Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/076495
PCT/US2021/053680
TITLE OF THE INVENTION
PREPARATION OF BENZIMIDAZOLONE DERIVATIVES AS NOVEL
DIACYLGLYCERIDE 0-ACYLTRANSFERASE 2 INHIBITORS
FIELD OF THE INVENTION
The present invention is directed to novel pharmaceutical compounds which
inhibit
diacylglyceride 0-acyltransferase 2 ("DGAT2-), and may be useful for
preventing, treating or
acting as a reversing agent for hepatic steatosis, nonalcoholic
steatohepatitis (NASH), fibrosis,
type-2 diabetes mellitus, obesity, hyperlipidemia, hypercholesterolemia,
atherosclerosis,
cognitive decline, dementia, cardiorenal diseases such as chronic kidney
diseases and heart
failure, and related diseases and conditions, as well as methods of making
such compounds and
pharmaceutical compositions comprising such a compound and a pharmaceutical
carrier.
BACKGROUND OF THE INVENTION
1 5 Triacylglycerols ("TGs") serve several functions in living organisms.
One such function
of TGs is in the storage of energy. TGs also play a role in the synthesis of
membrane lipids. TG
synthesis in cells may protect them from the potentially toxic effects of
excess fatty acid ("FA").
In enterocytes and hepatocytes, TGs are synthesized for the assembly and
secretion of
lipoproteins which transport FA between tissues. TGs play a role in the skin's
surface water
barrier, and TGs in adipose tissue provide insulation for organisms.
The glycerol phosphate and the monoacylglycerol pathways are the major
pathways for
the biosynthesis of TG. However, the last step in the synthesis of TG involves
the reaction of a
fatty acyl-CoA and diacylglycerol ("DAG") to form TG. The reaction is
catalyzed by acyl-
CoA:diacylglycerol acyltransferase ("DGAT") enzymes. There have been
identified two DGAT
enzymes, DGAT1 and DGAT2. Although DGAT1 and DGAT2 catalyze the same reaction,
they
differ significantly at the level of DNA and protein sequences. DGAT2 can
utilize endogenous
fatty acid to synthesize TG in in vitro assays, whereas DGAT1 appears to be
more dependent on
exogenous fatty acid (Yen et al., J. Lipid Research, 2008, 49, 2283).
Inactivation of DGAT2
impaired cytosolic lipid droplet growth, whereas inactivation of DGAT1 exerts
opposite effect.
(Li et at., Arterloscler. Thromb. Vase. Biol. 2015, 35, 1080).
DGAT2 is an integral membrane protein of the endoplasmic reticulum and is
expressed
strongly in adipose tissue and the liver. DGAT2 appears to be the dominant
DGAT enzyme
controlling TG homeostasis in vivo. DGAT2 deficient mice survive for only a
few hours after
1
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
birth. On the other hand, DGAT1 deficient mice are viable (Yen et al., J Lipid
Research, 2008,
49, 2283).
Despite this perinatal lethal phenotype, the metabolic role of DGAT2 has been
mostly
understood from effort exploiting anti-sense oligonucleotides (ASO) in
rodents. In this setting,
DGAT2 knockdown in oh/oh mice with a DGAT2 gene-specific ASO resulted in a
dose
dependent decrease in very low density lipoprotein ("VLDL") and a reduction in
plasma TG,
total cholesterol, and ApoB (Liu, et al., Biochim. Biophys Acta 2008, 1781,
97). In the same
study, DGAT2 anti sense oligonucleotide treatment of oh/oh mice showed a
decrease in weight
gain, adipose weight and hepatic TG content. Id. In another study, antisense
treatment of ob/ob
mice improved hepatic steatosis and hyperlipidemia (Yu, el al., Hepalology,
2005, 42, 362).
Another study showed that diet-induced hepatic steatosis and insulin
resistance was improved by
knocking down DGAT2 in rats. These effects seem to be unique to inhibition of
DGAT2, as
ASO against DGAT1 did not lead to similar beneficial effects. Although the
molecular
mechanism behind these observations remains uncertain, the collective data
suggest that
suppression of DGAT2 is associated with reduced expression of lipogenic genes
(SREBP1c,
ACC1, SCD1, and mtGPAT) and increased expression of oxidative/thermogenic
genes (CPT1,
UCP2) (Choi etal., J Bio. Chem., 2007, 282, 22678).
In light of the above, inhibitors of DGAT2 are useful for treating disease
related to the
spectrum of metabolic syndrome such as hepatic steatosis, non-alcoholic
steatohepatitis (NASH),
fibrosis, type-2 diabetes mellitus, obesity, hyperlipidemia,
hypercholesterolemia, atherosclerosis,
cognitive decline, dementia, cardiorenal diseases such as chronic kidney
diseases and heart
failure and related diseases and conditions.
DGAT2 inhibitor compounds are described in W02021064590, W02016036633,
W02016036636, W02016036638, W02018093696, W02018093698, W02013150416,
US20150259323, W02015077299, W02017011276, W02018033832, US201801628,
W02003053363.
SUMMARY OF THE INVENTION
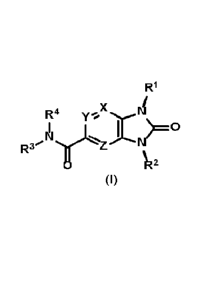
The present invention relates to compounds represented by Formula I:
x
R4
2
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
as well as pharmaceutically acceptable salts, esters, and prodrugs thereof,
which are DGAT2
inhibitors. Also provided are methods of making compounds of Formula I,
pharmaceutical
compositions comprising compounds of Formula I, and methods of using these
compounds to
treat hepatic steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, type-2
diabetes mellitus,
obesity, hyperlipi demi a, hyperchol esterol emi a, atherosclerosis, cognitive
decline, dementia,
cardiorenal diseases such as chronic kidney diseases and heart failure and
related diseases and
conditions, comprising administering a compound of Formula I to a patient in
need thereof.
DETAILED DESCRIPTION OF THE INVENTION
Formula I
The present disclosure is directed to compounds having structural Formula I:
Ri
R4 yl.X
/R2
or pharmaceutically acceptable salts thereof wherein:
X, Y, and Z are independently selected from N or C(R5);
R1 is
(1) phenyl unsubstituted or substituted with 1, 2, or 3 R6, or
(2) 5- or 6-membered heteroaryl containing 1, 2, 3 or 4 heteroatoms
independently
selected from N, 0, and S, wherein the heteroaryl is unsubstituted or
substituted
with 1, 2, or 3 R6, or
(3) 8- to 10- membered fused heteroaryl containing 1, 2, 3 heteroatoms
independently
selected from N, 0, and S, wherein the heteroaryl is unsubstituted or
substituted
with 1, 2, or 3 R6;
R2 is
(1) phenyl unsubstituted or substituted with 1, 2, or 3 R7,
(2) 5- or 6-membered heteroaryl containing 1, 2 or 3 heteroatoms
independently
selected from N, 0, and S, wherein the heteroaryl is unsubstituted or
substituted
with 1, 2, or 3 R7,
(3) C1_6alkyl unsubstituted or optionally mono-substituted or disubstituted
with
halogen, OH, CF3, or -CN,
3
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(4) (C3 -6) cycloalkyl unsubstituted or optionally mono-substituted or
disubstituted
with C1_3alkyl, halogen, OH, CF3, or CN,
(5) -(C3_6)alkylC(0)NH2,
(6) 4- to 6- membered heterocyclyl containing 1 or 2 heteroatoms
independently
selected from N, 0 and S wherein the heterocyclyl is unsubstituted or
substituted
by 1, 2, or 3 R7,
(7) -CH2-aryl unsubstituted or substituted by 1, 2, or 3 R7,
(8) -S02(C16)alkyl unsubstituted or substituted with 1, 2, or 3117, or
(9) -S02-aryl unsubstituted or substituted with 1, 2, or 3 le;
le is
(1) 4- to 7-membered heterocyclyl containing 1, 2, or 3 heteroatoms
independently
selected from N, 0 and S,
(2) 5- or 6-membered heteroaryl containing 1, 2 or 3 heteroatoms
independently
selected from N, 0, and S.
(3) -(C16)alkyl-heteroaryl, wherein the heteroaryl is a 5- or 6-membered
heteroaryl
containing 1, 2, or 3 heteroatoms independently selected from N, 0 and S,
(4) -(C16)alkyl-aryl,
(5) -(Cis)alkyl-heterocyclyl, wherein the heterocyclyl is a 3- to 6-
membered ring
containing 1 or 2 heteroatoms independently selected from N, 0 and S,
(6) -(C16)alkyl,
(7) -(C34cycl alkyl,
(8) -(C 1_6)hydroxyalkyl,
(9) -(C1_6)alkyl-S(0)2_NR8aR8b, or
(10) -(C1_6)alkyl-S(0)2-(C1_3)alkyl,
wherein each aryl, heteroaryl, cycloalkyl, or heterocyclyl is unsubstituted or
substituted with 1,
2, or 3 le, and wherein each alkyl is unsubstituted or substituted with 1, 2,
or 3 Rm,
le is
(1) hydrogen, or
(2) (C13)alkyl,
or le and le combine along with the nitrogen atom to which they are attached
to form a mono-
or bicyclic heterocyclyl ring containing 1 or 2 heteroatoms independently
selected from N, 0 and
S, wherein the heterocyclyl ring is unsubstituted or substituted by 1, 2, or 3
R",
when present, each R5 is selected from
4
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(1) hydrogen,
(2) (C16)alkyl,
(3) (C3_6)cycloalkyl,
(4) (Ci_6)haloalkyl,
(5) cyano, or
(6) halogen,
when present, each R6 is independently selected from
(1) cyano,
(2) halogen,
(3) -0C1_6alkyl,
(4) (C3_6)cycloalkyl, optionally substituted with halogen,
Cr_3alkyl, C1_6haloalky1, or
OH,
(5) -C(=0)NH2,
(6) -0(C3_6)cycloalkyl wherein the cycloalkyl is optionally
substituted with halogen,
C1_3alkyl, or OH,
(7) hydroxy,
(8) N(R11)2,
(9) (C1_6)haloalkyl-,
(10) -0(C1_6)haloalkyl,
(11) -S02(C1_6)alkyl,
(12) -SO2NH(Ci4alkyl,
(13) -SCi_balkyl,
(14) N(R11)C(0)R11,
(15) -SC1_6haloa1ky1, or
(16) (C1_6)alkyl,
when present, each R7 is independently selected from
(1) (C13)alkyl,
(2) halogen,
(3) (C1_6)alkoxy-,
(4) (C1-6)haloalkyl-, or
(5) hydroxy;
when present, lea and R8b are independently selected from
(1) hydrogen,
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(2) (C1_3)alkyl, or
(3) (C37)cycloalkyl;
when present, each R9 is independently selected from
(1) (C1_3)alkyl,
(2) (C1_3)hal alkyl -,
(3) oxo,
(4) (C36)cycloalkyl,
(5) N(R11)2,
(6) hydroxy,
(7) (C1_3)alkoxyl-,
(8) cyano, or
(9) halogen,
when present, RI is independently selected from
Cl) (C1_3)alkoxy-,
(2) hydroxy,
(3) halogen,
(4) (C1_3)haloalkyl-, or
(5) N(R11)2;
R", when present, is independently
(1) hydrogen, or
(2) (C13)alkyl;
In Embodiment 1 of this disclosure are compounds of Formula I, or a
pharmaceutically acceptable
salt of any of the foregoing, wherein R1 is
a) phenyl optionally substituted with one to three substituents independently
selected from
halogen, hydroxy, CN, C1_3alkyl, Ci_3haloalkyl, C3_6cycloalkyl, 0C1_3alkyl,
OCi_3haloalkyl,
0C3_6cycloalkyl, -SCi_3alkyl,
S(0)2Ci_3alkyl, and wherein the cycloalkyl
is optionally substituted with, halogen or OH,
b) a 6 membered heteroaryl containing one or two nitrogen atom optionally
substituted with
one to three substituents independently selected from halogen, hydroxy,
C1_6alky1, CI
-
6haloalkyl, C3_6cyc1oalkyl, 0C1_3alkyl, OCi_3haloalkyl, 0-C3_6cycloalkyl, or
CN, and
when the cycloalkyl is cyclopropyl, and the cyclopropyl is optionally
substituted with
halogen, or OH,
6
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
c) a 5 membered heteroaryl containing one to four nitrogen atoms or hetero
atoms
independently selected from N, 0, and S optionally substituted with one to two
substituents independently selected from with halogen, (C1_3)alkyl,
(C36)cycloalkyl, (C1-
3)haloalkyl-, OH or 0C1_3alky1; or
d) 8- to 10- membered fused heteroaryl containing 1, 2, 3 heteroatoms
independently
selected from N, 0, and S, wherein the heteroaryl is unsubstituted or
substituted with
halogen, (C13)alkyl, (C3_6)cyc10a1ky1, (C1_3)haloalkyl-, OH or OCi_3alkyl.
In Embodiment 2 of this disclosure are compounds of Formula I, or of
Embodiment 1, or a
pharmaceutically acceptable salt of any of the foregoing, wherein RI is not
alkyl
In Embodiment 3a of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
2, or a pharmaceutically acceptable salt of any of the foregoing, wherein
is
a) phenyl optionally substituted with halogen, Ci_3a1ky1, Ci3haloalkyl,
C3_6cycloalkyl, 0-C3_
6cyc10a1ky1, CN, NHC(0)C1_3alkyl, NC1_3a1ky1, C(0)NH2, SC1_3a1ky1,
S(0)2NHC1_3alkyl,
S(0)2Ci_3alkyl, OCi_3alkyl, or 0C1_3haloalkyl, wherein the cycloalkyl is
additionally
optionally substituted with halogen;
b) pyridyl optionally substituted with one to three substituents independently
selected from
halogen, Ci_3alkyl, C3_6cycloalkyl, OCi_3alkyl, or OCi_3haloalkyl;
c) 5 membered heteroaryl containing one to four nitrogen atoms or hetero atoms
independently selected from N, 0, and S optionally substituted with one to two
substituents
independently selected from with halogen, (Ci_3)alkyl, (C3_6)cycloalkyl,
OH or OC1_3alkyl, or
d) 8- to 10- membered fused heteroaryl containing 1, 2, 3 heteroatoms
independently selected
from N, 0, and S, wherein the heteroaryl is unsubstituted or substituted with
halogen, (CI -
3)alkyl, (C36)cycloalkyl, (Ci_3)haloalkyl-, OH or OCi_lalkyl.
In Embodiment 3b of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
2, or a pharmaceutically acceptable salt of any of the foregoing, wherein
is
e) phenyl optionally substituted with halogen, C1_3a1ky1, C3_6cyc1oa1ky1, 0-
C3_6cyc1oalkyl,
0C1_3alkyl, or OCi_3haloalkyl, wherein the cycloalkyl is additionally
optionally substituted
with halogen;
f) pyridyl optionally substituted with one to three substituents independently
selected from
halogen, Ci_3alkyl, C3_6cyc10a1ky1, 0c13 alkyl, or OCi_3haloalkyl;
g) 5 membered heteroaryl containing one to four nitrogen atoms or hetero atoms
independently selected from N, 0, and S optionally substituted with one to two
substituents
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
independently selected from with halogen, (Ci_3)alkyl, (C3_6)cycloalkyl,
(Ci_3)haloalkyl-,
OH or OC1_3alkyl, or
h) 8- to 10- membered fused heteroaryl containing 1, 2, 3 heteroatoms
independently selected
from N, 0, and S, wherein the heteroaryl is unsubstituted or substituted with
halogen, (Ci _
)alkyl, (C3_6)cycloalkyl, (C1_3)haloalkyl-, OH or OC1_3alkyl
In Embodiment 4a of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
3, or a pharmaceutically acceptable salt of any of the foregoing, wherein
is
a) phenyl optionally substituted with one to three substituents independently
selected from F,
CF3, CN, N(CH3)2, C(0)NH2, SCH3, S(0)2NHCH3, S(0)2CH3, OCH2CH3, OCH(CH3)2,
OCHF2, OCH2CHF2, OCF3, OCF2CHF2, NHC(0)CH3, cyclopropyl, 0-cyclopropyl,
wherein the cyclopropyl is additionally optionally substituted with 1-3 F,
b) pyridyl optionally substituted with one to three substituents independently
selected from
OC(F)2(CH3), OCH2CH3, OCH(CH3)2, OCHF2, OCH2CHF2, OCF3, OCF2CHF2,
OCF2CHF2, or cyclopropyl;
c) 5 membered heteroaryl containing one to four nitrogen atoms or hetero atoms
independently selected from N, 0, and S optionally substituted with one to two
substituents
independently selected from with halogen, (Ci_3)alkyl, (C3_6)cycloalkyl,
(Ci_3)haloalkyl-,
OH or OC1_3alkyl; or
d) 8-10 membered fused heteroaryl containing one to three heteroatoms selected
from N, 0,
or S atom optionally substituted with CH3.
In Embodiment 4b of this disclosure are compounds of Formula!, or any one of
Embodiments 1 -
3, or a pharmaceutically acceptable salt of any of the foregoing, wherein
is
a) phenyl optionally substituted with one to three substituents independently
selected from F,
OCH2CH3, OCH(CH3)2, OCHF2, OCH2CHF2, OCF3, OCF2CHF2, cyclopropyl, 0-
cyclopropyl, wherein the cyclopropyl is additionally optionally substituted
with 1-3 F,
b) pylidyl optionally substituted with one to three substituents independently
selected from
OC(F)2(CH3), OCH2CH3, OCHF2, OCH2CIIF2, OCF3, OCF2CHF2, or cyclopropyl;
c) 5 membered heteroaryl containing one to four nitrogen atoms or hetero atoms
independently selected from N, 0, and S optionally substituted with one to two
substituents
independently selected from with halogen, (Ci_3)alkyl, (C3_6)cycloalkyl,
(Ci_3)haloalkyl-,
OH or OCi_3alkyl; or
d) 8-10 membered fused heteroaryl containing one to three heteroatoms selected
from N, 0,
or S atom.
8
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
In Embodiment 5a of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein It'
is
F
40 * F F gh, FF * 0 F F
)---1- 4 IN .-.-F = )c_FF
* OF *
5.F._.
F
F at
F4_.F 0
0 F
F * ON.....c 4# oy 44 . 14 A * c F
F FN....0
F)....0p F F-1,..0
0
Fi p , pN N N ,_, F , = F )--
--. µ....1y. F.y-- õ___I HF2dr.. N h/11 p
F F
F F
0
F HN--ic
Kf .
0 H
* 0 H * ---N * \ *
NH2 * 3\ * tN\
-61
* Or *
alp ,
,or.
,
In Embodiment 5b of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein It'
is
F
r..0 40 0).....F F = Oy * 0 .. 0
F * .IF F---CF c 0) *
N. ---
C..-F
'
F A FN F
F+F 0 .....0 FN...0 \...0
F
r.0
fh, A = oNt? * F-{F pi Fl p , pN
/......\,Z F p. HF2d ; pN
F F
N,
, =====,, , , , , '
, ,
F
Fmto 4:1s2
In Embodiment 6a of this disclosure are compounds of Formula I, or any one of
Embodiments
1-4 or a pharmaceutically acceptable salt of any of the foregoing, wherein It'
is a phenyl
unsubstituted or substituted with 1, 2, or 3 substituents independently
selected from F, CF3, CN,
N(CH3)2, C(0)NH2, SCH3, S(0)2NHCH3, S(0)2CH3, OCHF2, OCF3, OCH(CH3)(CH3),
OCF2CHF2, OCH2C1-1F2, OCH2CH3, NHC(0)CH3, cyclopropyl, or 0-cyclopropyl,
wherein the
cyclopropyl is additionally optionally substituted with one to three halogen
atoms.
9
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In Embodiment 6b of this disclosure are compounds of Formula I, or any one of
Embodiments
1-4 or a pharmaceutically acceptable salt of any of the foregoing, wherein R'
is a phenyl
unsubstituted or substituted with 1, 2, or 3 substituents independently
selected from F, OCHE),
OCF3, OCH(CH3)(CH3), OCF2CHF2, OCH2CHF2, OCH2CH3, cyclopropyl, or 0-
cyclopropyl,
wherein the cyclopropyl is additionally optionally substituted with one to
three halogen atoms
In Embodiment 7a of this disclosure are compounds of Formula 1, or any one of
Embodiments
1-4 or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is a 5- or 6-
membered heteroaryl containing 1 N and wherein the heteroaryl is unsubstituted
or substituted
with 1, 2, or 3 substituents independently selected from OCF1F2, OCH2C113,
OCF2CFlF2,
OCH(CH3)2, OCH2CHF2, OCF2CH3, F, or cyclopropyl.
In Embodiment 7b of this disclosure are compounds of Formula I, or any one of
Embodiments
1-4 or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is a 5- or 6-
membered heteroaryl containing 1 N and wherein the heteroaryl is unsubstituted
or substituted
with 1, 2, or 3 substituents independently selected from OCHF2, OCH2C113, F,
or cyclopropyl.
In Embodiment 8 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
In Embodiment 9 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
F
F
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
In Embodiment 10 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
N
_..
F
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
In Embodiment 11 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
F,HCO N
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
In Embodiment 12 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
F
0
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
In Embodiment 13 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
r_o
HF2d
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In Embodiment 14 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
r-0
H F2d ;NI
4, or a pharmaceutically acceptable salt of any of the foregoing, wherein R1
is
In Embodiment 15a of this disclosure are compounds of Formula I, or
Embodiments 1-14, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
(1) 4 to 6 membered heteroaryl containing zero to one oxygen atoms and/or zero
to two
nitrogen atoms optionally mono-substituted or disubstituted with halogen, CI_
3haloalkyl, C1_3alkyl, or OH,
(2) Ci -6alkyl unsubstituted or optionally mono-substituted, disubstituted or
trisubstituted
with halogen, OH, CF3, or (C3_6)cycloalkyl, phenyl, C(0)NH2,
(3) C3 -6cycloalkyl optionally mono-substituted, disubstituted or
trisubstituted with Ci_
6alkyl, halogen, OH, or CF3,
(4) 4 to 6 membered heterocycle containing zero to one oxygen atoms and/or
zero to two
nitrogen atoms optionally mono-substituted or disubstituted with halogen, Ci_
3haloalkyl, C1_3alkyl, or OH,
(5) phenyl optionally substituted with C1_6alky1 or halogen, or
(6) S(0)2C1_6a1ky1, or S(0)2-phenyl.
In Embodiment 15b of this disclosure are compounds of Formula I, or
Embodiments 1-14, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
(1) 4 to 6 membered heteroaryl containing zero to one oxygen atoms and/or zero
to two
nitrogen atoms optionally mono-substituted or disubstituted with halogen, Ci_
3haloalkyl, C1_3alkyl, or OH,
(2) C 1 -6alkyl unsubstituted or optionally mono-substituted, disubstituted or
trisubstituted
with halogen, OH, CF3, or (C3_6)cycloalkyl,
(3) C3 -6cycloalkyl optionally mono-substituted, disubstituted or
trisubstituted with Ci_
6a1ky1, halogen, OH, or CF3,
(4) 4 to 6 membered heterocycle containing zero to one oxygen atoms and/or
zero to two
nitrogen atoms optionally mono-substituted or disubstituted with halogen, Ci_
3haloalkyl, C1_3alkyl, or OH,
(5) phenyl optionally substituted with Ci_6alky1 or halogen, or
(6) S(0)2Ci_6alkyl.
In Embodiment 16a of this disclosure are compounds of Formula I, or
Embodiments 1-15 or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
11
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(1) 5 or 6- membered heteroaryl containing 2 N atoms optionally substituted
with Ci_
3a1ky1 or halogen,
(2) phenyl substituted with halogen,
(3) Ci_6alkyl, or C3_6cycloalkyl wherein each alkyl or cycloalkyl is
optionally mono-
substituted, di substituted or tri substituted with C1_6alkyl, halogen,
C(0)NH2, OH,
CF3, or CN,
(4) 5 membered saturated heterocycle containing one oxygen atom, optionally
substituted
with OH,
(5) 6 membered saturated heterocycle containing zero or one oxygen atom and
zero or
one nitrogen atoms optionally mono-substituted or disubstituted with halogen,
C1_
3haloalkyl, or C1_3alkyl, or
(6) S(0)2C1_6a1ky1 or S(0)2-phenyl.
In Embodiment 16b of this disclosure are compounds of Formula I, or
Embodiments 1-15 or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
(1) 5 or 6- membered heteroaryl containing 2 N atoms optionally substituted
with Ci_
3a1ky1 or halogen,
(2) phenyl substituted with halogen,
(3) Ci_6alkyl, or C3_6cycloalkyl wherein each alkyl or cycloalkyl is
optionally mono-
substituted, disubstituted or trisubstituted with Ci -6alkyl, halogen, OH, CF
3 , or CN,
(4) 5 membered saturated heterocycle containing one oxygen atom, optionally
substituted
with OH,
(5) 6 membered saturated heterocycle containing zero or one oxygen atom and
zero or
one nitrogen atoms optionally mono-substituted or di substituted with halogen,
CI_
lhaloalkyl, or Ci_lalkyl, or
(6) S(0)2C1_6alkyl.
In Embodiment 17 of this disclosure are compounds of Foimula I, or Embodiments
1-16 or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
(1) phenyl substituted with halogen,
(2) C1_6a1kyl, or C3_6cycloalkyl wherein each alkyl or cycloalkyl is
optionally mono-
substituted, disubstituted or trisubstituted with Ci_6alkyl, halogen, OH, CF3,
or
(3) 6 membered saturated heterocycle containing zero or one oxygen atom and
zero or
one nitrogen atoms optionally mono-substituted or disubstituted with halogen,
C1 -
3haloalkyl, or Ci -3alkyl.
12
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In Embodiment 18a of this disclosure are compounds of Formula I, or
Embodiments 1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
C1_6alkyl, C3_6cycloalkyl, C1_6alkylOH, C1_6haloalkyl, phenyl, C3-
6heterocycle, C3-
6alkylC(0)Nth, S(0)2-phenyl, C1_6alkyl-phenyl, wherein the alkyl is optionally
substituted with
1-3 halogens or CF3, and wherein the phenyl is optionally substituted with
halogen.
In Embodiment 18b of this disclosure are compounds of Formula I, or
Embodiments 1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
C16alkyl, C3_6cycloalkyl, C16alkylOH, Ch6haloalkyl, phenyl, C3-6heterocycle,
wherein the alkyl
is optionally substituted with 1-3 halogens or CF3, and wherein the phenyl is
optionally
substituted with halogen.
In Embodiment 19 of this disclosure are compounds of Formula 1, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is phenyl
substituted with halogen.
In Embodiment 20 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is C1-
6alkyl.
In Embodiment 21 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is C1-
6alkyl optionally substituted with 1-3 halogen or OH.
In Embodiment 22 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is C1_
6haloalkyl.
In Embodiment 23 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is C1_
6alkyl-OH.
In Embodiment 24 of this disclosure are compounds of Foimula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is C3-
6cycloalkyl.
In Embodiment 25 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
13
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
1--F3F-F
."7.1NH2 .1CõCH2
\*, F
or
In Embodiment 26 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2is
In Embodiment 27 of this disclosure are compounds of Formula I, or Embodiments
1-16, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R2 is
In Embodiment 28 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-16, or a pharmaceutically acceptable salt of any of the foregoing, wherein
R2 is
In Embodiment 29 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-25, or a pharmaceutically acceptable salt of any of the foregoing, wherein
R3 is
(1) 4- to 7-membered heterocyclyl containing 1, 2 or 3 heteroatoms
independently
selected from N, 0 and S,
(2) 5- or 6-membered heteroaryl containing 1, 2 or 3 heteroatoms
independently
selected from N, 0, and S,
(3) -(C1_6)alkyl-heteroaryl, wherein the heteroaryl is a 5- or 6-membered
heteroaryl
containing 1, 2 or 3 heteroatoms independently selected from N, 0 and S,
(4) -(C16)alkyl-aryl,
(5) -(C1_6)alkyl-heterocyclyl, wherein the heterocyclyl is a 3- to 6-
membered ring
containing 1 or 2 heteroatoms independently selected from N, 0 and S,
(6) -(C16)alkyl,
(7) -(C3_6)cycloalkyl, or
(8) (C1_6)hydroxy al kyl,
wherein each aryl, heteroaryl, cycloalkyl, or heterocyclyl is unsubstituted or
substituted with 1,
2, or 3 R9, and wherein each alkyl is unsubstituted or substituted with 1, 2,
or 3 R3 .
In Embodiment 30 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-26, or a pharmaceutically acceptable salt of any of the foregoing, wherein
R3 is
14
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(1) 4- to 6-membered heterocyclyl containing 1, 2 or 3 heteroatoms
independently
selected from N, 0 and S,
(2) -(C36)cycloalkyl,
(3) (C1_6)hydroxyalkyl, or
wherein each aryl, heteroaryl, cycloalkyl, or heterocyclyl is unsubstituted or
substituted with 1,
2, or 3 R9, and wherein each alkyl is unsubstituted or substituted with 1, 2,
or 3 R1 .
In Embodiment 31 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
27, or a pharmaceutically acceptable salt of any of the foregoing, wherein R3
is
(1) 4 to 6 membered cycloalkyl optionally mono-substituted, disubstituted, or
trisubstituted with halogen, OH, C1_6alkyl, or Co_6alkyl(OH),
(2) 4 to 6 membered heterocyclyl containing 1 sulfur atom or 1 oxygen atom
optionally
mono-substituted, disubstituted, or trisubstituted with halogen, C1 -3alkyl,
Ci-
3alkyl(OH), or oxo, or
(3) Ci_6alkyl wherein the alkyl is optionally mono-substituted, disubstituted,
or
trisubstituted with halogen or OH.
In Embodiment 32 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-28 or a pharmaceutically acceptable salt of any of the foregoing, wherein R3
is
(1) 4 to 6 membered cycloalkyl optionally mono-substituted, disubstituted, or
trisubstituted with F, or OH,
(2) 4 to 6 membered heterocyclyl containing 1 sulfur atom optionally mono-
substituted,
di substituted, or trisubstituted with oxo, CHF2, or CH3,
(3) 5 or 6 membered heterocyclyl containing 1 oxygen atom optionally mono-
substituted,
di substituted, or tri substituted with CH3, or OH,
(4) C3_6alkyl-heterocyclyl, wherein the heterocyclyl is a S membered-
heterocyclyl
containing 1 oxygen atom optionally mono-substituted, disubstituted, or
trisubstituted
with OH,
(5) C1_6alkyl-heteroaryl, wherein the heteroaryl contains 2 nitrogen atoms
optionally
mono-substituted, disubstituted, or trisubstituted with CH2CH3,
(6) 5 membered heteroaryl containing 2 nitrogen atoms and 1 sulfur atoms
optionally
mono-substituted, disubstituted, or trisubstituted with CH3, or
(7) C3_6alkyl, wherein the alkyl is optionally mono-substituted,
disubstituted, or
trisubstituted with halogen, or OH.
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In Embodiment 33 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-28 or a pharmaceutically acceptable salt of any of the foregoing, wherein R3
is
(1) 4 to 6 membered cycloalkyl optionally substituted with F, or OH,
(2) 4 to 6 membered heterocyclyl containing 1 sulfur atom optionally mono-
substituted,
di substituted, or trisubstituted with oxo, or CH3,
(3) 5 or 6 membered heterocyclyl containing 1 oxygen atom optionally mono-
substituted,
di substituted, or trisubstituted with CH3, or OH, or
(4) C1_6alkyl, wherein the alkyl is optionally mono-substituted,
disubstituted, or
trisubstituted with halogen, or OH
In Embodiment 34a of this disclosure are compounds of Formula I, or any one of
Embodiments
1-29 or a pharmaceutically acceptable salt of any of the foregoing, wherein R3
is
(1) 4 to 6 membered cycloalkyl optionally mono-substituted, disubstituted, or
trisubstituted with F, or OH,
(2) 4 to 6 membered heterocyclyl containing 1 sulfur atom optionally mono-
substituted,
disubstituted, or trisubstituted with oxo, CHF2, or CH3,
(3) 5 or 6 membered heterocyclyl containing 1 oxygen atom optionally mono-
substituted,
di substituted, or trisubstituted with CH3, or OH,
(4) C1_6alkyl-heterocyclyl containing 1 oxygen atom optionally mono-
substituted,
di substituted, or trisubstituted with OH,
(5) Co_6alkyl-heteroaryl, wherein the heteroaryl contains 2 nitrogen atoms,
and 0-1 sulfur
atoms optionally mono-substituted, disubstituted, or trisubstituted with CH3,
CH7CH3, or
(6) Ct -6 al ky 1 , wherein the alkyl is optionally mono-substituted,
disubstituted, or
trisubstituted with halogen, or OH.
In Embodiment 34b of this disclosure are compounds of Formula I, or any one of
Embodiments
1-29 or a pharmaceutically acceptable salt of any of the foregoing, whet ein
RR is
(1) 4 to 6 membered cycloalkyl optionally mono-substituted, disubstituted, or
trisubstituted with F, or OH,
(2) 4 to 6 membered heterocyclyl containing 1 sulfur atom optionally mono-
substituted,
disubstituted, or trisubstituted with oxo, or CH3,
(3) 5 or 6 membered heterocyclyl containing 1 oxygen atom optionally mono-
substituted,
di substituted, or trisubstituted with CH3, or OH, or
(4) C1_6alkyl, wherein the alkyl is optionally mono-substituted,
disubstituted, or
16
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
trisubstituted with halogen, or OH.
In Embodiment 35a of this disclosure are compounds of Formula I, or any one of
Embodiments
1-29, or a pharmaceutically acceptable salt of any of the foregoing, wherein
le is
OH OH ,
cy OH
st
:
6
sek RH 0A ,c4
F
ofT, , oc_AH
, or
In Embodiment 35b of this disclosure are compounds of Formula I, or any one of
Embodiments
1-29, or a pharmaceutically acceptable salt of any of the foregoing, wherein
R3 is
OH
0h)N Rui F H 6\
RA
õse. ce 04
(5)
OH
OH
In Embodiment 36 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
29, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
In Embodiment 37 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
29, or a pharmaceutically acceptable salt of any of the foregoing, wherein le
is
di
In Embodiment 38 of this disclosure are compounds of Formula 1, or any one of
Embodiments 1-
29, or a pharmaceutically acceptable salt of any of the foregoing, wherein R3
is
0=es.
In Embodiment 39 of this disclosure are compounds of Formula I, or any one of
Embodiments 1-
29, or a pharmaceutically acceptable salt of any of the foregoing, wherein R3
is
(3,
17
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In Embodiment 40 of this disclosure are compounds of Formula I, or Embodiments
1-37, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R4 is H.
In Embodiment 41 of this disclosure are compounds of Formula I, or Embodiments
1-38, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R5 is H,
halogen, or CN
In Embodiment 42 of this disclosure are compounds of Formula I, or Embodiments
1-38, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R5 is H, F, Cl,
or CN
In Embodiment 43 of this disclosure are compounds of Formula I, or Embodiments
16-42, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R6 is
(1) cyano,
(2) halogen,
(3) -0C1_6alkyl,
(4) (C6)cycloalkyl, optionally substituted with halogen, Ci_3alkyl,
C1_6haloalkyl, or
OH,
(5) -C(=0)NH2,
(6) -O(C36)cycloalkyl wherein the cycloalkyl is optionally substituted with
halogen,
C13alkyl, or OH,
(7) hydroxy,
(8) N(R11)2,
(9) (C1_6)h al oalkyl
(10) -0(C1_6)hal alkyl -,
(11) -S02(C16)alkyl,
(12) -SO2NH(C 1_6)alkyl,
(13) -SC1_6alkyl,
(14) -SCi_61ta1oa1ky1, or
(15) (C1_6alkyl).
In Embodiment 44a of this disclosure are compounds of Formula I, or
Embodiments 15-42, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R6 is halogen,
hydroxy, CN, C1_3alkyl, C1-3haloalkyl, C3_6cycloalkyl, 0C1-3alkyl,
OC1_3haloalkyl, 0C3-
6cyc10a1ky1, S(0)2Ci_3alkyl, NHC(0)C1_3alkyl, NC1_6alkyl, C(0)NH2, SC1_6alkyl,
S(0)2NHC1_
6a1ky1, and wherein the cycloalkyl is optionally substituted with halogen.
In Embodiment 44b of this disclosure are compounds of Formula I, or
Embodiments 15-42, or a
18
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R6 is halogen,
hydroxy, CN, C13alkyl, Ci_3haloalkyl, C3_6cycloalkyl, OC13alkyl,
OC1_3haloalkyl, 0C3_
6cyc10a1ky1, S(0)2C1_3alkyl, and wherein the cycloalkyl is optionally
substituted with halogen.
In Embodiment 45 of this disclosure are compounds of Formula I, or Embodiments
15-42, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R6i s C1 _3a1 kyl,
C3_6cycloalkyl, 0-C3_6cycloalkyl, OC1_3a1ky1, OC1_3ha1oa1ky1, or
S(0)2C1_3alkyl, and wherein the
cycloalkyl is additionally optionally substituted with 1-3 F.
In Embodiment 46a of this disclosure are compounds of Formula I, or
Embodiments 15-42, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R6 is F, CF3,
OCHF2, OCF nCT-T CH OCH(CH cm-F CHF OCH CHF - - --2 -3, -3,2, 2 2, - -
-2 -- cyclopropyl, Of 0-
cyclopropyl, CN, N(CH3)2, C(0)NH2, SCH3, S(0)2NHCH3, NHC(0)CH3, OH, OCF2CHF2,
S(0)2CH3, and wherein the cyclopropyl is additionally optionally substituted
with one to three
halogen atoms.
In Embodiment 46b of this disclosure are compounds of Formula I, or
Embodiments 15-42, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein R6 is F,
OCHF2, OCF3, OCH2CH3, OCH(CH3)2, OCF2CHF2, OCH2CHF2, cyclopropyl, or 0-
cyclopropyl, and wherein the cyclopropyl is additionally optionally
substituted with one to three
halogen atoms.
In Embodiment 47 of this disclosure are compounds of Formula I, or Embodiments
1-15 and 29-
46, or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein R7 is
halogen, C1_6haloalkyl, or OH
In Embodiment 48 of this disclosure are compounds of Formula I, or Embodiments
1-15 and 29-
46, or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein R7i s
F, CF3, or OH.
In Embodiment 49 of this disclosure are compounds of Formula I, or Embodiments
1-45, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein Rs is H.
In Embodiment 50 of this disclosure are compounds of Formula I, or Embodiments
1-46, or a
class thereof, or a pharmaceutically acceptable salt of any of the foregoing,
wherein leb is H.
In Embodiment 51 of this disclosure are compounds of Formula I, or Embodiments
1-30, 40-50,
or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein R9 is =0,
OH, C1_6alkyl, C16haloalky, halogen, or C1_6alkylOH.
In Embodiment 52 of this disclosure are compounds of Formula I, or Embodiments
1-30, 40-50,
or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein R9 is 02,
19
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
=0, OH, Ci_6alkyl, Ci_6haloalky, or C1_6alkylOH.
In Embodiment 53a of this disclosure are compounds of Formula I, or
Embodiments 1-30, 40-50,
or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein R9 is 02,
=0, OH, CH3, CH2CITh, F, CF3, CHF2, or CH7OH.
In Embodiment 53b of this disclosure are compounds of Formula I, or
Embodiments 1-30, 40-50,
or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein R9 is =0,
OH, CH3, CH2CH3, F, CF3, or CH2OH
In Embodiment 54 of this disclosure are compounds of Formula I, or Embodiments
1-30, 40-53,
or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein Rl is =0,
OH, C1_6alkyl, C1_6haloalky, or C1_6alkylOH.
In Embodiment 55 of this disclosure are compounds of Formula I, or Embodiments
1-30, 40-53,
or a class thereof, or a pharmaceutically acceptable salt of any of the
foregoing, wherein RI is =0,
OH, CH3, CH2CH3, F, CF3, or CH2OH
In Embodiment 56 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein X
is N, Y is C(R5),
and Z is C(R5).
In Embodiment 57 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein X
is C(R5), Y is N,
and Z is C(R5).
In Embodiment 58 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein
Xis C(R5), Y is
C(R5) and Z is N.
In Embodiment 59 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein
X, Y, and Z are
C(R5).
In Entbodiment 60 of this disclosure are compounds of Foimula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein X
is N, Y is N and Z
is C(R5).
In Embodiment 61 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein
Xis N, Y is C(R5)
and Z is N.
In Embodiment 62 of this disclosure are compounds of Formula I, or any one of
Embodiments
1-51, or a pharmaceutically acceptable salt of any of the foregoing, wherein
Xis C(R5), Y is N
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
and Z is N.
In Embodiment 63, the present invention provides a compound as described in
any one of
Examples 1-129 as set forth below, or a pharmaceutically acceptable salt
thereof.
In Embodiment 64, the present invention is a compound, or a pharmaceutically
acceptable salt
thereof, which is
)..-0
* r_O
* F _
õup
0, iF
41* F/T--
F F F N
.D.C,...... I 1
, or
..:ZF
IRI 110I .
ni
co-8
In Embodiment 65, the present invention is a compound, or a pharmaceutically
acceptable salt
¨N 0 N F Fc __
...N 0
....y5_,..F F \ Fir F
_
'---
1 H
N 4 1 \f,0 H F ....,õ
H 1:001 1\ J,0
0.0- I )--- 0=sy t I
0= N
thereof, which is 8 & )-- C =I )--
, or
F
iil 0 rt0
.r--- L
c,. )--
i ,.......
In Embodiment 66, the present invention is a compound, or a pharmaceutically
acceptable salt
F 0
)---
F
11 (lb rq)c0
I0=1.....--- = )---
thereof, which is
In Embodiment 67, the present invention is a compound, or a pharmaceutically
acceptable salt
F
)..._0
49
F
Iii (1101 rsi0
=
n ,....'"
thereof, which is d .
21
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In Embodiment 68, the present invention is a compound, or a pharmaceutically
acceptable salt
F _
o=c1101 r\i/0
=
thereof, which is d' ¨
In Embodiment 69, the present invention is a compound, or a pharmaceutically
acceptable salt
* FiV.F
odiN
thereof, which is c?
In Embodiment 70, the present invention is a compound, or a pharmaceutically
acceptable salt
N
1101
=
thereof, which is - 8
In Embodiment 71, the present invention is a compound, or a pharmaceutically
acceptable salt
1101 k
thereof, which is de
In Embodiment 72, the present invention is a compound, or a pharmaceutically
acceptable salt
11 tNO
thereof, which is de
In Embodiment 73, the present invention is a compound, or a pharmaceutically
acceptable salt
aki 0
thereof, wh i ch is
In Embodiment 74, the present invention is a compound, or a pharmaceutically
acceptable salt
22
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
4
NI 1101 = C2'
IN
cI
thereof, which is
In Embodiment 75, the present invention is a compound, or a pharmaceutically
acceptable salt
....N 0
Fit \---
F
dH
N 4C) N(
0= i )."--
thereof, which is 8 .
