Note: Descriptions are shown in the official language in which they were submitted.
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
BIODEGRADABLE ELECTROCHEMICAL DEVICE WITH BARRIER LAYER
TECHNICAL FIELD
[001] The presently disclosed embodiments or implementations are directed to
biodegradable
electrochemical devices, solid aqueous electrolytes thereof, and moisture
barriers for the same.
BACKGROUND
[002] The number of batteries being produced in the world is continuously
increasing as a
consequence of the growing need for portable and remote power sources.
Particularly, a
number of new technologies require batteries to power embedded electronics.
For example,
embedded electronics, such as portable and wearable electronics, Internet of
Things (IoT)
devices, patient healthcare monitoring, structural monitoring, environmental
monitoring, smart
packaging, or the like, rely on batteries for power. While conventional
batteries may be
partially recycled, there are currently no commercially available batteries
that are
environmentally friendly or biodegradable. As such, an increase in the
manufacture and use of
conventional batteries results in a corresponding increase in toxic and
harmful waste in the
environment if not properly disposed of or recycled. In view of the foregoing,
there is a need
to develop biodegradable batteries; especially for applications that utilize
disposable batteries
for a limited time before being discarded.
[003] Further, to meet the demand for flexible, low-cost, medium or low
performance
batteries, all-printed batteries have been developed, that are commercially
available as single-
use disposable batteries. However, none of these all-printed batteries are
biodegradable.
[004] It is generally accepted that one of the greatest challenges to
producing biodegradable
batteries is the development of a biodegradable polymer electrolyte, which is
the main
polymer-based component of an all-printed battery. Moreover, the development
of such a
biodegradable polymer electrolyte that can also be printed using existing
printing technologies
is an additional challenge.
[005] Conventional biodegradable polymer electrolytes may often include a
combination of
a biodegradable polymer and a conductive salt. To obtain the biodegradable
polymer
electrolyte, the biodegradable polymer and the conductive salt are dissolved
in a solvent, and
then the solvent is subsequently evaporated at a relatively slow rate to
produce a solid polymer
electrolyte film. These conventional biodegradable polymer electrolytes often
suffer from low
ionic conductivity (e.g., less than about 10-5 S/cm at RT) at ambient
temperature due to the low
mobility of the ions in the biodegradable polymer. Sufficient conductivity,
however, may be
1
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
achieved when the polymer electrolyte is heated to a temperature sufficient
(i.e., an operational
temperature) to allow polymer chain mobility, thereby allowing the ions to
move more freely
through the polymer electrolyte structure. Sufficient conductivity may also be
achieved by
incorporating additives that suppress the crystallinity of the polymer
electrolyte, thereby
decreasing the operational temperature thereof. As such, biodegradable polymer
electrolytes
that may be operated with sufficient conductivity at room temperature is
limited.
[006] In addition to the foregoing drawbacks, conventional biodegradable
polymer
electrolytes also suffer from lengthy manufacturing processes due to the time
required to
evaporate the solvent during manufacture. For example, several hours of
evaporation aided by
vacuum and/or temperature are often required to evaporate the solvent to
prepare the
conventional biodegradable polymer electrolytes, thereby limiting the
compatibility of
conventional biodegradable polymer electrolytes with high-throughput printing
processes
where successive layers must be printed on top of each other in a matter of
minutes.
[007] While printable, biodegradable electrochemical devices, solid aqueous
electrolytes
thereof, and methods for synthesizing and fabricating the same are available,
the layers of
various materials, including current collectors, cathode/anode materials,
binders, adhesives,
and electrolyte need to be printed with high fidelity and accuracy.
Furthermore, the retention
of moisture or water content within the aqueous electrolyte is critical to
battery performance
via maintenance of solubilized salts for good ion conductivity and printed
biodegradable
batteries such as these suffer from shortened lifespan due to water losses via
evaporation
through the biodegradable substrate, such as polylactic acid (PLA) film. There
is a need to
improve the moisture retention rates of these batteries, and in particular,
the electrolyte layer,
by reducing the WVTR (water vapor transmission rate). At the same time, a
challenge remains
to achieve this sealing property to surround the electrochemical device whilst
maintaining or
reducing the non-biodegradable content of printed biodegradable batteries,
which is currently
not possible with the use of conventional relatively thick, non- biodegradable
foil 'pouches' to
seal printed batteries such as those found in Li-ion batteries.
SUMMARY
[008] The following presents a simplified summary in order to provide a basic
understanding
of some aspects of one or more embodiments of the present teachings. This
summary is not an
extensive overview, nor is it intended to identify key or critical elements of
the present
teachings, nor to delineate the scope of the disclosure. Rather, its primary
purpose is merely
2
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
to present one or more concepts in simplified form as a prelude to the
detailed description
presented later.
[009] An electrochemical device is disclosed. The electrochemical device also
includes an
anode and a cathode. The device also includes an electrolyte composition
disposed between
the anode and the cathode and a water vapor barrier which may include a
biodegradable
material, where the water vapor barrier is disposed to prevent water vapor
escaping from the
electrochemical device. The water vapor barrier further may include polylactic
acid. The water
vapor barrier further may include a metalized or aluminum metalized coating.
The water vapor
barrier further may further include multiple layers. The electrochemical
device further may
have a water vapor transmission rate (WVTR) less than or equal to 2 wt % over
24 hours.
[0010] Embodiments of the water vapor barrier may also include a biodegradable
material,
which may include a polymer. The water vapor barrier may also include a
metalized coating
disposed onto the biodegradable material. The water vapor barrier may include
a polymer
which may further include polylactic acid, a metalized or aluminum metalized
coating. The
water vapor barrier may also include multiple layers and may have a water
vapor transmission
rate (WVTR) less than or equal to 1 mg per cm2 over 24 hours.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] The accompanying drawings, which are incorporated in and constitute a
part of this
specification, illustrate embodiments of the present teachings. These and/or
other aspects and
advantages in the embodiments of the disclosure will become apparent and more
readily
appreciated from the following description of the various embodiments, taken
in conjunction
with the accompanying drawings of which:
[0012] Figure 1 illustrates an exploded view of an exemplary biodegradable
electrochemical
device in a side-by-side configuration, according to one or more embodiments
disclosed.
[0013] Figure 2 illustrates an exploded view of another exemplary
biodegradable
electrochemical device in a stacked configuration, according to one or more
embodiments
disclosed.
[0014] Figure 3 illustrates a cross-sectional view of a comparative example of
a fully
assembled surrogate for a biodegradable battery assembly having a water vapor
barrier,
according to one or more embodiments.
[0015] Figure 4 illustrates a cross-sectional view of the embodiment of a
fully assembled
surrogate for an electrochemical device assembly of Example 1, having a water
vapor barrier,
according to one or more embodiments.
3
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0016] Figure 5 illustrates a cross-sectional view of the embodiment of a
fully assembled
surrogate for an electrochemical device assembly of Example 2, having a water
vapor barrier,
according to one or more embodiments.
[0017] Figure 6 illustrates a cross-sectional view of the embodiment of a
fully assembled
surrogate for an electrochemical device assembly of Example 3, having a water
vapor barrier,
according to one or more embodiments.
