Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/066791
PCT/US2021/051583
COMPOSITION AND METHOD FOR PREVENTION AND TREATMENT OF
CUTANEOUS RADIATION INJURY
FIELD OF THE INVENTION
[0001]
This invention is directed to compounds, compositions, and methods for
treating a cutaneous condition or disorder induced by radiation exposure.
BACKGROUND OF THE INVENTION
[0002]
Cutaneous Radiation Injury (CRI) is damage to skin or underlying tissue in
animals, including mammals and humans, which is induced by, or results from,
exposure to
radiation from industrial or military-related sources, e.g., radioactive
materials used in
nuclear power plants or in nuclear weapons. Mild skin damage, e.g., sunburn
from
overexposure to the sun, is not generally considered to fall within the
definition of CRI;
however, the term CRI can include skin conditions, such as Radiation
Dermatitis, or other
conditions such as Radiation Proctopathy, Oral Mucositis, or Severe Oral
Mucositis, each of
which can be observed following medical radiation therapy administered to a
particular area
of the body.
[0003]
Radiation therapy in the medical field has been used successfully in
treating
locally or regionally advanced cancer and can be employed as a sole treatment
or adjunctive
to chemotherapy or surgery. The radiation exposure in such treatments,
however, may cause
severe burns of the skin and surrounding tissue as well as permanent changes
in
pigmentation. As many as 95% of patients treated with radiation therapy for
cancer will
experience a skin reaction. Certain skin reactions due to radiation treatments
administered
to breast cancer patients, for example, are referred to as "radiation
dermatitis."
[0004]
Head and neck cancer patients can suffer oral nnucositis due to damage
caused
to the epithelial cells lining the mouth following radiation treatments to
treat the head and
neck cancer. When ulcerative lesions form in the mouth due to radiation
treatment, or if the
patient becomes unable to swallow solid food, the condition is considered as
"Severe Oral
Mucositis," or "SOM."
1
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[0005]
Radiation therapy administered to the pelvic area can cause damage to
rectal
tissue. A variety of disorders resulting from radiation therapy targeted to
the pelvic area may
affect the anorectal region, and may involve one or more of inflammatory,
ischennic,
infectious, traumatic, or neoplastic pathologies. Symptoms of anorectal
disease include anal
or rectal pain, urgency to move the bowels, fecal incontinence, diarrhea,
rectal bleeding, and
difficulty with evacuation of the rectum. These conditions are referred to as
"radiation
proctitis," more recently and more accurately referred to as "radiation
proctopathy."
[0006]
In view of the prevalence of these conditions in cancer patients
undergoing
radiation therapy, several strategies and treatments have been proposed for
patients
suffering from radiation dermatitis, but not have shown great success. Aloe
vera and topical
Vitamin C have been tried without improvement in the results of irradiated
breast tissue.
[0007]
Other reports indicate that the prophylactic and ongoing use of topical
corticosteroid or a dexpanthenol-containing emollient can ameliorate, but not
prevent,
radiation dermatitis. Other reports have shown that moist skin care with 3%
urea lotion
delays the occurrence and reduces the grade of acute skin reactions in
percutaneously
irradiated patients with head and neck tumors. Negative side-effects from
corticosteroids are
well documented and their use is preferably avoided. Topical corticosteroids
have little to no
effect on pigmentation changes.
[0008]
Biafine and Lipidernn were shown to have no radioprotective effect, while
significant dernnato-cytoprotective effects were shown from the use of
amifostine in a
retrospective analysis.
[0009]
Misoprostol, a prostaglandin E(1) analog, has been found to be an
effective
radioprotector in animal studies preventing oncogenic transformation of Syrian
hamster
embryos exposed to radiation in utero. However, successful results were not
obtained in
humans.
[00010]
Attempts to prevent or treat radiation proctopathy or proctitis have also
been
largely unsuccessful. Many topical treatments used for anorectal or lower
bowel conditions
appear to be ineffective. For example, use of the anti-inflammatory agent 5-
anninosalicylic
acid (5-ASA), previously used to treat Inflammatory Bowel Disease (IBD), was
unsuccessful in
2
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
treating radiation proctopathy or proctitis, with and without a steroid such
as hydrocortisone.
Sucralfate had also been recommended for radiation proctopathy but has proven
ineffective.
[00011]
Topical therapies, such as nitroglycerin and calcium channel blockers,
were
also found ineffective for treating radiation proctopathy. Short chain fatty
acid enemas have
also been used to treat radiation proctopathy but are not readily available
and are difficult to
administer.
[00012]
Recently, antioxidants, such as Vitamin A, Vitamin C, and Vitamin E
formulated
in a suppository have been described for treating radiation proctopathy or
proctitis.
Suppositories are solid dosage forms comprising medications for placement in
the anus or
vagina for the treatment of certain systemic conditions but can be used for
localized
treatment of anorectal and gynecologic disorders. For example, one common use
of rectal
suppositories is for the treatment of constipation.
[00013]
Rectal suppositories are also used as an alternative form of drug delivery
in
patients that cannot receive medications by mouth, whereby the medication is
absorbed by
the mucus membrane and distributed systemically. Examples of these types of
rectal
suppositories include treatments for nausea and pain
[00014]
It has been proposed that damage to healthy tissue from radiation therapy
may involve an endothelial response associated with signaling from the plasma
membrane,
via the acid sphingomyelinase/cerannide pathway (Corre, I., et al., Intl. J.
Mol. Sci. (2013) 14,
22678-22696.)
Protecting against endothelial damage, for example, by inhibiting acid
sphingonnyelinase (ASMase) activity, is suggested as a means for limiting
radiation toxicity in
normal tissues. Corre, supra, at 22685. Although inhibitors of ASMase are
known, none have
been developed for topical administration, nor demonstrated to be effective
for prevention
or treatment of CRI, generally, or radiation dermatitis or radiation
proctopathy, specifically.
[00015]
Annitriptyline has been formulated in a suppository dosage form. However,
amitriptyline suppositories were only prepared for systemic delivery and to
treat depression
¨the known indication for amitriptyline. Another indication for amitriptyline
suppositories
for systemic delivery is insomnia, exploiting the sleep-inducing side effect
observed for
amitriptyline. Annitriptyline suppositories have not previously been known to
be
3
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
administered to patients undergoing, or that have undergone, pelvic radiation
therapy or for
treating radiation proctopathy or proctitis or any other in situ treatment
within the lower
intestinal tract.
[00016]
Tricyclic antidepressants, in general, have not previously been known to
be
used for treating anorectal disorders such as radiation proctopathy or
proctitis. Nor have
dosage forms comprising a tricyclic antidepressant such as annitriptyline been
formulated for
anal or rectal administration or in situ (or non-systemic) treatment of
radiation proctopathy
or proctitis. More specifically, annitriptyline has not previously been
formulated in a dosage
form, such as a suppository or other anorectal delivery device for localized
treatment of
radiation proctopathy or proctitis. Nor has a tricyclic antidepressant, such
as annitriptyline,
been formulated as a controlled release formulation to bypass release within a
low pH
environment (acidic pH of less than 7), such as in the stomach, such that the
drug release
occurs primarily in the intestine, preferably the lower intestine for in situ
delivery of the drug
to treat radiation proctopathy or proctitis.
[00017]
Selective serotonin reuptake inhibitors (SSRI's) are not known to be
available
in suppository form; nor are SSRI's known to be previously used for treatment
of radiation
proctopathy or proctitis.
[00018]
There are numerous anorectal diseases that may benefit from localized
administration of TCAs, such as annitriptyline or SSR1s, such as sertraline.
These conditions
include (but are not limited to), inflammatory bowel disease (IBD) including
ulcerative
proctitis and Crohn's disease, anal fissures, internal hemorrhoids, radiation
proctopathy, anal
and rectal neoplasms, anal warts, anal dysplasia, solitary rectal ulcer
syndrome, pruritis ani
and anorectal ischennia. These conditions represent a variety of significant
clinical problems
for which limited treatment options are currently available.
[00019]
The continued and increased use of radiation therapy in the treatment of
cancer, and lack of available preventive or therapeutic medications for CRI,
including radiation
dermatitis, or radiation proctopathy, is a longstanding unmet patient need
affecting millions
of patients.
4
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
BRIEF DESCRIPTION OF THE INVENTION
[00020]
The subject invention concerns a method for preventing, reducing the
incidence or recurrence of, inhibiting, treating, ameliorating or reducing the
severity of, or
reversing, Cutaneous Radiation Injury (CRI), including environmental exposure
to radiation
for industrial or military radioactive sources, or radiation dermatitis or
radiation proctopathy
resulting from therapeutic radiation procedures in cancer treatment. The term,
CRI, generally
includes radiation damage to the outer layer of the skin, or dernnis, but may
include damage
to deeper skin layers, such as the subdernnal layer or adipose layer.
Radiation dermatitis and
radiation proctopathy fall within the definition of CRI, but are generally
considered to be
conditions relating to damage of the dermis.
[00021]
A method according to the invention comprises topically administering or
applying to the skin surface of a patient suffering from CRI, including
radiation dermatitis or
radiation proctopathy, a composition comprising at least one acid
sphingomyelinase
(ASMase) inhibitor. A functional inhibitor of acid sphingomyelinase, known in
the art by the
acronym, FIASMA, includes certain selective serotonin reuptake inhibitors
(SSR1s), tricyclic
antidepressants (TCAs), antihistamines, antiarrhythmics, adrenergic receptor
blockers (ARBs),
beta blockers, and the like.
