Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/094124
PCT/US2021/057111
CO2 Separation Systems and Methods
CROSS REFERENCE TO RELATED APPLICATION
This application claims priority to and the benefit of U.S.
Provisional Patent Application Serial No. 63/106,729 filed October 28,
2020, entitled "Building Emission Processing and/or Sequestration
Systems and Methods"; U.S. Provisional Patent Application Serial No.
63/106,759 filed October 28, 2020, entitled "Building Emission
Processing and/or Sequestration Systems and Methods"; and U.S.
Provisional Patent Application Serial No. 63/106,862 filed October 28,
2020, entitled "Building Emission Processing and/or Sequestration
Systems and Methods", the entirety of each of which is incorporated by
reference herein.
TECHNICAL FIELD
The field of the invention relates to CO2 separation systems and
methods. In particular embodiments, the systems and/or methods can
separate CO2 and provide heat and/or power.
In particular
implementations, the heat and power can be provided to buildings.
Also, combustion products can be processed to separate CO2 from the
combustion products. Additionally, CO2 can be separated from air.
Further, power can be generated in the form of electricity while
separating CO2 from combustions products and/or air.
BACKGROUND
Carbon dioxide generation in buildings, particularly in large
metropolitan areas, is a significant contributor to carbon dioxide
generation overall.
Carbon dioxide is currently listed as a global
warming compound whose reduction is sought worldwide. The
generation of carbon dioxide is a necessary part of respiration, which
is a necessary part of life, but it is important to limit the generation of
carbon dioxide in an effort to address climate change. The present
1
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
disclosure provides power systems as well as power systems and
methods for building emission processing and sequestration systems
that can address carbon dioxide generation from combustion of fossil
fuels and proliferation thereof in metropolitan areas. Additionally, the
CO2 separation systems and methods of the present disclosure can
separate CO2 from combustion products and/or air, and, in some
embodiments, generated power in the form of electricity.
SUMMARY
Systems for separating CO2 from a combustion product are
provided. The systems can include: a combustion product stream; the
combustion stream comprising at least CO2 and N2; and a carbonate
electrochemical cell operatively aligned with the combustion stream
and configured to react the CO2 from the combustion product stream
and 02 to form carbonate ion and react the carbonate ion to form a CO2
product stream.
Methods for separating CO2 from a combustion product stream
are also provided. The methods can include: receiving a combustion
product stream comprising CO2 and N2, reacting the combustion
product stream to form carbonate ion; and reacting the carbonate ion
to form a CO2 product stream.
Systems for separating CO2 from air are also provided. The
systems can include: an air stream with the air stream comprising at
least CO2 and N2; and a carbonate fuel cell operatively aligned with the
air stream and configured to react the CO2 from the air stream and 02
to form carbonate ion and react the carbonate ion to form a CO2 product
stream.
Systems or methods for operating a combustion boiler within a
building are provided. The systems or methods can include: providing
air and fuel to a combustion burner; combusting the air and fuel within
the combustion burner; monitoring the amount of free oxygen in the
burner; and controlling the amount of air and fuel provided to the burner
2
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
to maintain a free oxygen amount of about 3%. The systems or
methods can include: combusting air and fuel within a burner to produce
flue gas having an oxygen concentration; and restricting air from the
flue gas by substantially eliminating tramp air within the conduit
operably aligned to convey flue gas from the burner.
Systems or methods for cooling flue gas from a combustion boiler
within a building are provided. The systems or methods can include
providing the flue gas to at least one economizer having at least one
set of cooling coils conveying the boiler feed water, the providing
cooling the flue gas and heating the boiler feed water.
Systems or methods for separating carbon dioxide from flue gas
generated from a combustion boiler within a building are provided. The
systems or methods can include: providing flue gas comprising less
than about 3% water; compressing the flue gas; and cooling the
compressor with a heat transfer fluid and providing the heat transfer
fluid to/from a chiller and/or a cooling tower. The systems or methods
can include: compressing the flue gas; and drying the flue gas using
nitrogen recovered during separation of carbon dioxide recovered from
the flue gas. The systems or methods can include: removing at least
some of the nitrogen from the flue gas to produce greater than about
95% carbon dioxide using a pressure swing adsorption assembly; and
using the nitrogen removed from the flue gas to remove water from the
flue gas before providing the flue gas to the pressure swing adsorption
assembly. The systems or methods can include: removing at least
some of the nitrogen from the flue gas to produce greater than about
95% carbon dioxide using a pressure swing adsorption assembly; and
providing at least some of the nitrogen removed from the flue gas to a
gas expander/generator. The systems or methods can include:
removing at least some of the nitrogen from the flue gas to produce
greater than 95% carbon dioxide using a pressure swing adsorption
assembly; and providing the at least some of the nitrogen removed from
the flue gas to both a dryer and an expander/generator, or to a dryer
3
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
and control valve. The control valve may or may not be equipped with
a silencer.
System or methods for cooling carbon dioxide separated from flue
gas generated from a combustion boiler within a building are provided.
The system or methods can include: separating nitrogen from flue gas
using a pressure swing adsorption assembly; expanding the nitrogen
through a turbine expander within the presence of a heat exchanger to
cool fluid within the heat exchanger; and transferring that cooled fluid
to another heat exchanger operably aligned with the carbon dioxide
product of the pressure swing adsorption assembly to cool the carbon
dioxide product.
System or methods for liquefying carbon dioxide separated from
flue gas generated from a combustion boiler within a building are
provided. The system or methods can include providing the gaseous
carbon dioxide through a sparge assembly into liquid carbon dioxide
within a storage vessel.
Buildings utilizing a carbon fuel source and generating carbon
emission upon combustion of the carbon fuel source are provided.
Building emissions can be operably coupled to a carbon capture
system, the system configured to separate and condense carbon
dioxide from the carbon emission. The system can be configured to
process the carbon emission and return heat to the building. The
system can be configured to process the carbon emission and generate
electricity. The system can be configured to process the carbon
emission and store electrical energy. The system can be configured to
dynamically control the combustion and capture systems to reduce
carbon combustion and increase carbon capture.
DRAWINGS
Embodiments of the disclosure are described below with
reference to the following accompanying drawings.
4
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
Fig. 1 is a carbon dioxide capture method and/or system
according to an embodiment of the disclosure.
Fig. 2 is a carbon dioxide capture method and/or system
according to another embodiment of the disclosure
Fig. 3A is an example boiler equipped with a free oxygen sensor
according to an embodiment of the disclosure.
