Note: Descriptions are shown in the official language in which they were submitted.
1
DESCRIPTION
Title of Invention: COMPOUND AND RADIOACTIVE LABELING COMPOUND
Technical Field
[0001]
The present invention relates to a compound and a radiolabeled compound.
Background Art
[0002]
Radiolabeled compounds, having a structure containing a radioactive nuclide,
have been used as a reagent for detecting a target molecule, a diagnostic
agent, or medicine
to treat a disease. For the purpose of further improving performance in
detecting a lesion
and therapeutic effect, studies are in progress on: improving specific
accumulation at a
target tissue and site; and reducing accumulation at a non-target tissue and
site.
[0003]
Patent Literature 1 discloses a derivative (T-ITK01169) that has, as an
albumin-
binding site, an added iodophenylbutyryl group that branches from a PSMA-617
structure.
It is also disclosed that this derivative targets and binds to prostate-
specific membrane
antigen (PSMA) and can be used in order to detect and treat prostate cancer.
[0004]
Patent Literature 2 discloses an albumin-binding PSMA inhibitor. It is also
disclosed that this inhibitor also targets and binds to PSMA as disclosed in
Patent Literature
1 and can be used in order to detect and treat prostate cancer.
Citation List
Patent Literature
[0005]
Patent Literature 1: WO 2019/075583 Al
Patent Literature 2: WO 2018/098390 Al
CA 03210294 2023- 8- 29
2
Summary of Invention
[0006]
In achieving: improvement in specific accumulation at a target tissue and
site; and
reduction in accumulation at a non-target tissue and site, it is important to
control
pharmacokinetics, such as retention in blood. As a method to effectively
control
pharrnacokinetics, chemical structures are desired to be further optimized. In
general, a
compound with a small molecular weight has poor retention in blood, which may
result in:
insufficient accumulation at a target tissue; and unintended accumulation at a
normal tissue.
In addition, the compounds disclosed in Patent Literature 1 and 2 undesirably
show much
unspecific accumulation at the kidney, still having had room for improvement
in this respect.
[0007]
Therefore, the present invention relates to a compound and a radiolabeled
compound capable of achieving both of: improvement in accumulation at a target
tissue;
and reduction in accumulation at a non-target tissue, particularly reduction
in accumulation
at the kidney.
[000S]
The present invention provides a compound including a structure having: a
chelating part capable of coordinating with a radioactive metal ion; a first
atomic group
containing an albumin-binding part; and a second atomic group containing a
PSMA
molecule-binding part,
wherein the first atomic group and the second atomic group are bonded with
each
other through the chelating part.
[0009]
The present invention provides a compound represented by the following general
formula (2):
[0010]
CA 03210294 2023- 8- 29
3
A2
(2)
B
wherein, in the formula (2), A2 represents a chelating part capable of
coordinating
with a radioactive metal ion;
B represents an atomic group containing an albumin-binding part;
C represents an atomic group containing a PSMA molecule-binding part;
A2 represents Neunpa. Octapa, or a derivative thereof;
Lc represents a linker structure; and
when A2 represents Octapa, Lc contains a polyethylene glycol structure.
[0011]
The present invention provides a radiolabeled compound comprising a
radioactive
metal ion and the compound which is coordinated with a radioactive metal ion.
[0012]
The present invention provides a method for manufacturing a radiolabeled
compound, the method including: coordinating the compound to a radioactive
metal ion to
obtain a radiolabeled compound.
Brief Description of Drawings
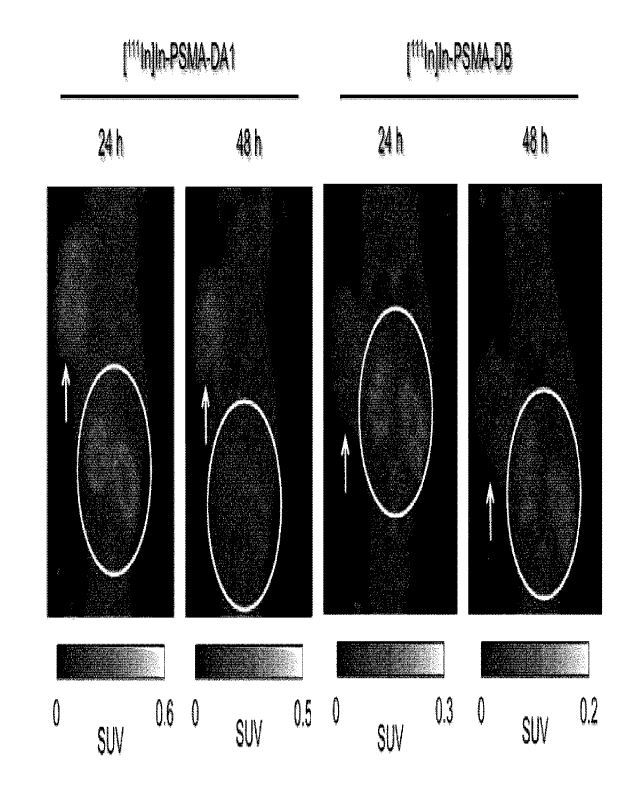
[0013]
[Fig.1] Fig. 1 is graphs showing the results of the cell-binding assay in
Example 1-1
([1111ri]Tn-PSMA-DA1) and Comparative Example 1-1 ([ui In]In-PSMA-DB).
[Fig. 2] Fig. 2 is a graph showing the results of the albumin-binding
experiments in
[1111n]In-PSMA-DA1 and [111-ln]Tn-PSMA-DB.
[Fig. 3] Fig. 3 is graphs showing the results of the internal radioactivity
distribution
experiments in [111Tn]Tn-PSMA-DA1 and [1111n]In-PSMA-DB.
[Fig. 4] Fig. 4 is SPECT/CT images in [1111n]In-PSMA-DA1 and [111In]lri-PSMA-
DB.
CA 03210294 2023- 8- 29
4
[Fig. 5] Fig. 5 is graphs showing changes in tumor volume and mouse body
weight in
Example 1-2 ([90Y]Y-PSMA-DA1) and Comparative Example 1-2 ([90Y]Y-PSMA-DB).
[Fig. 6] Fig. 6 is graphs showing changes in tumor volume and mouse body
weight in
Example 1-3 ([225Ac]Ac-PSMA-DA1) and Comparative Example 1-3 ([225Ac]Ac-PSMA-
D13).
[Fig. 7] Fig. 7 is an HPLC chart showing the results of stability in plasma in
Example 2
([111In]In-PtDA).
[Fig. 8] Fig. 8 is a graph showing the results of the cell-binding assay in
[111In]In-PtDA.
[Fig. 9] Fig. 9 is a graph showing the results of the albumin-binding
experiments in
[111In]Tri-PtDA
[Fig. 10]Fig. 10 is SPECT/CT images in [111In]In-PtDA.
[Fig. 11]Fig. 11 is graphs showing the results of the cell-binding experiments
in Example
3-1 ([111In]In-Octapa-2), Example 3-2 ([111In]In-Octapa-3), Example 3-3
([111In]In-
Neunpa-2), and Comparative Example 2-1 ([1111n]In-Octapa-1) and Comparative
Example
2-2 ([111In]In-Neunpa-1).
[Fig. 12]Fig. 12 is a graph showing the results of the albumin-binding
experiments in
Examples 3-1 to 3-3 and Comparative Examples 2-1 to 2-2.
[Fig. 13]Fig. 13 is graphs showing the results of the internal radioactivity
distribution
experiments in Examples 3-1 to 3-3 and Comparative Examples 2-1 to 2-2.
[Fig. 14]Fig. 14 is a SPECT/CT image in Example 3-1 ([111In]ln-Octapa-2).
[Fig. 15]Fig. 15 is a graph showing the results of the cell-binding assay in
Example 4-1
([111In]In-PSMA-NAT-DA1).
[Fig. 16]Fig. 16 is a graph showing the results of the albumin-binding
experiments in
Example 4-1 ([111Inffn-PSMA-NAT-DA1).
[Fig. 17]Fig. 17 is SPECT/CT images in Example 4-1 ([1111n]Tn-PSMA-NAT-DA1).
Description of Embodiments
100141
Hereinafter, the compound of the present invention and the radiolabeled
compound using the same will be described based on preferred embodiments
thereof. In
the following description, "T to U [V]" (T and U are arbitrary numbers, and
[V] is a unit.)
means "T [V] or more and U [V] or less" unless otherwise specified. In
addition, when
an asymmetric carbon atom is present in the structure, unless otherwise
specified, each
independently may be the S configuration or the R configuration.
CA 03210294 2023- 8- 29
5
[0015]
The compound of the present invention, roughly classified based on its
chemical
structure, includes a structure having three atomic groups: a chelating part
capable of
coordinating with a radioactive metal ion; a first atomic group containing an
albumin-
binding part; and a second atomic group containing a part binding to a
prostate-specific
membrane antigen (Hereinafter, it is also referred to as PSMA.) molecule.
Having such a chemical structure, the radiolabeled compound in which the
compound of the present invention is coordinated with a radioactive metal ion
is capable
of achieving both of: improvement in accumulation at a target tissue
expressing a PSMA
molecule; and reduction in accumulation at a non-target tissue, particularly
at the kidney.
The compound of the present invention is a precursor compound to be used for
labeling
with a radioisotope such as a radioactive metal, that is, preferably a
compound to be used
as a labeling precursor. The radioactive metal will be described later.
[0016]
In one embodiment, the compound of the present invention is a compound in
which a first atomic group containing an albumin-binding part is bonded with a
second
atomic group containing a PSMA molecule-binding part through a chelating part.
Such a compound is preferably represented by the following general formula
(1).
[0017]
C-(Lb)¨AiiLa)¨B (1)
[0018]
In the formula (1), A1 represents a chelating part capable of coordinating
with a
radioactive metal ion; B represents an atomic group containing an albumin-
binding part;
and C represents an atomic group containing a PSMA molecule-binding part.
La represents a linker structure.
Lb represents a linker structure that is identical to or different from La.
"in" and "n" each independently represent 0 (zero) or 1.
B or La is bonded to an arbitrary position of A1.
C or Lb is bonded to A1 at a position different from the position at which B
or La
CA 03210294 2023- 8- 29
6
is bonded to Ai.
[0019]
As described above, the compound in the embodiment, seen as its macroscopic
chemical structure, is preferably a compound in which the chelating part (in
the general
formula (1), represented by symbol Ai) is positioned at the center of the
structure, and the
first atomic group containing an albumin-binding part (in the general formula
(1),
represented by symbol B) and the second atomic group containing a PSMA
molecule-
binding part (in the general formula (1), represented by symbol C) are
linearly arranged
through the chelating part.
[0020]
In the general formula (1), each of "between A1 and B" and "between A1 and C",
independently, may be indirectly bonded via the linker structure, or may be
directly bonded
without the linker structure. In the formula (1), when each of "between Ai and
B" and
"between Ai and C" has a linker structure, and the linker structures may be
the same as or
different from each other.
Details of the linker structure will be described later.
[0021]
From the viewpoint that the radiolabeled compound made from the compound of
the present invention achieves, in higher level, both of: improvement in
accumulation at a
target tissue expressing a PSMA; and reduction in accumulation at a non-target
tissue,
particularly at the kidney, it is preferable in the formula (1) that: Ai has a
cyclic structure,
the cyclic structure has two or more nitrogen atoms, and each of the nitrogen
atoms is
connected with one another through two or more adjacent carbon atoms; or Ai
has an open
chain structure, the open chain structure has two or more nitrogen atoms, and
each of the
nitrogen atoms is connected with one another through two or more adjacent
carbon atoms.
[0022]
In the formula (1), when Ai has a cyclic structure, the skeleton of the cyclic
structure may be composed only of nitrogen atoms and carbon atoms, or may be
composed
of oxygen atoms in addition to nitrogen atoms and carbon atoms. The bonds
between
CA 03210294 2023- 8- 29
7
carbon atoms in the cyclic structure may be a chain or may form a cyclic
structure.
In the formula (1), when Ai has an open chain structure, the bonds between
carbon
atoms in the open chain structure may be divided by a nitrogen atom. The bonds
between
carbon atoms in the open chain structure may be a chain or may form a cyclic
structure.
[0023]
In the formula (1), when A1 has a cyclic structure or an open chain structure,
Ai
preferably has a nitrogen-bonding atomic group directly bonded with a nitrogen
atom
constituting the cyclic structure or the open chain structure. As a specific
example of the
nitrogen-bonding atomic group, an atomic group containing one or more groups
selected
from the group consisting of a carboxy group, a phosphate group, an amide
group, a
benzene ring, and a pyridine ring is preferable, and the atomic group is more
preferably a
chain.
Furthermore, in the formula (1), when Ai has a cyclic structure or an open
chain
structure, under the condition that: B is bonded to an arbitrary position of
Ai; and C is
bonded to an arbitrary position of Ai different from the bonding position of
B, it is
preferable that, when B is bonded to the nitrogen-bonding atomic group through
La or
without La, C is bonded to a position other than a nitrogen-bonding atomic
group to which
B is bonded through La or without La.
[0024]
Specifically, in the formula (1), the chelating part capable of coordinating
with a
radioactive metal represented by symbol Ai preferably has a structure derived
from a
compound represented by any one of the following formulas (Al) to (A9), and
more
preferably has a structure derived from a compound represented by the
following formula
(Al). That is, the compound of the present invention is preferably a
derivative of a
compound represented by any one of the following formulas (Al) to (A9), and
more
preferably a derivative of a compound represented by the following formula
(Al). These
structures can be appropriately selected according to the type ofradioactive
metal described
later. The chelating part having any of the structures achieves both of:
improvement in
accumulation at a target tissue expressing PSMA; and reduction in accumulation
at a non-
target tissue, particularly at the kidney.
CA 03210294 2023- 8- 29
8
[0025]
In the formula (1), examples of the chelating part represented by symbol Ai
include structures derived from the following compounds, but applicable
structures are not
limited thereto.
[0026]
<DOTA or derivative thereof>
= 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)
= 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrapropionic acid (DOTPA)
= 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylene phosphoric acid
(DOTMP)
= Hydroxypropyltetraazacyclododecane triacetic acid (HP-DO3A)
= (1R,4R,7R,10R)-a,a',a",cr-tetramethyl-1,4,7,10-tetraazacyclododecane-
1,4,7,10-tetraacetic acid (DOTAMA)
= 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane (DOTAA)
= 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(acetamidomethylene)
phosphonic acid (DOTA-A-AMP)
= Tetraazacyclododecane dimethane phosphonic acid (DO2P)
=a-(2-carboxyethyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetratetraacetic
acid
(DOTAGA)
[0027]
<HOPO or derivative thereof>
= N,N',N",N'"-tetra(1,2-dihydro-l-hydroxy-2-oxopyridine-6-carbonyl)-
1,5,10,14-
tetraazatetradodec an e (1,2-HOPO)
[0028]
<TETA, PEPA, or derivative thereof>
= 1,4,8,11-tetraa7acyclotetradecane-1,4,8,11-tetraacetic acid (TF,TA)
= 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetrapropionie acid (TETPA)
= 1,4,7,10,13-pentaazacyclopentadecane-N,N',N",N'",N"-pentaacetic acid
(PEPA)
CA 03210294 2023- 8- 29
9
[0029]
<Open chain structure (octapa, neunpa or derivative thereof)>
= Ethylenediaminetetraacetic acid (EDTA)
=
6,6'4(ethane-1,2-diylbis((carboxymethypazanediy1)) bis(methylene))
dipicolinic acid (T-Loctapa)
= 6,6'-( {9-hydroxy-1,5-bis(methoxycarbony1)-2,4-di(pyridine-2-y1)-3,7-
diazabicyclo[3.3.1]nonane-3,7-diyll bis(-methylene)) dipicolinic acid
(H2bispa2)
= 1,24 16-(carboxy)-pyridine-2-yll -methyl amino] ethane (H2dedpa)
-6-(1,4,10,13-tetraoxa-7,16-diazacyclooctadecane-N,N'-,dimethyl) picolinic
acid
(H2macropa)
=
N, N"-bis(6-carboxy-2-pyri dylm ethyl)-di ethyl en etri ac etic
acid (H5decapa)
= N,N'-(m ethyl enephosphon ate)-N,1\1'46-(m ethoxycarbonyl)pyri din e-2-
y1]-
methy1-1,2-di arninoethane (Hophospa)
= 6,6'-(((((4-i sothi ocyan ate
phen ethyl) azan ediyl) bis(ethane-2,1-diy1))
bis((carboxymethyl) azanediy1)) bis(methylene)) dipicolinic acid (p-SCN-Bn-
Rineunpa)
= 6,6'-(((((4-nitrophenethyl) azanediyl) bis(ethane-2,1-diy1))
bis((carboxymethyl)
azanediy1)) bis(methylene)) dipicolinic acid (p-NO2-Bn-H4neunpa)
= 6,6'-(((azanediylbis (ethane-2,1-diy1)) bis((carboxyrnethyl) azanediy1)
bis(methylene)) dipicolinic acid (H5neunpa)
[0030]
<NOTA or derivative thereof >
= 2[4,7-bis(carboxymethyl)-1,4,7-triazonan-1 -yl] acetic acid (NOTA)
[0031]
CA 03210294 2023- 8- 29
10
(Al) (A2)
R21\ /R22
R11\ / N22
R
_,N N,, R2c-N N"-R24
R1C __ / R14
(A3) (A4)
R31\ R R41\ ki
33
R32 PI., R42 R44
rs,34
R35 R45
(A5) (A6) (A7)
1-1 R51\ ny, /R52 R6s1F¨\ /R62
CO OD CNN
R66¨ R67
R4 ¨ N¨R4g R55 ¨N N¨R53 (N
sCill 0 0-) cN)
R54 Reg' \__/ R64
[0032]
In the formula (Al), R11, R12, R13, and Ri4 are each independently any of
groups
consisting of -(CH2)pCOOH, -(C1-12)pC5H5N, -(C112)pP03f12, -(CH2)pCONH2, and -
(CHCOOH)(CH2)pCOOH; and "p" is an integer of 0 or more and 3 or less.
[0033]
In the formula (A2), R21, R22, R23. and R24 each independently represent a
carboxy
group or a carboxyalkyl group having 2 or 3 carbon atoms.
[0034]
CA 03210294 2023- 8- 29
11
In the formula (A3), R31, R32, R33. and R34 each independently represent an
atomic
group haying a hydrogen atom and 2 or more and 10 or less carbon atoms and
optionally
containing a nitrogen atom or an oxygen atom; and R35 represents a hydrogen
atom, a
carboxy group, or a carboxyalkyl group haying 2 or 3 carbon atoms.
[0035]
In the formula (A4), R41, R47, R43, and R44 each independently represent an
atomic
group haying a hydrogen atom and 2 or more and 10 or less carbon atoms and
optionally
containing a nitrogen atom or an oxygen atom; and R45 represents a hydrogen
atom, a
carboxy group, or a carboxyalkyl group haying 2 or 3 carbon atoms.
[0036]
In the formula (A5), R48 and R49 each independently represent an atomic group
haying a hydrogen atom and 2 or more and 10 or less carbon atoms and
optionally
containing a nitrogen atom or an oxygen atom.
[0037]
In the formula (A6), R51, R52, R53, R54, and R55 each independently represent
an
atomic group haying a hydrogen atom and 2 or more and 10 or less carbon atoms
and
optionally containing a nitrogen atom or an oxygen atom.
[0038]
In the formula (A7), R61, 1(62, R63, 1(64, R65, and R66 each independently
represent
an atomic group haying a hydrogen atom and 2 or more and 10 or less carbon
atoms and
optionally containing a nitrogen atom or an oxygen atom; and 1(67 represents a
hydrogen
atom, a carboxy group, or a carboxyalkyl group haying 2 or 3 carbon atoms.
[0039]
CA 03210294 2023- 8- 29
12
(A8) (A9)
R81
1 72
R85 ____________________________________________
R87
N
D ______________________________________________________ R86
1µ83 R84
R73
R82
[0040]
In the folmula (A8), R71, R72 and R73 each independently represent an atomic
group having a hydrogen atom and 2 or more and 10 or less carbon atoms and
optionally
containing a nitrogen atom or an oxygen atom.
[0041]
In the formula (A9), R81 and Rg2 are each independently an alkyl group having
1
or more and 5 or less carbon atoms, and the terminal of the alkyl group may be
substituted
with a pyridyl group substituted with 1 or more carboxy groups; R87 is a
hydroxyl group or
the oxygen atom (=0) of a carbonyl group; R83 and R84 are a substituted or
unsubstituted
pyridinyl group; R85 and R86 are each independently -COO-Ra; and Ra is an
alkyl group
having 1 or more and 5 or less carbon atoms.
[0042]
Examples of the specific structure represented by the formula (Al) include
structures represented by the following founulas (A1-1) to (Al-?).
CA 03210294 2023- 8- 29
13
[0043]
(A1-1) (A1-2)
SCN li OMe
l'--\
HO cN N OH HONN.,1 OH
HO N/N( L
HO\ /N\_21\ i) OH
/ (OH
\µ
0 0 Cr- -Th
DOTA Me0-DOTA-NCS
CA 03210294 2023- 8- 29
14
[0044]
(A1-3) (A1-4)
OH 0
0 0
j-OH \\
HO-P-\ /--\ /-P\-OH
rõN HO/ EN N.,1 OH
L. N N.) 0 ..)
_c_/ HO-P z N\_/N \ p
HO 0 / \
HO OH
0 HO
DOTPA DOTMP
(A1-5) (A1-6)
? NH2 0
-N H2
r., Nr¨\rsr10, O r,N N.,1
/ /N N
L) L. N
N= H2N 0
/ 0 H2N
LPY DOTAM
CA 03210294 2023- 8- 29
15
[0045]
(A1-7)
H OT H
/-\
N N
OH
He'-'0 0 OH
DOTAGA
[0046]
Examples of the specific structure represented by the formula (A2) include
structures represented by the following formulas (A2-1) to (A2-2).
[0047]
(A2-1) (A2-2)
OH
n
OH C ) OH
LN N.)
0
HO
OH
TETA TETPA
[0048]
Examples of the specific structure represented by the formula (A3) include
structures represented by the following formulas (A3-1) to (A3-7).
[0049]
CA 03210294 2023- 8- 29
16
(A3-1) (A3-2) (A3-3)
02N
0
* *
OH
0 (AOH 0 0
Hok,,N \z"õN,...\11,0H HO*1_1 cAN3-0H
,- j-N OH
HO N \-µ
HOy 0 HO \ IN C
8-- 0
0 0 H0-µ OH r HO =
0
EDTA CHX-K-DTPA HBED
CA 03210294 2023- 8- 29
17
[0050]
(A3-4) (A3-5)
HOOC¨\ /¨COOH
\ N \
rNH HN
N/
COOH HOOCNI c
COOH HOOC
H4octapa H2dedpa
(A3-6) (A3-7)
NCS NO2
=
HOOC¨\ 7¨COOH HOOC¨\ /¨COOH
\ N NI \
rrN
N/
COOH HOOC)¨
COOH HOOC)¨
p-SCN-Bn-H4octapa p-NO2-Bn-H4octapa
[0051]
Examples of the specific structure represented by the formula (A4) include
structures represented by the following formulas (A4-1) to (A4-2).
CA 03210294 2023- 8- 29
18
[0052]
(A4-1) (A4-2)
NO2 NCS
11110
HOOC¨\ [COOH HOOC¨\ /¨\\ [COOH
N N N
\ N N2N
COOH HOOC COOH HOOC
p-NO2-Bn-H4neunpa p-SCN-Bn-H4neunpa
[0053]
Examples of the specific structure represented by the formula (A5) include
structures represented by the following formulas (A5-1) to (A5-3).
[0054]
(A5-1) (A5-2) (A5-3)
(:) -1A
0
HO_ õ.õ, a_0:1
Ccfl NCSNN
Nõ,)
(¨CU)
HOC
macropa macropa-NCS macropid
[0055]
Examples of the specific structure represented by the formula (A6) include a
structure represented by the following formula (A6-1).
CA 03210294 2023- 8- 29
19
[0056]
(A6-1)
H
PEPA
[0057]
Examples of the specific structure represented by the formula (A7) include
structures represented by the following formulas (A7-1) to (A7-2).
[0058]
(A7-1) (A7-2)
0
OH 0 OH o
NCS
OH N OH 0 OH N OH 0111 0\K¨ 1--N
N
HO NN,HO \--N W.-4r
HO
HO 0/ \-14
HO
0 0
HEHA HEHA-NCS
[0059]
Examples of the specific structure represented by the formula (A8) include
structures represented by the following formulas (A8-1) to (A8-3).
CA 03210294 2023- 8- 29
20
[0060]
(A8-1) (A8-2) (A8-3)
HOOC HOOc
t.... F-1 ,,--.õ,
I, /-1 ,----, iN N\
COOH
HOCC /NI N COOH
\-N-../ it¨COOH
LN/--\N ,COOH \-N---) /¨COOH
N¨,,, COOH
(,N COOH
L."COOH
0
0 NO2 NO2.
NOTA 3p-C-NETA 5p-C-N ETA
[0061]
Examples of the specific structure represented by the formula (A9) include
structures represented by the following formulas (A9-1) to (A9-4).
CA 03210294 2023- 8- 29
21
[0062]
(A9-1) (A9-2)
N I iv I
OH OH
Me00C¨"---N¨COOMe Me00C----1L--COOMe
OH
(,)\1 =-=
N N
HO HO
0 0
b1spa2 N2PY4
(A9-3) (A9-4)
I 0
Me00C¨"..-N¨COOMe
OH OH-===
Me00C¨r---*N¨COOMe
OH
N
===N I N
HO
0
Hbispala Hbi = b
spal
[0063]
Another embodiment of the compound of the present invention is described
below.