[00022]
SSRIs useful in the method of the invention include one or more of:
citalopram,
escitaloprann, fluoxetine, fluvoxannine, paroxetine, or sertraline. A
preferred SSRI is sertraline.
According to a preferred embodiment, the dosage form of the invention
comprises
annitriptyline at a dosage of between 0.1 mg ¨ 1000 mg; more preferably a dose
of about 1
mg ¨ 100 mg, and most preferably a dose of about 5 mg- 50 mg. It is
contemplated that a
preferred dose is about 10-50 mg of the SSRI, formulated in a 1-5% (w/w)
composition. The
dose can vary, being lower in dosage forms that more rapidly deliver drug to
the site, or higher
in dosage forms that slowly release drug.
[00023]
Tricyclic antidepressants (TCAs) useful in the method or composition of
the
invention include one or more of: annineptine, annitriptyline, annoxapine,
butriptyline,
clonniprannine, desiprannine, dibenzepin, dosulepin, doxepin (in combination
but not alone),
imipra mine, iprindole, lofepramine, maprotiline, norclomipramine, northiaden,
nortriptyline,
opiprannol, protriptyline, tianeptine, or trinniprannine. A preferred TCA is
annitriptyline.
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
According to one preferred embodiment, the dosage form of the invention
comprises
a nnitriptyline at a dosage of between 0.1 ¨ 1000 mg; more preferably a dose
of about 1 mg ¨
100 mg, and most preferably a dose of about 5 mg- 50 mg. A preferred dose is
about 10-50
mg of the TCA, formulated in a 1-5% (w/w) composition. The dose can vary,
being lower in
dosage forms that more rapidly deliver drug to the site, or higher in dosage
forms that slowly
release drug.
[00024] More specifically, the method of the invention
comprises the steps of:
- providing a topical composition comprising a pharmaceutically effective
amount of at
least one FIASMA as an active ingredient, or a combination of two or more
FIASMA
drugs,
- topically administering or applying an effective amount of the
composition to a patient
in need thereof prior to radiation therapy or following radiation therapy or
both.
[00025] In a method for preventing, reducing the incidence or
recurrence of, inhibiting,
treating, ameliorating or reducing the severity of, or reversing radiation
proctopathy, the
method can comprise the step of:
- anorectally administering to a patient in need thereof, such as a patient
undergoing
radiation treatment of the pelvic area or a patient suffering from radiation
proctopathy or proctitis, a dosage form comprising a pharmaceutically
effective
amount of one or more active ingredients, wherein at least one active
ingredient is a
FIASMA.
[00026] In a method for preventing, reducing the incidence or
recurrence of, inhibiting,
treating, ameliorating or reducing the severity of, or reversing oral
mucositis or severe oral
nnucositis, the method can comprise the step of:
- Providing a sublingual controlled release dosage form comprising a FIASMA
as an
active ingredient, and
- administering to a patient in need thereof, such as a patient undergoing
radiation
treatment for head and neck cancer, a controlled release sublingual dosage
form
comprising a pharmaceutically effective amount of one or more active
ingredients,
wherein at least one active ingredient is a FIASMA.
6
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00027]
Controlled release dosage forms for sublingual administration are known in
the art and can slow the dissolution of the dosage form so that release of the
drug is extended
overtime, retaining presence or increasing residence time of the active drug
in the oral cavity.
Such controlled release dosage forms can be a tablet, capsule, lozenge,
pastille, troche, or
thin film strip or the like as recognized be persons of ordinary sill in the
pharmaceutical arts.
[00028]
It would be understood that applying or administering the composition
prior
to radiation therapy can include retention of the composition at the location
during the
radiation therapy session. The method can be repeated for each radiation
therapy session
and can include administering or applying the composition at least one time
per week, several
times per week, daily, or multiple times daily, before, after, or between
radiation therapy
sessions.
[00029]
Alternatively, a method according to the subject invention can comprise
orally
administering a composition comprising an effective amount of at least one
FIASMA. Such
oral composition can be an immediate release or controlled release dosage
form. For
conditions affecting the oral cavity, such as oral nnucositis or severe oral
nnucositis, oral
dosage forms that dissolve in the oral cavity, and are not swallowed whole,
are preferred.
[00030]
In a patient undergoing radiation therapy in the pelvic area, radiation
proctopathy, a preferred method can comprise providing a solid suppository or
rectal plug
dosage form comprising a composition comprising a FIASMA and placing the
dosage form
within the rectal cavity for a period of time required for delivery of the
active ingredient to
the site.
[00031]
The subject invention also concerns a topical pharmaceutical composition
comprising as an active drug or active substance, at least one functional
inhibitor of acid
sphingonnyelinase (FIASMA), useful for preventing, reducing the incidence or
recurrence of,
treating, ameliorating or reducing the severity of, or reversing, Cutaneous
Radiation Injury
(CRI), including radiation dermatitis or radiation proctopathy.
[00032]
The invention can also include a suppository dosage form for use in
preventing,
reducing the incidence or recurrence of, treating, ameliorating or reducing
the severity of, or
reversing, radiation proctopathy. A suppository dosage form can comprise a
soluble base and
7
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
a TCA, e.g., amitriptyline, an SSRI, such as sertraline, or a combination of
one or more TCA or
SSRI, and can further include other pharmaceutically acceptable excipients as
conventionally
employed in suppository dosage forms.
[00033]
In the method employing a suppository or a rectal plug delivery device for
delivery of the FIASMA to the anorectal cavity and in situ administration of
the drug, the
dosage form can be provided with or incorporated into the rectal plug delivery
device as a
controlled release formulation, having one or more excipient that can retard
the release of
active drug incorporated into the dosage form or which is coated onto the
outer surface of
the dosage form or a particle, granule or bead within the dosage form
comprising the active
ingredient. A controlled release dosage form can also be provided for oral
administration
wherein the oral dosage form is formulated as a delayed release dosage form
which bypasses
the acidic environment in the stomach and releases active ingredient in
intestinal tract where
the pH is above 7Ø Such controlled release dosage forms can be formulated
using an enteric
coating on a tablet, provided within delayed release capsule or caplet,
formulated in a slow
release matrix composition, or a combination of the above.
[00034]
Where the dosage form is a rectal plug delivery device, the device can be
an
insoluble plug forming a housing which has an internal chamber for containing
therewithin a
composition comprising the FIASMA, which can be a TCA, an SSRI, or various
combinations
thereof. For example, the chamber of the rectal plug can be filled with a
viscous composition
comprising a TCA or can contain a suppository dosage form comprising the TCA
active
ingredient.
[00035]
An alternative embodiment of a rectal plug delivery device useful for
carrying
out the method of the invention comprises a porous compressible foam material
infused or
coated with a composition comprising the TCA, e.g., annitriptyline, the SSRI,
e.g., sertraline, or
combinations thereof. The devices described for the method of the invention
can contain an
effective dose of the FIASMA or FIASMA combination between about 0.1 mg to
about 1000
mg or greater.
[00036]
A composition in accordance with the invention can also include two or
more
active pharmaceutical ingredients formulated or mixed together in a single
fixed-dose
8
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
composition, such as a composition comprising two or more different TCAs, two
or more
different SSR1s, or one or more TCA in combination with one or more SSRI or
the like.
[00037]
A composition for use in a method of the invention can comprise between
about 0.1-1000 milligrams (mg) of a FIASMA. A topical composition useful in
accordance with
a method of the invention can be provided as a topical liquid, but is
preferably formulated as
a topical cream, gel, lotion, or ointment. One preferred FIASMA useful as an
active ingredient
in a composition and method of the invention is the SSRI, sertraline. Another
FIASMA useful
as an active ingredient in a composition and method of the invention is the
TCA, annitriptyline.
Other FIASMA compounds may be substituted or can be provided in combination in
a
composition or method of the invention.
[00038]
The topical dosage form can comprise a conventional and commercially
available pharmaceutical base composition pharmaceutically compatible with the
one or
more FIASMA used as the one or more active ingredients, and can further
include other
pharmaceutically acceptable excipients as conventionally employed in topical
or oral dosage
forms. These commercially available pharmaceutical bases can serve as a
vehicle or medium
for the active drug substance and can comprise one or more of an emollient,
penetration
enhancer, solvent, gelling agent, thickening agent or viscosity-enhancing
agent, preservative,
or other suitable excipient commonly used in topical preparations.
[00039]
Preferably, the FIASMA is provided in an immediate-release topical
formulation which allows targeted dosing and immediate delivery of the drug
from the
composition to the skin upon application or administration of the composition.
Retention of
the FIASMA component at the site of administration or application can be
preferred.
Accordingly, a composition of the invention may exclude a penetration enhancer
to maintain
the composition, and the active substance, on the surface of the skin for a
longer period of
time relative to a composition comprising a penetration enhancer which allows
or facilitates
penetration of the active substance into or below the skin where it can be
absorbed
systemically. Alternatively, in certain instances, a penetration enhancer may
be preferred
and employed within the composition.
9
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00040]
An oral dosage form of a composition can be immediate-release or may be
formulated with excipients, such as polymers, gels, gums, waxes, or the like,
or incorporated
into a matrix or comprising a coating to slow, delay, sustain, or extend
release of the active
ingredient from the composition following oral administration. For example, a
solid dosage
form for sublingual administration of the FIASMA in treating oral nnucositis
can be formulated
for dissolving within the oral cavity, and can include excipients which
control, delay, slow,
sustain or extend the release of the drug from the dosage form.