Fig. 3B is a configuration of example boilers operably coupled to
a plenum according to an embodiment of the disclosure.
Fig. 3C is an example boiler equipped with a free oxygen sensor
according to an embodiment of the disclosure.
Fig. 4A is a portion of a carbon dioxide capture method and/or
system according to an embodiment of the disclosure.
Fig. 4B is a portion of a carbon dioxide capture method and/or
system according to another embodiment of the disclosure.
Fig. 4C is a portion of a carbon dioxide capture method and/or
system according to another embodiment of the disclosure.
Fig. 5A is an example configuration of a component of a carbon
dioxide capture method and/or system according to an embodiment of
the disclosure.
Fig. 5B is another example configuration of a component of a
carbon dioxide capture method and/or system according to an
embodiment of the disclosure.
Fig. 6 is a portion of a carbon dioxide capture method and/or
system according to an embodiment of the disclosure.
Fig. 7 is a portion of a carbon dioxide capture method and/or
system according to an embodiment of the disclosure.
5
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
Fig. 8 is a is an example configuration of a component of a carbon
dioxide capture method and/or system according to an embodiment of
the disclosure.
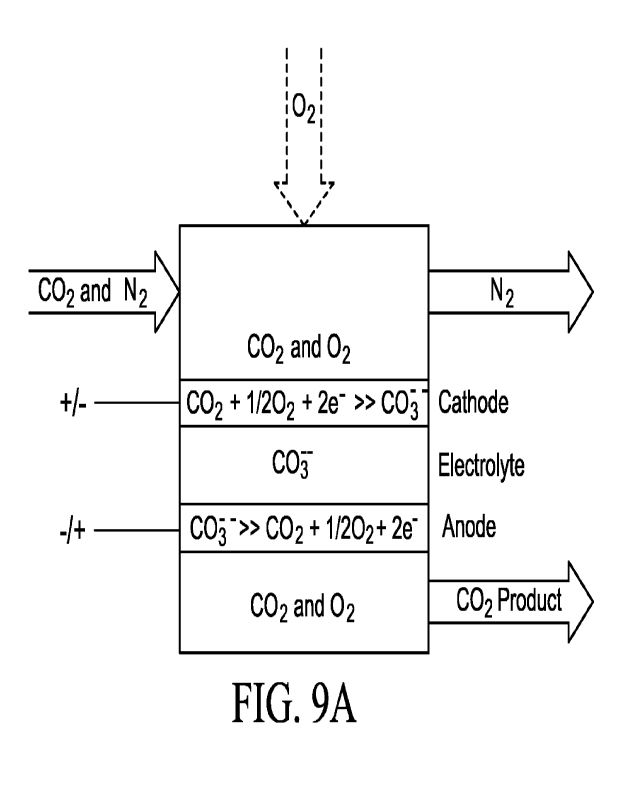
Fig. 9A is an example of a CO2 separation system according to
an embodiment of the disclosure.
Fig. 9B is an example CO2 separation system component
according to an embodiment of the disclosure configured to operate as
a carbonate pump.
Fig. 9C is an example CO2 separation system component
according to an embodiment of the disclosure configured to receive CO2
and N2 from a flue gas source and operate as a carbonate fuel cell.
Fig. 90 is an example CO2 separation system according to an
embodiment of the disclosure configured to receive CO2 and N2 from
an air source and operate as a carbonate fuel cell.
Fig. 10 is a portion of a carbon dioxide capture method and/or
system according to an embodiment of the disclosure.
Fig. 11 is a portion of a carbon dioxide capture method and/or
system according to another embodiment of the disclosure.
Fig. 12 is a portion of a carbon dioxide capture method and/or
system according to an embodiment of the disclosure.
Fig. 13A is a portion of a carbon dioxide capture method and/or
system according to an embodiment of the disclosure.
Fig. 13B is another portion of the carbon dioxide capture method
and/or system of Fig. 13A according to an embodiment of the
disclosure.
6
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
DESCRIPTION
The present disclosure will be described with reference to Figs.
1-13B. The systems and methods of the present disclosure can be
operated unattended and/or continuously within a building for up to ten
years with only minor periodic maintenance. Referring first to Fig. 1, a
system 10 is provided that includes a source of flue gas, such as a
boiler that combusts air and fuel to produce flue gas. Flue gas 12 can
include typical combustion products from heating and/or cooling
systems of a building. These buildings can be considered buildings
that are commercial, residential, and/or industrial. System 10 can rely
on combustion of fossil fuels. These fossil fuels can include oil, and/or
natural gas. Upon combustion of fuel, CO2 as part of flue gas can be
produced. In the case of natural gas combustion, system 10 can
generate at least about 10% CO2 and about 18% water. Systems and/or
methods of the present disclosure can include a portion 14 for
separation, a portion 16 for liquefaction, a portion 18 for storage, and
a portion 19 for transfer of CO2_
In accordance with example implementations, at least about 600
standard cubic feet per minute of building flue gas can be diverted to
the flue gas process stream where CO2 is separated and purified in
component 14 of system 10. This separation / purification component
can be an adsorption purification system, operated under conditions of
Pressure Swing (PSA), Vacuum Pressure Swing Adsorption (VPSA);
Temperature Swing (TSA), or Electrical Swing (ESA), or any
combination thereof. In accordance with example implementations, it
can be a Pressure Swing Adsorption system that is a multicomponent
adsorption system that includes multiple vessels containing layered
solid phase adsorbent materials coupled and/or configured to work in
concert to provide greater than 85% CO2 recovery. These
multicomponent adsorption systems can remove carbon dioxide from
an essentially "dry" flue gas stream to a purity of greater than 95% in
most cases, and in other cases, at least 99%. This purified carbon
dioxide gas can then be liquified with successive cooling and
7
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
compression steps to effect phase change to form liquid carbon dioxide
in liquefaction component 16, and then providing that liquified carbon
dioxide to a storage component 18 for scheduled removal as desirable.
In accordance with example implementations, this liquified carbon
dioxide can be transferred away in transfer component 19, and the
transfer can be provided to another source such as a storage facility
which can distribute the carbon dioxide for use in applications such as
concrete curing, waste water treatment, other carbon dioxide
sequestration methods, recycled for fire suppression systems,
industrial specialty gas, consumed in production of hybrid fuels and
organic intermediate chemicals, or for beverage carbonation, as a few
examples.
Referring next to Fig. 2, a building system 30 is shown having
system 32 therein. Flue gas 12 is provided to a series of portions of
system and/or methods 14, 16, 18, 19 and/or cooling tower 31 for the
capture of CO2 from flue gas generated by the building.