[0018] Figure 7 illustrates a cross-sectional view of an example of a
surrogate for an
electrochemical device assembly having a multilaminate enclosure structure, in
accordance
with the present disclosure.
[0019] Figure 8 illustrates a plot of water loss in milligrams per square
centimeter vs. time in
days for the comparative example of Figure 3 compared to examples 1 ¨ 5 of
Figures 4, 5, 6,
and 7, respectively.
[0020] It should be noted that some details of the figures have been
simplified and are drawn
to facilitate understanding of the present teachings rather than to maintain
strict structural
accuracy, detail, and scale.
DETAILED DESCRIPTION
[0021] The following description of various typical aspect(s) is merely
exemplary in nature
and is in no way intended to limit the disclosure, its application, or uses.
[0022] As used throughout, ranges are used as shorthand for describing each
and every value
that is within the range. Any value within the range may be selected as the
terminus of the
range. In addition, all references cited herein are hereby incorporated by
reference in their
entireties. In the event of a conflict in a definition in the present
disclosure and that of a cited
reference, the present disclosure controls.
[0023] Unless otherwise specified, all percentages and amounts expressed
herein and
elsewhere in the specification should be understood to refer to percentages by
weight. The
amounts given are based on the active weight of the material.
[0024] Additionally, all numerical values are "about" or "approximately" the
indicated value,
and take into account experimental error and variations that would be expected
by a person
having ordinary skill in the art. It should be appreciated that all numerical
values and ranges
disclosed herein are approximate values and ranges, whether "about" is used in
conjunction
therewith. It should also be appreciated that the term "about," as used
herein, in conjunction
with a numeral refers to a value that may be 0.01% (inclusive), 0.1%
(inclusive), 0.5%
(inclusive), 1% (inclusive) of that numeral, 2% (inclusive) of that
numeral, 3% (inclusive)
4
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
of that numeral, 5% (inclusive) of that numeral, 10% (inclusive) of that
numeral, or 15%
(inclusive) of that numeral. It should further be appreciated that when a
numerical range is
disclosed herein, any numerical value falling within the range is also
specifically disclosed.
[0025] As used herein, the term "or" is an inclusive operator, and is
equivalent to the term
"and/or," unless the context clearly dictates otherwise. The term "based on"
is not exclusive
and allows for being based on additional factors not described, unless the
context clearly
dictates otherwise. In the specification, the recitation of "at least one of
A, B, and C," includes
embodiments containing A, B, or C, multiple examples of A, B, or C, or
combinations of A/B,
A/C, B/C, A/B/B/ B/B/C, A/B/C, etc. In addition, throughout the specification,
the meaning
of "a," "an," and "the" include plural references. The meaning of "in"
includes "in" and "on."
[0026] Reference will now be made in detail to exemplary embodiments of the
present
teachings, examples of which are illustrated in the accompanying drawings.
Wherever possible,
the same reference numbers will be used throughout the drawings to refer to
the same, similar,
or like parts.
[0027] A biodegradable electrochemical device is disclosed herein. As used
herein, the term
"biodegradable" or "biodegradable material" may refer to a material,
component, substance,
device, or the like, capable of or configured to be decomposed by living
organisms, particularly
microorganisms in a landfill within a reasonable amount of time. The material,
component,
substance, device, or the like may be decomposed into water, naturally
occurring gases like
carbon dioxide and methane, biomass, or combinations thereof. As used herein,
the expression
"biodegradable electrochemical device" or "biodegradable device" may refer to
an
electrochemical device or a device, respectively, where at least one or more
components thereof
is biodegradable. In some instances, a majority or substantial number of the
components of
the biodegradable electrochemical device or the biodegradable device are
biodegradable. In
other instances, all of the polymer components of the biodegradable
electrochemical device or
the biodegradable device are biodegradable. For example, the polymers and/or
other organic-
based components of the electrochemical device are biodegradable while the
inorganic
materials of the electrochemical device disclosed herein, including the metals
and/or metal
oxides, may not be biodegradable. It should be appreciated that if all polymer
and/or organic-
based components of an electrochemical device are biodegradable, it is
generally accepted that
the complete electrochemical device is considered biodegradable. As used
herein, the term
"compostable" may refer to items that are able to be made into compost or
otherwise disposed
of in a sustainable or environmentally friendly manner. Compostable materials
may be
considered to be a subset category of biodegradable materials wherein
additional specific
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
environmental temperatures or conditions may be needed to break down a
compostable
material. While the term compostable is not synonymous with biodegradable,
they may be used
interchangeably in some instances, wherein the conditions necessary to break
down or
decompose a biodegradable material are understood to be similar to the
conditions necessary
to break down a compostable material. As used herein, the term or expression
"electrochemical
device" may refer to a device that converts electricity into chemical
reactions and/or vice-versa.
Illustrative electrochemical devices may be or include, but are not limited
to, batteries, die-
sensitized solar cells, electrochemical sensors, electrochromic glasses, fuel
cells, electrolysers,
or the like.
[0028] As used herein, the term or expression "environmentally friendly
electrochemical
device" or "environmentally friendly device" may refer to an electrochemical
device or device,
respectively, that exhibits minimal, reduced, or no toxicity to the ecosystems
or the
environment in general. In at least one embodiment, the electrochemical
devices and/or
components thereof disclosed herein are environmentally friendly.
[0029] As used herein, the term or expression "film" or "barrier layer" may
refer to a thin,
partially or substantially plastic and/or polymeric material that may be used
in various
electrochemical device components or parts, including, but not limited to
substrates,
connections, enclosures, barriers, or combinations thereof. Films as described
herein may be
rigid or flexible, depending upon the inherent physical properties or
dimensions of their
respective compositions. In at least one embodiment, these films or barrier
layers may be
environmentally friendly or biodegradable
[0030] As used herein, the term or expression "enclosure," "barrier," or
"water vapor barrier"
may refer to materials utilized in partially sealed, fully sealed or otherwise
used to prevent
moisture, water or other evaporable materials from entering or exiting via the
barrier of an
electrochemical device. In at least one embodiment, these enclosures may be
environmentally
friendly or biodegradable.
[0031] As used herein, the term or expression "metal layer" may refer to a
layer of metal on a
surface, film, substrate, or barrier layer. A metal layer, as described herein
may include
examples of metalized coatings, which may be deposited onto a surface by means
of a vapor
or chemical deposition process, as well as metal layers including metal foils,
metal films, or
other metal layers combined with or adhered to a surface, film, substrate, or
barrier layer.
[0032] In at least one embodiment, the biodegradable electrochemical device
disclosed herein
may include an anode, a cathode (i.e., a current collector and/or an active
layer), and one or
more electrolyte compositions (e.g., a biodegradable solid aqueous electrolyte
composition).
6
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
In another embodiment, the biodegradable electrochemical device may further
include one or
more substrates, one or more seals, one or more packages, one or more pouches,
one or more
enclosures, or combinations thereof.
