Note: Descriptions are shown in the official language in which they were submitted.
CA 03211079 2023-08-11
WO 2022/178177
PCT/US2022/016866
FREEZE-DRIED PLATELET DERIVATIVE COMPOSITIONS FOR TREATING
ANTIPLATELET-INDUCED COAGULOPATHY
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims priority to U.S. Provisional Application Serial
No. 63/150,338, filed on
February 17, 2021, U.S. Provisional Application Serial No. 63/275,937, filed
on November 4, 2021, U.S.
Provisional Application Serial No. 63/276,420, filed on November 5, 2021, U.S.
Provisional Application
Serial No. 63/264,227, filed on November 17, 2021, and U.S. Application Serial
No. 17/673,773, filed on
February 16, 2022, which is a continuation in part of U.S. application number
16/994,377, filed on August
14, 2020 and PCT/U52020/046525 filed on August 14,2020. U.S. application
number 16/994,377, filed on
August 14, 2020, claims priority to U.S. Provisional Application Serial No.
62,887,923, filed on August
16, 2019, and U.S. Provisional Application Serial No. 63,065,337, filed on
August 13, 2020.
PCT/U52020/046525 filed on August 14, 2020, claims priority to U.S.
Provisional Application Serial No.
62,887,923, filed on August 16, 2019, and U.S. Provisional Application Serial
No. 63,065,337, filed on
August 13, 2020. Each of the applications listed in this paragraph is
incorporated herein by reference in its
entirety.
FIELD OF THE INVENTION
[003] This disclosure relates to the use of platelet derivatives as a
treatment for anti-thrombotic agent-
induced coagulopathy. The use of antiplatelet agents can result in increased
bleeding potential.
BACKGROUND
[004] Antiplatelet drugs (also herein called antiplatelet agents) are common
in the U.S. adult population
and employ multiple mechanisms of inhibiting platelet action. Antiplatelet
drugs are used to treat and/or
prevent a number of cerebrovascular and cardiovascular diseases
[005] Antiplatelet drugs, however, are responsible for many adverse drug-
related events (ADEs).
Overdose and adverse events related to these drugs carry the risk of serious
bleeding and related
complications in the patient population. In addition, subjects treated with
antiplatelet drugs face additional
1
RECTIFIED SHEET (RULE 91) ISA/EP
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
complications for surgery, as a subject may need to be tapered off the drugs
before surgery, though cessation
of therapy could put the subject at an increased risk for heart attack,
stroke, or death.
[006] There is therefore a need in the art for the treatment of coagulopathy,
such as antiplatelet agent-
induced coagulopathy, as well as a need for a solution for preparing subjects
taking an anti-platelet drug
for surgery.
SUMMARY OF THE INVENTION
[007] Accordingly, the use of anti-thrombotic agents (i.e. antiplatelet agents
and/or anti-coagulants) can
result in increased bleeding potential of a subject. Here we demonstrate that
platelet derivatives can
circumvent or overcome this inhibition to restore hemostasis. Accordingly,
provided herein are platelet
derivatives, in illustrative embodiment freeze-dried platelet derivative
(FDPD) and compositions
comprising the same, that can reduce this increased bleeding potential of a
subject, and in certain illustrative
embodiments, circumvent or overcome this inhibition of platelets by such anti-
thrombotic agents, to restore
hemostasis.
[008] Provided herein are methods and compositions for treating a coagulopathy
in a subject. Such
methods can include administering to the subject in need thereof, for example
because they have been
administered an anticoagulant agent, an effective amount of a composition
including platelets, or in
illustrative embodiments platelet derivatives, and in further illustrative
embodiments FDPDs. Various
properties of exemplary embodiments of such FDPDs and methods, as well as
numerous additional aspects
and embodiments are provided herein.
[009] Further details regarding aspects and embodiments of the present
disclosure are provided
throughout this patent application. The preceding paragraphs in this Summary
section are not an exhaustive
list of aspects and embodiments disclosed herein. Sections and section headers
are for ease of reading and
are not intended to limit combinations of disclosure, such as methods,
compositions, and kits or functional
elements therein across sections.
BRIEF DESCRIPTION OF THE DRAWINGS
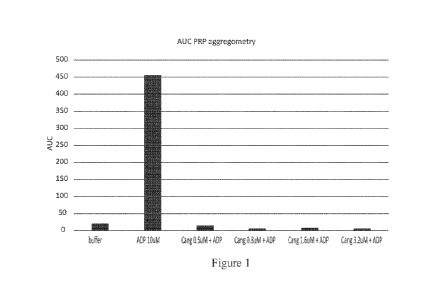
[0010] Figure 1 shows transmission light aggregometry of cangrelor ("Cang") in
platelet rich plasma,
expressed as the integrated aggregation curve, induced by 10 tiM adenosine
diphosphate (ADP) activation
with and without increasing concentrations of cangrelor.
[0011] Figure 2 shows the effect of cangrelor, ADP, or a combination thereof
on platelet occlusion using
T-TAS technology.
[0012] Figure 3 is a bar plot of the area under the curve (AUC) for data sets
from Figure 2. Replicate data
sets from Figure 2 are presented as averages.
2
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[0013] Figure 4 is a bar plot of the occlusion time for data sets from Figure
2. Replicate data sets from
Figure 2 are presented as averages.
[0014] Figure 5 shows the effect of FDPDs ("thromb"; 300,000/ L) supplemented
to platelet rich plasma
in the presence and absence of ADP and cangrelor, at 60, 90, or 115 minutes
post-rehydration on platelet
occlusion using T-TAS@ technology.
[0015] Figure 6 is a bar plot of the AUC for data sets from Figure 5.
Replicate data sets from Figure 5 are
shown as averages.
[0016] Figure 7 is a bar plot of the occlusion time for data sets from Figure
5. Replicate data sets from
Figure 5 are shown as averages.
[0017] Figure 8 is a bar plot of the AUC from aggregation experiments for
platelets (at a concentration of
250,000 platelets per L) treated with collagen (10 pg/mL) and various
concentrations of eptifibatide
("Epti").
[0018] Figure 9 shows the effect of eptifibatide at various concentrations on
whole blood using T-TAS@
technology.
[0019] Figure 10 shows the effect of FDPD ("Tsomes") supplementation
(approximately 200,000/ L) on
whole blood with and without various concentrations of eptifibatide using T-
TAS@ technology.
[0020] Figure 11 is a bar plot of the occlusion time for the data sets from
Figure 10.
[0021] Figure 12 is a bar plot of the AUC for the data sets from Figure 10.
[0022] Figure 13 shows that FDPDs (various lots) occlude in the presence of
eptifibatide in platelet-poor
plasma (PPP).
[0023] Figure 14 is a bar plot of the AUC for data sets from Figure 13.
Replicate data sets from Figure 13
are shown as averages.
[0024] Figure 15 is a bar plot of the occlusion time for the data sets from
Figure 13. Replicate data sets
from Figure 13 are shown as averages.
[0025] Figure 16 is a bar plot of the AUC from aggregation experiments for
platelets treated with collagen
(10 g/mL) or arachidonic acid ("AA"; 500 g/mL) with and without various
concentrations of aspirin
("ASA").
[0026] Figure 17 is a bar plot of the occlusion time for whole blood, whole
blood treated with various
concentrations of aspirin (ASA), and whole blood treated with various
concentrations of aspirin and
supplemented with FDPDs (approximately 200,000-400,000/ L) as measured by
response to collagen
coated plastic under shear using T-TAS@ technology.
[0027] Figure 18 shows the recovery of thrombus formation promoted by FDPDs in
whole blood in the
presence of ASA (200 micromolar), cangrelor (1 micromolar), AP2 6F1 (40
micrograms), as measured by
occlusion time on the T-TAS AR chip coated with thromboplastin and collagen.
3
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[0028] Figure 19 shows the recovery of thrombus formation promoted by FDPDs in
whole blood in the
presence of ASA (200 micromolar), cangrelor (1 micromolar) and 6F1 (40
micrograms/mL), as measured
by occlusion (pressure) over time.
[0029] Figure 20 shows the effect of FDPDs supplementation to aspirin-(ASA-
)inhibited whole blood
(500 micromolar) on the interaction with plastic immobilized porcine collagen
under high shear, as
measured by AUC.
[0030] Figure 21 shows the effect of FDPDs supplementation to aspirin-(ASA-
)inhibited whole blood
(500 micromolar) on the interaction with plastic immobilized porcine collagen
under high shear, as
measured by occlusion (pressure) over time.
[0031] Figure 22 shows the effect of FDPDs supplementation to aspirin-(ASA-
)inhibited whole blood
(100 micromolar) on the interaction with plastic immobilized porcine collagen
under high shear, as
measured by AUC.
[0032] Figure 23 shows the effect of FDPDs supplementation to aspirin-(ASA-
)inhibited whole blood
(100 micromolar) on the interaction with plastic immobilized porcine collagen
under high shear, as
measured by occlusion (pressure) over time.
[0033] Figure 24 shows the effect on peak thrombin of FDPD supplementation to
normal and aspirin-
inhibited plasma.
[0034] Figure 25A shows the effect of cangrelor alone or cangrelor plus FDPDs
on platelet occlusion
using T-TAS@ technology.
[0035] Figure 25B is a bar plot of the occlusion time for data sets from
Figure 25A.
[0036] Figure 26A shows the effect of tirofiban alone, or with random donor
platelets (RDP) or FDPDs
on platelet occlusion using T-TAS@ technology.
[0037] Figure 26B is a bar plot of the occlusion time for data sets from
Figure 26A.
[0038] Figure 27A shows the effect of eptifibatide alone, or with RDP or FDPDs
on platelet occlusion
using T-TAS@ technology.
[0039] Figure 27B is a bar plot of the occlusion time for data sets from
Figure 27A.
[0040] Figure 28A shows the effect of AP2 alone, or with RDP or FDPDs on
platelet occlusion using T-
TAS@ technology.
[0041] Figure 28B is a bar plot of the occlusion time for data sets from
Figure 28A.
[0042] Figure 29A shows the effect of FDPDs on PRP taken from a subject on
aspirin therapy using T-
TAS@ technology.
[0043] Figure 29B is a bar plot of the occlusion time for data sets from
Figure 29A.
[0044] Figure 30A shows the effect of FDPDs on PRP taken from a subject on
aspirin therapy on thrombin
generation.
4
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[0045] Figure 30B is a bar plot of thrombin generation parameters for PRP
taken from a subject on aspirin
therapy, with or without added FDPDs.
[0046] Figure 31A shows aggregometry of PRP taken from a subject on ibuprofen
therapy, with added
buffer, arachidonic acid, or collagen.
[0047] Figure 31B shows the effect of ADP on PRP taken from a subject on
ibuprofen therapy, with or
without FDPDs.
[0048] Figure 32 shows the effect of dosing FDPDs on the bleeding time of mice
treated with a
superpharmacologic dose of clopidogrel.
[0049] Figure 33A shows the effect of cangrelor and ticagrelor on the
occlusion time of Platelet Rich
Plasma (PRP) with and without FDPD
[0050] Figure 33B shows the effect of cangrelor and ticagrelor on the
occlusion time of Platelet Rich
Plasma (PRP) with and without FDPD
[0051] Figure 34 shows the tail snip test for depicting the ability of FDPDs
to restore bleeding time in
NOD-SCID mice treated with supra-pharmacologic clopidogrel.
[0052] Figure 35 shows the ability of FDPDs to restore bleeding time in New
Zealand White Rabbits
treated with supra-pharmacologic clopidogrel. Each line represents a different
rabbit dosed under identical
conditions and with the same dose.
[0053] Figure 36A shows that FDPDs are capable of catalyzing thrombin
generation in the presence of 25
ng/ml rivaroxaban.
[0054] Figure 36B shows that FDPDs are capable of partially recovering the
endogenous thrombin
potential in the presence of 25 ng/ml rivaroxaban.
[0055] Figure 37 shows the occlusion time was partially restored with the
addition of FDPDs into
rivaroxaban treated whole blood.
[0056] Figure 38A shows the activator thrombin potential by FDPDs in
rivaroxaban treated Octaplas.
[0057] Figure 38B shows the maximum thrombin concentration by FDPDs in
rivaroxaban treated
Octaplas.
[0058] Figure 38C shows the ATG lag time by FDPDs in rivaroxaban treated
Octaplas.
[0059] Figure 39A shows the activator thrombin potential by FDPDs in
rivaroxaban treated fresh Platelet
Rich Plasma (PRP).
[0060] Figure 39B shows the maximum thrombin concentration by FDPDs in
rivaroxaban treated fresh
Platelet Rich Plasma (PRP).
[0061] Figure 39C shows the ATG lag time by FDPDs in rivaroxaban treated
Platelet Rich Plasma (PRP).
[0062] Figure 40 shows the effect of FDPDs on time to clot in the presence of
anticoagulants using the
ACT test.
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[0063] Figure 41A shows the effect of 0.1 U heparin on thrombin generation, in
pooled normal plasma,
comparing apheresis units (APU) with FDPDs at 5K and 50K platelets per L.
[0064] Figure 41B shows the impact of 0.8 U/mL heparin reversed by 4 g/m1
protamine (1/2 of the
recommended reversal doses) with FDPDs at 10K and 50K platelets per L.
[0065] Figure 41C shows peak height of thrombin generation of samples which
were treated with heparin
and protamine as described on the x-axis.
[0066] Figure 42 shows the occlusion time with aspirin treatment in the
presence of FDPDs versus PRP
alone.
[0067] Figure 43 shows the occlusion time with both ticagrelor and aspirin in
the presence of FDPDs
versus PRP alone.
[0068] Figure 44A shows the aggregation response of FDPDs in the presence of
agonists, but in the
absence of fresh platelets.
[0069] Figure 44B shows the aggregation response of platelet-rich plasma (PRP)
in the presence of
agonists, but in the absence of fresh platelets.
[0070] Figure 44C shows the comparison of aggregation of FDPDs and PRP in the
presence of 20 M
ADP.
[0071] Figure 44D shows the comparison of aggregation of FDPDs and PRP in the
presence of 10 g/m1
collagen.
[0072] Figure 44E shows the comparison of aggregation of FDPDs and PRP in the
presence of 300 M
epinephrine.
[0073] Figure 44F shows the comparison of aggregation of FDPDs and PRP in the
presence of 1 mg/ml
ristocetin.
[0074] Figure 44G shows the comparison of aggregation of FDPDs and PRP in the
presence of 10 M
TRAP-6.
[0075] Figure 44H shows the comparison of aggregation of FDPDs and PRP in the
presence of 5 mg/ml
arachidonic acid.
[0076] Figure 45A shows that TRAP-6 peptide is capable of promoting platelet
activation by observing
expression of CD62P on the apheresis platelets.
[0077] Figure 45B shows that TRAP-6 peptide is not able to increase the
expression of CD62P on FDPDs.
[0078] Figure 46 shows the measurement of thrombospondin (TSP-1) by flow
cytometry in terms of mean
fluorescent intensity (MFI) in resting fresh platelets, activated fresh
platelets, and different lots of FDPDs.
[0079] Figure 47 shows the measurement of von Willebrand factor (vWF) by flow
cytometry in terms of
mean fluorescent intensity (MFI) in resting fresh platelets, activated fresh
platelets, and different lots of
FDPDs.
6
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[0080] Figure 48 shows the forward scatter (FSC) measured by flow cytometry of
apheresis platelets, and
FDPDs.
[0081] Figure 49 shows exemplary flow cytometry data of FDPDs unstained (dark
data points) or stained
(light data points) with an anti-CD-41 antibody.
[0082] Figure 50 shows an exemplary histogram of FDPDs incubated with annexin
V with (light data
points) and without (dark data points) calcium.
[0083] Figure 51 shows an exemplary histogram of FDPDs incubated with an anti-
CD62 antibody (light
data points) or with an isotype control (dark data points).
[0084] Figure 52 shows a plot of thrombin peak height for FDPDs in the
presence of PRP Reagent
containing tissue factor and phospholipids (solid line and long dashes) and
control cephalin (dots).
[0085] Figure 53A is a plot of the percent occupancy of particles of different
radii in human in-date stored
platelets (Batch J) and platelet derivatives (pre-lyophilization) derived
therefrom as determined by dynamic
light scattering (DLS).
[0086] Figure 53B is a plot of the percent occupancy of particles of different
radii in human in-date stored
platelets (Batch K) and platelet derivatives (pre-lyophilization) derived
therefrom as determined by DLS.
[0087] Figure 53C is a plot of the percent occupancy of particles of different
radii in human in-date stored
platelets (Batch L) and platelet derivatives (pre-lyophilization) derived
therefrom as determined by DLS.
[0088] Figure 54A is a plot of the percent occupancy of particles of different
radii in human in-date stored
platelets (Batch D) and platelet derivatives (pre-lyophilization) derived
therefrom as determined by DLS.
[0089] Figure 54B is a plot of the percent occupancy of particles of different
radii in human in-date stored
platelets (Batch E) and platelet derivatives (pre-lyophilization) derived
therefrom as determined by DLS.
[0090] Figure 54C is a plot of the percent occupancy of particles of different
radii in human in-date stored
platelets (Batch F) and platelet derivatives (pre-lyophilization) derived
therefrom as determined by DLS.
[0091] Figure 55 shows an exemplary histogram comparison of low-plasma FDPDs
unstained (black) or
stained with an isotype control antibody (dark gray) or a FITC-labeled 9F9
antibody (light gray), and a
table showing the mean fluorescence intensity for two replicates.
[0092] Figure 56 shows an exemplary histogram comparison of low-plasma FDPDs
unstained (black) or
stained with an anti-PAC-1 antibody (light gray), and a table showing the mean
fluorescence intensity for
two replicates.
DETAILED DESCRIPTION
[0093] Before embodiments of the present invention are described in detail, it
is to be understood that the
terminology used herein is for the purpose of describing particular
embodiments only, and is not intended
to be limiting. Unless defined otherwise, all technical and scientific terms
used herein have the same
7
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
meaning as commonly understood by one of ordinary skill in the art to which
the term belongs. Although
any methods and materials similar or equivalent to those described herein can
be used in the practice of the
present invention, the preferred methods and materials are now described. All
publications mentioned
herein are incorporated herein by reference to disclose and describe the
methods and/or materials in
connection with which the publications are cited. The present disclosure is
controlling to the extent it
conflicts with any incorporated publication.
[0094] As used herein, the singular forms "a", "an", and "the" include plural
referents unless the context
clearly dictates otherwise. Thus, for example, reference to "a saccharide"
includes reference to one or more
saccharides, and equivalents thereof known to those skilled in the art.
Furthermore, the use of terms that
can be described using equivalent terms include the use of those equivalent
terms. Thus, for example, the
use of the term "subject" is to be understood to include the terms "patient",
"person", "animal", "human",
and other terms used in the art to indicate one who is subject to a medical
treatment. The use of multiple
terms to encompass a single concept is not to be construed as limiting the
concept to only those terms used.
[0095] It is to be understood that the terminology used herein is for the
purpose of describing particular
embodiments only, and is not intended to be limiting. Further, where a range
of values is disclosed, the
skilled artisan will understand that all other specific values within the
disclosed range are inherently
disclosed by these values and the ranges they represent without the need to
disclose each specific value or
range herein. For example, a disclosed range of 1-10 includes 1-9,1-5, 2-10,
3.1-6, 1, 2, 3,4, 5, and so forth.
In addition, each disclosed range includes up to 5% lower for the lower value
of the range and up to 5%
higher for the higher value of the range. For example, a disclosed range of 4 -
10 includes 3.8 - 10.5. This
concept is captured in this document by the term "about".
[0096] It is appreciated that certain features of the invention, which are,
for clarity, described in the context
of separate embodiments, may also be provided in combination in a single
embodiment. Conversely,
various features of the invention, which are, for brevity, described in the
context of a single embodiment,
may also be provided separately or in any suitable sub-combination. All
combinations of the embodiments
pertaining to the invention are specifically embraced by the present invention
and are disclosed herein just
as if each and every combination was individually and explicitly disclosed. In
addition, all sub-
combinations of the various embodiments and elements thereof are also
specifically embraced by the
present invention and are disclosed herein just as if each and every such sub-
combination was individually
and explicitly disclosed herein.
[0097] As used herein, the term "coagulopathy" means any derangement of
hemostasis resulting in either
excessive bleeding or clotting. Coagulopathies caused by administration of an
anti-platelet or anti-coagulant
to a subject typically includes an increased bleeding potential. Thus, methods
herein for treating a
coagulopathy in illustrative embodiments, are methods for decreasing the
bleeding potential of a subject.
8
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[0098] As used herein, the term "platelet" can include whole platelets,
fragmented platelets, platelet
derivatives, or FDPDs. "Platelets" within the above definition may include,
for example, platelets in whole
blood, platelets in plasma, platelets in buffer optionally supplemented with
select plasma proteins, cold
stored platelets, dried platelets, cryopreserved platelets, thawed
cryopreserved platelets, rehydrated dried
platelets, rehydrated cryopreserved platelets, lyopreserved platelets, thawed
lyopreserved platelets, or
rehydrated lyopreserved platelets. "Platelets" may be "platelets" of mammals,
such as of humans, or such
as of non-human mammals.As used herein, "preparation agent" can include any
appropriate components.
In some embodiments, the preparation agent may comprise a liquid medium. In
some embodiments the
preparation agent may comprise one or more salts selected from phosphate
salts, sodium salts, potassium
salts, calcium salts, magnesium salts, and any other salt that can be found in
blood or blood products, or
that is known to be useful in drying platelets, or any combination of two or
more of these. In some
embodiments, the preparation agent comprises one or more salts, such as
phosphate salts, sodium salts,
potassium salts, calcium salts, magnesium salts, and any other salt that can
be found in blood or blood
products. Exemplary salts include sodium chloride (NaCl), potassium chloride
(KC1), and combinations
thereof. In some embodiments, the preparation agent includes from about 0.5 mM
to about 100 mM of the
one or more salts. In some embodiments, the preparation agent includes from
about 0.5 mM to about 100
mM (e.g., about 0.5 to about 2 mM, about 2 mM to about 90 mM, about 2 mM to
about 6 mM, about 50
mM to about 100 mM, about 60 mM to about 90 mM, about 70 to about 85 mM) of
the one or more salts.
In some embodiments, the preparation agent includes about 5 mM, about 75 mM,
or about 80 mM of the
one or more salts. In some embodiments, the preparation agent comprises one or
more salts selected from
calcium salts, magnesium salts, and a combination of the two, in a
concentration of about 0.5 mM to about
2 mM.
[0099] As used herein, "thrombosomes" (sometimes also herein called "Tsomes"
or "Ts", particularly in
the Examples and Figures) are platelet derivatives that have been treated with
an incubating agent (e.g., any
of the incubating agents described herein) and lyopreserved (i.e. freeze-
dried). Thus, thrombosomes are
freeze-dried platelet derivatives (FDPDs). In some cases, FDPDs can be
prepared from pooled platelets.
FDPDs can have a shelf life of 2-3 years in dry form at ambient temperature
and can be rehydrated with
sterile water within minutes for immediate infusion. One example of FDPDs are
THROMBOSOMES ,
which are in clinical trials for the treatment of acute hemorrhage in
thrombocytopenic patients and are a
product of Cellphire, Inc. In non-limiting illustrative embodiments, FDPD
compositions, or illustrative
freeze-dried platelet-derivative (i.e. "FDPD") compositions herein, such as
those prepared according to
Example 15 herein, are compositions that include a population of platelet
derivatives having a reduced
propensity to aggregate such that no more than 10% of the platelet derivatives
in the population aggregate
under aggregation conditions comprising an agonist but no platelets, and
wherein the platelet derivatives
9
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
have a potency of at least 1.5 thrombin generation potency units (TGPU) per
106 platelet derivatives. In
non-limiting illustrative embodiments, FDPD compositions, or illustrative FDPD
compositions herein, such
as those prepared according to Example 15 herein, are compositions that
include platelet derivatives,
wherein at least 50% of the platelet derivatives are CD 41-positive platelet
derivatives, wherein less than
15%, 10%, or in further, non-limiting illustrative embodiments less than 5% of
the CD 41-positive platelet
derivatives are microparticles having a diameter of less than 0.5 tim, and
wherein the platelet derivatives
have a potency of at least 0.5, 1.0 and in further, non-limiting illustrative
embodiments 1.5 thrombin
generation potency units (TGPU) per 106 platelet derivatives. In certain
illustrative embodiments, including
non-limiting examples of the illustrative embodiment in the preceding
sentence, the platelet derivatives are
between 0.5 and 2.5 um in diameter.
[00100] As used herein, an "anticoagulant" is an antithrombotic that does not
include antiplatelet agents.
Typically, agents that inhibit Factor Ha, VIIa, IX, Xa, XI, Tissue Factor, or
vitamin K-dependent synthesis
of clotting factors (e.g., Factor II, VII, IX, or X) or that activate
antithrombin (e.g., antithrombin III) are
considered to be anticoagulants. Other mechanisms of anticoagulants are known.
Anticoagulants include
dabigatran, argatroban, hirudin, rivaroxaban, apixaban, edoxaban,
fondaparinux, warfarin, heparin, and low
molecular weight heparins.
[00101] As used herein, an "antiplatelet agent" is an antithrombotic and does
not include anticoagulants.
Typically, agents that inhibit P2Y receptors (e.g., P2Y12), glycoprotein
Hb/IIIa (I.e. CD41), or that
antagonize thromboxane synthase or thromboxane receptors, are considered to be
antiplatelet agents. Other
mechanisms of antiplatelet agents are known. As used herein, aspirin is
considered to be an antiplatelet
agent but not an anticoagulant. Examples of antiplatelet agents include
aspirin (also called acetylsalicylic
acid or ASA), cangrelor (e.g., KENGREALO), ticagrelor (e.g., BRILINTAO),
clopidogrel (e.g.,
PLAVIXO), prasugrel (e.g., EFFIENTO), eptifibatide (e.g., INTEGRILINO),
tirofiban (e.g.,
AGGRASTATO), and abciximab (e.g., REOPROO). For the purpose of this
disclosure, antiplatelet agents
include agents that inhibit P2Y receptors (e.g., P2Y12), glycoprotein
IIb/IIIa, or that antagonize
thromboxane synthase or thromboxane receptors. Non-limiting examples of
thromboxane A2 antagonists
are aspirin, terutroban, and picotamide. Non-limiting examples of P2Y receptor
antagonists include
cangrelor, ticagrelor, elinogrel, clopidogrel, prasugrel, and ticlopidine. Non-
limiting examples of
glycoprotein IIb/IIIa include abciximab, eptifibatide, and tirofiban. NSAIDS
(e.g., ibuprofen) are also
considered to be antiplatelet agents for the purposes of this disclosure.
Other mechanisms of anti-platelet
agents are known. Antiplatelet agents also include PAR1 antagonists, PAR4
antagonists GPVI antagonists
and alpha2beta 1 collagen receptor antagonists. Non-limiting examples of PAR-1
antagonists include
vorapaxar and atopaxar. As used herein, aspirin is considered to be an
antiplatelet agent but not an
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
anticoagulant. Additional non-limiting examples of antiplatelet agents include
cilostazol, prostaglandin El,
epoprostenol, dipyridamole, treprostinil sodium, and sarpogrelate.
[00102] In some embodiments, an antiplatelet agent can be selected from the
group consisting of aspirin,
cangrelor, ticagrelor, clopidogrel, prasugrel, eptifibatide, tirofiban,
abciximab, and combinations thereof.
In some embodiments, an antiplatelet agent can be selected from the group
consisting of aspirin, cangrelor,
ticagrelor, clopidogrel, prasugrel, eptifibatide, tirofiban, abciximab,
terutroban, picotamide, elinogrel,
ticlopidine, ibuprofen, vorapaxar, atopaxar, and combinations thereof. In some
embodiments, an
antiplatelet agent can be selected from the group consisting of aspirin,
cangrelor, ticagrelor, clopidogrel,
prasugrel, eptifibatide, tirofiban, abciximab, terutroban, picotamide,
elinogrel, ticlopidine, ibuprofen,
vorapaxar, atopaxar, cilostazol, prostaglandin El, epoprostenol, dipyridamole,
treprostinil sodium,
sarpogrelate and combinations thereof. In some embodiments, the antiplatelet
agent can include multiple
antiplatelet agents, such as 2 (or more) of any of the antiplatelet agents
described herein. In some
embodiments, the antiplatelet agent can be aspirin and clopidogrel.
[00103] Cangrelor like clopidogrel, ticagrelor, and prasugrel, blocks the
P2Y12 (ADP) receptor on platelets.
Cangrelor can in some cases be used as a representative of this class of drug.
Cangrelor, unlike clopidogrel
and prasugrel, does not need hepatic metabolism to become biologically active.
[00104] Eptifibatide is a peptide therapeutic that blocks the fibrin binding
role of GPIIb-IIIa receptor on
platelets. The drug is typically administered via IV as a 180 g/kg bolus
followed by 2 g/kg/min
continuous infusion. The blood concentration of eptifibatide is typically
about 1-2 M. Bleeding times
generally return to normal within about 1 hour of drug stoppage.
[00105] Aspirin is an irreversible cyclooxygenase (COX) inhibitor. The COX
enzyme in platelets is
responsible for synthesis of thromboxane A2, prostaglandin E2 and prostacyclin
(PGI2). Aspirin
permanently inactivates the COX enzyme within platelets, and since platelets
do not have the nuclear
material to synthesize new enzyme, new platelets must be produced to overcome
the aspirin effect. Without
thromboxane A2, prostaglandin E2, and prostacyclin (PGI2) platelets are
limited in their pro-aggregation
activity. Many people are maintained on a low dose of aspirin to prevent
unwanted clotting events. Aspirin
bioavailability largely varies with administration route, with a single 500 mg
dose IV at peaks of 500 M
and the same dose orally at 44 M.
[00106] The antiplatelet class of drugs is widely used to prevent unwanted
clotting episodes that lead to
heart failure, stroke, and the like. In many cases, an antiplatelet drug may
need to be reversed or stopped,
or bleeding potential needs to be reduced in some other manner in a subject
who has an antiplatelet drug in
their blood stream, such that a bleeding potential of the subject is
increased. In the case of advanced notice,
as in a pre-planned surgery situation, the antiplatelet drug dose can
sometimes be stopped before the
surgery, preventing unwanted bleeding during surgery. In the case where an
antiplatelet agent needs
11
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
reversing quickly, reversal agents are typically not readily available, are
expensive, or carry significant risk
to the patient. In the case of need for rapid antiplatelet reversal, a
platelet transfusion is typically
administered, though the response to this is often only partial reversal. The
caveat of this course of reversal
is that the newly-infused platelets themselves are susceptible to circulating
drug antiplatelet activity
whereas, in some embodiments, compositions as described herein (e.g.,
including FDPDs) are not. In some
embodiments, compositions as described herein (e.g., including FDPDs) are an
active reversal agent. In
some embodiments, the hemostatic activity of compositions as described herein
(e.g., including FDPDs)
does not succumb to antiplatelet drugs.
[00107] Some exemplary antiplatelet agents and potential methods of reversal
are described below.
[00108] Acetylsalicylic acid (ASA; aspirin) - aspirin acts as a COX-1 blocker
in platelets, which renders
the platelet inactive by irreversibly inhibiting platelet-derived thromboxane
formation. Clinically, aspirin
is sometimes reversed by a platelet transfusion in emergency situations or by
stopping treatment where
surgery is scheduled in the future.
[00109] Clopidogrel (e.g., PLAVIX@) - clopidogrel acts as to prevent ADP from
binding to its receptor on
platelets. ADP binding leads to platelet shape change and aggregation.
Clopidogrel is non-reversible.
Clinically, clopidogrel is sometimes reversed by a platelet transfusion in
emergency situations or by
stopping treatment where surgery is scheduled in the future.
[00110] Cangrelor (e.g., KENGREAL@) - cangrelor acts to prevent ADP from
binding to its receptor on
platelets. ADP binding leads to platelet shape change and aggregation.
Clopidogrel is reversible and
platelet function is returned approximately 1 hour after stopping infusion.
Clinically it is generally
preferred when reversal is needed after a procedure.
[00111] Ticagrelor (e.g., BRILINTA@) - ticagrelor acts to prevent ADP from
binding to its receptor and
acts as an inverse agonist. Ticagrelor is reversible and platelet function can
return after approximately 72
hours of the last dosage. Reversal of action of ticagrelor can be affected by
the time after the last dose. If
the last dose was longer than 24 hours previous, then platelet transfusion can
sometimes be therapeutic to
reverse the results.
[00112] Effient (e.g., PRASUGREL@) - Effient acts to prevent ADP from binding
to its receptor and acts
as a non-reversable antagonist. It being a non-reversible antagonist, new
platelets must be formed to
overcoming its effect. Clinically Effient is reversed by a platelet
transfusion in emergency situations or by
stopping treatment where surgery is scheduled in the future.
[00113] Eptifibatide (Integrilin) -Eptifibatide acts to block the GpIIb/IIIa
and acts as a reversible antagonist.
Clinically, Integrilin is reversed by a platelet transfusion in emergency
situations or by stopping treatment
where surgery is scheduled in the future.
12
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00114] Platelets infusions are currently used as a treatment method for
antiplatelet drugs, but platelet
transfusions only act to dilute out the effect of these drugs. In some
embodiments, FDPDs are not reactive
to these drugs and maintain their ability to aid in clotting. This makes
treatment via FDPDs entirely unique
and introduces a new application for the product.
[00115] Platelet-derived products are not currently used as a treatment method
to counteract the activity of
an anti-thrombotic agent(s) (i.e. anticoagulant or antiplatelet drugs), when
such effects can have detrimental
consequences to a subject or pose an unacceptable risk to a subject, for
example during a surgical procedure
or as the results of a traumatic event. There are no currently approved
reversal agents for antiplatelet agents
or agents that otherwise reduce the bleeding potential of a subject, or
restore hemostasis after treatment
with an anti-coagulant and/or antiplatelet agent. As such, emergency
treatments (pre-op, trauma, and the
like) are typically require blanket precautions to avoid or mitigate
hemorrhage. Non-limiting examples
include infusion of plasma, red blood cells, and anti-fibrinolytics. Platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs) provided herein,
overcome this long-standing need,
and are an effective alternative or supplement to these general treatments or
risk-mitigation strategies.
[00116] The results provided in numerous Examples in the Examples section
herein demonstrate the impact
of a composition comprising FDPDs product in an in vitro model of a subject
taking antiplatelet drugs.
FDPD compositions and other lyophilized platelet products are designed for
infusion into a subject's
bloodstream following diagnosis of trauma or hemostatic failure. These anti-
platelet drugs utilize multiple
forms of platelet inhibition mechanisms which inhibit platelet response to
adenosine diphosphate (ADP),
arachidonic acid, fibrinogen and von Willebrand factor binding to name a few.
These include drugs like
aspirin, clopidogrel, ticagrelor, effient, cangrelor and eptifibatide.
Regardless of the mechanism, FDPDs
provided herein are able to decrease the bleeding potential of a subject
taking such anti-platelet agents, and
in some embodiments, restore normal hemostasis to the subject.
[00117] Without being bound by any particular theory, it is believed that
certain platelet derivatives, in
illustrative embodiments FDPDs provided herein, can work at least in part by
providing a procoagulant
negatively charged surface to augment thrombin generation above and beyond
that suppressed by the anti-
coagulants. Similarly, without being bound by any particular theory, it is
believed that certain platelet
derivatives, in illustrative embodiments FDPDs provided herein, can work at
least in part by binding to and
co-aggregating with circulating platelets. Thus, such FDPDs provide the
surprising property of being able
to reduce bleeding potential of a subject taking an anti-thrombotic agent
(i.e. anti-coagulant or anti-platelet
agent) despite having a reduced ability to aggregate and despite retaining at
least some if not all of the
surface markers that are targeted by at least some, if not all anti-platelet
agents.
[00118] Products and methods are described herein for controlling bleeding and
improving healing. The
compositions, products and methods described herein can also be used to
counteract the activity of any of
13
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
the antiplatelet agents disclosed herein (e.g., as non-limiting examples,
aspirin (also called acetylsalicylic
acid or ASA), cangrelor (e.g., KENGREALO), ticagrelor (e.g., BRILINTAO),
clopidogrel (e.g.,
PLAVIXO), prasugrel (e.g., EFFIENTO), eptifibatide (e.g., INTEGRILINO),
tirofiban (e.g.,
AGGRASTATO), or abciximab (e.g., REOPROO)). The products and methods disclosed
herein in certain
embodiments, are directed toward embodiments that can aid in the closure and
healing of wounds.
[00119] In certain aspects, provided herein, a composition comprising
platelets, or in illustrative
embodiments a composition comprising platelet derivatives, which in further
illustrative embodiments are
FDPDs, may be delivered to a wound on the surface of or in the interior of a
patient. In various
embodiments, a composition comprising platelets, or in illustrative
embodiments a composition comprising
platelet derivatives, which in further illustrative embodiments are FDPDs, or
in illustrative embodiments a
composition comprising platelet derivatives, which in further illustrative
embodiments are FDPDs can be
applied in selected forms including, but not limited to, adhesive bandages,
compression bandages, liquid
solutions, aerosols, matrix compositions, and coated sutures or other medical
closures. In embodiments, a
composition comprising platelets, or in illustrative embodiments a composition
comprising platelet
derivatives, which in further illustrative embodiments are FDPDs may be
administered to all or only a
portion of an affected area on the surface of a patient. In other embodiments,
a composition comprising
platelets, or in illustrative embodiments a composition comprising platelet
derivatives, which in further
illustrative embodiments are FDPDs may be administered systemically, for
example via the blood stream.
In embodiments, an application of the platelet derivative can produce
hemostatic effects for 2 or 3 days,
preferably 5 to 10 days, or most preferably for up to 14 days.
[00120] Some aspects provide a method of treating a coagulopathy in a subject,
the method comprising
administering to the subject in need thereof an effective amount of a
composition comprising platelets, or
in illustrative embodiments a composition comprising platelet derivatives,
which in further illustrative
embodiments are FDPDs. In some embodiments, the composition comprising FDPDs
further comprises
additional components, such as components that were present when such FDPDs
were freeze-dried. Such
additional components can include components of an incubating agent comprising
one or more salts, a
buffer, and in certain embodiments a cryoprotectant (also called a
lyophilizing agent) and/or an organic
solvent. For example, such compositions can comprise one or more saccharides,
as provided further herein,
which in illustrative embodiments include trehalose and in further
illustrative embodiments include
polysucrose.
[00121] Some aspects provide a method of treating a coagulopathy in a subject,
the method comprising
administering to the subject in need thereof an effective amount of a
composition prepared by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
14
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00122] In some embodiments of any of the methods described herein, the
coagulopathy is the result of the
presence of an antiplatelet agent in the blood of a subject.
[00123] Some aspects provide a method of treating coagulopathy in a subject,
wherein the subject has been
treated or is being treated with an antiplatelet agent, the method comprising
administering to the subject in
need thereof an effective amount of a composition comprising platelets, or in
illustrative embodiments a
composition comprising platelet derivatives, which in further illustrative
embodiments are FDPDs and an
incubating agent comprising one or more salts, a buffer, optionally a
cryoprotectant, and optionally an
organic solvent.
[00124] Some aspects provide a method of treating coagulopathy in a subject,
wherein the subject has been
treated or is being treated with an antiplatelet agent, the method comprising
administering to the subject in
need thereof an effective amount of a composition prepared by a process
comprising incubating platelets
with an incubating agent comprising one or more salts, a buffer, optionally a
cryoprotectant, and optionally
an organic solvent, to form the composition.
[00125] Some aspects provide a method of restoring normal hemostasis in a
subject, the method comprising
administering to the subject in need thereof an effective amount of a
composition comprising platelets, or
in illustrative embodiments a composition comprising platelet derivatives,
which in further illustrative
embodiments are FDPDs. In some embodiments, the composition comprising FDPDs
further comprises
additional components, such as components that were present when such FDPDs
were freeze-dried. Such
additional components can include components of an incubating agent comprising
one or more salts, a
buffer, and in certain embodiments a cryoprotectant (also called a
lyophilizing agent) and/or an organic
solvent. For example, such compositions can comprise one or more saccharides,
as provided further herein,
which in illustrative embodiments include trehalose and in further
illustrative embodiments include
polysucrose.
[00126] Some aspects provide a method of restoring normal hemostasis in a
subject, the method comprising
administering to the subject in need thereof an effective amount of a
composition prepared by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
[00127] Some aspects provide a method of restoring normal hemostasis in a
subject, wherein the subject
has been treated or is being treated with an antiplatelet agent, the method
comprising administering to the
subject in need thereof an effective amount of a composition comprising
platelets, or in illustrative
embodiments a composition comprising platelet derivatives, which in further
illustrative embodiments are
FDPDs. In some embodiments, the composition comprising FDPDs further comprises
additional
components, such as components that were present when such FDPDs were freeze-
dried. Such additional
components can include components of an incubating agent comprising one or
more salts, a buffer, and in
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
certain embodiments a cryoprotectant (also called a lyophilizing agent) and/or
an organic solvent. For
example, such compositions can comprise one or more saccharides, as provided
further herein, which in
illustrative embodiments include trehalose and in further illustrative
embodiments include polysucrose.
[00128] Some embodiments provide a method of restoring normal hemostasis in a
subject, wherein the
subject has been treated or is being treated with an antiplatelet agent, the
method comprising administering
to the subject in need thereof an effective amount of a composition prepared
by a process comprising
incubating platelets with an incubating agent comprising one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent, to form the composition.
[00129] Compositions as described herein can also be administered to prepare a
subject for surgery, in some
cases. For some patients taking an antiplatelet agent, it may be difficult or
impossible to reduce the dosage
of the antiplatelet agent before surgery (e.g., in the case of trauma or other
emergency surgery). For some
patients taking an antiplatelet agent, it may be inadvisable to reduce the
dosage of the antiplatelet agent
before surgery (e.g., if the patient would be at risk of a thrombotic event
(e.g., deep vein thrombosis,
pulmonary embolism, or stroke) if the dosage of the antiplatelet agent were
reduced over time.
[00130] Accordingly, some embodiments provide a method of preparing a subject
for surgery, the method
comprising administering to the subject in need thereof an effective amount of
a composition comprising
platelets, or in illustrative embodiments a composition comprising platelet
derivatives, which in further
illustrative embodiments are FDPDs. In some embodiments, the composition
comprising FDPDs further
comprises additional components, such as components that were present when
such FDPDs were freeze-
dried. Such additional components can include components of an incubating
agent comprising one or more
salts, a buffer, and in certain embodiments a cryoprotectant (also called a
lyophilizing agent) and/or an
organic solvent. For example, such compositions can comprise one or more
saccharides, as provided further
herein, which in illustrative embodiments include trehalose and in further
illustrative embodiments include
polysucrose.
[00131] Some embodiments provide a method of preparing a subject for surgery,
the method comprising
administering to the subject in need thereof an effective amount of a
composition prepared by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
[00132] Some embodiments provide a method of preparing a subject for surgery,
wherein the subject has
been treated or is being treated with an antiplatelet agent, the method
comprising administering to the
subject in need thereof an effective amount of a composition comprising
platelets, or in illustrative
embodiments a composition comprising platelet derivatives, which in further
illustrative embodiments are
FDPDs. In some embodiments, the composition comprising FDPDs further comprises
additional
components, such as components that were present when such FDPDs were freeze-
dried. Such additional
16
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
components can include components of an incubating agent comprising one or
more salts, a buffer, and in
certain embodiments a cryoprotectant (also called a lyophilizing agent) and/or
an organic solvent. For
example, such compositions can comprise one or more saccharides, as provided
further herein, which in
illustrative embodiments include trehalose and in further illustrative
embodiments include polysucrose.
[00133] Some embodiments provide a method of preparing a subject for surgery,
wherein the subject has
been treated or is being treated with an antiplatelet agent, the method
comprising administering to the
subject in need thereof an effective amount of a composition prepared by a
process comprising incubating
platelets with an incubating agent comprising one or more salts, a buffer,
optionally a cryoprotectant, and
optionally an organic solvent, to form the composition.
[00134] In some embodiments, a surgery can be an emergency surgery (e.g., in
the case of trauma) or a
scheduled surgery.
[00135] In some embodiments, treatment with an anticoagulant can be stopped
(e.g., in preparation for
surgery) in illustrative embodiments before the composition comprising
platelet derivatives is administered
to the subject. In some embodiments, treatment with an anticoagulant can
continue in illustrative
embodiments for a time period after the composition comprising platelet
derivatives is administered to the
subject. Such a time period can include 1, 2, 3, 4õ 5, 6, or 7 days, or 1, 2,
3, or 4 weeks, or 1, 2, or 3 months
or longer.
[00136] Some embodiments provide a method of ameliorating the effects of an
antiplatelet agent in a
subject, the method comprising administering to the subject in need thereof an
effective amount of a
composition comprising platelets, or in illustrative embodiments a composition
comprising platelet
derivatives, which in further illustrative embodiments are FDPDs and an
incubating agent comprising one
or more salts, a buffer, optionally a cryoprotectant, and optionally an
organic solvent.
[00137] Some aspects provide a method of ameliorating the effects of an
antiplatelet agent in a subject, the
method comprising administering to the subject in need thereof an effective
amount of a composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00138] In some embodiments, the effects of an antiplatelet agent may need to
be ameliorated due to an
incorrect dosage of an antiplatelet agent. For example, in some embodiments,
the effects of an antiplatelet
agent can be ameliorated following an overdose of the antiplatelet agent. In
some embodiments, the effects
of an antiplatelet agent may need to be ameliorated due to a potential for
interaction with another drug (e.g.,
a second antiplatelet agent). For example, in some embodiments, the effects of
an antiplatelet agent can be
ameliorated following an erroneous dosing of two or more drugs, at least one
of which is an antiplatelet
agent.
17
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00139] In some embodiments of any of the methods described herein, the
composition can further comprise
an active agent, such as an anti-fibrinolytic agent. Non-limiting examples of
anti-fibrinolytic agents include
e-aminocaproic acid (EACA), tranexamic acid, aprotinin, aminomethylbenzoic
acid, and fibrinogen. In
some embodiments, platelets or platelet derivatives can be loaded with an
active agent, such as an anti-
fibrinolytic agent.
[00140] Compositions comprising FDPDs herein, in certain embodiments have the
surprising property that
they can reduce bleeding potential and in illustrative embodiments, restore
hemostasis in a subject whose
blood has an elevated bleeding potential, independent of whether a laboratory
test for bleeding potential of
the subject is negative or positive after administration of the FDPDs. Such
elevated bleeding potential in
illustrative embodiments is typically because an effective amount of anti-
platelet agent was delivered to the
subject and is in the blood of the subject. Accordingly, in any of the aspects
herein, in some embodiments,
the composition comprising FDPDs has the property that it is capable of
reducing the bleeding potential of
the subject, independent of whether a post-administering evaluation of
bleeding potential, if performed,
yields a normal or abnormal result. In some embodiments such post-
administering evaluation comprises an
in vitro laboratory test performed on a sample taken or drawn at a time
period, for example, between 1 and
4, or 1 and 3, or 1 and 2 hours after administering the composition comprising
FDPDs to the subject. In
other embodiments of any of the aspects herein, wherein the composition
comprising FDPDs has the
property that it is capable of reducing the bleeding potential of a subject
such that normal hemostasis is
restored in a subject having an increased bleeding potential, independent of
whether a post-administering
evaluation of bleeding potential yields a normal or abnormal result. In some
embodiments, such post-
administering evaluation if performed, comprises an in vitro laboratory test
performed on a sample taken
or drawn at a time period, for example, between 1 and 4, or 1 and 3, or 1 and
2 hours after administering
the composition comprising FDPDs to the subject. The time period, can be for
example, within 0 minutes
and 72 hours, or between 10 minutes and 72 hours, or between 10 minutes and 48
hours, or between 10
minutes 24 hours, or between 10 minutes and 4 hours, or between 10 minutes and
1 hour, or between 10
minutes and 30 minutes, or between 30 minutes and 24 hours, or between 30
minutes and 4 hours, or
between 30 minutes and 1 hour after administering the composition comprising
the platelet derivatives (e.g.
FDPDs) to the subject. The lab test in certain embodiments, is one or more, or
two or more, or three or
more of the bleeding parameters disclosed herein.
