Note: Descriptions are shown in the official language in which they were submitted.
CA. 03191576 2023-02-10
-==1
=
DESCRIPTION
IRON ORE PELLETS AND METHOD FOR PRODUCING IRON ORE PELLETS
[TECHNICAL FIELD]
[0001]
The present invention relates to iron ore pellets and a method for producing
iron ore
pellets.
[BACKGROUND ART]
[0002]
As a blast furnace operation, a method is well-known in which pig iron is
produced
by: alternately stacking, in a blast furnace, a first layer containing an iron
ore raw material,
and a second layer containing coke; and injecting an auxiliary fuel into the
blast furnace from
a tuyere and melting the iron ore raw material by using resulting hot blasts.
In this method
for producing pig iron, the iron ore raw material, being supplied as iron ore
pellets, is reduced,
whereby the pig iron is produced. At this time, the coke mainly serves as a
spacer to secure
gas permeability.
[0003]
As a technique for improving reducibility of this iron ore raw material, iron
ore
pellets in which a volume of pores having a diameter of greater than or equal
to 10 inn is
greater than or equal to 0.01 cm3/g has been proposed (see Japanese Unexamined
Patent
Application, Publication No. S63-219534). With regard to these iron ore
pellets, by
controlling the volume of comparatively large pores , a decrease in crushing
strength is
inhibited and closure of the pores is prevented, whereby overall porosity is
increased.
[PRIOR ART DOCUMENTS]
[PATENT DOCUMENTS]
[0004]
Patent Document 1: Japanese Unexamined Patent Application, Publication No.
S63-219534
[SUMMARY OF THE INVENTION]
[PROBLEMS TO BE SOLVED BY THE INVENTION]
[0005]
In blast furnace operations, due to recent demands to decrease CO2, there is a
requirement for further decreasing coke consumption. To respond to this, a
method in which
the reducibility of the iron ore raw material is improved by increasing pores
to enlarge a
surface area thereof is conceivable. However, in the case of the above-
described
conventional iron ore pellets, since the porosity is controlled by the pores
having the
comparatively large diameter, it is difficult to enlarge the surface area per
unit weight of the
1
CA, 03191576 2023-02-10
iron ore pellets, even if the porosity is increased. In order to improve the
reducibility, it is
necessary to greatly increase a volume of the pores having the diameter of
greater than or
equal to 10 gm. In this case, the crushing strength of the iron ore pellets
tends to decrease.
Since the iron ore pellets are easily pulverized in the blast furnace when the
crushing strength
decreases, gas permeation resistance in the blast furnace increases, thereby
increasing the risk
of hindering stable operation of the blast furnace.
[0006]
In other words, with the conventional iron ore pellets, it is difficult to
further improve
the reducibility; thus, in order to further decrease the coke consumption,
iron ore pellets
having novel characteristic(s) are required.
[0007]
The present invention was made in view of the foregoing circumstances, and an
object of the present invention is to provide iron ore pellets having a
characteristic of enabling
a further decrease in the coke consumption in a blast furnace operation.
[MEANS FOR SOLVING THE PROBLEMS]
[0008]
As described above, in order to improve the reducibility of the iron ore
pellets, it is
necessary to increase the surface area per unit weight of the iron ore
pellets, while inhibiting a
decrease in the crushing strength. As a result of thorough investigation, the
present
inventors found that if porosity which results from large open pores having a
pore size of
greater than or equal to 4 gm is controlled, iron ore pellets having improved
reducibility can
be produced, thereby completing the present invention.
[0009]
More specifically, iron ore pellets according to one aspect of the present
invention
are iron ore pellets for use in a blast furnace operation, wherein a porosity
of the iron ore
pellets which results from large open pores having a pore size of greater than
or equal to 4 gm
is greater than or equal to 21%, and the iron ore pellets have a crushing
strength of greater
than or equal to 180 kg/P.
[0010]
The porosity of the iron ore pellets which results from the large open pores
having
the pore size of greater than or equal to 4 gm is set to greater than or equal
to the lower limit.
