[0046] Antibody: As used herein, the term "antibody" refers to a
polypeptide that
includes canonical immunoglobulin sequence elements sufficient to confer
specific binding to a
particular target antigen. As is known in the art, intact antibodies as
produced in nature are
approximately 150 kD tetrameric agents comprising two identical heavy chain
polypeptides
(about 50 kD each) and two identical light chain polypeptides (about 25 kD
each) that associate
with each other into what is commonly referred to as a "Y-shaped" structure.
Each heavy chain
8
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
comprises at least four domains (each about 110 amino acids long) ¨ an amino-
terminal variable
(VH) domain (located at the tips of the Y structure), followed by three
constant domains: CHL
CH2, and the carboxy-terminal CH3 (located at the base of the Y's stem). A
short region, known
as the "switch", connects the heavy chain variable and constant regions. The
"hinge" connects
CH2 and CH3 domains to the rest of the antibody. Two disulfide bonds in this
hinge region
connect the two heavy chain polypeptides to one another in an intact antibody.
Each light chain
comprises two domains ¨ an amino-terminal variable (VL) domain, followed by a
carboxy-
terminal constant (CL) domain, separated from one another by another "switch".
Intact antibody
tetramers comprise two heavy chain-light chain dimers in which the heavy and
light chains are
linked to one another by a single disulfide bond; two other disulfide bonds
connect the heavy
chain hinge regions to one another, so that the dimers are connected to one
another and a
tetramer is formed. Naturally-produced antibodies are also glycosylated,
typically on the CH2
domain. Each domain in a natural antibody has a structure characterized by an
"immunoglobulin
fold" formed from two beta sheets (e.g., 3-, 4-, or 5-stranded sheets) packed
against each other in
a compressed antiparallel beta barrel. Each variable domain contains three
hypervariable loops
known as "complementarity determining regions" (CDR1, CDR2, and CDR3) and four
somewhat invariant "framework" regions (FR1, FR2, FR3, and FR4). When natural
antibodies
fold, the FR regions form the beta sheets that provide the structural
framework for the domains,
and the CDR loop regions from both the heavy and light chains are brought
together in three-
dimensional space so that they create a single hypervariable antigen binding
site located at the tip
of the Y structure. The Fc region of naturally-occurring antibodies binds to
elements of the
complement system, and also to receptors on effector cells, including, for
example, effector cells
that mediate cytotoxicity. Affinity and/or other binding attributes of Fc
regions for Fc receptors
can be modulated through glycosylation or other modification. In some
embodiments, antibodies
produced and/or utilized in accordance with the present invention (e.g., as a
component of a
CAR) include glycosylated Fc domains, including Fc domains with modified or
engineered
glycosylation. In some embodiments, any polypeptide or complex of polypeptides
that includes
sufficient immunoglobulin domain sequences as found in natural antibodies can
be referred to
and/or used as an "antibody", whether such polypeptide is naturally produced
(e.g., generated by
an organism reacting to an antigen), or produced by recombinant engineering,
chemical
synthesis, or other artificial system or methodology. In some embodiments, an
antibody is
9
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
polyclonal. In some embodiments, an antibody is monoclonal. In some
embodiments, an
antibody has constant region sequences that are characteristic of mouse,
rabbit, primate, or
human antibodies. In some embodiments, antibody sequence elements are
humanized,
primatized, chimeric, etc, as is known in the art. Moreover, the term
"antibody", as used herein,
can refer in appropriate embodiments (unless otherwise stated or clear from
context) to any of
the art-known or developed constructs or formats for utilizing antibody
structural and functional
features in alternative presentation. For example, in some embodiments, an
antibody utilized in
accordance with the present invention is in a format selected from, but not
limited to, intact IgA,
IgG, IgE or IgM antibodies; bi- or multi- specific antibodies (e.g., Zybodies
, etc); antibody
fragments such as Fab fragments, Fab' fragments, F(ab')2 fragments, Fd'
fragments, Fd
fragments, and isolated CDRs or sets thereof; single chain Fvs; polypeptide-Fc
fusions; single
domain antibodies (e.g., shark single domain antibodies such as IgNAR or
fragments thereof);
cameloid antibodies; masked antibodies (e.g., Probodies ); Small Modular
ImmunoPharmaceuticals ("SMIPsTm"); single chain or Tandem diabodies (TandAbg);
VHI-Is;
Anticalinsg; Nanobodies minibodies; BiTE s; ankyrin repeat proteins or
DARPINsg;
Avimersg; DARTs; TCR-like antibodies;, Adnectinsg; Affilinsg; Trans-bodies ,
Affibodies ,
TrimerX ; MicroProteins; Fynomers , Centyrinsg; and KALBITOR s. In some
embodiments, an antibody may lack a covalent modification (e.g., attachment of
a glycan) that it
would have if produced naturally. In some embodiments, an antibody may contain
a covalent
modification (e.g., attachment of a glycan, a payload [e.g., a detectable
moiety, a therapeutic
moiety, a catalytic moiety, etc], or other pendant group [e.g., poly-ethylene
glycol, etc.].
[0047] Antibody agent: As used herein, the term "antibody agent" refers to
an agent that
specifically binds to a particular antigen. In some embodiments, the term
encompasses any
polypeptide or polypeptide complex that includes immunoglobulin structural
elements sufficient
to confer specific binding. Exemplary antibody agents include, but are not
limited to monoclonal
antibodies or polyclonal antibodies. In some embodiments, an antibody agent
may include one
or more constant region sequences that are characteristic of mouse, rabbit,
primate, or human
antibodies. In some embodiments, an antibody agent may include one or more
sequence
elements are humanized, primatized, chimeric, etc., as is known in the art. In
many
embodiments, the term "antibody agent" is used to refer to one or more of the
art-known or
developed constructs or formats for utilizing antibody structural and
functional features in
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
alternative presentation. For example, in some embodiments, an antibody agent
utilized in
accordance with the present invention is in a format selected from, but not
limited to, intact IgA,
IgG, IgE or IgM antibodies; bi- or multi- specific antibodies (e.g., Zybodies
, etc); antibody
fragments such as Fab fragments, Fab' fragments, F(ab')2 fragments, Fd'
fragments, Fd
fragments, and isolated CDRs or sets thereof; single chain Fvs; polypeptide-Fc
fusions; single
domain antibodies (e.g., shark single domain antibodies such as IgNAR or
fragments thereof);
cameloid antibodies; masked antibodies (e.g., Probodies ); Small Modular
ImmunoPharmaceuticals ("SMIPsTM"); single chain or Tandem diabodies (TandAbg);
VI-11-1s;
Anticalinsg; Nanobodies minibodies; BiTE s; ankyrin repeat proteins or
DARPINsg;
Avimersg; DARTs; TCR-like antibodies;, Adnectinsg; Affilinsg; Trans-bodies ;
Affibodiesg;
TrimerX ; MicroProteins; Fynomers , Centyrinsg; and KALBITOR s. In some
embodiments, an antibody agent may lack a covalent modification (e.g.,
attachment of a glycan)
that it would have if produced naturally. In some embodiments, an antibody
agent may contain a
covalent modification (e.g., attachment of a glycan, a payload [e.g., a
detectable moiety, a
therapeutic moiety, a catalytic moiety, etc], or other pendant group [e.g.,
poly-ethylene glycol,
etc.]. In many embodiments, an antibody agent is or comprises a polypeptide
whose amino acid
sequence includes one or more structural elements recognized by those skilled
in the art as a
complementarity determining region (CDR); in some embodiments an antibody
agent is or
comprises a polypeptide whose amino acid sequence includes at least one CDR
(e.g., at least one
heavy chain CDR and/or at least one light chain CDR) that is substantially
identical to one found
in a reference antibody. In some embodiments an included CDR is substantially
identical to a
reference CDR in that it is either identical in sequence or contains between 1-
5 amino acid
substitutions as compared with the reference CDR. In some embodiments an
included CDR is
substantially identical to a reference CDR in that it shows at least 85%, 86%,
87%, 88%, 89%,
90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 100% sequence identity
with the
reference CDR. In some embodiments an included CDR is substantially identical
to a reference
CDR in that it shows at least 96%, 96%, 97%, 98%, 99%, or 100% sequence
identity with the
reference CDR. In some embodiments an included CDR is substantially identical
to a reference
CDR in that at least one amino acid within the included CDR is deleted, added,
or substituted as
compared with the reference CDR but the included CDR has an amino acid
sequence that is
otherwise identical with that of the reference CDR. In some embodiments an
included CDR is
11
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
substantially identical to a reference CDR in that 1-5 amino acids within the
included CDR are
deleted, added, or substituted as compared with the reference CDR but the
included CDR has an
amino acid sequence that is otherwise identical to the reference CDR. In some
embodiments an
included CDR is substantially identical to a reference CDR in that at least
one amino acid within
the included CDR is substituted as compared with the reference CDR but the
included CDR has
an amino acid sequence that is otherwise identical with that of the reference
CDR. In some
embodiments an included CDR is substantially identical to a reference CDR in
that 1-5 amino
acids within the included CDR are deleted, added, or substituted as compared
with the reference
CDR but the included CDR has an amino acid sequence that is otherwise
identical to the
reference CDR. In some embodiments, an antibody agent is or comprises a
polypeptide whose
amino acid sequence includes structural elements recognized by those skilled
in the art as an
immunoglobulin variable domain. In some embodiments, an antibody agent is a
polypeptide
protein having a binding domain which is homologous or largely homologous to
an
immunoglobulin-binding domain. In some embodiments, an antibody agent is not
and/or does
not comprise a polypeptide whose amino acid sequence includes structural
elements recognized
by those skilled in the art as an immunoglobulin variable domain. In some
embodiments, an
antibody agent may be or comprise a molecule or composition which does not
include
immunoglobulin structural elements (e.g., a receptor or other naturally
occurring molecule which
includes at least one antigen binding domain).
[0048] Antibody fragment: As used herein, the term "antibody fragment"
refers to a
portion of an intact antibody and refers to the antigenic determining variable
regions of an intact
antibody. Examples of antibody fragments include, but are not limited to, Fab,
Fab', F(ab')2, and
Fv fragments, linear antibodies, scFv antibodies, and multispecific antibodies
formed from
antibody fragments and human and humanized versions thereof
[0049] Antibody heavy chain: As used herein, the term "antibody heavy
chain" refers to
the larger of the two types of polypeptide chains present in all antibody
molecules in their
naturally occurring conformations.
[0050] Antibody light chain: As used herein, the term "antibody light
chain" refers to the
smaller of the two types of polypeptide chains present in all antibody
molecules in their naturally
occurring conformations.
12
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0051] Synthetic antibody: As used herein, the term "synthetic antibody"
refers to an
antibody that is generated using recombinant DNA technology, such as, for
example, an antibody
expressed by a bacteriophage as described herein. The term should also be
construed to mean an
antibody which has been generated by the synthesis of a DNA molecule encoding
the antibody
and which DNA molecule expresses an antibody protein, or an amino acid
sequence specifying
the antibody, wherein the DNA or amino acid sequence has been obtained using
synthetic DNA
or amino acid sequence technology which is available and well known in the
art.
[0052] Antigen: As used herein, the term "antigen" or "Ag" refers to a
molecule that is
capable of provoking an immune response. This immune response may involve
either antibody
production, the activation of specific immunologically-competent cells, or
both. A skilled
artisan will understand that any macromolecule, including virtually all
proteins or peptides, can
serve as an antigen. Furthermore, antigens can be derived from recombinant or
genomic DNA.
A skilled artisan will understand that any DNA that comprises a nucleotide
sequences or a partial
nucleotide sequence encoding a protein that elicits an immune response encodes
an "antigen" as
that term is used herein. Furthermore, one skilled in the art will understand
that an antigen need
not be encoded solely by a full length nucleotide sequence of a gene. It is
readily apparent that
the present invention includes, but is not limited to, the use of partial
nucleotide sequences of
more than one gene and that these nucleotide sequences are arranged in various
combinations to
elicit the desired immune response. Moreover, a skilled artisan will
understand that an antigen
need not be encoded by a "gene" at all. It is readily apparent that an antigen
can be generated
synthesized or can be derived from a biological sample. Such a biological
sample can include,
but is not limited to a tissue sample, a tumor sample, a cell or a biological
fluid.
[0053] Anti-tumor effect: As used herein, the term "anti-tumor effect"
refers to a
biological effect which can be manifested by a decrease in tumor volume, a
decrease in the
number of tumor cells, a decrease in the number of metastases, an increase in
life expectancy, or
amelioration of various physiological symptoms associated with the cancerous
condition. An
"anti-tumor effect" can also be manifested by the ability of the peptides,
polynucleotides, cells
and antibodies of the invention in prevention of the occurrence of a tumor in
the first place.
[0054] Autologous: As used herein, the term "autologous" refers to any
material derived
from an individual to which it is later to be re-introduced into the same
individual.
13
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0055] Allogeneic: As used herein, the term "allogeneic" refers to any
material (e.g., a
population of cells) derived from a different animal of the same species.
[0056] Xenogenic: As used herein, the term "xenogeneic" refers to any
material (e.g., a
population of cells) derived from an animal of a different species.
[0057] Cancer: As used herein, the term "cancer" refers to a disease
characterized by the
rapid and uncontrolled growth of aberrant cells. Cancer cells can spread
locally or through the
bloodstream and lymphatic system to other parts of the body. Examples of
various cancers
include but are not limited to, breast cancer, prostate cancer, ovarian
cancer, cervical cancer, skin
cancer, pancreatic cancer, colorectal cancer, renal cancer, liver cancer,
brain cancer, lymphoma,
leukemia, lung cancer and the like. In certain embodiments, the cancer is
medullary thyroid
carcinoma.
[0058] Conservative sequence modifications: As used herein, the term
"conservative
sequence modifications" refers to amino acid modifications that do not
significantly affect or
alter the binding characteristics of an antibody containing the amino acid
sequence. Such
conservative modifications include amino acid substitutions, additions and
deletions.
Modifications can be introduced into an antibody compatible with various
embodiments by
standard techniques known in the art, such as site-directed mutagenesis and
PCR-mediated
mutagenesis. Conservative amino acid substitutions are ones in which an amino
acid residue is
replaced with an amino acid residue having a similar side chain. Families of
amino acid residues
having similar side chains have been defined in the art. These families
include amino acids with
basic side chains (e.g., lysine, arginine, histidine), acidic side chains
(e.g., aspartic acid, glutamic
acid), uncharged polar side chains (e.g., glycine, asparagine, glutamine,
serine, threonine,
tyrosine, cysteine, tryptophan), nonpolar side chains (e.g., alanine, valine,
leucine, isoleucine,
proline, phenylalanine, methionine), beta-branched side chains (e.g.,
threonine, valine,
isoleucine) and aromatic side chains (e.g., tyrosine, phenylalanine,
tryptophan, histidine). Thus,
one or more amino acid residues within the CDR regions of an antibody can be
replaced with
other amino acid residues from the same side chain family and the altered
antibody can be tested
for the ability to bind antigens using the functional assays described herein.
[0059] Co-stimulatory ligand: As used herein, the term "co-stimulatory
ligand" refers to
a molecule on an antigen presenting cell (e.g., an APC, dendritic cell, B
cell, and the like) that
14
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
specifically binds a cognate co-stimulatory molecule on a
monocyte/macrophage/dendritic cell,
thereby providing a signal which mediates a monocyte/macrophage/dendritic cell
response,
including, but not limited to, proliferation, activation, differentiation, and
the like. A co-
stimulatory ligand can include, but is not limited to, CD7, B7-1 (CD80), B7-2
(CD86), PD-L1,
PD-L2, 4-1BBL, OX4OL, inducible costimulatory ligand (ICOS-L), intercellular
adhesion
molecule (ICAM), CD3OL, CD40, CD70, CD83, HLA-G, MICA, MICB, HVEM, lymphotoxin
beta receptor, 3/TR6, ILT3, ILT4, HVEM, an agonist or antibody that binds Toll
ligand receptor
and a ligand that specifically binds with B7-H3. A co-stimulatory ligand also
encompasses, inter
alia, an antibody that specifically binds with a co-stimulatory molecule
present on a
monocyte/macrophage/dendritic cell, such as, but not limited to, CD27, CD28, 4-
1BB, 0X40,
CD30, CD40, PD-1, ICOS, lymphocyte function-associated antigen-1 (LFA-1), CD2,
CD7,
LIGHT, NKG2C, B7-H3, and a ligand that specifically binds with CD83.
[0060] Cytotoxic: As used herein, the term "cytotoxic" or "cytotoxicity"
refers to killing
or damaging cells. In one embodiment, cytotoxicity of the metabolically
enhanced cells is
improved, e.g. increased cytolytic activity of macrophages.
[0061] Effective amount: As used herein, "effective amount" and
"therapeutically
effective amount" are interchangeable, and refer to an amount of a compound,
formulation,
material, or composition, as described herein effective to achieve a
particular biological result or
provides a manufacturing, therapeutic or prophylactic benefit. Such results
may include, but are
not limited to, anti-tumor activity as determined by any means suitable in the
art.
[0062] Effector function: As used herein, "effector function" or
"effector activity" refers
to a specific activity carried out by an immune cell in response to
stimulation of the immune cell.
For example, an effector function of macrophages to engulf and digest cellular
debris, foreign
substances, microbes, cancer cells and other unhealthy cells by phagocytosis.
[0063] Encoding: As used herein, "encoding" refers to the inherent
property of specific
sequences of nucleotides in a polynucleotide, such as a gene, a cDNA, or an
mRNA, to serve as
templates for synthesis of other polymers and macromolecules in biological
processes having
either a defined sequence of nucleotides (i.e., rRNA, tRNA and mRNA) or a
defined sequence of
amino acids and the biological properties resulting therefrom. Thus, a gene
encodes a protein if
transcription and translation of mRNA corresponding to that gene produces the
protein in a cell
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
or other biological system. Both the coding strand, the nucleotide sequence of
which is identical
to the mRNA sequence and is usually provided in sequence listings, and the non-
coding strand,
used as the template for transcription of a gene or cDNA, can be referred to
as encoding the
protein or other product of that gene or cDNA.
[0064] Endogenous: As used herein "endogenous" refers to any material from
or
produced inside a particular organism, cell, tissue or system.
[0065] Exogenous: As used herein, the term "exogenous" refers to any
material
introduced from or produced outside a particular organism, cell, tissue or
system.
[0066] Expand: As used herein, the term "expand" refers to increasing in
number, as in
an increase in the number of cells, for example, monocytes, macrophages,
and/or dendritic cells.
In one embodiment, monocytes, macrophages, or dendritic cells that are
expanded ex vivo
increase in number relative to the number originally present in a culture. In
another
embodiment, monocytes, macrophages, or dendritic cells that are expanded ex
vivo increase in
number relative to other cell types in a culture. In some embodiments,
expansion may occur in
vivo. The term "ex vivo," as used herein, refers to cells that have been
removed from a living
organism, (e.g., a human) and propagated outside the organism (e.g., in a
culture dish, test tube,
or bioreactor).
[0067] Expression: As used herein, the term "expression" of a nucleic acid
sequence
refers to generation of any gene product from a nucleic acid sequence. In some
embodiments, a
gene product can be a transcript. In some embodiments, a gene product can be a
polypeptide. In
some embodiments, expression of a nucleic acid sequence involves one or more
of the following:
(1) production of an RNA template from a DNA sequence (e.g., by
transcription); (2) processing
of an RNA transcript (e.g., by splicing, editing, 5' cap formation, and/or 3'
end formation); (3)
translation of an RNA into a polypeptide or protein; and/or (4) post-
translational modification of
a polypeptide or protein.
[0068] Expression vector: As used herein, the term "expression vector"
refers to a vector
comprising a recombinant polynucleotide comprising expression control
sequences operatively
linked to a nucleotide sequence to be expressed. An expression vector
comprises sufficient cis-
acting elements for expression; other elements for expression can be supplied
by the host cell or
in an in vitro expression system. Expression vectors include all those known
in the art, such as
16
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
cosmids, plasmids (e.g., naked or contained in liposomes) and viruses (e.g.,
lentiviruses,
retroviruses, adenoviruses, and adeno-associated viruses).
[0069] Fragment: As used herein, the terms "fragment" or "portion" refers
to a structure
that includes a discrete portion of the whole, but lacks one or more moieties
found in the whole
structure. In some embodiments, a fragment consists of such a discrete
portion. In some
embodiments, a fragment consists of or comprises a characteristic structural
element or moiety
found in the whole. In some embodiments, a nucleotide fragment comprises or
consists of at
least 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 25, 30,
35, 40, 45, 50, 55, 60, 65,
70, 75, 80, 85, 90, 95, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200,
210, 220, 230, 240,
250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, or more monomeric units
(e.g., nucleic
acids) as found in the whole nucleotide. In some embodiments, a nucleotide
fragment comprises
or consists of at least about 5%, 10%, 15%, 20%, 25%, 30%, 25%, 40%, 45%, 50%,
55%, 60%,
65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, or more of the
monomeric units
(e.g., residues) found in the whole nucleotide. The whole material or entity
may in some
embodiments be referred to as the "parent" of the whole.
[0070] Homology: As used herein, the term "homology" refers to the overall
relatedness
between polymeric molecules, e.g., between nucleic acid molecules (e.g., DNA
molecules and/or
RNA molecules) and/or between polypeptide molecules. In some embodiments,
polymeric
molecules are considered to be "homologous" to one another if their sequences
are at least 25%,
30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 99%
identical. In some embodiments, polymeric molecules are considered to be
"homologous" to one
another if their sequences are at least 25%, 30%, 35%, 40%, 45%, 50%, 55%,
60%, 65%, 70%,
75%, 80%, 85%, 90%, 95%, or 99% similar (e.g., containing residues with
related chemical
properties at corresponding positions). As will be understood by those skilled
in the art, a variety
of algorithms are available that permit comparison of sequences in order to
determine their
degree of homology, including by permitting gaps of designated length in one
sequence relative
to another when considering which residues "correspond" to one another in
different sequences.
Calculation of the percent homology between two nucleic acid sequences, for
example, can be
performed by aligning the two sequences for optimal comparison purposes (e.g.,
gaps can be
introduced in one or both of a first and a second nucleic acid sequences for
optimal alignment
17
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
and non-corresponding sequences can be disregarded for comparison purposes).
In certain
embodiments, the length of a sequence aligned for comparison purposes is at
least 30%, at least
40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at
least 95%, or
substantially 100% of the length of the reference sequence. The nucleotides at
corresponding
nucleotide positions are then compared. When a position in the first sequence
is occupied by the
same nucleotide as the corresponding position in the second sequence, then the
molecules are
identical at that position; when a position in the first sequence is occupied
by a similar nucleotide
as the corresponding position in the second sequence, then the molecules are
similar at that
position. The percent homology between the two sequences is a function of the
number of
identical and similar positions shared by the sequences, taking into account
the number of gaps,
and the length of each gap, which needs to be introduced for optimal alignment
of the two
sequences.
[0071] Identity: As used herein, the term "identity" refers to the subunit
sequence
identity between two polymeric molecules particularly between two amino acid
molecules, such
as, between two polypeptide molecules. When two amino acid sequences have the
same residues
at the same positions; e.g., if a position in each of two polypeptide
molecules is occupied by an
Arginine, then they are identical at that position. The identity or extent to
which two amino acid
sequences have the same residues at the same positions in an alignment is
often expressed as a
percentage. The identity between two amino acid sequences is a direct function
of the number of
matching or identical positions; e.g., if half (e.g., five positions in a
polymer ten amino acids in
length) of the positions in two sequences are identical, the two sequences are
50% identical; if
90% of the positions (e.g., 9 of 10), are matched or identical, the two amino
acids sequences are
90% identical.
[0072] Substantial identity: As used herein, the term "substantial
identity" refers to a
comparison between amino acid or nucleic acid sequences. As will be
appreciated by those of
ordinary skill in the art, two sequences are generally considered to be
"substantially identical" if
they contain identical residues in corresponding positions. As is well known
in this art, amino
acid or nucleic acid sequences may be compared using any of a variety of
algorithms, including
those available in commercial computer programs such as BLASTN for nucleotide
sequences
and BLASTP, gapped BLAST, and PSI-BLAST for amino acid sequences. In some
18
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
embodiments, two sequences are considered to be substantially identical if at
least 50%, 55%,
60%, 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%
or
more of their corresponding residues are identical over a relevant stretch of
residues. In some
embodiments, the relevant stretch is a complete sequence. In some embodiments,
the relevant
stretch is at least 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75,
80, 85, 90, 95, 100, 125,
150, 175, 200, 225, 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500 or
more residues. In
the context of a CDR, reference to "substantial identity" typically refers to
a CDR having an
amino acid sequence at least 80%, preferably at least 85%, at least 90%, at
least 95%, at least
98% or at least 99% identical to that of a reference CDR.
[0073] Immune cell: As used herein, the term "immune cell," refers to a
cell that is
involved in an immune response, e.g., promotion of an immune response.
Examples of immune
cells include, but are not limited to, macrophages, monocytes, dendritic
cells, neutrophils,
eosinophils, mast cells, platelets, large granular lymphocytes, Langerhans'
cells, natural killer
(NK) cells, T-lymphocytes, or B-lymphocytes. A source of immune cells (e.g.,
macrophages,
monocytes, or dendritic cells) can be obtained from a subject.
[0074] Immune response: As used herein the term "immune response" refers
to a cellular
and/or systemic response to an antigen that occurs when lymphocytes identify
antigenic
molecules as foreign and induce the formation of antibodies and/or activate
lymphocytes to
remove the antigen.
[0075] Immunoglobulin: As used herein, the term "immunoglobulin" or "Ig,"
refers to a
class of proteins that function as antibodies. Antibodies expressed by B cells
are sometimes
referred to as a BCR (B cell receptor) or antigen receptor. The five members
included in this
class of proteins are IgA, IgG, IgM, IgD, and IgE. IgA is the primary antibody
that is present in
body secretions, such as saliva, tears, breast milk, gastrointestinal
secretions and mucus
secretions of the respiratory and genitourinary tracts. IgG is the most common
circulating
antibody. IgM is the main immunoglobulin produced in the primary immune
response in most
subjects. It is the most efficient immunoglobulin in agglutination, complement
fixation, and
other antibody responses, and is important in defense against bacteria and
viruses. IgD is an
immunoglobulin that has no known antibody function, but may serve as an
antigen receptor. IgE
19
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
is an immunoglobulin that mediates immediate hypersensitivity by causing
release of mediators
from mast cells and basophils upon exposure to allergen.
[0076] Isolated: As used herein, the term "isolated" refers to something
altered or
removed from the natural state. For example, a nucleic acid or a peptide
naturally present in a
living animal is not "isolated," but the same nucleic acid or peptide
partially or completely
separated from the coexisting materials of its natural state is "isolated." An
isolated nucleic acid
or protein can exist in substantially purified form, or can exist in a non-
native environment such
as, for example, a host cell.
[0077] Modified: As used herein, the term "modified" refers to a changed
state or
structure of a molecule or cell of the invention. Molecules may be modified in
many ways,
including chemically, structurally, and functionally. Cells may be modified
through the
introduction of nucleic acids.
[0078] Modulating: As used herein the term "modulating," refers to
mediating a
detectable increase or decrease in the level of a response and/or a change in
the nature of a
response in a subject compared with the level and/or nature of a response in
the subject in the
absence of a treatment or compound, and/or compared with the level and/or
nature of a response
in an otherwise identical but untreated subject. The term encompasses
perturbing and/or
affecting a native signal or response thereby mediating a beneficial
therapeutic response in a
subject, preferably, a human.