[00141] In any of the aspects herein, in some embodiments the composition
comprising platelet derivatives
(e.g. FDPDs) has the property that it is capable of reducing the bleeding
potential of a subject having an
elevated bleeding potential. Furthermore, the composition comprising FDPDs
typically has the additional
and surprising property, that after being administered to the subject in an
effective amount, for example for
reducing the bleeding potential of the subject, the subject may have an
abnormal value for one or more in
18
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
vitro lab tests, for example of one or more clotting parameters in a post-
administering evaluation performed
using an, or the in vitro laboratory test performed on a blood sample taken
between 15 minutes and 4 hours,
30 minutes and 4 hours, 1 hour and 4 hours, or taken between 15 minutes and 2
hours, 30 minutes and 2
hours, or 1 hour and 2 hours, or taken between 15 minutes and 1 hour or 30
minutes and 1 hour, after
administering the composition comprising FDPDs. In some subembodiments of this
embodiment, the
composition comprising FDPDs has the property that it is capable of reducing
the bleeding potential of a
subject to about or at a normal hemostasis or about or at the hemostasis level
of the subject when not taking
the antiplatelet agent. Yet, in these embodiments, the composition comprising
FDPDs retains the additional
and surprising property, that after being administered to the subject in the
effective amount, such a property
is independent of a post-adminstering lab test for bleeding potential. Thus,
in some embodiments, the
subject would have an abnormal value for the one or more clotting parameters
in a post-administering
evaluation performed using an, or the in vitro laboratory test performed on a
blood sample taken between
1 and 4 hours, or any of the time ranges recited immediately above, after
administering the composition
comprising FDPDs. It will be understood that in methods that include
compositions comprising FDPDs
with such properties, or any properties that include an evaluation or test, no
testing actually needs to be
performed to practice such methods unless such testing step is actually
recited as a step of the method.
[00142] Platelet derivatives, and especially FDPDs provided herein, for
example produced using methods
provided herein, in certain embodiments have a number of identified properties
as disclosed herein. For
example, in some embodiments the FDPDs comprise (detectable amounts of) the
biomolecule (e.g.
receptor) targeted by an anti-platelet reversal agent. For example, the FDPDs
can comprise one or more
biomolecules that are targeted by one or more anti-platelet reversal agents
that are administered or are being
administered to the same subject to which a composition comprising the FDPDs
is administered. In some
embodiments the receptor present on the FDPDs is selected from a P2Y receptor
(e.g., the P2Y12 receptor),
glycoprotein IIb (i.e. CD41)/IIIa (i.e. CD61), thromboxane synthase or
thromboxane receptors, PAR1,
PAR4, VPVI, or collagen receptor (e.g. alpha2betal collagen receptor). In
certain embodiments, at least
55%, 60%, 70%, 75%, 80%, 85%, 90%, or 95% of the platelet derivatives, in
illustrative embodiments
FDPDs, are positive for (i.e. have detectable levels of) a biomolecule
targeted by the anti-platelet agent
administered to the subject and/or detectable in the blood of the subject. As
a few noteworthy non-limiting
examples, the biomarker present on FDPDs can be CD41 or it can be the
CD41/CD61 complex. In some
embodiments, the CD41/CD61 complex on FDPDs is bound by fibrinogen (Factor 1).
[00143]In certain embodiments the composition comprising FDPDs comprises a
population of FDPDs
having a reduced propensity to aggregate such that no more than 2%, 3%, 4%,
5%, 7.5%, 10%, 12.5%,
15%, 17.5%, 20%, 22.5%, or 25% of the FDPDs in the population aggregate under
aggregation conditions
comprising an agonist but no platelets. In certain embodiments the FDPDs have
a potency of at least 1.2
19
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
(e.g., at least 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, or
2.5) thrombin generation potency units
(TGPU) per 106 particles.
[00144] In certain embodiments the FDPDs have one or more characteristics of
super-activated platelets.
Such characteristics can include one or more of the following:
[00145] A) the presence of thrombospondin (TSP) on their surface at a level
that is greater than on the
surface of resting platelets;
[00146] B) the presence of von Willebrand factor (vWF) on their surface at a
level that is greater than on
the surface of resting platelets; and
[00147] C) an inability to increase expression of a platelet activation marker
in the presence of an agonist
as compared to the expression of the platelet activation marker in the absence
of an agonist.
[00148] In some embodiments less than 5% of a population of FDPDs, and in
illustrative embodiments CD
41-positive FDPDs are microparticles having a diameter of less than 0.5
tim.Platelet derivatives herein have
been observed to have numerous surprising properties, as disclosed in further
detail herein. It will be
understood, as illustrated in the Examples provided herein, that although
platelet derivatives in some aspects
and embodiments are in a solid, such as a powder form, the properties of such
platelet derivatives can be
identified, confirmed, and/or measured when a composition comprising such
platelet derivatives is in liquid
form.
[00149] In some embodiments, the platelets or platelet derivatives (e.g.,
FDPDs) have a particle size (e.g.,
diameter, max dimension) of at least about 0.5 m (e.g., at least about at
least about 0.6 m, at least about
0.7 m, at least about 0.8 m, at least about 0.9 m, at least about 1.0 m,
at least about 1.2 m, at least
about 1.5 m, at least about 2.0 m, at least about 2.5 m, or at least about
5.0 m). In some embodiments,
the particle size is less than about 5.0 m (e.g., less than about 2.5 m,
less than about 2.0 m, less than
about 1.5 m, less than about 1.0 m, less than about 0.9 m, less than about
0.8 m, less than about 0.7
m, less than about 0.6 m, less than about 0.5 m, less than about 0.4 m, or
less than about 0.3 m). In
some embodiments, the particle size is from about 0.5 m to about 5.0 m
(e.g., from about 0.5 m to about
4.0 m, from about 0.5 m to about 2.5 m, from about 0.6 m to about 2.0 m,
from about 0.7 m to
about 1.0 m, from about 0.5 m to about 0.9 m, or from about 0.6 m to about
0.8 m).
[00150] In some embodiments, at least 50% (e.g., at least about 55%, at least
about 60%, at least about 65%,
at least about 70%, at least about 75%, at least about 80%, at least about
85%, at least about 90%, at least
about 95%, or at least about 99%) of platelets or platelet derivatives (e.g.,
FDPDs), have a particle size in
the range of about 0.5 m to about 5.0 m (e.g., from about 0.5 m to about
4.0 m, from about 0.5 m to
about 2.5 m, from about 0.6 m to about 2.0 m, from about 0.7 m to about
1.0 m, from about 0.5 m
to about 0.9 m, or from about 0.6 m to about 0.8 m). In some embodiments,
at most 99% (e.g., at most
about 95%, at most about 80%, at most about 75%, at most about 70%, at most
about 65%, at most about
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
60%, at most about 55%, or at most about 50%) of the platelets or platelet
derivatives (e.g., FDPDs), are in
the range of about 0.5 tim to about 5.0 tim (e.g., from about 0.5 tim to about
4.0 tim, from about 0.5 tim to
about 2.5 tim, from about 0.6 tim to about 2.0 tim, from about 0.7 tim to
about 1.0 tim, from about 0.5 tim
to about 0.9 tim, or from about 0.6 tim to about 0.8 tim). In some
embodiments, about 50% to about 99%
(e.g., about 55% to about 95%, about 60% to about 90%, about 65% to about 85,
about 70% to about 80%)
of the platelets or platelet derivatives (e.g., FDPDs) are in the range of
about 0.5 tim to about 5.0 tim (e.g.,
from about 0.5 tim to about 4.0 tim, from about 0.5 tim to about 2.5 tim, from
about 0.6 tim to about 2.0
tim, from about 0.7 tim to about 1.0 tim, from about 0.5 tim to about 0.9 tim,
or from about 0.6 tim to about
0.8 p.m).
[00151] Platelets or platelet derivatives (e.g., FDPDs) as described herein
can have cell surface markers.
The presence of cell surface markers can be determined using any appropriate
method. In some
embodiments, the presence of cell surface markers can be determined using
binding proteins (e.g.,
antibodies) specific for one or more cell surface markers and flow cytometry
(e.g., as a percent positivity,
e.g., using approximately 2.7x105 FDPDs/tit; and about 4.8 tit of an anti-CD41
antibody, about 3.3 tit of
an anti-CD42 antibody, about 1.3 tit of annexin V, or about 2.4 tit of an anti-
CD62 antibody). Non-limiting
examples of cell-surface markers include CD41 (also called glycoprotein IIb or
GPIIb, which can be
assayed using e.g., an anti-CD41 antibody), CD42 (which can be assayed using,
e.g., an anti-CD42
antibody), CD62 (also called CD62P or P-selectin, which can be assayed using,
e.g., an anti-CD62
antibody), phosphatidylserine (which can be assayed using, e.g., annexin V
(AV)), and CD47 (which is
used in self-recognition; absence of this marker, in some cases, can lead to
phagocytosis). The percent
positivity of any cell surface marker can be any appropriate percent
positivity. For example, populations of
platelet derivatives (e.g., FDPDs), such as those prepared by methods
described herein and included in
compositions herein, can have an average CD41 percent positivity of at least
55% (e.g., at least 60%, at
least 65%, at least 67%, at least 70%, at least 75%, at least 80%, at least
85%, at least 90%, or at least 95%).
In some embodiments, at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%
platelet derivatives
that are positive for CD 41 have a size in the range of 0.5-2.5 tim. In some
embodiments, at least 90%,
91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% platelet derivatives that are
positive for CD 41 have
a size in the range of 0.4-2.8 tim. In some embodiments, at least 90%, 91%,
92%, 93%, 94%, 95%, 96%,
97%, 98%, or 99% platelet derivatives that are positive for CD 41 have a size
in the range of 0.3-3 tim.
[00152] As another example, platelets or platelet derivatives (e.g., FDPDs),
such as those described herein,
can have an average CD42 percent positivity of at least 65% (e.g., at least
67%, at least 70%, at least 75%,
at least 80%, at least 85%, at least 90%, or at least 95%). In some
embodiments, at least 90%, 91%, 92%,
93%, 94%, 95%, 96%, 97%, 98%, or 99% platelet derivatives that are positive
for CD 42 have a size in the
range of 0.5-2.5 tim. In some embodiments, at least 90%, 91%, 92%, 93%, 94%,
95%, 96%, 97%, 98%, or
21
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
99% platelet derivatives that are positive for CD 42 have a size in the range
of 0.4-2.8 tim. In some
embodiments, at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
platelet derivatives that
are positive for CD 42 have a size in the range of 0.3-3 tim.
[00153] As another example, platelets or platelet derivatives (e.g., FDPDs),
such as those prepared by
methods described herein, can have an average CD62 percent positivity of at
least 10% (e.g., at least 15%,
at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at least 55%,
at least 60%, at least 65%, at least 70%, at least 75%, at least 80%, at least
82%, at least 83%, at least 84%,
at least 85%, at least 90%, or at least 95%). In some embodiments, at least
70%, 75%, 80%, 85%, 90%,
91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% platelet derivatives that are
positive for CD 62 have
a size in the range of 0.5-2.5 tim. In some embodiments, at least 70%, 75%,
80%, 85%, 90%, 91%, 92%,
93%, 94%, 95%, 96%, 97%, 98%, or 99% platelet derivatives that are positive
for CD 62 have a size in the
range of 0.4-2.8 tim. In some embodiments, at least 70%, 75%, 80%, 85%, 90%,
91%, 92%, 93%, 94%,
95%, 96%, 97%, 98%, or 99% platelet derivatives that are positive for CD 62
have a size in the range of
0.3-3 p.m.
[00154] As yet another example, platelets or platelet derivatives (e.g.,
FDPDs), such as those prepared by
methods described herein, can have an average annexin V positivity of at least
25% (e.g., at least 30%, at
least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at least
60%, at least 65%, at least 70%, at
least 75%, at least 80%, at least 85%, at least 90%, at least 95%, at least
97%, or at least 99%). In some
embodiments, at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%
platelet derivatives that are
positive for annexin V have a size in the range of 0.5-2.5 tim. In some
embodiments, at least 90%, 91%,
92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% platelet derivatives that are
positive for annexin V have a
size in the range of 0.4-2.8 tim. In some embodiments, at least 90%, 91%, 92%,
93%, 94%, 95%, 96%,
97%, 98%, or 99% platelet derivatives that are positive for annexin V have a
size in the range of 0.3-3 tim.
[00155] As another example, platelets or platelet derivatives (e.g., FDPDs),
such as those prepared by
methods described herein, can have an average CD47 percent positivity of at
least about 8% (e.g., at least
about 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, or 55%).
[00156] Platelets or platelet derivatives (e.g., FDPDs) as described herein
can be capable of generating
thrombin, for example, when in the presence of a reagent containing tissue
factor and phospholipids. For
example, in some cases, platelets or platelet derivatives (e.g., FDPDs) (e.g.,
at a concentration of about
4.8x103 particles/tit) as described herein can generate a thrombin peak height
(TPH) of at least 25 nM (e.g.,
at least 30 nM, 35 nM, 40 nM, 45 nM, 50 nM, 52 nM, 54 nM, 55 nM, 56 nM, 58 nM,
60 nM, 65 nM, 70
nM, 75 nM, or 80 nM) when in the presence of a reagent containing tissue
factor (e.g., at 0.25 pM, 0.5 pM,
1 pM, 2 pM, 5 pM or 10 pM) and optionally phospholipids. For example, in some
cases, platelets or platelet
derivatives (e.g., FDPDs) (e.g., at a concentration of about 4.8x103
particles/pL) as described herein can
22
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
generate a TPH of about 25 nM to about 100 nM (e.g., about 25 nM to about 50
nM, about 25 to about 75
nM, about 50 to about 100 nM, about 75 to about 100 nM, about 35 nM to about
95 nM, about 45 to about
85 nM, about 55 to about 75 nM, or about 60 to about 70 nM) when in the
presence of a reagent containing
tissue factor and (e.g., at 0.25 pM, 0.5 pM, 1 pM, 2 pM, 5 pM or 10 pM) and
optionally phospholipids. In
some cases, platelets or platelet derivatives (e.g., FDPDs) (e.g., at a
concentration of about 4.8x103
particles/tit) as described herein can generate a TPH of at least 25 nM (e.g.,
at least 30 nM, 35 nM, 40 nM,
45 nM, 50 nM, 52 nM, 54 nM, 55 nM, 56 nM, 58 nM, 60 nM, 65 nM, 70 nM, 75 nM,
or 80 nM) when in
the presence of PRP Reagent (cat# TS30.00 from Thrombinoscope), for example,
using conditions
comprising 20 tit of PRP Reagent and 80 tit of a composition comprising about
4.8 x 103particles/tit of
platelets or platelet derivatives (e.g., FDPDs). In some cases, platelets or
platelet derivatives (e.g., FDPDs)
(e.g., at a concentration of about 4.8x103 particles/tit) as described herein
can generate a TPH of about 25
nM to about 100 nM (e.g., about 25 nM to about 50 nM, about 25 to about 75 nM,
about 50 to about 100
nM, about 75 to about 100 nM, about 35 nM to about 95 nM, about 45 to about 85
nM, about 55 to about
75 nM, or about 60 to about 70 nM) when in the presence of PRP Reagent (cat#
TS30.00 from
Thrombinoscope), for example, using conditions comprising 20 tit of PRP
Reagent and 80 tit of a
composition comprising about 4.8 x 103partilces/tit of platelets or platelet
derivatives (e.g., FDPDs).
[00157] Platelets or Platelet derivatives (e.g., FDPDs) as described herein
can be capable of generating
thrombin, for example, when in the presence of a reagent containing tissue
factor and phospholipids. For
example, in some cases, platelets or platelet derivatives (e.g., FDPDs) can
have a potency of at least 1.2
(e.g., at least 1.3, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1, 2.2, 2.3, 2.4, or
2.5) thrombin generation potency units
(TGPU) per 106 particles. For example, in some cases, platelets or platelet
derivatives (e.g., FDPDs) can
have a potency of between 1.2 and 2.5 TPGU per 106 particles (e.g., between
1.2 and 2.0, between 1.3 and
1.5, between 1.5 and 2.25, between 1.5 and 2.0, between 1.5 and 1.75, between
1.75 and 2.5, between 2.0
and 2.5, or between 2.25 and 2.5 TPGU per 106 particles). TPGU can be
calculated as follows:
TGPU/million particles = [TPH in nM]* [Potency Coefficient in IU/(nM)] /
110.576 million particles in the
well]. Similarly, the Potency Coefficient for a sample of thrombin can be
calculated as follows: Potency
Coefficient = Calculated Calibrator Activity (IU)/ Effective Calibrator
Activity (nM). In some cases, the
calibrator activity can be based on a WHO international thrombin standard.
[00158] Platelets or platelet derivatives (e.g., FDPDs) as described herein
can be capable of clotting, as
determined, for example, by using a total thrombus-formation analysis system
(T-TASC)). In some cases,
platelets or platelet derivatives as described herein, when at a concentration
of at least 70x103particles/tit
(e.g., at least 73 x103, 100 x103, 150 x103, 173 x103, 200 x103, 250 x103, or
255 x103 particles/tit) can
result in a T-TAS occlusion time (e.g., time to reach kPa of 80) of less than
14 minutes (e.g., less than 13.5,
13, 12.5, 12, 11.5, or 11 minutes), for example, in platelet-reduced citrated
whole blood. In some cases,
23
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
platelets or platelet derivatives as described herein, when at a concentration
of at least 70x103 particles/pL
(e.g., at least 73 x103, 100 x103, 150 x103, 173 x103, 200 x103, 250 x103, or
255 x103 particles/tit) can
result in an area under the curve (AUC) of at least 1300 (e.g., at least 1380,
1400, 1500, 1600, or 1700), for
example, in platelet-reduced citrated whole blood.
[00159] Platelets or platelet derivatives (e.g., FDPDs) as described herein
can be capable of thrombin-
induced trapping in the presence of thrombin. In some cases, platelets or
platelet derivatives (e.g., FDPDs)
as described herein can have a percent thrombin-induced trapping of at least
5% (e.g., at least 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 67%, 70%, 75%, 85%, 90%, or
99%) in the
presence of thrombin. In some cases, platelets or platelet derivatives (e.g.,
FDPDs) as described herein can
have a percent thrombin-induced trapping of about 25% to about 100% (e.g.,
about 25% to about 50%,
about 25% to about 75%, about 50% to about 100%, about 75% to about 100%,
about 40% to about 95%,
about 55% to about 80%, or about 65% to about 75%) in the presence of
thrombin. Thrombin-induced
trapping can be determined by any appropriate method, for example, light
transmission aggregometry.
Without being bound by any particular theory, it is believed that the thrombin-
induced trapping is a result
of the interaction of fibrinogen present on the surface of the platelet
derivatives with thrombin.
[00160] Platelets For platelet derivatives (e.g., FDPDs) as described herein
can be capable of co-
aggregating, for example, in the presence of an aggregation agonist, and fresh
platelets. Non-limiting
examples of aggregation agonists include, collagen, epinephrine, ristocetin,
arachidonic acid, adenosine di-
phosphate, and thrombin receptor associated protein (TRAP). In some cases,
platelets or platelet derivatives
(e.g., FDPDs) as described herein can have a percent co-aggregation of at
least 5% (e.g., at least 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 67%, 70%, 75%, 85%, 90%, or
99%) in the
presence of an aggregation agonist, and fresh platelets. In some cases,
platelets or platelet derivatives (e.g.,
FDPDs) as described herein can have a percent co-aggregation of about 25% to
about 100% (e.g., about
25% to about 50%, about 25% to about 75%, about 50% to about 100%, about 75%
to about 100%, about
40% to about 95%, about 55% to about 80%, or about 65% to about 75%) in the
presence of an aggregation
agonist. Percent co-aggregation can be determined by any appropriate method,
for example, light
transmission aggregometry.
[00161] Platelet derivative compositions, which in certain illustrative
embodiments herein are FDPD
compositions, comprise a population of platelet derivatives (e.g. FDPDs)
having a reduced propensity to
aggregate under aggregation conditions comprising an agonist but no fresh
platelets, compared to the
propensity of fresh platelets and/or activated to aggregate under these
conditions. Platelet derivatives (e.g.,
FDPDs) as described herein in illustrative embodiments, display a reduced
propensity to aggregate under
aggregation conditions comprising an agonist but no fresh platelets, compared
to the propensity of fresh
platelets and/or activated platelets to aggregate under these conditions.
Surprisingly, such FDPDs have the
24
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
ability to increase clotting and aggregation of platelets in in vitro and in
vivo assays, in the presence of anti-
thrombotic agents such as anti-coagulants and anti-platelet agents, under
conditions where such anti-
thrombotic agents reduce clotting and/or aggregation, including in the
presence of two of such agents. It is
noteworthy that aggregation of platelet derivatives is different from co-
aggregation in that aggregation
conditions typically do not include fresh platelets, whereas co-aggregation
conditions include fresh
platelets. Exemplary aggregation and co-aggregation conditions are provided in
the Examples herein. Thus,
in some embodiments, the platelet derivatives as described herein have a
higher propensity to co-aggregate
in the presence of fresh platelets and an agonist, while having a reduced
propensity to aggregate in the
absence of fresh platelets and an agonist, compared to the propensity of fresh
platelets to aggregate under
these conditions. In some embodiments, a platelet derivative composition
comprises a population of platelet
derivatives having a reduced propensity to aggregate, wherein no more than 2%,
3%, 4%, 5%, 7.5%, 10%,
12.5%, 15%, 17.5%, 20%, 22.5%, or 25% of the platelet derivatives in the
population aggregate under
aggregation conditions comprising an agonist but no platelets, in illustrative
embodiments no fresh platelets.
In some embodiments, the population of platelet derivatives aggregate in the
range of 2-30%, 5-25%, 10-
30%, 10-25%, or 12.5-25% of the platelet derivatives under aggregation
conditions comprising an agonist
but no platelets, in illustrative embodiments no fresh platelets.
[00162] As provided in Examples herein, exemplary aggregation conditions and
related methods include
treating FDPD sample preparations at room temperature with an agonist at a
final agonist concentration of
20 tiM ADP, 0.5 mg/mL arachidonic acid, 10 tig/mL collagen, 200 tiM
epinephrine, lmg/mL ristocetin,
and 10 tiM TRAP-6 and measured by LTA, for example, 5 minutes after agonist
addition to the FDPD
sample, which can be compared to LTA measurements of the sample prior to
agonist addition.
[00163]In some embodiments, the platelet derivatives as described herein are
activated to a maximum
extent such that in the presence of an agonist, the platelet derivatives are
not able to show an increase in the
platelet activation markers on them as compared to the level of the platelet
activation markers which were
present prior to the exposure with the agonist. In some embodiments, the
platelet derivatives as described
herein show an inability to increase expression of a platelet activation
marker in the presence of an agonist
as compared to the expression of the platelet activation marker in the absence
of an agonist. In some
embodiments, the agonist is selected from the group consisting of collagen,
epinephrine, ristocetin,
arachidonic acid, adenosine di-phosphate, and thrombin receptor associated
protein (TRAP). In some
embodiments, the platelet activation marker is selected from the group
consisting of Annexin V, and CD
62. In some embodiments, the platelet derivatives as described herein show an
inability to increase
expression of Annexin V in the presence of TRAP. An increased amount of the
platelet activation markers
on the platelets indicates the state of activeness of the platelets. However,
in some embodiments, the platelet
derivatives as described herein are not able to increase the amount of the
platelet activation markers on
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
them even in the presence of an agonist. This property indicates that the
platelet derivatives as described
herein are activated to a maximum extent. In some embodiments, the property
can be beneficial where
maximum activation of platelets is required, because the platelet derivatives
as described herein is able to
show a state of maximum activation in the absence of an agonist.
[00164] Thrombospondin is a glycoprotein secreted from the a-granules of
platelets upon activation. In the
presence of divalent cations, the secreted protein binds to the surface of the
activated platelets and is
responsible for the endogenous lectin-like activity associated with activated
platelets. In some
embodiments, the platelet derivatives have the presence of thrombospondin (TSP-
1) on their surface at a
level that is greater than that presence on the surface of resting platelets,
activated platelets, or lyophilized
fixed platelets. In some embodiments, the platelet derivatives have the
presence of thrombospondin (TSP-
1) on their surface at a level that is at least 10%, 20%, 25%, 30%, 50%, 60%,
70%, 80%, 90%, or 100%
higher than on the surface of resting platelets, or lyophilized fixed
platelets. In some embodiments, the
platelet derivatives have the presence of thrombospondin (TSP-1) on their
surface at a level that is more
than 100% higher than on the surface of resting platelets, or lyophilized
fixed platelets. In some
embodiments, the platelet derivatives when analyzed for the binding of anti-
thrombospondin (TSP)
antibody to the platelet derivatives using flow cytometry exhibit at least 2
folds, 5 folds, 7 folds, 10 folds,
20 folds, 30 folds, 40 folds, 50 folds, 60 folds, 70 folds, 80 folds, 90
folds, or 100 folds higher mean
fluorescent intensity (MFI) in the absence of an agonist as compared to the
MFI of binding of anti-TSP
antibody to the resting platelets. In some embodiments, the platelet
derivatives when analyzed for the
binding of anti-thrombospondin (TSP) antibody to the platelet derivatives
using flow cytometry exhibit at
least 2 folds, 5 folds, 7 folds, 10 folds, 20 folds, 30 folds, or 40 folds
higher mean fluorescent intensity
(MFI) in the absence of an agonist as compared to the MFI of binding of anti-
TSP antibody to the
lyophilized fixed platelets. In some embodiments, the platelet derivatives
when analyzed for the binding of
anti-thrombospondin (TSP) antibody to the platelet derivatives using flow
cytometry exhibit 10-800 folds,
20-800 folds, 100-700 folds, 150-700 folds, 200-700 folds, or 250-500 folds
higher mean fluorescent
intensity (MFI) in the absence of an agonist as compared to the MFI of binding
of anti-TSP antibody to the
resting platelets. In some embodiments, the platelet derivatives when analyzed
for the binding of anti-
thrombospondin (TSP) antibody to the platelet derivatives using flow cytometry
exhibit at least 2 folds, 5
folds, 7 folds, 10 folds, 20 folds, 30 folds, or 40 folds higher mean
fluorescent intensity (MFI) in the absence
of an agonist as compared to the MFI of binding of anti-TSP antibody to the
active platelets. In some
embodiments, the platelet derivatives when analyzed for the binding of anti-
thrombospondin (TSP)
antibody to the platelet derivatives using flow cytometry exhibit 2-40 folds,
5-40 folds, 5-35 folds, 10-35
folds, or 10-30 folds higher mean fluorescent intensity (MFI) in the absence
of an agonist as compared to
the MFI of binding of anti-TSP antibody to the active platelets.
26
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00165] Von Willebrand factor (vWF)P is a multimeric glycoprotein that plays a
major role in blood
coagulation. vWF serves as a bridging molecule that promotes platelet binding
to sub-endothelium and
other platelets, thereby promoting platelet adherence and aggregation. vWF
also binds to collagens to
facilitate clot formation at sites of injury. In some embodiments, the
platelet derivatives as described herein
have the presence of von Willebrand factor (vWF) on their surface at a level
that is greater than that on the
surface of resting platelets, activated platelets, or lyophilized fixed
platelets. In some embodiments, the
platelet derivatives have the presence of von Willebrand factor (vWF) on their
surface at a level that is at
least 10%, 20%, 25%, 30%, 50%, 60%, 70%, 80%, 90%, or 100% higher than on the
surface of resting
platelets, or lyophilized fixed platelets. In some embodiments, the platelet
derivatives when analyzed for
the binding of anti-von Willebrand factor (vWF) antibody to the platelet
derivatives using flow cytometry
exhibits at least 1.5 folds, 2 folds, or 3 folds, or 4 folds higher mean
fluorescent intensity (MFI) in the
absence of an agonist as compared to the MFI of binding of anti-vWF antibody
to the resting platelets, or
lyophilized fixed platelets. In some embodiments, the platelet derivatives
when analyzed for the binding of
anti-von Willebrand factor (vWF) antibody to the platelet derivatives using
flow cytometry exhibits 2-4
folds, or 2.5-3.5 higher mean fluorescent intensity (MFI) in the absence of an
agonist as compared to the
MFI of binding of anti-vWF antibody to the resting platelets, or lyophilized
fixed platelets.
[00166] Platelet derivatives, in illustrative embodiments FDPDs, in further
illustrative aspects and
embodiments herein are surrounded by a compromised plasma membrane. In these
further illustrative
aspects and embodiments, the platelet derivatives lack an integrated membrane
around them. Instead, the
membrane surrounding such platelet derivatives (e.g. FDPDs) comprises pores
that are larger than pores
observed on living cells. Not to be limited by theory, it is believed that in
embodiments where platelet
derivatives have a compromised membrane, such platelet derivatives have a
reduced ability to, or are unable
to transduce signals from the external environment into a response inside the
particle that are typically
transduced in living platelets. Furthermore, such platelet derivatives (e.g.
FDPDs) are not believed to be
capable of mitochondrial activation or glycolysis.
[00167] A compromised membrane can be identified through a platelet
derivative's inability to retain more
than 50% of lactate dehydrogenase (LDH) as compared to fresh platelets, or
cold stored platelets, or
cryopreserved platelets. In some embodiments, the platelet derivatives are
incapable of retaining more than
35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of lactate dehydrogenase as
compared to lactate
dehydrogenase retained in fresh platelets, or cold stored platelets, or
cryopreserved platelets. In some
embodiments, the platelet derivatives exhibit an increased permeability to
antibodies. In some
embodiments, the antibodies can be IgG antibodies. The increased permeability
can be identified by
targeting IgG antibodies against a stable intracellular antigen. One non-
limiting type of stable intracellular
27
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
antigen is 13 tubulin. The compromised membrane of the platelet derivatives
can also be determined by flow
cytometry studies.
[00168] Platelet or platelet derivatives (e.g., FDPDs) as described herein can
retain some metabolic activity,
for example, as evidenced by lactate dehydrogenase (LDH) activity. In some
cases, platelets or platelet
derivatives (e.g., FDPDs) as described herein can retain at least about 10%
(e.g., at least about 12%, 15%,
20%, 25%, 30%, 35%, 40%, or 45%) of the LDH activity of donor apheresis
platelets. Without being bound
by any particular theory, it is believed that the addition of increasing
amounts of polysucrose increases the
amount of LDH activity remained (e.g., products of a preparation agent with 8%
polysucrose have more
retained LDH activity than products of a preparation agent with 4%
polysucrose). Similarly unbound by
any particular theory, it is believed that thermal treatment of a lyophilized
composition comprising platelets
or platelet derivatives (e.g., FDPDs) increases the amount of LDH activity
retained. As another example,
metabolic activity can be evidenced by retained esterase activity, such as the
ability of the cells to cleave
the acetate groups on carboxyfluorescein diacetate succinimidyl ester (CFDASE)
to unmask a fluorophore.
[00169] Clotting parameters of blood (e.g., the subject's blood) can be
assessed at any appropriate time
during the methods described herein. For example, one or more clotting
parameters of blood can be assessed
before administration of a composition comprising platelets, or in
illustrative embodiments a composition
comprising platelet derivatives, which in further illustrative embodiments are
FDPDs as described herein,
e.g., in order to determine the need for administration of a composition
comprising platelets or platelet
derivatives as described herein. For example, such clotting parameters can be
assessed using a pre-
administration evaluation or test ,such as an in vitro lab test. Such test can
be performed on a liquid sample,
for example a blood sample, taken within 7, 5, 3, 2, or 1 day, or within 12,
8, 6, 4, 2, or 1 hour before
administering a composition comprising platelet derivatives to the subject. As
another example, one or
more clotting parameters of blood can be assessed after administration of a
composition comprising
platelets or platelet derivatives as described herein, e.g., in order to
determine the effectiveness of the
administered composition, to determine whether additional administration of
the composition is warranted,
or to determine whether it is safe to perform a surgical procedure. Such post-
administering evaluation or
test can be performed on a liquid sample, for example a blood sample, taken
within 7, 5, 3, 2, or 1 day, or
within 12, 8, 6, 4, 2, or 1 hour after administering a composition comprising
platelet derivatives to the
subject.
[00170] Accordingly, any of the methods described herein can include steps of
assessing one or more
clotting parameters of blood before administration of a composition comprising
platelets or platelet
derivatives as described herein, assessing one or more clotting parameters of
blood after administration of
a composition comprising platelets or platelet derivatives as described
herein, or both.
28
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00171] Any appropriate method can be used to assess (or evaluate) clotting
parameters of blood. Non-
limiting examples of methods include the World Health Organization (WHO)
bleeding scale, prothrombin
time (PT) assay, thrombin generation (TGA; which can be used to generate
parameters such as, e.g., peak
thrombin, endogenous thrombin potential (ETP), and lag time),
thromboelastography (TEG), multiple
electrode aggregometry, light transmission aggregometry (LTA), activated
clotting time (ACT), P2Y12
Reaction Units (PRU) or Aspirin Reaction Units (ARU) tests, and partial
thromboplastin time (PTT or
aPTT).
[00172] The WHO bleeding scale was developed to help clinicians and
researchers assess bleeding,
particularly in the context of toxicity reporting in cancer treatment, but it
is also used in other contexts.
[00173] Prothrombin time (PT) is a measure of how long it takes blood to clot,
typically in the presence of
Tissue Factor. In some cases, PT can be affected by laboratory reagents, so a
normalized ratio (INR) is
more frequently used.
[00174] The activated partial thromboplastin time (aPTT) is a measure of how
long it takes blood to clot,
typically in the presence of an activator such as silica, celite, kaolin, or
ellagic acid. In some cases, aPTT
can be affected by laboratory reagents, so INR is sometimes used instead of or
in addition to aPTT.
[00175] The thrombin generation assay measured the production of thrombin
after sample activation via a
pro-coagulation agent resulting of thrombin enzymatic cleavage of a
fluorescent peptide and release of
fluorescent molecule. The peak thrombin is a measure of the maximum thrombin
produced, lag time, the
time to start of thrombin production, and ETP as the total thrombin
potentially produced.
[00176] In some embodiments, a patient can have a peak thrombin of about 60 nM
to about 170 nM, such
as about 65 nM to about 170 nM, such as about 65 nM to about 120 nM, such as
about 80 nM, before
administration of a composition comprising platelets or platelet derivatives
as described herein.
[00177] Thrombin clotting time (TCT) is a measure of how long it takes blood
to clot, after an excess of
thrombin has been added.
[00178] TEG assesses intrinsic hemostasis via plots of clot strength over
time. Calcium chloride (CaCl2) is
typically used as the initiating reagent. A TEG waveform (see, e.g., Figure
16) has multiple parameters that
can provide information about clotting.
R-time = reaction time (s) - time of latency from start of test to initial
fibrin formation.
K = kinetics (s) ¨ speed of initial fibrin formation, time taken to achieve a
certain level of clot
strength (e.g., an amplitude of 20 mm)
alpha angle = slope of line between R and K - measures the rate of clot
formation.
MA = maximum amplitude (mm) - represents the ultimate strength of the fibrin
clot.
= amplitude 30 minutes after maximum amplitude is reached- represents rate of
lysis phase.
29
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00179] In hypocoagulable blood states, R-time increases and MA decreases. R-
time typically provides a
broader response range than MA.
[00180] Multiple electrode aggregometery (MEA) can also be used to evaluate
clotting parameters of blood.
MEA measures changes in electrical impedance when platelets aggregate on metal
electrodes. Typically,
aggregation agonists such as ADP, epinephrine, collagen, or ristocetin are
used to initiate aggregation.
[00181] Light transmission aggregometry (LTA) is sometimes used to evaluate
clotting parameters of
blood; unaggregated blood allows little light to pass through, but aggregation
(typically initiated by an
agonist) results in an increase in aggregation.
[00182] In the Total Thrombus-formation Analysis System (T-TAS , FUJIMORI
KOGYO CO., LTD), the
sample is forced through collagen-coated microchannels using mineral oil.
Changes in pressure are used to
assess thrombus formation. The Occlusion Start Time is time it takes to reach
10 kPa, and the Occlusion
Time = time it takes to each A80 kPa using an AR chip (e.g., Zacros Item No,
TC0101). According to the
manufacturer, an AR chip can be used for analyzing the formation of a mixed
white thrombus consisting
chiefly of fibrin and activated platelets. It has a flow path (300 p.m wide by
50 tim high) coated with collagen
and tissue factors and can be used to analyze the clotting function and
platelet function. In comparison, a
PL chip can be used for analyzing the formation of a platelet thrombus
consisting chiefly of activated
platelets. A PL chip has a flow path coated with collagen only and can be used
to analyze the platelet
function.
[00183] The ACT assay is the most basic, but possibly most reliable, way to
measure clotting time (tAcT),
determined by a magnet's resistance to gravity as a clot forms around it.
Typical donor blood has a tACT
200-300s using only CaCl2.
[00184]VerifyNow measures platelet aggregation in PRU units (P2Y12 reaction
units) in the presence of
P2Y12 inhibitors (PRU)) or platelet dysfunction in ARU units (aspirin reaction
units) in the presence of
aspirin. The VerifyNow System is a turbidometric based optical detection
system utilising microbeads that
measures platelet induced aggregation as an increase in light transmittance
available from Werfren
(https://www.werfen.com).
[00185] The VerifyNow-P2Y12 Assay/VerifyNow PRU Test is a rapid test that uses
ADP to stimulate
platelets in the presence of PGE1 [Prostaglandin El] and which inhibits
activation downstream of a second
ADP receptor P2Y1 - making the assay more sensitive and specific for the
activity of the P2Y12 receptor
and of drugs that bind to the P2Y12 receptor. The assay system reagent is
designed to specifically measure
P2Y12 - mediated platelet aggregation.
[00186] VerifyNow Aspirin Assay methodology is very similar to the VerifyNow-
P2Y12
Assay! VerifyNow PRU Test where Arachidonic Acid is incorporated to measure
the response of the platelet
to Aspirin. In an individual on Aspirin, Aspirin irreversibly inhibits the COX-
1, the enzyme that converts
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
Arachidonic acid to Thromboxane A [TxA2] and which ultimately activates the
GPIIb/IIIa receptor
involved in platelet aggregation. In the presence of Aspirin the aggregation
does not occur.
[00187] Exemplary normal ranges for some of these clotting parameters are
shown below in Table El.
Typically, a value outside of the ranges shown below is considered to be
abnormal.
[00188] Table El
Evaluation Exemplary Normal Range
Bleeding (WHO scale) 0(4)
LTA (percent aggregation)
5 mon ADP 70 + 10(1)
2 tig/mL collagen 80 + 13(1)
1 mmol/L arachidonic acid 77 + 10(1)
2 mmol/L arachidonic acid 80 + 11(1)
5 mmol/L arachidonic acid 78 + 5(1)
TEG
1 mmol/L arachidonic acid (%
95 + 9(1)
aggregation)
MA (mm) 50 ¨ 60(6)
R-time (minutes) 7.5 ¨ 1 (6)
K (minutes) 3 ¨ 6(6)
Alpha angle (degrees) 45 ¨ 45(6)
PT (seconds) 10 ¨14(5)
aPTT (seconds) 22 ¨ 35(2)
TCT (seconds) 20 ¨ 30(2)
tACT (seconds) 200 - 300
MEA (Units)
ADP-induced 53 ¨ 122(3)
Arachidonic acid-induced 76 ¨ 136(3)
VerifyNow
PRU 180-376(7)
ARU 550-700(7)
(1) DiChiara, et al. "The effect of aspirin dosing on platelet function in
diabetic and nondiabetic patients: an
analysis from the aspirin-induced platelet effect (ASPECT) study." Diabetes
56.12 (2007): 3014-3019.(2)
Thrombosis Canada. Use And Interpretation Of Laboratory Coagulation Tests In
Patients Who Are
Receiving A New Oral Anticoagulant (Dabigatran, Rivaroxaban, Apixaban). 2013
(3) Beynon, et al. "Multiple electrode aggregometry in antiplatelet-related
intracerebral haemorrhage."
Journal of Clinical Neuroscience 20.12 (2013): 1805-1806.
(4) Rodeghiero, Francesco, et al. "Standardization of bleeding assessment in
immune thrombocytopenia:
report from the International Working Group." Blood 121.14 (2013): 2596-2606.
31
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
(5) Cleveland Clinic "Prothrombin Time (PT) test"
haps://my.clevelandclinic.org/health/diagnostics/17691-
prothrombin-time-pt-test#results-and-follow-up . Accessed 15 February 2021.
(6) Bose and Hravnak. "Thromboelastography: a practice summary for nurse
practitioners treating
hemorrhage." The Journal for Nurse Practitioners 11.7 (2015): 702-709.
(7) Regional Medical Laboratory "VerifyNow PRU testing".
https://www.rmlonline.com/site/sections/684.
Accessed 15 February 2021.
[00189] Some embodiments provide a method of increasing thrombin generation in
a subject, the method
comprising administering to the subject in need thereof an effective amount of
a composition comprising
platelets, or in illustrative embodiments a composition comprising platelet
derivatives, which in further
illustrative embodiments are FDPDs and an incubating agent comprising one or
more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00190] Some embodiments, provide a method of increasing thrombin generation
in a subject, the method
comprising administering to the subject in need thereof an effective amount of
a composition prepared by
a process comprising incubating platelets with an incubating agent comprising
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition.
[00191] Some embodiments provide a method of increasing peak thrombin in a
subject, the method
comprising administering to the subject in need thereof an effective amount of
a composition comprising
platelets, or in illustrative embodiments a composition comprising platelet
derivatives, which in further
illustrative embodiments are FDPDs and an incubating agent comprising one or
more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00192] Some embodiments provide a method of increasing peak thrombin in a
subject, the method
comprising administering to the subject in need thereof an effective amount of
a composition prepared by
a process comprising incubating platelets with an incubating agent comprising
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition.
[00193] In some embodiments, prior to the administering, the peak thrombin of
the subject was below 66
nM (e.g., below 64 nM, 62 nM, 60 nM, 55 nM, 50 nM, 45 nM, 40 nM, 35 nM, 30 nM,
25 nM, 20 nM, 15
nM, 10 nM, or 5 nM). In some embodiments, after the administering, the peak
thrombin of the subject is
above 66 nM (e.g., above 68 nM, 70 nM, 75 nM, 80 nM, 85 nM, 90 nM, 95 nM, 100
nM, 110 nM, 120 nM,
130 nM, 140 nM, or 150 nM). In some embodiments, after the administering, the
peak thrombin of the
subject is between 66 and 166 nM. Peak thrombin can be measured by any
appropriate method.
[00194] In some embodiments a composition as provided herein, or a composition
produced by a method
described herein can be administered to a subject because of an abnormal
result in an evaluation of one or
more clotting parameters, e.g., indicating that the subject is in a
hypocoagulable state.
32
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00195] Some embodiments include a method of treating a coagulopathy in a
subject that is being
administered or has been administered an antiplatelet agent, the method
including: (a) determining that the
subject has an abnormal result for evaluation of one or more clotting
parameters; and (b) after (a),
administering to the subject in need thereof an effective amount of a
composition including platelets or
platelet derivatives and an incubating agent including one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00196] Some embodiments include a method of treating a coagulopathy in a
subject that is being
administered or has been administered an antiplatelet agent, the method
including: (a) determining that the
subject an abnormal result for evaluation of one or more clotting parameters;
and (b) after (a), administering
to the subject in need thereof an effective amount of a composition prepared
by a process including
incubating platelets with an incubating agent including one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent, to form the composition.
[00197] Some embodiments include a method of treating a coagulopathy in a
subject that is being
administered or has been administered an antiplatelet agent, the method
including: administering to the
subject in need thereof an effective amount of a composition including
platelets or platelet derivatives and
an incubating agent including one or more salts, a buffer, optionally a
cryoprotectant, and optionally an
organic solvent, wherein before the administering, for example at the moment
before or immediately before
the administering, the subject has been determined to have an abnormal result
for evaluation of one or more
clotting parameters.
[00198] Some embodiments include a method of treating a coagulopathy in a
subject that is being
administered or has been administered an antiplatelet agent, the method
including: administering to the
subject in need thereof an effective amount of a composition prepared by a
process including incubating
platelets with an incubating agent including one or more salts, a buffer,
optionally a cryoprotectant, and
optionally an organic solvent, to form the composition, wherein before the
administering, for example at
the moment before or immediately before the administering, the subject has
been determined to have an
abnormal result for evaluation of one or more clotting parameters.
[00199] In some embodiments of any of the methods herein, a subject has been
administered an
antiplatelet agent or is being administered an antiplatelet agent in any
appropriate time frame. For example,
in some cases, a subject has been administered an antiplatelet agent and/or a
composition comprising
platelet derivatives, in illustrative embodiments FDPDs, before the effect of
a prior dose of the antiplatelet
agent wears off. For example, in some cases, a subject is being administered
an antiplatelet agent and the
effect of the antiplatelet agent has not worn off. As another example, in some
cases, a subject has been
administered an antiplatelet agent (e.g., the most recent dose) within about 1
week, about 5 days, about 3
days, about 36 hours, about 24 hours, about 18 hours, about 12 hours, about 8
hours, about 6 hours, about
33
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
4 hours, about 2 hours, or about 1 hour. As another example, in some cases, a
subject is being administered
an antiplatelet agent and the last dose (e.g., the most recent dose as
prescribed by a medical professional or
self-administered by the subject) was within about 1 week, about 5 days, about
3 days, about 36 hours,
about 24 hours, about 18 hours, about 12 hours, about 8 hours, about 6 hours,
about 4 hours, about 2 hours,
or about 1 hour.
[00200] In some embodiments of any of the methods herein, determination of an
abnormal result for the
evaluation of one or more clotting parameters can be at any appropriate time.
For example, determination
of an abnormal result for the evaluation of one or more clotting parameters
can be before the abnormal
result returns to a normal result. As another example, determination of an
abnormal result for the evaluation
of one or more clotting parameters can be within about 1 week, about 5 days,
about 3 days, about 36 hours,
about 24 hours, about 18 hours, about 12 hours, about 8 hours, about 6 hours,
about 4 hours, about 2 hours,
or about 1 hour of the administering.
[00201] In some embodiments, the method can further include determining the
result of the evaluation
one or more clotting parameters following the administering. For example, in
some embodiments, the
evaluation of the one or more clotting parameters following the administering
shows a normal result for at
least one of the one or more clotting parameters. In some embodiments, the
result of the evaluation of the
one or more clotting parameters following the administering is improved from
the result of the evaluation
of the one or more parameters prior to the administering.
[00202] In some cases, a subject might be administered an antiplatelet agent,
but they were not supposed
to be, for example, if a subject is confused, or if a medical error occurs. In
some such cases, a subject can
be administered any of the compositions provided herein, or a composition
produced by any of the methods
described herein.
[00203] Some embodiments include a method of treating a coagulopathy in a
subject, the method
including: (a) determining that the subject, contrary to medical instruction,
was administered an antiplatelet
agent; and (b) administering to the subject in need thereof an effective
amount of a composition including
platelets or platelet derivatives and an incubating agent including one or
more salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00204] Some embodiments include a method of treating a coagulopathy in a
subject, the method
including: (a) determining that the subject, contrary to medical instruction,
was administered an antiplatelet
agent; and (b) administering to the subject in need thereof an effective
amount of a composition prepared
by a process including incubating platelets with an incubating agent including
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition.
[00205] Some embodiments include a method of treating a coagulopathy in a
subject, the method
including: administering to the subject in need thereof an effective amount of
a composition including
34
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
platelets or platelet derivatives and an incubating agent including one or
more salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent, wherein the subject is
determined to have been
administered an antiplatelet agent contrary to medical instruction.
[00206] Some embodiments include a method of treating a coagulopathy in a
subject, the method
including: administering to the subject in need thereof an effective amount of
a composition prepared by a
process including incubating platelets with an incubating agent including one
or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is determined to have been administered an antiplatelet agent contrary to
medical instruction.
[00207] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: (a) determining that the subject, contrary to medical instruction,
was administered an antiplatelet
agent; and (b) administering to the subject in need thereof an effective
amount of a composition including
platelets or platelet derivatives and an incubating agent including one or
more salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00208] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: (a) determining that the subject, contrary to medical instruction,
was administered an antiplatelet
agent; and (b) administering to the subject in need thereof an effective
amount of a composition prepared
by a process including incubating platelets with an incubating agent including
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition.