In Embodiment 76, the present invention is a compound, or a pharmaceutically
acceptable salt
F--crYN )--F
F
rl # NO
0=
)---
thereof, which is c5'sd- 1
In Embodiment 77, the present invention is a compound, or a pharmaceutically
acceptable salt
..._N 0
Fi__.F
F
# N)=0
i
.1' = )---
thereof, which is oc5'
In Embodiment 78, the present invention is a compound, or a pharmaceutically
acceptable salt
N
F
ril Iiii NO
=r--- 1 ,)---
thereof, which is .:?.'" .
In Embodiment 79 of the invention, the compound of formula I, or a
pharmaceutically
acceptable salt thereof, is:
F),..._F
F)......F
F
0 p p N p
04.11 , so 1,4,. 0,5* NH i SO \C' 0... NH 1 = o p
si.H 110 'No
Ni-
8 6
5
5
2 c).-)ci..F
.-0)......
c
= i:N1 I NI*1
)---- 6: 1110 NO
N
2
I
= )--- si: 1110 NO
=
c,=.8=01-8 cr-8
' ' , '
23
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
41k 41
pe-Fc>rF 2_0\___c
F
HN
0,,... = ,,,_.
, - , , ,
0 Et0 N
Et0ON
r
9_0,7.
0
.
I I 1101 ..
,,,
)--- 0=r I : H
N
NH so , IP ,oF , , 0 F
i
)--- 0s-A- = )-- 0.65
cr%8 ---- 8 dr
Et0 F2HCO N Et0
N F2HCO N F A
/ N
pF
pF F
PF *
c.---Z- F F
_6H
H
N.,0 LN 1 ).... dNH 0
I N
9115 = =
i
../L- 0= i
0- =
& & .0)---- 01'8 *8
F2HCO F2HCO N _F
n_.01-
)F
r
1 H
N * H
1,1,0 LN * 0 1 13 NI
N * - 1\ 0
'
0=0 1 c3 I
=
& )-'. cf-g /L- cpii
F
)F H0,0 yroy 0
9--(\ F
(C) 9H H (61 9-1,1, 0 '..--F
HOFFk.s.'
/N HA
OH H00
NH I $11 i'll HA N
()
--- 1 )-- 4'..) =I )--- i )---
F ),...F
F
F, _F
Pr-rF
9.-rF
6 s 1 ill 'I: t 0 f * N (C'
Foil Ilp r,(0
I
= OH HN
' I
. 1_
7---
,d' ,
F F
F),.....F F
Yc),
,,. i * r,c) , * rs,.. o .. 1 iii 1,40
C
(;)H 1
6,,,z) efsb 1 4), (-3 1 r 1 )---- o--C-18 =I
b 02(c- L )
,
,
k _o
)...Ø2
P H
H 00 N)=0 0=c_.=N
0, , ii, isto (...N
=
I LAF . C;8/ I
IL"--F=F =
/
24
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
F F
),..0
* F
\r..0
.).-.0
* * ,c) H
N 110 N>=0 c,k1 110 N>=
00.------õ,. (=, =
,
6' cr-8
,
F
,.....F F
F)_-F FzHCO
* ).....F
F *
F
6.. 1 110 N>=0
I
= µ=-=-./ H
ci,,N
I
= *NO
A. I ... -!r=H
N *
I Nt.0
.)-- i
crr-h cr.8 -Ill F,../ = 0=
)"..--
6, V
Fz HCO FzH CO
F2HCO F2HCO
1
F 1 11 110 n?=0 1,1 * r?c=0 01 NO
0
)-' (,,;'8/1 = * I \ .-E-
1
1
2--
e, \./-= 0.,
O N
2¨. -8
,
0 F
N N
c)F
N
I ; r\i)( iiiNlkiTriC) 0
- Ort,..0\¨(2'
)---- õ,,,
(37/ ',./. ---- o i =
6' ).---
, ,
C = oc_.
F '.0 F>F y F
N F
N
N,....
0 N
0-iy.:Eir.(N Fl\lpNC)
/)---- 0.C'
/)----
2-- ,8
F
= VF
F F
Nõ, 0
* F>c(F 0
= F>F
.)----- 0
H I NI
OALyN,,IrCilg 0
H
oly,r: N
.., N
(P8 /"\--- *---
, , ,
\-- .
. 0\
\¨
N
S--1' I ;(D
..)---- 0
H I N
Oly NrCõ,,,X-- No 0
orty...:1,1rUN"' No
o,--8
..)--- /)--
ik o\__ at oy
N N N
LI
0 .g(;1:;00
=r"
)-------
)--- 6, --,...--
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
N
F = )F
NF O oy N . F
,
6..N ,...-
)---- -'('
)------ ---1 P,)---
a=8 -_,--....--=
40 o*8
, , ,
ci/N õe ri NI 1,4,0 dirlp r\j,0
/I-- /Is-
018 ci5=8
*
0
F).....F F)....E r F
F * ),....F
F * F *
INilirXr ? Id 110 r\O
Nr 6. i
= i
0=
./L ,)"....- 0-0 i "'I's' - =
6, -...../.
F21-100 F2HCO F2HCO
F * )....F
P *
P
F
IF
F
HN 0110 Nr, 0
H R, so
i\,,
0=r-- :
6 i , ,
0= õ,..._ 0= = )......
0c
., .
.)--
, ....-
, ef di (g
,
F2HCO 1-2FICO F2HCO F2HCO
* *
p ci P
,, 0 ,c, 0 ,,,0 ,,,,i * 11,..0 cH *
NI rµi,,0
_ i
0::0L 1 ,õ,)\ 0=6 L ,)-- 0=(....õ. 1 ,,)--- 0= =1
)---
6,
7
0)....F E = 0).... F
H
* r,(0 II * r4,0 Il 4 r,C)
1 lie Isi0
0=r 1 I 0=CF i N
=I Co).4.0(.. 1
e9 \ .===' 2----(OH
,$)--=6H
OF
H 0
N 40 Nt 14 140 Ni/CD rE4I 40 is I.
N/C)
0= r- 1 0=r- ,
,k
=
1).-f H d, ....- --(0 H O=d-
ef 4eLf0H 0=
,.....- = 4)-
6 H
N N
F cy 0 F
F_p..--0._
F_p---:___
rEsJI
O 0H 11 Mit ri)=0 H
r-' i
- =-....." ,)--14.0H 0=r 1 r' I r' I
0=
= ,Lf Oa ,....õ.. =
)==-f0H "L.-6H
& er ....." eso
`...., .
26
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
F * 0)CF F * )c-FF F * )c-FF F
r "F
F F
4 1 \e,0 140 4r \(
411 1 \ 1,0
1 r' 1 Lej =I
..)--.6H 0.3...-(OH
").-----(0 H -
7, ......," ..)...(0 H 0= 0=
C i
/ ' / /
F
F-5_r --''(F
F i. \--F F * F 1
F F
il 4 Nr*0 ril 410 Nr*0 ji41 41
0 r4I 0 iµ 0
i )---6H 0= (' 1 ,s`L-(OH ......." 0=U
I
=
).--'(OH OH
,,, ......,"
F F
F F F F
rhql 141 Ne)= o
r===^J 0 ,t 0 ,..-.) * ,. *
0 = Sõ... r'' 1
, -...- 1 4..1 ----(0 H 0 p,,,,, 1
.)--.1Z OH (:).?, ',..., = IL(0 H
/ u / / /
F
5--)--FO".....4:F 9.-"F )14:F 9- OF)r(F
_11 4o H 111 11. Nr)=0
CP 4 rs(C'
1=I
i'Lf0H = ,$)---"f0H 0=
4, '....e. ).--(OH 0=
e ''.'" = ,
....--(OH
F
N N F 1 ....2- 9
2 -N )
F-1)(F
õv....0s_ F /....:, 0.õ,....... F
F --.
6N 0 1\(C) F
. r\(0
1 c.,',):0
40 r\i,c,
H
dm
N I* .
r.,
= )¨OH = s$:L--(OH 01'8 0=
2---
d
F F
F
...N F--51)-- \-"' N y...).....F
F
F
* r \ j)=0 110 r\I o 4 o H F
1
. )--- 0=1-- I
8 cY
, ' , , ,
0
HN--k.
F * OH a 0
F__;-9-)-F FirN \---k,
F 11110 r,(0 F
H
N * i ,. 1.0 t 0 H
0=r-'.., ...õ I N 1/01 r\i/C)
ci-- =i )---
co)>:204.. i
N
(
llir * F
4* N
c....k1 * µ=0
is( H
N * 1,10
I 1
10=1J- = )--
. )--- 0= .
0,8 >-
)-- or: :
d'
, , '
27
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
F F * 0 F * r\f\ . H2 * S
\
liki I\ 11101 r\i0 H
N 1.1 I\C' 1101
r,i3O
(......,. i
= 2-..".
1 02( .......õ. õ)""==== 0=rj L
µ)"." 0-r' I 0=
'i /IL Ci--== F F -).-- Fµ.
III I!)
r *
0= H
CN I Ni10
= )s-- H
I
= =
)2
N t1101
i 02,,,. EN1 (116 1\1 F (:P H
' 0&d
0 1401
is
* I %
* ).._.F
F, sysC1)H pl...
2'.
1 1011 1,( HN 4 No riC-.)),N
4
H NO 1101
Nr, 0
0=c-. ,
4,õ_INH2
... )- 1
1 ,. 1 ,,.... 6,......
, , ,
,
F
).....F F)e..F
9- 101 p;C>_.F
* 1,1)0 0=r- I cab
H2
.0,,ILIN dp N.I...
(5, 4 , or
, .
In Embodiment 80 of the invention, the compound of formula I, or a
pharmaceutically
acceptable salt thereof, is:
(R) - 1,3-bis(4-fluoropheny1)-N-(3-methy1-1,1-dioxidotetrahydrothiophen-3-y1)-
2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3-(difluoromethoxy)pheny1)-3-(4-fluoropheny1)-N-(3-methyl-1,1-
dioxidothietan-3-y1)-2-oxo-
2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
(R) - 1-(3 -(difluoromethoxy)pheny1)-3-(4-fluoropheny1)-N-(3-methyl-1, 1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imidazole-5-
carb oxamide,
3-(3-(difluoromethoxy)pheny1)-1-(4-fluoropheny1)-N-(3-methyl-1,1-
dioxidothietan-3-y1)-2-oxo-
2,3-dihydro-1H-imidazo[4,5-b]pyridine-6-carboxami de,
3-i sopropyl-N-(3-methy1-1,1-dioxo-thietan-3-y1)-2-oxo-1-[3-
(trifluoromethoxy)phenyl]benzimidazole-5-carb oxamide,
1-(3-isopropoxypheny1)-3-isopropyl-N-(3-methy1-1,1-dioxo-thietan-3-y1)-2-oxo-
benzimidazole-
5-carboxamide,
3-i sopropyl -N-(3-methyl -1,1-di oxo-thi etan -3 -y1)-2-oxo-1 - [3-(1, 1,2,2-
tetrafluoroethoxy)phenylThenzimidazole-5-carboxamide,
1-[3-(2,2-difluoroethoxy)pheny1]-3-isopropyl-N-(3-methy1-1,1-dioxo-thietan-3-
y1)-2-oxo-
28
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
benzimidazole-5-carboxamide,
1-(3-ethoxypheny1)-3isopropyl-N-(3-methy1-1,1-di oxo-thi etan-3-y1)-2-oxo-b
enzimi dazol e-5-
carboxamide,
3-i sopropyl -N-(4-methy1-1,1-di oxo-thian-4-y1)-2-oxo-1- [3-
(trifluoromethoxy)phenyl ]benzimi dazol e-5-carb oxami de,
1- [3-(2,2-difluoroethoxy)phenyl] -3-i sopropyl-/V-(4-methyl -1,1-di oxo-thi
an-4-y1)-2-oxo-
benzimi dazole-5-carboxamide,
1- [4-(di fl uorom eth oxy)-2-pyri dyl ]-3 sopropyl -N-(3 -m ethyl -1,1-di oxo-
thi etan-3-y1)-2-oxo-
benzimidazole-5-carboxamide,
1-(3-cyclopropylpheny1)-34 sopropyl-N-(3-methy1-1,1-di oxo-thietan-3-y1)-2-oxo-
benzimidazole-
5-carb oxamide,
1- [3-(cycl opropoxy)phenyl ]-3-i sopropyl-N-(3-methy1-1,1-di oxo-thi etan-3-
y1)-2-oxo-
benzimi dazole-5-carboxamide,
1-(2-Ethoxy-5-fluoropyri di n-4-y1)-3-i sopropyl -N-(4-methy1-1,1-dioxi
dotetrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(2-ethoxy-5-fluoropyridin-4-y1)-3-i sopropyl-N-(3-methy1-1, 1-di oxi dothi
etan-3-y1)-2-oxo-2,3-
dihydro-1H-benzo [d]imidazole-5-carboxami de,
(5)- 1-(2-Ethoxy-5-fluoropyri din-4-y1)-3-i sopropyl-N-(3 -methyl-1,1-di oxi
dotetrahydrothiophen-
3 -y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
(R)- 1-(2-Ethoxy-5-fluoropyri din-4-y1)-3 sopropyl-N-(3-methy1-1,1-di oxi
dotetrahy drothi ophen-
3 -y1)-2-oxo-2,3-dihydro-1H-benzo[d] i mi dazol e-5-carboxami de,
1-(2-(Difluoromethoxy)-5-fluoropyridin-4-y1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-
2H-thi opyran-4-y1)-2-oxo-2,3-di hydro-1H-benzo[d]imi dazol e-5-carb oxami de,
1-(2-(difluoromethoxy)-5-fluoropyri din-4-y1)-34 sopropyl-N-(3-methyl -1,1-di
oxi dothi etan-3-y1)-
2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxami de,
1-(2-Ethoxy -541 uoi opyi idin-4-y1)-6-fluoi o-3-isopi opyl-N-(3-methy1-1,1-
dioxidothietan-3-y1)-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3-(2,2-Difluorocyclopropyl)pheny1)-3-isopropyl-N-(3-methyl-1,1-
dioxidothietan-3-y1)-2-oxo-
2,3-dihydro-1H-benzordlimidazole-5-carboxamide,
(R)- 1 -(3-(Difluoromethoxy)pheny1)-3 sopropyl-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-
y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(2-(Difluoromethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3-i sopropyl-N-(3-methy1-
1,1-
dioxidothietan-3-y1)-2-oxo-2,3-dihydro-1H-benzordlimidazole-5-carboxamide,
29
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
1-13 -(difluoromethoxy)phenyl] -3-isopropyl-N-(4-m ethyl-1,1 -di oxo-thi an-4-
y1)-2-oxo-
benzimi dazole-5-carboxamide,
1- [3 -(difluoromethoxy)phenyl] -3-i sopropyl-N-(3 -m ethyl-1,1 -di oxo-thi
etan-3-y1)-2-oxo-
benzimi dazole-5-carboxamide,
143 -(difluoromethoxy)phenyl]-N-(2-hydroxy-1,2-di methyl -propy1)-3 -isopropy1-
2-oxo-
benzimi dazole-5-carboxamide,
1- [3 -(difluoromethoxy)phenyl] -N-[(1S,2S)-2-hydroxycycl op enty1]-3 -
isopropy1-2-oxo-
benzi mi dazol e-5-carboxami de,
1- [3 -(difluoromethoxy)phenyl] -N- [(3R,4S)-3 -hydroxytetrahydropyran-4-yl] -
3-i sopropy1-2-oxo-
benzimidazole-5-carboxamide,
N-[(1 S,2R)-3 ,3-difluoro-2-hydroxy-cycl ohexyl] -1-[3-
(difluoromethoxy)phenyl] -3-isopropy1-2-
oxo-b enzimi dazole-5-carb oxami de,
1- [3 -(difluoromethoxy)phenyl] -N-[(1R,2R)-2-hydroxycycl ohexyl] -3-i
sopropy1-2-oxo-
benzimi dazole-5-carboxamide,
113 -(difluoromethoxy)phenyl] -3-i sopropyl-N-[(3S)-3 -methyl-1,1 -di oxo-thi
ol an-3-y1]-2-oxo-
benzimi dazole-5-carboxamide,
1- [3 -(difluoromethoxy)phenyl] -N-(3 ,3-di fluoro-l-methyl-cy cl obuty1)-3-
isopropy1-2-oxo-
benzimi dazole-5-carboxamide,
1- [3 -(difluoromethoxy)phenyl] 15)-1-(hydroxym ethyl)-2-methyl-propyl] -
3-i sopropy1-2-oxo-
benzimidazole-5-carboxamide,
143 -(difluoromethoxy)phenyl]-N-K1S,2,9-2-hydroxycycl oh exyl] -3-i sopropy1-2-
oxo-
benzimidazole-5-carboxamide,
1-(3-(difluoromethoxy)pheny1)-N-(3-m ethyl -1,1 -di oxi doth i etan-3 -y1)-2-
oxo-3 -(tetrahy dro-211-
pyran-4-y1)-2,3-dihydro-1H-benzo[d]imidazol e-5-carboxamide,
(R)-3 -Cycl obuty1-1-(3 -(difluoromethoxy)phenyl)-N-(3-methy1-1, 1-di oxi
dotetrahydrothi ophen-3-
y1)-2-oxo-2,3 -ditty o-1H-benzo[d]imidazole-5-cat boxamide,
(R)-3 -Cy cl openty1-1-(3-(difluoromethoxy)pheny1)-N-(3 -methyl-1,1 -di oxi
dotetrahy drothiophen-
3 -y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
1-(3 -(Difluoromethoxy)pheny1)-3-ethyl-N-(4-methyl-1,1-dioxi dotetrahydro-2H-
thi opyran-4-y1)-
2-oxo-2,3-dihydro-1H-benzordlimidazole-5-carboxami de,
1-(3 -(Difluoromethoxy)pheny1)-3-ethyl-N-(3-methy1-1,1-dioxi dothi etan-3-y1)-
2-oxo-2,3 -
dihydro-1H-benzo [d]imidazole-5-carboxami de,
3 -Cycl opropy1-1-(3-(difluoromethoxy)pheny1)-N-(4-methyl-1,1-dioxi
dotetrahydro-2H-
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
1-(3-(Difluoromethoxy)pheny1)-N-(4-methy1-1,1-dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-3-
(3,3,3-trifluoropropyl)-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3 -(Difluoromethoxy)pheny1)-/V--(3-methyl-1, 1-di oxi dothi etan-3 -y1)-2-
oxo-3-(2,2,2-
tri fluoroethyl )-2,3-di hydro-1H-benzo[d]imi dazole-5-carboxami de,
1-(3 -(difluoromethoxy)pheny1)-N-(4-m ethy1-1,1-dioxidotetrahy dro-2H-thi
opyran-4-y1)-2-oxo-3-
(2,2, 2-trifluoroethyl)-2,3-dihydro-1H-b enzo[d]imidazole-5-carboxamide,
(R)-1-(3-(di uorom eth oxy)pheny1)-3-(1, 1-di fl uoropropan-2-y1)-AT-(4-m
ethyl -1,1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imi
dazol e-5-carb oxami de,
(5)-143 -(difluoromethoxy)pheny1)-3-(1, 1-difluoropropan-2-y1)-N-(4-methy1-1,1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imi
dazol e-5-carb oxami de,
3 -(sec-buty1)-1-(3 -(difluoromethoxy)pheny1)-N-(3 -methyl-1,1-di oxidothietan-
3-y1)-2-oxo-2,3 -
dihydro-1H-benzo [d]imidazole-5-carboxami de,
(R)-3 -(sec-butyl)-1-(3-(difluoromethoxy)pheny1)-N-(3-methyl -1, 1-di oxi
dothi etan-3 -y1)-2-oxo-
2,3 -dihydro-1H-benzokilimidazole-5-carboxamide,
(S)-3-(sec-buty1)-1-(3-(di fluoromethoxy)pheny1)-N-(3 -m ethy1-1,1-di oxi
dothi etan-3 -y1)-2-oxo-
2,3 -dihydro-1H-benzordlimidazole-5-carboxamide,
1-(3 -(difluoromethoxy)pheny1)-3-(2-hy droxy-2-methylpropy1)-N-(3 -methyl-1,1-
di oxi dothi etan-
3 -y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
1-(5-(Difluoromethoxy)-2-fluoropheny1)-3-i sopropyl-N-(4-methyl-1, 1-di oxi
dotetrahy dro-2H-
thi opyran-4-y1)-2-oxo-2,3-di hydro-1H-benzo[d] i mi dazol e-5-carboxami de,
1-(5-(Difluoromethoxy)-2-fluoropheny1)-3-i sopropyl-N-(3-methy1-1, 1-di oxi
dothi etan-3 -y1)-2-
oxo-2,3-di hydro-1H-benzo [d]i m i dazole-5-carboxami de,
(5')-1-(5-(Difluoromethoxy)-2-fluoropheny1)-3-i sopropyl-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide,
(R)-1-(5-(Difl uoi omethoxy)-2-fl uoi opheny1)-3-isopi opyl-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide,
1-(5-(Difluoromethoxy)pyri din-3 -y1)-3-i sopropyl-N-(3-methy1-1, 1-di oxi
dothi etan-3-y1)-2-oxo-
2,3 -dihydro-1H-benzordlimidazole-5-carboxamide,
1-(5-Cy cl opropylpyridin-3-y1)-3-i sopropyl-N-(3-methy1-1, 1-di oxi dothi
etan-3-y1)-2-oxo-2,3-
dihydro-1H-benzo [d]imidazole-5-carboxami de,
1-i sopropyl-N-(4-methy1-1,1-dioxo-thian-4-y1)-2-oxo-3-[3-(1,1,2,2-
tetrafluoroethoxy)phenyllimidazo[4,5-b]pyridine-6-carboxamide,
31
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
3-13 -(difluoromethoxy)phenyl] -1-i sopropyl-N-(4-m ethy1-1,1-di oxo-thi an-4-
y1)-2-oxo-
imidazo [4,5-b] pyridine-6-carboxamide,
3 -[3-(difluoromethoxy)pheny1]-1-isopropyl-N-[(3S)-3-methyl-1,1-dioxo-thiolan-
3-y1]-2-oxo-
imidazo[4,5-b]pyridine-6-carboxamide,
3 -[3-(difluoromethoxy)pheny1]-1-i sopropyl-N-[(3R)-3-m ethyl -1,1-di oxo-thi
ol an-3 -y1]-2-oxo-
imidazo [4,5-b] pyridine-6-carboxamide,
1-i sopropyl -N-(3-methy1-1,1-di oxo-thietan-3 -y1)-2-oxo-3 - [3-
(tri fl uoromethoxy)phenyl ]imi dazo[4,5-h]pyri di ne-6-carb oxami de,
1-i sopropyl-N-(4-methy1-1,1-di oxo-thian-4-y1)-2-oxo-3 -[3-
(trifluoromethoxy)phenyl]imidazo[4,5-b]pyridine-6-carboxamide,
1-i sopropyl-N-(3-methyl-1,1-di oxo-thietan-3 -y1)-2-oxo-3 -[3-(1, 1,2,2-
tetrafluoroethoxy)phenyl]imidazo[4,5-b]pyri dine-6-carboxamide,
1-i sopropyl-N- [(3S)-3 -methyl-1, 1-dioxo-thiolan-3-y1]-2-oxo-3 4341, 1,2,2-
tetrafluoroethoxy)phenyllimidazo[4,5-b]pyri dine-6-carboxamide,
1-i sopropyl-N- [(3R)-3 -methyl-1,1-di oxo-thi ol an-3-y1]-2-oxo-3-[3 -
(1,1,2,2-
tetrafluoroethoxy)phenyl]imidazo[4,5-b]pyri dine-6-carboxamide,
3 -(3 -ethoxypheny1)-14 sopropyl-N-(3-methy1-1,i-di oxo-thietan-3-y1)-2-oxo-
imi dazo[4,5 -
b]pyridine-6-carboxamide,
3 -(3-ethoxypheny1)-14 sopropyl-N-[(3,9-3-methyl-1,1-dioxo-thi olan-3-y1]-2-
oxo-imi dazo[4,5-
b]pyridine-6-carboxamide,
3 -(3-eth oxypheny1)-1-i sopropyl-N-[(3R)-3-m ethyl -1,1-di oxo-thi ol an-3-
y1]-2-oxo-imi dazo[4,5-
b]pyridine-6-carboxamide,
3 -(3-eth oxypheny1)-1-i sopropyl-N--(4-m ethyl-1, 1-di oxo-thi an-4-y1)-2-oxo-
imi dazo[4,5-
b]pyridine-6-carboxamide,
3 -(3 -i sopropoxypheny1)-1 sopropyl-N-(3-methy1-1,1-dioxo-thietan-3-y1)-2-oxo-
imidazo[4,5-
b]pylidine-6-calboxamide,
3 -(3 -i sopropoxypheny1)-1 sopropyl-N-(4-methy1-1,1-dioxo-thian-4-y1)-2-oxo-
imidazo[4,5-
b]pyridine-6-carboxamide,
3 45-(difluoromethoxy)-2-fluoro-pheny1]-1-i sopropyl-N-(3 -methyl-1,1-dioxo-
thi etan-3 -y1)-2-
oxo-imidazo[4,5-b]pyridine-6-carboxamide,
3 - [5-(difluoromethoxy)-2-fluoro-phenyl] -1-i sopropyl-N-(4-methy1-1,1-dioxo-
thian-4-y1)-2-oxo-
imidazo[4,5-b]pyridine-6-carboxamide,
3- [3 -(difluoromethoxy)phenyl] -1-i sopropyl-N-(3 -m ethy1-1,1-di oxo-thi
etan-3-y1)-2-oxo-
32
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
imidazo[4,5-b]pyridine-6-carboxamide,
3 -(3 -(difluoromethoxy)pheny1)-1-i sopropyl-N-(3 -m ethy1-1,1-di oxi dothi
etan-3 -y1)-2-oxo-2,3-
dihydro-1H-imidazo[4,5-c] pyridine-6-carb oxamide,
3 -(3 -(difluoromethoxy)pheny1)-1-i sopropyl-N-(4-m ethy1-1,1-di oxi dotetrahy
dro-2H-thi opyran-4-
y1)-2-oxo-2,3 -di hydro-1H-i midazo[4,5-c]pyridine-6-carboxami de,
1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(3-methy1-1,1-dioxidothietan-3-y1)-
2-oxo-2,3-
dihydro-1H-imidazo[4,5-b]pyridine-5-carboxamide,
143 -(difluorom ethoxy)pheny1]-7-fluoro-3-i sopropyl -N-(3-m ethyl -1,1-di oxo-
thi etan-3-y1)-2-oxo-
benzimidazole-5-carboxamide,
143 -(difl uoromethoxy)pheny1]-7-fl uoro-3-i sopropyl-N-[(3S)-3-methy1-1,i-di
oxo-thi ol an-3 -y1]-
2-oxo-b enzimidazol e-5-carboxamide,
143 -(difluoromethoxy)pheny1]-7-fluoro-3-i sopropyl-N-[(3R)-3-methyl -1,1-di
oxo-thi olan-3-y1]-
2-oxo-b enzimidazol e-5-carboxamide,
1-13 -(difluoromethoxy)phenyl] -7-fluoro-3-i sopropyl-N-(4-methy1-1, 1-di oxo-
thi an-4-y1)-2-oxo-
benzimidazole-5-carboxamide,
(5)-1-(3-(Difluoromethoxy)pheny1)-6-fluoro-3-i sopropyl-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3-dihydro-1H-benzordlimidazole-5-
carboxamide,
(R)-1-(3-(Difluoromethoxy)pheny1)-6-fluoro-3-isopropyl-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide,
1-(3 -(Difluoromethoxy)pheny1)-6-fluoro-3-i sopropyl-N-(4-methyl-1, 1-di oxi
dotetrahydro-2H-
thi opyran-4-y1)-2-oxo-2,3-di hydro-1H-benzo[d] i mi dazol e-5-carboxami de,
(5)-1-(3-(Difluoromethoxy)pheny1)-4-fluoro-3-i sopropyl-N-(3-methy1-1,1-
di oxi dotetrahydrothi ophen-3-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imi dazol e-5-
carboxami de,
(R)-1-(3-(Difluoromethoxy)pheny1)-4-fluoro-34 sopropyl-N-(3-methy1-1,1-
di oxi dotetrahydrothi ophen-3-y1)-2-oxo-2,3 -dihydro-1H-b enzo [d] imi dazol
e-5-carb oxami de,
1-(3-(Difl uoi omethoxy)pheny1)-4-fluoro-3-i sop opyl-N-(4-methy1-1,1-
dioxidotth ally& o-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
(S)-6-Chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3-dihydro-1H-benzordlimidazole-5-
carboxamide,
4-chl oro-1-(3 -(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methy1-1, 1-di oxi
dotetrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
4-cy ano-1-(3-(di fluoromethoxy)pheny1)-3 sopropyl-N-(4-methyl-1,1-di oxi
dotetrahy dro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
33
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(R)- 1 -(5-(difluoromethoxy)-2-fluoropheny1)-3 -(3-hydroxy-3 -methylbutan-2-
y1)-N-(3 -methyl- 1, 1-
dioxidothietan-3 -y1)-2-oxo-2,3 -dihydro-1H-benzo[d]imidazole-5-carboxamide,
(5)-145 -(difluoromethoxy)-2-fluoropheny1)-3 -(3 -hydroxy-3 -methylbutan-2-y1)-
N-(3 -methyl-1, 1-
dioxidothietan-3 -y1)-2-oxo-2, 3 -dihydro-1H-benzo[d]imidazole-5-carboxamide,
(R) - 1 -(5-(difluoromethoxy)-2-fluoropheny1)-3-(3-hydroxy-3 -m ethyl butan-2-
y1)-/V-(4-m ethyl- 1 , 1 -
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzo [d] imi
dazol e- 5 -carboxamide,
(5)- i-(5 -(difluoromethoxy)-2-fluoropheny1)-3 -(3 -hydroxy-3 -methylbutan-2-
y1)-N-(4-methyl- 1,1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -di hydro-1 H-benzo[d]imi
dazol e- 5 -carboxami de,
(R)- i-(3 -(difluoromethoxy)pheny1)-3 -(3 -hydroxy-3 -methylbutan-2-y1)-N-(4-
methyl- 1, 1 -
di oxi dotetrahydro-2H-thi opy ran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzo[d]imi
dazol e- 5 -carboxamide,
(5)- i-(3 -(di fluoromethoxy)pheny1)-3-(3 -hydroxy-3 -methylbut an-2-y1)-N-(4-
methyl- 1, 1 -
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzo [d] imi
dazol e- 5 -carboxamide,
(R)- 1-(3 -(2,2-difluoroethoxy)pheny1)-3 -(3-hydroxy-3 -methylbutan-2-y1)-N-(4-
methyl- 1, 1 -
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzordlimi
dazol e- 5 -carboxamide,
(5)-i -(3 -(2, 2-difluoroethoxy)pheny1)-3-(3 -hydroxy-3 -methylbutan-2-y1)-N-
(4-methyl- 1, 1 -
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzo [d] imi
dazol e- 5 -carboxamide,
(R)- 1 -(2-ethoxy- 5 -fluoropyri di n-4-y1)-3 -(3 -hydroxy-3 -methylbutan-2-
y1)-N-(4-methyl- 1 , 1 -
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3 -dihydro- 1H-benzo[d]imidazole-
5 -carboxamide,
(5) - 1 -(2-ethoxy-5-fluoropyri din-4-y1)-3 -(3 -hydroxy-3 -methylbutan-2-y1)-
N-(4-methyl- 1, 1 -
2 0 di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzo
[d] imi dazol e- 5 -carboxamide,
(R)-6-fluoro- 1 -(2-fluoro-5-(trifluoromethoxy)pheny1)-3 -(3 -hydroxy-3-m
ethylbutan -2-y1)-N-(4-
methyl- 1, 1 -di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2, 3 -dihydro- 1H-
benzo [d]i mi dazol e-5-
carboxam i de,
(5)-6-fluoro- 1 -(2-fluoro-5 -(trifluoromethoxy)pheny1)-3 -(3 -hydroxy-3 -
methylbutan-2-y1)-N-(4-
2 5 methyl-1, 1 -di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3 -dihy dro-
1H-benzo [d]i mi dazol e-5-
cat boxamide,
(R)- 1 -(2-fl uoro-5 -(trifl uoromethoxy)pheny1)-3 -(3 -hydroxy -3 -
methylbutan-2-y1)-N-(4-methyl-
1 , 1 -dioxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3 -di hydro- 1H-b enzo
imidazole-5 -
carboxamide,
30 (5)-i -(2-fluoro-5-(trifluorom ethoxy)pheny1)-3 -(3-hydroxy-3 -
methylbutan-2-y1)-N-(4-methyl- 1, 1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro- 1H-b enzo[d]imi
dazol e- 5 -carboxamide,
(R)- 1 -(2-(2,2-difluoroethoxy)-5-fluoropyri din-4-y1)-3 -(3 -hydroxy-3 -
methylbutan-2-y1)-N-(4-
methyl- 1, 1 -di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2, 3 -dihydro- 1H-
benzo mi dazol e-5-
3 4
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
carboxamide,
(S) - 1-(2-(2,2-difluoroethoxy)-5-fluoropyridin-4-y1)-3-(3-hydroxy-3-
methylbutan-2-y1)-N-(4-
methyl-1, 1-di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihy dro-1H-benzo
[d]i mi dazol e-5-
carboxamide,
(R)-1-(5-(2,2-di fluoroethoxy)-2-fluoropheny1)-3-(3-hydroxy-3 -m ethyl butan-2-
yI)-N-(4-m ethyl -
1,1-dioxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-di hydro-1H-b enzo imi
dazol e-5 -
carboxamide,
(5')-1-(5-(2, 2-di fl uoroeth oxy)-2-fluoroph enyl )-6-fluoro-3 -(3-hydroxy-3 -
m ethyl butan-2-yI)-N-(4-
methyl-1, 1-di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihy dro-1H-benzo
[d] mi dazol e-5-
carboxamide,
(R)- 1-(2-(2,2-difluoroethoxy)-5-fluoropyri din-4-y1)-6-fluoro-3-(3-hydroxy-3-
methylbutan-2-y1)-
N-(4-methy1-1, 1-di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1
H -
benzo[d]imidazole-5-carboxamide,
(S)-1-(2-(2,2-difluoroethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3 -(3-hydroxy-3 -
methylbutan-2-yl)-
1-di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1 H -
benzo[d]imidazole-5-carboxamide,
(R)- 1-(5-(2,2-difluoroethoxy)-2-fluoropheny1)-6-fluoro-3 -(3-hydroxy-3-
methylbutan-2-y1)-N-(4-
methyl-1, 1-di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihy dro-1H-benzo
[d]i mi dazol e-5-
carboxamide,
(5')-1-(5-(2,2-difluoroethoxy)-2-fluoropheny1)-6-fluoro-3 -(3-hydroxy-3 -
methylbutan-2-y1)-N-(4-
m ethyl -1,1-di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-
benzo[d]i mi dazol e-5-
carboxamide,
(R)-3 -(3-hy droxy-3-m ethyl butan-2-y1)-N-(3-m ethyl -1,1-di oxi dothi etan -
3 -y1)-2-oxo-1-(3-
(1,1, 2,2-tetrafluoroethoxy)pheny1)-2,3 -di hydro-1H-b enzo[d] imi dazol e-5-
carboxamide,
(5)-3 -(3 -hydroxy-3 -m ethylbutan-2-y1)-N-(3-m ethy1-1,1-di oxidothietan-3 -
y1)-2-oxo-1-(3-(1,1,2,2-
ten afluoi oethoxy)pheny1)-2,3-dilly o-1H-benzoklimidazole-5-calboxamide,
(R)-3-(3-hy droxy-3-methylbutan-2-y1)-N-(4-methyl -1,1-di oxi dotetrahy dro-2H-
thiopyran-4-y1)-2-
oxo-1-(3 -(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-1H-benzo [d]imidazole-
5-carboxamide,
(S)-3 -(3 -hydroxy-3 -m ethylbutan-2-y1)-N-(4-m ethy1-1,1-di oxidotetrahydro-
2H-thi opyran-4-y1)-2-
oxo-1-(3-(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide,
(R)- 1-(2-ethoxy-5-fluoropyri di n-4-y1)-3-(3-hydroxy-3 -methylbutan-2-y1)-N-
(3-methy1-1,1-
dioxidothietan-3-y1)-2-oxo-2,3 -dihydro-1H-benzo[d]imidazole-5-carboxamide,
(5')-1-(2-ethoxy-5-fluoropyri din-4-y1)-3 -(3-hydroxy-3 -methylbutan-2-y1)-N-
(3 -methyl-1,1-
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
dioxidothietan-3-y1)-2-oxo-2,3-dihydro-1H-benzordlimidazole-5-carboxamide,
N-(4-(difluoromethyl)-1,1-dioxidotetrahydro-2H-thiopyran-4-y1)-1-(2-ethoxy-5-
fluoropyridin-4-
y1)-6-fluoro-3-isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
6-fluoro-3 sopropyl-N-(4-methy1-1,1-dioxi dotetrahydro-2H-thi opyran-4-y1)-2-
oxo-1-(4-(1, 1,2,2-
tetrafluoroethoxy)pyri din -2-y1)-2,3 -di hydro-1H-benzo[d]imi dazol e-5 -earb
oxami de,
1-(4-(2,2-difluoroethoxy)pyridin-2-y1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
3-i sopropyl -)V-(4-methyl- 1,1-di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-
1-(4-(1, 1,2,2-
tetrafluoroethoxy)pyridin-2-y1)-2,3 -dihydro-1H-benzo[d]imidazole-5 -carb
oxamide,
1-(4-(2,2-difl uoroethoxy)pyridin-2-y1)-6-fl uoro-3-isopropyl-N-(4-methy1-1,1-
di oxidotetrahydro-
2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carb oxamide,
1-(2-ethoxy-5-fluoropyridin-4-y1)-6-fluoro-3-i sopropyl -N-(3 -methyl-1,1-
dioxi dothi etan-3-y1)-2-
oxo-2,3-dihydro-1H-b enzo [a] imi dazole-5-carb oxami de,
1-(2-(difluoromethoxy)-5-fluoropyri din-4-y1)-6-fluoro-3-i sopropyl-N-(3-
methy1-1, 1-
dioxidothietan-3-y1)-2-oxo-2,3-dihydro-1H-benzordlimidazole-5-carboxamide,
1-(2-(difluoromethoxy)-5-fluoropyri din-4-y1)-6-fluoro-3-i sopropyl-N-(4-
methyl-1, 1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzordlimi
dazol e-5-carb oxami de,
1-(2-(2,2-difluoroethoxy)-5-fluoropyridin-4-y1)-6-fluoro-34 sopropyl-N-(4-
methy1-1,1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imi
dazol e-5-carb oxami de,
1-(4-acetami do-3 -hydroxypheny1)-3-i sopropyl -N-(3 -methyl-1,1-dioxi dothi
etan-3-y1)-2-oxo-2,3 -
di hydro-1H-benzo [d]i mi dazole-5-carboxami de,
3-i sopropyl -N-(3-methy1-1,1-dioxidothietan-3-y1)-1-(2-methylbenzo[d] oxazol -
4-y1)-2-oxo-2,3 -
di hydro-1H-benzo [d]i mi dazole-5-carboxami de,
3-isopropyl-N-(3-methyl- 1,1-dioxidothietan-3-y1)-1-(2-methylbenzo[d] oxazol -
6-y1)-2-oxo-2,3-
dihydro-1H-benzo [d]imidazole-5-carboxami de,
1-(3-fluoropheny1)-3-isopi opyl-N-(4-methy1-1,1-dioxidoteti ahydro-2H-thiopyi
an-4-y1)-2-oxo-
2,3 -dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(5-fluoro-2-i sopropoxypyri din-4-y1)-3 sopropyl-N-(4-methy1-1,1-di oxi
dotetrahy dro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d] imidazol e-5-carboxamide,
1-(3 -cyanopheny1)-3-isopropyl-N-(4-methy1-1,1-dioxi dotetrahydro-2H-thi
opyran-4-y1)-2-oxo-
2,3 -dihydro-1H-benzo[d]imidazole-5-carboxamide,
3-i sopropyl-N-(4-methy1-1,1-dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-1-(3-
(trifluoromethyl)pheny1)-2,3-dihydro-1H-benzo[dlimidazole-5-carboxamide,
36
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
1-(3-(dimethylamino)pheny1)-3-isopropyl-N-(4-methy1-1,1-dioxidotetrahydro-2H-
thiopyran-4-
y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3 -carb amoylpheny1)-3-i sopropyl-N-(4-methyl-1, 1-di oxi dotetrahy dro-2H-
thi opyran-4-y1)-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
3-i sopropyl -AT-(4-methyl -1,1-di oxi dotetrahydro-2H-thi opyran-4-y1)-1-(3-
(m ethylthio)pheny1)-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
3-i sopropyl-N-(4-methyl-1,1-dioxidotetrahydro-2H-thiopyran-4-y1)-1-(3 -(N -
methyl sul famoyl )pheny1)-2-oxo-2,3-dihydro-1H-benzo[d]imi dazol e-5-
carboxami de,
3-i sopropyl-N-(4-methy1-1,1-dioxidotetrahydro-2H-thiopyran-4-y1)-1-(3-
(methylsulfonyl)pheny1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3-(difluoromethoxy)pheny1)-3-(4-fluorobenzy1)-N-(4-methyl-1,1-
dioxidotetrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3-(difluoromethoxy)pheny1)-3-methyl-N-(3-methy1-1,1-dioxidothietan-3-y1)-2-
oxo-2,3-
dihydro-1H-benzo Vilimidazole-5-carboxami de,
1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(5-methy1-1,3,4-thiadiazol-2-y1)-2-
oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3-(difluoromethoxy)pheny1)-N43-hydroxytetrahydrofuran-3-yOmethyl)-3-
isopropyl-2-oxo-
2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide,
1-(3-(difluoromethoxy)pheny1)-N41-ethyl-1H-pyrazol-4-yl)methyl)-3-i sopropy1-2-
oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxamide,
(R)-3-(1-amino-l-oxopropan -2-y1)-1-(3 -(di fl uorom ethoxy)pheny1)-AT-(4-m
ethyl -1,1-
dioxidotetrahydro-211-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide,
(S)-3-(1-amino-1-oxopropan-2-y1)-1-(3-(di fluoromethoxy)pheny1)-N-(4-m ethyl -
1,1 -
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide,
or
1-(3-(difluot omethoxy)pheny1)-N-(4-methy1-1,1-dioxidotett
dro-2H-thi opy an-4-y1)-2-oxo-3-
(phenylsulfony1)-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide.