[0033] The biodegradable electrochemical devices disclosed herein may be
flexible. As used
herein, the term "flexible" may refer to a material, device, or components
thereof that is capable
of being bent around a predetermined radius of curvature without breaking
and/or cracking.
The biodegradable electrochemical devices and/or the components thereof
disclosed herein
may be bent around a radius of curvature of about 30 cm or less, about 20 cm
or less, about 10
cm or less, about 5 cm or less without breaking or cracking.
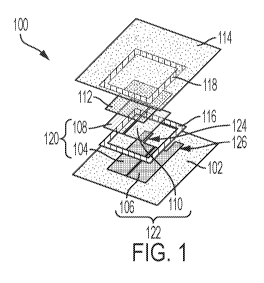
[0034] Figure 1 illustrates an exploded view of an exemplary biodegradable
electrochemical
device 100 in a side-by-side or coplanar configuration, according to one or
more embodiments.
As illustrated in Figure 1, the biodegradable electrochemical device 100 may
include a first
substrate 102, first and second current collectors 104, 106 disposed adjacent
to or on top of the
first substrate 102, an anode active layer 108 disposed adjacent to or on top
of the first current
collector 104, a cathode active layer 110 disposed adjacent to or on top of
the second current
collector 106, an electrolyte layer 112 disposed adjacent to or on top of the
anode active layer
108 and the cathode active layer 110, and a second substrate 114 disposed
adjacent to or on top
of the electrolyte composition 112. It should be appreciated that the first
current collector 104
and the anode active layer 108 may be collectively referred to herein as an
anode 120 of the
biodegradable electrochemical device 100. It should further be appreciated
that the second
current collector 106 and the cathode active layer 110 may be collectively
referred to herein as
a cathode 122 of the biodegradable electrochemical device 100. As illustrated
in Figure 1, the
anode 120 and the cathode 122 of the biodegradable electrochemical device 100
may be
coplanar such that the anode 120 and the cathode 122 are arranged along the
same X-Y plane.
[0035] In at least one embodiment, the biodegradable electrochemical device
100 may include
one or more seals (two are shown 116, 118) capable of or configured to seal or
hermetically
seal the current collectors 104, 106, the anode active layer 108, the cathode
active layer 110,
and the electrolyte composition 112 between the first and second substrates
102, 114 of the
biodegradable electrochemical device 100. For example, as illustrated in
Figure 1, the
biodegradable electrical device 100 may include two seals 116, 118 interposed
between the
first and second substrates 102, 114 and about the current collectors 104,
106, the anode active
layer 108, the cathode active layer 110, and the electrolyte composition 112
to seal or
hermetically seal the biodegradable electrochemical device 100. In another
embodiment, the
biodegradable electrochemical device 100 may be free or substantially free of
seals 116, 118.
7
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
For example, the substrates 102, 114 may be melted or bonded with one another
to seal the
biodegradable electrochemical device 100.
[0036] Figure 2 illustrates an exploded view of another exemplary
biodegradable
electrochemical device 200 in a stacked configuration, according to one or
more embodiments.
As illustrated in Figure 2, the biodegradable electrochemical device 200 may
include a first
substrate 202, a first current collectors 204 disposed adjacent to or on top
of the first substrate
102, an anode active layer 208 disposed adjacent to or on top of the first
current collector 204,
an electrolyte layer 212 disposed adjacent to or on top of the anode 108, a
cathode active layer
210 disposed adjacent to or on top of the electrolyte composition 212, a
second current collector
206 disposed adjacent to or on top of the cathode active layer 210, and a
second substrate 214
disposed adjacent to or on top of the second current collector 206. It should
be appreciated that
the first current collector 204 and the anode active layer 208 may be
collectively referred to
herein as an anode 220 of the biodegradable electrochemical device 200. It
should further be
appreciated that the second current collector 206 and the cathode active layer
210 may be
collectively referred to herein as a cathode 222 of the biodegradable
electrochemical device
200. As illustrated in Figure 2, the anode 220 and the cathode 222 of the
biodegradable
electrochemical device 200 may be arranged in a stacked configuration or
geometry such that
the anode 220 and the cathode 222 are disposed on top of or below one another.
[0037] In at least one embodiment, the biodegradable electrochemical device
200 may include
one or more seals (two are shown 216, 218) capable of or configured to
hermetically seal the
current collectors 204, 206, the anode active layer 208, the cathode active
layer 210, and the
electrolyte composition 212 between the first and second substrates 202, 214
of the
biodegradable electrochemical device 200. For example, as illustrated in
Figure 2, the
biodegradable electrical device 200 may include two seals 216, 218 interposed
between the
first and second substrates 202, 214 and about the current collectors 204,
206, the anode active
layer 208, the cathode active layer 210, and the electrolyte composition 212
to hermetically
seal the biodegradable electrochemical device 200. In another embodiment, the
biodegradable
electrochemical device 200 may be free or substantially free of seals 216,
218. For example,
the substrates 202, 214 may be melted or bonded with one another to seal the
biodegradable
electrochemical device 200.
[0038] As illustrated in Figures 1 and 2, each of the current collectors 104,
106, 204, 206 may
include a respective tab 124, 126, 224, 226 that may extend outside the seals
116, 118, 216,
218 to thereby provide connectivity.
8
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0039] In at least one embodiment, any one or more of the substrates 102, 114,
202, 214 of the
respective biodegradable electrochemical devices 100, 200 may be or include,
but is not limited
to, a biodegradable substrate. Illustrative biodegradable substrates may be or
include, but are
not limited to, one or more of polylactic acid (PLA), polylactic-co-glycolic
acid (PLGA), silk-
fibroin, chitosan, polycaprolactone (PCL), polyhydroxybutyrate (PHB), rice
paper, cellulose,
or combinations or composites thereof.
[0040] The biodegradable substrates of the respective biodegradable
electrochemical devices
100, 200 may be stable at temperatures of from about 50 C to about 150 C. As
used herein,
the term "stable" or "stability" may refer to the ability of the substrate to
resist dimensional
changes and maintain structural integrity when exposed to temperature of from
about 50 C to
about 150 C. For example, the biodegradable substrates may be capable of or
configured to
maintain structural integrity with dimensional changes of less than about 20%,
less than about
15%, or less than about 10% after exposure to temperatures of from about 50 C
to about 150 C.
In one example, each of the biodegradable substrates may be stable (e.g.,
dimensional changes
less than 20%) at a temperature of from about 50 C, about 60 C, about 70 C,
about 80 C,
about 90 C, about 100 C, or about 110 C to about 120 C, about 130 C, about 140
C, or about
150 C. In another example, each of the biodegradable substrates may be stable
at a temperature
of at least 100 C, at least 105 C, at least 110 C, at least 115 C, at least
120 C, at least 125 C,
at least 130 C, at least 135 C, at least 140 C, or at least 145 C. In at least
one embodiment,
the biodegradable substrates may be stable at temperatures of from about 50 C
to about 150 C
for a period of from about 5 mm to about 60 mm or greater. For example, the
biodegradable
substates may be stable at the aforementioned temperatures for a period of
time of from about
mm, about 10 mm, about 20 min, or about 30 mm to about 40 mm, about 45 min,
about 50
min, about 60 mm, or greater.