Only the open pores, which connect to an exterior of the pellets, contribute
to enlarging the
surface area of the iron ore pellets; thus, controlling the porosity which
results from these
open pores enables directly enlarging the surface area per unit weight of the
iron ore pellets,
which actually contributes to a reaction. Furthermore, due to the crushing
strength being
greater than or equal to the lower limit, the iron ore pellets are not easily
pulverized in the
blast furnace during the blast furnace operation. Thus, the iron ore pellets
are superior in
2
CA 03191576 2023-02-10
reducibility, consequently enabling further decreasing the coke consumption in
the blast
furnace operation.
[0011]
As referred to herein, the "porosity which results from the large open pores
having
the pore size of greater than or equal to 4 p.m" means an amount calculated in
accordance with
Co x A+4 / A [%],
wherein: co [%] denotes an open porosity, determined by using a mercury
intrusion
porosimeter (for example, "Autopore III 9400", manufactured by Shimadzu
Corporation); A
[cm3/g] denotes a total pore capacity per unit weight of the iron ore pellets;
and A+4 [cm3/g]
denotes a total pore capacity of pores having a pore size of greater than or
equal to 4 gm per
unit weight of the iron ore pellets. It is to be noted that the open porosity
means a proportion
accounted for by a volume of total open pores with respect to an apparent
volume of the iron
ore pellets.
[0012]
A content of fines having a grain size of less than or equal to 4.7 gm is
preferably
greater than or equal to 8% by mass. When the content of the fines having the
grain size of
less than or equal to 4.7 gm is greater than or equal to the lower limit, the
crushing strength
can be increased while improving the porosity which results from the large
open pores having
the pore size of greater than or equal to 4 p.m.
[0013]
The iron ore pellets preferably have an aggregate structure of fines. When the
iron
ore pellets thus have the aggregate structure of the fines, the crushing
strength can be
increased while improving the porosity which results from the large open pores
having the
pore size of greater than or equal to 4 gm. As referred to herein, the
"aggregate structure"
means a state in which a plurality of grains of dispersed fines gather to form
secondary
particles, and specifically means a state in which greater than or equal to 5,
and preferably
greater than or equal to 10 grains of the fines are in contact with each
other.
[0014]
A method for producing iron ore pellets according to another aspect of the
present
invention includes: a step of balling green pellets by adding, to an iron ore
raw material, water
for use in the balling; and a step of firing the green pellets, wherein a
viscosity of the water is
greater than or equal to 15 mPa.s.
[0015]
In the method for producing iron ore pellets, since the viscosity of the water
at the
time of balling the green pellets is greater than or equal to the lower limit,
the iron ore pellets
can be easily produced having: the porosity which results from the large open
pores having
the pore size of greater than or equal to 4 gm being greater than or equal to
21%; and the
crushing strength which is greater than or equal to 180 kg/P.
3
CA 03191576 2023-02-10
*-1 = -1
[0016]
As referred to herein, the "viscosity" means a value measured in accordance
with
JIS-Z8803: 2011 by using a rotary viscometer.
[0017]
The water preferably contains an organic binder, and a content of the organic
binder
in the green pellets is preferably greater than or equal to 0.01% by mass and
less than or equal
to 1.0% by mass. When the organic binder is thus contained in the water in a
content falling
within the above range, the aggregate structure of the fines can be formed in
the iron ore
pellets to be produced. Accordingly, the crushing strength of the iron ore
pellets can be
increased while improving the porosity of the iron ore pellets which results
from the large
open pores having the pore size of greater than or equal to 4 gm.
[EFFECTS OF THE INVENTION]
[0018]
As described above, the iron ore pellets of the present invention have the
characteristic of enabling further decreasing the coke consumption in the
blast furnace
operation. Furthermore, by carrying out the blast furnace operation using the
iron ore pellets
produced by using the method for producing iron ore pellets of the present
invention, the coke
consumption can be further decreased.
[BRIEF DESCRIPTION OF THE DRAWINGS]
[0019]
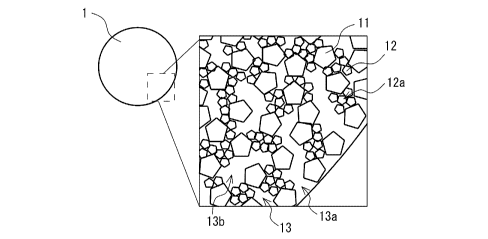
FIG. 1 is a schematic plan view and a partially enlarged cross sectional view
illustrating iron ore pellets according to one embodiment of the present
invention.
