Note: Descriptions are shown in the official language in which they were submitted.
CELL COMPOSITIONS DERIVED FROM DEDIFFERENTIATED REPROGRAMMED
CELLS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Patent Application No.
13/761,078,
entitled CELL COMPOSITIONS DERIVED FROM DEDIFFERENTIATED
REPROGRAMMED CELLS, filed February 6,2013.
SEOUENCE LISTING IN ELECTRONIC FORM
[0002] A copy of the sequence listing is available from the Canadian
Intellectual
Property Office.
FIELD OF THE INVENTION
[0003] This invention relates generally to the isolation, maintenance,
and use of cell
cultures. More specifically, it relates to cell compositions derived from
induced pluripotent stem
cells.
BACKGROUND
[0004] An important application of pluripotent cells is their use in
cell therapy.
Pluripotent stem cells include, but are not limited to, human embryonic stem
(hES) cells, human
embryonic germ (hEG) cells. Still other types of pluripotent cells exist, for
example,
dedifferentiated mouse and human stem cells, i.e. differentiated somatic adult
cells are
dedifferentiated to become pluripotent-like stem cells. These dedifferentiated
cells induced to
establish cells having pluripotency and growth ability similar to those of ES
cells are also called
"induced pluripotent stem (iPS) cells", "embryonic stem cell-like cells", "ES-
like cells", or
1
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
equivalents thereof. Such
cells are potentially viable alternative pluripotent cells. The
therapeutic application of iPS cells will require demonstrating that these
cells are stable and
show an appropriate safety profile in preclinical studies to treat diabetes
and other diseases.
Reprogramming of differentiated human somatic cells into a pluripotent state
allows for patient-
and disease-specific stem cells. Sec Takahashi, K. et al. Cell, 1-12, 2007 and
Ju, J. et al. Science
2007. Takahashi et al. and Ju et al. each introduced four genes into adult and
fetal/newborn
fibroblasts to generate the iPS cells: 004, Sox2, Klf4 and c-myc by Takahashi
et al.; 0ct4,
Sox2, Nanog and Lin28 by Ju et al. In either case, iPS cells had some
characteristics of hES
cells including, LIES cell morphology, marker expression, prolonged
proliferation, normal
karyotype, and pluripoteney.
[0005]
Although, iPS cells may provide a cell therapy¨based regenerative medicine
without the associated ethical controversy, the differentiation properties of
iPS cells, for
example, differentiation potential and efficiency of the differentiation in
vitro, are still unclear,
and a directed differentiation method for iPS cells has not been demonstrated.
Hence, there is a
need to determine and demonstrate detailed differentiation properties and the
directional
differentiation efficiencies of iPS cells.
SUMMARY OF THE INVENTION
[0006]
Embodiments described herein provide for cell compositions derived from
pluripotent cells, for example, dedifferentiated reprogrammed cells, such as
induced pluripotent
stem (iPS) cells.
[0007] One
embodiment provides for compositions and methods of making an in
vitro cell culture comprising human cells wherein at least about 15% of the
human cells are
definitive endoderm cells, wherein the definitive cells are derived from
dedifferentiated
genetically reprogrammed cells. In one aspect, the definitive endoderm cells
are multipotent
cells that can differentiate into cells of the gut tube or organs derived
therefrom.
[0008]
Another embodiment provides for compositions and methods of making an in
vitro cell culture comprising human cells wherein at least about 15% of the
human cells are
pancreatic-duodenal horneobox factor-1 (PDXI) positive foregut endoderm cells,
wherein the
PDXI positive forcgut endoderm cells arc derived from dedifferentiated
genetically
reprogrammed cells. In one aspect, the PDX1 positive foregut endoderm cells
are PDX1, SOX9,
PROX I and HNF6 co-positive
[0009] A
further embodiment provides for compositions and methods of making an
in vitro cell culture comprising human cells wherein at least about 15% of the
human cells are
2
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
pancreatic-duodenal homcobox factor-1 (PDX1) positive pancreatic progenitor
cells, wherein the
PDX1 positive pancreatic progenitor cells are derived from dedifferentiated
genetically
reprogrammed cells. In one aspect, thc PDX1 positive pancreatic progenitor
cells arc PDX1 and
NKX6.1 co-positive.
[0010] Still another embodiment provides for compositions and
methods of making
an vitro cell culture comprising human cells wherein at least about 15% of the
human cells are
Neurogenin 3 (NGN3) positive endocrine precursor cells, wherein the NGN3
endocrine
precursor cells are derived from dedifferentiated genetically reprogrammed
cells. In one aspect,
the NGN3 positive endocrine precursor cells are NGN3, PAX4 and NKX2.2 co-
positive.
Further embodiments of the cell culture compositions described herein include
in vitro
human pancreatic endoderm cell cultures comprising differentiated cells
derived from
dedifferentiated genetically reprogrammed cells and an ERBB receptor tyrosine
kinase activating
agent.
Additional embodiments described herein relate to a method for producing
insulin. In
some such embodiments, the method comprises the steps of (a) contacting at
least a foregut
endoderm cell culture and/or at least a PDX1 negative foregut endoderm cell
culture derived
from dedifferentiated genetically reprogrammed cells in vitro with an ERBB
receptor tyrosine
kinasc activating agent, thereby producing a pancreatic endoderm cell
population comprising
endocrine cell and non-endocrine cell sub-populations; and (b) transplanting
and maturing the
pancreatic endoderm cell population of step (a) or a cell subpopulation of
step (a) In vivo,
thereby obtaining insulin secreting cells, wherein the insulin secreting cells
secrete insulin in
response to glucose stimulation.
Still other embodiments described herein relate to a method for producing
insulin, the
method comprising the steps of: (a) contacting dedifferentiated genetically
reprogrammed cells
in vitro with a first medium comprising an agent that activates a TGEI3
receptor family member;
(b) culturing, in vitro, the cells of step (a) in a second medium lacking the
agent that activates a
TGE[3 receptor family member, thereby generating at least foregut endoderm
cells and/or at least
PDX1 negative foregut endoderm cells; (c) contacting the cells of step (b)
with an ERBB
receptor tyrosine kinase activating agent, thereby generating a cell
population comprising
endocrine cell and non-endocrine cell sub-populations; and (d) transplanting
and maturing the
cell population of step (c) or a cell subpopulation of step (c) in vivo,
thereby obtaining insulin
secreting cells, wherein the insulin secreting cells secrete insulin in
response to glucose
stimulation.Still other embodiments described herein relate to contacting a
population
comprising at least foregut endoderm cells, at least PDX1 negative foregut
endoderm cells,
3
Date Recue/Date Received 2023-09-08
and/or at least PDX1 positive pancreatic endoderm cells with an ERBB receptor
tyrosine kinase
activating agent, thereby generating a cell population capable of maturing to
glucose-responsive
insulin-secreting cells in vivo..
Still other embodiments described herein relate to contacting a population
comprising at
least foregut endoderm cells, at least PDX1 negative foregut endoderm cells,
and/or at least
PDX1 positive pancreatic endoderm cells with an ERBB receptor tyrosine kinase
activating
agent and a rho-kinase inhibitor, thereby generating a cell population capable
of maturing to
glucose-responsive insulin-secreting cells in vivo.
As used herein, the phrases "at least foregut endoderm cells," at least PDX1
negative
foregut endoderm cells," and "at least PDX1 positive pancreatic endoderm
cells" means that
some of or a portion of the cells in the cell population have differentiated
from iPSC to foregut
endoderm cells or beyond, from iPSC to PDX1 negative foregut endoderm cells or
beyond
and/or from iPSC to PDX1 positive pancreatic endoderm cells or beyond in their
differentiation
toward pancreatic islet cells.
As used herein, the phrases "some of" and/or "a portion of," when drawn to
cells in a
cell population, means that the cell population comprises at least 5%, at
least 10%, at least 15%,
at least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90%, at least 95% or at least more than 95% cells of the specified cell
type.
Aspects of the disclosure pertain to an in vitro human pancreatic endoderm
cell
population in a media comprising an ERBB receptor tyrosine kinase activating
agent.
Aspects of the disclosure pertains to use of a human pancreatic endoderm cell
population
in a media comprising an ERBB receptor tyrosine kinase activating agent for
the preparation of
glucose-responsive insulin secreting cells that secrete insulin in response to
glucose stimulation.
Aspects of the disclosure pertain to a method for generating a cell population
comprising
endocrine cell and non-endocrine cell subpopulations, said method comprising
the steps of: a.
contacting dedifferentiated genetically reprogrammed cells in vitro with a
first medium
comprising an agent that activates a TGFI3 receptor family member; b.
4
Date Recue/Date Received 2023-09-08
culturing, in vitro, the cells of step (a) in a second medium lacking the
agent that activates a
TG93 receptor family member, thereby generating foregut endoderm cells; and c.
contacting the
foregut endoderm cells of step (b) with an ERBB receptor tyrosine kinase
activating agent,
thereby generating a cell population comprising endocrine cell and non-
endocrine cell
subpopulations.
Aspects of the disclosure pertain to use of foregut endoderm cells in
combination with an
ERBB receptor tyrosine kinase activating agent in the preparation of glucose-
responsive insulin
secreting cells that secrete insulin in response to glucose stimulation.
Aspects of the disclosure pertain to use of foregut endoderm cells in
combination with an
ERBB receptor tyrosine kinase activating agent and a rho-kinase inhibitor in
the preparation of
glucose-responsive insulin secreting cells that secrete insulin in response to
glucose stimulation.
Aspects of the disclosure pertain to use of foregut endoderm cells in
combination with an
ERBB receptor tyrosine kinase activating agent in the preparation of a cell
population
comprising endocrine cell and non-endocrine cell subpopulations for maturation
in vivo to
produce glucose-responsive insulin-secreting cells that secrete insulin in
response to glucose
stimulation.
Aspects of the disclosure pertain to use of foregut endoderm cells in
combination with an
ERBB receptor tyrosine kinase activating agent and a rho-kinase inhibitor in
the preparation of a
cell population comprising endocrine cell and non-endocrine cell
subpopulations for maturation
in vivo to produce glucose-responsive insulin-secreting cells that secrete
insulin in response to
glucose stimulation.
Aspects of the disclosure pertain to use of a cell population comprising
endocrine cell and
non-endocrine cell subpopulations for producing insulin secreting cells in
vivo, wherein the
insulin secreting cells secrete insulin in response to glucose stimulation,
wherein the cell
population is generated according to a method comprising: a. contacting
dedifferentiated
genetically reprogrammed cells in vitro with a first medium comprising an
agent that activates a
TG93 receptor family member; b. culturing, in vitro, the cells of step (a) in
a second medium
lacking the agent that activates a TG93 receptor family member, thereby
generating foregut
endoderm cells; and c. contacting the foregut endoderm cells of step (b) with
4a
Date Recue/Date Received 2023-09-08
an ERBB receptor tyrosine kinase activating agent, thereby generating the cell
population
comprising endocrine cell and non-endocrine cell subpopulations.
Aspects of the disclosure pertain to a pancreatic progenitor cell population
comprising
endocrine cell and non-endocrine cell subpopulations, wherein the cell
population is derived
from dedifferentiated human reprogrammed pluripotent stem cells in a medium
comprising an
effective amount of growth factor, wherein the growth factor is an epidermal
growth factor
(EGF) family ligand or a fibroblast growth factor, and at least at least 30%
of the cell population
is the non-endocrine cell subpopulation.
Aspects of the disclosure pertain to use of a cell population as claimed
herein for the
production of insulin secreting cells in vivo, wherein the insulin secreting
cells secrete insulin in
response to glucose stimulation.
Aspects of the disclosure pertain to a device comprising a semi-permeable
membrane,
and a pancreatic progenitor cell population derived from dedifferentiated
genetically
reprogrammed pluripotent mammalian stem cells, wherein the semi-permeable
membrane
encapsulates the pancreatic progenitor cell population.
Aspects of the disclosure pertain to use of a device as claimed herein for the
production
of insulin secreting cells in vivo, wherein the insulin secreting cells
secrete insulin in response to
glucose stimulation.
Aspects of the disclosure pertain to use of endocrine and non-endocrine cell
populations
derived from dedifferentiated genetically reprogrammed pluripotent cells for
production of
insulin secreting cells in vivo, wherein the insulin secreting cells secrete
insulin in response to
glucose stimulation.
Aspects of the disclosure pertain to an in vitro method of producing human
pancreatic
progenitor cells, the method comprising culturing a population of human cells
comprising
foregut endoderm cells in a medium comprising an ErbB receptor tyrosine kinase
activating
agent, thereby generating human pancreatic progenitor cells.
Aspects of the disclosure pertain to use of human pancreatic progenitor cells
for
production of insulin secreting cells in vivo, wherein the insulin secreting
cells secrete insulin in
response to glucose stimulation, and wherein the human pancreatic progenitor
cells are produced
according to a method comprising culturing a population of
4h
Date Recue/Date Received 2023-09-08
human cells comprising foregut endoderm cells in a medium comprising an ErbB
receptor
tyrosine kinase activating agent, thereby generating human pancreatic
progenitor cells.
Aspects of the disclosure pertain to an in vitro human pancreatic endoderm
cell population
comprising differentiated cells derived from dedifferentiated genetically
reprogrammed cells and
an ERBB receptor tyrosine kinase activating agent.
Aspects of the disclosure pertain to a method for producing insulin, said
method
comprising the steps of: a. contacting a foregut endoderm cell culture derived
from dedifferentiated
genetically reprogrammed cells in vitro with an ERBB receptor tyrosine kinase
activating agent,
thereby producing a cell population comprising endocrine cell and non-
endocrine cell
subpopulations; and b. maturing the subpopulations of step (a) in vivo,
thereby obtaining insulin
secreting cells, wherein the insulin secreting cells secrete insulin in
response to glucose
stimulation.
Aspects of the disclosure pertain to a method for producing a cell population
comprising
endocrine cell and non-endocrine cell subpopulations that when matured in vivo
can mature into
insulin secreting cells that secrete insulin in response to glucose
stimulation , said method
comprising the steps of: a. contacting dedifferentiated genetically
reprogrammed cells in vitro with
a first medium comprising an agent that activates a TGFP receptor family
member; b. culturing, in
vitro, the cells of step (a) in a second medium lacking the agent that
activates a TGFP receptor
family member, thereby generating foregut endoderm cells; and c. contacting
the foregut
endoderm cells of step (b) with an ERBB receptor tyrosine kinase activating
agent, thereby
generating a cell population comprising endocrine cell and non-endocrine cell
subpopulations.
Aspects of the disclosure pertain to an in vitro human pancreatic endoderm
cell population
in a media comprising a heregulin.
Aspects of the disclosure pertain to use of a human pancreatic endoderm cell
population in
a media comprising a heregulin for the preparation of glucose-responsive
insulin secreting cells
that secrete insulin in response to glucose stimulation.
Aspects of the disclosure pertain to an in vitro human pancreatic endoderm
cell population
comprising differentiated cells derived from dedifferentiated genetically
reprogrammed cells and
a heregulin.
4c
Date Recue/Date Received 2023-09-08
BRIEF DESCRIPTION OF THE DRAWINGS
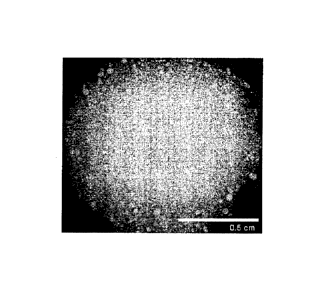
[0011] Figure 1 is a photographic image of an aggregate suspension
culture of
dedifferentiated reprogrammed cells or, also referred to herein, as iPS cells.
[0012] Figures 2A-L are bar graphs showing the relative gene expression
levels of
OCT4 (FIG.2A), BRACHYURY (FIG.2B), CER1 (FIG.2C), GSC (FIG.2D), FOXA2
(FIG.2E),
FOXA1 (FIG.2F), HNF6 (FIG.2G), PDX1 (FIG.2H), PTF1A (FIG.2I), NKX6.1 (FIG.2J),
NGN3
(FIG.2K) and INS (FIG.2L). Expression levels are normalized to the average
expression levels of
housekeeping genes, cyclophilin G and TATA Binding Protein (TBP) expression.
The graphs
depict fold upregulation over the lowest data point in the data set
[0013] Figures 3A-3D are photmicrographs of immunocytochemistry (ICC)
of human
iPS cell cultures from Stage 4 differentiation using antibodies specific for
(3A) PDX-1; (3B)
NKX6.1; (3C) PTF1A; and (3D) Dapi
4d
Date Recue/Date Received 2023-09-08
[0014]
Figures 4A-4D are pictures of immunocytochemistry (ICC) of iPS cell cultures
from Stage 4 differentiation using ligands specific for (4A) Glucagon; (4B)
Insulin; (4C)
Somatostatin; and (4D) Dapi.
Figures 5A-B are an array location map and array key provided in the Proteome
ProfilerTm
human phospho-RTK antibody arrays from R&D Systems. The location map in Figure
5A shows the
coordinates or location of the RTK antibodies. The identity or name of the RTK
family and
antibodies are described in the key, Figure 5B. The positive signals observed
on the developed film
can therefore be identified by overlaying a transparency as in Figure 5A and
identifying the signals
by referring to the coordinates on the overlay (Figure 5A) with the name of
the RTK in Figure 5B.
Figures 6A-D are an RTK array analyses of iPS cell¨derived pancreatic endoderm
cells
(PEC) under four different conditions (A, B, C and D as described in Example
5. Tyrosine
phosphorylation of certain RTKs are observed by the identification of high to
low-intensity signals.
IGF1R/IR and ERBB (EGFR) family members are identified or boxed.
Figures 7A-C are graphs showing the concentrations of human C-peptide and
insulin in sera
of implanted mice for experiments E2314, E2356 and E2380 (FIG.7A), E2347
(FIG.7B), and E2354
(FIG.7C) as indicated in Table 9. Mice implanted with PEC were analyzed at the
indicated post-
engraftment times for serum levels of human C-peptide at fasting, and 30 min
and 60 min after
intraperitoneal glucose administration. In FIG.7C, PEC was encapsulated with
cell encapsulation
devices (Encaptrai EN20, or EN20, ViaCyte, San Diego, CA) and in some
instances the devices had
micro-perforations (pEN20, ViaCyte, San Diego, CA). Such devices have been
described in U.S.
Patent No. 8,278,106.
Figures 8A and 8B are graphs showing the results of blood glucose analyses of
STZ-treated
mice for Experiment 12347. FIG.8A shows the blood glucose for each of the 13
mice (baseline with
and without heregulin) and FIG.8B shows the combined average measurements for
each treatment
(baseline with and without heregulin). Measurements of random non-fasting
blood glucose levels are
shown for the 13 mice implanted with iPEC grafts up to 14 days before they
were treated with STZ
(day 0), and for the same mice after STZ treatment and after the grafts were
explanted. STZ-treated
animals were given STZ about 26 weeks post graft transplant (day 0). At 28
weeks post graft
transplant, approximately 2 weeks after initiation of STZ-treatment, the iPEC
grafts were explanted
(removed). Nonfasting blood glucose measurements were collected over time for
each of the
animals.
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
DETAILED DESCRIPTION OF THE INVENTION
[0015] The present invention may be understood more readily by
reference to the
following detailed description of the preferred embodiments of the invention
and the Examples
included herein. However, before the present compounds, compositions, and
methods are
disclosed and described, it is to be understood that this invention is not
limited to specific cell
types, specific feeder cell layers, specific conditions, or specific methods,
etc., and, as such, may
vary. Numerous modifications and variations therein will be apparent to those
skilled in the art.
It is also to be understood that the terminology used herein is for the
purpose of describing
specific embodiments only and is not intended to be limiting.
Definitions
[0016] It will be appreciated that the numerical ranges expressed
herein include the
endpoints set forth and describe all integers between the endpoints of the
stated numerical range.
[0017] Unless otherwise noted, the terms used herein are to be
understood according
to conventional usage by those of ordinary skill in the relevant art. Also,
for the purposes of this
specification and appended claims, unless otherwise indicated, all numbers
expressing quantities
of ingredients, percentages or proportions of materials, reaction conditions,
and other numerical
values used in the specification and claims, are to be understood as being
modified in all
instances by the term "about." Accordingly, unless indicated to the contrary,
the numerical
parameters set forth in the following specification and attached claims are
approximations that
may vary depending upon the desired properties sought to be obtained by the
present invention.
At the very least, and not as an attempt to limit the application of the
doctrine of equivalents to
the scope of the claims, each numerical parameter should at least be construed
in light of the
number of reported significant digits and by applying ordinary rounding
techniques.
[0018] The practice of embodiments described herein employs, unless
otherwise
indicated, conventional techniques of cell biology, molecular biology,
genetics, chemistry,
microbiology, recombinant DNA, and immunology.
[0019] It is to be understood that as used herein and in the
appended claims, the
singular forms "a," "an," and "the," include plural referents unless the
context clearly indicates
otherwise. Thus, for example, reference to "a cell" includes one or more of
such different cells,
and reference to "the method" includes reference to equivalent steps and
methods known to those
of ordinary skill in the art that could be modified or substituted for the
methods described herein.
6
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0020] The term "cell" as used herein also refers to individual
cells, cell lines, or
cultures derived from such cells. A "culture" refers to a composition
comprising isolated cells of
the samc or a different type.
[0021] As used herein, the phrase "totipotent stem cells" refer to
cells having the
ability to differentiate into all cells constituting an organism, such as
cells that arc produced from
the fusion of an egg and sperm cell. Cells produced by the first few divisions
of the fertilized egg
can also be totipotent. These cells can differentiate into embryonic and
extraembryonic cell
types. Pluripotent stem cells, such as ES cells for example, can give rise to
any fetal or adult cell
type. However, alone they cannot develop into a fetal or adult animal because
they lack the
potential to develop extraembryonic tissue. Extraembryonic tissue is, in part,
derived from
extraembryonic endoderm and can be further classified into parietal endoderm
(Reichert's
membrane) and visceral endoderm (forms part of the yolk sac). Both parietal
and visceral
endoderm support developments of the embryo but do not themselves form
embryonic
structures. There also exist other extraembryonic tissue including
extraembryonic mesoderm and
extraemblyonic ectoderm.
[0022] In some embodiments, a "pluripotent cell" is used as the
starting material for
differentiation to endoderm-lineage, or more particularly, to pancreatic
endoderm type cells. As
used herein, "pluripotency" or "pluripotent cells" or equivalents thereof
refers to cells that are
capable of both proliferation in cell culture and differentiation towards a
variety of lineage-
restricted cell populations that exhibit multipotent properties, for example,
both pluripotent ES
cells and induced pluripotent stem (iPS) cells can give rise to each of the
three embryonic cell
lineages. Pluripotcnt cells, however, may not be capable of producing an
entire organism. That
is, pluripotent cells are not totipotent.
[0023] In certain embodiments, the pluripotent cells used as
starting material are stern
cells, including hES cells, hEG cells, iPS cells, even parthenogenic cells and
the like. As used
herein, "embryonic" refers to a range of developmental stages of an organism
beginning with a
single zygote and ending with a multicellular structure that no longer
comprises pluripotent or
totipotent cells other than developed gametic cells. In addition to embryos
derived by gamete
fusion, the term "embryonic" refers to embryos derived by somatic cell nuclear
transfer. Still in
another embodiment, pluripotent cells arc not derived or arc not immediately
derived from
embryos, for example, iPS cells are derived from a non-pluripotent cell, e.g.,
a multipotent cell
or terminally differentiated cell.
[0024] Human pluripotent stem cells can also be defined or
characterized by the
presence of several transcription factors and cell surface proteins including
transcription factors
7
Date Recue/Date Received 2023-09-08
Oct-4, Nanog, and Sox-2, which form the core regulatory complex ensuring the
suppression of genes that
lead to differentiation and the maintenance of pluripotency; and cell surface
antigens, such as the
glycolipids SSEA3, SSEA4 and the keratan sulfate antigens, Tra-1-60 and Tra-1-
81.
[00251 As used herein, the phrase "induced pluripotent stem cells," or
"iPS cells" or
"iPSCs", refer to a type of pluripotent stem cell artificially prepared from a
non-pluripotent cell, typically
an adult somatic cell, or terminally differentiated cell, such as a
fibroblast, a hematopoietic cell, a
myocyte, a neuron, an epidermal cell, or the like, by inserting certain genes
or gene products, referred to
as reprogramming factors. See Takahashi et al., Cell 131:861-872 (2007);
Wernig et al., Nature 448:318-
324 (2007); Park et al., Nature 451:141-146 (2008). Induced pluripotent stem
cells are substantially
similar to natural human pluripotent stem cells, such as hES cells, in many
respects including, the
expression of certain stem cell genes and proteins, chromatin methylation
patterns, doubling time,
embryoid body formation, teratoma formation, viable chimera formation, and
potency and
differentiability. Human iPS cells provide a source of pluripotent stem cells
without the associated use of
embryos.
[00261 Various methods can be employed to produce iPS cells, which are
described herein in
further detail below. However, all the methodologies employ certain
reprogramming factors comprising
expression cassettes encoding Sox-2, Oct-4, Nanog and optionally Lin-28, or
expression cassettes
encoding Sox-2, Oct-4, K1f4 and optionally c-myc, or expression cassettes
encoding Sox-2, Oct-4, and
optionally Esrrb. Nucleic acids encoding these reprogramming factors can be in
the same expression
cassette, different expression cassettes, the same reprogramming vector, or
different reprogramming
vectors. Oct-3/4 and certain members of the Sox gene family (Sox-1, Sox-2, Sox-
3, and Sox-15) are
crucial transcriptional regulators involved in the induction process whose
absence makes induction
impossible. Oct-3/4 (Pou5f1) is one of the family of octamer ("Oct")
transcription factors, and plays an
important role in maintaining pluripotency. For example, the absence of Oct-
3/4 in normally Oct-3/4+
cells, such as blastomeres and embryonic stem cells, leads to spontaneous
trophoblast differentiation;
whereas the presence of Oct-3/4 gives rise to the pluripotency and
differentiation potential of embryonic
stem cells. Also, other genes in the "Oct" family, for example, Octl and 0ct6,
do not induce pluripotency,
therefore this pluripotency induction process can be attributed to Oct-3/4.
Another family of genes
associated with maintaining pluripotency similar to Oct-3/4, is the Sox
family. However, the Sox family
is not exclusive to pluripotent cell types but is also associated with
multipotent and unipotent stem cells.
The Sox family has been found to work as well in the induction process.
Initial studies by Takahashi et
al., 2006 supra used Sox2. Since then, Soxl,
8
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Sox3, SoxI5, and Sox18 genes have also generated iPS cells. K1f4 of the Klf
family of genes
(Klf-1, Klf2, K1f4, and K1f5) was initially identified by Yamanaka et al. 2006
supra as a factor
for the generation of mouse iPS cells. Human iPS cells from S. Yamanaka were
used herein to
explore cell therapeutic applications of hIPS cells. However, Yu et al. 2007
supra reported that
Klf4 was not required and in fact failed to produce human iPS cells. Other
members of the Klf
family are capable generating iPS cells, including Klfl, K112 and Klf5.
Lastly, the Myc family
(C-myc, L-myc, and N-myc), proto-oncogenes implicated in cancer; c-myc was a
factor
implicated in the generation of mouse and human iPS cells, but Yu et al. (2007
supra reported
that c-myc was not required for generation of human iPS cells.
[0027] As used herein, -multipotency" or "multipotent cell" or
equivalents thereof
refers to a cell type that can give rise to a limited number of other
particular cell types. That is,
multipotent cells are committed to one or more embryonic cell fates, and thus,
in contrast to
pluripotent cells, cannot give rise to each of the three embryonic cell
lineages as well as to
extraembryonic cells. Multipotent somatic cells are more differentiated
relative to pluripotent
cells, but are not terminally differentiated. Pluripotent cells therefore have
a higher potency than
multipotent cells. Potency-determining factors that can reprogram somatic
cells or used to
generate iPS cells include, but are not limited to, factors such as Oct-4,
Sox2, FoxD3, UTF1,
Stella, Rexl, ZNF206, Sox15, Myb12, Lin28, Nanog, DPPA2, ESG1, 0tx2 or
combinations
thereof.
As used herein, "ERBB receptor tyrosine kinase activating agent" includes, but
is not
limited to, at least 16 different EGF family ligands that bind ERBB receptors:
EGF (epidermal
growth factor), AG or AREG (Amphiregulin), and TGF-Alpha (Transforming Growth
Factor-
Alpha), Btc (Betacellulin), HBEGF (Heparin-Binding EGF), and Ereg
(Epiregulin), Neuregulins
(or Heregulins) such as Neuregulin-1, -2, -3 and -4 (or Heregulin-1, -2, -3
and -4). However, the
instant invention contemplates any ligand that is capable of binding to any
one of the four ERBB
receptors or a combination thereof to induce formation of homo- and
heterodimer receptor
complexes leading to activation of the intrinsic kinase domain and subsequent
phosphorylation.
See also Table 11.
Some embodiments of the methods of producing insulin described herein can
include
treating an animal having diabetes, or controlling glucose concentration in
the blood of an
animal, by providing the animal with pancreatic endoderm cells that can mature
in vivo into
insulin producing cells that secrete insulin in response to glucose
stimulation.
[0028] One aspect described herein includes populations of
pluripotent or precursor
cells that are capable of selectively, and in some aspects selectively
reversibly, developing into
9
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
different cellular lineages when cultured under appropriate conditions. As
used herein, the term
"population" refers to cell culture of more than one cell having the same
identifying
characteristics. The term "cell lineage" refers to all of the stages of the
development of a cell
type, from the earliest precursor cell to a completely mature cell (i.e. a
specialized cell). A
"precursor cell" or "progenitor cell" can be any cell in a cell
differentiation pathway that is
capable of differentiating into a more mature cell. As such, a precursor cell
can be a pluripotent
cell, or it can be a partially differentiated multipotent cell, or reversibly
differentiated cell. The
term "precursor cell population" refers to a group of cells capable of
developing into a more
mature or differentiated cell type. A precursor cell population can comprise
cells that are
pluripotent, cells that are stem cell lineage restricted (i.e. cells capable
of developing into less
than all ectodermal lineages, or into, for example, only cells of neuronal
lineage), and cells that
are reversibly stem cell lineage restricted. Therefore, the term "progenitor
cell" or "precursor
cell" may be a "pluripotent cell" or "multipotent cell."
[0029] As used herein, the term "reprogramming", "reprogrammed" or
equivalents
thereof, refers to a process that confers on a cell a measurably increased
capacity to form
progeny of at least one new cell type, either in culture or in vivo, than it
would have under the
same conditions without reprogramming. in certain embodiments described
herein, somatic cells
are "reprogrammed" to pluripotent cells, hi certain aspects, somatic cells are
reprogrammed
when after sufficient proliferation, a measurable proportion of cells, either
in vivo or in an in
vitro cell culture, display phenotypic characteristics of the new pluripotent
cell type. Without
reprogramming, such somatic cells would not give rise to progeny displaying
phenotypic
characteristics of the new pluripotent cell type. if, even without
reprogramming, somatic cells
could give rise to progeny displaying phenotypic characteristics of the new
pluripotent cell type,
the proportion of progeny from these somatic cells displaying phenotypic
characteristics of the
new pluripotent cell type is measurably more than before reprogramming.
[0030] As used herein, the phrase "differentiation programming"
refers to a process
that changes a cell to form progeny of at least one new cell type with a new
differentiation status,
either in culture or in vivo, than it would have under the same conditions
without differentiation
reprogramming. This process includes differentiation, dedifferentiation and
transdifferentiation.
Hence, as used herein, the phrase "differentiation" refers to the process by
which a less
specialized cell becomes a more specialized cell type. in contrast, the phrase
"dedifferentiation"
refers to a cellular process in which a partially or terminally differentiated
cell reverts to an
earlier developmental stage, such as cell having pluripotency or multipotency.
In further contrast,
Date Recue/Date Received 2023-09-08
the phrase "transdifferentiation" refers to a process of transforming one
differentiated cell type into
another differentiated cell type.
[0031] As used herein, the terms "develop from pluripotent cells",
"differentiate from
pluripotent cells", "mature from pluripotent cells" or "produced from
pluripotent cells", "derived
from pluripotent cells", "differentiated from pluripotent cells" and
equivalent expressions refer to the
production of a differentiated cell type from pluripotent cells in vitro or in
vivo, e.g., in the case of
endocrine cells matured from transplanted PDX1 pancreatic endoderm cells in
vivo as described in
International Patent Application No. PCT/US2007/015536, entitled METHODS OF
PRODUCING
PANCREATIC HORMONES. All such terms refer to the progression of a cell from
the stage of
having the potential to differentiate into at least two different cellular
lineages to becoming a
specialized and terminally differentiated cell. Such terms can be used
interchangeably for the
purposes of the present application. Embodiments described herein contemplate
culture conditions
that permit such differentiation to be reversible, such that pluripotency or
at least the ability to
differentiate into more than one cellular lineage can be selectively regained.
[0032] The term "feeder cell" refers to a culture of cells that grows
in vitro and secretes
at least one factor into the culture medium, and that can be used to support
the growth of another cell
of interest in culture. As used herein, a "feeder cell layer" can be used
interchangeably with the term
"feeder cell." A feeder cell can comprise a monolayer, where the feeder cells
cover the surface of the
culture dish with a complete layer before growing on top of each other, or can
comprise clusters of
cells. In a preferred embodiment, the feeder cell comprises an adherent
monolayer.
[0033] As used herein, the terms "cluster" and "clump" or "aggregate"
can be used
interchangeably, and generally refer to a group of cells that have not been
dissociated into single
cells. The clusters may be dissociated into smaller clusters. This
dissociation is typically manual in
nature (such as using a Pasteur pipette), but other means of dissociation are
contemplated. Aggregate
suspension pluripotent or multipotent cell cultures are substantially as
described in International
Publications PCT/U52007/062755, titled COMPOSITIONS AND METHODS FOR CULTURING
DIFFERENTIAL CELLS and PCT/US2008/082356, titled STEM CELL AGGREGATE
SUSPENSION COMPOSITIONS AND METHODS OF DIFFERENTIATION THEREOF.
[0034] Similarly, embodiments in which pluripotent cell cultures or
aggregate
pluripotent suspension cultures are grown in defined conditions without the
use of feeder cells, are
"feeder-free". Feeder¨free culture methods increase scalability and
reproducibility of pluripotent cell
culture and reduces the risk of contamination, for example, by infectious
agents from the feeder cells
11
Date Recue/Date Received 2023-09-08
or other animal¨sourced culture components. Feeder-free methods are also
described in U.S. Patent
No. 6,800,480 to Bodnar et at. (assigned to Geron Corporation, Menlo Park,
California). However,
and in contrast to U.S. Patent No. 6,800,480 patent, embodiments described
herein, whether they be
pluripotent, multipotent or differentiated cell cultures, are feeder-free and
do not further contain an
endogenous or exogenous extracellular-matrix; i.e. the cultures described
herein are extracellular-
matrix-free as well as being feeder free. For example, in the U.S. Patent No.
6,800,480, extracellular
matrix is prepared by culturing fibroblasts, lysing the fibroblasts in situ,
and then washing what
remains after lysis. Alternatively, in U.S. Patent No. 6,800,480 extracellular
matrix can also be
prepared from an isolated matrix component or a combination of components
selected from collagen,
placental matrix, fibronectin, laminin, merosin, tenascin, heparin sulfate,
chondroitin sulfate,
dermatan sulfate, aggrecan, biglycan, thrombospondin, vitronectin, and
decorin. Embodiments
described herein neither produce an extracellular-matrix by growth of a feeder
or fibroblast layer and
lysing the cells to produce the extracellular-matrix; nor does it require
first coating the tissue culture
vessel with extracellular matrix component or a combination of extracellular-
matrix components
selected from collagen, placental matrix, fibronectin, laminin, merosin,
tenascin, heparin sulfate,
chondroitin sulfate, dermatan sulfate, aggrecan, biglycan, thrombospondin,
vitronectin, and decorin.
Hence, the aggregate suspension cultures described herein for pluripotent,
multipotent and
differentiated cells do not require a feeder layer, a lysed feeder or
fibroblast cell to produce an
extracellular matrix coating, an exogenously added extracellular matrix or
matrix component; rather
use of soluble human serum component as described in International Application
PCT/US2008/080516, titled METHODS AND COMPOSITIONS FOR FEEDER-FREE
PLURIPOTENT STEM CELL MEDIA CONTAINING HUMAN SERUM, overcomes the need for
either a feeder-cell or feeder monolayer, as well as overcoming the need for
an endogenous
extracellular-matrix from a feeder or fibroblast cell or from exogenously
added extracellular-matrix
components.
[0035] In preferred embodiments, culturing methods are free of animal-
sourced products.
In another preferred embodiment, the culturing methods are xeno-free. In even
more preferred
embodiments, one or more conditions or requirements for the commercial
manufacture of human cell
therapeutics met or exceeded by the culturing methods described herein.
[0036] The population of pluripotent cells can be further cultured in
the presence of
certain supplemental growth factors to obtain a population of cells that are
or will develop into
different cellular lineages, or can be selectively reversed in order to be
able to develop into
12
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
different cellular lineages. The term "supplemental growth factor" is used in
its broadest context
and refers to a substance that is effective to promote the growth of a
pluripotent cell, maintain the
survival of a cell, stimulate the differentiation of a cell, and/or stimulate
reversal of the
differentiation of a cell. Further, a supplemental growth factor may be a
substance that is
secreted by a feeder cell into its media. Such substances include, but are not
limited to,
cytokines, chemokines, small molecules, neutralizing antibodies, and proteins.
Growth factors
may also include intercellular signaling polypeptides, which control the
development and
maintenance of cells as well as the form and function of tissues. In preferred
embodiments, the
supplemental growth factor is selected from the group consisting of steel cell
factor (SCF),
oncostatin M (OSM), ciliary neurotrophic factor (CNTF), lnterleukin-6 (IL-6)
in combination
with soluble Interleukin-6 Receptor (IL-6R), a fibroblast growth factor (FGF),
a bone
morphogenetic protein (BMP), tumor necrosis factor (TNF), and granulocyte
macrophage colony
stimulating factor (GM-CSF).
[0037] In certain processes for producing the cells as described
herein, the growth
factors are removed from the cell culture or cell population subsequent to
their addition. For
example, the growth factor, such as Activin A, Activin B, GDF-8, or GDF-11 can
be added and
removed within about one day, about two days, about three days, about four
days, about five
days, about six days, about seven days, about eight days, about nine days or
about ten days after
their addition. In some embodiments, the differentiation factors are not
removed from the cell
culture.
[0038] Because the efficiency of the differentiation process can be
adjusted by
modifying certain parameters, which include but arc not limited to, cell
growth conditions,
growth factor concentrations and the timing of culture steps, the
differentiation procedures
described herein can result in about 5%, about 10%, about 15%, about 20%,
about 25%, about
30%, about 35%, about 40%, about 45%, about 50%, about 55%, about 60%, about
65%, about
70%, about 75%, about 80%, about 85%, about 90%, about 95%, or greater than
about 95%
conversion of pluripotent cells, which includes induced pluripotent cells, to
multipotent or
differentiated cells e.g., definitive endoderm, foregut endoderm, PDX1-
positive foregut
endoderm, PDX1-positive pancreatic endoderm or PDXUNKX6.1 co-positive
pancreatic
endoderm, endocrine precursor or NON3/NICX2.2 co-positive endocrine precursor,
and hormone
secreting endocrine cells or INS, GCG, GERL, SST, PP singly-positive endocrine
cells. In
processes in which isolation of preprimitive streak or mesendoderm cells is
employed, a
substantially pure preprimitive streak or mesendoderm cell population can be
recovered.
