Note: Descriptions are shown in the official language in which they were submitted.
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
Title:
System and Method for Minimally Invasive Treatment with Injectable Electrodes
Statement Regarding Related Applications
(001) This application claims priority to, and the full benefit of, US
provisional
application #63/079,275 filed on September 16, 2020; international application
#PCT/US20/061374 filed November 19, 2020; US provisional application
63/119,444 filed
November 30, 2020; US provisional application #63/153,223 filed February 24,
2021; US
provisional application #63/167,836 filed March 30, 2021; US provisional
application
#63/171,780 filed on April 7, 2021, US provisional application #63/184,656
filed on May 5, 2021
and international application PCT/1JS21/33007 filed on May 18, 2021. This
application also
incorporates in their entirety both international application #PCT/US20/061374
filed on November
1
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
19, 2020 (referred to herein as PCT '374) and international application
PCT/US21/33007, filed on
May 18, 2021 (referred to herein as PCT '007), as if both were set forth
herein.
Field of the Invention
(002) The field of the invention is minimally invasive treatment of tissue
with injectable
electrodes comprising wire structures.
Aspects of the Invention
(003) The present invention includes methods of treating a tissue target
with an array of
wire structure electrodes including those which are non-helical (rolled,
folded, extruded, twisted,
braided as in PCT '374) which are very mechanically compliant when injected
against or into
bodily tissue, or using a helical wire structure electrode (PCT '007) that is
less mechanically
compliant but able to form a bunching anchor 8 in a deterministic fashion when
injected against,
around, onto, or into biological tissue. Examples of the non-helical structure
are shown in Figs.
4-C, 8-10, and 29-30, and examples of the helical structure are in Figs. 11-
15, 18-A, 20, 22-28
(004) The wire structure electrodes of PCT '374 and PCT '007 have certain
similarities.
They all are made of fine wire. All are injectable through a dispenser (e.g.,
a needle) in a minimally
invasive procedure without an open cut down or even laparoscopy. They can be
injected in a linear
fashion when the needle is being retracted to form a linear path (or curved
out of a curved needle).
All can, to some extent, bend, flex and fold and integrate well with the
tissue and offer large surface
area to provide ample interface for energy exchange.
(005) Differences between helical and non-helical wire structure electrodes
can be used
by the clinician for different applications in ablation and other energy
transfer therapies. Non-
helical does not bend as deterministically around a corner and creates a
deterministic filling in that
it will fill a cavity but it will not necessarily widen the cavity in a way
that helical will. Non-helical
is more compressible than helical. Wire to air (compaction) inside the needle
(prior to deployment)
is around 60 to 70% for helical whereas the wire to air ratio inside the
needle for non-helical is
about 20 to 30%. Helical is mechanically stronger against deformation from
outside forces and
will tend to bend over instead of compressing during normal body movements
(not so during
removal). Non-helical is therefor a good choice near delicate structures that
a clinician may not
want to compress. Helical may compress a delicate structure if too much
helical is injected into a
2
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
cavity, thereby providing more pressure on its surrounding walls of said
cavity. Non-helical is
more easily conformable than helical. Helical can be easily injected from a
thin cannula and it will
form a more deterministic shape with rolling and folding over to form bunching
anchors 8. Non-
helical is less deterministic in its folding. Helical will not compress when
ejected from a needle
against mechanical pressure (say, when the dispenser is stationary) and form a
bunching anchor,
but non-helical will compress when ejected from a needle. Helical can help to
widen a cavity
during the injection, but non-helical will instead fold in on itself, making
it ideal around delicate
structures. Non-helical can be made to compress more in certain locations
during the placement to
concentrate wire there for either increasing charge injection or easing the
interfacing by having a
more densely packed non-helical in certain locations. Helical can be unzipped
coil-by-coil for easy
removal but non-helical does not unzip and is less easily removed in a chronic
stage once that
tissue has grown into it. Both though are easily removed if still in linear
shape on injection day
(meaning non-helical may compress but can still be pulled out before tissue in-
growth, so can
helical via unzipping even if it has formed an anchor. Helical can self-anchor
during the placement
procedure. This does not necessarily increase the wire density in that
location, but instead will
increase the cavity volume and thus target area for needle interfacing or
volume of cavity that
electrical energy may be deployed from into the tissue for stimulation or
ablation applications.
Brief Description of the Figures
(006) Note: This application incorporates two PCT applications and adopts
the reference
numbers from PCT/US21/33007 in the following figures filed in this
application.
(007) Fig. 1-A is an image of a prior art electroporation probe and Fig. 1-
B is the
companion generator, Nanoknife, by AngioDynamics.
(008) Fig. 2 contains four images of prior art devices. Fig. 2-A is an
image of a 3cm
Single Active-Tip (Covidien Cool-Tip). Fig. 2-B is a StarBurst Expandable
Electrode
(Angiodynamics). Fig. 2-C is a Cluster Tri-Electrode 2.5cm Active Tip
(Covidien Cool-Tip). Fig.
2-D is a LeVeen Expandable Anchor Electrode (Boston Scientific).
(009) Fig. 3-A is an image of cadaver tissue with the end of a prior art RF
ablation probe
inserted to a subcutaneous point in the tissue to be ablated. Fig. 3-B is an
image of the same cadaver
3
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
after ablation and removal of the probe and showing the pattern of ablation is
limited to near the
end of the probe.
(010) Fig.4-A is an image of cadaver tissue with a wire structure electrode
implanted
subcutaneously prior to RF ablation. Fig. 4-B shows the end of a gold wire in
the wire structure
electrode excised and clamped directly to an RF probe. Fig. 4-C is an image of
the ablation pattern
in the cadaver tissue from the wire structure electrode.
(011) Fig. 5 is a photo showing a comparison of ablation patterns from the
prior art device
in Fig. 3-B and from a wire structure electrode in Fig. 4-C for the same
duration and same amount
of RF energy.
(012) Fig. 6 is an ultrasound visualization of a pattern of RF ablation
with the present
invention for 20 watts and 120 seconds.
(013) Fig. 7-A is transdermal imaging of a subcutaneously implanted gold
wire structure
electrode in a J-hook shape, and Fig. 7-B is the same image with cross
sections 8-8, 9-9 and 10-10
labeled.
(014) Fig. 8 is an ultrasound image of the cross section V-V in Fig. 7-B
showing none of
the wire structure electrode, taken with a VEVO 3100 system and 45 MHz probe.
(015) Fig. 9 is an ultrasound image of the cross section W-W in Fig. 7-B
showing only
the long shaft of the J, taken with a VEVO 3100 system and 45 MHz probe.