[0079] Nucleic acid: As used herein, the term "nucleic acid" refers to a
polymer of at
least three nucleotides. In some embodiments, a nucleic acid comprises DNA. In
some
embodiments, a nucleic acid comprises RNA. In some embodiments, a nucleic acid
is single
stranded. In some embodiments, a nucleic acid is double stranded. In some
embodiments, a
nucleic acid comprises both single and double stranded portions. In some
embodiments, a
nucleic acid comprises a backbone that comprises one or more phosphodiester
linkages. In some
embodiments, a nucleic acid comprises a backbone that comprises both
phosphodiester and non-
phosphodiester linkages. For example, in some embodiments, a nucleic acid may
comprise a
backbone that comprises one or more phosphorothioate or 5'-N-phosphoramidite
linkages and/or
one or more peptide bonds, e.g., as in a "peptide nucleic acid". In some
embodiments, a nucleic
acid comprises one or more, or all, natural residues (e.g., adenine, cytosine,
deoxyadenosine,
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
deoxycytidine, deoxyguanosine, deoxythymidine, guanine, thymine, uracil). In
some
embodiments, a nucleic acid comprises one or more, or all, non-natural
residues. In some
embodiments, a non-natural residue comprises a nucleoside analog (e.g., 2-
aminoadenosine, 2-
thiothymidine, inosine, pyrrolo-pyrimidine, 3 -methyl adenosine, 5-
methylcytidine, C-5
propynyl-cytidine, C-5 propynyl-uridine, 2-aminoadenosine, C5-bromouridine, C5-
fluorouridine,
C5-iodouridine, C5-propynyl-uridine, C5 -propynyl-cytidine, C5-methylcytidine,
2-
aminoadenosine, 7-deazaadenosine, 7-deazaguanosine, 8-oxoadenosine, 8-
oxoguanosine, 0(6)-
methylguanine, 2-thiocytidine, methylated bases, intercalated bases, and
combinations thereof).
In some embodiments, a non-natural residue comprises one or more modified
sugars (e.g., 2'-
fluororibose, ribose, 2'-deoxyribose, arabinose, and hexose) as compared to
those in natural
residues. In some embodiments, a nucleic acid has a nucleotide sequence that
encodes a
functional gene product such as an RNA or polypeptide. In some embodiments, a
nucleic acid
has a nucleotide sequence that comprises one or more introns. In some
embodiments, a nucleic
acid may be prepared by isolation from a natural source, enzymatic synthesis
(e.g., by
polymerization based on a complementary template, e.g., in vivo or in vitro,
reproduction in a
recombinant cell or system, or chemical synthesis. In some embodiments, a
nucleic acid is at
least 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70,
75, 80, 85, 90, 95, 100,
110, 120, 130, 140, 150, 160, 170, 180, 190, 20, 225, 250, 275, 300, 325, 350,
375, 400, 425,
450, 475, 500, 600, 700, 800, 900, 1000, 1500, 2000, 2500, 3000, 3500, 4000,
4500, 5000 or
more residues long.
[0080] Operably linked: As used herein, the term "operably linked" refers
to functional
linkage between, for example, a regulatory sequence and a heterologous nucleic
acid sequence
resulting in expression of the latter. For example, a first nucleic acid
sequence is operably linked
with a second nucleic acid sequence when the first nucleic acid sequence is
placed in a functional
relationship with the second nucleic acid sequence. For instance, a promoter
is operably linked
to a coding sequence if the promoter affects the transcription or expression
of the coding
sequence. Generally, operably linked DNA sequences are contiguous and, where
necessary to
join two protein coding regions, in the same reading frame.
[0081] Overexpressed tumor antigen: As used herein, the term
"overexpressed" tumor
antigen or "overexpression" of a tumor antigen refers to an abnormal level of
expression of a
21
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
tumor antigen in a cell from a disease area like a solid tumor within a
specific tissue or organ of
the patient relative to the level of expression in a normal cell from that
tissue or organ. Patients
having solid tumors or a hematological malignancy characterized by
overexpression of the tumor
antigen can be determined by standard assays known in the art.
[0082] Polynucleotide: As used herein, the term "polynucleotide" refers to
a chain of
nucleotides. Furthermore, nucleic acids are polymers of nucleotides. Thus,
nucleic acids and
polynucleotides as used herein are interchangeable. One skilled in the art has
the general
knowledge that nucleic acids are polynucleotides, which can be hydrolyzed into
the monomeric
"nucleotides." The monomeric nucleotides can be hydrolyzed into nucleosides.
As used herein
polynucleotides include, but are not limited to, all nucleic acid sequences
which are obtained by
any means available in the art, including, without limitation, recombinant
means, i.e., the cloning
of nucleic acid sequences from a recombinant library or a cell genome, using
ordinary cloning
technology and PCRTM, and the like, and by synthetic means.
[0083] Polypeptide: As used herein, the term "polypeptide" refers to any
polymeric
chain of residues (e.g., amino acids) that are typically linked by peptide
bonds. In some
embodiments, a polypeptide has an amino acid sequence that occurs in nature.
In some
embodiments, a polypeptide has an amino acid sequence that does not occur in
nature. In some
embodiments, a polypeptide has an amino acid sequence that is engineered in
that it is designed
and/or produced through action of the hand of man. In some embodiments, a
polypeptide may
comprise or consist of natural amino acids, non-natural amino acids, or both.
In some
embodiments, a polypeptide may comprise or consist of only natural amino acids
or only non-
natural amino acids. In some embodiments, a polypeptide may comprise D-amino
acids, L-
amino acids, or both. In some embodiments, a polypeptide may comprise only D-
amino acids.
In some embodiments, a polypeptide may comprise only L-amino acids. In some
embodiments,
a polypeptide may include one or more pendant groups or other modifications,
e.g., modifying or
attached to one or more amino acid side chains, at the polypeptide's N-
terminus, at the
polypeptide's C-terminus, or any combination thereof. In some embodiments,
such pendant
groups or modifications may be selected from the group consisting of
acetylation, amidation,
lipidation, methylation, pegylation, etc., including combinations thereof. In
some embodiments,
a polypeptide may be cyclic, and/or may comprise a cyclic portion. In some
embodiments, a
22
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
polypeptide is not cyclic and/or does not comprise any cyclic portion. In some
embodiments, a
polypeptide is linear. In some embodiments, a polypeptide may be or comprise a
stapled
polypeptide. In some embodiments, the term "polypeptide" may be appended to a
name of a
reference polypeptide, activity, or structure; in such instances it is used
herein to refer to
polypeptides that share the relevant activity or structure and thus can be
considered to be
members of the same class or family of polypeptides. For each such class, the
present
specification provides and/or those skilled in the art will be aware of
exemplary polypeptides
within the class whose amino acid sequences and/or functions are known; in
some embodiments,
such exemplary polypeptides are reference polypeptides for the polypeptide
class or family. In
some embodiments, a member of a polypeptide class or family shows significant
sequence
homology or identity with, shares a common sequence motif (e.g., a
characteristic sequence
element) with, and/or shares a common activity (in some embodiments at a
comparable level or
within a designated range) with a reference polypeptide of the class; in some
embodiments with
all polypeptides within the class). For example, in some embodiments, a member
polypeptide
shows an overall degree of sequence homology or identity with a reference
polypeptide that is at
least about 30-40%, and is often greater than about 50%, 60%, 70%, 80%, 90%,
91%, 92%,
93%, 94%, 95%, 96%, 97%, 98%, 99% or more and/or includes at least one region
(e.g., a
conserved region that may in some embodiments be or comprise a characteristic
sequence
element) that shows very high sequence identity, often greater than 90% or
even 95%, 96%,
97%, 98%, or 99%. Such a conserved region usually encompasses at least 3-4 and
often up to 20
or more amino acids; in some embodiments, a conserved region encompasses at
least one stretch
of at least 2, 3, 4, 5, 6, 7, 8,9, 10, 11, 12, 13, 14, 15 or more contiguous
amino acids. In some
embodiments, a useful polypeptide may comprise or consist of a fragment of a
parent
polypeptide. In some embodiments, a useful polypeptide as may comprise or
consist of a
plurality of fragments, each of which is found in the same parent polypeptide
in a different
spatial arrangement relative to one another than is found in the polypeptide
of interest (e.g.,
fragments that are directly linked in the parent may be spatially separated in
the polypeptide of
interest or vice versa, and/or fragments may be present in a different order
in the polypeptide of
interest than in the parent), so that the polypeptide of interest is a
derivative of its parent
polypeptide.
23
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0084] Protein: As used herein, the term "protein" refers to a polypeptide
(i.e., a string of
at least two amino acids linked to one another by peptide bonds). Proteins may
include moieties
other than amino acids (e.g., may be glycoproteins, proteoglycans, etc.)
and/or may be otherwise
processed or modified. Those of ordinary skill in the art will appreciate that
a "protein" can be a
complete polypeptide chain as produced by a cell (with or without a signal
sequence), or can be a
characteristic portion thereof. Those of ordinary skill will appreciate that a
protein can
sometimes include more than one polypeptide chain, for example linked by one
or more disulfide
bonds or associated by other means. Polypeptides may contain L-amino acids, D-
amino acids, or
both and may contain any of a variety of amino acid modifications or analogs
known in the art.
Useful modifications include, e.g., terminal acetylation, amidation,
methylation, etc. In some
embodiments, proteins may comprise natural amino acids, non-natural amino
acids, synthetic
amino acids, and combinations thereof The term "peptide" is generally used to
refer to a
polypeptide having a length of less than about 100 amino acids, less than
about 50 amino acids,
less than 20 amino acids, or less than 10 amino acids. In some embodiments,
proteins are
antibodies, antibody fragments, biologically active portions thereof, and/or
characteristic
portions thereof.
[0085] Signal transduction pathway: As used herein, the term "signal
transduction
pathway" refers to the biochemical relationship between a variety of signal
transduction
molecules that play a role in the transmission of a signal from one portion of
a cell to another
portion of a cell. The phrase "cell surface receptor" includes molecules and
complexes of
molecules capable of receiving a signal and transmitting signal across the
plasma membrane of a
cell.
[0086] Single chain antibodies: As used herein, the term "single chain
antibodies" refers
to antibodies formed by recombinant DNA techniques in which immunoglobulin
heavy and light
chain fragments are linked to the Fv region via an engineered span of amino
acids. Various
methods of generating single chain antibodies are known, including those
described in U.S. Pat.
No. 4,694,778; Bird (1988) Science 242:423-442; Huston et al. (1988) Proc.
Natl. Acad. Sci.
USA 85:5879-5883; Ward et al. (1989) Nature 334:54454; Skerra et al. (1988)
Science
242:1038-1041.
24
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0087] Specifically binds: As used herein, the term "specifically binds,"
with respect to
an antigen binding domain, such as an antibody agent, refers to an antigen
binding domain or
antibody agent which recognizes a specific antigen, but does not substantially
recognize or bind
other molecules in a sample. For example, an antigen binding domain or
antibody agent that
specifically binds to an antigen from one species may also bind to that
antigen from one or more
species. But, such cross-species reactivity does not itself alter the
classification of an antigen
binding domain or antibody agent as specific. In another example, an antigen
binding domain or
antibody agent that specifically binds to an antigen may also bind to
different allelic forms of the
antigen. However, such cross reactivity does not itself alter the
classification of an antigen
binding domain or antibody agent as specific. In some instances, the terms
"specific binding" or
"specifically binding," can be used in reference to the interaction of an
antigen binding domain
or antibody agent, a protein, or a peptide with a second chemical species, to
mean that the
interaction is dependent upon the presence of a particular structure (e.g., an
antigenic
determinant or epitope) on the chemical species; for example, an antigen
binding domain or
antibody agent recognizes and binds to a specific protein structure rather
than to proteins
generally. If an antigen binding domain or antibody agent is specific for
epitope "A", the
presence of a molecule containing epitope A (or free, unlabeled A), in a
reaction containing
labeled "A" and the antigen binding domain or antibody agent, will reduce the
amount of labeled
A bound to the antibody.
[0088] Stimulation: As used herein, the term "stimulation," refers to a
primary response
induced by binding of a stimulatory molecule (e.g., an FcR complex, a TLR
complex, or a
TCR/CD3 complex), for example, with its cognate ligand thereby mediating a
signal
transduction event, such as, but not limited to, signal transduction via Fc
receptor machinery or
via a synthetic CAR. Stimulation can mediate altered expression of certain
molecules, such as
downregulation of TGF-beta, and/or reorganization of cytoskeletal structures,
and the like. As
used herein, the term "stimulatory molecule," refers to a molecule of a
monocyte, macrophage,
or dendritic cell that specifically binds with a cognate stimulatory ligand
present on an antigen
presenting cell. In some embodiments, a stimulatory molecule comprises an FcR
extracellular
domain comprising a CD64 (FcyRI), CD32a (FcyRIIa), CD32b (FcyRIIb), CD32c,
CD16a
(FcyRIIIa), CD16b (FcyRIIIb), FccRI, FccRII, FcaRI (CD89) or CD40 domain. In
some
embodiments, a stimulatory molecule comprises a TLR extracellular domain
comprising a
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, or TLR9 domain. As used
herein, the
term "stimulatory ligand," refers to a ligand that when present on an antigen
presenting cell (e.g.,
an aAPC, a macrophage, a dendritic cell, a B-cell, and the like) or tumor cell
can specifically
bind with a cognate binding partner (referred to herein as a "stimulatory
molecule") on a
monocyte, macrophage, or dendritic cell thereby mediating a response by the
immune cell,
including, but not limited to, activation, initiation of an immune response,
proliferation, and the
like. Stimulatory ligands are well-known in the art and encompass, inter al/a,
Toll-like receptor
(TLR) ligand, an anti-toll-like receptor antibody, an agonist, and an antibody
for a
monocyte/macrophage receptor. In addition, cytokines, such as interferon-
gamma, are potent
stimulants of macrophages.
[0089] Subject: As used herein, the term "subject" refers to an organism,
for example, a
mammal (e.g., a human, a non-human mammal, a non-human primate, a primate, a
laboratory
animal, a mouse, a rat, a hamster, a gerbil, a cat, or a dog). In some
embodiments a human
subject is an adult, adolescent, or pediatric subject. In some embodiments, a
subject is suffering
from a disease, disorder or condition, e.g., a disease, disorder, or condition
that can be treated as
provided herein, e.g., a cancer or a tumor listed herein. In some embodiments,
a subject is
susceptible to a disease, disorder, or condition; in some embodiments, a
susceptible subject is
predisposed to and/or shows an increased risk (as compared to the average risk
observed in a
reference subject or population) of developing the disease, disorder, or
condition. In some
embodiments, a subject displays one or more symptoms of a disease, disorder,
or condition. In
some embodiments, a subject does not display a particular symptom (e.g.,
clinical manifestation
of disease) or characteristic of a disease, disorder, or condition. In some
embodiments, a subject
does not display any symptom or characteristic of a disease, disorder, or
condition. In some
embodiments, a subject is a patient. In some embodiments, a subject is an
individual to whom
diagnosis and/or therapy is and/or has been administered.
[0090] Substantially purified: As used herein, the term "substantially
purified", for
example as applied to a cell, refers to a cell that is essentially free of
other cell types. A
substantially purified cell also refers to a cell which has been separated
from other cell types with
which it is normally associated in its naturally occurring state. In some
instances, a population of
substantially purified cells refers to a homogenous population of cells. In
other instances, this
26
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
term refers simply to cell that have been separated from the cells with which
they are naturally
associated in their natural state. In some embodiments, the cells are cultured
in vitro. In other
embodiments, the cells are not cultured in vitro.
[0091] Target: As used herein, the term "target" refers to a cell,
tissue, organ, or site
within the body that is the subject of provided methods, systems, and /or
compositions, for
example, a cell, tissue, organ or site within a body that is in need of
treatment or is preferentially
bound by, for example, an antibody (or fragment thereof) or a CAR.
[0092] Target site: As used herein, the term "target site" or "target
sequence" refers to a
genomic nucleic acid sequence that defines a portion of a nucleic acid to
which a binding
molecule may specifically bind under conditions sufficient for binding to
occur.
[0093] T cell receptor: As used herein, the term "T cell receptor" or
"TCR" refers to a
complex of membrane proteins that participate in the activation of T cells in
response to the
presentation of antigen. A TCR is responsible for recognizing antigens hound
to major
histocompatibility complex molecules A TCR comprises a heterodimer of an alpha
(a) and beta
(p) chain, although in some cells the ICR comprises gamma and delta (7/6)
chains. TCRs may
exist in alpha/beta and gamma/delta forms, which are structurally similar but
have distinct
anatomical locations and functions. Each chain comprises two extracellular
domains, a variable
and constant domain. In some embodiments, a TCR may be modified on any cell
comprising a
TCR, including, for example, a helper T cell, a cytotoxic I cell, a memory T
cell, regulatory
cell, natural killer T cell, and gamma delta T cell.
[0094] Therapeutic: As used herein, the term "therapeutic" refers to a
treatment and/or
prophylaxis. A therapeutic effect is obtained by suppression, remission, or
eradication of a
disease state.
[0095] Transfected: As used herein, the term "transfected" or
"transformed" or
"transduced" refers to a process by which exogenous nucleic acid is
transferred or introduced
into the host cell. A "transfected" or "transformed" or "transduced" cell is
one which has been
transfected, transformed or transduced with exogenous nucleic acid. The cell
includes the
primary subject cell and its progeny.
27
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0096] Treat: As used herein, the term "treat," "treatment," or
"treating" refers to partial
or complete alleviation, amelioration, delay of onset of, inhibition,
prevention, relief, and/or
reduction in incidence and/or severity of one or more symptoms or features of
a disease,
disorder, and/or condition. In some embodiments, treatment may be administered
to a subject
who does not exhibit signs or features of a disease, disorder, and/or
condition (e.g., may be
prophylactic). In some embodiments, treatment may be administered to a subject
who exhibits
only early or mild signs or features of the disease, disorder, and/or
condition, for example for the
purpose of decreasing the risk of developing pathology associated with the
disease, disorder,
and/or condition. In some embodiments, treatment may be administered to a
subject who
exhibits established, severe, and/or late-stage signs of the disease,
disorder, or condition. In
some embodiments, treating may comprise administering to an immune cell (e.g.,
a monocyte,
macrophage, or dendritic cell) or contacting an immune cell with a modulator
of a pathway
activated by in vitro transcribed mRNA.
[0097] Tumor: As used herein, the term "tumor" refers to an abnormal
growth of cells or
tissue. In some embodiments, a tumor may comprise cells that are precancerous
(e.g., benign),
malignant, pre-metastatic, metastatic, and/or non-metastatic. In some
embodiments, a tumor is
associated with, or is a manifestation of, a cancer. In some embodiments, a
tumor may be a
disperse tumor or a liquid tumor. In some embodiments, a tumor may be a solid
tumor.
[0098] Vector: As used herein, the term "vector" refers to a composition
of matter that
comprises an isolated nucleic acid and which can be used to deliver the
isolated nucleic acid to
the interior of a cell. Numerous vectors are known in the art including, but
not limited to, linear
polynucleotides, polynucleotides associated with ionic or amphiphilic
compounds, plasmids, and
viruses. Thus, the term "vector" includes an autonomously replicating plasmid
or a virus. The
term should also be construed to include non-plasmid and non-viral compounds
which facilitate
transfer of nucleic acid into cells, such as, for example, polylysine
compounds, liposomes, and
the like. Examples of viral vectors include, but are not limited to,
adenoviral vectors, adeno-
associated virus vectors, retroviral vectors, lentiviral vectors, and the
like.
[0099] Throughout this disclosure, various aspects of the invention can
be presented in a
range format. It should be understood that the description in range format is
merely for
convenience and brevity and should not be construed as an inflexible
limitation on the scope of
28
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
the invention. Accordingly, the description of a range should be considered to
have specifically
disclosed all the possible subranges as well as individual numerical values
within that range. For
example, description of a range such as from 1 to 6 should be considered to
have specifically
disclosed subranges such as from 1 to 3, from 1 to 4, from 1 to 5, from 2 to
4, from 2 to 6, from 3
to 6 etc., as well as individual numbers within that range, for example, 1, 2,
2.7, 3, 4, 5, 5.3, and
6. This applies regardless of the breadth of the range.
DETAILED DESCIPTION
Immune Cells
[0100] The present disclosure, among other things, provides modified
immune cells (e.g.,
macrophages, monocytes, or dendritic cells) comprising at least one chimeric
antigen receptor
(CAR) as described herein. Accordingly, in some embodiments, an immune cell
comprising at
least one CAR comprises: (a) an extracellular domain (e.g., an extracellular
domain as described
herein), (b) a transmembrane domain (e.g., a transmembrane domain as described
herein), and
(c) an intracellular domain (e.g., an intracellular domain as described
herein).
[0101] In some embodiments, a population of immune cells as described
herein
comprises monocytes, macrophages, dendritic cells, and/or precursors thereof.
In some
embodiments, a population of immune cells comprises a purified population of
monocytes,
macrophages, or dendritic cells, or a cell line.
[0102] In some embodiments, an immune cell is activated, e.g., an immune
cell exhibits
increased cytokine production, chemokine production, phagocytosis, cell
signaling, target cell
killing, and/or antigen presentation, e.g., relative to an inactive cell. In
some embodiments, an
activated immune cell exhibits changes in gene expression, e.g., an induction
of pro-
inflammatory gene expression (e.g., one, two, three, four, five, six, or seven
of TNF, IL-12, IFN,
GM-CSF, G-CSF, M-CSF, or IL-1), e.g., relative to an inactive cell. In certain
embodiments,
activated immune cells are undergoing cell division. In some embodiments,
targeted effector
activity of an immune cell is enhanced by inhibition of CD47 and/or SIRPa
activity. CD47
and/or SIRPa activity may be inhibited by treating an immune cell with an anti-
CD47 or anti-
SIRPa antibody or by any method known to those skilled in the art.
29
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0103] In some embodiments, immune cells (e.g., macrophages, monocytes,
or dendritic
cells) are obtained (e.g., isolated) from a subject. Immune cells may be
autologous or sourced
from allogeneic or universal donors. Cells can be obtained from a number of
sources including
peripheral blood mononuclear cells, bone marrow, lymph node tissue, spleen
tissue, umbilical
cord, tumors, and/or induced pluripotent stem cells, such as embryonic stem
cells (ESCs). In
certain embodiments, cells can be obtained from a unit of blood collected from
a subject using
any number of separation techniques known to a skilled artisan, such as Ficoll
separation. In
some embodiments, cells from circulating blood of a subject are obtained by
apheresis or
leukapheresis. Cells collected by apheresis may be washed to remove a plasma
fraction and
resuspended in a variety of buffers (e.g., phosphate buffered saline (PBS)) or
culture media). In
some embodiments, enrichment of immune cells (e.g. monocytes) comprises
plastic adherence.
In some embodiments, following enrichment, differentiation of immune cells
(e.g. monocytes)
comprises stimulation with GM-CSF. In some embodiments, a composition
comprising blood
cells (e.g., monocytes, lymphocytes, platelets, plasma, and/or red blood
cells), such as a
leukapheresis composition (e.g., a leukopak) is used for enrichment. In some
embodiments, a
leukapheresis composition (e.g., a leukopak) comprises a sample from a healthy
human donor.
In certain embodiments, apheresis of immune cells (e.g. monocytes) is followed
by mobilization
with GM-CSF. In certain embodiments, selection of immune cells (e.g.,
monocytes) comprises
CD14 positive selection using microbeads (e.g., MACS MicroBeads on a
CliniMACS Prodigy
device). In some embodiments, an immune cell precursor (e.g., precursors to
macrophages,
monocytes, or dendritic cells) is used in compositions and methods described
herein. Immune
cell precursors may be differentiated in vivo or ex vivo into immune cells.
Non-limiting
examples of precursor immune cells include hematopoietic stem cells, common
myeloid
progenitors, myeloblasts, monoblasts, promonocytes, or intermediates thereof.
For example,
induced pluripotent stem cells may be used to generate monocytes, macrophages,
and/or
dendritic cells. Induced pluripotent stem cells (iPSCs) may be derived from
normal human
tissue, such as peripheral blood, fibroblasts, skin, keratinocytes, or renal
epithelial cells.
Autologous, allogeneic, or universal donor iPSCs could be differentiated
toward a myeloid
lineage (e.g., a monocyte, macrophage, dendritic cell, or precursor thereof).
[0104] Immune cells (e.g., macrophages, monocytes, or dendritic cells) as
described
herein can be isolated from peripheral blood, for example, by lysing red blood
cells and
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
depleting lymphocytes and red blood cells, such as by centrifugation through a
PERCOLLTM
gradient. Alternatively, immune cells can be isolated from umbilical cord
tissue. A specific
subpopulation of immune cells can be further isolated by positive or negative
selection
techniques. In some embodiments, immune cells can be depleted of cells
expressing certain
antigens, including, but not limited to, CD34, CD3, CD4, CD8, CD56, CD66b,
CD19, or CD20.
In some embodiments, enrichment of an immune cell population, for example, by
negative
selection can be accomplished using a combination of antibodies directed to
surface markers
unique to the negatively selected cells. By way of non-limiting example, cell
selection can also
comprise negative magnetic immunoadherence or flow cytometry that uses a
cocktail of
monoclonal antibodies directed to cell surface markers present on negatively
selected cells.
[0105] During isolation of a desired population of immune cells (e.g.,
macrophages,
monocytes, or dendritic cells) as described herein by positive or negative
selection, immune cell
concentration and surface (e.g., particles, such as beads) can be varied. It
may be desirable to
significantly decrease volume in which beads and cells are mixed together to
ensure maximum
contact area of cells and beads.
[0106] In some embodiments, prior to administration, immune cells (e.g.,
macrophages,
monocytes, or dendritic cells) as described herein (e.g., comprising a CAR
described herein) are
treated with a pro-inflammatory agent. In some embodiments, treatment with a
pro-
inflammatory agent increases anti-tumor activity of immune cells described
herein. In some
embodiments, treatment with a pro-inflammatory agent promotes M1 phenotype
(e.g., a switch
from M2 to M1 phenotype) in immune cells described herein. In some
embodiments, a pro-
inflammatory agent comprises or is a CD40 agonist (e.g., CD4OL). In some
embodiments, a pro-
inflammatory agent comprises or is a 41BB-ligand agonists (e.g., 4-1BB).
[0107] In some embodiments, immune cells (e.g., macrophages, monocytes,
or dendritic
cells) as described herein (e.g., comprising a CAR described herein) are
administered to a subject
in combination with a pro-inflammatory agent. In some embodiments, immune
cells (e.g.,
macrophages, monocytes, or dendritic cells) as described herein (e.g.,
comprising a CAR
described herein) are administered to a subject substantially simultaneously,
before, or after a
pro-inflammatory agent. In some embodiments, administration with a pro-
inflammatory agent
increases anti-tumor activity of immune cells described herein. In some
embodiments,
31
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
administration with a pro-inflammatory agent promotes M1 phenotype (e.g., a
switch from M2 to
M1 phenotype) in immune cells described herein. In some embodiments, a pro-
inflammatory
agent comprises or is a CD40 agonist (e.g., CD4OL). In some embodiments, a pro-
inflammatory
agent comprises or is a 41BB-ligand agonists (e.g., 4-1BB).
Macrophages
[0108] Macrophages are immune cells specialized for detection,
phagocytosis, and
destruction of target cells, such as pathogens or tumor cells. Macrophages are
potent effectors of
the innate immune system and are capable of at least three distinct anti-tumor
functions:
phagocytosis of dead and dying cells, microorganisms, cancer cells, cellular
debris, or other
foreign substances; cytotoxicity against tumor cells; and presentation of
tumor antigens to
orchestrate an adaptive anti-tumor immune response.
[0109] Accumulating evidence suggests that macrophages are abundant in
the tumor
microenvironment of numerous cancers and can adopt a number of phenotypes that
are
collectively referred to as tumor-associated macrophages (TAMs). The
immunosuppressive
nature of the tumor microenvironment typically results in more M2-like TAMs,
which further
contribute to the general suppression of anti-tumor immune responses. Recent
studies, however,
have identified that TAMs are able to be "reprogrammed" via pro-inflammatory
signals, and that
the switch from a M2 phenotype to a more M1 phenotype is associated with
productive anti-
tumor immune responses. Inducing endogenous TAMs to switch to Ml-type cells
and
engineering macrophages that cannot be subverted into M2 would greatly enhance
anti-tumor
immunotherapy and represent a significant advance in the field.
[0110] In some embodiments, a macrophage comprises or is an
undifferentiated or MO
macrophage. In certain embodiments, a macrophage comprises or expresses one,
two, three,
four, five, or six of CD14, CD16, CD64, CD68, CD71, or CCR5. Exposure to
various stimuli
can induce MO macrophages to polarize into several distinct populations, which
may be
identified by macrophage phenotype markers, cytokine production, and/or
chemokine secretion.
