Note: Descriptions are shown in the official language in which they were submitted.
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
BCMA-TARGETED CHIMERIC ANTIGEN RECEPTORS
CROSS REFERENCE TO RELATED APPLICATIONS
The present application claims priority to U.S. Provisional Application Nos.
63/120,692 (filed on December 2, 2020), 63/153,666 (filed on February 25,
2021), and
63/212,289 (filed on June 18, 2021), and U.S. application No. 17/476,661
(filed on
September 16, 2021), each of which is hereby incorporated by reference in its
entirety.
SEQUENCE LISTING
This application contains a Sequence Listing which has been filed
electronically in
ASCII format and is hereby incorporated by reference in its entirety. Said
ASCII copy,
created on December 1,2021, is named 11299-008884-W01 ST25.txt and is 41 KB in
size.
TECHNICAL FIELD
The invention relates to the field of biomedicine, and more particularly to
chimeric antigen
receptors targeting BCMA, as well as preparation methods and applications
thereof.
BACKGROUND
BCMA is a B cell maturation antigen, also known as CD269 or TNFRSF17, and is a
member
of the tumor necrosis factor receptor superfamily. Its ligands are B cell
activating factor (BAFF)
and a proliferation-induced ligand (APRIL).
Binding of BCMA to BAFF and APRIL activates NF-kB and induces up-regulation of
anti-
apoptotic Bc1-2 members such as Bc1-xL or Bc1-2 and Mcl-1. The interaction
between BCMA and
its ligands regulates humoral immunity as well as the growth and
differentiation of B cells from
different aspects to maintain a stable and balanced environment in the human
body.
The expression of BCMA is restricted to B cell lines. It is expressed on
plasma blasts, plasma
cells and a portion of mature B cells, and increased at the differentiation of
terminal B cells. While
in most B cells, such as naive B cells, memory B cells and B cell germinal
centers and other organs,
BCMA is not expressed. It has been reported that the expression of BCMA is
important for long-
lived, fixed plasma cells in the bone marrow. Therefore, plasma cells in the
bone marrow are
reduced in BCMA-deficient mice, but plasma cell level in the spleen is not
affected. Mature B
1
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
cells can normally differentiate into plasma cells in BCMA knockout mice. The
BCMA knockout
mice looked normal and seemed healthy, and the number of B cells was normal,
but the plasma
cells could not survive for a long time.
BCMA is also highly expressed in malignant plasma cells, such as multiple
myeloma and
plasma cell leukemia. BCMA is also detected in HRS cells of patients with
Hodgkin's lymphoma.
In America, malignant tumors of blood system account for about 10% of all
malignant tumors, and
myeloma accounts for 15% of all malignant hematological tumors. According to
the literature, the
expression of BCMA is associated with progression of multiple myeloma disease.
The BCMA
gene is highly expressed in myeloma samples, but is low expressed in chronic
lymphocytic
leukemia, acute lymphocytic leukemia, and acute T-cell lymphocytic leukemia. B
cell lymphomas
were significantly increased in a mouse model overexpressing BCMA ligands BAFF
and APRIL.
Ligands that bind to BCMA have been shown to regulate the growth and survival
of multiple
myeloma cells expressing BCMA. The combination of BCMA with BAFF and APRIL can
make
malignant plasma cells survive. Therefore, loss of tumor cells expressing BCMA
and distribution
of the interaction between BCMA ligand and receptor can improve outcome in the
treatment of
multiple myeloma or other BCMA positive B cell lines malignant lymphoma.
Multiple myeloma, also known as plasmacytoma or Keller's disease, is a
malignant tumor of
the refractory B cell line, characterized by abnormal proliferation of plasma
cells. Plasma cells are
a type of leukocyte that is responsible for production of antibodies.
According to data released by
the National Cancer Institute in 2017, myeloma accounts for 1.8% of all tumor
cases, with a
mortality rate of 2.1%. The statistical results of 2010-2014 show that the
incidence rate is about
6.6 in 100,000 per year and the mortality rate is about 50%. Multiple myeloma
is a middle-aged
disease. The median age of onset in Europe and the United States is 68 years
old. There are more
males than females. The peak age of onset in China is 55-65 years old, and the
ratio of male to
female is 2.35:1. There is no confirmed epidemiological data on multiple
myeloma in China. It is
generally estimated that the incidence rate is similar to that in surrounding
southeast Asia and
Japan, about one in 100,000. Traditional treatments for multiple myeloma
include chemotherapy
and hematopoietic stem cell transplantation, but these methods have a high
recurrence rate.
Bortezomib (PS -341) is first proteasome inhibitor, which is approved by the
FDA in 2003 for the
treatment of relapsed refractory multiple myeloma, either alone or in
combination with existing
medications. The results were gratifying. The drug was also marketed in China
in 2005 and has
2
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
become one of the options for the treatment of multiple myeloma with
thalidomide and
dexamethasone. The treatment of multiple myeloma is usually combined. However,
if multiple
drugs are used at the same time, there are also negative effects of costly and
cumulative side
effects. There is still a clinical need to develop new methods for the
treatment of multiple myeloma.
Recently, immunotherapy, especially adoptive T-cell therapy, has shown strong
efficacy and
bright prospects in clinical trials for the treatment of malignant tumors of
the blood system. T cells
can be genetically modified to express a chimeric antigen receptor (CAR),
which includes an
antigen recognition portion and a T cell activation region. Using the antigen
binding properties of
monoclonal antibodies, CAR can redirect the specificity and reactivity of T
cell and target in a
non-MHC restricted manner. This non-MHC restricted antigen recognition allows
CAR-
expressing T cells to recognize antigen without antigen processing, thus
avoiding a major
mechanism of tumor escape. In addition, CAR does not produce dimers with alpha
chain and beta
chain of the endogenous TCR.
At present, two chimeric antigen receptor T cell therapy (CAR-T) products
targeting CD19
have been approved for the treatment of acute lymphoblastic leukemia in
children and young adult
patients and adult second-line or multi-line system therapy of recurrent or
refractory large B -cell
lymphoma. However, CD19 is rarely expressed in malignant plasma cells of
multiple myeloma.
There is an urgent need to develop a CAR-T product that targets BCMA for the
treatment of
multiple myeloma.
3
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
SUMMARY
It is an object of the present disclosure to provide chimeric antigen
receptors targeting
BCMA as well as preparation methods and applications thereof.
The present disclosure provides for a chimeric antigen receptor (CAR). The CAR
may
comprise: an anti-BCMA antigen-binding region which comprises a light chain
variable region
(VL) and a heavy chain variable region (VH).
VL may comprise three complementarity determining regions (CDRs), LCDR1, LCDR2
and LCDR3; VH may comprise three CDRs, HCDR1, HCDR2 and HCDR3.
In certain embodiments, LCDR1, LCDR2 and LCDR3 may have amino acid sequences
about 80% to about 100% identical to the amino acid sequences set forth in SEQ
ID NO: 17, SEQ
ID NO: 19, SEQ ID NO: 21, respectively. HCDR1, HCDR2 and HCDR3 may have amino
acid
sequences about 80% to about 100% identical to the amino acid sequences set
forth in SEQ ID
NO: 24, SEQ ID NO: 26, SEQ ID NO: 28, respectively.
In certain embodiments, LCDR1, LCDR2 and LCDR3 may have amino acid sequences
about 80% to about 100% identical to the amino acid sequences set forth in SEQ
ID NO: 31, SEQ
ID NO: 33, SEQ ID NO: 35, respectively. HCDR1, HCDR2 and HCDR3 may have amino
acid
sequences about 80% to about 100% identical to the amino acid sequences set
forth in SEQ ID
NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, respectively.
In certain embodiments, LCDR1, LCDR2 and LCDR3 may have amino acid sequences
about 80% to about 100% identical to the amino acid sequences set forth in SEQ
ID NO: 45, SEQ
ID NO: 47, SEQ ID NO: 49, respectively. HCDR1, HCDR2 and HCDR3 may have amino
acid
sequences about 80% to about 100% identical to the amino acid sequences set
forth in SEQ ID
NO: 52, SEQ ID NO: 54, SEQ ID NO: 56, respectively.
VL and VH of the CAR may have amino acid sequences about 80% to about 100%
identical to amino acid sequences set forth in (a) SEQ ID NO: 1 and SEQ ID NO:
2,
respectively; (a) SEQ ID NO: 3 and SEQ ID NO: 4, respectively; or (a) SEQ ID
NO: 5 and
SEQ ID NO: 6, respectively.
In certain embodiments, VL is located at the N-terminus of VH.
The anti-BCMA antigen-binding region may be a single-chain variable fragment
(scFv) that specifically binds BCMA.
4
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
The CAR may further comprise one or more of the following: (a) a signal
peptide,
(b) a hinge region, (c) a transmembrane domain, (d)
a co-stimulatory region, and (e) a
cytoplasmic signaling domain.
The co-stimulatory region may comprise a co-stimulatory region of (or may be
derived from) 4-1BB (CD137), CD28, 0X40, CD2, CD7, CD27, CD30, CD40, CD70,
CD134, PD1, Dap10, CDS, ICAM-1, LFA-1 (CD11a/CD18), ICOS (CD278), NKG2D,
GITR, TLR2, or combinations thereof.
The cytoplasmic signaling domain may comprise a cytoplasmic signaling domain
of
(or may be derived from) CD3.
The hinge region may comprise a hinge region of (or may be derived from) CD8,
CD28, CD137, Ig4, or combinations thereof.
The transmembrane domain may comprise a transmembrane domain of (or may be
derived from) CD8, CD28, CDR, CD45, CD4, CD5, CD9, CD16, CD22, CD33, CD37,
CD64, CD80, CD86, CD134, CD137, CD154, or combinations thereof.
The present disclosure provides for an immune cell expressing the present CAR.
The
immune cell may be a T cell or a natural killer (NK) cell. The immune cell may
be allogeneic
or autologous.
Also encompassed by the present disclosure is a nucleic acid encoding the
present
CAR.
The present disclosure further provides for a vector comprising the present
nucleic acid.
The present disclosure provides for a method of treating cancer. The method
may
comprise administering the immune cell to a subject in need thereof.
The cancer may be a hematologic cancer. The cancer may be a plasma-cell
malignancy.
The cancer may be a BCMA-positive malignancy. The cancer may be multiple
myeloma
(MM), or plasma cell leukemia.
The immune cell may be administered by infusion, injection, transfusion,
implantation,
and/or transplantation.
The immune cell may be administered intravenously, subcutaneously,
intradermally,
intranodally, intratumorally, intramedullary, intramuscularly, or
intraperitoneally.
The immune cell may be administered via intravenous infusion.
The subject may be a human.
5
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
The present disclosure provides for a method for treating cancer. The method
may comprise
administering the present immune cell to a subject in need of.
The chimeric antigen receptor (CAR) may generate an area under the curve (AUC)
ranging
from about 5.0e+05 copies/vg genomic DNA (copies/gDNA) to about 1.3e+07
copies/gDNA,
from about 5.0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.0e+07
copies/gDNA,
from about 5.0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.3e+07
copies/gDNA, or
from about 7.0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.0e+07
copies/gDNA, in
the blood of the subject in about 28 days after administration.
The chimeric antigen receptor (CAR) may generate a maximum plasma
concentration (Cmax)
ranging from about 5x104 copies/vg genomic DNA (copies/gDNA) to about 1.3x106
copies/gDNA, from about 5x105 copies/vg genomic DNA (copies/gDNA) to about
1.3x106
copies/gDNA, or from about 7.5x105 copies/vg genomic DNA (copies/gDNA) to
about 1x106
copies/gDNA, in the blood of the subject.
The CAR may have a Tmax ranging from about 12 days to about 25 days, from
about 14 days
to about 20 days, or from about 6 days to about 22 days.
In certain embodiments, the anti-BCMA antigen-binding region includes a light
chain
variable region (VI) comprising an amino acid sequence at least or about 70%,
at least or about
75%, at least or about 80%, at least or about 85%, at least or about 90%, at
least or about 95%, at
least or about 99%, at least or about 81%, at least or about 82%, at least or
about 83%, at least or
about 84%, at least or about 85%, at least or about 86%, at least or about
87%, at least or about
88%, at least or about 89%, at least or about 90%, at least or about 91%, at
least or about 92%, at
least or about 93%, at least or about 94%, at least or about 95%, at least or
about 96%, at least or
about 97%, at least or about 98%, at least or about 99%, or about 100%
identical to the amino acid
sequence set forth in SEQ ID NO: 1, SEQ ID NO: 3, or SEQ ID NO: 5.
In certain embodiments, the anti-BCMA antigen-binding region includes a heavy
chain
variable region (VH) comprising an amino acid sequence at least or about 70%,
at least or about
75%, at least or about 80%, at least or about 85%, at least or about 90%, at
least or about 95%, at
least or about 99%, at least or about 81%, at least or about 82%, at least or
about 83%, at least or
about 84%, at least or about 85%, at least or about 86%, at least or about
87%, at least or about
88%, at least or about 89%, at least or about 90%, at least or about 91%, at
least or about 92%, at
least or about 93%, at least or about 94%, at least or about 95%, at least or
about 96%, at least or
6
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
about 97%, at least or about 98%, at least or about 99%, or about 100%
identical to the amino acid
sequence set forth in SEQ ID NO: 2, SEQ ID NO: 4, or SEQ ID NO: 6.
A light chain variable region of the anti-BCMA antigen-binding region can
comprise one,
two, or three complementarity determining regions (CDRs) that are at least or
about 70%, at least
or about 75%, at least or about 80%, at least or about 85%, at least or about
90%, at least or about
95%, at least or about 99%, at least or about 81%, at least or about 82%, at
least or about 83%, at
least or about 84%, at least or about 85%, at least or about 86%, at least or
about 87%, at least or
about 88%, at least or about 89%, at least or about 90%, at least or about
91%, at least or about
92%, at least or about 93%, at least or about 94%, at least or about 95%, at
least or about 96%, at
.. least or about 97%, at least or about 98%, at least or about 99%, or about
100%, identical to the
CDRs of a light chain variable region of the BCMA-20 antibody (CDR1, CDR2 and
CDR3 as set
forth in position 24-34, position 50-56, position 89-97 of SEQ ID NO: 1,
respectively), or the
CDRs of a light chain variable region of the BCMA-CA8 antibody (CDR1, CDR2 and
CDR3 as
set forth in position 24-34, position 50-56, position 89-97 of SEQ ID NO: 3,
respectively), or the
.. CDRs of a light chain variable region of the BCMA-M06 antibody (CDR1, CDR2
and CDR3 as
set forth in position 24-34, position 50-56, position 89-97 of SEQ ID NO: 5,
respectively).
A heavy chain variable region of the anti-BCMA antigen-binding region can
comprise one,
two, or three complementarity determining regions (CDRs) that are at least or
about 70%, at least
or about 75%, at least or about 80%, at least or about 85%, at least or about
90%, at least or about
.. 95%, at least or about 99%, at least or about 81%, at least or about 82%,
at least or about 83%, at
least or about 84%, at least or about 85%, at least or about 86%, at least or
about 87%, at least or
about 88%, at least or about 89%, at least or about 90%, at least or about
91%, at least or about
92%, at least or about 93%, at least or about 94%, at least or about 95%, at
least or about 96%, at
least or about 97%, at least or about 98%, at least or about 99%, or about
100%, identical to the
CDRs of a heavy chain variable region of the BCMA-20 antibody (CDR1, CDR2 and
CDR3 as
set forth in position 31-35, position 50-66, position 99-110 of SEQ ID NO: 2,
respectively), or the
CDRs of a heavy chain variable region of the BCMA-CA8 antibody (CDR1, CDR2 and
CDR3 as
set forth in position 31-35, position 50-66, position 99-110 of SEQ ID NO: 4,
respectively), or the
CDRs of a heavy chain variable region of the BCMA-M06 antibody (CDR1, CDR2 and
CDR3 as
set forth in position 31-35, position 50-66, position 99-110 of SEQ ID NO: 6,
respectively).