(016) Fig. 10 is an ultrasound image of the cross section X-X in Fig. 7-B
showing the
long shaft and hook of the J, taken with a VEVO 3100 system and 45 MHz probe.
(017) Fig. 11 is an image (15.6x) of one embodiment of the injectable
electrode with the
helical wire structure after removal of the guidewire.
(018) Fig. 12 is a closer image (100x) of a middle portion of the helical
wire structure of
Fig. 11.
(019) Fig. 13 is a closer image (100x) of a rounded end of the helical wire
structure of
Fig. 11.
4
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(020) Fig. 14 is an image (300x) of a latitudinal cross-section of an
electrode comprising
a helical wire structure comprising a wire rope comprising 100 strands of 25
micron diameter gold
wire.
(021) Fig. 15 is the same electrode as in Fig. 14 before the 0.25mm
guidewire was
removed.
(022) Fig. 16 is an RF ablation experimental set-up.
(023) Fig. 17 is a DC ablation experimental set-up.
(024) Fig. 18 addresses post-experiment image-based temperature estimation
in
application of RF energy. Fig. 18-A is an image of an ablative zone effected
by a 2cm-length
helical wire structure electrode subjected to 40W power over minutes. Fig. 18-
B shows
temperature estimation based on RGB value found 2mm from electrode site
compared to RGB
value of control set across 25-70 degrees Celsius. Fig. 18-C shows temperature
estimation vs.
distance at sites 1-5mm from the center of the ablative zone.
(025) Fig. 19 shows prior electrode placement and heat dispersion ¨ bird's
eye view. Fig.
19-A shows linear electrode placement, radial heat dispersion for a small
(<3cm) tumor, and
complete ablation.
(026) Fig. 20 is an image of rat liver left lobe subject to lcm helical
wire structure
electrode placement and RFA at 20W over 1 minute, and the ablation pattern.
(027) Figs. 21-A, 21-B and 21-C are three schematics showing the benefit of
"around a
corner" procedures for a tumor (in dotted lines) shielded by a blood vessel
comparing prior art
ablation devices and outcomes with the present invention and outcome, compared
to the prior art.
(028) Fig. 22 contains four images of patterns of radiofrequency ablation
for linear helical
wire structures in tissue-mimicking polyacrylamide gel phantoms laced with a
thermochromic ink,
along with a temperature scale.
(029) Fig. 23 includes four images of ablation with a substantially linear
helical wire
structure electrode in cadaver tissue at differing energy and durations.
(030) Fig. 24 contain images of a hooked helical wire structure and
ablation patterns with
RF energy in tissue-mimicking polyacrylamide gel phantoms during different
durations.
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(031) Fig. 25 includes four images of ablation with a J-hook shaped helical
wire structure
electrode in cadaver tissue at differing energy and durations.
(032) Fig. 26-A, Fig. 26-B, Fig. 26-C and Fig. 26-D are images of two
linear helical wire
structures coupled to the negative terminal of a DC power supply for 0, 60,
300 and 600 seconds,
respectively, embedded into a tissue mimicking polyacrylamide gel phantom
laced with a pH
indicator.
(033) Fig. 27 is an image of electrolysis-induced local pH change and
oxygen gas
evolution surrounding multiple helical wire structure anodes embedded in pH
sensitive tissue-
mimicking phantom, the result of applied DC voltage between anodes and saline
bath return.
(034) Fig. 28 is a fluoroscopy image of a curved helical wire structure
embedded near
rodent liver, with a partially insulated stainless steel interfacing needle
approaching.
(035) Fig. 29 and Fig. 30 are images of non-helical wire structure
electrodes implanted
in tissue. (These are identical to Figs. 40 and 41 from PCT '374).
Further Aspects of the Invention:
(036) The wire structure electrodes disclosed in PCT '374 (non-helical) and
PCT '007
(helical) provide tools to solve several problems with the prior methods of
treating a tissue target
such as a tumor or a peripheral nerve.
(037) Prior art methods of ablation rely upon the transcutaneous insertion
of probes to the
tissue target so that energy conducted through the probes contacts the target
directly to generate
heat and destroy the tissue. Those methods are limited by the fact that probes
must be relatively
narrow to be inserted through the skin but their narrowness limits the size
and configuration of the
ablation pattern. These probes are straight (except for some which have
limited also by their
inability to extend "around corners" to hard to reach locations say around a
blood vessel.
(038) One aspect of the improved solution herein is to use implanted wire
structure
electrodes positioned at, on or in the tissue target, so that energy conducted
through probes passes
to the implanted electrodes which then progresses to the tissue target, where
the greater resistance
of the tissue creates heat for ablation. The helical and non-helical
electrodes are flexible, bendable,
stretchable, and the helical wire structure can take almost any shape, and as
such these electrodes
are configured to create larger and more complicated ablation patterns. The
helical especially can
6
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
create treatment patters around corners created by essential structures such
as blood vessels and
peripheral nerves which are not treatment targets. With these electrodes being
chronically
implantable, this solution also allows repeated procedures with thinner probes
(i.e., needles) so
that entry wounds caused by prior art probes are reduced, thereby producing
less collateral damage
and infection risk to healthy tissue of persons such as cancer patients who
are often
immunocompromised by chemotherapies and pharmaceuticals. More accurate and
complete
ablation can make the administration of chemotherapy more effective at lower
concentrations as a
result of increasing the permeability of tumor cell membranes which have been
subject to ablation
but not yet destroyed.
(039) The helical and non-helical wire structure electrodes can be utilized
for fiducial
marking of a tumor, follow up and tracking of the exact site of tumor and
treatment area. Multi-
functional long term retention provides ability to treat, mark and re-treat
without additional
injection or skin puncture. That is, the originally injected wire structure
electrode can remain in
the tissue, providing the clinician the option of additional ablation follow
up procedures,
commonly referred to as re-ablation procedures, without need for re-insertion
of a large diameter
conventional probe, but instead a thin probe may be used that connects to the
large surface area of
the wire structure electrode. In this sense, ablation with the wire structure
electrode offers: 1)
repeatable procedures with thin (needle) energy conductive probe, (2) much
larger surface area
and (3) customizable shape. Because wire structure electrodes can integrate
into the tissue and
dwell for extended periods, they open several long-term treatment
possibilities; re-treatment, in
cases when a tumor returns after initial acute ablation, without additional
injection or skin
puncture; re-treatment using energy coupling; and ongoing treatment with
continuous stimulation;
acute stimulation in conjunction with oral, IV, intra arterial chemotherapy
agents for enhanced
drug effectiveness; and chronic enhancement of drug uptake of oral, IV, intra
arterial
chemotherapy agents. Additionally, certain classes of tumors carry a known
risk of recurrence.
