Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/180155
PCT/EP2022/054633
1
Lithium nickel-based composite oxide as a positive electrode active material
for
rechargeable lithium-ion batteries
TECHNICAL FIELD AND BACKGROUND
This invention relates to a lithium nickel-based oxide positive electrode
active material for
solid-state batteries suitable for electric vehicle (EV) and hybrid electric
vehicle (HEV)
applications, comprising lithium nickel-based oxide particles comprising
zirconium (Zr).
A positive electrode active material is defined as a material which is
electrochemically active
in a positive electrode. By active material, it must be understood a material
capable to
capture and release Li ions when subjected to a voltage change over a
predetermined
period of time.
In the framework of the present invention, at% signifies atomic percentage.
The at% or
"atom percent" of a given element expression of a concentration means how many
percent
of all atoms in the claimed compound are atoms of said element. The
designation at% is
equivalent to mol% or "molar percent".
The weight percent (wt.%) of a first element E (Et1) in a material can be
converted from a
given atomic percent (at%) of said first element E (Eati) in said material by
applying the
following formula: E (E. at X Eawwt1 ¨ õ
x 100%, wherein the product of Eati with Eawl, Eawl
Ei-i(Eatix Eawi)
being the atomic weight (or molecular weight) of the first element E, is
divided by the sum
of Eat' x Eawi for the other elements in the material. n is an integer which
represents the
number of different elements included in the material.
It is an object of the present invention to provide a positive electrode
active material having
an improved first charge capacity of at least 160 mAh/g in the solid-state
battery.
SUMMARY OF THE INVENTION
This objective is achieved by providing a positive electrode active material
for solid-state
batteries, wherein the positive electrode active material comprises Li, M',
and oxygen,
wherein M' comprises:
- Ni in a content x between 50.0 mol% and 85.0 mol%, relative to M';
Co in a content y between 0.0 mol% and 40.0 mol%, relative to M';
Mn in a content z between 0.0 mol% and 40.0 mol%, relative to M',
- D in a content a between 0.0 mol% and 2.0 mol%, relative to the total
atomic
content of M', wherein D comprises at least one element of the group
consisting
of: Al, B, Ba, Ca, Cr, Fe, Mg, Mo, Nb, S, Si, Sr, Ti, Y, V. W, and Zn, and,
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
2
- Zr in a content b between 0.1 mol% and 5.0 mol%, relative to M',
- wherein x, y, z, a, and b are measured by ICP,
- wherein x+y+z+a+b is 100.0 mol%,
wherein the positive electrode active material has a Zr content ZrA defined as
6 ,
(x+y+z+b)
wherein the positive electrode active material has a Zr content ZrB, wherein
ZrB is
determined by XPS analysis, wherein ZrB is expressed as molar fractions
compared to the
sum of molar fractions of Co, Mn, Ni, and Zr, as measured by XPS analysis,
wherein the ratio ZrB / ZrA > 50.0,
wherein the positive electrode active material comprises secondary particles
having a
plurality of primary particles,
wherein said primary particle has an average diameter of between 170 nm to 340
nm as
determined by the method of this invention.
The present invention concerns the following embodiments:
Embodiment 1
In a first aspect, the present invention concerns a positive electrode active
material for
solid-state batteries, wherein the positive electrode active material
comprises Li, M', and
oxygen, wherein M' comprises:
- Ni in a content x between 50.0 mol% and 85.0 mol%, relative to M';
preferably Ni
in a content x between 50.0 mol% and 75.0% relative M';
- Co in a content y between 0.0 mol% and 40.0 mol%, relative to M';
- Mn in a content z between 0.0 mol% and 70.0 mol%, relative to M',
preferably Mn
in a content z between 0.0 mol% and 40.0 mol% relative to M',
- D in a content a between 0.0 mol% and 2.0 mol%, relative to the total
atomic
content of M', wherein D comprises at least one element of the group
consisting
of: Al, B, Ba, Ca, Cr, Fe, Mg, Mo, Nb, S, Si, Sr, Ti, Y, V. W, and Zn, and,
- Zr in a content b between 0.1 mol% and 5.0 mol%, relative to M',
- wherein x, y, z, a, and b are measured by ICP,
- wherein x+y+z+a+b is 100.0 mol%,
wherein the positive electrode active material has a Zr content ZrA defined as
__ 6 ,
(x+y+z+b)
wherein the positive electrode active material has a Zr content ZrB, wherein
ZrB is
determined by XPS analysis, wherein ZrB is expressed in percent as molar
fractions
compared to the sum of molar fractions of Co, Mn, Ni, and Zr, as measured by
XPS analysis,
wherein the ratio ZrB / ZrA > 50.0,
Note that ZrA is the Zr content of the positive electrode active material as
determined by
ICP and expressed as a fraction relative to the sum of the contents of Co, Ni,
Mn, and Zr.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
3
Preferably, the ZrB / ZrA ratio is at least 80, preferably at least 100, more
preferably at least
130. Preferably, the Zr B / ZrA ratio is at most 500, more preferably at most
300 and most
preferably at most 200.
Preferably the positive electrode active material comprises secondary
particles having a
plurality of primary particles and wherein said primary particles have an
average diameter
of between 170 nm to 340 nm, preferably between 200 nm to 340 nm, as
determined by
measuring primary particle size in an image taken by SEM.
More preferably said primary particles have an average diameter of at least
180 nm,
preferably 200 nm, preferably 220 nm, even more preferably of at least 225 nm.
Preferably said primary particles have an average diameter of at most 330 nm,
preferably of
at most 320 nm, more preferably of at most 300 nm, even more preferably of at
most 250
nm.
