Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/115355
PCT/US2021/060268
TITLE:
MULTIPURPOSE ACIDIC COMPOSITIONS AND METHODS OF
USE
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims priority under 35 U.S.C. 119 to U.S. Provisional
Application Ser. No. 63/198,956, filed November 25, 2020, and U.S. Serial No.
17/249,793, filed March 12, 2021, which are herein incorporated by reference
in its
entirety including without limitation, the specification, claims, and abstract
as well as any
figures, tables, or examples thereof.
This application is also related to U.S. Patent Application Sec. No.
17/249,784,
entitled Multipurpose Alkaline Compositions and Methods of Use, filed
concurrently
herewith. The entire contents of this patent application are hereby expressly
incorporated
herein by reference, including without limitation, the specification, claims,
and abstract, as
well as any figures, tables, or drawings thereof.
FIELD OF THE INVENTION
The invention relates to multipurpose acidic compositions for cleaning,
including
de-greasing, de-staining, and/or de-liming, and/or sanitizing. The acidic
compositions are
liquids are suitable for use as pre-sprays (i.e., spot treatment) to
beneficially remove
polymerized soils, remove hard water deposit (e.g., calcium carbonate), soap
scum, rust
and other stains (e.g., coffee and tea), including assisting in general
cleaning of difficult
soils, such as fats, oils, cosmetics, and other difficult soils. The acidic
compositions can be
used for pre-treatments for machine and manual warewash in order to enhance
performance of general-purpose products without the inclusion of costly
additives in
conventional specialty detergents. The acidic compositions can include at
least one organic
acid and a solvent or solvent system. If desired, the acidic compositions can
be PPE free
compositions. Methods for using the acidic compositions as pre-treatments,
soaks and/or
application in machine and manual warewash are also provided. Methods for
using the
acidic compositions for removing polymerized oils, carbonized soils, baked on
soils, fats,
oils, stains (e.g., coffee and tea), hard water scale/deposits, and cosmetics
are also
provided.
1
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
BACKGROUND OF THE INVENTION
Acidic cleaning compositions are often used for hard water and mineral deposit
removal, grout and tile cleaning, and the like. The acidic cleaning
compositions generally
attach and dissolve stains by breaking them down for removal. Acidic
compositions are not
generally used for general purpose cleaning or removal difficult soils such as
polymerized
soils. Instead, specialty alkaline detergents are more commonly formulated
with specialty
additives for treating these types of soils. Formulations containing these
specialty additives
are costly. They are also not needed for all markets and types of cleaning,
degreasing, de-
staining, de-liming and/or sanitizing. As a result, often specialty cleaning
compositions or
formulation to include certain specialty additives are not needed for all
applications and/or
markets.
There is use of acidic and alkaline compositions for use as paint strippers,
for
example benzyl alcohol and acids having a pH of about 2.5. However, it is more
effective
and common for alkaline paint strippers to be used at a pH higher than 7.0
with a
neutralized acid or alkaline source, a solvent, and a detergent. These paint
strippers are
used to remove old coatings that are difficult to remove by other methods. The
use of
acidic paint removers is known to work slowly to remove paints, often
requiring contact
overnight or for extended periods of time. These formulations require
hazardous use
precautions as well as they can be hazardous to health and safety.
It is therefore an object of this disclosure to provide a multipurpose acidic
composition combining acids and solvents that can be used as a pre-spray or
spot treatment
composition to remove difficult soils, including polymerized soils, de-stain,
remove hard
water deposits, remove soap scum, remove rust, and assist in general cleaning
of other
difficult soils and stains, including coffee and tea.
It is a further object of the disclosure to provide a multipurpose acidic
composition
that aids in general purpose cleaning of fats, oils, cosmetics, and other
common
institutional soils.
It is a further object of the disclosure to provide a multipurpose acidic
composition
that can be used as a pre-treatment for machine and manual warewash to enhance
or boost
performance of general-purpose products, thereby reducing the use of specialty
additives in
detergent compositions.
2
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
It is another object of this disclosure to formulate multipurpose acidic
compositions
that are PPE free products.
It is another object of this disclosure to formulate multipurpose acidic
compositions
that remove challenging soils including tea stains, coffee stains, hard water
scale/deposits,
polymerized oils, carbonized soils, baked on soils, fats, oils, cosmetics, and
others.
Other objects, aspects and advantages of this invention will be apparent to
one
skilled in the art in view of the following disclosure, the drawings, and the
appended
claims.
SUMMARY OF THE INVENTION
The present disclosure relates to multipurpose acidic cleaning compositions
and
uses thereof. In an embodiment, the composition comprises from about 1 wt-% to
about 50
wt-% of at least one acid source; from about 1 wt-% to about 50 wt-%
surfactant; and from
about I wt-% to about 50 wt-% solvent or solvent system; wherein a use
solution of the
composition has a pH between about 1 and about 5. The compositions provide
efficacy as
multipurpose cleaning and degreasing formulations that penetrate soils with
the acidic
formulations, namely pH less than about 6, and preferably between about 1 and
about 5.
In an embodiment, a method of cleaning and/or degreasing is provided. The
method
comprises: applying to a surface or object in need of cleaning and/or
degreasing the acidic
composition according to the disclosure herein, and removing soils, stains,
and/or hard
water deposits from the surface or object. In an embodiment, the applying to
the surface or
object is a multipurpose spot treatment, wherein the cleaning benefits are
degreasing, de-
liming and de-staining.
While multiple embodiments are disclosed, still other embodiments will become
apparent to those skilled in the art from the following detailed description,
which shows
and describes illustrative embodiments. Accordingly, the drawings and detailed
description are to be regarded as illustrative in nature and not restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
The patent or application file contains at least one drawing executed in
color.
Copies of this patent or patent application publication with color drawing(s)
will be
provided by the Office upon request and payment of the necessary fee.
3
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
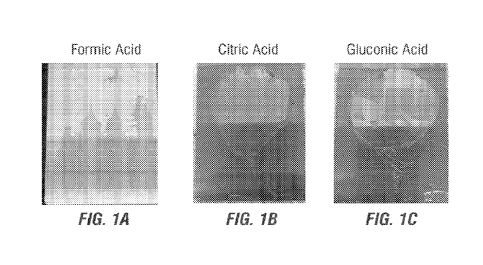
FIGS. 1A-1C show photographs of a drop test using Acidic Compositions
containing formic acid (FIG. 1A), citric acid (FIG. 1B), and gluconic acid
(FIG. 1C) on
stainless steel coupons for efficacy in speed to penetrate and remove corn oil
soil on the
coupons as described in Example 1.
FIGS. 2A-2C show photographs of a soak test using Acidic Compositions
containing formic acid (FIG. 1A), citric acid (FIG. 1B), and gluconic acid
(FIG. 1C) on
stainless steel coupons for efficacy in time to completely remove corn oil
soil on the
coupons as described in Example 1.
FIG. 3 shows a graph of the speed of removal of polymerized corn oil soils
from
coupons as described in Example 2.
FIG. 4 shows a graph of tea stain removal efficacy of a Control formulation
(alkaline degreaser composition) compared to an Acidic Composition containing
citric acid
following a 30 second, 1 minute and 2-minute soak as described in Example 3.
FIG. 5 shows a graph of red and black soil removal by a Control formulation
compared to various Acidic Compositions as described in Example 4.
FIG. 6 shows a graph of soap scum removal by the Control formulations compared
to various Acidic Compositions as described in Example 5.
FIGS. 7A-7E show photographs of soap scum removal from glass slides using
Acidic Compositions containing formic acid (FIG. 7A) and citric acid (FIG.
7B), an Acidic
Control (FIG. 7C), an Alkaline Control (FIG. 7D), and water (FIG. 7E) as
described in
Example 5.
FIGS. 8A-8B show photographs of stain removal using spot treatments containing
water (FIG. 8A) and citric acid (FIG. 8B) as described in Example 6.
FIGS. 9A-9B show photographs of polymerized corn oil removal using spot
treatments containing water (FIG. 9A) and citric acid (FIG. 9B) as described
in Example 6.
FIGS. 10A-10B show photographs of protein removal using spot treatments
containing water (FIG. 10A) and citric acid (FIG. 10B) as described in Example
6.
FIG. 11 shows a graph of tea stain removal, protein removal, and polymerized
corn
oil removal by spot treatment of the Acidic Compositions compared to a water
control as
described in Example 6.