[0041] In at least one embodiment, the biodegradable substrate is weldable,
bondable, and/or
permanently thermo-sealable without the use of an additional adhesive. For
example, the
biodegradable substrates of each of the substrates 102, 114, 202, 214 may be
weldable and/or
bondable with one another without the use of the respective seals 116, 118,
216, 218.
Illustrative biodegradable substrates that may be weldable and/or bondable
with one another
may be or include, but are not limited to, thermoplastics, such as polylactic
acid (PLA),
polylactides modified with a nucleating agent to enhance crystallinity, such
as polylactide
modified with nucleating agent D (PLA-D) and polylactide modified with
nucleating agent E
(PLA-E), polybutylene succinate (PBS), polybutylene adipate terephthalate
(PBAT), blends of
9
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
PLA and polyhydroxybutyrate (PHB), PHB-based blends, or the like, or
combinations thereof.
As used herein, the term or expression "bondable," "weldable," and/or
"permanently thermo-
sealable" may refer to an ability of a material (e.g., substrate) to heat seal
two surfaces with
one another or permanently join two surfaces with one another via heating or
melting.
[0042] The anode active layer 108, 208 of the respective biodegradable
electrochemical
devices 100, 200 may be or include, but is not limited to, one or more of zinc
(Zn), lithium
(Li), carbon (C), cadmium (Cd), nickel (Ni), magnesium (Mg), magnesium alloys,
zinc alloys,
or the like, or combinations and/or alloys thereof. Illustrative anode active
layers or materials
thereof may be or include, but are not limited, or the like, or combinations
thereof. In at least
one embodiment, the anode active layer may include zinc oxide (ZnO) in a
sufficient amount
to regulate or control H2 gassing.
[0043] In at least one embodiment, the anode active layer 108, 208 of the
respective
biodegradable electrochemical devices 100, 200 may be prepared or fabricated
from an anode
paste. For example, the anode active layer may be prepared from a zinc anode
paste. The
anode paste may be prepared in an attritor mill. In at least one embodiment,
stainless steel shot
may be disposed in the attritor mill to facilitate the preparation of the
anode paste. The anode
paste may include one or more metal or metal alloys, one or more organic
solvents, one or more
styrene-butadiene rubber binders, or combinations thereof. In an exemplary
embodiment, the
anode paste may include one or more of ethylene glycol, a styrene-butadiene
rubber binder,
zinc oxide (Zn0), bismuth (III) oxide (Bi203), Zn dust, or combinations
thereof. Illustrative
organic solvents are known in the art and may be or include, but are not
limited to, ethylene
glycol, acetone, NMP, or the like, or combinations thereof. In at least one
embodiment, any
one or more biodegradable binders may be utilized in lieu of or in combination
with a styrene-
butadiene rubber binder.
[0044] The cathode active layer 110, 210 of the respective biodegradable
electrochemical
devices 100, 200 may be or include, but are not limited to, one or more of
iron (Fe), iron (VI)
oxide, mercury oxide (Hg0), manganese (IV) oxide (Mn02), carbon (C), carbon-
containing
cathodes, gold (Au), molybdenum (Mo), tungsten (W), molybdenum trioxide
(Mo03), silver
oxide (Ag2O), copper (Cu), vanadium oxide (V205), nickel oxide (NiO), copper
iodide (Cu2I2),
copper chloride (CuC1), or the like, or combinations and/or alloys thereof. In
an exemplary
embodiment, the cathode active layer 110, 210 may include manganese (IV)
oxide. The carbon
and/or carbon-containing cathode active layers may be utilized in aqueous
metal-air batteries,
such as zinc air batteries.
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0045] In at least one embodiment, the cathode active layer 110, 210 may
include one or more
additives capable of or configured to at least partially enhance the
electronic conductivity of
the cathode active layer 110, 210. Illustrative additives may be or include,
but are not limited
to, carbon particles, such as graphite, carbon nanotubes, carbon black, or the
like, or the like,
or combinations thereof.
[0046] In at least one embodiment, the cathode active layer 110, 210 of the
respective
biodegradable electrochemical devices 100, 200 may be prepared or fabricated
from a cathode
paste. For example, the cathode active layer 110, 210 may be prepared from a
manganese (IV)
oxide cathode paste. The cathode paste may be prepared in an attritor mill. In
at least one
embodiment, stainless steel shot may be disposed in the attritor mill to
facilitate the preparation
of the cathode paste. The cathode paste may include one or more metal or metal
alloys, one or
more organic solvents (e.g., ethylene glycol), one or more styrene-butadiene
rubber binders, or
combinations thereof. In an exemplary embodiment, the cathode paste may
include one or
more of ethylene glycol, a styrene-butadiene rubber binder, manganese (IV)
oxide (Mn02),
graphite, or combinations thereof. Illustrative organic solvents are known in
the art and may
be or include, but are not limited to, ethylene glycol, acetone, NMP, or the
like, or combinations
thereof. In at least one embodiment, the one or more organic solvents may be
replaced or used
in combination with an aqueous solvent, such as water. For example, water may
be utilized in
combination with manganese (IV) oxide.
[0047] The anode and/or cathode paste may have a viscosity of from about 100
cP to about
1E6 cP. For example, the anode and/or cathode paste may have a viscosity of
from greater
than or equal to about 100 cP, greater than or equal to about 200 cP, greater
than or equal to
about 500 cP, greater than or equal to about 1,000 cP, greater than or equal
to about 1,500 cP,
greater than or equal to about 2,000 cP, greater than or equal to about 10,000
cP, greater than
or equal to about 20,000 cP, greater than or equal to about 50,000 cP, greater
than or equal to
about 1E5 cP, greater than or equal to about 1.5E5 cP, greater than or equal
to about 2E5 cP,
greater than or equal to about 3E5 cP, greater than or equal to about 4E5 cP,
greater than or
equal to about 5E5 cP, greater than or equal to about 6E5 cP, greater than or
equal to about 7E5
cP, greater than or equal to about 8E5 cP, or greater than or equal to about
9E5 cP. In another
example, the anode and/or cathode paste may have a viscosity of less than or
equal to about
200 cP, less than or equal to about 500 cP, less than or equal to about 1,000
cP, less than or
equal to about 1,500 cP, less than or equal to about 2,000 cP, less than or
equal to about 10,000
cP, less than or equal to about 20,000 cP, less than or equal to about 50,000
cP, less than or
equal to about 1E5 cP, less than or equal to about 1.5E5 cP, less than or
equal to about 2E5 cP,
11
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
less than or equal to about 3E5 cP, less than or equal to about 4E5 cP, less
than or equal to
about 5E5 cP, less than or equal to about 6E5 cP, less than or equal to about
7E5 cP, less than
or equal to about 8E5 cP, less than or equal to about 9E5 cP, or less than or
equal to about 1E6
cP.
[0048] In at least one embodiment, each of the anodes 120, 220 and the
cathodes 122, 222, or
the active layers 108, 110, 208, 210 thereof may independently include a
biodegradable binder.