A light chain variable region of the anti-BCMA antigen-binding region can
comprise one,
7
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
two, or three complementarity determining regions (CDRs) that are at least or
about 70%, at least
or about 75%, at least or about 80%, at least or about 85%, at least or about
90%, at least or about
95%, at least or about 99%, at least or about 81%, at least or about 82%, at
least or about 83%, at
least or about 84%, at least or about 85%, at least or about 86%, at least or
about 87%, at least or
about 88%, at least or about 89%, at least or about 90%, at least or about
91%, at least or about
92%, at least or about 93%, at least or about 94%, at least or about 95%, at
least or about 96%, at
least or about 97%, at least or about 98%, at least or about 99%, or about
100%, identical to the
CDRs of a light chain variable region of the BCMA-20 antibody (CDR1, CDR2 and
CDR3 as set
forth in SEQ ID NO: 17, SEQ ID NO: 19, SEQ ID NO: 21, respectively), or the
CDRs of a light
chain variable region of the BCMA-CA8 antibody (CDR1, CDR2 and CDR3 as set
forth in SEQ
ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 35, respectively), or the CDRs of a light
chain variable
region of the BCMA-M06 antibody (CDR1, CDR2 and CDR3 as set forth in SEQ ID
NO: 45,
SEQ ID NO: 47, SEQ ID NO: 49, respectively).
A heavy chain variable region of the anti-BCMA antigen-binding region can
comprise one,
two, or three complementarity determining regions (CDRs) that are at least or
about 70%, at least
or about 75%, at least or about 80%, at least or about 85%, at least or about
90%, at least or about
95%, at least or about 99%, at least or about 81%, at least or about 82%, at
least or about 83%, at
least or about 84%, at least or about 85%, at least or about 86%, at least or
about 87%, at least or
about 88%, at least or about 89%, at least or about 90%, at least or about
91%, at least or about
92%, at least or about 93%, at least or about 94%, at least or about 95%, at
least or about 96%, at
least or about 97%, at least or about 98%, at least or about 99%, or about
100%, identical to the
CDRs of a heavy chain variable region of the BCMA-20 antibody (CDR1, CDR2 and
CDR3 as
set forth in SEQ ID NO: 24, SEQ ID NO: 26, SEQ ID NO: 28, respectively), or
the CDRs of a
heavy chain variable region of the BCMA-CA8 antibody (CDR1, CDR2 and CDR3 as
set forth in
SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42, respectively), or the CDRs of a
heavy chain
variable region of the BCMA-M06 antibody (CDR1, CDR2 and CDR3 as set forth in
SEQ ID
NO: 52, SEQ ID NO: 54, SEQ ID NO: 56, respectively).
In certain embodiments, a light chain variable region of the anti-BCMA antigen-
binding
region includes three CDRs that are identical (e.g., 80% - 100% identical) to
CDRs of a heavy
chain variable region of the BCMA-20 antibody (CDR1, CDR2 and CDR3 as set
forth in position
24-34, position 50-56, position 89-97 of SEQ ID NO: 1), and a heavy chain
variable region of the
8
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
anti-BCMA antigen-binding region includes three CDRs that are identical (e.g.,
80% - 100%
identical) to CDRs of a heavy chain variable region of the BCMA-20 antibody
(CDR1, CDR2 and
CDR3 as set forth in position 31-35, position 50-66, position 99-110 of SEQ ID
NO: 2,
respectively).
In certain embodiments, a light chain variable region of the anti-BCMA antigen-
binding
region includes three CDRs that are identical (e.g., 80% - 100% identical) to
the CDRs of a light
chain variable region of the BCMA-20 antibody (CDR1, CDR2 and CDR3 as set
forth in SEQ ID
NO: 17, SEQ ID NO: 19, SEQ ID NO: 21, respectively), and a heavy chain
variable region of the
anti-BCMA antigen-binding region includes three CDRs that are identical (e.g.,
80% - 100%
identical) to the CDRs of a heavy chain variable region of the BCMA-20
antibody (CDR1, CDR2
and CDR3 as set forth in SEQ ID NO: 24, SEQ ID NO: 26, SEQ ID NO: 28,
respectively).
In certain embodiments, a light chain variable region of the anti-BCMA antigen-
binding
region includes three CDRs that are identical (e.g., 80% - 100% identical) to
CDRs of a light chain
variable region of the BCMA-CA8 antibody (CDR1, CDR2 and CDR3 as set forth in
position 24-
34, position 50-56, position 89-97 of SEQ ID NO: 3, respectively), and a heavy
chain variable
region of the anti-BCMA antigen-binding region includes three CDRs that are
identical (e.g., 80%
- 100% identical) to CDRs of a heavy chain variable region of the BCMA-CA8
antibody (CDR1,
CDR2 and CDR3 as set forth in position 31-35, position 50-66, position 99-110
of SEQ ID NO:
4, respectively).
In certain embodiments, a light chain variable region of the anti-BCMA antigen-
binding
region includes three CDRs that are identical (e.g., 80% - 100% identical) to
the CDRs of a light
chain variable region of the BCMA-CA8 antibody (CDR1, CDR2 and CDR3 as set
forth in SEQ
ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 35, respectively), and a heavy chain
variable region of
the anti-BCMA antigen-binding region includes three CDRs that are identical
(e.g., 80% - 100%
identical) to the CDRs of a heavy chain variable region of the BCMA-CA8
antibody (CDR1,
CDR2 and CDR3 as set forth in SEQ ID NO: 38, SEQ ID NO: 40, SEQ ID NO: 42,
respectively).
In certain embodiments, a light chain variable region of the anti-BCMA antigen-
binding
region includes three CDRs that are identical to CDRs of a light chain
variable region of the
BCMA-M06 antibody (CDR1, CDR2 and CDR3 as set forth in position 24-34,
position 50-56,
position 89-97 of SEQ ID NO: 5, respectively), and a heavy chain variable
region of the anti-
BCMA antigen-binding region includes three CDRs that are identical to CDRs of
a heavy chain
9
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
variable region of the BCMA-M06 antibody (CDR1, CDR2 and CDR3 as set forth in
position 31-
35, position 50-66, position 99-110 of SEQ ID NO: 6, respectively).
In certain embodiments, a light chain variable region of the anti-BCMA antigen-
binding
region includes three CDRs that are identical (e.g., 80% - 100% identical) to
the CDRs of a light
chain variable region of the BCMA-M06 antibody (CDR1, CDR2 and CDR3 as set
forth in SEQ
ID NO: 45, SEQ ID NO: 47, SEQ ID NO: 49, respectively), and a heavy chain
variable region of
the anti-BCMA antigen-binding region includes three CDRs that are identical
(e.g., 80% - 100%
identical) to the CDRs of a heavy chain variable region of the BCMA-M06
antibody (CDR1,
CDR2 and CDR3 as set forth in SEQ ID NO: 52, SEQ ID NO: 54, SEQ ID NO: 56,
respectively).
In certain embodiments, the CAR may comprise an amino acid sequence about 80%
to about
100%, about 85% to about 100%, about 90% to about 100%, about 95% to about
100%, at least
or about 70%, at least or about 75%, at least or about 80%, at least or about
85%, at least or about
90%, at least or about 95%, at least or about 99%, at least or about 81%, at
least or about 82%, at
least or about 83%, at least or about 84%, at least or about 85%, at least or
about 86%, at least or
about 87%, at least or about 88%, at least or about 89%, at least or about
90%, at least or about
91%, at least or about 92%, at least or about 93%, at least or about 94%, at
least or about 95%, at
least or about 96%, at least or about 97%, at least or about 98%, at least or
about 99%, or about
100%, identical to the amino acid sequence set forth in SEQ ID NO: 59, SEQ ID
NO: 61, or SEQ
ID NO: 63.
In certain embodiments, the nucleic acid encoding the CAR may comprise a
nucleic acid
sequence about 80% to about 100%, about 85% to about 100%, about 90% to about
100%, about
95% to about 100%, at least or about 70%, at least or about 75%, at least or
about 80%, at least or
about 85%, at least or about 90%, at least or about 95%, at least or about
99%, at least or about
81%, at least or about 82%, at least or about 83%, at least or about 84%, at
least or about 85%, at
least or about 86%, at least or about 87%, at least or about 88%, at least or
about 89%, at least or
about 90%, at least or about 91%, at least or about 92%, at least or about
93%, at least or about
94%, at least or about 95%, at least or about 96%, at least or about 97%, at
least or about 98%, at
least or about 99%, or about 100%, identical to the amino acid sequence set
forth in SEQ ID NO:
58, SEQ ID NO: 60, or SEQ ID NO: 62.
In certain embodiments, the CAR may generate an area under the curve (AUC)
ranging
from about 0.5e+06 copies4tg genomic DNA (copies/gDNA) to about 2e+07
copies/gDNA, from
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
about 5.0e+05 copies/vg genomic DNA (copies/gDNA) to about 1.3e+07
copies/gDNA, from
about 5.0e+05 copies/vg genomic DNA (copies/gDNA) to about 2e+07 copies/gDNA,
from about
5.0e+05 copies/vg genomic DNA (copies/gDNA) to about 1.5e+07 copies/gDNA, from
about
.0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.0e+07 copies/gDNA, from
about
5 5.0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.3e+07 copies/gDNA,
from about
7 .0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.0e+07 copies/gDNA,
from about
8.0e+06 copies/vg genomic DNA (copies/gDNA) to about 1.0e+07 copies/gDNA, from
about
0.5e+06 copies/vg genomic DNA (copies/gDNA) to about 4e+06 copies/gDNA, from
about
0.5e+06 copies/vg genomic DNA (copies/gDNA) to about 3.5e+06 copies/gDNA, from
about
le+06 copies/vg genomic DNA (copies/gDNA) to about 3.5e+06 copies/gDNA, from
about
1.2e+06 copies/vg genomic DNA (copies/gDNA) to about 3.2e+06 copies/gDNA, from
about
0.8e+06 copies/vg genomic DNA (copies/gDNA) to about 3.2e+06 copies/gDNA, from
about
1.6e+06 copies/vg genomic DNA (copies/gDNA) to about 3.2e+06 copies/gDNA, from
about
le+06 copies/vg genomic DNA (copies/gDNA) to about 2e+06 copies/gDNA, from
about 0.6e+06
copies/vg genomic DNA (copies/gDNA) to about 1.8e+06 copies/gDNA, from about
3e+06
copies/vg genomic DNA (copies/gDNA) to about 3.2e+06 copies/gDNA, from about
0.5e+06
copies/vg genomic DNA (copies/gDNA) to about 1.7e+06 copies/gDNA, from about
2e+06
copies/vg genomic DNA (copies/gDNA) to about 3.2e+06 copies/gDNA, from about
1.5e+06
copies/vg genomic DNA (copies/gDNA) to about 2e+06 copies/gDNA, or from about
le+06
copies/vg genomic DNA (copies/gDNA) to about 3.2e+06 copies/gDNA, in the blood
of the
subject in about 28 days after administration of the CAR to the subject. The
AUC may be a median
AUC.
In certain embodiments, the CAR generates a maximum plasma concentration
(Cmax)
ranging from about 5x104 copies/vg genomic DNA (copies/gDNA) to about 1.3x106
copies/gDNA, from about 5x104 copies/vg genomic DNA (copies/gDNA) to about
1.5x106
copies/gDNA, from about 5x105 copies/vg genomic DNA (copies/gDNA) to about
1.3x106
copies/gDNA, from about 7.5x105 copies/vg genomic DNA (copies/gDNA) to about
1x106
copies/gDNA, from about 7x105 copies/vg genomic DNA (copies/gDNA) to about
1x106
copies/gDNA, from about 8x105 copies/vg genomic DNA (copies/gDNA) to about
1x106
11
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
copies/gDNA, from about 7.5x105 copies/vg genomic DNA (copies/gDNA) to about
1.5x106
copies/gDNA, from about 7x105 copies/vg genomic DNA (copies/gDNA) to about
1.5x106
copies/gDNA, from about 8x105 copies/vg genomic DNA (copies/gDNA) to about
1.5x106
copies/gDNA, from about 0.8e+05 copies/vg genomic DNA (copies/gDNA) to about
3.5e+05
copies/gDNA, from about le+05 copies/vg genomic DNA (copies/gDNA) to about
3.5e+05
copies/gDNA, from about le+05 copies/vg genomic DNA (copies/gDNA) to about
1.6e+05
copies/gDNA, from about le+05 copies/vg genomic DNA (copies/gDNA) to about
3.3e+05
copies/gDNA, from about 0.8e+05 copies/vg genomic DNA (copies/gDNA) to about
1.5e+05
copies/gDNA, from about 0.8e+05 copies/vg genomic DNA (copies/gDNA) to about
2e+05
copies/gDNA, from about le+05 copies/vg genomic DNA (copies/gDNA) to about
2e+05
copies/gDNA, from about 2e+05 copies/vg genomic DNA (copies/gDNA) to about
3e+05
copies/gDNA, from about 2e+05 copies/vg genomic DNA (copies/gDNA) to about
3.5e+05
copies/gDNA, from about 2e+05 copies/vg genomic DNA (copies/gDNA) to about
2.5e+05
copies/gDNA, or from about le+05 copies/vg genomic DNA (copies/gDNA) to about
3e+05
copies/gDNA, in the blood of the subject after administration of the CAR to
the subject. The Cmax
may be a median Cmax.
In certain embodiments, the CAR has a Tma,, (the time it takes the CAR to
reach Cmax)
ranging from about 12 days to about 25 days, from about 14 days to about 20
days, from about 6
days to about 22 days, from about 3 days to about 20 days, from about 4 days
to about 18 days,
from about 5 days to about 17 days, from about 6 days to about 16 days, from
about 7 days to
about 15 days, from about 9 days to about 15 days, from about 10 days to about
15 days, from
about 10 days to about 14 days, from about 8 days to about 12 days, from about
6 days to about
14 days, from about 12 days to about 14 days, from about 8 days to about 11
days, from about 8
days to about 15 days, or from about 10 days to about 14 days. The Tmax may be
a median Tmax.
In certain embodiments, the CAR has a Tiast (the time corresponding to the
last
quantifiable CAR level) ranging from about 30 days to about 200 days, from
about 50 days to
about 150 days, from about 50 days to about 100 days, from about 60 days to
about 80 days, from
about 60 days to about 150 days, from about 80 days to about 150 days, from
about 50 days to
about 200 days, from about 50 days to about 60 days, from about 50 days to
about 80 days, from
about 50 days to about 100 days, from about 60 days to about 100 days, from
about 80 days to
12
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
about 100 days, from about 60 days to about 200 days, from about 80 days to
about 200 days, from
about 50 days to about 140 days, from about 60 days to about 140 days, or from
about 80 days to
about 140 days. The Tlast may be a median Tlast=
Specifically, it is an object of the present disclosure to provide a sequence
of BCMA targeted
chimeric antigen receptor as well as preparation method and activity
identification of a modified
T cell (CART-BCMA) thereof.
The present disclosure provides a chimeric antigen receptor structure for use
in the treatment
of BCMA positive B cell lymphoma.
In a first aspect, it provides a chimeric antigen receptor (CAR) (sequence),
and its antigen
binding domain is an antibody single chain variable region sequence that
targets extracellular
region of BCMA.
In another embodiment, the antigen binding domain is an antibody single chain
variable
region sequence that targets amino acid residues at positions 24 to 41 of the
BCMA sequence.
In another embodiment, the NCBI accession number of the BCMA sequence is
AY684975.1.
In another embodiment, the structure of the antigen binding domain is shown in
formula I as
below:
VL-VH (I)
wherein VH is an antibody heavy chain variable region; VL is an antibody light
chain variable
region; and "-" is a linker peptide or a peptide bond;
and, the amino acid sequence of VL is as shown in SEQ ID NO: 1, and the amino
acid
sequence of VH is as shown in SEQ ID NO: 2;
or, the amino acid sequence of VL is as shown in SEQ ID NO: 3, and the amino
acid sequence
of VH is as shown in SEQ ID NO: 4;
or, the amino acid sequence of VL is as shown in SEQ ID NO: 5, and the amino
acid sequence
of VH is as shown in SEQ ID NO: 6.
In another embodiment, the amino acid sequence of the linker peptide is as
shown in SEQ ID
NO: 10 or SEQ ID NO: 11.
In another embodiment, the antibody single chain variable region comprises a
human, mouse,
human-mouse chimeric antibody single chain variable region.