With the current state of the art, each recurrence must be re-treated carrying
similar or greater
procedural and surgical risks if it is even possible to re-intervene. The wire
structure electrodes
address this difficulty. Because the wire structure electrodes can remain
chronically in situ, they
can be used as a fiducial marker for precise re-evaluation of the target site
as well as repeated
treatments to address recurrent tumor growth. This greatly reduces the time,
trauma and cost of
repeated ablative insertions and/or resection procedures.
7
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(040) Tumors in complicated locations may be treated by deploying a helical
wire
structure in a (partial or complete) half moon shape, or any other complex
shape which a skillful
clinician can devise. Examples of such tumors are carcinoma on the outside of -
or surrounding of
- an organ, a blood vessel, or in a volume that itself is more of a shape of a
half moon than a round
sphere.
(041) Tissue targets include without limitation tumors of the liver,
kidney, lung, and bone,
as well as aberrant peripheral nerves. The present method employs electrodes
comprising thin,
highly conductive wires and may be loaded and deployed into or surrounding a
target tissue region
through a straight or nonlinear dispenser of sufficient length and diameter.
The device enables
tissue ablation of multiple shapes, adaptable to specific anatomy of a tumor
and its surrounding
vasculature or other critical structures (i.e. gallbladder, porta hepatis,
bile ducts). The wire
structure is capable of tissue ablation and repeated ablation through a number
of energy modalities,
including radiofrequency ablation (RF), direct current ablation (DC),
microwave ablation (MW),
laser light or high intensity focused ultrasound (HIFU).
(042) RFA is a minimally invasive procedure used to thermally destroy
tumors. Needle-
like ablation electrodes are inserted into or surrounding a tumor, with
electrical current (-500 kHz)
conducted between the electrode and large-surface dispersive electrodes placed
on patient skin, or
between electrodes in multipolar configurations. Electrical power is converted
into heat by induced
ionic vibrations, referred to as the joule effect. These vibrations cause cell
death over an affected
volume when subjected to temperatures above 60C for several minutes. Induced
high temperature
leads to intracellular protein denaturation, the disruption of membrane lipid
bilayers, and the
coagulative necrosis of tumor cells. RFA at lower power may induce mild
hyperthermia, whereby
tissue is heated above the body temperature to induce physiological effects
while not directly
producing substantial cell death. Temperatures of 40 to 45 degrees Celsius may
be maintained for
times up to 1 hour, in contrast to ablative hyperthermia, which achieves
temperatures greater than
55C for shorter durations of 15 to 20 minutes. Hyperthermia treatments may
result in physiological
(i.e. perfusion) or cellular (i.e. gene expression) changes which improve
therapeutic efficacy
through localized sensitization in conjunction with a chemotherapeutic. Power
may be cycled on
and off over an hour-long period as a method of avoiding the transition from
mild hyperthermia to
ablative hyperthermia.
8
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(043) RF current, typically a 500kHz alternating current, may be applied
through pushing
one or more partially-uninsulated thin (20-30 gauge) needles into the bulk of
the wire structure
electrode under image guidance. Strength of the metal-to-metal connection may
be verified by an
associated electrical control system, where there exists a lower impedance for
the helical wire
structure / ground connection compared to the partially-uninsulated needle /
ground connection.
Said electrical control systems prevent ablation until impedance between
helical wire structure and
ground, or impedance between helical wire structure and adjacent electrode, is
below 1000 ohms
or above 25 ohms. Needle-based current transfer may be a repeated procedure,
facilitated by the
secure anchoring of the wire-structure by tissue ingrowth.
(044) A generated coagulation zone in RFA is strongly limited by heat sinks
¨ fluid (e.g.
blood) flow through vessels near a tumor causes local pockets of convective
heating such that RFA
is unable to achieve consistent necrosis near the tumor. For example, in the
case of hepatocellular
carcinoma, traditionally risky locations for treatment are tumors attached to
vasculature
(perivascular) which are adjacent to extrahepatic vital organs or larger
intrahepatic vessels, which
can considerably alter the size of the ablation zone due to the heat-sink
effect, resulting in
aggressive recurrences after ablation. Intravascular tumor spread along the
peritumoral portal vein
contributes greatly to HCC recurrence and spread. The helical wire structure
is capable of
surrounding vasculature closely and in a user-customized manner. Delivery of
the helical wire
structure may be in parallel alignment with the vasculature or hooked around
the vasculature in a
C or J-shape. Deploying the helical wire structure circumferentially
(helically) around the vessel
allows regions of focal RFA heat deposition around a perivascular tumor,
essentially allowing for
ablation around comers.
(045) Coagulation zones in RFA are also strongly limited by roll-off, the
cessation of RF
power due to sudden increase in electrical impedance with the active electrode
surrounded by
desiccated tissue, which has an insulation effect. Ablation with saline
infusion through cannula
may limit coagulation on the electrode surface. Pulsing with RF at regular
intervals, and ramping
power from low wattages to mid-level power settings may help avoid charring as
a result of rapid
power delivery at the wire structure surface
(046) RFA's clinical efficacy is mediated by creation of a sufficient
ablative margin
surrounding tumors (-20%). Tumors may have irregular volumes that limit the
use of RFA
9
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
applicators, which may only produce spherical or minimally oblong ablation
zones. Controlled
delivery of the flexible, high geometric-surface-area (GSA) helical wire
structure enables the
creation of complex ablation geometries, more efficiently overlapping
unconventional / non-
spherical tumors. Controlled delivery of multiple helical wire structures
applied around, without
directly puncturing the tumor, further increases ablative volumes while
avoiding unintentional
scattering of tumor cells (tumor seeding.
(047) The primary goal is to impair the target tissue: in the case of
malignant tumors,
permanent destruction, but in the case of peripheral nerves, only temporary
impairment of neural
conduction for applications such as pain relief (sensory block, afferent nerve
fiber block),
reduction of spasticity (motor fiber block, efferent nerve fiber block), or
modulating the autonomic
function of an organ, organ system or an entire individual (autonomic nerve
block affecting either
autonomic afferents or efferents). In the case of cancer, the goal is
elimination of all viable
malignant (cancerous) cells in a designated tumor volume and to provide
immediate pain relief by
affecting the afferent innervation into cancerous tissues. As such, ablative
therapies are intended
to include a 0.3cm to lcm ablative margin of non-malignant tissue, in order to
minimize the chance
of local tumor progression or recurrence. Known risk factors of tumor
recurrence post-ablation
include an insufficient ablative margin, the presence of vasculature (e.g.
periportal hepatocellular
carcinomas (HCC) ), with the odds of tumor recurrence highly correlated with
larger, irregular
tumors. Larger tumors, typically defined as tumors over 3cm in diameter,
oftentimes require
multiple overlapping probes, applied in succession or simultaneously, to
successfully achieve a
sufficient ablative margin.