[0111] In some embodiments, a macrophage comprises or is a polarized
macrophage.
Under classical conditions of activation, MO macrophages can be exposed to pro-
inflammatory
signals, such as LPS, IFNy, and GM-CSF, and polarize into M1 macrophages.
Generally, M1
32
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
macrophages are associated with pro-inflammatory immune responses, such as Thl
and Th17 T
cell responses. Exposure to other stimuli can polarize macrophages into a
diverse group of
"alternatively activated" or M2 macrophages.
[0112] In some embodiments, a macrophage comprises or is an M1
macrophage. In
some embodiments, a macrophage expresses one or more markers of M1 macrophages
(e.g., 1, 2,
3,4, 5, 6, 7, 8,9, 10, 11, 12, 13, 14, 15, 16, 17, or 18 of CD86, CD80, MHC
II, IL-1R, TLR2,
TLR4, iNOS, SOCS3, CD83, PD-L1, CD69, MHC I, CD64, CD32, CD16, IL1R, a IFIT
family
member, or an ISG family member).
[0113] In some embodiments, a macrophage comprising or expressing at
least one CAR
described herein secretes relatively high levels of one or more inflammatory
cytokines (e.g., 1, 2,
3, 4, 5, 6, 7, 8, 9, 10, 11, or 12 of IL-1, TNF, IL-12, IL-18, IL-23, IFNa,
IFNO, IFNy, IL-2, IL-6,
IL-8, or IL33) or chemokines (e.g., one or both of CC or CXC chemokines)
(e.g., 1, 2, 3, 4, 5, 6,
7, 8,9, 10, 11, 12, 13, 14, 15, or 16 of the CXC chemokines; e.g., 1, 2, 3, 4,
5, 6, 7, 8,9, 10, 11,
12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, or 28 of the
CC chemokines; eg., one
of the CX3C chemokines, e.g., one or both of the C chemokines), e.g., relative
to a macrophage
without a CAR as described herein. In some embodiments, a macrophage
comprising or
expressing at least one CAR described herein stimulates an immune response
and/or
inflammation, e.g., relative to a macrophage without a CAR as described
herein. In some
embodiments, a macrophage comprises or is an M2 macrophage (e.g., an M2a, M2b,
M2c, and
M2d macrophage). An M2a macrophage can be induced by IL-4, IL-13, and/or
fungal infection.
An M2b macrophage can be induced by IL-1R ligands, an immune complex, and/or
LPS. An
M2c macrophage can be induced by IL-10 and/or TGFP. An M2d macrophage can be
induced
by IL-6 and/or adenosine. In some embodiments, a macrophage comprising or
expressing at
least one CAR described herein decreases an immune response in a subject,
e.g., relative to a
macrophage without a CAR as described herein. In some embodiments, a
macrophage expresses
one or more markers of M2 macrophages (e.g., one, two, or three of CD206,
CD163, or CD209).
In some embodiments, a macrophage comprising or expressing at least one CAR
described
herein exhibits increased secretion of one or more anti-inflammatory cytokines
(e.g., one or both
of IL-10 or TGF43), e.g., relative to a macrophage without a CAR as described
herein.
33
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0114] In some embodiments, a macrophage comprises at least one
upregulated M1
marker and/or at least one downregulated M2 marker. In some embodiments, at
least one M1
marker (e.g., HLA DR, CD86, CD80, PD-L1, CD83, CD69, MHC I, CD64, CD32, CD16,
IL1R,
an IFIT family member, and/or an ISG family member) is upregulated in a
macrophage. In some
embodiments, at least one M2 marker (e.g., CD206, CD163, and/or CD209) is
downregulated in
a macrophage.
[0115] In some embodiments, a macrophage comprising or expressing at
least one CAR
described herein exhibits increased phagocytosis, e.g., relative to a
macrophage without a CAR
as described herein. In some embodiments, a macrophage comprising or
expressing at least one
CAR described herein exhibits increased cytotoxicity against a tumor cell,
e.g., relative to a
macrophage without a CAR as described herein. In some embodiments, a
macrophage
comprising or expressing at least one CAR described herein exhibits increased
tumor antigen
presentation (e.g., post-phagocytosis presentation) and/or increased antigen
processing, e.g.,
relative to a macrophage without a CAR as described herein. In some
embodiments, a
macrophage comprising or expressing at least one CAR described herein exhibits
increased
tumor killing (e.g., by phagocytosis, lysis, apoptosis, or production of tumor
killing cytokines
(e.g., TNFa), e.g., relative to a macrophage without a CAR as described
herein.
[0116] In some embodiments, a macrophage comprising or expressing at
least one CAR
described herein exhibits one or both of increased expression of favorable
genes (e.g., CD80,
CD86, MHC-I, MHC-II, CD40, 41BBL, TNF, IFN-a, IFN-y, IL2, IL12, IL6, IL8,
IL lb,
and/or CXCL12) or decreased expression of unfavorable genes (e.g., CD163,
CD206, TGFP,
IL10, and/or IL4), e.g., relative to a macrophage without a CAR as described
herein. In some
embodiments, a macrophage comprising or expressing at least one CAR described
herein
exhibits increased production of ROS, e.g., relative to a macrophage without a
CAR as described
herein. In some embodiments, a macrophage comprising or expressing at least
one CAR
described herein exhibits metabolic reprogramming (e.g., of an interferon
signaling pathway,
TH1 pathway, PTEN signaling, PI3K signaling, MTOR signaling, TLR signaling,
CD40
signaling, 41BB signaling, 41BBL signaling, macrophage maturation signaling,
dendritic cell
maturation signaling, CD3-zeta signaling, FcR y signaling, CD64 signaling,
CD32a signaling,
CD32c signaling, CD16a signaling, TLR1 signaling, TLR2 signaling, TLR3
signaling, TLR4
34
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
signaling, TLR5 signaling, TLR6 signaling, TLR7 signaling, TLR8 signaling,
TLR9 signaling,
ALK signaling, AXL signaling, DDR2 signaling, EGFR signaling, EphAl signaling,
INSR
signaling, cMET signaling, MUSK signaling, PDGFR signaling, PTK7 signaling,
RET signaling,
ROR1 signaling, ROS1 signaling, RYK signaling, TIE2 signaling, TRK signaling,
VEGFR
signaling, CD40 signaling, CD19 signaling, CD20 signaling, 41BB signaling,
CD28 signaling,
0X40 signaling, GITR signaling, TREM-1 signaling, TREM-2 signaling, DAP12
signaling, MR
signaling, ICOS signaling, MyD88 signaling, V/I/LxYxxL/V signaling, SIRPa
signaling, CD45
signaling, Siglec-10 signaling, PD1 signaling, SHP-1 signaling, SHP-2
signaling, KIR-2DL
signaling, KIR-3DL signaling, NKG2A signaling, CD170 signaling, CD33
signaling, BTLA
signaling, CD32b signaling, SIRPI3 signaling, CD22 signaling, PIR-B signaling,
and/or LILRB1
signaling), e.g., relative to a macrophage without a CAR as described herein.
In some
embodiments, a macrophage comprising or expressing at least one CAR described
herein
exhibits induction of cell survival mechanisms, e.g., relative to a macrophage
without a CAR as
described herein. In some embodiments, a macrophage comprising or expressing
at least one
CAR described herein exhibits induction of cell death mechanisms, e.g.,
relative to a
macrophage without a CAR as described herein. In some embodiments, a
macrophage
comprising or expressing at least one CAR described herein exhibits one, two,
three, four, or five
of increased resistance to phagocytic checkpoints, increased expression of
chemokine receptors
to aid in trafficking, increased expression of chemokines to recruit other
immune cells, increased
expression of ECM degrading enzymes (e.g., MMPs to degrade tumor ECM and/or
exhibit anti
fibrotic activity), or increased proliferation, e.g., relative to a macrophage
without a CAR as
described herein. In some embodiments, a macrophage comprising or expressing
at least one
CAR described herein exhibits one, two, three, or four of improved duration of
CAR expression,
improved stability of the CAR on the cell surface, increased level of CAR
expression, or
decreased background activity of the CAR, e.g., relative to a macrophage
without a CAR as
described herein.
[0117] In some embodiments, a macrophage comprising or expressing at
least one CAR
described herein decreases infection (e.g., of an infectious agent) in a
subject, e.g., relative to a
macrophage without a CAR as described herein. In some embodiments, an
infectious agent
comprises or is a virus, a protozoa (e.g., trypanosome, malaria, or
toxoplasma), a bacteria (e.g.,
mycobacterium, salmonella, or listeria), a fungi (e.g., Candida), or a
combination thereof. In
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
some embodiments, a virus comprises hepatitis virus (e.g., hepatitis A,
hepatitis B, hepatitis C, or
hepatitis E), retrovirus, human immunodeficiency virus (e.g., HIV1 or HIV2), T
cell leukemia
virus, a Lymphotropic virus (e.g., HTLV1 or HTLV2), herpes simplex virus
(e.g., herpes
simplex virus type 1 or type 2), Epstein-Barr virus, cytomegalovirus,
varicella-zoster virus,
poliovirus, measles virus, Rubella virus, Japanese encephalitis virus, mumps
virus, influenza
virus, adenovirus, enterovirus, rhinovirus, coronavirus (e.g., severe acute
respiratory syndrome
(SARS) virus, Middle East respiratory syndrome (MERS) virus, or severe acute
respiratory
syndrome coronavirus 2 (SARS-CoV2)), Ebola virus, West Nile virus, or a
variant or
combination thereof.
[0118] In some embodiments, a macrophage comprising or expressing at
least one CAR
described herein decreases formation and/or degrades existing aggregates via
phagocytosis of at
least one protein aggregate in a subject (e.g., a subject having a
neurodegenerative disease, an
inflammatory disease, a cardiovascular disease, a fibrotic disease,
amyloidosis, or a combination
thereof), e.g., relative to a macrophage without a CAR as described herein. In
some
embodiments, a neurodegenerative disease is selected from the group consisting
of tauopathy, a-
synucleopathy, presenile dementia, senile dementia, Alzheimer's disease,
progressive
supranuclear palsy (PSP), Pick's disease, primary progressive aphasia,
frontotemporal dementia,
corticobasal dementia, Parkinson's disease, dementia with Lewy bodies, Down's
syndrome,
multiple system atrophy, amyotrophic lateral sclerosis (ALS), Hallervorden-
Spatz syndrome,
polyglutamine disease, trinucleotide repeat disease, and prion disease. In
some embodiments, an
inflammatory disease is selected from the group consisting of systemic lupus
erythematosus,
vasculitis, rheumatoid arthritis, periodontitis, ulcerative colitis,
sinusitis, asthma, tuberculosis,
Crohn's disease, chronic infection, hereditary periodic fever, a malignancy,
systemic
vasculitides, cystic fibrosis, bronchiectasis, epidermolysis bullosa, cyclic
neutropenia, an
immunodeficiency, Muckle-Wells (MWS) disease, and Familiar Mediterranean Fever
(FMF). In
some embodiments, amyloidosis is selected from the group consisting of Primary
Amyloidosis
(AL), Secondary Amyloidosis (AA), Familial Amyloidosis (ATTR) , Beta-2
Microglobulin
Amyloidosis, Localized Amyloidosis, Heavy Chain Amyloidosis (AH), Light Chain
Amyloidosis (AL), Primary Systemic Amyloidosis, ApoAI Amyloidosis, ApoAII
Amyloidosis,
ApoAIV Amyloidosis, Apolipoprotein C2 Amyloidosis, Apolipoprotein C3
Amyloidosis,
Corneal lactoferrin amyloidosis, Transthyretin-Related Amyloidosis, Dialysis
amyloidosis,
36
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
Fibrinogen amyloidosis, Lect2 amyloidosis (ALECT2), and Lysozyme amyloidosis.
In some
embodiments, a cardiovascular disease is selected from the group consisting of
atherosclerosis,
coronary artery disease, peripheral artery disease, hypertensive heart
disease, metabolic
syndrome, hypertension, cerebrovascular disease, and heart failure. In some
embodiments, a
fibrotic disease is selected from the group consisting of pulmonary fibrosis,
idiopathic
pulmonary fibrosis, cirrhosis, cystic fibrosis, scleroderma, cardiac fibrosis,
radiation-induced
lung injury, steatohepatitis, glomerulosclerosis, interstitial lung disease,
liver fibrosis,
mediastinal fibrosis, retroperitoneal cavity fibrosis, bone marrow fibrosis,
and skin fibrosis.
Monocytes
[0119] Monocytes are multipotent cells that circulate in the blood, bone
marrow, and
spleen, and generally do not proliferate when in a steady state. Monocytes can
vary in size
significantly in the range of about 10-30 [tm in diameter. A ratio of nucleus
to cytoplasm for a
monocyte can range from about 2:1 to about 1:1. Typically, monocytes comprise
chemokine
receptors and pathogen recognition receptors that mediate migration from blood
to tissues, such
as during an infection. Monocytes can produce inflammatory cytokines, take up
cells and/or
toxic molecules, and differentiate into dendritic cells or macrophages.
[0120] In some embodiments, a monocyte comprises or expresses one or more
phenotypic markers. Exemplarily phenotypic markers for human monocyte cells
include, but are
not limited to, CD9, CD11b, CD11c, CDw12, CD13, CD14, CD15, CDw17, CD31, CD32,
CD33, CD35, CD36, CD38, CD43, CD49b, CD49e, CD49f, CD63, CD64, CD65s, CD68,
CD84,
CD85, CD86, CD87, CD89, CD91, CDw92, CD93, CD98, CD101, CD102, CD111, CD112,
CD115, CD116, CD119, CDw121b, CDw123, CD127, CDw128, CDw131, CD147, CD155,
CD156a, CD157, CD162 CD163, CD164, CD168, CD171, CD172a, CD180, CD206,
CD131a1,
CD213 2, CDw210, CD226, CD281, CD282, CD284, and CD286. Exemplarily phenotypic
markers for mouse monocyte cells include, but are not limited to, CD11a,
CD11b, CD16, CD18,
CD29, CD31, CD32, CD44, CD45, CD49d, CD115, CD116, Cdw131, CD281, CD282,
CD284,
CD286, F4/80, and CD49b. In certain embodiments monocytes comprise one, two,
or three of
CD11b, CD14, or CD16. In certain embodiments, monocytes comprise CD14+ CD16-
monocytes, CD14+ CD16+ monocytes, or CD14- CD16+ monocytes.
37
CA 03187138 2022-12-13
WO 2021/263152
PCT/US2021/039168
[0121] In
some embodiments, a monocyte differentiates into a macrophage. In some
embodiments, a monocyte differentiates into a dendritic cell (DC). Monocytes
can be
differentiated into macrophages or DCs by any technique known in the art. For
example,
differentiation of monocytes into macrophages can be induced by macrophage
colony
stimulating factor (M-C SF). Differentiation of monocytes into DCs can be
induced by
granulocyte-macrophage colony stimulating factor (GM-CSF) in combination with
IL-4.
[0122] In
some embodiments, a monocyte comprising or expressing at least one CAR
described herein exhibits increased secretion of one or more cytokines (e.g.,
one, two, three,
four, five, six, or seven of TNF, IL-12, IFN, GM-CSF, G-CSF, M-CSF, or IL-1),
e.g., relative to
a monocyte without a CAR as described herein. In some embodiments, a monocyte
comprising
or expressing at least one CAR described herein exhibits increased
phagocytosis, e.g., relative to
a monocyte without a CAR as described herein. In some embodiments, a monocyte
comprising
or expressing at least one CAR described herein exhibits enhanced survival,
e.g., relative to a
monocyte without a CAR as described herein. In some embodiments, a monocyte
comprising or
expressing at least one CAR described herein exhibits enhanced differentiation
into macrophages
(e.g., M1 or M2 macrophages), e.g., relative to a monocyte without a CAR as
described herein.
In some embodiments, a monocyte comprising or expressing at least one CAR
described herein
exhibits enhanced differentiation into DCs (e.g., resident or migrating DCs
and/or in lymphoid
and non-lymphoid tissue), e.g., relative to a monocyte without a CAR as
described herein. In
some embodiments, a monocyte comprising or expressing at least one CAR
described herein
exhibits increased cytotoxicity against a tumor cell, e.g., relative to a
monocyte without a CAR
as described herein. In some embodiments, a monocyte comprising or expressing
at least one
CAR described herein exhibits increased tumor antigen presentation (e.g., post-
phagocytosis
presentation) and/or increased antigen processing, e.g., relative to a
monocyte without a CAR as
described herein. In some embodiments, a monocyte comprising or expressing at
least one CAR
described herein exhibits increased tumor killing (e.g., by phagocytosis,
lysis, apoptosis, or
production of tumor killing cytokines (e.g., TNFa), e.g., relative to a
monocyte without a CAR
as described herein.
[0123] In
some embodiments, a monocyte comprising or expressing at least one CAR
described herein exhibits one or both of increased expression of favorable
genes or decreased
38
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
expression of unfavorable genes, e.g., relative to a monocyte without a CAR as
described herein.
In some embodiments, a monocyte comprising or expressing at least one CAR
described herein
exhibits increased production of ROS, e.g., relative to a monocyte without a
CAR as described
herein. In some embodiments, a monocyte comprising or expressing at least one
CAR described
herein exhibits metabolic reprogramming, e.g., relative to a monocyte without
a CAR as
described herein. In some embodiments, a monocyte comprising or expressing at
least one CAR
described herein exhibits induction of cell survival mechanisms, e.g.,
relative to a monocyte
without a CAR as described herein. In some embodiments, a monocyte comprising
or
expressing at least one CAR described herein exhibits induction of cell death
mechanisms, e.g.,
relative to a monocyte without a CAR as described herein. In some embodiments,
a monocyte
comprising or expressing at least one CAR described herein exhibits one, two,
three, four, or five
of increased resistance to phagocytic checkpoints, increased expression of
chemokine receptors
to aid in trafficking, increased expression of chemokines to recruit other
immune cells, increased
expression of ECM degrading enzymes (e.g., MMPs to degrade tumor ECM and/or
exhibit anti
fibrotic activity), or increased proliferation, e.g., relative to a monocyte
without a CAR as
described herein. In some embodiments, a monocyte comprising or expressing at
least one CAR
described herein exhibits one, two, three, or four of improved duration of CAR
expression,
improved stability of the CAR on the cell surface, increased level of CAR
expression, or
decreased background activity of the CAR, e.g., relative to a monocyte without
a CAR as
described herein.
Dendritic Cells
[0124] Dendritic cells (DCs) are bone marrow-derived, specialized antigen
presenting
cells that are involved in initiating immune responses and maintaining
tolerance of the immune
system to self-antigens. Dendritic cells may be found in both lymphoid and non-
lymphoid
organs and are generally thought to arise from lymphoid or myeloid lineages.
[0125] In some embodiments, a DC comprises or expresses one or more
phenotypic
markers. Exemplarily phenotypic markers for DCs include, but are not limited
to, CD11 c,
CD83, CD1a, CD lc, CD141, CD207, CLEC9a, CD123, CD85, CD180, CD187, CD205,
CD281,
CD282, CD284, CD286 and partially CD206, CD207, CD208 and CD209.
39
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0126] Immature DCs can be characterized by a high capacity for antigen
capture, but
relatively low T cell stimulatory capability. Inflammatory mediators promote
DC maturation.
Once DCs reach the mature stage, there is a dramatic change in properties
relative to immature
DCs, such as a decrease in antigen capture ability and/or an increased ability
to stimulate T cells.
In some embodiments, a DC comprises or is an immature DC. In other
embodiments, a DC
comprises or is a mature DC.
[0127] Without wishing to be bound by theory, it is believed that
modification of a DC
cell to comprise or express at least one CAR described herein can allow mature
DCs to
simultaneously exhibit increased antigen capture ability and T cell
stimulation, e.g., relative to a
DC without a CAR described herein. In some embodiments, a DC comprising or
expressing at
least one CAR described herein mediates tumor antigen presentation, e.g.,
increased tumor
antigen presentation relative to a DC without a CAR as described herein. In
some embodiments,
a DC comprising or expressing at least one CAR described herein mediates tumor
T cell
stimulation, e.g., increased T cell stimulation relative to a DC without a CAR
as described
herein.
[0128] In some embodiments, a DC comprising or expressing at least one
CAR described
herein exhibits increased secretion of one or more cytokines (e.g., one, two,
three, four, five, six,
or seven of TNF, IL-12, IFN, GM-CSF, G-C SF, M-C SF, or IL-1), e.g., relative
to a DC without
a CAR as described herein. In some embodiments, a DC comprising or expressing
at least one
CAR described herein exhibits increased phagocytosis, e.g., relative to a DC
without a CAR as
described herein. In some embodiments, a DC comprising or expressing at least
one CAR
described herein exhibits increased tumor antigen presentation (e.g., post-
phagocytosis
presentation), increased antigen processing, increased antigen cross
presentation, increased T cell
priming, and/or stimulation of T cells, e.g., relative to a DC without a CAR
as described herein.
[0129] In some embodiments, a DC comprising or expressing at least one
CAR described
herein exhibits one or both of increased expression of favorable genes or
decreased expression of
unfavorable genes, e.g., relative to a DC without a CAR as described herein.
In some
embodiments, a DC comprising or expressing at least one CAR described herein
exhibits
increased production of ROS, e.g., relative to a DC without a CAR as described
herein. In some
embodiments, a DC comprising or expressing at least one CAR described herein
exhibits
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
metabolic reprogramming, e.g., relative to a DC without a CAR as described
herein. In some
embodiments, a DC comprising or expressing at least one CAR described herein
exhibits
induction of cell survival mechanisms, e.g., relative to a DC without a CAR as
described herein.
[0130] In some embodiments, a DC comprising or expressing at least one
CAR described
herein exhibits induction of cell death mechanisms, e.g., relative to a DC
without a CAR as
described herein. In some embodiments, a DC comprising or expressing at least
one CAR
described herein exhibits one, two, three, four, or five of increased
resistance to phagocytic
checkpoints, increased expression of chemokine receptors to aid in
trafficking, increased
expression of chemokines to recruit other immune cells, increased expression
of ECM degrading
enzymes (e.g., MMPs to degrade tumor ECM and/or exhibit anti fibrotic
activity), or increased
proliferation, e.g., relative to a DC without a CAR as described herein. In
some embodiments, a
DC comprising or expressing at least one CAR described herein exhibits one,
two, three, or four
of improved duration of CAR expression, improved stability of the CAR on the
cell surface,
increased level of CAR expression, or decreased background activity of the
CAR, e.g., relative to
a DC without a CAR as described herein.
Methods of Immune Cell Modification
[0131] In some embodiments, the present disclosure provides methods of
modifying an
immune cell, the methods comprising the steps of: (a) modifying a nucleic acid
encoding a
chimeric antigen receptor (CAR), (b) purifying the nucleic acid, and (c)
delivering the nucleic
acid to the immune cell, wherein the immune cell comprises a macrophage, a
monocyte or a
dendritic cell, and wherein the modified immune cell comprises a CAR. In some
embodiments,
the present disclosure provides methods of modifying an immune cell, the
methods comprising
the steps of: (a) modifying a messenger RNA (mRNA) encoding a chimeric antigen
receptor
(CAR), (b) purifying the mRNA, and (c) delivering the mRNA to the immune cell,
wherein the
immune cell comprises a macrophage, a monocyte or a dendritic cell, and
wherein the modified
immune cell comprises a CAR.
[0132] In some embodiments, the present disclosure provides methods
comprising
delivering a modified mRNA to an immune cell, wherein the mRNA comprises a
CAR. In some
embodiments, provided methods further comprise treating the immune cell with
an RNaseL
inhibitor, optionally before the delivering step. In some embodiments,
provided methods further
41
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
comprise a step of culturing an immune cell with a cytokine or immune
stimulating recombinant
protein (e.g., IFN-a, IFN-f3, IFN-y, TNFa, IL-6, STNGL, LPS, a CD40 agonist, a
4-1BB ligand,
a recombinant 4-1BB receptor, a TLR agonist, beta-glucan, IL-4, IL-13, IL-10,
TGF-f3, a
glucocorticoid, an immune complex, or a combination thereof). In some
embodiments, a
cytokine comprises IFN-f3.
Nucleic Acid Modifications
[0133] In some embodiments of the present disclosure, a nucleic acid
construct is or
comprises an mRNA. In some embodiments, mRNA according to the present
disclosure may be
synthesized as unmodified or modified mRNA. Typically, mRNAs are modified to
enhance
stability. Modifications of mRNA can include, for example, modifications of
the nucleotides of
the RNA. A modified mRNA according to the present disclosure can thus include,
for example,
backbone modifications, sugar modifications or base modifications. In some
embodiments, a
step of modifying an mRNA comprises causing the mRNA to include a modified
nucleotide, an
alteration to the 5' or 3' untranslated region (UTR), a cap structure, and/or
a poly(A) tail. In
some embodiments, mRNAs may be synthesized from naturally occurring
nucleotides and/or
nucleotide analogues (modified nucleotides) including, but not limited to,
purines (adenine (A),
guanine (G)) or pyrimidines (thymine (T), cytosine (C), uracil (U)), and as
modified nucleotide
analogues or derivatives of purines and pyrimidines, such as, e.g., 1-methyl-
adenine, 2-methyl-
adenine, 2-methylthio-N-6-isopentenyl-adenine, N6-methyl-adenine, N6-
isopentenyl-adenine, 2-
thio-cytosine, 3-methyl-cytosine, 4-acetyl-cytosine, 5-methyl-cytosine, 2,6-
diaminopurine, 1-
methyl-guanine, 2-methyl-guanine, 2,2-dimethyl-guanine, 7-methyl-guanine,
inosine, 1-methyl-
inosine, pseudouracil (5-uracil), dihydro-uracil, 2-thio-uracil, 4-thio-
uracil, 5-
carboxymethylaminomethy1-2-thio-uracil, 5-(carboxyhydroxymethyl)-uracil, 5-
fluoro-uracil, 5-
bromo-uracil, 5-carboxymethylaminomethyl-uracil, 5-methy1-2-thio-uracil, 5-
methyl-uracil, N-
uracil-5-oxyacetic acid methyl ester, 5-methylaminomethyl-uracil, 5-
methoxyaminomethy1-2-
thio-uracil, 5'-methoxycarbonylmethyl-uracil, 5-methoxy-uracil, uracil-5-
oxyacetic acid methyl
ester, uracil-5-oxyacetic acid (v), 1-methyl-pseudouracil, queosine, P-D-
mannosyl-queosine,
wybutoxosine, and phosphoramidates, phosphorothioates, peptide nucleotides,
methylphosphonates, 7-deazaguanosine, 5-methylcytosine and inosine. The
preparation of such
42
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
analogues is known to a person skilled in the art, e.g., from U.S. Patent No.
4,373,071, U.S.
Patent No. 4,401,796, U.S. Patent No. 4,415,732, U.S. Patent No. 4,458,066,
U.S. Patent No.
4,500,707, U.S. Patent No. 4,668,777, U.S. Patent No. 4,973,679, U.S. Patent
No. 5,047,524,
U.S. Patent No. 5,132,418, U.S. Patent No. 5,153,319, U.S. Patent No.
5,262,530 and U.S. Patent
No. 5,700,642, the disclosures of which are incorporated by reference in their
entirety.
[0134] In some embodiments, mRNAs of the present disclosure (e.g., mRNAs
encoding
CARs) may contain RNA backbone modifications. Typically, a backbone
modification is a
modification in which the phosphates of the backbone of the nucleotides
contained in the RNA
are modified chemically. Exemplary backbone modifications typically include,
but are not
limited to, modifications from the group consisting of methylphosphonates,
methylphosphoramidates, phosphoramidates, phosphorothioates (e.g. cytidine 5'-
0-(1-
thiophosphate)), boranophosphates, positively charged guanidinium groups etc.,
which
comprises replacing the phosphodiester linkage by other anionic, cationic or
neutral groups.
