Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/073011
PCT/US2021/071648
METHODS AND SYSTEMS TO IMPROVE THE SIGNAL TO NOISE RATIO OF
DNA METHYLATION PARTITIONING ASSAYS
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims the benefit of priority of US Provisional
Patent
Application No. 63/086,000, filed September 30, 2020, and US Provisional
Patent
Application No. 63/105,183, filed October 23, 2020, each of which is
incorporated by
reference herein in its entirety for all purposes.
FIELD OF THE INVENTION
[002] The present disclosure provides compositions and methods related to
analyzing nucleic acids, such as DNA, such as cell-free DNA. In some
embodiments, the
cell-free DNA is from a subject having or suspected of having cancer and/or
the cell-free
DNA includes DNA from cancer cells. In some embodiments, the DNA is
partitioned into a
plurality of partitioned sets based on the methylation status of the nucleic
acid molecules, and
at least a subset of at least one partitioned set is digested with at least
one methylation
sensitive restriction enzyme.
BACKGROUND
[003] Current methods of cancer diagnostic assays of cell-free nucleic
acids (e.g.,
cell-free DNA or cell-free RNA) may focus on the detection of tumor-related
somatic
variants, including single nucleotide variants (SNVs), copy number variations
(CNVs),
fusions, and indels (i.e., insertions or deletions), which are all mainstream
targets for liquid
biopsy. There is growing evidence that non-sequence modifications like
methylation status
and fragmentomic signal in cell-free DNA can provide information on the source
of cell-free
DNA and disease level. The non-sequence modifications of the cell-free DNA,
when
combined with somatic mutation calling, can yield a more comprehensive
assessment of
tumor status than that available from either approach alone.
[004] However, it has been challenging to develop accurate and sensitive
methods
for analyzing liquid biopsy material that provide detailed information
regarding nucleobase
modifications given the low concentration and heterogeneity of cell-free DNA.
Isolating and
processing the fractions of cell-free DNA useful for further analysis in
liquid biopsy
- 1 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
procedures is an important part of these methods. Accordingly, there is a need
for improved
methods and compositions for analyzing cell-free DNA, e.g., in liquid
biopsies.
SUMMARY
[005] The present disclosure aims to meet the need for
improved analysis of cell-
free DNA and/or provide other benefits. The present disclosure provides
methods,
compositions, and systems for analyzing nucleic acids. Accordingly, the
following exemplary
embodiments are provided. Embodiment 1 is a method for analyzing nucleic acid
molecules
in a biological sample, comprising:
a) partitioning at least a subset of the nucleic acid molecules in the
biological
sample, based on the methylation status of the nucleic acid molecules into a
plurality
of partitioned sets, wherein the biological sample comprises methylated
nucleic acid
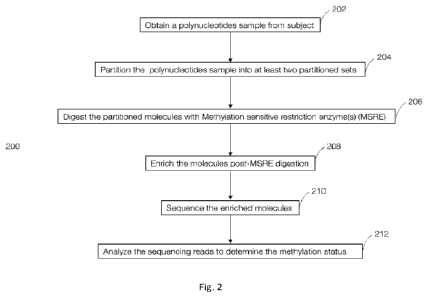
molecules and unmethylated nucleic acid molecules;
b) digesting at least a subset of the one or more partitioned sets in the
plurality
of partitioned sets with at least one methylation sensitive restriction
enzyme; and
c) determining methylation status at one or more genetic loci of the
nucleic acid
molecules in at least one of the partitioned sets.
[006] Embodiment 2 is a method for determining methylation
status of nucleic acid
molecules, comprising:
a) providing a biological sample of nucleic acid molecules, wherein the
nucleic
acid molecules comprises methylated nucleic acid molecules and unmethylated
nucleic acid molecules;
b) partitioning at least a subset of the nucleic acid molecules in the
biological
sample based on the methylation status of the nucleic acid molecules into a
plurality
of partitioned sets;
c) digesting at least a subset of the one or more partitioned sets in the
plurality
of partitioned sets with at least one methylation sensitive restriction
enzyme;
d) enriching at least a subset of the nucleic acid molecules in the
plurality of
partitioned sets for genomic regions of interest, wherein the at least a
subset of the
nucleic acid molecules comprises digested nucleic acid molecules in the one or
more
partitioned sets; and
e) determining methylation status at one or more genetic loci of the
nucleic acid
molecules in at least one of the partitioned sets.
- 2 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[007] Embodiment 3 is a method of analyzing nucleic acid molecules in a
biological sample, comprising:
a) partitioning at least a subset of the nucleic acid molecules in the
biological
sample, based on the methylation status of the nucleic acid molecules into a
plurality
of partitioned sets, wherein the biological sample comprises methylated
nucleic acid
molecules and unmethylated nucleic acid molecules and the plurality of
partitioned
sets comprises a first partitioned set and a second partitioned set, wherein
methylated
nucleic acid molecules are overrepresented in the first partitioned set
relative to the
second partitioned set;
b) digesting at least a subset of the first partitioned set in the
plurality of
partitioned sets with at least one methylation sensitive restriction enzyme;
and
c) capturing a first target region set comprising epigenetic target regions
from
at least a portion of a first partitioned set, and capturing a second target
region set
comprising epigenetic target regions from at least a portion of the second
partitioned
set.
[008] Embodiment 4 is the method of embodiment 3, wherein capturing the first
target region set comprises contacting the DNA of the first partitioned set
with a first set of
target-specific probes, and capturing the second target region set comprises
contacting the
DNA of the second partitioned set with a second set of target-specific probes.
[009] Embodiment 5 is the method of embodiment 3 or 4, further comprising
determining methylation status at one or more genetic loci of the nucleic acid
molecules in at
least one of the partitioned sets or target region sets.
[010] Embodiment 6 is the method of any one of the above embodiments, wherein
the genomic regions of interest, the first target region set, and/or the
second target region set
comprise sequence-variable target regions.
[011] Embodiment 7 is the method of any one of the above embodiments, further
comprising, prior to the digesting step, attaching one or more adapters to at
least one end of at
least a portion of the nucleic acid molecules in the plurality of partitioned
sets.
[012] Embodiment 8 is a method for determining methylation status of nucleic
acid
molecules, comprising:
a) providing a biological sample of nucleic acid molecules,
wherein the nucleic
acid molecules comprises methylated nucleic acid molecules and unmethylated
nucleic acid molecules;
- 3 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
b) partitioning at least a subset of the nucleic acid molecules in the
biological
sample based on the methylation status of the nucleic acid molecules into a
plurality
of partitioned sets;
c) attaching one or more adapters to at least one end of the nucleic acid
molecules in the plurality of partitioned sets;
d) digesting at least a subset of the one or more partitioned sets in the
plurality
of partitioned sets with at least one methylation sensitive restriction
enzyme;
e) enriching at least a subset of the nucleic acid molecules in the
plurality of
partitioned sets for genomic regions of interest; wherein the at least a
subset of the
nucleic acid molecules comprises digested nucleic acid molecules in the one or
more
partitioned sets; and
determining methylation status at one or more genetic loci of the nucleic acid
molecules in at least one of the partitioned sets.
[013] Embodiment 9 is the method of embodiment 7 or 8, wherein adapters are
attached to both ends of at least a portion of the nucleic acid molecules in
the plurality of
partitioned sets.
[014] Embodiment 10 is the method of embodiment 1, further comprising, prior
to
c), enriching at least a subset of the nucleic acid molecules in the plurality
of partitioned sets
for genomic regions of interest, wherein the at least a subset of the nucleic
acid molecules
comprises digested nucleic acid molecules in the one or more partitioned sets.
[015] Embodiment 11 is the method of any one of the preceding embodiments,
further comprising detecting presence or absence of cancer in the biological
sample.
[016] Embodiment 12 is the method of any one of the above embodiments, further
comprising determining a level of cancer in the biological sample
[017] Embodiment 13 is the method of any one of the above embodiments,
wherein determining the methylation status comprises sequencing at least a
subset of the
digested nucleic acid molecules.
[018] Embodiment 14 is the method of any one of embodiments 7-13, wherein the
one or more adapters comprises at least one tag.
[019] Embodiment 15 is the method of any one of the above embodiments,
wherein the methylation sensitive restriction enzyme selectively digests
nucleic acid
molecules that are unmethylated at the recognition site of the methylation
sensitive restriction
enzyme.
- 4 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[020] Embodiment 16 is the method of any one of the above embodiments,
wherein at least a portion of nucleic acid molecules are amplified and/or
sequenced after the
digesting step, and nucleic acid molecules that were digested by the
methylation sensitive
restriction enzyme are not amplified and/or are not sequenced.
[021] Embodiment 17 is the method of any one of the above embodiments
comprising digesting at least a subset of the one or more partitioned sets in
the plurality of
partitioned sets with at least two methylation sensitive restriction enzymes.
[022] Embodiment 18 is the method of embodiment 17, wherein the at least two
methylation sensitive restriction enzymes consist of two methylation sensitive
restriction
enzymes
[023] Embodiment 19 is the method of embodiment 17 or 18, wherein the
methylation sensitive restriction enzymes comprise or consist of BstUI and
HpaII.
[024] Embodiment 20 is the method of embodiment 17 or 18, wherein the
methylation sensitive restriction enzymes comprise or consist of HhaI and
AccII.
[025] Embodiment 21 is the method of embodiment 17 or 18, wherein the at least
two methylation sensitive restriction enzymes comprise or consist of three
methylation
sensitive restriction enzymes.
[026] Embodiment 22 is the method of embodiment 17 or 21, wherein the
methylation sensitive restriction enzymes comprise or consist of BstUI, Hpall
and Hin6I.
[027] Embodiment 23 is the method of any one of the above embodiments,
wherein the methylation sensitive restriction enzyme is selected from the
group consisting of
AatII, AccII, AciI, Aor13HI, Aor15HI, BspT104I, BssHII, BstUI, Cfr10I, ClaI,
CpoI,
Eco52I, HaeII, HapII, HhaI, Hin6I, HpaII, HpyCH4IV, MluI, MspI, NaeI, NotI,
NruI, NsbI,
PmaCI, Psp14061, PvuI, SacII, Salt, SmaI, and SnaBI.
[028] Embodiment 24 is the method of any one of embodiments 7-23, wherein the
one or more adapters are resistant to digestion by the methylation sensitive
restriction
enzymes.
[029] Embodiment 25 is the method of embodiment 24, wherein the one or more
resistant adapters comprise one or more methylated nucleotides, optionally
wherein the
methylated nucleotides comprise 5-methylcytosine and/or 5-
hydroxymethylcytosine.
[030] Embodiment 26 is the method of embodiment 24, wherein the one or more
resistant adapters comprise one or more nucleotide analogs resistant to
methylation sensitive
restriction enzymes.
- 5 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[031] Embodiment 27 is the method of embodiment 24, wherein the one or more
resistant adapter comprises a nucleotide sequence not recognized by
methylation sensitive
restriction enzymes.
[032] Embodiment 28 is the method of any one of embodiments 14-27, wherein
the tag comprises a molecular barcode.
[033] Embodiment 29 is the method of embodiment 28, wherein the molecular
barcodes attached to nucleic acid molecules in a first partitioned set of the
plurality of
partitioned sets are different from the molecular barcodes attached to nucleic
acid molecules
in a second partitioned set of the plurality of partitioned sets.
[034] Embodiment 30 is the method of embodiments 1-29, wherein a first
partitioned set of the plurality of partitioned sets is differentially tagged
from a second
partitioned set of the plurality of partitioned sets.
[035] Embodiment 31 is the method of embodiment 30, wherein a first partition
tag is attached to nucleic acid molecules in the first partitioned set and a
second partition tag
is attached to nucleic acid molecules in the second partitioned set.
[036] Embodiment 32 is the method of any one of the above embodiments,
wherein the methylated nucleic acid molecules comprise 5-methylcytosine and/or
5-
hydroxymethylcytosine.
[037] Embodiment 33 is the method of any one of embodiments 13-32, wherein
the sequencing is performed by a next generation sequencer.
[038] Embodiment 34 is the method of any one of the preceding embodiments,
wherein the biological sample is selected from the group consisting of a DNA
sample, an
RNA sample, a polynucleotide sample, a cell-free DNA sample, and a cell-free
RNA sample.
[039] Embodiment 35 is the method of any one of the preceding embodiments,
wherein the biological sample is a cell-free DNA sample.
[040] Embodiment 36 is the method of embodiment 35, wherein the cell-free DNA
is between 1 ng and 500 ng.
[041] Embodiment 37 is the method of any one of the preceding embodiments,
wherein the partitioning comprises partitioning the nucleic acid molecules
based on a
differential binding affinity of the nucleic acid molecules to a binding agent
that
preferentially binds to nucleic acid molecules comprising methylated
nucleotides.
[042] Embodiment 38 is the method of embodiment 37, wherein the binding agent
is a methyl binding domain (MBD) protein.
- 6 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[043] Embodiment 39 is the method of embodiment 37, wherein the binding agent
is an antibody that is specific to one or more methylated nucleotide bases.
[044] Embodiment 40 is the method of any one of embodiments 2-39, wherein the
genomic regions of interest or epigenetic target regions comprise
differentially methylated
regions for cancer detection.
[045] Embodiment 41 is the method of any one of embodiments 13-40, further
comprising, prior to the sequencing, amplifying at least a portion of the
nucleic acid
molecules.
[046] Embodiment 42 is the method of embodiment 41, wherein primers used in
the amplification comprise at least one sample index.
[047] Embodiment 43 is the method of any one of the above embodiments,
wherein the one or more genetic loci comprises a plurality of genetic loci.
[048] Embodiment 44 is the method of embodiment 43, wherein the plurality of
genetic loci comprises one or more genomic regions.
[049] In any of the foregoing embodiments, epigenetic target regions may be
captured from one or more, or each, of the partitioned sets. Any of the
methods may further
comprise quantifying captured epigenetic target regions, e.g., by sequencing
or quantitative
PCR. In some embodiments, the methods comprise capturing a first target region
set
comprising epigenetic target regions from at least a portion of a first
partitioned set, and
capturing a second target region set comprising epigenetic target regions from
at least a
portion of the second partitioned set. The first and second target region sets
may be the same
or different.
[050] The epigenetic target regions may comprise a hypermethylation
variable
target region set, e.g., comprising regions having a higher degree of
methylation in at least
one type of tissue than the degree of methylation in cell-free DNA from a
healthy subject.
Any of the methods may further comprise determining a presence, absence, or
likelihood of
cancer based at least in part on sequences or quantities of regions in the
hypermethylation
variable target region set. Any of the methods may further comprise
quantifying tumor DNA
in the sample based at least in part on sequences or quantities of regions in
the
hypermethylation variable target region set.
[051] The epigenetic target regions may comprise a hypomethylation variable
target region set, e.g., comprising regions having a lower degree of
methylation in at least one
type of tissue than the degree of methylation in cell-free DNA from a healthy
subject. Any of
- 7 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
the methods may further comprise determining a presence, absence, or
likelihood of cancer
based at least in part on sequences or quantities of regions in the
hypomethylation variable
target region set. Any of the methods may further comprise quantifying tumor
DNA in the
sample based at least in part on sequences or quantities of regions in the
hypomethylation
variable target region set.
[052] In any of the foregoing embodiments, sequence-variable target regions
may
be captured from one or more, or each, of the partitioned sets. Any of the
methods may
further comprise quantifying captured epigenetic target regions, e.g., by
sequencing or
quantitative PCR. DNA molecules corresponding to the sequence-variable target
region set
may be sequenced to a greater depth of sequencing than DNA molecules
corresponding to the
epigenetic target region set.
[053] In any of the foregoing embodiments, capturing target region sets may
comprise contacting DNA to be captured with a set of target-specific probes,
whereby
complexes of target-specific probes and DNA are formed. Capturing may further
comprise
separating the complexes from DNA not bound to target-specific probes, thereby
providing
captured DNA.
[054] In any of the foregoing embodiments, DNA may amplified before a
sequencing step, or DNA may be amplified before a capturing step.
[055] In any of the foregoing embodiments, the DNA may comprise DNA
obtained from a bodily fluid, optionally wherein the bodily fluid is plasma,
urine, lymph, or
spinal fluid. For example, the DNA may comprise cell-free DNA (cfDNA) obtained
from a
test subject.
[056] In any of the foregoing embodiments, the methylation-sensitive
restriction
enzyme may cleave an unmethylated CpG sequence. In any of the foregoing
embodiments,
the methylation-sensitive restriction enzyme may be one or more of AatII,
AccII, AciI,
Aor13HI, Aor15HI, BspT104I, BssHII, BstUI, Cfr10I, ClaI, CpoI, Eco52I, HaeII,
HapII,
HhaI, Hin6I, HpaII, HpyCH4IV, MluI, NaeI, NotI, NruI, NsbI, PmaCI, Psp14061,
PvuI,
SacII, Sall, SmaI, and SnaBI.
[057] In any of the foregoing embodiments, the method may further comprise
determining a likelihood that the subject has cancer. For example, wherein the
sequencing
may generates a plurality of sequencing reads; and the method may further
comprise mapping
the plurality of sequence reads to one or more reference sequences to generate
mapped
- 8 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
sequence reads, and processing the mapped sequence reads corresponding to the
sequence-
variable target region set and to the epigenetic target region set to
determine the likelihood
that the subject has cancer.
[058] In any of the foregoing embodiments, the test subject may have been
previously diagnosed with a cancer and received one or more previous cancer
treatments,
optionally wherein the cfDNA is obtained at one or more preselected time
points following
the one or more previous cancer treatments, and sequencing the captured set of
cfDNA
molecules, whereby a set of sequence information is produced. Such a method
may further
comprise detecting a presence or absence of DNA originating or derived from a
tumor cell at
a preselected timepoint using the set of sequence information. Such a method
may further
comprise determining a cancer recurrence score that is indicative of the
presence or absence
of the DNA originating or derived from the tumor cell for the test subject,
optionally further
comprising determining a cancer recurrence status based on the cancer
recurrence score,
wherein the cancer recurrence status of the test subject is determined to be
at risk for cancer
recurrence when a cancer recurrence score is determined to be at or above a
predetermined
threshold or the cancer recurrence status of the test subject is determined to
be at lower risk
for cancer recurrence when the cancer recurrence score is below the
predetermined threshold
Such a method may further comprise comparing the cancer recurrence score of
the test
subject with a predetermined cancer recurrence threshold, wherein the test
subject is
classified as a candidate for a subsequent cancer treatment when the cancer
recurrence score
is above the cancer recurrence threshold or not a candidate for a subsequent
cancer treatment
when the cancer recurrence score is below the cancer recurrence threshold.
[059] In another aspect, the present disclosure provides a system
comprising a
controller comprising or capable of accessing, computer readable media
comprising non-
transitory computer-executable instructions which, when executed by at least
one electronic
processor perform a method comprising: (a) partitioning at least a subset of
the nucleic acid
molecules in the biological sample, based on the methylation status of the
nucleic acid
molecules into a plurality of partitioned sets, wherein the biological sample
comprises
methylated nucleic acid molecules and unmethylated nucleic acid molecules; (b)
digesting at
least a subset of the one or more partitioned sets in the plurality of
partitioned sets with at
least one methylation sensitive restriction enzyme; and (c) determining
methylation status at
one or more genetic loci of the nucleic acid molecules in at least one of the
partitioned sets.
In some embodiment, the method further comprises further comprises, prior to
(c), enriching
- 9 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
at least a subset of the nucleic acid molecules in the plurality of
partitioned sets for genomic
regions of interest, wherein the at least a subset of the nucleic acid
molecules comprises
digested nucleic acid molecules in the one or more partitioned sets. In some
embodiments,
the method further comprises, prior to (b), attaching one or more adapters to
at least one end
of the nucleic acid molecules in the plurality of partitioned sets. In some
embodiments, the
method further comprises, prior to determining the methylation status,
enriching at least one
portion of the nucleic acid molecules in the plurality of partitioned sets;
wherein the at least
one portion of the nucleic acid molecules comprises digested nucleic acid
molecules in the
one or more partitioned sets.
[060] In another aspect, the present disclosure provides a system
comprising a
controller comprising or capable of accessing, computer readable media
comprising non-
transitory computer-executable instructions which, when executed by at least
one electronic
processor perform a method comprising: a) providing a biological sample of
nucleic acid
molecules, wherein the nucleic acid molecules comprises methylated nucleic
acid molecules
and unmethylated nucleic acid molecules; (b) partitioning at least a subset of
the nucleic acid
molecules in the biological sample based on the methylation status of the
nucleic acid
molecules into a plurality of partitioned sets; (c) digesting at least a
subset of the one or more
partitioned sets in the plurality of partitioned sets with at least one
methylation sensitive
restriction enzyme; (d) enriching at least a subset of the nucleic acid
molecules in the
plurality of partitioned sets for genomic regions of interest, wherein the at
least a subset of
the nucleic acid molecules comprises digested nucleic acid molecules in the
one or more
partitioned sets; and (e) determining methylation status at one or more
genetic loci of the
nucleic acid molecules in at least one of the partitioned sets. In some
embodiments, the
method further comprises, prior to (b), attaching one or more adapters to at
least one end of
the nucleic acid molecules in the plurality of partitioned sets.
[061] In another aspect, the present disclosure provides a system
comprising a
controller comprising or capable of accessing, computer readable media
comprising non-
transitory computer-executable instructions which, when executed by at least
one electronic
processor perform a method comprising: a) providing a biological sample of
nucleic acid
molecules, wherein the nucleic acid molecules comprises methylated nucleic
acid molecules
and unmethylated nucleic acid molecules; (b) partitioning at least a subset of
the nucleic acid
molecules in the biological sample based on the methylation status of the
nucleic acid
molecules into a plurality of partitioned sets; (c) attaching one or more
adapters to at least
- 10 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
one end of the nucleic acid molecules in the plurality of partitioned sets;
(d) digesting at least
a subset of the one or more partitioned sets in the plurality of partitioned
sets with at least one
methylation sensitive restriction enzyme; (e)enriching at least a subset of
the nucleic acid
molecules in the plurality of partitioned sets for genomic regions of
interest; wherein the at
least a subset of the nucleic acid molecules comprises digested nucleic acid
molecules in the
one or more partitioned sets; and (f) determining methylation status at one or
more genetic
loci of the nucleic acid molecules in at least one of the partitioned sets.
[062] In another aspect, the present disclosure provides a method for
determining
methylation status of nucleic acid molecules, comprising: (a) providing a
biological sample
of nucleic acid molecules, wherein the nucleic acid molecules comprises
methylated nucleic
acid molecules and unmethylated nucleic acid molecules; (b) partitioning at
least a subset of
the nucleic acid molecules in the biological sample based on the methylation
status of the
nucleic acid molecules into a plurality of partitioned sets; (c) attaching one
or more adapters
to at least one end of the nucleic acid molecules in the plurality of
partitioned sets; (d)
digesting at least a subset of the one or more partitioned sets in the
plurality of partitioned
sets with at least one methylation sensitive restriction enzyme; (e)enriching
at least a subset
of the nucleic acid molecules in the plurality of partitioned sets for genomic
regions of
interest; wherein the at least a subset of the nucleic acid molecules
comprises digested
nucleic acid molecules in the one or more partitioned sets; and (f)
determining methylation
status at one or more genetic loci of the nucleic acid molecules in at least
one of the
partitioned sets.
[063] In another aspect, the present disclosure provides a method for
determining
methylation status of nucleic acid molecules, comprising: (a) providing a
biological sample
of nucleic acid molecules, wherein the nucleic acid molecules comprises
methylated nucleic
acid molecules and unmethylated nucleic acid molecules; (b) partitioning at
least a subset of
the nucleic acid molecules in the biological sample based on the methylation
status of the
nucleic acid molecules into a plurality of partitioned sets; (c) digesting at
least a subset of the
one or more partitioned sets in the plurality of partitioned sets with at
least one methylation
sensitive restriction enzyme; (d) enriching at least a subset of the nucleic
acid molecules in
the plurality of partitioned sets for genomic regions of interest, wherein the
at least a subset
of the nucleic acid molecules comprises digested nucleic acid molecules in the
one or more
partitioned sets; and (e) determining methylation status at one or more
genetic loci of the
nucleic acid molecules in at least one of the partitioned sets. In some
embodiments, the
-11 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
method further comprises, prior to (b), attaching one or more adapters to at
least one end of
the nucleic acid molecules in the plurality of partitioned sets.
[064] In some embodiments, the method further comprises detecting presence or
absence of cancer in the biological sample. In some embodiments, the method
further
comprises determining a level of cancer in the biological sample, for example,
by
determining a level of DNA from cancer cells in the biological sample. In some
embodiments, determining the methylation status comprises sequencing at least
a subset of
the digested nucleic acid molecules. In some embodiments, the sequencing is
performed by a
next generation sequencer. In some embodiments, the one or more adapters
comprises at least
one tag. In some embodiments, the adapter is resistant to digestion by the
methylation
sensitive restriction enzymes. In some embodiments, the adapter comprises one
or more
methylated nucleotides (e.g., nucleotides comprising a methylated base). In
some
embodiments, the adapter comprises one or more nucleotide analogs resistant to
methylation
sensitive restriction enzymes (e.g., nucleotide analogs with a linkage
modification, such as
phosphorothioate). In some embodiments, the adapter comprises a nucleotide
sequence not
recognized by methylation sensitive restriction enzymes. In some embodiments,
the adapter
does not comprise any sequence recognized by methylation sensitive restriction
enzymes
used in the method. In some embodiments, the tag comprises molecular barcode.
In some
embodiments, the molecular barcodes attached to nucleic acid molecules in a
first partitioned
set is different from the molecular barcodes attached to nucleic acid
molecules in a second
partitioned set. In some embodiments, a first partitioned set is
differentially tagged with
respect to a second partitioned set. In some embodiments, a first partition
tag is attached to
nucleic acid molecules in a first partitioned set and a second partition tag
is attached to
nucleic acid molecules in a second partitioned set.
[065] In some embodiments, the method comprises digesting at least a subset
of
the one or more partitioned sets in the plurality of partitioned sets with at
least two
methylation sensitive restriction enzymes (MSREs). As used herein, reference
to two (or
more) MSREs means that two (or more) different MSREs with different properties
(e.g.,
different recognition sequences) are used. In some embodiments, the at least
two methylation
sensitive restriction enzymes consist of two methylation sensitive restriction
enzymes. In
some embodiments, the two methylation sensitive restriction enzymes comprise
BstUI and
HpaII. In some embodiments, the two methylation sensitive restriction enzymes
comprise
HhaI and AccII. In some embodiments, the at least two methylation sensitive
restriction
- 12 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
enzymes comprise three methylation sensitive restriction enzymes. In some
embodiments, the
three methylation sensitive restriction enzymes comprise BstUI, HpaII and
Hin6I. In some
embodiments, the methylation sensitive restriction enzyme is selected from the
group
consisting of AatII, AccII, AciI, Aor13HI, Aor15HI, BspT104I, BssHII, BstUI,
Cfr10I, ClaI,
CpoI, Eco52I, HaeII, HapII, HhaI, Hin6I, HpaII, HpyCH4IV, MluI, MspI, NaeI,
NotI, NruI,
NsbI, PmaCI, Psp14061, PvuI, SacII, Sall, SmaI, and SnaBI. In some
embodiments, at least
one MSRE selectively digests unmethylated nucleic acid molecules. In some
embodiments, at
least one MSRE selectively digests methylated nucleic acid molecules.
[066] In some embodiments, the methylated nucleotides comprise 5-
methylcytosine and/or 5-hydroxymethylcytosine. In some embodiments, the
biological
sample is selected from the group consisting of a DNA sample, an RNA sample, a
polynucleotide sample, a cell-free DNA sample, and a cell-free RNA sample. In
some
embodiments, the biological sample is a cell-free DNA sample. In some
embodiments, the
cell-free DNA is between 1 ng and 500 ng.
[067] In some embodiments, the partitioning comprises partitioning the
nucleic
acid molecules based on a differential binding affinity of the nucleic acid
molecules to a
binding agent that preferentially binds to nucleic acid molecules comprising
methylated
nucleotides (e.g., nucleotides comprising a methylated base) In some
embodiments, the
binding agent is a methyl binding domain (MBD) protein. In some embodiments,
the binding
agent is an antibody that is specific to one or more methylated nucleotide
bases. In some
embodiments, the genomic regions of interest comprise differentially
methylated regions for
cancer detection.
[068] In some embodiments, the method comprises further comprises, prior to
the
sequencing, amplifying at least a portion of the nucleic acid molecules (e.g.,
after the
digesting step, or after the enriching or capturing step). In some
embodiments, the primers
used in the amplification comprise at least one sample index. In some
embodiments, nucleic
acid molecules digested by a MSRE are not amplified. In some such embodiments,
essentially all nucleic acid molecules in a sample are amplified except the
nucleic acid
molecules digested by a MSRE.
[069] In some embodiments, the one or more genetic loci comprises plurality
of
genetic loci. In some embodiments, the plurality of genetic loci comprises one
or more
genomic regions.
- 13 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[070] In some embodiments, the method comprises digesting at least a subset of
the one or more partitioned sets in the plurality of partitioned sets with at
least two
methylation sensitive restriction enzymes. In some embodiments, the at least
two methylation
sensitive restriction enzymes consist of two methylation sensitive restriction
enzymes. In
some embodiments, the two methylation sensitive restriction enzymes comprise
BstUI and
HpaII. In some embodiments, the two methylation sensitive restriction enzymes
comprise
HhaI and AccII. In some embodiments, the at least two methylation sensitive
restriction
enzymes comprise three methylation sensitive restriction enzymes. In some
embodiments, the
three methylation sensitive restriction enzymes comprise BstUI, Hpall and
Hin6I. In some
embodiments, the methylation sensitive restriction enzyme is selected from the
group
consisting of AatII, AccII, AciI, Aor13HI, Aor15HI, BspT104I, BssHII, BstUI,
Cfr10I, ClaI,
CpoI, Eco52I, HaeII, HapII, HhaI, Hin6I, HpaII, HpyCH4IV, MluI, MspI, NaeI,
NotI, NruI,
NsbI, PmaCI, Psp14061, PvuI, SacII, Sall, SmaI, and SnaBI. In some
embodiments, at least
one MSRE selectively digests unmethylated nucleic acid molecules. In some
embodiments, at
least one MSRE selectively digests methylated nucleic acid molecules.
[071] In some embodiments of each and every aspect of the invention, the
results
of the systems and/or methods disclosed herein are used as an input to
generate a report The
report may be in a paper or electronic format. For example, information on the
presence or
absence of cancer, as determined by the methods or systems disclosed herein,
can be
displayed in such a report. Alternatively or additionally, the report may
comprise information
relating to the epigenetic rates of the epigenetic features, for example
whether they are above
or below the adjusted epigenetic rate threshold. The methods or systems
disclosed herein may
further comprise a step of communicating the report to a third party, such as
the subject from
whom the sample derived or a health care practitioner.
[072] The various steps of the methods disclosed herein, or the steps
carried out by
the systems disclosed herein, may be carried out at the same time or different
times, and/or in
the same geographical location or different geographical locations, e.g.
countries. The various
steps of the methods disclosed herein can be performed by the same person or
different
people.
[073] Additional aspects and advantages of the present disclosure will
become
readily apparent to those skilled in this art from the following detailed
description, wherein
only illustrative embodiments of the present disclosure are shown and
described. As will be
realized, the present disclosure is capable of other and different
embodiments, and its several
- 14 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
details are capable of modifications in various obvious respects, all without
departing from
the disclosure. Accordingly, the drawings and description are to be regarded
as illustrative in
nature, and not as restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
[074] The accompanying drawings, which are incorporated in and constitute a
part
of this specification, illustrate certain embodiments, and together with the
written description,
serve to explain certain principles of the methods, computer readable media,
and systems
disclosed herein. The description provided herein is better understood when
read in
conjunction with the accompanying drawings which are included by way of
example and not
by way of limitation. It will be understood that like reference numerals
identify like
components throughout the drawings, unless the context indicates otherwise. It
will also be
understood that some or all of the figures may be schematic representations
for purposes of
illustration and do not necessarily depict the actual relative sizes or
locations of the elements
shown.
[075] FIG. 1 is a schematic diagram of a methylation sensitive restriction
enzyme
(MSRE) digesting/cleaving the DNA as the restriction enzyme (RE) recognition
site contains
unmethylated nucleotides (top) and a schematic diagram of a methylation
sensitive restriction
enzyme (MSRE) not cleaving the DNA as the restriction enzyme (RE) recognition
site
contains a methylated nucleotide (bottom). Thus, Figure 1 shows one type of
MSRE, which
selectively digests recognition sites comprising unmethylated nucleotides and
generally does
not digest recognition sites comprising methylated nucleotides.
[076] FIG. 2 is a flow chart representation of a method for determining the
methylation status of nucleic acid molecules in a polynucleotide sample
obtained from a
subject according to an embodiment of the disclosure.
[077] FIG. 3 is a flow chart representation of a method for detecting the
presence
or absence of cancer in a subject according to an embodiment of the disclosure
[078] FIG. 4 is a schematic diagram of a method for detecting the presence or
absence of cancer in a subject according to certain embodiments of the
disclosure.
[079] FIG. 5 schematic diagram of an example of a system suitable for use with
some embodiments of the disclosure.
- 15 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[080] FIG. 6 shows the molecule count in the three partitions with and without
MSRE treatments in normal and diluted CRC samples.
[081] FIG. 7 shows CpG methylation quantification results obtained as
described
in Example 3 for three samples from subjects with early stage colorectal
cancer ("Early
CRC") and three healthy subjects ("Normal"). For the Early CRC plots, MAF
indicates
mutant allele fraction.
[082] FIGs. 8A-D show counts of positive and negative control molecules having
FspEI palindromic sites for the indicated enzyme and buffer conditions, as
described in
Example 4. Figs. 8A and 8C correspond to a first donor and Figs. 8B and 8D
correspond to a
second donor. Data points are distributed along the horizontal axis for
readability.
[083] FIGs. 9A-D show digestion efficiency and positive control molecule
counts
as described in Example 4.
[084] FIGs. 10A-J show hypomethylation variable target region ("Hypo VTR")
molecule counts (10A-E) or Hypo VTR/negative control molecule ratios (10F-J)
for the
indicated conditions as described in Example 5. Data points are distributed
along the
horizontal axis for readability. Triangles, circles, plus signs, and squares
indicate that the
source of the normal cfDNA was the first, second, third, or fourth of four
healthy donors,
respectively.
DEFINITIONS
[085] In order for the present disclosure to be more readily understood,
certain
terms are first defined below. Additional definitions for the following terms
and other terms
may be set forth through the specification. If a definition of a term set
forth below is
inconsistent with a definition in an application or patent that is
incorporated by reference, the
definition set forth in this application should be used to understand the
meaning of the term.
[086] As used in this specification and the appended claims, the singular
forms
"a", "an", and "the" include plural references unless the context clearly
dictates otherwise.
Thus, for example, a reference to "a method" includes one or more methods,
and/or steps of
the type described herein and/or which will become apparent upon reading this
disclosure and
so forth.
[087] It is also to be understood that the terminology used herein is for
the purpose
of describing particular embodiments only, and is not intended to be limiting.
Further, unless
defined otherwise, all technical and scientific terms used herein have the
same meaning as
- 16 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
commonly understood by one of ordinary skill in the art to which this
disclosure pertains. In
describing and claiming the methods, computer readable media, and systems, the
following
terminology, and grammatical variants thereof, will be used in accordance with
the
definitions set forth below.
[088] About: As used herein, "about" or "approximately" as applied to one or
more
values or elements of interest, refers to a value or element that is similar
to a stated reference
value or element. In certain embodiments, the term "about- or "approximately-
refers to a
range of values or elements that falls within 25%, 20%, 19%, 18%, 17%, 16%,
15%, 14%,
13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either
direction
(greater than or less than) of the stated reference value or element unless
otherwise stated or
otherwise evident from the context (except where such number would exceed 100%
of a
possible value or element).
[089] Adapter: As used herein, "adapter" refers to a short nucleic acid
(e.g., less
than about 500 nucleotides, less than about 100 nucleotides, or less than
about 50 nucleotides
in length) that is typically at least partially double-stranded and is
attached to either one end
or both ends (i.e., two adapters are attached to both ends of the nucleic acid
¨ one adapter at
end of the nucleic acid) of a given sample nucleic acid molecule. Adapters can
include
nucleic acid primer binding sites to permit amplification of a nucleic acid
molecule flanked
by adapters at both ends, and/or a sequencing primer binding site, including
primer binding
sites for sequencing applications, such as various next-generation sequencing
(NGS)
applications. Adapters can also include binding sites for capture probes, such
as an
oligonucleotide attached to a flow cell support or the like. Adapters can also
include a
nucleic acid tag as described herein. Nucleic acid tags are typically
positioned relative to
amplification primer and sequencing primer binding sites, such that a nucleic
acid tag is
included in amplicons and sequence reads of a given nucleic acid molecule.
Adapters of the
same or different sequences can be linked to the respective ends of a nucleic
acid molecule.
In some embodiments, the adapters of the same sequence is linked to the
respective ends of
the nucleic acid molecule except that the nucleic acid tag differs. In some
embodiments, the
adapter is a Y-shaped adapter in which one end is blunt ended or tailed as
described herein,
for joining to a nucleic acid molecule, which is also blunt ended or tailed
with one or more
complementary nucleotides and the other end of the Y-shaped adapter comprises
a non-
complementary sequence which does not hybridize to form a double-strand. In
still other
example embodiments, an adapter is a bell-shaped adapter that includes a blunt
or tailed end
- 17 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
for joining to a nucleic acid molecule to be analyzed. Other examples of
adapters include T-
tailed and C-tailed adapters.
[090] Amplify: As used herein, "amplify" or "amplification" in the context of
nucleic acids refers to the production of multiple copies of a polynucleotide,
or a portion of
the polynucleotide, typically starting from a small amount of the
polynucleotide (e.g., a single
polynucleotide molecule), where the amplification products or amplicons are
generally
detectable. Amplification of polynucleotides encompasses a variety of chemical
and
enzymatic processes. Amplification includes but is not limited to polymerase
chain reaction
(PCR).
[091] Barcode: As used herein, "barcode" in the context of nucleic acids
refers to
a nucleic acid molecule comprising a sequence that can serve as a identifier.
For example,
the barcode can serve as an identifier of the molecule (i.e., molecular
barcode), an identifier
of the sample (i.e., sample barcode) or an identifier of the partition (i.e.,
partition barcode).
The individual "barcode" sequences are typically added to each DNA fragment
during next-
generation sequencing (NGS) library preparation so that each read can be
identified and
sorted before the final data analysis.
[092] Cancer Type: As used herein, "cancer type" refers to a type or subtype
of
cancer defined, e.g., by hi stopathology. Cancer type can be defined by any
conventional
criterion, such as on the basis of occurrence in a given tissue (e.g., blood
cancers, central
nervous system (CNS), brain cancers, lung cancers (small cell and non-small
cell), skin
cancers, nose cancers, throat cancers, liver cancers, bone cancers, lymphomas,
pancreatic
cancers, bowel cancers, rectal cancers, thyroid cancers, bladder cancers,
kidney cancers,
mouth cancers, stomach cancers, breast cancers, prostate cancers, ovarian
cancers, lung
cancers, intestinal cancers, soft tissue cancers, neuroendocrine cancers,
gastroesophageal
cancers, head and neck cancers, gynecological cancers, colorectal cancers,
urothelial cancers,
solid state cancers, heterogeneous cancers, homogenous cancers), unknown
primary origin
and the like, and/or of the same cell lineage (e.g., carcinoma, sarcoma,
lymphoma,
cholangiocarcinoma, leukemia, mesothelioma, melanoma, or glioblastoma) and/or
cancers
exhibiting cancer markers, such as, but not limited to, Her2, CA15-3, CA19-9,
CA-125,
CEA, AFP, PSA, HCG, hormone receptor and NMP-22. Cancers can also be
classified by
stage (e.g., stage 1, 2, 3, or 4) and whether of primary or secondary origin.
- 18 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[093] Captured set: As used herein, a "captured set" of nucleic acids
refers to
nucleic acids that have undergone capture.
[094] Capturing: As used herein, "capturing" or "enriching" one or more
target
nucleic acids refers to preferentially isolating or separating the one or more
target nucleic
acids from non-target nucleic acids.
[095] Cell-Free Nucleic Acid: As used herein, "cell-free nucleic acid"
refers to
nucleic acids not contained within or otherwise bound to a cell or, in some
embodiments,
nucleic acids remaining in a sample following the removal of intact cells.
Cell-free nucleic
acids can include, for example, all non-encapsulated nucleic acids sourced
from a bodily fluid
(e.g., blood, plasma, serum, urine, cerebrospinal fluid (CSF), etc.) from a
subject Cell-free
nucleic acids include DNA (cfDNA), RNA (cfRNA), and hybrids thereof, including
genomic
DNA, mitochondrial DNA, circulating DNA, siRNA, miRNA, circulating RNA (cRNA),
tRNA, rRNA, small nucleolar RNA (snoRNA), Piwi-interacting RNA (piRNA), long
non-
coding RNA (long ncRNA), and/or fragments of any of these. Cell-free nucleic
acids can be
double-stranded, single-stranded, or a hybrid thereof. A cell-free nucleic
acid can be released
into bodily fluid through secretion or cell death processes, e.g., cellular
necrosis, apoptosis, or
the like. Some cell-free nucleic acids are released into bodily fluid from
cancer cells, e.g.,
circulating tumor DNA (ctDNA). Others are released from healthy cells. CtDNA
can be
non-encapsulated tumor-derived fragmented DNA. A cell-free nucleic acid can
have one or
more epigenetic modifications, for example, a cell-free nucleic acid can be
acetylated, 5-
methylated, and/or hydroxy methylated.