Preferably said primary particles have an average diameter of between 180 nm
and 330
nm, preferably between 200 nm and 320 nm, more preferably between 220 nm and
310
nm, even more preferably between 225 nm and 250 nm.
As appreciated by the skilled person the primary particle size is determined
by measuring
the particle size of the primary particle in an image taken by SEM.
Preferably, x > 55.0 mol% and more preferably x > 60.0 mol%
Preferably, x 80.0 mol% and more preferably x 75.0 mol%
Preferably, y > 0 mol % and more preferably y 5.0 mol and even more preferably
y
10.0 mol /c.
In another embodiment, said Ni in a content x is between 55 nnol /0 and 72
nnol /0 relative to
M' and said Co in a content y is between 0.0 mol% and 20.0 mol% relative to
M'.
In a preferred embodiment Ni is in a content x 55.0 mol%, preferably x L. 60.0
mol%,
more preferably x > 62.0 mol%. In a preferred embodiment, x < 80.0 mol%
preferably x
75.0 mol% and more preferably x 70.0 mol%.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
4
In a more preferred embodiment Ni is in a content x between 55.0 mol% x 80.0
mol%,
preferably 60.0 mol% x 75.0 mol%, more preferably 62.0 mol% x 70.0 mol%.
As appreciated by the skilled person the amount of Li and M', preferably Li,
Ni, Mn, Co, D
and Zr in the positive electrode active material is measured with Inductively
Coupled
Plasma-Optical Emission Spectroscopy (ICP-OES). For example, but not limiting
to the
invention, an Agilent ICP 720-ES is used in the ICP-OES analysis.
In a preferred embodiment Mn is in a content z > 0.0 mol%, more preferably z >
5.0 mol%,
and even more preferably z 8.0 mol%. In a preferred embodiment the content is
z 40.0
mol%, preferably z 30.0 mol%, and more preferably z 25.0 mol%. In a preferred
embodiment the content is 0.0 mol% < z 40.0 mol%, preferably 5.0 mol% z 30.0
mol%, more preferably 8.0 mol% z 25.0 mol%.
In a preferred embodiment Co is in a content y > 0.0 mol%, more preferably y
1.0 mol%,
and even more preferably y 3.0 mol%. In a preferred embodiment the content is
y
40.0 mol%, more preferably y 30.0 mol%, and even more preferably y 25.0 mol%.
In
a preferred embodiment the content is 0.0 mol% < y 40.0 mol%, preferably 1.0
mol%
y 30.0 mol%, more preferably 3.0 mol% y 25.0 mol%.
In a preferred embodiment D comprises at least one element of the group
consisting of: Al,
B, Ba, Ca, Cr, Fe, Mg, Mo, Nb, S, Si, Sr, Ti, Y, V. W, and Zn; preferably Al,
B, Cr, Nb, S, Si,
Ti, Y and W.
In a preferred embodiment D is in a content a > 0.0 mol%, more preferably a
0.25
mol%, and even more preferably a 0.5 mol%. In a preferred embodiment the
content is a
2.0 mol%, preferably a 1.75 mol%, and more preferably a
1.5 mol%. In a preferred
embodiment the content is 0.0 mol% < z 2.0 mol%, preferably 0.25m01% z 1.75
mol% and more preferably 0.5 mol% z 1.5 mol%.
As appreciated by the skilled person the secondary particles comprise of a
plurality of
primary particles, preferably more than 20 primary particles, preferably more
than 10
primary particles, most preferably more than 5 primary particles. Primary
particles are
particles which are individual crystals or which are formed of less than five,
and preferably
at most three, primary particles which are themselves individual crystals.
This can be
observed in proper microscope techniques like Scanning Electron Microscope
(SEM) by
observing grain boundaries.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
Embodiment 2
In a second embodiment, preferably according to the Embodiment 1, wherein the
Zr
content ZrA is b/(b+x+y+z) is at least 0.10 mol% and at most 1.00 mol%.
Preferably, the
Zr content ZrA is b/(b+x+y+z) is at least 0.20 mol% and at most 0.80 mol%.
Most
5 preferably the Zr content ZrA is b/(b+x+y+z) is at least 0.30 nnol /0 and
at most 0.70 mol%.
In an alternative but even preferred embodiment, the Zr content ZrA is
b/(b+x+y+z) is at
least 0.10 mol% and at most 1.50 mol%. Preferably, the Zr content ZrA is
b/(b+x+y+z) is
at least 0.20 mol% and at most 1.00 mol%. Most preferably the Zr content ZrA
is
b/(b+x+y+z) is at least 0.30 mol% and at most 0.90 mol%.
In a preferred embodiment, Zr is in a content b of at least 0.10 mol% and at
most 1.00
mol% relative M', more preferably at least 0.20 mol% and at most 0.80 mol%
relative to
M', most preferably at least 0.30 mol% and at most 0.70 mol% relative M'.
In an alternative but even preferred embodiment Zr is in a content b of at
least 0.10 mol%
and at most 1.50 mol% relative M', more preferably at least 0.20 mol% and at
most 1.00
mol% relative to M', most preferably at least 0.30 mol% and at most 0.90 mol%
relative M'.