The present invention includes the pharmaceutically acceptable salts of the
compounds
defined therein.
In one embodiment, the present invention is a composition comprising a
therapeutically
effective amount of at least one compound of formula I, or a pharmaceutically
acceptable salt
thereof.
37
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
The invention also provides a pharmaceutical composition comprising a
therapeutically
effective amount of at least one compound of Formula I, or a pharmaceutically
acceptable salt
thereof, and a pharmaceutically acceptable carrier.
The invention also provides a pharmaceutical composition comprising a
therapeutically
effective amount of at least one compound of formula I, or a pharmaceutically
acceptable salt
thereof, and a therapeutically effective amount of at least one other
pharmaceutically active
ingredient (such as, for example, a chemotherapeutic agent).
The invention also provides a pharmaceutical composition comprising a
therapeutically
effective amount of at least one compound of formula I, or a pharmaceutically
acceptable salt
thereof, and a therapeutically effective amount of at least one other
pharmaceutically active
ingredient (such as, for example, a chemotherapeutic agent), and a
pharmaceutically acceptable
carrier.
In one embodiment, the present invention provides a composition for treating
hepatic
steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, type-2 diabetes
mellitus, obesity,
hyperlipidemia, hypercholesterolemia, atherosclerosis, cognitive decline,
dementia, cardiorenal
diseases such as chronic kidney diseases or heart failure comprising an
acceptable carrier and a
compound of formula I or a pharmaceutically acceptable salt thereof.
In one embodiment, the present invention provides a composition for treating
hepatic
steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, type-2 diabetes
mellitus, obesity,
hyperlipidemia, hypercholesterolemia, atherosclerosis, cognitive decline,
dementia, cardiorenal
diseases such as chronic kidney diseases or heart failure, comprising a
compound of formula I, or
a pharmaceutically acceptable salt thereof.
In one embodiment, the present invention provides a composition for treating
hepatic
steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, type-2 diabetes
mellitus, obesity,
hyperlipidemia, hypercholesterolemia, atherosclerosis, cognitive decline,
dementia, cardiorenal
diseases such as chronic kidney diseases or heart failure, comprising a
compound of formula I, or
a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable
carrier.
In one embodiment, the present invention provides a method of treating hepatic
steatosis,
nonalcoholic steatohepatitis (NASH), fibrosis, type-2 diabetes mellitus,
obesity, hyperlipidemia,
hypercholesterolemia, atherosclerosis, cognitive decline, dementia,
cardiorenal diseases such as
chronic kidney diseases or heart failure in a subject in need of such
treatment, comprising a
administering to said subject a therapeutically effective amount of at least
one compound of
formula I, or a pharmaceutically acceptable salt thereof.
38
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In one embodiment, the present invention provides a method of treating hepatic
steatosis,
nonalcoholic steatohepatitis (NASH), fibrosis, type-2 diabetes mellitus,
obesity, hyperlipidemia,
hypercholesterolemia, atherosclerosis, cognitive decline, dementia,
cardiorenal diseases such as
chronic kidney diseases or heart failure in a patient in need thereof,
comprising administering to
said patient a therapeutically effective amount of at least one compound of
formula I, or a
pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable
carrier.
The methods of the invention include the administration of a pharmaceutical
composition
comprising at least one compound of the invention, or a pharmaceutically
acceptable salt thereof,
and a pharmaceutically acceptable carrier.
In another embodiment, the present invention includes a method of treating
NASH and/or
fibrosis, comprising administering to a patient in need thereof a compound of
formula I, or a
pharmaceutically acceptable salt thereof.
In another embodiment, the present invention includes a method of treating
NASH and/or
fibrosis, comprising administering to a patient in need thereof a compound of
formula I, or a
pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable
carrier.
In another embodiment, the present invention includes a method of treating
NASH and/or
fibrosis, comprising administering to a patient in need thereof a composition
comprising a
compound of formula I, or a pharmaceutically acceptable salt thereof.
In another embodiment, the present invention includes a method of treating
NASH and/or
fibrosis, comprising administering to a patient in need thereof a composition
comprising a
compound of formula I, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically
acceptable carrier.
In another embodiment, the present invention provides for the use of a
compound of
formula I, or a pharmaceutically acceptable salt thereof, in the manufacture
of a medicament for
treating NASH and/or fibrosis,
In another embodiment, the present invention includes the use of a compound of
formula
I, or a pharmaceutically acceptable salt thereof, for the preparation of a
medicament for the
treatment of NASH and/or fibrosis,
"Alkyl'' means branched- and straight-chain saturated aliphatic hydrocarbon
groups
having the specified number of carbon atoms when noted. If no number is
specified, 1-6 carbon
atoms are intended for linear and 3-7 carbon atoms for branched alkyl groups.
Examples of alkyl
groups include methyl, ethyl, propyl, isopropyl, cyclopropyl, butyl, sec- and
tert-butyl, pentyl,
hexyl, octyl, nonyl, and the like. For example, the term "Ci_6a1ky1" includes
all of "C1_4alkyl"
39
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
defined as follows, plus the linear or branched chain alkyl groups, including
all possible isomers,
having 5 or 6 carbon atoms. "Ci_6alkyl" means linear or branched chain alkyl
groups, including
all possible isomers, having 1, 2, 3, 4, 5 or 6 carbon atoms, and includes
each of the alkyl groups
within C1_6alkyl including each of the hexyl and pentyl isomers as well as n-,
iso-, sec- and tert-
butyl (butyl, i-butyl, s-butyl, t-butyl, collectively "C4alkyl"; Bu = butyl),
n- and i-propyl (propyl,
i-propyl, collectively "C3alkyl"; Pr = propyl), ethyl (Et) and methyl (Me).
Commonly used
abbreviations for alkyl groups are used throughout the specification, e.g.
methyl may be
represented by conventional abbreviations including "Me" or CH3 or a symbol
that is an
extended bond as the terminal group, e.g.
, ethyl may be represented by "Et" or CFLCH3,
propyl may be represented by "Pr" or CH2CH2CH3, butyl may be represented by
"Bu" or
HN HN
CH2CH2CH2CH3, etc. For example, the structures and have
equivalent meanings. C1_6 alkyl includes n-, iso-, sec- and t-butyl, n- and
isopropyl, ethyl and
methyl. If no number is specified, 1-6 carbon atoms are intended for linear or
branched alkyl
groups.
"Alkoxy" refers to an alkyl group linked to oxygen. Examples of alkoxy groups
include
methoxy, ethoxy, propoxy and the like.
-Aryl" refers to an aromatic monocyclic or multicyclic ring moiety comprising
6 to 14
ring carbon atoms. In one embodiment, an aryl group contains from about 6 to
10 ring carbon
atoms. Monocyclic aryl rings include, but are not limited to, phenyl.
Multicyclic rings include,
but are not limited to, naphthyl and bicyclic rings wherein phenyl is fused to
a C5_7cycloalkyl or
C5_7cycloalkenyl ring. Aryl groups may be optionally substituted with one or
more substituents
as defined herein. Bonding can be through any of the carbon atoms of any ring.
"Fused Aryl- refers to an aryl ring fused with heterocyclyl or cycloalkyl.
"Halogen" or "Halo" includes fluorine, chlorine, bromine and iodine.
"Cycloalkyl" refers to a non-aromatic mono-or multicyclic ring system
comprising about
3 to 10 ring carbon atoms. If no number of atoms is specified, 3-10 carbon
atoms are intended.
Cycloalkyl may also be fused, forming 1-3 carbocyclic rings. Non-limiting
examples of
monocyclic cycloalkyls include cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl
and cyclooctyl. A cycloalkyl group is unsubstituted or substituted with one or
more ring system
substituents which may be the same or different, and are as defined within.
The term C3_
ocycloalkyl" refers to a cycloalkyl group having 3 to 6 ring carbon atoms.
Thus, for example,
"C 3 -6 cycloalkyl- includes each of cyclopropyl, cyclobutyl, cyclopentyl, and
cyclohexyl. When
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
cycloalkyl is a sub stituent on an alkyl group, the cycloalkyl substituent can
be bonded to any
available carbon in the alkyl group. The following are illustrations of -
C3_6cycloalkyl substituents
on an alkyl group wherein the sub stituent is cyclopropyl in bold:
"Haloalkyl" refers to an alkyl group as defined within, wherein one or more of
the alkyl
group's hydrogen atoms has been replaced with a halogen. In one embodiment, a
haloalkyl group
has from 1 to 6 carbon atoms. Non-limiting examples of haloalkyl groups
include CH2F, CHF2,
CF3, CH2CF3, CH2CHF2, CF2CF3, CF2CHF2, CH2C1 and CC13. The term
"C1_6haloalkyl" or
"haloCi_6alkyl"refers to a haloalkyl group having from 1 to 6 carbons.
"Haloalkoxy," "haloalkyl-O" and derivatives such as "halo(C1_6)alkoxy" are
used
interchangeably and refer to halo substituted alkyl groups linked through the
oxygen atom.
Hal oalkoxy include mono- substituted as well as multiple halo substituted
alkoxy groups. For
example, trifluoromethoxy, chloromethoxy, and bromomethoxy are included as
well as
OCH2CF3, OCH2CHF2, OCF2CF3, and OCF2CHF2.
"Heterocyclyl," "heterocycle" or "heterocyclic" refers to monocyclic ring
structures in
which one or more atoms in the ring, the heteroatom(s), is an element other
than carbon.
Heteroatoms are typically 0, S or N atoms. A heterocycle containing more than
one heteroatom
may contain different heteroatoms. Bicyclic ring moieties include fused,
spirocyclic and bridged
bicyclic rings and may comprise one or more heteroatoms in either of the
rings. The ring
attached to the remainder of the molecule may or may not contain a heteroatom.
Either ring of a
bicyclic heterocycle may be saturated, partially unsaturated or unsaturated.
The heterocycle may
be attached to the rest of the molecule via a ring carbon atom, a ring oxygen
atom or a ring
nitrogen atom. Examples of heterocyclyl groups include: piperidine,
piperazine, morpholine,
pyrrolidine, tetrahydrofuran, azetidine, oxirane, or aziridine, and the like.
heterocyclyl," "bicyclic heterocycle" or -bicyclic heterocyclic" refers to a
heterocyclic ring fused to another ring system. The fusion may be bridged or
unbridged.
Except where noted, the term "Heteroaryl", as used herein, represents a stable
monocyclic, bicyclic or tricyclic ring of up to 10 atoms in each ring, wherein
at least one ring is
aromatic and contains from 1 to 4 heteroatoms selected from the group
consisting of 0, N and S.
Heteroaryl groups within the scope of this definition include but are not
limited to:
benzoimidazolyl, benzofuranyl, benzofurazanyl, benzopyrazolyl, benzotriazolyl,
41
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
benzothiophenyl, benzoxazolyl, carbazolyl, carbolinyl, cinnolinyl, furanyl,
imidazolyl, indolinyl,
indolyl, indolazinyl, indazolyl, isobenzofuranyl, isoindolyl, isoquinolyl,
isothiazolyl, isoxazolyl,
naphthpyridinyl, oxadiazolyl, oxazolyl, oxazoline, isoxazoline, pyranyl,
pyrazinyl, pyrazolyl,
pyridazinyl, pyridopyridinyl, pyridyl, pyrimidinyl, pyrrolyl, quinazolinyl,
quinolyl, quinoxalinyl,
tetrazolyl, tetrazolopyridyl, thiadiazolyl, thiazolyl, thienyl, triazolyl, di
hydrobenzoimi dazolyl,
dihydrobenzofuranyl, dihydrobenzothiophenyl, dihydrobenzoxazolyl,
dihydroindolyl,
dihydroquinolinyl, methylenedioxybenzene, benzothiazolyl, benzothienyl,
quinolinyl,
isoquinolinyl, oxazolyl, and tetra-hydroquinoline.
"Fused heteroaryl" is heteroaryl fused with an aryl or heteroaryl
"Oxo" means an oxygen linked to an atom by a double bond. An example of an oxo
group is a doubly bonded oxygen in a ketone, sulfoxide, sulfone and sulfate.
"Hydroxyalkyl" or "hydroxy(C1-3)alkyl" means an alkyl group having one or more
hydrogen atoms replaced by hydroxyl (-OH) groups
"Cyanoalkyl" means an alkyl group haying one or more hydrogen atoms replaced
by
cyano (-CN) groups.
"Hydroxyhaloalkyl" means an alkyl group having one or more hydrogen atoms
replaced
by hydroxyl (-OH) groups, and one or more hydrogen atoms replaced by a
halogen.
"Hydroxycycloalkyl" means a cyclic alkyl group having one or more hydrogen
atoms
replaced by hydroxyl (-OH) groups.
The term "composition" is intended to encompass a product comprising the
specified
ingredients in the specified amounts, as well as any product which results,
directly or indirectly,
from combination of the specified ingredients in the specified amounts.
The term "at least one" means one or more than one. The meaning of "at least
one" with
reference to the number of compounds of the invention is independent of the
meaning with
reference to the number of chemotherapeutic agents.
The term "chemotherapeutic agent" means a chug (medicament or pharmaceutically
active ingredient) for treating cancer (i.e., an antineoplastic agent).
The term "effective amount- means a "therapeutically effective amount". The
term
"therapeutically effective amount" means that amount of active compound or
pharmaceutical
agent that elicits the biological or medicinal response in a tissue, system,
animal or human that is
being sought by a researcher, veterinarian, medical doctor or other clinician.
The term "treating cancer" or "treatment of cancer" refers to administration
to a
mammal afflicted with a cancerous condition and refers to an effect that
alleviates the cancerous
42
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
condition by killing the cancerous cells, and also refers to an effect that
results in the inhibition
of growth and/or metastasis of the cancer.
Except where noted herein, the term "carbocycle" (and variations thereof such
as
"carbocyclic" or "carbocycly1") as used herein, unless otherwise indicated,
refers to a C3 to C6
monocyclic ring, e.g., C3_6 monocyclic carbocycle. The carbocycle may be
attached to the rest of
the molecule at any carbon atom which results in a stable compound. Saturated
carbocyclic rings
include, for example, "cycloalkyl" rings, e.g., cyclopropyl, cyclobutyl, etc.
Unsaturated
, 11811
carbocyclic rings include, for example
Except where noted, the term "saturated heterocycle" refers to a stable 4- to
7-membered
mono-cyclic heteroatom-containing ring system, and which consists of carbon
atoms and from
one to four heteroatoms independently selected from the group consisting of N,
0 and S, and
wherein the nitrogen and sulfur heteroatoms may optionally be oxidized, and
the nitrogen
heteroatom may optionally be quaternized. Especially useful are rings
containing one oxygen or
sulfur, one to four nitrogen atoms, or one oxygen or sulfur combined with one
or two nitrogen
atoms. The heterocyclic ring may be attached at any heteroatom or carbon atom
which results in
the creation of a stable structure. Representative examples include azetidine,
oxetane, thietane,
diazetidine, dioxetane, dithietane, pyrrolidine, tetrahydrofuran, thiolane,
imidazolidine,
pyrazolidine, oxazoli dine, isoxazolidine, thiazolidine, isothiazolidine,
dioxolane, dithiolane,
piperi dine, oxane, thiane, pi perazine, morpholine, thiomorpholine, di oxane,
dithiane, trioxane,
trithiane, azepane, oxepane, thiepane and homopiperazine For example, a
"saturated 6 membered
heterocycle" is a stable 6- membered mono-cyclic heteroatom-containing ring
system. An example
of a saturated 6 membered heterocycle is piperidine. Likewise, but not
limiting, a "saturated 4
membered heterocycle" is a stable 4- membered mono-cyclic heteroatom-
containing ring system.
Except where noted herein, the term "unsaturated heterocycle" refers to a
monocyclic
unsaturated heterocycle having a specified number of atom members (e.g., 5
membered),
including a specified number of heteroatoms (e.g., 1, 2, 3 or 4 heteroatoms
independently
selected from N, 0 or S), e.g., 5-membered rings containing one nitrogen
(pyrrole), one oxygen
(furan) or one sulfur (thiophene) atom, 5-membered rings containing one
nitrogen and one sulfur
(thiazole) atom, 5-membered rings containing one nitrogen and one oxygen
(oxazole or
isoxazole) atom, 5-membered rings containing two nitrogen (imidazole or
pyrazole) atoms, five-
membered aromatic rings containing three nitrogen (triazole) atoms, five-
membered aromatic
rings containing one oxygen, one nitrogen or one sulfur atom, five-membered
aromatic rings
43
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
containing two heteroatoms independently selected from oxygen, nitrogen and
sulfur (e.g.,
oxazole), Additional examples are thiophene, imidazole, isothiazole,
oxadiazole, and isoxazole.
For example, a "unsaturated 6 membered heterocycle" is a 6 membered ring
containing 6 atom
members including at least one heteroatom. Likewise, an "unsaturated 5
membered heterocycle"
is a 5 membered ring containing 5 atom members including at least one
heteroatom.
A "stable" compound is a compound which can be prepared and isolated and whose
structure and properties remain or can be caused to remain essentially
unchanged for a period of
time sufficient to allow use of the compound for the purposes described herein
(e.g., therapeutic
or prophylactic administration to a subject).
The compounds of the present disclosure are limited to stable compounds
embraced by
Formula I and its embodiments. For example, certain moieties as defined in
Formula I, may be
unsubstituted or substituted, and the latter is intended to encompass
substitution patterns (i.e.,
number and kind of substituents) that are chemically possible for the moiety
and that result in a
stable compound.
The term "substituted" means that one or more hydrogens on the designated atom
is
replaced with a selected from the indicated group, provided that the
designated atom's normal
valency under the existing circumstances is not exceeded, and that the
substitution results in a
stable compound. Where multiple substituent moieties are disclosed or claimed,
the substituted
compound can be independently substituted by one or more of the disclosed or
claimed
substituent moieties, singly or plurally. By independently substituted, it is
meant that the (two or
more) substituents can be the same or different. Combinations of substituents
and/or variables
are permissible only if such combinations result in stable compounds. If a sub
stituent is itself
substituted with more than one group, it is understood that these multiple
groups may be on the
same carbon or on different carbons, so long as a stable structure result. By
optionally
substituted, it is meant that compounds containing the specified optional
substituent(s) as well as
compounds that do not contain the optional substituent(s).
The wavy line -11-R-n-P , as used herein, indicates a point of attachment to
the rest of the
compound.
Where ring atoms are represented by variables such as "X", e.g.,
, the variables are
defined by indicating the atom located at the variable ring position without
depicting the ring
bonds associated with the atom. For example, when X in the above ring is
nitrogen, the
definition will show "N" and will not depict the bonds associated with it,
e.g., will not show
44
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
"=N-". Likewise, when X is a carbon atom that is substituted with bromide, the
definition will
show "C-Br" and will not depict the bonds associated with it, e.g., will not
show
¨C-Br
The invention also includes derivatives of the compound of formula I, acting
as prodrugs
and solvates. Any pharmaceutically acceptable pro-drug modification of a
compound of the
invention which results in conversion in vivo to a compound within the scope
of the invention is
also within the scope of the invention. Prodrugs, following administration to
the patient, are
converted in the body by normal metabolic or chemical processes, such as
through hydrolysis in
the blood, to the compound of formula I. Such prodrugs include those that
demonstrate enhanced
bioavailability, tissue specificity, and/or cellular delivery, to improve drug
absorption of the
compound of I. The effect of such prodrugs may result from modification of
physicochemical
properties such as lipophilicity, molecular weight, charge, and other
physicochemical properties
that determine the permeation properties of the drug.
For example, esters can optionally be made by esterification of an available
carboxylic
acid group or by formation of an ester on an available hydroxy group in a
compound. Similarly,
labile amides can be made Pharmaceutically acceptable esters or amides of the
compounds of
the invention may be prepared to act as pro-drugs which can be hydrolyzed back
to an acid (or -
COO- depending on the pH of the fluid or tissue where conversion takes place)
or hydroxy form
particularly in vivo and as such are encompassed within the scope of the
invention. Included are
those esters and acyl groups known in the art for modifying the solubility or
hydrolysis
characteristics for use as sustained-release or prodnig formulations. Examples
of
pharmaceutically acceptable pro-drug modifications include, but are not
limited to, -C1_6alkyl
esters and ¨C1_6alkyl substituted with phenyl esters.
"Celiteg" (Fluka) diatomite is diatomaceous earth, and can be referred to as
"celite".
When any variable (e.g., It' etc.) occurs more than one time in any
constituent or in Formula I or
other generic Formula herein, its definition on each occurrence is independent
of its definition at
every other occurrence. Combinations of substituents and/or variables are
permissible only if
such combinations result in stable compounds. In choosing compounds of the
present invention,
one of ordinary skill in the art will recognize that the various substituents,
i.e. RI etc., are to be
chosen in conformity with well-known principles of chemical structure
connectivity and
stability. Unless expressly stated to the contrary, substitution by a named
substituent is permitted
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
on any atom in a ring (e.g., aryl, a heteroaryl ring, or a saturated
heterocyclic ring) provided such
ring substitution is chemically allowed and results in a stable compound.
It should be noted that, if a discrepancy between the chemical name and
structure exists,
the structure is understood to dominate.
Compounds of structural Formula I may contain one or more asymmetric centers
and can
thus occur as racemates and racemic mixtures, single enantiomers,
diastereoisomeric mixtures
and individual diastereoisomers. Centers of asymmetry that are present in the
compounds of
Formula I can all independently of one another have S configuration or R
configuration. When
bonds to the chiral carbon are depicted as straight lines in the structural
Formula of the invention,
it is understood that both the (R) and (S) configurations of the chiral
carbon, and hence both
enantiomers and mixtures thereof, are embraced within the Formula. Similarly,
when a
compound name is recited without a chiral designation for a chiral carbon, it
is understood that
both the (R) and (S) configurations of the chiral carbon, and hence individual
enantiomers and
mixtures thereof, are embraced by the name. The production of specific
stereoisomers or
mixtures thereof may be identified in the Examples where such stereoisomers or
mixtures were
obtained, but this in no way limits the inclusion of all stereoisomers and
mixtures thereof from
being within the scope of the invention.
The compounds of this invention include all possible enantiomers and
diastereomers and
mixtures of two or more stereoisomers, for example mixtures of enantiomers
and/or
diastereomers, in all ratios. Thus, enantiomers are a subject of the invention
in enantiomerically
pure form, both as levorotatory and as dextrorotatory antipodes, in the form
of racemates and in
the form of mixtures of the two enantiomers in all ratios. In the case of a
cis/trans isomerism the
invention includes both the cis form and the trans form as well as mixtures of
these forms in all
ratios. The present invention is meant to comprehend all such stereo-isomeric
forms of the
compounds of structural Formula I.
Compounds of structural Formula I may be separated into their individual
diastereoisomers by, for example, fractional crystallization from a suitable
solvent, for example
Me0H or Et0Ac or a mixture thereof, or via chiral chromatography using an
optically active
stationary phase. Optionally a derivatization can be carried out before a
separation of
stereoisomers. The separation of a mixture of stereoisomers can be carried out
at an intermediate
step during the synthesis of a compound of formula I, or it can be done on a
final racemic
product. Absolute stereochemistry may be determined by X-ray crystallography
of crystalline
products or crystalline intermediates which are derivatized, if necessary,
with a reagent
46
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
containing an asymmetric center of known absolute configuration.
Alternatively, any
stereoisomer or isomers of a compound of Formula I may be obtained by
stereospecific synthesis
using optically pure starting materials or reagents of known absolute
configuration. The present
invention includes all such isomers, as well as salts, solvates (including
hydrates) and solvated
salts of such racemates, enantiomers, di astereomers and tautomers and
mixtures thereof.
If desired, racemic mixtures of the compounds may be separated so that the
individual
enantiomers are isolated. The separation can be carried out by methods well
known in the art,
such as the coupling of a racemic mixture of compounds to an enantiomerically
pure compound
to form a diastereomeric mixture, followed by separation of the individual
diastereoisomers by
standard methods, such as fractional crystallization or chromatography. The
coupling reaction is
often the formation of salts using an enantiomerically pure acid or base. The
diasteromeric
derivatives may then be converted to the pure enantiomers by cleavage of the
added chiral
residue. The racemic mixture of the compounds can also be separated directly
by
chromatographic methods utilizing chiral stationary phases, which methods are
well known in
the art.
For compounds of Formula I described herein which contain olefinic double
bonds,
unless specified otherwise, they are meant to include both E and Z geometric
isomers.
Some of the compounds described herein may exist as tautomers which have
different
points of attachment of hydrogen accompanied by one or more double bond
shifts. For example,
a ketone and its enol form are keto-enol tautomers. The individual tautomers
as well as mixtures
thereof are encompassed with compounds of Formula I of the present invention.
In the compounds of structural Formula I, the atoms may exhibit their natural
isotopic
abundances, or one or more of the atoms may be artificially enriched in a
particular isotope
having the same atomic number, but an atomic mass or mass number different
from the atomic
mass or mass number predominately found in nature. The present invention as
described and
claimed herein is meant to include all suitable isotopic variations of the
compounds of structural
Formula I and embodiments thereof For example, different isotopic forms of
hydrogen (H)
include protium (111) and deuterium (2H, also denoted herein as D). Protium is
the predominant
hydrogen isotope found in nature. Enriching for deuterium may afford certain
therapeutic
advantages, such as increasing in vivo half-life or reducing dosage
requirements, or may provide
a compound useful as a standard for characterization of biological samples.
Isotopically-enriched
compounds within structural Formula I, can be prepared without undue
experimentation by
conventional techniques well known to those skilled in the art or by processes
analogous to those
47
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
described in the Schemes and Examples herein using appropriate isotopically-
enriched reagents
and/or intermediates.
It will be understood that the compounds of structural Formula I may be
prepared as
pharmaceutically acceptable salts or as salts that are not pharmaceutically
acceptable when they
are used as precursors to the free compounds or their pharmaceutically
acceptable salts or in
other synthetic manipulations. The compounds of the present invention,
including the
compounds of the Examples, may also include all salts of the compounds of
Formulal which,
owing to low physiological compatibility, are not directly suitable for use in
pharmaceuticals but
which can be used, for example, as intermediates for chemical reactions or for
the preparation of
physiologically acceptable salts
The compounds of the present invention may be administered in the form of a
pharmaceutically acceptable salt. The term "pharmaceutically acceptable salt"
refers to salts
prepared from pharmaceutically acceptable non-toxic bases or acids including
inorganic or
organic bases and inorganic or organic acids.
Salts of basic compounds encompassed within the term "pharmaceutically
acceptable
salt" refer to non-toxic salts of the compounds of this invention which are
generally prepared by
reacting the free base with a suitable organic or inorganic acid.
Representative salts of basic
compounds of the present invention include, but are not limited to, the
following: acetate,
ascorbate, benzenesulfonate, benzoate, bicarbonate, bisulfate, bitartrate,
borate, bromide,
butyrate, camphorate, camphorsulfonate, camsylate, carbonate, chloride,
clavulanate, citrate,
dihydrochlori de, edetate, edi syl ate, estolate, esyl ate, fumarate,
gluceptate, gluconate, glutamate,
glycollylarsanilate, hexylresorcinate, hydrabamine, hydrobromide,
hydrochloride,
hydroxynaphthoate, iodide, isothionate, lactate, lactobionate, laurate,
malate, maleate, mandelate,
mesylate, methylbromide, methylnitrate, methyl sulfate, methanesulfonate,
mucate, napsylate,
nitrate, N-methylglucamine ammonium salt, oleate, oxalate, pamoate (embonate),
palmitate,
pantothenate, phosphate/diphosphate, polygalactmonate, piopionate, salicylate,
steal ate, sulfate,
subacetate, succinate, tannate, tartrate, teoclate, thiocyanate, tosylate,
triethiodide, valerate and
the like. Furthermore, where the compounds of the invention carry an acidic
moiety, suitable
pharmaceutically acceptable salts thereof include, but are not limited to,
salts derived from
inorganic bases including aluminum, ammonium, calcium, copper, ferric,
ferrous, lithium,
magnesium, manganic, mangamous, potassium, sodium, zinc, and the like. In one
embodiment,
the salts of acidic compounds are as follows, the ammonium, calcium,
magnesium, potassium,
and sodium salts.
48
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
With basic reagents such as hydroxides, carbonates, hydrogencarbonates,
alkoxides and
ammonia, organic bases or alternatively basic amino acids the compounds of the
formula I, form
stable alkali metal, alkaline earth metal or optionally substituted ammonium
salts.
Salts derived from pharmaceutically acceptable organic non-toxic bases include
salts of
primary, secondary, and tertiary amines, cyclic amines, dicyclohexyl amines
and basic ion-
exchange resins, such as arginine, betaine, caffeine, choline, N,N-
dibenzylethylenediamine,
diethylamine, 2-diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine,
ethylenediamine,
N-ethylmorpholine, N-ethylpiperi dine, glucamine, glucosamine, hi sti dine,
hydrabamine,
isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperidine,
polyamine resins,
procaine, purines, theobromine, triethylamine, trimethylamine, tripropylamine,
tromethamine,
and the like. Also, included are the basic nitrogen-containing groups may be
quaternized with
such agents as lower alkyl halides, such as methyl, ethyl, propyl, and butyl
chloride, bromides
and iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl; and diamyl
sulfates, long chain
halides such as decyl, lauryl, myristyl and stearyl chlorides, bromides and
iodides, aralkyl
halides like benzyl and phenethyl bromides and others.
The preparation of pharmacologically acceptable salts from compounds of the
formula I,
capable of salt formation, including their stereoisomeric forms is carried out
known methods, for
example, by mixing a compound of the present invention with an equivalent
amount and a
solution containing a desired acid, base, or the like, and then collecting the
desired salt by
filtering the salt or distilling off the solvent. The compounds of the present
invention and salts
thereof may form solvates with a solvent such as water, ethanol, or glycerol.
The compounds of
the present invention may form an acid addition salt and a salt with a base at
the same time
according to the type of substituent of the side chain
If the compounds of Formula I simultaneously contain acidic and basic groups
in the
molecule the invention also includes, in addition to the salt forms mentioned,
inner salts or
betaines (zwitterions). Salts can be obtained fr om the compounds of Formula I
by customary
methods which are known to the person skilled in the art, for example by
combination with an
organic or inorganic acid or base in a solvent or dispersant, or by anion
exchange or cation
exchange from other salts.
The present invention includes compounds of structural formula I, as well as
salts
thereof, particularly pharmaceutically acceptable salts, solvates of such
compounds and solvated
salt forms thereof, where such forms are possible unless specified otherwise.
49
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Furthermore, compounds of the present invention may exist in amorphous form
and/or
one or more crystalline forms, and as such all amorphous and crystalline forms
and mixtures
thereof of the compounds of Formula I, including the Examples, are intended to
be included
within the scope of the present invention. In addition, some of the compounds
of the instant
invention may form solvates with water (i.e., a hydrate) or common organic
solvents such as but
not limited to Et0Ac. Such solvates and hydrates, particularly the
pharmaceutically acceptable
solvates and hydrates, of the instant compounds are likewise encompassed
within the scope of
this invention, along with un-solvated and anhydrous forms.
Accordingly, the compounds within the generic structural Formula, embodiments
and
specific compounds described in the Examples and claimed herein encompass
salts, all possible
stereoisomers and tautomers, physical forms (e.g., amorphous and crystalline
forms), solvate and
hydrate forms thereof and any combination of these forms, as well as the
salts, pro-drug forms
thereof, and salts of pro-drug forms thereof, where such forms are possible
unless specified
otherwise,
The invention also relates to medicaments containing at least one compound of
the
Formula I and/or of a pharmaceutically acceptable salt of the compound of the
Formula I and/or
an optionally stereoisomeric form of the compound of the Formula I or a
pharmaceutically
acceptable salt of the stereoisomeric form of the compound of Formula I,
together with a
pharmaceutically acceptable vehicle, carrier, additive and/or other active
substances and
auxiliaries.
The medicaments according to the invention can be administered by oral,
inhalative,
rectal or transdermal administration or by subcutaneous, intraarticular,
intraperitoneal or
intravenous injection. Oral administration is preferred. Coating of stents
with compounds of the
Formula I and other surfaces which come into contact with blood in the body is
possible.
The invention also relates to a process for the production of a medicament,
which
comprises bringing at least one compound of the Formula I into a suitable
administration form
using a pharmaceutically acceptable carrier and optionally further suitable
active substances,
additives or auxiliaries.
The present invention also relates to processes for the preparation of the
compounds of
Formula I which are described in the following and by which the compounds of
the invention are
obtainable.
The terms "therapeutically effective (or efficacious) amount" and similar
descriptions
such as "an amount efficacious for treatment" are intended to mean that amount
of a
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
pharmaceutical drug that will alleviate the symptoms of the disorder,
condition or disease being
treated (i.e., disorder, condition or disease associated with DGAT2 activity)
in an animal or
human. The terms "prophylactically effective (or efficacious) amount" and
similar descriptions
such as "an amount efficacious for prevention" are intended to mean that
amount of a
pharmaceutical drug that will prevent or reduce the symptoms or occurrence of
the disorder,
condition or disease being treated (i.e., disorder, condition or disease
associated with DGAT2
activity) in an animal or human. The dosage regimen utilizing a compound of
the instant
invention is selected in accordance with a variety of factors including type,
species, age, weight,
sex and medical condition of the patient; the severity of the condition to be
treated; the potency
of the compound chosen to be administered, the route of administration, and
the renal and
hepatic function of the patient. A consideration of these factors is well
within the purview of the
ordinarily skilled clinician for the purpose of determining the
therapeutically effective or
prophylactically effective dosage amount needed to prevent, counter, or arrest
the progress of the
condition. It is understood that a specific daily dosage amount can
simultaneously be both a
therapeutically effective amount, e.g., for treatment of hepatic steatosis,
diabetes mellitus,
obesity, hyperlipidemia, hypercholesterolemia, and a prophylactically
effective amount, e.g., for
treatment of NASH.
Disorders, conditions and diseases which can be treated or prevented by
inhibiting
DGAT2 by using the compounds of Formula I are, for example, diseases such as
non-alcoholic
steatohepatitis (NASH), fibrosis, hyperlipidemia, type I diabetes, type II
diabetes mellitus,
cognitive decline, dementia, coronary heart disease, ischemic stroke,
restenosis, peripheral
vascular disease, intermittent claudication, myocardial infarction,
dyslipidemia, post-prandial
lipemi a, obesity, osteoporosis, hypertension, congestive heart failure, left
ventricular
hypertrophy, peripheral arterial disease, diabetic retinopathy, diabetic
nephropathy,
glomerulosclerosis, chronic renal failure, diabetic neuropathy, metabolic
syndrome, syndrome X,
coronary heart disease, angina pectoris, thrombosis, atherosclerosis,
myocardial infarction,
transient ischemic attacks, stroke, hyperglycemia, hyperinsulinemia,
hypertriglyceridemia,
hypertriglyceridemia, insulin resistance, impaired glucose tolerance, erectile
dysfunction, skin
and connective tissue disorders, hyper-apo B lipoproteinemia, non-alcoholic
fatty liver disease,
cardiorenal diseases such as chronic kidney diseases and heart failure, and
related diseases and
conditions.
The compounds of Formula I and their pharmaceutically acceptable salts can be
administered to animals, preferably to mammals, and in particular to humans,
as pharmaceuticals
51
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
by themselves, in mixtures with one another or in the form of pharmaceutical
preparations. The
term "patient" includes animals, preferably mammals and especially humans, who
use the instant
active agents for the prevention or treatment of a medical condition.
Administering of the drug to
the patient includes both self-administration and administration to the
patient by another person.
The patient may need, or desire, treatment for an existing disease or medical
condition, or may
be in need of or desire prophylactic treatment to prevent or reduce the risk
of occurrence of said
disease or medical condition. As used herein, a patient "in need" of treatment
of an existing
condition or of prophylactic treatment encompasses both a determination of
need by a medical
professional as well as the desire of a patient for such treatment.
Furthermore, a subject of the present invention are pharmaceutical
preparations (or
pharmaceutical compositions) which comprise as active component a
therapeutically effective
dose of at least one compound of Formula I and/or a pharmaceutically
acceptable salt thereof
and a customary pharmaceutically acceptable carrier, i.e., one or more
pharmaceutically
acceptable carrier substances and/or additives.
Thus, a subject of the invention is, for example, said compound and its
pharmaceutically
acceptable salts for use as a pharmaceutical, pharmaceutical preparations
which comprise as
active component a therapeutically effective dose of said compound and/or a
pharmaceutically
acceptable salt thereof and a customary pharmaceutically acceptable carrier,
and the uses of said
compound and/or a pharmaceutically acceptable salt thereof in the therapy or
prophylaxis of the
above mentioned syndromes as well as their use for preparing medicaments for
these purposes.
The pharmaceuticals according to the invention can be administered orally, for
example
in the form of pills, tablets, lacquered tablets, sugar-coated tablets,
granules, hard and soft gelatin
capsules, aqueous, alcoholic or oily solutions, syrups, emulsions or
suspensions, or rectally, for
example in the form of suppositories Administration can also be carried out
parenterally, for
example subcutaneously, intramuscularly or intravenously in the form of
solutions for injection
or infusion. Oilier suitable administration forms are, for example,
percutaneous or topical
administration, for example in the form of ointments, tinctures, sprays or
transdermal therapeutic
systems, or the inhalative administration in the form of nasal sprays or
aerosol mixtures, or, for
example, microcapsules, implants or rods. The preferred administration form
depends, for
example, on the disease to be treated and on its severity.
For the production of pills, tablets, sugar-coated tablets and hard gelatin
capsules it is
possible to use, for example, lactose, starch, for example maize starch, or
starch derivatives, talc,
stearic acid or its salts, etc. Carriers for soft gelatin capsules and
suppositories are, for example,
52
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
fats, waxes, semisolid and liquid polyols, natural or hardened oils, etc.
Suitable carriers for the
preparation of solutions, for example of solutions for injection, or of
emulsions or syrups are, for
example, water, physiologically sodium chloride solution, alcohols such as
ethanol, glycerol,
polyols, sucrose, invert sugar, glucose, mannitol, vegetable oils, etc. It is
also possible to
lyophilize the compounds of Formula I and their pharmaceutically acceptable
salts and to use the
resulting lyophilisates, for example, for preparing preparations for injection
or infusion. Suitable
carriers for microcapsules, implants or rods are, for example, copolymers of
glycolic acid and
lactic acid.
Suitable solid or galenical preparation forms are, for example, granules,
powders, coated
tablets, tablets, (micro)capsules, suppositories, syrups, juices, suspensions,
emulsions, drops or
injectable solutions and preparations having prolonged release of active
substance, in whose
preparation customary excipients such as vehicles, disintegrants, binders,
coating agents,
swelling agents, glidants or lubricants, flavorings, sweeteners and
solubilizers are used.
Frequently used auxiliaries which may be mentioned are magnesium carbonate,
titanium dioxide,
lactose, mannitol and other sugars, talc, lactose, gelatin, starch, cellulose
and its derivatives,
animal and plant oils such as cod liver oil, sunflower, peanut or sesame oil,
polyethylene glycol
and solvents such as, for example, sterile water and mono- or polyhydric
alcohols such as
glycerol.
Besides the active compounds and carriers, the pharmaceutical preparations can
also
contain customary additives, for example fillers, disintegrants, binders,
lubricants, wetting
agents, stabilizers, emulsifiers, dispersants, preservatives, sweeteners,
colorants, flavorings,
aromatizers, thickeners, diluents, buffer substances, solvents, solubilizers,
agents for achieving a
depot effect, salts for altering the osmotic pressure, coating agents or
antioxidants.
The dosage of the active compound of Formula I and/or of a pharmaceutically
acceptable
salt thereof to be administered depends on the individual case and is, as is
customary, to be
adapted to the individual circumstances to achieve an optimum effect. Thus, it
depends on the
nature and the severity of the disorder, condition or disease to be treated,
and also on the sex,
age, weight and individual responsiveness of the human or animal to be
treated, on the efficacy
and duration of action of the compounds used, on whether the therapy is acute
or chronic or
prophylactic, or on whether other active compounds are administered in
addition to compounds
of Formula I.
Combination Agents
53
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
The compounds of the present invention can be administered alone or in
combination
with one or more additional therapeutic agents disclosed herein or other
suitable agents,
depending on the condition being treated. Hence, in some embodiments the one
or more
compounds of the invention will be co-administered with other agents as
described above. When
used in combination therapy, the compounds described herein are administered
with the second
agent simultaneously or separately. This administration in combination can
include simultaneous
administration of the two agents in the same dosage form, simultaneous
administration in
separate dosage forms, and separate administration. That is, a compound of
Formula (I) and any
of the agents described above can be formulated together in the same dosage
form and
administered simultaneously. Alternatively, a compound of Formula (I) and any
of the agents
described above can be simultaneously administered, wherein both the agents
are present in
separate formulations. In another alternative, a compound of Formula (I) can
be administered
just followed by and any of the agents described above, or vice versa. In some
embodiments of
the separate administration protocol, a compound of Formula (I) and any of the
agents described
above are administered a few minutes apart, or a few hours apart, or a few
days apart.
As one aspect of the present invention contemplates the treatment of the
disease/conditions with a combination of pharmaceutically active compounds
that may be
administered separately, the invention further relates to combining separate
pharmaceutical
compositions in kit form. The kit comprises two separate pharmaceutical
compositions: a
compound of Formula (I), and a second pharmaceutical compound. The kit
comprises a
container for containing the separate compositions such as a divided bottle or
a divided foil
packet. Additional examples of containers include syringes, boxes, and bags.
In some
embodiments, the kit comprises directions for the use of the separate
components. The kit form
is particularly advantageous when the separate components are preferably
administered in
different dosage forms (e.g., oral, parenteral; IV, transdermal and
subcutaneous), are
administered at different dosage intervals, or when titration of the
individual components of the
combination is desired by the prescribing health care professional.
One or more additional pharmacologically active agents may be administered in
combination with a compound of Formula I. An additional active agent (or
agents) is intended to
mean a pharmaceutically active agent (or agents) that is active in the body,
including pro-drugs
that convert to pharmaceutically active form after administration, which are
different from the
compound of Formula I, and also includes free-acid, free-base and
pharmaceutically acceptable
salts of said additional active agents. Generally, any suitable additional
active agent or agents,
54
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
including but not limited to anti-hypertensive agents, anti-obetic, anti-
inflammatory, anti-
fibrotic, and anti-atherosclerotic agents such as a lipid modifying compound,
anti-diabetic agents
and/or anti-obesity agents may be used in any combination with the compound of
Formula I in a
single dosage formulation (a fixed dose drug combination), or may be
administered to the patient
in one or more separate dosage formulations which allows for concurrent or
sequential
administration of the active agents (co-administration of the separate active
agents).
