Note: Descriptions are shown in the official language in which they were submitted.
LOW SUGAR SPERM MEDIA AND COMPOSITIONS
The present invention relates to compositions comprising low sugar media, the
methods
of using these compositions to reduce trauma and stress on processed animal
sperm, the resulting
sperm and embryo products, and the methods of use of these products to
increase the quality,
quantity and viability of progeny and improved rates of births in animals.
BACKGROUND
Assisted reproductive technology (ART) includes such techniques as in vitro
fertilization
(IVF), artificial insemination (AI), intracytoplasmic sperm injection (ICSI)
(other techniques
using enucleated cells) and multiple ovulation and embryo transfer (MOET) (as
well as other
embryo transfer techniques) and is used across the animal kingdom. ART methods
are generally
expensive, time consuming and marginally successful given the inherent
fragility of gametes
when outside of their natural environments. Furthermore, the use of ART within
the animal
breeding industry in a commercially feasible manner is additionally
challenging due to the
limited availability of genetically desirable gametes (sperm or oocytes). One
way to lower the
cost of ART and to improve its commercial feasibility is to increase the
efficiency of the
involved processes by improving the viability and overall quality of gametes
used in ART.
Although there has been a growing interest in this field over the course of
the last decade or so,
there still remains a strong need to increase the overall quality of gametes
for use in ART,
especially when breeding focuses on pre-natal gender selection, including
improving gametes
viability, motility and fertility, as well as other longevity characteristics.
For example, in conventional Al, one problem limiting its commercial
application in
certain species is the need to use extremely high number of sperm per Al dose
to ensure
successful fertilization currently. In swine in particular, the need for
improved sperm quality is
especially strong since the typical dose of boar sperm required for successful
fertilization using
conventional artificial insemination techniques, such as intra-cervical
insemination, is currently 1
x i09 sperm to 3 x i09 sperm.
1
Date Recue/Date Received 2023-08-28
Processing gametes such as flushed oocytes or sperm, both conventional and sex
sorted,
before their use in ART may add a tremendous amount of stress on the gamete
cell(s) and often
negatively impacts their cellular integrity and membrane structure, which in
turn may be
reflected in decreased viability, motility and fertility. An example of
processing gametes prior to
their use in ART is the sorting of sperm based on sex (known as "gender
enrichment" or "sex
sorting"), which is a now commonly used procedure to minimize wasted births of
the wrong sex
for selective breeding in the livestock industry. In some species, however, it
is still cost
prohibitive and can represent a financial risk to those with smaller breeding
herds. Sex sorting
includes processes that physically separate X and Y bearing sperm from each
other into separate
subpopulations, as well as processes in which sperm bearing the undesired sex
chromosome in a
sperm sample are selectively killed, compromised, disabled, rendered immotile,
or otherwise
rendered infertile by, for example, laser ablation/photo damage techniques to
render a gender
enriched population of sperm.
The sex sorting process severely stresses and damages the cells and produces a
low
percentage of useful sperm, which although capable of fertilizing matured
oocytes, may have
reduced viability, motility and fertility compared to unprocessed cells.
Typically, sex sorting
involves many harsh steps including but not limited to: the initial collection
and handling of
sperm ejaculate, which naturally starts to deteriorate rapidly upon
collection; the staining of
sperm, which involves binding of an excitable dye to the DNA or a harmful
membrane selection
procedure; the physical sorting of the sperm using high energy fluorescence
that physically
energizes the dye that is bound to the DNA, forced orientation through a
narrow orifice, and
application of an electrical charge to the cell; the physical collection of
the cells into a receiving
container, which often shocks the fragile cell upon contact; the osmotic
stresses associated with
dilution of the sperm droplet in collection media; and the storage of the
sorted sperm usually by
freezing, which is well known to raise havoc with the cell's membrane systems.
Each step
places the processed sperm under abnormal stress that diminishes the overall
motility, viability
and/or fertility of the sperm. The result can lead to less efficient samples
for use in ART, such as
IVF and AT, and other types of subsequent or further processing.
2
Date Recue/Date Received 2023-08-28
Even non-sorted processed sperm exhibits significant losses in fertility,
viability and
motility when being collected, handled and transported without freezing, and
noticeably
experiences significant stress when mixed with cryoprotectant and frozen and
thawed. Many in
the field have tried to improve methods for the use on unsorted, conventional
semen to minimize
loss in the handling processes associated with in vitro handling, preservation
and use of semen
samples.
Regardless of the processing, sperm lose their potential to fertilize when
exposed to:
elevated temperatures, abnormal buffers, stains, altered pH systems, physical
pressurized
orientation as when forced through a nozzle or when oscillated to form drops
in a flow
cytometer, radiation used to illuminate the DNA binding dye, physical
stressors associated with
separation and collection techniques, cryoprotectants, freezing, thawing and
micromanipulation
by the handler.
There remains a continuing need to improve current methods of ART to reduce
the cost
and to make the procedures more dependable and commercially feasible to those
on a restricted
budget, especially for smaller breeders for whom sex-selection breeding may be
a high risk and
expensive option. One way in which researchers have attempted to increase the
viability,
motility and/or fertility of gametes for use in ART is through the use of
specialized media used
to store and/or process gametes. For example, in the sex sorting process,
sperm is typically
collected in an extender, stained in a staining solution, sorted with a sheath
fluid and finally
collected in a catch media. Exposure to each of these types of media may have
beneficial, or
deleterious, effects on the sperm depending on the constituents of the media.
For example,
current sperm media used in sex sorting typically include high concentrations
of one or more
sugar additives, which are often added as an energy source, but may actually
increase the
sensitivity of sperm to the stresses associated with processing and sorting.
While use of existing media has allowed commercially viable production of sex
sorted
sperm in certain species, there remains the need to further increase the
viability, motility and
fertility of sex sorted sperm in order to increase efficiency and lower costs.
Furthermore, while
sex sorting sperm is currently commercially viable in certain species, such as
bovines, in other
3
Date Recue/Date Received 2023-08-28
species, inefficiencies in the process preclude sex sorting in a commercially
viable manner at
present. Accordingly, there is a significant need for improved media for use
in processing and
sorting sperm.
SUMMARY OF THE INVENTION
A broad object of the present invention is to provide improvements in the
motility,
viability, fertility and overall integrity of processed sperm, particularly
sperm that undergo
analysis and/or sex sorting. In order to achieve such improvements, one aspect
of the present
invention broadly encompasses compositions comprising low sugar media and the
use of such
compositions during the cell sorting process. Other aspects of the invention
encompass methods
of using sperm processed with low sugar media in various ART procedures,
including but not
limited to, IVF, AT, ICSI and MOET.
A further aspect of the instant invention relates to the discovery that the
use of low sugar
media allows for freezing and thawing of media comprising a protein source,
such as egg yolk,
without any deleterious effects relative to media that has not been frozen. In
fact, the use of such
frozen-thawed media has been shown by the inventors to actually increase the
viability of
processed sperm. This discovery allows for the shipping of media in a frozen
state to a
destination where it can then be thawed and used. The ability to ship frozen
media with
perishable components such as egg yolk and other protein sources also allows
for the
standardization of media across various geographically separate lab
facilities, which can yield an
improvement in end product consistency and quality control among labs. A
further aspect of the
present invention broadly encompasses the use of such frozen-thawed low sugar
media
comprising a protein source, such as egg yolk, during the cell sorting
process.
In accordance with an aspect of the present invention, there is provided a
method of
processing sperm comprising freezing and thawing a media comprising less than
20 mM of sugar
additive and a protein source; and contacting sperm with said media.
4
Date Recue/Date Received 2023-08-28
In accordance with an aspect of the present invention, there is provided a
composition
comprising sperm and a media comprising less than 20 mM of sugar additive and
a protein
source, wherein said media has been frozen and thawed prior to addition of
said sperm.
In accordance with an aspect of the present invention, there is provided a
composition
comprising a gender enriched sperm population and a media comprising less than
20 mM of
sugar additive.
The term "sugar" as used herein refers to mono- or di- saccharides that are
generally
metabolized by mammalian sperm, e.g., glucose and fructose. The term "sugar
additive" as used
herein means sugar that is added to a media as a discrete compound and not as
a naturally
occurring component of another additive in the media such as egg yolk, seminal
fluid or milk.
4a
Date Recue/Date Received 2023-08-28
In certain embodiments of the invention, low sugar media comprises less than
about
50mM, 45mM, 40mM, 35mM, 30mM, 25mM, 20mM, 19mM, 18mM, 17mM, 16.5mM, 16mM,
15.5mM, 15mM, 14.5mM, 14mM, 13.5mM, 13mM, 12.5mM, 12mM, 11.5mM, 11mM,
10.9mM, 10.8mM, 10.7mM, 10.6mM, 10.5mM, 10.4mM, 10.3mM, 10.2mM, 10.1mM,
10.0mM,
10mM, 9.75mM, 9.5mM, 9.25mM, 9.0mM, 9mM, 5mM of sugar additive. In other
embodiments of the invention, low sugar media comprises about 1-5mM, 5-10mM,
10-15mM,
15-20mM, 20-25mM, 25-30mM, 35-40mM, or 45-50mM of sugar additive. In further
embodiments of the invention, low sugar media comprises about 1-5mM, 1-10mM, 1-
15mM, 1-
20mM, 1-25mM, 1-30mM, 1-35mM, 1-40mM, 1-45mM, or 1-50mM of sugar additive. In
yet
further embodiments of the invention, low sugar media comprises about 0.1ppm
to about 5mM,
about 0.1ppm to about 10mM, about 0.1ppm to about 15mM, about 0.1ppm to about
20mM,
about 0.1ppm to about 25mM, about 0.1ppm to about 30mM, about 0.1ppm to about
35mM,
about 0.1ppm to about 40mM, about 0.1ppm to about 45mM, or about 0.1ppm to
about 50mM of
sugar additive. In other embodiments of the invention, low sugar media
comprises about 1mM,
2mM, 3mM, 4mM, 4.1mM, 4.2mM, 4.3mM, 4.4mM, 4.5mM, 4.6mM, 4.7mM, 4.8mM, 4.9mM,
5mM, 5.0mM, 5.1mM, 5.2mM, 5.3mM, 5.4mM, 5.5mM, 5.6mM, 5.7mM, 5.8mM, 5.9mM,
6mM, 7mM, 8mM, 9mM, 9.1, 9.2, 9.3, 9.4, 9.5, 9.6, 9.7, 9.8, 9.9, 10mM, 10.0,
10.1, 10.2, 10.3,
10.4, 10.5, 10.6, 10.7, 10.8, 10.9, 11mM, 12mM, 13mM, 14mM, 15mM, 16mM, 17mM,
18mM,
19mM, 20mM, 25mM, 30mM, 35mM, 40mM, 45mM, or 50mM of sugar additive. In a
further
embodiment of the invention, low sugar media comprises about 1-10mM, 2-9mM, 3-
6mM, 4-
6mM, or 4-5mM of sugar additive. In yet another embodiment of the invention,
low sugar media
comprises about 5-15mM, 6-14mM, 7-13mM, 8-12mM, 9-11mM or 9-10mM of sugar
additive.
In yet another embodiment of the invention, low sugar media comprises no sugar
additive or at
most, trace amounts of sugar additive (i.e., no more than 0.1-20ppm of sugar
additive).
In certain embodiments of the invention, low sugar media comprises less than
about
50mM, 45mM, 40mM, 35mM, 30mM, 25mM, 20mM, 19mM, 18mM, 17mM, 16.5mM, 16mM,
15.5mM, 15mM, 14.5mM, 14mM, 13.5mM, 13mM, 12.5mM, 12mM, 11.5mM, 11mM,
10.9mM, 10.8mM, 10.7mM, 10.6mM, 10.5mM, 10.4mM, 10.3mM, 10.2mM, 10.1mM,
10.0mM,
10mM, 9.75mM, 9.5mM, 9.25mM, 9.0mM, 9mM, 5mM of sugar. In other embodiments of
the
invention, low sugar media comprises about 1-5mM, 5-10mM, 10-15mM, 15-20mM, 20-
25mM,
Date Recue/Date Received 2023-08-28
25-30mM, 35-40mM, or 45-50mM of sugar. In further embodiments of the
invention, low sugar
media comprises about 1-5mM, 1-10mM, 1-15mM, 1-20mM, 1-25mM, 1-30mM, 1-35mM, 1-
40mM, 1-45mM, or 1-50mM of sugar. In yet further embodiments of the invention,
low sugar
media comprises about 0.1ppm to about 5mM, about 0.1ppm to about 10mM, about
0.1ppm to
about 15mM, about 0.1ppm to about 20mM, about 0.1ppm to about 25mM, about
0.1ppm to
about 30mM, about 0.1ppm to about 35mM, about 0.1ppm to about 40mM, about
0.1ppm to
about 45mM, or about 0.1ppm to about 50mM of sugar. In other embodiments of
the invention,
low sugar media comprises about 1mM, 2mM, 3mM, 4mM, 4.1mM, 4.2mM, 4.3mM,
4.4mM,
4.5mM, 4.6mM, 4.7mM, 4.8mM, 4.9mM, 5mM, 5.0mM, 5.1mM, 5.2mM, 5.3mM, 5.4mM,
5.5mM, 5.6mM, 5.7mM, 5.8mM, 5.9mM, 6mM, 7mM, 8mM, 9mM, 9.1, 9.2, 9.3, 9.4,
9.5, 9.6,
9.7, 9.8, 9.9, 10mM, 10.0, 10.1, 10.2, 10.3, 10.4, 10.5, 10.6, 10.7, 10.8,
10.9, 11mM, 12mM,
13mM, 14mM, 15mM, 16mM, 17mM, 18mM, 19mM, 20mM, 25mM, 30mM, 35mM, 40mM,
45mM, or 50mM of sugar. In a further embodiment of the invention, low sugar
media comprises
about 1-10mM, 2-9mM, 3-6mM, 4-6mM, or 4-5mM of sugar. In yet another
embodiment of the
invention, low sugar media comprises about 5-15mM, 6-14mM, 7-13mM, 8-12mM, 9-
11mM or
9-10mM of sugar. In another embodiment of the invention, low sugar media
comprises no sugar
or at most, trace amounts of sugar (i.e., no more than 0.1-20ppm of sugar).
