Note: Descriptions are shown in the official language in which they were submitted.
CA 03192238 2023-02-15
DESCRIPTION
CURABLE RESIN COMPOSITION, FUEL CELL, AND SEALING METHOD
TECHNICAL FIELD
[0001]
The present invention relates to a curable resin composition,
a fuel cell, and a sealing method.
BACKGROUND ART
[0002]
In recent years, a fuel cell has attracted attention as a
new energy system for an automobile and a home. A fuel cell is a
power generator that produces electricity by chemically reacting
hydrogen and oxygen. In addition, a fuel cell is a clean next-
generation power generator because the fuel cell has high energy
efficiency during power generation and generates water through the
reaction of hydrogen and oxygen. There are four types of fuel
cells: a polymer electrolyte fuel cell, a phosphoric acid fuel
cell, a fused carbonate fuel cell, and a solid oxide fuel cell,
and among these, the polymer electrolyte fuel cell has a relatively
low operating temperature (around 80 C) but high power generation
efficiency and thus is expected in a use such as a power source
for an automobile, a power generator for a home, a compact power
source for an electronic device such as a cellphone, or an
emergency power source.
[0003]
As shown in Figure 1, a cell 1 of a polymer electrolyte fuel
cell has a structure including an electrolyte membrane electrode
assembly (MEA) 5 having a structure in which a polymer electrolyte
membrane 4 is sandwiched between an air electrode (cathode
electrode) 3a and a fuel electrode (anode electrode) 3b, a frame
6 that supports the MEA, and a separator 2 in which a gas flow
¨ 1 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
path is formed.
[0004]
In order to start up the polymer electrolyte fuel cell, it
is necessary to separately supply a hydrogen-containing fuel gas
to the anode electrode and an oxygen-containing oxidation gas to
the cathode electrode with these gases isolated from each other.
This is because if the isolation is insufficient and one gas mixes
with the other gas, the power generation efficiency may decrease.
Against this background, a sealing agent is often used for the
purpose of preventing leakage of a fuel gas, an oxidation gas, or
the like. Specifically, a sealing agent is used between adjacent
separators, between a separator and a frame, between a frame and
an electrolyte membrane or an MEA, or the like.
[0005]
As a sealing agent used in a polymer electrolyte fuel cell,
a polymer composition using a polyisobutylene-based polymer is
used because it is a rubber elastic body having excellent gas
permeability resistance, low moisture permeability, heat
resistance, and acid resistance. Specifically, Patent Literature
1 discloses a polymer composition that contains a telechelic
polyisobutylene polymer having a terminal acrylate group and a
reactive diluent and that exhibits excellent cured product
properties of high strength and high elongation.
Citation List
Patent Literature
[0006]
[Patent Literature 1] Japanese Patent Laid-Open No. 2-88614
SUMMARY OF INVENTION
Technical Problem
[0007]
¨ 2 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
In recent years, there has been a demand for further
shortening of the tact time in the step of applying and curing a
sealing agent at a production site.
Specifically, from the
viewpoint of productivity, there has been a demand for
compatibility with screen printing (see Japanese Patent Laid-Open
No. 2009-117314). However,
the polymer composition of Patent
Literature 1 uses a high-molecular-weight polymer in order to
improve cured product properties such as high strength and high
elongation, and thus repellence occurs from the adherend when the
polymer composition is applied by screen printing, or it is
difficult to eliminate an air bubble generated by screen printing,
and the polymer composition is not suitable for screen printing.
[0008]
The present invention has been made in view of the above
circumstances, and the purpose of the present invention is to
provide a curable resin composition that is compatible with
application by screen printing while maintaining cured product
properties of high strength and high elongation.
Means for Solving Problem
[0009]
Next, a summary of the present invention will be described.
[0010]
[1] A curable resin composition comprising the following (A)
to (C) components:
(A) component: a polyisobutylene resin comprising one or more
(meth)acryloyl groups and a -[CH2C(CH3)2]- unit
(B) component: a radical polymerization initiator
(C) component: an antifoaming agent comprising a silicone compound
having none of a methoxysilyl group, an ethoxysilyl group, and a
(meth)acryloyl group and not comprising an organic solvent.
[0011]
[2] The curable resin composition according to [1], wherein
....._ 3 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
the (A) component is a polyisobutylene resin represented by a
general formula (1):
[0012]
[Formula 1]
R2
R1 PiIS 0
C:-ICH1
R5
0
( )
[0013]
wherein R4 represents a monovalent or polyvalent aromatic
hydrocarbon group or a monovalent or polyvalent aliphatic
hydrocarbon group optionally having an aromatic ring, PIB
represents a polyisobutylene skeleton comprising the -[CH2C(CF13)2]-
unit, R4 represents a divalent hydrocarbon group having 2 to 6
carbon atoms optionally containing an oxygen atom, R2 and R2 each
independently represent a hydrogen atom or a monovalent
hydrocarbon group having 1 to 20 carbon atoms, R5 represents a
hydrogen atom, a methyl group, or an ethyl group, and n is any
integer of 1 to 6.
[0014]
[3] The curable resin composition according to [1] or [2],
wherein the curable resin composition comprises 0.1 to 10 parts
by mass of the (C) component per 100 parts by mass of the (A)
component.
[0015]
[4] The curable resin composition according to any one of
[1] to [3], wherein the curable resin composition further comprises
a monofunctional monomer as a (D) component.
[0016]
[5] The curable resin composition according to any one of
¨ 4 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
[1] to [4], wherein the curable resin composition does not comprise
an organic solvent.
[0017]
[6] The curable resin composition according to any one of
[1] to [5], wherein the silicone compound in the (C) component is
a compound having a structure of any of dimethylsiloxane,
methylphenylsiloxane, or diphenylsiloxane.
[0018]
[7] The curable resin composition according to any one of
[4] to [6], wherein the curable resin composition comprises 0.1
to 9 parts by mass of the (C) component per 100 parts by mass in
total of the (A) component and the (D) component.
[0019]
[8] The curable resin composition according to any one of
[1] to [7], wherein the (B) component is a radical
photopolymerization initiator or an organic peroxide.
[0020]
[9] A curable sealing agent for a fuel cell, comprising the
curable resin composition according to any of [1] to [8].
[0021]
[10] The curable sealing agent for a fuel cell according to
[9], wherein the curable sealing agent for a fuel cell is used for
any member selected from the group consisting of a separator, a
frame, an electrolyte membrane, a fuel electrode, an air electrode,
and an electrolyte membrane electrode assembly, which are members
of a fuel cell.
[0022]
[11] A cured product of the curable resin composition
according to any of [1] to [8].
[0023]
[12] A fuel cell comprising any seal selected from the group
consisting of a seal between adjacent separators in a fuel cell
5 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
and a seal between a frame of a fuel cell and an electrolyte
membrane or an electrolyte membrane electrode assembly, wherein
the any seal is the cured product according to [11].
[0024]
[13] A sealing method for sealing at least a portion between
at least two flanges of a part to be sealed having the at least
two flanges, wherein at least one of the flanges is permeable to
an active energy ray, the sealing method comprising: a step of
applying the curable resin composition according to any one of [1]
to [8] to a surface of at least one of the flanges; a step of
bonding the one flange to which the curable resin composition is
applied and another flange together via the curable resin
composition; and a step of irradiating the curable resin
composition with an active energy ray through the flange that is
permeable to the active energy ray to cure the curable resin
composition to seal at least a portion between the at least two
flanges.
[0025]
[14] A sealing method for sealing at least a portion between
at least two flanges of a part to be sealed having the at least
two flanges, the sealing method comprising: a step of applying the
curable resin composition according to any one of [1] to [8] to
at least one flange of the flanges; a step of irradiating the
applied curable resin composition with an active energy ray to
cure the curable resin composition to form a gasket made of a
cured product of the curable resin composition; and a step of
disposing another flange on the gasket and crimping the one flange
to which the curable resin composition is applied and the another
flange via the gasket to seal at least a portion between the at
least two flanges.
[0026]
[15] A sealing method for sealing at least a portion between
¨ 6 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
at least two flanges of a part to be sealed having the at least
two flanges, the sealing method comprising: a step of disposing a
mold for gasket formation on at least one flange of the flanges;
a step of injecting the curable resin composition according to any
of [1] to [8] into at least a portion of a gap between the mold
for gasket formation and the one flange on which the mold is
disposed; a step of irradiating the curable resin composition with
an active energy ray to cure the curable resin composition to form
a gasket made of a cured product of the curable resin composition;
a step of removing the mold from the one flange; and a step of
disposing another flange on the gasket and crimping the one flange
and the another flange via the gasket to seal at least a portion
between the at least two flanges.
Advantageous Effect of the Invention
[0027]
The present invention provides a curable resin composition
that is compatible with application by screen printing while
maintaining cured product properties of high strength and high
elongation.
BRIEF DESCRIPTION OF DRAWINGS
[0028]
[Figure 1] Figure 1 is a schematic cross-sectional view of
a single cell of a fuel cell.
[Figure 2] Figure 2 is a schematic diagram showing the whole of a
fuel cell.
DESCRIPTION OF EMBODIMENTS
[0029]
Hereinafter, details of the present invention will be
described. As used herein, "X to Y" is used in a sense that
includes the numerical values (X and Y) written before and after
_ 7 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
the word "to" as the lower limit value and the upper limit value,
respectively, and means "X or more and Y or less." In addition,
in the present invention, (meth)acrylate means both acrylate and
methacrylate.
[0030]
The curable resin composition of the present invention
includes (A) component: a polyisobutylene resin containing one or
more (meth)acryloyl groups and a -[CH2C(CF13)2]- unit, (B)
component: a radical polymerization initiator, and (C) component:
an antifoaming agent containing a silicone compound having none
of a methoxysilyl group, an ethoxysilyl group, and a (meth)acryloyl
group and not containing an organic solvent. According to the
present invention, a curable resin composition having high
strength and high elongation and suitable for screen printing is
provided. The term "suitable for screen printing" means having
properties such as no occurrence of repellence from an adherend
during application by screen printing and no occurrence of an air
bubble due to screen printing.
