Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/104227
PCT/US2021/059413
METHODS AND COMPOSITIONS FOR PRODUCING RECOMBINANT
COMPONENTS FOR USE IN FOOD AND OTHER PRODUCTS
RELATED APPLICATIONS
[0001]
This application claims priority to U.S. Provisional Patent Application
Serial No.
63/113,729, filed on November 13, 2020, and to U.S. Provisional Patent
Application Serial No.
63/175,278, filed on April 15, 2021, which are incorporated herein by
reference, in their
entireties.
FIELD OF THE INVENTION
[0002]
The present disclosure relates generally to methods and compositions for
producing
a recombinant component for use in a food or other product. The present
disclosure further
relates generally to compositions comprising the recombinant component
produced by such
methods and compositions.
BACKGROUND OF THE INVENTION
[0003]
Animal-derived food products (e.g., meat, milk, egg) are popular sources
of
nutrition. They comprise high-quality protein, essential minerals (e.g.,
calcium, phosphorus,
zinc, magnesium), and vitamins (e.g., riboflavin, vitamin A, vitamin B12). In
addition, many
such food products possess advantageous functional characteristics that permit
production of a
wide variety of derivative food products (e.g., yogurt, cheese, cream, ice
cream, butter,
mayonnaise).
[0004]
However, animal-derived food products comprise components (e.g., lactose,
allergens, saturated fats, cholesterol) that can cause unhealthy reactions in
humans. Moreover,
production of these food products involves animal husbandry, which has a
significant impact
on animal welfare and the environment, and which bears the potential for
contamination with
pesticide residues, heavy metals, aflatoxin Ml, and pathogens.
[0005]
These concerns have fueled development of alternatives to animal-derived
food and
other products (e.g., cosmetics, personal care products). Some such
alternatives comprise plant-
derived components (e.g., proteins, lipids, vitamins). Increasingly, however,
alternatives to
animal-derived food and other products are produced from components (e.g.,
proteins, lipids)
that are produced recombinantly (e.g., using recombinant host cells).
[0006]
The use of recombinant components in food and other products poses new
problems.
One such problem is that food and other products produced from recombinant
components
1
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
frequently contain a significant amount of such recombinant components (more
than was
typical in previous products in which recombinant components were utilized),
and that the use
of large amounts of recombinant components may be impacted by other, sometimes
undesired,
components that are simultaneous produced by the recombinant host cells from
which the
recombinant components are obtained, and that potentially co-purify with the
recombinant
component.
[0007]
One such other component are enzymes with activity that leads to the
release of free
fatty acids (FFAs; i.e., FFA releasing enzymes). FBA releasing enzymes can
hydrolyze bonds
in diglycerides, triglycerides, phospholipids, lipoproteins, and other
molecules to release FFAs.
Substrates for FFA releasing enzymes are comprised in a variety of food and
other products in
which recombinant components could be used. In some such food and other
products, the
release of FFAs can have detrimental effects, by, for example, producing
rancid aroma and/or
taste, interfering with formation of emulsions, having undesirable effects on
texture, interacting
with essential nutrients (e.g., vitamins) and thereby decreasing nutritional
content, and reducing
shelf-life. Production of FFAs in a food and other product may also have
beneficial effects, by,
for example, producing desired flavor and/or aroma profiles (e.g., flavor
profiles of aged
cheese), or making enzyme-modified cheese for use in processed cheese.
Challenges have thus
to be overcome, particularly with respect to FFA releasing enzyme activities,
in the production,
processing, and use of recombinant components for production of alternatives
to animal-
derived food and other products, such that, for example production of rancid
aroma and taste is
delayed, emulsion formation is not impacted, nutritional content is
maintained, texture is not
modulated, and shelf-life is not shortened; and/or desired flavor and/or aroma
profiles are
produced.
[0008]
Therefore, there exists a need for methods by which alternatives to animal-
derived
food and other products can be produced from recombinant components, as well
as for
compositions used in and obtained from such methods.
INCORPORATION BY REFERENCE
[0009]
All publications, patents, patent applications, sequences, database
entries, scientific
publications, and other references mentioned herein are incorporated by
reference in their
entireties to the same extent as if each individual publication, patent,
patent application,
sequence, database entry, scientific publication, or other reference was
specifically and
individually indicated to be incorporated by reference. To the extent the
material incorporated
2
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
by reference contradicts or is inconsistent with the present disclosure, the
present disclosure,
including definitions, will supersede any such material.
SUMMARY OF THE INVENTION
[0010]
In various aspects, provided herein is a recombinant host cell capable of
producing
a recombinant component, wherein the recombinant host cell comprises a
modulated
production and/or activity of a FFA releasing enzyme compared to production
and/or activity
of the FFA releasing enzyme comprised in a corresponding recombinant host
cell.
[0011]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from 1-1-A releasing
enzymes
comprising UniProt sequence# GORH85, GOR6T6, GOR6X2, G0R707, GOR7K1, GOR810,
GOR9D1, G0R9F9, G0R9J9, G0R9X3, GORBGO, GORBJO, GORBM4, GORBZ6, GORD16,
GORDK5, GORDU7, GOREM9, GOREZ4, GORFR3, GORFT3, GORG04, GORG60, GORGD5,
GORGN7, GORGQO, GORGQ7, GORHJ4, G0RI29, GORIJ9, GORIU1, GORIV5, GORJ76,
GORJC6, GORJYO, GORK83, GORKE6, GORKH7, GORKI9, GORKL4, GORL87, GORLBO,
GORLB7, GORLH4, GORLLO, GORLR3, GORM14, GORME5, GORMI3, GORNF8, GORPQ8,
GORQD1, GORQG3, GORQJ5, GORQN5, GORR42, GORRK3, GORRQ4, GORSK7, GORTR6.
GORTT4, GORUIO, GORUZ9, GORV93, GORW73, GORW77, GORWS1, GORWT9, GORWY5,
GORX82, GORX90, GORHQ7, GORVD2, or GOR8A6, or homologs thereof, or
combinations
thereof.
[0012]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from 1-1-A releasing
enzymes
comprising UniProt sequence# GORGQO, GORH85, GORMI3, GORLH4, GORIU1, GORBM4,
GOR9D1, GORFR3, GORG60, G0R6T6, G0R8N5, GORBJO, GORRQ4, GOREZ4, GORIJ9,
GOR6X2, GORJY0, GORR42, GORW77, GORQJ5, GORFT3, GOR810, G0RI29, GORL87,
GORLLO, GORGD5, or GORKH7, or homologs thereof, or combinations thereof.
[0013]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from FBA releasing
enzymes
comprising UniProt sequence# GORGQO, GORLH4, or G0RMI3, or homologs thereof,
or
combinations thereof.
[0014]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from FBA releasing
enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof.
3
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0015]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from FFA releasing
enzymes
comprising UniProt sequence# GORGQO or homologs thereof, or combinations
thereof.
[0016]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from FBA releasing
enzymes
comprising UniProt sequence# GORLH4 or homologs thereof, or combinations
thereof.
[0017]
The recombinant host cell of paragraph 1100101, wherein the FFA releasing
enzyme
comprises or consists of a FFA releasing enzyme selected from FBA releasing
enzymes
comprising UniProt sequence# GORMI3 or homologs thereof, or combinations
thereof.
[0018]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; and a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# G0R616, GOR6X2, G0R707, GOR7K1, GOR810, GOR9D1, G0R9F9, G0R9J9,
G0R9X3, GORBGO, GORBJO, GORBM4, GORBZ6, GORD16, GORDK5, GORDU7, GOREM9,
GOREZ4, GORFR3, GORFT3, GORG04, GORG60, GORGD5, GORGN7, GORGQO, GORGQ7,
GORHJ4, G0RI29, GORU9, GORIU1, GORIV5, GORJ76, GORJC6, GORJYO, GORK83,
GORKE6, GORKH7, GORKI9, GORKL4, GORL87, GORLBO, GORLB7, GORLH4, GORLLO,
GORLR3, GORM14, GORME5, GORMI3, GORNF8. GORPQ8, GORQD1, GORQG3, GORQJ5,
GORQN5, GORR42, GORRK3, GORRQ4, GORSK7, GOR1R6, G0R114, GOR U10, GORUZ9,
GORV93, GORW73, GORW77. G0RWS1, GORWT9, GORWY5, GORX82, GORX90,
GORHQ7, RORVD2, or GOR8A6, or homologs thereof, or combinations thereof.
[0019]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; and a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORGQO, GORH85, GORMI3, GORLH4, GORIU1, GORBM4, GOR9D1, GORFR3,
GORG60, GOR6T6, GOR8N5, GORBJO, GORRQ4, GOREZ4, GORU9, GOR6X2, GORJYO,
GORR42, GORW77, GORQJ5, GORFT3, G0R810, G0RI29, GORL87, GORLLO, GORGD5, or
GORKH7, or homologs thereof, or combinations thereof.
[0020]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; and a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
4
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
sequence# GORGQO, GORLH4, GORMI3, G0R707, GOR7K1, G0R810, GORFT3, GORG60,
G0RGD5, G0RI29, GOR119, GORKH7, GORKL4, G0RL87, GORLLO, G0RLR3, GORME5,
GORQJ5, GORRK3, GORSK7, GORWT9, or GORX82, or homologs thereof, or
combinations
thereof.
[0021]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; and a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORGQO, GORLH4, or GORMI3, or homologs thereof, or combinations
thereof.
[0022]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; and a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORMI3 or homologs thereof, or combinations thereof.
[0023]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from a first
FFA releasing
enzyme selected from FFA releasing enzymes comprising UniProt sequence# GORH85
or
homologs thereof, or combinations thereof; and a second FFA releasing enzyme
selected from
FFA releasing enzymes comprising UniProt sequence# GORGQO or homologs thereof,
or
combinations thereof.
[0024]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; and a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORLH4 or homologs thereof, or combinations thereof.
[0025]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORMI3 or homologs thereof, or combinations thereof; and a third 1-
PA releasing
enzyme selected from FFA releasing enzymes comprising UniProt sequence# GORGQO
or
homologs thereof, or combinations thereof.
[0026]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORM13 or homologs thereof, or combinations thereof; and a third FFA
releasing
enzyme selected from FFA releasing enzymes comprising UniProt sequence# GORLH4
or
homologs thereof, or combinations thereof.
[0027]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORGQO or homologs thereof, or combinations thereof; and a third FFA
releasing
enzyme selected from FFA releasing enzymes comprising UniProt sequence# GORLH4
or
homologs thereof, or combinations thereof.
[0028]
The recombinant host cell of paragraph [0010], wherein the FFA releasing
enzyme
comprises or consists of a first FFA releasing enzyme selected from FFA
releasing enzymes
comprising UniProt sequence# GORH85 or homologs thereof, or combinations
thereof; a
second FFA releasing enzyme selected from FFA releasing enzymes comprising
UniProt
sequence# GORMI3 or homologs thereof, or combinations thereof; a third FFA
releasing
enzyme selected from FFA releasing enzymes comprising UniProt sequence# GORGQO
or
homologs thereof, or combinations thereof; and a fourth FFA releasing enzyme
selected from
FFA releasing enzymes comprising UniProt sequence# GORLH4 or homologs thereof,
or
combinations thereof.
[0029]
The recombinant host cell of any of paragraphs [0010-0028], wherein the
modulated
production and/or activity of the FFA releasing enzyme comprises a decreased
production
and/or activity of the FFA releasing enzyme.
[0030]
The recombinant host cell of paragraph [0029], wherein the decreased
production
and/or activity of the FFA releasing enzyme is a decreased production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%.
[0031]
The recombinant host cell of any of paragraphs [0010-0030], wherein the
recombinant host cell is derived from a bacterium, yeast, or filamentous
fungus.
6
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0032]
The recombinant host cell of paragraph [0031], wherein the filamentous
fungus is
selected from Aspergillus (e,g., Aspergillus niger), Trichoderma (e,g,,
Trichoderma reesei,
Trichoderma citrinoviride), or Myceliophthora (e.g., Myceliophthora
thermophila).
[0033]
The recombinant host cell of any of paragraphs [0010-0032], wherein the
recombinant component is a recombinant protein.
[0034]
The recombinant host cell of paragraph [0033], wherein the recombinant
protein is
a recombinant milk protein.
[0035]
The recombinant host cell of paragraph [0034], wherein the recombinant
milk
protein is a recombinant casein.
[0036]
The recombinant host cell of paragraph [0034], wherein the recombinant
milk
protein is a recombinant whey protein.
[0037]
The recombinant host cell of paragraph [0034], wherein the recombinant
milk
protein is derived from cow, human, sheep, goat, or horse.
[0038]
In various aspects, provided herein is a method for producing a
composition that
comprises a recombinant component produced by a recombinant host cell capable
of producing
the recombinant component, wherein the method comprises modulating a
production and/or an
activity of a FFA releasing enzyme.
[0039]
The method of paragraph [0038], wherein the recombinant host cell is the
recombinant host cell of any of paragraphs [0010-0037].
[0040]
The method of paragraph [0038], wherein the modulating a production and/or
an
activity of a FFA releasing enzyme comprises decreasing a production and/or an
activity of the
FFA releasing enzyme.
[0041]
The method of paragraph [0040], wherein the decreasing the production
and/or
activity of the FFA releasing enzyme is a decreasing production and/or
activity of at least 10%,
at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%,
at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%,
at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%,
or 100%.
[0042]
The method of paragraphs 100401 or 1-00411, wherein the decreasing a
production
and/or an activity of the FFA releasing enzyme comprises adding to a
fermentation broth,
preparation, or composition an inhibitor of a FFA releasing enzyme.
[0043]
The method of paragraph [0042], wherein the inhibitor of the FFA releasing
enzyme
is an inhibitor of an FBA releasing enzyme that comprises a serine residue in
its catalytic
domain.
7
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0044] The method of paragraphs [0040] or [0041], wherein the
decreasing a production
and/or an activity of the FFA releasing enzyme comprises purifying the
recombinant
component away from the FFA releasing enzyme activity and/or purifying the FFA
releasing
enzyme away from the recombinant component.
[0045] The method of paragraph [0044], wherein the purifying the
FFA releasing enzyme
away from the recombinant component comprises use of an activity-based protein
profiling
(ABPP) small-molecule probe.
[0046] In various aspects, provided herein is a method for
producing a recombinant
component, wherein the method comprises fermenting the recombinant host cell
of any of
paragraphs [0010-0037] in a culture medium under conditions suitable for
production of the
recombinant component.
[0047] The method of paragraph [0046], wherein the method
further comprises purifying
the recombinant component to obtain a preparation comprising the recombinant
component;
and/or post-processing the recombinant component.
[0048] The method of paragraph [0047], wherein the purifying
comprises purifying to
obtain a preparation comprising the recombinant component at a purity of
greater than 90%.
[0049] The method of paragraph [0047], wherein the post-
processing comprises spray
drying or concentrating the recombinant component to obtain a powder.
[0050] In various aspects, provided herein is a composition
comprising a recombinant
component, wherein the composition is produced by the method of any of
paragraphs [0038-
0045].
[0051] The composition of paragraph [0050], wherein the
composition comprises between
about 0.1% and about 100% by dry mass of the recombinant component.
[0052] The composition of paragraph [0050], wherein the
composition is a food product.
[0053] The composition of paragraph [0052], wherein the
composition is a supplemented
food product.
[0054] The composition of paragraph [0053], wherein the
composition is a supplemented
dairy product.
[0055] The composition of paragraph [0053], wherein the
composition is a supplemented
egg product.
[0056] The composition of paragraph [0052], wherein the
composition is a substitute food
product.
[0057] The composition of paragraph [0056], wherein the
composition is a substitute dairy
product.
8
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0058]
The composition of paragraph [0057], wherein the composition is a
substitute egg
product.
[0059]
The composition of paragraph [0050], wherein the composition is a cosmetic
or
personal care product.
[0060]
The composition of any of paragraphs [0050-0059], wherein the recombinant
components is a recombinant protein.
[0061]
The composition of paragraph [0060], wherein the composition is
essentially free
of any protein other than the recombinant protein.
[0062]
The composition of paragraph [0060], wherein the composition is
essentially free
of any recombinant protein other than the recombinant protein.
[0063]
The composition of paragraph [0060], wherein the recombinant protein is a
recombinant milk protein.
[0064]
The composition of paragraph [0063], wherein the composition is
essentially free
of any protein other than the recombinant milk protein
[0065]
The composition of paragraph [0063], wherein the composition is
essentially free
of any recombinant protein other than the recombinant milk protein.
[0066]
In various aspects, provided herein is a recombinant host cell that
comprises a
recombinant expression construct encoding a FFA releasing enzyme, and that
comprises an
increased production and/or activity of the FFA releasing enzyme compared to a
corresponding
host cell.
[0067]
The recombinant host cell of paragraph [0066], wherein the FBA releasing
is
selected from FFA releasing enzymes comprising UniProt sequence# GORH85,
GOR6T6,
GOR6X2, G0R707, GOR7K1, GOR810, GOR9D1, GOR9F9, GOR9J9, GOR9X3, GORBGO,
GORBJO, GORBM4, GORBZ6, GORD16, GORDK5, GORD U7, GOREM9, GOREZ4, GORFR3,
GORFT3, GORG04, GORG60, GORGD5, GORGN7, GORGQO, GORGQ7, GORHJ4, G0RI29,
GOR119, GORIU1, GORIV5, GORJ76, GORJC6, GORJYO, GORK83, GORKE6, GORKH7,
GORKI9, GORKL4, GORL87, GORLBO, GORLB7, GORLH4, GORLLO, GORLR3, GORM14,
GORME5, GORMI3, GORNF8, GORPQ8, GORQD1, GORQG3, GORQJ5, GORQN5, GORR42,
GORRK3, GORRQ4, GORSK7, GORTR6, GORTT4, GORUIO, GORUZ9, GORV93, GORW73,
GORW77, G0RWS1, GORWT9, GORWY5, GORX82, GORX90, GORHQ7, GORVD2, or
GOR8A6, or homologs thereof.
[0068]
The recombinant host cell of paragraphs [0066] or [0067], wherein the
recombinant
host cell is derived from a bacterium, yeast, or filamentous fungus.
9
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0069]
The recombinant host cell of paragraph [0068], wherein the filamentous
fungus is
selected from Aspergillus (e,g., Aspergillus niger), Trichoderma (e,g,,
Trichoderma reesei,
Trichoderma citrinoviride), or Myceliophthora (e.g., Myceliophthora
thermophila).
[0070]
The recombinant host cell of any of paragraphs [0066-0069], wherein the
increased
production and/or activity of the FFA releasing enzyme is an increase in
production and/or
activity of at least 50%.
[0071]
In various aspects, provided herein is a method for producing a FFA
releasing
enzyme, wherein the method comprises: obtaining the recombinant host cell of
any of
paragraphs [0066-0070], culturing the recombinant host cell in a culture
medium under
conditions suitable for production and/or secretion of the FFA releasing
enzyme, and optionally
purifying the FFA releasing enzyme.
BRIEF DESCRIPTION OF THE FIGURES
[0072]
Example embodiments will be described and explained with additional
specificity
and detail through the use of the accompanying drawings in which:
[0073]
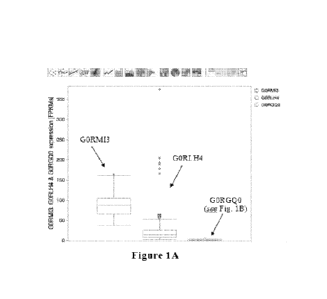
Figures 1A and 1B provide box-and-whisker plots showing results of RNAseci
analyses of Trichoderma reesei host cells producing recombinant protein (i.e.,
recombinant (3-
lactoglobulin) for presence of GORMI3, GORGQO, and GORLH4 transcripts, wherein
Figure
1B provides a detailed view of the GORGQO plot shown in Figure 1A, in
accordance with
various embodiments of the present invention. FPKMs = Fragments per kilobase
of transcript
per million mapped reads.
[0074]
Figure 2 is a bar plot showing para-phenyl (pNP) acyl ester hydrolyzing
activity
comprised in recombinant protein (i.e., recombinant f3-lactoglobulin)
preparations in absence
(Sample 1) or presence (Sample 2) of Thermo ActivX TAMRA-FP fiwiropliosphonaie
(inhibitor of hydrolases comprising a serine residue in their catalytic
domain), showing removal
of FFA releasing activity according to various representative embodiments of
the present
invention. Activity unit U/g = micromole pNP formation per hour per gram of
recombinant
protein; Acyl groups: C4 = butyrate, C8 = octanoate, C12= laurate, and C16 =
palmitate.
[0075]
Figure 3 is a map of a recombinant vector used for production of a
recombinant host
cell capable of producing a GORGQO, GORLH4, or GORMI3 protein, in accordance
with
various representative embodiments of the present invention.
[0076]
Figure 4 is a map of a recombinant vector used for production of a
recombinant host
cell capable of producing a GORGQO, GORLH4, or GORMI3 protein, in accordance
with
various representative embodiments of the present invention.
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0077]
Figure 5A shows a Western blot of recombinant GORMI3 protein comprised in
fermentation broths of 4 independent recombinant Pichia pastoris
transformants, Figure 5B
shows an SDS PAGE gel of recombinant GORGQO produced by 3 independent
recombinant
Trichoderma reesei transformants, and Figure 5C shows an SDS PAGE gel of
recombinant
GORLH4 produced by 3 independent recombinant Trichoderma reesei transformants,
in
accordance with various representative embodiments of the present invention.
[0078]
Figure 6 are photographs of UV-illuminated wells of 24-well plates
comprising
rhodamine B in presence (boxed) or absence (not boxed) of GORMI3, GORGQO, and
GORLH4
proteins, in accordance with various representative embodiments of the present
invention.
[0079]
Figure 7 is a map of a targeting vector used for production of a
recombinant host
cell comprising an eliminated FFA releasing activity of a GORH85, GORMI3,
GORGQO, and/or
GORLH4 protein, in accordance with various representative embodiments of the
present
invention.
DETAILED DESCRIPTION OF THE INVENTION
[0080]
The subsequent discussion of the invention is presented for purposes of
illustration
and description, and is not intended to limit the scope of the invention to
the embodiments
disclosed herein. As such, variations and modifications of the disclosed
embodiments are
within the scope of the invention, e.g., as may be within the skill and
knowledge of those in the
art after understanding the present disclosure. It is intended to obtain
rights which include
alternative embodiments to the extent permitted, including alternate,
interchangeable and/or
equivalent structures, functions, ranges or steps to those disclosed herein,
and without intending
to publicly dedicate any patentable subject matter. Unless defined otherwise,
all technical and
scientific terms used herein have the same meaning as is commonly understood
by one of
ordinary skill in the art to which this disclosure pertains. Further, unless
otherwise required by
context, singular terms shall include the plural, and plural terms shall
include the singular.
Definitions
[0081]
The terms "a" and "an" and the and similar references as used herein refer
to both
the singular and the plural (e.g., meaning at least one or "one or more"),
unless otherwise
indicated herein or clearly contradicted by context. For example, the term "a
compound" is
synonymous with the terms at least one compound" and "one or more compounds",
and may
refer to a single compound or to a plurality of compounds, including mixtures
thereof.
[0082]
The term "and/or" as used herein refers to multiple components in
combination with
or exclusive of one another. For example, "x, y, and/or z" may refer to "x"
alone, "y" alone, "z"
11
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
alone, "x, y, and z", "(x and y) or z", "(x and z) or y", "(y and z) or x", "x
and y" alone, "x and
z" alone, "y and z" alone, or "x or y or z".
[0083]
The term "at least" or "one or more" as used herein refers to one, two,
three, four,
five, six, seven, eight, nine, ten, at least one, at least two, at least
three, at least four, at least
five, at least six, at least seven, at least eight, at least nine, at least
ten, or more, or all of the
elements subsequently listed.
[0084]
The term "encoding" as used herein in context of a polynucleotide refers
to a
polynucleotide that comprises a coding sequence that when placed under the
control of
appropriate regulatory sequences is transcribed into mRNA that may be
translated into a
polypeptide. A coding sequence generally starts at a start codon (e.g., ATG)
and ends at a stop
codon (e.g., UAA, UAG and UGA). A coding sequence may contain a single open
reading
frame, or several open reading frames (e.g., separated by introns).
[0085]
The term "endogenous" as used herein refers to what is natively present in
the
context described. When used in reference to a protein that is produced by a
cell, the term
implies that the protein is natively produced by the cell. When used in
reference to a
polynucleotide that is comprised in a cell, the term implies that the
polynucleotide is natively
comprised in the cell (e.g., is present in the native cell; or is situated in
the same genomic
location in the native cell).
[0086]
The terms "1-TA releasing enzyme activity" or "activity of a FFA releasing
enzyme"
as used herein refer to the activity of an enzyme that can hydrolyze a bond
(e.g., hydrolase an
ester bond) that leads to the release of a free fatty acid (FFA). FFA
releasing enzymes are
designated with enzyme commission number (EC number) 3.1. The terms are used
interchangeably herein.
[0087]
The term "essentially free of" as used herein refers to the indicated
component being
either not detectable in the indicated composition by common analytical
methods, or to the
indicated component being present in such trace amount as to not be
functional. The term
"functional" as used in this context refers to not materially contributing to
properties of the
composition comprising the trace amount of the indicated component, or to not
having material
activity (e.g., chemical activity, enzymatic activity) in the indicated
composition comprising
the trace amount of the indicated component, or to not having health-adverse
effects upon use
or consumption of the composition comprising the trace amount of the indicated
component.
The term "materially contributing" as used herein refers to the indicated
component
contributing to an attribute of a composition to such extent that in the
absence of the component
(e.g., in a reference composition that is identical to the composition except
that it lacks the
12
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
indicated component) the attribute is at least 10%, at least 20%, at least
30%, at least 40%, or
at least 50% less present/active/measurable.
[0088]
The term "fermentation broth" as used herein refers to a culture
comprising a
recombinant host cell capable of producing a recombinant component.
[0089]
The term "filamentous fungus" as used herein refers to an organism from
the
filamentous form of the subdivision Eumycota and Oomycota (as defined by
Hawksworth et
al., In, Ainsworth and Bisby's Dictionary of The Fungi, 8th edition, 1995, CAB
International,
University Press, Cambridge, UK). A filamentous fungus is distinguished from a
yeast by its
hyphal elongation during vegetative growth.
[0090]
The term "fungus" as used herein refers to organisms of the phyla
Ascomycotas,
Basidiomycota, Zygomycota, and Chythridiomycota, Oomycota, and Glomeromycota.
It is
understood, however, that fungal taxonomy is continually evolving, and
therefore this specific
definition of the fungal kingdom may be adjusted in the future.