In certain embodiments of the invention, sex sorting of sperm may be
accomplished using
any process or device known in the art for cell analysis, sorting and/or
population enrichment
including but not limited to use of a flow cytometer or use of a microfluidic
chip. As
contemplated herein, sex sorting in addition to encompassing techniques for
physically
separating X and y bearing sperm from each other as with droplet sorting and
fluid switching
sorting, also encompasses techniques for gender enrichment in which sperm
bearing the
undesired sex chromosome are killed, immobilized, or otherwise rendered
infertile, such as by
use of laser ablation/photo damage techniques.
In one embodiment of the invention, low sugar media may comprise one or more
buffers,
including but not limited to carbonates, phosphates, citrates, acetates,
lactates, and combinations
thereof, or a solution containing a salt, a carbohydrate, or a combination
thereof can be employed
in some of the embodiments of the invention, such as, but not limited to,
Tris, TES, HEPES,
6
Date Recue/Date Received 2023-08-28
TALP, TCA, PBS, citrate, milk and derivatives thereof, as discussed in detail
in U.S. Patent
7,208,265.
In certain embodiments, low sugar media may comprise one or more chelators,
including
but not limited to, deferoxamine, deferasirox, penicillamine, alpha lipoic
acid, DMPS, DMSA,
dimercaprol and aminopolycarboxylic acids (complexones), including but not
limited to Fura-2,
IDA, NTA, EDTA, DTPA, EGTA, BAPTA, NOTA, DOTA and nicotianamine, and
derivatives
thereof.
In other embodiments, low sugar media may comprise one or more "organic stress
reducing" agents (OSRs), which may comprise an antioxidant, a vitamin or other
organic
molecule involved directly or indirectly in modulating physiological stresses
in the cell. In
certain embodiments, low sugar media may comprise one or more OSRs, each in
the
concentration range of 0.01 mg/ml to 5 mg/ml. Various OSRs can be used in the
context of the
current invention, including but not limited to: catalase, superoxide
dismutase (SOD), SOD
mimics, glutathione, glutathione reductase, glutathione peroxidase, pyruvate,
mercaptoethanol,
butylated hydroxytoluene (BHT), lipoic acid, flavins, quinones, vitamin K (and
related vitamers),
vitamin B12 (and related vitamers), with `vitamers' defined as compounds
having the same
vitamin activity (such as cobalamin, cyanocobalamin, methylcobalamin,
adenosylcobalamin,
hydroxocobalamin, and pseudo-B12), vitamin E (including its vitamers,
tocopherols (a, 13, y),
tocotrienols, and a-tocopheryl), alpha-ketoglutarate (also known as a-KG, AKG
or oxo-
glutarate) and various biological forms of AKG (such as arginine, aspartate,
lysine, and similar
derivatives), other compounds that regulate nitric oxide in the cell including
malondialdehyde
(MDA) and asymmetric dimethylarginine (ADMA), and biologically active
derivatives thereof.
Certain embodiments of the invention utilize concentrations of OSRs selected
from the
following ranges: 0.01 to 5.0 mg/ml; 0.01 to 0.25 mg/ml; 0.01 to 0.5 mg/ml;
0.01 to 1 mg/ml;
0.01 to 2.5 mg/ml; 0.01 to 5 mg/ml; 0.05 to 0.1 mg/ml; 0.05 to 1.0 mg/ml; 0.05
to 2.5 mg/ml; 0.1
to 0.25 mg/ml; 0.1 to 0.5 mg/ml; 0.1 to 1 mg/ml; 0.1 to 2.5 mg/ml; 0.1 to 5
mg/ml; 0.15 to 0.45
mg/ml; 0.15 to 0.5 mg/ml; 0.25 to 0.35 mg/m1;0.25 to 0.5 mg/ml; 0.25 to 1
mg/ml; 0.25 to 2.5
mg/ml; 0.25 to 5 mg/ml; 0.35 to 0.5 mg/ml; 0.35 to 1 mg/ml; 0.35 to 2.5 mg/ml;
0.35 to 5 mg/ml;
7
Date Recue/Date Received 2023-08-28
0.5 to 1mg/m1; 0.5 to 2.5 mg/ml; 0.5 to 5 mg/ml; 1 to 2.5 mg/ml; 1 to 5 mg/ml;
about 0.05
mg/ml; about 0.1 mg/ml; about 0.15 mg/ml; about 0.25 mg/ml; about 0.35 mg/ml;
about 0.45
mg/ml; and about 0.5 mg/ml.
In other embodiments, low sugar media may comprise one or more tricarboxylic
acid
cycle intermediates, including but not limited to, pyruvate, acetyl-CoA,
citrate, isocitrate, a-
ketoglutarate, succinyl-CoA, succinate, fumarate, malate, oxaloacetate, and
derivatives thereof,
including but not limited to isomers and acids. In a particular embodiment,
low sugar media
comprises two or more tricarboxylic acid cycle intermediates, including but
not limited to,
pyruvate, acetyl-CoA, citrate, isocitrate, a-ketoglutarate, succinyl-CoA,
succinate, fumarate,
malate, oxaloacetate, and derivatives thereof, including but not limited to
isomers and acids.
Certain embodiments of the invention utilize concentrations of a tricarboxylic
acid cycle
intermediate selected from the following ranges: 0.01 to 5.0 mg/ml; 0.01 to
0.25 mg/ml; 0.01 to
0.5 mg/ml; 0.01 to 1 mg/ml; 0.01 to 2.5 mg/ml; 0.01 to 5 mg/ml; 0.05 to 0.1
mg/ml; 0.05 to 1.0
mg/ml; 0.05 to 2.5 mg/ml; 0.1 to 0.25 mg/ml; 0.1 to 0.5 mg/ml; 0.1 to 1 mg/ml;
0.1 to 2.5 mg/ml;
0.1 to 5 mg/ml; 0.15 to 0.45 mg/ml; 0.15 to 0.5 mg/ml; 0.25 to 0.35 mg/m1;0.25
to 0.5 mg/ml;
0.25 to 1 mg/ml; 0.25 to 2.5 mg/ml; 0.25 to 5 mg/ml; 0.35 to 0.5 mg/ml; 0.35
to 1 mg/ml; 0.35 to
2.5 mg/ml; 0.35 to 5 mg/ml; 0.5 to 1mg/m1; 0.5 to 2.5 mg/ml; 0.5 to 5 mg/ml; 1
to 2.5 mg/ml; 1
to 5 mg/ml; about 0.05 mg/ml; about 0.1 mg/ml; about 0.15 mg/ml; about 0.25
mg/ml; about
0.35 mg/ml; about 0.45 mg/ml; and about 0.5 mg/ml.
In other embodiments, low sugar media may comprise one or more
cryoprotectants,
including but not limited to, propylene glycol, dimethyl sulfoxide, ethylene
glycol and glycerol,
or a combination thereof. In certain embodiments, low sugar media may comprise
a
concentration of cryoprotectant by percent volume selected from the following:
less than 1%; 1-
5%; 5%; 5 to 10%; 10%; 10 to 20%; 16.7%; 20%; 20 to 30%; or 30 to 40%.
In a further embodiment, low sugar media may comprise one or more
antioxidants,
including but not limited to catalase, superoxide dismutase (SOD), SOD mimics,
glutathione,
glutathione reductase, glutathione peroxidase, pyruvate, mercaptoethanol,
8
Date Recue/Date Received 2023-08-28
butylatedhydroxytoluene (BHT), lipoic acid, flavonoids, phenolic acids and
their esters,
quinines, vitamin A (and related vitamers), vitamin C (and related vitamers),
vitamin K (and
related vitamers), vitamin B12 (and related vitamers), with "vitamers" defined
as compounds
having the same vitamin activity (such as cobalamin, cyanocobalamin,
methylcobalamin,
adenosylcobalamin, hydroxocobalamin, and pseudo-B12), vitamin E (including its
vitamers,
tocopherols (a, 13, y), tocotrienols, and a-tocopheryl), a-ketoglutarate (also
known as a-KG, AKG
or oxo-glutarate) and various biological forms of AKG (such as arginine,
aspartate, lysine, and
similar derivatives), coenzyme Q, manganese, iodide, melatonin and carotenoid
terpenoids.
Certain embodiments of the invention utilize concentrations of an antioxidant
selected
from the following ranges: 0.01 to 5.0 mg/ml; 0.01 to 0.25 mg/ml; 0.01 to 0.5
mg/ml; 0.01 to 1
mg/ml; 0.01 to 2.5 mg/ml; 0.01 to 5 mg/ml; 0.05 to 0.1 mg/ml; 0.05 to 1.0
mg/ml; 0.05 to 2.5
mg/ml; 0.1 to 0.25 mg/ml ; 0.1 to 0.5 mg/ml; 0.1 to 1 mg/ml; 0.1 to 2.5 mg/ml;
0.1 to 5 mg/ml;
0.15 to 0.45 mg/ml; 0.15 to 0.5 mg/ml; 0.25 to 0.35 mg/m1;0.25 to 0.5 mg/ml;
0.25 to 1 mg/ml;
0.25 to 2.5 mg/ml; 0.25 to 5 mg/ml; 0.35 to 0.5 mg/ml; 0.35 to 1 mg/ml; 0.35
to 2.5 mg/ml; 0.35
to 5 mg/ml; 0.5 to lmg/m1; 0.5 to 2.5 mg/ml; 0.5 to 5 mg/ml; 1 to 2.5 mg/ml; 1
to 5 mg/ml; about
0.05 mg/ml; about 0.1 mg/ml; about 0.15 mg/ml; about 0.25 mg/ml; about 0.35
mg/ml; about
0.45 mg/ml; and about 0.5 mg/ml.
In yet another embodiment, low sugar media may comprise one or more protein
sources,
including but not limited to, egg yolk, egg yolk extract, milk (including heat
homogenized and
skim), milk extract, soy protein, soy protein extract, serum albumin, bovine
serum albumin,
human serum substitute supplement, seminal proteins, such as, for example,
whole seminal
plasma or seminal plasma extracts, and derivatives thereof. In certain
embodiments, low sugar
media may comprise a concentration of protein source by percent volume
selected from the
following: 1-5%; 5%; 5 to 10%; 10%; 10 to 20%; 16.7%; 20%; 20 to 30%; or 30 to
40%.
In a further embodiment of the invention, low sugar media may comprise one or
more
antimicrobial or antibiotic agents, including but not limited to, tylosin,
gentamicin, lincomycin,
spectinomycin, Linco-Spectin0 (lincomycin hydrochloride-spectinomycin),
penicillin,
streptomycin, ticarcillin, polymyxin B, and their derivatives. If included,
the antibiotics may be
9
Date Recue/Date Received 2023-08-28
present in a concentration of about 501.1g to about 8001.1g per ml of semen.
In another
embodiment low sugar media may comprise a detergent, including but not limited
to, an alkyl
ionic detergent such as sodiumdodecyl sulfate (SDS).
In another embodiment, low sugar media may comprise one or more salts,
including but
not limited to, NaCl, KC1, MgCl2, CaCl2 and any combination of a salt-forming
anion, including
but not limited to acetate (CH3C00-), carbonate (C032-), chloride (Cr),
citrate
(HOC(C00-)(CH2C00-)2), fluoride (F-), nitrate (NO3), nitrite (NO2), phosphate
(P043-) and
sulfate (S042-) and a salt-forming cation, including but not limited to,
ammonium (NH4),
calcium (Ca2+), iron (Fe2+ and Fe3+), magnesium (Mg2+), potassium (IC),
pyridinium (C5H5NH ),
quaternary ammonium NR4+ and sodium (Nat).
In yet another embodiment, low sugar media may comprise one or more growth
factors
including but not limited to transforming growth factors ("TGF"), such as, for
example, TG93-1
and TGFP-2, and insulin-like growth factors ("IGF"), such as for example, IGF-
1.
Another embodiment of the invention encompasses a staining solution comprising
low
sugar media and a DNA selective dye. DNA selective dyes for use with the
invention include
but are not limited to UV light excitable, selective dyes, such as Hoechst
33342 and Hoechst
33258 and visible light excitable dyes, such as SYBR-14 and bisbenzimide-
BODIPYO conjugate
6- { [3 -((2Z)-2- { [ 1 -(di fluorobory1)-3 ,5 -dimethyl- 1H-pyrrol-2y1]
methylene} -2H-pyrrol-5-
yl)propan oyl] amino 1 -N-[3- (methyl {3 - [( {4- [6- (4-m ethylpiperazin- 1-
y1) -1H,3 'H-
2,5 'bibenzimidazol-2 '- yl]phenoxy } ac etyl)amino]propyl }
amino)propyl]hexanami de . Each of
these dyes may be used alone or in combination. In another embodiment, the
staining solution
further comprises one or more dye quenchers, including but not limited to F&DC
red food dye
No. 40 and yellow food dye No. 4. In yet another embodiment, the staining
solution further
comprises one or more OSRs.
Date Recue/Date Received 2023-08-28
In one embodiment of the invention, the concentration of dye in the staining
solution is
from about 0.1 M to about 1.0M; from about 0.1 M to about 1000 M; from about
100 M to
about 500 M; from about 200 M to about 500 M; from about 300 M to about 450 M;
about
350 M; about 400 M; or about 450 M.
The pH of low sugar media of the invention may be maintained at any of a range
of pHs
suitable for the particular process and sperm type. In certain embodiments,
this will be in the
range of about 5.0 to about 9.0; in the range of 5.5 to 7.8; from about 5.0 to
about 7.0; from
about 6.0 to about 7.0; from about 6.0 to about 6.5; about 6.2, about 6.5;
about 6.6; about 6.7;
about 6.8; about 6.9; or about 7.0; from about 7.0 to about 9.0; from about
7.0 to about 8.0; from
about 7.0 to about 7.5; about 7.0; about 7.1; about 7.2; about 7.3; about
7.35; about 7.4; or about
7.5.