[00041]
According to a further aspect of the invention, the dosage form can
include a
second active, such as a TCA or SSRI ingredient, or can comprise a second
active which is not
a TCA or SSRI, for example, is an active ingredient in different class of
drug. In a preferred
embodiment, a composition comprises a first active ingredient which is a TCA
and a second
active ingredient which is a different TCA or is an SSRI or other class of
compound which is
not a TCA. For example, a second active ingredient can be an antioxidant, such
as Vitamin A,
Vitamin C, Vitamin D, or Vitamin E. Preferably, the second active in the
composition is not an
anti-inflammatory agent such as a non-steroidal anti-inflammatory drug (NSAID)
nor an
anesthetic agent, such as a local anesthetic, e.g., lidocaine.
[00042]
In another preferred embodiment, a composition of the invention comprises
a
first active ingredient which is an SSRI and a second active ingredient which
is a different SSRI,
or is a TCA or other class of compound which is not an SSRI. For example, a
second active
ingredient can be an antioxidant, such as Vitamin A, Vitamin C, Vitamin D, or
Vitamin E.
Preferably, the second active in the composition is not an anti-inflammatory
agent such as a
non-steroidal anti-inflammatory drug (NSAID) nor an
anesthetic agent, such as a local
anesthetic, e.g., lidocaine.
[00043]
Preferably, the method is carried out using a FIASMA formulated in a
topical
composition which can deliver an effective dose to a target area of the skin
which is directly
or collaterally exposed to radiation during radiation therapy or treatment.
For example, the
active ingredient can be formulated as a liquid composition and administered
as drops to the
target area of the skin. Alternatively, the FIASMA can be formulated in a
composition
comprising a thickening agent or viscosity-enhancing agent as a lotion, cream,
ointment or
gel for topical delivery of the FIASMA to the target skin area of the patient.
The topical
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
formulation can be rubbed onto or into the target area of the skin prior to or
following
radiation therapy.
[00044]
In the method employing a solid oral dosage form, the active ingredient
can be
provided as a particle, granule or bead and manufactured in the form of a
tablet, capsule,
caplet or the like, as would be readily understood in the art. The oral dosage
form can be
provided, for example, as a controlled release dosage form where the active
ingredient is
formulated in a delayed release dosage form, such as an enteric coated tablet,
delayed
release capsule or caplet, or can be formulated in a slow release matrix
composition.
Combinations of these controlled release dosage forms can also be employed.
[00045]
Thus, in accordance with the invention, the method for preventing,
reducing
the incidence or recurrence of, inhibiting, treating, ameliorating or reducing
the severity of,
or reversing, cutaneous radiation injury (CRI), comprises topically
administering to a target
area of skin of a patient in need thereof at least one time per day prior to,
during, and after
radiation treatment, 0.5-50 ml or more, depending on the size of the area to
be treated, a
composition comprising an effective amount, e.g., from about 0.5% to about 5%
w/w, of a
FIASMA, such as a TCA (e.g., annitriptyline) or an SSRI (e.g., sertraline.)
[00046]
The method for preventing, treating, or ameliorating radiation dermatitis
also
comprises topically administering to a target area of skin of a patient in
need thereof at least
one time per day prior to, during, and after radiation treatment, 0.5-50 ml or
more, depending
on the size of the area to be treated, a composition comprising an effective
amount, e.g., from
about 0.5% to about 5% w/w, of a FIASMA, such as a TCA (e.g., a nnitriptyline)
or an SSRI (e.g.,
se rtra line.)
[00047]
The method also includes preventing, treating, or ameliorating radiation
proctopathy, by topically administering or delivering to a target area a
patient in need thereof
at least one time per day prior to, during, and after radiation treatment, 0.5-
50 ml or more,
depending on the size of the area to be treated, a composition comprising an
effective
amount, e.g., from about 0.5% to about 5% w/w, of a FIASMA, such as a TCA
(e.g.,
annitriptyline) or an SSRI (e.g., sertraline.)
11
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00048]
A topical composition employed in the method can comprise 5-50 mg
annitriptyline per ml of the composition or can comprise 5-50 mg sertraline
per ml of the
composition or can comprise 10-40 mg annitriptyline per ml of the composition
and 10-40 mg
sertraline per ml of the composition.
[00049]
The above topical compositions can also include an antioxidant such as
Vitamin
A, Vitamin C, Vitamin D, and Vitamin E.
[00050]
Preferably, the composition used in the method of the invention is
formulated
as a topical dosage form which delivers the effective dose of active
ingredient in situ to the
target area of the patient. This can be carried out using a composition
formulated with a
pharmaceutically acceptable base to form a lotion, cream, ointment, or gel for
topical delivery
of the active ingredient to a target area of skin of the patient.
[00051]
Alternatively, the composition can comprise amitriptyline and a second
tricyclic antidepressant which is not annitriptyline, such as annineptine,
annitriptyline,
annoxapine, butriptyline, clonniprannine, desiprannine, dibenzepin, dosulepin,
doxepin (in
combination but not alone), inniprannine, iprindole, lofeprannine,
nnaprotiline,
norclonniprannine, northiaden, nortriptyline, opiprannol, protriptyline,
tianeptine, or
trimipramine.
[00052]
The composition comprising sertraline can comprise a second selective
serotonin reuptake inhibitor which is not sertraline, such as citaloprann,
escitaloprann,
fluoxetine, fluvoxannine, or paroxetine.
[00053]
The pharmaceutical composition of the invention can be provided as a
topical
pharmaceutical composition or can be an oral dosage form comprising an
effective amount
of the one or more active ingredient, such as annitriptyline, sertraline, or a
combination
thereof for delivery of the one or more active ingredients to the patient. In
a preferred
embodiment, a topical composition of the invention is free of, does not
include, or excludes,
an anti-inflammatory agent and an anesthetic agent.
[00054]
A preferred topical pharmaceutical composition comprises 10-20 mg
annitriptyline per ml of the composition, or 10-50 mg sertraline per ml of the
composition, or
12
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
15-20 mg amitriptyline per ml of the composition and 20-40 mg sertraline per
ml of the
composition. Any of the above can further comprise an effective amount of an
antioxidant
such as Vitamin A, Vitamin C, Vitamin D, or Vitamin E.
[00055]
An oral dosage form of the invention can be formulated as a controlled
release
oral dosage form.
[00056]
It would be understood that the method and compositions and dosage forms
of the invention can be useful for preventing, treating or ameliorating other
dermal conditions
such as atopic dermatitis, thermal burn, sunburn, dermatomyo-fibromas,
exposure-induced
wrinkles, or the like.
13
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
BRIEF DESCRIPTION OF THE DRAWINGS
[00057]
FIG. 1 shows perspective view of a rectal plug device having a projecting
member and a flange member, where the projecting member comprises a housing
depicted
as having pores and bounding a chamber for containing a soluble dosage form
comprising at
least one active ingredient, such as a TCA, an SSRI, or a combination thereof.
[00058]
FIG. 2 shows a rectal plug of FIG. 1 in cross-section, illustrating the
dosage
form disposed within the chamber.
[00059]
FIG. 3 shows a perspective view of an alternative embodiment of a rectal
plug
of the invention comprising a porous compressible foam material infused or
coated with a
composition comprising a TCA or SSRI or combination and is depicted in its
expanded
configuration after administration.
[00060]
FIG. 4 shows a perspective view of the porous compressible foam rectal
plug
of FIG. 3, in a compressed configuration prior to administration.
[00061]
Figure 5 shows the results upon examination of skin injury after 30 Gy
focal
irradiation. Panel A of FIG. 5 is a summary of the skin injury scores for
individual mice up to
4 weeks after 30 Gy. Panel B is graphical representation of the observed
changes in the
radiation skin injury score over time. Data are presented as mean +/- SEM. n=5
mice per
treatment group.
[00062]
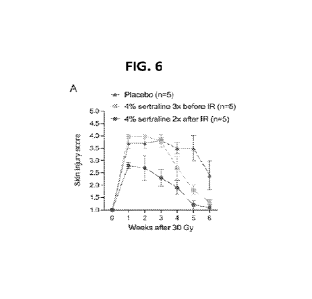
Figure 6 shows the results of examination of skin injury after 30 Gy focal
irradiation. Panel A of FIG. 6 is a summary of skin injury scores for mice
treated with 4%
sertraline 3 times before irradiation, 4% sertraline 2 times after irradiation
or placebo 5 times
before and after irradiation. Skin injury scores were examined every week for
up to 6 weeks
after irradiation. Panels B ¨ D of FIG. 6 illustrate comparisons of skin
injury scores over time
between cohorts of mice that received different treatments. Data are presented
as mean +/-
SEM. n=5 mice per treatment group. *P<0.05 by two-way ANOVA with Bonferroni
post-hoc
test.
14
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
DETAILED DESCRIPTION OF THE INVENTION
[00063]
The present invention provides an innovative method for preventing,
reducing
the incidence or recurrence of, inhibiting, treating, ameliorating or reducing
the severity of,
or reversing a skin condition in a mammal, including humans, and can employ
the use of a
novel composition or dosage form to carry out the method of treatment.
[00064]
Hereinafter, the terms "treat," "treating," or "treatment of" in relation
to
Cutaneous Radiation Injury, or "CRI," or a specific condition considered a
type of CRI, can
mean or refer to, or can be substituted by, any one of "preventing," "reducing
the incidence
of" "reducing the recurrence of," "inhibiting," "ameliorating," "reducing the
severity of,"
"reversing" or "treating" CRI. The specific term, "preventing," "reducing the
incidence of"
"reducing the recurrence of," "inhibiting," "ameliorating," "reducing the
severity of," or
"reversing" CRI is used where it is intended to designate that treatment only,
or a term may
be expressly excluded from "treatment" when intended not to mean that term.