[0091]
The term "heterologous" as used herein refers to not being natively
present in the
context described. When used in reference to a protein that is produced by a
cell, the term
implies that the protein is not natively produced by the cell. When used in
reference to a
polynucleotide that is comprised in a cell, the term implies that the
polynucleotide is not
natively comprised in the cell (e.g., is not present in the native cell; or is
not situated in the
genomic location in the native cell, whether or not the heterologous
polynucleotide is itself
endogenous (originating from the same cell or progeny thereof) or exogenous
(originating from
a different cell or progeny thereof)).
[0092]
The term "homolog" as used herein refers to a protein that comprises an
amino acid
sequence that is at least 40% (e.g., at least 40%, at least 45%, at least 50%,
at least 55%, at least
60%, at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at
least 90%, at least
91%, at least 92%, at least 93%, at least 94%, at least 95%, at least 96%, at
least 97%, at least
98%, at least 99%, at least 99.5%, or 100%) identical to a sequence of amino
acids of a similar
length (i.e., a length that is within +/- 20% of the length of the query amino
acid sequence)
comprised in a reference protein, and that has a functional property that is
similar (e.g., is within
50%, within 40%, within 30%, within 20%, or within 10% of) or identical to
that of the
reference protein. The term includes polymorphic variants, interspecies
homologs (e.g.,
orthologs), paralogs, and alleles of a protein, as well as variants that are
man-made using genetic
engineering techniques.
[0093]
The term "host cell" as used herein refers not only to the particular
subject cell but
to the progeny of such cell. Because certain modifications may occur in
succeeding generations
13
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
due to either mutation or environmental influences, such progeny may not, in
fact, be identical
to the subject cell, but are still included within the scope of the term "host
cell" as used herein.
[0094]
The terms "identity" or "identical" in the context of two or more
polynucleotide or
polypeptide sequences as used herein refer to the nucleotide or amino acid
residues that are the
same when the two or more polynucleotide or polypeptide sequences,
respectively, are aligned
for maximum correspondence. Depending on the application, the "identity" can
exist over a
region of the sequences being compared (e.g., over the length of a functional
domain) or over
the full length of the sequences. A "region" is considered to be a continuous
stretch of at least
6, 9, 14, 19, 24, 29, 34, 39, or more nucleotides, or of at least 2, 6, 10,
14, 18, 22, 26, 30, or
more amino acids. For comparison, typically one sequence acts as a reference
sequence to
which one or more test sequences are compared. When using a sequence
comparison algorithm,
test and reference sequences are input into a computer, subsequence
coordinates are designated,
if necessary, and sequence algorithm program parameters are designated. The
sequence
comparison algorithm then calculates the percent sequence identity for the
test sequence(s)
relative to the reference sequence, based on the designated program
parameters. Optimal
alignment of sequences for comparison can be conducted, e.g., by the local
homology algorithm
of Smith & Waterman, Adv. Appl. Math. 2:482 (1981), by the homology alignment
algorithm
of Needleman & Wunsch, J. Mol. Biol. 48:443 (1970), by the search for
similarity method of
Pearson & Lipman, Proc. Nat'l. Acad. Sci. USA 85:2444 (1988), by computerized
implementations of these algorithms (GAP, BESTFIT, PASTA, and TFASTA in the
Sequence
Analysis Software Package of the Genetics Computer Group (GCG), University of
Wisconsin
Biotechnology Center, which can be used with default parameters), or by visual
inspection (see
generally Ausubel et al., infra). One example of an algorithm that is suitable
for determining
percent sequence identity and sequence similarity is the BLAST algorithm (see,
for example,
Altschul et al. [1990] J. Mol. Biol. 215:403-410; Gish & States. [1993] Nature
Genet. 3:266-
272; Madden et al. [1996] Meth. Enzymol. 266:131-141; Altschul et al. [1997]
Nucleic Acids
Res. 25:3389-3402; Zhang 7 Madden. [1997] Genome Res. 7:649-656). Software for
performing BLAST analyses is publicly available through the National Center
for
Biotechnology Information.
[0095]
The terms "including," "includes," "having," "has," "with," or variants
thereof as
used herein are intended to be inclusive in a manner similar to the term
"comprising".
[0096]
The term Ain-modulated" as used herein in connection with a FFA releasing
enzyme
activity refers to a lack of alteration of an activity and/or expression of a
FFA releasing enzyme
14
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
(e.g., no change in a concentration of a FFA releasing enzyme or its enzymatic
activity towards
a substrate).
[0097]
The term "modulated" as used herein in connection with a FFA releasing
enzyme
activity refers to any alteration of an activity of a FFA releasing enzyme.
Such modulated FFA
releasing enzyme activity is typically due either to an increase or decrease
in a concentration
of a FFA releasing enzyme, or an increase or decrease in an enzymatic activity
of a FFA
releasing enzyme towards a substrate. For example, the term can refer to a
decrease in activity
by at least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at
least 35%, at least
40%, at least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at
least 70%, at least
75%, at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at
least 97%, at least
98%, at least 99%, or 100%. Alternatively, the term can refer to an increase
in activity by at
least 5%, at least 10%, at least 15%, at least 20%, at least 25%, at least
50%, at least 75%, at
least 100%, at least 150%, at least 200%, at least 300%, at least 400%, at
least 500%, at least
600%, at least 700%, at least 800%, at least 900%, or at least 1,000%.
[0098]
The term "native" as used herein refers to what is found in nature in its
unmodified
state (e.g., a cell that is not genetically modified by a human, and that is
maintained under
conditions [e.g., level of oxygenation, pH, salt concentration, temperature,
and nutrient (e.g.,
carbon, nitrogen, sulfur) availability] that are not defined by a human).
[0099]
The term "operably linked" as used herein refers to an arrangement of
elements that
allows them to be functionally related. For example, a promoter sequence is
operably linked to
a protein coding sequence if it controls the transcription of the protein
coding sequence, and a
secretion signal sequence is operably linked to a protein if the secretion
signal sequence directs
the protein through the secretion system of a cell. An "operably linked"
element may be in
contiguous linkage with another element, or act in trans or at a distance to
another element.
Non-limiting examples of functions that may be operably linked include control
of
transcription, control of translation, protein folding, and protein secretion.
[0100]
The term "one or more" as used herein refers to one, at least one, two,
three, four,
five, six, seven, eight, nine, ten, or more, or all of the elements
subsequently listed.
[0101]
The terms "optional" or "optionally" as used herein refer to a feature or
structure
being present or not, or an event or circumstance occurring or not. The
description includes
instances in which a feature or structure is present, instances in which a
feature or structure is
absent, instances in which an event or circumstance occurs, and instances in
which an event or
circumstance does not occur.
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0102]
The terms "plant protein", "animal protein", and "microbial protein" as
used herein
refer to polypeptides that comprise a sequence of at least 20 (e.g., at least
20, at least 30, at least
40, at least 50, at least 60, at least 70, at least 80, at least 90, at least
100, or at least 150, and
usually not more than 250) amino acids that is at least 80% (e.g., at least
80%, at least 85%, at
least 90%, at least 95%, at least 99%, 100%) identical to a sequence of amino
acids in a protein
natively found in a plant, animal, or microbe (i.e., unicellular organism,
including all bacteria,
archaea, unicellular protista, unicellular animals, unicellular plants,
unicellular fungi,
unicellular algae, protozoa, and chromista), respectively (i.e., a protein
that is native to a plant
cell, animal cell, or microbial cell, respectively).
[0103]
The term "polynucleotide" as used herein refers to a polymeric form of at
least 2
(e.g., at least 5, at least 10, at least 20, at least 30, at least 40, at
least 50, at least 100, at least
500, at least 1,000) nucleotides. The term includes both sense and antisense
strands of DNA
molecules (e.g., cDNA, genomic DNA, synthetic DNA) and RNA molecules (e.g.,
mRNA,
synthetic RNA), as well as analogs of DNA or RNA containing non-natural
nucleotide analogs,
non-native internucleoside bonds, and/or chemical modifications. A
polynucleotide may be
modified chemically or biochemically or may contain non-natural or derivatized
nucleotide
bases. Such modifications include, for example, labels; methylation;
substitution of one or more
of the naturally occurring nucleotides with an analog; internucleotide
modifications such as
uncharged linkages (e.g., methyl phosphonates, phosphotriesters,
phosphoramidates,
carbamates), charged linkages (e.g., phosphorothioates, phosphorodithioates),
pendent
moieties (e.g., polypeptides), intercalators (e.g., acridine, psoralen),
chelators, alkylators, and
modified linkages (e.g., alpha anomeric nucleic acids). Examples of modified
nucleotides are
described in the art (see, for example, Malyshev et al. 2014. Nature 509:385;
Li et al. 2014. J.
Am. Chem. Soc. 136:826). Also included are synthetic molecules that mimic
polynucleotides
in their ability to bind to a designated sequence via hydrogen bonding or
other chemical
interaction. Such molecules are known in the art and include, for example,
molecules in which
peptide linkages substitute for phosphate linkages in the backbone of the
molecule. Other
modifications may include, for example, analogs in which the ribose ring
contains a bridging
moiety or other structure such as the modifications found in "locked"
polynucleotides. A
polynucleotide may be in any topological conformation. For instance, a
polynucleotide may be
single- stranded, double-stranded, triple-stranded, quadruplexed, partially
double-stranded,
branched, hairpinned, circular, or in a padlocked conformation. The term
"polynucleotide
sequence" as used herein refers to a sequence of nucleotides that are
comprised in a
polynucleotide or of which a polynucleotide consists.
16
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0104]
The terms "polypeptide" and "protein" as used herein can be interchanged,
and refer
to a naturally-occurring or a naturally not occurring polymeric form of at
least 2 (e.g., at least
5, at least 10, at least 20, at least 30, at least 40, at least 50, at least
100) amino acids. A
"polypeptide" or "protein" may have an active structure or lack a functional
structure, comprise
coded and/or non-coded amino acids, comprise amino acids that occur in nature
and/or amino
acids that do not occur in nature, comprise chemically modified and/or
biochemically modified
and/or derivatized amino acids, comprise unmodified and/or modified peptide
backbones,
and/or be monomeric (i.e., having a single chain) or polymeric (i.e., having
of two or more
chains, which may be covalently or non-covalently associated). The term "amino
acid
sequence" as used herein refers to a sequence of amino acids that is comprised
in a
"polypeptide" or "protein", or of which a "polypeptide or "protein" consists.
[0105]
The term "preparation" as used herein refers to a preparation obtained
upon
separation of a recombinant component from one or more other components of a
fermentation
broth. For example, a preparation may be a clarified fermentation broth (i.e.,
a fermentation
broth from which cells and cell debris were removed).
[0106]
The term "promoter sequence" as used herein refers to a polynucleotide
that directs
transcription of a downstream polynucleotide in a cell. A promoter sequence
may include
necessary nucleotides near the start site of transcription, such as, in the
case of a polymerase II
type promoter, a TATA element. A promoter sequence may also optionally include
distal
enhancer or repressor elements, which may be located as much as several
thousand base pairs
from the start site of transcription.
[0107]
The term "purifying" or "purified" or "isolating" or "isolated" as used
herein refers
to a component being substantially separated from chemicals, cellular
components, and cells
(e.g., cell walls, membrane lipids, chromosomes, other proteins, other cells
in an organism) of
the source from which the component originated. The component may be at least
60% pure,
e.g., greater than 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 99% pure. The term
does not
require (albeit allows) that the component be separated from all chemicals,
cellular
components, and cells.
[0108]
The term "recombinant component" as used herein refers to a component that
is
produced recombinantly (i.e., is produced in a recombinant host cell, or is
synthesized from a
recombinant polynucleotide). Non-limiting examples of recombinant components
include
recombinant proteins (e.g., microbial proteins, plant proteins (e.g., pea
protein (e.g., legumin,
vicillin, covicillin), potato protein (e.g., tuberin, protease inhibitor
notate II)), animal proteins
(e.g., structural proteins (e.g., collagen, tropoelastin, elastin), milk
proteins, egg proteins (e.g.,
17
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
ovalbumin, hereinovomucoid, ovalbumin, ovotransferrin, G162M F167A ovomucoid,
ovoglobulin G2, ovoglobulin G3, ct-ovomucin, J3-ovomucin, lysozyme,
ovoinhibitor,
ovoglycoprotein, flavoprotein, ovomacroglobulin, ovostatin, cystatin, avidin,
ovalbumin
related protein X, ovalbumin related protein Y)), lipids, carbohydrates, small
molecules, food
additives (e.g., sweetening agents), food supplements (e.g., vitamins),
neutraceuticals, and
probiotics.
[0109]
The term "recombinant protein- as used herein refers to a protein that is
produced
in a recombinant host cell, or to a protein that is synthesized from a
recombinant polynucleotide.
[0110]
The term "recombinant host cell" as used herein refers to a host cell that
comprises
a recombinant polynucleotide. Thus, for example, a recombinant host cell may
produce a
polynucleotide or polypeptide not found in the native (non-recombinant) form
of the host cell,
or a recombinant host cell may produce a polynucleotide or polypeptide at a
level that is
different from that in the native (non-recombinant) form of the host cell. It
should be understood
that such term is intended to refer not only to the particular subject cell
but also to the progeny
of such a cell. Because certain modifications may occur in succeeding
generations due to either
mutation or environmental influences, such progeny may not be identical to the
subject cell,
but are still included within the scope of the term "recombinant host cell" as
used herein. A
recombinant host cell may be an isolated cell or cell line grown in culture or
may be a cell
which resides in a living tissue or organism.
[0111]
The term "recombinant polynucleotide" as used herein refers to a
polynucleotide
that is removed from its naturally occurring environment, or a polynucleotide
that is not
associated with all or a portion of a polynucleotide abutting or proximal to
the polynucleotide
when it is found in nature, or a polynucleotide that is operatively linked to
a polynucleotide that
it is not linked to in nature, or a polynucleotide that does not occur in
nature, or a polynucleotide
that contains a modification that is not found in that polynucleotide in
nature (e.g., insertion,
deletion, or point mutation introduced artificially, e.g., by human
intervention), or a
polynucleotide that is integrated into a chromosome at a heterologous site.
The term may be
used, e.g., to describe cloned DNA isolates, or a polynucleotide comprising a
chemically
synthesized nucleotide analog. A polynucleotide is also considered
"recombinant" if it contains
a genetic modification that does not naturally occur. For instance, an
endogenous
polynucleotide is considered a "recombinant polynucleotide" if it contains an
insertion,
deletion, or substitution of one or more nucleotides that is introduced
artificially (e.g., by human
intervention). Such modification can introduce into the polynucleotide a point
mutation,
substitution mutation, deletion mutation, insertion mutation, missense
mutation, frameshift
18
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
mutation, duplication mutation, amplification mutation, translocation
mutation, or inversion
mutation. The term includes a polynucleotide in a host cell's chromosome, as
well as a
polynucleotide that is not in a host cell's chromosome (e.g., a polynucleotide
that is comprised
in an episome). A recombinant polynucleotide in a host cell or organism may
replicate using
the in vivo cellular machinery of the host cell; however, such recombinant
polynucleotide,
although subsequently replicated intracellularly, is still considered
recombinant for purposes of
this invention.
[0112]
The term "regulatory element" as used herein refers a polynucleotide
sequence that
mediates, modulates, or regulates expression (e.g., transcription, post-
transcriptional events,
translation) of a polynucleotide to which the regulatory element is operably
linked. Non-
limiting examples of regulatory elements include promoter sequences,
termination sequences,
transcriptional start sequences, translational start sequences, translation
stop sequences,
enhancer sequences, activator sequences, response elements, protein
recognition sites,
inducible elements, protein binding sequences, 5' and 3' untranslated regions,
upstream
activation sequences (UAS), introns, operators (i.e., sequences of nucleic
acids adjacent to a
promoter that comprise a protein-binding domain where a repressor protein can
bind and reduce
or eliminate activity of the promoter), efficient RNA processing signals
(e.g., splicing signals,
polyadenylation signals), sequences that stabilize cytoplasmic mRNA, sequences
that enhance
translation efficiency (e.g., ribosome binding sites [e.g., Shine-Dalgarno
sequencesp,
sequences that enhance protein stability, sequences that enhance protein
secretion, and
combinations thereof.
[0113]
The terms "secrete," "secretion", and "secreted" as used herein refer to
the process
of export of a protein across a cellular membrane and/or cell wall of a cell
that produces the
protein to an extracellular milieu. As provided herein, such secretion may
occur actively or
passively.
[0114]
The term "secretion signal" as used herein refers to a peptide that is
operably linked
to the N-terminus of a protein, and that mediates the delivery of the protein
via the intracellular
secretory pathway of a host cell in which the protein is produced (i.e.,
synthesized) to the
exterior of the host cell. Typically, operable linkage of a recombinant
protein with a secretion
signal requires removal of a start codon of the polynucleotide sequence
encoding the
recombinant protein.
[0115]
The term two or more" as used herein refers to two, three, four, five,
six, seven,
eight, nine, ten, or more, or all of the elements subsequently listed.
19
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0116]
The term "vector" as used herein refers to a nuclei acid that can carry a
polynucleotide sequence to be introduced into a host cell. Non-limiting
examples of vectors
include cloning vectors, expression vectors, shuttle vectors, plasmids, phage
particles, viral
vectors, cosmids, bacterial artificial chromosomes (BACs), yeast artificial
chromosomes
(YACs), virus particles (e.g., comprising heterologous polynucleotides), DNA
constructs (e.g.,
produced by cloning or PCR amplification), and linear double-stranded
molecules (e.g., PCR
fragments). Certain vectors are capable of autonomous replication in a host
cell into which they
are introduced (e.g., vectors having an origin of replication which functions
in the host cell).
Other vectors may be integrated into the genome of a host cell upon
introduction into the host
cell, and are thereby replicated along with the host genome.
[0117]
The term "yeast" as used herein refers to any organism of the order
Saccharomycetales. Vegetative growth of yeast is by buddingiblebbing of a
unicellular thallus,
and carbon catabolism may be fermentative.
[0118]
Recitation of ranges of values herein are merely intended to serve as a
shorthand
method of referring individually to each separate value (fractional or
integral) falling within the
range inclusive of the recited minimum and maximum value, unless otherwise
indicated herein,
and each separate value is incorporated into the specification as if it were
individually recited
herein. Also, it should be understood that any numerical range recited herein
is intended to
include all sub-ranges subsumed therein. For example, a range of "1 to 10" is
intended to
include all sub-ranges between (and including) the recited minimum value of 1
and the recited
maximum value of 10, that is, having a minimum value equal to or greater than
1 and a
maximum value of less than or equal to 10. It should further be understood
that all ranges and
quantities described below are approximations and are not intended to limit
the invention.
[0119]
It should be understood that in any method disclosed herein the order of
steps or
order for performing certain actions is immaterial so long as the invention
remains operable.
Moreover, two or more steps or actions may be conducted simultaneously.
Method for Producing Composition Comprising Recombinant Component
[0120]
In various aspects, provided herein is a method for producing a
composition that
comprises a recombinant component (e.g., any of the recombinant components
disclosed
herein) produced by a recombinant host cell capable of producing the
recombinant component,
wherein the method comprises modulating an activity of a FFA releasing enzyme
(e.g., activity
of any one FFA releasing enzyme disclosed herein or activities of any
combination of two or
more FFA releasing enzymes disclosed herein).
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0121]
Modulating an activity of a FFA releasing enzyme in a method provided
herein may
occur in any single step or in any combination of two or more steps that
provide for a modulated
FFA releasing enzyme activity. Non-limiting examples of suitable steps
include: i) culturing
the recombinant host cell under fermentation conditions suitable for
modulating a FFA
releasing enzyme activity; ii) modulating a FFA releasing enzyme activity in a
fermentation
broth, a preparation, or a composition; iii) purifying the recombinant
component away from a
FFA releasing enzyme activity produced by the recombinant host cell and/or
purifying a FFA
releasing enzyme activity away from the recombinant component; and/or iv)
obtaining a
recombinant component produced by a recombinant host cell that comprises a
modulated FFA
releasing enzyme activity.
Fermentation Conditions Suitable for Modulating FFA Releasing Enzyme Activity
[0122]
The method according to any of the above may comprise: a) obtaining a
recombinant host cell that is capable of producing a recombinant component;
and b) culturing
the recombinant host cell in a culture medium under fermentation conditions
suitable for
production and/or secretion of the recombinant component and for modulating a
FFA releasing
enzyme activity.
[0123]
Conditions suitable for modulating a FBA releasing enzyme activity may be,
for
example, conditions under which the recombinant host cell modulates its
production of the FFA
releasing enzyme activity. Non-limiting examples of such conditions include a
suitable pH, a
suitable temperature, a suitable feed rate, a suitable pressure, a suitable
fluid shear force, a
suitable type and/or amount of a nutrient (e.g., a suitable carbon content, a
suitable nitrogen
content, a suitable phosphorus content), a suitable type and/or amount of a
culture supplement,
a suitable type and/or amount of a trace metal, and/or a suitable level of
oxygenation.
[0124]
A suitable pH may be a pH of between 2 and 7.5, 6.5, 6, 5,5, 5, 4.5, 4,
3.5, 3, or 2.5;
between 2.5 and 7.5, 6.5, 6, 5,5, 5, 4.5, 4, 3.5, or 3; between 3 and 7.5,
6.5, 6, 5,5, 5, 4.5, 4, or
3.5; between 3.5 and 7.5, 6.5, 6, 5,5, 5, 4.5, 4; between 4 and 7.5, 6.5, 6,
5,5, 5, 4.5; between
4.5 and 7.5, 6.5, 6, 5,5, 5; between 5 and 7.5, 6.5, 6, 5,5; between 5.5 and
7.5, 6.5, 6; between
6 and 7.5, 6.5; between 6.5 and 7.5, or 7; or between 7 and 7.5.
[0125]
A suitable type of culture supplement may be an anti-foam agent. Non-
limiting
examples of suitable anti-foam agents include Struktol J 673 A
(Schill&Seilacher GmbH,
Hamburg, Germany), Industrol DF204 (BASF Canada, Inc., Mississauga, Canada),
Polyglycol
P-2000 (Dow, Midland, MI), Hodag K-60K (Hodag Chemical Corp., Chicago, IL),
and Erol
DF6000K (PMC Ouvrie, Carvin, France), ACP 1500 (Dow Chemical Company, Midland,
MI),
Antifoam 204 (Sigma-Aldrich, St Louis, MO), SAG 471 (Momentive Performance
Materials
21
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
Inc., Waterford, NY), SAG 5693 (Momentive Performance Materials Inc.,
Waterford, NY),
SAG 710 (Momentive Performance Materials Inc., Waterford, NY), SAG 730 (Mom
end ve
Performance Materials Inc., Waterford, NY), silicone antifoams, Struktol J 647
(Schill&Seilacher, Hamburg, Germany), and sunflower oil.
Modulating FFA Releasing Enzyme Activity in Fermentation Broth, Preparation,
or
Composition
[0126]
The method according to any of the above may comprise: i) obtaining a
recombinant
host cell that is capable of producing a recombinant component; ii) culturing
the recombinant
host cell in a culture medium under conditions suitable for production and/or
secretion of the
recombinant component to obtain a fermentation broth comprising the
recombinant component;
iii) optionally purifying the recombinant component from the fermentation
broth to obtain a
preparation comprising the recombinant component; and iv) modulating a 1-1-A
releasing
enzyme activity in the fermentation broth, preparation, or composition
comprising the
recombinant component.
[0127]
A FFA releasing enzyme activity in a fermentation broth, preparation, or
composition may be modulated by, for example, adding to the fermentation
broth, preparation,
or composition a FFA releasing enzyme inhibitor. Non-limiting examples of
suitable FFA
releasing enzyme inhibitors include synthetic inhibitors (e.g., phosphonates,
boronic acid, lipid
analogues) and natural inhibitors (e.g., fl-lactones (such as valilactone,
ebelactone A & B, and
vibralactone), manno-oligosaccharides, acetylcholine esterase inhibitors,
cholinesterase
inhibitors, polyphenols, saponins, panclicins, hesperidin, caulerpenyne,
proanthocyanidin,
Orlistat (Xenical), carnosic acid, escin, crocin, crocetin, chlorogenic acid,
neochlorogenic acid,
feruloyquinic acid, e-polylysine, chitosan, chitin), isolated, for example,
from sources such as
Juniperus communis, Illicium religiosum, Panax japonicus rhizomes, Panax
ginseng, Panax
quinquefolius, Acanthopanax senticosus, Camellia sinensis var. sinensis,
Camellia sinensis var.
assamica, Kochia scoparia, Platycodi radix, Salacia reticulate, Nelumbo
nucifera, Salix
matsudana, Eriochloa villosa, Salacia reticulate, Scabiosa tschiliensis Gnm,
and Acanthopanax
sessiliflorous. In some embodiments, FFA releasing enzyme activity is
modulated by addition
to a fermentation broth, preparation, or composition of an inhibitor of FFA
releasing enzymes
that comprise a senile residue in their catalytic domain (e.g., inhibitor
Thermo ActivX
TAMRA-FP Iluorophosphonate). Non-limiting examples of FFA releasing enzymes
that
comprise a serine residue in their catalytic domain include GORGQO, GORH85,
GORMI3,
GORLH4, GORIU1, GORBM4, G0R9D1, GORFR3, GORG60, GOR6T6, GOR8N5, GORBJO,
22
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
GORRQ4, GOREZ4, GORU9, G0R6X2, GORJYO, G0RR42, G0RW77, GORQJ5, GORFT3,
G0R810, G0RI29, G0RL87, GORLLO, G0RGD5, and G0RKH7.
[0128]
A FFA releasing enzyme activity in a fermentation broth, preparation, or
composition also may be modulated by, for example, removing from and/or adding
to the
fermentation broth, preparation, or composition a cofactor required for
activity of a FFA
releasing enzyme and/or a cofactor required for activity of a FBA releasing
enzyme inhibitor,
respectively. Non-limiting examples of such cofactors include metals (e.g.,
divalent cations,
such as calcium), which may be removed, for example, with a chelating agent
such as
ethylenediaminetetraacetic acid (EDTA).
[0129]
A FFA releasing enzyme activity in a fermentation broth, preparation, or
composition also may be modulated by, for example, thermal or non-thermal
processing. Non-
limiting examples of thermal processing include pasteurizing and sterilizing.
Non-limiting
examples of non-thermal processing include high pressure pasteurizing (i.e.,
high-pressure
processing, HPP), ultrasonicating, pulse electric field processing, and
irradiating. In some
embodiments, a 1-TA releasing enzyme activity in a fermentation broth,
preparation, or
composition is essentially eliminated or reduced by incubating the
fermentation broth,
preparation, or composition at high temperature for a relatively short period
of time (e.g., at a
temperature of between 85 C and 90 C for between 5 and 10 min).