The function of the biodegradable binder is to anchor the particles of each of
the respective
layers together and provide adhesion to the substrate underneath, the
respective layers being
the anode current collector 104, 204, the cathode current collector 106, 206,
the anode active
layer 108, 208, the cathode active layer 110, 210, or combinations thereof.
Illustrative
biodegradable binders may be or include, but are not limited to, one or more
of chitosan,
polylactic-co-glycolic acid (PLGA), gelatin, xanthan gum, cellulose acetate
butyrate (CAB),
polyhydroxybutyrate (PHB), or a combinations thereof. In at least one
embodiment, any one
or more of the biodegradable polymers disclosed herein with regard to the
electrolyte
composition may also be utilized as the biodegradable binder of the anode 120,
220, the cathode
122, 222, components thereof, or any combination thereof. As further described
herein, the
one or more biodegradable polymers may be cross-linked. As such, the
biodegradable binders
utilized for the anode 120, 220, the cathode 122, 222, and/or the components
thereof, may
include the cross-linked biodegradable binders disclosed herein with regard to
the electrolyte
composition.
[0049] The electrolyte layer 112, 212 of each of the respective biodegradable
electrochemical
devices 100, 200 may be or include an electrolyte composition. The electrolyte
composition
may utilize biodegradable polymeric materials. The electrolyte composition may
be a solid,
aqueous electrolyte composition. The solid, aqueous electrolyte composition
may be or include
a hydrogel of a copolymer and a salt dispersed in and/or throughout the
hydrogel. The
copolymer may include at least two polycaprolactone (PCL) chains attached with
a polymeric
center block (CB). For example, the copolymer may be a block copolymer or a
graft copolymer
including at least two PCL chains coupled with the polymeric center block,
such as PCL-CB-
PCL. In another example, the copolymer may be a block copolymer or a graft
copolymer
including at least one or more of polylactic acid (PLA), polyglycolic acid
(PGA), polyethylene
imine (PEI) or combinations thereof, coupled with the polymeric center block.
[0050] The copolymer or the solids may be present in the hydrogel in an amount
of from about
weight % or greater to 90 weight % or less, based on a total weight of the
hydrogel (e.g., total
weight of solvent, polymer, and salt). For example, the copolymer may be
present in an amount
12
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
of from about 5 weight % or greater, 10 weight % or greater, 15 weight % or
greater, 20 weight
% or greater, 25 weight % or greater, 30 weight % or greater, 35 weight % or
greater, based on
a total weight of the hydrogel. In another example, the copolymer may be
present in an amount
of from 90 weight % or less, 80 weight % or less, 70 weight % or less, or 60
weight % or less,
based on a total weight of the hydrogel. In a preferred embodiment, the
copolymer or the solids
may be present in the hydrogel in an amount of from about 5 weight % to about
60 weight %,
about 5 weight % to about 50 weight %, about 20 weight % to about 40 weight %,
or about 30
weight %, based on a total weight of the hydrogel. In yet another preferred
embodiment, the
copolymer or the solids may be present in the hydrogel in an amount of from
greater than 30
weight % to 60 weight %, based on a total weight of the hydrogel.
[0051] The copolymer may be present in the hydrogel in an amount sufficient to
provide a
continuous film or layer that is free or substantially free of bubbles. The
copolymer may also
be present in the hydrogel in an amount sufficient to provide a viscosity of
from about 1,000
cP to about 100,000 cP. For example, the copolymer may be present in the
hydrogel in an
amount sufficient to provide a viscosity of from about 1,000 cP, about 5,000
cP, about 10,000
cP, or about 20,000 cP to about 30,000 cP, about 40,000 cP, about 50,000 cP,
about 75,000 cP,
about 90,000 cP, or about 100,000 cP.
[0052] The polymeric center block of the copolymer may be a biodegradable
polymer, thereby
improving or increasing biodegradability of the solid, aqueous electrolyte
composition. The
biodegradable polymer of the polymeric center block is preferably naturally
occurring. The
polymeric center block may be or include, or be derived from, a polymer, such
as a
biodegradable polymer, including at least two free hydroxyl groups available
for reaction with
E-caprolactone. As further described herein, the polymer including the at
least two free
hydroxyl groups may be reacted with E-caprolactone to form the copolymer.
Illustrative
polymers including at least two free hydroxyl groups that may be utilized to
form the polymeric
center block (CB) may be or include, but are not limited to, one or more of
polyvinyl alcohol
(PVA), a hydroxyl-bearing polysaccharide, a biodegradable polyester, a hydroxy
fatty acid
(e.g., castor oil), or the like, or combinations thereof. Illustrative
hydroxyl-bearing
polysaccharides may be or include, but are not limited to, starch, cellulose,
carboxymethyl
cellulose, methyl cellulose, hydroxyethyl cellulose, chitin, guar gum, xanthan
gum, agar-agar,
pullulan, amylose, alginic acid, dextran, or the like, or combinations
thereof. Illustrative
biodegradable polyesters may be or include, but are not limited to,
polylactide, polyglycolic
acid, polylactide-co-glycolic acid, polyitaconic acid, polybutylene succinate,
or the like, or
combinations thereof. In a preferred embodiment, the polymer center block may
be or include
13
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
one or more of polyvinyl alcohol (PVA), a hydroxyl-bearing polysaccharide, a
biodegradable
polyester, or a hydroxy fatty acid.
[0053] In at least one embodiment, the polymeric center block of the copolymer
may not be a
biodegradable polymer. For example, the polymeric center block of the
copolymer may be or
include, but is not limited to, polyethylene glycol (PEG), hydroxy-terminated
polyesters,
hydroxyl-terminated polyolefins, such as hydroxy-terminated polybutadiene, or
the like, or
combinations thereof.
[0054] The copolymer, including at least two polycaprolactone (PCL) chains
bonded to the
polymeric center block, may be a graft copolymer or a block copolymer. Whether
the
copolymer is a graft copolymer or a block copolymer may be at least partially
determined by
the number and/or placement of the at least two free hydroxyl groups of the
polymeric center
block. For example, reacting E-caprolactone with polymeric center blocks
having the hydroxyl
groups on monomers along a length of the polymeric center block chain forms
graft
copolymers. In another example, reacting E-caprolactone with polymeric center
blocks having
each of the hydroxyl groups at respective ends of the polymeric center blocks
forms block
copolymers. Illustrative block copolymers may be or include triblock
copolymers, tetrablock
copolymers, star block copolymers, or combinations thereof.
[0055] As discussed above, the electrolyte composition may be a solid, aqueous
electrolyte
composition including the hydrogel of the copolymer and the salt dispersed in
the hydrogel.
The salt of the hydrogel may be or include any suitable ionic salt known in
the art. Illustrative
ionic salts may be or include, but are not limited to, one or more of organic-
based salts,
inorganic-based salts, room temperature ionic liquids, deep eutectic solvent-
based salts, or the
like, or combinations or mixtures thereof. In a preferred embodiment, the
salts are or include
salts useable in zinc/manganese (IV) oxide (Zn/Mn02) electrochemistry.