Various embodiments of the present invention will be described in detail with
reference to the drawings, wherein like reference numerals represent like
parts throughout
4
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
the several views. Reference to various embodiments does not limit the scope
of the
invention. Figures represented herein are not limitations to the various
embodiments
according to the invention and are presented for exemplary illustration of the
invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The embodiments are not limited to particular acidic compositions and methods
of
using the same, which can vary and are understood by skilled artisans. It is
further to be
understood that all terminology used herein is for the purpose of describing
particular
embodiments only, and is not intended to be limiting in any manner or scope.
For
example, as used in this specification and the appended claims, the singular
forms "a," "an"
and "the" can include plural referents unless the content clearly indicates
otherwise.
Further, all units, prefixes, and symbols may be denoted in its SI accepted
form. Numeric
ranges recited within the specification are inclusive of the numbers within
the defined
range. Throughout this disclosure, various aspects are presented in a range
format. It
should be understood that the description in range format is merely for
convenience and
brevity and should not be construed as an inflexible limitation on the scope
of the
invention. Accordingly, the description of a range should be considered to
have
specifically disclosed all the possible sub-ranges as well as individual
numerical values
within that range (e.g.,1 to 5 includes 1, 1.5, 2, 2.75, 3, 3.80, 4, and 5).
So that the present invention may be more readily understood, certain terms
are
first defined. Unless defined otherwise, all technical and scientific terms
used herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which
embodiments of the invention pertain. Many methods and materials similar,
modified, or
equivalent to those described herein can be used in the practice of the
embodiments
without undue experimentation, but the preferred materials and methods are
described
herein. In describing and claiming the embodiments, the following terminology
will be
used in accordance with the definitions set out below.
The term "about," as used herein, refers to variation in the numerical
quantity that
can occur, for example, through typical measuring and liquid handling
procedures used for
making concentrates or use solutions in the real world; through inadvertent
error in these
procedures; through differences in the manufacture, source, or purity of the
ingredients
used to make the compositions or carry out the methods; and the like. The term
"about"
5
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
also encompasses amounts that differ due to different equilibrium conditions
for a
composition resulting from a particular initial mixture. Whether or not
modified by the
term "about", the claims include equivalents to the quantities.
The term "actives" or "percent actives" or "percent by weight actives" or
"actives
concentration" are used interchangeably herein and refers to the concentration
of those
ingredients involved in cleaning expressed as a percentage minus inert
ingredients such as
water or salts.
As used herein, the term "cleaning" refers to a method used to facilitate or
aid
in soil removal, bleaching, microbial population reduction, and any
combination
thereof.
As used herein, the term "free" refers to compositions completely lacking the
component or having such a small amount of the component that the component
does not
affect the performance of the composition. The component may be present as an
impurity
or as a contaminant and shall be less than 0.5 wt-%. In another embodiment,
the amount of
the component is less than 0.1 wt-% and in yet another embodiment, the amount
of
component is less than 0.01 wt-%.
The term "hard surface" refers to a solid, substantially non-flexible surface
such as
a countertop, tile, floor, wall, panel, window, plumbing fixture, kitchen and
bathroom
furniture, appliance, engine, circuit board, and dish. Hard surfaces may
include for
example, health care surfaces, food processing surfaces, bathroom surfaces,
and the like,
and may be interior or exterior.
The term "substantially similar cleaning performance" refers generally to
achievement by a substitute cleaning product or substitute cleaning system of
generally
the same degree (or at least not a significantly lesser degree) of cleanliness
or with
generally the same expenditure (or at least not a significantly lesser
expenditure) of
effort, or both, when using the substitute cleaning product or substitute
cleaning system
to address a typical soiling condition on a typical substrate as described
herein. This
degree of cleanliness may, depending on the particular cleaning product and
particular
substrate, correspond to a general absence of visible soils, or to some lesser
degree of
cleanliness.
6
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
The term "surfactant" or "surface active agent" refers to an organic chemical
that
when added to a liquid change the properties of that liquid at a surface.
The term "weight percent," "wt-%," "percent by weight," "% by weight," and
variations thereof, as used herein, refer to the concentration of a substance
as the weight of
that substance divided by the total weight of the composition and multiplied
by 100. It is
understood that, as used here, "percent," "%," and the like are intended to be
synonymous
with "weight percent," "wt-%, " etc.
The methods and compositions may comprise, consist essentially of, or consist
of
the components and ingredients as well as other ingredients described herein.
As used
herein, "consisting essentially of' means that the methods and compositions
may include
additional steps, components, or ingredients, but only if the additional
steps, components
or ingredients do not materially alter the basic and novel characteristics of
the claimed
methods and compositions.
Multipurpose acidic compositions
The multipurpose acidic compositions include at least one acid, surfactant(s),
a
solvent and/or solvent system, and water. The multipurpose alkaline
compositions can
include additional functional ingredients and can be provided as concentrate
or use
compositions. Exemplary multipurpose acidic compositions are shown in Table 1
in weight
percentage. The compositions are provided as concentrate compositions that can
be used
for pre-treatment, such as for direct application to a soil, or can be further
diluted in a
cleaning and/or sanitizing application. The multipurpose acidic compositions
are
beneficially formulated as concentrates (e.g.,First Exemplary Range) or can be
further
diluted to a use concentrate or ready-to-use (RTU) formulation (e.g., Third
Exemplary
Range).
TABLE 1
Material First Exemplary Second Exemplary Third
Exemplary
Range wt.-% Range wt.-% Range wt.-
%
Acid source(s) 1-50 1-25 1-10
Surfactant(s) 1-50 1-20 1-5
7
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
Solvent and/or Solvent 1-50 1-20 1-20
System
Water 10-90 20-90 40-90
Additional Functional 0-50 0-25 0-20
Ingredients
Total 100 100 100
According to embodiments the pH of the multipurpose acidic compositions use
solution is less than about 7, between about 1 to about 7, between about 2 to
about 7,
between about 2.5 to about 7, and preferably less than about 6. According to
preferred
embodiments the pH of the multipurpose acidic compositions use solution is
less than
about 6, or less than about 5, between about 1 to about 5, between about 1 to
about 4,
between about 2.5 to about 4, or between about 3 to about 4. The multipurpose
acidic
compositions provide significant safety benefits as a result of the pH above
about 2.5
and/or between about 3 to about 4, including the formulations not requiring
personal
protective equipment (PPE) for safe handling, while providing substantially
similar
cleaning efficacy, and in many embodiments superior cleaning efficacy to
traditional acidic
compositions, as well as providing additional cleaning and/or sanitizing
benefits. In other
aspects, the multipurpose acidic compositions provide superior degreasing
efficacy, along
with stain removal (e.g., difficult to remove stains such as tea, coffee, and
the like),
calcium carbonate and soap scum removal, rust removal, and aiding in further
general-
purpose cleaning of fats, oils, cosmetics and other difficult to remove soils.
In some embodiments, the wt-ratio of the solvent or solvent system to the acid
source is about 1:1. In other embodiments, the wt-ratio of the solvent or
solvent system to
the acid source is from about 4:1 to about 1:4to provide beneficial effects in
removing
difficult soils, such as polymerized corn oil.
Acid Source
The multipurpose acidic compositions include at least one acid source. Acid
sources can include organic acids, inorganic acid or a mixture thereof.
Examples of acid
sources include, for example, citric acid, formic acid, glycolic acid,
gluconic acid,
phosphoric acid, hydrochloric acid, sulfuric acid, nitric acid, acetic acid or
peroxycarboxylic acids. In an embodiment one or more organic acids are
included as the
8
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
acid source, including for example lactic acid, gluconic acid, formic acid,
citric acid, acetic
acid, oxalic acid, uric acid, malic acid, tartaric acid, or the like. A
variety of acids can be
formulated into the multipurpose acidic compositions to provide a desired pH
for the
compositions.
In some embodiments, the concentrate multipurpose alkaline compositions
comprise about 1 wt-% to about 50 wt-%, from about 1 wt-% to about 50 wt-%,
from
about 1 wt-% to about 30 wt-%, from about 1 wt-% to about 25 wt-%, from about
5 wt-%
to about 25 wt-%, from about 5 wt-% to about 20 wt-%, or from about 5 wt-% to
about 15
wt-% of the at least one acid source. It is to be understood that all values
and ranges
between these values and ranges are encompassed by the present invention as
well as
dilutions of the concentrate.
,S'urfactants
The multipurpose acidic compositions include at least one surfactant. Suitable
surfactants can include anionic, cationic, amphoteric, zwitterionic, and/or
nonionic
surfactants. The emulsifying properties of surfactants can be used for both a
concentrate
that can be diluted to create a usable cleaning and/or sanitizing product (use
dilution) and
the use dilution itself The surfactant or mixture of surfactants can have
foaming or
defoaming characteristics suitable for a desired cleaning and/or sanitizing
application. The
surfactant or surfactant system can be selected depending upon the particular
soil, e.g.,
polymerized soil, that is to be removed.