In another embodiment, the structure of the chimeric antigen receptor is shown
in formula II
as below:
13
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
S-VL-VH-H-TM-C-CD3 (II)
wherein,
S is an optional signal peptide;
H is a hinge region;
TM is a transmembrane domain;
C is a co-stimulatory signaling molecule;
CD3 is a cytoplasmic signaling sequence derived from CD3;
V H and V L are as described above.
In another embodiment, the S is a signal peptide of a protein selected from
the group
consisting of CD8, CD28, GM-CSF, CD4, CD137, or a combination thereof.
In another embodiment, the S is a signal peptide derived from CD8.
In another embodiment, the amino acid sequence of S is as shown in SEQ ID NO:
9.
In another embodiment, the H is a hinge region of a protein selected from the
group consisting
of CD8, CD28, CD137, or a combination thereof.
In another embodiment, the H is a hinge region derived from CD8.
In another embodiment, the amino acid sequence of H is as shown in SEQ ID NO:
12.
In another embodiment, the TM is a transmembrane region of a protein selected
from the
group consisting of CD28, CDR, CD45, CD4, CD5, CD8, CD9, CD16, CD22, CD33,
CD37,
CD64, CD80, CD86, CD134, CD137, CD154, or a combination thereof.
In another embodiment, the TM is a transmembrane region derived from CD8.
In another embodiment, the sequence of TM is as shown in SEQ ID NO: 13.
In another embodiment, the C is a co-stimulatory signaling molecule of a
protein selected
from the group consisting of 0X40, CD2, CD7, CD27, CD28, CD30, CD40, CD70,
CD134, 4-
1BB (CD137), PD1, Dap10, CDS, ICAM-1, LFA-1 (CD1 la/CD18), ICOS (CD278),
NKG2D,
GITR, TLR2, or a combination thereof.
In another embodiment, C is a co-stimulatory signaling molecule derived from 4-
1BB.
In another embodiment, the amino acid sequence of C is as shown in SEQ ID NO:
14.
In another embodiment, the amino acid sequence of CD3t is as shown in SEQ ID
NO: 15.
In a second aspect, it provides a nucleic acid molecule, encoding the chimeric
antigen receptor
(CAR) of the first aspect of.
In another embodiment, the nucleic acid molecule is isolated.
14
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
In a third aspect, it provides a vector, comprising the nucleic acid molecule
of the second
aspect.
In another embodiment, the vector is selected from the group consisting of
DNA, RNA,
plasmid, lentiviral vector, adenoviral vector, retroviral vector, transposon,
or a combination
.. thereof.
In another embodiment, the vector is a lentiviral vector.
In a fourth aspect, it provides a host cell, comprising the vector of the
third aspect or having
the exogenous nucleic acid molecule of the second aspect integrated into the
chromosome or
expressing the CAR of the first aspect.
In another embodiment, the cell is an isolated cell, and/or the cell is a
genetically engineered
cell.
In another embodiment, the cell is a mammalian cell.
In another embodiment, the cell is a T cell.
In a fifth aspect, it provides a method for preparing a CAR-T cell expressing
the CAR of the
first aspect, and the method comprises the steps of: transducing the nucleic
acid molecule of the
second aspect or the vector of the third aspect into a T cell, thereby
obtaining the CAR-T cell.
In a sixth aspect, it provides a preparation, comprising the chimeric antigen
receptor of the
first aspect, the nucleic acid molecule of the second aspect, the vector of
the third aspect, or the
cell of the fourth aspect, and a pharmaceutically acceptable carrier, diluent
or excipient.
In another embodiment, the preparation is a liquid preparation.
In another embodiment, the dosage form of the preparation is injection.
In another embodiment, the concentration of the CAR-T cells in the preparation
is 1 x 10 3-
1 x108 cells / ml, or 1 x 10 4-1x107 cells / ml.
In a seventh aspect, it provides the use of the chimeric antigen receptor of
the first aspect, the
nucleic acid molecule of the second aspect, the vector of the third aspect, or
the cell of the fourth
aspect, for the preparation of a medicine or a preparation for preventing
and/or treating tumor or
cancer.
In another embodiment, the tumor is selected from the group consisting of a
hematological
tumor, a solid tumor, or a combination thereof.
In one embodiment, the cancer is B cell lymphoma.
In another embodiment, the blood tumor is selected from the group consisting
of acute
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
myeloid leukemia (AML), multiple myeloma (MM), chronic lymphocytic leukemia
(CLL), acute
lymphoblastic leukemia (ALL), diffuse large B cell lymphoma (DLBCL), or a
combination
thereof.
In another embodiment, the solid tumor is selected from the group consisting
of gastric
cancer, peritoneal metastasis of gastric cancer, liver cancer, leukemia, renal
cancer, lung cancer,
small intestine cancer, bone cancer, prostate cancer, colorectal cancer,
breast cancer, large intestine
cancer, cervical cancer, ovarian cancer, lymphoma, nasopharyngeal carcinoma,
adrenal tumor,
bladder tumor, non-small cell lung cancer (NSCLC), glioma, endometrial cancer,
or a combination
thereof.
In another embodiment, the tumor is a BCMA positive tumor, such as a BCMA
positive B
cell lymphoma, multiple myeloma, or plasma cell leukemia.
In an eighth aspect, it provides a kit for the preparation of the cell of the
fourth aspect, the kit
comprises a container, and the nucleic acid molecule of the second aspect or
the vector of the third
aspect is located in the container.
In a ninth aspect, it provides a use of the cell of the fourth aspect, or the
preparation of the
sixth aspect for the prevention and/or treatment of cancer or tumor.
In a tenth aspect, it provides a method of treating a disease comprising
administering an
appropriate amount of the cell of the fourth aspect, or the preparation of the
sixth aspect, to a
subject in need of treatment.
In another embodiment, the disease is cancer or tumor.
It is to be understood that the various technical features of the present
disclosure mentioned
above and the various technical features specifically described hereinafter
(as in the Examples)
may be combined with each other within the scope of the present disclosure to
constitute a new
or preferred technical solution, which needs not be described one by one, due
to space
limitations.
16
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1A, 1B, and 1C show screening result of CART-BCMA comparative
examples. FIG.
lA shows detection of transfection efficiency of engineered T cell with
chimeric antigen receptors
targeting BCMA. The expression level of the CAR gene-encoded protein on the
surface of the T
cell membrane in CAR-BCMA cells cultured on day 6 was identified by Fc
fragment staining
method of recombinant human BCMA protein. 1* i05 of CART-BCMA cells cultured
on day 10
were cultured respectively with BCMA-positive K562-BCMA-B9 tumor cell line,
MM. 1S and
RPMI8226 tumor cell lines naturally expressing BCMA, and BCMA-negative K562
tumor cell
line, or without tumor cells, in 200 pi GT-551 medium for 18h at a ratio of
1:1. Then the expression
level of CD137 on the surface of T cell membrane (FIG. 1B) and the secretion
level of IFNy in the
culture supernatant was detected respectively (FIG. 1C).
Figure 2 shows structure of chimeric antigen receptor targeting BCMA. The
structure of
CAR includes a leader sequence, an antigen recognition sequence, a linker
region, a
transmembrane region, a co-stimulatory factor signal region, and a CD3
signaling region.
Figures 3A, 3B, and 3C show detection of transfection efficiency of engineered
T cell with
chimeric antigen receptors targeting BCMA. The expression levels of the CAR
gene-encoded
protein on the surface of the T cell membrane in CAR-BCMA cells cultured on
day 7 (FIG. 3A)
day 21 (FIG. 3B) and day 29 (FIG. 3C) were identified by Fc fragment staining
method of
recombinant human BCMA protein.
Figures 4A and 4B show the expression level of CD137 on the surface of T cell
membrane
(FIG. 4A) and the secretion level of IFNy in the culture supernatant (FIG.
4B). Specifically, lx105
of CART-BCMA cells cultured on day 7 were cultured respectively with BCMA-
positive K562-
BCMA-E7 tumor cell line and BCMA-negative K562 tumor cell line, or without
tumor cells, in
200 pi GT-551 medium for 18h at a ratio of 1:1. Then the expression level of
CD137 on the surface
of T cell membrane and the secretion level of IFNy in the culture supernatant
was detected
respectively.
Figure 5 shows detection of advanced apoptosis level of tumor cells induced by
CART-
BCMAs. Specifically, 1x104 CFSE-labeled BCMA-negative (NH929) or BCMA-positive
(NH929-BCMA) tumor cell lines were co-cultured respectively with corresponding
T cells in 100
pi of GT-551 medium for 4 h at a ratio as shown in the figure. The proportion
of PI-positive cells
17
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
in CFSE-positive cells was analyzed by flow cytometry after staining with 100
pi of 25% PI dye
for 15 min. The figure shows the statistical analysis of PI positive cells in
the corresponding co-
culture samples.
Figures 6A and 6B show detection of advanced apoptosis level of tumor cells
induced by
CART-BCMAs. FIG. 6A shows ratio of CART positive cells in the analyzed
samples, wherein
NT and BCMA-M06 were calculated at 60%. 1 x 10 4 CFSE-labeled BCMA-negative
(NH929)
or BCMA-positive (NH929-BCMA, MM.1S) tumor cell lines were co-cultured
respectively with
corresponding T cells in 100 pi of GT-551 medium for 4 h at a ratio as shown
in the figure. The
proportion of PI-positive cells in CFSE-positive cells was analyzed by flow
cytometry after
staining with 100 pi of 25% PI dye for 15 min. FIG. 6B shows the statistical
analysis of PI positive
cells in the corresponding co-culture samples.
Figures 7A and 7B show the inhibitory effect of CART-BCMAs on the
proliferation in vivo
of myeloma cell line RPMI-8226 in B-NDG mice. RPMI-8226 cells in logarithmic
growth phase
were collected, and 4.0x10 6 tumor cells were inoculated subcutaneously in the
right back of mice.
When the tumor volume reached about 120 mm3, the animals were randomly divided
into 4 groups
according to tumor volume. Then solvent control, 7.5x10 6 NT (T cells only)
and 7.5x10 6 CART-
BCMAs cells were injected through tail vein respectively. FIG. 7A shows that
single injection
of CART-BCMA-1 and CART-BCMA-20 through tail vein can effectively inhibit the
growth of
human myeloma RPMI-8226 cells. FIG. 7B shows that CART-BCMA-1 and CART-BCMA-20
can significantly prolong the survival of tumor-bearing mice with human
myeloma RPMI-8226
cells.
Figure 8 shows results the expression of BCMA, CR2, CXADR, DDR2, and MAG in
293T
cells after transfection of relevant plasmids by flow cytometry.
Figures 9A, 9B, and 9C show the cytokine detection results.
Figure 10 shows the killing ability of each cell to the target cell. Triangle:
BCMA-20 (C-
CAR088); square: positive control; circle: NT control (non-transfected (NT) T
cells).
Figure 11 shows the effect of soluble BCMA on cell killing activity.
Figure 12 shows the survival rate of the mice in each group.
Figure 13 shows the experimental process of phase I clinical study.
Figure 14 shows the clinical response of phase I clinical study.
Figure 15 shows the treatment condition of the patient of ID Z0203-00801C008.
18
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
Figures 16A and 16B show the treatment condition of patient of ID Z0203-
00701C001.
Figure 17 shows the expansion of C-CAR088 and the decrease of M-protein/sFLC
levels in
the blood.
Figures 18A-18C show the results of evaluating the binding specificity of the
scFv of C-
CAR088. Figure 18A shows the experimental scheme. Figure 18B shows the
structure of the anti-
BCMA CAR and scFv rabFc. Figure 18C shows the experimental results.
Figures 19A-19B show tissue cross reactivity IHC GLP study and validation.
HAdCC,
human primary adrenal cortical cells. HPTEC, human primary thyroid epithelial
cells. A549
BCMA OE, a stable strain overexpres sing BCMA.
Figures 20 shows an example of the CAR (C-CAR088) manufacture process. The
process
includes the usage of serum free media, a closed, modular integrated, semi-
automated system, and
digital monitoring. Stars indicate improved processes. Median vein to vein
time is about 17 days
(range: 13 to 83 days). Median manufacturing time is about 7 days (range: 5 to
10 days).
Figure 21 shows C-CAR088 phase I clinical study design (in treating relapsed
or refractory
multiple myeloma). Phase I, open-label, dose escalation and expansion studies
were conducted at
four medical centers. C-CAR088 is an embodiment of the present CAR which is
based on the
BCMA-20 antibody.
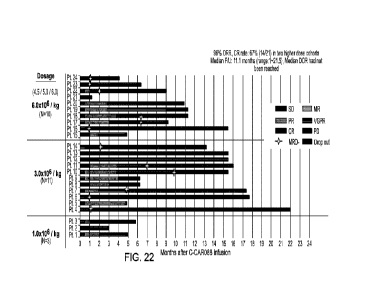
Figure 22 shows the C-CAR088 clinical response, including SD, MR, PR, CR,
VGPR, MRD,
and PD. SD: stable disease; PR: partial response; CR: complete response; PD:
progressive disease;
MR: minimal response; VGPR: very good partial response; MRD: minimal residual
disease.
Figure 23 shows the C-CAR088 clinical response, including CR/sCR, VGPR and PR.
Figure 24 shows the Kaplan Meyer estimation of progression-free survival (PFS)
for mid
and high dose group.
Figure 25A shows the expansion of C-CAR088 in the blood of the patients after
CAR
administration. Figure 25B shows the expansion of C-CAR088 in the blood of the
patients up to
the most recent visit. Figure 25C shows Cma,, levels in the blood of the
patients after CAR
administration. Figure 25D shows AUC levels in the blood of the patients after
CAR
administration. Figure 25E shows Tmax levels in the blood of the patients
after CAR administration.
Figure 25F shows Tiast levels in the blood of the patients after CAR
administration.
19
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
DETAILED DESCRIPTION
The present disclosure provides for chimeric antigen receptors (CARs)
targeting BCMA. In
certain embodiments, the CARs are based on four monoclonal antibodies: BCMA-1,
BCMA-20,
BCMA-CA8, and BCMA-M06. The present disclosure also provides for the analysis
and
identification of the expression levels of the CARs in primary T cells, in
vitro activation ability
and tumor cell killing efficacy of these chimeric antigen receptors. Studies
have shown that the
chimeric antigen receptors of the present disclosure target BCMA positive
cells and can be used
to treat BCMA positive B cell lymphoma, multiple myeloma, plasma cell leukemia
or other
diseases.
Specifically, the present disclosure identifies the correlation between the
expression time and
the expression intensity of different CAR structures on the surface of the
cell membrane after virus
infection, and further identifies the difference in expression of different
CAR structural proteins.
This finding suggests that different CAR structures exist a difference in the
expression level of
CAR protein on the membrane surface and the persistence of CART in vivo
activity under same
infection condition. After extensive screening, the CAR structure of the
present disclosure was
obtained. The results show that the protein encoded by the CAR structure of
the present disclosure
can be fully expressed and membrane-localized.
In the present disclosure, the preparation process of CAR-modified T cell
targeting BCMA
antigen is improved. In one embodiement, GT-551 serum-free medium supplemented
with 1%
human albumin is used to culture lymphocytes in vitro.
Term
The term "about" may refer to a value or composition within an acceptable
error range for a
particular value or composition as determined by those skilled in the art,
which will depend in part
on how the value or composition is measured or determined. The term "about" in
reference to a
numeric value may refer to 10% of the stated numeric value. In other words,
the numeric value
can be in a range of 90% of the stated value to 110% of the stated value.
The term "administering" refers to the physical introduction of a product of
the disclosure
into a subject using any one of various methods and delivery systems known to
those skilled in the
art, including, but not limited to, intravenous, intramuscular, subcutaneous,
intraperitoneal, spinal
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
or other parenteral administration, such as by injection or infusion.
The term "antibody" (Ab) may include, but is not limited to, an immunoglobulin
that
specifically binds an antigen and contains at least two heavy (H) chains and
two light (L) chains
linked by disulfide bonds, or an antigen binding parts thereof. Each H chain
contains a heavy chain
variable region (abbreviated herein as VH) and a heavy chain constant region.