[0135] In some embodiments, mRNAs of the present disclosure (e.g., mRNAs
encoding
CARs) may contain sugar modifications. A typical sugar modification is a
chemical
modification of the sugar of the nucleotides it contains including, but not
limited to, sugar
modifications chosen from the group consisting of 2'-deoxy-2'-fluoro-
oligoribonucleotide (2'-
fluoro-2'-deoxycytidine 5'-triphosphate, 2'-fluoro-2'-deoxyuridine 5'-
triphosphate), 2'-deoxy-2'-
deamine-oligoribonucleotide (2'-amino-2'-deoxycytidine 5'-triphosphate, 2'-
amino-2'-
deoxyuridine 5'-triphosphate), 2'-0-alkyloligoribonucleotide, 2'-deoxy-2'-C-
alkyloligoribonucleotide (2'-0-methylcytidine 5'-triphosphate, 2'-
methyluridine 5'-triphosphate),
2'-C-alkyloligoribonucleotide, and isomers thereof (2'-aracytidine 5'-
triphosphate, 2'-arauridine
5'-triphosphate), or azidotriphosphates (2'-azido-2'-deoxycytidine 5'-
triphosphate, 2'-azido-2'-
deoxyuridine 5'-triphosphate).
[0136] In some embodiments, mRNAs of the present disclosure (e.g., mRNAs
encoding
CARs) may contain modifications of the bases of the nucleotides (base
modifications). A
modified nucleotide which contains a base modification is also called a base-
modified
nucleotide. Examples of such base-modified nucleotides include, but are not
limited to, 2-
amino-6-chloropurine riboside 5'-triphosphate, 2-aminoadenosine 5'-
triphosphate, 2-thiocytidine
5'-triphosphate, 2-thiouridine 5'-triphosphate, 4-thiouridine 5'-triphosphate,
5-aminoallylcytidine
43
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
5'-triphosphate, 5-aminoallyluridine 5'-triphosphate, 5-bromocytidine 5'-
triphosphate, 5-
bromouridine 5'-triphosphate, 5-iodocytidine 5'-triphosphate, 5-iodouridine 5'-
triphosphate, 5-
methylcytidine 5'-triphosphate, 5-methyluridine 5'-triphosphate, 6-azacytidine
5'-triphosphate, 6-
azauridine 5'-triphosphate, 6-chloropurine riboside 5'-triphosphate, 7-
deazaadenosine 5'-
triphosphate, 7-deazaguanosine 5'-triphosphate, 8-azaadenosine 5'-
triphosphate, 8-
azidoadenosine 5'-triphosphate, benzimidazole riboside 5'-triphosphate, N1-
methyladenosine 5'-
triphosphate, N1-methylguanosine 5'-triphosphate, N6-methyladenosine 5'-
triphosphate, 06-
methylguanosine 5'-triphosphate, pseudouridine 5'-triphosphate, puromycin 5'-
triphosphate or
xanthosine 5'-triphosphate. In some embodiments, a modified nucleotide
comprises
pseudouridine (PsU), 5-methoxyuridine (5moU), 5-methylcytidine/pseudouridine
(5meC PsU),
Nl-methyl-pseudouridine (N1mPsU), or combinations thereof.
[0137] Typically, mRNA synthesis includes the addition of a "cap" on the
N-terminal
(5') end, and a "tail" on the C-terminal (3') end. The presence of the cap is
important in
providing resistance to nucleases found in most eukaryotic cells. The presence
of a "tail" serves
to protect the mRNA from exonuclease degradation.
[0138] Thus, in some embodiments, mRNAs of the present disclosure (e.g.,
mRNAs
encoding CARs) include a 5' cap structure. A 5' cap is typically added as
follows: first, an RNA
terminal phosphatase removes one of the terminal phosphate groups from the 5'
nucleotide,
leaving two terminal phosphates; guanosine triphosphate (GTP) is then added to
the terminal
phosphates via a guanylyl transferase, producing a 5' triphosphate linkage;
and the 7-nitrogen of
guanine is then methylated by a methyltransferase. Examples of cap structures
include, but are
not limited to, m7G(5')ppp (5'(A,G(5')ppp(5')A and G(5')ppp(5')G. In some
embodiments, a cap
comprises a Cap() structure. A cap() structures lack a 2'-0-methyl residue of
the ribose attached
to bases 1 and 2. In some embodiments, a cap comprises an AGCapl structure. An
AGCapl
structures has a 2'-0-methyl residue at base 2. In some embodiments, a cap
comprises a Cap2
structure. Cap2 structures have a 2'-0-methyl residue attached to both bases 2
and 3. In some
embodiments, a cap structure comprises AGCapl, m6AGCapl, or Anti-Reverse Cap
Analog
(ARCA). In some embodiments, a modified mRNA of the present disclosure
comprises an
m6AGCapl and modified nucleotides comprising pseudouridine (PsU).
44
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0139] In some embodiments, mRNAs of the present disclosure (e.g., mRNAs
encoding
CARs) include a 3' poly(A) tail structure. A poly(A) tail on the 3' terminus
of mRNA typically
includes about 10 to 400 adenosine nucleotides (e.g., about 100 to 400
adenosine nucleotides,
about 10 to 200 adenosine nucleotides, about 10 to 150 adenosine nucleotides,
about 10 to 100
adenosine nucleotides, about 20 to 70 adenosine nucleotides, or about 20 to 60
adenosine
nucleotides). In some embodiments, mRNAs include a 3' poly(C) tail structure.
A suitable
poly(C) tail on the 3' terminus of mRNA typically include about 10 to 200
cytosine nucleotides
(e.g., about 10 to 150 cytosine nucleotides, about 10 to 100 cytosine
nucleotides, about 20 to 70
cytosine nucleotides, about 20 to 60 cytosine nucleotides, or about 10 to 40
cytosine
nucleotides). A poly(C) tail may be added to a poly(A) tail or may be a
substitute for the
poly(A) tail.
[0140] In some embodiments, mRNAs of the present disclosure (e.g., mRNAs
encoding
CARs) include a 5' and/or 3' untranslated region. In some embodiments, a 5'
untranslated
region includes one or more elements that affect an mRNA's stability or
translation, for example,
an iron responsive element. In some embodiments, a 5' untranslated region may
be between
about 50 and 500 nucleotides in length.
[0141] In some embodiments, a 3' untranslated region includes one or more
of a
polyadenylation signal, a binding site for proteins that affect an mRNA's
stability of location in a
cell, or one or more binding sites for miRNAs. In some embodiments, a 3'
untranslated region
may be between 50 and 500 nucleotides in length or longer.
Nucleic Acid Delivery
[0142] The present disclosure, among other things, provides methods for
modifying an
immune cell (e.g., a monocyte, macrophage, or dendritic cell) comprising
delivering a nucleic
acid construct comprising one or more nucleic acid sequences encoding a
chimeric antigen
receptor (CAR) or a fragment thereof into an immune cell. Methods can comprise
delivering to
an immune cell (e.g., a monocyte, macrophage, or dendritic cell), a nucleic
acid construct
comprising one or more nucleic acid sequences encoding: (a) an extracellular
domain (e.g., an
extracellular domain as described herein), (b) a transmembrane domain (e.g., a
transmembrane
domain as described herein), and (c) an intracellular domain (e.g., an
intracellular domain as
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
described herein), such that an immune cell comprises a CAR comprising (a)-
(c). In some
embodiments, a nucleic acid construct comprising one or more nucleic acid
sequences further
encodes one, two, or three of: (d) an extracellular leader domain (e.g., an
extracellular leader
domain as described herein), (e) an extracellular hinge domain (e.g., an
extracellular hinge
domain as described herein), or (f) an intracellular co-stimulatory domain
(e.g., an intracellular
co-stimulatory domain as described herein).
[0143] A nucleic acid construct comprising one or more nucleic acid
sequences encoding
at least one CAR as described herein can be introduced into an immune cell
(e.g., a monocyte,
macrophage, or dendritic cell) by physical, chemical, or biological methods.
In some
embodiments, one or more physical, chemical, or biological methods of nucleic
acid delivery as
described herein can be used to introduce one or more nucleic acid sequences
encoding at least
one CAR as described herein and to introduce one or more nucleic acid
sequences that does not
encode a CAR. Physical methods for introducing a nucleic acid construct as
described herein
into an immune cell (e.g., a monocyte, macrophage, or dendritic cell) can
comprise
electroporation, calcium phosphate precipitation, lipofection, Viromer-
mediated transfection,
particle bombardment, microinjection, mechanotransduction (e.g., squeeze-type
technology), or a
combination thereof. A nucleic acid construct can be introduced into immune
cells using
commercially available methods, including electroporation (Amaxa Nucleofector-
II (Amaxa
Biosystems, Cologne, Germany), ECM 830 BTX (Harvard Instruments, Boston,
Mass.), Gene
Pulser II (BioRad, Denver, Colo.), Multiporator (Eppendort, Hamburg
Germany)), Maxcyte
STX (Maxcyte), Maxcyte VLX (Maxcyte), Maxcyte GT (Maxcyte), CliniMacs
Electroporator
(Miltenyi Biotec), or Neon Transfection System (Thermo Fisher). A nucleic acid
construct can
also be introduced into immune cells using mRNA transfection, e.g., cationic
liposome-mediated
transfection, lipofection, polymer encapsulation, peptide-mediated
transfection, or biolistic
particle delivery systems, such as "gene guns" (See, e.g., Nishikawa, et al.
Hum Gene Ther.,
12(8):861-70 (2001), which is hereby incorporated by reference in its
entirety).
[0144] Biological methods for introducing a nucleic acid construct as
described herein
into an immune cell (e.g., a monocyte, macrophage, or dendritic cell) include
use of DNA and
RNA vectors. Viral vectors, and especially retroviral vectors, have become
widely used for
inserting genes into mammalian cells (e.g., human cells). Viral vectors can
also be derived from
46
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
lentivirus, poxviruses, herpes simplex virus I, adenoviruses (e.g. Adf535), or
adeno-associated
viruses (See, e.g., U.S. Patent Nos. 5,350,674 and 5,585,362, which are hereby
incorporated by
reference in their entirety). Retroviral vectors, such as lentivirus, are
suitable tools to achieve
long-term gene transfer that allow for long-term, stable integration of a
transgene and its
propagation in daughter cells. In some embodiments, a lentiviral vector is
packaged with a Vpx
protein (e.g., as described in International Publication No. WO 2017/044487,
which is hereby
incorporated by reference in its entirety). In some embodiments, Vpx comprises
a virion-
associated protein (e.g., an accessory protein for viral replication). In some
embodiments, a Vpx
protein is encoded by human immunodeficiency virus type 2 (HIV-2). In some
embodiments, a
Vpx protein is encoded by simian immunodeficiency virus (Sly). In some
embodiments, an
immune cell as described herein (e.g., a monocyte, macrophage, or dendritic
cell) is transfected
with a lentiviral vector packaged with a Vpx protein. In some embodiments, Vpx
inhibits at least
one antiviral factor of an immune cell as described herein (e.g., a monocyte,
macrophage, or
dendritic cell). In some embodiments, a lentiviral vector packaged with a Vpx
protein exhibits
increased transfection efficiency of an immune cell as described herein (e.g.,
a monocyte,
macrophage, or dendritic cell), e.g., relative to a lentiviral vector not
packaged with a Vpx
protein. In some embodiments, an immune cell as described herein (e.g., a
monocyte,
macrophage, or dendritic cell) is one or both of electroporated or transfected
with at least one
Vpx mRNA prior to transfection with a viral vector (e.g., an adenoviral
vector, e.g., an Ad2
vector or an Ad5 vector (e.g., Ad5f35 adenoviral vector, e.g., a helper-
dependent Ad5F35
adenoviral vector)). Chemical means for introducing a nucleic acid construct
as described herein
into an immune cell (e.g., a monocyte, macrophage, or dendritic cell) include
colloidal dispersion
systems, macromolecule complexes, nanocapsules, microspheres, beads, and lipid-
based systems
(e.g., oil-in-water emulsions, micelles, mixed micelles, nanoparticles,
liposomes, and
lipofectamine-nucleic acid complexes).
[0145] In some embodiments, a system for delivery of a nucleic acid
construct as
described herein is a lipid-based system. A nucleic acid construct as
described herein may be
encapsulated in an aqueous interior of a liposome, interspersed within a lipid
bilayer, attached to
a liposome via a linking molecule, entrapped in a liposome, complexed with a
liposome,
dispersed in a solution or suspension comprising a lipid, mixed with a lipid,
complexed with a
micelle, or otherwise associated with a lipid. Lipids for use in methods
described herein may be
47
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
naturally occurring or synthetic lipids. Lipids can also be obtained from
commercial sources.
For example, dimyristyl phosphatidylcholine can be obtained from Sigma (St.
Louis, MO);
dicetyl phosphate can be obtained from K & K Laboratories (Plainview, NY);
cholesterol can be
obtained from Calbiochem-Behring; and dimyristyl phosphatidylglycerol can be
obtained from
Avanti Polar Lipids, Inc. (Birmingham, AL.). Stock solutions of lipids in
chloroform or
chloroform/methanol can be stored at about -20 C.
Nucleic Acid Purification
[0146] In some embodiments, methods of the present disclosure comprise a
step of
purifying nucleic acids (e.g., mRNAs encoding CARs). In some embodiments, a
step of
purifying nucleic acids (e.g., mRNAs encoding CARs) comprises the use of any
standard
purification method known in the art. In some embodiments, a step of purifying
nucleic acids
(e.g., mRNAs encoding CARs) comprises silica membrane purification, high
performance liquid
chromatography (HPLC), Dynabeads, LiC1 precipitation, phenol-chloroform
extraction, resin
based purification, polyA isolation, RNeasy, or a combination thereof. In some
embodiments, a
step of purifying nucleic acids (e.g., mRNAs encoding CARs) comprises silica
membrane
purification. In some embodiments, a step of purifying nucleic acids (e.g.,
mRNAs encoding
CARs) comprises high performance liquid chromatography (HPLC).
Treatment and Culturing of Immune Cells During Modification
[0147] In some embodiments, methods of the present disclosure comprise
one or more
steps of treating an immune cell (e.g., a monocyte, macrophage, or dendritic
cell) during the
process of modifying the immune cell.
[0148] In some embodiments, methods of the present disclosure comprise a
step of
treating an immune cell (e.g., a monocyte, macrophage, or dendritic cell) with
a modulator of a
pathway activated by in vitro transcribed mRNA. In vitro transcribed (IVT)
mRNA is
recognized by various endosomal innate immune receptors (Toll-like receptor 3
(TLR3), TLR7
and TLR8) and cytoplasmic innate immune receptors (protein kinase RNA-
activated (PKR),
retinoic acid-inducible gene I protein (RIG-I), melanoma differentiation-
associated protein 5
(MDA5) and 2'-5'-oligoadenylate synthase (OAS)). Signaling through these
different pathways
48
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
results in inflammation associated with type 1 interferon (IFN), tumor
necrosis factor (TNF),
interleukin-6 (IL-6), IL-12 and the activation of cascades of transcriptional
programs. Overall,
these create a pro-inflammatory microenvironment poised for inducing specific
immune
responses. Moreover, downstream effects such as slow-down of translation by
eukaryotic
translation initiation factor 2a (eIF2a) phosphorylation, enhanced RNA
degradation by
ribonuclease L (RNaseL), and overexpression and inhibition of replication of
self-amplifying
mRNA are of relevance for the pharmacokinetics and pharmacodynamics of IVT
mRNA.
[0149] In some embodiments, a modulator of a pathway activated by in
vitro transcribed
mRNA comprises an RNase inhibitor. In some embodiments, a modulator of a
pathway
activated by in vitro transcribed mRNA comprises an RNaseL, RNase T2 or RNasel
inhibitor.
In some embodiments, a modulator of a pathway activated by in vitro
transcribed mRNA
comprises an RNaseL inhibitor. In some embodiments, an RNaseL inhibitor
comprises
sunitinib. In some embodiments, an RNaseL inhibitor comprises ABCE1.
[0150] In some embodiments, treating an immune cell (e.g., a monocyte,
macrophage, or
dendritic cell) with an RNaseL inhibitor increases mRNA stability in a
modified immune cell
relative to mRNA stability in a modified immune cell of the same type that was
not treated with
an RNaseL inhibitor. In some embodiments, treating an immune cell (e.g., a
monocyte,
macrophage, or dendritic cell) with an RNaseL inhibitor increases CAR
expression in a modified
immune cell relative to CAR expression in a modified immune cell of the same
type that was not
treated with an RNaseL inhibitor. In some embodiments, treating an immune cell
(e.g., a
monocyte, macrophage, or dendritic cell) with an RNaseL inhibitor increases
effector activity in
a modified immune cell relative to effector activity in a modified immune cell
of the same type
that was not treated with an RNaseL inhibitor.
[0151] In some embodiments of the present disclosure, a step of treating
an immune cell
(e.g., a monocyte, macrophage, or dendritic cell) occurs before a step of
delivering an mRNA to
the immune cell.
[0152] In some embodiments, methods of the present disclosure comprise a
step of
culturing an immune cell (e.g., a monocyte, macrophage, or dendritic cell)
with a cytokine or
immune stimulating recombinant protein. In some embodiments, a cytokine
comprises IFN-a,
IFN-f3, IFN-y, TNFa, IL-6, STNGL, LPS, a CD40 agonist, a 4-1BB ligand,
recombinant 4-1BB,
49
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
a CD19 agonist, a TLR agonist (e.g., TLR-1, TLR-2, TLR-3, TLR-4, TLR-5, TLR-6,
TLR-7,
TLR-8 or TLR-9), TGF-(3 (e.g., TGF-(31, TGF- (32, or TGF-(33), a
glucocorticoid, an immune
complex, interleukin-1 alpha (IL-1a), IL-113, IL-2, IL-3, IL-4, IL-5, IL-6, IL-
7, IL-9, IL-10, IL-
12, IL-13, IL-14, IL-15, IL-16, IL-17, IL-18, IL-20, granulocyte-macrophage
colony-stimulating
factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), Leukemia
inhibitory factor
(LIF), oncostatin M (OSM), TNF-(3, CD154, lymphotoxin beta (LT-(3), an A
proliferation-
inducing ligand (APRIL), CD70, CD153, glucocorticoid-induced TNF receptor
ligand (GITRL),
tumor necrosis factor superfamily member 14 (TNFSF14), OX4OL (CD252), TALL-1
(Tumor
necrosis factor ligand superfamily member 13B - TNFSF13B), TNF-related
apoptosis-inducing
ligand (TRAIL), TNF-related weak inducer of apoptosis (TWEAK), TNF-related
activation-
induced cytokine (TRANCE), erythropoietin (Epo), thyroid peroxidase precursor
(Tpo), FMS-
related tyrosine kinase 3 ligand (FLT-3L), stem cell factor (SCF), macrophage
colony-
stimulating factor (M-CSF), merozoite surface protein (MSP), a Nucleotide-
binding
oligomerization domain-containing protein (NOD) ligand (e.g., NOD1, NOD2, or
NOD1/2
agonists), a RIG-I-like receptor (RLR) ligand (e.g., 5'ppp-dsRNA, 3p-hpRNA,
Poly(I:C), or
Poly(dA:dT)), a C-type lectin receptor (CLR) ligand (e.g., curdlan, (3-glucan,
HKCA, laminarin,
pustulan, scleroglucan, WGP dispersible, WGP soluble, zymosan, zymosan
depleted, furfurman,
b-GlcCer, GlcC14C18, HKMT, TDB, TDB-HS15, or TDM), a cyclic dinucleotide
sensor ligand
(e.g., C-Gas agonist or stimulator of interferon gene (STING) ligand), an
inflammasome inducer
(e.g., alum, ATP, CPPD crystals, hemozoin, MSU crystals, Nano-SiO2, Nigericin,
or TDB), an
aryl hydrocarbon (AhR) ligand (e.g., FICZ, indirubin, ITE, or L-kynurenine),
an alpha-protein
kinase 1 (ALPK1) ligand, a multi-PRR ligand, an NFKB/NFAT activator (e.g.,
concavalin A,
ionomycin, PHA-P, or PMA) or a combination thereof. In some embodiments, a
cytokine
comprises IFN-(3.
[0153] In some embodiments of the present disclosure, a step of culturing
an immune cell
(e.g., a monocyte, macrophage, or dendritic cell) occurs after a step of
delivering an mRNA to
the immune cell.
[0154] In some embodiments, culturing a modified immune cell (e.g., a
monocyte,
macrophage, or dendritic cell) with a cytokine or immune stimulating
recombinant protein
increases the viability of the modified immune cell relative to a modified
immune cell of the
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
same type that was not cultured with the cytokine or immune stimulating
recombinant protein.
In some embodiments, culturing a modified immune cell (e.g., a monocyte,
macrophage, or
dendritic cell) with a cytokine or immune stimulating recombinant protein
increases protein (e.g.,
CAR) expression of the modified immune cell relative to a modified immune cell
of the same
type that was not cultured with the cytokine or immune stimulating recombinant
protein. In
some embodiments, culturing a modified immune cell (e.g., a monocyte,
macrophage, or
dendritic cell) with a cytokine or immune stimulating recombinant protein
increases longevity of
protein (e.g., CAR) expression relative to a modified immune cell of the same
type that was not
cultured with the cytokine or immune stimulating recombinant protein. In some
embodiments,
culturing a modified immune cell (e.g., a monocyte, macrophage, or dendritic
cell) with a
cytokine or immune stimulating recombinant protein increases effector activity
of the modified
immune cell relative to a modified immune cell of the same type that was not
cultured with the
cytokine or immune stimulating recombinant protein. In some embodiments,
culturing a
modified immune cell (e.g., a monocyte, macrophage, or dendritic cell) with a
cytokine or
immune stimulating recombinant protein increases M1 polarization of the
modified immune cell
relative to a modified immune cell of the same type that was not cultured with
the cytokine or
immune stimulating recombinant protein.
Modified Immune Cells
[0155] In some embodiments, a modified immune cell is made by methods of
the present
disclosure.
[0156] In some embodiments, a modified immune cell exhibits increased
viability
relative to a modified immune cell of the same type comprising unmodified mRNA
encoding a
CAR. In some embodiments, a modified immune cell exhibits viability increased
at least 1%,
2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, or 50%
relative to a modified immune cell of the same type comprising unmodified mRNA
encoding a
CAR.
[0157] In some embodiments, a modified immune cell exhibits increased
expression of
an mRNA encoding a CAR relative to a modified immune cell of the same type
comprising
unmodified mRNA encoding the CAR. In some embodiments, a modified immune cell
exhibits
51
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
expression of an mRNA encoding a CAR increased at least 100%, 200%, 300%,
400%, 500%,
600%, 700%, 800%, 900%, 1000%, 1500%, or 2000%, relative to a modified immune
cell of the
same type comprising unmodified mRNA encoding the CAR. In some embodiments, a
modified
immune cell exhibits expression of an mRNA encoding a CAR increased at least
lx, 2x, 3x, 4x,
5x, 6x, 7x, 8x, 9x, 10x, 15x, or 20x, relative to a modified immune cell of
the same type
comprising unmodified mRNA encoding the CAR.
[0158] In some embodiments, a modified immune cell exhibits increased CAR
expression relative to a modified immune cell of the same type comprising
unmodified mRNA
encoding the CAR. In some embodiments, a modified immune cell exhibits CAR
expression
increased at least 100%, 200%, 300%, 400%, 500%, 600%, 700%, 800%, 900%,
1000%, 1500%,
or 2000%, relative to a modified immune cell of the same type comprising
unmodified mRNA
encoding the CAR. In some embodiments, a modified immune cell exhibits CAR
expression
increased at least lx, 2x, 3x, 4x, 5x, 6x, 7x, 8x, 9x, 10x, 15x, or 20x,
relative to a modified
immune cell of the same type comprising unmodified mRNA encoding the CAR.
[0159] In some embodiments, a modified immune cell exhibits increased
longevity of an
mRNA encoding a CAR relative to a modified immune cell of the same type
comprising
unmodified mRNA encoding the CAR. In some embodiments, a modified immune cell
exhibits
longevity of an mRNA encoding a CAR increased at least 12 hours, 24 hours, 36
hours, 48
hours, 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, 9 days, 10 days, 11
days, 12 days, 13 days,
14 days, 15 days, 16 days, 17 days, 18 days, 19 days, 20 days, 3 weeks, or 1
month, relative to a
modified immune cell of the same type comprising unmodified mRNA encoding the
CAR.
[0160] In some embodiments, a modified immune cell exhibits increased
longevity of a
CAR relative to a modified immune cell of the same type comprising unmodified
mRNA
encoding the CAR. In some embodiments, a modified immune cell exhibits
longevity of a CAR
increased at least 12 hours, 24 hours, 36 hours, 48 hours, 3 days, 4 days, 5
days, 6 days, 7 days, 8
days, 9 days, 10 days, 11 days, 12 days, 13 days, 14 days, 15 days, 16 days,
17 days, 18 days, 19
days, 20 days, 3 weeks, or 1 month, relative to a modified immune cell of the
same type
comprising unmodified mRNA encoding the CAR.
[0161] In some embodiments, a modified immune cell exhibits increased
effector activity
relative to a modified immune cell of the same type comprising unmodified mRNA
encoding a
52
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
CAR. In some embodiments, a modified immune cell exhibits effector activity
increased 5%,
10%, 15%, 20%, 25%, 50%, 75%, 100%, 200%, 300%, 400%, 500%, 600%, 700%, 800%,
900%, or 1000%, relative to a modified immune cell of the same type comprising
unmodified
mRNA encoding a CAR. In some embodiments, increased effector activity
comprises increased
cytokine production, chemokine production, phagocytosis, cell signaling,
target cell killing,
and/or antigen presentation.
[0162] In some embodiments, a modified immune cell exhibits increased M1
polarization
relative to a modified immune cell of the same type comprising unmodified mRNA
encoding a
CAR. In some embodiments, increased M1 polarization comprises increased levels
of an M1
marker comprising CD86, CD80, MHC II, IL-1R, TLR2, TLR4, iNOS, SOCS3, CD83, PD-
L1,
CD69, MHC I, CD64, CD32, CD16, IL1R, an IFIT family member, or an ISG family
member.
In some embodiments, a modified immune cell exhibits M1 polarization increased
5%, 10%,
15%, 20%, 25%, 50%, 75%, 100%, 200%, 300%, 400%, 500%, 600%, 700%, 800%, 900%,
or
1000%, relative to a modified immune cell of the same type comprising
unmodified mRNA
encoding a CAR.
[0163] In some embodiments, a modified immune cell exhibits decreased M2
polarization relative to a modified immune cell of the same type comprising
unmodified mRNA
encoding a CAR. In some embodiments, decreased M2 polarization comprises
decreased levels
of an M2 marker comprising CD206, CD163, or CD209. In some embodiments, a
modified
immune cell exhibits M2 polarization decreased 5%, 10%, 15%, 20%, 25%, 50%,
75%, 100%,
200%, 300%, 400%, 500%, 600%, 700%, 800%, 900%, or 1000%, relative to a
modified
immune cell of the same type comprising unmodified mRNA encoding a CAR.
Assays
[0164] A variety of assays may be performed to confirm presence of a
nucleic acid
construct as described herein in an immune cell (e.g., a monocyte, macrophage,
or dendritic cell).
For example, such assays include molecular biological assays well known to
those of skill in the
art, such as Southern and Northern blotting, RT-PCR, and PCR; and biochemical
assays, such as
detecting the presence or absence of a particular peptide, e.g., by
immunological means (ELISAs
and Western blots). Other assays of the present disclosure, include, for
example, fluorescence-
53
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
activated cell sorting (FACS), immunofluorescent microscopy, MSD cytokine
analysis, mass
spectrometry (MS), RNA-Seq and functional assays.
[0165] A variety of assays may be performed to determine various
characteristics of a
modified immune cell (e.g., a monocyte, macrophage, or dendritic cell), such
as, but not limited
to, immune cell viability, nucleic acid (e.g., mRNA) expression, nucleic acid
(e.g., mRNA)
longevity, protein (e.g., CAR) expression, protein (e.g., CAR) longevity,
effector activity, and
MI polarization.
[0166] All publications, patent applications, patents, and other
references mentioned
herein, including GenBank Accession Numbers, are incorporated by reference in
their entirety.