In certain embodiments, low sugar media may be combined with a sperm sample to
form
a sperm composition. The term "sperm sample" may comprise a processed semen
sample or an
unsorted, conventional semen sample. In some embodiments of the invention, the
sperm
composition comprising low sugar media is used with ART. Such ART techniques
involve
different levels of gamete cell processing which in the case of sperm can
entail, by example only
and is not limited to one or more of the following: artificially collecting a
semen sample from the
male animal that may involve natural, electronic or other types of sexual
stimulation; holding;
transporting; buffering with different pHs; chilling; warming; staining;
diluting; concentrating;
energetically exciting as with a laser; electronic charging; deflecting;
ablating to kill unwanted
cells usually with targeted lasers; sorting; collecting; shaking; oscillating;
magnetically
separating; oxygenating as associated with microchip sorting procedures;
labeling; precipitating;
centrifuging; resuspending; mixing; dialyzing; cryostabilizing; freezing;
vitrifying;
cryopreserving; thawing; culturing; inseminating; microinjecting; microfluidic
processing;
microchip processing; jet and air processing; flow cytometry processing; and
similar handling
techniques. Whereas a single processing step may exert only minimal stress on
a sperm, others
or a combination may add significant stress, often killing the cell. An
example is the sex sorting
process used to separate X- from Y-chromosome bearing cells; the sorting
process combines a
11
Date Recue/Date Received 2023-08-28
large number of independent stressful steps that compromise the overall
integrity of the sorted
sperm population.
In some embodiments of the invention, a sperm composition comprising a sperm
sample
and low sugar media can be used immediately or processed within the first few
minutes after the
sperm sample is added to the low sugar media for whatever processing step is
needed, whereby
the holding period would be in the range 2 sec to 3 min. In other embodiments,
holding periods
can be short, as in the range of a 3-15 minutes, moderate as in the range of
15 min to 1 hr; and
longer processing periods ranging up to about 8 hrs or overnight for extensive
processing such as
with sex sorting techniques. Transportation hold periods associated with
transporting unfrozen
sperm compositions can be much longer, extending up to a few days, which may
for example
occur if the sperm sample is collected, added to the low sugar media,
transported or shipped to
another location possibly by air, and further processed at the second location
as for sex sorting at
a designated facility. In other instances, the sperm composition might need to
be held for a few
days while a recipient female is hormonally prepped for artificial
insemination, as might occur if
a sample is mistakenly thawed and cannot be refrozen. The use of low sugar
media could
theoretically prolong these extended hold periods over what is currently
accepted in the art, and
could provide sufficient protection to the sperm in the sperm composition so
that they could
remain viable and fertile for up to a week or more.
In some embodiments of the invention, low sugar media is used several times
during a
complex processing procedure to minimize cell stress throughout the procedure.
In other
embodiments, low sugar media is used only at one or more particular steps
which are notably
harsh on the cells to help minimize stress and fatigue on the sperm. By way of
example, the
staining process during sex sorting is often performed at non-physiological pH
and at elevated
temperatures, both known to be harsh on the cells. Similarly, cryopreservation
is also extremely
harsh on the cells and disrupts cell membranes, both internal and external.
Following an
intensive multi-step sorting procedure, sex sorted sperm which are already
compromised are
even more susceptible to cryogenic and freeze processing.
12
Date Recue/Date Received 2023-08-28
A further embodiment of the present invention provides a method of improving
the
motility, viability and/or fertility of a sperm sample that has already
undergone a sorting process,
including but not limited to sex sorting, comprising the step of contacting or
adding a sorted
sperm sample to low sugar media that may also comprise at least one OSR in the
concentration
range of 0.01 mg/ml to 5 mg/ml to form a sperm composition.
Another broad object of the present invention is to improve the motility,
viability
(including longevity and ability to survive environmental stress) and
fertility of processed and/or
sorted sperm for use in ART such as IVF, AT, ICSI (as well as other techniques
using enucleated
cells), and MOET (as well as other embryo transfer techniques). Some
embodiments of the
invention encompass low sugar media comprising a sorted or processed sperm
sample, and
optionally at least one OSR in the range of 0.01 mg/ml to 5 mg/ml, for use in
ART. An
additional embodiment of the invention encompasses a frozen or vitrified sperm
composition
comprising low sugar media, a processed or sorted sperm sample, and
optionally, at least one
OSR in the concentration range of 0.01 mg/ml to 5 mg/ml.
A further embodiment of the invention resides in a method of making an embryo
comprising mixing at least one egg with at least one sperm contacted with low
sugar media,
which may also comprise at least one OSR in the concentration range of 0.01
mg/ml to 5 mg/ml.
The embryos produced by this method constitute a further embodiment of the
invention.
Another embodiment of the invention includes a method for inseminating an
organism
through an AT technique using a processed or sorted sperm sample contacted
with low sugar
media that may also comprise at least one OSR in the concentration of 0.01
mg/ml to 5 mg/ml.
The progeny of the organism that results from the aforementioned AT method
also constitutes an
embodiment of the invention. A further embodiment of the invention encompasses
a method for
recovering embryos that are produced from the aforementioned AT method.
Embodiments of the invention can include sperm, or spermatozoa, collected from
numerous species of male animals, and the invention should be understood not
to be limited to
the species of male animals described by the specific examples within this
application. Rather
13
Date Recue/Date Received 2023-08-28
the specific examples within this application are intended to be illustrative
of the varied and
numerous species of male animals from which semen can be collected and
utilized in certain
embodiments of the invention. Embodiments of the invention, for example, may
include the
sperm of humans as well as animals having commercial value for meat or dairy
production such
as swine, ovine, bovine, equine, deer, elk, buffalo, or the like (naturally
the mammals used for
meat or dairy production may vary from culture to culture). It may also
include the sperm of
various domesticated mammalian species encompassed by canines and felines, as
well as sperm
of primates, including but not limited to chimpanzees, gorillas, or humans and
the spermatozoa
from whales, dolphins and other marine mammals. It may also include frozen-
thawed sperm
from all the various mammals above-described and further, including but not
limited to, the
sperm of deceased donors, from rare or exotic mammals, zoological specimens,
or endangered
species.
A particular embodiment of the invention comprises a method of sorting a sperm
sample
to form one or more subpopulations comprising the steps of providing a sperm
sample, sorting
the sperm sample to form one or more subpopulations and using a low sugar
media during one or
more of the aforementioned sorting steps. In the context of sorting sperm
using a flow
cytometer, for example, low sugar media may be used in a diluent for diluting
sperm, a staining
solution for staining the sperm with, for example, a DNA selective dye, a
sheath fluid for
encapsulating the core stream containing the sperm as it passes through the
flow cytometer, a
catch media for receiving one or more of the sorted sperm subpopulations, or a
resuspension
media for resuspending processed sperm.
Another embodiment of the invention encompasses a method of sorting sperm
comprising staining the sperm with a staining solution; sorting the sperm
using a flow cytometer
with a sheath fluid into one or more subpopulations, wherein at least 60% of
sperm in one of the
one or more subpopulations bear X chromosomes or bear Y chromosomes; and
collecting the
one of the one or more subpopulations in a catch media; wherein the staining
solution, the sheath
fluid or the catch media is low sugar and may further comprise one or more
OSRs. In a certain
embodiment of the invention, any combination of the staining solution, the
sheath fluid and the
catch media may be sugar free and comprise one or more OSRs. In further
embodiments, the
14
Date Recue/Date Received 2023-08-28
one or more OSRs may be different or identical between the staining solution,
the sheath fluid
and the catch media, and in other embodiments, the one or more OSRs may be
identical between
the staining solution, the sheath fluid and the catch media. In a further
embodiment, a diluent or
resuspension media used for processing the sperm is also sugar free and may
also comprises one
or more OSRs. In a yet further embodiment, the staining solution, the sheath
fluid or the catch
media is low sugar and comprises a buffer, a chelator and a plurality
tricarboxylic acid cycle
intermediates.
In another embodiment of the invention, low sugar media, including but not
limited to
diluents, staining solutions, sheath fluids, catch media, and resuspension
media, shifts sperm
metabolism away from glycolytic metabolism and towards tricarboxylic acid
cycle metabolism.
The invention also encompasses a method of processing sperm comprising
contacting the
sperm with low sugar media comprising a cryoprotectant and optionally, one
more OSRs,
wherein the low sugar media comprising the cryoprotectant has been frozen and
thawed prior to
contacting with the sperm. A further embodiment of the inventions encompasses
low sugar
media comprising a cryoprotectant, wherein the low sugar media comprising the
cryoprotectant
has been frozen and thawed prior to addition of the sperm. An additional
embodiment of the
invention encompasses low sugar media used in processing sperm that comprises
at least one
antioxidant and/or OSR at the appropriate stock concentration to be present at
a final processing
concentration in the range of 0.01 mg/ml to 5 mg/ml in the sperm composition
at the time of
processing. In some embodiments, low sugar media can be used for different
processes
including but not limited to sperm collection, artificial insemination, sperm
sorting, in vitro
fertilization, embryo culture, as well as sperm and embryo freezing. In
particular embodiments,
low sugar media used in the sorting of sperm typically comprises one or more
buffers and/or
extenders (i.e., substances that preserve the viability and/or fertility of
sperm).
In another embodiment, the invention encompasses low sugar media that is cell-
free and
comprises a cryoprotectant, wherein the low sugar media has been frozen and
then thawed. In a
further embodiment, the low sugar media may also comprise one or more OSRs.
For purposes of
the aforementioned embodiments, the term "cell-free" means the media is free
of cells collected
Date Recue/Date Received 2023-08-28
from a subject (e.g., sperm and blood cells), processed cells and laboratory
grown or cultured
cells.
In a further embodiment, the invention encompasses a method of processing
sperm
comprising the steps of forming a stream comprising sperm and a first media;
determining a
property of said sperm in said stream; selecting sperm having a property of
interest from said
sperm in said stream; and collecting said sperm having said property of
interest in a second
media, wherein the first or second media comprise low sugar media. In certain,
embodiments,
the property of interest may be the presence of an X or Y chromosome. In a
further embodiment,
the first and second media are identical in terms of their components and
relative concentrations
of those components. In another embodiment, the first and second media are
different in terms
of their components and/or relative concentrations of those components. In yet
another
embodiment, the step of selecting sperm having a property of interest from
said sperm in said
stream comprises photo-damaging said sperm having said property of interest or
isolating said
sperm having said property of interest. In yet another embodiment, the
aforementioned method
further comprises the step of resuspending the sperm having the property of
interest (after, for
example, a cell concentrating step or centrifugation) in a third media
comprising low sugar
media.
In another embodiment, the invention encompasses a method of processing sperm
comprising freezing and thawing a media comprising low sugar media and a
protein source; and
contacting sperm with the media. In a particular embodiment, the protein
source comprises egg
yolk.
An additional embodiment of the invention encompasses a composition comprising
sperm and low sugar media and a protein source, wherein the media has been
frozen and thawed
prior to addition of the sperm. In yet a further embodiment, the protein
source comprises egg
yolk.
16
Date Recue/Date Received 2023-08-28
Another embodiment of the invention encompasses a composition comprising a
gender
enriched sperm population and low sugar media. In a further embodiment, the
composition
further comprises a protein source, such as egg yolk.
BRIEF DESCRIPTION OF THE DRAWINGS
In order that the invention may be more clearly understood, various
embodiments of the
present invention will now be described by way of example only with reference
to the
accompanying sheets of drawings wherein:
Figure 1 is a schematic representation of part of a flow cytometer
illustrating a method of sorting
a sperm sample into one or more subpopulations according to some embodiments
of the present
invention.
Figure 2 is a graphical depiction of the post-sort sperm motility of different
treatment groups in
Example 1 over time.
Figure 3 is a graphical depiction of the post-sort sperm motility of different
treatment groups in
Example 1.
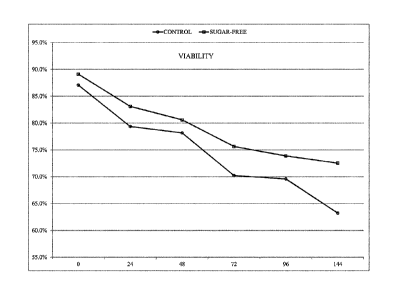
Figure 4 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 1 over time.
Figure 5 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 1.
Figure 6 is a graphical depiction of the post-sort sperm motility of different
treatment groups in
Example 2 over time.
Figure 7 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 2 over time.
17
Date Recue/Date Received 2023-08-28
Figure 8 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 2.
Figure 9 is a graphical depiction of the post-sort sperm motility of different
treatment groups in
Example 3 over time.
Figure 10 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 3 over time.
Figure 11 is a graphical depiction of the post-sort sperm motility of
different treatment groups in
Example 4 over time.
Figure 12 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 4 over time.
Figure 13 is a graphical depiction of the percent intact acrosomes of
different treatment groups in
Example 4 over time.
Figure 14 is a graphical depiction of the post-sort sperm motility of
different treatment groups in
Example 5 over time.
Figure 15 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 5 over time.
Figure 16 is a graphical depiction of the percent intact acrosomes of
different treatment groups in
Example 5 over time.
Figure 17 is a graphical depiction of the post-sort sperm motility of
different treatment groups in
Example 6 over time.
18
Date Recue/Date Received 2023-08-28
Figure 18 is a graphical depiction of the post-sort sperm viability of
different treatment groups in
Example 6 over time.
Figure 19 is a graphical depiction of the dead intact acrosomes of different
treatment groups in
Example 6 over time.
DETAILED DESCRIPTION OF THE INVENTION
The invention broadly encompasses methods and compositions comprising low
sugar
media for processing sperm. It has been discovered that the use of low sugar
media for
processing sperm improves the overall quality of the sorted sperm, including
but not limited to
increased motility, viability and fertility. It has further been discovered
that the addition of one
or more OSRs to low sugar media provides an additional improvement to the
quality of
processed sperm. Another aspect of the invention relates to the discovery that
the use of frozen-
thawed, low sugar catch media comprising a cryoprotectant improves the
viability of processed
sperm.
Processing steps to which sperm are commonly subjected, and with which the
invention
may be used, include, but are not limited to, collecting from a male animal,
which may involve
natural, electronic or other types of sexual stimulation; holding;
transporting; buffering; chilling;
warming; staining; diluting; concentrating; energetically exciting (as with a
laser, for example);
electronic charging; deflecting; ablating to kill unwanted cells usually with
targeted lasers;
sorting; collecting; shaking; oscillating; magnetically separating;
oxygenating as associated with
microchip sorting procedures; labeling; precipitating; centrifuging;
resuspending; mixing;
dialyzing; cryostabilizing; freezing; vitrifying; cryopreserving; thawing;
culturing; inseminating;
microinjecting; microfluidic processing; microchip processing; jet and air
processing; flow
cytometry processing; and similar handling techniques.