[0031]
<(A) Component>
The (A) component used in the present invention is not
particularly limited as long as it is a polymer having a
polyisobutylene skeleton containing one or more (meth)acryloyl
groups and an -[CH2C(C1-13)2]- unit. The (A) component may have, for
example, a -[CH2C(CH3)2]- unit (polyisobutylene skeleton), and may
be a polymer further containing a "different constitutional unit
other than the -[CH2C(CH3)2]-unit." The (A) component
appropriately contains a -[CH2C(CH3)2]- unit in an amount of, for
example, 70% by mass or more, preferably 75% by mass or more, and
more preferably 80% by mass or more, based on the total amount of
constitutional units. In addition, the (A) component
appropriately contains a -[CH2C(CH3)2]- unit in an amount of, for
¨ 8 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
example, 100% by mass or less, in another embodiment, 95% by mass
or less, and in yet another embodiment, 90% by mass or less. The
(A) component appropriately has preferably 1 to 12 (meth)acryloyl
groups, more preferably 2 to 8 (meth)acryloyl groups, further
preferably 2 to 4 (meth)acryloyl groups, and particularly
preferably 2 (meth)acryloyl groups. In the
present invention,
without being bound by theory, the "polymer" can be defined as,
for example, a compound having a structure with a repeating unit
of a monomer in the main chain of the polymer and consisting of
100 or more repeating units. In addition, the
(meth)acryloyl
group may be present on either of a side chain and/or a terminal
of the molecule, and is preferably present on a terminal of the
molecule from the viewpoint of rubber elasticity.
[0032]
The (A) component is preferably a polymer having a
polyisobutylene skeleton represented by the general formula (1)
from the viewpoint of obtaining a curable resin composition
exhibiting excellent cured product properties of high strength and
high elongation. Specific examples of the (A) component include
a polyisobutylene having a (meth)acryloyloxyalkoxyphenyl group.
The main skeleton of the (A) component in the present invention
is a polyisobutylene skeleton, and as a monomer constituting this
polyisobutylene skeleton, isobutylene is mainly used, and another
monomer may be copolymerized as long as it does not impair the
effects of the present invention. The (A) component is preferably
liquid at normal temperature (25 C) from the viewpoint of obtaining
a curable resin composition that is much more compatible with
application by screen printing.
[0033]
[Formula 2]
....._ 9 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
R2
-.^"PIB 0
. O-Ri 0 -C-C 7.7 C
R!)
R3
(1)
[0034]
wherein R1 represents a monovalent or polyvalent aromatic
hydrocarbon group or a monovalent or polyvalent aliphatic
hydrocarbon group optionally having an aromatic ring. The
monovalent or polyvalent aromatic hydrocarbon group is preferably
an aryl group having 6 to 18 carbon atoms or an arylene group
having 6 to 18 carbon atoms. Specific examples of the monovalent
or polyvalent aromatic hydrocarbon group include a phenyl group,
a phenylene group, a naphthyl group, a naphthylene group, a
biphenyl group, a tolyl group, a tolylene group, a xylyl group,
and a xylylene group. The
monovalent or polyvalent aliphatic
hydrocarbon group optionally having an aromatic ring is an alkyl
group having 6 to 18 carbon atoms, an alkylene group having 6 to
18 carbon atoms, an arylalkyl group having 6 to 18 carbon atoms,
or an alkylenearylenealkylene group having 6 to 18 carbon atoms.
Specific examples of the monovalent or polyvalent aliphatic
hydrocarbon group optionally having an aromatic ring include a -
C(CH3)2CH2C(CH3)2CH3 group, a -C(CH3)2CH2C(CH3)2CH2C(CH3)2- group, and
a group represented by a -C(CH3)2-C6H4-C(CH3)2- group (dicumyl
group). Among these, a polyvalent aromatic hydrocarbon group or
an aliphatic hydrocarbon group optionally having an aromatic ring
is preferable, a divalent group is particularly preferable, and
for example, a phenylene group or a dicumyl group (more preferably
p-dicumyl group) is preferable.
[0035]
PIB represents a polyisobutylene skeleton containing a -
¨ 10 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
[CH2C (CH3) 2] unit.
[0036]
R4 represents a divalent hydrocarbon group having 2 to 6
carbon atoms optionally containing an oxygen atom, and is
preferably a divalent hydrocarbon group having 2 or 3 carbon atoms.
Preferable examples of the divalent saturated hydrocarbon group
having 2 to 6 carbon atoms optionally containing an oxygen atom
include -CH2CH2-, -CH2CH2CH2-, -CH2CH2CH2CH2-, -CH2CH2CH2CH2CH2-, -
CH2CH2CH2CH2CH2CH2-, -CH2CH2-0-CH2CH2-, and -CH2CH(OH)CH2-. Among
these, -CH2CH2- is preferable.
[0037]
R2 and R2 each independently represent a hydrogen atom or a
monovalent hydrocarbon group having 1 to 20 carbon atoms, and are
each independently preferably a hydrogen atom. R5 represents a
hydrogen atom, a methyl group, or an ethyl group, and is preferably
a hydrogen atom or a methyl group. n is any integer of 1 to 6,
and particularly preferably an integer of 2 to 4.
[0038]
The molecular weight of the (A) component in the present
invention is not particularly limited, and the number average
molecular weight obtained by chromatographic measurement is, for
example, preferably 200 to 500,000, further preferably 1,000 to
100,000, and particularly preferably 3,000 to 50,000, from the
viewpoint of being compatible with application by screen printing
and having an excellent sealing property. The number
average
molecular weight was calculated by a standard polystyrene
conversion method using size permeation chromatography (SEC).
[0039]
The viscosity at 25 C of the (A) component in the present
invention is not particularly limited, and is, for example, 5 Pas
or more, preferably 50 Pas or more, more preferably 100 Pas or
more, further preferably 500 Pas or more, particularly preferably
¨ 11 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
1000 Pas or more, and, for example, 3000 Pas or less, preferably
2500 Pas or less, more preferably 2000 Pas or less, from the
viewpoint of workability and the like. A particularly preferable
viscosity is 1800 Pas or less. Unless
otherwise specified,
viscosity measurement was carried out using a cone-plate type
viscometer, and viscosity at 25 C was measured.
[0040]
The method for producing the (A) component is not
particularly limited, and a known method can be used. Examples
thereof include methods for obtaining the same by reacting a
hydroxyl group-terminated polyisobutylene with acryloyl chloride
or methacryloyl chloride, disclosed in Polymer Bulletin, Vol. 6,
pp. 135-141 (1981), T.P. Liao and J.P. Kennedy, and Polymer
Bulletin, Vol. 20, pp. 253-260 (1988), Puskas et al. In addition,
other examples of the method for producing the (A) component
include a method for obtaining the same by reacting a hydroxyl
group-terminated polyisobutylene with a compound having a
(meth)acryloyl group and an isocyanate group; a method for
obtaining the same by reacting a hydroxyl group-terminated
polyisobutylene with a compound having an isocyanate group and a
compound having a (meth)acryloyl group and a hydroxyl group; and
a method for obtaining the same by reacting a hydroxyl group-
terminated polyisobutylene with (meth)acrylic acid or a lower
ester of (meth)acrylic acid using a dehydration esterification
method or an ester exchange method.
[0041]
The method for producing the polyisobutylene represented by
the general formula (1) is not particularly limited, and preferable
examples thereof include a method involving reacting the halogen-
terminated polyisobutylene disclosed in Japanese Patent Laid-Open
No. 2013-216782 with a compound having a (meth)acryloyl group and
a phenoxy group as represented by the general formula (2). In
¨ 12 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
addition, the halogen-terminated polyisobutylene can be obtained
by a known method, and can be obtained, for example, by cationic
polymerization and more preferably by living cationic
polymerization.
[0042]
[Formula 3]
17,
0
,¨() i(1C c
R' (2)
[0043]
wherein R2, R3, R4, and R5 may be as defined in the above
general formula (1). Specifically,
R4 represents a divalent
hydrocarbon group having 2 to 6 carbon atoms and optionally
containing an oxygen atom. R2 and R3 each independently represent
a hydrogen atom or a monovalent hydrocarbon group having 1 to 20
carbon atoms. R5 represents a hydrogen atom, a methyl group, or
an ethyl group. Examples of the compound represented by the above
general formula (2) include phenoxymethyl (meth)acrylate,
phenoxyethyl (meth)acrylate, phenoxypropyl (meth)acrylate,
phenoxybutyl (meth)acrylate, and phenoxypentyl (meth)acrylate, and
preferable examples include phenoxyethyl (meth)acrylate,
phenoxypropyl (meth)acrylate, phenoxybutyl (meth)acrylate, and
phenoxypentyl (meth)acrylate.
[0044]
<(B) Component>
The (B) component that can be used in the present invention
is a radical polymerization initiator. Examples of
the (B)
component include a radical photopolymerization initiator and an
organic peroxide (thermal radical polymerization initiator). The
curing form of the radical curable resin composition of the present
invention can be selected from photo-curing, heat curing, or redox
¨ 13 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
curing by selecting the (B) component of the present invention.
For example, if "photocurability" is to be imparted to the radical
curable resin composition, a radical photopolymerization initiator
may be selected, and if "curing by heating or curing by a redox
reaction" is to be imparted thereto, an organic peroxide may be
selected.
[0045]
The amount of the (B) component of the present invention
blended is not particularly limited, and is, for example, 0.1 to
30 parts by mass, preferably 0.5 to 20 parts by mass, more
preferably 0.3 to 18 parts by mass, further preferably 1 to 15
parts by mass, and particularly preferably 1.5 to 10 parts by mass,
per 100 parts by mass of the (A) component. It is preferable for
the amount to be within the above range from the viewpoint of
being able to have cured product properties of high strength and
high elongation.
[0046]
The radical photopolymerization initiator, which is one (B)
component that can be used in the present invention, is not limited
as long as it is a compound that generates a radical upon
irradiation with light (active energy ray). Here, the
active
energy ray includes all light in a broad sense such as a radiation
such as an a ray or a p ray, an electromagnetic wave such as a y
ray or an X ray, an electron beam (EB), an ultraviolet ray having
a wavelength of about 100 to 400 nm, and a visible ray having a
wavelength of about 400 to 800 nm, and is preferably an ultraviolet
ray. Examples of the radical photopolymerization initiator as the
(B) component include an acetophenone-based radical
photopolymerization initiator, a benzoin-based radical
photopolymerization initiator, a benzophenone-based radical
photopolymerization initiator, a thioxanthone-based radical
photopolymerization initiator, an acylphosphine oxide-based
¨ 14 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
radical photopolymerization initiator, and a titanocene-based
radical photopolymerization initiator, and among these, an
acetophenone-based radical photopolymerization initiator and an
acylphosphine oxide-based radical photopolymerization initiator
are preferable from the viewpoint of obtaining a cured product
having high strength and high elongation. In addition, these may
be used singly or in combinations of two or more.