[096] Cellular Nucleic Acids: As used herein, "cellular nucleic acids"
means
nucleic acids that are disposed within one or more cells from which the
nucleic acids have
originated, at least at the point a sample is taken or collected from a
subject, even if those
nucleic acids are subsequently removed (e.g., via cell lysis) as part of a
given analytical
process.
[097] Corresponding to a target region set: As used herein, "corresponding to
a
target region set means that a nucleic acid, such as cfDNA, originated from a
locus in the
target region set or specifically binds one or more probes for the target-
region set.
[098] Coverage: As used herein, the terms "coverage", "total molecule
count", or
"total allele count" are used interchangeably. They refer to the total number
of DNA
molecules at a particular genomic position in a given sample.
- 19 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[099] Deoxyribonucleic Acid or Ribonucleic Acid: As used herein,
"deoxyribonucleic acid" or "DNA" refers to a natural or modified nucleotide
which has a
hydrogen group at the 2'-position of the sugar moiety. DNA typically includes
a chain of
nucleotides comprising four types of nucleotide bases; adenine (A), thymine
(T), cytosine
(C), and guanine (G). As used herein, "ribonucleic acid" or "RNA" refers to a
natural or
modified nucleotide which has a hydroxyl group at the 2'-position of the sugar
moiety. RNA
typically includes a chain of nucleotides comprising four types of nucleotide
bases; A, uracil
(U), G, and C. As used herein, the term "nucleotide" refers to a natural
nucleotide or a
modified nucleotide. Certain pairs of nucleotides specifically bind to one
another in a
complementary fashion (called complementary base pairing). In DNA, adenine (A)
pairs
with thymine (T) and cytosine (C) pairs with guanine (G). In RNA, adenine (A)
pairs with
uracil (U) and cytosine (C) pairs with guanine (G). When a first nucleic acid
strand binds to
a second nucleic acid strand made up of nucleotides that are complementary to
those in the
first strand, the two strands bind to form a double strand. As used herein,
"sequencing data,"
"nucleic acid sequencing information,- "sequence information," "nucleic acid
sequence,"
"nucleotide sequence", "genomic sequence," "sequence read" or "sequencing
read" denotes
any information or data that is indicative of the order and identity of the
nucleotide bases
(e.g., adenine, guanine, cytosine, and thymine or uracil) in a molecule (e.g.,
a whole genome,
whole transcriptome, exome, oligonucleotide, polynucleotide, or fragment) of a
nucleic acid
such as DNA or RNA. It should be understood that the present teachings
contemplate
sequence information obtained using all available varieties of techniques,
platforms or
technologies, including, but not limited to: capillary electrophoresis,
microarrays, ligation-
based systems, polymerase-based systems, hybridization-based systems, direct
or indirect
nucleotide identification systems, pyrosequencing, ion- or pH-based detection
systems, and
electronic signature-based systems.
[0100] Digestion efficiency: As used herein, -digestion efficiency" or
"cutting
efficiency" refers to the efficiency of restriction enzyme digestion. The
digestion efficiency
can be calculated based on the number of control molecules observed upon
digesting with
restriction enzyme and number of control molecules observed in the absence of
restriction
enzyme digestion. The MSRE digestion efficiency can be calculated by:
Efficiency = 1 -
(number of negative control moleculesimsRE] / number of negative control
molecules[Mock] ).
The MDRE (an MSRE that preferentially cleaves methylated DNA, also referred to
as a
methylation-dependent restriction enzyme) digestion efficiency can be
calculated by-
- 20 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
Efficiency = 1 - (number of positive control moleculesimpREi / number of
positive control
moleculesimocki).
[0101] DNA sequence: As used herein, "DNA sequence" or "sequence" refers to
"raw sequence reads" and/or "consensus sequences." Raw sequence reads are the
output of a
DNA sequencer, and typically include redundant sequences of the same parent
molecule, for
example after amplification. "Consensus sequences" are sequences derived from
redundant
sequences of a parent molecule intended to represent the sequence of the
original parent
molecule. Consensus sequences includes the base identity at a single position.
In some
embodiments, consensus sequence can represent a single nucleotide base at a
particular
genomic position. In some embodiments, consensus sequence can represent a
string of
nucleotide bases at a plurality of genomic positions. Consensus sequences can
be produced
by voting (wherein each majority nucleotide, e.g., the most commonly observed
nucleotide at
a given base position, among the sequences is the consensus nucleotide) or
other approaches
such as comparing to a reference genome. Consensus sequences can be produced
by tagging
original parent molecules with unique or non-unique molecular tags, which
allow tracking of
the progeny sequences (e.g., after amplification) by tracking of the tag
and/or use of sequence
read internal information. Examples of tagging or barcoding, and uses of tags
or barcodes, are
provided in, for example, U.S. Patent Pub. Nos. 2015/0368708, 2015/0299812,
2016/0040229, and 2016/0046986, each of which is entirely incorporated herein
by
reference.
[0102] Enriched sample: As used herein, "enriched sample" refers to a sample
that
has been enriched for specific regions of interest. The sample can be enriched
by amplifying
regions of interest or by using single-stranded DNA/RNA probes or double
stranded DNA
probes that can hybridize to nucleic acid molecules of interest (e.g.,
SureSelect probes,
Agilent Technologies). In some embodiments, an enriched sample refers to a
subset or
portion of the processed sample that is enriched, where the subset or portion
of the processed
sample being enriched contains nucleic acid molecules from a sample of cell-
free
polynucleotides or polynucleotides.
[0103] Epigenetic characteristic: As used herein, "epigenetic characteristic"
refers
to any directly observable measure of the DNA molecule that can be used in the
analysis of
the epigenetic feature of that DNA molecule. For example, if the epigenetic
feature is
methylation, then the epigenetic characteristic of the DNA molecule can refer
to, but not
limited to, the partitioning of the DNA molecule, number of CpG residues in
the DNA
- 21 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
molecule and the location (or offset) of the DNA molecule. For example, if the
epigenetic
feature is fragmentomic signal, then the epigenetic characteristics can be,
but not limited to,
length of the cfDNA molecules, the location (or offset) of the cfDNA molecule
¨ start and/or
end positions of the cfDNA molecules.
[0104] Epigenetic feature: As used herein, "epigenetic feature" refers to any
parameter that may manifest a non-sequence modification of nucleic acids and
also includes
chromatin modifications. These modifications do not change the sequence of the
DNA. The
epigenetic features can include, but not limited to, methylation state;
fragmentomic signal;
position/distribution of nucleosome, CTCF proteins, transcription start sites,
regulatory
proteins and any other proteins that may bind to the DNA.
[0105] Epigenetic target region set: As used herein, "epigenetic target region
set"
refers to a set of target regions that may manifest non-sequence modifications
in neoplastic
cells (e.g., tumor cells and cancer cells) and non-tumor cells (e.g., immune
cells, cells from
tumor microenvironment). These modifications do not change the sequence of the
DNA.
Examples of non-sequence modifications changes include, but not limited to,
changes in
methylation (increases or decreases), nucleosome distribution, CTCF binding,
transcription
start sites, regulatory protein binding regions and any other proteins that
may bind to the
DNA. For present purposes, loci susceptible to neoplasia-, tumor-, or cancer-
associated focal
amplifications and/or gene fusions may also be included in an epigenetic
target region set
because detection of a change in copy number by sequencing or a fused sequence
that maps
to more than one locus in a reference genome tends to be more similar to
detection of
exemplary epigenetic changes discussed above than detection of nucleotide
substitutions,
insertions, or deletions, e.g., in that the focal amplifications and/or gene
fusions can be
detected at a relatively shallow depth of sequencing because their detection
does not depend
on the accuracy of base calls at one or a few individual positions. For
example, the epigenetic
target region set can comprise a set of target regions for analyzing the
fragment length or
fragment end point location distribution. In some embodiments, the epigenetic
target region
set includes one or more genomic regions, where the epigenetic state (e.g.,
methylation state)
of cfDNA molecules in these regions is unchanged in cancer, but their
presence/quantity in
blood indicates increased, aberrant presentation of cfDNA from certain tissue
(e.g. cancer
origin) into circulation. The terms "epigenetic" and "epigenomic" are used
interchangeably
herein.
- 22 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0106] Fragmentomic signal: As used herein, "fragmentomic signal" refers to
the
distribution of the cfDNA fragment sizes and cfDNA fragment positions at a
particular
genomic region. Fragmentomic signal can include, but not limited to, cfDNA
fragment
lengths, start and/or end positions of the cfDNA molecule (fragments' size
coverage).
Fragmentomic signal can also include the frequency at which a DNA molecule
endpoint
occurs at genomic location (at a specific position or region of interest
surrounding the
specific position). Fragmentomic signal can also include the nucleosomal
positioning of
DNA molecules. In some embodiments, the fragmentomic signal includes DNA
molecule's
endpoint information, but does not necessarily include a length parameter of
the DNA
molecule).
[0107] Genomie region: As used herein, "genomic region" refers to any region
(e.g., range of base pair locations) of a genome, e.g., a chromosome, a
chromosome arm, a
gene, or an exon. A genomic region may be a contiguous or a non-contiguous
region. A
"genetic locus" (or "locus") can be a portion or entirety of a genomic region
(e.g., a gene, a
portion of a gene, or a single nucleotide of a gene). In some embodiments, the
size of the
genomic region comprises up to a length of a chromosome/chromosome arm or a
topologically associated domain (TAD). Tn some embodiments, the size of the
genomic
region can be limited to the biological activity of the region (e.g.,
transcriptional unit or
regulatory unit).
[0108] Hypermethylation: As used herein, "hypermethylation- refers to an
increased level or degree of methylation of nucleic acid molecule(s) relative
to the other
nucleic acid molecules within a population (e.g., sample) of nucleic acid
molecules. In some
embodiments, hypermethylation refers to an increased level or degree of
methylation of
nucleic acid m ol ecul e(s) from a particular genomic region in tumor samples
relative to the
degree of methylation of nucleic acid molecules form the same genomic region
in non-tumor
samples. In some embodiments, hypermethylated DNA can include DNA molecules
comprising at least 1 methylated residue, at least 2 methylated residues, at
least 3 methylated
residues, at least 5 methylated residues, at least 10 methylated residues, at
least 20 methylated
residues, at least 25 methylated residues, or at least 30 methylated residues.
[0109] Hypomethylation: As used herein, "hypomethylation" refers to a
decreased
level or degree of methylation of nucleic acid molecule(s) relative to the
other nucleic acid
molecules within a population (e.g., sample) of nucleic acid molecules. In
some
embodiments, hypomethylated DNA includes unmethylated DNA molecules. In some
- 23 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
embodiments, hypomethylation refers to an decreased level or degree of
methylation of
nucleic acid molecule(s) from a particular genomic region in tumor samples
relative to the
degree of methylation of nucleic acid molecules form the same genomic region
in non-tumor
samples. In some embodiments, hypomethylated DNA can include DNA molecules
comprising 0 methylated residues, at most 1 methylated residue, at most 2
methylated
residues, at most 3 methylated residues, at most 4 methylated residues, or at
most 5
methylated residues.
[0110] Methylation: As used herein, "methylation" or "DNA methylation" can
refer
to the presence of a methyl group to the cytosine at a CpG site (cytosine-
phosphate-guanine
site - i.e., a cytosine followed by a guanine in a 5' -> 3' direction of the
nucleic acid
sequence). In some embodiments, DNA methylation comprises addition of a methyl
group to
adenine, such as in N6-methyladenine. In some embodiments, DNA methylation is
5-
methylation (modification of the 5th carbon of the 6-carbon ring of cytosine).
In some
embodiments, 5-methylation comprises addition of a methyl group to the 5C
position of the
cytosine to create 5-methylcytosine (m5c). In some embodiments, methylation
comprises a
derivative of m5c. Derivatives of m5c include, but are not limited to, 5-
hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC), and 5-
caryboxylcytosine (5-caC)
In some embodiments, DNA methylation is 3C methylation (modification of the
3rd carbon
of the 6-carbon ring of cytosine). In some embodiments, 3C methylation
comprises addition
of a methyl group to the 3C position of the cytosine to generate 3-
methylcytosine (3mC).
Methylation can also occur at non CpG sites, for example, methylation can
occur at a CpA,
CpT, or CpC site. DNA methylation can change the activity of methylated DNA
region. For
example, when DNA in a promoter region is methylated, transcription of the
gene may be
repressed. DNA methylation is critical for normal development and abnormality
in
methylation may disrupt epigenetic regulation. The disruption, e.g.,
repression, in epigenetic
regulation may cause diseases, such as cancer. Promoter methylation in DNA may
be
indicative of cancer.
[0111] Methylation sensitive restriction enzyme (MSRE): As used herein,
"methylation sensitive restriction enzyme" or "MSRE" refers to a restriction
enzyme that is
sensitive to the methylation status of the DNA (e.g. cytosine methylation)
i.e., the presence or
absence of methyl group in a nucleotide base alters the rate at which the
enzyme cleaves the
target DNA. In some embodiments, the methylation sensitive restriction enzymes
do not
cleave the DNA if a particular nucleotide base is methylated at the
recognition sequence. For
- 24 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
example, HpaII is a methylation sensitive restriction enzyme with a
recognition sequence
"CCGG" and it does not cleave DNA if the second cytosine in the recognition
sequence is
methylated. In some embodiments, the methylation sensitive restriction enzymes
cleave the
DNA if a particular nucleotide base is methylated at the recognition sequence.
For example,
SgeI is a methylation sensitive restriction enzyme with a recognition sequence
"5''CNNG(N)9" and it cleaves DNA if the cytosine in the recognition sequence
is methylated
(5mC). As another example, FspEI is a methylation sensitive restriction enzyme
with a
recognition sequence "C5mC(N)12" and it cleaves DNA if the indicated cytosine
in the
recognition sequence is methylated (5111C). FIG. 1 is a schematic diagram of a
methylation
sensitive restriction enzyme (MSRE) digesting/cleaving the DNA as the
restriction enzyme
(RE) recognition site contains unmethylated nucleotides (top) and a schematic
diagram of a
methylation sensitive restriction enzyme (MSRE) not cleaving the DNA as the
restriction
enzyme (RE) recognition site (dashed box) contains a methylated nucleotide
(bottom) at a
position that affects activity of the MSRE. In some embodiments, the enzymatic
activity of a
MSRE is at least 10, 20, 50, or 100-fold higher on a methylated recognition
site relative to an
unmethylated version of the same recognition site. In some embodiments, the
enzymatic
activity of a MSRE is at least 10, 20, 50, or 100-fold higher on an
unmethylated recognition
site relative to a methylated version of the same recognition site.
[0112] Methylation status: As used herein, "methylation status- can refer to
the
presence or absence of methyl group on a DNA base (e.g. cytosine) at a
particular genomic
position in a nucleic acid molecule. It can also refer to the degree of
methylation in a nucleic
acid sequence (e.g., highly methylated, low methylated, intermediately
methylated or
unmethylated nucleic acid molecules). The methylation status can also refer to
the number of
nucleotides methylated in a particular nucleic acid molecule.
[0113] Mutation: As used herein, "mutation" refers to a variation from a known
reference sequence and includes mutations such as, for example, single
nucleotide variants
(SNVs), and insertions or deletions (indels). A mutation can be a germline or
somatic
mutation. In some embodiments, a reference sequence for purposes of comparison
is a
wildtype genomic sequence of the species of the subject providing a test
sample, typically the
human genome.
[0114] Neoplasm: As used herein, the terms -neoplasm" and -tumor" are used
interchangeably. They refer to abnormal growth of cells in a subject. A
neoplasm or tumor
- 25 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
can be benign, potentially malignant, or malignant. A malignant tumor is a
referred to as a
cancer or a cancerous tumor.
[0115] Next-Generation Sequencing. As used herein, "next-generation
sequencing"
or "NGS" refers to sequencing technologies having increased throughput as
compared to
traditional Sanger- and capillary electrophoresis-based approaches, for
example, with the
ability to generate hundreds of thousands of relatively small sequence reads
at a time. Some
examples of next-generation sequencing techniques include, but are not limited
to,
sequencing by synthesis, sequencing by ligation, and sequencing by
hybridization. In some
embodiments, next-generation sequencing includes the use of instruments
capable of
sequencing single molecules. Example of commercially available instruments for
performing
next-generation sequencing include, but are not limited to, NextSeq, HiSeq,
NovaSeq,
MiSeq, Ion PGM and Ion GeneStudio S5.
[0116] Nucleic Acid Tag: As used herein, "nucleic acid tag" refers to a short
nucleic
acid (e.g., less than about 500 nucleotides, about 100 nucleotides, about 50
nucleotides, or
about 10 nucleotides in length), used to distinguish nucleic acids from
different samples (e.g.,
representing a sample index), distinguish nucleic acids from different
partitions (e.g.,
representing a partition tag) or different nucleic acid molecules in the same
sample (e.g.,
representing a molecular barcode), of different types, or which have undergone
different
processing. The nucleic acid tag comprises a predetermined, fixed, non-random,
random or
semi-random oligonucleotide sequence. Such nucleic acid tags may be used to
label different
nucleic acid molecules or different nucleic acid samples or sub-samples.
Nucleic acid tags
can be single-stranded, double-stranded, or at least partially double-
stranded. Nucleic acid
tags optionally have the same length or varied lengths. Nucleic acid tags can
also include
double-stranded molecules having one or more blunt-ends, include 5' or 3'
single-stranded
regions (e.g., an overhang), and/or include one or more other single-stranded
regions at other
locations within a given molecule. Nucleic acid tags can be attached to one
end or to both
ends of the other nucleic acids (e.g., sample nucleic acids to be amplified
and/or sequenced).
Nucleic acid tags can be decoded to reveal information such as the sample of
origin, form, or
processing of a given nucleic acid. For example, nucleic acid tags can also be
used to enable
pooling and/or parallel processing of multiple samples comprising nucleic
acids bearing
different molecular barcodes and/or sample indexes in which the nucleic acids
are
subsequently being deconvolved by detecting (e.g., reading) the nucleic acid
tags. Nucleic
acid tags can also be referred to as identifiers (e.g. molecular identifier,
sample identifier).
- 26 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
Additionally, or alternatively, nucleic acid tags can be used as molecular
identifiers (e.g., to
distinguish between different molecules or amplicons of different parent
molecules in the
same sample or sub-sample). This includes, for example, uniquely tagging
different nucleic
acid molecules in a given sample, or non-uniquely tagging such molecules. In
the case of
non-unique tagging applications, a limited number of tags (i.e., molecular
barcodes) may be
used to tag each nucleic acid molecule such that different molecules can be
distinguished
based on their endogenous sequence information (for example, start and/or stop
positions
where they map to a selected reference genome, a sub-sequence of one or both
ends of a
sequence, and/or length of a sequence) in combination with at least one
molecular barcode.
Typically, a sufficient number of different molecular barcodes are used such
that there is a
low probability (e.g., less than about a 10%, less than about a 5%, less than
about a 1%, or
less than about a 0.1% chance) that any two molecules may have the same
endogenous
sequence information (e.g., start and/or stop positions, subsequences of one
or both ends of a
sequence, and/or lengths) and also have the same molecular barcode.
[0117] Partitioning: As used herein, -partitioning" refers to physically
separating or
fractionating a mixture of nucleic acid molecules in a sample based on a
characteristic of the
nucleic acid molecules The partitioning can be physical partitioning
of molecules
Partitioning can involve separating the nucleic acid molecules into groups or
sets based on
the level of epigenetic feature (for e.g., methylation). For example, the
nucleic acid molecules
can be partitioned based on the level of methylation of the nucleic acid
molecules. In some
embodiments, the methods and systems used for partitioning may be found in PCT
Patent
Application No. PCT/US2017/068329, which is hereby incorporated by reference
in its
entirety. Following partitioning, the groups or sets of separated or
fractionated nucleic acid
molecules are also referred to herein as fractions, partitions, or partitioned
sets.
[0118] Partitioned Net: As used herein, "partitioned set" or "partition"
refers to a set
of nucleic acid molecules partitioned into a set or group based on the
differential binding
affinity of the nucleic acid molecules or proteins associated with the nucleic
acid molecules
to a binding agent. The binding agent binds preferentially to the nucleic acid
molecules
comprising nucleotides with epigenetic modification. For example, if the
epigenetic
modification is methylation, the binding agent can be a methyl binding domain
(MBD)
protein. In some embodiments, a partitioned set can comprise nucleic acid
molecules
belonging to a particular level or degree of epigenetic feature (for e.g.,
methylation). For
example, the nucleic acid molecules can be partitioned into three sets ¨ one
set for highly
- 27 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
methylated nucleic acid molecules (hyper partitioned set or hypermethylated
partitioned set),
a second set for low methylated nucleic acid molecules (hypo partitioned set
or
hypomethylated partitioned set), and a third set for intermediate methylated
nucleic acid
molecules (intermediate partitioned set or intermediately methylated
partitioned set). In
another example, the nucleic acid molecules can be partitioned based on the
number of
methylated nucleotides - one partitioned set can have nucleic acid molecules
with nine
methylated nucleotides, and another partitioned set can have unmethylated
nucleic acid
molecules (zero methylated nucleotides).
[0119] Polynucleotide: As used herein, "polynucleotide", "nucleic acid",
"nucleic
acid molecule", or "oligonucleotide" refers to a linear polymer of nucleosides
(including
deoxyribonucleosides, ribonucleosides, or analogs thereof) joined by inter-
nucleosidic
linkages. Typically, a polynucleotide comprises at least three nucleosides.
Oligonucleotides
often range in size from a few monomeric units, e.g., 3-4, to hundreds of
monomeric units.
Whenever a polynucleotide is represented by a sequence of letters, such as
"ATGCCTG", the
nucleotides are in 5' 3' order from left to right, and in the case of
DNA, "A" denotes
deoxyadenosine, "C" denotes deoxycytidine, "G" denotes deoxyguanosine, and "T"
denotes
deoxythymi dine, unless otherwise noted The letters A, C, G, and T may be used
to refer to
the bases themselves, to nucleosides, or to nucleotides comprising the bases.
[0120] Processing: As used herein, "processing" refers to a set of steps used
to
generate a library of nucleic acids that is suitable for sequencing. The set
of steps can include,
but are not limited to, partitioning, end repairing, addition of sequencing
adapters, tagging,
and/or PCR amplification of nucleic acids.
[0121] Quantitative measure: As used herein, "quantitative measure- refers to
an
absolute or relative measure. A quantitative measure can be, without
limitation, a number, a
statistical measurement (e.g., frequency, mean, median, standard deviation, or
quantile), or a
degree or a relative quantity (e.g., high, medium, and low). A quantitative
measure can be a
ratio of two quantitative measures. A quantitative measure can be a linear
combination of
quantitative measures. A quantitative measure may be a normalized measure.
[0122] Reference Sequence: As used herein, "reference sequence" refers to a
known sequence used for purposes of comparison with experimentally determined
sequences.
For example, a known sequence can be an entire genome, a chromosome, or any
segment
thereof. A reference sequence can align with a single contiguous sequence of a
genome or
- 28 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
chromosome or chromosome arm or can include non-contiguous segments that align
with
different regions of a genome or chromosome. Examples of reference sequences
include, for
example, human genomes, such as, hg19 and hg38.
[0123] Restriction enzyme: As used herein, "restriction enzyme" is an enzyme
that
recognizes and cleaves the DNA at or near a specific recognition site.
[0124] Sample: As used herein, "sample" means anything capable of being
analyzed
by the methods and/or systems disclosed herein.
[0125] Sequencing: As used herein, "sequencing" refers to any of a number of
technologies used to determine the sequence (e.g., the identity and order of
monomer units)
of a biomolecule, e.g., a nucleic acid such as DNA or RNA. Examples of
sequencing
methods include, but are not limited to, targeted sequencing, single molecule
real-time
sequencing, exon or exome sequencing, intron sequencing, electron microscopy-
based
sequencing, panel sequencing, transistor-mediated sequencing, direct
sequencing, random
shotgun sequencing, Sanger dideoxy termination sequencing, whole-genome
sequencing,
sequencing by hybridization, pyrosequencing, duplex sequencing, cycle
sequencing, single-
base extension sequencing, solid-phase sequencing, high-throughput sequencing,
massively
parallel signature sequencing, emulsion PCR, co-amplification at lower
denaturation
temperature-PCR (COLD-PCR), multiplex PCR, sequencing by reversible dye
terminator,
paired-end sequencing, near-term sequencing, exonuclease sequencing,
sequencing by
ligation, short-read sequencing, single-molecule sequencing, sequencing-by-
synthesis, real-
time sequencing, reverse-terminator sequencing, nanopore sequencing, 454
sequencing,
Solexa Genome Analyzer sequencing, SOLjDTM sequencing, MS-PET sequencing, and
a
combination thereof. In some embodiments, sequencing can be performed by a
gene
analyzer such as, for example, gene analyzers commercially available from
Illumina, Inc.,
Pacific Biosciences, Inc., or Applied Biosystems/Thermo Fisher Scientific,
among many
others.
[0126] Sequence Information: As used herein, "sequence information" in the
context of a nucleic acid polymer means the order and identity of monomer
units (e.g.,
nucleotides, etc.) in that polymer.
[0127] Sequence-variable target region set: As used herein "sequence-variable
target region set" refers to a set of target regions that may exhibit changes
in sequence such
as nucleotide substitutions, insertions, deletions, or gene fusions or
transpositions in
- 29 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
neoplastic cells (e.g., tumor cells and cancer cells). In some embodiments, a
nucleotide
substitution is a single nucleotide variation.
[0128] Somatic Mutation: As used herein, the terms "somatic mutation" or
"somatic variation" are used interchangeably. They refer to a mutation in the
genome that
occurs after conception. Somatic mutations can occur in any cell of the body
except germ
cells and accordingly, are not passed on to progeny.
[0129] Specifically binds: As used herein, "specifically binds" in the context
of an
probe or other oligonucleotide and a target sequence means that under
appropriate
hybridization conditions, the oligonucleotide or probe hybridizes to its
target sequence, or
replicates thereof, to form a stable probe:target hybrid, while at the same
time formation of
stable probe:non-target hybrids is minimized. Thus, a probe hybridizes to a
target sequence or
replicate thereof to a sufficiently greater extent than to a non-target
sequence, to enable
capture or detection of the target sequence. Appropriate hybridization
conditions are well-
known in the art, may be predicted based on sequence composition, or can be
determined by
using routine testing methods (see, e.g., Sambrook et al., Molecular Cloning,
A Laboratory
Manual, 2nd ed. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY,
1989) at
1.90-1.91, 7.37-7.57, 9.47-9.51 and 11.47-11.57, particularly 9.50-9.51,
11.12-11.13,
11.45-11.47 and 11.55-11.57, incorporated by reference herein).
[0130] Subject: As used herein, "subject" refers to an animal, such as a
mammalian
species (e.g., human) or avian (e.g., bird) species, or other organism, such
as a plant. More
specifically, a subject can be a vertebrate, e.g., a mammal such as a mouse, a
primate, a
simian or a human. Animals include farm animals (e.g., production cattle,
dairy cattle,
poultry, horses, pigs, and the like), sport animals, and companion animals
(e.g., pets or
support animals). A subject can be a healthy individual, an individual that
has or is suspected
of having a disease or a predisposition to the disease, or an individual in
need of therapy or
suspected of needing therapy. The terms "individual" or "patient" are intended
to be
interchangeable with "subject". For example, a subject can be an individual
who has been
diagnosed with having a cancer, is going to receive a cancer therapy, and/or
has received at
least one cancer therapy. The subject can be in remission of a cancer. As
another example,
the subject can be an individual who is diagnosed of having an autoimmune
disease. As
another example, the subject can be a female individual who is pregnant or who
is planning
on getting pregnant, who may have been diagnosed of or suspected of having a
disease, e.g.,
a cancer, an auto-immune disease.
- 30 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0131] Target-region set: As used herein, "target-region set" or "set of
target
regions" or "target regions" or "target regions of interest" or "regions of
interest" or
"genomic regions of interest" refers to a plurality of genomic loci or a
plurality of genomic
regions targeted for capture and/or targeted by a set of probes (e.g., through
sequence
complementarity).
[0132] Tumor fraction: As used herein, "tumor fraction" refers to the
proportion of
ciDNA molecules that originated from tumor cells for a given sample, or sample-
region pair.
[0133] The terms "or a combination thereof' and "or combinations thereof' as
used
herein refers to any and all permutations and combinations of the listed terms
preceding the
term. For example, "A, B, C, or combinations thereof' is intended to include
at least one of:
A, B, C, AB, AC, BC, or ABC, and if order is important in a particular
context, also BA, CA,
CB, ACB, CBA, BCA, BAC, or CAB. Continuing with this example, expressly
included are
combinations that contain repeats of one or more item or term, such as BB,
AAA, AAB,
BBC, AAABCCCC, CBBAAA, CABABB, and so forth. The skilled artisan will
understand
that typically there is no limit on the number of items or terms in any
combination, unless
otherwise apparent from the context.
[0134] "Or" is used in the inclusive sense, i.e., equivalent to "and/or,"
unless the
context requires otherwise.
DETAILED DESCRIPTION
[0135] Certain embodiments of the invention are described herein. While the
invention will be described in conjunction with such embodiments, it will be
understood that
they are not intended to limit the invention to those embodiments. On the
contrary, the
invention is intended to cover all alternatives, modifications, and
equivalents, which may be
included within the invention as defined by the appended claims.
[0136] Numeric ranges are inclusive of the numbers defining the range.
Measured
and measurable values are understood to be approximate, taking into account
significant
digits and the error associated with the measurement. Also, the use of
"comprise",
"comprises", "comprising", "contain", "contains", "containing", "include",
"includes", and
"including" are not intended to be limiting. It is to be understood that both
the foregoing
general description and detailed description are exemplary and explanatory
only and are not
restrictive of the teachings.
- 31 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0137] Unless specifically noted in the above specification, embodiments in
the
specification that recite "comprising" various components are also
contemplated as
"consisting of' or "consisting essentially of' the recited components;
embodiments in the
specification that recite "consisting of' various components are also
contemplated as
"comprising" or "consisting essentially of' the recited components; and
embodiments in the
specification that recite "consisting essentially of' various components are
also contemplated
as -consisting of' or -comprising" the recited components (this
interchangeability does not
apply to the use of these terms in the claims).
[0138] The section headings used herein are for organizational purposes and
are not
to be construed as limiting the disclosed subject matter in any way. In the
event that any
document or other material incorporated by reference contradicts any explicit
content of this
specification, including definitions, this specification controls.
I. Overview
[0139] Cancer formation and progression may arise from both genetic
modification
and epigenetic features of deoxyribonucleic acid (DNA). The present disclosure
provides
methods and systems for analyzing DNA, such as cell-free DNA (cfDNA). The
present
disclosure provides methods and systems for reducing signal to noise ratio of
methylation
partitioning assays.
[0140] Without wishing to be bound by any particular theory, cells in or
around a
cancer or neoplasm may shed more DNA than cells of the same tissue type in a
healthy
subject. As such, the distribution of tissue of origin of certain DNA samples,
such as cfDNA,
may change upon carcinogenesis. Thus, for example, an increase in the level of
hypermethylation variable target regions that show lower methylation in
healthy cfDNA than
in at least one other tissue type can be an indicator of the presence (or
recurrence, depending
on the history of the subject) of cancer. Similarly, an increase in the level
of hypomethylation
variable target regions in the sample can be an indicator of the presence (or
recurrence,
depending on the history of the subject) of cancer.
[0141] Additionally, cancer can be indicated by non-sequence modifications,
such
as methylation. Examples of methylation changes in cancer include local gains
of DNA
methylation in the CpG islands at the TSS of genes involved in normal growth
control, DNA
repair, cell cycle regulation, and/or cell differentiation. This
hypermethylation can be
associated with an aberrant loss of transcriptional capacity of involved genes
and occurs at
least as frequently as point mutations and deletions as a cause of altered
gene expression.
- 32 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
DNA methylation profiling can be used to detect regions of the genome with
different extents
of methylation ("differentially methylated regions" or "DMRs"), such as
aberrant
methylation, that arises during development or by disease, for example, cancer
or any cancer-
associated disease. For example, regions that are normally hypermethylated or
hypomethylated in a given sample type (e.g., cfDNA from the bloodstream) but
which may
show an abnormal degree of methylation that correlates to a neoplasm or
cancer, e.g.,
because of unusually increased contributions of tissues to the type of sample
(e.g., due to
increased shedding of DNA in or around the neoplasm or cancer) and/or from
extents of
methylation can be detected using DNA methylation profiling.
[0142] In some embodiments, DNA methylation comprises addition of a methyl
group to a cytosine residue at a CpG site (cytosine-phosphate-guanine site
(i.e., a cytosine
followed by a guanine in a 5' -> 3' direction of the nucleic acid sequence).
In some
embodiments, DNA methylation comprises addition of a methyl group to an
adenine residue,
such as in N6-methyladenine. In some embodiments, DNA methylation is 5-
methylation
(modification of the 5th carbon of the 6-carbon ring of cytosine). In some
embodiments, 5-
methylation comprises addition of a methyl group to the 5C position of the
cytosine residue
to create 5-methylcytosine (m5c or 5-mC or 5mC) Tn some embodiments,
methylation
comprises a derivative of m5c. Derivatives of m5c include, but are not limited
to, 5-
hydroxymethylcytosine (5-hmC or 5hmC), 5-formylcytosine (5-fC), and 5-
caryboxylcytosine
(5-caC). In some embodiments, DNA methylation is 3C methylation (modification
of the 3rd
carbon of the 6-carbon ring of the cytosine residue). In some embodiments, 3C
methylation
comprises addition of a methyl group to the 3C position of the cytosine
residue to generate 3-
methylcytosine (3mC). Methylation can also occur at non-CpG sites, for
example,
methylation can occur at a CpA, CpT, or CpC site. DNA methylation can change
the activity
of methylated DNA region. For example, when DNA in a promoter region is
methylated,
transcription of the gene may be repressed. DNA methylation is critical for
normal
development and abnormality in methylation may disrupt epigenetic regulation.
The
disruption, e.g., repression, in epigenetic regulation may cause diseases,
such as cancer.
Promoter methylation in DNA may be indicative of cancer.
[0143] Methylation profiling can involve determining methylation patterns
across
different regions of the genome. For example, after partitioning molecules
based on extent of
methylation (e.g., relative number of methylated nucleotides per molecule) and
sequencing,
the sequences of molecules in the different partitions can be mapped to a
reference genome.
- 33 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
This can show regions of the genome that, compared with other regions, are
more highly
methylated or are less highly methylated. In this way, genomic regions, in
contrast to
individual molecules, may differ in their extent of methylation.
[0144] Combining the signals obtained from methylation profiling with the
signals
obtained from somatic variations (e.g., SNV, indel, CNV, and gene fusions)
facilitate the
detection of cancer.
[0145] Nucleic acid molecules in a sample may be fractionated or partitioned
based
on methylation status of the nucleic acid molecules. Partitioning nucleic acid
molecules in a
sample can increase a rare signal. For example, a genetic variation present in
hypermethylated DNA but less (or not) present in hypomethylated DNA can be
more easily
detected by partitioning a sample into hypermethylated and hypomethylated
nucleic acid
molecules. By analyzing multiple fractions of a sample, a multi-dimensional
analysis of a
single molecule can be performed and hence, greater sensitivity can be
achieved. Partitioning
may include physically partitioning nucleic acid molecules into subsets or
groups based on
the presence or absence of one or more methylated nucleotides (e.g.,
nucleotides comprising
a methylated base). A sample may be fractionated or partitioned into one or
more partitioned
sets based on a characteristic that is indicative of differential gene
expression or a disease
state. A sample may be fractionated based on a characteristic, or combination
thereof that
provides a difference in signal between a normal and diseased state during
analysis of nucleic
acids, e.g., cell free DNA ("cfDNA"), non-cfDNA, tumor DNA, circulating tumor
DNA
("ctDNA") and cell free nucleic acids ("cfNA").
[0146] In some embodiments, the sample can be partitioned into two or more
partitioned sets (e.g. at least 3, 4, 5, 6, or 7 partitioned sets) based on
the differential binding
affinity of the methylated nucleic acid molecules to a binding agent (i.e., a
binding agent that
binds to methylated nucleotides (e.g., nucleotides comprising a methylated
base)). Examples
of binding agents include, but not limited to methyl binding domain (MBDs) and
methyl
binding proteins (MBPs). Examples of MBPs contemplated herein include, but are
not
limited to:
(a) MeCP2 and MBD2 are proteins preferentially binding to 5-methyl-cytosine
over
unmodified cytosine;
(b) RPL26, PRP8 and the DNA mismatch repair protein MTIS6 preferentially bind
to
5-hydroxymethyl-cytosine over unmodified cytosine;
- 34 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
(c) FOXKl, FOXK2, FOXP1, FOXP4, and FOXI3 preferentially bind to 5-formyl-
cytosine over unmodified cytosine (Iurlaro et al., Genome Biol. 14, R119
(2013));
and
(d) Antibodies specific to one or more methylated nucleotide bases.
[0147] In such embodiments, nucleic acids overrepresented in a modification
bind
to the agent at a greater extent than nucleic acids underrepresented in the
modification.
Alternatively, nucleic acids having modifications may bind in an all or
nothing manner. But
then, various levels of modifications may be sequentially eluted from the
binding agent.
[0148] For example, in some embodiments, partitioning can be binary or based
on
degree/level of methylation. For example, all methylated fragments can be
partitioned from
unmethylated fragments using methyl-binding domain proteins (e.g., MethylMiner
Methylated DNA Enrichment Kit (ThermoFisher Scientific)). Subsequently,
additional
partitioning may involve eluting fragments having different levels of
methylation by
adjusting the salt concentration in a solution with the methyl-binding domain
and bound
fragments. As salt concentration increases, fragments having greater
methylation levels are
eluted.
[0149] Compared to standard methylation analysis methods (e.g. bisulfite
sequencing), methylation-partitioning method is highly efficient in recovering
analyte
molecules and enables simultaneous detection of somatic alterations. However,
as the method
identifies a molecule's methylation level by partitioning, the sensitivity and
specificity of the
method is challenged by methylated/unmethylated molecules partitioning
incorrectly (e.g.
unmethylated molecules partitioning into the hyper partitioned set). This
technical noise,
from molecule mis-partitioning, of the methylation partitioning assay limits
the performance
of the assay. In order to increase the signal to noise ratio of a methylation
partitioning assay,
specific partitioned sets can be subjected to a methylation-sensitive
restriction enzyme (RE)
digestion reaction to specifically remove the incorrectly partitioned
molecules. For example,
methylation-sensitive restriction enzymes (MSREs), that only cleave
unmethylated molecules
bearing the RE recognition site, can be applied to the hyper partitioned set
to selectively
cleave and remove (from assay process) only the unmethylated molecules that
were
incorrectly partitioned. Thus, from reducing the number of unmethylated
molecules in the
hyper partitioned set, the sensitivity and specificity of the assay is
improved, which in turn,
improves the ability to detect the presence or absence of circulating tumor
DNA (ctDNA).
- 35 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0150] The present disclosure provides methods and systems for improving
sensitivity and specificity of the DNA methylation partitioning assays. These
methods and
systems can be used in various applications, such as predicting prognosis,
diagnosis,
monitoring, recurrence, and/or relapse of cancer.
[0151] Accordingly, in one aspect, the present disclosure provides a method
for
analyzing nucleic acid molecules in a biological sample, comprising. (a)
partitioning at least
a subset of the nucleic acid molecules in the biological sample, based on the
methylation
status of the nucleic acid molecules into a plurality of partitioned sets,
wherein the biological
sample comprises methylated nucleic acid molecules and unmethylated nucleic
acid
molecules; (b) digesting at least a subset of the one or more partitioned sets
in the plurality of
partitioned sets with at least one methylation sensitive restriction enzyme;
and (c)
determining methylation status at one or more genetic loci of the nucleic acid
molecules in at
least one of the partitioned sets.
[0152] In some embodiments, the method further comprises detecting the
presence
or absence of cancer in the biological sample. In some embodiments, the method
comprises,
determining a level of cancer in the biological sample, for example, by
determining a level of
DNA from cancer cells in the biological sample. In some embodiments, the
method further
comprises, prior to digesting, attaching one or more adapters to at least one
of the end (i.e., 5'
and/or 3' ends) of the nucleic acid molecules in the plurality of partitioned
sets. In some
embodiments, determining the methylation status comprises sequencing at least
a subset of
the digested nucleic acid molecules. In some embodiments, the method further
comprises,
prior to determining the methylation status, enriching at least a subset of
the nucleic acid
molecules in the plurality of partitioned sets for genomic regions of
interest, wherein the at
least a subset of the nucleic acid molecules comprises digested nucleic acid
molecules in the
one or more partitioned sets. In some such embodiments, the genomic regions of
interest
comprise epigenetic target region sets. In some such embodiments, the methods
comprise
enriching or capturing a first epigenetic target region set from at least a
portion of a first
partitioned set and enriching or capturing a second epigenetic target region
set from at least a
portion of a second partitioned set.