In addition, the materials, methods, and examples are illustrative only and
not intended to be
limiting. Unless otherwise defined, all technical and scientific terms used
herein have the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although methods and materials similar or equivalent to those
described herein can be
used in the practice or testing of the present invention, suitable methods and
materials are
described herein.
Chimeric Antigen Receptors (CAR)
[0167] The term "chimeric antigen receptor" or "CAR," as used herein,
refers to an
artificial cell surface receptor that is engineered to be expressed on an
immune effector cell and
specifically targets a cell and/or binds an antigen. CARs may be used, for
example, as a therapy
with adoptive cell transfer. For example, in some embodiments, monocytes,
macrophages and/or
dendritic cells are removed from a patient (e.g., from blood, tumor or ascites
fluid) and modified
so that they express a receptor specific to a particular form of antigen. In
some embodiments,
CARs have been expressed with specificity to an antigen, for example, a tumor
associated
antigen. In some embodiments, a CAR comprises an extracellular domain, a
transmembrane
domain and an intracellular domain.
[0168] In some embodiments, a modified immune cell, for example, a
modified
macrophage, monocyte, or dendritic cell, is generated by expressing a CAR
therein. In some
embodiments, an immune cell comprises a CAR comprising an extracellular
domain, a
54
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
transmembrane domain, and an intracellular domain, wherein the immune cell
comprises a
macrophage, monocyte, or dendritic cell.
[0169] In some embodiments, a CAR may further comprise one or more of:
one or more
extracellular leader domains, one or more extracellular hinge domains and one
or more
intracellular co-stimulatory domains.
[0170] In some embodiments, a CAR comprises a spacer domain or hinge
between an
extracellular domain and a transmembrane domain (e.g., a CD8 or CD28 hinge
domain). In
some embodiments, a CAR comprises a spacer domain or hinge between an
intracellular domain
and a transmembrane domain. As used herein, the term "spacer domain" or
"hinge" refers to any
oligo- or polypeptide that functions to link a transmembrane domain to either
an extracellular
domain or to an intracellular domain in a polypeptide chain. In some
embodiments, a spacer
domain or hinge may comprise up to 300 amino acids, preferably 10 to 100 amino
acids and
most preferably 25 to 50 amino acids. In some embodiments, a short oligo- or
polypeptide
linker, preferably between 2 and 10 amino acids in length, may form a linkage
between a
transmembrane domain and an intracellular domain of a CAR. An example of a
linker includes a
glycine-serine doublet.
[0171] In some embodiments, an immune cell comprising a CAR may comprise
one or
more control systems including, but not limited to: a safety switch (e.g., an
on switch, and off
switch, a suicide switch), a logic gate, for example an AND gate (e.g., two or
more CARs, each
of which lacks one or more signaling domains such that activation of both/all
CARs is required
for full immune cell (e.g., macrophage, monocyte, or dendritic cell)
activation or function), an
OR gate (e.g., two or more CARs, each with an intracellular domain such as
CD3C and a co-
stimulatory domain), and/or a NOT gate (e.g., two or more CARs, one of which
includes an
inhibitory domain that antagonizes the function of the other CAR[s]).
[0172] The present disclosure also provides immune cells comprising a
nucleic acid
sequence (e.g., an isolated nucleic acid sequence) encoding a CAR, wherein the
nucleic acid
sequence comprises a nucleic acid sequence encoding an extracellular domain, a
nucleic acid
sequence encoding a transmembrane domain and a nucleic acid sequence encoding
an
intracellular domain, wherein the cell is a monocyte, macrophage or a
dendritic cell that
expresses the CAR.
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0173] In some embodiments, a CAR comprises an extracellular domain is
operably
linked to another domain of the CAR, such as a transmembrane domain or an
intracellular
domain, for expression in an immune cell. In some embodiments, a nucleic acid
encoding an
extracellular domain is operably linked to a nucleic acid encoding a
transmembrane domain and
the nucleic acid encoding the transmembrane domain is operably linked to a
nucleic acid
encoding an intracellular domain.
[0174] In some embodiments, an effector activity of an immune cell
comprising a CAR
is directed against a target cell comprising an antigen that specifically
binds an antigen binding
domain of the CAR. In some embodiments, a targeted effector activity directed
against a target
cell is or comprises phagocytosis, targeted cellular cytotoxicity, antigen
presentation, or cytokine
secretion.
[0175] In some embodiments, a CAR described herein comprises at least one
domain
(e.g., an extracellular domain, a transmembrane domain, and/or an
intracellular domain) that
inhibits anti-phagocytic signaling in an immune cell described herein (e.g., a
macrophage,
monocyte, or dendritic cell). In some embodiments, a CAR described herein
improves effector
activity of an immune cell described herein (e.g., a macrophage, monocyte, or
dendritic cell),
e.g., by enhancing inhibition of CD47 and/or SIRPa activity. In some
embodiments, a CAR
described herein binds CD47, e.g., and serves as a dominant negative receptor,
inhibiting SIRPa
activity (e.g., a CD47 sink). In some embodiments, a CAR described herein that
binds SIRPa,
e.g., comprises an activating receptor (e.g., comprises a CD3z intracellular
domain). In some
embodiments, a CAR described herein inhibits at least one interaction of CD47
and SIRPa. In
some embodiments, a CAR is or comprises a phagocytic logic gate.
[0176] In some embodiments, an immune cell described herein (e.g.,
comprising or
expressing a CAR described herein) comprises or expresses at least one variant
or fragment of:
SIRPa (e.g., a dominant negative SIRPa or a high-affinity engineered variant
of SIRPa (e.g.,
CV1)), 5F9 scFv, B6H12 scFv (e.g., a humanized B6H12 scFv), PD1 (e.g., a
dominant negative
PD1 or HAC-I), anti-PD1 scFv (e.g., E27 or durvalumab), Siglec-10, Siglec-9,
Siglec-11, and/or
SHP-1. In some embodiments, a variant or fragment comprises a mutated
intracellular domain.
In some embodiments, a variant or fragment does not comprise or express at
least one
intracellular domain (e.g., an immune cell comprises or expresses an anti-CD47
scFv, CD8 hinge
56
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
domain, and CD8 transmembrane). In some embodiments, an immune cell described
herein
(e.g., comprising or expressing a CAR described herein) comprises a dominant
negative receptor,
e.g., blocking an inhibitory checkpoint.
[0177] In some embodiments, a CAR described herein further comprises a
cleavage
peptide (e.g., a P2A, F2A, E2A and/or T2A peptide) and at least one second CAR
comprising at
least one inhibitory domain of anti-phagocytic signaling. In some embodiments,
at least one
second CAR comprises a SIRPa (e.g., a high-affinity engineered variant of
SIRPa (e.g., CV1)),
5F9 scFv, B6H12 scFv (e.g., a humanized B6H12 scFv), or a CD47 binding
extracellular domain
or a fragment thereof In some embodiments, at least one second CAR comprises a
SIRPa
transmembrane domain or a fragment thereof. In certain embodiments, a second
CAR further
comprises a hinge domain (e.g., a CD8 hinge domain). In certain embodiments,
at least one
second CAR comprises: (i) a leader sequence (e.g., a CD8 leader); ii) an
extracellular domain
(e.g., a SIRPa, CV1, 5F9 scFv, or B6H12 scFv (e.g., a humanized B6H12 scFv)
extracellular
domain); and ii) a transmembrane domain (e.g., a SIRPa transmembrane domain).
In some
embodiments, a CAR described herein further comprises a cleavage peptide
(e.g., a P2A peptide)
and at least one marker protein (e.g., CD20 or a fragment thereof, CD19 or a
fragment thereof,
NGFR or a fragment thereof, a synthetic peptide, and/or a fluorescent
protein).
[0178] In some embodiments, an immune cell described herein (e.g.,
comprising or
expressing a CAR described herein) comprises or expresses one or more
phosphatase dead
domains (e.g. a phosphatase dead Shpl, phosphatase dead 72-5ptase (INPP5E),
phosphatase
dead Shp2, and/or phosphatase dead SHIP-1 domain) and/or a constitutively
active kinase
domain (e.g., a constitutively active LYN domain). In some embodiments, a CAR
described
herein further comprises a cleavage peptide (e.g., a P2A, F2A, E2A and/or T2A
peptide) and one
or more phosphatase dead domains (e.g. a phosphatase dead Shpl, phosphatase
dead 72-5ptase
(INPP5E), phosphatase dead Shp2, and/or phosphatase dead SHIP-1 domain) and/or
a
constitutively active kinase domain (e.g., a constitutively active LYN
domain).
Extracellular Domains
The present disclosure provides chimeric antigen receptors (CAR) comprising
extracellular
domains. In some embodiments, an extracellular domain comprises an Fc receptor
(FcR)
57
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
extracellular domain. In some embodiments, an extracellular domain comprises a
toll-like
receptor (TLR) extracellular domain. In some embodiments, an extracellular
domain comprises
a leader domain. In some embodiments, an extracellular domain comprises an
antigen binding
domain. In some embodiments, an extracellular domain comprises a hinge domain.
In some
embodiments, an extracellular domain comprises one or more of an FcR
extracellular domain, a
TLR extracellular domain, a leader domain, an antigen binding domain and a
hinge domain. In
some embodiments, an extracellular domain may be a domain that is endogenous
to a particular
immune cell type (e.g., a modified immune cell as provided herein). In some
embodiments, an
extracellular domain may be a domain that is not endogenous to a particular
immune cell type
(e.g., a modified immune cell as provided herein).
FcR Extracellular Domains
[0179] In some embodiments, an FcR extracellular domain comprises a full-
length FcR
extracellular domain. In some embodiments, an FcR extracellular domain
comprises a portion of
a full-length FcR extracellular domain. In some embodiments, an FcR
extracellular domain (or
portion thereof) is or comprises a human FcR extracellular domain. In some
embodiments, an
FcR extracellular domain may be a domain that is endogenous to a particular
immune cell type
(e.g., a modified immune cell as provided herein). In some embodiments, an FcR
extracellular
domain may be a domain that is not endogenous to a particular immune cell type
(e.g., a
modified immune cell as provided herein). In some embodiments, an FcR
extracellular domain
comprises a CD64 (FcyRI), CD32a (FcyRIIa), CD32b (FcyRIIb), CD32c, CD16a
(FcyRIIIa),
CD16b (FcyRIIIb), FccRI, FccRII, or FcaRI (CD89) domain.
TLR Extracellular Domains
[0180] In some embodiments, a TLR extracellular domain comprises a full-
length TLR
extracellular domain. In some embodiments, a TLR extracellular domain
comprises a portion of
a full-length TLR extracellular domain. In some embodiments, a TLR
extracellular domain (or
portion thereof) is or comprises a human TLR extracellular domain. In some
embodiments, a
TLR extracellular domain may be a domain that is endogenous to a particular
immune cell type
(e.g., a modified immune cell as provided herein). In some embodiments, a TLR
extracellular
domain may be a domain that is not endogenous to a particular immune cell type
(e.g., a
58
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
modified immune cell as provided herein). In some embodiments, a TLR
extracellular domain
comprises a TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, or TLR9 domain.
Leader Domains
[0181] In some embodiments, a CAR comprises one or more extracellular
leader
domains. In some embodiments, a nucleic acid encoding a CAR comprises a
nucleic acid
sequence encoding an extracellular leader domain, but the extracellular leader
domain is cleaved
from the CAR before the CAR is expressed in an immune cell. In some
embodiments, an
extracellular leader domain is or comprises a human extracellular leader
domain. In some
embodiments, an extracellular leader domain may be a domain that is endogenous
to a particular
immune cell type (e.g., a modified immune cell as provided herein). In some
embodiments, an
extracellular leader domain may be a domain that is not endogenous to a
particular immune cell
type (e.g., a modified immune cell as provided herein). In some embodiments,
an extracellular
leader domain comprises a CD8 extracellular leader domain. In some
embodiments, an
extracellular leader domain comprises a leader domain from a stimulatory or co-
stimulatory
domain (e.g., a TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, ALK,
AXL,
DDR2, EGFR, EphAl, INSR, cMET, MUSK, PDGFR, PTK7, RET, ROR1, ROS1, RYK, TIE2,
TRK, VEGFR, CD40, CD19, CD20, 41BB, CD28, 0X40, GITR, TREM-1, TREM-2, DAP12,
MR, ICOS, MyD88 domain).
Antigen Binding Domains
[0182] In some embodiments, a CAR comprises an antigen binding domain
that binds to
an antigen, for example, on a target cell. In some embodiments, a CAR
comprises an antigen
binding domain that binds to an antigen associated with viral infection,
bacterial infection,
parasitic infection, autoimmune disease, and/or cancer cells. In some
embodiments, an antigen
binding domain recognizes an antigen that acts as a cell surface marker on a
target cell associated
with a particular disease state.
[0183] In some embodiments, an antigen binding domain binds to a tumor
antigen, such
as an antigen that is specific for a tumor or cancer of interest. In some
embodiments a tumor
antigen comprises one or more antigenic cancer epitopes. In some embodiments,
a tumor
antigen comprises CD19; CD123; CD22; CD30; CD171; CS-1 (also referred to as
CD2 subset 1,
CRACC, SLAMF7, CD319, and 19A24); C-type lectin-like molecule-1 (CLL-1 or
CLECL1);
59
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
CD33; epidermal growth factor receptor variant III (EGFRvIII); ganglioside G2
(GD2);
ganglioside GD3 (aNeu5Ac(2-8)aNeu5Ac(2-3 )bD G alp ( I -4)bD GI ( I - I )C
er); TNF receptor
family member B cell maturation (BCMA); Tn antigen ((Tn Ag) or (GalNAca-
Ser/Thr));
prostate-specific membrane antigen (PSMA); Receptor tyrosine kinase-like
orphan receptor 1
(ROR1); Fms-Like Tyrosine Kinase 3 (FLT3); Tumor-associated glycoprotein 72
(TAG72);
CD38; CD44v6; Carcinoembryonic antigen (CEA); Epithelial cell adhesion
molecule (EPCAM);
B7H3 (CD276); KIT (CD117); Interleukin-13 receptor subunit alpha-2 (IL-13Ra2
or CD213A2);
Mesothelin; Interleukin 11 receptor alpha (IL-11Ra); prostate stem cell
antigen (PSCA); Protease
Serine 21 (Testisin or PRSS21); vascular endothelial growth factor receptor 2
(VEGFR2);
Lewis(Y) antigen; CD24; Platelet-derived growth factor receptor beta (PDGFR-
beta); Stage-
specific embryonic antigen-4 (SSEA-4); CD20; Folate receptor alpha; Receptor
tyrosine-protein
kinase ERBB2 (Her2/neu); Mucin 1, cell surface associated (MUC1); epidermal
growth factor
receptor (EGFR); neural cell adhesion molecule (NCAM); Prostase; prostatic
acid phosphatase
(PAP); elongation factor 2 mutated (ELF2M); Ephrin B2; fibroblast activation
protein alpha
(FAP); insulin-like growth factor 1 receptor (IGF-I receptor), carbonic
anhydrase IX (CAIX);
Proteasome (Prosome, Macropain) Subunit, Beta Type, 9 (LMP2); glycoprotein 100
(gp100);
oncogene fusion protein consisting of breakpoint cluster region (BCR) and
Abelson murine
leukemia viral oncogene homolog 1 (Abl) (bcr-abl); tyrosinase; ephrin type-A
receptor 2
(EphA2); Fucosyl GM1; sialyl Lewis adhesion molecule (sLe); ganglioside GM3
(aNeu5Ac(2-
3)bDGa1p(1-4)bDGIcp(1-1)Cer); transglutaminase 5 (TGS5); high molecular weight-
melanoma-
associated antigen (HMWMAA); o-acetyl-GD2 ganglioside (0AcGD2); Folate
receptor beta;
tumor endothelial marker 1 (TEM1/CD248); tumor endothelial marker 7-related
(TEM7R);
claudin 6 (CLDN6); thyroid stimulating hormone receptor (TSHR); G protein-
coupled receptor
class C group 5, member D (GPRC5D); chromosome X open reading frame 61
(CXORF61);
CD97; CD179a; anaplastic lymphoma kinase (ALK); Polysialic acid; placenta-
specific 1
(PLAC1); hexasaccharide portion of globoH glycoceramide (GloboH); mammary
gland
differentiation antigen (NY-BR-1); uroplakin 2 (UPK2); Hepatitis A virus
cellular receptor 1
(HAVCR1); adrenoceptor beta 3 (ADRB3); pannexin 3 (PANX3); G protein-coupled
receptor 20
(GPR20); lymphocyte antigen 6 complex, locus K 9 (LY6K); Olfactory receptor
51E2
(OR51E2); TCR Gamma Alternate Reading Frame Protein (TARP); Wilms tumor
protein
(WT1); Cancer/testis antigen 1 (NY-ESO-1); Cancer/testis antigen 2 (LAGE-1a);
Melanoma-
CA 03187138 2022-12-13
WO 2021/263152
PCT/US2021/039168
associated antigen 1 (MAGE-A1); ETS translocation-variant gene 6, located on
chromosome 12p
(ETV6-AML); sperm protein 17 (SPA17); X Antigen Family, Member 1A (XAGE1);
angiopoietin-binding cell surface receptor 2 (Tie 2); melanoma cancer testis
antigen-1 (MAD-
CT-1); melanoma cancer testis antigen-2 (MAD-CT-2); Fos-related antigen 1;
tumor protein p53
(p53); p53 mutant; prostein; surviving; telomerase; prostate carcinoma tumor
antigen-1 (PCTA-1
or Galectin 8), melanoma antigen recognized by T cells 1 (MelanA or MART 1);
Rat sarcoma
(Ras) mutant; human Telomerase reverse transcriptase (hTERT); sarcoma
translocation
breakpoints; melanoma inhibitor of apoptosis (ML-IAP); ERG (transmembrane
protease, serine
2 (TMPRSS2) ETS fusion gene); N-Acetyl glucosaminyl-transferase V (NA17);
paired box
protein Pax-3 (PAX3); Androgen receptor; Cyclin Bl; v-myc avian
myelocytomatosis viral
oncogene neuroblastoma derived homolog (MYCN); Ras Homolog Family Member C
(RhoC);
Tyrosinase-related protein 2 (TRP-2); Cytochrome P450 1B1 (CYP1B1); CCCTC-
Binding
Factor (Zinc Finger Protein)-Like (BORIS or Brother of the Regulator of
Imprinted Sites),
Squamous Cell Carcinoma Antigen Recognized By T Cells 3 (SART3); Paired box
protein Pax-5
(PAX5); proacrosin binding protein sp32 (0Y-TES1); lymphocyte-specific protein
tyrosine
kinase (LCK); A kinase anchor protein 4 (AKAP-4); synovial sarcoma, X
breakpoint 2 (55X2);
Receptor for Advanced Glycation Endproducts (RAGE-1); renal ubiquitous 1
(RU1); renal
ubiquitous 2 (RU2); legumain; human papilloma virus E6 (HPV E6); human
papilloma virus E7
(HPV E7); intestinal carboxyl esterase; heat shock protein 70-2 mutated (mut
hsp70-2); CD79a;
CD79b; CD72; Leukocyte-associated immunoglobulin-like receptor 1 (LAIR1); Fc
fragment of
IgA receptor (FCAR or CD89); Leukocyte immunoglobulin-like receptor subfamily
A member 2
(LILRA2); CD300 molecule-like family member f (CD300LF); C-type lectin domain
family 12
member A (CLEC12A); bone marrow stromal cell antigen 2 (BST2); EGF-like module-
containing mucin-like hormone receptor-like 2 (EMR2); lymphocyte antigen 75
(LY75);
Glypican-3 (GPC3); Fc receptor-like 5 (FCRL5); or immunoglobulin lambda-like
polypeptide 1
(IGLL1). In certain embodiments, a tumor antigen comprises ERBB2 (Her2/neu).
In certain
embodiments, a tumor antigen comprises PSMA. In certain embodiments, a tumor
antigen
comprises Mesothelin.
[0184] In
some embodiments, an antigen binding domain binds to a misfolded protein
antigen or a protein of a protein aggregate, such as a protein that is
specific for a disease/disorder
of interest. In some embodiments, the disease/disorder is a neurodegenerative
disease/disorder,
61
CA 03187138 2022-12-13
WO 2021/263152
PCT/US2021/039168
an inflammatory disease/disorder, a cardiovascular disease/disorder, a
fibrotic disease/disorder,
or amyloidosis (e.g., mediated by protein aggregates of immunoglobulin light
chains or of
transthyretin). In some embodiments, the neurodegenerative disease/disorder is
selected from
the group consisting of tauopathy, asynucleopathy, presenile dementia, senile
dementia,
Alzheimer's disease (mediated by protein aggregates ofbeta-amyloid),
Parkinsonism linked to
chromosome 17 (FTDP-17), progressive supranuclear palsy (PSP), Pick' s
disease, primary
progressive aphasia, frontotemporal dementia, corticobasal dementia,
Parkinson's disease,
Parkinson's disease with dementia, dementia with Lewy bodies, Down syndrome,
multiple
system atrophy, amyotrophic lateral sclerosis (ALS), Hallervorden-Spatz
syndrome,
polyglutamine disease, trinucleotide repeat disease, Familial British
dementia, Fatal Familial
Insomnia, Gerstmann-Straussler-Scheinker Syndrome, Hereditary cerebral
hemorrhage with
amyloidosis (Icelandic) (HCHW A-I), Sporadic Fatal Insomnia (sFI), Variably
Protease-
Sensitive Prionopathy (VPSPr), Familial Danish dementia, and prion disease
(such as
Creutzfeldt-Jakob disease, CJD and Variant Creutzfeldt-Jakob Disease (vCJD)).
[0185] In
some embodiments, an antigen binding domain comprises any domain that
binds to an antigen. In some embodiments, an antigen binding domain is or
comprises a
monoclonal antibody, a polyclonal antibody, a synthetic antibody, a human
antibody, a
humanized antibody, a non-human antibody, or any fragment thereof, for example
an scFv. In
some embodiments, an antigen binding domain is or comprises an aptamer, a
darpin, a centyrin,
a naturally occurring or synthetic receptor, an affibody, or other engineered
protein recognition
molecule. In some embodiments, an antigen binding domain is or comprises a
mammalian
antibody or a fragment thereof. In some embodiments, an antigen binding domain
is derived, in
whole or in part, from the same species in which the CAR will ultimately be
used. For example,
for use in humans, an antigen binding domain of a CAR comprises a human
antibody, a
humanized antibody, or a fragment thereof (e.g. a scFv). In some embodiments,
an antigen
binding domain may be a domain that is endogenous to a particular immune cell
type (e.g., a
modified immune cell as provided herein). In some embodiments, an antigen
binding domain
may be a domain that is not endogenous to a particular immune cell type (e.g.,
a modified
immune cell as provided herein).
62
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0186] In some embodiments, a CAR comprises one or more antigen binding
domains.
In some embodiments, a CAR comprises two or more antigen binding domains. In
some
embodiments, a CAR is a bispecific CAR. In some embodiments, an immune cell
comprises two
or more different CARs comprising one or more antigen binding domains. In some
embodiments, an immune cell comprising a bispecific CAR and/or comprising two
or more
different CARs comprising one or more antigen binding domains can reduce off-
target and/or
on-target off-tissue effects by requiring that two antigens are present. In
some embodiments, an
immune cell comprises a bispecific CAR and/or comprises two or more different
CARs
comprising one or more antigen binding domains, wherein the CARs provide
distinct signals that
in isolation are insufficient to mediate activation of the modified cell, but
are synergistic
together, stimulating activation of the modified cell. In some embodiments,
such a construct
may be referred to as an 'AND' logic gate.
[0187] In some embodiments, an immune cell comprising a bispecific CAR
and/or
comprising two or more different CARs comprising one or more antigen binding
domains can
reduce off-target and/or on-target off-tissue effects by requiring that one
antigen is present and a
second, normal protein antigen is absent before the cell's activity is
stimulated. In some
embodiments, such a construct may be referred to as a 'NOT' logic gate. In
contrast to AND
gates, NOT gated CAR-modified cells are activated by binding to a single
antigen. However, the
binding of a second receptor to the second antigen functions to override the
activating signal
being perpetuated through the CAR. Typically, such an inhibitory receptor
would be targeted
against an antigen that is abundantly expressed in a normal tissue but is
absent in tumor tissue.
Hinge Domains
[0188] In some embodiments, a CAR comprises one or more extracellular
hinge
domains. In some embodiments, an extracellular hinge domain is or comprises a
human
extracellular hinge domain. In some embodiments, an extracellular hinge domain
may be a
domain that is endogenous to a particular immune cell type (e.g., a modified
immune cell as
provided herein). In some embodiments, an extracellular hinge domain may be a
domain that is
not endogenous to a particular immune cell type (e.g., a modified immune cell
as provided
herein). In some embodiments, one or more extracellular hinge domains comprise
a CD8a
extracellular hinge domain or an IgG4 or a CD28 extracellular hinge domain. In
some
63
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
embodiments, an extracellular hinge domain optimizes the physicochemical
parameters of a
CAR, e.g., optimal size relative to tumor antigen (e.g., allowing for
exclusion of inhibitory
molecules), optimal flexibility, optimal protein folding, optimal protein
stability, optimal
binding, optimal homodimerization, and/or lack of homodimerization.
Transmembrane Domains
[0189] In some embodiments, a CAR comprises a transmembrane domain, for
example,
that connects an extracellular domain to an intracellular domain. In some
embodiments, a
transmembrane domain is naturally associated with one or more other domain(s)
of a CAR. In
some embodiments, a transmembrane domain can be modified to avoid binding to
transmembrane domains of other surface membrane proteins, in order to minimize
interactions
with other members of a receptor complex. In some embodiments, a transmembrane
domain
may be derived either from a naturally-occurring or from a synthetic source.
In some
embodiments a transmembrane domain is derived from a naturally-occurring
membrane-bound
or transmembrane protein. In some embodiments, a transmembrane domain is or
comprises a
human transmembrane domain. In some embodiments, a transmembrane domain may be
a
domain that is endogenous to a particular immune cell type (e.g., a modified
immune cell as
provided herein). In some embodiments, a transmembrane domain may be a domain
that is not
endogenous to a particular immune cell type (e.g., a modified immune cell as
provided herein).
In some embodiments, a transmembrane domain comprises a CD8a, CD64, CD32a,
CD32c,
CD16a, TRL1, TLR2, TLR3, TRL4, TLR5, TLR6, TLR7, TLR8, TLR9, ALK, AXL, DDR2,
EGFR, EphAl, INSR, cMET, MUSK, PDGFR, PTK7, RET, ROR1, ROS1, RYK, TIE2, TRK,
VEGFR, CD40, CD19, CD20, 41BB, CD28, 0X40, GITR, TREM-1, TREM-2, DAP12, MR,
ICOS, MyD88, CD3-zeta, FcR y, V/I/LxYxxL/V, SIRPa, CD45, Siglec-10, PD1, SHP-
1, SHP-2,
KIR-2DL, KIR-3DL, NKG2A, CD170, CD33, BTLA, CD32b, SIRPI3, CD22, PIR-B,
LILRB1,
CD36, or Syk transmembrane domain.
FcR Transmembrane Domains
[0190] In some embodiments, an FcR transmembrane domain comprises a full-
length
FcR transmembrane domain. In some embodiments, an FcR transmembrane domain
comprises a
portion of a full-length FcR transmembrane domain. In some embodiments, an FcR
64
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
transmembrane domain is or comprises a human FcR transmembrane domain, or
portion thereof
In some embodiments, an FcR transmembrane domain may be a domain that is
endogenous to a
particular immune cell type (e.g., a modified immune cell as provided herein).
In some
embodiments, an FcR transmembrane domain may be a domain that is not
endogenous to a
particular immune cell type (e.g., a modified immune cell as provided herein).
In some
embodiments, an FcR transmembrane domain comprises a CD64 (FcyRI), CD32a
(FcyRIIa),
CD32b (FcyRIIb), CD32c, CD16a (FcyRIIIa), CD16b (FcyRIIIb), Feat', FccRII, or
FcaRI
(CD89) domain.