13
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCMJS2014/015156
[0039]
Various cell compositions derived from pluripotcnt stem cells are described
herein. One embodiment includes iPS cells and cells derived therefrom. Still
other processes and
compositions related to but distinct from the embodiments described herein can
be found in
United States Provisional Patent Application No. 60/532,004, entitled
DEFINITIVE
ENDODERM, filed December 23, 2003; U.S. Provisional Patent Application Number
60/566,293, entitled PDX1 EXPRESSING ENDODERM, filed April 27, 2004; U.S.
Provisional
Patent Application Number 60/586,566, entitled CHEMOKINE CELL SURFACE RECEPTOR
FOR THE ISOLATION OF DEFINITIVE ENDODERM, filed July 9, 2004; U.S. Provisional
Patent Application Number 60/587,942, entitled CHEMOKINE CELL SURFACE RECEPTOR
FOR THE ISOLATION OF DEFINITIVE ENDODERM, filed July 14, 2004; U.S. Patent
Application Number 11/021,618, entitled DEFINITIVE ENDODERM, filed December
23, 2004
and U.S. Patent Application Number 11/115,868, entitled PDX1 EXPRESSING
ENDODERM,
filed April 26, 2005; U.S. Patent Application Number 11/165,305, entitled
METHODS FOR
IDENTIFYING FACTORS FOR DIFFERENTIATING DEFINITIVE ENDODERM, filed June
23, 2005; U.S. Provisional Patent Application Number 60/730,917, entitled PDX1-
EXPRESSING DORSAL AND VENTRAL FOREGUT ENDODERM, filed October 27, 2005;
U.S. Provisional Patent Application Number 60/736,598, entitled MARKERS OF
DEFINITIVE
ENDODERM, filed November 14, 2005; U.S. Provisional Patent Application Number
60/778,649, entitled INSULIN-PRODUCING CELLS AND METHOD OF PRODUCTION,
filed March 2, 2006; U.S. Provisional Patent Application Number 60/833,633,
entitled
IN CELLS
AND METHOD OF PRODUCTION, filed July 26, 2006; U.S.
Provisional Patent Application Number 60/852,878, entitled ENRICHMENT OF
ENDOCRINE
PRECURSOR CELLS, IMMATURE PANCREATIC ISLET CELLS AND MATURE
PANCREATIC ISLET CELLS USING NCAM, filed October 18, 2006; -U.S. Patent
Application
Number 11/588,693, entitled PDX1-EXPRESSING DORSAL AND VENTRAL FOREGUT
ENDODERM, filed October 27, 2006; U.S. Patent Application Number 11/681,687,
entitled
ENDOCRINE PRECURSOR CELLS, PANCREATIC HORMONE-EXPRESSING CELLS
AND METHODS OF PRODUCTION, filed March 2, 2007; U.S. Patent Application Number
11/773,944, entitled METHODS OF PRODUCING PANCREATIC HORMONES, filed July 5,
2007; U.S. Patent Application Number 60/972,174, entitled METHODS OF TREATMENT
FOR
DIABETES, filed September 13, 2007; U.S. Patent Application Number 11/860,494,
entitled
METHODS FOR INCREASING DEFINITIVE ENDODERM PRODUCTION, filed September
24, 2007; U.S. Patent Application Number 60/977,349, entitled CELL SURFACE
MARKERS
OF HUMAN EMBRYONIC STEM CELLS AND CANCER STEM CELLS, filed October 3,
14
Date Recue/Date Received 2023-09-08
2007; and U.S. Patent Application Number 12/099,759, entitled METHODS OF
PRODUCING
PANCREATIC HORMONES, filed April 8, 2008; and U.S. Patent Application Number
12/107,020, entitled METHODS FOR PURIFYING ENDODERM AND PANCREATIC
ENDODERM CELLS DERIVED FORM HUMAN EMBRYONIC STEM CELLS, filed April
21, 2008.
[0040] General methods for production of endoderm lineage cells derived
from hES
cells are described in related U.S. applications as indicated above, and
D'Amour et al. 2005 Nat
Biotechnol. 23:1534-41 and D'Amour et al. 2006 Nat Biotechnol. 24(11):1392-
401. D'Amour et
al. describe a 5 step differentiation protocol: stage 1 (results in mostly
definitive endoderm
production), stage 2 (results in mostly PDX1-negative foregut endoderm
production), stage 3
(results in mostly PDX1-positive foregut endoderm production), stage 4
(results in mostly
pancreatic endoderm or pancreatic endocrine progenitor production) and stage 5
(results in
mostly hormone expressing endocrine cell production.
[0041] The term "trophectoderm" refers to a multipotent cell having the
relative high
expression of markers selected from the group consisting of HAND1, Eomes,
MASH2, ESXL1,
HCG, KRT18, PSG3, SFXN5, DLX3, PSX1, ETS2, and ERRB genes as compared to the
expression levels of HAND!, Eomes, MASH2, ESXL1, HCG, KRT18, PSG3, SFXN5,
DLX3,
PSX1, ETS2, and ERRB in non-trophectoderm cells or cell populations.
[0042] "Extraembryonic endoderm" refers to a multipotent cell having
relative high
expression levels of markers selected from the group consisting of SOX7,
SOX17, THBD,
SPARC, DAB1, or AFP genes as compared to the expression levels of SOX7, SOX17,
THBD,
SPARC, DAB1, or AFP in non-extraembryonic endoderm cells or cell populations.
[0043] The term "Preprimitive streak cells" refers to a multipotent
cell having
relative high expression levels of the FGF8 and/or NODAL marker genes, as
compared to
BRACHURY low, FGF4 low, SNA11 low, SOX17 low, FOXA2 low, SOX7 low and SOX1
low.
[0044] The term "Mesendoderm cell" refers to a multipotent cell having
relative high
expression levels of brachytuy, FGF4, SNAI1 MIXL1 and/or WNT3 marker genes, as
compared
to SOX17 low, CXCR4 low, FOXA2 low, SOX7 low and SOX! low.
Date Recue/Date Received 2023-09-08
[0045] The term "Definitive endoderm (DE)" refers to a multipotent
endoderm
lineage cell that can differentiate into cells of the gut tube or organs
derived from the gut tube.
In accordance with certain embodiments, the definitive endoderm cells are
mammalian cells, and
in a preferred embodiment, the definitive endoderm cells are human cells. In
some embodiments
of the present invention, definitive endoderm cells express or fail to
significantly express certain
markers. In some embodiments, one or more markers selected from SOX17, CXCR4,
MIXL1,
GATA4, HNF313, GSC, FGF17, VWF, CALCR, FOXQ1, CMKOR1 and CRTP1 are expressed
in
definitive endoderm cells. In other embodiments, one or more markers selected
from OCT4,
alpha-fetoprotein (AFP), Thrombomodulin (TM), SPARC, SOX7 and HNF4alpha are
not
expressed or significantly expressed in definitive endoderm cells. Definitive
endoderm cell
populations and methods of production thereof are also described in U.S.
Application Number
11/021,618, entitled DEFINITIVE ENDODERM, filed December 23, 2004.
[0046] Still other embodiments relate to cell cultures termed "PDX1-
negative foregut
endoderm cells" or "foregut endoderm cells" or equivalents thereof. In some
embodiments, the
foregut endoderm cells express SOX17, HNF1f3 (HNF1B), liNF4alpha (HNF4A) and
FOXA1
markers but do not substantially express PDX1, AFP, SOX7, or SOX1. PDX1-
negative foregut
endoderm cell populations and methods of production thereof are also described
in U.S.
Application Number 11/588,693, entitled PDX1-expressing dorsal and ventral
foregut endoderm,
filed October 27, 2006.
[0047] Other embodiments described herein relate to cell cultures of
"PDX1-positive,
dorsally-biased, foregut endoderm cells" (dorsal PDX1-positive foregut
endoderm cells) or just
"PDX1-positive endoderm." In some embodiments, the PDX1-positive endoderm
cells express
one or more markers selected from Table 1 and/or one or more markers selected
from Table 2,
also described in related U.S. Application 11/588,693 entitled PDX1 EXPRESSING
DOSAL
AND VENTRAL FOREGUT ENDODERM, filed October 27, 2006, and also U.S.
Application
Number 11/115,868, entitled PDX1-expressing endoderm, filed April 26, 2005.
16
Date Recue/Date Received 2023-09-08
P
FD
,
Table 1 - Markers expressed in both dorsal and ventral PDX1-positive foregut
endoderm
--b--
0
P
CD Gene_Symbol Unigene LocusLink OMIM SeqDerivedFrom Gene
Descriptor b.)
c>
cl, ANX. A4 Hs.422986 307 ______ 106491
NM 001153 annexin A4 .
4,
O -...
0. ASCL1 11024672 429 100790 DC(X)1 achaete-
scute complex-like 1 (Drosophila)
NP
<
_______________________________________________________________________________
________________________ ,
CD
t
cl- BNC1 Hs.459153 646 601930 NM 001717
basonuclin 1 --.1
l=-)
IN)
0 C1Oorf30 Hs.498740 222389 AW195407
Chromosome 10 open reading frame 30
tv
Le" C2orf23 Hs.368884 65055 609139 BE535746
chromosome 2 open reading frame 23
o
`. C9orf150 Hs.445356 286343 AI972386
chromosome 9 open reading frame 150
o
oc CDH6 Hs.171054 1004 603007 BC000019
cadherin 6, type 2, K-cadherin (fetal kidney)
DACH1 Hs.129452 1602 603803 A1650353
dachshund hornolog 1 (Drosophila)
DUSP9 Hs.144879 1852 300134 NM 001395 dual
specificity phosphatase 9
ELMODI Hs.495779 55531 AL359601 ELMO
domain containing 1
FLJ21462 fis Hs.24321 AW236803 CDNA
clone 1MAGE:5273964, partial cds
FLJ22761 Hs.522988 80201 W81116
hypothetical protein FLJ22761 g
GABRA2 Hs.116250 2555 137140 NM_000807 mma-
aminobu "c_acid (GABA A receptor, alp_ha 2 _ 0 _ _
_
0
GFtIA3 Hs.377070 2892 _305915 BC032004
glutamate receptor, ionotrophic,
AMPA 3 .
_
_______________________________________________________________________________
________________________ - w
11NF4G Hs.241529 3174 605966 AI916600
hepatocyte nuclear factor 4, gamma p
0
0
IDH2 Hs.513141 3418 147650 U52144
isocitrate dehydrogenase 2 (NADP+), mitochondrial m
u,
1
IL6R IIs.135087 3570 147880 AV700030
interleukin 6 receptor 3
KCNJ2 Ils.1547 3759 170390 AF153820
potassium inwardly-rectifying
channel, subfamily .1, member 2 P
Ul
ICLF3 Hs.298658 51274 AA130132 Kruppel-
like factor 3 (basic)
-
LGALS3 Hs.531081 3958 153619 AW085690 Lectin,
galactoside-binding, soluble, 3 (galectin 3)
LGALS3 /// lectin,
galactoside-binding, soluble, 3 (galectin 3) Ill galectin-3
GALIG Hs.531081 39581/1 153619 BCC01120
internal gene
LIPC Hs.188630 3990 151670 NM 000236
lipase, hepatic
Meisl , myeloid ecotropic viral integration site 1 homolog
MEIS1 Hs.526754 4211 601739 NM_002398 (mouse)
-0
NR2F1 Hs.519445 7025 132890 AI951185 Nuclear
receptor subfamily 2, group F, member 1 n
ONECUT2 Hs.194725 9480 604894 NM_004852 one cut
domain, family member 2
PAPPA IIs.494928 5069 176385 AA148534
pregnancy-associated plasma protein
A, pappalysin 1 t=-)
PDE3B IIs.445711 5140 602047 NM_000753
, phosphodiesterase 3B, cGMP-
inhibited c;
-
_______________________________________________________________________________
_______________________________________
PGPEP1 Hs.131776 54858 NM_017712
pyroglutamyl-peptidase I '.---
PMS2L1 Hs.520575 5379 605038 D38503
postmeiotic segegation increased 2-like 1 'II
I-,
cm
17
ci,
=
Gene_Symbol Unigene LocusLink OMIM SeqDerivedFrom Gene Descriptor
0
serine (or cysteine) proteinase inhibitor, clade F
L.4
(alpha-2 antiplasmin, pigment epithelium derived factor),
SERPINF2 Hs 159509 5345 262850 NM_000934 member 2
cp
SLC27A2 Hs.11729 11001 603247 NM
003645 solute carrier family 27 (fatty acid transporter), member 2
SLN Hs.334629 6588 602203 NM_003063
Sarcolip in
SRY (sex determining region Y)-box 9 (campomelic dysplasia,
Le"
SOX9 Hs.2316 6662 114290 NM
000346 autosomal sex-reversal)
sulfotransferase family, cytosolic, 2A, dehydroepiandrosterone
SULT2A1 Hs.515835 6822 125263 U08324 (DHEA)-
preferring, member 1
Tissue factor pathway inhibitor (lipoprotein-associated
TFPI Hs.516578 7035 152310 BF511231
coagulation inhibitor)
ZHX1 Hs.521264 11244 604764 A1123518
zinc fingers and homeoboxes 1
ZNF467 Hs.112158 168544 BE549732 zinc
finger protein 467
ZNF503 Hs.195710 84858 AA603467 zinc
finger protein 503
Hs.142869 AI935586
Transcribed locus
PG
-0
18
P
FD
,
O Table 2 - Markers expressed in dorsally-biased PDX1-positive foregut
endoderm
--b--
P 0
[4
CD
0
4.,
\
CD
1,
CD .
NO
< Gene_Symbol Unigene LocusLink OMIM SeqDerivedFrom Gene Descriptor 0
sa, ADORA2A Hs.197029 135 102776 NM 000675 adenosine
A2a receptor --4
l=-)
IN)
0 AMSH-LP Hs.16229 57559 A1638611
associated molecule with the SH3 domain of STAM(AMSH) like protein
6_)
Le" BA1AP2L1 Hs.489237 55971 AA62840) BAll-
associated protein 2-like 1
o
'F' CD47 Hs.446414 961 601028 BG230614 CD47
antigen (Rh-related antigen, integrin-associated signal transducer)
o
ci0 CHN2 Hs.203663 1124 602857 AK026415 Cbimerin
(chimaerin) 2
CLDN3 Hs.25640 1365 602910 BE791251
claudin 3
CPVL Hs.233389 54504 NM 031311
carboxypeptidase, vitellogenic-likell carboxypeptidase, vitellogenic-like
CREB3L1 Hs.405961 90993 AFO-55009 cAMP
responsive element binding protein 3-like 1
DACT1 Hs.48950 51339 607861 NM 016651
dapper homolog 1, antagonist of ll-catenin (xenopus)
DPP6 Hs.490684 1804 126141 AW071705
Dipeptidylpeptidase 6
ELF3 Hs.67928 1999 602191 AF017307
E74-like factor 3 (ets domain
transcription factor, epithelial-specific) g
ENPP2 Hs.190977 5168 601060 L35594
ectonucleotide pyrophosphataselphosphodiesterase 2 (autotaxin) 0
0
0
EPB41L1 Hs.437422 2036 602879 AA912711
erythrocyte membrane protein band 4.1-like 1 W
6
W
FAM46C Hs.356216 54855 AL046017 family
with sequence similarity 46, member C p
0
family with sequence similarity 49, member A HI family with sequence
0
m
FAM49A Hs.467769 81553 NM_030797 similarity
49, member A 0
1
0
FL330596 Hs.81907 133686 A1453203
hypothetical protein FLJ30596
I
P
HOXA1 Hs.67397 3198 142955 S79910
homeo box Al 0
HOXA3 Hs.533357 3200 142954 AW137982 home box
A3
HOXB2 Hs.514289 3212 142967 NM 002145 homeo box
B2
LAF4 Hs.444414 3899 601464 AW085565 Lymphoid
nuclear protein related to AF4
L0C283658 Hs.87194 283658 AA233912
hypothetical protein L0C283658
MAF Hs.134859 4094 177075 AF055376 v-maf
musculoaponeurotic fibrosarcoma oncogene homolog (avian)
MAG Hs.515354 4099 159460 X98405 myelin
associated glycoprotein
MYCPBP Hs.513817 10260 600382 BE268538
c-tnyc promoter binding protein
NR4A2 Hs.165258 4929 168600 / NM 006186 nuclear
receptor subfamily 4, group A, member 2 IV
NRXiN3 Hs.368307 9369 600567 A1129949 Leurexin 3
n
NSE1 Hs.260855 151354 A1601101 NSE1
PCGF5 Hs.500512 84333 AL045882 polycomb
group ring fingers sa
PDEllA Iis.130312 50940 604961 AB038041
phosphodiesterase 11A c;
c,
PDE5A Hs.370661 8654 603310 BF221547
Phosphodiesterase 5A, eGMP-specific -O-
PGA3 5/70 169710 A1570199 pepsinogen
3, group I (pepsinogen A) .
tit
PLN Hs.170839 5350 115200 NM_002667
Phospholamban
cm
o,
19
P
FD
,
- e =
P 0
cp Gene_Symbol Unigene LocusLink OIVIIM SeqDerivedFrom Gene
Descriptor Ls.)
o
prostaglandin 12 (prostacyclin) syathase 1// prostaglandin 12 (prostacyciin)
.
4,
O -...
0. PTGIS Hs.302085 5740 145500 NM_000961 synthase
...
< RARB Hs.436538 5915 180220
NM_000965 retinoic acid receptor, 13 0
RGN Hs.77854 9104 300212 D3I815
regucalcin (senescence marker protein-
30) --..
l=-)
IN)
o RND1 Hs,124940 27289 609038
U69563 Rho family GTPase 1
i.)
Le" SFRP5 Hs.279565 6425 604158 NM 003015 wereted
frizzled-related protein 5
o
'F' SGICL Hs.380877 23678 607591 AV690865
wrumtglueocortieoid regulated kinase-like
o
00 SLC16A10 Hs.520321 117247 607550 N30257
solute carrier family 16 (rnonocarboxylic add transporters), member 10
SLC16A2 Ils.75317 6567 300095 NM_006517
solute carrier family 16 (monocarboxylic acid transporters), member 2
SLC1A3 Hs.481918 6507 600111 NM 004172 solute
carrier family I (glial high affinity glutamate transporter), member 3
SLC30A4 Hs.162989 7782 602095 NM-013309 solute
carrier family 30 (zinc transporter), member 4
SLICK Hs.4200 I 6 343450 A1732637 sodium- and
chloride-activated ATP-sensitive potassium channel
SLITRK4 Hs.272284 139065 AL080239 SLIT and
NTRK-like family, member 4
ST8SIA3 Hs.298923 51046 NM_015879 ST8 alpha-N-
acetyl-neuraminide alpha-2,8-sialyltransferase 3 g
wingless-type MMTV integration site family, member 5A /// wingless-type
.
0
0
WNT5A Hg 152213 7474 164975 AI968085 MMTV
integration site family, member 5A W
6
XPR1 Hs.227656 9213 605237 M089744 xenotropic
and polytropic retrovirus receptor 0
p
Hs.535688 AK001582 CDNA
FL110720 fig, clone NT2RP3001116 .
0
..
Hs.127009 A1935541 Transcribed
locus 0
1
0
Hs.4749 ALI37310 CDNA
FLJ31660 fig, clone NT2RI2004410
1
F.=
Ul
IV
n
L..,
z;
1-
4,
"---
-
tn
1¨i
cm
o,
[0048]
The PDX1-positive foregut endoderm cells, such as those produced according to
the methods described herein, are progenitors which can be used to produce
fully differentiated
pancreatic hormone secreting or endocrine cells, e.g., insulin-producing n-
cells. In some
embodiments of the present invention, PDX1-positive foregut endoderm cells are
produced by
differentiating definitive endoderm cells that do not substantially express
PDX1 (PDX1-negative
definitive endoderm cells; also referred to herein as definitive endoderm) so
as to form PDX1-
positive foregut endoderm cells.
[0049] As
used herein, "pancreatic endoderm," "pancreatic epithelial," "pancreatic
epithelium" (all can be abbreviated "PE") "pancreatic progenitor," "PDX-1
positive pancreatic
endoderm or equivalents thereof, such as pancreatic endoderm cells ("PEC"),
are all precursor or
progenitor pancreatic cells. PEC as described herein is a progenitor cell
population after stage 4
differentiation (about day 12-14) and includes at least two major distinct
populations: i) pancreatic
progenitor cells that express NIOC6.1 but do not express CHGA (or CHGA
negative, CHGA-); and
ii) polyhormonal endocrine cells that express CHGA (CHGA positive, CHGA+).
Without being
bound by theory, the cell population that expresses NIOC6.1 but not CHGA is
hypothesized to be the
more active or therapeutic component of PEC, whereas the population of CHGA-
positive
polyhormonal endocrine cells is hypothesized to further differentiate and
mature in vivo into
glucagon-expressing islet cells. See Kelly et al. (2011) Cell-surface markers
for the isolation of
pancreatic cell types derived from human embryonic stem cells, Nat Biotechnol.
29(8):750-756,
published online 31 July 2011 and Schulz et al. (2012), A Scalable System for
Production of
Functional Pancreatic Progenitors from Human Embryonic Stem Cells, PLosOne
7(5): 1-17, e37004.
Still, sometimes, pancreatic endoderm cells are used without reference to PEC
as described
just above, but to refer to at least stages 3 and 4 type cells in general. The
use and meaning will be
clear from the context. Pancreatic endoderm derived from pluripotent stem
cells, and at least hES and
hIPS cells, in this manner are distinguished from other endodermal lineage
cell types based on
differential or high levels of expression of markers selected from PDX1,
NKX6.1, PTF1A, CPA1,
cMYC, NGN3, PAX4, ARX and NICX2.2 markers, but do not substantially express
genes which are
hallmark of pancreatic endocrine cells, for example, CHGA, INS, GCG, GHRL,
SST, MAFA,
PCSK1 and GLUT1. Additionally, some "endocrine progenitor cells" expressing
NGN3 can
differentiate into other non-pancreatic structures (e.g., duodenum). In one
embodiment, the NGN3
expressing endocrine progenitor described herein differentiates into mature
pancreatic lineage cells,
e.g., pancreatic endocrine cells. Pancreatic endoderm or endocrine progenitor
cell populations and
21
Date Recue/Date Received 2023-09-08
methods thereof are also described in U.S. Patent Application Number
11/773,944, entitled Methods
of producing pancreatic hormones, filed July 5, 2007, and U.S. Patent
Application Number
12/107,020, entitled METHODS FOR PURIFYING ENDODERM AND PANCREATIC
ENDODERM CELLS DERIVED FORM HUMAN EMBRYONIC STEM CELLS, filed April 21,
2008.
[0050] As used herein, "endocrine precursor cell" refers to a
multipotent cell of the
definitive endoderm lineage that expresses neurogenin 3 (NEUROG3) and which
can further
differentiate into cells of the endocrine system including, but not limited
to, pancreatic islet hormone-
expressing cells. Endocrine precursor cells cannot differentiate into as many
different cell, tissue
and/or organ types as compared to less specifically differentiated definitive
endoderm lineage cells,
such as PDX1-positive pancreatic endoderm cell.
[0051] As used herein, "pancreatic islet hormone-expressing cell,"
"pancreatic endocrine
cell," or equivalents thereof refer to a cell, which has been derived from a
pluripotent cell in vitro,
which can be polyhormonal or singly-hormonal. The endocrine cells can
therefore express one or
more pancreatic hormones, which have at least some of the functions of a human
pancreatic islet cell.
Pancreatic islet hormone-expressing cells can be mature or immature. Immature
pancreatic islet
hormone-expressing cells can be distinguished from mature pancreatic islet
hormone-expressing cells
based on the differential expression of certain markers, or based on their
functional capabilities, e.g.,
glucose responsiveness.
[0052] Many stem cell media culture or growth environments are
envisioned in the
embodiments described herein, including defined media, conditioned media,
feeder-free media,
serum-free media and the like. As used herein, the term "growth environment"
or "milieu" or
equivalents thereof is an environment in which undifferentiated or
differentiated stem cells (e. g.,
primate embryonic stem cells) will proliferate in vitro. Features of the
environment include the
medium in which the cells are cultured, and a supporting structure (such as a
substrate on a solid
surface) if present. Methods for culturing or maintaining pluripotent cells
and/or differentiating
pluripotent cells are also described in PCT/US2007/062755 entitled
COMPOSITIONS AND
METHODS USEFUL FOR CULTURING DIFFERENTIABLE CELLS, filed February 23, 2007;
U.S. Application Number 11/993,399, entitled EMBRYONIC STEM CELL CULTURE
COMPOSITIONS AND METHODS OF USE THEREOF, filed December 20, 2007; and U.S.
Application Number 11/875,057, entitled Methods and compositions for feeder-
free pluripotent stem
cell media containing human serum, filed October 19, 2007.
22
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0053] The term "essentially" or "substantially" means either a de
minimus or a
reduced amount of a component or cell present in any cell aggregate suspension
type, e.g., cell
aggregates in suspension described herein are "essentially or substantially
homogenous",
"essentially or substantially homo-cellular" or are comprised of "essentially
hES cells",
"essentially or substantially definitive endoderm cells", "essentially or
substantially foregut
endoderm cells", "essentially or substantially PDXI-negative foregut endoderm
cells",
"essentially or substantially PDX1-positive pre-pancreatic endoderm cells",
"essentially or
substantially PDX1-positive pancreatic endoderm or progenitor cells",
"essentially or
substantially PDX1-positive pancreatic endoderm tip cells", "essentially or
substantially
pancreatic endocrine precursor cells", "essentially or substantially
pancreatic endocrine cells"
and the like.
[0054] With respect to cells in cell cultures or in cell
populations, the term
"substantially free of" means that the specified cell type of which the cell
culture or cell
population is free, is present in an amount of less than about 10%, less than
about 9%, less than
about 8%, less than about 7%, less than about 6%, less than about 5%, less
than about 4%, less
than about 3%, less than about 2% or less than about 1% of the total number of
cells present in
the cell culture or cell population.
[0055] Cell cultures can be grown in medium containing reduced
scrum or
substantially free of serum or no serum. Under certain culture conditions,
serum concentrations
can range from about 0% (v/v) to about 10% (v/v). For example, in some
differentiation
processes, the serum concentration of the medium can be less than about 0.05%
(v/v), less than
about 0.1% (v/v), less than about 0.2% (v/v), less than about 0.3% (v/v), less
than about 0.4%
(v/v), less than about 0.5% (v/v), less than about 0.6% (v/v), less than about
0.7% (v/v), less than
about 0.8% (v/v), less than about 0.9% (v/v), less than about 1% (v/v), less
than about 2% (v/v),
less than about 3% (v/v), less than about 4% (v/v), less than about 5% (v/v),
less than about 6%
(v/v), less than about 7% (v/v), less than about 8% (v/v), less than about 9%
(v/v) or less than
about 10% (v/v). In some processes, preprimitive streak cells are grown
without serum or
without serum replacement. In still other processes, preprimitive streak cells
are grown in the
presence of B27. In such processes, the concentration of B27 supplement can
range from about
0.1% (v/v) to about 20% (v/v).
[0056] in still other processes, immature pancreatic islet hormone-
expressing cells
are grown in the presence of B27. In such processes, the concentration of B27
supplement can
range from about 0.1% (v/v) to about 20% (v/v) or in concentrations greater
than about 20%
(v/v). In certain processes, the concentration of B27 in the medium is about
0.1% (v/v), about
23
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
0.2% (v/v), about 0.3% (v/v), about 0.4% (v/v), about 0.5% (v/v), about 0.6%
(v/v), about 0.7%
(v/v), about 0.8% (v/v), about 0.9% (v/v), about 1% (v/v), about 2% (v/v),
about 3% (v/v), about
4% (v/v), about 5% (v/v), about 6% (v/v), about 7% (v/v), about 8% (v/v),
about 9% (v/v), about
10% (v/v), about 15% (v/v) or about 20% (v/v). Alternatively, the
concentration of the added
B27 supplement can be measured in terms of multiples of the strength of a
commercially
available B27 stock solution. For example, B27 is available from Invitrogen
(Carlsbad, CA) as a
50X stock solution. Addition of a sufficient amount of this stock solution to
a sufficient volume
of growth medium produces a medium supplemented with the desired amount of
B27. For
example, the addition of 10 ml of 50X B27 stock solution to 90 ml of growth
medium would
produce a growth medium supplemented with 5X B27, The concentration of B27
supplement in
the medium can be about 0.1X, about 0.2X, about 0.3X, about 0.4X, about 0.5X,
about 0.6X,
about 0.7X, about 0.8X, about 0.9X, about 1X, about 1.1X, about 1.2X, about
1.3X, about 1.4X,
about 1.5X, about 1.6X, about 1.7X, about 1.8X, about 1.9X, about 2X, about
2.5X, about 3X,
about 3.5X, about 4X, about 4.5X, about 5X, about 6X, about 7X, about 8X,
about 9X, about
10X, about 11X, about 12X, about 13X, about 14X, about 15X, about 16X, about
17X, about
18X, about 19X, about 20X and greater than about 20X.
[0057] As used herein, "exogenously added," compounds such as
growth factors,
differentiation factors, and the like, in the context of cultures or
conditioned media, refers to
growth factors that are added to the cultures or media to supplement any
compounds or growth
factors that may already be present in the culture or media. For example, in
some embodiments,
cells cultures and or cell populations do not include an exogenously-added
retinoid.
[0058] As used herein, "retinoid" refers to retinol, retinal or
retinoic acid as well as
derivatives of any of these compounds. In a preferred embodiment, the retinoid
is retinoic acid.
[0059] By "FGF family growth factor," "a fibroblast growth factor"
or "member of
the fibroblast growth factor family" is meant an FGF selected from the group
consisting of
FGF1, FGF2, FGF3, FGF4, FGF5, FGF6, FGF7, FGFR, FGF9, FGF10, FGF11, FGF12,
FGF13,
FGF14, FGF15, FGF16, FGF17, FGF18, FGF19, FGF20, FGF21, FGF22 and F0F23. In
some
embodiments, "FGF family growth factor," "a fibroblast growth factor" or
"member of the
fibroblast growth factor family" means any growth factor having homology
and/or function
similar to a known member of the fibroblast growth factor family.
[0060] As used herein, "expression" refers to the production of a
material or
substance as well as the level or amount of production of a material or
substance. Thus,
determining the expression of a specific marker refers to detecting either the
relative or absolute
24
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
amount of the marker that is expressed or simply detecting the presence or
absence of the
marker.
[0061] As used herein, "marker" refers to any molecule that can be
observed or
detected. For example, a marker can include, but is not limited to, a nucleic
acid, such as a
transcript of a specific gene, a polypeptidc product of a gene, a non-gene
product polypeptidc, a
glycoprotein, a carbohydrate, a glycolipid, a lipid, a lipoprotein or a small
molecule (for
example, molecules having a molecular weight of less than 10,000 arnu).
[0062] For most markers described herein, the official Human Genome
Organization
(HUGO) gene symbol is provided. Such symbols, which are developed by the HUGO
Gene
Nomenclature Committee, provide unique abbreviations for each of the named
human genes and
gene products. These gene symbols are readily recognized and can easily be
associated with a
corresponding unique human gene and/or protein sequence by those of ordinary
skill in the art.
[0063] In accordance with the I-RJGO designations, the following
gene symbols are
defined as follows: GHRL - ghrelin; 1APP - islet amyloid polypeptide; INS -
insulin; GCG -
glucagon; ISL1 - ISL1 transcription factor; PAX6 - paired box gene 6; PAX4 -
paired box gene
4; NEUROG3 - neurogenin 3 (NGN3); NKX2-2 - NKX2 transcription factor related,
locus 2
(NKX2.2); NKX6-1 - NKX6 transcription factor related, locus 1 (NKX6.1); IPF1 -
insulin
promoter factor 1 (PDX1); ONECUT1 - one cut domain, family member 1 (I-INF6);
HLXB9 -
homeobox B9 (HB9); TCF2 - transcription factor 2, hepatic (HNF1b); FOXA1-
forkhead box
Al; HGF - hepatocyte growth factor; IGF1 - insulin-like growth factor 1;
POU5F1 - POU
domain, class 5, transcription factor 1 (OCT4); NANOG - Nanog homeobox; SOX2 -
SRY (sex
determining region Y)-box 2; CDH1 - cadherin 1, type 1, E-cadherin (ECAD); T -
brachyury
homolog (BRACH); FGF4 - fibroblast growth factor 4; WNT3 - wingless-type MMTV
integration site family, member 3; SOX17 - SRY (sex determining region Y)-box
17; GSC -
goosecoid; CER1 - (cerberus 1, cysteine knot superfamily, homolog (CER); CXCR4
-
chemokine (C-X-C motif) receptor 4; FGF17 - fibroblast growth factor 17; FOXA2
- forkhead
box A2; SOX7 - SRY (sex determining region Y)-box 7; SOX1 - SRY (sex
determining region
Y)-box 1; AFP - alpha-fetoprotein; SPARC - secreted protein, acidic, cysteine-
rich (osteonectin);
and THBD - tlffombomodulin (TM), NCAM - neural cell adhesion molecule; SYP -
synaptophysin; ZIC1 - Zic family member 1; NEF3 - ncurofilament 3 (NFM); SST -
somatostatin; MAFA - v-maf musculoaponeurotic fibrosarcoma oncogenc homolog A;
MAFB -
v-maf musculoaponeurotic fibrosarcoma oncogene homolog B; SYP - synaptophysin;
CHGA -
chromogranin A (parathyroid secretory protein 1).
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0064] The following provides the full gene names corresponding to
non-HUGO
gene symbols as well as other abbreviations that may be used herein: SS ¨
somatostatin (SOM);
PP ¨ pancreatic polypeptidc; C-peptidc ¨ connecting peptide; Ex4 ¨ exendin 4;
MC ¨
nicotinamide and DAPT ¨ N4N-(3,5-difluorophenacety1)-L-alanyIJ-S-phenylglycine
t-butyl
ester; RA ¨ retinoic acid; RPM1 ¨ Roswell Park Memorial Institute medium; CMRL
¨
Connaught Medical Research Labs medium; FBS ¨ fetal bovine serum; NBPIO - NCAM
binding
protein 10; PTFla ¨ pancreas specific transcription factor la.
[0065] The progression of pluripotent cells to various multipotent
and/or
differentiated cells can be monitored by determining the relative expression
of genes, or gene
markers, characteristic of a specific cell, as compared to the expression of a
second or control
gene, e.g., housekeeping genes. In some processes, the expression of certain
markers is
determined by detecting the presence or absence of the marker. Alternatively,
the expression of
certain markers can be determined by measuring the level at which the marker
is present in the
cells of the cell culture or cell population. In such processes, the
measurement of marker
expression can be qualitative or quantitative. One method of quantitating the
expression of
markers that are produced by marker genes is through the use of quantitative
PCR (Q-PCR).
Methods of performing Q-PCR are well known in the art. Other methods which are
known in
the art can also be used to quantitate marker gene expression. For example,
the expression of a
marker gene product can be detected by using antibodies specific for the
marker gene product of
interest.
[0066] In some processes, the higher expression of the following
genes are indicative
of certain populations of cells, for example: SOX17, SOX7, AFP or THBD are
indicative of
extraembryonic endoderm; NODAL and/or FGF8 are indicative of preprimitive
strealc;
brachyury, FGF4, SNAI1 and/or WNT3 are indicative of mesendoderrn; CER, GSC,
CXCR4,
SOX17 and FOXA2 are indicative of definitive endoderm cells; SOX17, FOXA2,
FOXA1,
HNF1B and EINF4A are indicative of foregut endoderm (or PDX1-negative
endoderm); PDX1,
HNF6, SOX9 and PROX1 are indicative PDX1-positive endoderm; PDX1, NKX6.1,
PTFA1,
CPA and cMYC are indicative of pancreatic epithelium (PE or pancreatic
progenitor); NGN3,
PAX4, ARX and NK.X2.2 are indicate of endocrine precursor cells; and INS, GCG,
GHRL, SST
and PP arc indicative of the various endocrine cells; relative high MAFA to
MAFB gene
expression is indicative of insulin secreting endocrine cell; and relative
high expression of
MAFB to MAFA gene expression is indicative of glucagon secreting endocrine
cells.
[0067] The terms fibroblast growth factor 7 (FGF7) and keratinocyte
growth factor
(KGF) are synonymous.
26
Date Recue/Date Received 2023-09-08
Methods for production of induced pluripotent stem (iPS) cells
[0068] Embodiments described herein are not limited to any one type of
iPS cell or any
one method of producing the iPS cell. Embodiments are not limited or dependent
on levels of
efficiency of production of the iPS cells, because various methods exist.
Embodiments described
herein apply to differentiation of iPS cells into endoderm-lineage cells and
uses thereof.
[0069] Viral, nonviral and nonintegrating viral methods for generating
induced
pluripotent stem cells (iPSCs) using adenovirus, plasmids or excision of
reprogramming factors using
Cre-loxP3, or piggyBAC transposition have been described. See Stadtfeld, M.,
et al., Science 322,
945-949 (2008); Okita, K. et al., Science 322, 949-953 (2008); Kaji, K. et al.
Nature 458, 771-775
(2009); Soldner, F. et al. Cell 136, 964-977 (2009); and Woltjen, K. et at
Nature 458, 766-770
(2009. Also, see U.S. Patent Application number 20100003757 to Mack, A. et al.
(published January
7, 2010) and No.: PCT/US2009/037429 to Shi et al. These methods, however, have
low
reprogramming efficiencies (<0.003%), and may leave residual vector sequences
despite excision,
which limits their therapeutic applications. For example, viral integration in
the host genome and
over expression of the above transcription factors has been associated with
tumorigenesis; and a
residual transgene expression is potentially the feature which distinguishes
ES cells and iPS cells.
See Solder, F. et al., Cell 136:964-977 (2009); Foster et al., Oncogene
24:1491-1500 (2005); and
Hochedlinger, K. et al., Cell 121:465-477 (2005)..
[0070] In other embodiments of the invention, methods for generating
iPSCs include
episomal vectors derived from the Epstein-Barr virus. See Yu, J. et al.
Science 324, 797-801 (2009)
and U.S. Application 20100003757to Mack, A. et al. published on January 7,
2010. These methods
require three separate plasmids carrying a combination of seven factors,
including the oncogene
SV40.
[0071] In another embodiment of the invention, methods for generating
iPSCs include
protein-based iPSCs from mouse and human fetal and neonatal cells. See Zhou,
H. et al. Cell Stem
Cell 4, 381-384 (2009); and Kim, D. et al. Cell Stem Cell 4, 472-476 (2009).
These methodologies
are accomplished using a chemical treatment (e.g. valproic acid in the case of
Thou et al. 2009 supra)
or many rounds of treatment (Kim et al. 2009, supra).
[0072] In another embodiment of the invention, minicircle vectors or
plasmids, which
are supercoiled DNA molecules that lack a bacterial origin of replication and
antibiotic resistance
genes, can be used. See Chen, Z.-Y. et al., Mot Ther. 8, 495-500 (2003); Chen,
Z.-Y. et al., Hum.
27
Date Recue/Date Received 2023-09-08
Gene Ther. 16, 126-131(2005); and Jia, F. et al., Nature Methods Advance
Publication Online 7
February 2010. These methodologies generate iPSCs with higher transfection
efficiencies and longer
ectopic expression because they have lower activation of exogenous silencing
mechanisms.
[0073]
Still in another embodiment of the invention, iPS cells can be generated from
human patients with various diseases including, diabetic patients, ALS, spinal
muscular dystrophy
and Parkinson patients. See Maehr et al. PNAS USA 106(37):15768-73 (2009);
Dimos et al.,
Science, 321:1218-21 (2008); Ebert et al. Nature 457:277-80 (2009); Park et
al. Cell 134:877-886
(2008); and Soldner et al., Cell 136:964-977. At least one advantage of
producing hIPS cells from
patients with specific diseases is that the cell derived would contain the
genotype and cellular
responses of the human disease. Also, see Table 3 listing at least some
existing human iPS cell lines.