Purifying Recombinant Component Away from FFA Releasing Enzyme Activity
[0130]
'the method according to any of the above may comprise: i) obtaining a
recombinant
host cell capable of producing a recombinant component; ii) culturing the
recombinant host cell
in a culture medium under conditions suitable for production and/or secretion
of the
recombinant component to obtain a fermentation broth comprising the
recombinant component;
and iii) purifying the recombinant component away from a FFA releasing enzyme
activity
and/or purifying the FFA releasing enzyme away from the recombinant component
to obtain a
preparation comprising the recombinant component.
[0131]
Purifying a recombinant component away from a FFA releasing enzyme
activity
may be accomplished on the basis of any one property that differentiates the
recombinant
component from a FFA releasing enzyme, or on the basis of a combination of two
or more such
properties used in succession (e.g., separation based on charge followed by
separation based on
hydrophobicity, separation based on hydrophobicity followed by separation
based on pH
stability) or in parallel (e.g., separation based on pH stability and
thermolability/thermostability, separation based on pH stability and affinity
to a specific
23
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
molecule, separation based on solubility and pH stability and/or
thermolability/thermostability
and/or pI).
[0132]
For example, a fermentation broth or preparation comprising the
recombinant
component may be heated to a temperature at which a FFA releasing enzyme is
denatured and
precipitates out of solution but at which the recombinant component remains
structurally intact
and soluble. The precipitated FFA releasing enzyme may subsequently be
separated from the
soluble recombinant component by any suitable method, including 1-g
sedimentation,
accelerated sedimentation via centrifugation, and/or a variety of filtration
techniques, including
but not limited to depth filtration or tangential flow filtration, using
filter pads, sheets, or
membranes.
[0133]
As a further example, a suitable chromatographic support may be added to a
fermentation broth or preparation comprising the recombinant component, and
conditions (e.g.,
pH and/or ionic strength) may be adjusted such that the FFA releasing enzyme
or the
recombinant component (but not both) binds to the chromatographic support
(e.g., based on
charge or hydrophobicity), leaving behind the soluble recombinant component or
FFA
releasing enzyme in an unbound portion, respectively. Non-limiting examples of
suitable
chromatographic supports include phenyl sepharose, butyl sepharose, and octyl
sepharose. The
chromatographic support with bound FFA releasing enzyme or recombinant
component may
subsequently be separated from the soluble recombinant component or FFA
releasing enzyme,
respectively, by any suitable method known in the art, including but not
limited to 1-g
sedimentation, centrifugation, or filtration. Alternatively, the
chromatographic support may be
a stationary support (e.g., adsorbent in a column) through which the
fermentation broth or
preparation comprising the recombinant component is made to travel, and the
recombinant
component is obtained in the unbound portion whereas the FFA releasing enzyme
binds to the
chromatographic support, or the recombinant component is bound to the
chromatographic
support whereas and the FFA releasing enzyme remains in the unbound portion,
and the bound
recombinant component is subsequently released from the chromatograph support
by adjusting
conditions.
[0134]
As a further example, a counterion or ion-exchange resin or sodium acid
salt may
be added to a fermentation broth or preparation comprising the recombinant
component, and
the pH and/or ionic strength of the fermentation broth or preparation may be
adjusted such that
the counterion or ion-exchange resin or sodium acid salt forms a complex with
the recombinant
component or the FFA releasing enzyme, leaving behind a soluble FFA releasing
enzyme or
recombinant component, respectively. The complex may subsequently be isolated
by any
24
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
suitable method known in the art, including 1-g sedimentation, accelerated
sedimentation via
centrifugation, and/or a variety of filtration techniques, including but not
limited to depth
filtration or tangential flow filtration, using filter pads, sheets, or
membranes. In embodiments
in which the recombinant component is complexed to the counterion or ion-
exchange resin or
sodium acid salt, the recombinant component may be extracted from the complex
by adjusting
conditions (e.g., adjusting pH and/or ionic strength).
[0135]
As a further example, the pH of a fermentation broth or preparation
comprising the
recombinant component may be adjusted such that a FFA releasing enzyme is
denatured and
precipitates out of solution, leaving behind soluble recombinant component.
The precipitated
PM releasing enzyme may subsequently be separated from the soluble recombinant
component by any suitable method, including but not limited to 1-g
sedimentation, accelerated
sedimentation via centrifugation, and/or a variety of filtration techniques,
including but not
limited to depth filtration or tangential flow filtration, using filter pads,
sheets, or membranes.
[0136]
As a further example, an activity-based protein profiling (ABPP) small-
molecule
probe may be added to a fermentation broth or preparation comprising the
recombinant
component. The probe may bind to an Fl-A releasing enzyme that comprises a
serine residue
in its catalytic domain. Upon binding of the ABPP to an FBA releasing enzyme,
the bound FFA
releasing enzyme may be immobilized via a second specific affinity binding,
which may result
in removal of the FFA releasing enzyme from the recombinant component. Non-
limiting
examples of ABPPs include biotinylated fluorophosphonates (e.g., ActivX"
desthiobiotin-
fluorophosphonate serine hydrolase probe (DTB-FP)), wherein the phosphonate
moiety
covalently may bind to a nucleophilic serine in the catalytic domain of a FFA
releasing enzyme,
and wherein the biotin moiety may interact with avidinylated agarose and
thereby allow for
immobilization of the reacted FFA releasing enzyme (e.g., to agarose beads)
and physical
separation via centrifugation, filtration, and/or other soluble-insoluble
separation method.
FBA Releasing Enzyme Activity
[0137]
A modulated FFA releasing enzyme activity as provided herein (e.g., a
modulated
FM releasing enzyme activity comprised in a fermentation broth or preparation
comprising a
recombinant component, or comprised in a recombinant host cell provided herein
capable of
producing a recombinant component) may be a modulated activity of any one FFA
releasing
enzyme or any combination of two or more FFA releasing enzymes.
[0138]
Non-limiting examples of suitable FFA releasing enzymes include carboxylic
ester
hydrolases (EC number 3.1.1), phosphoric diester hydrolases (EC number 3.1.4),
exoribonucleases (EC number 3.1.13), carboxylesterases (EC number 3.1.1.1),
arylesterases
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
(EC number 3.1.1.2), triacylglycerol lipases (EC number 3.1.1.3),
phospholipases A2 (EC
number 3.1.1.4), lysophospholipases (EC number 3.1.1.5), acetylesterases (EC
number
3.1.1.6), acetylcholinesterases (EC number 3.1.1.7), glycerophosphocholine
phosphodiesterases (EC number 3.1.4.2), phospholipases C (EC number 3.1.4.3),
phospholipases D (EC number 3.1.4.4), phosphoinositide phospholipases C (EC
number
3.1.4.11), glycosylphosphatidylinositol phospholipases D (EC number 3.1.4.50),
N-
acetylphosphatidylethanolamine-hydrolyzing phospholipases D (EC number
3.1.4.54),
pectinesterases (EC number 3.1.1.11), gluconolactonases (EC number 3.1.1.17),
acylglycerol
lipases (EC number 3.1.1.23), 3-oxoadipate enol-lactonases (EC number
3.1.1.24), 1,4-
lactonases (EC number 3.1.1.25), galactolipases (EC number 3.1.1.26),
phospholipases Al (EC
number 3.1.1.32), lipoprotein lipases (EC number 3.1.1.34), cephalosporin-C
deacetylases (EC
number 3.1.1.41), carboxymethylenebutenolidases (EC number 3.1.1.45), 2-pyrone-
4,6-
dicarboxylate lactonases (EC number 3.1.1.57), acetylxylan esterases (EC
number 3.1.1.72),
feruloyl esterases (EC number 3.1.1.73), cutinases (EC number 3.1.1.74),
hormone-sensitive
lipases (EC number 3.1.1.79), palmitoyl-protein hydrolases (EC number
3.1.2.22), epoxide
hydrolases (EC number 3.3.2.3), ceramidases (EC number 3.5.1.23), leukotriene-
A4 hydrolases
(EC number 3.3.2.6), hepoxilin-epoxide hydrolase (EC number 3.3.2.7), limonene-
1,2-epoxide
hydrolases (EC number 3.3.2.8), microsomal epoxide hydrolase (EC number
3.3.2.9), soluble
epoxide hydrolase (EC number 3.3.2.10), cholesterol-5,6-oxide hydrolase (EC
number
3.3.2.11), fatty acid amide hydrolase (EC number 3.5.1.99), and lipoxygenases
(E.C. 1.13.11.).
[0139] Non-limiting examples of suitable FFA releasing enzymes
include any of the FFA
releasing enzymes listed in Table 1, and homologs thereof, as well as FFA
releasing enzymes
that comprise any one of the InterPro domains listed in Table 2.
[0140] Table 1 ¨ Exemplary FFA Releasing Enzymes (UniProt
Sequence IDs)
A0A2T4AZ21 A2 QUQ1 G0R733 GORES5 GORL78 GORSX2
A0A2T4B 235 A2 QV27 G0R765 GOREU9 G0RL87 G0RT28
A0A2T4BBP9 A2 QV39 G0R7A1 GOREVO GORLA5 GORT33
A0A2T4BCL7 A2 QV40 GOR7C 6 GOREW1 GORLB 0 GORT48
A0A2T4B FY3 A2 QV44 GOR7E2 GOREZ4 GORLB7 GORT83
A0A2T4B JB 5 A2 QVF5 G0R7I3 GORF39 GORLFO GORTAO
A0A2T4BJD9 A2QVJ4 G0R7I6 GORF50 GORLF5 GORTF8
A0A2T4BNI9 A2 QW25 GOR7K1 GORES 5 GORLH4 GORTHO
A2Q818 A2QW83 GOR7K4 GORF60 GORLJ6 GORTIO
A2Q8F7 A2 QX56 GOR7P7 GORFF4 GORLLO GORTJO
A2Q8R7 A2 QX92 G0R7T1 GORFG1 GORLR3 GORTK4
26
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
A2 Q8U6 A2 QXD2 GOR7V3 GORFK7 GORLW2 GORTR6
A2 Q9L0 A2 Q Y19 GOR810 GORFN9 GORM14 GORTT4
A2 QAC4 A2 Q YCO G0R858 GORFR3 GORM75 GORTU6
A2 QAD7 A2 QYFO G0R862 GORFT3 GORME1 GORTX4
A2 QAH7 A2 QYK5 G0R882 GORFV5 GORME5 GORUB 4
A2 QBC9 A2 QYS 7 GOR8A6 GORFVV1 GORMI3 GORUFO
A2 QB G8 A2 QYU7 GOR8F3 GORFX4 GORMI7 GORUF6
A2 QBH3 A2 QZ17 GOR8H0 GORFY5 GORMJO GORUH2
A2 QBI I5 A2 QZ72 GOR8H3 GORFZ5 GORMK6 GORUIO
A2 QBH8 A2 QZB 2 GOR8N5 GORGO4 GORMNO GORULO
A2QBK3 A 2 QZB7 GOR8Z3 GORG60 GORMS9 GORUQ7
A2QBP1 A 2 QZE3 G0R908 GORG87 GORMU2 GORUS 8
A2 QC75 A 2 QZI3 G0R932 GORGA2 GORMV2 GORUUO
A2 QCMO A2 QZK9 G0R976 GORGD5 GORMW 2 GOR U X0
A2 QCT1 A2 QZN6 GOR9B 7 GORGG2 GORMX3 GORUY2
A2 QE05 A2 QZRO GOR9D1 GORGK1 GORMZ1 GORUZ9
A2 QE77 A2 QZW3 GOR9F9 GORGL4 GORN71 GORV27
A2 QEH4 A2 QZXO GOR9J9 GORGN7 GORNC 1 GORV66
A2 QEJ 2 A2 QZX4 GOR9K4 GORGQO GORNE8 GORV80
A2 QEW9 A2 QZY6 GOR9R2 GORGQ7 GORNF6 GORV93
A2 QF42 A2R032 GOR9S 9 GORGR3 GORNF8 GORV93
A2 QF54 A2R088 GOR9U0 GORGU9 GORNI2 GORVC2
A2 QF64 A2R098 GOR9X3 GORGV1 GORNI4 GORVD2
A2 QFE9 A 2R0H9 GOR9X6 GORGW7 GORNL1 GORVJ5
A2 Q G33 A2ROP4 GORA08 GORH18 GORN L9 GORVK4
A2 Q G70 A2ROZ6 GORA23 GORH28 GORN PO GOR V K8
A2 Q GD9 A2R199 GORA68 GORH34 GORNR1 GORVN2
A2 QH22 A2R1N7 GORAB 1 GORH85 GORNS 6 GORVN3
A2 QH76 A2R1P3 GORAC5 GORHA2 GORNS 9 GORVR8
A2 QHB7 A2R1R5 GORAEO GORHA7 GORNV2 GORVT9
A2 QHE2 A2R1X8 GORAQO GORHB 2 GORNV2 GORVW2
A2 QHE9 A2R234 GORAQ2 GORHB 9 GORNX7 GORVW9
A2 Q132 A2R256 GORAQ9 GORHG1 GORNX9 GORVY3
A2 QIAO A2R273 GORB 17 GORHG3 GORNY4 GORVZ5
A2 QIE4 A2R274 GOR B 25 GORHH9 GOR P27 GOR WOO
A2 QII0 A2R2I5 GOR B33 GORHJ4 GORPQ8 GORW44
A2 QIR3 A2R2M3 GORB 46 GORHL 8 GORPU6 GORW65
A2 Q K82 A2R2W3 GORB 49 GORHQ7 GORPX6 GORW73
A2 Q K84 A2R350 GORB72 GORHZ4 GORQB 2 GORW77
27
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
A2 Q K90 A2R496 GORB A4 G0RI29
GORQD1 GORW78
A2 Q KQ5 A2R4Z2 GORB A7 G0RI53
GORQE9 GORW83
A2QKZ8 A2R502 GORBB 4 GORIB 6 GORQF9 GORWB 8
A2QL89 A2R5R4 GORB C8 GORID1 GORQG3 GORWC3
A2 QL90 A2R5R5 GORB GO GORIEO GORQJ5 GORWG2
A2 QLAO A2R5 V7 GORB H2 GORIFO
GORQJ7 GORWH5
A2QM14 A2R689 GORBJO GORIF9 GORQJ8 GORWI8
A2 QMI7 A2R6H5 GORBJ9 GORIJ9
GORQJ9 GORWN3
A2 QMK5 A2R6I6 GORB
KO GORIL6 GORQL6 GORWP3
A2 QN29 A2R6I8 GORB
L2 GORIU1 GORQMO GORWS 1
A2QN56 A2R6L8 GORBM4 GORIV5 GOR QN5 GORWT9
A2 QNFO A 2R709 GORB R4 GORIWO GORQW6 GORWW6
A2 QNW9 A2R775 GORB V8 GORIY7 GORQY3 GORWY5
A2 QPC2 A2R780 GORBZ6 GORJ 62
GORRO2 GORX05
A2 QPJ6 A2R7H4 GORC98 GORJ65
GORRO3 GORX23
A2 QPY4 A2R7J0 GORCA4 GORJ76
GORR14 GORX29
A2 QRK1 A2R835 GORCDO GORJ81
GORR30 GORX49
A2 QRK3 A2R845 GORCG3 GORJC6 GORR42 GORX82
A2 QRP8 A2R8C2 GORCI8
GORJK1 GORR69 GORX90
A2QS21 A2R8M8 GORCP2 GORJP3 GORR96 GORXG3
A2QS22 A2R8R3 GORCVO GORJ Q1 GORRB 6 GORXI5
A2QS33 A2R8R4 GORCV3 GORJW8 GORRD1 GORXL4
A2QS46 A2R8Z3 GORCY9 GORJYO GORRG3 GORXP9
A2QS56 A 2R9C0 GORD04 GORK83 GORRH7 G2 Q0K1
A2QS66 A2RAP4 GORD16 GORK88 GORRJ 7 02Q379
A2QSJ9 A2RB F9 GORD43 GORK89 GORRK3 G2 QH51
A2QST4 A5AB CO GORD44 GORKE6 GORRL3 G2 QL32
A2QSX2 A5ABC3 GORDB2 GORKG4 GORRQ1 032129
A2QSY5 A5ABE5 GORDB5 GORKH6 GORRQ4 P37957
A2QT47 A5ABE6 GORDDO GORKH7 GORRR1 P37967
A2QT57 A5ABE8 GORDE1 GORKI9 GORRS 5 P42969
A2 QT66 A5AB H9 GORDJ1 GORKJ8 GORRS 8 Q79F14
A2QT70 A5AB K1 GORD K5 GORKL2 GORRV4
A2 QT75 A5ABZ1 GORDN5 GORKL4 GORRZ7
A2 QT90 E2PS RO GOR DU7 GORKL9 GOR S43
A2 QTIO E2PT14 GORDV8 GORKQ8 GOR S48
A2 QTI9 E2PT22 GORDWO GORKT8 GORS C7
A2 QTZO GOR6T6 GORE70 GORKT9 GORS K7
A2QUA5 GOR6X2 GOREE8 GORKW8 GORS M4
28
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
A2QUC1 GOR6X7 GOREM1 GORKX7 GORSP4
A2QUD7 GOR6Z2 GOREM4 GORL22 GORSS3
A2QUE3 G0R707 GOREM9 GORL38 GORSW4
[0141]
Table 2: InterPro Numbers of Exemplary FFA Releasing Enzyme Catalytic
Domains
: ¨ ¨ - -- ,-- ---: -
! IPR002168 IPR017915 IPR003187 ! IPR038885
. IPR008265
:
: lPR033140 lPR001087 IPR004961 : lPR039097
: IPR033112
: IPR000675 IPR001531 IPR007000 : IPR039180
! IPR033113
! lPR002641 lPR003633 1PR007942 ! lPR008947
.. : 1PR001423
:
! IPR002642 IPR007751 lPR009613 ! IPR036541
: lPR009535
: IPR002921 IPR008475 IPR010711 : IPR033560
: IPR026605
: 1PRO01711 1PR010468 1PR011402 : 1PR033562
: 1PR028382
: IPR001736 IPR013818 IPR012354 : IPR033902
: IPR028407 :
:
! IPR000909 IPR014815 IPR016272 ! IPR034315
IPR032075 :
: lPR005592 lPR015359 IPR016338 : lPR037737
: IPR032588
! IPR006693 IPRO16090 IPRO16445 ! IPR038875
; IPR033556 !
: IPR025202 IPRO17913 IPR016674 : IPR001211
: IPR033903 :
:
: IPR003140 IPR017914 IPR017186 : IPR001981
: IPR033906 :
:
! IPRO11150 IPRO24632 lPRO17766 ! IPRO02330
: lPRO35547 !
, , i
.:
:1PR015679 1PR025920 1PR017767 : 1PR002331
: 1PR035669 :
.
.
IPRO16555 IPRO29002 IPR017769 IPRO02333
: IPR000734 1
! IPRO21771 IPRO32093 IPR020009 : IPRO02334
! IPR001028
:
! IPR001192 IPR032341 IPR025483 ! IPR036444
IPR036691 !
:
: lPR024884 lPR016035 IPR002918 : lPR005152
: IPR015141 :
IPR001446 IPR004126 lPR001885 IPR000801
! 1PR027433 :
: lPR020833 lPR020834 IPR013819 : lPR000907
! IPR001246 1
[0142]
Non-limiting examples of combinations of two or more FFA releasing enzymes
include: one or more cutinases and one or more other carboxylic ester
hydrolases; one or more
lysophospholipases and one or more other carboxylic ester hydrolases; one or
more
triacylglycerol lipases and one or more other carboxylic ester hydrolases; one
or more
phospholipases A2 and one or more other carboxylic ester hydrolases; one or
more
phospholipases C and one or more other carboxylic ester hydrolases; one or
more
acetylxylanesterase and one or more other carboxylic ester hydrolases; one or
more
extracellular lipase-like proteins and one or more other carboxylic ester
hydrolases; one or more
acetylesterases and one or more other carboxylic ester hydrolases; one or more
GDSL lipases
and one or more other carboxylic ester hydrolases; one or more alpha/beta
hydrolases and one
29
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
or more other carboxylic ester hydrolases; one or more transcription factors
regulating
expression of a FFA releasing enzyme and one or more other carboxylic ester
hydrolases; one
or more cutinases and one or more lysophospholipases; one or more cutinases
and one or more
triacylglycerol lipases; one or more cutinases and one or more phospholipases
A2; one or more
cutinases and one or more phospholipases C; one or more cutinases and one or
more
acetylxylanesterases; one or more cutinases and one or more extracellular
lipase-like proteins;
one or more cutinases and one or more acetylesterases; one or more cutinases
and one or more
GDSL lipases; one or more cutinases and one or more alpha/beta hydrolases; one
or more
cutinases and one or more transcription factors regulating expression of a FFA
releasing
enzyme; one or more lysophospholipases and one or more triacylglycerol
lipases; one or more
lysophospholipases and one or more phospholipases A2; one or more
lysophospholipases and
one or more phospholipases C; one or more lysophospholipases and one or more
acetylxylanesterases; one or more lysophospholipases and one or more
extracellular lipase-like
proteins; one or more lysophospholipases and one or more acetylesterases; one
or more
lysophospholipases and one or more GDSL lipases; one or more
lysophospholipases and one
or more alpha/beta hydrolases; one or more lysophospholipases and one or more
transcription
factors regulating expression of a FFA releasing enzyme; one or more
triacylglycerol lipases
and one or more phospholipases A2; one or more triacylglycerol lipases and one
or more
phospholipases C; one or more triacylglycerol lipases and one or more
acetylxylanesterases;
one or more triacylglycerol lipases and one or more extracellular lipase-like
proteins; one or
more triacylglycerol lipases and one or more acetylesterases; one or more
triacylglycerol
lipases and one or more GDSL lipases; one or more triacylglycerol lipases and
one or more
alpha/beta hydrolases; one or more triacylglycerol lipases and one or more
transcription factors
regulating expression of a FFA releasing enzyme; one or more phospholipases A2
and one or
more phospholipases C; one or more phospholipases A2 and one or more
acetylxylanesterases;
one or more phospholipases A2 and one or more extracellular lipase-like
proteins; one or more
phospholipases A2 and one or more acetylesterases; one or more phospholipases
A2 and one
or more GDSL lipases; one or more phospholipases A2 and one or more alpha/beta
hydrolases;
one or more phospholipases A2 and one or more transcription factors regulating
expression of
a FFA releasing enzyme; one or more phospholipases C and one or more
acetylxylanesterases;
one or more phospholipases C and one or more extracellular lipase-like
proteins; one or more
phospholipases C and one or more acetylesterases; one or more phospholipases C
and one or
more GDSL lipases; one or more phospholipases C and one or more alpha/beta
hydrolases; one
or more phospholipases C and one or more transcription factors regulating
expression of a FFA
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
releasing enzyme; one or more acetylxylanesterase and one or more
extracellular lipase-like
proteins; one or more acetylxylanesterase and one or more acetylesterases; one
or more
acetylxylanesterase and one or more GDSL lipases; one or more
acetylxylanesterase and one
or more alpha/beta hydrolases; one or more acetylxylanesterase and one or more
transcription
factors regulating expression of a FBA releasing enzyme; one or more
acetylesterases and one
or more GDSL lipases; one or more acetylesterases and one or more alpha/beta
hydrolases; one
or more acetylesterases and one or more transcription factors regulating
expression of a FFA
releasing enzyme; one or more GDSL lipases and one or more alpha/beta
hydrolases; one or
more GDSL lipases and one or more transcription factors regulating expression
of a FFA
releasing enzyme; one or more alpha/beta hydrolases and one or more
transcription factors
regulating expression of a FFA releasing enzyme; one or more cutinases and one
or more
lysophospholipases and one or more other carboxylic ester hydrolases; one or
more cutinases
and one or more triacylglycerol lipases and one or more other carboxylic ester
hydrolases; one
or more cutinases and one or more phospholipases A2 and one or more other
carboxylic ester
hydrolases; one or more cutinases and one or more phospholipases C and one or
more other
carboxylic ester hydrolases; one or more cutinases and one or more
acetylxylanesterases and
one or more other carboxylic ester hydrolases; one or more cutinases and one
or more
extracellular lipase-like proteins and one or more other carboxylic ester
hydrolases; one or more
cutinases and one or more acetylesterases and one or more other carboxylic
ester hydrolases;
one or more cutinases and one or more GDSL lipases and one or more other
carboxylic ester
hydrolases; one or more cutinases and one or more alpha/beta hydrolases and
one or more other
carboxylic ester hydrolases; one or more cutinases and one or more
transcription factors
regulating expression of a FFA releasing enzyme and one or more other
carboxylic ester
hydrolases; one or more lysophospholipases and one or more triacylglycerol
lipases and one or
more other carboxylic ester hydrolases; one or more lysophospholipases and one
or more
phospholipases A2 and one or more other carboxylic ester hydrolases; one or
more
lysophospholipases and one or more phospholipases C and one or more other
carboxylic ester
hydrolases; one or more lysophospholipases and one or more
acetylxylanesterases and one or
more other carboxylic ester hydrolases; one or more lysophospholipases and one
or more
extracellular lipase-like proteins; one or more lysophospholipases and one or
more
acetylesterases and one or more other carboxylic ester hydrolases; one or more
lysophospholipases and one or more GDSL lipases and one or more other
carboxylic ester
hydrolases; one or more lysophospholipases and one or more alpha/beta
hydrolases and one or
more other carboxylic ester hydrolases; one or more lysophospholipases and one
or more
31
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
transcription factors regulating expression of a FFA releasing enzyme and one
or more other
carboxylic ester hydrolases; one or more triacylglycerol lipases and one or
more phospholipases
A2 and one or more other carboxylic ester hydrolases; one or more
triacylglycerol lipases and
one or more phospholipases C and one or more other carboxylic ester
hydrolases; one or more
triacylglycerol lipases and one or more acetylxylanesterases and one or more
other carboxylic
ester hydrolases; one or more triacylglycerol lipases and one or more
extracellular lipase-like
proteins and one or more other carboxylic ester hydrolases; one or more
triacylglycerol lipases
and one or more acetylesterases and one or more other carboxylic ester
hydrolases; one or more
triacylglycerol lipases and one or more GDSL lipases and one or more other
carboxylic ester
hydrolases; one or more triacylglycerol lipases and one or more alpha/beta
hydrolases and one
or more other carboxylic ester hydrolases; one or more triacylglycerol lipases
and one or more
transcription factors regulating expression of a FFA releasing enzyme and one
or more other
carboxylic ester hydrolases; one or more phospholipases A2 and one or more
phospholipases
C and one or more other carboxylic ester hydrolases; one or more
phospholipases A2 and one
or more acetylxylanesterase and one or more other carboxylic ester hydrolases;
one or more
phospholipases A2 and one or more extracellular lipase-like proteins and one
or more other
carboxylic ester hydrolases; one or more phospholipases A2 and one or more
acetylesterases
and one or more other carboxylic ester hydrolases; one or more phospholipases
A2 and one or
more GDSL lipases, and one or more other carboxylic ester hydrolases; one or
more
phospholipases A2 and one or more alpha/beta hydrolases and one or more other
carboxylic
ester hydrolases; one or more phospholipases A2 and one or more transcription
factors
regulating expression of a FFA releasing enzyme and one or more other
carboxylic ester
hydrolases; one or more phospholipases C and one or more acetylxylanesterase
and one or more
other carboxylic ester hydrolases; one or more phospholipases C and one or
more extracellular
lipase-like proteins; one or more phospholipases C and one or more
acetylesterases and one or
more other carboxylic ester hydrolases; one or more phospholipases C and one
or more GDSL
lipases and one or more other carboxylic ester hydrolases; one or more
phospholipases C and
one or more alpha/beta hydrolases and one or more other carboxylic ester
hydrolases; one or
more phospholipases C and one or more transcription factors regulating
expression of a FFA
releasing enzyme and one or more other carboxylic ester hydrolases; one or
more
acetylxylanesterase and one or more extracellular lipase-like proteins and one
or more other
carboxylic ester hydrolases; one or more acetylxylanesterase and one or more
acetylesterases
and one or more other carboxylic ester hydrolases; one or more
acetylxylanesterase and one or
more GDSL lipases and one or more other carboxylic ester hydrolases; one or
more
32
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
acetylxylanesterase and one or more alphafbeta hydrolases and one or more
other carboxylic
ester hydrolases; one or more acetylxylanesterase and one or more
transcription factors
regulating expression of a FFA releasing enzyme and one or more other
carboxylic ester
hydrolases; one or more acetylesterases and one or more GDSL lipases and one
or more other
carboxylic ester hydrolases; one or more acetylesterases and one or more
alpha/beta hydrolases
and one or more other carboxylic ester hydrolases; one or more acetylesterases
and one or more
transcription factors regulating expression of a FFA releasing enzyme and one
or more other
carboxylic ester hydrolases; one or more GDSL lipases and one or more
alpha/beta hydrolases
and one or more other carboxylic ester hydrolases; one or more GDSL lipases
and one or more
transcription factors regulating expression of a FFA releasing enzyme and one
or more other
carboxylic ester hydrolases; one or more alpha/beta hydrolases and one or more
transcription
factors regulating expression of a FFA releasing enzyme and one or more other
carboxylic ester
hydrolases; one or more cutinases and one or more lysophospholipases and one
or more
triacylglycerol lipases; one or more cutinases and one or more
lysophospholipases and one or
more phospholipases A2; one or more cutinases and one or more
lysophospholipases and one
or more phospholipases C; one or more cutinases and one or more
lysophospholipases and one
or more acetylxylanesterases; one or more cutinases and one or more
lysophospholipases and
one or more extracellular lipase-like proteins; one or more cutinases and one
or more
lysophospholipases and one or more acetylesterases; one or more cutinases and
one or more
lysophospholipases and one or more GDSL lipases; one or more cutinases and one
or more
lysophospholipases and one or more alpha/beta hydrolases; one or more
cutinases and one or
more lysophospholipases and one or more transcription factors regulating
expression of a FFA
releasing enzyme; one or more cutinases and one or more triacylglycerol
lipases and one or
more phospholipases A2; one or more cutinases and one or more triacylglycerol
lipases and
one or more phospholipases C; one or more cutinases and one or more
triacylglycerol lipases
and one or more acetylxylanesterases; one or more cutinases and one or more
triacylglycerol
lipases and one or more extracellular lipase-like proteins; one or more
cutinases and one or
more triacylglycerol lipases and one or more acetylesterases; one or more
cutinases and one or
more triacylglycerol lipases and one or more GDSL lipases; one or more
cutinases and one or
more triacylglycerol lipases and one or more alpha/beta hydrolases; one or
more cutinases and
one or more triacylglycerol lipases and one or more transcription factors
regulating expression
of a FFA releasing enzyme; one or more cutinases and one or more
phospholipases A2 and one
or more phospholipases C; one or more cutinases and one or more phospholipases
A2 and one
or more acetylxylanesterases; one or more cutinases and one or more
phospholipases A2 and
33
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
one or more extracellular lipase-like proteins; one or more cutinases and one
or more
phospholipases A2 and one or more acetylesterases; one or more cutinases and
one or more
phospholipases A2 and one or more GDSL lipases; one or more cutinases and one
or more
phospholipases A2 and one or more alpha/beta hydrolases; one or more cutinases
and one or
more phospholipases A2 and one or more transcription factors regulating
expression of a FFA
releasing enzyme; one or more cutinases and one or more phospholipases C and
one or more
acetylxylanesterases; one or more cutinases and one or more phospholipases C
and one or more
extracellular lipase-like proteins; one or more cutinases and one or more
phospholipases C and
one or more acetylesterases; one or more cutinases and one or more
phospholipases C and one
or more GDSL lipases; one or more cutinases and one or more phospholipases C
and one or
more alpha/beta hydrolases; one or more cutinases and one or more
phospholipases C and one
or more transcription factors regulating expression of a FFA releasing enzyme;
one or more
cutinases and one or more acetylxylanesterases and one or more extracellular
lipase-like
proteins; one or more cutinases and one or more acetylxylanesterases and one
or more
acetylesterases; one or more cutinases and one or more acetylxylanesterases
and one or more
GDSL lipases; one or more cutinases and one or more acetylxylanesterases and
one or more
alpha/beta hydrolases; one or more cutinases and one or more
acetylxylanesterases and one or
more transcription factors regulating expression of a 141-A releasing enzyme;
one or more
cutinases and one or more extracellular lipase-like proteins and one or more
acetylesterases;
one or more cutinases and one or more extracellular lipase-like proteins and
one or more GDSL
lipases; one or more cutinases and one or more extracellular lipase-like
proteins and one or
more alpha/beta hydrolases; one or more cutinases and one or more
extracellular lipase-like
proteins and one or more transcription factors regulating expression of a FFA
releasing enzyme;
one or more cutinases and one or more acetylesterases and one or more GDSL
lipases; one or
more cutinases and one or more acetylesterases and one or more alpha/beta
hydrolases; one or
more cutinases and one or more acetylesterases and one or more transcription
factors regulating
expression of a FFA releasing enzyme; one or more cutinases and one or more
GDSL lipases
and one or more alpha/beta hydrolases; one or more cutinases and one or more
GDSL lipases
and one or more transcription factors regulating expression of a FFA releasing
enzyme; and
one or more cutinases and one or more alpha/beta hydrolase fold domain-
containing proteins
and one or more transcription factors regulating expression of a I-1-A
releasing enzyme.