Anionic surfactants suitable for use with the multipurpose alkaline
compositions
include alkylbenzene sulfonates, such as linear alkylbenzene sulfonates, alkyl
carboxylates,
paraffin sulfonates and secondary n-alkane sulfonates, sulfosuccinate esters
and sulfated
linear alcohols. Additional sulfonated anionics include alkyl sulfonates or
disulfonates,
alkyl aryl sulfonates, alkyl naphthalene sulfonates, alkyl diphenyl oxide
disulfonates, and
the like. In an embodiment linear alkylbenzene sulfonates (LAS) or linear
alkylbenzene
sulfonic acids (LABSA) are preferred as the anionic surfactant.
Zwitterionic or amphoteric surfactants suitable for use with the multipurpose
alkaline compositions include beta-N-alkylaminopropionic acids, n-alkyl-beta-
iminodipropionic acids, imidazoline carboxylates, n-alky-betaines, amine
oxides,
sulfobetaines and sultaines.
9
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
Nonionic surfactants suitable for use with the multipurpose alkaline
compositions
include alcohol alkoxylates having EO, PO and BO blocks, fatty acid
alkoxylate, alkyl
phenol alkoxylates, and polyether (also known as polyalkylene oxide,
polyoxyalkylene or
polyalkylene glycol) compounds. More particularly, the polyether compounds are
generally polyoxypropylene or polyoxyethylene glycol compounds. Typically, the
surfactants suitable for use with the multipurpose alkaline compositions are
synthetic
organic polyoxypropylene (P0)-polyoxyethylene (EO) block copolymers. These
surfactants have a diblock polymer comprising an E0 block and a PO block, a
center block
of polyoxypropylene units (PO), and having blocks of polyoxyethylene grated
onto the
polyoxypropylene unit or a center block of EO with attached PO blocks.
Cationic surfactants suitable for use with the multipurpose alkaline
compositions
can include alkylamines and their salts, alkyl imidazolines, ethoxylated
amines, and
quaternaries, such as alkylbenzyldimethylammonium salts, alkyl benzene salts,
heterocyclic ammonium salts, tetra alkylammonium salts, and the like.
Cationics further
include compounds containing at least one long carbon chain hydrophobic group
and at
least one positively charged nitrogen. The long carbon chain group may be
attached
directly to the nitrogen atom by simple substitution; or more preferably
indirectly by a
bridging functional group or groups in so-called interrupted alkylamines and
amido
amines. Such functional groups can make the molecule more hydrophilic and/or
more
water dispersible, more easily water solubilized by co-surfactant mixtures,
and/or water
soluble. For increased water solubility, additional primary, secondary or
tertiary amino
groups can be introduced, or the amino nitrogen can be quaternized with low
molecular
weight alkyl groups. Further, the nitrogen can be a part of branched or
straight chain
moiety of varying degrees of unsaturation or of a saturated or unsaturated
heterocyclic
ring. In addition, cationic surfactants may contain complex linkages having
more than one
cationic nitrogen atom. Additional description can be in "Surfactant
Encyclopedia",
Cosmetics & Toiletries, Vol. 104 (2) 86-96 (1989) and U.S. Patent No.
9,663,431, which
are herein incorporated by reference in its entirety.
Amphoteric surfactants suitable for use with the multipurpose alkaline
compositions include derivatives of aliphatic secondary and tertiary amines,
in which the
aliphatic radical may be straight chain or branched and wherein one of the
aliphatic
substituents contains from about 8 to 18 carbon atoms and one contains an
anionic water
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
solubilizing group, e.g., carboxy, sulfo, sulfato, phosphato, or phosphono.
Amphoteric
surfactants are subdivided into two major classes known to those of skill in
the art and
described in "Surfactant Encyclopedia" Cosmetics & Toiletries, Vol. 104 (2) 69-
71 (1989)
and U.S. Patent No. 9,663,431, which are herein incorporated by reference in
its entirety.
The first class includes acyl/di alkyl ethylenediamine derivatives (e.g. 2-
alkyl hydroxyethyl
imidazoline derivatives) and their salts. The second class includes N-
alkylamino acids and
their salts. Some amphoteric surfactants can be envisioned as fitting into
both classes.
Surfactants that can be used include anionic, cationic, amphoteric,
zwitterionic,
and/or nonionic surfactants, which are commercially available from a number of
sources.
For a discussion of surfactants, see Kirk-Othmer, Encyclopedia of Chemical
Technology,
Third Edition, volume 8, pages 900-912. Surfactants can be used alone or in
combination.
In an embodiment, nonionics and anionics are used in combination. The semi-
polar
nonionic, cationic, amphoteric and zwitterionic surfactants can be employed in
combination with nonionics or anionics. The above examples are merely specific
illustrations of the numerous surfactants which can find application within
the scope of the
multipurpose alkaline compositions. It should be understood that the selection
of particular
surfactants or combinations of surfactants can be based on a number of factors
including
compatibility with the surface to be cleaned at the intended use concentration
and the
intended environmental conditions including temperature and pH.
In a preferred embodiment, the surfactant is an anionic alkylbenzene
sulfonate. In
an embodiment, the surfactant is a linear alkyl benzene sulfonate and is
combined with the
solvent (e.g., benzyl alcohol) for a preferred acidic composition.
In some embodiments, the multipurpose acidic compositions comprise from about
1 wt-% to about 50 wt-%, from about 1 wt-% to about 40 wt-%, from about 1 wt-%
to
about 30 wt-%, from about 1 % to about 20 wt-% of surfactant, from about 1 %
to about 10
wt-% of surfactant or from about 1 % to about 5 wt-% of surfactant. It is to
be understood
that all values and ranges between these values and ranges are encompassed by
the present
invention.
Solvents and Solvent Systems
The multipurpose acidic compositions include at least one solvent or a solvent
system. In various embodiments, the multipurpose acidic compositions may
include a
solvent that also functions as a cleaning agent. The solvent or solvent system
can be used
11
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
for enhancing the cleaning properties of the multipurpose acidic composition
as well as to
provide emulsifying properties of a given composition For example, the solvent
system
may keep hydrophilic and hydrophobic components of the specific composition
from
separating. The emulsifying properties can be used for both a concentrate that
can be
diluted to create a usable cleaning product (use solution) and the use
dilution itself.