The heavy chain
constant region contains three constant domains, CH1, CH2, and CH3. Each light
chain contains
a light chain variable region (abbreviated herein as VL) and a light chain
constant region. The light
chain constant region contains a constant domain CL. The VH and VL regions can
be further
subdivided into hypervariable regions called complementarity determining
regions (CDR), which
are interspersed within more conservative regions called framework regions
(FR). Each VH and
VL contains three CDRs and four FRs, which are arranged from amino terminal to
carboxy
terminal in the following order: FR1, CDR1, FR2, CDR2, FR3, CDR3, FR4. The
variable regions
of the heavy and light chains contain a binding domain that interacts with an
antigen.
Chimeric antigen receptors (CARs)
The chimeric antigen receptors (CARs) of the present disclosure may comprise
an
extracellular domain, a transmembrane domain, and an intracellular domain. The
extracellular
domain comprises a target-specific binding element (also known as an antigen
binding domain).
The intracellular domain includes a co-stimulatory signaling region and a
chain. The co-
stimulatory signaling region refers to a part of the intracellular domain that
includes a co-
stimulatory molecule. The co-stimulatory molecule is a cell surface molecule
required for efficient
response of lymphocytes to antigens, rather than an antigen receptor or its
ligand.
A linker can be incorporated between the extracellular domain and the
transmembrane
domain of the CAR, or between the cytoplasmic domain and the transmembrane
domain of the
CAR. As used herein, the term "linker" generally refers to any oligopeptide or
polypeptide that
plays a role of linking the transmembrane domain to the extracellular domain
or the cytoplasmic
domain in a polypeptide chain. The linker may comprise 0-300 amino acids, 2-
100 amino acids,
or 3-50 amino acids.
In one embodiment, the extracellular domain of the CAR comprises an antigen
binding
domain targeting BCMA. When the CAR of the present disclosure is expressed in
T cell, antigen
recognition can be performed based on antigen binding specificity. When the
CAR binds to its
21
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
associated antigen, it affects tumor cell, causing tumor cell to fail to grow,
to death or to be affected
otherwise, causing the patient's tumor burden to shrink or eliminate. The
antigen binding domain
may be fused to the intracellular domain from one or more of the co-
stimulatory molecules and
the chain. The antigen binding domain may be fused with an intracellular
domain of a
combination of a 4-1BB signaling domain and a CD3t signaling domain.
As used herein, the "antigen binding domain" and "single-chain antibody
fragment" may refer
to a Fab fragment, a Fab' fragment, a F (ab') 2 fragment, or a single Fv
fragment that has antigen-
binding activity. The Fv antibody contains the heavy chain variable region and
the light chain
variable region of the antibody, but has no constant region. The Fv antibody
has the smallest
antibody fragment with all antigen-binding sites. Generally, Fv antibodies
also include a
polypeptide linker between the VH and VL domains, and can form the structure
required for
antigen binding. The antigen binding domain is usually a scFv (single-chain
variable fragment).
The size of scFv is typically 1/6 of a complete antibody. The single-chain
antibody may be an
amino acid chain sequence encoded by a nucleotide chain. In certain
embodiments, the scFv may
comprise an antibody which specifically recognizes the extracellular region of
BCMA, such as
amino acid residues at positions 24 to 41 of the BCMA sequence. The antibody
may be a single
chain antibody.
As for the hinge region and the transmembrane region (transmembrane domain),
the CAR
can be designed to comprise a transmembrane domain fused to the extracellular
domain of the
CAR. In one embodiment, a transmembrane domain that is naturally associated
with one of the
domains in the CAR is used. In some embodiments, transmembrane domains may be
selected or
modified by amino acid substitutions to avoid binding such domains to the
transmembrane domain
of the same or different surface membrane proteins, thereby minimizing the
interaction with other
members of the receptor complexes.
The intracellular domain in the CAR comprises the signaling domain of 4-1BB
and the
signaling domain of CD3c
In certain embodiments, the CAR structure of the present disclosure comprises
a signal
peptide, an antigen recognition sequence (antigen-binding domain), a linker
region, a
transmembrane region, a co-stimulatory factor signal region, and a CD3zeta
signaling region
chain portion). The order of connection is as follows:
[CD8 SHVL-Linker-VHHhinge-CD8TM[44-1BBHCD3zetal
22
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
In certain embodiments, the sequence selected in the present disclosure is as
follows:
(1) The signal peptide is a signal peptide sequence derived from CD8:
MALPVTALLLPLALLLHAARP (SEQ ID NO: 9)
(2) light chain (VL) sequence of single-chain variable region derived from
BCMA-1
antibody:
DIVLTQSPPSLAMSLGKRATISCRASESVTILGSHLIHWYQQKPGQPPTLLIQLASNV
QTGVPARFSGS GSRTDFTLTIDPVEEDDVAVYYCLQSRTIPRTFGGGTKLEIK (SEQ ID
NO: 7)
(3) heavy chain (VH) sequence of single-chain variable region derived from
BCMA-1
antibody:
QIQLVQSGPELKKPGETVKISCKASGYTFTDYSINWVKRAPGKGLKWMGWINTTETREPA
YAYDFRGRFAFSLETSASTAYLQINNLKYEDTATYFCALDYSYAMDYWGQGTSVTVSS
(SEQ ID NO: 8)
Among them, BCMA-1 is an antibody sequence contained in a published Car-T
sequence,
and is used as a control in the present application.
(4) light chain (VI) sequence of single-chain variable region derived from
BCMA-20
antibody:
DIQMTQSPSSLSASVGDRVTITCRASCIGISNYLNWYQQKPGKAPKPLIYYTSNLCIS
GVPSRFSGSGSGT DYTLTISSLQPEDFATYYCMGCITISSYTFGQGTKLEI (SEQ ID NO: 1)
Length
VI, Residues of SEQ (number of
Sequence
Region ID NO: 1
amino acid
residues)
DIQMTQSPSSLSASVGDRVTITC (SEQ ID
LFR1 1-23 23
NO: 16)
CDR-L1 RAS QGISNYLN (SEQ ID NO: 17) 24- 34 11
LFR2 WYQQKPGKAPKPLIY (SEQ ID NO: 18) 35 - 49 15
CDR-L2 YTSNLQS (SEQ ID NO: 19) 50 - 56 7
GVPSRFS GS GS GTDYTLTIS SLQPEDFATY
LFR3 57 - 88 32
YC (SEQ ID NO: 20)
23
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
CDR-L3 MGQTISSYT (SEQ ID NO: 21) 89 - 97 9
LFR4 FGQGTKLEI (SEQ ID NO: 22) 98 - 106 9
(5) heavy chain (VH) sequence of single-chain variable region derived from
BCMA-20
antibody:
EVQLVESGGGLVQPGGSLRLSCAAS GFTFSNFDMAWVRQAPGKGLVWVSSITTG
ADHAIYADSVKGRFTISRDNAKNTLYLQMNSLRAEDTAVYYCVRHGYYDGYHLFDY
WGQGTLVTVSS (SEQ ID NO: 2)
Length
VII Residues of
(number of
Sequence
Region SEQ ID NO: 2 amino
acid
residues)
HFR1 EVQLVESGGGLVQPGGSLRLSCAASGF 1 -30 30
TFS (SEQ lD NO: 23)
CDR- NFDMA (SEQ ID NO: 24) 31 - 35 5
H1
HFR2 WVRQAPGKGLVWVS (SEQ ID NO: 25) 36 - 49 14
CDR- SITTGADHAIYADSVKG (SEQ ID NO: 26) 50 - 66 17
H2
HFR3 RFTISRDNAKNTLYLQMNSLRAEDTAV 67 - 98 32
YYCVR (SEQ ID NO: 27)
CDR-
HGYYDGYHLFDY (SEQ ID NO: 28) 99- 110 12
H3
HFR4 WGQGTLVTVSS (SEQ ID NO: 29) 111 - 121 11
(6) light chain (VL) sequence of single-chain variable region derived from
BCMA-CA8
antibody:
DIQLTQTTSSLSASLGDRVTISCSASTTTSNYLNWYQQKPDGTVELVIYYTSNLHG
GGPSRFSGSGSGTDYSLTIGYLEPEDVATYYCCICIYRKLPWTFGGGSKLEIKR (SEQ ID
NO: 3)
24
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
Length
(number
VI, Residues of SEQ
Sequence of
amino
Region ID NO: 3
acid
residues)
LFR1 DIQLTQTTSSLSASLGDRVTISC (SEQ ID 1 - 23 23
NO: 30)
CDR-L1 SASTTTSNYLN (SEQ ID NO: 31) 24 - 34 11
LFR2 WYQQKPDGTVELVIY (SEQ ID NO: 32) 35 - 49 15
CDR-L2 YTSNLHG (SEQ ID NO: 33) 50- 56 7
LFR3 GGPSRFSGSGSGTDYSLTIGYLEPEDV 57 - 88 32
ATYYC (SEQ ID NO: 34)
CDR-L3 QQYRKLPWT (SEQ ID NO: 35) 89 - 97 9
LFR4 FGGGSKLEIKR (SEQ ID NO: 36) 98 - 108 11
(7) heavy chain (VH) sequence of single-chain variable region derived from
BCMA-CA8
antibody:
EVQLQQSGAVLARPGASVKMSCKGSGYTFTNYWMHWVKQRPGQGLEWIGATY
RGHSDTYYNCIKFKGKAKLTAVTSTSTAYMELSSLTNEDSAVYYCTRGAIYNGYDVLD
NWGQGTLVTVSS (SEQ ID NO: 4)
Length
(number
VII Residues of SEQ
Sequence of
amino
Region ID NO: 4
acid
residues)
HFR1 EVQLQQSGAVLARPGASVKMSCKGSG 1 -30 30
YTFT (SEQ ID NO: 37)
CDR-H1 NYWMH (SEQ ID NO: 38) 31 -35 5
HFR2 WVKQRPGQGLEWIG (SEQ ID NO: 39) 36 - 49 14
CDR-H2 ATYRGHSDTYYNQKFKG (SEQ ID NO: 50 - 66 17
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
40)
HFR3 KAKLTAVTSTSTAYMELSSLTNEDSAV 67 - 98
32
YYCTR (SEQ ID NO: 41)
CDR-H3
GAIYNGYDVLDN (SEQ ID NO: 42) 99- 110
12
HFR4 WGQGTLVTVSS (SEQ ID NO: 43) 111 - 121
11
(8) light chain (VL) sequence of single-chain variable region derived from
BCMA-M06
antibody:
DIQMTQSPSSLSASVGDRVTITCSASCIDISNYLNWYQQKPGKAPKLLIYYTSNLHS
GVPSRFS GS GS GTDFTLTISSLQPEDFATYYCCICIYRKLPWTFGQGTKLEIKR (SEQ ID
NO: 5)
Length
VL Residues of SEQ (number of
Sequence
Region ID NO: 5 amino
acid
residues)
LFR1 DIQMTQSPSSLSASVGDRVTITC (SEQ 1 - 23 23
ID NO: 44)
CDR-L1 SASQDISNYLN (SEQ ID NO: 45) 24 - 34 11
LFR2 WYQQKPGKAPKLLIY (SEQ ID NO: 46) 35 - 49 15
CDR-L2 YTSNLHS (SEQ ID NO: 47) 50 - 56 7
LFR3 GVPSRFSGSGSGTDFTLTISSLQPEDF 57 - 88 32
ATYYC (SEQ ID NO: 48)
CDR-L3 QQYRKLPWT (SEQ ID NO: 49) 89 - 97 9
LFR4 FGQGTKLEIKR (SEQ ID NO: 50) 98 - 108 11
(9) heavy chain (VH) sequence of single-chain variable region derived from
BCMA-M06
antibody:
QVQLVQSGAEVKKPGSSVKVSCKASGGTFSNYWMHWVRQAPGQGLEWMGATY
RGHSDTYYNOKFKGRVTITADKSTSTAYMELSSLRSEDTAVYYCARGAIYDGYDVLD
26
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
NWGQGTLVTVSS (SEQ ID NO: 6)
Length
(number
VII Residues of SEQ
Sequence
of amino
Region ID NO: 6
acid
residues)
HFR1 QVQLVQSGAEVKKPGSSVKVSCKASG 1 -30 30
GTFS (SEQ ID NO: 51)
CDR-H1 NYWMH (SEQ ID NO: 52) 31 -35 5
HFR2 WVRQAPGQGLEWMG (SEQ ID NO: 53) 36 - 49 14
CDR-H2 ATYRGHSDTYYNQKFKG (SEQ ID NO: 50 - 66 17
54)
HFR3 RVTITADKSTSTAYMELSSLRSEDTAV 67 - 98 32
YYCAR (SEQ ID NO: 55)
CDR-H3
GAIYDGYDVLDN (SEQ ID NO: 56) 99- 110 12
HFR4 WGQGTLVTVSS (SEQ ID NO: 57) 111 - 121 11
(10) The linker sequence between heavy chain and light chain of BCMA-1 single-
chain
variable region is:
GSTSGSGKPGSGEGSTKG (SEQ ID NO: 10)
(11) The linker sequence between heavy chain and light chain of BCMA-20, BCMA-
CA8,
and BCMA-M06 single-chain variable region is:
GGGGSGGGGSGGGGS (SEQ ID NO: 11)
(12) Sequence of hinge region and linker region:
FVPVFLPAKPTTTPAPRPPTPAPTIAS QPLSLRPEACRPAAGGAVHTRGLDFACD
(SEQ ID NO: 12)
(13) The transmembrane region is a transmembrane region sequence of CD8
(CD8TM)
antigen:
IYIWAPLAGTCGVLLLSLVITLYC (SEQ ID NO: 13)
(14) The co-stimulatory factor signal region is derived from the sequence of 4-
1BB
27
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
cytoplasmic signaling motif:
KRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCEL (SEQ ID NO: 14)
(15) The signaling region of CD3 zeta is derived from the sequence of
immunorecceptor
tyrosine-based activation motif (ITAM) of CD3zeta in the TCR complex:
RVKFSRSADAPAYQQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPQRRK
NPQEGLYNELQKDKMAEAYS EIGMKGERRRGKGHDGLY QGLS TAT KDTYD ALHMQA
LPPR (SEQ ID NO: 15)
In certain embodiments, the nucleic acid encoding the CAR (derived from the
BCMA-20 antibody) may have the following sequence (SEQ ID NO: 58):
atggccttaccagtgaccgccttgctcctgccgctggccttgctgctccacgccgccaggccggacatccagatgaccc
agtcccc
ctcctccctgtccgcctccgtgggcgaccgggtgaccatcacctgccgggcctcccagggcatctccaactacctgaac
tggtacc
agcagaagcccggcaaggcccccaagcccctgatctactacacctccaacctgcagtccggcgtgccctcccggttctc
cggctc
cggctccggcaccgactacaccctgaccatctcctccctgcagcccgaggacttcgccacctactactgcatgggccag
accatct
cctcctacaccttcggccagggcaccaagctggagatcaagggtggcggtggctcgggcggtggtgggtcgggtggcgg
cgga
tctgaggtgcagctggtggagtccggcggcggcctggtgcagcccggcggctccctgcggctgtcctgcgccgcctccg
gcttc
accttctccaacttcgacatggcctgggtgcggcaggcccccggcaagggcctggtgtgggtgtcctccatcaccaccg
gcgccg
accacgccatctacgccgactccgtgaagggccggttcaccatctcccgggacaacgccaagaacaccctgtacctgca
gatgaa
ctccctgcgggccgaggacaccgccgtgtactactgcgtgcggcacggctactacgacggctaccacctgttcgactac
tggggc
cagggcaccctggtgaccgtgtcctccttcgtgccggtcttcctgccagcgaagcccaccacgacgccagcgccgcgac
cacca
acaccggcgcccaccatcgcgtcgcagcccctgtccctgcgcccagaggcgtgccggccagcggcggggggcgcagtgc
aca
cgagggggctggacttcgcctgtgatatctacatctgggcgcccttggccgggacttgtggggtccttctcctgtcact
ggttatcac
cctttactgcaaacggggcagaaagaaactcctgtatatattcaaacaaccatttatgagaccagtacaaactactcaa
gaggaagat
ggctgtagctgccgatttccagaagaagaagaaggaggatgtgaactgagagtgaagttcagcaggagcgcagacgccc
ccgc
gtaccagcagggccagaaccagctctataacgagctcaatctaggacgaagagaggagtacgatgttttggacaagaga
cgtggc
cgggaccctgagatggggggaaagccgagaaggaagaaccctcaggaaggcctgtacaatgaactgcagaaagataaga
tgg
cggaggcctacagtgagattgggatgaaaggcgagcgccggaggggcaaggggcacgatggcctttaccagggtctcag
taca
gccaccaaggacacctacgacgcccttcacatgcaggccctgccccctcgctaa
In certain embodiments, the CAR (derived from the BCMA-20 antibody) may have
28
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
the following amino acid sequence (SEQ ID NO: 59):
MALPVTALLLPLALLLHAARPDIQMTQSPSSLSASVGDRVTITCRASQGISNYLNWY
QQKPGKAPKPLIYYTSNLQS GVPSRFS GS GS GTDYTLTIS SLQPEDFATYYCMGQTISS
YTFGQGTKLEIKGGGGSGGGGS GGGGSEVQLVESGGGLVQPGGSLRLSCAASGFTFS
NFDMAWVRQAPGKGLVWVS S ITTGADHAIYADSVKGRFTISRDNAKNTLYLQMNS
LRAEDTAVYYCVRHGYYDGYHLFDYWGQGTLVTVSSFVPVFLPAKPTTTPAPRPPT
PAPTIAS QPLS LRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLS LVITLY
CKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYQ
QGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMA
EAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR
In certain embodiments, the nucleic acid encoding the CAR (derived from the
BCMA-
CA8 antibody) may have the following sequence (SEQ ID NO: 60):
atggccttaccagtgaccgccttgctcctgccgctggccttgctgctccacgccgccaggccggatatccagctgaccc