[0143]
In a method according to any of the above, the fermentation broth or
preparation
comprising a recombinant component, or the recombinant host cell capable of
producing a
recombinant component, may be a combination and/or level of FFA releasing
enzyme activities
34
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
that provides a desired profile of FFA releasing enzyme activities (Le., FFA
releasing enzyme
activity profile) that is useful for production of a desired composition. In
some such
embodiments, the FFA releasing enzyme activity profile may be optimized to
provide a desired
flavor, aroma, texture, emulsification, nutritional content, and/or shelf-life
of the composition.
[0144]
A suitable number and/or combination of FFA releasing enzymes of which
activity
must be modulated in a method according to any of the above may be identified
by methods
known in the art. For example, FFA releasing enzymes may be isolated by
methods known in
the art (e.g., employing affinity chromatography, zymogram assays, gel
electrophoresis) and
tested in vitro to determine which one or which combination of two or more FFA
releasing
enzymes provides a substantial amount of degradation of a specific
diglycerides, triglyceride,
phospholipid, or lipoprotein. Also, recombinant host cells capable of
producing a recombinant
component (e.g., any recombinant component disclosed herein) may be obtained
that comprise
a modulated activity in any one or any combination of two or more FFA
releasing enzymes,
and degree of reduction or elimination of lipid degradation of a composition
comprising the
recombinant component produced by each such recombinant host cell may be
measured by
methods known in the art.
[0145]
FFA releasing enzyme activity may be measured using an enzyme assay. For
example, enzyme activity may be determined by production of a colorimetric
reaction product
or a product that may be detected (e.g., FFAs and/or glycerol produced from an
esterase-
catalyzed hydrolysis of a triglyceride), using, for example, PAGE gel,
spectrophotometer,
imaging, UV/Vis, light, and HPLC.
[0146]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85, GOR6T6, GOR6X2, G0R707, GOR7K1, GOR810, GOR9D1, GOR9F9, GOR9J9,
GOR9X3, GORBGO, GORBJO, GORBM4, GORBZ6, GORD16, GORDK5, GORDU7, GOREM9,
GOREZ4, GORFR3, GORFT3, GORG04, GORG60, GORGD5, GORGN7, GORGQO, GORGQ7,
GORHJ4, G0RI29, GORIJ9, GORIU1, GORIV5, GORJ76, GORJC6, GORJYO, GORK83,
GORKE6, GORKH7, GORKI9, GORKL4, GORL87, GORLBO, GORLB7, GORLH4, GORLLO,
GORLR3, GORM14, GORME5, GORMI3, GORNF8, GORPQ8, GORQD1, GORQG3, GORQJ5,
GORQN5, GORR42, GORRK3, GORRQ4, GORSK7, GORTR6, GORTT4, GORUIO, GORUZ9,
GORV93, GORW73, GORW77. GORWS1, GORWT9, GORWY5, GORX82, GORX90,
GORHQ7, GORVD2, and GOR8A6, and homologs thereof, and combinations thereof;
wherein
the modulated production and/or activity of the FFA releasing enzyme is a
decrease in
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%.
[0147]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORGQO, GORH85, GORMI3, GORLH4, GORIU1, GORBM4, GOR9D1, GORFR3, GORG60,
GOR6T6, GOR8N5, GORBJO, GORRQ4, GOREZ4, GORIJ9, GOR6X2, GORJYO, G0RR42,
GORW77, GORQJ5, GORFT3, GOR810, G0RI29, GORL87, GORLLO, GORGD5, and GORKH7,
and homologs thereof, and combinations thereof; wherein the modulated
production and/or
activity of the FFA releasing enzyme is a decrease in production and/or
activity of at least 10%,
at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%,
at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%,
at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%,
or 100%.
[0148]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
CIOKH85, GOKGQ0, GOKLH4, and GOKMI3, and homologs thereof, and combinations
thereof;
wherein the modulated production and/or activity of the FFA releasing enzyme
is a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%.
[0149]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; wherein the modulated
production
and/or activity of the FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%. at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
36
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0150]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORGQO and homologs thereof, and combinations thereof; wherein the modulated
production
and/or activity of the FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
[0151]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORLH4 and homologs thereof, and combinations thereof; wherein the modulated
production
and/or activity of the FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
[0152]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORMI3 and homologs thereof, and combinations thereof; wherein the modulated
production
and/or activity of the FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
[0153]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GOR6T6, GOR6X2, G0R707, GOR7K1, GOR810,
GOR9D1, GOR9F9, GOR9J9, GOR9X3, GORBGO, GORBJO, GORBM4, GORBZ6, GORD16,
37
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
GORDK5, GORDU7, GOREM9, GOREZ4, GORFR3, GORFT3, GORG04, GORG60, GORGD5,
GORGN7, GORGQO, GORGQ7, GORHJ4, GORI29, GORIJ9, GORIU1, GORIV5, G0R.176,
GORJC6, GORJ YO, GORK83, GORKE6, GORKH7, GORK19, GORKL4, GORL87, GORLBO,
GORLB7, GORLH4, GORLLO, GORLR3, GORM14, GORME5, GORMI3, GORNF8, GORPQ8,
GORQD1, GORQG3, GORQJ5, GORQN5, GORR42, GORRK3, GORRQ4, GORSK7, GORTR6,
GORTT4, GORUIO, GORUZ9, GORV93, GORW73, GORW77, G0RWS1, GORWT9, GORWY5,
GORX82, GORX90, GORHQ7, RORVD2, and GOR8A6, and homologs thereof, and
combinations thereof; wherein the modulated production and/or activity of the
first FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; and
wherein the modulated production and/or activity of the second FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%,.
[0154]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORGQO, GORH85, GORMI3, GORLH4, GORIU1,
GORBM4, GOR9D1, GORFR3, GORG60, GOR6T6, GOR8N5, GORBJO, GORRQ4, GOREZ4,
GOR119, GOR6X2, GORJYO, GORR42, GORW77, GORQJ5, GORFT3, GOR810, G0RI29,
GORL87, GORLLO, GORGD5, and GORKH7, and homologs thereof, and combinations
thereof;
wherein the modulated production and/or activity of the first FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; and wherein
and the modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
38
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%.
[0155]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORGQO, GORLH4, GORMI3, G0R707, GOR7K1,
GOR810, GORFT3, GORG60, GORGD5, G0RI29, GORU9, GORKH7, GORKL4, GORL87,
GORLLO, GORLR3, GORME5, GORQJ5, GORRK3, GORSK7, GORWT9, and GORX82, and
homologs thereof, and combinations thereof; wherein the modulated production
and/or activity
of the first FFA releasing enzyme is a decrease in production and/or activity
of at least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%; and wherein the modulated production and/or activity of the second FFA
releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0156]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORGQO, GORLH4, and GORMI3, and homologs
thereof, and combinations thereof; wherein the modulated production and/or
activity of the first
141-A releasing enzyme is a decrease in production and/or activity of at least
10%, at least 15%,
at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%,
at least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%,
at least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; and
wherein the modulated production and/or activity of the second FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
39
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%,
[0157]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORGQO, GORLH4, GORMI3, G0R707, GOR7K1,
GOR810, GORFT3, GORG60, GORGD5, G0RI29, GORU9, GORKH7, GORKL4, GORL87,
GORLLO, GORLR3, GORME5, GORQJ5, GORRK3, GORSK7, GORWT9, and GORX82, and
homologs thereof, and combinations thereof; and comprise an un-modulated
production and/or
activity of a third FFA releasing enzyme selected from the group consisting of
1-1-A releasing
enzymes comprising UniProt sequence# GOR6T6, GOR6X2, GOR8N5, GOR9D1, GOR9X3,
GORBJO, GORBM4, GOREM9, GOREZ4, GORFR3, GORHJ4, GORIU1, GORJYO, GORR42,
GORV93, GORRQ4, GORX90, and GORW77, and homologs thereof, and combinations
thereof;
wherein the modulated production and/or activity of the first FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; and wherein
the modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%.
[0158]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORIU1 and homologs thereof, and
combinations
thereof; and a modulated production and/or activity of a third FFA releasing
enzyme selected
from the group consisting of FFA releasing enzymes comprising UniProt
sequence# GORHJ4
and homologs thereof, and combinations thereof; and a modulated production
and/or activity
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
of a fourth FFA releasing enzyme selected from the group consisting of FFA
releasing enzymes
comprising UniProt sequence# G0RR42, GORQ1-5, GORQD1, GOREZ4, GORSK7, GORV93,
GOR9D1, GOR6T6, GORLLO, GORGD5, GORFR3, GORHQ7, GORG60, GOR810, GORL87,
GOR119, GORME5, GORRQ4, GORX82, GORGQO, GORLR3, GORLH4, GORFT3, GORWY5,
GOR9F9, GORVD2, GOR8A6, GOR9X3, GORBM4, GORHJ4, GOREM9, GORIU1, GORX90,
GOR8N5, GOR6X2, GORK83, GORKH7, G0RI29, GORKI9, GORJ76, GORBJO, GORJY0,
GORM13, GORW77, GORRK3, and GORLBO, and homologs thereof, and combinations
thereof;
wherein the modulated production and/or activity of the first FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; wherein the
modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%; and wherein the modulated production
and/or activity
of the third FFA releasing enzyme is a decrease in production and/or activity
of at least 10%,
at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%,
at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%,
at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%,
or 100%.
[0159]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORMI3 and homologs thereof, and
combinations
thereof; wherein the modulated production and/or activity of the first FFA
releasing enzyme is
a decrease in production and/or activity of at least 10%, at least 15%, at
least 20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; and wherein
the modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
41
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%.
[0160]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of a first FFA releasing enzyme selected
from the group
consisting of FFA releasing enzymes comprising UniProt sequence# GORH85 and
homologs
thereof, and combinations thereof; and a modulated production and/or activity
of a second FFA
releasing enzyme selected from the group consisting of FFA releasing enzymes
comprising
UniProt sequence# GORGQO and homologs thereof, and combinations thereof;
wherein the
modulated production and/or activity of the first FFA releasing enzyme is a
decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the second FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%. at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
[0161]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORLH4 and homologs thereof, and
combinations
thereof; wherein the modulated production and/or activity of the first FFA
releasing enzyme is
a decrease in production and/or activity of at least 10%, at least 15%, at
least 20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; and wherein
the modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
42
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%.
[0162]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORMI3 and homologs thereof, and
combinations
thereof; and a modulated production and/or activity of a third FFA releasing
enzyme selected
from the group consisting of FFA releasing enzymes comprising UniProt
sequence# GORGQO
and homologs thereof, and combinations thereof; wherein the modulated
production and/or
activity of the first FFA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%; wherein the modulated production and/or activity of the second
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%;
and wherein the
modulated production and/or activity of the third FFA releasing enzyme is a
decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%.
[0163]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORMI3 and homologs thereof, and
combinations
thereof; and a modulated production and/or activity of a third FFA releasing
enzyme selected
from the group consisting of FFA releasing enzymes comprising UniProt
sequence# GORLH4
43
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
and homologs thereof, and combinations thereof; wherein the modulated
production and/or
activity of the first FFA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%; wherein the modulated production and/or activity of the second
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%;
and wherein the
modulated production and/or activity of the third FFA releasing enzyme is a
decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%.
[0164]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORGQO and homologs thereof, and
combinations
thereof; and a modulated production and/or activity of a third FFA releasing
enzyme selected
from the group consisting of FFA releasing enzymes comprising UniProt
sequence# GORLH4
and homologs thereof, and combinations thereof; wherein the modulated
production and/or
activity of the first FBA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%; wherein the modulated production and/or activity of the second
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%;
and wherein the
modulated production and/or activity of the third FFA releasing enzyme is a
decrease in
44
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%.
[0165]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a first FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85 and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a second FFA releasing enzyme selected from the group consisting
of FFA releasing
enzymes comprising UniProt sequence# GORMI3 and homologs thereof, and
combinations
thereof; and a modulated production and/or activity of a third FFA releasing
enzyme selected
from the group consisting of FFA releasing enzymes comprising UniProt
sequence# GORGQO
and homologs thereof, and combinations thereof; and a modulated production
and/or activity
of a fourth FFA releasing enzyme selected from the group consisting of FFA
releasing enzymes
comprising UniProt sequence# GORLH4 and homologs thereof, and combinations
thereof;
wherein the modulated production and/or activity of the first FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; wherein the
modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%; wherein the modulated production
and/or activity of
the third FFA releasing enzyme is a decrease in production and/or activity of
at least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%; and wherein the modulated production and/or activity of the fourth FFA
releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0166]
A modulated FFA releasing enzyme activity according to any of the above
may
comprise or consist of a modulated production and/or activity of a FFA
releasing enzyme
selected from the group consisting of FFA releasing enzymes comprising UniProt
sequence#
GORH85, GORGQO, GORLH4, and GORMI3, and homologs thereof, and combinations
thereof;
and a modulated production and/or activity of a transcription factor selected
from the group
consisting of transcription factors comprising UniProt sequence# GORT83,
GORRR1, GORIF9,
G0R765, GORRJ7, G0R932, GORB V8, GOREE8, GORLFO, and GORBH2, and homologs
thereof, and combinations thereof; wherein the modulated production and/or
activity of the FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; and
wherein the modulated production and/or activity of the transcription factor
is a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%.
Recombinant Host Cell Comprising Modulated FFA Releasing Enzyme Activity
[0167]
The method according to any of the above may comprise: i) obtaining a
recombinant
host cell capable of producing a recombinant component, wherein the
recombinant host cell
comprises a modulated FFA releasing enzyme activity compared to the FFA
releasing enzyme
activity comprised in a corresponding recombinant host cell (i.e., a
recombinant host cell that
is identical to the recombinant host cell that is being compared to the
"corresponding
recombinant host cell" except that the FFA releasing enzyme activity of the
"corresponding
recombinant host cell" is not modulated); and ii) culturing the recombinant
host cell in a culture
medium under conditions suitable for production and/or secretion of the
recombinant
component.
[0168]
Accordingly, in various aspects, provided herein is a recombinant host
cell that is
capable of producing a recombinant component (i.e., that comprises an
expression constructed
provided herein) and that comprises a modulated FFA releasing enzyme activity
(e.g., activity
of any one FFA releasing enzyme disclosed herein or activities of any
combination of two or
more FFA releasing enzymes disclosed herein) compared to the FFA releasing
enzyme activity
comprised in a corresponding recombinant host cell.
46
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0169]
A FFA releasing enzyme activity comprised in a recombinant host cell may
be
modulated by any means. For example, a FFA releasing enzyme activity may be
modulated by
a genetic modification in the recombinant host cell that modulates or
essentially eliminates
expression (i.e., production of an active protein) of the FFA releasing
enzyme, or that modulates
or essentially eliminates activity of the FFA releasing enzyme. Non-limiting
examples of
genetic modifications that may be comprised in a recombinant host cell
according to any of the
above include: i) a genetic modification in a polynucleotide sequence that
encodes an
endogenous FFA releasing enzyme or a functional part thereof (e.g., a
catalytic domain),
wherein the genetic modification modulates or essentially eliminates activity
of the endogenous
141-A releasing enzyme; ii) a genetic modification that introduces a
polynucleotide sequence
that encodes a heterologous FFA releasing enzyme, wherein the genetic
modification
modulates or essentially eliminates activity of the heterologous FFA releasing
enzyme; iii) a
genetic modification in a regulatory element or a functional part thereof
(i.e., a part that is
sufficient for the function of the regulatory element) that drives expression
of a FFA releasing
enzyme, wherein the genetic modification modulates or essentially eliminates
expression of the
_EPA releasing enzyme; iv) a genetic modification in a coding sequence that
encodes a protein
required for expression of a FFA releasing enzyme (e.g., a transcription
factor [e.g., any of the
transcription factors shown in Table 3, and homologs thereof], a post-
translational modification
enzyme required for production of an active form of a FFA releasing enzyme) or
for activity of
a FFA releasing enzyme (e.g., an inhibitor, an activator, a co-enzyme), or a
functional part
thereof (e.g., DNA binding domain of a transcription factor, catalytic domain
of a post-
translational modification enzyme), wherein the genetic modification modulates
or essentially
eliminates activity of the protein required for expression or activity of the
FFA releasing
enzyme and thereby modulates or essentially eliminates expression of the FFA
releasing
enzyme; v) a genetic modification in a regulatory element or a functional part
thereof that drives
expression of a protein required for expression or activity of a FFA releasing
enzyme, wherein
the genetic modification modulates or essentially eliminates expression of the
protein required
for expression or activity of the FFA releasing enzyme and thereby modulates
or essentially
eliminates expression of the FFA releasing enzyme; and/or vi) a genetic
modification that
introduces a polynucleotide sequence that encodes a heterologous protein that
regulates
expression of a FFA releasing enzyme (e.g., a transcription factor [e.g., any
of the transcription
factors shown in Table 3, and homologs thereof], a post-translational
modification enzyme
required for production of an active form of a FFA releasing enzyme) or
activity of a FFA
releasing enzyme (e.g., an inhibitor, an activator, a co-enzyme) wherein the
genetic
47
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
modification provides for production of the heterologous protein and thereby
modulates or
essentially eliminates activity of the FFA releasing enzyme.
[0170] Table 3 ¨ Exemplary Transcription Factors (UniProt
Sequence IDs)
GORX49 GORRJ7 A2R2J1 G2Q2Z5
GORHG1 G0R932 A2R903 G2Q816
GORBV8 GOREE8 GORLFO GORBH2
GORT83 GORIF9 GORRR1 G0R765
[0171] The recombinant host cell according to any of the above
may be derived from any
wild type unicellular organism, including any bacterium, yeast, filamentous
fungus, archaea,
unicellular protista, unicellular animals, unicellular plants, unicellular
algae, protozoa, and
chromista, or from a genetic variant (e.g., mutant) thereof, as well as from
any generally
recognized as safe (GRAS) industrial host cell.
[0172] Non-limiting examples of suitable bacteria include
firmicutes, cyanobacteria (blue-
green algae), oscillatoriophcideae, bacillales, lactobacillales,
oscillatoriales, bacillaceae,
lactobacillaceae, and members of any of the following genera, and derivatives
and crosses
thereof: Acinetobacter, Acetobacter (e.g., Acetobacter suboxydans, Acetobacter
xylinum),
Actinoplane (e.g., Actinoplane missouriensis), Arthrospira (e.g., Arthrospira
platensis,
Arthrospira maxima), Bacillus (e.g., Bacillus cereus, Bacillus coagulans,
Bacillus
licheniformis, Bacillus stearothermophilus, Bacillus subtilis), Escherichia
(e.g., Escherichi a
coli), Lactobacillus (e.g., Lactobacillus acidophilus, Lactobacillus
bulgaricus), Lactococcus
(e.g., Lactococcus lactis, Lactococcus lactis Lancefield Group N,
Lactobacillus reuteri),
Leuconosioc (e.g., Leuconostoc citrovorum, Leuconostoc dextranicum,
Leuconostoc
mesenteroides), Micrococcus (e.g., Micrococcus lysodeikticus), Rhodococcus
(e.g.,
Rhodococcus opacus, Rhodococcus opacus strain PD630), Spirulina, Streptococcus
(e.g.,
Streptococcus cremoris, Streptococcus lactis, Streptococcus lactis subspecies
diacetylactis,
Streptococcus thermophilus), Streptomyces (e.g., Streptomyces chattanoogensis,
Streptomyces
griseus, Streptomyces natalensis, Streptomyces olivaceus, Streptomyces
olivochromogenes,
Streptomyces rubiginosus), Tetrahymena (e.g., Tetrahymena thermophile,
Tetrahymena
hegewischi, Tetrahymena hyperangularis, Tetrahymena malaccensis, Tetrahymena
pigmentosa, Tetrahymena pyriformis, Tetrahymena vorax ), and Xanthomonas
(e.g.,
Xanthomonas campestris).