Exemplary solvents and solvent systems may include one or more different
solvents including aromatic alcohols, alkanol amines, ether amines, glycol
ethers, esters
and mixtures thereof. Representative solvents may include acetamidophenol,
acetanilide,
acetophenone, 2-acetyl-1-methylpyrrole, benzyl acetate, benzyl alcohol, methyl
benzyl
alcohol, alpha phenyl ethanol, benzyl benzoate, benzyloxyethanol, ethylene
glycol phenyl
ether (commercially available as "DOWANOL EPh" from Dow Chemical Co.),
propylene
glycol phenyl ether (commercially available as "DOWANOL PPh" from Dow Chemical
Co.), amyl acetate, amyl alcohol, butanol, 3-butoxyethy1-2-propanol, butyl
acetate, n-butyl
propionate, cyclohexanone, di acetone alcohol, di ethoxyethanol, di ethylene
glycol methyl
ether, diisobutyl carbinol, diisobutyl ketone, dimethyl heptanol, dipropylene
glycol tert-
butyl ether, ethanol, ethyl acetate, 2-ethylhexanol, ethyl propionate,
ethylene glycol methyl
ether acetate, hexanol, isobutanol, isobutyl acetate, isobutyl heptyl ketone,
isophorone,
isopropanol, isopropyl acetate, methanol, methyl amyl alcohol, methyl n-amyl
ketone, 2-
methy1-1-butanol, methyl ethyl ketone, methyl isobutyl ketone, 1-pentanol, n-
pentyl
propionate, 1-propanol, n-propyl acetate, n-propyl propionate, propylene
glycol ethyl ether,
tripropylene glycol methyl ether (commercially available as DOWANOL TPM from
Dow
Chemical Co.), tripropylene glycol n-butyl ether (commercially available as
DOWANOL
TPNB from Dow Chemical Co.), diethylene glycol n-butyl ether acetate
(commercially
available as Butyl CARBITOL acetate from Dow Chemical Co.), diethylene glycol
monobutyl ether (commercially available as Butyl CARBITOL from Dow Chemical
Co.),
ethylene glycol n-butyl ether acetate (commercially available as Butyl
CELLOSOLVE
acetate from Dow Chemical Co.), ethylene glycol monobutyl ether (commercially
available as Butyl CELLOSOLVE from Dow Chemical Co.), dipropylene glycol
monobutyl ether (commercially available as Butyl DIPROPASOL from Dow Chemical
Co.), propylene glycol monobutyl ether (commercially available as Butyl
PROPASOL
from Dow Chemical Co.), ethyl 3-ethoxypropionate (commercially available as
UCAR
Ester EEP from Dow Chemical Co.), 2,2,4-Trimethy1-1,3-Pentanediol
Monoisobutyrate
12
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
(commercially available as UCAR Filmer IBT from Dow Chemical Co.), diethylene
glycol
monohexyl ether (commercially available as Hexyl CARBITOL from Dow Chemical
Co.),
ethylene glycol monohexyl ether (commercially available as Hexyl CELLOSOLVE
from
Dow Chemical Co.), diethylene glycol monomethyl ether (commercially available
as
Methyl CARBITOL from Dow Chemical Co.), diethylene glycol monoethyl ether
(commercially available as CARBITOL from Dow Chemical Co.), ethylene glycol
methyl
ether acetate (commercially available as Methyl CELLOSOLVE acetate from Dow
Chemical Co.), ethylene glycol monomethyl ether (commercially available as
Methyl
CELLOSOLVE from Dow Chemical Co.), dipropylene glycol monomethyl ether
(commercially available as Methyl DIPROPASOL from Dow Chemical Co.), propylene
glycol methyl ether acetate (commercially available as Methyl PROPASOL acetate
from
Dow Chemical Co.), propylene glycol monomethyl ether (commercially available
as
Methyl PROPASOL from Dow Chemical Co.), diethylene glycol monopropyl ether
(commercially available as Propyl CARBITOL from Dow Chemical Co.), ethylene
glycol
monopropyl ether (commercially available as Propyl CELLOSOLVE from Dow
Chemical
Co.), dipropylene glycol monopropyl ether (commercially available as Propyl
DIPROPASOL from Dow Chemical Co.) and propylene glycol monopropyl ether
(commercially available as Propyl PROPASOL from Dow Chemical Co.).
Representative
dialkyl carbonates include dimethyl carbonate, diethyl carbonate, dipropyl
carbonate,
diisopropyl carbonate and dibutyl carbonate. Representative oils include
benzaldehyde,
pinenes (alphas, betas, etc.), terpineols, terpinenes, carvone,
cinnamealdehyde, borneol and
its esters, citrals, ionenes, jasmine oil, limonene, dipentene, linalool and
its esters.
Representative dibasic esters include dimethyl adipate, dimethyl succinate,
dimethyl
glutarate, dimethyl malonate, diethyl adipate, diethyl succinate, diethyl
glutarate, dibutyl
succinate, dibutyl glutarate and products available under the trade
designations DBE,
DBE- 3, DBE-4, DBE-5, DBE-6, DBE-9, DBE-IB, and DBE-ME from DuPont Nylon.
Representative phthalate esters include dibutyl phthalate, diethylhexyl
phthalate and
diethyl phthalate.
Preferred solvents for wetting of soils, such as difficult to remove soils,
such as
polymerized non-trans-fat soils, include benzyl alcohol, dibasic esters,
essential oils,
dialkyl carbonates, ethylene glycol monobutyl ether, diethylene glycol
monobutyl ether,
ethylene glycol phenyl ether, propylene glycol phenyl ether and mixtures
thereof
13
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
According to an embodiment, the solvent or solvent system includes at least
one
aromatic alcohol (e.g., benzyl alcohols, phenyl alcohols). Preferably the
aromatic alcohol
solvent system is benzyl alcohol. The solvent may further include solvents in
similar
limited water solubility range as benzyl alcohol, including for example
benzyloxyethanol
and/or benzyloxypropanol.
According to a further embodiment, the solvent system may include benzyl
acetate,
benzyl alcohol, methyl benzyl alcohol, alpha phenyl ethanol, benzyl benzoate,
benzyloxyethanol and/or the like. Additional description of solvent systems
that may be
included in the compositions are disclosed in U.S. Patent Publication No.
2010/0317559,
incorporated herein by reference in its entirety.
In some embodiments, the multipurpose acidic compositions include from about 1
wt-% to about 50 wt-%, from about 1 wt-% to about 40 wt-%, from about 1 wt-%
to about
30 wt-%, from about 1 wt-% to about 20 wt-%, or from about 1 wt-% to about 20
wt-% of
a solvent system It is to be understood that all values and ranges between
these values and
ranges are encompassed by the present invention.
Additional Functional Ingredients
The components of the multipurpose acidic compositions can further be combined
with various functional components suitable for uses disclosed herein. In some
embodiments, the multipurpose acidic compositions including the at least one
acid,
surfactant(s), solvent and/or solvent system, and water make up a large
amount, or even
substantially all of the total weight of the compositions. For example, in
some
embodiments few or no additional functional ingredients are disposed therein.
In other embodiments, additional functional ingredients may be included in the
multipurpose acidic compositions. The functional ingredients provide desired
properties
and functionalities to the compositions. For the purpose of this application,
the term
"functional ingredient" includes a material that when dispersed or dissolved
in a use and/or
concentrate solution, such as an aqueous solution, provides a beneficial
property in a
particular use. Some particular examples of functional materials are discussed
in more
detail below, although the particular materials discussed are given by way of
example only,
and that a broad variety of other functional ingredients may be used. For
example, many
of the functional materials discussed below relate to materials used in
cleaning. However,
other embodiments may include functional ingredients for use in other
applications.
14
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
In some embodiments, the multipurpose acidic compositions may include
hydrotropes or couplers, neutralizing agents, optical brighteners, defoaming
agents, anti-
redeposition agents, bleaching agents, solubility modifiers, buffers, tracers,
dispersants,
metal protecting agents, soil antiredeposition agents, stabilizing agents,
corrosion
inhibitors, chelating/sequestrating agents, enzymes, aesthetic enhancing
agents including
fragrances and/or dyes, additional rheology and/or solubility modifiers or
thickeners,
buffers, solvents, additional cleaning agents and the like.
In some embodiments, the multipurpose acidic compositions may include one or
more of a buffer or pH adjuster (i.e., alkalinity source), dye (i.e., for
product
safety/identification), fragrance, thickener, corrosion inhibitor and/or
enzyme.
In embodiments, the additional ingredients can be pre-formulated with the
multipurpose alkaline compositions or added to the use solution before, after,
or
substantially simultaneously with the addition of the compositions.
Additionally, the
compositions can be used in conjunction with one or more conventional cleaning
and/or
sanitizing agents or compositions.
In preferred embodiments, the composition does not include polysaccharide
polymers and a homo/copolymer of vinylpyrrolidone. In preferred embodiments,
the
composition does not include cationic surfactants. In preferred embodiments,
the
composition does not include strong acids.
According to embodiments, the various additional functional ingredients may be
provided in the compositions in the amount from about 0 wt-% and about 50 wt-
%, from
about 0 wt-% and about 40 wt-%, from about 0 wt-% and about 30 wt-%, from
about 0 wt-
% and about 25 wt-%, from about 0 wt-% and about 20 wt-%, 0.1 wt-% and about
50 wt-
%, from about 0.1 wt-% and about 40 wt-%, from about 0.1 wt-% and about 30 wt-
%, from
about 0.1 wt-% and about 25 wt-%, from about 0.1 wt-% and about 20 wt-%, from
about
0.1 wt-% and about 10 wt-%, from about 0.1 wt-% and about 5 wt-%, from about 1
wt-%
and about 50 wt-%, from about 1 wt-% and about 40 wt-%, from about 1 wt-% and
about
wt-%, from about 1 wt-% and about 25 wt-%, from about 1 wt-% and about 20 wt-
%,
from about 1 wt-% and about 10 wt-%, or from about 1 wt-% and about 5 wt-%. In
30 addition, without being limited according to the invention, all ranges
recited are inclusive
of the numbers defining the range and include each integer within the defined
range.
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
Hydrotropes
The multipurpose acidic compositions can optionally include a hydrotrope as an
additional functional ingredient. Hydrotropes aid in compositional stability
and aqueous
formulation. Functionally speaking, the suitable hydrotrope couplers which can
be
employed are non-toxic and retain the active ingredients in aqueous solution
throughout
the temperature range and concentration to which a concentrate or any use
solution is
exposed. Without being limited to a particular mechanism of action or
embodiment, as the
multipurpose acidic compositions increase in acid concentration the hydrotrope
can be
used to increase the pH of the acidic composition to the desired pH range,
such as pH
between about 2.5 and about 4, or between about 3 and about 4.