FIG. 2 is a flow chart illustrating a method for producing iron ore pellets
according to
an other embodiment of the present invention.
FIG. 3 is a schematic view illustrating a structure of a production apparatus
used in
the method for producing the iron ore pellets illustrated in FIG. 2.
FIG. 4 is a graph illustrating a relationship between crushing strength, and a
porosity
which results from large open pores having a pore size of greater than or
equal to 4 gm in
EXAMPLES.
FIG. 5 is a schematic cross sectional view illustrating a structure of a
furnace for a
large-scale reduction under load test, used for investigating reduction
percentages in
EXAMPLES.
FIG. 6 is a graph illustrating a temperature profile for heating a sample-
packed bed at
the time of investigating the reduction percentages in EXAMPLES.
FIG. 7 is a graph illustrating a relationship between a temperature of the
sample-packed bed, and a flow rate of gas supplied.
4
CA 03191576 2023-02-10
e
¨e
FIG. 8 is a graph illustrating a relationship between the reduction
percentage, and the
porosity which results from the large open pores having the pore size of
greater than or equal
to 4 gm in EXAMPLES.
[DESCRIPTION OF EMBODIMENTS]
[0020]
Hereinafter, the iron ore pellets according to the one embodiment of the
present
invention, and the method for producing iron ore pellets according to the
other embodiment of
the present invention are described.
[0021]
Iron Ore Pellets
Iron ore pellets 1 shown in FIG. 1 are iron ore pellets for use in a blast
furnace
operation. Iron ore pellets are a product which is obtained from a pellet
feed, iron ore fines,
and auxiliary material(s) as needed, and is made with characteristics suitable
for a blast
furnace (for example, size, strength, reducibility, and the like) in order to
improve quality.
[0022]
As shown in FIG. 1, the iron ore pellets 1 are constituted mainly from coarse
grains
11, serving as the pellet feed, and fines 12, being a pulverized raw material
of iron ore, and
numerous pores 13 are formed in an interior thereof. As described above, the
iron ore pellets
1 may contain the auxiliary material(s). Examples of such auxiliary
material(s) include
limestone, dolomite, and the like.
[0023]
A size of the iron ore pellets 1 is appropriately decided in accordance with,
e.g., the
blast furnace to be used, and for example, a grain size may be greater than or
equal to 10 mm
and less than or equal to 25 mm.
[0024]
As the coarse grains 11, for example, coarse grains prepared from a blend of
one or a
plurality of brands of fine-grain pellet feed may be used. The coarse grains
11 are grains
having a grain size of greater than or equal to 45 gm, and it is preferred
that coarse grains
having a grain size of less than or equal to 0.5 mm account for greater than
or equal to 90%
by mass of a total of the coarse grains 11. When the proportion of the coarse
grains 11
accounted for by the coarse grains having a grain size of less than or equal
to 0.5 mm is less
than the lower limit, a surface area may be insufficient, whereby the
reducibility at the time of
the blast furnace operation may decrease.
[0025]
The fines 12 which may be used are, for example, fines prepared by
pulverizing, with
a pulverizer, the pellet feed for use as the coarse grains 11. The fines 12
are grains having a
grain size of less than 45 gm, and of these, the lower limit of a content of
the fines 12 having
a grain size of less than or equal to 4.7 gm is, with respect to the total of
the iron ore pellets 1,
CA 03191576 2023-02-10
preferably 8% by mass, more preferably 10% by mass, and still more preferably
20% by mass.
When the content of the fines 12 having the grain size of less than or equal
to 4.7 pm is
greater than or equal to the lower limit, the crushing strength can be
increased while
improving the porosity which results from the large open pores having the pore
size of greater
than or equal to 4 pm. On the other hand, the upper limit of the content of
the fines 12
having the grain size of less than or equal to 4.7 pm is not particularly
limited, and may be,
for example, 50% by mass.