For example,
a method for "reducing the incidence of" may be used to mean only reducing the
incidence
of CRI and not "inhibiting" CRI. Alternatively, "a method for treating CRI,
excluding reducing
the occurrence of CRI" can mean a method for preventing, reducing the
incidence of,
inhibiting, ameliorating, reducing the severity of, or reversing CRI.
[00065]
The term "Cutaneous Radiation Injury," or "CRI" is accepted in the medical
field as being defined as damage to skin or underlying tissue in animals,
including mammals
and humans, which is induced by, or results from, exposure to radiation from
industrial or
military-related sources, e.g., radioactive materials used in nuclear power
plants or in nuclear
weapons, or in medical radiation therapy. Therefore, the term CRI can include
skin conditions
such as Radiation Dermatitis or Radiation Proctopathy, observed following
medical radiation
therapy. Mild skin damage, e.g., sunburn from overexposure to the sun, is not
generally
considered to fall within the definition of CRI.
[00066]
"Radiation dermatitis" refers to a disorder of the skin resulting from
radiation
therapy of an area of the body. For example, radiation therapy is a known and
accepted
treatment for cancerous breast tissue in women and men. Inflammation of the
skin covering
the targeted cancerous tissue, and exposed to the radiation, can result in
damage to the skin.
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
These signs and symptoms are known as radiation dermatitis. The term is used
herein as it is
clinically understood by dermatologists and other physicians that treat such
disorders.
[00067]
"Radiation proctopathy" or its synonym, "radiation proctitis," refers to a
disorder of the rectum causing alteration of rectal function, including
inflammation of the
lining of the rectum, resulting from radiation therapy of the pelvic area.
Radiation therapy of
the pelvic area is a known and accepted treatment procedure for cancer of
urogenital tissue,
for example, treating prostate cancer in men, or treating cervical cancer in
women. The term
is used herein as it is clinically understood by physicians that treat such
disorders
[00068]
"Oral mucositis" refers to a condition that results from radiation therapy
used
in treating head and neck cancers, A more severe condition aptly termed
"Severe Oral
Mucositis" or "SOM" can often be associated with ulcerative tissue in the
mouth or when
patients cannot swallow food or drink. Oral nnucositis is a common,
debilitating complication
of cancer treatments, particularly radiation. It can lead to several problems,
including pain,
nutritional problems as a result of inability to eat, and increased risk of
infection due to open
sores in the nnucosa.
[00069]
One embodiment of the invention concerns a method for treating Cutaneous
Radiation Injury (CRI) by administering an effective amount or dose of a
Functional Inhibitor
of Acid Sphingomyelinase (FIASMA). Examples of FIASMAs include certain
selective serotonin
reuptake inhibitors (SSR1s), tricyclic antidepressants (TCAs), antihistamines,
antiarrhythnnics,
antipsychotics, antidiarrheals, adrenergic receptor blockers (ARBs), beta
blockers, Estrogen
Receptor Modulators, and the like.
[00070]
Examples of SSRIs useful in accordance with the subject invention are
citaloprann, escitaloprann, fluoxetine, fluvoxannine, paroxetine, and
sertraline. Preferred SSRIs
useful in accordance with the subject invention include fluoxetine,
fluvoxannine, paroxetine,
and sertraline.
[00071]
Examples of TCAs useful in accordance with the subject invention are
annineptine, annitriptyline, annoxapine, butriptyline, clonniprannine,
desiprannine, dibenzepin,
dosulepin, doxepin (in combination but not alone), imiprannine, iprindole,
lofepramine,
nnaprotiline, norclonniprannine, northiaden, nortriptyline, opipramol,
protriptyline,
16
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
tianeptine, and trimipramine. Preferred TCAs useful in accordance with the
subject invention
are annitriptyline, desiprannine, and inniprannine.
[00072]
The invention includes the use of at least one FIASMA, such as a TCA or
and
SSRI and can include the use of at least two different FIASMAs from the same
class of drug or
at least two different FIASMAs from different classes of drug. For example,
the subject
invention can include treatment of CRI using at least two TCAs, at least one
TCA and at least
one SSRI, or at least one TCA or SSRI and at least one additional active
ingredient which is not
a TCA or SSRI.
[00073]
A preferred TCA for use in accordance with the subject invention is
annitriptyline. Amitriptyline, marketed in the United States under the brand
name ELAVIL
(AstraZeneca PLC, Cambridge England) is primarily used to treat a number of
mental illnesses,
including major depressive disorder, anxiety disorders, and less commonly
attention deficit
hyperactivity disorder (ADHD) and bipolar disorder.
Other uses include prevention
of migraines, treatment of neuropathic pain such as fibronnyalgia and
postherpetic neuralgia,
and less commonly insomnia.
[00074]
A preferred SSRI for use in accordance with the subject invention is
sertraline.
Sertraline, marketed in the United States under the brand name Zoloft
(Pfizer, New York, NY
USA) is primarily used to treat depression, panic attacks, obsessive
compulsive disorder, post-
traumatic stress disorder, social anxiety disorder (social phobia), and a
severe form of
premenstrual syndrome (prernenstruEll dysphoric disorder).
[00075]
Annitriptyline and sertra line have not previously been known to be useful
for
prevention or treatment of radiation dermatitis.
[00076]
In a preferred embodiment, at least one FIASMA can be incorporated into a
topical pharmaceutical composition for administration by placement or
application onto the
surface of the skin for the prevention, amelioration, or treatment of a dermal
condition or
disorder, such as radiation dermatitis. This composition can be administered
once daily, or
more or less frequently as prescribed by the patient's physician or healthcare
provider.
17
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00077]
Topical compositions or preparations as dosage forms are well known in the
art and are used conventionally in medical treatments. A preferred embodiment
in
accordance with the subject invention is to incorporate a FIASMA into a
pharmaceutically
compatible base composition to make a topical lotion, cream, gel, or ointment
comprising the
at least one FIASMA as an active pharmaceutical ingredient (API) or agent.
Another
embodiment of the invention includes incorporating at least two different
FIASMAs, e.g., a
combination of at least one TCA and at least one SSRI, as active agents into a
pharmaceutically
compatible base composition to make a topical lotion, cream, gel, or ointment.
A preferred
embodiment comprises the one or more active ingredients thoroughly mixed into
a
pharmaceutically acceptable base to provide a homogeneous mixture. For active
ingredients
that incompletely solubilize in the base, a suspension can be formed, wherein
the suspension
comprises an active ingredient which is thoroughly mixed to evenly disperse
the active
throughout the base. The composition of the invention can include other
pharmaceutically
acceptable excipients as conventionally employed in topical dosage forms.
[00078]
In another embodiment of the invention, the active ingredient, such as one
or
more TCA or SSRI can be formulated for oral administration. In one embodiment,
the oral
dose is provided as an immediate release dosage form. In one embodiment, the
oral dose is
provided as controlled release dosage form.
[00079]
In one embodiment, an oral dosage form for sublingual administration
of the invention includes a pharmaceutical composition in unit dosage form
(e.g., a lozenge,
a pill, a tablet, a film, or a strip) comprising at least one FIASMA and in a
preferred
embodiment is prepared as a controlled release formulation. Regarding the size
of the unit
dosage form, e.g., lozenges, the simplicity and cost of production will also
be taken into
account, as well as the preferences of consumers. The size of the sublingual
dosage form can
vary from approximately 5 mm to 20 mm, or from approximately 5 mm to 18 mm, or
from
approximately 7 mm to 18 mm, or from approximately 10 mm to 15 mm. If the
sublingual
dosage form has a round or almost round shape, then the size range is a
suitable diameter
value. The thickness of the lozenges in most cases will be from 1 mm to 10 mm,
and more
generally from 3 mm to 5 mm.
18
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00080]
A sublingual dosage form can be in the form of monolayer or bilayer. A
sublingual dosage form having soluble nnucoadhesive properties can be made by
pressing
powders, including nnucoadhesive hydrocolloids. As an example, an adhesive
pastil can be
made by mixing dry powders, including gum arabic for adhesive properties and
at least one
FIASMA as an active ingredient. Residence time in the oral cavity for the
sublingual dosage
forms can be optimized according to the dissolution rate and total time of
dissolution for the
product. For example a sublingual controlled release lozenge or film can be
prepared to have
a residence time in the oral cavity of at least one minute, preferably between
one to 30
minutes, and more preferably 5 -15 minutes after administration.
[00081] In another aspect, the invention features a pharmaceutical composition
in unit dosage form
formulated for sublingual administration, the unit dosage form including from
5 to 50 mg of a FIASMA
(e.g., a dosage form comprising 5 mg, 10 mg, 15 mg, 20 mg, 25 mg, 30 mg, 35
mg, 40 mg, 45 mg, or SO
mg of sertraline.
[00082]
Controlled release oral dosage forms include delayed release formulations
created by coating a solid dosage form with a polymeric film, which is
insoluble in the acidic
environment of the stomach, and soluble in the neutral environment of the
small intestine.
[00083]
Composition of the invention may include conventional additives or other
excipients, such as plasticizers, pigments, colorants, stabilizing agents,
glidants, etc.
[00084]
The present invention provides a method for preventing, ameliorating, or
treating CRI by administration of a FIASMA. The method includes a step of
providing a
composition comprising between about 0.001 - 100 grams of active ingredient
and delivering
the drug to a patient in need thereof, either directly to the skin as a
topical composition, such
as a cream or lotion or ointment, or formulated in an oral dosage form.