[0153] In another aspect, the present disclosure provides a method for
determining
methylation status of nucleic acid molecules, comprising: (a) providing a
biological sample
of nucleic acid molecules, wherein the nucleic acid molecules comprises
methylated nucleic
acid molecules and unmethylated nucleic acid molecules; (b) partitioning at
least a subset of
- 36 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
the nucleic acid molecules in the biological sample based on the methylation
status of the
nucleic acid molecules into a plurality of partitioned sets; (c) digesting at
least a subset of the
one or more partitioned sets in the plurality of partitioned sets with at
least one methylation
sensitive restriction enzyme; (d) enriching at least a subset of the nucleic
acid molecules in
the plurality of partitioned sets for genomic regions of interest, wherein the
at least a subset
of the nucleic acid molecules comprises digested nucleic acid molecules in the
one or more
partitioned sets; and (e) determining methylation status at one or more
genetic loci of the
nucleic acid molecules in at least one of the partitioned sets. In some such
embodiments, the
genomic regions of interest comprise epigenetic target region sets. In some
such
embodiments, the methods comprise enriching or capturing a first epigenetic
target region set
from at least a portion of a first partitioned set and enriching or capturing
a second epigenetic
target region set from at least a portion of a second partitioned set.
[0154] In some embodiments, the method further comprises detecting the
presence
or absence of cancer in the biological sample. In some embodiments, the method
comprises,
determining a level of cancer in the biological sample, for example, by
determining a level of
DNA from cancer cells in the biological sample. In some embodiments, the
method further
comprises, prior to digesting, attaching one or more adapters to at least one
of the end (i e , 5'
and/or 3' ends) of the nucleic acid molecules in the plurality of partitioned
sets. In some
embodiments, determining the methylation status comprises sequencing at least
a subset of
the digested nucleic acid molecules.
[0155] FIG. 2 illustrates an example embodiment of a method 200 for
determining
the methylation status of nucleic acid molecules in a polynucleotide sample
obtained from a
subject. In 202, a polynucleotide sample is obtained from the subject. In some
embodiments,
the polynucleotide sample is a DNA sample is obtained from a tumor tissue
biopsy. In some
embodiments, the polynucleotide sample is a cell-free DNA (cfDNA) sample
obtained from
blood. In 204, the polynucleotide sample is partitioned into at least two
partitioned sets. In
some embodiments, the partitioning comprises partitioning the nucleic acid
molecules based
on a differential binding affinity of the polynucleotides to a binding agent
that preferentially
binds to polynucleotides comprising methylated nucleotides (e.g., nucleotides
comprising a
methylated base). Examples of binding agents include, but are not limited to,
methyl binding
domain (1VMDs) and methyl binding proteins (MBPs) Examples of MBPs
contemplated
herein are listed above.
(e) Antibodies specific to one or more methylated nucleotide bases.
- 37 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0156] Partitioning can refer to physically separating or fractionating the
nucleic
acid molecules based on a characteristic of the nucleic acid molecules. The
partitioning can
be physical partitioning of molecules. Partitioning can involve separating the
nucleic acid
molecules into groups or sets based on the level of methylation of the nucleic
acid molecules.
In some embodiments, the methods and systems used for partitioning may be
performed as
described by PCT Patent Application W02018/119452, which is hereby
incorporated by
reference in its entirety. In those embodiments, the nucleic acids are
partitioned based on the
different levels of methylation (e.g., different number or frequency of
methylated nucleotides
(e.g., nucleotides comprising a methylated base)). In some embodiments, the
nucleic acids
can be partitioned into two or more partitioned sets (e.g., at least 3, 4, 5,
6, or 7 partitioned
sets). For example, the nucleic acid molecules can be partitioned into three
sets ¨ one set for
highly methylated nucleic acid molecules (hyper partitioned set or
hypermethylated
partitioned set), a second set for low methylated nucleic acid molecules (hypo
partitioned set
or hypomethylated partitioned set), and a third set for intermediate
methylated nucleic acid
molecules (intermediate partitioned set or intermediately methylated
partitioned set). In some
embodiments, the partitioned sets are representatives of nucleic acids having
different levels
of methylation (over representative or under representative of modifications).
Over
representation and under representation can be defined by the number of
methylated
nucleotides present in a DNA molecule (e.g., cfDNA molecule) relative to the
median
number of methylated nucleotides per strand in a population. For example, if
the median
number of 5-methylcytosine nucleotides in nucleic acid molecules in a sample
is 2, a nucleic
acid molecule including more than two 5-methylcytosine residues is over-
represented and a
nucleic acid with 1 or zero 5-methylcytosine residues is under-represented.
The effect of the
affinity separation is to enrich for nucleic acids that are over-represented
in a modification
(i.e., methylation level) in a bound phase and for nucleic acids that are
under-represented in a
modification in an unbound phase (i.e., in solution). The nucleic acids in the
bound phase
can be eluted before subsequent processing.
[0157] In 206, the nucleic acid molecules in at least one partitioned set are
digested
with at least one methylation sensitive restriction enzyme (MSRE). In some
embodiments,
the nucleic acids in at least one partitioned set are digested with at least
two MSREs. In some
embodiments, two MSREs are used for digesting the nucleic acid molecules in at
least one
partitioned set. In some embodiments, the two MSREs are BstUI and HpaII. In
some
embodiments, the two MSREs are HhaI and AccII In some embodiments, three MSREs
are
- 38 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
used for digesting the nucleic acid molecules in at least one partitioned set.
In some
embodiments, the three MSREs are BstUI, HpaII and Hin6I. In some embodiments,
the
MSRE is selected from the group consisting of AatII, AccII, AciI, Aor13HI,
Aor15HI,
BspT104I, BssHII, BstUI, Cfr10I, ClaI, CpoI, Eco52I, HaeII, HapII, HhaI,
Hin6I, HpaII,
HpyCH4IV, MluI, MspI, NaeI, NotI, NruI, NsbI, PmaCI, Psp14061, PvuI, SacII,
Sall, SmaI,
and SnaBI. In some embodiments, any commercially available MSRE can be used
(MSREs
provided by Takara Bio USA Inc., New England Biolabs Inc. and/or Thermo
Fisher
Scientific Inc. can be used).
[0158] In some embodiments, FspEI is used for digesting the nucleic acid
molecules
in at least one other partitioned set (e.g., a hypomethylated partition). In
some embodiments,
BstUI, HpaII and Hin6I are used for digesting the nucleic acid molecules in at
least one
partitioned set (e.g., a hypermethylated partition) and FspEI is used for
digesting the nucleic
acid molecules in at least one other partitioned set (e.g., a hypomethylated
partition). In
embodiments involving an intermediately methylated partition, the nucleic acid
molecules
therein may be digested with at least one methylation sensitive restriction
enzyme that
preferentially cleaves methylated or unmethylated DNA. In some embodiments,
the nucleic
acid molecules in an intermediately methylated partition are digested with the
same MSRE(s)
as the hypermethylated partition. For example, the intermediately methylated
partition may
be pooled with the hypermethylated partition and then the pooled partitions
may be subjected
to digestion. In some embodiments, the nucleic acid molecules in an
intermediately
methylated partition are digested with the same MSRE(s) as the hypomethylated
partition.
For example, the intermediately methylated partition may be pooled with the
hypomethylated
partition and then the pooled partitions may be subjected to digestion.
[0159] In some embodiments, prior to restriction digestion with MSRE, at least
one
adapter is attached to at least one end of the nucleic acid molecules (i.e.,
5' and/or 3' ends of
the DNA molecule). In some such embodiments, adapters are attached to both
ends of the
nucleic acid molecules. In other embodiments, after the digestion but prior to
enriching in
208, at least one adapter is attached to at least one end of the nucleic acid
molecules. In some
embodiments, the adapter is resistant to digestion by the methylation
sensitive restriction
enzymes. In some embodiments, the adapter comprises one or more methylated
nucleotides
(e.g., nucleotides comprising a methylated base). In some embodiments, the
methylated
nucleotides can be 5-methylcytosine and/or 5-hydroxymethylcytosine. In some
embodiments,
the adapter comprises one or more nucleotide analogs resistant to methylation
sensitive
- 39 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
restriction enzymes. In some embodiments, the adapter comprises a nucleotide
sequence not
recognized by methylation sensitive restriction enzymes. In some embodiments,
the tags may
be provided as components of adapters. In some embodiments, the tag comprises
molecular
barcode (i.e., molecule identifier). In some embodiments, the tag attached to
nucleic acid
molecules in one partitioned set is different from the tag attached to nucleic
acid molecules in
the other partitioned set(s). In some embodiments, one partitioned set is
differentially tagged
from the other partitioned set(s). Differential tagging of the partitioned
sets helps in keeping
track of the nucleic acid molecules belonging to a particular partitioned set.
The nucleic acid
molecules in different partitioned sets receive different tags that can
distinguish members of
one partitioned set from another. The tags linked to nucleic acid molecules of
the same
partition set can be the same or different from one another. But if different
from one another,
the tags can have part of their sequence in common so as to identify the
molecules to which
they are attached as being of a particular partitioned set. For example, if
the molecules of the
sample are partitioned into two partitioned sets ¨ P1 and P2, then the
molecules in P1 can be
tagged with Al, A2, A3, and so forth, and the molecules in P2 can be tagged
with B 1, B2,
B3, and so forth. Such a tagging system allows distinguishing the partitioned
sets and
between the molecules within a partitioned set. In some embodiments, the tag
comprises
partition tag (i.e., partition identifier). In such embodiments, the nucleic
acid molecules
within a partitioned set receive the same partition tag and is different from
the partition tag
attached to the nucleic acid molecules of the other partitioned set(s).
[0160] In 208, after MSRE digestion, the nucleic acid molecules in the one or
more
partitioned sets can be enriched for genomic regions of interest. In some
embodiments, the
genomic regions of interest can comprise differentially methylated regions for
cancer
detection. In 210, at least a subset of the enriched molecules is sequenced by
a next
generation sequencer. In 212, the sequencing reads generated by the sequencer
are then
analyzed using bioinformatic tools/algorithms to determine the number of
molecules in the
one or more partitioned sets, which in turn is used to determine the
methylation status at one
or more genetic loci of the nucleic acid molecules in at least one partitioned
sets. In some
embodiments, the one or more genetic loci can comprise multiple genetic loci.
In some
embodiments, the one or more genetic loci can comprise one or more genomic
regions. In
some embodiments, the genomic regions can be promoter region of genes. In some
embodiments, prior to sequencing, the nucleic acid molecules can be amplified
via PCR
amplification In some embodiments, the primers used in the amplification can
comprise at
- 40 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
least one sample index.
[0161] In some embodiments, the method can further comprise, detecting the
presence or absence of cancer in the subject based on the methylation status
at one or more
genetic loci of the nucleic acid molecules in at least one partitioned set. In
some
embodiments, the method further comprises, determining a level of cancer in
the
polynucleotide sample, for example, by determining a level of DNA from cancer
cells in the
polynucleotide sample.
[0162] In another aspect, the present disclosure provides a method for
determining
methylation status of nucleic acid molecules, comprising: (a) providing a
biological sample
of nucleic acid molecules, wherein the nucleic acid molecules comprises
methylated nucleic
acid molecules and unmethylated nucleic acid molecules; (b) partitioning at
least a subset of
the nucleic acid molecules in the biological sample based on the methylation
status of the
nucleic acid molecules into a plurality of partitioned sets; (c) attaching one
or more adapters
to at least one end of the nucleic acid molecules in the plurality of
partitioned sets; (d)
digesting at least a subset of the one or more partitioned sets in the
plurality of partitioned
sets with at least one methylation sensitive restriction enzyme; (d) enriching
at least a subset
of the nucleic acid molecules in the plurality of partitioned sets for genomic
regions of
interest; wherein the at least a subset of the nucleic acid molecules
comprises digested
nucleic acid molecules in the one or more partitioned sets; and (e)
determining methylation
status at one or more genetic loci of the nucleic acid molecules in at least
one of the
partitioned sets. In some such embodiments, the genomic regions of interest
comprise
epigenetic target region sets. In some such embodiments, the methods comprise
enriching or
capturing a first epigenetic target region set from at least a portion of a
first partitioned set
and enriching or capturing a second epigenetic target region set from at least
a portion of a
second partitioned set.
[0163] In some embodiments, the method further comprises detecting the
presence
or absence of cancer in the biological sample. In some embodiments, the method
comprises,
determining a level of cancer in the biological sample, for example, by
determining a level of
DNA from cancer cells in the biological sample. In some embodiments, the
method further
comprises, prior to digesting, attaching one or more adapters to at least one
of the end (i.e., 5'
and/or 3' ends) of the nucleic acid molecules in the plurality of partitioned
sets. In some
embodiments, determining the methylation status comprises sequencing at least
a subset of
the digested nucleic acid molecules.
- 41 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0164] FIG. 3 illustrates an example embodiment of a method 300 for detecting
the
presence or absence of cancer in a subject according to an embodiment of the
disclosure. In
302, a polynucleotide sample is obtained from the subject. In some
embodiments, the
polynucleotide sample is a DNA sample is obtained from a tumor tissue biopsy.
In some
embodiments, the polynucleotide sample is a cell-free DNA (cfDNA) sample
obtained from
blood (e.g., from plasma). In 304, the polynucleotide sample is partitioned
into at least two
partitioned sets. In some embodiments, the partitioning comprises partitioning
the nucleic
acid molecules based on a differential binding affinity of the polynucleotides
to a binding
agent that preferentially binds to polynucleotides comprising methylated
nucleotides (e.g.,
nucleotides comprising a methylated base). Examples of binding agents include,
but are not
limited to, methyl binding domain (MBDs) and methyl binding proteins (MBPs).
Examples
of MBPs contemplated herein are listed above.
[0165] In some embodiments, the nucleic acids can be partitioned into two or
more
partitioned sets (e.g., at least 3, 4, 5, 6, or 7 partitioned sets). In some
embodiments, the
partitioned sets are representatives of nucleic acids having different levels
of methylation
(over representative or under representative of modifications). For example,
the nucleic acid
molecules can be partitioned into three sets ¨ one set for highly methylated
nucleic acid
molecules (hyper partitioned set or hypermethylated partitioned set), a second
set for low
methylated nucleic acid molecules (hypo partitioned set or hypomethylated
partitioned set),
and a third set for intermediate methylated nucleic acid molecules
(intermediate partitioned
set or intermediately methylated partitioned set).
[0166] In 306, the nucleic acid molecules in the one or more partitioned sets
are
attached with adapters, wherein the adapter comprises at least one tag and is
attached to at
least one end of the nucleic acid molecules (i.e., 5' and/or 3' ends of the
DNA molecule). In
some embodiments, the adapter is resistant to digestion by the methylation
sensitive
restriction enzymes. In some embodiments, the adapter comprises one or more
methylated
nucleotides (e.g., nucleotides comprising a methylated base). In some
embodiments, the
methylated nucleotides can be 5-methylcytosine and/or 5-hydroxymethylcytosine.
In some
embodiments, the adapter comprises one or more nucleotide analogs resistant to
methylation
sensitive restriction enzymes. In some embodiments, the adapter comprises a
nucleotide
sequence not recognized by methylation sensitive restriction enzymes. In some
embodiments,
the adapter does not comprise a nucleotide sequence recognized by the
methylation sensitive
restriction enzyme(s) used in the method. In some embodiments, the adapter
comprises one
- 42 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
or more modifications (e.g., a linkage modification, such as phosphorothioate)
that inhibits
cleavage by the methylation sensitive restriction enzyme(s). In some
embodiments, the tags
may be provided as components of adapters. In some embodiments, the tag
comprises
molecular barcode (i.e., molecule identifier). In some embodiments, the tag
attached to
nucleic acid molecules in one partitioned set is different from the tag
attached to nucleic acid
molecules in the other partitioned set(s). In some embodiments, one
partitioned set is
differentially tagged from the other partitioned set(s). Differential tagging
of the partitioned
sets helps in keeping track of the nucleic acid molecules belonging to a
particular partitioned
set. The nucleic acid molecules in different partitioned sets receive
different tags that can
distinguish members of one partitioned set from another. The tags linked to
nucleic acid
molecules of the same partition set can be the same or different from one
another. But if
different from one another, the tags can have part of their sequence in common
so as to
identify the molecules to which they are attached as being of a particular
partitioned set. For
example, if the molecules of the sample are partitioned into two partitioned
sets ¨ P1 and P2,
then the molecules in P1 can be tagged with Al, A2, A3, and so forth, and the
molecules in
P2 can be tagged with Bl, B2, B3, and so forth. Such a tagging system allows
distinguishing
the partitioned sets and between the molecules within a partitioned set. In
some
embodiments, the tag comprises partition tag (i.e., partition identifier). In
such embodiments,
the nucleic acid molecules within a partitioned set receive the same partition
tag and is
different from the partition tag attached to the nucleic acid molecules of the
other partitioned
set(s). In some embodiments, the tag sequences used do not comprise a
nucleotide sequence
recognized by the methylation sensitive restriction enzyme(s) used in the
method.
[0167] In 308, the nucleic acid molecules in at least one partitioned set is
digested
with at least one methylation sensitive restriction enzyme (MSRE). In some
embodiments,
the nucleic acids in at least one partitioned set is digested with at least
two MSREs. In some
embodiments, two MSREs are used for digesting the nucleic acid molecules in at
least one
partitioned set. In some embodiments, the two MSREs are BstUI and HpaII. In
some
embodiments, the two MSREs are HhaI and AccII. In some embodiments, three
MSREs are
used for digesting the nucleic acid molecules in at least one partitioned set.
In some
embodiments, the three MSREs are BstUI, HpaII and Hin6I. In some embodiments,
the
MSRE is selected from the group consisting of AatII, AccII, AciI, Aor13HI,
Aor15HI,
BspT104I, BssHII, BstUI, Cfr10I, ClaI, CpoI, Eco52I, Haen, HapII, HhaI, Hin6I,
HpaII,
HpyCH4TV, MU, MspI, NaeT, NotT, NruI, NsbI, PmaCT, Psp1406I, PvuT, SacIT,
Salt, SmaT,
- 43 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
and SnaBI. In some embodiments, any commercially available MSRE can be used
(MSREs
provided by Takara Bio USA Inc., New England Biolabs Inc. and/or Thermo
Fisher
Scientific Inc. can be used).
[0168] In 310, after MSRE digestion, the nucleic acid molecules in the one or
more
partitioned sets can be enriched for genomic regions of interest. In some
embodiments, the
genomic regions of interest can comprise differentially methylated regions for
cancer
detection. In 312, at least a subset of the enriched molecules is sequenced by
a next
generation sequencer. In 314, the sequencing reads generated by the sequencer
are then
analyzed using bioinformatic tools/algorithms to determine the number of
molecules in the
one or more partitioned sets, which in turn is used to determine the
methylation status at one
or more genetic loci of the nucleic acid molecules in at least one partitioned
sets. In some
embodiments, the one or more genetic loci can comprise multiple genetic loci.
In some
embodiments, the one or more genetic loci can comprise one or more genomic
regions. In
some embodiments, the genomic regions can be promoter region of genes. In some
embodiments, prior to sequencing, the nucleic acid molecules can be amplified
via PCR
amplification. In some embodiments, the primers used in the amplification can
comprise at
least one sample index Tn some embodiments, nucleic acid molecules digested by
a MSRE
are not amplified. In some such embodiments, essentially all nucleic acid
molecules in a
sample are amplified except the nucleic acid molecules digested by a MSRE.
[0169] In some embodiments, the method can further comprise, detecting the
presence or absence of cancer in the subject based on the methylation status
at one or more
genetic loci of the nucleic acid molecules in at least one partitioned set. In
some
embodiments, the method further comprises, determining a level of cancer in
the
polynucleotide sample, for example, by determining a level of DNA from cancer
cells in the
polynucleotide sample.
[0170] FIG. 4 illustrates an exemplary workflow to detect the presence or
absence
of cancer according to certain embodiments of the disclosure beginning with a
cfDNA
sample, in which cfDNA is isolated from the blood sample and the cfDNA sample
comprises
cfDNA molecules belonging to cancer hypermethylated DMR regions and
unmethylated
control regions; the cfDNA is partitioned using a methyl-binding domain
protein (MBD) into
hypo methylated, residual (i.e., intermediately methylated), and hyper
methylated partitioned
sets; each partitioned set is subjected to molecular barcoding to
distinguishably tag DNA
from the hypo, residual, and hyper partitioned sets; the hyper partitioned set
is digested with
- 44 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
two MSREs - HhaI and AccI, cleaving the unmethylated cfDNA molecules at the RE
recognition site; and then partitioned sets (including the MSRE digested hyper
partitioned
set) are pooled, captured, amplified, and sequenced. In some embodiments,
nucleic acid
molecules digested by a MSRE are not amplified. In some such embodiments,
essentially all
nucleic acid molecules in a sample are amplified except the nucleic acid
molecules digested
by a MSRE.
[0171] In some embodiments, MSREs are chosen to maximize the number of
methylation biomarker sequences (i.e., DMRs) targeted. In some embodiments, if
two or
more MSREs are used in a single digestion, the enzyme buffers should be
compatible
(verified by the vendor and/or tested empirically). Additionally, MSREs should
have a
mechanism to inactivate their activity that is compatible with downstream
assay processing.
For example, if MSRE digestion is performed prior to ligation, heat
inactivation ( >65 C) of
MSRE would not be appropriate as it would denature dsDNA, rendering it
incompatible with
adapter ligation reaction.
[0172] In some embodiments, the methylation sensitive restriction enzymes that
do
not cleave the DNA if a particular nucleotide base is methylated at the
recognition sequence
can be used. Such MSREs can be used in hyper partition only in order to remove
unmethylated molecules that partitioned incorrectly in the hyper partition;
thereby improving
methylated nucleic acid molecule detection specificity. In some embodiments,
the
methylation sensitive restriction enzymes that cleave the DNA if a particular
nucleotide base
is methylated at the recognition sequence can be used. Such MSREs can be used
in hypo
partition in order to remove methylated molecules that partitioned incorrectly
in the hypo
partition; thereby improving unmethylated nucleic acid molecules detection
specificity. In
some embodiments, both hyper (and residual) and hypo partitions are digested
with MSREs
such that (i) MSRE(s) that cleave DNA if there are unmethylated nucleotide(s)
at the
recognition site are used in hyper (and residual) partition and (ii) MSRE(s)
that cleave DNA
if there are methylated nucleotide(s) at the recognition site are used in the
hypo partition.
[0173] In some embodiments, after adapter ligation, if more than one partition
(e.g.
hyper and residual) is to be digested with same MSREs, the digestions can be
performed on
each partition separately or the partitions can be combined and digested in
one reaction. In
some embodiments, performing the digestion separately on each partition can be
advantageous if necessary enzyme performance (efficiency, specificity) can
only be achieved
using with separate reactions. In some embodiments, combining the partitions
and then
- 45 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
performed MSRE digestion can be beneficial in reducing assay's cost of goods
sold - COGS
(SPRI beads, enzymes, PCR plates, pipetting tips, etc.) and for streamlining
the scaled,
automated assay (i.e. single digestion reaction per sample).
[0174] In some embodiments, if MSRE digestion (where MSRE cleaves
unmethylated DNA at the recognition site) is performed prior to the ligation
of adapters, the
cleaved fragments of the molecules can be retained and the ends of molecules
matching RE
recognition sites are used to identify a unmethylated molecule in the hyper
partition. In such
embodiments, when analyzing cfDNA sample, if there is genomic DNA
contamination, then
genomic DNA can be cleaved by MSRE (prior to adapter ligation) and can lead to
genomic
DNA contamination. This can be avoided by performing adapter ligation prior to
MSRE
digestion.
[0175] In some embodiments, all the partitioned sets or a subset of all the
partitioned sets can be sequenced. In some embodiments, only the one or more
partitioned
sets for which MSRE digestion was performed can be sequenced to analyze
nucleic acid
molecules in the cancer DMRs.
[0176] In some embodiments, the polynucleotide sample is partitioned into two
partitioned sets. In some embodiments, the polynucleotide sample is
partitioned into three
partitioned sets. In some embodiments, MSRE digestion is performed to nucleic
acid
molecules in hyper partition and hypo partition, wherein the MSRE used in
hyper partition
cleaves DNA if the recognition site has unmethylated nucleotides and the MSRE
used in
hypo partition cleaves DNA if the recognition site has methylated nucleotides.
This enables
simultaneous sensitive detection of hyper and hypo DMRs.
[0177] In some embodiments, the polynucleotide sample is between 1 ng and 500
ng. In some embodiments, the polynucleotide sample is less than 500 ng. In
some
embodiments, the polynucleotide sample is selected from the group consisting
of a DNA
sample, an RNA sample, a cell-free DNA sample, and a cell-free RNA sample. In
some
embodiments, the polynucleotide sample is a cfDNA sample obtained from the
blood of the
subject. In some embodiments, the polynucleotide sample is a DNA sample
obtained from
the tumor tissue biopsy.
- 46 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
General Features of the Methods
A. Samples
[0178] A sample can be any biological sample isolated from a subject. Samples
can
include body tissues, whole blood, platelets, serum, plasma, stool, red blood
cells, white
blood cells or leucocytes, endothelial cells, tissue biopsies (e.g., biopsies
from known or
suspected solid tumors), cerebrospinal fluid, synovial fluid, lymphatic fluid,
ascites fluid,
interstitial or extracellular fluid (e.g., fluid from intercellular spaces),
gingival fluid,
crevicular fluid, bone marrow, pleural effusions, cerebrospinal fluid, saliva,
mucous, sputum,
semen, sweat, and urine. Samples may thus be bodily fluids, such as blood and
fractions
thereof, and urine. Such samples can include nucleic acids shed from tumors.
The nucleic
acids can include DNA and RNA, and can be in double- and single-stranded
forms. In some
embodiments, a sample comprises cell-free DNA. A sample can be in the form
originally
isolated from a subject or can have been subjected to further processing to
remove or add
components, such as cells, enrich for one component relative to another, or
convert one form
of nucleic acid to another, such as RNA to DNA or single-stranded nucleic
acids to double-
stranded. Thus, for example, a bodily fluid for analysis can be plasma or
serum containing
cell-free nucleic acids, e.g., cell-free DNA (cfDNA).
[0179] A sample can be isolated or obtained from a subject and transported to
a site
of sample analysis. The sample may be preserved and shipped at a desirable
temperature,
e.g., room temperature, 4 C, -20 C, and/or -80 C. A sample can be isolated
or obtained
from a subject at the site of the sample analysis. The subject can be a human,
a mammal, an
animal, a companion animal, a service animal, or a pet. The subject may have a
cancer. The
subject may not have cancer or a detectable cancer symptom. The subject may
have been
treated with one or more cancer therapy, e.g., any one or more of
chemotherapies. The
subject may be in remission. The subject may or may not be diagnosed of being
susceptible
to cancer or any cancer-associated genetic mutations/disorders.
[0180] In some embodiments, the sample volume of bodily fluid taken from a
subject depends on the desired read depth for sequenced regions. Examples of
volumes are
about 0.4-40 milliliters (mL), about 5-20 mL, about 10-20 mL. For example, the
volume can
be about 0.5 mL, about 1 mL, about 5 mL, about 10 mL, about 20 mL, about 30
mL, about 40
mL, or more milliliters. A volume of sampled plasma is typically between about
5 mL to
about 20 mL.
- 47 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0181] The sample can comprise various amounts of nucleic acid. Typically, the
amount of nucleic acid in a given sample is equates with multiple genome
equivalents. For
example, a sample of about 30 nanograms (ng) DNA can contain about 10,000
(104) haploid
human genome equivalents and, in the case of cfDNA, about 200 billion (2 x
1011) individual
polynucleotide molecules. Similarly, a sample of about 100 ng of DNA can
contain about
30,000 haploid human genome equivalents and, in the case of cfDNA, about 600
billion
individual molecules.
[0182] In some embodiments, a sample comprises nucleic acids from different
sources, e.g., from cells and from cell-free sources (e.g., blood samples,
etc.). Typically, a
sample includes nucleic acids carrying mutations. For example, a sample
optionally
comprises DNA carrying germline mutations and/or somatic mutations. Typically,
a sample
comprises DNA carrying cancer-associated mutations (e.g., cancer-associated
somatic
mutations).
[0183] Example amounts of cell-free nucleic acids in a sample before
amplification
typically range from about 1 femtogram (fg) to about 1 microgram (fig), e.g.,
about 1
picogram (pg) to about 200 nanograms (ng), about 1 ng to about 100 ng, about
10 ng to about
1000 ng. In some embodiments, a sample includes up to about 600 ng, up to
about 500 ng,
up to about 400 ng, up to about 300 ng, up to about 200 ng, up to about 100
ng, up to about
50 ng, or up to about 20 ng of cell-free nucleic acid molecules. Optionally,
the amount is at
least about 1 fg, at least about 10 fg, at least about 100 fg, at least about
1 pg, at least about
pg, at least about 100 pg, at least about 1 ng, at least about 10 ng, at least
about 100 ng, at
least about 150 ng, or at least about 200 ng of cell-free nucleic acid
molecules. In some
embodiments, the amount is up to about 1 fg, about 10 fg, about 100 fg, about
1 pg, about 10
pg, about 100 pg, about 1 ng, about 10 ng, about 100 ng, about 150 ng, or
about 200 ng of
cell-free nucleic acid molecules. In some embodiments, methods include
obtaining between
about 1 fg to about 200 ng cell-free nucleic acid molecules from samples.
[0184] Cell-free nucleic acids typically have a size distribution of between
about
100 nucleotides in length and about 500 nucleotides in length, with molecules
of about 110
nucleotides in length to about 230 nucleotides in length representing about
90% of molecules
in the sample, with a mode of about 168 nucleotides length (in samples from
human subjects)
and a second minor peak in a range between about 240 nucleotides to about 440
nucleotides
in length In some embodiments, cell-free nucleic acids are from about 160
nucleotides to
- 48 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
about 180 nucleotides in length, or from about 320 nucleotides to about 360
nucleotides in
length, or from about 440 nucleotides to about 480 nucleotides in length.
[0185] In some embodiments, cell-free nucleic acids are isolated from bodily
fluids
through a partitioning step in which cell-free nucleic acids, as found in
solution, are separated
from intact cells and other non-soluble components of the bodily fluid. In
some
embodiments, partitioning includes techniques such as centrifugation or
filtration.
Alternatively, cells in bodily fluids may be lysed, and cell-free and cellular
nucleic acids may
be processed together. Generally, after addition of buffers and wash steps,
cell-free nucleic
acids may be precipitated with, for example, an alcohol. In some embodiments,
additional
clean-up steps are used, such as silica-based columns to remove contaminants
or salts. Non-
specific bulk carrier nucleic acids, for example, are optionally added
throughout the reaction
to optimize aspects of the example procedure, such as yield. After such
processing, samples
typically include various forms of nucleic acids including double-stranded
DNA, single-
stranded DNA and/or single-stranded RNA. Optionally, single-stranded DNA
and/or single-
stranded RNA are converted to double-stranded forms so that they are included
in subsequent
processing and analysis steps.
[0186] Double-stranded DNA molecules in a sample and single stranded nucleic
acid molecules that have been converted to double stranded DNA molecules can
be linked to
adapters at either one end or both ends. Typically, double stranded molecules
are blunt ended
by treatment with a polymerase with a 5'-3' polymerase and a 3 '-5'
exonuclease (or proof
reading function), in the presence of all four standard nucleotides. Klenow
large fragment and
T4 polymerase are examples of suitable polymerase. The blunt ended DNA
molecules can be
ligated with at least partially double stranded adapter (e.g., a Y shaped or
bell-shaped
adapter). Alternatively, complementary nucleotides can be added to blunt ends
of sample
nucleic acids and adapters to facilitate ligation. Contemplated herein are
both blunt end
ligation and sticky end ligation. In blunt end ligation, both the nucleic acid
molecules and the
adapter tags have blunt ends. In sticky-end ligation, typically, the nucleic
acid molecules bear
an "A" overhang and the adapters bear a "T" overhang.
B. Partitioning, Adding Adapters, Tagging
[0187] In another embodiment, a partitioning scheme can be performed using the
following exemplary procedure. Nucleic acids are linked at both ends to Y-
shaped adapters
including primer binding sites and tags. The molecules are amplified. The
amplified
- 49 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
molecules are then fractionated by contact with an antibody preferentially
binding to 5-
methylcytosine to produce two partitions. One partition includes original
molecules lacking
methylation and amplification copies having lost methylation. The other
partition includes
original DNA molecules with methylation. The partition including original DNA
molecules
with methylation is subjected to a procedure that affects a first nucleobase
in the DNA
differently from a second nucleobase in the DNA of the first partition,
wherein the first
nucleobase is a modified or unmodified nucleobase, the second nucleobase is a
modified or
unmodified nucleobase different from the first nucleobase, and the first
nucleobase and the
second nucleobase have the same base pairing specificity. The two partitions
are then
processed and sequenced separately with further amplification of the
methylated partition.
The sequence data of the two partitions can then be compared. In this example,
tags are not
used to distinguish between methylated and unmethylated DNA but rather to
distinguish
between different molecules within these partitions so that one can determine
whether reads
with the same start and stop points are based on the same or different
molecules.
[0188] Tags may be incorporated into or otherwise joined to adapters by
chemical
synthesis, ligation (e.g., blunt-end ligation or sticky-end ligation), or
overlap extension
polymerase chain reaction (PCR), among other methods. Such adapters may be
ultimately
joined to the target nucleic acid molecule. In other embodiments, one or more
rounds of
amplification cycles (e.g., PCR amplification) are generally applied to
introduce sample
indexes to a nucleic acid molecule using conventional nucleic acid
amplification methods.
The amplifications may be conducted in one or more reaction mixtures (e.g., a
plurality of
microwells in an array). Molecular barcodes and/or sample indexes may be
introduced
simultaneously, or in any sequential order. In some embodiments, molecular
barcodes and/or
sample indexes are introduced prior to and/or after sequence capturing steps
are performed.
In some embodiments, only the molecular barcodes are introduced prior to probe
capturing
and the sample indexes are introduced after sequence capturing steps are
performed. In some
embodiments, both the molecular barcodes and the sample indexes are introduced
prior to
performing probe-based capturing steps. In some embodiments, the sample
indexes are
introduced after sequence capturing steps are performed. In some embodiments,
molecular
barcodes are incorporated to the nucleic acid molecules (e.g. cfDNA molecules)
in a sample
through adapters via ligation (e.g., blunt-end ligation or sticky-end
ligation). In some
embodiments, sample indexes are incorporated to the nucleic acid molecules
(e.g. cfDNA
molecules) in a sample through overlap extension polymerase chain reaction
(PCR).
Typically, sequence capturing protocols involve introducing a single-stranded
nucleic acid
- 50 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
molecule complementary to a targeted nucleic acid sequence, e.g., a coding
sequence of a
genomic region and mutation of such region is associated with a cancer type.
[0189] In some embodiments, the tags may be located at one end or at both ends
of
the sample nucleic acid molecule. In some embodiments, tags are predetermined
or random
or semi-random sequence oligonucleotides. In some embodiments, the tags may be
less than
or equal to about 500, 200, 100, 50, 20, 10, 9, 8, 7, 6, 5, 4, 3, 2, or 1
nucleotides in length.
The tags may be linked to sample nucleic acids randomly or non-randomly.
[0190] In some embodiments, each sample is uniquely tagged with a sample index
or a combination of sample indexes. In some embodiments, each nucleic acid
molecule of a
sample or sub-sample is uniquely tagged with a molecular barcode or a
combination of
molecular barcodes. In other embodiments, a plurality of molecular barcodes
may be used
such that molecular barcodes are not necessarily unique to one another in the
plurality (e.g.,
non-unique molecular barcodes). In these embodiments, molecular barcodes are
generally
attached (e.g., by ligation) to individual molecules such that the combination
of the molecular
barcode and the sequence it may be attached to creates a unique sequence that
may be
individually tracked. Detection of non-unique molecular barcodes in
combination with
endogenous sequence information (e.g., the beginning (start) and/or end (stop)
genomic
location/position corresponding to the sequence of the original nucleic acid
molecule in the
sample, start and stop genomic positions corresponding to the sequence of the
original
nucleic acid molecule in the sample, the beginning (start) and/or end (stop)
genomic
location/position of the sequence read that is mapped to the reference
sequence, start and stop
genomic positions of the sequence read that is mapped to the reference
sequence, sub-
sequences of sequence reads at one or both ends, length of sequence reads,
and/or length of
the original nucleic acid molecule in the sample) typically allows for the
assignment of a
unique identity to a particular molecule. In some embodiments, beginning
region comprises
the first 1, first 2, the first 5, the first 10, the first 15, the first 20,
the first 25, the first 30 or at
least the first 30 base positions at the 5' end of the sequencing read that
align to the reference
sequence. In some embodiments, the end region comprises the last 1, last 2,
the last 5, the
last 10, the last 15, the last 20, the last 25, the last 30 or at least the
last 30 base positions at
the 3' end of the sequencing read that align to the reference sequence. The
length, or number
of base pairs, of an individual sequence read are also optionally used to
assign a unique
identity to a given molecule. As described herein, fragments from a single
strand of nucleic
- 51 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
acid having been assigned a unique identity, may thereby permit subsequent
identification of
fragments from the parent strand, and/or a complementary strand.
[0191] In some embodiments, molecular barcodes are introduced at an expected
ratio of a set of identifiers (e.g., a combination of unique or non-unique
molecular barcodes)
to molecules in a sample. One example format uses from about 2 to about
1,000,000 different
molecular barcode sequences, or from about 5 to about 150 different molecular
barcode
sequences, or from about 20 to about 50 different molecular barcode sequences,
ligated to
both ends of a target molecule. Alternatively, from about 25 to about
1,000,000 different
molecular barcode sequences may be used. For example, 20-50 x 20-50 molecular
barcode
sequences (i.e., one of the 20-50 different molecular barcode sequences can be
attached to
each end of the target molecule) can be used. Such numbers of identifiers are
typically
sufficient for different molecules having the same start and stop points to
have a high
probability (e.g., at least 94%, 99.5%, 99.99%, or 99.999%) of receiving
different
combinations of identifiers. In some embodiments, about 80%, about 90%, about
95%, or
about 99% of molecules have the same combinations of molecular barcodes.
[0192] In some embodiments, the assignment of unique or non-unique molecular
barcodes in reactions is performed using methods and systems described in, for
example,
U.S. Patent Application Nos. 20010053519, 20030152490, and 20110160078, and
U.S.
Patent Nos. 6,582,908, 7,537,898, 9,598,731, and 9,902,992, each of which is
hereby
incorporated by reference in its entirety. Alternatively, in some embodiments,
different
nucleic acid molecules of a sample may be identified using only endogenous
sequence
information (e.g., start and/or stop positions, sub-sequences of one or both
ends of a
sequence, and/or lengths).
[0193] In certain embodiments described herein, a population of different
forms of
nucleic acids (e.g., hypermethylated and hypomethylated DNA in a sample) can
be physically
partitioned prior to analysis, e.g., sequencing, or tagging and sequencing.
For example, in
some embodiments, the partitioning comprises separating nucleic acid molecules
into
partition sets based on a differential binding affinity of the nucleic acid
molecules to a
binding agent that preferentially binds to nucleic acid molecules comprising
methylated
nucleotides. In some embodiments, partitioned sets are modified by, for
example, digesting at
least a subset of at least one partitioned set with a MSRE. This approach can
be used to
determine, for example, whether hypermethylation variable epigenetic target
regions show
hypermethylation characteristic of tumor cells or hypomethylation variable
epigenetic target
- 52 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
regions show hypomethylation characteristic of tumor cells. Additionally, by
partitioning a
heterogeneous nucleic acid population, one may increase rare signals, e.g., by
enriching rare
nucleic acid molecules that are more prevalent in one fraction (or partition)
of the population.
For example, a genetic variation present in hyper-methylated DNA but less (or
not) in
hypomethylated DNA can be more easily detected by partitioning a sample into
hyper-
methylated and hypo-methylated nucleic acid molecules. By analyzing multiple
fractions of a
sample, a multi-dimensional analysis of a single locus of a genome or species
of nucleic acid
can be performed and hence, greater sensitivity can be achieved.
[0194] In some instances, a heterogeneous nucleic acid sample is partitioned
into
two or more partitions (e.g., at least 3, 4, 5, 6 or 7 partitions). In some
embodiments, each
partition is differentially tagged ¨ i.e., each partition can have a different
set of molecular
barcodes. Tagged partitions can then be pooled together for collective sample
prep and/or
sequencing. The partitioning-tagging-pooling steps can occur more than once,
with each
round of partitioning occurring based on a different characteristics (examples
provided
herein) and tagged using differential tags that are distinguished from other
partitions and
partitioning means.