agaccac
aagcagcctgagcgcctccctgggcgacagggtgaccattagctgcagcgccagccaggacatcagcaactacctgaac
tggta
ccagcagaagcccgacggcaccgtggagctcgtgatctactacacctccaacctgcacagcggcgtgcccagcaggttc
tctggc
agcggcagcggcaccgactacagcctgaccatcggctatctggagcccgaggacgtcgccacctactactgccagcagt
acagg
aagctgccctggaccttcggcggaggctctaagctggagattaagcgtggtggcggtggctcgggcggtggtgggtcgg
gtggc
ggcggatctgaggtgcagctgcagcagagcggcgccgtgctggccaggcccggagctagcgtgaagatgagctgcaagg
gca
gcggctacaccttcaccaactactggatgcactgggtgaaacagaggcccggccagggactggagtggatcggcgccac
ctaca
ggggccacagcgacacctactacaaccagaagttcaagggcaaggccaagctgaccgccgtgacctcaaccagcaccgc
ctac
atggaactgagcagcctgaccaacgaggacagcgccgtctattactgcaccaggggcgccatctacaacggctacgacg
tgctg
gacaattggggccagggaacactagtgaccgtgtccagcttcgtgccggtcttcctgccagcgaagcccaccacgacgc
cagcg
ccgcgaccaccaacaccggcgcccaccatcgcgtcgcagcccctgtccctgcgcccagaggcgtgccggccagcggcgg
ggg
gcgcagtgcacacgagggggctggacttcgcctgtgatatctacatctgggcgcccttggccgggacttgtggggtcct
tctcctgt
cactggttatcaccctttactgcaaacggggcagaaagaaactcctgtatatattcaaacaaccatttatgagaccagt
acaaactact
caagaggaagatggctgtagctgccgatttccagaagaagaagaaggaggatgtgaactgagagtgaagttcagcagga
gcgca
gacgcccccgcgtaccagcagggccagaaccagctctataacgagctcaatctaggacgaagagaggagtacgatgttt
tggac
aagagacgtggccgggaccctgagatggggggaaagccgagaaggaagaaccctcaggaaggcctgtacaatgaactgc
aga
29
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
aagataagatggcggaggcctacagtgagattgggatgaaaggcgagcgccggaggggcaaggggcacgatggccttta
ccag
ggtctcagtacagccaccaaggacacctacgacgcccttcacatgcaggccctgccccctcgctaa
In certain embodiments, the CAR (derived from the BCMA-CA8 antibody) may have
the following amino acid sequence (SEQ ID NO: 61):
MALPVTALLLPLALLLHAARPDIQLTQTTS S LS ASLGDRVTIS CS AS QDISNYLNWYQ
QKPDGTVELVIYYTSNLHS GVPSRFS GS GS GTDYSLTIGYLEPEDVATYYCQQYRKLP
WTFGGGS KLEIKRGGGGS GGGGS GGGGSEVQLQQS GAVLARPGASVKMSCKGS GY
TFTNYWMHWVKQRPGQGLEWIGATYRGHSDTYYNQKFKGKAKLTAVTS TS TAYM
ELS S LTNEDSAVYYCTRGAIYNGYDVLDNWGQGTLVTVS S FVPVFLPAKPTTTPAPR
PPTPAPTIAS QPLS LRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLS LVIT
LYCKRGRKKLLYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFS RS ADAPA
YQQGQNQLYNELNLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDK
MAEAYSEIGMKGERRRGKGHDGLYQGLSTATKDTYDALHMQALPPR
In certain embodiments, the nucleic acid encoding the CAR (derived from the
BCMA-
M06 antibody) may have the following sequence (SEQ ID NO: 62):
atggccttaccagtgaccgccttgctcctgccgctggccttgctgctccacgccgccaggccggacatccagatgaccc
agagccctagct
cactgagcgccagcgtgggcgacagggtgaccattacctgctccgccagccaggacatcagcaactacctgaactggta
ccagcagaag
cccggcaaggcccccaagctgctgatctactacacctccaacctgcactccggcgtgcccagcaggttcagcggaagcg
gcagcggca
ccgatttcaccctgaccatctccagcctgcagcccgaggacttcgccacctactactgccagcagtacaggaagctccc
ctggactttcggc
cagggcaccaaactggagatcaagcgtggtggcggtggctcgggcggtggtgggtcgggtggcggcggatctcaggtgc
agctggtcc
agagcggcgccgaagtgaagaagcccggcagctccgtgaaagtgagctgcaaggccagcggcggcaccttcagcaacta
ctggatgc
actgggtgaggcaggcccccggacagggcctggagtggatgggcgccacctacaggggccacagcgacacctactacaa
ccagaagt
tcaagggccgggtgaccatcaccgccgacaagagcaccagcaccgcctacatggaactgagcagcctcaggagcgagga
caccgctg
tgtattactgcgccaggggcgccatctacgacggctacgacgtgctggacaactggggccagggcacactagtgaccgt
gtccagcttcg
tgccggtcttcctgccagcgaagcccaccacgacgccagcgccgcgaccaccaacaccggcgcccaccatcgcgtcgca
gcccctgtc
cctgcgcccagaggcgtgccggccagcggcggggggcgcagtgcacacgagggggctggacttcgcctgtgatatctac
atctgggcg
cccttggccgggacttgtggggtccttctcctgtcactggttatcaccctttactgcaaacggggcagaaagaaactcc
tgtatatattcaaac
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
aaccatttatgagaccagtacaaactactcaagaggaagatggctgtagctgccgatttccagaagaagaagaaggagg
atgtgaactgag
agtgaagttcagcaggagcgcagacgcccccgcgtaccagcagggccagaaccagctctataacgagctcaatctagga
cgaagagag
gagtacgatgttttggacaagagacgtggccgggaccctgagatggggggaaagccgagaaggaagaaccctcaggaag
gcctgtaca
atgaactgcagaaagataagatggcggaggcctacagtgagattgggatgaaaggcgagcgccggaggggcaaggggca
cgatggcc
tttaccagggtctcagtacagccaccaaggacacctacgacgcccttcacatgcaggccctgccccctcgctaa
In certain embodiments, the CAR (derived from the BCMA-BCMA-M06 antibody)
may have the following amino acid sequence (SEQ ID NO: 63):
MALPVTALLLPLALLLHAARPDIQMTQS PS S LS AS VGDRVTITCS AS QDISNYLNWYQQ
KPGKAPKLLIYYTS NLHS GVPS RFS GS GS GTDFTLTIS S LQPEDFATYYCQQYRKLPWTF
GQGTKLEIKRGGGGSGGGGSGGGGS QVQLVQSGAEVKKPGSSVKVSCKASGGTFSNY
WMHWVRQAPGQGLEWMGATYRGHS DTYYNQKFKGRVTITADKS TS TAYMELS S LRS
EDTAVYYCARGAIYDGYDVLDNWGQGTLVTVS S FVPVFLPAKPTTTPAPRPPTPAPTIAS
QPLSLRPEACRPAAGGAVHTRGLDFACDIYIWAPLAGTCGVLLLSLVITLYCKRGRKKL
LYIFKQPFMRPVQTTQEEDGCSCRFPEEEEGGCELRVKFSRSADAPAYQQGQNQLYNEL
NLGRREEYDVLDKRRGRDPEMGGKPRRKNPQEGLYNELQKDKMAEAYSEIGMKGERR
RGKGHDGLYQGLSTATKDTYDALHMQALPPR
Chimeric antigen receptor T cells (CAR-T cells)
As used herein, the terms "CAR-T cell", "CAR-T", and "CART", may be used
interchangeably.
The present disclosure relates to the construction of a chimeric antigen
receptor structure
targeting BCMA, a preparation method of a chimeric antigen receptor engineered
T cell targeting
BCMA, and activity identification thereof.
Vector
The nucleic acid sequences coding for the desired molecules can be obtained
using
recombinant methods known in the art, such as, for example by screening
libraries from cells
expressing the gene, by deriving the gene from a vector known to include the
same, or by isolating
directly from cells and tissues containing the same, using standard
techniques. Alternatively, the
31
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
gene of interest can be produced synthetically.
The present disclosure also provides vectors in which the expression cassette
of the present
disclosure is inserted. Vectors derived from retroviruses such as the
lentivirus are suitable tools to
achieve long-term gene transfer since they allow long-term, stable integration
of a transgene and
its propagation in daughter cells. Lentiviral vectors have the advantage over
vectors derived from
onco-retroviruses such as murine leukemia viruses in that they can transduce
non-proliferating
cells, such as hepatocytes. They also have the advantage of low
immunogenicity.
In brief summary, the expression cassette or nucleic acid sequence is
typically and operably
linked to a promoter, and incorporated into an expression vector. The vectors
can be suitable for
replication and integration in eukaryotes. Typical cloning vectors contain
transcription and
translation terminators, initiation sequences, and promoters useful for
regulation of the expression
of the desired nucleic acid sequence.
The expression constructs of the present disclosure may also be used for
nucleic acid immune
and gene therapy, using standard gene delivery protocols. Methods for gene
delivery are known in
the art. See, e.g., U.S, Pat. Nos. 5,399,346, 5,580,859, 5,589,466,
incorporated by reference herein
in their entireties. In another embodiment, the disclosure provides a gene
therapy vector.
The nucleic acid can be cloned into a number of types of vectors. For example,
the nucleic
acid can be cloned into a vector including, but not limited to a plasmid, a
phagemid, a phage
derivative, an animal virus, and a cosmid. Vectors of particular interest
include expression vectors,
replication vectors, probe generation vectors, and sequencing vectors.
Further, the expression vector may be provided to a cell in the form of a
viral vector. Viral
vector technology is well known in the art and is described, for example, in
Sambrook et al, (2001
, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, New
York), and in
other virology and molecular biology manuals. Viruses, which are useful as
vectors include, but
are not limited to, retroviruses, adenoviruses, adeno-associated viruses,
herpes viruses, and
lentiviruses. In general, a suitable vector contains an origin of replication
functional in at least one
organism, a promoter sequence, convenient restriction endonuclease sites, and
one or more
selectable markers, (e.g., WO 01/96584; WO 01/29058; and U.S, Pat. No. 6,326,
193).
A number of viral based systems have been developed for gene transfer into
mammalian
cells. For example, retroviruses provide a convenient platform for gene
delivery systems. A
selected gene can be inserted into a vector and packaged in retroviral
particles using techniques
32
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
known in the art. The recombinant virus can then be isolated and delivered to
cells of the subject
either in vivo or ex vivo. A number of retroviral systems are known in the
art. In some
embodiments, adenovirus vectors are used. A number of adenovirus vectors are
known in the art.
In one embodiment, lentivirus vectors are used.
Additional promoter elements, e.g., enhancers, regulate the frequency of
transcriptional
initiation. Typically, these are located in the region 30-110 bp upstream of
the start site, although
a number of promoters have recently been shown to contain functional elements
downstream of
the start site as well. The spacing between promoter elements frequently is
flexible, so that
promoter function is preserved when elements are inverted or moved relative to
one another. In
the thymidine kinase (tk) promoter, the spacing between promoter elements can
be increased to 50
bp apart before activity begins to decline. Depending on the promoter, it
appears that individual
elements can function either cooperatively or independently to activate
transcription.
One example of a suitable promoter is the immediate early cytomegalovirus
(CMV)
promoter sequence. This promoter sequence is a strong constitutive promoter
sequence capable of
driving high levels of expression of any polynucleotide sequence operatively
linked thereto.
Another example of a suitable promoter is Elongation Growth Factor-la (EF-
1a). However, other
constitutive promoter sequences may also be used, including, but not limited
to the simian virus
40 (SV40) early promoter, mouse mammary tumor virus (MMTV), human
immunodeficiency
virus (HIV) long terminal repeat (LTR) promoter, MoMuLV promoter, an avian
leukemia virus
promoter, an Epstein-Barr virus immediate early promoter, a Rous sarcoma virus
promoter, as well
as human gene promoters such as, but not limited to, the actin promoter, the
myosin promoter, the
hemoglobin promoter, and the creatine kinase promoter. Further, either
constitutive promoters or
inducible promoters may be used. The use of an inducible promoter provides a
molecular switch
capable of turning on expression of the polynucleotide sequence which it is
operatively linked
when such expression is desired, or turning off the expression when expression
is not desired.
Examples of inducible promoters include, but are not limited to a
metallothionein promoter, a
glucocorticoid promoter, a progesterone promoter, and a tetracycline promoter.
In order to assess the expression of a CAR polypeptide or portions thereof,
the expression
vector to be introduced into a ceil can also contain either a selectable
marker gene or a reporter
gene or both to facilitate identification and selection of expressing cells
from the population of
cells sought to be transfected or infected through viral vectors. In other
aspects, the selectable
33
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
marker may be carried on a separate piece of DNA and used in a co-
transfection procedure. Both
selectable markers and reporter genes may be flanked with appropriate
regulatory sequences to
enable expression in the host cells. Useful selectable markers include, for
example, antibiotic-
resistance genes, such as neo and the like.
Reporter genes are used for identifying potentially transfected cells and for
evaluating the
functionality of regulatory sequences. In general, a reporter gene is a gene
that is not present in or
expressed by the recipient organism or tissue and that encodes a polypeptide
whose expression is
manifested by some easily detectable property, e.g., enzymatic activity.
Expression of the reporter
gene is assayed at a suitable time after the DNA has been introduced into the
recipient cells.
Suitable reporter genes may include genes encoding luciferase, beta-
galactosidase,
chloramphenicol acetyl transferase, secreted alkaline phosphatase, or the
green fluorescent protein
gene (e.g., Ui-Tei et al., 2000 FEBS Letters 479: 79-82). Suitable expression
systems are well
known and may be prepared using known techniques or obtained commercially. In
general, the
construct with the minimal 5' flanking region showing the highest level of
expression of reporter
gene is identified as the promoter. Such promoter regions may be linked to a
reporter gene and
used to evaluate agents for the ability to modulate promoter- driven
transcription.
Methods of introducing and expressing genes into a cell are known in the art.
In the context
of an expression vector, the vector can be readily introduced into a host
cell, e.g., mammalian,
bacterial, yeast, or insect cell by any method in the art. For example, the
expression vector can be
transferred into a host cell by physical, chemical, or biological means.
Physical methods for introducing a polynucleotide into a host cell include
calcium phosphate
precipitation, lipofection, particle bombardment, microinjection,
electroporation, and the like.
Methods for producing cells comprising vectors and/or exogenous nucleic acids
are well-known
in the art. See, for example, Sambrook et al. (2001, Molecular Cloning: A
Laboratory Manual,
Cold Spring Harbor Laboratory, New York). A method for the introduction of a
polynucleotide
into a host cell is calcium phosphate transfection.