[00085]
According to one preferred embodiment, the FIASMA contained in the topical
dosage form is a nnitriptyline provided at a dosage of between 1 ¨ 1000 mg;
more preferably
a dose of about 5 mg ¨ 150 mg, and most preferably a dose of about 10-100 mg,
administered
as about 0.5% to about 5% w/w topical composition. For example, a composition
comprising
1% to 5% active ingredient (10-50 mg of active ingredient per 1 ml of the
composition) can be
administered in amounts of about 0.5 ml to about 5 ml per topical application.
Preferably,
19
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
the composition is topically administered in about 1 ml to about 2 ml amounts
to deliver 10-
100 mg of the active ingredient to the skin for preventing or treating CRI.
[00086]
One preferred dose is administering at least 1 ml of a composition
comprising
1% of a FIASMA as an active ingredient according to the subject invention.
Another preferred
dose is administering at least 1 ml of a 2% w/w composition comprising a
FIASMA active
ingredient according to the subject invention. Yet another preferred dose is
administering at
least 1 ml of a 3% w/w composition comprising a FIASMA active ingredient
according to the
subject invention. Still another preferred dose is administering at least 1 ml
of a 4% w/w
composition comprising a FIASMA active ingredient according to the subject
invention. And
a further preferred dose is administering at least 1 ml of a 5% w/w
composition comprising a
FIASMA active ingredient according to the subject invention. It would be
understood that the
concentration of drug can be provided at incremental increases of about 0.1 %.
As a non-
limiting illustration, the FIASMA can be provided in a concentration of 1.1%,
1.2%, 1.3%, etc.,
up to 5%, as desired to obtain the intended effect without negative side-
effects.
[00087]
The dose contained within the dosage form can vary, being lower in dosage
forms that more rapidly deliver drug to the site, or higher in dosage forms
that slowly release
drug to the site. Dose variation can also depend on the active ingredient or
ingredients
contained within the composition. For example, a composition comprising more
than one
FIASMA can include each active at less than 1%, such as 0.1%-0.9%, preferably
about 0.5% of
each active, such that the final composition comprises 1% to less than 2%
total active
ingredient in the composition. This can advantageously lower the dose of each
active
ingredient being administered to the patient while providing an equivalent or
substantially
equivalent preventive or therapeutic effect. It is also contemplated that a
fixed dose
combination product comprising at least one TCA and at least one SSRI can
provide a
synergistic effect, wherein the efficacy is greater than the expected additive
effect from the
respective active ingredients used alone.
[00088]
In one embodiment, the FIASMA is provided in an oral dosage form as a
controlled-release formulation which allows delivery of the drug over a period
of time as
compared to an immediate-release formulation which provides delivery of all
the drug from
the dosage form as soon as it is administered. Controlled release formulations
known in the
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
art include the use of coatings, such as enteric-coated tablets, beads, or
pellets, ion exchange
resins, waxes, alginates, gelling agents, such as cellulosic hydrogels (e.g.,
hydroxypropyl
methyl cellulose, or HPMC) or polymeric acrylannides (e.g., CARBOPOL)
formulated with an
active agent, and a suitable vehicle.
[00089]
The present invention provides a method for treating anorectal disorders
by in
situ administration of an active pharmaceutical ingredient of the invention,
such as a TCA,
e.g., annitriptyline, an SSRI, e.g., sertraline, or combinations thereof. The
method includes a
step of providing a composition comprising between about 0.001 - 100 grams of
the active
ingredient, and delivering the drug directly to the anorectal area, either as
a topical
composition, such as a cream or ointment, or formulated in a suppository or
rectal plug
dosage form. According to a preferred embodiment, a TCA contained within the
suppository
or rectal plug dosage form is annitriptyline at a dosage of between 1 ¨ 1000
milligrams (mg);
more preferably a dose of about 5 mg ¨ 150 mg, and most preferably a dose of
about 10-100
mg, where one preferred dosage is a suppository or other dosage form
comprising about 50
mg. In another preferred embodiment, an SSRI contained within the suppository
or rectal
plug dosage form is sertraline at a dosage of between 1 ¨ 1000 milligrams
(mg); more
preferably a dose of about 5 mg ¨ 150 mg, and most preferably a dose of about
10-100 mg,
where one preferred dosage is a suppository or other dosage form comprising
about 50 mg.
The dose contained within the suppository or rectal plug can vary, being lower
in dosage
forms that more rapidly deliver drug to the site, or higher in dosage forms
that slowly release
drug to the site. In addition, where two or more active pharmaceutical
ingredient are
provided in a fixed dose combination suppository product, the dose of each of
the active
ingredients may be reduced within the dosage form and thereby advantageously
lower the
risk or incidence of side effects.
[00090]
Preferably, the method comprises providing a solid suppository or rectal
plug
dosage form comprising a composition having a TCA or SSRI as an active
ingredient and
placing the dosage form within the rectal cavity for a period of time required
for delivery of
the active ingredient to the site. The composition provided within the rectal
plug delivery
device can be a suppository.
21
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00091]
Alternatively, the drug can be delivered using a rectal delivery device
such as
a rectal plug containing an effective dose of active ingredient, such as a
FIASMA, or a
combination more than one FIASMA. In one embodiment of a rectal plug device
according to
the invention, the plug device comprises an insoluble porous housing forming a
chamber for
containing the active ingredient. The active ingredient can be provided as a
soluble
suppository disposed within a chamber portion formed within the porous rectal
plug housing.
The suppository is exposed to bodily fluids when administered to cause
dissolution and in situ
delivery of the active ingredient from the suppository. In a preferred
embodiment, the
suppository is formulated as a controlled release formulation. Alternatively,
the active
ingredient can be formulated as a viscous, controlled release gel, ointment,
or cream for filling
the chamber within the rectal plug housing.
[00092]
Other types of rectal plugs are known and can be adapted for use in
accordance with the subject invention. For example, rectal plugs formed of a
porous sponge-
like polymeric material, such as polyurethane foam, are known for use in fecal
incontinence.
Such rectal plugs are marketed under the name PERISTEENTm Anal Plug (Coloplast
Corp.,
North Minneapolis, MN, USA). The polyurethane foam can be infused with a
solution
comprising an effective amount of a FIASMA can be adsorbed onto the material
for delivery
when inserted into the rectal cavity. These commercially available rectal
plugs generally are
provided in a compressed configuration for ease of insertion and are wrapped
with a water-
soluble film which dissolves following insertion and can expand to an extended
configuration.
Such compression and expansion are not required for in situ delivery of drug
in accordance
with the subject invention
[00093]
FIGs. 1 and 2 illustrate one example of a rectal plug delivery device for
use in
accordance with the invention. FIG. 1 shows a rectal plug delivery device 100
comprising an
elongate member 101 which forms the proximal end of the device which is
inserted into and
resides within the rectal cavity during delivery of the active ingredient. The
rectal plug
delivery device can comprise a distal flange 102 to prevent the entirety of
the rectal plug
delivery device from being taken up into the rectal cavity, thereby holding
the rectal plug
device in place during administration of the active ingredient. The active
ingredient, such as
a TCA or SSRI, can be provided as a pharmaceutical composition which is housed
within a
cavity (not shown) formed within the elongate member. The proximal end of the
elongate
22
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
member 101 comprises pores 103 for allowing bodily fluids to enter the cavity
formed within
the elongate member, and to allowing egress of the active ingredient from the
composition
contained within the elongate member. The rectal plug delivery device can have
an extension
piece connected to the distal flange, such as a strap or string, to facilitate
removal of the rectal
plug delivery device, functioning similarly to removal of a tampon.
[00094]
FIG. 2 shows a sectioned view of the rectal plug delivery device 100 of
FIG. 1,
illustrating the cavity formed within the proximal end of the elongate member
101, which can
contain the composition 104 comprising active pharmaceutical ingredient. As
illustrated, the
composition can be formed as a suppository, which dissolves or melts when
administered
within the rectal cavity. Alternatively, the composition can be an amorphous,
or unformed,
composition comprising a gel, cream, or ointment. Preferably, the composition
is provided
as a controlled-release composition, preferably an extended-release
composition, which
releases the active ingredient from the composition over time.
[00095]
In another embodiment, the dosage form comprises an anal plug comprising a
soft, compressible, porous material (e.g., a foam rubber or polymeric sponge-
like material)
wherein the active ingredient is infiltrated or infused within, or coated on,
the porous
compressible plug material. This embodiment is illustrated in FIGs. 3 and 4.
[00096]
FIG. 3 shows a rectal plug delivery device 200 comprising a soft,
compressible,
porous material in an expanded configuration. The rectal plug delivery device
200 comprises
at its proximal end a drug delivery component 201 which expands when inserted
into the
rectal cavity, and conforms to the shape of the rectal cavity. Connected to
the drug delivery
component is an extension piece 202, such as a strap or string, to facilitate
removal of the
rectal plug delivery device, functioning similarly to removal of a tampon.
[00097]
The composition comprising active ingredient according to the invention
can
be infused or infiltrated into the porous material forming the delivery
component 201, as a
liquid or gel, cream, or ointment. The composition comprising the active
ingredient can be
provided as a controlled-release composition, preferably an extended-release
composition,
which releases the active ingredient over time.
23
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[00098]
FIG. 4 shows the rectal plug delivery device 200 of FIG. 3, wherein drug
delivery
component 201 is provided in compressed form, for ease of insertion into the
rectal cavity.
The compressed drug delivery component 201 can include a water-soluble wrapper
surrounding the drug delivery component 201. Following insertion of the rectal
plug delivery
device embodiment 200 into the rectal cavity, bodily fluids dissolve the
wrapper and allow
the drug delivery component 201 to expand to its expanded configuration as
shown in FIG. 3.