Illustrative salts may
be or include, but are not limited to, zinc chloride (ZnC12), ammonium
chloride (NH4C1),
sodium chloride (NaCl), phosphate-buffered saline (PBS), sodium sulfate
(Na2SO4), zinc
sulfate (ZnSO4), manganese sulfate (MnSO4), magnesium chloride (MgCl2),
calcium chloride
(CaCl2), ferric chloride (FeCl3), lithium hexafluorophosphate (LiPF6),
potassium hydroxide
(KOH), sodium hydroxide (NaOH), or the like, or combinations thereof. In a
preferred
embodiment, the salt of the electrolyte composition may be or include ammonium
chloride
(NH4C1), zinc chloride (ZnC12), or a combination or mixture thereof. In
another embodiment,
the salt may be or include alkali metal salts, such as sodium hydroxide
(NaOH), ammonium
hydroxide (NH4OH), potassium hydroxide (KOH), or combinations or mixtures
thereof.
14
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0056] The salt may be present in an amount capable of, configured to, or
sufficient to provide
ionic conductivity. For example, the salt may be present in the hydrogel in an
amount or
concentration of at least 0.1M, more preferably at least 0.5M, even more
preferably at least
2M, even more preferably at least 4M. The salt may be present in the hydrogel
at a
concentration of 10M or less, more preferably 6M or less. In another example,
the salt may be
present in the hydrogel in an amount of from about 3M to about 10M, about 4M
to about 10M,
about 5M to about 9M, or about 6M to about 8M. In an exemplary implementation,
the salts
included ammonium chloride and zinc chloride, where ammonium chloride is
present in an
amount of from about 2.5M to about 3M, about 2.8M to about 2.9M, or about
2.89M, and
where zinc chloride is present in an amount of from about 0.5M to 1.5M, about
0.8M to about
1.2M, or about 0.9M.
[0057] In at least one embodiment, the electrolyte composition may include one
or more
additives. The one or more additives may be or include, but are not limited
to, biodegradable
or environmentally friendly nanomaterials. The biodegradable nanomaterials may
be capable
of or configured to provide and/or improve structural strength of the
electrolyte layer or the
electrolyte composition thereof without sacrificing flexibility of the
electrolyte layer or the
electrolyte composition thereof. Illustrative biodegradable nanomaterials of
the additives may
be or include, but are not limited to, polysaccharide-based nanomaterials,
inorganic
nanomaterials, or the like, or combinations thereof. Illustrative
polysaccharide-based
nanomaterials may be or include, but are not limited to, one or more of
cellulose nanocrystals,
chitin nanocrystals, chitosan nanocrystals, starch nanocrystals or the like,
or combinations or
mixtures thereof. Illustrative inorganic nanomaterials may be or include, but
are not limited
to, one or more of silicon oxides (e.g., fumed silica), aluminum oxides,
layered silicates or
lime, or combinations or mixtures thereof. Illustrative layered silicates may
be or include, but
are not limited to, one or more of bentonite, kaolinite, dickite, nacrite,
stapulgite, illite,
halloysite, montmorillonite, hectorite, fluorohectorite, nontronite,
beidellite, saponite,
volkonskoite, magadiite, medmontite, kenyaite, sauconite, muscovite,
vermiculite, mica,
hydromica, phegite, brammalite, celadonite, or combinations or mixtures
thereof.
[0058] The one or more additives may be present in an amount of from at least
0.1 weight %,
based on a total weight of the hydrogel. For example, the one or more additive
may be present
in an amount of at least 0.1 weight %, at least 0.5 weight %, or at least 1
weight %, based on a
total weight of the hydrogel. The one or more additives may also be present in
an amount of
40 weight % or less, based on a total weight of the hydrogel. For example, the
one or more
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
additives may be present in an amount of 40 weight % or less, 20 weight % or
less, or 10 weight
% or less, based on a total weight of the hydrogel.
[0059] In at least one embodiment, the electrolyte composition may include an
aqueous
solvent. For example, the electrolyte composition may include water. In at
least one
embodiment, the electrolyte composition may include a co-solvent. For example,
the
electrolyte composition may include water and an additional solvent.
Illustrative co-solvents
may be or include, but are not limited to, one or more of ethylene glycol,
propylene glycol,
diethylene glycol, dipropylene glycol, or combinations thereof. The cosolvent
may include
water in an amount greater than about 20%, greater than about 30%, greater
than about 40%,
greater than about 50% to greater than about 60%, greater than about 70%,
greater than about
80%, greater than about 85%, or greater than about 90%, by total weight or
volume of the
aqueous solvent of the electrolyte composition.
[0060] In at least one embodiment, the electrolyte composition includes the
hydrogel of the
copolymer and the salt dispersed in the hydrogel, a solvent (e.g., water or
water and a co-
solvent), one or more photoinitiators, the optional one or more additives, or
combinations
thereof. For example, the electrolyte composition includes the hydrogel of the
copolymer, the
salt dispersed in the hydrogel, the solvent, the one or more additives, or
combinations or
mixtures thereof. In at least one embodiment, the electrolyte composition
consists of or
consists essentially of the hydrogel of the copolymer, the salt dispersed in
the hydrogel, and
the solvent (e.g., water or water and a cosolvent). In another embodiment, the
electrolyte
composition consists of or consists essentially of the hydrogel of the
copolymer, the salt
dispersed in the hydrogel, the solvent, and the one or more additives. The
solvent, which may
be water or a combination of water and a cosolvent, may provide the balance of
the hydrogel.
Suitable electrolyte compositions and processes and procedures for producing
the same are
disclosed in International Application No. PCT/U52020/046932, the disclosure
of which is
hereby incorporated herein by reference in its entirety.
[0061] As previously discussed, the electrolyte layer 112, 212 of the
respective biodegradable
electrochemical devices 100, 200 may be or include the solid, aqueous
electrolyte composition.
The solid, aqueous electrolyte composition may have sufficient mechanical and
electrochemical properties necessary for a commercial printed battery or a
commercially useful
printed battery. For example, the solid, aqueous electrolyte composition may
have a Young's
modulus or storage modulus of greater than about 0.10 Megapascals (MPa),
greater than about
0.15 MPa, or greater than about 0.20 MPa, thereby providing the solid, aqueous
electrolyte
composition with sufficient strength while maintaining sufficient flexibility
to prevent
16
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
breakage under stress. The solid, aqueous electrolyte composition may have a
Young's
modulus of less than or equal to about 100 MPa, less than or equal to about 80
MPa, less than
or equal to about 60 MPa, or less.
[0062] As used herein, the term or expression "Yield strength" may refer to a
maximum stress
a material can experience or receive before the material begins to deform
permanently. The
solid, aqueous electrolyte composition may have a Yield strength of from about
5 kPa or
greater. For example, the solid, aqueous electrolyte composition may have a
Yield strength of
from about 5 kPa or greater, about 8 kPa or greater, about 10 kPa or greater,
about 12 kPa or
greater, about 15 kPa or greater, or about 20 kPa or greater.