Referring next to Figs. 3A-3C, example boiler configurations are
shown as part of the systems and/or methods of the present disclosure.
Referring first to Fig. 3A, a boiler 40 is shown generating combustion
42 in the presence of a free oxygen sensor 43. Combustion 42
generates flue gas 44 which is provided to a boiler exhaust 45.
Referring to Fig. 3B, boiler exhaust is operatively coupled with a plenum
48. In this depicted configuration, multiple boilers are shown, each with
an exhaust 45 and 46, for example, each exhaust operatively coupled
to plenum 48.
Referring next to Fig. 3C, a boiler configured with the systems
and/or methods of the present disclosure is depicted. Accordingly, air
60 and fuel 62 can be provided to the combustion burner, the mix of
which and accordingly the burn of which is controlled by combustion
controller 66 which is operably connected with free oxygen sensor 43.
Accordingly, boiler feed water 52 is received by the combustion boiler
and heated to hot water or steam 50 which is used to heat the building
8
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
and/or building systems such as water heater 58. Water heater system
58 can be configured to receive potable water for heating and/or
industrial process water for heating.
In accordance with example implementations, control 66 can
utilize sensor 43 to monitor the amount of free oxygen in the combustion
burner and maintain the amount of free oxygen to about 3%. About 3%
free oxygen can include free oxygen from 3 to 7 %. In accordance with
example implementations, combustion can generate flue gas 44. The
composition of (wet) flue gas 44 can be controlled to include at least
about 8% carbon dioxide. About 10% carbon dioxide can include
carbon dioxide from 9 to 11 % of the flue gas (dry basis) from
combustion of natural gas_ System 10 can be utilized to combust fuels
other than natural gas which may dictate other optimal CO2 flue gas
concentrations. Accordingly, system 10 can be configured to utilize
multiple fuels.
The systems and/or methods of the disclosure can include
separating the carbon dioxide from the flue gas, liquefying the carbon
dioxide after separating the carbon dioxide from the flue gas, liquefying
the separated carbon dioxide after separating the carbon dioxide from
the flue gas, storing the carbon dioxide after liquefying the carbon
dioxide, and/or transporting the carbon dioxide after storing the carbon
dioxide.
Referring to both Figs. 3B and 30, systems and/or methods for
operating the combustion boiler within the building are provided that
can include combusting air and fuel within the burner to produce flue
gas 44 having an oxygen concentration; and restricting air from the flue
gas by substantially eliminating tramp air within the conduit operably
aligned to convey flue gas from the burner. In accordance with example
implementations, in the case of multiple boilers as shown in Fig_ 3B,
exhausts 45 and 46 can be operatively aligned with plenum 48.
Exhausts not in use, such as 46, can be a source of tramp air to the
plenum. In accordance with example implementations, the systems
9
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
and/or methods of the present disclosure can include providing fluid
communication between the operating burner of one boiler and the
plenum while restricting fluid communication between the plenum and
an idle burner of the other operating boiler.
In at least one
configuration, a door or divider 47 can be provided and operable to
eliminate tramp air from the exhaust of the idle burner.
In accordance with at least one aspect of the present disclosure,
real time control of the combustion source, or boiler, can achieve higher
efficiency to reduce consumption of natural gas or fuel, for example,
while increasing the concentration of carbon dioxide in the flue gas.
This may be considered counter intuitive to increase the concentration
of carbon dioxide in the flue gas when the systems and/or methods of
the present disclosure are being utilized to reduce carbon emissions
from a building. However, increasing carbon dioxide concentration can
provide the benefit of decreasing fuel consumption by reducing heat
loss through the exhaust. Adjusting combustion to control free oxygen
to 3% can give a higher efficiency burn. In accordance with example
implementations, through combustion control, it is desirable to
approach the 12% concentration value of 002, when burning natural
gas, and achieve at least about 10% carbon dioxide concentration in
the flue gas (dry basis). This is at least one feature of the disclosed
building emission processing systems and/or methods and can be
utilized as one of the initial steps in carbon capture.
Within the building, boiler operation can be dictated by
responding to the need for hot water or steam by controlling the
combustion burner to various predetermined firing rates; 1) an off
condition, 2) a low fire rate, and/or 3) a high fire rate. These rates may
have been established on older boilers through calibrated mechanical
linkages, for example. Recognizing that cyclic boiler operation will vary
widely from hour to hour, day to day, and season to season, it is desired
to establish automatic control of the flame rate continuously across the
entire boiler load range, while also controlling free oxygen as discussed
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
above. The systems and/or methods of the present disclosure can be
configured to reduce on-off cycles by extending boiler run time at a
reduced flame rate, increasing the life on the boilers, and providing a
more continuous flow of flue gas to the separation, liquefaction, storage
and/or transport systems and/or methods of present disclosure.
Accordingly, the boiler and system controls (for example Fig. 12)
can achieve higher building thermal efficiency, while creating optimal
conditions for flue gas supply to the systems and methods of the
present disclosure.
Referring next to Figs. 4A-C, multiple portions of systems and
methods are depicted for separating water from flue gas as well as
cooling the flue gas. Referring first to Figs. 4A-4C, three different
configurations of systems and/or methods for cooling flue gas from a
combustion boiler within a building are depicted. Referring first to Fig.
4A, flue gas 44 can proceed to a combination non-condensing and
condensing economizer 60a. Flue gas 44 first proceeds to a non-
condensing configuration in which boiler feed water 52 is provided
through a conduit, set of conduits, and/or coils and flue gas is cooled
and the boiler feed water heated. Accordingly, methods for cooling flue
gas from a combustion boiler within a building are provided. Upon
heating the boiler feed water, it can be provided to the boiler thus
lowering the necessary energy required to heat the feed water to hot
water and/or steam.
Additionally, the economizer can be configured for condensing.
Accordingly, a conduit, set of conduits, or coils 54 can be configured to
convey potable or industrial process water that is received from a utility
for example. This water can have the temperature close to that of
ground water as it is conveyed through typically underground pipes.
Accordingly, the water has a substantially different temperature than
the flue gas, even after being partially cooled in the non-condensing
economizer. The providing of the flue gas to these conduits can remove
water from the flue gas thus creating a water condensate effluent 53.
