Note: Descriptions are shown in the official language in which they were submitted.
METHOD FOR ENZYMATIC AND/OR MICROBIAL PROCESSING OF WASTE COMPRISING
RECIRCULATION OF PROCESS WATER
FIELD
The present invention relates to a method for continuous or batch processing
of waste in a
bioreactor resulting in a bioliquid, which method comprises recirculation of
process water obtained
from the downstream processing of said bioliquid. The waste can for instance
be sorted or unsorted
municipal solid waste (MSW) subject to enzymatic and/or microbial degradation
in a continuous or
batch bioreactor. The process water can for example be bioliquid, reject water
obtained from an
anaerobic digestion (AD) process, evaporation from digestate or wash water and
any combinations
thereof. Water from external sources may also be added to the reactor in
addition to the
recirculation of any process water.
BACKGROUND
There is a great interest to employ methods in which the energy stored within
waste comprising
organic material is utilized to the fullest. Agricultural material/waste,
household waste and
municipal waste are examples of sources containing a high content of dry
matter and a certain
content of organic material, where it is of high interest to utilize the
stored energy within the organic
material instead of just disposing the waste. Considerable interest has arisen
in development of
efficient and environmentally friendly methods of processing solid waste, to
maximize recovery of
their inherent energy potential and, also, recovery of recyclable materials.
One significant challenge
in "waste to energy" processing has been the heterogeneous nature of waste,
such as MSW.
The commonly used methods for treatment and subsequent disposal of waste such
as household,
agricultural or municipal waste include among others incineration, landfill,
burning, dumping and
composting, where the method of choice often depends on e.g. the content of
organic material
compared to the content of nonorganic material. However, these methods do not
directly provide
an optimum utilization of the energy stored within the organic material.
Pre-sorting may sometimes be provided by the consumers or by the waste station
and this reduces
the pollution released by e.g. incineration and simplifies the degradation of
the organic waste into
CA 03196334 2023- 4- 20
1
valuable end-products. However, pre-sorting may not be efficient in separating
all non-
biodegradable material such as metal and glass from the organic waste.
In methods, such as the one described below, wherein the organic contents of
the waste are
liquefied while maintaining the non-organic contents in their solid phase, and
afterwards separate
the solid and the liquid phases, pre-sorting may simplify the process but is
not a necessity.
An environmentally friendly waste processing method is a biologically based
method, such as the
method currently applied by Renescience, wherein waste comprising organic
matter, such as
ordinary unsorted or sorted/partially sorted household waste, is mixed with
water, enzymes and
optionally microorganisms in order to dissolve all organic waste such as food
waste, cardboard,
paper, labels and similar, and turn it into a bio-liquid that can be used for
production of for example
biogas via an anaerobic digestion process. The liquefaction process wherein
the waste is subjected
to enzymatic and/or microbial treatment requires water in order to provide a
bioliquid that can be
used for further processing as an energy source. Moreover, the subsequent
processing steps of the
bioliquid and/or the solid fractions may also require addition of water
depending on the features of
the specific process.
The method according to the present invention is based on the method currently
applied by
Renescience and is suitable for processes wherein waste comprising organic
matter has been
subject to enzymatic degradation and/or microbial fermentation producing a
bioliquid and various
solid fractions. Examples of such waste treatment processes are disclosed in
W02006056838,
W02007036795, W02011032557, W02013185778, W02014198274, W02016030480,
W02016030472, W02016050893, W02017/174093, which is hereby expressly
incorporated by
reference in entirety.
Enzymatic treatment offers unique advantages over "autoclave" methods for
liquefaction of
degradable organic components. Using enzymatic liquefaction, processing of
waste, such as MSW,
can be conducted in a continuous manner, using comparatively cheap equipment
and non-
pressurized reactions run at comparatively low temperatures. In the context of
the present
invention liquefaction includes "to make fluid" and "to dissolve into water".
CA 03196334 2023- 4- 20
2
Enzymatic liquefaction may sometimes require thermal pre-treatment to a
comparatively high
temperature of 120 C or higher, in part to affect a "sterilization" of waste
such as unsorted MSW
and also so that degradable organic components can be softened and paper
products "pulped."
However, high temperature pre-treatment can be actively detrimental, since
this kills ambient
microorganisms which are thriving in the waste. Thus, in a preferred
embodiment the waste is not
pre-treated at high temperature i.e. above 90 C. In one preferred embodiment
the waste is mixed
with pre-heated water to a temperature in the range of 60 C to 75 *C,
preferably 70 C or preferably
around 70 C. This water is preferably fully supplied by re-circulated process
water, such as water
from dewatering the digestate i.e. reject water. Alternatively, or in addition
the process water
recirculated is clean condensate water.
In addition to improving "organic capture" from enzymatic treatment,
concurrent microbial
fermentation using any combination of lactic acid bacteria, or acetate-,
ethanol-, formate-,
butyrate-, lactate-, pentanoate- or hexanoate- producing microorganisms, "pre-
conditions" the
bioliquid so as to render it more efficient as a substrate for further
processing, such as biomethane
production. Microbial fermentation produces bioliquid having a generally
increased percentage of
dissolved compared with suspended solids, relative to bioliquid produced by
enzymatic liquefaction
alone. Higher chain polysaccharides are generally more thoroughly degraded due
to microbial "pre-
conditioning". Concurrent microbial fermentation and enzymatic treatment
degrades biopolymers
into readily usable substrates and, further, achieves metabolic conversion of
primary substrates to
short chain carboxylic acids such as glucose, xylose, arabinose, lactate,
acetate and/or ethanol. The
conversion of the organic matter in the waste according to a process of the
invention normally at
least include lactic acid producing bacteria. The resulting bioliquid
comprising a high percentage of
sugars and other soluble degradation products provides a biomethane substrate,
a substrate
suitable for anaerobic digestion for the production of biogas. A bioliquid
comprising a higher
amount of acids will contribute to a faster anaerobic degradation process.
Anaerobic digestion is a series of biological processes in which
microorganisms break down
biodegradable material in the absence of oxygen. One of the end products is
biogas, which can be
CA 03196334 2023- 4- 20
3
combusted to generate electricity and heat, or can be processed into renewable
natural gas and
transportation fuels. A range of anaerobic digestion technologies exists in
the state of the art for
converting waste, such as municipal solid waste, municipal wastewater solids,
food waste, high
strength industrial wastewater and residuals, fats, oils and grease (FOG), and
various other organic
waste streams into biogas. Many different anaerobic digester systems are
commercially available,
and the skilled person will be familiar with how to apply and optimize the
anaerobic digestions
process. The metabolic dynamics of microbial communities engaged in anaerobic
digestion are
complex. In typical anaerobic digestion (AD) for production of methane biogas,
biological processes
mediated by microorganisms achieve four primary steps ¨ hydrolysis of
biological macromolecules
into constituent monomers or other metabolites; acidogenesis, whereby short
chain hydrocarbon
acids and alcohols are produced; acetogenesis, whereby available nutrients are
catabolized to acetic
acid, hydrogen and carbon dioxide; and methanogenesis, whereby acetic acid and
hydrogen are
catabolized by specialized archaea to methane and carbon dioxide. Apart from
the production of
valuable output, AD also produces a digestate, sometimes called "raw effluent"
or "AD effluent".
These terms can be used interchangeably and refers to the waste product from
anaerobic digestion.
The digestate comprises both solids and liquids and these fractions may be
used for various
purposes. In a preferred embodiment the liquid digestate may be hygienized
e.g. by heating to 60
to 75 C, preferably 65 C to 70 C or above 70 C for 60-80 minutes, preferably
60-70 minutes,
preferably about 60 minutes e.g. to prevent pathogenic microbes to pass on
from the AD digestion.
Solid-liquid separations can for instance be done by decantation,
centrifugation and/or
sedimentation. In a preferred embodiment the liquid digestate, which may be
hygienized as
described above, is dewatered e.g. through a decanter centrifuge, which
produce reject water,
which is re-circulated into the process according to the invention. In case
the of a hygienization of
liquid digestate the reject water resulting from a solid-liquid separation of
the digestate will be
about 70 C and could be used in the pre-treatment of the waste as described
above. Usually, the
liquid digestate has alkaline pH, and comprises mainly water, but also dry
suspended solids and
dissolved matter such as salts. "Process water" is defined as the liquid
fraction obtained after one
or more solid-liquid separations in a process downstream of the enzymatic
and/or microbial
treatment of waste, such as water resulting from dewatering of digestate from
an AD process, which
is also defined as "reject water". Thus, process water comprises reject water.
Process water may
CA 03196334 2023- 4- 20
4
comprise various salts, dissolved matter and live microorganisms. Preferably
the process water is
reject water obtained from an anaerobic digestion (AD) process, evaporation
from digestate or wash
water.
Recirculation of process water in waste treatment processes as such have been
applied in various
treatment process.
EP341547261 discloses anaerobic digestion of organic matter wherein the
digestion effluent from
the digester is separated into a fiber fraction and a liquid fraction, and
wherein each fraction may
be recirculated into the digester.
WO 2020/03194 Al discloses recirculation of digestate flow wherein pH and
electric conductivity is
controlled by an algorithm in order to maintain pH at 5.2 ¨ 5.7 in the
digester.
US 1011885162 discloses anaerobic digestion of biologic waste matter. In a
first step, waste is mixed
with liquid and is subjected to AD. In the second step, the digestate is
dewatered by pressing
followed by another separation step and finally recirculation of the separated
liquid effluent into
the first AD step.
W02015/004146 discloses an anaerobic digestion process wherein the effluent
may be
recirculated.
The above documents relate to recirculation of water from AD processes. None
of the documents
describe methods wherein recirculation of process water, which is water
originating from an
enzymatic waste treatment process, such as an reject water obtained from
anaerobic digestion,
relates to recirculation of the process water back into a previous step
wherein the previous step is
subjecting waste, such as MSW to enzymatic and/or microbial treatment in a
bioreactor.
The present invention relates to a method wherein process water from an
enzymatic and/or
microbial liquefaction processing of waste followed by subsequent downstream
processing of the
CA 03196334 2023- 4- 20
5
bioliquid is recirculated into the bioreactor. The process water can be
recirculated into the
bioreactor optionally without any hygienization. In the present method, the
amount of water from
external sources such as tap water and water from natural sources needed in
the liquefaction
process of waste comprising organic matter can be reduced meaning the spared
tap water is
available for other purposes in nature and in society.
SUMMARY OF THE INVENTION
The present invention is a method for continuous or batch processing of waste
in a bioreactor,
wherein process water from one or more downstream processes is recirculated
into the bioreactor.
The advantages of recirculating water include but is not limited to
distribution of enzymes into the
MSW, mechanical flushing of organics into bioliquid, dissolvement of organics
dried onto surfaces,
salt dilution and/or transfer of organics and salts out of the waste
undergoing treatment in the
bioreactor.
The process water is obtained from one or more downstream process steps. For
instance, the
process water can be bioliquid collected directly from the enzymatic and/or
microbial liquefaction
process or the process water can be obtained from other downstream processes
such as from an
anaerobic digestion (AD) process, washing steps of solid waste or process
water obtained by
evaporation derived from various downstream processes. The downstream
processes may be
utilising the bioliquid produced in said bioreactor for biogas production or
for other energy derivable
products. In one embodiment, the process water is reject water obtained from
an anaerobic
digestion (AD) process, evaporation from digestate or wash water. Water from
external sources
may also be added to the reactor in addition to the recirculation of any
process water.
The method of the invention is a method for continuous or batch processing of
waste comprising:
a) subjecting waste to an enzymatic and/or microbial treatment in a bioreactor
b) subjecting the treated waste from step a) to one or more separation
step(s),
whereby a bioliquid and a solid fraction is provided;
c) subjecting said bioliquid and/or solid fraction to downstream processing
providing
process water;
CA 03196334 2023- 4- 20
6
d) adding the process water obtained from step c) and optionally water from an
external water source to the bioreactor in step a).
For a liquefaction process that runs continuously at a pH that meets the
optimal enzymatic and
microbial conditions, the pH is normally around between pH 2 ¨ 6.5 in the
bioreactor. It has been
found here that recirculation of the process water obtained from step c) or a
fraction thereof meets
these requirements in general but that the best conditions seem to be met if
the pH of the process
water to be recirculated into the reactor is of a similar pH, preferably a
between pH 3.5 to 6. The pH
of the process water will depend on the process it is derived from. Thus, in
some embodiments it is
preferred to adjust the pH to between 3.5 and 6 before recirculating the
process water into the
bioreactor. For example, if the process water is reject water obtained from an
anaerobic digestion
process it will normally have a pH around 8 to 9. If basic reject water from
an AD process is to be
recirculated into the bioreactor, it is preferred that pH of the reject water
is adjusted to a pH
between 3.5 to 6.0 prior to being recirculated, to ensure that the pH of the
liquefaction process in
the bioreactor is remains at an optimum level below 6.5, preferably below 5.5.
Alternatively,
repetitive pH adjustments in the bioreactor after steady state conditions have
been established is
preferred, or if no adjustment of the pH of the process water e.g. reject
water prior to adding it into
the bioreactor is made the process water may be added in batches or on
continuously to the
bioreactor. In one embodiment of the invention the process water is added to
step a) in batches,
such that pH in the reactor is between pH 3.5-6.
In some embodiment the process water may have to undergo hygienization at 70 C
for 60 minutes.
The process water added to the process of the invention will then be 60-70 C
and could be used
directly in pre-treating the waste as described above.
The waste can be any kind of waste comprising at least some organic matter and
can be sorted or
unsorted. Waste such as municipal solid waste is particularly useful because
it comprises both
organic matter and live microorganisms that may contribute to the combined
enzymatic and
microbial degradation. Water from external sources may optionally be added to
the bioreactor in
addition to the process water. Water from external sources may be water
obtained from natural
CA 03196334 2023- 4- 20
7
sources such as rivers, lakes and ponds; water reservoirs; tap water; and any
combination thereof.
Means that further stimulate the enzymatic and microbial degradation of waste
may be added. Such
means include enzyme compositions; feed for the microorganisms such as
carbohydrates; addition
of microorganisms such as lactic acid producing bacteria; temperature
regulations; mixing or
rotation devices.
The method according to the present invention can be performed within a single
waste processing
plant comprising one or more bioreactors and/or one or more downstream process
reactors, such
as AD digesters that are part of the same waste treatment loop; or the process
water may be
obtained from one or more different and possibly independent enzymatic and/or
microbial waste
processing and/or biogas production sites.
It is shown herein that process water can be recirculated from downstream
processes into the
bioreactor while keeping the bioliquid production process in the bioreactor in
a steady state
wherein both the enzymatic and microbial processes are active and the
retention time and
accordingly cost are optimized. Preferably the process water has a pH between
3.5 and 6 when
being recirculated into the bioreactor to avoid a delaying impact on the
enzymatic and/or microbial
liquefaction process in the bioreactor. The examples disclosed herein shows
that both the enzymatic
activity and the activity of the microorganisms producing the valuable organic
acids in the bioliquid
required as feed to the AD biogas production is upheld at a continuous rate in
the bioreactor when
process water such as reject water is added to the bioreactor. Whereas the pH
optimum of
enzymatic compositions that are added to the waste are known beforehand, the
pH optimum for
the enzymes derived from the natural microbial flora of the waste is unknown.
Also, whereas the
pH optimum of the microbial blends being added to the waste is known
beforehand, the identity of
the natural occurring microorganisms in the microbial flora of the waste and
thus the pH optimum
of these organisms are unknown and will vary depending on the natural
microbial flora of the waste
being processed.
CA 03196334 2023- 4- 20
8
BRIEF DESCRIPTION OF FIGURES
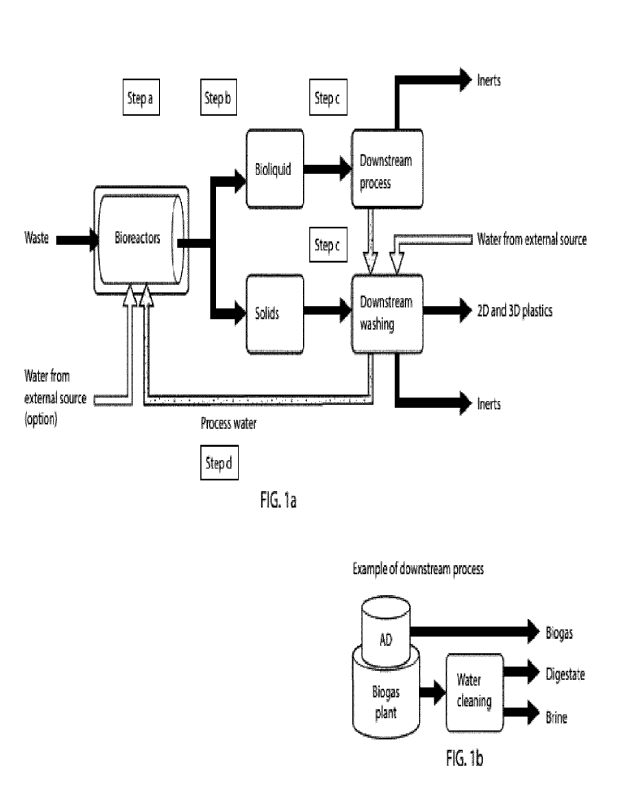
Figure 1: la is a schematic drawing of steps in a waste treatment process and
recirculation of
process water comprising step a), b), c) and d) according to the invention. lb
is a schematic drawing
of an example of a downstream process.
Figure 2: Graph showing pH as a function of fermentation time.
Figure 3: Graph showing pH as a function of fermentation time, reject water
added with or without
pH adjustment.
Figure 4: Graph showing pH as a function of fermentation time, sequential
removal of material from
the fermenter followed by addition of reject water.
Figure 5: Graph showing pH as a function of fermentation time, addition of
reject water.
Figure 6: Bar chart showing the proportion of lactic acid producing bacteria
of the total amount of
bacteria during fermentation with addition of reject water.
Figure 7a to 7d: Pie charts showing the proportion of the most dominant
species of lactic acid
producing bacteria of the total amount of bacteria during fermentation with
addition of reject
water.
Figure 8a to 8d: Pie charts showing the proportion of specific species of
lactic acid producing
bacteria during fermentation with addition of reject water.
Figure 9: Bar chart showing the proportion of archaea of the total amount of
bacteria during
fermentation with addition of reject water.
Figure 10: Methane yield as a function of time.
Figure 11: pH profile the fermentation of MSW model substrate in a fermenter
with the addition of
NH4FIC03 and ammonia.
Figure 12. Distribution of the lactic acid bacteria and other bacterial
species over the course of the
fermentation of MSW model substrate in a rotating horizontal reactor with
addition of reject water
and glucose.
DEFINITIONS
As used herein, the following terms have the following meaning:
CA 03196334 2023- 4- 20
9
"About" as used herein, usually with reference to a quantitative number or
range, may refer to +/-
1, 2,5 or even 10% in relative terms of the number or range referred to. In
the context of the present
invention, the terms "about", "around", "approximately" or the symbol "¨" can
be used
interchangeably and are meant to comprise variations generally accepted in the
field, e.g.
comprising analytical errors and the like.
The term "comprising" is to be interpreted as specifying the presence of the
stated parts, steps,
features, components, or the like, but does not exclude the presence of one or
more additional
parts, steps, features, components etc. For example, a composition comprising
a chemical
compound may thus comprise additional chemical compounds.
It can differ in one or more atoms, functional groups, or substructures, which
are replaced with
other atoms, groups, or substructures. A structural analogue can be imagined
to be formed, at least
theoretically, from the other compound.
"Batch" refers to any defined amount of waste delivered from a specified
geographic area to the
waste plant. The amount of waste in a "batch" and the size of the geographic
area will vary from
plant to plant and depend of the specific renovation-collecting system and the
specific size of the
plant, such as a large scale plant. Each batch may be treated separately in
each of step a), b) and c)
of the present method or several batches may be treated continuously or at
least having overlapping
retention times at one or more step(s). Typically, a batch will be the amount
of waste loaded into
the waste plant by a single truck which usually comprises between 15 ¨ 20 m3
waste disposal per
load. Several batches from trucks may be collected, stored and entered into
the treatment plant as
one large batch. In such circumstances, the batch will usually comprise
between 40 ¨ 6000 m3
waste.
"Batch process" The treatment can be carried out as a batch process or series
of batch processes.
The treatment can be carried out as a fed batch or continuous process, or
series of fed batch or
continuous processes, where the municipal solid waste material is fed
gradually to, for example, a
treatment solution containing an enzyme composition. The treatment may be a
"continuous
CA 03196334 2023- 4- 20
process" in which an MSW material and an enzyme composition are added at
different intervals
throughout the treatment and the hydrolysate is removed at different intervals
throughout the
treatment. The removal of the hydrolysate may occur prior to, simultaneously
with, or after the
addition of the cellulosic material and the cellulolytic enzymes composition.
"Bioreactor" refers to any manufactured or engineered device or system that
supports a biologically
active environment. A bioreactor may be a vessel in which a chemical process
is carried out which
involves organisms or biochemically active substances derived from such
organisms. This process
can either be aerobic or anaerobic. These bioreactors may be cylindrical or
not, ranging in size from
liters to cubic meters, and are often made of stainless steel. For the purpose
of the present
invention, "bioreactor" comprises any facility, container or environment
providing the appropriate
conditions to perform the present invention.
"Bioliquid" is the liquefied and/or saccharified degradable components
obtained by enzymatic
treatment of waste comprising organic matter. Bioliquid also refers to the
liquid fraction obtained
by enzymatic and/or microbial treatment of waste comprising organic matter
once separated from
non-fermentable solids. Bioliquid comprises water and organic substrates such
as protein, fat,
galactose, ma nnose, glucose, xylose, arabinose, lactate, acetate, ethanol
and/or other components,
depending on the composition of the waste (the components such as protein and
fat can be in a
soluble and/or insoluble form). Bioliquid comprises also fibers, ashes and
inert impurities. The
resulting bioliquid comprising a high percentage of solubles, provides a
substrate for gas production,
a substrate suitable for anaerobic digestion e.g. for the production of
biogas.
"Cellulolytic background composition (CBC) or Cellulolytic Enzyme Blend" means
an enzyme
composition comprising a mixture of two or more cellulolytic enzymes. The CBC
may comprise two
or more cellulolytic enzymes selected from: i) an Aspergillus fumigatus
cellobiohydrolase I; (ii) an
Aspergillus fumigatus cellobiohydrolase II; (iii) an Aspergillus fumigatus
beta-glucosidase or variant
thereof; and (iv) a Penicillium sp. GH61 polypeptide having cellulolytic
enhancing activity; or
homologs thereof. The CBC may further comprise one or more enzymes selected
from: (a) an
Aspergillus fumigatus xylanase or homolog thereof, (b) an Aspergillus
fumigatus beta-xylosidase or
CA 03196334 2023- 4- 20
11
homolog thereof; or (c) a combination of (a) and (b) (as described in further
detail in WO
2013/028928). The major activities of the CBC may comprise: endo-1,4-beta-
glucanases (E.C.
3.2.1.4); endo-1,4-beta-xylanases (E.C. 3.2.1.8); endo-1,4-beta-mannanase
(E.C. 3.2.1.78), beta-
ma nnosidase (E.0 3.2.1.25), whereas other enzymatic activities may also be
present in the CBC such
as activity from glucanases, glucosidases, cellobiohydrolase I
cellobiohydrolase II; beta-glucosidase;
beta-xylosidase; beta-L-arabinofuranosidase; amyloglucosidase; alpha-amylase;
acetyl xylan
esterase. The CBC may be any CBC described in WO 2013/028928 (the content of
which is hereby
incorporated by reference). The CBC may be from T. reesei. The CBC may be from
Myceliophtora
thermophilae. The CBC may be Cellic CTec3 obtainable from Novozymes A/S
(Bagsvaerd,
Denmark). Cellulolytic enzyme activity can be determined by measuring the
increase in
production/release of sugars during hydrolysis of a cellulosic material by
cellulolytic enzyme(s)
under the following conditions: 1 -50 mg of cellulolytic enzyme proteinig of
cellulose in pre-treated
corn stover (PCS) (or other pre-treated cellulosic material) for 3-7 days at a
suitable temperature
such as 40 C-80 C, e.g., 40 C, 45 C, 50 C, 55 C, 60 C, 65 C, 70 C, 75 C, or 80
C, and a suitable pH,
such as 4-9, e.g., 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, or 9.0,
compared to a control treatment
without addition of cellulolytic enzyme protein.