TLR Transmembrane Domains
[0191] In some embodiments, a TLR transmembrane domain comprises a full-
length
TLR transmembrane domain. In some embodiments, a TLR transmembrane domain
comprises a
portion of a full-length TLR transmembrane domain. In some embodiments, a TLR
transmembrane domain is or comprises a human TLR transmembrane domain, or
portion thereof
In some embodiments, a TLR transmembrane domain may be a domain that is
endogenous to a
particular immune cell type (e.g., a modified immune cell as provided herein).
In some
embodiments, a TLR transmembrane domain may be a domain that is not endogenous
to a
particular immune cell type (e.g., a modified immune cell as provided herein).
In some
embodiments, a TLR transmembrane domain comprises a TLR1, TLR2, TLR3, TLR4,
TLR5,
TLR6, TLR7, TLR8, or TLR9 domain.
Intracellular Domains
[0192] In some embodiments, a CAR comprises one or more intracellular
domains. In
some embodiments, an intracellular domain is or comprises a human
intracellular domain, or
portion thereof. In some embodiments, an intracellular domain may be a domain
that is
endogenous to a particular immune cell type (e.g., a modified immune cell as
provided herein).
In some embodiments, an intracellular domain may be a domain that is not
endogenous to a
particular immune cell type (e.g., a modified immune cell as provided herein).
In some
embodiments, an intracellular domain and/or other cytoplasmic domain of a CAR
is responsible
for activation of the cell in which the CAR is expressed (e.g., an immune
cell). In some
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
embodiments, an intracellular domain of a CAR is responsible for signal
activation and/or
transduction in an immune cell comprising said CAR.
[0193] In some embodiments, an intracellular domain of a CAR includes at
least one
domain responsible for signal activation and/or transduction. In some
embodiments, an
intracellular domain is or comprises at least one of a co-stimulatory molecule
and a signaling
domain. In some embodiments, an intracellular domain of a CAR comprises dual
signaling
domains. In some embodiments, an intracellular domain of a CAR comprises more
than two
signaling domains.
[0194] In some embodiments, an intracellular domain comprises a
cytoplasmic portion of
a surface receptor. In some embodiments, an intracellular domain comprises a
co-stimulatory
molecule. In some embodiments, an intracellular domain comprises a molecule
that acts to
initiate signal transduction in an immune cell.
[0195] In some embodiments, an intracellular domain of a CAR includes any
portion of
one or more co-stimulatory molecules, such as at least one signaling domain
from CD3, Fc
epsilon RI gamma chain, any derivative or variant thereof, any synthetic
sequence thereof that
has the same functional capability, and any combination thereof
FcR Intracellular Domains
[0196] In some embodiments, an FcR intracellular domain comprises a full-
length FcR
intracellular domain. In some embodiments, an FcR intracellular domain
comprises a portion of
a full-length FcR intracellular domain. In some embodiments, an FcR
intracellular domain is or
comprises a human FcR intracellular domain, or portion thereof. In some
embodiments, an FcR
intracellular domain may be a domain that is endogenous to a particular immune
cell type (e.g., a
modified immune cell as provided herein). In some embodiments, an FcR
intracellular domain
may be a domain that is not endogenous to a particular immune cell type (e.g.,
a modified
immune cell as provided herein). In some embodiments, an FcR intracellular
domain comprises
a CD64 (FcyRI), CD32a (FcyRIIa), CD32b (FcyRIIb), CD32c, CD16a (FcyRIIIa),
CD16b
(FcyRIIIb), Feat', FccRII, or FcaRI (CD89) domain.
TLR Intracellular Domains
66
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0197] In some embodiments, a TLR intracellular domain comprises a full-
length TLR
intracellular domain. In some embodiments, a TLR intracellular domain
comprises a portion of a
full-length TLR intracellular domain. In some embodiments, a TLR intracellular
domain is or
comprises a human TLR intracellular domain, or portion thereof. In some
embodiments, a TLR
intracellular domain may be a domain that is endogenous to a particular immune
cell type (e.g., a
modified immune cell as provided herein). In some embodiments, a TLR
intracellular domain
may be a domain that is not endogenous to a particular immune cell type (e.g.,
a modified
immune cell as provided herein). In some embodiments, a TLR intracellular
domain comprises a
TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, or TLR9 domain.
Signaling Domains
[0198] In some embodiments, a CAR comprises one or more intracellular
signaling
domains. In some embodiments, an intracellular signaling domain is or
comprises a human
intracellular signaling domain, or portion thereof. In some embodiments, a
signaling domain
may be a domain that is endogenous to a particular immune cell type (e.g., a
modified immune
cell as provided herein). In some embodiments, a signaling domain may be a
domain that is not
endogenous to a particular immune cell type (e.g., a modified immune cell as
provided herein).
[0199] In some embodiments, one or more intracellular signaling domains
comprise a
CD3-zeta, FcR y, CD64, CD32a, CD32c, CD16a, TLR1, TLR2, TLR3, TLR4, TLR5,
TLR6,
TLR7, TLR8, TLR9, ALK, AXL, DDR2, EGFR, EphAl, INSR, cMET, MUSK, PDGFR, PTK7,
RET, ROR1, ROS1, RYK, TIE2, TRK, VEGFR, CD40, CD19, CD20, 41BB, CD28, 0X40,
GITR, TREM-1, TREM-2, DAP12, MR, ICOS, MyD88, V/I/LxYxxL/V, SIRPa, CD45,
Siglec-
10, PD1, SHP-1, SHP-2, KIR-2DL, KIR-3DL, NKG2A, CD170, CD33, BTLA, CD32b,
SIRPp,
CD22, PIR-B, LILRB1,Syk, 41BB ligand (41BBL; TNFSF9), CD27, OX4OL, CD32b,
CD11b,
ITGAM, SLAMF7, CD206, CD163, CD209, Dectin-2, or one or more cytokine receptor
signaling domains (e.g., an IL1R, an IL2R, an IL3R, an IL4R, an IL5R, an IL6R,
an IL7R, an
IL8R, an IL9R, an IL1OR, an 11,11R, an IL12R, an IL13R, an 11,14R, an 11,15R,
an IL17R, an
IFNaR, an IFNgR, an TNFR, an CSF1R, an CSF2R, Dap10, CD36, Dectin-1, or ICOSL
intracellular signaling domain)
[0200] In some embodiments, an intracellular domain of a CAR comprises
dual signaling
domains, such as 41BB, CD28, ICOS, TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7,
TLR8,
67
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
TLR9, TLR10, TLR11, CD116 receptor beta chain, CSF1-R, LRP1/CD91, SR-Al, SR-
A2,
MARCO, SR-CL1, SR-CL2, SR-C, SR-E, CR1, CR3, CR4, dectin 1, DEC-205, DC-SIGN,
CD14, CD36, LOX-1, CD11b, together with any of the signaling domains listed in
the above
paragraph in any combination.
Co-stimulatory Domains
[0201] As used herein, a "co-stimulatory molecule" or "co-stimulatory
domain" refers to
a molecule in an immune cell that is used to heighten or dampen an initial
stimulus. For
example, pathogen-associated pattern recognition receptors, such as TLR or the
CD47/SIRPa
axis, are molecules on immune cells that, respectively, heighten or dampen an
initial stimulus.
In some embodiments, a co-stimulatory domain comprises TCR, CD3 zeta, CD3
gamma, CD3
delta, CD3 epsilon, CD86, common FcR gamma, FcR beta (Fc Epsilon Rib), CD79a,
CD79b,
Fcgamma RITa, DAP10, DAP12, T cell receptor (TCR), CD27, CD28, 4-1BB (CD137),
0X40,
CD30, CD40, PD-1, ICOS, lymphocyte function-associated antigen-1 (LFA-1), CD2,
CD7,
LIGHT, NKG2C, B7-H3, a ligand that specifically binds with CD83, CDS, ICAM-1,
GITR,
BAFFR, HVEM (LIGHTR), SLAMF7, NKp80 (KLRF1), CD127, CD160, CD19, CD4,
CD8alpha, CD8beta, IL2R beta, IL2R gamma, IL7R alpha, ITGA4, VLA1, CD49a,
ITGA4, IA4,
CD49D, ITGA6, VLA-6, CD49f, ITGAD, CD11d, ITGAE, CD103, ITGAL, CD11 a, LFA-1,
ITGAM, CD11b, ITGAX, CD11 c, ITGB1, CD29, ITGB2, CD18, LFA-1, ITGB7, TNFR2,
TRANCE/RANKL, DNAM1 (CD226), SLAMF4 (CD244, 2B4), CD84, CD96 (Tactile),
CEACAM1, CRTAM, Ly9 (CD229), CD160 (BY55), PSGL1, CD100 (SEMA4D), CD69,
SLAMF6 (NTB-A, Ly108), SLAM (SLAMF1, CD150, IP0-3), BLAME (SLAMF8), SELPLG
(CD162), LTBR, LAT, GADS, SLP-76, PAG/Cbp, NKp44, NKp30, NKp46, NKG2D, other
co-
stimulatory molecules described herein, any derivative, variant, or fragment
thereof, any
synthetic sequence of a co-stimulatory molecule that has the same functional
capability, and any
combinations thereof
[0202] In some embodiments, a co-stimulatory domain may be a domain that
is
endogenous to a particular immune cell type (e.g., a modified immune cell as
provided herein).
In some embodiments, a co-stimulatory domain may be a domain that is not
endogenous to a
particular immune cell type (e.g., a modified immune cell as provided herein).
68
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0203] As used herein, a "co-stimulatory signal" refers to a signal,
which in combination
with a primary signal, such as activation of a CAR on an immune cell, leads to
activation of the
immune cell.
Cleavage Peptides
[0204] As used herein, a cleavage peptide refers to a peptide that can
induce the cleaving
of a recombinant protein in a cell. In some embodiments, a cleavage peptide is
a 2A peptide. In
some embodiments, a cleavage peptide is or comprises a P2A, F2A, E2A or T2A
peptide. In
some embodiments, a nucleic acid as described herein comprises one or more
nucleic acid
sequences encoding one or more cleavage peptides. In some embodiments, a
nucleic acid
comprising a nucleic acid sequence encoding a cleavage peptide also comprises
one or more
nucleic acid sequences encoding one or more intracellular domains and one or
more nucleic acid
sequences comprising one or more peptide agents, wherein translation of the
nucleic acid results
in a protein comprising one or more intracellular domains separated from one
or more peptide
agents by a cleavage peptide. In some embodiments, a first promoter is
operably linked to one or
more nucleic acids encoding a CAR and a second promoter is operably linked to
one or more
nucleic acids encoding a peptide agent. In some embodiments, a nucleic acid
sequence
comprising a CAR, and optionally one or more peptide agents, further comprises
an internal
ribosome entry site (IRES) sequence. An IRES sequence may be any viral,
chromosomal or
artificially designed sequence that initiates cap-independent ribosome binding
to mRNA
facilitates the initiation of translation.
Peptide Agents
[0205] As used herein, a peptide agent refers to a peptide co-expressed
with a CAR in an
immune cell. In some embodiments, a peptide agent is co-expressed with a CAR
to ensure
stoichiometric balance and optimal signaling of a CAR. In some embodiments, a
peptide agent
forms a homodimer with an identical peptide agent. In some embodiments, a
peptide agent
forms a heterodimer with a different peptide agent. In some embodiments, a
nucleic acid as
described herein comprises one or more nucleic acid sequences encoding one or
more peptide
agents. In some embodiments, a peptide agent is or comprises an FcR gamma
chain.
In some embodiments, a peptide agent comprises any peptide, protein, receptor,
secreted
antibody or a fragment thereof (e.g., an scFv, Fab, Fab', F(ab')2, Fc, or
nanobody). In some
69
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
embodiments, a peptide agent comprises one or more cytokines (e.g., one or
more of IL-1, IL-2,
IL-6, IL-8, TNF-a, IFNa, IFNb, IFN-y, GMCSF, or MC SF), CD4O-L, dominant
negative SIRPa,
dominant negative PD1, dominant negative CD45, dominant negative SIGLEC 10, or
dominant
negative LILRB.
Fc Receptors (FcR)
[0206] In some embodiments, a CAR comprises one or more antigen binding
domains
and an FcR extracellular domain, and/or the transmembrane domain of the CAR
comprises an
FcR transmembrane domain, and/or the intracellular domain of the CAR comprises
an FcR
intracellular domain. In some embodiments, a CAR comprises, from N-terminus to
C-terminus,
one or more extracellular binding domains, an FcR extracellular domain, an FcR
transmembrane
domain, and an FcR intracellular domain. In some embodiments, one or more of
the FcR
extracellular domain, the FcR transmembrane domain and the FcR intracellular
domain is or
comprises a human FcR domain. In some embodiments, an FcR extracellular
domain, an FcR
transmembrane domain and an FcR intracellular domain together comprise a full-
length FcR. In
some embodiments, an FcR extracellular domain, an FcR transmembrane domain and
an FcR
intracellular domain together comprise a portion of a full-length FcR. In some
embodiments, an
FcR extracellular domain comprises a portion of a full-length FcR
extracellular domain. In some
embodiments, an FcR transmembrane domain comprises a portion of a full-length
FcR
transmembrane domain. In some embodiments, an FcR intracellular domain
comprises a portion
of a full-length FcR intracellular domain.
Toll-Like Antigen Receptors (TLR)
[0207] In some embodiments, a CAR comprises one or more antigen binding
domains
and a toll-like receptor (TLR) extracellular domain and/or the transmembrane
domain of the
CAR comprises a TLR transmembrane domain and/or the intracellular domain of
the CAR
comprises a TLR intracellular domain. In some embodiments, a CAR comprises,
from N-
terminus to C-terminus, one or more extracellular binding domains, a TLR
extracellular domain,
a TLR transmembrane domain, and a TLR intracellular domain. In some
embodiments, one or
more of the TLR extracellular domain, the TLR transmembrane domain and the TLR
intracellular domain is or comprises a human TLR domain. In some embodiments,
a TLR
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
extracellular domain, a TLR transmembrane domain and a TLR intracellular
domain together
comprise a full-length TLR. In some embodiments, a TLR extracellular domain, a
TLR
transmembrane domain and a TLR intracellular domain together comprise portion
of a full-
length TLR. In some embodiments, a TLR extracellular domain comprises a
portion of a full-
length TLR extracellular domain. In some embodiments, a TLR transmembrane
domain
comprises a portion of a full-length TLR transmembrane domain. In some
embodiments, a TLR
intracellular domain comprises a portion of a full-length TLR intracellular
domain.
Nucleic Acid Constructs
[0208] The present disclosure, among other things, provides nucleic acid
molecules
encoding at least one CAR described herein or a fragment thereof An immune
cell can
comprise a nucleic acid molecule (e.g., an exogenous nucleic acid molecule)
encoding at least
one CAR described herein. In some embodiments, a nucleic acid molecule
encoding at least one
CAR comprises: (a) an extracellular domain (e.g., an extracellular domain as
described herein),
(b) a transmembrane domain (e.g., a transmembrane domain as described herein),
and (c) an
intracellular domain (e.g., an intracellular domain as described herein).
[0209] Unless otherwise specified, a "nucleotide sequence encoding an
amino acid
sequence" includes all nucleotide sequences that are degenerate versions of
each other and that
encode the same amino acid sequence. The phrase nucleotide sequence that
encodes a protein or
an RNA may also include introns to the extent that the nucleotide sequence
encoding the protein
may in some version contain an intron(s). The term "encoding" refers to the
inherent property of
specific sequences of nucleotides in a polynucleotide, such as a gene, a cDNA,
or an mRNA, to
serve as templates for synthesis of other polymers and macromolecules in
biological processes
having either a defined sequence of nucleotides (e.g., rRNA, tRNA and mRNA) or
a defined
sequence of amino acids and the biological properties resulting therefrom.
Thus, a gene, cDNA,
or RNA, encodes a protein if transcription and translation of mRNA
corresponding to that gene
produces the protein in a cell or other biological system. Both the coding
strand, the nucleotide
sequence of which is identical to the mRNA sequence and is usually provided in
sequence
listings, and the non-coding strand, used as the template for transcription of
a gene or cDNA, can
be referred to as encoding the protein or other product of that gene or cDNA.
71
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0210] The term "operably linked" or "transcriptional control" refers to
functional
linkage between a regulatory sequence and a heterologous nucleic acid sequence
resulting in
expression of the heterologous nucleic acid sequence. For example, a first
nucleic acid sequence
is operably linked with a second nucleic acid sequence when the first nucleic
acid sequence is
placed in a functional relationship with the second nucleic acid sequence. For
instance, a
promoter is operably linked to a coding sequence if the promoter affects the
transcription or
expression of the coding sequence. Operably linked DNA sequences can be
contiguous with
each other and, e.g., where necessary to join two protein coding regions, are
in the same reading
frame.
[0211] Nucleic acid molecules encoding at least one CAR described herein
or a fragment
thereof can be a DNA molecule, an RNA molecule, or a combination thereof In
some
embodiments, a nucleic acid molecule comprises or is a messenger RNA (mRNA)
transcript
encoding at least one CAR described herein or a fragment thereof In some
embodiments, a
nucleic acid molecule comprises or is a DNA construct encoding at least one
CAR described
herein or a fragment thereof.
[0212] In some embodiments, all or a fragment of a CAR described herein
is encoded by
a codon optimized nucleic acid molecule, e.g., for expression in a cell (e.g.,
a mammalian cell).
A variety of codon optimization methods are known in the art, e.g., as
disclosed in US Patent
Nos. 5,786,464 and 6,114,148, each of which is hereby incorporated by
reference in its entirety.
[0213] Expression of nucleic acids as described herein may be achieved by
operably
linking a nucleic acid encoding a CAR polypeptide or fragment thereof to a
promoter in an
expression vector. Exemplary promoters (e.g., constitutive promoters) include,
but are not
limited to, an elongation factor-la promoter (EF-1a) promoter, immediate early
cytomegalovirus
(CMV) promoter, ubiquitin C promoter, phosphoglycerokinase (PGK) promoter,
simian virus 40
(5V40) early promoter, mouse mammary tumor virus (MMTV) promoter, human
immunodeficiency virus (HIV) long terminal repeat (LTR) promoter, Moloney
murine leukemia
virus (MoMuLV) promoter, an avian leukemia virus promoter, an Epstein-Barr
virus immediate
early promoter, a Rous sarcoma virus promoter, an actin promoter, a myosin
promoter, a
hemoglobin promoter, or a creatine kinase promoter. Examples of inducible
promoters include,
but are not limited to a metallothionine promoter, a glucocorticoid promoter,
a progesterone
72
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
promoter, and a tetracycline promoter. A vector can also comprise additional
promoter elements,
e.g., enhancers, to regulate the frequency of transcriptional initiation.
[0214] In some embodiments, a vector comprising a nucleic acid molecule
encoding at
least one CAR as described herein or a fragment thereof comprises or is a
viral vector. Viral
vector technology is well known in the art and is described (e.g., in Sambrook
et al., 2012,
MOLECULAR CLONING: A LABORATORY MANUAL, volumes 1-4, Cold Spring Harbor
Press, NY). Examples of viral vectors include, but are not limited to,
adenoviral vectors, adeno-
associated viral vectors, or retroviral vectors (e.g., a lentiviral vector or
a gammaretroviral
vector). In some embodiments, a vector comprises a lentiviral vector (e.g., as
described in US
Patent No. 9,149,519 or International Publication No. WO 2017/044487, each of
which is hereby
incorporated by reference in its entirety).
[0215] In some embodiments, a viral vector comprises an adenoviral
vector.
Adenoviruses are a large family of viruses containing double stranded DNA.
They replicate
within the nucleus of a host cell, using the host's cell machinery to
synthesize viral RNA, DNA
and proteins. Adenoviruses are known in the art to affect both replicating and
non-replicating
cells, to accommodate large transgenes, and to code for proteins without
integrating into the host
cell genome. In some embodiments, an adenoviral vector comprises an Ad2 vector
or an Ad5
vector (e.g., Ad5f35 adenoviral vector, e.g., a helper-dependent Ad5F35
adenoviral vector).
[0216] In some embodiments, a viral vector is an adeno-associated virus
(AAV) vector.
AAV systems are generally well known in the art (see, e.g., Kelleher and Vos,
Biotechniques,
17(6):1110-17 (1994); Cotten et al., P.N.A.S. U.S.A., 89(13):6094-98 (1992);
Curiel, Nat
Immun, 13(2-3):141-64 (1994); Muzyczka, Curr Top Microbiol Immunol, 158:97-129
(1992);
and Asokan A, et al., Mol. Ther., 20(4):699-708 (2012)). Methods for
generating and using
recombinant AAV (rAAV) vectors are described, for example, in U.S. Pat. Nos.
5,139,941 and
4,797,368.
[0217] Several AAV serotypes have been characterized, including AAV1,
AAV2, AAV3
(e.g., AAV3B), AAV4, AAV5, AAV6, AAV7, AAV8, AAV9, AAV10, and AAV11, as well
as
variants thereof. Generally, any AAV serotype may be used to deliver at least
one CAR
described herein. In some embodiments, an AAV serotype has a tropism for a
particular tissue.
73
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0218] In some embodiments, CRISPR/Cas9 system has recently been shown to
facilitate
high levels of precise genome editing using adeno associated viral (AAV)
vectors to serve as
donor template DNA during homologous recombination (HR).
[0219] In some embodiments, a vector comprises a gammaretroviral vector
(e.g., as
described in Tobias Maetzig et al., "Gammaretroviral Vectors: Biology,
Technology and
Application" Viruses. 2011 Jun; 3(6): 677-713, which is hereby incorporated by
reference in its
entirety). Exemplary gammaretroviral vectors include Murine Leukemia Virus
(MLV), Spleen-
Focus Forming Virus (SFFV), and Myeloproliferative Sarcoma Virus (MPSV), and
vectors
derived therefrom.
[0220] In some embodiments, a vector comprises two or more nucleic acid
sequences
encoding a CAR, e.g., at least one CAR described herein, and a second CAR,
e.g., a different
CAR described herein. In some embodiments, two or more nucleic acid sequences
encoding a
CAR and a second CAR are encoded by a single nucleic molecule, e.g., in same
frame and as a
single polypeptide chain. In some embodiments, two or more CARs are separated
by one or
more cleavage peptide sites (e.g., an auto-cleavage site or a substrate for an
intracellular
protease). In certain embodiments, a cleavage peptide comprises a porcine
teschovirus-1 (P2A)
peptide, Thosea asigna virus (T2A) peptide, equine rhinitis A virus (E2A)
peptide, foot-and-
mouth disease virus (F2A) peptide, or a variant thereof
[0221] In some embodiments, a vector comprises at least one nucleic acid
sequence
encoding a CAR, e.g., at least one CAR described herein, and at least one
nucleic acid encoding
at least one gene co-expressed with a CAR, e.g., a cytokine described herein
(e.g., TNF, IL-12,
IFN, GM-CSF, G-CSF, M-CSF, and/or IL-1) or a stimulatory ligand described
herein (e.g., CD7,
B7-1 (CD80), B7-2 (CD86), PD-L1, PD-L2, 4-1BBL, OX4OL, ICOS-L, ICAM, CD3OL,
CD40,
CD4OL, CD70, CD83, HLA-G, MICA, MICB, HVEM, lymphotoxin beta receptor, 3/TR6,
ILT3,
ILT4, HVEM, an agonist or antibody that binds Toll ligand receptor, and/or a
B7-H3 ligand).
Pharmaceutical Compositions
[0222] The present disclosure, among other things, provides
pharmaceutical
compositions comprising immune cells as described herein (e.g., macrophages,
monocytes, or
74
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
dendritic cells) in combination with one or more pharmaceutically or
physiologically acceptable
carriers, diluents, or excipients.
[0223] In some embodiments, a pharmaceutical composition of the present
disclosure
comprises one or more modified mRNA, wherein the one or more modified mRNA
comprise a
modified nucleotide, an alteration to the 5' or 3' untranslated region (UTR),
a cap structure, a
poly A tail, or a combination thereof, and one or more RNaseL inhibitors. In
some
embodiments, a pharmaceutical composition comprises one or more modified mRNA
comprising an AGCapl or m6AGCap1 cap structure. In some embodiments, a
pharmaceutical
composition comprises one or more modified mRNA comprising a modified
nucleotide
comprising pseudouridine (PsU), 5-methoxyuridine (5moU), 5-
methylcytidine/pseudouridine
(5meC PsU), or Ni-methyl-pseudouridine (N1mPsU). In some embodiments, a
pharmaceutical
composition comprises sunitinib. In some embodiments, a pharmaceutical
composition
comprises ABCE1. In some embodiments, a pharmaceutical composition comprises a
macrophage transfected with mRNA comprising an m6-AGCap1 and PsU
modifications,
wherein the mRNA encodes a CAR, wherein the macrophage was cultured with IFN-
f3 and
culturing the macrophage with IFN-f3 enhanced CAR expression, CAR persistence,
CAR
macrophage function, an M1 phenotype, resistance to M2-inducing factors, or a
combination
thereof relative to a macrophage transfected with an identical mRNA but not
cultured with IFN-
r3.
[0224] When "a therapeutically effective amount, "an immunologically
effective
amount," "an anti-immune response effective amount," or "an immune response-
inhibiting
effective amount" is indicated, a precise amount of a pharmaceutical
composition comprising
immune cells as described herein (e.g., macrophages, monocytes, or dendritic
cells) can be
determined by a physician with consideration of individual differences in age,
weight, immune
response, and condition of the patient (subject).
[0225] Pharmaceutical compositions comprising immune cells as described
herein (e.g.,
macrophages, monocytes, or dendritic cells) may comprise buffers, such as
neutral buffered
saline or phosphate buffered saline (PBS); carbohydrates, such as glucose,
mannose, sucrose,
dextrans, or mannitol; proteins, polypeptides, or amino acids (e.g., glycine);
antioxidants;
chelating agents, such as EDTA or glutathione; adjuvants (e.g., aluminum
hydroxide); serum and
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
preservatives, such as cryoprotectant. In some embodiments, a pharmaceutical
composition is
substantially free of contaminants, e.g., there are no detectable levels of a
contaminant (e.g., an
endotoxin).
[0226] Pharmaceutical compositions described herein may be administered
in a manner
appropriate to the disease, disorder, or condition to be treated or prevented.
Quantity and
frequency of administration will be determined by such factors as condition of
a patient, and type
and severity of a patient's disease, disorder, or condition, although
appropriate dosages may be
determined by clinical trials.
[0227] Pharmaceutical compositions described herein may be in a variety
of forms.
These include, for example, liquid, semi-solid and solid dosage forms, such as
liquid solutions
(e.g., injectable and infusible solutions), dispersions or suspensions,
liposomes, and
suppositories. Preferred compositions may be injectable or infusible
solutions. Pharmaceutical
compositions described herein can be formulated for administration
intravenously,
subcutaneously, intradermally, intratumorally, intranodally, intramedullary,
intramuscularly,
transarterially, or intraperitoneally.
[0228] In some embodiments, a pharmaceutical composition described herein
is
formulated for parenteral (e.g., intravenous, subcutaneous, intraperitoneal,
or intramuscular)
administration. In some embodiments, a pharmaceutical composition described
herein is
formulated for intravenous infusion or injection. In some embodiments, a
pharmaceutical
composition described herein is formulated for intramuscular or subcutaneous
injection.
Pharmaceutical compositions described herein can be formulated for
administered by using
infusion techniques that are commonly known in immunotherapy (See, e.g.,
Rosenberg et al.,
New Eng. J. of Med. 319:1676, 1988, which is hereby incorporated by reference
in its entirety).
[0229] As used herein, the terms "parenteral administration" and
"administered
parenterally" refer to modes of administration other than enteral and topical
administration,
usually by injection or infusion, and include, without limitation,
intravenous, intramuscular,
intraarterial, intrathecal, intracapsular, intraorbital, intracardiac,
intradermal, intraperitoneal,
transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular,
subarachnoid, intraspinal,
epidural, intratumoral, and intrasternal injection and infusion.