11
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
This water proceeding through the conduits can be heated and provided
to a water heating system 58 (Fig. 3C) as water heating system water
intake 54, heated and received through outlet 56. Accordingly, the
amount of energy needed to heat the water within water heating system
58 is less for at least the reason the water received for heating does
not need to be heated from the lower temperature associated with
typical utility water, rather it had been preheated. In accordance with
an alternative configuration, and with reference to Fig. 4B, one set of
coils 52 can be associated with one economizer 60b, and another set
of coils 54 can be associated with another economizer 65a. In this
configuration, economizer 60b can be a non-condensing economizer
and economizer 65a can be configured as a condensing economizer.
In accordance with another embodiment of the disclosure, a diverter 64
can be operably coupled to the economizers as shown in Figs. 4A-40.
In accordance with example implementations, the cooled flue gas can
be provided from diverter 64 using a blower. The systems and/or
methods can control the amount of flue gas to be processed using the
diverter. In accordance with example implementations, the current
system in accordance with Fig. 4C is going to receive 450 Standard
Cubic Feet per Minute (SC FM) to 500 SCFM of wet flue gas 44. This
diverter can be controlled by the overall master system (Fig. 12) which
can control the motor operated butterfly valve within the diverter. The
master system can also collect gas temperature and flow data, and
operate the blower as shown in Fig. 6.
Accordingly, where an economizer is down process stream from
a diverter, a blower may precede the economizer. In accordance with
example implementations, the wet flue gas is at least about 8% carbon
dioxide and/or at least about 3% free oxygen prior to entering the first
economizer. The systems and/or methods of the present disclosure
can utilize economizers configured as shown in Figs. 5A and 5B for
example, and the methods can include additional separation as well as
liquefaction, storage, and transport.
12
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
It has been determined that flue gas from the boiler may have a
water content of approximately 18%, and a temperature ranging up to
350 F. Prior to separation of CO2, this water can be substantially
removed from the flue gas. This involves dropping the flue gas
temperature below dewpoint and allowing water to condense out as a
liquid. As the water content of the flue gas lowers, so does the
dewpoint, requiring yet additional cooling to continue removing the
water. This cooling can result in flue gas condensates.
Flue gas condensates tend to be slightly acidic (at pH<=5) which
is a condition that can damage some building plenums due to
construction materials (such as carbon steel) which are not acid
resistant. In these cases, gas must be removed from the plenum and
condensed in external heat exchangers having acid resistant stainless
steel components. Additionally, depending on condenser design, some
amount of micro-liquid droplets may remain in the gas stream. These
micro-liquid droplets can be referred to as acid aerosols which can be
present at ppm levels. The present disclosure contemplates the
removal of acid aerosols. These systems and/or methods include wet
wall heat exchangers, impingers or mists eliminators with inert
reticulated carbon or metal foam, and precipitators for example.
In accordance with the above, the non-condensing economizer
can operate above dew point temperature, preventing any liquid
condensate from forming. Without condensation, this economizer can
be compatible with most plenum construction materials.
As described above, a condensing economizer can be provided
downstream of the diverter (Fig. 40) which extracts flue gas from the
plenum and directs it on to the condensing economizer. Condensate
from this condensing economizer can be chemically neutralized before
proceeding to the building drain as shown by 75 in Fig. 6.
Referring next to Fig. 6, flue gas drying can continue with a
blower 68 to increase pressure of flue gas from the diverter. This
13
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
blower 68 can support flow through the heat exchanger/condenser 70
which can include a water outlet 71 operatively coupled to an acid
neutralizer assembly 75. Heat exchanger 70 can be configured to cool
the gas below dewpoint to condense out most water leaving less than
about 3% water or as low as approximately 0.2% water.
Heat exchanger 70 can be a tube and shell configuration, cooled
by an external water/glycol loop provided from a chiller and/or water
from the building cooling tower for example. As shown, the water
removed from the system at heat exchanger 70 can be slightly acidic,
and it is anticipated that the water can be neutralized before proceeding
to a Publicly Owned Treatment Works (POTW) or through a sewer
system. Additionally, some water will remain in the process stream as
small micro droplets, mist, or acidic aerosols which will be minimized
or removed with special heat exchanger designs, mist eliminator,
impingement devices, or possibly a precipitator. These components
may produce additional condensate or effluent which can be treated
before proceeding to a POTW.
After a preponderance of water has been removed, and acidic
aerosols mitigated, the cooled flue gas 72 can continue on to a
compressor to increase pressure of the flue gas to an optimum level of
approximately 100 psig, or lower, as dictated by the PSA system
specification. Since compression raises process gas dew point, the
compressor may produce additional condensate or effluent.
Referring next to Fig. 7, compressor 74 can receive flue gas 72.
Compressor 74 can be an "oil free" compressor to eliminate
downstream product contamination, and the compressor can be
configured with variable frequency drives (VFD's) to respond to variable
gas flows. Compression can raise the temperature and dew point of
the flue gas, so a second heat exchanger 76 can be utilized to lower
the temperature of the flue gas to less than 40 C. At this stage, the gas
can have less than about 0.2% water which can exist as a vapor, the
14
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
gas can be less than 40 C temperature, and can be about 100 psig in
pressure.
Referring to Fig. 7, the systems and/or methods for separating
carbon dioxide from flue gas generated from a combustion boiler within
a building can include providing flue gas 72 having less than about 3%
water; compressing the flue gas; and cooling compressor 74 with a heat
transfer fluid 90 and providing the heat transfer fluid to/from a chiller
and/or a cooling tower.
In accordance with another example implementation, mist
eliminator subsystem 89 can be provided which can produce an effluent
53. During water removal from flue gas, liquid condensate as effluent
at other points in the process or system can be produced. This effluent
can be slightly acidic (approx. 5 pH) and can be neutralized before
being provided to the building drain.
A very small amount of this slightly acidic condensate remains
entrained in process gas as mist or acid aerosols. In order to remove
these liquid micro-droplets, mist eliminator sub-system 89 can be
added just prior to the compressor inlet. This subsystem can be an
electrostatic unit, wet walled heat exchanger, or a passive impinger
arrangement comprised of reticulated metal or carbon foam, wire mesh
pad, or other material designed with a tortuous gas path causing mist
particles to strike surface areas, nucleate and drain from the system by
gravity. By reducing or eliminating acid aerosols, the mist eliminator
solution can significantly prevent harmful corrosion in downstream
components of the process gas stream. Accordingly, the mist eliminator
can produce effluent 53 which can be neutralized and provided to a
drain.