Any hydrotrope coupler may be used provided it does not react with the other
components of the composition or negatively affect the performance properties
of the
composition. Representative classes of hydrotropic coupling agents or
solubilizers which
can be employed include anionic surfactants such as alkyl sulfates and alkane
sulfonates,
linear alkyl benzene (including linear alkylbenzene sulfonates (LAS)) or
naphthalene
sulfonates, secondary alkane sulfonates, alkyl ether sulfates or sulfonates,
alkyl phosphates
or phosphonates, dialkyl sulfosuccinic acid esters, sugar esters (e.g.,
sorbitan esters), amine
oxides (mono-, di-, or tri-alkyl) and C8-C10 alkyl glucosides. Preferred
coupling agents
include aromatic sulfonates such as the alkyl benzene sulfonates (e.g., xylene
sulfonates)
or naphthalene sulfonates, aryl or alkaryl phosphate esters or their
alkoxylated analogues
having 1 to about 40 ethylene, propylene or butylene oxide units or mixtures
thereof Other
preferred hydrotropes include nonionic surfactants of C6-C24 alcohol
alkoxylates
(alkoxylate means ethoxylates, propoxylates, butoxylates, and co-or-terpolymer
mixtures
thereof) (preferably C6-C14 alcohol alkoxylates) having 1 to about 15 alkylene
oxide
groups (preferably about 4 to about 10 alkylene oxide groups); C6-C24
alkylphenol
alkoxylates (preferably C8-C10 alkylphenol alkoxylates) having 1 to about 15
alkylene
oxide groups (preferably about 4 to about 10 alkylene oxide groups); C6-C24
alkylpolyglycosides (preferably C6-C20 alkylpolyglycosides) having 1 to about
15
glycoside groups (preferably about 4 to about 10 glycoside groups); C6-C24
fatty acid
ester ethoxylates, propoxylates or glycerides; and C4-C12 mono or
dialkanolamides. A
preferred hydrotrope is sodium xylene sulfonate (SXS).
16
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
In embodiments employing a hydrotrope, the multipurpose acidic compositions
include from about 0.1 wt-% to about 20 wt-%, from about 0.1 wt-% to about 10
wt-%,
from about 0.5 wt-% to about 10 wt-%, from about 0.5 wt-% to about 8 wt-%, or
from
about 0.5 wt-% to about 5 wt-% of hydrotrope. It is to be understood that all
values and
ranges between these values and ranges are encompassed by the present
invention
Use Solutions
According to an embodiment, a use dilution of the concentrate multipurpose
acidic
compositions can range from a RTU formulation that does not require further
dilution to
about 1:10 dilution of the concentrate to solvent. Dilution ranges in between
are also
suitable. More preferably, a use dilution of about 1:3 to about 1:6 is
obtained from the
concentrate composition. As one skilled in the art will ascertain as a result
of the disclosure
herein, a use solution can be generated according to the particular needs of a
user and its
application.
In some embodiments, a dilution step may be initially employed to provide a
water
source to the concentrated composition suitable for generating a use solution
or use
composition. In some aspects, the concentrated multipurpose cleaning
composition may be
diluted at a dilution factor between approximately 1 to about 22 ounces liquid
concentrate
per gallon of water diluent, from about 1 to about 12 ounces liquid
concentrate per gallon
of water diluent, or from about 8 to about 10 ounces liquid concentrate per
gallon of water
diluent. In some aspects, the dilution step occurs at or near a point of use,
and may include
for example use of a water source that is provided using an aspirator or other
dilution
mechanism known to the art. In other aspects, when the cleaning composition is
employed
in a diluted (or a use solution or composition) formulation no further
dilution is required by
a user.
Methods of Use
The multipurpose acidic compositions are suited for cleaning, sanitizing
and/or
disinfecting various surfaces and objects. Multipurpose compositions, as the
name implies,
are intended to be used on multiple types of surfaces and multiple types of
soils. The
multipurpose acidic compositions are efficacious in cleaning and removing
soils from such
surfaces and objects, including for example difficult to remove soils,
including
polymerized soil, carbonized soil, baked on soil, and/or other fat soils.
These often include
polymerized fat soils, such as polymerized zero trans-fat soils including corn
oil.
17
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
While an understanding of the mechanism is not necessary to practice the
methods of
use described herein, it is contemplated that, in some embodiments, the
solvent or solvent
system (e.g., benzyl alcohol) provides a limited water-soluble alcohol
providing
hydrophobicity that adds affinity towards greasy soils and acts as a
plasticizer. The
soils, upon contact with the multipurpose acidic compositions, swell and lose
adhesion
from the substrate, providing a unique cleaning approach in comparison to the
use of
caustic degreasers and/or other alkaline control compositions.
Beneficially, the multipurpose acidic compositions have a higher pH than
traditional acidic compositions while providing substantially similar cleaning
efficacy.
In embodiments, the compositions have a pH less than about 4. Beneficially,
the pH of
the composition in use solution is less than about 4, from about 1 to about 4,
or from
about 2 to about 4. In other embodiments the pH of the compositions in a use
solution
is from about 2.5 to about 4, or from about 3 to about 4. The compositions
provide
significant safety benefits as a result of the pH range while providing
substantially
similar cleaning efficacy, and in many embodiments superior cleaning efficacy
to
traditional acidic compositions (or even in comparison to traditional alkaline
degreasing compositions).
According to preferred embodiments, the compositions having a pH above
about 2.5 do not require PPE, while unexpectedly providing the same or
substantially
similar cleaning efficacy for soil removal as compositions having alkaline pH,
such as
above about 11.5 and/or compositions including hydroxide (i.e., caustic)
alkalinity
sources.
The multipurpose acidic compositions act quickly to remove soils, such as
polymerized fat soils. The fast penetrating of the soils allows the
compositions to be used a
pretreatment that does not require extended dwell or pretreatment time. In an
embodiment,
the compositions achieve degreasing action within about approximately 5
seconds to a few
minutes of contact to a soiled surface or object. According to a preferred
embodiment,
application of the compositions result in soil removal within about seconds
without
requiring substantial mechanical action or excessive temperatures. The methods
result in
cleaning efficacy that is at least substantially similar to with the use of a
hydroxide-based
and corrosive, highly alkaline compositions, demonstrating an unexpected
beneficial
application of use of the multipurpose acidic compositions. In a further
embodiment, the
18
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
methods of cleaning and/or degreasing result in the compositions penetrating
soils more
quickly than an alkaline control composition. As referred to herein, an
alkaline control
composition can include either a hydroxide-based alkaline composition or a non-
hydroxide
composition including pH above 11.5 and/or requires use of PPE.
The multiuse acidic compositions are particularly well suited for use as a
multipurpose de-greasing, de-liming (i.e., hard water spots), and de-staining
composition.
The de-staining can include removal of difficult stains such as tea and coffee
stains. These
multipurpose benefits are particularly useful as a multipurpose kitchen spot
treatment.
Beneficially, such multipurpose benefits provide a single cleaning application
instead of
formulating detergents to remove stains, polymerized soils (also including
carbonized soils
and fats), and hard water spots.
In some embodiments, the de-staining of surfaces or objects with the
multipurpose
acidic composition is achieved within less than about 10 minutes, less than
about 5
minutes, less than about 4 minutes, less than about 3 minutes, less than 90
seconds, less
than about 60 seconds, less than about 45 seconds, or less than about 30
seconds of
contacting time.
In some embodiments, the soil removal of surfaces or objects with the
multipurpose
acidic composition is achieved within less than about 10 minutes, less than
about 5
minutes, less than about 4 minutes, less than about 3 minutes, less than about
2 minutes,
less than about 60 seconds, or less than about 45 seconds of contacting time.
Exemplary industries in which the present methods can be used include but are
not
limited to: food service industry; food and beverage industry; consumer
degreasing
applications; oil processing industry; industrial agriculture and ethanol
processing; and the
pharmaceutical manufacturing industry. Suitable use for the compositions and
methods of
the invention may include, for example, oven cleaner, including microwave
ovens, general
degreaser, fryer degreaser, smokehouse cleaner, floor cleaner, exhaust hood
cleaner, drain
cleaner, floor finish remover, floor cleaner, fryer cleaner, pot and pan
cleaner, carpet
spotter, pharmaceutical and cosmetics cleaner, instrument cleaner, tar
remover, and the
like.