This information was derived from the literature and publically available
databases including for
example the National Institutes of Health (NIH) Stem Cell Registry, the Human
Embryonic Stem
Cell Registry and the International Stem Cell Registry located at the
University of Massachusetts
Medical School, Worcester, Massachusetts, USA. These databases are
periodically updated as cell
lines become available and registration obtained.
Embodiments of the compositions and methods described herein contemplate the
use of
various differentiable primate pluripotent stem cells including human
pluripotent stem cells such as
hESC, including but not limited to, CyT49, CyT212, CyT203, CyT25,
(commercially available at
least at the time of filing of this instant application from ViaCyte Inc.
located at 3550 General
Atpmics Court, San Deigo CA 92121) BG01, BG02 and MEL1, and induced
pluripotent stem (iPS)
cells such as iPSC-482c7 and iPSC-603 (Cellular Dynamics International, Inc.,
Madison, Wisconsin)
and iPSC-G4 (hereinafter "G4") and iPSC-B7 (hereinafter, "B7") (Shinya
Yamanaka, Center for iPS
Cell Research, Kyoto University); studies using G4 and B7 are described in
detail herein. Certain of
these human pluripotent stem cells are registered with national registries
such as the National
Institutes of Health (NIH) and listed in the NIH Human Stem Cell Registry
(e.g., CyT49 Registration
No. #0041). Information on CyT49, other available cell lines can also be found
on the worldwide
web at stemcells.nih.gov/research/registry. Still other cell lines, e.g., BG01
and BGOlv, are sold and
distributed to third parties by WiCell , an affiliate of the Wisconsin
International Stem Cell (W1SC)
Bank (Catalog name, BG01) and ATCC (Catalog No. SCRC-2002), respectively.
While other cell
lines described herein may not be registered or distributed by a biological
repository, such as
WiCell or ATCC, such cell lines are available to the public directly or
indirectly from the principle
investigators, laboratories and / or institutions. Public requests for cell
lines and reagents, for
28
Date Recue/Date Received 2023-09-08
example, are customary for those skilled in the art in the life sciences.
Typically, transfer of these
cells or materials is by way of a standard material transfer agreement between
the proprietor of the
cell line or material and the recipient. These types of material transfers
occur frequently in a research
environment, particularly in the life sciences. In fact, Applicant has
routinely transferred cells since
the time they were derived and characterized, including CyT49 (2006), CyT203
(2005), Cyt212
(2009), CyT25 (2002), BG01 (2001), BG02 (2001), BG03 (2001) and BGOlv (2004),
through such
agreements with commercial and non-profit industry partners and collaborators.
The year in
parenthesis next to each cell line in the previous list indicates the year
when the cell lines or materials
became publically available and immortal (e.g. cell banks were made) and thus
destruction of another
embryo has not been performed or required since the establishment of these
cell lines in order to
make the compositions and practice the methods described herein.
In August 2006, Klimanskaya et al. demonstrated that hESC can be derived from
single
blastomeres, hence keeping the embryo intact and not causing their
destruction. Biopsies were
performed from each embryo using micromanipulation techniques and nineteen
(19) ES-cell-like
outgrowths and two (2) stable hESC lines were obtained. These hESC lines were
able to be
maintained in an undifferentiated state for over six (6) months, and showed
normal karyotype and
expression of markers of pluripotency, including Oct-4, SSEA-3, SSEA-4, TRA-1-
60, TRA-1-81,
Nanog and Alkaline Phosphatase. These hESC can differentiate and form
derivatives of all three (3)
embryonic germ layers both in vitro and form in teratomas in vivo. These
methods to create new
stem cell lines without destruction of embryos addresses the ethical concerns
of using human
embryos. See Klimanskaya et at. (2006) Nature 444:481-5, Epub 2006 Aug 23.
However,
Klimanskaya et al. co-cultured the derived hESC line with other hESC. Later,
in 2008, Chung Y. et
al., were able to obtain hES cell lines again from a single blastomere but
without co-culture with
hESC. See Chung Y. et al., Cell Stem Cell 2008, 2(2), 113-117. Thus the
compositions and methods
described herein, and in particular, the such compositions and methods as
related to induced
pluripotent stem cells or genetically dedifferentiated pluripotent stem cells,
do not require the
destruction of a human embryo
29
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Tables 3 and 4 are non-exhaustive lists of certain iPSC and hESCs,
respectively, which
are available worldwide for research and/or commercial purposes, and are
suitable for use in the
methods and compositions of the present invention. The information in Tables 3
and 4 were
derived from the literature and publically available databases including, for
example, the
National Institutes of Health (NIH) Human Stem Cell Registry, the Human
Embryonic Stem Cell
Registry and the International Stem Cell Registry located at the University of
Massachusetts
Medical School, Worcester, Massachusetts, USA. These databases are
periodically updated as
cell lines become available and registration obtained.
Human iPSC described herein (at least iPSC-603 and iPSC-482-c7) were provided
by
Cellular Dynamics international, Inc. (Madison, Wisconsin, USA).
Table 3: Listing of human induced pluripotent stem (hIPS) cell lines
University of 1. IPS(FORESKIN)-1 (Normal; 46XY; Yu, J., et al.
[Thomson' Science.
Wisconsin ¨ Madison 2007 Induced pluripotent stem cell lines derived
from human somatic
(USA) cells 318(5858):1917-20.)
2. IPS(FORESKIN)-2 (Normal; 46XY; Yu, J., et al. [Thomson] Science.
2007 Induced pluripotent stem cell lines derived from human somatic
cells 318(5858):1917-20.)
3. IPS(FORESKIN)-3 (Normal; 46XY; Yu, J., et al. [Thomson] Science.
2007 Induced pluripotent stem cell lines derived from human somatic
cells 318(5858):1917-20.)
4. WS(FORESKIN)-4 (Normal; 46XY; Yu, J., et al. [Thomson] Science.
2007 Induced pluripotent stem cell lines derived from human somatic
cells 318(5858):1917-20.)
5. IPS(IMR90)-1 (Normal; 46XX; Yu, J., et al. [Thomson] Science. 2007
Induced pluripotent stem cell lines derived from human somatic cells
318(5858):1917-20.)
6. IPS(IMR90)-2 (Normal; 46XX; Yu, J., et al. [Thomson] Science. 2007
Induced pluripotent stem cell lines derived from human somatic cells
318(5858):1917-20.)
7. IPS(IMR90)-3 (Normal; 46XX; Yu, J.. et al. [Thomson] Science. 2007
Induced pluripotent stem cell lines derived from human somatic cells
318(5858):1917-20.)
8. IPS(IMR90)-4 (Normal; 46XX; Yu, J., et al. [Thomson] Science. 2007
Induced pluripotent stem cell lines derived from human somatic cells
318(5858):l 917-20.)
9. IPS-SMA-3.5 (Normal; 46XY; Type 1 Spinal Muscular Atrophy; Ebert,
A. D., et al. 2009. Induml pluripotent stem cells from a spinal
muscular atrophy patient Nature. 457:277-80)
10.IPS-SMA-3.6 (Normal; 46XY; Type 1 Spinal Muscular Atrophy; Ebert,
A. D., et al. 2009. Induced pluripotent stern cells from a spinal
Date Reeue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
muscular atrophy patient Nature. 457:277-80)
11.IPS-WT (Normal; 46XX; Type I Spinal Muscular Atrophy; Ebert, A.
D., et al. 2009. Induced pluripotent stem cells from a spinal muscular
atrophy patient Nature. 457:277-80)
University of 1. IPS-1 (Kammbayaram, S. et al. 2009. Directed
Differentiation of
California, Los Human-Induced Pluripotent Stem Cells Generates
Active Motor
Angeles (USA) NeuronsStem Cells. 27:806-811; Lowry, W. E., et al.
2008. Generation
of human induced pluripotent stem cells from dermal fibroblasts Proc
Natl Acad Sci U S A. [05:2883-8)
2. IPS-2 (Karumbayaram, S. et al. 2009. Directed Differentiation of
Human-Induced Pluripotent Stem Cells Generates Active Motor
NeuronsStem Cells. 27:806-811; Lowry, W. E., et al. 2008. Generation
of human induced pluripotent stem cells from dermal tibroblastsProc
Natl Acad Sci U S A. 105:2883-8)
3. IPS-5 (Lowry, W. E., et al. 2008. Generation of human induced
pluripotent stern cells from dermal fibroblasts Proc Natl Acad Sci U S
A. 105:2883-8)
4. IPS-7 (Lowry, W. E., et al. 2008. Generation of human induced
pluripotent stem cells from delinal fibroblasts Proc Natl Acad Sci U S
A. 105:2883-8)
5. IPS-18 (Karumbayaram, S. et al. 2009. Directed Differentiation of
Human-Induced Pluripotent Stem Cells Generates Active Motor
NeuronsStem Cells, 27:806-811; Lowry, W. E., et al. 2008. Generation
of human induced pluripotent stem cells from denial fibroblastsProc
Natl Acad Sci U S A. 105:2883-8)
6. IPS-24 (Lowry, W. E., et al. 2008. Generation of human induced
pluripotent stem cells from dermal fibroblasts Proc Natl Acall Sci U S
A. 105:2883-8)
7. IPS-29 (Lowry, W. E., et al. 2008. Generation of human induced
pluripotent stem cells from dermal fibroblasts Proc Nall Acad Sci U S
A. 105:2883-8)
Mt. Sinai Hospital 1. 60 (Woltjen, K. et al. 2009. PiggyBac
transposition reprograms
(Samuel Lunenfeld fibroblasts to induced pluripotent stem cells
Nature, 458(7239):766-70)
Research Institute;
USA) 2. 61 (Woltjen, K. et al. 2009. PiggyBac
transposition reprograms
fibroblasts to induced pluripotent stern cells Nature. 458(7239):766-70)
3. 66 (Woltjen, K. et al. 2009. PiggyBac transposition reprograms
fibroblasts to induced pluripotent stem cells Nature 458(7239):766-70)
4. 67 (Woltjen, K. et al, 2009. PiggyBac transposition reprograms
fibroblasts to induced pluripotent stem cells Nature 458(7239):766-70)
5. HIPSC117 (Kaji K, et al. 2009 Virus-free induction of pluripotency
and subsequent excision of reprogramming factors Nature
458(7239):771-5)
6. HIPSC121 (Kaji K, et al. 2009 Virus-free induction of pluripotency
and subsequent excision of reprogramming factors Nature
458(7239):771-5)
31
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
7. HIPSC122 (Kaji K, et al. 2009 Virus-free induction of pluripotency
and subsequent excision of reprogramming factors Nature
458(7239):771-5)
Children's Hospital - 1. 55 1-IPS8 (Park HI, et al. 2008. Reprogramming of
human somatic cells
Boston (USA) to plurilxiteney with defined factors Nature
451:141-6).
2. ADA-IPS2 ((ADA-SCID) Adenosine Deaminase Deficiency-related
Severe Combined Immunodeficiency (GG(>AGG, exon 7, ADA
gene); Park, I. H. et al. 2008. Disease-Specific Induced Pluripotent
Stem Cells Cell 134(5):877-86)
3. ADA-IPS3 ((ADA-SCID) Adenosine Deaminase Deficiency-related
Severe Combined Immunodeficiency (GGG>AGG, exon 7, ADA
gene); Park, I. H. et al. 2008, Disease-Specific Induced Pluripotent
Stem Cells Ce11134(5):877-86)
4. BJI -TPSI (Park, I. H. et al. 2008. Disease-Specific Induced Pluripotent
Stem Cells Cell 134(5):877-86)
5. BMD-IPS1 (Male; (BMD) Becker Muscular Dystrophy (Unidentified
mutation in dystrophin); Park, I. H. et al. 2008, Disease-Specific
Induced Pluripotent Stem Cells Cell 134(5):877-86)
6. BMD-IPS4 (Normal; 46XY, (BMD) Becker Muscular Dystrophy
(Unidentified mutation in dystrophin); Park, I. H. et al. 2008, Disease-
Specific induced Pluripotent Stem Cells Cell 134(5):877-86)
7. DH1CH 6-IPS1 (Normal; 46XY; Park, I. H. et at. 2008. Disease-
Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
8. DHICF32-IPS2 (Male; Park, I. H. et al. 2008. Disease-Specific Induced
Pluripotent Stem Cells Cell 134(5):877-86)
9. DR 1F-IPS3-3(Normal; 46XY; Park, I. H. et al. 2008. Disease-Specitic
Induced Pluripotent Stem Cells Cell 134(5):877-86)
10.DMD-IPS1 ((Normal; 46XY; DMD) Duchenne Muscular Dystrophy
(Deletion of exon 45-52, dystrophin gene; Park, I. H. et at. 2008.
Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
11.DMD-IPS2 (Male; (DMD) Duchenne Muscular Dystrophy (Deletion of
exon 45-52, dystrophin gene; Park, I. H. et al. 2008. Disease-Specific
Induced Pluripotent Stem Cells Cell 134(5):877-86)
12.DS1-IPS4 (Male; Down syndrome (Trisomy 21); Park, I. H. eta!, 2008.
Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
13.DS2-IPS1 (Male; Down syndrome (Trisomy 21); Park,!. H. et al. 2008.
Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
14.DS2-IPSI0 (Male; Down syndrome (Trisomy 21); Park, I. H. et al.
2008. Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-
86)
15.0D-IPS1(Male; (GD) Gaucher Disease type III (AAC > AGC, exon 9,
G-insertion, nucleotide 84 of cDNA, GBA gene; Park, I. H. et at. 2008.
Disease-Specific induced Pluripotent Stem Cells Cell 134(5):877-86)
16.GD-IPS3 (Male; (GD) Gauchcr Disease type III (AAC > AGC, exon 9,
G-insertion, nucleotide 84 of cDNA, GBA gene; Park, L H. et al. 2008.
32
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
17.HFIB2-IPS2 (Park, I. ii., et al. 2008. Generation of human-induced
pluripotent stem cells Nat Protoc. 3:1180-6 ; Park, I. It et al, 2008.
Reprogramming of human somatic cells to pluripotency with defined
factors Nature 451:141-6)
18.HFIB2-IPS4 (Park, I. H., et al. 2008. Generation of human-induced
pluripotent stem cells Nat Protoc. 3:1180-6 ; Park, I. H. et al. 2008.
Reprogramming of human somatic cells to pluripotency with defined
factors Nature 451:141-6)
19.HFIB2-IPS5 (Park, I. H., etal. 2008. Generation of human-induced
pluripotent stem cells Nat Protoc. 3:1180-6 ; Park, I. H. et al. 2008.
Reprogramming of human somatic cells to pluripotency with defined
factors Nature 451:141-6)
20.JDM-IPS1 (Normal, 46XX; Juvenile diabetes mellitus (multifactorial);
Park, 1. H. et al. 2008. Disease-Specific induced Pluripotent Stem Cells
Cell 134(5):877-86)
21.JDM-IPS1 (Normal, 46XX; Juvenile diabetes mellitus (multifactorial);
Park, I. H. et al. 2008. Disease-Specific Induced Pluripotent Stem Cells
Cell 134(5):877-86)
22.JDM-IPS2 (Female; Juvenile diabetes mellitus (multifactorial); Park, I.
H. et al. 2008, Disease-Specific Induced Pluripotent Stem Cells Cell
134(5):877-86)
23.JDM-IPS3 (Female; Juvenile diabetes mellitus (multifactorial); Park, I.
H. et al. 2008, Disease-Specific Induced Pluripotent Stem Cells Cell
134(5):877-86)
24.LNSC-IPS2 (Female; Lesch-Nyhan syndrome (carrier, heterozygosity
of 1-1FIC11; Park, I. H. et at. 2008. Disease-Specific Induced Pluripotent
Stem Cells Cell 134(5):877-86)
25.MRC5-IPS7 (Male; Park, I. H. et al. 2008. Disease-Specific Induced
Pluripotent Stem Cells Ce11134(5):877-86)
26.MRC5-1PS 12 (Normal; 46XY; Park, 1. H. etal. 2008. Disease-Specific
Induced Pluripotent Stem Cells Cell 134(5):877-86)
27.MRC5-IPS1 (Male; Park, I. H. et at 2008. Disease-Specific Induced
Pluripotent Stem Cells Cell 134(5):877-86)
28.PD-1PS1 (Male; Parkinson disease (multifactorial); Park, 1. H. et al,
2008. Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-
86)
29.SBDS-IPS1 (Male; Swaehman-Bodian-Diamond syndrome (IV2 +
21>C and IV3 - 1G>A, SBDS gene; Park, I. H. et al. 2008. Disease-
Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
30.SBDS-IPS2
31.SBDS-IPS3 (Normal; 46XY; Swachman-Bodian-Diamond syndrome
(I12 + 2T>C, and IV3 - 1G>A, SBDS gene; Park, I. H. et al. 2008.
Disease-Specific Induced Pluripotent Stem Cells Cell 134(5):877-86)
Harvard University 1. A29a (46XX; (ALS) Amyotrophic Lateral Sclerosis
(L144F [Leu144 >
33
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
(USA) Phe] dominant allele of the superoxide dismutase
(SOD1) gene;
Caucasian; Dimos, J. T., et al. 2008. Induced pluripotent stem cells
generated from patients with ALS can be differentiated into motor
neurons Science. 321:1218-21)
2. A29b (46XX; (ALS) Amyotrophic Lateral Sclerosis (L144F [Leu144 >
Phe] dominant allele of the superoxide dismutase (SOD1) gene;
Caucasian; Dimos, J. T., et al. 2008. Induced pluripotent stem cells
generated from patients with ALS can be differentiated into motor
neurons Science. 321:1218-21)
3. A29c (46XX; (ALS) Amyotrophic Lateral Sclerosis (L144F [Leu144 >
Phe] dominant allele of the superoxide dismutase (SOD1) gene;
Caucasian; Dirnos, J. T., et al. 2008. Induced pluripotent stem cells
generated from patients with ALS can be differentiated into motor
neurons Science 321:1218-21)
Salk Institute (USA) 1. HAIR-IPS1 (Aasen, T., et al [Belmonte, J. C,]
2008. Efficient and rapid
generation of induced pluripotent stem cells from human keratinocytes
Nat Biotechnol 26:1276-84)
2. HAIR-IPS2 (Aasen, T,, et al [Belmonte, J. C,]2008. Efficient and rapid
generation of induced pluripotent stem cells from human keratinocytes
Nat Biotechnol 26;1276-84)
Royan Institute (Iran) 1. R.1.H.iPSC.1(OCT4, Sox2, KLF4, e-Myc; Human
fibroblasts)
2. BOM.I.HIPSC.1 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
3. FHC.1.H.iP SC.3 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
4. GSD.1.H.iP SC.7 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
5. TYR.1.H.iP SC.1 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
6. HER.1.H.iPSC.I. (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
7. R.1.1-LiPSC.4 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
8. R.1.H.iPSC.9 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
9. RP2.H3PSC.3 (OCT4, Sox2, KLF4, c-Myc; IN cells)
10.LCA.1.H.iPSC.1 (OCT4, Sox2, KLF4, c-Myc; iPS cells)
11.USII, 1 .H.iP SC.6 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
12.RP.1.11.iPSC.2 (OCT4, Sox2, KLF4, c-Myc; Human fibroblasts)
13.ARMD.1.11.iPSC.2 (OCT4, Sox2, KLF4, e-Myc; Human fibroblasts)
14.LIION.1.1UPSC.5 (OC1'4, Sox2, KLF4, c-Myc; iPS cells)
15.CNS.1.H.iPSC.10 (0CT4, Sox2, KLF4, c-Myc; iPS cells)
16.CNS.2.H.iPSC.7 (OCT4, Sox2, KLF4, c-Myc; iPS cells)
Centre of Regenerative 1. KiPS4F-1 (OCT4, Sox2, KLF4, c-Myc; human foreskin
keratinocytes;
Medicine in Barcelona 46XY)
(Spain)
2. KiPS3F-7 (OCT4, Sox2, KLF4); human foreskin keratinocytes)
3. KiPS4F-8 (OCT4, Sox2, KLF4, c-Myc human foreskin keratinocytes;
46XY)
4. cFA404-KiPS4F-1 (OCT4, Sox2, KLF4, c-Myc; Epidermal
keratinocytes; 46XY)
5. cFA404-KiPS4F-3 (OCT4, Sox2, KLF4, c-Myc; Epidermal
keratinocytes; 46XY)
34
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCT/US2014/015156
Universite Paris-Sud 11 1. PB03 (0ct4, Sox2, Lin28, Nanog; Primary
Amniocytes; 46XX;
(France) Lentivirus)
2. PB04 (0c14, Sox2, Lin28, Nanog; Primary Amniocytes; B-Thalassemia
affected; 46XY; Lentivirus)
3. PB05-1 (Oct4, Sox2, Lin28, Nanog; Primary Amniocytes; B-
Thalassernia affected; 46XY; Lentivirus)
4. PB05 (0c14, Sox2, Lin28, Nanog; Primary Amniocytes; B-Thalassemia
affected; 46XY; Lentivirus)
5. PB06 (004, Sox2, Lin28, Nanog; Primary Amniocytes; Down
Syndrome; 47XY, +21; Lentivirus)
6. PB06-1 (0ct4, Sox2, Lin28, Nanog; Primary Amniocytes; Down
Syndrome; 47XY, +21; Lentivirus)
7. PB07 (OCT4, Sox2, KI,F4, c-Myc; Primary Amniocytes; 46XY;
Retrotivirus)
8. PB08 (0CT4, Sox2, KLF4, c-Myc; Primary Amniocytes; 46XY;
Retrotivirus)
9, P1309 (0ct4, Sox2, Lin28, Nanog; Primary Amniocytes; 46XY;
Lentivirus)
10.PB10 (0ct4, Sox2; Primary Arnniocytes46XY, Lentivirus)
Kyoto University 1. 201131 (human fibroblast; 46XX)
(Japan)
2. 201B2 (human fibroblast; 46XX)
3. 201B3 (human fibroblast; 46XX)
4. 201136 (human fibroblast; 46XX)
5. 201B7 (human fibroblast; 46XX)
6. 243H1 (human fibroblast)
7. 243H7 (human fibroblast)
8. 246B1 (Normal, 46XX)
9. 24612 (Normal, 463(X)
10.246B3 (Normal, 463(X)
11.246B4 (Normal, 463(X)
12.246135 (Normal, 463(X)
13.246B6 (Normal, 46XX)
14.24631 (human fibroblast; Takahashi, K., et al. 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
15.246C13 (human fibroblast; Takahashi, K., et al. 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
16.24634 (human fibroblast; Takahashi, K., et al. 2007. Induction of
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
pluripotent stem cells from adult human fibroblasts by defined factors
Cell, 131:861-72)
17.246G5 (human fibroblast; Takahashi, K., et at. 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
18.246G6 (human fibroblast; Takahashi, K., et at. 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
19.253F1 (Normal, 46XX; Takahashi, K,, et al, 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
20.253F2 (Normal, 46XX; Takahashi, K., et al. 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
21.253F3 (Normal, 46XX; Takahashi, K., et al. 2007. Induction of
pluripotent stern cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
22.253F4 (Normal, 46XX; Takahashi, K., et al. 2007. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors
Cell. 131:861-72)
23.253F5 (Normal, 46XX; Takahashi, K., et al. 2007. Induction of
pluripotent stem cells from adult human tibroblasts by delmed tactors
Cell. 131:861-72)
Shanghai Institutes for 24.HAFDC-TPS-6 (Li C., et al. 2009 Pluripotency can
be rapidly and
Biological Sciences efficiently induced in human amniotic fluid-derived
cells Hum Mol
(China) Genet. 2009 Nov 15;18(22):4340-9)
25.IPS-S (Liao, J., et al. 2008. Enhanced efficiency of generating induced
pluripotent stem (iPS) cells from human somatic cells by a combination
of six transcription factors Cell Res. 18:600-3)
[0074] Another advantage is that such hIPS cells would be an
immunologically
matched autologous cell population; and patient-specific cells would allow for
studying origin
and progression of the disease. Thus, it is possible to understand the root
causes of a disease,
which can provide insights leading to development of prophylactic and
therapeutic treatments for
the disease.
Pluripotent human embryonic stem (hES) cells
[0075] Some embodiments are directed to methods for deriving
definitive endoderm
cells and ultimately any endoderm-lineage derived cell type, including but not
limited to, foregut
endoderm, pancreatic endoderm, endocrine precursor cells and/or pancreatic
islet hormone-
expressing cells using human embryonic stem (hES) cells as the starting
material. These hES
36
Date Recue/Date Received 2023-09-08
cells can be cells that originate from the morula, embryonic inner cell mass
or those obtained from
embryonic gonadal ridges. Human embryonic stem cells can be maintained in
culture in a
pluripotent state without substantial differentiation using methods that are
known in the art. Such
methods are described, for example, in US Patent Nos. 5,453,357, 5,670,372,
5,690,926 5,843,780,
6,200,806 and 6,251,671.
[0076] In some processes, pluripotent stem cells, e.g. hES cells, are
maintained on a
feeder layer. In such processes, any feeder layer which allows pluripotent
cell to be maintained in a
pluripotent state can be used. One commonly used feeder layer for the
cultivation of human
embryonic stem cells is a layer of mouse fibroblasts. More recently, human
fibroblast feeder layers
have been developed for use in the cultivation of pluripotent cell (see US
Patent Application
Publication No. 2002/0072117). Alternative processes permit the maintenance of
pluripotent cells
without the use of a feeder layer. Methods of maintaining pluripotent cells
under feeder-free
conditions have been described in US Patent Application Publication No.
2003/0175956.
[0077] The pluripotent cells described herein can be maintained in
culture either with or
without serum, with or without extracellular matrix, with or without FGF. In
some pluripotent cell
maintenance procedures, serum replacement is used. These and other methods for
culturing and
differentiation pluripotent or multipotent cells, respectively, are described
in PCT/U52007/062755,
filed February 23, 2007, and titled Compositions and methods for culturing
differential cells and
PCT/US2008/080516, filed October 20, 2008, and titled Methods and compositions
for feeder-free
pluripotent stem cell media containing human serum.
[0078] The invention described herein is useful with all hES cell
lines, and at least those
listed in Table 4. This information was derived from the literature and
publically available databases
including for example the National Institutes of Health (NTH) Stem Cell
Registry, the Human
Embryonic Stem Cell Registry and the International Stem Cell Registry located
at the University of
Massachusetts Medical School, Worcester, Massachusetts, USA. These databases
are periodically
updated as cell lines become available and registration obtained. As of the
filing date of this
application there were 254 iPSC lines listed with the Internation Stem Cell
Registry and 1211 hESC
lines. Table 4 below is not inclusive of all hESC and iPSC that are listed,
but rather, are examples of
the pluripotent stem cells potentially available.
37
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
Table 4: Listing of human embryonic stem (hES) cell lines
Institution (Country) Name
U.S.A.
BresaGen, Inc., Athens, Georgia (USA) BGOI, B002, BG03; BG04; BGO lv
Invitrogen (USA) BGOlv/hOG
CyThera, Inc., San Diego, California (USA)
CyT49, CyT203, CyT25
Geron Corporation, Menlo Park, California GE01 , GE07, GEN, GE13, GE14,
GE91, GE92 (HI,
(USA) H7, H9, H13, H14, 119.1, 119.2)
University of California, San Francisco, UC01, UCO6 (11SF-1, HSF-6);
UCSFB1, UCSF132,
Calithmia (USA) UCSFB3, UCSFB4, UCSFB5, UCSFB6, UCSFB7,
UCSFB8, UCSFB9 & UCSF1310
Wisconsin Alumni Research Foundation, WAOI, WA07, WA09, WA13, WAI4 (H1,
H7,1-19,
Madison, Wisconsin (USA) H13,1-114)
Children's Hospital Corporation (USA) CHB-1, CHB-2 CHB-3 CHB-4, CHB-5, CHB-
6,
CHB-8, CHB-9, CHB-10, CHB-11 & CHB-12
The Rockefeller University (USA) RUES1, RUES2 & RUES3
Harvard University (USA) HUES1, HUES2, HUES3,HUES4, HUES5,HUES6,
HUES7, HUES8, HUES9, HUES10, H1JES11,
HUES12, HUES13, HUES14, HUES15, 111JES16,
HUES17, HUES18, HUE S19, HUES20, HUES21,
HUES22, HUES23, HUES24, HUES25, HUES26,
H1JES27; HUES28; HUES48; HUES49; 111JES53;
11UES55 & HUES 56
Mt Sinai Hospital-Samuel Lunenfeld Research
CAI 84 CA/
Institute (USA)
Children's Memorial Hospital (USA) CM-1, CM-2, CM-5, CM-6, CM-7, CM-8, CM-
11,
CM-12, CM-13, CM-14, CM-16
The University of Texas Health Science Center
CR1 & CR2
at Houston (USA)
California Stem Cell, Inc. (USA) CSCI4
University of Connecticut School of
CSC14, CT1, CT2, CT3, & CT4
Medicine/Dentistry (USA)
The Third Affiliated Hospital of Guangzhou FY-3PN; FY-hES-1; FY-hES-3; FY-
hES-5; FY-hES-
Medical College (USA) 7 & FY-hES-8
Advanced Cell Technology, Inc. (USA) MA 01; MA 09; MA 42; MA 50; MA135; NED
1;
NED 2; NED 3 & NED 4
Stanford University (USA) MESS
New York University School of Medicine NYUES1; NYUES2; NYUES3; NY1JES4;
NYUES5;
(USA) NYUES6 & NYUES7
Reprogenetics, LLC (USA) RN.I7
University of California, Los Angeles (USA) UCLA]; UCLA2 & UCLA3
Eastern Virginia Medical School (USA) ES-76; ES-78-1; ES-78-2
Reproductive Genetics Institute (USA) RG-222; RG-230; RG-249; RG-308; RG-
313;
38
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
RG-148; DYSTROPHIA IVIYOTONICA 1 (DM1),
affected, 46,XY;
RG-153; DYSTROPHIA MYOTONICA I (DM1),
affected, 46,XX;
RG-170; MUSCULAR DYSTROPHY, BECKER
TYPE (BMD), affected, 46,XY;
RG-186; HUNTINGTON DISEASE (HD), affected,
46,XX;
RG-I 94; HUNTINGTON DISEASE (HD), affected,
46,XY;
RG-233; HEMOGLOBIN B LOCUS (HBB), affected
(HbS/HbS - sickle cell anemia), 46,XX;
RG-245; EMERY-DREIFUSS MUSCULAR
DYSTROPHY, X-LINKED (EDMD), carrier,
47,XXY;
RG-246; EMERY-DRE1FUSS MUSCULAR
DYSTROPHY, X-LINKED (EDMD), affected,
46,XY;
RG-271; TORSION DYSTONIA 1 ( DYT1),
AUTOSOMAL DOMINANT, affected (N/GAG del),
46,XY;
RG-283; MUSCULAR DYSTROPHY, DUCHENNE
TYPE (DMD), affected, 46,XY;
RG-288; CYSTIC FIBROSIS (CF), affected
(de1taF508/deltaF508), 46,XY;
RG-289; CYSTIC FIBROSIS (CF), affected
(deltaF508/deltaF508), 46,XX;
RG-301; MUSCULAR DYSTROPHY, DUCHENNE
TYPE( DMD) affected, 46,XY;
RG-302; MUSCULAR DYSTROPHY, DUCHENNE
TYPE (DMD), carrier, 46,XX;
RG-315; NEUROFIBROMATOSIS, TYPE I (NF I),
affected (Rl9 47X/N), 46,XY;
RG-316; TUBEROUS SCLEROSIS, TYPE1(TSC I),
affected (N/IVS7+1 G-A);
RG-316; TUBEROUS SCLEROSIS, TYPE 1(TSCI),
affected (N/IVS7+1 G-A);
RG-320; TUBEROUS SCLEROSIS, TYPE 1(TSCI),
affected (N/IVS7+1 G-A);
RG-326; POPL1TEAL PTERYGIUM SYNDROME
(PPS),affected (R84H/N), 46,XY;
RG-328; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY 1A( FSHD), affected,
46,XY;
RG-330; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY lA (FSHD), affected,
46,XY;
RG-333; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY IA (FSHD), affected,
46,XX;
RG-356; HEMOGLOBIN ALPHA LOCUS (HBA),
affected (-alpha /--), 46,XX;
39
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCT/US2014/015156
RG-357; EMERY-DREIFUSS MUSCULAR
DYSTROPHY, X-LINKED (EDMD), affected,
46,XY;
RG-358; EMERY-DREIFUSS MUSCULAR
DYSTROPHY, X-LINKED (EDMD), affected,
46,XY;
RG-399; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY IA (FSHD), affected,
46,XX;
RG-401; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY lA (FSHD), affected,
46,XX;
RG-402; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY IA (FSHD), affected,
46,XX;
RG-403; FACIOSCAPULOHUMERAL
MUSCULAR DYSTROPHY IA (FSHD), affected;
RG-404; SPINAL MUSCULAR ATROPHY, TYPE I
(SMA1), affected, 46,XY;
RG-406; TORSION DYSTONIA 1, AUTOSOMAL
DOMINANT (DYT1), affected (N/GAG del);
RG-413; BREAST CANCER, FAMILIAL
(BRCA2),affected (N/IVS7 GT del) & MULTIPLE
ENDOCRINE NEOPLASIA, TYPE I (MEN!),
affected (N/3036 4bp del);
RG-414; MULTIPLE ENDOCRINE NEOPLASIA,
TYPE I (MEND, affected (N/3036 4bp del);
RG-415; HUNTINGTON DISEASE (HD), affected;
RG-416; CYSTIC FIBROSIS (CF), affected
(deltaF508/1717-1 G-A);
RG-417; CYSTIC FIBROSIS (CF), affected
(deltaF508/1717-1 G-A);
RG-418; HEMOGLOBIN 13 LOCUS (HBB), affected
(cd8+G /619del);
RG-420: FIF,MOGLORIN B LOCIJS (111113), affected
(cd8+G/619de1);
RG-422; CYSTIC FIBROSIS (CF), affected
(NI 303K/deltaF508);
RG-423; CYSTIC FIBROSIS (CF), carrier
(N/de1taF508);
RG-424; MULTIPLE ENDOCRINE NEOPLASIA,
TYPE 2 (MEN2B), affected (M918T/N);
RG-426; PELIZAEUS-MERZBACHER DISEASE
(PMLD), affected;
RG-428; TUBEROUS SCLEROSIS, TYPE I (TSC I ),
affected (N/IVS7+1 G-A);
South American
Instituto de Riociencias, Silo Paulo (Brazil) BR-I
Middle East
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
Technion-Israel Institute of Technology, Haifa
TE03, TE04, TE06 (13 14 I 6)
(Israel)
Hadassah University Hospital (Israel) HAD 1; HAD 2; HAD 3; HAD 4; HAD 5;
HAD 6
Hebrew University of Jerusalem HEFX1
Technion - Israel Institute of Technology 13; 132;13.3; 14; 16; 16.2; J3;
J3.2
Royan Institute (Iran) ARMD,1.H.iPSC.2; BOM.1 .H.iP SC.1;
CNS.I.RiPSC.10; CNS.2,HAPSC.7;
FHC. 1 .1-linC.3; G31). I 1-1.1PSC.7; HER 1.1-1.iPSC. I ;
LCA.1.HiPSC.1; LIION.1.11313SC.5; R.1.11.iPSC.1;
R.1.H.iPSC.4; R.1.H.iPSC.9; Royan Hl; Royan H10;
Royan H2; Royan H3; Royan H4; Royan 115; Royan
H6; Royan H7; Royan H8; Royan H9; RP.1.H.iPSC.2;
RP2.R.iPSC.3; TYR.1.HiPSC.1; USR.I.H.iPSC.6
Europe
Cellartis AB, Gotenbcrg SA001, SA002 (Sahlgrenska 1, Sahlgrenska 2);
SA002.2; SA003;
(Sweden) AS034.1; AS034.1.1; AS034.2; AS038; AS046; FC018;
ASo85; AS094;
SA111; SA121; SA142; SA167; SA181; SA191; 5A196; 5A202; SA203;
SA211; SA218; SA240; SA279; SA348; SA352; SA399; SA461; SA502;
SA506; SA521; SA540; SA611
Karolinska Institutet (Sweden) HS181; HS207; HS235; HS237; HS293; HS306;
HS346; HS351; 11S356;
HS360; HS361; HS362; HS363; HS364; HS366; HS368; HD380; HS382;
113400, HS401, If S402, 113415, H3420, 1-13422, 11S426, H3429, 113429A,
EIS429B; HS429C; HS429D; HS475; HS480; 11S481; HS539
Goteborg University, Goteborg
SA04¨SA19 (Sahlgrenska 4¨Sahlgrenska 19)
(Sweden)
Karolinska Institute, Stockholm KA08, KA09, KA40, KA41, KA42, KA43 (hICM8,
hICM9, hICM40,
(Sweden) hlUM41, h1CM42, h1CM4:3)
Geneva University (Switzerland) CII-ES1
University of Basel
CH-13S3; CH-ES3; CH-ESS
(Switzerland)
Roslin Cells Lid (UK) RC2; RC3; RC4; RCS
University of Newcastle upon
NCL-1; NCL-2; NCL-3; NCL-4; NCL-5; NCL-6; NCL-7; NCL-8; NCL-9
Tyne (UK)
Roslin Institute (Edinburgh) &
RII1; RI12; 11113; R114; 11115; RH& , ,
Creron Corporation (UK)
University of Manchester (UK) Man 2
King's College London (UK) KCL-001 (formerly WT3)
The University of Sheffield,
SHEF-1; SHEF-2; SHEF-3; SHEF-4; SHEF-5; SHEF-6; SHEF-7; SHEF-8
Sheffield (UK)
Universities of Edinburgh &
Oxford; University of Edi-1; Edi-2; Edi-3; Edi-4
Cambridge (UK)
Roslin Cells Ltd, Roslin
Institute, Universities of
RCM-1; RC-1; RC-2; RC-3; RC-4; RC-5; RC-6; RC-7; RC-8; RC-9; RC-
Edinburgh & Manchester, 10
Central Manchester &
41
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
Manchester Children's
University Hospitals NHS Trust
(UK)
King's College London & Guy's
Hospital Trust/ Charitable KCL-003-CF I (formerly CFI); KCL-005-HD1; KCL008-
HD-2; KCL009-
Foundation of Guy's & St trans-1; KCL-001 (WT-3); KCL-001 (WT-4)
Thomas (UK)
Stem Cell Sciences Ltd,
Australia (SCS) & Australian MIL-l; MEL-2; MEL-3; MEL-4
Stem Cell Centre (ASCC)
University of Edinburgh (UK) CB660
Axordia Ltd. (UK) Shef-1; Sbef-2; Shef-3; Shef-4; Shel-5; Shef-6;
Shef-7
University of Nottingham (UK) Nott-1; Nott-2
Centre of Regenerative Medicine ES-2; ES-3; ES-4; ES-5; ES-6; ES-7; ES-8; ES-
9; ES-10; ES-1 [EM;
in Barcelona (Spain) cFA404-KiPS4F-1; cFA404-KiPS4F-3; KiPS3F-7; KiPS4F-
1; KiPS4F-8
Principe Felipe Centro de VAL-3; VAL-4; VAL-5; VAL-6M; VAL-7; VAL-8; VAL-9;
VAL-10B
Investigacion (Spain)
Universite Libre de Bruxelles ERA-1; ERA2; ERA-3; ERAMUC-1; ERAMUC-1
(Belgium)
Vrijc Universiteit Brussel VUB01; VUB02; VUB06; 'VUB07; VUB03_DM1; VUB04
CF;
(Belgium) VUB05 HD; VUB08 MFS; VUB09 FSHD; VUB10 SCA77;
VUBICFXS; VUB1 FXS; VUB147, VUB19 DM1;¨VUB2O_CMTIA;
VUB22 13 CF; VUB23_6I;
VUB24_DM1; VU-26; VUB27;
VUB28_HD_MFS
Central Manchester and Man 1; Man 2
Manchester Children's
University Hospitals NHS (UK)
Universite Paris-Sud 11 (France) CL01; CL02; CL03; PB04; PB05; PB05-1; PB06;
PB06-1; PB07; PB08;
PB09; PB10
INSERM (France) OSCAR; STR-1-155-HD; STR-I-171-GLA; STR-I-189-
FRAXA; STR-I-
203-CFTR; STR-I-209-MEN2a; STR-I-211-MEN2a; STR-1-221-Sca2;
STR-I-229-MTMX; STR-I-231-MTMX; STR-I-233-FRAXA; STR -1-251 -
CFTR; STR-I-30 1.-MFS; STR-I-305-APC; STR-1-315-CMT1a; STR4-347-
FRAXA; STR-I-355-APC; STR-1-359-APC
Masaryk University (Czech CCTL 6; CCTL 8; CCTL 9; CCTL 10; CCTL 12; CCTL
13; CCRL 14
Republic)
Aalborg University (Denmark) CLS1; CLS2; CLS3; CLS4
University of Copenhagen LRB001; LRB002; LRB003; LRB004; LRB005; LRB006;
LRB007;
(Denmark) LRB008; LRB009; LRB010; LRB011; LRB013; LR13014;
LRB016;
LR13017; LRB018;
University of Southern Denmark KMEB1; 1CMEB2; KMEB3; KMEB4; KMEB
University of Helsinki (Finland) FES21; FES22; FES29; FES30; FES61; FES75
University of Tampere (Finland) Regea 06/015; Regea 06/040; Regea 07/027;
Regea 07/046; Regea 08/013;
Regea 08/017; Regea 08/023; Regea 08/056
Leiden University Medical HESC-NL1; HESC-NL2; HESC-NL3; HESC-NL4
Center (Netherlands)
Russian Academy of Sciences ESM01; ESM02; ESM03;
(Russia)
Instanbul Memorial Hospital MINE: NS-2; NS-3; NS-4; NS-5; NS-6; NS-7; NS-8;
NS-9; NS-10; OZ-1;
(Turkey) OZ-2; OZ-3; OZ-4; OZ-5; OZ-6; OZ-7; OZ-8
42
Date Reeue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTMS2014/015156
Australia
Monash University (Australia) Envy
Prince of Wales Hospital, E1C1; E1C2; El C3; E1C4; Endeavour 1; Endeavour
2; hES3.1; hES3.2;
Sydney (Australia) hES3.3
Sydney IVE Limited (Australia) SIVF01; STVF03; SIVF05; SIVF06; SIVF07; SIVF08;
SIVF09; SIVF10;
SIVF11; SIVF12; SIVE 13
Asia
Kyoto University (Japan) 201B1; 201B2; 201B3; 201B6; 201B7; 243H1; 243H7;
24601; 24603;
24604; 24605; 24606; khES-1; khES-2; kliES-3;
Singapore Stem Cell Consortium ESI-013; ESI-014; ESI-017; ESI-027; ESI-035;
ESI-049; ESI-051; ESI-053
ES Cell International Pte Ld ESO I, ES02, ES03, ES04, ES05, ES06 (HES-1,
HES-2, HES-3, HES-4,
(Singapore) HES-5, HES-6
Maria Biotech Co. Ltd. -Maria MBOI, MB02, MB03; MB04; MB05; MB06; MB07; MB08;
MB09
Infertility Hospital Medical
Institute, Seoul (Korea)
MizMedi Hospital Seoul MI01 (Miz-hblS1); Miz-hES2; Miz-hES3; Miz-hES4; Miz-
hES5; Miz-
National University, Seoul hES6; Miz-hES7; Miz-hES8; Miz-hES9; Miz-hES10;
lvliz-hES11; Miz-
(Korea) hES12; Miz-hES13; Miz-hES14; Miz-bES15;
Pochon CHA University College CHA-hES3; CHA-hES4
of Medicine (Korea)
Seoul National university SNLThES1; SNUhES2; SNIThES3; SNUhES4; SNUhES11;
SNUhES16
(Korea)
National Centre for Biological NCO 1, NCO2, NC03 (FCNCBS1, FCNCBS2,
FCNCBS3); BJN-hem19;
Sciences/Tata Institute of BJN-hem20
Fundamental Research,
Bangalore (India)
Reliance Life Sciences, Mumbai RL05, RL07, RLIO, RL13, RL15, RL20, RL21 (RLS
ES 05, RLS ES 07,
(India) RLS ES 10,
National Institute for Research in KIND-1; KIND-2
Reproductive Health (India)
Tata Institute of Fundamental FCNCBS1; FCNCBS2; FCNCBS3
Research (India)
Kaohsiung Medical University Ti; T2; T3; T4; T5
(Taiwan)
Central South University (China) chESC-3 (H3); chESC-8;chESC-20; chESC-22;
EBNAl+H9
Graduate University of Chinese hPES-1; hPES-2
Academy of Sciences (China) ,
Huazhong University of Science hES-8; hES18
and Technology (China)
Peking University Third Hospital 134; E7; PKUl; PKU2
(China)
Shanghai Jiao Tong University SHbES1
School of Medicine (China)
Shanghei Second Medical RH; SH2; SH4; SH7; SH28; SIDS; SH35a; 5H38; SH39;
SH42
University (China)
Sun Yat-sen University (China) CHES-1; SYSU-1; SYSU-2
Sun Yat-sen University Second CHE-1; CHE-2; CHE-3
Affiliated Hospital (China)
The Third Affiliated Hospital of FY-hES-5; FY-hES-9; FY-hES-10;; FY-hES-11
43
Date Recue/Date Received 2023-09-08
Guangzhou Medical College
(China)
Pluripotent dedifferentiated somatic cells
[0079] Recently, studies using certain nuclear reprogramming factors
have allowed
pluripotent stem cells or pluripotent-like stem cells to be derived from a
patient's own somatic cells.