[0063] The solid, aqueous electrolyte composition may be electrochemically
stable for both
the anode active layers 108, 208 and cathode active layers 110, 210 of the
respective
biodegradable electrochemical devices 100, 200. For example, the solid,
aqueous electrolyte
composition may maintain a stable open circuit voltage over an extended period
of time,
thereby demonstrating electrochemical stability towards both the anode active
layers 108, 208
and cathode active layers 110, 210 of the respective biodegradable
electrochemical devices
100, 200. In at least one embodiment, the solid, aqueous electrolyte
composition may be
electrochemically stable in contact with the electrode layers for at least one
month, at least two
months, at least three months, at least four months, at least five months, at
least six months, at
least one year, or more.
[0064] The solid, aqueous electrolyte composition disclosed herein may be
utilized in any
electrochemical device, such as an electrochemical cell, a battery, and/or the
biodegradable
electrochemical devices 100, 200 disclosed herein. In a preferred embodiment,
the solid,
aqueous electrolyte composition may be utilized in a battery including a Zn
anode active layer
and a Mn02 cathode active layer.
[0065] The current collectors 104, 106, 204, 206 of the respective
biodegradable
electrochemical devices 100, 200 may be capable of or configured to receive,
conduct, and
deliver electricity. Illustrative current collectors 104, 106, 204, 206 may be
or include, but are
not limited to, silver, such as silver microparticles and silver
nanoparticles, carbon, such as
carbon black, graphite, carbon fibers, carbon nanoparticles, such as carbon
nanotubes,
graphene, reduced graphene oxide (RGO), or the like, or any combination
thereof.
METHODS
[0066] Embodiments of the present disclosure may provide methods for
fabricating an
electrochemical device, such as the biodegradable electrochemical devices 100,
200 disclosed
17
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
herein. The method may include providing a biodegradable substrate. The method
may also
include depositing an electrode and/or electrode composition adjacent or on
the biodegradable
substrate. Depositing the electrode may include depositing and drying a
current collector of
the electrode, and depositing and drying an active layer (i.e., anode or
cathode material)
adjacent or on the current collector. The method may also include drying the
electrode and/or
electrode composition. The electrode composition may be dried thermally (e.g.,
heating). The
method may also include depositing a biodegradable, radiatively curable
electrolyte
composition on or adjacent the electrode composition. The method may further
include
radiatively curing the biodegradable radiatively curable electrolyte
composition. The
biodegradable radiatively curable electrolyte composition may be radiatively
cured before or
subsequent to drying the electrode composition. The biodegradable substrate
may be thermally
compatible with the optional thermal drying. For example, the biodegradable
substrate may be
dimensionally stable (e.g., no buckling and/or curling) when thermally drying.
The method
may include depositing a second electrode and/or electrode composition on or
adjacent the
biodegradable, radiatively curable electrolyte composition. In at least one
embodiment, each
of the first and second electrode compositions is a metal foil composition.
The metal foil
composition of the first electrode may be different from the metal foil
composition of the
second electrode.
[0067] In at least one embodiment, the electrochemical device, all of the
components thereof,
or substantially all of the components thereof are fabricated via a printing
process. The printing
process may include depositing, stamping, spraying, sputtering, jetting,
coating, layering, or
the like. For example, the one or more current collectors, the one or more
electrode
compositions, the biodegradable, radiatively curable electrolyte composition,
or combinations
thereof may be deposited via the printing process. Illustrative printing
processes may be or
include, but are not limited to, one or more of screen printing, inkjet
printing, flexography
printing (e.g. stamps), gravure printing, off-set printing, airbrushing,
aerosol printing,
typesetting, roll-to-roll methods, or the like, or combinations thereof. In a
preferred
embodiment, the components of the electrochemical device are printed via
screen printing.
[0068] In at least one embodiment, radiatively curing the biodegradable
radiatively curable
electrolyte composition includes exposing the electrolyte composition to a
radiant energy. The
radiant energy may be ultraviolet light. Exposing the biodegradable
radiatively curable
electrolyte composition to the radiant energy may at least partially crosslink
the biodegradable
radiatively curable electrolyte composition, thereby forming a hydrogel. The
biodegradable
radiatively curable electrolyte composition may be radiatively cured at room
temperature. In
18
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
at least one embodiment, the biodegradable radiatively curable electrolyte
composition is cured
at an inert atmosphere. For example, the biodegradable radiatively curable
electrolyte
composition may be cured under nitrogen, argon, or the like. In another
embodiment, the
biodegradable radiatively curable electrolyte composition may be cured in a
non-inert
atmosphere.
[0069] In at least one embodiment, the biodegradable radiatively curable
electrolyte
composition may be radiatively cured in a period of time from about 5 ms to
about 100 ms.
For example, the biodegradable radiatively curable electrolyte composition may
be radiatively
cured in a period of time from about 5 ms, about about 10 ms, about 15 ms,
about 20 ms, about
30 ms, about 40 ms, or about 50 ms to about 60 ms, about 70 ms, about 80 ms,
about 85 ms,
about 90 ms, about 95 ms, or about 100 ms. The period of time sufficient to
radiatively cure
the biodegradable radiatively curable electrolyte composition may be at least
partially
determined by a power output of the UV light.
[0070] In at least one embodiment, the method may also include depositing an
adhesive, such
as a biodegradable adhesive, to thereby provide the seals 116, 118, 216, 218
of the respective
biodegradable electrochemical devices 100, 200. For example, the method may
include
depositing a layer of the adhesive to couple the substrates or part of the
substrates (e.g., area
around the tabs 124, 126, 224, 226), of the electrochemical device with one
another. In some
embodiments, the adhesive may be a hot-melt adhesive. In another embodiment,
the
electrochemical device may be free or substantially free from any adhesive.
For example, the
biodegradable substrate may be weldable and/or heat-sealable without the use
of an additional
adhesive.
[0071] In at least one embodiment, the biodegradable substrate may be a
continuous web, or
may be supported by a continuous web. As used herein, the term "web" may refer
to a moving
supporting surface, such as a conveyor belt. In at least one example, a
plurality of
electrochemical devices are simultaneously printed as independent or linked
elements or
components on the continuous web. For example, respective components of the
plurality of
electrochemical devices may be simultaneously printed as independent or linked
components
on the continuous web as an array in a parallel process. As used herein, the
term or expression
"linked elements" or "linked components" may refer to elements or components,
respectively,
of the electrochemical device that are physically touching, overlapping, or
otherwise contacting
one another. Illustrative linked elements may be or include an active layer
(e.g., cathode active
layer or anode active layer) disposed adjacent to or on top of a current
collector layer, a current
19
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
collector layer and a copper tape tab, or an electrolyte layer on top of an
active cathode/anode
layer.
[0072] In at least one embodiment of an exemplary biodegradable
electrochemical device, the
solid aqueous electrolytes thereof, and methods for synthesizing and
fabricating the same are
available, the layers of various materials, including current collectors,
cathode/anode materials,
binders, adhesives, and electrolyte need to be printed with high fidelity and
accuracy.