(048) It is also desirable for ablative therapies to be highly precise in
their effect to
preserve as much normal tissue as possible. In hepatocellular carcinoma,
functional hepatic reserve
is a primary predictor for long-term patient survival. Well-planned ablative
therapies serve to
minimize damage to surrounding cirrhotic parenchyma. Preservation of nephrons
in the context of
renal cell carcinomas, or epithelial cells of the lung in the context of
adenocarcinomas, are equally
important for positive patient outcomes. The ability to preserve critical
structures surrounding a
tumor remains a challenge for ablation modalities.
(049) RF, MW, laser light, or ultrasound acoustic waves are the most common
sources of
clinical hyperthermic ablation, generating temperatures in excess of 60
degrees Celsius. Aside
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
from high-intensity focused ultrasound (H1FU), these energies are applied from
an generated
connected to needle-like applicators inserted into or surrounding the tumor.
Typical lesions
generated may be modeled as three-dimensional spheroids, with the major axis
of the lesion
aligned parallel to the applicator shaft, and two minor axes of the lesion
lying perpendicular to the
shaft. Though the lesion's major axis may be controlled by selecting different
lengths of
uninsulated applicator tips, necrosing along the minor axis is more
cumbersome. Attempts to
overcome this limitation have involved increasing the probe gauge, using
several applicators in
combination or multiple probes per applicator, and the use of expandable
applicators.
(050) As a micron-scale conductor, the wire within the helical wire
structure electrode
will locally produce an electric field and effective zone of heat conduction
and transmission into
the surrounding target tissue with target tissue fluids, and also acts as a
bulk conductor, which also
produces an electric field and effective zone of heat conduction. The bulk
conduction
(electrical/thermal) and radiation of the helical wire structure electrode
therefore is the
combination of both macro and micro-scale properties.
(051) Direct current does not ablate tissue like RF which is generally
associated with heat
generated that kills tissue. Direct current kills not with heat but by
changing the pH in the vicinity
of the electrode to get cells to leak their contents as the change in acidity
leading to the change in
pH messes with the cell walls and the metabolism of the cells whose cell walls
it does not damage
right away
(052) Electrolytic ablation / lesioning is a non-thermal technique in which
a local pH
change is created following application of direct current. This method has
been applied towards
the treatment of lung, liver, and pancreatic tumors. It has also been applied
in the field of controlled
nerve ablation, with nerves lesioned by DC experiencing a rapidly reduced
conduction (nerve
block). Applied low-voltage DC (<50V) between two or more electrodes results
in electrolysis,
generating hydrogen (hydronium, H30+) ions at the anode and hydroxide ions at
the cathode.
Anode: 2H20 <-> 02 + 4H+ + 4e
Cathode: 2 H20 + 2e- <-> H2 + 20H-
(053) Electrolysis also induces the movement of sodium cations towards the
cathode and
chloride anions towards the anode. This results in the production of sodium
hydroxide and
11.
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
hydrogen near the cathode, and hydrochloric acid, oxygen, and chlorine near
the anode. The
regions surrounding the anode become acidic (pH < 6), while the region
surrounding the cathode
becomes alkaline (pH > 9), resulting in non-thermal cell death (pH < 4.8, pH >
10.6). Additional
contributors to cell death in vivo include the generation of reactive oxygen
species, though their
effect is secondary to that of pH-driven cell death.
(054) Electrolytic ablations / lesionings offer a great deal of increased
precision, shaping
well defined ablation margins due to the introduction of toxic levels of acid
and base. Selective
alteration of the local microenvironment makes it well suited as a modality
for the treatment of
complex tissue shapes. Helical wire structures are able to be placed precisely
in user-tailored
conformations, making it well suited to treat complex tumor shapes. Ease of
multiple placements,
such as in the potential case of a multiple helical wire structures placed as
cathode returns,
surrounding a single anode of tailored shape, maximizes the potential of
electrolytic lesioning as
a potential treatment.
(055) The use of the helical wire structures as an embedded, indwelling
implant increases
the clinical relevance of electrolytic treatment by permitting the re-
lesioning of complex margins
without the need for multiple repeated probe insertions.
(056) Measuring in-situ tissue electrical resistance and buffering capacity
will further
enhance precise lesioning. Physiologic buffering in-vivo will limit the spread
of acidic and basic
species following treatment completion. Electrolytic ablation may be further
mediated by the flow
of blood through a tissue, delivering additional buffering species and
removing generated acid /
base ions, further emphasizing the importance of a flexible wire structure
capable of navigating
around vasculature.
(057) Electroporation, or electro-permeabilization, is the application of
short pulses of
strong electric fields to cells and tissues. External electric fields increase
transmembrane potential,
inducing the formation of nanopores, called poration. Applied voltages of up
to lkV across
electrodes introduces reversible electroporation, the formation of temporary
pores in the cell
membrane. Reversible electroporation has many documented applications in gene
and drug
delivery, where the permeabilization of the cell membrane allows the entry of
molecules that
would not otherwise penetrate it. Irreversible electroporation (IRE), applying
voltages of up to
3kV, results in permanent disruption of the lipid bilayer and loss of cell
homeostasis. The use of
12
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
small electrodes and short, repetitive electric field pulses results in a
nonthermal apoptotic, as
opposed to necrotic cell death, with a well-demarcated region of ablation and
sharp boundaries
between treated and untreated zones. IRE spares critical structures such as
bile ducts, nerves, blood
vessels. Pore formation does not occur significantly in tissue with higher
collagenous content or
elastic fiber contents. It affects only the membrane of living cells, and does
not cause the
denaturation or coagulation of proteins typical of thermal ablation. IRE is
insensitive to the heat-
sink effect. IRE generators may deliver up to 3kV of energy in up to 100
pulses (an electric field
gradient in a 40cm3 volume of at least 800V/cm is considered the threshold for
irreversible
electroporation), with two or more monopolar probes or a single bipolar probe
used at a time to
create ellipsoid ablation zones. Multi-bipolar configurations increase the
size of predictable
margins. Current IRE procedures are rapid - however, they require general
anesthesia and
paralytics, and require synchronization of voltage pulsing with the refractory
period of the cardiac
cycle to avoid arrhythmias.