In this embodiment, differences from the embodiment described above will be
mainly
described. Unless specifically described, the description of the embodiment
described
above is appropriately applied thereto.
[0064]
CA 03210294 2023- 8- 29
22
The compound of the present invention in another embodiment, seen as its
macroscopic chemical structure, is a compound in which a chelating part is
bonded so that
it branches from between a first atomic group containing an albumin-binding
part and a
second atomic group containing a PSMA molecule-binding part.
Specifically, it is preferable that the first atomic group containing an
albumin-
binding part and the second atomic group containing a PSMA molecule-binding
part are
bonded to each other so that they make a branch, through the linker structure
bonded with
the chelating part.
Such a compound is preferably represented by the following general formula
(2).
[0065]
A2
(2)
CB
[0066]
In the formula (2), A2 represents a chelating part capable of coordinating
with a
radioactive metal ion; B represents an atomic group containing an albumin-
binding part;
and C represents an atomic group containing a PSMA molecule-binding part.
A2 represents Neunpa. Octapa, or a derivative thereof.
L, represents a linker structure.
When A2 represents Octapa, Lc contains a polyethylene glycol structure.
[0067]
From the viewpoint that the radiolabeled compound achieves, in higher level,
both
of: improvement in accumulation at a target tissue expressing PSMA; and
reduction in
accumulation at a non-target tissue, particularly at the kidney, it is
preferable in the formula
(2) that A2 has an open chain structure, the open chain structure has two or
more nitrogen
atoms, and each of the nitrogen atoms is connected with one another through
two or more
adjacent carbon atoms.
[0068]
In the formula (2), when A2 has an open chain structure, the bonds between
carbon
CA 03210294 2023- 8- 29
23
atoms in the open chain structure may be divided by a nitrogen atom. The bonds
between
carbon atoms in the open chain structure may be a chain or may form a cyclic
structure.
Such A2 is more preferably a compound represented by the above-described
formula (A3)
or formula (A4), or a derivative thereof.
[0069]
Hereinafter, what is applicable to each of the above-described embodiments
will
be described.
Unless otherwise specified, the following descriptions are appropriately
applied to
the descriptions of the general fonnula (1) and the general fonnula (2), and
may be
appropriately employed in combination.
[0070]
In the formula (1) or (2), the position represented by symbol B is an atomic
group
containing an albumin-binding part, which has an affinity to albumin,
preferably serum
albumin, more preferably human serum albumin, and has a chemical structure
capable of
reversibly binding to albumin. The compound of the present invention,
containing such a
structure, can enhance retention in blood and reduce accumulation at the
kidney when the
compound is labeled with a radioactive metal and administered to a living
body.
[0071]
Specifically, the compound having an albumin-binding part in the molecule is
easily bonded to albumin in blood, and the compound bonded to albumin no
longer
undergoes glomerular filtration in the kidney, thereby reducing migration into
the kidney
or urine; and excretion to improve retention in blood. As a result,
accumulation at the
kidney, a normal tissue, is reduced and the compound can be further improved
in
transferability into a target tissue, such as a tumor tissue expressing PSMA.
[0072]
When the compound represented by the formula (1) is used, the distance between
the albumin-binding part and the PSMA molecule-binding part can be
appropriately
secured, so that both affinity to albumin and affinity to a PSMA molecule can
be provided.
In particular, the albumin-binding part is disposed at one structural terminal
of the
CA 03210294 2023- 8- 29
24
compound of the present invention and the PSMA molecule-binding part is
disposed at the
other structural terminal of the compound of the present invention. Thus it is
possible to
sufficiently secure the distance between the albumin-binding part and PSMA
molecule-
binding part, which is further advantageous in that both affinity to albumin
and affinity to
a PSMA molecule can be achieved in higher level.
[0073]
Alternatively, when the compound represented by the formula (2) is used, the
linker structure represented by Lc can appropriately secure the distance
between the
albumin-binding part and the PSMA molecule-binding part, so that both affinity
to albumin
and affinity to a PSMA molecule can be provided.
As will be described later, when a structure containing a polyethylene glycol
group
and containing neither a cyclohexyl group nor a naphthyl group is adopted as
the linker
structure represented by Lc in the formula (2), it is possible to secure an
appropriate distance
between the albumin-binding part and the PSMA molecule-binding part, which is
further
advantageous in that both affinity to albumin and affinity to a PSMA molecule
can be
achieved in higher level.
[0074]
In the formula (1) or the formula (2), examples of the structure of the
albumin-
binding part include a structure derived from one or more of y-glutamic acid,
a substituted
or unsubstituted phenylbutyric acid, a fat, hematin, bilirubin, clofibric
acid, clofibrate,
carotenoid, a compound having a steroid skeleton, a compound having an
ibuprofen
skeleton, a linear or branched, saturated or unsaturated hydrocarbon having 13
or more and
20 or less carbon atoms, a cyanine dye, a dye having a sulfonate group, a
diazo dye,
pentamethine cyanin dye, blue dextran, bromocresol green, Evans blue, and a
derivative
thereof, and structures disclosed in WO 2005/117984 A2, WO 2010/127336 Al, or
WO
2010/172844. In addition to or in place of this, an antibody or peptide
capable of binding
to albumin (for example, the peptide described in WO 2007/106120 A2 and the
like) can
also be used as the albumin-binding part.
[0075]
Among them, from the viewpoint of obtaining a compound applicable to a living
CA 03210294 2023- 8- 29
25
body and reducing accumulation at an unintended normal organ such as the
kidney, as the
structure of the albumin-binding part, it is preferable to use one or more of
a substituted or
unsubstituted phenylbutyric acid, Evans blue, and derivatives thereof, or an
antibody or
peptide capable of binding to albumin.
[0076]
Examples of the substituted or unsubstituted phenylbutyric acid applicable as
the
albumin-binding part include a structure represented by the following formula
(B1):
[0077]
0
(B1)
[0078]
wherein, in the formula (B1), R represents a hydrogen atom, a halogen atom, or
an alkyl group having 1 or more and 5 or less carbon atoms; and the wavy line
part
represents a part bonding to another structure.
In the formula (B1), R is preferably a hydrogen atom, an iodine atom, a
bromine
atom, or a methyl group.
[0079]
Examples of Evans blue and a derivative thereof applicable as the albumin-
binding
part include a structure represented by the following formula (B2):
CA 03210294 2023- 8- 29
26
[0080]
Rbl Rb2 Rb3 Rbei OH NH2
SO3H
FN 1\17. (B2)
Rbg Rb11
Rb5 Rb6 Rb7 Rb8 Rb1 0 SO3H
[0081]
wherein, in the formula (B2), Rbl to Rbil each independently represent a
hydrogen
atom, a halogen atom, a hydroxyl group, a cyano group, a substituted or
unsubstituted alkyl
group having 1 or more and 6 or less carbon atoms, or a substituted or
unsubstituted alkoxy
group having 1 or more and 6 or less carbon atoms; and the wavy line part
represents a part
bonding to another structure.
In the formula (B2), it is preferable that both Rbi and Rb4 are a methyl
group, and
all of Rb2, Rt,3, and Rb5 to Rbll are a hydrogen atom.
[0082]
As the antibody capable of binding to albumin, an immunoglobulin having a
class
of IgG, IgA, IgM, IgD, and IgE may be used, or an antibody fragment (for
example, a Fab
fragment) may be used as long as the antibody has affinity to albumin. From
the
viewpoint of reducing accumulation at an unintended tissue such as the liver,
the antibody
capable of binding to albumin to be used as the albumin-binding part is
preferably a Fab
fragment having a smaller molecular weight.
[0083]
Examples of the peptide capable of binding to albumin include a peptide
containing the sequences shown in WO 2007/106120 A2, and specifically include
a peptide
containing the following peptide sequences, but are not limited to these
sequences.
In the following peptide sequences, an amino acid is represented in one-letter
notation, and the left side of the paper represents the N-terminal, and the
right side of the
paper represents the C-terminal.
= LCLRDWGCLW (SEQ ID NO: 1)
CA 03210294 2023- 8- 29
27
= DICLPRWGCLWW (SEQ ID NO: 2)
= MEDICLPRWGCLWGD (SEQ TD NO: 3)
= QRLMEDICLPRWGCLWEDDE (SEQ ID NO: 4)
= QGLIGDICLPRWGCLWGRSV (SEQ ID NO: 5)
= QGLIGDICLPRWGCLWGRSVK (SEQ ID NO: 6)
= EDICLPRWGCLWEDD (SEQ ID NO: 7)
= RLMEDICLPRWGCLWEDD (SEQ ID NO: 8)
= MEDICLPRWGCLWEDD (SEQ ID NO: 9)
= MEDICLPRWCiCLWED (SEQ ID NO: 10)
= RLMEDTCLARWGCLWEDD (SEQ ID NO: 11)
= EVRSFCTRWPAEKSCKPLRG (SEQ ID NO: 12)
= RAPESFVCYWETICFERSEQ (SEQ ID NO: 13)
[0084]
In the formula (1) or the formula (2), the PSMA molecule-binding part
represented
by symbol C is an atomic group containing a PSMA molecule-binding part that
has affinity
to a PSMA molecule expressed in a tissue that causes a disease such as cancer
and has a
chemical structure capable of reversibly binding to the PSMA molecule. The
compound
of the present invention, containing such a structure, can efficiently
accumulate at a tissue
to be treated or diagnosed to enhance the efficiency of treatment or diagnosis
when the
compound is labeled with a radioactive metal and administered to a living
body.
[0085]
PSMA is a membrane-bound protein that is accelerated to express particularly
in
prostate cancer. Although PSMA expresses in a smaller amount in a normal
tissue
including the prostate, PSMA is accelerated to express in a higher amount as
the grade of
prostate cancer increases. Therefore, PSMA is one of the useful target
molecules in the
present invention, and is particularly useful as a target for diagnosis and
treatment of
prostate cancer.
[0086]
Examples of cancer expressing a PSMA molecule include prostate cancer. The
prostate cancer may be primary or metastatic.
CA 03210294 2023- 8- 29
28
[0087]
The chemical structure of the PSMA molecule-binding part can be appropriately
selected according to a target tissue and the amount of a PSMA molecule
expressed in the
tissue. Specifically, a structure having affinity to a PSMA molecule can be
adopted as the
PSMA molecule-binding part. Examples of the structure having affinity to a
PSMA
molecule include one or more of low molecular weight compounds, peptides,
antibodies,
and antibody fragments, such as Fab fragment.
[0088]
In the above fatmula (1) or (2), the PSMA molecule-binding part preferably has
a
structure represented by the following formula (Cl) or is preferably an
antibody or peptide
capable of binding to a PSMA molecule. The compound of the present invention,
containing such a structure, can efficiently accumulate at a tissue to be
treated or diagnosed
to enhance the efficiency of treatment or diagnosis when the compound is
labeled with a
radioactive metal and administered.
[0089]
HOOCyõ f A..NH
0
/a A. k ib (Cl)
HOOC COOH
[0090]
In the formula (Cl), "a" and "b" each independently represent an integer of 1
or
more and 7 or less;
and in the formula (Cl), the wavy line part represents a part bonding to Ai or
Lb
in the formula (1) or Le in the formula (2).
[0091]
As the antibody capable of binding to a PSMA molecule, an immunoglobulin
having a class of IgG, IgA, IgM, IgD, and IgE may be used, or an antibody
fragment (for
example, a Fab fragment) may be used as long as the antibody has affinity to a
PSMA
CA 03210294 2023- 8- 29
29
molecule. From the viewpoint of reducing accumulation at an unintended tissue
such as
the liver, the antibody capable of binding to a PSMA molecule to be used as
the PSMA
molecule-binding part is preferably a Fab fragment having a smaller molecular
weight.
[0092]
When the compound of the present invention has a structure of the formula (I),
it
is more preferable to have a structure represented by the following general
formula (IS),
and it is also more preferable to have a structure represented by the
following general
formula (1T).
Such a structure makes it possible to have sufficient hetero atoms capable of
coordinating with a radioactive metal in the structure. Therefore, complex
formation
efficiency can be enhanced when a radioactive metal is coordinated with the
compound of
the present invention. In addition, since degree of freedom in molecular
mobility is
increased within the albumin-binding part and the PSMA molecule-binding part,
both
affinity to albumin and affinity to a PSMA molecule can be achieved in higher
level.
Furthermore, unintended accumulation at a normal tissue, such as the liver and
kidney, is
reduced.
CA 03210294 2023- 8- 29
30
[0093]
0
C Ai iLa
(iS)
= R
0
HOOC ¨( )¨
LbAii La
H 41, R (1T)
HOOC N OOH
H H
=
[0094]
In the formula (1S) and the formula (1T), Ai each independently represents a
chelating part capable of coordinating with a radioactive metal ion.
In the formula (is), C represents an atomic group containing a PSMA molecule-
binding part.
In the formula (1S) and the formula (1T), La each independently represents a
linker
structurc.
In the formula (1S) and the formula (1T), Lb each independently represents a
linker
structure that is identical to or different from La.
In the formula (1S) and the formula (1T), "m" and "n" each independently
represent 0 (zero) or 1.
In the formula (1S) and the formula (IT), the same description as in the above
formula (B 1 ) is appropriately applied.
In the formula (IS) and the formula (IT), when "n" is I, La is bonded to an
arbitrary position of Ai .
In the formula (1S) and the formula (1T), when "n" is 0, the carbon atom of
the
carbonyl group bonded to the phenylalkyl group is bonded to an arbitrary
position of Al.
In the formula (1S) and the formula (1T), when "m" is 1, Lb bonds to Ai at a
position different from the position at which La is bonded to Al.
In the formula (1S) and the formula (IT), when "m" is 0, C or the nitrogen
atom
CA 03210294 2023- 8- 29
31
is bonded to Ai at a position different from the position at which La is
bonded to Ai.
[0095]
When the compound of the present invention has the structure of the formula
(1),
it is more preferable to have a structure represented by the following general
formula (3).
Such a structure makes it possible to have sufficient hetero atoms capable of
coordinating with a radioactive metal in the structure. Therefore, complex
formation
efficiency can be enhanced when a radioactive metal is coordinated with the
compound of
the present invention. In addition, since degree of freedom in molecular
mobility is
increased within the albumin-binding part and the PSMA molecule-binding part,
both
affinity to albumin and affinity to a PSMA molecule can be achieved in higher
level.
Furthermore, unintended accumulation at a normal tissue, such as the liver and
kidney, is
reduced.
[0096]
HOOC) 0
Rci N
(3)
0
COOH
[0097]
In the formula (3), one of RB1 and RB2 is an atomic group containing an
albumin-
binding part, and the other is a hydrogen atom, a hydroxyl group, or a carboxy
group; and
one of Rci and Rc2 is an atomic group containing a PSMA molecule-binding part,
and the
other is a hydrogen atom, a hydroxyl group, or a carboxy group.
Among them, in the formula (3), it is preferable that Rni is an atomic group
containing an albumin-binding part, Rci is an atomic group containing a PSMA
molecule-
binding part, and both RB2 and Rc2 are a hydroxyl group.
Alternatively, in the formula (3), it is also preferable that RB2 is an atomic
group
containing an albumin-binding part, RC2 is an atomic group containing a PSMA
molecule-
CA 03210294 2023- 8- 29
32
binding part, and RBI_ and Rci are both a hydrogen atom or each independently
a
carboxyalkyl group having 1 to 5 carbon atoms.
In any of the embodiments described above, it is preferable that the albumin-
binding part is disposed at one structural terminal of the compound and the
PSMA
molecule-binding part is disposed at the other structural terminal of the
compound, and
therefore, seen as the macroscopic chemical structure of the compound of the
present
invention, the chelating part, the albumin-binding part, and the PSMA molecule-
binding
part are almost linearly disposed.
[0098]
In particular, in the formula (3), the atomic group containing an albumin-
binding
part is preferably an atomic group containing the structure represented by the
formula (B1)
or the formula (B2) described above as the albumin-binding part. Specific
examples of
such a chemical structure are shown in the following formulas (3-1) to (3-4).
hi the following formulas (3-1) and (3-2), Li each independently represents an
alkyl group having 1 or more and 8 or less carbon atoms and containing a
carboxy group.
In the following formulas (3-3) and (3-4), each independently, "g" represents
an
integer of 1 or more and 5 or less; and "h" represents 0 or 1.
[0099]
Furthermore, in the following formulas (3-1) to (3-4), regarding the
descriptions
of R, Rbi to Rbii, and Rci and RC2, the descriptions regarding the above-
described formulas
(B1) and (B2), and formula (3) are appropriately applied independently.
That is, R is a hydrogen atom, a halogen atom, or an alkyl group having 1 or
more
and 5 or less carbon atoms, and preferably R is a hydrogen atom, an iodine
atom, a bromine
atom, or a methyl group.
Rh] to Rbi I each independently represent a hydrogen atom, a halogen atom, a
hydroxyl group, a cyano group, a substituted or unsubstituted alkyl group
having 1 or more
and 6 or less carbon atoms, or a substituted or unsubstituted alkoxy group
having 1 or more
and 6 or less carbon atoms. Preferably, both Rb1 and Rb4 represent a methyl
group, and all
of Rb2, Rb3, and Rb5 to Rb11 represent a hydrogen atom.
One of Rci and RC2 is an atomic group containing a PSMA molecule-binding part,
and the other is a hydrogen atom, a hydroxyl group, or a carboxyalkyl group
having 1 to 5
carbon atoms.
CA 03210294 2023- 8- 29
33
[0100]
0
HOOC \
0 R
1 0 Li ¨N
Rci r N Thi¨ N../ H (3-1)
H
N N
RC2 4¨ N J
0
COOH
HOOC \
1 0
Rci (¨Nm )_ NH Rbl R2 Rb3 Rb4 OH NH2
N N ¨I \ H
NN SO3H
Li ¨ N
R02 ______________ N?-4.s,......N......,) (3-
2)
0
Rb5 Rb6 Rb7 R Rbg
COOH Rbio SO3HRbi 1
H.
CA 03210294 2023- 8- 29
34
[0101]
H 00C \
1 0
Rci OH
RC2 Qy0
R N ) (3-3)
0
COOH
HOOC
0
Rci N OH
N 0
H Rbl Rb2 Rb3 Rt,4
OH NH2
RC2 g
SO3H
0
0)¨N
(34)
COOH Rbg Rb11
Rb5 Rb6, Rb7 RbEl Rb10 S031-I
[0102]
The compound of the present invention having each of the structures related to
the
formulas (1) and (3) can be manufactured, for example, by the method and
synthetic route
described in Examples described later.
[0103]
When the compound of the present invention has the structure of the formula
(2),
it is more preferable that, in the formula (2), B has a structure represented
by the formula
(B1) and C has a structure represented by the formula (Cl). Specifically, it
is more
preferable to have the structure of the following formula (2S).
Such a structure makes it possible to have sufficient hetero atoms capable of
coordinating with a radioactive metal in the structure. Therefore, complex
formation
efficiency can be enhanced when a radioactive metal is coordinated with the
compound of
the present invention. In addition, since degree of freedom in molecular
mobility is
increased within the albumin-binding part and the PSMA molecule-binding part,
both
affinity to albumin and affinity to a PSMA molecule can be achieved in higher
level.
Furthermore, unintended accumulation at a normal tissue, such as the liver and
kidney, is
reduced. In addition, the complex formation needs less production time,
thereby further
CA 03210294 2023- 8- 29
35
improving production efficiency and radiochemical yield of the radiolabeled
compound.
[0104]
A2
0
HOO N ¨Lc (2S)
R
[0105]
In the formula (2S), A2 represents Neunpa, Octapa, or a derivative thereof.
In the formula (2S), Le represents a linker structure.
In the formula (2S), R represents a hydrogen atom, a halogen atom, or an alkyl
group having 1 or more and 5 or less carbon atoms.
In the formula (2S), "a" and "b" each independently represent an integer of 1
or
more and 7 or less.
In the formula (2S), when A2 represents Octapa, Lc contains a polyethylene
glycol
structure.
[0106]
The compound of the present invention having each of the structures related to
the
formulas (2) and (2S) can be manufactured, for example, by the method and
synthetic route
described in Examples described later.
[0107]
The compound of the present invention, preferably in a condition that it has
been
dissolved in an aqueous solution such as a solvent and a buffer solution, is
reacted with a
radioactive metal ion to coordinate the compound in each of the embodiments to
a
radioactive metal ion, thereby obtaining the radiolabeled compound. This
radiolabeled
compound is a radioactive metal complex in which the chelating part of the
compound is
coordinated to a radioactive metal ion.
CA 03210294 2023- 8- 29
36
[0108]
When the compound represented by the formula (1) is used in obtaining a
radiolabeled compound, it is preferable that B in the formula (1) has a
structure represented
by the formula (B1). Specifically, in obtaining a radiolabeled compound, it is
more
preferable to use a compound represented by the formula (1S). Thereby, complex
formation efficiency can be enhanced.
[0109]
From the viewpoint of enhancing complex formation efficiency, the radioactive
metal to be reacted with the compound is preferably used in the faun of an
ionizable
radioactive metal compound, and more preferably used in the form of a
radioactive metal
ion (Hereinafter, these forms are also collectively referred to as
"radioactive metal source".).
As the radioactive metal source, for example, a radioactive metal ion-
containing liquid in
which a radioactive metal ion is dissolved or dispersed in a solvent mainly
composed of
water can be used.
[0110]
From the viewpoint of enhancing the efficiency of complex formation with a
radioactive metal regardless of the combination of the compound's chelating
part and the
radioactive metal, the complex is formed preferably by heating and reacting
the compound
and the radioactive metal. Under such reaction conditions, even when a
radioactive metal
nuclide emitting a low-energy radiation difficult to detect or a-rays is used,
complex
formation can proceed well. Therefore, a target radiolabeled compound can be
obtained
in high yield.
[0111]
In obtaining the radiolabeled compound, the order of adding the compound and
the radioactive metal source is not limited as long as the compound and the
radioactive
metal ion can form a complex. For example, it is possible that one of the
compound and
the radioactive metal source is added to a reaction vessel containing a
solvent, and then the
other is added to cause a reaction. It is also possible that a solution in
which one of the
compound and the radioactive metal source is dissolved in a solvent is added
with the other
to cause a reaction. Alternatively, they may be simultaneously added to a
reaction vessel
CA 03210294 2023- 8- 29
37
containing a solvent to cause a reaction.
[0112]
The reaction conditions for obtaining the radiolabeled compound can be, for
example, the following conditions. As the solvent used in this step, for
example, water,
saline, or a buffer solution, such as a sodium acetate buffer solution, an
ammonium acetate
buffer solution, a phosphate buffer solution, a phosphate buffer saline
solution, a Iris buffer
solution, a HEPES buffer solution, and a tetramethylammonium acetate buffer
solution can
be used. The reaction temperature may be, for example, room temperature (25 C)
or
under heating conditions.
[0113]
As the radioactive metal source, for example, a solution in which a
radioactive
metal ion is dispersed in a solvent mainly composed of water can be used.
[0114]
The amount of the reaction liquid in this step is not particularly limited,
but from
the viewpoint of practicality in the production step, 0.01 mL to 100 mL is
practical at the
start of this step. In addition, it is preferable that the concentrations of
the compound and
the radioactive metal ion in the reaction liquid are each independently 1 i_tM
to 100 [tM at
the start of this step from the viewpoint of the yield of an intended
radiolabeled compound.
[0115]
The obtained radiolabeled compound may be used as it is, or may be purified
using
a filtration filter, a membrane filter, a column packed with various fillers,
chromatography,
or the like.
If necessary, in the subsequent steps, a solvent mainly containing water and
other
pharmaceutically acceptable components may be added to the radiolabeled
compound to
form a radioactive pharmaceutical composition containing the radiolabeled
compound as
an active ingredient. The radioactive pharmaceutical composition can be
produced, for
example, by dissolving the radiolabeled compound produced by the above-
described
method in a solvent that is mainly composed of water and is substantially
isotonic with a
living body. The radioactive pharmaceutical composition is administered to a
living body
CA 03210294 2023- 8- 29
38
orally, or parenterally, for example, intravenously, subcutaneously,
intraperitoneally, and
intramuscularly, and is used for treatment and diagnosis of a disease,
detection of a lesion,
or the like.
[0116]
As the radioactive metal coordinated in the radiolabeled compound in an ionic
state, a metal nuclide that emits radiation of a-rays, 13-rays, y-rays, or a
combination thereof
can be used. Examples of the nuclide of such a radioactive metal include a
radioisotope
of an alkali metal, an alkaline earth metal, a lanthanoid, an actinoid, a
transition metal, or a
metal other than these metals.
Among them, it is preferable to use 44^c,
51Cr, '7Co, 58Co, 60Co, 59Fe,64Cu,67cu,
67Ga, 68Ga, "Sr, "Zr, 90Y, 99111Tc, 102Ru, 111In, 152Sm, 165Dy, 166ti-0,
177Lu, 186Re, 188Re, 197Hg,
198A.u, 201T1, 203Hg, 212pb, 212Bi, 213Bi, 225 AAC ,
or 227Th as a radioactive metal nuclide from
the viewpoint of being commercially available and improving complex formation.
These
radioactive metals can be produced by a conventional method. These
radionuclides are
preferably obtained as a solution containing a radioactive metal in an ionized
state.