"Commercially available cellulase preparation optimized for biomass
conversion" refers to a
commercially available mixture of enzyme activities which is sufficient to
provide enzymatic
treatment of biomass such as lignocellulosic biomass and which usually
comprises endocellulase
(endoglucanase), exocellulase (exoglucanase), endoxylanase, acetyl xylan
esterase, xylosidase
and/or beta-glucosidase activities. The term "optimized for biomass
conversion" refers to a product
development process in which enzyme mixtures have been selected and/or
modified for the specific
purpose of improving yields and/or reducing enzyme consumption in treatment of
biomass to
fermentable sugars. A commercially available cellulase preparation optimized
for biomass
conversion can be used, such as one that is e.g. provided by GENENCORTm (now
DuPont), DSM or
NOVOZYMESTm. Usually, such compositions comprise cellulase(s) and/or
hemicellulase(s), such as
one or more of exoglucanases, endoglucanases, endoxylanases, xylosidases,
acetyl xylan esterases
and beta-glucosidases, including any combination thereof. Such enzymes can
e.g. be isolated from
fermentations of genetically modified Trichoderma reesei, such as, for
example, the commercial
CA 03196334 2023- 4- 20
12
cellulase preparation sold under the trademark ACCELLERASE TRIOrm from DuPont
(and/or
GENENCOR). A commercially available cellulase preparation optimized for
biomass conversion that
can be used is provided by NOVOZYMESTm and comprises exoglucanases,
endoglucanases,
endoxylanases, xylosidases, acetyl xylan esterases and beta-glucosidases, such
as, for example, the
commercial cellulase preparations sold under either of the trademarks Cellic
CTec2 or Cellic CTec3
from NOVOZYMESTm.
The term "Cellulase(s)" is meant to comprise one or more enzymes capable of
degrading cellulose
and/or related compounds. Cellulase is any of several enzymes commonly
produced by fungi,
bacteria, and protozoans that catalyse cellulolysis, the decomposition of
cellulose and/or related
polysaccharides. Cellulase can also be used for any mixture or complex of
various such enzymes,
that act serially or synergistically to decompose cellulosic material.
Cellulases break down the
cellulose molecule into monosaccharides ("simple sugars") such as beta-
glucose, and/or shorter
polysaccharides and oligosaccharides. Specific reactions may comprise
hydrolysis of the 1,4-beta-D-
glycosidic linkages in cellulose, hemicellulose, lichenin, and cereal beta-D-
glucans. Several different
kinds of cellulases are known, which differ structurally and mechanistically.
Synonyms, derivatives,
and/or specific enzymes associated with the name "cellulase" comprise endo-1,4-
beta-D-glucanase
(beta-1,4-glucanase, beta-1,4-endoglucan hydrolase, endoglucanase D, 1,4-
(1,3,1,4)-beta-D-glucan
4-glucanohydrolase), carboxymethyl cellulase (CMCase), avicelase,
celludextrinase, cellulase A,
cellulosin AP, alkali cellulase, cellulase A 3, 9.5 cellulase, and pancellase
SS.
Cellulases can also be classified based on the type of reaction catalysed,
where endocellulases (EC
3.2.1.4) randomly cleave internal bonds at amorphous sites that create new
chain ends,
exocellulases or cellobiohydrolases (EC 3.2.1.91) cleave two to four units
from the ends of the
exposed chains produced by endocellulase, resulting in tetra-, tri-or
disaccharides, such as
cellobiose. Exocellulases are further classified into type I - that work
processively from the reducing
end of the cellulose chain, and type II - that work processively from the
nonreducing end. Cellobiases
(EC 3.2.1.21) or beta-glucosidases hydrolyse the exocellulase product into
individual
monosaccharides. Oxidative cellulases depolymerize cellulose by radical
reactions, as for instance
cellobiose dehydrogenase (acceptor). Cellulose phosphorylases depolymerize
cellulose using
phosphates instead of water.
CA 03196334 2023- 4- 20
13
Chemical oxygen demand (COD) is an indicative measure of the amount of oxygen
that can be
consumed by reactions in a measured solution. It is commonly expressed in mass
of oxygen
consumed over volume of solution which in SI units is milligrams per litre
(mg/L). A COD test can be
used to easily quantify the amount of organics in water. The most common
application of COD is in
quantifying the amount of oxidizable pollutants found in surface water (e.g.
lakes and rivers) or
wastewater. COD is useful in terms of water quality by providing a metric to
determine the effect
an effluent will have on the receiving body, much like biochemical oxygen
demand (BOD).
The term "Hemicellulase(s)" is meant to comprise one or more enzymes capable
and/or
contributing to breaking down hemicellulose, one of the major components of
plant cell walls. Some
of the main polysaccharides that constitute hemicellulose are believed to be
xylan, arabinoxylan,
xyloglucan, glucuronoxylan and glucomannan. In the context of the present
invention, the term
"hemicellulase(s)" is meant to comprise: xyla nase(s), xylosidase(s),
arabinoxylanase(s),
xyloglucanase(s), glucoronoxylanase(s), glucomannanase(s), and/or esterase(s),
including any
combination thereof.
The term "Xylanase(s)" is meant to comprise one or more enzymes capable of
degrading xylan
and/or related compounds. Xylanase is any of several enzymes produced e.g. by
microorganisms
such as yeast that catalyse decomposition of xylan and/or related
polysaccharides. Xylanase can
also be used for any mixture or complex of various such enzymes that act
serially or synergistically
to decompose xylanosic material. Synonyms, derivatives, and specific enzymes
associated with the
name "xylanase" may comprise EC 3.2.1.8, endo-(1->4)-beta-xylan 4-
xylanohydrolase, endo-1,4-
xyla nase, endo-1,4-beta-xylanase, beta-1,4-xylanase, endo-1,4-beta-D-
xylanase, 1,4-beta-xylan
xylanohydrolase, beta-xylanase, beta-1,4-xylan xylanohydrolase, beta-D-
xylanase and/or xylosidase
capable of degrading xylan, such as beta-1,4-xylan into xylose, thus
contributing to breaking down
hemicellulose, one of the major components of plant cell walls.
"Xylosidase" as used herein is intended to comprise the enzyme xylan 1,4-beta-
xylosidase (E.C.
3.2.1.37) which is also named xylobiase, beta-xylosidase, exo-1,4-beta-D-
xylosidase or 4-beta-D-
CA 03196334 2023- 4- 20
14
xylan xylohydrolase. This enzyme catalyses the hydrolysis of (1-4)-beta-D-
xylans removing
successive D-xylose residues from the non-reducing termini of the substrate,
e.g. hemicellulose and
the disaccharide xylobiose. One unit of beta-xylosidase is defined as 1.0
mole of p-nitrophenolate
anion produced per minute at 40 C, pH 5 from 1 mM p-nitrophenyl- beta-D-
xyloside in 100 mM
sodium citrate containing 0.01 % TWEEN 20.
The term "Arabinoxylanase(s)" is meant to comprise one or more enzymes capable
of degrading
a ra binoxyla n and/or related compounds, comprising e.g.
glucuronoarabinoxylan endo-1,4-beta-
xylanase (EC 3.2.1.136), feraxan endoxylanase, feraxanase,
endoarabinoxylanase, glucuronoxylan
xylohydrolase, glucuronoxylanase, glucuronoxylan xylanohydrolase,
glucuronoarabinoxylan 1,4-
beta-D-xyla nohydrolase), and glucuronoarabinoxylan 4-beta-D-xylanohydrolase.
Glucurono-
arabinoxylan 4-beta-D-xylanohydrolase is believed to endohydrolyse (1->4)-beta-
D-xylosyl links in
some glucuronoarabinoxylans. It also believed that this enzyme possesses a
high activity towards
feruloylated arabinoxylans. (Nishitani, K.; Nevins, D.J. (1988). "Enzymic
analysis of feruloylated
arabinoxylans (Feraxan) derived from Zea mays cell walls. I. Purification of
novel enzymes capable
of dissociating Feraxan fragments from Zea mays coleoptile cell wall". Plant
Physiol. 87: 883-890.)
The term "Xyloglucanase(s)" is meant to comprise one or more enzymes capable
of degrading
xyloglucan and/or related compounds, comprising e.g. xyloglucan-specific endo-
beta-1,4-glucanase
(EC 3.2.1.151), which is an enzyme that is believed to catalyse the chemical
reaction:
xyloglucan + H20 4 xyloglucan oligosaccharides. This enzyme belongs to the
family of hydrolases,
specifically those glycosidases that hydrolyse 0- and S-glycosyl compounds.
The systematic name of
this enzyme class is [(1->6)-alpha-D-xylo]-(1->4)-beta-D-glucan
glucanohydrolase. Other names in
common use may include XEG, xyloglucan endo-beta-1,4-glucanase, xyloglucanase,
xyloglucanendohydrolase, XH, and 1,4-beta-D-glucan glucanohydrolase.
The term "Glucuronoxylanase(s)" is meant to comprise one or more enzymes
capable of degrading
glucuronoxylan and/or related compounds.
CA 03196334 2023- 4- 20
The term "Glucomannanase(s)" is meant to comprise one or more enzymes capable
of degrading
glucomannanase and/or related compounds.
The term "Esterase(s)" is meant to comprise one or more enzymes capable of
splitting an ester in
an acid and an alcohol. Examples of esterases comprise acetylesterases and
feroyl esterase.
The term "Acetylesterase(s)" is meant to comprise an enzyme capable of
splitting off acetyl groups.
An acetylesterase (EC 3.1.1.6) is an enzyme that catalyses the chemical
reaction:
acetic ester + H20 4 alcohol + acetate. This enzyme belongs to the family of
hydrolases, specifically
those acting on carboxylic ester bonds. The systematic name of this enzyme
class is acetic-ester
acetylhydrolase. Other names in common use include C-esterase (in animal
tissues), acetic ester
hydrolase, chloroesterase, p-nitrophenyl acetate esterase, and Citrus
acetylesterase.
The terms "Feroyl esterase(s)" and "Feruloyl esterase(s)" can be used
interchangeably, and is/are
meant to comprise an enzyme that catalyses the chemical reaction feruloy1-
(poly-, oligo-, or mono-
)polysaccharide + H20 .4 ferulic acid + (poly-, oligo-, or mono-)saccharide.
Feroyl esterase belongs
to the family of hydrolases, specifically those acting on carboxylic ester
bonds. The systematic name
of this enzyme class is feruloyl esterase (EC 3.1.1.73); other names may
include ferulic acid esterase
(FAE), hydroxycinnamoyl esterase, hemicellulase accessory enzyme, and
cinnamoyl ester hydrolase
(cinnAE).
Suitable enzymes, such as cellulases, hemicellulase(s) including xylanases,
and or esterases, can be
expressed in suitable hosts using methods known in the art. Such enzymes are
also commercially
available, either in pure form or in enzyme cocktails. Specific enzyme
activities can be purified from
commercially available enzyme cocktails, again using methods known in the art -
see e.g. Sorensen
et al. (2005) "Efficiencies of designed enzyme combinations in releasing
arabinose and xylose from
wheat arabinoxylan in an industrial fermentation residue" (Enzyme and
Microbial Technology 36
(2005) 773-784), where a Trichoderma reesei beta-xylosidase is purified from
Celluclast (Finizym),
and further commercial enzyme preparations are disclosed.
CA 03196334 2023- 4- 20
16
Conducting a treatment/process "at" a dry matter level refers to the dry
matter content of the
feedstock at the start of said treatment, unless indicated otherwise.
Likewise, conducting a
treatment/process "at" a pH refers to the pH of the aqueous content of the
biomass at the start of
said treatment, unless indicated otherwise.
In the context of the present invention, the term "pH- and/or temperature-
adjusted" is meant to
comprise pH and/or temperature adjustments in order to allow an enzymatic
treatment and/or
fermentation to take place under suitable pH and/or temperature conditions.
"Dry matter" also appearing as "DM", refers to total solids, both soluble and
insoluble, and
effectively means "non-water content." Dry matter content is measured by
drying at between 40
and 120 C, preferably between 50 and 105 C, until constant weight is
achieved.
"Pre-treatment" commonly refers to the use of water, either as hot liquid,
vapour steam or
pressurized steam comprising high temperature liquid or steam or both, to
"cook" biomass, at
temperatures of 120 C or higher, either with or without addition of acids or
other chemicals. In the
context of the present invention, "hydrothermal pre-treatment" is meant to
comprise methods, unit
operations and/or processes relating to softening lignocellulosic biomass by
the use of temperature
and water, and usually, also, pressure, aiming at providing a pre-treated
biomass suitable for
enzymatic digestion. Pre-treatment in context of the present invention may be
the step of mixing
the waste with about 70 C water (preferably recirculated) prior to
(alternatively subsequent or
simultaneously with) liquefaction of the waste with enzymes and/or
microorganisms.
"Solid/liquid separation" refers to an active mechanical process, and/or unit
operation(s), whereby
liquid is separated from solid by application of some force through e.g.
pressing, centrifugation,
sedimentation, decanting or the like. Commonly, a solid/liquid (sip separation
provides a liquid and
solid fraction.
In the context of the present invention, unless indicated otherwise, "%"
indicates % weight/weight
(w/w).
CA 03196334 2023- 4- 20
17
An "effective amount" of one or more isolated enzyme preparations is an amount
where
collectively the enzyme preparation used achieves sufficient solubilization of
waste to provide a
solution comprising a high percentage of sugars and other soluble degradation
products, a substrate
suitable for anaerobic digestion e.g. for the production of biogas. The
effective amount can be
determined by use of a solubilization test as described herein.
"Solubilization test" is a test applied in order to find out how much of a
given enzymatic
composition should be added to the waste for sufficient enzymatic treatment. A
solubilization test
of the selected enzyme composition on MSW model substrate can be applied to
identify an
optimum enzymatic solubilization process. The solubilization of the waste,
such as municipal solid
waste, can be determined by applying the below testing method:
Solubilization laboratory test I
A model substrate consisting of 41% mixed food waste of vegetable origin, 13%
mixed food waste
of animal origin and 46% mixed cellulosic waste is shredded, mixed and milled
several times until
homogeneous, passed through a 3 mm screen, divided into smaller portions and
stored frozen at
-18 C.
A set of pre-tared 50 mL centrifuge tubes, each containing 1.500 0.010 g TS
(Total Solids at 60 *C)
of the above-mentioned model substrate in a 50 mM Sodium acetate buffer pH
4.50 0.05, are
added various amounts of the enzyme to test (typically 5 ¨ 60 mg El'ig TS of
model substrate) for a
final total weight of 20.000 0.025 g in each tube.
The tubes are closed with tight fitting lids and the reaction mixtures are
incubated at 50 1 C for
24 hours 10 minutes with agitation by inverting the test tubes (end-over-
end) at 10.0 0.5
revolutions per minute.
Immediately after finished incubation the tubes are centrifuged at 2100 10 G
for 10 minutes, and
immediately after centrifugation (and within less than 5 minutes) the
supernatant is decanted into
another set of pre-tared tubes. The first set of tubes (including lids), with
the residual undissolved
model substrate, and the second set of tubes, with the decanted supernatant
containing the
CA 03196334 2023- 4- 20
18
solubilized model substrate, are weighed on a 4 decimal analytical balance and
then left to dry at
60 1 *C for 6 days in a well-ventilated drying cabinet.
After drying the tubes (including lids) are weighed again, the TS amounts in
pellet and supernatant
are determined and the mass balance is calculated as:
Mass balance% = ((TS pellet + TS supernatant ¨ TS Enzyme) / TS model
substrate) * 100%
The mass balance based on TS model substrate (1.500 0.010 g), to assure for
no loss of material
and proper drying, will typically be in the interval of 95-105%.
Based on the Total amount and TS amount of the decanted supernatant, TS% in
the decanted
supernatant is calculated as:
TS% = (TS decanted supernatant /Total decanted supernatant) * 100%
Finally, the solubilization is calculated as:
Solubilization% = (((TS% * Residual water / (1 - TS%)) ¨ TS Enzyme) / TS model
substrate) * 100%
By calculating solubilization based on TS% of the decanted supernatant and the
Residual water
amount (weight of decanted supernatant and residual wet pellet subtracted
weight of the empty
tubes and TS of model substrate), the liquid phase that is trapped in the
centrifugation pellet will
also be accounted for.
A graph of solubilization versus enzyme dose will show the characteristics of
enzyme efficacy
(maximum solubilization at high enzyme dosages) and enzyme potency (dose
required for obtaining
a certain level of solubilization).
¨ Enzyme efficacy may typically be 35-70% solubilization, depending on the
model substrate
composition and the enzyme composition to test. Dose in use may typically be
defined to
obtain 85-95% of the efficacy.
The current invention appears well suited for industrial applications,
including large-scale industrial
applications. In some embodiments, methods of the invention are practiced
using at least about
CA 03196334 2023- 4- 20
19
100, 200, 500 kg waste per hour. In some embodiments, at least 1, 5, 10, 15,
20, 25, 50, or 100 tons
(t) waste can be processed per h.
In the context of the present invention, the term "anaerobic digestion" is
meant to comprise
biological processes in which microorganisms break down biodegradable material
in the absence of
oxygen. One of the end products may be biogas, which can e.g. be combusted to
generate electricity
and/or heat. Biogas can also be used, either directly or after upgrading, as
renewable natural gas
and/or transportation fuels. Biogas can be injected into a natural gas and/or
biogas grid.
In the context of the present invention, the term "anaerobic digestion system"
refers to a
fermentation system comprising one or more digesters operated under controlled
aeration
conditions in which methane gas is produced in each of the reactors comprising
the system.
Methane gas is produced to the extent that the concentration of metabolically
generated dissolved
methane in the aqueous phase of the fermentation mixture within the "anaerobic
digestion system"
is saturated at the conditions used and methane gas is emitted from the
system. The "anaerobic
digestion system" may be a fixed filter system. A "fixed filter anaerobic
digestion system" refers to
a system in which an anaerobic digestion consortium is immobilized, optionally
within a biofilm, on
a physical support matrix.
The terms "fermenter" and "digester" can be used interchangeably. "Digester"
is commonly used
for anaerobic digestions, often in the context of biogas production.
Likewise, the terms "fermentation" and "digestion" can be used
interchangeably. "Digestion" is
commonly used for anaerobic digestions, often in the context of biogas
production.
In the context of the present invention, the term "digestate" or "AD effluent"
is defined as the
residual output from an anaerobic digestion (AD) used for biogas production.
Anaerobic digesters
sustainably treat organic waste from municipal, industrial, and/or
agricultural operations with
microorganisms under anaerobic conditions for production of biogas. Usually,
the "digestate" has
alkaline pH, and comprises mainly water, but also suspended solids and
dissolved matter such as
salts which may include both inorganic salts and organic salts.
CA 03196334 2023- 4- 20
"Reject water" is defined as the liquid fraction obtained after one or more
solid-liquid separations
of the AD digestate and is accordingly the term applied to denote process
water obtained from an
AD process. The one or more solid liquid separations can comprise one or more
of decantation,
centrifugation, filtering, flocculation, pressing and sedimentation. Like the
AD digestate, reject
water has an alkaline pH, and comprises dissolved matter, such as salts which
may include both
inorganic salts and organic salts. Reject water may also comprise some
suspended matter and live
microorganisms from the AD process. Such water may be subject to hygienization
and/or other
purification steps in accordance with national requirements e.g. Animal
Byproduct legislation (the
APB) prior to being released from the AD plant.
"Wash water" is defined as any water stream used for washing of any solid
fraction obtained after
solid-liquid separation of the bioreactor effluent. Examples of wash water are
water used for
washing of the 2D fraction (flat materials such as textiles, plastic film,
undigested cardboard) and/or
the 3D fraction (metals and solid plastic), the wash water used for washing
the 2D and 3D fractions
may be the reject water from dewatering the digestate, as illustrated in
figure la. However, the
spent wash water from the washing units may be used as process water in the
bioreactors. As this
water is of low pH and contain the washed-off organics and left-over enzymes,
the pH adjusting acid
consumption, the bioreactor process and e.g. biogas yield will benefit from
the re-use of spent wash
water in the bioreactors. Other examples of wash water are water used for
washing of inerts, metals
and/or plastics. Wash water can in one embodiment be diluted bioliquid.
"Water from external sources" includes water obtained from any source wherein
said water has
not previously been subjected to any steps in an enzymatic and/or microbial
waste treatment
process. Thus, water from external sources comprise tap water, wastewater that
has not been
subjected to an enzymatic and/or microbial waste treatment process, and water
from natural
sources.
"Water from natural sources" is water obtained from natural sources such as
rivers, lakes and
ponds.
CA 03196334 2023- 4- 20
21
"Hygienization" refers to a process that reduces certain microbial activity.
Various hygienization
processes applied by national or regional authorities for reducing or
eliminating the microbial
content in waste is for example described and compared in Liu X., Lendormi T.,
Lanoiselle1.-L., 2018,
A review of hygienization technology of biowastes for anaerobic digestion:
effect on pathogen
inactivation and methane production, Chemical Engineering Transactions, 70,
529-534.
The term "pH-adjusted process water" is meant to comprise process water after
a pH adjustment
step, usually after addition of acid to provide a less alkaline pH.
"Process water" Process water may comprise water that is recycled from an
industrial process,
wherein e.g. waste undergo an enzymatic and/or microbial treatment, such as a
process according
to the present invention including wash water, reject water and bioliquid.
Process water is of lower
quality than drinking water such as in terms of e.g. any one of organic and/or
inorganic salt(s),
microbial organisms / plate counts, suspended solids, DM, and/or pH, including
any combination
thereof. Process water may be adjusted in terms of mineral/salt content, pH
and the like. Process
water includes bioliquid, reject water and wash water as described above. In
one embodiment
process water is water that is not external water, e.g. tap water.
"Tap water" is defined as any type of fresh water including municipal water,
water from rainwater-
collecting cisterns, water from village pumps or town pumps and water carried
from streams, rivers,
or lakes.
In the context of the present invention, the term "lactic acid producing
bacteria" comprises both
bacteria of the lactic acid bacteria order "LAB" where the currently accepted
taxonomy is based on
the List of Prokaryotic names with Standing in Nomenclature (LPSN) - an online
database that
maintains information on the naming and taxonomy of prokaryotes, following the
taxonomy
requirements and rulings of the International Code of Nomenclature of
Bacteria. The phylogeny of
the order is based on 16S rRNA-based LTP release 106 by 'The All-Species
Living Tree' Project. In
addition to bacteria belonging to the LAB order, the term "lactic acid
producing bacteria" used
CA 03196334 2023- 4- 20
22
herein also comprises bacteria that do not belong to the LAB order, but that
are nevertheless
capable of producing lactic acid.
"Solubles" refers to the degradation products obtained from the enzymatic
and/or microbial
treatment of waste, sometimes referred to as microbial metabolites. The
solubles are accordingly
present in the bioliquid and normally is a mixture of substrates such as
protein, fat, galactose,
mannose, glucose, xylose, arabinose, lactate, acetate, ethanol and/or other
components,
depending on the composition of the waste (the components such as protein and
fat can be in a
soluble and/or insoluble form). The solubles / microbial metabolites provide a
substrate for gas
production, a substrate suitable for anaerobic digestion e.g. for the
production of biogas.
"Waste" comprises, sorted and unsorted, municipal solid waste (MSW),
agriculture waste, hospital
waste, industrial waste, e.g., waste fractions derived from industry such as
restaurant industry, food
processing industry, general industry; waste fractions from paper industry;
waste fractions from
recycling facilities; waste fractions from food or feed industry; waste
fraction from the medicinal or
pharmaceutical industry; waste fractions from hospitals and clinics, waste
fractions derived from
agriculture or farming related sectors; waste fractions from processing of
sugar or starch rich
products; contaminated or in other ways spoiled agriculture products such as
grain, potatoes and
beets not exploitable for food or feed purposes; or garden refuse.