[0047]
Examples of the acetophenone-based radical
photopolymerization initiator include, but are not limited to,
diethoxyacetophenone, 2-hydroxy-
2-methyl-l-phenyl-propan-l-one,
benzyl dimethyl ketal, 4-(2-hydroxyethoxy)phenyl-(2-hydroxy-2-
propyl)ketone, 1-hydroxy-cyclohexyl-phenyl-ketone, 2-methy1-2-
morpholino(4-thiomethylphenyl)propan-l-one, 2-benzy1-2-
1 5 dimethylamino-1-(4-morpholinophenyl)butanone, and 2-hydroxy-2-
methy1-1-[4-(1-methylvinyl)phenyl]propanone oligomer. Examples
of a commercially available product of the acetophenone-based
radical photopolymerization initiator include Omnirad ((R),
hereinafter the same applies) 184, Omnirad 1173, Omnirad 2959,
Omnirad 127, and ESACURE (R) KIP-150 (manufactured by IGM Resins
B.V.).
[0048]
Examples of the acylphosphine oxide-based radical
photopolymerization initiator include, but are not limited to,
bis(2,4,6-trimethylbenzoy1)-phenyl-phosphine oxide and 2,4,6-
trimethylbenzoyl-diphenyl-phosphine oxide. Examples of a
commercially available product of the acylphosphine oxide-based
radical photopolymerization initiator include Omnirad TPO, Omnirad
819, and Omnirad 819DW (manufactured by IGM Resins B.V.).
[0 04 9]
The organic peroxide, which is one (B) component that can be
used in the present invention, is a compound that generates a
¨ 15 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
radical species upon heating or a redox reaction. Here, the
heating is suitably carried out, for example, at a temperature of
50 C or more, preferably at a temperature of 80 C or more, and more
preferably at a temperature of 100 C or more. A compound that
generates a radical species upon heating is also particularly
referred to as a thermal radical polymerization initiator. The
redox reaction is also referred to as an oxidation-reduction
reaction, and is a phenomenon in which an oxidation-reduction
reaction is caused by a radical species released from an organic
peroxide. Use of a redox reaction is preferable because it can
generate a radical species at room temperature. The
organic
peroxide as the (B) component is not particularly limited, and
examples thereof include a ketone peroxide such as methyl ethyl
ketone peroxide, cyclohexanone peroxide, 3,3,5-
trimethylcyclohexanone peroxide, methylcyclohexanone peroxide,
methyl acetoacetate peroxide, or acetylacetone peroxide; a
peroxyketal such as 1,1-bis(t-
butylperoxy)-3,3,5-
trimethylcyclohexane, 1,1-bis(t-butylperoxy)cyclohexane, 2,2-
bis(t-butylperoxy)octane, n-butyl-4,4-bis(t-butylperoxy)valerate,
or 2,2-bis(t-butylperoxy)butane; a hydroperoxide such as t-butyl
hydroperoxide, cumene hydroperoxide,
diisopropylbenzene
hydroperoxide, p-menthane hydroperoxide, 2,5-dimethylhexane-2,5-
dihydroperoxide, or 1,1,3,3-tetramethylbutyl hydroperoxide; a
dialky1 peroxide such as di-t-butyl peroxide, t-butylcumyl
peroxide, dicumyl peroxide, a,a'-bis(t-butylperoxy-m-
isopropyl)benzene, 2,5-dimethy1-2,5-di(t-butylperoxy)hexane, or
2,5-dimethy1-2,5-di(t-butylperoxy)hexyne-3; a diacy1 peroxide
such as acetyl peroxide, isobutyryl peroxide, octanoyl peroxide,
decanoyl peroxide, lauroyl peroxide, 3,5,5-trimethylhexanoyl
peroxide, succinic acid peroxide, benzoyl peroxide, 2,4-
dichlorobenzoyl peroxide, or m-toluoyl peroxide; a
peroxydicarbonate such as diisopropyl peroxydicarbonate, di-2-
- 16 ¨
Date Recue/Date Received 2023-02-15
CA 0312313 2023-02-15
ethylhexyl peroxydicarbonate, di-n-propyl peroxydicarbonate, bis-
(4-t-butylcyclohexyl) peroxydicarbonate, dimyristyl
peroxydicarbonate, di-2-ethoxyethyl
peroxydicarbonate,
dimethoxyisopropyl peroxydicarbonate, di(3-methy1-3-methoxybutyl)
peroxydicarbonate, or dially1 peroxydicarbonate; a peroxyester
such as t-butyl peroxyacetate, t-butyl peroxyisobutyrate, t-butyl
peroxypivalate, t-butyl peroxyneodecanoate, cumyl
peroxyneodecanoate, t-butyl peroxy-2-ethylhexanoate, t-butyl
peroxy-3,5,5-trimethylhexanoate, t-butyl peroxylaurate, t-butyl
peroxybenzoate, di-t-butyl peroxyisophthalate, 2,5-dimethy1-2,5-
di(benzoylperoxy)hexane, t-butyl peroxymaleic acid, t-butyl
peroxyisopropyl carbonate, cumyl peroxyoctoate, t-hexyl
peroxyneodecanoate, t-hexyl peroxypivalate, t-butyl
peroxyneohexanoate, t-hexyl peroxyneohexanoate, or cumyl
peroxyneohexanoate; and acetylcyclohexylsulfonyl peroxide and t-
butyl peroxyallyl carbonate. These organic peroxides may be used
singly, or a plurality thereof may be used in combination. Among
these, cumene hydroperoxide is preferably used from the viewpoint
of cured product properties of high strength and high elongation.
[0050]
When an organic peroxide is used as the (B) component, a
curing accelerator can be blended for the purpose of accelerating
a redox reaction. Such a curing accelerator is not particularly
limited, and preferable examples thereof that can be used include
saccharin (o-benzoic sulfimide), a hydrazine compound, an amine
compound, a mercaptan compound, and a transition metal-containing
compound.
[0051]
Examples of the hydrazine compound include 1-acetyl-2-
phenylhydrazine, 1-acetyl-2(p-tolyl)hydrazine, 1-benzoy1-2-
phenylhydrazine, 1-(1',1',1'-trifluoro)acety1-2-phenylhydrazine,
1,5-diphenyl-carbohydrazine, 1-formy1-2-phenylhydrazine, 1-
-- 17 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
acetyl-2-(p-bromophenyl)hydrazine, 1-acety1-2-
(p-
nitrophenyl)hydrazine, 1-acetyl-2-
(2'-phenylethylhydrazine),
ethylcarbazate, p-nitrophenylhydrazine, and p-
trisulfonylhydrazide.
[0052]
Examples of the amine compound include a heterocyclic
secondary amine such as 2-
ethylhexylamine, 1,2,3,4-
tetrahydroquinone, or 1,2,3,4-tetrahydroquinaldine; a
heterocyclic tertiary amine such as quinoline, methylquinoline,
quinaldine, quinoxaline, or phenazine; an aromatic tertiary amine
such as N,N-dimethyl-para-toluidine, N,N-dimethyl-anisidine, or
N,N-dimethylaniline; and an azole-based compound such as 1,2,4-
triazole, oxazole, oxadiazole, thiadiazole, benzotriazole,
hydroxybenzotriazole, benzoxazole, 1,2,3-benzothiadiazole, or 3-
mercaptobenzotriazole.
[0053]
Examples of the mercaptan compound include n-dodecyl
mercaptan, ethyl mercaptan, butyl mercaptan, tris-[(3-
mercaptopropionyloxy)-ethyl]-isocyanurate,
pentaerythritol
tetrakis(3-mercaptopropionate), dipentaerythritol hexakis(3-
mercaptopropionate), trimethylolpropane tris(3-
mercaptopropionate), trimethylolpropane tristhioglycolate, and
pentaerythritol tetrakisthioglyco late.
[0054]
As the transition metal-containing compound, a metal chelate
complex salt is preferably used. Examples
thereof include
pentadione iron, pentadione cobalt, pentadione copper,
propylenediamine copper, ethylenediamine copper, iron naphthenate,
nickel naphthenate, cobalt naphthenate, copper naphthenate, copper
octanoate, iron hexanoate, iron propionate, and acetylacetone
vanadium.
[0055]
¨ 18 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
The above curing accelerators may be used singly, or a
plurality thereof may be used in combination. Among these, a
mixture of saccharin, a hydrazine-based compound, an amine-based
compound, and a transition metal-containing compound is more
preferable because the mixture exhibits a good curing acceleration
effect.
[0056]
<(C) Component>
The (C) component of the present invention is an antifoaming
agent containing a silicone compound having none of a methoxysilyl
group, an ethoxysilyl group, and a (meth)acryloyl group, and not
containing an organic solvent. That is, the antifoaming agent
does not contain an organic solvent and contains a silicone
compound having none of a methoxysilyl group, an ethoxysilyl group,
and a (meth)acryloyl group. The present invention has a remarkable
effect of being able to provide a curable resin composition that
is compatible with application by screen printing while
maintaining cured product properties of high strength and high
elongation, by selecting the (C) component of the present invention
from among many antifoaming agents present and combining the (C)
component with the other components of the present invention.
Examples of the silicone compound include a compound containing -
Si-O-Si- (siloxane bond), and more specific examples include a
compound having a structure of
dimethylsiloxane,
methylphenylsiloxane, diphenylsiloxane, or the like. In addition,
the silicone compound in the (C) component is a silicone compound
having none of a methoxysilyl group, an ethoxysilyl group, and a
(meth)acryloyl group at a terminal or a side chain in one molecule
of the silicone compound and preferably a compound having at least
one of the terminals capped with a trimethylsily1 group, from the
viewpoint of small repellence when the curable resin composition
is applied to an adherend by screen printing while maintaining the
-- 19 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
tensile strength of the cured product. In
addition, the (C)
component is characterized by not containing an organic solvent
from the viewpoint of small repellence when the curable resin
composition is applied to an adherend by screen printing while
maintaining the tensile strength of the cured product. As used
herein, the organic solvent means an organic compound that is
liquid at 25 C, other than the silicone compound, the (B) component,
and the (D) component, and examples thereof include propylene
glycol and diisobutyl ketone. In addition, as will be described
later, the curable resin composition according to the present
invention preferably does not contain an organic solvent, and
because of this as well, the (C) component does not contain an
organic solvent.