[0026]
The iron ore pellets 1 preferably have an aggregate structure 12a of the fines
12. As
shown in FIG. 1, in the iron ore pellets 1, a plurality of grains of the fines
12 gather to come
in contact with each other, forming secondary particles. In other words, in
the iron ore
pellets 1, there are regions in which a density of the fines 12 is higher than
elsewhere. When
the fines 12 thus have the aggregate structure 12a, strength of this
aggregated site increases,
whereby the crushing strength of the iron ore pellets 1 improves. On the other
hand, due to
the aggregating, the fines 12 become localized and regions in which the fines
12 are not
present are also localized, whereby a volume of one pore 13, described later,
tends to increase.
Consequently, the number of open pores 13a having a large pore size increases.
Therefore,
when the iron ore pellets 1 thus have the aggregate structure 12a of the fines
12, the crushing
strength can be increased while improving the porosity which results from the
large open
pores having the pore size of greater than or equal to 4 pm.
[0027]
There are two types of the pores 13, being: open pores 13a which connect to an
exterior of the iron ore pellets 1; and closed pores 13b which are confined in
the interior of the
pellets. In other words, as shown in the enlarged cross sectional view of FIG.
1, while a part
of the open pores 13a comes in contact with a surface of the iron ore pellets
1, the closed
pores 13b are enclosed by the coarse grains 11 and the fines 12. Generally,
the porosity is
decided based on a volume ratio of the total pores 13, being a total of the
open pores 13a and
the closed pores 13b, but the porosity which results from the open pores 13a
is important in
order to improve the reducibility of the iron ore raw materials since, of the
pores 13 of the
iron ore pellets 1, only the open pores 13a come in contact with a reducing
gas in the blast
furnace.
[0028]
Furthermore, in a case in which the porosity is at a certain level, a surface
area of the
iron ore pellets 1 increases as the pore size of the open pores 13a decreases.
However, when
the pore size of the open pores 13a is small, dispersion of the reducing gas
in the interior of
the open pores 13a may be difficult. Thus, it is considered necessary for the
pore size of the
open pores 13a to be greater than or equal to the certain level. On the other
hand, when the
porosity increases, the crushing strength of the ire ore pellets 1 decreases,
which may lead to a
6
CA 03191576 2023-02-10
disadvantage in which pulverization tends to occur in the blast furnace.
[0029]
As a result of thorough investigation, the present inventors found that if the
porosity
which results from the large open pores 13a having the pore size of greater
than or equal to 4
gm is controlled, the reducibility of the iron ore pellets 1 can be improved.
In other words,
the lower limit of the porosity which results from the large open pores 13a
having the pore
size of greater than or equal to 4 gm is 21%, more preferably 23%, and still
more preferably
25%. When the porosity which results from the open pores 13a is less than the
lower limit,
improvement of the reducibility of the iron ore pellets 1 may be insufficient,
whereby
sufficiently decreasing the coke consumption in the blast furnace operation
may fail.
[0030]
On the other hand, since increased porosity results in decreased crushing
strength, the
upper limit of the porosity which results from the open pores 13a is set to
fall within a range
not being below a certain value. The lower limit of this crushing strength is
180 kg/P, more
preferably 190 kg/P, and still more preferably 200 kg/P. When the crushing
strength is less
than the lower limit, the iron ore pellets 1 may be easily pulverized in the
blast furnace,
whereby the blast furnace operation may be difficult.
[0031]
The lower limit of a total open pore volume of the large open pores 13a having
the
pore size of greater than or equal to 4 tm is preferably 0.06 cm3/g, and more
preferably 0.07
cm3/g. When the total open pore volume is greater than or equal to the lower
limit, the
reducibility of the iron ore pellets 1 can be improved.
[0032]
Furthermore, an open pore size leading to a maximum change percentage of the
open
pore volume is preferably greater than or equal to 7 gm, and more preferably
greater than or
equal to 8 gm. When the open pore size is greater than or equal to the lower
limit, the
reducibility of the iron ore pellets 1 can be improved.
[0033]
Advantages
The porosity of the iron ore pellets 1 which results from the large open pores
13a
having the pore size of greater than or equal to 4 gm is set to greater than
or equal to 21%.