Examples of additional active agents which may be employed include but are not
limited
to angiotensin converting enzyme inhibitors (e.g., alacepril, benazepril,
captopril, ceronapril,
cilazapril, delapril, enalapril, enalaprilat, fosinopril, imidapril,
lisinopril, moveltipril, perindopril,
quinapril, ramipril, spirapril, temocapril, or trandolapril), angiotensin II
receptor antagonists
(e.g., losartan i.e., COZAAR , valsartan, candesartan, olmesartan,
telmesartan and any of these
drugs used in combination with hydrochlorothiazide such as HYZAAR e), neutral
endopeptidase inhibitors (e.g., thiorphan and phosphoramidon), aldosterone
antagonists,
aldosterone synthase inhibitors, renin inhibitors (e.g. urea derivatives of di-
and tri-peptides,
amino acids and derivatives, amino acid chains linked by non-peptidic bonds,
di- and tri-peptide
derivatives, peptidyl amino diols and peptidyl beta-aminoacyl aminodiol
carbamates; also, and
small molecule renin inhibitors including diol sulfonamides and, N-morpholino
derivatives, N-
heterocyclic alcohols and pyrolimidazolones; also, pepstatin derivatives and
fluoro- and chloro-
derivatives of statone-containing peptides, enalkrein, RO 42-5892, A 65317, CP
80794, ES
1005, ES 8891, SQ 34017, aliskiren (2(S),4(S),5(S),7(S)-N-(2-carbamoy1-2-
methylpropy1)-5-
amino-4-hydroxy-2,7-diisopropy1-844-methoxy-3-(3-methoxypropoxy)-pheny1]-
octanamid
hemifumarate) SPP600, SPP630 and SPP635), endothelin receptor antagonists,
phosphodiesterase-5 inhibitors (e.g. sildenafil, tadalfil and vardenafil),
vasodilators, calcium
channel blockers (e.g., amlodipine, nifedipine, veraparmil, diltiazem,
gallopamil, niludipine,
nimodipins, nicardipine), potassium channel activators (e.g., nicorandil,
pinacidil, cromakalim,
minoxidil, apiilkalim, lopiazolam), diuretics (e.g., hydrochloi othiazide),
sympatholitics, beta-
adrenergic blocking drugs (e.g., propranolol, atenolol, bisoprolol,
carvedilol, metoprolol, or
metoprolol tartate), alpha adrenergic blocking drugs (e.g., doxazosin,
prazosin or alpha
methyldopa) central alpha adrenergic agonists, peripheral vasodilators (e.g.
hydralazine); lipid
lowering agents e.g., HMG-CoA reductase inhibitors such as simvastatin and
lovastatin which
are marketed as ZOCOR and MEVACOR in lactone pro-drug form and function as
inhibitors
after administration, and pharmaceutically acceptable salts of dihydroxy open
ring acid HMG-
CoA reductase inhibitors such as atorvastatin (particularly the calcium salt
sold in LIPITORg),
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
rosuvastatin (particularly the calcium salt sold in CRESTORV), pravastatin
(particularly the
sodium salt sold in PRAVACHOLe), fluvastatin (particularly the sodium salt
sold in
LESCOLg), cerivastatin, and pitavastatin; a cholesterol absorption inhibitor
such as ezetimibe
(ZETIA8) and ezetimibe in combination with any other lipid lowering agents
such as the HMG-
CoA reductase inhibitors noted above and particularly with simvastatin
(VYTORINR) or with
atorvastatin calcium; niacin in immediate-release or controlled release forms,
and/or with an
I-IMG-CoA reductase inhibitor; niacin receptor agonists such as acipimox and
acifran, as well as
niacin receptor partial agonists; anti-cholesterol agents such as PCSK9
inhibitors (alirocumab,
evolocumab), NexletolTm (bempedoic acid, ACL inhibitor), and Vascepag
(Icosapent ethyl);
metabolic altering agents including insulin and insulin mimetics (e.g.,
insulin degludec, insulin
glargine, insulin lispro), dipeptidyl peptidase-IV (DPP-4) inhibitors (e.g.,
sitagliptin, alogliptin,
omarigliptin, linagliptin, vildagliptin); insulin sensitizers, including (i)
13-klotho/FGFR1
activating monoclonal antibody (e.g. MK-3655), pan FGFR1-4/KLB modulators,
FGF19
analogue (e.g. Aldafermin) (ii) PPARy agonists, such as the glitazones (e.g.
pioglitazone, AMG
131, CHS 131, 1VIBX2044, mitoglitazone, lobeglitazone, IDR-105, rosiglitazone,
and
balaglitazone), and other PPAR ligands, including (1) PPARa/y dual agonists
(e.g.ZYH2, ZYHL
GFT505, chiglitazar, muraglitazar, aleglitazar, sodelglitazar, and
naveglitazar); (2) PPARa
agonists such as fenofibric acid derivatives (e.g., gemfibrozil, clofibrate,
ciprofibrate,
fenofibrate, bezafibrate), (3) selective PPARy modulators (SPPARyM's), (e.g.,
such as those
disclosed in WO 02/060388, WO 02/08188, WO 2004/019869, WO 2004/020409, WO
2004/020408, and WO 2004/066963); (4) PPARy partial agonists, (5) PPAR a/5
dual agonists
(e.g. Elafibranor ); (iii) biguanides, such as metformin and its
pharmaceutically acceptable salts,
in particular, metformin hydrochloride, and extended-release formulations
thereof, such as
GlumetzaTM, FortametTM, and GlucophageXRTM; and (iv) protein tyrosine
phosphatase-1B (PTP-
1B) inhibitors (e.g., ISIS-113715 and TTP814); insulin or insulin analogs
(e.g., insulin detemir,
insulin glulisine, insulin degludec, insulin glargine, insulin lispro and
inhalable formulations of
each); leptin and leptin derivatives and agonists; amylin and amylin analogs
(e.g., pramlintide);
sulfonylurea and non-sulfonylurea insulin secretagogues (e.g., tolbutamide,
glyburide, glipizide,
glimepiride, mitiglinide, meglitinides, nateglinide and repaglinide); a-
glucosidase inhibitors
(e.g., acarbose, voglibose and miglitol); glucagon receptor antagonists (e.g.,
MK-3577, MK-
0893, LY-2409021 and KT6-971); incretin mimetics, such as GLP-1, GLP-1
analogs,
derivatives, and mimetics; and GLP-1 receptor agonists (e.g., dulaglutide,
semaglutide,
albiglutide, exenatide, liragluti de, lixisenatide, taspogluti de, CJC-1131,
and B I M-51077,
56
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
including intranasal, transdermal, and once-weekly formulations thereof), bile
acid sequestering
agents (e.g., colestilan, colestimide, col esevalam hydrochloride, colestipol,
cholestyramine, and
dialkylaminoalkyl derivatives of a cross-linked dextran), acyl CoA:cholesterol
acyltransferase
inhibitors, (e.g., avasimibe); antiobesity compounds; agents intended for use
in inflammatory
conditions, such as aspirin, non-steroidal anti-inflammatory drugs or NSAIDs,
glucocorticoids,
and selective cyclooxygenase-2 or COX-2 inhibitors; glucokinase activators
(GKAs) (e.g.,
AZD6370); inhibitors of 1113-hydroxysteroid dehydrogenase type 1, (e.g., such
as those disclosed
in U.S. Patent No. 6,730,690, and LY-2523199); CETP inhibitors (e.g.,
anacetrapib, torcetrapib,
and evacetrapib)., inhibitors of fructose 1,6-bisphosphatase, (e.g., such as
those disclosed in U.S.
Patent Nos. 6,054,587, 6,110,903, 6,284,748, 6,399,782, and 6,489,476),
inhibitors of acetyl
CoA carboxylase-1 or 2 (ACC1 or ACC2); AMP-activated Protein Kinase (AMPK)
activators;
other agonists of the G-protein-coupled receptors: (i) GPR-109, (ii) GPR-119
(e.g., 1VB3X2982
and PSN821), and (iii) GPR-40 (e.g., TAK875); SSTR3 antagonists (e.g., such as
those disclosed
in WO 2009/001836); neuromedin U receptor agonists (e.g., such as those
disclosed in WO
2009/042053, including, but not limited to, neuromedin S (NMS)); SCD
modulators (e.g.
Aramchol); GPR-105 antagonists (e.g., such as those disclosed in WO
2009/000087); SGLT
inhibitors (e.g., ASP1941, SGLT-3, SGLT-2 such as empagliflozin,
dapagliflozin, canagliflozin,
and ertugliflozin, BI-10773, remogloflozin, TS-071, tofogliflozin,
ipragliflozin, and LX-4211);
inhibitors of acyl coenzyme A carboxylase (ACC, MK-4074); inhibitors of
diacylglycerol
acyltransferase 1 and 2 (DGAT-1 and DGAT-2); inhibitors of fatty acid
synthase; inhibitors of
acyl coenzyme A:monoacylglycerol acyltransferase 1 and 2 (MGAT-1 and MGAT-2);
agonists
of the TGR5 receptor (also known as GPBAR1, BG37, GPCR19, GPR131, and M-BAR);
ileal
bile acid transporter inhibitors; bile acid modulators; PACAP, PACAP mimetics,
and PACAP
receptor 3 agonists; IL-lb antibodies, (e.g., X0MA052 and canakinumab);, anti-
fibrotic and/or
anti-inflammatory agents (CCR2/CCR5 dual receptor antagonist (e.g.
cenicriviroc); galectin 3
inhibitor (e.g. belapectin, GB-1107, GB-1211), siRNA against HSP 47 (e.g. BMS-
986263),
NSAID derived from pirfenidone (e.g. hydronidone), A3AR agonist (e.g.
namodenoson,
FM101); TGFTX4 (e.g. nitazoxanide); 5-lipoxygenase inhibitor (e.g.
tipelukast), Bifunctional
urate inhibitor (e.g. ACQT1127), adiponectin receptor agonist (e.g. ALY688),
TNF receptor
antagonist (e.g. atrosimab), Autotaxin inhibitor (e.g. BLD-0409, TJC 0265, TJC
0316), CCL24
blocking monoclonal antibody (e.g. CM101), IL-11 inhibitor (e.g. ENx 108A),
LPA1 receptor
antagonist (e.g. EPGN 696), Dual JAK1/2 inhibitor (e.g. EX 76545), GPR
antagonist (e.g.
GPR91 antagonist), Integrin avf31, avI33 and avf36 inhibitor (e.g. IDL 2965),
NLRP3 antagonist
57
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(e.g. IFM-514), inflammasome inhibitors (e.g. JT194, JT349), Cell membrane
permeability
inhibitor (e.g. Larazotide), CCR5 antagonist (e.g. Ieronlimab), TNF inhibitor
(e.g. LIVNate),
integrin avI36 inhibitor (e.g. MORF beta6), NLRP inflammasome antagonists,
siRNA (e.g. OLX
701), dual TFG13/Hedgehog inhibitor (e.g. Oxy 200), GPR40 agonist/GPR84
antagonist (e.g.
PBI-4547), neutrophil elastase inhibitor (e.g. PIP-303), integrin inhibitor
(e.g. PLN-1474),
TGFI31 modulator (e.g.PRM-151), CCK receptor antagonist (e.g. proglumide),
LOXL2 inhibitor
(e.g. PXS-5338K, PXS-5382A), IL-11 inhibitors, MPYS protein inhibitor (e.g.
eGAS/STING
antagonists), kinase inhibiting RNase, membrane protein mAbs, tumor necrosis
factor inhibitor,
NRF2 activator (e.g. SCO 116), SSA() inhibitor (e.g. TERN 201), TRAIL2 agonist
(e.g.
TLY012), IL-6 receptor antagonist (e.g. TZLS 501), A0C3 inhibitor (e.g. UD-
014),
SSAO/VAP-1 inhibitor, TREM2); anti-oxidant (e.g. vitamin E); anti-inflammatory
agents (e.g.
norfloxacin, ciprofloxacin, ceftriaxone); coagulation modifiers (e.g. anti-
coagulants, anti-platelet
agents, pentoxifylline, vitamin K, DDAVP); ; dual GIP and GLP-1 receptor
agonist (e.g.
tirzepetide); dual GLP-1/GRA (e.g. cotadutide, ALT-801, DD 01, G49, PB-718);
dual GLP-1
(e.g. CT 868); GLP-1/GRA/GIP triple agonist (e.g. HM15211); GRP120
stimulant/inflammasome modulator/PPARy dual agonist (e.g. KDT501); GLP-1/FGF21
(e.g.
YH25724); GLP-1 agonist (e.g. Ozempic (semaglutide sc), XW 003); selective
thyroid hormone
receptor43 agonist (e.g. resmetirom); apoptosis modulators (JNK-1 inhibitor
(e.g. CC-90001),
Peroxidase inhibitor (e.g AZM198), ASK-1 inhibitor (e.g. CS-17919, SRT 015));
erythropoietin-stimulating agents (erythropoietin receptor agonist (e.g.
cibinetide)); glucose
pathway modulators (SGLT-2 inhibitor (e.g. Forxiga, Farxiga (dapagliflozin)),
dual SGLT-1/2
inhibitor (e.g. licogliflozin), Glucose-6-P dehydrogenase inhibitor (e.g.
fluasterone) LAPS
glucagon combo (e.g. H1V114320), SGLT-1 inhibitor (e.g. SGL5213)); immune
modulators
(TLR4 inhibitor (e.g. GBK-233), immunomodulatory polyclonal antibody (e.g. IMM-
124E),
TLR4 antagonist (e.g. JKB-122), CD3 monoclonal antibody (e.g.foralumab), TLR4
antagonist
(e.g. JKB 133), TLR4 inhibitor (e.g. mosedipimod), Macrophage inhibitor via
CD206 targeting
(e.g. MT2002), TLR2/4 antagonist (e.g. VB-201, VB-703), immunomodulatory
polyclonal
antibody (e.g. IMM-124E)); incretin-based therapies (GLP-1 agonist (e.g.
Ozempic (semaglutide
sc), XW 003), GLP-1/glucagon dual receptor agonist (e.g. H1V412525A), prandial
insulin (e.g.
ORMD 0801)); lipid modulators (AMPK Activator/ Glutathione transferase (e.g.
oltipraz), THR-
beta agonist (e.g. resmetirom, VK2809, MGL-3745, ALG-009, ASC41, CNPT-101101,
TERN
501), IBAT inhibitor (e.g. elobixibat, CJ 14199), omega-6- fatty acid (e.g.
epeleuton), FASN
inhibitor (e.g. TV112640, FT 4101, FT 8225), ANGPTL3 inhibitor (e.g.
vupanorsen), PNPLA3
58
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
inhibitor (e.g. AZD2693), RAS domain kinase inhibitor (e.g. BioE1115), NTCP
inhibitor (e.g.
bulevirtide), P2Y13 receptor agonist (e.g. CER-209), omega-3 fatty acid,
HSD171313 inhibitor;
metabolism modulators (FXR agonist (e.g. Ocaliva (obeticholic acid), IOT022),
recombinant
variant of FGF19 (e.g. aldafermin), bi-specific FGFR1/KLB antibody (e.g.
BFKB8488A),
mTOT modulator (e.g. MSDC-0602K), pegylated analog of FGF21 (e.g.
pegbelfermin, BMS-
986171), non-bile FXR agonist (e.g. cilofexor, EDP-305, EYP 001, tropifexor,
MET409, AGN-
242256, AGN-242266, EDP 297, HPG 1860, MET642, RDX023, TERN 101), ACC
inhibitor
(e.g. firsocostat, PF-05221304), ketohexokinase inhibitor (e.g. PF-06835919),
AMPK activator
(e.g. PXL770, MSTM 101, 0304), bile acid modulator (e.g. Albiero), FGF21
analog (e.g.
B1089-100), MOTSc analog (e.g. CB4211), cyclophilin inhibitor (e.g. CRV 431),
FGF19 (e.g.
DEL 30), mitochondrial uncoupler (e.g. GEN 3026), FXR/GPCR dual agonist (e.g.
INT-767),
Cysteamine derivative (e.g. KB-GE-001), dual amylin and calcitonin receptor
agonist (e.g. KBP-
089), transient FXR agonist (e.g. M 1217), anti-beta-klotho (KLB)-FGFR1c
receptor complex
mAb (e.g. MK3655), GDF15 analog (e.g. NGM395), cyclophilin inhibitor (e.g.
NV556), LXR
modulator (e.g. PX 329, PX 655, PX 788), LXR inverse agonist (e.g. PX016),
deuterated
obeticholic acid (e.g. ZG 5216)); PPAR modulators (dual PPARa/y agonist (e.g.
elafibranor),
PPAR pan agonist (e.g. lanifibranor), PPARa agonists (e.g. Parmodia), PPARy
agonist (e.g. CHS
131), MPC inhibitor (e.g. PXL065), PPAR 6/y agonist (e.g.T3D 959)); RAAS
mIMModulators
(mineralocorticoid receptor antagonist (e.g. apararenone, eplerenone,
spironolactone),
angiotensin receptor blocker (e.g. losartan potassium)); neurotransmitter
modulators
(cannabinoid receptor modulator, CB1 receptor antagonist (e.g. CRB-4001, IM-
102,
nimacimab), TPH1 inhibitor (e.g. CU 02), GPR120 agonist (e.g. KBR2001),
combination of
cannabinoid and botanical anti-inflammatory compound (e.g. SCN 002)); PDE
Modulator
(PDE4 inhibitor (e.g. ART 648)); CYP2E1 inhibitor (e.g. SNP-610); cell
therapies (e.g.
HepaStem)and bromocriptine mesylate and rapid-release formulations thereof, or
with other
drugs beneficial for the prevention or the treatment of the above-mentioned
diseases including
nitroprusside and diazoxide the free-acid, free-base, and pharmaceutically
acceptable salt forms
of the above active agents where chemically possible.
The present invention includes the pharmaceutically acceptable salts of the
compounds
defined herein, including the pharmaceutically acceptable salts of all
structural formulas,
embodiments and classes defined herein.
Dosages of the Compounds of Formula (I)
59
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
If the patient is responding, or is stable, after completion of the therapy
cycle, the therapy
cycle can be repeated according to the judgment of the skilled clinician. Upon
completion of the
therapy cycles, the patient can be continued on the compounds of the invention
at the same dose
that was administered in the treatment protocol. This maintenance dose can be
continued until
the patient progresses or can no longer tolerate the dose (in which case the
dose can be reduced
and the patient can be continued on the reduced dose).
Those skilled in the art will recognize that the actual dosages and protocols
for
administration employed in the methods of the invention may be varied
according to the
judgment of the skilled clinician. The actual dosage employed may be varied
depending upon the
requirements of the patient and the severity of the condition being treated.
Determination of the
proper dosage for a particular situation is within the skill of the art. A
determination to vary the
dosages and protocols for administration may be made after the skilled
clinician considers such
factors as the patient's age, condition and size, as well as the severity of
the condition being
treated and the response of the patient to the treatment.
The dosage regimen utilizing a compound of the instant invention is selected
in
accordance with a variety of factors including type, species, age, weight, sex
and medical
condition of the patient; the severity of the condition to be treated; the
potency of the compound
chosen to be administered; the route of administration; and the renal and
hepatic function of the
patient. A consideration of these factors is well within the purview of the
ordinarily skilled
clinician for the purpose of determining the therapeutically effective or
prophylactically effective
dosage amount needed to prevent, counter, or arrest the progress of the
condition. It is
understood that a specific daily dosage amount can simultaneously be both a
therapeutically
effective amount, e.g., for treatment of an oncological condition, and a
prophylactically effective
amount, e.g., for prevention of an oncological condition.
While individual needs vary, determination of optimal ranges of effective
amounts of the
compound of the invention is within the skill of the art. For administration
to a human in the
curative or prophylactic treatment of the conditions and disorders identified
herein, for example,
typical dosages of the compounds of the present invention can be about 0.05
mg/kg/day to about
50 mg/kg/day, for example at least 0.05 mg/kg, at least 0.08 mg/kg, at least
0.1 mg/kg, at least
0.2 mg/kg, at least 0.3 m4/kg, at least 0.4 mg/kg, or at least 0.5 mg/kg, and
preferably 50 mg/kg
or less, 40 mg/kg or less, 30 mg/kg or less, 20 mg/kg or less, or 10 mg/kg or
less, which can be
about 2.5 mg/day (0.5 mg/kg x 5 kg) to about 5000 mg/day (50 mg/kg x 100 kg),
for example.
For example, dosages of the compounds can be about 0.1 mg/kg/day to about 50
mg/kg/day,
GO
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
about 0.05 mg/kg/day to about 10 mg/kg/day, about 0.05 mg/kg/day to about 5
mg/kg/day, about
0.05 mg/kg/day to about 3 mg/kg,/day, about 0.07 mg/kg/day to about 3
mg/kg/day, about 0.09
mg/kg/day to about 3 mg/kg/day, about 0.05 mg/kg/day to about 0.1 mg/kg/day,
about 0.1
mg/kg/day to about 1 mg/kg/day, about 1 mg/kg/day to about 10 mg/kg/day, about
1 mg/kg/day
to about 5 mg/kg/day, about 1 mg/kg/day to about 3 mg/kg/day, about 3 mg/day
to about 500
mg/day, about 5 mg/day to about 250 mg/day, about 10 mg/day to about 100
mg/day, about 3
mg/day to about 10 mg/day, or about 100 mg/day to about 250 mg/day. Such doses
may be
administered in a single dose or may be divided into multiple doses.
Pharmaceutical Compositions
The compounds of Formula I and their pharmaceutically acceptable salts can be
administered to animals, preferably to mammals, and in particular to humans,
as pharmaceuticals
by themselves, in mixtures with one another or in the form of pharmaceutical
compositions. The
term "subject- or "patient" includes animals, preferably mammals and
especially humans, who
use the instant active agents for the prevention or treatment of a medical
condition.
Administering of the compound of Formula Ito the subject includes both self-
administration and administration to the patient by another person. The
subject may need, or
desire, treatment for an existing disease or medical condition, or may be in
need of or desire
prophylactic treatment to prevent or reduce the risk of occurrence of said
disease or medical
condition. As used herein, a subject "in need" of treatment of an existing
condition or of
prophylactic treatment encompasses both a determination of need by a medical
professional as
well as the desire of a patient for such treatment.
Methods for the safe and effective administration of most of these agents are
known to
those skilled in the art. In addition, their administration is described in
the standard literature.
If the patient is responding, or is stable, after completion of the therapy
cycle, the therapy
cycle can be repeated according to the judgment of the skilled clinician. Upon
completion of the
therapy cycles, the patient can be continued on the compounds of the invention
at the same dose
that was administered in the treatment protocol. This maintenance dose can be
continued until
the patient progresses or can no longer tolerate the dose (in which case the
dose can be reduced
and the patient can be continued on the reduced dose).
Those skilled in the art will recognize that the actual dosages and protocols
for
administration employed in the methods of the invention may be varied
according to the
judgment of the skilled clinician. The actual dosage employed may be varied
depending upon the
requirements of the patient and the severity of the condition being treated.
Determination of the
61
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
proper dosage for a particular situation is within the skill of the art. A
determination to vary the
dosages and protocols for administration may be made after the skilled
clinician takes into
account such factors as the patient's age, condition and size, as well as the
severity of the
condition being treated and the response of the patient to the treatment.
The amount and frequency of administration of the compound of formula I, and
any
additional agents will be regulated according to the judgment of the attending
clinician
(physician) considering such factors as age, condition and size of the patient
as well as severity
of the condition being treated.
The compounds of the invention are also useful in preparing a medicament that
is useful
in treating NASH and fibrosis.
The instant compounds are also useful in combination with therapeutic,
chemotherapeutic
and anti-cancer agents for the treatment of hepatic cellular carcinoma.
Combinations of the
presently disclosed compounds with therapeutic, chemotherapeutic and anti-
cancer agents are
within the scope of the invention. Examples of such agents can be found in
Cancer Principles
and Practice of Oncology by V.T. Devita and S. Hellman (editors), 9th edition
(May 16, 2011),
Lippincott Williams & Wilkins Publishers. A person of ordinary skill in the
art would be able to
discern which combinations of agents would be useful based on the particular
characteristics of
the drugs and the cancer involved. Such agents include the following: estrogen
receptor
modulators, programmed cell death protein 1 (PD-1) inhibitors, programmed
death-ligand 1 (PD-
Li) inhibitors, androgen receptor modulators, retinoid receptor modulators,
cytotoxic/cytostatic
agents, antiproliferative agents, prenyl -protein transferase inhibitors, HMG-
CoA reductase
inhibitors and other angiogenesis inhibitors, HIV protease inhibitors, reverse
transcriptase
inhibitors, inhibitors of cell proliferation and survival signaling,
bisphosphonates, aromatase
inhibitors, siRNA therapeutics, y-secretase inhibitors, agents that interfere
with receptor tyrosine
kinases (RTKs) and agents that interfere with cell cycle checkpoints.
The chemotherapeutic agent can be administered according to therapeutic
protocols well
known in the art. It will be apparent to those skilled in the art that the
administration of the
chemotherapeutic agent can be varied depending on the cancer being treated and
the known
effects of the chemotherapeutic agent on that disease. Also, in accordance
with the knowledge of
the skilled clinician, the therapeutic protocols (e.g., dosage amounts and
times of administration)
can be varied in view of the observed effects of the administered therapeutic
agents on the
patient, and in view of the observed responses of the cancer to the
administered therapeutic
agents. The particular choice of chemotherapeutic agent will depend upon the
diagnosis of the
62
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
attending physicians and their judgment of the condition of the patient and
the appropriate
treatment protocol.
The initial administration can be made according to established protocols
known in the
art, and then, based upon the observed effects, the dosage, modes of
administration and times of
administration can be modified by the skilled clinician
The determination of the order of administration, and the number of
repetitions of
administration of the chemotherapeutic agent during a treatment protocol, is
well within the
knowledge of the skilled physician after evaluation of the condition being
treated and the
condition of the patient
Thus, in accordance with experience and knowledge, the practicing physician
can modify
each protocol for the administration of a chemotherapeutic agent according to
the individual
patient's needs, as the treatment proceeds. All such modifications are within
the scope of the
present invention.
The agent can be administered according to therapeutic protocols well known in
the art. It
will be apparent to those skilled in the art that the administration of the
anti-cancer agent can be
varied depending on the cancer being treated and the known effects of the anti-
cancer agent on
that disease.
The initial administration can be made according to established protocols
known in the
art, and then, based upon the observed effects, the dosage, modes of
administration and times of
administration can be modified by the skilled clinician.
The particular choice of agent will depend upon the diagnosis of the attending
physicians
and their judgment of the condition of the patient and the appropriate
treatment protocol.
The determination of the order of administration, and the number of
repetitions of
administration of the agent during a treatment protocol, is well within the
knowledge of the
skilled physician after evaluation of the cancer being treated and the
condition of the patient.
Thus, in accordance with experience and knowledge, the practicing physician
can modify
each protocol for the administration of an anti-cancer agent according to the
individual patient's
needs, as the treatment proceeds. All such modifications are within the scope
of the present
invention.
The attending clinician, in judging whether treatment is effective at the
dosage
administered, will consider the general well-being of the patient as well as
more definite signs
such as relief of cancer-related symptoms (e.g., pain), inhibition of tumor
growth, actual
shrinkage of the tumor, or inhibition of metastasis. Size of the tumor can be
measured by
63
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
standard methods such as radiological studies, e.g., CAT or MRI scan, and
successive
measurements can be used to judge whether or not growth of the tumor has been
retarded or even
reversed. Relief of disease-related symptoms such as pain, and improvement in
overall condition
can also be used to help judge effectiveness of treatment.
The compounds, compositions and methods provided herein are useful for the
treatment
of cancer. Cancers that may be treated by the compounds, compositions and
methods disclosed
herein include, but are not limited to: Liver: hepatoma (hepatocellular
carcinoma),
cholangiocarcinoma, hepatoblastom a, angiosarcoma, hepatocellular adenoma,
hemangioma.
PD-1 inhibitors include pembrolizumab (lambrolizumab), nivolumab and
MPDL3280A.
PDL- inhibitors include atezolizumab, avelumab, and durvalumab.
The invention further relates to a method of treating hepatic cellular
carcinoma in a
human patient comprising administration of a compound of the invention (i.e.,
a compound of
Formula I) and a PD-1 antagonist to the patient. The compound of the invention
and the PD-1
antagonist may be administered concurrently or sequentially.
In particular embodiments, the PD-1 antagonist is an anti-PD-1 antibody, or
antigen
binding fragment thereof. In alternative embodiments, the PD-1 antagonist is
an anti-PD-Li
antibody, or antigen binding fragment thereof. In some embodiments, the PD-1
antagonist is
pembrolizumab (KEYTRUDATm, Merck & Co., Inc., Kenilworth, NJ, USA), nivolumab
(OPDIVOTM, Bristol-Myers Squibb Company, Princeton, NJ, USA), cemiplimab
(LIBTAY0Tm,
Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA), atezolizumab
(TECENTRIQTm,
Genentech, San Francisco, CA, USA), durvalumab (IMFINZITm, AstraZeneca
Pharmaceuticals
LP, Wilmington, DE), or avelumab (BAVENCIOTm, Merck KGaA, Darmstadt, Germany).
In some embodiments, the PD-1 antagonist is pembrolizumab. In particular sub-
embodiments, the method comprises administering 200 mg of pembrolizumab to the
patient
about every three weeks. In other sub-embodiments, the method comprises
administering 400 mg
of pembrolizumab to the patient about every six weeks.
In further sub-embodiments, the method comprises administering 2 mg/kg of
pembrolizumab to the patient about every three weeks. In particular sub-
embodiments, the
patient is a pediatric patient.
In some embodiments, the PD-1 antagonist is nivolumab. In particular sub-
embodiments,
the method comprises administering 240 mg of nivolumab to the patient about
every two weeks.
In other sub-embodiments, the method comprises administering 480 mg of
nivolumab to the
patient about every four weeks.
64
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
In some embodiments, the PD-1 antagonist is cemiplimab. In particular
embodiments, the
method comprises administering 350 mg of cemiplimab to the patient about every
3 weeks.
In some embodiments, the PD-1 antagonist is atezolizumab. In particular sub-
embodiments, the method comprises administering 1200 mg of atezolizumab to the
patient about
every three weeks.
In some embodiments, the PD-1 antagonist is durvalumab. In particular sub-
embodiments, the method comprises administering 10 mg/kg of durvalumab to the
patient about
every two weeks.
In some embodiments, the PD-1 antagonist is avelumab. In particular sub-
embodiments,
the method comprises administering 800 mg of avelumab to the patient about
every two weeks.
A compound of the instant invention, or a pharmaceutically acceptable salt
thereof, may
also be useful for treating cancer in combination with the following
therapeutic agents:
pembrolizumab (Keytrudag), abarelix (Plenaxis depot ); aldesleukin (Prokineg);
Aldesleukin
(Proleuking); Alemtuzumabb (Campathg); alitretinoin (Panreting); allopurinol
(Zyloprimg);
altretamine (Hexaleng); amifostine (Ethyolg); anastrozole (Arimidexg); arsenic
trioxide
(Trisenoxg); asparaginase (Elsparg); azacitidine (Vidazag); bevacuzimab
(Avasting);
bexarotene capsules (Targreting); bexarotene gel (Targreting); bleomycin
(Blenoxaneg);
bortezomib (Velcadeg); busulfan intravenous (Busulfexg); busulfan oral
(Mylerang);
calusterone (Methosarbg); capecitabine (Xelodag); carboplatin (Paraplating);
carmustine
(BCNUg, BiCNUg); carmustine (Gliadelg); carmustine with Polifeprosan 20
Implant (Gliadel
Wafer ); celecoxib (Celebrexg); cetuximab (Erbituxg); chlorambucil
(Leukerang); cisplatin
(Platinolg); cladribine (Leustating, 2-CdAg); clofarabine (Clolarg);
cyclophosphamide
(Cytoxan , Neosarg); cyclophosphamide (Cytoxan Injection ); cyclophosphamide
(Cytoxan
Tablet ); cytarabine (Cytosar-U ); cytarabine liposomal (DepoCytg);
dacarbazine (DTIC-
2 5 Dome ); dactinomycin, actinomycin D (Cosmegeng); Darbepoetin alfa
(Aranespg);
daunorubicin liposom al (DanuoXomeg); daunorubicin, daunomycin
(Daunorubicing);
daunorubicin, daunomycin (Cerubidineg); Denileukin diftitox (Ontakg);
dexrazoxane
(Zinecardg); docetaxel (Taxotereg); doxorubicin (Adriamycin PFSg); doxorubicin
(Adriamycing, Rubexg); doxorubicin (Adriamycin PFS Injection ); doxorubicin
liposomal
(Doxilg); dromostanolone propionate (Dromostanoloneg); dromostanolone
propionate
(Masterone injection ); Elliott's B Solution (Elliott's B Solution );
epirubicin (Ellenceg);
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Epoetin al fa (epogeng); erlotinib (Tarcevag); estramustine (Emcytg); etoposi
de phosphate
(Etopophosg), etoposide, VP-16 (Vepesidg); exemestane (Aromasing); Filgrastim
(Neupogeng); floxuridine (intraarterial) (FUDRg); flu darabine (Fludarag); flu
orouracil, 5-FU
(Adrucilg); fulvestrant (Faslodexg), gefitinib (Iressag), gemcitabine
(Gemzarg), gemtuzumab
ozogamicin (Mylotargg); goserelin acetate (Zoladex Implant ); goserelin
acetate (Zoladexg),
histrelin acetate (Histrelin implant ); hydroxyurea (Hydreag); Ibritumomab
Tiuxetan
(Zevaling); idarubicin (Idamycing); ifosfamide (IF'EXS); imatinib mesylate
(Gleevecg);
interferon alfa 2a (Roferon Ag); Interferon alfa-2b (Intron Ag); irinotecan
(Camptosarg);
lenalidomide (Revlimide); letrozole (Femarag); leucovorin (Wellcovoring,
Leucovoring);
Leuprolide Acetate (Eligardg); levamisole (Ergamisolg); lomustine, CCNU
(CeeBUg);
meclorethamine, nitrogen mustard (Mustargeng); megestrol acetate (Megaceg);
melphalan, L-
PAM (Alkerang); mercaptopurine, 6-INSP (Purinetholg); mesna (Mesnexg); mesna
(Mesnex
tabs ); methotrexate (Methotrexateg); methoxsalen (Uvadexg); mitomycin C
(Mutamycing);
mitotane (Lysodreng), mitoxantrone (Novantroneg), nandrolone phenpropionate
(Durabolin-
50g); nelarabine (Arranong); Nofetumomab (Verlumag); Oprelvekin (Neumegag);
oxaliplatin
(El oxati n8); paclitaxel (Paxene8); paclitaxel (Taxo18); paclitaxel protein-
bound particles
(Abraxaneg); palifermin (Kepivanceg); pamidronate (Arediag); pegademase
(Adagen
(Pegademase Bovine) ); pegaspargase (Oncasparg); Pegfilgrastim (Neulastag);
pemetrexed
di sodium (Alimtag); pentostatin (Nipentg); pipobroman (Vercyteg); plicamycin,
mithramycin
(Mithracing); porfimer sodium (Photofring); procarbazine (Matulaneg),
quinacrine
(Atabrineg); Rasburicase (Elitekg); Rituximab (Rituxang); Ridaforolimus;
sargramostim
(Leukineg); Sargramostim (Prokineg); sorafenib (Nexavarg); streptozocin
(Zanosarg);
sunitinib maleate (Sutentg); talc (Sclerosolg); tamoxifen (Nolvadexg);
temozolomide
(Temodarg); teniposide, VM-26 (Vumong); testolactone (Teslacg); thioguanine, 6-
TG
(Thioguanineg); thiotepa (Thioplexg); topotecan (Hycamting); toremifene
(Farestong);
Tositumomab (Bexxarg); Tositumomab/I-131 tositumomab (Bexxarg); Trastuzumab
(Hercepting); tretinoin, ATRA (Vesanoidg); Uracil Mustard (Uracil Mustard
Capsules );
valrubicin (Val star ); vinblastine (Velbang); vincristine (Oncoving);
vinorelbine (Navelbineg);
vorinostat (Zolinzag) and zoledronate (Zometag), or a pharmaceutically
acceptable salt thereof
66
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Methods for Making the Compounds of Present Invention
The following examples are provided so that the invention might be more fully
understood. Unless otherwise indicated, the starting materials are
commercially available. They
should not be construed as limiting the invention in any way.
Several methods for preparing the compounds of this invention are described in
the
following Schemes and Examples. Starting materials and intermediates are
purchased, made
from known procedures, or as otherwise illustrated. Some frequently applied
routes to the
compounds of Formula I are also described by the Schemes as follows. In some
cases, the order
of carrying out the steps of reaction schemes may be varied to facilitate the
reaction or to avoid
unwanted reaction products. For stereoisomers, enantiomer A refers to the
faster/ earlier eluting
enantiomer and enantiomer B refers to the slower/ later eluting enantiomer at
the point of
separation and this nomenclature is maintained through the remainder of a
synthetic sequence for
a given enantiomeric series regardless of the possibility that subsequent
intermediates and final
compounds may have the same or opposite orders of elution.
Throughout the synthetic schemes and examples, abbreviations and acronyms may
be
used with the following meanings unless otherwise indicated:
ACN = acetonitrile
BP0 = Benzoyl peroxide
Brett phos Pd G3
CDI = 1 ,11-Carbonyl di irnidazol e
Cu(OAc)2 = copper acetate
DMF = dimethylformamide
DMS = dimethyl sulfide
DCM = dichloromethane
DIPEA = N,N-Diisopropylethylamine
DPPA ¨ Diphenylphosphoiy1 azide
dppf = 1,1'-bis(diphenylphosphino)ferrocene
Et = ethyl
Et0Ac = ethyl acetate
EDC = N-(3-Dimethylaminopropy1)-N'-ethylcarbodiimide hydrochloride
DCE = 1,2-dichloroethane
RP HPLC = Reverse Phase High Pressure Liquid Chromatography
h or hrs = hour or hours
67
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
HATU = 14bis(dimethylamino)rnethylenel- 1,2,3-triazolor4,5-
blpyridinium 3-wdd-
hexafluorophosphate
Hex = hexanes
HOBT = Hydroxybenzotriazole
IPA = i so-Propanol
Me = methyl
mCPBA = meta-chloroperoxybenzoic acid
MgSat = magnesium sulfate
MP-cyanoborohydride = macroporous polymer-supported cyanoborohydride
rt or RT = room temperature
nBurLI = Tetra-n-butylammonium iodide
NBS = N-bromosuccinimide
NCS = N-Chlorosuccinimide
NIS = N-Iodosuccinimide
PdC12(dppf) = bis(diphenylphosphino)ferrocene]dichloropalladium(II)
SFC = supercritical fluid chromatography
SM = Starting material
tBuBrettPhos = 2-(Di-tert-butylphosphino)-2',4',6'- triisopropy1-3,6-dimethoxy-
1,1'-biphenyl
THF ¨ tetrahydrofuran
TCMS = Methyltrichlorosilane
TFA = Trifluoroacetic acid
Xphos = 2-Dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl
XPhos Pd 62 = chl oro(2-di cycl ohexylphosphi no-2' ,4',6' -triisopropyl-1,1' -
bipheny1)[2-(2' -
amino-1,1' -biphenyl)]palladium(II)
Also, aq. is aqueous, TLC is thin layer chromatography; Ts is tosyl; UV is
ultraviolet; W
is watts; wt. % is percentage by weight; x g is times gravity; [ID] is the
specific rotation of
polarized light at 589 nm; C is degrees Celsius; % w/v is percentage in
weight of the former
agent relative to the volume of the latter agent.
LCMS conditions: column: SUPELCO Ascentis Express C18 3x100 mm, 2.7 urn.
Solvent
system: A - 0.05% TFA in water and B- 0.05% TFA in Acetonitrile.
Gradient condition: 10 - 99%B in 3.5 min.
GENERAL SYNTHETIC SCHEMES
68
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
While the present invention has been described in conjunction with the
specific examples
set forth above, many alternatives, modifications and variations thereof will
be apparent to those
of ordinary skill in the art. In some cases, the order of carrying out the
steps of the reaction
schemes may be varied to facilitate the reaction or to avoid unwanted reaction
products. All such
alternatives, modifications and variations are intended to fall within the
spirit and scope of the
present invention.
[General Scheme 1]
R1
R1
X F/C 1 X OJH X gl H
111-NH2 Zn
Z NO2 1,4'n 1").%Z NO2 1-4n yLe. NH2
R1 Ri
X g R2-1 X /4
1-4 1-5
R1 R4 R1
X r4 X rsi
R3 r(i R4 Y'
sI 0 -0..
H Ose z*L.
IR2 tz2
1-6 1
Compounds of formula I were prepared from I- I with R1-NI-I2 via SNA,-,
followed by reduction
of the nitro group to the amino group to yield 1-3. Cyclization with
carbonyldiimidazole
provided 1-4, and subsequent 5N2 with R2-I yielded 1-5. Saponification of 1-5
provided the
corresponding carboxylic acid (1-5), and subsequent amide coupling with the
appropriate amines
provided compounds of formula (I) as described by the general scheme. The
order of steps for
some examples may be varied to facilitate the syntheses.
[General Scheme 2]
69
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
X NO2 R2-NH x NO2
x NH,
2
Zn
1eLZ4k=F 14Q1c1-7 NHIV
1401(1-z- NH
2-1 2-2 2-3
0 H R1-Br or R1-B(01-0 or )3_Ri RI
CBI
N X NI
cr
Xre0 _______________________________________________________________________
Yiµ 10
R2 R2
2-4 2-5
121 R4 121
X 14 R3 R4 " x
v= Y'
0
HO,e1 zeJ., 0 R3lkyLl Z.1")
k2 k2
2-6
Compounds of formula I were also prepared from 2-1 with R2-NH2 via SNA,-,
followed by
reduction of the nitro group to the amino group to yield 2-3. Reaction with
carbodiimidazole
(CDI) formed the cyclized product 2-4, and C¨C coupling with the corresponding
R1-bromide,
R1-boronic acid, or R1--boronic ester species afforded 2-5. Hydrolysis of the
ester in 2-5 to the
carboxylic acid provided 2-6, which was then coupled to the requisite amine to
form the
compounds of formula (I) as described by the general scheme. The order of
steps for some
examples may be varied to facilitate the syntheses.