[0173] Non-limiting examples of suitable yeast include members
of any of the following
genera, and derivatives and crosses thereof: Candida (e.g., Candida albicans,
Candida etchellsii,
48
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
Candida guilliermondii, Candida humilis, Candida lipolytica, Candida
orthopsilosis, Candida
palmioleophila, Candida pseudotropical is, Candida sp., Candida utilis,
Candida versatilis),
Cladosporium, Cryptococcus (e.g., Cryptococcus terricolus, Cryptococcus
curvatus),
Debaryomyces (e.g., Debaryomyces hansenii), Endomyces (e.g., Endomyces
vemalis),
Endomycopsis (e.g., Endomycopsis vernalis), Eremothecium (e.g., Eremothecium
ashbyii),
Hansenula (e.g., Hansenula sp., Hansenula polymorpha), Kluyveromyces (e.g.,
Kluyveromyces
sp., Kluyveromyces lactis, Kluyveromyces marxianus var. lactis, Kluyveromyces
marxianus,
Kluyveromyces thermotolerans), Lipomyces (e.g., Lipomyces starkeyi, Lipomyecs
lipofer),
Ogataea (e.g., Ogataea minuta), Pichia (e.g., Pichia sp., Pichia pastoris,
Pichia finlandica, Pichia
trehalophila, Pichia koclamae, Pichia membranaefaciens, Pichia minuta, Pichia
lindneri),
Pichia opuntiae, Pichia thermotolerans, Pichia salictaria, Pichia guercuum,
Pichia pijperi,
Pichia stiptis, Pichia methanolica), Rhodosporidium (e.g., Rhodosporidium
toruloides),
Rhodotorula (e.g., Rhodotorula sp., Rhodotorula gracilis, Rhodotorula
glutinis, Rhodotorula
graminis), Saccharomyces (e.g., Saccharomyces sp., Saccharomyces b ay an us ,
Saccharomyces
beticus, Saccharomyces cerevisiae, Saccharomyces chevalieri, Saccharomyces
diastaticus,
Saccharomyces ellipsoideus, Saccharomyces exiguus, Saccharomyces florentinus,
Saccharomyces fragilis, Saccharomyces pastorianus, Saccharomyces pombe,
Saccharomyces
sake, Saccharomyces u v arum), Sporobolomyces (e.g., Sporobolomyces roseus),
Sporidiobolus
(e.g., Sporidiobolus johnsonii, Sporidiobolus salmonicolor), Trichosporon
(e.g., Trichosporon
cacaoliposimilis, Trichosporon oleaginosus sp. nov., Trichosporon
cacaoliposimilis sp. nov..
Trichosporon gracile, Trichosporon dulcitum, Trichosporon jirovecii,
Trichosporon
insectorum), Xanthophyllomyces (e.g., Xanthophyllomyces dendrorhous), Yarrowia
(e.g.,
Yarrowia lipolytica), and Zygosaccharomyces (e.g., Zygosaccharomyces rouxii).
[0174]
Non-limiting examples of suitable filamentous fungi include holomorphic,
teleomorphic, and anamorphic forms of fungi, including members of any of the
following
genera, and derivatives and crosses thereof: Acremonium (e.g., Acremonium
alabamense),
Aspergillus (e.g., Aspergillus acu lea tu s, Aspergillus awamori, Aspergillus
clavatu s,
Aspergillus flavus, Aspergillus foetidus, Aspergillus fumigatus, Aspergillus
japonicus,
Aspergillus nidulans, Aspergillus niger, Aspergillus niger var. awamori,
Aspergillus ochraceus,
Aspergillus oryzae, Aspergillus sojae, Aspergillus terreus, as well as
Emericella, Neosartorya,
and Petromyces species), Aureobasidium, Canariomyces, Chaetomium,
Chaetomidium,
Corynascus, Chrysosporium (e.g., Chrysosporium botryoides, Chrysosporium
carmichaeli,
Chrysosporium crassitunicatum, Chrysosporium europae, Chrysosporium
evolceannui,
Chrysosporium farinicola, Chrysosporium fastidium, Chrysosporium filiforme,
Chrysosporium
49
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
georgiae, Chrysosporium globiferum, Chrysosporium globiferum var. articulatum,
Chrysosporium globiferum var. niveum, Chrysosporium hirundo, Chrysosporium hi
spanicum,
Chrysosporium holmii, Chrysosporium indicum, Chrysosporium iops, Chrysosporium
keratinophilum, Chrysosporium kreiselii, Chrysosporium kuzurovianum,
Chrysosporium
lignorum, Chrysosporium obatum, Chrysosporium lucknowense, Chrysosporium
lucknowense
Garg 27K, Chrysosporium medium, Chrysosporium medium var. spissescens,
Chrysosporium
mephiticum, Chrysosporium merdarium, Chrysosporium merdarium var. roseum,
Chrysosporium minor, Chrysosporium pannicola, Chrysosporium parvum,
Chrysosporium
parvum var. crescens, Chrysosporium pilosum, Chrysosporium pseudomerdarium,
Chrysosporium pyriformis, Chrysosporium queenslandicum, Chrysosporium sigleri,
Chrysosporium sulfureum, Chrysosporium synchronum, Chrysosporium tropicum,
Chrysosporium undulatum, Chrysosporium vallenarense, Chrysosporium
vespertilium,
Chrysosporium zonatum), Coonemeria, Cunninghamella (e.g., Cunninghamella
ehinulata),
Dactylomyces, Emericella, Filibasidium, Fusarium (e.g., Fusarium moniliforme,
Fusarium
venenatum, Fusarium oxysporum, Fusarium graminearum, Fusarium proliferatum,
Fusarium
verticiollioides, Fusarium culmorum, Fusarium crookwellense, Fusarium poae,
Fusarium
sporotrichioides, Fusarium sambuccinum, Fusarium torulosum, as well as
associated
Gibberella teleomorphic forms thereof), Gibberella, Humicola, Hypocrea,
Lentinula,
Malbranchea (e.g., Malbranchea filamentosa), Magnaporthe, Malbranchium,
Melanocarpus,
Mortierella (e.g., Mortierella alpina 1S-4, Mortieralla isabelline, Mortierrla
vinacea,
Mortieralla vinaceae var. raffinoseutilizer), Mucor (e.g., Mucor miehei Cooney
et Emerson
(Rhizomucor miehei (Cooney & R. Emerson)) Schipper, Mucor pusillus Lindt,
Mucor
circinelloides Mucor mucedo), Myceliophthora (e.g., Myceliophthora
thermophila),
Myrothecium, Neocallimastix, Neurospora (e.g., Neurospora crassa),
Paecilomyces,
Penicillium (e.g., Penicillium chrysogenum, Pennicillium iilacinum,
Penicillium roquefortii),
Phenerochaete, Phlebia, Piromyces, Pythium, Rhizopus (e.g., Rhizopus niveus),
Schizophyllum, Scytalidium, Sporotrichum (e.g., Sporotrichum cellulophilum),
Stereum,
Talaromyces, Thermoascus, Thermomyces, Thielavia (e.g., Thielavia terrestris),
Tolypocladium, and Trichoderma (e.g., Trichoderma harzianum, Trichoderma
koningii,
Trichoderma longibrachiatum, Trichoderma reesei, Trichoderma atroviride,
Trichoderma
virens, Trichoderma citrinoviride, Trichoderma viride).
[0175]
The recombinant host cell according to any of the above may be selected
from the
group consisting of: a recombinant Trichoderma reesei host cell (i.e., a
recombinant host cell
derived from a Trichoderma reesei strain) comprising a modulated activity of a
FFA releasing
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
enzyme or of a combination of two or more FFA releasing enzymes, a recombinant
Aspergillus
niger host cell (i.e., a recombinant host cell derived from a Aspergillus
niger strain) comprising
a modulated activity of a FFA releasing enzyme or of a combination of two or
more FFA
releasing enzymes, a recombinant Trichoderma citrinovi ride host cell (i.e., a
recombinant host
cell derived from a Trichoderma citrinoviride strain) comprising a modulated
activity of a FFA
releasing enzyme or of a combination of two or more FFA releasing enzymes, and
a
recombinant Myceliophthora thermophila host cell (i.e., a recombinant host
cell derived from
a Myceliophthora thermophila strain) comprising a modulated activity of a FFA
releasing
enzyme or of a combination of two or more FFA releasing enzymes.
[0176]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
1-1-A releasing enzymes comprising UniProt sequence# GORH85, GOR6T6, GOR6X2,
G0R707,
GOR7K1, GOR810, GOR9D1, GOR9F9, GOR9J9, GOR9X3, GORBGO, GORBJO, GORBM4,
GORBZ6, GORD16, GORDK5, GORDU7, GOREM9, GOREZ4, GORFR3, GORFT3, GORG04,
GORG60, GORGD5, GORGN7, GORGQO, GORGQ7, GORHJ4, G0RI29, GORIJ9, GORIU1,
GORIV5, GORJ76, GORJC6, GORJYO, GORK83, GORKE6, GORKH7, GORKI9, GORKL4,
GORL87, GORLBO, GORLB7, GORLH4, GORLLO, GORLR3, GORM14, GORME5, GORMI3,
GORNF8, GORPQ8, GORQD1, GORQG3, GORQJ5, GORQN5, GORR42, GORRK3, GORRQ4,
GORSK7, GORTR6, GORTT4, GORUIO, GORUZ9, GORV93, GORW73, GORW77, GORWS1.
GORW19, GORW Y5, GORX82, GORX90, GORHQ7, GORVD2, and GOR8A6, and homologs
thereof, and combinations thereof; wherein the modulated production and/or
activity of the FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%.
[0177]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
141-A releasing enzymes comprising UniProt sequence# GORGQO, GORH85, GORMI3,
GORLH4, GORIU1, GORBM4, GOR9D1, GORFR3, GORG60, GOR6T6, GOR8N5, GORBJO,
GORRQ4, GOREZ4, GOR119, GOR6X2, GORJYO, GORR42, GORW77, GORQJ5, GORFT3,
GOR810, G0RI29, GORL87, GORLLO, GORGD5, and GORKH7, and homologs thereof, and
combinations thereof; wherein the modulated production and/or activity of the
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
51
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0178]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
FBA releasing enzymes comprising UniProt sequence# GORH85, GORGQO, GORLH4, and
GORMI3, and homologs thereof, and combinations thereof; wherein the modulated
production
and/or activity of the FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
[0179]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
141-A releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0180]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
141-A releasing enzymes comprising UniProt sequence# GORGQO and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0181]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
FBA releasing enzymes comprising UniProt sequence# GORLH4 and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
52
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0182]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
FBA releasing enzymes comprising UniProt sequence# GORMI3 and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
FFA releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0183]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
1-A releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GOR6T6, GOR6X2, G0R707, GOR7K1, GOR810, GOR9D1, GOR9F9, GOR9J9,
GOR9X3, GORBGO, GORBJO, GORBM4, GORBZ6, GORD16, GORDK5, GORDU7, GOREM9,
GOREZ4, GORFR3, GORFT3, GORG04, GORG60, GORGD5, GORGN7, GORGQO, GORGQ7,
GORHJ4, G0RI29, GORIJ9, GORIU1, GORIV5, GORJ76, GORJC6, GORJYO, GORK83.
GORKE6, GORKH7, GORKI9, GORKL4, GORL87, GORLBO, GORLB7, GORLH4, GORLLO,
GORLR3, GORM14, GORME5, GORMI3, GORNF8. GORPQ8, GORQD1, GORQG3, GORQJ5,
GORQN5, GORR42, GORRK3, GORRQ4, GORSK7, GORTR6, GORTT4, GORUIO, GORUZ9,
GORV93, GORW73, GORW77, G0RWS1, GORWT9, GORWY5, GORX82, GORX90,
GORHQ7, RORVD2, and GOR8A6, and homologs thereof, and combinations thereof;
wherein
the modulated production and/or activity of the first FFA releasing enzyme is
a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the second FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%. at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%,.
53
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0184]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
+A releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORGQO, GORH85, GORMI3, GORLH4, GORIU1, GORBM4, GOR9D1, GORFR3,
GORG60, GOR6T6, GOR8N5, GORBJO, GORRQ4, GOREZ4, GOR1,19, GOR6X2, GORJYO,
G0RR42, G0RW77, GORQJ5, GORFT3, G0R810, G0RI29, G0RL87, GORLLO, GORGD5, and
GORKH7, and homologs thereof, and combinations thereof; wherein the modulated
production
and/or activity of the first FFA releasing enzyme is a decrease in production
and/or activity of
at least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%,
at least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%,
at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%,
at least 99%, or 100%; and wherein and the modulated production and/or
activity of the second
FFA releasing enzyme is a decrease in production and/or activity of at least
10%, at least 15%,
at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%,
at least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%,
at least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%.
[0185]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second
PM releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORGQO, GORLH4, GORM13, G0R707, GOR7K1, GOR810, GORFT3, GORG60,
GORGD5, G0RI29, GORIJ9, GORKH7, GORKL4, GORL87, GORLLO, GORLR3, GORME5,
GORQJ5, GORRK3, GORSK7, GORWT9, and GORX82, and homologs thereof, and
combinations thereof; wherein the modulated production and/or activity of the
first FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; and
wherein the modulated production and/or activity of the second FFA releasing
enzyme is a
decrease in production and/or activity of at least 10%, at least 15%, at least
20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
54
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0186]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
+A releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORGQO, GORLH4, and GORMI3, and homologs thereof, and combinations
thereof; wherein the modulated production and/or activity of the first FFA
releasing enzyme is
a decrease in production and/or activity of at least 10%, at least 15%, at
least 20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; and wherein
the modulated
production and/or activity of the second FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%.
[0187]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
PA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORGQO, GORLH4, GORMI3, G0R707, GOR7K1, GOR810, GORFT3, GORG60,
GORGD5, G0RI29, GORIJ9, GORKH7, GORKL4, GORL87, GORLLO, GORLR3, GORME5,
GORQJ5, GORRK3, GORSK7, GORWT9, and GORX82, and homologs thereof, and
combinations thereof; and comprise an un-modulated production and/or activity
of a third FFA
releasing enzyme selected from the group consisting of FFA releasing enzymes
comprising
UniProt sequence# GOR6T6, GOR6X2, GOR8N5, GOR9D1, GOR9X3, GORBJO, GORBM4,
GOREM9, GOREZ4, GORFR3, GORHJ4, GORIU1, GORJYO, GORR42, GORV93, GORRQ4,
GORX90, and GORW77, and homologs thereof, and combinations thereof; wherein
the
modulated production and/or activity of the first FFA releasing enzyme is a
decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the second FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%.
[0188]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second
PM releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORIU1 and homologs thereof, and combinations thereof; and a
modulated
production and/or activity of a third FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORHJ4 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a fourth
FFA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORR42, GORQJ5, GORQD1, GOREZ4, GORSK7, GORV93, GOR9D1, GOR6T6,
GORLLO, GORGD5, GORFR3, GORHQ7, GORG60, GOR810, GORL87, GORIJ9, GORME5,
GORRQ4, GORX82, GORGQ0, GORLR3, GORLH4, GORP13, GORW Y5, GOR91-9, GORVD2,
GOR8A6, GOR9X3, GORBM4, GORHJ4, GOREM9, GORIU1, GORX90, GOR8N5, GOR6X2.
GORK83, GORKH7, G0RI29, GORKI9, GORJ76, GORBJO, GORJYO, GORMI3, GORW77,
GORRK3, and GORLBO, and homologs thereof, and combinations thereof; wherein
the
modulated production and/or activity of the first FFA releasing enzyme is a
decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; wherein the modulated
production and/or
activity of the second FFA releasing enzyme is a decrease in production and/or
activity of at
least 10%, at least 15%, at least 20%, at least 25%, at least 30%, at least
35%, at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least
97%, at least 98%, at
least 99%, or 100%; and wherein the modulated production and/or activity of
the third FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
56
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%.
[0189]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second
FFA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORMI3 and homologs thereof, and combinations thereof; wherein the
modulated
production and/or activity of the first FFA releasing enzyme is a decrease in
production and/or
activity of at least 10%, at least 15%, at least 20%, at least 25%, at least
30%, at least 35%, at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
96%, at least 97%, at
least 98%, at least 99%, or 100%; and wherein the modulated production and/or
activity of the
second FFA releasing enzyme is a decrease in production and/or activity of at
least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%.
[0190]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of a first FFA releasing enzyme selected from the group consisting of FFA
releasing enzymes
comprising UniProt sequence# GORH85 and homologs thereof, and combinations
thereof; and
a modulated production and/or activity of a second FFA releasing enzyme
selected from the
group consisting of FFA releasing enzymes comprising UniProt sequence# GORGQO
and
homologs thereof, and combinations thereof; wherein the modulated production
and/or activity
of the first FFA releasing enzyme is a decrease in production and/or activity
of at least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%; and wherein the modulated production and/or activity of the second FFA
releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
57
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0191]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
+A releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORLH4 and homologs thereof, and combinations thereof; wherein the
modulated
production and/or activity of the first FFA releasing enzyme is a decrease in
production and/or
activity of at least 10%, at least 15%, at least 20%, at least 25%, at least
30%, at least 35%, at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
96%, at least 97%, at
least 98%, at least 99%, or 100%; and wherein the modulated production and/or
activity of the
second FFA releasing enzyme is a decrease in production and/or activity of at
least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%.
[0192]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
PA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORM13 and homologs thereof, and combinations thereof; and a
modulated
production and/or activity of a third FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORGQO and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
first FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; wherein
the modulated production and/or activity of the second FFA releasing enzyme is
a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
58
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the third FFA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%.
[0193]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
1-A releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORMI3 and homologs thereof, and combinations thereof; and a
modulated
production and/or activity of a third FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORLH4 and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
first FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%. at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; wherein
the modulated production and/or activity of the second FFA releasing enzyme is
a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the third FFA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%.
[0194]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
+A releasing
59
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORGQO and homologs thereof, and combinations thereof; and a
modulated
production and/or activity of a third FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORLH4 and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
first FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; wherein
the modulated production and/or activity of the second FFA releasing enzyme is
a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the third FFA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%.
[0195]
'the recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORH85 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
+A releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORMI3 and homologs thereof, and combinations thereof; and a
modulated
production and/or activity of a third FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORGQO and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a fourth
FFA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORLH4 and homologs thereof, and combinations thereof; wherein the
modulated
production and/or activity of the first FFA releasing enzyme is a decrease in
production and/or
activity of at least 10%, at least 15%, at least 20%, at least 25%, at least
30%, at least 35%, at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
96%, at least 97%, at
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
least 98%, at least 99%, or 100%; wherein the modulated production and/or
activity of the
second FFA releasing enzyme is a decrease in production and/or activity of at
least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%; wherein the modulated production and/or activity of the third FFA
releasing enzyme is
a decrease in production and/or activity of at least 10%, at least 15%, at
least 20%, at least 25%,
at least 30%, at least 35%, at least 40%, at least 45%, at least 50%, at least
55%, at least 60%,
at least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%,
at least 96%, at least 97%, at least 98%, at least 99%, or 100%; and wherein
the modulated
production and/or activity of the fourth FFA releasing enzyme is a decrease in
production
and/or activity of at least 10%, at least 15%, at least 20%, at least 25%, at
least 30%, at least
35%, at least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at
least 65%, at least
70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100%.
[0196]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a FFA releasing enzyme selected from the group
consisting of
1-1-A releasing enzymes comprising UniProt sequence# GORH85, GORGQO, GORLH4,
and
GORMI3, and homologs thereof, and combinations thereof; and a modulated
production and/or
activity of a transcription factor selected from the group consisting of
transcription factors
comprising UniProt sequence# GORT83, GORRR1, GORIF9, G0R765, GORRJ7, G0R932,
GORBV8, GOREE8, GORLFO, and GORBH2, and homologs thereof, and combinations
thereof;
wherein the modulated production and/or activity of the FFA releasing enzyme
is a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the transcription factor is a decrease in production and/or
activity of at least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%.
[0197]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
61
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
of FFA releasing enzymes comprising UniProt sequence# GORGQO and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second
FFA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORLH4 and homologs thereof, and combinations thereof; wherein the
modulated
production and/or activity of the first FFA releasing enzyme is a decrease in
production and/or
activity of at least 10%, at least 15%, at least 20%, at least 25%, at least
30%, at least 35%, at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
96%, at least 97%, at
least 98%, at least 99%, or 100%; and wherein the modulated production and/or
activity of the
second FFA releasing enzyme is a decrease in production and/or activity of at
least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%.
[0198]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of a first FFA releasing enzyme selected from the group consisting of FFA
releasing enzymes
comprising UniProt sequence# GORGQO and homologs thereof, and combinations
thereof; and
a modulated production and/or activity of a second FFA releasing enzyme
selected from the
group consisting of FFA releasing enzymes comprising UniProt sequence# GORM13
and
homologs thereof, and combinations thereof; wherein the modulated production
and/or activity
of the first FFA releasing enzyme is a decrease in production and/or activity
of at least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%; and wherein the modulated production and/or activity of the second FFA
releasing
enzyme is a decrease in production and/or activity of at least 10%, at least
15%, at least 20%,
at least 25%, at least 30%, at least 35%, at least 40%, at least 45%, at least
50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%,
at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%.
[0199]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORLH4 and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second 1-
+A releasing
62
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# G0RMI3 and homologs thereof, and combinations thereof; wherein the
modulated
production and/or activity of the first FFA releasing enzyme is a decrease in
production and/or
activity of at least 10%, at least 15%, at least 20%, at least 25%, at least
30%, at least 35%, at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
96%, at least 97%, at
least 98%, at least 99%, or 100%; and wherein the modulated production and/or
activity of the
second FFA releasing enzyme is a decrease in production and/or activity of at
least 10%, at
least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%, at
least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%, at
least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least
98%, at least 99%, or
100%.
[0200]
The recombinant host cell according to any of the above may comprise a
modulated
production and/or activity of a first FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORGQO and homologs
thereof, and
combinations thereof; and a modulated production and/or activity of a second
PPA releasing
enzyme selected from the group consisting of FFA releasing enzymes comprising
UniProt
sequence# GORLH4 and homologs thereof, and combinations thereof; and a
modulated
production and/or activity of a third FFA releasing enzyme selected from the
group consisting
of FFA releasing enzymes comprising UniProt sequence# GORMI3 and homologs
thereof, and
combinations thereof; wherein the modulated production and/or activity of the
first FFA
releasing enzyme is a decrease in production and/or activity of at least 10%,
at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95%, at least 96%, at least 97%, at least 98%, at least
99%, or 100%; wherein
the modulated production and/or activity of the second FFA releasing enzyme is
a decrease in
production and/or activity of at least 10%, at least 15%, at least 20%, at
least 25%, at least 30%,
at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%,
at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least
95%, at least 96%,
at least 97%, at least 98%, at least 99%, or 100%; and wherein the modulated
production and/or
activity of the third FFA releasing enzyme is a decrease in production and/or
activity of at least
10%, at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at
least 40%, at least
45%, at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at
least 75%, at least
63
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at
least 98%, at least
99%, or 100%.
Method for Obtaining Recombinant Host Cell
[0201]
In various aspects, provided herein is a method for obtaining a
recombinant host cell
according to any of the above, wherein the method comprises any combination,
in any order, of:
i) obtaining a polynucleotide according to any of the below, or a recombinant
expression
construct according to any of the below, or a recombinant vector according to
any of the below;
ii) introducing the polynucleotide, expression construct, or recombinant
vector into a host
cell (e.g., any of the host cells disclosed herein) to obtain a recombinant
host cell capable of
producing a recombinant component; iii) genetically modifying a host cell
(e.g., any of the host
cells disclosed herein) to modulate or essentially eliminate production and/or
activity of a FFA
releasing enzyme (e.g., any one of the FFA releasing enzymes disclosed herein
or any
combination of two or more FFA releasing enzymes disclosed herein) to obtain a
recombinant
host cell comprising a modified or essentially eliminated FFA releasing enzyme
activity; iv)
introducing the polynucleotide, expression construct, or recombinant vector
into a recombinant
host cell comprising a modified or essentially eliminated FFA releasing enzyme
activity; and/or
v) genetically modifying a recombinant host cell capable of producing a
recombinant
component to modulate or essentially eliminate production and/or activity of a
FFA releasing
enzyme (e.g., any one of the FFA releasing enzymes disclosed herein or any
combination of
two or more PIA releasing enzymes disclosed herein).
Polynucleotide
[0202]
The polynucleotide, expression construct, and/or recombinant vector may be
obtained by any suitable method known in the art, including, without
limitation, direct chemical
synthesis and cloning.
[0203]
The polynucleotide may comprise: i) an optional secretion signal sequence
(i.e., a
sequence that encodes a peptide that mediates the delivery of a nascent
protein attached to the
peptide to the exterior of the cell in which the nascent protein is
synthesized; e.g., a
polynucleotide sequence encoding any of the secretion signals disclosed
herein), and ii) a
recombinant protein coding sequence (i.e., a polynucleotide sequence encoding
a recombinant
protein (e.g., any of the recombinant proteins provided herein), optionally
comprising a tag
polypeptide (e.g., any of the tag polypeptides disclosed herein)), wherein: a)
the optional
secretion signal sequence is operably linked in sense orientation to the
recombinant protein
coding sequence (i.e., the optional secretion signal sequence and the
recombinant protein
64
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
coding sequence are positioned such that transcription and translation
produces a recombinant
protein comprising the optional secretion signal).
Secretion Signal Sequence
[0091] The recombinant expression construct according to any of
the above may optionally
comprise any secretion signal sequence that is active in a recombinant host
cell according to
any of the below.
[0092] The optional secretion signal sequence may encode a
secretion signal that mediates
translocation of the nascent recombinant protein into the ER post-
translationally (i.e., protein
synthesis precedes translocation such that the nascent recombinant protein is
present in the cell
cytosol prior to translocating into the ER) or co-translationally (i.e.,
protein synthesis and
translocation into the ER occur simultaneously).
[0093] Non-limiting examples of suitable secretion signal
sequences include secretion
signal sequences that are functional in a bacterial host cell, including
secretion signal sequences
of genes encoding any of the following proteins: PelB, OmpA, Bla, PhoA, PhoS,
MalE, LivK,
LivJ, Mg1B, AraF, AmpC, RbsB, MerP, CpdB, Lpp, LamB, OmpC, PhoE, OmpF, To1C,
BtuB,
and LutA, and functional parts and combinations thereof.
[0094] Non-limiting examples of suitable secretion signal
sequences include secretion
signal sequences that are functional in a fungal host cell, including
secretion signal sequences
of genes encoding any of the following proteins: CBH1, CBH2, EGLI, EGL2, XYN1,
XYN2,
BXL1, HH31, HPB2, GLAA, AMYA, AMYC, AAMA, alpha mating factor, SUC2, PH05,
INV, AMY, LIP, PIR, OST1, and fi-glucosidase, and functional parts and
combinations
thereof.
Recombinant Protein Coding Sequence
[0204] The recombinant protein coding sequence may encode any
recombinant protein.
[0205] Non-limiting examples of recombinant proteins include
milk proteins.
[0206] The term "milk protein" as used herein refers to a whey
protein or a casein.