Biological methods for introducing a polynucleotide of interest into a host
cell include the
use of DNA and RNA vectors. Viral vectors, and especially retroviral vectors,
have become the
most widely used method for inserting genes into mammalian, e.g., human cells.
Other viral
vectors can be derived from lentivirus, poxviruses, herpes simplex virus I,
adenoviruses and adeno-
associated viruses, and the like. See, for example, U.S. Pat, Nos. 5,350,674
and 5,585,362.
34
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
Chemical means for introducing a polynucleotide into a host cell include
colloidal dispersion
systems, such as macromolecule complexes, nanocapsules, microspheres, beads,
and lipid-based
systems including oil-in-water emulsions, micelles, mixed micelles, and
liposomes. An exemplary
colloidal system for use as a delivery vehicle in vitro and in vivo is a
liposome (e.g., an artificial
membrane vesicle).
In the case where a non-viral delivery system is utilized, an exemplary
delivery vehicle is a
liposome. The use of lipid formulations is contemplated for the introduction
of the nucleic acids
into a host cell (in vitro, ex vivo or in vivo). In another aspect, the
nucleic acid may be associated
with a lipid. The nucleic acid associated with a lipid may be encapsulated in
the aqueous interior
of a liposome, interspersed within the lipid bilayer of a liposome, attached
to a liposome via a
linking molecule that is associated with both the liposome and the
oligonucleotide, entrapped in a
liposome, complexed with a liposome, dispersed in a solution containing a
lipid, mixed with a
lipid, combined with a lipid, contained as a suspension in a lipid, contained
or complexed with a
micelle, or otherwise associated with a lipid. Lipid, lipid/DNA or
lipid/expression vector
associated compositions are not limited to any particular structure in
solution. For example, they
may be present in a bilayer structure, as micelles, or with a "collapsed"
structure. They may also
simply be interspersed in a solution, possibly forming aggregates that are not
uniform in size or
shape. Lipids are fatty substances which may be naturally occurring or
synthetic lipids. For
example, lipids include the fatty droplets that naturally occur in the
cytoplasm as well as the class
of compounds which contain long-chain aliphatic hydrocarbons and their
derivatives, such as fatty
acids, alcohols, amines, amino alcohols, and aldehydes.
In one embodiment, the vector is a lentiviral vector.
Compositions
The disclosure provides a composition comprising the immune cell (e.g., CAR-T
cell), and
a pharmaceutically acceptable carrier, diluent and/or excipient. In one
embodiment, the
composition is a liquid composition. For example, the composition is an
injectable composition.
In certain embodiments, the concentration of the CAR-T cells in the
composition is 1 x 103-1x108
cells/ml, or 1 x 104-1x107 cells/ml.
In one embodiment, the composition may comprise buffers such as neutral
buffered saline,
phosphate buffered saline and the like; carbohydrates such as glucose,
mannose, sucrose or
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
dextrans, mannitol; proteins; polypeptides or amino acids such as glycine;
antioxidants; chelating
agents such as EDTA or glutathione; adjuvants (e.g., aluminum hydroxide); and
preservatives. The
composition may be formulated for intravenous administration.
Therapeutic application
The disclosure comprises therapeutic applications using cells (e.g., T cells)
transduced with
a lentiviral vector (LV) encoding the expression cassette of the disclosure.
The transduced T cells
can target the tumor cell marker BCMA, synergistically activate T cells, and
cause T cell immune
responses, thereby significantly increasing the killing efficiency against
tumor cells.
Thus, the present disclosure also provides a method for stimulating a T cell-
mediated
immune response to a target cell population or tissue in a mammal comprising
the step of
administering to the mammal a CAR-T cell of the disclosure.
In one embodiment, the present disclosure comprises a class of cell therapies,
wherein T
cells from autologous patient (or heterologous donor) are isolated, activated
and genetically
modified to generate CAR-T cells, and then injected into the same patient. The
probability of graft
versus host disease in the way is extremely low, and antigens are recognized
by T cells in a non-
MHC-restricted manner. In addition, one CAR-T can treat all cancers that
express the antigen.
Unlike antibody therapies, CAR-T cells are able to replicate in vivo resulting
in long-term
persistence that can lead to sustained tumor control
In one embodiment, the CAR-T cells of the disclosure can undergo robust in
vivo T cell
expansion and can persist for an extended amount of time. In addition, the CAR
mediated immune
response may be part of an adoptive immunotherapy approach in which CAR-
modified T cells
induce an immune response specific to the antigen binding moiety in the CAR.
For example, an
anti-BCMA CAR-T cell elicits an immune response specific against cells
expressing BCMA.
Although the data disclosed herein specifically disclose lentiviral vector
comprising BCMA
scFv, hinge and transmembrane domain, and 4-1BB and CD3t signaling domains,
the disclosure
should be construed to include any number of variations for each of the
components of the
construct as described elsewhere herein.
Cancers that may be treated include tumors that are unvascularized or largely
unvascularized, and tumors that are vascularized. Cancers may include non-
solid tumors (such as
hematological tumors, for example, leukemias and lymphomas) or solid tumors.
Types of cancers
36
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
to be treated with the CARs include, but are not limited to, carcinoma,
blastoma, and sarcoma, and
certain leukemia or lymphoid malignancies, benign and malignant tumors, and
malignancies e.g.,
sarcomas, carcinomas, and melanomas. Adult tumors/cancers and pediatric
tumors/cancers are
also included.
Hematologic cancers are cancers of the blood or bone marrow. Examples of
hematological
(or hematogenous) cancers include leukemias, including acute leukemias (such
as acute
lymphocytic leukemia, acute myelocytic leukemia, acute myelogenous leukemia
and myeloblasts,
promyeiocytic, myelomonocytic, monocytic and erythroleukemia), chronic
leukemias (such as
chronic myelocytic (granulocytic) leukemia, chronic myelogenous leukemia, and
chronic
lymphocytic leukemia), polycythemia vera, lymphoma, Hodgkin's disease, non-
Hodgkin's
lymphoma (indolent and high grade forms), multiple myeloma, Waldenstrom's
macroglobulinemia, heavy chain disease, myelodysplastic syndrome, hairy cell
leukemia and
myelodysplasia.
Solid tumors are abnormal masses of tissue that usually do not contain cysts
or liquid areas.
Solid tumors can be benign or malignant. Different types of solid tumors are
named for the type
of cells that form them (such as sarcomas, carcinomas, and lymphomas).
Examples of solid tumors,
such as sarcomas and carcinomas, include fibrosarcoma, myxosarcoma,
liposarcoma,
mesothelioma, malignant lymphoma, pancreatic cancer and ovarian cancer.
The CAR-modified T cells may also serve as a type of vaccine for ex vivo
immunization
and/or in vivo therapy in a mammal. Preferably, the mammal is a human.
With respect to ex vivo immunization, at least one of the following occurs in
vitro prior to
administering the cell into a mammal: i) expanding the cells, ii) introducing
a nucleic acid encoding
a CAR to the cells, and/or iii) cryopreservation of the cells.
Ex vivo procedures are well known in the art and are discussed more fully
below. Briefly,
cells are isolated from a mammal (such as a human) and genetically modified
(i.e., transduced or
transfected in vitro) with a vector expressing a CAR disclosed herein. The CAR-
modified cell can
be administered to a mammalian recipient to provide a therapeutic benefit. The
mammalian
recipient may be a human and the CAR-modified cell can be autologous with
respect to the
recipient. Alternatively, the cells can be allogeneic, syngeneic or xenogeneic
with respect to the
recipient.
In addition to using a cell-based vaccine in terms of ex vivo immunization,
the present
37
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
disclosure also provides compositions and methods for in vivo immunization to
elicit an immune
response directed against an antigen in a patient.
The present disclosure provides methods for treating tumors comprising
administering to a
subject in need thereof, a therapeutically effective amount of the CAR-
modified T cells.
The CAR-modified T cells of the present disclosure may be administered either
alone, or as
a pharmaceutical composition in combination with diluents and/or with other
components such as
IL-2, IL-17 or other cytokines or cell populations. Briefly, pharmaceutical
compositions of the
present disclosure may comprise a target cell population as described herein,
in combination with
one or more pharmaceutically or physiologically acceptable carriers, diluents
or excipients. Such
compositions may comprise buffers such as neutral buffered saline, phosphate
buffered saline and
the like; carbohydrates such as glucose, mannose, sucrose or dextrans,
mannitol; proteins;
polypeptides or amino acids such as glycine; antioxidants; chelating agents
such as EDTA or
glutathione; adjuvants (e.g., aluminum hydroxide); and preservatives.
Compositions of the present
disclosure may be formulated for intravenous administration.
Pharmaceutical compositions of the present disclosure may be administered in a
manner
appropriate to the disease to be treated (or prevented). The quantity and
frequency of
administration will be determined by such factors as the condition of the
patient, and the type and
severity of the patient's disease, although appropriate dosages may be
determined by clinical trials.
When "an immunologically effective amount", "an anti-tumor effective amount",
"an tumor-
inhibiting effective amount", or "therapeutic amount" is indicated, the
precise amount of the
compositions of the present disclosure to be administered can be determined by
a physician with
consideration of individual differences in age, weight, tumor size, extent of
infection or metastasis,
and condition of the patient (subject). It can generally be stated that a
pharmaceutical
composition comprising the T cells described herein may be administered at a
dosage of 104
to 109 cells/kg body weight, or 105 to 106 cells/kg body weight, including all
integer values
within those ranges. T cell compositions may also be administered multiple
times at these
dosages. The cells can be administered by using infusion techniques that are
commonly known in
immunotherapy (see, e.g., Rosenberg et alõ New Eng. J. of Med. 319: 1676,
1988). The optimal
dosage and treatment regime for a particular patient can readily be determined
by one skilled in
the art of medicine by monitoring the patient for signs of disease and
adjusting the treatment
accordingly.
38
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
The administration of the subject compositions may be carried out in any
convenient manner,
including by aerosol inhalation, injection, ingestion, transfusion,
implantation or transplantation.
The compositions described herein may be administered to a patient
subcutaneously,
intradermaliy, intratumorally, intranodally, intramedullary, intramuscularly,
by intravenous
injection, or intraperitoneally. In one embodiment, the T cell compositions of
the present
disclosure are administered to a patient by intradermal or subcutaneous
injection. In another
embodiment, the T cell compositions of the present disclosure may be
administered by intravenous
injection. The compositions of T cells may be injected directly into a tumor,
lymph node, or site
of infection.
In certain embodiments of the present disclosure, cells activated and expanded
using the
methods described herein, or other methods known in the art where T cells are
expanded to
therapeutic levels, are administered to a patient in conjunction with (e.g.,
before, simultaneously
or following) any number of relevant treatment modalities, including but not
limited to treatment
with agents such as antiviral therapy, cidofovir and interleukin-2, Cytarabine
(also known as ARA-
I 5
C) or natalizumab treatment for MS patients or efalizumab treatment for
psoriasis patients or other
treatments for PML patients. In further embodiments, the T cells of the
disclosure may be used in
combination with chemotherapy, radiation, immunosuppressive agents, such as
cyclosporin,
azathioprine, methotrexate, mycophenolate, and FK506, antibodies, or other
immunotherapeutic
agents. In a further embodiment, the cell compositions of the present
disclosure are administered
to a patient in conjunction with (e.g., before, simultaneously or following)
bone marrow
transplantation, or the use of chemotherapy agents such as, fludarabine,
external-beam radiation
therapy (XRT), cyclophosphamide. For example, in one embodiment, subjects may
undergo
standard treatment with high dose chemotherapy followed by peripheral blood
stem cell
transplantation. In certain embodiments, following the transplant, subjects
receive an infusion of
the expanded immune cells of the present disclosure. In an additional
embodiment, expanded cells
are administered before or following surgery.
The dosage of the above treatments to be administered to a patient will vary
with the precise
nature of the condition being treated and the recipient of the treatment. The
scaling of dosages for
patient administration can be performed according to art-accepted practices.
In genera1,1x106 to
1x101 of the modified T cells of the disclosure (e.g., CAR-T-BCMA cells) can
be applied to
patients by means of, for example, intravenous infusion each treatment or each
course of treatment.
39
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
The main advantages of the present disclosure include:
(a) As for the chimeric antigen receptor of the present disclosure, the
extracellular antigen
binding domain is a specific anti-BCMA scFv; the CARs have shown a great
ability of killing
tumor cells with low cytotoxicity and low side effects.
(b) The chimeric antigen receptor provided by the disclosure can achieve
stable expression
and membrane localization of CAR protein after T cells are infected by viral
vectors (e.g.,
lentiviruses) carrying the CAR gene.
(c) The CAR-modified T cells of the present disclosure have a longer survival
time in vivo
and strong anti-tumor effect. The scFv used in the present disclosure may be a
humanized or
human-derived antibody, and is less likely to produce specific immunological
rejection.
The present disclosure will be further illustrated below with reference to the
specific
examples. It is to be understood that these examples are for illustrative
purposes only and are not
intended to limit the scope of the invention. For the experimental methods in
the following
examples the specific conditions of which are not specifically indicated, they
are performed under
routine conditions, e.g., those described by Sambrook. et al., in Molecule
Clone: A Laboratory
Manual, New York: Cold Spring Harbor Laboratory Press, 1989, or as instructed
by the
manufacturers, unless otherwise specified. Percentages and parts are by weight
unless otherwise
stated.
Example 1 Construction of lentiviral expression vector
The full-length DNA synthesis and cloning were commissioned by Shanghai Boyi
Biotechnology Co., Ltd to achieve the construction of coding plasmids. The
pWPT lentiviral
vector was selected as a cloning vector, and the cloning sites were BamH I and
Sal I sites. The
specific sequence is as described above.
Example 2 Preparation of CAR-T cell
(1) Mononuclear cells (PBMCs) were isolated from venous blood of healthy
people by
density gradient centrifugation.
(2) On day 0, PBMCs were seeded in a cell culture flask previously coated with
CD3
monoclonal antibody (OKT3) at a final concentration of 5 [tg/mL and
Retronectin (purchased from
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
TAKARA) at a final concentration of 10 1.tg/mL. The medium was GT-551 cell
culture medium
containing 1% human albumin. Recombinant human interleukin 2 (IL-2) was added
to the medium
at a final concentration of 1000 U/mL. The cells were cultured in an CO2
incubator with a saturated
humidity of 5% at 37 C.
(3) On day 1, the supernatant of the cultured PBMCs was slowly aspirated and
discarded.
New GT-551 cell culture medium containing 1% human albumin was added, and
recombinant
human interleukin 2 (IL-2) was added to the medium at a final concentration of
1000 U/mL. The
cells were continuously cultured in an CO2 incubator with a saturated humidity
of 5% at 37 C.
(4) On day 3, fresh medium, concentrated and purified CAR-BCMAs lentivirus
solution,
protamine sulfate (12 ug/ml), and IL-2 (at a final concentration of 1000 U/mL)
were added. After
12 hours of infection in a 5% CO2 incubator at 37 C, the culture medium was
discarded, fresh
medium was added, and cultivation was continued in a 5% CO2 incubator at 37
C.
(5) Starting from day 6, CAR-BCMAs cells can be taken for the corresponding
activity
assay.
Example 3 Detection of the integration rate of the CAR gene in the T cell
genome and
the expression level of the encoded protein thereof on the membrane surface
0.5x106 of CART-BCMAs cell samples cultured on day 7 (Fig. 3A), day 21 (Fig.
3B) and
day 29 (Fig. 3C) in Example 2 were taken, respectively. The expression level
of CAR-BCMA
protein on the surface of T cell membrane was analyzed by flow cytometry after
Fc fragment
staining of recombinant human BCMA protein.
The result is shown in Figures 3A, 3B and 3C, and four CAR structures designed
in the
present disclosure can be expressed in their corresponding modified T cells
and complete the cell
membrane surface localization.