INTERMEDIATES
INTERMEDIATE -1: Methyl 3-isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylate
NO2 NO2 NH2
STEP A o (101 F 0 NH STEP B
===-o * NH STEP C
[10 NO
INT-1
STEP A: Methyl 3-(isopropylamino)-4-nitrobenzoate
To a stirred solution of methyl 3-fluoro-4-nitrobenzoate (4 g, 20.09 mmol) in
DMSO (50 mL)
was added propan-2-amine (1.140 g, 19.28 mmol) and DIPEA (7.02 mL, 40.2 mmol).
The
reaction mixture was stirred at 100 'V for 12 h, then diluted with water and
Et0Ac. The layers
were separated, and the aqueous layer was extracted Et0Ac. The combined
organic extracts were
washed with brine, dried over Na2SO4 (s) and concentrated in vacuo. The
residue was purified by
flash silica gel column chromatography eluting with 5% Et0Ac/PE to give the
title compound.
LC/MS = 239.2 [M+1]
STEP B: 4-Amino-3-(isopropylamino)benzoate
To a stirred solution of methyl 3-(isopropylamino)-4-nitrobenzoate (4.4 g,
18.47 mmol) in
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Me0H (60 mL) was added Pd/C (0.983 g, 9.23 mmol). The reaction mixture was
stirred at 25 C
for 12 h under 1 atm of hydrogen, then filtered and washed with DCM\IVIe0H
(VW=10:1). The
filtrate was concentrated under reduced pressure to give the title compound.
LC/MS = 209.3
[M+ 1].
STEP C: Methyl 3-i sopropy1-2-oxo-2,3-di hydro-1H-benzo[d]imi dazol e-5-
carboxyl ate
To a stirred solution of methyl 4-amino-3-(isopropylamino)benzoate (3.9 g,
18.73 mmol) in THE'
(50 mL) was added Et3N (7.83 mL, 56.2 mmol) and CDI (21.26 g, 131 mmol). The
reaction
mixture was stirred at 25 C for 12 hours under a nitrogen atmosphere, then
diluted with water
and Et0Ac. The layers were separated, and the aqueous layer was extracted with
Et0Ac. The
combined organic extracts were concentrated in vacno. The residue was diluted
with water. The
mixture was stirred at RT for thirty minutes, then filtered, and the filter
cake was concentrated
under reduced pressure to afford the title compound. 1H NAIR (500 MHz,
Chloroform-ci) 6 10.09
(s, 1H), 7.86 ¨ 7.79 (m, 2H), 7.18 ¨ 7.08 (m, 1H), 4.76 (hept, J = 7.0 Hz,
1H), 3.93 (s, 3H), 1.59
(d, J = 7.0 Hz, 6H). LC/MS = 235.2 [M+1].
EXAMPLES
The following experimental procedures detail the preparation of specific
examples of the instant
disclosure. The examples are for illustrative purposes only and are not
intended to limit the scope
of the instant disclosure in any way.
EXAMPLE 1: (R)-1,3-bis(4-fluoropheny1)-N-(3-methy1-1,1-
dioxidotetrahydrothiophen-3-y1)-2-
oxo-2,3-dihydro-1H-benzokilimi dazol e-5-carboxami de
NI/N
0 STEP A N,i(D STEP B Ho STEP C
0
0
401 1,(0
6
EX-1
STEP A: methyl 1,3-bis(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-benzo[a]imidazole-
5-
carboxylate
To a stirred solution of methyl 2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylate (470 mg,
2.446 mmol) and (4-fluorophenyl)boronic acid (5133 mg, 36.7 mmol) in DMF (20
mL) was
added Cu(OAc)2 (533 mg, 2.93 mmol) and pyridine (2 mL, 2.446 mmol) at 15 C.
After the
addition was finished, the reaction mixture was stirred at 15 C for 16 h,
then poured into H20
and was extracted with DCM. The combined organic layers were washed with
brine, dried over
71
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Na2SO4, filtered and concentrated in vacuo. The residue was purified by column
chromatography
on silica (0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 381
[M+ll].
STEP B: 1,3-bis(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylic acid
To a stirred solution of methyl 1,3-bis(4-fluoropheny1)-2-oxo-2,3-dihydro-IH-
benzo[d]imidazole-5-carboxylate (60 mg, 0.158 mmol) in Me0H (1 mL), THF (3 mL)
and water
(1 mL) was added lithium hydroxide hydrate (10 mg, 0.238 mmol) at 15 C. After
the addition
was finished, the reaction mixture was stirred at 50 C for 2 h, then
concentrated in vacuo. The
residue was dissolved in H20 and 1N HCl was added to adjust the solution to pH
= 4. The
mixture was extracted with DCM, and combined organic layers were washed with
brine, dried
over Na2SO4, filtered and concentrated in vacua to afford the title compound.
LC/MS = 367
[M+1].
STEP C: (R)-1,3-bis(4-fluoropheny1)-N-(3-methy1-1, 1-dioxidotetrahydrothiophen-
3-y1)-2-oxo-
2,3-dihydro-1H-benzo[c/]imidazole-5-carboxamide
To a stirred solution of 1,3-bis(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-
benzordlimidazole-5-
carboxylic acid (50 mg, 0.136 mmol) and (R)-3-amino-3-
methyltetrahydrothiophene 1,1-dioxide
(25 mg, 0.168 mmol) in DMF (1.5 mL) was added N-ethyl-N-isopropylpropan-2-
amine (53 mg,
0.410 mmol) and 2-(3H-[1,2,3]triazolo[4,5-b]pyridin-3-y1)-1,1,3,3-
tetramethylisouronium
hexafluorophosphate(V) (78 mg, 0.205 mmol) at 15 C. After the addition was
finished, the
reaction mixture was stirred at 15 C for 16 h. The crude was purified
directly by mass triggered
reverse phase HFILC (ACN/water with 0.1% TFA modifier) to afford the title
compound. LC/MS
= 498 [M+1 ]. 1H NMR (400 MHz, DMSO-d6, ppm) 6 8.31 (s, 1H), 7.68 (dt, J =
5.0, 8.5 Hz,
5H), 7.51 - 7.41 (m, 5H), 7.11 (d, J = 8.0 Hz, 1H), 3.90 (m, 1H), 3.27 (hr d,
J = 9.0 Hz, 2H), 3.16
(m, 1H), 2.80 - 2.60 (m, 1H), 2.17 (m, 1H), 1.53 (s, 3H). Human DGAT2 IC50 =
573 nM
EXAMPLE 2: 1-(3-(difluoromethoxy)pheny1)-3-(4-fluoropheny1)-N-(3-methyl-1,1-
dioxidothietan-3-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide
72
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
rash NO2 Ali NH2
NO2
WI NH =-" kill" NH
0 * i\(0
STEP A
= STEP B
= STEP C
F
1101
STEP D P STEP E STEP F
*,,,0
EX-2
STEP A: methyl 3-((4-fluorophenyl)amino)-4-nitrobenzoate
To a stirred solution of methyl 3-fluoro-4-nitrobenzoate (4.8 g, 24.10 mmol)
in DMSO (150 mL)
was added 4-fluoroaniline (2.68 g, 24.10 mmol) and DIEA (8.42 mL, 48.2 mmol)
at 15 C. After
the addition was finished, the reaction mixture was stirred at 100 C for 16
h. The mixture was
poured into H20 and filtered. The filter cake was dissolved in ethyl acetate
and concentrated in
vacno to afford the title compound. LC/MS = 291 [M+1].
STEP B: methyl 4-amino-3-((4-fluorophenyl)amino)benzoate
To a solution of Pd-C (600 mg, 5.64 mmol) in Me0H (150 mL) was added methyl 3-
((4-
fluorophenyl)amino)-4-nitrobenzoate (5.4 g, 18.60 mmol) at 15 C. The mixture
was purged with
H2 stirred under 1 atm of H2 at 15 C for 16 h, then filtered and concentrated
in vacuo. The
residue was purified by column chromatography on silica (0-100% Et0Ac/hexanes)
to afford the
title compound. LC/MS = 261 [M+1].
STEP C: methyl 3-(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylate
To a stirred solution of methyl 4-amino-3((4-fluorophenyl)amino)benzoate (2 g,
7.68 mmol) in
TUT' (80 mL) was added CDI (4.98 g, 30.7 mmol) at 15 C After the addition was
finished, the
reaction mixture was stirred at 15 C for 16 h, then poured into H20 and
extracted with DCM.
The combined organic layers were washed with brine, dried over Na2SO4,
filtered and
concentrated in 1)acuo . The residue was purified by column chromatography on
silica (0-100%
Et0Ac/hexanes) to afford the title compound (1.37 g). LC/MS = 287 [M+1].
STEP D: methyl 1-(3-(difluoromethoxy)pheny1)-3-(4-fluoropheny1)-2-oxo-2,3-
dihydro-1H-
benzo[dlimidazole-5-carboxylate
To a stirred solution of methyl 3-(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-
carboxylate (400 mg, 1.397 mmol) and 2-(3-(difluoromethoxy)pheny1)-4,4,5,5-
tetramethy1-1,3,2-
dioxaborolane (4 g, 14.81 mmol) in DMF (20 mL) was added Cu(OAc)2(305 mg,
1.677 mmol)
73
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
and pyridine (3 mL, 37.2 mmol) at 15 C. After the addition was finished, the
reaction mixture
was stirred at 75 C for 48 h, then poured into H20 and extracted with DCM.
The combined
organic layers were washed with brine, dried over Na2SO4, then filtered and
concentrated in
vacno. The residue was purified by column chromatography on silica (0-100%
Et0Ac/hexanes)
to afford the title compound (200 mg). LC/MS = 429 [M+1].
STEP E: 1-(3 -(difluorom eth oxy)ph eny1)-3 -(4-fluoropheny1)-2-oxo-2,3 -di hy
dro-1H-
benzo[d]imidazole-5-carboxylic acid
To a stirred solution of methyl 1-(3-(difluoromethoxy)pheny1)-3-(4-
fluoropheny1)-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylate (200 mg, 0.467 mmol) in THE (6 mL)
and water
(2 mL) was added lithium hydroxide hydrate (78 mg, 1.868 mmol) at 15 C. The
reaction
mixture was stirred at 15 C for 16 h, then concentrated in vacuo and
dissolved in H20. HC1 (1N
in water) was added to the mixture until pH = 4. The mixture was extracted
with DCM. The
combined organic layers were washed with brine, dried over Na2SO4, filtered
and concentrated
in yam() to afford the title compound. LC/MS = 415 [M+1].
STEP F: 1-(3 -(difluoromethoxy)pheny1)-3 -(4-fluoropheny1)-N-(3 -methyl -1,1-
di oxidothietan-3 -
y1)-2-oxo-2,3 -dihydro-1H-benzo[d]imidazole-5 -carboxamide
To a stirred solution of 1-(3-(difluoromethoxy)pheny1)-3-(4-fluoropheny1)-2-
oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylic acid (35 mg, 0.084 mmol) and 2-
(3H11,2,3]triazolo[4,5-
b]pyridin-3-y1)-1,1,3,3-tetramethylisouronium hexafluorophosphate(V) (64 mg,
0.168 mmol) in
DAV (1.5 mL) was added N-ethyl-N-isopropylpropan-2-amine (0.070 mL, 0.422
mmol) at 15 C.
After the mixture was stirred for 15 min, 3-amino-3-methylthietane 1,1-dioxide
(14 mg, 0.104
mmol) was added to the mixture. After the addition was finished, the reaction
mixture was stirred
at 15 C for 2 h. The crude mixture was purified by mass triggered reverse
phase HPLC
(ACN/water with 0.1% TFA modifier) to afford the title compound. LC/MS = 532
[M+l]. 1H
NMR (400 MHz, DMSO-d6, ppm) 6 7.60-7.53 (m, 4H), 7.51 - 7.45 (m, 2H), 7.40 (t,
J = 2.0 Hz,
1H), 7.30 - 7.26 (m, 2H), 7.26 - 7.21 (m, 2H), 7.18- 7.14 (m, 1H), 6.76 -6.49
(m, 1H), 4.62 - 4.50
(m, 2H), 4.21 - 4.09 (m, 2H), 1.25 (br d, J = 3.5 Hz, 3H). Human DGAT2 IC50 =
1.8 nM
By using procedures similar to those described in Example 2 with appropriate
amine and
(hetero)arylhalide reagents, the following compound was synthesized. These
compounds were
characterized by LC/MS.
Example Structure Name
LCMS [M+11 Human DGAT2
IC50 (nM)
74
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
3 F
).....F (R) - 1 -(3- 546 2.4
(thfluoromethoxy)pheny1)-3 -(4-
)
fluoropheny1)-N-(3 -methyl- 1, I-
ll (101 i\f`" dioxidotetrahydrothiophen-3-
e=6 I
cf, y1)-2-oxo-2,3-dihydro-1 H-
benzo[d]imidazole-5-
carboxamide
EXAMPLE 4: 3-(3-(difluoromethoxy)pheny1)-1-(4-fluoropheny1)-Y-(3-methyl -1,1-
dioxidothietan-3-y1)-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridine-6-carboxamide
F,....F
N NH, H
N N
N....xr NH, O
, -. .
.....0 ...... B STEP A p
yc ...
STEP B .'''
STEP C N p
q ¨ c 1 ;
q
F F..-F
),...F
_ fk _
STEP D N STEP E N c)
,.._ ..._
Neo
q at
q
EX-4
STEP A: methyl 6-amino-5-((4-fluorophenyl)amino)nicotinate
To a stirred solution of methyl 6-amino-5-bromonicotinate (300 mg, 1.298 mmol)
in TT-IF (8
mL) were added 4-fluoroaniline (1443 mg, 12.98 mmol), Cs2CO3 (1269 mg, 3.90
mmol) and
Brettphos Pd G3 (118 mg, 0.130 mmol) in glovebox at 25 C. The reaction mixture
was stirred at
70 C for 16 h, then poured into H20 and extracted with DCM (x 3). The
combined organic
layers were washed with brine, dried over Na2SO4, filtered and concentrated in
vacuo. The
residue was purified by column chromatography on silica (0-100% Et0Ac/hexanes)
to afford the
title compound. LC/MS = 262 [M-F1].
STEP B: methyl 1-(4-fluoropheny1)-2-oxo-2,3-dihydro-IH-imidazo[4,5-b]pyridine-
6-
carboxylate
To a stirred solution of methyl 6-amino-5-((4-fluorophenyl)amino)nicotinate
(822 mg, 3.15
mmol) in THF (30 mL) was added CDI (2041 mg, 12.59 mmol) at 25 C. The reaction
mixture
was stirred at 25 C for 16 h, then poured into H20 and extracted with DCM The
combined
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
organic layers were washed with brine, dried over Na2SO4, filtered and
concentrated in vacuo.
The residue was purified by column chromatography on silica (0-100%
Et0Ac/hexanes) to
afford the title compound. LC/MS = 288 [M+1].
STEP C: methyl 3-(3-(difluoromethoxy)pheny1)-1-(4-fluoropheny1)-2-oxo-2,3-
dihydro-1H-
imidazo[4,5-h]pyridine-6-carboxylate
To a stirred solution of methyl 1-(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-
imidazo[4,5-
b]pyridine-6-carboxylate (720 mg, 2.507 mmol) and 2-(3-
(difluoromethoxy)pheny1)-4,4,5,5-
tetramethy1-1,3,2-dioxaborolane (5416 mg, 20.05 mmol) in DMF (15 mL) were
added Cu(OAc)2
(683 mg, 3.76 mmol) and pyridine (4.04 mL, 50.1 mmol) at 25 C. The reaction
mixture was
stirred at 80 C for 16 h (open to air with a drying tube), then poured into
H20 and extracted with
DCM. The combined organic layers were washed with brine, dried over Na2SO4,
filtered and
concentrated in vacuo. The residue was purified by column chromatography on
silica (0-100%
Et0Ac/hexanes) to afford the title compound. LC/MS = 430 [M+1].
STEP D: 3-(3-(difluoromethoxy)pheny1)-1-(4-fluoropheny1)-2-oxo-2,3-dihydro-1H-
imidazo[4,5-b]pyridine-6-carboxylic acid
To a stirred solution of methyl 3-(3-(difluoromethoxy)pheny1)-1-(4-
fluoropheny1)-2-oxo-2,3-
dihydro-1H-imidazo[4,5-b]pyridine-6-carboxylate (100 mg, 0.233 mmol) in THE (1
mL) was
added lithium hydroxide hydrate (30 mg, 0.715 mmol) at 25 C. The reaction
mixture was stirred
at 25 C for 0.5 h, then concentrated in vacuo and dissolved in H20. HC1 (1N
in water) was
added to the mixture until pH = 4. Then, the mixture was extracted with DCM (x
3). The
combined organic layers were washed with brine, dried over Na2SO4, then
filtered and
concentrated in vacuo to afford the title compound. LC/MS = 416 [M+1].
STEP E: 3-(3 -(di fluorom eth oxy)ph eny1)-1 -(4-fluoropheny1)-N-(3 -m ethyl -
1,1-di oxidothietan-3-
y1)-2-oxo-2,3 -dihydro-1H-imidazo[4,5-b]pyridine-6-carboxamide
To a stirred solution of 3-(3-(difluoromethoxy)pheny1)-1-(4-fluoropheny1)-2-
oxo-2,3-dihydro-
1H-imidazo[4,5-b]pyridine-6-carboxylic acid (95 mg, 0.229 mmol) and 2-(3H-
[1,2,3]triazolo[4,5-b]pyridin-3-y1)-1,1,3,3-tetramethylisouronium
hexafluorophosphate(V) (130
mg, 0.343 mmol) in DMF (2 ml) was added N-ethyl-N-isopropylpropan-2-amine
(0.114 ml,
0.686 mmol) at 25 C. After the addition was finished, the reaction mixture was
stirred at 25 C
for 5 mins, followed by addition of 3-amino-3-methylthietane 1,1-dioxide (38
mg, 0.281 mmol).
The reaction mixture was stirred at 25 C for 1.5 h. The crude mixture was
purified directly by
mass triggered reverse phase HPLC (ACN/water with 0.1% TFA modifier) to afford
the title
compound as the TFA salt. LC/MS = 533 [M+1]. 1E1 NNIR (500MHz, METHANOL-d4,
ppm) 6
76
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
= 8.64 (d, J=1.8 Hz, 1H), 7.86 (d, J=1.8 Hz, 1H), 7.76 - 7.61 (m, 5H), 7.45 -
7.37 (m, 2H), 7.34 -
7.28 (m, 1H), 7.12 - 6.78 (m, 1H), 4.58 (d, J=14.6 Hz, 2H), 4.27 - 4.19 (m,
2H), 1.83 (s, 3H).
Human DGAT2 IC50 = 8.5 nM
EXAMPLE 5: 1-(2-(Difluoromethoxy)-5-fluoropyri di n-4-y1)-3 sopropyl-/V-(4-m
ethyl-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo [d] imidazole-
5-carboxamide
F
EN1 N C)sy=F STEP A STEP B
0 * F,V ...e 1101 \II
)=--.
1:
HO
5:0<\11.12 STEP C 110
11101 j/0 O.
EX-5
STEP A: methyl 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-3-isopropy1-2-oxo-
2,3-dihydro-
1H-benzo[d]imidazole-5-carboxylate
At 20 C, to 20-mL vial was charged 4-bromo-2-(difluoromethoxy)-5-
fluoropyridine (279 mg,
1.15 mmol), methyl 3-isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylate (150
mg, 0.640 mmol), copper(I) iodide (122 mg, 0.640 mmol), (1R,2R)-N1,N2-
dimethylcyclohexane-1,2-diamine (182 mg, 1.28 mmol), tripotassium phosphate
(408 mg, 1.92
mmol), and 1,4-dioxane (6.4 mL) was added. The vial was evacuated and refilled
with N2 thrice,
and the mixture was stirred at 100 C for 7 h. Then, the volatiles were
evaporated, and the
residue was purified by flash silica gel chromatography (0 to 80% Et0Ac in
hexanes) to afford
the title compound. LC/MS = 396 [M+1].
STEP B: 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylic acid
At 20 C, to a stirred mixture of methyl 1-(2-(difluoromethoxy)-5-
fluoropyridin-4-y1)-3-
isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (39 mg, 0.099
mmol) in THE
(0.4 mL) and Me0H (0.1 mL) was added lithium hydroxide hydrate (33.1 mg, 0.789
mmol) in
Water (0.4 mL), and the mixture was stirred at 50 "V for 1 h. Then, HC1 (1 N
solution in water)
(0.79 mL, 0.79 mmol) was added, and the mixture stirred vigorously. The
volatiles were
evaporated to afford the title compound. LC/MS = 382 [M+1].
STEP C: 1-(2-(Difluoromethoxy)-5-fluoropyridin-4-y1)-3-isopropyl-N-(4-methy1-
1,1-
77
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
At 20 C, to a stirred mixture of 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-
3-isopropy1-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylic acid (19 mg, 0.050 mmol) and
HATU (37.9
mg, 0.100 mmol) in DMF (1.0 mL) was added DIPEA (44 jil, 0.25 mmol), followed
by 4-amino-
4-methyltetrahydro-2H-thiopyran 1,1-dioxide (12 mg, 0.075 mmol). The mixture
was stirred at
20 C for 2 h, then purified directly by reverse phase HPLC (55 to 95% MeCN in
water) to
afford the title compound. 1f1 N1VIR (500 1\41-Iz, DMSO-d6) 6 8.61 (s, 1H),
7.89 (s, 1H), 7.80 (s,
1H), 7.73 (tõI = 72.4 Hz, 1H), 7.68 (dõI = 8.4 Hz, 1H), 7.55 (dõI = 4.8 Hz,
1H), 7.14 (ddõI =
8.3, 2.4 Hz, 1H), 4.71 (hept, J= 6.6 Hz, 1H), 3.21 ¨3.11 (m, 2H), 3.10 ¨ 3.03
(m, 2H), 2.85 ¨
2.76 (m, 2H), 2.08 ¨ 1.96 (m, 2H), 1.55 (d, J= 6.9 Hz, 6H), 1.44 (s, 3H).
LC/1\4S = 527 [M+1].
Human DGAT2 IC50 = 34 nM.
By using procedures similar to those described in Example 5 with appropriate
aryl bromide and
amine reagents, the following compounds were synthesized. These compounds were
characterized by LC/MS.
Example Structure Name LC/MS
Human
[M+1]
DGAT2
IC50
(nM)
6 40, 1-(3-isopropoxypheny1)-3- 473
88
isopropyl-N-(3-methy1-1,1-
.
6' 0
dioxo-thietan-3-y1)-2-oxo-
c4.B benzimidazole-5-carboxamide
7 * 3-isopropyl-N-(3-methyl-1,1- 531
16
dioxo-thietan-3-y1)-2-oxo-1-
6,11 * 11,0
. [i-(1,1,2,2-
crt tetrafluoroethoxy)phenyl]benzi
midazole-5-carboxamide
78
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
8 = F 1-[3 -(2,2- 495
43
(3\
difluoroethoxy)phenyl] -3 -
jirl i 1101 il i sopropyl -N-(3 -methyl-1,1-
)---
ol-B di oxo-thi etan-3-y1)-2-oxo-
benzimidazole-5-carboxamide
9 ao, o\....... 1-(3-
ethoxypheny1)-3- 459 4.3
i sopropyl -N-(3 -methyl-1,1-
)--- di oxo-thi etan-3-y1)-2-oxo-
al-8
benzimidazole-5 -carboxamide
*%..._F 3 -i sopropyl-N-(4-m ethyl-1,1- 527 153
F.
dioxo-thian-4-y1)-2-oxo-1-[3-
[110 o
o...õ...õ.. 1 )-- (trifluoromethoxy)phenyl]benz
i midazol e-5-carboxami de
11 4t, 0 1-[3 -(2,2- 523
15
difluoroethoxy)phenyl] -3 -
Fil 1101 r\i/
i sopropyl-N-(4-methy1-1,1 -
6, .....--
di oxo-thi an-4-y1)-2-oxo-
benzimidazole-5-carboxamide
12
9.1.....F 1- [4-(difluoromethoxy)-2- 481
122
pyri dy1]-3-i sopropyl-N-(3-
6
11 110 r.i -- =1 )-- m ethyl -1,1-di ox o-thi etan -3 -
y1)-2-oxo-benzimidazole-5-
carboxamide
13 * 4 1-(3-cyclopropylpheny1)-3- 455
17
i sopropyl -N-(3 -methyl-1,1-
cf 1 0 1,10
di oxo-thi etan-3-y1)-2-oxo-
cf-8
benzimidazole-5 -carboxamide
14 * c 143 -(cyclopropoxy)pheny11-3- 471
15
i sopropyl -N-(3 -methyl-1,1-
Jr; io ,o
r,
i
)-- di oxo-thi etan-3 -y1)-2-oxo-
cr=8 benzimidazole-5 -carboxamide
79
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
15 r p, 1-(2-Ethoxy-5-fluoropyridin-4-
505 6.7
F y1)-3-isopropyl-N-(4-methyl-
1, 1-dioxidotetrahydro-2H-
O.r- 1 ,......
, 6........_ thiopyran-4-y1)-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-
carboxamide
16 Et0 N 1-(2-ethoxy-5-fluoropyridin-4- 477
32
.....1
F y1)-3-isopropyl-N-(3-methyl-
Y )
ji i 110 rvio 1,1-dioxidothietan-3- 1 -2-
)--- oxo-2,3-dihydro-1H-
o1.8
benzo[d]imidazole-5-
carboxamide
17 Et c
(5)- 1-(2-Ethoxy-5- 491
122
z
F fluoropyridin-4-y1)-3-
isopropyl-IV-(3-methyl-1,1-
o
de dioxidotetrahydrothiophen-3-
y1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-S-
carboxamide
18 Et (R)-1-(2-Ethoxy-5- 491
28
pNF fluoropyridin-4-y1)-3-
6H
N 110 r\f isopropyl-N- 3-meth 1-1,1-
( 3'
)
i ---
o= =
de dioxidotetrahydrothiophen-3-
y1)-2-oxo-2,3-dihydro-1H-
benzokilimidazole-5-
carboxamide
19 =N3-
isopropyl--(3-methyl-1,1- 498 100
)c...FF
dioxo-thietan-3-y1)-2-oxo-1_
6_,
N,
i
)--- [3-
=
at
(trifluoromethoxy)phenyl]benz
imidazole-5-carboxamide
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
20 F2Fico N
1-(2-(difluoromethoxy)-5- 499
76
pF fluoropyridin-4-y1)-3-
ci. =I )---- i sopropyl -N-(3 -methyl-1,1-
o=8 di oxidothietan-3 -y1)-2-oxo-
2,3 -dihydro-1H-
benzo[d]i mi dazol e-5-
carboxami de
21 Et0 N 1-(2-Ethoxy-5-fl uoropyri din-4-
495 31
.....l
F F
y1)-6-fluoro-3-isopropyl-N-(3-
ci.,11 101 0
1\l' methyl-1, 1-dioxi dothi etan-3 -
.
i
/)-- y1)-2-oxo-2,3-dihydro-1H-
o1.8
benzo[d]imidazole-5-
carboxamide
22 F A i-(3-(2,2- 490
66
F
. Difluorocyclopropyl)pheny1)-
3 -i sopropyl -/V-(3 -m ethyl-1,1 -5>
di oxidothietan-3 -y1)-2-oxo-
2,3 -di hydro-1H-
benzo[d]imidazole-5-
carboxamide
23 F2Hco (R)- 1-(3 - 494
63
(Difluoromethoxy)pheny1)-3-
H
N 40 re0 i sop ropyl -N- (3 -methyl-1, 1 -
e di oxidotetrahy drothiophen-3 -
y1)-2-oxo-2,3 -dihydro-1H-
benzokilimidazole-5-
carboxamide
24 F,HCO.pN 1-(2-(Difluoromethoxy)-5-
517 66
F F fluoropyridin-4-y1)-6-fluoro-3-
61 1 0 N,c>
i sopropyl -N-(3 -methyl-1,1-
. )--
01-8 di oxidothietan-3 -y1)-2-oxo-
2,3 -di hy dro-1H-
benzo[d]i mi dazol e-5-
81
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
carboxamide
EXAMPLE 25: 1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-
2H-thi opyran-4-y1)-2-oxo-2,3-di hydro- 1H-b enzo[d]imi dazol e-5 -carb oxami
de
0 F 411
0 F 0T
F
T
0
=====
NO2+ 1411 STEP A
NH STEP B
NH
(110 NH2
-0" NO2 .0"o 110 NH
2
*
STEP C STEP D STEP E
0 *I 1,(3 HO
0 * r\(()
=
STEP F
HN 1\10
de=
EX-26
STEP A: Methyl 4-((3-(difluorom ethoxy)phenyl)amino)-3-nitrobenzoate
To a stirred solution of methyl 4-fluoro-3-nitrobenzoate (7.0 g, 35.2 mmol) in
DMSO (176 ml)
were added 3-(difluoromethoxy)aniline (4.34 ml, 35.2 mmol) and DIPEA (12.28
ml, 70.3 mmol)
at RT. The reaction was heated to 100 C and stirred overnight. The reaction
was cooled to RT.
Water was added, and the resulting solid was collected by vacuum filtration
and dissolved in
ethyl acetate. The aqueous fraction was extracted three times with ethyl
acetate. The combined
organic fractions were washed with brine, dried over Na2SO4 (s), filtered, and
concentrated under
reduced pressure to afford the title compound. LC/MS = 339 1M+1].
STEP B: Methyl 3-amino-4-((3-(difluoromethoxy)phenyl)amino)benzoate
At RT, zinc (10.44 g, 160 mmol) was added to a stirred mixture of methyl 4-((3-
(difluoromethoxy)phenyl)amino)-3-nitrobenzoate (10.8 g, 31.9 mmol) in methanol
(106 ml),
THF (53.2 ml), and saturated aqueous ammonium chloride (53.2 m1). The reaction
was stirred
for two hours at RT, then filtered over Celite. The celite was washed with
methanol. The mixture
was concentrated, Et0Ac was added, and the water layer was separated. The
organic layer was
dried with Na2SO4 (s), filtered, and concentrated under reduced pressure. The
crude residue was
purified by flash silica gel column chromatography (0 to 100% Et0Ac in
hexanes) to afford the
title compound. LC/MS = 309 [M+1].
STEP C: 1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-dihydro-1H-benzo[c]imidazole-5-
82
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
carboxylate
At RT, methyl 3-amino-4-((3-(difluoromethoxy)phenyl)amino)benzoate (5.90 g,
19.14 mmol)
was dissolved in THF (191 m1). CDI (15.52 g, 96 mmol) was added, and the
reaction was stirred
at RT for 16hrs. Water and Et0Ac were added, the layers were separated, and
the aqueous layer
was extracted three times with Et0Ac. The combined organic layers were dried
with Na2SO4 (s),
filtered, and concentrated under reduced pressure. The crude residue was
purified by flash silica
gel column chromatography (0 to 100% Et0Ac in hexanes) to afford the title
compound. LC/MS
=335 [M+1
STEP D: 1-(3-(difluoromethoxy)pheny1)-3-i sopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-
5-carboxylate
At RT, methyl 1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-
carboxylate (0.93 g, 2.78 mmol) was dissolved in DMF (27.8 ml) and cesium
carbonate (2.72 g,
8.35 mmol) was added. 2-iodopropane (0.833 ml, 8.35 mmol) was added, and the
mixture was
stirred under a nitrogen atmosphere, heated to 80 C, and stirred at that
temperature overnight.
Water and ethyl acetate were added, the layers were separated, and the aqueous
layer was
extracted three times with Et0Ac. The combined organic layers were washed with
brine, dried
with MgSO4 (s), filtered, and concentrated under reduced pressure. The crude
residue was
purified by flash silica gel column chromatography (0 to 100% Et0Ac in
hexanes) to afford the
title compound. LC/MS ¨ 377 [M-F1].
STEP E: 1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-
5-carboxylic acid
To a stirred solution of methyl 1-(3-(difluoromethoxy)pheny1)-3-i sopropy1-2-
oxo-2,3-dihydro-
1H-benzo[d]imidazole-5-carboxyl ate (937 mg, 2 490 mmol) in TI-IF (4.15 ml)
and methanol
(4.15 ml) at RT was added a solution of lithium hydroxide (298 mg, 12.45 mmol)
in water (4.15
m1). The reaction was stirred at RT, then, the reaction mixture was acidified
to pH = 1 with 1 N
aqueous HC1. Et0Ac was added, and the layers were separated. The aqueous layer
was extracted
with Et0Ac three times, and the combined organic layers were dried with MgSO4
(s), filtered,
and concentrated under reduced pressure to afford the title compound. LC/MS =
363 [M+1].
STEP F 1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methyl-1,1-
dioxidotetrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide
At RT, to a stirred solution of -(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-
2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylic acid (36 mg, 0.099 mmol), HATU (41.6 mg, 0.109
mmol), and
4-amino-4-methyltetrahydro-2H-thiopyran 1,1-dioxide (17.84 mg, 0.109 mmol) in
D1VIF (0.994
83
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
ml) was added DIPEA (0.052 ml, 0.298 mmol. The reaction was stirred at RT for
16hrs. The next
morning, the reaction was complete as determined by LCMS, and the DMf was
evaporated
under reduced pressure. The crude residue was purified by flash silica gel
column
chromatography (0 to 100% Et0Ac in hexanes followed by 0 to 30% Acetone in
DCM) to afford
the title compound. 1H N1VIR (500 MHz, Methanol-d4) 7.82 (d, J = 1.4 Hz, 1H),
7.66 ¨ 7.60
(m, 2H), 7.42 (dd, J = 8.0, 1.1 Hz, 11-1), 7.36 (t, J = 2.0 Hz, 1H), 7.28 (dd,
J = 8.2, 2.0 Hz, 1H),
7.15 (d, J = 8.2 Hz, 1H), 6.94 (t, J = 73.6 Hz, 1H), 4.84 ¨ 4.75 (m, 1H), 3.29
¨ 3.21 (m, 2H), 3.02
(d, J = 13.7 Hz, 2H), 2.91 (d, J = 14.7 Hz, 2H), 2.25 ¨2.14 (m, 2H), 1.64 (d,
J = 7.0 Hz, 6H),
1.55 (s, 3H). LC/MS = 508 [M+1]. Human DGAT2 IC50= 23nM.
By using procedures similar to those described in Example 25 with appropriate
reagents, the
following compounds were synthesized. These compounds were characterized by
LC/MS.
Example Structure Name LCMS
DGAT2
[M+11 IC50 (nM)
26 F F 1{3-
(difluoromethoxy)phenyl] -3- 481 54
isopropyl-N-(3-methy1-1,1-dioxo-
I
. thietan-3 -y1)-2-oxo-benzimidazol e-5-
carboxami de
cf-8
27 F F 1{3-(difluoromethoxy)phenyll -N-(2-
448 >10000
= hydroxy-1,2-d imethyl -propy1)-3-
OH H *
= isopropy1-2-oxo-b enzimidazo le -5 -
carboxamide
28 F 1{3-
(difluoromethoxy)phenyl] -N- 446 779
HOO [(1S,25)-2-hydroxycyclopentyl] -3 -
HA 110 0
isopropyl-2-oxo-b enzimidazo le -5 -
carboxami de
29 91_-F 1{3-
(difluoromethoxy)phenyll -N- 462 1256
(?H FN [(3R,z1S)-3-hydroxytetrahydropyran-4-
Or 1 y1]-3-isopropy1-2-oxo-benzimidazole-
5-earboxamide
84
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
30 0 F N-[(1S,2R)-3,3-difluoro-2-hydroxy- 496
31
HOFti c:y r
cyc1ohexy1J-143 -
HA 110 ,\
1 )-- (difluoromethoxy)phenyl] -3 -i sopropyl-
2-oxo-benzimidazole-5-carboxamide
31 F....F 143-(difluoromethoxy)pheny1]-N-
460 106
* [(1R,2R)-2-hydroxycyclohexyll -3 -
OH
Id 11101 N .1,0 isopropyl-2-oxo-benzimidazole-5 _
a- i )-- carboxamide
32 yF 1{3-(difluoromethoxy)phenyll -3- 494
96
51)--- i sopropyl -N-K3S)-3-m ethyl -1,1-di oxo-
thiolan-3-yll -2-oxo-benzimidazole-5-
--
o_j/C I )
carboxamide
33 F)._F
1{3-(difluoromethoxy)phenyl] -N-(3,3- 466 182
* difluoro-1-methyl-cyclobuty1)-3-
H 111)11 0 isopropyl-2-oxo-benzimidazole-5 -
....0,N i
)---- carboxamide
F
34 F
y 1{3-(difluoromethoxy)phenyll-N- 448
62
. R LS)-1-(hydroxymethyl)-2-methyl-
OH H
propyli -3 -isopropy1-2-oxo-
(111 1,1,0
2
benzimidazole-5 -carboxamide
35 F).....F 1{3-(difluoromethoxy)pheny1]-N-
460 71
ilk [(1S,25)-2-hydroxycyc1ohexy1l -3-
(r ri 00 rstoo isopropy1-2-oxo-benzimidazole-5 -
carboxamide
36 )F 1-(3-(difluoromethoxy)pheny1)-N-(3-
522 19.4
4*. methy1-1,1-dioxidothietan-3-y1)-2-oxo-
c? 00 o
N. 3 -(tetrahydro-2H-pyran-4-y1)-2,3-
1 dihydro-1H-benzo[d]imidazole-5-
.
crt
o carboxamide
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
37 F o (R)-3-Cyclobuty1-1-(3- 506
64
....p
(difluoromethoxy)pheny1)-N-(3 -
g 1101 o methy1-1,1-dioxidotetrahydrothiophen-
c-5 i
3 -y1)-2-oxo-2,3-dihydro-1H-
Abenzo[d]imidazole-5-carboxamide
38 F\,_0 (R)-3 -Cyclopenty1-1-(3- 520
24
(difluoromethoxy)pheny1)-N-(3-
il methy1-1,1-dioxidotetrahydrothiophen-
c5 i
b 3 -y1)-2-oxo-2,3-dihydro-1H-
A benzo [d] imidazole-5 -carboxamide
39 F
\....0 1-(3-(Di fluoromethoxy)pheinyl)-3-
494 109
Ff pethyl-N-(4-methy1-1,1-
H 1101 r)- dioxidotetrahydro-2H-thiopyran-4-y1)-
o=r--,. 1 )
6, _ 2-oxo-2,3-dihydro-1H-
benzo [d] imidazole-5 -carboxamide
40 5.....0) 1 -(3-(Difluorom ethoxy)pheny1)-3 -
466 194
ethyl-N-(3 -methy1-1,1-dioxidothietan-
c
c,µ./=\ ,IRil *I i\e 3 -y1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxamide
41 F
C3' /Mk 3 -Cyclopropy1-1-(3- 506
211.4
(difluoromethoxy)pheny1)-N-(4-
H
N so N,,, methyl-1,1-dioxidotetrahydro-2H-
0=d 1 h). thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-
d'
benzold] imidazole-5 -carboxam ide
42 50 1 -(3-(Difluorom ethoxy)pheny1)-N-(4-
562 404.9
...o
m ethyl -1,1 -di oxi dotetrahydro-2H-
1:00 r\e() thiopyran-4-y1)-2-oxo-3 -(3,3,3 -
o= 1 trifluoropropy1)-2,3-dihydro-1H-
a. ....0-
F
F benzo [d] imidazole-5-carboxamide
43 F0 1-(3-(Difluoromethoxy)pheny1)-N-(3- 520
202
methy1-1,1-dioxidothietan-3 -y1)-2-oxo-
r 3 -(2,2,2-trifluoroethyl)-2,3 -dihydro-
o vi 1101 isf
µ..i.F.F 1H-benzo imidazole -5 - carboxamide
86
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
44 1 -(3-(difluoromethoxy)pheny1)-N-(4-
548 87
methyl-1,1 -dioxid otetrahydro -2H-
thiopyran-4-y1)-2-oxo -3 -(2,2,2-
Lf.FF trifluoroethyl)-2,3-dihydro-1H-
d,
benzo[d]imidazole-5-carboxam i de
45a 1 -(3-(Difluorom ethoxy)phcny1)-3 -(1,
1- 544 12
difluoropropan-2-y1)-N-(4-methyl-1, 1-
010 j/o dioxidotetrahydro-2H-thiopyran-4-y1)-
r- 2-oxo-2,3-dihydro-1H-
61=
benzo [d] imidazole-5 -carboxamide
(faster eluting on AD-H column, 15%
Et0H/C 02)
45b 1 -(3-(Difluorom ethoxy)pheny1)-3 -(1,
1- 544 101
difluoropropan-2-y1)-N-(4-mcthyl-1, 1-
dioxidotetrahydro-2H-thiopyran-4-y1)-
(10
1 2-oxo-2,3-dihydro-1H-
&= benzokijimidazole-5-carboxam i de
(slower eluting on AD-H column, 15%
Et0H/C 02)
46a 3 -(sec-buty1)-1-(3 - 494
81
(difluoromethoxy)pheny1)-N-(3-
1101 methyl-1,1 -dioxidothi etan-3 -y1)-2-
oxo-
2,3-dihydro-1H-benzo[imidazole-5-
o-t
carboxamide (Faster eluting on IC-H
column, 40%/60% ethanol/CO2)
46b )F 3 -(sec-buty1)-1-(3 - 494
92
(difluoromethoxy)pheny1)-N-(3-
.
c--A-EN1 0
methyl-1,1 -dioxidothi etan-3 -y1)-2-oxo-
2,3 -dihydro-1H-benzo [d] imidazo le-5 -
cPB
carboxamide (slower eluting on IC-H
column, 40%/60% ethanol/CO2)
47 0
HNic 1 -(4-acetam i do-3-liydroxyph eny1)-3-
487 >10000
fikOH isopropyl-N-(3 -methyl-1,1-
dioxidothietan-3 -y1)-2-oxo-2,3 -
0O
dihydro-1H-benzo[d]imidazole-5-
o>
carboxami de
87
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
48 * O)...... 3 -isopropyl-N-(3 -methyl-1, 1-
469 >10000
N
dioxidothietaa-3 -y1)-1 -(2-
cki 1 110 11, o
methylbenzo [d]oxazol-4-y1)-2-oxo-
.)--
at 2,3 -dihydro-1H-benzo [a] imidazo le-5 -
carbox am i de
49 NI, 3 -i sopropyl-N-(3 -methyl-1, 1-
469 >10000
= dioxidothietan-3 -y1)-1 -(2-
"I 1101 methylbenzo [a] oxazol-6-y1)-2-oxo-
2,3 -dihydro-1H-benzo [d] imidazo le-5 -
ct
carboxami de
50 go F 1-(3-fl uoropheny1)-3-isopropyl -AT-(4-
460 >9990
methyl-1,1 -dioxidotctrahydro -2H-
riNH ,,,/
thiopyran-4-y1)-2-oxo -2,3 -dihydro-1H-
o. =
benzo[d]imidazole-5-earboxamidc
51 õV.-NI
)..... 1 -(5-fluoro-2-i sop ropoxypyridin-4-y1)- 519 251
3-isopropyl-N-(4-methyl-1, 1-
11 1110 )=0
i N)--. dioxidotetrahydro-2H-thiop yran-4-y1)-
2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxam i de
52 * :::" 1 -(3-cyanopheny1)-3 -1 sopropyl-N-(4-
467 >9990
Op
methyl-1,1 -dioxidotctrahydro -2H-
o
..r-J 1 )_=_. thiopyran-4-y1)-2-oxo -2,3 -dihydro-1H-
(5, _
benzo[d]imidazole-5-carboxamide
53 F
fAk F 3 -i sopropyl-N-(4-methy1-1, 1- 510
1834
dioxidotetrahydro-2H-thiopyran-4-y1)-
2-oxo-1-(3-(trifluoromethyl)pheny1)-
i )--
,75' '"' 2,3 -dihydro-1H-benzo [di imidazo le-5 -
carboxami de
54 . Nf, 1 -(3-(dimethylamino)pheny1)-3 - 485
1324
isopropyl-N-(4-methy1-1,1-
11 110 o
dioxidotetrahydro-2H-thiopyran-4-y1)-
o=r........