[00209] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: administering to the subject in need thereof an effective amount of
a composition including
platelets or platelet derivatives and an incubating agent including one or
more salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent, wherein the subject is
determined to have been
administered an antiplatelet agent contrary to medical instruction.
[00210] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: administering to the subject in need thereof an effective amount of
a composition prepared by a
process including incubating platelets with an incubating agent including one
or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is determined to have been administered an antiplatelet agent contrary to
medical instruction.
[00211] In some embodiments of any of the methods herein, determining that the
subject has been
administered an antiplatelet agent contrary to medical instruction can be at
any appropriate time. For
example, determining that the subject has been administered an antiplatelet
agent contrary to medical
instruction can be before the antiplatelet agent wears off. As another
example, determining that the subject
has been administered an antiplatelet agent contrary to medical instruction
can be within about 1 week,
about 5 days, about 3 days, about 36 hours, about 24 hours, about 18 hours,
about 12 hours, about 8 hours,
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
about 6 hours, about 4 hours, about 2 hours, or about 1 hour of the
administering a composition provided
herein or a composition produced by a method described herein.
[00212] Administration of the antiplatelet agent can include any appropriate
method, including self-
administering by the subject or administering by a medical professional.
[00213] Medical instruction can be any appropriate method, including verbal
instruction, written
instruction, or both verbal and written instruction.
[00214] In some cases, a subject may have been administered or is being
administered a second agent that
affects (e.g., decreases) platelet function. For example, such an
administration can put the subject into a
hypercoagulable state.
[00215] Some embodiments include a method of treating a coagulopathy in a
subject, the method including:
(a) determining that the subject was administered an antiplatelet agent and a
second agent that decreases
platelet function; and (b) administering to the subject in need thereof an
effective amount of a composition
including platelets or platelet derivatives and an incubating agent including
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00216] Some embodiments include a method of treating a coagulopathy in a
subject, the method including:
(a) determining that the subject was administered an antiplatelet agent and a
second agent that decreases
platelet function; and (b) administering to the subject in need thereof an
effective amount of a composition
prepared by a process including incubating platelets with an incubating agent
including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent, to
form the composition.
[00217] Some embodiments include a method of treating a coagulopathy in a
subject, the method including:
administering to the subject in need thereof an effective amount of a
composition including platelets or
platelet derivatives and an incubating agent including one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent, wherein the subject is
determined to have been
administered an antiplatelet agent and a second agent that decreases platelet
function.
[00218] Some embodiments include a method of treating a coagulopathy in a
subject, the method including:
administering to the subject in need thereof an effective amount of a
composition prepared by a process
including incubating platelets with an incubating agent including one or more
salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent, to form the composition,
wherein the subject is
determined to have been administered an antiplatelet agent and a second agent
that decreases platelet
function.
[00219] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: (a) determining that the subject was administered an antiplatelet
agent and a second agent that
decreases platelet function; and (b) administering to the subject in need
thereof an effective amount of a
36
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
composition including platelets or platelet derivatives and an incubating
agent including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent.
[00220] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: (a) determining that the subject was administered an antiplatelet
agent and a second agent that
decreases platelet function; and (b) administering to the subject in need
thereof an effective amount of a
composition prepared by a process including incubating platelets with an
incubating agent including one or
more salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00221] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: administering to the subject in need thereof an effective amount of
a composition including
platelets or platelet derivatives and an incubating agent including one or
more salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent, wherein the subject is
identified as having been
administered an antiplatelet agent and a second agent that decreases platelet
function.
[00222] Some embodiments include a method of restoring normal hemostasis in a
subject, the method
including: administering to the subject in need thereof an effective amount of
a composition prepared by a
process including incubating platelets with an incubating agent including one
or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is identified as having been administered an antiplatelet agent and a second
agent that decreases platelet
function.
[00223] In some embodiments, administration of the second agent is stopped
(for example, if the benefits
of stopping the second agent outweigh the costs of stopping the second agent).
In some embodiments,
administration of the second agent is continued (for example, if removal of
the second agent would be
detrimental to the subject, as can be the case with certain medications, such
as antidepressants).
[00224] The second agent can be any appropriate second agent that affects
(e.g., decreases) platelet
function. For example, a second agent can include (or be selected from the
group consisting of) an
antihypertensive, a proton pump inhibitor, or a combination thereof. As
another example, a second agent
can include (or be selected from the group consisting of) a chemotherapeutic
agent, an antibiotic, a
cardiovascular agent, a H2 antagonist, a neuropsychiatric agent, or a
combination thereof. In some
embodiments, the second agent can include (or be) an antidepressant (e.g., a
selective serotonin reuptake
inhibitor (SSRI), a serotonin antagonist and reuptake inhibitor (SARI), a
serotonin and norepinephrine
reuptake inhibitor (SNRI), or a combination thereof). In some embodiments, the
second agent is not an
anticoagulant.
[00225] In some embodiments of any of the methods provided herein,
administration of the antiplatelet
agent is stopped. In some embodiments of any of the methods provided herein,
administration of the
antiplatelet agent is continued.
37
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00226] In cases, such as certain emergency situations, it can be impossible
to timely determine whether
a subject is being administered an antiplatelet agent. In some such cases, a
composition provided herein or
a composition produced by a method provided herein can be administered to a
subject to prevent a
coagulopathy. In some embodiments, a composition provided herein or a
composition produced by a
method provided herein can be administered to a subject to mitigate the
potential for a coagulopathy in the
subject.
[00227] Some embodiments include a method of preventing or mitigating the
potential for a coagulopathy
in a subject, the method including: (a) determining that information regarding
whether the subject was
administered an antiplatelet agent is unavailable; and (b) administering to
the subject an effective amount
of a composition including platelets or platelet derivatives and an incubating
agent including one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent.
[00228] Some embodiments include a method of preventing or mitigating the
potential for a coagulopathy
in a subject, the method including: (a) determining that information regarding
whether the subject was
administered an antiplatelet agent is unavailable; and (b) administering to
the subject an effective amount
of a composition prepared by a process including incubating platelets with an
incubating agent including
one or more salts, a buffer, optionally a cryoprotectant, and optionally an
organic solvent, to form the
composition.
[00229] Some embodiments include method of preventing or mitigating the
potential for a coagulopathy in
a subject, the method including: administering to the subject in need thereof
an effective amount of a
composition including platelets or platelet derivatives and an incubating
agent including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent,
wherein the subject has been
determined to be a subject for which information regarding whether the subject
was administered an
antiplatelet agent is unavailable.
[00230] Some embodiments include a method of preventing or mitigating the
potential for a coagulopathy
in a subject, the method including: administering to the subject in need
thereof an effective amount of a
composition prepared by a process including incubating platelets with an
incubating agent including one or
more salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition,
wherein the subject has been determined to be a subject for which information
regarding whether the subject
was administered an antiplatelet agent is unavailable.
[00231] In some embodiments of any of the methods herein, determining that
information regarding
whether the subject was administered an antiplatelet agent is unavailable can
be at any appropriate time.
For example, determining that information regarding whether the subject was
administered an antiplatelet
agent is unavailable can be within about 1 week, about 5 days, about 3 days,
about 36 hours, about 24 hours,
about 18 hours, about 12 hours, about 8 hours, about 6 hours, about 4 hours,
about 2 hours, or about 1 hour
38
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
of the administering a composition provided herein or a composition produced
by a method described
herein.
[00232] There are several reasons that information regarding whether the
subjected was administered an
antiplatelet agent is unavailable. For example, a reason can include that the
subject cannot be identified,
that the medical history of the subject is unavailable, that the subject is in
need of emergency treatment,
that the subject is in need of emergency surgery, that the subject is having
emergency surgery, or a
combination thereof.
[00233] In some embodiments of any of the methods provided herein, the method
can further include
determining that the subject has an abnormal result for one or more
evaluations of clotting parameters. In
some embodiments of any of the methods provided herein the subject has been
determined to have an
abnormal result for one or more evaluations of clotting parameters.
[00234] In some cases, before an abnormal result was determined, the subject
was previously identified
as having a normal result for at least one of the one or more clotting
parameters.
[00235] In some embodiments of any of the methods provided herein, the method
can further include
determining the result of the evaluation one or more clotting parameters
following the administering of a
composition provided herein or a composition produced by a method provided
herein. In some such cases,
the evaluation of the one or more clotting parameters following the
administering shows a normal result,
such as defined in Table El for at least one of the one or more clotting
parameters. In some embodiments,
the result of the evaluation of the one or more clotting parameters following
the administering is improved
from the result of the evaluation of the one or more parameters prior to the
administering.
[00236] In some embodiments, the subject is identified as having an abnormal
result for one or more
evaluations of clotting parameters during surgery (e.g., emergency surgery or
scheduled surgery)
[00237] An evaluation of one or more clotting parameters can be any
appropriate evaluation of clotting
parameters, such as any of the evaluations of clotting parameters provided
herein. In some embodiments,
an evaluation of clotting parameters can be selected from the group consisting
of the World Health
Organization (WHO) bleeding scale, prothrombin time (PT) assay, international
normalized ratio (INR),
thrombin generation (TGA), thromboelastography (TEG), multiple electrode
aggregometry (MEA), light
transmission aggregometry (LTA), activated clotting time (ACT), VerifyNow, and
partial thromboplastin
time (e.g., PTT or aPTT), subparameters thereof, and a combination of any
thereof.
[00238] In some embodiments, the one or more clotting parameters includes an
evaluation of bleeding
(e.g., performed based on the World Health Organization (WHO) bleeding scale).
In some embodiments,
before the administering, the subject has bleeding of grade 2, 3, or 4 based
on the WHO bleeding scale. In
some embodiments, after the administering, the subject has bleeding of grade 0
or 1 based on the WHO
bleeding scale. In some embodiments, after the administering, the subject has
bleeding of one grade less,
39
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
based on the WHO bleeding scale, than before the administering. In some
embodiments, after the
administering, the subject has bleeding of two grades less, based on the WHO
bleeding scale, than before
the administering. In some embodiments, after the administering, the subject
has bleeding of three grades
less, based on the WHO bleeding scale, than before the administering.
[00239] In some embodiments, the one or more clotting parameters includes an
evaluation of prothrombin
time (PT). In some embodiments the abnormal results for PT comprises a PT of
greater than about 14
seconds (e.g., greater than about 15 seconds, 18 seconds, 20 seconds, 25
seconds, or more). In some
embodiments, after the administering, the subject has a decrease in PT of at
least 1 second (e.g., at least 2,
3, 4, 5, 6, 7, 8, 9, 10, or more, seconds). In some embodiments, after the
administering, the subject has a
normal PT, such as defined in Table El.
[00240] In some embodiments, the one or more clotting parameters includes an
evaluation of activated
partial thromboplastin time (aPTT). In some embodiments, the abnormal result
for aPTT comprises an
aPTT of greater than about 40 seconds (e.g., greater than about 43 seconds, 45
seconds, 50 seconds, 55
seconds, 60 seconds, 65 seconds, 70 seconds, or more). In some embodiments,
after the administering, the
subject has a decrease in aPTT of at least 5 seconds (e.g., at least 10, 15,
20, 25, 30, or more, seconds). In
some embodiments, after the administering, the subject has a normal aPTT, such
as defined in Table El.
[00241] In some embodiments, the one or more clotting parameters includes an
evaluation of thrombin
clot time (TCT). In some embodiments, the abnormal result for TCT comprises a
TCT of greater than about
35 seconds (e.g., greater than about 38 seconds, 40 seconds, 45 seconds, 50
seconds, 55 seconds, 60
seconds, or more). In some embodiments, after the administering, the subject
has a decrease in TCT of at
least 5 seconds (e.g., at least 10, 15, 20, 25, 30, or more, seconds. In some
embodiments, after the
administering, the subject has a normal TCT, such as defined in Table El.
[00242] In some embodiments, the evaluation of the one or more clotting
parameters includes
thromboelastography (TEG). In some embodiments, the abnormal result for TEG
comprises a maximum
amplitude (MA) of less than about 50 mm (e.g., less than about 48 mm, 45 mm,
40 mm, 35 mm, or less).
In some embodiments, after the administering, the subject has an increase in
MA of at least 5 mm (e.g., at
least 10, 15, 20, 25, 30, or more, mm). In some embodiments, after the
administering, the subject has a
normal MA, such as defined in Table El. In some embodiments, the abnormal
result for TEG comprises a
percent aggregation (in the presence of 1 mmol/L arachidonic acid) of less
than about 85% (e.g., less than
about 83%, 80%, 75%, 70%, or less). In some embodiments, after the
administering, the subject has an
increase in percent aggregation (in the presence of 1 mmol/L arachidonic acid)
of at least 2 percentage
points (e.g., at least, 3, 5, 8, 10, 12, 15, 18, 20, or more, percentage
points). In some embodiments, after the
administering, the subject has a normal percent aggregation (in the presence
of 1 mmol/L arachidonic acid),
such as defined in Table El. In some embodiments, the TEG is used to evaluate
adenosine diphosphate-
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
induced platelet-fibrin clot strength. In some embodiments, the TEG is used to
evaluate arachidonic acid-
induced platelet-fibrin clot strength.
[00243] In some embodiments, the evaluation of one or more clotting parameters
includes VerifyNow. In
some embodiments, abnormal result for VerifyNow comprises a P2Y12 reaction
unit (PRU) of less than
about 195 (e.g., less than about 190, 185, 180, 170, 165, 160, 155, or less),
In some embodiments, after the
administering, the subject has an increase in PRU of at least 25 (e.g., at
least 30, 35, 40, 45, 50, 75, 100, or
more). In some embodiments, after the administering, the subject has a normal
PRU, such as defined in
Table El. In some embodiments, the abnormal result for VerifyNow comprises an
Aspirin Reaction Unit
(ARU) of less than about 550 (e.g., less than about 540, 530, 520, 510, 500,
490, 480, 470, or less). In
some embodiments, after the administering, the subject has an increase in ARU
of at least 25 (e.g., at least
30, 35, 40, 45, 50, 75, 100, or more). In some embodiments, after the
administering, the subject has a normal
ARU, such as defined in Table El.
[00244] In some embodiments, the evaluation of one or more clotting parameters
includes multiple
electrode aggregometry (MEA). In some embodiments the abnormal result for MEA
comprises an abnormal
result for ADP-induced platelet activity. In some embodiments the abnormal
result for MEA comprises a
result of less than about 50 units (U) (e.g., less than about 48, 45, 40, 35,
or less, U) for ADP-induced
platelet activity. In some embodiments, after the administering, the subject
has an increase in ADP-induced
platelet activity by at least 5 U (e.g., at least 8, 10, 15, 20, or more U).
In some embodiments, after the
administering, the subject has a normal value for ADP-induced platelet
activity, such as defined in Table
El. In some embodiments, the abnormal result for MEA comprises an abnormal
result for arachidonic acid-
induced platelet activity. In some embodiments, the abnormal result for MEA
comprises a result of less
than about 70 units (U) (e.g., less than about 68, 65, 60, 55, 50, 45, or
less, U) for arachidonic acid-induced
platelet activity. In some embodiments, after the administering, the subject
has an increase in arachidonic
acid-induced platelet activity by at least 5 (e.g., at least 8, 10, 15, 20, or
more, units). In some embodiments,
after the administering, the subject has a normal value for arachidonic acid-
induced platelet activity, such
as defined in Table El.
[00245] In some embodiments, the evaluation of one or more clotting parameters
includes light
transmission aggregometry (LTA). In some embodiments, the abnormal result for
LTA includes, in the
presence of 5 mon adenosine diphosphate, a percent aggregation of less than
about 60% (e.g., less than
about 58%, 55%, 50%, 45%, or less). In some embodiments, the abnormal result
for LTA includes, in the
presence of 2 tig/mL collagen, a percent aggregation of less than about 65%
(e.g., less than about 63%,
60%, 55%, 50%, 45%, or less). ). In some embodiments, the abnormal result for
LTA includes, in the
presence of 1 mmol/L arachidonic acid, a percent aggregation of less than
about 65% (e.g., less than about
63%, 60%, 55%, 50%, 45%, or less). ). In some embodiments, the abnormal result
for LTA includes, in
41
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
the presence of 2 mmol/L arachidonic acid, a percent aggregation of less than
about 69% (e.g., less than
about 67%, 65%, 60%, 55%, 50%, 45%, or less). ). In some embodiments, the
abnormal result for LTA
includes, in the presence of 5 mmol/L arachidonic acid, a percent aggregation
of less than about 73% (e.g.,
less than about 70%, 65%, 60%, 55%, 50%, or less). In some embodiments, after
the administering, the
subject has an increase in percent aggregation (in the presence of 5 mon
adenosine diphosphate) of at
least 2 percentage points (e.g., at least 3, 5, 8, 10, 12, or more, percentage
points). In some embodiments,
after the administering, the subject has a normal percent aggregation (in the
presence of 5 mon adenosine
diphosphate), such as defined in Table El. In some embodiments, after the
administering, the subject has
an increase in percent aggregation (in the presence of 2 tig/mL collagen) of
at least 2 percentage points
(e.g., at least 3, 5, 8, 10, 12, or more, percentage points). In some
embodiments, after the administering, the
subject has a normal percent aggregation (in the presence of 2 tig/mL
collagen), such as defined in Table
El. In some embodiments, after the administering, the subject has an increase
in percent aggregation (in
the presence of 1 mmol/L arachidonic acid) of at least 2 percentage points
(e.g., at least 3, 5, 8, 10, 12, or
more, percentage points). In some embodiments after the administering, the
subject has a normal percent
aggregation (in the presence of 1 mmol/L arachidonic acid), such as defined in
Table El. In some
embodiments, after the administering, the subject has an increase in percent
aggregation (in the presence of
2 mmol/L arachidonic acid) of at least 2 percentage points (e.g., at least 3,
5, 8, 10, 12, or more, percentage
points). In some embodiments, after the administering, the subject has a
normal percent aggregation (in the
presence of 2 mmol/L arachidonic acid), such as defined in Table El. In some
embodiments, after the
administering, the subject has an increase in percent aggregation (in the
presence of 5 mmol/L arachidonic
acid) of at least 2 percentage points (e.g., at least, 3, 5, 8, 10, 12, or
more, percentage points). In some
embodiments, after the administering, the subject has a normal percent
aggregation (in the presence of 5
mmol/L arachidonic acid), such as defined in Table El.
[00246]In some embodiments, an additional antiplatelet agent reversal agent
can be administered to a
subject in addition to a composition provided herein or a composition produced
by a method described
herein. The additional antiplatelet agent reversal agent can be administered
in any order with the
composition provided herein or the composition produced by a method provided
herein. In some
embodiments, the administering of the composition occurs concurrently with
administering of the
additional antiplatelet agent reversal agent. In some embodiments, the
administering of the composition
occurs after administering of the additional antiplatelet agent reversal
agent. In some embodiments, the
administering of the composition occurs before administering of the additional
antiplatelet agent reversal
agent.
[00247]In one aspect of any of the embodiments herein, the subject does not
have cancer.
42
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00248] An "effective amount" as used herein is an amount of the composition
that comprises an amount
of platelets, typically platelet derivatives, which in illustrative
embodiments are FDPDs, effective in
treating the subject. Such treating, for example with respect to methods for
treating a coagulopathy or
methods for counteracting the effect of an anti-thrombotic agent (i.e. an anti-
platelet agent or an anti-
coagulant) of a subject herein reduces the bleeding potential of the subject.
In some embodiments, the
bleeding potential can be reduced to such an extent that normal hemostasis is
restored for the subject, such
as to a level for that subject without any anti-platelet agent in their body,
at least for a period of time. Thus,
in some embodiments, an effective amount of a composition comprising platelet
derivatives, for example
FDPDs, is an amount that results in reduced bleeding potential of a subject,
which in some embodiments
results in normal hemostasis, for any period of time. In some embodiments, the
bleeding potential is
reduced for at least 10, 20, 30, 40, 50 or 60 minutes after being administered
a dose of an effective amount
of the platelet derivatives, for example the FDPDs, or a second, third,
fourth. Fifth, or sixth dose of
composition comprising platelet derivatives that each, or two or more, or all,
cumulatively add up to an
effective dose.
[00249] Such an amount of platelets or typically platelet derivatives (e.g.,
FDPDs) includes any
appropriate dosage of a composition comprising the platelet derivatives as
described herein that can be
administered to the subject, in illustrative embodiments that results in
reduced bleeding potential of a
subject. For example, in some embodiments, a dose of a composition comprising
platelets or platelet
derivatives (e.g., FDPDs) can include between about or exactly 1.0 x 107 to
1.0 x 10" particles (e.g.
FDPDs)/kg of a subject, 1.0 x 107 to 1.0 x 1010 particles (e.g. FDPDs)/kg of
a subject, 1.6 x 107 to 1.0 x
1010 particles (e.g. FDPDs/kg of subject, 1.6 x 107 to 5.1 x 10 particles
(e.g. FDPDs/kg of a subject, 1.6 x
107 to 3.0 x 10 particles (e.g. FDPDs)/kg of a subject, 1.6 x 107 to 1.0 x 10
particles (e.g. FDPDs)/kg of a
subject, 1.6 x 107 to 5.0 x 10' particles (e.g. FDPDs)/kg of a subject,1.6 x
107 to 1.0 x 10' particles (e.g.
FDPDs)/kg of a subject, 1.6 x107 to 5.0 x 10 particles (e.g. FDPDs)/kg of a
subject, 5.0 x 107 to 1.0 x 108
particles (e.g. FDPDs)/kg of a subject, 1.0 x 10' to 5.0 x 10' particles (e.g.
FDPDs)/kg of a subject, 5.0 x
10' to 1.0 x 10 particles (e.g. FDPDs)/kg of a subject, 1.0 x 109 to 5.0 x 10
particles (e.g. FDPDs)/kg of a
subject, or 5.0 x 109 to 1.0 x 1010 particles (e.g. FDPDs)/kg of a subject).
In some embodiments an effective
amount of a composition comprising FDPDs is an activity-based amount that has
a potency between 250
and 5000 TGPU per kg of a subject. Such activity-based amount can be combined
with any of the particle
number ranges/kg in this paragraph.
[00250] In certain embodiments, any of the dose ranges provided above, and in
illustrative embodiments
those that include less than 1 x 10" particles/kg, can be administered more
than 1 time to a subject. For
example, a dose range of between 1.0 x 107 particles to about 1.0 x 1010
particles, can be administered
between 2 and 10 times, or between 2 and 8 times, or between 2 and 6 times, or
between 3 and 8 times, or
43
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
between 3 and 6 times, or between 4 and 6 times in a timeframe between within
1, 2, 3, 4, 5, or 7 days from
the first dose.
[00251] In some embodiments of the methods herein, the composition is
administered topically. In some
embodiments, topical administration can include administration via a solution,
cream, gel, suspension,
putty, particulates, or powder. In some embodiments, topical administration
can include administration via
a bandage (e.g. an adhesive bandage or a compression bandage) or medical
closure (e.g., sutures, staples));
for example the platelet derivatives (e.g., lyopreserved platelets (e.g.,
FDPDs)) can be embedded therein or
coated thereupon), as described in PCT Publication No. W02017/040238 (e.g.,
paragraphs 1101314069]),
corresponding to U.S. Patent Application Serial number 15/776,255, the
entirety of which is herein
incorporated by reference.
[00252] In some embodiments of the methods herein, the composition is
administered parenterally. In
some illustrative embodiments of the methods herein, the composition is
administered intravenously. In
some embodiments of the methods herein, the composition is administered
intramuscularly. In some
embodiments of the methods herein, the composition is administered
intrathecally. In some embodiments
of the methods herein, the composition is administered subcutaneously. In some
embodiments of the
methods herein, the composition is administered intraperitoneally.
[00253] In some embodiments of the methods herein, the composition is dried
prior to the administration
step. In illustrative embodiments of the methods herein, the composition is
freeze-dried prior to the
administration step. Such FDPD composition in illustrative embodiments, is
prepared according to methods
provided in the Examples herein. In illustrative embodiments of the methods
herein, the composition is
rehydrated following the drying or freeze-drying step, for example within 24,
12, 8, 6, 4, 3, 2, or 1 hour, or
within 30, 20. 15, 10, or 5 minutes before being administered to a subject.
[00254] In some embodiments, the antiplatelet agent is selected from the group
consisting of aspirin (also
called acetylsalicylic acid or ASA); a P2Y12 inhibitor such as cangrelor
(e.g., KENGREALC,), ticagrelor
(e.g., BRILINTAC,), clopidogrel (e.g., PLAVIVD), or prasugrel (e.g., EFFIENT
); a glycoprotein IIb/IIIa
inhibitor such as eptifibatide (e.g., INTEGRILINC), tirofiban (e.g.,
AGGRASTATC,), or abciximab (e.g.,
REOPROC)); supplements such as herbal supplements; or a combination of any
thereof. Examples of
supplements include ginger, ginseng, ginkgo, green tea, kava, saw palmetto,
boldo (Peumus boldus),
Danshen (Salvia miltiorrhiza), Dong quai (Angelica sinensis) papaya (Carica
papaya), fish oil, and vitamin
E. Examples of herbal supplements include ginger, ginseng, and ginkgo.
The prescribing information for each of the FDA-approved anticoagulants
provided herein is incorporated
by reference in its entirety. Such prescribing information includes, for
example:
44
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00255] HIGHLIGHTS OF PRESCRIBING INFORMATION for DURLAZA@ (aspirin), Revised
12/2019.
[00256] HIGHLIGHTS OF PRESCRIBING INFORMATION for KENGREAL@ (cangrelor),
Revised: 6/2015.
[00257] HIGHLIGHTS OF PRESCRIBING INFORMATION for BRILINTA@ (ticagrelor),
Revised: 09/2016.
[00258] HIGHLIGHTS OF PRESCRIBING INFORMATION for PLAVIX@ (clopidogrel
bisulfate), Revised: August 2010.
[00259] HIGHLIGHTS OF PRESCRIBING INFORMATION for EFFIENT@ (prasugrel),
Revised
09/2011.
[00260] HIGHLIGHTS OF PRESCRIBING INFORMATION for INTEGRILIN@ (eptifibatide),
Revised 03/2013.
[00261] HIGHLIGHTS OF PRESCRIBING INFORMATION for AGGRASTAT@ (tirofiban),
Revised 08/2016.
[00262] PRESCRIBING INFORMATION for REOPRO@ (abciximab), Revision Date:
11/1997.
[00263] APPROVAL PACKAGE for TICLID@ (ticlopidine hydrochloride), Revised
03/2001.
[00264] DESCRIPTION - MOTRIN@ (ibuprofen), Effective Date 1/2007.
[00265] HIGHLIGHTS OF PRESCRIBING INFORMATION for ZONTIVITYTm (vorapaxar),
Revised 5/2014.
[00266] HIGHLIGHTS OF PRESCRIBING INFORMATION for PLETAL@ (cilostazol),
Revised
5/2017.
[00267] HIGHLIGHTS OF PRESCRIBING INFORMATION for VELETRI@ (epoprostenol),
Revised 06/2012.
[00268] PRESCRIBING INFORMATION for PERSANTINE@ (dipyridamole), Revised
12/2019.
[00269] HIGHLIGHTS OF PRESCRIBING INFORMATION for REMODULIN@
(acenocoumarol), 12/2014.
[00270] In some embodiments, the antiplatelet agent is aspirin. In some
embodiments, the antiplatelet
agent is cangrelor (e.g., KENGREAL@). In some embodiments, the antiplatelet
agent is ticagrelor (e.g.,
BRILINTA@). In some embodiments, the antiplatelet agent is clopidogrel (e.g.,
PLAVIX@). In some
embodiments, the antiplatelet agent is prasugrel (e.g., EFFIENT@). In some
embodiments, the antiplatelet
agent is eptifibatide (e.g., INTEGRILIN@). In some embodiments, the
antiplatelet agent is tirofiban (e.g.,
AGGRASTAT@). In some embodiments, the antiplatelet agent is abciximab (e.g.,
REOPRO@). In some
embodiments, the antiplatelet agent is terutroban. In some embodiments, the
antiplatelet agent is
picotamide. In some embodiments, the antiplatelet agent is elinogrel. In some
embodiments, the antiplatelet
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
agent is ticlopidine. In some embodiments, the antiplatelet agent is
ibuprofen. In some embodiments, the
antiplatelet agent is vorapaxar. In some embodiments, the antiplatelet agent
is atopaxar. In some
embodiments, the antiplatelet agent is cilostazol. In some embodiments, the
antiplatelet agent is
prostaglandin El. In some embodiments, the antiplatelet agent is epoprostenol.
In some embodiments, the
antiplatelet agent is dipyridamole. In some embodiments, the antiplatelet
agent is treprostinil sodium. In
some embodiments, the antiplatelet agent is sarpogrelate. In some embodiments,
the antiplatelet agent is a
supplement. In some embodiments, the antiplatelet agent is an herbal
supplement.
[00271] In some embodiments, the antiplatelet agent was last administered at a
dosage and timepoint
relative to the time that the composition comprising platelet derivatives is
administered to a subject, such
that the blood of the subject comprises the antiplatelet agent, in
illustrative embodiments, a detectable
quantity of the antiplatelet agent. Typically, such agent is present in the
blood of the subject at the time the
subject is administered a dose of the composition comprising the composition
comprising platelet
derivatives, and is present in the blood of the subject in an amount that is
sufficient to increase the bleeding
potential of the subject. For example, the anti-platelet agent is present at a
concentration that is sufficient
to yield an abnormal value for one or more clotting parameters, for example in
an in vitro test. In some
embodiments, the antiplatelet agent is present in the subject at the time the
composition comprising the
FDPDs is administered at a level that increases the bleeding potential of the
subject. In some embodiments,
the antiplatelet agent is present at a Cmax within 15, 30 or 45 minutes, or
within 1, 2, 3, 4, 6, or 8 hours of
the time the composition comprising the FDPDs is administered or the time the
first or last dose of the
composition comprising the FDPDs is administered.
[00272] In some embodiments, antiplatelet agent comprises aspirin at a dosage
of about 80 mg to about
700 mg (e.g., 80 mg, 100 mg, 150 mg, 200 mg, 250 mg, 300 mg, 400 mg, 500 mg,
600 mg, or 700 mg),
once, twice, three times, or four times a day. In some embodiments, the
antiplatelet agent comprises aspirin,
and the subject achieved a C. of about 3 to about 25 mg/L (e.g., about 3 to
about 5 mg/L for a dose of
about 100 mg, about 10 to about 15 mg/L for a dose of about 300 mg, or about
20 to about 25 mg/L for a
dose of about 500 mg). See also, e.g., Nagelschmitz, J et al.
"Pharmacokinetics and pharmacodynamics of
acetylsalicylic acid after intravenous and oral administration to healthy
volunteers." Clinical Pharmacology
: Advances and Applications vol. 651-9. 19 Mar. 2014, doi:10.2147/CPAA.S47895.
In some embodiments,
the antiplatelet agent comprises aspirin, and before the administering of a
composition provided herein or
a composition produced by a method described herein, the subject had a ARU of
about 350 to about 549
ARU (e.g., about 400 to about 500 ARU). In some embodiments, the antiplatelet
agent comprises aspirin,
and after the administering of a composition provided herein or a composition
produced by a method
described herein, the subject had a ARU of about 550 to about 700 ARU (e.g.,
about 600 to about 700
ARU). In some embodiments, the antiplatelet agent comprises aspirin, and
before the administering of a
46
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
composition provided herein or a composition produced by a method described
herein, the subject had a
high on-treatment platelet reactivity (HPR) using MEA of greater than about 30
units (e.g., greater than
about 33, 35, 40, 45, 50, or more units). See, e.g., Kriiger, Jan-Christopher,
et al. "Monitoring ASA and
P2Y12-specific platelet inhibition¨comparison of conventional (single) and
multiple electrode
aggregometry." Scandinavian journal of clinical and laboratory investigation
74.7 (2014): 568-574.
[00273] In some embodiments, the antiplatelet agent comprises cangrelor at an
initial dosage of about 25
to about 35 pig/kg body weight of the subject (e.g., about 30 pig/kg body
weight of the subject) or a following
dosage of about 3 to about 5 pg/kg/min body weight of the subject (e.g., about
4 lug/kg/min body weight of
the subject). In some embodiments, the antiplatelet agent comprises cangrelor,
and the subject achieved a
C. of about 400 to about 500 ng/mL. See, e.g., the manufacturer's fact sheet
for KENGREAL found at
haps
://resources.chiesiusa.com/Kengreal/KENGREAL_Dosing_and_Administration_Fact_She
et.pdf . In
some embodiments, the antiplatelet agent comprises cangrelor, and before the
administering of a
composition provided herein or a composition produced by a method described
herein, the subject had a
maximum amplitude (TEG-ADP) of less than about 50 mm (e.g., less than about 48
mm, 45 mm, 40 mm,
or less). In some embodiments, the antiplatelet agent comprises cangrelor, and
after the administering of a
composition provided herein or a composition produced by a method described
herein, the subject had a
maximum amplitude (TEG-ADP) of at least about 50 mm (e.g., at least 53 mm, 55
mm, 50 mm, 60 mm, 65
mm, 70 mm, or more). See, e.g., Corliss BM, Polifka AJ, Harris NS, Hoh BL, Fox
WC. Laboratory
assessments of therapeutic platelet inhibition in endovascular neurosurgery:
comparing results of the
VerifyNow P2Y12 assay to thromboelastography with platelet mapping. J
Neurosurg. 2018 Nov
1;129(5):1160-1165. doi: 10.3171/2017.6.JNS17535. PMID: 29271717.
[00274] In some embodiments, the antiplatelet agent comprises ticagrelor at an
initial dosage of about
170 to about 190 mg (e.g., about 180 mg), or a following dosage in a first
year of treatment of about 80 to
about 100 mg (e.g., about 90 mg) twice daily, or a following dosage in a
second year of treatment of about
50 to about 70 mg twice daily (e.g., about 60 mg), optionally in combination
with aspirin. In some
embodiments, the antiplatelet agent comprises ticagrelor, and the subject
achieved a C. of about 550 to
about 650 ng/mL (e.g., about 600 mg/L). See, e.g., Teng R, Muldowney S, Zhao
Y, Berg JK, Lu J, Khan
ND. Pharmacokinetics and pharmacodynamics of ticagrelor in subjects on
hemodialysis and subjects with
normal renal function. Eur J Clin Pharmacol. 2018 Sep;74(9):1141-1148. doi:
10.1007/s00228-018-2484-
7. Epub 2018 May 30. PMID: 29850937; PMCID: PMC6096709. In some embodiments,
the antiplatelet
agent comprises ticagrelor, and before the administering of a composition
provided herein or a composition
produced by a method described herein, the subject had a PRU of less than
about 180 (e.g., less than about
175, 170, 165, 160, 155, 150, or less). In some embodiments, the antiplatelet
agent comprises ticagrelor,
and after the administering of a composition provided herein or a composition
produced by a method
47
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
described herein, the subject had a PRU of at least 180 (e.g., at least 180,
185, 190, 195, 200, 225, 250, 275,
300, 350, or more). In some embodiments, the antiplatelet agent comprises
ticagrelor, and after the
administering of a composition provided herein or a composition produced by a
method described herein,
the subject had a PRU of about 180 to about 376 PRU (e.g., about 200 to about
300 PRU). In some
embodiments, the antiplatelet agent comprises ticagrelor, and before the
administering of a composition
provided herein or a composition produced by a method described herein, the
subject had a maximum
amplitude (TEG-ADP) of less than about 50 mm (e.g., less than about 48 mm, 45
mm, 40 mm, or less). In
some embodiments, the antiplatelet agent comprises ticagrelor, and after the
administering of a composition
provided herein or a composition produced by a method described herein, the
subject had a maximum
amplitude (TEG-ADP) of at least about 50 mm (e.g., at least 53 mm, 55 mm, 50
mm, 60 mm, 65 mm, 70
mm, or more). See, e.g., Corliss BM, Polifka AJ, Harris NS, Hoh BL, Fox WC.
Laboratory assessments of
therapeutic platelet inhibition in endovascular neurosurgery: comparing
results of the VerifyNow P2Y12
assay to thromboelastography with platelet mapping. J Neurosurg. 2018 Nov
1;129(5):1160-1165. doi:
10.3171/2017.6.JN517535. PMID: 29271717.
[00275] In some embodiments, the antiplatelet agent comprises clopidogrel at
an initial dosage of about
275 to about 325 mg (e.g., about 300 mg), or a following dosage of about 70 to
about 80 mg (e.g., about 75
mg) once daily, optionally in combination with aspirin. In some embodiments,
the antiplatelet agent
comprises clopidogrel, and the subject achieved a C. of about 1 to about 40
mg/L (e.g., about 1 to about
15 ng/mL for a dosage of about 75 mg, or about 1 to about 40 ng/mL for a
dosage of about 300 mg). See,
e.g., Karainiewicz-Lada, Marta et al. "Clinical pharmacokinetics of
clopidogrel and its metabolites in
patients with cardiovascular diseases." Clinical Pharmacokinetics vol. 53,2
(2014): 155-64.
doi:10.1007/s40262-013-0105-2. In some embodiments, the antiplatelet agent
comprises clopidogrel, and
before the administering of a composition provided herein or a composition
produced by a method
described herein, the subject had a PRU of less than about 180 (e.g., less
than about 175, 170, 165, 160,
155, 150, or less). In some embodiments, the antiplatelet agent comprises
clopidogrel, and after the
administering of a composition provided herein or a composition produced by a
method described herein,
the subject had a PRU of at least 180 (e.g., at least 180, 185, 190, 195, 200,
225, 250, 275, 300, 350, or
more). In some embodiments, the antiplatelet agent comprises clopidogrel, and
after the administering of a
composition provided herein or a composition produced by a method described
herein, the subject had a
PRU of about 180 to about 376 PRU (e.g., about 200 to about 300 PRU). In some
embodiments, the
antiplatelet agent comprises clopidogrel, and before the administering of a
composition provided herein or
a composition produced by a method described herein, the subject had a high on-
treatment platelet reactivity
(HPR) using MEA of greater than about 47 units (e.g., greater than about 48,
50, 55, or more units). See,
e.g., Kriiger, Jan-Christopher, et al. "Monitoring ASA and P2Y12-specific
platelet inhibition¨comparison
48
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
of conventional (single) and multiple electrode aggregometry." Scandinavian
journal of clinical and
laboratory investigation 74.7 (2014): 568-574. In some embodiments, the
antiplatelet agent comprises
clopidogrel, and before the administering of a composition provided herein or
a composition produced by
a method described herein, the subject had a maximum amplitude (TEG-ADP) of
less than about 50 mm
(e.g., less than about 48 mm, 45 mm, 40 mm, or less). In some embodiments, the
antiplatelet agent
comprises clopidogrel, and after the administering of a composition provided
herein or a composition
produced by a method described herein, the subject had a maximum amplitude
(TEG-ADP) of at least about
50 mm (e.g., at least 53 mm, 55 mm, 50 mm, 60 mm, 65 mm, 70 mm, or more). See,
e.g., Corliss BM,
Poliflca AJ, Harris NS, Hoh BL, Fox WC. Laboratory assessments of therapeutic
platelet inhibition in
endovascular neurosurgery: comparing results of the VerifyNow P2Y12 assay to
thromboelastography with
platelet mapping. J Neurosurg. 2018 Nov 1;129(5):1160-1165. doi:
10.3171/2017.6.JN517535. PMID:
29271717.
[00276] In some embodiments, the antiplatelet agent comprises prasugrel at an
initial dosage of about 50
to about 70 mg (e.g., about 60 mg), or a following dosage of about 3 to about
12 mg (e.g., about 5 mg or
about 10 mg) once daily, optionally in combination with aspirin. In some
embodiments, the antiplatelet
agent comprises prasugrel, and the subject achieved a C. of about 200 to about
525 ng/mL (e.g., about
330 to about 350 ng/mL for a dose of about 20 mg). See, e.g., Umemura, Kazuo,
and Takayuki Iwaki. "The
Pharmacokinetics and Pharmacodynamics of Prasugrel and Clopidogrel in Healthy
Japanese
Volunteers." Clinical Pharmacology in Drug Development vol. 5,6
(2016): 480-487.
doi:10.1002/cpdd.259. In some embodiments, the antiplatelet agent comprises
prasugrel, and before the
administering of a composition provided herein or a composition produced by a
method described herein,
the subject had a PRU of less than about 180 (e.g., less than about 175, 170,
165, 160, 155, 150, or less). In
some embodiments, the antiplatelet agent comprises prasugrel, and after the
administering of a composition
provided herein or a composition produced by a method described herein, the
subject had a PRU of at least
180 (e.g., at least 180, 185, 190, 195, 200, 225, 250, 275, 300, 350, or
more). In some embodiments, the
antiplatelet agent comprises prasugrel, and after the administering of a
composition provided herein or a
composition produced by a method described herein, the subject had a PRU of
about 180 to about 376 PRU
(e.g., about 200 to about 300 PRU). In some embodiments, the antiplatelet
agent comprises prasugrel, and
before the administering of a composition provided herein or a composition
produced by a method
described herein, the subject had a maximum amplitude (TEG-ADP) of less than
about 50 mm (e.g., less
than about 48 mm, 45 mm, 40 mm, or less). In some embodiments, the
antiplatelet agent comprises
prasugrel, and after the administering of a composition provided herein or a
composition produced by a
method described herein, the subject had a maximum amplitude (TEG-ADP) of at
least about 50 mm (e.g.,
at least 53 mm, 55 mm, 50 mm, 60 mm, 65 mm, 70 mm, or more). See, e.g.,
Corliss BM, Poliflca AJ, Harris
49
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
NS, Hoh BL, Fox WC. Laboratory assessments of therapeutic platelet inhibition
in endovascular
neurosurgery: comparing results of the VerifyNow P2Y12 assay to
thromboelastography with platelet
mapping. J Neurosurg. 2018 Nov 1;129(5):1160-1165. doi:
10.3171/2017.6.JNS17535. PMID: 29271717.
In some embodiments, the antiplatelet agent comprises prasugrel, and before
the administering of a
composition provided herein or a composition produced by a method described
herein, the subject had a
high on-treatment platelet reactivity (HPR) using MEA of greater than about 47
units (e.g., greater than
about 48, 50, 55, or more units). See, e.g., Kriiger, Jan-Christopher, et al.
"Monitoring ASA and P2Y12-
specific platelet inhibition¨comparison of conventional (single) and multiple
electrode aggregometry."
Scandinavian journal of clinical and laboratory investigation 74.7 (2014): 568-
574.
[00277] In some embodiments, antiplatelet agent comprises eptifibatide at an
initial dosage of about 170
to about 190 mcg/kg body weight of the subject (e.g., about 180 mcg/kg body
weight of the subject),
optionally a second initial dosage of about 170 to about 190 mcg/kg body
weight of the subject (e.g., about
180 mcg/kg body weight of the subject), or a following dose of about 1 to
about 2 mcg/kg body weight of
the subject/min (e.g., about 1.5 mcg/kg body weight of the subject/min.
[00278] In some embodiments the antiplatelet agent comprises tirofiban at an
initial dosage of about 0.3
to about 0.5 g/kg body weight of the subject/min (e.g., about 0.5 g/kg body
weight of the subject/min)
for about 30 minutes, or a following dosage of about 0.1 g/kg body weight of
the subject/min.
[00279] In some embodiments, the antiplatelet agent comprises abciximab at an
initial dosage of about
0.2 to about 0.3 mg/kg body weight of the subject (e.g., about 0.25 mg/kg body
weight of the subject), or a
following dosage of about 0.10 to about 0.15 g/kg body weight of the
subject/min (e.g., about 0.125 g/kg
body weight of the subject/min). In some embodiments, the antiplatelet agent
comprises abciximab at an
initial dosage of about 0.2 to about 0.3 mg/kg body weight of the subject
(e.g., about 0.25 mg/kg body
weight of the subject), or a following dosage of about 8 to about 10 g/min
(e.g., about 9 g/min).
[00280] In some embodiments, the antiplatelet agent comprises ticlopidine at a
dosage of about 240 to
about 260 mg (e.g., about 250 mg) twice per day.
[00281] In some embodiments, the antiplatelet agent comprises ibuprofen at a
dosage of about 100 to
about 600 mg (e.g., about 100 mg, 200 mg, 250 mg, 300 mg, 400 mg, 500 mg, or
600 mg) once, twice,
three times, or four times per day.
[00282] In some embodiments, the antiplatelet agent comprises vorapaxar at a
dosage of about 2 to about
3 mg (e.g., about 2.5 mg) once per day, optionally with aspirin or
clopidogrel.
[00283] In some embodiments, the antiplatelet agent comprises cilostazol at a
dosage of about 40 to about
110 mg (e.g., about 50 mg, 75 mg, or 100 mg) twice daily.
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00284] In some embodiments, the antiplatelet agent comprises epoprostenol at
an initial dosage of about
2 ng/kg body weight of the subject/min, or a following dosage of about 4, 6,
8, 10, 12, 14, 16, 18, or 20
ng/kg body weight of the subject/min.
[00285] In some embodiments, the antiplatelet agent comprises dipyridamole at
a dosage of about 60 to
about 110 mg (e.g., about 75 mg or 100 mg) four times daily.
[00286] In some embodiments, the antiplatelet agent comprises treprostinil
sodium at a dosage of about
0.5 to about 3.0 ng/kg body weight of the subject/min (e.g., about 0.625 ng/kg
body weight of the
subject/min about 1.25 ng/kg body weight of the subject/min or about 2.5 ng/kg
body weight of the subject
per min).
[00287] In some embodiments, rehydrating the composition comprising platelet
derivatives comprises
adding to the platelet derivatives (e.g. FDPDs) an aqueous liquid. In some
embodiments, the aqueous liquid
is water. In some embodiments, the aqueous liquid is an aqueous solution
(e.g., a buffer). In some
embodiments, the aqueous liquid is a saline solution. In some embodiments, the
aqueous liquid is a
suspension.
[00288] In some embodiments, the rehydrated platelet derivatives (e.g., FDPDs)
have coagulation factor
levels showing all individual factors (e.g., Factors VII, VIII and IX)
associated with blood clotting at 40
international units (IU) or greater.
[00289] In some embodiments, the platelet derivatives (e.g., FDPDs) have less
than about 10%, such as
less than about 8%, such as less than about 6%, such as less than about 4%,
such as less than about 2%,
such as less than about 0.5% crosslinking of platelet membranes via proteins
and/or lipids present on the
membranes. In some embodiments, the rehydrated platelet derivatives (e.g.,
FDPDs), have less than about
10%, such as less than about 8%, such as less than about 6%, such as less than
about 4%, such as less than
about 2%, such as less than about 0.5% crosslinking of platelet membranes via
proteins and/or lipids present
on the membranes.
[00290] In some embodiments, the platelets, typically platelet derivatives,
and in illustrative embodiments
FDPDs, have a particle size (e.g., diameter, max dimension) of at least about
0.2 tim (e.g., at least about 0.3
at least about 0.4 tim, at least about 0.5 tim, at least about 0.6 tim, at
least about 0.7 tim, at least about
0.8 tim, at least about 0.9 tim, at least about 1.0 tim, at least about 1.2
tim, at least about 1.5 tim, at least
about 2.0 tim, at least about 2.5 tim, or at least about 5.0 tim). In some
embodiments, the particle size is
less than about 5.0 tim (e.g., less than about 2.5 tim, less than about 2.0
tim, less than about 1.5 tim, less
than about 1.0 tim, less than about 0.9 tim, less than about 0.8 tim, less
than about 0.7 tim, less than about
0.6 tim, less than about 0.5 tim, less than about 0.4 tim, or less than about
0.3 tim). In some embodiments,
the particle size is from about 0.3 tim to about 5.0 tim (e.g., from about 0.4
tim to about 4.0 tim, from about
51
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
0.5 m to about 2.5 m, from about 0.6 m to about 2.0 m, from about 0.7 m
to about 1.0 m, from
about 0.5 m to about 0.9 m, or from about 0.6 m to about 0.8 m).