Fig. 4 further shows extension piece 202, which can be a strap or string, to
facilitate removal
of the rectal plug delivery device.
[00099]
Preferably, the active ingredient is provided in a controlled-release
formulation which allows delivery of the drug over a period of time as
compared to an
immediate-release formulation which provides delivery of all the drug from the
dosage form
as soon as it is administered. Controlled release formulations known in the
art include the
use of coatings, such as enteric-coated tablets, beads, or pellets, ion
exchange resins, waxes,
alginates, gelling agents, such as cellulosic hydrogels (e.g., hydroxypropyl
methyl cellulose, or
HPMC) or polymeric acrylannides (e.g., CARBOPOL ) formulated with an active
agent, and a
suitable vehicle which melts or dissolves in rectal fluids. Such formulations
and compositions,
as well as methods of manufacturing suppositories or other controlled-release
compositions
are well known in the art
[000100]
In a method according to the invention, treating a CRI such radiation
dermatitis
employs a composition comprising at least one FIASMA, such as a TCA, e.g.,
annitriptyline,
and/or at least one second FIASMA, such as an SSRI, e.g., sertraline. The
composition
employed in the method of the invention can be provided in a topical dosage
form. In
addition, the subject invention comprises a method for treating, ameliorating
or preventing
a skin disorder or condition such radiation dermatitis using an oral dosage
form comprising at
least one TCA such as annitriptyline, and/or at least one SSRI such as
sertraline.
[000101]
Preferably, the method comprises providing a topical dosage form
comprising
a composition having at least one FIASMA as an active ingredient, in a
composition, and
placing an effective amount of the composition onto a target area of skin to
be treated, for a
period of time required for delivery of the active ingredient to the site. In
one preferred
embodiment, the active ingredient or ingredients can be formulated as a
viscous, controlled
24
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
release gel, ointment, or cream for administration to the skin. A topical
composition of the
invention preferably comprises about 0.1% concentration (1 nnennl of the
composition) up to
about 5% concentration (50 nnennl of the composition) of each active
ingredient provided;
for example, 0.1%. 0.2%, 0.3%, 0.4%, .05%, 0.6%, 0.7%, 0.8%, 0.9%, 1.0% and
1.1%, 1.2%,
1.3%, and the like in 0.1 % increments up to about 5.0%. A typical dose is
about 0.5 ml to
about 2 ml, preferably about 1 ml, but is not so limited, and is bounded only
by the area of
skin needed to be treated, such that a dose of 5 ml or greater can be applied.
[000102]
According to one embodiment of the invention, the method of the invention
comprises the step of:
topically administering an effective dose of one or more active ingredients,
namely a
drug which is a FIASMA, to the skin of a patient in need thereof, such as a
patient who
is suffering from CRI following exposure to environmental radiation or a
patient that
will undergo or is undergoing medical radiation therapy and is suffering from
radiation
dermatitis or radiation proctopathy.
[000103]
In a method for preventing, reducing the incidence or recurrence of,
inhibiting,
treating, ameliorating or reducing the severity of, or reversing oral
nnucositis or severe oral
nnucositis, the method can comprise the step of:
- Providing a sublingual controlled release dosage form comprising a FIASMA
as an
active ingredient, and
- administering to a patient in need thereof, such as a patient undergoing
radiation
treatment for head and neck cancer, a controlled release sublingual dosage
form
comprising a pharmaceutically effective amount of one or more active
ingredients,
wherein at least one active ingredient is a FIASMA.
[000104]
In a method for treating radiation proctopathy, the method of the
invention
comprises the step of:
anorectally administering an effective dose of one or more FIASMA drugs as an
active
ingredient, preferably a drug in the class selected from a TCA, an SSRI, or a
combination
thereof, to the rectal cavity or rectal tissue of a patient in need thereof,
such as a patient
suffering from radiation proctopathy or proctitis resulting from medical
radiation
treatment.
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000105]
A preferred method for treating radiation proctopathy comprises
anorectally
administering an effective dose of a FIASMA as an active ingredient, such as
the TCA,
annitriptyline or the SSRI, sertraline. Compositions comprising a fixed dose
combination two
or more different FIASMAs, or one or more FIASMA with an active ingredient
which is not a
FIASMA can also be used.
In one preferred example, the method is carried out using
annitriptyline formulated as a composition and provided in dosage form which
is capable of
delivering an effective dose in situ to the rectal cavity of the patient. For
example,
annitriptyline can be formulated as a liquid and administered as an enema, or
can be
formulated with a thickening agent or viscosity-enhancing agent to provide a
lotion, cream,
ointment or gel for topical delivery of amitriptyline to an anorectal area and
rectal cavity of
the patient.
[000106]
Alternatively, the method can employ a semi-solid or solid dosage form,
such
as a suppository or a rectal plug delivery device for delivery of the active
pharmaceutical
ingredient to the anorectal cavity and in situ administration of the drug.
Preferably, the
dosage form provided with or incorporated into the rectal plug delivery device
is a controlled
release formulation, having excipients that can delay or retard the release of
active drug
incorporated into the dosage form or coated onto the outer surface of the
dosage form.
[000107]
Where the dosage form is a rectal plug delivery device, the device can be
an
insoluble plug forming a housing which has an internal chamber for containing
therewithin a
composition comprising the active pharmaceutical ingredient in accordance with
the
invention
For example, the chamber of the rectal plug can be filled with a viscous
composition comprising the FIASMA active ingredient or can contain a
suppository dosage
form comprising a FIASMA as the active ingredient.
[000108]
An alternative embodiment of a rectal plug delivery device useful for
carrying
out the method of the invention comprises a porous compressible foam material
infused or
coated with a composition comprising the active pharmaceutical ingredient in
accordance
with the subject invention. The devices described for the method of the
invention can contain
an effective dose of the FIASMA provided in mounts of between about 0.1 mg to
about 1000
mg or greater.
26
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000109]
The method of the invention can further include the administration to a
patient in need thereof of at least one active ingredient and an additional or
second active
ingredient which is not a FIASMA. Preferably, the additional active is
formulated together
with the at least one TCA, at least one SSRI, or a combination TCA and SSRI in
a fixed-dose
combination drug product.
[000110]
One preferred embodiment of composition having a first and second active
comprising a TCA or an SSRI and an anti-inflammatory, anesthetic agent, or an
antioxidant.
The anti-inflammatory can be a steroid or a non-steroidal anti-inflammatory
(NSAID).
Alternatively, the second active in the composition is not an anti-
inflammatory agent, wherein
the composition excludes a steroid compound or the composition excludes a non-
steroidal
anti-inflammatory drug (NSAID). The anesthetic agent can be a local anesthetic
commonly
used for topical administration, such as lidocaine. The antioxidant can be,
for example,
Vitamin A, Vitamin C, Vitamin D, and Vitamin E.
[000111]
The subject invention includes a composition for treating radiation
proctopathy comprising providing an effective amount of a FIASMA formulated as
a
controlled release formulation for in situ delivery of the active ingredient
to the rectal cavity.
In one preferred embodiment, the composition can be formulated as a controlled
release
suppository containing at least one TCA or at least one SSRI, or at least one
TCA and one SSRI,
as the active ingredient or ingredients. The composition can include an
additional drug which
is not a FIASMA as described herein.
[000112]
The active pharmaceutical ingredient contained in a suppository or rectal
delivery device can be administered prior to, during, or following pelvic
radiation therapy. A
preferred administration includes inserting the suppository or rectal delivery
device into the
rectal cavity and allowing the suppository or rectal delivery device to remain
within the rectal
cavity until delivery of the entire dose is completed. A preferred suppository
in accordance
with the invention can slowly dissolve and remain for a period of up to about
24 hours, i.e.,
daily use. A rectal plug delivery device can be retained within the rectal
cavity for a period
of up to about 48 hours but is preferred to include a drug formulation which
delivers the dose
of the active ingredient or ingredients within about 24 hours. This provides
for daily use
27
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
following a bowel movement. Alternatively, the rectal plug can be temporarily
removed for
bowel movements and re-inserted.
[000113]
The suppository containing active pharmaceutical ingredient is placed to
reside within the rectal cavity for the time period required for dissolution.
Since the
suppositories are fully dissolvable, release of active ingredient from the
suppository will be
achieved after residence of the suppository in the rectum. The released active
ingredient will
occur in high concentrations at the site of delivery, thus enhancing the
effectiveness of this
therapy for anorectal disorders.
[000114]
According to an alternative embodiment, the suppository may comprise or
consist of other drugs or supplements, such as antioxidants, including vitamin
E and vitamin
C and natural antioxidants such as fish oils, green tea, cranberry, etc. These
may be used as
distinct suppository preparations or as additional components of the
suppositories of the
invention.
[000115]
These uses and in any of the embodiments of the invention, a suppository
form
of these agents is used as a clinical treatment for chronic diseases of the
anus and rectum.
[000116]
Any form of the active ingredients contemplated by the invention that is
placed in a suppository form for the treatment of anorectal diseases is within
the confines of
the invention. Additionally, the incorporation of active pharmaceutical
ingredients described
herein in combination with other active or inactive ingredients into
suppositories to treat
anorectal diseases is embodied within this invention. Additionally, the
incorporation of any
FIASMA within the suppositories as a means of treating anorectal disorders is
embodied in
the invention. Finally, other agents, such as anti-inflannnnatories,
anesthetics, herbals, or
other vitamins may be included in the suppositories to enhance the efficacy of
the active
ingredient or ingredients. Substances utilized to produce the suppositories
include any fatty
(or oleaginous) bases and/or water soluble (or miscible) bases.