Regardless of how sperm are to be ultimately utilized, the initial processing
step is
typically collection of a sperm sample from a male. Generally, the sperm
sample is collected
into an extender or diluent designed to sustain the cells until further
processing or use.
19
Date Recue/Date Received 2023-08-28
Alternatively, semen is collected and then subsequently diluted with an
extender after collection.
One embodiment of the invention encompasses an extender or diluent that
comprises low sugar
media, which can be used as a storage media or with ART.
Whereas a single processing step, such as collection, may exert only minimal
stress on
sperm, others or a combination may add significant stress, often killing the
cells. An example is
the sex sorting process used to separate X- from Y-chromosome bearing cells;
the sorting
process combines a large number of independent stressful steps that compromise
the overall
integrity of the sorted sperm population. Accordingly, in a particular
embodiment of the
invention, low sugar media is used in the sorting process, including but not
limited to the staining
solution, sheath fluid and catch media, and in the cryopreservation of such
processed cells.
I. Collecting Sperm
It is contemplated that intact viable bovine, porcine, equine, ovine, cervine,
murine or
other mammalian sperm, may be collected and contacted with low sugar media.
Various
methods of collection of viable sperm are known and include, for example, the
gloved-hand
method, use of an artificial vagina, and electro-ejaculation. As an example, a
bovine sperm
sample, typically containing about 0.5 to about 10 billion sperm per
milliliter, may be collected
directly from the source mammal, or from more than one source mammal of the
same species,
into a vessel containing low sugar media to form a sperm composition. The low
sugar media
may optionally comprise one or more OSRs, which may be present as constituents
of the low
sugar media prior to contacting with the sperm, or which may be added to the
sperm
composition, each OSR in the concentration range of 0.01 mg/ml to 5 mg/ml.
Once the sperm composition is in the laboratory, various quality checks can be
conducted, including checking the motility (e.g., via CASA System), viability
(e.g., via flow
cytometer), morphology (e.g., via microscopy) and concentration (e.g., via
NucleoCounter).
Sperm compositions that pass these quality checks can then be prepared for
further processing,
such as sorting. A comparison of viewing chambers and slides can be done in a
variety of IVOS
instruments, which for example only can be a Hamilton-Thorne IVOS (Hamilton-
Thorne,
Date Recue/Date Received 2023-08-28
Beverly, MA). Instrument settings may be set as follows: image capture; frames
per second =
60; number of frames = 30; cell detection; minimum contrast = 50; minimum cell
size = 5;
defaults, cell size = 5; cell intensity = 50; progressive cells, path velocity
= 50 um/s; straightness
> 70%; slow cells (um/s); average path velocity (VAP, <30 um/s), straight-line
velocity (VSL,
<15 um/s). The CASA motility variables measured can be a percentage of total
motile sperm
(motile), percentage of progressively motile sperm (progressive), VAO, VSL,
curvilinear
velocity (VCL, um/s), average lateral head displacement (ALK, um) and the
number of times the
sperm head crosses the mean path/s (BCF, Hz), straight-line sperm motility
(STR, %), and linear
sperm motility (UN, %). See for instance, Lenz, RW, et al., J AnimSci (2011)
89:383-388.
Various OSRs can be used in the context of the current invention, including
but not
limited to catalase, superoxide dismutase (SOD), SOD mimics, glutathione,
glutathione
reductase, glutathione peroxidase, pyruvate, mercaptoethanol, butylated
hydroxytoluene (BHT),
lipoic acid, flavins, quinines, vitamin K (and related vitamers), vitamin B12
(and related
vitamers), with `vitamers' defined as compounds having the same vitamin
activity (such as
cobalamin, cyanocobalamin, methylcobalamin, adenosylcobalamin,
hydroxocobalamin, and
pseudo-B12), vitamin E (including its vitamers, tocopherols (a, 13, y),
tocotrienols, and a-
tocopheryl), alpha-ketoglutarate (also known as a-KG, AKG or oxo-glutarate)
and various
biological forms of AKG (such as arginine, aspartate, lysine, and similar
derivatives), other
compounds that regulate nitric oxide in the cell including malondialdehyde
(MDA) and
asymmetric dimethylarginine (ADMA); and biologically active derivatives
thereof.
Alternatively, the semen sample may be collected into an empty vessel and then
subsequently contacted with low sugar media within several minutes to hours
after collection to
form the sperm composition. In addition to a buffer, the sperm composition may
also contain a
range of additives, including but not limited to the aforementioned OSRs,
chelators, tricarboxylic
acid cycle intermediates, cryoprotectants, sterols, lipids, fatty acids,
protein sources, antibiotics,
growth factors, caproic acid, catalase, Caprogen (caproic acid, catalase, and
5% egg yolk)
detergents, including alkyl ionic detergents such as sodiumdodecyl sulfate
(SDS). It should be
noted that certain substances may be classified in one or more of the above
listed categories of
21
Date Recue/Date Received 2023-08-28
additives. For example, citrate may be considered both a tricarboxylic acid
cycle intermediate
and a buffer.
Exemplary buffers for use in the invention include, but are not limited to,
carbonates,
phosphates, citrates, acetates, lactates, and combinations thereof. Specific
buffers that may be
used include, but are not limited to, Tris, TES, Pipes, HEPES, TALP, TCA, PBS,
citrate, milk
and derivatives thereof, which are discussed in detail in U.S. Patent
7,208,265.
Exemplary chelators for use in the invention include, but are not limited to,
deferoxamine, deferasirox, penicillamine, alpha lipoic acid, DMPS, DMSA,
dimercaprol and
aminopolycarboxylic acids (complexones), including but not limited to Fura-2,
IDA, NTA,
EDTA, DTPA, EGTA, BAPTA, NOTA, DOTA and nicotianamine, and derivatives
thereof.
Exemplary tricarboxylic acid cycle intermediates for use in the invention
include, but are
not limited to, pyruvate, acetyl-CoA, citrate, isocitrate, a-ketoglutarate,
succinyl-CoA, succinate,
fumarate, malate, oxaloacetate, and derivatives thereof, including but not
limited to isomers and
acids. In a particular embodiment, low sugar media comprises two or more
tricarboxylic acid
cycle intermediates, including but not limited to, pyruvate, acetyl-CoA,
citrate, isocitrate, a-
ketoglutarate, succinyl-CoA, succinate, fumarate, malate, oxaloacetate, and
derivatives thereof,
including but not limited to isomers and acids.
Exemplary cryoprotectants for use in the invention include but are not limited
to
propylene glycol, dimethyl sulfoxide, ethylene glycol and glycerol, or a
combination thereof. In
certain embodiments, low sugar media may comprise a concentration of
cryoprotectant by
percent volume (w/v) selected from the following: 1-5%; 5%; 5 to 10%; 10%; 10
to 20%; 16.7%;
20%; 20 to 30%; or 30 to 40%.
Exemplary protein sources for use in the invention include egg yolk, egg yolk
extract,
milk (including heat homogenized and skim), milk extract, soy protein, soy
protein extract,
serum albumin, bovine serum albumin, human serum substitute supplement,
seminal proteins,
22
Date Recue/Date Received 2023-08-28
such as, for example, whole seminal plasma or seminal plasma extracts (see,
for example, Parks
et al., Sperm Membrane Phospholipid Peroxidation and Fragmentation: Effects on
Sperm
Function and Role of Seminal Plasma PAF-Acetylhydrolase, Proceedings of the
16th Technical
Conference on Artificial Insemination & reproduction, 1996), and combinations
thereof. In
certain embodiments, low sugar media may comprise a concentration of protein
source by
percent volume selected from the following: 1-5%; 5%; 5 to 10%; 10%; 10 to
20%; 16.7%; 20%;
20 to 30%; or 30 to 40%. Albumin, and more particularly bovine serum albumin
(BSA), is a
commonly used protein source. For example, if included, BSA may be present in
the sperm
composition in an amount of, less than about 5.0% (w/v); less than about 2%
(w/v); less than
about 1% (w/v); or about 0.1% (w/v).
The use of a protein source, such BSA, alone may initiate the process of
capacitation in a
percentage of the sperm in the composition. It is generally preferred that
this process take place
in the female reproductive tract. Therefore, in order to inhibit the
initiation of capacitation
during dilution, as well as during subsequent processing step such as staining
and sorting, an
alternative protein source or a protein substitute may be included in the
sperm composition. The
alternative protein source or protein substitute possess the advantageous
effects of a typical
protein source, such as BSA, in addition to the ability to inhibit the
initiation of capacitation in a
larger percentage of the cells in the sperm composition. Examples of a
alternative protein
sources includes human serum substitute supplement (SSS) (Irvine Scientific,
Santa Ana, CA)
and cholesterol enhanced BSA, while an example of a protein substitute
includes a polyvinyl
alcohol, such as for example, a low to medium viscosity polyvinyl alcohol
generally of a
molecular weight of about 30,000 to about 60,000. Generally, if included,
these compositions
will be present in the same amounts as disclosed above with respect to BSA,
with the total
albumin content of the buffer or buffered solution generally not exceeding
about 5.0% (w/v).
An antibiotic may be included in the sperm composition in order to inhibit
bacterial
growth. Exemplary antibiotics include, for example, tylosin, gentamicin,
lincomycin,
spectinomycin, Linco-Spectin0 (lincomycin hydrochloride-spectinomycin),
penicillin,
streptomycin, ticarcillin, polymyxin B, or any combination thereof. If
included, the antibiotics
23
Date Recue/Date Received 2023-08-28
may be present in a concentration of about 501.1g to about 8001.1g per ml of
semen, regardless of
whether the semen is neat, buffered, or contains additional substances, such
as for example, any
of the additives mentioned herein. The Certified Semen Services (CSS) and
National Association
of Animal Breeders (NAAB) have promulgated guidelines regarding the use of
antibiotics with
respect to sperm collection and use.
A growth factor may be added to the sperm composition in order to help
maintain the
viability of the sperm. Exemplary growth factors include, for example,
transforming growth
factors ("TGF"), such as, for example, TG93-1 and TG93-2, and insulin-like
growth factors
("IGF"), such as for example, IGF-1. Generally, TGF may be present in the
sperm composition
in the form of TG93-1 in a concentration of about 0.1ng/L to about 101.1g/L or
as TG93-2 in a
concentration of about 0.1ng/L to about 200ng/L, and IGF may be present in the
sperm
composition in the form of IGF-1 in a concentration of about 0.1 ng/L to about
501.1g/L. The use
of such growth factors is well known in the art and is disclosed, for example,
in U.S. Patent
Application Publication No. 2003/0157473.
Once collected, the sperm may be stored in low sugar media for a desired
period of time
or alternatively, may be used and/or further processed within several hours.
In either event, the
cells may be used, for example, in a staining process, a sorting process, or a
fertilization process.
It is contemplated that any such further use and/or processing of the sperm
may utilize low sugar
media.
II. Sorting of Collected Sperm
A. Staining of the Cells
One embodiment of the invention encompasses the use of low sugar media in a
staining
solution for sperm. A process of staining sperm typically comprises the
formation of a staining
solution containing intact viable sperm and a dye, sometimes referred to as a
label. In this aspect
of the invention, the low sugar media may be contacted with the sperm to form
a sperm
24
Date Recue/Date Received 2023-08-28
composition, and then the sperm composition contacted with a DNA selective dye
to form the
staining solution. Alternatively, a DNA selective dye may be added to a low
sugar media to
form a staining solution, with sperm subsequently added to the staining
solution.
In this embodiment, the sperm source may be neat semen, or alternatively, a
sperm-
containing semen derivative obtained by centrifugation or the use of other
means to separate
semen into fractions.
The pH of the staining solution may be maintained at any of a range of pHs;
typically this
will be in the range of about 5.0 to about 9.0, or in the range of 5.5 to 7.8.
The staining solution
may be maintained at a slightly acid pH, i.e., from about 5.0 to about 7Ø
Typically, the pH is
from about 6.0 to about 7.0; from about 6.0 to about 6.5; about 6.2, about
6.5; about 6.6; about
6.7; about 6.8; about 6.9; or about 7Ø Alternatively, the staining solution
may be maintained at
a slightly basic pH, i.e., from about 7.0 to about 9Ø Typically, the pH is
about 7.0 to about 8.0;
about 7.0 to about 7.5; about 7.0; about 7.1; about 7.2; about 7.3; about
7.35; about 7.4; or about
7.5.
The staining solution may be formed by using one or more UV or visible light
excitable,
DNA selective dyes as previously described in U.S. Patent No. 5,135,759 and WO
02/41906.
Exemplary UV light excitable, selective dyes include Hoechst 33342 and Hoechst
33258.
Exemplary visible light excitable dyes include SYBR-14 and bisbenzimide-
BODIPYO conjugate
6-{ [3-((2Z)-2-{ [1-(difluorobory1)-3,5-dimethy1-1H-pyrrol-2y1] methylene} -2H-
pyrrol-5-
yl)propanoyl] amino 1 -N-[3- (methyl {3- [({4- [6- (4-methylpiperazin-1-y1) -
1H,3'H-
2,5'bibenzimidazol-2'- yl]phenoxylacetyl)amino]propyllamino)propyl]hexanamide
("BBC")
described in WO 02/41906. Each of these dyes may be used alone or in
combination;
alternatively, other cell permeant UV and visible light excitable dyes may be
used, alone or in
combination with the aforementioned dyes, provided the dye does not
detrimentally affect the
viability of the sperm to an unacceptable degree when used in concentrations
which enable
sorting or enrichment as described elsewhere.
Date Recue/Date Received 2023-08-28
The staining solution may also comprise a dye quencher in addition to a DNA
selective
dye. Staining protocols for sex sorting, or even bulk sorting, sperm typically
rely upon the
inclusion of F&DC red food dye No. 40 ("red food dye No. 40" or "red 40")
and/or yellow food
dye No. 4 as quenching dyes. The maximal absorbance wavelengths of these
quenching dyes
overlaps the maximal emissions wavelengths of fluorescent dyes, including
Hoechst 33342 when
bound to nuclear or chromosomal DNA. Because red food dye No. 40 and yellow
food dye No.