"Municipal solid waste" (MSW) refers to waste fractions which are typically
available in a city, but
that need not come from any municipality per se, i.e., MSW refers to every
solid waste from any
municipality but not necessarily being the typical household waste ¨ could be
waste from airports,
universities, campus, canteens, general food waste, among others. MSW may be
any combination
of one or more of cellulosic, plant, animal, plastic, metal, or glass waste
including, but not limited
to, any one or more of the following: Garbage collected in normal municipal
collections systems,
optionally processed in a central sorting, shredding or pulping device, such
as e.g., a Dewaster or
a reCulture ; solid waste sorted from households, including both organic
fractions and paper rich
fractions; Generally, municipal solid waste in the Western part of the world
normally comprise one
or more of: animal food waste, vegetable food waste, newsprints, magazines,
advertisements,
CA 03196334 2023- 4- 20
23
books, office paper, other clean paper, paper and carton containers, other
cardboard, milk cartons
and alike, juice cartons and other carton with alu-foil, kitchen tissues,
other dirty paper, other dirty
cardboard, soft plastic, plastic bottles, other hard plastic, non-recyclable
plastic, yard waste, flowers
etc., animals and excrements, diapers and tampons, cotton sticks etc., other
cotton etc., wood,
textiles, shoes, leather, rubber etc., office articles, empty chemical
bottles, plastic products,
cigarette buts, other combustibles, vacuum cleaner bags, clear glass, green
glass, brown glass, other
glass, aluminium containers, alu-trays, a lu-foil (including tealight candle
foil), metal containers (-Al),
metal foil (-Al), other sorts of metal, soil, rocks, stones and gravel,
ceramics, cat litter, batteries
(button cells, alkali, thermometers etc.), other non-combustibles and fines.
The word "Renescience" refers to a company under Orsted, a Danish founded
provider of energy
solutions. Renescience applies an enzymatic and/or microbial waste processing
technology for
treating waste comprising organic matter and turning the liquefied organics
from the waste into
energy.
DETAILED DESCRIPTION
With the present invention, it has surprisingly been found that process water
such as bioliquid, wash
water and reject water obtained from AD processes, can be re-used as a source
of water in a
bioreactor wherein waste is subject to enzymatic and/or microbial degradation.
This reduces the
amount of water otherwise required in the enzymatic and/or microbial
degradation of the waste. It
is shown herein that the process water need not necessarily be subject to
hygienization prior to
being added into a bioreactor. If the process water e.g. reject water has been
subjected to
hygienization, this water, which is about 70 C, may preferably be recycled and
mixed with the waste
prior to liquefaction. In order for the liquefaction process to proceed
continuously in the bioreactor,
it has been found that the pH in the bioreactor is preferably within pH 2 to
6.5, such as between 3.5
and 6, or between 3 and 5. The pH in the bioreactor can be monitored
continuously or
discontinuously. Thus, in one embodiment the pH of the reject water is
adjusted to between 3.5
and 6 by addition of acid and/or by reducing the ammonium content The process
water may be
added together with or mixed with water from external sources such as tap
water/purified water.
CA 03196334 2023- 4- 20
24
It is shown herein that during combined enzymatic and microbial treatment of
waste with addition
of tap water (pH 7) and without any regulation of the pH in the bioreactor,
acidification is observed
during the first 24 hours. It was found that the acidification in these
experiments were mainly due
to the formation of lactic acid. After about 24 hours pH was found to drop to
about 4.8 and the
production rate of lactic acid decreased significantly. After 48hrs the
production of lactate halted.
It was found herein that the pH in the bioreactor wherein the pH had
stabilised at around 4.8
increased when reject water from an AD process was added to the bioreactor.
This increase in pH
had a negative impact on the enzymatic and/or microbial liquefaction of the
waste and led to
bioliquid with fewer organic acids and hence of a poorer quality for further
processing into valuable
energy sources. As also shown herein, several smaller additions of reject
water, waste and enzymes
was found to be a faster way to achieve the pH of around 4.8. Without being
bound by a specific
theory, it is believed that faster acidification was achieved by adding
smaller additions of reject
water and is the result of mainly two factors: 1) there is already an
established lactic acid community
when the reject water is added. 2) the presence of bioliquid with low pH
limits the pH increase due
to the addition of the reject water, which in turn allows the enzymes to
function more efficiently in
the conversion of the added IVISW. Accordingly, in a preferred embodiment of
the invention the
process water is added in batches to keep pH within pH 3.5-6 if no adjustment
of the pH of the
process water is made prior to adding it into the bioreactor, in order to
provide a continued
production of solubles.
The reject water from dewatering digestate mainly comprises archaea when it is
entered into the
bioreactor without prior hygienization. This is not a problem for the
liquefaction process if the lactic
acid bacteria can outcompete the archaea population entering the bioreactor.
It is shown here that
when reject water is entered into the bioreactor, the lactic acid population
declines but
subsequently re-establishes whereas the archaea population declines steadily
upon entry into the
bioreactor. Thus, the use of non-hygienized process water such as reject
water, comprising e.g.
archaea, does not inhibit the liquefaction process.
CA 03196334 2023- 4- 20
In conclusion, the disclosed examples demonstrate that process water, such as
reject water which
is a waste product from anaerobic digestion comprising, inter alia, a
significant amount of salts and
archaea, can be used successfully instead of external water, in the enzymatic
and/or microbial
conversion of organic matter in waste such as carbohydrate(s) to solubles such
as organic acids, e.g.
lactic acid, acetic acid and succinic acid. Consequently, it is now possible
to conduct an enzymatic
and/or microbial treatment of waste on a commercial scale, where a significant
fraction of the water
is recycled, thus reducing the overall water consumption of the process.
The method of the invention is a method for continuous or batch processing of
waste comprising:
a) subjecting waste to an enzymatic and/or microbial treatment in a bioreactor
b) subjecting the treated waste from step a) to one or more separation
step(s),
whereby a bioliquid and a solid fraction is provided;
c) subjecting said bioliquid and/or solid fraction to downstream processing
providing
process water;
d) adding the process water obtained from step c) and optionally water from an
external water source to the bioreactor in step a).
The method according to the first aspect of the present invention can be
performed within a single
waste processing plant comprising one or more bioreactors and/or one or more
downstream
processing steps such as AD digesters that are part of the same waste
treatment loop; or the process
water may be obtained from one or more different and possibly independent
waste processing
and/or biogas production sites.
Step a)
The enzymatic and/or microbial treatment of the waste in step a) is performed
in a reactor, here
denoted a bioreactor. The treatment is performed by adding one or more enzymes
and by the
bacteria present in the waste. Optionally, standard, cultivated, or
manipulated yeast, bacteria, or
any other microorganism capable of converting the organic matter present in
the waste into
compositions suitable for subsequent biogas production in an anaerobic
digestion process may be
added to the bioreactor. The enzymes are supplied in either native form or in
form of microbial
CA 03196334 2023- 4- 20
26
organisms expressing the enzymes. Depending on the dry matter content of the
waste, water will
usually have to be added to the process. In the context of the present
invention at least part of such
added water will be recycled water e.g. process water.
The enzymatic and/or microbial treatment in step a) may be performed by adding
one or more
enzymes, supplied in either native form and/or in form of microbial organisms
giving rise to the
expression of such enzymes; and/or by the bacteria present in the waste and/or
optionally by adding
standard, cultivated, or manipulated yeast, bacteria, or any other
microorganism capable of
converting the organic matter present in the waste into organic acids or other
compositions, such
as lactic acid, 3-hydroxypropionic acid (3-HPA), 1,4-butanediol (BDO),
butanedioic acid (succinic
acid), ethane-1,2-diol (ethylene glycol), butanol or 1,2-propanediol
(propylene glycol), suitable for
subsequent biogas production in an anaerobic digestion process.
In one embodiment, the method of the invention is a method wherein said
enzymatic and/or
microbial treatment in step a) is performed by adding enzymes, supplied in
either native form or in
form of microbial organisms giving rise to the expression of such enzymes,
and/or by the bacteria
present in the waste and optionally by adding standard, cultivated, or
manipulated yeast, bacteria,
or any other microorganism capable of producing biochemicals, ethanol, or
biogas.
Microorganisms that may be added to the bioreactor in step a) include yeasts,
and/or fungi and/or
bacteria.
Other microorganisms that may be added to the bioreactor in step a) include
bacteria that can
efficiently ferment hexose and pentose including but not limited to
cellobiose, glucose, xylose and
arabinose to short chain organic acids including but not limited to citric
acid, lactic, formic acid,
acetic acid, butyric acid, valeric acid, isovaleric acid and propionic acid as
well as alcohols including
but not limited to ethanol.
Other microorganisms that may be added to the bioreactor in step a) include
fermenting organisms
such as Bacillus sp., e.g. Bacillus coagulans; Candida sp., such as C.
sonorensis, C. methanosorbosa,
CA 03196334 2023- 4- 20
27
C. diddensiae, C. parapsilosis, C. naedodendra, C. blankii, C. entomophilia,
C. brassicae, C.
pseudotropicalis, C. boidinii, C. utilis, and C. scehatae; Clostridium sp.,
such as C. acetobutylicum, C.
thermocellum, and C. phytofermentans; Escherichia sp., such as E. coll.,
especially E. coil strains that
have been genetically modified to improve the yield of ethanol, bioethanol or
lactic acid; Geobacillus
sp.; Hansenula sp., such as Hansenula anomala; Klebsiella sp., such as K.
oxytoca; Kluyveromyces
sp., such as K. marxianus, K. lactis, K. thermotolerans, and K. fragilis;
Schizosaccharomyces sp., such
as S. pombe; Thermoanaerobacter sp., such as Thermoanaerobacter
saccharolyticum; and
Zymomonas sp., such as Zymomonas mobil/s. Lactobacillus sp., e.g.
Lactobacillus delbrueckii subsp.
Bulgaricus, Lactobacillus ultunensis, Lactobacillus senmaizukei, Lactobacillus
equicursoris,
Lactobacillus tucceti, Lactobacillus bra ntae, Lactobacillus parakefiri,
Lactobacillus crispatus,
Lactobacillus intermedius, Lactobacillus mucosae, Lactobacillus agili,s
Lactobacillus equi,
Lactobacillus delbrueckii, Lactobacillus frumenti, Lactobacillus letivazi,
Lactobacillus thailandensis,
Lactobacillus helveticus, Lactobacillus apis, Lactobacillus acidifarinae,
Lactobacillus gallinarum,
Lactobacillus kalixensis, Lactobacillus hayakitensis Lactobacillus gastricus,
Lactobacillus
homohiochii, Lactobacillus guizhouensis, Lactobacillus intestinalis,
Lactobacillus hilgardii,
Lactobacillus iners, Lactobacillus brevis, Lactobacillus fermentum,
Lactobacillus or/s. Lactobacillus
coleohominis, Lactobacillus panis, Lactobacillus acidophilus, Lactobacillus
ruminis, Lactobacillus
suebicus, Lactobacillus pobuzihil, Lactobacillus similis, Lactobacillus
rhamnosus, Lactobacillus
manihotivorans, Lactobacillus nodensis, Lactobacillus aviaries, Lactobacillus
vagina/is, Lactobacillus
namurensis, Lactobacillus rossiae, Lactobacillus buchneri, Lactobacillus
jensenii, Lactobacillus
parabrevis, Lactobacillus equigenerosi, Lactobacillus oligofermentans,
Lactobacillus farciminis,
Lactobacillus johnsonii, Lactobacillus parabuchneri, Lactobacillus
parabuchneri, Lactobacillus
hamsteri, Lactobacillus pentosus, Lactobacillus bobalius, Lactobacillus
Alimentarius, Lactobacillus
crustorum, Lactobacillus pontis, Lactobacillus salivarius, Lactobacillus
taiwanensis Lactobacillus
antri, Lactobacillus siliginis, Lactobacillus kitasatonis, Lactobacillus
camelliae, Lactobacillus
secaliphilus, Lactobacillus ingluviei, Pediococcus spp., Fructobacillus
pseudoficulneus, Lactobacillus
gigeriorum, Pediococcus sp., e.g. Pediococcus argentinicus, Pediococcus
stilesii, Pediococcus
cellicola, Streptococcus spp., Alkalibacterium spp., Leuconostoc spp.,
Enterococcus spp.,
Tetragenococcus sp., e.g. Tetragenococcus doogicus, Weissella spp.,
Streptococcus fryi, Oenococcus
spp., Enterococcus cecorum, Vagococcus teuberi, Streptococcus bovis,
Lactobacillus faeni,
CA 03196334 2023- 4- 20
28
Pediococcus acidilactici, Leuconostoc camosum, Lactobacillus japonicus,
Trichococcus spp.,
Weissella minor, Weissella salipiscis, Facklamia, Vagococcus, Enterococcus
camelliae, Streptococcus
infantarius, Aerococcus viridans, Lactococcus fujiensis, Alkalibacterium
subtropicum, Weissella
viridescens, Lactobacillus amylolyticus, Facklamia tabacinasalis,
Streptococcus dentirousetti,
Streptococcus vestibularis, Desemzia incerta, Pediococcus parvulus,
Streptococcus dentapri,
Granulicatella elegans, Enterococcus columbae, Aerococcus urinaeequi,
Pediococcus siamensis,
Weissella soli, Aerococcus spp., Enterococcus rotate, Streptococcus miller,
Camobacterium
inhibens, Streptococcus ursoris, Desemzia spp., Vagococcus penaei,
Streptococcus castoreus,
Enterococcus asini, Enterococcus lactis, Weissella paramesenteroides,
Melissococcus spp.,
Vagococcus fluvialis, Lactobacillus versmoldensis, Streptococcus gallinaceus,
Enterococcus
hawaiiensis, Leuconostoc palmae, Pediococcus inopinatus, Tetragenococcusspp.,
Facklamia
languida, Lactococcus spp., Abiotrophia defective, Weissella thailandensis,
Facklamia hominis,
Lactobacillus paracasei, Streptococcus halichoeri, Streptococcus equinus,
Enterococcus gilvus,
Enterococcus inusitatus, Streptococcus alactolyticus, Enterococcus
aquimarinus, Camobacterium
mobile, Streptococcus parasanguinis, Streptococcus tigurinus, Streptococcus
luteciae, Granulicatella
adiacens, Lactococcus sp., e.g. Lactococcus raffinolactis, Carnobacterium
maltaromaticum,
Enterococcus avium, Streptococcus peroris, Streptococcus plurextorum,
Lactobacillus
vaccinostercus, Streptococcus troglodytae, Tetragenococcus solitaries,
Weissella hanii,
Camobacterium spp., Lactococcus garvieae, Lactococcus lactis, Streptococcus
lactarius,
Marinilactibacillus psychrotolerans, Camobacterium funditum, Leuconostoc
mesenteroides,
Leuconostoc pseudomesenteroides, Enterococcus haemoperoxidus, Enterococcus
gallinarum,
Enterococcus italicus, Aerococcus christensenii, Streptococcus didelphis,
Streptococcus orisratti,
Alkalibacterium iburiense, Lactobacillus collinoides, Trichococcus
flocculiformis, Aerococcus
sanguinicola Lactobacillus amylovorus, Leuconostoc gelidum, Leuconostoc
gasicomitatum,
Granulicatella spp., Leuconostoc kimchi, Leuconostoc argentinum, Streptococcus
sanguinis,
Streptococcus pseudopneumoniae, Weiss&la koreensis, Fructobacillus spp.,
Leuconostoc garlicum,
Weissella ciboria, Leuconostoc citreum, Lactobacillus zymae, Fructobacillus
fructosus, Leuconostoc
inhae, Lactobacillus reuteri, Lactobacillus hammesii, Lactobacillus nantensis,
Lactobacillus
paralimentarius, Streptococcus thermophilus, Leuconostoc lactis, Weissella
confuse, Lactobacillus
acetotolerans, Lactobacillus otakiensis, Fructobacillus ficulneus,
Lactobacillus kefiri, Lactobacillus
CA 03196334 2023- 4- 20
29
zeae, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus pantheris,
Marinilactibacillus
piezotolerans, Lactobacillus acidipiscis, Lactobacillus malefermentans,
Lactobacillus gasseri,
Lactobacillus parafarraginis, Carnobacterium gallinarum, Vagococcus
camiphilus, Streptococcus
parauberis, Lactobacillus sanfranciscensis, Carnobacterium divergens,
Streptococcus oralis,
Streptococcus infantis, Enterococcus casseliflavus, Streptococcus
oligofermentans, Lactobacillus
kefiranofaciens, Streptococcus australis, Pediococcus claussenii,
Alkalibacterium psychrotolerans,
Enterococcus durans, Vagococcus salmoninarum, Vagococcus lutrae, Enterococcus
faecalis
Carnobacterium viridans, Lactobacillus kisonensis, Pediococcus pentosaceus,
Enterococcus mundtii,
Enterococcus sulfureus, Enterococcus silesiacus, Lactobacillus kimchi,
Fructobacillus tropaeol,i
Abiotrophia spp., Streptococcus anginosus, Pediococcus ethanolidurans.
The fermenting microorganisms may have been genetically modified to provide
the ability to
ferment pentose sugars, such as xylose utilizing, arabinose utilizing, and
xylose and arabinose co-
utilizing microorganisms.
The fermenting organisms may comprise one or more polynucleotides encoding one
or more
cellulolytic enzymes, hemicellulolytic enzymes, and accessory enzymes
described herein.
The microorganisms present in the waste or added to the bioreactor, may
produce fermentable
sugars and organic acid or other compositions, such as lactic acid, 3-
hydroxypropionic acid (3-HPA),
1,4-butanediol (BDO), butanedioic acid (succinic acid), ethane-1,2-diol
(ethylene glycol), butanol or
1,2-propanediol (propylene glycol), that may be used as feed in a subsequent
anaerobic digestion
process. These organic acids or other compositions further include acetate,
propionate and
butyrate. Waste that is suitable for treatment normally comprises, at least,
lactic acid producing
bacteria.
When microorganisms are added and/or the waste is inoculated prior to the
enzymatic and/or
microbial degradation in step a) one or more species of lactic acid producing
bacteria can be used.
It will be readily understood by one skilled in the art that a bacterial
preparation used for inoculation
may comprise a community of different organisms. One or more naturally
occurring bacteria which
CA 03196334 2023- 4- 20
exist in any given geographic region and which are adapted to thrive in waste,
such as MSW, from
that region, can be used. As is well known in the art, lactic acid producing
bacteria are ubiquitous
and will typically comprise a major component of any naturally occurring
bacterial community
within waste, such as MSW.
In a preferred embodiment, the microbial treatment in step a) is performed by
a microbial
composition wherein the majority of the living microorganisms are lactic acid
producing bacteria
including e.g. Bacillus coagulans.
The microbial treatment in step a) may be performed by a microbial composition
wherein at least
10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%,
85%, 90%, 95% or
99% of the living microorganisms are lactic acid producing bacteria.
The treatment step a) may comprise contacting the waste with live lactic acid
bacteria and/or other
microorganisms including fermenting organisms such as Bacillus coagulans
concentration of
approximately 1x105CFU/ml, 1x106CFU/ml, 1x107CFU/ml, 9x107CFU/m11.0x109 or
1.0x109CFU/ml.
When these microorganisms are added to the waste, these should be added at a
concentration of
at least 1x105CFU/ml, 1x106CFU/ml, 1x107CFU/ml, 9x107CFU/ml, 1.0x108, 1.0x109
or
1.0x1019CFU/ml. In a preferred embodiment these microorganisms are added to
and/or present in
the waste at a concentration of 1x106CFU/ml to 9x106CFU/ml. In a preferred
embodiment these
microorganisms are already present in the waste and no additional
microorganisms are added.
In one embodiment of the method of the invention, the treatment in step a)
comprises contacting
the waste with a live lactic acid bacteria concentration of at least 1.0 x
106, 1.0 x 107, 1.0 x 108 or 1.0
x 109 CFU/L.
In one embodiment of the method of the invention, the treatment in step a)
comprises adding
microorganisms to the waste at a concentration of 1.0 x 106, 1.0 x 107, 1.0 x
108, 1.0 x 109 or 1.0 x
1019CFU/L.
CA 03196334 2023- 4- 20
31
The treatment step a) may comprise addition of cellulase activity by
inoculation with one or more
microorganism(s) that exhibits extracellular cell ulase activity.
In step a) the waste may be treated with an enzyme composition. Suitable
enzyme compositions
are well known in the art and are commercially available e.g. such as
cellulolytic background
composition.
In order to find out how much enzyme of a given enzymatic composition may be
added, a
solubilization test of the enzyme composition on model waste may be applied to
provide an
optimum enzymatic solubilization process.
It is not only the amount of enzymes to be added at the initiation of the
process that can be
determined by a solubilization test. A solubilization test can also be applied
when determining the
total enzymatic performance including enzyme optionally added to the
bioreactor in step d) as being
comprised in the process water. Regardless of whether the enzymes are added as
fresh enzymes to
the waste or as "re-usable" enzymes comprised in process water or as mixtures
of fresh and re-used
enzymes, the enzyme efficacy in the reactor may typically be 35-70%
solubilization of model
substrate in the laboratory assay, depending on the model substrate
composition and the enzyme
composition being tested.
When added to the process the cellulolytic background composition (CBC) may
comprise a
commercial cellulolytic enzyme preparation. Examples of commercial
cellulolytic enzyme
preparations suitable for use in the method according to the present invention
include but is not
limited to, for example, CELLIC CTec (Novozymes A/S), CELLIC CTec2
(Novozymes A/S), CELLIC
CTec3 (Novozymes A/S), CELLUCLAST (Novozymes A/S), NOVOZYMTm 188 (Novozymes
A/S),
SPEZYMETm CP (Genencor Int.), ACCELLERASETM TRIO (DuPont), FILTRASE NL (DSM);
METHAPLUS
S/L 100 (DSM), ROHAMENTIm 7069 W (Rohm GmbH), or ALTERNAFUEL CMAX3Tm (Dyadic
International, Inc.).
CA 03196334 2023- 4- 20
32
When the enzyme composition comprises further enzymatic activity apart from
the activities
present in the CBC, such enzyme activity may be added from individual sources
or together as part
of enzyme blends. Suitable blends include but are not limited to the
commercially available enzyme
compositions Cellulase PLUS, Xylanase PLUS, BrewZyme LP, FibreZyme G200 and
NCE BG PLUS from
Dyadic International (Jupiter, FL, USA) or Optimash BG from Genencor
(Rochester, NY, USA).
The CBC may comprise the following enzymatic activities:
Cellobiohydrolase I:
Endo-1,4-beta-glucanase
Beta-glucosidase
Endo-1,4-beta-xylanase
Beta-xylosidase
Beta-L-arabinofuranosidase
Amyloglocosidase
Alpha-amylase
Acetyl xyl a n esterase
The cellulolytic enzyme preparation may be added in an amount effective from
about 0.5 to about
10 wt. % of solids, e.g., about 1 to about 5 wt. % of solids when the waste
has a composition
corresponding to the MSW model substrate (41% of fraction I (food waste of
plant origin), 13% of
fraction II (food waste of animal origin) and 46% of fraction III (cartons,
paper, wood and textiles)),
which mimics the organic fraction of MSW from Northern European households. In
MSW from other
geographic areas, the composition of the MSW may not match the model substrate
and in such
case, the enzymatic performance should be tested on suitable model substrate
by applying solubility
test such as the test disclosed herein order to identify the amount of enzyme
required to obtain
sufficient solubilization.
Other enzymes which may be added e.g. in addition to the CBC are listed below.