[000117]
In particularly preferred embodiments, the medication contained in the
suppository is annitriptyline. In the preferred embodiment, the suppository is
composed of
fatty (or oleaginous) bases and/or water soluble (or miscible) bases. However,
other bases
may be employed in the invention to allow for the passage of the medication
into the rectum.
28
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
More generally, any form of suppository base may be used to construct the
devise. In
addition, a variety of TCAs may be incorporated into the suppository to allow
direct
application of these substances to the rectum and anus. Other aforementioned
substances
may also be included in the suppositories to enhance their efficacy in
treating anorectal
disorders. The contents of the suppository may also consist of a variety of
TCAs, either alone
or in combination with annitriptyline, depending on the goal of treatment.
[000118]
Variable doses of annitriptyline may be utilized, depending on the
condition
being treated. For example, the rectal dose of annitriptyline used for the
treatment of
radiation proctopathy is 25 - 100nng a day. Both lower and higher doses than
these would
initially be employed in the construction of the devise. The optimal dosage to
treat these
conditions will be determined based on clinical studies.
[000119]
However, following appropriate clinical evaluation of this treatment,
either
larger or smaller doses of annitriptyline may ultimately be used for treating
radiation
proctopathy as well as other anorectal disorders. Dosing for a nnitriptyline
and other TCAs in
suppositories are anticipated to be lower than oral doses for the treatment of
anorectal
diseases because these agents will be directly applied to the affected areas.
However,
because the delivery by suppository or rectal device does not need to be
ingested and the
dosage forms can be larger in size, higher doses than typically employed in
oral dosage forms
may also be utilized if so determined safe and effective based on further
clinical experience
or studies
[000120]
Preferably, the method for treating CRI is carried out using a FIASMA,
e.g, a
TCA such as annitriptyline or an SSRI, such as sertraline, wherein the TCA or
SSRI or both are
prepared as a composition and provided in a dosage form which is capable of
delivering an
effective dose in situ to the skin of the patient. For example, annitriptyline
can be formulated
as a liquid and administered in liquid form to the target area of the skin, or
can be formulated
with a thickening agent or viscosity-enhancing agent to provide a lotion,
cream, ointment or
gel for topical delivery of annitriptyline to target area of the skin of a
patient in need thereof,
such as a patient who will undertake radiation therapy or who is suffering
from radiation
dermatitis.
29
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000121]
Alternatively, the method can employ administration of a solid oral dosage
form.
[000122]
The method of the invention can further include the administration to a
patient in need thereof of at least one FIASMA and an additional active
ingredient which is
not a FIASMA. Preferably, the additional active is formulated together with
the at least one
FIASMA in a fixed-dose combination drug product for topical administration to
the skin.
[000123]
One preferred embodiment of a composition of the invention having a first
and second active comprises an SSRI as a first active ingredient and a TCA or
an antioxidant
as a second active ingredient. The antioxidant can be, for example, Vitamin A,
Vitamin C,
Vitamin D, and Vitamin E. A further embodiment of a composition of the
invention can
comprise a TCA, an SSRI, and an antioxidant. Examples of the active
ingredients are
annitriptyline as the TCA, sertraline, as the SSRI, and Vitamin D as the
antioxidant.
[000124]
Variable doses of the one or more active ingredient described herein may
be
utilized, depending on the condition being treated. For example, the topical
dose of
annitriptyline used for the prevention or treatment of radiation dermatitis is
lmg ¨ 100 mg a
day.
[000125]
The optimal dosage and area to be treated to prevent or treat these
conditions
will be determined based on clinical studies. However, following appropriate
clinical
evaluation of this treatment, either larger or smaller doses of annitriptyline
or sertraline may
ultimately be used for preventing or treating radiation dermatitis as well as
other
dermatological inflammatory disorders.
EXAMPLES
Example 1¨ Use of a Composition Comprising a TCA
[000126]
A patient undergoing or scheduled to undergo radiation therapy for
treatment
of a 25 mm breast tumor will be provided a composition which is an ointment
comprising 1%-
2% amitriptyline in a pharmaceutically acceptable base. The area of the skin
over the tumor,
which is or will be exposed to the radiation during the radiation therapy
procedure will be
identified and can be marked.
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000127]
The healthcare worker or patient will administer the composition by
applying
approximately 1 ml of the composition to the area of the skin exposed or
expected to be
affected by the radiation at least one time per day. Administration of the
composition will be
repeated at least daily, or up to five times daily during the radiation
treatment regimen for a
period of at least one week. For example, a patient undergoing a radiation
therapy regimen
five times per week (Monday through Friday), may apply cream to the treated
area 90
minutes before radiation therapy each day and then at bedtime, for seven days.
So before
and after the radiation treatment Monday to Friday and then twice a day on the
weekends
when they are not getting radiation. This is repeated for 5 or 6 weeks.
[000128]
Expected result: radiation dermatitis is prevented or ameliorated or
reversed
by the administration of the composition.
Example 2 ¨ Determining efficacy of single active v a plurality of actives
[000129]
Five patient groups undergoing or scheduled to undergo radiation therapy
for
treatment of a 25 mm breast tumor will be provided a composition which is an
ointment
comprising either:
= 1% amitriptyline in a pharmaceutically acceptable base;
= 1% sertraline in a pharmaceutically acceptable base;
= 1% amitriptyline and 1% sertraline in a pharmaceutically acceptable base;
= 0.5% amitriptyline and 0.5% sertraline in a pharmaceutically acceptable
base; and
= Pharmaceutically acceptable base, alone (placebo).
[000130]
The area of the skin over the tumor, which is or will be exposed to the
radiation
during the radiation therapy procedure will be identified and can be marked.
The healthcare
worker or patient will administer the composition by applying approximately 1
ml of the
composition to the area of the skin exposed or to be exposed to the radiation
at least one
time per day before radiation treatment. Administration of the composition
will be repeated
at least daily, or up to five times daily following the radiation treatment
for a period of one
week.
31
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000131]
Expected results: The efficacy for each composition comprising an active
ingredient will be determined by scoring the level of radiation dermatitis
present in each
group of patients. A determination of whether the efficacy of using
amitriptyline and
sertraline in combination exhibits an additive or synergistic effect can be
determined by
comparing whether the radiation dermatitis score for the composition
comprising 1%
annitriptyline and 1% sertraline is less than, equal to, or greater than the
effect of the 1%
annitriptyline composition, alone, and the 1% sertraline composition, alone.
The radiation
dermatitis score for the fixed dose combination composition comprising 0.5%
annitriptyline
and 0.5% sertraline in a pharmaceutically acceptable base can provide
information on efficacy
of lower doses of each active, in combination, compared to higher doses of
each active, alone,
in a composition.
Example 3 ¨ Treatment of radiation dermatitis in radiated mice
[000132] Purpose:
Determining effects of topical reformulation of sertraline, as a
protector for radiation-induced skin injury.
[000133]
Materials and Methods: Radiation-induced dermatitis experiments were
conducted in 10-week-old female C57BL/6J mice (The Jackson Laboratory). An
approximately
3 x 3 cm area on the dorsal skin of mice was shaved prior to drug treatment.
Mice were
randomly assigned to receive topical treatment of 4% sertraline or vehicle
(n=5 mice per
cohort) and all investigators were blinded as to topical treatment.
[000134]
The study compared active drug-containing composition to a placebo
(vehicle).
Both the active drug-containing composition and placebo were prepared by a
third party and
provided to us for use in the study. The preparation of compositions is
summarized as
follows:
4% Sertraline Cream ¨4% (w/w) Sertraline (as a hydrochloride salt) in an oil-
in-water
vanishing cream base comprising hexylene glycol, purified water, isopropyl
palnnitate,
caprylic/capric triglyceride, propylene glycol, ceteareth 20, cetearyl
alcohol, glyceryl
stearate, PEG 100 stea rate, dinnethicone, octyldodecanol, lecithin,
ethylhexylglycerin,
and phenoxyethanol.
32
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
Placebo Cream ¨ An oil-in-water vanishing cream base comprising purified
water,
isopropyl palnnitate, caprylic/capric triglyceride, propylene glycol,
ceteareth 20,
cetearyl alcohol, glyceryl stearate, PEG 100 stearate, dinnethicone,
octyldodecanol,
lecithin, ethylhexylglycerin, and phenoxyethanol.
Both products were packaged in white polypropylene jars with white
polypropylene
closures with polyvinyl chloride disc liners, labeled for external use only,
storage at
controlled room temperature (20 C to 25 C) and with instruction to not freeze.
[000135] The formulation is further described in Composition
Table, below:
Composition Table.
Rviciv.a . .. ....
i.s.conpotitkot 4%. Setatane
4.".esi=nowent tot % wfv$ 2200
...
Se:traioeNd 665:lg.-NJ 4,48
9.85i ..
He.nriet Citycol NG1 Tf 364 5.4:X31
11.trt.
PEW:ma:1'i P.93 09 20:a Ki-521 ..
Sertrawe HE:1 MW
',ie:Irtkiim F8 MW 306.23
:n5tzliz.:th)os
Add Settft03..e HO to a e&ss
2?. Add .xyÃen-f.?Wytt"A.
T,:ti3r-zdel.r.3ing 4
CI A& PFJ Crown bkne aiiloot :; to the Oitss nto$taf and tIrx.