[00291] In some embodiments, at least 50% (e.g., at least about 55%, at least
about 60%, at least about
65%, at least about 70%, at least about 75%, at least about 80%, at least
about 85%, at least about 90%, at
least about 95%, or at least about 99%) of platelets, or in illustrative
embodiments platelet derivatives (e.g.,
FDPDs), have a particle size in the range of about 0.3 m to about 5.0 m
(e.g., from about 0.4 m to about
4.0 m, from about 0.5 m to about 2.5 m, from about 0.6 m to about 2.0 m,
from about 0.7 m to
about 1.0 m, from about 0.5 m to about 0.9 m, or from about 0.6 m to about
0.8 m). In some
embodiments, at most 99% (e.g., at most about 95%, at most about 80%, at most
about 75%, at most about
70%, at most about 65%, at most about 60%, at most about 55%, or at most about
50%) of the platelets, or
in illustrative embodiments platelet derivatives (e.g., FDPDs), are in the
range of about 0.3 m to about 5.0
m (e.g., from about 0.4 m to about 4.0 m, from about 0.5 m to about 2.5 m,
from about 0.6 m to
about 2.0 m, from about 0.7 m to about 1.0 m, from about 0.5 m to about
0.9 m, or from about 0.6
m to about 0.8 m). In some embodiments, about 50% to about 99% (e.g., about
55% to about 95%, about
60% to about 90%, about 65% to about 85, about 70% to about 80%) of the
platelets, or in illustrative
embodiments platelet derivatives (e.g., FDPDs) are in the range of about 0.3
m to about 5.0 m (e.g., from
about 0.4 m to about 4.0 m, from about 0.5 m to about 2.5 m, from about
0.6 m to about 2.0 m,
from about 0.7 m to about 1.0 m, from about 0.5 m to about 0.9 m, or from
about 0.6 m to about 0.8
m).
[00292] In some illustrative embodiments, a microparticle can be a particle
having a particle size (e.g.,
diameter, max dimension) of less than about 0.5 m (less than about 0.45 m or
0.4 m) In some cases, a
microparticle can be a particle having a particle size of about 0.01 m to
about 0.5 m (e.g., about 0.02 m
to about 0.5 m).
[00293] Compositions comprising platelets or platelet derivatives (e.g.,
FDPDs), such as those prepared
according to methods described herein, can have a microparticle content that
contributes to less than about
5.0% (e.g., less than about 4.5%, 4.0%, 3.5%, 3.0%, 2.5%, 2.0%, 1.5%, 1.0%, or
0.5%) of the total
scattering intensity of all particles from about 1 nm to about 60,000 nm in
radius in the composition. In
some embodiments, the platelet derivative composition comprises a population
of platelet derivatives
comprising CD41-positive platelet derivatives, wherein less than 15%, 10%,
7.5%, 5%, 4.5%, 4%, 3.5%,
3%, 2.5%, 2%, 1.5%, 1%, 0.5%, or 0.1% of the CD41-positive platelet
derivatives are microparticles having
a diameter of less than 1 m, 0.9 m, 0.8 m, 0.7 m, 0.6 m, 0.5 m, 0.4 m,
0.3 m, 0.2 m, or 0.1 m,
which in certain illustrative embodiments are less than 0.5 m. In some
embodiments, the platelet derivative
composition comprises a population of platelet derivatives comprising CD42-
positive platelet derivatives,
wherein less than 15%, 10%, 7.5%, 5%, 4.5%, 4%, 3.5%, 3%, 2.5%, 2%, 1.5%, 1%,
0.5%, or 0.1% of the
52
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
CD42-positive platelet derivatives are microparticles having a diameter of
less than 1 m, 0.9 m, 0.8 m,
0.7 m, 0.6 m, 0.5 m, 0.4 m, 0.3 m, 0.2 m, or 0.1 m, which in certain
illustrative embodiments are
less than 0.5 m. In some embodiments, the platelet derivative composition
comprises a population of
platelet derivatives comprising CD61-positive platelet derivatives, wherein
less than 15%, 10%, 7.5, 5%,
4.5%, 4%, 3.5%, 3%, 2.5%, 2%, 1.5%, 1%, 0.5%, or 0.1% of the CD61-positive
platelet derivatives are
microparticles having a diameter of less than 1 m, 0.9 m, 0.8 m, 0.7 m,
0.6 m, 0.5 m, 0.4 m, 0.3
m, 0.2 m, or 0.1 m, which in certain illustrative embodiments are less than
0.5 m. In some illustrative
embodiments, the microparticles have a diameter of less than 0.5 m. In some
embodiments of any of the
aspects and embodiments herein that include a platelet derivative composition
in a powdered form, the
diameter of the microparticles is determined after rehydrating the platelet
derivative composition with an
appropriate solution. In some embodiments, the amount of solution for
rehydrating the platelet derivative
composition is equal to the amount of buffer or preparation agent present at
the step of freeze-drying. As
used herein, a content of microparticles "by scattering intensity" refers to
the microparticle content based
on the scattering intensity of all particles from about 1 nm to about 60,000
nm in radius in the composition.
The microparticle content can be measured by any appropriate method, for
example, by dynamic light
scattering (DLS). In some cases, the viscosity of a sample used for DLS can be
at about 1.060 cP (or adjusted
to be so), as this is the approximate viscosity of plasma. In some
embodiments, the platelet derivative
composition as per any aspects, or embodiments comprises a population of
platelet derivatives, and
microparticles, wherein the numerical ratio of platelet derivatives to the
microparticles is at least 90:1, 91:1,
92:1, 93:1, 94:1, 95:1, 96:1, 97:1, 98:1, or 99:1. In some embodiments, the
platelet derivatives have a
diameter in the range of 0.5-2.5 m, and the microparticles have a diameter
less than 0.5 m.
[00294] In some embodiments, platelets are isolated, for example in a liquid
medium, for example prior
to processing to form platelet derivatives, or prior to directly administering
to a subject.
[00295] In some embodiments, platelets are donor-derived platelets. In some
embodiments, platelets are
obtained by a process that comprises an apheresis step. In some embodiments,
platelets are pooled platelets.
[00296] In some embodiments, platelets are pooled from a plurality of donors.
Such platelets pooled from
a plurality of donors may be also referred herein to as pooled platelets. In
some embodiments, the donors
are more than 5, such as more than 10, such as more than 20, such as more than
50, such as up to about 100
donors. In some embodiments, the donors are from about 5 to about 100, such as
from about 10 to about
50, such as from about 20 to about 40, such as from about 25 to about 35.
Pooled platelets can be used to
make any of the compositions described herein.
[00297] In some embodiments, platelets are derived in vitro. In some
embodiments, platelets are derived
or prepared in a culture. In some embodiments, preparing the platelets
comprises deriving or growing the
platelets from a culture of megakaryocytes. In some embodiments, preparing the
platelets comprises
53
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
deriving or growing the platelets (or megakaryocytes) from a culture of human
pluripotent stem cells
(PCSs), including embryonic stem cells (ESCs) and/or induced pluripotent stem
cells (iPSCs).
[00298] Accordingly, in some embodiments, platelets are prepared prior to
treating a subject as described
herein. In some embodiments, the platelets are lyophilized. In some
embodiments, the platelets are
cryopreserved.
[00299] In some embodiments, the platelets or pooled platelets may be
acidified to a pH of about 6.0 to
about 7.4 prior to the incubation with the incubating agent. In some
embodiments, the method comprises
acidifying the platelets to a pH of about 6.5 to about 6.9. In some
embodiments, the method comprises
acidifying the platelets to a pH of about 6.6 to about 6.8. In some
embodiments, the acidifying comprises
adding to the pooled platelets a solution comprising Acid Citrate Dextrose
(ACD).
[00300] In some embodiments, tangential flow filtration (TFF) is used to
process platelets for making
platelet derivatives, in illustrative embodiments FDPDs, for use in aspects
here. For example, TFF can be
used for concentration and/or buffer or other solution exchange, such that
platelets are suspended at an
appropriate concentration range in an appropriate medium, for example an
incubating agent and/or a
lyophilizing agent, or an incubating agent which is or comprises a
lyophilizing agent, for example before
the composition is dried to form platelet derivatives, or in illustrative
embodiments, before the platelet
composition is freeze-dried to form FDPDs.
[00301] In some embodiments, the method can include an initial dilution step,
for example, a starting
material (e.g., an unprocessed blood product (e.g., donor apheresis material
(e.g., pooled donor apheresis
material)) can be diluted with a preparation agent (e.g., any of the
preparation agents described herein) to
form a diluted starting material. In some cases, the initial dilution step can
include dilution with a
preparation agent with a mass of preparation agent equal to at least about 10%
of the mass of the starting
material (e.g., at least about 15%, 25%, 50%, 75%, 100%, 150%, or 200% of the
mass of the starting
material. In some embodiments, an initial dilution step can be carried out
using the TFF apparatus.
[00302] In some embodiments, the method can include concentrating (e.g.,
concentrating platelets) (e.g.,
concentrating a starting material or a diluted starting material) to form a
concentrated platelet composition.
For example, concentrated can include concentrating to a about 1000 x 103 to
about 4000 x 103 platelets/pL
(e.g., about 1000 x 103 to about 2000 x 103, about 2000 x 103 to about 3000 x
103, or about 4000 x 103
platelets/pL). In some embodiments, a concentration step can be carried out
using the TFF apparatus.
[00303] The concentration of platelets or platelet derivatives (e.g., FDPDs)
can be determined by any
appropriate method. For example, a counter can be used to quantitate
concentration of blood cells in
suspension using impedance (e.g., a Beckman Coulter AcT 10 or an AcT diff 2).
[00304] In some embodiments, TFF can include diafiltering (sometimes called
"washing") of a starting
material, a diluted starting material, a concentrated platelet composition, or
a combination thereof. In some
54
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
embodiments, diafiltering can include washing with at least 2 (e.g., at least
3, 4, 5, 6, 7, 8, 9, 10, or more)
diavolumes. In some embodiments, TFF can include buffer exchange. In some
embodiments, a buffer can
be used in TFF. A buffer can be any appropriate buffer. In some embodiments,
the buffer can be a
preparation agent (e.g., any of the preparation agents described herein). In
some embodiments, the buffer
can be the same preparation agent as was used for dilution. In some
embodiments, the buffer can be a
different preparation than was used for dilution. In some embodiments, a
buffer can include a lyophilizing
agent, including a buffering agent, a base, a loading agent, optionally a
salt, and optionally at least one
organic solvent such as an organic solvent selected from the group consisting
of ethanol, acetic acid,
acetone, acetonitrile, dimethylformamide, dimethyl sulfoxide, dioxane,
methanol, n-propanol, isopropanol,
tetrahydrofuran (THF), N-methyl pyrrolidone, dimethylacetamide (DMAC), or
combinations thereof. A
buffering agent can be any appropriate buffering agent. In some embodiments, a
buffering agent can be
HEPES ((4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). A base can be any
appropriate base. In
some embodiments, a base can be sodium bicarbonate. In some embodiments, a
saccharide can be a
monosaccharide. In some embodiments, a loading agent can be a saccharide. In
some embodiments, a
saccharide can include sucrose, maltose, trehalose, glucose (e.g., dextrose),
mannose, or xylose. In some
embodiments, a monosaccharide can be trehalose. In some embodiments, the
loading agent can include
polysucrose. A salt can be any appropriate salt. In some embodiments, a salt
can be selected from the group
consisting of sodium chloride (NaCl), potassium chloride (KC1), or a
combination thereof.
[00305] In some embodiments, a membrane with a pore size of about 0.1 m to
about 1 m (e.g., about
0.1 m to about 1 m, about 0.1 m to about 0.5 m, about 0.2 to about 0.45
m, about 0.45 to about 1
m, about 0.1 m, about 0.2 m, about 0.45 m, about 0.65 m, or about 1 m)
can be used in TFF. A
membrane can be made from any appropriate material. In some cases, a membrane
can be a hydrophilic
membrane. In some embodiments, a membrane can be a hydrophobic membrane. In
some embodiments, a
membrane with a nominal molecular weight cutoff (NMWCO) of at least about 100
kDa (e.g., at least about
200, 300 kDa, 500 kDa, or 1000 kDa) can be used in TFF. The TFF can be
performed with any appropriate
pore size within the range of 0.1 m to 1.0 m with the aim of reducing the
microparticles content in the
composition and increasing the content of platelet derivatives in the
composition. A skilled artisan can
appreciate the required optimization of the pore size in order to retain the
platelet derivatives and allow the
microparticles to pass through the membrane. The pore size in illustrative
embodiments, is such that the
microparticles pass through the membrane allowing the TFF-treated composition
to have less than 5%
microparticles. The pore size in illustrative embodiments is such that a
maximum of platelet derivatives
gets retained in the process allowing the TFF-treated composition to have a
concentration of the platelet
derivatives in the range of 100 x 103 to 20,000 x 10g. The pore size during
the TFF process can be exploited
to obtain a higher concentration of platelet derivatives in the platelet
derivative composition such that a
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
person administering the platelet derivatives to a subject in need has to
rehydrate/reconstitute fewer vials,
therefore, being efficient with respect to time and effort during the process
of preparing such platelet
derivatives for a downstream procedure, for example a method of treating
provided herein. TFF can be
performed at any appropriate temperature. In some embodiments, TFF can be
performed at a temperature
of about 20 C to about 37 C (e.g., about 20 C to about 25 C, about 20 C
to about 30 C, about 25 C
to about 30 C, about 30 C to about 35 C, about 30 C to about 37 C, about
25 C to about 35 C, or
about 25 C to about 37 C). In some embodiments, TFF can be carried out at a
flow rate (e.g., a circulating
flow rate) of about 100 ml/min to about 800 ml/min (e.g., about 100 to about
200 ml/min, about 100 to
about 400 ml/min, about 100 to about 600 ml/min, about 200 to about 400
ml/min, about 200 to about 600
ml/min, about 200 to about 800 ml/min, about 400 to about 600 ml/min, about
400 to about 800 ml/min,
about 600 to about 800 ml/min, about 100 ml/min, about 200 ml/min, about 300
ml/min, about 400 ml/min,
about 500 ml/min, about 600 ml/min, about 700 ml/min, or about 800 ml/min).
[00306] In some embodiments, TFF can be performed until a particular endpoint
is reached, forming a
TFF-treated composition. An endpoint can be any appropriate endpoint. In some
embodiments, an endpoint
can be a percentage of residual plasma (e.g., less than or equal to about 50%,
40%, 30%, 20%, 15%, 10%,
9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%,
0.2%, or 0.1% of
residual plasma). In some embodiments, an endpoint can be a relative
absorbance at 280 nm (A280). For
example, an endpoint can be an A280 (e.g., using a path length of 0.5 cm) that
is less than or equal to about
50% (e.g., less than or equal to about 40%, 30%, 20%, 15%, 10%, 9%, 8%, 7%,
6%, 5%, 4%, 3%, 2%, 1%,
0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, or 0.1%) of the A280 (e.g.,
using a path length of 0.5
cm) prior to TFF (e.g., of a starting material or of a diluted starting
material). In some embodiments, an
A280 can be relative to a system that measures 7.5% plasma = 1.66 AU. In some
embodiments, an
instrument to measure A280 can be configured as follows: a 0.5cm gap flow cell
can be attached to the
filtrate line of the TFF system. The flow cell can be connected to a
photometer with fiber optics cables
attached to each side of the flow cell (light source cable and light detector
cable). The flow cell can be made
with a silica glass lens on each side of the fiber optic cables. Apart from
the relative protein concentration
of proteins in the aqueous medium, the protein concentration in the aqueous
medium can also be measured
in absolute terms. In some embodiments, the protein concentration in the
aqueous medium is less than or
equal to 15%, or 14%, or 13%, or 12%, or 11%, or 10%, or 9%, or 8%, or 7%, or
6%, or 5%, or 4%, or 3%,
or 2%, or 1%, or 0.1%, or 0.01%. In some exemplary embodiments, the protein
concentration is less than
3% or 4%. In some embodiments, the protein concentration is in the range of
0.01-15%, or 0.1-15%, or 1-
15%, or 1-10%, or 0.01-10%, or 3-12%, or 5-10% in the TFF-treated composition.
In some embodiments,
an endpoint can be an absolute A280 (e.g., using a path length of 0.5 cm). For
example, an endpoint can be
an A280 that is less than or equal to 2.50 AU, 2.40 AU, 2.30 AU, 2.20 AU, 2.10
AU, 2.0 AU, 1.90 AU, 1.80
56
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
AU, or 1.70 AU (e.g., less than or equal to 1.66, 1.6, 1.5, 1.4, 1.3, 1.2,
1.1, 1.0, 0.9, 0.8, 0.7, 0.6, 0.5, 0.4,
0.3, 0.2, or 0.1 AU) (e.g., using a path length of 0.5 cm). In some
embodiments, a percentage of residual
plasma, a relative A280, or an A280 can be determined based on the aqueous
medium of a composition
comprising platelets and an aqueous medium. In some embodiments, a percentage
of residual plasma can
be determined based on a known correlation to an A280. In some embodiments, an
endpoint can be a platelet
concentration, as TFF can include concentration or dilution of a sample (e.g.,
using a preparation agent).
For example, an endpoint can be a platelet concentration of at least about
2000 x 103 platelets/tit (e.g., at
least about 2050 x 103, 2100 x 103, 2150 x 103, 2200 x 103, 2250 x 103, 2300 x
103, 2350 x 103, 2400 x 103,
2450 x 103, or 2500 x 103 platelets/tit). As another example, an endpoint can
be a platelet concentration of
about 1000 x 103 to about 2500 platelets/tit (e.g., about 1000 x 103 to about
2000 x 103, about 1500 x 103
to about 2300 x 103, or about 1700 x 103 to about 2300 x 103 platelets/tit).
In some embodiments, an
endpoint can be a concentration of platelets in the TFF-treated composition
are at least 100 x 103
platelets/tit, 200 x 103 platelets/tit, 400 x 103 platelets/tit, 1000 x 103
platelets/tit, 1250 x 103 platelets/tit,
1500 x 103 platelets/tit, 1750 x 103 platelets/tit, 2000 x 103 platelets/tit,
2250 x 103 platelets/tit, 2500 x
103 platelets/tit, 2750 x 103 platelets/tit, 3000 x 103 platelets/tit, 3250 x
103 platelets/tit, 3500 x 103
platelets/tit, 3750 x 103 platelets/tit, 4000 x 103 platelets/tit, 4250 x 103
platelets/tit, 4500 x 103
platelets/tit, 4750 x 103 platelets/tit, 5000 x 103 platelets/tit, 5250 x 103
platelets/tit, 5500 x 103
platelets/tit, 5750 x 103 platelets/tit, 6000 x 103 platelets/tit, 7000 x 103
platelets/tit, 8000 x 103
platelets/tit, 9000 x 103 platelets/tit, 10,000 x 103 platelets/tit, 11,000 x
103 platelets/tit, 12,000 x 103
platelets/tit, 13,000 x 103 platelets/tit, 14,000 x 103 platelets/tit, 15,000
x 103 platelets/tit, 16,000 x 103
platelets/tit, 17,000 x 103 platelets/tit, 18,000 x 103 platelets/tit, 19,000
x 103 platelets/tit, 20,000 x 103
platelets/pt. In some embodiments, the platelets or platelet derivatives in
the TFF-treated composition is
in the range of 100 x 103 ¨ 20,000 x 103 platelets/tit, or 1000 x 103 ¨ 20,000
x 103 platelets/tit, or 1000 x
103 ¨ 10,000 x 103 platelets/tit, or 500 x 103 ¨ 5,000 x 103 platelets/tit, or
1000 x 103 ¨ 5,000 x 103
platelets/tit, or 2000 x 103¨ 8,000 x 103 platelets/tit, or 10,000 x 103¨
20,000 x 103 platelets/tit, or 15,000
x 103 ¨ 20,000 x 103 platelets/pt.
[00307] In some embodiments, an endpoint can include more than one criterion
(e.g., a percentage of
residual plasma and a platelet concentration, a relative A280 and a platelet
concentration, or an absolute
A280 and a platelet concentration).
[00308] Typically, a TFF-treated composition is subsequently lyophilized,
optionally with a thermal
treatment step, to form a final blood product (e.g., platelets, cryopreserved
platelets, FDPDs. However, in
some cases, a TFF-treated composition can be considered to be a final blood
product.
[00309] In some embodiments, a blood product can be prepared using
centrifugation of a blood product
(e.g., an unprocessed blood product (e.g., donor apheresis material (e.g.,
pooled donor apheresis material)),
57
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
or a partially processed blood product (e.g., a blood product that has
undergone TFF)). In some
embodiments, a blood product can be prepared without centrifugation of a blood
product (e.g., an
unprocessed blood product (e.g., donor apheresis material), or a partially
processed blood product (e.g., a
blood product that has undergone TFF)). Centrifugation can include any
appropriate steps. In some
embodiments, centrifugation can include a slow acceleration, a slow
deceleration, or a combination thereof.
In some embodiments, centrifugation can include centrifugation at about 1400 x
g to about 1550 x g (e.g.,
about 1400 to about 1450 x g, about 1450 to about 1500 x g, or 1500 to about
1550 x g, about 1400 x g,
about 1410 x g, about 1430 x g, about 1450 x g, about 1470 x g, about 1490 x
g, about 1500 x g, about 1510
x g, about 1530 x g, or about 1550 x g). In some embodiments, the duration of
centrifugation can be about
min to about 30 min (e.g., about 10 to about 20 min, about 20 to about 30 min,
about 10 min, about 20
min, or about 30 min).
[00310] In some embodiments, a final blood product can be prepared using both
TFF and centrifugation
(e.g., TFF followed by centrifugation or centrifugation followed by TFF).
[00311] Also provided herein are compositions prepared by any of the methods
described herein.
[00312] In some embodiments, a composition as described herein can be analyzed
at multiple points
during processing. In some embodiments, a starting material (e.g., donor
apheresis material (e.g., pooled
donor apheresis material)) can be analyzed for antibody content (e.g., HLA or
HNA antibody content). In
some embodiments, a starting material (e.g., donor apheresis material (e.g.,
pooled donor apheresis
material)) can be analyzed for protein concentration (e.g., by absorbance at
280 nm (e.g., using a path length
of 0.5 cm)). In some embodiments, a composition in an intermediate step of
processing (e.g., when protein
concentration reduced to less than or equal to 75% (e.g., less than or equal
to 70%, 65%, 60%, 55%, 50%,
45%, 40%, 35%, 30%, 25%, 20%, 15%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or
less) of the
protein concentration of an unprocessed blood product) can be analyzed for
antibody content (e.g., HLA or
HNA antibody content). In some embodiments, the antibody content (e.g., HLA or
HNA antibody content)
of a blood product in an intermediate step of processing can be at least 5%
reduced (e.g., 10%, 20%, 30%,
40%, 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, or more,
reduced) compared to
the antibody content of the starting material. In some embodiments, a final
blood product (e.g., (e.g.,
platelets, cryopreserved platelets, FDPDs can be analyzed for antibody content
(e.g., HLA or HNA antibody
content). In some embodiments described herein, a final blood product can be a
composition that includes
platelets and an aqueous medium. In some embodiments, the antibody content
(e.g., HLA or HNA antibody
content) of a final blood product (e.g., (e.g., platelets, cryopreserved
platelets, FDPDs can be at least 5%
reduced (e.g., 10%, 20%, 30%, 40%, 50%, 60%, 70%, 75%, 80%, 85%, 90%, 95%,
96%, 97%, 98%, 99%,
or more, reduced) compared to the antibody content of the starting material.
In some embodiments, a final
blood product can have no detectable level of an antibody selected from the
group consisting of HLA Class
58
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
I antibodies, HLA Class II antibodies, and HNA antibodies. In some
embodiments, the aqueous medium of
a composition as described herein can be analyzed as described herein.
[00313] In some embodiments, the platelets are isolated prior to the
incubation with the incubating agent
and/or lyophilizing agent. In some embodiments, the incubating agent is or
comprises a lyophilizing agent
as disclosed in more detail herein. In some embodiments, the method further
comprises isolating platelets
by using centrifugation. In some embodiments, the centrifugation occurs at a
relative centrifugal force
(RCF) of about 1000 x g to about 2000 x g. In some embodiments, the
centrifugation occurs at relative
centrifugal force (RCF) of about 1300 x g to about 1800 x g. In some
embodiments, the centrifugation
occurs at relative centrifugal force (RCF) of about 1500 x g. In some
embodiments, the centrifugation
occurs for about 1 minute to about 60 minutes. In some embodiments, the
centrifugation occurs for about
minutes to about 30 minutes. In some embodiments, the centrifugation occurs
for about 30 minutes.
[00314] An incubating agent can include any appropriate components. In some
embodiments, the
incubating agent may comprise a liquid medium. In some embodiments the
incubating agent may
comprise one or more salts selected from phosphate salts, sodium salts,
potassium salts, calcium salts,
magnesium salts, and any other salt that can be found in blood or blood
products, or that is known to
be useful in drying platelets, or any combination of two or more of these.
[00315] In some embodiments, the incubating agent comprises one or more salts,
such as phosphate salts,
sodium salts, potassium salts, calcium salts, magnesium salts, and any other
salt that can be found in blood
or blood products. Exemplary salts include sodium chloride (NaCl), potassium
chloride (KC1), and
combinations thereof. In some embodiments, the incubating agent includes from
about 0.5 mM to about
100 mM of the one or more salts. In some embodiments, the incubating agent
includes from about 0.5 mM
to about 100 mM (e.g., about 0.5 to about 2 mM, about 2 mM to about 90 mM,
about 2 mM to about 6 mM,
about 50 mM to about 100 mM, about 60 mM to about 90 mM, about 70 to about 85
mM) about of the one
or more salts. In some embodiments, the incubating agent includes about 5 mM,
about 60 mM, about 65
mM, about 70 mM, about 75 mM, or about 80 mM of the one or more salts. In some
embodiments, the
incubating agent comprises one or more salts selected from calcium salts,
magnesium slats, and a
combination of the two, in a concentration of about 0.5 mM to about 2 mM.
[00316] Preferably, these salts are present in the composition comprising
platelets or platelet
derivatives, such as freeze-dried platelets, at an amount that is about the
same as is found in whole
blood.
[00317] In some embodiments, the incubating agent further comprises a carrier
protein. In some
embodiments, the carrier protein comprises human serum albumin, bovine serum
albumin, or a
combination thereof. In some embodiments, the carrier protein is present in an
amount of about 0.05%
to about 1.0% (w/v).
59
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00318] The incubating agent may be any buffer that is non-toxic to the
platelets and provides
adequate buffering capacity to the solution at the temperatures at which the
solution will be exposed
during the process provided herein. Thus, the buffer may comprise any of the
known biologically
compatible buffers available commercially for example phosphate buffers such
as phosphate buffered
saline (PBS), bicarbonate/carbonic acid buffers such as sodium-bicarbonate
buffer, N-2-
hydroxyethylpiperazine-N'-2- ethanesulfonic acid (HEPES), and tris-based
buffers such as tris-
buffered saline (TBS). Likewise, it may comprise one or more of the following
buffers: propane- 1,2,3-
tricarboxylic (tricarballylic); ben Rnepentacarboxylic; maleic; 2,2-
dimethylsuccinic; EDTA; 3,3-
dimethylglutaric; bi s (2 -hydroxyethyl)imino-
tris(hydroxymethyl)-methane (B IS - TRIS) ;
ben Rnehexacarboxylic (mellitic); N-(2- acetamido)imino-diacetic acid (ADA);
butane-1,2,3,4-
tetracarboxylic; pyrophosphoric; 1,1-cyclopentanediacetic (3,3 tetramethylene-
glutaric acid);
piperazine-1,4-bis-(2-ethanesulfonic acid) (PIPES); N-(2-acetamido )-2-
amnoethanesulfonic acid
(ACES); 1, 1 -cycl ohexanediacetic ; 3, 6-endomethylene- 1,2,3 ,6-
tetrahydrophthal ic acid (EMTA;
ENDCA); imidaQcole;; 2- (aminoethyl)trimethylammonium chloride (CHOLAMINE);
N,N-bis(2-
hydroxyethyl)-2-aminoethanesulfonic acid (BES); 2-methylpropane-1,2,3-
triscarboxylic (beta-
methyltricarballylic ); 2-(N-morpholino)propane-sulfonic acid (MOPS);
phosphoric; and N-
tris(hydroxymethyl)methy1-2-amminoethane sulfonic acid (TES). In some
embodiments, the incubating
agent includes one or more buffers, e.g., N-2-hydroxyethylpiperazine-N'-2-
ethanesulfonic acid (HEPES),
or sodium-bicarbonate (NaHCO3). In some embodiments, the incubating agent
includes from about 5 to
about 100 mM of the one or more buffers. In some embodiments, the incubating
agent includes from about
to about 50 mM (e.g., from about 5 mM to about 40 mM, from about 8 mM to about
30 mM, about 10
mM to about 25 mM) about of the one or more buffers. In some embodiments, the
incubating agent includes
about 10 mM, about 20 mM, about 25 mM, or about 30 mM of the one or more
buffers.
[00319] In some embodiments, the incubating agent includes one or more
saccharides, such as
monosaccharides and disaccharides, including sucrose, maltose, trehalose,
glucose, mannose, dextrose, and
xylose. In some embodiments, the saccharide is a monosaccharide. In some
embodiments, the saccharide
is a disaccharide. In some embodiments, the saccharide comprises a
monosaccharide, a disaccharide, or a
combination thereof. In some embodiments, the saccharide is a non-reducing
disaccharide. In some
embodiments, the saccharide comprises sucrose, maltose, trehalose, glucose
(e.g., dextrose), mannose, or
xylose. In some embodiments, the saccharide comprises trehalose. In some
embodiments, the incubating
agent comprises a starch. In some embodiments, the incubating agent includes
polysucrose, a polymer of
sucrose and epichlorohydrin. In some embodiments, the incubating agent
includes from about 10 mM to
about 1,000 mM of the one or more saccharides. In some embodiments, the
incubating agent includes from
about 50 to about 500 mM of the one or more saccharides. In embodiments, one
or more saccharides is
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
present in an amount of from 10 mM 10 to 500 mM. In some embodiments, one or
more saccharides is
present in an amount of from 50 mM to 200 mM. In embodiments, one or more
saccharides is present in an
amount from 100 mM to 150 mM. In some embodiments, the one or more saccharides
is the lyophilizing
agent; for example, in some embodiments, the lyophilizing agent comprises
trehalose, polysucrose, or a
combination thereof.
[00320] In some embodiments the composition comprising platelets or platelet
derivatives, (e.g., FDPDs),
may comprise one or more of water or a saline solution. In some embodiments
the composition comprising
platelets or platelet derivatives, such as freeze-dried platelets, may
comprise DMSO.
[00321] In some embodiments, the incubating agent comprises an organic
solvent, such as an alcohol
(e.g., ethanol). In such an incubating agent, the amount of solvent can range
from 0.1 % to 5.0 % (v/v).
In some embodiments, the organic solvent can range from about 0.1 % (v/v) to
about 5.0 % (v/v), such as
from about 0.3 % (v/v) to about 3.0 % (v/v), or from about 0.5 % (v/v) to
about 2 % (v/v).
[00322] In some embodiments, suitable organic solvents include, but are not
limited to alcohols, esters,
ketones, ethers, halogenated solvents, hydrocarbons, nitriles, glycols, alkyl
nitrates, water or mixtures
thereof. In some embodiments, suitable organic solvents includes, but are not
limited to methanol, ethanol,
n-propanol, isopropanol, acetic acid, acetone, methyl ethyl ketone, methyl
isobutyl ketone, methyl acetate,
ethyl acetate, isopropyl acetate, tetrahydrofuran, isopropyl ether (IPE), tert-
butyl methyl ether, dioxane
(e.g., 1,4-dioxane), acetonitrile, propionitrile, methylene chloride,
chloroform, toluene, anisole,
cyclohexane, hexane, heptane, ethylene glycol, nitromethane,
dimethylformamide, dimethyl sulfoxide, N-
methyl pyrrolidone, dimethylacetamide, and combinations thereof. In some
embodiments the organic
solvent is selected from the group consisting of ethanol, acetic acid,
acetone, acetonitrile,
dimethylformamide, dimethyl sulfoxide (DMSO), dioxane, methanol, n-propanol,
isopropanol,
tetrahydrofuran (THF), N-methyl pyrrolidone, dimethylacetamide (DMAC), or
combinations thereof. In
some embodiments, the organic solvent comprises ethanol, DMSO, or a
combination thereof. The presence
of organic solvents, such as ethanol, can be beneficial in the processing of
platelets, platelet derivatives, or
FDPDs (e.g., freeze-dried platelet derivatives).
[00323] In some embodiments the incubating agent is incubated into the
platelets in the presence of an
aqueous medium. In some embodiments the incubating agent is incubated in the
presence of a medium
comprising DMSO.
[00324] In some embodiments, one or more other components may be incubated in
the platelets.
Exemplary components may include Prostaglandin El or Prostacyclin and or
EDTA/EGTA to prevent
platelet aggregation and activation during the incubating process.
[00325] Non-limiting examples of incubating agent compositions that may be
used are shown in Tables
1-5.
61
CA 03211079 2023-08-11
WO 2022/178177
PCT/US2022/016866
Table 1
Buffer
Concentration (mM
Component unless otherwise
specified)
NaCl 75.0
KC1 4.8
HEPES 9.5
NaHC 03 12.0
Dextrose 3
Trehalose 100
Ethanol (optional) 1% (v/v)
Table 2
Buffer A
Concentration (mM
Component unless specified
otherwise)
CaCl2 1.8
MgCl2 1.1
KC1 2.7
NaCl 137
NaH2PO4 0.4
HEPES 10
D-glucose 5.6
pH 6.5
Table 3
Buffer B
62
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
Concentration (mM
Component unless otherwise
specified)
Buffer and Salts Table 4 (below)
BSA 0.35%
Dextrose 5
pH 7.4
Table 3. Buffer B can used when incubating platelets, e.g.,
for flow cytometry. Such an incubation can be done at
room temperature in the dark. Albumin is an optional
component of Buffer B.
Table 4
Concentration of HEPES and of Salts in Buffer B
Concentration (mM
Component unless otherwise
specified)
HEPES 25
NaCl 119
KC1 5
CaCl2 2
MgCl2 2
glucose 6 g/1
[00326] Table 4 is another exemplary incubating agent. The pH can be
adjusted to 7.4 with
NaOH. Albumin is an optional component of Buffer B.
63
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00327] Table 5.
Tyrode's HEPES Buffer (plus PGE1)
Component Concentration (mM)
CaCl2 1.8
MgCl2 1.1
KC1 2.7
NaCl 137
NaH2PO4 0.4
HEPES 10
D-glucose 5.6
pH 6.5
Prostaglandin El
1 Wail
(PGE1)
[00328] Table 5 is another exemplary incubating agent.
[00329] In some embodiments, platelets (e.g., apheresis platelets, platelets
isolated from whole blood,
pooled platelets, or a combination thereof) are incubated with the incubating
agent for different durations
at or at different temperatures from 15-45 C, or about 37 C.
[00330] In some embodiments, platelets (e.g., apheresis platelets, platelets
isolated from whole blood,
pooled platelets, or a combination thereof) form a suspension in an incubating
agent and/or a lyophilizing
agent, or an incubating agent that is or comprises a lyophilizing agent,
comprising a liquid medium at a
concentration from 10,000 platelets/ L to 10,000,000 platelets/ L, such as
50,000 platelets/ L to 2,000,000
platelets/ L, such as 100,000 platelets/ L to 500,000 platelets/pL, such as
150,000 platelets/pL to 300,000
platelets/ L, such as 200,000 platelets/ L.
[00331] The platelets (e.g., apheresis platelets, platelets isolated from
whole blood, pooled platelets, or a
combination thereof) may be incubated with the incubating agent, for example
that is or comprises a
lyophilizing agent, for different durations, such as, for example, for at
least about 5 minutes (mins) (e.g., at
least about 20 mins, about 30 mins, about 1 hour (hr), about 2 hrs, about 3
hrs, about 4 hrs, about 5 hrs,
about 6 hrs, about 7 hrs, about 8 hrs, about 9 hrs, about 10 hrs, about 12
hrs, about 16 hrs, about 20 hrs,
about 24 hrs, about 30 hrs, about 36 hrs, about 42 hrs, about 48 hrs, or at
least about 48 hrs. In some
embodiments, the platelets may be incubated with the incubating agent for no
more than about 48 hrs (e.g.,
no more than about 20 mins, about 30 mins, about 1 hour (hr), about 2 hrs,
about 3 hrs, about 4 hrs, about
64
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
hrs, about 6 hrs, about 7 hrs, about 8 hrs, about 9 hrs, about 10 hrs, about
12 hrs, about 16 hrs, about 20
hrs, about 24 hrs, about 30 hrs, about 36 hrs, or no more than about 42 hrs).
In some embodiments, the
platelets may be incubated with the incubating agent for from about 10 mins to
about 48 hours (e.g., from
about 20 mins to about 36 hrs, from about 30 mins to about 24 hrs, from about
1 hr to about 20 hrs, from
about 2 hrs to about 16 hours, from about 10 mins to about 24 hours, from
about 20 mins to about 12 hours,
from about 30 mins to about 10 hrs, or from about 1 hr to about 6 hrs. In some
embodiments, the platelets,
the platelet derivatives, or the FDPDs are incubated with the incubating agent
for a period of time of 5
minutes to 48 hours, such as 10 minutes to 24 hours, such as 20 minutes to 12
hours, such as 30 minutes to
6 hours, such as 1 hour minutes to 3 hours, such as about 2 hours.
[00332] In some embodiments, the platelets (e.g., apheresis platelets,
platelets isolated from whole blood,
pooled platelets, or a combination thereof) are incubated with the incubating
agents at different
temperatures. In embodiments, incubation is conducted at 37 C. In certain
embodiments, incubation is
performed at 4 C to 45 C, such as 15 C to 42 C. For example, in embodiments,
incubation is performed
at 35 C to 40 C (e.g., 37 C) for 110 to 130 (e.g., 120) minutes and for as
long as 24-48 hours. In some
embodiments, the platelets are incubated with the incubating agent for
different durations as disclosed
herein, and at temperatures from 15-45 C, or about 37 C.
[00333] In some embodiments, platelets (e.g., apheresis platelets, platelets
isolated from whole blood,
pooled platelets, or a combination thereof) are loaded with one or more active
agents. In some embodiments,
the platelets can be loaded with an anti-fibrinolytic agent. Non-limiting
examples of anti-fibrinolytic agents
include e-aminocaproic acid (EACA), tranexamic acid, aprotinin,
aminomethylbenzoic acid, and
fibrinogen.
[00334] Loading platelets (e.g., apheresis platelets, platelets isolated from
whole blood, pooled platelets,
or a combination thereof) with an active agent (e.g., an anti-fibrinolytic
agent) can be performed by any
appropriate method. See, for example, PCT Publication Nos. W02020113090A1,
W02020113101A1,
W02020113035A1, and W02020112963A1. Generally, the loading includes contacting
the platelets with
the anti-fibrinolytic agent. In some embodiments, the loading can be performed
by combining the active
agent with the incubating agent. In some embodiments, the loading can be
performed in a separate step
from the incubating step. For example, the loading can be performed in a step
prior to the incubation step.
In some such embodiments, the active agent can be supplied to the platelets as
a solution or suspension in
any of the incubation agents described herein, which may or may not be the
same as the incubating agent
used in the incubating step. In some embodiments, the loading step can be
performed during the incubation
step. In some such embodiments, the active agent can be added to the
incubation agent (e.g., as a solid or
in a solution or suspension) during the incubation). In some embodiments, the
loading step can be
performed in a step following the incubation step. In some such embodiments,
be supplied to the platelets
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
as a solution or suspension in any of the incubation agents described herein,
which may or may not be the
same as the incubating agent used in the incubating step.
[00335] An active agent can be applied to the platelets in any appropriate
concentration. In some
embodiments, an active agent can be applied to the platelets (e.g., as part of
the incubating agent or another
solution or suspension) in a concentration of about 1 tiM to about 100 mM
(e.g., about 1 tiM to about 10
m, about 1 tiM to about 50 tiM, about 1 tiM to about 100 tiM, about 1 tiM to
about 500 tiM, about 1 tiM
to about 1 mM, about 1 tiM to about 10 mM, about 1 tiM to about 25 mM, about 1
tiM to about 50 mM,
about 1 tiM to about 75 mM, about 10 tiM to about 100 mM, about 50 tiM to
about 100 mM, about 100
tiM to about 100 mM, about 500 tiM to about 100 mM, about 1 mM to about 100
mM, about 10 mM to
about 100 mM, about 25 mM to about 100 mM, about 50 mM to about 100 mM, about
75 mM to about 100
mM, about 10 M to about 100 mM, about 200 tiM to about 1 mM, about 800 tiM to
about 900 tiM, about
400 tiM to about 800 tiM, about 500 tiM to about 700 tiM, about 600 tiM, about
5 mM to about 85 mM,
about 20 mM to about 90 mM, about 25 mM to about 75 mM, about 30 mM to about
90 mM, about 35 mM
to about 65 mM, about 40 mM to about 60 mM, about 50 mM to about 60 mM, about
40 mM to about 70
mM, about 45 mM to about 55 mM, or about 50 mM).
[00336] In some embodiments, the method further comprises drying the
platelets. In some embodiments,
the drying step comprises lyophilizing the platelets. In some embodiments, the
drying step comprises
freeze-drying the platelets. In some embodiments, the method further comprises
rehydrating the platelets
obtained from the drying step.
[00337] In some embodiments, the platelets are cold stored, cryopreserved, or
lyophilized (e.g., to
produce FDPDs) prior to use in therapy or in functional assays.
[00338] Any known technique for drying platelets can be used in accordance
with the present disclosure,
as long as the technique can achieve a final residual moisture content of less
than 5%. Preferably, the
technique achieves a final residual moisture content of less than 2%, such as
1%, 0.5%, or 0.1%. Non-
limiting examples of suitable techniques are freeze-drying (lyophilization)
and spray-drying. A suitable
lyophilization method is presented in Table A. Additional exemplary
lyophilization methods can be found
in U.S. Patent No. 7,811,558, U.S. Patent No. 8,486,617, and U.S. Patent No.
8,097,403. An exemplary
spray-drying method includes: combining nitrogen, as a drying gas, with a
incubating agent according to
the present disclosure, then introducing the mixture into GEA Mobile Minor
spray dryer from GEA
Processing Engineering, Inc. (Columbia MD, USA), which has a Two-Fluid Nozzle
configuration, spray
drying the mixture at an inlet temperature in the range of 150 C to 190 C, an
outlet temperature in the
range of 65 C to 100 C, an atomic rate in the range of 0.5 to 2.0 bars, an
atomic rate in the range of 5 to
13 kg/hr, a nitrogen use in the range of 60 to 100 kg/hr, and a run time of 10
to 35 minutes. The final step
66
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
in spray drying is preferentially collecting the dried mixture. The dried
composition in some embodiments
is stable for at least six months at temperatures that range from -20 C or
lower to 90 C or higher.
[00339] Table A: Exemplary
Lyophilization Protocol
Step Temp. Set Type Duration Pressure Set
Freezing Step Fl -50 C Ramp Var N/A
F2 Hold 3 Hrs
-50 C N/A
Vacuum Pulldown F3 -500 Hold Var N/A
Primary Dry P1 -400 Hold 1.5 Hrs 0 mT
P2 -35 Ramp 2 Hrs 0 mT
P3 -25 Ramp 2 Hrs 0 mT
P4 -17 C Ramp 2 Hrs 0 mT
P5 0 C Ramp 1.5 Hrs 0 mT
P6 27 C Ramp 1.5 Hrs 0 mT
P7 27 C Hold 16Hr s 0 mT
Secondary Dry Si 27 C Hold > 8Hrs 0 mT
[00340] In some embodiments, the step of drying the platelets that are
obtained as disclosed herein, to
produce platelet derivatives for use in any of the aspects or embodiments
herein, such as the step of freeze-
drying the platelets that are obtained as disclosed herein, to produce FDPDs
for use in any of the aspects or
embodiments herein comprises incubating the platelets with a lyophilizing
agent (e.g., a non-reducing
disaccharide). Accordingly, in some embodiments, the methods for preparing
platelets further comprise
incubating the platelets with a lyophilizing agent. In some embodiments the
lyophilizing agent is a
saccharide. In some embodiments the saccharide is a disaccharide, such as a
non-reducing disaccharide.
[00341] In some embodiments, the platelets are incubated with a
lyophilizing agent for a sufficient
amount of time and at a suitable temperature to incubate the platelets with
the lyophilizing agent. In some
embodiments, the incubating agent is the lyophilizing agent. Non-limiting
examples of suitable lyophilizing
agents are saccharides, such as monosaccharides and disaccharides, including
sucrose, maltose, trehalose,
glucose (e.g., dextrose), mannose, and xylose. In some embodiments, non-
limiting examples of
lyophilizing agent include serum albumin, dextran, polyvinyl pyrrolidone
(PVP), starch, and
67
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
hydroxyethyl starch (HES). In some embodiments, exemplary lyophilizing agents
can include a high
molecular weight polymer. By "high molecular weight" it is meant a polymer
having an average
molecular weight of about or above 70 kDa and up to 1,000,000 kDa. Non-
limiting examples are
polymers of sucrose and epichlorohydrin (e.g., polysucrose). In some
embodiments, the lyophilizing
agent is polysucrose. Although any amount of high molecular weight polymer can
be used as a
lyophilizing agent, it is preferred that an amount be used that achieves a
final concentration of about
3% to 10% (w/v), such as 3% to 7%, for example 6%.
[00342] An exemplary saccharide for use in the compositions disclosed
herein is trehalose. Regardless
of the identity of the saccharide, it can be present in the composition that
is dried to form platelet derivatives,
or freeze-dried to form, FDPDs, and in rehydrated compositions of such dried
platelet derivatives and
FDPDs, in any suitable amount. For example, it can be present in an amount of
1 mM to 1 M. In
embodiments, it is present in an amount of from 10 mM 10 to 500 mM. In some
embodiments, it is present
in an amount of from 20 mM to 200 mM. In embodiments, it is present in an
amount from 40 mM to 100
mM. In various embodiments, the saccharide is present in different specific
concentrations within the ranges
recited above, and one of skill in the art can immediately understand the
various concentrations without the
need to specifically recite each herein. Where more than one saccharide is
present in the composition, each
saccharide can be present in an amount according to the ranges and particular
concentrations recited above.
[00343] Within the process provided herein for making the compositions
provided herein, addition
of the lyophilizing agent can be the last step prior to drying. However, in
some embodiments, the
lyophilizing agent is added at the same time or before other components of the
composition, such as a
salt, a buffer, optionally a cryoprotectant, or other components. In some
embodiments, the lyophilizing
agent is added to the incubating agent, thoroughly mixed to form a drying
solution, dispensed into a
drying vessel (e.g., a glass or plastic serum vial, a lyophilization bag), and
subjected to conditions that
allow for drying of the solution to form a dried composition.
[00344] The step of incubating the platelets with a cryoprotectant can
include incubating the platelets
for a time suitable for loading, as long as the time, taken in conjunction
with the temperature, is sufficient
for the cryoprotectant to come into contact with the platelets and,
preferably, be incorporated, at least to
some extent, into the platelets. In embodiments, incubation is carried out for
about 1 minute to about 180
minutes or longer.
[00345] The step of incubating the platelets with a cryoprotectant can
include incubating the platelets
and the cryoprotectant at a temperature that, when selected in conjunction
with the amount of time allotted,
is suitable for incubating. In general, the composition is incubated at a
temperature above freezing for at
least a sufficient time for the cryoprotectant to come into contact with the
platelets. In embodiments,
incubation is conducted at 37 C. In certain embodiments, incubation is
performed at 20 C to 42 C. For
68
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
example, in embodiments, incubation is performed at 35 C to 40 C (e.g., 37 C)
for 110 to 130 (e.g., 120)
minutes.
[00346] In various embodiments, the lyophilization bag is a gas-permeable
bag configured to allow
gases to pass through at least a portion or all portions of the bag during the
processing. The gas-
permeable bag can allow for the exchange of gas within the interior of the bag
with atmospheric gas
present in the surrounding environment. The gas-permeable bag can be permeable
to gases, such as
oxygen, nitrogen, water, air, hydrogen, and carbon dioxide, allowing gas
exchange to occur in the
compositions provided herein. In some embodiments, the gas-permeable bag
allows for the removal
of some of the carbon dioxide present within an interior of the bag by
allowing the carbon dioxide to
permeate through its wall. In some embodiments, the release of carbon dioxide
from the bag can be
advantageous to maintaining a desired pH level of the composition contained
within the bag.