[0195] Examples of characteristics that can be used for partitioning include
sequence length, methylation level, nucl eosom e binding, sequence mismatch,
immunoprecipitation, and/or proteins that bind to DNA. Resulting partitions
can include one
or more of the following nucleic acid forms: single-stranded DNA (ssDNA),
double-stranded
DNA (dsDNA), shorter DNA fragments and longer DNA fragments. In some
embodiments,
partitioning based on a cytosine modification (e.g., cytosine methylation) or
methylation
generally is performed and is optionally combined with at least one additional
partitioning
step, which may be based on any of the foregoing characteristics or forms of
DNA. In some
embodiments, a heterogeneous population of nucleic acids is partitioned into
nucleic acids
with one or more epigenetic modifications and without the one or more
epigenetic
modifications. Examples of epigenetic modifications include presence or
absence of
methylation; level of methylation; type of methylation (e.g., 5-methylcytosine
versus other
types of methylation, such as adenine methylation and/or cytosine
hydroxymethylation); and
association and level of association with one or more proteins, such as
histones.
Alternatively, or additionally, a heterogeneous population of nucleic acids
can be partitioned
into nucleic acid molecules associated with nucleosomes and nucleic acid
molecules devoid
of nucleosomes. Alternatively, or additionally, a heterogeneous population of
nucleic acids
- 53 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
may be partitioned into single-stranded DNA (ssDNA) and double-stranded DNA
(dsDNA).
Alternatively, or additionally, a heterogeneous population of nucleic acids
may be partitioned
based on nucleic acid length (e.g., molecules of up to 160 bp and molecules
having a length
of greater than 160 bp).
[0196] In some embodiments, a population of nucleic acids is partitioned into
two or
more different partitions. Each partition is representative of a different
nucleic acid form, and
a first partition comprises DNA with a cytosine modification in a greater
proportion than a
second partition. Each partition is distinctly tagged. The first partition is
subjected to a
procedure that affects a first nucleobase in the DNA differently from a second
nucleobase in
the DNA of the first partition, wherein the first nucleobase is a modified or
unmodified
nucleobase, the second nucleobase is a modified or unmodified nucleobase
different from the
first nucleobase, and the first nucleobase and the second nucleobase have the
same base
pairing specificity. The tagged nucleic acids are pooled together prior to
sequencing.
Sequence reads are obtained and analyzed, including to distinguish the first
nucleobase from
the second nucleobase in the DNA of the first partition, in silico. Tags are
used to sort reads
from different partitions. Analysis to detect genetic variants can be
performed on a partition-
by-partition level, as well as whole nucleic acid population level For
example, analysis can
include in silico analysis to determine genetic variants, such as CNV, SNV,
indel, fusion in
nucleic acids in each partition. In some instances, in silico analysis can
include determining
chromatin structure. For example, coverage of sequence reads can be used to
determine
nucleosome positioning in chromatin. Higher coverage can correlate with higher
nucleosome
occupancy in genomic region while lower coverage can correlate with lower
nucleosome
occupancy or nucleosome depleted region (NDR).
[0197] Samples can include nucleic acids varying in modifications including
post-
replication modifications to nucleotides and binding, usually noncovalently,
to one or more
proteins.
[0198] In an embodiment, the population of nucleic acids is one obtained from
a
serum, plasma or blood sample from a subject suspected of having neoplasia, a
tumor, or
cancer or previously diagnosed with neoplasia, a tumor, or cancer. The
population of nucleic
acids includes nucleic acids having varying levels of methylation. Methylation
can occur
from any one or more post-replication or transcriptional modifications. Post-
replication
modifications include modifications of the nucleotide cytosine, particularly
at the 5-position
- 54 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
of the nucleobase, e.g., 5-methylcytosine, 5-hydroxymethylcytosine, 5-
formylcytosine and 5-
carboxylcytosine.
[0199] Agents used in the partitioning, such as binding agents, can be
antibodies
with the desired specificity, natural binding partners or variants thereof
(Bock et al., Nat
Biotech 28: 1106-11114 (2010); Song et al., Nat Biotech 29: 68-72 (2011)), or
artificial
peptides selected e.g., by phage display to have specificity to a given
target.
[0200] Examples of binding agents contemplated herein include methyl binding
domain (MBDs) and methyl binding proteins (MBPs) as described herein,
including proteins
such as MeCP2 and antibodies preferentially binding to 5-methylcytosine. Where
an antibody
is used to immunoprecipitate methylated DNA, the methylated DNA may be
recovered in
single-stranded form. In such embodiments, a second strand can be synthesized.
Hypermethylated (and optionally intermediately methylated) partitions may then
be contacted
with an MSRE that does not cleave hemi-methylated DNA but cleaves unmethylated
DNA,
such as HpaII, BstUI, or Hin6i. Alternatively or in addition, hypomethylated
(and optionally
intermediately methylated) partitions may then be contacted with an MSRE that
cleaves
hemi-methylated DNA but does not cleave unmethylated DNA.
[0201] Likewise, partitioning of different forms of nucleic acids can be
performed
using histone binding proteins which can separate nucleic acids bound to
histones from free
or unbound nucleic acids. Examples of histone binding proteins that can be
used in the
methods disclosed herein include RBBP4, RbAp48 and SANT domain peptides.
[0202] Although for some binding agents and some nucleic acid modifications,
binding to the agent may occur in an essentially all or none manner depending
on whether a
nucleic acid bears a modification, the separation may be one of degree. In
such instances,
nucleic acids overrepresented in a modification bind to the agent at a greater
extent that
nucleic acids underrepresented in the modification. Alternatively, nucleic
acids having
modifications may bind in an all or nothing manner. But then, various levels
of modifications
may be sequentially eluted from the binding agent.
[0203] For example, in some embodiments, partitioning can be binary or based
on
degree/level of modifications. For example, all methylated fragments can be
partitioned from
unmethylated fragments using methyl-binding domain proteins (e.g.,
MethylMinder
Methylated DNA Enrichment Kit (ThermoFisher Scientific). Subsequently,
additional
partitioning may involve eluting fragments having different levels of
methylation by
adjusting the salt concentration in a solution with the methyl-binding domain
and bound
- 55 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
fragments. As salt concentration increases, fragments having greater
methylation levels are
eluted.
[0204] In some instances, the final partitions are representative of nucleic
acids
having different extents of modifications (overrepresentative or
underrepresentative of
modifications). Overrepresentation and underrepresentation can be defined by
the number of
modifications born by a nucleic acid relative to the median number of
modifications per
strand in a population. For example, if the median number of 5-methylcytosine
residues in
nucleic acid in a sample is 2, a nucleic acid including more than two 5-
methylcytosine
residues is overrepresented in this modification and a nucleic acid with 1 or
zero 5-
methylcytosine residues is underrepresented. The effect of the affinity
separation is to enrich
for nucleic acid molecules overrepresented in a modification in a bound phase
and for nucleic
acid molecules underrepresented in a modification in an unbound phase (i.e. in
solution). The
nucleic acid molecules in the bound phase can be eluted before subsequent
processing.
[0205] When using MethylMiner Methylated DNA Enrichment Kit (ThermoFisher
Scientific) DNA comprising various levels of methylation can be partitioned
using sequential
elutions. For example, a hypomethylated partition (no methylation) can be
separated from a
methylated partition by contacting the nucleic acid population with the MBD
from the kit,
which is attached to magnetic beads. The beads are used to separate out the
methylated
nucleic acids from the non- methylated nucleic acids. Subsequently, one or
more elution steps
are performed sequentially to elute nucleic acids having different levels of
methylation. For
example, a first set of methylated nucleic acids can be eluted at a salt
concentration of 160
mM or higher, e.g., at least 150 mM, at least 200 mM, 300 mM, 400 mM, 500 mM,
600 mM,
700 mM, 800 mM, 900 mM, 1000 mM, or 2000 mM. After such methylated nucleic
acids are
eluted, magnetic separation is once again used to separate higher level of
methylated nucleic
acids from those with lower level of methylation. The elution and magnetic
separation steps
can repeat themselves to create various partitions such as a hypomethylated
partition
(representative of no methylation), a methylated partition (representative of
low level of
methylation), and a hyper methylated partition (representative of high level
of methylation).
[0206] In some methods, nucleic acids bound to an agent used for partitioning
are
subjected to a wash step. The wash step washes off nucleic acids weakly bound
to the binding
agent. Such nucleic acids can be enriched in nucleic acids having the
modification to an
extent close to the mean or median (i.e., intermediate between nucleic acids
remaining bound
to the solid phase and nucleic acids not binding to the solid phase on initial
contacting of the
sample with the agent).
- 56 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0207] The partitioning results in at least two, and sometimes three or more
partitions of nucleic acids with different extents of a modification. While
the partitions are
still separate, the nucleic acids of at least one partition, and usually two
or three (or more)
partitions are linked to nucleic acid tags, usually provided as components of
adapters, with
the nucleic acids in different partitions receiving different tags that
distinguish members of
one partition from another. The tags linked to nucleic acid molecules of the
same partition
can be the same or different from one another. But if different from one
another, the tags may
have part of their code in common so as to identify the molecules to which
they are attached
as being of a particular partition.
[0208] For further details regarding partitioning nucleic acid samples based
on
characteristics such as methylation, see W02018/119452, which is incorporated
herein by
reference.
[0209] In some embodiments, the nucleic acid molecules can be partitioned into
different partitions based on the nucleic acid molecules that are bound to a
specific protein or
a fragment thereof and those that are not bound to that specific protein or
fragment thereof.
[0210] Nucleic acid molecules can be partitioned based on DNA-protein binding.
Protein-DNA complexes can be partitioned based on a specific property of a
protein.
Examples of such properties include various epitopes, modifications (e.g.,
histone
methylation or acetylation) or enzymatic activity. Examples of proteins which
may bind to
DNA and serve as a binding agent for partitioning may include, but are not
limited to, protein
A and protein G. Any suitable method can be used to partition the nucleic acid
molecules
based on protein bound regions. Examples of methods used to partition nucleic
acid
molecules based on protein bound regions include, but are not limited to, SDS-
PAGE,
chromatin-immuno-precipitation (Ch1P), heparin chromatography, and
asymmetrical field
flow fractionation (AF4).
[0211] In general, elution is a function of the number of methylated sites per
nucleic
acid molecule, with molecules having more methylation eluting under increased
salt
concentrations. To elute the DNA into distinct populations or partitions based
on the extent of
methylation, one can use a series of elution buffers of increasing NaCl
concentration. Salt
concentration can range from about 100 nm to about 2500 mM NaCl. In one
embodiment, the
process results in three (3) partitions. Molecules are contacted with a
solution at a first salt
concentration and comprising a molecule comprising a methyl binding domain,
which
molecule can be attached to a capture moiety, such as streptavidin. At the
first salt
concentration a population of molecules will bind to the MBD and a population
will remain
- 57 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
unbound. The unbound population can be separated as a "hypomethylated"
population. For
example, a first partition representative of the hypomethylated form of DNA is
that which
remains unbound at a low salt concentration, e.g., 100 mM or 160 mM. A second
partition
representative of intermediate methylated DNA is eluted using an intermediate
salt
concentration, e.g., between 100 mM and 2000 mM concentration. This is also
separated
from the sample. A third partition representative of hypermethylated form of
DNA is eluted
using a high salt concentration, e.g., at least about 2000 mM.
[0212] Partitioning procedures may result in imperfect sorting of DNA
molecules
among the resulting partitions or fractions. For example, a minority of the
molecules in a
hypomethylated partition may be highly modified (e.g., hypermethylated),
and/or a minority
of the molecules in a hypermethylated partition may be unmodified or mostly
unmodified
(e.g., unmethylated or mostly unmethylated). Such molecules are considered
nonspecifically
partitioned. The methods described herein comprise steps that can reduce
technical noise
from nonspecifically partitioned DNA, e.g., by degrading it and/or by
converting certain
bases such that nonspecifically partitioned DNA can be identified following
sequencing.
Thus, the methods described herein can provide improved sensitivity and/or
streamlined
analysis.
[0213] In some instances, each partitioned set (representative of a different
nucleic
acid form) is differentially tagged with molecular barcodes, and the
partitioned sets are
pooled together prior to sequencing. In other instances, the different forms
are separately
sequenced.
[0214] In some embodiments, the nucleic acid molecules (from the sample of
polynucleotides, e.g., after partitioning) may be tagged with sample indexes
and/or molecular
barcodes (referred to generally as -tags"). Tags can be used to label nucleic
acids of
partitions so as to correlate the tag (or tags) with a specific partition.
Alternatively, tags can
be used in embodiments of the invention that do not employ a partitioning
step. Tags or
indexes can be molecules, such as nucleic acids, containing information that
indicates a
feature of the molecule with which the tag is associated. For example,
molecules can bear a
sample tag or sample index (which distinguishes molecules in one sample from
those in a
different sample), a partition tag (which distinguishes molecules in one
partition from those
in a different partition) or a molecular tag/molecular barcode/barcode (which
distinguishes
different molecules from one another (in both unique and non-unique tagging
scenarios). In
certain embodiments, a tag can comprise one or a combination of barcodes. In
some
- 58 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
embodiments, the barcode has, for example, between 10 and 100 nucleotides. A
collection of
barcodes can have degenerate sequences or can have sequences having a certain
hamming
distance, as desired for the specific purpose. So, for example, a molecular
barcode can be
comprised of one barcode or a combination of two barcodes, each attached to
different ends
of a molecule. Additionally or alternatively, for different partitions and/or
samples, different
sets of molecular barcodes, molecular tags, or molecular indexes can be used
such that the
barcodes serve as a molecular tag through their individual sequences and also
serve to
identify the partition and/or sample to which they correspond based the set of
which they are
a member.
[0215] Tagging strategies can be divided into unique tagging and non-unique
tagging strategies. In unique tagging, all or substantially all of the
molecules in a sample bear
a different tag, so that reads can be assigned to original molecules based on
tag information
alone. Tags used in such methods are sometimes referred to as "unique tags".
In non-unique
tagging, different molecules in the same sample can bear the same tag, so that
other
information in addition to tag information is used to assign a sequence read
to an original
molecule. Such information may include start and stop coordinate, coordinate
to which the
molecule maps, start or stop coordinate alone, etc. Tags used in such methods
are sometimes
referred to as "non-unique tags". Accordingly, it is not necessary to uniquely
tag every
molecule in a sample. It suffices to uniquely tag molecules falling within an
identifiable class
within a sample. Thus, molecules in different identifiable families can bear
the same tag
without loss of information about the identity of the tagged molecule.
[0216] In certain embodiments of non-unique tagging, the number of different
tags
used can be sufficient that there is a very high likelihood (e.g., at least
99%, at least 99.9%, at
least 99.99% or at least 99.999% that all molecules of a particular group bear
a different tag.
It is to be noted that when barcodes are used as tags, and when barcodes are
attached, e.g.,
randomly, to both ends of a molecule, the combination of barcodes, together,
can constitute a
tag. This number, in term, is a function of the number of molecules falling
into the calls. For
example, the class may be all molecules mapping to the same start-stop
position on a
reference genome. The class may be all molecules mapping across a particular
genetic locus,
e.g., a particular base or a particular region (e.g., up to 100 bases or a
gene or an exon of a
gene). In certain embodiments, the number of different tags used to uniquely
identify a
number of molecules, z, in a class can be between any of 2*z, 3*z, 4*z, 5*z,
6*z, 7*z, 8*z,
- 59 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
9*z, 10*z, 11 *z, 12*z, 13*z, 14*z, 15*z, 16*z, 17*z, 18*z, 19*z, 20*z or
100*z (e.g., lower
limit) and any of 100,000*z, 10,000*z, 1000*z or 100*z (e.g., upper limit).
[0217] For example, in a sample of about 5 ng to 30 ng of cell free DNA, one
expects around 3000 molecules to map to a particular nucleotide coordinate,
and between
about 3 and 10 molecules having any start coordinate to share the same stop
coordinate.
Accordingly, about 50 to about 50,000 different tags (e.g., between about 6
and 220 barcode
combinations) can suffice to uniquely tag all such molecules. To uniquely tag
all 3000
molecules mapping across a nucleotide coordinate, about 1 million to about 20
million
different tags would be required.
[0218] Generally, assignment of unique or non-unique tags barcodes in
reactions
follows methods and systems described by US patent applications 20010053519,
20030152490, 20110160078, and U.S. Pat. No. 6,582,908 and U.S. Pat. No.
7,537,898 and
US Pat. No. 9,598,731. Tags can be linked to sample nucleic acids randomly or
non-
randomly.
[0219] In some embodiments, the tagged nucleic acids are sequenced after
loading
into a microwell plate. The microwell plate can have 96, 384, or 1536
microwells. In some
cases, they are introduced at an expected ratio of unique tags to microwells.
For example, the
unique tags may be loaded so that more than about 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 20, 50, 100,
500, 1000, 5000, 10000, 50,000, 100,000, 500,000, 1,000,000, 10,000,000,
50,000,000 or
1,000,000,000 unique tags are loaded per genome sample. In some cases, the
unique tags may
be loaded so that less than about 2, 3, 4, 5, 6, 7, 8, 9, 10, 20, 50, 100,
500, 1000, 5000, 10000,
50,000, 100,000, 500,000, 1,000,000, 10,000,000, 50,000,000 or 1,000,000,000
unique tags
are loaded per genome sample. In some cases, the average number of unique tags
loaded per
sample genome is less than, or greater than, about 1, 2, 3, 4, 5, 6, 7, 8, 9,
10, 20, 50, 100, 500,
1000, 5000, 10000, 50,000, 100,000, 500,000, 1,000,000, 10,000,000, 50,000,000
or
1,000,000,000 unique tags per genome sample.
[0220] A preferred format uses 20-50 different tags (e.g., barcodes) ligated
to both
ends of target nucleic acids. For example 35 different tags (e.g., barcodes)
ligated to both
ends of target molecules creating 35 x 35 permutations, which equals 1225 for
35 tags. Such
numbers of tags are sufficient so that different molecules having the same
start and stop
points have a high probability (e.g., at least 94%, 99.5%, 99.99%, 99.999%) of
receiving
different combinations of tags. Other barcode combinations include any number
between 10
- 60 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
and 500, e.g., about 15x15, about 35x35, about 75x75, about 100x100, about
250x250, about
500x500.
[0221] In some cases, unique tags may have predetermined or random or semi-
random sequences. In other cases, a plurality of barcodes may be used such
that barcodes are
not necessarily unique to one another in the plurality. In this example,
barcodes may be
ligated to individual nucleic acid molecules such that the combination of the
barcode and the
sequence it may be ligated to creates a unique sequence that may be
individually tracked. As
described herein, detection of non-unique barcodes in combination with
sequence data of
beginning (start) and end (stop) portions of sequence reads may allow
assignment of a unique
identity to a particular molecule. The length or number of base pairs, of an
individual
sequence read may also be used to assign a unique identity to such a molecule.
As described
herein, fragments from a single strand of nucleic acid having been assigned a
unique identity,
may thereby permit subsequent identification of fragments from the parent
strand.
[0222] In some embodiments, adapters, e.g., adapters comprising tags, are
added to
the nucleic acids after partitioning the nucleic acids, in other embodiments
adapters may be
added to the nucleic acids prior to partitioning the nucleic acids. In some
such methods, a
population of nucleic acids bearing a modification to different extents (e.g.,
0, 1, 2, 3, 4, 5 or
more methyl groups per nucleic acid molecule) is contacted with adapters
before partitioning
of the population depending on the extent of the modification. Adapters are
attached to either
one end or both ends of nucleic acid molecules in the population. In some
embodiments, the
adapters include different tags of sufficient numbers that the number of
combinations of tags
results in a low probability e.g., 95, 99 or 99.9% of two nucleic acids with
the same start and
stop points receiving the same combination of tags. Adapters, whether bearing
the same or
different tags, can include the same or different primer binding sites, but
preferably adapters
include the same primer binding site. In some embodiments, the nucleic acids
are amplified
from primers binding to the primer binding sites within the adapters after
partitioning.
Following amplification, the different partitions can then be subject to
further processing
steps, which may include further (e.g., clonal) amplification, and sequence
analysis, in
parallel but separately. Sequence data from the different partitions can then
be compared.
[0223] In some embodiments, a single tag can be used to label a specific
partition.
In some embodiments, multiple different tags can be used to label a specific
partitioned set.
In embodiments employing multiple different tags to label a specific
partition, the set of tags
used to label one partition can be readily differentiated from the set of tags
used to label other
- 61 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
partitions. In some embodiments, a tag can be multifunctional ¨ i.e., it can
simultaneously act
as a molecular identifier (i.e., molecular barcode), partition identifier
(i.e., partition tag) and
sample identifier (i.e., sample index). For example, if there are four DNA
samples and each
DNA sample is partitioned into three partitions, then the DNA molecules in
each of the
twelve partitions (i.e., twelve partitions for the four DNA samples in total)
can be tagged with
a separate set of tags such that the tag sequence attached to the DNA molecule
reveals the
identity of the DNA molecule, the partition it belongs to and the sample from
which it was
originated. In some embodiments, a tag can be used both as a molecular barcode
and as a
partition tag. For example, if a DNA sample is partitioned into three
partitions, then DNA
molecule in each partition is tagged with a separated set of tags such that
the tag sequence
attached to a DNA molecule reveals the identity of the DNA molecule and the
partition it
belongs to. In some embodiments, a tag can be used both as a molecular barcode
and as a
sample index. For example, if there are four DNA samples, then DNA molecules
in each
sample with be tagged with a separate set of tags that can be distinguishable
from each
sample such that the tag sequence attached to the DNA molecule serves as a
molecule
identifier and as a sample identifier.
[0224] Tn some embodiments, the tags may have additional functions, for
example
the tags can be used to index sample sources or used as unique molecular
identifiers (which
can be used to improve the quality of sequencing data by differentiating
sequencing errors
from mutations, for example as in Kinde et al., Proc Nat'l Acad Sci USA 108:
9530-9535
(2011), Kou et al., PLoS ONE,11: e0146638 (2016)) or used as non-unique
molecule
identifiers, for example as described in US Pat. No. 9,598,731. Similarly, in
some
embodiments, the tags may have additional functions, for example the tags can
be used to
index sample sources or used as non-unique molecular identifiers (which can be
used to
improve the quality of sequencing data by differentiating sequencing errors
from mutations).
[0225] In one embodiment, partition tagging comprises tagging molecules in
each
partition with a partition tag. After re-combining partitions (e.g., to reduce
the number of
sequencing runs needed and avoid unnecessary cost) and sequencing molecules,
the partition
tags identify the source partition. In another embodiment, different
partitions are tagged with
different sets of molecular tags, e.g., comprised of a pair of barcodes. In
this way, each
molecular barcode indicates the source partition as well as being useful to
distinguish
molecules within a partition. For example, a first set of 35 barcodes can be
used to tag
- 62 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
molecules in a first partition, while a second set of 35 barcodes can be used
tag molecules in
a second partition.
[0226] In some embodiments, after partitioning and tagging with partition
tags, the
molecules may be pooled for sequencing in a single run. In some embodiments, a
sample tag
is added to the molecules, e.g., in a step subsequent to addition of partition
tags and pooling.
Sample tags can facilitate pooling material generated from multiple samples
for sequencing
in a single sequencing run.
[0227] Alternatively, in some embodiments, partition tags may be correlated to
the
sample as well as the partition. As a simple example, a first tag can indicate
a first partition of
a first sample; a second tag can indicate a second partition of the first
sample; a third tag can
indicate a first partition of a second sample; and a fourth tag can indicate a
second partition of
the second sample.
[0228] While tags may be attached to molecules already partitioned based on
one or
more epigenetic characteristics, the final tagged molecules in the library may
no longer
possess that epigenetic characteristic. For example, while single stranded DNA
molecules
may be partitioned and tagged, the final tagged molecules in the library are
likely to be
double stranded. Similarly, while DNA may be subject to partition based on
different levels
of methylation, in the final library, tagged molecules derived from these
molecules are likely
to be unmethylated. Accordingly, the tag attached to molecule in the library
typically
indicates the characteristic of the "parent molecule" from which the ultimate
tagged molecule
is derived, not necessarily to characteristic of the tagged molecule, itself.
[0229] As an example, barcodes 1, 2, 3, 4, etc. are used to tag and label
molecules in
the first partition; barcodes A, B, C, D, etc. are used to tag and label
molecules in the second
partition; and barcodes a, b, c, d, etc. are used to tag and label molecules
in the third partition.
Differentially tagged partitions can be pooled prior to sequencing.
Differentially tagged
partitions can be separately sequenced or sequenced together concurrently,
e.g., in the same
flow cell of an Illumina sequencer.
[0230] After sequencing, analysis of reads to detect genetic variants can be
performed on a partition-by-partition level, as well as a whole nucleic acid
population level.
Tags are used to sort reads from different partitions. Analysis can include in
silico analysis to
determine genetic and epigenetic variation (one or more of methylation,
chromatin structure,
etc.) using sequence information, genomic coordinates length, coverage and/or
copy number.
- 63 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
In some embodiments, higher coverage can correlate with higher nucleosome
occupancy in
genomic region while lower coverage can correlate with lower nucleosome
occupancy or a
nucleosome depleted region (NDR).
C. Digesting Nucleic Acid Molecules with Restriction Enzymes
[198] In some embodiments, a partition or partitioned set (e.g., a first,
second, or
third partitioned set prepared by partitioning a sample as described herein,
such as on the
basis of a level of a cytosine modification, such as methylation, e.g., 5-
methylation) is
digested by contacting the partition or partitioned set with a methylation
sensitive restriction
enzyme (MSRE). In some embodiments where partitioning is performed on the
basis of a
cytosine modification, the first partition is the partition with a higher
level of the
modification; the second partition is the partition with a lower level of the
modification; and,
when present, the third partition has a level of the modification intermediate
between the first
and second partitions.
[199_1 As discussed above, partitioning procedures may result in imperfect
sorting of
DNA molecules among the partitions. The choice of MSRE can be made so as to
degrade
nonspecifically partitioned DNA. For example, the second partition can be
contacted with a
MSRE that selectively digests methylated nucleic acid molecules. This can
degrade
nonspecifically partitioned DNA in the second partition (e.g., methylated DNA)
to produce a
treated second partition. Alternatively or in addition, the first partition
can be contacted with
a MSRE that selectively digests unmethylated nucleic acid molecules, thereby
degrading
nonspecifically partitioned DNA in the first partition to produce a treated
first partition.
Degradation of nonspecifically partitioned DNA in either or both of the first
or second
partitions is proposed as an improvement to the performance of methods that
rely on accurate
partitioning of DNA on the basis of a cytosine modification, e.g., to detect
the presence of
aberrantly modified DNA in a sample, to determine the tissue of origin of DNA,
and/or to
determine whether a subject has cancer. For example, such degradation may
provide
improved sensitivity and/or simplify downstream analyses.
[200] In a contacting a partition with a nuclease, such as a MSRE, one or more
nucleases can be used. In some embodiments, a partition is contacted with a
plurality of
nucleases. The partition may be contacted with the nucleases sequentially or
simultaneously.
Simultaneous use of nucleases may be advantageous when the nucleases are
active under
similar conditions (e.g., buffer composition) to avoid unnecessary sample
manipulation.
- 64 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
Contacting the second partition with more than one MSRE can more completely
degrade
nonspecifically partitioned hypermethylated DNA. Similarly, contacting the
first partition
with more than one MSRE can more completely degrade nonspecifically
partitioned
hypomethylated and/or unmethylated DNA.
[2011 In some embodiments, a MSRE that selectively digests methylated nucleic
acid molecules comprises one or more of Mspn, LpnPI, FspEI, or MerBC. In some
embodiments, at least two MSREs that selectively digest methylated nucleic
acid molecules
are used. In some embodiments, at least three MSREs that selectively digest
methylated
nucleic acid molecules are used.
[202] In some embodiments, a MSRE that selectively digests unmethylated
nucleic
acid molecules comprises one or more of AatII, AccII, AciI, Aor13HI, Aorl5HI,
BspT 104I,
BssHII, BstUI, Cfr10I, ClaI, CpoI, Eco52I, HaeII, HapII, HhaI, Hin6I, HpaII,
HpyCH4IV,
MluI, MspI, NaeI, NotI, NruI, NsbI, PmaCI, Psp1406I, PvuI, SacII, Sall, SmaI,
and SnaBI. In
some embodiments, at least two MSREs are used that selectively digest
unmethylated nucleic
acid molecules. In some embodiments, at least three MSREs are used that
selectively digest
unmethylated nucleic acid molecules. In some embodiments, the MSREs comprise
BstUI and
HpaII. In some embodiments, the two MSREs comprise HhaI and AccII. In some
embodiments, the MSREs comprise BstUI, Hpall and Hin6I.
[203] In some embodiments, a partition is contacted with a nuclease as
described
above after a step of tagging or attaching adapters to both ends of the DNA.
The tags or
adapters can be resistant to cleavage by the nuclease using any of the
approaches described
above. In this approach, cleavage can prevent the nonspecifically partitioned
molecule from
being carried through the analysis because the cleavage products lack tags or
adapters at both
ends.
[204] Alternatively, a step of tagging or attaching adapters can be performed
after
digestion with a nuclease as described above Cleaved molecules can be then
identified in
sequence reads based on having an end (point of attachment to tag or adapter)
corresponding
to a nuclease recognition site. Processing the molecules in this way can also
allow the
acquisition of information from the cleaved molecule, e.g., observation of
somatic mutations.
When tagging or attaching adapters after contacting the partition with a
nuclease, and low
molecular weight DNA such as cfDNA is being analyzed, it may be desirable to
remove high
molecular weight DNA (such as contaminating genomic DNA) from the sample
before the
- 65 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
contacting step. It may also be desirable to use nucleases that can be heat-
inactivated at a
relatively low temperature (e.g., 65 C or less, or 60 C or less) to avoid
denaturing DNA, in
that denaturation may interfere with subsequent ligation steps.
[205] Where a sample is partitioned into three partitions, including a third
partition
containing intermediately methylated molecules, the third partition is in some
embodiments
contacted with a MSRE, e.g., an MSRE that selectively digests selectively
digests
unmethylated nucleic acid molecules. Such a step may have any of the features
described
elsewhere herein with respect to contacting steps, and may be performed before
or after a
step of tagging or attaching adapters as discussed above. In some embodiments,
the first and
third partitions are combined before being contacted with a MSRE. Such a step
may have any
of the features described elsewhere herein with respect to contacting steps,
and may be
performed before or after a step of tagging or attaching adapters as discussed
above. In some
embodiments, the first and third partitions are differentially tagged before
being combined.
pm] In some embodiments, where a sample is partitioned into three partitions,
including a third partition containing intermediately methylated molecules,
the third partition
is in some embodiments contacted with an MSRE that selectively digests
methylated nucleic
acid molecules. Such a step may have any of the features described elsewhere
herein with
respect to contacting steps, and may be performed before or after a step of
tagging or
attaching adapters as discussed above. In some embodiments, the second and
third partitions
are combined before being contacted with the MSRE. Such a step may have any of
the
features described elsewhere herein with respect to contacting steps, and may
be performed
before or after a step of tagging or attaching adapters as discussed above. In
some
embodiments, the second and third partitions are differentially tagged before
being combined.
[2o71 In some embodiments, the DNA is purified after being contacted with the
nuclease, e.g., using SPRI beads. Such purification may occur after heat
inactivation of the
nuclease. Alternatively, purification can be omitted; thus, for example, a
subsequent step
such as amplification can be performed on the partition containing heat-
inactivated nuclease.
In another embodiment, the contacting step can occur in the presence of a
purification reagent
such as SPRI beads, e.g., to minimize losses associated with tube transfers.
After cleavage
and heat inactivation, the SPRI beads can be re-used for cleanup by adding
molecular
crowding reagents (e.g., PEG) and salt.
D. Amplification
- 66 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[0231] Sample nucleic acids may be flanked by adapters and amplified by PCR
and
other amplification methods using nucleic acid primers binding to primer
binding sites in
adapters flanking a DNA molecule to be amplified. In some embodiments,
amplification
methods involve cycles of extension, denaturation, and annealing resulting
from
thermocycling, or can be isothermal as, for example, in transcription mediated
amplification.
Other examples of amplification methods that may be optionally utilized
include the ligase
chain reaction, strand displacement amplification, nucleic acid sequence-based
amplification,
and self-sustained sequence-based replication.
[0232] Typically, the amplification reactions generate a plurality of non-
uniquely or
uniquely tagged nucleic acid amplicons with molecular barcodes and sample
indexes at size
ranging from about 150 nucleotides (nt), to about 700 nt, from 250 nt to about
350 nt, or from
about 320 nt to about 550 nt. In some embodiments, the amplicons have a size
of about 180
nt. In some embodiments, the amplicons have a size of about 200 nt.
[0233] In some embodiments, the present methods comprise dsDNA ligations with
T-tailed and C-tailed adapters, which result in amplification of at least 50,
60, 70 or 80% of
double stranded nucleic acids before linking to adapters. Preferably the
present methods
increase the amount or number of amplified molecules relative to control
methods performed
with T-tailed adapters alone by at least 10, 15 or 20%
[0234] In some embodiments, nucleic acid molecules digested by a MSRE are not
amplified. In some such embodiments, essentially all nucleic acid molecules in
a sample are
amplified except the nucleic acid molecules digested by a MSRE.
E. Enrichment/Capturing
[208] In some embodiments, methods disclosed herein comprise capturing or
enriching one or more target regions of nucleic acid molecules. Capture may be
performed
using any suitable approach known in the art. In some embodiments, capturing
comprises
contacting the DNA to be captured with a set of target-specific probes, for
example, probes as
described herein. Capturing may be performed on one or more partitions
prepared during
methods disclosed herein. In some embodiments, DNA is captured from at least
the first
partition or the second partition, e.g., at least the first partition and the
second partition.
Capturing may be performed on any, any two, or all subsets of a partition or
partitioned set.
In some embodiments, the partitions are differentially tagged (e.g., as
described herein) and
then pooled before undergoing capture.
- 67 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[2o91 The capturing step may be performed using conditions suitable for
specific
nucleic acid hybridization, which generally depend to some extent on features
of the probes
such as length, base composition, etc. Those skilled in the art will be
familiar with
appropriate conditions given general knowledge in the art regarding nucleic
acid
hybridization. In some embodiments, complexes of target-specific probes and
DNA are
formed.
[210] In some embodiments, a method described herein comprises capturing cfDNA
obtained from a test subject for a plurality of sets of target regions. The
target regions
comprise epigenetic target regions, which may show differences in methylation
levels and/or
fragmentation patterns depending on whether they originated from a tumor or
from healthy
cells. The target regions also comprise sequence-variable target regions,
which may show
differences in sequence depending on whether they originated from a tumor or
from healthy
cells. The capturing step produces a captured set of cfDNA molecules. In some
embodiments,
the cfDNA molecules corresponding to the sequence-variable target region set
are captured at
a greater capture yield in the captured set of cfDNA molecules than cfDNA
molecules
corresponding to the epigenetic target region set. For additional discussion
of capturing steps,
capture yields, and related aspects, see W02020/160414, which is incorporated
herein by
reference for all purposes.
[211] In some embodiments, a method described herein comprises contacting
cfDNA obtained from a test subject with a set of target-specific probes,
wherein the set of
target-specific probes is configured to capture cfDNA corresponding to the
sequence-variable
target region set at a greater capture yield than cfDNA corresponding to the
epigenetic target
region set.
[2121 It can be beneficial to capture cfDNA corresponding to the sequence-
variable
target region set at a greater capture yield than cfDNA corresponding to the
epigenetic target
region set because a greater depth of sequencing may be necessary to analyze
the sequence-
variable target regions with sufficient confidence or accuracy than may be
necessary to
analyze the epigenetic target regions. The volume of data needed to determine
fragmentation
patterns (e.g., to test for perturbation of transcription start sites or CTCF
binding sites) or
fragment abundance (e.g., in hypermethylated and hypomethylated partitions) is
generally
less than the volume of data needed to determine the presence or absence of
cancer-related
sequence mutations Capturing the target region sets at different yields can
facilitate
- 68 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
sequencing the target regions to different depths of sequencing in the same
sequencing run
(e.g., using a pooled mixture and/or in the same sequencing cell).
[213] In various embodiments, the methods further comprise sequencing the
captured cfDNA, e.g., to different degrees of sequencing depth for the
epigenetic and
sequence-variable target region sets, consistent with the discussion herein.
[214] In some embodiments, complexes of target-specific probes and DNA are
separated from DNA not bound to target-specific probes. For example, where
target-specific
probes are bound covalently or noncovalently to a solid support, a washing or
aspiration step
can be used to separate unbound material. Alternatively, where the complexes
have
chromatographic properties distinct from unbound material (e.g., where the
probes comprise
a ligand that binds a chromatographic resin), chromatography can be used.
[215] As discussed in detail elsewhere herein, the set of target-specific
probes may
comprise a plurality of sets such as probes for a sequence-variable target
region set and
probes for an epigenetic target region set. In some such embodiments, the
capturing step is
performed with the probes for the sequence-variable target region set and the
probes for the
epigenetic target region set in the same vessel at the same time, e.g., the
probes for the
sequence-variable and epigenetic target region sets are in the same
composition. This
approach provides a relatively streamlined workflow. In some embodiments, the
concentration of the probes for the sequence-variable target region set is
greater that the
concentration of the probes for the epigenetic target region set.
[216] Alternatively, the capturing step is performed with the sequence-
variable
target region probe set in a first vessel and with the epigenetic target
region probe set in a
second vessel, or the contacting step is performed with the sequence-variable
target region
probe set at a first time and a first vessel and the epigenetic target region
probe set at a second
time before or after the first time. This approach allows for preparation of
separate first and
second compositions comprising captured DNA corresponding to the sequence-
variable
target region set and captured DNA corresponding to the epigenetic target
region set. The
compositions can be processed separately as desired (e.g., to fractionate
based on methylation
as described elsewhere herein) and recombined in appropriate proportions to
provide material
for further processing and analysis such as sequencing.
- 69 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
2171 In some embodiments, the DNA is amplified. In some embodiments,
amplification is performed before the capturing step. In some embodiments,
amplification is
performed after the capturing step.
[218] In some embodiments, adapters are included in the DNA. This may be done
concurrently with an amplification procedure, e.g., by providing the adapters
in a 5' portion
of a primer, e.g., as described above. Alternatively, adapters can be added by
other
approaches, such as ligation.
[219] In some embodiments, tags, which may be or include barcodes, are
included in
the DNA, e.g., included in adapters added to the DNA. Tags can facilitate
identification of
the origin of a nucleic acid. For example, barcodes can be used to allow the
origin (e.g.,
subject) whence the DNA came to be identified following pooling of a plurality
of samples
for parallel sequencing. This may be done concurrently with an amplification
procedure, e.g.,
by providing the barcodes in a 5' portion of a primer, e.g., as described
above. In some
embodiments, adapters and tags/barcodes are provided by the same primer or
primer set. For
example, the barcode may be located 3' of the adapter and 5' of the target-
hybridizing
portion of the primer. Alternatively, barcodes can be added by other
approaches, such as
ligation, optionally together with adapters in the same ligation substrate.
[220] Additional details regarding amplification, tags, and barcodes are
discussed in
other sections herein, which can be combined to the extent practicable with
any of the
embodiments set forth herein.
[221] In some embodiments, sequences are enriched prior to sequencing the
nucleic
acids. Enrichment may be optionally performed for specific target regions or
may be
performed nonspecifically ("target sequences"). In some embodiments, targeted
regions of
interest may be enriched/captured with nucleic acid capture probes ("baits"),
such as a target
region probe set, selected for one or more bait set panels using a
differential tiling and
capture scheme. A differential tiling and capture scheme generally uses bait
sets of different
relative concentrations to differentially tile (e.g., at different
"resolutions") across genomic
regions associated with the baits, subject to a set of constraints (e.g.,
sequencer constraints
such as sequencing load, utility of each bait, etc.), and capture the targeted
nucleic acids at a
desired level for downstream sequencing. These targeted genomic regions of
interest
optionally include natural or synthetic nucleotide sequences of the nucleic
acid construct. In
some embodiments, biotin-labeled beads with probes to one or more regions of
interest can
- 70 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
be used to capture target sequences, and optionally followed by amplification
of those
regions, to enrich for the regions of interest. In some embodiments, the
nucleic acid capture
probes can be single-stranded RNA or double-strand DNA molecules.
222] Sequence capture typically involves the use of oligonucleotide probes
that
hybridize to the target nucleic acid sequence. In some embodiments, a probe
set strategy
involves tiling the probes across a region of interest. Such probes can be,
for example, from
about 60 to about 120 nucleotides in length. The set can have a depth (e.g.,
depth of
coverage) of about 2X, 3X, 4X, 5X, 6X, 7X, 8X, 9X, 10X, 15X, 20X, SOX, or more
than
SOX. The effectiveness of sequence capture generally depends, in part, on the
length of the
sequence in the target molecule that is complementary (or nearly
complementary) to the
sequence of the probe.
[223] In some embodiments, a first target region set is captured from the
first
partition, comprising at least epigenetic target regions. The epigenetic
target regions captured
from the first partition may comprise hypermethylation variable target
regions. In some
embodiments, the hypermethylation variable target regions are CpG-containing
regions that
are unmethylated or have low methylation in cfDNA from healthy subjects (e.g.,
below-
average methylation relative to bulk cfDNA). In some embodiments, the
hypermethylation
variable target regions are regions that show lower methylation in healthy
cfDNA than in at
least one other tissue type. Without wishing to be bound by any particular
theory, cancer cells
may shed more DNA into the bloodstream than healthy cells of the same tissue
type. As such,
the distribution of tissue of origin of cfDNA may change upon carcinogenesis.