[0057]
The silicone compound contained in the (C) component
preferably contains a dimethylpolysiloxane structure having a
siloxane bond (Si-O-Si) and a methyl group. By
containing a
dimethylpolysiloxane structure in the silicone compound contained
in the (C) component, the desired effect of the present invention
can be more exerted, and a curable resin composition suitable for
screen printing can be obtained.
[0058]
The silicone compound contained in the (C) component is
preferably silicone oil, which is a type of silicone compound.
Therefore, the (C) component is preferably an oil-type antifoaming
agent.
[0059]
The viscosity at 25 C of the silicone compound contained in
the (C) component is preferably in the range of 1 to 500 mm2/s,
and more preferably in the range of 10 to 350 mm2/s. When the
viscosity at 25 C of the silicone compound is in the above range,
the effect as an antifoaming agent can be suitably exerted. In
¨ 20 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
addition, the viscosity at 25 C of the (C) component is also
preferably in the range of 1 to 500 mm2/s, and more preferably in
the range of 10 to 350 mm2/s, from the viewpoint of exerting the
effect as an antifoaming agent.
[0060]
In the (C) component, the silicone compound is preferably
contained as a main component. As used herein, the main component
means that the content thereof is 75% by mass or more based on the
total mass of the (C) component. In addition, in the (C) component,
the upper limit of the content of the silicone compound is 100%
by mass based on the total mass of the (C) component. The content
of the silicone compound in the (C) component is preferably 80%
by mass or more, more preferably 90% by mass or more, and further
preferably 95% by mass or more, based on the total mass of the
antifoaming agent.
[0061]
The (C) component may contain an additive as long as the
additive does not impair a property of the antifoaming agent.
Examples of the additive include a wax such as polyethylene wax,
and hydrophobic silica. For example, the content of the additive
in the (C) component is 25% by mass or less, preferably 20% by
mass or less, more preferably 15% by mass or less, further
preferably 10% by mass or less, and particularly preferably 5% by
mass or less. The lower limit of the content of the additive in
the (C) component is 0% by mass based on the total mass of the (C)
component.
[0062]
Examples of a commercially available product of the (C)
component of the present invention include BYK-1799 (manufactured
by BYK-Chemie GmbH) and KF-96 series (manufactured by Shin-Etsu
Chemical Co., Ltd.) .
[0063]
¨ 21 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
The amount of (C) added is not particularly limited, and the
content of the (C) component is, for example, in the range of 0.1
to 10 parts by mass, preferably in the range of 0.15 to 5 parts
by mass, more preferably in the range of 0.15 to 4.5 parts by mass,
further preferably in the range of 0.2 to 4 parts by mass,
particularly preferably in the range of 0.2 to 3 parts by mass,
and further preferably in the range of 0.5 to 2.5 parts by mass,
per 100 parts by mass of the (A) component. In addition, the
content of the (C) component is preferably in the range of 0.1 to
9 parts by mass, more preferably in the range of 0.2 to 7 parts
by mass, further preferably in the range of 0.3 to 5 parts by mass,
particularly preferably in the range of 0.3 to 3 parts by mass,
and most preferably in the range of 0.3 to 1.5 parts by mass, per
100 parts by mass in total of the (A) component and the (D)
component described later. Within the above range, it is possible
to obtain a curable resin composition that is much more compatible
with application by screen printing while maintaining cured
product properties of high strength and high elongation.
[0064]
<(D) Component>
Further, the curable resin composition of the present
invention can further contain a monofunctional monomer as a (D)
component. The (D) component, when combined with other components
(components (A) to (C)) of the present invention, is much more
compatible with application by screen printing, and can maintain
cured product properties. Examples of the (D) component include
a (meth)acrylate monomer having an alkyl group having 5 to 30
carbon atoms or a (meth)acrylate monomer having an alicyclic
hydrocarbon group having 5 to 30 carbon atoms. Among these, a
(meth)acrylate monomer having an alkyl group having 5 to 30 carbon
atoms and a (meth)acrylate monomer having an alicyclic hydrocarbon
group having 5 to 30 carbon atoms are particularly preferably used
-- 22 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
in combination from the viewpoint of being much more compatible
with application by screen printing and being able to maintain
cured product properties.
[0065]
Examples of the (meth)acrylate monomer having an alkyl group
having 5 to 30 carbon atoms include 2-ethylhexyl (meth)acrylate,
octyl (meth)acrylate, isooctyl (meth)acrylate, decyl
(meth)acrylate, dodecyl (meth)acrylate, isodecyl (meth)acrylate,
lauryl (meth)acrylate, n-octadecyl (meth)acrylate, isooctadecyl
(meth)acrylate, nonadecane (meth)acrylate, isostearyl
(meth)acrylate, and stearyl (meth)acrylate, and among these,
preferable examples include 2-ethylhexyl (meth)acrylate, isooctyl
(meth)acrylate, isodecyl (meth)acrylate, lauryl (meth)acrylate,
isooctadecyl (meth)acrylate, isostearyl (meth)acrylate, and
stearyl (meth)acrylate. The
(meth)acrylate monomer having an
alkyl group having 5 to 30 carbon atoms is preferably a
(meth)acrylate monomer having an alkyl group having 6 to 20 carbon
atoms, and preferably a (meth)acrylate monomer having an alkyl
group having 8 to 20 carbon atoms. Such (D) components can be
used singly or as a mixture of two or more. A
commercially
available product of the (meth)acrylate monomer having an alkyl
group having 5 to 30 carbon atoms is not particularly limited, and
examples thereof include 5R335, 5R395, 5R440, 5R489D, 5R313, 5R324,
and 5R493D (manufactured by Sartomer), and S-1800A (manufactured
by SHIN-NAKAMURA CHEMICAL Co., Ltd.).
[0066]
In addition, examples of the (meth)acrylate monomer having
an alicyclic hydrocarbon group having 5 to 30 carbon atoms include
cyclohexyl (meth)acrylate, trimethylcyclohexyl (meth)acrylate, t-
butylcyclohexyl (meth)acrylate, dicyclopentanyl (meth)acrylate,
dicyclopentenyl (meth)acrylate, dicyclopentenyloxy (meth)acrylate,
isobornyl (meth)acrylate, adamantyl (meth)acrylate, and
-- 23 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
dicyclopentenyl di(meth)acrylate. Among these,
trimethylcyclohexyl (meth)acrylate, t-
butylcyclohexyl
(meth)acrylate, dicyclopentanyl (meth)acrylate, dicyclopentenyl
(meth)acrylate, dicyclopentenyloxy (meth)acrylate, and isobornyl
(meth)acrylate are preferable. These can be used singly or as a
mixture of two or more. The (meth)acrylate monomer having an
alicyclic hydrocarbon group having 5 to 30 carbon atoms is
preferably a (meth)acrylate monomer having an alicyclic
hydrocarbon group having 6 to 20 carbon atoms, and preferably a
(meth)acrylate monomer having an alicyclic hydrocarbon group
having 8 to 20 carbon atoms. A commercially available product of
the (meth)acrylate monomer having an alicyclic hydrocarbon group
having 5 to 30 carbon atoms is not particularly limited, and
examples thereof include SR506 and SR423 (both manufactured by
Sartomer), IBX and IBX-A (both manufactured by Kyoeisha Chemical
Co., Ltd.), and FA-511AS, FA-512AS, FA-513AS, FA-512M, FA-512MT,
and FA-513M (all manufactured by Showa Denko Materials Co., Ltd.).
[0067]
The amount of the (D) component blended is preferably 5 to
500 parts by mass, more preferably in the range of 10 to 300 parts
by mass, further preferably in the range of 12 to 200 parts by
mass, particularly preferably in the range of 30 to 150 parts by
mass, and most preferably in the range of 40 to 100 parts by mass,
per 100 parts by mass of the (A) component. Within the above
range, it is possible to provide a curable resin composition that
is much more compatible with application by screen printing and
can be photocured in a short time. In addition, when two or more
(D) components are combined, the total amount thereof is regarded
as the content of the (D) component. In
addition, when a
(meth)acrylate monomer having an alkyl group having 5 to 30 carbon
atoms and a (meth)acrylate monomer having an alicyclic hydrocarbon
group having 5 to 30 carbon atoms are used in combination, the
-- 24 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
mass ratio (x:y) between the (meth)acrylate monomer having an
alkyl group having 5 to 30 carbon atoms (x) and the (meth)acrylate
monomer having an alicyclic hydrocarbon group having 5 to 30 carbon
atoms (y) is, for example, in the range of 1:99 to 99:1, preferably
10:90 to 90:10, and particularly preferably in the range of 15:85
to 85:15.
[0068]
<Optional component>
To the curable resin composition of the present invention,
an additive such as an oligomer or a polymer having a
(meth)acryloyl group (not containing the (A) component of the
present invention), an inorganic filler, a curing accelerator, a
storage stabilizer, an antioxidant, a light stabilizer, a
plasticizer, a pigment, a flame retardant, or a surfactant can be
added as long as the object of the present invention is not
impaired.
[0069]
The oligomer or polymer having a (meth)acryloyl group (not
containing the (A) component of the present invention) is not
particularly limited, and examples thereof include a urethane
(meth)acrylate having a polybutadiene skeleton, a urethane
(meth)acrylate having a hydrogenated polybutadiene skeleton, a
urethane (meth)acrylate having a polycarbonate skeleton, a
urethane (meth)acrylate having a polyether skeleton, a urethane
(meth)acrylate having a polyester skeleton, a urethane
(meth)acrylate having a castor oil skeleton, an isoprene-based
(meth)acrylate, a hydrogenated isoprene-based (meth)acrylate,
epoxy (meth)acrylate, and a (meth)acrylic group-containing acrylic
polymer, and among these, a urethane (meth)acrylate having a
polybutadiene skeleton, a urethane (meth)acrylate having a
hydrogenated polybutadiene skeleton, a urethane (meth)acrylate
having a castor oil skeleton, an isoprene-based (meth)acrylate,
-- 25 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
and a hydrogenated isoprene-based (meth)acrylate are preferable
from the viewpoint of excellent compatibility with the (A)
component and the (B) component of the present invention. In the
present invention, the "oligomer" refers to a compound having a
structure with a repeating unit of a monomer in the main chain and
consisting of 2 to 100 repeating units. In addition, these may
be used singly or in combinations of two or more.