In a preferred embodiment the Zr content ZrB is more than 0.25 mol%,
preferably more
than 0.50 mol%, most preferably more than 0.60 mol%. In a preferred embodiment
ZrB is
less than 2.0 mol%, preferably less than 1.5 mol%, more preferably less than
1.0 mol%. In
a preferred embodiment ZrB is between 0.25 mol% and 2.0 mol%, preferably
between 0.50
mol /0 and 1.5 mol%, most preferably between 0.60 mol% and 1.0 mol%. As
appreciated
by the skilled person ZrB is expressed as molar fraction, as measured by XPS
analysis
compared to the sum of molar fractions of Co, Mn, Ni, and Zr, as measured by
XPS analysis.
Embodiment 3
In a third embodiment, according to Embodiment 1 to 2, said material has a
secondary
particle median size D50 of at least 2 pm, and preferably of at least 5 pm as
determined by
laser diffraction particle size analysis.
Preferably, said material has a secondary particle median size D50 of at most
15 pm, and
preferably of at most 13 pm as determined by laser diffraction particle size
analysis.
For example, but not limiting to the invention, the laser diffraction particle
size analysis is
performed by a Malvern Mastersizer 3000.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
6
Embodiment 4
In a fourth embodiment, according to Embodiment 1 to 3, said material has a
carbon
content of at least 600 ppm, preferably of at least 650 ppm, more preferably
of at least 750
ppm and most preferably at least 900 ppm as determined by carbon analyzer.
Preferably, said material has a carbon content of at most 5000 ppm, preferably
of at most
3000 ppm, more preferably of at most 1500 ppm and most preferably of at most
1100 ppm
as determined by carbon analyzer.
Embodiment 5
In a fifth embodiment, according to any Embodiment 1 to 4, wherein the
thickness of Zr >
10 nm. Preferably, the thickness is 15 nm and more preferably 20 nm as
determined
by TEM-EDS measurement. Preferably the thickness of Zr is 100 nm, preferably
the
thickness is 50 nm, more preferably the thickness is 30 nm. Preferably the
thickness of
Zr is between 10 nm and 100 nm, preferably between 15 nm and 50 nm, more
preferably
between 22 nm and 28 nm.
In the framework of this invention, the (minimum) thickness of the surface
layer is defined
as the shortest distance between a first point located at a periphery of a
cross-section of a
particle and a second point located in a line defined between said first point
and a geometric
center (or centroid) of said particle, wherein the difference of content of Zr
measured by
TEM-EDS at the second point location (Zr2) and at any location between said
second point
location and the center of the particle is around 0.1 at%.
The content of Zr at the second point location (Zr2) is constant: it can be
superior to 0 at%
and must be inferior or equal to 5.0% of a first content of Zr at the first
point location (Zr).
Said second content of Zr2 is equal to a content of Zr at a third point
location (Zr3) in said
line and said third point is located at any location between the geometric
center of said
particle and the second point location.
In other words, the thickness of the surface layer corresponds to a minimal
distance D
defined as:
D (in nm) = Lzri ¨ Lzr2
wherein Lzriis a first point location at the periphery of a particle, LZr2 is
a second point
location in a line defined between said first point location and a geometric
center of said
particle as illustrated in Figure 2,
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
7
wherein a second content of Zr measured by TEM-EDS at the second point
location Lzr2 is
superior or equal to 0 at% and inferior or equal to 5.0% of a first content of
Zr (Zr)
measured at the first point location. Said second content of Zr (Zr2) is
defined as:
Zr2 (in at%) = Zr3 + 0.1 at%, and optionally
Zri ¨ Zr2 > 10.0 at%
Zr3 is a third content of Zr (in at%) at a third point location (Lzr3) in said
line, said third
point is located at any location between the geometric center of said particle
and the second
point location Lzr2.
When Zr2 and Zr3 are superior to 0.0 at%, the second and third content of Zr
corresponds
to the content of Zr, measured by TEM-EDS, present as a dopant in the core of
the particles
according to the invention.
The TEM-EDS protocol is applied as follows:
1) A cross-sectional TEM lamella of the lithium transition metal-based oxide
particles is
extracted by cutting the particle sample using a Ga ion beam so as to obtain a
prepared sample.
2) The prepared sample (a cross section of particle) is scanned with a TEM/EDS
line
scan from the external edge of the surface layer to the center of a lithium
transition
metal-based oxide particle, so as to provide a quantitative element analysis
of the
cross-section.
3) The Zr content detected by EDS are normalized by the total atomic content
of Ni,
Mn, Co, and Zr in the scanned lamella.
4) The measured line scan of Zr/(Ni+Mn+Co+Zr) is then plotted as a function of
a
linear distance in a cross section of said particle.
The aforementioned steps 1) to 4) are repeated as many times as there are
particles to be
analyzed.
The aforementioned TEM-EDS measurement is performed on at least one particle.
When
more than one particle is measured, the Zr/(Ni+Mn+Co+Zr) are numerically
averaged.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
8
For example, but not limiting to the invention, TEM-EDS measurement is
performed with a a
Tecnai G2 F30 S-TWIN (FEI) with ELITE T 70 detector (EDAX).
As appreciated by the skilled person, for any of the Embodiments 1 to 5, Zrp
is expressed
as molar fraction, as measured by XPS analysis compared to the sum of molar
fractions of
Co, Mn, Ni, and Zr, as measured by XPS analysis; in particular Zr 3 is the
molar fractions of
Zr measured in a region of a secondary particle of the positive electrode
active material
according to invention defined between a first point of an external edge of
said particle and
a second point at a distance from said first point, said distance separating
said first to said
second point being equal to a penetration depth of said XPS, said penetration
depth D being
comprised between 1.0 to 10.0 nm. In particular, the penetration depth is the
distance
along an axis perpendicular to a virtual line tangent to said external edge
and passing
trough said first point.