76
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0230] Pharmaceutical compositions comprising immune cells as described
herein may
be administered at a dosage of about 104 to about 109 cells/kg body weight
(e.g., about 105 to
about 106 cells/kg body weight), including all integer values within those
ranges. In some
embodiments, a dose of immune cells as described herein (e.g., macrophages,
monocytes, or
dendritic cells) comprises at least about 1 x 106, about 1.1 x 106, about 2 x
106, about 3.6 x 106,
about 5 x 106, about lx 107, about 1.8 x 107, about 2 x 107, about 5 x 107,
about 1 x 108, about 2
x 108, about 5 x 108, about 1 x 109, about 2 x 109, or about 5 x 109 cells.
Pharmaceutical
compositions described herein may also be administered multiple times at a
certain dosage. An
optimal dosage and treatment regime for a particular patient can readily be
determined by one
skilled in the art by monitoring a patient for signs of a disease, disorder,
or condition and
adjusting treatment accordingly.
[0231] It may be desired to administer pharmaceutical compositions
comprising immune
cells (e.g., macrophages, monocytes, or dendritic cells) as described herein
to a subject and then
subsequently redraw blood (or have apheresis performed), activate collected
immune cells, and
reinfuse a subject with activated immune cells. This process can be performed
multiple times,
e.g., every few weeks. Immune cells (e.g., macrophages, monocytes, or
dendritic cells) can be
activated from blood draws of from about 10 cc to about 400 cc. In some
embodiments, immune
cells (e.g., macrophages, monocytes, or dendritic cells) are activated from
blood draws of about
20 cc, about 30 cc, about 40 cc, about 50 cc, about 60 cc, about 70 cc, about
80 cc, about 90 cc,
or about 100 cc. Without being bound by theory, methods comprising multiple
blood draw and
reinfusions as described herein may select for certain immune cell
populations. In some
embodiments, pharmaceutical compositions comprising immune cells as described
herein (e.g.,
macrophages, monocytes, or dendritic cells) are administered in combination
with (e.g., before,
simultaneously, or following) a second therapy. For example, a second therapy
can include, but
is not limited to antiviral therapy (e.g., cidofovir, interleukin-2,
Cytarabine (ARA-C), or
natalizumab), chimeric antigen receptor-T cell (CAR-T) therapy, T-cell
receptor (TCR)-T cell
therapy, chemotherapy, radiation, an immunosuppressive agent (e.g.,
cyclosporin, azathioprine,
methotrexate, mycophenolate, FK506 antibody, or glucocorticoids), an
antagonist (e.g., one or
more of a PD-1 antagonist, a PD-Li antagonist, CTLA4 antagonist, CD47
antagonist, SIRPa
antagonist, CD40 agonists, CSF1/CSF1R antagonist, or a STING agonist), or an
immunoablative
agent (e.g., an anti-CD52 antibody (e.g., alemtuzumab), an anti-CD3 antibody,
cytoxin,
77
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
fludaribine, cyclosporin, FK506, rapamycin, mycophenolic acid, a steroid,
FR901228, or
irradiation).
[0232] In some embodiments, pharmaceutical compositions comprising immune
cells as
described herein (e.g., macrophages, monocytes, or dendritic cells) are
administered in
combination with (e.g., before, simultaneously, or following) bone marrow
transplantation or
lymphocyte ablative therapy using a chemotherapy agent (e.g., fludarabine,
external-beam
radiation therapy (XRT), cyclophosphamide, or Rituxan). In certain
embodiments, subjects
undergo standard treatment with high dose chemotherapy followed by peripheral
blood stem cell
transplantation. In certain embodiments, following transplant, subjects
receive an infusion of a
pharmaceutical composition comprising immune cells as described herein.
Pharmaceutical
compositions described herein may be administered before or following surgery.
[0233] A dosage of any aforementioned therapy to be administered to a
subject will vary
with a disease, disorder, or condition being treated and based on a specific
subject. Scaling of
dosages for human administration can be performed according to art-accepted
practices. For
example, a dose of alemtuzumab will generally be about 1 mg to about 100 mg
for an adult,
usually administered daily for a period of between about 1 day to about 30
days, e.g., a daily
dose of about 1 mg to about 10 mg per day (e.g., as described in U.S. Patent
No. 6,120,766,
which is hereby incorporated by reference in its entirety).
Methods of Treatment
[0234] The present disclosure, among other things, provides methods of
treating a
disease or disorder (e.g., a disease or a disorder described herein) in a
subject comprising
delivering a pharmaceutical composition comprising immune cells as described
herein (e.g.,
macrophages, monocytes, or dendritic cells). In some embodiments, a
therapeutically effective
amount of a pharmaceutical composition described herein is administered to a
subject having a
disease or disorder. Pharmaceutical compositions as described herein can be
for use in the
manufacture of a medicament for treating a disease or disorder in a subject or
stimulating an
immune response in a subject.
[0235] A subject to be treated with methods described herein can be a
mammal, e.g., a
primate, e.g., a human (e.g., a patient having, or at risk of having, a
disease or disorder described
78
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
herein). In some embodiments, immune cells (e.g., macrophages, monocytes, or
dendritic cells)
may be autologous, allogeneic, or xenogeneic with respect to a subject.
Pharmaceutical
compositions as described herein can be administered to a subject in
accordance with a dosage
regimen described herein, alone or in combination with one or more therapeutic
agents,
procedures, or modalities.
[0236] Pharmaceutical composition comprising immune cells as described
herein (e.g.,
macrophages, monocytes, or dendritic cells) can be used to treat or prevent a
disease associated
with a tumor or cancer, a neurodegenerative disease or disorder, an
inflammatory disease or
disorder, a cardiovascular disease or disorder, a fibrotic disease or
disorder, a disease associated
with amyloidosis, and a combination of thereof.
[0237] A method of treating (e.g., one or more of reducing, inhibiting,
or delaying
progression of) a cancer or a tumor in a subject with a pharmaceutical
composition comprising
immune cells as described herein (e.g., macrophages, monocytes, or dendritic
cells) is provided.
A subject can have an adult or pediatric form of cancer. A cancer may be at an
early,
intermediate, or late stage, or a metastatic cancer. A cancer can include, but
is not limited to, a
solid tumor, a hematological cancer (e.g., leukemia, lymphoma, or myeloma,
e.g., multiple
myeloma), or a metastatic lesion. Examples of solid tumors include
malignancies, e.g., sarcomas
and carcinomas, e.g., adenocarcinomas of the various organ systems, such as
those affecting the
lung, breast, ovarian, lymphoid, gastrointestinal (e.g., colon), anal,
genitals and genitourinary
tract (e.g., renal, urothelial, bladder cells, prostate), pharynx, CNS (e.g.,
brain, neural or glial
cells), head and neck, skin (e.g., melanoma, e.g., a cutaneous melanoma),
pancreas, and bones
(e.g., a chordoma).
[0238] In some embodiments, a cancer is chosen from a lung cancer (e.g.,
a non-small
cell lung cancer (NSCLC) (e.g., a non-small cell lung cancer (NSCLC) with
squamous and/or
non-squamous histology, or a NSCLC adenocarcinoma), or a small cell lung
cancer (SCLC)), a
skin cancer (e.g., a Merkel cell carcinoma or a melanoma (e.g., an advanced
melanoma)), an
ovarian cancer, a mesothelioma, a bladder cancer, a soft tissue sarcoma (e.g.,
a
hemangiopericytoma (HPC)), a bone cancer (a bone sarcoma), a kidney cancer
(e.g., a renal
cancer (e.g., a renal cell carcinoma)), a liver cancer (e.g., a hepatocellular
carcinoma), a
cholangiocarcinoma, a sarcoma, a myelodysplastic syndrome (MDS), a prostate
cancer, a breast
79
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
cancer (e.g., a breast cancer that does not express one, two or all of
estrogen receptor,
progesterone receptor, or Her2/neu, e.g., a triple negative breast cancer), a
colorectal cancer (e.g.,
a relapsed colorectal cancer or a metastatic colorectal cancer, e.g., a
microsatellite unstable
colorectal cancer, a microsatellite stable colorectal cancer, a mismatch
repair proficient
colorectal cancer, or a mismatch repair deficient colorectal cancer), a
nasopharyngeal cancer, a
duodenal cancer, an endometrial cancer, a pancreatic cancer, a head and neck
cancer (e.g., head
and neck squamous cell carcinoma (HNSCC)), an anal cancer, a gastro-esophageal
cancer, a
thyroid cancer (e.g., anaplastic thyroid carcinoma), a cervical cancer (e.g.,
a squamous cell
carcinoma of the cervix), a neuroendocrine tumor (NET) (e.g., an atypical
pulmonary carcinoid
tumor), a lymphoproliferative disease (e.g., a post-transplant
lymphoproliferative disease), a
lymphoma (e.g., T-cell lymphoma, B-cell lymphoma, or a non-Hogdkin lymphoma),
a myeloma
(e.g., a multiple myeloma), or a leukemia (e.g., a myeloid leukemia or a
lymphoid leukemia).
[0239] In some embodiments, a cancer is a brain tumor, e.g., a
glioblastoma, a
gliosarcoma, or a recurrent brain tumor. In some embodiments, a cancer is a
pancreatic cancer,
e.g., an advanced pancreatic cancer. In some embodiments, a cancer is a skin
cancer, e.g., a
melanoma (e.g., a stage II-IV melanoma, an HLA-A2 positive melanoma, an
unresectable
melanoma, or a metastatic melanoma), or a Merkel cell carcinoma. In some
embodiments, a
cancer is a renal cancer, e.g., a renal cell carcinoma (RCC) (e.g., a
metastatic renal cell
carcinoma). In some embodiments, a cancer is a breast cancer, e.g., a
metastatic breast
carcinoma or a stage IV breast carcinoma, e.g., a triple negative breast
cancer (TNBC). In some
embodiments, a cancer is a virus-associated cancer. In some embodiments, a
cancer is an anal
canal cancer (e.g., a squamous cell carcinoma of the anal canal). In some
embodiments, a cancer
is a cervical cancer (e.g., a squamous cell carcinoma of the cervix). In some
embodiments, a
cancer is a gastric cancer (e.g., an Epstein Barr Virus (EBV) positive gastric
cancer, or a gastric
or gastro-esophageal junction carcinoma). In some embodiments, a cancer is a
head and neck
cancer (e.g., an HPV positive and negative squamous cell cancer of the head
and neck
(SCCHN)). In some embodiments, a cancer is a nasopharyngeal cancer (NPC). In
some
embodiments, a cancer is a colorectal cancer, e.g., a relapsed colorectal
cancer, a metastatic
colorectal cancer, e.g., a microsatellite unstable colorectal cancer, a
microsatellite stable
colorectal cancer, a mismatch repair proficient colorectal cancer, or a
mismatch repair deficient
colorectal cancer.
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0240] In some embodiments, a cancer is a hematological cancer. In some
embodiments,
a cancer is a leukemia, e.g., acute myeloid leukemia, chronic myeloid
leukemia, acute
lymphoblastic leukemia, chronic lymphocytic leukemia, chronic leukemia, or
acute leukemia. In
some embodiments, a cancer is a lymphoma, e.g., Hodgkin lymphoma (HL), non-
Hodgkin's
lymphoma, lymphocytic lymphoma, or diffuse large B cell lymphoma (DLBCL)
(e.g., a relapsed
or refractory HL or DLBCL). In some embodiments, a cancer is a myeloma, e.g.,
multiple
myeloma.
[0241] Pharmaceutical composition comprising immune cells as described
herein (e.g.,
macrophages, monocytes, or dendritic cells) can be used to enhance or modulate
an immune
response in a subject. In one embodiment, a pharmaceutical composition
described herein
enhances, stimulates, or increases an immune response in a subject (e.g., a
subject having, or at
risk of, a disease or disorder described herein). In certain embodiments, a
subject is, or is at risk
of being, immunocompromised. For example, a subject is undergoing or has
undergone a
chemotherapeutic treatment and/or radiation therapy.
[0242] In some embodiments, a subject has, or is at risk of, developing
an inflammatory
disorder (e.g., a chronic or acute inflammatory disorder). In some
embodiments, a subject has,
or is at risk, of developing an autoimmune disease or disorder. Exemplary
autoimmune diseases
that can be treated with methods described herein include, but are not limited
to, Alzheimer's
disease, asthma (e.g., bronchial asthma), an allergy (e.g., an atopic
allergy), Acquired
Immunodeficiency Syndrome (AIDS), atherosclerosis, Behcet's disease, celiac,
cardiomyopathy,
Crohn's disease, cirrhosis, diabetes, diabetic retinopathy, eczema,
fibromyalgia, fibromyositis,
glomerulonephritis, graft vs. host disease (GVHD), Guillain-Barre syndrome,
hemolytic anemia,
multiple sclerosis, myasthenia gravis, osteoarthritis, polychondritis,
psoriasis, rheumatoid
arthritis, sepsis, stroke, vasculitis, ventilator-induced lung injury,
transplant rejection, Raynaud's
phenomena, Reiter's syndrome, rheumatic fever, sarcoidosis, scleroderma,
Sjogren's syndrome,
ulcerative colitis, uveitis, vitiligo, or Wegener's granulomatosis.
[0243] Administration of pharmaceutical compositions described herein may
be carried
out in any convenient manner (e.g., injection, ingestion, transfusion,
inhalation, implantation, or
transplantation). In some embodiments, a pharmaceutical compositions described
herein is
administered by injection or infusion. Pharmaceutical compositions described
herein may be
81
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
administered to a patient transarterially, subcutaneously, intravenously,
intradermally,
intratumorally, intranodally, intramedullary, intramuscularly, or
intraperitoneally. In some
embodiments, a pharmaceutical composition described herein is administered
parenterally (e.g.,
intravenously, subcutaneously, intraperitoneally, or intramuscularly). In some
embodiments, a
pharmaceutical composition described herein is administered by intravenous
infusion or
injection. In some embodiments, a pharmaceutical composition described herein
is administered
by intramuscular or subcutaneous injection. Pharmaceutical compositions
described herein may
be injected directly into a site of inflammation, a local disease site, a
lymph node, an organ, a
tumor, or site of infection in a subject.
[0244] The disclosure is further illustrated by the following example. An
example is
provided for illustrative purposes only. It is not to be construed as limiting
the scope or content
of the disclosure in any way.
EXAMPLES
[0245] The following examples are provided so as to describe to the
skilled artisan how
to make and use methods and compositions described herein, and are not
intended to limit the
scope of the present disclosure.
Methods
Differentiation of Monocytes to Macrophages
[0246] In exemplary methods of the present disclosure, normal donor
apheresis-derived
leukopacs were subjected to elutriation using an Elutra Cell Separation System
(Terumo BCT) or
cell separation using a CliniMACS Prodigy (Miltenyi Biotec) or CliniMACS Plus
(Miltenyi
Biotec) to reduce erythrocytes, platelets, lymphocytes and granulocytes.
Monocytes were
enriched using elutriation or positively selected using MACS CD14+ selection
(Miltenyi Biotec)
according to the manufacturer's instructions. The pre-selection and post-
selection (positive and
negative fraction) purity and viability were tested using flow cytometry.
Selected CD14+
monocytes were differentiated into macrophages for up to 7 days.
Differentiated macrophages
were harvested at day 5-7 and kept frozen in freezing media or used fresh for
experiments. In
some cases, cells were utilized post-selection in the monocyte state.
82
CA 03187138 2022-12-13
WO 2021/263152
PCT/US2021/039168
Electroporation
[0247] In exemplary methods of the present disclosure, fresh or thawed
human
macrophages were thawed and cultured at 37 C overnight one day prior to
electroporation.
Macrophages were collected and washed with PBS. Viable cells were counted with
the NC200.
For macrophages transfected using a Neon Transfection System, 50nM-300nM of
mRNA per
le+6 macrophages were mixed on ice in electroporation buffer. Macrophages were
electroporated with a Neon Transfection System or with a Maxcyte
Electroporation system.
Cells were kept on ice after for 10 minutes after the electroporation. Cells
were collected,
counted, and cultured in macrophage culture media for further use. Cells were
then transferred
to a plate and placed at 37 C for 15 minutes for recovery. Cells were plated
in 6 well plates with
macrophage culture media for further use.
Fluorescence-Activated Cell Sorting (FACS)
[0248]
Viability was assessed using Live/Dead Fixable Aqua Dead Cell Staining Kit
(Thermo) or similar reagents. Additionally, cell viability was assessed using
the NC-200
(Chemometec).
[0249] Primary human macrophages were tested for CAR-HER2 expression
using a two-
step staining protocol: human HER2/ ERBB2 Protein-His tag (Sino Biological
Inc, 10004-
HO8H-100) primary stain followed by Human TruStain FcX (Biolegend, 422302) and
Anti-His
Tag APC (R&D Systems, IC050A) secondary stain. TruStain FcX (Biolegend,
422302) was
used for FACS staining of monocytes, macrophages, or monocytic cell lines
expressing Fc
receptors. Macrophage purity was tested using the following panel: Anti-CD lib
PE (Biolegend,
301306), Anti-CD14 BV711 (Biolegend, 301838), Anti-CD3 FITC (eBioscience, 11-
0038-42),
Anti-CD19 PE-CY7 (eBioscience, 25-0198-42), Anti-CD66b PerCP-CY5.5 (Biolegend,
305108), Anti-CD56 BV605 (Biolegend, 318334), and Live/Dead Fixable Aqua (L/D
aqua)
Dead Cell Stain Kit (ThermoFisher, L34957). The same panel was used for
testing the monocyte
purity post CD14 MACS selection, prior to seeding for differentiation. M1/M2
markers on
primary human macrophages were detected with the following panel: Anti-CD11B
PE
(Biolegend, 301306), Anti-CD80 BV605 (Biolegend, 305225), Anti-CD86 BV711
(Biolegend,
83
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
305440), Anti CD206 BV421 (Biolegend, 321126), Anti CD163 APC-CY7 (Biolegend,
333622), anti HLA-DR BV785 (Biolegend, 307642), Anti-HLA ABC PE/CY7
(Biolegend,
311430) and Live/Dead Fixable Aqua Dead Cell Stain Kit.
Functional Assays
Co-Culture of Target Cancer Cells and CAR Macrophages
[0250] Target cells such as HER2+ breast cancer cells (CRL2351) or HER2+
ovarian
cancer cells stably expressing NucLight Green GFP (Sartorious #4475) were
used. Additional
cell lines were evaluated that expressed the cognate antigen targeted by the
CAR, as described
within below. CAR macrophages were co-cultured with target cells at indicated
ratios for
indicated periods of time. To quantify an anti-tumor effect in vitro, the
relative numbers of
target cancer cells were measured using the Incucyte live imaging microscope
(Sartorius) which
monitors the green fluorescent intensity of cultured wells roughly every one
hour. The anti-
tumor activity of CAR macrophages was compared to control macrophages or
target cells alone.
Graphpad Prism was used to graph data and perform statistical analysis.
Treatment of CAR Macrophages with Interferon (IF1V)
[0251] After the transfection of CAR mRNA, macrophages were cultured in
macrophage
culture media with 10-300ng/mL of recombinant human IFN-a, IFN-f3, or IFN-y
(peprotech) for
4 to 24 hours, as indicated. IFN containing media was washed off after the
indicated time. IFN-
primed CAR macrophages were used for further analysis.
Treatment of CAR Macrophages with Pro-Inflammatory Factors
[0252] After the transfection of CAR mRNA, 2e+6 macrophages were cultured
in
macrophage culture media with varying concentrations of pro-inflammatory
mediators.
Exemplary pro-inflammatory meditators include: TLR1/2 agonists (e.g.,
Pam3CSK4), TLR3
agonists (e.g., Poly(I:C)), TLR4 agonists (e.g., LPS-EK standard
(Lipopolysaccharide from
Escherichia coli K12)), TLR5 agonists (e.g., FLA-ST standard (Flagellin from
S. Ophimurium)),
TLR6/2 agonists (e.g., FSL-1), TLR7 agonists (e.g., Imiquimod), TLR8 agonists
(e.g.,
ssRNA40/LyoVec), TLR9 agonists (e.g., CpG oligonucleotides), IFN-y 20 ng/mL,
TNF-a 20
ng/mL, 0-Glucan, recombinant human CD40-ligand, recombinant human 41BB-ligand,
84
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
recombinant human 41BB receptor, TNFa, IL6, IL12, STING agonists, cGAS
agonists, and
other pro-inflammatory mediators (cytokines, agonists, antibodies, small
molecules, peptides) for
4 to 48 hours. The media was washed off after the indicated time and the
primed CAR
macrophages were used for further analysis.
Evaluation of Macrophage Phenotype (M1/M2)
[0253] M1/M2 markers on primary human macrophages were detected with the
following panel: Anti-CD11B PE (Biolegend, 301306), Anti-CD80 BV605
(Biolegend, 305225),
Anti-CD86 BV711 (Biolegend, 305440), Anti CD206 BV421 (Biolegend, 321126),
Anti CD163
APC-CY7 (Biolegend, 333622), anti HLA-DR BV785 (Biolegend, 307642), Anti HLA
ABC
PE/CY7 (Biolegend, 311430) and Live/Dead Fixable Aqua Dead Cell Stain Kit.
Cytokine
production (IL12, IFN-y, TNF-a, IL6, IL8, ILlb, MCP-1, ILI , IL4, IL13, and
other human
cytokines) was evaluated in supernatant collected from macrophages (control or
CAR) treated as
described below using the MSD instrument per manufacturers recommendations
(Meso Scale
Discovery). In some cases, macrophages were co-cultured with antigen-bearing
target cells at
indicated effector to target ratios.
Detection of mCherry Expression
[0254] The transfection efficiency, persistence of expression, and
intensity of expression
of the fluorescent reporter gene mCherry in human monocytes or macrophages was
evaluated
using real time live microscopy on the Incucyte (Sartorius) for the indicated
period of time.
Treatment of Macrophages with Sunitinib
[0255] Macrophages were treated with 0-10pM sunitinib (an RNAse-L
inhibitor) for 2
hours prior to mRNA electroporation or transfection. The level of expression
and persistence of
expression of the encoded transgene was assessed using methods described
above.
Real time PCR
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0256] Real time PCR of the encoded transgene (such as a CAR) was
performed using
standard methods. In brief, RNA was isolated using Ambion RiboPure RNA
Purification Kit
(AM1924, Thermo Fisher) and reverse transcribed using iScript RT Supermix for
RT-qPCR
(1708841, Bio-Rad). For quantitative PCR, template cDNA, primers, Taqman Gene
Expression
primer/probe and Taqman Gene Expression Master Mix (4369016, Applied
Biosystems) were
used according to the manufacturer's instructions.
Determination of Duration of CAR Expression
Flow Cytometry
[0257] In exemplary methods of the present disclosure, 50,000 CAR
macrophages were
plated into the wells of a 96 well plate. In order to measure anti-HER2 CAR
expression, His-
tagged recombinant HER2 protein, along with a buffer such as PBS supplemented
with BSA,
was added to the cells and allowed to incubate. The cells were then spun down
at 300 x g for 5
minutes and the supernatant was then removed. Fc-receptors were then blocked
using an Fc
blocking solution, such as Human TruStain FcX (BioLegend, Cat 422302) for five
minutes in
PBS. Following Fc blocking, staining for cell viability and other surface
markers can be
accomplished. For example, cell viability can be determined using LIVE/DEADTM
Fixable
Aqua Dead Cell Stain Kit (Invitrogen, Cat L34957). In addition, anti-His
antibodies, such as His
Tag APC-conjugated Antibody (R&D Systems, Cat IC050A) were added. Expression
of the
CAR was determined through flow cytometry, first gating on a single cell
population, followed
by selection of live cells, and finally measuring the APC fluorescence of the
cells. Cells
expressing the CAR will be brighter in the APC channel than control cells
which have not been
exposed to the anti-His antibody. The brightness of the CAR-positive cells
determined the
extent of expression, while repeated measures over time allowed for tracking
the expression of
the CAR over time.
Immunofluorescence (IF) Microscopy
[0258] In exemplary methods of the present disclosure, cells were
cultured as appropriate
on a glass slide. Media was then removed and the cells were washed three times
with PBS. Cell
were then fixed using 4% paraformaldehyde or methanol. The incubation time in
the fixative
depended on the identity of the fixative. Once the appropriate amount of time
passed, the
86
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
fixative was removed and the cells were washed again three times with PBS. If
intracellular
staining was desired, cells were then incubated in 1% Triton X-100
(ThermoFisher, Cat BP151-
100) in PBS and then washed three times in PBS. Blocking solution, such as BSA
in PBS, was
then added to the cells and allowed to block for 60 minutes. The blocking
solution was then
removed and the cells were washed. Fluorochrome-conjugated antibodies were
diluted
according to the manufacturer's instructions and allowed to bind to the cells
overnight. The
antibody solution was then removed and the cells were washed. Finally, a
mounting solution,
such as ProLongTM Diamond Antifade Mountant with DAPI (Invitrogen, Cat
P36966), was
applied to the cells and the coverslip placed on top. This was allowed to dry
for 24 hours before
imaging. Imaging was performed via fluorescence microscopy. Cells expressing
CAR were
brighter than cells not expressing the CAR in the appropriate channel for the
fluorophore.
RT-PCR
[0259] In exemplary methods of the present disclosure, macrophages were
lysed and the
RNA collected through the use of a 1 step kit RTPCR kit (SuperScriptTM III
Platinum Tm One-
Step qRT-PCR Kit, Invitrogen Cat 11732-020) by following the manufacturer's
instructions.
Primers specific to the CAR were used in the assay. Macrophages with mRNA for
the CAR
showed a signal in the RTPCR assay, whereas untransduced macrophages did show
the signal.
Determination of CAR Effector Cell Functionality
[0260] In exemplary methods of the present disclosure, a CAR transgene
was introduced
into monocytes or macrophages via electroporation/transfection with a nucleic
acid (e.g., DNA,
mRNA, or chemically modified mRNA) or through viral transduction with a
lentiviral,
adenoviral or alternative viral vector. Expression of a CAR is confirmed using
antigen specific-
staining via flow cytometry, real-time PCR, or fluorescent microscopy. These
techniques can
also be used to determine the intensity and kinetics of expression of a CAR.
CAR constructs that
express on the surface of macrophages are tested for activity in a tumor
phagocytosis assay
and/or tumor killing assay against a target-positive cell line. Constructs
that cause phagocytosis
and/or killing of the target cells were tested for cytokine secretion,
chemokine secretion, immune
cell recruitment ability, phenotypic alteration (i.e. self-polarization to
M1/M2) and T cell
stimulation/antigen presentation functionality.
87
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
Phagocytosis Assays
Flow Phagocytosis Assay (Cells)
[0261] In exemplary methods of the present disclosure, target positive
and target negative
tumor cells were labeled with CellTraceTm CF SE Cell Proliferation Kit
(Invitrogen, Cat C34554)
according to the manufacturer's instructions or the cells have been engineered
to express a
fluorescent protein (e.g., GFP). CAR-expressing and control macrophages were
then plated in a
U-bottom 96 well plate at a 1:1 macrophage:tumor cell ratio and cultured for 4
hours. At the end
of incubation, cells were removed from the wells and stained for flow
cytometry. The panel
included a viability dye and a macrophage-specific marker, such as CD11b. Upon
gating for live
cells, cells that were CD11b/CF SE double positive were macrophages presumed
to have
phagocytosed a cell. CAR macrophages should have an increased percentage of
double positive
cells when cultured with the target positive tumor cells compared to
macrophages without the
CAR. The specificity of the target-based phagocytosis was tested by using a
control of
macrophages cultured with cells negative for the target.
Flow Phagocytosis Assay (Beads)
[0262] In exemplary methods of the present disclosure, polystyrene beads
were
functionalized with the target of the CAR or an irrelevant protein. In
addition, these beads were
labeled with pHrodoTM Red, SE (Invitrogen, Cat P36600), which is a pH-reactive
dye. Upon
acidification, the dye increased its level of fluorescence. The beads were
then cultured with
CAR macrophages and untransduced macrophages. After a period of time, the
macrophages
were removed and stained for flow cytometry using a viability dye and a
macrophage specific
marker, such as CD11b. Upon gating for live cells, cells that were
CD11b/pHrodo double
positive were considered macrophages presumed to have phagocytosed a bead. CAR
macrophages should have an increased percentage of double positive cells when
cultured with
the target positive beads compared to macrophages without the CAR. The
specificity of the
target-based phagocytosis was tested by using a control of macrophages
cultured with beads
negative for the target.