Examples of bacterial endoglucanases that can be used in the enzymatic
degradation processes in
step a) include, but are not limited to one or more of: Acidothermus
cellulolyticus endoglucanase
(WO 91/05039; WO 93/15186; U.S. Patent No. 5,275,944; WO 96/02551; U.S. Patent
No. 5,536,655;
CA 03196334 2023- 4- 20
33
WO 00/70031; WO 05/093050), Erwinia carotovara endoglucanase (Saarilahti et
al., 1990, Gene 90:
9-14), Thermobifida fusca endoglucanase Ill (WO 05/093050), and Thermobifida
fusca
endoglucanase V (WO 05/093050).
Examples of fungal endoglucanases that can be used in the enzymatic
degradation process in step
a) include, but are not limited to one or more of: Trichoderma reesei
endoglucanase I (Penttila et
al., 1986, Gene 45: 253-263, Trichoderma reesei Cel7B endoglucanase I
(GenBank:M15665),
Trichoderma reesei endoglucanase II (Saloheimo et al., 1988, Gene 63:11-22),
Trichoderma reesei
Cel5A endoglucanase II (GenBank:M19373), Trichoderma reesei endoglucanase III
(Okada et al.,
1988, Appl. Environ. Microbial. 64: 555-563, GenBank:AB003694), Trichoderma
reesei
endoglucanase V (Saloheimo et al., 1994, Molecular Microbiology 13: 219-228,
GenBank:Z33381),
Aspergillus aculeatus endoglucanase (0oi et al., 1990, Nucleic Acids Research
18: 5884), Aspergillus
kawachii endoglucanase (Sakamoto et al., 1995, Current Genetics 27: 435-439),
Fusarium
oxysporum endoglucanase (GenBank:L29381), Humicola grisea var. thermoidea
endoglucanase
(GenBank:AB003107), Melanocarpus albomyces endoglucanase (GenBank:MAL515703),
Neurospora crassa endoglucanase (GenBank:XM_324477), Humicola insoiens
endoglucanase V.
Myceliophthora thermophila CBS 117.65 endoglucanase, Thermoascus aurantiacus
endoglucanase
I (GenBank:AF487830), Trichoderma reesei strain No. VTT-D-80133 endoglucanase
(GenBank:M15665), and Penicillium pinophilum endoglucanase (WO 2012/062220)
and
endoglucanases produced by Aspergillus niger.
Examples of cellobiohydrolases that can be used in the enzymatic degradation
processes in step a)
include, but are not limited to one or more of: Aspergillus aculeatus
cellobiohydrolase II (WO
2011/059740), Aspergillus fumigatus cellobiohydrolase I (WO 2013/028928),
Aspergillus fumigatus
cellobiohydrolase II (WO 2013/028928), Chaetomium thermophilum
cellobiohydrolase I,
Chaetomium thermophilum cellobiohydrolase II, Humicola insolens
cellobiohydrolase I,
Myceliophthora thermophila cellobiohydrolase II (WO 2009/042871), Penicillium
occitanis
cellobiohydrolase I (GenBank:AY690482), Talaromyces emersonii
cellobiohydrolase I
(GenBank:AF439936), Thielavia hyrcanie cellobiohydrolase II (WO 2010/141325),
Thielavia
terrestris cellobiohydrolase II (CEL6A, WO 2006/074435), Trichoderma reesei
cellobiohydrolase I,
CA 03196334 2023- 4- 20
34
Trichoderma reesei cellobiohydrolase II, and Trichophaea saccata
cellobiohydrolase II (WO
2010/057086).
Examples of beta-glucosidases that can be used in the enzymatic degradation
processes in step a)
include, but are not limited to one or more of: beta-glucosidases from
Aspergillus aculeatus
(Kawaguchi et al., 1996, Gene 173: 287-288), Aspergillus fumigatus (WO
2005/047499), Aspergillus
niger (Dan et al., 2000, J. Biol. Chem. 275: 4973-4980), Aspergillus oryzae
(WO 02/095014),
Penicillium brasilianum IBT 20888 (WO 2007/019442 and WO 2010/088387),
Thielavia terrestris
(WO 2011/035029), and Trichophaea saccata (WO 2007/019442).
Other useful endoglucanases, cellobiohydrolases, and beta-glucosidases are
disclosed in numerous
Glycosyl Hydrolase families using the classification according to Henrissat,
1991, Biochem. J. 280:
309-316, and Henrissat and Bairoch, 1996, Biochem. J. 316: 695-696.
Any "Auxiliary Activity 9 polypeptide" or "AA9" polypeptide can be used as a
component of the
enzyme composition.
Examples of AA9 polypeptides that can be used in the enzymatic degradation
processes in step a)
include, but are not limited to one or more of: AA9 polypeptides from
Thielavia terrestris (WO
2005/074647, WO 2008/148131, and WO 2011/035027), Thermoascus aurantiacus (WO
2005/074656 and WO 2010/065830), Trichoderma reesei (WO 2007/089290 and WO
2012/149344), Myceliophthora thermophila (WO 2009/085935, WO 2009/085859, WO
2009/085864, WO 2009/085868, and WO 2009/033071), Aspergillus fumigatus (WO
2010/138754),
Penicillium pinophilum (WO 2011/005867), Thermoascus sp. (WO 2011/039319),
Penicillium sp.
emersoni (WO 2011/041397 and WO 2012/000892), Thermoascus crustaceous (WO
2011/041504),
Aspergillus aculeatus (WO 2012/125925), Thermomyces lanuginosus (WO
2012/113340, WO
2012/129699, WO 2012/130964, and WO 2012/129699), Aurantiporus alborubescens
(WO
2012/122477), Trichophaea saccata (WO 2012/122477), Penicillium thomii (WO
2012/122477),
Talaromyces stipitatus (WO 2012/135659), Humicola insolens (WO 2012/146171),
Malbranchea
cinnamomea (WO 2012/101206), Talaromyces leycettanus (WO 2012/101206), and
Chaetomium
CA 03196334 2023- 4- 20
thermophilum (WO 2012/101206), and Talaromyces thermophilus (WO 2012/129697
and WO
2012/130950).
Examples of proteases that can be used in the enzymatic degradation processes
in step a) may be
derived from the genus Bacillus, such as e.g. Bacillus amyloliquefaciens such
as e.g. the protease
encoded by SEQ ID NO:1 as disclosed in W017076421, or a protease having at
least 60%, e.g., at
least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 91%, at least
92%, at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at
least 98%, at least 99%,
or 100% sequence identity to SEQ ID NO: 1 as disclosed in W017076421.
Examples of lipases that can be used in the enzymatic degradation processes in
step a) may be
derived from the genus Thermomyces sp. such as e.g. Thermomyces lanuginosus
such as e.g. the
lipase encoded by SEQ ID NO: 2 as disclosed in W017076421 (or a lipase having
at least 60%, e.g.,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 91%, at least
92%, at least 93%, at least 94%, at least 95%, at least 96%, at least 97%, at
least 98%, at least 99%,
or 100% sequence identity to SEQ ID NO: 2 as disclosed in W017076421) or a
lipase derived from
the genus Humicola sp. such as e.g. Humicola insolens.
Examples of beta-glucanases that can be used in the enzymatic degradation
processes in step a)
may be derived from a member of the genus Aspergillus such as e.g. Aspergillus
aculeatus such as
e.g. the beta-glucanase encoded by the sequence encoded by SEQ ID NO: 4 as
disclosed in
W017076421 or homologs thereof (e.g., a beta-glucanase having at least 60%,
e.g., at least 65%, at
least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
91%, at least 92%, at least
93%, at least 94%, at least 95%, at least 96%, at least 97%, at least 98%, at
least 99%, or 100%
sequence identity to SEQ ID NO: 4 as disclosed in W017076421).
Examples of pectate 'stases that can be used in the enzymatic degradation
processes in step a) may
form part of a multicomponent enzyme composition comprising pectate lyase,
xylanase and
cellulase activities such as e.g. Novozym 81243TM
CA 03196334 2023- 4- 20
36
Examples of mannanases that can be used in the enzymatic degradation processes
in step a) may
be an amylase may be an alpha-amylase derived from the genus Rhizomucor such
as e.g. Rhizomucor
push/us such as e.g. the alpha-amylase encoded by SEQ ID NO: 5 as disclosed in
W017076421or
homologs thereof (e.g., an alpha-amylase having at least 60%, e.g., at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 91%, at least
92%, at least 93%, at least
94%, at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or
100% sequence identity
to SEQ ID NO: 5 as disclosed in W017076421).
The enzymatic treatment of the biodegradable parts of the waste optionally
concurrently with
microbial fermentation according to step a) may be performed at a temperature
above 20 C and up
to 75 C resulting in liquefaction and/or saccharification of biodegradable
parts of the waste and
accumulation of sugars and other soluble degradation products.
The method according to treatment step a) may be performed at a temperature
between 20 and
75 C, 30 C and 70 C, 40 C and 65 C, 45 C and 65 C.
In a preferred embodiment of the method of the invention, the treatment step
a) is performed at a
temperature between 20 and 75 C, 30 C and 70 C, 40 C and 60 C, 45 and 55 C, or
around 50 C.
One preferred temperature range is the optimal temperature for most current
suitable enzyme
compositions, that is between 50 C - 56 C, such as 53 C. Another preferred
temperature range is
the temperature optimum of other suitable enzyme compositions, that is between
55 C - 57 C.
It can be advantageous to adjust the temperature of the waste such as MSW
prior to initiation of
enzymatic treatment. As is well known in the art, cellulases and other enzymes
typically exhibit an
optimal temperature range. While examples of enzymes isolated from extreme
thermophilic
organisms are certainly known, having optimal temperatures on the order of 60
C or even 70 C,
enzyme optimal temperature ranges typically fall within the range 35 C to 55
C. Enzymatic
treatment may be conducted within the temperature range 30 C to 35 C, or 35 C
to 40 C, or 40 C
CA 03196334 2023- 4- 20
37
to 45 C, or 45 C to 50 C, or 50 C to 55 C, or 55 C to 60 C, or 60 C to 65 C,
or 65 C to 70 C, or 70 C
to 75 C.
As used herein, the temperature to which waste such as MSW is heated is the
highest average
temperature of waste such as MSW achieved within the reactor. The highest
average temperature
may not necessarily be maintained for the entire period. The heating reactor
may comprise different
zones such that heating occurs in stages at different temperatures. Heating
may be achieved using
the same reactor in which enzymatic treatment is conducted. The object of
heating is simply to
render the majority of cellulosic waste and a substantial fraction of the
plant waste in a condition
optimal for enzymatic treatment. To be in a condition optimal for enzymatic
treatment, waste
should ideally have a temperature and water content appropriate for the enzyme
activities used for
enzymatic treatment.
It can be advantageous to agitate during heating to achieve evenly heated
waste. Agitation further
achieves the introduction of mechanical energy to create shear forces in the
waste and the waste
mix. Agitation can comprise free-fall mixing, such as mixing in a reactor
having a chamber that
rotates along a substantially horizontal axis or in a mixer having a rotary
axis lifting the waste such
as MSW or in a mixer having horizontal shafts or paddles lifting the waste
such as MSW. Agitation
can comprise one or more of shaking, stirring or conveyance through a
transport screw conveyor.
The agitation may continue after waste such as MSW has been heated to the
desired temperature.
The bioreactor in step a) may be adapted to process more than 1; 5; 10; 15;
20; 25; 30; 35; 40; 45;
or 50 t waste/h.
The method of the invention can be applied at any plant scale. It has been
tested in small scale at
laboratory tests, in medium scale at test plants and at large scale waste
processing plants. In one
embodiment, the filling volume of the bioreactor in step a) is larger than 10,
50, 100, 150, 200, 250,
300, 350, 400, 450 or 500 m3 during operation and can be much larger, such as
6000 m3 and is
suitable plants that process more than 5, 10, 15, 20, 25, 30, 35, 40, 45 or 50
tons of waste per hour.
CA 03196334 2023- 4- 20
38
The waste, e.g. MSW, may have a Dry Matter (DM) content in the range 10%-90%;
20%-85%; 30%-
80%; 40%-75%; 50%-70%; or 55%-65 % (w/w); and/or around 10%; 15%; 20%; 25%;
30%; 35%; 40%;
45%; 50%; 55%; 60%; 65%; 70%; 75%; 80%; 85%; or 90% (w/w). The amount of water
added in step
a) depends on the amount of dry matter of the waste, when the dry matter
content is low the need
of adding water to the process of step a is also low. When the dry matter
content is above XX %
addition of water to process a) is needed. Addition of water to process of
step a) may always be
beneficial, and usually, addition of water to the process of step a) is
necessary, due to the dry matter
content in the waste. In the context of the present invention at least part of
the water added to step
a) is process water.
In one embodiment of the invention the amount of dry matter content is above
60 %, such as at
least 70%, such as at least 75%, such as at least 80%, such as at least 85%,
such as at least 90%, such
as at least 95%, such as at least 97%, such as at least 98% or even such as at
least 99% dry matter.
The dry matter may be measured as follows: prepare crucibles by adding 1.5 g
of mineral based
(heat resistant) litter e.g. cat litter. Heat in furnace for 1 hour at 550 C
then allow to cool to 200 C
before transferring the crucibles into a desiccator filled with silica gel
using metal tongues. Allow to
cool to room temperature. Weigh the crucible Wcrucible and add 25 g of sample
and reweigh and note
the weight W.*. Place crucible on a suitable tray and place in the preheated
oven and heat at 105
C for 24 hours. Take crucibles from the oven and return them into the
desiccator. When cooled to
room temperature weigh crucible plus contents and note the weight Wdry. Dry
matter (DM) is
calculated as DM= ((w
õ ¨ dry- Wcrucible/ Wsample Wcrucible) * 100).
The DM content of the waste may be determined at different points in time. The
DM content of the
waste may be measured or assessed (i) before entry into the bioreactor in step
a), such as in the
waste pit or transfer station; (ii) at the onset of said enzymatic and/or
microbial treatment of the
waste entering the bioreactor in step a); and/or (iii) before provision of the
bioliquid obtained in
step a) through one or more solid/liquid separation step(s).
CA 03196334 2023- 4- 20
39
Thus, the DM content of the waste may be measured or assessed at one or more
of the following
points in time: (i) before entry into the bioreactor in step a); ii) at the
onset of said enzymatic and/or
microbial treatment of the waste entering the bioreactor in step a); (iii)
before provision of the
bioliquid obtained in step a) through one or more solid/liquid separation
step(s).
As a practical matter, notwithstanding some variability in the composition of
the waste being
processed, it is convenient to add a relatively constant mass ratio of water
(which includes aqueous
solution) in step a). For instance, when the waste to be treated is municipal
solid waste (MSW) it
will be convenient to add between 0.8 and 1.8 kg water per kg MSW, or between
0.5 and 2.5 kg
water per kg MSW, or between 1.0 and 3.0 kg water per kg MSW. As a result, the
actual non-water
content of the waste (or MSW) during processing may vary within the
appropriate range.
In order for the enzymatic and/or microbial liquefaction of the waste in the
bioreactor in step a) to
provide a bioliquid comprising an optimum amount of short chain carboxylic
acids and sugars such
as glucose, xylose, arabinose, lactic acid/lactate, acetic acid/acetate and/or
ethanol, the pH in the
bioreactor should generally remain within a pH range of between pH 3 ¨ 6.5.
Step b)
Step b) is a separation step where the bioliquid is separated from the non-
degradable solid waste
fractions. Clean and efficient use of the degradable component of waste, such
as MSW, combined
with recycling typically requires some method of sorting or separation to
separate degradable from
non-degradable material. The separation in step b) may be performed by any
means known in art,
such as in a ballistic separator, washing drums and/or hydraulic presses. In
one embodiment the
separation is performed before the enzymatic treatment. Separation of liquid
and solids can e.g.
be done in different presses (such as screw and/or piston presses) or e.g.
using a simpler sieve
function. A ballistic separator is typically used to separate the solids into
20 and 3D fractions and
only secondarily a liquid separation.
Step b) can be conducted one or more times before, during or after enzymatic
and/or microbial
treatment in a bioreactor, wherein said step b) while being conducted during
the enzymatic and/or
CA 03196334 2023- 4- 20
microbial treatment may, in one embodiment, occur after said enzymatic
treatment but prior to
said microbial treatment in a bioreactor.
Separation of liquefied and/or saccharified, fermentable parts of the waste
from non-fermentable
solids can be achieved by a variety of means. Using one separation operation
or a combination of
at least two different separation operations, including but not limited to
screw press operations,
ballistic separator operations, vibrating sieve operations, or other
separation operations known in
the art may be applicable.
The separation is typically performed by one or more separation steps means,
which could be
exemplary performed by those of one or more ballistic separator(s), sieve(s),
washing drum(s),
presses and/or hydraulic press(es). The one or more separation means separate
the waste such as
MSW treated with enzyme and/or microbial action, into the bioliquid, a
fraction of 2D materials
non-biodegradable (flat materials such as textiles, plastic film, undigested
cardboard), and a fraction
of 3D materials (including metals and solid plastic). Inert material, which is
sand and glass is typically
removed e.g. sieved from the bioliquid. Metals are typically removed from all
mentioned fractions.
The process water obtained from one or more of these downstream processing of
the solid waste
derived from step a) i.e. the wash water obtained from the washing drums can
be recirculated into
step a) in the method according to the present invention.
Step c)
In Step c), the bioliquid and/or the solid fraction(s) obtained in step b) is
processed further. The
bioliquid is normally processed further for being applicable for use in
methods for providing energy
or biochemicals, such methods including thermo-chemical conversion of the
solubilized waste to
electricity, heat, methanol, hydrogen, dimethyl ether, petrol, bio-diesel
and/or bio-chemical
conversion of the solubilized waste to biogas, hydrogen, bio-ethanol, bio-
diesel and the like.
The one or more solid fraction(s) exiting the bioreactor are normally
subjected to one or more
washing steps prior to any subsequent reuse processes of the solid waste.
CA 03196334 2023- 4- 20
41
The 2D fraction of the solid fraction obtained in step b) can be further
separated into recyclables
and/or residuals such as SRF (Solid Recovered Fuel), RDF (Refused Derived
Fuel) and/or inerts. The
3D fraction can also be further separated into recyclables and/or residuals
such as metals, 3D plastic
and/or RDF.
The 3D fraction (such as cans and plastic bottles) does not bind large amounts
of bioliquid, so a
single washing step is often sufficient to clean the 3D fraction. The 2D
fraction (textiles and foils as
examples) typically binds a significant amount of bioliquid. Therefore, the 2D
fraction is typically
pressed using e.g. a screw press, washed and pressed again to optimize the
recovery of bioliquid
and to obtain a cleaner and drier 2D fraction.
Wash water is any water stream used for washing of any solid fraction obtained
after the solid-liquid
separation in step b). Examples of wash water are water used for washing of
the 2D fraction and/or
the 3D fraction. Other examples of wash water are water used for washing of
inerts, metals and/or
plastics. Wash water can also be diluted bioliquid obtained from step a) in
the method according to
the present invention. In one embodiment, the wash water is water used for
washing the 2D and/or
the 3D fractions.
In a preferred embodiment of the method of the invention, the downstream
processing in step c) is
a washing process of solid waste such as washing of the 2D solid waste
fraction or of the 3D solid
waste fraction. The water from different downstream washing processes of solid
waste may be
joined to provide the process water as shown in figure la.
In another preferred embodiment of the method of the invention, the downstream
processing in
step c) is an evaporation process wherein water from dewatering the digestate
(e.g. resulting from
an AD process) or from another downstream process is evaporated and collected.
The process water
e.g. reject water or wash water treated in an evaporator system will result in
a clean water
condensate and a nutrient rich liquid termed Brine. The clean water, which is
also according to the
definition of the invention process water, may be reused for washing recovered
material in the 2D
and 3D mechanical waste treatment stages or may be recirculated back to the
reactor.
CA 03196334 2023- 4- 20
42
In another preferred embodiment of the invention, the downstream processing in
step c) is a
collection of the bioliquid or a part of the bioliquid obtained in step b).
In one preferred embodiment the further processing of bioliquid in step c) is
anaerobic digestion
(AD). Anaerobic digestion (AD) is a series of biological processes in which
microorganisms break
down biodegradable material in the absence of oxygen. One of the end products
is biogas, which
can be combusted to generate electricity and/or heat, or can be processed into
renewable natural,
biomethane gas and/or transportation fuels. A range of anaerobic digestion
technologies exists in
the state of the art for converting waste, such as municipal solid waste,
municipal waste water
solids, food waste, high strength industrial wastewater and residuals, fats,
oils and grease (FOG),
and various other organic waste streams into biogas. Many different anaerobic
digester systems are
commercially available, and the skilled person will be familiar with how to
apply and optimize the
anaerobic digestions process. The metabolic dynamics of microbial communities
engaged in
anaerobic digestion are complex.
In typical anaerobic digestion (AD) for production of methane biogas,
biological processes mediated
by microorganisms achieve four primary steps ¨ hydrolysis of biological
macromolecules into
constituent monomers or other metabolites; acidogenesis, whereby short chain
hydrocarbon acids
and alcohols are produced; acetogenesis, whereby available nutrients are
catabolized to acetic acid,
hydrogen and carbon dioxide; and methanogenesis, whereby acetic acid and
hydrogen are
catabolized by specialized archaea to methane and carbon dioxide. The
hydrolysis step is typically
rate-limiting and dependent on the biomass type. In the bioliquid it is the
methanogens that limits
the processing rate. From AD is furthermore obtained digestate, comprising a
solid fraction and a
liquid fraction (reject water).
It is well known in the art, that the conversion of biodegradable organic
material e.g., in waste into
CI-14 and CO2 during the enzymatic and/or microbial treatment followed by
anaerobic digestion
process is facilitated by three major groups of microorganisms. The fermenting
microorganisms
converts the organic material to short-chain fatty acids (such as lactic acid)
through hydrolysis by
CA 03196334 2023- 4- 20
43
e.g. extracellular enzymes and subsequent fermentation of the hydrolyzed
products. Other products
of the fermentation process are acetic acid, alcohols, CO2 and H2. The end
products from the
fermenting and the acidogenic bacteria (lactic acid, formic acid, acetic acid,
and H2) are converted
to CH4 and CO2 by methane producing microorganisms. The methane producing
microorganisms
comprise microorganisms belonging to the archaea domain.
When the further processing in step c) is anaerobic digestion, the anaerobic
digestion may comprise
one or more digesters operated under controlled aeration conditions,
eliminating or minimizing the
available oxygen, in which methane gas is produced in each of the digesters
comprising the system.
The AD reactor(s) can, but need not, be part of the same waste processing
plant as the bioreactor
in step a) and can, but need not, be connected to the bioreactor in step a).
Moreover, the AD process
may be in the form of a fixed filter system. A fixed filter anaerobic
digestion system is a system in
which an anaerobic digestion consortium is immobilized, optionally within a
biofilm, on a physical
support matrix.
In a preferred embodiment of the method of the invention, the downstream
processing in step c) is
anaerobic digestion providing an anaerobic digestion effluent, resulting in
reject water from
dewatering the digestate.
Since the process water may comprise microorganisms, it may be desirable to
sanitize the process
water prior to re-circulating the process water into the bioreactor in step
a). This may for instance
be the case if the process water is obtained from a digestate i.e. reject
water where the microbial
flora is different from the microbial flora in the bioreactor.
In one embodiment of the method of the invention, the process water obtained
in step c) is subject
to hygienization before being subjected to step d).
Additionally, the process water may be added to the reactor in batches to keep
pH within 3.5-6.
In one embodiment of the method of the invention, the process water obtained
in step c) is added
in step a) in batches, such that pH in the reactor is between pH 3.5-6.
CA 03196334 2023- 4- 20
44
Hygienization is any process decimating the concentration of reference
organisms by at least 6
decades and can also achieved by means of thermal treatment, filtering,
ultraviolet treatment,
electrification, treatment at 50 C for more than one hour, among other means.
The Animal By-
product Protocol (EU Regulation No 142/2011) describes hygienization at 70 *C
for 60 minutes.
In one embodiment, the method of the invention comprises a hygienization step
wherein the
process water obtained in step c) is subjected to hygienization. In a
preferred embodiment, the
hygienization comprises treatment of process water at a temperature in the
range of 60 C to 75 C,
preferably 70 C or preferably around 70 C for at least one hour, preferably
for 60-80 minutes,
preferably 60-70 minutes or preferably about 60 minutes.