Example 4 Detection of the in vitro activation ability of CAR-BCMAs
Cell activation level indicator proteins CD137 and IFNy was detected using
CART-BCMAs
cells cultured on day 7 in Example 2. lx105 of CART-BCMA cells cultured on day
7 were cultured
respectively with BCMA-positive K562-BCMA+E7 tumor cell line and BCMA-negative
K562
tumor cell line, or without tumor cells, in 200 Ill GT-551 medium for 18h at a
ratio of 1:1. Then
the expression level of CD137 on the surface of T cell membrane was detected
by flow cytometry
41
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
and the secretion level of IFN7 in the culture supernatant was detected by
ELISA.
The results are shown in Fig. 4A and Fig. 4B, the expression of CD137 was
detected on the
surface of four CART cells, and the expression of IFN7 was detected in the
culture supernatant.
Among them, CAR-BCMA-20 shows best CD137 activation level and IFN7 release
level. CART-
S BCMA-M06 which is constructed based on humanized M06 antibody sequence
shows a weaker
level of CD137 activation but a higher level of IFN7 release than CART-BCMA-
CA8 which is
constructed based on mouse antibody sequence.
Additionally, C-CAR088 induced higher levels of IFNI, release (Figure 4B) and
CD137 expression (Figure 4A) in BCMA-positive tumor cells, compared to CARs
based on
BCMA-1, BCMA-CA8 and BCMA-M06.
CART-BCMA-20 induced greater apoptosis of BCMA-positive tumor cells than
CART-BCMA-1 (positive control), CART-BCMA-M06 and CART-BCMA-CA8 (Figure 5).
Example 5 Detection of advanced apoptosis activity of tumor cells induced by
CART-
BCMAs cells
(1) The CART-BCMAs cells cultured on day 17 in Example 2 were mixed
respectively with
1x104CFSE-labeled BCMA-negative cells (NH929) or BCMA-positive self-
constructed cells
(NH929-BCMA overexpressing tumor cell line) at a ratio of 1:1, 2.5:1, 5:1,
10:1, 20:1 (as shown
in Fig. 5). The mixed cells were co-cultured in 100 pi GT-551 medium for 4 h,
and then stained
with 100 pi 25% PI dye for 15 min. The proportion of PI positive cells in CFSE
positive cells was
analyzed by flow cytometry.
(2) The CART-BCMAs cells cultured on day 22 in Example 2 were mixed
respectively with
1x104 CFSE-labeled BCMA-negative cells (NH929), BCMA-positive self-constructed
cells
(NH929-BCMA overexpressing tumor cell line) or MM.1S cell line naturally
expressed BCMA at
a ratio of 1:1, 5:1, 10:1, 10:1,40:1 (as shown in Fig. 6B). The mixed cells
were co-cultured in 100
pi GT-551 medium for 4 h, and then stained with 100 pi 25% PI dye for 15 min.
The proportion
of PI positive cells in CFSE positive cells was analyzed by flow cytometry.
The results are shown in Fig. 5 and Figures 6A and 6B, all four CART cells can
induce
apoptosis of BCMA-positive tumor cells. Among them, CART-BCMA-20 can induce
advanced
apoptosis of BCMA-positive tumor cells better than CART-BCMA-1. The ability of
CART-
BCMA-M06 and CART-BCMA-CA8 to induce advanced apoptosis of BCMA-positive tumor
42
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
cells is similar.
Example 6 Inhibition of CART-BCMAs on RPMI-8226 myeloma xenograft model
RPMI-8226 cells in logarithmic growth phase were collected, and 4.0x106 tumor
cells were
inoculated subcutaneously in the right back of 6-8 week old B-NDG mice. When
the tumor volume
reached about 120 mm3, the animals were randomly divided into 4 groups
according to tumor
volume, so that the tumor volume difference of each group was less than 10% of
the mean value.
Then the solvent control, 7.5x10 6 NT and 7.5x10 6 CART-BCMAs cells were
injected through
tail vein respectively.
The results are shown in Figures 7A and 7B, compared with the control group,
single
injection of CART-BCMA-1 and CART-BCMA-20 through tail vein can effectively
inhibit the
growth of human myeloma RPMI-8226 cells (relative tumor proliferation rate
%T/CRTV<40%,
P<0.05), and can significantly prolong the survival time of human myeloma-
bearing mice (median
survival of the control group is 23 days, median survival of the CART-BCMAs
treatment group >
33 days). There was no significant difference in tumor proliferation rate and
median survival
between the mice treated with CART-BCMA-1 and CART-BCMA-20.
For in vivo studies, B-NDG mice were xenografted with human myeloma RPMI-
8226 cells. When the tumor volume reached about 120 mm3, the solvent control,
non-
transfected (NT) T cells (a negative control) or CART-BCMAs cells were
injected through
the tail vein of the mice.
Compared with the control group, a single injection of CART-BCMA-1 and CART-
BCMA-20 effectively inhibited the growth of the myeloma cells, and
significantly prolonged
the survival time of human myeloma-bearing mice: median survival of the CART-
BCMA-
treated groups was >33 days, while median survival of the control group was 23
days. There
was no significant difference in the tumor proliferation rate and median
survival between the
mice treated with CART-BCMA-1 and the mice treated with CART-BCMA-20 (Figures
7A
and 7B).
The results suggest that BCMA-20 (C-CAR088) had high in vivo anti-tumor
efficacy.
Comparative example
In the screening process of the chimeric antigen receptor of the present
application, the
43
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
inventors tested a large number of candidate sequences, which are illustrated
below with examples.
The antibodies to be screened include: BCMA-1, BCMA-2, BCMA-69, BCMA-72, BCMA-
2A1, BCMA-1E1, BCMA-J22.9, BCMA-20, BCMA-CA8, and BCMA-M06. Structures of
chimeric antigen receptor targeting BCMA were constructed on the basis of the
above antibodies.
Among them, BCMA-1 and BCMA-2 are published Car-T sequences and used as a
positive control
for screening. CAR-T cells were prepared in the same way as in Example 2, and
detected in the
same way as in Examples 3 and 4.
The results are shown in Figures 1A, 1B and 1C, which are the experimental
results of two
batches of CAR-T cells. The expression of Car-T was detected with BCMA-Fc
fusion protein. It
can be seen that there is a high expression in primary T cells, as seen in
Fig. 1A. In Fig. TB, it can
be seen that BCMA-1, BCMA-20, BCMA-1E1, BCMA-CA8, BCMA-M06, and BCMA-J22.9
can be activated by the BCMA antigen. Fig. 1C shows that the activated BCMA-1,
BCMA-20,
BCMA-1E1, BCMA-CA8, BCMA-M06, and BCMA-J22.9 CAR-Ts can produce higher levels
of
IFN-y. The results show that the CAR-T functions obtained by BCMA-1, BCMA-20,
CA8 and
M06 are similar, so BCMA-20, CA8 and MO6CAR-T were further analyzed and
studied.
Example 7 Membrane protein array experiment
The light chain variable region of SEQ ID NO: 1 and the heavy chain variable
region of SEQ
ID NO: 2 were used to prepare a single chain antibody B20-scFv-rabFc, and
membrane protein
array experiment was performed.
20 ug/mL of B20-scFv-rabFc was added to HEK293T cell array transiently
transfected with
5344 membrane proteins, respectively. Flow cytometry found that, under this
test condition, B20-
scFv-rabFc may cross-recognize with TNFRSF17 (Q02223), MAG (P20916), CR2
(P20023),
CXADR (P78310) and DDR2 (Q16832), wherein TNFRSF17, i.e., BCMA is the specific
target of
B20-scFv, and MAG, CR2, CXADR and DDR2 are suspected non-specific targets.
To further confirm whether the suspected target can cause the activation of
CAR-T,
CBM.BCMA CAR-T was co-cultured with 293T cells transfected with BCMA, CR2,
CXADR,
DDR2, and MAG, respectively. The IFNy, TNF, IL-2 and other cytokines in co-
culture supernatant
were detected. 293T cells transfected with empty vector were used as a
negative control, and 293T
cells transfected with BCMA were used as a positive control.
Figure 8 shows results the expression of BCMA, CR2, CXADR, DDR2, and MAG in
293T
44
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
cells after transfection of relevant plasmids by flow cytometry.
The cytokine detection results are shown in Figures 9A, 9B and 9C. Only the
BCMA
expressed on 293T cells can induce CBM. BCMA CAR-T cells to produce large
amounts of
IFNy/TNF/IL-2, while the other four non-specific surface markers cannot
activate CBM. BCMA
CAR-T cells.
Figure 8 and Figures 9A-9C show whole genome membrane proteome array and
validation.
TNFRSF17, MAG, CR2, CXADR and DDR2 were identified with moderate binding with
B-20
scFv rabFc at the concentrations of 20.0 1.tg/mL. Only cells expressing
TNFRSF17(BCMA) can
induce BCMA-CAR-T cells producing a large number of cytokines (IFNy/TNF/IL-2).
Specific membrane staining was observed on human lymphocytes in thymus,
spleen, lymph
nodes, bone marrow and scattered lymphocytes in thyroid gland, adrenal gland
at the
concentrations of 20.0m/mL (Table 1 and Figures 19A-19B).
Table 1
Staining site Tissue 5.0 j.tg/mL 20.0 itg/mL
Nucleus Esophagus, mucosal 1/3a, +b 1/3, +
layer
Cytoplasm Stomach, mucosal 1/3, + 2/3, +
layer
Kidney, cortex 1/3, + 2/3, +
Bladder, mucosal - 1/3, +
layer
Membrane Thyroid - 3/3, +
Thymus, cortex, 3/3, + 3/3, +
medulla
Spleen, splenic cord 2/3, + 3/3, +¨++
Lymph node, medulla 3/3, + 3/3, +
Adrenal gland, cortex - 3/3, +
Bone marrow - 3/3, +
a) number of positive staining in 3 donors.
b) staining intensity of positive cells and percentage of positive cells in
the cells of the same type.
"-" No stained cells,
"+" weakly positive staining, < 10% cells are stained,
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
"++" moderately positive staining, 11%-50% cells are stained.
Example 8 In vitro anti-tumor activity of C-CAR088 cells
The chimeric antigen receptor BCMA-20 (hereafter named C-CAR088) was selected
for
subsequent experiments. C-CAR088 cells, NT cells (non-transfected T cells,
used as negative
control) and positive control cells (Bluebird bb2121) were co-cultured with
BCMA negative cells
(NH929) or BCMA positive cells (NH929-BCMA) at different effect target ratios,
The killing
ability of each cell to the target cell was analyzed.
The results are shown in Figure 10. C-CAR088 cells and positive control cells
only have a
strong killing effect on NH929-BCMA cells, but no significant killing effect
on negative target
cells NH929. The non-transfected group (NT) also have no significant killing
effect on the target
cells.
T cells with C-CAR088, non-transfected (NT) T cells (a negative control) and
positive
control cells (Bluebird bb2121) were co-cultured with BCMA negative cells
(NH929) or
BCMA positive cells (NH929-BCMA) at different effect/target ratios. Then the
cytotoxicity
was assayed.
Figure 10 shows that C-CAR088 T cells and the positive control demonstrated
strong
cytotoxicity on NH929-BCMA cells, but not NH929 cells.
Example 9 Effect of soluble BCMA on cell killing activity
In the co-cultivation system of C-CAR088 cells with BCMA negative target cells
A549 and
BCMA positive target cells A549-BCMA-2E9, 100 ng/ml and 500 ng/ml soluble BCMA
protein
was added respectively to detect its effect on CD137 expression.
The results are shown in Figure 11. Soluble BCMA protein have no effect on the
upregulation of CD137 expression, indicating that soluble BCMA does not block
the specific
recognition of CAR and target antigen. In the test of in vitro cell killing
ability and ELISA of IFN-
y release, the killing effect of C-CAR088 cells on target cells and the
release of IFN-y were reduced
by 500ng/m1 soluble BCMA protein, but there was no significant difference. The
above results
indicate that the cell killing activity of C-CAR088 cells is basically
unaffected by soluble BCMA
Example 10 Dose dependent effect of C-CAR088 cells
46
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
6 week old B-NDG mice (half male and half female) were selected and
intraperitoneally
injected with 2.5x106 human multiple myeloma cells RPMI-8226. Mice with
similar tumor burden
were selected and divided into 5 groups, and were injected with 2.5x106 C-
CAR088 cells (low-
dose group), 5x106 C-CAR088 cells (medium-dose group), 1x107 C-CAR088 cells
(high-dose
group), T cells and vehicle (with cryoprotectant (CBMG C-CFMC) as vehicle),
respectively. The
experiment lasted 54 days. During the experiment, the tumor burden of the mice
was evaluated
every 5 days. At the end of the experiment, the survival rate of the mice was
calculated.
The results are shown in Figure 12. Single administration of doses of 5x106
cells /mouse and
lx 107 cells /mouse C-CAR088 cells can effectively inhibit the growth of B-NDG
mouse xenograft
tumor of human myeloma RPMI-8226 cell. The relative tumor proliferation rates
were 6.12% and
0.75%, respectively (p <0.05). At the end of the experiment, the survival rate
of tumor-bearing
mice in the middle-dose group was 91.7% (11/12). No death occurred in the
tumor-bearing mice
in the high-dose group, and the survival rate was 100.0% (12/12), having a
significant difference
(p <0.05) compared with the vehicle group (survival rate 58.3%). The above
results indicate that
C-CAR088 exhibits dose dependent in vivo anti-tumor activity.
Example 11 Phase I clinical study of C-CAR088
With the approval of the Ethics Committee, a total of 15 volunteers conducted
phase I
clinical trials. The key eligibility criteria for volunteer are as follows:
patients with multiple
myeloma aged 18-75 years old, MM cells express BCMA, have measurable MM, and
have
received 2 prior lines of therapy for MM and have received treatment with PI
and IMiD, have
adequate hepatic, renal, cardiac and hematopoietic function.
The experimental process is shown in Figure 13.
The treatment results are shown in Figure 14 and Table 2. Two patients were
evaluated at
week 2, one SD and one CR. The remaining 13 patients were evaluated at week 4
and the objective
response rate ORR reached 100%. In all patients, there were 3 CR, 5 VGPR
(including 1
Daratumumab-resistant patient), and 6 PR.
47
Table 2 Clinical response
Follow Serum M Clinical ressonse
0
Research
Best t..)
o
Patient ID up protien
t..)
t..)
center Baseline 2w 4w 8w
12w 16w 20w 6 m ORR
length /sFLC type
,z
,z
t..)
1.8g/L 6.2g/L 7.0g/L
(...)
Z0203-00801C001 6m IgG 71.0g/L 42.0g/L 26.3g/L
16.4g/L 45g/L VGPR
UIF(-) UIF(-) UIF(-)
75 mg/L
3050mg/ SIF(-)
Z0203-00801C003 12w k 3250mg/L
170mg/L 960mg/L PD VGPR
Jiangsu L UIF(+)
Provincial UPEP(-)
P
People's SPEP(-
) SPEP(-) SPEP(-) SPEP(-) -
Hospital Z0203-00801C004 6m IgG 46.5g/L 7.9g/L 9.0g/L UIF(-
) UIF(-) UIF(-) UIF(-) 21.6g/L VGPR
cc
SIF(+) SIF(+) SIF(+) SIF(+) "
2
Z0203-00801C008 20w IgG 26.1g/L 9.1g/L 4.8g/L 4.6g/L
9g/L* 10g/L* 17g/L * PD PR
,
Z0203-00801C010 4e IgG 55.7g/L 45.4g/L 23.3g/L
PR
Z0203-00801C011 3w IgG 22.5g/L 18.8g/L
SD
SPEP(-)
Z0203-01301C001 8w IgA 16.7g/L 7.4g/L 3.6g/L UIF(-)
VGPR
Tianjin
SIF(+) 1-d
n
Hematology Z0203-01301C003 4w IgG 50.2g/L 27.1g/L 15.0g/L
PR
Hospital SPEP(-) SPEP(-)
cp
t..)
o
t..)
Z0203-01301C006 4w X 760mg/L UIF(-) UIF(-)
VGPR
O-
o,
SIF(+) SIF(+)
.6.
,-,
o
Peking Z0203-00701C001 4w IgG 17.4g/L 10.8g/L 5.3g/L
PR
Union Z0203-00701C002 4w IgG 21.7g/L 15.8g/L 7.9g/L
PR
Medical
College
0
t..)
o
Hospital
t..)
t..)