(058) Creation of membrane nanopores allow permeability of agents such as
chemotherapy drugs or macromolecules which would not otherwise cross the cell
membrane, thus
allowing for an effect upon the cell where there would otherwise be none. This
allows for
augmented drug/genetic delivery systems. Additionally, if enough power is
transferred in a
controlled field, irreversible membrane poration occurs (hence irreversible
electroporation) with
subsequent cell death. This is analogous to ablation, but without the thermal
effects which may
damage the tissue structure and scaffolding (significant vessels, ducts,
critical structures).
Lowering chemo load for therapeutic benefit (Irreversible/Reversible
electroporation mediated
increased cellular permeability) DC or rapid AC concept); Low level/chronic
stimulation (external
stimulator); DC or rapid short burst AC stimulation to induce damage to cancer
cell membranes,
allowing chemotherapy drugs to be better absorbed at potentially lower
concentrations; Chemical
cancer therapies require cancerous cells to uptake enough drugs to ensure
their destruction. Some
chemotherapeutic drugs that would otherwise be effective as a treatment may
not have activity due
to reduced tumor cellular uptake. Thus, drug doses that are required for
tumoricidal effect may
cause global damage to the surrounding healthy tissue and thus leads to
unacceptable toxicity in
the subject. Using specific energy delivery methods such as passing DC energy
through helical
wire structures causes damage to the cellular membrane of affected local
cells, effectively making
the cells more permeable. This technique, with well-placed electrodes, make
tumor cells more
13
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
susceptible to lower concentrations of cancer treatment drugs, improving
effectiveness, reducing
negative side effects and reducing cost.
(059) Reversible Electroporation (RE), Irreversible Electroporati on (IRE)
and
Electrolytic lesioning (or electrolytic ablation, EA) are emerging non-thermal
focal therapies. Both
electroporation and electrolytic lesioning operate on the principle of an
applied DC voltage.
Electrolytic treatments are an area of active research in the fields of both
tumor ablation and nerve
blocks, with studies using bipolar-configured linear electrodes to cause
chemical species evolution
near the electrode surface, causing a pH-mediated localized necrosis. Prior to
causing a larger
volume localized necrosis, pH-mediated large volume changes (i.e. 2 to lOmm
radially away from
the wire structure electrode) will first cause a neural blocking effect on
afferent nerves transmitting
and/or processing pain and other sensations from or through the pH-mediated
localized volume as
well as on efferent nerves transmitting and/or processing action / motor
information to, from or
through the pH-mediated localized target volume. This temporary reduction of
neural activity, akin
to a temporary block of neural activity, may be used as a diagnostic tool as
well as a tool to
determine the optimal charge delivered as direct current injected over
treatment time to ensure
sufficient but not over treating the target and adjacent untargeted tissues.
If so desired, the direct
current injection may be partially or fully reversed in either charge amount
injected or in time
current has been applied prior to allow for a partial temporary and a partial
permanent nerve or
target tissue effect as the outcome of one treatment event. Irreversible
electroporation typically
requires a combination of probes, with energy delivered between two probes at
a time. Recorded
voltages of up to lkV are determined reversible electroporation, inducing
temporary nanopores in
the cell membrane to more easily introduce genes or drugs. Recorded voltages
of lkV through
3kV form permanent pores which induce local apoptotic cell death. Current
electroporation
applicators are 19 gauge needles with 1-4cm exposed active tips, placed
parallel to one another 1-
2 centimeters apart. Fig. 1-A is an image of a prior art electroporation probe
and Fig. 1-B is the
companion generator, Nanoknife, by AngioDynamics.
(060) Fig. 2 contains four images of prior art devices. Fig. 2-A is an
image of a 3cm
Single Active-Tip (Covidien Cool-Tip). Fig. 2-B is a StarBurst Expandable
Electrode
(Angiodynamics). Fig. 2-C is a Cluster Tr-Electrode 2.5cm Active Tip (Covidien
Cool-Tip). Fig.
2-D is a LeVeen Expandable Anchor Electrode (Boston Scientific).
14
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(061) Fig. 3-A is an image of cadaver tissue with the end of a prior art RF
ablation probe
inserted to a subcutaneous point in the tissue to be ablated. Fig. 3-B is an
image of the same cadaver
after ablation and removal of the probe and showing the pattern of ablation is
limited to near the
end of the probe.
(062) Fig. 4-A is an image of cadaver tissue with a wire structure
electrode implanted
subcutaneously prior to RF ablation. Fig. 4-B shows the end of a gold wire in
the wire structure
electrode excised and clamped directly to an RF probe. Fig. 4-C is an image of
the ablation pattern
in the cadaver tissue from the wire structure electrode.
(063) Fig. 5 is a photo showing a comparison of ablation patterns from the
prior art device
in Fig. 3-B and from a wire structure electrode in Fig. 4-C for the same
duration and same amount
of RF energy. The prior art device pattern is shown by axes A (16.7mm) and B
(10.6mm) with an
approximate total area of 139mm2, and the present invention's pattern is shown
by axes C
(33.6mm) and B (23.0mm) with an approximate total are of 607mm2.
(064) Fig. 6 is an ultrasound visualization of a pattern of RF ablation
with the present
invention for 20 watts and 120 seconds.
(065) Fig. 7-A is transdermal imaging of a subcutaneously implanted gold
wire structure
electrode in a J-hook shape, and Fig. 7-B is the same image with cross
sections 8-8, 9-9 and 10-10
labeled.
(066) Fig. 8 is an ultrasound image of the cross section 8-8 in Fig. 7-B
showing none of
the wire structure electrode, taken with a VEVO 3100 system and 45 MI-Iz
probe.
(067) Fig. 9 is an ultrasound image of the cross section 9-9 in Fig. 7-B
showing only the
long shaft of the J, taken with a VEVO 3100 system and 45 MHz probe.
(068) Fig. X is an ultrasound image of the cross section X-X in Fig. 7-B
showing the
long shaft and hook of the J, taken with a VEVO 3100 system and 45 MI-Iz
probe.
(069) The present invention uses, in one embodiment, a flexible multi-
stranded helical
wire structure of materials which display desirable thermal conductivity,
electrical conductivity,
and heat capacity, making the device suitable for efficient electrical
coupling, heat transfer, or
electric field distribution. The manufacturing of a helical wire structure
electrode outside the body
may involve steps of rolling and / or folding. Individual strand diameters in
the wire structure
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
preferentially range from 25 ¨ 75 microns. Incorporating strands of greater
thicknesses is a method
of mechanical optimization for increasing rigidity of the helical coil, or
creating permanent
curvatures at certain points along the length of the wire structure upon
deployment.