[0207] The term "casein" as used herein refers to a polypeptide
that comprises a sequence
of at least 20 (e.g., at least 20, at least 30, at least 40, at least 50, at
least 60, at least 70, at least
80, at least 90, at least 100, at least 150) amino acids that is at least 40%
(e.g., at least 40%, at
least 45%, at least 50%, at least 55%, at least 60%, at least 65%, at least
70%, at least 75%, at
least 80%, at least 85%, at least 90%, at least 95%, at least 99%, 100%)
identical to a sequence
of amino acids in a casein natively found in a mammal-produced milk (i.e., a
casein that is
native to a milk produced by a mammal; e.g., a native casein). Examples of
caseins include 13-
casein, K-casein, a-S1-casein, and a-S2-casein. Accordingly, the terms "I3-
casein", "K-casein",
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
"a-S1-casein", and "a-S2-casein" as used herein refer to a polypeptide that
comprises a
sequence of at least 20 (e.g., at least 20, at least 30, at least 40, at least
50, at least 60, at least
70, at least 80, at least 90, at least 100, at least 150) amino acids that is
at least 40% (e.g., at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
99%, 100%) identical
to a sequence of amino acids in a I3-casein, x-casein, a-S1-casein, and a-S2-
casein, respectively,
natively found in a mammal-produced milk (e.g., Bos taurus I3-casein (amino
acids 16 to 224
of UniProt sequence P02666), Bos taurus x-casein (amino acids 22 to 190 of
UniProt sequence
P02668), Bos taut-us ct-S1-casein (amino acids 16 to 214 of UniProt sequence
P02662), and
Bos taurus a-S2-casein (amino acids 16 to 222 of UniProt sequence P02663),
respectively).
[0208]
The term "whey protein" as used herein refers to a polypeptide that
comprises a
sequence of at least 20 (e.g., at least 20, at least 30, at least 40, at least
50, at least 60, at least
70, at least 80, at least 90, at least 100, at least 150) amino acids that is
at least 40% (e.g., at
least 40%, at least 45%, at least 50%, at least 55%, at least 60%, at least
65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
99%, 100%) identical
to a sequence of amino acids in a whey protein natively found in a mammal-
produced milk
(i.e., a whey protein that is native to a milk produced by a mammal; e.g., a
native whey protein).
Examples of whey proteins include a-lactalbumin, 13-lactoglobulm,
lactotransferrin,
lactoferricin, serum albumin protein, lactoperoxidase protein, and
glycomacropeptide.
Accordingly, the terms "a-lactalbumin", 13-lactoglobulin", "lactotransferrin",
"lactoferricin",
"serum albumin", "lactoperoxidase", and "glycomacropeptide" as used herein
refer to a
polypeptide that comprises a sequence of at least 20 (e.g., at least 20, at
least 30, at least 40, at
least 50, at least 60, at least 70, at least 80, at least 90, at least 100, at
least 150) amino acids
that is at least 40% (e.g., at least 40%, at least 45%, at least 50%, at least
55%, at least 60%, at
least 65%, at least 70%, at least 75%, at least 80%, at least 85%, at least
90%, at least 95%, at
least 99%, 100%) identical to a sequence of amino acids in an cc-lactalbumin,
0-lactoglobulin,
lactotransferrin, lactoferricin, serum albumin, lactoperoxidase, and
glycomacropeptide (GMP),
respectively, natively found in a mammal-produced milk (e.g., Bos taurus a-
lactalbumin
(amino acids 20-142 of UniProt sequence P00711), Bos taurus 13-lactoglobulin
(amino acids
17-178 of UniProt sequence P02754), Bus taurus lactotransferrin (amino acids
20 to 708 of
UniProt sequence P24627), Bus taurus lactoferricin (amino acids 36 to 60 of
UniProt sequence
P24627), Bos taurus serum albumin (amino acids 25 to 607 of UniProt sequence
P02769), Bos
66
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
tuurus lactoperoxidase (amino acids 101 to 712 of UniProt sequence P80025),
and Bos tuurus
glycomacropeptide (GMP; amino acids 127 to 190 of UniProt sequence P02668),
respectively).
[0209]
The milk protein may have an amino acid sequence that is identical to or a
homolog
of an amino acid sequence of a native milk protein found in any mammalian
species, including
but not limited to cow, human, sheep, mouflon, goat, buffalo, camel, horse,
donkey, alpaca,
yak, llama, lemur, panda, guinea pig, squirrel, bear, macaque, gorilla,
chimpanzee, mountain
goat, monkey, ape, cat, dog, wallaby, rat, mouse, elephant, opossum, rabbit,
whale, baboons,
gibbons, orangutan, mandrill, pig, wolf, fox, lion, tiger, and echidna.
Recombinant Expression Construct
[0210]
The recombinant expression construct may comprise: i) a promoter sequence
(e.g.,
a polynucleotide sequence for any of the promoters disclosed herein), ii) the
polynucleotide of
any of the above, and iii) a termination sequence (e.g., a polynucleotide
sequence for any of the
terminators disclosed herein); wherein: a) the promoter sequence is operably
linked in sense
orientation to the optional secretion signal sequence and the recombinant
protein coding
sequence of the polynucleotide (i.e., the promoter sequence and the optional
secretion signal
sequence and the recombinant protein coding sequence of the polynucleated are
positioned such
that the promoter sequence is effective in mediating or regulating
transcription of the optional
secretion signal sequence and the recombinant protein coding sequence), and b)
the one or more
terminator sequences are operably linked to the recombinant protein coding
sequence (i.e., the
recombinant protein coding sequence and the one or more terminator sequences
are positioned
such that the one or more terminator sequences are effective in terminating
transcription of the
recombinant protein coding sequence).
[0211]
The recombinant expression construct may further comprise an operably
linked
sequence encoding for an affinity purification tag, such that the expressed
recombinant protein
includes a peptide sequence for affinity purification. Such affinity
purification tag may be
operably linked such that when expressed the affinity purification tag is
present either at or
toward the amino terminus, the carboxy terminus, or both. Such affinity
purification tag may
be a maltose binding protein (MBP) tag, a glutathione-S-transferase (GST) tag,
a poly(His) tag,
a hexa(His) tag, a FLAG-tag, a V5-tag, a VS V-tag, an E-tag, an NE-tag, a
hemagglutinin (Ha)-
tag, and a Myc-tag.
[0212]
The recombinant expression construct may further comprise a sequence for
integration by homologous (i.e., targeted integration) or nonhomologous
recombination into
the genome of a host cell. The recombinant expression construct may comprise
at least 10, at
least 25, at least 50, at least 100, at least 250, at least 500, at least 750,
at least 1,000, or at least
67
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
10,000 base pairs that have sufficient identity with a target sequence in the
genome of the host
cell to enhance the probability of homologous recombination of the recombinant
expression
construct. Such homologous sequence may be non-coding or coding.
[0213]
The optional secretion signal sequence and/or recombinant protein coding
sequence
comprised in the recombinant expression construct according to any of the
above may be
codon-optimized for expression in the recombinant host cell according to any
of the above.
[0214]
The recombinant expression construct according to any of the above may be
generated upon integration of a fragment of the recombinant expression
construct into the
genome of a host cell (e.g., the genome of the recombinant host cell according
to any of the
above). For example, a polynucleotide according to any of the above may be
stably integrated
within the genome of a host cell such that one or more regulatory elements of
an endogenous
gene locus become operably linked to the recombinant protein coding sequence,
thereby
generating the recombinant expression construct according to any of the above.
Promoter Sequence
[0215]
The recombinant expression construct according to any of the above may
comprise
any promoter sequence that is active in a recombinant host cell according to
any of the below.
[0216]
The promoter sequence may be a constitutive promoter sequence (i.e., a
promoter
sequence that is active under most environmental and developmental
conditions), or an
inducible or repressible promoter sequence (i.e., a promoter sequence that is
active only under
certain environmental or developmental conditions ie.g., in presence or
absence of certain
factors, such as, but not limited to, carbon (e.g., glucose, galactose,
lactose, sucrose, cellulose,
sophorose, gentiobiose, sorbose, disaccharides that induce the cellulase
promoters, starch,
tryptophan, thiamine, methanol), phosphate, nitrogen, or other nutrient;
temperature; pH;
osmolarity; heavy metals or heavy metal ions; inhibitors; stress; catabolites;
and combinations
thereof]).
[0217]
The promoter sequence may consist of a single promoter sequence, or of two
or
more promoter sequences (e.g., combination of two or more promoters or
functional parts
thereof arranged in sequence, combination of an inducible and a constitutive
promoter). The
two or more promoter sequences may be identical, or at least two of the two or
more promoter
sequences cannot be identical.
[0218]
The promoter sequence may comprise or consist of a bidirectional promoter
sequence (i.e., a polynucleotide that initiates transcription in both
orientations by recruiting
transcription factors, for example generated by fusing two identical or
different promoters in
opposite directions).
68
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0219]
Non-limiting examples of suitable promoter sequences include promoter
sequences
that are functional in a bacterial host cell, including T7 promoter, T5
promoter, Tac promoter,
pL/pR promoter, phoA promoter, lac U V5 promoter, trc promoter, trp promoter,
cstA promoter,
xylA promoter, manP promoter, malA promoter, lacA promoter, aprE promoter,
AaprE
promoter, srfA promoter, p43 promoter, ylbA promoter, GB promoter, veg
promoter, PG1
promoter, PG6 promoter, i\PL promoter, 2\,PR promoter, and spa promoter, and
functional parts
and combinations thereof.
[0220]
Non-limiting examples of suitable promoter sequences include promoter
sequences
that are functional in a fungal host cell, including xlnA promoter, xynl
promoter, xyn2
promoter, xyn3 promoter, xyn4 promoter, bxl 1 promoter, cbhl promoter, cbh2
promoter, egll
promoter, eg12 promoter, eg13 promoter, eg14 promoter, eg15 promoter, glaA
promoter, agdA
promoter, gpdA promoter, gpdl promoter, AOX1 promoter, GAP1 promoter, MET3
promoter,
EN01 promoter, GPD1 promoter, PDC1 promoter, TEF1 promoter, AXE1 promoter,
CIP1
promoter, GH61 promoter, PKI1 promoter, RP2 promoter, ADH1 promoter, CUP1
promoter,
GAL1 promoter, PGK1 promoter, YPT1 promoter, LAC4 promoter, LAC4-PB 1
promoter.
FLD1 promoter, MOX promoter, DAS1 promoter, DAS2 promoter, GAP1 promoter, STR3
promoter, ADH3 promoter, GUT2 promoter, CYC1 promoter, TDH3 promoter, PGL1
promoter, ADH2 promoter, HXT7 promoter, CLB1 promoter, and PHO5 promoter, and
functional parts and combinations thereof.
Termination Sequence
[0221]
The recombinant expression construct according to any of the above may
comprise
any termination sequence that is active in a recombinant host cell according
to any of the below.
[0222]
Non-limiting examples of suitable termination sequences include
termination
sequences of adhl, amaA, amdS, amyA, aoxl, cbhl, cbh2, cyc2, egll, eg12, gall,
gapl, glaA,
gpdl, gpdA, pdcl, pgkl, tefl, tpsl, trpC, xynl, xyn2, xyn3, and xyn4 genes,
and functional
parts and combinations thereof.
[0223]
The termination sequence may consist of a single termination sequence, or
of two
or more termination sequences, wherein the two or more termination sequences
may be
identical, or at least two of the two or more termination sequences may be not
identical. The
termination sequence may consist of a bidirectional termination sequence.
Additional Regulatory Elements
[0224]
The recombinant expression construct according to any of the above may
further
comprise additional regulatory elements.
69
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0225]
Non-limiting examples of regulatory elements include promoter sequences,
termination sequences, transcriptional start sequences, translational start
sequences, translation
stop sequences, enhancer sequences, activator sequences, response elements,
protein
recognition sites, inducible elements, protein binding sequences, 5' and 3'
untranslated regions
(e.g., a 3' untranslated region comprising a poly-adenylation signal),
upstream activation
sequences (UAS), introns, operators (i.e., sequences of nucleic acids adjacent
to a promoter that
comprise a protein-binding domain where a repressor protein can bind and
reduce or eliminate
activity of the promoter), efficient RNA processing signals (e.g., splicing
signals,
polyadenylation signals), sequences that stabilize cytoplasmic mRNA, sequences
that enhance
translation efficiency (e.g., ribosome binding sites [e.g., Shine-Dalgarno
sequencesp,
sequences that enhance protein stability, sequences that enhance protein
secretion, and
combinations thereof.
Recombinant Vector
[0226]
The recombinant vector may comprise a recombinant expression construct
according to any of the above.
[0227]
The recombinant vector may comprise a single recombinant expression
construct
according to any of the above, or two or more recombinant expression
constructs according to
any of the above, which may be identical or at least two of which may be not
identical (e.g.,
differ from each other in a promoter sequence, a secretion signal, a protein
coding sequence, a
termination sequence, and/or an additional regulatory element). In embodiments
in which the
recombinant vector comprises two or more recombinant expression constructs,
the two or more
recombinant expression constructs may encode the same recombinant protein. In
some such
embodiments, the two or more recombinant expression constructs encoding the
same
recombinant protein differ from each other in a promoter sequence, secretion
signal sequence,
termination sequence, and/or additional regulatory element.
[0228]
The recombinant vector may further comprise one or more other elements
suitable
for propagation of the recombinant vector in a recombinant host cell. Non-
limiting examples
of such other elements include origins of replication and selection markers.
Origins of
replication and selection markers are known in the art, and include bacterial
and fungal origins
of replication (e.g., AMA1, ANSI). Selection markers may be resistance genes
(i.e.,
polynucleotides that encode proteins that enable host cells to detoxify an
exogenously
added compound [e.g., an antibiotic compound]),
auxotrophic
markers (i.e., polynucleotides that encode proteins that permit a host cell to
synthesize an
essential component (usually an amino acid) while grown in media that lacks
that essential
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
component), or color markers (i.e., genes that encode proteins that can
produce a color). Non-
limiting examples of suitable selection markers include amdS (acetamidase),
argB (omithine
carbamoyltransferase), bar (phosphinothricin acetyltransferase), hph
(hygromycin
phosphotransferase), niaD (nitrate reductase), pyrG (orotidine 5'-phosphate
decarboxylase), sC
(sulfate adenyltransferase), trpC (anthranilate
synthase), and ble (bleomycin-
type antibiotic resistance), and derivatives thereof. The selection marker may
comprise an
alteration that decreases production of the selective marker, thus increasing
the number of
copies needed to permit a recombinant host cell comprising the recombinant
vector to survive
under selection. Selection may also be accomplished by co-transformation,
wherein the
transformation is carried out with a mixture of two vectors and the selection
is made for one
vector only.
[0229]
The recombinant vector may further comprise sequences for integration by
homologous (i.e., targeted integration) or nonhomologous recombination into
the genome of a
host cell. The recombinant expression construct may comprise at least 10, at
least 25, at least
50, at least 100, at least 250, at least 500, at least 750, at least 1,000, or
at least 10,000 base
pairs that have sufficient identity with a target sequence in the genome of
the host cell to
enhance the probability of homologous recombination of the recombinant
expression construct.
Such homologous sequence may be non-coding or coding.
Genetic Modification
[0230]
A genetic modification may consist of, for example, an insertion, a
substitution, a
duplication, a rearrangement and/or a deletion of one or more nucleotides in a
genome of a cell.
A genetic modification may, for example, introduce a stop codon; remove a
start codon; insert
a frame-shift of the open reading frame; or create a point mutation, missense
mutation,
substitution mutation, deletion mutation, frameshift mutation, insertion
mutation, duplication
mutation, amplification mutation, translocation mutation, or inversion
mutation.
[0231]
Methods for genetically modifying a host cell are well known in the art,
and include,
without limitation, random mutagenesis and screening, site-directed
mutagenesis, PCR
mutagenesis, insertional mutagenesis, chemical mutagenesis (using, for
example,
hydroxylamine, N-methyl-N'-nitro-N-nitrosoguanidine
(MNNG), N-methyl-N'-
nitrosogaunidine (NTG), 0-methyl hydroxylamine, nitrous acid, ethyl methane
sulphonate
(EMS), sodium bisulphite, formic acid, nucleotide analogues), irradiation
(e.g., ultraviolet (UV)
irradiation), deletion of coding or non-coding nucleotide sequences,
homologous
recombination, FLP/FRT recombination, gene disruption, CRISPR gene editing,
and gene
conversion. Such methods include introduction into a host cell of a
recombinant polynucleotide
71
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
that comprises a polynucleotide sequence that is complementary to a
polynucleotide sequence
encoding a protein of interest (e.g., a FFA releasing enzyme, a protein that
drives expression
and/or regulates activity of a FFA releasing enzyme), that encodes a RNAi
construct that is
specific to a protein of interest, or that encodes a heterologous inhibitor or
activator of a FFA
releasing enzyme.
[0232]
The modulated production and/or activity of a FFA releasing enzyme in the
recombinant host cell according to any of the above may be evaluated using any
suitable
method known in the art, such as assays that are carried out at the RNA level
and, most suitable,
at the protein level, or by use of functional bioassays that measure the
production or activity of
the secretion-related protein. Non-limiting examples of such assays include
Northern blotting,
dot blotting (DNA or RNA), RT-PCR (reverse transcriptase polymerase chain
reaction), in situ
hybridization, Southern blotting, enzyme activity assays, immunological assays
(e.g.,
immunohistochemical staining, immunoassays, Western blotting, ELISA), and free
thiol assays
(e.g., for measuring production of protein comprising free cys [eine
residues). The production
and/or activity of a FFA releasing enzyme in the recombinant host cell
according to any of the
above may be compared to that of a corresponding recombinant host cell (i.e.,
an identical
recombinant host cell that is also capable of producing the recombinant
component (i.e.,
comprises the same expression construct) but that does not comprise the
genetic modification
that modulates or essentially eliminates the FFA releasing enzyme activity)
evaluated by the
same method.
Introducing Polynucleotide, Recombinant Expression Construct, or Recombinant
Vector into
Host Cell
[0233]
Methods for introducing a polynucleotide, recombinant expression
construct, or
recombinant vector into a host cell are well-known in the art. Non-limiting
examples of such
methods include calcium phosphate transfection, dendrimer transfection,
liposome transfection
(e.g., cationic liposome transfection), cationic polymer transfection, DEAE-
dextran
transfection, cell squeezing, sonoporation, optical transfection, protoplast
fusion, protoplast
transformation, impalefection, hyrodynamic delivery, gene gun, magnetofection,
viral
transduction, electroporation, and chemical transformation (e.g., using PEG).
[0234]
Methods for identifying a recombinant host cell are well-known in the art,
and
include screening for expression of a drug resistance or auxotrophic marker
encoded by the
polynucleotide, recombinant expression construct, or recombinant vector that
permits selection
for or against growth of cells, or by other means (e.g., detection of light
emitting peptide
comprised in the polynucleotide, recombinant expression construct, or
recombinant vector,
72
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
molecular analysis of individual recombinant host cell colonies [e.g., by
restriction enzyme
mapping, PCR amplification, Southern analysis, or sequence analysis of
isolated
extrachromosomal vectors or chromosomal integration sites]).
[0235]
Production of the recombinant protein by the recombinant host cell
according to any
of the above may be evaluated using any suitable method known in the art, such
as assays that
are carried out at the RNA level and, most suitable, at the protein level, or
by use of functional
bioassays that measure the production or activity of the recombinant protein.
Non-limiting
examples of such assays include Northern blotting, dot blotting (DNA or RNA),
RT-PCR
(reverse transcriptase polymerase chain reaction), RNA-Seq, in situ
hybridization, Southern
blotting, enzyme activity assays, immunological assays (e.g.,
immunohistochemical staining,
immunoassays, Western blotting, ELISA), and free thiol assays (e.g., for
measuring production
of protein comprising free cysteine residues)
Method for Producing a Recombinant Component
[0236]
In various aspects, provided herein is a method for producing the
recombinant
component according to any of the above, wherein the method comprises:
fermenting a
recombinant host cell according to any of the above in a culture medium under
conditions
suitable for production of the recombinant component.
[0237]
The method may further comprise: purifying the recombinant component from
the
fermentation broth to obtain a preparation comprising the recombinant
component; and/or post-
processing the recombinant component.
Fermenting
[0238]
Suitable conditions for producing the recombinant component are typically
those
under which the recombinant host cell according to any of the above can grow
and/or
remain viable, and produce the recombinant component.
[0239]
Non-limiting examples of suitable conditions include a suitable culture
medium
(e.g., a culture medium having a suitable nutrient content [e.g., a suitable
carbon content, a
suitable nitrogen content, a suitable phosphorus content], a suitable
supplement content, a
suitable trace metal content, a suitable pH), a suitable temperature, a
suitable feed rate, a
suitable pressure, a suitable level of oxygenation, a suitable fermentation
duration (i.e., volume
of culture media comprising the recombinant host cells), a suitable
fermentation volume (i.e.,
volume of culture media comprising the recombinant host cells), and a suitable
fermentation
vessel.
[0240]
Suitable culture media include all culture media in which the recombinant
host
cell can grow and/or remain viable, and produce the recombinant component.
Typically, the
73
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
culture medium is an aqueous medium that comprises a carbon source, an
assimilable nitrogen
source (i.e., a nitrogen-containing compound capable of releasing nitrogen in
a form suitable
for metabolic utilization by the recombinant host cell), and a phosphate
source.
[0241]
Non-limiting examples of carbon sources include monosaccharides,
disaccharides,
polysaccharides, acetate, ethanol, methanol, glycerol, methane, and
combinations thereof. Non-
limiting examples of monosaccharides include dextrose (glucose), fructose,
galactose, xylose,
arabinose, and combinations thereof. Non-limiting examples of disaccharides
include sucrose,
lactose, maltose, trehalose, cellobiose, and combinations thereof. Non-
limiting examples of
polysaccharides include starch, glycogen, cellulose, amylose, hemicellulose,
maltodextrin, and
combinations thereof.
[0242]
Non-limiting examples of assimilable nitrogen sources include anhydrous
ammonia, ammonium sulfate, ammonium hydroxide, ammonium nitrate, diammonium
phosphate, monoammonium phosphate, ammonium pyrophosphate, ammonium chloride,
sodium nitrate, urea, peptone, protein hydrolysates, corn steep liquor, corn
steep solids, spent
grain, spent grain extract, and yeast extract. Use of ammonia gas is
convenient for large
scale operations, and may be employed by bubbling through the aqueous ferment
(fermentation
medium) in suitable amounts. At the same time, such ammonia may also be
employed to assist
in pH control.
[0243]
The culture medium may further comprise an inorganic salt, a mineral
(e.g.,
magnesium, calcium, potassium, sodium; e.g., in suitable soluble assimilable
ionic and
combined forms), a metal or transition metal (e.g., copper, manganese,
molybdenum, zinc, iron,
boron, iodine; e.g., in suitable soluble assimilable form), a vitamin, and any
other nutrient or
functional ingredient (e.g., a protease [e.g., a plant-based protease] that
can prevent degradation
of the recombinant component, a protease inhibitor that can reduce the
activity of a protease
that can degrade the recombinant component, and/or a sacrificial protein that
can siphon away
protease activity, an anti-foaming agent, an anti-microbial agent, a
surfactant, an emulsifying
oil).
[0244]
Suitable culture media are available from commercial suppliers or may be
prepared
according to published compositions (e.g., in catalogues of the American Type
Culture
Collection).
[0245]
A suitable pH may be a pH of between about 2 and about 8 (e.g., a pH of
between
2 and 8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2, 5.1, 5, 4.9, 4.8, 4.7, 4.6, 4.5,
4, 3.5, 3, or 2.5; between
2.5 and 8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2, 5.1, 5, 4.9, 4.8, 4.7, 4.6,
4.5, 4, 3.5, or 3; between 3
and 8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2, 5.1, 5, 4.9, 4.8, 4.7, 4.6, 4.5, 4,
or 3.5; between 3.5 and
74
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2, 5.1, 5, 4.9, 4.8, 4.7, 4.6, 4.5, or 4;
between 4 and 8, 7.5, 7,
6.5, 6, 5.5, 5.4, 5.3, 5.2, 5.1, 5, 4.9, 4.8, 4.7, 4.6, or 4.5; between 4.5
and 8, 7.5, 7, 6.5, 6, 5.5,
5.4, 5.3, 5.2, 5.1, 5, 4.9, 4.8, 4.7, or 4.6; between 4.6 and 8, 7.5, 7, 6.5,
6, 5.5, 5.4, 5.3, 5.2, 5.1,
5, 4.9, 4.8, or 4.7; between 4.7 and 8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2,
5.1, 5,4.9, or 4.8; between
4.8 and 8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2, 5.1, 5, or 4.9; between 4.9 and
8, 7.5, 7, 6.5, 6, 5.5,
5.4, 5.3, 5.2, 5.1, or 5; between 5 and 8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, 5.2,
or 5.1; between 5.1 and
8, 7.5, 7, 6.5, 6, 5.5, 5.4, 5.3, or 5.2; between 5.2 and 8, 7.5, 7, 6.5, 6,
5.5, 5.4, or 5.3; between
5.3 and 8, 7.5, 7, 6.5, 6, 5.5, or 5.4; between 5.4 and 8, 7.5, 7, 6.5, 6, or
5.5; between 5.5 and 8,
7.5, 7, 6.5, or 6; between 6 and 8, 7.5, 7, or 6.5; between 6.5 and 8, 7.5, or
7; between 7 and 8,
or 7.5; or between 7.5 and 8).
[0246]
A suitable temperature may be a temperature of between about 20 C and
about 46 C
(e.g., between 20 C and 46 C, 44 C, 42 C, 40 C, 38 C, 36 C, 34 C, 32 C, 30 C,
28 C, 26 C,
24 C, or 22 C; between 22 C and 46 C, 44 C, 42 C, 40 C, 38 C, 36 C, 34 C, 32
C, 30 C,
28 C, 26 C, or 24 C; between 24 C and 46 C, 44 C, 42 C, 40 C, 38 C, 36 C, 34
C, 32 C,
30 C, 28 C, or 26 C; between 26 C and 46 C, 44 C, 42 C, 40 C, 38 C, 36 C, 34
C, 32 C.
30 C, or 28 C; between 28 C and 46 C, 44 C, 42 C, 40 C, 38 C, 36 C, 34 C, 32
C, or 30 C;
between 30 C and 46 C, 44 C, 42 C, 40 C, 38 C, 36 C, 34 C, or 32 C; between 32
C and
46 C, 44 C, 42 C, 40 C, 38 C, 36 C, or 34 C; between 36 C and 46 C, 44 C, 42
C, 40 C, or
38 C; between 38 C and 46 C, 44 C, 42 C, or 40 C; between 40 C and 46 C, 44 C,
or 42 C.
between 42 C and 46 C or 44 C; or between 44 C and 46 C).