Due to the amount of salt in the digestate and due to the presence of
microbial organisms in the
digestate, it was expected that addition of reject water would primarily
possess a negative impact
on the enzymatic and/or microbial treatment in the bioreactor. However,
surprisingly process water
which comprise reject water has shown not to diminish the liquefaction process
in the reactor and
therefore such water can be recycled continuously during the process of the
invention.
Step d)
In step d) the process water obtained from step c) and optionally water from
an external water
source is added to the bioreactor in step a).
Water from an external water source may be mixed with the process water prior
to the addition
into the bioreactor or the process water and the water from an external source
may be added
separately at the same time or at different points in time and the addition
may be continuous or at
different frequencies and volumes depending on the amount of waste and the
composition of the
waste in the bioreactor.
Water from external sources includes water obtained from any source wherein
said water has not
previously been subjected to any steps in an enzymatic and/or microbial waste
treatment process.
CA 03196334 2023- 4- 20
Thus, water from external sources comprise tap water, wastewater that has not
been subjected to
an enzymatic and/or microbial waste treatment process, and water from natural
sources such as
rivers and lakes.
In one embodiment of the method of the invention, the external water in step
d) is selected from
natural sources such as rivers, lakes and ponds; water reservoirs; tap water;
and any combination
thereof.
The pH of the process water and optionally water from an external source can
optionally be adjusted
by any known means such as by the addition of acid or base if the pH of the
process water is not
within the optimum pH range of the liquefaction process in step a).
In one embodiment, the pH of the process water is adjusted by adding acid
until pH of the process
water is between pH 3.5 ¨ 6, such as pH 3.5, pH 4, pH 4.5, pH 5, pH 5.5 and pH
6 or any pH value in
between these pH values.
Any acid could be used to adjust pH. Preferably, the acid is an organic acid
since such acids are less
likely to accumulate water soluble salts in the recirculation loop.
The pH of the process water could be adjusted to pH 3.5 - 6 by addition of a
base if the pH of the
process water and optionally water from an external source is below pli 3.
When the process water is provided by an AD process, then the pH of the
process water, i.e. the
reject water can be adjusted by reduction of the ammonia concentration.
Reduction of the ammonia
concentration could be the only pH adjustment or reduction of the ammonia
concentration could
be in addition to adjusting the pH by the addition of an acid.
The means for reducing the ammonia concentration could be any suitable means
available in the
art.
CA 03196334 2023- 4- 20
46
In one embodiment of the method of the invention, the downstream processing in
step c) is an
anaerobic digestion process and the pH of the reject water is adjusted by
addition of acid and/or by
reducing the ammonium content prior to being added to the bioreactor in step
a). The ammonium
content can for instance be reduced by means of evaporation or by using
specialized ammonium
extraction equipment.
In some embodiments of the present invention, the process water and optionally
water from one
or more external sources is continuously entered into a bioreactor with
ongoing enzymatic and/or
microbial treatment of waste.
In other embodiments of the present invention, the process water and
optionally water from one
or more external sources is discontinuously entered into a bioreactor with
ongoing combined
enzymatic and microbial treatment of waste. That is, the process water and
optionally water from
an external source is entered into the bioreactor when required optionally
subject to the monitored
pH in the bioliquid present in the bioreactor. In one embodiment of the
invention the process water
is added to step a) in batches, such that pH in the reactor is between pH 3.5-
6. Thus, even when
adding process water, the pH is kept within the optimum range of pH 3.5-6.
As will be readily understood by one skilled in the art, the capacity to
render solid components into
a liquid slurry is increased with increased water content. For instance,
effective pulping of paper
and cardboard, which comprise a substantial fraction of MSW in some countries,
is typically
improved where water content is increased. Water content provides a medium in
which the
microbial preparation can propagate and which dissolves metabolites.
Furthermore, enzyme
activities may exhibit diminished activity when treatment is conducted under
conditions with low
water content. For example, cellulases typically exhibit diminished activity
in treatment mixtures
that have non-water content higher than about 10% by weight. In the case of
cellulases, which
degrade paper and cardboard, an effectively linear inverse relationship has
been reported between
substrate concentration and yield from the enzymatic reaction per gram
substrate, see Kristensen
et al. 2009.
CA 03196334 2023- 4- 20
47
The waste to be processed, such as e.g. MSW, may, in a preferred embodiment,
have a non-water
content of between above 10% or more and below 45%. In another preferred
embodiment, the
waste to be processed may have a water content of 40-85%. Waste such as MSW
may often
comprise a considerable amount of water. However, the water content may be
adjusted in order to
achieve appropriate non-water content.
In one embodiment of the method of the invention, the flow rate of the
addition of process water
and optionally water from an external source in step d) into the bioreactor in
step a) is essentially
constant and/or essentially proportional, to the amount of waste, having
between 1:1 and 3:1 of
water:waste proportion.
In situations where the downstream process in step c) is an AD process
providing a digestate or an
alkaline fraction thereof, the reject water, resulting from dewatering the
digestate, will comprise
microorganisms involved in the AD process.
It has surprisingly been found here, that adding process water comprising
reject water obtained in
step c), wherein the reject water has not been subject to hygienization prior
to entry into a
bioreactor in step a) did not abolish the continued liquefaction process.
Thus, in some embodiments of the method according to the present invention,
the reject water
obtained in step c) comprises methanogenic microorganisms, such as archaea,
from the anaerobic
digestion.
Likewise, in some embodiments of the method according to the present
invention, the reject water
has not been subjected to a hygienization step prior to adding process water
comprising the reject
water obtained in step c) to the bioreactor in step d).
In some embodiments of the method according to the present invention, the
methanogenic
microorganisms comprised in the reject water obtained in step c) is
exterminated upon adding the
CA 03196334 2023- 4- 20
48
process water comprising the reject water obtained in step c) to the enzymatic
and/or microbial
treatment in step d).
The process water and optionally water from an external source, may be added
to a bioreactor in
step d) at different points in time either continuously or discontinuously
before and/or during the
enzymatic and/or microbial treatment of waste. Preferably, the treatment in
step a) is at a steady
state when the process water and optionally water from an external source is
entered into the
bioreactor in step d).
In one embodiment of the method according to the invention, process water and
optionally water
from an external source, is added to said bioreactor in step d) before the
enzymatic and/or microbial
treatment of the waste is initiated.
In another embodiment of the method according to the invention, process water
and optionally
water from an external source, is added to said bioreactor in step d) during
the enzymatic and/or
microbial treatment of the waste.
In yet another embodiment of the method according to the invention, process
water and optionally
water from an external source, is added to said bioreactor in step d) before
and during the
enzymatic and/or microbial treatment of the waste.
One skilled in the art will readily be able to determine an appropriate
quantity of water content, to
add to waste in adjusting water content in a bioreactor wherein enzymatic
and/or microbial
degradation is taking place, such as in step a) of the present invention.
Typically, as a practical
matter, notwithstanding some variability in the composition of the waste being
processed, it is
convenient to add a relatively constant mass ratio of water (which includes
aqueous solution). For
instance, when the waste to be treated is municipal solid waste (MSW) it will
be convenient to add
between 0.8 and 1.8 kg water per kg MSW, or between 0.5 and 2.5 kg water per
kg MSW, or
between 1.0 and 3.0 kg water per kg MSW. As a result, the actual non-water
content of the waste
CA 03196334 2023- 4- 20
49
(or MSW) during processing may vary within the appropriate range, e.g. between
above 10% or
more and below 45%.
In one embodiment of the method according to the present invention, the flow
rate of process
water and optionally water from an external source into the bioreactor in step
d) is essentially
constant and/or essentially proportional to the amount of waste entering said
bioreactor.
The amount of wash water, bioliquid or tap water/purified water that is added
to the bioreactor
together with or in addition to process water obtained in step c), depend both
on the compositions
of the waste such as the dry matter content and the ratio of organic matter to
inorganic or non-
biodegradable matter and on the salt and microbial content of the process
water. Thus, in
embodiments of the method according to the present invention, more than 1.0,
2.5, 5, 10, 15, 20,
25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90, 95, or 98% (w/w) of
the water added to said
waste in step d) is process water, optionally including water from an external
source.
The ratio between the process water obtained in step c) to water from an
external source will have
to be optimized to the specific waste and composition of process water.
However, this should be
possible for the skilled person using routine tests for determining the DM of
the waste and for
determining the salt concentration of the process water and the level of
microbial activity in the
process water. Thus, in embodiments of the method according to the present
invention, the ratio
of process water obtained in step c) to water from an external source is in
the range of 0.01-0.1;
0.1-0.25; 0.25-0.50; 0.50-1.0; 1.0-2.0; 2.0-4.0; 4.0-6.0; 6.0-8.0; 8.0-10; 10-
20, 20-40; 40-60; 60-80;
or 90-100.
The dry matter (DM) content of the process water obtained in step c) entered
into the bioreactor in
step d) depends on the specific separation steps performed in step b) and on
the content of
insoluble matter of the process water prior to entry into the bioreactor. In
the method according to
the present invention it is preferred that the process water obtained in step
c) has a DM content
(w/w %) of 1.0 or more; 1.5 or more; 2.0 or more; 2.5 or more; 3.0 or more;
3.5 or more; 4.0 or
CA 03196334 2023- 4- 20
more; 4.5 or more; 5.0 or more; 5.5 or more; 6.0 or more; 6.5 or more; 7.0 or
more; 7.5 or more; or
8.0 or more.
Similarly, the salt content of the process water obtained in step c) entered
into the bioreactor in
step d) depends on the composition of the waste treated in step a), on the
specific washing and
separation steps applied and on the content of insoluble matter of the process
water prior to entry
into the bioreactor. In the method according to the present invention the
process water obtained
in step c) may have an organic salt content and/or an inorganic salt content
(w/w %) of 1.5 or less;
3 or less; 4.5 or less; 6 or less; 7.5 or less; 9 or less; 10.5 or less; 12 or
less; 13.5 or less; 15 or less;
16.5 or less; 18 or less; 19 or less; 19.5 or less; or 20 or less.
In the method according to the present invention, the process water obtained
in step c) may
comprise salts such as one or more of ammonium, nitrate, nitrite, phosphate,
chloride, sodium,
potassium, sulfate, iron, calcium, carbonate, bicarbonate, magnesium or other
salts.
In one embodiment, in the method according to the present invention, the
process water obtained
in step c) comprises one or more of the following elements: up to 3800 mg-N/kg-
FW
ammonia/ammonium; up to 4700 mg-N/kg-FW nitrogen; up to 280 mg-P/kg-FW
phosphorus; up
to 3300 mg/kg-FW water soluble chlorine; up to 3300 mg/kg-FW water soluble
sodium; up to 2900
mg/kg-FW potassium; up to 430 mg/kg-FW sulphur; up to 590 mg/kg-FW iron: up to
6000 mg/kg-
FW calcium; up to 3 mg/kg-FW nickel; up to 3 mg/kg-FW lead; up to 3 mg/kg-FW
zinc.
In another embodiment, in the method according to the present invention, the
process water
obtained in step c) comprises one or more of the following elements: up to
8000 mg-N/kg-FW
ammonia/ammonium; up to 10000mg-N/kg-FW nitrogen; up to 600 mg-P/kg-FW
phosphorus; up to
7000 mg/kg-FW water soluble chlorine; up to 7000 mg/kg-FW water soluble
sodium; up to 6000
mg/kg-FW potassium; up to 900 mg/kg-FW sulphur; up to 1200 mg/kg-FW iron: up
to 12000 mg/kg-
FW calcium; up to 6 mg/kg-FW nickel; up to 6 mg/kg-FW lead; up to 6 mg/kg-FW
zinc.
CA 03196334 2023- 4- 20
51
The method according to the present invention has proven effective both in
small scale experiments
where the bioreactor in step a) is small as well as in large scale waste
processing reactors.
Accordingly, in one embodiment of the method according to the invention, the
filling volume of the
bioreactor in step a) is larger than 10; 50; 100; 150; 200; 250; 300; 350;
400; 450; or 500 m3 during
operation.
In one embodiment of the method according to the invention, the bioreactor in
step a) is adapted
to process more than 5; 10; 15; 20; 25; 30; 35; 40; 45; or 50 t waste/h.
In one embodiment of the method according to the invention, wherein the waste,
e.g. MSW, has a
DM content in the range 10-90; 20-85; 30-80; 40-75; 50-70; or 55-65 % (w/w);
and/or around 10;
15; 20; 25; 30; 35; 40; 45; 50; 55; 60; 65; 70; 75; 80; 85; or 90% (w/w).
The DM content of the waste may be determined at different points in time. In
the method
according to the present invention, the DM content of the waste may be
measured or assessed (i)
before entry into the bioreactor in step a); (ii) at the onset of said
combined enzymatic and microbial
treatment of the waste entering the bioreactor in step a); and/or (iii) before
provision of the
bioliquid obtained in step a) through one or more solid/liquid separation
step(s).
In one embodiment, the DM content of the waste is measured or assessed (i)
before entry into the
bioreactor in step a).
In one embodiment, the DM content of the waste is measured or assessed (ii) at
the onset of said
enzymatic and/or microbial treatment of the waste entering the bioreactor in
step a).
In one embodiment, the DM content of the waste is measured or assessed (iii)
before provision of
the bioliquid obtained in step a) through one or more solid/liquid separation
step(s).
In one embodiment, the DM content of the waste is measured or assessed at one
or more of the
following points in time: (i) before entry into the bioreactor in step a); ii)
at the onset of said
enzymatic and/or microbial treatment of the waste entering the bioreactor in
step a); (iii) before
provision of the bioliquid obtained in step a) through one or more
solid/liquid separation step(s).
CA 03196334 2023- 4- 20
52
In one embodiment of the method according to the invention, reject water
obtained in step c) or
an alkaline fraction thereof, is provided by the use of a digester such as
disclosed in
W02016/050893 or W02017/174093.
Waste
Any waste comprising a mixture of biodegradable and non-biodegradable material
could be used in
the method of the invention.
In one embodiment, in the method according to the present invention, the waste
comprises both
biodegradable and non-biodegradable material.
In preferred embodiments of the method according to the present invention,
said waste is selected
from one or more of unsorted municipal solid waste, centrally sorted municipal
solid waste, source
sorted municipal solid waste from households, municipal solid waste processed
by shredding or
pulping, organic fractions and paper rich fractions, Refuse-Derived-Fuel
fractions and municipal
solid waste wherein the biodegradable material in said waste comprises a
combination of one or
more items selected from: food residues, paper, cardboard, and fines.
Relevant types of mono- and/or polysaccharide containing waste that is
suitable for being processed
by the enzymatic and/or microbial treatment in step a) according to the
present invention may
include:
Waste fractions derived from households such as e.g.:
= Unsorted municipal solid waste (MSW)
= MSW processed in some central sorting, shredding or pulping device such
as e.g.
Dewaster or reCulture
= Solid waste sorted from households, including both organic fractions and
paper rich
fractions
= RDF (Refuse-Derived-Fuel) fractions
Waste fractions derived from the industry such as e.g.:
CA 03196334 2023- 4- 20
53
= General industry waste fractions containing paper or other organic
fractions now
being treated as household waste
= Waste fraction from paper industry, e.g. from recycling facilities
= Waste fractions from food and feed industry
= Waste fraction from the medicinal industry
Waste fractions derived from agriculture or farming related sectors such as
e.g.:
= Waste fractions from processes including sugar or starch rich products
such as
potatoes and beet
= Contaminated or in other ways spoiled agriculture products such as
grain, potatoes
and beet not exploitable for food or feed purposes
= Garden refuse
= Manure, or manure derived products
Waste fractions derived from municipal, county or state related or regulated
activities such as e.g.:
= Sludge from wastewater treatment plants
= Fiber or sludge fractions from biogas processing
= General waste fractions from the public sector containing paper or other
organic
fractions.
In one embodiment, the dry matter content of the mono- and/or polysaccharide
containing waste
fraction in the enzymatic treatment and fermentation processes in step a) is
above 20%, such as 20-
100%, such as 20-50%, such as 20-45%, such as 20-40% and such as 20-80% and
also such as 80-
100%, preferably 90-100%, most preferably about 95%.
Waste, such as MSW, is typically heterogeneous. Statistics that provide firm
basis for comparisons
between countries concerning composition of waste materials are not widely
known. Standards and
operating procedures for correct sampling and characterization remain
unstandardized. Indeed,
only a few standardized sampling methods have been reported. At least in the
case of household
waste, the composition exhibits seasonal and geographical variation, even over
small distances of
CA 03196334 2023- 4- 20
54
200-300 km. As a general rule, the dry weight of modern urban waste from
Western Europe typically
comprises on the order of from 10 to 25% by weight of "vegetable and food
waste". In China, in
contrast, the relative proportions of "food waste" are typically increased by
a factor of at least two
relatives to MSW from Western Europe.
Municipal solid waste may, in particular, comprise one or more of kitchen
putrescible, garden
putrescible, paper, card, plastics, miscellaneous combustible and non-
combustible matters, textiles,
glass, ceramics, metals, and electronic devises. Generally, municipal solid
waste in the Western part
of the world normally comprise one or more of: animal food waste, vegetable
food waste,
newsprints, magazines, advertisements, books and phonebooks, office paper,
other clean paper,
paper and carton containers, other cardboard, milk cartons and alike, juice
cartons and other carton
with alu-foil, kitchen tissues, other dirty paper, other dirty cardboard, soft
plastic, plastic bottles,
other hard plastic, non-recyclable plastic, yard waste, flowers etc., animals
and excrements, diapers
and tampons, cottonsticks etc., other cotton etc., wood, textiles, shoes,
leather, rubber etc., office
articles, empty chemical bottles, plastic products, cigarette buts, other
combustibles, vacuum
cleaner bags, clear glass, green glass, brown glass, other glass, aluminium
containers, alu-trays, a lu-
foil (including tealight candle foil), metal containers (-Al), metal foil (-
Al), other sorts of metal, soil,
rocks, stones and gravel, ceramics, cat litter, batteries (button cells,
alkali, thermometers etc.), other
non-combustibles and fines.
The waste that can be processed in the present invention may be sorted or
unsorted.
In one embodiment, the waste subject to combined enzymatic and microbial
treatment in step a) is
unsorted waste, such as unsorted MSW.
In another embodiment, the waste subject to combined enzymatic and microbial
treatment in step
a) is sorted MSW.
In a preferred embodiment of the present invention, the waste subject to
enzymatic and/or
microbial treatment in step a) is MSW.
CA 03196334 2023- 4- 20
Typically, unsorted MSW may comprise organic waste, including one or more of
food and kitchen
waste; paper- and/or cardboard-containing materials; recyclable materials,
including glass, bottles,
cans, metals, and certain plastics; burnable materials; and inert materials,
including ceramics, rocks,
and debris.
Waste subject to enzymatic and/or microbial treatment in step a) such as MSW,
can be source-
separated organic waste comprising predominantly fruit, vegetable and/or
animal waste. A variety
of different sorting systems may be applied to MSW, for instance source
sorting, where individual
households dispose of different waste materials separately. Source sorting
systems are currently in
place in some municipalities in Austria, Germany, Luxembourg, Sweden, Belgium,
the Netherlands,
Spain and Denmark. Alternatively, industrial sorting systems may be applied at
the large-scale plant
prior to subjecting the waste to the combined enzymatic and microbial
treatment. Means of
mechanical sorting and separation may include any methods known in the art
including but not
limited to the systems described in U52012/0305688; W02004/101183;
W02004/101098;
W02001/052993; W02000/0024531; W01997/020643; W01995/0003139; CA2563845;
US5465847.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) is derived from or comprises
any one or more of
waste from household, industry, agriculture, farming, county, or state
activities.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) comprises 10-100%
biodegradable material on a
dry basis. In another embodiment of the method according to the present
invention, the waste
subject to enzymatic and/or microbial treatment in step a) comprises 10-20%
biodegradable
material on a dry basis, 20-30% biodegradable material on a dry basis,
30-40% biodegradable material on a dry basis, 40-50% biodegradable material on
a dry basis,
50-60% biodegradable material on a dry basis, 60-70% biodegradable material on
a dry basis,
70-80% biodegradable material on a dry basis, 80-90% biodegradable material on
a dry basis,
CA 03196334 2023- 4- 20
56
90-100% biodegradable material on a dry basis, or any combination of these
intervals.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) comprises 20-30% biodegradable
material on a dry
basis.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) comprises 10-100%
biodegradable material on a
wet basis.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) comprises 25-60% such as 35-
50% biodegradable
material on a wet basis.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) is selected from one or more
of unsorted municipal
solid waste, centrally sorted municipal solid waste, source sorted municipal
solid waste from
households, municipal solid waste processed by shredding or pulping, organic
fractions and paper
rich fractions, Refuse-Derived-Fuel fractions.
In one embodiment of the method according to the present invention, the
biodegradable material
in waste subject to enzymatic and/or microbial treatment in step a) comprises
a combination of one
or more items selected from: food residues, paper, cardboard, and fines.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) is sorted municipal solid
waste not comprising
items selected from one or more of the following: domestic appliances, glass,
ceramics, batteries,
newsprints, magazines, advertisements, books, plastics, fabrics, textiles,
yard waste, electrical and
electronic equipment, chemicals, pharmaceuticals, metals.
CA 03196334 2023- 4- 20
57
In one embodiment of the method according to the present invention, one or
more of the following
groups of items are removed from the waste prior to the enzymatic and/or
microbial treatment in
step a): leaves, grasses, wood, fabrics, stones, plastics, metals.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) is selected from one or more
of general industry
waste fractions containing paper or other organic fractions, waste fractions
from paper industry or
recycling facilities, waste fractions from food and feed industry, waste
fractions from the medicinal
industry.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) is selected from one or more
of agriculture or
farming, waste fractions from processes of sugar or starch rich products,
contaminated or spoiled
agricultural products not exploitable for food or feed purposes, manure,
manure derived products.
In one embodiment of the method according to the present invention, the waste
subject to
enzymatic and/or microbial treatment in step a) is selected from one or more
of waste fractions
derived from municipal, county or state related or regulated activities,
sludge from waste water
treatment plants, fiber or sludge fractions from biogas processing, general
waste fractions from the
public sector containing paper or other organic fractions.
To summarize, it is shown herein that in order to keep the bioliquid
production from waste in a
steady state wherein both the enzymatic and/or microbial processes are active
and the retention-
time and accordingly cost are optimized, process water and optionally water
from external sources
can added to the bioreactor. This is surprising because the difference between
using tap water (as
is normally done in the combined enzymatic and microbial liquefaction of
waste) and process water
such as reject water obtained from an digestate which comprises a large amount
of salts, other
solubles and live microorganisms, was expected to be huge. However, the
examples disclosed
herein surprisingly shows that both the enzymatic activity and the activity of
the microorganisms
CA 03196334 2023- 4- 20
58
producing the valuable organic acids required as feed to for instance an AD
biogas production is
upheld in the bioreactor when process water is added to the bioreactor.
Numbered embodiments
Relevant embodiments of the current invention may also be found in the
following section, termed
"numbered embodiments".
1. A method for continuous or batch processing of waste
comprising:
a) subjecting waste to an enzymatic and/or microbial treatment in a bioreactor
b) subjecting the treated waste from step a) to one or more separation
step(s),
whereby a bioliquid and a solid fraction is provided;
c) subjecting said bioliquid and/or fraction to downstream processing
providing
process water;
d) adding the process water obtained from step c) and optionally water from an
external water source to the bioreactor in step a).
2. Method according to embodiment 1, wherein the downstream processing in step
c)
providing said process water is selected from one or more of an anaerobic
digestion process,
washing of a solid waste fraction, evaporation and collection of bioliquid.
3. Method according to embodiment 1, wherein the process water is from an
anaerobic
digestion process and/or washing of a solid waste fraction.
4. Method according to the previous embodiments wherein, the pH of the process
water
obtained in step c) is adjusted to between 3.5 and 6 prior to step c).