Thus, an
increase in the level of hypermethylation variable target regions in the first
partition can be an
indicator of the presence (or recurrence, depending on the history of the
subject) of cancer.
[2241 In some embodiments, a second target region set is captured from the
second
partition, comprising at least epigenetic target regions. The epigenetic
target regions may
comprise hypomethylation variable target regions. In some embodiments, the
hypomethylation variable target regions are CpG-containing regions that are
methylated or
have high methylation in cfDNA from healthy subjects (e.g., above-average
methylation
relative to bulk cfDNA). In some embodiments, the hypomethylation variable
target regions
are regions that show higher methylation in healthy cfDNA than in at least one
other tissue
type. Without wishing to be bound by any particular theory, cancer cells may
shed more
DNA into the bloodstream than healthy cells of the same tissue type As such,
the distribution
of tissue of origin of cfDNA may change upon carcinogenesis. Thus, an increase
in the level
- 71 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
of hypomethylation variable target regions in the second partition can be an
indicator of the
presence (or recurrence, depending on the history of the subject) of cancer.
[225] In some embodiments, the enriched DNA molecules (or the captured set)
may
comprise DNA corresponding to a sequence-variable target region set and an
epigenetic
target region set. In some embodiments the quantity of captured sequence-
variable target
region DNA is greater than the quantity of the captured epigenetic target
region DNA, when
normalized for the difference in the size of the targeted regions (footprint
size). In some
embodiments, the compositions, methods and systems described in PCT Patent
Application
No. PCT/US2020/016120, which is hereby incorporated by reference in its
entirety.
[226] Alternatively, first and second captured sets may be provided,
comprising,
respectively, DNA corresponding to a sequence-variable target region set and
DNA
corresponding to an epigenetic target region set. The first and second
captured sets may be
combined to provide a combined captured set.
[227_1 In a captured set comprising DNA corresponding to the sequence-variable
target region set and the epigenetic target region set, including a combined
captured set as
discussed above, the DNA corresponding to the sequence-variable target region
set may be
present at a greater concentration than the DNA corresponding to the
epigenetic target region
set, e.g., a 1.1 to 1.2-fold greater concentration, a 1.2-to 1.4-fold greater
concentration, a 1.4-
to 1.6-fold greater concentration, a 1.6- to 1.8-fold greater concentration, a
1.8- to 2.0-fold
greater concentration, a 2.0- to 2.2-fold greater concentration, a 2.2- to 2.4-
fold greater
concentration a 2.4- to 2.6-fold greater concentration, a 2.6- to 2.8-fold
greater concentration,
a 2.8- to 3.0-fold greater concentration, a 3.0- to 3.5-fold greater
concentration, a 3.5- to 4.0,
a 4.0- to 4.5-fold greater concentration, a 4.5- to 5.0-fold greater
concentration, a 5.0- to 5.5-
fold greater concentration, a 5.5- to 6.0-fold greater concentration, a 6.0-
to 6.5-fold greater
concentration, a 6.5- to 7.0-fold greater, a 7.0- to 7.5-fold greater
concentration, a 7.5- to 8.0-
fold greater concentration, an 8.0- to 8.5-fold greater concentration, an 8.5-
to 9.0-fold greater
concentration, a 9.0- to 9.5-fold greater concentration, 9.5- to 10.0-fold
greater concentration,
a 10- to 11-fold greater concentration, an 11- to 12-fold greater
concentration a 12- to 13-fold
greater concentration, a 13- to 14-fold greater concentration, a 14- to 15-
fold greater
concentration, a 15- to 16-fold greater concentration, a 16- to 17-fold
greater concentration, a
17- to 18-fold greater concentration, an 18- to 19-fold greater concentration,
a 19- to 20-fold
greater concentration, a 20- to 30-fold greater concentration, a 30- to 40-
fold greater
concentration, a 40- to 50-fold greater concentration, a 50- to 60-fold
greater concentration, a
- 72 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
60- to 70-fold greater concentration, a 70- to 80-fold greater concentration,
a 80- to 90-fold
greater concentration, or a 90- to 100-fold greater concentration. The degree
of difference in
concentrations accounts for normalization for the footprint sizes of the
target regions, as
discussed in the definition section.
a. Epigenetic target region set
[228] The epigenetic target region set may comprise one or more types of
target
regions likely to differentiate DNA from neoplastic (e.g., tumor or cancer)
cells and from
healthy cells, e.g., non-neoplastic circulating cells. Exemplary types of such
regions are
discussed in detail herein. In some embodiments, methods according to the
disclosure
comprise determining whether cfDNA molecules corresponding to the epigenetic
target
region set comprise or indicate cancer-associated epigenetic modifications
(e.g.,
hypermethylation in one or more hypermethylation variable target regions; one
or more
perturbations of CTCF binding; and/or one or more perturbations of
transcription start sites)
and/or copy number variations (e.g., focal amplifications). The epigenetic
target region set
may also comprise one or more control regions, e.g., as described herein.
[229] In some embodiments, the epigenetic target region set has a footprint of
at
least 100 kbp, e.g., at least 200 kbp, at least 300 kbp, or at least 400 kbp.
In some
embodiments, the epigenetic target region set has a footprint in the range of
100-20 Mbp,
e.g., 100-200 kbp, 200-300 kbp, 300-400 kbp, 400-500 kbp, 500-600 kbp, 600-700
kbp, 700-
800 kbp, 800-900 kbp, 900-1,000 kbp, 1-1.5 Mbp, 1.5-2 Mbp, 2-3 Mbp, 3-4 Mbp, 4-
5 Mbp,
5-6 Mbp, 6-7 Mbp, 7-8 Mbp, 8-9 Mbp, 9-10 Mbp, or 10-20 Mbp. In some
embodiments, the
epigenetic target region set has a footprint of at least 20 Mbp.
i. Hypermethylation variable target regions
[230] In some embodiments, the epigenetic target region set comprises one or
more
hypermethylation variable target regions. In general, hypermethylation
variable target regions
refer to regions where an increase in the level of observed methylation
indicates an increased
likelihood that a sample (e.g., of cfDNA) contains DNA produced by neoplastic
cells, such as
tumor or cancer cells. For example, hypermethylation of promoters of tumor
suppressor
genes has been observed repeatedly. See, e.g., Kang et al., Genome Biol. 18:53
(2017) and
references cited therein. In another example, as discussed above,
hypermethylation variable
target regions can include regions that do not necessarily differ in
methylation in cancerous
- 73 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
tissue relative to DNA from healthy tissue of the same type, but do differ in
methylation (e.g.,
have more methylation) relative to cfDNA that is typical in healthy subjects.
[231] An extensive discussion of methylation variable target regions in
colorectal
cancer is provided in Lam et al., Biochim Biophys Acta. 1866:106-20 (2016).
These include
VIM, SEPT9, ITGA4, OSM4, GATA4 and NDRG4. An exemplary set of hypermethylation
variable target regions comprising the genes or portions thereof based on the
colorectal
cancer (CRC) studies is provided in Table 1. Many of these genes likely have
relevance to
cancers beyond colorectal cancer; for example, TP53 is widely recognized as a
critically
important tumor suppressor and hypermethylation-based inactivation of this
gene may be a
common oncogenic mechanism.
Table 1. Exemplary hypermethylation target regions (genes or portions thereof)
based on
CRC studies.
Gene Name Additional Chromosome
Gene
Name
VIM chrl 0
SEPT9 chr7
CYCD2 CCND2 chri 2
TFPI2 chr7
GATA4 chr8
RARB2 RARB chr3
pl6INK4a CDKN2A chr9
MGMT MGMT chrl 0
APC chr5
NDRG4 chr16
HLTF chr3
HPP1 TMEFF2 chr2
hMLH1 MLH1 chr3
RAS SF lA RAS SF 1 chr3
CDH13 chr16
IGFBP3 chr7
ITGA4 chr2
- 74 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
12321 In some embodiments, the hypermethylation variable target regions
comprise
a plurality of genes or portions thereof listed in Table 1, e.g., at least
10%, 20%, 30%, 40%,
50%, 60%, 70%, 80%, 90 '/0, or 100% of the genes or portions thereof listed
in Table 1. For
example, for each locus included as a target region, there may be one or more
probes with a
hybridization site that binds between the transcription start site and the
stop codon (the last
stop codon for genes that are alternatively spliced) of the gene. In some
embodiments, the
one or more probes bind within 300 bp upstream and/or downstream of the genes
or portions
thereof listed in Table 1, e.g., within 200 or 100 bp.
12331 Methylation variable target regions in various types of lung cancer are
discussed in detail, e.g., in Ooki et al., Clin. Cancer Res. 23:7141-52
(2017); Belinksy, Annu.
Rev. Physiol. 77:453-74 (2015); Hulbert et al., Clin. Cancer Res. 23:1998-2005
(2017); Shi et
al., BMC Genomics 18:901 (2017); Schneider et al., BMC Cancer. 11:102 (2011);
Lissa et al.,
Transl Lung Cancer Res 5(5):492-504 (2016); Skvortsova et al., Br. J. Cancer.
94(10):1492-
1495 (2006); Kim et al., Cancer Res. 61:3419-3424 (2001); Furonaka et al.,
Pathology
International 55:303-309 (2005); Gomes et al., Rev. Port. Pneumol. 20:20-30
(2014); Kim et
al., Oncogene. 20:1765-70 (2001); Hopkins-Donaldson et al., Cell Death Differ.
10:356-64
(2003); Kikuchi et al., Clin Cancer Res 11.2954-61 (2005); Heller et al.,
Oncogene 25.959-
968 (2006); Licchesi et al., Carcinogenesis. 29:895-904 (2008); Guo et al.,
Clin. Cancer Res.
10:7917-24 (2004); Palmisano et al., Cancer Res. 63:4620-4625 (2003); and
Toyooka et al.,
Cancer Res. 61:4556-4560, (2001). In an example, hypermethylation variable
target regions
can include regions that do not necessarily differ in methylation in cancerous
tissue relative
to DNA from healthy tissue of the same type, but do differ in methylation
(e.g., have more
methylation) relative to cfDNA that is typical in healthy subjects. Where, for
example, the
presence of a cancer results in increased cell death such as apoptosis of
cells of the tissue type
corresponding to the cancer, such a cancer can be detected at least in part
using such
hypermethylation variable target regions. In some embodiments,
hypermethylation variable
target regions include one or more genomic regions, where the cfDNA molecules
in those
regions do not differ in methylation state in cancer subjects relative to
cfDNA from healthy
subjects, but the presence/increased quantity of hypermethylated cfDNA in
those regions is
indicative of a particular tissue type (e.g., cancer origin) and is presented
as cfDNA with
increased apoptosis (e.g. tumor shedding) into circulation.
[234] An exemplary set of hypermethylation variable target regions comprising
genes or portions thereof based on the lung cancer studies is provided in
Table 2. Many of
- 75 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
these genes likely have relevance to cancers beyond lung cancer; for example,
Casp8
(Caspase 8) is a key enzyme in programmed cell death and hypermethylation-
based
inactivation of this gene may be a common oncogenic mechanism not limited to
lung cancer.
Additionally, a number of genes appear in both Tables 1 and 2, indicating
generality.
Table 2. Exemplary hypermethylation target regions (genes or portions thereof)
based on
lung cancer studies.
Gene Name Chromosome
MARCH11 chr5
TAC1 chr7
TCF21 chr6
SHOX2 chr3
p16 chr3
Casp8 chr2
CDH13 chr16
MGMT chrl 0
MLH1 chr3
MSH2 chr2
TSLC 1 cln 1 1
APC chr5
DKK1 chrl 0
DKK3 chrl 1
LKB1 chrl 1
WIF 1 chr12
RUNX3 chrl
GATA4 chr8
GATA5 chr20
PAX5 chr9
E-Cadherin chrl 6
H-Cadherin chr16
[235] Any of the foregoing embodiments concerning target regions identified in
Table 2 may be combined with any of the embodiments described above concerning
target
- 76 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
regions identified in Table 1. In some embodiments, the hypermethylation
variable target
regions comprise a plurality of genes or portions thereof listed in Table 1 or
Table 2, e.g., at
least 10%,
20%, 30%, 40%, 50%, 60%, 70%, 80%, 90 '/0, or 100% of the genes or portions
thereof listed in Table 1 or Table 2.
[236] Additional hypermethylation target regions may be obtained, e.g., from
the
Cancer Genome Atlas. Kang et al., Genome Biology 18.53 (2017), describe
construction of a
probabilistic method called Cancer Locator using hypermethylation target
regions from
breast, colon, kidney, liver, and lung. In some embodiments, the
hypermethylation target
regions can be specific to one or more types of cancer. Accordingly, in some
embodiments,
the hypermethylation target regions include one, two, three, four, or five
subsets of
hypermethylation target regions that collectively show hypermethylation in
one, two, three,
four, or five of breast, colon, kidney, liver, and lung cancers.
237] In some embodiments, where different epigenetic target regions are
captured
from the first and second partitions, the epigenetic target regions captured
from the first
partition comprise hypermethylation variable target regions.
Hypomethylation variable target regions
[238] Global hypomethylation is a commonly observed phenomenon in various
cancers. See, e.g., Hon et al., Genome Res. 22:246-258 (2012) (breast cancer);
Ehrlich,
Epigenomics 1:239-259 (2009) (review article noting observations of
hypomethylation in
colon, ovarian, prostate, leukemia, hepatocellular, and cervical cancers). For
example,
regions such as repeated elements, e.g., LINE1 elements, Alu elements,
centromeric tandem
repeats, pericentromeric tandem repeats, and satellite DNA, and intergenic
regions that are
ordinarily methylated in healthy cells may show reduced methylation in tumor
cells.
Accordingly, in some embodiments, the epigenetic target region set includes
hypomethylation variable target regions, where a decrease in the level of
observed
methylation indicates an increased likelihood that a sample (e.g., of cfDNA)
contains DNA
produced by neoplastic cells, such as tumor or cancer cells. In an example,
hypomethylation
variable target regions can include regions that do not necessarily differ in
methylation state
in cancerous tissue relative to DNA from healthy tissue of the same type, but
do differ in
methylation (e.g., are less methylated) relative to cfDNA that is typical in
healthy subjects.
Where, for example, the presence of a cancer results in increased cell death
such as apoptosis
of cells of the tissue type corresponding to the cancer, such a cancer can be
detected at least
- 77 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
in part using such hypomethylation variable target regions. In some
embodiments,
hypomethylation variable target regions include one or more genomic regions,
where the
cfDNA molecules in those regions do not differ in methylation state in cancer
subjects
relative to cfDNA from healthy subjects, but the presence/increased quantity
of
hypomethylated cfDNA in those regions is indicative of a particular tissue
type (e.g., cancer
origin) and is presented as cfDNA with increased apoptosis (e.g. tumor
shedding) into
circulation.
[239] In some embodiments, hypomethylation variable target regions include
repeated elements and/or intergenic regions. In some embodiments, repeated
elements
include one, two, three, four, or five of LINE' elements, Alu elements,
centromeric tandem
repeats, pericentromeric tandem repeats, and/or satellite DNA.
[240] Exemplary specific genomic regions that show cancer-associated
hypomethylation include nucleotides 8403565-8953708 and 151104701-151106035 of
human chromosome 1, e.g., according to the hg19 or hg38 human genome
construct. In some
embodiments, the hypomethylation variable target regions overlap or comprise
one or both of
these regions.
[241] In some embodiments, where different epigenetic target regions are
captured
from the first and second partitions, the epigenetic target regions captured
from the second
partition comprise hypomethylation variable target regions.
CTCF binding regions
[242] CTCF is a DNA-binding protein that contributes to chromatin organization
and often colocalizes with cohesin. Perturbation of CTCF binding sites has
been reported in a
variety of different cancers. See, e.g., Katainen et al., Nature Genetics,
doi:10.1038/ng.3335,
published online 8 June 2015; Guo et al., Nat. Commun. 9:1520 (2018). CTCF
binding
results in recognizable patterns in ctDNA that can be detected by sequencing,
e.g., through
fragment length analysis. For example, details regarding sequencing-based
fragment length
analysis are provided in Snyder et al., Cell 164:57-68 (2016); WO 2018/009723;
and
US20170211143A1, each of which are incorporated herein by reference.
- 78 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
1-2431 Thus, perturbations of CTCF binding result in variation in the
fragmentation
patterns of cfDNA. As such, CTCF binding sites represent a type of
fragmentation variable
target regions.
244] There are many known CTCF binding sites. See, e.g., the CTCFBSDB (CTCF
Binding Site Database), available on the Internet at insulatordb.uthsc.edu/;
Cuddapah et al.,
Genome Res. 19.24-32 (2009), Martin et al., Nat. Struct. Mol. Biol. 18.708-14
(2011), Rhee
et al., Cell. 147:1408-19 (2011), each of which are incorporated by reference.
Exemplary
CTCF binding sites are at nucleotides 56014955-56016161 on chromosome 8 and
nucleotides
95359169-95360473 on chromosome 13, e.g., according to the hg19 or hg38 human
genome
construct.
[245] Accordingly, in some embodiments, the epigenetic target region set
includes
CTCF binding regions. In some embodiments, the CTCF binding regions comprise
at least
10, 20, 50, 100, 200, or 500 CTCF binding regions, or 10-20, 20-50, 50-100,
100-200, 200-
500, or 500-1000 CTCF binding regions, e.g., such as CTCF binding regions
described above
or in one or more of CTCFBSDB or the Cuddapah et al., Martin et al., or Rhee
et al. articles
cited above.
246] In some embodiments, at least some of the CTCF sites can be methylated or
unmethylated, wherein the methylation state is correlated with the whether or
not the cell is a
cancer cell. In some embodiments, the epigenetic target region set comprises
at least 100 bp,
at least 200 bp, at least 300 bp, at least 400 bp, at least 500 bp, at least
750 bp, at least 1000
bp upstream and/or downstream regions of the CTCF binding sites.
iv. Transcription start sites
247] Transcription start sites may also show perturbations in neoplastic
cells. For
example, nucleosome organization at various transcription start sites in
healthy cells of the
hematopoietic lineage¨which contributes substantially to cfDNA in healthy
individuals¨
may differ from nucleosome organization at those transcription start sites in
neoplastic cells.
This results in different cfDNA patterns that can be detected by sequencing,
for example, as
discussed generally in Snyder et al., Cell 164:57-68 (2016); WO 2018/009723;
and
US20170211143A1. In another example, transcription start sites that do not
necessarily differ
epigenetically in cancerous tissue relative to DNA from healthy tissue of the
same type, but
do differ epigenetically (e.g., with respect to nucleosome organization)
relative to cfDNA that
is typical in healthy subjects. Where, for example, the presence of a cancer
results in
- 79 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
increased cell death such as apoptosis of cells of the tissue type
corresponding to the cancer,
such a cancer can be detected at least in part using such transcription start
sites.
[2.48] Thus, perturbations of transcription start sites also result in
variation in the
fragmentation patterns of cfDNA. As such, transcription start sites also
represent a type of
fragmentation variable target regions.
[249] Human transcriptional start sites are available from DBTSS (DataBase of
Human Transcription Start Sites), available on the Internet at dbtss.hgcjp and
described in
Yamashita et al., Nucleic Acids Res. 34(Database issue): D86-D89 (2006), which
is
incorporated herein by reference.
1-25o1 Accordingly, in some embodiments, the epigenetic target region set
includes
transcriptional start sites. In some embodiments, the transcriptional start
sites comprise at
least 10, 20, 50, 100, 200, or 500 transcriptional start sites, or 10-20, 20-
50, 50-100, 100-200,
200-500, or 500-1000 transcriptional start sites, e.g., such as
transcriptional start sites listed
in DBTSS. In some embodiments, at least some of the transcription start sites
can be
methylated or unmethylated, wherein the methylation state is correlated with
the whether or
not the cell is a cancer cell. In some embodiments, the epigenetic target
region set comprises
at least 100 bp, at least 200 bp, at least 300 bp, at least 400 bp, at least
500 bp, at least 750 bp,
at least 1000 bp upstream and/or downstream regions of the transcription start
sites.
v. Copy number variations; focal amplifications
[251] Although copy number variations such as focal amplifications are somatic
mutations, they can be detected by sequencing based on read frequency in a
manner
analogous to approaches for detecting certain epigenetic changes such as
changes in
methylation. As such, regions that may show copy number variations such as
focal
amplifications in cancer can be included in the epigenetic target region set
and may comprise
one or more of AR, BRAF, CCND1, CCND2, CCNE1, CDK4, CDK6, EGFR, ERBB2,
FGFR1, FGFR2, KIT, KRAS, MET, MYC, PDGFRA, PIK3CA, and RAF1. For example, in
some embodiments, the epigenetic target region set comprises at least 2, 3, 4,
5, 6, 7, 8, 9, 10,
11, 12, 13, 14, 15, 16, 17, or 18 of the foregoing targets.
iv. Methylation control regions
[252] It can be useful to include control regions to facilitate data
validation. In some
embodiments, the epigenetic target region set includes control regions that
are expected to be
- 80 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
methylated or unmethylated in essentially all samples, regardless of whether
the DNA is
derived from a cancer cell or a normal cell. In some embodiments, the
epigenetic target
region set includes control hypomethylated regions that are expected to be
hypomethylated in
essentially all samples. In some embodiments, the epigenetic target region set
includes
control hypermethylated regions that are expected to be hypermethylated in
essentially all
samples.
b. Sequence-variable target region set
[253] In some embodiments, the sequence-variable target region set comprises a
plurality of regions known to undergo somatic mutations in cancer (referred to
herein as
cancer-associated mutations). Accordingly, methods may comprise determining
whether
cfDNA molecules corresponding to the sequence-variable target region set
comprise cancer-
associated mutations.
[254] In some embodiments, the sequence-variable target region set targets a
plurality of different genes or genomic regions ("panel") selected such that a
determined
proportion of subjects having a cancer exhibits a genetic variant or tumor
marker in one or
more different genes or genomic regions in the panel. The panel may be
selected to limit a
region for sequencing to a fixed number of base pairs. The panel may be
selected to sequence
a desired amount of DNA, e.g., by adjusting the affinity and/or amount of the
probes as
described elsewhere herein. The panel may be further selected to achieve a
desired sequence
read depth. The panel may be selected to achieve a desired sequence read depth
or sequence
read coverage for an amount of sequenced base pairs. The panel may be selected
to achieve a
theoretical sensitivity, a theoretical specificity, and/or a theoretical
accuracy for detecting one
or more genetic variants in a sample.
[255] Probes for detecting the panel of regions can include those for
detecting
genomic regions of interest (hotspot regions) as well as nucleosome-aware
probes (e.g.,
KRAS codons 12 and 13) and may be designed to optimize capture based on
analysis of
cfDNA coverage and fragment size variation impacted by nucleosome binding
patterns and
GC sequence composition. Regions used herein can also include non-hotspot
regions
optimized based on nucleosome positions and GC models.
[256] Examples of listings of genomic locations of interest may be found in
Table 3
and Table 4. In some embodiments, a sequence-variable target region set used
in the methods
of the present disclosure comprises at least a portion of at least 5, at least
10, at least 15, at
- 81 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
least 20, at least 25, at least 30, at least 35, at least 40, at least 45, at
least 50, at least 55, at
least 60, at least 65, or 70 of the genes of Table 3. In some embodiments, a
sequence-variable
target region set used in the methods of the present disclosure comprises at
least 5, at least 10,
at least 15, at least 20, at least 25, at least 30, at least 35, at least 40,
at least 45, at least 50, at
least 55, at least 60, at least 65, or 70 of the SNVs of Table 3. In some
embodiments, a
sequence-variable target region set used in the methods of the present
disclosure comprises at
least 1, at least 2, at least 3, at least 4, at least 5, or 6 of the fusions
of Table 3. In some
embodiments, a sequence-variable target region set used in the methods of the
present
disclosure comprise at least a portion of at least 1, at least 2, or 3 of the
indels of Table 3. In
some embodiments, a sequence-variable target region set used in the methods of
the present
disclosure comprises at least a portion of at least 5, at least 10, at least
15, at least 20, at least
25, at least 30, at least 35, at least 40, at least 45, at least 50, at least
55, at least 60, at least
65, at least 70, or 73 of the genes of Table 4. In some embodiments, a
sequence-variable
target region set used in the methods of the present disclosure comprises at
least 5, at least 10,
at least 15, at least 20, at least 25, at least 30, at least 35, at least 40,
at least 45, at least 50, at
least 55, at least 60, at least 65, at least 70, or 73 of the SNVs of Table 4.
In some
embodiments, a sequence-variable target region set used in the methods of the
present
disclosure comprises at least 1, at least 2, at least 3, at least 4, at least
5, or 6 of the fusions of
Table 4. In some embodiments, a sequence-variable target region set used in
the methods of
the present disclosure comprises at least a portion of at least 1, at least 2,
at least 3, at least 4,
at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at
least 11, at least 12, at least
13, at least 14, at least 15, at least 16, at least 17, or 18 of the indels of
Table 4. Each of these
genomic locations of interest may be identified as a backbone region or hot-
spot region for a
given panel. An example of a listing of hot-spot genomic locations of interest
may be found
in Table 5. The coordinates in Table 5 are based on the hg19 assembly of the
human genome,
but one skilled in the art will be familiar with other assemblies and can
identify coordinate
sets corresponding to the indicated exons, introns, codons, etc. in an
assembly of their choice.
In some embodiments, a sequence-variable target region set used in the methods
of the
present disclosure comprises at least a portion of at least 1, at least 2, at
least 3, at least 4, at
least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least
11, at least 12, at least 13,
at least 14, at least 15, at least 16, at least 17, at least 18, at least 19,
or at least 20 of the genes
of Table 5. Each hot-spot genomic region is listed with several
characteristics, including the
associated gene, chromosome on which it resides, the start and stop position
of the genome
representing the gene's locus, the length of the gene's locus in base pairs,
the exons covered
- 82 -
CA 03193090 2023- 3- 17
WO 2022/073011 PCT/US2021/071648
by the gene, and the critical feature (e.g., type of mutation) that a given
genomic region of
interest may seek to capture.
Table 3
Point Mutations (SNVs) and Indels
Fusions
AKTI ALK APC AR ARAF ARIDIA ALK
ATM BRAF BRCA1 BRCA2 C CN D1 C CN D2 FGFR2
CCNE1 CDH1 CDK4 CDK6 CDKN2A CDKN2B FGFR3
C'TNNB1 EGFR ERBB2 ESR1 EZH2 FBXW7 NTRK1
FGFRI FGFR2 FGFR3 GATA3 GNAll GNAQ RET
GNAS HNF 1 A HRAS IDHI IDH2 JAK2 RO SI
JAK3 KIT KRA S MAP2K1 MAP2K2 MET
MLH1 MPL MYC NF1 NFE2L2 NOTCH1
NPMI NRAS NTRK1 PDGFRA PIK3 CA PTEN
PTPN11 RAF1 RB 1 RET RHEB RHOA
RIT I ROS I SMAD4 SMO SRC STK 1 I
TERT TP53 TSCI VHL
Table 4
Point Mutations (SNVs) and Indels
Fusions
AKTI ALK APC AR ARAF ARIDIA ALK
ATM BRAF BRCAI BRCA2 C CNDI CCND2 FGFR2
CCNE I CDH I CDK4 CDK6 C DKN2A DDR2 FGFR3
CTNNBI EGFR ERBB2 ESRI EZH2 FB XW7 NTRK1
FGFRI FGFR2 FGFR3 GATA3 GNAll GNAQ RET
GNAS HNF 1 A HRAS IDHI IDH2 JAK2 ROSI
JAK3 KIT KRA S MAP2K1 MAP2K2 MET
MLH1 MPL MY C NF1 NFE2L2 NOTCH'
NPM1 NRA S NTRK1 PDGFRA PIK3 CA PTEN
PTPN11 RAF1 RB 1 RET RHEB RHOA
RITI ROS 1 SMAD4 SMO MAPKI STK11
TERT TP53 TSC I VHL MA PK3 MTOR
NTRK3
Table 5
Exons/
Gene Start Stop Length Introns
Chromosome Position Position (bp) Covered Feature
ALK chr2 29446405 29446655 250 intron 19 Fusion
ALK chr2 29446062 29446197 135 intron 20 Fusion
ALK chr2 29446198 29446404 206 exon 20 Fusion
ALK chr2 29447353 29447473 120 intron 19 Fusion
ALK chr2 29447614 29448316 702 intron 19 Fusion
ALK chr2 29448317 29448441 124 exon 19 Fusion
ALK chr2 29449366 29449777 411 intron 18 Fusion
ALK chr2 29449778 29449950 172 exon 18 Fusion
BRAF chr7 140453064 140453203 139 exon 15 BRAF V600
- 83 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
CTNNB1 chr3 41266007 41266254 247 exon 3 S37
exons 18 G719 and
EGFR chr7 55240528 55240827 299 and 19
deletions
EGFR chr7 55241603 55241746 143 exon 20
Insertions/1790M
EGFR chr7 55242404 55242523 119 exon 21 L858R
ERBB2 chr17 37880952 37881174 222 exon 20
Insertions
V534, P535,
L536, Y537,
ESR1 chr6 152419857 152420111 254 exon 10 D538
FGFR2 chr10 123279482 123279693 211 exon 6 S252
GATA3 chrl 0 8111426 8111571 145 exon 5 SS /
Indels
GATA3 chr10 8115692 8116002 310 exon 6 SS /
Indels
GNAS chr20 57484395 57484488 93 exon 8 R844
IDH1 chr2 209113083 209113394 311 exon 4 R132
IDH2 chr15 90631809 90631989 180 exon 4 R140, R172
KIT chr4 55524171 55524258 87 exon 1
KIT chr4 55561667 55561957 290 exon 2
KIT chr4 55564439 55564741 302 exon 3
KIT chr4 55565785 55565942 157 exon 4
KIT chr4 55569879 55570068 189 exon 5
KIT chr4 55573253 55573463 210 exon 6
KIT chr4 55575579 55575719 140 exon 7
KIT chr4 55589739 55589874 135 exon 8
KIT chr4 55592012 55592226 214 exon 9
exons 10 557, 559, 560,
KIT chr4 55593373 55593718 345 and 11 576
exons 12
KIT chr4 55593978 55594297 319 and 13 V654
KIT chr4 55595490 55595661 171 exon 14 T670,
S709
KIT chr4 55597483 55597595 112 exon 15 D716
KIT chr4 55598026 55598174 148 exon 16 L783
C809, R815,
D816, L818,
D820, S821F,
KIT chr4 55599225 55599368 143 exon 17 N822,
Y823
KIT chr4 55602653 55602785 132 exon 18 A829P
KIT chr4 55602876 55602996 120 exon 19
KIT chr4 55603330 55603456 126 exon 20
- 84 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
KIT chr4 55604584 55604733 149 exon 21
KRAS chr12 25378537 25378717 180 exon 4 A146
KRAS chrl 2 25380157 25380356 199 exon 3 Q61
KRAS chr12 25398197 25398328 131 exon 2 G12/G13
exon 13,
exon 14,
intron
13,
MET chr7 116411535 116412255 720 intron 14 MET
exon 14 SS
NRAS chrl 115256410 115256609 199 exon 3 Q61
NRAS chrl 115258660 115258791 131 exon 2 G12/G13
PIK3CA chr3 178935987 178936132 145 exon 10 E545K
PIK3CA chr3 178951871 178952162 291 exon 21 H1047R
PTEN chr10 89692759 89693018 259 exon 5 R130
SMAD4 chr18 48604616 48604849 233 exon 12 D537
TERT chr5 1294841 1295512 671 promoter
chr5:1295228
Q331, R337,
TP53 chr17 7573916 7574043 127 exon 11 R342
TP53 chr17 7577008 7577165 157 exon 8 R273
TP53 chr17 7577488 7577618 130 exon 7 R248
TP53 chr17 7578127 7578299 172 exon 6 R213/Y220
TP53 chr17 7578360 7578564 204 exon 5 R175 /
Deletions
TP53 chr17 7579301 7579600 299 exon 4
12574
(total
target
region)
16330
(total
probe
coverage)
[257] Additionally, or alternatively, suitable target region sets are
available from the
literature. For example, Gale et al., PLoS One 13: e0194630 (2018), which is
incorporated
herein by reference, describes a panel of 35 cancer-related gene targets that
can be used as
part or all of a sequence-variable target region set. These 35 targets are
AKT1, ALK, BRAF,
CCND1, CDK2A, CTNNB1, EGFR, ERBB2, ESR1, FGFR1, FGFR2, FGFR3, FOXL2,
- 85 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
GATA3, GNAll, GNAQ, GNAS, HRAS, IDHL IDH2, KIT, KRAS, MED12, MET, MYC,
NFE2L2, NRAS, PDGFRA, PIK3CA, PPP2R1A, PTEN, RET, STK11, TP53, and U2AF1.
[258] In some embodiments, the sequence-variable target region set comprises
target
regions from at least 10, 20, 30, or 35 cancer-related genes, such as the
cancer-related genes
listed above.
[259] In some embodiments, the sequence-variable target region set has a
footprint
of at least 50 kbp, e.g., at least 100 kbp, at least 200 kbp, at least 300
kbp, or at least 400 kbp.
In some embodiments, the sequence-variable target region set has a footprint
in the range of
100-2000 kbp, e.g., 100-200 kbp, 200-300 kbp, 300-400 kbp, 400-500 kbp, 500-
600 kbp,
600-700 kbp, 700-800 kbp, 800-900 kbp, 900-1,000 kbp, 1-1.5 Mbp or 1.5-2 Mbp.
In some
embodiments, the sequence-variable target region set has a footprint of at
least 2 Mbp.
c. Collections of target-specific probes
[260] In some embodiments, a collection of target-specific probes is used in
methods described herein. In some embodiments, the collection of target-
specific probes
comprises target-binding probes specific for a sequence-variable target region
set and target-
binding probes specific for an epigenetic target region set. In some
embodiments, the capture
yield of the target-binding probes specific for the sequence-variable target
region set is higher
(e.g., at least 2-fold higher) than the capture yield of the target-binding
probes specific for the
epigenetic target region set. In some embodiments, the collection of target-
specific probes is
configured to have a capture yield specific for the sequence-variable target
region set higher
(e.g., at least 2-fold higher) than its capture yield specific for the
epigenetic target region set.
[2611 In some embodiments, the capture yield of the target-binding probes
specific
for the sequence-variable target region set is at least 1.25-, 1.5-, 1.75-, 2-
, 2.25-, 2.5-, 2.75-,
3-, 3.5-, 4-, 4.5-, 5-, 6-, 7-, 8-, 9-, 10-, 11-, 12-, 13-, 14-, or 15-fold
higher than the capture
yield of the target-binding probes specific for the epigenetic target region
set. In some
embodiments, the capture yield of the target-binding probes specific for the
sequence-
variable target region set is 1.25- to 1.5-, 1.5- to 1.75-, 1.75- to 2-, 2- to
2.25-, 2.25- to 2.5-,
2.5- to 2.75-, 2.75- to 3-, 3- to 3.5-, 3.5- to 4-, 4- to 4.5-, 4.5- to 5-, 5-
to 5.5-, 5.5- to 6-, 6- to
7-, 7-to 8-, 8-to 9-, 9-to 10-, 10- to 11-, 11- to 12-, 13- to 14-, or 14- to
15-fold higher than
the capture yield of the target-binding probes specific for the epigenetic
target region set.
[262] In some embodiments, the collection of target-specific probes is
configured to
have a capture yield specific for the sequence-variable target region set at
least 1.25-, 1.5-,
- 86 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
1.75-, 2-, 2.25-, 2.5-, 2.75-, 3-, 3.5-, 4-, 4.5-, 5-, 6-, 7-, 8-, 9-, 10-, 11-
, 12-, 13-, 14-, or 15-
fold higher than its capture yield for the epigenetic target region set. In
some embodiments,
the collection of target-specific probes is configured to have a capture yield
specific for the
sequence-variable target region set is 1.25- to 1.5-, 1.5- to 1.75-, 1.75- to
2-, 2- to 2.25-, 2.25-
to 2.5-, 2.5- to 2.75-, 2.75- to 3-, 3- to 3.5-, 3.5- to 4-, 4- to 4.5-, 4.5-
to 5-, 5- to 5.5-, 5.5- to
6-, 6-to 7-, 7-to 8-, 8-to 9-, 9-to 10-, 10- to 11-, 11- to 12-, 13- to 14-,
or 14- to 15-fold
higher than its capture yield specific for the epigenetic target region set.
[263] The collection of probes can be configured to provide higher capture
yields for
the sequence-variable target region set in various ways, including
concentration, different
lengths and/or chemistries (e.g., that affect affinity), and combinations
thereof. Affinity can
be modulated by adjusting probe length and/or including nucleotide
modifications as
discussed below.
[264] In some embodiments, the target-specific probes specific for the
sequence-
variable target region set are present at a higher concentration than the
target-specific probes
specific for the epigenetic target region set. In some embodiments,
concentration of the
target-binding probes specific for the sequence-variable target region set is
at least 1.25-, 1.5-
1.75-, 2-, 2.25-, 2.5-, 2.75-, 3-, 3.5-, 4-, 4.5-, 5-, 6-, 7-, 8-, 9-, 10-, 11-
, 12-, 13-, 14-, or 15-
fold higher than the concentration of the target-binding probes specific for
the epigenetic
target region set. In some embodiments, the concentration of the target-
binding probes
specific for the sequence-variable target region set is 1.25- to 1.5-, 1.5- to
1.75-, 1.75- to 2-,
2- to 2.25-, 2.25- to 2.5-, 2.5- to 2.75-, 2.75- to 3-, 3- to 3.5-, 3.5- to 4-
, 4- to 4.5-, 4.5- to 5-,
5-to 5.5-, 5.5-to 6-, 6-to 7-, 7-to 8-, 8-to 9-, 9-to 10-, 10-to 11-, 11-to 12-
, 13-to 14-, or
14- to 15-fold higher than the concentration of the target-binding probes
specific for the
epigenetic target region set. In such embodiments, concentration may refer to
the average
mass per volume concentration of individual probes in each set.
[265] In some embodiments, the target-specific probes specific for the
sequence-
variable target region set have a higher affinity for their targets than the
target-specific probes
specific for the epigenetic target region set. Affinity can be modulated in
any way known to
those skilled in the art, including by using different probe chemistries. For
example, certain
nucleotide modifications, such as cytosine 5-methylation (in certain sequence
contexts),
modifications that provide a heteroatom at the 2' sugar position, and LNA
nucleotides, can
increase stability of double-stranded nucleic acids, indicating that
oligonucleotides with such
modifications have relatively higher affinity for their complementary
sequences See, e.g.,
- 87 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
Severin etal., Nucleic Acids Res. 39: 8740-8751 (2011); Freier etal., Nucleic
Acids Res. 25:
4429-4443 (1997); US Patent No. 9,738,894. Also, longer sequence lengths will
generally
provide increased affinity. Other nucleotide modifications, such as the
substitution of the
nucleobase hypoxanthine for guanine, reduce affinity by reducing the amount of
hydrogen
bonding between the oligonucleotide and its complementary sequence. In some
embodiments, the target-specific probes specific for the sequence-variable
target region set
have modifications that increase their affinity for their targets. In some
embodiments,
alternatively or additionally, the target-specific probes specific for the
epigenetic target
region set have modifications that decrease their affinity for their targets.
In some
embodiments, the target-specific probes specific for the sequence-variable
target region set
have longer average lengths and/or higher average melting temperatures than
the target-
specific probes specific for the epigenetic target region set. These
embodiments may be
combined with each other and/or with differences in concentration as discussed
above to
achieve a desired fold difference in capture yield, such as any fold
difference or range thereof
described above.
[266] In some embodiments, the target-specific probes comprise a capture
moiety.
The capture moiety may be any of the capture moieties described herein, e g ,
biotin In some
embodiments, the target-specific probes are linked to a solid support, e.g.,
covalently or non-
covalently such as through the interaction of a binding pair of capture
moieties. In some
embodiments, the solid support is a bead, such as a magnetic bead.
12671 In some embodiments, the target-specific probes specific for the
sequence-
variable target region set and/or the target-specific probes specific for the
epigenetic target
region set are a bait set as discussed above, e.g., probes comprising capture
moieties and
sequences selected to tile across a panel of regions, such as genes.
[268] In some embodiments, the target-specific probes are provided in a single
composition. The single composition may be a solution (liquid or frozen).
Alternatively, it
may be a lyophilizate.
[269] Alternatively, the target-specific probes may be provided as a plurality
of
compositions, e.g., comprising a first composition comprising probes specific
for the
epigenetic target region set and a second composition comprising probes
specific for the
sequence-variable target region set. These probes may be mixed in appropriate
proportions to
provide a combined probe composition with any of the foregoing fold
differences in
- 88 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
concentration and/or capture yield. Alternatively, they may be used in
separate capture
procedures (e.g., with aliquots of a sample or sequentially with the same
sample) to provide
first and second compositions comprising captured epigenetic target regions
and sequence-
variable target regions, respectively.
ii. Probes specific for epigenetic target regions
[270] The probes for the epigenetic target region set may comprise probes
specific
for one or more types of target regions likely to differentiate DNA from
neoplastic (e.g.,
tumor or cancer) cells from healthy cells, e.g., non-neoplastic circulating
cells. Exemplary
types of such regions are discussed in detail herein, e.g., in the sections
above concerning
captured sets. The probes for the epigenetic target region set may also
comprise probes for
one or more control regions, e.g., as described herein.