An example compressor is depicted in Fig. 8. The heat transfer
fluid can be water for example, and the water of the chiller can be
cooled within a cooling tower of the building before returning spent heat
transfer fluid to the chiller. Accordingly, the systems and/or methods of
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
the present disclosure can include additional separation, liquefaction,
storage, and/or transport. This is just one example of the heat
generating components of the system that can be cooled with chiller
and/or cooling tower heat transfer fluid. Over 70% of the cooling
requirement for the systems and/or methods of the present disclosure
can come from heat generated in compressors and/or pumps, and from
heat exchangers on the liquefaction skid. Each of these components
can be provided with a water cooling circuit supplied from a local chiller
or directly from the central chiller. The local chiller can be water cooled
with a water loop coming from the central chiller or from cooling water
from the building cooling tower. The central chiller can be designed to
prioritize heat transfer in the following order: a) domestic hot water
makeup; b) cooling tower; c) exchange with outside air, for example.
Referring again to Fig. 7, after compression the flue gas can be
provided to a dryer 78, such as a desiccant dryer. Dryer 78 can be
operatively engaged with a nitrogen feed, such as a sweep feed,
configured to regenerate spent desiccant. Typically, the dryer is a two-
chamber cycling device, wherein one chamber is drying while the other
chamber is re-generated for drying, and those cycles continue. The
nitrogen can be provided to spent desiccant in one chamber while the
other chamber is drying flue gas. Accordingly, systems and/or methods
for separating carbon dioxide from flue gas generated from a
combustion boiler within a building are provided that can include drying
the flue gas using nitrogen recovered during separation of carbon
dioxide recovered from the flue gas. This recovered nitrogen can be
conveyed from the pressure swing adsorption assembly BO via conduit
92 to dryer 78 and then exhausted through the stack 86. In accordance
with example implementations, the dried flue gas can be provided for
additional separation, liquefaction, storage, and/or transport.
From the dryer, the flue gas 79, containing less than 10 ppm
water, can proceed to pressure swing adsorption (PSA) assembly 80.
This pressure swing adsorption assembly can provide greater than 85%
16
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
CO2 recovery, at greater than 95% purity, at 1 psig, from ambient to
about 100Q C.
Maximum CO2 output flow at this point can be
approximately 40 SC FM. The remainder of the flue gas, mostly nitrogen
may continue under pressure, and/or be split with a portion returning to
dryer 78. Another portion of the nitrogen can proceed to a turbine
expander 82/generator 93 which can provide electrical energy 94 and
a cold output gas, at near ambient pressure. Additionally, a control
valve 84 equipped with a silencer can be operationally aligned in
parallel with expander 82/generator 93.
Accordingly, methods for separating carbon dioxide from flue gas
generated from a combustion boiler within a building are provided that
can include removing at least some of the nitrogen from the flue gas to
produce greater than about 95% carbon dioxide 78 using a pressure
swing adsorption assembly 80. Nitrogen removed from the flue gas can
be used to remove water from the flue gas before providing the flue gas
to the pressure swing adsorption assembly, in dryer 78, for example.
Alternatively, or additionally, at least some of the nitrogen removed from
the flue gas can be provided to a gas expander/generator. Alternatively,
or additionally one part of the nitrogen from the PSA can be provided
to a control valve equipped with a silencer and providing another part
to the expander/generator.
In accordance with example
implementations, the systems and/or methods of the present disclosure
can include separating the nitrogen into parts and providing one part to
the dryer and another part to the expander/generator. In one example
implementation, the one part is about a third of the nitrogen from the
pressure swing adsorption assembly.
In accordance with an example implementation, during the PSA
process a small amount of rejected gas can be produced containing
both CO2 and nitrogen. Rather than purging this gas it can be recycled
back through the compressor via recycle line 81 in order to enhance
the overall recovery of 002.
17
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
Systems and/or methods are also provided for cooling carbon
dioxide separated from flue gas generated from a combustion boiler
within a building using the nitrogen exhaust of a PSA. The systems
and/or methods can include separating nitrogen from flue gas using
pressure swing adsorption assembly 80, and expanding the nitrogen
through a turbine within the presence of a heat exchanger 92 to cool
fluid within heat exchanger 92; and transferring that cooled fluid to
another heat exchanger 100 operably aligned with the carbon dioxide
product of the pressure swing adsorption assembly to cool the carbon
dioxide product 78. The turbine can be part of a generator 93, for
example, or may be provided to cool exchanger 92.
Typically, the nitrogen gas exiting the PSA can be at least 85 psig.
with a flow exceeding 80% of the rated system flow. In accordance with
example implementations, the nitrogen may be processed and saved
as a marketable product. With regard to the electricity generation, grid
compatible power conversion will be needed. The turbine generator
will have a 500 Hz output which is not compatible with a 60Hz grid.
Therefore, it is envisioned that appropriate power conversion will be
specified. This can be rectification followed by DC to AC multi phase
inverter with proper safety features in case of a building power outage.
After use in the turbine generator, and in the CO2 heat exchanger, the
nitrogen waste gas can proceed back to the exhaust stack or plenum.
Referring to Figs. 9A-9B, different embodiments of CO2
separation systems and/or methods are depicted that can be used
alone or in combination with some or all of the components of the
systems and/or methods of Fig. 1.
For example, referring to Fig. 9A, an electrochemical cell is
shown that includes a cathode configured to receive for reaction CO2
and 02. This CO2 and 02 may be received from the same or different
streams. For example, the CO2 can be received from a flue gas stream
or may be received from an air stream. The flue gas stream may be
CO2 and N2, it may also contain 02, it may also contain H20 in a range
18
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
of concentrations. For example, the stream may be considered wet or
dry, with a wet stream containing H20 from the combustion process that
generated the flue gas. The 02 may be received as part of a flue gas
stream, an air stream and/or as part of an 02 stream.
Exiting the cathode side of the cell can be an N2 stream. This N2
stream may also contain CO2 that did not react to form carbonate ion
(0032-). For example, it may contain CO2, H20, and/or 02. This N2
stream will contain less CO2 than the stream exposed to the cathode.
Upon exposure to the cathode and electrical coupling, the CO2 is
reacted to form the carbonate ion which is conveyed through the
carbonate electrolyte to the anode where the electrical coupling returns
the 0032- to CO2 and 02 as a CO2 product. As will be detailed below,
this system can be implemented in a variety of ways; for example, as a
carbonate ion pump (Fig. 9B), as flue gas carbonate fuel cell (Fig. 9C),
and/or as fuel cell (Fig. 9D). In one or more of these implementations,
CO2 can be removed/separated/sequestered from one or more streams.