[0247]
A suitable feed rate may be a feed rate of between about 0.01 g and about
0.2 g
glucose equivalent per g dry cell weight (DCW) per hour.
[0248]
A suitable pressure may be a pressure of between 0 psig and about 50 psig
(e.g.,
between 0 psig and 50 psig, 40 psig, 30 psig, 20 psig, or 10 psig; between 10
psig and 50 psig,
40 psig, 30 psig, or 20 psig; between 20 psig and 50 psig, 40 psig, or 30
psig; between
30 psig and 50 psig, or 40 psig; or between 40 psig and 50 psig).
[0249]
A suitable oxygenation may be an aeration rate of between about 0.1
volumes of
oxygen per liquid volume in the fermentor per minute (vvm) and about 2.1 vvm
(e.g., between
0.1 vvm and 2.1 vvm, 1.9 vvm, 1.7 vvm, 1.5 vvm, 1.3 vvm, 1.1 vvm, 0.9 vvm, 0.7
vvm,
0.5 vvm, or 0.3 vvm; between 0.3 vvm and 2.1 vvm, 1.9 vvm, 1.7 vvm, 1.5 vvm,
1.3 vvm,
1.1 vvm, 0.9 vvm, 0.7 vvm, or 0.5 vvm; between 0.5 vvm and 2.1 vvm, 1.9 vvm,
1.7 vvm,
1.5 vvm, 1.3 vvm, 1.1 vvm, 0.9 vvm, or 0.7 vvm; between 0.7 vvm and 2.1 vvm,
1.9 vvm.
1.7 vvm, 1.5 vvm, 1.3 vvm, 1.1 vvm, or 0.9 vvm; between 0.9 vvm and 2.1 vvm,
1.9 vvm,
1.7 vvm, 1.5 vvm, 1.3 vvm, or 1.1 vvm; between 1.1 vvm and 2.1 vvm, 1.9 vvm,
1.7 vvm,
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
1.5 vvm, or 1.3 vvm; between 1.3 vvm and 2.1 vvm, 1.9 vvm, 1.7 VVM, or 1.5
vvm; between
1.5 vvm and 2.1 vvm, 1.9 vvm, or 1.7 vvm; between 1.7 vvm and 2.1 vvm or 1.9
vvm; or
between 1.9 vvm and 2.1 vvm).
[0250]
A suitable fermentation duration may be a fermentation duration of between
about
hours and about 500 hours (e.g., between 10 hours and 500 hours, 400 hours,
300 hours, 200
hours, 100 hours, 50 hours, 40 hours, 30 hours, or 20 hours; between 20 hours
and 500 hours,
400 hours, 300 hours, 200 hours, 100 hours, 50 hours, 40 hours, or 30 hours;
between 30 hours
and 500 hours, 400 hours, 300 hours, 200 hours, 100 hours, 50 hours, or 40
hours; between 40
hours and 500 hours, 400 hours, 300 hours, 200 hours, 100 hours, or 50 hours;
between 50
hours and 500 hours, 400 hours, 300 hours, 200 hours, or 100 hours; between
100 hours and
500 hours, 400 hours, 300 hours, or 200 hours; between 200 hours and 500
hours, 400 hours.
or 300 hours; between 300 hours and 500 hours, or 400 hours; or between 400
hours and 500
hours).
[0251]
A suitable fermentation volume may be between about 1 L and about
10,000,000 L
(e.g., between 1 L and 10,000,000 L, 5,000,000 L, 1,000,000 L, 500,000L,
100,000 L, 50,000
L, 10, 000 L, 5,000 L, 1,000 L, 500 L, 100 L, 50 L, or 10 L; between 10 L and
10,000,000 L,
5,000,000 L, 1,000,000 L, 500,000L, 100,000 L, 50,000 L, 10, 000 L, 5,000 L,
1,000 L, 500 L,
100 L, or 50 L; between 50 L and 10,000,000 L, 5,000,000 L, 1,000,000 L,
500,000L, 100,000
L, 50,000 L, 10, 000 L, 5,000 L, 1,000 L, 500 L, or 100 L; between 100 L and
10,000,000 L,
5,000,000 L, 1,000,000 L, 500,000L, 100,000 L, 50,000 L, 10, 000 L, 5,000 L,
1,000 L, or 500
L; between 500 L and 10,000,000 L, 5,000,000 L, 1,000,000 L, 500,000L, 100,000
L, 50,000
L, 10, 000 L, 5,000 L, or 1,000 L; between 1,000 L and 10,000,000 L, 5,000,000
L, 1,000,000
L, 500,000L, 100,000 L, 50,000 L, 10, 000 L, or 5,000 L; between 5,000 L and
10,000,000 L,
5,000,000 L, 1,000,000 L, 500,000L, 100,000 L, 50,000 L, or 10, 000 L; between
10,000 L and
10,000,000 L, 5,000,000 L, 1,000,000 L, 500,000L, 100,000 L, or 50,000 L;
between 50,000 L
and 10,000,000 L, 5,000,000 L, 1,000,000 L, 500,000L, or 100,000 L; between
100,000 L and
10,000,000 L, 5,000,000 L, 1,000,000 L, or 500,000L; between 500,000 L and
10,000,000 L,
5,000,000 L, or 1,000,000 L; between 1,000,000 L and 10,000,000 L, or
5,000,000 L; or
between 5,000,000 L and 10,000,000 L).
[0252]
A suitable fermentation vessel may be any fermentation vessel known in the
art.
Non-limiting examples of suitable fermentation vessels include culture plates,
shake
flasks, fermentors (e.g., stirred tank fermentors, airlift fermentors, bubble
column fermentors,
fixed bed bioreactors, laboratory fermentors, industrial fermentors, or any
combination
76
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
thereof), used at any suitable scale (e.g., small-scale, large-scale) and in
any process (e.g., solid
culture, submerged culture, batch, fed-batch, or continuous-flow).
Purification and Post-processing
[0253]
Methods for purifying a recombinant component (e.g., from a fermentation
broth)
to obtain a preparation comprising the recombinant component are well-known in
the art, and
may be adapted to purify the recombinant produced by a recombinant host cell
according to
any of the above.
[0254]
A recombinant component may be purified on the basis of its molecular
weight, for
example, by size exclusion/exchange chromatography, ultrafiltration through
membranes, gel
permeation chromatography (e.g., preparative disc-gel electrophoresis), or
density
centrifugation.
[0255]
A recombinant component also may be purified on the basis of its surface
charge or
hydrophobicity/hydrophilicity, for example, by isoelectric precipitation,
anion/cation exchange
chromatography, isoelectric focusing (IEF), or reverse phase chromatography.
[0256]
A recombinant component also may be purified on the basis of its
solubility, for
example, by ammonium sulfate precipitation, isoelectric precipitation,
surfactants, detergents,
or solvent extraction.
[0257]
A recombinant component also may be purified on the basis of its affinity
to another
molecule, for example, by affinity chromatography, reactive dyes, or
hydroxyapatite. Affinity
chromatography may include the use of an antibody having a specific binding
affinity for the
recombinant component, or nickel NTA for a His-tagged recombinant protein, or
a lectin to
bind to a sugar moiety on a recombinant protein, or any other molecule that
specifically binds
the recombinant component. The recombinant component may carry an epitope or
peptide tag
that facilitates purification. An epitope or peptide tag may be removed
following isolation of
the recombinant component (e.g., via protease cleavage).
[0258]
In embodiments in which the recombinant component according to any of the
above
is secreted by the recombinant host cell according to any of the above, the
recombinant
component may be purified directly from the culture medium. In other
embodiments, the
recombinant component may be purified from a cell lysate.
[0259]
The recombinant component may be purified to obtain a preparation
comprising the
recombinant component at a purity of greater than 30%, greater than 35%,
greater than 40%,
greater than 45%, greater than 50%, greater than 55%, greater than 60%,
greater than 65%,
greater than 70%, greater than 75%, greater than 80%, greater than 85%,
greater than 90%,
greater than 95%, greater than 97%, or greater than 99% relative to other
components
77
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
comprised in the fermentation broth; or to at least 2-fold, at least 3-fold,
at least 4-fold, at least
5-fold, at least 6-fold, at least 7-fold, at least 8-fold, at least 9-fold, or
at least 10-fold greater
abundancy relative to other components comprised in the fermentation broth; or
to a purity of
greater than 30%, greater than 35%, greater than 40%, greater than 45%,
greater than 50%,
greater than 55%, greater than 60%, greater than 65%, greater than 70%,
greater than 75%,
greater than 80%, greater than 85%, greater than 90%, greater than 95%,
greater than 97%, or
greater than 99% by mass.
[0260]
The identity of the recombinant component may be confirmed and/or
quantified by
high performance liquid chromatography (HPLC), Western blot analysis, Eastern
blot analysis,
polyacrylamide gel electrophoresis, capillary electrophoresis, formation of an
enzyme product,
disappearance of an enzyme substrate, and 2-dimensional mass spectroscopy (2D-
MS/MS)
sequence identification.
[0261]
The recombinant component may be spray dried or concentrated via
evaporation
(e.g., to obtain a powder).
Composition Comprising Recombinant Component
[0262]
In various aspects, provided herein is a composition that comprises or
consists
essentially of a recombinant component produced by a recombinant host cell
according to any
of the above and/or a method according to any of the above, wherein the
composition comprises
a modulated FFA releasing enzyme activity (e.g., activity of any one FFA
releasing enzyme
disclosed herein or activities of any combination of two or more FFA releasing
enzymes
disclosed herein) compared to the FFA releasing enzyme activity in a
corresponding
composition (i.e., a composition that is identical to the composition that is
compared to the
"corresponding composition " except that the method by which the
"corresponding
composition" is produced does not comprise at least one step in which a FFA
releasing enzyme
activity is modulated as provided herein.
[0263]
The composition may comprise between about 0.1% and about 100% (e.g.,
between
0.1% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%,
35%,
30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%,
1%,
0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, or 0.2%; between 0.2% and 100%, 95%,
90%,
85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%,
14%,
13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%,
0.6%, 0.5%,
0.4%, or 0.3%; between 0.3% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%,
55%,
50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%,
6%,
5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, or 0.4%; between 0.4% and
100%, 95%,
78
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%,
15%,
14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%,
0.6%,
or 0.5%; between 0.5% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%,
50%,
45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%,
5%,
4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, or 0.6%; between 0.6% and 100%, 95%, 90%,
85%, 80%,
75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%,
12%,
11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, or 0.7%; between
0.7% and
100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%,
25%,
20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%,
or
0.8%; between 0.8% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%,
45%,
40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%,
3%,
2%, 1%, or 0.9%; between 0.9% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%,
60%,
55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%,
7%,
6%, 5%, 4%, 3%, 2%, or 1%; between 1% and 100%, 95%, 90%, 85%, 80%, 75%, 70%,
65%,
60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%,
8%, 7%, 6%, 5%, 4%, 3%, or 2%; between 2% and 100%, 95%, 90%, 85%, 80%, 75%,
70%,
65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%,
10%,
9%, 8%, 7%, 6%, 5%, 4%, or 3%; between 3% and 100%, 95%, 90%, 85%, 80%, 75%,
70%,
65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%,
10%,
9%, 8%, 7%, 6%, 5%, or 4%; between 4% and 100%, 95%, 90%, 85%, 80%, 75%, 70%,
65%,
60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%,
8%, 7%, 6%, or 5%; between 5% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%,
60%,
55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%,
7%,
or 6%; between 6% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%,
45%,
40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, 8%, or 7%; between
7%
and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%,
30%,
25%, 20%, 15%, 14%, 13%, 12%, 11%, 10%, 9%, or 8%; between 8% and 100%, 95%,
90%,
85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%,
14%,
13%, 12%, 11%, 10%, or 9%; between 9% and 100%, 95%, 90%, 85%, 80%, 75%, 70%,
65%,
60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, 11%, or 10%;
between 10% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%,
40%,
35%, 30%, 25%, 20%, 15%, 14%, 13%, 12%, or 11%; between 11% and 100%, 95%,
90%,
85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%,
14%,
13%, or 12%; between 12% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%,
55%,
79
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 14%, or 13%; between 13% and 100%,
95%,
90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, 20%,
15%,
or 14%; between 14% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%,
50%,
45%, 40%, 35%, 30%, 25%, 20%, or 15%; between 15% and 100%, 95%, 90%, 85%,
80%,
75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, 25%, or 20%; between 20% and
100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 35%, 30%, or
25%; between 25% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%,
45%,
40%, 35%, or 30%; between 30% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%,
60%,
55%, 50%, 45%, 40%, or 35%; between 35% and 100%, 95%, 90%, 85%, 80%, 75%,
70%,
65%, 60%, 55%, 50%, 45%, or 40%; between 40% and 100%, 95%, 90%, 85%, 80%,
75%,
70%, 65%, 60%, 55%, 50%, or 45%; between 45% and 100%, 95%, 90%, 85%, 80%,
75%,
70%, 65%, 60%, 55%, or 50%; between 50% and 100%, 95%, 90%, 85%, 80%, 75%,
70%,
65%, 60%, or 55%; between 55% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, or
60%;
between 60% and 100%, 95%, 90%, 85%, 80%, 75%, 70%, or 65%; between 65% and
100%,
95%, 90%, 85%, 80%, 75%, or 70%; between 70% and 100%, 95%, 90%, 85%, 80%, or
75%;
between 75% and 100%, 95%, 90%, 85%, or 80%; between 80% and 100%, 95%, 90%,
or
85%; or between 85% and 100%, 95%, 90%; between 90% and 100% or 95%, or
between 95%
and 100%) by dry mass of the recombinant component.
[0264]
At standard ambient temperature and conditions (i.e., 20-30 C and 0.95-
1.05 atm),
the composition according to any of the above may be a fluid, semi-solid
(e.g., gelatinous),
solid, or powder. The powder may comprise a moisture content of less than 20%,
less than
15%, less than 10%, less than 7%, less than 5%, less than 3%, or less than 1%;
or between
about 0.1% and about 20% (e.g., between 0.1% and 20%, 15%, 10%, 5%, or 1%;
between 1%
and 20%, 15%, 10%, or 5%; between 5% and 20%, 15%, or 10%; between 10% and
20%, or
15%; or between 15% and 20%). The powder may be used in powder form, or the
powder may
be reconstituted with a hydrating agent prior to use, or the powder may be
mixed with other dry
components (e.g., flour, sugar, minerals, pH or ionic strength adjusting
agents) before a
hydrating agent is added to the mixture.
[0265]
The composition according to any of the above may be a variety of
products,
including, for example, an encapsulate (e.g., a product that encapsulates a
therapeutic or
nutraceutical for delivery [e.g., a micro- or nano-particle (e.g., a bead, a
micelle, a capsule, a
hydrogel), a composition with industrial utility (e.g., a dielectric), an
adhesive (i.e., a material
that forms an adhesive bond; e.g., glue, wallpaper adhesive, wood adhesive,
paper adhesive,
cork adhesive, chipboard adhesive, surgical/medical glue, cement, mucilage,
paste), a coating
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
or facing (e.g., glossy coating, protective coating, varnish, coating for
medical tablet, paper
coating, painting, leather finishing, textile coating), a spray, a paint or
ink or pigment binder
for ink, a hard plastic (e.g., bottle, button, window, pen), a medium hard
plastic (e.g., bottle,
fiber [e.g., yarn1), a soft plastic (e.g., bag, wrap, edible film, waterproof
film, contact lens,
packaging material), a fabric (e.g., textile, carpet, curtain, clothing), an
industrial polymer
(i.e., compound used in the manufacture of synthetic industrial materials), a
medical diagnostic
(see, for example, J. Berger et al. 2004. Europ J of Pharm and Biopharm 57:19,
respectively),
a gel (e.g., hydrogel for controlled release of a therapeutic, hydrogel for
immobilizing a protein
(e.g., enzyme)), an implant (e.g., bone-replacing composite, material
supporting nerve repair,
scaffold for growing cells, prosthetic implant), an article of clothing (e.g.,
shoe), a lubricant, a
piece of furniture, a paper (e.g., paper sheet, paper label, packaging paper,
photographic
support), a household item (e.g., pot, bowl, plate, cup), and a biological
scaffold (i.e., a structure
that mimics a biological matrix, sutures, bone-replacing material, material
supporting nerve
repair, scaffold for growing cells, prosthetic implant, membrane for promoting
wound healing,
wound dressing, tissue-engineering scaffolding).
Food Product
[0266] The composition according to any of the above may be a
food product.
[0267] The term "food product" as used herein refers to a
composition that can be ingested
by a human or an animal for dietary purposes (i.e., without ill health effects
but with significant
nutritional and/or caloric intake due to uptake of digested material in the
gastrointestinal tract),
including a domesticated animal (e.g., dog, cat), farm animal (e.g., cow, pig,
horse), and wild
animal (e.g., non-domesticated predatory animal). The term includes
compositions that may be
combined with or added to one or more other ingredients to make a food product
that can be
ingested by a human or an animal.
[0268] The food product may be a supplemented food product
(i.e., a conventional food
product that is supplemented with the recombinant component according to any
of the above),
or may be a substitute food product (i.e., a food product that resembles a
conventional food
product and that can be used in place of the conventional food product),
selected from any of
the food product categories defined by the National Health and Nutrition
Examination Survey
(NHANES).
[0269] Non-limiting examples of NHANES food product categories
include snack foods
and gums (e.g., snack bars, crackers, salty snacks from grain products,
chewing gums); breads,
grains, and pastas (e.g., oat breads and rolls, cornbread, corn muffins,
tortillas, flour and dry
mixes, biscuits, multi-grain breads and rolls, whole wheat breads and rolls,
pastas, rye breads
81
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
and rolls, cracked wheat breads and rolls, white breads and rolls); beverages
(e.g., beers and
ales, beverage concentrates, beverages, energy drinks, sports drinks, fluid
replacements, soft
drinks, carbonated beverages, juices, wines, beers, cocktails, nutrition
drinks, nutrition
powders, protein-enriched beverages, coffee, tea); sweets and desserts (e.g.,
cakes, candies,
chips, cookies, cobblers, pastries, ices or popsicles, muffins, pies, sugar
replacements or
substitutes, syrups, honey, jellies, jams, preserves, salads, crepes, Danish,
breakfast pastries,
doughnuts); breakfast foods (e.g., cereal grains, cereal, rice, French toast,
pancakes, waffles,
coffee cake); salad dressings, oils, sauces, condiments (e.g., cooking fats,
vegetable oils, salad
dressings, tomato sauces, gravies); potatoes (e.g., potato salad, potato
soups, chips and sticks,
fried potatoes, mashed potatoes, stuffed potatoes, puffs); and soups (e.g.,
vegetable soups,
vegetable broths), meals, main dishes, proteins (e.g., meat substitutes), and
seafoods.
[0270]
The food product according to any of the above may be a supplemented dairy
product (i.e., a conventional dairy product that is supplemented with the
recombinant
component according to any of the above) or a substitute dairy product (i.e.,
a food product that
resembles a conventional dairy product). The term "dairy product" as used
herein refers to milk
(e.g., whole milk [at least 3.25% milk fat], partly skimmed milk [from 1% to
2% milk fat], skim
milk [less than 0.2% milk fat], cooking milk, condensed milk, flavored milk,
goat milk, sheep
milk, dried milk, evaporated milk, milk foam), and products derived from milk,
including but
not limited to yogurt (e.g., whole milk yogurt [at least 6 grams of fat per
170 g], low-fat yogurt
[between 2 and 5 grams of fat per 170 g], nonfat yogurt [0.5 grams or less of
fat per 170 g],
greek yogurt [strained yogurt with whey removed], whipped yogurt, goat milk
yogurt, Labneh
[labne], sheep milk yogurt, yogurt drinks [e.g., whole milk Kefir, low-fat
milk Kefir], Lassi),
cheese (e.g., whey cheese such as ricotta; pasta filata cheese such as
mozzarella; semi-soft
cheese such as Havarti and Muenster; medium-hard cheese such as Swiss and
Jarlsberg and
halloumi; hard cheese such as Cheddar and Parmesan; washed curd cheese such as
Colby and
Monterey Jack; soft ripened cheese such as Brie and Camembert; fresh cheese
such as cottage
cheese, feta cheese, cream cheese, paneer, and curd), processed cheese,
processed cheese food,
processed cheese product, processed cheese spread, enzyme-modulated cheese;
cold-pack
cheese), dairy-based sauces (e.g., salad dressing, bechamel sauce, fresh
sauces, frozen sauces,
refrigerated sauces, shelf stable sauces), dairy spreads (e.g., low-fat
spread, low-fat butter),
cream (e.g., dry cream, heavy cream, light cream, whipping cream, half-and-
half, coffee
whitener, coffee creamer, sour cream, creme fraiche), frozen confections
(e.g., ice cream,
smoothie, milk shake, frozen yogurt, sundae, gelato, custard), dairy desserts
(e.g., fresh,
refrigerated, or frozen), butter (e.g., whipped butter, cultured butter),
dairy powders (e.g., whole
82
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
milk powder, skim milk powder, fat-filled milk powder (i.e., milk powder
comprising plant fat
in place of all or some animal fat), infant formula, milk protein concentrate
(e.g., milk protein
concentrate, whey protein concentrate, demineralized whey protein concentrate,
13-
lactoglobulin concentrate, a-lactalbumin concentrate, glycomacropeptide
concentrate, casein
concentrate), milk protein isolate (e.g., milk protein isolate, whey protein
isolate, demineralized
whey protein isolate, 0-lactoglobulin protein isolate, cc-lactalbumin protein
isolate,
glycomacropeptide protein isolate, casein protein isolate), nutritional
supplements, texturizing
blends, flavoring blends, coloring blends, ready-to-drink or ready-to-mix
products (e.g., fresh,
refrigerated, or shelf stable dairy protein beverages, weight loss beverages,
nutritional
beverages, sports recovery beverages, and energy drinks), puddings, gels,
chewables, crisps,
bars (e.g., nutrition bars, protein bars), and fermented dairy products (e.g.,
yoghurt, cheese, sour
cream, cultured buttermilk, cultured butter, cultured butter oil).
[0271]
The food product according to any of the above may be a supplemented
animal meat
or animal meat product (i.e., a conventional animal meat or animal meat
product that is
supplemented with the recombinant component according to any of the above
produced by the
recombinant host cell according to any of the above and/or a method according
to any of the
above) or a substitute animal meat or animal meat product (i.e., a food
product that resembles
a conventional animal meat or animal meat product). Non-limiting examples of
animal meats
and animal meat products include flesh obtained from skeletal muscle or from
other organs
(e.g., kidney, heart, liver, gallbladder, intestine, stomach, bone marrow,
brain, thymus, lung,
tongue), or parts thereof, obtained from an animal. The animal meat may be
dark or white meat.
Non-limiting examples of animals from which animal meat or animal meat product
can be
obtained include cattle, lamb, mutton, horse, poultry (e.g., chicken, duck,
goose, turkey), fowl
(e.g., pigeon, dove, grouse, partridge, ostrich, emu, pheasant, quail), fresh
or salt water fish
(e.g., catfish, tuna, spearfish, shark, halibut, sturgeon, salmon, bass,
muskie, pike, bowfin, gar,
eel, paddlefish, bream, carp, trout, walleye, snakehead, crappie, sister,
mussel, scallop, abalone,
squid, octopus, sea urchin, cuttlefish, tunicate), crustacean (e.g., crab,
lobster, shrimp,
barnacle), game animal (e.g., deer, fox, wild pig, elk, moose, reindeer,
caribou, antelope, zebra,
squirrel, marmot, rabbit, bear, beaver, muskrat, opossum, raccoon, armadillo,
porcupine, bison,
buffalo, boar, lynx, bobcat, bat), reptile (e.g., snakes, turtles, lizards,
alligators, crocodiles), any
insect or other arthropod, rodent (nutria, guinea pig, rat, mice, vole,
groundhog, capybara),
kangaroo, whale, and seal. The animal meat or animal meat product may be
ground, chopped,
shredded, or otherwise processed, and uncooked, cooking, or cooked.
83
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0272]
The food product according to any of the above may be a supplemented egg
product
(i.e., a conventional egg or egg product that is supplemented with the
recombinant component
according to any of the above) or a substitute egg or egg product (i.e., a
food product that
resembles a conventional egg or egg product). Non-limiting examples of eggs or
egg products
include whole egg (e.g., liquid whole egg, spray-dried whole egg, frozen whole
egg), egg white
(e.g., liquid egg white, spray-dried egg white, frozen egg white), egg yolk,
egg dishes, egg
soups, mixtures made with egg whites, mixtures made with egg substitutes,
mayonnaise,
custard, and salad dressings.
[0273]
Resemblance of a substitute food product provided herein to a conventional
food
product may be due to any physical attribute, chemical/biological attribute,
sensory attribute,
and functional attribute, and any combination thereof.
[0274]
The food product according to any of the above may be a pet food or animal
feed.
[0275]
The food product according to any of the above may be essentially free of
any
protein other than a recombinant protein or recombinant proteins comprised in
the composition
according to any of the above.
[0276]
The food product according to any of the above may be essentially free of
any
recombinant protein other than a recombinant protein or recombinant proteins
contained in the
composition according to any of the above.
[0277]
The food product according to any of the above may be essentially free of
any
recombinant milk protein other than a recombinant protein or recombinant
proteins contained
in the composition according to any of the above.
[0278]
The food product according to any of the above may be essentially free of
a
component found in a mammal-produced milk (e.g., cow milk, goat milk, sheep
milk, human
milk, buffalo milk, yak milk, camel milk, llama milk, alpaca milk, horse milk,
donkey milk),
or may comprise a lower concentration of at least one component found in a
mammal-produced
milk. Non-limiting examples of components found in mammal-derived milk include
lactose,
saturated fat, cholesterol, native milk proteins, and native milk lipids. The
food product may be
essentially free of any milk protein other than a recombinant milk protein or
recombinant milk
proteins contained in the composition according to any of the above.
[0279]
The food product according to any of the above may be essentially free of
a
component obtained from an animal (i.e., a component that is native to an
animal, including
animal products [i.e., parts of an animal that are consumables or typically
prepared for
consumption by humans; e.g., animal meat, animal fat, animal blood], animal
byproducts [i.e.,
products that are typically not consumable by themselves but are the
byproducts of slaughtering
84
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
animals for consumption; e.g., animal bones, animal carcasses, and
constituents isolated
therefrom], products produced by an animal [e.g., mammal-derived milk, chicken
eggs, bee
honey], and consumables produced therefrom [e.g., gelatin, rennet, whey
proteins extracted
from mammal-derived milk, casein extracted from mammal-derived milk, milk
lipid extracted
from mammal-derived milk, animal lipids, animal proteins]), or comprise 2% or
less by mass
of such component.