- 1 N)__
(3,
2-oxo-2,3-dihydro-1H-
benzo [di imidazole-5 -carb oxam ide
88
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
55 0 1-(3-
carbamoylpheny1)-3-isopropyl-N- 485 >9990
# H2
(4-m ethy1-1,1-dioxidotetrahydro-2H-
,-
..r-...__õ.. thiopyran-4-y1)-2-oxo-2,3 -dihydro-1H-
de _
benzo[d]imidazole-5-earboxamide
56 /Ai 8\ 3 -isopropyl-N-(4-methy1-1, 1- 488
367
*
dioxidotetrahydro-211-thiopyran-4-y1)-
o
o.r i rr¨ 1-(3-(methylthio)pheny1)-2-oxo-2,3 -
(3, ....-
dihydro-1H-benzo[d]imidazole-5-
carboxamide
57 2_1_1\ 3 -isopropyl-N-(4-methy1-1, 1- 535
>9990
di oxi dotetrahydro-2H-thi opyran-4-y1)-
CI" 1 40 r,- 1-(3-(N-methylsulfam oyl)pheny1)-2-
6'
oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxamide
58 * i_ 3-isopropyl-N-(4-methyl-1, 1- 520
>9990
dioxidotetrahydro-2H-thiopyran-4-y1)-
1101
1-(3-(methylsolfonyl)pheny1)-2-oxo-
0.rj
de _
2,3 -dihydro-1H-benzo [di imidazole-5 -
carboxami de
59 F....F 1-(3-(difluoromethoxy)pheny1)-3-(4-
574 38
5)- fluorobenzy1)-N-(4-methyl-1,1-
0.d dioxidotetrahydro-2H-thiopyran-4-y1)-
d' IP F 2-oxo-2,3-dihydro-1H-
benzo [di imidazole-5 -carb oxamide
60 F,_0 1-(3-(difluoromethoxy)pheny1)-3- 452
1086
methyl-N-(3 -methyl-1,1-
dioxidothietan-3-y1)-2-oxo-2,3-
cP*C 1 % dihydro-1H-benzord]imidazole-5-
earboxamide
61 9--)....F 1-(3-(difluoromethoxy)pheny1)-3-
460 >9990
11 100 Isc' isopropyl-N-(5 -methyl-1,3,4-
.....<1_, 1 )......
thiadiazol-2-y1)-2-oxo-2,3 -dihy dro-
1H-benzo[d] imidazo1e-5-carboxamide
89
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
62
1-(3-(difluoromethoxy)pheny1)-N-((3- 462 >9990
0(..01H
hydroxytetrahydrofuran-3-yl)methyl)-
HN 0111 r\O
3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxamide
63 n
1-(3-(difluoromethoxy)pheny1)-N-((1- 470 >9990
ra,rexl
ethyl-1H-pyrazol-4-y1)methyl)-3-
HN
lsopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxamide
EXAMPLE 64: 1-(3-(difluoromethoxy)pheny1)-3-(2-hydroxy-2-methylpropy1)-N-(3-
methyl-
1,1-dioxidothietan-3-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide
*
410
* STEP A * STEP B STEP C
HO 110 \i/0
401 N)=0
cP8
LtH
EX-64
STEP A: 1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylic
acid
To a stirred solution of methyl 1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate (211 mg, 0.631 mmol) in THF (2 mL), Me0H (2mL)
and
water (2 mL) was added lithium hydroxide hydrate (79 mg, 1.894 mmol) at 25 C.
After the
addition was finished, the reaction mixture was stirred at 50 C for 5 h, then
concentrated in
men . The residue was dissolved in H20, and HC1 (1N in water) was added to the
mixture until
pH = 4. Then the mixture was extracted with DCM (x 3). The combined organic
layers were
washed with brine, dried over Na2SO4, then filtered and concentrated in vacuo
to afford the title
compound. LC/MS = 321 [M+1].
STEP B: 1-(3 -(difluoromethoxy)pheny1)-N-(3 -methyl-1, 1-dioxidothietan-3 -y1)-
2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxamide
To a stirred solution of 1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylic acid (179 mg, 0.559 mmol) and 2-(3H-
[1,2,3]triazolo[4,5-
b]pyri di n-3-y1)-1,1,3,3-tetram ethyl i souroni um hexafl uorophosphate(V)
(319 mg, 0.838 mmol) in
DMF (5 mL) was added N-ethyl-N-isopropylpropan-2-amine (0.278 ml, 1.677 mmol)
at 25 C.
After the addition was finished, the reaction mixture was stirred at 25 C for
5 mins, and 3-
amino-3-methylthietane 1,1-dioxide (83 mg, 0.615 mmol) was added. After the
addition was
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
finished, the reaction mixture was stirred at 50 C for 2 h. The crude mixture
was purified
directly by mass triggered reverse phase HPLC (ACN/water with 0.1% TFA
modifier) to afford
the title compound. LC/MS = 438 [M+1].
STEP C: 1-(3 -(difluoromethoxy)pheny1)-3 -(2-hydroxy-2-methylpropy1)-N-(3 -
methyl-1, 1-
di oxi dothi etan-3-y1)-2-ox o-2,3 -di hy dro-1H-benzo[d]i mi dazol e-5-
carboxami de
A flask was charged with 1-(3-(difluoromethoxy)pheny1)-N-(3-methy1-1,1-
dioxidothietan-3-y1)-
2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxamide (39 mg, 0.089 mmol), 1-
chloro-2-
methylpropan-2-ol (48.4 mg, 0.446 mmol), and cesium carbonate (87 mg, 0.267
mmol) in DMF
(2 mL). The reaction mixture was bubbled with a stream of N2 for 2 mins, then
was stirred at
90 C for 16 h under N? atmosphere. The crude mixture was directly purified by
mass triggered
reverse phase 1-1PLC (ACN/water with 0.1% TFA modifier) to afford the title
compound. LC/MS
= 510 [M-F1]. 1H NMR (500MHz, METHANOL-d4, ppm) 67.90 (s, 1H), 7.71 -7.62 (m,
2H),
7.47 (d, J = 7.5 Hz, 1H), 7.41 (s, 1H), 7.32 (br d, J = 8.5 Hz, 1H), 7.18 (d,
J= 8.5 Hz, 1H), 7.13 -
6.79 (m, 1H), 4.61 (m, 2H), 4.27 (m, 2H), 4.01 (s, 2H), 1.87 (s, 3H), 1.37 (s,
6H). Human
DGAT2 IC50 = 35.6 nM
EXAMPLE 65: 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-(3-hydroxy-3-methylbutan-
2-y1)-N-
(3 -methyl -1, 1-di oxidothietan-3 -y1)-2-oxo-2,3 -dihydro-1H-benzo[d]imi
dazole-5 -carboxamide
NH,
0
<:Fi STEP A ,2,0 STEP B 0 (111 NH
STEP C
(110 NH2 )11) NH ¨Y.- (so
-*pH =
F )."'F F (3).-F
STEP D STEP E STEP F
O 11011 rs(0 HO 1/01 rsf 0
H
)¨OH )OH
EX-65a and 65b
STEP A: methyl 4-bromo-34(3-hydroxy-3-methylbutan-2-yl)amino)benzoate
To a mixture of methyl 3-amino-4-bromobenzoate (1 g, 4.35 mmol) in DMF (15 mL)
was added
3-hydroxy-3-methylbutan-2-one (0.666 g, 6.52 mmol) at 20 C. The resulting
mixture was
degassed and backfilled with N2 (three times), followed by addition of TMCS
(1.161 ml, 13.04
mmol) and sodium cyanoborohydride (0.410 g, 6.52 mmol) at 0 C under N2. The
resulting mixture
was stirred under N2 at 20 C for 1 h, then poured into H20 and extracted with
Et0Ac (x 3). The
combined organic layers were washed with brine, dried over Na2SO4, then
filtered and
concentrated under reduced pressure. The residue was purified by flash column
chromatography
on silica (0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 316 [M-
P1].
91
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
STEP B: methyl 4-amino-3-((3-hydroxy-3-methylbutan-2-yl)amino)benzoate
A 8 mL of tube was charged with methyl 4-bromo-3-((3-hydroxy-3-methylbutan-2-
yl)amino)benzoate (300 mg, 0.949 mmol),ammonia hydrate (66.5 mg, 1.898 mmol),
K3PO4 (242
mg, 1.139 mmol), BPPO (37.2 mg, 0.095 mmol), copper(I) iodide (18.07 mg, 0.095
mmol) and
DMSO (4 mL) in glovebox at 20 C. Then the reaction was sealed and stirred at
90 C for 24 h.
The mixture was poured into H20, then extracted with Et0Ac (x 3). The combined
organic layers
were washed with brine, dried over Na2SO4, then filtered and concentrated
under reduced pressure.
The residue was purified by flash column chromatography on silica (0-100%
Et0Ac/hexanes) to
afford the title compound. LC/MS = 253 [M+1].
STEP C: methyl 3-(3-hydroxy -3 -methylb utan-2-y1)-2-oxo-2,3 -dihy dro-1H-b
enzo[d]imi dazol e-5-
carboxylate
A mixture of methyl 4-amino-3-((3-hydroxy-3-methylbutan-2-yl)amino)benzoate
(110 mg, 0.436
mmol) and di(1H-imidazol-1-yl)methanone (353 mg, 2.180 mmol) in THF (3 mL) was
stirred at
30 C for 12 h. The mixture was poured into H20, then extracted with Et0Ac (x
3). The combined
organic layers were washed with brine, dried over Na2SO4, then filtered and
concentrated under
reduced pressure. The residue was purified by flash column chromatography on
silica (0-100%
Et0Ac/hexanes) to afford the title compound. LC/1VIS = 279 [M+1].
STEP D: methyl 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-(3-hydroxy-3-
methylbutan-2-y1)-2-
oxo-2,3 -di hy dro-1H-b enzo [d]i mi dazol e-5 -carb oxyl ate
To a mixture of 2-(5-(difluoromethoxy)-2-fluoropheny1)-4,4,5,5-tetramethy1-
1,3,2-dioxaborolane
(279 mg, 0.970 mmol) in DMF (5 mL) was added pyridine (0.521 ml, 647 mmol),
methyl 3-(3-
hydroxy-3-methylbutan-2-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylate (90 mg,
0.323 mmol), copper(ii) acetate (70.5 mg, 0 388 mmol). The mixture was stirred
at 80 C for 16 h
open to air under a drying tube. The mixture was poured into H20, then
extracted with Et0Ac (x
3). The combined organic layers were washed with brine, dried over Na2SO4,
filtered and
concentrated under reduced pressure. The residue was purified by flash column
chromatography
on silica (0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 439
[M+1].
STEP E: 1-(5-(di fluoromethoxy)-2-fluoropheny1)-3 -(3 -hydroxy-3-methylbutan-2-
y1)-2-oxo-2,3-
dihy dro-1H-b enzo kilimidazole-5-carboxylic acid
To a solution of methyl 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-(3-hydroxy-3-
methylbutan-2-
y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (90 mg, 0.205 mmol)
in Me0H (3
mL)/ Water (1.00 mL) was added lithium hydroxide hydrate (17.23 mg, 0.411
mmol). The reaction
mixture was stirred at 40 C for 3 h, then concentrated under reduced
pressure. The concentrated
92
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
mixture was dissolved in H20. HCl (1N in water) was added to the mixture until
pH = 4. Then the
mixture was extracted with Et0Ac (x 3). The combined organic layers were
washed with brine,
dried over Na2SO4, then filtered and concentrated under reduced pressure to
afford the title
compound. LC/MS = 425 [M+1] .
STEP F: 1-(5-(difluorom ethoxy)-2-fluoroph eny1)-3 -(3 -hydroxy-3 -m ethyl
butan-2-y1)-N-(3-
methyl-1, 1-dioxidothi etan-3 -y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
A 8 mL of tube was charged with 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-(3-
hydroxy-3-
methylbutan-2-y1)-2-oxo-2,3 -di hy dro-1H-b enzo [d]i midazol e-5-carboxylic
acid (60 mg, 0.141
mmol), DIVA_ (0.074 ml, 0.424 mmol), HATU (81 mg, 0.212 mmol), 3-amino-3-
methylthietane
1,1-dioxide (28.7 mg, 0.212 mmol) and DMF (1 mL) at 25 C. The mixture was
stirred at 25 C
for 0.5 h, then poured into H20 and extracted with Et0Ac (x 3). The combined
organic layers were
washed with brine, dried over Na2SO4, then filtered and concentrated under
reduced pressure. The
residue was purified by flash column chromatography on silica (0-100%
Et0Ac/hexanes) to afford
the title compound. LC/MS = 542 [M+1].
The mixture of the two stereoisomers was purified by chiral SFC (IC-H column,
40%/60%
ethanol/CO2). EX-65a (faster eluting): LC/MS = 542 [M+1]. 1H NAIR (500 MHz,
METHANOL-
d4) 6 7.99 (br s, 1H), 7.53 (br d, J = 7.48 Hz, 1H), 7.21 - 7.45 (m, 3H), 6.58
- 7.03 (m, 2H), 4.43 -
4.56 (m, 3H), 4.13 (br d, J = 14.95 Hz, 2H), 1.73 (s, 3H), 1.58 (br d, J =
7.02 Hz, 3H), 1.25 - 1.35
(m, 3H), 1.00 - 1.21 (m, 3H). Human DGAT2 1050 - 53 nM. EX-65b (slower
eluting): LC/MS -
542 [M+1]. 1H NMR (500 MHz, METHANOL-d4) 6 7.96 (br s, 1H), 7.53 (br d, J =
7.48 Hz, 1H),
7.23 -7.45 (m, 3H), 6.59 - 6.98 (m, 2H), 4.46 - 4.54 (m, 3H), 4.13 (br d, J =
14.80 Hz, 2H), 1.67 -
1.82 (m, 3H), 1.58 (br d, J = 7.17 Hz, 3H), 1.29 (s, 3H), 1.13 (br s, 3H).
Human DGAT2 1050= 0.7
nM.
By using procedures similar to those described in Example 65 with appropriate
amine reagents,
the following compounds were synthesized. These compounds were characterized
by LC/MS.
Example Structure Name LCMS
DGAT2
[M+1]
1050 (nM)
66a
F * 1-(5-(difluoromethoxy)-2- 570 1.1
"I Nc) fluoropheny1)-3-(3-hydroxy-3-
methylbutan-2-y1)-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro-1H-
93
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
benzo [a] imidazole-5 -carboxamide
(faster eluting on Cellulose 2 column,
5-40% Et0H/CO2)
66b F = OF 1-(5-(difluoromethoxy)-2- 570
16.9
* N fluoropheny1)-3-(3 -hydroxy-3 -
r.)
(=s, methylbutan-2-y1)-N-(4-methyl-1,1-
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro-1H-
benzo[d] imidazole-5 -carboxamide
(slower eluting on Cellulose 2 column,
5-40% Et0H/CO2)
67a 1-(3-(difluoromethoxy)pheny1)-3-(3- 552
1.6
IR; OID re,c) hydroxy-3-methylbutan-2-y1)-N-(4-
methy1-1,1-dioxidotetrahydro-2H-
2,
thiopyran-4-y1)-2-oxo-2,3-dihydro-
1H-benzo [d]imidazole-5-
carboxamide
(faster eluting on Chiral Pak IC-3
column, 40% iPr01-1/CO2)
67b 1-(3-(difluoromethoxy)pheny1)-3-(3- 552
211
rl rs( hydroxy-3-methylbutan-2-y1)-N-(4-
C-' methy1-1,1-dioxidotctrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-
1H-benzo[d]imidazole-5-
carboxamide
(slower eluting on Chiral Pak IC-3
column, 40% iPrOT-I/CO2)
68a 1-(3-(2,2-difluoroethoxy)pheny1)-3-
566 4.1
4 rsf (3 -hydroxy-3 -methylbutan-2-y1)-N-
(4-methy1-1,1-dioxidotetrahydro-2H-
thiopyran-4-y1)-2-oxo-2,3-dihydro-
1H-benzo [d] imidazole-5-
carbox arni de
(faster eluting on Chiral Pak IC-3
column, 40% Et0H/CO2)
94
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
68b 1 -(3-(2,2-difluoroethoxy)pheny1)-3 -
566 1.8
H 4111 Nc)
I re¨ (3 -hydroxy-3 -methylbutan-2-y1)-1V-
06,. = ,./Lf0H (4-methyl-I, 1 -dioxidotetrahydro-2H-
thiopyran-4-y1)-2-oxo -2,3 -dihydro-
1 H-benzo [d] imi dazol e-5-
carboxami de
(slower eluting on Chiral Pak IC-3
column, 40% Et0H/CO2)
69a 1 -(2-ethoxy-5 -fluoropyridin-4-y1)-3 -
549 9.7
(3 -hydroxy-3 -methylbutan-2-y1)-N-
/1 rs
0= (4-ni ethyl-1, 1 -di oxi dotetrahydro-
2H-
thiopyran-4-y1)-2-oxo -2,3 -dihydro-
1H-benzo [d] imidazole-5-
carboxami de
(faster eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
69b FiN)--0 1 -(2-eth oxy-5 -fl uoropyri din -4-y1)-
3 - 549 3.9
4 rs (3 -hydroxy-3 -methylbutan-2-y1)-N-
0=rj. OH (4-methyl-I, 1 -dioxidotetrahydro-2H-
d, _
thiopyran-4-y1)-2-oxo -2,3 -dihydro-
1H-benzo [d] imidazole-5-
carboxami de
(slower eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
70a F F
6-fluoro- 1 -(2-fluoro -5 -
PF 606
1.0
NI 40 r\?= (trifluoromethoxy)pheny1)-3-(3-
hydroxy-3 -methylbutan-2-y1)-N-(4-
methyl-1, 1 -dioxidotetrahydro -2H-
thiopyran-4-y1)-2-oxo -2,3 -dihydro-
1H-benzo[d]imidazole-5-
carboxamide
(slower eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
70b C\....F 6-fluoro-1-(2-fluoro-5-
' 606
30.4
r\ (trifluoromethoxy)pheny1)-3-(3-
hydroxy-3-methylbutan-2-y1)-N-(4-
2,
methyl-1,1 -dioxid otetrahydro -2H-
th i opyran-4-y1)-2-oxo-2,3-dihydro-
1H-benzo [d] imidazole-5-
carboxamide
(faster eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
71a F 0µ._F 1-(2-fluoro-5- 588 71
,145y,
'F
(trifluoromethoxy)plieny1)-3-(3-
I hydroxy-3-methylbutan-2-y1)-N-(4-
07, = )--(OH
methyl-1,1 -dioxidotetrahydro -2H-
thiopyran-4-y1)-2-oxo -2,3-dihydro-
1H-benzo [d] imidazole-5-
carboxamide
(slower eluting on Cellulose 2 column,
5-40% Et0H/CO2)
71b F me!=JIL (c_F 1-(2-fluoro-5- 588
5.4
'F
r)
(trifluoromethoxy)pheny1)-3-(3_ ,,(0
hydroxy-3-methylbutan-2-y1)-N-(4-
2,
methyl-1,1 -dioxidotetrahydro -2H-
thiopyran-4-y1)-2-oxo -2,3-dihydro-
1H-benzo [d] imidazole-5-
carboxamide
(faster eluting on Cellulose 2 column,
5-40% Et0H/CO2)
72a F_ 1-(2-(2,2-difluoroethoxy)-5- 585
6.4
411 fluoropyridin-4-y1)-3-(3-hydroxy-3 _
.... methylbutan-2-y1)-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro-1H-
benzo [d] imidazole-5-carboxamide
(faster eluting on Chiral Pak 1G-3
column, 5-40 % Me0H/CO2)
96
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
72b 1 -(2-(2,2-difluoroethoxy)-5 - 585
6.6
H 110 N=0
fluoropyridin-4-y1)-3-(3 -hydroxy-3 -
(DO,- = )--(01-1 methylbutan-2-y1)-N-(4-methy1-1, 1 -
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-di hydro -1 H-
benzo [61] imidazole-5 -carboxamide
(slower eluting on Chiral Pak IG-3
column, 5% Me0H/CO2)
73a F 1 -(5-(2,2-difluoroethoxy)-2- 584
2.6
NizEs.
N>= fluoropheny1)-3 -(3 -hydroxy-3 -
(
m ethyl butan -2-y1)-N--(4-rn ethyl -1 ,1 -
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro -1 H-
benzo [d] imidazole-5 -carboxamide
(faster eluting on Chiral Pak IG-3
column, 5-40% Et0H/CO2)
73b F 1 -(5-(2,2-difluoroetlioxy)-2- 584
3.3
N455
H ,=0 fluoropheny1)-6-fluoro-3-(3 -hydroxy-
r,,,N
OH 3 -methylbutan-2-y1)-N-(4-methyl- 1, 1 -
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro -1 H-
benzo [d] imidazole-5 -carboxamide
(slower eluting on Chiral Pak IG-3
column, 5-40% Et0H/CO2)
74a 1 -(2-(2,2-difluoroethoxy)-5 - 603
6.1
"1
fluoropyridin-4-y1)-6-fluoro-3 -(3 -
1.
hydroxy-3 -methylbutan-2-y1)-N-(4-
methyl-1, 1 -dioxidotetrahydro -2H-
thiopyran-4-y1)-2-oxo -2,3 -dihydro-
1H-benzo [ d[imidazole -5 -
carboxamide
(faster eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
97
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
74b 1-(2-(2,2-difluoroethoxy)-5- 603
6.5
fluoropyridin-4-y1)-6-fluoro-3-(3-
hydroxy-3-methylbutan-2-y1)-N-(4-
methyl-1,1 -dioxid otetrahydro -2H-
th i opyran-4-y1)-2-oxo-2,3-dihydro-
1H-benzo [d] imidazole-5-
carboxamide
(slower eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
75a 4*, 1-(5-(2,2-difluoroethoxy)-2- 602
1.5
41) 11,0 fluoropheny1)-6-fluoro-3-(3-hydroxy-
o= )(OH 3-methylbutan-2-y1)-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro-1H -
benzo [d] imidazole-5-carboxamide
(faster eluting on Cellulose 2 column,
5-40% Et0H/CO2)
75b F o 1-(5-(2,2-difluoroethoxy)-2- 602
2.4
1.1 fluoropheny1)-6-fluoro-3-(3-hydroxy-
o= )--(oH 3-methylbutan-2-y1)-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-
y1)-2-oxo-2,3-dihydro-1H-
benzo [d] imidazole-5-carboxamide
(slower eluting on Cellulose 2 column,
5-40% Et0H/CO2)
76a 3-(3-hydroxy-3-methylbutan-2-y1)-N- 574
80
H Nc= (3 -m ethy1-1, 1-dioxidothietan-3 -y1)-2-
oxo-1-(3 -(1, 1,2,2-
tetrafluoroethoxy)pheny1)-2,3-
dihydro-1H-benzord]imidazole-5-
carboxamide
(faster eluting on Chiral Pak AD-3
column, 5-40% Et0H/CO2)
98
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
76b p-oj 3 -(3-hydroxy-3-m ethylbutan-2-y1)-N- 574
7.9
,
011)
(3 -m ethy1-1, 1-dioxidothietan-3-y1)-2-
0 H
OX0 - 1-(3 -(1, 1,2,2-
tetrafluoro ethoxy)pheny1)-2,3 -
di hydro-1H-ben zo [d] m idazole-5-
carboxamide
(slower eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
77a 9_,),.; 3 -(3-hydroxy-3-m ethylbutan-2-y1)-N- 602
5.6
-F
(4-m ethy1-1, 1-dioxidotetrahydro-2H-
= = th opyran-4-y1)-2-oxo -1 -(3 -
(1,1,2,2-
tetrafluoro ethoxy)pheny1)-2,3 -
dihydro-1H-benzo [d] im idazole-5-
carboxamide
(faster eluting on Cellulose 2 column,
5-40% Et0H/CO2)
77b 3 -(3-hydroxy-3-m ethylbutan-2-y1)-N-
602 9.8
F'T
( (4-m ethy1-1, 1-dioxi
I, dotetrahydro-2H-
)(ohi02), = thiopyran-4-y1)-2-oxo -1 -(3 -(1,1,2,2-
tetrafluoro ethoxy)pheny1)-2,3 -
dihydro-1H-benzo[d] im idazole-5-
carboxamide
(slower eluting on Cellulose 2 column,
5-40% Et0H/CO2)
78a Fiy"o 1 -(2-ethoxy-5-fluoropyridin-4-y1)-3 -
521 123
(3 -hydroxy-3 -methylbutan-2-y1)-N-
O 140 No
oKis. (3 -m ethy1-1, 1-dioxidothietan-3-y1)-2-
oxo-2,3-dihydro -1H-
benzo [d] imidazole-5 -carboxamide
(faster eluting on Chiral Pak AD-3
column, 40% Et0H/CO2)
78b FiyNo 1 -(2-ethoxy-5-fluoropyridin-4-y1)-3 -
521 13
(3 -hydroxy-3 -methylbutan-2-y1)-N-
O No
(3 -m ethy1-1, 1-dioxidothietan-3-y1)-2-
= \40H
oxo-2,3-dihydro -1H-
benzold] imidazole-5 -carboxamide
99
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(slower eluting on Chiral Pak AD-3
column, 40% Et01-1/CO2)
EXAMPLE 79: 1-(5-(Difluoromethoxy)-2-fluoropheny1)-3-isopropyl-A/-(4-methyl-
1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
F
F
STEP B
Me0
rst.No STEP A
Me0 (001 r\f0
FF
F F4t,
STEP C H
1101 1\(
HO * 1,10
e _
EX-79
STEP A: Methyl 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
At 20 C, to a stirred mixture of 2-(5-(difluoromethoxy)-2-fluoropheny1)-
4,4,5,5-tetramethyl-
1,3,2-dioxaborolane (2.15 g, 7.47 mmol), methyl 3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-5-carboxylate (0.700 g, 2.99 mmol), and diacetoxycopper
(1.09 g, 5.98
mmol) in DCE (14.9 ml) was added triethylamine (1.67 ml, 12.0 mmol), and the
mixture was
stirred at 95 C for 32 h. Then, the volatiles were evaporated, and the
residue was purified by
flash silica gel chromatography (0 to 100% Et0Ac in hexanes) to afford the
title compound.
LC/MS = 395 [M+1].
STEP B: 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-5-carboxylic acid
At 20 C, to a stirred mixture of methyl 1-(5-(difluoromethoxy)-2-
fluoropheny1)-3-isopropyl-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (990 mg, 2.51 mmol) in THE
(5.71 mL)
and Me0H (1.14 mL) was added lithium hydroxide hydrate (316 mg, 7.53 mmol) in
Water (5.71
mL), and the mixture was stirred vigorously at 50 C for 5 h. Then, the
mixture was neutralized
to pH 7 with HC1 (aqueous, 12 N) (628 pi, 7.53 mmol), the volatiles were
evaporated, and the
aqueous layer was extracted with Et0Ac. The organic layer was washed with
brine and dried
over Na2SO4 (s), and the volatiles evaporated to afford the title compound.
LC/MS = 381 [M+1].
100
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
STEP C: 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
At 20 C, to a stirred mixture of 1-(5-(difluoromethoxy)-2-fluoropheny1)-3-
isopropy1-2-oxo-2,3-
dihydro-IH-benzo[d]imidazole-5-carboxylic acid (690 mg, 1.81 mmol) and HATU
(1.00g, 2.63
mmol) in DMF (5.00 mL) and ethyl acetate (3.00 mL) was added 4-amino-4-
methyltetrahydro-
2H-thiopyran 1,1-dioxide hydrochloride (600 mg, 3.00 mmol), followed by DIPEA
(0.951 mL,
5.44 mmol). The mixture was stirred at 20 C for 1 h, ten partitioned between
Et0Ac and LiOH
(aqueous, 10 wt%). The organic layer was separated, washed with LiOH (aqueous,
10 wt%),
brine, dried over Na2SO4 (s), and the volatiles evaporated. The residue was
purified by flash
silica gel chromatography (0 to 100% Et0Ac in hexanes) to afford the title
compound. 11-INMIR
(500 MHz, DMSO-d6) 6 7.85 (s, 1H), 7.79 (s, 1H), 7.66 (d, J= 8.3 Hz, 1H), 7.62
(t, J= 9.4 Hz,
1H), 7.57 (dd, J= 6.0, 3.0 Hz, 1H), 7.46¨ 7.40 (m, 1H), 7.30 (t, J= 73.6 Hz,
1H), 6.93 (d, J=
8.3 Hz, 1H), 4.71 (hept, J= 6.9 Hz, 1H), 3.17 (t, J= 13.2 Hz, 2H), 3.06 (d, J=
13.6 Hz, 2H),
2.81 (d, J= 14.5 Hz, 2H), 2.02 (t, J= 12.9 Hz, 2H), 1.55 (d, J= 6.9 Hz, 6H),
1.44 (s, 3H).
LC/MS = 526 [M+1]. Human DGAT2 IC50= 2.1 nM.
By using procedures slimier to those described in Example 79 with appropriate
amine and
(hetero)arylhalide reagents, the following compounds were synthesized. These
compounds were
characterized by LC/MS.
Example Structure Name LCMS
Human
11\4+11
DGAT2
1050 (nM)
80 F,Hce 1-(5-(Difluoromethoxy)-2- 498
7.7
F fluoropheny1)-3-isopropyl-N-(3-
0,,e\ 11101 o methy1-1,1-dioxidothietan-3-y1)-2-oxo-
o%
2,3-dihydro-1H-benzo [d] imi dazole-5 -
carboxamide
81 F,HCO (9- 1-(5-(Difluoromethoxy)-2- 512
6.3
F fluoropheny1)-3-tsopropyl-N-(3 -
3 110 methy1-1,1-dioxidotetrahydrothiophen-
o
3-y1)-2-oxo -2,3-d ihydro-1
benzo lc/Jim idazole-5-earboxamide
101
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
82 F2HCO (R)-1-(5-(Difluoromethoxy)-2- 512 5.7
fluoropheny1)-3 -isopropyl-N-(3-
o methy1-1,1-dioxidotetrahydrothiophen-
o*6
3-y1)-2-oxo-2,3 -dihydro-1H-
benzo [d]im idazol e-5-earboxami de
83 F,HCO 145 -(Difluoromethoxy)pyridin-3-y1)- 481 150
pm
3-isopropyl-N-(3-methy1-1,1-
1101 Ne dioxidothietan-3-y1)-2-oxo-2,3-
5:
dihydro-1H-benzo [d]imidazole-5-
carboxamide
84 1-(5-Cycl opropylpyridin -3 -y1)-3 - 455 71
ApN isopropyl-N-(3-methy1-1,1 -
N dioxidothietan-3-y1)-2-oxo-2,3-
..r I dihydro-1H-benzo [d]imidazole-5-
8 carboxamide
EXAMPLE 85: 3-( 1-amino-l-oxoprop an-2-y1)-1-(3-(difluorom ethoxy)pheny1)-N-(4-
m ethy1-1, 1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imi dazol
e-5 -carb oxamide
STEP A STEP B
1
1 10 14,0 (101
,\
C
( 0
0 =:
0= I
0=r,6, _ OH ,NH2
EX-85a and EX-85b
STEP A: 2-(3 -(3 -(difluoromethoxy)pheny1)-6-((4-methyl-1, 1-di oxi
dotetrahydro-2H-thi opyran-4-
yl)carb amoy1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imidazol-1 -yl)propanoi c acid
Sodium hydride (20.62 mg, 0.516 mmol) was added to the mixture of 1-(3-
(di fluorom ethoxy)pheny1)-N-(4-m ethyl-1,1 -di oxi d otetrahy dro-2H-
thiopyran-4-y1)-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxami de (160 mg, 0.344 mmol) in MAI (2 ml)
at 0 C
under N2. The mixture was stirred at 0 C for 0.5 h. Then ethyl 2-
bromopropanoate (93 mg, 0.516
mmol) was added to the mixture. The mixture was stirred at 15 C for 12 h
under N2. LCMS
showed that the desired product was formed. The mixture was poured into NH4C1,
then the mixture
was extracted with DCM (x 3). The combined organic layers were washed with
brine, dried over
Na2SO4, then filtered and concentrated under reduced pressure to afford the
title compound.
LC/MS = 538 [M+ 1 ].
STEP B: 3-(1-amino-l-oxopropan-2 -y1)- 1-(3 -(difluoromethoxy)pheny1)-N-(4-
methyl-1, 1 -
102
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
di oxidotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imidazol
e-5 -carb oxamide
To a solution of 2-(3-(3-(difluoromethoxy)pheny1)-6-((4-methyl-1,1-
dioxidotetrahydro-2H-
thiopyran-4-y1)carbamoy1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-y1)propanoic
acid (200 mg,
0.372 mmol), DIEA (0.195 ml, 1.116 mmol), HATU (212 mg, 0.558 mmol) in DMF (3
ml) was
added NI-14C1 (25 mg, 0.467 mmol). The mixture was stirred at 15 C for 1 h.
LCMS showed that
the desired product was formed. The mixture was poured into H20, then the
mixture was extracted
with DCM (x 3). The combined organic layers were washed with brine, dried over
Na2SO4, then
filtered and concentrated under reduced pressure. The residue was purified by
column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LCNIS = 537
[M+1]. The mixture of the two stereoisomers 3-(1-amino-l-oxopropan-2-y1)-1-(3-
(di fluorom ethoxy)pheny1)-N-(4-m ethy1-1,1-di oxi dotetrahy dro-2H-thi opyran-
4-y1)-2-oxo-2,3 -
dihydro-1H-benzo[d]imidazole-5-carboxamide (100 mg) was purified by chiral SFC
(IC-H
column, 40%/60% ethanol/CO2). Isomer EX-85a (faster eluting): LC/MS = 537
[M+1]. IHNMR
(400 MHz, METHANOL-d4) 6 7.70 (d, J=1.43 Hz, 1H), 7.61-7.67 (m, 2H), 7.44-7.48
(m, 1H),
7.41 (t, J=2.03 Hz, 1H), 7.29 (dd, J=2.09, 8.29 Hz, 1H), 6.75-7.18 (m, 2H),
5.28 (q, J=7.27 Hz,
1H), 3.19-3.28 (m, 2H), 2.99-3.02 (m, 2H), 2.84-2.93 (m, 2H), 2.14-2.23 (m,
2H), 1.79 (d, J=7.39
Hz, 3H), 1.53 (s, 3H). Human DGAT2 IC50 = 551 nM. Isomer EX-85b (slower
eluting): LC/MS
= 537 [M+l]. 1HNMR (400 MHz, 1VIETHANOL-d4) 67.70 (d, J=1.43 Hz, 1H), 7.61-
7.67 (m, 2H),
7.46 (d, J-7.99 Hz, 1H), 7.41 (t, J-1.97 Hz, 1H), 7.29 (dd, J-2.09, 8.29 Hz,
1H), 6.75-7.18 (m,
2H), 5.28 (q, J=7.39 Hz, 1H), 3.19-3.28 (m, 2H), 2.99-3.02 (m, 2H), 2.84-2.94
(m, 2H), 2.15-2.24
(m, 2H), 1.79 (d, J=7.39 Hz, 3H), 1.53 (s, 3H). Human DGAT2 IC50 = 1752 nM.
EXAMPLE 86: 1-(3 -(di fluorom eth oxy)ph eny1)-7V-(4-m ethyl -1, 1 -di oxi
dotetrahydro-211-
thiopyran-4-y1)-2-oxo-3 -(phenyl sulfony1)-2,3 -di hydro-1H-b enzo[d]imi
dazole-5 -carb oxamide
STEP A 9-0rF STEP B c:\r )..-F STEP G
11100 HO IP isj H
[11101
H EM EM ENI=
rµEM
CY'
STEP B 9--)--F STEP E
H 1110
rh,1 110 Nõ(:)
or 1 H
EX-86
STEP A: methyl 1-(3 -(di fluoromethoxy)pheny1)-2- oxo-3 -((2-(trim ethyl
silyl)ethoxy)methyl)-2,3 -
103
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
di hy dro-1H-b enzo [d]i mi dazol e-5 -carboxyl ate
To a solution of methyl 1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylate (500 mg, 1.496 mmol) in THF (10 ml) was added
NaH (90 mg,
2.244 mmol) at 0 C under N2. The mixture was stirred at 0 C for 0.5 h. Then
2-
(trimethylsilyl)ethoxymethyl chloride (374 mg, 2.244 mmol) was added dropwi se
to the mixture.
The mixture was stirred at 15 C for 15 h under N2. LCMS showed that the
desired product was
formed. The mixture was poured into NH4C1, then extracted with DCM (x 3). The
combined
organic layers were washed with brine, dried over Na2SO4, then filtered and
concentrated under
reduced pressure. The residue was purified by column chromatography on silica
(0-100%
Et0Ac/hexanes) to afford the title compound. LC/MS = 465 [M+1].
STEP B: 1-(3-(difluoromethoxy)pheny1)-2-oxo-342-(trimethylsilypethoxy)methyl)-
2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylic acid
To a solution of methyl 1-(3-(difluoromethoxy)pheny1)-2-oxo-3-((2-
(trimethylsilyl)ethoxy)methyl)-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate
(590 mg, 1.270
mmol) in Me0H (2 ml), THF (2 ml) and H20 (1 ml) was added Li0H.H20 (107 mg,
2.54
mmol). The mixture was stirred at 15 C for 12 h. LCMS showed that the desired
product was
formed. The mixture was concentrated under reduced pressure and was dissolved
in H20. HC1
(1N in water) was added to the mixture until pH = 4. Then the mixture was
extracted with DCM
(x 3). The combined organic layers were washed with brine, dried over Na2SO4,
then filtered and
concentrated under reduced pressure to afford the title compound. LC/MS = 451
[M+1].
STEP C: 1-(3-(difluorom eth oxy)ph eny1)-/V-(4-m ethyl -1,1-di oxi
dotetrahydro-2H-thi opyran-4-
y1)-2-oxo-3 -((2-(trimethyl silyl)ethoxy)methyl)-2,3 -di hydro- 1H-b enzo
[d]imi dazol e-5 -
carboxam i de
To a solution of 1-(3 -(di flu oromethoxy)pheny1)-2- oxo-3 -((2-(trim ethyl si
lyl)ethoxy)methyl)-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylic acid (500 mg, 1.110 mmol) in DMF (10
ml) was
added N-ethyl-N-isopropylpropan-2-amine (430 mg, 3.33 inmol), 2-
(3H41,2,3]triazolo[4,5-
b]pyridin-3-y1)-1,1,3,3-tetramethylisouronium hexafluorophosphate(V) (633 mg,
1.665 mmol)
and 4-amino-4-methyltetrahydro-2H-thiopyran 1,1-dioxide hydrochloride (266 mg,
1.332 mmol).
The reaction was stirred at 15 C for 1 h. LCMS showed that the desired
product was formed. The
mixture was poured into H20, then extracted with DCM (x 3). The combined
organic layers were
washed with brine, dried over Na2SO4, then filtered and concentrated under
reduced pressure. The
residue was purified by column chromatography on silica (0-100% Et0Ac/hexanes)
to afford the
title compound. LC/MS = 596 [M-Fil=
104
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
STEP D: 1-(3-(difluorom ethoxy)pheny1)-N-(4-methy1-1, 1-di oxi dotetrahy dro-
2H-thi opyran-4-
y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imidazole-5-carb oxamide
To a solution of 1-(3-(difluoromethoxy)pheny1)-N-(4-methy1-1,1-
dioxidotetrahydro-2H-
thi opyran-4-y1)-2-oxo-3 -((2-(trimethyl silyl)ethoxy)m ethyl)-2,3 - di hy dro-
1H-b enzo [d]imidazol e-
5-carboxamide (790 mg, 1.326 mmol) in THE (10 ml) was added TBAF (4 ml, 4.00
mmol). The
mixture was stirred at 65 C for 15 h. LCMS showed that the desired product
was formed. The
mixture was poured into H20, then extracted with DCM (x 3). The combined
organic layers were
washed with brine, dried over Na2SO4, then filtered and concentrated under
reduced pressure. The
residue was purified by column chromatography on silica (0-100% Et0Ac/hexanes)
to afford the
title compound. LC/MS = 466 [M+1].
STEP E: 1-(3 -(difluorom ethoxy)pheny1)-N-(4-methy1-1, 1-di oxi dotetrahy dro-
2H-thi opyran-4-y1)-
2-oxo-3 -(phenylsulfony1)-2,3 -dihydro-1H-benzo[d]imidazol e-5-carb oxamide
Sodium hydride (10 mg, 0.250 mmol) was added to the mixture of 1-(3-
(difluoromethoxy)pheny1)-
N-(4-methy1-1, 1-di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihy dro-
1H-
benzo[d]imidazole-5-carboxamide (70 mg, 0.150 mmol) in DMF (2 ml) at 0 C
under N2. The
mixture was stirred at 0 C for 0.5 h. Then benzenesulfonyl chloride (54 mg,
0.306 mmol) was
added to the mixture. The mixture was stirred at 15 C for 12 h under N2. LCMS
showed that the
desired product was formed. The mixture was poured into NH4C1, then extracted
with DCM (x 3).