4 differentially permeate membrane-compromised sperm and overlap the emission
spectra of the
DNA selective fluorescent dye, FRET (florescence resonance energy transfer)
between the light
leaving the DNA-stain complex and the dead quenching dye reduces the overall
detected
intensity of the light emitted from membrane compromised sperm. The quenched,
or dampened,
fluorescence from these cells provide fewer photons to the detectors resulting
in a distinctly
lower signal. This distinctly lower signal results in a noticeable separated
subpopulation which
allows the exclusion ("gating out") of the membrane compromised sperm during
the sorting
procedure. Since membrane compromised sperm comprises largely non-viable
sperm, excluding
these cells from the analysis results in an enriched sperm subpopulation with
respect to viability
in the sex sorted subpopulation.
The staining solution may be formed using fluorescent polyamides, and more
specifically
polyamides with a fluorescent label or reporter conjugated thereto. Such
labels will fluoresce
when bound to nucleic acids. Examples of polyamides with a fluorescent label
or reporter
attached thereto include, for example, those disclosed in Best et al., Proc.
Natl. Acad. Sci. USA,
15 100(21): 12063-12068 (2003); Gygi, et al., Nucleic Acids Res., 30(13): 2790-
2799 (2002);
U.S. Patent No. 5,998,140; U.S. Patent No. 6,143,901; and U.S. Patent No.
6,090,947.
Fluorescent nucleotide sequences may also be used to label the sperm. Such
nucleotide
sequences fluoresce when hybridized to a nucleic acid containing a target or
complementary
sequence, but are otherwise nonfluorescent when in a non-hybridized state.
Such
oligonucleotides are disclosed, for example, in U.S. Patent Application
Publication No.
2003/0113765.
26
Date Recue/Date Received 2023-08-28
Sex specific antibodies may also be used to label the sperm in a staining
solution. In this
embodiment, for example, a sex specific antibody may be conjugated with a
fluorescent moiety
(or equivalent reporter molecule). Because the antibody binds to antigens
present on only an X
chromosome-bearing or, alternatively, a Y chromosome-bearing cell, such cells
can be
selectively identified based upon their fluorescence (versus the
nonfluorescence of an unlabeled
cell). Moreover, more than one sex specific antibody, each antibody having a
different
fluorescent moiety attached thereto, may be used simultaneously. This allows
for differentiation
of X chromosome-bearing and Y chromosome-bearing cells based upon the
differing
fluorescence of each.
Luminescent, color-selective nanocrystals may also be used to label sperm in a
staining
solution. Also referred to as quantum dots, these particles are well known in
the art, as
demonstrated by U.S. Patent No. 6,322,901 and U.S. Patent No. 6,576,291. These
nanocrystals
have been conjugated to a number of biological materials, including for
example, peptides,
antibodies, nucleic acids, streptavidin, and polysaccharides, (see, for
example, U.S. Patent Nos.
6,207,392; 6,423,551; 5,990,479, and 6,326,144), and have been used to detect
biological targets
(see, for example, U.S. Patent Nos. 6,207,392 and 6,247,323).
The concentration of the DNA selective or of any other type of dye in the
staining
solution is a function of a range of variables which include the permeability
of the cells to the
selected dye, the temperature of the staining solution, the amount of time
allowed for staining to
occur, the concentration of sperm, and the degree of enrichment desired in the
subsequent sorting
or enrichment step. In general, the dye concentration is preferably sufficient
to achieve the
desired degree of staining in a reasonably short period of time without
substantially detrimentally
affecting sperm viability. For example, the concentration of Hoechst 33342,
Hoechst 33258,
SYBR-14, or BBC in the staining solution will generally be between about
0.111M and about
1.0M; from about 0.111M to about 100011M; from about 100jiM to about 50011M;
from about
200jiM to about 50011M; or from about 300jiM to about 45011M. Accordingly,
under one set of
staining conditions, the concentration of Hoechst 33342 is about 35011M. Under
another set of
27
Date Recue/Date Received 2023-08-28
staining conditions, the concentration of Hoechst 33342 is about 400 M. Under
still another set
of staining conditions the concentration is about 450 M.
As another example, the concentration of a fluorescent polyamide, such as for
example,
those described in U.S. Application Publication No. 2001/0002314, will
generally be between
about 0.1 M and about 1mM; about 11.1M to aboutlmM; about 511M to about 100 M;
or about
M.
Optionally, the staining solution may also contain additives to enhance sperm
quality.
Exemplary additives include one or more OSRs, an antibiotic, a growth factor
or a composition
which regulates oxidation/reduction reactions intracellularly and/or
extracellularly as discussed
above with respect to cell sample collection. These additives may be added to
the collection
fluid in accordance therewith.
Once formed, the staining solution may be maintained at any of a range of
temperatures;
typically, this will be within a range of about 4 C to about 50 C. For
example, the staining
solution may be maintained at a relatively low temperature, i.e., a
temperature of about 4 C to
about 30 C; in this embodiment, the temperature is about 20 C to about 30 C;
from about 25 C
to about 30 C; or about 28 C. Alternatively, the staining solution may be
maintained within an
intermediate temperature range, i.e., a temperature of about 30 C to about 39
C; in this
embodiment, the temperature is at about 34 C to about 39 C; about 35 C; or
about 37 C. In
addition, the staining solution may be maintained within a relatively high
temperature range, i.e.,
a temperature of about 40 C to about 50 C; in this embodiment, the temperature
is from about
41 C to about 49 C; from about 41 C to about 45 C; from about 41 C to about 43
C; or about
41 C. Selection of a preferred temperature generally depends upon a range of
variables,
including for example, the permeability of the cells to the dye(s) being used,
the concentration of
the dye(s) in the staining solution, the amount of time the cells will be
maintained in the staining
solution, and the degree of enrichment desired in the sorting or enrichment
step.
Uptake of dye by the sperm in the staining solution is allowed to continue for
a period of
time sufficient to obtain the desired degree of DNA staining. That period is
typically a period
28
Date Recue/Date Received 2023-08-28
sufficient for the dye to bind to the DNA of the sperm such that X and Y
chromosome-bearing
sperm may be sorted or enriched based upon the differing and measurable
fluorescence intensity
between the two. Generally, this will be no more than about 24 hours; no more
30 than about 10
hours; no more than about 2 hours; no more than about 90 minutes; no more than
about 60
minutes; or from about 5 minutes to about 60 minutes. In a particular
embodiment, the period is
about 30 minutes or about 55 minutes.
The length of the staining period and the temperature at which staining occurs
are related
such that the longer the period of staining, the lower the temperature of
staining temperature may
be. For example, in one embodiment, the staining may occur at a relatively low
temperature and
for a period of about 3 hours to about 24 hours. Alternatively, the staining
may occur at an
intermediate temperature and for a period of about one half hour to about 3
hours. In addition,
staining may occur at a relatively high temperature and for a period of about
10 minutes to about
90 minutes. In a particular embodiment, staining may occur at a temperature of
about 4 C for a
period of about 24 hours. In another embodiment, staining may occur at a
temperature of about
18 C for a period of about 4 hours. In yet another embodiment, staining may
occur at a
temperature of about 41 C for a period of about 30 minutes. In another
embodiment, staining
may occur at a temperature of about 35 C for a period of about 55 minutes.
Accordingly, in one
embodiment, a staining solution is formed comprising low sugar media, sperm
and a dye in a
concentration from about 100 M to about 450 M, and the staining mixture is
held for a period
of time at a temperature of about 28 C; about 35 C; or about 41 C. In another
embodiment, the
period of time is about 30 minutes; about 55 minutes; or about 3 hours.
B. Sorting or Enriching of the Stained Sperm
Some embodiments include use of one or more OSRs as pre-mixed components of
the
prepared buffers, extenders, stains, catch fluids, and/or cryo-extenders used
in the sex sorting
procedure. In some cases, when the sorting of sperm is not going to involve
sex sorting, a
quenching dye without the need for a DNA staining dye may be required, in
which case the OSR
will only be present with the quenching dye to form the stained sample.
Commonly used and
well known sorting methods include flow cytometry systems, as exemplified by
and described in
29
Date Recue/Date Received 2023-08-28
U.S. Patent Nos. 5,135,759, 5,985,216, 6,071,689, 6,149,867, and 6,263,745;
International Patent
Publications WO 99/33956 and WO 01/37655; and U.S. Patent Application Serial
No.10/812,351 (corresponding International Patent Publication WO 2004/088283).
When
sorting according to such methods, the sperm are introduced into the nozzle of
a flow cytometer
in a sample fluid. In one embodiment, therefore, the sample fluid may comprise
low sugar
media and the stained sperm.
As noted above, in certain embodiments of the invention, sex sorting of sperm
may be
accomplished using any process or device known in the art for cell analysis,
sorting and/or
population enrichment including but not limited to use of a flow cytometer or
use of a
microfluidic chip, and encompasses techniques for physically separating X and
Y bearing sperm
from each other, as with droplet sorting and fluid switching sorting, and
techniques for gender
enrichment in which sperm bearing the undesired sex chromosome are killed,
immobilized, or
otherwise rendered infertile, such as by use of laser ablation/photo-damage
techniques.
Generally, in certain embodiments, devices used with the invention determine a
property
of sperm based on fluorescence emitted by the sperm when passed before a
source of
illumination such as a laser beam. The presence or absence of an X- or Y-
bearing chromosomes
are examples of such a property. Other properties include but are not limited
to viability,
motility, intact or damaged membranes, genetic defects, the presence or
absence of specific
genes or gene markers, morphology and fertility. In certain embodiments, the
user decides the
property or properties the device will analyze and select for, referred to as
a property, or
properties, of interest. For example, in one embodiment of the invention, if
the property of
interest is the presence of a Y-bearing chromosome in sperm, the devices used
with the invention
can analyze sperm and then select the sperm having the property of interest
by, for example,
photo-damaging/laser ablating the sperm having the property of interest.
Alternatively, the
devices used with the invention can photo-damage/laser ablate the sperm
without the property of
interest, leaving the sperm having the property of interest in tact. In other
embodiments, the
devices of invention can select sperm having the property of interest by
isolating the sperm
having the property of interest, by for example, separating, or sorting, the
sperm having the
property of interest from sperm without the property of interest. Devices that
may be used with
Date Recue/Date Received 2023-08-28
the invention include but are not limited to those disclosed above as well as
in US Patent
Application Publication No. US 2008/0153087 at paragraphs 23-87; paragraphs 99-
148; and
Figures 1-5; and in US Patent No. 8,206,987 at column 28, line 17 to column
93, line 35; column
126, line 54 to column 130, line 3; and Figures 135-138.
In addition to being used in the sample fluid, low sugar media can be used as
the sheath
fluid used to surround the stream of sample fluid as it travels through a flow
cytometer or
microfluidic chip, for example. Generally, the sheath fluid may be introduced
into a nozzle of a
flow cytometer using pressurized gas or by a syringe pump. The pressurized gas
is often carbon
dioxide or nitrogen. In certain embodiments of the invention, a stream
containing sperm to be
analyzed may be comprised of a sample fluid and a sheath fluid, or a sample
fluid alone.
Optionally, the sample fluid or sheath fluid may also contain an additive,
such as, one or
more OSRs, an antibiotic or a growth factor, as discussed above with respect
to cell sample
collection. Each of these additives may be added to either fluid in accordance
therewith.
One embodiment of a low sugar sheath fluid comprises
Tris(hydroxymethylaminomethane), sodium-citrate dihydrate and citric acid
anhydrous. To
prepare this low sugar sheath fluid, regardless of the intended volume of
sheath fluid desired, the
entire quantity of Tris and sodium-citrate are mixed with approximately 95% of
the desired
volume of ddH20. Three quarters of the citric acid is also concurrently added.
A titration with
the remaining quantity of citric acid is performed to reach a specific pH of
6.80. After pH
adjustment, ddH20 is added until an osmolarity of 300 mOsm is obtained. One or
more OSRs
may also be added to this sheath fluid, typically in the range of 0.01 to 5.0
mg/ml.
Figure 1 illustrates, in schematic form, part of a flow cytometer used to sort
a sperm
composition to form one or more subpopulations, the flow cytometer being
generally referenced
as 10. In this particular embodiment of the invention, sex sorting is taking
place so the
subpopulations are X-chromosome bearing sperm and Y-chromosome bearing sperm.
31
Date Recue/Date Received 2023-08-28
The flow cytometer 10 of Figure 1 can be programmed by an operator to generate
two
charged droplet streams, one containing X-chromosome bearing sperm, charged
positively, 12,
one containing Y-chromosome bearing sperm, charged negatively 13 while an
uncharged
undeflected stream of dead cells 14 simply goes to waste.
An operator may also choose to program the flow cytometer in such a manner,
that both
the X- and Y-chromosome bearing sperm are collected using a "high purity sort"
(in other words
only live X- and Y-chromosome bearing sperm are collected) or to program the
flow cytometer
to collect both the X- and Y-chromosome bearing sperm using an "enriched sort"
(in other words
it will collect droplets containing live cells that were not previously sorted
and excluding all
initial dead cells again by the use of Boolean Gate logic available with the
computer that controls
the flow cytometer). The Boolean Gate logic can also be used to collect only
one of either the X-
or Y-chromosome bearing sperm.
Initially, a stream of sperm under pressure, is deposited into the nozzle 15
from the sperm
source 11 in a manner such that they are able to be coaxially surrounded by a
sheath fluid
supplied to the nozzle 15 under pressure from a sheath fluid source 16. An
oscillator 17 which
may be present can be very precisely controlled via an oscillator control
mechanism 18, creating
pressure waves within the nozzle 15 which are transmitted to the coaxially
surrounded sperm
stream as it leaves the nozzle orifice 19. As a result, the exiting coaxially
surrounded sperm
stream 20 could eventually and regularly form droplets 21.
The charging of the respective droplet streams is made possible by the cell
sensing
system 22 which includes a laser 23 which illuminates the nozzle exiting
stream 20, and the light
emission of the fluorescing stream is detected by a sensor 24. The information
received by the
sensor 24 is fed to a sorter discrimination system 25 which very rapidly makes
the decision as to
whether to charge a forming droplet and if so which charge to provide the
forming drop and then
charges the droplet 21 accordingly.