[0070]
To the curable resin composition of the present invention,
an inorganic filler may be added to such an extent that the storage
stability is not impaired, for the purpose of improving the elastic
modulus, the fluidity, or the like of the cured product. Specific
examples thereof include an inorganic powder and a metallic powder.
Examples of the inorganic powder filler include glass, fumed silica,
alumina, mica, a ceramic, silicone rubber powder, calcium
carbonate, aluminum nitride, carbon powder, kaolin clay, a dry
clay mineral, and dry diatomaceous earth. The amount
of the
inorganic powder blended is preferably about 0.1 to 100 parts by
mass per 100 parts by mass of the (A) component.
[0071]
Fumed silica can be blended for the purpose of adjusting the
viscosity of the curable resin composition or improving the
mechanical strength of the cured product.
Preferably, those
subjected to hydrophobization treatment with an organochlorosilane,
a polyorganosiloxane, hexamethyldisilazane, or the like can be
used. Specific examples of fumed silica include a commercially
available product such as trade name AEROSIL (R) R974, R972, R972V,
R972CF, R805, R812, R8125, R816, R8200, RY200, RX200, RY200S, or
R202 manufactured by Nippon Aerosil Co., Ltd.
[ 072]
An antioxidant may be added to the curable resin composition
of the present invention. Examples of the antioxidant include a
¨ 26 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
quinone-based compound such as P-naphthoquinone, 2-methoxy-1,4-
naphthoquinone, methylhydroquinone, hydroquinone, hydroquinone
monomethyl ether, mono-tert-butylhydroquinone, 2,5-di-tert-
butylhydroquinone, p-benzoquinone, 2,5-diphenyl-p-benzoquinone,
or 2,5-di-tert-butyl-p-benzoquinone; a phenol-based compound such
as phenothiazine, 2,2-methylene-bis(4-methy1-6-tert-butylphenol),
catechol, tert-butyl catechol, 2-butyl-4-hydroxyanisole, 2,6-di-
tert-butyl-p-cresol, 2-tert-
buty1-6-(3-tert-buty1-2-hydroxy-5-
methylbenzy1)-4-methylphenyl acrylate, 2-[1-(2-hydroxy-3,5-di-
tert-pentylphenyl)ethy11-4,6-di-tert-pentylphenyl acrylate, 4,4'-
butylidenebis(6-tert-buty1-3-methylphenol), 4,4'-thiobis(6-tert-
buty1-3-methylphenol), 3,9-bis(2-
[3-(3-tert-buty1-4-hydroxy-5-
methylphenyl)propionyloxy]-1,1-dimethylethy11-2,4,8,10-
tetraoxaspiro[5,5]undecane, pentaerythritol tetrakis[3-(3,5-di-
tert-butyl-4-hydroxyphenyl)propionate], thiodiethylenebis[3-(3,5-
di-tert-buty1-4-hydroxyphenyl)propionate], octadecy1-
3-(3,5-di-
tert-buty1-4-hydroxyphenyl)propionate, N,N'-hexane-1,6-diylbis[3-
(3,5-di-tert-buty1-4-hydroxyphenyl)propionamide],
benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-C7-C9
side chain alkyl ester, 2,4-dimethy1-6-(1-methylpentadecyl)phenol,
diethyl [[3,5-
bis(1,1-dimethylethyl)-4-
hydroxyphenyllmethyllphosphonate,
3,3',3",5,5',5"-hexa-tert-
butyl-a,a',a"-(mesitylene-2,4,6-tolyl)tri-p-cresol, calcium
diethylbis[[3,5-bis(1,1-dimethylethyl)-4-
hydroxyphenyllmethyllphosphonate, 4,6-bis(octylthiomethyl)-o-
cresol, ethylenebis(oxyethylene)bis[3-(5-tert-buty1-4-hydroxy-m-
tolyl)propionate],
hexamethylenebisf3-(3,5-di-tert-buty1-4-
hydroxyphenyl)propionate, 1,3,5-
tris(3,5-di-tert-buty1-4-
hydroxybenzy1)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-
tris[(4-tert-buty1-3-hydroxy-2,6-xylyl)methyl]-1,3,5-triazine-
2,4,6(1H,3H,5H)-trione, a reaction product of N-phenylbenzenamine
and 2,4,6-trimethylpentene, 2,6-di-
tert-buty1-4-(4,6-
- 27 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
bis(octylthio)-1,3,5-triazin-2-ylamino)phenol, picric acid, or
citric acid; a phosphorus compound such as tris(2,4-di-tert-
butylphenyl) phosphite, tris(2-
[[2,4,8,10-tetra-tert-
butyldibenzo[d,f1[1,3,2]dioxaphosphepin-6-yl]oxylethyllamine,
bis(2,4-di-tert-butylphenyl)pentaerythritol diphosphite, bis[2,4-
bis(1,1-dimethylethyl)-6-methylphenyi] ethyl ester phosphorous
acid,
tetrakis(2,4-di-tert-butylphenyl)[1,1-bispheny1]-4,4'-
diylbisphosphonite, or 6-[3-(3-
tert-buty1-4-hydroxy-5-
methylphenyl)propoxy]-2,4,8,10-tetra-tert-
butyldibenzo[d,f](1,3,2]dioxaphosphepin; a sulfur-based compound
such as dilauryl 3,3'-thiodipropionate, dimyristyl 3,3'-
thiodipropionate, distearyl 3,3'-
thiodipropionate,
pentaerythrityl tetrakis(3-laurylthiopropionate), or 2-
mercaptobenzimidazole; an amine-based compound such as
phenothiazine; a lactone-based compound; and a vitamin E-based
compound. Among these, a phenol-based compound is suitable.
[0073]
A light stabilizer may be added to the curable resin
composition of the present invention. Examples
of the light
stabilizer include a hindered amine-based compound such as
bis(2,2,6,6-tetramethy1-4-piperidyl)sebacate,
bis(1,2,2,6,6-
pentamethy1-4-piperidyl)sebacate, 4-
benzoyloxy-2,2,6,6-
tetramethylpiperidine, 1-(2-[3-
(3,5-di-tert-buty1-4-
hydroxyphenyl)propionyloxy]ethy11-4-[3-(3,5-di-tert-buty1-4-
hydroxyphenyl)propionyloxy]-2,2,6,6-tetramethylpiperidine,
1,2,2,6,6-pentamethy1-4-piperidinyl-methacrylate, bis(1,2,2,6,6-
pentamethy1-4-piperidinyi)[(3,5-bis(1,1-dimethylethyl)-4-
hydroxyphenyllmethyl]butylmalonate, decanedioic acid bis(2,2,6,6-
tetramethy1-1(octyloxy)-4-piperidinyl) ester, a reaction product
of 1,1-dimethylethyl hydroperoxide and octane, N,N',N",N'"-
tetrakis-(4,6-bis-(butyl-(N-methy1-2,2,6,6-tetramethylpiperidin-
4-yl)amino)-triazin-2-y1)-4,7-diazadecane-1,10-diamine, a
¨ 28 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
polycondensate of dibutylamine/1,3,5-triazine/N,N'-bis(2,2,6,6-
tetramethy1-4-piperidy1-1,6-hexamethylenediamine and N-(2,2,6,6-
tetramethy1-4-piperidyl)butylamine, poly[[6-
(1,1,3,3-
tetramethylbutyl)amino-1,3,5-triazine-2,4-diyl][(2,2,6,6-
tetramethy1-4-piperidyl)imino]hexamethylene[(2,2,6,6-
tetramethy1-4-piperidyl)imino]], a polymer of dimethyl succinate
and 4-hydroxy-2,2,6,6-tetramethyl-1-piperidine ethanol, 2,2,4,4-
tetramethy1-20-(J3-lauryloxycarbonyl)ethyl-7-oxa-3,20-
diazadispiro[5.1.11.2]heneicosan-21-one, Falanine, N-(2,2,6,6-
tetramethy1-4-piperidiny1)-dodecyl ester/tetradecyl ester, N-
acety1-3-dodecy1-1-(2,2,6,6-tetramethyl-4-
piperidinyl)pyrrolidine-2,5-dione, 2,2,4,4-
tetramethy1-7-oxa-
3,20-diazadispiro[5,1,11,2]heneicosan-21-one, 2,2,4,4-
tetramethy1-21-oxa-3,20-diazadicyclo-[5,1,11,2]-heneicosane-20-
propanoic acid dodecyl ester/tetradecyl ester, propanedioic acid,
[(4-methoxypheny1)-methylene]-bis(1,2,2,6,6-pentamethy1-4-
piperidinyl) ester, a higher fatty acid ester of 2,2,6,6-
tetramethy1-4-piperidinol, 1,3-benzenedicarboxamide, or N,N'-
bis(2,2,6,6-tetramethy1-4-piperidinyl); a benzophenone-based
compound such as octabenzone; a benzotriazole-based compound such
as 2-(2H-
benzotriazol-2-y1)-4-(1,1,3,3-tetramethylbutyl)phenol,
2-(2-hydroxy-5-methylphenyl)benzotriazole, 2-[2-
hydroxy-3-
(3,4,5,6-tetrahydrophthalimido-methyl)-5-
methylphenyl]benzotriazole, 2-(3-tert-
buty1-2-hydroxy-5-
methylpheny1)-5-chlorobenzotriazole, 2-(2-hydroxy-3,5-di-tert-
pentylphenyl)benzotriazole, a reaction product of methyl 3-(3-(2H-
benzotriazol-2-y1)-5-tert-buty1-4-hydroxyphenyl)propionate and
polyethylene glycol, or 2-(2H-benzotriazol-2-y1)-6-dodecy1-4-
methylphenol; a benzoate-based compound such as 2,4-di-tert-
butylpheny1-3,5-di-tert-buty1-4-hydroxybenzoate; and a triazine-
based compound such as 2-(4,6-dipheny1-1,3,5-triazin-2-y1)-5-
[(hexyl)oxy]phenol. A hindered
amine-based compound is
¨ 29 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
particularly preferable.