[00347] In some embodiments, the container of the process herein is a gas-
permeable container
that is closed or sealed. In some embodiments, the container is a container
that is closed or sealed and
a portion of which is gas-permeable. In some embodiments, the surface area of
a gas-permeable portion
of a closed or sealed container (e.g., bag) relative to the volume of the
product being contained in the
container (hereinafter referred to as the "SA/V ratio") can be adjusted to
improve pH maintenance of
the compositions provided herein. For example, in some embodiments, the SA/V
ratio of the container
can be at least about 2.0 cm2/mL (e.g., at least about 2.1 cm2/mL, at least
about 2.2 cm2/mL, at least
about 2.3 cm2/mL, at least about 2.4 cm2/mL, at least about 2.5 cm2/mL, at
least about 2.6 cm2/mL, at
least about 2.7 cm2/mL, at least about 2.8 cm2/mL, at least about 2.9 cm2/mL,
at least about 3.0 cm2/mL,
at least about 3.1 cm2/mL, at least about 3.2 cm2/mL, at least about 3.3
cm2/mL, at least about 3.4
cm2/mL, at least about 3.5 cm2/mL, at least about 3.6 cm2/mL, at least about
3.7 cm2/mL, at least about
3.8 cm2/mL, at least about 3.9 cm2/mL, at least about 4.0 cm2/mL, at least
about 4.1 cm2/mL, at least
about 4.2 cm2/mL, at least about 4.3 cm2/mL, at least about 4.4 cm2/mL, at
least about 4.5 cm2/mL, at
least about 4.6 cm2/mL, at least about 4.7 cm2/mL, at least about 4.8 cm2/mL,
at least about 4.9 cm2/mL,
or at least about 5.0 cm2/mL . In some embodiments, the SA/V ratio of the
container can be at most
about 10.0 cm2/mL (e.g., at most about 9.9 cm2/mL, at most about 9.8 cm2/mL,
at most about 9.7
cm2/mL, at most about 9.6 cm2/mL, at most about 9.5 cm2/mL, at most about 9.4
cm2/mL, at most about
9.3 cm2/mL, at most about 9.2 cm2/mL, at most about 9.1 cm2/mL, at most about
9.0 cm2/mL, at most
about 8.9 cm2/mL, at most about 8.8 cm2/mL, at most about 8.7 cm2/mL, at most
about 8.6, cm2/mL at
most about 8.5 cm2/mL, at most about 8.4 cm2/mL, at most about 8.3 cm2/mL, at
most about 8.2 cm2/mL,
at most about 8.1 cm2/mL, at most about 8.0 cm2/mL, at most about 7.9 cm2/mL,
at most about 7.8
cm2/mL, at most about 7.7 cm2/mL, at most about 7.6 cm2/mL, at most about 7.5
cm2/mL, at most about
7.4 cm2/mL, at most about 7.3 cm2/mL, at most about 7.2 cm2/mL, at most about
7.1 cm2/mL, at most
69
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
about 6.9 cm2/mL, at most about 6.8 cm2/mL, at most about 6.7 cm2/mL, at most
about 6.6 cm2/mL, at
most about 6.5 cm2/mL, at most about 6.4 cm2/mL, at most about 6.3 cm2/mL, at
most about 6.2 cm2/mL,
at most about 6.1 cm2/mL, at most about 6.0 cm2/mL, at most about 5.9 cm2/mL,
at most about 5.8
cm2/mL, at most about 5.7 cm2/mL, at most about 5.6 cm2/mL, at most about 5.5
cm2/mL, at most about
5.4 cm2/mL, at most about 5.3 cm2/mL, at most about 5.2 cm2/mL, at most about
5.1 cm2/mL, at most
about 5.0 cm2/mL, at most about 4.9 cm2/mL, at most about 4.8 cm2/mL, at most
about 4.7 cm2/mL, at
most about 4.6 cm2/mL, at most about 4.5 cm2/mL, at most about 4.4 cm2/mL, at
most about 4.3 cm2/mL,
at most about 4.2 cm2/mL, at most about 4.1 cm2/mL, or at most about 4.0
cm2/mL . In some
embodiments, the SA/V ratio of the container can range from about 2.0 to about
10.0 cm2/mL (e.g.,
from about 2.1 cm2/mL to about 9.9 cm2/mL, from about 2.2 cm2/mL to about 9.8
cm2/mL, from about
2.3 cm2/mL to about 9.7 cm2/mL, from about 2.4 cm2/mL to about 9.6 cm2/mL,
from about 2.5 cm2/mL
to about 9.5 cm2/mL, from about 2.6 cm2/mL to about 9.4 cm2/mL, from about 2.7
cm2/mL to about 9.3
cm2/mL, from about 2.8 cm2/mL to about 9.2 cm2/mL, from about 2.9 cm2/mL to
about 9.1 cm2/mL,
from about 3.0 cm2/mL to about 9.0 cm2/mL, from about 3.1 cm2/mL to about 8.9
cm2/mL, from about
3.2 cm2/mL to about 8.8 cm2/mL, from about 3.3 cm2/mL to about 8.7 cm2/mL,
from about 3.4 cm2/mL
to about 8.6 cm2/mL, from about 3.5 cm2/mL to about 8.5 cm2/mL, from about 3.6
cm2/mL to about 8.4
cm2/mL, from about 3.7 cm2/mL to about 8.3 cm2/mL, from about 3.8 cm2/mL to
about 8.2 cm2/mL,
from about 3.9 cm2/mL to about 8.1 cm2/mL, from about 4.0 cm2/mL to about 8.0
cm2/mL, from about
4.1 cm2/mL to about 7.9 cm2/mL, from about 4.2 cm2/mL to about 7.8 cm2/mL,
from about 4.3 cm2/mL
to about 7.7 cm2/mL, from about 4.4 cm2/mL to about 7.6 cm2/mL, from about 4.5
cm2/mL to about 7.5
cm2/mL, from about 4.6 cm2/mL to about 7.4 cm2/mL, from about 4.7 cm2/mL to
about 7.3 cm2/mL,
from about 4.8 cm2/mL to about 7.2 cm2/mL, from about 4.9 cm2/mL to about 7.1
cm2/mL, from about
5.0 cm2/mL to about 6.9 cm2/mL, from about 5.1 cm2/mL to about 6.8 cm2/mL,
from about 5.2 cm2/mL
to about 6.7 cm2/mL, from about 5.3 cm2/mL to about 6.6 cm2/mL, from about 5.4
cm2/mL to about 6.5
cm2/mL, from about 5.5 cm2/mL to about 6.4 cm2/mL, from about 5.6 cm2/mL to
about 6.3 cm2/mL,
from about 5.7 cm2/mL to about 6.2 cm2/mL, or from about 5.8 cm2/mL to about
6.1 cm2/mL.
[00348] Gas-permeable closed containers (e.g., bags) or portions thereof
can be made of one or
more various gas-permeable materials. In some embodiments, the gas-permeable
bag can be made of
one or more polymers including fluoropolymers (such as polytetrafluoroethylene
(PTFE) and
perfluoroalkoxy (PFA) polymers), polyolefins (such as low-density polyethylene
(LDPE), high-
density polyethylene (HDPE)), fluorinated ethylene propylene (FEP),
polystyrene, polyvinylchloride
(PVC), silicone, and any combinations thereof.
[00349] In some embodiments, dried platelets or platelet derivatives (e.g.,
FDPDs) can undergo
heat treatment. Heating can be performed at a temperature above about 25 C
(e.g., greater than about
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
40 C, 50 C, 60 C, 70 C, 80 C or higher). In some embodiments, heating is
conducted between about
70 C and about 85 C (e.g., between about 75 C and about 85 C, or at about 75 C
or 80 C). The
temperature for heating can be selected in conjunction with the length of time
that heating is to be
performed. Although any suitable time can be used, typically, the lyophilized
platelets are heated for
at least 1 hour, but not more than 36 hours. Thus, in embodiments, heating is
performed for at least 2
hours, at least 6 hours, at least 12 hours, at least 18 hours, at least 20
hours, at least 24 hours, or at
least 30 hours. For example, the lyophilized platelets can be heated for 18
hours, 19 hours, 20 hours,
21 hours, 22 hours, 23 hours, 24 hours, 25 hours, 26 hours, 27 hours, 28
hours, 29 hours, or 30 hours.
Non-limiting exemplary combinations include: heating the dried platelets or
platelet derivatives (e.g.,
FDPDs) for at least 30 minutes at a temperature higher than 30 C; heating the
dried platelets or platelet
derivatives (e.g., FDPDs) for at least 10 hours at a temperature higher than
50 C; heating the dried
platelets or platelet derivatives (e.g., FDPDs) for at least 18 hours at a
temperature higher than 75 C;
and heating the dried platelets or platelet derivatives (e.g., FDPDs) for 24
hours at 80 C. In some
embodiments, heating can be performed in sealed container, such as a capped
vial. In some
embodiments, a sealed container be subjected to a vacuum prior to heating. The
heat treatment step,
particularly in the presence of a cryoprotectant such as albumin or
polysucrose, has been found to
improve the stability and shelf-life of the freeze-dried platelets. Indeed,
advantageous results have
been obtained with the particular combination of serum albumin or polysucrose
and a post-
lyophilization heat treatment step, as compared to those cryoprotectants
without a heat treatment step.
A cryoprotectant (e.g., sucrose) can be present in any appropriate amount
(e.g. about 3% to about 10%
by mass or by volume of the platelets or platelet derivatives (e.g., FDPDs).
[00350] In some embodiments, the platelets or platelet derivatives (e.g.,
FDPDs) prepared as disclosed
herein by a process comprising incubation with an incubating agent have a
storage stability that is at least
about equal to that of the platelets prior to the incubation.
[00351] In some embodiments, the method further comprises cryopreserving
the platelets or platelet
derivatives prior to administering the platelets or platelet derivatives
(e.g., with an incubating agent, e.g.,
an incubating agent described herein).
[00352] In some embodiments, the method further comprises drying a
composition comprising platelets
or platelet derivatives, (e.g., with an incubating agent e.g., an incubating
agent described herein) prior to
administering the platelets or platelet derivatives (e.g., FDPDs). In some
embodiments, the method may
further comprise heating the composition following the drying step. In some
embodiments, the method may
further comprise rehydrating the composition following the freeze-drying step
or the heating step.
[00353] In some embodiments, the method further comprises freeze-drying a
composition comprising
platelets or platelet derivatives (e.g., with an incubating agent e.g., an
incubating agent described herein)
71
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
prior to administering the platelets or platelet derivatives (e.g., FDPDs) In
some embodiments, the method
may further comprise heating the composition following the freeze-drying step.
In some embodiments, the
method may further comprise rehydrating the composition following the freeze-
drying step or the heating
step.
[00354] In some embodiments, the method further comprises cold storing the
platelets, platelet
derivatives, or the FDPDs prior to administering the platelets, platelet
derivatives, or FDPDs (e.g., with an
incubating agent, e.g., an incubating agent described herein).
[00355] Storing conditions include, for example, standard room temperature
storing (e.g., storing at a
temperature ranging from about 20 to about 30 C) or cold storing (e.g.,
storing at a temperature ranging
from about 1 to about 10 C). In some embodiments, the method further comprises
cryopreserving, freeze-
drying, thawing, rehydrating, and combinations thereof, a composition
comprising platelets or platelet
derivatives (e.g., FDPDs) (e.g., with an incubating agent e.g., an incubating
agent described herein) prior
to administering the platelets or platelet derivatives (e.g., FDPDs). For
example, in some embodiments, the
method further comprises drying (e.g., freeze-drying) a composition comprising
platelets or platelet
derivatives (e.g., with an incubating agent e.g., an incubating agent
described herein) (e.g., to form FDPDs)
prior to administering the platelets or platelet derivatives (e.g., FDPDs). In
some embodiments, the method
may further comprise rehydrating the composition obtained from the drying
step.
[00356] In some embodiments, provided herein is a composition comprising
platelets or platelet
derivatives (e.g., FDPDs), polysucrose and trehalose made by the process of
obtaining fresh platelets,
optionally incubating the platelets in DMSO, isolating the platelets by
centrifugation, resuspending the
platelets in an incubating agent which comprises trehalose and ethanol thereby
forming a first mixture,
incubating the first mixture, mixing polysucrose with the first mixture,
thereby forming a second mixture,
and lyophilizing the second mixture to form a freeze dried composition
comprising platelets or platelet
derivatives (e.g., FDPDs), polysucrose and trehalose.
[00357] In some embodiments, provided herein is a method of making a freeze-
dried platelet
composition comprising platelets or platelet derivatives (e.g., FDPDs),
polysucrose and trehalose
comprising obtaining fresh platelets, optionally incubating the platelets in
DMSO, isolating the platelets by
centrifugation, resuspending the platelets in a incubating agent which
comprises trehalose and ethanol
thereby forming a first mixture, incubating the first mixture, mixing
polysucrose with the first mixture,
thereby forming a second mixture, and lyophilizing the second mixture to form
a freeze-dried composition
comprising platelets or platelet derivatives (e.g., FDPDs), polysucrose and
trehalose.
[00358] In some embodiments, provided herein is a process for making freeze-
dried platelets, the
process comprising incubating isolated platelets in the presence of at least
one saccharide under the
following conditions: a temperature of from 20 C. to 42 C for about 10
minutes to about 180 minutes,
72
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
adding to the platelets at least one cryoprotectant, and lyophilizing the
platelets, wherein the process
optionally does not include isolating the platelets between the incubating and
adding steps, and optionally
wherein the process does not include exposing the platelets to a platelet
activation inhibitor. The
cryoprotectant can be a polysugar (e.g., polysucrose). The process can further
include heating the
lyophilized platelets at a temperature of 70 C to 80 C for 8 to 24 hours.
The step of adding to the platelets
at least one cryoprotectant can further include exposing the platelets to
ethanol. The step of incubating
isolated platelets in the presence of at least one saccharide can include
incubating in the presence of at least
one saccharide. The step of incubating isolated platelets in the presence of
at least one saccharide can
include incubating in the presence of at least one saccharide. The conditions
for incubating can include
incubating for about 100 minutes to about 150 minutes. The conditions for
incubating can include
incubating for about 110 minutes to about 130 minutes. The conditions for
incubating can include
incubating for about 120 minutes. The conditions for incubating can include
incubating at 35 C to 40 C.
The conditions for incubating can include incubating at 37 C. The conditions
for incubating can include
incubating at 35 C to 40 C. for 110 minutes to 130 minutes. The conditions
for incubating can include
incubating at 37 C for 120 minutes. The at least one saccharide can be
trehalose, sucrose, or both trehalose
and sucrose. The at least one saccharide can be trehalose. The at least one
saccharide can be sucrose.
[00359] In some embodiments, provided herein is a method of preparing
freeze-dried platelets, the
method including providing platelets, suspending the platelets in a salt
buffer that includes about 100 mM
trehalose and about 1% (v/v) ethanol to make a first composition, incubating
the first composition at about
37 C. for about 2 hours, adding polysucrose (e.g., polysucrose 400) to a
final concentration of about 6%
(w/v) to make a second composition, lyophilizing the second composition to
make freeze-dried platelets,
and heating the freeze-dried platelets at 80 C for 24 hours.
[00360] Specific embodiments disclosed herein may be further limited in the
claims using "consisting
of' or "consisting essentially of' language.
[00361] Exemplary Embodiments
[00362] Provided in this Exemplary Embodiments section are non-limiting
exemplary aspects and
embodiments provided herein and further discussed throughout this
specification. For the sake of brevity
and convenience, all of the aspects and embodiments disclosed herein, and all
of the possible combinations
of the disclosed aspects and embodiments are not listed in this section.
Additional embodiments and aspects
are provided in other sections herein. Furthermore, it will be understood that
embodiments are provided
that are specific embodiments for many aspects and that can be combined with
any other embodiment, for
example as discussed in this entire disclosure. It is intended in view of the
full disclosure herein, that any
individual embodiment recited below or in this full disclosure can be combined
with any aspect recited
73
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
below or in this full disclosure where it is an additional element that can be
added to an aspect or because
it is a narrower element for an element already present in an aspect. Such
combinations are sometimes
provided as non-limiting exemplary combinations and/or are discussed more
specifically in other sections
of this detailed description.
[00363] Provided herein in one aspect is a method of treating a coagulopathy
in a subject, the method
including administering to the subject in need thereof an effective amount of
a composition including
platelets, or in illustrative embodiments platelet derivatives, and in further
illustrative embodiments FDPDs,
thereby treating the coagulopathy. In illustrative embodiments, the
composition comprising the platelet
derivatives is administered such that the bleeding potential of the subject is
reduced, and in illustrative
embodiments such that normal hemostasis is restored in the subject.
[00364] In one aspect, provided herein is a method of treating a coagulopathy
in a subject, the method
including administering to the subject in need thereof an effective amount of
a composition prepared by a
process including incubating platelets with an incubating agent including one
or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, and in
illustrative embodiments freeze-
drying the incubated platelets, to form the composition, wherein the
composition includes platelet
derivatives, and in illustrative embodiments FDPDs, thereby treating the
coagulopathy. In illustrative
embodiments, the composition comprising the platelet derivatives is
administered such that the bleeding
potential of the subject is reduced, and in illustrative embodiments such that
normal hemostasis is restored
in the subject.
[00365] In one aspect, provided herein is a method of restoring normal
hemostasis in a subject, the method
including administering to the subject in need thereof an effective amount of
a composition including
platelets, or in illustrative embodiments platelet derivatives, and in further
illustrative embodiments FDPDs,
thereby treating the coagulopathy. In illustrative embodiments, the
composition comprising the platelet
derivatives is administered such that the bleeding potential of the subject is
reduced, and in illustrative
embodiments such that normal hemostasis is restored in the subject.
[00366] In one aspect, provided herein is a method of restoring normal
hemostasis in a subject, the method
including administering to the subject in need thereof an effective amount of
a composition prepared by a
process including incubating platelets with an incubating agent including one
or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, and in
illustrative embodiments freeze-
drying the incubated platelets, to form the composition, wherein the
composition comprises platelet
derivatives, and in further illustrative embodiments FDPDs, thereby treating
the coagulopathy. In
illustrative embodiments, the composition comprising the platelet derivatives
is administered such that the
bleeding potential of the subject is reduced, and in illustrative embodiments
such that normal hemostasis is
restored in the subject.
74
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00367] In one aspect, provided herein is a method of preparing a subject for
surgery, the method including
administering to the subject in need thereof an effective amount of a
composition including platelets, or in
illustrative embodiments platelet derivatives, and in further illustrative
embodiments FDPDs. Various
properties of exemplary embodiments of such FDPDs are provided herein, thereby
treating the
coagulopathy. In illustrative embodiments, the composition comprising the
platelet derivatives is
administered such that the bleeding potential of the subject is reduced, and
in illustrative embodiments such
that normal hemostasis is restored in the subject. Implementations can include
one or more of the following
features. The surgery can be an emergency surgery. The surgery can be a
scheduled surgery.
[00368] In one aspect, provided herein is a method of preparing a subject for
surgery, the method including
administering to the subject in need thereof an effective amount of a
composition prepared by a process
including incubating platelets with an incubating agent including one or more
salts, a buffer, optionally a
cryoprotectant, and optionally an organic solvent, and in illustrative
embodiments freeze-drying the
incubated platelets, to form the composition, wherein the composition includes
platelet derivatives, and in
further illustrative embodiments FDPDs, thereby treating the coagulopathy. In
illustrative embodiments,
the composition comprising the platelet derivatives is administered such that
the bleeding potential of the
subject is reduced, and in illustrative embodiments such that normal
hemostasis is restored in the subject.
Various properties of exemplary embodiments of such FDPDs are provided herein.
Implementations can
include one or more of the following features. The surgery can be an emergency
surgery. The surgery can
be a scheduled surgery.
[00369] In one aspect, provided herein is a method of ameliorating the effects
of an antiplatelet agent in a
subject, the method including administering to the subject in need thereof an
effective amount of a
composition platelets, or in illustrative embodiments platelet derivatives,
and in further illustrative
embodiments FDPDs, thereby treating the coagulopathy. In illustrative
embodiments, the composition
comprising the platelet derivatives is administered such that the bleeding
potential of the subject is reduced,
and in illustrative embodiments such that normal hemostasis is restored in the
subject.
[00370] In one aspect, provided herein is a method of ameliorating the effects
of an antiplatelet agent in a
subject, the method including administering to the subject in need thereof an
effective amount of a
composition prepared by a process including incubating platelets with an
incubating agent including one or
more salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition,
wherein the composition includes platelet derivatives, and in further
illustrative embodiments FDPDs,
thereby treating the coagulopathy. In illustrative embodiments, the
composition comprising the platelet
derivatives is administered such that the bleeding potential of the subject is
reduced, and in illustrative
embodiments such that normal hemostasis is restored in the subject.
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00371] In one aspect, provided herein is a method of treating a coagulopathy
in a subject, or of restoring
hemostasis in a subject, or of reducing bleeding potential of a subject that
is being administered, or has been
administered, an antiplatelet agent, the method comprising: administering to
the subject in need thereof an
effective amount of a composition comprising platelet derivatives, thereby
treating the coagulopathy. In
illustrative embodiments, the platelet derivatives are freeze-dried platelet
derivatives (FDPDs). In further
illustrative embodiments, the composition comprising the platelet derivatives
is administered such that the
bleeding potential of the subject is reduced, and in illustrative embodiments
such that normal hemostasis is
restored in the subject.
[00372] In another aspect, provided herein is a method of treating a
coagulopathy in a subject, or of restoring
hemostasis in a subject, or of reducing bleeding potential of a subject,
wherein the subject is being
administered, or has been administered, an antiplatelet agent, the method
comprising administering to the
subject in need thereof an effective amount of the composition comprising
FDPDs, wherein the composition
comprising FDPDs comprises a population of FDPDs having a reduced propensity
to aggregate such that
no more than 10% of the FDPDs in the population aggregate under aggregation
conditions comprising an
agonist but no platelets, thereby treating the coagulopathy. In further
illustrative embodiments, the
composition comprising the FDPDs is administered such that the bleeding
potential of the subject is
reduced, and in illustrative embodiments such that normal hemostasis is
restored in the subject.
[00373] In another aspect, provided herein is a method of preventing or
mitigating the potential for a
coagulopathy in a subject, the method comprises: (a) determining that
information regarding whether the
subject was administered an antiplatelet agent is unavailable; and (b)
administering to the subject an
effective amount of a composition comprising freeze-dried platelet derivatives
(FDPDs). In some
embodiments of such a method, information regarding whether the subject was
administered an antiplatelet
agent is unavailable for a reason comprising that the subject cannot be
identified. In some embodiments of
the method, information regarding whether the subject was administered an
antiplatelet agent is unavailable
for a reason comprising that the medical history of the subject is
unavailable. In further embodiments
information regarding whether the subject was administered an antiplatelet
agent is unavailable for a reason
comprising that the subject is in need of emergency treatment.
[00374] In another aspect, provided herein is a method of treating a
coagulopathy in a subject or of
reducing the bleeding potential of a subject, or of restoring hemostasis in a
subject, wherein the method
comprises: administering to the subject in need thereof an effective amount of
a composition comprising
platelet derivatives, in illustrative embodiments, FDPDs, wherein the subject
before the administering the
composition comprising platelet derivatives, was administered an antiplatelet
agent and a second agent that
decreases platelet function, thereby treating the coagulopathy. In further
illustrative embodiments, the
composition comprising the platelet derivatives is administered such that the
bleeding potential of the
76
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
subject is reduced, and in illustrative embodiments such that normal
hemostasis is restored in the subject.
In illustrative embodiments, before the administering of the composition
comprising FDPDs the subject
was in need thereof because of an increased risk of bleeding due to, or as a
result of being administered the
anti-platelet agent and the second agent.
[00375] In another aspect, provided herein is a composition comprising freeze-
dried platelet derivatives
(FDPDs) for treating a coagulopathy in a subject, wherein the treating
comprises:
administering to the subject in need thereof, an effective amount of the
composition comprising FDPDs
such that the bleeding potential, or risk of bleeding of the subject is
reduced,
wherein the subject was administered an antiplatelet agent and a second agent
that decreases
platelet function, and
wherein the subject is in need thereof because of an increased potential for,
or risk of bleeding due
to, or as a result of being administered the antiplatelet agent and the second
agent,
thereby treating the coagulopathy.
[00376] In another aspect, provided herein is a composition comprising freeze-
dried platelet derivatives
(FDPDs) for treating a coagulopathy in a subject having an increased potential
for, or risk of bleeding as a
result of being administered or having been administered an anticoagulant,
wherein the treating comprises:
administering to the subject having the increased potential for, or risk of
bleeding, an effective
amount of the composition comprising FDPDs such that the bleeding potential or
risk of bleeding of the
subject is reduced,
wherein the composition comprising FDPDs comprises a population of FDPDs
having a reduced
propensity to aggregate such that no more than 10% of the FDPDs in the
population aggregate under
aggregation conditions comprising an agonist but no platelets,
thereby treating the coagulopathy.
[00377] In some embodiments, for example of aspects wherein a subject was
administered the antiplatelet
agent and the second agent that decreases platelet function, such a method
further comprises before the
administering the composition comprising FDPDs, determining that the subject
was administered the
antiplatelet agent and the second agent that decreases platelet function. In
some embodiments, the
antiplatelet agent is a first antiplatelet agent and the second agent is a
second antiplatelet agent. In some
embodiments, the first antiplatelet agent and the second anti-platelet agent
are each different antiplatelet
agents selected from aspirin, cangrelor, ticagrelor, clopidogrel, prasugrel,
eptifibatide, tirofiban, abciximab,
terutroban, picotamide, elinogrel, ticlopidine, ibuprofen, vorapaxar,
atopaxar, cilostazol, prostaglandin El,
epoprostenol, dipyridamole, treprostinil sodium, and sarpogrelate. In some
embodiments, the first
antiplatelet agent and the second anti-platelet agent have different
mechanisms of action. In some
77
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
embodiments, the first antiplatelet agent and the second anti-platelet agent
are each different antiplatelet
agents selected from aspirin, cangrelor, ticagrelor, clopidogrel, prasugrel,
eptifibatide, tirofiban, abciximab,
terutroban, picotamide, elinogrel, ticlopidine, ibuprofen, vorapaxar,
atopaxar, cilostazol, prostaglandin El,
epoprostenol, dipyridamole, treprostinil sodium, and sarpogrelate.
[00378] In some embodiments of any of the aspects herein, before,
immediately before, at the moment
before, at the moment of, and/or at an initial time of, the administering of
the composition comprising
platelet derivatives, for example FDPDs, the subject was or is at an increased
risk of bleeding due to being
administered or having been administered the anti-platelet agent. Furthermore,
the subject can be at an
increased risk of bleeding at 7, 6, 5, 4, 3, 2, or 1 day, 12 hours, 8 hours, 4
hours, 2 hours, 1 hour or 45, 30,
15, 10, 5, 4, 3, 2, or 1 minute before the administering of the composition
comprising the platelet
derivatives. In some optional embodiments, this is confirmed by laboratory
testing. However, in some
embodiments no laboratory testing of bleeding risk or any clotting parameter
is performed 7, 6, 5, 4, 3, 2,
or 1 day or sooner before and/or after the administering of the composition
comprising the platelet
derivatives. Bleeding risk is typically decreased after administration of an
effective dose of the composition
comprising platelet derivatives, in illustrative embodiments FDPDs.
Furthermore, the subject may remain
at an increased risk of bleeding even after the administering of the
composition comprising platelet
derivatives (e.g. FDPDs), for example for 1, 2, 3, 4, 5, 10, 15, 20, 30, or 45
minutes, or 1, 2, 3, 4, 5, or 8
hours, or longer after the administering, depending on how long it takes for
the FDPDs to decrease the risk
in the subject after they are administered. Furthermore, in some embodiments,
the administration of the
composition comprising the platelet derivatives (e.g. FDPDs) decreases but
does not completely resolve
the increased risk of bleeding in the subject.
[00379] In some embodiments, for example of aspects wherein a subject was
administered the antiplatelet
agent and the second agent that decreases platelet function, administration of
the second agent is stopped,
for example before administrating the composition comprising the platelet
derivatives. In other
embodiments of such aspects, administration of the second agent is continued,
for example after
administering the composition comprising the platelet derivatives.
[00380] In certain embodiments of any of the aspects provided herein, the
method further comprises before
administering the composition comprising platelet derivatives, determining in
a pre-administering
evaluation, that the subject has an abnormal value for one or more clotting
parameters. The pre-
administration evaluation, in illustrative embodiments, is an in vitro
laboratory test.
[00381] In certain embodiments of any of the aspects provided herein, the
antiplatelet agent is selected from
aspirin, cangrelor, ticagrelor, clopidogrel, prasugrel, eptifibatide,
tirofiban, abciximab, terutroban,
picotamide, elinogrel, ticlopidine, ibuprofen, vorapaxar, atopaxar,
cilostazol, prostaglandin El,
epoprostenol, dipyridamole, treprostinil sodium, and sarpogrelate. In other
embodiments, the antiplatelet
78
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
agent is selected from cangrelor, ticagrelor, abciximab, terutroban,
picotamide, elinogrel, ibuprofen,
vorapaxar, atopaxar, cilostazol, prostaglandin El, epoprostenol, dipyridamole,
treprostinil sodium, and
sarpogrelate.
[00382] In certain embodiments of any of the aspects provided herein, the
FDPDs comprise (detectable
amounts of) a biomolecule (e.g. receptor) targeted by the anti-platelet
reversal agent that was administered
or is being administered to the subject. In some embodiments the receptor is
selected from a P2Y receptor
(e.g., the P2Y12 receptor), glycoprotein IIb (i.e. CD41), glycoprotein lila
(CD61), the glycoprotein IIb/IIIa
complex, thromboxane synthase or thromboxane receptors, PAR1, PAR4, VPVI, or
collagen receptor (e.g.
alpha2beta1 collagen receptor). Provided in other sections herein are examples
of anti-platelet agents that
target these specific biomolecules. In certain embodiments, at least 55%, 60%,
70%, 75%, 80%, 85%, 90%,
or 95% of the platelet derivatives, in illustrative embodiments FDPDs, are
positive for (i.e. have detectable
levels of) a biomolecule targeted by the anti-platelet agent administered to
the subject and/or detectable in
the blood of the subject. As noteworthy non-limiting examples, the anti-
platelet agent inhibits the
glycoprotein CDIIb/IIIa complex, and at least 55%, 60%, 70%, 75%, 80%, 85%,
90%, or 95% of the platelet
derivatives, in illustrative embodiments FDPDs, are CD41 positive (i.e.
comprise detectable CD41) and/or
are positive for the CDIIb/IIIa complex.
[00383] In certain embodiments of any of the aspects provided herein, the
composition comprising
FDPDs comprises a population of FDPDs having a reduced propensity to aggregate
such that no more than
2%, 3%, 4%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20%, 22.5%, or 25% of the FDPDs
in the population
aggregate under aggregation conditions comprising an agonist but no platelets.
In certain embodiments of
any of the aspects provided herein, including for example, embodiments where
the composition comprises
FDPDs comprises a population of FDPDs having a reduced propensity to aggregate
such that no more than
10% of the FDPDs in the population aggregate under aggregation conditions
comprising an agonist but no
platelets, the FDPDs have a potency of at least 1.2 (e.g., at least 1.3, 1.4,
1.5, 1.6, 1.7, 1.8, 1.9, 2.0, 2.1, 2.2,
2.3, 2.4, or 2.5) thrombin generation potency units (TGPU) per 106 particles.
For example, in some cases,
platelets or platelet derivatives (e.g., FDPDs) can have a potency of between
1.2 and 2.5 TPGU per 106
particles (e.g., between 1.2 and 2.0, between 1.3 and 1.5, between 1.5 and
2.25, between 1.5 and 2.0,
between 1.5 and 1.75, between 1.75 and 2.5, between 2.0 and 2.5, or between
2.25 and 2.5 TPGU per 106
particles).
[00384] In certain embodiments of any of the aspects provided herein,
including for example,
embodiments where the composition comprises FDPDs comprises a population of
FDPDs having a reduced
propensity to aggregate such that no more than 10% of the FDPDs in the
population aggregate under
aggregation conditions comprising an agonist but no platelets, the FDPDs have
having one or more
characteristics of a super-activated platelet selected from
79
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00385] A) the presence of thrombospondin (TSP) on their surface at a level
that is greater than on the
surface of resting platelets;
[00386] B) the presence of von Willebrand factor (vWF) on their surface at
a level that is greater than
on the surface of resting platelets; and
[00387] C) an inability to increase expression of a platelet activation
marker in the presence of an
agonist as compared to the expression of the platelet activation marker in the
absence of an agonist.
[00388] In certain embodiments of any of the aspects provided herein wherein
the composition comprising
FDPDs comprises a population of FDPDs comprising CD 41-positive platelet
derivatives, including non-
limiting embodiments where the population comprises FDPDs have a reduced
propensity to aggregate such
that no more than 10% of the FDPDs in the population aggregate under
aggregation conditions comprising
an agonist but no platelets, the FDPDs have an inability to increase
expression of a platelet activation marker
in the presence of an agonist as compared to the expression of the platelet
activation marker in the absence
of the agonist. In some embodiments of such methods, the FDPDs have a potency
of at least 1.5 thrombin
generation potency units (TGPU) per 106 platelet derivatives. In some
embodiments of such methods, less
than 5% of the CD 41-positive FDPDs are microparticles having a diameter of
less than 0.5 m.
[00389] In certain embodiments of any of the aspects provided herein,
including non-limiting embodiments
where the population comprises FDPDs have a reduced propensity to aggregate
such that no more than 10%
of the FDPDs in the population aggregate under aggregation conditions
comprising an agonist but no
platelets, the FDPDs further have one or both of: the presence of
thrombospondin (TSP) on their surface at
a level that is greater than on the surface of resting platelets; and the
presence of von Willebrand factor
(vWF) on their surface at a level that is greater than on the surface of
resting platelets.
[00390] In certain embodiments of any of the aspects provided herein, the
composition comprising FDPDs
comprises a population of FDPDs comprising
[00391] a population of platelet derivatives comprising CD 41-positive
platelet derivatives, wherein less
than 5% of the CD 41-positive platelet derivatives are microparticles having a
diameter of less than 0.5 m,
and comprising platelet derivatives having one or more, or in illustrative
embodiments all of the following
characteristics:
[00392] a reduced propensity to aggregate such that no more than 10% of the
platelet derivatives in the
population aggregate under aggregation conditions comprising an agonist but no
platelets;
[00393] an inability to increase expression of a platelet activation marker in
the presence of an agonist as
compared to the expression of the platelet activation marker in the absence of
the agonist;
[00394] the presence of thrombospondin (TSP) on their surface at a level that
is greater than on the surface
of resting platelets;
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00395] the presence of von Willebrand factor (vWF) on their surface at a
level that is greater than on the
surface of resting platelets; and
[00396] a potency of at least 1.5 thrombin generation potency units (TGPU) per
106 platelet derivatives.
[00397] In some embodiments of any aspects herein, the platelet derivatives,
and in illustrative
embodiments FDPDs, are surrounded by a compromised plasma membrane. In such
embodiments, the
platelet derivatives lack an integrated membrane around them. In such
embodiments, the platelet derivatives
are not surrounded by an integrated membrane. Instead, the membrane comprises
pores that are larger than
pores observed on living cells. Thus, such platelet derivatives have a reduced
ability to, or are unable to
transduce signals from the external environment into a response inside the
particle that are typically
transduced in living platelets. Furthermore, such platelet derivatives (e.g.
FDPDs) are not believed to be
capable of mitochondrial activation or glycolysis.
[00398] In some embodiments of any aspects herein, the effective amount of the
composition comprising
FDPDs is between 1.0 X 107 to 1.0 X 10" particles or FDPDs/kg of the subject.
In some embodiments of
any of the aspects herein, the effective amount of the composition comprising
FDPDs is between 1.6 X 107
to 5.1 X 109 particles or FDPDs/kg of the subject. In some embodiments of any
of the aspects herein, which
can be combined with either of the above embodiments with ranges of particles
or FDPDs/kg, the effective
amount of the composition comprising FDPDs is an amount that has a potency
between 250 and 5000
TGPU per kg of the subject. Further examples of effective amounts are provided
in a different section
herein.
[00399] In one aspect, provided herein is a method of treating a coagulopathy
in a subject, or of restoring
hemostasis in a subject, or of reducing bleeding potential of a subject that
is being administered, or has been
administered, an antiplatelet agent, the method comprising: administering to
the subject in need thereof an
effective amount of a composition comprising platelet derivatives, thereby
treating the coagulopathy,
wherein the composition comprising FDPDs has the property that it is capable
of reducing the bleeding
potential of the subject, independent of whether a post-administering
evaluation of bleeding potential, if
performed, would yield a normal or abnormal result.
[00400] In any of the aspects herein, in some embodiments, the composition
comprising FDPDs has the
property that it is capable of reducing the bleeding potential of the subject,
independent of whether a post-
administering evaluation of bleeding potential, if performed, would yield a
normal or abnormal result. In
some optional embodiments, which in some embodiments is performed and in some
embodiments is not
performed, such post-administering evaluation comprises an in vitro laboratory
test performed on a sample
taken or drawn in a time period after administering the composition comprising
FDPDs to the subject. In
other embodiments of any of the aspects herein, wherein the composition
comprising FDPDs has the
property that it is capable of reducing the bleeding potential of a subject
having an increased bleeding
81
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
potential, and in some embodiments an abnormal value for one or more clotting
parameters in an in vitro
laboratory test, such that normal hemostasis is restored in the subject,
independent of whether a post-
administering evaluation of bleeding potential, if performed would yield a
normal or abnormal result. In
some embodiments, such post-administering evaluation comprises an in vitro
laboratory test performed on
a sample taken or drawn in a time period after administering the composition
comprising FDPDs to the
subject. The time period, can be for example, within 0 minutes and 72 hours,
or between 10 minutes and
72 hours, or between 10 minutes and 48 hours, or between 10 minutes 24 hours,
or between 10 minutes and
4 hours, or between 10 minutes and 1 hour, or between 10 minutes and 30
minutes, or between 30 minutes
and 24 hours, or between 30 minutes and 4 hours, or between 30 minutes and 1
hour after administering
the composition comprising the platelet derivatives (e.g. FDPDs) to the
subject. For example, the time
period in certain embodiments is between 1 and 4 hours after administering the
composition comprising
the platelet derivatives (e.g. FDPDs). In some embodiments of any of the
aspects herein, a pre or post
administration of the composition comprising platelet derivatives is not
performed, for example during the
recited time periods above.
[00401] In any of the aspects herein, in some embodiments the composition
comprising platelet derivatives
(e.g. FDPDs) has the property that it is capable of reducing the bleeding
potential of a subject having an
increased or elevated bleeding potential. In some embodiments, such increased
or elevated bleeding
potential can be determined by abnormal value for one or more clotting
parameters in an in vitro laboratory
test performed on a sample taken within 0 minutes and 72 hours, or between 10
minutes and 72 hours, or
between 10 minutes and 48 hours, or between 10 minutes 24 hours, or between 10
minutes and 4 hours, or
between 10 minutes and 1 hour, or between 10 minutes and 30 minutes, or
between 30 minutes and 24
hours, or between 30 minutes and 4 hours, or between 30 minutes and 1 hour
before administering the
composition comprising the platelet derivatives (e.g. FDPDs). Furthermore, the
composition comprising
FDPDs typically has the additional and surprising property, that after being
administered to the subject in
an effective amount, for example for reducing the bleeding potential of the
subject, the subject has an
abnormal value for the one or more in vitro lab tests, for example of one or
more clotting parameters in a
post-administering evaluation performed using an, or the in vitro laboratory
test performed on a blood
sample taken between 15 minutes and 4 hours, 30 minutes and 4 hours, 1 hour
and 4 hours, or taken between
15 minutes and 2 hours, 30 minutes and 2 hours, or 1 hour and 2 hours, or
taken between 15 minutes and 1
hour or 30 minutes and 1 hour, after administering the composition comprising
FDPDs. In some
subembodiments of this embodiment, the composition comprising FDPDs has the
property that it is capable
of reducing the bleeding potential of a subject to about or at a normal
hemostasis or about or at the
hemostasis level of the subject when not taking the antiplatelet agent. Yet,
in these embodiments, the
composition comprising FDPDs retains the additional and surprising property,
that after being administered
82
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
to the subject in the effective amount, such a property is independent of a
post-adminstering lab test for
bleeding potential. Thus, in some embodiments, the subject would have an
abnormal value for the one or
more clotting parameters in a post-administering evaluation performed using
an, or the in vitro laboratory
test performed on a blood sample taken between 1 and 4 hours, or any of the
time ranges recited immediately
above, after administering the composition comprising FDPDs. It will be
understood that in methods that
include compositions comprising FDPDs with such properties, or any properties
that include an evaluation
or test, no testing actually needs to be performed to practice such methods
unless such testing step is actually
recited as a method step.
[00402] In any of the aspects herein, in illustrative embodiments the
composition comprising platelet
derivatives or FDPDs further comprises additional components, such as
components that were present when
such platelet derivatives were dried, or FDPDs were freeze-dried. Such
additional components can include
components of an incubating agent comprising one or more salts, a buffer, and
in certain embodiments a
cryoprotectant (also called a lyophilizing agent) and/or an organic solvent.
For example, such compositions
can comprise one or more saccharides, as provided further herein, which in
illustrative embodiments
include trehalose and in further illustrative embodiments include polysucrose.
[00403] In any of the aspects herein, in some embodiments the FDPDs are
prepared using centrifugation.
In some illustrative embodiments, the FDPDs are prepared using TFF, in further
illustrative embodiments
without isolating platelets by centrifugation during the process.
[00404] In some embodiments of any of the aspects herein, the method further
includes determining the
value of one or more clotting parameters in a post-administering evaluation,
wherein the post-administering
evaluation is performed following the administering. In some embodiments the
post-administering
evaluation of the one or more clotting parameters shows a normal value for at
least one of the one or more
clotting parameters. In further embodiments the method the result of the post-
administering evaluation of
the one or more clotting parameters is improved from the result of the
evaluation of the one or more
parameters prior to the administering.
[00405] In further embodiments of the method the administering of the
antiplatelet agent contrary to
medical instruction is self-administering by the subject, is administered by
another, or is administering by
a medical professional.
[00406] In some embodiments of any of the aspects or embodiments herein that
include a second agent,
typically a second agent that decreases platelet function, the second agent is
selected from the group
consisting of an antihypertensive, a proton pump inhibitor, and a combination
thereof. In some
embodiments the second agent is selected from the group consisting of a
chemotherapeutic agent, an
antibiotic, a cardiovascular agent, a H2 antagonist, a neuropsychiatric agent
and a combination thereof. In
some embodiments the second agent comprises an antidepressant. In further
embodiments the
83
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
antidepressant is selected from the group consisting of a selective serotonin
reuptake inhibitor (SSRI), a
serotonin antagonist and reuptake inhibitor (SARI), a serotonin and
norepinephrine reuptake inhibitor
(SNRI), and a combination thereof. In some embodiments the second agent is not
an anticoagulant.
[00407] In any of the aspects herein, in some embodiments, administration of
the antiplatelet agent is
stopped before or when a composition comprising platelet derivatives (e.g.
FDPDs) is administer to a
subject. In some aspects of the method, administration of the antiplatelet
agent is continued after a
composition comprising platelet derivatives (e.g. FDPDs) is administer to a
subject.
[00408] In any of the aspects herein, in some embodiments further comprise
determining that the subject
has an abnormal value for one or more clotting parameters in a pre-
administering evaluation. In some
aspects of the method, the method comprises determining the value of one or
more clotting parameters in a
post-administering evaluation. In some embodiments the post-administering
evaluation of the one or more
clotting parameters shows a normal result for at least one of the one or more
clotting parameters. In some
embodiments the result of the post-administering evaluation of the one or more
clotting parameters is
improved from the result of the evaluation of the one or more parameters prior
to the administering.
[00409] In any of the aspects herein, in some embodiments, the subject is
identified as having an abnormal
result for one or more pre-administering evaluations of clotting parameters
during surgery. In some
embodiments the surgery is an emergency surgery. In some embodiments the
surgery is a scheduled
surgery.
[00410] In any of the aspects herein, in some embodiments, the clotting
parameters includes an
evaluation of bleeding. In some embodiments the evaluation of bleeding is
performed based on the World
Health Organization (WHO) bleeding scale. In some embodiments of the method,
before administering,
the subject has bleeding of grade 2, 3, or 4 based on the WHO bleeding scale;
In some embodiments of the
method, after administering, the subject has bleeding of grade 0 or 1 based on
the WHO bleeding scale. In
some embodiments, after the administering, the subject has bleeding of one
grade less, based on the WHO
bleeding scale, than before the administering. In some embodiments, after the
administering, the subject
has bleeding of two grades less, based on the WHO bleeding scale, than before
the administering. In some
embodiments, after the administering, the subject has bleeding of three grades
less, based on the WHO
bleeding scale, than before the administering.
[00411] In any of the aspects herein, in some embodiments, evaluation of
the clotting parameters
includes an evaluation of prothrombin time (PT). In some embodiments, abnormal
results for PT comprises
a PT of greater than about 14 seconds. In some embodiments, after the
administering, the subject has a
decrease in PT of at least 1, 2, 3, 4, 5, or more, seconds. In some
embodiments, after the administering, the
subject has a normal PT.
84
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00412] In any of the aspects herein, in some embodiments, the one or more
clotting parameters
includes an evaluation of activated partial thromboplastin time (aPTT). In
some embodiments, the
abnormal result for aPTT comprises an aPTT of greater than about 40 seconds.
In some embodiments, after
the administering, the subject has a decrease in aPTT of at least 5, 10, 15,
20, or more, seconds. In some
embodiments, after the administering, the subject has a normal aPTT.
[00413] In any of the aspects herein, in some embodiments, the one or more
clotting parameters includes
an evaluation of thrombin clot time (TCT). In some embodiments, the abnormal
result for TCT comprises
a TCT of greater than about 35 seconds. In some embodiments, after the
administering, the subject has a
decrease in TCT of at least 5, 10, 15, 20, or more, seconds. In some
embodiments, after the administering,
the subject has a normal TCT.
[00414] In any of the aspects herein, in some embodiments, the evaluation
of the one or more clotting
parameters is measured using thromboelastography (TEG). In some embodiments,
the abnormal result for
TEG comprises a maximum amplitude (MA) of less than about 50 mm. In some
embodiments, after the
administering, the subject has an increase in MA of at least 5, 10, 15, 20, or
more, mm. In some
embodiments, after the administering, the subject has a normal MA.
[00415] In any of the aspects herein, in some embodiments, the abnormal
result for TEG comprises a
percent aggregation (in the presence of 1 mmol/L arachidonic acid) of less
than about 85%. In some
embodiments, after the administering, the subject has an increase in percent
aggregation (in the presence of
1 mmol/L arachidonic acid) of at least 2, 3, 5, 8, 10, 12, or more, percentage
points. In some embodiments,
after the administering, the subject has a normal percent aggregation (in the
presence of 1 mmol/L
arachidonic acid). In some embodiments, the TEG is used to evaluate adenosine
diphosphate-induced
platelet-fibrin clot strength. In some aspects of the method, the TEG is used
to evaluate arachidonic acid-
induced platelet-fibrin clot strength.
[00416] In any of the aspects herein, in some embodiments, the evaluation
of one or more clotting
parameters is measured using an P2Y12 Reaction Units (PRU) or Aspirin Reaction
Units (ARU) test
method.
[00417] In any of the aspects herein, in some embodiments, the abnormal
result of the P2Y12 reaction
unit test method comprise a PRU of less than about 195, or less than about
180. In some embodiments,
after the administering, the subject has an increase in PRU of at least 25,
50, 75, 100, or more. In some
embodiments, after the administering, the subject has a normal PRU.