The external edge of the particle is, in the framework of this invention, the
boundary or
external limit distinguishing the particle from its external environment.
Therefore, XPS analysis provides atomic content of elements in an uppermost
layer of a
particle with a penetration depth of about 10.0 nnn from an outer boundary of
the particle.
The outer boundary of the particle is also referred to as "surface". In the
framework of the
present invention, at% signifies atomic percentage. The at% or "atomic
percent" of a given
element expression of a concentration means how many percent of all atoms in
the
concerned compound are atoms of said element. The designation at% is
equivalent to mol /0
or "molar percent". For example, but not limiting to the invention, XPS
analysis is carried
out with a Thermo K-a-P spectrometer (Thermo Scientific).
The present invention concerns a use of the positive electrode active material
according to
any of the preceding Embodiments 1 to 5 in a battery.
The present invention is also inclusive of a process for manufacturing the
positive electrode
active material according to any of the preceding Embodiments 1 to 5,
comprising the
steps of:
- preparing a lithium transition metal-based compound,
- mixing said lithium transition metal-based compound with a source of Zr,
preferably Zr alkoxide in lithium alkoxide containing alcohol solvent, thereby
obtaining a mixture, and
- removing volatile phases including solvent, preferably by vacuum heating,
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
9
heating the mixture in an oxidizing atmosphere in a furnace at a temperature
between 350 C and less than 500 C, preferably at most 450 C, for a time
between 1 hour and 20 hours so as to obtain the positive electrode active
material
powder according to the present invention.
In a preferred embodiment the lithium transition metal-based compound is a
lithium nickel-
based oxide compound.
In a preferred embodiment of the method the lithium transition metal-based
oxide
compound comprising Li, M' and oxygen, wherein M' comprises Ni, Mn, Co and D,
wherein D
is at least one element of the group consisting of: Al, B, Ba, Ca, Cr, Fe, Mg,
Mo, Nb, S, Si,
Sr, Ti, Y, V. W, and Zn; preferably Al, B, Cr, Nb, S, Si, Ti, Y, W
Preferably the lithium transition metal oxide powder used is also typically
prepared
according to a lithiation process, that is the process wherein a mixture of a
transition metal
precursor and a lithium source is heated at a temperature preferably of at
least 500 C.
Typically, the transition metal precursor is prepared by coprecipitation of
one or more
transition metal sources, such as salts, and preferably sulfates of the M'
elements Ni, Mn
and/or Co, in the presence of an alkali compound, such as an alkali hydroxide
e.g. sodium
hydroxide and/or ammonia.
Preferably the method comprises a further step, before heating said mixture,
of drying said
mixture, preferably by means of vacuum heating.
n a preferred embodiment of the method, the source of Zr is a Zr-alkoxide,
preferably Zr-
ethoxide, Zr-propoxide or Zr-butoxide, more preferably Zr-propoxide. In a
preferred
embodiment the Zr-alkoxide is mixed as a solid with the mixture.
Alternatively, and more
preferably, the Zr alkoxide is mixed as a solution with the slurry, wherein
the solution
comprises the Zr-alkoxide and a further alcohol, wherein the alkoxide group is
a conjugate
base of the further alcohol. For example, the Zr-alkoxide is Zr-propoxide,
which is dissolved
in propanol. Typically, the solution comprises 50-90 wt.% of the Zr-alkoxide
by total weight
of the solution. Examples of such a solution are a 70 wt.% Zr-propoxide in 1-
propanol or a
80 wt.% Zr-butoxide in 1-butanol.
Preferably, the alcohol solvent is methanol, ethanol, propanol or butanol,
preferably
ethanol.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
The present invention is also inclusive of a solid-state battery comprising
the positive
electrode active material according to any of the preceding Embodiments 1 to
5, preferably
the solid-state battery comprises a sulfide based solid electrolyte, more
preferably the sulfide
based solid electrolyte comprises Li, P and S. Typically, the following sulfur
containing
5 compounds of Li6PS5CI (LPSCL), Thio-LISICON Li 3.25Ge0.25P0.75S4) f Li 2S
P2SHjC I f LiC2S Si S2f
Li I 2S S2f LHD2S0jC I f Li C2S S2f Li I 2S iS2f LiI-Li2S-P2S5, LiI¨Li2SP205,
LiI-Li3PO4-P2S5,
Li2S-P2S5, Li3PS4, Li7P3S11f Li I Li 2S B2S3 f Li 3Par Li 2S S iS2f
3PO4Li 2S iS2f LiPO4-Li2S-SiS2õ
Li1oGeP2S12, Li9.54511.74P1.44511.7C10.3, and/or Li7P3S11 may be suitably
used.
10 BRIEF DESCRIPTION OF THE FIGURES
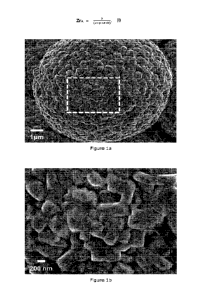
Figure la. SEM image shows secondary particle of CEX2.2 comprising plurality
of primary
particles. Dotted line shows the area to be captured in order to obtain the
average primary
particle diameter.
Figure lb. SEM image of CEX2.2 to obtain the average primary particle
diameter.
Figure lc. SEM image of EX1 to obtain the average primary particle diameter.
Figure id. SEM image of EX3 to obtain the average primary particle diameter
Figure 2. XPS spectra showing Zr peak of EX1 and CEX2.2.