Since only the open pores 13a, which connect to the exterior of the pellets,
contribute to
enlarging the surface area of the iron ore pellets 1, directly enlarging the
surface area per unit
weight of the iron ore pellets 1, which actually contributes to the reaction,
is enabled by
controlling the porosity which results from these open pores 13a. Furthermore,
due to the
crushing strength being greater than or equal to 180 kg/P, the iron ore
pellets 1 are resistant to
pulverization in the blast furnace during the blast furnace operation. Thus,
the iron ore
7
CA 03191576 2023-02-10
pellets 1 are superior in reducibility, consequently enabling further
decreasing the coke
consumption in the blast furnace operation.
[0034]
Method for Producing Iron Ore Pellets
The method for producing iron ore pellets shown in FIG. 2 includes: a balling
step
Si; a firing step S2; and a cooling step S3, and enables producing the iron
ore pellets 1 of the
present invention, shown in FIG. 1. The method for producing iron ore pellets
may be
carried out by using, for example, a production apparatus with a grate kiln
system (hereinafter,
may be also merely referred to as "production apparatus 2"), shown in FIG. 3.
The
production apparatus 2 includes: a pan pelletizer 3; a traveling grate furnace
4; a kiln 5; and
an annular cooler 6.
[0035]
Balling Step
In the balling step Si, green pellets P are balled by adding water for use in
the balling
to an iron ore raw material. Specifically, the water is added to the iron ore,
and then this
water-containing iron ore is charged into the pan pelletizer 3, serving as the
pelletizer, and
rolled to produce the green pellets P, having a ball shape.
[0036]
The iron ore is constituted from the coarse grains 11 and the fines 12 which
constitute the iron ore pellets I. Although surface characteristics of the
iron ore vary greatly
depending upon a mining region and a pulverizing/transporting method, the
surface
characteristics of the iron ore are not particularly limited in the present
method for producing
iron ore pellets.
[0037]
The water constitutes bridges between particles of the iron ore. Strength of
the
green pellets P balled in the balling step Si is maintained due to an adhesion
force acting
between the particles, resulting from this bridging. In other words, a bond
between the
particles is expressed by means of surface tension of the water between the
particles, and the
adhesion force between the particles is ensured by a value obtained by
multiplying the surface
tension by the number of points of contact between the particles.
[0038]
In the method for producing iron ore pellets, the lower limit of a viscosity
of the
water is 15 mPa.s, more preferably 30 mPa.s, and still more preferably 100
mPa.s. When
the viscosity of the water is less than the lower limit, the crushing strength
of the iron ore
pellets 1 to be produced may be insufficient. On the other hand, the upper
limit of the
viscosity of the water is not particularly limited, and may be, for example,
10,000 mPa.s.
[0039]
The water preferably contains an organic binder. As the organic binder, an
organic
8
CA 03191576 2023-02-10
v
binder having a molecular weight of preferably greater than or equal to 104
and less than or
equal to 108, and more preferably a substance having a molecular weight of
greater than or
equal to 104 and less than or equal to 106 is used, and in particular,
examples thereof include
cornstarch, tapioca, potato, guar beans, and the like.
[0040]
It is to be noted that with regard to blending of this organic binder, in a
case in which
the iron ore has sufficiently retained water, the organic binder alone is
preferably added in
accordance with an amount of the retained water. Conversely, in a case in
which the iron ore
has not sufficiently retained water, the water is preferably added in a state
of being brought to
a desired viscosity by blending the organic binder with water. In an
intermediate case, the
amount of water retained in the iron ore is considered, and a blending amount
of the organic
binder is decided such that the viscosity of the water added has the desired
viscosity. In this
case, the blending of the organic binder may be carried out with respect to
moisture retained
by the iron ore. In other words, the adding of water to the iron ore, and the
blending of the
organic binder may be carried out concurrently.
[0041]
The lower limit of a content of the organic binder in the green pellets P is
preferably
0.01% by mass, and more preferably 0.1% by mass. On the other hand, the upper
limit of
the content of the organic binder is preferably 1.0% by mass, and more
preferably 0.2% by
mass. When the content of the organic binder is less than the lower limit, the
aggregate
structure 12a of the fines 12 may not be sufficiently formed in the iron ore
pellets 1 to be
produced, whereby the crushing strength may be insufficient. On the other
hand, when the
content of the organic binder is greater than the upper limit, the porosity of
the iron ore pellets
1 which results from the large open pores having the pore size of greater than
or equal to 4 1..tm
may increase and tend toward saturation, whereby the effects may be
insufficient with respect
to a rise in raw material cost.