[0074]
An adhesion-imparting agent may be added to the curable resin
composition of the present invention. Examples of the adhesion-
imparting agent include 3-methacryloxypropylmethyldimethoxysilane,
3-methacryloxypropyltrimethoxysilane, 3-
methacryloxypropylmethyldiethoxysilane, 3-
methacryloxypropyltriethoxysilane, 3-
acryloxypropyltrimethoxysilane, methacryloxyoctyltrimethoxysilane,
vinyltrimethoxysilane, vinyltrichlorosilane, vinyltriethoxysilane,
vinyl-tris(P-methoxyethoxy)silane, y-chloropropyltrimethoxysilane,
13-(3,4-epoxycyclohexyl)ethyltrimethoxysilane, 7-
glycidoxypropyltrimethoxysilane, y-mercaptopropyltrimethoxysilane,
y-aminopropyltriethoxysilane, N-13-
(aminoethyl)-7-
1 5 aminopropyltrimethoxysilane, N-13-
(aminoethyl)-7-
aminopropylmethyldimethoxysilane, y-ureidopropyltriethoxysilane,
hydroxyethyl methacrylate phosphate, methacryloxyoxyethyl acid
phosphate, methacryloxyoxyethyl acid phosphate monoethylamine half
salt, and 2-hydroxyethyl methacrylic acid phosphate. Among these,
hydroxyethyl methacrylate phosphate, methacryloxyoxyethyl acid
phosphate, methacryloxyoxyethyl acid phosphate monoethylamine half
salt, 2-hydroxyethyl methacrylic acid phosphate, and the like are
preferable. The
content of the adhesion-imparting agent is
preferably 0.05 to 30 parts by mass, and further preferably 0.2
to 10 parts by mass, per 100 parts by mass of the (A) component.
[0075]
The curable resin composition of the present invention is
preferably one not containing an organic solvent from the viewpoint
of small repellence when the curable resin composition is applied
to an adherend by screen printing while maintaining the tensile
strength of the cured product. The
organic solvent means an
organic compound that is liquid at 25 C, other than the silicone
¨ 30 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
compound, the (B) component, the (C) component, and the (D)
component, and examples thereof include propylene glycol and
diisobutyl ketone.
[0076]
The viscosity at 25 C of the curable resin composition of
the present invention is not particularly limited, and is, for
example, 0.1 Pas or more, preferably 0.2 Pas or more, more
preferably 0.5 Pas or more, further preferably 1 Pas or more,
particularly preferably 2 Pas or more, and, for example, 100 Pas
or less, preferably 50 Pas or less, more preferably 20 Pas or
less, from the viewpoint of workability and the like. A
particularly preferable viscosity is 10 Pas or less. Unless
otherwise specified, viscosity measurement was carried out using
a cone-plate type viscometer, and viscosity at 25 C was measured.
[0077]
The curable resin composition of the present invention can
be produced by a conventionally known method. For example, the
curable resin composition can be produced by blending
predetermined amounts of the (A) component to the (C) component
and mixing these using mixing means such as a mixer at a
temperature of preferably 10 to 70 C for preferably 0.1 to 5 hours.
In addition, the curable resin composition is preferably produced
in a light-shielded environment.
[0078]
<Application method>
As a method for applying the curable resin composition of
the present invention to an adherend, a method such as dispensing,
spraying, inkjet, screen printing, gravure printing, dipping, or
spin coating can be used as an automatic application machine, and
among these, the curable resin composition of the present invention
is most suitable for screen printing because the curable resin
composition exerts the effect of eliminating an air bubble
-- 31 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
generated during screen printing. The curable resin composition
of the present invention is preferably liquid at 25 C from the
viewpoint of applicability.
[0079]
<Curing method>
The light source for curing the curable resin composition of
the present invention by irradiating the same with light such as
ultraviolet light or visible light is not particularly limited,
and examples thereof include a low-pressure mercury lamp, a medium-
pressure mercury lamp, a high-pressure mercury lamp, an ultra-high
pressure mercury lamp, a black light lamp, a microwave-excited
mercury lamp, a metal halide lamp, a sodium lamp, a halogen lamp,
a xenon lamp, an LED, a fluorescent lamp, sunlight, and an electron
beam irradiation apparatus. The dosage of light irradiation is
preferably 3 kJ/m2 or more, and more preferably 5 kJ/m2 or more,
from the viewpoint of the properties of the cured product, and is
preferably 100 kJ/m2 or less, more preferably 80 kJ/m2 or less,
and particularly preferably 60 kJ/m2 or less, from the viewpoint
of the tact time in the curing step.
[0080]
<Cured product>
The cured product of the present invention is obtained by
curing the curable resin composition of the present invention by
irradiating the same with an ultraviolet ray according to the
above curing method. The cured product of the present invention
can be obtained by any curing method as long as the curable resin
composition of the present invention is cured.
[0081]
<Use and sealing agent>
A use for which the curable resin composition of the present
invention or a cured product thereof is suitably used is a curable
sealing agent. In the present invention, the sealing agent also
¨ 32 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
includes a use such as an adhesive, a coating agent, a casting
agent, or a potting agent. For use in such a use, the curable
resin composition of the present invention is preferably liquid
at 25 C.
[0082]
The curable resin composition of the present invention or a
cured product thereof is a rubber elastic body excellent in low
gas permeability (gas barrier property), low moisture permeability,
heat resistance, acid resistance, flexibility, and the like, and
thus examples of a specific use of the sealing agent include a
fuel cell, a solar cell, a dye-sensitized solar cell, a lithium
ion battery, an electrolytic capacitor, a liquid crystal display,
an organic EL display, an electronic paper, an LED, a hard disk
drive, a photodiode, and an optical communication/circuit, an
electric wire/cable/optical fiber, an optical isolator, a
laminated body such as an IC card, a sensor, a substrate, and a
pharmaceutical/medical device/instrument. The
curable resin
composition of the present invention is rapidly cured by
irradiation with an active energy ray such as an ultraviolet ray,
and has an excellent gas barrier property, and thus among the
above uses, a fuel cell use is particularly preferable.
[0083]
<Fuel cell>
A fuel cell is a power generator that produces electricity
by chemically reacting hydrogen and oxygen. In addition, there
are four types of fuel cells: a polymer electrolyte fuel cell, a
phosphoric acid fuel cell, a fused carbonate fuel cell, and a
solid oxide fuel cell, and among these, the polymer electrolyte
fuel cell has a relatively low operating temperature (around 80 C)
but high power generation efficiency and thus is used for a use
such as a power source for an automobile, a power generator for a
home, a compact power source for an electronic device such as a
¨ 33 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
cellphone, or an emergency power source.
[0084]
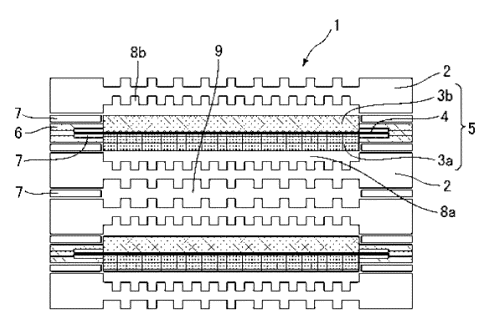
As shown in Figure 1, a cell 1 of a representative polymer
electrolyte fuel cell has a structure including an electrolyte
membrane electrode assembly (MEA) 5 having a structure in which a
polymer electrolyte membrane 4 is sandwiched between an air
electrode 3a and a fuel electrode 3b, a frame 6 that supports the
MEA, and a separator 2 in which a gas flow path is formed. In
addition, when starting up the polymer electrolyte fuel cell, a
fuel gas (hydrogen gas) and an oxidation gas (oxygen gas) are
supplied through an oxidation gas flow path 8a and a fuel gas flow
path 8b. In addition, cooling water flows through a flow path 9
for the purpose of mitigating heat generation during power
generation. A package obtained by stacking several hundred cells
is referred to as a cell stack 10 as shown in Figure 2.
[0085]
When a fuel gas (hydrogen gas) is supplied to a fuel
electrode and an oxidation gas (oxygen gas) is supplied to an
oxygen electrode (air electrode), the following reaction occurs
at each electrode, and on the whole, a reaction that produces
water (H2 + 1/202 -* H20) occurs. To describe it in detail, as
described below, a proton (H+) generated at the fuel electrode
diffuses through the solid polymer membrane and moves to the oxygen
electrode side, and water (H20) generated by reaction of the proton
with oxygen is discharged from the oxygen electrode side.
Fuel electrode (anode electrode): H2 - -2H+ + 2e
Oxygen electrode (cathode electrode): 1/202 + 21-1+ + 2e- -* H20
In order to start up the polymer electrolyte fuel cell, it
is necessary to separately supply a hydrogen-containing fuel gas
to the anode electrode and an oxygen-containing oxidation gas to
the cathode electrode with these gases isolated from each other.
This is because if the isolation is insufficient and one gas mixes
¨ 34 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
with the other gas, the power generation efficiency may decrease.
Against this background, a sealing agent is often used for the
purpose of preventing leakage of a fuel gas, an oxidation gas, or
the like. Specifically, a sealing agent is used between adjacent
separators, between a separator and a frame, between a frame and
an electrolyte membrane or an MEA, or the like.
[0086]
An example of the polymer electrolyte membrane is a cation
exchange membrane having ion conductivity, and a preferable
example is a fluorine-based polymer having a sulfonic acid group
in that it is chemically stable and resistant to operation at a
high temperature. Examples of a commercially available product
thereof include Nafion (R) manufactured by DuPont, FLEMION (R)
manufactured by AGC Inc., and Aciplex (R) manufactured by Asahi
Kasei Corporation. Usually, the polymer electrolyte membrane is
difficult to adhere, but can be adhered by using the curable resin
composition of the present invention.
[0087]
[Formula 4]
¨ 35 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
i F F -1
I I
* f CF2CF2)¨C¨C 1 it
n 1
I
0
IF ....1 x
F2C
I
F¨C ¨ 0 ¨CF2CF2¨SO3- H+
1
CF3
Nafion (R)
[0088]
The fuel electrode is referred to as a hydrogen electrode or
an anode, and a known one is used. For example, one having a
catalyst such as platinum, nickel, or ruthenium supported on carbon
Is used. In addition, the air electrode is referred to as an
oxygen electrode or a cathode, and a known one is used. For
example, one having a catalyst such as platinum or an alloy
supported on carbon is used. The surface of each electrode may
include a gas diffusion layer that serves to diffuse a gas and to
retain moisture in the electrolyte. A known material is used as
the gas diffusion layer, and examples thereof include carbon paper,
carbon cloth, and carbon fiber.
[0089]
As shown in Figure 1, the separator 2 has flow paths having
fine irregularities through which a fuel gas and an oxidation gas
pass and are supplied to the electrodes. In
addition, the
separator is made of aluminum, stainless steel, titanium, graphite,
carbon, or the like.