These cells are also called induced pluripotent stern (iPS) cells. The present
invention describes
various iPS cell lines provided by Shinya Yamanaka, Kyoto University. However,
other iPS cell
lines, for example, those described by James Thomson et al. Al. are by the
invention herein. See U.S.
Publication 20090047263, International Publication W02005/80598, U.S.
Publication 20080233610
and International Publication W02008/11882. Thus, as used herein, "induced
pluripotent stem (iPS)
cells" means cells having properties similar to other pluripotent stem cells,
e.g., hES cells, hEG cells,
pPS (primate pluripotent stem) cells, parthenogenic cells and the like.
[0080] Nuclear programming factors are described in U.S. Publication
20090047263,
International Publication W02005/80598, U.S. Publication 20080233610 and
International
Publication W02008/11882 and were used to induce reprogramming of a
differentiated cell without
using eggs, embryos, or ES cells. Methods for preparing induced iPS cells from
somatic cells by
using the nuclear reprogramming factor similar to that used and described in
the present invention are
not particularly limited. In preferred embodiments, the nuclear reprogramming
factor contacts the
somatic cells under an environment in which the somatic cells and induced
pluripotent stem cells can
proliferate. An advantage of the certain embodiments described herein is that
an induced pluripotent
stem cell can be prepared by contacting a nuclear reprogramming factor with a
somatic cell in the
absence of eggs, embryos, or embryonic stem (ES) cells. By using a nuclear
reprogramming factor,
the nucleus of a somatic cell can be reprogrammed to obtain an iPS cell or an
"ES-like cell."
[0081] Pluripotent stem cells described herein, whether it be hES cells
or iPS cells, may
express any number of pluripotent cell markers, including but not limited to:
alkaline phosphatase
(AP); ABCG2; stage specific embryonic antigen-1 (SSEA-1); SSEA-3; SSEA-4; TRA-
1-60; TRA-1-
81; Tra-2-49/6E; ERas/ECAT5, E-cadherin; .13.III-tubulin; .alpha.-smooth
muscle actin (. alpha.-
SMA); fibroblast growth factor 4 (Fgf4), Cripto, Daxl; zinc finger protein
296 (Zfp296); N-acetyltransferase-1 (Nail); (ES cell associated transcript 1
(ECAT1);
ESG1/DPPA5/ECAT2; ECAT3 ; ECAT6; ECAT7; ECAT8; ECAT9; ECAT10; ECAT15-1;
ECAT15-2; Fth117; Sal 14; undifferentiated embryonic cell transcription factor
(Utfl); Rex 1; p53;
G3PDH; telomerase, including TERT; silent X chromosome genes; Dnmt3a; Dnmt3b;
TRIM28; F-
44
Date Recue/Date Received 2023-09-08
box containing protein 15 (Fbx15); Nanog/ECAT4; 0ct3/4; Sox2; Klf4; c-Myc;
Esrrb; TDGF1;
GABRB3; Z1p42, FoxD3; GDF3; CYP25A1; developmental pluripotency-associated 2
(DPPA2); T-
cell lymphoma breakpoint 1 (Tell); DPPA3/Stella; DPPA4 and the like. It is
understood that the
present invention is not limited to those markers listed herein, and
encompasses markers such as cell
surface markers, antigens, and other gene products including ESTs, RNA
(including microRNAs and
antisense RNA), DNA (including genes and cDNAs), and portions thereof.
[0082] In one embodiment, the iPS cell lines used herein contain the
following nuclear
reprogramming factor genes: an Oct family gene, a Klf family gene, and a Sox
family gene. In one
i PS cell line, each of the following three kinds of genes are provided:
0ct3/4, Klf4, and Sox2. Other
iPS cell lines gene products of each of the following three kinds of genes
were employed: an Oct
family gene, a Klf family gene, and a Myc family gene, e.g., 0ct3/4, Klf4 and
c-Myc. Accordingly,
it is understood that the nuclear reprogramming factor can be with or without
the Myc family gene.
[0083] The nuclear reprogramming factors described herein and also
known in the art,
can be used to generate iPS cells from differentiated adult somatic cells, and
is not limited by the
type of somatic cells to be reprogrammed, i.e., any kind of somatic cell may
be reprogrammed or
dedifferentiated. Because reprogramming a somatic does not require an egg
and/or embryo, an iPS
cell can be a mammalian cell, therefore, providing an opportunity to generate
patient- or disease-
specific pluripotent stem cells.
Aggregate suspension of pluripotent stem cells
[0084] In contrast to previously known methods of tissue engineering
which are based
on seeding individual cells into polymer scaffolds, matrices and/or gels,
embodiments described
herein can use cell aggregate suspensions formed from pluripotent stem cell,
single cell suspensions
or differentiated single cell suspensions derived therefrom. Methods of
processing and/or manufacturing of stem cell aggregate suspension and
differentiation of cells
thereof is described in International Applications PCT/1JS2007/062755 and
PCT/US2008/082356.
[0085] In one embodiment, methods are provided for producing
pluripotent stem cell
aggregate suspensions from a single cell suspension of pluripotent stem cell
cultures, e.g. hES or iPS
cell cultures. The pluripotent stem cell can be initially cultured on
fibroblast feeders or can be
feeder-free. Methods of isolating hES cells and culturing such on human feeder
cells is described in
U.S. Patent No. 7,432,104.
Date Recue/Date Received 2023-09-08
[0086] As used herein, "single cell suspension" or equivalents thereof
refers to a
pluripotent, multipotent or terminally differentiated single cell suspension,
or a single cell suspension
derived from a pluripotent or multipotent cell, by any mechanical or chemical
means. Several
methods exist for dissociating cell clusters to form single cell suspensions
from primary tissues,
attached cells in culture, and aggregates, e.g., physical forces (mechanical
dissociation such as cell
scraper, trituration through a narrow bore pipette, fine needle aspiration,
vortex disaggregation and
forced filtration through a fine nylon or stainless steel mesh), enzymes
(enzymatic dissociation such
as trypsin, collagenase, Accutase' and the like), or a combination of both.
Further, methods and
culture media conditions capable of supporting single-cell dissociation of
pluripotent, multipotent or
differentiated cells are useful for expansion, cell sorting, and defined
seeding for multi-well plate
assays and enable automatization of culture procedures and clonal expansion.
[0087] Other embodiments provide for methods of producing pluripotent
stem cell
aggregate suspensions directly into a differentiation media, e.g., a
differentiating media containing an
agent, preferably a TGFP family member, which is capable of activating a TGFP
family of receptor,
e.g., Activin A, Activin B, GDF-8, GDF-11, and Nodal. Growth factors for
production of endoderm
is described in International Application No., PCT/US2008/065686. Methods of
producing cell
aggregate suspension in a differentiation media can be distinguished from
other methods, also
described herein, which provide for production of cell aggregate suspension
cultures in a pluripotent
stem cell media, e.g., StemPro 0 hES SFM (Invitrogen) based on the heregulin-
based DC-HAIF
media described in PCT/1JS2007/062755
. [0088] Embodiments described herein relate to methods for generating a
pluripotent cell
aggregate in suspension from a pluripotent adherent culture, by culturing a
pluripotent cell in an
adherent growth culture condition which allows for expansion in an
undifferentiated state;
disassociating the adherent pluripotent cell culture into a single cell
suspension culture; contacting
the single cell suspension culture with a first differentiating culture
condition which allows for
formation of hES-derived cell aggregates in suspension by agitating the single
cell suspension
culture until such a period of time when the single cell suspension culture
forms a pluripotent -
derived cell aggregate in suspension, and thereby generating a pluripotent -
derived cell aggregate in
suspension. In preferred embodiments, agitation of the single cell suspension
culture is performed by
rotation at about 80 rpm to 160 rpm. In certain other embodiments described
herein, a rho-kinase
inhibitor is used to facilitate pluripotent stem cell aggregation, growth,
proliferation, expansion
and/or cell mass.
46
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0089] Embodiments described herein also relate to methods for
generating a
pluripotent -derived cell aggregate in suspension from a pluripotent -derived
single cell
suspension, by culturing a hES cell in an adherent growth culture condition
which allows for
expansion in an undifferentiated state; contacting the undifferentiated hES
cell with a first
differentiating culturing condition suitable for differentiating the hES cell
and resulting in an
adherent pluripotent-derived cell; disassociating the adherent hES-derived
cell into a single cell
suspension culture; contacting the single cell suspension culture with a
second differentiating
culture condition which allows for formation of hES-derived cell aggregates in
suspension by
agitating the single cell suspension culture until such a period of time when
the single cell
suspension culture forms a pluripotent -derived cell aggregate in suspension,
and thereby
generating a pluripotent -derived cell aggregate in suspension. In preferred
embodiments,
agitation of the single cell suspension culture is performed by rotation at
about 80 rpm to 160
rpm.
[0090] In preferred embodiments, manufacturing-scale suspension
cultures utilize
continuous perfusion of media as a method for maintaining cell viability while
maximizing cell
density. In this context, media exchange contributes fluid shear to the
culture affecting adherent
cells and suspended aggregates differently. Immobile adherent cells are
subject to fluid shear
stress as the media flows tangentially across the cell surface. In contrast,
suspended aggregates
experience significantly less shear stress across the aggregate surface, as
aggregates are free to
tumble in response to applied shear force. It is expected that prolonged shear
stress will be
detrimental to adherent pluripotent cells and that the suspended aggregate
format is preferred for
optimal survival and function. Thus, based on a need for an efficient
manufacturing process for
production of pluripotent stem cells and/or multipotent progenitor cells
derived from pluripotent
stern cells and the above observed mechanics relating to shear rate and shear
stress, embodiments
described herein provide, for the first time, methods of manufacturing for the
production of
pluripotent stem cells and/or multipotent progenitor cells derived from
pluripotent stem cells in
suspension format, in particular, cell aggregate suspension format.
[0091] In yet another embodiment, hES cell aggregate suspensions
were cultured in a
media substantially free of serum and further in the absence of exogenously
added fibroblast
growth factor (POT). This is distinguished from U.S. Patent No. 7,005,252 to
Thomson, J.,
which requires culturing hES cells in a media without scrum but containing
exogenously added
growth factors, including FGF. In some embodiments, iPS cell aggregate
suspensions are
cultured in a media substantially free of serum and / or further in the
absence of exogenously
added fibroblast growth factor (FGF).
47
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0092] In contrast to cell aggregates produced by previously known
methods that
may vary in both size and shape, the cell aggregates and methods described
herein have a narrow
size and shape distribution, i.e., the cell aggregates arc substantially
uniform in size and/or shape.
The size uniformity of the cell aggregates is critical for differentiation
performance and the
culture homogeneity. Applying basic mass transport analysis to the aggregates,
it is expected that
diffusion of oxygen and nutrients into the center of large aggregates will be
slow compared to
diffusion into smaller aggregates, assuming equal permeability. As
differentiation of aggregated
ES cells, or iPS cells, into pancreatic lineage cells is dependent on the
temporal application of
specific growth factors, a culture with a mixture of aggregates of different
diameters is likely to
be de-synchronized as compared to a uniform (size and shape) culture of cell
aggregates. This
mixture of cell aggregates gives rise to heterogeneity and may result in poor
differentiation
performance and ultimately not lend itself to being amenable to manufacturing,
scale-up, and
production. Hence, as used herein, the phrase "substantially uniform" or
"substantially uniform
in size and shape" or equivalents thereof, refers to the spread in uniformity
of the aggregates and
is not more than about 20%. In another embodiment, the spread in uniformity of
the aggregates is
not more than about 15%, 10% or 5%.
[0093] Although the exact number of cells per aggregate is not
critical, it will be
recognized by those skilled in the art that the size of each aggregate (and
thus the number of cells
per aggregate) is limited by the capacity of oxygen and nutrients to diffuse
to the central cells,
and that this number may also vary depending on cell type and the nutritive
requirements of that
cell type. Cell aggregates may comprise a minimal number of cells (e.g., two
or three cells) per
aggregate, or may comprise many hundreds or thousands of cells per aggregate.
Typically, cell
aggregates comprise hundreds to thousands of cells per aggregate. For purposes
of the present
invention, the cell aggregates are typically from about 50 microns to about
600 microns in size,
although, depending on cell type, the size may be less or greater than this
range. In one
embodiment, the cell aggregates are from about 50 microns to about 250 microns
in size, or
about 75 to 200 microns in size, and preferably they are about 100 to 150
microns in size.
[0094] Still other methods describe making embryoid bodies (EBs).
As used herein,
the term "embryoid bodies", "aggregate bodies" or equivalents thereof, refer
to aggregates of
differentiated and undifferentiated cells that appear when ES cells overgrow
in monolaycr
cultures, or are maintained in suspension cultures in undefined media or are
differentiated via
non-directed protocols towards multiple germ layer tissues. That is, EBs are
not formed from a
single cell suspension of pluripotent stem cells as described herein; nor are
EBs formed from
48
Date Recue/Date Received 2023-09-08
adherent cultures of pluripotent-derived multipotent cells. These features
alone make the present
invention clearly distinguished from an embryoid body.
[0095] In contrast to embryoid bodies, which are a mixture of
differentiated and
undifferentiated cells and typically consist of cells from several germ layers
and go through random
differentiation, the cell aggregates described herein are essentially or
substantially homo-cellular,
existing as aggregates of pluripotent, multipotent, bipotent, or unipotent
type cells, e.g., embryonic
cells, definitive endoderm, foregut endoderm, PDX1 positive pancreatic
endoderm, pancreatic
endocrine cells and the like.
[0096] Embodiments described herein address the above problems by
providing a cost
efficient manufacturing process or methods capable of reproducibly producing
cell aggregates that
are substantially uniform in size and shape using a process that can easily be
applied to large-scale
manufacturing. In one particular embodiment, the differentiable cells are
expanded in a suspension
culture, using the cell media of the present invention. In another particular
embodiment, the
differentiable cells can be maintained and expanded in suspension, i.e., they
remain undifferentiated
or are prevented from further differentiating. The term "expand" in the
context of cell culture is used
as it is in the art, and refers to cellular proliferation and increase the
number of cells, preferably
increase in number of viable cells. In a specific embodiment, the cells are
expanded in a culture
suspension by culturing for more than about one day, i.e., about 24 hours. In
a more specific
embodiment, the cells are expanded in a suspension culture by culturing for at
least 1, 2, 3, 4, 5, 6, 7
days, or at least 2, 3, 4, 5, 6, 7, 8 weeks.
[0097] Still other embodiments of the invention provide for methods of
producing cell
aggregate suspensions formed from differentiated pluripotent stem cell
cultures e.g., cells produced
from the pluripotent cells described herein, and including cells from stages
1, 2, 3, 4 and 5 as
described in d'Amour 2005 and 2006, supra. Hence, methods for making the cell
aggregates
described herein are not limited to any one pluripotent or multipotent cell or
cell stage, rather the
manner of use and need for cell type optimization will dictate which methods
are preferred.
[0098] The methods described herein for producing aggregate suspension
cultures of
pluripotent cells, e.g., hES or iPS cells, and cells derived from other
pluripotent cell sources, for
example, embryonic germ or parthenote cells, are substantially as described in
PCT/US2007/062755,
filed February 23, 2007, and titled Compositions and methods for culturing
differential cells and
PCT/US2008/080516, filed October 20, 2008, and titled Methods and compositions
for feeder-free
pluripotent stem cell media containing human serum.
49
Date Recue/Date Received 2023-09-08
[0099] The methods described herein in no way require first coating the
culturing vessels
with an extracellular matrix, e.g., as described in U.S. Patent 6,800,480 to
Bodnar et al. and assigned
to Geron Corporation. In some embodiments described herein, iPS cells can be
cultured in the same
way that other pluripotent stem cells, e.g., hES and iPS cells, are cultured
using soluble human serum
as substantially described in U.S. Application, PCT/US2008/080516, filed
October 20, 2008, and
titled Methods and compositions for feeder-free pluripotent stem cell media
containing human serum.
[0100] The methods described herein in no way require exogenously added
fibroblast
growth factor (FGF) supplied from a source other than just a fibroblast feeder
layer as described in
U.S. Patent No. 7,005,252 to Thomson, J. and assigned to the Wisconsin Alumni
Research
Foundation (WARF).
Multivotent and Differentiated Cell Compositions
[0101] Cell compositions produced by the methods described herein
include cell cultures
comprising pluripotent stem cells, preprimitive streak, mesendoderm,
definitive endoderm, foregut
endoderm, PDX1-postiive foregut endoderm, PDX1-positive pancreatic endoderm or
PDX1/NKX6.1
co-positive pancreatic endoderm, endocrine precursor or NGN3/NKX2.2 co-
positive endocrine
precursor, and hormone secreting endocrine cells or INS, GCG, GHRL, SST, PP
singly-positive
endocrine cells, wherein at least about 5-90% of the cells in culture are the
preprimitive streak,
mesendoderm, definitive endoderm, foregut endoderm, PDX1-postiive foregut
endoderm, PDX1-
positive pancreatic endoderm or PDX1/NKX6.1 co-positive pancreatic endoderm,
endocrine
precursor or NGN3/NKX2.2 co-positive endocrine precursor, and hormone
secreting endocrine cells
or INS, GCG, GHRL, SST, PP singly-positive endocrine cells produced.
[0102] Some embodiments described herein relate to compositions, such
as cell
populations and cell cultures that comprise both pluripotent cells, such as
stem cells and iPS cells,
and multipotent cells, such as preprimitive streak, mesendoderm or definitive
endoderm, as well as
more differentiated, but still potentially multipotent, cells, such as PDX1-
postiive foregut endoderm,
PDX1-positive pancreatic endoderm or PDX1/NIOC6.1 co-positive pancreatic
endoderm, endocrine
precursor or NGN3/NKX2.2 co-positive endocrine precursor, and hormone
secreting endocrine cells
or INS, GCG, GHRL, SST, PP singly-positive endocrine cells. For example, using
the methods
described herein, compositions comprising mixtures of pluripotent stem cells
and other multipotent
or differentiated cells can be produced. In some embodiments, compositions
comprising at least
about 5 multipotent or differentiated cells for about every 95
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
pluripotent cells are produced. In other embodiments, compositions comprising
at least about 95
multipotent or differentiated cells for about every 5 pluripotent cells are
produced. Additionally,
compositions comprising other ratios of multipotent or differentiated cells to
pluripotent cells are
contemplated. For example, compositions comprising at least about 1
multipotent or
differentiated cell for about every 1,000,000 pluripotent cells, at least
about 1 multipotent or
differentiated cell for about every 100,000 pluripotent cells, at least about
1 multipotent or
differentiated cell for about every 10,000 pluripotent cells, at least about 1
multipotent or
differentiated cell for about every 1000 pluripotent cells, at least about 1
multipotent or
differentiated cell for about every 500 pluripotent cells, at least about 1
multipotent or
differentiated cell for about every 100 pluripotent cells, at least about 1
multipotent or
differentiated cell for about every 10 pluripotent cells, at least about 1
multipotent or
differentiated cell for about every 5 pluripotent cells, and up to about every
1 pluripotent cell and
at least about 1,000,000 multipotent or differentiated cell for about every 1
pluripotent cell are
contemplated.
[0103] Some embodiments described herein relate to cell cultures or
cell populations
comprising from at least about 5% multipotent or differentiated cell to at
least about 99%
multipotent or differentiated cells. In some embodiments the cell cultures or
cell populations
comprise mammalian cells. In preferred embodiments, the cell cultures or cell
populations
comprise human cells. For example, certain specific embodiments relate to cell
cultures
comprising human cells, wherein from at least about 5% to at least about 99%
of the human cells
are multipotent or differentiated cell. Other embodiments relate to cell
cultures comprising
human cells, wherein at least about 5%, at least about 10%, at least about
15%, at least about
20%, at least about 25%, at least about 30%, at least about 35%, at least
about 40%, at least
about 45%, at least about 50%, at least about 55%, at least about 60%, at
least about 65%, at
least about 70%, at least about 75%, at least about 80%, at least about 85%,
at least about 90%,
at least about 95%, at least about 98%, at least about 99%, or greater than
99% of the human
cells are multipotent or differentiated cells. In embodiments where the cell
cultures or cell
populations comprise human feeder cells, the above percentages are calculated
without respect to
the human feeder cells in the cell cultures
[0104] Pluripotent, multipotent or differentiated cells arc capable
of differentiating,
or further differentiating, into preprimitive streak, mesendoderm, definitive
endoderm cells as
well as cells, tissues and/or organs derived therefrom. Mesendoderm cells are
capable of
differentiating into mesoderm cells and/or definitive endoderm cells as well
as cells, tissues
and/or organs derived from either of these lineages. In some embodiments, the
preprimitive
51
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
streak cells are converted, through a mesendoderm intermediate, into
terminally differentiated
cells of either the mesoderm or definitive endoderm lineages. For example,
such processes can
provide the basis for efficient production of human endodcrmal derived
tissues, such as pancreas,
liver, lungs, stomach, intestine, thyroid, parathyroid, thymus, pharynx,
gallbladder and urinary
bladder. Importantly, production of preprimitive streak cells and/or
mescndoderm cells is an
early step in differentiation of a pluripotent stem cell to a functional
insulin-producing 13-cell. As
another example, preprimitive streak cell and/or mesendoderm cell
differentiation can provide
the basis for efficient production of human mesodermal derived tissues, such
as blood cells,
cardiovascular tissues, skeletal tissues as well as other structural and
connective tissues.
[0105] The compositions and methods described herein have several
useful features.
For example, the cell cultures and cell populations comprising, multipotent
cells, e.g.,
preprimitive streak cells and/or mesendoderm cells as well as the methods for
producing such
cell cultures and cell populations, are useful for modeling the early stages
of human
development. Furthermore, the compositions and methods described herein can
also serve for
therapeutic intervention in disease states, such as diabetes mellitus. For
example, since
preprimitive streak cells and/or mesendoderm cells serve as the source for
only a limited number
of tissues, they can be used in the development of pure tissue or cell types.
In some processes for
producing preprimitive streak cells, the pluripotent cells used as starting
material are pluripotent
stem cells, e.g., hES, hEG or iPS cells.
Trophectoderm Cells
[0106] Using the methods described herein, compositions comprising
trophectoderm
cells substantially free of other cell types can be produced. In some
embodiments described
herein, the trophectoderm cell populations or cell cultures produced by the
methods described
herein substantially have high expression of markers selected from the group
consisting of
HAND 1, Fumes, MASH2, ESXL1, HCG, KRT18, PSG3, SFXN5, DLX3, PSX1, ETS2, and
ERRB genes as compared to the expression levels of HAND I , Eomes, MASH2,
ESXL1,
KRT18, PSG3, SFXN5, DLX3, PSX1, ETS2, and ERRS in non-trophectoderm cells or
cell
populations.
Preprimitive Streak Cells
[0107] Using the methods described herein, compositions comprising
preprimitive
streak cells substantially free of other cell types can be produced. In some
embodiments
described herein, the preprimitive streak cell populations or cell cultures
produced by the
52
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
methods described herein are substantially express FGF8 and/or NODAL marker
genes as
compared to BRACHURYlow, FGF4 low, SNAIL low, SOX17 low, FOXA2 low, SOX7 low
and SOX1 low.
Extraembryonic Cells
[0108] Using
the methods described herein, compositions comprising extraembryonic
cells substantially free of other cell types can be produced. Primitive,
visceral and parietal
endoderm cells are extraembryonic cells. Primitive endoderm cells give rise to
visceral and
parietal endoderm cells. Visceral endoderm cells are endoderm cells that form
part of the yolk
sac. Visceral endoderm cells function in both nutrient uptake and transport.
Parietal endoderm
cells are contiguous with an extraembryonic tissue known as Reichert's
membrane. One of the
roles of parietal endoderm cells is to produce basement membrane components.
Together,
visceral endoderm cells and parietal endoderm cells form what is often
referred to as
extraembryonic endoderm. As the name suggests, extraembryonic endoderm cells
do not give
rise to embryonic structures formed during development. In contrast,
definitive endoderm cells
and other endoderm-lineage or pancreatic-lineage cells described herein are
embryonic or
derived from embryonic cells and give rise to tissues that are derived from
the gut tube that
forms during embryonic development. In
some embodiments described herein, the
extraembryonic cell populations or cell cultures produced by the methods
described herein
substantially have high expression of markers selected from the group
consisting of SOX7,
S0X17, THBD, SPARC, DAB1, HI F4alpha or AFP genes as compared to the
expression levels
of at least SOX7, SOX17, THBD, SPARC, DAB!, or AFP, which is not expressed in
other types
of cells or cell populations, for example, definitive endoderm.
Mensendoderm Cells
[0109] Using
the methods described herein, compositions comprising mesendoderm
cells substantially free of other cell types can be produced. In some
embodiments described
herein, the mesendoderm cell populations or cell cultures produced by the
methods described
herein substantially have high expression of markers selected from the group
consisting of
FGF4, SNAI1 MIXL1 and/or WNT3 marker genes, as compared to SOX17 low, CXCR4
low,
FOXA2 low, SOX7 low and SOX1 low.
53
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Screening methods
[0110] In some embodiments, screening methods are employed to
obtain certain cell
populations comprising pluripotem, multipotcrit and/or differentiated cells,
such as human
pluripotent stem cells, induced pluripotent stem cells, preprimitive streak
cells, in ensendoderm
cells, definitive endoderm cells, foregut endoderm or PDX1-negative foregut
endoderm cells,
PDX1-postiive foregut endoderm or PDX1-positive pancreatic endoderm cells or
pancreatic
progenitor cells, endocrine precursor cells, and/or endocrine cells. The cell
population is then
provided with a candidate differentiation factor. At a first time point, which
is prior to or at
approximately the same time as providing the candidate differentiation factor,
expression of a
marker is determined. Alternatively, expression of the marker can be
determined after providing
the candidate differentiation factor. At a second time point, which is
subsequent to the first time
point and subsequent to the step of providing the candidate differentiation
factor to the cell
population, expression of the same marker is again determined. Whether the
candidate
differentiation factor is capable of promoting the differentiation of the
pancreatic precursor cells
is determined by comparing expression of the marker at the first time point
with the expression
of the marker at the second time point. If expression of the marker at the
second time point is
increased or decreased as compared to expression of the marker at the first
time point, then the
candidate differentiation factor is capable of promoting the differentiation
of pancreatic
progenitor cells.
[0111] Sonic embodiments of the screening methods described herein
utilize cell
populations or cell cultures which comprise human definitive endoderm, PDX-1
negative foregut
endoderm, PDX-1 positive foregut endoderm, PDX-1 positive pancreatic endoderm,
or
pancreatic progenitor or endocrine precursor cells. For example, the cell
population can be a
substantially purified population of PDX-1-positivei pancreatic endoderm or
pancreatic
progenitor cells. For example, the cell population can be an enriched
population of human
pancreatic progenitor cells, wherein at least about 50% to 97% of the human
cells in the cell
population are human pancreatic progenitor cells, the remainder comprising of
endocrine
precursor or endocrine cells and other cell types.
[0112] In embodiments of the screening methods described herein,
the cell
population is contacted or otherwise provided with a candidate (test)
differentiation factor. The
candidate differentiation factor can comprise any molecule that may have the
potential to
promote the differentiation of any of the above-mentioned cells, e.g.. human
pancreatic
progenitor cells. In some embodiments described herein, the candidate
differentiation factor
comprises a molecule that is known to be a differentiation factor for one or
more types of cells.
54
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
In alternate embodiments, the candidate differentiation factor comprises a
molecule that is not
known to promote cell differentiation. In preferred embodiments, the candidate
differentiation
factor comprises a molecule that is not known to promote the differentiation
of human pancreatic
progenitor cells.
[0113] In some embodiments of the screening methods described
herein, the
candidate differentiation factor comprises a small molecule. In preferred
embodiments, a small
molecule is a molecule having a molecular mass of about 10,000 amu or less.
[0114] In other embodiments described herein, the candidate
differentiation factor
comprises a large molecule, e.g., a polypeptide. The polypeptide can be any
polypeptide
including, but not limited to, a glycoprotein, a lipoprotein, an extracellular
matrix protein, a
cytokine, a chemokine, a peptide hormone, an interleukin or a growth factor.
Preferred
polypeptides include growth factors.
[0115] In some embodiments of the screening methods described
herein, the
candidate differentiation factors comprise one or more growth factors selected
from the group
consisting of Amphiregulin, B-lymphocyte stimulator, IL-16, Thymopoietin,
TRAIL/Apo-2, Pre
B cell colony enhancing factor, Endothelial differentiation-related factor 1
(EDF1), Endothelial
monocyte activating polypeptide IT, Macrophage migration inhibitory factor
(M1F), Natural killer
cell enhancing factor (NKEFA), Bone morphogenetic protein 2, Bone
morphogenetic protein 8
(osteogeneic protein 2), Bone morphogenic protein 6, Bone morphogenic protein
7, Connective
tissue growth factor (CTGF), CGI-149 protein (neuroendocrine differentiation
factor), Cytokine
A3 (macrophage inflammatory protein I-alpha), Gliablastoma cell
differentiation-related protein
(GBDR1), Elepatoma-derived growth factor, Neuromcdin U-25 precursor, Vascular
endothelial
growth factor (VEGF), Vascular endothelial growth factor B (VEGF-B), 1-cell
specific
RANTES precursor, thymic dendritic cell-derived factor 1, Transferrin,
Interleukin-1 (IL 1),
Interleukin-2 (IL 2), Interleukin-3 (IL 3), Interleukin-4 (IL 4), Interleukin-
5 (IL 5), Interleukin-6
(IL 6), Interleukin-7 (IL 7), Interleukin-8 (IL 8), Interleukin-9 (IL 9),
Interleukin-10 (IL 10),
Interleukin-11 (IL 11), Interleukin-12 (IL 12), Interleukin-13 (IL 13),
Granulocyte-colony
stimulating factor (G-CSF), Granulocyte macrophage colony stimulating factor
(GM-CSF),
Macrophage colony stimulating factor (M-CSF), Erythropoietin, Thrombopoietin,
Vitamin D3,
Epidermal growth factor (EGF), Brain-derived neurotrophic factor, Leukemia
inhibitory factor,
Thyroid hormone, Basic fibroblast growth factor (bFGF), aFGF, FGF-4, FGF-6,
FGF-
7/Keratinocyte growth factor (KGF), Platelet-derived growth factor (PDGF),
Platelet-derived
growth factor-BB, 13 nerve growth factor, activin A, Transforming growth
factor 131 (TGF131),
Interferon-a, Interferon-13, Interferon-y, Tumor necrosis factor- a, Tumor
necrosis factor- 0,
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Burst promoting activity (BPA), Erythroid promoting activity (EPA), PGE2,
insulin growth
factor-1 (IGF-1), IGF-II, Neutrophin growth factor (NGF), Neutrophin-3,
Neutrophin 4/5,
Ciliary neurotrophic factor, Glial-dcrivcd ncxin, Dexuncthasonc, [3-
mcreaptocthanol, Rctinoic
acid, Butylated hydroxyanisole, 5-azacytidine, Amphotericin B, Ascorbic acid,
Ascrorbate,
isobutylxanthinc, indomethacin, P-glycerolphosphate,
nicotinamidc, DMS 0,
Thiazolidinediones, TWS119, oxytocin, vasopressin, melanocyte-stimulating
hormone,
corticortropin, lipotropin, thyrotropin, growth hormone, prola.ctin,
luteinizing hormone, human
chorionic gonadotropin, follicle stimulating hormone, corticotropin-releasing
factor,
gonadotropin-releasing factor, prolactin-releasing factor, prolactin-
inhibiting factor, growth-
hormone releasing factor, somatostatin, thyrotropin-releasing factor,
calcitonin gene-related
peptide, parathyroid hormone, glucagon-like peptide 1, glucose-dependent
insulinotropic
polypeptide, gastrin, secretin, cholecystokinin, motilin, vasoactive
intestinal peptide, substance
P. pancreatic polypeptide, peptide tyrosine, neuropeptide tyrosine, insulin,
glucagon, placental
lactogen, relaxin, angiotensin II, calctriol, atrial natriuretic peptide, and
melatonin. thyroxine,
triiodothyronine, calcitonin, estradiol, estrone, progesterone, testosterone,
cortisol,
corticosterone, aldosterone, epinephrine, norepinepherine, androstiene,
calcitriol, collagen,
.Dexamethasone, il-mercaptoethanol, Retinoic acid, Butylated hydroxyanisole, 5-
azacytidine,
Amphotericin B, Ascorbic acid, Ascrorbate, isobutylxanthine, indomethacin, [3-
glycerolphosphate, nicotinamide, DMSO, Thiazolidinediones, and TWS119.
[0116] In
some embodiments of the screening methods described herein, the
candidate differentiation factor is provided to the cell population in one or
more concentrations.
In some embodiments, the candidate differentiation factor is provided to the
cell population so
that the concentration of the candidate differentiation factor in the medium
surrounding the cells
ranges from about 0.1 ng/ml to 10 mg/ml. In some embodiments, the
concentration of the
candidate differentiation factor in the medium surrounding the cells ranges
from about 1 ng/ml to
about 1 mg/ml. In other embodiments, the concentration of the candidate
differentiation factor
in the medium surrounding the cells ranges from about 10 ng/ml to about 100
g/ml. In still
other embodiments, the concentration of the candidate differentiation factor
in the medium
surrounding the cells ranges from about 100 ng/ml to about 10 g/ml. In
preferred
embodiments, the concentration of the candidate differentiation factor in the
medium
surrounding the cells ranges from about 5 ng/ml to 1000 g/ml.
[0117] In
some embodiments, steps of the screening methods described herein
comprise determining expression of at least one marker at a first time point
and a second time
point. In some of these embodiments, the first time point can be prior to or
at approximately the
56
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
same time as providing the cell population with the candidate differentiation
factor.