Incucyte (Cells)
88
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0263] In exemplary methods of the present disclosure, target-positive
tumor cells
expressing a fluorescent protein like GFP were cultured with CAR macrophages
and
untransduced macrophages in a 96 well plate. The ratio between the effector
macrophages and
target tumor cells was varied from 10:1 E:T to 1:10 E:T, along with a 0:1 E:T
target cell only
control. The number of macrophages was kept constant at 10e3 macrophages per
well. The
change in fluorescence over time, measured every four hours, was measured to
determine the
amount of tumor cell killing occurring the culture. In addition, image
analysis techniques were
used to determine the location of macrophages in the culture and determine the
number of
macrophages that phagocytosed a tumor cell.
Incucyte (Beads)
[0264] In exemplary methods of the present disclosure, pHrodo
functionalized beads
bearing the protein target for the CAR were added to both CAR macrophages and
untransduced
macrophages in the wells of a 96 well plate. The macrophages were plated at a
concentration of
20e3 per well and the beads are added at a 5:1 bead to macrophage ratio. The
fluorescence of the
pHrodo was measured for five hours, with measurements every 30 minutes. The
ratio of
increase in fluorescence between the initial time point and the 1 hour time
point was used to
determine the amount of phagocytosis occurring. The CAR macrophages were
expected to have
a higher change in fluorescence than the untransduced controls.
Mouse Models
___ Xenograft Mouse Model
[0265] In exemplary methods of the present disclosure, NSGS (NSG-SGM3)
mice were
challenged with SKOV3, a HER2+ human ovarian cancer, via intraperitoneal
administration in
order to model peritoneal carcinomatosis. Mice were treated with human CAR
macrophages that
were generated via mRNA electroporation with or without IFN-0 priming (or
negative controls).
Tumor burden was monitored via bioluminescent imaging.
Syngeneic Mouse Model
[0266] BALB-C immunocompetent mice were engrafted with CT26-HER2, a model
of
HER2+ colorectal carcinoma, via subcutaneous injection. Once tumors engrafted,
mice were
89
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
treated with CAR macrophages that were generated via mRNA electroporation with
or without
IFN-f3 priming (or negative controls). Tumor burden was monitored via caliper
measurements of
the tumor mass.
Example 1: Effect of mRNA Modifications on Expression in and Viability of
Primary
Human Macrophages
[0267] Donor macrophages (HC153444 and/or HC156308) were differentiated
from
CD14+ monocytes and mCherry mRNA was electroporated or transfected. mCherry
mRNA
included exemplary modifications such as variant cap structures comprising
AGCapl,
m6AGCap1, or Anti-Reverse Cap Analog (ARCA) and modified nucleotides
comprising
pseudouridine (PsU), 5-methoxyuridine (5moU), or 5-
methylcytidine/pseudouridine (5meC
PsU). mRNA expression and cell viability were detected by FACS on day 1 or day
15 post-
transfection.
[0268] mCherry mRNA variants did not impact macrophage viability when
electroporated, however, macrophages transfected with mRNA ranged in viability
from 36% to
70%, depending on the variant (Figure 1A). The percentage of mCherry
expressing cells was
almost 100% in electroporated cells and 70-90% in transfected cells (not
shown). The mean
fluorescent intensity of mCherry (representing the number of mCherry proteins
expressed per
cell) was dependent on the mRNA modification as well as transfection methods
(Figure 1B).
AGCapl generated greater mCherry intensity in electroporated cells, while PsU
modification and
HPLC purification generated greater mCherry intensity in transfected cells.
[0269] As shown in Figure 1C, the persistence of mCherry RNA was
dependent on the
mRNA modifications and on the mRNA delivery method used. mCherry mRNA
persisted
longer in transfected macrophages when compared to electroporated macrophages.
mRNA
comprising an AGCapl persisted better than m6AGCap1 mRNA in electroporated
cells. HPLC
purification had a greater impact on transfected cells than on electroporated
cells.
Example 2: Effect of CAR mRNA Modifications on the Viability of Macrophages
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0270] Donor macrophages (HC153444) were differentiated from CD14+
monocytes and
the macrophages were transfected with mRNA encoding a CAR (CAR mRNA) using
electroporation or transfection. mRNA encoding a CAR comprising a HER2
extracellular
domain and a CD3zeta intracellular domain was used. CAR expression and cell
viability were
detected by FACS on day 1.
[0271] As shown in Figure 2A, exemplary viability of macrophages
electroporated with
mRNA was >80% for all mRNA modifications evaluated. Macrophages transfected
with
Viromer mRNA had a viability of >90% for most of the CAR-expressing
macrophages and
untreated macrophages, though viability was 65% for macrophages transfected
with mRNA
comprising m6AGCapland PsU modifications and mRNA comprising 5moU
modifications.
Additionally, as shown in Figure 2B, for electroporated macrophages, CAR mRNA
comprising
m6AGCapl and PsU modifications led to the highest CAR expression. For
macrophages
transfected with mRNA, CAR mRNA comprising m6AGCapl, N1mPsU, and/or PsU
modifications led to the highest CAR expression. As shown in Figure 2C, the
optimization of
the mRNA 5'Cap to an m6AGCapl and the modification of uracil to pseudouracil
(PsU) led to
an approximately 3x increase in CAR expression on human macrophages.
Example 3: Effect of CAR mRNA Modifications on Macrophage Function
[0272] Human macrophages were differentiated from CD14+ monocytes and the
macrophages were electroporated with mRNA encoding a HER2 CAR (CAR mRNA).
Fluorescent labeled HER2+ breast cancer cells (CRL2351) were co-cultured with
the CAR
macrophages two days after they were transfected with CAR mRNA at an Effector
(CAR
macrophage) to Target (cancer cell) ratio of 5:1. CRL2351 cell growth was
monitored every
four hours by its fluorescence.
[0273] As shown in Figure 3A, macrophages that had been transfected with
HER2 CAR
mRNA comprising m6AGCaplPsU modifications showed strong killing activity
comparable to
macrophages transduced with Ad5f35. Macrophages that had been transfected with
HER2 CAR
mRNA comprising m6AGCaplPsU modifications showed the best target cell killing
activity,
compared to macrophages transfected with HER2 CAR mRNA comprising 5moU
modifications
(Figures 3B-3D). These results also show that macrophages transfected with the
optimal mRNA
91
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
modification had greater antitumor activity at lower effector (CAR-M) to
target (cancer cell)
ratios.
Example 4: M1 Polarization Enhances mRNA Persistence and Macrophage/CAR-
Macrophage Function
[0274] Human macrophages were electroporated with HER2 CAR mRNA
comprising
m6AGCap1 and PsU modifications. Cells were cultured with cytokines that induce
an M1
phenotype such as IFN-alpha, IFN-beta, IFN-gamma, IFN-gamma plus
lipopolysaccharide
(LPS), TNF-alpha, IL-6 or a STING ligand (STING-L) for up to 48 hours and then
cytokines
were washed off and fresh media was added. CAR expression and M1 marker
expression were
measured on days 2 and 7 after transfection. Fluorescent labeled HER2+ breast
cancer cells
(CRL2351) were co-cultured with HER2 CAR macrophages two days after the
macrophages
were transfected. Cancer cell growth was monitored via its fluorescence on an
Incucyte live
imaging microscope every four hours. The Effector (CAR macrophage) to Target
(cancer cell)
ratio was 5:1.
[0275] As shown in Figure 4A, the interferon cytokines tested did not
lead to lowered
viability of CAR transfected macrophages on day 2. Surprisingly, macrophages
treated with
IFN-f3 demonstrated higher CAR expression than macrophages treated with
control media, IFN-
a or IFN- y (Figure 4B). As shown in Figure 4C, treatment of macrophages with
the interferon
cytokines did not lead to lowered viability of CAR transfected macrophages on
Day 7.
Surprisingly, macrophages treated with IFN-f3 demonstrated higher CAR
expression than
macrophages treated with control media, IFN- a, or IFN- y ¨ demonstrating that
IFN- 0 improves
the duration of expression of mRNA encoded transgenes, such as a CAR, in human
macrophages
(Figure 4D). Additionally, treatment of mRNA transfected CAR macrophages with
IFN-a, IFN-
(3, or IFN-y led to induction of an M1 phenotype (based on CD86 expression;
Figure 4E) and
reduction of M2 markers (based on CD163 expression; Figure 4F). IFN-f3 led to
the strongest
M1 phenotype of the interferons evaluated, and the M1 phenotype persisted for
at least 7 days
post treatment (Figure 4E).
92
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
Example 5: Effect of IFN Treatment on CAR Expression, CAR Macrophage Function,
MI
Phenotype Markers and Cytokine Production
[0276] Five different mRNA modifications were also tested to determine if
interferon
treatment differentially affected macrophages transfected with mRNA comprising
different
modifications. Human macrophages were electroporated with HER2 CAR mRNA
comprising
different mRNA modifications. CAR expression and M1 markers were detected by
Flow
Cytometry (Attune) on day four after electroporation. Day 4 CAR macrophage
cells were then
co-cultured with a Nuc-Light labeled, HER2+ breast cancer cell line (CRL2351)
at an Effector
(CAR macrophage) to Target (cancer cell) ratio of 5:1. Cancer cell growth was
monitored via its
fluorescence on an Incucyte live imaging microscope every four hours.
[0277] As shown in Figure 5A, macrophages transfected with all evaluated
chemistries
with or without IFN-f3 treatment demonstrated high viability. Macrophages
transfected with
AGCapl or m6AGCap1 and PsU or N1mPsU modified CAR mRNA demonstrated the
highest
level of expression, and IFN-f3 modestly improved the percentage of cells
expressing CAR for all
modifications on day 4 (Figure 5B). However, IFN-f3 treatment led to a marked
increase in the
number of CARs expressed per cell based on CAR1VIFI for all mRNA modifications
evaluated,
with m6AGCap1/PsU co-modified mRNA leading to the highest expression (Figure
5C).
[0278] To further evaluate the impact of IFN-f3 on CAR persistence, human
macrophages
electroporated with m6AGCap1/PsU mRNA encoding a HER2 CAR were evaluated on
day 2
and day 7. IFN-f3 treatment led to a significantly improved CAR expression
rate on day 7 as
compared to CAR macrophages not treated with IFN-f3 (Figure 6).
[0279] In order to validate the effect of IFN-f3 treatment on improved
CAR expression,
and to optimize the IFN-f3 concentration, macrophages transfected with
M6AGCap1/PsU
modified mRNA were treated with 0, 3, 10, 30, or 10Ong/mL IFN-f3 for 4 hours
and the viability,
CAR percentage, and CAR1VIFI were evaluated on day 4 and day 7 post
electroporation. A
dose-dependent effect of IFN-f3 on CAR expression by human macrophages was
observed
(Figure 7A). As described in Example 4, treatment of CAR macrophages with IFN-
f3 induced
an M1 phenotype, so further experiments were performed to determine if the
effect was dose
dependent. As shown in Figure 7B, induction of M1 markers CD80, CD86, and HLA-
DR was
IFN-f3 dose-dependent.
93
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0280] Given that macrophage phenotype is considered plastic, and
immunosuppressive
cytokines such as IL-10 are known to induce an M2 phenotype, the impact of IL-
10 treatment on
IFN-f3 treated or untreated HER2 CAR mRNA transfected macrophages was
evaluated. IFN-f3
treated CAR macrophages resisted the effects of IL-10 and did not express the
M2 marker
CD163, while instead retaining expression of the M1 marker CD86 48 hours post-
treatment
(Figure 8A) and 7 days post-treatment with IL-10 (Figure 8B). IFN-f3 primed
CAR
macrophages resisted other M2 inducing factors as well.
[0281] To evaluate the anti-tumor function of macrophages transfected
with mRNA
encoding a HER2 CAR with or without priming with IFN-f3, untransduced (UTD) or
CAR
macrophages were primed with 0, 3, 10, 30, or 100 ng/mL IFN-f3 for 4 hours or
20 hours. These
effector cells were then co-cultured with the HER2+ breast cancer cell line
CRL2351-GFP at an
effector to target ratio of 3:1 or 1.5:1 and anti-tumor activity was measured
based on GFP
expression using an Incucyte live imaging microscope. IFN-f3 priming led to an
improved ability
for CAR macrophages to kill cancer cells (Figure 9A). To evaluate whether IFN-
f3 treatment
improved CAR macrophage anti-tumor activity with mRNAs comprising
modifications, the anti-
tumor activity of macrophages electroporated with mRNA comprising unique
modifications with
or without IFN-f3 treatment was evaluated. IFN-f3 treatment led to improved
anti-tumor activity
for all CAR macrophages except those transfected with 5moU mRNA (Figure 9B).
To evaluate
whether the improved mRNA-transfected CAR macrophage anti-tumor effect was
universal to
all interferons or only interferon beta, macrophages electroporated with
M6AGCap1/PsU mRNA
were treated with IFN-alpha, beta, or gamma and evaluated for their cancer
cell killing ability.
CAR macrophages treated with IFN-f3 led to the best cancer cell killing, with
a greater effect
than IFN-a or IFN-y (Figure 9C).
[0282] To evaluate whether interferon treatment of macrophages improved
other anti-
tumor functions, the cytokine secretion of interferon treated or untreated
mRNA transfected
HER2 CAR macrophages after co-culture with HER2+ breast cancer cells was
evaluated.
Human macrophages were electroporated with 150nM HER2 CAR mRNA comprising
m6AGCap1 and PsU modifications. HER2+ breast cancer cells (CRL2351) were co-
cultured
with CAR macrophages after the macrophages were transfected with the CAR mRNA
at an
Effector (CAR macrophage) to Target (cancer cell) ratio of 3:1. Supernatant
was collected 48
94
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
hours after the cancer cells and macrophages were co-cultured and cytokine
levels were
measured using a Meso Scale Discovery (MSD) instrument. As shown in Figure 10,
treatment
of macrophages with IFN-a, IFN-0, or IFN-y led to increased secretion of the
cytokines IL-6, IL-
8 and TNFa from the macrophages.
[0283] Additional studies were performed to determine if treatment with
interferons
could further improve CAR mRNA persistence in macrophages and if CAR
macrophage
functionality could be extended. Human macrophages were electroporated with
300nM CAR
mRNA comprising m6AGCap1 and PsU modifications. Cells were cultured with
20ng/mL IFNs
for 24 hours and then the cells were washed to remove cytokines. CAR
expression was detected
by Flow Cytometry (Attune) on day 2 after transfection. Cells were then co-
cultured with a Nuc-
Light labeled, HER2+ breast cancer cell line (CRL2351) at the Effector to
Target ratio of 3:1.
Cancer cell growth was monitored via its fluorescence on an Incucyte live
imaging microscope.
CAR expression and M1 markers in macrophages were detected by Flow Cytometry
(Attune) on
day 7 after transfection.
[0284] As shown in Figure 11A, two days after transfection with CAR mRNA,
CAR
macrophage viability and CAR expression were very high except in macrophages
that had been
treated with IFN-y. Additionally, as shown in Figure 11B and Figure 11C, IFN
treatment
enhanced the target cell killing activity of CAR macrophages. Figure 11C shows
target cell
killing after cancer cells and macrophages had been co-cultured for 72 hours.
Treatment with
IFN also impacted macrophage viability, CAR expression, M1 marker expression,
and CAR
macrophage functionality. As shown in Figure 12A, treatment of transfected
macrophages with
IFN-0 lead to increased cell viability, HER2 CAR expression and expression of
M1 markers
CD80, CD86 and HLA-DR relative to macrophages that hadn't been treated with an
interferon at
the later day 7 timepoint. Figure 12A shows that all macrophages had high
viability at day 7,
but IFN-0 treated macrophages expressed the highest level of CAR by a
significant margin.
Figure 12B shows that out of all CAR macrophages that were tested in a cancer
cell killing
assay 7 days post-electroporation, those treated with IFN-0 led to the highest
level of cancer
killing (greatest decrease in tumor growth). Seven days after macrophages were
electroporated,
they were co-cultured with target cancer cells for 72 hours. As shown in
Figure 12C, all
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
interferons improved the cancer cell killing activity of CAR macrophages
compared to CAR
macrophages that weren't treated with an interferon.
Example 6: Transfected Macrophages are Sensitive to IFNy
[0285] Human macrophages were transfected with mCherry mRNA comprising
m6AGCap1 and PsU or N1mPsU modifications and were then cultured for one day
with
different doses of IFN-y. mCherry expression was monitored on an Incucyte live
imaging
microscope. As shown in Figure 13A, IFN-y reduced mCherry mRNA expression when
macrophages were transfected with mRNA.
[0286] It has been previously shown that in vitro transcribed mRNA is
recognized by
various endosomal innate immune receptors (e.g., Toll-like receptor (TLR) 3,
TLR7 and TLR8)
and cytoplasmic innate immune receptors (protein kinase RNA-activated (PKR),
retinoic acid-
inducible gene I protein (RIG-I), melanoma differentiation-associated protein
5 (MDA5) and 2'-
5'-oligoadenylate synthase (OAS)). Signaling through these different pathways
results in
inflammation associated with type 1 interferon (IFN), tumor necrosis factor
(TNF), interleukin-6
(IL-6), IL-12 and the activation of cascades of transcriptional programs.
Overall, these create a
pro-inflammatory microenvironment poised for inducing specific immune
responses. Moreover,
downstream effects such as decreased translation by eukaryotic translation
initiation factor 2a
(eIF2a) phosphorylation, enhanced RNA degradation by ribonuclease L (RNaseL),
overexpression and inhibition of self-amplifying mRNA replication are all of
relevance for the
pharmacokinetics and pharmacodynamics of IVT mRNA.
[0287] Activation of IFN-y via the TLR pathways can activate 2'-5'-
oligoadenylate
synthase (OAS), which produces 2'-5'-oligoadenylate (2-5A), which in turn can
activate RNaseL,
leading to RNA degradation and apoptosis.
Example 7: Effect of RNaseL Inhibitors, Sunitinib and ABCE1, on CAR
Macrophages
[0288] In order to determine whether an RNaseL inhibitor could rescue IFN-
y-induced
instability of transfected mRNA, human macrophages were treated with 111M
sunitinib (an
RNaseL inhibitor) two hours prior to the transfection of mCherry mRNA
comprising
96
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
m6AGCap1 and PsU modifications. Transfected cells were then cultured for one
day with
different dosages of IFN-y. mCherry expression was monitored on an Incucyte
live imaging
microscope. As shown in Figure 14A, sunitinib rescued the IFN-y-induced
degradation of
mRNA.
[0289] In order to evaluate if RNAse L inhibition can improve the anti-
tumor function of
mRNA-transfected CAR macrophages, macrophages were transfected with mRNA
comprising
modifications and pre-treated with sunitinib prior to being evaluated as
effector cells in a cancer
cell killing assay. CAR macrophage pre-treated with 1nM sunitinib led to
higher cancer cell
killing than CAR macrophages not pre-treated with sunitinib or the
untransfected control
macrophages that were treated or untreated with sunitinib (Figure 14B). The
improved cancer
killing ability of sunitinib-primed CAR macrophages in a 48 hour CRL2351
breast cancer cell
killing assay is shown in Figure 14C.
[0290] The effect of another RNaseL inhibitor (RU I or ABCE1) was also
tested to further
validate the concept. Human macrophages were co-transfected with mRNA encoding
mCherry
comprising m6AGCap 1 and PsU modifications and with mRNA encoding ABCE1.
[0291] As shown in Figure 15, ABCE1 co-expression significantly improved
the
expression of the mRNA-encoded transgene of interest 48 hours post-
electroporation. The
viability of macrophages co-transfected with ABCE1 was not impacted and
remained high.
ABCE1 co-transfection increased mCherry expression by roughly 2-fold, and pre-
treating with
sunitinib further enhanced this effect.
Example 8: Effect of Genetically Encoded RNaseL Inhibitor on CAR macrophages
[0292] In order to evaluate if other genetically encoded RNaseL
inhibitors could improve
CAR expression, the co-transfection of CAR mRNA comprising modifications and
mRNA
encoding NS1 was evaluated. NS1 is a gene derived from Influenza A that
encodes the NS1A
protein. As shown in Figure 16, co-transfection of CAR mRNA with ABCE1 or CAR
mRNA
with NS1 increased the level of CAR expression when compared to co-
transfection of CAR with
the control gene mCherry. NS1 co-transfection led to higher CAR expression
than ABCE1 co-
transfection.
97
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0293] To evaluate the mechanism by which ABCE1 and/or NS1 impact CAR
expression, the decay of electroporated mRNA encoding a HER2 CAR comprising
modifications
was evaluated by RT-qPCR. The relative abundance of CAR mRNA in macrophages
transfected
with CAR plus mCherry, CAR plus ABCE1, or CAR plus NS1 mRNA at 8 hours was
compared
to the CAR mRNA abundance at 2 hours. As shown in Figure 17, levels of CAR
mRNA co-
transfected with mCherry (a negative control) dropped by more than 50% in 6
hours, while levels
of CAR mRNA co-transfected with ABCE1 or NS1 mRNA continued to rise.
Example 9: Priming of Macrophages
[0294] To generate Her2-Zeta CAR expressing macrophages, primary human
macrophages were suspended in EP buffer (MaxCyte) containing 300nM mRNA
(TriLink) at a
concentration of 90x106 cells/mL. 100 L of cell mixture was added to an
electroporation
cassette (0C100x2; MaxCyte) and electroporated using the Experimental T cell 1
setting. Cells
were removed from the cassette, plated in 3mL of TexMACS media (Miltenyi
Biotech)
containing 20% FBS (Gibco) on UpCell plates (Thermo Scientific), and incubated
overnight at
37 C and 5% CO2.
[0295] Recombinant CD40 ligand (Peprotech), 4-1BB ligand (Enzo Life
Sciences), and
4-1BB receptor (Peprotech) were re-suspended in molecular grade water to a
stock concentration
of 100 g/mL. Stock solution was then used to create working solutions in PBS
ranging from 2 ¨0.002 g/mL. 1004, of working solution was added to wells in a
96-well plate and left at room
temperature for 4 hours.
[0296] Plates were removed from the incubator and left at room
temperature for 30
minutes. Cells were detached from plate, counted using the NC-200 automated
cell counter
(Chemomtech), and re-suspended in TexMACS media with 10% FBS. Protein coated
plates
were washed 2x with PBS, followed by addition of macrophages in a final volume
of 1004,
TexMACS media with 10% FBS. Plates were incubated for 3 h at 37 C and 5% CO2.
After 3
hours, 10,000 CRL-2351 cells expressing nuclear GFP were added to each well.
Final
concentration of GM-CSF was lOng/mL in all wells. Cell lysis was detected
using the Incucyte
(Essen Bioscience). Tumor cell death was calculated by integrated GFP
intensity per well
relative to time 0.
98
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
[0297] For detection of cell surface proteins, macrophages were plated
onto wells coated
with agonist molecules in a final volume of 2004, TexMACS media + 10% FBS +
lOng/mL
GM-CSF and incubated for 3 days at 37 C and 5% CO2. Cells were incubated in
300 L
Accutase (Sigma) for 30min and transferred to 96-well round bottom plate for
staining. Cells
were incubated in FACS buffer containing 20 g/mL Her2-His for 20 min at RT,
followed by
incubation in Human TruStain FcX for 10 min at RT. Surface protein staining
was done using
the following panel: CD8O-FITC, CD86-PE, CD163-APC-Cy7, CD206-BV421, anti-His-
APC,
Aqua Live/Dead. Detection of surface protein expression was completed using
the Attune NxT
flow cytometer (Thermo Fischer).
[0298] Treatment of CAR macrophages with CD4OL significantly improved the
tumor
killing ability of macrophages and CAR macrophages (Figure 18A). Priming with
CD4OL also
induced an M1 phenotype in macrophages transfected with CAR mRNA (Figure 18B).
Treatment with 4-1BB and 4-1BBL caused similar results, although less potent
than CD4OL
(Figures 19A-19B and Figures 20A-B). These results indicate that pre-treatment
or priming of
CAR macrophages with CD40 agonists, such as CD4OL, may result in increased
efficacy and
that a combination therapy comprising CAR macrophages with CD40 agonists may
have
increased efficacy.
Example 10: Effect of mRNA Modifications on Human Monocytes
[0299] The delivery of CAR mRNA comprising modifications to human
monocytes was
also evaluated. Specifically, mRNA encoding a HER2 CAR comprising M6AGCap1 and
PsU
modifications was electroporated into monocytes derived from five human
donors. As shown in
Figure 21, high CAR expression was achieved both in terms of intensity and
percentage.
Example 11: Efficacy of CAR-Macrophages Generated via mRNA Electroporation in
Xenograft Solid Tumor Mouse Model
[0300] In order to evaluate the efficacy of CAR-macrophages generated by
mRNA
electroporation in a xenograft solid tumor mouse model, five groups were used.
The study
groups included an untreated group, a mock treated group, a mock treated group
with IFN-f3, an
99
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
mRNA CAR-macrophage (mRNA-CAR) treated group and an mRNA CAR-macrophage with
IFN-0 (mRNA-CAR + IFNb) treated group.
[0301] NSGS mice were injected with 6e5 SKOV3 cells (n=5 mice per group).
Mice
were subsequently treated with 8e6 macrophages via intraperitoneal injection
on day 0, 4, and 8
as shown by arrows in Figure 22. Tumor burden was measured by bioluminescent
imaging
using an IVIS imaging system (Perkin Elmer). Mice were imaged every 2-3 days.
[0302] As shown in Figure 22, mice treated with mRNA CAR-macrophages or
IFN-0-
primed mRNA CAR-macrophages demonstrated suppressed tumor growth as compared
to
controls.
Example 12: Efficacy of CAR-Macrophages Generated via mRNA Electroporation in
Syngeneic Solid Tumor Mouse Model
[0303] In order to evaluate the efficacy of CAR-macrophages generated by
mRNA
electroporation in a syngeneic solid tumor mouse model, five groups were used.
The study
groups included an untreated group, a mock treated group, a mock treated group
with IFN-0, an
mRNA CAR-macrophage (mRNA CAR) treated group and an mRNA CAR-macrophage with
IFN-0 (mRNA CAR + IFNb) treated group.
[0304] BALB/c mice were subcutaneously injected with 7.5e5 CT26-HER2 colon
cancer
cells (n=12-13 mice per group). 12 days after tumor injection (once average
tumor mass was 50
mm3), approximately 3e6 macrophages were injected intratumorally. Additional
macrophages
were injected on day16 and day 20 (approximately 3e6 per mouse per injection,
shown by arrows
in Figure 23). Tumor volumes were measured using calipers bi-weekly and tumor
volume was
estimated using the following formula: Tumor volume = length x width^2/2,
where length
represents the largest tumor diameter and width represents the perpendicular
tumor diameter.
[0305] As shown in Figure 23, nearly 80% of mice that received IFN-0
treated mRNA
CAR-macrophages rejected their tumors while around 60% of mice with mock, IFN-
0 treated
mock, or mRNA CAR- macrophage rejected tumors. IFN-0-primed mRNA CAR-
macrophages
significantly suppressed tumor growth as compared to negative controls.
Notably, in the context
of an immunocompetent mouse model, IFN-0-primed mRNA CAR-macrophages
outperformed
100
CA 03187138 2022-12-13
WO 2021/263152
PCT/US2021/039168
mRNA CAR-macrophages or IFN-P-primed control macrophages, demonstrating the
synergy
between CAR engineering and IFN-f3 priming.
101
CA 03187138 2022-12-13
WO 2021/263152 PCT/US2021/039168
EQUIVALENTS
[0306] It is to be appreciated by those skilled in the art that various
alterations,
modifications, and improvements to the present disclosure will readily occur
to those skilled in
the art. Such alterations, modifications, and improvements are intended to be
part of the present
disclosure, and are intended to be within the spirit and scope of the
invention. Accordingly, the
foregoing description and drawing are by way of example only and any invention
described in
the present disclosure if further described in detail by the claims that
follow.
[0307] Those skilled in the art will appreciate typical standards of
deviation or error
attributable to values obtained in assays or other processes as described
herein. The publications,
websites and other reference materials referenced herein to describe the
background of the
invention and to provide additional detail regarding its practice are hereby
incorporated by
reference in their entireties.
102