[0117]
When the radiolabeled compound is used for the purpose of treating a disease,
it
is preferable to use an a-ray-emitting nuclide or a 13--ray-emitting nuclide
as the radioactive
metal from the viewpoint of enhancing therapeutic effect. The a-ray-emitting
nuclide
may be any nuclide that emits a-rays in the disintegration process of the
radioactive metal.
Specifically, 212.13.i, 213Bi, 225Ac, 227Th or the like is preferably used.
The nuclide is more
preferably 227Th or 225Ac, and still more preferably 225Ac.
The p--ray-emitting nuclide may be any nuclide that emits P--rays in the
disintegration process of the radioactive metal. Specifically, 9Fe, 60co,
64cu, 67-u,
89Sr,
90y5 9917C, 103R115 153sm, 165Dy, 166H0, 177Lu, 186Re, 188-e,
K 198AU, 203Hg, 212pb, 212Bi, 213Bi,
or the like is preferably used, more preferably 64cti, 67-u,
L. "Sr, 9017, 177Lu, 186Re, or 188Re is
used, and still more preferably 90Y is used.
[0118]
hi addition, when the radiolabeled compound is used for the purpose of
diagnosis
of a disease or detection of a lesion, it is preferable to use a f3-ray-
emitting nuclide, an
CA 03210294 2023- 8- 29
39
electron-capturing disintegration nuclide, or a 7-ray-emitting nuclide as the
radioactive
metal from the viewpoint of improving diagnostic performance. The ft'-ray-
emitting
nuclide maybe a nuclide that emits positrons in the disintegration process of
the radioactive
metal, and 44Sc, 58Co, 68Ga, 64Cu, 89Zr, or the like is preferably used, and
'Cu or 89Zr is
more preferably used.
The electron-capturing disintegration -nuclide may be any nuclide that emits
Auger
electrons or characteristic X-rays in the disintegration process of the
radioactive metal, and
51Cr, "Co, ssco, 64cu, 67Ga, 68^a,
0 89Zr, 111In, 186Re, 197-1-1g, 201T1, or the like is preferably
used.
The 7-ray-emitting nuclide may be a nuclide that emits y-rays through y-decay,
and 68Ga, 99mTc, or 201T1 is preferably used as a nuclide that emits 7-rays
through 7-decay.
[0119]
When the radioactive metal to be coordinated in the radioactive metal complex
in
an ionic state is selected on the basis of ionic radius, examples of the
radioactive metal
having an ionic radius of about 70 to 130 pm include "Cu, 67Cu, 67Ga, 68Ga,
89Zr, 90y, 99mTc,
103Ru, "'in, 153Sm, 165Dy, 166-1-1r -ro,
177tu, 86Re, 188Re, 198Au, 201T1, 197-Fig, 203Hg, 212pb, 212Bi,
213Bi, and 22'Ac, which can fano a complex of the radioactive metal ion with
the compound
of the present invention having the chelating part preferably having a
structure represented
by the formula (Al) to (A9).
[0120]
For example, when the radiolabeled compound is used for the purpose of
treating
a disease and 225AC is used as the radioactive metal, as the compound of the
present
invention, a compound having a chelating part having a structure represented
by any of the
formulas (Al), (A3) to (A5), or (A7) is preferably used, and a compound having
a chelating
part having a structure represented by the formulas (Al), (A3), or (A4) is
more preferably
used. When 9017 is used as the radioactive metal, as the compound of the
present invention,
a compound having a chelating part having a structure represented by any of
the formulas
(Al) to (A3) or (A8) is preferably used, and a compound having a chelating
part having a
structure represented by the formula (Al) is more preferably used.
When the radiolabeled compound is used for the purpose of diagnosing a disease
or detecting a lesion and 89Zr is used as the radioactive metal, as the
compound of the
present invention, a compound having a chelating part having a structure
represented by
CA 03210294 2023- 8- 29
40
any of the formulas (Al), (A3), or (A4) is preferably used, and a compound
having a
chelating part having a structure represented by the formula (A 1 ) is more
preferably used.
When "Ga or 11 'In is used as the radioactive metal, as the compound of the
present
invention, a compound having a chelating part having a structure represented
by any of the
formulas (Al) to (A4) or (A9) is preferably used, and a compound having a
chelating part
having a structure represented by the formula (Al) is more preferably used.
[0121]
Regarding the bond between the chelating part and the atomic group containing
the albumin-binding part, the chelating part and the albumin-binding part may
be directly
bonded without the later-described linker structure, or the chelating part and
the atomic
group containing the albumin-binding part may be indirectly bonded through the
later-
described linker structure.
Similarly, regarding the bond between the chelating part and the atomic group
containing the PSMA molecule-binding part, the chelating part and the PSMA
molecule-
binding part may be directly bonded without the later-described linker
structure, or the
chelating part and the atomic group containing the PSMA molecule-binding part
may be
indirectly bonded through the later-described linker structure.
Whether the above "directly" form or "indirectly" form is employed, it is
preferable to bond with each other through an amide bond from the viewpoint of
achieving
both easiness of synthesis and stability of chemical structure.
[0122]
The linker structure of La or Lb in the formula (1) or Lc in the formula (2)
is
independently preferably a structure derived from a compound capable of
forming an amide
bond or an ether bond. Specific examples thereof include a structure derived
from: an L-
form or D-form amino acid, such as an acidic amino acid, including glutamic
acid and
aspartic acid, and a basic amino acid, including lysine; a dicarboxylic acid,
such as oxalic
acid and malonic acid; a diamine, such as ethylenediamine; a polyethylene
glycol group;
an amino acid having a structure containing an alicyclie of 5 to 10 carbon
atoms or an
aromatic of 6 to 14 carbon atoms; and the like. These can be employed alone or
by
connecting more than one through an amide bond or an ether bond. The above-
described
structures may be each independently unsubstituted or substituted with various
substituents.
When the structure derived from an amino acid or the like is included as the
above-
CA 03210294 2023- 8- 29
41
described linker structure, for example, for the purpose of controlling
kinetics in a living
body, peptide linkers described in WO 2017/150549 Al, WO 2019/065774 Al, WO
2019/221269 Al, WO 2020/075746 Al, WO 2020/145227 Al, WO 2020/145228 Al, and
the like can be used.
[0123]
When the structure derived from ethylene glycol is contained as the above-
described linker structure, an indirect bond through a linker structure
represented by the
following formula (P) is also preferable. The structure is derived from
ethylene glycol.
In the formula (P), "k" is preferably an integer of 2 or more and 10 or less,
more preferably
an integer of 2 or more and 8 or less, and still more preferably an integer of
2 or more and
or less.
[0124]
(P)
[0125]
These linker stnictures may be composed of one kind of linker structure, or
one
kind of linker structure may be repeated, or a plurality kinds of linker
structure may be
combined, and these may be bonded in a linear or branched manner.
[0126]
In particular, when the compound represented by the formula (2) is used, it is
preferable that the linker structure of Lc contains a polyethylene glycol
structure represented
by the formula (P) and contains neither a cyclohexyl group nor a naphthyl
group. Such a
structure makes it possible to provide the chemical structure with high
mobility and to
appropriately secure the distance between the albumin-binding part and the
PSMA
molecule-binding part, so that both affinity to albumin and affinity to a PSMA
molecule
can be effectively provided.
[0127]
CA 03210294 2023- 8- 29
42
When the chelating part and the albumin-binding part are "indirectly" bonded
with
each other or the chelating part and the PSMA molecule-binding part are
"indirectly"
bonded with each other, another form thereof may be a connection through a
known
coupling method. For example, it is possible to make a connection through a
click
reaction. Structures bonded in these ways are also encompassed by the linker
structures
herein.
Hereinafter, a case where a click reaction is used to bond the chelating part
and the
PSMA molecule-binding part will be described as an example. In this case, each
of the
chelating part and the PSMA molecule-binding part has an atomic group capable
of a click
reaction, and these atomic groups react with each other so that the chelating
part and the
PSMA molecule-binding part can be bonded to each other. That is, a click
reaction is
performed between a first atomic group the chelating part has and a second
atomic group
the PSMA molecule-binding part has.
[0128]
In the present invention, the combination of atomic groups capable of a click
reaction is appropriately selected according to the type of click reaction.
Examples
thereof include a combination of an alkyne and an azide, a combination of
1,2,4,5-tetrazine
and an alkene, and the like. Regarding these atomic groups, the first atomic
group has to
have one of the atomic groups, and the second atomic group has to have an
atomic group
that can be combined with the first atomic group. From the viewpoint of
achieving both
of: stability of the chelating part and the PSMA molecule-binding part; and
improvement
in the bonding efficiency thereof, it is preferable that: the first atomic
group is an alkyne
and the second atomic group is an azide; or the first atomic group is 1,2,4,5-
tetrazine and
the second atomic group is an alkene. Specific examples of the click reaction
by such a
combination of atomic groups include Huisgen cycloaddition reaction and
reverse electron
request type Diels-Alder reaction.
[0129]
As shown in the following formulas, specific examples of the combination of
atomic groups capable of click-reaction include: a combination of an atomic
group (the
formula (11a)) containing dibenzoclooctyne (DBCO) as the alkyne of the first
atomic group
and an atomic group (the formula (12a)) containing an azide group as the azide
of the
second atomic group; and a combination of an atomic group (the formula (11b))
containing
CA 03210294 2023- 8- 29
43
1,2,4,5-tetrazine as the first atomic group and an atomic group (the formula
(12b))
containing trans-cyclooctene (TCO) as the alkene of the second atomic group.
[0130]
R1
0
(11a) (12a) --R2
N" N
Dibenzocyclooctyne Azide
[0131]
(In the formula (11 a), Ri represents a chelating part; and in the formula
(12a), R2
represents a PSMA molecule-binding part.)
CA 03210294 2023- 8- 29
44
[0132]
R3
R6
(11b) N (12b) #0.
11
trans-cyclooctene
R4
1.2.4.5-tetrazine
[0133]
(In the formula (11b), one of R3 and R4 represents a chelating part or a PSMA
molecule-binding part, and the other represents a hydrogen atom, a methyl
group, a phenyl
group, or a pyridyl group; in the formula (12b), R5 represents a chelating
part or a PSMA
molecule-binding part.)
[0134]
When the chelating part and the PSMA molecule-binding part are bonded with
each other through a click reaction in the present invention, the order of
adding them is not
limited as long as a click reaction can proceeds. For example, it is possible
that one of the
chelating part and the PSMA molecule-binding part is added to a reaction
vessel containing
a solvent, and then the other is added to cause a reaction. It is also
possible that a
dispersion in which one of the chelating part and the PSMA molecule-binding
part is
dispersed in a solvent is added with the other to cause a reaction.
Alternatively, they may
be simultaneously added to a reaction vessel containing a solvent to cause a
reaction.
[0135]
In each of the above-described embodiments, examples of the substituent that
can
be substituted in each atomic group, each structure, and each chemical
structure of the
compound and the radiolabeled compound include a halogen atom, a saturated or
unsaturated alkyl group, a hydroxy group, an aldehyde group, a carboxy group,
an acyl
group, an amino group, a nitro group, an ester group, an isothiocyanate group,
a thioxy
group, a cyano group, an amide group, an imide group, a phosphate group, a
phenyl group,
a benzyl group, a pyridyl group, and a naphthyl group. These substituents may
be one
alone or a group in which two or more substituents are combined.
CA 03210294 2023- 8- 29
45
[0136]
Although the present invention has been described above based on the preferred
embodiments thereof, the present invention is not limited to the above-
described
embodiments. For example, in each of the above-described embodiments, a
compound
having one chelating part, one albumin-binding part, and one PSMA molecule-
binding part
has been described. However, as long as the present invention is exhibited, at
least one of
the albumin-binding part and the PSMA molecule-binding part may be contained
at a
plurality of positions in one chemical structure.
Examples
[0137]
Hereinafter, the present invention will be described in more detail with
reference
to Examples. However, the scope of the present invention is not limited to
such examples.
In the following Examples, unless otherwise specified, NMR is always with JNM-
AL400 FT-NMR apparatus, which is manufactured by JEOL Ltd., using
tetramethylsilane
(TMS) as an internal standard substance, and setting TMS resonance to 0.00
ppm. All
chemical shifts were in ppm on the delta scale (6) and signal microfission was
indicated
using abbreviations (s: singlet, d: doublet, t: triplet, m: multiplet, br:
broad).
In mass spectrometry, LCMS 2020 (manufactured by SHIMADZU
CORPORATION) was used when MS was performed, and LCMS-IT-TOF (manufactured
by SHIMADZU CORPORATION) was used when HRMS was performed.
[0138]
[Examples 1-1 to 1-4 and Comparative Examples 1-1 to 1-3]
In the examples, two compounds (PSMA-DA1 and PSMA-DB) whose target
molecule is PSMA were synthesized. Next, radiolabeled compounds were obtained,
in
which each of the compounds was coordinated with a 1111n ion, a 90Y ion, or a
'Ac ion as
a radioactive metal. Details are described below.
PSMA-DA1, used in Examples 1-1 to 1-4, has a chemical structure linearly
including a PSMA molecule-binding part and an albumin-binding part through a
chelating
part, as represented by the general formula (1). In PSMA-DA1, the chelating
part and the
PSMA molecule-binding part are directly bonded to each other by an amide bond,
and the
chelating part and the atomic group containing the albumin-binding part are
indirectly
bonded to each other through a lysine-derived linker structure.
CA 03210294 2023- 8- 29
46
PSMA-DB, used in Comparative Examples 1-1 to 1-3, has a structure containing
a chelating part and a PSMA molecule-binding part, but does not contain an
albumin-
binding part.
[0139]
<Examples 1-1 to 1-4>
The outlines of synthesis routes in Examples 1-1 to 1-4 are defined as
synthesis
routes (V-1) and (V-2), and are shown below.
[0140]
Scheme (V-1) NH2
Co2tBu
CO2Me 0
0
1 H2Isr;-'-'-'-'N tBuO2C-'NA N
CO2tBu
tBuO2C)
H H
H
tB \u02C (----N--) EDC-HCI EDC-HCI
HOAt HOAt
HO2CN N Et3N Et-IN CO2H 1.-
a
N---) CO2tBu DMF DMF
KCO2tBu
1
r,CO2H
2 0 HO C sl (...__HO2CN)Th
),
HN,-,-0
_________________________ ).- 1 HH02C
0 CO2H 0 air
I
6 N HCI
1111111
N leWN
CO2H 0
c-N---).--c21:1-ILH H
<CO2H
(PS MA-DA1)
[0141]
CA 03210294 2023- 8- 29
47
Scheme (V-2)
rco2H
Ho2c ,I
1' -02C)
[iv In]InC13 HNY0 H (D2C f------N--
I
(PSMA-DA1) ______________________ 0 HN),...-,,N1cõ).,. 0 002H
N 111 3F 1 0
Acetate buffer
CO2H 0 /
H
\--N-----) CO2- H
CO2-
r
Lill
Inpn-PSMA-DA1
r.co2H
HO C I -02C)
[90Y]YCI3 HN'0
H 02C ,,,..-----N--\\
,. I
(PSMA-DA1) ______________________ 0 HNy...õ..-...,Nli.,),\ ,,, / 0
cO2H 0
VAcetate buffer N
.),,,,- NNwN
CO2H 0
c_--N---) CO2- H H
C)2-
[NY]Y-PSMA-DA1
r,CO2H
HO2C ,I
) ' -02C
[225AC]ACCI3 HN'0
H 02C (-------NTh
,. I
CO2H 0
(PSMA-DA1) ______________________ 0. HNI.,..,,,..,,Nli,,..), \
VI
01-13COOH- N 225AC3'N NWN
CH3COONH4 buffer CO2H 0
c¨N----) 002- H H
Cc02-
[225AC]Ac-PSMA-DA1
(õCO2H
HO C sl -02C
[89Zr]Zr(ox)2 HN 0
Y )
H 2C c------NTh ab. I
0 co2H 0
(PSMA-DA1) ________________________ HNIõ....-.._,N.irj....õ,,
Wgentistic acid- I'
CH3COOH-CH3COONH4 CO2H 0 c--N) H H
butter CO2-
CO2-
[89Zr]Zr-PSMA-DA1
[0142]
(1) Synthesis of PSMA-DA1 (Compound 1)
Compound 1 was synthesized from 1,4,7,10-tetraazacyclododecane in 3 steps
according to the method of Chem Commun. 2008, 28, 3248-3250. This compound 1
(20
mg, 0.026 mmol) was dissolved in N,N-dimethylformamide (DMF) (2 mL), and
methyl-
N6-(4-(4-iodophenyl)butanoy1)-L-lysinate (11 mg, 0.0254 mmol), 1-ethy1-3-(3-
dimethylaminopropy1)-carbodiimide (EDC) hydrochloride (7.0 mg, 0.037 mmol), 1-
hydroxy-7-azabenzotriazole (HOAt) (5.0 mg, 0.037 mmol), and triethylamine (5
L, 0.036
mmol) were added thereto, and then the mixture was stirred at room temperature
for 24
hours. Thereafter, (S)-di-tert-butyl 2-(3-((S)-6-amino-1-tert-
butoxy-1-oxohexan-2-
CA 03210294 2023- 8- 29
48
yl)ureido) pentanedioate (13 mg, 0.0267 mmol), EDC hydrochloride (7.0 mg,
0.037 mmol),
HOAt (5.0 mg, 0.037 mmol) and triethylarnine (5 iL, 0.036 mmol) were added
thereto, and
the mixture was stirred at room temperature for 72 hours. After removing the
solvent, 6
N hydrochloric acid (3 mL) was added to the residue, the mixture was stirred
at 40 C for
24 hours, and then purified by reverse phase IIPLC under the following
conditions to obtain
the desired compound (PSMA-DA1). The yield and MS were as follows.
[0143]
Purification conditions: Cosmosil 5C18-AR-IT column (10 x 250 mm); mobile
phase: MeCN/H20/trifluoroacetic acid (TFA) [10/90/0.1 (0 mm) to 100/0/0.1 (90
min)];
flow rate: 4 mL/min.
Recovery amount: 1.0 mg (Yield: 3%; calculated from the amount of substance of
PSMA-DA1 obtained relative to the amount of substance of compound 1).
MS(EST): m/z1250.5[M+H].
[0144]
(2)1111n labeling (Example 1-1)
A solution (3.7 MBq, 100 L) of [111In]TnC13 and a DMSO solution of PSMA-DA1
(1 mM, 10 }_tL) were added to an acetate buffer (0.1 M, pH 5.5, 200 pL), and
the mixture
was allowed to stand at 90 C for 30 minutes. Thereafter, the reaction solution
was
purified by reverse phase HPLC under the following conditions to obtain the
desired
radiolabeled compound ([111In]In-PSMA-DA1).
The radioactivity of the obtained radiolabeled compound was measured with a
Curie meter, and the percentage to the radioactivity of the solution of
[111In]InCl3 used in
the reaction was defined as radiochemical yield (%).
In addition, a part of the HPLC preparative liquid of the radiolabeled
compound
was analyzed under the same HPLC conditions as the purification conditions,
and the
percentage of the area value of the radiolabeled compound to the area value of
all detected
peaks was defined as radiochemical purity (%).
As a result, the radiochemical yield was 61 to 90%, and the radiochemical
purity
was 95% or more.
[0145]
CA 03210294 2023- 8- 29
49
Purification conditions for 1111n labeling: Cosmosil 5C18-PAQ column (4.6 x
250
mm); mobile phase: MeCN/H20/TFA [20/80/0.1 (0 min) to 50/50/0.1 (30 min) or
5/95/0.1
(0 to 10 min), 5195/0.1 (10 min) to 35/65/0.1 (40 min)]; flow rate: 1 mL/min.
[0146]
The compound in which PSMA-DA1 is coordinated to non-radioactive In can be
produced, for example, by the following method. The obtained In complex was
used to
identify the HPLC retention time of the radiolabeled compound.
PSMA-DA1 (1 mg) and indium (ITT) chloride anhydrous (2 mg) were dissolved in
dimethyl sulfoxide (DMSO) (100 L), and 2-(N-morpholino) ethanesulfonic acid
buffer
(0.1 M, pH 5.6, 9001..IL) was added. The reaction solution was stirred at 60 C
for 12 hours,
and then the solution was purified through reverse phase HPLC according to the
following
method to obtain a compound having the following MS.
Purification conditions: Cosmosil 5C18-AR-TI column (10 x 250 mm); mobile
phase: MeCN/1-120/TFA [10/90/0.1 (0 min) to 100/0/0.1 (90 min)]; flow rate: 4
mL/min.
MS(EST): m/71362.4[M+H].
[0147]
(3)9 Y labeling (Example 1-2)
A solution (65-118 MBq, 10 1AL) of [90Y]l7C13 and a DMSO solution of PSMA-
DA1 (1 rnM, 10 L) were added to an acetate buffer (0.1 M, pH 5.5, 200 uL),
and the
mixture was allowed to stand at 90 C for 30 minutes. Thereafter, the reaction
solution
was purified by reverse phase HPLC under the following conditions to obtain
the target
radiolabeled compound ([9 Y]Y-PSMA-DA1).
The radiochemical yield was measured in the same manner as in Example 1-1.
As the HPLC conditions used for the analysis of radiochemical purity, the
following
purification conditions for 90Y labeling were used.
As a result, the radiochemical yield was 49 to 79%, and the radiochemical
purity
was 95% or more.
[0148]
Purification conditions for "Y labeling: Cosmosil 5C18-PAQ column (4.6 x 250
mm); mobile phase: MeCN/H20/TFA [20/80/0.1 (0 min) to 50/50/0.1 (30 min) or
5/95/0.1
CA 03210294 2023- 8- 29
50
(0 to 10 min), 5/95/0.1 (10 mM) to 35/65/0.1 (40 min)]; flow rate: 1 mL/min.
[0149]
(4) 225AC labeling (Example 1-3)
To a 0.2 M hydrochloric acid solution (1.5 MBq, 10 },t.L) of [225Ac]AcC13, a
0.1 M
acetic acid-ammonium acetate buffer solution (pH 5.5, 170 }_tL) and a DMSO
solution (2.0
mM, 10 ifL) of PSMA-DA1 were added, and the mixture was allowed to stand at 70
C
for 1 hour. H20 (800 4-) was added to the reaction solution, and the mixture
was passed
through an Oasis 14LB Light column. H20 (10 mL) was passed through the column,
and
then 70% Et0H (0.5 mL) was passed through the column to obtain a purified
solution.
The purified solution was heated and distilled off to dryness, and then a 158
mM acetic
acid-sodium acetate buffer containing 5% ethanol (pH 6.5) was added to obtain
a solution
for administration of the target radiolabeled compound ([225Ac]Ac-PSMA-DA1).
The radiochemical yield was measured by the following method.
The
radioactivity of the obtained radiolabeled compound was measured with a gamma
ray
spectrometer, and the percentage to the radioactivity of the solution of
[225Ac]AcC13 used
in the reaction was defined as radiochemical yield (%).
The radiochemical purity of the obtained radiolabeled compound was measured
by the following method. That is, apart of the solution of the radiolabeled
compound was
analyzed by TLC (iTLC-SG; mobile phase: mixed solution of MeCN/H20 = 1 : 1),
and the
percentage of the area value of the radiolabeled compound to the area value of
all detected
peaks was defined as radiochemical purity (%).
As a result, the radiochemical yield was 49%, and the radiochemical purity was
87%.
[0150]
(5)89Zr labeling (Example 1-4)
A 1.0 M hydrochloric acid solution (2.2 MBq, 10 pt) of [89Zr]Zr(0x)2 was
dispensed into a Type Plus vial (2R) (manufactured by SCHOTT) and heated to
110 C,
and the solvent was distilled off under an Ar gas flow for about 40 minutes.
To the vial
were added 0.1 mol/L hydrochloric acid (100 1.1L), 300 mM gentisic acid-0.78 M
acetic
acid-sodium acetate buffer (pH 5.5, 50 iL), a PSMA-DA1-DMS0 solution (2 mM, 75
pt),
and DMSO (75 lit), and the vial was allowed to stand at 70 C for 1 hour to
obtain the target
radiolabeled compound ([89Zr]Zr-PSMA-DA1).
CA 03210294 2023- 8- 29
51
The radiochemical purity of the obtained radiolabeled compound was measured in
the same manner as in Example 1-3. As a result, the radiochemical purity was
96%.
[0151]
<Comparative Examples 1-1 to 2-3>
The outlines of synthesis routes in Comparative Examples 1-1 to 2-3 are
defined
as synthesis routes (VT-1) and (VT-2), and are shown below.
[0152]
CA 03210294 2023- 8- 29
52
Scheme (VI-1) NH2
002t1311
L..
- 0
E SOtBuO2C-""NA N CO2tBu
tBuO2C\ H H H2N
i tBuO2C EDC-HCI EDC-HCI
_ v r"¨NTh I-10At HOAt
Et3N Et3N
HO2CN N(-0O2H r- r
c--N-. CO2tBu DMF DM F
<
CO2tBu
1
r....0O2H
HO2C sl HO2S
HN,,..0 i
1 H HO2C e=----N¨N
_______________________ r HNõr"...,õ..--..,...õAIN r 0
--1----'11'N 4
6 N HCI N
CO2H 0
<CO2H
(PSMA-DB)
[0153]
CA 03210294 2023- 8- 29
53
Scheme (VI-2)
rco2H
-02c)
[1111n]InC13 H - rs
(PSMA-DB)
Acetate buffer HN0 '32 -NTh
602H 02-
(002-
[1111n]ln-PSMA-DB
r_co2H
Ho2c
-020)
[9:11YCI3 HN'r0
H -1D2C
ir).LI\J
0
Acetate buffer N 9ay3', N
CO2H 0
0_H
C1)2-
(9 M-PSMA-DB
rco2H
H02c1,1 -o2c
[225Ac]AcC13 HN,O - 2C1\
H ?