For example, and with reference to Fig. 9B, a portion of a carbon
dioxide capture method and/or system is shown configured with a
carbonate ion pump. As shown, the carbonate ion can be produced
electrochemically on the surface of a cathode electrode by reacting
carbon dioxide and oxygen from the air in the presence of electrons.
The CO2 can be obtained from flue gas in wet or dry form, and the 02
can be part of the flue gas or provided from an 02 source, from air for
example. The flue gas can contain N2 which will not react at the cathode
and proceed to the gas stack along with any other components that did
not react (e.g., H20, 02, 002)
The electrochemical equation is: CO2 +1/202 + 2e- , 003-
Multiple cells can be configured into stacks whereby CO2 and 02 are
supplied through respective manifolds.
Once the carbonate ion is produced at the cathode, a solid or
liquid electrolyte can be in ionic communication with the cathode
19
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
providing a pathway for carbonate ions to move to the associated
anode. This electrochemical activity is the basis for forming and
transporting carbonate ions and for separating CO2 from the original
cathode gas mixture.
Upon reaching the anode, electrons can be removed from the
carbonate ion(s) causing dissociation back to CO2 and 1/2 02.
In accordance with another implementation of the present
disclosure, CO2 can be separated from flue gas using a membrane
alone or in combination with other separation techniques. The
membrane separation can utilize solvent absorption and/or polymeric
based membranes with appropriate permeability and selectivity for
002. The polymeric membranes can include mixed polymeric
membranes as well. Additional membranes can include carbon and/or
inorganic membranes. CO2 separation can be performed using
membranes configured to perform Knudson diffusion, molecular
sieving, solution-diffusion separation, surface diffusion and/or capillary
condensation.
As shown in Figs. 9B-9D, 02 can be generated from the CO2
product stream, for example during liquefaction. This 02 can be
provided to the cathode as part of the 02 source. The amount of the
02 being provided to the cathode can be monitored and/or adjusted as
desired to operate the cell for optimal CO2 separation.
In accordance with one embodiment of the disclosure and with
reference to Figs 90-9D, a carbonate fuel cell configuration can be
provided with fuel, particularly hydrocarbon fuel sufficient to form
syngas (H2 and CO). The syngas can be formed from natural gas
(CH4H2 and CO), and/or other hydrocarbon materials including, but
not limited to coal. The cathode can be exposed to CO2 and 02 as
described above to form the carbonate ion which can be exposed to the
syngas at the anode to form CO2 and H20 as well as electrons to
provide power output in the form of heat and electricity. This system
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
can be part of a building design, replacing a power generator or boiler
within a building, and/or as a stand alone system to provide heat and
power.
The natural gas can be provided as part of the fuel cell and this
natural gas can be tapped into from the intake to an existing building.
Accordingly, while using natural gas which can be directly reformed to
syngas at operational temperatures, heat and power can be generated
electrochemically and this heat and power can be provided to the
building thereby offsetting part or all of the building's thermal and power
needs without natural gas combustion, thus, drastically reducing CO2
emissions.
For example, the system can be paired with a common boiler
system that is configured to combust natural gas. Accordingly, both the
boiler and the system of the ion pump can be configured to receive
natural gas. Therefore, the system of Fig. 9C can provide heat and
electrical power while the boiler provides thermal power in the form of
steam. The system of Fig. 9C can be operationally aligned with CO2
capture and purification systems as well.
Accordingly, the fuel cell can receive flue gas at the cathode and
CO2 is electrochemically purified and made available for separation and
liquefaction. As shown, flue gas can be provided though a tortuous
path to maximize CO2 exposure to the cathode electrode surface area.
This flue gas may be provided directly from the combustion boiler or it
may be treated as described with reference to Figs. 4-6 prior to being
exposed to the cathode side of the fuel cell. Multiple cells can be
configured in stacks with appropriate gas manifolds. This particular
embodiment can offer increased performance in higher CO2
concentrations thus allowing for less pretreatment of flue gas before
CO2 separation.
Upon reaching the anode, electrons can be removed from the
carbonate ion(s) causing reformation of CO2 and 02. In accordance
21
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
with Figs. 9A-9D, the stream comprising CO2 and 02 can be provided
to liquefaction as described, for example, herein with reference to Fig.
10. Non condensable oxygen can be separated and fed back to the
Cathode side of the pump. As shown electrical energy can be provided
to operate the pump. The pump can also utilize heat from the flue gas.
Accordingly, while using natural gas which is directly reformed to
syngas at operational temperatures, heat and power can be generated
electrochemically and this heat and power can be provided to the
building thereby offsetting part or all of the building's thermal and power
needs and without natural gas combustion.
Accordingly, syngas can be introduced at the anode and
carbonate ions react exothermically to form more CO2 and water vapor.
At this stage a large substantial amount of the resulting gas can be
purified CO2. The concept further teaches removal of water vapor
through condensation followed by CO2 liquefaction.
Accordingly, systems for separating CO2 from a combustion
product are provided that can include a combustion product stream.
This combustion product stream can be a wet stream or a dry stream
that contains CO2 and N2, or for example CO2, 02, H20, and/or N2. The
system can include a carbonate ion pump or carbonate electrochemical
cell operatively aligned with the combustion stream and configured to
react the CO2 and 02 from the combustion product stream to form
carbonate ion and react the carbonate ion to form a CO2 product
stream.
The carbonate electrochemical cell can include a cathode
configured to receive electrons from a power supply and react those
electrons with the CO2 and 02 of the combustion product stream to form
the carbonate ion. This carbonate ion can be C032-, for example and
can be a component of a carbonate electrolyte. The cell can also
include an anode configured to react the carbonate ion and form CO2,
22
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
02 and electrons. Accordingly, the system can include a cathode and
anode about a carbonate electrolyte.
In accordance with additional embodiments, an 02 source can be
operatively coupled to the cathode of the carbonate electrochemical
cell. In this configuration, the cathode can be configured to be exposed
to CO2 from the combustion stream and 02 from the 02 source. Further
embodiments can utilize a syngas source operatively coupled to the
anode to provide a carbonate fuel cell. In this configuration, the anode
can be configured to receive the carbonate ion and the syngas and form
the CO2 product stream.
The system can include a catalytic burner operatively coupled to
the CO2 product stream as well as a heat exchanger operatively
coupled to the catalytic burner and configured to remove H20 from the
CO2 product stream. The heat exchanger can be operatively coupled
to a heat recovery loop with an additional conduit configured to provide
CO2 to the cathode.