(070) Fig. 11 is an image (15.6x) of one embodiment of the injectable
electrode with the
helical wire structure after removal of the guidewire. The wire rope is 100
strands of 25 diameter
micron gold wire and the helical wire structure has an approximate outer
diameter of 0.75mm.
Overall length is approximately 2cm and is made from 6 meters of continuous
gold wire. Fig. 12
is a closer image (100x) of a middle portion of the helical wire structure of
Fig. 11. Fig. 13 is a
closer image (100x) of a rounded end of the helical wire structure of Fig. Y.
Fig. 14 is an image
(300x) of a latitudinal cross-section of an electrode comprising a helical
wire structure comprising
a wire rope comprising 100 strands of 25 micron diameter gold wire. Fig. 15 is
the same electrode
as in Fig. 14 before the 0.25mm guidewire was removed.
(071) The helical wire structure, loaded into a needle (linear or curved
passive introducer)
or flexible catheter (steerable, active introducer), is capable of deployment
via the use of a
bendable plunger, sufficiently dense hydrogel, or similar methods of pushing
the coil within the
needle such that the coil exists at a constant, predictable rate. Insertion of
the needle or flexible
catheter may create a void in soft tissue by displacement. Bodily fluid (e.g.
blood) ingress into the
void is a natural consequence of the insertion of an electrode, and may be
accompanied by the
introduction of saline or a hydrogel, or gaseous microbubbles in the context
of contrast-enhanced
ultrasound. The helical wire structure inside the needle or flexible catheter
may be combined with
liquid, gel, gas, or a mixture of the three, to pass around or through
crevices between the helix and
the wall of the introducer, or through the centerline of the helix itself.
Hypertonic saline injection
in the context of ablation increases ionicity and conduction within the tumor,
thereby increasing
ablative volumes while preventing tissue desiccation that would otherwise
limit ablation volume.
(072) One or more helical wire electrodes may be injected centrally within
the tumor site,
or at multiple oblique sites tangential / adjacent to the targeted tumor to
create different polar
arrangements for selective heating or application of an electric field across
wire electrodes. Once
placed, the helical wire structure is intended to reside within the tissue.
High contrast / radiopacity
exhibited by the helical wire structure enables accurate localization of the
tumor and previous
treatment area under computed tomography or fluoroscopy. Re-interfacing and
retreatment
16
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
through the helical wire structure can be accomplished through electrical
coupling with an external
energy source, and subsequent hyperthermal or non-thermal modes of ablation.
(073) Both hyperthermic and non-thermal ablation modes require high spatial
targeting
accuracy. Predictability is crucial in balancing the critical need to apply
irreversible damage to a
whole tumor with reduction of harm to surrounding critical structures. A
variety of models exist
which seek to mimic properties of biological tissue as simple, highly
reproducible methods of
evaluating device performance and creating therapy protocols, thereby
eliminating the need for
animal and human subjects. Ex vivo tissues are the most common method of
assessing a treatment
volume. However, the heterogeneous nature of ex-vivo tissue makes reproducible
characterization
difficult. Assessments of ablation therapies using ex-vivo tissues require
cutting, staining, and
subjective observation of tissue. Other efforts have focused on the creation
of tissue-mimicking
phantoms for quantitative and predictive measures of device performance. In
evaluating
hyperthermic ablation modes, proposed formulations have used agarose and
polyacrylamide gels
incorporated with heat-sensitive materials such as bovine serum albumin and
thermochromic
compounds (liquid crystals, leuco dyes, or permanent color change inks). Gels
such as agarose and
polyacrylamide (PAG) have desirable properties, including melting points
higher than those
achieved through ablation, and the ability to be doped with materials to mimic
properties such as
electrical and thermal conductivity. The use of polyacrylamide gels altered
with sodium chloride
and permanent color changing dyes allows for visualization and quantitative
assessment of heat-
affected zones caused by an electrode. The use of polyacrylamide gels altered
with pH indicators
(i.e. phenol red) allows for visualization and quantitative assessment of
locoregional pH changes
surrounding an electrode. Temperatures as well as acidity may be backtraced
through post-
experimental image analyses and verified through the placement of
thermocouples, fiber-optic
thermometers, or micro-pH electrodes. This allows for the creation of models
that predict
temperature or pH change in response to supplied power for specific amounts of
time, aiding in
therapeutic planning for both thermal (RFA, MW, Laser, HIFU) and non-thermal
(IRE, EA) modes
of ablation.
(074) Fig. 16 is an RF ablation experimental set-up. The helical wire
structure electrode
is either injected via dispenser or may be pre-embedded into a temperature
sensitive gel phantom.
Connection between RF generator and helical wire structure may be made via
direct contact using
a 30g needle.
17
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(075) Fig. 17 is a DC ablation experimental set-up. The helical wire
structure electrode is
either injected via a dispenser or may be pre-embedded in a pH sensitive gel
phantom. The negative
terminal of the DC supply is attached to a helical wire structure electrode
via direct contact (30g
needle). The positive terminal is attached to the saline-filled grounding
plate for return.
(076) Fig. 18 addresses post-experiment image-based temperature estimation
in
application of RF energy. Fig. 18-A is an image of an ablative zone effected
by a 2cm-length
helical wire structure electrode subjected to 40W power over two minutes. Fig.
18-B shows
temperature estimation based on RGB value found 2mm from electrode site
compared to RGB
value of control set across 25-70 degrees Celsius. Fig. 18-C shows temperature
estimation vs.
distance at sites 1-5mm from the center of the ablative zone.
(077) Fig. 19 shows prior art electrode placement and heat dispersion¨
bird's eye view.
Fig. 19-A shows linear electrode placement, radial heat dispersion for a small
(<3cm) tumor, and
complete ablation. Fig. 19-B shows linear electrode placement, radial heat
dispersion for a large
(>3 cm) tumor, but incomplete ablation. Fig. 19-C shows multiple probe
placement (intratumoral)
required to cover larger spheroid tumor. Fig. 19-D shows alternative multi-
probe arrangement,
inconsistent tumor margin for intratumoral placement. Fig. 19-E shows "no
touch" ablation, probes
arranged outside the tumoral space, with current/heat diffusion running
between each electrode to
achieve consistent tumor margin.