(PSMA-DB) _____________________________________________________ 0
CH3COOH-CH3C00 NH4 buffer t FIN "'AC'4 140
CO2H 0
\--N--) 02-
Kc02-
[2 =
25AciAc-PSMA-DB
[0154]
(1) Synthesis of PSMA-DB
Compound 1(35 mg, 0.045 mmol) synthesized in the same manner as in Examples
1-1 to 1-4 was dissolved in DMF (2 mL). Then (S)-di-tert-butyl 2-(34(S)-6-
amino-1-tert-
butoxy- 1 -oxohexan-2-yOureido) pentanedioate (22 mg, 0.045 mmol), EDC
hydrochloride
(10 mg, 0.052 mmol), HOAt (7.0 mg, 0.051 mmol), and triethylamine (7 uL, 0.050
mmol)
were added thereto, and the mixture was stirred at room temperature for 24
hours.
Thereafter, aniline (5 j.tL, 0.055 mmol), EDC hydrochloride (10 mg, 0.052
mmol), I-10At
(7.0 mg, 0.051 mmol), and triethylamine (7 i.tL, 0.050 mmol) were added
thereto, and the
mixture was stirred at room temperature for 24 hours. After the solvent was
removed,
TFA (1.8 mL), triisopropylsilane (100 !IL), and H20 (100 [I,L) were added to
the residue,
and the mixture was stirred at room temperature for 24 hours. After removing
the solvent,
the residue was purified by reverse phase HPLC under the following conditions
to obtain
the desired compound (PSMA-DB). The yield and MS were as follows.
CA 03210294 2023- 8- 29
54
[0155]
Purification conditions: Cosrnosil 5C18-AR-TI column (10 x 250 mm); mobile
phase: MeCN/H20/TFA [5/95/0.1 (0 to 10 minutes), 5/95/0.1 (10 minutes) to
35/65/0.1 (40
minutes)]; flow rate: 4 mL/min.
Recovery amount: 5.0 mg (Yield: 12%; calculated from the amount of substance
of PSMA-DB obtained relative to the amount of substance of compound 1).
MS(ESI)m/z925.3 [M+H]-1.
[0156]
(2)1111n labeling (Comparative Example 1-1)
The desired radiolabeled compound ([111In]In-PSMA-DB) was obtained in the
same manner as in Example 1-1 except that PSMA-DB was used instead of PSMA-
DA1.
The radiochemical yield and the radiochemical purity were measured in the same
manner as described in Example 1-1. As the purification conditions and HPLC
conditions
used to measure the radiochemical purity, the following conditions were used.
As a result, the radiochemical yield was 61 to 90%, and the radiochemical
purity
was 95% or more.
[0157]
The compound in which PSMA-DB is coordinated to non-radioactive in can be
produced, for example, by the following method. The obtained In complex was
used to
identify the HPLC retention time of the radiolabeled compound.
A solution of PSMA-DB (1 eq) in H20/MeCN/TFA (49.95/49.95/0.1, 300 uL) was
added with indium (III) chloride anhydrous (10 eq). After stirring at room
temperature
for 18 hours, the solution was purified by reverse phase HPLC.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [5/95/0.1 (0 to 10 minutes), 5/95/0.1 (10 minutes) to
35/65/0.1 (40
minutes)]; flow rate: 1 mL/min.
[0158]
(3)90Y labeling (Comparative Example 1-2)
The desired radiolabeled compound ([90Y]Y-PSMA-DB) was obtained in the same
manner as in Example 1-2 except that PSMA-DB was used instead of PSMA-DAl.
CA 03210294 2023- 8- 29
55
The radiochemical yield and radiochemical purity were measured in the same
manner as described in Example 1-1. The radiochemical purity was measured
under the
HPLC conditions shown in the purification conditions of the above-described
compound
in which PSMA-DB was coordinated to non-radioactive In.
As a result, the radiochemical yield was 49 to 79%, and the radiochemical
purity
was 95% or more.
[0159]
(4) 225Ac labeling (Comparative Example 1-3)
The desired radiolabeled compound ([225Ac]Ac-PSMA-DB) was obtained in the
same manner as in Example 1-3 except that PSMA-DB was used instead of PSMA-
DAl.
The radiochemical yield and radiochemical purity were measured in the same
manner as described in Example 1-3.
As a result, the radiochemical yield was 48%, and the radiochemical purity was
83%.
[0160]
<Evaluation of stability in plasma>
A saline (20 1.1L) solution of [ill In]In-PSMA-DA1 (370 kBq) or [90Y]Y-PSMA-
DA1 (3.7 MBq) was added to mouse plasma (200 1.1,L), and the mixture was
allowed to
stand at 37 C for 24 hours (n = 3). Then, MeCN (200 lit) was added, and the
mixture
was centrifuged at 10,000 x g for 5 minutes. The supernatant was filtered, and
the filtrate
was analyzed by reverse phase HPLC under the following conditions.
(Analytical conditions: Cosmosil 508-PAQ column (4.6 x 250 mm); mobile
phase: MeCN/H20/TFA [20/80/0.1 (0 min) to 50/50/0.1 (30 mint flow rate: 1
mL/min.)
As a result, 95% or more of all the labeling compounds were stably present in
mouse plasma even after standing at 37 C for 24 hours.
[0161]
<Binding assay using cultured cells>
LNCaP cells (PSMA-positive, human prostate cancer) and PC-3 cells (PSMA-
negative, human prostate cancer) were used. These cells were purchased from
American
Type Culture Collection and DS Biomedical, respectively. Each of the cells
were cultured
CA 03210294 2023- 8- 29
56
in RPM' 1640, which is manufactured by NACALAI TESQUE, INC. and contains
antibiotics (penicillin and streptomycin) and 10% inactivated fetal bovine
serum, at 37 C
under 5% CO2.
[0162]
The LNCaP cells and PC-3 cells were each seeded on a 12 well plate at 4.0 x
105
cells/well, and left standing at 37 C under 5% CO2 for 48 hours.
The culture medium was removed, and an assay medium (0.5% FBS-containing
RPMI 1640 medium) solution (1 mL) containing [1111n]In-PSMA-DA1 or [111-In]In-
PSMA-
DB (37 kBq) was added. Thereafter, the plate was allowed to stand at 37 C
under 5%
CO2 for 1 hour.
In the inhibition assays, after removing the culture medium, an assay medium
(1
mL) solution containing: [111In]In-PSMA-DA1 or rl'In]In-PSMA-DB (37 kBq); and
2-
(phosphonomethyl) pentanedioic acid (2-PMPA) (PSMA inhibitor; final
concentration: 100
M) was added. Thereafter, the plate was allowed to stand at 37 C under 5% CO2
for 1
hour.
After removing the assay medium, each well was washed with an assay medium
(1 mL) containing neither the radiolabeled compound nor 2-PMPA, and the cells
were lysed
with 1 N aqueous sodium hydroxide solution (200 i_LL x 2).
The radioactivity for each of the assay medium and the cell lysate was
measured
with a gamma counter. Separately, the total protein concentration in the cell
lysate was
calculated using BCA Protein Assay Kit, which is manufactured by Thermo Fisher
Scientific K.K. The value (% ID/mg protein) obtained by dividing the
percentage (% ID)
of the sample's radioactivity amount to the added radioactivity amount by the
total protein
amount was calculated for each sample.
The data were expressed as mean value standard deviation. The significant
difference test was performed using Student's t-test and one-way analysis of
variance
(ANOVA) test with Dunnet's post-hoc test, and p < 0.05 was defined as having a
significant
difference.
[0163]
The results of the evaluation of binding to cultured cells are shown in Fig.
I. The
higher the value, the higher the abundance of the radiolabeled compound, which
indicates
that the compound is highly accumulated.
CA 03210294 2023- 8- 29
57
[1111n]ln-PSMA-DA1 and [1111n]ln-PSMA-DB showed high binding ability to
LNCaP cells compared to PC-3 cells and the binding was significantly reduced
by adding
an excess amount of the PSMA inhibitor (2-PMPA). These results showed that
[111In]In-
PSMA-DA1 and [111In]ln-PSMA-DB specifically bind to highly PSMA-expressing
cells.
[0164]
<Evaluation of binding to albumin>
A PBS solution (37 kBq, 50 uL) of [111In]In-PSMA-DA1 or [111In]In-PSMA-DB
was added to 200 IA_ of PBS, mouse plasma, human plasma, or a human serum
albumin
(HSA) solution (45 mg/mL) in PBS, respectively, and the mixtures were allowed
to stand
at 37 C for 10 minutes. Thereafter, the reaction liquids were added to a spin
column
(Sephadex G-100; manufactured by Cytiva), and centrifuged at 1500 x g at 4 C
for 2
minutes. After separation, the radioactivity for each of the column and the
eluate was
measured with a gamma counter.
The data were expressed as mean value standard deviation. The significant
difference test was performed using Student's t-test and one-way analysis of
variance
(ANOVA) test with Dunnet's post-hoc test, and p < 0.05 was defined as having a
significant
difference.
[0165]
The results of the evaluation of binding to albumin are shown in Fig. 2. The
higher the value, the higher the binding ability to albumin.
When a compound to be evaluated binds to albumin to form a composite, the
compound passes through the column because of the increased molecular size.
However,
when it does not bind to albumin, it is retained on the gel in the column.
When [nlIn]In-PSMA-DA1 and [1111n]ln-PSMA-DB were left to stand in PBS and
then applied to the column, no significant radioactivity was observed in the
eluate. On
the other hand, when left to stand in mouse plasma, human plasma, and the HSA
solution,
the radioactivity in the eluate of [111In]In-PSMA-DA1 was significantly higher
than that of
[1 11 In]In-PSIVEA-DB, indicating that [1 uir]in-PSMA-DA1 binds to plasma
albumin.
[0166]
<Evaluation of internal radioactivity distribution using LNCaP or PC-3 tumor-
transplanted
CA 03210294 2023- 8- 29
58
mouse>
The animal test was performed in compliance with the guidelines of Kyoto
University Animal Experiment Committee. Male CB17/IcrJcl-Prkdc"'d mice were
purchased from CLEA Japan, Inc. The animals were kept under 12 h/12 h,
day/night cycle
conditions and fed ad libitum with food and water. LNCaP cells (1.0 x 107
cells/mouse)
or PC-3 cells (1.0 x 107 cells/mouse) were suspended in a mixture of PBS and
Matrigel
manufactured by Corning Life Sciences (1 : 1, 150 }..t.L) and the suspension
was
subcutaneously transplanted to the right shoulder of mice under isoflurane
anesthesia.
Thereafter, the mice were kept for 40 to 60 days.
LNCaP or PC-3 tumor-implanted mice were administered saline solutions (185
kBq, 1001_1) of [1111n]ln-PSMA-DA1 or [111In]1n-PSMA-DB from the tail vein (n
= 3 each).
Mice were euthanized 1, 4, 24, 48, 96, and 192 hours after administration.
Thereafter,
blood and each organ were recovered, and the mass and radioactivity of the
organs were
measured.
The percentage (% ID) of the radioactivity amount to the administered
radioactivity amount (injected dose) is shown as the value (% ID/g) divided by
the blood
mass or the organ mass (g). The higher the value of %ID/g, the higher the
abundance of
the radiolabeled compound, which indicates that the compound is highly
accumulated at
the target organ.
[0167]
The results (Mean value + standard deviation, each n = 3) in LNCaP tumor-
transplanted mice and [111In]In-PSMA-DA1 are shown in Table I and Fig. 3
below.
The results (Mean value standard deviation, each n = 3) in LNCaP tumor-
transplanted mice and [11 I Tn]in-PSMA-DB are shown in Table 2 and Fig. 3
below.
[111Ir]In-PSMA-DA1 showed high accumulation at the LNCaP tumor (9.41 to
12.6% ID/g; 1 to 24 hours after administration). In addition, retention in
blood was shown
(14.0% TD/g; 24 hours after administration), and 48 hours after
administration, the
tumor/kidney ratio exceeded 1. On the other hand, [111In]In-PSMA-DB less
accumulated
at the tumor than ["[In]ln-PSMA-DA1 at any time point, showing lower
tumor/kidney ratio.
From these results, it has been clear that [111In]in-PSMA-DA1 highly
accumulates
at a highly PSMA-expressing tumor and exhibits a more excellent internal
distribution than
that of [111in]In-PSMA-DB.
CA 03210294 2023- 8- 29
59
[0168]
[Table 1]
['' In]Tn-PSM A-
1 h 4h 24h 48h 96h 192h
DA1
Blood 31.1 3.99 26.9 9.56 14.0
3.27 4.18 + 0.56 0.76 0.58 0.16 0.03
Spleen 8.56 + 2.90 9.52 + 4.28 6.01
2.33 4.28 0.79 2.42 1.54 2.69 0.83
Pancreas 3.34 0.40 2.48 1.03 2.12
0.49 1.06 + 0.12 0.64 0.24 0.46 0.08
Stomach (% ID) 0.52 0.02 0.51 0.05 0.40 0.09 0.25
0.05 0.08 0.03 0.05 0.01
Intestine 2.63 0.42 3.17 0.73 1.53
0.44 0.96 + 0.25 0.75 0.44 0.12 0.03
Kidney 34.3 + 7.79 63.5 + 13.9 27.1 9.25
9.04 + 2.70 3.51 2.54 1.33 + 0.42
Liver 4.95 0.27 4.20 1.51 2.64 0.38
1.59 0.14 1.10 0.27 0.69 0.12
Heart 7.93 2.10 7.10 1.82 4.26 0.89
1.66 + 0.23 0.75 0.33 0.41 0.08
Lung 16.2 3.86 17.9 6.62 10.5
2.58 4.17 0.26 1.55 0.86 0.75 0.22
Brain 0.56 + 0.06 0.52 + 0.15 0.36 +
0.11 0.16 + 0.01 0.07 + 0.03 0.05 + 0.01
Muscle 2.02 + 0.17 2.15 + 1.09 1.38 +
0.39 0.54 + 0.10 0.23 + 0.09 0.15 + 0.05
LNCaP Tumor 9.41 2.30 12.1 4.04 12.6 2.15
8.82 1.26 5.98 1.87 4.69 1.32
CA 03210294 2023- 8- 29
60
[0169]
[Table 2]
[1111n1Tn-PSM A-
1 h 4 h 24 h 48 h 96 h 192
h
DB
Blood 3.33 + 0.21 2.00 + 0.29 0.68 + 0.17
0.27 + 0.16 0.14 + 0.04 0.07 + 0.03
Spleen 3.05 0.53 2.39 0.05 3.37 0.75
2.63 0.83 .. 2.82 0.87 .. 3.46 0.32
Pancreas 0.74 0.25 0.54 0.22 0.71 0.10
0.71 0.17 .. 0.85 0.39 .. 1.04 0.18
Stomach (% TD) 0.10 + 0.04 0.13 + 0.03 0.16 0.05
0.07 0.02 0.10 + 0.03 0.05 0.00
Intestine 0.59 + 0.14 1.05 + 0.10 1.00 + 0.44
0.79 + 0.21 0.41 0.17 0.13 + 0.02
Kidney 59.2 + 35.2 22.7 + 9.69 13.2 + 2.41
8.58 + 2.02 7.04 + 3.28 3.47 + 1.24
Liver 0.91 0.20 0.95 0.05 1.55 0.32
1.28 0.16 1.59 0.57 1.20 0.09
Heart 0.87 + 0.33 0.60 + 0.08 0.43 + 0.07
0.32 + 0.05 0.40 + 0.12 0.33 + 0.01
Lung 2.24 + 0.60 1.56 + 0.07 0.97 + 0.15
0.66 + 0.24 .. 0.70 + 0.15 .. 0.67 + 0.05
Brain 0.06 0.01 0.05 0.00 0.07 0.02
0.03 0.02 0.09 0.05 0.04 0.00
Muscle 0.23 w 0.68 0.21 0.04 0.21 0.07
0.15+0.06 0.14 w 0.08 0.14+0.09
LNCaP Tumor 3.36 0.04 2.50 0.50 2.09 0.39
1.69 0.77 .. 1.05 0.03 .. 0.83 0.11
[0170]
The results (Mean value standard deviation, each n = 3) in PC-3 tumor-
transplanted mice and [1111n]In-PSMA-DA1 are shown in Table 3 below.
The results (Mean value
standard deviation, each n = 3) in PC-3 tumor-
transplanted mice and [1111n]in-PSMA-DB are shown in Table 4 below.
Both rInfIn-PSMA-DA1 and [ln In]In-PSMA-DB less accumulated at the PC-3
tumor than the result of accumulation at the LNCaP tumor at the same time
point, showing
that both of the radiolabeled compounds selectively accumulate at the PSMA-
positive
tumor.
[0171]
[Table 3]
[111Tn]in-PSMA-DA1 24 h
Blood 5.73 + 0.67
Spleen 2.52 0.12
Pancreas 1.37 + 0.04
Stomach (% ID) 0.27 0.08
Intestine 1.37 + 0.22
Kidney 9.56 1.55
Liver 1.60 0.12
Heart 1.90 0.22
Lung 4.74 0.67
Brain 0.17 0.04
Muscle 0.59 + 0.02
PC-3 Tumor 2.69 0.30
CA 03210294 2023- 8- 29
61
[0172]
[Table 4]
[1111n1Tn-PSM A-DB 111
Blood 1.92 0.27
Spleen 2.57 0.39
Pancreas 0.44 0.06
Stomach (V TD) 0.12 0.04
Intestine 0.48 0.04
Kidney 49.5 12.6
Liver 0.62 0.03
Heart 0.49 0.05
lung 1.36 0.23
Brain 0.06 0.00
Muscle 0.21 0.08
PC-3 Tumor 0.61 0.06
[0173]
<SPECT/CT using LNCaP tumor-transplanted mouse>
LNCaP tumor-implanted mice prepared by the method described above were
administered saline solutions (1.9 to 3.0 MBq, 150 1..t.L) of [iii In]ii-PSMA-
DA1 or
[iii
In]In-PSMA-DB from the tail vein. SPECT/CT was performed 24 and 48 hours after
administration with FX3300 pre-clinical imaging system, which is manufactured
by
Gamma Medica-Ideas. Imaging was performed using a pinhole collimator with a
diameter of 1.0 mm at a rotation radius of 35 mm, a projection time of 70
seconds, and the
number of projections of 32 times under isoflurane anesthesia. After SPECT, CT
(Tube
voltage: 60 kV, Tube current: 350 A) was performed. Image reconstruction was
performed on the projection data of SPECT through three-dimensional ordered
subset
expectation maximization method (8 subsets, 5 iterations).
[0174]
SPECT/CT results are shown in Figure 4. In the figure, the part indicated by
the
arrow is the location of the tumor, and the part indicated by the circle is
the location of the
kidney. The higher the SUV, the higher the radioactivity accumulation.
In SPECT/CT imaging using [111n]Tn-PSMA-DA1, significant radioactivity
accumulation was observed at the LNCaP tumor (the arrow in the figure) 24 and
48 hours
after administration. High radioactivity accumulation was also observed at the
kidney
(the circle in the figure), but the radioactivity signal was almost the same
as at the tumor
CA 03210294 2023- 8- 29
62
48 hours after administration. On the other hand, in SPECT/CT imaging using
[1111n]ln-
PSMA-DB, radioactivity accumulation was also observed at the LNCaP tumor (the
arrow
in the figure), but the accumulation was lower than at the kidney (the circle
in the figure).
From these results, it has been indicated that [1111n]ln-PSMA-DA1 can clearly
draw the highly PSMA-expressing tumor through SPECT and is superior to
[1111n]In-
PSMA-DB.
[0175]
<Evaluation of suppressing tumor growth by "Y-labeling compound>
LNCaP tumor-implanted mice were administered saline solutions (100 uL) of
[90Y]Y-PSMA-DA1 (3.7 MBq) or [90Y]Y-PSMA-DB (3.7 MBq) obtained by the method
described above from the tail vein (n = 7). As the control group, LNCaP tumor-
implanted
mice were administered 100 tt1_, of saline from the tail vein (n = 7).
After administration of the "Y-labeling compound, the tumor volume and body
weight were measured 3 times per week. The tumor volume was calculated based
on the
calculation formula "(tumor volume) = [(long side) x (short side)2/2]".
The tumor volumes on the day when administration of the "Y-labeling compound
was started were 63.1 8.7, 66.1 30.7, and 66.7 25.7 mm3 for the groups
administered
[9 Y]Y-PSMA-DA1, [90Y]Y-PSMA-DB, and saline, respectively.
[0176]
The results of this evaluation are shown in Fig. 5.
When the LNCaP tumor-transplanted mice were administered [9 Y]Y-PSMA-DA1
and [93Y]Y-PSMA-DB, a significant difference in tumor volume was observed 9
days and
33 days after administration, respectively, as compared with the saline-
administered group.
Compared to [90Y]Y-PSMA-DB, the tendency that [90Y]Y-PSMA-DA1 suppresses tumor
growth was observed. The body weight of the mice was slightly
reduced by
administration of [90Y]Y-PSMA-DA1, but then recovered to a value equivalent to
that of
the saline-administered group.
[0177]
<Evaluation of suppressing tumor growth by 225Ac-labeling compound>
[22'Ac]Ac-PSMA-DA1 (20 kBq) or [225Ac]Ac-PSMA-DB (20 kBq) obtained by
CA 03210294 2023- 8- 29
63
the above method was dissolved in 5% ethanol-containing acetate buffer (158
mM, pH 6.5,
100 LL), and the solution was administered to LNCaP tumor-transplanted mice
from the
tail vein (n = 6 or 5). As the control group, 100 1..IL of 5% ethanol-
containing acetate buffer
(158 mM, pH 6.5) was administered to LNCaP tumor-transplanted mice from the
tail vein
(n = 4).
After administration of the 225Ac-labeling compound, the tumor volume and body
weight were measured 2 times per week. The tumor volumes on the day when
administration of 225Ac-labeling compound was started were 75.3 26.0, 80.6
20.8, and
91.1 15.9 mm3 for the groups administered [225Ac]Ac-PSMA-DA1, [225Ac]Ac-PSMA-
DB, and 5% ethanol-containing acetate buffer, respectively.
[0178]
The results of this evaluation are shown in Fig. 6.
When the LNCaP tumor-transplanted mice were administered [225Ac]Ac-PSMA-
DA1 and [225Ac]Ac-PSMA-DB, tumor growth was significantly suppressed as
compared
with the saline-administered group. In particular, in [225
A ]Ac-PSMA-DA 1 -administered
group, the tumor hardly grew, and growth suppression was continuously observed
until 6
weeks after administration.
The body weight reduction likely due to an influence of [225Ac]Ac-PSMA-DA1
administration was transient. The body weight reduction likely due to an
influence of
[225
A ]Ac-PSMA-DB administration was temporarily accelerated, but there was no sip
of
recovery thereafter.
[0179]
[Example 2]
In the Example, synthesized was a compound (PtDA) that has a structure
containing (((S)-5-amino-1-carboxypentyl)carbamoy1)-L-glutamic acid (in the
fotinula
(Cl), "a" is 2, and "b" is 4.), whose target molecule is PSMA, as the PSMA
molecule-
binding part, and utilizes a click reaction between an azide group and
dibenzocyclooctyne
(DBCO) to bond the chelating part and the PSMA molecule-binding part. Next,
radiolabeled compounds were obtained respectively, in which these compounds
were
coordinated with a 1111n ion as a radioactive metal. The outline of the
synthesis route is
shown below as synthesis routes (VIII-1) to (VIII-4).
The compound used in Example 2 has a structure containing the chelating part,
the
CA 03210294 2023- 8- 29
64
PSMA molecule-binding part, and the albumin-binding part. There are two
synthesis
routes (see synthesis route (VIII-4)) to coordinate with a "'In ion, and the
resulting
radiolabeled compounds are the same in either route.
In PtDA, the chelating part and the PSMA molecule-binding part are indirectly
bonded to each other through a chemical structure derived from the click
reaction and a
polyethylene glycol group, and the chelating part and the atomic group
containing the
albumin-binding part are indirectly bonded to each other through a lysine-
derived linker
structure.
[0180]
CA 03210294 2023- 8- 29
65
Scheme (1/111-1)
I
4111Ir
1;02%u
tert-Elutyl trichloroacetimidate
BF=OEt.,
CH,C1,
71
= GO2to
2% 'WA
5% Triisopropylsilane
CH2C12
72
4-01-lodophenyl)butanoic acid 0 gO2iBu
EDC.HCI F
HOAt
EtAl
Anhydrous DMF
111. 73
CO2,112
Fiperidine
DUE HzieWN
74
[0181]
CA 03210294 2023- 8- 29
66
Scheme (VIII-2)
.BNO2C) .13P.r02C
tBuO2C CN,,,i. )
o 74 hauoic (--ri-="")
No 0 Fis. 0 I
N N CONN
--).. Hi) N illi
MN.....C.)1,..N...-1W.N
0
hyDdirePEAus DMF 0
CN......./ 24,,, N
(COAtu
75 <mph,
76
41 vuo2c
)
ADIBO=NH II . NN t u02 0r.......) yo 1-
0 CO241.0 0 ah I
COW
WI
_N.. .4,14
N
OPER
0.,;,,,,,,,,,,,,,H,
Althydrous DMF # ....r.'''