5. Method according to the previous embodiments, wherein the downstream
processing in
step c) is an anaerobic digestion process providing reject water.
6. Method according to embodiment 5, wherein the pH of the reject water is
adjusted to
between 3.5 and 6 by addition of acid and/or by reducing the ammonium content.
CA 03196334 2023- 4- 20
59
7. Method according to embodiment 5 or 6 wherein the process water obtained
from said
anaerobic digestion process is subject to hygienization before being subjected
to step
8. Method according to the previous embodiments, wherein process water is
added to step a)
in batches, such that pH in the reactor is between pH 3.5-6.
9. Method according to the previous embodiments, wherein the external water in
step d) is
selected from water obtained from natural sources such as rivers, lakes and
ponds; water
reservoirs; tap water, and any combination thereof.
10. Method according to the previous embodiments, wherein the filling volume
of the
bioreactor in step a) is larger than 10, 50, 100, 150, 200, 250, 300, 350,
400, 450 or 500 m3
during operation and wherein it is adapted to process more than 5, 10, 15, 20,
25, 30, 35,
40, 45 or 50 tons of waste per hour.
11. Method according to previous embodiments wherein said waste is selected
from one or
more of unsorted municipal solid waste, centrally sorted municipal solid
waste, source
sorted municipal solid waste from households, municipal solid waste processed
by shredding
or pulping, organic fractions and paper rich fractions, Refuse-Derived-Fuel
fractions and
municipal solid waste wherein the biodegradable material in said waste
comprises a
combination of one or more items selected from: food residues, paper,
cardboard, and fines.
12. Method according to the previous embodiments wherein said enzymatic and/or
microbial
treatment in step a) is performed by adding enzymes, supplied in either native
form or in
form of microbial organisms giving rise to the expression of such enzymes,
and/or by the
bacteria present in the waste and optionally by adding standard, cultivated,
or manipulated
yeast, bacteria, or any other microorganism capable of producing biochemicals,
ethanol, or
biogas.
CA 03196334 2023- 4- 20
13. Method according to the previous embodiments wherein the treatment in step
a) comprises
contacting the waste with a live lactic acid bacteria concentration of at
least 1.0 x 106, 1.0 x
107, 1.0 x 108 or 1.0 x 109 CFU/L.
14. Method according to the previous embodiments, wherein the treatment in
step a)
comprises adding microorganisms to the waste at a concentration of 1.0 x 106,
1.0 x 107, 1.0
x 108, 1.0 x 109 or 1.0 x 1010 CFU/L.
15. Method according to the previous embodiments wherein treatment step a) is
performed at
a temperature between 20 and 75 C, 30 C and 70 C, 40 C and 60 C, 45 and 55 C,
or around
50 C.
16. Method according to the previous embodiments wherein the flow rate of the
addition of
process water and optionally water from an external source in step d) into the
bioreactor in
step a) is essentially constant and/or essentially proportional, to the amount
of waste,
having between 1:1 and 3:1 of water:waste proportion.
17. Method according to previous embodiments wherein said waste is selected
from one or
more of unsorted municipal solid waste, centrally sorted municipal solid
waste, source
sorted municipal solid waste from households, municipal solid waste processed
by shredding
or pulping, organic fractions and paper rich fractions, Refuse-Derived-Fuel
fractions and
municipal solid waste wherein the biodegradable material in said waste
comprises a
combination of one or more items selected from: food residues, paper,
cardboard, and fines.
18. The method according to the previous embodiments, wherein said enzymatic
and/or
microbial treatment in step a) is performed by adding enzymes, supplied in
either native
form or in the form of microbial organisms giving rise to the expression of
such enzymes;
and by the bacteria present in the waste and optionally by adding standard,
cultivated, or
manipulated yeast, bacteria, or any other microorganism capable of producing
biochemicals,
ethanol, or biogas.
CA 03196334 2023- 4- 20
61
19. The method according to any one of the preceding embodiments, wherein the
treatment in
step a) is accomplished by one or more species of lactic acid producing
bacteria, acetate-
producing bacteria, propionate-producing bacteria, or butyrate-producing
bacteria,
including any combination thereof.
20. The method according to any one of the preceding embodiments, wherein the
treatment
step a) comprises contacting the waste with a live lactic acid bacteria
concentration of at
least 1.0 x 106, 1.0 x 107, 1.0 x 109 or 1.0 x 109 CFU/L.
21. The method according to any one of the preceding embodiments, wherein the
treatment
step a) comprises addition of cellulase activity by inoculation with one or
more
microorganism(s) that exhibits extracellular cellulase activity.
22. The method according to any one of the preceding embodiments, wherein the
treatment
step a) is performed at a temperature between 20 and 75 C, 30 C and 70 C, 40 C
and 60 C,
45 and 55 C, or around 50 C.
23. The method according to any one of the previous embodiments, wherein the
microbial
treatment in step a) is performed by a microbial composition wherein the
majority of the
living microorganisms are lactic acid producing bacteria.
24. The method according to any one of the previous embodiments, wherein the
process water
is obtained from a downstream AD process and comprises methanogenic
microorganisms
from the anaerobic digestion.
25. The method according to any one of the previous embodiments, wherein
process water is
obtained from a downstream AD process and has not been subjected to a
hygienization step
prior to step d).
CA 03196334 2023- 4- 20
62
26. The method according to any one of the previous embodiments wherein said
process water
and optionally water from an external source is added to said bioreactor in
step d) before
and/or during the combined enzymatic and microbial treatment of the waste in
step a).
27. The method according to any one of the preceding embodiments, wherein one
or more
carbohydrates, such as one or more carbohydrate(s) selected from poly-, oligo-
, di-, or
monosaccharide(s), including any combination thereof is added to the
bioreactor in step a)
in addition to adding said process water and optionally water from an external
source.
28. The method according to any one of the preceding embodiments, wherein the
flow rate of
process water and optionally the flow rate of water from an external water
source into the
bioreactor in step d) is added regularly or irregularly.
29. The method according to any one of the preceding embodiments, wherein more
than 1.0,
2.5, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 90,
95, or 98% (w/w) of the
water added to said waste in step a) is process water obtained in step c)
30. The method according to any one of the preceding embodiments, wherein the
ratio of
process water obtained in step c) to water from an external source in step d)
is in the range
of 0.01-0.1; 0.1-0.25; 0.25-0.50; 0.50-1.0; 1.0-2.0; 2.0-4.0; 4.0-6.0; 6.0-
8.0; 8.0-10; 10-20, 20-
40; 40-60; 60-80; or 90-100.
31. The method according to any one of the preceding embodiments, wherein
process water
obtained in step c) has a DM content (w/w %) of 1.0 or more; 1.5 or more; 2.0
or more; 2.5
or more; 3.0 or more; 3.5 or more; 4.0 or more; 4.5 or more; 5.0 or more; 5.5
or more; 6.0
or more; 6.5 or more; 7.0 or more; 7.5 or more; or 8.0 or more.
32. The method according to any one of the preceding embodiments, wherein the
process water
obtained in step c) has a salt content (w/w %) of 1.0 or more; 1.5 or more;
2.0 or more; 2.5
or more; 3.0 or more; 3.5 or more; 4.0 or more; 4.5 or more; 5.0 or more; 5.5
or more; or
6.0 or more; 6.5 or more; 7.0 or more; 7.5 or more; or 8.0 or more.
CA 03196334 2023- 4- 20
63
33. The method according to any one of the preceding embodiments, wherein the
process water
obtained in step c) comprises salts comprising one or more of ammonium,
nitrate, nitrite,
phosphate, chloride, sodium, potassium, sulfate, iron, calcium, nickel, lead
and zinc.
34. The method according to any of the preceding embodiments, wherein the
process water is
obtained from an AD process providing an digestate comprising one or more of
the following
elements: up to 3800 mg-N/kg-FW ammonia/ammonium; up to 4700 mg-N/kg-FW
nitrogen;
up to 280 mg-P/kg-FW phosphorus; up to 3300 mg/kg-FW water soluble chlorine;
up to 3300
mg/kg-FW water soluble sodium; up to 2900 mg/kg-FW potassium; up to 430 mg/kg-
FW
sulphur; up to 590 mg/kg-FW iron: up to 6000 mg/kg-FW calcium; up to 3 mg/kg-
FW nickel;
up to 3 mg/kg-FW lead; up to 3 mg/kg-FW zinc.
35. The method according to any one of the preceding embodiments, wherein the
filling volume
of the bioreactor in step a) is larger than 10; 50; 100; 150; 200; 250; 300;
350; 400; 450; or
500 m3 during operation.
36. The method according to any one of the preceding embodiments, wherein the
bioreactor in
step a) is adapted to process more than 5; 10; 15; 20; 25; 30; 35; 40; 45; or
50 tons of
waste/h.
37. The method according to any one of the preceding embodiments, wherein the
waste, e.g.
MSW, has a DM content in the range 10-90; 20-85; 30-80; 40-75; 50-70; or 55-65
% (w/w);
and/or around 10; 15; 20; 25; 30; 35; 40; 45; 50; 55; 60; 65; 70; 75; 80; 85;
or 90% (w/w).
38. The method according to any of the preceding embodiment, wherein the DM
content of the
waste is measured or assessed (i) before entry into the bioreactor in step a);
(ii) at the onset
of said combined enzymatic and microbial treatment of the waste entering the
bioreactor in
step a); and/or (iii) before provision of the bioliquid obtained in step a)
through one or more
solid/liquid separation step(s).
39. The method according to any one of the preceding embodiments, wherein the
process water
is obtained from an AD process using of one or more anaerobic digester(s)
comprising
CA 03196334 2023- 4- 20
64
attachment means for microbial biofilms, such as a device comprising a carrier
matrix, such
as a digester disclosed in W02016050893 or W02017/174093.
40. The method according to any one of the preceding embodiments, wherein the
waste
comprises both biodegradable and non-biodegradable material.
41. The method according to any one of the preceding embodiments, wherein said
waste is
unsorted or sorted municipal solid waste (MSW).
42. The method according to any one of the preceding embodiments, wherein said
waste is
derived from or comprises any one or more of waste from household, industry,
agriculture,
farming, county, or state activities.
43. The method according to any one of the preceding embodiments, wherein said
waste
comprises 10-100% biodegradable material on a dry basis.
44. The method according to any one of the preceding embodiments, wherein said
waste
comprises 20-30% biodegradable material on a dry basis.
45. The method according to any one of the preceding embodiments, wherein said
waste
comprises 10-100% biodegradable material on a wet basis.
46. The method according to any one of the preceding embodiments, wherein said
waste
comprises 25-60% such as 35-50% biodegradable material on a wet basis.
47. The method according to any one of the preceding embodiments, wherein said
waste is
selected from one or more of unsorted municipal solid waste, centrally sorted
municipal
solid waste, source sorted municipal solid waste from households, municipal
solid waste
processed by shredding or pulping, organic fractions and paper rich fractions,
Refuse-
Derived-Fuel fractions.
CA 03196334 2023- 4- 20
48. The method according to any one of the preceding embodiments wherein the
biodegradable
material in said waste municipal solid waste comprises a combination of one or
more items
selected from: food residues, paper, cardboard, and fines.
49. The method according to any one of the preceding embodiments wherein said
waste is
sorted municipal solid waste not comprising items selected from one or more of
the
following: domestic appliances, glass, ceramics, batteries, newsprints,
magazines,
advertisements, books, plastics, fabrics, textiles, yard waste, electrical and
electronic
equipment, chemicals, pharmaceuticals, metals.
50. The method according to any one of the preceding embodiments, wherein one
or more of
the following groups of items are removed from the waste prior to the combined
enzymatic
and microbial treatment in step a): leaves, grasses, wood, fabrics, stones,
plastics, metals.
51. The method according to any one of the preceding embodiments, wherein said
waste is
selected from one or more of general industry waste fractions containing paper
or other
organic fractions, waste fractions from paper industry or recycling
facilities, waste fractions
from food and feed industry, waste fractions from the medicinal industry.
52. The method according to any one of the preceding embodiments, wherein said
waste is
selected from one or more of agriculture or farming, waste fractions from
processes of sugar
or starch rich products, contaminated or spoiled agricultural products not
exploitable for
food or feed purposes, manure, manure derived products.
53. The method according to any one of the preceding embodiments, wherein said
waste is
selected from one or more of waste fractions derived from municipal, county or
state related
or regulated activities, sludge from waste water treatment plants, fiber or
sludge fractions
from biogas processing, general waste fractions from the public sector
containing paper or
other organic fractions.
54. The method according to any one of the preceding embodiments, wherein said
waste is
subjected to pre-treatment prior to step a).
CA 03196334 2023- 4- 20
66
55. The method according to embodiment 54, wherein said pre-treatment is one
or more of:
acid hydrolysis, steam explosion, oxidation, extraction with alkali,
extraction with ethanol,
sorting, shredding, pulping, pressure, size fractionation, bag opening, free
fall mixing, stirring
or rotation.
56. The method according to any of embodiments 54 to 55, wherein sorted or
unsorted MSW is
size fractionated into fractions, providing a fraction with a size range of
e.g. 0 to 60 cm,
and/or providing an oversize fraction (bulk refuse refraction), such as a
fraction comprising
waste with a size exceeding 60 or more cm.
57. The method according to any of the previous embodiments wherein said pre-
treatment is a
non-pressurised pre-treatment for up to 120 min with a temperature ranging
between 60-
110 C and a steam admission of up to 2 kg/kg dry matter.
58. The method according to any of the previous embodiments wherein the waste
with a dry
matter content above 20% is processed mechanically, e.g. by free fall mixing
while subjected
to pre-treatment and/or to step a).
EXAMPLES
General Methods and Materials used in examples
This part describes the general methods and materials used for the examples
presented in this
application. If deviated from the general methods and materials, this will be
specified in the
example. A schematic overview of the waste treatment process and the
recirculation of the process
water is shown in Figure la. An example of a downstream process is shown in
Figure lb. In the
following examples, the process water from a downstream process being
recirculated into the
bioreactor was reject water obtained from an AD process as shown in Figure lb.
Preparation of municipal solid waste ("model MSW") model substrate
CA 03196334 2023- 4- 20
67
In the below examples wherein MSW model substrate was used, 50 kg "model MSW"
was prepared
in order to mimic the composition of real municipal solid waste. The model
substrate was prepared
essentially as disclosed in e.g. W02016/030480.
The model substrate consisted of 3 fractions:
- 41% vegetable fraction (cf. Table 1)
- 13% protein/fat fraction (animal origin) (cf. Table 2) and
- 46% cellulosic fraction (cf. Table 3).
Table 1: Vegetable fraction of MSW model substrate (for production of 50 kg
model MSW)
% of vegetable fraction Weight in kg
Onions 7.5 1.538
Carrots 7.5 1.538
Potatoes 6.3 1.292
Leeks 4.4 0.902
Salad 3.2 0.656
Frozen peas 4.4 0.902
Tomatoes 3.2 0.656
Cucumber 3.2 0.656
Red cabbage 3.2 0.656
Mushrooms 3.2 0.656
Oatmeal 3.2 0.656
Cornflakes 4.4 0.902
Apples, bananas, oranges, lemons, pears 4.4 0.902
Remoulade 3.2 0.656
Ketchup 3.2 0.656
Rye bread 6.3 1.292
White bread 9.5 1.948
Cake 3.2 0.656
Flowers 1 0.205
Coffee grounds 1 0.205
Boiled rice 3 0.615
Boiled pasta 3 0.615
Celery 3 0.615
Brussels sprout, kale 3.5 0.718
Beans, lentils 1 0.205
Broccoli 0.25 0.051
Cauliflower 0.25 0.051
Green beans 0.25 0.051
Pineapple 0.15 0.031
CA 03196334 2023- 4- 20
68
% of vegetable fraction Weight
in kg
SUM 99.9 20.480
Table 2: Protein/fat fraction (animal origin) of MSW model substrate (for
production of 50 kg model
MSW)
% of protein/fat fraction (animal
origin) Weight
in kg
Roasted pork 6 0.388
Dog/cat food 6 0.388
Liver pate 5 0.323
Salami 5 0.323
Mortadella 5 0.323
Liver sausage 5 0.323
Ham 5 0.323
Rolled sausage 5 0.323
Hotwings 10 0.647
Spareribs 5.5 0.356
Fat of animal origin with spices 10 0.647
Cheese 4 0.259
Ymer (soured whole milk) 10 0.647
Eggs 3 0.194
Shrimps 3 0.194
Herring 5 0.323
Ground beef 1.5 0.097
Chicken whole 2 0.129
Chicken filet 4 0.259
SUM 100 6.467
Table 3: Cellulose fraction of MSW model substrate (for production of 50 kg
model MSW)
% of cellulose fraction Weight in kg
Milk cartons 30.0 6.9
Newspapers 8.0 1.8
Magazines 2.8 0.6
Advertising materials 9.7 2.2
Phone books 0.7 0.2
Printing paper 2.2 0.5
Gift wrapping 6.2 1.4
Cardboard 9.8 2.3
Paper towel 22.5 5.2
Cotton pads 1.7 0.4
Wood 1.2 0.3
Textiles (dishtowels) 5.3 1.2
CA 03196334 2023- 4- 20
69
SUM 100 123.0
Enzymes
Cellic CTec3Tm was purchased from Novozymes A/S.
CTec3Tm is a state-of-the-art cellulase
and hemicellulase complex comprising GH61 compounds and beta-glucosidases. In
addition,
another enzyme composition with enzymatic activities similar to CTec3 was
tested.
The amount of enzymatic composition to be added to the waste for sufficient
enzymatic treatment
was determined by a solubilization test, as in the definition "Solubilization
test" described above.
It is believed that similar results would be obtained using other commercially
available cellulase
preparations optimized for biomass conversion, such as Cellic CTec2rm
(Novozymes A/S) and
ACCELLERASE 1500TM (Genencor).
CTec2 and Cellic CTec3 as well as ACCELLERASE 1500
each contains endoxylanase activity over 200 U/g, xylosidase activity at
levels over 85 U/g, B-L-
arabinofuranosidase activity at levels over 9 U/g, amyloglucosidase activity
at levels over 15 U/g,
and alpha-amylase activity at levels over 2 U/g according to our assessment.
Simpler isolated
cellulase preparations may also be effectively used to practice methods of the
invention.
Reiect water
Reject water was provided as follows. As part of a feasibility study Dutch
household waste was
transported to Copenhagen and processed at the Renescience demonstration
facility at Amager,
Denmark. The waste was entered into a bioreactor with a length of 16 m and a
diameter of 2.5 m
resulting in a volume of 78.5 m3 for combined enzymatic and microbial
treatment at 50 C with a
total load (waste + water) of 13.5 tons. The retention time in the bioreactor
was 20.6 hours, the tap-
water: MSW ratio was 1.9, and the enzyme dose was 0.97% w/w
CTec3 purchased from
Novozymes A/S). The produced bioliquid underwent treatment in a CSTR
(Continued Stirred
Reactor) at the Technical University of Denmark. The pilot-scale CSTR was a
mobile SEAD anaerobic
digester provided by VEOLIA BiothaneTM. The SEAD anaerobic digester was a 500
Liter tank (00.6
x 2.1 m) where the biological anaerobic digestion took place. The contents of
the AD-tank were
mixed due to the reinjection of the biogas at the bottom of the reactor (230
L/h), and a recirculation
pump (2-6 m3/h). The recirculated fraction of the digestate was reinjected
through a nozzle, which
CA 03196334 2023- 4- 20
applied shear forces and facilitated the disintegration of particulate matter.
The Biothane SEAD
digester was equipped with an internal vertical tubing from top to bottom of
the digester. The inner
tubing was perforated at the bottom and allowed circulation of digester
content to spread
throughout the digester tank.
The SEAD digester was equipped with on-line monitoring of pH and temperature
and was connected
to the feeding pump and the sampling outlet. The digestate was discharged by
overflow to a settling
tank (0 0.25 x 0.8 m) where sludge and water were passively separated. The
lower fraction of the
digestate in the settler tank was recycled back to the main digester tank, the
supernatant was
discharged by overflow. The feedstock was stored in a 100 Liter tank, which
was constantly agitated.
A 5 mm mesh prevented the introduction of too large particles into the feed
tank.
The resulting digestate from the SEAD was stored frozen, then thawed and
centrifugated to allow
separation of solid digestate from the remaining reject water on a Thermo
Scientific SL4OR
centrifuge (4700 rpm, 15 minutes, 4C) to precipitate most of the suspended
solids like cells,
remaining fibers and inorganics.
The pH of the thus obtained reject water was 8.35 and the alkalinity total was
determined by manual
titration of 50 mL sample of reject water with 5 mL of 1 M HCI to bring the pH
of the resulting
solution below 4. In industrial scale, the process should operate under
conditions where the
digestate and/or a fraction thereof such as reject water is re-circulated to
the bioreactor. This will
result in a higher concentration of salts and could also increase the
alkalinity of the reject water. In
order to obtain a reject water with higher salt concentrations a suitable
amount of reject water
were placed in a heated open vessel until the volume of the reject water had
been reduced to about
half of the initial volume. This was the reject water that was used in the
following examples unless
otherwise mentioned in the examples 1 to 5.
Example 1: Liquefaction of MSW model substrate
Model system for waste liquefaction
For the Lab scale experiments in Examples 1, 2, 3, 4, and 5, fermentations
were performed in
SartoriusTM 1 L or 5 L fermenters equipped with mechanical stirrer, heating
mantle, cooling tower
for exhaust gases and pH-meter, The filling degree of the fermenter comprising
MSW model
CA 03196334 2023- 4- 20
71
substrate, enzymes and water and/or reject water was approximately 80-90 %
(v/v). The
temperature was kept at 50 C using an electrical heating mantle and the
stirring was 600 rpm except
for the first hour where more vigorous stirring was used to initiate the
solubilization (1200 rpm).
The added components (solids and liquids) were not pre-heated prior to
addition into the
fermenter.
Sample acquisition
Samples of about 10 mL were withdrawn from the fermenters as indicated in the
tables below and
subjected to centrifugation to remove solids (3600 rpm for 10 minutes). The
supernatant was
subsequently heat-treated to inactivate the enzyme (100 C, 10 minutes). The
samples were
subsequently subjected to a second centrifugation (4000 rpm for 10 minutes)
and filtered through
a 0.2 ilm PTFE filter (PhenexTM) and subsequently subjected to HPLC analysis.
HPLC analysis
The concentrations of relevant compounds such as sugars, organic acids and
ethanol were
measured using an UltiMate 3000 HPLC (Thermo Scientific DionexTM) equipped
with a refractive
index detector (Shodex(R) RI-101) and a UV detector at 250 nm. The separation
was performed on
a Rezex RHM monosaccharide column (PhenomenexTM) at 80 C with 5 mM H2504 as
eluent at a
flow rate of 0.6 mi./minute. The results were analyzed using the Chromeleon
software program
(DionexTM).
Standard liquefaction of MSW model substrate using de-ionized water
Liquefaction of MSW model substrate was carried out using 166 g MSW model
substrate, 1 L de-
ionized water and 4 g Cellic CTec3 (Novozymes A/S). First water and MSW model
substrate was
heated to 50 C while stirring (300 rpm). Upon reaching the desired
temperature, Cellic Ctec3 was
added (4 g) and the stirring increased to 1200 rpm for 5 minutes and
thereafter to 900 rpm for 1
hour. After 1 hour stirring was decreased to 600 rpm until the end of the
experiment. The content
of glucose, xylose, lactate, acetate was measured using HPLC at time points
16, 24, 40, 48 and 64 as
shown in Table 4.
CA 03196334 2023- 4- 20
72
Table 4: Data obtained using 166 g MSW model substrate, 4 g Cellic CTec3 and
de-ionized water
(all in g/L).
Time (h) Glucose Xylose Lactate Acetate pH
16 14.1 1.6 4.5 0.2 4.91
24 14.1 1.9 6.4 0.2 4.77
40 14.2 2.2 7.5 0.3 4.60
48 15.5 2.5 8.7 0.4 4.57
64 13.1 2.3 8.3 0.5 4.49
Figure 2 shows pH measured in the fermenters as a function of time. There is a
clear acidification
during the first 24 hours which is mainly due to the formation of lactic acid.