[0042]
The lower limit of a content of moisture in the green pellets P is preferably
7.0% by
mass, and more preferably 8.0% by mass. On the other hand, the upper limit of
the content
of moisture is preferably 11.0% by mass, and more preferably 10.0% by mass.
When the
content of moisture is less than the lower limit, the bridge structure,
resulting from water,
between the particles of the iron ore may be insufficient, whereby the
crushing strength may
be insufficient. Conversely, when the content of moisture is greater than the
upper limit, the
porosity of the iron ore pellets 1 which results from the large open pores
having the pore size
of greater than or equal to 4 gm may not sufficiently increase.
[0043]
Firing Step
In the firing step S2, the green pellets P are fired. In the firing step S2,
the traveling
9
CA 03191576 2023-02-10
grate furnace 4 and the kiln 5 are used.
[0044]
Traveling grate furnace
As shown in FIG. 3, the traveling grate furnace 4 has: a traveling grate 41; a
drying
chamber 42; a dehydrating chamber 43: and a preheating chamber 44.
[0045]
The traveling grate 41 is configured to be endless, and the green pellets P
placed on
this traveling grate 41 can be transferred to the drying chamber 42, the
dehydrating chamber
43, and the preheating chamber 44, in this order.
[0046]
In the drying chamber 42, the dehydrating chamber 43, and the preheating
chamber
44, the green pellets P are subjected to: drying by blowing a heating gas G1
downward;
dehydrating; and preheating, whereby preheated pellets H are obtained having
strength,
imparted to the green pellets P, sufficient to resist the rotation in the kiln
5.
[0047]
Specifically, the following procedure is followed. First, in the drying
chamber 42,
the green pellets P are dried at an atmospheric temperature of about 250 C.
Next, in the
dehydrating chamber 43, the green pellets P after the drying are heated to
about 450 C in
order to mainly decompose and remove combined water in the iron ore.
Furthermore, in the
preheating chamber 44, the green pellets P are heated to about 1,100 C,
whereby carbonate
contained in limestone, dolomite, and/or the like is degraded to remove carbon
dioxide, and
magnetite in the iron ore is oxidized. Accordingly, the preheated pellets H
are obtained.
[0048]
As shown in FIG. 3, the heating gas GI used in the dehydrating chamber 43 is
reused
as the heating gas G1 in the drying chamber 42. Similarly, the heating gas G1
in the
preheating chamber 44 is reused as the heating gas G1 in the dehydrating
chamber 43, and a
combustion exhaust gas G2 used in the kiln 5 is reused as the heating gas G1
in the preheating
chamber 44. By thus reusing the heating gas G 1, which is on the downstream
side and has a
high temperature, and the combustion exhaust gas G2, heating cost of the
heating gas G1 can
be decreased. It is to be noted that burner(s) may be provided in each chamber
to control the
temperature of the heating gas G1. In FIG. 3, burners 45 are provided in the
dehydrating
chamber 43 and the preheating chamber 44. Furthermore, the heating gas GI used
in the
drying chamber 42 is finally discharged from a smokestack C.
[0049]
Kiln
The kiln 5 is directly connected to the traveling grate furnace 4, and is a
rotary
furnace having a sloped cylindrical shape. The kiln 5 fires the preheated
pellets H which are
discharged from the preheating chamber 44 of the traveling grate furnace 4.
Specifically, the
CA 03191576 2023-02-10
=
preheated pellets H are fired at a temperature of about 1,200 C by combustion
with a kiln
burner (not shown in the figure) provided on an outlet side of the kiln 5.
Accordingly,
high-temperature iron ore pellets 1 are obtained.
[0050]
In the kiln 5, as air for combustion, an atmosphere serving as a cooling gas
G3 used
in the annular cooler 6 is used. Furthermore, the high-temperature combustion
exhaust gas
G2 used for firing the preheated pellets H is sent to the preheating chamber
44 as the heating
gas Gl.