The combined organic layers were washed with brine, dried over Na2SO4, then
filtered and
concentrated under reduced pressure. The residue was purified by mass
triggered reverse phase
HPLC (ACN/water with 0.1% NH4HCO3 modifier) to afford the title compound.
LC/MS = 606
[M+1]. 114NMR (500 MHz, METHANOL-d4) 6 8.44 (d, .1=1.53 Hz, 1H), 8.17-8.21 (m,
2H), 7.73-
7.79 (m, 2H), 7.58-7.67 (m, 3H), 7.27-7.34 (m, 3H), 6.76-7.09 (m, 2H), 3.23-
3.29 (m, 2H), 3.02-
3.05 (m, 2H), 2.89-2.92 (m, 2H), 2.18-2.25 (m, 2H), 1.56 (s, 3H). Human DGAT2
IC50 = >9990
nM.
EXAMPLE 87: 1-i sopropyl-N-(4-m ethy1-1, 1-di oxo-thi an-4-y1)-2-oxo-3 - [3-
(1, 1,2,2-
tetrafluoroethoxy)phenyl]imidazo[4,5-b]pyri dine-6-carb oxamide
105
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
F F
0
KLF
N.... CI F
No2 iv N H STEP B
N NH
NH, STEP A ...00...trUINO,0110,UINH,
0µ 0µ
* FinF
STEP C STEP D STEP E
.= 1 ; r."00
==0 0 I r,C)
Ho
_ F
UF)1(F
STEP F
1F.41 I .õ.",
6,.
EX-87
STEP A: Methyl 5-nitro-6-((3-(1,1,2,2-
tetrafluoroethoxy)phenyl)amino)nicotinate
At RT, methyl 6-chloro-5-nitronicotinate (0.206 g, 095 mmol) was dissolved in
dioxane (4.75
mL), and 3-(1,1,2,2-tetrafluoroethoxy)aniline (0.209 g, 1.00 mmol) and DIPEA
(0.199 mL, 1.140
mmol) were added. The reaction was heated to 100 C for 1 hour. The reaction
was then cooled to
RT, and the dioxane was removed under reduced pressure to afford the title
compound. LC/MS =
390 [M-F1].
STEP B: Methyl 5-amino-64(3-(1,1,2,2-tetrafluoroethoxy)phenypamino)nicotinate
At RT, methyl 5-nitro-6-((3-(1,1,2,2-tetrafluoroethoxy)phenyl)amino)nicotinate
(370 mg, 0.951
mmol) was dissolved in THF (3.80 mL), methanol (1.901 mL), and saturated
aqueous
ammonium chloride (3.80 mL). Zinc (311 mg, 4.75 mmol) was added, and the
reaction was
stirred at RT for 20 minutes. The reaction was filtered through Celite, and
the THF and methanol
were evaporated under reduced pressure. Et0Ac and water were added, and the
layers were
separated. The aqueous layer was extracted three times with ethyl acetate. The
combined organic
layers were washed with brine, dried with MgSO4 (s), filtered, and
concentrated under reduced
pressure to afford the title compound. LCN1S = 360 [M+l].
STEP C: Methyl 2-oxo-3-(3-(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-1H-
imidazo[4,5-
b]py ri dine-6-carboxyl ate
At RT, methyl 5-amino-6-43-(1,1,2,2-tetrafluoroethoxy)phenyl)amino)nicotinate
(341 mg, 0.95
mmol) was dissolved in THF (9.50 mL) and di(1H-imidazol-1-yl)methanone (462
mg, 2.85
mmol) was added. The reaction was stirred at RT for 16 hrs. The next morning,
water was added,
and the THF was removed under reduced pressure. Ethyl acetate was added, and
the layers were
106
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
separated. The aqueous layer was extracted three times with ethyl acetate, and
the combined
organic layers were washed with brine. The organic fraction was then dried
with MgSO4 (s),
filtered, and concentrated under reduced pressure. The crude residue was
purified by flash silica
gel column chromatography (0 to 100% Et0Ac in hexanes), then the combined
fractions were
taken up in Et0Ac and washed with 1 N HC1 and brine, then dried with MgSO4
(s), filtered, and
concentrated to afford the title compound. LC/MS = 386 [A4+1] .
STEP D: Methyl 1-i sopropy1-2-oxo-3-(3-(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-
dihydro-1H-
imi dazo[4,5-b]pyri dine-6-carboxyl ate
At RT, methyl 2-oxo-3-(3-(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-1H-
imidazo[4,5-
b]pyridine-6-carboxylate (187.5 mg, 0.487 mmol) and cesium carbonate (634 mg,
1.947 mmol)
were suspended in DMF (2.433 m1). 2-iodopropane (0.097 ml, 0.973 mmol) was
added, and the
reaction was heated to 80 C and stirred for one hour. Then, the reaction was
cooled to RT and
water was added, followed by Et0Ac. The layers were separated, and the aqueous
layer was
extracted with Et0Ac three times. The combined organic layers were washed with
brine, dried
with MgSO4 (s), filtered, and concentrated under reduced pressure. The crude
residue was
purified by flash silica gel column chromatography (0 to 10% Et0Ac in DCM) to
afford the title
compound. LC/MS = 428 [M+l].
STEP E: 1-isopropy1-2-oxo-3-(3-(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-
1H-
imidazo[4,5-b]pyridine-6-carboxylic acid
At RT, methyl 1-isopropy1-2-oxo-3-(3-(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-
dihydro-1H-
imidazo[4,5-b]pyridine-6-carboxylate (144 mg, 0.337 mmol) was dissolved in THF
(1.444 mL)
and methanol (0.481 mL). A solution of lithium hydroxide (40.3 mg, 1.685 mmol)
in Water
(1.444 mL) was added. The reaction was stirred overnight at RT. The next
morning, 1 N aqueous
HC1 was added to a pH of 0. Ethyl acetate and water were added, the layers
were separated, and
the aqueous layer was extracted with ethyl acetate three times. The combined
organic layers were
washed with brine, dried with MgSO4 (s), filtered, and concentrated under
reduced pressure to
afford the title compound. LC/MS = 414 [M+1].
STEP F: 1-isopropyl-N-(4-methy1-1,1-dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-
3-(3-
(1,1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-1H-imidazo[4,5-b]pyridine-6-
carboxamide
At RT, 1-i sopropy1-2-oxo-3-(3-(1, 1,2,2-tetrafluoroethoxy)pheny1)-2,3-dihydro-
1H-imidazo[4,5-
b]pyridine-6-carboxylic acid (31.1 mg, 0.075 mmol), 4-amino-4-methyltetrahydro-
2H-thiopyran
1,1-dioxide (13.5 mg, 0.083 mmol), and HATU (31.5 mg, 0.083 mmol) were
dissolved in DMF
(0.75 mL). DIPEA (0.04 mL, 0.226 mmol) was added, and the reaction was stirred
at RT for
107
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
16hrs. The crude residue was purified by mass triggered reverse phase HPLC
(ACN/water with
0.1% formic acid modifier) to afford the title compound. 1H NMR (500 MHz,
Methanol-d4) 6
8.52 (d, J = 1.5 Hz, 1H), 8.04 (d, J = 1.5 Hz, 1H), 7.75 ¨ 7.71 (m, 1H), 7.68
(s, 1H), 7.63 (t, J =
8.2 Hz, 1H), 7.35 (d, J = 7.7 Hz, 1H), 6.36 (t, J = 52.6 Hz, 2H), 4.84¨ 4.75
(m, 1H), 3.30 ¨ 3.25
(m, 2H), 3.08 ¨ 2.99 (m, 2H), 2.94 ¨ 2.86 (m, 2H), 2.27 ¨ 2.17 (m, 2H), 1.63
(d, J = 6.9 Hz, 6H),
1.55 (s, 3H). LC/MS = 559 [M+1]. Human DGAT2 1050= 20 nM.
By using procedures similar to those described in Example 87 with appropriate
reagents, the
following compounds were synthesized. These compounds were characterized by
LC/MS.
Example Structure Name
LCMS DGAT2
[M-hl] IC50
(nM)
88 OF
3[3-(difluoromethoxy)pheny1]-1- 510 62
i sopropyl-N-(4-methy1-1,1-dioxo-
o
lici(X)--N/ thian-4-y1)-2-oxo-imidazo[4,5-
b]pyridine-6-carboxamide
89 343-(difluoromethoxy)pheny1]-1- 495
288
isopropyl-N-[(3S)-3-methyl-1,1-
40)1((XN
dioxo-thiolan-3-y1]-2-oxo-
imidazo[4,5-b]pyridine-6-
carboxamide
90 o\FF
3[3-(difluoromethoxy)pheny1]-1- 495 193
isopropyl-N-[(3R)-3-methyl-1,1-40<iji(GC
dioxo-thiolan-3-y1]-2-oxo-
imidazo[4,5-b]pyridine-6-
carboxamide
91 =0\___F 1-isopropyl-N-(3-methyl-1,1- 499
355
dioxo-thietan-3-y1)-2-oxo-3-[3-
jp9
(trifiuoromethoxy)phenyl]imidazo
[4,5-b]pyridine-6-carboxamide
108
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
92 = %__.F I -
isopropyl-N-(4-methy1-1, 1- 527 236
di oxo-thi an-4-y1)-2-oxo-3
(trifluoromethoxy)phenyl]imidazo
[4,5-b] pyridine-6-carboxamide
93 0 \ F 1 -
isopropyl-N-(3-methy1-1, 1- 531 24
Fir
di oxo-thi etan-3-y1)-2-oxo-3 -3-
yr-C.:;XN (1,1,2,2-
c)=-8 tetrafluoroethoxy)phenyl]imidazo[
4,5-b]pyridine-6-carboxami de
94 F
p-V F , -isopropyl-N-R3S)-3-methy1-1, 1- 545 108
% N dioxo-thiolan-3 -y1]-2-oxo-3
0--\
(1,1,2,2-
tetrafluoroethoxy)phenyl]imidazo[
4,5-b]pyridine-6-carboxami de
95 F
p-V F , -isopropyl-N-R3R)-3-methy1-1, 1- 545 .. 23
N dioxo-thiolan-3 -y1]-2-oxo-3
(1,1,2,2-
tetrafluoroethoxy)phenyl]imidazo[
4,5-b]pyridine-6-carboxami de
96 3-(3-
ethoxypheny1)-1-isopropyl-N- 459 93
(3 -methy1-1,1-di oxo-thi etan-3 -y1)-
irLX. No
2-oxo-imidazo[4,5-b]pyridine-6-
8 carboxamide
97 *O 3-
(3-ethoxypheny1)-1-isopropyl-N- 473 154
[(3S)-3-methy1-1,1-dioxo-thi ol an-
0<, -
3-y1]-2-oxo-imidazo[4,5-
b]pyridine-6-earboxamide
98 *0 3-
(3-ethoxypheny1)-1-isopropyl-N- 473 102
[(3R)-3-methy1-1,1-dioxo-thiolan-
010<1,2
3-y1]-2-oxo-imidazo[4,5-
b]pyridine-6-carboxamide
109
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
99 *O 3-(3-ethoxypheny1)-1-isopropyl-N- 487
52
(4-methyl-i1-dioxo-thian-4-y1)-2-
o=r oxo-imidazo[4,5-b]pyridine-6-
carboxamide
100 =oy 3-(3-isopropoxypheny1)-1- 473 692
rirN isopropyl-N-(3-methyl-1, 1-di oxo-
thietan-3-y1)-2-oxo-imidazo[4,5-
o=-8
h]pyridine-6-carboxamide
101 * 3-(3-isopropoxypheny1)-1- 501
506
i sopropyl-N-(4-methy1-1, 1-di oxo-
thian-4-y1)-2-oxo-imidazo[4,5-
d,
h]pyridine-6-carboxamide
102 F 3-[5-(difluoromethoxy)-2-fluoro- 499
148
pheny1]-1-isopropyl-N-(3 -methyl-
1,1-di oxo-thi etan-3 -y1)-2-oxo-
oer imidazo[4,5-b]pyridine-6-
carboxami de
103
F 3-[5-(difluoromethoxy)-2-fluoro- 528
74
, pheny1]-1-isopropyl-N-(4-methyl-
põ
N'
1,1-di oxo-thi an-4-y1)-2-oxo-
e=
imidazo[4,5-b]pyridine-6-
carboxamide
104 = 3-[3-(difluoromethoxy)pheny1]-1- 481
94
isopropyl-N-(3-methyl-1, 1-di oxo-
thietan-3-y1)-2-oxo-imidazo[4,5-
cr.,V h]pyridine-6-carboxamide
EXAMPLE 105: 3 -(3 -(difluoromethoxy)pheny1)-1-i sopropyl-N-(3 -methyl-1, 1 -
dioxi dothietan-3 -
y1)-2-oxo-2,3-dihydro-1H-imidazo[4,5-c]pyridine-6-carboxamide
110
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
.No2 NH2
NO2 ____________________
c STEP A 0, STEP B STEP C :11
STEP D
I NH NH
I CI CI
CI I
* OrF OrF OrF
* OrF
STEP E STEP F STEP G N
CI N?=o N. rµto
H No0 i,Np
r
EX-105
STEP A: 2-chloro-N-isopropy1-5-nitropyridin-4-amine
To a solution of 2,4-dichloro-5-nitropyridine (300 mg, 1.555 mmol) in THF (6
mL) was added
propan-2-amine (110 mg, 1.865 mmol) and Et3N (0.433 mL, 3.11 mmol). The
reaction mixture
was stirred at 25 C for 3 h, then poured into sat. NH4C1, and extracted with
DCM (x 3). The
combined organic layers were washed with brine, dried over Na2SO4, filtered
and concentrated
in vacuo. The residue was purified by column chromatography on silica (0-100%
Et0Ac/hexanes) to afford the title compound. LC/MS = 216 [M+1].
STEP B: 6-chloro-N-isopropylpyridine-3,4-diamine
To a solution of 2-chloro-N-isopropyl-5-nitropyridin-4-amine (300 mg, 1.391
mmol) in Me0H
(4 mL) was added iron (194 mg, 3.48 mmol) and ammonium chloride (0.186 mL,
3.48 mmol).
The reaction mixture was stirred at 65 C for 13 h, then poured into sat.
NH4C1, and extracted
with DCM (x 3). The combined organic layers were washed with brine, dried over
Na2SO4,
filtered and concentrated in vacuo. The residue was purified by column
chromatography on silica
(0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 186 [M+1].
STEP C: 6-chloro-1-isopropy1-1H-imidazo[4,5-c]pyridin-2(3H)-one
To a solution of 6-chloro-N4-isopropylpyridine-3,4-diamine (200 mg, 1.077
mmol) in THF (5
mL) was added Et3N (0.450 mL, 3.23 mmol) and CDI (873 mg, 5.39 mmol). The
reaction
mixture was stirred at 25 C for 12 h under a Ni balloon, then poured into
sat. N1H4C1 and
extracted with DCM (x 3). The combined organic layers were washed with brine,
dried over
Na2SO4, filtered and concentrated in vacuo. The residue was purified by column
chromatography
on silica (0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 212
[M+1].
STEP D: 6-chloro-3-(3-(difluoromethoxy)pheny1)-1-isopropy1-1H-imidazo[4,5-
cipyridin-2(3H)-
one
To a solution of 6-chloro-1-isopropy1-1H-imidazo[4,5-c]pyridin-2(31/)-one (190
mg, 0.898
mmol) in DMF (3 mL) was added 2-(3-(difluoromethoxy)pheny1)-4,4,5,5-
tetramethy1-1,3,2-
111
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
dioxaborolane (1212 mg, 4.49 mmol), Pyridine (1.452 mL, 17.95 mmol), 4A
molecular sieve
(200 mg) and Cu(OAc)2 (326 mg, 1.795 mmol). The mixture was stirred at 80 C
for 13 h, open
to air with a drying tube, then poured into sat. NH4C1 and extracted with DCM
(x 3). The
combined organic layers were washed with brine, dried over Na2SO4, then
filtered and
concentrated in vacua The residue was purified by column chromatography on
silica (0-100%
Et0Ac/hexanes) to afford the title compound. LC/MS = 354 [M+1].
STEP E: ethyl 3-(3-(difluoromethoxy)pheny1)-1-isopropy1-2-oxo-2,3-dihydro-1H-
imidazo[4,5-
c]pyridine-6-carboxyl ate
To a solution of 6-chloro-3-(3-(difluoromethoxy)pheny1)-1-isopropy1-1H-
imidazo[4,5-c]pyridin-
2(311)-one (300 mg, 0.848 mmol) in Et0H (20 mL) was added potassium acetate
(0.159 mL,
2.54 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II)
(124 mg, 0.170
mmol). The mixture was stirred at 100 C for 16 h under a CO balloon, then
poured into sat.
NH4C1 and extracted with DCM (x 3). The combined organic layers were washed
with brine,
dried over Na2SO4, filtered and concentrated in vacuo. The residue was
purified by column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 392
[M+1].
STEP F: 3-(3-(difluoromethoxy)pheny1)-1-isopropy1-2-oxo-2,3-dihydro-1H-
imidazo[4,5-
c]pyridine-6-carboxylic acid
To a solution of ethyl 3-(3-(difluoromethoxy)pheny1)-1-isopropy1-2-oxo-2,3-
dihydro-1H-
imidazo[4,5-c]pyridine-6-carboxylate (217 mg, 0.554 mmol) in Me0H (4 mL) and
water (0.4
mL) was added lithium hydroxide monohydrate (46.5 mg, 1.109 mmol). The mixture
was stirred
at 25 C for 8 h, then concentrated in vacuo and dissolved in H20. HC1 (1N in
water) was added
to the mixture until pH = 4. The mixture was extracted with DCM (x 3). The
combined organic
layers were washed with brine, dried over Na2SO4, filtered and concentrated in
vacuo to afford
the title compound. LC/MS = 364 [M+1].
STEP G: 3-(3-(difluoromethoxy)pheny1)-1-isopiopyl-N-(3-methyl-1,1-
dioxidothietan-3-y1)-2-
oxo-2,3-dihydro-1H-imidazo[4,5-c]pyridine-6-carboxamide
To a solution of 3-(3-(difluoromethoxy)pheny1)-1-isopropy1-2-oxo-2,3-dihydro-
1H-imidazo[4,5-
c]pyridine-6-carboxylic acid (30 mg, 0.083 mmol) in DWIF (2 mL) was added DIEA
(0.072 ml,
0.413 mmol) and HATU (47.1 mg, 0.124 mmol). The mixture was stirred at 25 C
for 0.5 h.
Then, 3-amino-3-methylthietane 1,1-dioxide (14.51 mg, 0.107 mmol) was added to
the mixture
followed by stirring at 25 C for 1 h. The crude mixture was purified directly
by mass triggered
reverse phase HPLC (ACN/water with 0.1% TFA modifier) to afford the title
compound as the
112
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
TFA salt. LC/MS = 481 [M+1]. 1H NMIR (400 MHz, METHANOL-d4, ppm) 6 8.33 (s,
1H), 8.12
(s, 1H), 7.58-7.71 (m, 1H), 7.42-7.54 (m, 2H), 7.31 (dd, J= 1.6, 8.4 Hz, 1H),
6.71-7.14 (m, 1H),
4.76-4.84 (m, 1H), 4.65 (br d, J = 14.8 Hz, 2H), 4.18-4.29 (m, 2H), 1.84 (s,
3H), 1.63 (d, J = 7.2
Hz, 6H). Human DGAT2 IC50= 1804 nM.
By using procedures similar to those described in Example 72 with appropriate
reagents, the
following compounds were synthesized. These compounds were characterized by
LC/MS.
Examp Structure Name LCMS
Human
le [M+l]
DGAT2
IC50
(nM)
106 = 3-(3-(difluoromethoxy)pheny1)-1-
509 69.8
1 N isopropyl-N-(4-methyl-1,1-
1I N)=0
dioxidotetrahydro-2H-thiopyran-4-
--..-
y1)-2-oxo-2,3-dihydro-1H-
imidazo[4,5-c]pyridine-6-
carboxamide
EXAMPLE 107: 1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(3-methy1-1,1-
dioxidothietan-3-
y1)-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridine-5-carboxamide
N
STEP A 3, CIreXN1. STEP B XXI STEP C
STEP D
C1'...LIAC I
4* F ai F
CI re
STEP E STEP F
1\
EX-107
STEP A: 6-chloro-N-isopropy1-3-nitropyridin-2-amine
Propan-2-amine (2.66 mL, 31.1 mmol) was added to a solution of 2,6-dichloro-3-
nitropyridine (3
g, 15.55 mmol) in absolute Et0H (30 mL). The resulting mixture was stirred at
15 C for 20 min,
then concentrated in vacno. The residue was purified by column chromatography
on silica (0-
100% Et0Ac/hexanes) to afford the title compound. LC/MS = 217 [M+ 1 . 1H NMR
(400 MHz,
113
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
METHANOL-d4, ppm) 6 8.41 (d, J = 8.61 Hz, 1H), 6.68 (d, J = 8.61 Hz, 1H), 4.35
- 4.51 (m,
1H), 1.31 (d, J = 6.26 Hz, 6H).
STEP B: 6-chloro-N2-isopropylpyridine-2,3-diamine
The NH4C1 (2.98 g, 55.6 mmol) of H20 (10 mL) was added to 6-chloro-N-isopropy1-
3-
nitropyridin-2-amine (2 g, 9.27 mmol) in Me0H (20 mL) solution, and then iron
(2.59 g, 46.4
mmol) was added to the above mixed solution. The resulting mixture was stirred
at 75 C for 13
h, then poured into sat. NaHCO3 and was extracted with DCM (x 3). The combined
organic
layers were washed with brine, dried over Na2SO4, then filtered and
concentrated in vacuo. The
residue was purified by column chromatography on silica (0-100% Et0Ac/hexanes)
to afford the
title compound. LC/MS = 186 [M+1]. 11-INMIR (400 MHz, METHANOL-d4, ppm) 6 6.75
(d, J
= 7.83 Hz, 1H), 6.32 (d, J = 7.58 Hz, 1H), 4.11 - 4.27 (m, 1H), 1.20 (d, J =
6.36 Hz, 6H).
STEP C: 5-chloro-3-isopropy1-1H-imidazo[4,5-b]pyridin-2(3H)-one
A mixture of 6-chloro-N2-isopropylpyridine-2,3-diamine (400 mg, 2.155 mmol)
and CDI (1747
mg, 10.77 mmol) in TI-IF (5 mL) was stirred at 20 C for 2 h. The mixture was
poured into sat.
NaHCO3 and extracted with DCM (x 3). The combined organic layers were washed
with brine,
dried over Na2SO4, filtered and concentrated in vacno. The residue was
purified by column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 213
[M+1].
STEP D: 5-chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-1H-imidazo[4,5-
b]pyridin-2(3H)-
one
To a solution of 5-chloro-3-isopropyl-1H-imidazo[4,5-h]pyridin-2(3H)-one (280
mg, 1.323
mmol) and 2-(3-(difluoromethoxy)pheny1)-4,4,5,5-tetramethy1-1,3,2-
dioxaborolane (1787 mg,
6.61 mmol) in DMF (4 mL) was added diacetoxycopper (288 mg, 1 588 mmol) and
pyridine
(2.131 mL, 26.5 mmol) at 80 C. The mixture was stirred for 5 h, then poured
into sat. NaHCO3
and extracted with DCM (x 3). The combined organic layers were washed with
brine, dried over
Na2SO4, then filtered and concentrated in vacua. The residue was purified by
column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 355
[M+1].
STEP E: 1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-1H-
imidazo[4,5-
b]pyridine-5-carboxylic acid
A 50 mL flask was charged with 5-chloro-1-(3-(difluoromethoxy)pheny1)-3-
isopropy1-1H-
imidazo[4,5-b]pyridin-2(3H)-one (100 mg, 0.283 mmol), K2CO3 (78 mg, 0.565
mmol),
diacetoxypalladium (19.04 mg, 0.085 mmol), propane-1,3-
diylbis(dicyclohexylphosphonium)
114
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(69.2 mg, 0.113 mmol), DMSO (2 ml) and H20 (0.2 mL) at 20 'C. The mixture was
degassed
and backfilled with CO (three times), then stirred under CO (pressure: 15 psi)
at 110 C for 5 h.
The mixture was poured into water and extracted with DCM (x 3). The combined
organic layers
were washed with brine, dried over Na2SO4, filtered and concentrated in vacuo
to afford the title
compound. LC/MS = 364 [M+1].
STEP F: 1-(3-(difluoromethoxy)pheny1)-3-i sopropyl-N-(3 -methyl-1, 1-
dioxidothi etan-3 -y1)-2-
oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridine-5-carboxamide
A 8 mL tube was charged with 1-(3-(difluoromethoxy)pheny1)-3-isopropyl-2-oxo-
2,3-dihydro-
1H-imidazo[4,5-b]pyridine-5-carboxylic acid (30 mg, 0 083 mmol), DMA (0.043
mL, 0.248
mmol), 3-amino-3-methylthietane 1,1-dioxide (16.74 mg, 0.124 mmol), HATU (47.1
mg, 0.124
mmol) and DMF (0.5 mL) at 20 C and stirred for 0.5 h. The crude mixture was
purified by mass
triggered reverse phase HPLC (ACN/water with 0.1% TFA modifier) to afford the
title
compound as the TFA salt. LC/MS = 481 [M+l]. 1E1 NMIR (500 MHz, METHANOL-d4,
ppm) 6
8.99 (s, 1H), 7.91 (d, J = 8.24 Hz, 1H), 7.61 - 7.70 (m, 1H), 7.55 (d, J =
8.09 Hz, 1H), 7.47 (dd, J
= 0.99, 8.01 Hz, 1H), 7.43 (s, 1H), 7.30 (dd, J = 1.75, 8.32 Hz, 1H), 6.80 -
7.15 (m, 1H), 5.14 (d,
J = 6.89 Hz, 1H), 4.69 (br d, J = 14.50 Hz, 2H), 4.29 (d, J = 14.80 Hz, 2H),
1.89 (s, 3H), 1.69 (d,
J = 6.87 Hz, 6H). Human DGAT2 IC50 = 1083 nA/1
EXAMPLE 108: 1- [3 -(difluoromethoxy)pheny1]-7-fluoro-34 sopropyl -N-(3 -
methyl-1, 1-dioxo-
thietan-3-y1)-2-oxo-benzimidazole-5-carboxamide
F 0
0 F 0 F
T
0 up
NO2 F
+ I* T. STEP A,.
H STEP B
NH
STEP C
0 ulp
NH2 __OUP' NO2 NH,
F F F
STEP D STEP E
0
(161 1\1)=0 0 *
HO
F
STEP F
101 N>
0=4-j=
EX-108
STEP A: Methyl 4-((3-(difluoromethoxy)phenyl)amino)-3-fluoro-5-nitrobenzoate
At RT, methyl 3,4-difluoro-5-nitrobenzoate (761 mg, 3.50 mmol), 3-
(difluoromethoxy)aniline
115
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(0.432 ml, 3.50 mmol), and DIPEA (1.224 ml, 7.01 mmol) were dissolved in DMSO
(17.5 mL).
The mixture was heated to 100 C and stirred overnight. The reaction was
cooled to RT and
water was added. Et0Ac was added, and the layers were separated. The aqueous
layer was
extracted three times with Et0Ac. The combined organic layers were washed with
brine, dried
with MgSO4 (s), filtered, and concentrated under reduced pressure. The crude
residue was
purified by flash silica gel chromatography (0 to 100% Et0Ac) to afford the
title compound.
LC/MS = 357 [M+l].
STEP B: Methyl 3-amino-44(3-(difluoromethoxy)phenyl)amino)-5-fluorobenzoate
At RT, methyl 4-((3-(difluoromethoxy)phenyl)amino)-3-fluoro-5-nitrobenzoate
(911 mg, 2.56
mmol) was dissolved in THF (5.11 ml), and methanol (2.56 ml) and saturated
aqueous
ammonium chloride (5.11 m1). Zinc (836 mg, 12.8 mmol) were added. The reaction
was stirred
for 30 minutes at RT, then filtered through Celite, and the celite was washed
with Et0Ac. The
mixture was concentrated under reduced pressure. Water and Et0Ac were added,
and the layers
were separated. The aqueous layer was extracted three times with Et0Ac. The
combined organic
layers were washed with brine, dried with MgSO4 (s), filtered, and
concentrated under reduced
pressure. The crude residue was purified by flash silica gel column
chromatography (0 to 100%
Et0Ac in Hexanes) to afford the title compound. LC/MS = 327 [M+1].
STEP C: Methyl 1-(3-(difluoromethoxy)pheny1)-7-fluoro-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylate
At RT, methyl 3-amino-4-((3-(difluoromethoxy)phenyl)amino)-5-fluorobenzoate
(777 mg, 2.381
mmol) was dissolved in THF (23.81 ml). di(1H-imidazol-1-yl)methanone (1545 mg,
9.53 mmol)
was added and the reaction was stirred overnight. The next day, water was
added and the THE
was removed under reduced pressure. Et0Ac was added, the layers were
separated, and the
aqueous layer was extracted three times with Et0Ac. The combined organic
layers were washed
with brine, dried with MgSO4 (s), filtered, and concentrated under reduced
pressure. The crude
residue was purified by flash silica gel column chromatography (0 to 50% EtOac
in DCM) to
afford the title compound. LC/MS = 353 [M+1].
STEP D: Methyl 1-(3-(difluoromethoxy)pheny1)-7-fluoro-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
At RT, methyl 1-(3-(difluoromethoxy)pheny1)-7-fluoro-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylate (176 mg, 0.500 mmol) and cesium carbonate (651
mg, 1.998
mmol) were suspended in DMF (2.5 mL). 2-iodopropane (100 tl, 0.999 mmol) was
added, and
the reaction was heated to 80 C for two days. The reaction was cooled to RT,
then water and
116
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Et0Ac were added. The layers were separated and the aqueous layer was
extracted with Et0Ac
three times. The combined organic layers were washed with brine, dried with
MgSO4 (s),
filtered, and concentrated under reduced pressure. The crude residue was
purified by flash silica
gel column chromatography (0 to15% Et0Ac in DCM) to afford the title compound.
LC/MS =
395 [M+1].
STEP E: 1-(3-(difluoromethoxy)pheny1)-7-fluoro-3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-5-carboxylic acid
At RT, to a stirred solution of methyl 1-(3-(difluoromethoxy)pheny1)-7-fluoro-
3-isopropy1-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (0.198 g, 0.500 mmol)
dissolved in TEIF
(0.714 ml) and methanol (2.143 m1). was added a solution of lithium hydroxide
(0.060 g, 2.500
mmol) in water (2.143 m1). The reaction was stirred at RT for 16 hrs. Aqueous
hydrochloric acid
(1 N, 10 ml, 10.00 mmol) was added until the pH = 1, and Et0Ac was added. The
layers were
separated, and the aqueous layer was extracted three times with Et0Ac. The
combined organic
layers were washed with brine, dried with MgSO4 (s), filtered, and
concentrated under reduced
pressure to afford the title compound. LC/MS = 381 [M+1].
STEP F: 1-(3-(difluoromethoxy)pheny1)-7-fluoro-3-isopropyl-N-(3-methy1-1,1-
dioxidothietan-
3-y1)-2-oxo-2,3-dihydro-1H-benzordlimidazole-5-carboxamide
At RT, DIPEA (0.262 mL, 1.499 mmol) was added to a stirred solution of 1-(3-
(difluoromethoxy)pheny1)-7-fluoro-3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-
carboxylic acid (190 mg, 0.500 mmol), 3-amino-3-methylthietane 1,1-dioxide
hydrochloride (94
mg, 0 550 mmol), and HATU (209 mg, 0.550 mmol in DMF (5.00 mL). DIPEA (0.262
mL,
1.499 mmol) was added, and the reaction was stirred at RT. Then, the DMF was
removed under
reduced pressure. The crude residue was purified twice by flash silica gel
column
chromatography (0 to 100% Et0Ac in hexanes followed by 0 to 30% Acetone in
DCM) to afford
the title compound. 1H NMR (500 MHz, Methanol-d4) 6 7.74 (d, J = 1.3 Hz, 1H),
7.58 (t, J = 8.2
Hz, 1H), 7.49 (dd, J ¨ 12.0, 1.3 Hz, 1H), 7.38 (d, J¨ 7.9 Hz, 1H), 7.34 (d, J
¨ 1.9 Hz, 1H), 7.29
(dd, J = 8.3, 2.1 Hz, 1H), 6.93 (t, J = 73.7 Hz, 1H), 4.79 (hept, J = 6.9 Hz,
1H), 4.65 ¨4.58 (m,
2H), 4.30 ¨ 4.22 (m, 2H), 1.86 (s, 3H), 1.65 (d, J = 7.0 Hz, 6H). LC/MS = 498
[M+1]. Human
DGAT2 IC50 = 12 nM
By using procedures similar to those described in Example 108 with appropriate
amine reagents,
the following compounds were synthesized. These compounds were characterized
by LC/MS.
Example Structure Name
LCMS DGAT2
117
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
[M+1] IC50
(nM)
109
F____F 1[3-(difluoromethoxy)pheny1]-7- 513 9.0
F lik fluoro-3-i sopropyl -N-[(3S)-3-
. H 0 o methyl-1,1-dioxo-thiolan-3-y1]-2-
oz.
<5'0 I N
)"----- oxo-benzimidazole-5-carboxamide
110 FF 143-(difl uorom efhoxy)plienyl ]-7-
513 7 0
F . fluoro-3-isopropyl-N-[(3R)-3-
LH
N so NI,C) methy1-1,1-dioxo-thiolan-3-y1]-2-
0_, i *--- oxo-benzimidazole-5-carboxamide
d'
111 1-[3-(difluoromethoxy)pheny1]-7-
527 68
F . )...._F
F so fluoro-3-isopropyl-N-(4-methyl-
i o
N
/)---- 1, 1-di oxo-thi an-4-y1)-2-oxo-
c3, _
benzimidazole-5-carboxamide
EXAMPLE 112: 1-(3-(Difluoromethoxy)pheny1)-6-fluoro-3-isopropyl-N-(4-methy1-
1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[c]imidazole-5-
carboxamide
H
F
F F NO2 F aiiiii NH2
Ali Nisto
STEP C
diasID N 2 STEP A 46 STEP B
EtO0C WI NH -1.- EtO0C 111111111 NH EtO0C 41111fr. t
EtO0C F /,
/...
F.....F F......F F,HCO
I.
STEP D F STEP E F STEP F F
EtO0C HOOC 0 N(C' _)....
. 1,10
..zr 1
2'....
EX-79
STEP A: Ethyl 2-fluoro-5-(isopropylamino)-4-nitrobenzoate
At 20 C, to a stirred mixture of ethyl 2,5-difluoro-4-nitrobenzoate (1.00 g,
4.33 mmol) in
acetonitrile (8.65 ml) was added propan-2-amine (1.11 ml, 13.0 mmol), and the
mixture was stirred
at 40 nC for 12 h. Then the volatiles were evaporated to afford the title
compound. LC/MS = 271
[M+1].
STEP B: Ethyl 4-amino-2-fluoro-5-(isopropylamino)benzoate
At 0 C, to a stirred mixture of ethyl 2-fluoro-5-(isopropylamino)-4-
nitrobenzoate (1.17 g, 4.33
118
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
mmol) in THE (8.66 m1). and Me0H (4.33 ml) saturated ammonia hydrochloride
aqueous
solution (8.66 ml), was added zinc (1.13 g, 17.3 mmol). The mixture was
stirred at 20 C for 1 h,
then filtered over sand, and the filtrate concentrated. The residue was
partitioned between Et0Ac
and water. The organic layer was separated, washed with brine, dried over
Na2SO4 (s), and the
volatiles evaporated to afford the title compound. LC/MS = 241 [M+1].
STEP C: Ethyl 6-fluoro-3-isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxylate
At 20 C, to a stirred mixture of ethyl 4-amino-2-fluoro-5-
(isopropylamino)benzoate (1040 mg,
4.33 mmol) in TT-IF (8.66 mL) was added 1,1'-carbonyldiimidazole (2.81 g, 17.3
mmol). The
mixture was stirred at 20 C for 16 h, then was filtered, and the volatiles
were removed. The
residue was purified by flash silica gel chromatography (0 to 100% Et0Ac in
hexanes) to afford
the title compound. LC/MS = 267 [M+1].
STEP D: Ethyl 1-(3-(difluoromethoxy)pheny1)-6-fluoro-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
At 20 C, to a stirred mixture of ethyl 6-fluoro-3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-5-carboxylate (100 mg, 0.376 mmol), and (3-
(difluoromethoxy)phenyl)boronic acid (85 mg, 0.451 mmol) was added DCE (1.25
mL) and
copper (II) acetate (82 mg, 0.451 mmol). The mixture was stirred at 50 C for
3 d. Then the
volatiles were evaporated, and the residue was purified by flash silica gel
chromatography (0 to
70% Et0Ac in hexanes) to afford the title compound. LC/MS ¨ 409 [M+1].
STEP E: 1-(3-(Difluoromethoxy)pheny1)-6-fluoro-3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-5-carboxylic acid
At 20 C, to a stirred mixture of ethyl 1-(3-(difluoromethoxy)pheny1)-6-fluoro-
3-isopropy1-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (58 mg, 0.142 mmol) in TT-
IF (1.22 mL)
and Me0H (0.40 mL) was added lithium hydroxide hydrate (23.8 mg, 0.568 mmol)
in water
(1.22 mL). The mixture was stirred at 20 C for 1 h, then, 1N HC1 (1 mL,
aqueous) was added,
and the mixture was partitioned between Et0Ac and water. The organic layer was
separated,
washed with brine, dried over Na2SO4(s), filtered, and the volatiles
evaporated to afford the title
compound. LC/MS = 381 [M+1].
STEP F: 1-(3-(Difluoromethoxy)pheny1)-6-fluoro-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
At 20 C, to a stirred mixture of 1-(3-(difluoromethoxy)pheny1)-6-fluoro-3-
isopropy1-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylic acid (50 mg, 0.13 mmol) and HATU (65
mg, 0.17
mmol) in Miff (0.9 mL) were added 4-amino-4-methyltetrahydro-2H-thiopyran 1,1-
dioxide
119
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
hydrochloride (53 mg, 0.26 mmol), then DIPEA (92 Ill, 0.53 mmol). The mixture
was stirred at
20 C for 1 h, then partitioned between LiC1 (lOwt%, aqueous, 10 mL) and Et0Ac
(10 mL). The
organic layer was separated, washed with brine, dried over Na2SO4 (s), and the
volatiles
evaporated. The residue was purified by flash silica gel chromatography (0 to
100% Et0Ac in
hexanes), then lyophilized, to afford the title compound. 1H N1VFR (500 MHz,
DMSO-d6) 6 8.08
(s, 11-1), 7.64 (t, J= 8.2 Hz, 11-1), 7.50 (d, J= 5.7 Hz, 1H), 7.43 (dd, J=
8.0, 1.1 Hz, 1H), 7.38 (t,
J= 2.1 Hz, 1H), 7.34 (t, J= 73.8 Hz, 1H), 7.29 (dd, J= 8.3, 2.3 Hz, 1H), 7.06
(d, J= 10.0 Hz,
1H), 4.70 (heptõI = 7.1 Hz, 1H), 3.18 ¨3.09 (m, 2H), 3.09¨ 3.01 (m, 2H), 2.72¨
2.65 (m, 2H),
2.03 ¨ 1.94 (m, 2H), 1.51 (d, J= 6.9 Hz, 6H), 1.44 (s, 3H). LC/MS = 526 [M+1].
Human
DGAT2 1050 = 73.8 nM.
By using procedures similar to those described in Example 112 with appropriate
reagents, the
following compounds were synthesized. These compounds were characterized by
LC/MS.
Example Structure Name LC MS DGAT2
[M+11 ICs
(nM)
113 F2HCO (R)-1-(3-(Difluoromethoxy)pheny1)-6- 512
78
F
fluoro-3-isopropyl-N-(3-methy1-1,1 -
FNI di oxi dotetrahydrothi ophen-3-1)-2-oxo-
= 2-- 2,3 -dihydro-1H-benzo [d]imidazole-
5-
ee
carboxamide
114 F2HCO (S)- 1-(3-(Difluoromethoxy)pheny1)-6-
512 80
F fluoro-3-isopropyl-N-(3-methy1-1,1 -
O 1101 dioxidotetrahydrothiophen-3-y1)-2-
=
oxo-2,3 -di hydro-1H-
b enzo [d] imidazole-5 -carboxamide
EXAMPLE H5: 1-(2-(2,2-difluoroethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3-
isopropyl-N-(4-
methyl-1, 1-di oxi dotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihy dro-1H-benzo
[d]imi dazol e-5-
carboxamide
120
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
\I O. j
NO2 NO2 NH,
\ F
F _,.STEP A # NH 1,...TEP B * NH F-
01 -0 F STEP C F
up,
NH
F
AF \-AF
STEP 0 STEP E F NI STEP F
0 0HO 1\(C'
r-
---
EX-115
STEP A: methyl 2-fluoro-5-(isopropylamino)-4-nitrobenzoate
A mixture of methyl 2,5-difluoro-4-nitrobenzoate (20 g, 92 mmol), propan-2-
amine (7.08 g, 120
mmol), and potassium carbonate (12.73 g, 92 mmol) in THF (200 ml) was stirred
at 40 C for 15
h. The mixture was poured into H20, then extracted with Et0Ac (x 3). The
combined organic
layers were washed with brine, dried over Na? SO4, then filtered and
concentrated under reduced
pressure. The residue was purified by column chromatography on silica (0-100%
Et0Ac/hexanes) to afford the title compound. LC/MS = 257 [M+1].
STEP B: methyl 4-amino-2-fluoro-5-(isopropylamino)benzoate
A mixture of methyl 2-fluoro-5-(isopropylamino)-4-nitrobenzoate (13.8 g, 53.9
mmol) and Pd/C
(2.5 g, 2.349 mmol) in THF (20 ml) was stirred at 50 C for 2 h under H2 15
psi. The suspension
was filtered through a pad of Celite and the filter cake was washed with Et0Ae
(30 mL). The
combined filtrates were concentrated. The residue was purified by column
chromatography on
silica (0-100% Et0Ac/hexanes) to afford the title compound LC/MS = 227 [M+1]_
STEP C: methyl 442-(2,2-difluoroethoxy)-5-fluoropyri di n-4-yl)amino)-2-fluoro-
5-
(isopropylamino)benzoate
A mixture of methyl 4-amino-2-fluoro-5-(isopropylamino)benzoate (4.5 g, 19.89
mmol),
difluoroethoxy)-5-fluoro-4-iodopyridine (7.23 g, 23.87 mmol) in THF (50 mL)
was added Cs2CO3
(19.44 g, 59.7 mmol), Brettphos Pd G3 (1.5 g, 1.655 mmol) at 20 'C. Then the
mixture was heated
to 80 C under N2 for 15 h then poured into H20, and extracted with Et0Ac (x
3). The combined
organic layers were washed with brine, dried over Na2SO4, then filtered and
concentrated under
reduced pressure. The residue was purified by column chromatography on silica
(0-100%
Et0Ac/hexanes) to afford the title compound. LC/MS = 402 [M+1].