A characteristic of X-chromosome bearing sperm is that they absorb more
fluorochrome
dye than Y-chromosome bearing sperm because of the presence of more DNA, and
as such, the
32
Date Recue/Date Received 2023-08-28
amount of light emitted by the laser excited absorbed dye in the X-chromosome
bearing sperm
differs from that of the Y-chromosome bearing sperm and this difference
communicates to the
sorter discrimination system 25 the type of charge to apply to the individual
droplets which
theoretically contain only a single X- or Y-chromosome bearing sperm. Dead
cells (or those
about to die) typically absorb the quenching dye which is communicated to the
sorter
discrimination system 25 not to apply a charge to the droplets containing such
cells.
The charged or uncharged droplet streams then pass between a pair of
electrostatically
charged plates 26, which cause them to be deflected either one way or the
other or not at all
depending on their charge into respective collection vessels 28 and 29 to form
respectively a
gender enriched population of X-chromosome bearing and a gender enriched Y-
chromosome
bearing sperm having a DNA selective dye associated with their DNA. The
uncharged non-
deflected sub-population stream containing dead cells (or those about to die)
go to the waste
container 30.
Alternatively, the cells of a sperm composition may be sorted or enriched
using laser
steering. This is often referred to as optical trapping or holographic optical
trapping. Generally,
tightly focused laser light, such as, for example, light focused by a
microscope lens, will have a
steep intensity gradient. Optical traps use the gradient forces of a beam of
light to trap particles
based upon the dielectric constant of the beam. To minimize its energy, a
particle having a
dielectric constant greater than the surrounding medium will move to a region
of an optical trap
where the electric field is highest. Such devices and methods are described,
for example, in WO
2004/012133, U.S. Patent No. 6,416,190 and related applications and patents.
The cells of the
sperm composition may be sorted accordingly into separate populations, wherein
the
spermatozoa of the populations comprises a certain percent X chromosome
bearing or Y
chromosome bearing sperm. Laser ablation/photo damage or fluid switching may
also be used to
create gender enriched populations.
Any of the steps of the cell sorting process may be carried out within a
temperature range
selected from the group consisting of about 5 C to about 15 C; about 15 C to
about 20 C; about
33
Date Recue/Date Received 2023-08-28
20 C to about 25 C; about 25 C to about 30 C; about 30 C to about 35 C; about
35 C to about
40 C and about 40 C to about 45 C.
Furthermore, it is contemplated that sorted or gender enriched sperm of the
invention
may comprise at least about 65% X chromosome bearing or Y chromosome bearing
sperm, at
least about 70% X chromosome bearing or Y chromosome bearing sperm, at least
about 75% X
chromosome bearing or Y chromosome bearing sperm, at least about 80% X
chromosome
bearing or Y chromosome bearing sperm, at least about 85% X chromosome bearing
or Y
chromosome bearing sperm, at least about 90% X chromosome bearing or Y
chromosome
bearing sperm, at least about 95% X chromosome bearing or Y chromosome bearing
sperm, at
least about 98% X chromosome bearing or Y chromosome bearing sperm, or at
least about 99%
X chromosome bearing or Y chromosome bearing sperm.
C. Collection of the Sorted Cells into Catch Media
Once sorted, the sorted cells are collected in a vessel that contains a catch
media.
Generally, the purpose of the catch media includes providing a fluid support
for the cells. In one
aspect of the invention, the catch media may comprise low sugar media.
Optionally, the catch
media may further comprise any of the additives as discussed above with
respect to cell sample
collection, including but not limited to one or more OSRs, cryoprotectants,
sterols, lipids, fatty
acids and protein sources. If included in the catch media, the sterols,
lipids, and fatty acids may
be, for example, cholesterol.
Exemplary protein sources include milk (including heat homogenized and skim),
milk
extract, egg yolk, egg yolk extract, soy protein and soy protein extract. Such
proteins may be
used in a concentration from about 1% (v/v) to about 30% (v/v), preferably
from about 10%
(v/v) to about 20% (v/v), and more preferably about 10% (v/v).
Optionally, the catch media may also contain additives such as, an antibiotic,
a growth
factor or one or more OSRs, as discussed above with respect to cell sample
collection. Each of
these additives may be added to the catch media in accordance therewith.
34
Date Recue/Date Received 2023-08-28
One embodiment of a low sugar catch media is prepared by adding the
appropriate
amount of egg yolk to media comprising Tris(hydroxymethylaminomethane), sodium
citrate
dehydrate and citric acid anhydrous, mixing and then allowing the mixture set
overnight at 5 C.
The following day the mixture is centrifuged at 5000g for 1.5h. After
centrifugation, the top
layer of the supernatant is discarded, and the remaining supernatant, which
comprises the low
sugar catch media, is collected. Finally, one or more OSRs may also be added
to the low sugar
catch media, typically in the range of 0.01 to 5.0 mg/ml. The low sugar catch
media should then
be stored at 5 C until use. Alternatively, the low sugar catch media can be
frozen prior to
storage and/or shipping. It should be understood that sugar or sugar additive
may be added
during any of the aforementioned steps for preparing low sugar media including
prior to use,
prior to freezing or subsequent to thawing of the media.
The sorted cells may be collected into a vessel containing or coated with a
cryoextender
comprising low sugar media, a cryoprotectant and optionally, any of the
additives as discussed
above with respect to cell sample collection. Accordingly, in one particular
embodiment, the
sorted cells are collected into a cryoextender comprising low sugar media and
a suitable
cry oprotectant.
D. Cryopreservation of the Sorted Cells
Once the sperm have been sorted and caught in collection vessels, they may be
used for
inseminating female mammals. This can occur almost immediately, requiring
little additional
treatment of the sperm. In such an instance, the sperm may be stored in their
current state for a
period of time necessary to, for example, transport them to the location where
the insemination is
to take place. The sperm may, therefore, be stored and transported in, for
example, the catch
media.
Likewise, the sperm may be concentrated to a density appropriate for the
particular
mammalian species, for example, a density of about 10 x 106 sperm/ml to about
120 x 106
sperm/ml, in a low sugar media and subsequently stored and transported. The
selected density
Date Recue/Date Received 2023-08-28
depends upon factors such as those discussed below with respect to
fertilization, including the
species of mammal from which the sperm were obtained. Such a range of
densities based upon
the species of mammal from which the sperm were obtained are well known to
those of skill in
the art.
Likewise, the sperm may also be cooled or frozen for use at a later date. In
such
instances, the sperm may benefit from the addition of a cryoextender to
minimize the impact
upon viability or post-thaw motility as a result of cooling or freezing.
A protein source may be added to provide support to the sperm. Examples of
common
protein sources include milk (including heat homogenized and skim), milk
extract, egg yolk, egg
yolk extract, soy protein and soy protein extract. Such proteins may be found
in a concentration
from about 3% (v/v) to about 30% (v/v); from about 10% (v/v) to about
20% (v/v); or about 20% (v/v).
A cryoprotectant is preferably included in the cryoextender to lessen or
prevent cold
shock or to maintain fertility of the sperm. Numerous cryoprotectants are
known in the art.
Selection of a cryoprotectant suitable for use with a given extender may vary,
and depends upon
the species from which the sperm to be frozen were obtained. Examples of
suitable
cryoprotectants include, for example, glycerol, dimethyl sulfoxide, ethylene
glycol, propylene
glycol, trehalose, Trilady10, and combinations thereof. If included,
generally, these
cryoprotectants are present in the cryoextender in an amount, by percent
volume, of about 1-5%;
5%; about 5 to 10%; about 10%; about 10 to 20%; about 16.7%; about 20%; about
20 to 30%; or
about 30 to 40%.
Optionally, the cryoextender may also contain additives as discussed above
with respect
to cell sample collection, including but not limited to an antibiotic, a
growth factor, or one or
more OSRs. Each of these additives may be added to the cryoextender in
accordance therewith.
36
Date Recue/Date Received 2023-08-28
The following method of freezing porcine sperm can be used with the invention,
but is
presented by way of example only¨any suitable cryopreservation method known in
the art can
be used.
After sorting, 50 ml tubes containing the sex sorted sperm (each with 20
million cells)
can be divided into tubes of 15 ml, with approximately 12 ml of a sex-select
sperm sample in
each tube, each containing approximately 10 million sex sorted sperm. Theses
tubes can be
centrifuged at 3076 g at 21 C for 4 minutes. The supernatant decanted, and the
pellet can remain
with some of the supernatant in approximately 50 jil.
To each pellet, a first cooling medium that may comprise a solution of 20% egg
yolk
and/or lactose can then be added at room temperature. The motility of the
sperm can then be
checked. If acceptable, the tubes can be taken to a programmable temperature
control machine
(PolyScience ¨ MiniTube) or can be manually handled to decrease the
temperature from about
21 C to about 5 C over a period of about 2 hours. After the timed temperature
shift with the
cells at 5 C a freezing medium is added to the cells, which may comprise egg-
yolk, lactose,
glycerol and EquexPasteStem, or may just comprise a cryoprotectant such as
glycerol, or the
cryoprotectant with an osmotic stabilizer which is previously cooled to 5 C is
added to the
samples. After 10 minutes, the sex sorted sperm composition can be placed in
artificial
insemination straws, and the straws then exposed to liquid nitrogen vapors
(approximately 4 cm
from the liquid nitrogen) for a short period of time (e.g. 10 minutes) or
cryopreserved in
programmable freezer and then placed directly into the liquid nitrogen for
long term
preservation.
When the sex sorted sperm compositions are ready for use, the straws can be
unfrozen by
thawing/warming the straws (e.g. place in a water bath set at about 37 C for
about 1 minute or
50 for 20 seconds). Post-thaw, motility and viability of the sperm can then
be analyzed at 30,
90 and/or 150 minutes for standard comparisons.
37
Date Recue/Date Received 2023-08-28
III. Storage of the Collected Cells
A. Storage Period
Once the sperm have been collected from the source male, regardless of whether
they are
optionally sorted thereafter, the sperm may be stored for a period of time in
low sugar media.
The period of storage is dependent upon several factors, including for
example, the temperature
at which the cells are stored, the number of cells within the storage
container, whether the sperm
are sorted or unsorted or have been previously been subjected to other
processes such as
cryopreservation, the method of fertilization for which the cells will be
used, and the female
mammal being fertilized.
Generally, for example, the sperm may be stored for several hours, such as for
example,
2, 4, 8, 12, or 24 hours; for several days, such as for example 1, 2, 3, 4, 5,
6, or 7 days; several
weeks, such as for example, 1, 2, 3, or 4 weeks; or several months, such as
for example, 1, 2, or 3
months. Typically, sperm may be stored for several hours to several days at a
temperature of
about 0 C to about 30 C; for several days to several weeks at a temperature of
about -4 C to
about 5 C; and for several weeks to several months at a temperature of about -
196 C (in liquid
nitrogen vapor) to about -4 C. For porcine sperm, for example, a sample can be
held at a
temperature of 0-39 C (typically 16-17 C) for between about 12 hours to about
18 hours while it
is being shipped from the collection point to the point of further processing
or use. In other
embodiments of the invention, the sample can be held at a temperature of 0-39
C (typically 16-
17 C) for more than 18 hours.
B. Storage Temperature
The sperm, whether sorted or unsorted, may be stored at a range of different
temperatures. Selection of a storage temperature is dependent upon several
factors, such as for
example, the length of time for which the sperm will be stored, the
concentration of sperm within
the storage container, whether the sperm are sorted or unsorted, the method of
fertilization for
38
Date Recue/Date Received 2023-08-28
which the sperm will be used, and the female being fertilized. All of these
factors affect the
number of sperm that will remain viable during the storage period. By way of
example,
generally the greater the length of time for which the sperm may be stored,
the lower the
temperature at which the sperm may be stored. In certain species, a decrease
in temperature
generally permits a greater percentage of the stored sperm to remain viable
over a longer period
of time. In other species, such as with porcine sperm, this may not be true.
Accordingly, sperm may be stored at a temperature of about -196 C to about 30
C. For
example, sperm may be stored at a relatively low storage temperature, i.e., a
temperature range
of about -196 C to about -4 C; in this embodiment, the temperature is
typically from about -
12 C to about -4 C; from about -10 C to about -4 C; or about -4 C.
Alternatively, the sperm
may be stored at an intermediate storage temperature, i.e., a temperature
range of about -4 C to
about 5 C; in this embodiment, the temperature is typically at about -3 C to
about 5 C; about
0 C to about 5 C; or about 5 C. In addition, the sperm may be stored at a
moderately high
storage temperature, i.e., a temperature range of about 5 C to about 30 C; in
this embodiment,
the temperature is typically from about 10 C to about 25 C; from about 12 C to
about 23 C;
from about 15 C to about 20 C; or about 18 C.
C. Storage Container
The sperm composition may be stored in a range of different containers. While
the
containers may vary in size, generally suitable containers will be capable of
containing the sperm
composition; that is to say, the containers will be constructed of a material
that is not susceptible
to leaking or deterioration as a result of contact with fluids generally, and
sperm compositions
specifically, regardless of whether such contact occurs on the inside or
outside of the container.
Examples of suitable containers include, for example, flasks, beakers, test
tubes, ampules, and
other such containers that are generally constructed of glass, plastic, or
other similar materials. In
a particular embodiment, the container is of a type of construction that is
used in the
insemination of a female, such as for example, an elongated container. Such
elongated
containers may generally have a length to diameter ratio of about 1000:1 to
about 100:1; a length
to diameter ratio of about 900:1 to about 200:1; a length to diameter ratio of
about 800:1 to about
39
Date Recue/Date Received 2023-08-28
300:1; a length to diameter ratio of about 700:1 to about 400:1; a length to
diameter ratio of
about 600:1 to about 400:1; a length to diameter ratio of about 500:1 to about
400:1; and in one
particular embodiment, a length to diameter ratio of about 450:1. Such
elongated containers may
generally have a volume of 10 about 0.1cc to about 100cc, the volume of the
container selected to
be used being based upon the species of mammal from which the semen was
collected. For
example, the volume of such elongated containers may be from about 0.1cc to
about 0.7cc,
preferably a volume of about 0.2cc to about 15 0.6cc, more preferably a volume
of about 0.23cc
to about 0.5cc, and most preferably a volume of about 0.3cc to about 0.4cc.