After about 24 hours
pH has dropped to about 4.8 and the production rate of lactic acid had
decreased significantly (1.9
eL generated in 8 h (16-24) vs. 2.3 g/L generated in 24 h (24-48). After 48h
the production of lactate
halts and the pH was 4.57. After 64 hours the pH reached 4.49 and the
concentrations of glucose
and lactic acid were 13,1 gn. and 8.3 g/L, respectively. We decided to
investigate if it was possible
to increase the production of lactic acid by continuously neutralizing the
formed lactic acid using
reject water (cf. (B) and (C) below) i.e. increasing the pH in the bioreactor
by adding alkaline reject
water.
Example 2: Effect of adding reject water to the bioreactor with and without
prior pH adjustment
The same experimental set-up and conditions as in Example 1 was applied.
However, in order to
determine the effect of reject water with a pH about 8.3 (prepared as
described in the introductory
section of the examples) on the fermentation of MSW model substrate, a series
of experiments
using reject water instead of de-ionized water with neutral pH were performed.
One fermenter was
run using reject water (1 L), MSW model substrate (166 g) and 4 g Cellic
Ctec3. In three other
fermenters, the reject water was titrated prior to entry into the fermenter to
pH 7, pH 6, or pH 5,
respectively using acetic anhydride, which is assumed to be hydrolyzed in the
solution to acetic acid.
CA 03196334 2023- 4- 20
73
Table 5 Data obtained using 4 g Celli& Ctec3 and reject water (all in g/L).
Time (h) Glucose Xylose Lactate Acetate pH
0.5hr 1.8 1.2 0.1 0.1 7.29
16.5 0.9 1.1 5.1 0.7 6.67
24.5 1.4 0.3 3.0 0.5 6.31
41.5 2.0 0.5 4.3 0.7 5.99
48.5 4.4 1.0 5.9 1.2 5.80
64.5 3.7 1.2 7.3 1.3 5.48
72.5 3.6 1.2 7.4 1.3 5.41
88.5 3,0 1.3 8.3 1.5 5.28
161.5 0,2 1.1 12.4 1.1 5.00
Table 6 Data obtained using 4 g Cellic Ctec3 and reject water pH adjusted to
7.0 (all in g/L).
Time (h) Glucose Xylose Lactate Acetate pH
0.5 4.3 2.0 0.1 0.6 7.03
16.5 4.3 1.3 2.5 1.8 6.67
24.5 2.1 0.5 2.2 1.0 6.07
41.5 2.8 1.0 11.2 2.1 5.23
48.5 2.2 1.1 12.0 2.1 5.11
64.5 1.0 1.3 13.9 2.1 4.90
72.5 0.2 1.3 14.0 2.0 4.89
88.5 0.2 1.4 14.7 2.0 4.84
161.5 1.0 1.6 15.5 2.4 4.85
Table 7 Data obtained using 4 g Cellice Ctec3 and reject water pH adjusted to
6.0 (all in g/L).
Time (h) Glucose Xylose Lactate Acetate pH
0,5 8.7 3.0 0.1 3.5 6.14
16.5 12.2 2.1 6.3 3.8 5.64
CA 03196334 2023- 4- 20
74
Time (h) Glucose Xylose Lactate Acetate pil
24.5 6.3 1.2 5.6 2.2 4.95
41.5 10.8 2.4 12.1 3.9 4.64
48.5 10.9 2.4 12.1 3.9 4.62
64.5 11.1 2.5 11.9 4.0 4.64
72.5 11.0 2.5 11.9 3.7 4.65
88.5 10.8 2.5 12.0 3.8 4.65
Table 8 Data obtained using 4 g Cellic Ctec3 and reject water pH adjusted to
5.0 (all in g/L).
Time (h) Glucose Xylose Lactate Acetate pH
0.5hr 10.1 3.3 0.1 6.1 5.48
16.5 17.3 4.3 0.3 6.8 5.59
24.5 8.1 1.9 2.2 3.6 5.15
41.5 14.8 2.8 8.1 7.0 4.86
48.5 14.6 2.9 8.2 7.1 4.86
64.5 14.5 3.0 8.1 7.3 4.87
72.5 14.1 2.9 7.7 6.6 4.90
88.5 14.2 2.9 - 7.7 6.7 4.94
Figure 3 shows the pH during the fermentations of IVISW model substrate using
reject water with or
without pH adjustment prior to fermentation.
When using reject water without pH adjusting the production of lactic acid is
slow and it takes about
60 hours before reaching pH 5.5. This is believed to be caused by a lower
enzymatic activity at higher
pH which in turn may generate less glucose for microbial growth, thus delaying
the production of
lactic acid. By pH adjusting the reject water the process is accelerated
although in all cases it is
slower than with tap water (neutral pH) cf. Example 1.
Example 3: Effect of pH upon addition of reject water
CA 03196334 2023- 4- 20
Reject water (prepared as described in the above introductory section to the
Examples) was added
portion-wise to the bioreactor.
The fermentation was started using 250 g of reject water, 1.0 g Cellic Ctec3
and 41 g MSW model
substrate. The initial pH in the 1L SartoriusT* fermentor was >7. After 26
hours pH had decreased
below 5.0 (this is termed time = 0 hours). HPLC analysis showed both glucose
(5.2 g/L) and lactate
(6.7 g/L) present at time = 0 hours. Reject water, Cellic Ctec3 and MSW was
added at various time
points (indicated as the number of hours after time = 0 hours) as described in
Table 9 below. At time
= 15 hours the concentration of lactate was 13.1 g/L and glucose 4.1 gA,
respectively. At time = 39
hours the concentration of lactic acid was 14.3 g/L and glucose 4.2 FA,
respectively.
Table 9: outline of MSW, reject water and CTec3 addition at time 0, 15 and 39
hours and
corresponding pH values measured in the fermenter
Time (hours after pH before Amount of MSW model pH after
time =0) addition* substrate, reject water addition**
and CTec3
0 <5 41 g MSW model substrate > 7
250 ml reject water
1 g Cellic Ctec3TM
<5 41 g MSW model substrate > 7
250 ml reject water
1 g Cellic Ctec3TM
39 <5
* pH measured at the indicated time just before addition of MSW model
substrate, reject water and
CTec3 TM
15 ** pH measured at the indicated time just after addition of MSW model
substrate, reject water and
CTec3 TM
The experiment shows that several smaller additions of reject water could be a
faster way to achieve
the acidification compared to a single addition of a large volume of reject
water. We believe the
faster acidification was achieved by adding smaller additions of reject water
and is the result of
mainly two factors: 1) there is already an established soluble producing
community when the reject
water is added. 2) the presence of bioliquid with low pH limits the pH
increase due to the addition
of the reject water, which in turn allows the enzymes to function more
efficiently in the conversion
of the added MSW. Accordingly, repetitive pH adjustments of the fermenter
after appropriate
CA 03196334 2023- 4- 20
76
conditions have been established are beneficial for the ability of the system
to return to a state with
low pH and a microbial community capable of producing desired solubles.
Example 4: Addition of reject water with pH 8.3
The fermentation was started using 250 g of reject water, 1.0 g Cellic Ctec3
and 41 g MSW model
substrate. The initial pH in the 1 L SartoriusTM fermenter was >7. After 26
hours, the pH was below
5Ø Then pH control was set to pH=6.0 and reject water with a pH of 8.3 that
was obtained as
described in the introductory section to the Examples was automatically pumped
into the system
to continuously keep pH at 6Ø After 24 hours, the addition of reject water
automatically ceased
because the pH did not drop below 6, assumingly because there was no more
sugar available for
the microbial conversion. A total of 433 mL of reject water had been added
during the first 24 h of
this experiment. Then 1.0 g Cellic Ctec3 and 41 g MSW model substrate were
added. After 30 h an
additional 464 mL of reject water had been added automatically in order to
keep the pH constant.
The experiment shows that continuous addition of reject water provides the
benefit of maintaining
pH of the reaction mixture in the optimal range (pH 4.0-6.0) for both
enzymatic degradation and
production of solubles.
From the amount of MSW model substrate (41 g) about 6 g of solubles is
expected to be produced
which corresponds to 0.070 moles when the soluble is lactic acid. For the
reject water, a titration
indicated a concentration of about 0.1 M which corresponds to 700 mL. In this
experiment, we used
a total of 897 mL reject water. This indicates that there is a direct
correlation between the amount
of MSW processed in the reaction and the volume of reject water added.
Obviously, this correlation
is dependent both on the content of available sugar in the MSW (i.e. food
waste and cellulosic
material) as well as the alkalinity of the reject water used.
Example 5: Modelling a continuous process by sequential removal of bioliquid
and waste and
addition of aliquots of reject water, MSW and enzyme
The fermentation was started in a 5 Liter fermenter with 3 Liter de-ionized
water, 500 g MSW model
substrate and 12 g Cellic Ctec3TM. The next day pH had dropped to 4.3 and the
concentration of
CA 03196334 2023- 4- 20
77
glucose was 8.0 g/L and lactate 8.8 g/L, respectively. This is indicated as
time = 0 hours (cf. Figure
4). Material (both liquids and solids) was removed from the fermenter at
various time points after
time= 0 and replaced with MSW model substrate, reject water and Cellic Ctec3
as listed in Table
below.
5
Table 10: outline of MSW/glucose, reject water and CTec3 addition at various
time points and
corresponding pH values measured in the fermenter.
Time (hours after pH before Amount of material Amount of
model pH after
time =0) addition* removed from the MSW/glucose,
reject addition**
fermentor (g) water and CTec3
2 4.3 750 83 g MSW model
substrate 4.6
500 ml reject water
2 g Cellic Ctec3TM
6.5 4.6 500 83 g MSW model
substrate 4.9
500 ml reject water
I 2 g Cellic Ctec3TM
11.25 4.9 500 83 g MSW model
substrate 5.34
500 ml reject water
2 g Cellic Ctec3TM
14.5 5.29 1000 166 g MSW model 6.1
substrate
500 ml reject water
4 g Cellic Ctec3TM
24.5 4.8 500 83 g MSW model
substrate 5.1
500 ml reject water
2 g Cellic Ctec3TM
27.5 4.74 500 83 g MSW model
substrate 4.81
500 ml reject water
1 2 g Cellic Ctec3TM
31.5 4.62 500 166 g MSW model
5.15
substrate
1000 ml reject water
4 g Cellic Ctec3TM
37.5 4.53 1500 250 g MSW model
5.55
substrate
1500 ml reject water
4 g Cellic Ctec3TM
47.5 4.52 500 83 g MSW model
substrate 4.72
500 ml reject water
I 2 g Cellic Ctec3TM
49.5 4.68 500 83 g MSW model
substrate 4.96
CA 03196334 2023- 4- 20
78
Time (hours after pH before Amount of material Amount of
model pH after
time =0) addition* removed from the MSW/glucose,
reject addition**
fermentor (g) water and CTec3
500 ml reject water
2 g Cellic Ctec3TM
52.5 4.8 0 83 g glucose
5.02
500 ml reject water
2 g Cellic Ctec3TM
55.5 4.74
* pH measured at the indicated time just before addition of MSW model
substrate, reject water and
CTec3 TM
* * pH measured at the indicated time just after addition of MSW model
substrate, reject water and
CTec3rm
The fact that pH continued to increase until time = 14.5 hours without a
significant pH drop between
the new additions of reject water shows that the microbial conversion of
glucose to inter alia lactic
acid was very slow, which in turn suggest that the soluble producing microbial
population has not
been established.
The pH value at 47.5 hours indicates that at such low pH (4.72) the population
of soluble producing
bacteria again has diminished and therefore the expected decrease in pH is
very slow.
The data point at 52.5 hours and the following rapid decrease in pH shows that
the soluble
producing microbial community has now increased in size again and the system
is capable of
neutralizing the reject water rapidly.
We surmised that the MSW model substrate could be replaced by a more direct
source of glucose.
This could be important in cases where the amount of reject water is large but
the amount of waste
that can be added to the reactor is limited or the waste has a low content of
organics. At time = 52.5
at pH 4.80 we tested addition of 20 g of glucose together with 500 mL reject
water. After three
hours the pH valued had dropped to 4.74.
Example 6: Fermentation of MSW model substrate in a rotating horizontal
reactor with addition of
reject water
CA 03196334 2023- 4- 20
79
An enzyme composition having similar enzymatic activities to Cellic CTec3 was
purchased from
Novozymes A/S and stored at ¨20 C. The enzymes were thawed prior to use.
Superfloc C498HMW
was purchased from Kemira. MSW model substrate was prepared as described in
the introductory
section to the Examples. Reject water was obtained from a large-scale waste-
treatment plant
comprising 2500 m3 intake tank, four 4500 m3 AD digesters, one 2500 m3 post
storage tank at the
Northwich Renescience plant on November 16, 2017. The supernatant of the
reject water obtained
after anaerobic digestion of food waste (naturally pre-hydrolyzed, approx. 25%
dry matter, 90% of
the dry matter was convertible) followed by flocculation using a 0.3% solution
of Superfloc
C498HMW polymer (polymer flow 1220 L/h with 23 m3/h feed) and decantation
using decanter
centrifuges without any additional heat treatment at the anaerobic digestion
system.
A stainless-steel rotating horizontal reactor (total volume 63 L, length 2 m)
was filled with MSW
model substrate (2 kg), tap water (5 L) and an enzyme composition having
similar enzymatic
activities to Cellic CTec3 purchased from Novozymes A/S (32 g) in tap water (1
L). The mixture was
mixed at 50 C under constant rotation (4 rpm). After 48 h, tap water (3 L),
MSW model substrate
(1 kg) and a solution of an enzyme composition having similar enzymatic
activities to Cellic CTec3
purchased from Novozymes A/S (16 g) in tap water (400 mL) were added and the
mixing was
continued for 24 h. MSW model substrate (1 kg), reject water (3 L) and a
solution of an enzyme
composition having similar enzymatic activities to Cellic CTec3 purchased from
Novozymes A/S (16
g) in tap water (400 mL) were subsequently added and the mixing was continued
for 24 h. Then, 10
kg of the reactor content were removed through the outfeeder (located opposite
to the infeed end
of the reactor). Thereafter, MSW model substrate (1.5 kg), reject water (4 L)
and a solution of an
enzyme composition having similar enzymatic activities to Cellic CTec3
purchased from Novozymes
A/S (24 g) in tap water (400 mL) were added to the reactor and the mixing was
continued for 93 h.
The progress of the fermentation was monitored by inline pH measurements and
HPLC analysis to
determine the concentration of glucose, xylose, arabinose, lactate, acetate
and ethanol in the
samples taken from the outfeeder at the time-points shown in the below Table
11.
Figure 5 shows the pH profile of the fermentation of MSW model substrate in
the rotating horizontal
reactor at 50 C (pH probe was mounted approx. 1.75 m away from the infeed end
of the reactor).
CA 03196334 2023- 4- 20
Table 11. Concentration (in g per kg of dry matter) of selected compounds
formed during the
fermentation of MSW model substrate in rotating horizontal reactor at 50 C.
Sample Time (h) Glucose Xylose Arabinose Lactate Acetate
ID
Si 0.67 39.80 4.60 0.00 3.72
2.57
S2 1.25 39.52 4.65 0.00 3.65
2.52
53 1.75 39.89 35.65 0.00 3.66 2.54
54 2.75 38.86 33.70 0.00 3.53 2.42
55 18.75 23.86 25.74 0.00 34.62 7.54
56 20.25 23.85 25.90 0.00 41.27 8.15
57 24.25 22.74 24.55 0.00 51.83 9.94
58 43.25 25.77 22.70 0.00 92.33 16.94
59 44.00 30.89 23.34 0.00 94.56 13.58
510 45.75 37.03 24.35 0.00 96.78 14.23
511* 48.00 45.98 27.73 0.00 108.21 15.62
512 49.75 48.65 27.74 0.00 106.77 15.65
513 50.50 51.19 28.68 0.00 109.44 15.57
514 66.25 64.03 26.14 0.00 113.91 15.91
515 69.92 66.17 26.95 0.00 119.39 16.70
516** 72.50 65.99 26.65 0.00 117.50 16.86
517 74.50 66.97 26.81 0.00 116.45 16.34
518*** 90.50 78.59 32.53 1.46 128.12 16.47
519 98.75 61.83 35.09 1.51 159.26 14.54
520 162.75 28.48 30.70 1.34 218.21 16.68
521 169.58 32.76 31.09 1.37 220.66 17.23
522 189.25
*After 48h, tap water (3 L), MSW model substrate (1 kg) and a solution of an
enzyme composition
having similar enzymatic activities to Cellic CTec3 purchased from Novozymes
A/S (16 g) in tap
water (400 mL) were added
** After 72h, MSW model substrate (1 kg), reject water (3 L) and a solution of
an enzyme
composition having similar enzymatic activities to Cellic CTec3 purchased from
Novozymes A/5 (16
g) in tap water (400 mL) were added
***After 96h, 10 kg of the reactor content were removed and MSW model
substrate (1.5 kg), reject
water (4 L) and a solution of an enzyme composition having similar enzymatic
activities to
Cellic CTec3 purchased from Novozymes A/S (24 g) in tap water (400 mL) were
added.
CA 03196334 2023- 4- 20
81
The addition of reject water (up to 56 vol. %) did not have a negative
influence on the fermentation
process that had been started using tap water. This is demonstrated by the
continuous decrease of
pH and the increase of lactate and sugars concentration after the addition of
reject water.
Example 7: Determination of microflora
The samples listed in the table 12 below were the samples that were obtained
from the mini reactor
in Example 6. The samples were analyzed using the DNeasy PowerSoil Kit
purchased from
QIAGEN. The Quick-Start Protocol from June 2016 provided by the manufacturer
QIAGEN was
followed.
Table 12
Sample Date of Duration after Conc. of Type of Conc.
of
ID sample start
of purified DNA index used purified DNA
experiment [h] 1st PCR [ng/ I] 2nd
PCR
[nevi]
S3 17-11-2017 1.75 2 S505/727 8.60
S6 28-11-2017 20.25 3.4 S506/727 10.00
S12* 29-11-2017 49.75 1.5 S507/728 10.60
S16** 30-11-2017 72.5 2.4 S508/728 12.70
S18*** 01-12-2017 90.5 4.7 S510/728 8.50
S20 04-12-2017 162.75 4 S511/728 9.22
S22 05-12-2017 189.25 2 4.52
DNA purification, 15t PCR, and 2nd PCR conducted on 12-12-2017, 13-12-2017,
and 14-12-2017,
respectively.
*After 48h, tap water (3 L), MSW model substrate (1 kg) and a solution of an
enzyme composition
having similar enzymatic activities to Cellic CTec3 purchased from Novozymes
A/S (16 g) in tap
water (400 mL) were added
** After 72h, MSW model substrate (1 kg), reject water (3 L) and a solution of
an enzyme
composition having similar enzymatic activities to Cellic CTec3 purchased from
Novozymes A/S (16
g) in tap water (400 mL) were added
***After 96h, 10 kg of the reactor content were removed and MSW model
substrate (1.5 kg), reject
water (4 L) and a solution of an enzyme composition having similar enzymatic
activities to
Cellic CTec3 purchased from Novozymes A/S (24 g) in tap water (400 mL) were
added.
CA 03196334 2023- 4- 20
82
DNA purification:
0.25 g of sample was added to a PowerBead Tube (QIAGEN). The tube was gently
vortexed and
mixed. A volume of 60 I of solution Cl was added and inverted several times
or vortexed briefly.
The PowerBead Tubes were secured horizontally using a vortex Adapter tube
holder. The tubes
were vortexed at maximum speed for 10 min. Tubes were centrifuged at 10,000 x
g for 30 s. The
supernatant was transferred to a clean 2 ml collection tube. 250 I of
solution C2 was added and
vortexed for 5 s. Incubation was done at 4 *C for 5 min. The tubes were
centrifuged for 1 min at
10,000 x g. Avoiding the pellet, up to 600 I of supernatant was transferred
to a clean 2 ml collection
tube. 200 I of Solution C3 was subsequently added and vortexed briefly.
Incubation was done at 4
*C for 5 min. The tubes were centrifuged for 1 min at 10,000 x g. Avoiding the
pellet, up to 750 p.I of
supernatant was added to a clean 2 ml collection tube. The solution of C4 was
shaken to be mixed
and 1200 p.I was added to the supernatant. The solution was vortexed for 5 s.
675 p.1 was loaded
onto an MB Spin Column and centrifuged at 10,000 g for 1 min. Flow through was
discarded. The
last step was repeated twice. 500 I of solution C5 was added. Centrifugation
was done for 30 s at
10,000 x g. Flow through was discarded. Centrifugation was done again for 1
min at 10,000 x g. The
MB Spin Column was spaced into a clean 2 ml collection tube. 50 ,1 of
Solution C6 was added to the
centre of the white filter membrane. Centrifugation was done at room
temperature for 30 s at
10,000 x g. The MB Spin Column was discarded. At this point the DNA was ready
for downstream
applications.
Preparation of DNA for sequencing:
After the DNA purification, further preparative steps were conducted before
sequencing on the
IIlumina MiSeq systemTM. The procedure provided by the manufacturer IIlumina
was followed:
(https://support.illumina.comiclownloads/16s_metagenomic_sequencing_library_pre
paration.ht
ml accessed 11-01-2018,16S Metagenomic Sequencing Library Preparation,
Preparing 16S
Ribosomal RNA Gene Amp!icons for the Illumina MiSeq System, Part # 15044223
Rev. B.)
The 165 library preparation workflow was as follows: 1) 1st Stage of PCR, 2)
PCR Clean-Up, 3) 2nd
Stage PCR, 4) PCR Clean-Up 2, 5) Library Quantification and Normalization, and
6) Library Denaturing
an MiSeq Sample Loading.
CA 03196334 2023- 4- 20
83
The purified DNA samples were prepared using the 16S PCR step described in the
procedure.
Primers were added to the solution and PCR was performed. The solution was
subsequently indexed
according to the Index PCR procedure using index primers. After index PCR the
solutions were
purified according to the PCR Clean-Up 2 procedure. The solution was
subsequently purified by
removing any free primers and primer dimer species in the PCR Clean-Up 2 step.
The DNA library
was quantified by using fluorometric quantification method using dsDNA binding
dyes. The DNA
concentrations were calculated based on analysis made with a Qibit 3=0TM= The
library was
normalized and pooled. The pooled libraries were denatured and loaded onto a
MiSeq system. After
the loading of samples, the MiSeq system provided an on-instrument secondary
analysis using 16S
metagenomics database. As known in the art, the determination of a specific
species may depend
on the specific database applied and may in fact include more than one species
or may refer to a
species that can/will/may be classified differently in another database.
However, regardless of the
database applied and the possible specific species identification applied by
various databases, for
the present purpose, the distinction between lactic acid producing bacteria
and non-lactic acid
producing bacteria should be the same.
Conclusions
Figure 6 describes the % of lactic acid producing bacteria (comprising
bacteria of the lactic acid
bacteria order "LAB" where the currently accepted taxonomy is based on the
List of Prokaryotic
names with Standing in Nomenclature (LPSN) - an online database that maintains
information on
the naming and taxonomy of prokaryotes, following the taxonomy requirements
and rulings of the
International Code of Nomenclature of Bacteria. The phylogeny of the order is
based on 16S rRNA-
based LTP release 106 by 'The All-Species Living Tree' Project. The lactic
acid producing bacteria
referred to here also comprises bacteria that do not belong to the LAB order,
but that are
nevertheless capable of producing lactic acid) compared to all other bacterial
species present in the
samples at seven different timepoints in the experiment (termed S3, S6, S12,
S16, S18, S20 and S22
and defined in Table 12).
CA 03196334 2023- 4- 20
84
1.75 hours after waste, de-ionized water and enzymes were added to the mini
reactor (53) 33% of
the bacterial population in the reactor was comprised of lactic acid producing
bacteria. While all
other bacterial species made up the remaining 67%.