,-,
SIF- SIF- SIF- SIF- SIF- S IF-
o
o
Z0203-00601C002 12w X, 187mg/L
3.28mg/L UIF- UIF- UIF- UIF- UIF- UIF- sCR t..)
(...)
MRD- MRD- MRD- MRD- MRD- MRD-
SIF- SIF- SIF- S IF-
Daupei Z0203-00601C004 4w lc 209mg/L UIF-
UIF- UIF- UIF- sCR
Hospital
MRD- MRD- MRD- MRD-
Z0203-00601C005 4w IgG 28.27g/L 24.3g/L 8.6g/L
PR P
S IF-
,
-p.
.
Z0203-00601C006 2W lc 121mg/L UIF-
sCR .
3
"
MRD-
2
,
,
1-d
n
1-i
cp
t..)
o
t..)
,-,
O-
o
,-,
4,.
,-,
o
CA 03201008 2023-05-05
WO 2022/119923 PCT/US2021/061410
The treatment-emerging adverse events are shown in Table 3. Only one patient
occurred
grade 3 cytokine release syndrome in 15 patients. No neurotoxicity events and
no dose-limiting
toxicity (DLTs) were observed in the dose escalation. The cytopenias is mostly
related to Cy/Flu
lymphodepletion. It should be noted that the occurrence of a certain degree of
cytokine release
syndrome after treatment also illustrates the effectiveness of CART treatment
from the side. None
of the 15 patients had particularly serious cytokines, and C-CAR088 has better
safety.
Table 3 Treatment-Emerging Adverse Events
Treatment-Emerging Adverse Events n (%) Overall n (%) Grade 3/4
Cytokine release syndrome 14 (93) 1(7)
Neutropenia 15 (100) 15 (100)
Thrombocytopenia 13 (87) 11(73)
Anemia 12 (80) 6(40)
Increased AST 5(33) 3(20)
Infection 3(20) 3(20)
Figure 15 shows the treatment condition of the patient of ID Z0203-00801C008.
Figure 16
shows the treatment condition of patient of ID Z0203-00701C001. The PET-CT
images of the
cancer lesions for one patient show that abnormal plasma cells in the bone
marrow were
significantly decreased after the C-CAR088 treatment (Figure 15). Figures 16A
and 16B show that
majority of the PCs were abnormal (>90% were CD451o/-), were BCMA+ and clonal
for Kappa
light chain at the beginning of the experiment (Baseline). Figure 16B shows
that after 14 or 28
days of BCMA CAR-T treatment, abnormal PC in BM were significantly decreased,
especially
Kappa + PC, which decreased from the baseline level of 85.2% to 0%.
Figure 17 shows the expansion of C-CAR088 and the decrease of M-protein/sFLC
levels in
the blood. The results showed that C-CAR088 cells expanded effectively after
injection, and the
level of M-protein/sFLC markers continued to decrease. The M-marker level of
one patient was
CA 03201008 2023-05-05
WO 2022/119923 PCT/US2021/061410
dropped to 0 on day 14.
Summary of observations for C-CAR088 was as follow.
= In preclinical studies, C-CAR088 showed antitumor activity both in vitro
and in vivo.
= Early C-CAR088 trial results in patients with r/r MM support preclinical
findings, showed
promising efficacy and manageable safety profile.
= The early clinical efficacy signal at low, suboptimal dose was
encouraging.
= Compared to KarMMa data, our current dose level from infused patients was
well below the
optimal dose of bb2121. 53% (8/15) were recently dosed (at ¨4 weeks).
= Dose dependence was observed based on PK profile. C-CAR088 was well
tolerated in patients.
Example 12 An Anti-BCMA CAR T-Cell Therapy (C-CAR088) Showed Promising Safety
and Efficacy Profile in Treating Relapsed or Refractory Multiple Myeloma (r/r
MM)
Our studies demonstrated that C-CAR088 was considerably more cytotoxic towards
tumor cells both in vitro and in vivo, compared to other anti-BCMA CARs.
We conducted a clinical trial of an anti-BCMA CAR (BCMA-20, also termed "C-
CAR088") in treating relapsed/refractory multiple myeloma (R/R MM) in
patients.
Clinical trials are evaluated along a number of different criteria. Two key
measures
are overall response rate (ORR) and complete response rate (CR). When compared
with other
anti-BCMA CARs, the CARs of the claimed method offer better therapeutic
efficacy in a
clinical trial, as reflected by high response rates (95% overall response rate
or ORR, and 67%
complete response rate or CR) in treating relapsed/refractory multiple myeloma
(R/R MM).
Even when compared with JNJ-4528 (CARTITUDE-1), the percentage of adverse
events,
such as neurotoxicity, in the C-CAR088 was significantly lower. Specifically,
for C-
CAR088, only 4% patients experienced grade 1 neurotoxicity which resolved
spontaneously.
Thus, the CAR-T cells of the claimed method offered a favorable safety
profile.
C-CAR088 demonstrated a manageable safety profile.
= Most cases of CRS were grade 1/2 with median time to onset of 6 days.
= Neurotoxicity (ICANS) was infrequent and generally low-grade with 1 grade
1 event.
51
CA 03201008 2023-05-05
WO 2022/119923 PCT/US2021/061410
Dose dependent responses occurred early and deepened.
= ORR: 95.7%, with 43.5% CR rate at median time to CR: 1.6 months (range:
0.5-9.5).
Median time to response: 0.5 month.
= At median 6.2 months follow up, 65.1% patients were progression-free at 6
months
In preclinical studies, C-CAR088 showed very good in vitro and in vivo anti-
tumor activity
and target specificity. Clinical trial results in 24 patients with r/r MM
showed strong therapeutic
index with promising efficacy and manageable safety profile.
We conducted a clinical trial of an anti-BCMA CAR (BCMA-20, also termed "C-
CAR088") in treating relapsed/refractory multiple myeloma (R/R MM) in
patients. The patients'
baseline demographics and clinical characteristics prior to the start of our
anti-BCMA CAR
treatment are shown in Table 4.
The median age of the patients dosed was 60 years (range: 45-74 years). The
median
number of prior lines of therapy was 4 (ranging from 2-12 prior therapies).
All patients had
received prior treatment with IMiDs (immunomodulatory drug) and proteasome
inhibitors. 25%
patents were previously treated with anti-CD38 monoclonal antibody, while 25%
patents had
received autologous hematopoietic stem cell transplant.
Table 4 Summary of baseline clinical characteristics of the patients
Characteristic N=24 Characteristic N=24
Median age, yrs (range) 60 (45-74) High Risk Cytogenetics*, n (%)
= Age > 65, n (%) 9
(37.5) .0 3 (12.5)
.1 5(20.8)
Male/Female, n 14/10
.2 11
(45.8)
MM subtype, n (%)
.3 2 (8.3)
= IgG 14 (58.3)
=Unknown 3 (12.5)
= IgA 3(12.5)
= IgD 1(42)
Median number of prior lines of therapy, n 4 (2-12)
= Light Chain 6 (25)
(range)
Prior regimens, n (%)
=
Prior PIs and IIVIiDs 24 (100)
52
CA 03201008 2023-05-05
WO 2022/119923 PCT/US2021/061410
=
Prior ASCT 6(25.0)
=
Prior CD38 mAb 6 (25.0)
ECOG PS, n (%) Received bridging therapy, n (%) 4
(16.7)
= 0 15 (62.5)
= 1 9(37.5)
ISS stage, n (%)
= I 7(29.2)
= II 11 (45.8)
= III 6(25)
* Including 1q21 gain, del (17p), t (4;14) and t (14;16).
The clinical protocol, as well as the key inclusion criteria, is shown in
Figure 21.
Specifically, qualified subjects were enrolled, and the collected peripheral
blood leukocytes were
used to produce the CAR-T cells (C-CAR088). The CAR-T cells were then frozen
and stored
below -135 C until use. For CAR-T treatment, the CAR-T cells were thawed, and
administration
completed within 30-45 minutes.
At -5 and -3 days before the CAR-T infusion, the patients received
lymphodepletion
pretreatment, including fludarabine (30 mg/m2/d, intravenous, once per day for
three days), and
cyclophosphamide (300 mg/m2/d, intravenous, once per day for three days).
Approximately 72 hours after lymphodepletion, the patients were administered
1.0-6.0 x
106 CAR-T cells/kg on day 0 as 3 + 3 dose escalation. Follow-ups with the
patients were carried
out after the infusion starting on day 1 through month 24.
The primary objectives included safety: rated of dose limiting toxicities;
incidence and
severity of treatment-emergent adverse events (CTCAEV5.0). Secondary
objectives included
efficacy: IMWG 2016 ORR; DOR; PFS; OS. Exploratory objectives included CAR-T
expansion
and persistence.
As shown in Figure 22, an overall response rate (ORR, including CR and PR) of
our anti-
BCMA CAR-T trial is 96%. The best overall response (BOR) included 12 stringent
complete
responses (sCRs), 2 complete responses (CR), 8 very good partial responses
(VGPRs) and 1 partial
response (PR). The complete response (CR) is 67%. (SD: stable disease; PR:
partial response; CR:
53
CA 03201008 2023-05-05
WO 2022/119923 PCT/US2021/061410
complete response; PD: progressive disease; MR: minimal response; VGPR: very
good partial
response; MRD: minimal residual disease.)
The CR/sCR, VGPR and PR for overall and each dose group are shown in Table 5
and
Figure 23. In the 3.0-6.0 x106 CAR-T cells/kg dose groups, 14/21 (66.7%)
patients achieved a
CR/sCR and all (14/14) patients achieved MRD negative by flow cytometry at 10-
4 -10-6.
Table 5
1.0 x 106/kg 3.0 x 106/kg 4.5-6.0 x 106/kg
Overall
Response (n=3) (n=11) (n=10)*
(n=24)
(low dose) (mid dose) (high dose)
ORR, n (%) 23 (95.8%) 3 (100%) 11(100%) 9(90.0%)
CRR, n (%) 13 (54.2%) 0 6 (54.5%) 7 (70.0%)
*1 patient received 4.5 x 106/kg and 1 received 5.0 x 106/kg.
Table 6 below compares the C-CAR088 trial with those of Munshi et al. (KarMMa:
Idecabtagene Vicleucel), Mailankody et al. (EVOLVE: Orvacabtagene Autoleucel)
and
Madduri et al. (CARTITUDE-1: JNJ-4528).
Table 6 Comparison of C-CAR088 clinical trial with other BCMA-specific CARs
KarMMa: EVOLVE: CARTITUDE-1: C-CAR088
Idecabtagene Orvacabtagene JNJ-4528 (n=24)
Vicleucel Autoleucel (n=97)
(n=128) (n=62)
Median age, yrs (range) 61(33-78) 61(33-77) 61(43-78) 60 (45-74)
High risk cytogenetics 35% 41%* 24% 75%*
Tumor burden in >50% PC: 51 - >60% PC: 22 >50% PC:
BM, % 17
Extramedullary PCs 39% 23% 13% 8%
Median prior lines of 6 (3-16) 6 (3-18) 6 (3-18) 4 (2-12)
therapies, No. (range)
54
CA 03201008 2023-05-05
WO 2022/119923 PCT/US2021/061410
Triple refractory, % 84% 94% 88% 25%
Bridging therapy 88% 63% 17%
CRS: all / grade 3 84%/6% 89%/3% 95%/5% 92%/4%
ICANS: all / grade 3 17%/3% 13%/3% 21%/10% 4%/0
(Neurotoxicity)
ORR 73% 92% 97% 95%
sCR/CR 33% 36% 67% 67%
Evaluable patients 94% 84% 55% 83%
with MRD neg > 105
PFS, mos 8.8 9.3+ 12-mo
PFS: 6-mo PFS:
76.6% 78.7%#
* Includes +1q21. + PFS in lowest dose cohort (300 x 106 cells). # Results in
3-6 x 106
CAR-T cell/ kg dose cohort.
ICANS: immune effector cell-associated neurotoxicity syndrome.
See, Munshi et al., Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted
CAR
T-cell therapy, in patients with relapsed and refractory multiple myeloma
(RRMM): Initial
KarMMa results, Journal of Clinical Oncology, 2020, 38(15) suppl., Abstract
8503;
Mailankody et al., Orvacabtagene autoleucel (orva-cel), a B-cell maturation
antigen
(BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory
multiple
myeloma (RRMM): update of the phase 1/2 EVOLVE study (NCT03430011), Journal of
Clinical Oncology, 2020, 38(15) suppl., Abstract 8504; Madduri et al.,
CARTITUDE-1:
Phase lb/2 Study of Ciltacabtagene Autoleucel, a B-Cell Maturation
Antigen¨Directed
Chimeric Antigen Receptor T Cell Therapy, in Relapsed/Refractory Multiple
Myeloma, 62nd
ASH Annual Meeting and Exposition, December 5-8, 2020, Abstract 177.
For our clinical trial of C-CAR088, the Kaplan Meier progression-free survival
(PFS)
estimates include a 6-month PFS of 78.7% for the mid- and high-dose group,
with 95% confidence
intervals (CIs) of 62.1% - 99.7%. Median duration of response (DOR) was not
reached. See Table
7 and Figure 24.
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
Table 7
Time 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
(month)
n.risk 21 19 19 18 16 16 13 13 10 9 8 8 5 3 2 1 1 1 1
n.event 1 0 0 0 1 2 0 0 1 0 0 0 0 0 0 0 0 0 0
The time course of the CAR copies in the blood of the patients is shown in
Figures 25A
and 25B. Thus, the CAR levels were maintained in the blood after
administration.
C-CAR088 treatment was well tolerated. The patients' adverse reactions
(adverse
events, AEs) were recorded (Tables 8 and 9). There was only 1 (4.2%) grade
cytokine release
syndrome (CRS). Neurotoxicity was observed only in one patient (4.2%) which
resolved
spontaneously, with no grade neurotoxicity. Cytopenia, such as neutropenia
and
thrombocytopenia, was mostly related to the fludarabine/cyclophosphamide
(Cy/Flu)
lymphodepletion. No dose-limiting toxicities were observed, and all adverse
events were
reversible. These demonstrated that our anti-BCMA CAR had an excellent safety
profile.
Table 8
Grade 3
AEs (25%), n (%) Any Grade (n=24)
(n=24)
Neutropenia 24 (100%) 22 (91.7%)
Lymphopenia 22 (91.7%) 20 (83.3%)
Thrombocytopenia 22 (91.7%) 9 (37.5%)
Anemia 20 (83.3%) 12 (50.0%)
Elevated AST/ALT 13 (54.2%) / 8 (33.3%) 4 (16.7%) / 0
(0)
Infection 12 (50.0%) 6 (25.0%)
Table 9
Grade 3
CRS & Neurotoxicity * Any Grade (n=24)
(n=24)
CRS, n (%) 22 (91.7%) 1(4.2%)
= Median days to
onset, d (range) 6(1-11)
56
CA 03201008 2023-05-05
WO 2022/119923
PCT/US2021/061410
= Median days to
resolution, d (range) 5 (2-9)
= Treated with
Tocilizumab, n (%) 6 (26.1)
= Treated with
Steroids, n (%) 2 (8.7)
Neurotoxicity, n (%) 1 (4.2%) 0 (0)
= Days to onset, d
8
= Days to
resolution, d 1
*ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity
Associated with Immune Effector Cells 2019.
CRS: Cytokine release syndrome
The scope of the present invention is not limited by what has been
specifically shown and
described hereinabove. Those skilled in the art will recognize that there are
suitable alternatives
to the depicted examples of materials, configurations, constructions and
dimensions. Numerous
references, including patents and various publications, are cited and
discussed in the description
of this invention. The citation and discussion of such references is provided
merely to clarify the
description of the present invention and is not an admission that any
reference is prior art to the
invention described herein. All references cited and discussed in this
specification are incorporated
herein by reference in their entirety. Variations, modifications and other
implementations of
what is described herein will occur to those of ordinary skill in the art
without departing from the
spirit and scope of the invention. While certain embodiments of the present
invention have been
shown and described, it will be obvious to those skilled in the art that
changes and modifications
may be made without departing from the spirit and scope of the invention. The
matter set forth
in the foregoing description and accompanying drawings is offered by way of
illustration only and
not as a limitation.
57