Pecie 4: 4oz. wilit*z PP Jo s.h3t= Wit h Whit:0 PP *jon-w :..ap and
Ps.T. di.sc in e3-791,109
SKS Part W.I. (1651-2.1. TT
I
[000136] As noted in the Composition Table, the 4% sertraline
composition is prepared
as the free base. The amount of sertraline HCI added to the formulation is
adjusted to
compensate for the HCI salt.
[000137] PENCreann is a commercially available oil-in-water
vanishing base purchased
from Hunnco (https://www.hunnco.conn/pharnnaceuticals/pencreann/). The NDC
code of the
cream is 0395-6010-56.
[000138] Mice received topical treatment once per day for 12
days starting at 2 days
before irradiation. Dermatitis was induced by delivering a single dose of 30
Gy X-rays to a 1 x
1 cm area of the shaved skin using an image-guided small animal irradiation X-
Rad 225 Cx.
33
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000139] The
development of radiation-induced dermatitis was examined before
irradiation and every week for 4 weeks after irradiation by a single observer
who was blinded
to the treatment groups. Skin reaction to radiation was assessed according to
a semi-
quantitative score system established previously for preclinical studies ¨
scoring ranges from
1.0 to 5.5 (0.5 increments) based on erythema, desquannation (dry and moist),
necrosis and
loss of dernnis (Table 1).
Table Seirsi.quantitative Skin dAnla.,ge scores
SCORE SK N CHANGES
.0 No. effect
Ls M 3.1irmi thyththrm,.:thik, dry 4:M.
Modth-the .Eryth,Errsa, thy 1,kir,
Marksfd :,,,rylA-1.:Nna,: thy thzw.Kimiltign:
3.0 Dry desqitmthattn.. n-thlimth thy
I:rue:Mg
3.5 Dy dtsquartlatiQn. dfy st3parkil
mithiTsa3
ath titykst. th!,,,:thsamato3, mader3... scabWrIg
45 ,th,s.thwrtzt4ths, d=Mr; MZSI
Op iFNbthes.:5: skfr: hmr,
S.5 Nz-crosis
[000140] Results:
During the first two weeks after 30 Gy irradiation, all of the mice
that received treatment with 4% sertraline cream had a skin injury score of
4.5, while the mice
that received vehicle treatment had less severe scores of 3.0¨ 3.5 (Figure 1,
panels A and B).
Scratching behaviors and sequelae were noted in the 4% sertraline cohort.
[000141] On day 21
after irradiation, both treatment cohorts had skin injury scores of
3.0 ¨ 3.5. At the end of the experiment on day 28 after irradiation, mice that
received
treatment of 4% sertraline had less severe skin injury scores ranging from 1.5
¨ 2.0 with an
average score of 1.7.
[000142] In contrast,
vehicle treated mice maintained higher skin injury scores ranging
from 1.5 ¨3.5 with an average score of 3.0 (Figure 5, panels A and B).
34
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
[000143] Discussion:
Our preliminary results show that skin injury of mice that
received 4% sertraline was substantially improved 4 weeks after 30 Gy, whereas
skin injury of
vehicle treated mice remained severe. The causes of higher injury scores for
the mice treated
with 4% sertraline during the first two weeks after irradiation are under
investigation, but
literature search has confirmed that in mice, topical sertraline trigger a
significant itch-evoked
scratching in mice as shown in previous published animal studies (Neuron 87,
124-138, July 1,
2015). Repeat of this experiment with removal of cream after 30 minutes
substantially
reduced the scratching behavior and initial skin injury score while still
showing similar benefit
after 2 weeks as the data shown above. Therefore, it is believed that the
early toxicity noted
at week 1 was related to self-injury due to sertraline-induced scratching.
[000144]
Conclusion: The results surprisingly suggest that a 4% FIASMA composition,
e.g., 4% sertraline topical composition, is efficacious against radiation-
induced skin injury.
Example 4 ¨ Comparison of pre-irradiation and post-irradiation treatments
[000145]
The experiment presented in Example 3 was repeated in mice to compare 4%
sertraline administered pre-irradiation treatment and post irradiation
treatment. 4%
annitriptyline was also tested.
[000146]
The same scoring chart shown in Example 3 was used to evaluate the
irradiated
sites on the mice.
[000147] Methods:
Radiation-induced dermatitis experiments were conducted
using 10-week-old female C57BL/6J mice (The Jackson Laboratory). An
approximately 3 x 3
cm area on the dorsal skin of mice was shaved prior to drug treatment. A
single dose of 30 Gy
X-rays was delivered to a 1 x 1 cm area of the skin using an image-guided
small animal
irradiator X-Rad 225 Cx to induce dermatitis. Mice were randomized by cage to
receive topical
treatment of 4% sertraline cream once a day for 3 days before irradiation, 4%
sertraline cream
once a day for 2 days starting at 24 hours after irradiation or placebo once a
day for 5 days
(before and after irradiation).
[000148]
The development of radiation-induced dermatitis was examined every week
for 6 weeks after irradiation by two observers who were blinded to the
treatment cohorts
(S.H. and S.S). Skin reaction to radiation was assessed according to a semi-
quantitative score
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
system established previously for preclinical studies - scoring ranges from
1.0 to 5.5 (0.5
increments) based on erythema, desquannation (dry and moist), necrosis and
loss of dernnis.
[000149] The score results at 1 week, 2 weeks, 3 weeks and 4
weeks post irradiation
treatment (IR) are shown in Table 2, below:
TABLE 2 - Semiquantitative Skin Damage Scores
A. 4% sertraline cream applied 48 hr., 24 hr., and 30 min. prior to
irradiation treatment
Mouse No. 1 week post IR 2 weeks post IR 3 weeks post IR
4 weeks post IR
6632 4.00 4.00 3.50 2.00
6633 4.00 4.00 4.00 2.00
6634 4.00 4.00 4.50 4.50
6635 4.00 4.00 3.00 2.50
6636 4.00 4.00 4.00 2.50
B. Vehicle-only cream applied 48 hr., 24 hr., and 30 min. prior and 24 hr. and
48 hr. post
irradiation treatment
Mouse No. 1 week post IR 2 weeks post IR 3 weeks post IR
4 weeks post IR
6637 4.00 4.00 4.00 3.00
6638 3.50 3.50 3.50 3.00
6639 4.00 4.00 3.50 3.50
6640 4.00 4.00 4.00 4.00
6641 3.00 3.00 4.00 4.00
C. 4% sertraline cream applied 24 hr. and 48 hr. post irradiation treatment
Mouse No. 1 week post IR 2 weeks post IR 3 weeks post IR
4 weeks post IR
6642 2.50 4.00 2.50 2.00
6643 3.00 3.00 3.00 1.50
6644 2.50 3.50 3.00 3.00
6645 3.00 1.50 1.50 1.50
6646 3.00 1.50 1.50 1.50
[000150] RESULTS. At 4 weeks post-irradiation treatment, 9 of
10 sertraline-
treated mice showed lower scores (better improvement) than vehicle-only
treated mice.
Generally, the 4% sertraline cream performed better when administered post-
irradiation
treatment than pre-irradiation treatment; however, pre-irradiation treatment
exhibited
some preventive effects. 4% sertraline cream administered 24 hr. and 48 hr.
post-irradiation
treatment also showed an earlier response, showing lower scores within one
week of
irradiation treatment, and continuing to show improved scores through the
fourth week after
irradiation treatment.
[000151] These results are graphically represented in FIG. 6,
panels A-D. Overall, mice
that received 4% sertraline 2 times after irradiation showed accelerated
recovery from acute
radiation dermatitis starting at the 1st week after irradiation (Figure 6, A
and 2). The skin
36
CA 03193398 2023- 3- 21
WO 2022/066791
PCT/US2021/051583
injury scores for the 2x after group were significantly lower than the placebo
group at week
3 to 6 after 30 Gy (Figure 6, panel B). On the other hand, the skin injury
scores for the 3x
before group were significantly lower than the placebo group only at week 5
after 30 Gy
(Figure 6, panel C). Remarkably, mice that received 4% sertraline 3 times
before irradiation
showed significantly slower recovery from acute radiation dermatitis compared
with mice
that received 4% sertraline 2 times after irradiation (Figure 6, panel D).
[000152]
Notably, administration of 4% arnitriptyline cream and 4% fluoxetine cream
had deleterious effects on the mice and this arm of the experiment was
stopped. This
suggests that a 4% cream formulation comprising a tricyclic antidepressant
(TCA) is an
excessive dose and that lower doses are required to determine their efficacy.
[000153]
The foregoing description of the invention is illustrative only and is not
intended to limit the scope of the invention to the precise terms set forth.
Further, although
the invention has been described in detail with reference to certain
illustrative embodiments,
variations and modifications exist within the scope and spirit of the
invention as described
and defined in the following claims.
[000154]
The above disclosure and example generally describe the present invention
and is provided for purposes of illustration and is not intended to limit the
scope of the
invention. The invention described herein may be practiced in the absence of
any element or
elements, limitation or limitations which is not specifically disclosed
herein. Thus, for
example, in each instance herein, any of the terms "comprising," "consisting
essentially of,"
and "consisting of" may be replaced with either of the other two terms. The
terms and
expressions are used as terms of description and not of limitation, and there
is no intention
in the use of such terms and expressions of excluding any equivalents of the
features shown
and described or portions thereof, but it is recognized that various
modifications are possible
within the scope of the invention claimed. Thus, it should be understood that
although the
present invention has been specifically disclosed by preferred embodiments and
optional
features, modification and variation of the concepts herein disclosed may be
resorted to by
those skilled in the art, and that such modifications and variations are
considered to be within
the scope of this invention as defined by the claims.
37
CA 03193398 2023- 3- 21