<
COilko 77
IN-HydroxysuccInimIde
COMU WA
76 ______________________________
DIPEA Thioanlsole
Anhydrous MeCN Trilsopropylsliane
41 HO,C
A01130-NH
I I
IliN'")*C. 0 co2n
z 0 i
Anhydrous DMF
(
002N
ADA(78)
0
NH 2 fe=0
0 Heilfs"'¨'=
4**=====,61
0 . '
COOtlu N.,...0)Ø34.............H. )
L_ 0 L-
0 a c00t0u
- 0
Anhydrous DMF
nENOOC'^..1)1 / 1 C00.13u ..,1,..: X
'BuO0C ri 0 COO'Hu
7A
0
NW 8,-......***ot=-==)13
COON
TFA
Triisopropylsilan=
HOOC NAN COOH
H H
PSMA-ligand azkle (7B)
[0182]
CA 03210294 2023- 8- 29
67
Scheme (VIII-3)
7A TFA
Anhydrous DMF Thioanisok
Triisopropylsilane
H20
HOOC ji¨Nk_ss%_
HP1)..." 0
HOOC
HOOC N
I
HN PI:, I N
Usirs,õõkr. 0 õrib.
HOOC)-.'\¨COOH * 0 cõ.NjT00:r
COON
PSMA-trlazole-DOTADG-ALB (PtDA) (7C)
[0183]
Scheme (VIII-4)
<Route A>
PSMA-ligand
[1111n]InC13 azide
ADA (78) _3. rill
lnpn-ADA [111Inpn-PtDA
<Route B>
PSMA-ligand
azide [1111npnC13
PtDA ____
ADA (78) (7c) ) riln]ln-PtDA
[0184]
<Synthesis of compound 71>
N2-[(9H-fluoren-9-ylmethoxy)carbony1]-1\16-[(4-methylphenyl)diphenylmethyl]-
L-lysine (1000 mg, 1.6 mmol) was dissolved in dichloromethane (10 mL), and
then tert-
butyl trichloroacetimidate (699.5 mg, 3.2 mmol) and BF3.0Et2 (25 ilL) were
added thereto.
The reaction solution was stirred at room temperature for 42 hours. After the
solvent was
removed, the residue was washed with H20 (100 mL) and extracted with ethyl
CA 03210294 2023- 8- 29
68
acetate/hexane (1/5, 100 ml. x 2). The organic layer was dried with sodium
sulfide and
filtered. The filtrate was distilled off under reduced pressure, and then the
residue was
purified by medium-pressure column chromatography (ethyl acetate/hexane). The
recovery amount, NMR spectrum, and MS were as follows.
[0185]
Recovery amount: 569 mg (Yield: 52%; calculated from the amount of substance
of Compound 71 obtained relative to the amount of substance of N2-[(9T-T-
fluoren-9-
ylinethoxy)carbonyl]-N6-[(4-methylphenyl)diphenylmethyl]-L-lysine).
tH_
NMR(400MHz,CDC13)67.69(d,J=7.3Hz,2H),7.56(d,J=7.8-
Hz,2H),7.45(d,J=7.8Hz,4H),7.3
3(d,J=8.2Hz,4H),7.23(m,6H),7.13(m,21-
3),7.04(d,J=7.8Hz,2H),5.39(d,J=8.2Hz,1H),4.35(d
,J=6.9Hz,2H),4.25(m,1H),4.18(t,J=6.9Hz,1H),2.26(s,3H),2.12(m,2H),1.77(m,1H),1.5
9(m,
1H),1.49(m,4H),1.44(s,9H).1'C-
NMR(100MHz,CDC13)6171 .6,155.7,146.3 (2C),143.7(2C),143
.2,141.1(2C),135.4,128.4-
128.3 (8C),127.6-
127.5
(6C),126.9(2C),126.0(2C),125.0(2C),119.8(2C),81.7,70.5,66.7,60.2,47.1,43.2,32.7
,3
0.4,27.8(3C),22.8,20.7.
HRMS(EST):m/7681.3677[M+H]+.
[0186]
<Synthesis of compound 72>
Compound 71(569 mg, 0.84 mmol) was dissolved in a mixture of trifluoroacetic
acid (TFA) (1001aL), triisopropylsilane (250 FL), and dichloromethane (4.65
mL), and the
mixture was stirred at room temperature for 6 hours. The solvent was removed,
and then
the residue was purified by medium-pressure column chromatography
(methanol/chloroform). The recovery amount NMR spectrum, and MS were as
follows.
[0187]
CA 03210294 2023- 8- 29
69
Recovery amount: 355 mg (Yield: 100%; calculated from the amount of substance
of compound 72 obtained relative to the amount of substance of compound 71).
NMR(400MHz,CDC13)67.71 (d,J=7 .6Hz,2H),7 .56(d,J=7.1Hz,2H),7 .35(t,J=7.
6Hz,2H),7 .2
7(t,J=7.6Hz,2H),5.72(d,J=8.0Hz,1H),4.33(d,J=7.1Hz,2H),4.16(t,J=6.6Hz,2H),3.38(s
,2H),
1.77-1.56(m,4H),1.43(s,9H),1.27-1.13(m,2H).13C-
NMR(100MHz,CDC13)6171.5,156.2,143.6(2C),141.1(2C),127.6(2C),127.0(2C),125.0(2C
),119.8(2C),82 .3,66.9,54.1,50.0,46.9,39.5,31.1,27.7(3C),26.8,22 .1 .
HRMS(ESI):m/z425.2437[M+H]+.
[0188]
<Synthesis of compound 73>
4-(4-iodophenyl) butanoic acid (364 mg, 1.25 mmol) was dissolved in N,N-
dimethylformamide (DMF) (3 mL), then 1-ethyl-3-(3-dimethylaminopropy1)-
carbodiimide
(FDC) hydrochloride (320 mg, 1.7 mmol) and 1-hydroxy-7-azabenzotriazole (HOAt)
(228
mg, 1.7 mmol) were added, and the mixture was stirred at 0 C for 15 minutes.
Then,
compound 72 (355 mg, 0.84 mmol) and triethylamine (169 mg, 1.7 mmol) were
added, and
the mixture was stirred at 0 C. After 15 hours, the reaction solution was
washed with H20
(100 mi.), and extracted with ethyl acetate/hexane (1/5, 100 mi. x 2). The
organic layer
was dried with sodium sulfide, and filtered. The filtrate was distilled off
under reduced
pressure, and then the residue was purified by medium-pressure column
chromatography
(ethyl acetate/hexane). The recovery amount, NMR spectrum, and MS were as
follows.
[0189]
Recovery amount: 226 mg (Yield: 39%; calculated from the amount of substance
of compound 73 obtained relative to the amount of substance of compound 72).
11T-NMR(400M117,CDC13)67.73(d,J=7.3117,211),7.60-
7.50(m,6H),7.37(0=7.3Hz,2H),7.28(t,J=7.3Hz,2H),6.83(d,J=8.2Hz,2H),4.38-
4.27(rn,2H),4.25-4.16(m,2H),3.21-3.14(m,2H),2.48(m,2H),2.08(0=7.3Hz,2H),1.91 -
1.84(m,2H),1.73-1.57(m,2H),1.47-1.29(m,13H).13C-
CA 03210294 2023- 8- 29
70
NMR(100MHz,CDC13)6172.8,171.5,156.0,143.5(2C),141.0-
140.9(3C),137.1(2C),130.3(2C),127.5(2C),126.9(2C),124.9(2C),119.8(2C),90.8,82.0
,66.8
,53 .9,46.9,38.9,35.4,34 .4,32.1,28.6,27 .9(3C),26.7,22.2.
HRMS(ESI):m/z697.2130[M+H]'.
[0190]
<Synthesis of compound 74>
Piperidine (1 mL) was added to a DMF (4 mL) solution of compound 73. After
stirring at room temperature for 2 hours, the solution was washed with 1420
(100 mL) and
extracted with ethyl acetate/hexane (1/5, 100 mL x 2). The organic layer was
dried with
sodium sulfide and filtered. The filtrate was distilled off under reduced
pressure, and then
the residue was purified by medium-pressure column chromatography
(methanol/chloroform). The recovery amount, NMR spectrum, and MS were as
follows.
[0191]
Recovery amount: 133 mg (Yield: 87%; calculated from the amount of substance
of compound 74 obtained relative to the amount of substance of compound 73).
11 I-NMR(400M11z,CDC13)67 .58(d,J=8.21-17,21 I),6.92(d,J=8.2I Tz,2TI),3 .31 -
3.27(m,1H),3.23(m,2H),2.58(m,2H),2 .13(m,211),1.96-1.88(m,2H),1.74-
1.65(m,2H),1.56-
1.48(m,2H),1.44(s,9H),1.43-1.40(m,2H).13C-
NMR(100MHz,CDC13)o1
75.2,172.3,141.1,137.3(2C),130.5(2C),90.9,80.9,54.7,39.1,35.6,
34.6,34.3,29.2,28.0(3C),26.8,22.9.
HRMS(ESI):m/z475.1453[M+H]'.
[0192]
<Synthesis of compound 75>
As in the examples described above, compound 75 was synthesized in 3 steps
from
1,4,7,10-tetraazacyclododecane (Chem Commun. 2008, 28, 3248-3250).
CA 03210294 2023- 8- 29
71
[0193]
<Synthesis of compound 76>
N-[1-(cyano-2-ethoxy-2-oxoethylideneaminooxy)dirnethylamino (morpholino)]
uronium hexafluorophosphate (COMU) (176 mg, 0.41 mmol) was added to a solution
of
compound 75 (317 mg, 0.41 mmol) in DMF (2 mL), and the mixture was stirred at
0 C for
15 minutes. N,N-diisopropylethylamine (DIPEA) (53 mg, 0.41 mmol) was added to
the
reaction solution, the mixture was further stirred at 0 C for 15 minutes, and
then compound
74 (163 mg, 0.34 mmol) was added thereto. The mixture was stirred at room
temperature
for 12 hours, and then the solution was purified by reverse phase HPLC under
the following
conditions. The recovery amount and MS were as follows.
Purification conditions: Cosmosil 5C18-AR-II column (20 x 250 mm); mobile
phase: MeCN/H20/TFA [30/70/0.1 (0 min) to 90/10/0.1 (40 min)]; flow rate: 5
mL/min.
Recovery amount: 229 mg (Yield: 55%; calculated from the amount of substance
of compound 76 obtained relative to the amount of substance of compound 75).
HRMS(ESI)m/z1229.6182[M+H]t
[0194]
<Synthesis of compound 7'7>
COMU (147 mg, 0.34 mmol) was added to a solution of compound 76 (106 mg,
0.086 mmol) in DMF (0.6 mL), and the mixture was stirred at 0 C for 15
minutes. DIPE A
(89 mg, 0.69 mmol) was added to the reaction solution, and the mixture was
further stirred
at 0 C for 15 minutes, and then dibenzocyclooctyneamine (ADIBO-NH2) (36 mg,
0.22
mmol) was added thereto. The mixture was stirred at room temperature for 12
hours, and
then the solution was purified by reverse phase HPLC under the following
conditions.
The recovery amount and MS were as follows.
Purification conditions: Cosmosil 5C18-AR-II column (20 x 250 mm); mobile
phase: MeCN/H20/TFA [20/80/0.1 (0 min) to 90/10/0.1 (35 min)]; flow rate: 5
mL/min.
Recovery amount: 51 mg (Yield: 40%; calculated from the amount of substance
of compound 77 obtained relative to the amount of substance of compound 76).
HRMS(ESI):m/z1487 .7351 [M+H]t
[0195]
CA 03210294 2023- 8- 29
72
<Synthesis of compound 78 (ADA)>
COW' (70 mg, 0.16 mmol) was added to a solution of compound 76 (50 mg,
0.041 mmol) in MeCN (0.4 mL), and the mixture was stirred at 0 C for 15
minutes.
DIPEA (89 mg, 0.69 mmol) was added to the reaction solution, the mixture was
further
stirred at 0 C for 15 minutes, and then N-hydroxysuccinimide (19 mg, 0.16
mmol) was
added. The reaction solution was stirred at room temperature for 24 hours, and
then the
solution was washed with H20 (100 mL) and extracted with ethyl acetate/hexane
(1/5, 100
mL X 2). The organic layer was dried with sodium sulfide and filtered. The
filtrate was
distilled off under reduced pressure, then half of the residue was dissolved
in a mixture
(95/3/2, 2 mL) of TFA/thioanisole/triisopropylsilane, and the mixture was
stirred at room
temperature for 11 hours. After the solvent was removed, the residue was
dissolved in
DMF (0.4 mL) and triethylamine (10 !IL), and then ADIBO-NH2 (6.2 mg, 0.023
mmol)
was added. The reaction solution was stirred at room temperature for 24 hours,
and then
the solution was purified by reverse phase HPLC under the following
conditions. The
recovery amount and MS were as follows.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 90/10/0.1 (40 min)]; flow rate: 1
mL/min.
Recovery amount: 6.7 mg (Yield: 27%; calculated from the amount of substance
of compound 78 obtained relative to the amount of substance of compound 76).
HRMS(ESI):m/z1207 .4213 [M+H]+.
[0196]
<Synthesis of compound 7A>
(S)-di-tert-butyl
2-(3-((S)-6-amino-1-tert-butoxy-l-ox ohexan-2-yl)ureido)
pentanedioate (31 mg, 0.064 mmol) was dissolved in DMF (0.5 mL), and then 2,5-
dioxopyrrolidin-1-yl 1-azido-3,6,9,12-tetraoxapentadecan-15-oate 1 (25 mg,
0.064 mmol)
was added thereto. After stirring at room temperature for 12 hours, the
solution was
purified by reverse phase HPLC under the following conditions. The recovery
amount,
NMR spectrum, and MS were as follows.
[0197]
Purification conditions: Cosmosil 5C18-AR-TI column (10 x 250 mm); mobile
phase: MeCN/H20/TFA [30/70/0.1 (0 min) to 90/10/0.1 (30 min)]; flow rate: 4
mL/min.
Recovery amount: 35 mg (Yield: 72%; calculated from the amount of substance
CA 03210294 2023- 8- 29
73
of compound 7A obtained relative to the amount of substance of (S)-di-tert-
butyl 2-(3-((S)-
6-amino-l-tert-butox y-1-ox oh ex an-2-yl)urei do) pentanedioate).
1H-NMR(400MHz,CDC13)64 .26-4.23 (m ,2H),3 .75 (t,J=5.2Hz,2H),3 .69-
3.61(m,14H),3 .41(t,J=5.2Hz,2H),3.38-
3.16(m,2H),2 .59(0=5 .2Hz,2H),2.34(dt,J=2 .3,7.5Hz,2H),2.11-
1.60(m,4H),1.54(t,J=7.0Hz,2H),1.48-1.40(m,27H),1.39-1.26(m,2H).13C-
NMR(100MHz,CDC13)6174.3(2C),172.8(2C),158.4,82.7,82.4,81.2,70.4(2C),70.3,70.1,7
0.
0(2C),69.9,66.8,53.7,
53.3,50.5,39.2,35.8,31.5,31.3,28.2,27.9(3C),27.8(7C),21.9.
HRMS(ESI):m/z761.4656[M+H]+.
[0198]
<Synthesis of compound 7B>
Compound 7A (23 mg, 0.030 mmol) was dissolved in TFA (950 i_it) and
triisopropylsilane (50 1,1), and the mixture was stirred at room temperature
for 4 hours.
After the solvent was removed, the residue was purified by reverse phase HPLC
under the
following conditions. The recovery amount, NMR spectrum, and MS were as
follows.
Purification conditions: Cosmosil 5C18-AR-TT column (10 x 250 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 40/60/0.1 (30 min)]; flow rate: 4
mL/min.
Recovery amount: 11 mg (Yield: 63%; calculated from the amount of substance
of compound 7B obtained relative to the amount of substance of compound 7A).
1H
NMR(400MHz,CDC13)67 .52(s,1H),6.37(s,2H),4 .39(s,1H),4.31(s,1H),3 .66(m,16H),3
.40(s,
211),2.53-1.23(m,1411).
HR M S(ESI): 593 .2779[M+Hr .
[0199]
<Synthesis of compound 7C (PtDA)>
Compound 77 (20 mg, 0.013 mmol) was dissolved in DMF (0.6 mL), and then
compound 7A (35 mg, 0.046 mmol) was added thereto. The mixture was stirred at
room
CA 03210294 2023- 8- 29
74
temperature for 12 hours, and then the solvent was removed. TFA (1.9 mL),
thioanisole
(60 pt), triisopropylsilane (20 pL), and H20 (20 pL) were added to the
residue, and the
mixture was stirred at room temperature for 10 hours. After the solvent was
removed, the
residue was purified by reverse phase HPLC under the following conditions. The
recovery amount and MS were as follows.
Purification conditions: Cosmosil 5C18-AR-11 column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 mm) to 90/10/0.1 (40 min)]; flow rate: 1
mL/min.
Recovery amount: 1.0 mg (Yield: 4.2%; calculated from the amount of substance
of compound 7C(PtDA) obtained relative to the amount of substance of compound
77).
HRMS(ESI):m/z900.3488[M+2H]2'.
[0200]
<Synthesis of [1" In]ln-ADA through route A>
A solution (9.2 MBq, 100 L) of [111In]InC13 and a solution of compound 78 in
dimethyl sulfoxide (DMSO) (0.60 mM, 7 L) were added to a 2-(N-
morpholino)ethanesulfonic acid (MES) buffer (0.1 M, pH 5.7, 100 A), and the
mixture
was allowed to stand at 90 C for 5 minutes. Thereafter, the reaction solution
was purified
by reverse phase HPLC under the following conditions.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 70/30/0.1(30 min)]; flow rate: 1
mL/min.
The radiochemical yield and radiochemical purity were measured in the same
manner as described in Example 1-1. The HPLC conditions used to measure the
radiochemical purity was the same as the purification conditions.
The radiochemical conversion rate (the ratio of the radioactivity of [111
In]In-ADA
to the radioactivity of [111In]InC13) and the radiochemical yield are shown in
Table 5 below.
[0201]
<Synthesis of [1" Tn]In-PtDA through route A>
To a phosphate buffer saline (PBS)/DMS0 mixture (9/1, 200 pL) or DMSO (200
[LL), [iiiIn]In-ADA (0.80 MBq) was added, and compound 7B (0.2 mg) was further
added.
After standing at 37 C for 10 or 30 minutes, the reaction solution was
purified by reverse
phase HPLC under the following conditions.
CA 03210294 2023- 8- 29
75
Purification conditions: Cosmosil 5C18-AR-I1 column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 70/30/0.1 (30 mint flow rate: 1
mL/min.
The radiochemical yield and radiochemical purity were measured in the same
manner as described in Example 1-1. The HPLC conditions used to measure the
radiochemical purity was the same as the purification conditions.
The radiochemical conversion rate (the ratio of the radioactivity of [111In]In-
PtDA
to the radioactivity of [111 In]In-ADA) and the radiochemical yield are shown
in Table 5
below.
[0202]
<Synthesis of [111In]In-PtDA through route B>
A solution (2.1 MI3q, 100 uL) of [1111n]InC13 and a solution of compound 7C in
DMSO (0.56 mM, 2 uL) were added to a MES buffer (0.1 M, pH 5.7, 150 pt), and
the
mixture was allowed to stand at 90 C for 5 minutes. Thereafter, the reaction
solution was
purified by reverse phase HPLC under the following conditions.
Purification conditions: Cosmosil 5C18-AR-I1 column (4.6 x 150 mm); mobile
phase: MeCN/I-120/TFA [10/90/0.1 (0 min) to 70/30/0.1 (30 mint flow rate: 1
mL/min.
The radiochemical yield and radiochemical conversion rate were measured in the
same manner as described in Example 1-1. The HPLC conditions used to measure
the
radiochemical conversion rate was the same as the purification conditions.
The radiochemical purity (the ratio of the radioactivity of [111In]In-PtDA to
the
radioactivity of [1111n]InC13) and the radiochemical yield are shown in Table
5 below.
[0203]
[Table 5]
Radiochemical
Reaction
Radiochemical
Compound Solvent conversion rate
conditions (%) yield
(%)
[111 k]ln- AD A MES buffer 90 C, 5 min > 95 54.9
9.3
Route A PBS 37 C, 10 min >95 55.3
1.6
[ I I TrifIn-P tD A
DMSO 37 C, 30 min > 95 66.7
7.2
Route B [111In]In-PtDA MES buffer 90 C, 5 min
> 95 47.4 14.7
[0204]
CA 03210294 2023- 8- 29
76
In addition, In-ADA and In-PtDA in which non-radioactive In is coordinated can
be produced by the following method. The obtained In complex was used to
identify the
HPLC retention time of the radiolabeled compound.
<Synthesis of non-radioactive In-ADA>
Compound 78 (1 eq) was dissolved in acetate buffer (1.0 M, pH 5.0, 100 L),
and
indium (III) chloride anhydrous (10 eq) was added. The reaction solution was
stirred at
90 C for 5 minutes, and then the solution was purified by reverse phase HPLC.
Purification conditions: Cosmosil 5C18-AR-H column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/30.1 (0 mm) to 90/10/0.1 (40 mm)]; flow rate: 1
mL/min.
MS(ESI):m/z1319.3[M+H]+.
[0205]
<Synthesis of non-radioactive In-PtDA>
A solution of compound 7C (0.5 mg, 1.25 mmol) in H20/MeCN/TFA
(49.95/49.95/0.1, 300 1.1L) was added with indium (III) chloride anhydrous
(0.62 mg, 2.8
1..ano1). After stirring at room temperature for 18 hours, the solution was
purified by
reverse phase HPLC.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 90/10/0.1(40 mint flow rate: 1
mi,/min.
Recovery amount: 0.05 mg (Yield: 9.4%; calculated from the amount of substance
of non-radioactive In-PtDA obtained relative to the amount of substance of
compound 7C).
HRMS(ESI):m/z956.2893[M+2H]2.
[0206]
<Evaluation of distribution coefficient>
[111In]In-PtDA (111 kBq) was added to a mixture of PBS (pH 7.4, 3 mL) and 1-
octanol (3 mL), and the mixture was dispersed by vortexing for 2 minutes and
then
centrifuged at 4000 x g for 5 minutes. 1 mL was recovered respectively from
the 1-
octanol layer and the PBS layer, and the radioactivity of each layer was
measured with a
gamma counter (n = 3).
CA 03210294 2023- 8- 29
77
As the calculation formula, "(Distribution coefficient) = Logio[(radioactivity
of 1-
octanol layer [kBq])/(radioactivity of PBS layer [kBq])]" was used.
As a result, LogP of [111In]ln-PtDA was "-3.08 0.02".
[0207]
<Evaluation of stability in plasma>
A saline (204) solution of [111In]In-PtDA (259 kBq) was added to mouse plasma
(200 4), and the mixture was allowed to stand at 37 C for 24 hours (n = 3).
MeCN (400
1AL) was added thereto, and the mixture was centrifuged at 10,000 x g for 5
minutes. The
supernatant was filtered, and the filtrate was analyzed by reverse phase HPLC
under the
following conditions.
Analytical conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/1170/TFA [10/90/0.1 (0 mm) to 70/30/0.1 (30 min)]; flow rate: 1
mL/min.
As a result, as shown in Fig. 7, 95% or more of [111In]Tn-PtDA was stably
present
in mouse plasma even after standing at 37 C for 24 hours.
[0208]
<Evaluation of binding using cultured cell line>
In the same manner as in Example 1-1 described above, LNCaP cells and PC-3
cells were cultured and cell-binding assay were performed. Evaluation was
carried out
by the same experimental procedure and statistical method as in Example 1-1,
except that
0.5% FBS-containing RPMI 1640 medium (1 mL) containing [111In]ln-PtDA (37 kBq)
was
used.
[0209]
The results are shown in Fig. 8.
[111In]In-PtDA showed high binding ability to LNCaP cells (15% ID/mg protein)
compared to PC-3 cells (0.15% ID/mg protein) and the bond was significantly
reduced
(0.36% TD/mg protein) by adding an excess amount of the PSMA inhibitor (2-
PMPA).
These results showed that [111In]ln-PtDA specifically binds to PSMA-positive
cells.
[0210]
<Evaluation of binding to albumin>
CA 03210294 2023- 8- 29
78
As in Example 1-1 described above, PBS, mouse plasma, human plasma, and
human albumin were used to evaluate binding to albumin. Evaluation was carried
out by
the same experimental procedure and statistical method as in Example 1-1,
except that a
PBS solution (37kBq,501iL) of [mIn]In-PtDA was used.
[0211]
The results are shown in Fig. 9.
When [111In]In-PtDA was allowed to stand in PBS and then applied to the
column,
little radioactivity was detected in the eluate (6.9%). On the other hand,
when allowed to
stand in mouse plasma, human plasma, and a human albumin solution, high
radioactivity
was observed in the eluate (67.2, 79.0, and 92.4% respectively). Therefore it
was shown
that [1nIn]In-PtDA binds to plasma albumin.
[0212]
<Evaluation of internal radioactivity distribution using LNCaP tumor-
transplanted mouse>
LNCaP tumor-implanted mice prepared by the same method as in Example 1-1
were administered a saline solution (241 kBq/100 !IL) of [111In]In-PtDA from
the tail vein
(n = 3). Mice were euthanized 1, 24, and 48 hours after administration.
Thereafter,
blood and each organ were recovered, and the mass and radioactivity of the
organs were
measured.
The percentage (% TD) of the radioactivity amount to the administered
radioactivity amount (injected dose) is shown as the value (% ID/g) divided by
the blood
mass or the organ mass (g). The higher the value of %ID/g, the higher the
abundance of
the radiolabeled compound, which indicates that the compound is highly
accumulated at
the target organ.