-- 36 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
[0090]
The frame supports and reinforces the electrolyte membrane
or the MEA, which is a thin membrane, so that the electrolyte
membrane or the MEA is not broken. Examples of the material of
the frame include a thermoplastic resin such as polyvinyl chloride,
polyethylene naphthalate, polyethylene
terephthalate,
polypropylene, or polycarbonate. In addition, in order to bond
members together using the curable resin composition of the present
invention or a cured product thereof, the frame is preferably made
of a material that transmits light.
[0091]
The fuel cell of the present invention is a fuel cell
characterized by being sealed using the curable resin composition
of the present invention or a cured product thereof. Examples of
a member that requires sealing in a fuel cell include a separator,
a frame, an electrolyte membrane, a fuel electrode, an air
electrode, and an MEA. Examples
of a more specific sealing
location include one between adjacent separators, one between a
separator and a frame, and one between a frame and an electrolyte
membrane or an MEA. The main
purpose of sealing "between a
separator and a frame" or "between a polymer electrolyte membrane
or an MEA and a frame" is to prevent gas mixing and leakage, and
the purpose of sealing between adjacent separators is to prevent
gas leakage and to prevent cooling water from leaking outside from
the cooling water flow path. The acid
generated from the
electrolyte membrane produces a strong acid atmosphere, and thus
the sealing is required to have acid resistance.
[0092]
<Sealing method>
The sealing method using the curable resin composition of
the present invention is not particularly limited, and
representative examples thereof include FIPG (form-in-place
¨ 37 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
gasketing), CIPG (cure-in-place gasketing), MIPG (mold-in-place
gasketing), and liquid injection molding.
[0093]
FIPG is a method involving applying the curable resin
composition of the present invention to a flange of a part to be
sealed using an automatic application apparatus or the like, and
irradiating the curable resin composition with an active energy
ray such as an ultraviolet ray from the flange side that is
permeable to light, in the state of the flange being bonded to
another flange, to cure the curable resin composition to adhere
and seal the flanges. More specifically, the method is a sealing
method for sealing at least a portion between at least two flanges
of a part to be sealed having the at least two flanges, wherein
at least one of the flanges is permeable to an active energy ray,
the sealing method including: a step of applying the above curable
resin composition to a surface of at least one flange of the
flanges; a step of bonding the one flange (application surface)
to which the curable resin composition is applied and another
flange together via the curable resin composition; and a step of
irradiating the curable resin composition with an active energy
ray through the flange that is permeable to the active energy ray
to cure the curable resin composition to seal at least a portion
between the at least two flanges.
[0094]
CIPG is a method involving bead-applying the curable resin
composition of the present invention to a flange of a part to be
sealed using a screen printing application apparatus, an automatic
application apparatus, or the like, irradiating the curable resin
composition with an active energy ray such as an ultraviolet ray
to cure the curable resin composition to form a gasket, and bonding
the flange and another flange together to compress and seal the
flanges. More specifically, the method is a sealing method for
¨ 38 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
sealing at least a portion between at least two flanges of a part
to be sealed having the at least two flanges, the sealing method
including: a step of applying the above curable resin composition
to at least one flange of the flanges; a step of irradiating the
applied curable resin composition with an active energy ray to
cure the curable resin composition to form a gasket made of a
cured product of the curable resin composition; and a step of
disposing another flange on the gasket and crimping the one flange
to which the curable resin composition is applied and the another
flange via the gasket to seal at least a portion between the at
least two flanges.
[0095]
MIPG is a method involving pressing a mold against a flange
of a part to be sealed in advance, injecting a curable resin
composition into a cavity formed between the mold made of a
material that is permeable to light and the flange, irradiating
the curable resin composition with an active energy ray such as
an ultraviolet ray to photocure the curable resin composition to
form a gasket, and bonding the flange and another flange together
to compress and seal the flanges. The mold is preferably made of
a material that is permeable to light, and specific examples of
the material include glass, polymethyl methacrylate (PMMA),
polycarbonate, a cycloolefin polymer, and an olefin. In addition,
in order to make it easy to remove the gasket from the mold after
gasket formation, it is preferable to apply a release agent such
as a fluorine-based release agent or a silicone-based release
agent to the mold in advance. More specifically, the method is a
sealing method for sealing at least a portion between at least two
flanges of a part to be sealed having the at least two flanges,
the sealing method including: a step of disposing a mold for gasket
formation on at least one flange of the flanges; a step of
injecting the above curable resin composition into at least a
¨ 39 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
portion of a gap between the mold for gasket formation and the one
flange on which the mold is disposed; a step of irradiating the
curable resin composition with the active energy ray to cure the
curable resin composition to form a gasket made of a cured product
of the curable resin composition; a step of removing the mold from
the one flange; and a step of disposing another flange on the
gasket and crimping the one flange and the another flange via the
gasket to seal at least a portion between the at least two flanges.
[0096]
The liquid injection molding is a method involving pouring
the curable resin composition of the present invention under a
specific pressure into a mold made of a material that is permeable
to light, irradiating the curable resin composition with an active
energy ray such as an ultraviolet ray to photocure the curable
resin composition to form a gasket, and bonding the flange and
another flange together to compress and seal the flanges. The
mold is preferably made of a material that is permeable to light,
and specific examples of the material include glass, PMMA,
polycarbonate, a cycloolefin polymer, and an olefin. In addition,
in order to make it easy to remove the gasket from the mold after
gasket formation, it is preferable to apply a release agent such
as a fluorine-based release agent or a silicone-based release
agent to the mold in advance.
Examples
[0097]
The present invention will be described in more detail with
reference to Examples below, but the present invention is not
limited to these Examples.
[0 0 98]
<Production of al>
Production of polyisobutylene having acryloyloxyethoxyphenyl
¨ 40 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
group (al)
The inside of a 5 L separable flask was purged with nitrogen, then
200 mL of n-hexane and 2000 mL of butyl chloride were added, and
the resulting mixture was cooled to -70 C in a nitrogen atmosphere
with stirring. Next, 840 mL (9 mol) of isobutylene, 12 g (0.05
mol) of p-dicumyl chloride and 1.1 g (0.012 mol) of 2-
methylpyridine were added. After the reaction mixture was cooled
to -70 C, 5.0 mL (0.05 mol) of titanium tetrachloride was added to
initiate polymerization. Three hours after the initiation of
polymerization, 40 g of phenoxyethyl acrylate (Light Acrylate PO-
A, manufactured by Kyoeisha Chemical Co., Ltd.) and 110 ml of
titanium tetrachloride were added. After that, after continuing
stirring at -70 C for 4 hours, 1000 ml of methanol was added to
terminate the reaction.
[0099]
The supernatant liquid was separated from the reaction
solution and the solvent and the like were distilled off, then the
product was dissolved in 3000 ml of n-hexane, washed with 3000 ml
of pure water three times, and reprecipitated from methanol, then
the solvent was distilled off under reduced pressure, and the
resulting polymer was vacuum-dried at 80 C for 24 hours to obtain
a polyisobutylene having an acryloyloxyethoxyphenyl group (al).
[0100]
The al contains a -[CH2C(CF13)2]- unit and contains two
acryloyl groups. More specifically, al is a polymer represented
by the general formula (1) wherein R4 represents a dicumyl group,
PIB represents a polyisobutylene skeleton, R4 represents a
hydrocarbon group having 2 carbon atoms, R2 and R2 each
independently represent a hydrogen atom, and R5 is a hydrogen atom.
The number average molecular weight (chromatographic method, in
terms of polystyrene) of the al component was 11,100, and the
viscosity (25 C) of the al component was 1550 Pas.
¨ 41 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
[0101]
<Preparation of curable resin composition>
= Example 1
100 parts by mass of the polyisobutylene having an
acryloyloxyethoxyphenyl group (al) as the (A) component of the
present invention, 7.8 parts by mass of bis(2,4,6-
trimethylbenzoy1)-phenyl-phosphine oxide (manufactured by IGM
Resins B.V., Omnirad 819) as (bl) of the (B) component of the
present invention, 0.9 parts by mass of an antifoaming agent (BYK-
1 0 1799 manufactured by BYK-Chemie GmbH, kinematic viscosity (25 C)
158 mm2/s) containing no organic solvent and containing a silicone
compound having none of a methoxysilyl group, an ethoxysily1 group,
and a (meth)acryloyl group and a hydrophobic solid as (cl) of the
(C) component, 65 parts by mass of isobornyl acrylate (IBX-A
manufactured by Kyoeisha Chemical Co., Ltd.) as (d1) of the (D)
component, and 22 parts by mass of lauryl acrylate (L-A
manufactured by Kyoeisha Chemical Co., Ltd.) as (d2) were added,
and these was mixed using a planetary mixer under light shielding
at normal temperature (25 C) for 60 minutes to obtain Example 1,
which was a curable resin composition containing no organic solvent.
[0102]
= Example 2
Example 2 containing no organic solvent was prepared and
obtained in the same manner as in Example 1, except that in Example
1, the amount of the (cl) component was changed from 0.9 parts by
mass to 1.8 parts by mass.
[0103]
= Example 3
Example 3 containing no organic solvent was prepared and
obtained in the same manner as in Example 1, except that in Example
1, the amount of the (cl) component was changed from 0.9 parts by
mass to 3.6 parts by mass.
¨ 42 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
[0104]
= Example 4
Example 4 containing no organic solvent was prepared and
obtained in the same manner as in Example 1, except that in Example
1, dimethylpolysiloxane capped with trimethylsilyl groups at both
terminals containing no organic solvent and having none of a
methoxysilyl group, an ethoxysilyl group, and a (meth)acryloyl
group (KF-96-100cps manufactured by Shin-Etsu Chemical Co., Ltd.,
kinematic viscosity (25 C): 100 mm2/s) was used as the (c2)
component instead of the (cl) component.
[0105]
= Comparative Example 1
Comparative Example 1 containing no organic solvent was
prepared and obtained in the same manner as in Example 1, except
that in Example 1, (cl) was omitted.
[0106]
= Comparative Example 2
Comparative Example 2 containing no organic solvent was
prepared and obtained in the same manner as in Example 1, except
that in Example 1, an antifoaming agent containing no organic
solvent and containing an organic polymer that was not a silicone
compound (BYK-1790 manufactured by BYK-Chemie GmbH) was used as a
(c'1) component instead of the (cl) component.