[00418] In any of the aspects herein, in some embodiments, the abnormal
result of the Aspirin Reaction
Unit test method comprise an ARU of less than about 550, or less than about
500. In some embodiments,
after the administering, the subject has an increase in ARU of at least 25,
50, 75, 100, or more. In some
embodiments, after the administering, the subject has a normal ARU.
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00419] In any of the aspects herein, in some embodiments, the one or more
clotting parameters is
measured using multiple electrode aggregometry (MEA). In some embodiments, the
abnormal result using
MEA comprises an abnormal result for ADP-induced platelet activity. In some
embodiments, the abnormal
result for MEA comprises a result of less than about 50 units (U) for ADP-
induced platelet activity. In some
embodiments, after the administering, the subject has an increase in ADP-
induced platelet activity by 5, 10,
15, 20, or more units. In some embodiments, after the administering, the
subject has a normal value for
ADP-induced platelet activity. In some embodiments, the abnormal result for
MEA comprises an abnormal
result for arachidonic acid-induced platelet activity. In some embodiments,
the abnormal result for MEA
comprises a result of less than about 70 units (U) for arachidonic acid-
induced platelet activity. In some
embodiments, after the administering, the subject has an increase in
arachidonic acid-induced platelet
activity by 5, 10, 15, 20, or more units. In some embodiments, after the
administering, the subject has a
normal value for arachidonic acid-induced platelet activity.
[00420] In any of the aspects herein, in some embodiments, the one or more
clotting parameters is
measured using light transmission aggregometry (LTA).
[00421] In any of the aspects herein, in some embodiments, the abnormal
result for LTA comprises
one or more of the following: (a) in the presence of 5 mon adenosine
diphosphate, a percent aggregation
of less than about 60%; (b) in the presence of 2 tig/mL collagen, a percent
aggregation of less than about
65%; (c) in the presence of 1 mmol/L arachidonic acid, a percent aggregation
of less than about 65%; (d)
in the presence of 2 mmol/L arachidonic acid, a percent aggregation of less
than about 69%; or (e) in the
presence of 5 mmol/L arachidonic acid, a percent aggregation of less than
about 73%.
[00422] In any of the aspects herein, in some embodiments, after the
administering, the subject has an
increase in percent aggregation (in the presence of 5 mon adenosine
diphosphate) of at least 2, 3, 5, 8,
10, 12, or more, percentage points. In some embodiments, after the
administering, the subject has a normal
percent aggregation (in the presence of 5 mon adenosine diphosphate).
[00423] In any of the aspects herein, in some embodiments, after the
administering, the subject has an
increase in percent aggregation (in the presence of 2 tig/mL collagen) of at
least 2, 3, 5, 8, 10, 12, or more,
percentage points. In some embodiments, after the administering, the subject
has a normal percent
aggregation (in the presence of 2 tig/mL collagen).
[00424] In any of the aspects herein, in some embodiments,
[00425] the administering, the subject has an increase in percent aggregation
(in the presence of 1 mmol/L
arachidonic acid) of at least 2, 3, 5, 8, 10, 12, or more, percentage points.
In some embodiments, after the
administering, the subject has a normal percent aggregation (in the presence
of 1 mmol/L arachidonic acid).
In some embodiments, after the administering, the subject has an increase in
percent aggregation (in the
presence of 2 mmol/L arachidonic acid) of at least 2, 3, 5, 8, 10, 12, or
more, percentage points. In some
86
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
embodiments, after the administering, the subject has a normal percent
aggregation (in the presence of 2
mmol/L arachidonic acid). In some embodiments, after the administering, the
subject has an increase in
percent aggregation (in the presence of 5 mmol/L arachidonic acid) of at least
2, 3, 5, 8, 10, 12, or more,
percentage points. In some embodiments, after the administering, the subject
has a normal percent
aggregation (in the presence of 5 mmol/L arachidonic acid).
[00426] In any of the aspects herein, in some embodiments, the method
further comprises administering
to the subject an additional antiplatelet agent reversal agent. In some
embodiments, the administering of
the composition occurs concurrently with administering of the additional
antiplatelet agent reversal agent.
In some embodiments, the administering of the composition occurs after
administering of the additional
antiplatelet agent reversal agent. In some embodiments, the administering of
the composition occurs before
administering of the additional antiplatelet agent reversal agent.
[00427] In any of the aspects herein, in some embodiments, the composition
further comprises an anti-
fibrinolytic agent. In some embodiments, the anti-fibrinolytic agent is
selected from the group consisting
of e-aminocaproic acid (EACA), tranexamic acid, aprotinin, aminomethylbenzoic
acid, fibrinogen, and a
combination thereof. In some embodiments, the platelets or platelet
derivatives are loaded with the anti-
fibrinolytic agent.
[00428] In In any of the aspects herein, in some embodiments, administering
comprises administering
topically, parenterally, intravenously, intramuscularly, intrathecally,
subcutaneously, intraperitoneally, or a
combination thereof.
[00429] In any of the aspects herein, in some embodiments, the composition
is dried prior to the
administration step. In some embodiments, the composition is rehydrated
following the drying step.
[00430] In any of the aspects herein, in some embodiments, the composition
is freeze-dried prior to the
administration step. In some embodiments, the composition is rehydrated
following the freeze-drying step.
[00431] In any of the aspects herein, in some embodiments, the incubating
agent comprises one or more
salts selected from phosphate salts, sodium salts, potassium salts, calcium
salts, magnesium salts, and a
combination of two or more thereof. In some embodiments, the incubating agent
comprises a carrier
protein. In some embodiments the incubating agent comprises a buffer that
comprises HEPES, sodium
bicarbonate (NaHCO3), or a combination thereof.
[00432] In any of the aspects herein, in some embodiments, the composition
comprises one or more
saccharides. In some embodiments, the one or more saccharides comprise
trehalose. In some embodiments,
the one or more saccharides comprise polysucrose. In some embodiments, the one
or more saccharides
comprise dextrose.
[00433] In some aspects of the method, the composition comprises an organic
solvent.
87
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00434] In some embodiments of any of the aspects herein, the antiplatelet
agent is present in the subject
at the time the composition comprising the FDPDs is administered at a level
that increases the bleeding
potential of the subject. In some embodiments, the antiplatelet agent is
present at a Cmax within 15, 30 or
45 minutes, or within 1, 2, 3, 4, 6, or 8 hours of the time the composition
comprising the FDPDs is
administered or the time the first or last dose of the composition comprising
the FDPDs is administered
[00435] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises aspirin that
has been administered or is being administered at a dosage of about 80 mg to
about 700 mg, once, twice,
three times, or four times a day. In some embodiments, the antiplatelet agent
comprises aspirin, that has
been administered, or is being administered, to the subject such that the
subject achieved a C. of about 3
to about 25 mg/L.
[00436] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises cangrelor
that has been administered, or is being administered, to the subject at an
initial dosage of about 25 to about
30 pig/kg body weight of the subject or a following dosage of about 3 to about
5 tig/kg/min body weight of
the subject. In some embodiments, the antiplatelet agent comprises cangrelor
that has been administered,
or is being administered, to the subject such that the subject achieved a C.
of about 400 to about 500
ng/mL.
[00437] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises ticagrelor
that has been administered, or is being administered, to the subject at an
initial dosage of about 170 to about
190 mg, or a following dosage in a first year of treatment of about 80 to
about 100 mg twice daily, or a
following dosage in a second year of treatment of about 50 to about 70 mg
twice daily, optionally in
combination with aspirin. In some embodiments the antiplatelet agent comprises
ticagrelor, that has been
administered, or is being administered, to the subject such that the subject
achieved a C. of about 550 to
about 650 ng/mL.
[00438] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises clopidogrel
that has been administered, or is being administered, to the subject at an
initial dosage of about 275 to about
325 mg, or a following dosage of about 70 to about 80 mg once daily,
optionally in combination with
aspirin. In some embodiments, the antiplatelet agent comprises clopidogrel,
that has been administered or
is being administered to the subject such that the subject achieved a C. of
about 6 to about 20 ng/mL.
[00439] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises prasugrel
that has been administered, or is being administered, to the subject at an
initial dosage of about 50 to about
70 mg, or a following dosage of about 3 to about 12 once daily, optionally in
combination with aspirin. In
some embodiments, the antiplatelet agent comprises prasugrel that has been
administered, or is being
administered, to the subject such that the subject achieved a C. of about 200
to about 525 ng/mL.
88
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00440] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises eptifibatide
that has been administered, or is being administered, to the subject at an
initial dosage of about 170 to about
190 mcg/kg body weight of the subject, optionally a second initial dosage of
about 170 to about 190 mcg/kg
body weight of the subject, or a following dose of about 1 to about 2 mcg/kg
body weight of the subject/min.
[00441] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises tirofiban
that has been administered, or is being administered, to the subject at an
initial dosage of about 0.3 to about
0.5 pig/kg body weight of the subject/min for about 30 minutes, or a following
dosage of about 0.1 pig/kg
body weight of the subject/min.
[00442] In any of the aspects herein, in some embodiments, antiplatelet
agent comprises abciximab that
has been administered, or is being administered, to the subject at an initial
dosage of about 0.2 to about 0.3
mg/kg body weight of the subject, or a following dosage of about 0.10 to about
0.15 pig/kg body weight of
the subject/min. In some embodiments, the antiplatelet agent comprises
abciximab that has been
administered, or is being administered, to the subject at an initial dosage of
about 0.2 to about 0.3 mg/kg
body weight of the subject, or a following dosage of about 8 to about 10
tig/min.
[00443] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises ticlopidine
that has been administered, or is being administered, to the subject at a
dosage of about 240 to about 260
mg twice per day.
[00444] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises ibuprofen
that has been administered, or is being administered, to the subject at a
dosage of about 100 to about 600
mg once, twice, three times, or four times per day.
[00445] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises vorapaxar
that has been administered, or is being administered, to the subject at a
dosage of about 2 to about 3 mg
once per day, optionally with aspirin or clopidogrel.
[00446] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises cilostazol
that has been administered, or is being administered, to the subject at a
dosage of about 40 to about 110 mg
twice daily.
[00447] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises
epoprostenol that has been administered, or is being administered, to the
subject at an initial dosage of about
2 ng/kg body weight of the subject/min, or a following dosage of about 4, 6,
8, 10, 12, 14, 16, 18, or 20
ng/kg body weight of the subject/min.
[00448] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises
dipyridamole that has been administered, or is being administered, to the
subject at a dosage of about 60 to
about 110 mg four times daily.
89
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00449] In any of the aspects herein, in some embodiments, the antiplatelet
agent comprises treprostinil
sodium that has been administered, or is being administered, to the subject at
a dosage of about 0.5 to about
1.3 ng/kg body weight of the subject/min.
[00450] In any of the aspects herein, in some embodiments, some aspects of
the method, the subject
does not have cancer.
[00451] Any of the method aspects herein, can be uses for a composition
comprising platelet derivatives
(e.g. FDPDs) provided herein, or uses for a kit comprising such composition,
as set out in the following
aspects that include such "use" language. It will be understood that where
such aspects refer to FDPDs,
they could refer to platelet derivatives instead. It will be further
understood that such aspects can include
any of the elements provided herein for method aspects, and any of the
embodiments provided herein. For
example, administering of an effective amount of composition comprising
platelet derivatives (e.g. FDPDs)
can be such that the bleeding potential of the subject is reduced, and in
illustrative embodiments normal
hemostasis is restored. Where such aspects refer to a disorder, the disorder
in illustrative embodiments, is
a bleeding disorder. Such disorder can be identified, for example, because a
sample from a subject having
such disorder yields an abnormal value for one or more clotting parameters.
[00452] In one aspect, provided herein is a use of a composition comprising
platelet derivatives, in
illustrative embodiments freeze-dried platelet derivatives (FDPDs), in the
manufacture of a kit for treating
a coagulopathy in a subject that is being administered or has been
administered an antiplatelet agent,
wherein the use of the kit comprises: (a) determining in a pre-administering
evaluation, that the subject has
an abnormal value for one or more clotting parameters; and (b) after (a),
administering to the subject in
need thereof an effective amount of a composition comprising freeze-dried
platelet derivatives (FDPDs).
In some embodiments, the use further comprises before the administering,
determining that the subject was
administered an anti-platelet agent. In some embodiments, the use further
comprises before the
administering, determining that information regarding whether the subject was
administered an antiplatelet
agent is unavailable. In some embodiments, the use further comprises
determining that the subject has an
abnormal value for one or more clotting parameters in a pre-administering
evaluation before the
administering of the composition comprising freeze-dried platelet derivatives.
In some embodiments, the
antiplatelet agent is a first antiplatelet agent and the second agent is a
second antiplatelet agent.
[00453] In one aspect, provided herein is a use of a composition comprising
platelet derivatives, in
illustrative embodiments freeze-dried platelet derivatives (FDPDs), in the
manufacture of a kit for treating
a coagulopathy in a subject that is being administered or has been
administered an antiplatelet agent,
wherein the use of the kit comprises: administering to the subject in need
thereof an effective amount of the
FDPDs, wherein the composition comprising FDPDs comprises a population of
FDPDs having a reduced
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
propensity to aggregate such that no more than 10% of the FDPDs in the
population aggregate under
aggregation conditions comprising an agonist but no platelets.
[00454] In one aspect, provided herein is a use of a composition comprising
platelet derivatives, in
illustrative embodiments freeze-dried platelet derivatives (FDPDs), in the
manufacture of a kit for treating
a coagulopathy in a subject, wherein the use of the kit comprises:
administering to the subject in need
thereof an effective amount of a composition comprising freeze-dried platelet
derivatives (FDPDs), wherein
the subject was administered an antiplatelet agent and a second agent that
decreases platelet function.
[00455] In one aspect, provided herein is a use of a composition comprising
platelet derivatives, in
illustrative embodiments freeze-dried platelet derivatives (FDPDs), of any
aspect provided herein, in the
manufacture of a kit for treating coagulopathy in a subject that is being
administered or has been
administered an antiplatelet agent.
[00456] In one aspect, provided herein is a use of a composition comprising
platelet derivatives, in
illustrative embodiments freeze-dried platelet derivatives (FDPDs), of any
aspect provided herein, in the
preparation of a medicament for use in treating coagulopathy in a subject that
is being administered or has
been administered an antiplatelet agent.
[00457] In one aspect, provided herein is a composition comprising platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs), of any aspect provided
herein, for use in the
manufacture of a kit for treating coagulopathy in a subject that is being
administered or has been
administered an antiplatelet agent.
[00458] In one aspect, provided herein is a composition comprising platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs), of any aspect provided
herein, for use as a
medicament for treating coagulopathy in a subject that is being administered
or has been administered an
antiplatelet agent.
[00459] In one aspect, provided herein is a composition comprising platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs), of any aspect provided
herein, for use in the
treatment of coagulopathy in a subject that is being administered or has been
administered an antiplatelet
agent.
[00460] In one aspect, provided herein is a composition comprising platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs), of any aspect provided
herein, for use in the
manufacture of a kit for treating a disorder in a subject that is being
administered or has been administered
an antiplatelet agent.
[00461] In one aspect, provided herein is a composition comprising platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs), of any aspect provided
herein, for use as a
91
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
medicament for treating a disorder in a subject that is being administered or
has been administered an
antiplatelet agent.
[00462] In one aspect, provided herein is a composition comprising platelet
derivatives, in illustrative
embodiments freeze-dried platelet derivatives (FDPDs), of any aspect provided
herein, for use in the
treatment of a disorder in a subject that is being administered or has been
administered an antiplatelet agent.
[00463] In any of the aspects provided herein that include a composition
comprising platelet derivatives,
in illustrative embodiments freeze-dried platelet derivatives (FDPDs), for use
in the treatment of a disorder,
or in the manufacture of a kit, or as a medicament, the disorder is selected
from the group consisting of
alopecia areata, Von Willebrand Disease, hemophilia, thrombasthenia,
thrombocytopenia,
thrombocytopenic purpura, trauma, or a combination thereof.
[00464] In any of the aspects provided herein that include a composition
comprising platelet derivatives,
in illustrative embodiments freeze-dried platelet derivatives (FDPDs), for use
in the treatment of
coagulopathy or a disorder, or in the manufacture of a kit, or as a
medicament, the antiplatelet agent is
selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel, eptifibatide,
tirofiban, abciximab, terutroban, picotamide, elinogrel, ticlopidine,
ibuprofen, vorapaxar, atopaxar,
cilostazol, prostaglandin El, epoprostenol, dipyridamole, treprostinil sodium,
sarpogrelate, and a
combination thereof.
[00465] In any of the aspects provided herein that include a composition
comprising platelet derivatives,
in illustrative embodiments freeze-dried platelet derivatives (FDPDs), for use
in the treatment of
coagulopathy or a disorder, or in the manufacture of a kit, or as a
medicament, wherein the treatment, or
the use of the kit, or the medicament comprises: administering to the subject
in need thereof an effective
amount of the FDPDs, wherein the composition comprising FDPDs comprises a
population of FDPDs
having a reduced propensity to aggregate such that no more than 10% of the
FDPDs in the population
aggregate under aggregation conditions comprising an agonist but no platelets.
In some embodiments, the
FDPDs have a potency of at least 1.5 thrombin generation potency units (TGPU)
per 106 platelet derivatives.
[00466] In any of the aspects provided herein that include a composition
comprising platelet derivatives,
in illustrative embodiments freeze-dried platelet derivatives (FDPDs), for use
in the treatment of
coagulopathy or a disorder, or in the manufacture of a kit, or as a
medicament, wherein at least 50% of the
FDPDs are CD 41-positive platelet derivatives, wherein less than 5% of the CD
41-positive FDPDs are
microparticles having a diameter of less than 0.5 m, and wherein the FDPDs
have a potency of at least 1.5
thrombin generation potency units (TGPU) per 106 platelet derivatives.
[00467] In any of the aspects provided herein that include a composition
comprising platelet derivatives,
in illustrative embodiments freeze-dried platelet derivatives (FDPDs), for use
in the treatment of
coagulopathy or a disorder, or in the manufacture of a kit, or as a
medicament, wherein the FDPDs have
92
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
one or more characteristics of a super-activated platelet selected from A) the
presence of thrombospondin
(TSP) on their surface at a level that is greater than on the surface of
resting platelets; B) the presence of
von Willebrand factor (vWF) on their surface at a level that is greater than
on the surface of resting platelets;
and C) an inability to increase expression of a platelet activation marker in
the presence of an agonist as
compared to the expression of the platelet activation marker in the absence of
an agonist.
[00468] In any of the aspects provided herein that include a composition
comprising platelet derivatives,
in illustrative embodiments freeze-dried platelet derivatives (FDPDs), for use
in the treatment of
coagulopathy or a disorder, or in the manufacture of a kit, or as a
medicament, wherein the effective amount
of the composition comprising FDPDs is between 1.0 X 107 to 1.0 X 1011/kg of
the subject. In some
embodiments, the effective amount of the composition comprising FDPDs is
between 1.6 X 107 to 5.1 X
109/kg of the subject. In some embodiments, the effective amount of the
composition comprising FDPDs
is between an amount that has a potency between 250 and 5000 TGPU per kg of
the subject. In some
embodiments, the effective amount of the composition comprising FDPDs is
between an amount that has a
potency between 300 to 3800 TGPU per kg.
[00469] Further exemplary aspects and embodiments are provided as follows:
[00470] Provided herein in some embodiments is a method of treating a
coagulopathy in a subject, the
method including administering to the subject in need thereof an effective
amount of a composition
including platelets or platelet derivatives and an incubating agent including
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00471] In some embodiments, provided herein is a method of treating a
coagulopathy in a subject, the
method including administering to the subject in need thereof an effective
amount of a composition
prepared by a process including incubating platelets with an incubating agent
including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent, to
form the composition.
[00472] In some embodiments, provided herein is a method of restoring
normal hemostasis in a subject,
the method including administering to the subject in need thereof an effective
amount of a composition
including platelets or platelet derivatives and an incubating agent including
one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00473] In some embodiments, provided herein is a method of restoring
normal hemostasis in a subject,
the method including administering to the subject in need thereof an effective
amount of a composition
prepared by a process including incubating platelets with an incubating agent
including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent, to
form the composition.
[00474] In some embodiments, provided herein is a method of preparing a
subject for surgery, the
method including administering to the subject in need thereof an effective
amount of a composition
including platelets or platelet derivatives and an incubating agent including
one or more salts, a buffer,
93
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
optionally a cryoprotectant, and optionally an organic solvent.
Implementations can include one or more of
the following features. The surgery can be an emergency surgery. The surgery
can be a scheduled surgery.
[00475] In some embodiments, provided herein is a method of preparing a
subject for surgery, the
method including administering to the subject in need thereof an effective
amount of a composition
prepared by a process including incubating platelets with an incubating agent
including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent, to
form the composition.
Implementations can include one or more of the following features. The surgery
can be an emergency
surgery. The surgery can be a scheduled surgery.
[00476] In some embodiments of the above methods, the subject has been
treated or is being treated
with an antiplatelet agent. In some embodiments, treatment with the
antiplatelet agent can be stopped. In
some embodiments, treatment with the antiplatelet agent can be continued.
[00477] In some embodiments, provided herein is a method of ameliorating
the effects of an antiplatelet
agent in a subject, the method including administering to the subject in need
thereof an effective amount of
a composition including platelets or platelet derivatives and an incubating
agent including one or more salts,
a buffer, optionally a cryoprotectant, and optionally an organic solvent.
[00478] In some embodiments, provided herein is a method of ameliorating
the effects of an antiplatelet
agent in a subject, the method including administering to the subject in need
thereof an effective amount of
a composition prepared by a process including incubating platelets with an
incubating agent including one
or more salts, a buffer, optionally a cryoprotectant, and optionally an
organic solvent, to form the
composition.
[00479] In some embodiments, the effects of the antiplatelet agent can be
the result of an overdose of
the antiplatelet agent.
[00480] In some embodiments, the antiplatelet agent can be selected from
the group consisting of
aspirin, cangrelor, ticagrelor, clopidogrel, prasugrel, eptifibatide,
tirofiban, abciximab, and a supplement.
[00481] Some embodiments of any of the methods herein can include one or
more of the following
features. Administering can include administering topically. Administering can
include administering
parenterally. Administering can include administering intravenously.
Administering can include
administering intramuscularly. Administering can include administering
intrathecally. Administering can
include administering subcutaneously. Administering can include administering
intraperitoneally. The
composition can be dried prior to the administration step. The composition can
be rehydrated following
the drying step. The composition can be freeze-dried prior to the
administration step. The composition can
be rehydrated following the freeze-drying step. The incubating agent can
include one or more salts selected
from sodium salts, potassium salts, calcium salts, magnesium salts, and a
combination of two or more
thereof. The incubating agent can include a carrier protein. The buffer can
include HEPES, sodium
94
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
bicarbonate (NaHCO3), or a combination thereof. The composition can include
one or more saccharides.
The one or more saccharides can include trehalose. The one or more saccharides
can include polysucrose.
The one or more saccharides can include dextrose. The composition can include
an organic solvent. The
platelets or platelet derivatives can include FDPDs.
[00482] Further non-limiting example embodiments are provided in numbered
form as follows:
[00483] Embodiment 1 is a method of treating a coagulopathy in a subject,
the method comprising
administering to the subject in need thereof an effective amount of a
composition comprising platelets or
platelet derivatives and an incubating agent comprising one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00484] Embodiment 2 is a method of treating a coagulopathy in a subject,
the method comprising
administering to the subject in need thereof an effective amount of a
composition prepared by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
[00485] Embodiment 3 is a method of restoring normal hemostasis in a
subject, the method comprising
administering to the subject in need thereof an effective amount of a
composition comprising platelets or
platelet derivatives and an incubating agent comprising one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00486] Embodiment 4 is a method of restoring normal hemostasis in a
subject, the method comprising
administering to the subject in need thereof an effective amount of a
composition prepared by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
[00487] Embodiment 5 is a method of preparing a subject for surgery, the
method comprising
administering to the subject in need thereof an effective amount of a
composition comprising platelets or
platelet derivatives and an incubating agent comprising one or more salts, a
buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00488] Embodiment 6 is a method of preparing a subject for surgery, the
method comprising
administering to the subject in need thereof an effective amount of a
composition prepared by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
[00489] Embodiment 7 is the method of any one of embodiments 5-6, wherein
the surgery is an
emergency surgery.
[00490] Embodiment 8 is the method of any one of embodiments 5-6, wherein
the surgery is a scheduled
surgery.
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00491] Embodiment 9 is the method of any one of embodiments 1-8, wherein
the subject has been
treated or is being treated with an antiplatelet agent.
[00492] Embodiment 10 is the method of embodiment 9, wherein treatment with
the antiplatelet agent
is stopped.
[00493] Embodiment 11 is the method of embodiment 9, wherein treatment with
the antiplatelet agent
is continued.
[00494] Embodiment 12 is a method of ameliorating the effects of an
antiplatelet agent in a subject, the
method comprising administering to the subject in need thereof an effective
amount of a composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00495] Embodiment 13 is a method of ameliorating the effects of an
antiplatelet agent in a subject, the
method comprising administering to the subject in need thereof an effective
amount of a composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00496] Embodiment 14 is the method of embodiment 12 or embodiment 13,
wherein the effects of the
antiplatelet agent are the result of an overdose of the antiplatelet agent.
[00497] Embodiment 15 is the method of any one of embodiments 1-14, wherein
the composition
further comprises an anti-fibrinolytic agent.
[00498] Embodiment 16 is the method of embodiment 15, wherein the anti-
fibrinolytic agent is selected
from the group consisting of e-aminocaproic acid (EACA), tranexamic acid,
aprotinin,
aminomethylbenzoic acid, fibrinogen, and a combination thereof.
[00499] Embodiment 17 is the method of embodiment 15 or embodiment 16,
wherein the platelets or
platelet derivatives are loaded with the anti-fibrinolytic agent.
[00500] Embodiment 18 is the method of any one of embodiments 9-16, wherein
the antiplatelet agent
is selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel, eptifibatide,
tirofiban, abciximab, a supplement, and a combination thereof.
[00501] Embodiment 19 is the method of any one of embodiments 9-16, wherein
the antiplatelet agent
is selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel, eptifibatide,
tirofiban, abciximab, terutroban, picotamide, elinogrel, ticlopidine,
ibuprofen, vorapaxar, atopaxar, and a
combination thereof.
[00502] Embodiment 20 is the method of any one of embodiments 9-16, wherein
the antiplatelet agent
is selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel, eptifibatide,
tirofiban, abciximab, terutroban, picotamide, elinogrel, ticlopidine,
ibuprofen, vorapaxar, atopaxar,
96
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
cilostazol, prostaglandin El, epoprostenol, dipyridamole, treprostinil sodium,
sarpogrelate, and a
combination thereof.
[00503] Embodiment 21 is the method of any one of embodiments 1-20, wherein
administering
comprises administering topically.
[00504] Embodiment 22 is the method of any one of embodiments 1-20, wherein
administering
comprises administering parenterally.
[00505] Embodiment 23 is the method of any one of embodiments 1-20, wherein
administering
comprises administering intravenously.
[00506] Embodiment 24 is the method of any one of embodiments 1-20, wherein
administering
comprises administering intramuscularly.
[00507] Embodiment 25 is the method of any one of embodiments 1-20, wherein
administering
comprises administering intrathecally.
[00508] Embodiment 26 is the method of any one of embodiments 1-20, wherein
administering
comprises administering subcutaneously.
[00509] Embodiment 27 is the method of any one of embodiments 1-20, wherein
administering
comprises administering intraperitoneally.
[00510] Embodiment 28 is the method of any one of embodiments 1-27, wherein
the composition is
dried prior to the administration step.
[00511] Embodiment 29 is the method of embodiment 28, wherein the
composition is rehydrated
following the drying step.
[00512] Embodiment 30 is the method of any one of embodiments 1-28, wherein
the composition is
freeze-dried prior to the administration step.
[00513] Embodiment 31 is the method of embodiment 30, wherein the
composition is rehydrated
following the freeze-drying step.
[00514] Embodiment 32 is the method of any one of embodiments 1-31, wherein
the incubating agent
comprises one or more salts selected from phosphate salts, sodium salts,
potassium salts, calcium salts,
magnesium salts, and a combination of two or more thereof.
[00515] Embodiment 33 is the method of any one of embodiments 1-32, wherein
the incubating agent
comprises a carrier protein.
[00516] Embodiment 34 is the method of any one of embodiments 1-33, wherein
the buffer comprises
HEPES, sodium bicarbonate (NaHCO3), or a combination thereof.
[00517] Embodiment 35 is the method of any one of embodiments 1-34, wherein
the composition
comprises one or more saccharides.
97
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00518]
Embodiment 36 is the method of embodiment 35, wherein the one or more
saccharides
comprise trehalose.
[00519]
Embodiment 37 is the method of embodiment 35 or embodiment 36, wherein the one
or more
saccharides comprise polysucrose.
[00520]
Embodiment 38 is the method of any one of embodiments 35-37, wherein the one
or more
saccharides comprise dextrose.
[00521]
Embodiment 39 is the method of any one of embodiments 1-38, wherein the
composition
comprises an organic solvent.
[00522]
Embodiment 40 is the method of any of embodiments 1-39, wherein the platelets
or platelet
derivatives comprise FDPDs.
[00523]
Embodiment 41 is a method of treating a coagulopathy in a subject that is
being administered
or has been administered an antiplatelet agent, the method comprising:
[00524]
(a) determining that the subject has an abnormal result for evaluation of one
or more
clotting parameters; and
[00525]
(b) after (a), administering to the subject in need thereof an effective
amount of a
composition comprising platelets or platelet derivatives and an incubating
agent comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent.
[00526]
Embodiment 42 is a method of treating a coagulopathy in a subject that is
being administered
or has been administered an antiplatelet agent, the method comprising:
[00527]
(a) determining that the subject an abnormal result for evaluation of one or
more clotting
parameters; and
[00528]
(b) after (a), administering to the subject in need thereof an effective
amount of a
composition prepared by a process comprising incubating platelets with an
incubating agent comprising
one or more salts, a buffer, optionally a cryoprotectant, and optionally an
organic solvent, to form the
composition.
[00529]
Embodiment 43 is a method of treating a coagulopathy in a subject that is
being administered
or has been administered an antiplatelet agent, the method comprising:
[00530]
administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, wherein before
the administering, the subject
has been determined to have an abnormal result for evaluation of one or more
clotting parameters.
[00531]
Embodiment 44 is a method of treating a coagulopathy in a subject that is
being
administered or has been administered an antiplatelet agent, the method
comprising:
98
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00532]
administering to the subject in need thereof an effective amount of a
composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition,
wherein before the administering, the subject has been determined to have an
abnormal result for evaluation
of one or more clotting parameters.
[00533]
Embodiment 45 is the method of any one of embodiments 41-44, further
comprising
determining the result of the evaluation one or more clotting parameters
following the administering.
[00534]
Embodiment 46 is the method of embodiment 45, wherein the evaluation of the
one or more
clotting parameters following the administering shows a normal result for at
least one of the one or more
clotting parameters.
[00535]
Embodiment 47 is the method of embodiment 45, wherein the result of the
evaluation of the
one or more clotting parameters following the administering is improved from
the result of the evaluation
of the one or more parameters prior to the administering.
[00536] Embodiment 48 is a method of treating a coagulopathy in a subject,
the method comprising:
[00537]
(a) determining that the subject, contrary to medical instruction, was
administered an
antiplatelet agent; and
[00538]
(b) administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00539] Embodiment 49 is a method of treating a coagulopathy in a subject,
the method comprising:
[00540]
(a) determining that the subject, contrary to medical instruction, was
administered an
antiplatelet agent; and
[00541]
(b) administering to the subject in need thereof an effective amount of a
composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00542] Embodiment 50 is a method of treating a coagulopathy in a subject,
the method comprising:
[00543]
administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, wherein the
subject is determined to have
been administered an antiplatelet agent contrary to medical instruction.
[00544] Embodiment 51 is a method of treating a coagulopathy in a subject,
the method comprising:
[00545]
administering to the subject in need thereof an effective amount of a
composition prepared
by a process comprising incubating platelets with an incubating agent
comprising one or more salts, a buffer,
99
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is determined to have been administered an antiplatelet agent contrary to
medical instruction.
[00546]
Embodiment 52 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00547]
(a) determining that the subject, contrary to medical instruction, was
administered an
antiplatelet agent; and
[00548]
(b) administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00549]
Embodiment 53 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00550]
(a) determining that the subject, contrary to medical instruction, was
administered an
antiplatelet agent; and
[00551]
(b) administering to the subject in need thereof an effective amount of a
composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00552]
Embodiment 54 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00553]
administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, wherein the
subject is determined to have
been administered an antiplatelet agent contrary to medical instruction.
[00554]
Embodiment 55 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00555]
administering to the subject in need thereof an effective amount of a
composition prepared
by a process comprising incubating platelets with an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is determined to have been administered an antiplatelet agent contrary to
medical instruction.
[00556]
Embodiment 56 is the method of any one of embodiments 48-55, wherein the
administering of
the antiplatelet agent contrary to medical instruction is self-administering
by the subject.
[00557]
Embodiment 57 is the method of any one of embodiments 48-55, wherein the
administering of
the antiplatelet agent contrary to medical instruction is administering by a
medical professional.
[00558]
Embodiment 58 is the method of any one of embodiments 48-57, wherein the
medical
instruction is verbal instruction by a medical professional.
100
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00559]
Embodiment 59 is the method of any one of embodiments 48-57, wherein the
medical
instruction is written instruction.
[00560] Embodiment 60 is a method of treating a coagulopathy in a subject,
the method comprising:
[00561]
(a) determining that the subject was administered an antiplatelet agent and a
second agent
that decreases platelet function; and
[00562]
(b) administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00563] Embodiment 61 is a method of treating a coagulopathy in a subject,
the method comprising:
[00564]
(a) determining that the subject was administered an antiplatelet agent and a
second agent
that decreases platelet function; and
[00565]
(b) administering to the subject in need thereof an effective amount of a
composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00566] Embodiment 62 is a method of treating a coagulopathy in a subject,
the method comprising:
[00567] administering to the subject in need thereof an effective amount of
a composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, wherein the
subject is determined to have
been administered an antiplatelet agent and a second agent that decreases
platelet function.
[00568] Embodiment 63 is a method of treating a coagulopathy in a subject,
the method comprising:
[00569]
administering to the subject in need thereof an effective amount of a
composition prepared
by a process comprising incubating platelets with an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is determined to have been administered an antiplatelet agent and a second
agent that decreases platelet
function.
[00570]
Embodiment 64 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00571]
(a) determining that the subject was administered an antiplatelet agent and a
second agent
that decreases platelet function; and
[00572]
(b) administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent.
[00573]
Embodiment 65 is a method of restoring normal hemostasis in a subject, the
method
comprising:
101
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00574]
(a) determining that the subject was administered an antiplatelet agent and a
second agent
that decreases platelet function; and
[00575]
(b) administering to the subject in need thereof an effective amount of a
composition
prepared by a process comprising incubating platelets with an incubating agent
comprising one or more
salts, a buffer, optionally a cryoprotectant, and optionally an organic
solvent, to form the composition.
[00576]
Embodiment 66 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00577]
administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, wherein the
subject is identified as having
been administered an antiplatelet agent and a second agent that decreases
platelet function.
[00578]
Embodiment 67 is a method of restoring normal hemostasis in a subject, the
method
comprising:
[00579]
administering to the subject in need thereof an effective amount of a
composition prepared
by a process comprising incubating platelets with an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
is identified as having been administered an antiplatelet agent and a second
agent that decreases platelet
function.
[00580]
Embodiment 68 is the method of any one of embodiments 60-67, wherein
administration of the
second agent is stopped.
[00581]
Embodiment 69 is the method of any one of embodiments 60-67, wherein
administration of the
second agent is continued.
[00582]
Embodiment 70 is the method of any one of embodiments 60-69, wherein the
second agent is
selected from the group consisting of an antihypertensive, a proton pump
inhibitor, and a combination
thereof.
[00583]
Embodiment 71 is the method of any one of embodiments 60-69, wherein the
second agent is
selected from the group consisting of a chemotherapeutic agent, an antibiotic,
a cardiovascular agent, a H2
antagonist, a neuropsychiatric agent and a combination thereof.
[00584]
Embodiment 72 is the method of any one of embodiments 60-69, wherein the
second agent
comprises an antidepressant.
[00585]
Embodiment 73 is the method of embodiment 72, wherein the antidepressant is
selected from
the group consisting of a selective serotonin reuptake inhibitor (SSRI), a
serotonin antagonist and reuptake
inhibitor (SARI), a serotonin and norepinephrine reuptake inhibitor (SNRI),
and a combination thereof.
102
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00586]
Embodiment 74 is the method of any one of embodiments 60-73, wherein the
second agent is
not an anticoagulant.
[00587]
Embodiment 75 is the method of any one of embodiments 41-74, wherein
administration of the
antiplatelet agent is stopped.
[00588]
Embodiment 76 is the method of any one of embodiments 41-74, wherein
administration of the
antiplatelet agent is continued.
[00589]
Embodiment 77 is a method of preventing or mitigating the potential for a
coagulopathy in a
subject, the method comprising:
[00590]
(a) determining that information regarding whether the subject was
administered an
antiplatelet agent is unavailable; and
[00591]
(b) administering to the subject an effective amount of a composition
comprising platelets
or platelet derivatives and an incubating agent comprising one or more salts,
a buffer, optionally a
cryoprotectant, and optionally an organic solvent.
[00592]
Embodiment 78 is a method of preventing or mitigating the potential for a
coagulopathy in a
subject, the method comprising:
[00593]
(a) determining that information regarding whether the subject was
administered an
antiplatelet agent is unavailable; and
[00594]
(b) administering to the subject an effective amount of a composition prepared
by a process
comprising incubating platelets with an incubating agent comprising one or
more salts, a buffer, optionally
a cryoprotectant, and optionally an organic solvent, to form the composition.
[00595]
Embodiment 79 is a method of preventing or mitigating the potential for a
coagulopathy in a
subject, the method comprising:
[00596]
administering to the subject in need thereof an effective amount of a
composition
comprising platelets or platelet derivatives and an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, wherein the
subject has been determined to
be a subject for which information regarding whether the subject was
administered an antiplatelet agent is
unavailable.
[00597]
Embodiment 80 is a method of preventing or mitigating the potential for a
coagulopathy in a
subject, the method comprising:
[00598]
administering to the subject in need thereof an effective amount of a
composition prepared
by a process comprising incubating platelets with an incubating agent
comprising one or more salts, a buffer,
optionally a cryoprotectant, and optionally an organic solvent, to form the
composition, wherein the subject
has been determined to be a subject for which information regarding whether
the subject was administered
an antiplatelet agent is unavailable.
103
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00599] Embodiment 81 is the method of any one of embodiments 77-80,
wherein information
regarding whether the subject was administered an antiplatelet agent is
unavailable for a reason comprising
that the subject cannot be identified.
[00600] Embodiment 82 is the method of any one of embodiments 77-81,
wherein information
regarding whether the subject was administered an antiplatelet agent is
unavailable for a reason comprising
that the medical history of the subject is unavailable.
[00601] Embodiment 83 is the method of any one of embodiments 77-82,
wherein information
regarding whether the subject was administered an antiplatelet agent is
unavailable for a reason comprising
that the subject is in need of emergency treatment.
[00602] Embodiment 84 is the method of any one of embodiments 77-83,
wherein information
regarding whether the subject was administered an antiplatelet agent is
unavailable for a reason comprising
that the subject is in need of emergency surgery.
[00603] Embodiment 85 is the method of any one of embodiments 77-84,
wherein information
regarding whether the subject was administered an antiplatelet agent is
unavailable for a reason comprising
that the subject is having emergency surgery.
[00604] Embodiment 86 is the method of any one of embodiments 48-85,
wherein the method further
comprises determining that the subject has an abnormal result for one or more
evaluations of clotting
parameters.
[00605] Embodiment 87 is the method of any one of embodiments 48-86,
wherein the subject has been
determined to have an abnormal result for one or more evaluations of clotting
parameters.
[00606] Embodiment 88 is the method of any one of embodiments 84-87,
wherein the subject was
previously identified as having a normal result for at least one of the one or
more clotting parameters.
[00607] Embodiment 89 is the method of any one of embodiments 84-88,
further comprising
determining the result of the evaluation one or more clotting parameters
following the administering.
[00608] Embodiment 90 is the method of embodiment 89, wherein the
evaluation of the one or more
clotting parameters following the administering shows a normal result for at
least one of the one or more
clotting parameters.
[00609] Embodiment 91 is the method of embodiment 89, wherein the result of
the evaluation of the
one or more clotting parameters following the administering is improved from
the result of the evaluation
of the one or more parameters prior to the administering.
[00610] Embodiment 92 is the method of any one of embodiments 41-47 or 86-
91, wherein the subject
is identified as having an abnormal result for one or more evaluations of
clotting parameters during surgery.
[00611] Embodiment 93 is the method of embodiment 92, wherein the surgery
is an emergency surgery.
[00612] Embodiment 94 is the method of embodiment 92, wherein the surgery
is a scheduled surgery.
104
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00613] Embodiment 95is the method of any one of embodiments 41-47 or 86-
94, wherein the one or
more clotting parameters includes an evaluation of bleeding.
[00614] Embodiment 96 is the method of embodiment 95, wherein the
evaluation of bleeding is
performed based on the World Health Organization (WHO) bleeding scale.
[00615] Embodiment 97 is the method of embodiment 96, wherein before the
administering, the subject
has bleeding of grade 2, 3, or 4 based on the WHO bleeding scale.
[00616] Embodiment 98 is the method of embodiment 97, wherein after the
administering, the subject
has bleeding of grade 0 or 1 based on the WHO bleeding scale.
[00617] Embodiment 99 is the method of embodiment 96, wherein after the
administering, the subject
has bleeding of one grade less, based on the WHO bleeding scale, than before
the administering.
[00618] Embodiment 100 is the method of embodiment 96, wherein after the
administering, the subject
has bleeding of two grades less, based on the WHO bleeding scale, than before
the administering.
[00619] Embodiment 101 is the method of embodiment 96, wherein after the
administering, the subject
has bleeding of three grades less, based on the WHO bleeding scale, than
before the administering.
[00620] Embodiment 102 is the method of any one of embodiments 41-47 or 86-
101, wherein the one
or more clotting parameters includes an evaluation of prothrombin time (PT).
[00621] Embodiment 103 is the method of embodiment 102, wherein the
abnormal results for PT
comprises a PT of greater than about 14 seconds.
[00622] Embodiment 104 is the method of embodiment 102 or embodiment 103,
wherein after the
administering, the subject has a decrease in PT of at least 1, 2, 3, 4, 5, or
more, seconds.
[00623] Embodiment 105 is the method of any one of embodiments 102-104,
wherein after the
administering, the subject has a normal PT.
[00624] Embodiment 106 is the method of any one of embodiments 41-47 or 86-
105, wherein the one
or more clotting parameters includes an evaluation of activated partial
thromboplastin time (aPTT).
[00625] Embodiment 107 is the method of embodiment 106, wherein the
abnormal result for aPTT
comprises an aPTT of greater than about 40 seconds.
[00626] Embodiment 108 is the method of embodiment 106 or embodiment 107,
wherein after the
administering, the subject has a decrease in aPTT of at least 5, 10, 15, 20,
or more, seconds.
[00627] Embodiment 109 is the method of any one of embodiments 106-108,
wherein after the
administering, the subject has a normal aPTT.
[00628] Embodiment 110 is the method of any one of embodiments 41-47 or 86-
109, wherein the one
or more clotting parameters includes an evaluation of thrombin clot time
(TCT).
[00629] Embodiment 111 is the method of embodiment 110, wherein the
abnormal result for TCT
comprises a TCT of greater than about 35 seconds.
105
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00630] Embodiment 112 is the method of embodiment 110 or embodiment 111,
wherein after the
administering, the subject has a decrease in TCT of at least 5, 10, 15, 20, or
more, seconds.
[00631] Embodiment 113 is the method of any one of embodiments 110-111,
wherein after the
administering, the subject has a normal TCT.
[00632] Embodiment 114 is the method of any one of embodiments 41-47 or 86-
113, wherein the
evaluation of the one or more clotting parameters includes thromboelastography
(TEG).
[00633] Embodiment 115 is the method of embodiment 114, wherein the
abnormal result for TEG
comprises a maximum amplitude (MA) of less than about 50 mm.
[00634] Embodiment 116 is the method of embodiment 114 or embodiment 115,
wherein after the
administering, the subject has an increase in MA of at least 5, 10, 15, 20, or
more, mm.
[00635] Embodiment 117 is the method of any one of embodiments 114-116,
wherein after the
administering, the subject has a normal MA.
[00636] Embodiment 118 is the method of any one of embodiments 114-117,
wherein the abnormal
result for TEG comprises a percent aggregation (in the presence of 1 mmol/L
arachidonic acid) of less than
about 85%.
[00637] Embodiment 119 is the method of embodiment 118, wherein after the
administering, the subject
has an increase in percent aggregation (in the presence of 1 mmol/L
arachidonic acid) of at least 2, 3, 5, 8,
10, 12, or more, percentage points.
[00638] Embodiment 120 is the method of embodiment 118 or embodiment 119,
wherein after the
administering, the subject has a normal percent aggregation (in the presence
of 1 mmol/L arachidonic acid).
[00639] Embodiment 121 is the method of any one of embodiments 105-120
wherein the TEG is used
to evaluate adenosine diphosphate-induced platelet-fibrin clot strength.
[00640] Embodiment 122 is the method of any one of embodiments 105-120,
wherein the TEG is used
to evaluate arachidonic acid-induced platelet-fibrin clot strength.
[00641] Embodiment 123 is the method of any one of embodiments 41-47 or 86-
122, wherein the
evaluation of one or more clotting parameters includes VerifyNow.
[00642] Embodiment 124 is the method of embodiment 123, wherein the
abnormal result for VerifyNow
comprises a P2Y12 reaction unit (PRU) of less than about 195, or less than
about 180.
[00643] Embodiment 125 is the method of embodiment 123 or embodiment 124,
wherein after the
administering, the subject has an increase in PRU of at least 25, 50, 75, 100,
or more.
[00644] Embodiment 126 is the method of any one of embodiments 123-125,
wherein after the
administering, the subject has a normal PRU.
106
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00645]
Embodiment 127 is the method of any one of embodiments 123-126, wherein the
abnormal
result for VerifyNow comprises an Aspirin Reaction Unit (ARU) of less than
about 550, or less than about
500.
[00646]
Embodiment 128 is the method of embodiment 126 or embodiment 127, wherein
after the
administering, the subject has an increase in ARU of at least 25, 50, 75, 100,
or more.
[00647]
Embodiment 129 is the method of any one of embodiments 126-128, wherein after
the
administering, the subject has a normal ARU.
[00648]
Embodiment 130 is the method of any one of embodiments 41-47 or 86-129,
wherein the one
or more clotting parameters includes multiple electrode aggregometry (MEA).
[00649]
Embodiment 131 is the method of embodiment 130, wherein the abnormal result
for MEA
comprises an abnormal result for ADP-induced platelet activity.
[00650]
Embodiment 132 is the method of embodiment 131, wherein the abnormal result
for MEA
comprises a result of less than about 50 units (U) for ADP-induced platelet
activity.
[00651]
Embodiment 133 is the method of embodiment 131 or embodiment 132, wherein
after the
administering, the subject has an increase in ADP-induced platelet activity by
5, 10, 15, 20, or more units.
[00652]
Embodiment 134 is the method of any one of embodiments 131-133, wherein after
the
administering, the subject has a normal value for ADP-induced platelet
activity.
[00653]
Embodiment 135 is the method of any one of embodiments 131-134, wherein the
abnormal
result for MEA comprises an abnormal result for arachidonic acid-induced
platelet activity.
[00654]
Embodiment 136 is the method of embodiment 135, wherein the abnormal result
for MEA
comprises a result of less than about 70 units (U) for arachidonic acid-
induced platelet activity.
[00655]
Embodiment 137 is the method of embodiment 135 or embodiment 136, wherein
after the
administering, the subject has an increase in arachidonic acid-induced
platelet activity by 5, 10, 15, 20, or
more units.