[0280]
A variety of recipes exist for preparing a food product, and any such
recipe may be
used to produce a food product according to any of the above. The recombinant
component
may be used in such recipe in purified/isolated form or comprised in a
fermentation broth or
preparation obtained by a method according to any of the above.
Cosmetic or Personal Care Product
[0281]
The composition according to any of the above may be a cosmetic or
personal care
product.
[0282]
The term "cosmetic or personal care product" as used herein refers to a
composition
that upon application to a body surface (i.e., an exposed area of a human
body, such as skin,
hair, nail, tooth, and tissues of the oral cavity [e.g., gums]) confers a
perceived or actual
beautifying or hygienizing effect. Non-limiting examples of cosmetic or
personal care products
include anti-wrinkling treatments (i.e., compositions used for tensioning
[e.g., smoothing out
of skin, reducing wrinkles in skin, removing fine lines in skin1), anti-aging
treatments (i.e.,
compositions used for removing signs of aging [e.g., wrinkles, fine lines,
manifestations of
photodamage (e.g., sun spots)1), sun protection (i.e., compositions used to
protect against UV
exposure), anti-burn treatments (i.e., compositions used for soothing burns
[e.g., sunburnsp,
anti-acne treatments (i.e., compositions that are effective in the treatment
of acne and/or the
symptoms associated therewith), skin cleansers (i.e., compositions used for
cleaning skin and/or
skin pores [e.g., nose strips for pore cleaning]), anti-dandruff treatments
(i.e., compositions
used for reducing or eliminating dandruff), anti-body odor treatments (i.e.,
compositions used
for reducing or eliminating body odor), self-tanning treatments (i.e.,
compositions used for
darkening skin color), skin whitening treatments (i.e., compositions used for
bleaching/depigmenting skin color), hair coloring treatments (i.e.,
compositions used for
coloring hair), lotions (e.g., skin lotions, body care lotions, wash lotions,
moisture retention
lotions, pre-shave lotions, after-shave lotions), pastes (e.g., washing
pastes), ointments, balms,
salves, masks, creams (e.g., water in oil creams, oil in water creams, day
creams, night creams,
eye creams, skin creams, face creams, anti-wrinkle creams, sun protection
creams, moisture
retention creams, after-shave creams, skin bleaching creams, self-tanning
creams, vitamin
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
creams, moisturizing creams, massage creams), milks (e.g., body milks,
cleansing milks), gels
(e.g., anhydrous gels, shower gels), Eau de Toilette, soaps (e.g., transparent
soaps, luxurious
soaps, deodorant soaps, cream soaps, baby soaps, skin protection soaps,
abrasive soaps,
syndets, pasty soaps, soft soaps, peeling soaps), skin peeling treatments,
liquid washes, shower
and bath preparations (e.g., wash lotions, shower baths, shower gels, foam
baths, oil baths,
scrub preparations), foams (e.g., shaving foams, foam baths), deodorants, hair
care products
(e.g., shampoos, conditioners, hair mousses, hair colorants, hair sprays,
rinse-off lotions, hair
gels, hair emulsions, hair laquers, hair tonics), lip glosses, sprays (e.g.,
hair sprays, pump
sprays, sprays containing blowing agents), treatments for skin defects (e.g.,
dermatitis, weals,
chaps, blemishes, cracks, scars, freckles, moles, rashes, blisters, pustules),
toners, cleaning
tissues, sanitary towels, tampons, nappies, repellents, make-up products
(e.g., studio pigments,
mascara, eye shadows, eyeliners, eye liner pens, rouges, face powders, eyebrow
pencils,
lipsticks, foundations, tinted creams, concealer sticks, blemish sticks,
blushes), sticks (e.g.,
lipsticks, concealer sticks, blemish sticks), hair removing agents, hand
cleaning products,
intimate hygiene products, foot care products, baby care products, and oral
hygiene products
(e.g., chewing gums, mouthwashes, toothpastes, gum-cleaning agents, denture
adhesives,
denture fixatives).
[0283]
The cosmetic or personal care product according to any of the above may be
essentially free of any protein other than the recombinant protein or the
recombinant proteins
contained in the composition according to any of the above.
[0284]
The cosmetic or personal care product according to any of the above may be
essentially free of any recombinant protein other than the recombinant protein
or the
recombinant proteins contained in the composition according to any of the
above.
[0285]
The cosmetic or personal care product according to any of the above may be
essentially free of any recombinant milk protein other than the recombinant
protein or the
recombinant proteins contained in the composition according to any of the
above.
[0286]
The cosmetic or personal care product according to any of the above may be
essentially free of a component found in a mammal-produced milk (e.g., cow
milk, goat milk,
sheep milk, human milk, buffalo milk, yak milk, camel milk, llama milk, alpaca
milk, horse
milk, donkey milk), or may comprise a lower concentration of at least one
component found in
a mammal-produced milk. Non-limiting examples of components found in mammal-
derived
milk include lactose, saturated fat, cholesterol, native milk proteins, and
native milk lipids. The
cosmetic or personal care product may be essentially free of any milk protein
other than the
86
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
milk protein comprised in the recombinant protein or the recombinant proteins
contained in the
composition according to any of the above.
[0287]
The cosmetic or personal care product according to any of the above may be
essentially free of a component obtained from an animal (i.e., a component
that is native to an
animal, including animal products [i.e., parts of an animal that are
consumables or typically
prepared for consumption by humans; e.g., animal meat, animal fat, animal
blood], animal
byproducts [i.e., products that are typically not consumable by themselves but
are the
byproducts of slaughtering animals for consumption; e.g., animal bones, animal
carcasses, and
constituents isolated therefrom], products produced by an animal [e.g., mammal-
derived milk,
chicken eggs, bee honey], and consumables produced therefrom [e.g., gelatin,
rennet, whey
proteins extracted from mammal-derived milk, casein extracted from mammal-
derived milk,
milk lipid extracted from mammal-derived milk, animal lipids, animal
proteins1), or comprise
2% or less by mass of such component.
[0288]
The cosmetic or personal care product according to any of the above may be
essentially free of a component derived from petroleum.
Recombinant Host Cell Producing FFA Releasing Enzyme Activity
[0289]
In various aspects, provided herein is a recombinant host cell that
comprises a
recombinant expression construct encoding a FFA releasing enzyme, and that
comprises an
increased production and/or activity of the FFA releasing enzyme compared to a
corresponding
host cell (i.e., a host cell that is essentially identical to the recombinant
host cell except that it
does not comprise the recombinant expression construct encoding the FFA
releasing enzyme).
[0290]
The recombinant host cell may be derived from any wild type unicellular
organism,
including any bacterium, yeast, filamentous fungus, archaea, unicellular
protista, unicellular
animals, unicellular plants, unicellular algae, protozoa, and chromista, or
from a genetic variant
(e.g., mutant) thereof, as well as from any generally recognized as safe
(GRAS) industrial host
cell, and including any organism disclosed herein (e.g., Triehoderma reesei,
Aspergillus niger,
Trichoderma ciirinoviride,Nlyceliophihora thermophila).
[0291]
The recombinant host cell according to any of the above may comprise an
increased
production and/or activity of a FFA releasing enzyme activity selected from
the group
consisting of FFA releasing enzymes comprising UniProt sequence# GORH85,
GOR6T6,
GOR6X2, G0R707, GOR7K1, GOR810, GOR9D1, GOR9F9, GOR9J9, GOR9X3, GORBGO,
GORBJO, GORBM4, GORBZ6, GORD16, GORDK5, GORDU7, GOREM9, GOREZ4, GORFR3,
GORFT3, GORG04, GORG60, GORGD5, GORGN7, GORGQO, GORGQ7, GORHJ4, G0RI29,
GOR119, GORIU1, GORIV5, GORI76, GORJC6, GORTY0, GORK83, GORKE6, GORKH7,
87
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
GORKI9, GORKL4, GORL87, GORLBO, GORLB7, GORLH4, GORLLO, GORLR3, GORM14,
GORME5, GORMI3, GORNF8, GORPQ8, GORQD1, GORQG3, GORQ1-5, GORQN5, GORR42,
GORRK3, GORRQ4, GORSK7, GORTR6, GORTT4, GORUIO, GORUZ9, GORV93, GORW73,
GORW77, GORWS1, GORWT9, GORWY5, GORX82, GORX90, GORHQ7, GORVD2, and
GOR8A6, and homologs thereof, and combinations thereof; wherein the increased
production
and/or activity of the FFA releasing enzyme is an increase in production
and/or activity of at
least 5%, at least 10%, at least 15%, at least 20%, at least 25%, at least
50%, at least 75%, at
least 100%, at least 150%, at least 200%, at least 300%, at least 400%, at
least 500%, at least
600%, at least 700%, at least 800%, at least 900%, or at least 1,000%.
[0292]
In various aspects, provided herein is a method for obtaining a
recombinant host cell
that comprises a recombinant expression construct encoding a FFA releasing
enzyme, wherein
the method comprises: i) obtaining a polynucleotide according to any of the
above, or a
recombinant expression construct according to any of the above, or a
recombinant vector
according to any of the above, wherein the recombinant protein coding sequence
of the
polynucleotide, recombinant expression construct, or recombinant vector
encodes a FFA
releasing enzyme activity selected from the group consisting of 1-1-A
releasing enzymes
comprising UniProt sequence# GORH85, GOR6T6, GOR6X2, G0R707, GOR7K1, GOR810,
GOR9D1, GOR9F9, GOR9J9, GOR9X3, GORBGO, GORBJO, GORBM4, GORBZ6, GORD16,
GORDK5, GORDU7, GOREM9, GOREZ4, GORFR3, GORFT3, GORG04, GORG60, GORGD5,
GORGN7, GORGQO, GORGQ7, GORHJ4, G0R129, GURU 9, GORIU1, GOR1V5, GORJ76.
GORJC6, GORTY0, GORK83, GORKE6, GORKH7, GORKI9, GORKL4, GORL87, GORLBO.
GORLB7, GORLH4, GORLLO, GORLR3, GORM14, GORME5, GORMI3, GORNF8, GORPQ8,
GORQD1, GORQG3, GORQJ5, GORQN5, GORR42, GORRK3, GORRQ4, GORSK7, GORTR6,
GORTT4, GORUIO, GORUZ9, GORV93, G0RW73, GORW77, GORWS1, GORWT9, GORW Y5,
GORX82, GORX90, GORHQ7, GORVD2, and GOR8A6, and homologs thereof; and ii)
introducing the polynucleotide, expression construct, or recombinant vector
into a host
cell (e.g., any of the host cells disclosed herein; using any of the methods
disclosed herein) to
obtain a recombinant host cell comprising an increased production and/or
activity of the FFA
releasing enzyme.
[0293]
In various aspects, provided herein is a method for producing a FFA
releasing
enzyme, wherein the method comprises: obtaining a recombinant host cell that
comprises a
recombinant expression construct encoding the FFA releasing enzyme, and that
comprises an
increased production and/or activity of the FFA releasing enzyme compared to a
corresponding
host cell; culturing the recombinant host cell in a culture medium under
conditions suitable for
88
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
production and/or secretion of the FFA releasing enzyme; and optionally
purifying the FFA
releasing enzyme.
EXAMPLES
[0294]
The following examples are included to illustrate specific embodiments of
this
disclosure. The techniques disclosed in the examples represent techniques
discovered by the
inventors to function well in the methods and processes of this disclosure;
however, those of
skill in the art should, in light of the present disclosure, appreciate that
many changes can be
made in the specific embodiments that are disclosed and still obtain a like or
similar result
without departing from the spirit and scope of the disclosure. Therefore, all
matter set forth or
shown in the examples is to be interpreted as illustrative and not in a
limiting sense.
Example 1: Expression Analysis of Recombinant Protein Preparations
[0295]
The presence of GORMI3, GORGQO and GORLH4 mRNA transcripts in various
recombinant Trichoderma reesei host cells (i.e., various Trichoderma reesei
host cells
comprising a recombinant expression construct encoding Bos taurus 13-
lactoglobulin) was
determined by fermenting the recombinant Trichoderma reesei host cells in 2 L
tanks under a
variety of conditions suitable for production and secretion of the recombinant
P-lactoglobulin.
Biomass samples were taken at various time points during fermentations, and
flash frozen in
liquid nitrogen. RNA was extracted from the samples, the RNA quality was
checked with
agarose gel electrophoresis, and the RNA was submitted for RNA sequencing and
read
processing/analysis. Expression levels were assessed for transcripts encoding
GORMI3,
GORGQO, and GORLH4 proteins.
[0296]
As shown in Figures 1A and 1B, fermentations of recombinant Trichoderma
reesei
host cells producing recombinant protein showed detectable expression of
GORMI3, GORGQO,
and GORLH4 transcripts.
Example 2: Removal of FFA Releasing Activity via Inhibition
[0297]
A recombinant Trichoderma reesei host cell capable of producing a
recombinant13-
lactoglobulin and comprising an essentially eliminated cutinase (e.g., cutl
(UniProt#
G0RH85)) activity ("cutinase knockout recombinant host cell") was generated
and fermented
as described in PCT patent publication W02020/081789. Recombinant 13-
lactoglobulin was
isolated from clarified fermentation broths of the recombinant Trichoderma
reesei host cell
based on charge (i.e., electrostatic interaction), and spray dried to obtain a
13-lactoglobulin
powder preparation.
89
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
[0298] To remove from the 13-lactog1obulin powder preparation
activity of 1-1-A releasing
enzymes that comprise a serine residue, such as GORMI3, GORGQO, and GORLH4
proteins,
the powder preparation was dissolved in water to a final concentration of 40
g/L proteins. Two
1-mL samples of the solution were taken as a Sample 1 and a Sample 2. To
Sample 1 was added
20 .1_, of dimethyl sulfoxide (DMSO) to a final concentration of 2%. To
Sample 2 was added
20 litL of 0.1 mM phosphonate substrate inhibitor Thermo ActivX TAMRA-FP
fluorophosphonate (ThermoFisher Scientific catalog number 88318; a member of
the
phosphonate inhibitors of hydrolases comprising a nucleophilic serine in their
active site
(Simon & Cravatt. 2010. J. Biol. Chem. 285(15):11051-11055)) in DMSO to a
final
concentration of 2 'LEM Thermo ActivX TAMRA-FP fluorophosptionate and 2% DMSO.
The
samples were incubated for 18.5 hours at 21 C.
[0299] Para-phenyl (pNP) acyl ester hydrolyzing enzyme
activities comprised in Sample 1
and Sample 2 were determined by first diluting each sample with equal volume
of 0.4 M 4-(2-
hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7, then diluting
serially up to
1/128 fold with 0.2 M HEPES pH7, mixing with equal volumes of 0.3 mM pNP acyl
ester
(pNP-butyrate, pNP-octanoate, pNP-laurate, or pNP-palmitate) in 10% DMSO, and
incubating
at 30 C. The reaction mixtures were measured hourly (from 1 to 6 hours) for
absorbance at 348
nm by a SpectraMax M3 plate reader from Molecular Devices (San Jose,
California) in UV-
star plates from Greiner Bio-one (Monroe, North Carolina; catalogue number
G55801).
[0300] As shown in Figure 2, Sample 1 produced para-nitrophenol
from the hydrolysis of
the four pNP acyl esters, which co-produced corresponding FFAs and is
indicative of the
presence of a lipase (as an esterase) activity in Sample 1. Such generation of
1-FAs (i.e., fatty
acid ester hydrolyzing enzyme activity) was essentially eliminated in Sample
2.
Example 3: Removal of Esterase Activity via Purification
[0301] A recombinant Trichoderma reesei host cell capable of
producing a recombinant 13-
lactoglobulin and comprising an essentially eliminated cutinase (e.g., cutl
(UniProt#
GORH85)) activity ("cutinase knockout recombinant host cell") was generated
and fermented
as described in PCT patent publication W02020/081789. Recombinant P-
lactoglobulin was
isolated from clarified fermentation broths of the recombinant Trichoderma
reesei host cell
based on charge (i.e., electrostatic interaction), and spray dried to obtain a
recombinant 13-
lactoglobulin powder preparation.
[0302] The recombinant 0-lactoglobulin powder preparation was re-
dissolved in water to
40 or 200 g/L concentration, and 2.5 or 7.5 mL, respectively, of the solution
was reacted with
80 or 20 vit of 0.1 mM ActivXTM desthiobiotin-fluorophosphonate serine
hydrolase probe
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
(DTB-FP, catalog number 88317, ThermoFisher Scientific, Waltham, MA) in DMSO
at 21 C.
DTB-FP covalently binds FFA releasing enzymes that comprise a nucleophilic
serine in their
active sites by bonding to the phosphorus and the side chain oxygen of the
serine. After 8 hours,
100 1_, of hydrated PierceTM high capacity streptavidin agarose (SA-A;
ThermoFisher
Scientific, Waltham, MA) was added, and the reaction suspension was incubated
at 21 C (FFA
releasing enzymes with attached DTB-FP bind to SA-A via biotin-avidin
binding). After 21
hours, the suspension was centrifuged for 2 minutes at 1,000 relative
centrifugal force (rce,
and the supernatant (comprising the recombinant fl-lactoglobulin) was
separated from the pellet
(comprising bound FFA releasing enzymes that comprise a serine residue in
their catalytic
domain, such as GORMI3, GORGQO, and GORLH4 proteins).
Example 4: Production of Recombinant GORMI3, GORGQO and GORLH4 Proteins
[0303]
For recombinant GORLH4 and GORGQO protein production in Trichoderma
reesei,
recombinant vectors were constructed using genetic engineering methods known
in the art. The
general structure of the recombinant vectors is shown in Figure 3. The
recombinant vectors
comprised a recombinant expression construct comprising a protein coding
sequence
encoding GORLH4 or GORGQO protein, operably linked to a N-terminal GORLH4 or
GORGQO
native secretion signal sequence, respectively, and under control of a pSES
promoter and a pdc1
terminator. The recombinant expression construct further comprised a
polynucleotide encoding
a synthetic transcription factor to drive expression of the GORLH4 or GORGQO
expression
constructs. 'the recombinant vector further comprised a polynucleotide that
could direct
integration of the recombinant expression construct at the egll locus in the
genome of
a Trichoderma reesei host cell, selection markers for selection of bacterial
and/or fungal
transfonnants, and a bacterial origin of replication. The bacterial selection
markers and origin
of replication were removed from the recombinant vector via restriction enzyme
digestion prior
to transformation of the recombinant vector into a Trichoderma reesei host
cell.
[0304]
For recombinant GORMI3 protein production in Pichia pastoris, a
recombinant
vector was constructed using genetic engineering methods known in the art. The
general
structure of the recombinant vector is shown in Figure 4. The recombinant
vector comprised a
recombinant expression construct comprising a protein coding sequence encoding
GORMI3
protein, operably linked to a N- terminal pre-pro secretion signal sequence of
the alpha mating
factor of Saccharomyces cerevisiae and to a C-terminal 6x-His tag, under
control of an A0X1
methanol-inducible promoter and terminator. The recombinant vector further
comprised a
selection marker for selection of bacterial and/or fungal transformants, and a
bacterial origin of
replication. The bacterial selection markers and origin of replication were
removed from the
91
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
recombinant vector via restriction enzyme digestion prior to transformation of
the recombinant
vector into a Pichia pastoris host cell.
[0305]
The recombinant vectors were transformed into Trichoderma reesei or Pichia
pastoris host cells (e.g., by using a heat-shock protocol), and transformants
were selected by
growth on minimal media or antibiotics for positive selection. The
transformants were grown
in expression media in 24-well plates, and supernatants were harvested for
further analysis.
Recombinant host cells that comprised an integrated copy of a recombinant
expression
construct according to any of the above, and that secreted recombinant GORMI3,
GORLH4, or
GORGQ protein, were identified by SDS-PAGE or Western blot gel analyses of
fermentation
broth samples.
[0306]
The recombinant GORMI3 protein was purified from fermentation broth using
an
affinity column (e.g., a HisTrap HP column (GE Healthcare, Piscataway, NJ)),
and then eluted
at 0.16 mg/mL concentration (as determined by Bradford assay) in buffered
solution of 50 mNI
Tris-HCL, 150 mM NaCL, 10% glycerol at pH8.
[0307]
The recombinant GORGQO and GORLH4 proteins were not purified, but rather
supernatants from 5 day old shake flasks were used in subsequent experiments.
Shake flasks
were inoculated with 1x108 spores into 100m1 of shake flask minimal media.
[0308]
As shown in Figures 5A-5C, the recombinant strains produced GORMI3,
GORGQO,
and GORLH4 proteins.
Example 5: Analysis of FFA Releasing Activity of GORMI3, GORGQO, and GORLH4
Proteins
Using Lipolytic Assay
[0309]
141-A releasing activities of GORMI3, GORGQO, and GORLH4 proteins were
demonstrated using a method based on detection of fluorescent signal caused by
FFA binding
and concentration of rhodamine B (Kouger & Jaeger. 1987. Appl Env Microbiol.
53(1):211-
213). To this end, wells of a 24-well culture plate were each filled with 3 mL
of an agar gel
comprising 1% weight by volume agar, 10 mL/L of sunflower coconut oil blend,
and 5 mg/L
rhod amine B. Onto the agar was then pipetted a 10 uL sample of the GORMI3
protein
preparation of Example 3, a 20 uL sample of the GORGQO or GORLH4 culture
supernatant of
Example 3, or an equivalent volume of a negative control (i.e., supernatant of
a Trichoderma
reesei strain that did not comprise any of the expression constructs of
Example 3). The 24-well
plate was incubated at 30 C for 48 hours, and then illuminated with a UV
transilluminator to
determine whether FFAs were present.
[0310]
As shown in Figure 6, fluorescent signal was observed for wells containing
GORMI3, GORGQO, or GORLH4 protein, but not from negative control wells,
indicating that
92
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
GORMI3, GORGQO, and GORLH4 proteins can cause enzymatic release of FFAs from
sunflower/coconut oil substrate.
Example 6: Analysis of FFA Releasing Activity of GORMI3 Protein Using Ice
Cream Rancidity
Assay
[0311]
FBA releasing activities of GORMI3 protein was demonstrated by evaluating
ice
creams produced from a preparation comprising the protein for rancidity. To
this end, an ice
cream mix was produced that comprised sugar, maltodextrin, salt, minerals,
gum, and Bus
taurus whey protein isolate dissolved in water. To this mix, 16% by mass of
lipids (i.e.,
emulsified mono- and diglycerides obtained from soy, melted coconut oil, and
melted
sunflower oil) were added, and the mixture was blended at 20,000 rpm for 30
seconds in an
Ultraturrax (IKA Works, Wilmington, NC). The blend was transferred to a Hot
Mix Pro
container equipped with scrape surface paddle, and incubated with a hold temp
of 82 C and
hold time of 25 seconds, on 90 rpm. The finished ice cream base was
pasteurized and
homogenized (2-stage: 180 /30 bar = 210 bar), before approximately 207 g of it
was added to
a 250 mL Schott bottle, and incubated in a water bath at 55 C. To the blend
was added 15ug of
GORMI3 protein, and the mixture was mixed using an Ultraturrax at 16,000 rpm
for at least 60
seconds, and then incubated in a water bath at 55 C. After 4 hours, the
samples were cooled
and stored at 4 C for 7-8 days. The ice cream was evaluated by sensory experts
at time points
0 hour, 4 hours, 24 hours, and 6-8 days. Samples that comprised the GORMI3
protein were
found to have rancid smell and/or taste.
Example 7: Analysis of FFA Releasing Activity of GORMI3 Protein Using Yogurt
Rancidity
Assay
[0312]
FBA releasing activities of GORMI3 protein was demonstrated by evaluating
yogurts produced from preparations comprising the proteins for rancidity. To
this end, a first
mixture of 50 mg of YCX-11 yogurt culture (Chr. Hansen Inc., Horsholm,
Denmark) and 80 g
of whole milk was mixed on a stir plate at low speed for at least 10 min or
until granules were
fully hydrated. A second mixture of 24 ug purified GORMI3 protein and 30 g of
whole milk
was mixed in an IKA Ultra Turrax TubeDrive (IKA Works, Wilmington, NC) for 3
min at
2,000 rpm. An 18.5 g aliquot of the first mixture was added to the second
mixture (final
concentration of 0.025% w/w/ yogurt culture), and the combined mixture was
mixed in the IKA
Ultra Turrax TubeDrive for 1 min at 2000 rpm. The sample was then poured into
a clean 80
mL glass Weck jar and seaedl with 60 mm lid and gasket, and incubated at 44 C
for 4 hours (or
until a pH of 4.6 +/- 0.1 was reached) to obtain a yogurt. After 24 hours,
after 3-4 days, and
after 6-8 days, the yogurt was evaluated by sensory experts for the presence
of rancid smell
93
CA 03198703 2023- 5- 12
WO 2022/104227
PCT/US2021/059413
and/or taste. From the 24-hour time point onwards, yogurts produced using the
GORMI3 protein
powders were judged as rancid.
Example 8: Removal of Esterase Activity via Genetic Modification
[0313]
Recombinant Trichoderma reesei host cells capable of producing a
recombinant 13-
lactoglobulin and comprising essentially eliminated FFA releasing activities
of GORH85 and
GORMI3 (double deficient); GORH85, GORMI3, and GORGQO (triple deficient);
GORH85,
GORMI3, and GORLH4 (triple deficient); GORH85, GORMI3 and GORLH4 (triple
deficient);
and GORH85, GORMI3, GORGQO, and GORLH4 (quadruple deficient) were generated by
transforming protoplasts of a Trichoderma reesei strain capable of producing a
recombinant 13-
lactoglobulin ("corresponding recombinant host cell") with polynucleotides
(targeting vectors)
engineered to integrate by homologous recombination a selectable marker into
genes encoding
GORH85, GORMI3, GORGQO, and/or GORLH4 proteins. The general structure of the
targeting
vector is shown in Figure 7. The targeting vector comprised a selective marker
(pyr4 gene,
which enables growth without uracil supplementation) flanked by polynucleotide
sequences
that are homologous to the upstream and downstream polynucleotide sequences
flanking the
genes open reading frame of each gene in the Trichoderma reesei genome.
Multiple gene
replacements were completed by targeting the loci sequentially, and by
recycling marker after
each round.
[0314]
Transformants were selected on minimal media, and then screened by PCR to
identify a lipase knockout recombinant host cell. Lipase knock out recombinant
host cells were
"cured" of the selective marker by plating cells to 5-fluoroorotic acid
containing media.
"Cured" cells were taken for sequential rounds of transformation/curing until
a strain in which
all four gene open reading frames were knocked out was obtained.
[0315]
The final knockout recombinant host cell and the corresponding recombinant
host
cell were fermented in a culture medium suitable for growth of the recombinant
host cells and
for production and secretion of the recombinant P-lactoglobulin.
What is claimed is:
94
CA 03198703 2023- 5- 12