[0213]
The results (Mean value standard deviation, each n = 3) in LNCaP tumor-
transplanted mice and [111T-n]Tn-PtDA are shown in Table 6 below.
[111In]In-PtDA highly accumulated at the LNCaP tumor (16.0 and 18.7% ID/g; 24
and 48 hours after administration, respectively) and showed retention in blood
(6.33 to
19.8% ID/g; 1 to 48 hours after administration). One hour after
administration,
accumulation at the kidney was 37.2% ID/g, which was significantly lower than
[1111n]In-
CA 03210294 2023- 8- 29
79
PSMA-1&T (kidney: 191% 1D/g) and [68Ga]Ga-PSMA-11 (kidney: 139% 1D/g). each of
which was a known radiolabeled compound whose target molecule was PSMA (for
example, EJNMMI Res, 2012, 2, 23, J Nucl Med, 2017, 58,235-242).
[0214]
[Table 6]
[1 "Inpri-PLIDA 1 h 24 h 48 h
Blood 19.8 1 2.74 12.5 1.47 6.33
= 1.53
Spleen 15.3 4.89 9.69 1 0.92 10.5
= 5.62
Pancreas 2.31 1 0.14 2.03 0.48 1.12
= 0.39
Stomach (% ID) 0.791 0.19 0.46 1 0.23 0.29
= 0.20
Intestine 2.66 + 0.28 1.52 1 0.41 0.74
= 0.28
Kidney 37.2 1 6.85 68.0 1 2.82 55.9
= 6.39
Liver 3.51 + 0.38 3.36 + 0.77 2.28
= 0.75
Heart 5.49 + 0.77 3.95 1 0.87 1.98
= 0.41
Lung 11.9+ 1.57 8.63+ 1.31 6.16=
1.56
Brain 0.36 0.05 0.30 0.05 0.20
= 0.07
Muscle 1.95 + 0.30 1.28 0.34 0.81
= 0.40
LNCaP Tumor 2.18 + 0.17 16.0 + 9.49 18.7
= 5.21
[0215]
<Evaluation of SPECT/CT using tumor-transplanted mouse>
LNCaP cells (1.0 x 107 cells/mouse) were suspended in a mixture of PBS and
Matrigel (1 : 1, 150 !AL) and subcutaneously transplanted to the right
shoulder of a male
CB17/IcrJcl-Prkdcs" mouse kept in the same manner as in Example 1-1 under
isoflurane
anesthesia. In addition, a suspension of PC-3 cells (1.0 x 107 cells/mouse) in
a mixture of
PBS and Matrigel (1 : 1, 150 L) was subcutaneously transplanted into the left
shoulder of
the same mouse under isoflurane anesthesia. Thereafter, the mouse was kept for
40 to 60
days. in this way, a tumor-transplanted mouse in which LNCaP cells and PC-3
cells were
simultaneously transplanted into one mouse was obtained.
[0216]
The tumor-transplanted mouse was then administered saline solution (2.7 MBq,
100 !AL) of [1111n]in-PtDA from the tail vein. SPECT/CT was performed 24 and
48 hours
after administration with FX3300 pre-clinical imaging system, which is
manufactured by
Gamma Medica-Ideas. Imaging was performed using a pinhole collimator with a
diameter of 1.0 mm at a rotation radius of 35 mm, a projection time of 70
seconds, and the
CA 03210294 2023- 8- 29
80
number of projections of 32 times under isoflurane anesthesia. After SPECT, CT
(Tube
voltage: 60 kV, Tube current: 350 liA) was performed. Image reconstruction was
performed on the projection data of SPECT through three-dimensional ordered
subset
expectation maximization method (8 subsets, 5 iterations).
[0217]
SPECT/CT results are shown in Figure 10. The higher the SUV, the higher the
radioactivity accumulation.
In SPECT/CT imaging using [in Tr]in-PtDA, high radioactivity accumulation was
observed at the LNCaP tumor 24 and 48 hours after administration, but almost
no
radioactive signal was observed in the PC-3 tumor. This result showed that
[111In]In-PtDA
was able to clearly draw the PSMA-positive tumor.
[0218]
[Examples 3-1 to 3-3 and Comparative Examples 2-1 to 2-2]
In this example, compounds whose target molecule was PSMA were synthesized.
Next, radiolabeled compounds were obtained in which each of the compounds were
coordinated with a 1111n ion as a radioactive metal. The outline of the
synthesis routes is
shown below as synthesis routes (X-1) to (X-3).
The compound (Octapa-2, Octapa-3 and Neunpa-2) used in Examples 3-1 to 3-3
have a chemical structure in which the chelating part, the PSMA molecule-
binding part,
and the albumin-binding part are bonded with each other in a branched manner
through the
linker structure as shown in the general formula (2).
The compounds (Octapa-1 and Neunpa-1) used in Comparative Examples 2-1 and
2-2 had a structure containing the chelating part and the PSMA molecule-
binding part, and
not containing the albumin-binding part.
[0219]
CA 03210294 2023- 8- 29
81
Scheme (X-1)
o 1. LiAIH4
dry IF-IFNH2
NH2 _________________________________ ).-- NH2 ¨0.- ¨).-
101 NH2 2. Nitration mixture 02N
H2SO4 101
NO2 NH2
0 e ________________________________________ 0 0,e,0
, i , , Fir4
. c
¨)- ) 0 N N-0_) _),... ) OiN N¨\ 0 (
Me0H
( iN
0 0 _________________________________________________________________
4¨
,7-
0 7c 0 0 7c o
102 103
NO2 NO2
NO2
01 J-,Br 101 0 SH
01
tBuO
.-- Ns NS ¨).-
\ /--\ IV ¨N /--\
Na2CO3
K2CO3 (k¨ NH N HN-0
\/ _____________________ H
Ns-NH
N-Ns MeCN 0 N N¨=\
o THF
0 ( ) 0 )
0
104 105 o( 106
NO2 NH2
1.1 le
Brj-NOtBu 0 0 0 0
H2
____________________________________ 3.- ) 0 cN N N¨µ 0 (
11,....d/C /10 iN N N-0_\)(
Na2CO3 Me0H
N1 \
DMF N N1 N
/7-0 04 /-ICIL '04
0 0 0 o
107 108
[0220]
CA 03210294 2023- 8- 29
,-,
.
u.,
iv
i--i
o
i.,
LID
e.
iv
o
iv
0'
iv
,o
Scheme (X-2)
o 0
H 0 H
H
.Ernoc Enloe H IR,.N.1õ--....,--,.,NTO
N' Fi l¨\--i
X.'
N
FiN FN
zO
103 Orin 1. Piperidine
-, H
_.... DME
EDC HCI 2. Succinic anhydride
0 HOAt DIPEA
i
r
j 0
Urea motif
TIPS
for 113: HIBTU
DIPEA
___________________________________________________ r 0
õ...
0 0
......õ0 H20 0 NH
01õOH
Et,N 0 THE O DCM
11%. 0
0
OF NH NH
DCM ,...---.1
R,f, Rtn for 114: EDC HCI 1 N-11.. N N
.,....,. .11, L.r0
--i N
HOAt
109 I'll 111 or 112 Et,N 1
0 H I-1 0.
0H H H 0H1
0CM
115: Octapa-1
113 or 114
116: Neunpa-1
,rna
Hiv ma , OH
R
mit o 0 H
NW.
EDC HCI 1. Piperidine
DME FIN
r- 0
2. 4-(4-iodoph i
enyl)butyric acid s t 1% TFA HN
Urea motif H HN 0
2. Succinic anhydrde
=
0 _....
103 or 108
EDC HCI
si¨' NH HOAt S
õ3¨NH EDC HCI 2¨NH DIPEA
HOAt 0 NH
0
Ot =Emoc Et3N i0,, `prim HOAt o im THF ." /¨ NH
Et2N
1101
OH DCM NH Et3N NH W 0
DCM
DCM IR!i NI-1
I LI'l 0
I Fq'i
NAN 0
117 or 118 119 or120 1
121 or 122
,_.0 H H
-1-
T.--
o oo
123 or 124
H HN = Rxi substituent Rx2 substituent
Urea motif
TFA
'
0 NH
TIPS
H20 c:,...:
SO
+
NH2
Ot_ _1/0
Oy.'-.NAN 9,JEE:43 ,-1 ,--
..,,¨,, /¨
OH H H OH :¨)-0 N N /0*).14"¨NNI¨Y
HO N N /OH HO N 11...¨\N ,OH 0111 0
125: Octapa-2
126: Neunpa-2 \ .,,N
N
\ N N 1.=-=".µN N
LfD
_,,_..0 H H 0..õ
_
_
0 0 NO OH HO
H HO 1 r-
0 7( 0 0 0
0 0 0
109, 111, 113, 117, 11, 112, 114, 118,
115, 125 116, 126
119, 121, 123 125, 122, 124
.................................................................. .
...................... . .........
n
>
o
u,
n,
,
o
,..,
LID
.1,
n,
o
n,
L."
93
n,
Lc,
C
IN.)
Scheme (X-3)
IV
1.--,
0
=
,Mtt Mft
HI's NW. HI)
) _____________________________________________________________________ 1.-
HI)
TEITA 1. Piperidine
BF, E120 1. 5% TFA 41' DMF
) *
DCM s 2. 4-(4-iodophenyl)butyric acid
=-14t1 EDC HCI
04-111 ---',
0=%. 'Fmoc 0 Fmoc HOAt Fmoc HOLTh--**--aOH
0, ,¨NH
OH Et3N EDC 1-ICI
DCM 41 HOAt
Et3N
127 128 DMF 129 M.
õp-OH
0
,Mtt ...,
HN cl=I C)Th< 0
....1,1 I 01-0,1
1. Piperidine
DMF
2. 128 >I.:I< N 0 H 1.
1% TFA . N
2. Succinic anhydride >CI( N
IP' 0
H 0
.'.¨NH EDC HCI
0 'Fmoc HOAt
010õ1.< H HN,C DIP EA
THE 0
cr),rtlyis celtri< 111-)LIHNr}L H
H Et3N
RA DGM
117
Ll
LI
00
`-' H H
µ.'10 H H CAD
130 Cr)b- tu 0
* I Oj'0 0 11101 1
131
+
..--
0 ...--- 1 0 ,..N
I CITOH
N T)< -I-
a,....... N OH
0
0 .õ,......õ.õ......r
H Urea motif
,._
0 N WILI"'"'","'`,"N"ir,ANCt:IH TEA õ OH
N.,1 1111)-111 N'Ay''''.."-AY'"-Alif NH
HBTU TIPS ,....f.0
DIP EA 0
DCM ...,õ Icsyl, clerk H HNT: 0 H
0. NH I-100
-.)-INYO.").'0H H HN 0 H 0NH
LO
I
HOH,cyrY,,
\
OyJ
OH
0...,
>r
L H H
O H H
L. NM
Le.,......-,.............N
0'17'0 0 lill I
0A-OH 0 10 I
132
133: Octapa-3
84
[0222]
<Synthesis of compound 101>
L-phenylalanine amide (985 mg, 6.0 mmol) was dissolved in tetrahydrofuran
(THF) (20 mL), and a THF (10 mL) solution of LiA1H4 (1139 mg, 30 mmol) was
slowly
added at 0 C. The reaction solution was stirred at 60 C for 24 hours, and then
H20 (1.2
mL), a 15% aqueous sodium hydroxide solution (1.2 mL), and H20 (3.6 mL) were
added
in this order. The reaction solution was stirred at room temperature for 1
hour, and then
filtered through celite, and the filtrate was distilled off under reduced
pressure.
Concentrated sulfuric acid (5 mL) was added to the residue, and then a mixture
of
concentrated nitric acid (400 tit) and concentrated sulfuric acid (400 L) was
added. The
mixture was neutralized after being stirred at room temperature for 5 hours,
and extracted
with chloroform (50 mL x 3). The organic layer was dried with sodium sulfide,
and
filtered. The filtrate was distilled off under reduced pressure and the
residue was purified
by medium-pressure column chromatography (methanol/chloroform).
Recovery amount: 830 mg (Yield: 71%; calculated from the amount of substance
of compound 101 obtained relative to the amount of substance of L-
phenylalanine amide).
MS(ESI)rniz:196.1 [M+H] .
[0223]
<Synthesis of compound 102>
Compound 102 was synthesized in 3 steps from compound 101 according to a
previously reported method (J Am Chem Soc. 2013,135, 12707-12721, J Chem Soc
Dalt
Trans. 2014, 43, 7176-7190).
[0224]
<Synthesis of compound 103>
Compound 102 (288 mg, 0.36 mmol) was dissolved in methanol (5 mL), and
palladium carbon (30 mg) was added. The reaction solution was stirred under
hydrogen
atmosphere for 3 hours, and then filtered through celite, and the filtrate was
distilled off
under reduced pressure. The residue was purified by medium-
pressure column
chromatography (methanol/chloroform).
Recovery amount: 150 mg (Yield: 54%; calculated from the amount of substance
CA 03210294 2023- 8- 29
85
of compound 103 obtained relative to the amount of substance of compound 102).
1H-NMR(400MHz,CDC13)67.82(d,J=6.9Hz,2H),7.70-
7.60(m ,4H),6.86(d,J=7 .6Hz,2H),6.54(d,J=7.6Hz,2H),4.07(m,5H),3.40-3.23
(m,5H),2.91-2.89(m,2H),2 .73-2.69(m,1H),2.53-2
.50(m,2H),1.62(s,18H),1.40(s,18H).
MS(EST)m/z: 806.4 [M+H]+.
[0225]
<Synthesis of compound 104>
Compound 104 was synthesized according to a previously reported method
(Bioconjug. Chem. 2017, 28, 2145-2159).
[0226]
<Synthesis of compound 105>
To a solution of compound 104 (729 mg, 1.17 mmol) in MeCN (40 mL), t-
butylbromoacetic acid (396 uL, 2.69 mmol) and sodium carbonate (285 mg, 2.69
mmol)
were added, and the mixture was stirred at 60 C for 24 hours. After the
temperature was
returned to room temperature, the reaction solution was filtered, and the
filtrate was
distilled off under reduced pressure. The residue was purified by medium-
pressure
column chromatography (hexane/ethyl acetate).
Recovery amount: 566 mg (Yield: 57%; calculated from the amount of substance
of compound 105 obtained relative to the amount of substance of compound 104).
1H-NMR(400MHz,CDC13)68.14(d,J=8.7Hz,2H),8 .05-8.02(m,214),7 .71 -
7.67(m,4H),7.62-7 .58(m,2H),7.37(d,J=8.7Hz,2H),4.08(s,4H),3.41
(t,J=7.0Hz,4H),2.86-
2.77(m,8H),1 .34(s,1 8H).
MS(EST)m/z: 851.2 [M+11]
[0227]
CA 03210294 2023- 8- 29
86
<Synthesis of compound 106>
Thiophenol (253 [IL, 1.53 mmol) and potassium carbonate (212 mg, 1.53 mmol)
were added to a solution of compound 105 (566 mg, 0.67 mmol) in THF (20 mL),
and the
mixture was stirred at 50 C for 50 hours. After the temperature was returned
to room
temperature, the reaction solution was filtered, and the filtrate was
distilled off under
reduced pressure. The residue was purified by medium-pressure column
chromatography
(methanol/chloroform).
Recovery amount: 213 mg (Yield: 66%; calculated from the amount of substance
of compound 106 obtained relative to the amount of substance of compound 105).
1H-NMR(400MHz,CDC13)68.14(d,J=8.2Hz,2H),7.42-
7.37(m,2H),3 .26(s,4H),2 .91-2 .65(m,12H),1.47(s,18H).
MS(ESI)m/z:481 .3 [M+H]+.
[0228]
<Synthesis of compound 107>
To a solution of compound 106 (213 mg, 0.44 mmol) in N,N-dimethylformamide
(DMF) (15 mL), t-butyl-6-(bromomethyl) picolinate (264 mg, 0.97 mmol) and
sodium
carbonate (103 mg, 0.97 mmol) were added, and the mixture was stirred at 60 C
for 24
hours. After the temperature was returned to room temperature, the reaction
solution was
filtered, and the filtrate was distilled off under reduced pressure. The
residue was purified
by medium-pressure column chromatography (methanol/chloroform).
Recovery amount: 224 mg (Yield: 60%; calculated from the amount of substance
of compound 107 obtained relative to the amount of substance of compound 106).
1H-NMR(400MHz,CDC13)68 .06(d,J=8.5Hz,2H),7 .88-7.86(m,2H),7.75-
7.73(m,4H),7 .23 (d,J=8.7117,2H),3.99(s,4H),3.30(s,4H),2.74-
2.63(m,12H),1.62(s,18H),1.45(s,1811).
MS(ESI)m/z: 863.5 [M+H]+.
[0229]
CA 03210294 2023- 8- 29
87
<Synthesis of compound 108>
A similar reaction to the synthesis of the compound 103 was performed to
obtain
50.7 mg (Yield: 23%; calculated from the amount of substance of compound 108
obtained
relative to the amount of substance of compound 107) of compound 108 from
compound
107.
1H-NMR(400MHz,CDC13).37 .86(d,J=7 .1Hz,2H),7 .78-
7.72(m,4H),6.85(d,J=8.2,2H),6.56(d,J=8 .2,2H),4.00(s,4H),3 .32(s,4H),2 .76-
2.55(m,12H),1.62(s,18H),1.44(s,18H).MS(ESI)m/z: 833 .4[M+H]+.
[0230]
<Synthesis of compound 109>
Compound 103 (56.6 mg, 0.073 mmol), 1-ethy1-3-(3-dimethylaminopropy1)-
carbodiimide (EDC) hydrochloride (28.8 mg, 0.15 mmol), 1-hydroxy-7-
azabenzotriazole
(HO.At) (20.4 mg, 0.15 mmol), and triethylamine (21 11L, 0.15 mmol) were added
to a
dichloromethane (7 mL) solution of 6-(Fmoc-amino) hexanoic acid (31.1 mg,
0.088 mmol),
and the mixture was stirred at room temperature for 3 hours. The solvent was
distilled off
under reduced pressure, and then the residue was purified by medium-pressure
column
chromatography (methanol/chloroform).
Recovery amount: 30.2 mg (Yield: 37%; calculated from the amount of substance
of compound 109 obtained relative to the amount of substance of compound 103).
HRMS(ESI)m/z:1111.6170[M+11] .
[0231]
<Synthesis of compound 110>
A similar reaction to the synthesis of compound 109 was performed to obtain
17.7
mg (Yield: 25%; calculated from the amount of substance of compound 110
obtained
relative to the amount of substance of compound 108) of compound 110 from
compound
108.
HRMS(ESI)m/z:1168.6372[M+H]t
CA 03210294 2023- 8- 29
88
[0232]
<Synthesis of compound 111>
Compound 109 (30.2 mg, 0.027 mmol) was dissolved in a mixture of piperidine
(1 mL) and DMF (4 mL), and the mixture was stirred at room temperature for 2.5
hours.
Ethyl acetate and H20 were added to the reaction liquid, and then the organic
layer was
dried with sodium sulfide and filtered. The filtrate was distilled off under
reduced
pressure, and then THF (5 mL) was added to the residue. Succinic anhydride (11
mg, 0.11
mmol) and N,N-diisopropylethylamine (DIPEA) (19 !IL, 0.11 mmol) were added,
and the
mixture was stirred at room temperature for 3 hours. The solvent was distilled
off under
reduced pressure, and then the residue was purified by medium-pressure column
chromatography (methanol/chloroform).
Recovery amount: 9.7 mg (Yield: 37%; calculated from the amount of substance
of compound 111 obtained relative to the amount of substance of compound 109).
HRMS(ESI)m/z:989.5600[M+H]+.
[0233]
<Synthesis of compound 112>
A similar reaction to the synthesis of compound 111 was performed to obtain
17.7
mg (yield: 95%) of compound 112 from compound 110.
1-1121V1 S(EST)m/z: 1046 .6139 [1\4-4-T]t
[0234]
<Synthesis of compound 113>
To a solution of compound 111(6.2 mg, 6.3 [imol) in dichloromethane (5 mL),
(S)-di-tert-butyl 2-(3-((S)-6-amino-1-tert-butoxy-1-oxohexan-2-yl)ureido)
pentanedioate
(6.2 mg, 13 itmol), 1 -[bi s(dim ethylamino)m ethyl en e]-1H-benzotriazolium 3-
oxide
hexafluorophosphate (HBTU) (5.0 mg, 13 iamol), and DTPEA (10 1AL, 0.57 iAmol)
were
added, and the mixture was stirred at room temperature for 26 hours. The
solvent was
distilled off under reduced pressure, and the residue was purified by medium-
pressure
column chromatography (methanol/chloroform).
Recovery amount: 4.5 mg (Yield: 49%; calculated from the amount of substance
CA 03210294 2023- 8- 29
89
of compound 113 obtained relative to the amount of substance of compound 111).
HRMS(ESI)m/z:729.9404[M+2H]t
[0235]
<Synthesis of compound 114>
A solution of compound 112 (19.3 mg, 0.018 mmol) in dichloromethane (5 mL)
was added with (S)-di-tert-butyl 2-(3-((S)-6-amino-1-tert-butoxy-l-oxohexan-2-
yl)ureido)
pentanedioate (17.6 mg, 0.036 mmol), EDC hydrochloride (6.9 mg, 0.036 mmol),
HOAt
(4.9 mg, 0.036 mmol), and triethylamine (5 1.1L, 0.036 mmol). The reaction
solution was
stirred at room temperature for 6 hours, and the solvent was distilled off
under reduced
pressure. The residue was purified by medium-pressure column chromatography
(methanol/chloroforni).
Recovery amount: 15.6 mg (Yield: 57%; calculated from the amount of substance
of compound 114 obtained relative to the amount of substance of compound 112).
HRMS(ESI)m/z :758 .4704[M+2H]t
[0236]
<Synthesis of compound 115 (Octapa-1)>
Compound 113 (11.4 mg, 7.8 litmol) was dissolved in a mixture of
trifluoroacetic
acid (TFA)/H20/triisopropylsilane (95/2.5/2.5, 2 mL), and the solution was
stirred at room
temperature for 3 hours. After the solvent was removed, the residue was
purified by
reverse phase HPLC under the following conditions.
Purification conditions: Cosmosil 5C18-AR-H column (4.6 x 150 mm); mobile
phase: MeCN/1-120/TFA [10/90/0.1 (0 min) to 30/70/0.1 (30 mint flow rate: 1
mL/min.
Recovery amount: 2.2 mg (Yield: 27%; calculated from the amount of substance
of compound 115 obtained relative to the amount of substance of compound 113).
1-IRMS(EST)m/7: 1066.4325 [M-44] .
[0237]
<Synthesis of compound 116 (Neunpa-1)>
CA 03210294 2023- 8- 29
90
A similar reaction to the synthesis of compound 115 was performed to obtain
1.8
mg (Yield: 43%) of compound 116 from compound 114.
HRMS(EST)m/z : 1123 .4924 [M+H]t
[0238]
<Synthesis of compound 117>
Compound 103 (42.3 mg, 0.055 mmol), EDC hydrochloride (21.1 mg, 0.11 mmol),
HOAt (15.0 mg, 0.11 mmol), and triethylamine (15 [IL, 0.11 mmol) were added to
a
dichloromethane (5 ml_.) solution of N2-[(9T-T-fluoren-9-ylmethoxy)carbonyl]-
N6-[(4-
methylphenyl)diphenylmethyl]-L-lysine (40.9 mg, 0.065 mmol). The reaction
solution
was stirred at room temperature for 3 hours, and the solvent was distilled off
under reduced
pressure. The residue was purified by medium-pressure column chromatography
(methanol/chloroform).
Recovery amount: 69.3 mg (Yield: 91%; calculated from the amount of substance
of compound 117 obtained relative to the amount of substance of N2-[(9H-
fluoren-9-
ylmethoxy)carbony1]-N6-[(4-methylphenyl)diphenylmethyl]-L-lysine).
HRMS(EST)m/7: 1382 .7418 [M+H].
[0239]
<Synthesis of compound 118>
A similar reaction to the synthesis of compound 117 was performed to obtain
123
mg (Yield: 77%; calculated from the amount of substance of compound 118
obtained
relative to the amount of substance of compound 108) of compound 118 from
compound
108.
HR M S(ESI)rn/z : 1439.8029 [M+H]'.
[0240]
<Synthesis of compound 119>
Compound 117 (115 mg, 0.083 mmol) was dissolved in a mixture of piperidine (1
mL) and DMF (4 inL), and the mixture was stirred at room temperature for 2.5
hours.
CA 03210294 2023- 8- 29
91
Ethyl acetate and H20 were added to the reaction liquid. Thereafter. the
organic layer was
dried with sodium sulfide and filtered. The filtrate was distilled off under
reduced
pressure, and the residue was dissolved in dichloromethane (5 mL), and 4-(4-
iodophenyl)
butanoic acid (49.3 mg, 0.17 mmol), EDC hydrochloride (32.6 mg, 0.17 mmol),
HOAt
(23.1 mg, 0.17 mmol), and triethylamine (20 uL, 0.17 mmol) were added. The
reaction
liquid was stirred at room temperature for 7 hours, and then the solvent was
distilled off
under reduced pressure. The residue was purified by medium-
pressure column
chromatography (methanol/chloroform).
Recovery amount: 62.4 mg (Yield: 52%; calculated from the amount of substance
of compound 119 obtained relative to the amount of substance of compound 117).