Methods for separating CO2 from a combustion product stream
can include receiving a combustion product stream comprising CO2 and
N2; reacting the combustion product stream to form carbonate ion; and
reacting the carbonate ion to form a CO2 product stream. Electrons
can be provided to form the carbonate ion, and electrons can be
removed to form the CO2 product stream.
Additionally, syngas can be provided to react with the carbonate
ion to form the CO2 product stream, and natural gas can be provided to
form the syngas. Embodiments of this method can generate a net
positive electrical potential upon forming the CO2 product stream.
With reference to Fig. 9D, a system for separating CO2 from air
is provided. The system can include an air stream; the air stream can
include at least CO2 and N2, and a carbonate fuel cell operatively
aligned with the air stream and configured to react the CO2 from the air
stream and 02 to form carbonate ion and react the carbonate ion to
23
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
form a CO2 product stream. An 02 source can be operatively coupled
to the cathode of the carbonate fuel cell, with the cathode being
configured to react CO2 from the combustion stream and/or 02 from the
source. As shown, this 02 source can be from the CO2 liquefaction
process. A natural gas source can be operatively coupled to the anode
of the carbonate fuel cell, with the anode configured to receive the
carbonate ion and the natural gas and form the CO2 product stream.
In some implementations, a portion of CO2 can be returned to the
cathode as required, with remaining CO2 sent to liquefaction as
described with reference to Figs. 10-13B.
Referring next to Fig. 10, in another series of components of the
present disclosure, the >95% pure CO2 78 can be cooled and
compressed in sequential steps as shown in heat exchangers 104,
compressors 106 and 108, and heat exchanger 110 with compressors
operatively engaged with cooling transfer fluid 90 to approach the
phase change state for liquefaction. In accordance with example
implementations, the >95% pure CO2 can have a temperature coming
out of the PSA of as high as 100 C. As described, a heat exchanger
can be provided to lower the temperature of the gas to a sufficient
temperature and then compress the gas to a higher pressure. In
accordance with example implementations, heat removed from this CO2
stream can be transferred through external water / glycol cooling loops
back to a heat management system which will support preheating of
makeup water as shown in Fig. 10. It can also be provided to raise the
temperature of nitrogen gas coming off of the PSA prior to expansion
through the turbine. This can improve turbine efficiency by allowing full
use of nitrogen flow before exceeding the COLD temperature output
limit. This is just one of several examples of utilizing heat from system
components at other portions of the system to derive a more efficient
overall system. In accordance with Fig 11, there is a stepwise cooling
and compression sequence of the CO2 gas, which drives towards a final
24
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
state of 311 psig and 0 F, at which point phase change occurs and the
CO2 becomes a liquid.
Referring next to Fig. 11, a CO2 liquefaction and storage system
and/or method is shown wherein CO2 gas 112 is sparged inside a vessel
113 such as an insulated vessel. Example insulated vessels can
include but are not limited to vacuum jacketed liquid storage tanks.
Within this vessel, gas 112 can be converted to a liquid 114.
In
accordance with example implementations, gas 112 can be provided to
sparge assembly 118 where it is provided as sparged gas 120 which
liquefies upon sparging into liquid 114.
Vapor 116 at the top of vessel 113 is managed by a refrigeration
system 122 which cools vapor 116, which condenses back to liquid 114,
which returns back into vessel 113. In accordance with example
configurations, system 122 can be configured as a loop in fluid
communication with vessel 113 wherein vapor CO2 116 enters system
122 and returns to vessel 113 as a liquid CO2 114. In at least one
configuration, system 122 is configured as a low temperature
condenser equipped with an evaporator.
In accordance with an additional embodiment, vessel 113 can be
configured with a controlled venting subsystem to facilitate removal of
non-condensable gases while minimizing loss of CO2. Inlet gas to the
CO2 liquefaction system can be a high concentration of CO2 preferably
>95%. Remaining gases, such as nitrogen and oxygen, can be
considered as non-condensable gases in the liquefaction process. In
addition, a very small subset of impurity gases remain which are
miscible with liquid CO2. These impurities must be measured
accurately in order to qualify the liquid CO2 product in accordance with
commercial standards, such as ISBT, the international beverage
guideline. Both the controlled venting subsystem and the purity
analytical system can account for non-condensable gases which can
dissolve into the liquid.
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
Without preprocessing by a distillation tower, non-condensable
gases in the continuous feed to liquefaction can build up in the storage
system vapor space 116. Without removal, these non-condensable
gases can continue to build pressure in the vapor space of the storage
tank causing some of the gas to dissolve into the liquid, thus
contaminating the liquid. In addition, excessive pressure in the tank
can inhibit both the gas feed system and the refrigeration system which
manages vapor and re-condenses CO2 as liquid back into the tank. The
venting system can be controlled to manage tank vapor space in
conjunction with the refrigeration unit to release non-condensable
gases, reduce pressure buildup, while minimizing loss of CO2 vapor.
Instrumentation (see for example, Fig. 11) at the tank can be configured
to acquire and provide data regarding vapor pressure, dissolved gas,
vapor composition, gas flow, etc. to the processor which can operate
solenoid valves on vent lines to enact controlled release of tank vapor
within strict parameters.
In the event of building power loss, the superior insulation of a
vacuum jacketed tank, for example, may maintain liquid CO2 for at least
30 days. In accordance with example implementations, the building
itself may be able to tap into vessel 113 for a supply of CO2 to extinguish
fires; for example, fires related to electronic components that require
CO2 extinguishing methods.
With reference to Figs. 1, 12, 13A, and 13B, in accordance with
other example implementations, a CO2 removal and/or delivery system
is provided that can include off-take management using one or more
vehicles provided in concert with CO2 removal and/or delivery needs as
provided by system control. For example, a removal and/or delivery
truck 200 can be provided which transfers CO2 directly from vessel 113
via a transfer pump 202 into a liquid CO2 tank affixed to truck 200. The
system can be configured to generate CO2 pick up times based on
numerous parameters, such as: vessel 113 capacity, system 10 CO2
generation, legal date/time pickup windows, and/or CO2 delivery needs.
26
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
With respect to CO2 delivery needs, it is contemplated that such high
purity CO2 can be delivered to a user directly without being warehoused
or the need for additional purification. Just one example of direct
delivery can be delivery to a wastewater treatment plant. In any case,
however, offtake analytics can be provided to qualify the product with
the ability to issue a Certificate of Analysis before transport.