Alternatively, in some embodiments, the first time point is subsequent to
providing the cell
population with thc candidate differentiation factor. In some embodiments,
expression of a
plurality of markers is determined at a fil'St time point.
[0118] In addition to determining expression of at least one marker
at a first time
point, some embodiments of the screening methods described herein contemplate
determining
expression of at least one marker at a second time point, which is subsequent
to the first time
point and which is subsequent to providing the cell population with the
candidate differentiation
factor. In such embodiments, expression of the same marker is determined at
both the first and
second time points. In some embodiments, expression of a plurality of markers
is determined at
both the first and second time points. In such embodiments, expression of the
same plurality of
markers is determined at both the first and second time points. In some
embodiments, marker
expression is determined at a plurality of time points, each of which is
subsequent to the first
time point, and each of which is subsequent to providing the cell population
with the candidate
differentiation factor. In certain embodiments, marker expression is
determined by Q-PCR. In
other embodiments, marker expression is determined by immunocytochemistry.
[0119] In certain embodiments of the screening methods described
herein, the marker
having its expression determined at the first and second time points is a
marker that is associated
with the differentiation of pancreatic progenitor cells to cells which are the
precursors of
terminally differentiated cells which make up pancreatic islet tissues. Such
cells can include
immature pancreatic islet hormone-expressing cells.
[0120] In some embodiments of the screening methods described
herein, sufficient
time is allowed to pass between providing the cell population with the
candidate differentiation
factor and determining marker expression at the second time point. Sufficient
time between
providing the cell population with the candidate differentiation factor and
determining
expression of the marker at the second time point can be as little as from
about 1 hour to as much
as about 10 days. In some embodiments, the expression of at least one marker
is determined
multiple times subsequent to providing the cell population with the candidate
differentiation
factor. In some embodiments, sufficient time is at least about 1 hour, at
least about 6 hours, at
least about 12 hours, at least about 16 hours, at least about 1 day, at least
about 2 days, at least
about 3 days, at least about 4 days, at least about 5 days, at least about 6
days, at least about 7
days, at least about 8 days, at least about 9 days, at least about 10 days, at
least about 11 days, at
least about 12 days, at least about 13 days, at least about 14 days, at least
about 1 week, at least
57
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
about 2 weeks, at least about 3 weeks, at least about 4 weeks, at least about
5 weeks, at least
about 6 weeks, at least about 7 weeks, or at least about 8 weeks.
[0121] In some embodiments of the methods described herein, it is
further
determined whether the expression of the marker at the second time point has
increased or
decreased as compared to the expression of this marker at the first time
point. An increase or
decrease in the expression of the at least one marker indicates that the
candidate differentiation
factor is capable of promoting the differentiation of the endocrine precursor
cells. Similarly, if
expression of a plurality of markers is determined, it is further determined
whether the
expression of the plurality of markers at the second time point has increased
or decreased as
compared to the expression of this plurality of markers at the first time
point. An increase or
decrease in marker expression can be determined by measuring or otherwise
evaluating the
amount, level or activity of the marker in the cell population at the first
and second time points.
Such determination can be relative to other markers, for example housekeeping
gene expression,
or absolute. In certain embodiments, wherein marker expression is increased at
the second time
point as compared with the first time point, the amount of increase is at
least about 2-fold, at
least about 5-fold, at least about 10-fold, at least about 20-fold, at least
about 30-fold, at least
about 40-fold, at least about 50-fold, at least about 60-fold, at least about
70-fold, at least about
80-fold, at least about 90-fold, at least about 100-fold or more than at least
about 100-fold. In
some embodiments, the amount of increase is less than 2-fold. In embodiments
where marker
expression is decreased at the second time point as compared with the first
time point, the
amount of decrease is at least about 2-fold, at least about 5-fold, at least
about 10-fold, at least
about 20-fold, at least about 30-fold, at least about 40-fold, at least about
50-fold, at least about
60-fold, at least about 70-fold, at least about 80-fold, at least about 90-
fold, at least about 100-
fold or more than at least about 100-fold. In some embodiments, the amount of
decrease is less
than 2-fold.
Monitoring the Production of Multipotent or Differentiated Cells
[0122] The progression of pluripotent cells to multipotent cells to
further multipotent
cells or differentiated cells, such as pancreatic progenitors or hormone
endocrine secreting cells,
can be monitored by determining the expression of markers characteristic of
the specific cells,
including genetic markers and phenotypic markers such as, the expression of
islet hormones and
the processing of proinsulin into insulin and C peptide in endocrine cells. In
some processes, the
expression of certain markers is determined by detecting the presence or
absence of the marker.
Alternatively, the expression of certain markers can be determined by
measuring the level at
58
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
which the marker is present in the cells of the cell culture or cell
population. For example, in
certain processes, the expression of markets characteristic of immature
pancreatic islet hormone-
expressing cells as well as the lack of significant expression of markers
characteristic of
pluripotent cells, definitive endoderm, foregut endoderm, PDX1-positive
foregut endoderm,
endocrine precursor, extraembryonic endoderm, mesoderm, ectoderm, mature
pancreatic islet
hormone-expressing cells and/or other cell types is determined.
[0123] As described in connection with monitoring the production of
other less
differentiated cell types of the definitive endoderm lineage, qualitative or
semi-quantitative
techniques, such as blot transfer methods and immunocytochemistry, can be used
to measure
marker expression. Alternatively, marker expression can be accurately
quantitated through the
use of technique such as Q-PCR. Additionally, it will be appreciated that at
the polypeptide
level, many of the markets of pancreatic islet hormone-expressing cells are
secreted proteins. As
such, techniques for measuring extracellular marker content, such as ELISA,
may be utilized.
[0124] For example, in one embodiment, PDX1 is a marker gene that
is associated
with PDX1-positive foregut endoderm. As such, in some embodiments of the
present invention,
the expression of PDX1 is determined. In other embodiments, the expression of
other markers,
which are expressed in PDX1-positive foregut endoderm, including, but not
limited to, SOX17,
HNF6, SOX9 and PROX1 is also determined. Since PDX1 can also be expressed by
certain
other cell types (that is, visceral endoderm and certain neural ectoderm),
some embodiments of
the present invention relate to demonstrating the absence or substantial
absence of marker gene
expression that is associated with visceral endoderm and/or neural ectoderm.
For example, in
some embodiments, the expression of markers, Which are expressed in visceral
endoderm and/or
neural cells, including, but not limited to, SOX7, AFP, SOX I, ZIC I and/or
NFM is determined.
[0125] In some embodiments, PDX1-positive foregut endoderm cell
cultures
produced by the methods described herein are substantially free of cells
expressing the SOX7,
AFP, SOX1, ZIC1 or NFM marker genes. In certain embodiments, the PDX1-positive
foregut
endoderm cell cultures produced by the processes described herein are
substantially free of
visceral endoderm, parietal endoderm and/or neural cells.
[0126] The developmental progression of the pluripotent cells
described herein (e.g.,
cells produced as a result of Stages or Steps 1-5 as described in D'Amour et
at. 2006, supra) can
be monitored by determining the expression of markers characteristic of each
hES-derived or
iPS-derived cell type along the developmental pathway. In some processes, the
identification
and characterization of a hES-derived or iPS-derived cell type is by
expression of a certain
marker or different expression levels and patterns of more than one marker.
That is, the presence
59
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
or absence, the high or low expression, of one or more the marker(s) typifies
and identifies a
cell-type. Also, certain markers can have transient expression, whereby the
marker is highly
expressed during one stage of development and poorly expressed in another
stage of
development. The expression of certain markers can be determined by measuring
the level at
which the marker is present in the cells of the cell culture or cell
population as compared to a
standardized or normalized control marker. In such processes, the measurement
of marker
expression can be qualitative or quantitative. One method of quantitating the
expression of
markers that are produced by marker genes is through the use of quantitative
PCR (Q-PCR).
Methods of performing Q-PCR are well known in the art.
[0127] In some embodiments of the present invention, the presence,
absence and/or
level of expression of a marker is determined by quantitative PCR (Q-PCR). For
example, the
amount of transcript produced by certain genetic markers, such as S0X17,
CXCR4, OCT4, AFP,
TM, SPARC, SOX7, CDX2, MINH, GATA 4, FINF3[3, FINF4alpha, GSC, FGF17, VWF,
CALCR, FOXQ I, CMKOR1, CRIP I and other markers described herein is determined
by
quantitative Q-PCR.
[0128] In other embodiments, immunohistochemistry is used to detect
the proteins
expressed by the above-mentioned genes. In still other embodiments, Q-PCR can
be used in
conjunction with immunohistochemical techniques or flow cytometry techniques
to effectively
and accurately characterize and identify cell types and determine both the
amount and relative
proportions of such markers in a subject cell type. In one embodiment, Q-PCR
can quantify
levels of RNA expression in a cell culture containing a mixed population of
cells. However, Q-
PCR cannot provide or qualify whether the subject markers or proteins arc co-
expressed on the
same cell. In another embodiment, Q-PCR is used in conjunction with flow
cytometry methods
to characterize and identify cell types. Thus, by using a combination of the
methods described
herein, and such as those described above, complete characterization and
identification of
various cell types, including endoderm lineage type cells, can be accomplished
and
demonstrated.
[0129] For example, in one preferred embodiment, pancreatic
progenitors or
pancreatic endoderm or PDX-1 positive pancreatic endoderm, expresses at least
PDX1, Nloc6.1,
PTF1A, CPA and/or cMYC as demonstrated by Q-PCR and / or ICC, but such a cell
at least co-
expresses PDX1 and Nkx6.1 as demonstrated by ICC and does not express other
markers
including SOX17 CXCR4, or CER, to be identified as a PDX1-positive expressing
cell.
Similarly, for proper identification of a mature hormone secreting pancreatic
cell, in vitro or in
vivo, for example, there is demonstrated that C-peptide (a product of proper
processing of pro-
Date Recue/Date Received 2023-09-08
insulin in a mature and functioning cell) and insulin are co-expressed by ICC
in the insulin
secreting cell.
[0130]
Still, other methods which are known in the art can also be used to quantitate
marker gene expression. For example, the expression of a marker gene product
can be detected by
using antibodies specific for the marker gene product of interest (e.g., e.g.
Western blot, flow
cytometry analysis, and the like). In certain processes, the expression of
marker genes characteristic
of hES-derived cells as well as the lack of significant expression of marker
genes characteristic of
hES-derived cells. Still further methods for characterizing and identifying
hES-derived cells types
are described in related applications as indicated above.
[0131]
Amplification probe/primer combinations suitable for use in amplification
assays
include the following: Insulin (INS) (GenBank NM_000207): primers
AAGAGGCCATCAAGCAGATCA (SEQ ID NO: 1); CAGGAGGCGCATCCACA (SEQ ID NO:
2); Nkx6.1 (NM_006168): primers CTGGCCTGTACCCCTCATCA (SEQ ID NO: 3);
CTTCCCGTCTTTGTCCAACAA (SEQ ID NO: 4); Pdxl (NM_000209): primers
AAGTCTACCAAAGCTCACGCG (SEQ ID NO: 5); GTAGGCGCCGCCTGC (SEQ ID NO: 6);
Ngn3 (NM 020999): primers GCTCATCGCTCTCTATTCTTTTGC (SEQ ID NO: 7);
GGTTGAGGCGTCATCCTTTCT (SEQ ID NO: 8); FOXA2 (HNF3B) (NM_021784): primers
GGGAGCGGTGAAGATGGA (SEQ ID NO: 9); TCATGTTGCTCACGGAGGAGTA (SEQ ID
NO: 10); Glucagon (GCG) (NM_002054): primers AAGCATTTACTTTGTGGCTGGATT (SEQ ID
NO: 11); TGATCTGGATTTCTCCTCTGTGTCT (SEQ ID NO: 12); HNF6 (NM_030712): primers
CGCTCCGCTTAGCAGCAT (SEQ ID NO: 13); GTGTTGCCTCTATCCTTCCCAT (SEQ ID NO:
14); HNF4Alpha (NM_000457): primers GAAGAAGGAAGCCGTCCAGA (SEQ ID NO: 15);
GACCTTCGAGTGCTGATCCG (SEQ ID NO: 16); Sox17 (NM_022454): primers
GGCGCAGCAGAATCCAGA (SEQ ID NO: 17); NNNNNNNNNNNNNNN NNNNN (SEQ ID
NO: 18); HLxB9 (NM_005515): primers CACCGCGGGCATGATC (SEQ ID NO: 19);
ACTTCCCCAGGAGGTTCGA (SEQ ID NO: 20); Nkx2.2 (NM_002509): primers
GGCCTTCAGTACTCCCTGCA (SEQ ID NO: 21); GGGACTTGGAGCTTGAGTCCT (SEQ ID
NO: 22); PTF la (NM_178161): primers GAAGGTCATCATCTGCCATCG (SEQ ID NO: 23)
GGCCATAATCAGGGTCGCT
(SEQ ID NO: 24); SST (NM 001048): primers
CCCCAGACTCCGTCAGTTTC (SEQ ID NO: 25); TCCGTCTGGTTGGGTTCAG (SEQ ID NO:
26); PAX6 (NM_000280): primers CCAGAAAGGATGCCTCATAAAGG (SEQ ID NO: 27);
TCTGCGCGCCCCTAGTTA (SEQ ID NO: 28); 0ct4 primers: TGGGCTCGAGAAGGATGTG
61
Date Recue/Date Received 2023-09-08
(SEQ ID NO: 29) GCATAGTCGCTGCTTGATCG (SEQ ID NO: 30); MIXL1 primers
CCGAGTCCAGGATCCAGGTA (SEQ ID NO: 31) CTCTGACGCCGAGACTTGG (SEQ ID NO:
32); GATA4 primers CCTCTTGCAATGCGGAAAG (SEQ ID NO: 33)
CGGGAGGAAGGCTCTCACT (SEQ ID NO: 34); GSC primers
GAGGAGAAAGTGGAGGTCTGGTT (SEQ ID NO: 35) CTCTGATGAGGACCGCTTCTG (SEQ
ID NO: 36); CER primers ACAGTGCCCTTCAGCCAGACT (SEQ ID NO: 37)
ACAACTACTTITTCACAGCMCGT (SEQ ID NO: 38); AFP primers
GAGAAACCCACTGGAGATGAACA (SEQ ID NO: 39) CTCATGGCAAAGTTCTTCCAGAA
(SEQ ID NO: 40); SOX1 primers ATGCACCGCTACGACATGG (SEQ ID NO: 41)
CTCATGTAGCCCTGCGAGTTG (SEQ ID NO: 42); ZIC1 primers
CTGGCTGTGGCAAGGTCTTC (SEQ ID NO: 43) CAGCCCTCAAACTCGCACTT (SEQ ID NO:
44); NFM primers ATCGAGGAGCGCCACAAC (SEQ ID NO: 45) TGCTGGATGGTGTCCTGGT
(SEQ ID NO: 46). Other primers are available through ABI Taqman including
FGF17
(Hs00182599_m1), VWF (Hs00169795_m1), CMKOR1 (Hs00604567_m1), CRIP1
(Hs00832816_g1), FOXQ I (Hs00536425_s1), CALCR (Hs00156229_m1) and CHGA
(Hs00154,111 m1).
Summary of the production of PDX1-positive pancreatic endoderm (Stages 1 to 4)
and insulin
production in vivo
[0132] The methods for production of certain endoderm-lineage and
pancreatic
endoderm-lineage cells are provided herein, and discussed elsewhere in related
applications such as
U.S. Application No. 11/773,944, entitled METHODS OF PRODUCING PANCREATIC
HORMONES, filed July 5, 2007, which is a continuation-in-part of U.S. Patent
Application Number
11/681,687, entitled ENDOCRINE PRECURSOR CELLS, PANCREATIC HORMONE-
EXPRESSING CELLS AND METHODS OF PRODUCTION, filed March 2, 2007.
[0133] Briefly, the directed differentiation methods herein for
pluripotent stem cells, for
example, hES and iPS cells, can be described into at least four or five
stages. Stage 1 is the
production of definitive endoderm from pluripotent stem cells and takes about
2 to 5 days, preferably
2 or 3 days. Pluripotent stem cells are suspended in media comprising RPMI , a
TGFI3 superfamily
member growth factor, such as Activin A, Activin B, GDF-8 or GDF-11
(100ng/m1), a Wnt family
member or Wnt pathway activator, such as Wnt3a (25ng/m1), and alternatively a
rho-kinase or
ROCK inhibitor, such as Y-27632 (10 tiM) to enhance growth, survival and
proliferation as well as
promoting cell-cell adhesion. After about 24 hours, the media is exchanged for
media comprising
62
Date Recue/Date Received 2023-09-08
RPMI with serum, such as 0.2% FBS, and a TGF(3 superfamily member growth
factor, such as
Activin A, Activin B, GDF-8 or GDF-11 (10Ong/m1), and alternatively a rho-
kinase or ROCK
inhibitor for another 24 (day 1) to 48 hours (day 2). Alternatively, after
about 24 hours in a medium
comprising Activin / Wnt3a, the cells are cultured during the subsequent 24
hours in a medium
comprising Activin alone (i.e., the medium does not include Wnt3a).
Importantly, production of
definitive endoderm requires cell culture conditions low in serum content and
thereby low in insulin
or insulin-like growth factor content. See McLean et al. (2007) Stem Cells 25:
29-38. McLean et al.
also show that contacting hES cells with insulin in concentrations as little
as 0.2 g/m1 at Stage 1 can
be detrimental to the production of definitive endoderm. Still others skilled
in the art have modified
the Stage 1 differentiation of pluripotent cells to definitive endoderm
substantially as described here
and in D'Amour et al. (2005), for example, at least, Agarwal et al., Efficient
Differentiation of
Functional Hepatocytes from Human Embryonic Stem Cells, Stem Cells (2008)
26:1117-1127;
Borowiak et al., Small Molecules Efficiently Direct Endodermal Differentiation
of Mouse and
Human Embryonic Stem Cells, (2009) Cell Stem Cell 4:348-358; and Brunner et
al., Distinct DNA
methylation patterns characterize differentiated human embryonic stem cells
and developing human
fetal liver, (2009) Genome Res. 19:1044-1056. Proper differentiation,
specification, characterization
and identification of definitive are necessary in order to derive other
endoderm-lineage cells.
Definitive endoderm cells at this stage co-express SOX17 and IANF313 (FOXA2)
and do not
appreciably express at least HNF4alpha, HNF6, PDX1, SOX6, PROX1, P ________
1A, CPA, cMYC,
NKX6.1, NGN3, PAX3, ARX,NKX2.2, INS, GSC, GHRL, SST, or PP.
[0134]
Stage 2 takes the definitive endoderm cell culture from Stage 1 and produces
foregut endoderm or PDX1-negative foregut endoderm by incubating the
suspension cultures with
RPMI with low serum levels, such as 0.2% FBS, in a 1:1000 dilution of ITS,
25ng KGF (or FGF7),
and alternatively a ROCK inhibitor for 24 hours (day 2 to day 3) to enhance
growth, survival,
proliferation and promote cell-cell adhesion. After 24 hours (day 3 to day 4),
the media is exchanged
for the same media minus a TGFf3 inhibitor, but alternatively still a ROCK
inhibitor to enhance
growth, survival and proliferation of the cells, for another 24 (day 4 to day
5) to 48 hours (day 6). A
critical step for proper specification of foregut endoderm is removal of TGF13
family growth factors.
Hence, a TGFI3 inhibitor can be added to Stage 2 cell cultures, such as 2.5 M
TGFI3 inhibitor no.4 or
M SB431542, a specific inhibitor of activin receptor-like kinase (ALK), which
is a TGF13 type I
receptor. Foregut endoderm or PDX1-negative foregut endoderm cells produced
from Stage 2 co-
express SOX17, HNF113 and HNF4alpha and do not
63
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
appreciably co-express at least S0X17 and HNF3P (FOXA2), nor FINF6, PDX1,
S0X6,
PROX1, PTF1A, CPA, cMYC, NKX6.1, NGN3, PAX3, ARX, NKX2.2, INS, GSC, GHRL,
SST, or PP, which arc hallmark of definitive endoderm, PDX1-positive
pancreatic endoderm or
pancreatic progenitor cells or endocrine precursors as well as singly or poly
hormonal type cells.
[0135] Stage
3 (days 5-8) takes the foregut endoderm cell culture from Stage 2 and
produces a PDX1-positive foregut endoderm cell by DMEM or RPM1 in 1% B27,
0.2504
KAAD cyclopamine, a retinoid, such as 0,2 M retinoie acid (RA) or a retinoic
acid analog such
as 3nM of TTNPB, and 50ng/mL of Noggin for about 24 (day 7) to 48 hours (day
8).
Specifically, Applicants have used DMEM-high glucose since about 2003 and all
patent and
non-patent disclosures as of that time employed DMEM-high glucose, even if not
mentioned as
"DMEM-high glucose" and the like. This is, in part, because manufacturers such
as Gibco did
not name their DMEM as such, e.g. DMEM (Cat.No 11960) and Knockout DMEM (Cat.
No
10829). It is noteworthy, that as of the filing date of this application,
Gibco offers more DMEM
products but still does not put "high glucose" in certain of their DMEM
products that contain
high glucose e.g. Knockout DMEM (Cat. No. 10829-018). Thus, it can be assumed
that in each
instance DMEM is described, it is meant DMEM with high glucose and this was
apparent by
others doing research and development in this field. Again, a ROCK inhibitor
or rho-lcinase
inhibitor such as Y-27632 can be used to enhance growth, survival,
proliferation and promote
cell-cell adhesion. PDXI-positive foregut cells produced from Stage 3 co-
express PDX1 and
HNF6 as well as SOX9 and PROX, and do not appreciably co-express markers
indicative of
definitive endoderm or foregut endoderm (PDX1-negative foregut endoderm) cells
or PDX1-
positive foregut endoderm cells as described above in Stages 1 and 2.
[0136] Stage
4 ((lays 8-14) takes the media from Stage 3 and exchanges it for media
containing DMEM in 1% vol/vol 327 supplement, plus 50rtg/mL KGF and 50ng/mL of
EGF and
sometimes also 50ng/mL Noggin. Again, a ROCK inhibitor such as Y-27632 can be
used to
enhance growth, survival, proliferation and promote cell-cell adhesion. PDX1-
positive
pancreatic endoderm cells produced from Stage 4 co-express at least PDX1 and
Nkx6.1 as well
as PTF1A, and do not appreciably express markers indicative of definitive
endoderm or foregut
endoderm (PDX1-negative foregut endoderm) cells as described above in Stages
1, 2 and 3.
[0137]
Alternatively, the cells from Stage 4 can be further differentiated in Stage 5
to
produce endocrine precursor or progenitor type cells and / or singly and poly-
hormonal
pancreatic endocrine type cells from Stage 4 cells in a medium containing DMEM
in 1% vol/vol
B27 supplement for about 1 to 6 days (days 15-20). Endocrine precursors
produced from Stage 5
co-express at least NGN3 and PAX4 as well as Nkx2.2, and do not appreciably
express markers
64
Date Recue/Date Received 2023-09-08
indicative of definitive endoderm or foregut endoderm (PDX1-negative foregut
endoderm) or PDX1-
positive pancreatic endoderm or progenitor cells as described above in Stages
1, 2, 3and 4.
[0138]
PDX1-positive pancreatic endoderm produced from Stage 4 are loaded and
wholly contained in a macro-encapsulation device and transplanted in a
patient, and the PDX1-
positive pancreatic endoderm cells mature into pancreatic hormone secreting
cells, e.g., insulin
secreting cells, in vivo. Encapsulation of the PDX1-positive pancreatic
endoderm cells and
production of insulin in vivo is described in detail in U.S. Application No.
12/618,659 (the '659
Application"), entitled Encapsulation of pancreatic lineage cells derived from
human pluripotent
stem cells, filed November 13, 2009. The '659 Application claims the benefit
of priority to
Provisional Patent Application Number 61/114,857, entitled ENCAPSULATION OF
PANCREATIC
PROGENITORS DERIVED FROM HES CELLS, filed November 14, 2008; and U.S.
Provisional
Patent Application Number 61/121,084, entitled ENCAPSULATION OF PANCREATIC
ENDODERM CELLS, filed December 9, 2008.
[0139]
The methods, compositions and devices described herein are presently
representative of preferred embodiments and are exemplary and are not intended
as limitations on the
scope of the invention. Changes therein and other uses will occur to those
skilled in the art which are
encompassed within the spirit of the invention and are defined by the scope of
the disclosure.
Accordingly, it will be apparent to one skilled in the art that varying
substitutions and modifications
may be made to the invention disclosed herein without departing from the scope
and spirit of the
invention.
[0140]
For example, Activin A, a member of the TGFP superfamily of growth factors or
signaling proteins, is used to produce definitive endoderm from pluripotent
cells, e.g., hES cells and
iPS cellsõ however, other TGFP super family members can be used, for example
GDF-8 and GDF-
11, to produce definitive endoderm such as those described in International
Application
PCT/US2008/065686, entitled Growth factors for production of definitive
endoderm, filed June 3,
2008.
[0141]
Retinoic acid (RA) is used to differentiate PDX1-negative foregut endoderm
cells
in Stage 2 to PDX1-positive foregut cells in Stage 3. However, other retinoids
or retinoic acid
analogues such as 4-
[(E)-2-(5,6,7,8-tetrahydro -5,5,8,8-tetramethy1-2-napthaleny1)-1-
propenyl]benzoic acid (or TTNPB) and similar analogs (e.g., 4-HBTTNPB) can be
used.
[0142]
Noggin is a protein for example that inactivates members of the TGFP
superfamily signaling proteins, such as bone morphogenetic protein-4 (BMP4).
However, other
Date Recue/Date Received 2020-04-16
Date Recue/Date Received 2023-09-08
BMP4 inhibitors such as Chordin and Twisted Gastrulation (Tsg) or anti-BMP
neutralizing
antibodies can prevent BMP binding to its cell surface receptors, thereby
effectively inhibiting the
BMP signaling. Alternatively, the gene for human Noggin has been cloned and
sequenced. See U.S.
Patent No. 6,075,007. Analysis of the Noggin sequence shows a carboxy terminal
region having
homology to a Kunitz-type protease inhibitor, indicating that potentially
other Kunitz-type protease
inhibitors may have a similar effect on inhibiting BMP.
[0143] Lastly, the macro-encapsulation devices described herein and in
U.S. Application
No. 12/618,659, are again only exemplary and are not intended as limitations
on the scope of the
invention. Particularly, changes to the device design such as size of the
device, plurality of chambers
or subcompartments in the device, or plurality of ports, or even mechanisms
for loading and
extracting the device are all encompassed within the spirit of the invention.
Hence, it will be
apparent to one skilled in the art that varying substitutions and
modifications not only to the
described differentiation methods herein but to the encapsulation device as
well may be made to the
invention disclosed herein without departing from the scope and spirit of the
invention.
Production and Compositions of Definitive Endoderm (Stage 1)
[0144] In some processes, differentiation to definitive endoderm is
achieved by
providing to the pluripotent cell culture a growth factor of the TGFP
superfamily in an amount
sufficient to promote differentiation to definitive endoderm. Growth factors
of the TGFP
superfamily which are useful for the production of definitive endoderm are
selected from the
Nodal/Activin, GDF-8, -9, -10, -11 and the like or BMP subgroups. In some
preferred differentiation
processes, the growth factor is selected from the group consisting of Nodal,
Activin A, Activin B,
GDF-8, GDF-11 and BMP4. Additionally, the growth factor Wnt3a, other Wnt
family members, and
Wnt pathway activators are useful for the production of definitive endoderm
cells. In certain
differentiation processes, combinations of any of the above-mentioned growth
factors can be used.
This and other methods are described in detail in U.S. Patent No. 7,510.876.
[0145] With respect to some of the processes for the differentiation of
pluripotent cells to
definitive endoderm cells, the above-mentioned growth factors are provided to
the cells so that the
growth factors are present in the cultures at concentrations sufficient to
promote differentiation of at
least a portion of the pluripotent cells to definitive endoderm cells. In some
processes, the above-
mentioned growth factors are present in the cell culture at a concentration of
at least about 5 ng/ml, at
least about 10 ng/ml, at least about 25 ng/ml, at least about 50 ng/ml, at
least about 75 ng/ml, at least
66
Date Recue/Date Received 2023-09-08
about 100 ng/ml, at least about 200 ng/ml, at least about 300 ng/ml, at least
about 400 ng/ml, at least
about 500 ng/ml, at least about 1000 ng/ml, at least about 2000 ng/ml, at
least about 3000 ng/ml, at
least about 4000 ng/ml, at least about 5000 ng/ml or more than about 5000
ng/ml.
[0146] In certain processes for the differentiation of pluripotent
cells to definitive
endoderm cells, the above-mentioned growth factors are removed from the cell
culture subsequent to
their addition. For example, the growth factors can be removed within about
one day, about two
days, about three days, about four days, about five days, about six days,
about seven days, about
eight days, about nine days or about ten days after their addition. In a
preferred process, the growth
factors are removed about four days after their addition.
[0147] In some embodiments of the processes described herein, defmitive
endoderm
cells are enriched, isolated and/or purified prior to further differentiation.
In such embodiments,
definitive endoderm cells can be enriched, isolated and/or purified using any
known method. In
preferred embodiments, the definitive endoderm cells are enriched, isolated
and/or purified using one
or more of the methods described in U.S. Patent Application Number 11/021,618,
entitled
DEFINITIVE ENDODERM, filed December 23, 2004, and U.S. Provisional Patent
Application
Number 60/736,598, entitled MARKERS OF DEFINITIVE ENDODERM, filed November 14,
2005.
[0148] In a preferred embodiment, the definitive endoderm cells
produced and described
herein have relatively high levels of CER, GSC, CXCR4, SOX17 and FOXA2 gene
expression when
normalized and compared to levels of control genes, such as housekeeping
genes.
Production and Compositions of PDX1-Negative Foregut Endoderm (Stage 2)
[0149] Definitive endoderm cells can be specified toward pancreatic
differentiation by
further differentiation of these cells to produce PDXI-negative foregut
endoderm cells. In some of
the differentiation processes described herein, cell cultures as well as
enriched or purified cell
populations comprising definitive endoderm cells can be used for further
differentiation to cell
cultures and/or enriched cell populations comprising PDX1-negative foregut
endoderm cells.
67
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0150] Typically, definitive endoderm cells are differentiated to
PDX1-negative
foregut endoderm cells by reducing or eliminating or removing TGFP superfamily
growth factor
signaling in a cell culture or cell population of SOX17-positive definitive
endoderm cells. In
some embodiments, reducing or eliminating TGFP superfamily growth factor
signaling is
mediated by diluting or removing an exogenously added TGFP superfamily growth
factor, such
as activin A, from the cell culture or cell population of definitive endoderm.
In other
embodiments, TGFP superfamily growth factor signaling is reduced or eliminated
by providing
the definitive endoderm cells with a compound that blocks TGFP superfamily
growth factor
signaling, such as follistatin and/or noggin. In some embodiments, TGFP
superfamily growth
factor signaling can be reduced or eliminated for about one day, about two
days, about three
days, about four days, about five days, about six days, about seven days,
about eight days, about
nine days, about ten days or greater than about ten days subsequent to the
differentiation of the
human pluripotent cells to definitive endoderm cells.
[0151] In some embodiments, differentiation of definitive endoderm
cells to foregut
endoderm cells is enhanced by providing the definitive endoderm cell culture
or cell population
with an FGF-family growth factor and/or a hedgehog pathway inhibitor. In such
embodiments
the FGF-family growth factor and/or hedgehog pathway inhibitor is provided at
about one day,
about two days, about three days, about four days, about five days, about six
days, about seven
days, about eight days, about nine days, about ten days or greater than about
ten days subsequent
to reducing or eliminating TGF13 superfamily growth factor signaling in the
definitive endoderm
cell culture. In a preferred embodiment, the FGF-family growth factor and/or
hedgehog pathway
inhibitor is provided at about the same time as reducing or eliminating TGFP
superfilmily growth
factor signaling in the defmitive endoderm cell culture.
[0152] In a preferred embodiment, the FGF-family growth factor
provided to the
definitive endoderm cell culture or cell population is FGF10 and/or FGF7.
However, it will be
appreciated that other FGF-family growth factors or FCF-family growth factor
analogs or
mimetics may be provided instead of or in addition to FGF10 and/or FGF7. For
example, an
FGF-family growth factor selected from the group consisting of FGF1, FGF2,
FGF3, and the like
up to and including FGF23 may be provided. In such embodiments, the FGF-family
growth
factor and/or the FGF-family growth factor analog or mimetic is provided to
the cells of a cell
culture such that it is present at a concentration of at least about 10 ng/ml,
at least about 25
ng/ml, at least about 50 ng/ml, at least about 75 ng/ml, at least about 100
ng/ml, at least about
200 ng/ml, at least about 300 ng/ml, at least about 400 ng/ml, at least about
500 ng/ml, or at least
about 1000 ng/ml.
68
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCT/US2014/015156
[0153] In other preferred embodiments, the hedgehog inhibitor is
KAAD-
cyclopamine. However, it will be appreciated that other hedgehog inhibitors
can be used. Such
inhibitors include, but arc not limited to, ICAAD-cyclopaminc analogs,
jcrvinc, jervinc analogs,
hedgehog pathway blocking antibodies and any other inhibitors of hedgehog
pathway function
known to those of ordinary skill in the art. When used alone or in conjunction
with FGF-family
growth factor, the hedgehog inhibitor can be provided at a concentration of at
least about 0.01
M to 50 M.
[0154] In a preferred process for the production of a population of
PDX1-negative
foregut endoderm cells from definitive endoderm cells, TGF13 superfamily
growth factor
signaling is reduced or eliminated for about two day subsequent to the
differentiation of a
substantial portion of human pluripotent cells to definitive endoderm (for
example, after a three
day, four or five day differentiation protocol as described in the examples
below). At about the
same time, the cell culture or cell population of definitive endoderm cells is
provided noggin and
KAAD-cyclopamine, e.g., 50 ng/ml of Noggin and 0.25 M. ICAAD-cyclopamine in
DMEM
medium in the presence of 2 niM RA or 3nM TTNPB.
[0155] In some embodiments, the PDX1-negative foregut endoderm
cells can be
further differentiated to PDXI-positive foregut endoderm cells by contacting
the cells with a
medium comprising, or otherwise providing to the cells, a retinoid, such as
retinoic acid (RA).
In some embodiments, the retinoid is provided to the cells of a cell culture
such that it is present
at a concentration of at least about 1 nM to 50 M. In such embodiments, the
retinoid is
provided to the cells at about one day, about two days, about three days,
about four days, about
five days, about six days, about seven days, about eight days, about nine
days, about ten days or
greater than about ten days subsequent to reducing or eliminating TGF13
superfamily growth
factor signaling in the definitive endoderm cell culture. In a preferred
embodiment, from about
0.05 M RA to about 2 M RA is provided to the PDX-1 negative foregut endoderm
cell culture
about 2 to 3 days subsequent to reducing or eliminating TGFp superfamily
growth factor
signaling.
[0156] In some of the differentiation processes described herein,
the above-
mentioned differentiation factors are removed from the cell culture subsequent
to their addition.
For example, the above-mentioned differentiation factors can be removed within
about one day,
about two days, about three days, about four days, about five days, about six
days, about seven
days, about eight days, about nine days or about ten days after their
addition.
[0157] In a preferred embodiment, the PDX1-negative foregut
endoderm cells
produced and described herein have relatively high levels of S0X17, FOXA1,
HNF1B and
69
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
HNF4A gene expression when normalized to levels of control genes, such as
housekeeping
genes. In particular, levels of 1-INF4A gene expression does not substantially
increase until
removal of TG93 superfamily growth factor signaling (e.g., Activin) from a,
definitive endoderm
culture, for example.
Production and Compositions of PDX1-Positive Foregut Endoderm (Stage 3)
[0158] PDX1-negative foregut endoderm cells can be further
specified toward
pancreatic differentiation by further differentiation of these cells to
produce PDX1-positive
foregut endoderm cells or PDX1-positive pancreatic endoderm or equivalents
thereof. In some
of the differentiation processes described herein, cell cultures as well as
enriched or purified cell
populations comprising definitive endoderm cells can be used for further
differentiation to cell
cultures and/or enriched cell populations comprising PDX1-positive foregut
endoderm cells.
[01591 Typically, PDX1 -negative foregut endoderm cells are
differentiated to PDX1-
positive foregut endoderm cells by providing to a cell culture comprising
SOX17-positive
foregut endoderm cells a retinoid, such as retinoic acid (RA). In some of the
differentiation
processes, PDX1-negative foregut endoderm cells in culture are also provided
with a member of
the fibroblast growth factor family either prior to or about the same time as
the addition of RA.
A preferred fibroblast growth factor is FGF-7. In another preferred process,
the fibroblast
growth factor comprises any fibroblast growth factor or a ligand that
stimulates or otherwise
interacts with the fibroblast growth factor 2 receptor Mb (FGER2(IIIb)). In
even more preferred
processes, the FGF family growth factor is used in conjunction with a hedgehog
pathway
inhibitor. A preferred hedgehog pathway inhibitor is KAAD-cyclopamine. In
especially
preferred differentiation processes. FGF-10 and/or KAAD-cyclopamine are
provided to a cell
culture comprising PDX1-negative definitive endoderm cells in the presence of
RA. In certain
processes, BMP4 may be included with FGF 10 and/or KAAD-cyclopamine in the
presence of
RA. In some processes, the retinoid is used in conjunction with a member of
the TGFP
superfamily of growth factors and/or Connaught Medical Research Labs medium
(CRML
medium) (lnvitrogen, Carlsbad, CA).
[0160] With respect to some of the embodiments of differentiation
processes
described herein, the retinoid and/or a combination of the above-mentioned
differentiation
factors are provided to the cells so that these factors are present in the
cell culture or cell
population at concentrations sufficient to promote differentiation of at least
a portion of the
PDX1-negative foregut endoderm cell culture or cell population to PDX1-
positive foregut
endoderm cells.
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCT/US2014/015156
[0161] hi other processes, FGF-10 is provided to the cells of a
cell culture such that it
is present at a concentration of at least about 1 ng/ml, at least about 2
ng/ml, at least about 5
ng/ml, at least about 10 ng/ml, at least about 25 ng/ml and up to 50 M. In
some embodiments
of the present invention, a fibroblast growth factor or a ligand that
stimulates or otherwise
interacts with the fibroblast growth factor 2 receptor Mb (FGFR2(II1b) is
provided either alone
or in combination with the hedgehog pathway inhibitor.
[0162] In a preferred process for the production of a population of
PDX1-positive
foregut endoderm cells from PDX1-negative foregut endoderm cells, a cell
culture or an enriched
cell population of PDX1-negative foregut endoderm cells is provided with FGF-
7, KAAD-
cyclopamine and retinoic acid (RA), e.g., 25 ng/ml of FGF-7 and 0.2 i.tM KAAD-
cyclopamine in
CMRL medium in the presence of 2 p.M RA.
[0163] In some processes described herein, TGFI3 signaling factor,
e.g., activin A
and/or activin B, GDF-8, GDF-11 and the like arc provided to the cell culture
along with the
retinoid and/or the fibroblast growth factor and the hedgehog inhibitor. For
example, in such
processes, activin A and/or activin B and/or GDF-8 and/or GDF-11 is provided
to the cell culture
at a concentration of at least about 5 ng/ml, at least about 10 ngiml, at
least about 25 ng/ml, at
least about 50 ng/ml, at least about 75 ng/ml, at least about 100 ng/ml, at
least about 200 ng/ml,
at least about 300 ng/ml, at least about 400 ng/ml, at least about 500 ng/ml,
or at least about 1000
ng/ml.