After 20.25 hours (S6), the amount of LAB had increased to 55% of the
population while other
bacterial species had decreased to 45%.
At 49.75 hours (512) and 72.5 hours (516) lactic acid producing bacteria
dominated the population
with 79% and 76%, respectively, compared to 21% and 24% of other bacterial
species, respectively.
After 72.5 hours (516) reject water was added. At 91 hours (518), the total
amount of lactic acid
producing bacteria had decreased to 51%, while other bacterial species had
increased to 49%.
Interestingly, after 162.75 hours (S20) and 189.25 hours (S22), the presence
of lactic acid producing
bacteria had increased again to 77% and 65%, respectively. Other bacterial
species had decreased
to 23% and 35%, respectively.
Thus, even after the introduction of many other bacterial species and higher
pH via reject water and
a decrease in lactic acid producing bacteria population size after 91 hours,
lactic acid producing
bacteria were surprisingly still able to regain the lead in terms of
population size within the
bioreactor compared to other bacterial species. Even after significant change
of the environment in
the bioreactor lactic acid producing bacteria were surprisingly still the main
bacterial contributor.
Figures 7a ¨ 7d describe the bacterial species that dominate the population
compared to all other
bacteria present in the samples at seven different timepoints in the
experiment (termed 53, 56, 512,
S16, S18, 520 and S22 and defined in Table 12). Figures 7a to g are described
below.
7a) 1.75 hours after waste, de-ionized water and enzymes were
added to the mini reactor
(53), Calothrix parietina dominated the bacterial population with 29% compared
to all other
bacterial species (71%).
After 20.25 hours (56), the bacterial population was dominated by Bacillus
coagulans
with 55% compared to 45% or other bacterial species. Note that this was equal
to the entire amount
of lactic acid producing bacteria (Figure 6) in this sample. B. coagulans is a
well-known producer of
lactic acid bacteria (T. Michelson et al., 2006, Enzyme and Microbial
Technology).
CA 03196334 2023- 4- 20
7b) At 49.75 hours (512) the population of B. coagulans had increased to
75% compared
to 25% of other bacterial species. Note again that B. coagulans represented
almost the entire lactic
acid producing bacteria population (Figure 6)
At 72.5 hours (516) the population of B. coagulans was stable at 73% again
representing the vast majority of the entire lactic acid producing bacteria
population. Reject water
was added after this sample.
7c) Reject water was added at 91 hours (518). The population of B.
coagulans decreased
to 51% making up the majority of the entire lactic acid producing bacteria
population. This shows
that, B. coagulans was struggling with the addition of reject water.
Indeed, after 162.75 hours (S20) the bacterial population was dominated by
Lactobacillus ultunensis with 53 % Note that, apart from L. ultunensis the LAB
comprised further
24% of other lactic acid producing bacteria species, suggesting that other LAB
species than L.
ultunensis can thrive in the bioreactor environment after addition of reject
water.
7d) After 189.25 hours (522), L. ultunensis was still the dominating
species at 44%.
It is highly surprising that lactic acid producing bacteria can survive an
environment comprising
reject water. Surprisingly the bacterial population changed from one dominant
lactic acid producing
bacteria species to another as a consequence of the change of the bioreactor
environment caused
by the addition of reject water.
Figures 8a to 8d describe the five most dominating species in the population
compared to all other
bacterial species present in the samples at the same seven timepoints in the
experiment (termed
53, 56, S12, S16, 518, S20 and S22 as defined in Table 12). "Unclassified"
denotes a bacterial species
the methodology is unable to classify, possibly a novel species not present in
any database. Figures
8a to 8d are described below.
8a) 1.75 hours after waste, de-ionized water and enzymes were
added to the mini reactor
(S3), Calothrix parietina dominated the bacterial population with 29%,
followed by 5% of
Leuconostoc sp. and 5% unclassified bacteria. Weissella viridescens and
Leuconostoc mesenteriodes
were present at 4% and 3% respectively. Other bacterial species comprised 54%
of the total
CA 03196334 2023- 4- 20
86
bacteria. Leuconostoc sp., W. viridescens and L. mesenteriodes are all lactic
acid producing bacteria.
None of these remained as a dominant species of the population over time (see
below).
After 20.25 hours (56), the bacterial population was dominated by B. coagulans
with
55% and Bacillus thermoamylovorans at 17%. Unclassified bacteria, Bacillus sp.
and
Sporolactobacillus putidus were present in the amount of 8%, 3% and 2%,
respectively. Other
bacterial species comprised 15% of the total bacteria. There was a clear
bacterial population shifts
compared to Figure 7a. All top species had changed i.e. bacteria able to
survive in the bioreactor
environment increased in population size. The bioreactor population was
dominated by lactic acid
producing bacteria. Note that S. putidus is a lactic acid producing bacteria.
8b) At 49.75 hours (S12) the population of B. coagulans had increased to
75%. This was
followed by "Unclassified bacteria" (6%), Bacillus sp. (3%), S. putidus and
Enterococcus lactis (2%)
and other bacterial species at 12%. Note that several of the top species from
Figure 8a are still
present showing that the population is stabilizing. Note that E. lactis is a
lactic acid producing
bacteria.
At 72.5 hours (516) the population of B. coagulans was stable at 73% again
representing the vast majority of the entire lactic acid producing bacteria
population. This was
followed by "Unclassified bacteria" (11%), Bacillus sp. (3%), Lactobacillus
ultunensis (2%), S. putidus
(1%) and other bacterial species at 11%. Note that L. ultenensis is an lactic
acid producing bacteria.
This showed that the bioreactor population was stable. Reject water was added
after this sample.
8c) At 91 hours (S18) the population of B. coagulans decreased to 51%. The
stable
conditions were interrupted by the reject water and this affected the
bacterial population
significantly. "Unclassified bacteria" (13%), Aminobacterium sp. (7%), S.
putidus (3%),
Anaerobaculum sp. (2%) and other bacterial species at 24% were also present.
Except for B.
coagulans and "Unclassified bacteria" the other dominant species changed
compared to Figure 8b
due to the changing environment in the bioreactor. Other bacterial species
increased from 10% to
24% suggesting a shift in the entire population.
Indeed, after 162.75 hours (520) the entire bacterial population was dominated
by
another lactic acid producing bacteria species, Lactobacillus ultunensis with
53 % B. coagulans had
decreased to 8%, again suggesting that the new environment in the bioreactor
was not favoring this
CA 03196334 2023- 4- 20
87
species. "Unclassified bacteria" (8%), Lactobacillus sp. (3%), Aminobacterium
sp. (3%), and other
bacterial species at 25% were also present. The entire population made a shift
towards becoming
stable again with new dominating species.
8d) After 189.25 hours (522), L. ultunensis was still the
dominating species at 45% followed
by "Unclassified" bacteria (15%), B. coagulans (5%), Aminobacterium sp. (4%),
Lactobacillus
delbrueckii (3%) and other bacterial species at 28%. Note that L. delbrueckii
is a lactic acid producing
bacteria.
It is highly surprising that lactic acid producing bacteria can survive an
environment comprising
reject water. Surprisingly the bacteria population changed from one dominating
LAB species to
another as a consequence of the change of the bioreactor environment caused by
the addition of
reject water.
Example 8: comparison of Anaerobic Digestion of Open and Closed loop Bioliquid
As mentioned in the introductory section to the Examples, the AD-effluent
obtained from anaerobic
digestion was obtained using bioliquid produced from Municipal Solid Waste in
the Renescience
demonstration scale at the Amager Resource Center, Denmark. This Renescience
demonstration
plant did not comprise bioliquid utilization means and water make-up units and
is accordingly a
plant wherein no recirculation of water is applied. For the purposes of this
example, this is termed
an "open loop" plant. Consequently, the resulting concentrations of salts and
ammonia in the
bioliquid obtained in this demonstration plant (which may be mentioned as
"Renescience bioliquid"
for the purpose of this example, being said term not limited to the scope of
the present example)
and further used for anaerobic digestion were lower than those expected in
bioliquid obtained from
a plant wherein water other than tap water is added to the bioreactor. A
"closed-loop" plant is
accordingly a waste treating plant wherein the combined enzymatic and
microbial treatment of
waste comprises recirculation of water.
Both modelling and experimental results within the field of anaerobic
digestion show that the
conversion rates and biomethane yields are negatively affected by sodium
concentration
(Hierholtzer, A et al 2012; Modelling sodium inhibition on the anaerobic
digestion process; Water
Science and Technology; 1565-1573). In Fact, the cited study shows that there
can be perceived
CA 03196334 2023- 4- 20
88
inhibitory effects on anaerobic digestion already after addition of 0.083
mol/L (1.91 g/L) of sodium.
In analogous manner, the increase of ammonia concentrations usually results in
a loss of the
methanogenic activity, as well as decrease in the biomass growth (Chen, Y et
al .2008; Inhibition of
anaerobic digestion process: a review. Bioresource Technology, 99(10), 4044-
64).
Since the soluble salt and ammonia concentrations have great potential to
negatively affect the
convertibility of the substrates within anaerobic digestion, experiments were
performed to
compare the anaerobic digestion of bioliquid with different concentrations of
sodium, ammonia and
calcium.
Experimental Description:
In this study, conventional continuous stirred tank reactors (CSTR) for biogas
production were
operated with Renescience bioliquid and with Renescience bioliquid
supplemented with sodium,
calcium, chlorine, and ammonia; respectively, to compare AD from open-loop
bioliquid with AD of
closed-loop bioliquid in agreement with predicted mass balance wherein the
water is re-circulated.
Continuous tests were performed for at least 4 full retentions at a hydraulic
retention time (HRT) of
days, which can be the nominal flow rate expected for operation of full-scale
anaerobic digesters
treating the bioliquid downstream of a process according to step a) of the
present invention.
Materials:
20 1. The initial seed (inoculum) for the anaerobic digestion was from
Foulum Biogas, a plant
in Denmark for converting manure and fibre rich residues from agricultural
straw into biogas by
anaerobic digestion.
2. Renescience bioliquid obtained from combined enzymatic and microbial
treatment of
Dutch Municipal Solid Waste from 2016, produced in a demonstration facility in
Amager, Denmark.
The bioliquid was screened through a 2 mm screen mesh to reduce clogging in
the lab scale CSTRSs.
3. Two 10 litre biogas reactors: a CSTR to treat Renescience bioliquid with
enhanced salts
and ammonia concentration (closed loop) and the second with Renescience
bioliquid comprising
lower concentration of salts and ammonia (open loop) as positive control.
4. Tedlar gas sampling bags US atmospheric gas methods and GC
quantification of
methane production.
CA 03196334 2023- 4- 20
89
5. Gas Flow measuring device. Bioprocess Control, Sweden.
Methods:
The experiment was conducted through continuous feeding of the two 10 litre
CSTR reactors which
were previously seeded with inoculum and spiked for a week with bioliquid to
maintain active
microorganisms. The agitation of the active volume content was achieved
through active
recirculation through a peristaltic pump. The process of the rectors was held
in the mesophilic
temperature range at 38 C achieved with heating jacket in each reactor.
Samples were taken
periodically inside the reactor through a sampling port.
The closed-Loop CSTR digester was used to convert bioliquid supplemented with
sodium chloride,
calcium bicarbonate and ammonia to resemble the expected concentration of the
high salts AD feed
from the mass balance calculations. The substrate was also screened through a
2 mm kitchen mesh
sieve to remove larger particles and reduce the risk of clogging of the
recirculation of the lab-scale
pumps. Characterization of the substrate was based on the following expected
salt and ammonia
concentrations being in accordance with the NREL (National Renewable Energy
Laboratory, US)
guidelines.
The salt and ammonia concentrations were adjusted to meet the expected salt
and ammonia
concentrations of a bioliquid substrate from a closed-loop system:
Na: 2.3 ei ; Cl: 2,3 g/L ; TAN (Total Ammonia Nitrogen): 2,5 eL ; Ca: 5.1 eL.
The ramp up for all the reactors started at 50 days HRT and the feed rate was
increased until
achieving 20 days HRT in a period of 5 days. When the target HRT was achieved,
the reactors were
operated for at least 6 total retention times. Operation of the reactors for
different entire retentions
ensures that the digesting media of the biomethane reactors have been replaced
completely. After
having started the second retention of the experiment, corresponding with day
25 of operation, the
collected effluent was stored as representative effluent of the process to
quantify the salt and
ammonia concentrations of the reactor. The obtained yields in the reactor fed
with bioliquid
resembling "closed loop" bioliquid were compared to the yields obtained in the
control reactor fed
with bioliquid resembling "open loop" bioliquid, i.e. bioliquid without the
addition of salts and
ammonia.
CA 03196334 2023- 4- 20
Biogas produced during the anaerobic digestion process was quantitatively
monitored by a gas flow
device obtained from Bioprocess Control AB (Lund, Sweden). Gas composition was
determined via
gas chromatography, (model GC82 Mikrolab Aarhus A/S, Denmark. Chemical oxygen
demand (COD)
and volatile fatty acids (VFAs) in the digestate were quantified with Hach
LCK514 COD and LCK365
Organic Acid cuvette test using a DR 3900 spectrophotometer (Hach, Dusseldorf,
Germany).
Results:
A continuous biogas test was performed to estimate the biogas yield of the
Renescience bioliquid
with increased salt and ammonia concentrations to simulate a full-scale
Renescience AD process
comprising a closed loop. As control, the Renescience bioliquid was produced
in the open loop demo
scale Renescience pilot plant in Amager, without salt addition was also tried
out at the same process
conditions ("Digester A").
The production of methane from the CSTR reactors during the four retention
times of 20 HRT is
shown in Figure 10. The data shows that it was possible to produce methane
using the concentrated
closed-loop bioliquid as substrate for anaerobic digestion. The trend of the
methane production
during 20 days HRT indicated that the process remained stable during the whole
operation of the
reactor. This experiment shows that increased concentration of Na, Cl, Ca and
ammonia
corresponding to the expected salt and ammonia concentrations in bioliquid
obtained from a
closed-loop plant surprisingly did not have a detrimental effect of the
anaerobic digestion of the
Renescience bioliquid at the studied conditions.
Example 9: Fermentation of MSW model substrate in a fermenter at pH above 6
Ammonium bicarbonate and ammonia solution used in the experiment were obtained
from Sigma
Aldrich.
A 1-L Sartorius fermenter was filled with MSW model substrate (166 g) and tap
water (900 mL).
NH4FICO3 (13 g) and ammonia (aqueous, 25%, 2 mL) were dissolved in tap water
(100 mL) and the
solution was added into the fermenter. The mixture was brought to 50 *C under
constant stirring
(500 rpm). After the mixture had reached 50 C, an enzyme composition having
similar enzymatic
activities to Cellic CTec3 purchased from Novozymes A/S (4 g) was added into
the fermenter and
the fermentation was conducted at 50 C under constant stirring (500 rpm).
CA 03196334 2023- 4- 20
91
Table 13. Concentration (in g/L) of selected compounds formed during the
fermentation of MSW
model substrate in a fermenter with the addition of NH4FIC03 and ammonia
Time (h) Glucose Xylose Lactate Acetate Propionate Butyrate Formate pH
1.5 0.69 0.00 1.28 _ 0.53 0.00 0.00 0.00
7.80
3.4 0.77 0.00 1.31 0.59 0.00 0.00 0.00
7.88
5.2 0.81 0.00 1.30 0.58 0.00 0.00 0.00
7.95
22.0 0.18 0.00 2.75 0.86 0.00 0.00 0.29
7.94
24.1 0.19 0.00 3.11 0.97 0.00 0.00 0.45
7.90
29.2 0.14 0.13 3.56 1.16 0.00 0.00 0.75
7.68
45.2 0.12 0.62 5.78 2.29 0.00 0.09 2.48
6.98
51.7 0.00 0.88 5.77 2.49 0.00 0.19 2.81
6.57
68.2 0.00 1.07 5.60 3.23 0.10 0.80 3.34
6.15
_
75.7 0.00 0.94 4.82 3.17 0.00 1.48 3.38
6.17
92.2 0.00 0.16 1.57 2.60 0.00 4.35 3.50
6.45
100.2 0.00 0.00 0.23 2.53 0.11 5.68 3.55
6.66
165.4 0.00 0.00 0.00 3.99 0.24 6.03 3.29
6.46
171.2 0.00 0.00 0.00 3.93 0.20 5.82 3.26
6.49
189.4 0.00 0.00 0.00 4.35 0.27 5.86 2.94
6.49
Conclusion. As can be seen from Figure 11 and Table 13, pH during the
experiment was always above
6 which led to the disappearance of lactic acid and to the production of some
other organic acids
(formic, propionic and butyric). These newly formed acids are undesirable in
the bioliquid because
they might create difficulties for the AD process. Thus, this experiment
demonstrated that the pH
of the fermentations should not be above pH 6.
Example 10: Fermentation of MSW model substrate in a fermenter at constant pH
3.5
HCI (aqueous, 4 M) used in the experiment were obtained from Sigma Aldrich.
A 1-L Sartorius fermenter was filled with MSW model substrate (166 g) and
distilled water (1 L). pH
of the mixture was adjusted to 3.5 with a 4 M aqueous solution of HCl. The
mixture was brought to
50 C under constant stirring (600 rpm). After the mixture had reached 50 C, an
enzyme composition
having similar enzymatic activities to Cellic CTec3 purchased from Novozymes
A/S (4 g) was added
into the fermenter and the fermentation was conducted at 50 C under constant
stirring (600 rpm)
CA 03196334 2023- 4- 20
92
at constant pH of 3.5 (which was maintained automatically by the fermenter by
adding a 4 M
aqueous solution of HCl).
Table 14. Concentration (in g/L) of selected compounds formed during the
fermentation of MSW
model substrate at constant pH of 3.5
Time (h) Glucose Xylose Lactate Acetate Propionate Butyrate Formate pH
0 1.52 0.00 0.18 0.23 0.00 0.00 0.00
3.50
1.8 8.86 2.06 0.21 0.25 0.00 0.00 0.00
3.50
20.8 15.74 3.50 0.28 0.27 0.00 0.00 0.00
3.50
45.6 17.77 3.68 0.33 0.22 0.00 0.00 0.00
3.50
139.6 20.35 3,49 0.37 0,27 0.00 0.00 0.00
3.50
Conclusion. The experiment showed that at pH 3.5 there was almost no lactate,
acetate and other
carboxylates produced that is an indication the preferred bacterial activity
is still present at pH 3.5
but is reduced compared to the bacterial activity at a pH between 3,5 and 6.
Example 11: Fermentation of MSW model substrate in a rotating horizontal
reactor with addition
of reject water and glucose
Table 15. Composition of MSW model substrate used in the experiment
Component Weight (kg)
Rye bread 0.8
Apples 0.6
Potatoes 2.8
Spread cheese 0.2
Meat sausages 1.6
Newspapers 1.8
Magazines 0.6
Juice cartons 1.6
Saw dust 0.8
Viscose clothes 1.2
Plastic bag (LDPE) 3.2
- Hard plastic 4.8
The components were passed three times through a shredder (Frandsen lndustri
findeler type 5500)
and the resulting mixture was passed once through a pulveriser (Retsch sm300
with a 6-disc rotor).
Dry matter of the thus obtained MSW model substrate was 77%.
CA 03196334 2023- 4- 20
93
MSW model substrate (1 kg, Table X), tap water (3 L) and a solution of an
enzyme composition
having similar enzymatic activities to Cellic CTec3 purchased from Novozymes
A/S (23 g) in tap
water (1 L) were added into a stainless-steel rotating horizontal reactor
(total volume 63 L, length
2 m). The mixture was mixed at 50 C under constant rotation (4 rpm). 24 h
after the beginning of
the experiment, reject water (4 L), MSW model substrate (1,2 kg) and an enzyme
composition
having similar enzymatic activities to Cellic CTec3 purchased from Novozymes
A/S (24 g) were
added. 47 h after the beginning of the experiment, an enzyme composition
having similar enzymatic
activities to Cellic CTec3 purchased from Novozymes A/S (20 g) was added. 49 h
after the beginning
of the experiment, MSW model substrate (1 kg), tap water (2 kg), reject water
(1 kg) and an enzyme
composition having similar enzymatic activities to Cellic CTec3 purchased from
Novozymes A/S
(20 g) were added. 117 h after the beginning of the experiment, 5 kg of the
reactor content were
removed through the outfeeder. 121 h after the beginning of the experiment,
glucose (200 g) was
added into the reactor.
The progress of the fermentation was monitored by pH measurements and HPLC
analysis to
determine the concentration of sugars, organic acids and ethanol in the
samples taken from the
outfeeder at the time-points shown in Table 16.
Table 16. Concentration (in g per kg of dry matter) of selected compounds
formed during the
fermentation of MSW model substrate with addition of reject water and glucose
in rotating
horizontal reactor at 50 C.
Sample Time
Glucose Xylose Lactate Acetate Butyrate Formate Ethanol pH
ID (h)
_
Si
18.50 0.16 2.66 13.43 6.26 0.19 0.00 0.32 4.88-
52
22.58 0.07 2.44 10.71 8.41 0.25 0.00 0.38 4.96
S3* 25.50 3.87 2.48 9.72 6.88 0.18 0.00 0.23 7.84
S4
27.50 0.16 1.87 14.38 7.92 0.21 1.14 0.16 7.67
55
42.50 0.06 0.05 0.00 15.63 13,06 4.41 1.94 6.93
56
44.75 0.00 0.06 0.00 17.32 13.31 4.15 1,82 6.90
57** 49.25 0.11 0.08 0.45 19.48 13.10 3.76 1,37 6.82
S8
51.00 1.70 0.72 3.18 16.62 9.53 2.89 0,98 6.81
S9 114.50 0.12 0.10 0.63 13.31 30.51
4.55 5,03 5.26
S10*** 122.25 61.15 0.00 0.55 14.60 31.40
3.75 4,74 5.25
S11 123.00 52.31 0.00 0.64 13.56 28.24
3.37 4.33 5.25
CA 03196334 2023- 4- 20
94
Sample Time
Glucose Xylose Lactate Acetate Butyrate Formate Ethanol pH
ID (h)
$12 138.50 36.64 0.38 10.82 19.05
26.71 2.83 2.90 5.00
$13 145.50 29.09 0.36 17.62 20.46
26.91 3.00 2.87 4.85
$14 163.58 23.36 0.40 28.22 22.66
29.08 3.16 3.33 4.64
$15 194.00 22.83 1.92 30.84 23.65
29.27 4.60 3.45 4.88
* - after 24 h, reject water (4 L), MSW model substrate (1,2 kg) and an enzyme
composition having
similar enzymatic activities to Cellic CTec3 purchased from Novozymes A/S (24
g) were added, ** -
after 47 h, an enzyme composition having similar enzymatic activities to
Cellic CTec3 purchased
from Novozymes A/S(20 g) were added. After 49 h, MSW model substrate (1 kg),
tap water (2 kg),
reject water (1 kg) and an enzyme composition having similar enzymatic
activities to Cellic CTec3
purchased from Novozymes A/S (20 g) were added, *** - after 117 h, 5 kg of the
reactor content
were removed from the outfeeder. After 121 h, glucose (200 g) was added.
Also, the population of LAB relative to the presence of other bacterial
species was measured as
described in Example 7. As is shown in Figure 12, the percentage of LAB
relative to other bacterial
species dropped severely, when pH was increased. At 26 hours, where pH had
increased to
approximately 7.84, the presence of LAB was reduced and remained low until
after 49 hours where
the pH was still close to pH 7. At 139 hours, where the pH had dropped to
around pH 5, the
percentage of LAB had increased to approximately 90% relative to other
bacterial species.
Conclusions:
The fermentation proceeded successfully with MSW model substrate that is
different from the MSW
model substrate used in the other examples. It is also shown that when the
process operates at pH
>6 for the prolonged amount of time, butyric acid is produced which is
undesired for the AD process.
Moreover, a shift in pH to above 6 changes the composition of the microflora
wherein the relative
% of the bacterial population producing valuable solubles for energy
production is significantly
reduced.
CA 03196334 2023- 4- 20