[271] In some embodiments, the probes for the epigenetic target region set
have a
footprint of at least 100 kbp, e.g., at least 200 kbp, at least 300 kbp, or at
least 400 kbp. In
some embodiments, the epigenetic target region set has a footprint in the
range of 100-20
Mbp, e.g., 100-200 kbp, 200-300 kbp, 300-400 kbp, 400-500 kbp, 500-600 kbp,
600-700 kbp,
700-800 kbp, 800-900 kbp, 900-1,000 kbp, 1-1.5 Mbp, 1.5-2 Mbp, 2-3 Mbp, 3-4
Mbp, 4-5
Mbp, 5-6 Mbp, 6-7 Mbp, 7-8 Mbp, 8-9 Mbp, 9-10 Mbp, or 10-20 Mbp. In some
embodiments, the epigenetic target region set has a footprint of at least 20
Mbp.
a. Hypermethylation variable target regions
[272] In some embodiments, the probes for the epigenetic target region set
comprise
probes specific for one or more hypermethylation variable target regions.
Hypermethylation
variable target regions may also be referred to herein as hypermethylated
DMIts
(differentially methylated regions). The hypermethylation variable target
regions may be any
of those set forth above. For example, in some embodiments, the probes
specific for
hypermethylation variable target regions comprise probes specific for a
plurality of loci listed
in Table 1, e.g., at least 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, or
100% of the
loci listed in Table 1. In some embodiments, the probes specific for
hypermethylation
variable target regions comprise probes specific for a plurality of loci
listed in Table 2, e.g.,
at least 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, or 100% of the loci
listed in
Table 2. In some embodiments, the probes specific for hypermethylation
variable target
regions comprise probes specific for a plurality of loci listed in Table 1 or
Table 2, e.g., at
least 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, or 100% of the loci listed
in Table 1
or Table 2. In some embodiments, for each locus included as a target region,
there may be
- 89 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
one or more probes with a hybridization site that binds between the
transcription start site and
the stop codon (the last stop codon for genes that are alternatively spliced)
of the gene. In
some embodiments, the one or more probes bind within 300 bp of the listed
position, e.g.,
within 200 or 100 bp. In some embodiments, a probe has a hybridization site
overlapping the
position listed above. In some embodiments, the probes specific for the
hypermethylation
target regions include probes specific for one, two, three, four, or five
subsets of
hypermethylation target regions that collectively show hypermethylation in
one, two, three,
four, or five of breast, colon, kidney, liver, and lung cancers.
b. Hypomethylation variable target regions
1273] In some embodiments, the probes for the epigenetic target region set
comprise
probes specific for one or more hypomethylation variable target regions.
Hypomethylation
variable target regions may also be referred to herein as hypomethylated DMRs
(differentially methylated regions). The hypomethylation variable target
regions may be any
of those set forth above. For example, the probes specific for one or more
hypomethylation
variable target regions may include probes for regions such as repeated
elements, e.g., LINE1
elements, Alu elements, centromeric tandem repeats, pericentromeric tandem
repeats, and
satellite DNA, and intergenic regions that are ordinarily methylated in
healthy cells may
show reduced methylation in tumor cells.
[274] In some embodiments, probes specific for hypomethylation variable target
regions include probes specific for repeated elements and/or intergenic
regions. In some
embodiments, probes specific for repeated elements include probes specific for
one, two,
three, four, or five of LINE1 elements, Alu elements, centromeric tandem
repeats,
pericentromeric tandem repeats, and/or satellite DNA.
[2751 Exemplary probes specific for genomic regions that show cancer-
associated
hypomethylation include probes specific for nucleotides 8403565-8953708 and/or
151104701-151106035 of human chromosome 1. In some embodiments, the probes
specific
for hypomethylation variable target regions include probes specific for
regions overlapping or
comprising nucleotides 8403565-8953708 and/or 151104701-151106035 of human
chromosome 1.
c. CTCF binding regions
[276] In some embodiments, the probes for the epigenetic target region set
include
probes specific for CTCF binding regions. In some embodiments, the probes
specific for
- 90 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
CTCF binding regions comprise probes specific for at least 10, 20, 50, 100,
200, or 500
CTCF binding regions, or 10-20, 20-50, 50-100, 100-200, 200-500, or 500-1000
CTCF
binding regions, e.g., such as CTCF binding regions described above or in one
or more of
CTCFBSDB or the Cuddapah et al., Martin et al., or Rhee et al. articles cited
above. In some
embodiments, the probes for the epigenetic target region set comprise at least
100 bp, at least
200 bp at least 300 bp, at least 400 bp, at least 500 bp, at least 750 bp, or
at least 1000 bp
upstream and downstream regions of the CTCF binding sites.
d. Transcription start sites
[2771 In some embodiments, the probes for the epigenetic target region set
include
probes specific for transcriptional start sites. In some embodiments, the
probes specific for
transcriptional start sites comprise probes specific for at least 10, 20, 50,
100, 200, or 500
transcriptional start sites, or 10-20, 20-50, 50-100, 100-200, 200-500, or 500-
1000
transcriptional start sites, e.g., such as transcriptional start sites listed
in DBTSS. In some
embodiments, the probes for the epigenetic target region set comprise probes
for sequences at
least 100 bp, at least 200 bp, at least 300 bp, at least 400 bp, at least 500
bp, at least 750 bp,
or at least 1000 bp upstream and downstream of the transcriptional start
sites.
e. Focal amplifications
[278] As noted above, although focal amplifications are somatic mutations,
they can
be detected by sequencing based on read frequency in a manner analogous to
approaches for
detecting certain epigenetic changes such as changes in methylation. As such,
regions that
may show focal amplifications in cancer can be included in the epigenetic
target region set,
as discussed above. In some embodiments, the probes specific for the
epigenetic target region
set include probes specific for focal amplifications. In some embodiments, the
probes specific
for focal amplifications include probes specific for one or more of AR, BRAF,
CCND1,
CCND2, CCNE1, CDK4, CDK6, EGFR, ERBB2, FGFR1, FGFR2, KIT, KRAS, MET,
MYC, PDGFRA, PIK3CA, and RAF1. For example, in some embodiments, the probes
specific for focal amplifications include probes specific for one or more of
at least 2, 3, 4, 5,
6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, or 18 of the foregoing targets.
f. Control regions
279] It can be useful to include control regions to facilitate data
validation. In some
embodiments, the probes specific for the epigenetic target region set include
probes specific
for control methylated regions that are expected to be methylated in
essentially all samples
In some embodiments, the probes specific for the epigenetic target region set
include probes
- 91 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
specific for control hypomethylated regions that are expected to be
hypomethylated in
essentially all samples.
ii. Probes specific for sequence-variable target
regions
[280] The probes for the sequence-variable target region set may comprise
probes
specific for a plurality of regions known to undergo somatic mutations in
cancer. The probes
may be specific for any sequence-variable target region set described herein.
Exemplary
sequence-variable target region sets are discussed in detail herein, e.g., in
the sections above
concerning captured sets.
[281] In some embodiments, the sequence-variable target region probe set has a
footprint of at least 0.5 kb, e.g., at least 1 kb, at least 2 kb, at least 5
kb, at least 10 kb, at least
20 kb, at least 30 kb, or at least 40 kb. In some embodiments, the epigenetic
target region
probe set has a footprint in the range of 0.5-100 kb, e.g., 0.5-2 kb, 2-10 kb,
10-20 kb, 20-30
kb, 30-40 kb, 40-50 kb, 50-60 kb, 60-70 kb, 70-80 kb, 80-90 kb, and 90-100 kb.
In some
embodiments, the sequence-variable target region probe set has a footprint of
at least 50 kbp,
e.g., at least 100 kbp, at least 200 kbp, at least 300 kbp, or at least 400
kbp. In some
embodiments, the sequence-variable target region probe set has a footprint in
the range of
100-2000 kbp, e.g., 100-200 kbp, 200-300 kbp, 300-400 kbp, 400-500 kbp, 500-
600 kbp,
600-700 kbp, 700-800 kbp, 800-900 kbp, 900-1,000 kbp, 1-1.5 Mbp or 1.5-2 Mbp.
In some
embodiments, the sequence-variable target region set has a footprint of at
least 2 Mbp.
282] In some embodiments, probes specific for the sequence-variable target
region
set comprise probes specific for at least a portion of at least 5, at least
10, at least 15, at least
20, at least 25, at least 30, at least 35, at least 40, at least 45, at least
50, at least 55, at least
60, at least 65, or at 70 of the genes of Table 3. In some embodiments, probes
specific for the
sequence-variable target region set comprise probes specific for the at least
5, at least 10, at
least 15, at least 20, at least 25, at least 30, at least 35, at least 40, at
least 45, at least 50, at
least 55, at least 60, at least 65, or 70 of the SNVs of Table 3. In some
embodiments, probes
specific for the sequence-variable target region set comprise probes specific
for at least 1, at
least 2, at least 3, at least 4, at least 5, or 6 of the fusions of Table 3.
In some embodiments,
probes specific for the sequence-variable target region set comprise probes
specific for at
least a portion of at least 1, at least 2, or 3 of the indels of Table 3. In
some embodiments,
probes specific for the sequence-variable target region set comprise probes
specific for at
least a portion of at least 5, at least 10, at least 15, at least 20, at least
25, at least 30, at least
35, at least 40, at least 45, at least 50, at least 55, at least 60, at least
65, at least 70, or 73 of
- 92 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
the genes of Table 4. In some embodiments, probes specific for the sequence-
variable target
region set comprise probes specific for at least 5, at least 10, at least 15,
at least 20, at least
25, at least 30, at least 35, at least 40, at least 45, at least 50, at least
55, at least 60, at least
65, at least 70, or 73 of the SNVs of Table 4. In some embodiments, probes
specific for the
sequence-variable target region set comprise probes specific for at least 1,
at least 2, at least
3, at least 4, at least 5, or 6 of the fusions of Table 4. In some
embodiments, probes specific
for the sequence-variable target region set comprise probes specific for at
least a portion of at
least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least
7, at least 8, at least 9, at
least 10, at least 11, at least 12, at least 13, at least 14, at least 15, at
least 16, at least 17, or 18
of the indels of Table 4. In some embodiments, probes specific for the
sequence-variable
target region set comprise probes specific for at least a portion of at least
1, at least 2, at least
3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at
least 10, at least 11, at least
12, at least 13, at least 14, at least 15, at least 16, at least 17, at least
18, at least 19, or at least
20 of the genes of Table 5.
[283] In some embodiments, the probes specific for the sequence-variable
target
region set comprise probes specific for target regions from at least 10, 20,
30, or 35 cancer-
related genes, such as AKT1, AT,K, BRAF, CCND1, CDK2A, CTNN111, EGFR, ERRI12,
ESR1, FGFR1, FGFR2, FGFR3, FOXL2, GATA3, GNAll, GNAQ, GNAS, BRAS, IDH1,
IDH2, KIT, KRAS, 1VIED12, MET, MYC, NFE2L2, NRAS, PDGFRA, PIK3CA, PPP2R1A,
PTEN, RET, STK11, TP53, and U2AF1.
F. Sequencing
[284] Sample nucleic acids, optionally flanked by adapters, with or without
prior
amplification are generally subjected to sequencing. Sequencing methods or
commercially
available formats that are optionally utilized include, for example, Sanger
sequencing, high-
throughput sequencing, pyrosequencing, sequencing-by-synthesis, single-
molecule
sequencing, nanopore-based sequencing, semiconductor sequencing, sequencing-by-
ligation,
sequencing-by-hybridization, RNA-Seq (IIlumina), Digital Gene Expression
(Helicos), next-
generation sequencing (NGS), Single Molecule Sequencing by Synthesis (SMSS)
(Helicos),
massively-parallel sequencing, Clonal Single Molecule Array (Solexa), shotgun
sequencing,
Ion Torrent, Oxford Nanopore, Roche Genia, Maxim-Gilbert sequencing, primer
walking,
sequencing using PacBio, SOLiD, Ion Torrent, or Nanopore platforms. Sequencing
reactions
- 93 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
can be performed in a variety of sample processing units, which may include
multiple lanes,
multiple channels, multiple wells, or other means of processing multiple
sample sets
substantially simultaneously. Sample processing units can also include
multiple sample
chambers to enable the processing of multiple runs simultaneously.
[285] In some embodiments, a sequencing step is performed on a library
comprising
captured set of target regions, which may comprise any of the target region
sets described
herein. In some embodiments, a sequencing step is performed on a library
comprising a
partition that has not undergone capture/enrichment (e.g., a whole genome
sample). For
example, target regions may be captured from the first partition and the
second sample and
then sequenced; or target regions may be captured from the first partition and
combined with
the second partition after processing such as contacting and tagging steps; or
target regions
may be captured from the second partition and combined with the first
partition after
processing such as contacting and tagging steps; or both the first and second
partitions may
be processed and combined without undergoing capture/enrichment.
[286] The sequencing reactions can be performed on one or more nucleic acid
fragment types or regions containing markers of cancer or of other diseases.
The sequencing
reactions can also be performed on any nucleic acid fragment present in the
sample. The
sequence reactions may be performed on at least about 5%, 10%, 15%, 20%, 25%,
30%,
40%, 50%, 60%, 70%, 80%, 90%, 95%, 99%, 99.9%, or 100% of the genome. In other
cases, sequence reactions may be performed on less than about 5%, 10%, 15%,
20%, 25%,
30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, 99%, 99.9%, or 100% of the genome.
Sequence coverage can performed on at least 5, 10, 20, 70, 100% of the genome,
at least 200
or 500 different genes, or up to 5000, 2500, 1000, 500 or 100 different genes.
[2871 Simultaneous sequencing reactions may be performed using multiplex
sequencing techniques. In some embodiments, cell-free polynucleotides are
sequenced with
at least about 1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000, 9000, 10000,
50000, or
100,000 sequencing reactions.
In other embodiments, cell-free polynucleotides are
sequenced with less than about 1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000,
9000,
10000, 50000, or 100,000 sequencing reactions.
Sequencing reactions are typically
performed sequentially or simultaneously. Subsequent data analysis is
generally performed
on all or part of the sequencing reactions. In some embodiments, data analysis
is performed
on at least about 1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000, 9000, 10000,
50000, or
100,000 sequencing reactions. In other embodiments, data analysis may be
performed on
- 94 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
less than about 1000, 2000, 3000, 4000, 5000, 6000, 7000, 8000, 9000, 10000,
50000, or
100,000 sequencing reactions. An example of a read depth is from about 1000 to
about
50000 reads per locus (e.g., base position). Another example of a read depth
has at least
50000 reads per locus (e.g., base position).
1. Differential depth of sequencing
[288] In some embodiments, nucleic acids corresponding to the sequence-
variable
target region set are sequenced to a greater depth of sequencing than nucleic
acids
corresponding to the epigenetic target region set. For example, the depth of
sequencing for
nucleic acids corresponding to the sequence variant target region set may be
at least 1.25-,
1.5-, 1.75-, 2-, 2.25-, 2.5-, 2.75-, 3-, 3.5-, 4-, 4.5-, 5-, 6-, 7-, 8-, 9-,
10-, 11-, 12-, 13-, 14-, or
15-fold greater, or 1.25- to 1.5-, 1.5- to 1.75-, 1.75- to 2-, 2- to 2.25-,
2.25- to 2.5-, 2.5- to
2.75-, 2.75- to 3-, 3- to 3.5-, 3.5- to 4-, 4- to 4.5-, 4.5- to 5-, 5- to 5.5-
, 5.5- to 6-, 6- to 7-, 7-
to 8-, 8-to 9-, 9-to 10-, 10- to 11-, 11- to 12-, 13- to 14-, 14- to 15-fold,
or 15- to 100-fold
greater, than the depth of sequencing for nucleic acids corresponding to the
epigenetic target
region set. In some embodiments, said depth of sequencing is at least 2-fold
greater. In some
embodiments, said depth of sequencing is at least 5-fold greater. In some
embodiments, said
depth of sequencing is at least 10-fold greater. In some embodiments, said
depth of
sequencing is 4- to 10-fold greater. In some embodiments, said depth of
sequencing is 4- to
100-fold greater. Each of these embodiments refer to the extent to which
nucleic acids
corresponding to the sequence-variable target region set are sequenced to a
greater depth of
sequencing than nucleic acids corresponding to the epigenetic target region
set.
1289] In some embodiments, the captured DNA corresponding to the sequence-
variable target region set and the captured DNA corresponding to the
epigenetic target region
set are sequenced concurrently, e.g., in the same sequencing cell (such as the
flow cell of an
Illumina sequencer) and/or in the same composition, which may be a pooled
composition
resulting from recombining separately captured sets or a composition obtained
by capturing
the cfDNA corresponding to the sequence-variable target region set and the
captured DNA
corresponding to the epigenetic target region set in the same vessel.
G. Additional Features of Certain Methods
a. Subjecting a sample or partition to a procedure that affects a
first nucleobase in the DNA differently from a second nucleobase
in the DNA
- 95 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
1-29o1 Methods disclosed herein may comprise a step of subjecting a sample or
first
partition to a procedure that affects a first nucleobase in the DNA
differently from a second
nucleobase in the DNA of the first partition, wherein the first nucleobase is
a modified or
unmodified nucleobase, the second nucleobase is a modified or unmodified
nucleobase
different from the first nucleobase, and the first nucleobase and the second
nucleobase have
the same base pairing specificity (e.g., while the second partition is
contacted with a MSRE
according to any of the embodiments described elsewhere herein). In some
embodiments, if
the first nucleobase is a modified or unmodified adenine, then the second
nucleobase is a
modified or unmodified adenine; if the first nucleobase is a modified or
unmodified cytosine,
then the second nucleobase is a modified or unmodified cytosine; if the first
nucleobase is a
modified or unmodified guanine, then the second nucleobase is a modified or
unmodified
guanine; and if the first nucleobase is a modified or unmodified thymine, then
the second
nucleobase is a modified or unmodified thymine (where modified and unmodified
uracil are
encompassed within modified thymine for the purpose of this step). Such a
procedure can be
used to identify nucleotides in the partition that have or lack certain
modifications, such as
methylation.
[291] Tn some embodiments, the first nucleobase is a modified or unmodified
cytosine, then the second nucleobase is a modified or unmodified cytosine. For
example, first
nucleobase may comprise unmodified cytosine (C) and the second nucleobase may
comprise
one or more of 5-methylcytosine (mC) and 5-hydroxymethylcytosine (hmC).
Alternatively,
the second nucleobase may comprise C and the first nucleobase may comprise one
or more of
mC and hmC. Other combinations are also possible, as indicated, e.g., in the
Summary above
and the following discussion, such as where one of the first and second
nucleobases
comprises mC and the other comprises hmC.
292] In some embodiments, the procedure that affects a first nucleobase in the
DNA differently from a second nucleobase in the DNA of the first partition
comprises
bisulfite conversion. Treatment with bisulfite converts unmodified cytosine
and certain
modified cytosine nucleotides (e.g. 5-formyl cytosine (fC) or 5-
carboxylcytosine (caC)) to
uracil whereas other modified cytosines (e.g., 5-methylcytosine, 5-
hydroxylmethylcystosine)
are not converted. Thus, where bisulfite conversion is used, the first
nucleobase comprises
one or more of unmodified cytosine, 5-formyl cytosine, 5-carboxylcytosine, or
other cytosine
forms affected by bisulfite, and the second nucleobase may comprise one or
more of mC and
hmC, such as mC and optionally hmC. Sequencing of bisulfite-treated DNA
identifies
- 96 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
positions that are read as cytosine as being mC or hmC positions. Meanwhile,
positions that
are read as T are identified as being T or a bisulfite-susceptible form of C,
such as
unmodified cytosine, 5-formyl cytosine, or 5-carboxylcytosine. Performing
bisulfite
conversion on a first partition as described herein thus facilitates
identifying positions
containing mC or hmC using the sequence reads obtained from the first
partition. For an
exemplary description of bisulfite conversion, see, e.g., Moss et al., Nat
Commun. 2018; 9:
5068.
[293] In some embodiments, the procedure that affects a first nucleobase in
the
DNA differently from a second nucleobase in the DNA of the first partition
comprises
oxidative bisulfite (Ox-BS) conversion. This procedure first converts hmC to
fC, which is
bisulfite susceptible, followed by bisulfite conversion. Thus, when oxidative
bisulfite
conversion is used, the first nucleobase comprises one or more of unmodified
cytosine, fC,
caC, hmC, or other cytosine forms affected by bisulfite, and the second
nucleobase comprises
mC. Sequencing of Ox-BS converted DNA identifies positions that are read as
cytosine as
being mC positions. Meanwhile, positions that are read as T are identified as
being T, hmC,
or a bisulfite-susceptible form of C, such as unmodified cytosine, fC, or hmC.
Performing
Ox-RS conversion on a first partition as described herein thus facilitates
identifying positions
containing mC using the sequence reads obtained from the first partition. For
an exemplary
description of oxidative bisulfite conversion, see, e.g., Booth et al.,
Science 2012; 336: 934-
937.
[294] In some embodiments, the procedure that affects a first nucleobase in
the
DNA differently from a second nucleobase in the DNA of the first partition
comprises Tet-
assisted bisulfite (TAB) conversion. In TAB conversion, hmC is protected from
conversion
and mC is oxidized in advance of bisulfite treatment, so that positions
originally occupied by
mC are converted to U while positions originally occupied by hmC remain as a
protected
form of cytosine. For example, as described in Yu et al., Cell 2012; 149: 1368-
80, f3-glucosyl
transferase can be used to protect hmC (forming 5-
glucosylhydroxymethylcytosine (ghmC)),
then a TET protein such as mTet1 can be used to convert mC to caC, and then
bisulfite
treatment can be used to convert C and caC to U while ghmC remains unaffected.
Thus,
when TAB conversion is used, the first nucleobase comprises one or more of
unmodified
cytosine, fC, caC, mC, or other cytosine forms affected by bisulfite, and the
second
nucleobase comprises hmC. Sequencing of TAB-converted DNA identifies positions
that are
read as cytosine as being hmC positions. Meanwhile, positions that are read as
T are
- 97 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
identified as being T, mC, or a bisulfite-susceptible form of C, such as
unmodified cytosine,
fC, or caC. Performing TAB conversion on a first partition as described herein
thus facilitates
identifying positions containing hmC using the sequence reads obtained from
the first
partition.
[295] In some embodiments, the procedure that affects a first nucleobase in
the
DNA differently from a second nucleobase in the DNA of the first partition
comprises Tet-
assisted conversion with a substituted borane reducing agent, optionally
wherein the
substituted borane reducing agent is 2-picoline borane, borane pyridine, tert-
butylamine
borane, or ammonia borane. In Tet-assisted pic-borane conversion with a
substituted borane
reducing agent conversion, a TET protein is used to convert mC and hmC to caC,
without
affecting unmodified C. caC, and fC if present, are then converted to
dihydrouracil (DHU) by
treatment with 2-picoline borane (pic-borane) or another substituted borane
reducing agent
such as borane pyridine, tert-butylamine borane, or ammonia borane, also
without affecting
unmodified C. See, e.g., Liu et al., Nature Biotechnology 2019; 37:424-429
(e.g., at
Supplementary Fig. 1 and Supplementary Note 7). DHU is read as a T in
sequencing. Thus,
when this type of conversion is used, the first nucleobase comprises one or
more of mC, fC,
caC, or hmC, and the second nucleobase comprises unmodified cytosine.
Sequencing of the
converted DNA identifies positions that are read as cytosine as being
unmodified C positions.
Meanwhile, positions that are read as T are identified as being T, mC, fC,
caC, or hmC.
Performing TAP conversion on a first partition as described herein thus
facilitates identifying
positions containing unmodified C using the sequence reads obtained from the
first partition.
This procedure encompasses Tet-assisted pyridine borane sequencing (TAPS),
described in
further detail in Liu et al. 2019, supra.
[296] Alternatively, protection of hmC (e.g., using PGT) can be combined with
Tet-
assisted conversion with a substituted borane reducing agent. hmC can be
protected as noted
above through glucosylation using pGT, forming ghmC. Treatment with a TET
protein such
as mTet1 then converts mC to caC but does not convert C or ghmC. caC is then
converted to
DHU by treatment with pic-borane or another substituted borane reducing agent
such as
borane pyridine, tert-butylamine borane, or ammonia borane, also without
affecting
unmodified C or ghmC. Thus, when Tet-assisted conversion with a substituted
borane
reducing agent is used, the first nucleobase comprises mC, and the second
nucleobase
comprises one or more of unmodified cytosine or hmC, such as unmodified
cytosine and
optionally hmC, fC, and/or caC. Sequencing of the converted DNA identifies
positions that
- 98 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
are read as cytosine as being either hmC or unmodified C positions. Meanwhile,
positions
that are read as T are identified as being T, fC, caC, or mC. Performing TAP
S13 conversion on
a first partition as described herein thus facilitates distinguishing
positions containing
unmodified C or hmC on the one hand from positions containing mC using the
sequence
reads obtained from the first partition. For an exemplary description of this
type of
conversion, see, e.g., Liu et al., Nature Biotechnology 2019; 37:424-429.
12971 In some embodiments, the procedure that affects a first nucleobase in
the
DNA differently from a second nucleobase in the DNA of the first partition
comprises
chemical-assisted conversion with a substituted borane reducing agent,
optionally wherein
the substituted borane reducing agent is 2-picoline borane, borane pyridine,
tert-butylamine
borane, or ammonia borane. In chemical-assisted conversion with a substituted
borane
reducing agent, an oxidizing agent such as potassium perruthenate (KRu04)
(also suitable for
use in ox-BS conversion) is used to specifically oxidize hmC to fC. Treatment
with pic-
borane or another substituted borane reducing agent such as borane pyridine,
tert-butylamine
borane, or ammonia borane converts fC and caC to DHU but does not affect mC or
unmodified C. Thus, when this type of conversion is used, the first nucleobase
comprises one
or more of hmC, fC, and caC, and the second nucleobase comprises one or more
of
unmodified cytosine or mC, such as unmodified cytosine and optionally mC.
Sequencing of
the converted DNA identifies positions that are read as cytosine as being
either mC or
unmodified C positions. Meanwhile, positions that are read as T are identified
as being T, fC,
caC, or hmC. Performing this type of conversion on a first partition as
described herein thus
facilitates distinguishing positions containing unmodified C or mC on the one
hand from
positions containing hmC using the sequence reads obtained from the first
partition. For an
exemplary description of this type of conversion, see, e.g., Liu et al.,
Nature Biotechnology
2019; 37:424-429.
[298] In some embodiments, the procedure that affects a first nucleobase in
the
DNA differently from a second nucleobase in the DNA of the first partition
comprises
APOBEC-coupled epigenetic (ACE) conversion. In ACE conversion, an AID/APOBEC
family DNA deaminase enzyme such as APOBEC3A (A3A) is used to deaminate
unmodified
cytosine and mC without deaminating hmC, fC, or caC. Thus, when ACE conversion
is used,
the first nucleobase comprises unmodified C and/or mC (e.g., unmodified C and
optionally
mC), and the second nucleobase comprises hmC. Sequencing of ACE-converted DNA
identifies positions that are read as cytosine as being hmC, fC, or caC
positions. Meanwhile,
- 99 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
positions that are read as T are identified as being T, unmodified C, or mC.
Performing ACE
conversion on a first partition as described herein thus facilitates
distinguishing positions
containing hmC from positions containing mC or unmodified C using the sequence
reads
obtained from the first partition. For an exemplary description of ACE
conversion, see, e.g.,
Schutsky et al., Nature Biotechnology 2018; 36: 1083-1090.
[299] In some embodiments, procedure that affects a first nucleobase in the
DNA
differently from a second nucleobase in the DNA of the first partition
comprises enzymatic
conversion of the first nucleobase, e.g., as in EM-Seq. See, e.g., Vaisvila R,
et al. (2019) EM-
seq: Detection of DNA methylation at single base resolution from picograms of
DNA.
bioRxiv; DOT: 10.1101/2019.12.20.884692, available
at
www.biorxiv.org/content/10.1101/2019.12.20.884692v1. For example, TET2 and T4-
I3GT
can be used to convert 5mC and 5hmC into substrates that cannot be deaminated
by a
deaminase (e.g., APOBEC3A), and then a deaminase (e.g., APOBEC3A) can be used
to
deaminate unmodified cytosines converting them to uracils.
poo] In some embodiments, the procedure that affects a first nucleobase in the
DNA differently from a second nucleobase in the DNA of the first partition
comprises
separating DNA originally comprising the first nucleobase from DNA not
originally
comprising the first nucleobase. In some such embodiments, the first
nucleobase is hmC.
DNA originally comprising the first nucleobase may be separated from other DNA
using a
labeling procedure comprising biotinylating positions that originally
comprised the first
nucleobase. In some embodiments, the first nucleobase is first derivatized
with an azide-
containing moiety, such as a glucosyl-azide containing moiety. The azide-
containing moiety
then may serve as a reagent for attaching biotin, e.g., through Huisgen
cycloaddition
chemistry. Then, the DNA originally comprising the first nucleobase, now
biotinylated, can
be separated from DNA not originally comprising the first nucleobase using a
biotin-binding
agent, such as avidin, neutravidin (deglycosylated avidin with an isoelectric
point of about
6.3), or streptavidin. An example of a procedure for separating DNA originally
comprising
the first nucleobase from DNA not originally comprising the first nucleobase
is hmC-seal,
which labels hmC to form 13-6-azide-glucosy1-5-hydroxymethylcytosine and then
attaches a
biotin moiety through Huisgen cycloaddition, followed by separation of the
biotinylated
DNA from other DNA using a biotin-binding agent. For an exemplary description
of hmC-
seal, see, e.g., Han et al., Mol. Cell 2016; 63: 711-719. This approach is
useful for identifying
fragments that include one or more hmC nucleobases.
- 100 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[301] In some embodiments, following such a separation, the method further
comprises differentially tagging each of the DNA originally comprising the
first nucleobase,
the DNA not originally comprising the first nucleobase, and the DNA of the
second partition.
The method may further comprise pooling the DNA originally comprising the
first
nucleobase, the DNA not originally comprising the first nucleobase, and the
DNA of the
second partition following differential tagging. The DNA originally comprising
the first
nucleobase, the DNA not originally comprising the first nucleobase, and the
DNA of the
second partition may then be sequenced in the same sequencing cell while
retaining the
ability to resolve whether a given read came from a molecule of DNA originally
comprising
the first nucleobase, DNA not originally comprising the first nucleobase, or
DNA of the
second partition using the differential tags.
[302] In some embodiments, the first nucleobase is a modified or unmodified
adenine, and the second nucleobase is a modified or unmodified adenine. In
some
embodiments, the modified adenine is N6-methyladenine (mA). In some
embodiments, the
modified adenine is one or more of N6-methyladenine (mA), N6-
hydroxymethyladenine
(hmA), or N6-formyladenine (fA).
Pm Techniques comprising methylated DNA immunoprecipitation (MeDIP) can
be used to separate DNA containing modified bases such as mA from other DNA.
See, e.g.,
Kumar et al., Frontiers Genet. 2018; 9: 640; Greer et al., Cell 2015; 161: 868-
878. An
antibody specific for mA is described in Sun et al., Bioessays 2015; 37:1155-
62. Antibodies
for various modified nucleobases, such as forms of thymine/uracil including
halogenated
forms such as 5-bromouracil, are commercially available. Various modified
bases can also be
detected based on alterations in their base-pairing specificity. For example,
hypoxanthine is a
modified form of adenine that can result from deamination and is read in
sequencing as a G.
See, e.g., US Patent 8,486,630; Brown, Genomes, 2nd Ed., John Wiley & Sons,
Inc., New
York, N.Y., 2002, chapter 14, "Mutation, Repair, and Recombination."
b. Subjects
[304] In some embodiments, the nucleic acid molecules, such as DNA (e.g.,
cfDNA)
are obtained from a subject having a cancer. In some embodiments, DNA (e.g.,
cfDNA) is
obtained from a subject suspected of having a cancer. In some embodiments, DNA
(e.g.,
cfDNA) is obtained from a subject having a tumor. In some embodiments, DNA
(e.g.,
cfDNA) is obtained from a subject suspected of having a tumor. In some
embodiments, DNA
- 101 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
(e.g., cfDNA) is obtained from a subject having neoplasia. In some
embodiments, DNA (e.g.,
cfDNA) is obtained from a subject suspected of having neoplasia. In some
embodiments,
DNA (e.g., cfDNA) is obtained from a subject in remission from a tumor,
cancer, or
neoplasia (e.g., following chemotherapy, surgical resection, radiation, or a
combination
thereof). In any of the foregoing embodiments, the cancer, tumor, or neoplasia
or suspected
cancer, tumor, or neoplasia may be of the lung, colon, rectum, kidney, breast,
prostate, or
liver. In some embodiments, the cancer, tumor, or neoplasia or suspected
cancer, tumor, or
neoplasia is of the lung. In some embodiments, the cancer, tumor, or neoplasia
or suspected
cancer, tumor, or neoplasia is of the colon or rectum. In some embodiments,
the cancer,
tumor, or neoplasia or suspected cancer, tumor, or neoplasia is of the breast.
In some
embodiments, the cancer, tumor, or neoplasia or suspected cancer, tumor, or
neoplasia is of
the prostate. In any of the foregoing embodiments, the subject may be a human
subject.
c. Quantification
[3051 In some embodiments, epigenetic target regions captured from one or more
of
the first partition, the treated first partition, or the treated second
partition are quantified. For
example, hypomethylation variable target regions may be quantified in the
treated second
partition, and/or hypermethylation variable target regions may be quantified
in the first
partition or treated first partition. Quantification may be by any appropriate
technique, e.g.,
quantitative amplification such as quantitative PCR. In some embodiments,
quantification is
based on sequencing data (e.g., number of sequencing reads or number of unique
molecules
sequenced).
[306] Quantification of epigenetic target regions as discussed above can be
used for
determining a presence, absence, or likelihood of cancer in a subject. For
example, a
determination of the presence or absence of cancer can be based, at least in
part, on whether
the amount of hypermethylation variable target regions in the first partition
or treated first
partition and/or the amount of hypomethylation variable target regions in the
treated second
partition exceeds a predetermined threshold. In some embodiments, such an
amount can be
used together with other data collected from the sample, e.g., the presence of
mutations
and/or other epigenetic features described elsewhere herein such as
perturbations of
transcription start sites and/or CTCF binding sites.
d. Pooling of DNA from first and second partitions or portions
thereof
- 102 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[307] In some embodiments, the methods comprise preparing a pool comprising at
least a portion of the DNA of the second partition (e.g., the hypomethylated
partition) and at
least a portion of the DNA of the first partition (e.g., the hypermethylated
partition). Target
regions, e.g., including epigenetic target regions and/or sequence-variable
target regions, may
be captured from the pool. The steps of capturing a target region set from at
least a portion of
a partition described elsewhere herein encompass capture steps performed on a
pool
comprising DNA from the first and second partitions. A step of amplifying DNA
in the pool
may be performed before capturing target regions from the pool. The capturing
step may
have any of the features described elsewhere herein.
[308] The epigenetic target regions may show differences in methylation levels
and/or fragmentation patterns depending on whether they originated from a
tumor or from
healthy cells, or what type of tissue they originated from, as discussed
elsewhere herein. The
sequence-variable target regions may show differences in sequence depending on
whether
they originated from a tumor or from healthy cells.
[309] Analysis of epigenetic target regions from the hypomethylated partition
may
be less informative in some applications than analysis of sequence-variable
target-regions
from the hypermethylated and hypomethylated partitions and epigenetic target
regions from
the hypermethylated partition. As such, in methods where sequence-variable
target-regions
and epigenetic target regions are being captured, the latter may be captured
to a lesser extent
than one or more of the sequence-variable target-regions from the
hypermethylated and
hypomethylated partitions and epigenetic target regions from the
hypermethylated partition.
For example, sequence-variable target regions can be captured from the portion
of the
hypomethylated partition not pooled with the hypermethylated partition, and
the pool can be
prepared with some (e.g., a majority, substantially all, or all) of the DNA
from the
hypermethylated partition and none or some (e.g., a minority) of the DNA from
the
hypomethylated partition. Such approaches can reduce or eliminate sequencing
of epigenetic
target regions from the hypomethylated partition, thereby reducing the amount
of sequencing
data that suffices for further analysis.
113101 In some embodiments, including a minority of the DNA of the
hypomethylated partition in the pool facilitates quantification of one or more
epigenetic
features (e.g., methylation or other epigenetic feature(s) discussed in detail
elsewhere herein),
e g , on a relative basis
- 103 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[311] In some embodiments, the pool comprises a minority of the DNA of the
hypomethylated partition, e.g., less than about 50% of the DNA of the
hypomethylated
partition, such as less than or equal to about 45%, 40%, 35%, 30%, 25%, 20%,
15%, 10%, or
5% of the DNA of the hypomethylated partition. In some embodiments, the pool
comprises
about 5%-25% of the DNA of the hypomethylated partition. In some embodiments,
the pool
comprises about 10%-20% of the DNA of the hypomethylated partition. In some
embodiments, the pool comprises about 10% of the DNA of the hypomethylated
partition. In
some embodiments, the pool comprises about 15% of the DNA of the
hypomethylated
partition. In some embodiments, the pool comprises about 20% of the DNA of the
hypomethylated partition.
[312] In some embodiments, the pool comprises a portion of the hypermethylated
partition, which may be at least about 50% of the DNA of the hypermethylated
partition. For
example, the pool may comprise at least about 55%, 60%, 65%, 70%, 75%, 80%,
85%, 90%,
or 95% of the DNA of the hypermethylated partition. In some embodiments, the
pool
comprises 50-55%, 55-60%, 60-65%, 65-70%, 70-75%, 75-80%, 80-85%, 85-90%, 90-
95%,
or 95-100% of the DNA of the hypermethylated partition. In some embodiments,
the second
pool comprises all or substantially all of the hypermethylated partition
[313] In some embodiments, the methods comprise preparing a first pool
comprising
at least a portion of the DNA of the hypomethylated partition. In some
embodiments, the
methods comprise preparing a second pool comprising at least a portion of the
DNA of the
hypermethylated partition. In some embodiments, the first pool further
comprises a portion of
the DNA of the hypermethylated partition. In some embodiments, the second pool
further
comprises a portion of the DNA of the hypomethylated partition. In some
embodiments, the
first pool comprises a majority of the DNA of the hypomethylated partition,
and optionally
and a minority of the DNA of the hypermethylated partition. In some
embodiments, the
second pool comprises a majority of the DNA of the hypermethylated partition
and a
minority of the DNA of the hypomethylated partition. In some embodiments
involving an
intermediately methylated partition, the second pool comprises at least a
portion of the DNA
of the intermediately methylated partition, e.g., a majority of the DNA of the
intermediately
methylated partition. In some embodiments, the first pool comprises a majority
of the DNA
of the hypomethylated partition, and the second pool comprises a majority of
the DNA of the
hypermethylated partition and a majority of the DNA of the intermediately
methylated
partition.
- 104 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[314] In some embodiments, the methods comprise capturing at least a first set
of
target regions from the first pool, e.g., wherein the first pool is as set
forth in any of the
embodiments above. In some embodiments, the first set comprises sequence-
variable target
regions. In some embodiments, the first set comprises hypomethylation variable
target
regions and/or fragmentation variable target regions. In some embodiments, the
first set
comprises sequence-variable target regions and fragmentation variable target
regions. In
some embodiments, the first set comprises sequence-variable target regions,
hypomethylation
variable target regions and fragmentation variable target regions. A step of
amplifying DNA
in the first pool may be performed before this capture step. In some
embodiments, capturing
the first set of target regions from the first pool comprises contacting the
DNA of the first
pool with a first set of target-specific probes. In some embodiments, the
first set of target-
specific probes comprises target-binding probes specific for the sequence-
variable target
regions. In some embodiments, the first set of target-specific probes
comprises target-binding
probes specific for the sequence-variable target regions, hypomethylation
variable target
regions and/or fragmentation variable target regions.
[315] In some embodiments, the methods comprise capturing a second set of
target
regions or plurality of sets of target regions from the second pool, e g ,
wherein the first pool
is as set forth in any of the embodiments above. In some embodiments, the
second plurality
comprises epigenetic target regions, such as hypermethylation variable target
regions and/or
fragmentation variable target regions. In some embodiments, the second
plurality comprises
sequence-variable target regions and epigenetic target regions, such as
hypermethylation
variable target regions and/or fragmentation variable target regions. A step
of amplifying
DNA in the second pool may be performed before this capture step. In some
embodiments,
capturing the second plurality of sets of target regions from the second pool
comprises
contacting the DNA of the first pool with a second set of target-specific
probes, wherein the
second set of target-specific probes comprises target-binding probes specific
for the
sequence-variable target regions and target-binding probes specific for the
epigenetic target
regions. In some embodiments, the first set of target regions and the second
set of target
regions are not identical. For example, the first set of target regions may
comprise one or
more target regions not present in the second set of target regions.