(078) Fig. 20 is an image of rat liver left lobe subject to lcm helical
wire structure
electrode placement and RFA at 20W over 1 minute. The arrows show the extent
of the ablation:
width of ¨3mm, and length of ¨15 mm.
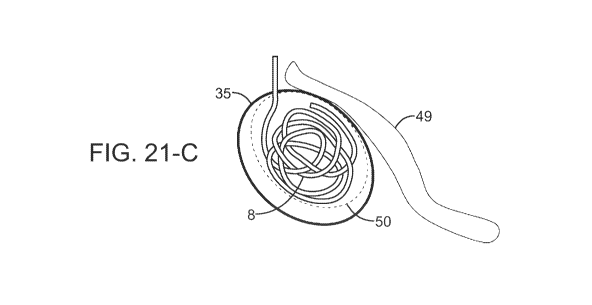
(079) Fig. 21 contains three schematics for "around a corner" procedures for a
tumor (in
dotted lines) shielded by a blood vessel comparing prior art ablation devices
and outcomes with
the present invention and outcome. Fig. 21-A is a schematic of a prior art
single probe and its
pattern (oval with solid line) which affects only part of the tumor. Fig. 21-B
is a schematic of a
prior art single probe with a starburst and its pattern (circle with solid
line) which affects only part
of the tumor. Fig. 21-C is a schematic of a helical wire structure electrode
and its pattern (tilted
larger oval with solid line) which affects all of the tumor. By introducing
the helical wire structure
into a tumor in the shape of the tumor and close to the center line of the
tumor (while staying far
enough away from vital structure such as the blood vessel that is not to be
damaged by the
18
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
application of RF ablation for example), a treatment may be provided to a
patient that would
otherwise not be possible to treat the tumor and leave the blood vessel
intact. Similar scenarios are
a complex shaped tumor in difficult regions to access with a "straight"
ablation probe.
(080) Fig. 22 contains four images of patterns of radiofrequency ablation
for linear helical
wire structures in tissue-mimicking polyacrylamide gel phantoms laced with a
thermochromic ink,
along with a temperature scale. Fig. 22-A received 10 watts for 60 seconds,
Fig. 22-B received 10
watts for 120 seconds, Fig. 22-C received 20 watts for 60 seconds, and Fig. 22-
D received 20 watts
for 120 seconds.
(081) Fig. 23 includes four images of ablation with a substantially linear
helical wire
structure electrode in cadaver tissue as follows: Fig. 23-A, 20 watts for 60
seconds; Fig. 23-B, 20
watts for 120 seconds; Fig. 23-C, 40 watts for 60 seconds; and Fig. 23-D, 40
watts for 120 seconds.
(082) Fig. 24 contains images of curved ablation patterns with RF energy in
tissue-
mimicking polyacrylamide gel phantoms. Fig. 24-A is a photo of a helical wire
structure in "hook"
conformation prior to implantation. Fig. 24-B shows the implanted helical wire
structure of Fig.
24-A and the affected pattern after being subjected to 40 watts of over 60
seconds. Fig. 24-C shows
the same implanted helical wire structure subjected to 40 watts over 120
seconds, showing a larger
affected pattern than in Fig. 24-B. The focal ablative region expands from the
center of curvature
of the implanted helical wire structure.
(083) Fig. 25 includes four images of ablation with a J-hook shaped helical
wire structure
electrode in cadaver tissue as follows: Fig. 25-A, 20 watts for 60 seconds;
Fig. 25-B, 20 watts for
120 seconds; Fig. 25-C, 40 watts for 60 seconds; and Fig. 25-D, 40 watts for
120 seconds.
(084) Fig. 26-A, Fig. 26-B, Fig. 26-C and Fig. 26-D are images of two
linear helical wire
structures coupled to the negative terminal of a DC power supply for 0, 60,
300 and 600 seconds,
respectively, embedded into a tissue mimicking polyacrylamide gel phantom
laced with a pH
indicator. The gel phantom is in a container surrounded by saline, to which
the positive terminal
of the power supply is connected such that the saline acts as the return. by
DC is supplied over
the course of 600 seconds and a pH decrease due to the production of acidic
species (H+, HC1 in
particular) is observed across both helical wire structures emanating
radially, alongside oxygen
gas evolution (bubbles), Through use of a battery or a DC power supply set to
a constant potential,
the negative terminal may be connected via needle or partially-insulated clip
to anode (helical wire
19
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
structure). The cathode (positive terminal) may be connected to an adjacent
site, either a
conductive bath or an tangentially placed electrode.
(085) In ablation, lidocaine is often injected at the ablation location
prior to applying
ablation. With the implanted helical wire structure electrode which is
implanted prior to the
ablation procedure, instead of lidocaine injection the clinician may pre-treat
with slowly ramped
DC to block the sensory innervation of the tissue prior to DC Ablation or RF
ablation.
(086) Fig. 27 is an image of electrolysis-induced local pH change and
oxygen gas
evolution surrounding multiple helical wire structure anodes embedded in pH
sensitive tissue-
mimicking phantom, the result of applied DC voltage between anodes and saline
bath return.
Helical wire structure, electrolysis-induced local pH decreases at anode
(left) and pH increases at
cathode (right), accompanied by surrounding oxygen and hydrogen gas evolution
at anode and
cathode, respectively, are the result of applied DC between anode and cathode
return. The structure
of the anode emphasizes that the helical wire structure is capable of non-
linear pH change
geometries.
(087) Fig. 28 is a fluoroscopy image of a curved helical wire structure
embedded near
rodent liver, with a partially insulated stainless steel interfacing needle
approaching.
(088) Amplitude and gradient of a generated field depends on the applied
voltage and the
distance between the electrodes. The tailored delivery of the highly
conductive helical wire
structure allows the user to introduce electrodes in a close arrangement to
either irreversibly or
temporarily electroporate tissue. Helical wire structures are also not
restricted to fixed geometries,
again, making it simple to ensure that the target is entirely enclosed within
an applied field. Being
an indwelling device with a predictable field output allows repeated
interfacing and sensitization
of tissue through reversible electroporation to a combination with chemical
therapeutics, prior to
a hyperthermic or non-thermal ablative treatment. Sensitization using
electroporation through the
helical wire structure decreases the required load of drug, reducing side
effects and cost.
(089) Energy may be applied to the helical wire structure through a
specially designed
high voltage generator and a secure, well insulated needle interface, as
described. Design of power
systems for electroporation devices requires a great deal of attention to
safety due to the high
energies accumulated in capacitors and from the delivery of high electrical
currents to the patient
- both operator and patient are at some risk of electrocution if energy
release to the patient is not
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
reliably controlled. The resistive load of a biological tissue varies, and
depends on the physical
properties of the electrodes. In the case of the helical wire structure, this
may be difficult to assess
without a preliminary test. Existing electroporation devices which measure a
load of more than
50A will interrupt the pulse sequence, under the assumption that a short
circuit or sparking is
occurring between electrodes.