Figure 3. TEM-EDS analysis result of Zr/(Ni+Mn+Co+Zr) of EX1 (x-axis: distance
where 0 is
the starting point of the surface layer, y-axis: element in atomic ratio)
DETAILED DESCRIPTION
In the drawings and the following detailed description, preferred embodiments
are described
so as to enable the practice of the invention. Although the invention is
described with
reference to these specific preferred embodiments, it will be understood that
the invention
is not limited to these preferred embodiments. The invention includes numerous
alternatives, modifications and equivalents that are apparent from
consideration of the
following detailed description and accompanying drawings.
A) ICP analysis
The amount of Li, Ni, Mn, Co, and Zr in the positive electrode active material
powder is
measured with the Inductively Coupled Plasma (ICP) method by using an Agilent
ICP 720-
ES (Agilent Technologies, https://www.agilent.com/cs/library/brochures/5990-
6497E1\P/020720-725 ICP-OES LR.pdf). 2 grams of powder sample is dissolved
into 10 mL
of high purity hydrochloric acid (at least 37 wt.% of HCI with respect to the
total weight of
solution) in an Erlenmeyer flask. The flask is covered by a glass and heated
on a hot plate
at 380 C until complete dissolution of the precursor. After being cooled to
room
temperature, the solution of the Erlenmeyer flask is poured into a 250 mL
volumetric flask.
Afterwards, the volumetric flask is filled with deionized water up to the 250
ni-IL mark,
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
11
followed by complete homogenization. An appropriate amount of solution is
taken out by
pipette and transferred into a 250 mL volumetric flask for the 2' dilution,
where the
volumetric flask is filled with internal standard and 10% hydrochloric acid up
to the 250 mL
mark and then homogenized. Finally, this 50 mL solution is used for ICP
measurement.
B) SEM (Scanning Electron Microscope) analysis
The morphology of positive electrode active materials is analyzed by a
Scanning Electron
Microscopy (SEM) technique. The measurement is performed with a JEOL JSM 7100F
(https://www.jeolbenelux.com/JEOL-BV-News/jsm-7100f-thermal-field-emission-
electron-
microscope) under a high vacuum environment of 9.6x10-5 Pa at 25 C.
C) Particle size
Cl) Secondary particle size analysis
The particle size distribution (PSD) of the positive electrode active material
powder is
measured by laser diffraction particle size analysis using a Malvern
Mastersizer 3000 with a
Hydro MV wet dispersion accessory (https://www.malvernpanalytical.com/en/
products/product-range/mastersizer-range/mastersizer-3000#overview) after
having
dispersed each of the powder samples in an aqueous medium. In order to improve
the
dispersion of the powder, sufficient ultrasonic irradiation and stirring is
applied, and an
appropriate surfactant is introduced. D50 is defined as the particle size at
50% of the
cumulative volume% distributions obtained from the Malvern Mastersizer 3000
with Hydro
MV measurements.
C2) Primary particle size analysis
The diameter of primary particle is calculated by using Image] software
(Image] 1.52a,
National Institutes of Health, USA) according to the following steps:
Step 1) Open the file containing SEM image of positive electrode active
material with 10,000
times magnification wherein the image is taken at the center part of a
secondary particle.
Example of such image is shown in Figure la wherein the dotted line shows the
area to be
captured corresponding to Figure lb.
Step 2) Set scale according to the SEM magnification.
Step 3) Draw lines following primary particle edges using polygon selections
tool for at least
50 particles. The particles at the edges of image is to be excluded if
truncated.
Step 4) Measure the area of the drawn primary particles selected from Set
Measurements
and Area box.
Step 5) Calculate the particle diameter of each measured area by assuming the
particle in
the spherical shape following d = 2 x = and obtain the average primary
particle diameter
TE
for at least 50 particles.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
12
D) X-ray photoelectron spectroscopy analysis
In the present invention, X-ray photoelectron spectroscopy (XPS) is used to
analyze the
surface of positive electrode active material powder particles. In XPS
measurement, the
signal is acquired from the first few nanonneters (e.g. 1 nnn to 10 nnn) of
the uppermost part
of a sample, i.e. surface layer. Therefore, all elements measured by XPS are
contained in
the surface layer.
For the surface analysis of positive electrode active material powder
particles, XPS
measurement is carried out using a Thermo K-a+ spectrometer (Thermo
Scientific,
https://www.thermofisher.com/order/catalog/product/IQLAADGAAFFACVMAHV).
Monochromatic Al Ka radiation (hu=1486.6 eV) is used with a spot size of 400
pm and
measurement angle of 45 . A wide survey scan to identify elements present at
the surface
is conducted at 200 eV pass energy. Cis peak having a maximum intensity (or
centered) at
a binding energy of 284.8 eV is used as a calibrate peak position after data
collection.
Accurate narrow scans are performed afterwards at 50 eV for at least 10 scans
for each
identified element to determine the precise surface composition.
Curve fitting is done with CasaXPS Version2.3.19PR1.0 (Casa Software,
http://www.casaxps.com/) using a Shirley-type background treatment and
Scofield
sensitivity factors. The fitting parameters are according to Table la. Line
shape GL(30) is
the Gaussian/Lorentzian product formula with 70% Gaussian line and 30%
Lorentzian line.
LA(a, 13, m) is an asymmetric line-shape where a and 13 define tail spreading
of the peak and
m define the width.
Table la. XPS fitting parameter for Ni2p3, Mn2p3, Co2p3, and Zr3d.