HRMS(ESI)m/z:1432.6529[M+H]t
[0241]
<Synthesis of compound 120>
A similar reaction to the synthesis of compound 119 was performed to obtain
84.2
mg (Yield: 66%; calculated from the amount of substance of compound 120
obtained
relative to the amount of substance of compound 118) of compound 120 from
compound
118.
HRMS(ESI)m/7:1489.7260[M+H]t
[0242]
<Synthesis of compound 121>
Compound 119 (62.4 mg, 0.044 mmol) was dissolved in 1% TFA-containing
dichloromethane (5 mL) and stirred at room temperature for 2 hours. After the
solvent
was removed, the residue was dissolved in THF (5 mL), and then succinic
anhydride (8.1
mg, 0.088 mmol) and DIPEA (15 }IL, 0.088 mmol) were added. The solution was
stirred
at room temperature for 4 hours, and then the solvent was removed. The residue
was
purified by medium pressure column chromatography (methanol/chloroform).
Recovery amount: 65 mg (Yield: 100%; calculated from the amount of substance
of compound 121 obtained relative to the amount of substance of compound 119).
CA 03210294 2023- 8- 29
92
HRMS(ESI)m/z :1276.5478 [M+H]+.
[0243]
<Synthesis of compound 122>
A similar reaction to the synthesis of compound 121 was performed to obtain
39.1
mg (Yield: 35%; calculated from the amount of substance of compound 122
obtained
relative to the amount of substance of compound 120) of compound 122 from
compound
120.
HRMS(FST)m/7:1333 .5464 [M-41] .
[0244]
<Synthesis of compound 123>
A solution of compound 121 (65 mg, 0.051 mmol) in dichloromethane (5 mt.) was
added with (S)-di-tert-butyl 2-(34(S)-6-amino-l-tert-butoxy-1-oxoliexan-2-
yOureido)
pentanedioate (37.3 mg, 0.077 mmol), EDC hydrochloride (19.2 mg, 0.10 mmol),
HOAt
(13.6 mg, 0.10 mmol), and triethylamine (15 L, 0.10 mmol). The reaction
solution was
stirred at room temperature for 5 hours, and the solvent was distilled off
under reduced
pressure.
The residue was purified by medium-pressure column chromatography
(methanol/chloroform).
Recovery amount: 54.3 mg (Yield: 61%; calculated from the amount of substance
of compound 123 obtained relative to the amount of substance of compound 121).
HRM S(ESI)m/z : 873 .4248 [M+2H]+.
[0245]
<Synthesis of compound 124>
A similar reaction to the synthesis of compound 123 was performed to obtain
22.3
mg (Yield: 25%; calculated from the amount of substance of compound 124
obtained
relative to the amount of substance of compound 122) of compound 124 from
compound
122.
HRMS(ESI)m/z:902.4312[M+2H]+.
CA 03210294 2023- 8- 29
93
[0246]
<Synthesis of compound 125 (Octapa-2)>
Compound 123 (9.6 mg, 6.6 umol) was dissolved in a mixture of
TFA/H20/triisopropylsilane (95/2.5/2.5, 1 mL), and the mixture was stirred at
room
temperature for 2 hours. After removing the solvent, the residue was purified
by reverse
phase HPLC under the following conditions.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [20/80/0.1 (0 min) to 50/50/0.1 (30 min)]; flow rate: 1
mL/min.
Recovery amount: 1.3 mg (Yield: 17%; calculated from the amount of substance
of compound 125 obtained relative to the amount of substance of compound 123).
HRMS(ESI)m/z:677.2144[M-h2H]
[0247]
<Synthesis of compound 126 (Neunpa-2)>
A similar reaction to the synthesis of compound 125 was performed to obtain
2.0
mg (Yield: 47%; calculated from the amount of substance of compound 126
obtained
relative to the amount of substance of compound 124) of compound 126 from
compound
124.
HRMS(ESI)m/z:705.74021[M+2H]+.
[0248]
<Synthesis of compound 127>
To a solution of N2-[(9T-T-fluoren-9-
ylmethoxy)carbonyl]-1\16-[(4-
methylphenyl)diphenylmethyl]-L-lysine (625 mg, 1.0 mmol) in dichloromethane
(20 mL),
tert-butyl trichloroacetimidate (437 mg, 2.0 mmol) and BF3.0Et2 (20 L, 0.16
mmol) were
added. The reaction solution was stirred overnight at room temperature, and
then the
solvent was distilled off under reduced pressure. The residue was purified by
medium-
pressure column chromatography (hexane/ethyl acetate).
Recovery amount: 322 mg (Yield: 47%; calculated from the amount of substance
of compound 127 obtained relative to the amount of substance of N2-[(9H-
fluoren-9-
ylmethoxy)carbony1]-N6-[(4-methylphenyl)diphenylmethy1]-L-lysine).
CA 03210294 2023- 8- 29
94
1H-NMR(400MHz,CDC13)67.71-
7.69(m,2H),7.56(d,J=7.21-17,21-1),7.46(d,J=8.11-17,41-1),7.35-7.30(m,4H),7.26-
7.21(m,6H),7.15-
7.11 (m,21-1),7.04(d,J=8 .1Hz,2H),5 .38(d,J=8.7Hz,1H),4.35 (d,J=6.4Hz,2H),4.28-
4.23(m,1H),4 .18(t,J=7.5Hz,1H),2.26(s,3H),2.12-2.11(m,2H),1.81-1
.75(m,1H),1.59-
1.44(rn,14H).
MS(ESI)m/z:681.4[M+1-1] .
[0249]
<Synthesis of compound 128>
Compound 127 (200 mg, 0.47 mmol) was dissolved in 5% TFA-containing
dichloromethane (10 rnL), and the mixture was stirred at room temperature for
3 hours.
The solvent was removed, and then the residue was purified by medium-pressure
column
chromatography (methanol/chloroform). The obtained amine was dissolved in DMF
(10
mL), and 4-(4-iodophenyl) butanoic acid (273 mg, 0.94 mmol), FIX hydrochloride
(180
mg, 0.94 mmol), HOAt (123 mg, 0.94 mmol), and triethylamine (130 uL, 0.94
mmol) were
added thereto. The reaction liquid was stirred overnight at room temperature,
and then
ethyl acetate and 1-120 were added thereto. The organic layer was dried with
sodium
sulfide, and filtered. The filtrate was distilled off under reduced pressure,
and then the
residue was purified by medium-pressure column chromatography
(methanol/chloroform).
[0250]
Recovery amount: 161 mg (Yield: 50%; calculated from the amount of substance
of compound 128 obtained relative to the amount of substance of compound 127).
1H-NMR(400MHz,CDC13)67.76(d,J=7.5Hz,2H),7.60-
7.53(m,4H),7.39(0-7.5Hz,2H),7.30(0-7.5Hz,2H),6.92-6.86(m,2H),4.40-
4.09(m,4H),3 .27-3 .19(m,2H),2.52(t,J=7 .8Hz,2H),1.93-1.80(m,3H),1.71-1.62(m,
1H),1.52-
1.36(rn,13H).
MS(F,ST)m/z:697.2[M+T-T]t
CA 03210294 2023- 8- 29
95
[0251]
<Synthesis of compound 129>
Compound 128 (473 mg, 0.68 mmol) was dissolved in a mixture of piperidine (2
mL) and DMF (8 mL), and the mixture was stirred at room temperature for 1
hour. Ethyl
acetate and H20 were added to the reaction liquid. Thereafter, the organic
layer was dried
with sodium sulfide and filtered. The filtrate was distilled off under reduced
pressure, and
the residue was purified by medium-pressure column chromatography
(methanol/chloroform). The obtained amine was dissolved in DMF (10 mL), and
3,6,9-
trioxaundecanedioic acid (91.1 mg, 0.41 mmol), EDC hydrochloride (78.6 mg,
0.41 mmol),
HUM (55.8 mg, 0.41 mmol), and triethylamine (57 L, 0.41 mmol) were added. The
reaction liquid was stirred at room temperature for 5 hours, and then ethyl
acetate and H20
were added. The organic layer was dried with sodium sulfide, and filtered. The
filtrate
was distilled off under reduced pressure, and the residue was then purified by
medium-
pressure column chromatography (methanol/chloroform).
[0252]
Recovery amount: 87.9 mg (Yield: 37%; calculated from the amount of substance
of compound 129 obtained relative to the amount of substance of compound 128).
1H-NMR(400MHz,CD30D)67.58(d,J=8.7Hz,2H),6.96(d,J=7.5,2H)4.41-4.29(m,2H),4.09-
4.02(m,211),3.85-
3 .73(m,8H),3 .60(q,J=7 .21-17,2H),3 .35(s,2H),3.10(t,J=4.0Hz,2H),2 .55(0=7
.5Hz,2H),2.16-
2.14(m,2H),1.89-1.81(11,2H),1.41(s,9H),1.32-1.27(m,3H),1.18(0=7.0Hz,2H).
MS(EST)m/z:701.2[M+Na]t
[0253]
<Synthesis of compound 130>
Compound 117 (400 mg, 0.29 mmol) was dissolved in a mixture of piperidine (1
mL) and DMF (4 mL), and the mixture was stirred at room temperature for 2.5
hours.
Ethyl acetate and H20 were added to the reaction liquid. Thereafter, the
organic layer was
dried with sodium sulfide and filtered. The filtrate was distilled off under
reduced
pressure, and the residue was purified by medium-pressure column
chromatography
CA 03210294 2023- 8- 29
96
(methanol/chloroform). The obtained amine was dissolved in DMF (10 mL), and
compound 129 (170 mg, 0.25 mmol), EDC hydrochloride (95.9 mg, 0.50 mmol), HOAt
(68.1 mg, 0.50 mmol), and triethylarnine (69 1..t1., 0.50 mmol) were added.
The reaction
liquid was stiffed at room temperature for 2 hours, and then ethyl acetate and
H20 were
added. The organic layer was dried with sodium sulfide, and filtered. The
filtrate was
distilled off under reduced pressure, and then the residue was purified by
medium-pressure
column chromatography (methanol/chloroform).
Recovery amount: 272 mg (Yield: 60%; calculated from the amount of substance
of compound 130 obtained relative to the amount of substance of compound 117).
HRMS(ESI)m/z:911.4302[M+2Hr.
[0254]
<Synthesis of compound 131>
Compound 130 (272 mg, 0.15 mmol) was dissolved in 1% TFA-containing
dichloromethane (3 mL), and the mixture was stirred at room temperature for
1.5 hours.
After the solvent was removed, the residue was dissolved in THF (5 mL), and
succinic
anhydride (30 mg, 0.30 mmol) and DTPEA (52 uL, 0.30 mmol) were added. The
reaction
solution was stirred at room temperature for 2 hours, and the solvent was
distilled off under
reduced pressure. The residue was purified by medium-pressure column
chromatography
(methanol/chlorofonn).
Recovery amount: 230 mg (Yield: 92%; calculated from the amount of substance
of compound 131 obtained relative to the amount of substance of compound 130).
HRMS(EST)m/z:832.8701[M+2H]t
[0255]
<Synthesis of compound 132>
(S)-di-tert-butyl
2-(34(S)-6-amin o-1 -tert-butox y-1-ox oh ex an-2-yl)urei do)
pentanedioate (63.4 mg, 0.13 mmol), HBTU (83.4 mg, 0.22 mmol) and DTPEA (38
uL,
0.22 mmol) were added to a solution of compound 131 (186 mg, 0.11 mmol) in
dichloromethane (5 mL), and the mixture was stirred at room temperature for
1.5 hours.
The solvent was distilled off under reduced pressure, and the residue was
purified by
CA 03210294 2023- 8- 29
97
medium-pressure column chromatography (methanol/chloroform).
Recovery amount: 80.4 mg (Yield: 34%; calculated from the amount of substance
of compound 132 obtained relative to the amount of substance of compound 131).
HRMS(ESI)m/z :1068.0419 [M+2H].
[0256]
<Synthesis of compound 133 (Octapa-3)>
Compound 132 (10 mg, 4.7 nmol) was dissolved in a mixture of
TFA/1-T20/triisopropylsilane (95/2.5/2.5, 2 mi..), and the solution was
stirred overnight at
room temperature. After the solvent was removed, the residue was purified by
reverse
phase HPLC under the following conditions.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [20/80/0.1 (0 min) to 50/50/0.1 (30 min)]; flow rate: 1
mL/min.
Recovery amount: 2.3 mg (Yield: 29%; calculated from the amount of substance
of compound 133 obtained relative to the amount of substance of compound 132).
HRMS(ESI)m/z : 843 .2900[M+2H]
[0257]
<Production of radiolabeled compound with 111In-labeling (Examples 3-1 to 3-2,
and
Comparative Example 2-1)>
For each of the three Octapa derivatives (Octapa-1 to -3), a solution (100
ilL) of
[11 lln]InC13 was mixed with an acetate buffer (pH 5.5, 10 mM, 350 i_tL), and
an aqueous
solution (< 10% DMSO, 10 1iM, 50 pi-) of the labeling precursor (Compound 115,
125, or
33) was added. After left to stand at room temperature for 15 minutes, the
reaction
solution was purified by reverse phase HPLC under the following conditions.
Purification conditions: Cosmosil 5C18-MS-II column (4.6 x 150 mm); mobile
phase: MeCN/acetate buffer (pH 4.5, 10 mM) [10/90 (0 min) to 30/70 (30 min) or
20/80 (0
min) to 50/50 (30 min)]; flow rate: 1 rnL/min.
The radiochemical yield and radiochemical purity were measured in the same
manner as described in Example 1-1. The HPLC conditions used to measure the
radiochemical purity was the same as the purification conditions.
CA 03210294 2023- 8- 29
98
[0258]
As a result, the following three kinds of radiolabeled compound were obtained
with a radiochemical yield of 55 to 93% and a radiochemical purity of 99% or
more.
= Comparative Example 2-1: [1111n]1n-Octapa-1
= Example 3-1: [111In]In-Octapa-2
= Example 3-2: [1111n]ln-Octapa-3
[0259]
<Production of radiolabeled compound with 1111n-labeling (Example 3-3 and
Comparative
Example 2-2>
For the two Neunpa derivatives (Neunpa-1 and -2), a solution (100 lit) of
[111In]InC13 was mixed with an acetate buffer (pH 4.0, 10 mM, 350 [iL), and an
aqueous
solution (< 10% DMSO, 10 LIM, 50 lit) of the labeling precursor (compound 116
or 126)
was added. The mixture was allowed to stand at room temperature for 15
minutes, and
then the reaction solution was purified by reverse phase HPLC under the
following
conditions.
Purification conditions: Cosmosil 5C18-AR-II column (4.6 x 150 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 30/70/0.1 (30 mm) or 20/80/0.1 (0
min) to
50/50/0.1 (30 min)]; flow rate: 1 mL/min.
The radiochemical yield and radiochemical purity were measured in the same
manner as described in Example 1-1. The HPLC conditions used to measure the
radiochemical purity was the same as the purification conditions.
[0260]
As a result, the following two kinds of radiolabeled compound were obtained
with
a radiochemical yield of 55 to 93% and a radiochemical purity of 99% or more.
= Comparative Example 2-2: [111In]In-Neunpa-1
= Example 3-3: [111In]In-Neuripa-2
[0261]
<Binding assay using cultured cells>
LNCaP cells and PC-3 cells cultured in the same method as in Example 1-1 were
each seeded on a 12 well plate at 4.0 x 105 cells/well, and left standing at
37 C under 5%
CA 03210294 2023- 8- 29
99
CO2 for 48 hours.
The culture medium was removed, and an assay medium (0.5% FBS-containing
RPMI 1640 medium) solution (1 mL) containing the radiolabeled compound (37 kBq
each)
in Examples 3-1 to 3-3 or Comparative Examples 2-1 to 2-2 was added.
Thereafter, the
plate was allowed to stand at 37 C under 5% CO2 for 1 hour.
In the inhibition experiments, after removing the culture medium, an assay
medium (1 mL) containing: the radiolabeled compound described above; and 2-
(phosphonomethyl) pentanedioic acid (2-PMPA) (PSMA inhibitor; final
concentration: 100
iõtM) was added. Thereafter, the plate was allowed to stand at 37 C under 5%
CO2 for 1
hour.
After removing the assay medium, each well was washed with assay medium (1
mL), and the cells were lysed with 1 N aqueous sodium hydroxide solution (200
uL x 2).
Radioactivity of each of the cell lysates was measured with a gamma counter.
Separately, the total protein concentration in the cell lysate was calculated
using BCA
Protein Assay Kit, which is manufactured by Thermo Fisher Scientific K.K. The
value
(% ID/mg protein) obtained by dividing the percentage (% ID) of the sample's
radioactivity
amount to the added radioactivity amount by the total protein amount was
calculated for
each sample.
The data were expressed as mean value standard deviation. The significant
difference test was performed using Student's t-test and one-way analysis of
variance
(ANOVA) test with Dunnet's post-hoc test, and p < 0.05 was defined as having a
significant
difference.
[0262]
The results of the evaluation of binding to cultured cells are shown in Fig.
11.
The higher the value, the higher the abundance of the radiolabeled compound,
which
indicates that the compound is highly accumulated.
The radiolabeled compounds in Examples 3-1 to 3-3 and Comparative Examples
2-1 to 2-2 showed high binding ability to PSMA-positive LNCaP cells compared
to PSMA-
negative PC-3 cells, and the bonds were significantly reduced by adding an
excess amount
of the PSMA inhibitor (2-PMPA). Therefore, it was shown that the radiolabeled
compounds of Examples and Comparative Examples specifically bind to the PSMA
positive-cells, respectively.
CA 03210294 2023- 8- 29
100
[0263]
<Binding assay to albumin>
As in Examples described above, PBS and human albumin were used to evaluate
binding to albumin. Evaluation was performed by the same experimental
procedure and
statistical method as in Example 1-1 except that PBS solutions (37 kBq, 501_1
each) of the
radiolabeled compounds in Examples 3-1 to 3-3 and Comparative Examples 2-1 to
2-2 were
used, respectively.
[0264]
The results are shown in Fig. 12.
The radioactivity ratio of the eluates of the HSA solutions using Examples 3-1
to
3-3 ([111In]ln-Octapa-2, [111In]In-Octapa-3, and [11 11n]ln-Neunpa-2) were
significantly
higher than the radioactivity ratio of the PBS eluates.
On the other hand, the radioactivity ratio of the eluates of the NSA solutions
of
Comparative Examples 2-1 to 2-2 ([111In]ln-Octapa-1 and [1111n]ln-Neunpa-1)
was low as
in the case of PBS. These results showed that each of the compounds and
radiolabeled
compounds used in Examples 3-1 to 3-3 had a binding ability to albumin.
[0265]
<Evaluation of internal radioactivity distribution using tumor-transplanted
mouse>
LNCaP tumor-implanted mice prepared by the same method as in Example 1-1
were administered a saline solution (259 kBq/10011L each) of each of the
radiolabeled
compounds in Examples 3-1 to 3-3 or Comparative Examples 2-1 to 2-2 from the
tail vein
(n = 2 to 3). Mice were euthanized 1, 4, 24, 48, 96, and 192 hours after
administration.
Thereafter, blood and each organ were recovered, and the mass and
radioactivity of the
organs were measured.
The percentage (% ID) of the radioactivity amount to the administered
radioactivity amount (injected dose) is shown as the value (% ID/g) divided by
the blood
mass or the organ mass (g). The higher the value of %ID/g, the higher the
abundance of
the radiolabeled compound, which indicates that the compound is highly
accumulated at
the target organ.
[0266]
CA 03210294 2023- 8- 29
101
The results (Mean value + standard deviation, each n = 2 to 3) in LNCaP tumor-
transplanted mouse and each of the radiolabeled compounds are shown in Tables
7 to 11
and Figure 13 below.
It was confirmed that, compared to Comparative Examples 2-1 to 2-2, each of
the
radiolabeled compounds of Examples 3-1 to 3-3 had high retention in blood and
tumor
accumulation while accumulation at the kidney was reduced to the same extent.
This was
particularly remarkable in Example 3-1
In]In-Octapa-2), Example 3-2 ([111In]In-
Octapa-3), and Example 3-3 ([thlIn]In-Neunpa-2).
[0267]
= Comparative Example 2-1 (11111In]In-Octapa-1; Mean value standard
deviation,
n = 3 for each, and n = 2 only for the point of 192 hours).
[0268]
[Table 7]
[111in]in-
1 h 4h 24h 48h 96h 192h
Octapa-1
Blood 0.48 + 0.09 0.21 + 0.18 0.02 0.00 +
0.00 0.02 + 0.01 0.00
Spleen 10.9 w 5.59 3.55 + 1.75 2.48 w 1.44
1.64 + 0.53 2.06 + 0.88 0.85
Pancreas 0.62 + 0.19 0.24 + 0.07 0.12 + 0.03
0.05 + 0.01 0.04 + 0.00 0.05
Stomach (% TO) 0.08 + 0.01 0.37 + 0.22 0.03 + 0.01 0.07
+ 0.04 0.03 + 0.01 0.01
Intestine 0.40 0.13 3.50 1.21 0.27 0.08
0.52 + 0.33 0.13 0.05 0.02
Kidney 322 + 42.8 112 + 54.6 24.9 + 10.9 7.48
+ 1.23 7.24 + 3.00 2.16
Liver 0.36 + 0.13 0.13 + 0.02 0.06 + 0.01
0.04 + 0.01 0.08 + 0.02 0.05
Heart 0.32 + 0.08 0.18 + 0.03 0.04 + 0.01
0.02 + 0.03 0.03 + 0.00 0.01
Lung 0.99 + 0.22 0.23 + 0.10 0.13 + 0.06
0.06 + 0.02 0.09 + 0.03 0.04
Brain 0.02 0.00 0.02 0.01 0.04 0.04
0.01 0.00 0.01 0.00 0.01
Muscle 0.20 0.01 0.10 0.02 0.01 0.01
0.01 0.02 0.03 0.04 0.02
LNCaP Tumor 9.72 1.65 4.73 + 0.40 2.00 0.61
0.58 + 0.11 0.72 0.11 0.36
[0269]
= Example 3-1 ([111In]In-Octapa-2; Mean value I standard deviation, and
each n =
3).
[0270]
[Table 8]
h 4h 24h 48h 96h 192h
Octapa-2
CA 03210294 2023- 8- 29
102
Blood 14.4 + 1.81 6.73 + 1.63 0.84 =
0.27 0.26 + 0.23 0.01 + 0.01 0.01 0.01
Spleen 18.5 4.39 13.2 2.66 1.97 =
0.90 1.13 0.70 0.61 0.04 0.44 0.12
Pancreas 1.73 + 0.18 1.59 + 0.94 0.19 =
0.05 0.13 + 0.06 0.06 + 0.02 0.08 + 0.02
Stomach C/0
0.51 + 0.22 0.23 + 0.02 0.21 = 0.15 0.16 + 0.14 0.04 + 0.02
0.01 + 0.01
ID)
Intestine 1.55 0.21 1.65 1 0.37 0.82 =
0.68 0.68 1 0.32 0.45 0.29 0.27 1 0.18
Kidney 121 14.9 177 35.7 55.3 =
19.3 22.4 14.5 3.27 0.26 1.76 0.70
Liver 2.75 0.54 1.67 0.47 0.33 =
0.02 0.32 0.10 0.22 0.01 0.20 + 0.05
Heart 3.98 + 0.51 1.89 0.49 0.33 =
0.05 0.18 + 0.12 0.07 + 0.02 0.06 0.02
Lung 10.1 1.34 5.02W 0.89 0.74 =
0.12 0.45 0.18 0.14 0.08 0.07 1 0.02
Brain 0.20 1 0.03 0.14 1 0.02 0.03 =
0.01 0.02 0.01 0.01 0.00 0.01 0.01
Muscle 1.44 0.24 0.63 0.19 0.07 =
0.02 0.04 0.05 0.05 0.02 0.01 0.01
LNCaP Tumor 12.5 0.26 13.7W 3.24 8.64 = 2.27 8.10 2.67
4.48 0.78 1.76 + 0.45
[02711
= Example 3-2 ([" Iln]In-Octapa-3; Mean value + standard deviation, and
each n =
3).
CA 03210294 2023- 8- 29
103
[0272]
[Table 9]
[111inlIn-Octapa-
1 h 4h 24h 48h 96h 192h
3
Blood 27.2 + 2.92 19.3 3.84 7.20 0.50 3.88
1.13 0.63 0.32 0.03 0.00
Spleen 8.56 + 0.95 7.42 + 0.88 2.87 + 0.40 1.76
0.39 0.88 0.32 0.31 0.11
Pancreas 2.59 0.26 2.17 0.15 1.24 0.16 0.60
0.13 0.22 0.06 0.07 0.01
Stomach (% TD) 0.49 + 0.04 0.78 + 0.28 0.25 + 0.08 0.13 +
0.04 0.06 + 0.03 0.01 0.00
Intestine 2.60 0.48 3.23 0.18 1.23 0.28 0.62
0.14 0.29 0.09 0.05 0.01
Kidney 57.1 + 17.4 100 6.33 26.4 + 2.50 17.3 +
5.57 4.33 2.06 0.27 + 0.02
Liver 4.32 + 0.40 3.36 0.39 1.73 0.09 1.42
0.08 0.70 0.25 0.35 0.04
Heart 6.53 + 0.66 5.98 0.93 2.50 0.17 1.37
0.18 0.43 0.17 0.12 0.02
lung 16.7 + 1.41 14.1 2.06 5.85 0.39 3.18
0.68 0.78 0.29 0.12 0.04
Brain 0.40 + 0.05 0.31 0.07 0.16 + 0.01 0.12 +
0.02 0.07 + 0.02 0.02 + 0.01
Muscle 1.51 + 0.23 1.26 + 0.18 0.61 + 0.04 0.26
0.05 0.08 w 0.03 0.01 W 0.02
LNCaP Tumor 7.79 + 1.11 11.3 w 2.39 8.04 0.38 7.79 +
2.72 4.17 w 0.80 1.31 + 0.49
[0273]
= Comparative Example 2-2 ([111Tn]In-Neunpa-1; Mean value standard
deviation,
n = 3 for each, and n = 2 only for the point of 192 hours).