Referring next to Fig. 12, plant, process and field level
components of a control system are shown. In accordance with an
example implementation, an example overall control system is provided
that shows combustion emission and control, MASTER PLC controller,
the diverter, the compression, dryer, separation, cooling and
compression, refrigeration / storage, and the providing of food grade
CO2. These systems are also coupled to utility systems of electricity,
natural gas, and water. These control systems exemplify a basic
Network Architecture Diagram. The MASTER PLC controls the entire
plant with Ethernet loop connections and with Internet IP protocol
communications to the Local Packaged controllers, and through direct
connection and control to the digital and analog I/O field
instrumentation level. The HMI server gathers data from the MASTER
PLC, manages plant real time displays, executes logging, data
management applications, and communicates through the secure
firewall to external users. Also implied is the Engineering Development
workstation which maintains all operational software and updates which
are periodically downloaded to the MASTER PLC.
Referring to Figs. 13A and 13B, an example implementation of
the systems and/or methods is disclosed which details the sequence of
the different components and processes described herein, as well as
additional thermal management components that are associated with
the building. As can be seen throughout the Figures and accompanying
description, there are multiple places for heat to be transferred from
different components of the disclosed system to existing building
systems. For example, as shown, chillers can be in the building, as
27
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
well as existing cooling towers. These active cooling components can
be operably coupled with heat being removed from process components
via individual cooling loops. In accordance with example
implementations heat, sometimes referred to as waste heat, can be
transferred to building systems which can use extra heat to operate
more efficiently. Therefore, regarding waste heat from the disclosed
system, design preference is to transfer waste heat, firstly to building
steam and hot water makeup systems, secondly to the building cooling
tower, and finally to an appropriate chiller with heat exchange to air.
As shown in Figs 12 and 13A-B, a thermal management system
(see, e.g., MASTER PLC, controllers, etc.) can conserve use of fuel
such as natural gas in the boiler by optimizing the combustion with the
combustion controller, control water removal from the flue gas with the
front end controller, perform additional separation with the dryer and
PSA with the separation controller, liquefy and store CO2 with the
liquefaction/storage controller, and dictate off-take to a pickup and/or
delivery truck with the off-take controller.
These and additional
controllers can work to control boiler feed water, potable and/or
industrial water, chiller water, and/or cooling tower water, as well as
nitrogen expansion cooling to reduce and/or eliminate heat loads in the
system. Accordingly, flue gas can be cooled for water knockout, and
heat generating electrical components such as compressors, blowers,
pumps, and fans can be cooled as well.
Additionally, localized gas analytic instruments can be configured
to provide localized CO2 and 02 concentration measurements in near
real time. By locating gas sampling instruments / sensors directly at
sampling points within subsystems like the PSA subsystem, small
streams of sample gas can be pumped through sensor caps just inches
away from the process gas to be measured. This innovation provides
near instantaneous measurements at sub second sampling rates from
multiple devices simultaneously. Each measurement device can be
configured to prepare and format data for immediate transmission to
28
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
the master controller using standard communication protocols.
Individual sensor devices can be uniquely addressed by the master
controller over a common hardwire connection (ethernet, RS232,
RS485, etc.).
As indicated above, in order to meet commercial requirements for
transporting and marketing liquid 002, off-take analytics can be
provided that are integrated into the system in order to certify off-take
weight of liquid CO2 removed, and to qualify CO2 product purity within
required commercial standards. This off-take analytical system can be
configured to issue a Certificate of Analysis for the product CO2 at the
time it is transferred out of the building, or from an intermediate storage
and processing facility. In addition, the off-take analytical system can
be configured to document all information to officially account for all off-
take transactions.
In accordance with an example implementation, a set of
electronic load cells can be placed underneath each storage tank to
accurately measure weight of the tank and its contents. The system
will make a difference calculation to certify weight of liquid CO2
removed.
In accordance with another implementation, the analytical system
can be configured to measure product impurities within exacting
standards. Just before product transfer the analytical system will
automatically take a small liquid CO2 sample from the storage tank,
vaporize it, and then flow the sample gas to a state of the art FTIR
spectrum analyzer or to a field grade gas chromatograph system. The
FTIR spectrum analyzer will be equipped with procedures and chemical
spectral libraries sufficient to identify and measure all "impurities"
shown on the customers contractual purity specification for liquid CO2.
Such specifications usually stipulate the ISBT beverage guideline along
with one or more additional compounds of importance to the customer.
At least one advantage of the FTIR analytical system is that it will be
configured to operate automatically, not requiring manual assistance,
29
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
while providing several times more measurement fidelity than required
by the ISBT guideline. It is generally understood that FTIR systems
cannot measure chemical compounds which do not exhibit a molecular
dipole. This does not apply to impurities of interest, since they all
exhibit molecular dipoles, with several degrees of motion (observable
f requen cies).
Additionally, in a separate embodiment the FTIR system can also
be connected to the "front end" to accurately measure impurities of flue
gas from the boiler system.
In accordance with example implementations, the systems and/or
methods of the present disclosure can include an energy storage
system that can be configured to include a power conversion
component and/or a battery or battery bank component. As one
example, energy can be generated via turbine expansion of the nitrogen
and this energy can be converted and stored within the building. The
energy may be converted and provided directly to system components,
for example compressors, and/or provided to the system components
after storage, thus lowering building energy demand. Additionally, the
energy may be provided to the power grid associated with the building
itself.
In accordance with example implementations, using the MASTER
PLC, energy generated with the system can be utilized during "peak
demand" times (when, for example electricity rates are higher) and/or
when the building is utilizing a "peak" amount of power. During these
times, the MASTER PLC is monitoring building demand and then modify
the system parameters to efficiently use energy storage and/or change
carbon dioxide separation, liquefaction, storage, and/or transport to
lower energy consumption during "peak demand" thus providing energy
cost savings_
Example implementations of the systems and/or methods of the
present disclosure can provide not only a carbon capture system but
CA 03200451 2023- 5- 29
WO 2022/094124
PCT/US2021/057111
also an improvement in overall building energy efficiency (both thermal
and electrical) while lessening CO2 emissions.
Example
implementations can include lowering carbon fuel consumption through
optimizing boiler combustion, providing warmer boiler feed water thus
requiring less energy to heat the boiler feed water, warming potable or
process water thus requiring less energy to the heat the potable or
process water, generating electrical energy and using same to power
system components, and/or using building cooling towers to reduce
building thermal load, etc., which individually and/or collectively can be
part of systems that dramatically improve building efficiency.
31
CA 03200451 2023- 5- 29