Element Sensitivity Fitting range Defined peak(s) Line
shape
factor (eV)
Ni 14.61 851.3 0.1- Ni2p3, Ni2p3 satellite
LA(1.33, 2.44, 69)
869.4 0.1
Mn 9.17 639.9 0.1- Mn2p3, Mn2p3 satellite
GL(30)
649.5 0.1
Co 12.62 775.8 0.4- Co2p3-1, Co2p3-2, Co2p3
GL(30)
792.5 0.4 satellite
Zr 7.04 178.0-188.5 Zr3d3, Zr3d1
GL(30)
For Zr and Co peaks, constraints are set for each defined peak according to
Table lb.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
13
Table lb. XPS fitting constraints for peaks fitting.
Fitting range FWHM
Element Defined peak constraint constraint Area
constraint
(eV) (eV)
Zr3d1 184.0-188.5 Equal to Zr3d3 66.7%
of Zr3d3
Zr
Zr3d3 178.0-184.0 0.5-4.0 No
constraint set
Co2p3-1 776.0-780.9 0.5-4.0 No
constraint set
Co Co2p3-2 781.0-785.0 0.5-4.0 No
constraint set
Co2p3 satellite 785.1-792.0 0.5-6.0 No
constraint set
The Zr surface contents as determined by XPS are expressed as a molar fraction
of Zr in the
surface layer of the particles divided by the total content of Ni, Mn, Co, and
Zr in said surface
layer. It is calculated as follows:
Zr (at%)
fraction of Zr = ZrB =
NI (at /o) + Mn (at /o) + Co (at /) + Zr (at /o)
E) Sulfide solid-state battery testing
El) Sulfide solid-state battery preparation
Positive electrode preparation:
For the preparation of a positive electrode, a slurry contains positive
electrode active
material powder, Li-P-S based solid electrolyte, carbon (Super-P. Timcal), and
binder (RC-
10, Arkema) ¨ with a formulation of 64.0 : 30.0 : 3.0 : 3.0 by weight ¨ in
butyl acetate
solvent is mixed in Ar-filled glove box. The slurry is casted on one side of
an aluminum foil
followed by drying the slurry coated foil in a vacuum oven to obtain a
positive electrode.
The obtained positive electrode is punched with a diameter of 10 nm wherein
the active
material loading amount is around 4 mg/cm2.
Negative electrode preparation:
For the preparation of negative electrode, Li foil (diameter 3 mm, thickness
100 pm) is
placed centered on the top of In foil (diameter 10 nnn, thickness 100 pm) and
pressed to
form Li-In alloy negative electrode.
Separator
For the preparation of separator which also has a function of the solid
electrolyte in a
battery, the Li-P-S based solid electrolyte is pelletized with a pressure of
250 MPa to obtain
100 pm pellet thickness.
Cell assembly
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
14
A sulfide solid-state battery is assembled in an argon-filled glovebox with
such order from
bottom to top: positive electrode comprising Al current collector with the
coated part on the
top - separator - negative electrode with Li side on the top ¨ Cu current
collector. The
stacked components are pressed together with a pressure of 250 MPa and placed
in an
external cage to prevent air exposure.
E2) Testing method
The testing method is a conventional "constant cut-off voltage" test. The
conventional cell
test in the present invention follows the schedule shown in Table 2. Each cell
is cycled at
60 C using a Toscat-3100 computer-controlled galvanostatic cycling station
(from Toyo).
The schedule uses a 1C current definition of 160 mA/g. The initial charge
capacity (CQ1)
and discharge capacity (DQ1) are measured in constant current mode (CC) at C
rate of 0.1
C in below voltage range:
- 4.2 V to 2.5 V (Li/Lit) or 3.6 V to 1.9 V (InLi/Li+) for CEX1.1, EX1,
CEX2.1,
CEX2.2, CEX3, and EX2.
- 4.3 V to 2.5 V (Li/Li) or 3.7 V to 1.9 V (InLi/Li+) for CEX4 and EX3.
The irreversible capacity IRRQ is expressed in % as follows:
(CQ1 ¨ DQ1)
IRRQ (%) = ________________________________________ CQ1 x 100
Table 2. Cycling schedule for sulfide solid-state battery testing method
Charge Discharge
C End Rest V/Li metal C End Rest
V/Li metal
Rate current (min) (V) Rate current (min)
(V)
0.1 - 30 4.2 0.1 - 30
2.5
0.1 - 30 4.3 0.1 - 30
2.5
F) TEM (Transmission Electron Microscope) analysis
To examine the Zr distributions within a lithium transition metal-based oxide
particle, cross-
sectional TEM lamellas of particles are prepared by Nova Nano SEM200 (FEI). A
Ga ion
beam is used with 30kV voltage and 30pA-7nA current. The obtained etched
sample has a
dimension of 5x8pm with 100nm thickness. Using the prepared (etched) sample,
the
surface property from the top to the center of the lithium transition metal-
based oxide
particle is analyzed by TEM and energy-dispersive X-ray spectroscopy (EDS).
The TEM-EDS
line scan is performed on a Tecnai G2 F30 S-TWIN (FEI) with ELITE T 70
detector (EDAX).
An EDS analysis of the lithium transition metal-based oxide particle provides
the
quantitative element analysis of the cross-section. Zr content is normalized
by the total
atomic fraction of Ni, Mn, Co, and Zr.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
The invention is further illustrated by the following (non-limitative)
examples:
Comparative Example 1
5 CEX1 is obtained through a solid-state reaction between a lithium source
and a transition
metal-based source running as follows:
1) Co-precipitation: a transition metal-based oxidized hydroxide precursor
with metal
composition of Ni0.64Mno.1.7Coo.20 is prepared by a co-precipitation process
in a large-scale
continuous stirred tank reactor (CSTR) with mixed nickel-manganese-cobalt
sulfates,
10 sodium hydroxide, and ammonia.