[00656]
Embodiment 138 is the method of any one of embodiments 135-137, wherein after
the
administering, the subject has a normal value for arachidonic acid-induced
platelet activity.
[00657]
Embodiment 139 is the method of any one of embodiments 41-47 or 86-138,
wherein the one
or more clotting parameters includes light transmission aggregometry (LTA).
[00658]
Embodiment 140 is the method of embodiment 139, wherein the abnormal result
for LTA
comprises one or more of the following:
[00659]
(a) in the presence of 5 mol/L adenosine diphosphate, a percent aggregation
of less than
about 60%;
[00660] (b) in the presence of 2 usimL collagen, a percent aggregation of
less than about 65%;
107
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00661]
(c) in the presence of 1 mmol/L arachidonic acid, a percent aggregation of
less than about
65%;
[00662]
(d) in the presence of 2 mmol/L arachidonic acid, a percent aggregation of
less than about
69%; or
[00663]
(e) in the presence of 5 mmol/L arachidonic acid, a percent aggregation of
less than about
73%.
[00664]
Embodiment 141 is the method of embodiment 140, wherein after the
administering, the
subject has an increase in percent aggregation (in the presence of 5 mon
adenosine diphosphate) of at
least 2, 3, 5, 8, 10, 12, or more, percentage points.
[00665]
Embodiment 142 is the method of embodiment 140 or embodiment 141, wherein
after the
administering, the subject has a normal percent aggregation (in the presence
of 5 mon adenosine
diphosphate).
[00666]
Embodiment 143 is the method of embodiment 140, wherein after the
administering, the
subject has an increase in percent aggregation (in the presence of 2 tig/mL
collagen) of at least 2, 3, 5, 8,
10, 12, or more, percentage points.
[00667]
Embodiment 144 is the method of embodiment 140 or embodiment 143, wherein
after the
administering, the subject has a normal percent aggregation (in the presence
of 2 tig/mL collagen).
[00668]
Embodiment 145 is the method of embodiment 140, wherein after the
administering, the
subject has an increase in percent aggregation (in the presence of 1 mmol/L
arachidonic acid) of at least 2,
3, 5, 8, 10, 12, or more, percentage points.
[00669]
Embodiment 146 is the method of embodiment 140 or embodiment 145, wherein
after the
administering, the subject has a normal percent aggregation (in the presence
of 1 mmol/L arachidonic acid).
[00670]
Embodiment 147 is the method of embodiment 140, wherein after the
administering, the
subject has an increase in percent aggregation (in the presence of 2 mmol/L
arachidonic acid) of at least 2,
3, 5, 8, 10, 12, or more, percentage points.
[00671]
Embodiment 148 is the method of embodiment 140 or embodiment 147, wherein
after the
administering, the subject has a normal percent aggregation (in the presence
of 2 mmol/L arachidonic acid).
[00672]
Embodiment 149 is the method of embodiment 140, wherein after the
administering, the
subject has an increase in percent aggregation (in the presence of 5 mmol/L
arachidonic acid) of at least 2,
3, 5, 8, 10, 12, or more, percentage points.
[00673]
Embodiment 150 is the method of embodiment 140 or embodiment 149, wherein
after the
administering, the subject has a normal percent aggregation (in the presence
of 5 mmol/L arachidonic acid).
[00674]
Embodiment 151 is the method of any one of embodiments 41-150, wherein the
method further
comprises administering to the subject an additional antiplatelet agent
reversal agent.
108
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00675] Embodiment 152 is the method of embodiment 151, wherein the
administering of the
composition occurs concurrently with administering of the additional
antiplatelet agent reversal agent.
[00676] Embodiment 153 is the method of embodiment 151, wherein the
administering of the
composition occurs after administering of the additional antiplatelet agent
reversal agent.
[00677] Embodiment 154 is the method of embodiment 151, wherein the
administering of the
composition occurs before administering of the additional antiplatelet agent
reversal agent.
[00678] Embodiment 155 is the method of any one of embodiments 41-154,
wherein the composition
further comprises an anti-fibrinolytic agent.
[00679] Embodiment 156 is the method of embodiment 155, wherein the anti-
fibrinolytic agent is
selected from the group consisting of e-aminocaproic acid (EACA), tranexamic
acid, aprotinin,
aminomethylbenzoic acid, fibrinogen, and a combination thereof.
[00680] Embodiment 157 is the method of embodiment 155 or embodiment 156,
wherein the platelets
or platelet derivatives are loaded with the anti-fibrinolytic agent.
[00681] Embodiment 158 is the method of any one of embodiments 41-157,
wherein the antiplatelet
agent is selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel,
eptifibatide, tirofiban, abciximab, a supplement, and a combination thereof.
[00682] Embodiment 159 is the method of any one of embodiments 41-157,
wherein the antiplatelet
agent is selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel,
eptifibatide, tirofiban, abciximab, terutroban, picotamide, elinogrel,
ticlopidine, ibuprofen, vorapaxar,
atopaxar, and a combination thereof.
[00683] Embodiment 160 is the method of any one of embodiments 41-157,
wherein the antiplatelet
agent is selected from the group consisting of aspirin, cangrelor, ticagrelor,
clopidogrel, prasugrel,
eptifibatide, tirofiban, abciximab, terutroban, picotamide, elinogrel,
ticlopidine, ibuprofen, vorapaxar,
atopaxar, cilostazol, prostaglandin El, epoprostenol, dipyridamole,
treprostinil sodium, sarpogrelate, and a
combination thereof.
[00684] Embodiment 161 is the method of any one of embodiments 41-160,
wherein administering
comprises administering topically, parenterally, intravenously,
intramuscularly, intrathecally,
subcutaneously, intraperitoneally, or a combination thereof.
[00685] Embodiment 162 is the method of any one of embodiments 41-161,
wherein the composition
is dried prior to the administration step.
[00686] Embodiment 163 is the method of embodiment 162, wherein the
composition is rehydrated
following the drying step.
[00687] Embodiment 164 is the method of any one of embodiments 41-161,
wherein the composition
is freeze-dried prior to the administration step.
109
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00688] Embodiment 165 is the method of embodiment 164, wherein the
composition is rehydrated
following the freeze-drying step.
[00689] Embodiment 166 is the method of any one of embodiments 41-165,
wherein the incubating
agent comprises one or more salts selected from phosphate salts, sodium salts,
potassium salts, calcium
salts, magnesium salts, and a combination of two or more thereof.
[00690] Embodiment 167 is the method of any one of embodiments 41-166,
wherein the incubating
agent comprises a carrier protein.
[00691] Embodiment 168 is the method of any one of embodiments 41-167,
wherein the buffer
comprises HEPES, sodium bicarbonate (NaHCO3), or a combination thereof.
[00692] Embodiment 169 is the method of any one of embodiments 41-168,
wherein the composition
comprises one or more saccharides.
[00693] Embodiment 170 is the method of embodiment 169, wherein the one or
more saccharides
comprise trehalose.
[00694] Embodiment 171 is the method of embodiment 169 or embodiment 170,
wherein the one or
more saccharides comprise polysucrose.
[00695] Embodiment 172 is the method of any one of embodiments 169-171,
wherein the one or more
saccharides comprise dextrose.
[00696] Embodiment 173 is the method of any one of embodiments 41-172,
wherein the composition
comprises an organic solvent.
[00697] Embodiment 174 is the method of any one of embodiments 41-173,
wherein the platelets or
platelet derivatives comprise FDPDs.
[00698] Embodiment 175 is the method of any one of embodiments 41-174,
wherein the antiplatelet
agent comprises aspirin at a dosage of about 80 mg to about 700 mg, once,
twice, three times, or four times
a day.
[00699] Embodiment 176 is the method of any one of embodiments 41-175,
wherein the antiplatelet
agent comprises aspirin, and the subject achieved a Cmax of about 3 to about
25 mg/L.
[00700] Embodiment 137 is the method of any one of embodiments 41-176,
wherein the antiplatelet
agent comprises 177 at an initial dosage of about 25 to about 30 pig/kg body
weight of the subject or a
following dosage of about 3 to about 5 lug/kg/min body weight of the subject.
[00701] Embodiment 178 is the method of any one of embodiments 41-177,
wherein the antiplatelet
agent comprises cangrelor, and the subject achieved a Cmax of about 400 to
about 500 ng/mL.
[00702] Embodiment 179 is the method of any one of embodiments 41-178,
wherein the antiplatelet
agent comprises ticagrelor at an initial dosage of about 170 to about 190 mg,
or a following dosage in a first
110
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
year of treatment of about 80 to about 100 mg twice daily, or a following
dosage in a second year of
treatment of about 50 to about 70 mg twice daily, optionally in combination
with aspirin.
[00703] Embodiment 180 is the method of any one of embodiments 41-179,
wherein the antiplatelet
agent comprises ticagrelor, and the subject achieved a Cmax of about 550 to
about 650 ng/mL.
[00704] Embodiment 181 is the method of any one of embodiments 41-180,
wherein the antiplatelet
agent comprises clopidogrel at an initial dosage of about 275 to about 325 mg,
or a following dosage of
about 70 to about 80 mg once daily, optionally in combination with aspirin.
[00705] Embodiment 182 is the method of any one of embodiments 41-181,
wherein the antiplatelet
agent comprises clopidogrel, and the subject achieved a Cmax of about 6 to
about 20 ng/mL.
[00706] Embodiment 183 is the method of any one of embodiments 41-182,
wherein the antiplatelet
agent comprises prasugrel at an initial dosage of about 50 to about 70 mg, or
a following dosage of about 3
to about 12 once daily, optionally in combination with aspirin.
[00707] Embodiment 184 is the method of any one of embodiments 41-183,
wherein the antiplatelet
agent comprises prasugrel, and the subject achieved a Cmax of about 200 to
about 525 ng/mL.
[00708] Embodiment 185 is the method of any one of embodiments 41-184,
wherein the antiplatelet
agent comprises eptifibatide at an initial dosage of about 170 to about 190
mcg/kg body weight of the
subject, optionally a second initial dosage of about 170 to about 190 mcg/kg
body weight of the subject, or
a following dose of about 1 to about 2 mcg/kg body weight of the subject/min.
[00709] Embodiment 186 is the method of any one of embodiments 41-185,
wherein the antiplatelet
agent comprises tirofiban at an initial dosage of about 0.3 to about 0.5 g/kg
body weight of the subject/min
for about 30 minutes, or a following dosage of about 0.1 g/kg body weight of
the subject/min.
[00710] Embodiment 187 is the method of any one of embodiments 41-186,
wherein the antiplatelet
agent comprises abciximab at an initial dosage of about 0.2 to about 0.3 mg/kg
body weight of the subject,
or a following dosage of about 0.10 to about 0.15 g/kg body weight of the
subject/min.
[00711] Embodiment 188 is the method of any one of embodiments 41-187,
wherein the antiplatelet
agent comprises abciximab at an initial dosage of about 0.2 to about 0.3 mg/kg
body weight of the subject,
or a following dosage of about 8 to about 10 g/min.
[00712] Embodiment 189 is the method of any one of embodiments 41-188,
wherein the antiplatelet
agent comprises ticlopidine at a dosage of about 240 to about 260 mg twice per
day.
[00713] Embodiment 190 is the method of any one of embodiments 41-189,
wherein the antiplatelet
agent comprises ibuprofen at a dosage of about 100 to about 600 mg once,
twice, three times, or four times
per day.
111
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00714] Embodiment 191 is the method of any one of embodiments 41-190,
wherein the antiplatelet
agent comprises vorapaxar at a dosage of about 2 to about 3 mg once per day,
optionally with aspirin or
clopidogrel.
[00715] Embodiment 192 is the method of any one of embodiments 41-191,
wherein the antiplatelet
agent comprises cilostazol at a dosage of about 40 to about 110 mg twice
daily.
[00716] Embodiment 193 is the method of any one of embodiments 41-192,
wherein the antiplatelet
agent comprises epoprostenol at an initial dosage of about 2 ng/kg body weight
of the subject/min, or a
following dosage of about 4, 6, 8, 10, 12, 14, 16, 18, or 20 ng/kg body weight
of the subject/min.
[00717] Embodiment 194 is the method of any one of embodiments 41-193,
wherein the antiplatelet
agent comprises dipyridamole at a dosage of about 60 to about 110 mg four
times daily.
[00718] Embodiment 195 is the method of any one of embodiments 41-194,
wherein the antiplatelet
agent comprises treprostinil sodium at a dosage of about 0.5 to about 1.3
ng/kg body weight of the
subject/min.
[00719] Embodiment 196 is the method of any one of claims 1-195, wherein
the subject does not have
cancer.
112
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
EXAMPLES
[00720] Example 1 ¨ P21(12 inhibitors
[00721] Cangrelor, like clopidogrel, ticagrelor, and prasugrel, blocks the
P2Y12 (ADP) receptor on
platelets. Cangrelor is used here as a representative of this class of drug.
[00722] FDPDs were prepared consistent with the procedure in Example 4.
Transmission light
aggregometry and T-TAS@ experiments were carried out according to Example 4.
[00723] The effect of cangrelor on the aggregation of platelets in
platelet-rich plasma (PRP; taken
from humans as whole blood and processed to isolate platelets in plasma
without white blood cells (WBC)
or red blood cells (rbc) was evaluated by transmission light aggregometry.
Aggregation of platelets (platelet
rich plasma) in response to agonist-induced activation showed complete
inhibition of 10 tiM adenosine
diphosphate (ADP)-induced aggregation by cangrelor at therapeutic
concentration of 0.5 tiM-3.5 iM
(Figure 1). All doses of cangrelor investigated completely eliminated ADP-
induced platelet aggregation in
PRP.
[00538] The effect of cangrelor on platelet occlusion under shear was
evaluated by T-TASa Fresh
platelet rich plasma (platelet concentration 278,000/1jL; PRP generally has a
platelet concentration of about
200,000/ L to about 300,000/ L) stimulated in vitro with 10 tiM ADP occluded
earlier under high shear
than unstimulated platelets (PRP) as determined by AR chip (collagen and
tissue thromboplastin) using T-
TAS@ technology (Figure 2). Cangrelor alone (1 tiM) did not exhibit inhibition
on occlusion, but when
combined with ADP (10 tiM), platelet adhesion and occlusion was essentially
eliminated. These results are
further illustrated in Figures 3 and 4. Without being bound by any particular
theory, it is believed that this
pattern is observed because platelets have other ADP receptors not blocked by
cangrelor that respond to
ADP and cause shape change and aggregation where the ADP receptor P2Y12
blocking inhibits collagen
binding, and, accordingly, the platelets may bind each other due to ADP
stimulation but may be prevented
from binding collagen on the coated chip.
[00539] In Figure 3, the area under the curve (AUC) values (derived from
data in Figure 2;
replicates are averaged and plotted once) are indicative of a combined value
of how quickly the thrombus
happened in time and how substantial the thrombus is when it does happen. PRP
AUC was increased with
ADP stimulation. Cangrelor had little effect on AUC value, but when combined
with ADP stimulation, the
AUC dropped close to zero.
[00540] In Figure 4, the time to occlusion of the AR T-TAS@ chip with drug
treatment was
evaluated. PRP occluded the chip channel at approximately 20 minutes, and
stimulation of platelets with
113
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
ADP decreased that time. Cangrelor had little effect on occlusion times, but
addition of ADP stimulation
to PRP sample inhibited occlusion essentially completely.
[00541] In the presence of cangrelor with ADP stimulation at the
concentrations shown to be
inhibitory of platelets, thrombosomes or FDPDs ("thromb" in Figures 5-7) were
not inhibited, indicating
that FDPDs can aid in a clot formation even in the presence of cangrelor at
therapeutic levels.
[00542] The effect of cangrelor on FDPDs under shear was evaluated by T-
TAS@. Figure 5 shows
that FDPDs (after 60, 90, or 115 minutes of rehydration, as indicated) retain
hemostatic function in the
absence or presence of cangrelor (1 uM), with ADP (10 uM) present. Unlike
platelets, thrombosome
occlusion of the T-TAS@ AR Chip is unaffected by the antiplatelet effect of
cangrelor + ADP. This suggests
FDPDs will maintain expected function when infused into patients receiving
cangrelor and similar agents.
These results are further illustrated in Figures 6 and 7.
[00543] In Figure 6, the AUC values (derived from the data in Figure 5)
are indicative of thrombus
formation. There was no effect of cangrelor + ADP on thrombosome adhesion and
occlusion of the T-
TAS@ AR Chip in plasma; FDPDs caused a thrombus formation regardless of
cangrelor and ADP. The
same dose of cangrelor and ADP completely inhibited freshly harvested
platelets.
[00544] In Figure 7, the time to occlusion (derived from the data in
Figure 5) of the FDPDs on AR
T-TAS@ chip with drug treatment was evaluated. There was no effect from
cangrelor + ADP on
thrombosome time to occlusion using the T-TAS@ AR Chip in plasma. The same
dose of cangrelor and
ADP completely inhibited freshly harvested platelets.
[00545] Example 2. GPIIb-IIIa inhibitors.
[00546] The results that follow demonstrate the impact of FDPDs in an in
vitro model of a patient
taking a GPIIb-IIIa inhibitor. Eptifibatide, a common antiplatelet drug,
competitively inhibits the GPIIb-
IIIa receptor on platelets which interact with fibrinogen and von Willebrand
factor.
[00547] Eptifibatide is a peptide therapeutic that blocks the fibrin
binding role of GPIIb-IIIa
receptor on platelets. The drug is typically administered via IV as a 180
g/kg bolus followed by 2
g/kg/min continuous infusion. The blood concentration of eptifibatide is
typically about 1-2 M. Bleeding
time generally returns to normal within about 1 hour of drug stoppage.
[00548] FDPDs were prepared consistent with the procedure in Example 4.
Transmission light
aggregometry and T-TAS@ experiments were carried out according to Example 4.
[00549] The aggregation of platelets (in platelet rich plasma) was
evaluated using transmission light
aggregometry. Eptifibatide completely inhibited collagen-induced (10 g/mL)
platelet aggregation in PRP
at all concentrations tested, as detected by light transmission aggregometry
in PRP. (Figure 8).
114
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00550] The effect of FDPDs on shortening clotting times while in the
presence of eptifibatide was
also studied. The ability of FDPDs to recover occlusion times was studied on
the T-TAS@ system. The T-
TAS@ system measures occlusion time under shear forces with collagen and
thromboplastin stimulation.
The whole blood profile of occlusion and AUC on the AR T-TAS@ chip lengthened
and decreased,
respectively, with eptifibatide. Eptifibatide extended the occlusion time of
whole blood on the T-TAS@
AR Chip in a dose-dependent manner. In this experiment, whole blood occluded
at 8 minutes, and the
occlusion time was extended to 16 minutes with 6 tiM eptifibatide (Figure 9).
FDPDs reversed the
inhibitory effect of eptifibatide on thrombus formation. Eptifibatide
inhibition of whole blood occlusion on
the T-TAS@ AR Chip was reversed by the addition of FDPDs at approximately
200,000/ L (N=3). When
FDPDs (approximately 200k/tit) were added to the sample of whole blood
inhibited with eptifibatide, the
time to occlusion decreased to 'normal' at 9 minutes (Figure 10).
[00551] The area under the curve values with thrombosome treatment also
increased with FDPDs
compared to that of normal whole blood samples. Figure 11 demonstrates the
time to of occlusion of the
FDPDs on AR T-TAS@ chip with drug treatment; eptifibatide inhibition of T-TAS@
AR Chip occlusion
was nearly entirely reversed by the addition of FDPDs (200,000/ L; N=3). In
Figure 12, the area under the
curve values were indicative of thrombus formation, where FDPDs returned
inhibition by eptifibatide to
normal levels; eptifibatide inhibition of platelet adhesion to and occlusion
of the T-TAS@ AR Chip is
overcome by addition of FDPDs (200,000/ L; N=3).
[00552] FDPDs, unlike platelets, are not inhibited in their ability to
occlude under shear in the
presence of eptifibatide (Figure 13). Figure 13 shows profiles of thrombus
formation of various lots of
thrombsomes on AR T-TAS@ system were unchanged with eptifibatide treatment.
FDPDs in platelet poor
plasma (PPP) were flowed through the T-TAS@ AR Chip with and without 6 uM
eptifibatide. There was
no effect of eptifibatide on thrombosome adhesion and occlusion. All
thrombosome concentrations were
approximately 300,000/ L.
[00553] The AUC and occlusion values by T-TAS for FDPDs (approximately
300,000/ L) in
plasma was the same with and without eptifibatide (Figure 14-15). Figure 14
shows the area under the curve
values were indicative of thrombus formation, and no changes were observed
with eptifibatide in platelet-
poor plasma. There was no effect of 6 uM eptifibatide on AUC of FDPDs T-TAS@
AR Chip occlusion.
Figure 15 shows the time to occlusion of the FDPDs on AR T-TAS@ chip was
unchanged with eptifibatide.
There was no significant influence from 6 tiM eptifibatide on FDPDs occlusion
time of the T-TAS@ AR
Chip in platelet-poor plasma.
[00554] Example 3. COX inhibitors.
115
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00555] The results that follow demonstrate the impact of FDPDs in an in
vitro model of a patient
taking a COX inhibitor. Aspirin, a common antiplatelet drug, blocks the COX1
enzyme in platelets. COX1
is responsible for converting arachidonic acid to prostaglandin.
[00556] Aspirin is an irreversible cyclooxygenase (COX) inhibitor. The COX
enzyme in platelets
is responsible for synthesis of thromboxane A2, prostaglandin E2, and
prostacyclin (PGI2). Aspirin
permanently inactivates the COX enzyme within platelets, and since platelets
do not have the nuclear
material to synthesize new enzyme, new platelets must be produced to overcome
the aspirin effect. Without
thromboxane A2, prostaglandin E2 and prostacyclin (PGI2) platelets are limited
in their pro-aggregation
activity. Many people are maintained on a low dose of aspirin to prevent
unwanted clotting events. Aspirin
bioavailability largely varies with administration route, with a single 500 mg
dose IV at peaks of 500 M
and the same dose orally at 44 M.
[00557] FDPDs were prepared consistent with the procedure in Example 4.
Transmission light
aggregometry and T-TAS@ experiments were carried out according to Example 4.
[00558] Platelets will aggregate with collagen and arachidonic acid
stimulation. Stimulation by
arachidonic acid can be completely inhibited whereas collagen stimulation
aggregation can only be partially
inhibited at concentrations of 100-400 M aspirin (Figure 16). Figure 16 shows
light transmission
aggregometry in PRP with collagen (10 ug/mL) and arachidonic acid (AA; 500
ug/mL), which induced
platelet aggregation, and that aggregation was inhibited by all doses of
aspirin (ASA) tested. Aspirin
eliminated arachidonic acid induced platelet aggregation entirely. The PL chip
system on the T-TAS@ was
used to emulate in vitro platelet binding and aggregation due to the exposure
of collagen in the vasculature
under shear conditions. This action of platelets was largely limited in the
presence of 100 and 500 M of
aspirin but can be at least partially returned in the presence of FDPDs
(approximately 200,000 to
400,000/ L; Figure 17). Figure 17 shows via area under the curve measurement
of whole blood that
thrombus formation on the PL T-TAS@ chip was inhibited by aspirin with partial
return of thrombus
formation with FDPDs.
[00559] Example 4. Protocols
[00560] Generation of FDPDs. FDPDs were prepared consistent with the
procedures described in
U.S. Patent Nos. 8,486,617 (such as, e.g., Examples 1-5) and 8,097,403 (such
as, e.g., Examples 1-3),
incorporated herein by reference in their entirety.
[00561] Transmission Light Aggregometry
[00562] Plasma samples with platelet or FDPDs or combination of both are
loaded into cuvettes
and placed into the aggregometry chambers. The chambers warm the sample and
provide constant stirring.
The initiation of aggregation can be done by multiple types of inhibitor
agents not limited to thrombin,
116
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
ADP, collagen and any agent know to stimulate platelet aggregation. The
samples can also have been taken
as ex-vivo, or in-vitro supplemented with inhibitors. The instrument begins
the assay by first recording the
light transmission previous to stimulation for 2 minutes. The stimulant of
interest is then introduced by the
technician and the change in light transmission is recorded overtime. The
increase in light transmission
corresponds to increase in platelet aggregation.
[00563] Evaluation by T-TAS@ using an AR chip. AR chips are characterized
by a single channel
containing collagen and tissue factor; they can be used to analyze clotting
and platelet function.
[00564] The T-TAS@ instrument was prepared for use according to the
manufacturer's instructions.
AR Chips (Diapharma Cat. # TC0101) and AR Chip Calcium Corn Trypsin Inhibitor
(CaCTI; Diapharma
Cat. # TR0101) were warmed to room temperature. 300 uL of rehydrated FDPDs
were transferred to a 1.7
mL microcentrifuge tube and centrifuged at 3900 g x 10 minutes to pellet. The
FDPDs pellet was
resuspended in George King (GK) pooled normal human plasma or autologous
plasma with or without
autologous platelets to a concentration of approximately 100,000- 450,000/uL,
as determined by AcT
counts (Beckman Coulter AcT Diff 2 Cell Counter). 20 uL of CaCTI with 480 uL
of FDPDs sample in GK
plasma were mixed with gentle pipetting. The sample was loaded and run on the
T-TAS@ according to the
manufacturer's instructions.
[00565] Evaluation by T-TAS@ using a PL chip
[00566] PL chips are run similarly to AR chips but this chip is only coated
with collagen alone.
[00567] Thrombin Generation
[00568] Reagent Preparation. For thrombin generation, the following materials
were used from
manufacturers, as follows: FluCa Kit (Diagnostica Stago, Cat. No. 86197),
Thrombin calibrator
(Diagnostica Stago, Cat. No. 86197), PRP Reagent (Diagnostica Stago, Cat. No.
86196), OCTOPLAS@, a
solvent detergent treated human pooled plasma (Octapharma, Cat. No. 8-68209-
952-04). All frozen
reagents were thawed in a 37 C water bath before use. All reagents were
rehydrated with sterile water
using the volume printed on the reagent labels. Approximately 2 min after
rehydration, the reagents were
mixed by inverting vials 5 times, so no chunks or powder left; vortexing was
not used. This procedure was
repeated approximately 10 minutes after rehydration. All reagents were
incubate at room temperature for
another approximately 10 minutes (total of approximately 20 min after
rehydration). A 30% solution of
OCTOPLAS@ was prepared by mixing 4.66m1 of FDPDs control buffer (Table B) with
2m1 of
OCTOPLAS .
[00569] Table B. FDPDs Control Buffer
Concentration
Component (mg/mL, except where otherwise indicated)
117
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
NaC1 6.08
KC1 0.28
HEPES 2.47
NaHCO3 0.77
Dextrose 0.41
Trehalose 28.83
Ethanol 0.76% (v/v)
[00570] Sample Analysis - Plate preparation and testing. For experiments
containing FDPDs, a FDPDs
dilution series was generated (dilutions of 194.4K, 64.8K, 21.6K, and 7.2K per
tit were typically used; cell
counts are determined by flow cytometry) for each the experimental FDPDs and
the reference FDPDs.
FDPDs were rehydrated unless indicated otherwise. The highest-concentration
dilution (e.g., 194.4k
FDPDs) was prepared by combining FDPDs, OCTAPLAS@, and FDPDs Control Buffer.
The rest of the
dilution series was prepared by serial 1:3 dilutions in OCTAPLAS (D. For each
test sample, 20 uL of PRP
reagent was added to each sample well (of Immulon 2HB Clear, round-bottom 96-
well plate (VWR, Cat.
No. 62402-954)) and 20 uL of Thrombin Calibrator was added to each calibrator
well. To each sample well
and calibrator well, 80 uL of the each of the FDPDs dilution series was added.
Continue until the last
dilution. The plate was then incubated in the Fluoroskan Ascent 96 well
fluorescent plate reader
(Thrombinoscope) (ThermoFisher Scientific) for 10 minutes. During this
incubation phase, the FluCa
solution was prepared by adding 40 L of FluCa substrate to the 1.6m1 of thawed
Fluo-Buffer, vortexing,
and returning the solution to the water bath. When incubation was complete,
the FluCa solution was added
to the Fluroskan instrument according to the manufacturer' s instructions. The
plate fluorescence was
monitored for 75 minutes at an interval of 20 seconds and a temperature of 40-
41 C.
[00571] Example 5.
[00572] Additional experiments were carried out with cangrelor and
aspirin. FDPDs were prepared
consistent with the procedure in Example 4. Transmission light aggregometry, T-
TAS@, and thrombin
generation experiments were carried out according to Example 4.
[00573] The effect of FDPDs on the recovery of thrombus formation was
evaluated using T-TAS@
technology and an AR chip. Figure 18 shows the occlusion time of whole blood
treated with various
combinations of FDPDs (at a concentration of 250,000 FDPDs per tit), aspirin
(200 tiM), cangrelor (1
tiM), anti-Integrin alpha-2 (CD49B) antibody 6F1 (40 pig; see
dshb.biology.uiowa.edu/integrin-alpha-2-
alpha2beta1?sc=7&category=-107 for product/manufacturer information), and anti-
GPIIb/IIIa receptor
118
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
antibody AP2 (20 ug/mL; see kerafast.com/product/2010/anti-glycoprotein-
gpiiiagpiib-complex-ap-2-
antibody for product/manufacturer information). Figure 19 shows the occlusion
over time of untreated
whole blood and whole blood treated with FDPDs (at a concentration of 250,000
FDPDs per tiL), a mixture
containing 6F1 (40 ug/mL; anti-CD49b), ASA (aspirin; 200 uM), and cangrelor (1
uM); or a combination
thereof.
[00574] The effect of FDPDs on the recovery of thrombus formation was also
evaluated using T-
TAS@ technology and a PL chip. Figure 20 shows the occlusion time of whole
blood treated only with
buffer, aspirin (500 tiM), or aspirin (500 tiM) and FDPDs (at a concentration
of 250,000 FDPDs per L).
Figure 21 shows the occlusion over time of whole blood, whole blood treated
with aspirin (500 tiM), or
aspirin (500 tiM) and FDPDs (250,000/ L). Figures 22 and 23 show similar
experimental data using 100
tiM aspirin instead of 500 tiM aspirin.
[00575] The effect of aspirin treatment (concentration) on thrombin
generation was measured.
FDPDs were evaluated at concentrations 1450, 1150, 850, 650, 450, 150, 50, and
0 k/uL in PPP from
patients taking baby aspirin daily and standard plasma (INR = 1). Figure 24
shows that the peak thrombin
value of the aspirin plasma in absence of FDPDs was below the normal range
(about 45 nM; normal range
is about 66-166 nM), but with FDPDs addition, it came back to being within the
normal range at even the
lowest FDPDs concentration used (50 k/iut). The values again were saturated at
about 800 k FDPDs and
went up to 220 nM ¨ 5 times the value of this plasma in absence of FDPDs
(increase from 45 to 220 nM).
[00576] Example 6. FDPDs Reversed Prolonged PRP Occlusion Times Induced by
Cangrelor
[00577] Additional experiments were carried out with cangrelor. FDPDs were
prepared consistent
with the procedure in Example 4. T-TAS@ was carried out according to Example
4.
[00578] Figures 25A and 25B show that platelet rich plasma treated with
10Ong/mL cangrelor and
ADP extended occlusion times from 19 to 26 minutes on the T-TAS@ flow system
(collagen and tissue
factor coated channel). The addition of 150k/tiL FDPDs decreased the time back
to 15.3 minutes.
[00579] Example 7. FDPDs but not Random Donor Platelets (RDP) Reversed
Extended
Occlusion Times Induced by tirofiban in PRP
[00580] Additional experiments were carried out with tirofiban. FDPDs were
prepared consistent
with the procedure in Example 4. T-TAS@ was carried out according to Example
4. Random donor platelets
were prepared from whole blood.
[00581] Figures 26A and 26B show that platelet rich plasma treated with
10Ong/mL tirofiban
extended occlusion times from 18.43 to no occlusion on the T-TAS@ flow system
(collagen and tissue
119
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
factor coated channel). The addition of 150k/ L of FDPDs decreased the time
back to 12.94 minutes but
RDP only partially recovered at the same count.
[00582] Example 8. FDPDs but not Random Donor Platelets Reversed Extended
Occlusion
Times Induced by Eptifibatide in PRP
[00583] Additional experiments were carried out with eptifibatide. FDPDs
were prepared
consistent with the procedure in Example 4. T-TAS@ was carried out according
to Example 4. Random
donor platelets were prepared from whole blood.
[00584] Figures 27A and 27B show that platelet rich plasma treated with 9
M eptifibatide extended
occlusion times from 18.43to over 30 minutes on the T-TAS@ flow system
(collagen and tissue factor
coated channel). The addition of 150k/ L of FDPDs decreased the time back to
11.56 minutes but not
occlusion seen with same number of RDP.
[00585] Example 9. FDPDs Reversed Extended Occlusion Times Induced by AP2
(anti-
Gpllb/IIIa) in PRP
[00586] Additional experiments were carried out with AP2. FDPDs were
prepared consistent with
the procedure in Example 4. T-TAS@ was carried out according to Example 4.
Random donor platelets
were prepared from whole blood.
[00587] Figures 28A and 28B show that platelet rich plasma treated with 10
g/mL AP-2 extended
occlusion times from 18.43 to over 30 minutes on the T-TAS@ flow system
(collagen and tissue factor
coated channel). The addition of 150k/ L of FDPDs decreased the time back to
13.14 minutes and occlusion
was seen at 17.43 minutes same number of RDP.
[00588] Example 10. FDPDs Reversed Prolonged Occlusion in PRP from
Subjects on Aspirin
Therapy
[00589] Additional experiments were carried out with aspirin. FDPDs were
prepared consistent
with the procedure in Example 4. T-TAS@ was carried out according to Example
4. Random donor platelets
were prepared from whole blood. The subject was on a standard dose of 81
mg/day of aspirin.
[00590] Figures 29A and 29B show that platelet rich plasma taken from an
aspirin patient failed to
occlude on the T-TAS@ flow system (collagen and tissue factor coated channel).
The addition of 200k/ L
of FDPDs returned to normal occlusion time to 16 minutes.
120
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00591] Example 11. FDPDs Restore Thrombin Generation in Ex-vivo Aspirin
Platelet Rich
Plasma
[00592] Additional experiments were carried out with aspirin. FDPDs were
prepared consistent
with the procedure in Example 4. Thrombin generation was carried out according
to Example 4.
[00593] Figure 30A shows Thrombin generation of platelet rich plasma from
aspirin patient verses
normal stimulated with PRP reagent was reversed with 50k/it of FDPDs. Figure
30B shows the change
from and return to normal thrombin production, time to peak production, and
lag time in three repeat aspirin
ex-vivo samplings with FDPDs (50k/tit). (n=3 thrombosome lots, n=2
individuals).
[00594] Example 12. FDPDs Restore Hemostasis in PRP from Subject on NSAID
Ibuprofen
Therapy.
[00595] Additional experiments were carried out with ibuprofen, an NSAID.
FDPDs were prepared
consistent with the procedure in Example 4. Aggregometry and T-TAS@ were
carried out according to
Example 4.
[00596] Platelet rich plasma was taken from subject on 800 mg ibuprofen.
Figure 31A shows that
a lack of aggregation in response to arachidonic acid confirms NSAID presence
in the PRP. Figure 31B
shows occlusion on the T-TAS@ flow system (collagen and tissue factor coated
channel); PRP from the
ibuprofen patient demonstrated occlusion, while addition of ADP abolished
occlusion. The addition of
150k/tiL thrombosome restored occlusion.
[00597] Example 13. FDPDs Restore Bleeding Time in NOD-SCID Mice Treated
with
Supra-pharmacologic Clopidogrel
[00598] Additional experiments were carried out with clopidogrel. FDPDs
were prepared
consistent with the procedure in Example 4.
[00599] The mouse was treated with clopidogrel for 5 days. The mouse was
anesthetized, the tail
end was snipped off followed by FDPDs being immediately administered. The time
from tail snip to tail
stop bleeding was recorded by visual inspection.
[00600] NOD/SCID mice were treated with ¨ 3 times the clinical dose of
clopidogrel for 5 days
then assessed in the tail-snip bleed model. The bleed time (min) was extended
to 17.8 minutes with
clopidogrel treatment verses untreated at 9 minutes (data not shown).
Treatment with 8 L/gram of FDPDs
(1.8 x 10"9 particles/mL at 200 tit) decreased bleeding to 12.31 minutes
(Figure 32).
121
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00601] Example 14. Reproducibly of FDPD reversal of ticagrelor and
cangrelor inhibition of
occlusion
[00602] Human freeze-dried platelet derivatives (FDPDs), also called
lyophilized human platelets
(LHP), were prepared according to the procedure in Example 15. T-TAS@
experiments, to measure
occlusion time, using AR chips were carried out according to Example 4.
[00603] The effect of cangrelor and ticagrelor on occlusion time of
Platelet Rich Plasma (PRP) with
and without FDPDs was assessed using the T-TAS@ assay. The concentrations of
the agents were as
follows: cangrelor at 1 M, ticagrelor at 500ng/mL, FDPDs at 150k/ L, and ADP
at 2 M. The results are
shown in FIGs 33A and 33B. Adenosine diphosphate (ADP) stimulated PRP samples
showed occlusion at
19.5 1.5 minutes (n=4) which increased to 28.0 3.0 minutes with cangrelor
(n=4) or 28.0 3.0 minutes
with ticagrelor (n=5) treatment (Figures 33A-33B). The addition of 150k/ L
lyophilized human platelets
to P2Y12-inhibited PRP reduced time to thrombus formation to lower than PRP
alone; 15.5 0.5 minutes
in the presence of cangrelor (n=3) versus 17.5 1.5 minutes in the presence of
ticagrelor (n=5) (Figure 33).
The number of trials per each assay is listed as the "(n=_)" values.
[00604] These results demonstrate that occlusion of platelets on the T-
TAS@ AR Chip in the
presence of human FDPDs is not affected by the antiplatelet effect of
cangrelor and ticagrelor. These results
suggest that human FDPDS will maintain expected function when infused into
patients receiving cangrelor,
ticagrelor, and/or similar agents.
[00605] Example 15. Tangential Flow Filtration (TFF) Method of Platelet
Derivative
Preparation
[00606] Apheresis platelets underwent tangential flow filtration in
accordance with a standard
operating procedure, including the following process steps: platelet dilution,
platelet concentration and
platelet washing.
[00607] The platelet donor units were initially pooled into a common
vessel. The platelets may or
may not be initially diluted with an acidified washing buffer (e.g., a control
buffer) to reduce platelet
activation during processing. The platelets can undergo two processing
pathways; 1) either washed with
control buffer until a desired residual component is reached (e.g., donor
plasma) before being concentrated
to a final product concentration or 2) the platelets are concentrated to a
final product concentration before
being washed with control buffer until a desired residual component is reached
(e.g., donor plasma). TFF
processed platelets are then filled into vials, lyophilized and thermally
treated.
[00608] One particular protocol follows.
[00609] For all steps of the TFF process in this Example, Buffer F was
used. The process was
carried out at a temperature of 18-24 C.
122
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00610] Buffer F
Component Value ( 1%)
HEPES 7.6 mM
NaC1 60 mM
KC1 3.84 mM
Dextrose 2.4 mM
NaHCO3 9.6 mM
Trehlao se 80 mM
Ethanol 0.8%
Poly sucro se 6% (w/v)
pH 6.6-6.8
[00611] Platelets were loaded onto the TFF (PendoTECH controller system
(PendoTECH Princeton,
NJ; https://www.pendotech.com), which was prepared with a Repligen TFF
Cassette (XPM45L01E). The
TFF process was performed using a membrane with a pore size of 0.45 tim. The
platelets were diluted with
an equal weight ( 10%) of Buffer F. The platelets were concentrated to about
2250 x 103 cells/vIL ( 250 x
103) and then washed with approximately 2 diavolumes (DV) of Buffer F. The
target plasma percentage
was typically less than 15% relative plasma (as determined by plasma protein
content). Removal of plasma
proteins was monitored through 280 nm UV absorbance against known
correlations.
[00612] In some cases, samples were drawn at UV absorbance readings
correlating to about 51%
relative plasma volume, about 8.1% relative plasma volume, about 6.0% relative
plasma volume, and about
1.3% relative plasma volume. Low volume aliquots were sampled throughout each
processing step with the
about 6.0% and under samples.
[00613] Following washing, if the concentration of the cells was not 2000 x
103 cells/vIL ( 300 x 103),
the cells were either diluted with Buffer F or were concentrated to fall
within this range. Under all
circumstances whenever the cells were contacted with the Buffer F, it was done
at a temperature in the
range of 18-24 C. For a better clarity, the cells were loaded with the
reagents of the Buffer F at a temperature
in the range of 18-24 C. The cells were typically then freeze-dried (I.e.
lyophilized) and subsequently
heated (thermally treated) at 80 C for 24 hours, thereby forming Freeze-dried
platelet derivatives (FDPDs),
which are also called THROMBOSOMES when prepared by Cellphire, Inc. For
clinical or commercial
use.
[00614] The lyophilization procedure used to prepare the human FDPDs is
presented in Table LA2.
123
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00615] Table LA2
LYOPHILIZER RECIPE
30g Fill 10g Fill
Freezing Freezing
Ramp -40 0C Omins onaTorr Ramp -40"C 0 min,., 0 inTorr
to to
Hold a.t -40 ''C 180 0 naTorr Hold a.t -40"C 180 0 in:Tarr
min s mins
Final Freezing Final Freezing
Hold at -40 '"C Omins 100 inTorr Hold at -40 0 mins li,U
niforr
Primary Drying Primary Drying
Ramp .5 '12 420 0 inTorr Ramp -10 360 0 in'Torr
to mins to "C mins
HoM at .5 "C: 1200 0 niTorr Hold at -10 360 o rifforr
mins 'C mins
Ramp +5'C 120 0 niTon: Ramp +S"C 180 0 niTorr
to mins to mins
:Hold at +S"C 1380 0 rifirOlT :Hold at +S"C :360 0 inTorr
mins mins
Ramp .+30 C 300 0 tri'll'arr Ramp .4-30 300 0 inTorr
to mins to "C mins
Hold at +30 720 0 tnTorr Hold at +30 720mi ris 0
niTorr
mins
Hold at +30'"C 720 200 ITITon Hold at +30' 720mins 200 niTotT
mins C
Hold at -1-.30`'C 60 mins 0 in'torr Hold at. +30 60 tni D S 0
ITITOrr
"C
Secondary Drying Secondary Drying
Hold. at +30 C 9999 0 niTOIT Hold at +10 9999 0 IT3Torr
mins 'C mins
Total Recipe Time la -85 hours Total Recipe Time la -54 hours
[00616] To perform studies such as thrombin generation studies (TGPU), and
aggregation studies,
FDPDs were typically rehydrated with water over 10 minutes at room
temperature. In general, the
rehydration volume is equal to the volume used to fill each vial with cells
prior to drying. The platelet
derivatives which were heated (thermally treated) after lyophilization are
also referred to as baked FDPDs.
Whereas the FDPDs which were not heated (thermally treated) after
lyophilization are referred to as
unbaked FDPDs.
124
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00617] Human FDPDs, obtained after lyophilization in the form of a powder
can be used for
commercial applications, such as providing the human FDPDs (e.g.
THROMBOSOMESC) in dried form
in vials to, for example, a medical practitioner who can rehydrate the vials
with an appropriate amount of a
liquid.
[00618] Example 16. FDPDS Restore Bleeding Time in NOD-SCID Mice Treated
with Supra-
pharmacologic Clopidogrel
[00619] Human FDPDs were prepared consistent with the procedure in Example
15.
[00620] Mice were treated with clopidogrel at 5mg/kg for 3 days. The mice
were anesthetized, the
tail end was snipped off at lmm diameter and submerged in warm saline and time
to clot recorded. Animals
were syringe injected, into a vein or artery, with saline or 1.6 x 109/kg
FDPDs, at the same time the tail
was snipped, and the tail snip trial commenced. The time from tail snip to
tail stop bleeding was recorded
by visual inspection of cessation of blood loss.
[00621] The results for this experiment are shown in FIG. 34. The bleed
time (seconds) was
extended to 1661 +/- 257.9 seconds with clopidogrel treatment verses untreated
at 758.6 +/- 623.4 seconds.
Treatment with 1.6 x 109/kg FDPDS decreased bleeding to 417.9 +/- 166.6
seconds. One-way ANOVA
software, was utilized for analysis of the data.
[00622] These results demonstrate that human FDPDs are resistant to
clopidogrel effects and
restore hemostasis in a mice tail snip model. Human FDPDs have the potential
to be an effective clinical
tool to stop bleeding in a patient population being treated with clopidogrel.
[00623] Example 17. FDPDs Restore Bleeding Time in New Zealand White
Rabbits Treated
with Supra-pharmacologic Clopidogrel
[00624] Additional experiments were carried out with clopidogrel treated
New Zealand White
Rabbits. Human FDPDs were prepared consistent with the procedure in Example
15.
[00625] Rabbits were treated with clopidogrel at 23mg/kg for 5 days. The
rabbits were anesthetized,
the ear was bled and time to clot recorded. Animals were treated, injected
into a vein or artery, with saline
or 1.6 x 109/kg cf human FDPDs, at the same time the ear was bled, and the ear
bleed trial commenced.
The time from ear bleed to stop bleeding was recorded by visual inspection.
[00626] Results of this experiment are shown in FIG. 35. The bleed time
(seconds) was extended
to 206.8 +/- 104.2 seconds with clopidogrel treatment verses untreated at 87.1
+/- 18.2 seconds. Treatment
with 1.6 x 109/kg FDPDS decreased bleeding to 417.9 +/- 166.6 seconds (Figure
35). One-way ANOVA
was utilized for analysis of the data.
125
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00627] These results demonstrate that human FDPDs are resistant to
clopidogrel effects and
restore hemostasis in a rabbit ear bleed model. Lyophilized human platelets
have the potential to be an
effective clinical tool to stop bleeding in a patient population being treated
with clopidogrel.
[00628] Example 18. FDPDs Contribution to Thrombin Generation in the
Presence of 25
ng/mL Rivaroxaban Treated OCTAPLAS PRPs Reagent
[00629] Human FDPDs were prepared according to the method as described in
Example 15. The
OCTAPLAS@ plasma used in this example is a solvent/detergent treated, pooled
human plasma available
from Octapharma USA, Inc., 117W. Century Road Paramus, NJ 07652;
www.octapharmausa.com.
[00630] A Thrombin Generation Assay (TGA) was performed to detect thrombin
generation and
endogenous thrombin potential in (1) OCTAPLAS@ with platelet rich plasma (PRP)
and incrementally
increasing FDPD concentration (0, 10, 20, 40, 80, 160) x 103/iut and (2)
OCTAPLAS@ with platelet rich
plasma reagent (PRP), 25 ng/mL rivaroxaban, and incrementally increasing FDPD
concentration (0, 10, 20,
40, 80, 160) x 103/ L. A 25 ng/mL dose of Rivaroxaban is within the
physiological dose range and is an
effective dose to inhibit thrombin generation.
[00631] The results of this experiment are shown in FIGs. 36A and 36B.
These results demonstrated
that even low doses of FDPDs are capable of catalyzing some thrombin
generation (FIG. 36A) and partially
recovering the endogenous thrombin potential (FIG. 36B) in the presence of 25
ng/mL dose of rivaroxaban
in OCTAPLAS@ with PRP reagent.
[00632] Example 19. Addition of FDPDs Shows Partial Recovery of Occlusion
Time of 25
ng/mL Rivaroxaban Treated Whole Blood (WB)
[00633] Occlusion time was measured on the Total Thrombin formation
Analysis System (T-
TAS@) 01 using AR chips (Collagen and Tissue FactorF stimulant). The T-TAS@
01(Diapharma@,
https://diapharma.com) instrument was prepared for use according to the
manufacturer's instructions. AR
Chips (Diapharma # 19001) and Calcium Corn Trypsin Inhibitor (CaCTI; Diapharma
Cat. # TR0101) were
warmed to 37 C or room temperature, respectively. Whole blood was collected in
sodium citrate tubes 30
minutes prior to the start of the assay. FDPDS were rehydrated, counted
(Beckman Coulter AcT Diff 2 Cell
Counter), and added to whole blood at the indicated final concentration.