Alternatively or in
addition, the second set of target regions may comprise one or more target
regions not present
in the first set of target regions. In some embodiments, at least one
hypermethylation variable
target region is captured from the second pool but not from the first pool In
some
- 105 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
embodiments, a plurality of hypermethylation variable target regions are
captured from the
second pool but not from the first pool. In some embodiments, the first set of
target regions
comprises sequence-variable target regions and/or the second set of target
regions comprises
epigenetic target regions. In some embodiments, the first set of target
regions comprises
sequence-variable target regions, and fragmentation variable target regions;
and the second
set of target regions comprises epigenetic target regions, such as
hypermethylation variable
target regions and fragmentation variable target regions. In some embodiments,
the first set of
target regions comprises sequence-variable target regions, fragmentation
variable target
regions, and comprises hypomethylati on variable target regions; and the
second set of target
regions comprises epigenetic target regions, such as hypermethylation variable
target regions
and fragmentation variable target regions.
[316] In some embodiments, the first pool comprises a majority of the DNA of
the
hypomethylated partition and a portion of the DNA of the hypermethylated
partition (e.g.,
about half), and the second pool comprises a portion of the DNA of the
hypermethylated
partition (e.g., about half). In some such embodiments, the first set of
target regions
comprises sequence-variable target regions and/or the second set of target
regions comprises
epigenetic target regions The sequence-variable target regions and/or the
epigenetic target
regions may be as set forth in any of the embodiments described elsewhere
herein.
f. Capture moieties, bait sets
[317] As discussed above, nucleic acids in a sample can be subject to a
capture step,
in which molecules having target sequences are captured for subsequent
analysis. Target
capture can involve use of a bait set comprising oligonucleotide baits, such
as target specific
probes, labeled with a capture moiety, such as biotin or the other examples
noted below. The
probes can have sequences selected to tile across a panel of regions, such as
genes. In some
embodiments, a bait set can have higher and lower capture yields for sets of
target regions
such as those of the sequence-variable target region set and the epigenetic
target region set,
respectively, as discussed elsewhere herein. Such bait sets are combined with
a sample under
conditions that allow hybridization of the target molecules with the baits.
Then, captured
molecules are isolated using the capture moiety. For example, a biotin capture
moiety by
bead-based streptavidin. Such methods are further described in, for example,
U.S. patent
9,850,523, issuing December 26, 2017, which is incorporated herein by
reference.
- 106 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[318] Capture moieties include, without limitation, biotin, avidin,
streptavidin, a
nucleic acid comprising a particular nucleotide sequence, a hapten recognized
by an
antibody, and magnetically attractable particles. The extraction moiety can be
a member of a
binding pair, such as biotin/streptavidin or hapten/antibody. In some
embodiments, a capture
moiety that is attached to an analyte is captured by its binding pair which is
attached to an
isolatable moiety, such as a magnetically attractable particle or a large
particle that can be
sedimented through centrifugation. The capture moiety can be any type of
molecule that
allows affinity separation of nucleic acids bearing the capture moiety from
nucleic acids
lacking the capture moiety. Exemplary capture moieties are biotin which allows
affinity
separation by binding to streptavidin linked or linkable to a solid phase or
an oligonucleotide,
which allows affinity separation through binding to a complementary
oligonucleotide linked
or linkable to a solid phase.
H. Analysis
[3191 In some embodiments, a method described herein comprises identifying the
presence of DNA produced by a tumor (or neoplastic cells, or cancer cells).
[320] In some embodiments, the methods herein comprise analyzing nucleic acid
molecules in which at least some of the nucleic acids include one or more
modified cytosine
residues, such as 5-methylcytosine and any of the other modifications
described previously.
In some such methods, after partitioning, the partitions of nucleic acids are
contacted with
adapters including one or more cytosine residues modified at the 5C position,
such as 5-
methylcytosine. In some embodiments, all cytosine residues in such adapters
are also
modified, or all such cytosines in a primer binding region of the adapters are
modified.
Adapters attach to both ends of nucleic acid molecules in the population. In
some
embodiments, the adapters include different tags of sufficient numbers that
the number of
combinations of tags results in a low probability e.g., 95, 99 or 99.9% of two
nucleic acids
with the same start and stop points receiving the same combination of tags.
The primer
binding sites in such adapters can be the same or different, but are
preferably the same. After
attachment of adapters, the nucleic acids are amplified from primers binding
to the primer
binding sites of the adapters. The amplified nucleic acids are split into
first and second
aliquots. The first aliquot is assayed for sequence data with or without
further processing.
The sequence data on molecules in the first aliquot is thus determined
irrespective of the
initial methylation state of the nucleic acid molecules The nucleic acid
molecules in the
second aliquot are subjected to a procedure that affects a first nucleobase in
the DNA
- 107 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
differently from a second nucleobase in the DNA, wherein the first nucleobase
comprises a
cytosine modified at the 5 position, and the second nucleobase comprises
unmodified
cytosine. This procedure may be bisulfite treatment or another procedure that
converts
unmodified cytosines to uracils. The nucleic acids subjected to the procedure
are then
amplified with primers to the original primer binding sites of the adapters
linked to nucleic
acid. Only the nucleic acid molecules originally linked to adapters (as
distinct from
amplification products thereof) are now amplifiable because these nucleic
acids retain
cytosines in the primer binding sites of the adapters, whereas amplification
products have lost
the methylation of these cytosine residues, which have undergone conversion to
uracils in the
bisulfite treatment. Thus, only original nucleic acid molecules in the
populations, at least
some of which are methylated, undergo amplification. After amplification,
these nucleic
acids are subject to sequence analysis. Comparison of sequences determined
from the first
and second aliquots can indicate among other things, which cytosines in the
nucleic acid
population were subject to methylation.
[321] Such an analysis can be performed using the following exemplary
procedure.
After partitioning, methylated DNA is linked to Y-shaped adapters at both ends
including
primer binding sites and tags The cytosines in the adapters are modified at
the 5 position
(e.g., 5-methylated). The modification of the adapters serves to protect the
primer binding
sites in a subsequent conversion step (e.g., bisulfite treatment, TAP
conversion, or any other
conversion that does not affect the modified cytosine but affects unmodified
cytosine). After
attachment of adapters, the DNA molecules are amplified. The amplification
product is split
into two aliquots for sequencing with and without conversion. The aliquot not
subjected to
conversion can be subjected to sequence analysis with or without further
processing. The
other aliquot is subjected to a procedure that affects a first nucleobase in
the DNA differently
from a second nucleobase in the DNA, wherein the first nucleobase comprises a
cytosine
modified at the 5 position, and the second nucleobase comprises unmodified
cytosine. This
procedure may be bisulfite treatment or another procedure that converts
unmodified cytosines
to uracils. Only primer binding sites protected by modification of cytosines
can support
amplification when contacted with primers specific for original primer binding
sites. Thus,
only original molecules and not copies from the first amplification are
subjected to further
amplification. The further amplified molecules are then subjected to sequence
analysis.
Sequences can then be compared from the two aliquots. As in the separation
scheme
- 108 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
discussed above, nucleic acid tags in adapters are not used to distinguish
between methylated
and unmethylated DNA but to distinguish nucleic acid molecules within the same
partition.
[322] Sequencing may generate a plurality of sequence reads or reads. Sequence
reads or reads may include sequences of nucleotide data less than about 150
bases in length,
or less than about 90 bases in length. In some embodiments, reads are between
about 80
bases and about 90 bases, e.g., about 85 bases in length. In some embodiments,
methods of
the present disclosure are applied to very short reads, e.g., less than about
50 bases or about
30 bases in length. Sequence read data can include the sequence data as well
as meta
information. Sequence read data can be stored in any suitable file format
including, for
example, VCF files, FASTA files, or FASTQ files.
[323] FASTA may refer to a computer program for searching sequence databases,
and the name FASTA may also refer to a standard file format. FASTA is
described by, for
example, Pearson & Lipman, 1988, Improved tools for biological sequence
comparison,
PNAS 85:2444-2448, which is hereby incorporated by reference in its entirety.
A sequence
in FASTA format begins with a single-line description, followed by lines of
sequence data.
The description line is distinguished from the sequence data by a greater-than
(">") symbol
in the first column. The word following the ">" symbol is the identifier of
the sequence, and
the rest of the line is the description (both are optional). There may be no
space between the
">" and the first letter of the identifier. It is recommended that all lines
of text be shorter
than 80 characters. The sequence ends if another line starting with a ">"
appears; this
indicates the start of another sequence.
[324] The FASTQ format is a text-based format for storing both a biological
sequence (usually nucleotide sequence) and its corresponding quality scores.
It is similar to
the FASTA format but with quality scores following the sequence data. Both the
sequence
letter and quality score are encoded with a single ASCII character for
brevity. The FASTQ
format is a de facto standard for storing the output of high throughput
sequencing instruments
such as the Illumina Genome Analyzer, as described by, for example, Cock et
al. ("The
Sanger FASTQ file format for sequences with quality scores, and the
Solexa/Illumina
FASTQ variants," Nucleic Acids Res 38(6):1767-1771, 2009), which is hereby
incorporated
by reference in its entirety.
[325] For FASTA and FASTQ files, meta information includes the description
line
and not the lines of sequence data. In some embodiments, for FASTQ files, the
meta
- 109 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
information includes the quality scores. For FASTA and FASTQ files, the
sequence data
begins after the description line and is present typically using some subset
of IUPAC
ambiguity codes optionally with "¨". In an embodiment, the sequence data may
use the A, T,
C, G, and N characters, optionally including "¨" or U as-needed (e.g., to
represent gaps or
uracil).
p261 In some embodiments, the at least one master sequence read file and the
output file are stored as plain text files (e.g., using encoding such as
ASCII; ISO/IEC 646;
EBCDIC; UTF-8; or UTF-16). A computer system provided by the present
disclosure may
include a text editor program capable of opening the plain text files. A text
editor program
may refer to a computer program capable of presenting contents of a text file
(such as a plain
text file) on a computer screen, allowing a human to edit the text (e.g.,
using a monitor,
keyboard, and mouse). Examples of text editors include, without limitation,
Microsoft Word,
emacs, pico, vi, BBEdit, and TextWrangler. The text editor program may be
capable of
displaying the plain text files on a computer screen, showing the meta
information and the
sequence reads in a human-readable format (e.g., not binary encoded but
instead using
alphanumeric characters as they may be used in print or human writing).
[3271 While methods have been discussed with reference to FASTA or FASTQ
files, methods and systems of the present disclosure may be used to compress
any suitable
sequence file format including, for example, files in the Variant Call Format
(VCF) format.
A typical VCF file may include a header section and a data section. The header
contains an
arbitrary number of meta-information lines, each starting with characters
'44', and a TAB
delimited field definition line starting with a single '4' character. The
field definition line
names eight mandatory columns and the body section contains lines of data
populating the
columns defined by the field definition line. The VCF format is described by,
for example,
Danecek et al. ("The variant call format and VCF tools," Bioinformatics
27(15):2156-2158,
2011), which is hereby incorporated by reference in its entirety. The header
section may be
treated as the meta information to write to the compressed files and the data
section may be
treated as the lines, each of which can be stored in a master file only if
unique.
P28] Some embodiments provide for the assembly of sequence reads. In assembly
by alignment, for example, the sequence reads are aligned to each other or
aligned to a
reference sequence. By aligning each read, in turn to a reference genome, all
of the reads are
positioned in relationship to each other to create the assembly. In addition,
aligning or
mapping the sequence read to a reference sequence can also be used to identify
variant
- 110 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
sequences within the sequence read. Identifying variant sequences can be used
in
combination with the methods and systems described herein to further aid in
the diagnosis or
prognosis of a disease or condition, or for guiding treatment decisions.
[329] In some embodiments, any or all of the steps are automated.
Alternatively,
methods of the present disclosure may be embodied wholly or partially in one
or more
dedicated programs, for example, each optionally written in a compiled
language such as
C++, then compiled and distributed as a binary. Methods of the present
disclosure may be
implemented wholly or in part as modules within, or by invoking functionality
within,
existing sequence analysis platforms. In some embodiments, methods of the
present
disclosure include a number of steps that are all invoked automatically
responsive to a single
starting queue (e.g., one or a combination of triggering events sourced from
human activity,
another computer program, or a machine). Thus, the present disclosure provides
methods in
which any or the steps or any combination of the steps can occur automatically
responsive to
a queue. "Automatically" generally means without intervening human input,
influence, or
interaction (e.g., responsive only to original or pre-queue human activity).
[330] The methods of the present disclosure may also encompass various forms
of
output, which includes an accurate and sensitive interpretation of a subject's
nucleic acid
sample. The output of retrieval can be provided in the format of a computer
file. In some
embodiments, the output is a FASTA file, a FASTQ file, or a VCF file. The
output may be
processed to produce a text file, or an XML file containing sequence data such
as a sequence
of the nucleic acid aligned to a sequence of the reference genome. In other
embodiments,
processing yields output containing coordinates or a string describing one or
more mutations
in the subject nucleic acid relative to the reference genome. Alignment
strings may include
Simple UnGapped Alignment Report (SUGAR), Verbose Useful Labeled Gapped
Alignment
Report (VULGAR), and Compact Idiosyncratic Gapped Alignment Report (CIGAR) (as
described by, for example, Ning et al., Genome Research 11(10):1725-9, 2001,
which is
hereby incorporated by reference in its entirety). These strings may be
implemented, for
example, in the Exonerate sequence alignment software from the European
Bioinformatics
Institute (Hinxton, UK).
[331] In some embodiments, a sequence alignment is produced¨such as, for
example, a sequence alignment map (SAM) or binary alignment map (BAM) file¨
compri sing a CIGAR string (the SAM format is described, e g_, by Li et al.,
"The Sequence
Alignment/Map format and SAMtools," Bioinformatic,s, 25(16):2078-9, 2009,
which is
-111 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
hereby incorporated by reference in its entirety). In some embodiments, CIGAR
displays or
includes gapped alignments one-per-line. CIGAR is a compressed pairwise
alignment format
reported as a CIGAR string. A CIGAR string may be useful for representing long
(e.g.,
genomic) pairwise alignments. A CIGAR string may be used in SAM format to
represent
alignments of reads to a reference genome sequence.
[332] A CIGAR string may follow an established motif. Each character is
preceded
by a number, giving the base counts of the event. Characters used can include
M, I, D, N,
and S (M=match; I=insertion; D=deletion; N=gap; S=substitution). The CIGAR
string
defines the sequence of matches and/or mismatches and deletions (or gaps). For
example, the
CIGAR string 21\'ID3M2D2M may indicate that the alignment contains 2 matches,
1 deletion
(number 1 is omitted in order to save some space), 3 matches, 2 deletions, and
2 matches.
[333] In some embodiments, a nucleic acid population is prepared for
sequencing by
enzymatically forming blunt-ends on double-stranded nucleic acids with single-
stranded
overhangs at one or both ends. In these embodiments, the population is
typically treated with
an enzyme having a 5'-3' DNA polymerase activity and a 3'-5' exonuclease
activity in the
presence of the nucleotides (e.g., A, C, G, and T or U). Examples of enzymes
or catalytic
fragments thereof that may be optionally used include Klenow large fragment
and T4
polymerase. At 5' overhangs, the enzyme typically extends the recessed 3' end
on the
opposing strand until it is flush with the 5' end to produce a blunt end. At
3' overhangs, the
enzyme generally digests from the 3' end up to and sometimes beyond the 5' end
of the
opposing strand. If this digestion proceeds beyond the 5' end of the opposing
strand, the gap
can be filled in by an enzyme having the same polymerase activity that is used
for 5'
overhangs. The formation of blunt ends on double-stranded nucleic acids
facilitates, for
example, the attachment of adapters and subsequent amplification.
[334] In some embodiments, nucleic acid populations are subjected to
additional
processing, such as the conversion of single-stranded nucleic acids to double-
stranded nucleic
acids and/or conversion of RNA to DNA (e.g., complementary DNA or cDNA). These
forms
of nucleic acid are also optionally linked to adapters and amplified.
[335] With or without prior amplification, nucleic acids subject to the
process of
forming blunt-ends described above, and optionally other nucleic acids in a
sample, can be
sequenced to produce sequenced nucleic acids. A sequenced nucleic acid can
refer either to
the sequence of a nucleic acid (e.g., sequence information) or a nucleic acid
whose sequence
- 112 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
has been determined. Sequencing can be performed so as to provide sequence
data of
individual nucleic acid molecules in a sample either directly or indirectly
from a consensus
sequence of amplification products of an individual nucleic acid molecule in
the sample.
[336] In some embodiments, double-stranded nucleic acids with single-stranded
overhangs in a sample after blunt-end formation are linked at both ends to
adapters including
barcodes, and the sequencing determines nucleic acid sequences as well as in-
line barcodes
introduced by the adapters. The blunt-end DNA molecules are optionally ligated
to a blunt
end of an at least partially double-stranded adapter (e.g., a Y-shaped or bell-
shaped adapter).
Alternatively, blunt ends of sample nucleic acids and adapters can be tailed
with
complementary nucleotides to facilitate ligation (for e.g., sticky-end
ligation).
[337_1 The nucleic acid sample is typically contacted with a sufficient number
of
adapters that there is a low probability (e.g., less than about 1% or 0.1%)
that any two copies
of the same nucleic acid receive the same combination of adapter barcodes from
the adapters
linked at both ends. The use of adapters in this manner may permit
identification of families
of nucleic acid sequences with the same start and stop points on a reference
nucleic acid and
linked to the same combination of barcodes. Such a family may represent
sequences of
amplification products of a nucleic acid in the sample before amplification.
The sequences of
family members can be compiled to derive consensus nucleotide(s) or a complete
consensus
sequence for a nucleic acid molecule in the original sample, as modified by
blunt-end
formation and adapter attachment. In other words, the nucleotide occupying a
specified
position of a nucleic acid in the sample can be determined to be the consensus
of nucleotides
occupying that corresponding position in family member sequences. Families can
include
sequences of one or both strands of a double-stranded nucleic acid. If members
of a family
include sequences of both strands from a double-stranded nucleic acid,
sequences of one
strand may be converted to their complements for purposes of compiling
sequences to derive
consensus nucleotide(s) or sequences. Some families include only a single
member
sequence. In this case, this sequence can be taken as the sequence of a
nucleic acid in the
sample before amplification. Alternatively, families with only a single member
sequence can
be eliminated from subsequent analysis.
[338] Nucleotide variations (e.g., SNVs or indels) in sequenced nucleic acids
can be
determined by comparing sequenced nucleic acids with a reference sequence. The
reference
sequence is often a known sequence, e g , a known whole or partial genome
sequence from a
subject (e.g., a whole genome sequence of a human subject). The reference
sequence can be
- 113 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
an external reference sequence, for example, hG19 or hG38. The sequenced
nucleic acids
can represent sequences determined directly for a nucleic acid in a sample, or
a consensus of
sequences of amplification products of such a nucleic acid, as described
above. A
comparison can be performed at one or more designated positions on a reference
sequence.
A subset of sequenced nucleic acids can be identified including a position
corresponding with
a designated position of the reference sequence when the respective sequences
are maximally
aligned. Within such a subset it can be determined which, if any, sequenced
nucleic acids
include a nucleotide variation at the designated position, and optionally
which if any, include
a reference nucleotide (e.g., same as in the reference sequence). If the
number of sequenced
nucleic acids in the subset including a nucleotide variant exceeding a
selected threshold, then
a variant nucleotide can be called at the designated position. The threshold
can be a number,
such as at least 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 sequenced nucleic acids
within the subset
including the nucleotide variant, or it can be a ratio, such as at least about
0.5, 1, 2, 3, 4, 5,
10, 15, or 20, of sequenced nucleic acids within the subset that include the
nucleotide variant,
among other possibilities. The comparison can be repeated for any designated
position of
interest in the reference sequence. Sometimes a comparison can be performed
for designated
positions occupying at least about 20, 100, 200, or 300 contiguous positions
on a reference
sequence, e.g., about 20-500, or about 50-300 contiguous positions.
[339] Additional details regarding nucleic acid sequencing, including the
formats
and applications described herein, are also provided in, for example, Levy et
al., Annual
Review of Genomics and Human Genetics, 17: 95-115 (2016), Liu et al., J. of
Biomedicine
and Biotechnology, Volume 2012, Article ID 251364:1-11 (2012), Voelkerding et
al.,
Clinical Chem., 55: 641-658 (2009), MacLean et al., Nature Rev. Microbiol., 7:
287-296
(2009), Astier et al., J Am Chem Soc., 128(5):1705-10 (2006), U.S. Pat. No.
6,210,891, U.S.
Pat. No. 6,258,568, U.S. Pat. No. 6,833,246, U.S. Pat. No. 7,115,400, U.S.
Pat. No.
6,969,488, U.S. Pat. No. 5,912,148, U.S. Pat. No. 6,130,073, U.S. Pat. No.
7,169,560, U.S.
Pat. No. 7,282,337, U.S. Pat. No. 7,482,120, U.S. Pat. No. 7,501,245, U.S.
Pat. No.
6,818,395, U.S. Pat. No. 6,911,345, U.S. Pat. No. 7,501,245, U.S. Pat. No.
7,329,492, U.S.
Pat. No. 7,170,050, U.S. Pat. No. 7,302,146, U.S. Pat. No. 7,313,308, and U.S.
Pat. No.
7,476,503, each of which is hereby incorporated by reference in its entirety.
- 114 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
I. Exemplary Workflows
[340] Exemplary workflows are provided herein. In some embodiments, some or
all
features of the partitioning and library preparation workflows may be used in
combination
with each other and with other features of the methods described herein.
a. Partitioning
[341] In some embodiments, sample nucleic acid molecules, such as DNA (e.g.,
between 5 and 200 ng) is mixed with methyl binding domain (MBD) buffer and
magnetic
beads conjugated with MBD proteins and incubated overnight. Methylated DNA
(hypermethylated DNA) binds the MBD protein on the magnetic beads during this
incubation. Non-methylated (hypomethylated DNA) or less methylated DNA
(intermediately
methylated) is washed away from the beads with buffers containing increasing
concentrations
of salt. For example, one, two, or more fractions containing non-methylated,
hypomethylated,
and/or intermediately methylated DNA may be obtained from such washes.
Finally, a high
salt buffer is used to elute the heavily methylated DNA (hypermethylated DNA)
from the
MBD protein. In some embodiments, these washes result in three partitions
(hypomethylated
partition, intermediately methylated fraction and hypermethylated partition)
of DNA having
increasing levels of methylation.
[342] In some embodiments, the three partitions of DNA are desalted and
concentrated in preparation for the enzymatic steps of library preparation.
b. Library preparation
[343] In some embodiments (e.g., after concentrating the DNA in the
partitions), the
partitioned DNA is made ligatable, e.g., by extending the end overhangs of the
DNA
molecules are extended, and adding adenosine residues to the 3' ends of
fragments and
phosphorylating the 5' end of each DNA fragment. DNA ligase and adapters are
added to
ligate each partitioned DNA molecule with an adapter on each end. These
adapters contain
partition tags (e.g., non-random, non-unique barcodes) that are
distinguishable from the
partition tags in the adapters used in the other partitions. Either before or
after making the
portioned DNA ligatable and performing the ligation, at least one partition
(e.g., the
hypermethylated partition, or the hypermethylated partition and the
intermediately
methylated partition if applicable) is digested with an MSRE (e.g., an MSRE
that
preferentially cleaves unmethylated DNA, such as one or more, or each of
HpaII, BstUI and
Hin6i). Optionally, the hypomethylated partition may be digested with an MSRE
that
- 115 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
preferentially cleaves methylated DNA, such as FspEI. Optionally, the
hypermethylated
partition may be subjected to a procedure that affects a first nucleobase in
the DNA
differently from a second nucleobase in the DNA, such as any of those
described herein.
Where the procedure that affects a first nucleobase in the DNA differently
from a second
nucleobase in the DNA further partitions the hypermethylated partition, the
ligation of
adapters should be performed after the procedure so that the sub-partitions of
the
hypermethylated partition can be differentially tagged. Then, the three (or
more) partitions
are pooled together and are amplified (e.g., by PCR, such as with primers
specific for the
adapters).
[344] Following PCR, amplified DNA may be cleaned and concentrated prior to
enrichment. The amplified DNA is contacted with a collection of probes
described herein
(which may be, e.g., biotinylated RNA probes) that target specific regions of
interest. The
mixture is incubated, e.g., overnight, e.g., in a salt buffer. The probes are
captured (e.g., using
streptavidin magnetic beads) and separated from the amplified DNA that was not
captured,
such as by a series of salt washes, thereby enriching the sample. After the
enrichment, the
enriched sample is amplified by PCR. In some embodiments, the PCR primers
contain a
sample tag, thereby incorporating the sample tag into the DNA molecules In
some
embodiments, DNA from different samples is pooled together and then multiplex
sequenced,
e.g., using an Illumina NovaSeq sequencer.
J. Compositions comprising captured nucleic acid molecules
[345] Provided herein is a combination comprising first and second populations
of
DNA, wherein the second population comprises fragments of DNA with ends, or
attached
tags or adapters, at a recognition site of at least one MSRE, which may be any
one or any
combination of the MSREs described herein. In some embodiments, the first and
second
populations are differentially tagged. The first population may comprise or be
derived from
DNA with a cytosine modification in a greater proportion than the second
population. The
first population may comprise a form of a first nucleobase originally present
in the DNA with
altered base pairing specificity and a second nucleobase without altered base
pairing
specificity, wherein the form of the first nucleobase originally present in
the DNA prior to
alteration of base pairing specificity is a modified or unmodified nucleobase,
the second
nucleobase is a modified or unmodified nucleobase different from the first
nucleobase, and
the form of the first nucleobase originally present in the DNA prior to
alteration of base
pairing specificity and the second nucleobase have the same base pairing
specificity. In some
- 116 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
embodiments, the cytosine modification is cytosine methylation. In some
embodiments, the
first nucleobase is a modified or unmodified cytosine and the second
nucleobase is a
modified or unmodified cytosine. The first and second nucleobase may be any of
those
discussed herein or with respect to subjecting the first partition to a
procedure that affects a
first nucleobase in the DNA differently from a second nucleobase in the DNA of
the first
partition. In some embodiments, the first population comprises fragments of
DNA with ends,
or attached tags or adapters, at a recognition site of at least one MSRE,
which may be any
one or any combination of the MSREs described herein.
[346] In some embodiments, the first population comprises a sequence tag
selected
from a first set of one or more sequence tags and the second population
comprises a sequence
tag selected from a second set of one or more sequence tags, and the second
set of sequence
tags is different from the first set of sequence tags. The sequence tags may
comprise
barcodes.
[347] In some embodiments, the first population comprises protected hmC, such
as
glucosylated hmC.
[348] In some embodiments, the first population was subjected to any of the
conversion procedures discussed herein, such as bisulfite conversion, Ox-BS
conversion,
TAB conversion, ACE conversion, TAP conversion, TAPSI3 conversion, or CAP
conversion.
In some embodiments, the first population was subjected to protection of hmC
followed by
deamination of mC and/or C.
[349] In some embodiments of the combination, the first population comprises
or
was derived from DNA with a cytosine modification in a greater proportion than
the second
population and the first population comprises first and second subpopulations,
and the first
nucleobase is a modified or unmodified nucleobase, the second nucleobase is a
modified or
unmodified nucleobase different from the first nucleobase, and the first
nucleobase and the
second nucleobase have the same base pairing specificity. In some embodiments,
the second
population does not comprise the first nucleobase. In some embodiments, the
first nucleobase
is a modified or unmodified cytosine, and the second nucleobase is a modified
or unmodified
cytosine, optionally wherein the modified cytosine is mC or hmC. In some
embodiments, the
first nucleobase is a modified or unmodified adenine, and the second
nucleobase is a
modified or unmodified adenine, optionally wherein the modified adenine is mA.
- 117 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[350] In some embodiments, the first nucleobase (e.g., a modified cytosine) is
biotinylated. In some embodiments, the first nucleobase (e.g., a modified
cytosine) is a
product of a Huisgen cycloaddition to 13-6-azide-glucosy1-5-
hydroxymethylcytosine that
comprises an affinity label (e.g., biotin).
[351] In any of the combinations described herein, the captured DNA may
comprise
cfDNA.
[352] The captured DNA may have any of the features described herein
concerning
captured sets, including, e.g., a greater concentration of the DNA
corresponding to the
sequence-variable target region set (normalized for footprint size as
discussed above) than of
the DNA corresponding to the epigenetic target region set. In some
embodiments, the DNA
of the captured set comprises sequence tags, which may be added to the DNA as
described
herein. In general, the inclusion of sequence tags results in the DNA
molecules differing from
their naturally occurring, untagged form. The combination may further comprise
a probe set
described herein or sequencing primers, each of which may differ from
naturally occurring
nucleic acid molecules. For example, a probe set described herein may comprise
a capture
moiety, and sequencing primers may comprise a non-naturally occurring label.
III. Computer Systems
[3531 Methods of the present disclosure can be implemented using, or with the
aid
of, computer systems. For example, such methods, which may comprise: (a)
providing a
biological sample of nucleic acid molecules, wherein the nucleic acid
molecules comprises
methylated nucleic acid molecules and unmethylated nucleic acid molecules; (b)
partitioning
at least a subset of the nucleic acid molecules in the biological sample based
on the
methylation status of the nucleic acid molecules into a plurality of
partitioned sets; (c)
digesting at least a subset of the one or more partitioned sets in the
plurality of partitioned
sets with at least one methylation sensitive restriction enzyme; (d) enriching
at least a subset
of the nucleic acid molecules in the plurality of partitioned sets for genomic
regions of
interest, wherein the at least a subset of the nucleic acid molecules
comprises digested nucleic
acid molecules in the one or more partitioned sets; and (e) determining
methylation status at
one or more genetic loci of the nucleic acid molecules in at least one of the
partitioned sets,
which in turn is used to determine the presence or absence of cancer in a
subject.
[354] FIG. 5 shows a computer system 501 that is programmed or otherwise
configured to implement the methods of the present disclosure. The computer
system 501 can
- 118 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
regulate various aspects sample preparation, sequencing, and/or analysis. In
some examples,
the computer system 501 is configured to perform sample preparation and sample
analysis,
including nucleic acid sequencing.
[355] In some embodiments, the method further comprises obtaining a plurality
of
sequence reads generated by a nucleic acid sequencer from the sequencing;
mapping the
plurality of sequence reads to one or more reference sequences to generate
mapped sequence
reads; and processing the mapped sequence reads to determine the likelihood
that the subject
has cancer.
[356] The computer system 501 includes a central processing unit (CPU, also
"processor" and "computer processor" herein) 505, which can be a single core
or multi core
processor, or a plurality of processors for parallel processing. The computer
system 501 also
includes memory or memory location 510 (e.g., random-access memory, read-only
memory,
flash memory), electronic storage unit 515 (e.g., hard disk), communication
interface 520
(e.g., network adapter) for communicating with one or more other systems, and
peripheral
devices 525, such as cache, other memory, data storage, and/or electronic
display adapters.
The memory 510, storage unit 515, interface 520, and peripheral devices 525
are in
communication with the CPU 505 through a communication network or bus (solid
lines),
such as a motherboard. The storage unit 515 can be a data storage unit (or
data repository) for
storing data. The computer system 501 can be operatively coupled to a computer
network 530
with the aid of the communication interface 520. The computer network 530 can
be the
Internet, an internet and/or extranet, or an intranet and/or extranet that is
in communication
with the Internet. The computer network 530 in some cases is a
telecommunication and/or
data network. The computer network 530 can include one or more computer
servers, which
can enable distributed computing, such as cloud computing. The computer
network 530, in
some cases with the aid of the computer system 501, can implement a peer-to-
peer network,
which may enable devices coupled to the computer system 501 to behave as a
client or a
server.
[357] The CPU 505 can execute a sequence of machine-readable instructions,
which
can be embodied in a program or software. The instructions may be stored in a
memory
location, such as the memory 510. Examples of operations performed by the CPU
405 can
include fetch, decode, execute, and writeback.
- 119 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
113581 The storage unit 515 can store files, such as drivers, libraries, and
saved
programs. The storage unit 515 can store programs generated by users and
recorded sessions,
as well as output(s) associated with the programs. The storage unit 515 can
store user data,
e.g., user preferences and user programs. The computer system 501 in some
cases can include
one or more additional data storage units that are external to the computer
system 501, such
as located on a remote server that is in communication with the computer
system 501 through
an intranet or the Internet. Data may be transferred from one location to
another using, for
example, a communication network or physical data transfer (e.g., using a hard
drive, thumb
drive, or other data storage mechanism).
[359] The computer system 501 can communicate with one or more remote
computer systems through the network 530. For embodiment, the computer system
501 can
communicate with a remote computer system of a user (e.g., operator). Examples
of remote
computer systems include personal computers (e.g., portable PC), slate or
tablet PC's (e.g.,
Apple iPad, Samsung Galaxy Tab), telephones, Smart phones (e.g., Apple
iPhone,
Android-enabled device, Blackberry ), or personal digital assistants. The user
can access the
computer system 501 via the network 530.
[360] Methods as described herein can be implemented by way of machine (e.g.,
computer processor) executable code stored on an electronic storage location
of the computer
system 501, such as, for example, on the memory 510 or electronic storage unit
515. The
machine executable or machine-readable code can be provided in the form of
software.
During use, the code can be executed by the processor 505. In some cases, the
code can be
retrieved from the storage unit 515 and stored on the memory 510 for ready
access by the
processor 505. In some situations, the electronic storage unit 515 can be
precluded, and
machine-executable instructions are stored on memory 510.
[361] In an aspect, the present disclosure provides a non-transitory computer-
readable medium comprising computer-executable instructions which, when
executed by at
least one electronic processor, perform at least a portion of a method
comprising: (a)
providing a biological sample of nucleic acid molecules, wherein the nucleic
acid molecules
comprises methylated nucleic acid molecules and unmethylated nucleic acid
molecules; (b)
partitioning at least a subset of the nucleic acid molecules in the biological
sample based on
the methylation status of the nucleic acid molecules into a plurality of
partitioned sets; (c)
digesting at least a subset of the one or more partitioned sets in the
plurality of partitioned
sets with at least one methylation sensitive restriction enzyme; (d) enriching
at least a subset
- 120 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
of the nucleic acid molecules in the plurality of partitioned sets for genomic
regions of
interest, wherein the at least a subset of the nucleic acid molecules
comprises digested nucleic
acid molecules in the one or more partitioned sets; and (e) determining
methylation status at
one or more genetic loci of the nucleic acid molecules in at least one of the
partitioned sets,
which in turn is used to detect the presence or absence of cancer in a
subject.
[362] The code can be pre-compiled and configured for use with a machine have
a
processer adapted to execute the code or can be compiled during runtime. The
code can be
supplied in a programming language that can be selected to enable the code to
execute in a
pre-compiled or as-compiled fashion.
[363] Aspects of the systems and methods provided herein, such as the computer
system 501, can be embodied in programming. Various aspects of the technology
may be
thought of as "products" or "articles of manufacture" typically in the form of
machine (or
processor) executable code and/or associated data that is carried on or
embodied in a type of
machine readable medium. Machine-executable code can be stored on an
electronic storage
unit, such memory (e.g., read-only memory, random-access memory, flash memory)
or a
hard disk. "Storage" type media can include any or all of the tangible memory
of the
computers, processors or the like, or associated modules thereof, such as
various
semiconductor memories, tape drives, disk drives and the like, which may
provide non-
transitory storage at any time for the software programming.
[364] All or portions of the software may at times be communicated through the
Internet or various other telecommunication networks. Such communications, for
example,
may enable loading of the software from one computer or processor into
another, for
example, from a management server or host computer into the computer platform
of an
application server. Thus, another type of media that may bear the software
elements includes
optical, electrical, and electromagnetic waves, such as those used across
physical interfaces
between local devices, through wired and optical landline networks, and over
various air-
links. The physical elements that carry such waves, such as wired or wireless
links, optical
links, or the like, also may be considered as media bearing the software. As
used herein,
unless restricted to non-transitory, tangible "storage" media, terms such as
computer or
machine "readable medium" refer to any medium that participates in providing
instructions to
a processor for execution.
- 121 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[365] Hence, a machine-readable medium, such as computer-executable code, may
take many forms, including but not limited to, a tangible storage medium, a
carrier wave
medium or physical transmission medium. Non-volatile storage media include,
for example,
optical or magnetic disks, such as any of the storage devices in any
computer(s) or the like,
such as may be used to implement the databases, etc. shown in the drawings.
Volatile storage
media include dynamic memory, such as main memory of such a computer platform.
Tangible transmission media include coaxial cables; copper wire and fiber
optics, including
the wires that comprise a bus within a computer system. Carrier-wave
transmission media
may take the form of electric or electromagnetic signals, or acoustic or light
waves such as
those generated during radio frequency (RF) and infrared (IR) data
communications.
Common forms of computer-readable media therefore include for example: a
floppy disk, a
flexible disk, hard disk, magnetic tape, any other magnetic medium, a CD-ROM,
DVD or
DVD-ROM, any other optical medium, punch cards, paper tape, any other physical
storage
medium with patterns of holes, a RAM, a ROM, a PROM and EPROM, a FLASH-EPROM,
any other memory chip or cartridge, a carrier wave transporting data or
instructions, cables or
links transporting such a carrier wave, or any other medium from which a
computer may read
programming code and/or data. Many of these forms of computer readable media
may be
involved in carrying one or more sequences of one or more instructions to a
processor for
execution.
[366] The computer system 501 can include or be in communication with an
electronic display 535 that comprises a user interface (UI) 540 for providing,
for example,
one or more results of sample analysis. Examples of UIs include, without
limitation, a
graphical user interface (GUI) and web-based user interface.
[367] Additional details relating to computer systems and networks, databases,
and
computer program products are also provided in, for example, Peterson,
Computer Networks:
A Systems Approach, Morgan Kaufmann, 5th Ed. (2011), Kurose, Computer
Networking: A
Top-Down Approach, Pearson, 7th Ed. (2016), Elmasri, Fundamentals of Database
Systems,
Addison Wesley, 6th Ed. (2010), Coronel, Database Systems: Design,
Implementation, &
Management, Cengage Learning, 11th Ed. (2014), Tucker, Programming Languages,
McGraw-Hill Science/Engineering/Math, 2nd Ed. (2006), and Rhoton, Cloud
Computing
Architected: Solution Design Handbook, Recursive Press (2011), each of which
is hereby
incorporated by reference in its entirety.
IV. Applications
- 122 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
A. Cancer and Other Diseases
[368] The present methods can be used to diagnose presence or absence of
conditions, particularly cancer, in a subject, to characterize conditions (e g
, staging cancer or
determining heterogeneity of a cancer), monitor response to treatment of a
condition, effect
prognosis risk of developing a condition or subsequent course of a condition.
The present
disclosure can also be useful in determining the efficacy of a particular
treatment option.
Successful treatment options may increase the amount of copy number variation
or rare
mutations detected in subjects blood if the treatment is successful as more
cancers may die
and shed DNA. In other examples, this may not occur. In another example,
perhaps certain
treatment options may be correlated with genetic profiles of cancers over
time. This
correlation may be useful in selecting a therapy. In some embodiments,
hypermethylation
variable epigenetic target regions are analyzed to determine whether they show
hypermethylation characteristic of tumor cells or cells that do not ordinarily
contribute
significantly to cfDNA and/or hypomethylation variable epigenetic target
regions are
analyzed to determine whether they show hypomethylation characteristic of
tumor cells or
cells that do not ordinarily contribute significantly to cfDNA
P691 Additionally, if a cancer is observed to be in remission after treatment,
the
present methods can be used to monitor residual disease or recurrence of
disease
[370] In some embodiments, the methods and systems disclosed herein may be
used
to identify customized or targeted therapies to treat a given disease or
condition in patients
based on the classification of a nucleic acid variant as being of somatic or
germline origin.
Typically, the disease under consideration is a type of cancer. Non-limiting
examples of such
cancers include biliary tract cancer, bladder cancer, transitional cell
carcinoma, urothelial
carcinoma, brain cancer, gliomas, astrocytomas, breast carcinoma, metaplastic
carcinoma,
cervical cancer, cervical squamous cell carcinoma, rectal cancer, colorectal
carcinoma, colon
cancer, hereditary nonpolyposis colorectal cancer, colorectal adenocarcinomas,
gastrointestinal stromal tumors (GISTs), endometrial carcinoma, endometrial
stromal
sarcomas, esophageal cancer, esophageal squamous cell carcinoma, esophageal
adenocarcinoma, ocular melanoma, uveal melanoma, gallbladder carcinomas,
gallbladder
adenocarcinoma, renal cell carcinoma, clear cell renal cell carcinoma,
transitional cell
carcinoma, urothelial carcinomas, Wilms tumor, leukemia, acute lymphocytic
leukemia
(ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL),
chronic
myeloid leukemia (CML), chronic myelomonocytic leukemia (C1VEML), liver
cancer, liver
- 123 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
carcinoma, hepatoma, hepatocellular carcinoma, cholangiocarcinoma,
hepatoblastoma, Lung
cancer, non-small cell lung cancer (NSCLC), mesothelioma, B-cell lymphomas,
non-
Hodgkin lymphoma, diffuse large B-cell lymphoma, Mantle cell lymphoma, T cell
lymphomas, non-Hodgkin lymphoma, precursor T-lymphoblastic lymphoma/leukemia,
peripheral T cell lymphomas, multiple myeloma, nasopharyngeal carcinoma (NPC),
neuroblastoma, oropharyngeal cancer, oral cavity squamous cell carcinomas,
osteosarcoma,
ovarian carcinoma, pancreatic cancer, pancreatic ductal adenocarcinoma,
pseudopapillary
neoplasms, acinar cell carcinomas. Prostate cancer, prostate adenocarcinoma,
skin cancer,
melanoma, malignant melanoma, cutaneous melanoma, small intestine carcinomas,
stomach
cancer, gastric carcinoma, gastrointestinal stromal tumor (GIST), uterine
cancer, or uterine
sarcoma. Type and/or stage of cancer can be detected from genetic variations
including
mutations, rare mutations, indels, copy number variations, transversions,
translocations,
inversion, deletions, aneuploidy, partial aneuploidy, polyploidy, chromosomal
instability,
chromosomal structure alterations, gene fusions, chromosome fusions, gene
truncations, gene
amplification, gene duplications, chromosomal lesions, DNA lesions, abnormal
changes in
nucleic acid chemical modifications, abnormal changes in epigenetic patterns,
and abnormal
changes in nucleic acid 5-methylcytosine.