In a particular embodiment, the elongated container is what is commonly
referred to in
the artificial insemination industry as a straw, having a volume of about
0.23cc and a length to
diameter ratio of about 133:1. In another particular embodiment, the elongated
container is what
is commonly referred to in the artificial insemination industry as a straw,
having a volume of
about 0.5cc and a length to diameter ratio of about 67:1. Typically containers
of these volumes
are used for the storage of bovine sperm.
Alternatively, the volume of the elongated containers may be from about lcc to
about
100cc; about lOcc to about 75cc; about 15cc to about 50cc; about 20cc, to
about 40cc; or a
volume of about 25cc to about 30cc. In a particular embodiment, the elongated
container is what
is commonly referred to in the artificial insemination industry as a straw,
having a volume of
about 25cc and a length to diameter ratio of about 445:1. Typically,
containers of this volume
are used for the storage of porcine sperm.
The advantage of storing the sperm composition in a straw is that the
composition may
remain stored therein until it is to be used for insemination of a female, at
which time the
contents of the straw may be placed into the uterus of a female.
IV. Fertilization or Insemination
Another aspect of the present invention is the fertilization of an egg or
insemination of a
female, generally employing the novel process for storing spermatozoa as
described above.
Date Recue/Date Received 2023-08-28
Once a sperm composition has been formed as discussed in greater detail above
with
respect to the collection of a sperm sample, the sperm composition may be used
to inseminate a
female. Insemination may be performed according to any of a number of ART
methods well
known to those of skill in the art. These methods include, for example,
artificial insemination,
including standard artificial insemination, deep uterine insemination and
laparoscopic
insemination, and other methods well known to those of skill in the art. For
example, a sperm
composition comprising low sugar media and one or more OSRs may be used to
inseminate a
female, such as for example, by artificial insemination. In a particular
embodiment, the sperm
composition may be in an elongated container for use in the insemination of a
female mammal.
Alternatively, the sperm composition may be used to fertilize an egg, and more
particularly, an egg in vitro, such as for example, by microinjection,
including intracytoplasmic
sperm injection (ICSI), and other methods well known to those in the art. The
fertilized egg may
thereafter be introduced into the uterus of a female by any of a number of
means well known to
those of skill in the art, such as for example embryo transplant. In another
aspect of the
invention, zygotes and/or embryos from artificially inseminated females can be
recovered and
then cultured and/or cryopreserved/vitrified.
Insemination of a female mammal or fertilization of an egg in vitro (followed
by
introduction of the fertilized egg into the uterus of a female) using a sperm
composition may
occur shortly after formation of the sperm composition, such as for example,
within about 120
hours; within about 96 hours; within about 72 hours; within about 48 hours,
and in a particular
embodiment, within about 24 hours after formation of the sperm composition. In
such instances,
generally the sperm compositions may not have been cryopreserved prior to
insemination of a
female or fertilization of an egg in vitro (i.e., the composition is fresh or
comprises fresh sperm)
instead it may have been refrigerated at temperatures of about 4 C to about 25
C; about 10 C to
about 25 C; about 15 C to about 20 C; or about 18 C. Alternatively, the sperm
composition may
be cryopreserved and then thawed prior to insemination of a female or
fertilization of an egg in
vitro (i.e., the dispersion is frozen/thawed or comprises frozen/thawed
sperm). Typically, in such
an instance, the cryopreserved sperm composition will be thawed immediately,
such as, for
41
Date Recue/Date Received 2023-08-28
example, within about 15 minutes, before insemination of a female or
fertilization of an egg in
vitro. Alternatively, the cryopreserved dispersion may be thawed over a period
of time or
thawed and subsequently stored for a period of time, such as for example less
than about 5 days;
less than about 2 days; less than about 1 day; or less than about 12 hours.
V. Freezing Low Sugar Media Comprising a Cryoprotectant
One aspect of the instant invention relates to the discovery that low sugar
media
comprising a protein source, such as egg yolk, that is frozen and thawed prior
to use in
processing sperm, increases the viability of the processed sperm. A specific
application of this
discovery encompasses the use of such frozen-thawed low sugar media during the
cell sorting
process and, in particular, as a catch fluid.
Additionally, prior to this discovery, media comprising a protein source, such
as egg
yolk, was typically made at the facility where it was to be used due to the
fact that freezing such
media prior to use in sperm processing generally resulted in lower quality
sperm. Accordingly,
the discovery has an additional benefit in that the use of low sugar media
allows for the shipping
of such media to a destination in a frozen state, where it can then be thawed
and used without
any deleterious effects compared to media with higher sugar content that has
not been frozen.
To the extent the same type of sperm processing is being carried out in
multiple, geographically
distant lab facilities, media with higher sugar content comprising a protein
source such as egg
yolk becomes a variable in processing since the perishable constituents of the
media, such as the
egg yolk, must generally be obtained locally. Accordingly, the ability to ship
frozen media
comprising perishable and otherwise highly variable components, such as egg
yolk, from a
centralized manufacturing facility allows for better quality control across
multiple,
geographically separate lab facilities where the media will be used.
EXAMPLE 1
The following Example demonstrates the effect of freezing and thawing low
sugar catch
media prior to use in sorting boar sperm.
42
Date Recue/Date Received 2023-08-28
A. Collection
The sperm-rich fraction of ejaculates from ten boars was collected into a
collection bag
using the gloved-hand technique. The motility and morphology of each ejaculate
was evaluated
by CASA whereas sperm morphology was evaluated by a trained technician
counting a
minimum of 100 cells. Ejaculates with greater than 85% motile cells and
greater than 80%
normal morphological cells were diluted in a Tris-citrate media (maintained at
36 C) to form a
sperm composition having a sperm concentration of 100 million cells/mL. Each
sperm
composition was then placed in a semen tube, bottle or bag and cooled to 17 C
prior to transport
to the sorting facility in thermal coolers containing frozen gel packs..
B. Sex Sorting
At the sorting facility, the sperm composition was checked for motility and
morphology.
1/1 mL of Hoechst 33342 (5mg/m1 in MiliQ water; Ref: B-2261) was added to
normal sperm
compositions to form a staining solution. The staining solution was then
incubated for 55
minutes at 35 C. After incubation the staining solution was placed in a dark
place at room
temperature (21-22 C). Immediately prior to sorting, the staining solution was
filtered
(CellTricks of 0.50m) and either 1111 of yellow food dye No. 6 or red food dye
(25mg/m1
inMiliQ water) was added to the solution to form a sample fluid. The sample
fluid was then
sorted in a Genesis III flow cytometer (CytonomeST, Boston, MA) with an event
rate at 20-
25,000mi11ion/sec. Sorted sperm were collected in 50m1 tubes containing 2.5m1
of catch media
for every 20mi11ion cells, centrifuged at 2400g for 3 minutes and then
resuspended in a Tris-
citrate media.
C. Experimental Conditions
In this Example, sorted sperm were collected into three types of catch media:
a low sugar
"Control" media, containing Tris, sodium citrate, citric acid and 16.7% egg
yolk; a low sugar
"Freeze" media, containing Tris, sodium citrate, citric acid and 16.7% egg
yolk, and which was
43
Date Recue/Date Received 2023-08-28
frozen and then thawed prior to use; and a "+Sugar" media, containing
fructose, Tris, citric acid
and 16.7% egg yolk.
After resuspension, motility (assessed by CASA) and viability (assessed by
LIVE/DEAD
Viability/Cytotoxicity Kit; Molecular Probes, Inc.) of the sorted sperm were
determined at 0, 24,
48, 72, 96 and 144 hours. Results for each treatment group ("Trt") are shown
in Tables 1
through 4 below. There was not a treatment by time effect so values at each
time were pooled in
Tables 2 and 4.
Table 1.
Motility (PSE = 3.41)
Trt Oh 24h 48h 72h 96h 144h
Control 91.84 91.75 88.02 85.33 80.11 64.82
+Sugar 91.19 90.48 86.41 84.23 79.62 55.15
Freeze 92.83 92 89.5 87.42 81.42 63.55
Table 2.
Trt Motility SE
Control 82.69 1.03
+Sugar 80.47 1.03
Freeze 82.84 1.03
Variable P Value
Boar <0.0001
Trt 0.214
Time <0.0001
Trt*Time 0.814
44
Date Recue/Date Received 2023-08-28
Table 3.
Viability (PSE = 1.93)
Trt Oh 24h 48h 72h 96h 144h
Control 87.07 87.74 79.87 78.54 76.32 66.43
+Sugar 86.76 84.43 79.87 78.54 76.32 66.43
Freeze 90.85 89.41 85.85 84.07 79.74 71.07
Table 4.
Trt Viability SE
Control 80.26 0.60
+Sugar 78.33 0.62
Freeze 82.31 0.61
Variable P Value
Boar <0.0001
Trt <0.0001
Time <0.0001
Trt*Time 0.47
EXAMPLE 2
The following Example compares the post-sort motility, viability and
quantitative sorting
parameters (peak to valley ration [PVR], sort rate and event rate) in boar
semen using sheath
fluid and catch media containing Tris, sodium citrate and citric acid (the
"Low Sugar"
treatment), and sheath fluid and catch media containing fructose, Tris and
citric acid (the
"Control").
The sperm-rich fraction of ejaculates from eight boars (two replicates) and
five boars
(tested one time) were collected and then sex sorted using the methods
described in Example
Date Recue/Date Received 2023-08-28
1(A) and 1(B) above. Sperm were sorted using either Low Sugar media or the
Control media.
After resuspension, motility (assessed by CASA) and viability (assessed by
LIVE/DEAD
Viability/Cytotoxicity Kit; Molecular Probes, Inc.) of the sorted sperm were
determined at 0, 24,
48, 72, 96 and 144 hours. Results for motility and viability are shown in
Tables 5 through 7
below. There was not a treatment by time effect for viability so data was
pooled and expressed in
Table 7. The Low Sugar treatment had 32% higher PVR, 23% faster event rate and
a 15% faster
sort rate.
Table 5.
Motility (PSE = 1.44)
Trt Oh 24h 48h 72h 96h 144h
Control 90.04 89.05 87.01 81.35 79.2 62.78
Low Sugar 92.08 92.07 90.10 83.74 80.58 72.87
Variable P Value
Boar <0.0001
Trt <0.0001
Time <0.0001
Trt*Time 0.03
Rep <0.0001
Table 6.
Viability (PSE = 1.36)
Trt Oh 24h 48h 72h 96h 144h
Control 87.11 79.35 78.16 70.21 69.59 63.21
Low sugar 89.11 83.11 80.59 75.63 73.87 72.53
Variable P Value
Boar 0.0019
Trt <0.0001
46
Date Recue/Date Received 2023-08-28
Time <0.0001
Trt*Time 0.08
Rep <0.0001
Table 7
Trt Viability SE
Control 74.60 0.59
Low sugar 79.14 0.59
EXAMPLE 3
The following Example compares the post-sort motility and viability in boar
semen when
using media without a sugar additive, media without a sugar additive
supplemented with
SexedUltraTM (ST Reproductive Technologies, LLC, 2014), or media containing a
sugar
additive, in the various processing steps for sex sorting sperm. (SexedUltraTM
is purported to
contain less than 1g/m1 of at least two of the following OSRs: vitamin B12,
vitamin B12
vitamers, vitamin E, vitamin E vitamers, tocopherols, tocotrienols, a-
tocoperyl, alpha
ketoglutarate, and derivatives thereof.)
The sperm-rich fraction of ejaculates from six boars (two replicates) were
collected using
the methods described in Example 1(A) above, except that the diluent employed
varied
depending on the treatment group, as shown in Table 8. The sperm were sorted
using the
methods described in Example 1(B), except that the resuspension media varied
depending on the
treatment group, as shown in Table 8. The sheath fluid, catch media and
resuspension media
utilized in each treatment group are shown in Table 8 ("EY" = egg yolk). The
media indicated in
Table 8 comprised the components as follows.
"BTS" media was purchased from a commercial company and the relative
ingredients are
not known. However, it is a published formula from Pursel and Johnson (1975)
that includes
37.0 g/L of glucose; 6.0 g/L of sodium citrate; 1.25 g/L of sodium
bicarbonate; 1.25 g/L of
47
Date Recue/Date Received 2023-08-28
EDTA; and 0.75 g/L of KC!. Purse! VG and Johnson LA. 1975. Freezing of boar
spermatozoa:
fertilizing capacity with concentrated semen and a new thawing extender. J.
Anim. Sci..40:99-
102.
"PBS" media contained 8 g/L of NaCl; 0.201 g/L of KC!; 0.204 g/L of KII2PO4;
1.149
g/L of Na2}1PO4; 0.058 g/L of penicillin; and 0.05 g/L of streptomycin
sulphate.
"TTG" media contained 50 g/L of TES; 6.8 g/L of Tris; and 6 g/L of glucose.
"Tris-citrate" media contained Tris, sodium citrate and citric acid.
"Tris-citrate + Glucose" media contained Tris, sodium citrate, citric acid and
glucose.
"Tris-citrate + SexedU!traTM" media contained Tris, sodium citrate, citric
acid and
SexedU!traTM.
Table 8.
Treatment Diluent Sheath Catch Resuspension
1 BTS PBS TTG with BTS
16.7% EY
2 BTS Tris-citrate Tris-citrate with BTS
16.7% EY
3 BTS Tris-citrate Tris-citrate + BTS
Glucose with
16.7% EY
4 Tris-citrate + Tris-citrate Tris-citrate + Tris +
SexedU!traTM SexedUltraTm SexedU!traTM
with 16.7% EY
48
Date Recue/Date Received 2023-08-28
BTS Tris-citrate Tris-citrate + BTS
SexedUltraTM
with 16.7% EY
After resuspension, motility (assessed by CASA) and viability (assessed by
LIVE/DEAD
Viability/Cytotoxicity Kit; Molecular Probes, Inc.) of the sorted sperm in the
five treatment
groups were determined at 0, 24, 48, 72, 96 and 120 hours. Results for
motility and viability are
shown in Tables 9 and 10 below.
Table 9.