As a further benefit, the multipurpose acidic compositions are also able to
remove
other soils from surfaces or objects beyond the polymerized fat soils. In an
additional
embodiment, the multipurpose acidic compositions can be used in any other
methods
19
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
seeking to remove polymerized soils, stains and/or hard water scaling without
requiring the
use of hydroxide-based or corrosive formulations, such as removing polymerized
or cross-
linked films from floors and other finishes. In such an embodiment, methods of
use of the
composition as a floor stripper and/or floor cleaner may be employed. In an
embodiment,
methods of use include removing soils from interior and/or exterior floors. In
such an
embodiment, the floor may be made of various materials including for example
concrete,
for example outside a drive thru wherein oil and grease soils may be present.
In a further
embodiment, methods of using the composition as a multipurpose formulation are
employed, unexpectedly demonstrating efficacy in non-traditional applications
of a non-
hydroxide alkalinity composition.
The present methods can also be used to remove soils other than polymerized
soils. Such other soils include, but are not limited to, starch, cellulosic
fiber, protein,
simple carbohydrates, and combinations of any of these soil types with mineral
complexes. Examples of specific food soils that are effectively removed using
the
present methods include, but are not limited to, soils generated in the
manufacture and
processing meat, poultry, vegetables and fruit, bakery goods, soft drinks,
brewing and
fermentation residues, soils generated in sugar beet and cane processing and
processed
foods containing these ingredients and associated ingredients such as juices,
sauces,
and condiments (e.g., fruit juices, ketchup, tomato sauce, barbeque sauce).
These soils
can develop on environmental surfaces such as walls and floors, freezers and
cooling
systems, heat exchange equipment surfaces, conveyor surfaces and on other
surfaces
during the manufacturing and packaging process.
The multipurpose acidic compositions can be further employed as a bathroom
cleaner. It is beneficial in that the multipurpose cleaning capability of the
compositions further removes soils that can be found in bathroom applications.
For
example, hard water deposits, soap scum (e.g., calcium stearate and other soap
scum soils)
and/or rust can be removed from the surface or object being cleaned with the
multipurpose
acidic compositions. The compositions can be used to remove stains, soil, hard
water and
the like from any conventional bathroom surfaces including but not limited to,
toilets,
shower stalls, racks, curtains, shower doors, bathing appliances, shower bars,
bathtubs,
bidets, sinks, etc., as well as countertops, walls, floors, etc. Additional
hard surfaces which
may be cleaned using the compositions, include for example, counter tops,
tile, floors,
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
walls, windows, fixtures, kitchen furniture, appliances, and the like. The
various hard
surfaces suitable for cleaning include for example, glass; metals; plastics
e.g., polyester,
vinyl; fiberglass, Formica, Corn, refractory materials such as: glazed and
unglazed tile,
brick, porcelain, ceramics as well as stone including marble, granite, and
other stones
surfaces; and other hard surfaces known to the industry.
Acidic compositions having a low pH (e.g., below about 3) are particularly
well
suited for cleaning soap scum, scale (i.e., hard water stains and lime scale
as may also be
used to refer to such stains commonly found in bathrooms) and/or other
residues as is
commonly found in bathrooms due to is triprotic acid strength when formulated
at pH
values less than about 3. The removal of soap scum and scale requires the
strength of an
acid to effectively clean due to the presence of calcium and magnesium salts
and soap
residues. Similarly, the acid component is needed to treat hard water stains,
which are
mineral stains caused by the deposition of salts, such as calcium or magnesium
carbonates,
frequently present in hard water. Still further, the strength of acidic
products are further
needed for removing soap scum stains, which include the residues of fatty acid
soaps
which are often based on alkaline salts of low fatty acids known to
precipitate in hard
water due to the presence of metal salts therein leaving an undesirable
residue upon such
surfaces. In an embodiment, it is unexpected that the acidic compositions have
a pH higher
than those typically used as bathroom cleaners (conventional pH <2.5 or <2).
Without
being limited to a particular mechanism of action, the combination of the
acid, surfactant
and solvent or solvent systems provides the benefits in cleaning without
requiring lower
pH.
The multipurpose acidic compositions can be further employed as an
antimicrobial composition. The antimicrobial efficacy can be employed for
sanitizing
and/or disinfecting cleaning composition. Beneficially, the combination of the
one or
more acid sources with the anionic surfactant (e.g., LAS) can provide
sanitizing
benefits. Use for sanitizing provides antimicrobial efficacy against a broad
spectrum of
microorganisms, providing broad spectrum bactericidal and fungistatic
activity. For
example, the broad-spectrum activity can include activity against wide range
of different
types of microorganisms (including both aerobic and anaerobic microorganisms,
gram
positive and gram negative microorganisms), including bacteria, yeasts, molds,
fungi,
algae, and other problematic microorganisms. Sanitizing methods can be used to
achieve
21
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
any suitable reduction of the microbial population in and/or on the surface or
object,
including reducing the microbial population by at least one logio, at least
two logio, at least
three logio, at least four logio, or at least five logio. Without limiting the
scope of invention,
the numeric ranges are inclusive of the numbers defining the range and include
each
integer within the defined range. In some embodiments, the multipurpose acidic
compositions are employed at pH (e.g., less than about 4) that sanitizing
efficacy is
achieved at least at a concentration of about 500 ppm surfactant (e.g., LAS).
In embodiments, the compositions can be used as a concentrate or a use
solution.
In embodiments, the compositions can be used as a pretreatment, soak, or
spray. The composition or use solutions thereof can be applied using a variety
of
methods and conventional application techniques, which will vary depending
upon the
application as a soak, spray, or the like. These methods can operate on an
object,
surface, or the like, by contacting the object or surface with the
composition. Contacting
can comprise any of numerous methods for applying a liquid, such as spraying
the
compound, immersing the object in the compound, foam or gel treating the
object with the
compound, or a combination thereof. Without being limited to the contacting
method, a
concentrate or use composition can be applied to or brought into contact with
an object or
surface by any conventional method or apparatus for applying a liquid
composition to an
object. For example, the surface can be wiped with, sprayed with, foamed on,
and/or
immersed in the compositions, or use compositions made from the concentrated
compositions. The liquid compositions can be sprayed, foamed, or wiped onto a
surface;
the compound can be caused to flow over the surface, or the surface can be
dipped into the
compound. Contacting can be manual or by machine.
A particularly well-suited method for applying or contacting the compositions
to a
stained or soiled surface is through the use of a manually operated spray-
dispensing
container. The spray-dispensing container preferably includes a spray nozzle,
a dip tube
and associated pump dispensing parts, providing convenient application to
stained or soiled
surfaces or objects.
The various methods include a step of contacting a surface in need of cleaning
and/or degreasing with the compositions for a sufficient amount of time such
that the
composition penetrates into the soil to be removed. The length of time
required for
22
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
soil penetration will depend on the thickness of the soil as well as the
relative
polymerization level of the soil. In such cases, it is preferable that the
composition
includes a high foaming surfactant system or a thickening system so that the
composition does not dry out and remains hydrated on the surface for an
extended
period of time.
The multipurpose acidic compositions can be in contact with a surface or
object for
a sufficient amount of time to clean the surface or object. In an aspect, the
surface or
object is contacted with the composition for at least about 10 seconds, 30
seconds, 1
minute, at least about 10 minutes, or between about 10 minutes and about 20
minutes. The
contact time is also provided at a sufficiently acidic pH to provide the
multipurpose
efficacy, including at a pH less than about 6, less than about 5, and
preferably less than
about 4. In still other embodiments the contact time is also provided at a RTU
or use
concentration of the multipurpose acidic compositions from about 1 wt-% to
about 20 wt-
%, including all ranges therebetween.
The methods can further optionally include a step of wiping off the treated
surface
or object with a rag, towel, sponge, or other item (e.g., a disposable paper
towel or
sponge). In other embodiments this step is not require, as the surface or
object may be
placed into a washing machine or ware washing machine for further treatment
with a
detergent composition. In some embodiments involving heavy soil deposits or
stains, the
composition may be left on the soiled surface until it has effectively
loosened the soil
deposits or stains, after which it may be wiped off, rinsed off, or otherwise
removed. For
particularly heavy deposits of such undesired stains, multiple applications
may also be
used.
The methods can further optionally include using mechanical force during the
contacting step. For example, for removing certain soils or stains from the
surface or object
additional force may need to be applied, e.g., applying a water source and/or
mechanical
force to assist in removing soils.
The methods can further optionally include a step of rinsing off the treated
surface
or object with water. In yet other embodiments the composition is wiped off
the soiled
surface, effectively removing the soils and any remaining composition. In
further aspects,
there is no need for a rinse step.