2) Mixing: the transition metal-based oxidized hydroxide precursor and LiOH as
a lithium
source are homogenously mix with a lithium to metal M' (Li/M') ratio of 1.03
in an industrial
blending equipment to obtain a mixture.
3) First heating: the mixture from Step 2) is heated at 830 C for 10 hours
under an oxygen
15 atmosphere. The heated powder is crushed, classified, and sieved so as
to obtain an
intermediate product.
4) Second heating: the intermediate product from Step 3) is heated at 350 C
for 6 hours
under an oxygen atmosphere so as to obtain CEX1.1 having M' comprising Ni, Mn,
and Co in
a ratio Ni: Mn: Co of 0.638: 0.165: 0.197 as obtained by ICP. CEX1 has a D50
of 10 pm.
Optionally, a source of dopant can be added in the co-precipitation process in
Step 1) or in
the mixing step in the Step 2) together with lithium source. A certain element
can be added
as a dopant, for instance, to improve the electrochemical properties of the
positive electrode
active material.
Example 1
EX1 is obtained through a solid-state reaction between a lithium source and a
transition
metal-based source running as follows:
1) Co-precipitation: a transition metal-based oxidized hydroxide precursor
with metal
composition of Ni0.64Mno.17Coo.200 is prepared by a co-precipitation process
in a large-scale
continuous stirred tank reactor (CSTR) with mixed nickel-manganese-cobalt
sulfates,
sodium hydroxide, and ammonia.
2) Mixing: the transition metal-based oxidized hydroxide precursor and LiOH as
a lithium
source are homogenously mix with a lithium to metal M' (Li/M') ratio of 1.03
in an industrial
blending equipment to obtain a mixture.
3) First heating: the mixture from Step 2) is heated at 830 C for 10 hours
under an oxygen
atmosphere. The heated powder is crushed, classified, and sieved so as to
obtain an
intermediate product.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
16
4) Wet mixing: Step 4a) to Step 4c) below is applied to introduce Zr into the
positive
electrode active material
Step 4a) Zr solution preparation: 0.6 mol% of Zr from Zr-propoxide (70 wt% Zr-
propoxide
in n-propanol solution), 1.2 mol% of Li-ethoxide powder, each with respect to
the total
molar contents of Ni, Mn, and Co in the intermediate product, and ethanol
solvent are
mixed to form a solution. The amount of ethanol solvent is 55 wt.% of the
total weight of
the designated intermediate product to mix in the Step 4b).
Step 4b) Mixing: intermediate product obtained from Step 3) is mixed with Zr
solution
prepared in Step 4a) for 20 minutes in a heatable reactor.
Step 4c) Heating: 70 C heat is applied to reactor in Step 4b) while at the
same time reactor
is connected to a vacuum pump to evaporate volatile phases. The product
obtained from
this step is a dried powder.
5) Second heating: the dried powder from Step 4c) is heated at 350 C for 6
hours under an
oxygen atmosphere so as to obtain EX1 having M' comprising Ni, Mn, Co and Zr
in a ratio
Ni: Mn: Co: Zr of 0.635: 0.163: 0.196: 0.006 as obtained by ICP. EX1 has a D50
of 10 pm.
Comparative Example 2
CEX2.1 is obtained through the same procedure as CEX1, except that the first
heating
temperature in Step 3) is 860 C.
CEX2.2 is obtained through the same procedure as EX1, except that the first
heating
temperature in Step 3) is 860 C.
Comparative Example 3
CEX3 is obtained through the same procedure as CEX1, except that the first
heating
temperature in Step 3) is 795 C.
Example 2
EX2 is obtained through the same procedure as EX1, except that the first
heating
temperature in Step 3) is 795 C.
Comparative Example 4
CEX4 is obtained through a solid-state reaction between a lithium source and a
transition
metal-based source running as follows:
1) Co-precipitation: a transition metal-based oxidized hydroxide precursor
with metal
composition of Ni0.83Mno.12Coo.05 is prepared by a co-precipitation process in
a large-scale
continuous stirred tank reactor (CSTR) with mixed nickel-manganese-cobalt
sulfates,
sodium hydroxide, and ammonia.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
17
2) First mixing: the transition metal-based oxidized hydroxide precursor and
LiOH as a
lithium source are homogenously mix with a lithium to metal M' (Li/M') ratio
of 0.96 in an
industrial blending equipment to obtain a mixture.
3) First heating: the mixture from Step 2) is heated at 765 C for 10 hours
under an oxygen
atmosphere. The heated powder is crushed, classified, and sieved so as to
obtain an
intermediate product.
4) Second mixing: the heated powder from Step 3) and LiOH as a lithium source
are
homogenously mix with a lithium to metal M' (Li/M') ratio of 1.02 in an
industrial blending
equipment to obtain a mixture.
5) Second heating: the mixture from Step 4) is heated at 730 C for 10 hours
under an
oxygen atmosphere so as to obtain CEX4 having M' comprising Ni, Mn, and Co in
a ratio Ni:
Mn: Co of 0.829: 0.121: 0.050 as obtained by ICP. CEX4 has a D50 of 6 pm.
Example 3
EX3 is obtained through following steps:
1) Wet mixing: Step la) to Step 1c) below is applied to introduce Zr into the
positive
electrode active material
Step la) Zr solution preparation: 0.5 mol% of Zr from Zr-propoxide (70 wt% Zr-
propoxide
in n-propanol solution), 1.0 mol% of Li-ethoxide powder, each with respect to
the total
molar contents of Ni, Mn, and Co in the intermediate product, and ethanol
solvent are
mixed to form a solution. The amount of ethanol solvent is 55 wt.% of the
total weight of
the designated CEX4 to mix in the Step lb).