[0164] In some processes, the differentiation factors and/or CRML
medium is
provided to the PDX1-negative foregut endoderm cells at about one day, two
days, three days, at
about four days, at about five days, at about six days, at about seven days,
at about eight days, at
about nine days, at about ten days or at about greater than ten days
subsequent to the initiation of
differentiation from pluripotent cells. In preferred processes,
differentiation factors and/or
CRML medium is provided to the PDX1-negative foregut endoderm cells at about
three or four
or five days subsequent to the initiation of differentiation from pluripotent
stem cells.
[0165] In certain processes described herein, the above-mentioned
differentiation
factors are removed from the cell culture subsequent to their addition. For
example, the above-
mentioned differentiation factors can be removed within about one day, about
two days, about
three days, about four days, about five days, about six days, about seven
days, about eight days,
about nine days or about ten days after their addition.
[0166] These and other methods are described in detail in U.S.
Provisional Patent
Application No. 60/730,917, entitled PDX1- EXPRESSING DORSAL AND VENTRAL
FOREGUT ENDODERM, filed October 27, 2005, and PDX1-positive foregut endoderm
cells
71
Date Recue/Date Received 2023-09-08
can be found in U.S. Patent Application No. 11/115,868, entitled PDX1
EXPRESSING
ENDODERM, filed April 26, 2005_
[0167] In a preferred embodiment, the PDX1-positive foregut endoderm
cells produced
and described herein have relatively high levels of PDX1, NKX6.1, PTF1A, CPA
and cMYC gene
expression when normalized and compared to levels of control genes, such as
housekeeping genes.
Production and Compositions of PDX1-Positive Pancreatic Endoderm (Stage 4)
[0168] Production and compositions of PDX1-positive pancreatic endoderm
or
"pancreatic epithelium" or more generally pancreatic progenitors is described
in detail in U.S.
Application No. 12/167,227, entitled Methods of producing pancreatic hoimones,
filed July 2, 2008,
which issued May 19, 2009 as U.S. Patent No. 7,534,608. The 12/167,227
application is a
continuation of U.S. Application No. 11/773,944, entitled METHODS OF PRODUCING
PANCREATIC HORMONES, filed July 5, 2007, which is a continuation-in-part of
U.S. Patent
Application Number 11/681,687, entitled ENDOCRINE PRECURSOR CELLS, PANCREATIC
HORMONE-EXPRESSING CELLS AND METHODS OF PRODUCTION, filed March 2, 2007.
[0169] Example 22 of the 12/167,227 application describes in detail
various
modifications of the cell culture conditions described herein in Stages 1 to
4. In general, Stage 3
PDX1-positive foregut endoderm type cells are cultured in media containing
DMEM in 1% vol/vol
B27 supplement plus Noggin, or another BMP4 specific inhibitor for about 1 to
3 days, or about 1 to
4 days, or 1 to 5 days, or about 1 to 6 days. At the end of Stage 4, a PDX1-
positive pancreatic
endoderm is produced, which cell at least co-expresses PDX1 and Nkx6.1 as well
as PTF1A. The
cell culture does not appreciably express cells indicative of singly or poly-
hormonal endocrine cells
of Stage 5, or definitive endoderm cells of Stage 1, or PDX1-negatiave foregut
endoderm cells of
Stage 2, or PDX1-positive foregut endoderm cells of Stage 3.
Production and Compositions of Endocrine Precursor Cells (Stage 5)
[0170] Some embodiments described herein relate to methods of producing
endocrine
precursor cells starting from pluripotent cells. As described above, endocrine
precursor cells can be
produced by first differentiating pluripotent cells to produce definitive
endoderm cells then further
differentiating the definitive endoderm cells to produce PDX1-positive foregut
endoderm cells. In
such embodiments, PDX1-positive foregut endoderm cells are further
differentiated to
72
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCT/US2014/015156
multipotent endocrine precursor cells, which are capable of differentiating
into human pancreatic
islet hormone-expressing cells.
[0171] In one
embodiment, PDX1-positive foregut endoderm cells are differentiated
to endocrine precursor cells by continuing the incubation of PDX I-positive
foregut endoderm
cells in the presence of a retinoid, such as retinoic acid, for an amount of
time sufficient to
produce endocrine precursor cells. In some embodiments, the amount of time
sufficient for the
production of endocrine precursor cells ranges from about 1 hour to about 10
days subsequent to
the expression of the PDX1 marker in a portion of the cells in the cell
culture. In some
embodiments, the retinoid is maintained in the cell culture for about 1 hour,
about 2 hours, about
4 hours, about 6 hours, about 8 hours, about 10 hours, about 12 hours, about
16 hours, about 1
day, about 2 days, about 3 days, about 4 days, about 5 days, about 6 days,
about 7 days, about 8
days, about 9 days, about 10 days or greater than about 10 days subsequent to
the expression of
the PDX1 marker in a portion of the cells in the cell culture.
[0172] In
some processes described herein, the concentration of retinoid used to
differentiate PDX1-positive foregut endoderm cells in the cell culture or cell
population to
endocrine precursor cells ranges from about 1 nM to about 100 M.
[0173] In
some preferred embodiments, differentiation from PDX1-positive foregut
endoderm cells to pancreatic endocrine precursor cells is mediated by
providing a cell culture or
cell population comprising human PDX1-positive foregut endoderm cells with a
gamma
secretase inhibitor. In a preferred embodiment, the gamma secretase inhibitor
is N1N-(3,5-
Diflurophenacetyl-L-alany1)]-S-phenylglycine t-Butyl Ester (DAPT).
[0174] In
other embodiments, the gamma secretase inhibitor is provided at the start of
the differentiation process, for example, at the pluripotent stage, and
remains in the cell culture
throughout the differentiation to pancreatic islet hormone-expressing cells.
In still other
embodiments, the gamma secretase inhibitor is added subsequent to the
initiation of
differentiation but prior to differentiation to the PDX1-positive foregut
endoderm stage. In
I referred embodiments, the gamma secretase inhibitor is provided to the cell
culture or cell
population at about the same time as providing the differentiation factors
which promote the
conversion of definitive endoderm to PDX1-positive endoderm. In
other preferred
embodiments, the gamma secretase inhibitor is provided to the cell culture or
cell population
after a substantial portion of the cells in the cell culture or cell
population have differentiated to
PDX1-positive forcgut endoderm cells.
[0175] With
respect to some embodiments regarding the differentiation of PDX1-
positive foregut endoderm cells to endocrine precursor cells, the gamma
secretase inhibitor is
73
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
provided to the cells so that it is present in the cell culture or cell
population at concentrations
sufficient to promote differentiation of at least a portion of the PDX1-
positive cells to endocrine
precursor cells. In some embodiments, the gamma secretase inhibitor is present
in the cell
culture or cell population at a concentration ranging from about 0.01 M to
about 1000 M. In
preferred embodiments, the gamma secretase inhibitor is present in the cell
culture or cell
population at a concentration ranging from about 0.1 M to about 100 M.
[0176] In certain embodiments of the processes for producing
endocrine precursor
cells as described herein, the gamma secretase inhibitor is provided after one
or more previously
provided differentiation factors have been removed from the cell cultures. For
example, the one
or more previously provided differentiation factors can be removed about 1 day
to 10 days or
more than about 10 days prior to the addition of the gamma secretase
inhibitor. In other
embodiments, the gamma secretasc inhibitor is provided to cell cultures or
cell populations
comprising one or more differentiation factors that were previously provided
or provided at
about the same time as the gamma secretase inhibitor. In preferred
embodiments, differentiation
factors that were previously provided or provided at about the same time as
the gamma secretase
inhibitor include, but are not limited to, FGF-10, KAAD-cyclopamine, activin
A, activin B,
GDF-8, GDF-11, BMP4 and/or RA.
[0177] In some embodiments of the invention described herein,
exendin 4 is provided
to the differentiating cell culture or cell population at about the same time
as the gamma
secretase inhibitor. In certain embodiments, exendin 4 is provided so as to be
in present in the
cell culture or cell population at a concentration of at least about 0.1
ng/ml, to 1000 ng/ml.
[0178] In a preferred process for the production of endocrine
precursor cells from
PDXI -positive foregut endoderm cells, a cell culture or cell population of
PDXI -positive foregut
endoderm cells is provided with 3 M DAFT and 40 ng/ml exendin 4. In
especially preferred
embodiments, the cells are differentiated in CMRL. In another especially
preferred process, for
the production of a endocrine precursor cells from PDX1-positive foregut
endoderm cells, a cell
culture or cell population of PDX1-positive foregut endoderm cells is provided
with 3 M DAPT
and 40 ng/ml exendin 4 in the presence of 2 M RA.
[0179] It will be appreciated that NGN3, NKX2.2 and/or PAX4 marker
expression is
induced over a range of different levels in endocrine precursor cells
depending on the
differentiation conditions. As such, in some embodiments described herein, the
expression of the
NGN3, NKX2.2 and/or PAX4 marker in endocrine precursor cells or cell
populations is at least
about 2-fold higher to at least about 10,000-fold higher than the expression
of the NGN3,
NKX2.2 and/or PAX4 marker in non-endocrine precursor cells or cell
populations, for example
74
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
pluripotent stem cells, definitive endoderm cells, PDX1-positive foregut
endoderm cells,
immature pancreatic islet hormone-expressing cells, mature pancreatic islet
hormone-expressing
cells, extraembryonic endoderm cells, mesoderm cells and/or ectoderm cells. In
other
embodiments, the expression of the 1 GN3, NKX2.2 and/or PAX4 marker in
endocrine precursor
cells or cell populations is at least about 4-fold higher, at least about 6-
fold higher to 10,000-fold
higher than the expression of the NGN3, NKX2.2 and/or PAX4 marker in non-
endocrine
precursor cells or cell populations, for example pluripotent stem cells,
definitive endoderm cells,
PDX1-positive forego endoderm cells, immature pancreatic islet hormone-
expressing cells,
mature pancreatic islet hormone-expressing cells, extraembryonic endoderm
cells, mesoderm
cells and/or ectoderm cells. In some embodiments, the expression of the NGN3,
NKX2.2 and/or
PAX4 marker in endocrine precursor cells or cell populations is infinitely
higher than the
expression of the NGN3, NKX2.2 and/or PAX4 marker in non-endocrine precursor
cells or cell
populations, for example pluripotent cells like iPS cells and hES cells,
definitive endoderm cells,
PDXI-positive foregut endoderm cells, immature pancreatic islet hormone-
expressing cells,
mature pancreatic islet hormone-expressing cells, extraembryonic endoderm
cells, mesoderm
cells and/or ectoderm cells.
[0180] Further embodiments of the present invention relate to
compositions, such as
cell cultures or cell populations, comprising human cells, including human
endocrine precursor
cells, wherein the expression of the NGN3 marker is greater than the
expression of the AFP,
S0X7, SOXI, ZICI, NFM, MAFA, SYP, CHGA, ThTS, GCG, SST, GHRL, and/or PAX6
marker
in at least about 2% of the human cells. In other embodiments, the expression
of the NGN3
marker is greater than the expression of the AFP, SOX7, SOXI, ZIC1, NFM, MAFA,
SYP,
CHGA, INS, GCG, SST, GHRL, and/or PAX6 marker in at least about 5% to 98% of
the human
cells. In some embodiments, the percentage of human cells in the cell cultures
or populations,
wherein the expression of NGN3 is greater than the expression of the AFP,
SOX7, SOX I, ZIC1,
NFM, MAFA, SYP, CHGA, INS, GCG, SST, GHRL, and/or PAX6 marker, is calculated
without regard to feeder cells.
[0181] It will be appreciated that some embodiments of the present
invention relate to
compositions, such as cell cultures or cell populations, comprising human
endocrine precursor
cells, wherein the expression of NKX2.2 and/or PAX4 is greater than the
expression of the AFP,
SOX7, SOX1, ZICI, NFM, MAFA, SYP, CHGA, INS, GCG, SST, GHRL, and/or PAX6
marker
in from at least about 2% to greater than at least about 98% of the human
cells. In some
embodiments, the expression of NKX2.2 and/or PAX4 is greater than the
expression of the AFP,
SOX7, SOXI, ZIC1, NFM, MAFA, SYP, CHGA, INS, GCG, SST, GHRL, and/or PAX6
marker
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
in at least about 5% of the human cells to 98% of the human cells. In some
embodiments, the
percentage of human cells in the cell cultures or populations, wherein the
expression of NKX2.2
and/or PAX4 is greater than the expression of thc AFP, SOX7, SOX1, ZIC1, NFM,
MAFA,
SYP, CHGA, IS, GCG, SST, GHRL, and/or PAX6 marker, is calculated without
regard to
feeder cells.
[0182] Additional embodiments of the present invention relate to
compositions, such
as cell cultures or cell populations, comprising mammalian cells
differentiated from definitive
endoderm in vitro, such as human cells differentiated from definitive endoderm
in vitro, wherein
the expression of the NGN3, NKX2.2 and/or PAX4 marker is greater than the
expression of the
AFP, SOX7, SOX, Z1C1, NFM, MAFA, SYP, CHGA, INS, GCG, SST, GHRL, and/or PAX6
marker in at least about 2% of the cells differentiated from definitive
endoderm in vitro. In other
embodiments, the expression of the NGN3, NKX2.2 and/or PAX4 marker is greater
than the
expression of the AFP, SOX7, SOX1, ZIC1, NFM, MAFA, SYP, CHGA, INS, GCG, SST,
GHRL, and/or PAX6 marker in at least about 5% of the cells differentiated from
definitive
endoderm in vitro to 98% of the cells differentiated from definitive endoderm
in vitro.
[0183] Using the processes described herein, compositions
comprising endocrine
precursor cells substantially free of other cell types can be produced. In
some embodiments of
the present invention, the endocrine precursor cell populations or cell
cultures produced by the
methods described herein are substantially free of cells that significantly
express the AFP,
S0X7, SOX1, ZIC1 and/or NFM markers. In some embodiments, the endocrine
precursor cell
populations of cell cultures produced by the methods described herein are
substantially free of
cells that significantly express the AFP, SOX7, SOX1, ZICI, NFM, MAFA, SYP,
CHGA, INS,
GCG, SST, GHRL, and/or PAX6 markers.
[0184] In one embodiment of the present invention, a description of
a endocrine
precursor cell based on the expression of markers is, NGN3 high, NKX2.2 high,
PAX4 high,
AFP low, SOX7 low, SOX I low, ZIC1 low NFM low, MAFA low; SYP low; CHGA low;
NS
low, GCG low, SST low, GHRL low and/or PAX6 low.
Production of Immature Pancreatic Islet Hormone-Expressing Cells
[0185] Embodiments described herein relate to methods of producing
immature
pancreatic islet hormone-expressing cells starting from pluripotent cells. As
described above,
immature pancreatic islet hormone-expressing cells can be produced by first
differentiating
pluripotent cells to produce definitive endoderm cells, differentiating the
definitive endoderm
cells to produce foregut endoderm cells, differentiating foregut endoderm to
produce PDX1-
76
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
positive foregut endoderm cells and then further differentiating the PDX1-
positive foregut
endoderm cells to produce endocrine precursor cells. In some embodiments, the
process is
continued by allowing the endocrine precursor cells to further differentiate
to immature
pancreatic islet hormone-expressing cells.
[0186] In some embodiments of the present invention,
differentiation from endocrine
precursor cells to immature pancreatic islet hormone-expressing cells proceeds
by continuing the
incubation of a culture of endocrine precursor cells with a gamma secretase
inhibitor for a
sufficient time that the cells stop substantially expressing NGN3, and start
expressing PAX6, and
to permit the cells to become competent to express at least one pancreatic
islet cell hormone. In
some embodiments, the gamma secretase inhibitor is removed about I day, about
2 days, about 3
days, about 4 days, about 5 days, about 6 days, about 7 days, about 8 days,
about 9 days, about
days or more than about 10 days after the induction of endocrine precursor
cells. In a
preferred embodiment, the gamma secretase inhibitor is N4N-(3,5-
Diflurophenacetyl-L-alany1)]-
S-phenylglycine t-Butyl Ester (DAPT).
[0187] Certain processes for the production of immature pancreatic
islet hormone-
expressing cells disclosed herein are mediated by providing a cell culture or
cell population
comprising human endocrine precursor cells with one or more factors selected
from the group
consisting of nicotinamide, exendin 4, bepatocyte growth factor (HGF), insulin-
like growth
factor-1 (IGF 1). In some embodiments, all four of the above-described factors
are provided
together. In some embodiments, one or more of the above-described factors are
provided to the
cell culture prior to the differentiation of endocrine precursor cells and
remain present in the cell
culture during the differentiation of at least a portion of the cells in the
cell culture to endocrine
precursor cells. In other embodiments, one or more of the above-described
factors are provided
to the cell culture at or about the time of differentiation of a substantial
portion of the cells to
endocrine precursor cells and remain present in the cell culture until at
least a substantial portion
of the cells have differentiated into immature pancreatic islet hormone-
expressing cells. In some
embodiments of the present invention, one or more of the above-described
factors are provided
at the start of the differentiation process, for example, at the pluripotent
cell stage, and remain in
the cell culture throughout the differentiation to immature pancreatic islet
hormone-expressing
cells.
[0188] in some processes for the production of immature pancreatic
islet hormone-
expressing cells disclosed herein, nicotinamide, nicotinamide-adenine
dinucleotide (NAD), or
nicotinic acid is provided to the cells so that it is present in the cell
culture or cell population at
concentrations sufficient to promote differentiation of at least a portion of
the endocrine
77
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
precursor cells to immature pancreatic islet hormone-expressing cells. In some
embodiments,
nicotinamide is present in the cell culture or cell population at a
concentration of at least about
0.1 mM, at least about 0.5 mM, to 1000 mM.
[0189] In other processes for the production of immature pancreatic
islet hormone-
expressing cells disclosed herein, exendin 4 is provided to the cells so that
it is present in the cell
culture or cell population at concentrations sufficient to promote
differentiation of at least a
portion of the endocrine precursor cells to immature pancreatic islet hormone-
expressing cells.
In some embodiments, exendin 4 is present in the cell culture or cell
population at a
concentration of at least about 1 ng/ml at least about 5 ng/ml to 1000 ng/ml.
[0190] In still other processes for the production of immature
pancreatic islet
hormone-expressing cells disclosed herein, HGF is provided to the cells so
that it is present in
the cell culture or cell population at concentrations sufficient to promote
differentiation of at
least a portion of the endocrine precursor cells to immature pancreatic islet
hormone-expressing
cells. In some embodiments, HGF is present in the cell culture or cell
population at a
concentration of at least about 1 ng/ml at least about 5 ng/ml to 1000 ng/ml.
[0191] In yet other processes for the production of immature
pancreatic islet
hormone-expressing cells disclosed herein, IGF I is provided to the cells so
that it is present in
the cell culture or cell population at concentrations sufficient to promote
differentiation of at
least a portion of the endocrine precursor cells to immature pancreatic islet
hormone-expressing
cells. In some embodiments, IGP1 is present in the cell culture or cell
population at a
concentration of at least about 1 ng/ml to 1000 ng/ml.
[0192] In certain embodiments of the processes for producing
immature pancreatic
islet hormone-expressing cells as described herein, one or more of
nicotinamide, exendin 4, HGF
and IGF1 are provided after one or more previously provided differentiation
factors have been
removed from the cell cultures. In other embodiments, one or more of
nicotinamide, exendin 4,
HGF and IGF1 are provided to cell culture or cell population comprising one or
more
differentiation factors that were previously provided or provided at about the
same time as one or
more of nicotinamide, exendin 4, HGF and IGF1. In preferred embodiments,
differentiation
factors that were previously provided or provided at about the same time as
one or more of
nicotinamide, exendin 4, HOF and IGFI include, but arc not limited to, DAFT,
FOF-10, KAAD-
cyclopamine, activin A, activin B, BMP4 and/or RA.
[0193] Further embodiments of the present invention relate to
compositions, such as
cell cultures or cell populations, comprising human cells, including human
immature pancreatic
islet hormone-expressing cells, wherein the expression of the MAFB, SYP, CHGA,
NKX2.2,
78
Date Recue/Date Received 2023-09-08
ISL1, PAX6, NEUROD, PDX1, HB9, GHRL, IAPP, INS GCG, SST, PP, and/or C-peptide
marker is
greater than the expression of the NGN3, MAFA, MOX1, CER, POU5F1, APP, SOX7,
SOX1, ZIC1
and/or NFM marker in at least about 2% of the human cells. In other
embodiments, the expression of
the MAFB, SYP, CHGA, NICX2.2, ISL1, PAX6, NEUROD, PDX1, HB9, GHRL, IAPP INS
GCG,
SST, PP, and/or C-peptide marker is greater than the expression of the NGN3,
MAFA, MOX1, CER,
POU5F1, APP, SOX7, SOX1, ZIC1 and/or NFM marker in at least about 5% of the
human cells, in
at least about 10% of the human cells to 95% of the human cells or in at least
about 98% of the
human cells.
[0194] In some embodiments, the percentage of human cells in the cell
cultures or
populations, wherein the expression of MAFB, SYP, CHGA, NKX2.2, ISL1, PAX6,
NEUROD,
PDX1, HB9, GHRL, IAPP, INS GCG, SST, PP, and/or C-peptide is greater than the
expression of
the NGN3, MAFA, MOX1, CER, POU5F1, AFP, SOX7, SOX1, ZIC1 and/or NFM marker, is
calculated without regard to feeder cells.
[0195] In certain embodiments of the present invention, cell cultures
and/or cell
populations comprising immature pancreatic islet hormone-expressing cells also
include a medium
which comprises one or more secreted hormones selected from gihrelin, insulin,
somatostatin and/or
glucagon. In other embodiments, the medium comprises C-peptide. In a preferred
embodiment, the
concentration of one or more secreted hormones or C-peptide in the medium
ranges from at least
about 1 pmol of ghrelin, insulin, somatostatin, glucagon or C-peptide/gg of
cellular DNA to at least
about 1000 picomoles (pmol) of ghrelin, insulin, somatostatin, glucagon or C-
peptide/m of cellular
DNA.
Method of producing insulin in vivo
[0196] In some embodiments, in vitro-derived pancreatic progenitor
cells or PDX-1-
positive pancreatic endoderm type cells or equivalents thereof described-above
are transplanted into
a mammalian subject. These methods are described in detail in International
Application
PCT/US2007/015536, titled Methods of producing pancreatic hormones. In a
preferred embodiment,
the mammalian subject is a human subject. Particularly preferred subjects are
those that have been
identified as having a condition which limits the ability of the subject to
produce sufficient levels of
insulin in response to physiologically high blood glucose concentrations. A
range of blood glucose
levels that constitutes a physiologically high blood glucose level for any
particular mammalian
species can be readily determined by those of ordinary skill in the art. Any
persistent blood glucose
79
Date Recue/Date Received 2023-09-08
level that results in a recognized disease or condition is considered to be a
physiologically high blood
glucose level.
[0197] Additional embodiments of the present invention relate to an in
vivo insulin
secreting cell that is derived from an in vitro pluripotent stem cell or
progeny thereof, e.g.,
multipotent cells, such as PDX-1 positive foregut endoderm cell, a PDX-1
positive pancreatic
endoderm or pancreatic progenitor cell, an endocrine precursor, such as an
NGN3 positive endocrine
precursor, or a functional differentiated hormone secreting cell, such as an
insulin, glucagon,
somatistatin, ghrelin, or pancreatic polypeptide secreting cell. Any of the
above-described terminally
differentiated or multipotent cells can be transplanted into the host, or
mammal, and mature into
physiologically functional hormone secreting cells, such as insulin secreting
cells, in response to host
blood glucose levels. In preferred embodiments the cell does not form a
teratoma in vivo, and if so
formed, remains localized to the area of transplant and can be easily excised
or removed. In
especially preferred embodiments, the cell does not contain any karyotypic
abnormality during the in
vitro differentiation process, or when transplanted into the mammal in vivo,
or when maturing and
developing into functional islets in vivo.
[0198] Further, although embodiments described herein relate to an
engineered or
genetically recombinant pluripotent cell, multipotent or differentiated cell
derived from the
pluripotent cell, such as a human iPS cell, based on the description provided
herein, it is anticipated
that because iPS cells demonstrate similar physiology and gene marker
expression profiles to that of
hES cells and hES-derived cells, they will have similar physiological
characteristics in vivo.
Method of encapsulating pancreatic progenitors
[0199] In some embodiments, the pluripotent, multipotent and
differentiated cell
composition described herein can be encapsulated in a biological and/or non-
biological mechanical
device, where the encapsulated device separates and/or isolates the cell
compositions from the host.
[0200] Methods of encapsulation are described in detail in U.S.
Application 61/114,857,
filed November 14, 2008, titled Encapsulation of pancreatic progenitors
derived from hES celLS, and
U.S. Application No. 61/121,086 filed December 12, 2008, titled Encapsulation
of pancreatic
endoderm cells and which describes encapsulation of pancreatic endoderm cells
using a semi-
permeable membrane, e.g., a TheracyteTm or Gore device.
[0201] In one embodiment, hES-derived cells are encapsulated using a
bio-compatible
polyethylene glycol (PEG). PEG-based encapsulation is described in more detail
in U.S. Patent No.
Date Recue/Date Received 2023-09-08
7,427,415, titled Implantation of encapsulated biological materials for
treating diseases; U.S. Patent
No. 6,911,227, entitled Gels for encapsulation of biological materials; and
U.S. Patent Nos.
6,911,227, 5,529,914, 5,801,033, 6,258,870, entitled Gels for encapsulation of
biological materials,
and which describes conformal coating of cell aggregates using polyethylene
glycol.
[0202] In one embodiment, the encapsulation device contains the
pluripotent derived
cells, for example, PDX-1 positive foregut endoderm cell, a PDX-1 positive
pancreatic endoderm or
progenitor cell, an endocrine precursor, such as an NGN3 positive endocrine
precursor, or a
functional differentiated hormone secreting cell, such as an insulin,
glucagon, somatistatin, ghrelin,
or pancreatic polypeptide secreting cell, in a semipermeable membrane that
prevents passage of the
transplanted cell population, retaining them in the device, while at the same
time permitting passage
of certain secreted polypeptides, e.g., insulin, glucagon, somatistatin,
ghrelin, pancreatic polypeptide
and the like. Alternatively, the device has a plurality of membranes,
including a vascularizing
membrane.
Use of agents to enhance and promote growth, survival, proliferation and cell-
cell adhesion of human
pluripotent stem cells, e.g., hES cells and iPS cells
[0203] Cellular regulation can be affected through the transduction of
extracellular
signals across the membrane that, in turn, modulates biochemical pathways
within the cell. Protein
phosphorylation represents one course by which intracellular signals are
propagated from molecule
to molecule resulting finally in a cellular response. These signal
transduction cascades are highly
regulated and often overlapping as evidenced by the existence of many protein
kinases as well as
phosphatases. It has been reported that in humans, protein tyrosine kinases
are known to have a
significant role in the development of many disease states including diabetes,
cancer and have also
been linked to a wide variety of congenital syndromes. Serine threonine
kinases, e.g., Rho kinases,
are a class of enzymes, which if inhibited can have relevance to the treatment
of human disease,
including diabetes, cancer, and a variety of inflammatory cardiovascular
disorders and AIDS. The
majority of inhibitors identified to date act at the ATP-binding site. Such
ATP-competitive inhibitors
have demonstrated selectivity by virtue of their ability to target the more
poorly conserved areas of
the ATP-binding site.
[0204] The Rho kinase family of small GTP binding proteins contains at
least 10
members including Rho A-E and G, Rac 1 and 2, Cdc42, and TC10. The inhibitors
are often referred
to as ROK or ROCK inhibitors or Rho-kinase inhibitors, and they are used
interchangeably herein.
81
Date Recue/Date Received 2023-09-08
The effector domains of RhoA, RhoB, and RhoC have the same amino acid sequence
and appear to
have similar intracellular targets. Rho kinase operates as a primary
downstream mediator of Rho and
exists as two isoforms: a (ROCK2) and 3 (ROCK1). Rho kinase family proteins
have a catalytic
(kinase) domain in their N-terminal domain, a coiled-coil domain in its middle
portion, and a putative
pleckstrin-homology (PH) domain in their C-terminal region. The Rho-binding
domain of ROCK is
localized in the C-terminal portion of the coiled-coil domain and the binding
of the GTP-bound form
of Rho results in enhancement of kinase activity. The Rho/Rho-kinase-mediated
pathway plays an
important role in the signal transduction initiated by many agonists,
including angiotensin II,
serotonin, thrombin, endothelin-1, norepinephrine, platelet-derived growth
factor, ATP/ADP and
extracellular nucleotides, and urotensin II_ Through the modulation of its
target effectors/substrates
Rho kinase plays an important role in various cellular functions including
smooth muscle
contraction, actin cytoskeleton organization, cell adhesion and motility and
gene expression. By
virtue of the role that Rho kinase proteins play in mediating a number of
cellular functions perceived
to be associated with the pathogenesis of arteriosclerosis, inhibitors of
these kinases may also be
useful for the treatment or prevention of various arteriosclerotic
cardiovascular diseases and involved
in endothelial contraction.
[0205] In
some embodiments, agents which promote and/or support cell growth,
survival, proliferation and cell-cell adhesion are added to various cell
culture media conditions,
including but not limited to, Rho-kinase inhibitors Y-27632, Fasudil (also
referred to as HA1077), H-
1152P and ITS (insulin/transferrin/selenium; Gibco), Wf-536, Y-30141
(described in U.S. Pat. No.
5,478,838) and derivatives thereof, and antisense nucleic acid for ROCK, RNA
interference inducing
nucleic acid (for example, siRNA), competitive peptides, antagonist peptides,
inhibitory antibodies,
antibody-ScFV fragments, dominant negative variants and expression vectors
thereof. Further, since
other low molecular compounds are known as ROCK inhibitors, such compounds or
derivatives
thereof can be also used in the present invention (for example, refer to
United State Patent
Application Nos. 20050209261, 20050192304, 20040014755, 20040002508,
20040002507,
20030125344 and 20030087919, and International Patent Publication
Nos.2003/062227,
2003/059913, 2003/062225, 2002/076976 and 2004/039796.
82
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
[0206] In the present invention, a combination of one or two or
more of the ROCK
inhibitors can also be used. These agents function, in part, by promoting re-
association of
dissociated hES cell, iPS or differentiated cell cultures, e.g., definitive
endoderm, foreE;ut
endoderm, pancreatic endoderm, pancreatic epithelium, pancreatic progenitor
populations,
endocrine progenitors and populations and the like. Likewise, the agents can
function when cell
dissociation is not performed. Increase in growth, survival, proliferation and
cell-cell adhesion of
the human pluripotent stem cells was achieved independent of whether the cells
were produced
from cell aggregates in suspension or from adherent plate cultures (with or
with no extracellular
matrix components, with or without serum, with or without fibroblast feeders,
with or without
FGF, with or without Activin). increase in survival of these cell populations
facilitates and
improves purification systems using a cell-sorter and, therefore allows
improved recovery of the
cells. Use of Rho kinase inhibitors such as Y27632 may allow for expansion of
differentiated cell
types as well by promoting their survival during serial passaging dissociated
single cells or from
cryogenic preservation. Although, Rho kinase inhibitors such as Y27632 have
been tested on
human pluripotent stem cells (e.g., hES and iPS cells) and differentiated
cells thereof, Rho kinase
inhibitors can be applied to other cell types, for example, in general,
epithelial cells including but
not limited to intestinal, lung, thymus, kidney as well as neural cell types
like pigmented retinal
epithelium.
[0207] The concentration of the ROCK inhibitor in the cell culture
medium is not
limited to that described in the examples below so long as the concentration
can achieve the
desired effects such as enhancing, increasing, and / or promoting growth,
survival, proliferation
and cell-cell adhesion of cells is achieved. One skilled in the art will
recognize that optimization
of various ROCK inhibitors under various conditions may be necessary. For
example, when
employing Y-27632 a preferable concentration can range from about 0.01 to
about 1000 M,
more preferably about 0.1 to about 100 j.tM, and even more preferably about
1.0 to about 50 M,
and most preferably about 5 to 20 M. When Fasudil/FIA1077 is used, it can be
used at about
two or three-fold the aforementioned Y-27632 concentration. When H-1152 is
used, it can be
used at about fraction, e.g., about 1/10th, 1/20th, 1130th, 1/40th, 1/50th or
1/60th of the amount of
the aforementioned Y-27632 concentration. The concentration of ROCK-inhibitor
used will
depend, in part, on the bioactivity and potentcy of the inhibitor and the
conditions in which they
arc used.
[0208] The time and period for treating with the ROCK inhibitor may
or may not be
limited depending on the desired effects such as the enhancing, increasing,
and / or promoting
growth, survival, proliferation (cell mass) and cell-cell adhesion. However,
addition of a ROCK
83
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCT/US2014/015156
inhibitor may also affect differentiation in surprising ways as better
described in Example 7. The
Examples below describe human pluripotent stem cell cultures and / or
differentiated cell
cultures treated for about 12 hours, 24 hours, 48 hours, or more.
[0209] The
density of the human pluripotent stern cell cultures treated with the
ROCK inhibitor is also not limited as far as it is a density at which the
desired effects such as the
enhancing, increasing, and / or promoting growth, survival, proliferation and
cell-cell adhesion
of cells is achieved. The cell density of the seeded cells may be adjusted
depending on a variety
of factors, including but not limited to the use of adherent or suspension
cultures, the specific
recipe of the cell culture media used, the growth conditions and the
contemplated use of the
cultured cells, Examples of cell culture densities include, but are not
limited to, 0.01 x 105
cells/ml, 0.05 x 105 cellsiml, 0.1 x 105 cells/ml, 0.5 x 105 cells/ml, 1.0 x
105 cells/ml, 1.2 x 105
cells/ml, 1.4 x 105 cells/ml, 1.6 x 105 cells/ml, 1.8 x 105 cells/ml, 2.0 x
105 cells/ml, 3.0 x 105
cells/ml, 4.0 x 105 cells/ml, 5.0 x 105 cells/ml, 6.0 x 105 cells/ml, 7.0 x
105 cells/ml, 8.0 x 105
cells/ml, 9.0 x 105 cells/ml, or 10.0 x 105 cells/ml, or more, e.g., up to 5 x
107 cells/ml, or more,
or any value in between, have been cultured with good cell growth, survival,
proliferation and
cell-cell adhesion.
Use of agents which activate TGFP receptor family members
[0210] Still
in another embodiment, agents that activate TGFP receptor family
member include members of the TGFP super family of growth factors, are
described herein. As
used herein, "TGFP superfarnily member" or equivalents thereof refers to over
30 structurally
related proteins including subfamilies including TGFP1, TGFP2, TF-P3, GDF-15,
GDF-9, BMP-
15, BMP-16, BMP-3, GDF-10, BMP-9, BMP-10, GDF-5,
GDF-7, BMP-5, BMP-6,
BMP-7, BMP-8, BMP-2, BMP-4, GDF-3, GDF-1, GDF 11, GDF8, Activins I3C, PE, I3A
and 13B,
BMP-14, GDF-14, MIS, Inhibin alpha, Lefty 1, Lefty2, GDNF, Neurteurin,
Persephin and
Artemin. See Chang et at (2002) Endocrine Rev. 23(6):787-823.
[0211] A TGFP
family member can be replaced by, or used in conjunction with, a
TGFP signaling pathway activator, which is a compound that stimulates one or
more of the
pulypeptides or interactions that participate in transducing or otherwise
effectuating changes in
the properties of a cell in response to a TGFP family member. A TG93 signaling
pathway
includes TGF13 family members themselves. TGFP super family members transmit
signals to the
nucleus by signaling through type II and I serine-threonine kinase receptors
and intracellular
effectors known as Smads. These receptors fall into two subfamilies known as
type I and type II
receptors that act cooperatively to bind ligand and transduce signal (Attisano
et al., Mol Cell Biol
84
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
16 (3), 1066-1073 (1996)). Ligands bind to type land 11 receptors on the cell
surface, promoting
activation of the type I receptor via phosphorylation. This activated complex
in turn activates
intracellular Smads, which assemble multi-subunit complexes that regulate
transcription.
Members of the TGFbeta super family are divided into two signaling subgroups:
those
functionally related to TGF13/Activin and those related to the BMP/GDF
subfamily. Most TGFI3
ligands are thought to bind first to a type II receptor and this ligand/type
II receptor complex then
recruits or phosphorylates a type I receptor (Mathews, L S, Endocr Rev 15:310-
325 (1994);
Massague, Nature Rev: Mol Cell Biol. 1, 169-178 (2000)). The type II receptor
kinase by
phosphorylating and activating the type I receptor kinase, which then
phosphorylates and
activates the Smad proteins, The TGFP/Activin ligands bind to T6193 and
Activin type 11
receptors and can activate Smad-2 and -3. Nodal and Lefty signal through this
Activin-type
pathway. The BMP/GDF ligands bind to BMP type II receptors and can activate
Smads 1, 5, and
8. See Derynck, R et al. Cell 95, 737-740 (1998)). Upon ligand stimulation,
Smads move into the
nucleus and function as components of transcription complexes.
[0212] TGFI3 signaling is regulated positively and negatively
though various
mechanisms. Positive regulation amplifies signals to a level sufficient for
biological activity.
TGFI3 superfamily ligands bind to a type IT receptor, which recruits and
phosphorylates a type
receptor. The type 1 receptor then phosphorylates receptor-regulated SMADs (R-
SMADs e.g.,
SMAD1, SMAD2, SMAD3, SMAD5, and SMAD8) which can now bind common mediator
Smad or co-SMAD. R-SMAD/coSMAD complexes accumulate in the nucleus where they
ac,t as
transcription factors and participate in the regulation of target gene
expression. For example,
Growth differentiation factors include 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, and 15.
And in one preferred
embodiment, GDF8 and GDF11, are TGFI3 family members that are also TGF13
signaling
pathway activators (positive regulation), and act by binding to the
extracellular ligand binding
domain portion of the ActR1I receptor and then forming a complex with ActRI,
leading to the
inhibition of the Smad7 negative regulator and phosphorylation of the
Smad2/Smad3 complex.
The Smad2/Smad3 complex associates with Smad4 to regulate expression of
certain genes.
[0213] Negative regulation of TGFI3 signaling occurs at the
extracellular, membrane,
cytoplasmic and nuclear levels. Signal transduction can be interrupted in
order to repress
signaling initiated by TGFP. This can be accomplished by any means known in
the art in which
the interaction between the TGFI3 receptor(s) and the SMAD protein is
antagonized or prevented,
including proteins that block or compete with TGFI3 receptor and SMAD protein
interactions.
Alternatively, the transcription or translation of TGF13 receptor or SMAD
protein can be altered
by any means known in the art in order to prevent signal transmission along
the signaling
Date Recue/Date Received 2023-09-08
pathway. Positive and negative regulation of various TGFI3 member proteins is
further exemplified
by a more detail description of some of the factors below.
[0214] As with the use of any agent, the concentration of any TGFI3
super family
member in the cell culture medium is not limited to that described in the
examples below so long as
the concentration can achieve the desired effects such as to activate a TGF13
receptor family member,
for example. For example, when employing Activins, e.g., Activin A and/or B,
or GDF8 and GDF-
11, a preferable concentration can range from about 10 to about 300 nM, more
preferably about 50 to
about 200 nM, and even more preferably about 75 to about 150 nM, and most
preferably about 100
to 125 nM. One of ordinary skill in the art can readily test any concentration
and using standard
techniques in the art can determine the efficacy of such concentration, e.g.,
evaluating differentiation
by determining expression and non-expression of a panel of gene makers for any
cell type.
[0215] These and other growth factors used to differentiate human
pluripotent stem cells
is described in more detail in U.S. Application 12/132,437, entitled Growth
factors for production of
definitive endoderm, filed June 3, 2008.
[0216] Having generally described this invention, a further
understanding can be
obtained by reference to certain specific examples which are provided herein
for purposes of
illustration only, and are not intended to be limiting.