23
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
The compositions can be applied following a step of heating the composition to
a temperature of about 40 F or above, 40 F to about 130 F. In other
embodiments, the
methods provide for soil removal from surfaces or objects at an ambient or
room
temperature, e.g., about 50 F to about 100 F. It is preferred in various
embodiments
that neither the surface or object nor the composition is heated before the
contacting
step. In still other cases, methods provide for soil removal from surfaces or
objects at
colder temperature, e.g., about 25 F. to about 50 F. In other cases, the
methods may
require applying to surfaces or objects that range in temperature from 0 F to
about
200 F.
The compositions and methods described herein beneficially remove stains
and/or soils and/or lime (hard water deposits) by at least about 70%, by at
least about
80%, and preferably at least about 90% or at least about 95%. Beneficially,
the
composition and methods described herein provide substantially similar or
superior
cleaning efficacy compared to hydroxide-based and corrosive, highly alkaline
compositions.
In exemplary embodiments, the compositions and methods beneficially remove
stains from various surfaces and provide at least about 80% stain removal, and
preferably at least about 90% stain removal or at least about 95% stain
removal. In still
further embodiments, the compositions and methods beneficially remove 100% of
stains from the treated surface. These performance benefits exceed those
achieved
from hydroxide-based and corrosive, highly alkaline compositions.
In further exemplary embodiments, the compositions and methods beneficially
remove soils from various surfaces and provide at least about 80% soil
removal, and
preferably at least about 90% soil removal or at least about 95% soil removal.
In still
further embodiments, the compositions and methods beneficially remove 100% of
soil
from the treated surface.
In still further exemplary embodiments, the compositions and methods
beneficially remove lime scale (hard water deposits) from various surfaces and
provide
at least about 70% lime scale removal, at least about 75% lime scale removal,
at least
about 80% lime scale removal, and preferably at least about 90% lime scale
removal
from the treated surface.
24
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
EXAMPLES
Embodiments of the present invention are further defined in the following non-
limiting Examples. It should be understood that these Examples, while
indicating certain
embodiments of the invention, are given by way of illustration only. From the
above
discussion and these Examples, one skilled in the art can ascertain the
essential
characteristics of this invention, and without departing from the spirit and
scope thereof,
can make various changes and modifications of the embodiments of the invention
to adapt
it to various usages and conditions. Thus, various modifications of the
embodiments of the
invention, in addition to those shown and described herein, will be apparent
to those skilled
in the art from the foregoing description. Such modifications are also
intended to fall
within the scope of the appended claims.
The Alkaline Control and Acidic Compositions utilized in the Examples are
shown
in Table 2-3:
Table 2
Acidic Formic Citric Gluconic
Control Acid Acid Acid
Strong acid(s) 10-15
Organic acid(s) 5-7 4.5
Block copolymer
surfactant (Plyoxyethylne
Plyoxypropylne bl.pol.)
<0.5
Benzyl Alcohol 10 8 8
Anionic surfactant
(Dodecyl Benz Sulfonic
Acid, 96%) 3.5 2.5 2.5
Formic Acid, 90% 5
Gluconic Acid 4.5
SXS, 40% 1 1
Dyes <1
Water Zeolite Softened Remainder Remainder Remainder Remainder
Total wt-% 100 100 100 100
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
Table 3
Alkaline Control
Description Wt-%
Non-hydroxide alkalinity 1-10
Anionic surfactant 0.5-5
Solvent 5-15
Aminocarboxylate
Chel ant 0
Additional functional
ingredient <1
Water Zeolite Softened Remainder
Total wt-% Composition 100
EXAMPLE 1
An alkaline control formulation (see Alkaline Control in Table 3) used for
removing grease stains and polymerized soils, such as corn oil soils, was
compared to an
Acidic Composition containing a combination of a solvent and an organic acid
(see Acidic
Composition in Table 2) to assess additional performance benefits. Initial
assessment of
the Acidic Composition was completed on soiled coupons with polymerized corn
oil.
Additional testing was completed on tea stains, to determine if the Acidic
Composition
containing the combination of solvent and an organic acid could expand
performance
benefits beyond greasy soil removals.
Preparation of polymerized Corn Oil Panels. Corn oil soils were prepared onto
3 x
5-inch stainless steel (304 grade) panels by lightly coating corn oil using a
2-inch
polyurethane brush. The panels are rectangular flat sheets of stainless steel
to simulate the
surface of vertical surfaces surrounding grilling equipment where vaporized
grease collects
and coats. Protecting coating is removed from the coupons before they are
cleaned, rinsed
and any residue removed before the coupons are dried. The panels were coated
with the
polymerized corn oil. They were coated evenly to ensure no streaks of bare
steel remained
and any excess oil was removed using only the weight of the brush.
Approximately 0.12 g
+/- 0.01 g corn oil was applied to the coupon.
26
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
Panels were then placed on an aluminum tray and cooked in a preheated 375 F
oven for approximately 20 minutes (rotating the tray at 10, 15 and 20 minutes)
until the
polymerized oil was no longer tacky and exhibited a light amber color. After
approximately 10 minutes of cooking the oil begins to polymerize and thicken
and smoke
evolves from the oil. The tray is rotated to ensure panels were evenly heated
in oven. The
coupons were then allowed to cool overnight at ambient temperature, and placed
on a rack
with the coated side angled down to reduce any dust accumulation. The coupons
are cured
after resting for 24 hours at room temperature before testing with the Control
and Acidic
Composition.
A first test comparing the various Acidic Compositions dropped onto coupons
using a pipet measured the time in seconds for the cleaning composition to
penetrate the
corn oil soil on the coupons. FIG. 1A shows the efficacy of formic acid; FIG.
1B shows the
efficacy of citric acid; and FIG. 1C shows the efficacy of gluconic acid-
containing Acidic
Compositions. All three formulations showed at least similar efficacy to a
Control.
Importantly the measured time to penetrate and remove the corn oil (i.e.,
degreasing) was
less than 1 minute for all acidic formulations as shown in FIGS. 1A-1C.
A second test compared the various Acidic Compositions for a soaking
application
of the chemistries onto the coupons. The coupons were submerged into a test
solution of
the chemistry being evaluated and the amount of time required for complete
soil removal
was measured. The efficacy of the compositions is shown in FIGS. 2A-2C where a
1-
minute soak time removed the polymerized corn oil.
EXAMPLE 2
Additional testing of the Acidic Compositions to remove polymerized corn oil
from
coupons was completed. The methodology of Example 1 for the polymerized corn
oil soils
was used with the chemistry dropped onto the coupons and pH was adjusted using
MEA.
The coupons were contacted with the various Acidic Compositions at pH between
2-4 to
assess the impact of pH on corn oil removal, namely the speed of removal. The
efficacy of
the compositions is shown in FIG. 3, showing that the lower pH formulations
provide
faster penetration and removal of the polymerized corn oil soils from the
coupons. The pH
of 3-4 provide complete removal, however, for spot treatments where contact
time is as
27
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
minimal as possible before an application, such as a ware wash application,
the Acidic
Compositions having a pH <4 may be preferred.
EXAMPLE 3
Methods for assessing tea-stained tile cleaning performance were performed
using
the Alkaline Compositions compared to Control (as outlined in Example 1). The
testing of
the Citric Acid Compositions against tea stains demonstrates ability to treat
and remove
one of the hardest stains in the warewash process. The composition of tea is
complex with
oxidized polyphenols (tannins) bridged by calcium silicates in its structure
of the stain on a
surface. The evaluated method is used to create the stain on white ceramic
tiles and then
try to remove it by using a standard automated dish machine with a known
concentration
of detergent. Performance is evaluated by comparison between sets of tiles
using both
visual and image manipulation methods.
Initially, tiles were washed in standard dish machine with a highly alkaline
detergent containing a high concentration of chelants. Cycles on the dish
machine are run
until the tiles are fully clean. Tiles are then ready to be soiled.
To prepare tiles for testing, a tea bath was filled with 17 grain hard water
and
heated to 180 F using a steam line. 150 Lipton black tea bags were added and
agitated for
about 5 minutes. The tea bags were removed while squeezing the liquid out of
them into
the broth. The temperature in the bath was then decreased to about 155-160 F.
Then the
airline leading to the tea bath was turned on. A set of tiles was added to a
rack in a dipper
so that the tiles were dipped 25 times for a period of 1 minute each time in
the solution and
1 minute out of solution for each dip. If necessary, deionized water was added
to the dipper
to replace any water loss by evaporation. The tiles were then allowed to air
dry for 3 days
(or baked in an oven at 180 F for 2 hours before testing).