Rivaroxaban (Cayman Chemical
cat #16043) was dissolved in 100% DMSO to make a 10 ug/mL stock solution and
added to the sample at
the indicated final concentration, yielding a final DMSO content of 0.25%,
480uL of sample was mixed
with 20uL of calcium CTI reagent and run on the AR chip on T-TAS@ 01.
126
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00634] FDPDs were prepared according to the procedures of Example 15. The
TAS@ 01 assays
using AR chips general method and OCTAPLAS@ plasma are described in Example 18
above. The TAS@
01 assays were run with no rivaroxaban, 25 ng/mL rivaroxaban, and 25 ng/mL
rivaroxaban and 20k/tiL
FDPDs.
[00635] The pressure over time is shown in Figure 37 with pressure
increase being indicative of
occlusion. The results show that Occlusion time was partially restored with
the addition of FDPDs into
rivaroxaban treated whole blood.
[00636] Example 20. The Addition of FDPDs to Rivaroxaban Treated OCTAPLAS
or
Rivaroxaban Treated Fresh PRP Show the Recovery of Fibrin and Thrombin
Generation Using
Thrombodynamics Analyser System (T2T)
[00637] The Thrombodynamics @ Analyser System (T2T) (Diapharma@,
https://diapharma.com) was used to detect thrombin generation and fibrin
formation. Human FDPDs
were prepared according to the procedure in Example 15.
[00638] Thrombodynamics set-up and sample prep were performed according to
manufacturer's
recommendation EXCEPT a phospholipid reagent was not added. The experiment was
performed with
either OCTAPLAS@ or fresh PRP. OCTAPLAS@ and PRP were each incubated with
300ng/mL
rivaroxaban for 2 minutes, FDPDSs were added to the sample, and then the
sample was added to Reagent
1 (contact pathway inhibitor and thrombin fluorescent substrate of the T2T
assay); 20k/tiL FDPDSs were
added for OCTAPLAS@ run and 2k/it for PRP run. The sample was incubated at 37
C for 3 min (PRP)
or 15 min (OCTAPLAS@ ) according to instrument protocol. Following the
incubation, the sample was
added into a cuvette containing TF-coated plastic insert and the run was
started. The concentrations of the
components in the fresh PRP run are decreased in order to see the dynamic
response when using fresh
platelets versus OCTAPLAS@, a detergent-treated plasma.
[00639] This results of this experiment showed recovery of fibrin and
thrombin generation by
FDPDs in rivaroxaban treated Octaplas (Figures 38A-38C). The results also
showed the partial recovery of
fibrin and thrombin generation in a rivaroxaban-treated fresh PRP sample
(Figures 39A-39C) at the
physiological ratios of endogenous platelets: FDPDs FDPDS FDPDS that would be
expected if using
clinically.
[00640] Example 21 Addition of FDPDSs Rescues Clot Forming Capacity of a
Heparin
Treated Sample with less than Clinically Suggested Dose of Protamine
127
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00641] This experiment assessed how clinical proportions of Heparin and
Protamine affect FDPD
function. FDPDs were prepared according to the procedure in Example 15. An
Activating Clotting Time
(ACT) assay was performed to measure time to clot and TGA was performed to
measure thrombin
generation. An initial Heparin titration run was performed using ACT to find
the minimum Heparin dose
needed to reach abnormal clot time. Results showed a minimum of 0.8U/mL
Heparin is needed to reach
abnormal clot formation.
[00642] Figure 40 shows the effect of FDPDs on time to clot in the
presence of anticoagulants using
the ACT test. Activated clotting time was measured using pooled normal plasma
with heparin (H) and
protamine (P) as labeled on the x-axis; 1/2 P, P, and 3/2 P represent
protamine doses of 4, 8, and 12 g/ml,
respectively. FDPDs, (referred to as "Tsomes" in FIG. 40) were added at 10, 25
or 50 K/ 1. Samples labeled
"0" Tsomes had an equal volume of control buffer added to pooled normal
plasma. "K" in the legend stands
for 103 FDPDS. All samples were run in duplicate. N = 2. The experiment was
repeated on separate days
to improve statistical relevance.
[00643] Figure 41 A-C show that FDPDs retain thrombin generation peak in
the presence of heparin
and protamine using TGA. Figure 41A shows the effect of 0.1 U heparin on
thrombin generation, in pooled
normal plasma, comparing apheresis units (APU) with FDPDs at 5K and 50K
platelets per L. Figure 41B
shows the impact of 0.8 U/mL heparin reversed by 4 g/m1 protamine (1/2 of the
recommended reversal
doses) with FDPDs at 10K and 50K platelets per L. TGA of Figure 41A and
Figure 41B is initiated by
PRP reagent containing a mixture of phospholipids and tissue factor. The
dashed line in Figures 41A and
41B denotes the typical thrombin peak seen in this assay. Figure 41C shows
peak height of thrombin
generation of samples which were treated with heparin and protamine as
described on the x-axis. Empty
(white fill) bars represent samples that were diluted with control buffer, and
filled bars represent samples
that were treated with FDPDs. All samples were run in triplicate. The
experiment was repeated on separate
days to improve statistical relevance.
[00644] The results of this experiment demonstrate that if there is a less
than clinical dose of
protamine present in a heparin treated sample, then FDPDs impose a dose
dependent decrease of time to
clot (ACT) and increase of thrombin peak height (TGA)
[00645] Example 22. Reproducibly of FDPD reversal of aspirin (ASA)
inhibition of
occlusion.
[00646] Human freeze-dried platelet derivatives (FDPDs) were prepared
according to the procedure
in Example 15. T-TAS@ experiments using AR chips were carried out according to
Example 4.
[00647] The effect of aspirin on the occlusion time of PRP with and
without FDPDs was assessed
using a T-TAS assay. The concentrations of the agents are as follows: PRP at
30K/ L, aspirin at 500 M,
128
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
and FDPDs at 20k/iut. The results are shown in FIG 42 PRP samples showed
occlusion of >30 minutes
with aspirin treatment versus a PRP alone time of 25:56 minutes; the addition
of 20k/it FDPDs to P2Y12-
inhibited PRP reduced time to thrombus formation to lower than PRP alone;
23:29 minutes in the presence
of aspirin (Figure 42).
[00648] These results demonstrate that the occlusion of platelets on the T-
TAS@ AR Chip in the
presence of human FDPDs is unaffected by the antiplatelet effect of aspirin.
This suggests that human
FDPDS will maintain expected function when infused into patients receiving
aspirin and/or similar agents.
[00649] Example 23. Reproducibly of FDPD reversal of ticagrelor and
aspirin (ASA)
combined inhibition of occlusion.
[00650] Human freeze-dried platelet derivatives (FDPDs) were prepared
according to the procedure
in Example 15. T-TAS@ experiments using AR chips were carried out according to
Example 4.
[00651] The effect of ticagrelor and aspirin together on the occlusion
time of PRP with and without
FDPDs was assessed using a T-TAS assay. The concentrations of the agents are
as follows: PRP at 50K/it,
ticagrelor at 1.5 g/mL, aspirin at 500 M, and FDPDs at 50k/it. The results are
shown in FIG 43. The
addition of 20k/it FDPDs to the combined ticagrelor and aspirin treated PRP
reduced time to thrombus
formation to 19:36 minutes versus a >30 minutes occlusion time in the presence
of combined ticagrelor and
aspirin alone.
[00652] These results demonstrate that the occlusion effect of platelets
on the T-TAS@ AR Chip in
the presence of human FDPDs unaffected by the combined antiplatelet effect of
ticagrelor and aspirin. This
suggests that human FDPDS will maintain expected function when infused into
patients receiving combined
ticagrelor and aspirin treatment.
[00653] Example 24. Inability of FDPDs to aggregate in the presence of
agonists and absence
of fresh platelets
[00654] Light transmission aggregometry (LTA) was used to observe FDPD
aggregation in the
presence of known platelet aggregation agonists. The FDPD aggregation data was
compared to aggregation
data of fresh platelets.
[00655] FDPDs, also referred as "TFF-FDPDs", were produced by the TFF
method described in
Example 15. Fresh platelets in Platelet Rich Plasma (PRP) were prepared from
whole blood collected in
acid-citrate-dextrose (ACD) collection tubes (BD Vacutainer ACD Solution A
Blood Collection Tubes ref#
364606). Platelet rich plasma (PRP) was prepared by centrifugation of ACD-
whole-blood at 180g for 15
minutes at 22 C using a Beckman Coulter Avanti J-15R centrifuge. Platelet poor
plasma (PPP) was
prepared by centrifugation of ACD-whole-blood at 2000g for 20 minutes at 22 C.
129
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00656] For sample preparation for aggregometry studies, PRP was diluted
with PPP to a platelet
concentration (plt count) of 250,000 plts/uL. Platelet count was determined
using a Coulter Ac=T diff2
Hematology Analyzer. TFF FDPDs, lyophilized and thermally treated, were
prepared using tangential flow
filtration as described in Example 15. A 30mL vial of FDPDs was rehydrated
using 30 mL of cell culture
grade water (Corning Cat# 25-055-CI). The vial was incubated at room
temperature for a total of 10
minutes. During the 10-minute rehydration period, the vial was gently swirled
at 0, 5, and 10 minutes to
promote dissolution of the lyophilizate. The aggregometry studies as per the
present Example was carried
out in the absence of fresh platelets. Therefore, the aggregometry studies
supported only aggregation ability
of the FDPDs, but not the co-aggregation ability. For sample preparation for
aggregometry studies,
rehydrated FDPDs were diluted in a buffer to a platelet count of 250,000/ L.
FDPDs sample preparations
used for ristocetin aggregation studies were composed of 20% citrated plasma
(George King Bio-Medical,
Inc. Pooled Normal Plasma product# 0010-1) and buffer. Light transmission
aggregometry (LTA)
(Bio/Data PAP-8E Platelet Aggregometer catalog# 106075) at 37 C was used to
observe the aggregation
response of FDPDs (Fig. 44A) and PRP samples (Fig. 44B) from a final
concentration of 20 M ADP, 10
tig/mL collagen, 200 M epinephrine (ADP, collagen, and epinephrine reagents
from Helena Laboratories
Platelet Aggregation Kit cat. # 5369), 0.5 mg/mL arachidonic acid (Helena
Arachidonic Acid Reagent cat.),
lmg/mL ristocetin (Helena Ristocetin for Aggregation Assays cat.), and 10 M
thrombin receptor activator
peptide 6 (TRAP-6) (Sigma Aldrich Cat# T1573-5MG). PPP, buffer, or buffer with
20% citrated plasma
were used as blanks for the PRP, FDPDs, and FDPDs with 20% citrated plasma
samples, respectively. Prior
to agonist treatment, 2251iL of FDPDs or PRP sample was reverse pipetted in a
test tube containing a stir
bar. The test tube was then placed into the aggregometer's non-stirred
incubation well for 1 minute. The
sample was then placed into a stirred incubation well for 1 minute. The sample
was then placed into the
stirred test well and the aggregation test was initiated. After 1-minute of
baseline observation the sample
was treated with agonist and the aggregation response was recorded. Using the
same procedure as the test
runs, a negative control of 25 L buffer was included simultaneously with all
runs to determine spontaneous
baseline-aggregation responses of all sample groups.
[00657] FDPD sample preparations in 1.7 mL microcentrifuge tubes, at room
temperature, were
treated with an agonist at a final agonist concentration of 20 M ADP, 0.5
mg/mL arachidonic acid, 10
tig/mL collagen, 200 M epinephrine, lmg/mL ristocetin, and 10 M TRAP-6 or 25
L buffer. FDPD
counts were determined prior to and 5-minutes after agonist treatment. ADP
(Fig. 44C), collagen (Fig.
44D), epinephrine (Fig. 44E), ristocetin (Fig. 44F), and TRAP-6 (Fig. 44G) did
not cause an aggregation
response in TFF FDPDs when measured by LTA. TFF FDPDs' response from the
aforementioned agonists
was equivalent to baseline aggregation values that would be obtained from no
agonist or a negative control
of buffer. When TFF FDPDs were treated with arachidonic acid (AA) and observed
by LTA (Fig. 44H)
130
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
there was an apparent aggregation response, however after visual inspection of
the aggregometry cuvette it
was observed that the solution had become visibly clear and aggregates were
not observed, indicating that
the apparent aggregation response was from lysis of FDPDs and not AA induced
aggregation. Determining
aggregation by cell count for TFF FDPDs produced similar results to the LTA
results for all agonists.
Agonists' functionality was confirmed by performing LTA on fresh PRP (Fig.
44B). ADP, arachidonic
acid, collagen, epinephrine, ristocetin and TRAP-6 caused normal aggregation
profiles and magnitudes that
are representative of a strong aggregation response in PRP. The aggregation
response from epinephrine in
PRP was reduced, however epinephrine was still able to elicit an aggregation
response that was above
baseline aggregation. The negative control of buffer in PRP indicated that the
PRP was not activated prior
to agonists additions. Visual inspection of the PRP samples after the
aggregation tests indicated that no cell
lysis had occurred and platelet aggregates were visually observed in the
aggregation cuvettes for all
agonists, indicating that all aggregations responses were from platelet
aggregation. The aggregation
percentage of FDPDs and fresh PRP observed in the presence of the afore-
mentioned agonists have been
captured in Table 6.
[00658] Table 6
TFF FDPDs PRP %Aggregation
Agonist
%Aggregation (n=3) (n=2)
20 uM ADP 1% 66%
0.5 mg/mL
28%* 73%
Arachidonic Acid
ug/mL Collagen 2% 83%
300 uM
1% 11%
Epinephrine
1 mg/mL
0% 98%
Ristocetin
101JM TRAP-6 1% 73%
25 uL Buffer 1% 2%
* - Due to lysis of FDPDs and not aggregation
131
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00659] Example 25. FDPDs are maximally activated - Binding of Annexin V
to FDPDs in the
presence of TRAP
[00660] FDPDs, prepared using the TFF process and treated with TRAP-6,
were tested for the
presence of phosphatidylserine (PS), indicative of an activated platelet, on
the surface of the FDPDs. The
presence of PS was assessed by analysis of Annexin V (AV) binding to the
FDPDs.
[00661] One 30 mL vial of FDPDs prepared using the TFF process as
described in the Example 15
was rehydrated using 30 mL of cell culture grade water (Corning Cat# 25-055-
C1). After water was added
to the vial, the vial was incubated for 10 minutes at room temperature. Gentle
swirling of the vial was
performed every 2 minutes during the 10-minute period to promote full
dissolution of the cake. Once the
FDPDs were fully rehydrated, two 475 L aliquots were transferred to two
separate 1.7 mL microcentrifuge
tubes. Twenty-five microliters of HEPES Modified Tryode's Albumin buffer
(HMTA) (Cellphire RGT-
004) was added to the sample in the first tube to generate FDPDs without TRAP-
6. Twenty-five microliters
of 400 M Thrombin Receptor Activating Peptide 6 (TRAP-6) (Sigma Aldrich Cat#
T1573-5MG) was
added to the second tube to generate FDPDs with TRAP-6. The final
concentration of TRAP-6 during
incubation was 20 M. Both tubes were inverted 5 times to mix and incubated at
room temperature for 10
minutes.
[00662] After incubation with HMTA buffer or TRAP-6, the samples were
further diluted 1:20 by
adding 10 L of the FDPD sample to 190 L HMTA. These diluted samples of FDPDs
incubated with
HMTA and FDPDs incubated with TRAP-6 were both stained in 1.7 mL
microcentrifuge tubes as follows:
unstained control samples were generated by combining 10 L of FDPDs and 20 L
HMTA; calcium free
control samples were generated by combining 10 L of FDPDs, 5 L of Annexin V
¨ ACP (BD Pharmingen
Cat# 550475), and 15 L HMTA; Annexin V (AV) stained test samples were
generated by combining 10
L of FDPDs, 5 L of AV ¨ ACP ,and 15 L HMTA supplemented with 9 mM CaCl2
(Cellphire RGT-012
Lot# LAB-0047-21). The final concentration of CaCl2 in the AV-stained test
samples was 3 mM. All stained
samples for both FDPDs incubated with HMTA and FDPDs incubated with TRAP-6
were generated in
triplicate. The samples were incubated at room temperature, protected from
light, for 20 minutes.
[00663] After incubation, 500 L of HEPES buffered saline (HBS) (Cellphire
RGT-017) was added
to all unstained control and calcium free control samples. Five hundred
microliters of HBS supplemented
with 3 mM CaCl2 was added to the AV-stained test samples. One hundred
microliters from each sample
was transferred to an individual well in a 96 well plate, and the samples were
analyzed using an Agilent
Quanteon flow cytometer.
[00664] TRAP-6 activity was confirmed by measuring CD62P expression in
human apheresis
platelets with and without exposure to TRAP-6. Two 475 L aliquots of
apheresis platelets were transferred
to two separate 1.7 mL microcentrifuge tubes. Twenty-five microliters of HMTA
buffer was added to the
132
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
sample in the first tube to generate apheresis platelets without TRAP-6.
Twenty-five microliters of 400 M
TRAP-6 was added to the second tube to generate FDPDs with TRAP-6. The final
concentration of TRAP-
6 during incubation was 20 M. Both tubes were inverted 5 times to mix and
incubated at room temperature
for 10 minutes.
[00665] After incubation with HMTA buffer or TRAP-6, the samples were
further diluted 1:20 by
adding 10 L of apheresis platelets to 190 L HMTA. These diluted samples of
apheresis platelets
incubated with HMTA and apheresis platelets incubated with TRAP-6 were both
stained in 1.7 mL
microcentrifuge tubes as follows: unstained control samples were generated by
combining 10 L of
apheresis platelets and 20 L HMTA; Anti-CD62P stained test samples were
generated by combining 10
L of apheresis platelets, 5 L of anti-CD62P-PE antibody (BD Pharmingen Cat#
550561 Lot# 6322976),
and 15 L HMTA. All stained samples for both apheresis platelets incubated
with HMTA and apheresis
platelets incubated with TRAP-6 were generated in triplicate. The samples were
incubated at room
temperature, protected from light, for 20 minutes.
[00666] After incubation, 500 L of phosphate buffered saline (PBS)
(Corning Cat# 21-040-CV1)
was added to all samples. One hundred microliters from each sample was
transferred to an individual well
in a 96 well plate, and the samples were analyzed using an Agilent Quanteon
flow cytometer.
[00667] FDPDs manufactured using the TFF process were incubated with
either TRAP-6 or buffer
and stained with Annexin V (AV) to determine the relative presence of
phosphatidylserine (PS). Apheresis
platelets were used to confirm TRAP-6 activity (Fig. 45A, and Table 7), and
increased expression of CD62P
after the exposure to TRAP-6 confirms that TRAP-6 is capable of promoting
platelet expression. PS
expression on the exterior membrane leaflet is a hallmark of platelet
activation and increases in membrane
expression of PS result in greater amounts of AV binding. Unstained samples
and samples stained with AV
but without the addition of calcium were analyzed on the flow cytometer as
negative controls. Unstained
samples generated little to no fluorescent signal, indicating that FDPDs were
not auto fluorescent at the
wavelength selected to measure AV (Fig. 45B). The calcium free control samples
also generated little to
no fluorescent signal. Since AV binding to PS is dependent on the presence of
calcium ions, a lack of signal
from the calcium free control samples demonstrates that the AV-ACP conjugate
was not associating with
the FDPD membrane in a nonspecific manner. All samples stained with AV in the
presence of calcium
provided a strong fluorescent signal that was, on average, approximately 695
times brighter than the
unstained controls. This result indicates that all FDPDs samples were
expressing, or comprised, PS.
Incubating the FDPDs with TRAP-6 did not cause a notable increase in AV
binding as measured by mean
fluorescent intensity (MFI) (Fig. 45B). The average MFI values for FDPDs
incubated with buffer and
FDPDs incubated with TRAP-6 were 68,179 and 68,783, respectively (Table 8).
[00668] Table 7
133
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
Apheresis Platelet CD62P MFI
Sample Type - TRAP +TRAP
Unstained 100 107
CD62P Stained 2,351 126,598
[00669] Table 8
FDPD Annexin V MFIs
Sample Type - TRAP +TRAP
Unstained 98 99
Calcium Free Control 203 198
AV Stained 68,179 68,783
[00670] FDPDs, manufactured using the TFF process, were shown to contain
phosphatidylserine
(PS) on the membrane as evident by the binding of Annexin V (AV) to the FDPDs.
The binding of AV to
activated platelets is a calcium dependent binding and therefore the calcium
ion dependency of AV binding
to the rehydrated FDPDs provides further support that the AV conjugate was not
associating with the
membrane of the FDPD in a nonspecific manner.
[00671] While TRAP-6 was shown to activate apheresis platelets, as evident
by increased CD62P
expression, and increased the binding of AV to the activated platelet, it was
not the case for the FDPDs.
The FDPDs with or without a TRAP-6 incubation exhibited same high level of AV
binding, and indicate
that TRAP-6 does not promote further surface expression of PS for FDPDs,
likely because the FDPDs are
maximally activated during the lyophilization and/or rehydration process, and
further stimulation/activation
is not possible.
[00672] Example 26. Presence of Thrombospondin (TSP1) on the surface of
the FDPDs
[00673] Thrombospondin (TSP1), a glycoprotein typically found to coat
external membranes of
activated platelets, was found to coat FDPDs without activation. The presence
of TSP1 was detected by
fluorescence of anti-Thrombospondin-1 (TSP-1) antibody.
134
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00674] Fresh platelet rich plasma (PRP) was isolated by centrifuging
whole blood collected in acid
citrate dextrose (ACD) at 180g for 10 minutes. Isolated PRP was centrifuged
again at 823g for an additional
minutes. The plasma was then removed and discarded, and the platelet pellet
was resuspended in HEPES
Modified Tyrode' s Albumin (HMTA) buffer. An aliquot of the resulting washed
platelet sample was
activated by incubated the platelets at room temperature for 10 minutes in the
presence of 2 mM GPRP
peptide (BaChem Cat# H-1998.0025), 2 mM CaCl2, 0.5 U/mL thrombin (EDM
Millipore Cat# 605190-
1000U), and 0.5 tig/mL collagen (ChronoPar Cat# 385). A separate aliquot of
washed platelets was set
aside to be used as a resting negative control.
[00675] All samples of FDPDs were manufactured using the TFF process as
described in Example
15. The FDPDs studied in this example were baked FDPDs which were heated after
lyophilization at 80 C
for 24 hours. All vials were rehydrated using the appropriate amount of cell
culture grade water. After water
was added, the vials were incubated for 10 minutes at room temperature. Gentle
swirling of the vials was
performed every 2 minutes during the 10-minute period to promote full
dissolution of the cake. Once
rehydrated, samples of FDPDs from each vial, along with samples from both the
resting and activated fresh
washed platelet aliquots, were diluted 1:500 in triplicate using phosphate
buffered saline (PBS) (Corning
Cat# 21-040-CV). The diluted samples were analyzed on the Quanteon flow
cytometer and the
concentrations of the platelets and FDPDs were determined. Based on these
concentrations, an aliquot of
each FDPDs or fresh platelet sample was diluted to a concentration of 100,000
FDPDs per microliter.
[00676] Stained samples from each vial of FDPDs and the resting and
activated fresh platelets were
generated by adding 10 tit of diluted FDPDs or platelets to 20 tit of HMTA
containing 4 tig/mL of anti-
Thrombospondin-1 (TSP-1) antibody (Santa Cruz Biotech Cat# sc-59887 AF594).
Unstained control
samples were generated by adding 10 tiL of diluted FDPDs or platelets to 20
tiL of HMTA. All The samples
were incubated at room temperature, protected from light, for 20 minutes.
After incubation, 500 tit of PBS
was added to all samples. One hundred microliters from each sample were
transferred to an individual well
in a 96 well plate, and the samples were analyzed using an Agilent Quanteon
flow cytometer.
[00677] Unstained samples of fresh platelets and FDPDs generated little to
no fluorescent signal,
indicating that the samples were not auto fluorescent at the wavelength
selected to measure TSP-1
expression or presence. Binding of the anti-TSP-1 antibody to fresh platelets
increased slightly after
activation with collagen and thrombin as shown by an increase in mean
fluorescent intensity (MFI) when
analyzed using flow cytometry (1,223 vs 3,306). Expression or presence of TSP-
1 on FDPD samples varied
from lot to lot with an average MFI value of 91,448 (Fig. 46). For all FDPD
samples tested, the fluorescent
signal was significantly higher than the signal generated by either resting or
fresh platelets, indicating high
amounts of TSP-1 may be bound to the surface of rehydrated FDPDs. This data
suggests that the FDPDs
135
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
without the requirement of an activation step exhibit properties which in
certain embodiments and
applications are superior to activated platelet properties.
[00678] Example 27. Presence of von Willebrand factor (vWF) on the surface
of the FDPDs
[00679] Fresh platelet rich plasma (PRP) was isolated by centrifuging
whole blood collected in acid
citrate dextrose (ACD) at 180g for 10 minutes. Isolated PRP was centrifuged
again at 823g for an additional
minutes. The plasma was then removed and discarded, and the platelet pellet
was resuspended in HEPES
Modified Tyrode' s Albumin (HMTA) buffer. An aliquot of the resulting washed
platelet sample was
activated by incubating the platelets at room temperature for 10 minutes in
the presence of 2 mM GPRP
peptide (BaChem Cat# H-1998.0025), 2 mM CaCl2 ), 0.5 U/mL thrombin (EDM
Millipore Cat# 605190-
1000U), and 0.5 tig/mL collagen (ChronoPar Cat# 385). A separate aliquot of
washed platelets was set
aside to be used as a resting negative control. All samples of FDPDs were
prepared using the TFF process
as described in the Example 15. The FDPDs studied in this example were baked
FDPDs which were heated
after lyophilization at 80 C for 24 hours. All vials were rehydrated using the
appropriate amount of cell
culture grade water (Corning Cat# 25-055-CI). After water was added, the vials
were incubated for 10
minutes at room temperature. Gentle swirling of the vials was performed every
2 minutes during the 10-
minute period to promote full dissolution of the cake. Once rehydrated,
samples of FDPDs from each vial,
along with samples from both the resting and activated fresh washed platelet
aliquots, were diluted 1:500
in triplicate using phosphate buffered saline (PBS). The diluted samples were
analyzed on the Quanteon
flow cytometer and the concentrations were determined. Based on these
concentrations, an aliquot of each
FDPDs or fresh platelet sample was diluted to a concentration of 100,000 FDPDs
per microliter.
[00680] Prior to staining, the anti-Von Willebrand Factor antibody (Novus
Biologicals Cat# NBP2-
54379PE) was diluted by a factor of 10. Stained samples from each vial of
FDPDs and the resting and
activated fresh platelets were generated by adding 10 tit of diluted FDPDs or
platelets to 10 tit of diluted
antibody and 10 tiL of HMTA. Unstained control samples were generated by
adding 10 tiL of diluted
FDPDs or platelets to 20 tiL of HMTA. All The samples were incubated at room
temperature, protected
from light, for 20 minutes. After incubation, 500 tit of PBS was added to all
samples. One hundred
microliters from each sample was transferred to an individual well in a 96
well plate, and the samples were
analyzed using an Agilent Quanteon flow cytometer.
[00681] Unstained samples of fresh platelets and FDPDs generated little to
no fluorescent signal,
indicating that the samples were not auto fluorescent at the wavelength
selected to measure vWF expression
or presence. Binding of the anti-vWF antibody to fresh platelets increased
after activation with collagen
and thrombin as shown by an increase in mean fluorescent intensity (MFI) when
analyzed using flow
cytometry (4,771 vs 19,717). Expression or presence of vWF on FDPD samples
varied from lot to lot with
136
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
an average MFI value of 13,991 (Fig. 47). For all FDPD samples tested, the
fluorescent signal fell between
the signals generated by resting and activated platelets. This suggests that
vWF is present on the surface of
rehydrated FDPDs, and that the amount of vWF present is greater than that seen
on resting platelets. The
data suggests that even in the absence of any activation, the FDPDs exhibit
properties that is superior to
resting platelets and similar to the activated platelets.
[00682] Example 28. FDPDs - compromised membrane
[00683] Membrane integrity of FDPDs, either heated at 80 C for 24hours
(baked FDPDs) or not
heated (unbaked FDPDs) after lyophilization, was tested. The baked and unbaked
FDPDs of the standard
formulation were analyzed by forward scatter against pre-lyophilization
material and by the use of an
antibody against a stable intracellular antigen, I3-tubulin, to determine if
FDPDs were permeable to IgGs
(150 kDa). Forward scatter is a flow cytometry measurement of laser scatter
along the path of the laser.
Forward scatter (FSC) is commonly used as an indication of cell size as larger
cells will produce more
scattered light. However, forward scatter also can indicate the membrane
integrity of the sample via optical
density (i.e., light transmission); a cell with less cytosolic material and a
porous membrane would transmit
more light (have a lower FSC) than the same cell if intact, despite being the
same size.
[00684] The FDPDs of Example 15 were studied to determine if FDPDs were
permeable to IgGs
(150 kDa) by the use of an antibody against a stable intracellular antigen, I3-
tubulin. Fresh platelets, unbaked
FDPDs, and baked FDPDs were fixed and stained with anti-I3 tubulin IgG with
and without cell
permeabilization. Fresh platelets showed a dramatic increase in IgG binding
with permeabilization, whereas
both baked and unbaked FDPDs showed no change in response to permeabilization
(Table 9). Results from
fresh platelets and FDPDs that were fixed and then either permeabilized with
0.2% Triton-X 100 or not
permeabilized and then stained with anti-I3 tubulin IgG conjugated to the
fluorophore AF594. Unstained
samples are included for background fluorescence.
[00685] Table 9
Sample Mean FSC-H AF594
MFI
Platelets Unstained 120,301 115
Platelets 118,782 636
Permeabilized Platelets 49,062 9,009
Unbaked FDPDs Unstained 23,140 75
Unbaked FDPDs 23,280 546
Permeabilized Unbaked 7,069 562
FDPDs
Baked FDPDs Unstained 49,740 362
Baked FDPDs 49,587 2,720
Permeabilized Baked FDPDs 27,527 2,523
137
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
[00686] The IgG binding studies suggest that the membrane integrity of
FDPDs is severely
impaired such that large molecules can pass through the cell membrane. Of
additional note,
permeabilization induced decreases in forward scatter value, corroborating the
proposed relationship
between membrane integrity and optical density for particles of the same size.
[00687] Additionally, the mean intensity of forward light scattering of
FDPDs prepared by TFF
method as described in Example 15 was compared to in-date human platelet
apheresis units. The method is
as described below.
[00688] All samples of FDPDs were manufactured using the TFF process. All
vials were rehydrated
using the appropriate amount of cell culture grade water (Corning Cat# 25-055-
CI). After water was added,
the vials were incubated for 10 minutes at room temperature. Gentle swirling
of the vials was performed
every 2 minutes during the 10-minute period to promote full dissolution of the
cake. Once rehydrated,
samples of FDPDs from each vial, along with samples from both in-date human
platelet apheresis units,
were diluted 1:500 in triplicate using phosphate buffered saline (PBS)
(Corning Cat# 21-040-CV). The
diluted samples were acquired on the Quanteon flow cytometer and the
concentrations were determined.
Based on these concentrations, an aliquot of each FDPDs or apheresis platelet
sample was diluted to a
concentration of 100,000 FDPDs per microliter in HEPES Modified Tyrode's
Albumin (HMTA) buffer
(Cellphire RGT-004).
[00689] Unstained samples of FDPDs and human apheresis platelets
containing 106 total cells in
HMTA were diluted with 500 tiL of PB. One hundred microliters from each sample
were transferred to an
individual well in a 96 well plate, and the samples were analyzed using an
Agilent Quanteon flow
cytometer.
[00690] The mean intensity is depicted in Fig. 48. It can be observed that
the mean intensity of
forward light scattering measured with flow cytometry is distinctly lower
(about 50%) for FDPDs as
compared to the apheresis plasma. Therefore, corroborating with the previous
result of Table 9 that a cell
with less cytosolic material and a porous membrane would transmit more light
(have a lower FSC) than the
same cell if intact, despite being the same size.
[00691] The overall results suggest that membrane integrity is
substantially degraded in FDPDs;
the platelet intracellular contents have been released (e.g. LDH) and large
molecules can enter the cellular
cytosol (e.g. anti I3-tubulin IgG). The plasma membrane of FDPDs is likely
damaged by the drying
(sublimation) or rehydration processes as freezing in cryopreserved platelets
appears to be insufficient to
induce severe membrane dysfunction. These results also imply that signal
transduction from the outside of
the cell is not possible in FDPDs, which is corroborated by lack of
aggregation response (as observed in
Example 24). Baking, although it produced an increase in optical density, did
not appear to improve
138
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
membrane integrity significantly (e.g., IgG I3-tubulin binding). The results
discussed in the present example
thus show that the platelet derivatives as disclosed herein have a compromised
plasma membrane.
[00692] Example 29. Surface Markers and Thrombin Generation.
[00693] FDPDs batch were produced by the TFF method described in Example
15 and assayed for
cell surface marker expression or presence or absence using flow cytometry.
[00694] Flow cytometry was used to assess FDPDs for expression or presence
or presence of CD41,
CD62, and phosphatidylserine (PS). Samples included approximately 270,000/ L
FDPDs during staining
and were diluted approximately 1:34 before the sample was analyzed in the
cytometer. FDPD samples
were rehydrated and diluted 1:2 in deionized water. A stock of anti-CD41 was
diluted by adding 47.6 tit
of antibody to 52.4 tiL of HMTA. Samples stained with anti-CD41 were made by
adding 10 tit of diluted
FDPDs to 10 tit HMTA and 10 tit of diluted CD41 antibody. An anti-CD62 master
mix was prepared by
combining 12 tit anti-CD62 with 23.8 tiL anti-CD41 and 64.2 tit of HMTA. An
isotype control mix was
made in the same manner. Samples stained with anti-CD62 were made by adding 10
tit of diluted FDPDs
to 20 tit of the anti-CD62 master. The isotype master mix was used to make
isotype control samples in the
same manner. An annexin V (AV) master mix was prepared by combining 11.7 tiL
of AV with 83.3 tit of
anti-CD41 and 80 tit of HMTA. Sample stained with AV were made by adding 20
tiL of diluted FDPDs
containing 50 mM GPRP to 20 tit of HMTA containing 15 mM CaCl2 and 20 tiL of
the AV master mix.
Negative gating control samples were made in the same manner using HMTA
without calcium to prevent
AV binding to PS. All samples were incubated at room temperature for 20
minutes. After incubation 1 mL
HBS was added to all samples. HBS used to dilute AV test samples contained 5
mM CaCl2. Anti-CD41
binding was used to identify the population of interest. CD62 and PS
expression or presence was assessed
by anti-CD62 and AV binding within the CD41 positive population.
[00695] Glycoprotein IIb (GPIIb, also known as antigen CD41) expression or
presence was assayed
using an anti-CD41 antibody (4.8 viL, Beckman Coulter part #IM1416U). The
assayed FDPDs
demonstrated CD41 positivity (Table 10; Fig. 49)
[00696] Table 10.
Batch CD41 Positivity (%)
1 81.5
2 79.4
3 85.7
4 78.2
81.5
6 84.0
7 78.5
139
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
Mean 81.3
[00697] Phosphatidylserine (PS) expression or presence was assayed using
annexin V (AV) (1.3
tiL, BD Biosciences Cat. No. 550475). AV is a calcium-dependent phospholipid
binding protein. The
assayed FDPDs demonstrated AV positivity (Table 11; Fig. 50).
[00698] Table 11.
Batch AV Positivity (%)
1 96.7
2 89.9
3 95.3
4 95.4
95.9
6 96.2
7 93.5
Mean 94.7
[00699] P-selectin (also called CD62P) expression or presence was assayed
using an anti-
CD62P antibody (2.4 uL, BD Biosciences Cat. No. 550888). The assayed FDPDs
demonstrated
CD62 positivity (Table 12, Fig. 51)
[00700] Table 12.
B Datch C 62 Positivity (%)
1 94.2
2 93.1
3 89.8
4 92.4
5 92.5
6 87.3
7 90.7
Mean 91.4
[00701] Thrombin generation was measured at 4.8x103 FDPDs/ 1 in the
presence of PRP Reagent
containing tissue factor and phospholipids using the below protocol. On
average, the Thrombin Peak Height
(TPH) for a FDPDs sample was 60.3 nM. Cephalin was used as a positive control.
(Table 13; Fig. 52)
[00702] For each vial tested, a rehydrated sample of FDPDs was diluted to
7,200 particles per tiL
based on the flow cytometry particle count using 30% solution of Octaplas in
control buffer. In a 96 well
plate, sample wells were generated by adding 20 tiL of PRP reagent
(Diagnostica Stago Catalog No. 86196)
and 80 tiL of diluted FDPDs. Calibrator wells were generated by adding 20 tiL
of Thrombin Calibrator
140
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
reagent (Diagnostica Stago Catalog No. 86197) to 80 iuL of diluted FDPDs. The
plate was loaded into the
plate reader and incubated in the dark at 40 C for 10 minutes. During sample
incubation, FluCa solution
was prepared by adding 40 iuL of FluCa substrate (Diagnostica Stago Catalog
No. 86197) to 1.6 mL of
Fluo-Buffer (Diagnostica Stago Catalog No. 86197) warmed to 37 C and vortexed
to mix. The FluCa
solution was aspirated in to the dispensing syringe and 20 iut was
mechanically dispensed in to each
reaction well, bringing the final FDPDs concentration in each well to 4,800
particles per iuL and starting
the thrombin generation reaction. Thrombin generation was measured via
fluorescence in each well over
the course of 75 minutes.
[00703] An exemplary step-by-step protocol follows:
[00704] Open CAT software; set up instrument; and prepare PRP reagent
(including Tissue Factor
and some phospholipids), calibrator, and fluo-buffer and fluo-substrate
according to manufacturer
guidelines.
[00705] Thaw Octaplas and TGA dilution buffer in 37 C water bath for 10
minutes.
[00706] Add thawed Octaplas to TGA dilution buffer to create a buffer
containing 30% Octaplas.
[00707] Use the 30% Octaplas mix to dilute reconstituted cephalin 1:50 to
be used as a positive
control.
[00708] Rehydrate FDPDs with cell culture grade water for 10 minutes then
dilute with 30%
Octaplas to 7,200 FDPDs/ L.
[00709] Using a multichannel pipette, add 20 iuL of PRP reagent to each
test well. Add 20 iut of
Calibrator to each calibration well.
[00710] Add 80 iuL of sample to each test and calibration well. Add 80 iut
of 30% Octaplas to
negative control wells and 1:50 cephalin to positive control wells.
[00711] Insert plate into tray and incubate for 10 minutes at 40 C. After
incubation, dispense fluo-
buffer and fluo-substrate mixture (including a fluorescent-labeled peptide,
that when cleaved by thrombin,
generates a fluorescent signal) into active wells.
[00712] Read plate for 75 minutes at 20 s intervals to capture full
thrombin generation profile.
[00713] Table 13.
Batch TPH (nM)
1 61.5
2 71.4
3 67.8
4 52.0
60.2
6 54.7
141
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
7 54.4
Mean 60.3
[00714] Data from these assays is summarized in Table 14.
[00715] Table 14.
TFF Batches
Average AV Average CD62
Average Average CD41 Positivity (0.5iim - Positivity
(0.5iim -
Batch TPH (nM) Positivity 2.5m)1 2.5m)1
Batch B 61.5 81.5 96.7 94.2
Batch C 71.4 79.4 89.9 93.1
Batch D 67.8 85.7 95.3 89.8
Batch E 52.0 78.2 95.4 92.4
Batch F 60.2 81.5 95.9 92.5
Batch G 54.7 84.0 96.2 87.3
Batch H 54.4 78.5 93.5 90.7
Mean 60.3 81.3 94.7 91.4
[00716] 'Particle diameter as assessed using sizing beats on the flow
cytometry forward scatter.
[00717] Example 30. Microparticle Content Reduction
[00718] The microparticle content of human in-date stored platelets
(hIDSP) compared to FDPDs
prepared according to Example 15 (but not lyophilized) were compared using
dynamic light scattering. The
results are shown in Figures 53A-C and Table 15. Figures 53A-C are histograms
that are normalized to a
relative intensity so that the sum of the intensity of each data point equals
1Ø For example, if a particular
data point has a y-axis value of 0.1 then it can be typically interpreted that
the data point makes up 10% of
the scattering intensity of the sample.
[00719] A pool of the apheresis units used to manufacture a batch of FDPDs
was made for analysis.
This sample type is denoted as "hIDSP." A lmL aliquot of this hIDSP (human In-
Date Stored Platelets)
pool was taken for dynamic light scattering (DLS; Thrombolux - Light Integra)
analysis. A sample from
this aliquot was then drawn into a capillary and inserted into the DLS
instrument. The capillary sat in the
instrument for 1 minute to allow the temperature and movement to equilibrate.
The internal temperature of
the machine is 37 C. After 1 minute of equilibration, the viscosity setting
for the sample was chosen. The
DLS instrument has a built-in viscosity setting for samples that are in
plasma, such as apheresis units. This
viscosity setting was used for hIDSP samples. The viscosity of this setting is
1.060cP (centipoise). After
the plasma viscosity setting was selected, the sample was analyzed. From the
same hIDSP aliquot, a 2' and
3rd sample were drawn into a capillary and analyzed with this hIDSP protocol,
for triplicate analysis.
142
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
Microparticle percentage was then determined from the data."Pre-Lyo" samples
are an in-process sample
from the FDPDs manufacturing process. This sample type is the material taken
right before lyophilization.
A viscosity measurement of the sample was taken in order to analysis these
samples with DLS. The
viscometer (Rheosense tiVISC) has a built-in oven that is used to bring the
sample to the temperature of
the DLS instrument (37 C). Prior to viscosity analysis of the sample the oven
must be heated to 37 C. To
determine the viscosity of the pre-lyo sample a 400-350 L sample was drawn
into a syringe and inserted
into the viscometer. After inserting the sample into the viscometer, the
instrument temperature needs to
reach 37 C again. After the oven reaches 37 C the sample was analyzed with all
settings on AUTO except
for "Measurement Volume" which was set to 400 L. This viscosity was used for
the DLS measurement of
the same sample. A lmL aliquot of this pre-lyo sample was taken for dynamic
light scattering (DLS;
Thrombolux - LightIntegra) analysis. A sample from this aliquot was then drawn
into a capillary and
inserted into the DLS instrument. The capillary sat in the instrument for 1
minute to allow the temperature
and movement to equilibrate. The internal temperature of the machine is 37 C.
After 1 minute of
equilibration, the previously measured viscosity was put into the viscosity
setting of the DLS instrument.
After the viscosity was entered, the sample was analyzed. From the same pre-
lyo aliquot, a 2' and 3'
sample were drawn into a capillary and analyzed with this Pre-Lyo Protocol,
for triplicate analysis.
Microparticle percentage was then determined from the data.
[00720] FDPDs were rehydrated according to standard protocol and diluted
1:5 in a mixture of
SeraSub (CST Technologies, Inc.) and ACD. The SeraSub/ACD diluent consists of
a 1:9 dilution of ACD
in SeraSub. lmL of the 1:5 dilution of FDPDs was prepared for analysis by DLS.
A sample of the FDPDs
dilution was drawn into the capillary and inserted into the DLS instrument.
The capillary sat in the
instrument for 1 minute to allow the temperature and movement to equilibrate.
The internal temperature of
the machine is 37 C. After 1 minute of equilibration, the viscosity setting
for the sample was chosen. The
viscosity used for the sample was 1.200cP. After the viscosity was entered,
the sample was analyzed. A 2',
3rd, and 4th sample were drawn into a capillary and analyzed with this FDPDs
protocol, for quadruplicate
analysis. Microparticle percentage was then determined from the data (and
platelet radius where
applicable).
[00721] Table 15.
Batch Number hIDSP %MP Pre-Lyo %MP
Batch J 9.47% 0.49%
Batch K 7.55% 0.65%
143
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
Batch L 7.73% 0.59%
Average 8.25% 0.58%
[00722] In additional experiments, the microparticle content of human in-date
stored platelets (hIDSP)
compared to rehydrated FDPDs prepared according to Example 15 were compared
using dynamic light
scattering (DLS). The results are shown in Figures 54A-C and Table 16.
[00723] Table 16.
Batch hIDSP FDPDs
Number % MP %MP
Batch D 7.43% 2.82%
B atch E 5.95% 3.40%
Batch F 12.39% 2.37%
Average 8.59% 2.86%
[00724] Example 31. 9F9 and PAC-1 binding.
[00725] Aggregation of activated platelets is mediated by the formation of
the GPIIb/IIIa complex,
which can bind to fibrinogen (also called Factor 1) and form a clot.
GPIIb/IIIa is a platelet fibrinogen
receptor also known as CD41/CD61 complex. In this process, ADP promotes the
active form of the
GPIIb/IIIa complex. Antibody 9F9 binds to fibrinogen associated with the cell
membrane. The presence of
fibrinogen on the cell membrane is thus indicative of FDPDs capable of forming
clots.
[00726] A vial of FDPDs prepared according to Example 15 was rehydrated using
10 mL of deionized
water. An aliquot of FDPDs was diluted to a final concentration of 1 x105
particles/ L using HMTA
(HEPES Modified Tyrode's Albumin). Samples were prepared as shown in Table 17.
Unstained samples
were prepared by adding 10 iuL of diluted FDPDs to 20 iut of HMTA. FITC
isotype control samples were
prepared by adding 10 iuL of diluted FDPDs to 10 iut of the isotype control
antibody (BD Biosciences Cat.
No. 555748) and 10 iut of HMTA. Samples stained with 9F9 were prepared by
adding 10 iuL of diluted
FDPDs to 10 iuL of the 9F9 antibody (BD Biosciences Cat. No. 340507 and 10 iut
of HMTA. Samples
stained with PAC-1 were prepared by adding 10 iut of diluted FDPDs to 5 iuL of
the isotype control
antibody and 15 iuL of HMTA. All samples were prepared in duplicated using a
total of 1 x 106 particles
per reaction mixture. Samples were incubated at room temperature for 20
minutes away from open light.
After incubation, all samples were diluted with lmL of HBS and analyzed using
the ACEA NovoCyte flow
144
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
cytometer. The fluorescent signal generated by PAC-1 was used to determine the
expression or presence of
activated GPIIb/IIIa receptors without bound fibrinogen. The fluorescent
signal from 9F9 was used to
determine binding of fibrinogen to the surface receptors on FDPDs.
[00727] HTMA (HEPES modified Tyrode's albumin).
Component Concentration
(mM, except where
otherwise indicated)
HEPES 9.5
NaCl 145.0
KC1 4.8
NaHCO3 12.0
Dextrose 5.0
Bovine Serum Albumin 0.35% w/v
[00728] Table 17.
Unstained FITC Iso 9F9 PAC-1
Cells (uL) 10 10 10 10
HMTA (uL) 20 10 10 15
Antibody (uL) 0 10 10 5
[00729] The samples were assayed by flow cytometry, and it was
demonstrated that there is surface-
bound fibrinogen post rehydration (Fig. 55), while the anti-PAC-1 antibody
shows no significant binding
(Fig. 56). This is further evidence that the FDPDs prepared by TFF include
fibrinogen bound to the active
form of GPIIb/GPIIIa, as PAC-1 binds to the same complex.
[00730] Although the foregoing description is directed to the preferred
embodiments of the
invention, it is noted that other variations and modifications will be
apparent to those skilled in the art, and
may be made without departing from the spirit or scope of the invention.
Moreover, features described in
connection with one embodiment of the invention may be used in conjunction
with other embodiments,
even if not explicitly stated above. Furthermore, one having ordinary skill in
the art will readily understand
that the invention as discussed above may be practiced with steps in a
different order, and/or with hardware
elements in configurations which are different than those which are disclosed.
Therefore, although the
145
CA 03211079 2023-08-11
WO 2022/178177 PCT/US2022/016866
invention has been described based upon these preferred embodiments, it would
be apparent to those of
skill in the art that certain modifications, variations, and alternative
constructions would be apparent, while
remaining within the spirit and scope of the invention. Embodiments of the
invention so claimed are
inherently or expressly described and enabled herein. In order to determine
the metes and bounds of the
invention, therefore, reference should be made to the appended claims.
146