[0051]
Cooling Step
In the cooling step S3, the high-temperature iron ore pellets 1 obtained in
the firing
step S2 are cooled. In the cooling step S3, the annular cooler 6 is used. The
iron ore
pellets 1 cooled in the cooling step S3 are accumulated and used in the blast
furnace
operation.
[0052]
In the annular cooler 6, the iron ore pellets 1 can be cooled by blowing the
atmosphere serving as the cooling gas G3 by using a blowing apparatus 61,
while transferring
the high-temperature iron ore pellets 1 discharged from the kiln 5.
[0053]
It is to be noted that the cooling gas G3, which was used in the annular
cooler 6,
resulting in an increase in temperature, is sent to the kiln 5 and used as the
air for combustion.
[0054]
Advantages
In the method for producing iron ore pellets, the viscosity of the water at
the time of
balling the green pellets P being greater than or equal to 15 mPa.s enables
easily producing
the iron ore pellets 1 of the present invention having: the porosity which
results from the large
open pores having the pore size of greater than or equal to 4 gm being greater
than or equal to
21%; and the crushing strength which is greater than or equal to 180 kg/P.
[0055]
Other Embodiments
It is to be noted that the present invention is not limited to the above-
described
embodiments.
[0056]
In the above-described embodiments, the case in which the iron ore pellets are
constituted from the coarse grains and the fines is described, but the iron
ore pellets being
constituted from only the coarse grains, or only the fines also falls within
the intended scope
of the present invention.
[EXAMPLES]
11
CA 03191576 2023-02-10
[0057]
Hereinafter, the present invention is explained in further detail by way of
Examples,
but the present invention is not in any way limited to these Examples.
[0058]
Example 1, Example 2, Comparative Example 1
In accordance with the method for producing iron ore pellets shown in FIG. 2,
iron
ore pellets of Example 1, Example 2, and Comparative Example 1 were produced.
[0059]
Balling Step
As the water, a water containing an organic binder was employed in Example 1
and
Example 2, and a content of the organic binder was 0.1% by mass in Example 1
and 0.2% by
mass in Example 2. As a result, a viscosity of the water used in the balling
was 17.4 mPa.s
in Example 1, and 31.7 mPa.s in Example 2. The organic binder used was a
starch-type
organic binder (an organic binder obtained by adding bentonite in a content of
10% by mass,
as an excluded amount, to a raw material being a mixture of 60% by mass
cornstarch, 30% by
mass tapioca, and 10% by mass potato). Furthermore, measurement of the
viscosity was
performed in accordance with JIS-Z8803: 2011 by using a rotary viscometer.
[0060]
On the other hand, the water of Comparative Example 1 was water not containing
an
organic binder. A viscosity of the water was 1 mPa.s.
[0061]
After adding the water to the iron ore raw material and mixing, ball-shaped
green
pellets were produced by: charging a resulting mixture into a pan pelletizer
with a diameter of
40 cm, a pan angle of 48 , a rotation speed of 30 rpm, and a rim height of 95
mm; and
rolling.
[0062]
Firing Step
The green pellets were charged into a furnace and fired for 15 min at a
temperature
of 1,210 C. It is to be noted that as an atmosphere, a mixture of 1 L of N2
gas and 3 L of air
was employed. Furthermore, each of a heating time period and a cooling time
period was 10
min.
[0063]
A porosity which results from the large open pores having the pore size of
greater
than or equal to 4 gm, and a crushing strength were measured for the iron ore
pellets of each
of Example 1, Example 2, and Comparative Example 1. The porosity which results
from the
large open pores was calculated in accordance with
Eo x A+4/ A [%],
wherein: So [%] denotes an open porosity, determined by using a mercury
intrusion
12
CA 03191576 2023-02-10
' )
porosimeter (for example, "Autopore III 9400", manufactured by Shimadzu
Corporation); A
[cm3/g] denotes a total pore capacity per unit weight of the iron ore pellets;
and A+4 [cm3/g]
denotes a total pore capacity of pores having a pore size of greater than or
equal to 4 m per
unit weight of the iron ore pellets. The crushing strength was determined by
using a
well-known crushing strength tester consisting of a turn table on which a
sample is to be
placed, a driving apparatus, and a load cell. The results are shown in FIG. 4.