[371] Genetic data can also be used for characterizing a specific form of
cancer.
Cancers are often heterogeneous in both composition and staging. Genetic
profile data may
allow characterization of specific sub-types of cancer that may be important
in the diagnosis
or treatment of that specific sub-type. This information may also provide a
subject or
practitioner clues regarding the prognosis of a specific type of cancer and
allow either a
subject or practitioner to adapt treatment options in accord with the progress
of the disease.
Some cancers can progress to become more aggressive and genetically unstable.
Other
cancers may remain benign, inactive or dormant. The system and methods of this
disclosure
may be useful in determining disease progression.
[372] Further, the methods of the disclosure may be used to characterize the
heterogeneity of an abnormal condition in a subject. Such methods can include,
e.g.,
generating a genetic profile of extracellular polynucleotides derived from the
subject,
wherein the genetic profile comprises a plurality of data resulting from copy
number
variation and rare mutation analyses. In some embodiments, an abnormal
condition is cancer.
In some embodiments, the abnormal condition may be one resulting in a
heterogeneous
genomic population. In the example of cancer, some tumors are known to
comprise tumor
- 124 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
cells in different stages of the cancer. In other examples, heterogeneity may
comprise
multiple foci of disease. Again, in the example of cancer, there may be
multiple tumor foci,
perhaps where one or more foci are the result of metastases that have spread
from a primary
site.
[373] The present methods can be used to generate or profile, fingerprint or
set of
data that is a summation of genetic information derived from different cells
in a
heterogeneous disease. This set of data may comprise copy number variation,
epigenetic
variation, and mutation analyses alone or in combination.
[374] The present methods can be used to diagnose, prognose, monitor or
observe
cancers, or other diseases. In some embodiments, the methods herein do not
involve the
diagnosing, prognosing or monitoring a fetus and as such are not directed to
non-invasive
prenatal testing. In other embodiments, these methodologies may be employed in
a pregnant
subject to diagnose, prognose, monitor or observe cancers or other diseases in
an unborn
subject whose DNA and other polynucleotides may co-circulate with maternal
molecules.
[375] Non-limiting examples of other genetic-based diseases, disorders, or
conditions that are optionally evaluated using the methods and systems
disclosed herein
include achondroplasia, alpha-1 antitrypsin deficiency, antiphospholipid
syndrome, autism,
autosomal dominant polycystic kidney disease, Charcot-Marie-Tooth (CMT), cri
du chat,
Crohn's disease, cystic fibrosis, Dercum disease, down syndrome, Duane
syndrome,
Duchenne muscular dystrophy, Factor V Leiden thrombophilia, familial
hypercholesterolemia, familial Mediterranean fever, fragile X syndrome,
Gaucher disease,
hemochromatosis, hemophilia, holoprosencephaly, Huntington's disease,
Klinefelter
syndrome, Marfan syndrome, myotonic dystrophy, neurofibromatosis, Noonan
syndrome,
osteogenesis imperfecta, Parkinson's disease, phenylketonuria, Poland anomaly,
porphyria,
progeria, retinitis pigmentosa, severe combined immunodeficiency (SCID),
sickle cell
disease, spinal muscular atrophy, Tay-Sachs, thalassemia, trimethylaminuria,
Turner
syndrome, velocardiofacial syndrome, WAGR syndrome, Wilson disease, or the
like.
[376] In some embodiments, a method described herein comprises detecting a
presence or absence of DNA originating or derived from a tumor cell at a
preselected
timepoint following a previous cancer treatment of a subject previously
diagnosed with
cancer using a set of sequence information obtained as described herein. The
method may
- 125 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
further comprise determining a cancer recurrence score that is indicative of
the presence or
absence of the DNA originating or derived from the tumor cell for the test
subject.
[377] Where a cancer recurrence score is determined, it may further be used to
determine a cancer recurrence status. The cancer recurrence status may be at
risk for cancer
recurrence, e.g., when the cancer recurrence score is above a predetermined
threshold. The
cancer recurrence status may be at low or lower risk for cancer recurrence,
e.g., when the
cancer recurrence score is above a predetermined threshold. In particular
embodiments, a
cancer recurrence score equal to the predetermined threshold may result in a
cancer
recurrence status of either at risk for cancer recurrence or at low or lower
risk for cancer
recurrence.
[378_1 In some embodiments, a cancer recurrence score is compared with a
predetermined cancer recurrence threshold, and the test subject is classified
as a candidate for
a subsequent cancer treatment when the cancer recurrence score is above the
cancer
recurrence threshold or not a candidate for therapy when the cancer recurrence
score is below
the cancer recurrence threshold. In particular embodiments, a cancer
recurrence score equal
to the cancer recurrence threshold may result in classification as either a
candidate for a
subsequent cancer treatment or not a candidate for therapy.
[379] The methods discussed above may further comprise any compatible feature
or
features set forth elsewhere herein, including in the section regarding
methods of determining
a risk of cancer recurrence in a test subject and/or classifying a test
subject as being a
candidate for a subsequent cancer treatment.
B. Methods of determining a risk of cancer recurrence in a test subject
and/or classifying a test subject as being a candidate for a subsequent
cancer treatment
[380] In some embodiments, a method provided herein is a method of determining
a
risk of cancer recurrence in a test subject. In some embodiments, a method
provided herein is
a method of classifying a test subject as being a candidate for a subsequent
cancer treatment.
[381_1 Any of such methods may comprise collecting DNA (e.g., originating or
derived from a tumor cell) from the test subject diagnosed with the cancer at
one or more
preselected timepoints following one or more previous cancer treatments to the
test subject.
The subject may be any of the subjects described herein. The DNA may be cfDNA.
The
DNA may be obtained from a tissue sample.
- 126 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[382] Any of such methods may comprise capturing a plurality of sets of target
regions from DNA from the subject, wherein the plurality of target region sets
comprises a
sequence-variable target region set and an epigenetic target region set,
whereby a captured set
of DNA molecules is produced. The capturing step may be performed according to
any of the
embodiments described elsewhere herein.
[383] In any of such methods, the previous cancer treatment may comprise
surgery,
administration of a therapeutic composition, and/or chemotherapy.
[384] Any of such methods may comprise sequencing the captured DNA molecules,
whereby a set of sequence information is produced. The captured DNA molecules
of the
sequence-variable target region set may be sequenced to a greater depth of
sequencing than
the captured DNA molecules of the epigenetic target region set.
[385] Any of such methods may comprise detecting a presence or absence of DNA
originating or derived from a tumor cell at a preselected timepoint using the
set of sequence
information. The detection of the presence or absence of DNA originating or
derived from a
tumor cell may be performed according to any of the embodiments thereof
described
elsewhere herein.
p86] Methods of determining a risk of cancer recurrence in a test subject may
comprise determining a cancer recurrence score that is indicative of the
presence or absence,
or amount, of the DNA originating or derived from the tumor cell for the test
subject. The
cancer recurrence score may further be used to determine a cancer recurrence
status. The
cancer recurrence status may be at risk for cancer recurrence, e.g., when the
cancer
recurrence score is above a predetermined threshold. The cancer recurrence
status may be at
low or lower risk for cancer recurrence, e.g., when the cancer recurrence
score is above a
predetermined threshold. In particular embodiments, a cancer recurrence score
equal to the
predetermined threshold may result in a cancer recurrence status of either at
risk for cancer
recurrence or at low or lower risk for cancer recurrence.
[387] Methods of classifying a test subject as being a candidate for a
subsequent
cancer treatment may comprise comparing the cancer recurrence score of the
test subject with
a predetermined cancer recurrence threshold, thereby classifying the test
subject as a
candidate for the subsequent cancer treatment when the cancer recurrence score
is above the
cancer recurrence threshold or not a candidate for therapy when the cancer
recurrence score
is below the cancer recurrence threshold. In particular embodiments, a cancer
recurrence
- 127 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
score equal to the cancer recurrence threshold may result in classification as
either a
candidate for a subsequent cancer treatment or not a candidate for therapy. In
some
embodiments, the subsequent cancer treatment comprises chemotherapy or
administration of
a therapeutic composition.
[388] Any of such methods may comprise determining a disease-free survival
(DFS)
period for the test subject based on the cancer recurrence score, for example,
the DFS period
may be 1 year, 2 years, 3, years, 4 years, 5 years, or 10 years.
[389] In some embodiments, the set of sequence information comprises sequence-
variable target region sequences, and determining the cancer recurrence score
may comprise
determining at least a first subscore indicative of the amount of SNVs,
insertions/deletions,
CNVs and/or fusions present in sequence-variable target region sequences.
[390] In some embodiments, a number of mutations in the sequence-variable
target
regions chosen from 1, 2, 3, 4, or 5 is sufficient for the first subscore to
result in a cancer
recurrence score classified as positive for cancer recurrence. In some
embodiments, the
number of mutations is chosen from 1, 2, or 3.
[391] In some embodiments, the set of sequence information comprises
epigenetic
target region sequences, and determining the cancer recurrence score comprises
determining
a second subscore indicative of the changes in the epigenetic features in the
epigenetic target
region sequences e.g., methylation of hypermethylation variable target regions
and/or
perturbed fragmentation of fragmentation variable target regions, where
"perturbed" means
different from DNA found in a corresponding sample from a healthy subject. In
some such
embodiments, the determining the cancer recurrence score comprises determining
a second
subscore indicative of the amount of molecules (obtained from the epigenetic
target region
sequences) that represent an epigenetic state different from DNA found in a
corresponding
sample from a healthy subject (e.g., cfDNA found in a blood sample from a
healthy subject,
or DNA found in a tissue sample from a healthy subject where the tissue sample
is of the
same type of tissue as was obtained from the test subject). These abnormal
molecules (i.e.,
molecules with an epigenetic state different from DNA found in a corresponding
sample from
a healthy subject) may be consistent with epigenetic changes associated with
cancer, e.g.,
methylation of hypermethylation variable target regions and/or perturbed
fragmentation of
fragmentation variable target regions.
- 128 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[3921 In some embodiments, a proportion of molecules corresponding to the
hypermethylation variable target region set and/or fragmentation variable
target region set
that indicate hypermethylation in the hypermethylation variable target region
set and/or
abnormal fragmentation in the fragmentation variable target region set greater
than or equal
to a value in the range of 0.001%-10% is sufficient for the second subscore to
be classified as
positive for cancer recurrence. The range may be 0.001%-1%, 0.005%-1%, 0.01%-
5%,
or 0.01%-1%.
[393] In some embodiments, any of such methods may comprise determining a
fraction of tumor DNA from the fraction of molecules in the set of sequence
information that
indicate one or more features indicative of origination from a tumor cell.
This may be done
for molecules corresponding to some or all of the epigenetic target regions,
e.g., including
one or both of hypermethylation variable target regions and fragmentation
variable target
regions (hypermethylation of a hypermethylation variable target region and/or
abnormal
fragmentation of a fragmentation variable target region may be considered
indicative of
origination from a tumor cell). This may be done for molecules corresponding
to sequence
variable target regions, e.g., molecules comprising alterations consistent
with cancer, such as
SNVs, indels, CNVs, and/or fusions The fraction of tumor DNA may be determined
based
on a combination of molecules corresponding to epigenetic target regions and
molecules
corresponding to sequence variable target regions.
[394] Determination of a cancer recurrence score may be based at least in part
on the
fraction of tumor DNA, wherein a fraction of tumor DNA greater than a
threshold in the
range of 10-11 to 1 or 10-10 to 1 is sufficient for the cancer recurrence
score to be classified
as positive for cancer recurrence. In some embodiments, a fraction of tumor
DNA greater
than or equal to a threshold in the range of 10-10 to 10-9, 10-9 to 10-8, 10-8
to 10-7, 10-7
to 10-6, 10-6 to 10-5, 10-5 to 10-4, 10-4 to 10-3, 10-3 to 10-2, or 10-2 to 10-
1 is
sufficient for the cancer recurrence score to be classified as positive for
cancer recurrence. In
some embodiments, the fraction of tumor DNA greater than a threshold of at
least 10-7 is
sufficient for the cancer recurrence score to be classified as positive for
cancer recurrence. A
determination that a fraction of tumor DNA is greater than a threshold, such
as a threshold
corresponding to any of the foregoing embodiments, may be made based on a
cumulative
probability. For example, the sample was considered positive if the cumulative
probability
that the tumor fraction was greater than a threshold in any of the foregoing
ranges exceeds a
- 129 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
probability threshold of at least 0.5, 0.75, 0.9, 0.95, 0.98, 0.99, 0.995, or
0.999. In some
embodiments, the probability threshold is at least 0.95, such as 0.99.
[395] In some embodiments, the set of sequence information comprises sequence-
variable target region sequences and epigenetic target region sequences, and
determining the
cancer recurrence score comprises determining a first subscore indicative of
the amount of
SNVs, insertions/deletions, CNVs and/or fusions present in sequence-variable
target region
sequences and a second subscore indicative of the amount of abnormal molecules
in
epigenetic target region sequences, and combining the first and second
subscores to provide
the cancer recurrence score. Where the first and second subscores are
combined, they may be
combined by applying a threshold to each subscore independently (e.g., greater
than a
predetermined number of mutations (e.g., > 1) in sequence-variable target
regions, and
greater than a predetermined fraction of abnormal molecules (i.e., molecules
with an
epigenetic state different from the DNA found in a corresponding sample from a
healthy
subject; e.g., tumor) in epigenetic target regions), or training a machine
learning classifier to
determine status based on a plurality of positive and negative training
samples.
113961 In some embodiments, a value for the combined score in the range of -4
to 2
or -3 to 1 is sufficient for the cancer recurrence score to be classified as
positive for cancer
recurrence.
[397] In any embodiment where a cancer recurrence score is classified as
positive
for cancer recurrence, the cancer recurrence status of the subject may be at
risk for cancer
recurrence and/or the subject may be classified as a candidate for a
subsequent cancer
treatment.
[398] In some embodiments, the cancer is any one of the types of cancer
described
elsewhere herein, e.g., colorectal cancer.
C. Therapies and Related Administration
[399] In certain embodiments, the methods disclosed herein relate to
identifying and
administering customized therapies to patients given the status of a nucleic
acid variant as
being of somatic or germline origin. In some embodiments, essentially any
cancer therapy
(e.g., surgical therapy, radiation therapy, chemotherapy, and/or the like) may
be included as
part of these methods. Typically, customized therapies include at least one
immunotherapy
(or an immunotherapeutic agent). Immunotherapy refers generally to methods of
enhancing
- 130 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
an immune response against a given cancer type. In certain embodiments,
immunotherapy
refers to methods of enhancing a T cell response against a tumor or cancer.
[400] In certain embodiments, the status of a nucleic acid variant from a
sample
from a subject as being of somatic or germline origin may be compared with a
database of
comparator results from a reference population to identify customized or
targeted therapies
for that subject. Typically, the reference population includes patients with
the same cancer or
disease type as the test subject and/or patients who are receiving, or who
have received, the
same therapy as the test subject. A customized or targeted therapy (or
therapies) may be
identified when the nucleic variant and the comparator results satisfy certain
classification
criteria (e.g., are a substantial or an approximate match).
[4011 In certain embodiments, the customized therapies described herein are
typically administered parenterally (e.g., intravenously or subcutaneously).
Pharmaceutical
compositions containing an immunotherapeutic agent are typically administered
intravenously. Certain therapeutic agents are administered orally. However,
customized
therapies (e.g., immunotherapeutic agents, etc.) may also be administered by
methods such
as, for example, buccal, sublingual, rectal, vaginal, intraurethral, topical,
intraocular,
intranasal, and/or intraauricular, which administration may include tablets,
capsules,
granules, aqueous suspensions, gels, sprays, suppositories, salves, ointments,
or the like.
D. Kits
[402] Also provided are kits comprising the compositions as described herein.
The
kits can be useful in performing the methods as described herein. The kits
comprise at least
one MSRE. In some embodiments, a kit also comprises a first reagent for
partitioning a
sample into a plurality of partitions as described herein, such as any of the
partitioning
reagents described elsewhere herein. In some embodiments, a kit comprises a
second reagent
for subjecting the first partition to a procedure that affects a first
nucleobase in the DNA
differently from a second nucleobase in the DNA of the first partition,
wherein the first
nucleobase is a modified or unmodified nucleobase, the second nucleobase is a
modified or
unmodified nucleobase different from the first nucleobase, and the first
nucleobase and the
second nucleobase have the same base pairing specificity (e.g., any of the
reagents described
elsewhere herein for converting a nucleobase such as cytosine or methylated
cytosine to a
different nucleobase). The kit may comprise the first and second reagents and
additional
elements as discussed below and/or elsewhere herein
- 131 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[403] Kits may further comprise a plurality of oligonucleotide probes that
selectively hybridize to least 5, 6, 7, 8, 9, 10, 20, 30, 40 or all genes
selected from the group
consisting of ALK, APC, BRAF, CDKN2A, EGFR, ERBB2, FBXVV7, KRAS, MYC,
NOTCH1, NRAS, PIK3CA, PTEN, RBI, TP53, MET, AR, ABL1, AKT1, ATM, CDH1,
CSFIR, CTNNB1, ERBB4, EZH2, FGFR1, FGFR2, FGFR3, FLT3, GNAll, GNAQ, GNAS,
HNF1A, HRAS, 1DHL IDH2, JAK2, JAK3, KDR, KIT, MLH1, MPL, NPM1, PDGFRA,
PROC, PTPN11, RET,SMAD4, SMARCB1, SMO, SRC, STK11, VHL, TERT, CCND1,
CDK4, CDKN2B, RAF1, BRCA1, CCND2, CDK6, NF1, TP53, ARID 1 A, BRCA2,
CCNEL ESR1, RIT1, GATA3, MAP2K1, RHEB, ROS1, ARAF, MAP2K2, NFE2L2,
RHOA, and NTRK1 . The number genes to which the oligonucleotide probes can
selectively
hybridize can vary. For example, the number of genes can comprise 1 , 2, 3, 4,
5, 6, 7, 8, 9,
10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28,
29, 30, 31, 32, 33, 34,
35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, or
54. The kit can
include a container that includes the plurality of oligonucleotide probes and
instructions for
performing any of the methods described herein.
[404] The oligonucleotide probes can selectively hybridize to exon regions of
the
genes, e.g., of the at least 5 genes. In some cases, the oligonucleotide
probes can selectively
hybridize to at least 30 exons of the genes, e.g., of the at least 5 genes. In
some cases, the
multiple probes can selectively hybridize to each of the at least 30 exons.
The probes that
hybridize to each exon can have sequences that overlap with at least 1 other
probe. In some
embodiments, the oligoprobes can selectively hybridize to non-coding regions
of genes
disclosed herein, for example, intronic regions of the genes. The oligoprobes
can also
selectively hybridize to regions of genes comprising both exonic and intronic
regions of the
genes disclosed herein.
[405] Any number of exons can be targeted by the oligonucleotide probes. For
example, at least 1, 2, 3,4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19, 20, 21, 22, 23,
24, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, 100, 105, 110,
115, 120, 125,
130, 135, 140, 145, 150, 155, 160, 165, 170, 175, 180, 185, 190, 195, 200,
205, 210, 215,
220, 225, 230, 235, 240, 245, 250, 255, 260, 265, 270, 275, 280, 285, 290õ
295, 300, 400,
500, 600, 700, 800, 900, 1,000, or more, exons can be targeted.
[406] The kit can comprise at least 4, 5, 6, 7, or 8 different library
adaptors having
distinct molecular barcodes and identical sample barcodes. The library
adaptors may not be
sequencing adaptors. For example, the library adaptors do not include flow
cell sequences or
- 132 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
sequences that permit the formation of hairpin loops for sequencing. The
different variations
and combinations of molecular barcodes and sample barcodes are described
throughout, and
are applicable to the kit. Further, in some cases, the adaptors are not
sequencing adaptors.
Additionally, the adaptors provided with the kit can also comprise sequencing
adaptors. A
sequencing adaptor can comprise a sequence hybridizing to one or more
sequencing primers.
A sequencing adaptor can further comprise a sequence hybridizing to a solid
support, e.g., a
flow cell sequence. For example, a sequencing adaptor can be a flow cell
adaptor. The
sequencing adaptors can be attached to one or both ends of a polynucleotide
fragment. In
some cases, the kit can comprise at least 8 different library adaptors having
distinct molecular
barcodes and identical sample barcodes. The library adaptors may not be
sequencing
adaptors. The kit can further include a sequencing adaptor having a first
sequence that
selectively hybridizes to the library adaptors and a second sequence that
selectively
hybridizes to a flow cell sequence. In another example, a sequencing adaptor
can be hairpin
shaped. For example, the hairpin shaped adaptor can comprise a complementary
double
stranded portion and a loop portion, where the double stranded portion can be
attached {e.g. ,
ligated) to a double-stranded polynucleotide. Hairpin shaped sequencing
adaptors can be
attached to both ends of a polynucleotide fragment to generate a circular
molecule, which can
be sequenced multiple times. A sequencing adaptor can be up to 10, 11, 12, 13,
14, 15, 16,
17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35,
36, 37, 38, 39, 40, 41,
42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60,
61, 62, 63, 64, 65, 66,
67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85,
86, 87, 88, 89, 90, 91,
92, 93, 94, 95, 96, 97, 98, 99, 100, or more bases from end to end. The
sequencing adaptor
can comprise 20-30, 20-40, 30-50, 30-60, 40-60, 40-70, 50-60, 50-70, bases
from end to end.
In a particular example, the sequencing adaptor can comprise 20-30 bases from
end to end. In
another example, the sequencing adaptor can comprise 50-60 bases from end to
end. A
sequencing adaptor can comprise one or more barcodes. For example, a
sequencing adaptor
can comprise a sample barcode. The sample barcode can comprise a pre-
determined
sequence. The sample barcodes can be used to identify the source of the
polynucleotides. The
sample barcode can be at least 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19,
20, 21, 22, 23, 24, 25, or more (or any length as described throughout)
nucleic acid bases,
e.g., at least 8 bases. The barcode can be contiguous or non-contiguous
sequences, as
described above.
- 133 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
114071 The library adaptors can be blunt ended and Y-shaped and can be less
than or
equal to 40 nucleic acid bases in length. Other variations of the can be found
throughout and
are applicable to the kit.
EXAMPLE S
[408] The following examples are provided to illustrate certain aspects of the
disclosed methods. The examples do not limit the disclosure.
Example 1: Reduction of Technical Noise by Digestion of Nonspecifically
Partitioned
DNA
[409] A pool of cfDNA from two healthy normal samples was combined, from
which 18.6ng was used as input to a MBD-partitioning assay described herein.
To a subset of
the samples, cfDNA from a colorectal cancer sample (CRC) with 0.5% MAF
(somatic allele
fraction) was added, resulting in a diluted CRC sample with 0.16% MAF. Three
sets of
normal samples and diluted CRC samples were used in the assay. The three sets
of samples
were then partitioned using MBD protein into three partitions (hypermethylated
(hyper),
intermediate (residual), and hypomethylated (hypo) partitions). Following
cleanup, the
cfDNA molecules in each partition was ligated with partition-specific adapters
comprising
molecular barcodes. The molecular barcodes used in hyper and residual
partition are selected
such that they do not have MSRE recognition sites, so they are not digested in
the
downstream processing (irrespective of cfDNA methylation state). Post-
ligation, ligation
cleanups were performed. Following the ligation cleanup, the hyper and
residual partitions
were subjected to MSRE digestion reactions. A first set of the samples (normal
and diluted
CRC samples) were treated with BstUI and HpaII and another set of the samples
were treated
BstUI, HpaII and Hin6I enzymes. The third set of samples were run through a
mock digest
(no MSREs) in the MBD-partitioning assay as a control. After the MSRE
digestion, the
enzymes were heat inactivated (65C, 20min) and cleaned up using SPRI beads.
After the
digest cleanups, the hyper, residual and (non-digested) hypo partitions
(adapter-ligated
cfDNA) were combined and processed through an NGS assay workflow comprising
PCR
amplification; enrichment of molecules in genomic regions of interest; pooling
of samples
thereby allowing multiplexed sequencing and sequencing the pooled sample using
NovaSeq.
In an alternative procedure the hypo partition may additionally be contacted
with one or more
MSREs having a methylated recognition site to cleave nonspecifically
partitioned DNA in
the hypo partition.
- 134 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[4101 Fig. 6 clearly shows the increase in cancer methylation signal at DMRs
relative to the technical noise from unmethylated molecules in normal samples
when the
MSRE digestion was applied. In the negative control regions (where the DNA
molecules are
non-methylated at almost all times irrespective of the disease state) shown in
Fig. 6, "a"
clearly indicates that it was clear that the MSRE digestion removes the
unmethylated
molecules that mis-partitioned into the hyper partition ¨ 90 molecules were
partitioned into
hyper partition in the mock digestion whereas in BstUI, HpaII and Hin6I
digestion the
molecule count was reduced to 10. In the classification DMRs shown in Fig. 6,
cfDNA
molecules were removed by much higher proportion in normal samples (b; 350
100) than
diluted CRC samples (c; 1500 4 1100) upon digestion with MSREs.
Example 2: Analysis of cfDNA to detect the presence of absence of a tumor
[411] A set of patient samples are analyzed by a blood-based NGS assay at
Guardant
Health (Redwood City, CA, USA) to detect the presence or absence of cancer.
cfDNA is
extracted from the plasma of these patients. cfDNA of the patient samples is
then combined
with methyl binding domain (MBD) buffers and magnetic beads conjugated with an
MBD
protein and incubated overnight. Methylated cfDNA (if present, in the cfDNA
sample) is
bound to the MBD protein during this incubation. Non-methylated or less
methylated DNA is
washed away from the beads with buffers containing increasing concentrations
of salt.
Finally, a high salt buffer is used to wash the heavily methylated DNA away
from the MBD
protein. These washes result in three partitions (hypomethylated, residual
methylation and
hypermethylated partitions) of increasingly methylated cfDNA.
[412] Optionally, the cfDNA molecules in the hypermethylated partition are
subjected to enzymatic modification (EM) with whereby unmodified cytosines,
but not mC
and hmC, undergo deamination, thereby marking nonspecifically partitioned
hypomethylated
molecules in the first partition by conversion of unmodified cytosines to
uracils.
[413] After concentrating the cfDNA in the partitions, the end overhangs of
the
partitioned cfDNA are extended, and adenosine residues are added to the 3'
ends of the
cfDNA fragment by the polymerase during the extension. The 5' end of each
fragment is
phosphorylated. These modifications make the partitioned cfDNA ligatable. DNA
ligase and
adapters are added to ligate each partitioned cfDNA molecule with an adapter
on each end.
These adapters contain non-unique molecular barcodes and each partition is
ligated with
- 135 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
adapters having non-unique molecular barcodes that is distinguishable from the
barcodes in
the adapters used in the other partitions.
[414] The cfDNA in the hypomethylated partition is contacted with one or more
MSREs having a methylated recognition site. The enzymes cleave at least a
portion of
nonspecifically partitioned DNA in the hypomethylated partition. Alternatively
or in
addition, the cfDNA in the hypermethylated partition is contacted with one or
more MSREs
having an unmethylated recognition site. The enzymes cleave at least a portion
of
nonspecifically partitioned DNA in the hypermethylated partition.
[415] After ligation, the four partitions are pooled together and are
amplified by
PCR. Molecules that were cleaved by the one or more MSREs do not undergo
exponential
amplification because they do not have an adapter on each end.
[416] Following PCR, amplified DNA is washed and concentrated prior to
enrichment. Once concentrated, the amplified DNA is combined with a salt
buffer and
biotinylated RNA probes that comprise probes for a sequence-variable target
region set and
probes for an epigenetic target region set and this mixture is incubated
overnight. The probes
for the sequence-variable region set has a footprint of about 50 kb and the
probes for the
epigenetic target region set has a footprint of about 500 kb. The probes for
the sequence-
variable target region set comprise oligonucleotides targeting at least a
subset of genes
identified in Tables 3-5 and the probes for the epigenetic target region set
comprises
oligonucleotides targeting a selection of hypermethylation variable target
regions,
hypomethylation variable target regions, CTCF binding target regions,
transcription start site
target regions, focal amplification target regions and methylation control
regions.
[417] The biotinylated RNA probes (hybridized to DNA) are captured by
streptavidin magnetic beads and separated from the amplified DNA that are not
captured by a
series of salt based washes, thereby enriching the sample. After enrichment,
an aliquot of the
enriched sample is sequenced using Illumina NovaSeq sequencer. The sequence
reads
generated by the sequencer are then analyzed using bioinformatic
tools/algorithms. The
molecular barcodes are used to identify unique molecules as well as for
deconvolution of the
sample into molecules that were differentially MBD-partitioned. The method
described in
this example, apart from providing information on the overall level
methylation (i.e.,
methylated cytosine residues) of a molecule based on its partition, including
with increased
accuracy and/or confidence due to the cleavage of nonspecifically partitioned
cfDNA in the
- 136 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
hypomethylated partition, can also provide a higher resolution information
about the location
of methylated cytosines based on the conversion of unmethylated cytosines in
the
hypermethylated partition. The sequence-variable target region sequences are
analyzed by
detecting genomic alterations such as SNVs, insertions, deletions and fusions
that can be
called with enough support that differentiates real tumor variants from
technical errors (for
e.g., PCR errors, sequencing errors). The epigenetic target region sequences
are analyzed
independently to detect methylation status of cfDNA molecules in regions that
have been
shown to be differentially methylated, e.g., in potentially cancerous tissue
compared to
healthy cfDNA. Finally, the results of both analysis are combined to produce a
final tumor
present/absent call.
Example 3: Analysis of methylation at single nucleotide resolution in cfDNA
samples from healthy subjects and subjects with early-stage colorectal cancer
14181 Samples of cfDNA from healthy subjects and subjects with early-stage
colorectal cancer were analyzed as follows. cfDNA was partitioned using MBD to
provide a
hypermethylated partition, an intermediate partition, and a hypomethylated
partition. The
partitioned DNA of each partition was ligated to adapters and subjected to an
EM-seq
conversion procedure whereby unmodified cytosines, but not mC and hmC, undergo
deamination, although in an alternative procedure the partitioned DNA of the
hypermethylated partition could be contacted with a MSRE having an
unmethylated
recognition site as described herein. Following such deamination, the
partitions were
prepared for sequencing and subjected to whole-genome sequencing. Each
partition was
sequenced separately, although in an alternative procedure the partitions
could be
differentially tagged (e.g., after partitioning and before EM-seq conversion,
or after
partitioning and EM-seq conversion and before further preparation for
sequencing), pooled,
and processed sequenced in parallel.
[419] Sequence data from hypermethylation variable target regions was isolated
bioinformatically, although in an alternative procedure target regions could
be enriched in
vitro before sequencing. Per-base methylation for the hypermethylation
variable target
regions was quantified as shown in Fig. 7, which shows the number of
methylated CpG per
molecule in the hypermethylation variable target regions from the
hypermethylated partition.
The x-axis indicates the total number of CpGs per molecule, such that points
along the
diagonal represent molecules with methylation at every CpG. Thus, it was
possible to analyze
methylation at single-base resolution and quantify per base methylation and
partial molecule
- 137 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
methylation of the MBD-partitioned material. The samples from subjects with
colorectal
cancer exhibited much higher overall methylation in these regions than samples
from healthy
subj ects.
Example 4: Analysis of MDRE-Digested cfDNA
[420] Multiple aliquots of cfDNA from two healthy donors were isolated and
subjected to MBD-based partitioning of methylated cfDNA. The hypomethylated
cfDNA
partition was then subjected to ligation of NGS adapters onto the cfDNA
molecules. Ligated
cfDNA from each donor was then subjected to digestion with an MSRE that
preferentially
cleaves methylated DNA, also referred to as a methylation-dependent
restriction enzyme
(MDRE) digestion. The MDREs used were FspEI, LpnPI, MspJI, or SgeI, or a
'mock'
digestion (no enzyme added to digestion) or an undigested condition in which
the MDRE
reaction was skipped as control reactions. After the MDRE step, the
hypomethylated cfDNA
partition was amplified in a universal PCR in which DNA that had been cleaved
by the
MDRE was not exponentially amplified because adapters were not present at each
end. The
PCR products were then subjected to enrichment of targeted genomic regions
using a hybrid
capture panel, amplified in a second PCR, and sequenced by NGS. The hybrid
capture panel
targets include 'positive control (ctrl)' and 'negative control (ctrl)'
regions of the genome for
enrichment. Positive control regions are CpG-dense regions of genome that are
found to be
ubiquitously highly methylated (>85% methylation by bisulfite-seq) in all
human tissues
including blood and cancerous tissue. Conversely, negative control regions are
ubiquitously
unmethylated (<15% methylation) in all human tissues. From the NGS analysis,
the number
of positive control molecules (i.e., molecules in the positive control
regions) and negative
control molecules (i.e., molecules in the negative control regions) sequenced
in all the
conditions are compared to estimate MDRE sensitivity and specificity,
respectively. Figs.
8A-B show that the FspEI enzyme treatment reduced the number of positive
control
molecules >100-fold compared to the 'mock' condition, demonstrating ¨99%
sensitivity with
respect to digestion of methylated molecules. Figs. 8C-D show that the FspEI
treatment does
not meaningfully reduce the negative control molecules, indicating high
specificity with
FspEI digestion (does not digest unmethylated molecules). Note that MspJI
shows some
sensitivity, but poor specificity compared to FspEI, while LpnI and SgeI show
little/no
sensitivity.
[421] The MDRE digestion efficiency was calculated using molecules with
different
recognition sites and number of sites per molecule. Digestion efficiency is
calculated as 1-
- 138 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
[number of positive control molecules in MDRE condition]/[number of positive
control
molecules in the mock condition]. The general recognition sequence of FspEI
that includes a
5mCpG is C5mCGH (H = A, C, or T), with cleavage occurring 12-16 bases
downstream. The
FspEI palindromic site C5mCGG contains two FspEI recognition sites on the top
and bottom
strands in opposite directions. The general 5mCpG-containing consensus is
5"'CpGNR, which
can overlap with the FspEI consensus. Figs. 9A-D show that digestion
efficiency increases
with the minimum number of C5mCGH or C5mCGG sites per molecule and is more
efficient at
the palindromic site (C5mCGG). Positive control molecules with at least one
C5mCGG or at
least two C5 m CGH sites were cleaved with 95% efficiency.
[422] Additionally, digestion with FspEI and MspJI simultaneously or
sequentially
was tested. Sequential digestion with the two MDREs (FspEI then MspJI) had the
highest
efficiency. It is possible that in the simultaneous digestion (FspEI and
MspJI), MspJI
sometimes binds to the DNA but does not cleave (lower individual efficiency),
thus sterically
blocking the FspEI activity. Although FspEI then MspJI has higher overall
efficiency than
FspEI alone here, FspEI alone has better cleavage specificity. Thus, in
different
circumstances, digestion with FspEI alone or with FspEI then MspJI may be
preferable. Note
that with higher numbers of minimum sites there are fewer positive control
molecules
observed (Figs. 9C-D) and thus the digestion efficiency estimate becomes more
noisy.
Example 5: Detection of tumor DNA following MDRE treatment
[423] cfDNA isolated from four healthy donors was used to create 'normal' and
simulated 'cancer' cfDNA samples. The donor samples were used neat as
'normals' and
spiked with the cfDNA of a colorectal cancer (CRC) patient to create a 'cancer
sample. The
circulating tumor DNA fraction of the CRC cfDNA sample had been previously
measured
and was used to spike a calculated amount of CRC cfDNA into the normal donor
cfDNA
such that the resulting 'cancer' sample contained 0.5% circulating tumor DNA (-
0.5% CRC"
in Figs. 10A-J). All the samples were subjected to MBD-based partitioning,
splitting the
cfDNA into hypermethylated and hypomethylated cfDNA partitions. The
hypomethylated
cfDNA partition was then ligated to NGS adapters. Ligated cfDNA from each
donor was
then subjected to a MDRE digestion with either FspEI, MspJI or FspEI+MspJI. A
'mock
digestion' (no enzyme added to digestion reaction) and 'no digestion'
condition (skip MDRE
reaction altogether) served as control reactions. After the MDRE step, the non-
digested
hypomethylated partition cfDNA was amplified in a universal PCR, then
subjected to
enrichment of targeted genomic regions using a hybrid capture panel, and then
amplified in a
- 139 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
2nd PCR and sequenced by NGS. The hybrid capture panel targets include
hypomethylation
variable target regions and 'negative control (ctrl)' regions of the genome
for enrichment.
Negative control regions are CpG-dense regions of genome that are found to be
ubiquitously
lowly methylated (<15% methylation by bisulfite-seq) in all human tissues
including blood
and cancerous tissue. The hypomethylation variable target regions are genomic
regions
annotated in literature as having reduced methylation percentage in CRC tissue
compared to
healthy colon tissue and blood. From the NGS analysis, the number of
hypomethylation
variable target region molecules with 2 CCGG sites or more (which should be
digested with
high efficiency by the MDRE) is compared between 'normal' and 'cancer' samples
across all
the digestion conditions (Figs. 10A-E). The ratios of the hypomethylation
variable target
region molecule counts were also compared to the negative control molecule
counts, which
normalizes for varying cfDNA input amounts that can affect the hypomethylation
variable
target region molecule counts (Figs. 10F-J). No resolvable detection of the
hypomethylation
variable target region cancer signals was observed in the no MDRE digestion
conditions ('no
digestion' and 'mock digestion'). That is, the hypomethylation variable target
region
molecules and the normalized ratio levels were indistinguishable (not
significantly different)
between the 'cancer' and 'normal' samples (this is marked by the horizontal
arrows in Figs.
10C, E, H, and J). Conversely, when there was an MDRE treatment, a shift
(increase) in the
hypomethylation variable target region counts and normalized ratio was
detected in the
'cancer' as compared to the 'normal' samples (marked by upward right arrow in
Figs. 10A,
B, D, F, G, and I). Thus, the MDRE treatment enables detection of a cancer
hypomethylation
variable target region signal in the 'cancer' samples at 0.5% CRC ctDNA, that
are not
detectable by the MBD-partitioning assay alone.
* * *
[424] While preferred embodiments of the present invention have been shown and
described herein, it will be obvious to those skilled in the art that such
embodiments are
provided by way of example only. It is not intended that the invention be
limited by the
specific examples provided within the specification. While the invention has
been described
with reference to the aforementioned specification, the descriptions and
illustrations of the
embodiments herein are not meant to be construed in a limiting sense. Numerous
variations,
changes, and substitutions will now occur to those skilled in the art without
departing from
the invention. Furthermore, it shall be understood that all aspects of the
invention are not
- 140 -
CA 03193090 2023- 3- 17
WO 2022/073011
PCT/US2021/071648
limited to the specific depictions, configurations or relative proportions set
forth herein which
depend upon a variety of conditions and variables. It should be understood
that various
alternatives to the embodiments of the disclosure described herein may be
employed in
practicing the invention. It is therefore contemplated that the disclosure
shall also cover any
such alternatives, modifications, variations or equivalents. It is intended
that the following
claims define the scope of the invention and that methods and structures
within the scope of
these claims and their equivalents be covered thereby.
[425] While the foregoing disclosure has been described in some detail by way
of
illustration and example for purposes of clarity and understanding, it will be
clear to one of
ordinary skill in the art from a reading of this disclosure that various
changes in form and
detail can be made without departing from the true scope of the disclosure and
may be
practiced within the scope of the appended claims. For example, all the
methods, systems,
computer readable media, and/or component features, steps, elements, or other
aspects
thereof can be used in various combinations.
[426] All patents, patent applications, websites, other publications or
documents,
accession numbers and the like cited herein are incorporated by reference in
their entirety for
all purposes to the same extent as if each individual item were specifically
and individually
indicated to be so incorporated by reference If different versions of a
sequence are
associated with an accession number at different times, the version associated
with the
accession number at the effective filing date of this application is meant.
The effective filing
date means the earlier of the actual filing date or filing date of a priority
application referring
to the accession number, if applicable. Likewise, if different versions of a
publication,
website or the like are published at different times, the version most
recently published at the
effective filing date of the application is meant, unless otherwise indicated.
- 141 -
CA 03193090 2023- 3- 17