To assess the ability of Citric Acid Compositions to better remove soil,
stained tiles
were submerged into beakers of various cleaning compositions. Before the tiles
were
washed, the amount of soil on the tiles was noted by taking pre-cleaned
pictures and visual
assessments of the tiles. Beakers of test solutions were prepared as the
concentrate RTU
(no further dilution). The solutions were stirred at 100 rpm. The tea-stained
tiles were
dipped into the respective beaker for 30 seconds, 1 minute, and 2 minutes.
Thereafter the
28
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
tile was visually analyzed and then quantified using imaging software to
assess the
cleanliness of the tile.
The Alkaline Control versus Citric Acid Composition efficacy is shown on FIG.
4
along with the % removal measurements shown in Table 4 and again summarized in
Table 5.
Table 4
Soak
Treatment time initial final %removal
Alkaline
Control 30 sec 75 77.9 17.06%
Citric Acid
Composition 30 sec 69.4 90.3 92.48%
Alkaline
Control 1 min 74.7 76.3 9.25%
Citric Acid
Composition 1 min 73.7 91.3 96.17%
Alkaline
Control 2 min 75.9 79.2 20.50%
Citric Acid
Composition 2 min 77.1 91.3 95.30%
Table 5
Treatment 30 sec 1 min 2 min
Alkaline Control 17.06% 9.25% 20.50%
Citric Acid
Composition 92.48% 96.17% 95.30%
The test results show that the Citric Acid Composition performs substantially
better
than the Alkaline Control.
EXAMPLE 4
Mechanical degreasing efficacy of the various Acidic Compositions compared to
the acidic Control, Alkaline Control (as outlined in Example 1) and a negative
control (DI
water) was assessed using red and black soils. The preparation of and testing
for each of
red and black soils is described.
Black Soil Preparation. A black soil including about 50 grams mineral spirits,
about
5 grams mineral oil, about 5 grams motor oil, about 2.5 grams black pigment
dispersion
and about 37.5 grams bandy black clay was prepared. A plurality of 3" x 3"
white vinyl
29
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
tiles were soiled on the back, grooved side with approximately 0.75 grams of
the black test
soil using a 3" foam brush. The tiles were allowed to dry at room temperature
overnight.
The next day, the tiles were placed into a soaking tray containing about 200
grams of the
cleaning compositions for about 2 minutes.
Red Soil Preparation. A red soil consisting of lard, oil, protein, and iron
(III) oxide
(for color) was prepared. About 30 grams of lard was combined with about 30
grams of
corn oil, about 15 grams of whole powdered egg, and about 1.5 grams of Fe2O3.
The back,
grooved sides of a plurality of 3" x 3" white vinyl tiles were soiled with
approximately 0.75
grams of the red soil using a 3" foam brush. The tiles were allowed to dry at
room
temperature overnight. It is believed that this incubation period allowed the
bonds holding
the triglycerides and proteins together in the soil to begin to crystallize
and interlink. The
next day, the tiles were placed into a soaking tray containing about 200 grams
of a test
composition for about 1 minute.
soiled .1,"
92A ¨ 2434 \
= 3.384
soiled L ¨24.74 ,
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/ITS2021/060268
1
washed
( 92.1 - 24.74 )
3.381n ____________________________________
washed L :1, -2474.
soiled - washed 100
percent soil removal
soiled L'
The Control versus Acidic Compositions efficacy results are shown on FIG. 5
with
performance of the Acidic Compositions surpassing the Alkaline Control (as
well as DI
water as a negative control).
EXAMPLE 5
Soap Scum removal efficacy of a Formic Acid composition and a Citric Acid
composition was compared to an Alkaline Control composition, an Acidic Control
composition, and water. The formulas for the compositions utilized are shown
in Tables 2-
3.
Soap Scum Soil Preparation. Soap scum soil was prepared by mixing
approximately 82 grams of DI water, 1.5 grams of casein protein, 3 grams of
Ivory brand
soap, 0.40 grams Crisco, 0.30 grams Kahn clay, and 12.8 grams of a hardness
solution
containing calcium and magnesium chloride and sodium bicarbonate. This mixture
was
adjusted to a pH of 8.75. Approximately 0.50 grams of the prepared soap scum
soil was
spread onto a plurality of glass slides and allowed to dry. After drying, the
slides were
baked for 30 minutes at 200 C, and then allowed to cool.
The soil removal test was conducted using a Gardner Straightline Apparatus
with a
synthetic sponge. The synthetic sponge was saturated with about 300 grams of
the test
compositions, wrung out, and then 25 grams of the test composition was applied
to one
side of the sponge. The slides were then placed into the Gardner and sprayed
lightly with
the test composition. The test composition was allowed to dwell on the slide
for 30
31
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
seconds. The slides were then scrubbed with about 2 pounds of pressure with
the
moistened synthetic sponge for 15 cycles. The slides were then rinsed with DI
water and
dried overnight at room temperature.
Percent soil removed, by weight, was then determined. A graph of percent soil
removal by composition is depicted in FIG. 6. Visual images of the glass
slides after the
soil removal test are shown in FIGS. 7A-7E. As shown in FIG. 6 and FIGS. 7A-
7E, the
Formic Acid composition and the Citric Acid composition perform substantially
better that
the Alkaline Control, the Acidic Control, and water.
EXAMPLE 6
Spot treatment efficacy of the Citric Acid and Formic Acid compositions
according
to the invention was compared to a control composition of water. The
formulations for the
acidic compositions are according to Table 2. The water used is 5 gpg water.
Tea-stained tiles were prepared according to the procedure described in
Example 3.
The tiles were sprayed with the test compositions and the compositions were
allowed to
dwell on each tile for one minute (i.e., a presoak). Then the tiles were
washed in a Hobart
A1\4-15 dishwashing machine in a single cycle with 10 drops of a commercially
available
alkaline detergent composition (60-100 wt-% sodium hydroxide, Alkaline
Detergent) using
5 gpg water, and a regular, non-foaming trigger spray.
Photographs were taken of the tiles before and after the wash. The Citric Acid
composition outperformed water. The Citric Acid composition removed
significantly more
of the soil, as shown by comparing FIGS. 8A (water) and 8B (Citric Acid
Composition).
Similar testing was done to compare spot treatment for polymerized corn oil
soil
removal. Panels soiled with corn oil were prepared as outlined in Example 1.
The panels
were sprayed with either the Citric Acid composition or water and each was
allowed to
dwell on the panel for one to two minutes. Panels were either sprayed with a
non-foaming
sprayer with 1 minute of dwell time wherein the panels were oriented
vertically, or the
panels were sprayed with a foaming trigger sprayer and oriented horizontally.
The panels
were then washed in a Hobart AM-15 dishwashing machine in a single cycle with
10 drops
of Alkaline Detergent and 5 gpg water. Photographs of the panels were taken
after the
cleaning was complete. The Citric Acid composition removed a significant
amount of the
polymerized corn oil with a two-minute treatment, as shown in FIG. 9B.
32
CA 03196534 2023- 4- 24
WO 2022/115355
PCT/US2021/060268
Similar testing was done to compare spot treatment for protein removal. Soil
preparation. The panels were sprayed with either the Formic Acid composition
or water
and each was allowed to dwell on the panel for one minute. The panels were
then washed
in a Hobart AM-15 dishwashing machine for 10 cycles using 10 drops of Alkaline
Detergent and 5 gpg water. Photographs were taken of the panels after cleaning
in the
dishwashing machine. The Formic Acid composition outperformed the control
formulation
as shown in FIGS. 10A and 10B.
For each of the tests outlined in this Example, percent soil removed was
calculated.
A graph of the results is shown in FIG. 11. As demonstrated in FIG. 11, the
Acidic
Compositions outperform the water control spot test for each type of stain and
soil.
It is to be understood that while the invention has been described in
conjunction
with the detailed description thereof, the foregoing description is intended
to illustrate, and
not limit the scope of the invention, which is defined by the scope of the
appended claims.
Other embodiments, advantages, and modifications are within the scope of the
following
claims. In addition, the contents of all patent publications discussed supra
are incorporated
in their entirety by this reference.
The features disclosed in the foregoing description, or the following claims,
or the
accompanying drawings, expressed in their specific forms or in terms of a
means for
performing the disclosed function, or a method or process for attaining the
disclosed result,
as appropriate, may, separately, or in any combination of such features, be
utilized for
realizing the invention in diverse forms thereof.
33
CA 03196534 2023- 4- 24