[0107]
= Comparative Example 3
Comparative Example 3 containing an organic solvent was
prepared and obtained in the same manner as in Example 1, except
that in Example 1, an antifoaming agent containing diisobutyl
ketone as an organic solvent and containing a silicone compound
having none of a methoxysilyl group, an ethoxysilyl group, and a
(meth)acryloyl group (BYK-066N manufactured by BYK-Chemie GmbH)
was used as a (c'2) component instead of the (cl) component.
¨ 43 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
[0108]
= Comparative Example 4
Comparative Example 4 containing an organic solvent was
prepared and obtained in the same manner as in Example 1, except
that in Example 1, an antifoaming agent containing propylene glycol
as an organic solvent and containing a silicone compound having
none of a methoxysilyl group, an ethoxysilyl group, and a
(meth)acryloyl group (BYK-067A manufactured by BYK-Chemie GmbH)
was used as a (c'3) component instead of the (cl) component.
[0109]
= Comparative Example 5
Comparative Example 5 containing no organic solvent was
prepared and obtained in the same manner as in Example 1, except
that in Example 1, a silicone oligomer containing no organic
solvent and having an acryloyl group and a methoxy group in a side
chain (KR-513 manufactured by Shin-Etsu Chemical Co., Ltd.) was
used as a (c'4) component instead of the (cl) component.
[0110]
The test methods used in the Examples and the Comparative
Examples in Table 1 are as follows. "-" in the table means being
unmeasured.
[0111]
(1) Evaluation of repellability during screen printing
Each curable resin composition was applied by printing onto
a polytetrafluoroethylene sheet using a SUS mesh screen printing
plate having an opening of 110 m in a 25 C environment using a
manual squeegee. It was visually checked whether or not each
curable resin composition was repelled on the
polytetrafluoroethylene sheet. Results are summarized in Table 1.
The evaluation was such that the rating "Pass" was given when
there was no repellence on the polytetrafluoroethylene sheet, and
the rating "Fail" was given when there was clear repellence.
-- 44 --
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
Polytetrafluoroethylene has a surface tension equivalent to that
of the electrolyte membrane.
[0112]
(2) Evaluation of antifoaming property during screen
printing
Each curable resin composition was applied by printing onto
a polytetrafluoroethylene sheet using a SUS mesh screen printing
plate having an opening of 110 m in a 25 C environment using a
manual squeegee. After that, the time required for an air bubble
to disappear from the printed layer (thickness: 50 m) made of the
curable resin composition was visually checked and was used as the
antifoaming time (seconds). In
addition, time measurement was
started immediately after application by printing. Results are
summarized in Table 1. The antifoaming time is preferably within
60 seconds, more preferably within 50 seconds, and particularly
preferably within 40 seconds, from the viewpoint of line tact.
[0113]
(3) Measurement of hardness
The thickness of each curable resin composition is set to 1
mm, and the composition is cured by irradiating the composition
with an ultraviolet ray having an integrated light quantity of 45
kJ/m2 to manufacture a sheet-shaped cured product. While keeping
the impression surface of a type A durometer (hardness tester)
parallel to a test piece (six sheet-shaped cured products stacked
and set to a thickness of 6 mm), the impression surface is pressed
with a force of 10 N to bring the impression surface and the sample
into close contact. The maximum value is read at the time of
measurement, and the maximum value is defined as the "hardness."
Details comply with JIS K 6253 (2012). The hardness is preferably
15 or more, and more preferably 20 or more.
[0114]
(4) Method for measuring elongation rate of cured product
¨ 45 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
The thickness of each curable resin composition is set to 1
mm, and the composition is cured by irradiating the composition
with an ultraviolet ray having an integrated light quantity of 45
kJ/m2 to manufacture a sheet-shaped cured product. The cured
product is punched using a No. 3 dumbbell to manufacture a test
piece, and marked lines at an interval of 20 mm are drawn on the
test piece.
[0115]
The test piece is fixed to a chuck in the same manner as in
the measurement of tensile strength, and pulled at a pull speed
of 500 ram/min until the test piece is cut. At the
time of
measurement, the test piece is stretched to widen the interval
between the marked lines, and thus the interval between the marked
lines is measured using a vernier caliper until the test piece is
cut. The proportion of elongation based on the initial marked
line interval is defined as the "elongation rate (%)." Evaluation
is carried out based on the following criteria, and results are
shown in Table 1. The elongation rate is preferably 300% or more,
and more preferably 410% or more, from the viewpoint of high
elongation.
[0116]
(5) Tensile strength measurement
The thickness of a curable resin composition is set to 1 mm,
and the composition is cured by irradiating the composition with
an ultraviolet ray having an integrated light quantity of 45 kJ/m2
to manufacture a sheet-shaped cured product. The cured product
is punched using a No. 3 dumbbell to manufacture a test piece.
Both ends of the test piece are fixed to a chuck such that the
long axis of the test piece and the center of the chuck are aligned.
The test piece is pulled at a pull speed of 500 ram/min to measure
the maximum load. The strength at the maximum load is defined as
the "tensile strength (MPa)." Details comply with JIS K 6251
¨ 46 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
(2010). The tensile strength is preferably 3.9 MPa or more, and
more preferably 4.2 MPa or more.
[0117]
[Table 1]
(2)
(1) Evaluation
Evaluation of
of antifoaming (3) (4) (5) Tensile
repellability property Hardness Elongation strength
during during (A) rate (c/o) (MPa)
screen screen
printing printing
(second)
Example 1 Pass 40 29 500 4.2
Example 2 Pass 20 29 575 5.7
Example 3 Pass 10 28 510 4.4
Example 4 Pass 40 30 581 5.5
Comparative
Fail 120 29 560 4.6
Example 1
Comparative
Fail 120 - - -
Example 2
Comparative
Fail 40 28 465 3.5
Example 3
Comparative
Fail 30 29 500 3.8
Example 4
Comparative
Fail 10 33 405 3.6
Example 5
[0118]
According to Examples 1 to 4 in Table 1, it can be seen that
the present invention is a curable resin composition that is
compatible with application by screen printing while maintaining
cured product properties of high strength and high elongation.
[0119]
In addition, Comparative Example 1 in Table 1 is a curable
resin composition not containing (cl) of the (C) component of the
¨ 47 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
present invention, and had the following results: there was
repellence from the adherend when applied by screen printing and
the antifoaming property was inferior. In addition, Comparative
Example 2 is a curable resin composition using (c'1) instead of
(cl) of the (C) component of the present invention, and had the
following results: there was repellence from the adherend when
applied by screen printing and the antifoaming property was
inferior. In addition, Comparative Examples 3 and 4 are a curable
resin composition using "(c'2) or (c'3), which was an antifoaming
agent containing a solvent," instead of (cl) of the (C) component
of the present invention, and had the following results: there was
repellence from the adherend when applied by screen printing and
the tensile strength of the cured product was inferior. In
addition, Comparative Example 5 is a curable resin composition
using (c'4) instead of (cl) of the (C) component of the present
invention, and had the following results: there was repellence
from the adherend when applied by screen printing and the tensile
strength and the elongation rate of the cured product were inferior.
[0120]
Further, (6) viscosity measurement and (7) moisture
permeability (water vapor barrier property) were evaluated as
follows.
[0121]
(6) Viscosity measurement
The viscosity (Pas) of a curable resin composition was
measured using a cone-plate type viscometer (manufactured by
Brookfield) based on the following measurement conditions.
Evaluation is carried out based on the following criteria, and
results are shown in Table 2. The viscosity is preferably 100
Pas or less, more preferably 0.1 to 50 Pas, and particularly
preferably in the range of 0.2 to 10 Pas.
[0122]
¨ 48 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
From the results in Table 2, it was found that the
viscosities of Examples 1 to 4 and Comparative Examples 1 and 3
to 5 were comparable.
[0123]
[Measurement conditions]
Cone-type CPE-52, shear rate of 10 (us), temperature of 25 C.
[0124]
[Table 2]
Comparati Comparati Comparati Comparati Comparati
Examp Examp Examp Examp
ye ye ye ye ye
le 1 1e2 1e3 1e4
Example 1 Example 2 Example 3 Example 4 Example 5
(6)
Viscosi
5.3 5.5 5.2 5.5 5.4 4.9 6.4 5.3
ty
(Pas)
[0125]
(7) Moisture permeability (water vapor barrier property)
The curable resin composition of Example 2 or 4 was poured
into a frame of 200 mm x 200 mm x 1.0 mm. After that, the curable
resin composition was irradiated with an ultraviolet ray for 20
seconds using an ultraviolet irradiator such that the integrated
light quantity was 45 kJ/m2 to prepare a sheet-shaped cured product
having a thickness of 1.0 mm. 5 g of calcium chloride (anhydrous)
was placed in an aluminum cup having an opening having a diameter
of 30 mm, and was set in the cup in such a way as to cover the
cured product. The "initial total weight" (g) was measured, then
the cup was left in a constant temperature and humidity bath kept
at an ambient temperature of 40 C and a relative humidity of 95%
for 24 hours, the "total weight after leaving" (g) was measured,
and the moisture permeability (g/m2.24h) was calculated and
evaluated based on the following evaluation criteria. Both
Examples 2 and 4 were rated as Pass. The detailed test method
complied with JIS Z 0208-1976. In the case of use as a curable
¨ 49 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
sealing agent for a fuel cell, the moisture permeability is
preferably less than 10 g/m2.24 h.
[0126]
[Evaluation Criteria]
Pass: Moisture permeability of less than 10 g/m2.24 h
Fail: Moisture permeability of 10 g/m2.24 h or more
[0127]
The present application is based on Japanese Patent
Application No. 2020-146349 filed on August 31, 2020, the
disclosure of which is incorporated herein by reference in its
entirety.
Industrial Applicability
[0128]
The present invention has been made in view of the above
circumstances, and is a curable resin composition that is
compatible with application by screen printing while maintaining
cured product properties of high strength and high elongation and
thus can be used for various sealing uses. In particular, the
present invention is industrially useful because it is effective
.. as a curable sealing agent for a fuel cell.
Reference Signs List
[0129]
1: cell of polymer electrolyte fuel cell
2: separator
3a: air electrode (cathode)
3b: fuel electrode (anode)
4: polymer electrolyte membrane
5: electrolyte membrane electrode assembly (MEA)
6: frame
7: adhesive or sealing agent
8a: oxidation gas flow path
8b: fuel gas flow path
¨ 50 ¨
Date Recue/Date Received 2023-02-15
CA 03192238 2023-02-15
9: cooling water flow path
10: cell stack
11: polymer electrolyte fuel cell
¨ 51 ¨
Date Recue/Date Received 2023-02-15