EXAMPLES
[0217] It should also be understood that the foregoing relates to
preferred embodiments
of the present invention and that numerous changes may be made therein without
departing from the
scope of the invention. The invention is further illustrated by the following
examples, which are not
to be construed in any way as imposing limitations upon the scope thereof. On
the contrary, it is to
be clearly understood that resort may be had to various other embodiments,
modifications, and
equivalents thereof, which, after reading the description herein, may suggest
themselves to those
skilled in the art without departing from the spirit of the present invention
and/or the scope of the
appended claims.
EXAMPLE 1
DIFFERENTIATION OF HUMAN IPS CELLS TO PANCREATIC PROGENITORS
AND ENDOCRINE CELLS VIA DEFINITIVE ENDODERM AND ENDODERM
INTERMEDIATES
86
Date Recue/Date Received 2023-09-08
[0218] Human induced pluripotent stem (iPS) cells were differentiated
in suspension
aggregates using a four (4) stage procedure over the course of about 2 weeks
(or 14 days) to generate
a population of pancreatic cell types including pancreatic progenitors,
endocrine progenitors and
hormone expressing endocrine cells. Human iPS cell lines employed herein were
provided by S.
Yamanaka, Kyoto University, Japan and Cellular Dynamics International, Inc.
(CDI).
[0219] The iPS cells described herein were first provided by Shinja
Yamanaka and later
by CDI. Undifferentiated iPS cells were grown on mitotically inactivated mouse
embryo fibroblasts
or preferably feeder-free (no fibroblast feeder cell layer) in DMEM/F 12
containing 20% Knockout
serum replacement. Differentiation was initiated by dissociating the
undifferentiated iPS cells to
single cells using accutase, cell samples were taken for RNA isolation &
analysis. The cells were
resuspended at 1-2 million cells per milliliter in RPMI + 0.2% vol/vol FBS
containing 1:5000
dilution of insulin-transferrin-selenium (ITS), activin A (10Ong/mL), wnt3a
(50ng/mL), and rho-
kinase or ROCK inhibitor, Y-27632, at 10 uM, placed into an ultra-low
attachment 6-well plate,
placed on a rotation platform and rotated at about 100rpm. Cultures were
rotated at 100rpm for the
remainder of the differentiation process with daily media exchange. Growth,
passaging and
proliferation of iPSC is substantially as described in U.S. Patent Nos.
7,961,402 and 8,211,699.
[0220] The methods described herein for producing aggregate suspension
cultures of
pluripotent cells, e.g., hES or iPS cells, and cells derived from pluripotent
cells, are as substantially
described in PCT/US2007/062755, filed February 23, 2007, and titled
Compositions and methods for
culturing differential cells and PCT/US2008/080516, filed October 20, 2008,
and titled Methods and
compositions for feeder-free pluripotent stem cell media containing human
serum.
[0221] The methods described herein can be facilitated by first coating
the culturing
vessels with an extracellular matrix, e.g., as described in U.S. Patent
6,800,480 to Bodnar et al. and
assigned to Geron Corporation. The methods as with other methods for culturing
other pluripotent
stem cells, e.g., hES and iPS cells, can be cultured using soluble human serum
as substantially
described in U.S. Application, PCT/US2008/080516, filed October 20, 2008, and
titled Methods and
compositions for feeder-free pluripotent stem cell media containing human
serum.
[0222] The methods described herein can be facilitated by exogenously
added fibroblast
growth factor (FGF) supplied from a source other than just a fibroblast feeder
layer as
87
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCMJS2014/015156
described in U.S. Patent No. 7,005,252 to Thomson, J. and assigned to the
Wisconsin Alumni
Research Foundation (WARF), which is herein inspirited by reference in its
entirety.
[0223] During
about the first 24 hours of rotation, the single cells adhered to each
other formed cell aggregates, and sufficient cell samples were taken for RNA
isolation &
analysis. The cell aggregates ranged from about 60 microns to 120 microns in
diameter. About
1 day (or 24 hours) after the iPS cell samples were put on the rotation
platform, the cultures were
fed with RPMI + 0.2% vol/vol FBS containing 1:5000 dilution of ITS, activin A
(100-
200ngimL), and Wnt3a (50-10Ong/mL, or about one day (time 0 to day 1) and an
additional day
or in the same media but without the Wnt3a (day 1 to day 2). Daily cell
samples were taken for
RNA isolation and analysis. After 2 days of differentiation, the cultures were
fed RPM1 + 0.2%
vol/vol FBS containing 1:1000 dilution of ITS, KGF (or FGF7, 25ng/mL), and
TGF13 inhibitor
no.4 (2.5 uM) for one day (or 24 hours, day 2 to day 3). For the next two days
(day 3 to day 5)
the iPS cell aggregate suspensions were fed with the same growth factor
cocktail media, with the
exception that the TG93 inhibitor was removed from the culture media. Again,
cell samples
were taken for RNA isolation at the end of this stage (stage 2, or day 5). For
stage 3 (day 5 to
day 8), the cell culture media was changed to DMEM + 1% vol/vol B27 supplement
containing
TTNPB [4- [E-
2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethy1-2-naphthaleny1)-1-propenyl]benzoic
acid] (3nM), KAAD-cyclopamine (0.241M) and noggin (50ng/mL). Again, cell
samples were
taken for RNA isolation & analysis at the end this stage (stage 3, day 8). For
stage 4 (days 8 to
day14), the media was changed to DMEM + 1% vol/vol B27 supplement containing
Noggin
(50ng/mL), KGF (50ng/mL) and EGF (50ng/mL). Again, cell samples were taken for
RNA
isolation & analysis at the end of stage 4 (or day 14).
[0224] Real-
time PCR was performed to measure the gene expression for various
marker genes during the course of differentiation. Gene expression of the
specific markers or
genes was first normalized to the average expression levels of housekeeping
genes, cyclophilin G
and TATA Binding Protein (TBP) expression. Normalized relative gene expression
was then
displayed in the bar graphs relative to the expression level in the
undifferentiated iPS cells and
thus represents the fold up-regulation for the various differentiation
markers. For OCT4, gene
expression was normalized to set the lowest sample in the data set (day 14)
and thus represents
the fold down-regulation during the course of differentiation.
[0225] Figure
2A-L are bar graphs showing the relative gene expression of the
identified gene (e.g., 0ct4, Brachyury, Cerl, GSC, FOXA2, FOXA1, HNF6, PDX1,
PTF1A,
NIOC6.1, NGN3 and INS) relative to the expression level of the same gene in
the
undifferentiated iPS cells. The expression level of the genes were normalized
to a set of
88
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
housekeeping genes (control) and comparing the gene expression level at the
two different time
points indicated whether the there was up- or down-regulation for that gene or
expression
marker. For OCT4 (FIG.2A), the gene expression was normalized and the lowest
level
expression sample was set at 1 (day 14). Hence, as indicated by FIG.2A, the
relative expression
levels of OCT4 represent the fold down-regulation (Y axis) during the course
of differentiation
(X axis, stage 0 to 4, or day 010 day 14).
[0226] As shown in Figure 2A, OCT4 (POU5F1) is expressed at high
levels in the
undifferentiated iPS cells and exhibits subsequent down regulation during the
course of
differentiation (day 0 to day 14). By day 14, the OCT4 expression levels were
more than 3000-
fold decreased from the expression levels observed in undifferentiated cells.
In contrast, there
was a transient up-regulation of brachyury gene (BRACHYURY, FIG.2B) expression
during the
first 2 days (day 1 and day 2). Transient up-regulation of brachyury was a
result of the directed
differentiation of pluripotent/iPS cell into mesendoderm by the application of
activin A and
wnt3a. The mesendoderm was further differentiated into definitive endoderm
during days 2 and
3 by continued exposure to activin A was indicated by the up-regulation of
CER1, GSC and
FOXA2 by the end of stage 1 at day 3 (FIG. 2C-E). During stage 2, the
definitive endoderm was
further directed to differentiate to gut tube endoderm as indicated by the up-
regulation of
FOXA1, maintenance of FOXA2 expression and down regulation of CER1 and GSC by
day 6 of
differentiation (FIG. 2C-F). During stage 3, upon exposure to retinoid,
cyclopamine and noggin,
the gut tube endoderm was further directed to differentiate to posterior
forgut/PDXI-expressing
endoderm as indicated by the up-regulation of HNF6 and PDX1 by day 8 (FIG. 2G-
H). During
stage 4, upon exposure to KGF and EGF, the posterior forgut/PDX1-expressing
endoderm was
further directed to differentiate to pancreatic progenitors, endocrine
progenitors and hormone
expressing endocrine cells as indicated by the up-regulation of PTF1A, NKX6-1,
NGN3 and INS
by day 14 (Figure 2I-L).
EXAMPLE 2
RHO-KINASE INHIBITORS PROMOTE GROWTH, SURVIVAL, PROLIFERATION
AND CELL-CELL ADHESION OF IPS CELLS
[0227] Methods for differentiating various hES and iPS cell lines
arc substantially as
described herein and in Example 1. In addition to the culture conditions as
described for Stages
1, 2, 3, 4 and 5, apoptotic inhibitor and/ or Rho-kinase or ROCK inhibitor was
added to the
culture media to enhance and promote growth, survival, proliferation and cell-
cell adhesion
during differentiation. Typically about 10 nM of a Rbo-kinase inhibitor, for
example, Y-27632
89
Date Recue/Date Received 2023-09-08
was added to the cell cultures at each of the stages. Alternatively, a Rho-
kinase inhibitor was added
to at least Stages 1 and 2 and stages 4 and 5, or any combination thereof. The
morphology and gene
marker expression profiles of the differentiated iPS suspension (aggregates)
cell cultures are
substantially similar to that of suspension cell cultures derived from hES
cells.
[0228] Figures 3 and 4 show immunocytochemistry (ICC) of iPS cell
cultures from
Stages 4 & 5, respectively. Figure 3 shows a cell aggregate from Stage 4
expressing typical gene
markers characteristic of PDX1-positive pancreatic endoderm (also referred to
as pancreatic
epithelium or pancreatic progenitors) including PDX1 / NICX6.1 co-positive
cells. Although not
shown in FIG. 3, Stage 4 cells do not express hormone secreting proteins or
gene markers more
typical of Stage 5 cells such as insulin (INS), glucagon (GCG), somatostatin
(SST) and pancreatic
polypeptide (PP). Figure 4 shows cell aggregate of hormone expressing cells
from Stage 5. These
ICC results were further confirmed using QPCR. However, because QPCR is a
total population
study of the total level of RNA expressed in the sample or cell culture, it
cannot definitively show
that any one cell expresses multiple markers.
EXAMPLE 3
ENCAPSULATION OF IPS-DERIVED PANCREATIC PROGENITORS
[0229] To date, methods for production of IPS cells and sources for
production of IPS
cells have been reported. However, there is no sufficient description of
differentiating any iPS cell to
any functioning differentiated cell for potential use in a cell therapy to
treat a particular disease, for
example, diabetes.
[0230] To determine whether the Stage 4 PDX1-positive pancreatic
endoderm or
pancreatic progenitor cell cultures derived from human iPS cells were fully
capable of developing
and maturing in vivo to glucose sensitive insulin secreting cells, the
pancreatic progenitor populations
substantially as described in Examples 1 and 2 were loaded into macro-
encapsulating devices
substantially similar to that described in U.S. Application 12/618,659,
entitled Encapsulation of
pancreatic lineage cells derived from human pluripotent stem cells, filed
November 13, 2009; and
U.S. Patent Nos. 7,534,608 and 7,695,965 entitled METHODS OF PRODUCING
PANCREATIC
HORMONES. In brief, about 5-10-20 111, gravity settled cell suspension
aggregates were loaded into
each device, having substantially about 3 x 106 cells.
[0231] The encapsulated cells in the device were then prepared for
implantation into a
mammal, for example an immuno-compromised SCID/Bg mice, but can be implanted
in larger
Date Recue/Date Received 2023-09-08
animals including rats, pigs, monkey or human patient. Methods of implanting
the encapsulated cells
and device are substantially as that described U.S. Patent Application No.
12/618,659, U.S. Patent
Nos. 7,534,608 and 7,695,965, including pancreatic progenitor cells implanted
on a GELFOAM
matrix and implanted under the epididymal fat pad (EFP).
[0232] No immuno-suppression was necessary in these studies, however,
immtmo-
suppression may be required for certain mammals for an initial interim period
until the progenitors
inside the device fully mature and are responsive to glucose. In some mammals
immuno-suppression
regimens may be for about 1,2, 3, 4, 5, 6 or more weeks, and will depend on
the mammal.
[0233] The transplanted cells were allowed to differentiate and further
mature in vivo. To
determine whether the transplanted cells had normal physiological function as
a naturally occurring
beta cell for example, levels of human insulin will be determined by testing
levels of human C-
peptide. Human C-peptide is cleaved or processed from human pro-insulin,
hence, the detection of
human C-peptide specifically, and not endogenous mouse C-peptide, indicates
that insulin secretion
is derived from the grafted (exogenous) cells. The animals with implants will
be tested for levels of
human C-peptide about every two, three or four weeks by injecting them with a
bolus of arginine or
glucose, preferably glucose. The then mature beta cells (derived from
differentiated pluripotent iPS
cells) should be physiologically functional and responsive to glucose not
unlike naturally occurring
or endogenous beta cells. Typically amounts of human C-peptide above 50 pM or
the average basal
(thereshold) level, is an indicator of function of the now beta cells from the
transplanted progenitors.
[0234] Similar to that described in Kroon et al. (2008) supra U.S.
Application
12/618,659, U.S. Patent Nos. 7,534,608; 7,695,965 and 7,993,920, the
encapsulated pancreatic
progenitors derived from hIPS cells are expected to mature into functioning
pancreatic islet clusters
having endocrine, acinar and ductal cells not unlike that in naturally
occurring islets. It is also
anticipated that purified or enriched pancreatic progenitors derived from hIPS
cells before
transplantation will also mature and develop into functioning pancreatic
islets and produce insulin in
vivo. Certain embodiments for purifying and enriching various differentiated
cell populations is
described in more detail in U.S. Application 12/107,020, entitled Methods for
purifying endodeint
and pancreatic endoderm cells derived from hES cells, filed April 8, 2008, now
U.S. Patent
8,338,170. It is further anticipated that pancreatic progenitors derived from
hIPS cells which have
been cryopreserved can be thawed and adapted in culture before transplantation
and mature and
produce insulin in vivo accordingly. And that hypoglycemia can be ameliorated
in diabetic induced
animals having the transplanted pancreatic progenitors derived from hIPS
cells.
91
Date Recue/Date Received 2023-09-08
[0235] In summary, wholly encapsulated pancreatic progenitor cells
derived from hIPS
cells in a macro-encapsulating device mature into physiologically functional
pancreatic islets and are
expected to produce insulin in response to glucose in vivo.
EXAMPLE 4
PANCREATIC PROGENITOR AND HORMONE SECRETING CELL
COMPOSITIONS
[0236] Differentiated hIPS cell populations were analyzed using flow
cytometry for their
content of PDX1-positive pancreatic endoderm or pancreatic progenitor cells
(at stage 4); and
endocrine or endocrine precursor cells (at stage 5) as shown in Tables 5a, 5b
and 6, respectively.
Table 5b is the same data set as that in Table 5a, but presented similar to
that of Table 9 for
comparison. Table 5a populations overlap each other, e.g. the total cell
number is greater than 100%
because the total PDX1+ and NKX6.1+ numbers overlap with that of the
NICX6.1+/PDX1+/CHGA-
cell population (5th column of Table 5a). Table 5b, includes the PDX1+ only
and triple negative
(residual) data, which is not shown in Table 5a. Certain of these iPEC grafts
as well as others using
substantially similar formulations did get implanted into animals to determine
in vivo function,
however, levels of human serum C-peptide was not sufficiently robust for any
potential therapeutic
purpose (data not shown). Values shown are the percentage of total cells which
belong to a given
population. The numbers of the pancreatic progenitors (NKX6.1(+) /PDX1(+)
/ChromograninA(-))
and a very small population of NKX6.1+/PDX1-/CHGA- are in the suspension cell
aggregates were
consistent with that observed in pancreatic progenitor cell suspension
aggregates derived from hES
cells and aggregated at the ESC stage as described in U.S. Application
12/264,760, entitled STEM
CELL AGGREGATE SUSPENSIONCOMPOSITIONS AND METHODS OF DIFFERENTIATION
THEREOF, filed November 4, 2008. Levels of endocrine and / or endocrine
precursor cells were also
substantially consistent with that obtained in hES-derived cell cultures in
U.S. Application
12/107,020, entitled Methods for purifying endoderm and pancreatic endoderm
cells derived from
hES cells, filed April 8, 2008. Similar to hES-derived cell suspension
aggregates, varying the
concentrations of different growth factors in the culture medium at certain
stages of differentiation
(e.g., stage 4) should increase and/or decrease certain populations of
pancreatic endoderm, endocrine,
PDX1-positive endoderm or non-pancreatic cell types.
92
Date Recue/Date Received 2023-09-08
Table 5a: Stage 4 Pancreatic Progenitor Cell Compositions (Percent of total
cells)
Exp. # iPS PDX1+ NIM.1+ Pancreatic Endoderm Endocrine
Cell (NKX6.1(+) /PDX1(+) (ChromograninA+)
line /ChromograninA(-))
1 G4 56.4 39.2 33.3 12.7
2 B7 88.3 _ 40.9 30.4 42.3
3 B7 84.1 53.1 38.8 51.8
4 B7 94.0 43.7 32.7 49.5
Table 5b: Stage 4 Pancreatic Progenitor Cell Compositions (Percent of total
cells)
PEC
CHGA- CHGA-
NKX6.1+ CHGA- NICX6.1-
CHGA+
PDX1+ or - NKX6.1- PDX1-
Exp. # Conditions (Pancreatic PDX1+ (Triple
(Endocrine)
Progenitors, (PDX1+ negative;
>96% only) residual
PDX1+) cells)
1 Baseline 12.7 33.3 10.6 42.7
2 Baseline 42.3 30.4 18.5 7.9
3 Baseline 51.8 38.8 8.4 0.5
4 Baseline 49.5 32.7 16.3 1.2
Table 6: Stage 5 Endocrine Cell Compositions (Percent of total cells)
Exp. # iPS Cell Line Insulin + Glucagon+ Somatostatin+
B7 15.9 15.0 12.1
6 B7 17.4 15.9 10.5
EXAMPLE 5
PEC RECEPTOR TYROSINE KINASES
The above described methods are substantially similar to those described in
Table 7 below,
adapted from Schulz et al., (2012), supra. These and other methods described
herein can be found in
Applicant's many patent and non-patent publications including U.S. Patent Nos.
7,964,402;
8,211,699; 8,334,138; 8,008,07; and 8,153,429.
93
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCT/1IS2014/015156
Table 7: Standard Manufacturing Method for Making Pancreatic Endoderm Cells
(PEC)
Derived from hESC
Tiine point Roller 6-well
(day) Stage Media Condition Bottle tray
(1-4) Speed Speed
(rpm) ..................................................... (rpm)
1i-1 hEsc XF . 5- I 0 . 95
di) 1 x0.2FBS-1TS1:5000 A100 W50 5-10 95
dl r0.2FBS-ITS1 :5000 A1.00 5-10 95
d2 -72 i0 2MS-Tr5E1000 .10.5 7pfmtrawS-10 95
,
.:.:x0.2FRs4T s tl 000 i(25 54.10 95
r0.2.FB11-ITS1.:.1000 1C25 .5-10 .. 105...
d5 3 db-CIT3 N50 5-10 105
d6 db-CIT3 N50 5-10 105
d7 db-CTT3 N50 5-10 105
.48 4 43.4450 K561.50 . 540 1(.15
d9 db-N0 K50 E.50 5-10 95
= d10 db-N50 K50 E50 (or no
feed) 5-10- 95
di 1 db-tsbu.'cil K50 E50 $4 0 95
. ,
' d12 db-N5Ø0c4 E50 $40 95 ..
hESC Agg.: hESC aggregates; XF HA: Di4EM/1-12 containing GlutaMAX,
supplemented with 10% v/v of Xeno-
free KnockOut Senun Replacement, 1% v/v non-essential amino acids, 0.1 mM 2-
mercaptoethanol, 1% v/v
penicillin/streptomycin (all from Life Technologies), 10 ng/mL heregulin-113
(Peprotech) and 10 ng/mL activin A
(R&D Systems); SP: StemPre* hESC SFM (Life Technologies); r0.2FES: RPMI 1640
(Mediatech); 0.2% FES
(HyClone), lx G1utaMAX-1 (Life Technologies), 1% v/v penicillin/streptomycin;
ITS: Insulin-Transferrin-
Selenium (Life Technologies) diluted 1:5000 or 1:1000; MOO: 100 ng/mL
recombinant human Activin A (R&D
Systems); W50: 50 ng/mL recombinant mouse Wnt3A (R&D Systems); K25: 25 ng/mL
recombinant human KGF
(R&D Systems); IV: 2.5 M TGF-11 RI Kinase inhibitor IV (EMD Bioscience); db:
DMEM HI Glucose (HyClone)
supplemented with 0.5x B-27 Supplement (Life Technologies), lx GlutaMAX, and
1% v/v penicillin/streptomycin;
CTT3: 0.25 pM ICAAD-Cyclopamine (Toronto Research Chemicals) and 3 nM TTNPB
(Sigma-Aldrich); N50: 50
ng,'mL recombinant human Noggin (R&D Systems); K50: 50 ng/mL recombinant human
KGE (R&D Systems);
E50: 50 ng/mL recombinant human EGF (R&D Systems); no feed: indicates that
cells were not re-fed on the
indicated day; db, DMEM (high-(Ilucose)
When the above methods were applied to iPS cells and the pancreatic
progenitors
transplanted in animals, Applicant did not consistently obtain the same robust
in vivo function as
compared to when the same methods were applied to hESC and hES-derived
pancreatic
progenitors. This was surprising given iPS cells are human pluripotent stem
cells that have the
morphology and gene-expression pattern of hESCs and can form both embryoid
bodies in vitro
and teratomas in vivo, indicating that they can form cells from all three germ
layers. See at least
for example Yu et al. (2007); U.S. Patent Application Publication No.
2009/0047263, International
Patent Application Publication No. W02005/80598; U.S. Patent Application
Publication No.
2008/0233610; and International Patent Application Publication No.
W02008/11882, supra. These
references describe that iPS cells meet the defining criteria for ESC. Hence,
there is an
expectation that iPS cells can substitute for ESCs in an in vitro
differentiation protocol that
94
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
yields hES-derived pancreatic progenitor cells that further mature and develop
into fully
functioning glucose responsive cells in vivo. However, given the inconsistent
in vivo functioning
data using the above methods, Applicants sought to explore a differentiation
media formulation
unique to pancreatic progenitors and/or pancreatic endoderm cells (PEC) i.e.,
stage 4 derived
cells from hiPSC (or "iPEC") that are capable of providing substantially
similar robust levels of
in vivo function which has been consistently observed for PEC derived from
hESC.
Applicants previously reported that endocrine (CHGA+ cells) cells present in
PEC are
polyhormonal endocrine cells and are not the sub-population of cells in PEC
that give rise to
islets having glucose-responsive insulin-secreting cells in vivo. See Kelly
et. al.(2011) supra.
Rather it is the non-endocrine cell population (CHGA- cells), especially those
that co-express
NIOC6.1 and PDX-1, that are believed to be the PEC that actually give rise to
the functioning
islets in vivo. Thus, Applicant's explored whether modulating, changing or
shifting the relative
ratios of endocrine and non-endocrine sub-populations might affect later in
vivo function.
Previous efforts to decipher receptor-ligand signaling in hESC successfully
identified
growth factors that promoted self-renewal and enabled the development of
defined media culture
conditions. See Wang et al (2007) supra. Wang et al. identified heregulin-113
as the ligand that
bound to ERBB3 and induced dimerization with ERBB2 to affect self-renewal of
hESC in that
context. ERBB is a receptor tyrosine kinase (RTK) and RTK are widely expressed
transmembrane proteins that act as receptors for growth factors and other
extracellular signaling
molecules. Upon ligand binding, they undergo tyrosine phosphorylation at
specific residues in
the cytoplasmic tail and setting off a signaling cascade for the binding of
other protein substrates
involved in RTK-mediated signal transduction. RTK function in several
developmental
processes, including regulating cell survival, proliferation, and motility and
their role in cancer
formation is well documented. ERBB tyrosine kinase receptors were also known
to be expressed
throughout the developing fetal human pancreas although specific roles of
certain ERBB
receptors and their ligands are unknown. See Mari-Anne Huotari et al. (2002)
ERRB Signaling
Regulates Lineage Determination of Developing Pancreatic Islet Cells in
Embryonic Organ
Culture, Endocrinology 143(11): 4437-4446.
Because of the role of ERBB RTK signaling in pluripotent stem cell self-
renewal and
their expression in fetal human pancreas as demonstrated by Wang et al. (2007)
supra and ERBB
RTK expression in the human fetal pancreas, Applicants then turned to
investigate the potential
activation of RTK in in vitro pancreatic endoderm cells (PEC) derived from
hESC in an effort to
identify receptors and ligands that might improve PEC specification during
differentiation, or
expansion via promotion of self-renewal, or some other unknown mechanism which
promotes
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
maturation to physiologically functioning islet hormone secreting cells in
vivo. PEC were
generated in suspension in differentiating aggregates, substantially as
described in Table 7,
except with the following modifications.
Four PEC samples were generated for RTK blotting analysis. A "steady state"
sample of
PEC aggregates in db-N50 K50 E50 was collected at the end of stage 4 (or d13).
A "starved"
sample represented d12 PEC aggregates that were fed with db (DMEM high-glucose
or DMEM
high-glucose supplemented with 0.5x B-27 Supplement (Life Technologies)) media
alone (no
growth factors) and collected on d13. Two "pulsed" samples were fed and
cultured in db media
on d12, then on d13 fed with either db-K50 E50 media, or db media containing
2% FBS, for 15
minutes prior to harvesting. Such conditions were intended to detect RTKs that
were active in
stage 4 conditions, and what response could be elicited with a pulse of KGF,
EGF and insulin
(present in the B27 supplement), or serum. The serum pulse was intended as a
broad-spectrum,
growth factor stimuli, potentially identifying RTKs that are present on PEC
and can be activated,
but are not stimulated with the present stage 4 conditions.
RTK analysis was performed essentially as described previously in Wang et al,
(2007)
supra. Briefly, Proteome ProfilerTM human phospho-RTK antibody arrays (R&D
Systems) were
used according to the manufacturer's instructions. Protein lysates were
prepared in 1% NP-40,
20 mlvl Tris-HC1 (pH 8.0), 137 mM NaC1, 10% glycerol, 2.0 mM EDTA, 1.0 mM
sodium
orthovanadate, 10 pg/mL Aprotinin, and 10 pg/ml Leupeptin. 500 lug fresh
protein lysates were
incubated overnight with nitrocellulose membranes dotted with duplicate spots
for 42 anti-RTK
antibodies and 5 negative control antibodies, as well as 8 anti-
phosphotyrosine positive control
spots (Figure 5A). The arrayed antibodies capture the extracellular domains of
both
phosphorylated and unphosphorylated RTKs, and bound phospho-RTKs are detected
with a pan
anti-phospho-tyrosine antibody conjugated to horseradish peroxidase (HRP)
using
chemiluminescence. See figure 5 for the RTK array layout as well as Table 8
below for the
listing of RTK in the array.
Table 8: Listing of Receptor Tyrosine Kinase (RTK) for RTK Analysis of PEC
Receptor Receptor
RTK RTK
Family Family
EGF R EGFR ROR ........... ROR2
EGF R ERBB2 Tie Tie-1
EGF R ERBB 3 -------------------------- Tie ------------ Tie-2
EGF R j EREll34 I NGF R TrkA
FGF R FGF R1 NGF R TrkB
4
FGF R FGF R2A NGF R TriC
96
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
FGF R FGF R3 VEGF R VEGF R1
FGF R FGF R4 VEGF R VEGF R2
Insulin R Insulin R .. VEGF R VEGF R3
Insulin R IGF-1R I MuSK MuSK
Axl I Axl j Eph R E phA 1
Axl Dtk Eph R EphA2
Axl Met Eph R EphA3
HOF R HGF R Eph R EphA4
_
HGF R MSP R Eph R EphA6
PDGF R PDGF Ra Eph R EphA7
PDGF R PDGF Rb Eph R -- EphB 1
PDGF R SCF Ft Eph R EphB2
PDGF R Fit-3 -------------------------- Eph] EphB3
PDGF R M-CSF R Eph R EphB4
RET c-Ret Eph R EphB6
ROR ROR 1 Insulin R ALK
Analysis of the RTK blots (Figure 6A) indicated that the insulin- and IGF1-
Receptors
(IR, IGF1R, respectively) were phosphorylated and activated in all conditions,
similar to that
observed previously with hESC. See Wang et at (2007) supra. The EGF receptor
(EGFR, also
known as ERBB1) was phosphorylated in steady state conditions, which was
expected given the
presence of EGF in the stage 4 medium. Indeed, low-level phosphorylation of
ERBB2 was
detected in both the steady state and starved conditions. Phosphorylation of
both EGFR and
ERBB2 was elevated in each of the pulsed conditions, confirming the capability
of the assay to
detect activation in response to a pulse of ligand. Phosphorylated VEGFR3 was
also detected in
all conditions and was elevated in the pulsed samples. This suggested that PEC
produces an
endogenous VEGFR3 ligand, possible candidates being VEGF-C and D. The serum
pulse
appeared to activate additional receptors, including low levels of ERBB3
phosphorylation. The
detection of phosphorylated ERBB2/3 is suggestive that a heregulin-like EGF-
family member
could activate signaling in PEC. TIE-2 is one of two angiopoietin receptors
and appeared to be
phosphorylated at a low level in response to serum. Angiopoietin 1 and
Angiopoietin 4 are
known to be activating ligands of Tie-2, whereas Angiopoietin 2 and
Angiopoeitin 3 function as
context dependent competitive antagonists. The HGF-receptor (HGFR) was also
phosphorylated
in response to the serum pulse, suggesting that hepatocyte growth factor could
also elicit
signaling in PEC. Finally, while low-level phosphorylation of the ephrin B2
RTK (EPHB2) was
detected, ephrin/Eph signaling is a membrane bound cell-cell signaling system
and not likely to
be exploited easily in PEC differentiation. Interestingly, ERBB4 was not
phosphorylated. RTK
analysis therefore highlighted several receptors that are phosphorylated in
PEC, or can become
97
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
phosphorylated in response to different conditions, e.g. serum. These results
suggest that several
soluble ligands may elicit RTK signaling in PEC and potentially impact cell
proliferation,
differentiation and/or specification, and therefore, potentially affect later
maturation into
functioning pancreatic islets in vivo.
EXAMPLE 6
HEREGULIN AND FGF2 GROWTH FACTORS AFFECT PEC DERIVED FROM hESC
COMPOSITIONS
In view of the RTK analyses, which demonstrated that certain RTK were
activated (or
phosphorylated) under certain conditions as described above in Example 5, and
because it
appeared that at least ERBB2 and ERBB3 were activated in PEC (after 13 days of
differentiation
from stages 1-4), Applicant sought to determine the effect of heregulin when
applied to stage
and 4 cells.
Preliminary studies were performed using Heregulin and FGF. In certain of
these
studies, Rho-kinase inhibitor, Y-27632, was included. These preliminary
studies showed that
treatment of pluripotent stern cells for one day at stage 1 with 1 Ong/mL
Heregulin-113 (the same
concentration and heregulin isomer as disclosed in Wang et al. (2007))
increased the cell
aggregate size of the hES-derived cell aggregates in suspension culture as
compared to the
aggregate size of the hES-derived cell aggregates in suspension culture
without lieregulin-113
(Hrg113). An increase in cell aggregate size is advantageous in that it
results in higher cell mass
for later implantation and testing for function in animals. In addition,
aggregate disk size
increased when Hrg113 was increased from lOng/mL to 50ng/mL at stage 3. This
result was also
observed when 5Ong/mL of another growth factor, FGF2, was used at stage 3 as
compared to
cultures in the absence of FGF2. An increase in cell aggregate size was also
observed when the
stage 3 cultures were exposed to additional days of FGF2 exposure, e.g. 3 days
of 50ng/mL
FGF2 as compared to 2 days.
Table 9 provides a summary of the flow cytometry analysis of PEC cells treated
with
Hrg113 and FGF2 at stage 3 and/or 4. The endocrine cells are denoted as CHGA
positive (or
CHGA+) cells and the non-endocrine cells are denoted as CHGA negative (or CHGA-
) cells.
The endocrine (CHGA+) and non-endocrine cells (CHGA-) may stain positive for
other markers,
e.g., positive for PDX1 and/or NKX6.1. Cells which do not stain with any of
the tested markers
are denoted as triple negative cells or residual cells (CHGA-/NKX6.1-/PDX1).
98
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCT/US2014/015156
Table 9: Flow Cytometry Analysis of PEC Derived From hESC and Treated With
Heregulin and/or FGF2
PEC
CHGA-, CHGA-
NKX6.1+, NKX6.1-
Treatment CHGA+)
PDX1+ PDX1-
(Endocrine
(Non-
endocrine) (Triple Negative/
Residual Cells)
No Hrg &No FGF2 32.9 54.01 13.1
Stg 3 Hrg10 30.3 61.2 8.55
Stg 3Hrg50 28.9 64.2 6.9
2d Stg 3 FGF2-50 11.9 79 9.15
3d Stg 3 FGF2-50 0.33 76.9 22.7
Hg, Heregutin-fl; FGF2, Fibroblast growth factor 2; Hrg10, lOng/mL Heregulin-
10; Hrg50,
50ng/mL Heregu1in-113; 2d FG1-50, 50ng/mL of FGF2 for 2 days at stage 3; 3d
FGF2-50,
5Ong/mL of FGF2 for 3 days at stage 3
To determine whether the increase in cell aggregate size affected the PEC sub-
populations, the composition of the PEC populations was analyzed by flow
cytometry. As
compared to the control cultures, whereby no Hrg113 and FGF2 were used to
differentiate the
cells, the PEC non-endocrine sub-population (CHGA-) increased from 54.01% to
61.2% with the
addition of lOng/mL Hrg113 at stage 3, and increased from 54.01% to 64.2% with
the addition of
501ig/mL Hrg113 at stage 3. The endocrine sub-population (CHGA+) was not
significantly
affected with the treatment of lOngimL Hrglii but more so with 5Ong/mL.
Meanwhile, the
relative levels of residual cells did decrease and more so with 50ng/mL
Hrg113. So, the increase
in cell aggregate size with Hrg113 treatment was mostly attributed to the
increase in non-
endocrine sub-populations relative to the endocrine and residual sub-
populations.
The effect of FGF2 in the stage 3 cultures was similar but even more
pronounced than
that for Hrgl 13. For example, the PEC non-endocrine sub-population (CHGA-)
increased as it
did for Hrg113. The major effect of FGF2 in these cultures was the substantial
decrease in the
endocrine sub-population. In some instances, these cells were almost non-
detectable with 3
days of treatment (32.9% to 0.33%). Hence, the increase in cell aggregate size
for cultures
treated with FGF2 was mostly attributed to the increase in non-endocrine, and
in some instances,
residual cell sub-populations (13.1% to 22.7% for 3 days at stage 3).
Thus, heregulin and/or FGF2 appear to play a role in the specification of
cells in PEC
populations. This is surprising given that Wang et al (2007) supra reported
that beregulin alone
played a role in cell renewal when used in the context with pluripotent stem
cells.
99
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCTI1JS2014/015156
EXAMPLE 7
METHODS FOR IMPROVING /1V VIVO GRAFT FUNCTION OF PEC BY
TREATMENT OF IPS-DERIVED CELL CULTURES WITH HEREGULIN
Because the methods according to Table 7 when applied to iPSC to produce iPEC
did not
provide robust in vivo function in animals, Applicants explored other methods
for iPEC
production. Changes to the standard method as set forth in Table 7 include,
but are not limited
to: optimization of the number of times any iPSC is passaged; modulating
levels of BMP
signaling; modulating iPSC suspension aggregation parameters during expansion
and
differentiation (e.g. shear force, rotation speed and the like); optimization
of the concentrations,
time of use and duration of use of growth factors, such as Wnt, Activin and
rho-kinase inhibitors;
and treatment with other growth factors at various stages 1 through 4 of the
differentiation
protocol as candidates for improving cell mass, proliferation,
differentiation, survival and the
like (e.g. ERBB ligands), These many iterative experiments were tested alone,
or in combination,
to determine how differentiation methods for iPSC during stages 1-4 could be
optimized. Such
optimized differentiation methods produce iPEC populations that when grafted,
resulted in
robust glucose-responsive insulin-secreting cells in vivo similar to those
observed and reported
for hESC. Table 10 below describes the baseline conditions, with and without
heregulin, that
were demonstrated to differentiate iPSC to iPEC, Which later matured to
glucose-responsive islet
cells in vivo. The baseline conditions were similar to those described in
Examples 1, 2 and 5 as
well as Table 7 herein, except that heregulin was added at stages 3 and 4.
Although 30nWmL of
Hrgli3 was used, concentrations ranging from lOng/mL to 5Ong/mL, or even
greater than
5Ong,/mL are suitable. Also, addition of a rho-kinase inhibitor, Y-27632, was
maintained in the
differentiation cultures as described in Example 2.
Table 10: Comparison of Baseline and Heregulin Differentiation Media
Formulations for
Making Pancreatic Endoderm Cells (PEC) Derived from iPSC
!IRiPaPPM"W""!!"""91"W"Ilf""9"""MIPM tiirMgrIFf"W"'IrrIr"Wrra"""Mii!!!!
Baseline (No Heregulin) (1-4) Baseline With ileregulin
INC
ikil=20ASKOIMIOxikaimminioiimmiAggan2914.4W1OWAV1Oimmummaii
r0.2FBS-ITS1:5000 A100 V. Y10
Sl.:5000/1/2.100õ Y1Ø r0,2EB Sl :5000 A100. YIO
1111100000-4000in5 Ytelifi;Ed===:Iiiiifillita2kt3ealltiNI081111111111
db- CTT3 N50 3 db- CTT3 N50 H30
db- CTT3 N50 db- CTT3 N50 1130
100
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172
PCT/1JS2014/015156
db- CIT3 N50 db- CTT3 N50 1330
tib- N5 K50 50 YIO '4.. .
................................................. = =1=::4, =
$14 YIII dbOK5QJ5O H3ft
" .
feed:
iPSC Aggs: iPSC aggregates; KSR: knock-out serum (Life Technologies); F10: 10
ng/mL bEGF (R&D Systems);
ehm2sli;
A10: 10 ng/mL Activin A (R&D Systems); A100: 100 ng/mL Activin A; r0.2FBS:
RPMI 1640, (Meth. aTtech ,
FBS (IlyClone), ix GiutaMAX-1 (Life Technologies), 1% v/v
penicillin/streptomycin; IhTS: InsAuletm-.hraAlf(eRr.kri:D:
Selenium (Life Technologies) diluted 15000 or 1:1000; A100; 100 ng/mL
recombinant muman do mine
Systems); K25: 25 ng/mL recombinant human KGE (R&D Systems); CTT3: 0.25 g
(Toronto Research Chemicals) and 3 nM TTNPB (Sigma-Aldrich); N50: 50 ng/mL
recombinant human fa*, gin
(R&D Systems); K50: 50 ng/mL recombinant human KGE (R&D Systems); E50: 5
recombinant human
EGE (R&D Systems); Y10: 10uM Y-27632; stock 20mM, 2000X; 1130: 30 ng/mL
IIereguglif n (stock 10Oug/m1); db,
DMEM (high-Glucose)
To determine the effect of the addition of heregulin or heregulin and a rho-
kinase
inhibitor on stage 3 and 4 cell subpopulations, iPEC populations were analyzed
by flow
cytometry. . Table 11 provides a summary of the flow cytometry analysis of
various iPEC
populations using the formulations set forth in Table 10, as well as such
formulations having
been modified by increasing the Activin concentration to 200nWmL. In addition,
Table 11
shows the general conditions used for each set of experiments (baseline with
or without
heregulin) and the relative percentages of the types of cells in the iPEC
population (endocrine,
non-endocrine, PDX1 only and triple negative or residual cell sub-
populations). Table 11 also
discloses data regarding in vivo function of the cells produced in each
experiment
Table 11: iPEC Compositions from Heregulin Treated iPS-derived Cell Cultures
PEC
CHGA-
CHGA, CHGA-
NKX6,1-
Exp. CHGA+ 1.+ NICX6.1- PDX.1.-
In Vivo
No. Conditi NIKX.6.
ons PDX1+
(Endocrine) PDX1+ (Triple Function
(Non- negative/
(PDX1 only)
endocrine) residual
cells)
-hiPSC 19.83 65.59 ; 11.32 3.20
E2314 FIG. 7A
Hg30 St 3+4 9.00 64.21 16.83 9.88
....................................... = .......