[0274]
[Table 10]
In h 4h 24h 48h 96h 192h
Neunpa-1
Blood 2.30 + 0.28 1.24 + 0.15 0.20 + 0.02 0.09 +
0.03 0.06 + 0.02 0.01
Spleen 6.83 + 1.70 4.76+ 1.17 2.74 + 0.07 2.62 +
0.79 2.47 + 0.60 1.05
Pancreas 0.57 + 0.14 0.50 + 0.13 0.28 + 0.03 0.38 +
0.06 0.32 + 0.02 0.37
Stomach (% TD) 0.17 + 0.02 0.11 + 0.03 0.05 + 0.00 0.09 +
0.06 0.07 + 0.03 0.02
Intestine 0.47 0.03 0.66 0.08 0.32 0.02 0.32
0.06 0.16 0.07 0.06
Kidney 214 53.0 125 + 89.2 13.5 0.43 25.2
17.1 15.1 4.76 2.79
Liver 0.60 W 0.07 0.69 W 0.05 0.69 W 0.05 0.80+0.13
0.77+0.07 0.63
Heart 0.70 0.03 0.47 0.08 0.21 0.01 0.18
0.05 0.28 0.06 0.22
lung 1.75 0.18 1.03 0.13 0.34 0.05 0.32 +
0.04 0.39 + 0.06 0.20
Brain 0.05 0.00 0.04 0.00 0.02 0.01 0.03
0.01 0.03 0.00 0.03
Muscle 0.28 + 0.06 0.11 + 0.04 0.10 + 0.00 0.10 +
0.03 0.09 + 0.03 0.07
LNCaP Tumor 1.06 + 0.21 0.69 + 0.30 0.28 + 0.02 0.30 +
0.11 0.32 + 0.09 0.15
CA 03210294 2023- 8- 29
104
[0275]
= Example 3-3 ([111In]In-Neunpa-2; Mean value standard deviation, and
each n
=3).
[0276]
[Table 11]
LINN_ lb 4h 24h 48h 96h 192h
Neunp a-2
Blood 13.6W 0.68 5.73W 0.26 2.48W 0.07
0.20W 0.02 0.13W 0.12 0.02W 0.02
Spleen 16.9 2.52 8.16 3.89 2.67 0.59
0.83 0.20 0.47 0.23 0.88 0.34
Pancreas 1.61 0.42 0.82 0.05 0.65 0.05
0.25 0.09 0.18 0.12 0.29 0.11
Stomach (% TD) 0.23 0.06 0.29 0.18 0.13 0.06
0.06 0.03 0.03 0.01 0.02 0.01
Intestine 1.22 + 0.30 1.05 + 0.29 0.68 = 0.11
0.23 + 0.05 0.17 + 0.10 0.10 + 0.05
Kidney 90.1 = 5.81 126 17.6 101 + 18.7
11.7 + 3.83 9.71 + 10.8 1.83 + 0.67
Liver 2.23 0.34 1.29 0.02 1.00 0.15
0.58 0.23 1.30 1.30 0.64 0.24
Heart 3.74 + 0.04 1.78 0.15 0.79 0.08
0.14 + 0.03 0.14 + 0.11 0.22 0.10
Lung 8.99 1.68 4.14 0.61 2.26 0.29
0.40 0,10 0.36 0.24 0.29 0.07
Brain 0.18 0.00 0.12 0.01 0.08 0.01
0.03 0.00 0.03 0.02 0.02 0.00
Muscle 1.09 w 0.25 0.61 w 0.11 0.27 = 0.11
0.05 = 0.04 0.03 w 0.02 0.08 0.00
LNCaP Tumor 7.44 0.18 8.57 0.61 8.61 1.06
4.92 0.70 3.35 0.90 5.16 0.23
[0277]
<Evaluation of SPECT/CT using tumor-transplanted mouse>
A mouse transplanted simultaneously with tumors of LNCaP cells and PC-3 cells
was obtained in the same manner as in Example 2, and then a saline solution
(7.3 MBq,
150 !IL) of Example 3-1 ([[ l'In]Tn-Octapa-2) was administered from the tail
vein.
SPECT/CT was performed 24 hours after administration in the same manner as in
Example
2 and the obtained image was reconstructed.
[0278]
SPECT/CT results are shown in Figure 14.
In SPECT/CT imaging using [111In]in-Octapa-2, 24 hours after administration,
high radioactivity accumulation was observed in the LNCaP tumor (the solid
arrow in the
figure), but radioactivity signal was hardly observed in the PC-3 tumor (the
dashed arrow
in the figure). Radioactivity accumulation at the kidney (the circle in the
figure) was also
observed. This result showed that [iti in]In-Octapa-2 was capable of clearly
drawing the
highly PSMA-expressing tumor.
CA 03210294 2023- 8- 29
105
[0279]
<Examples 4-1 to 4-2>
In Examples 4-1 to 4-2, synthesized was a compound (PSMA-NAT-DA1) that has
a structure containing (((S)-5-amino-1 -carboxypentyl)carbamoy1)-L-glutarnic
acid (In the
formula (Cl), "a" is 2, and "b" is 4.), whose target molecule is PSMA, as the
PSMA
molecule-binding part, and has a chemical structure linearly containing the
PSMA
molecule-binding part and the albumin-binding part through the chelating part
as shown in
the general formula (1). Next, radiolabeled compounds were obtained, in which
PSMA-
NAT-DA1 was coordinated with a in ion or a 225Ac ion each as a radioactive
metal.
Details are described below.
PSMA-NAT-DA1 has a structure containing the chelating part, the PSMA
molecule-binding part, and the albumin-binding part.
In PSMA-NAT-DA1, the chelating part and the PSMA molecule-binding part are
indirectly bonded to each other through a chemical structure derived from
trans-4-
aminocyclohexane-1 -carboxylic acid and naphthylglycine, and the chelating
part and the
atomic group containing the albumin-binding part are indirectly bonded to each
other
through a lysine-derived linker structure.
[0280]
The outline of the synthesis route in Examples 4-1 to 4-2 is shown below.
[0281]
CA 03210294 2023- 8- 29
106
1
co2teu a i --....
202:
H2N.;.--õ,----..õ----,N I ----
tBuO2Cõi H
tBu02C 7"-NTh COMU
Nµ,...r.õ-002H ___________________________________________ DIPEA
HO2C"--'"--"-ANN1 DP
DMF
c-N--.../ CO2tBu
LCO2tBu
1
tBuO2C,1
IBu02C r-N-Th
0 CO21Bu __ 0 7N----1 I
HO2C----."--"-CN Ny--,,....),NN)L-".." ____ )
c--N--) CO2tBu H H
1-..
co2teu
203
[0282]
tBuo2c,
1
113u 2 (---N N---\N)-_(....5-N---WC 2tBu N 0 0 I
203: ao2c
c¨N--) CO2iBu H H
00 LCO2tBu
0 H n T - HBTU TEA
C0 DIPEA TIPS IIII-LNy.0 0 H -
,NH2 DMF I. H20
HNye.,002tBu
602tBu
204
040
H i 0 HO2C,...
HO2C1:0--.....-....õNõ...;,N)L0 1.1 HO2C 1......,-44,--) argh I
0 CO2H 0
It.
HN.,e.0
F 8 H =,Ny--,..),,
HN.T....,CO2H 0 c....N.__.) CO2H H H
CO2H
l'CO2H
205
[0283]
(1) Synthesis of PSMA-NAT-DA1
PSMA-NAT-DA1 was synthesized according to the following procedures.
CA 03210294 2023- 8- 29
107
[0284]
<Synthesis of compounds 202 and 203>
Compound 1 was synthesized in the same manner as described in Example 1-1.
Compound 202 was synthesized in 4 steps from N2-[(9H-fluoren-9-
ylmethoxy)carbonyl]-N6-[(4-methylphenyl)diphenylmethyl]-L-lysine according to
a
previously reported method (Chem Commun. 2021, 57, 6432-6435).
N-[1-(cyan o-2-eth oxy-2-oxo ethyliden eaminooxy)dimethyl amino (morpholino)]
uronium hexafluorophosphate (COMU) (72.4 mg, 0.17 mmol) and N,N-
diisopropylethylamine (DIPEA) (95 1AL, 0.17 mmol) were added under ice cooling
to a
solution of compound 1 (131 mg, 0.17 mmol) in N,N-dimethylformamide (DMF) (0.4
mL),
and the mixture was stirred for 15 minutes. Compound 202 (66.8 mg, 0.14 mmol)
was
added, and the mixture was stirred at room temperature for 5 days. Then, the
solution was
purified by reverse phase HPLC to obtain compound 203.
Purification conditions: Cosmosil 5C18-AR-I1 column (10 x 250 mm); mobile
phase: MeCN/H20 [3/7 (0 min) to 9/1 (60 min)]; flow rate: 5 mL/min.
MS(ES1):m/z1230[M+Hr
[0285]
<Synthesis of compound 204>
Compound 204 was obtained in 9 steps from Fmoc-Lys (ivDde)-OH with
reference to the method previously reported (J. Med. Chem. 2016, 59, 1761-
1775, Mol.
Pharm. 2018, 15, 3502-3511).
In the chemical formula of compound 204, the chemical structure represented by
symbol Gp represents a resin.
[0286]
<Synthesis of compound 205>
Compound 203 (49.6 mg, 0.044 mmol), 1-[bis(dimethylamino)methylene]-111-
benzotriazolium 3-oxid hexafluorophosphate (HBTU) (16.7 mg, 0.44 mmol), and
D1PEA
(188A, 0.44 mmol) were added to compound 204, to which a resin was bonded, and
the
mixture was shaken in DMF overnight. After washing with DMF, trifluoroacetic
acid
(TFA)/triisopropylsilane/H20 (95 : 2.5 : 2.5, 2 mL) was added. After shaking
for 3 hours,
CA 03210294 2023- 8- 29
108
the solution was purified by reverse phase 1-1PLC under the following
conditions to obtain
the desired compound 205 (PSMA-NAT-DA1).
Purification conditions: Cosrnosil 5C15-AR-II column (4.6 x 250 mm); mobile
phase: MeCN/H20/TFA [10/90/0.1 (0 min) to 90/10/0.1(40 min)]; flow rate: 5
mL/min.
Recovery amount: 11 mg (16%; calculated from the amount of substance of
compound 205 obtained relative to the amount of substance of compound 203).
MS(ESI)m/z:MS(ESI)m/7793[M+2H]2+.
[0287]
(2)1111n labeling (Example 4-1)
The desired radiolabeled compound ([1' 11n] In-P SMA-NAT-DA1) was obtained
according to the following procedures.
[0288]
A solution (3.7 MBq, 100 L) of [111In]InC13 and a dimethyl sulfoxide solution
(1
g/AL, 5 pt) of compound 205 were added to a 2-morpholinoethanesulfonic acid
buffer
(0.1 M, pH 5.7, 150 1,,LL), and the mixture was allowed to stand at 90 C for
30 minutes.
Thereafter, the reaction solution was purified by reverse phase HPLC to obtain
a
radiolabeled compound ([1111n]In -P SMA-NAT-D Al ).
[0289]
CA 03210294 2023- 8- 29
109
400
H H020,1
HO2C dab, I
0 CO2H 0
HNyn,CO2H 0
CO2H
1')CO2H
206 (PSMA-NAT-DA1)
040
H E 0 -02C,,
H HO2C
dab, I
MES buffer HNY0 o H o cop
N 1110+ 0
(pH 5.7,0.1 M) 0
CO2H
CO2H
CO2-
.111
Inpn-PSMA-NAT-DA1
[0290]
The radiochemical purity of the obtained [IiiIn]In-PSMA-NAT-DA1 was
measured by the following method. That is, a part of the HPLC preparative
liquid of
[HlIn]In-PSMA-NAT-DA1 was analyzed again under the following HPLC conditions,
and
the percentage of the area value of [111In]In-PSMA-NAT-DA1 to the area value
of all
detected peaks was defined as radiochemical purity (%).
[0291]
The radiochemical yield was measured in the same manner as described in
Example 1-1. HPLC conditions used for measuring the radiochemical purity are
shown
below.
As a result, the radiochemical yield was 9%, and the radiochemical purity was
95% or more. When the same synthesis was performed again, the radiochemical
yield
was improved to 60%.
Purification conditions: Cosmosil 5C,8-AR-II column (4.6 X 150 mm); mobile
phase: MeCN/1120/TFA [10/90/0.1 (0 mm) to 90/10/0.1 (40 mm)]; flow rate: 1
mL/min.
[0292]
(3) 225Ac labeling (Example 4-2)
The desired radio labeled compound ([225Ac]Ac-PSMA-NAT-DA1) was obtained
according to the following procedures.
CA 03210294 2023- 8- 29
110
[0293]
To a 0.2 mol/L hydrochloric acid solution (1.0 MBq, 20 tiL) of [225 c,
A ]ACC13
dispensed in a Protein LoBind Tube 1.5mL (manufactured by EPPENDORF), 0.1 M
acetic
acid-ammonium acetate buffer (pH 5.5, 170 RL) and a DMSO solution (2.0 mM/L,
10 tit)
of PSMA-NAT-DAI were added, and the mixture was allowed to stand at 70 C for 1
hour.
H20 (0.8 mL) was added to the reaction solution, and the mixture was passed
through an
Oasis HLB Light column. After H20 (10 mL) was passed through the column, 70%
Et0H
(0.5 mL) was passed through the column to obtain a 70% ethanol solution of the
desired
radiolabeled compound ([225Ac]Ac-PSMA-NAT-DA1).
040
H = o
_
HN 0 0 1.1-1L0,,Nir.õ)..
CO2H = I
H
CO2H H
CO2H
LCO2H
205 (PSMA-NAT-DA1)
H -02C
[225AciAcC13 N
HO2. CO2H =
4111
HN 0 0 H
CH3C0OH-CH3C00N H4 buffer
(pH 5.5, 0.1 M) H Nyfr.,CO2H
002H H
CO2H
LCO2-
[225Ac]Ac-PSMA-NAT-DA1
[0294]
The radiochemical yield was measured by the following method.
The
radioactivity of the obtained radiolabeled compound was measured with a gamma
ray
spectrometer, and the percentage to the radioactivity of the solution of
[225Ac]AcC13 used
in the reaction was defined as radiochemical yield (%).
[0295]
The radiochemical purity of the obtained radiolabeled compound was measured
by the following method. That is, apart of the solution of the radiolabeled
compound was
analyzed by TLC (iTLC-SG; mobile phase: mixed solution of H20/MeCN = 1 : 1),
and the
percentage of the area value of the radiolabeled compound to the area value of
all detected
peaks was defined as radiochemical purity (%). As a result, the radiochemical
yield was
CA 03210294 2023- 8- 29
111
35%, and the radiochemical purity was 86%.
[0296]
<Binding assay using cultured cells>
LNCaP cells and PC-3 cells cultured in the same method as in Example 1-1 were
each seeded on a 12 well plate at 4.0 x 105 cells/well, and left standing at
37 C under 5%
CO2 for 48 hours.
The culture medium was removed, and an assay medium (0.5% FBS-containing
RPMI 1640 medium) solution (1 mL) containing [1111n]In-PSMA-NAT-DA1(8.5 kBq)
was
added. Thereafter, the plate was allowed to stand at 37 C under 5% CO2 for 1
hour.
In the inhibition experiments, after removing the culture medium, an assay
medium (1 mL) solution containing: [1111n]In-PSMA-NAT-DA1 (37 kBq); and 2-
(phosphonomethyl) pentanedioic acid (2-PMPA) (PSMA inhibitor; final
concentration: 100
[tM) was added. Thereafter, the plate was allowed to stand at 37 C under 5%
CO2 for 1
hour.
[0297]
After removing the assay medium, each well was washed with assay medium (1
mL) containing neither the radiolabeled compound nor 2-PMPA, and the cells
were lysed
with 1 N aqueous sodium hydroxide solution (200 !AL x 2).
The radioactivity for each of the assay medium and the cell lysate was
measured
with a gamma counter. Separately, the total protein concentration in the cell
lysate was
calculated using BCA Protein Assay Kit, which is manufactured by Thermo Fisher
Scientific K.K. The value (% ID/mg protein) obtained by dividing the
percentage (% ID)
of the sample's radioactivity amount to the added radioactivity amount by the
total protein
amount was calculated for each sample.
The data were expressed as mean value standard deviation. The significant
difference test was performed using Student's t-test and one-way analysis of
variance
(ANOVA) test with Dunnet's post-hoc test, and p < 0.05 was defined as having a
significant
difference.
[0298]
The results of the evaluation of binding to cultured cells are shown in Fig.
15.
CA 03210294 2023- 8- 29
112
The higher the value, the higher the abundance of the radiolabeled compound,
which
indicates that the compound is highly accumulated.
[111In]In-PSMA-NAT-DA1 showed high binding ability to LNCaP cells compared
to PC-3 cells, and the bond was significantly reduced by adding an excess
amount of the
PSMA inhibitor (2-PMPA). These results showed that [111In]In-
PSMA-NAT-DA1
specifically binds to highly PSMA-expressing cells.
[0299]
<Binding assay to albumin>
A phosphate buffered saline (PBS) solution (37 kBq, 50 L) of [1111n]In-PSMA-
NAT-DA1 was added to 200 1.1.L of a PBS solution (45 mg/mL) of human serum
albumin
(HSA), mouse plasma, human plasma, or PBS, and the mixture was allowed to
stand at
37 C for 10 minutes. Thereafter, the reaction liquid was added to a spin
column
(Sephadex G-50; manufactured by Cytiva), and centrifuged at 1500 x g at 4 C
for 2 minutes.
After separation, the radioactivity of the column and the eluate was measured
with a gamma
counter.
The data were expressed as mean value standard deviation. The significant
difference test was perfatined using Student's t-test and one-way analysis of
variance
(ANOVA) test with Dunnet's post-hoc test, and p < 0.05 was defined as having a
significant
difference.
[0300]
The results of the evaluation of binding to albumin are shown in Fig. 16. The
higher the value, the higher the binding ability to albumin.
It is considered that: when a compound to be evaluated binds to albumin to
form
a composite, the compound passes through the column because of the increased
molecular
size; and when it does not bind to albumin, it is retained on the gel in the
column.
When [111In]in-PSMA-NAT-DA1 was allowed to stand in PBS and then applied
to the column, no significant radioactivity was observed in the eluate. On the
other hand,
when left standing in mouse plasma, human plasma, and the BSA solution, the
radioactivity
in the eluate of [111In]ln-PSMA-NAT-DA1 was significantly high, suggesting
that [1111n]In-
PSMA-NAT-DA1 binds to plasma albumin.
CA 03210294 2023- 8- 29
113
[0301]
<Evaluation of internal radioactivity distribution using LNCaP or PC-3 tumor-
transplanted
mouse>
The animal test was perfonned in compliance with the guidelines of Kyoto
University Animal Experiment Committee.
Male C1317/1-crIcl-Prkdcsc1d mice were
purchased from CLEA Japan, Inc. The animals were kept under 1211/12 h,
day/night cycle
conditions and fed ad libitum with food and water. LNCaP cells (4.3 x 106
cells/mouse)
or PC-3 cells (3.3 x 106 cells/mouse) were suspended in a mixture of PBS and
Matrigel
manufactured by Coming Life Sciences (1 : 1, 150 L) and the suspension was
subcutaneously transplanted to the right shoulder of CB17/Tcdcl-Prkdcscld mice
under
isoflurane anesthesia. Thereafter, the mice were kept for 40 to 60 days.
LNCaP or PC-3 tumor-transplanted mice were administered a saline solution (259
kBci, 100 L) of [1111n]In-PSMA-NAT-DA1 from the tail vein (n = 3 each). Mice
were
euthanized 1, 4, 24, 48, 96, and 192 hours after administration. Thereafter,
blood and each
organ were recovered, and the mass and radioactivity of the organs were
measured.
[0302]
LNCaP tumor-transplanted mice were administered [111In]In-PSMA-NAT-DA1,
and the pharrnacokinetics (% ID/g) was evaluated. The results are shown in
Table 12
(Mean value standard deviation, n = 3).
[0303]
[Table 12]
lb 4h 24h 48h 9611 192h
NAT-DA1
Blood 39.8 2.91 38.8 8.25 10.9 5.55 ..
12.3 + 1.56 .. 4.07 1.37 .. 2.47 0.52
Spleen 31.2 1 2.84 23.0 3.16 13.5 + 8.18 ..
17.8 + 1.05 .. 6.33 1 5.25 .. 3.77 1 0.54
Pancreas 7.32 1.27 5.85 1.34 2,15 1.39
2.26 + 0.15 2.60 3.18 0.94 0.32
Stomach TD) 0.94 + 0.35 1.32 + 0.19 0.28
+ 0.16 0.44 + 0.09 0.19 + 0.10 0.12 + 0.01
Intestine 3.40 0.24 3.47 0.42 1.26 0.70
1.39 0.06 0.86 0.54 0.55 0.15
Kidney 41.5 4.27 66.2 13.0 37.1 + 19.3
79.0 + 6.78 22.0 14.9 11.9 + 2.08
Liver 8.24 + 2.38 8.38 + 2.27 2.73 + 1.08
4.49 + 0.71 2.31 + 0.89 2.25 + 0.45
Heart 10.8 + 1.31 12.2 + 2.44 3.14 + 1.52 ..
4.68 + 0.92 .. 2.10 + 0.83 .. 1.19 + 0.24
Lung 28.1 + 8.65 26.5 + 5.70 8.76 + 4.02
11.1 + 2.24 4.55 + 2.33 2.81 + 0.36
Brain 0.63 0.03 0.83 0.20 0.27 0.13
0.45 0.03 0.18 0.04 0,26 0.02
Muscle 2.82 + 0.55 3.69 0.83 1.23 0.36
1.48 + 0.31 0.59 0.09 0.33 + 0.05
LNCaP Tumor 23.0 1 2.20 43.6 1 8.49 51.9 +
39.3 131.6 + 22.0 56.2 1 35.6 35.0 1 27.3
CA 03210294 2023- 8- 29
114
[0304]
[111In]li-PSMA-NAT-DA1 showed high accumulation at the LNCaP tumor (23.0
to 131.6% ID/g; 1 to 48 hours after administration). In addition, retention in
blood was
exhibited (12.3% ID/g; 48 hours after administration), and 24 hours after
administration,
the tumor/kidney ratio exceeded 1. These results revealed that [111In]in-PSMA-
NAT-DA1
has high accumulation at the highly PSMA-expressing tumor.
[0305]
A PC-3 tumor-transplanted mouse was administered [1111n]in-PSMA-NAT-DA1,
and the pharmacokinetics (% ID/g) was evaluated. The results are shown in
Table 13
(Mean value standard deviation, n = 3).
[0306]
[Table 13]
24 h
Blood 5.73 1.10
Spleen 6.94 4.55
Pancreas 1.25 1 0.04
Stomach (% TD) 0.15 0.03
Intestine 0.68 0.07
Kidney 27.9 6.05
Liver 1.74 937
Heart 2.67 0.58
Lung 7.41 1.13
Brain 0.22 0.03
Muscle 0.83 0.26
PC-3 Tumor 3.21 1.00
10307]
[111In]In-PSMA-NAT-DA1 accumulation at the PC-3 tumor showed a significantly
lower value than that of accumul ation at the LNCaP tumor at the same time
point, indicating
that the radiolabeled compounds selectively accumulate at the PSMA-positive
tumor.
[0308]
<SPECT/CT using LNCaP tumor-transplanted mouse>
A LNCaP tumor-implanted mouse prepared by the method described above was
CA 03210294 2023- 8- 29
115
administered a saline solution (1.57 to 2.59 MBq, 150 .1_,) of [1111n]In-PSMA-
NAT-DA1
from the tail vein. SPECT/CT was performed 24, 48, and 96 hours after
administration
with FX3300 pre-clinical imaging system, which is manufactured by Gamma Medica-
Ideas.
Imaging was performed using a pinhole collimator with a diameter of 1.0 mm at
a rotation
radius of 35 mm, a projection time of 70 seconds, and the number of projecti
ons of 32 times
under isoflurane anesthesia. After SPECT, CT (Tube voltage: 60 kV, Tube
current: 350
liA) was performed. Image reconstruction was performed on the projection data
of
SPECT through three-dimensional ordered subset expectation maximization method
(8
subsets, 5 iterations).
[0309]
SPECT/CT results are shown in Figure 17. In the figure, the part indicated by
the arrow is the location of the tumor, and the part indicated by the circle
is the location of
the kidney. The higher the SUV, the higher the radioactivity accumulation.
In the SPECT/CT imaging, significant radioactivity accumulation was observed
at
the I_NCaP tumor (the arrow in the figure) 24 and 48 hours after
administration.
Radioactivity accumulation was also observed at the kidney (the solid circles
in the figure)
24 and 48 hours after administration, but was hardly observed 96 hours after
administration
(the broken line circles in the figure). From these results, it has been
indicated that
Inftn-PSMA-NAT-DA1 can clearly draw the highly PSMA-expressing tumor through
SPECT and is superior to [1111n]ln-PSMA-DB.
CA 03210294 2023- 8- 29