STEP D: methyl 1-(2-(2,2-di fluoroethoxy)-5-fluoropyri din-4-y1)-6-fluoro-3
sopropy1-2-oxo-2,3-
di hy dro-1H-b enzo [d]i mi dazol e-5 -carboxyl ate
A mixture of methyl 4-02-(2,2-difluoroethoxy)-5-fluoropyridin-4-yl)amino)-2-
fluoro-5-
121
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
(isopropylamino)benzoate (6.42 g, 16.00 mmol) and CDI (12.97 g, 80 mmol), DIEA
(8.38 ml, 48.0
mmol) in THF (70 mL) was stirred at 70 C for 40 h, then poured into H20, and
extracted with
Et0Ac (x 3). The combined organic layers were washed with brine, dried over
Na2SO4, then
filtered and concentrated under reduced pressure. The residue was purified by
column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 428
[M+1].
STEP E: 1-(2-(2,2-difluoroethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3-isopropy1-2-
oxo-2,3-
di hy dro-1H-b enzo [d]i mi dazol e-5- carb oxyl i c acid
A mixture of lithium hydroxide hydrate (1.304 g, 31.1 mmol) and methyl 1-(2-
(2,2-
difluoroethoxy)-5 -ft uoropyridin-4-y1)-6-fluoro-3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-5-carboxylate (6.64 g, 15.54 mmol) in TI-IF (15 mL) and
water (15 mL) was
stirred at 35 C for 2 h. The mixture was concentrated under reduced pressure
and the resulting
concentrate was dissolved in H20 and the pH was adjusted to 4 by addition of
HC1 (1N in water).
Then the mixture was extracted with Et0Ac (x 3). The combined organic layers
were washed with
brine, dried over Na2SO4, then filtered and concentrated under reduced
pressure to afford the title
compound. LC/MS = 414 [M+1].
STEP F: 1-(2-(2,2-difluoroeth oxy)-5-fluoropyri din-4-y1)-6-fluoro-3-isopropyl-
N-(4-methyl- 1, 1-
di oxidotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihydro-1H-b enzo[d]imidazol
e-5-carb oxamid e
A 100 mL eggplant shaped bottle was charged with 1-(2-(2,2-difluoroethoxy)-5-
fluoropyridin-4-
y1)-6-fluoro-3-isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylic
acid (6.62 g,
16.02 mmol), DIEA (8.39 ml, 48.0 mmol), HATU (9.13 g, 24.02 mmol), 4-amino-4-
methyltetrahydro-2H-thiopyran 1,1-dioxide (3.92 g, 24.02 mmol) and DMF (40 ml)
at 20 C, then
the mixture was stirred at 20 C for 0.5 h. The mixture was poured into H20,
then extracted with
Et0Ac (x 3). The combined organic layers were washed with brine, dried over
Na2SO4, then
filtered and concentrated under reduced pressure. The residue was purified by
column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS ¨ 559
[M+1]. 1H NMX (400 MHz, METHANOL-d4) 8.32 (d, J = 1.96 Hz, 1H), 8.05 (br s,
1H), 7.58
(d, J = 5.48 Hz, 1H), 7.17 (d, J = 4.70 Hz, 1H), 6.95 (dd, J = 2.35, 10.17 Hz,
1H), 5.98 - 6.45 (m,
1H), 4.73 (s, 1H), 4.58 (m, 2H), 3.13 - 3.27 (m, 2H), 2.99 (m, 2H), 2.81 (m,
2H), 2.11 -2.25 (m,
2H), 1.59 (m, 6H), 1.54 (s, 3H). Human DGAT2 IC50 = 4.1 nM.
By using procedures similar to those described in Example 115 with appropriate
reagents, the
following compounds were synthesized. These compounds were characterized by
LC/MS.
122
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
Example Structure Name LCMS
DGAT2
[M+1] ICso
(nM)
116 F o 1-(2-ethoxy-5-fluoropyri din-4-
495 93
F y1)-6-fluoro-3-isopropyl-N-(3-
110
methyl-1,1 -di oxi dothi etan-3 -
8
y1)-2-oxo-2,3 -dihydro-1H-
benzo[d] imi dazol e-5-
carb oxami de
117 c N-(4-(difluoromethyl)-1,1- 559
27
F F di oxi dotetrahy dro-2H-
3ri
thiopyran-4-y1)-1-(2-ethoxy-5-
8 fluoropyri din-4-y1)-6-fluoro-3 -
i sopropy1-2-oxo-2,3 -di hy dro-
1H-benzo[d]imidazole-5 -
carb oxami de
118 6-fluoro-3-isopropyl-N-(4- 577
5.5
F-54.FF
methyl-1,1 -di oxi dotetrahy dro-
0.0 ill I
= 2H-thi opy ran-4-y1)-2-oxo-1 -(4-
(1,1,2,2-
tetrafluoroethoxy)pyri din-2-y1)-
2,3 -dihydro-1H-
benzo[d] imi dazol e-5-
carb oxami de
119 1-(4-(2,2- 523
51
di fluoroethoxy)pyri din-2-y1)-3-
i sopropyl-N-(4-methy1-1, 1-
(101
), di oxi dotetrahy dro-2H-
_
thi opyran-4-y1)-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-
5-carboxamide
123
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
120 3-isopropyl-N-(4-methyl-L1- 559
3.2
F--54.F F
9- dioxidotetrahydro-2H-
r-j 110 thiopyran-4-y1)-2-oxo-1-(4-
0.k.).-.- (1,1,2,2-
tetrafluoroethoxy)pyridin-2-y1)-
2,3-dihydro-1 H -
benzo[d]imi dazol e-5-
carboxamide
121 F)
1-(4-(2,2- 541
92
_
difluoroethoxy)pyridin-2-y1)-6-
?-
fluoro-3-isopropyl-N-(4-methyl-
1110
1, 1-di oxi dotetrahy dro-2H-
thiopyran-4-y1)-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-
5-carboxamide
EXAMPLE 122 : 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3-
isopropyl-N-(4-
methyl-1, 1- di oxi dotetrahy dro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihy dro-1H-b
enzo [d] imi dazol e-5-
carboxamide
N
NH2
N F F
F STEP A I F- :s"1-0H STEP B 00
NH STEPC F ravu NH STEP D
0
NH
0
0
)--"FFF F
F STEP E F STEP F F
\ HO \( *,
,f)=0
)_... . õ..
EX-122
STEP A: 5-fluoro-4-iodopyridin-2-ol
2,5-difluoro-4-iodopyridine (25 g, 104 mmol) was added to con. HC1 (12 N in
water) (100 mL),
dioxane (100 mL) and H20 (100 mL). The mixture was heated to 110 C and stirred
at 110 C for
16 h. The reaction was concentrated to dryness to afford the title compound,
which was used into
next step directly. LC/MS = 240 [M+l].
STEP B: 2-(difluoromethoxy)-5-fluoro-4-iodopyridine
124
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Chlorodifluoromethane (43.4 g, 502 mmol) was added to a mixture of 5-fluoro-4-
iodopyridin-2-
ol (24 g, 100 mmol) and Cs2CO3 (110 g, 338 mmol) in DMF (300 mL) at 25 C for
25 min, then
the mixture was stirred at 50 C for 1 h. The mixture was poured into sat.
NH4C1, then extracted
with Et0Ac (x 3). The combined organic layers were washed with brine, dried
over Na2SO4,
then filtered and concentrated under reduced pressure. The residue was
purified by column
chromatography on silica (0-3% Et0Ac/hexanes) to afford the title compound.
LC/MS = 290
[M+1].
STEP C: methyl 4-((2-(difluorom ethoxy)-5-fluoropyri din-4-yl)amino)-2-fluoro-
5-
(isopropylamino)benzoate
To a mixture of methyl 4-amino-2-fluoro-5-(isopropylamino)benzoate (4.5 g,
19.89 mmol), 2-
(difluoromethoxy)-5-fluoro-4-iodopyridine (6.9 g, 23.87 mmol) in THF (50 mL)
was added
Cs2CO3 (19.4 g, 59.7 mmol), BRETTPHOS PD G3 (1.5 g, 1.655 mmol) at 20 C. The
resulting
mixture was heated to 80 C under N2 for 15 h, then poured into H20 and
extracted with Et0Ac
(x 3). The combined organic layers were washed with brine, dried over Na2SO4,
then filtered and
concentrated under reduced pressure. The residue was purified by column
chromatography on
silica (0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 388 [M+1].
STEP D: methyl 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3-
isopropy1-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylate
A mixture of methyl 442-(difluoromethoxy)-5-fluoropyridin-4-yl)amino)-2-fluoro-
5-
(isopropylamino)benzoate (6.35 g, 16.39 mmol) and CDI (13.29 g, 82 mmol), DIEA
(8.6 mL,
49.2 mmol) in THF (80 mL) was stirred at 70 C for 40 h, then poured into H20,
and extracted
with Et0Ac (x 3). The combined organic layers were washed with brine, dried
over Na2SO4,
then filtered and concentrated under reduced pressure. The residue was
purified by column
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 414
[M+li].
STEP E. 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-6-fluoro-3-isopropy1-2-
oxo-2,3-dihydro-
1H-benzo[d]imidazole-5-carboxylic acid
To a solution of methyl 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-6-fluoro-
3-isopropyl- 2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (6.4 g, 15.48 mmol) in THF
(50 mL)/Water
(50 mL) was added lithium hydroxide monohydrate (1.95 g, 46.5 mmol). The
reaction mixture
was stirred at 40 C for 2 hrs, then concentrated under reduced pressure. The
residue was dissolved
in H20, and the pH was adjusted to 4 by the addition of HC1 (1N in water)..
Then the mixture was
extracted with Et0Ac (x 3). The combined organic layers were washed with
brine, dried over
125
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
Na2SO4, then filtered and concentrated under reduced pressure to afford the
title compound.
LC/MS = 400 [M+1].)
STEP F: 1-(2-(difluorom ethoxy)-5-fluoropyri din-4-y1)-6-fluoro-3 sopropyl-N-
(4-methy1-1,1-
di oxi dotetrahydro-2H-thi opyran-4-y1)-2-oxo-2,3 -dihy dro-1H-b enzo[d]imi
dazol e-5-carb oxami de
A tube was charged with 1-(2-(difluoromethoxy)-5-fluoropyridin-4-y1)-6-fluoro-
3-isopropyl- 2-
oxo-2,3-dihydro-11-1-benzo[d]imidazole-5-carboxylic acid (6.15 g, 15.4 mmol),
DIEA (13.5 mL,
77 mmol), HATU (8.78 g, 23.10 mmol), 4-amino-4-methyltetrahydro-2H-thiopyran
1,1-dioxide
(3.02 g, 18 48 mmol) and DMF (80 mL) at 20 C. After stirring at 20 C for 1
h, the mixture was
poured into H20, and extracted with Et0Ac (x 3). The combined organic layers
were washed with
brine, dried over Na2SO4, then filtered and concentrated under reduced
pressure. The residue was
purified by column chromatography on silica (0-100% Et0Ac/hexanes) and mass
triggered reverse
phase HPLC (ACN/water) to afford the title compound. LC/MS = 545 [M+1].IFINMIt
(400 MHz,
DMSO-d6) 6 8.57 (d, J=1.47 Hz, 1H), 8.08 (s, 1H), 7.68-7.88 (m, 1H), 7.50 (dd,
J=2.32, 5.26 Hz,
2H), 7.17 (dd, J=2.08, 9.90 Hz, 1H), 4.67-4.71 (m, 1H), 3.07-3.16 (m, 2H),
2.99-3.07 (m, 2H),
2.64-2.75 (m, 2H), 1.96-2.12 (m, 2H), 1.48 (d, J=6.85 Hz, 6H), 1.41 (s, 3H).
Human DGAT2 1050
= 56 nM.
By using procedures similar to those described in Example 122 with appropriate
amine reagents,
the following compounds was synthesized. These compounds were characterized by
LC/MS.
Example Structure Name LCMS
DGAT2
[M+1] IC50 (nM)
123
1-(2-(difluoromethoxy)-5- 517
73
F fluoropyridin-4-y1)-6-fluoro-3-
c.rF1 110 i sopropyl -N-(3 -methyl-1,1-
cfls di oxi dothi etan-3 -y1)-2-oxo-2,3 -
di hy dro-lI I-benzo [d]imi dazol e-5-
carb oxami de
EXAMPLE 124: (S)-1-(3 -(Di fluorom ethoxy)pheny1)-4-fluoro-3 -isopropyl-N-(3 -
m ethyl -1, 1-
di oxidotetrahydrothi ophen-3-y1)-2-oxo-2,3 -dihydro-1H-b enzo [dlimidazol e-5-
carb oxamide
126
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
diThh NO2 NO2 riali NO2
STEP A
STEP B
HOOC 41111.-F F Me00C F Me00C 4111" NH
NH, STEP E
STEP C STEP D
Me00C (16I NH -a"- Me00C (16
F2 HCO
FF
49
* STEP F STEP G _ H
r\(o_
Me00C 14
=1\1,(3 1
HOOC
EX-124
STEP A: Methyl 2,3-difluoro-4-nitrobenzoate
At 20 C, to a stirred mixture of 2,3-difluoro-4-nitrobenzoic acid (490 mg,
2.41 mmol) in Me0H
(2.41 mL) was added sulfuric acid (51A 111, 0.965 mmol). The mixture was
stirred at 80 C for
24 h., then cooled to 20 C, concentrated, and partitioned between Et0Ac and
water. The organic
layer was separated, washed with water and brine, dried over Na2SO4 (s),
filtered, and the
volatiles were evaporated to afford the title compound. LCNIS = 218 [M+1].
STEP B: Methyl 2-fluoro-3-(isopropylamino)-4-nitrobenzoate
At 20 C, to a stirred mixture of methyl 2,3-difluoro-4-nitrobenzoate (0.521
g, 2.40 mmol) in
acetonitrile (4.80 mL) was added propan-2-amine (0.452 mL, 5.28 mmol), and the
mixture was
stirred at 40 C for 12 h. The volatiles were evaporated to afford the title
compound. LC/MS =
257 [M+1].
STEP C: Methyl 4-amino-2-fluoro-3-(isopropylamino)benzoate
At 0 C, to a stirred mixture of methyl 2-fluoro-3-(isopropylamino)-4-
nitrobenzoate (0.615 g,
2.40 mmol) in THF (4.80 ml) and Me0H (2.40 ml), and saturated ammonia
hydrochloride
aqueous solution (4.80 ml), was added zinc (0.471 g, 7.20 mmol). The mixture
was stirred at
C for 1 h, then filtered over sand, and the filtrate was concentrated. The
residue was
partitioned between Et0Ac and water. The organic layer was separated, washed
with brine, dried
over Na2SO4 (s), and the volatiles evaporated to afford the title compound.
LC/MS = 227 [M+1].
20 STEP D: Methyl 4-fluoro-3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylate
At 20 C, to a stirred mixture of methyl 4-amino-2-fluoro-3-
(isopropylamino)benzoate (543 mg,
2.40 mmol) in THF (4.80 mL) was added 1,1'-carbonyldiimidazole (1.17 g, 7.20
mmol), and the
mixture was stirred at 20 C for 16 h. The volatiles were evaporated. The
residue was purified by
flash silica gel column chromatography (0 to 100% Et0Ac in hexanes) to afford
the title
compound. LC/MS = 253 [M+1].
127
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
STEP E: Methyl 1-(3-(difluoromethoxy)pheny1)-4-fluoro-3-isopropy1-2-oxo-2,3-
dihydro-1H-
enzo[d]imidazole-5-carboxylate
At 20 C, to a stirred mixture of methyl 4-fluoro-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate (100 mg, 0.396 mmol), (3-
(difluoromethoxy)phenyl)boronic
acid (112 mg, 0.595 mmol), was added DCE (1.32 mL) and copper (II) acetate
(108 mg, 0.595
mmol). The mixture was stirred at 50 C for 3 days, then the volatiles were
evaporated, and the
residue was purified by flash silica gel column chromatography (0 to 50% Et0Ac
in hexanes) to
afford the title compound. LC/MS = 395 [M+1].
STEP F: 1-(3-(Difluoromethoxy)pheny1)-4-fluoro-3-isopropyl-2-oxo-2,3-dihydro-
lH-
benzo[d]imidazole-5-carboxylic acid
At 20 C, to a stirred mixture of methyl 1-(3-(difluoromethoxy)pheny1)-4-
fluoro-3-isopropy1-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (74 mg, 0.188 mmol) in THF
(0.80 mL)
and Me0H (0.27 mL) was added lithium hydroxide hydrate (31.5 mg, 0.751 mmol)
in Water
(0.80 mL). The mixture was stirred at 20 C for 1 h, then IN HC1 (1 mL,
aqueous) was added,
and the mixture was partitioned between Et0Ac and water. The organic layer was
separated,
washed with brine, dried over Na2SO4 (s), filtered, and the vol atiles
evaporated to afford the title
compound. LC/MS = 381 [M+1].
STEP G: (5)-1-(3-(Difluoromethoxy)pheny1)-4-fluoro-3-i sopropyl-N-(3 -methyl-
I, 1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
At 20 C, to a stirred mixture of 1-(3-(difluoromethoxy)pheny1)-4-fluoro-3-
isopropy1-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylic acid (20 mg, 0.053 mmol) and HATU
(30.0 mg,
0.079 mmol) in DMF (0.53 mL) were added DIPEA (36.7 ill, 0.210 mmol), then (S)-
3-amino-3-
methyltetrahydrothiophene 1,1-dioxide hydrochloride (14.7 mg, 0.079 mmol). The
mixture was
stirred at 20 C for 1 h, then purified by mass triggered reverse phase HPLC
(C18, 40 to 80%
ACN in water, 0.1% FA modifier) to afford the title compound. 1HNMR (500 MHz,
DMSO-d6)
6 8.54 (s, 1H), 7.65 (1, J¨ 8.1 Hz, 1H), 7.42 (d, J¨ 8.1 Hz, 1H), 7.37 (s,
1H), 7.31 (d, J¨ 8.9 Hz,
1H), 7.31 (t, J= 73.7 Hz, 1H), 7.26 ¨ 7.22 (m, 1H), 6.94 (d, J= 8.2 Hz, 1H),
4.85 (hept, J= 6.9
Hz, 1H), 3.94 (d, J= 13.6 Hz, 1H), 3.32 (dd, J= 8.9, 5.3 Hz, 1H), 3.20 (d, J=
13.7 Hz, 1H), 2.65
(dd, J= 12.8, 6.0 Hz, 1H), 2.20 (dt, J= 13.7, 9.3 Hz, 1H), 1.57 (s, 3H), 1.50
(d, J= 6.8 Hz, 6H).
One proton could not be located due to overlap with solvent or water peaks.
LC/MS = 512
[M+1]. Human DGAT2 IC50 = 191 nM.
By using procedures similar to those described in Example 124 with appropriate
amine reagents,
128
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
the following compounds were synthesized. These compounds were characterized
by LC/MS.
Example Structure Name LCMS DGAT2
[M+l] IC50 (nM)
125 F2Hco (R)- 1-(3- 512
203
41k (Difluoromethoxy)pheny1)-4-
1:10 1,1, o fluoro-3-i sopropyl-N-(3-methyl-
1 , 1-di oxi dotetrahydrothi ophen-3-
d'
y1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-
carboxamide
126 F2Hco 1-(3-(Difluoromethoxy)pheny1)- 526
257
. 4-fluoro-3-isopropyl-N-(4-
1110 0 methy1-1,1-dioxidotetrahydro-
o.rJõ 1 )---
2H-thi opyran-4-y1)-2-oxo-2,3-
dihydro-1H-benzo Mimi dazol e-5-
carb oxami de
EXAMPLE 127: (5)-6-Chloro-1-(3 -(difluoromethoxy)pheny1)-3-i sopropyl-N-(3 -
methyl-1,1-
dioxidotetrahydrothiophen-3-y1)-2-oxo-2,3 -dihy dro-1H-b enzo[d]imidazole-5-
carboxami de
IPCI F CI NH OCH F2
CI F
STEP A STEP B STEP C
_31..._
Me00C * NO2 -31.--
HOOC * NO2 Me00C ISPI NO2
F....F
OCHF2
* . 0CHF2
*
Cl NH CI STEP E CI
Me00C * NH2 STEP D
Me00C 161 N'''() -111.-
Me00C
H
)----
F F2HCO
).....F
49
4.t CI
STEP F CI STEP G H
* IN(C)
_2.... * r\e0 _,.... do
,
)
)---
HOOC = --- 0=
et
EX-127
STEP A: Methyl 2-chloro-4-fluoro-5-nitrobenzoate
129
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
At 20 C, to a stirred mixture of 2-chloro-4-fluoro-5-nitrobenzoic acid (1.00
g, 4.55 mmol) in
Me0H (4.55 ml) was added sulfuric acid (0.097 ml, 1.82 mmol). The mixture was
stirred at 80
C for 24 h, then concentrated, and partitioned between Et0Ac and water. The
organic layer was
separated, washed with water and brine, dried over Na2SO4 (s), and filtered.
The volatiles were
evaporated to afford the title compound. LC/MS = 234 [M+1].
STEP B: Methyl 2-chloro-4-((3-(difluoromethoxy)phenyl)amino)-5-nitrobenzoate
At 20 C, to a stirred mixture of methyl 2-chloro-4-fluoro-5-nitrobenzoate
(1.06 g, 4.55 mmol) in
Acetonitrile (9.10 ml) was added 3-(difluoromethoxy)aniline (0.668 ml, 5.46
mmol) and DIPEA
(0.874 ml, 5.01 mmol). The mixture was stirred at 80 C for 16 h. Then, the
volatiles were
evaporated to afford the title compound. LC/MS = 373 [M+1].
STEP C: Methyl 5-amino-2-chloro-4-((3-(difluoromethoxy)phenyl)amino)benzoate
At 0 C, to a stirred mixture of methyl 2-chloro-4-((3-
(difluoromethoxy)phenyl)amino)-5-
nitrobenzoate (1.696 g, 4.55 mmol) in THF (9.10 ml) and Me0H (4.55 ml), and
saturated
ammonia hydrochloride aqueous solution (9.10 ml), was added zinc (0.892 g,
13.65 mmol). The
mixture was stirred at 20 C for 1 h, then filtered, and was partitioned
between Et0Ac and water.
The organic layer was separated, washed with brine, dried over Na2SO4 (s), and
the volatiles
evaporated to afford the title compound. LC/1\4S = 343 [M+1].
STEP D: Methyl 6-chloro-1-(3-(difluoromethoxy)pheny1)-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylate
At 20 C, to a stirred mixture of methyl 5-amino-2-chloro-4-((3-
(difluoromethoxy)phenyl)amino)benzoate (1.56 g, 4.55 mmol) in THF (15.0 ml)
was added 1,1'-
carbonyldiimidazole (2.21 g, 13.7 mmol). The mixture was stirred at 20 C for
16 h. Then, the
volatiles were evaporated, and the residue was purified by flash silica gel
chromatography (0 to
100% Et0Ac in hexanes) to afford the title compound. LC/MS = 369 [M+1].
STEP E: Methyl 6-chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
At 20 C, to a stirred mixture of methyl 6-chloro-1-(3-
(difluoromethoxy)pheny1)-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylate (93.0 mg, 0.252 mmol) in DMF (2.52
mL) were
added 2-iodopropane (49.0 Ill, 0.504 mmol) and Cs2CO3 (247 mg, 0.757 mmol).
The mixture
was stirred at 80 C for 2 h, then partitioned between Et0Ac and water. The
organic layer was
separated, washed with brine, and the solvents evaporated to afford the title
compound. LC/MS =
411 [M+1].
STEP F: 6-Chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-
1H-
130
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
benzo[d]imidazole-5-carboxylic acid
At 20 C, to a stirred mixture of methyl 6-chloro-1-(3-
(difluoromethoxy)pheny1)-3-isopropy1-2-
oxo-2,3-dihydro-1H-benzo[d]imidazole-5-carboxylate (104 mg, 0.253 mmol) in THE
(1.09 mL)
and Me0H (0.36 mL) was added lithium hydroxide hydrate (42.5 mg, 1.01 mmol) in
Water (1.09
mL). The mixture was stirred at 20 C for 1 h. Then, 1N HC1 (1 mL, aqueous)
was added, and
the mixture was partitioned between Et0Ac and water. The organic layer was
separated, washed
with brine, dried over Na2SO4(s), filtered, and the volatiles evaporated to
afford the title
compound. LC/MS = 397 [M+1].
STEP G: 6-Chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
At 20 C, to a stirred mixture of 6-chloro-1-(3-(difluoromethoxy)pheny1)-3-
isopropy1-2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-5-carboxylic acid (30 mg, 0.076 mmol) and HATU
(43.1 mg,
0.113 mmol) in DMF (0.76 mL) was added DIPEA (52.8 ill, 0.302 mmol), then (4-
amino-4-
methyltetrahydro-2H-thiopyran 1,1-dioxide (18.5 mg, 0.113 mmol). The mixture
was stirred at
20 C for 1 h, then directly purified by mass triggered reverse phase HPLC
(C18, 40 to 70%
ACN in water, 0.1% FA modifier) to afford the title compound. 1H NMR (500 MHz,
DMSO-d6)
6 8.25 (s, 1H), 7.65 (t, J= 8.1 Hz, 1H), 7.49 (s, 1H), 7.41 (d, J= 8.1 Hz,
1H), 7.36 (s, 1H), 7.31
(t, J= 73.7 Hz, 1H), 7.31 (d, J= 8.5 Hz, 1H), 7.10 (s, 1H), 4.69 (hept, J= 6.7
Hz, 1H), 3.24 (t, J
¨ 12.8 Hz, 2H), 3.04 (d, J¨ 13.5 Hz, 2H), 2.68 (d, J¨ 13.7 Hz, 2H), 1.98 (t,
J¨ 13.5 Hz, 2H),
1.51 (d, J= 6.8 Hz, 6H), 1.46(s, 3H). LC/MS = 542 [M 1]. Human DGAT2 IC50 =
112 nM
EXAMPLE 128: 4-chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methy1-
1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
131
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
NH, NO2 Ail NO2 distsb NH,
Br 11" F STEP A
Br F STEP B
Br WI N-1=== STEP C
Br 411111billi
H H
111 =F
STEP D 11 STEP F 0 1,(0 STEP E
Br N..00
I Br
I
STEP G STEP H
II NI/0
L 1101 \C' I
1
EX-128
STEP A: 1-bromo-2-chloro-3-fluoro-4-nitrobenzene
To a solution of 4-bromo-3-chloro-2-fluoroaniline (5 g, 22.28 mmol) in toluene
(70 mL) was
added mCPBA (19.22 g, 89 mmol). The mixture was stirred for 12 h at 50 C,
then poured into
sat. Na2S03 (80 mL) and stirred at 25 C for 10 min. The pH value of the
solution was adjusted
to pH = 10 by adding HCl (1N) and the mixture was extracted with DCM (50 mL).
The
combined organic layers were washed with brine (20 mL), dried over M./ SO4,
filtered and
concentrated in vacuo to afford the title compound. 1H NMR (400 MHz,
CHLOROFORM-d) 6
7.23-7.29 (m, 1H), 7.09-7.20 (m, 1H).
STEP B: 3-bromo-2-chloro-N-isopropy1-6-nitroaniline
Solid K2CO3 (6.52 g, 47.2 mmol) was added to a mixture of 1-bromo-2-chloro-3-
fluoro-4-
nitrobenzene (6 g, 23.58 mmol) and propan-2-amine (1.951 g, 33.0 mmol) in DMI
(40 mL) at
25 C. The mixture was heated at 60 C for 3 h, then poured into sat. NaHCO3
and extracted
with DCM (x 3). The combined organic layers were washed with brine, dried over
Na2SO4,
filtered and concentrated in vacuo. The residue was purified by column
chromatography on silica
(0-100% Et0Ac/hexanes) to afford the title compound LC/MS = 293 and 295 [M+1]
STEP C: 5-bromo-6-chloro-N-isopropylbenzene-1,2-diamine
Iron powder (1.5 g, 26.9 mmol) was added to a mixture of ammonium chloride
(1.676 g, 31.3
mmol) and 3-bromo-2-chloro-N-isopropyl-6-nitroaniline (2.3 g, 7.84 mmol) in
THF (10 mL),
water (10 mL) and Et0H (10 mL). The mixture was stirred at 60 C for 2 h, then
poured into sat.
NaHCO3 and extracted with DCM (x 3). The combined organic layers were washed
with brine,
dried over Na2SO4, filtered and concentrated in vacno. The residue was
purified by column
132
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
chromatography on silica (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 263
and 265 [M+1].
STEP D: 6-bromo-7-chloro-1-isopropy1-1H-benzo[d]imidazol-2(311)-one
A mixture of 5-bromo-6-chloro-N1-isopropylbenzene-1,2-diamine (1.2 g, 4.55
mmol) and CDI
(4.43 g, 27.3 mmol) in TI-W (1 5 mL) was stirred at 60 C for 12 h. TLC showed
that the starting
material was used up and 2 new spots appeared. The mixture was poured into H20
and extracted
with DCM (x 3). The combined organic layers were washed with brine, dried over
Na2SO4,
filtered and concentrated in vacuo. The residue was purified by column
chromatography on silica
(0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 289 and 291
[M+1].
STEP E: 5-bromo-4-chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-1H-
benzo[a]imidazol-
2(3H)-one
To a solution of 6-bromo-7-chloro-1-isopropy1-1H-benzo[d]imidazol-2(311)-one
(300 mg, 1.036
mmol) in DAW (4 mL) was added 2-(3-(difluoromethoxy)pheny1)-4,4,5,5-
tetramethy1-1,3,2-
dioxaborolane (1g, 3.70 mmol), Pyridine (1.676 mL, 20.72 mmol), 4A molecular
sieve (200 mg)
and diacetoxycopper (376 mg, 2.072 mmol). The mixture was stirred at 80 C for
13 h, open to
air with a drying tube. LCMS showed the starting material was consumed
completely. The
mixture was poured into H20 and was extracted with DCM (x 3). The combined
organic layers
were washed with brine, dried over Na2SO4, then filtered and concentrated in
vacuo. The residue
was purified by column chromatography on silica (0-100% Et0Ac/hexanes) to
afford the title
compound (300 mg). LC/MS = 431 and 433 [M+1].
STEP F: ethyl 4-chl oro-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-2-oxo-2,3-di
hydro-1 H-
benzo[d]imidazole-5-carboxylate
Pd(dppf)C12 (50.9 mg, 0.069 mmol) was added to the mixture of 5-bromo-4-chloro-
1-(3-
(difluoromethoxy)pheny1)-3-isopropy1-1H-benzo[d]imidazol-2(311)-one (300 mg,
0.695 mmol)
and potassium acetate (205 mg, 2.085 mmol) in dry Et0H (10 mL) at 25 C under
CO (15 psi).
The mixture was stirred at 80 "V for 15 h. under CO (15 psi), then
concentrated, and the residue
was purified by column chromatography on silica (0-100% Et0Ac/hexanes) to
afford the title
compound. LC/MS = 425 [M+1].
STEP G: lithium 4-chloro-1-(3-(difluoromethoxy)pheny1)-34 sopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
Lithium hydroxide monohydrate (12 mg, 0.286 mmol) was added to a mixture of
ethyl 4-chloro-
1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-
carboxylate (40 mg, 0.094 mmol) in Me0H (3 mL) and water (0.4 mL). The mixture
was stirred
133
CA 03195014 2023- 4- 5
WO 2022/076495 PCT/US2021/053680
at 25 C for 12 h, then lyophilized to afford the title compound. LC/MS = 397
[1\4+11.
STEP H: 4-chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo [d] imidazole-
5-carboxamide
4-amino-4-methyltetrahydro-2H-thiopyran 1,1-dioxide (10 mg, 0.061 mmol) was
added to a
mixture of lithium 4-chl oro-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-2-oxo-
2,3-di hydro-1H-
benzo[d]imidazole-5-carboxylate (30 mg, 0.074 mmol), HATU (56.7 mg, 0.149
mmol) and
DIEA (0.052 mL, 0.298 mmol) in DMF (1 mL). The mixture was stirred at 25 C
for 15 min,
then purified by mass triggered reverse phase HPLC (ACN/water with 0.1% TFA
modifier) to
afford the title compound. LC/MS = 542 [M+1]. Human DGAT2 IC50= 1381 nM
EXAMPLE 129: 4-cyano-1-(3-(difluoromethoxy)pheny1)-3-isopropyl-N-(4-methy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
NO2 NO2 NH2
(110 STEP A STEP B ci (00 N STEP C (00
c, CINL
N /Ls
OrF OrF OrF
STEP D STEP E STEP F
110 tõ 0
0 (1101 110 0
CI i\
N n N N
STEP C
=
0 . N
d'
EX-129
STEP A: 6-chloro-2-(isopropylamino)-3-nitrobenzonitrile
Isopropylamine (1.421 mL, 16.59 mmol) was added to a mixture of 2,6-dichloro-3-
nitrobenzonitrile (3 g, 13.82 mmol) in THF (20 mL). The mixture was stirred at
0 C for 2 h,
then poured into sat. NI-14C1 and extracted with DCM (x 3). The combined
organic layers were
washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The
residue was
purified by column chromatography on silica (0-100% Et0Ac/hexanes) to afford
the title
compound. LC/1\4S = 240 [M+1].
STEP B: 3-amino-6-chloro-2-(isopropylamino)benzonitrile
Iron powder (0.932 g, 16.69 mmol) was added to a mixture of 6-chloro-2-
(isopropylamino)-3-
134
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
nitrobenzonitrile (1 g, 4.17 mmol) and ammonium chloride (0.339 mL, 6.34 mmol)
in THF (5
mL). The mixture was stirred at 60 C for 5 h, then poured into sat. NH4C1 and
extracted with
DCM (x 3). The combined organic layers were washed with brine, dried over
Na2SO4, filtered
and concentrated in vacuo. The residue was purified by flash silica gel column
chromatography
(0-100% Et0Ac/hexanes) to afford the title compound. LC/MS = 210 [M+1].
STEP C: 5-chloro-3-isopropy1-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-4-
carbonitrile
Triethylamine (0.598 ml, 4.29 mmol) was added to a mixture of 3-amino-6-chloro-
2-
(isopropylamino)benzonitrile (300 mg, 1.431 mmol) and CDT (1160 mg, 7.15 mmol)
in TI-IF (3
mL). The mixture was stirred at 25 C for 24 h, then concentrated in vacno.
The crude product
was poured into sat. NH4C1 and extracted with Et0Ac (x 3). The combined
organic layers were
washed with brine, dried over Na2SO4, filtered and concentrated in vacuo.
Then, the crude
product was poured into H20 and stirred at 25 C for 3 min (white solid
appeared). The mixture
was filtered to afford the title compound. LC/MS = 236 [M+1].
STEP D: 5-chloro-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-
1H-
benzo[d]imidazole-4-carbonitrile
Pyridine (0.820 mL, 10.18 mmol) was added to a mixture of 5-chloro-3-isopropy1-
2-oxo-2,3-
dihydro-1H-benzo[d]imidazole-4-carbonitrile (120 mg, 0.509 mmol), (3-
(difluoromethoxy)phenyl)boronic acid (478 mg, 2.55 mmol) and diacetoxycopper
(111 mg, 0.611
mmol) in DMF (15 mL). The mixture was stirred at 50 C for 12 h, then poured
into sat. NH4C1
and extracted with DCM (x 3). The combined organic layers were washed with
brine, dried over
Na2SO4, filtered and concentrated in vacuo. The residue was purified by flash
silica gel column
chromatography (0-100% Et0Ac/hexanes) to afford the title compound. LC/MS =
378 [M+1].
STEP E: ethyl 4-cyano-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
Pd(dppf)C12 (58.1 mg, 0.079 mmol) was added to a mixture of potassium acetate
(0.149 mL,
2.382 mmol) and 5-chloio-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-4-carbonitrile (300 mg, 0.794 mmol) in Et0H (20 mL) and the
solution was
stirred at 100 C for 18 h under CO (15 psi balloon). The mixture was poured
into sat. NH4C1
and was extracted with DCM (x 3). The combined organic layers were washed with
brine, dried
over Na2SO4, then filtered and concentrated in vacuo. The residue was purified
by flash silica gel
column chromatography (0-100% Et0Ac/hexanes) to afford the title compound.
LC/MS = 416
[M+1].
135
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
STEP F: lithium 4-cyano-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-
dihydro-1H-
benzo[d]imidazole-5-carboxylate
Ethyl 4-cyano-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-5-carboxylate (70 mg, 0.169 mmol) was added to a mixture of
lithium
hydroxide hydrate (14.14 mg, 0.337 mmol) in Me01-1 (1 mL) and water (0.1 mL).
The mixture
was stirred at 25 C for 1 h and was freeze-dried to afford the title
compound. LC/MS = 388
[M+1].
STEP G: 4-cyano-1-(3-(difluorom ethoxy)pheny1)-3 sopropyl -N-(4-m ethy1-1,1-
dioxidotetrahydro-2H-thiopyran-4-y1)-2-oxo-2,3-dihydro-1H-benzo[d]imidazole-5-
carboxamide
4-cyano-1-(3-(difluoromethoxy)pheny1)-3-isopropy1-2-oxo-2,3-dihydro-1H-
benzo[d]imidazole-
5-carboxylic acid (17.08 mg, 0.044 mmol) was added to a mixture of 4-amino-4-
methyltetrahydro-2H-thiopyran 1,1-dioxide (6 mg, 0.037 mmol), HATU (28.0 mg,
0.074 mmol)
and DIEA (0.026 mL, 0.147 mmol) in DMF (5 mL) and the mixture was stirred at
25 C for 1 h.
The crude mixture was purified directly by mass triggered reverse phase HPLC
(ACN/water with
0.1% TFA modifier) to afford the title compound. LC/MS = 533 [M+1]. 1H NMIt
(400 MHz,
1VIETHANOL-d4, ppm) 8.41 (s, 1H), 7.55-7.71 (m, 1H), 7.34-7.40 (m, 2H), 7.31
(t, J= 7.58
Hz, 3H), 6.73-7.14 (m, 1H), 5.39 (td, J = 6.82, 13.51 Hz, 1H), 3.33-3.42 (m,
2H), 2.98 (br d, J =
12.96 Hz, 2H), 2.83 (br d, J = 14.67 Hz, 2H), 2.18 (br t, J = 13.45 Hz, 2H),
1.69 (d, J = 6.85 Hz,
6H), 1.54 (s, 3H). Human DGAT2 IC50 ¨ 248 nM
ASSAYS
Insect cell expression and membrane preparation
Sf-9 insect cells were maintained in Grace's insect cell culture medium with
10 % heated-
inactivated fetal bovine serum, 1 % Pluronic F-68 and 0.14 ug/m1Kanamycine
sulfate at 27 C in
a shaker incubator. After infection with untagged baculovirus expressing human
DGAT2
(hDGAT2) at multiplicity of infection (MOI) 3 for 48 hours, cells were
harvested. Cell pellets
were suspended in buffer containing 10 mM Tris-HC1 pH 7.5, 1 mM EDTA, 250 mM
sucrose
and Complete Protease Inhibitor Cocktail (Sigma Aldrich), and sonicated on
ice. Cell debris
were removed by centrifugation at 2000 x g for 15 minutes. Membrane fractions
were isolated by
ultracentrifugation (100,000 x g), resuspended in the same buffer, and frozen
(- 80 C) for later
use. The protein concentration was determined with the PierceTM BCA Protein
Assay Kit
(Thermo Fisher Scientific). Expression of protein levels was analyzed by
immunoblotting with
rabbit anti-DGAT2 antibody (Abcam, ab102831) and donkey anti-rabbit IgG H&L
Alexa Fluor
136
CA 03195014 2023- 4- 5
WO 2022/076495
PCT/US2021/053680
647 (Abcam, ab150075) followed by detection using Typhoon FLA9000 (GE
Healthcare).
LC/MS/MS analysis method
LC/MS/MS analyses were performed using Thermal Fisher's LX4-TSQ Vantage
system. This
system consists of an Agilent binary high-performance liquid chromatography
(HPLC) pump
and a TSQ Vantage triple quadrupole MS/MS instrument. For each sample, 2 jit,
samples from
the top organic layer of in-plate liquid-liquid extraction were injected onto
a Thermo Betabasic
C4 column (2.1 mm x 20 mm, 5 nm particle size). The samples were then eluted
using the
following conditions; mobile phase: Isopropanol: acetonitrile/10mM ammonium
formate =
50/35/15 (v/v/v), flow rate: 0.8 mL/min, temperature: 25 C. Data was acquired
in positive mode
using a heated electrospray ionization (1-1ESI) interface. The operational
parameters for the TSQ
Vantage MS/MS instrument were a spray voltage of 3000 V. capillary temperature
of 280 C,
vaporizer temperature 400 C, sheath gas 45 arbitrary unit, Aux gas 10
arbitrary units, S-lens 165
and collision gas 1.0mTorr. Standard reference material (SRM) chromatograms of
13 C 18-tri ol ei n
(Q1: 920.8>Q3:621.3) and internal standard 13 C21-triolein (Q1:
923.8>Q3:617.3) were collected
for 33 sec. The peak area was integrated by Xcalibur Quan software. The ratio
between the
13Cistriolein generated in the reaction and spiked in internal standard 13C21-
triolein was used to
generate percentage inhibition and 1C5o values. Compound percentage inhibition
was calculated
by the following formula: Inhibition %=1-[(compound response ¨ low
control)/(high control ¨
low control)] x 100%. Potent compounds were titrated and ICso were calculated
by 4 parameter
sigmoidal curve fitting formula.
DGAT2 enzymatic activity assay
DGAT2 activity was determined by measuring the amount of enzymatic
productI3C18-triolein
(HC-1,2,3-Tri(cis-9-octadecenoyl)glycerol) using the membrane prep mentioned
above. The
assay was carried out in ABgene 384-well assay plates in a final volume of 25
IL.LL at rt. The
assay mixture contained the following: assay buffer (100 mM Tris=Cl, pH 7.0,
20 mM MgC12,
5% ethanol), 25 [1..M of diolein, 5 uM of 13C oleoyl-CoA and 8 ng/p..L of
DGAT2 membrane.
137
CA 03195014 2023- 4- 5