(090) MW ablation is a thermal technique which creates an electromagnetic
field
surrounding a monopolar electrode, inducing homogeneous heating and
coagulative necrosis. It
heats rapidly, reaching higher temperatures than other hyperthermia methods
(RF), and can treat
larger ablation areas compared to monopolar RF. It achieves higher
temperatures faster compared
to RF, and is less sensitive to the heat-sink effect. However, it is difficult
to define a reliable end-
point to set the amount of energy deposition during MW. Multiple linear
applicators increase the
risk of injuries and complications resulting from over-ablating. Helical wire
structure, indwelling,
serves as a fiducial marker for clinicians to easily locate and re-evaluate
the target site for required
repeat treatments.
(091) HIFU is used to cauterize tissue using 5W/cm2 or greater power. HIFU
has
difficulty as a standalone therapy, due to poor rates of complete ablation,
resulting in higher rates
of recurrence. A helical wire coil as a chronically indwelling implant allows
easy relocation and
accurate targeting for repeat treatments. HIFU introduces biological effects
upon tissue (most
commonly thermal effects and ablation). Typically this occurs with specific
energy deposition of
5W/cm2 and greater. Additional biological effects within the sub thermal
envelope may enhance
drug/gene delivery mechanisms similar to electroporation as discussed herein.
Currently HIFU as
an ablation technique is not complete enough to be completely curative in
treating malignant
tumors. HIFU has a good use case for benign tumors such as fibroid tumors of
the uterus, because
it can be useful to mechanically shrink down the bulk of the tumor. However,
while HIFU can
shrink a tumor through partial tissue destruction, studies of effectiveness
indicate it is unlikely to
achieve complete ablation. The failure mode of ablation as a treatment for
malignant tumors can
be due to microscopic disease that we can't image well enough to see. Non-
fully ablated malignant
tumors almost always regenerate and regrow. One embodiment of the present
invention resonates
external ultrasound off the solid focal point of the wire structure electrode
to create thermal energy
and thus strengthen the ablative ability of the ultrasound signal. Fiducial
marking and visibility
provides for accurate tracking for recurrence and retreatment at the target
site.
21
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
(092) The present invention in other embodiment includes temporary
placement of the
helical or non-helical wire structure electrodes.
Further Aspects of the Invention
(093) In addition to the foregoing, the invention also includes additional
aspects as
follows.
A method of treating a tissue target in a body comprising the steps of
implanting a wire
structure electrode in, on or near the tissue target, said wire structure
electrode being
biocompatible and capable of chronic implantation in said body, providing
grounding means
exterior to said body, contacting the wire structure electrode through a
percutaneous probe, said
probe being energy conductive, and delivering energy through said probe to
said tissue target,
said energy being sufficient to lesion or destroy said tissue target, and
withdrawing the probe
from the body. In another embodiment the method further comprises at the end a
step of imaging
the wire structure electrode as implanted as a fiducial marker and then
repeating all steps after
implanting the wire structure electrode. When the tissue target is a
peripheral nerve, one
embodiment of the method is a new step after imaging of gradually ramping up
direct current to
the peripheral nerve from zero mA to 0.1mA in less than 30 seconds and
thereafter increasing as
needed to prevent pain. Using the same method, the wire structure electrode
comprises a helical
wire structure or a non-helical wire structure, the latter being selected from
the group consisting
of rolled, folded, extruded and the like. The energy delivered through the
probe may be selected
from the group of radiofrequency current, microwave energy, direct current and
changing
magnetic field inducing alternating currents, and is sufficient to heat said
tissue target to at least
60 degrees C. In another embodiment said energy delivered through the probe is
direct current
and is sufficient to lesion said target tissue through an induced pH 4 or less
or 10 or more. In yet
another embodiment of the method, energy delivered to the probe is current and
produces
reversible electroporation, irreversible electroporation or electrolytic
lesioning.
In yet another aspect, the amplitude of the direct current to achieve the
lasting pH change
is reached by initially slowly ramping the DC from zero mA to 0.1 mA in less
than 10 seconds,
thereafter increasing the slow rate as applicable such that the patient does
not report sensation of
DC applications to avoid the need for pharmacological anesthetic agents
consisting of lidocaine,
22
Date Recue/Date Received 2023-03-16
CA 03195583 2023-03-16
WO 2022/060425 PCT/US2021/033265
marcaine, or similar as the means of reducing pain during the application of
DC to block pain
perception while applying treatment to the tissue.
The invention also embodies a system to treat a neurological condition
comprising an
implantable folded wire structure intended to be left in place chronically
that can fold up or
bunch up or fold in when brought in mechanical contact with the biological
target tissue or
surrounding tissues, an percutaneous interfacing device intended for the
temporary penetration of
skin to conduct energy from outside of the body to the implanted folded wire
structure inside the
body, the interfacing device having a skin penetrating insulated portion and
an uninsulated
portion, both portions intended to penetrate the skin, the insulated portion
penetrating the outer
layers of the skin and the uninsulated portion intended to interface with the
chronically placed
folded wire structure, a connector to an energy signal generation device, and
a signal generation
device providing the energy, and the signal generation device providing the
energy being a
radiofrequency generator, a microwave generator, a direct current generator.
In another
embodiment of the system, the signal generation device providing the energy
being a direct
current and a radiofrequency generator, the device being capable to first
generate a ramped direct
current to sensory block neural tissue in the vicinity of the implanted folded
wire structure
electrode, and second being able to provide a radiofrequency energy to ablate
target tissue in the
vicinity of the implanted folded wire structure electrode.
The invention also is a method of treating cancerous tissue by applying an
electrical
energy signal to cancerous tissue with the aid of a chronically implanted
folded wire structure in
close timed proximity to the application of chemotherapy agents to a cancer
patient, the
treatment consisting of multiple applications of either treating cancerous
tissue with RF, MW, or
DC energy alone, or treating cancerous tissue with RF, MW, or DC energy in
close time
proximity with therapeutic agents such as chemotherapeutic drugs.
Another method is included within the invention wherein the implanted folded
wire
structure enables easy repeat treatment via RF, MW or DC energy that is being
delivered by a 24
gauge or smaller needle, allowing for a patient-friendly repeat treatment.
23
Date Recue/Date Received 2023-03-16