BL h1PSC 56.51 36.15 5.45 1.80 Not
E2344
Hg30 St 3+4 36.13 49.00 11.23 235
transplanted.
=
E2347 BL - hIPSC 49.78 37.16 10.97 2.11 FIG. 7B&
Hg30 St 3+4 17.27 68.91 : 12.30 1.68 8A-I1
--------------------------------------- = ---------------------------
101
Date Recue/Date Received 2023-09-08
41.16 38.18 12.08 9.10
BL - hIPSC
E2380 FIG. 7A
Hg30 St 3+4
45.91 L 29.72 17.24 7.03
BL-hESC
33.39 6201. 2.89 1.75
E2354 Hg30 St3+4 FIG.7C
16.18 73.00 8.10 1.86
-hESC
BL, baseline conditions; hIPSC, human induced pluripotent stem cells; Hg30, 30
ng/mI, eregulin-1l St
3+4, Stages 3 and 4; hESC, human embryonic stem cells, CHGA, chromogranin A
Under certain conditions, the ratio of subpopulations of cells in the PEC
(hESC, E2354) and
iPEC (E2314, E2344, E2347) populations were altered. For example, sometimes,
the percentage of
endocrine (CHGA+) cells decreased and the percentage of non-endocrine cells
(CHGA-
/NKX6.1+/PDX1+) increased as compared to the baseline (no heregulin)
conditions. Although it
appeared that heregulin was responsible for changing the proportions of
endocrine cells relative to
non-endocrine cells in these PEC and iPEC populations, in experiment #2380
(E2380), the level of
endocrine (CHGA+) cells increased rather than decreased with the addition of
heregulin.
To determine whether the change in the composition of PEC and iPEC populations
affected
in vivo function, PEC and iPEC grafts from most of the experiments described
in Table 11 were
transplanted into mice substantially as previously described herein and in
Applicant's other patent
and non-patent publications, including Schulz et al. (2012) and Kroon et al
(2008), supra and U.S.
Patents Nos. 7,534,608; 7,695,965; 7,993,920 and 8,278,106, supra. Briefly,
PEC and iPEC
populations were wholly encapsulated with a biodegrabale semi-permeable cell
encapsulation device,
some of which included micro perforations. The devices were manufactured by
Applicant and are
described in detail in U.S. Patent No. 8,278,106, entitled ENCAPSULATION OF
PANCREATIC
CELLS FROM HUMAN PLURIPOTENT STEM CELLS, filed November 13, 2009. Glucose
stimulated insulin secretion (GSIS) assays were performed starting from about
56 days post-implant.
Blood was collected prior to (fasting) and at combinations of 30 and/or 60
minutes after glucose
administration. Graft function was assessed by measuring human C-peptide
concentrations in the
serum in response to glucose administration.
The amount of human C-peptide released into the serum is indicative of the
amount of insulin
released. C-peptide is a short 31 amino acid peptide connecting or linking A
and B-chains of
proinsulin and preproinsulin, which is secreted by functioning beta or insulin
secreting cells. As
discussed previously by ICroon et al. (2008) supra and others, human C-peptide
102
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
measurements are appropriate for assessing the release of de novo¨generated
insulin by the
implanted cells. Hence, levels of human C-peptide in the serum of these
animals is a measure of
the in vivo function of the mature PEC and iPEC grafts. Human C-peptide was
detected in the
serum by at least 8 weeks post-implant. With additional weeks of implant and
fasting, glucose-
stimulated C-peptide levels increased with the peak levels of C-peptide
shifting from 60 minutes
to 30 minutes post-glucose administration, which is indicative of a more rapid
response to
glucose challenge as the insulin cells mature. There were a few mice that
failed to exhibit
function, or were sacrificed due to poor health; however, these mice were in
cohorts that
otherwise exhibited high-functioning animals, thus suggesting a failure of
engraftment rather
than an inability of the implanted cells to differentiate and function.
Figures 7A-C show human C-peptide levels in the serum post glucose
administration for
all of the experiments indicated in Table 11 except E2344. Figures 7A-C show
that as compared
to baseline controls, those grafts resulting from heregulin treatment, in
general, had higher levels
of serum human C-peptide. For example, in FIG. 7A, in experiment 2380, there
is about a 5-fold
increase (933pM : 200pM at 60 minutes post glucose administration) in the
grafts resulting from
the heregulin treatment as compared to those prepared without heregulin
(baseline). Heregulin
seem to have lesser effect on PEC produced from hESC, since experiment 2354
(FIG.7C) does
not show higher levels of scrum C-peptide in those grafts resulting from
heregulin treatment as
compared to the baseline controls. Further, when comparing PEC derived from
hESC (CyT203)
and iPEC derived from iPSC, the iPEC grafts have comparable function In vivo
to the PEC grafts
(e.g. compare FIG.7A and FIG. 7B (iPSC grafts) with FIG.7C (CyT203 hESC). As
such, the
iPEC grafts arc as robust as the PEC grafts. Also, the relative ratios of
endocrine to non-
endocrine cells, which appeared to affect some of the iPEC populations (e.g.
E2314, E2347 and
E2354), did not appear to affect in vivo function because iPEC from E2380,
which did not have
the same shift in endocrine and non-endocrine subpopulations, also showed good
function (see
FIG.7A-C).
In addition to being tested for glucose-stimulated insulin secretion, the
mature iPEC
grafts were tested to determine whether they alone were able to maintain
euglycemia, similar to
euglycemia maintained by PEC derived from hESC, if the host animal's beti
cells were
destroyed. This involved destroying the beta cells of the implanted mouse
using the beta cell
toxin, streptozotocin (STZ), which exhibits greater cytotoxicity against
murine beta cells as
compared to human beta cells. Measurements of random non-fasting blood glucose
were taken
for each mouse before and after STZ-treatment. Upon explant of the iPEC graft
on day 13 post-
STZ treatment, hyperglycemia resumed (note the spike in blood glucose), which
demonstrates
103
Date Recue/Date Received 2023-09-08
the control of glycemia by the iPEC graft rather than the endogenous mouse
pancreas(see FIG. 8A
and FIG. 8B.
In addition there appeared to be a synergistic effect when heregulin and a rho-
kinase inhibitor
were provided during stages 1-4 of differentiation (see Table 10). For
example, iPSC treated with
heregulin at stages 3 and 4 without a rho-kinase inhibitor resulted in visibly
poor cell mass such that
it made implantation impossible. Further support for synergy of heregulin and
a rho-kinase inhibitor
was evident in some of the experiments, e.g. E2356, E2380, whereby baseline
conditions with a rho-
kinase inhibitor alone did not function as robustly as a graft with rho-kinase
inhibitor and heregulin
(see Figure 7A and B). It appears that treatment with heregulin and a rho-
kinase inhibitor were not
additive because addition of heregulin alone provided insufficient cell mass
for transplant and
addition of a rho-kinase inhibitor alone (baseline conditions) had poor in
vivo function. As such, the
provision of heregulin alone or a rho-kinase inhibitor alone is not
substantially similar to the sum
effect of the two combined. That is, alone neither results in robust glucose
responsiveness in vivo but
combined they produce glucose responsiveness similar to that of hES-derived
cells. Accordingly, it
appeared that the provision of both heregulin and a rho-kinase inhibitor is
synergistic since their
combined effect is greater than the sum of the effect of each separately. That
is, the rho-kinase
inhibitor and heregulin treated iPEC matured in vivo exhibiting glucose-
stimulated insulin secretion,
and were able to maintain euglycemia in a diabetes mouse model (see FIG.7A-B
and FIG.8A-B).
ERBB functionality requires ligand binding, receptor dimerization, and
receptor trafficking.
Variability in each process may produce differential regulation of the
receptors and the downstream
signals they control. For example, distinct ERBB ligands bind ERBB receptors
with different
affinities, thereby altering the patterns and dynamics of ERBB dimer
formation. Table 12 shows the
many possible different combinations of ligands and receptor binding
complexes. Reviews relating
to the complexity of this system are provided by Oda, et al. (2005). A
comprehensive pathway map
of epidermal growth factor receptor signaling, MoL Syst. Biol., 1 (2005) and
Lazzara et al. (2009)
Quantitative modeling perspectives on the ERBB system of cell regulatory
processes, Experimental
Cell Research 315(4): 717-725.
Table 12: ERRB Receptor Tyrosine ICinases and Their Ligands
ERRB Receptor Tyrosine Kinases
ErbB-1 I .............................. ErbB-2 ErbB-3 ErbB-4
t t
LIGANDS EGF x
TGFa ..
HB- x ....... L x
104
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
EGF
EPR x X
EPG .....................................
b-Cell ---------------------------------------------- X
AR
Hrgl x X
-
x X
Hrg3 ------------------------------------------------ X
Hrg4 ! )(
ERRB Receptor Tyrosine Masses: ErbB1 (also named Hen, or epidermal growth
factor receptor, EGFR); ErbB2 (also named
human epidermal growth factor receptor, or Her2; or Neu); ErbB3 (also named ,
Her3), ErbB4 (also named Her4), ERBB Uganda: EGF,
epidermal growth factor; TGFa, transforming growth factor ec; HB-EGF, heparin-
binding EGE-like growth factor; E'ER, epiregulin; EYG,
Epigen; AR, amphiregulin, Hrgl, heregulin-1 or neuregulin-1; Hrg2, heregulin-2
or neuregulin-2; Hrg3 , beregulin-3 or neuregulin-3; Hrg4,
heregulin4 or neuregulin-4; heregulir; is used interchangeably with
neuregulin.
Huotari et al. suggested that neuregulin-4 may modulate the relative levels of
the
endocrine cell subpopulations by increasing the number of somatostatin (delta)
cells at the
expense of glucagon (alpha) cells, and that ncuregulin-4 did not affect the
ratio of exocrine (e.g.,
amylase) to endocrine (e.g., B-insulin, oc-glucagon, 15-somatostatin, PP-
pancreatic polypeptide)
cells. These studies, however, were performed by incubating neuregulin-4 on
whole mount
organ tissue cultures obtained from day E12.5 mice. These mouse explant cell
populations were
differentiated further than the stage 3 (e.g. PDX1 negative foregut endoderm)
and/or stage 4
(PDX1 positive foregut endoderm) cell populations described herein. Neuregulin-
4 only binds to
ERBB4 RTK such that only the endocrine sub-population of the whole mount mouse
culture can
be modulated by neuregulin-4 in this context. Thus, treatment of the stage 3
(PDX1 negative
foregut endoderm) and/or stage 4 (PDX1 positive foregut endoderm) cells as
described herein
with a different ERBB ligand, e.g. Hrgl, would not be expected to modulate the
relative
endocrine subpopulation as in Huotari because Hrgl has already been shown to
bind to ERBB3
and induce dimerization of ERBB2/3. However, due to the low-level expression
of ERBB2 and
3 in PEC as shown in FIG.6, it was unclear whether stages 3 and 4 type cells
would express low
or high levels of ERBB2 and 3 to bind to Hrgl.
Further, in a different context, Applicant had described that Hrgl bound to
ERRB 2/3 and
promoted self-renewal of pluripotent stem cells (see Wang et al (2007).
Although it is possible
that Hrg I may act in the same capacity in the context of stage 3 and 4,
Applicant has previously
described that most of the cell expansion for production of PEC occurs at the
pluripotent stem
cell stage (stage 0). During stage 0 the hESC are grown, passaged and expanded
for about two
(2) weeks. Thus, most of the cell expansion or self-renewal to produce the
cell expansion does
not occur during stages 1-4. See Schulz et al. (2012) supra. Also, assuming
that ERRB2/3 is
105
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
present during stages 3 and 4, one might expect heregulin to have the same
effect as with
pluripotent stem cells (self renewal) as opposed to impacting directed
differentiation_ The
difference in function appears then to depend on the context, that is,
pluripotcnt stem cells versus
an endoderm or pancreatic-lineage cell type.
In summary, providing hcregulin or heregulin and a rho-kinasc inhibitor in
vitro to
foregut endoderm (stage 3) and PDX1 expressing pancreatic endoderm cells (end
of stage 3 and
stage 4) produced PEC and iPEC populations, that when transplanted, mature and
develop into
glucose responsive insulin-secreting cells in vivo (see Figures 7 and 8). Such
use of heregulin or
heregulin and a rho-kinase inhibitor has been reported here for the first
time. Such use and effect
are not discernible from that previously described in the patent or non-patent
literature.
[0237] It
will be appreciated that the Q-PCR results described herein can be further
confirmed by immunocytochemistry (ICC), and be readily performed by those of
ordinary skill
in the art.
[0238] The
methods, compositions, and devices described herein are presently
representative of preferred embodiments and are exemplary and are not intended
as limitations
on the scope of the invention. Changes therein and other uses will occur to
those skilled in the
art which are encompassed within the spirit of the invention and are defined
by the scope of the
disclosure. Accordingly, it will be apparent to one skilled in the art that
varying substitutions
and modifications may be made to the invention disclosed herein without
departing from the
scope and spirit of the invention.
[0239] As
used in the claims below and throughout this disclosure, by the phrase
"consisting essentially of" is meant including any elements listed after the
phrase, and limited to
other elements that do not interfere with or contribute to the activity or
action specified in the
disclosure for the listed elements. Thus, the phrase "consisting essentially
of' indicates that the
listed elements are required or mandatory, but that other elements are
optional and may or may
not be present depending upon whether or not they affect the activity or
action of the listed
elements. Also, it will be appreciated that in embodiments where numerical
values, such as
amounts, concentrations, percentages, proportions or ranges, are recited the
value that is referred
to can be "at least about" the numerical value, "about" the numerical value or
"at least" the
numerical value.
[0239] EMBODIMENTS
Embodiment 1. An in vitro human pancreatic endoderm cell culture.
Embodiment 2. The
cell culture of embodiment 1, wherein the pancreatic
endoderm cells are derived from pluripotent cells.
106
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 3. The cell culture of embodiment 1 or 2, wherein the
cell culture is
in contact with an ERBB tyrosine kinase receptor activating agent.
Embodiment 4. The cell culture of embodiment 3, wherein the ERBB
tyrosine
kinase receptor activating agent is EGF (epidemial growth factor), .AREG
(Amphiregulin), TGF-
Alpha (Transforming Growth Factor-Alpha), Btc (Betacellulin), HBEGF (Heparin-
Binding
EGF), Ereg (Epiregulin), Neuregulins or Heregulins.
Embodiment 5. The cell culture of any one of embodiments 1-4,
wherein the
pancreatic endoderm cells comprise pancreatic progenitor cells and
polyhormonal endocrine
cells.
Embodiment 6. The cell culture of embodiment 5, Wherein the
pancreatic
progenitor cells express NKX6.1 but do not express CHGA.
Embodiment 7. The cell culture of embodiment 5, wherein the
polyhormonal
endocrine cells express CHGA.
Embodiment 8. The cell culture of any of embodiments 1-4, wherein
the pancreatic
endoderm cells comprise CHGA-positive and CHGA-negative cells.
Embodiment 9. The cell culture of any one of embodiments 1-8,
wherein at least
30% of the pancreatic endoderm cells are CHGA -negative cells.
Embodiment 10. The cell culture of any of embodiments 1-9, wherein
at least 50%
of the pancreatic endoderm cells are PDX1-positive cells.
Embodiment 11. The cell culture of embodiment 3, wherein the ERBB
tyrosine
kinase receptor activating agent is heregulin-4.
Embodiment 12. The cell culture of any one of embodiments 1-11,
wherein the cell
culture is in contact with a fibroblast growth factor (FGF).
Embodiment 13. The cell culture of embodiment 12, wherein the FGF is
FGF-7.
Embodiment 14. The cell culture of any one of embodiments 1-13,
wherein the cell
culture is in contact with an ERBB tyrosine kinase receptor activating agent
and a FGF.
Embodiment 15. The cell culture of any one of embodiments 1-15,
wherein the cell
culture is in contact with an ERBB tyrosine kinase receptor activating agent
and a rho-kinase
inhibitor.
Embodiment 16. The cell culture of any one of embodiments 1-16,
wherein the ccll
culture is in contact with an ERBB tyrosine kinase receptor activating agent,
a FGF and a rho-
kinase inhibitor.
Embodiment 17. An in vitro human pancreatic endoderm population.
107
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 18. The cell population of embodiment 17, wherein the
pancreatic
endoderm cells are derived from pluripotent cells.
Embodiment 19. The cell population of embodiment 18, wherein the
pluripotent
cells are human embryonic stern cells or dedifferentiated genetically
reprogrammed cells.
Embodiment 20. The cell population of any one of embodiments 17-19,
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent.
Embodiment 21. The cell population of embodiment 20, wherein the
ERBB tyrosine
kinase receptor activating agent is EGF (epidermal growth factor), AREG
(Amphiregulin), TGF-
Alpha (Transforming Growth Factor-Alpha), Btc (Betacellulin), HBEGF (Heparin-
Binding
EGF), Ereg (Epiregulin), Neuregulins or Heregulins.
Embodiment 22. The cell population of any one of embodiments 17-21,
wherein the
pancreatic endoderm cells comprise pancreatic progenitor cells and
polyhormonal endocrine
cells.
Embodiment 23. The cell population of embodiment 22, wherein the
pancreatic
progenitor cells express NKX6.1 but do not express CHGA.
Embodiment 24. The cell population of embodiment 22, wherein the
polyhormonal
endocrine cells express CHGA.
Embodiment 25. The cell population of any of embodiments 17-24,
wherein the
pancreatic endoderm cells comprise CHGA-positive and CHGA-negative cells.
Embodiment 26. The cell population of any one of embodiments 17-25,
wherein at
least 30% of the pancreatic endoderm cells are CHGA-negative cells.
Embodiment 27. The cell population of any of embodiments 17-26,
wherein at least
50% of the pancreatic endoderm cells are PDX1-positive.
Embodiment 28. The cell population of embodiment 20, wherein the
ERBB tyrosine
kinase receptor activating agent is heregulin-4.
Embodiment 29. The cell population of any one of embodiments 17-28,
wherein the
cell culture is in contact with a fibroblast growth factor (FGF).
Embodiment 30. The cell population of embodiment 29, wherein the FGF
is FGF-7.
Embodiment 31. The cell population of any one of embodiments 17-30,
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent and a FGF.
Embodiment 32. The cell population of any one of embodiments 17-30,
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent and a rho-kinase
inhibitor.
108
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 33. The cell population of any one of embodiments 17-20,
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent, a FGF and a
rho-kinase inhibitor.
Embodiment 34. An in vitro cell population comprising human
pancreatic endoderm
cells in contact with an ERBB tyrosine kinase receptor activating agent.
Embodiment 35. The cell population of embodiment 34, wherein the
pancreatic
endoderm cells are derived from pluripotent cells.
Embodiment 36. The cell population of embodiment 35, wherein the
pluripotent
cells are human embryonic stem cells or dedifferentiated genetically
reprogrammed cells.
Embodiment 37. The cell population of any one of embodiments 34-36,
wherein the
ERBB tyrosine kinase receptor activating agent is EGF (epidermal growth
factor), AREG
(Amphiregulin), TGF-Alpha (Transforming Growth Factor-Alpha), Btc
(Betacellulin), HBEGF
(Heparin-Binding EGF), Ereg (Epiregulin), Neuregulins or Heregulins.
Embodiment 38. The cell population of any one of embodiments 34-37,
wherein the
pancreatic endoderm cells comprise pancreatic progenitor cells and
polyhormonal endocrine
cells.
Embodiment 39. The cell population of embodiment 38, wherein the
pancreatic
progenitor cells express N10(6.1 but do not express CHGA.
Embodiment 40. The cell population of embodiment 38, wherein the
polyhormonal
endocrine cells express CHGA.
Embodiment 41. The cell population of any of embodiments 34-40,
wherein the
pancreatic endoderm cells comprise CHGA-positive and CHGA-negative cells.
Embodiment 42. The cell population of any one of embodiments 34-41,
wherein at
least 30% of the pancreatic endoderm cells are CHGA-negative cells.
Embodiment 43. The cell population of any of embodiments 34-42,
wherein at least
50% of the pancreatic endoderm cells are PDX1-posative.
Embodiment 44. The cell population of any one of embodiments 34-36,
wherein the
ERBB tyrosine kinase receptor activating agent is heregulin-4.
Embodiment 45. The cell population of any one of embodiments 34-44,
wherein the
human pancreatic endoderm cells are in contact with a fibroblast growth factor
(FGF).
Embodiment 46. The cell population of embodiment 45, wherein the FGF
is FGF-7.
Embodiment 47. The cell population of any one of embodiments 34-46,
wherein the
human pancreatic endoderm cells are in contact with an ERBB tyrosine kinase
receptor
activating agent and a FGF.
109
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 48. The
cell population of any one of embodiments 34-47, wherein the
human pancreatic endoderm cells are in contact with an ERBB tyrosine kinase
receptor
activating agent and a rho-kinasc inhibitor.
Embodiment 49. The
cell population of any one of embodiments 34-48, wherein the
human pancreatic endoderm cells arc in contact with an ERBB tyrosine kinase
receptor
activating agent, a FGF and a rho-kinase inhibitor.
Embodiment 50. A
method for producing insulin, said method comprising the steps
of:
a. contacting foregut endoderm cells with an ERBB tyrosine kinase receptor
activating agent, thereby producing a cell population comprising pancreatic
endoderm; and
b. transplanting and maturing the pancreatic endoderm of step (a) in vivo,
thereby obtaining insulin secreting cells, wherein the insulin secreting cells
secrete insulin in
response to glucose stimulation.
Embodiment 51. A
method for producing insulin, said method comprising the steps
of:
a. contacting foregut endoderm cells derived from dedifferentiated
genetically
reprogrammed cells in vitro with art ERBB tyrosine kinase receptor activating
agent, thereby
producing a cell population comprising endocrine and non-endocrine sub-
populations; and
b. transplanting and maturing the sub-populations of step (a) in vivo,
thereby
obtaining insulin secreting cells, wherein the insulin secreting cells secrete
insulin in response to
glucose stimulation.
Embodiment 52. A
method for producing insulin, said method comprising the steps
of:
a.
transplanting and maturing pancreatic endoderm cells in vivo, thereby
obtaining insulin secreting cells, wherein the insulin secreting cells secrete
insulin in response to
glucose stimulation.
Embodiment 53. The
method of embodiment 52, wherein the pancreatic endoderm
cells are made by contacting foregut endoderm cells with an ERBB tyrosine
kinase receptor
activating agent.
Embodiment 54. The
method of any one of embodiments 50-52 and 53, wherein the
ERBB tyrosine kinasc receptor activating agent is EGF (epidermal growth
factor), AREG
(Amphiregulin), TGF-Alpha (Transforming Growth Factor-Alpha), Btc
(Betacellulin), HBEGF
(Heparin-Binding EGF), Ereg (Epiregulin), Neuregulins or Heregulins.
110
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 55. The method of any one of embodiments 50-52 and 53-54,
wherein
the foregut endoderm cells are further in contact with a fibroblast growth
factor (FGF).
Embodiment 56. The method of embodiment 55, wherein the FGF is FGF-
7.
Embodiment 57. The method of any one of embodiments 50-52 and 53-56,
wherein
the foregut endoderm cells are further in contact with a rho-kinase inhibitor.
Embodiment 58. The method of any one of embodiments 50-52 and 53-57,
wherein
the foregut endoderm cells are further in contact with rho-kinase inhibitor
and a FGF.
Embodiment 59. The method of any one of embodiments 57-58, wherein
the rho-
kinase inhibitor is selected from the group consisting of Y-27632, Fasudil, H-
1152P, Wf-536, Y-
30141, antisense nucleic acids for ROCK, RNA interference inducing nucleic
acid, competitive
peptides, antagonist peptides, inhibitory antibodies, antibody-ScFV fragments,
dominant
negative variants, derivatives and expression vectors thereof.
Embodiment 60. The method of any one of embodiments 57-59, wherein
the rho-
kinase inhibitor is selected from the group consisting of Y-27632, Fasudil, H-
I152P, Wf-536 and
Y-30141 and derivatives thereof.
Embodiment 61. The method of any one of embodiments 57-60, wherein
the Rho-
kinase inhibitor is selected from the group consisting of Y-27632, Fasudil and
H-1152P, and
derivatives thereof.
Embodiment 62. The method of embodiment 50, wherein the pancreatic
endoderm
comprises endocrine and non-endocrine cell sub-populations.
Embodiment 63. The method of embodiment 62 wherein the endocrine
cell sub-
population is a CHGA positive (CHGA+) cell.
Embodiment 64. The method of embodiment 62, wherein the non-
endocrine cell
sub-population is CHGA negative (CHGA-)
Embodiment 65. The method of embodiment 62, wherein the non-
endocrine cell
sub-population expresses NKX6.1.
Embodiment 66. The method of embodiment 50, wherein at least 30% of
the
pancreatic endoderm is CHGA-negative.
Embodiment 67. The method of embodiment 50, wherein at least 50% of
the
pancreatic endoderm is PDX1-positivc.
Embodiment 68. A method for producing insulin, said method
comprising the steps
of:
a. contacting dedifferentiated genetically reprogrammed cells
in vitro with a first
medium comprising an agent that activates a TGFI3 receptor family member;
111
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
b. culturing, in vitro, the cells of step (a) in a second medium lacking
the agent
that activates a TGFI3 receptor family member, thereby generating foregut
endoderm cells;
c. contacting the foregut endoderm cells of (b) with an ERBB tyrosine
kinase
receptor activating agent, thereby generating a cell population comprising an
endocrine and non-
endocrine cell sub-populations; and
d. transplanting and maturing the cell populations of (c) in vivo, thereby
obtaining insulin secreting cells, wherein the insulin secreting cells secrete
insulin in response to
glucose stimulation.
Embodiment 69. The method of embodiment 68, wherein the non-
endocrine cell is a
CHGA negative (CHGA-) cell.
Embodiment 70. The method of any one of embodiments 68-69, wherein
the
endocrine cell is a CHGA positive (CHGA+) cell.
Embodiment 71. The method of embodiment 69, wherein the CHGA
negative
(CHGA-) cell expresses NKX6.1.
Embodiment 72. The method of embodiment 69, wherein the foregut
endoderm
cells of (b) are contacted with a rho-kinase inhibitor.
Embodiment 73. The method of embodiment 69, wherein the foregut
endoderm
cells of (b) are contacted with a FGF.
Embodiment 74. The method of embodiment 69, wherein the foregut
endoderm
cells of (b) are contacted with a rho-kinase inhibitor and a FGF.
Embodiment 75. The method of any of embodiments 72-74, wherein the
rho-kinase
inhibitor is selected from the group consisting of Y-27632, Fasudil, H-1152P,
Wf-536, Y-30141,
antisense nucleic acids for ROCK, RNA interference inducing nucleic acid,
competitive
peptides, antagonist peptides, inhibitory antibodies, antibody-WV fragments,
dominant
negative variants, derivatives and expression vectors thereof.
Embodiment 76. A method of increasing the percentage of non-
endocrine cells in a
pancreatic endoderm cell population compared to endocrine cells comprising.
contacting foregut endoderm cells with an ERBB tyrosine kinase receptor
activating
agent, thereby increasing the percentage of non-endocrine cells in a
pancreatic endoderm cell
population
Embodiment 77. The method of embodiment 76, wherein the foregut
endoderm
cells are contacted with a FGF.
Embodiment 78. The method of embodiment 76, wherein the foregut
endoderm
cells are contacted with a rho-lcinase inhibitor.
112
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 79. The method of embodiment 76, wherein the foregut
endoderm
cells are further contacted with a FGF and a rho-kinase inhibitor.
Embodiment 80. A method for improving the glucose responsiveness of
implanted
pancreatic endoderm comprising:
contacting foregut endoderm cells with an ERBB tyrosine kinasc receptor
activating
agent, thereby generating a cell population comprising pancreatic endoderm;
and
transplanting and maturing the pancreatic endoderm in vivo, thereby obtaining
insulin
secreting cells, wherein the insulin secreting cells secrete insulin better
than insulin secreting
cells derived from pancreatic endoderm made without contacting foregut
endoderm cells with an
ERBB tyrosine kinase receptor activating agent.
Embodiment 81. The method of embodiment 80, wherein the pancreatic
endoderm
cells are derived from dedifferentiated genetically reprogrammed cells.
Embodiment 82. A method for producing insulin comprising (a)
contacting live
cells with an ERBB tyrosine kinase receptor activating agent and (b)
transplanting and maturing
the cells at the end of step a, thereby obtaining insulin secreting cells,
wherein the insulin
secreting cells secrete insulin in response to glucose stimulation.
Embodiment 83. The method of embodiment 82, wherein the live cells
are foregut
endoderm or PDX1 negative foregut endoderm.
Embodiment 84. The method of any one of embodiments 82-83, wherein
the live
cells are derived from dedifferentiated genetically reprogrammed cells.
Embodiment 85. A cell population comprising endocrine and non-
endocrine cell
sub-populations.
Embodiment 86. The cell population of embodiment 85, wherein the
endocrine and
non-endocrine cell sub-populations are derived from pluripotent cells.
Embodiment 87. The cell population of embodiment 86, wherein the
pluripotent
cells are human embryonic stem cells or dedifferentiated genetically
reprogrammed cells.
Embodiment 88. The cell population of any one of embodiments 85-87,
wherein the
ERBB tyrosine kinase receptor activating agent is EGF (epidermal growth
factor), AREG
(Amphiregulin), TGF-Alpha (Transforming Growth Factor-Alpha), Btc
(Betacellulin), HBEGF
(Heparin-Binding EGF), Ercg (Epiregulin), Ncuregulins or Hercgulins.
Embodiment 89. The cell population of any of embodiments 85-88,
wherein the
non-endocrine cells express NKX6.1 but do not express CHGA.
Embodiment 90. The cell population of any of embodiments 85-89,
wherein the
endocrine cells express CHGA.
113
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 91. The cell population of any one of embodiments 85-90,
wherein at
least 30% of the cells are CHGA-negative cells.
Embodiment 92. The cell population of any of embodiments 85-91,
wherein at least
50% of the cells are PDX I -positive.
Embodiment 93. The cell population of any one of embodiments 85-92,
wherein the
ERBB tyrosine kinase receptor activating agent is heregulin-4.
Embodiment 94. The cell population of any one of embodiments 85-93,
wherein the
cell culture is in contact with a fibroblast growth factor (FGF).
Embodiment 95. The cell population of embodiment 94, wherein the FGF
is FGF-7.
Embodiment 96. The cell population of any one of embodiments 85-95
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent and a FGF.
Embodiment 97. The cell population of any one of embodiments 85-96,
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent and a rho-kinase
inhibitor.
Embodiment 98. The cell population of any one of embodiments 85-97,
wherein the
cell culture is in contact with an ERBB tyrosine kinase receptor activating
agent, a FGF and a
rho-kinase inhibitor.
Embodiment 99. A method for producing insulin, the method comprising
the steps
of: (a) contacting dedifferentiated genetically reprogrammed cells in vitro
with a first medium
comprising an agent that activates a TGFP receptor family member; (b)
culturing, In vitro, the
cells of step (a) in a second medium lacking the agent that activates a TGFP
receptor family
member, thereby generating at least a foregut endoderm or at least a PDX1
negative foregut
endoderm cells; (c) contacting the cells of (b) with an ERBB tyrosine kinase
receptor activating
agent, thereby generating a cell population comprising endocrine and non-
endocrine cell sub-
populations; and (d) transplanting and maturing the cell populations of (c) in
vivo, thereby
obtaining insulin secreting cells, wherein the insulin secreting cells secrete
insulin in response to
glucose stimulation.
Embodiment 100. A method for producing insulin, the method comprising
the steps
of (a) contacting a PDX1-positive cell with an ERBB tyrosine kinase receptor
activating agent
and (b) transplanting and maturing the cell population of (a) in vivo, thereby
obtaining insulin
secreting cells, Wherein the insulin secreting cells secrete insulin in
response to glucose
stimulation.
Embodiment 101. The method of embodiments 68 or 99, wherein a rho-
kinase
inhibitor is added at step a, b or c.
114
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 102. The method of embodiments 68 or 99, wherein a rho-
kinase
inhibitor is added at step a, b and c.
Embodiment 103. The method of any one of embodiments 68 or 99-100,
wherein a
rho-kinase inhibitor is added at step a and b.
Embodiment 104. The method of embodiments 68 or 99, wherein a ERBB
tyrosine
kinase receptor activating agent is added at step a or c.
Embodiment 105. The method of embodiments 68 or 99, wherein a ERBB
tyrosine
kinase receptor activating agent is added at step a and c.
Embodiment 106. A method of generating a cell population capable of
maturing to
glucose-responsive insulin-secreting cells in vivo comprising: contacting a
population of at least
foregut endoderm, at least PDX1 negative foregut endoderm, or at least a
population of PDX1
positive pancreatic endoderm cells with an ERBB receptor tyrosine kinase
activating agent,
thereby generating a cell population capable of maturing to glucose-responsive
insulin-secreting
cells in vivo
Embodiment 107. A method for producing pancreatic endoderm, said
method
comprising the steps of:
a. contacting foregut endoderm cells with an ERBB tyrosine
kinase receptor
activating agent, thereby producing a cell population comprising pancreatic
endoderm.
Embodiment 108. The method of embodiment 107, further comprising
contacting the
foregut endoderm cells with a fibroblast growth factor (FGF).
Embodiment 109. The method of embodiment 108, wherein the FGF is FGF-
7.
Embodiment 110. The method of any one of embodiments 107-109, further
comprising contacting the foregut endoderm cells with a rho-kinase inhibitor.
Embodiment 111. The method of any one of embodiments 107-109, further
comprising contacting the foregut endoderm cells' with a rho-kinase inhibitor
and a FGF.
Embodiment 112. The method of any one of embodiments 107-111, wherein
at least
30% of the pancreatic endoderm in CHGA-negative.
Embodiment 113. The method of any one of embodiments 107-112, wherein
at least
50% of the pancreatic endoderm in PDX1-positive.
Embodiment 114. The method of any one of embodiments 50, 76, 107-113,
wherein
the foregut endoderm cells cell arc derived from pluripotent cells.
Embodiment 115. The method of any one of embodiments 50, 76, 107-114,
wherein
the pluripotent cells are human embryonic stem cells or dedifferentiated
genetically
reprogrammed cells.
115
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 116. The cell culture of embodiment 1, wherein the
pluripotent cells are
human embryonic stem cells or dedifferentiated genetically reprogrammed cells.
Embodiment 117. An in vitro human pancreatic endoderm cell population
comprising
differentiated cells derived from dedifferentiated genetically reprogrammed
cells and an ERBB
receptor tyrosine kinase activating agent
Embodiment 118. The pancreatic endoderm cell population of embodiment
117,
wherein the ERRB receptor tyrosine kinase activating agent comprises an EGF
growth factor or
ligand.
Embodiment 119. The pancreatic endoderm cell population of embodiment
118,
wherein the EGF growth factor or ligand comprises a heregulin isoform selected
from a group
consisting of heregulin-1, heregulin-2, and heregulin -3 and heregulin -4.
Embodiment 120. The pancreatic endoderm cell population of embodiment
119,
wherein the heregulin isoform comprises heregulin-1 and heregulin-4.
Embodiment 121. The pancreatic endoderm cell population of embodiment
119,
wherein ligand the heregulin isoform comprises heregulin-4.
Embodiment 122. A method for producing insulin, said method
comprising the steps
of:
a. contacting a forcgut endoderm cell culture derived from dedifferentiated
genetically reprogrammed cells in vitro with an ERBB receptor tyrosine kinase
activating agent,
thereby producing a cell population comprising endocrine cell and non-
endocrine cell
subpopulations; and
b. maturing the subpopulations of step (a) in vivo, thereby obtaining
insulin
secreting cells, wherein the insulin secreting cells secrete insulin in
response to glucose
stimulation.
Embodiment 123. The method of embodiment 122 further comprising
contacting the
foregut endoderm cell culture with a rho-kinase inhibitor.
Embodiment 124. The method of embodiment 123, wherein the rho-kinase
inhibitor
is selected from the group consisting of Y-27632, Fasudil, H-1 152P, Wf-536, Y-
30141, antisense
nucleic acids for ROCK, RNA interference inducing nucleic acids, competitive
peptides,
antagonist peptides, inhibitory antibodies, antibody-WV fragments, dominant
negative variants,
derivatives thereof and expression vectors thereof.
Embodiment 125. The method of embodiment 123, wherein the rho-kinase
inhibitor
is selected from the group consisting of Y-27632, Fasudil, H-1152P, Wf-536, Y-
30141 and
derivatives thereof.
116
Date Recue/Date Received 2023-09-08
CA 02898431 2015-07-15
WO 2014/124172 PCTMS2014/015156
Embodiment 126. The method of embodiment 125, wherein the Rho-kinase
inhibitor
is selected from the group consisting of Y-27632, Fasudil, H-1152P and
derivatives thereof
Embodiment 127. A method for producing insulin, said method
comprising the steps
of:
a. contacting dedifferentiated genetically reprogrammed cells in vitro with
a first
medium comprising an agent that activates a TG93 receptor family member;
b. culturing, in vitro, the cells of step (a) in a second medium lacking
the agent
that activates a TGFP receptor family member, thereby generating foregut
endoderm cells;
c. contacting the foregut endoderm cells of step (b) with an ERBB receptor
tyrosine kinase activating agent, thereby generating a cell population
comprising endocrine cell
and non-endocrine cell subpopulations; and
d. maturing the cell subpopulations of step (c) in vivo, thereby obtaining
insulin
secreting cells, wherein the insulin secreting cells secrete insulin in
response to glucose
stimulation.
Embodiment 128. The method of embodiment 127, wherein the non-
endocrine cells
are CHGA negative (CHGA-) cells.
Embodiment 129. The method of embodiment 127, wherein the endocrine
cells are
CHGA positive (CHGA+) cells.
Embodiment 130. The method of embodiment 127, wherein the CHGA
negative
(CHGA-) cells further expresses NKX6.1.
Embodiment 131. The method of embodiment 127, further comprising
contacting the
foregut endoderm cells with a rho-kinase inhibitor.
Embodiment 132. The method of embodiment 131, wherein the rho-kinase
inhibitor
is selected from the group consisting of Y-27632, Fasudil, H-1152P, Wf-536, Y-
30141, antisense
nucleic acids for ROCK, RNA interference inducing nucleic acid, competitive
peptides,
antagonist peptides, inhibitory antibodies, antibody-ScFV fragments, dominant
negative variants,
derivatives thereof and expression vectors thereof.
117
Date Recue/Date Received 2023-09-08