Step lb) Mixing: CEX4 is mixed with Zr solution prepared in Step 4a) for 20
minutes in a
heatable reactor.
Step 1c) Heating: 70 C heat is applied to reactor in Step 4b) while at the
same time reactor
is connected to a vacuum pump to evaporate volatile phases. The product
obtained from
this step is a dried powder.
5) Heating: the dried powder from Step 1c) is heated at 350 C for 6 hours
under an oxygen
atmosphere so as to obtain EX3 having M' comprising Ni, Mn, Co and Zr in a
ratio Ni: Mn:
Co: Zr of 0.826: 0.120: 0.050: 0.005 as obtained by ICP. EX3 has a D50 of 6
pm.
Comparative Example 5
CEX5 is obtained through the same procedure as CEX1, except that the first
heating
temperature in Step 3) is 750 C.
Example 4
EX4 is obtained through the same procedure as EX1, except that the first
heating
temperature in Step 3) is 750 C.
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
18
Comparative Example 6
CEX6 is obtained through the same procedure as CEX1, except that the first
heating
temperature in Step 3) is 750 C.
Example 5
EX5 is obtained through the same procedure as EX1, except that the first
heating
temperature in Step 3) is 750 C.
Table 3. Summary of the primary particle diameter, composition, and the
corresponding
electrochemical properties of example and comparative examples.
Average
Electrochemical
Carbon ICP XPS
XPS/ICP
primary
property
particle
ID
diameter Ni DQ1
IRRQ
(PPrn) ZrA* ZrB*
ZrB/ZrA
(nm) (mol%) (mAh/g) (mAh/g)
CEX1 270 249 63.8 0.00 0.00 n/a** 137.3 25.9
EX1 270 1077 63.5 0.0059
0.94 158.6 173.5 6.7
CEX2.1 371 178 63.8 0.00 0.00 n/a** 126.1 29.5
CEX2.2 371 549 63.2 0.0059
0.73 123.1 151.0 12.4
CEX3 231 195 65.0 0.00 0.00 n/a** 145.1 17.0
EX2 231 1062 64.7 0.0059 0.83 142.3
176.6 8.3
CEX4 292 358 82.9 0.00 0.00 n/a** 119.3 30.0
EX3 292 1497 82.6 0.48 0.77 161.6 164.6 19.5
CEX5 190 149 65.0 0.00 0.00 n/a** 146.0 17.2
EX4 190 1001 65.1 0.0059 0.59 101.6
172.5 6.6
CEX6 192 238 64.9 0.00 0.00 n/a** 120.3 23.3
EX5 192 1076 65.1 0.0059 0.68 114.6
171.7 8.5
* Relative to molar contents of Ni, Mn, Co, and Zr
** Not applicable because ZrA is 0.
Table 3 summarizes the primary particle diameter, composition, and the
corresponding
electrochemical properties of example and comparative examples. The average
primary
particle diameter of EX1, EX2, EX3, EX4, and EX5 are 270, 231, 293, 190, and
192 nm,
respectively. These average diameters are smaller than the average primary
particle
diameters of CEX2.1 and CEX2.2 which are 371 nm. The primary particle SEM
images of
CEX2.2, EX1, and EX3 are shown in Figure lb, lc, and id, respectively. The
images contain
CA 03209722 2023- 8- 24
WO 2022/180155
PCT/EP2022/054633
19
drawn lines and number to identify primary particle in order to obtain the
average primary
particle diameter.
In the Table 3, the XPS analysis result of EX1, CEX2.2, and EX3 showing Zr
atomic ratio
(equivalent with molar ratio) with respect to the total atomic fraction of Ni,
Mn, Co, and Zr
(ZrB). The table also compares the result with that of ICP. The Zrb higher
than 0 indicates
said Zr is presence in the surface of the positive electrode active material
as associated with
the XPS measurement which signal is acquired from the first few nanometers
(e.g. 1 nm to
nm) of the uppermost part of a sample, i.e. surface layer. On the other hand,
Zr atomic
10 ratio obtained from ICP measurement (ZrA) is from the entire particles.
Therefore, the ratio
of XPS to ICP (ZrB/ZrA) higher than 1 indicates said elements Zr presence
mostly on the
surface of the positive electrode active material. The higher ZrB/ZrA value
corresponds with
the more Zr presence in the surface of positive electrode active material.
ZrB/ZrA in EX1 and
EX3 are higher than that of CEX2.2 ZrB/ZrA. The representative of XPS spectra
showing
Zr3d5 and 3d3 peaks of CEX2.2 and EX1 are displayed in Figure 2.
Figure 3 shows the TEM-EDS measurement of EX1 (x-axis: distance where 0 is the
starting
point of the surface layer, y-axis: element in atomic ratio). Zr thickness
from the
measurement is 25.8 nm.
Carbon content in positive electrode active material after Zr treatment is
higher comparing
with before treatment which associated with a better electrochemical
performance of active
material. Carbon is originated from the Zr alkoxide compound used in the
treatment.
The combination of average primary particle diameter in the range of 170 nm to
340 nm
and ZrB/ZrA higher than 50.0, preferably higher than 100.0, can achieve the
objective of the
present invention, which is to provide a positive electrode active material
having an
improved first charge capacity of at least 160 mAh/g in the solid-state
battery.
CA 03209722 2023- 8- 24