[0064]
From the results in FIG. 4, it is revealed that the present method for
producing iron
ore pellets, in which the organic binder was added and the viscosity of the
water was greater
than or equal to 15 mPa.s, enables easily producing the iron ore pellets in
which the porosity
which results from the large open ore pores having the pore size of greater
than or equal to 4
m is greater than or equal to 21%, and the crushing strength is greater than
or equal to 180
kg/P. In contrast, it is revealed that with the iron ore pellets of
Comparative Example 1, in
which the viscosity of the water is less than 15 mPa.s, both the porosity
which results from
the large open pores having the pore size of greater than or equal to 4 m and
the crushing
strength are low.
[0065]
Reduction Percentage
Using the iron ore pellets of Example 1, Example 2, and Comparative Example 1,
a
large-scale reduction under load test was conducted simulating a peripheral
part of a blast
furnace to investigate the reduction percentage.
[0066]
Fig. 5 illustrates a furnace for a large-scale reduction under load test 7
used in this
experiment. A graphite crucible 71 to be packed with a sample was configured
to have an
inner diameter of 85 mm. A sample-packed bed 72 was constituted of, from the
top, an
upper coke layer 72a (20 mm in height), an iron ore layer 72b (150 mm in
height), and a
lower coke layer 72c (40 mm in height). The iron ore layer 72b was a mixture
of sintered
iron ore (16 to 19 mm in grain size), the iron ore pellets (11.2 to 13.2 mm in
grain size), and
lump iron ore (16 to 19 mm in grain size).
[0067]
While heating the sample-packed bed 72 with a temperature profile shown in
Fig. 6
by using an electric furnace 73, gas (reducing gas) of a composition shown in
Fig. 7 was
supplied thereto. The gas was supplied from a gas inlet pipe 74 provided in a
lower portion
of the furnace for a large-scale reduction under load test 7, and discharged
from a gas outlet
pipe 75 provided in an upper portion. A total feed rate of the gas was 51.3
NL/min, and
temperature control was carried out by two thermocouples 76. In addition, a
load applied to
the sample-packed bed 72 was 1 kgf/cm2. The load was applied by applying a
weight of a
weight 78 via a loading rod 77.
13
CA 03191576 2023-02-10
'
[0068]
Under the aforementioned conditions, the rise in temperature and the supply of
gas
were stopped when the temperature of the sample-packed bed 72 reached 1,250
C, and the
reduction percentage was calculated from a difference between the pre-
reduction weight and
the post-reduction weight of the sample-packed bed 72.
[0069]
The measurement of the reduction percentage was performed twice. The results
are
shown in FIG. 8. In the graph in FIG. 8, results of each of two trials are
shown with bars,
and average values thereof are shown with dots. From the results in FIG. 8, it
is revealed
that using the iron ore pellets of the present invention results in the
reduction percentage
being increased, and a further decrease in the coke consumption in the blast
furnace operation
being enabled.
[INDUSTRIAL APPLICABILITY]
[0070]
The iron ore pellets of the present invention have the characteristic of
enabling
further decreasing the coke consumption in the blast furnace operation.
Furthermore,
conducting the blast furnace operation using the iron ore pellets produced by
using the
method for producing iron ore pellets of the present invention enables further
decreasing the
coke consumption.
[Explanation of the Reference Symbols]
[0071]
1 Iron ore pellet
11 Coarse grain
12 Fines
12a Aggregate structure
13 Pore
13a Open pore
13b Closed pore
2 Production apparatus
3 Pan pelletizer
4 Traveling grate furnace
41 Traveling grate
42 Drying chamber
43 Dehydrating chamber
44 Preheating chamber
45 Burner
Kiln
6 Annular cooler
14
CA 03191576 2023-02-10
61 Blowing apparatus
7 Furnace for large-scale reduction under load test
71 Graphite crucible
72 Sample-packed bed
72a Upper coke layer
72b Iron ore layer
72c Lower coke layer
73 Electric furnace
74 Gas inlet pipe
75 Gas outlet pipe
76 Thermocouple
77 Loading rod
78 Weight
P Green pellet
H Preheated pellet
G1 Heating gas
G2 Combustion exhaust gas
G3 Cooling gas
C Smokestack