Motility
Treatment Oh* 24h 48h 72h 96h 120h
1 86.48 82.12 57.23 20.17 9.20 5.29
2 85.08 84.33 77.36 63.39 49.22 35.88
3 84.93 83.27 69.37 61.88 56.73 46.88
4 90.70 89.68 85.87 80.47 76.88 71.37
5 87.22 86.65 82.53 74.50 64.88 50.42
*Oh = Evaluation of cells immediately after sorting, centrifugation and
resuspension.
Pooled standard error = 4.32
P value = trt, time, boar, trt*time <0.0001
Table 10.
Viability
Treatment Oh* 24h 48h 72h 96h 120h
1 76.75 61.00 15.67 11.25 10.83
2 77.92 72.83 63.33 61.42 50.08
3 77.92 69.08 60.50 57.50 55.50
4 85.92 84.50 81.83 78.33 70.83
49
Date Recue/Date Received 2023-08-28
80.00 78.33 74.75 56.33 56.33
*Oh = Evaluation of cells immediately after sorting, centrifugation and
resuspension.
Pooled standard error = 4.77
P value = trt, time, boar, trt*time <0.0001
EXAMPLE 4
The following Example compares the post-sort motility and viability in boar
semen when
using media without a sugar additive, media without a sugar additive
supplemented with
SexedUltraTM (ST Reproductive Technologies, LLC, 2014), or media containing a
sugar
additive, in the various processing steps for sex sorting sperm.
The sperm-rich fraction of ejaculates from two boars were collected using the
methods
described in Example 1(A) above, except that the diluent employed varied
depending on the
treatment group, as shown in Table 11. The sperm were sorted using the methods
described in
Example 1(B), except that the resuspension media varied depending on the
treatment group, as
shown in Table 11. The sheath fluid, catch media and resuspension media
utilized in each
treatment group are shown in Table 11 ("EY" = egg yolk). The media indicated
in Table 11
comprised the components as follows:
"BTS"; "PBS"; "TTG"; "Tris-citrate"; "Tris-citrate + Glucose"; and "Tris-
citrate +
SexedUltraTM" media contained the components described for each of those media
in Example 3,
above.
"TCAl" media contained 29.2 g/L of Tris; 11.6 g/L of fumaric acid; and 2000jiL
of
HCL.
"TCA2" media contained 31.2 g/L of Tris; 11.6 g/L of maleic acid; and 4000jiL
of HCL.
Table 11.
Trt Dilution Sheath Catch Resuspension
Date Recue/Date Received 2023-08-28
1 BTS PBS TTG with 20% EY BTS
Monsanto with 20%
2 BTS Monsanto EY BTS
Tris-citrate with 20%
3 BTS Tris-citrate EY BTS
Tris-citrate + Glucose
4 BTS Tris-citrate with 20% EY BTS
BTS TCA1 TCA1 with 20% EY BTS
6 BTS TCA2 TCA2 with 20% EY BTS
Tris-citrate +
SexedUltraTM with
7 Tris Tris-citrate 20% EY Tris-citrate
After resuspension, motility (assessed by CASA), viability (assessed by
LIVE/DEAD
Viability/Cytotoxicity Kit; Molecular Probes, Inc.) and percent intact
acrosomes (PIA) of the
sorted sperm in the seven treatment groups were determined at 0, 24, 48, 72,
96 and 120 hours.
Results for motility, viability and PIA are shown in Tables 12, 13 and 14,
respectively.
Table 12.
Motility Motility Motility Motility Motility Motility
Trt (Oh) (24h) (48h) (72h) (96) (120h) PSE
1 91.65 72.3 15.65 0 0 0 9.38
2 29.6 0 0 0 0 0
51
Date Recue/Date Received 2023-08-28
3 71.15 12.15 0 0 0 0
4 77.15 48.55 4.95 1.6 0 0
67.45 50.25 31.4 11.9 1.05 0
6 71 53.6 13.65 10.15 6 2.4
7 84.05 79.45 75.35 65.05 48.9 38.05
Variable P Value
trt <0.0001
Time <0.0001
Tretime 0.02
Table 13.
Viability Viability Viability Viability Viability Viability
Trt (Oh) (24h) (48h) (72h) (96) (120h) PSE
1 84.5 59.5 62 46 0 0 8.82
2 66.5 61.5 0 0 0 0
3 71.5 60.5 51 0 0 0
4 67 45 46 44.5 22 0
5 49.5 55.5 50.5 41.5 25 23.5
6 62 67.5 54.5 47.5 22.5 22.5
7 90.5 83.5 76.5 69 72.5 76.5
Variable P Value
trt <0.0001
Time <0.0001
Tretime 0.0009
Bull 0.04
52
Date Recue/Date Received 2023-08-28
Table 14 PIA PIA PIA PIA PIA PIA
Trt (Oh) (24h) (48h) (72h) (96) (120h) PSE
1 97.5 96 92.5 89.5 . . 4.44
2 93.5 96 96 . . .
3 96 96.5 91.5 . . .
4 94.5 85 88 86 86.43 .
83.5 84 69.5 87 90.43 80.43
6 90.5 92 89.5 92 91.57 79.57
7 92.5 95.5 90.5 88.5 84.5 91.5
Variable P Value
trt 0.02
Time 0.27
Trt*time 0.6
Bull 0.08
EXAMPLE 5
The following Example compares the post-sort motility, viability and PIA in
boar semen
when using catch media without a sugar additive, media without a sugar
additive supplemented
with SexedUltraTM (ST Reproductive Technologies, LLC, 2014), or media
containing a sugar
additive, with or without egg yolk.
The sperm-rich fraction of ejaculates from six boars were collected using the
methods
described in Example 1(A) above, except that the diluent employed constituted
the BTS media
described in Example 3. The sperm were sorted using the methods described in
Example 1(B),
except that the resuspension media also constituted BTS. The catch media
utilized in each
treatment group are shown in Table 15 ("EY" = egg yolk). The media indicated
in Table 15
comprised the components as follows:
53
Date Recue/Date Received 2023-08-28
"BTS"; "PBS"; "TTG"; "Tris-citrate"; "Tris-citrate + Glucose"; and "Tris-
citrate +
SexedUltraTM" media contained the components described for each of those media
in Example 3,
above.
"Tris-citrate + Glucose + SexedUltraTM" contained Tris, sodium citrate, citric
acid,
glucose and SexedUltraTM.
Table 15.
Trt Dilution Sheath Catch Resuspension
Tris-citrate + Glucose +
1 BTS Tris-citrate SexedUltraTM BTS
Tris-citrate +
SexedUltraTM with
2 BTS Tris-citrate % EY BTS
Tris-citrate + Glucose
3 BTS Tris-citrate with EY BTS
4 BTS Tris-citrate Tris-citrate with EY BTS
Tris-citrate + Glucose +
BTS Tris-citrate SexedUltraTM BTS
Tris-citrate +
6 BTS Tris-citrate SexedUltraTM BTS
After resuspension, motility (assessed by CASA), viability (assessed by
LIVE/DEAD
Viability/Cytotoxicity Kit; Molecular Probes, Inc.) and DIA (assessed by flow
cytometry) of the
54
Date Recue/Date Received 2023-08-28
sorted sperm in the 6 treatment groups were determined at 0, 24, 48 and 72
hours. Results for
motility, viability and PIA are shown in Tables 16, 17 and 18, respectively.
Table 16.
Motility Motility Motility
trt (Oh) (24h) Motility (48h) (72h) PSE
1 82.55 79.17 15.2 0 2.74
2 85.62 82.58 27.08 2.03
3 84.45 84.37 19.48 2.48
4 86.05 82.88 18.9 5.6
0 0 0 0
6 0 0 0 0
Variable P Value
Boar 0.11
Trt <0.0001
Time <0.0001
Trt*Time <0.0001
Table 17.
Viability Viability Viability
trt (Oh) (24h) Viability (48h) (72h) PSE
1 66.17 64.83 38.17 26.67 3.95
2 75.83 67.33 47.33 28
3 68.83 61.67 40.83 23.67
4 73.33 66.5 34 23.83
5 18.17 13.67 9 8.5
6 5.17 8 9.5 5
Date Recue/Date Received 2023-08-28
Variable P Value
Boar 0.002
Trt <0.0001
Time <0.0001
Trt*Time <0.0001
Table 18.
trt PIA (0) PIA (24) PIA (48) PIA (72) PSE
1 93.33 85.83 84.83 80 2.59
2 91.67 88.17 84.5 77
3 91.5 86.83 85.67 77.33
4 94.5 87.67 85.17 72.83
87 88.67 83 74.83
6 82 90.17 78.17 74
Variable P Value
Boar 0.1
Trt 0.08
Time <0.0001
Trt*Time 0.236
EXAMPLE 6
Example 6 compares the post-sort motility, viability and dead intact acrosomes
(DIA) in
boar semen when using sheath fluid and catch media containing increasing
amounts of a sugar
additive (OmM up to +50mM glucose).
56
Date Recue/Date Received 2023-08-28
The sperm-rich fraction of ejaculates from four boars were collected using the
methods
described in Example 1(A). Two replicates were run for this Example. The sperm
were sorted
using the methods described in Example 1(B). The sheath and catch media
utilized in each
treatment group are shown in Table 19 ("EY" = egg yolk). The media indicated
in Table 19
comprised the components as follows:
"Tris-citrate" and "Tris-citrate + SexedUltraTM" media contained the
components described for
each of those media in Example 3, above.
Table 19.
Trt Sheath Catch
Tris-citrate +
SexedUltraTM +
Control Tris-citrate 20% EY
Tris-citrate +
SexedUltraTM +
Tris-citrate + 1mM Glucose +
1mM 1mM Glucose 20% EY
Tris-citrate +
SexedUltraTM +
Tris-citrate + 5mM Glucose +
5mM 5mM Glucose 20% EY
Tris-citrate +
SexedUltraTM +
Tris-citrate + 10mM Glucose +
10mM 10mM Glucose 20% EY
Tris-citrate +
SexedUltraTM +
Tris-citrate + 25mM Glucose +
25mM 25mM Glucose 20% EY
57
Date Recue/Date Received 2023-08-28
Tris-citrate +
SexedUltraTM +
Tris-citrate + 50mM Glucose +
50mM 50mM Glucose 20% EY
After resuspension, motility (assessed by CASA), viability (assessed by
LIVE/DEAD
Viability/Cytotoxicity Kit; Molecular Probes, Inc.) and DIA (assessed by flow
cytometry) of the
sorted sperm in the five treatment groups were determined at 0, 24, 48, 72 and
96 hours. Pooled
results for motility, viability and DIA for the two replicates are shown in
Tables 20, 21 and 22
below.
Table 20.
Trt Motility Oh Motility 24h Motility 48h Motility 72h Motility
96h
Control 88.32 80.59 70.7 71.82 63.96
1mM 85.49 79.13 76.12 75.1 66.06
5mM 85 80.22 80.05 75.87 69.75
10mM 87.58 82.65 77.82 76.95 73.67
25mM 89.54 82.47 80.85 78.15 69.2
50mM 89.78 81 78.19 75.35 68.76
PSE = 1.84
Variable P Value
trt <0.0001
time <0.0001
Boar <0.0001
trt*time 0.25
58
Date Recue/Date Received 2023-08-28
Table 21.
Trt Viability Oh Viability 24h Viability 48h Viability 72h Viability
96h
Control 87.03 84.55 82 81 81.63
1mM 86.2 83.84 81.16 83.28 82.77
5mM 87.55 86.03 84.36 85.68 86.54
10mM 88.77 87.46 85.02 86.53 86.57
25mM 89.61 87.41 84.1 84.82 81.6
50mM 91.07 89.19 86.72 88.82 86.96
PSE = 1.02
Variable P Value
trt <0.0001
Time <0.0001
boar 0.47
trt*time 0.8
Table 22.
Trt DIA Oh DIA 24h DIA 48h DIA 72h DIA 96h
Control 8 12.1 14.29 15.26 15.61
1mM 8.83 12.79 14.92 13.35 14.35
5mM 8.01 11.42 12.78 11.72 10.82
10mM 7.29 10.55 11.68 10.58 10.49
25mM 6.68 9.82 11.34 11.07 14.39
50mM 6.33 8.7 10.33 9.04 10.5
PSE = 0.81
Variable P Value
trt <0.0001
59
Date Recue/Date Received 2023-08-28
Time <0.0001
boar <0.0001
trt*time 0.21
EXAMPLE 7
The following Example compares the post-sort viability of cryopreserved bovine
semen
when using TRIS-based low sugar media ("Low Sugar" treatment) or TRIS-based
media
containing a sugar additive ("Control"), as the sheath media and the catch
media.
Semen from five bulls was collected and transported to a sorting facility in a
TRIS -based
holding media. The semen was prepared for sorting on the same day as
collection. Semen was
sorted as follows. In the Low Sugar treatment, the TRIS-based sheath media and
the TRIS-based
catch media comprised 10mM glucose. In the Control, the TRIS-based sheath
media comprised
78mM of fructose and the TRIS-based catch media comprised 56mM of fructose.
After sorting,
sperm was cryopreserved in AT straws. Cryopreserved straws (n= 10/treatment)
were thawed
and analyzed for total motility and progressive motility via CASA. Straws with
the highest,
lowest and median values for total motility were pooled and analyzed for
viability using
propidium iodide analysis on a flow cytometer. Post-thaw viability for the Low
Sugar treatment
was 67.53% and for the Control was 62.79% (P Value < 0.02, and bull P Value <
0.0001).
As can be easily understood from the foregoing, the basic concepts of the
present
invention may be embodied in a variety of ways. The invention involves
numerous and varied
embodiments using sex sorted sperm to increase the genetic progress of a line,
including, but not
limited to, the best mode of the invention.
As such, the particular embodiments or elements of the invention disclosed by
the
description or shown in the figures or tables accompanying this application
are not intended to be
limiting, but rather exemplary of the numerous and varied embodiments
generically
encompassed by the invention or equivalents encompassed with respect to any
particular element
thereof. In addition, the specific description of a single embodiment or
element of the invention
Date Recue/Date Received 2023-08-28
may not explicitly describe all embodiments or elements possible; many
alternatives are
implicitly disclosed by the description and figures.
61
Date Recue/Date Received 2023-08-28