Furthermore, retention of moisture within the aqueous electrolyte is critical
to battery
performance via maintenance of solubilized salts for good ion conductivity and
printed
biodegradable or compostable batteries such as these suffer from shortened
lifespan due to
water losses via evaporation through the biodegradable substrate, which may be
a polylactic
acid (PLA) film. Such electrochemical devices may have biodegradable polymeric
composite
film enclosure pouches that have a biodegradable barrier layer. Illustrative
biodegradable
enclosure materials may be or include, but are not limited to, one or more of
polylactic acid
(PLA), polylactic-co-glycolic acid (PLGA), silk-fibroin, chitosan,
polycaprolactone (PCL),
polyhydroxybutyrate (PHB), rice paper, cellulose, or combinations or
composites thereof.
[0073] In at least one embodiment, flexible biodegradable electrochemical
devices including
an anode, a cathode and an electrolyte composition comprising a crosslinked,
biodegradable
polymeric material that is radiatively curable prior to being crosslinked,
printed between the
anode and the cathode, may have biodegradable moisture or water vapor barriers
or barrier
layers forming an enclosure, film or pouch around the external portion of an
electrochemical
device to prevent moisture present within the aqueous electrolyte materials
from evaporating.
In such embodiments, since the entire electrochemical device is biodegradable,
the device may
have prolonged service life due to the improved water vapor barrier or
moisture barrier layer
properties of the enclosure pouch and be biodegradable and/or biodegradable
once its service
life is over. The function of the biodegradable water vapor barrier or
enclosure is to provide a
moisture barrier layer to impede the evaporation of water from aqueous
electrolyte
compositions within the electrochemical device, thus extending service life of
the
electrochemical device. It should be noted, in reference to water vapor
barriers or moisture
barrier layers described herein, that while certain embodiments of
electrochemical devices may
have a substantial amount of water or moisture, that other solvents or
evaporable materials may
also be conducive to prolonged and acceptable operation of an electrochemical
device enclosed
within water vapor barriers of the present disclosure.
[0074] The biodegradable water vapor barrier of the respective biodegradable
electrochemical
devices may be stable at temperatures of from about 50 C to about 150 C. As
used herein, the
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
term "stable" or "stability" may refer to the ability of the substrate to
resist dimensional changes
and maintain structural integrity when exposed to temperature of from about 50
C to about
150 C. For example, the biodegradable water vapor barrier may be capable of or
configured
to maintain structural integrity with dimensional changes of less than about
20%, less than
about 15%, or less than about 10% after exposure to temperatures of from about
50 C to about
150 C. In one example, each of the biodegradable water vapor barriers may be
stable (e.g.,
dimensional changes less than 20%) at a temperature of from about 50 C, about
60 C, about
70 C, about 80 C, about 90 C, about 100 C, or about 110 C to about 120 C,
about 130 C,
about 140 C, or about 150 C. In another example, each of the biodegradable
water vapor
barriers may be stable at a temperature of at least 100 C, at least 105 C, at
least 110 C, at least
115 C, at least 120 C, at least 125 C, at least 130 C, at least 135 C, at
least 140 C, or at least
145 C. In at least one embodiment, the biodegradable water vapor barriers may
be stable at
temperatures of from about 50 C to about 150 C for a period of from about 5 mm
to about 60
mm or greater. For example, the biodegradable water vapor barriers may be
stable at the
aforementioned temperatures for a period of time of from about 5 min, about 10
mm, about 20
mm, or about 30 mm to about 40 mm, about 45 mm, about 50 mm, about 60 mm, or
greater.
[0075] In at least one embodiment, the biodegradable water vapor barrier
material is weldable,
bondable, and/or permanently thermo-sealable without the use of an additional
adhesive. For
example, the biodegradable water vapor barriers described herein for
electrochemical device
enclosures may be weldable and/or bondable with one another without the use of
the respective
seals. Illustrative biodegradable water vapor barrier materials that may be
weldable and/or
bondable with one another may be or include, but are not limited to,
thermoplastics, such as
polylactic acid (PLA), polylactides modified with a nucleating agent to
enhance crystallinity,
such as polylactide modified with nucleating agent D (PLA-D) and polylactide
modified with
nucleating agent E (PLA-E), polybutylene succinate (PBS), polybutylene adipate
terephthalate
(PBAT), blends of PLA and polyhydroxybutyrate (PHB), PHB-based blends, or the
like, or
combinations thereof. As used herein, the term or expression "bondable,"
"weldable," and/or
"permanently thermo-sealable" may refer to an ability of a material (e.g.,
substrate) to heat seal
two surfaces with one another or permanently join two surfaces with one
another via heating
or melting.
[0076] In some embodiments, the biodegradable enclosures, pouches, or water
vapor barriers
may be made from metallized, biodegradable polylactic acid (PLA) film, such as
an aluminum
metalized polylactic acid film. The metal surface layer providing the
metallization may be
21
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
aluminum. In certain embodiments, the metallization layer may include
aluminum, other
suitable metals or alloys, ceramics, clays, hybrid materials of inorganic-
organic biopolymers,
and combinations thereof. Alternative embodiments may have multiple layers of
metal, metal
on an inner layer of a multilayer film, an outer layer, or both. The PLA film
may be biaxially
oriented to improve physical properties of the enclosure pouch. Still other
embodiments may
have additives incorporated into the film, providing enhanced moisture barrier
properties.
Biodegradable enclosures, pouches, or water vapor barriers for electrochemical
devices may
have single layer, or multiple layers with combinations of one or more
materials in alternate
embodiments. Metalized layer films or barriers can provide a thickness of from
about 1 nm to
about 100 nm, from about 5 nm to about 50 nm, or from about 10 nm to about 40
nm. Single
layer films or barriers in certain examples may have an overall thickness from
about 1 pm to
about 100 pm, from about 40 pm to about 80 pm, or from about 50 pm to about 75
pm.
Metallized layers of water vapor barriers may have a thickness from about 0.5
nm to about 100
nm, from about 5 nm to about 50 nm, or from about 5 nm to about 25 nm over a
base film layer
such as PLA.
[0077] In certain examples, a biodegradable enclosure, pouch, or water vapor
barrier may be
made of a multilayer composite constructed by either extruding or laminating a
biodegradable
polymer on each side of a thin metal foil, such as aluminum. In contrast to a
metallized polymer
layer in a biodegradable enclosure, a thin and continuous metal layer within
such a multilayered
laminated composite can provide a robust barrier layer coupled with one or
more biodegradable
enclosure layers. An advantage of such a multilayered laminated structure is
that the continuous
metal film forms a layer that can more effectively prevent water permeation
through the
composite, as well as providing an option to provide thicker metal or aluminum
layers or
multiple metal layers. Thus, a metal layer providing water vapor barrier can
be from about 1
pm to about 200 pm, or from about 5 pm to about 150 pm, or from about 10 pm to
about 100
pm. Metal foil layers in accordance with the present disclosure are not as
prone to issues such
as pinholes in the aluminum or metal layer, which can be more prevalent in
metal films formed
from sputtering or other deposition methods as described herein. The presence
of pinholes in
metal layers or other barrier layers within a biodegradable enclosure, pouch,
or water vapor
barrier can in some instances allow some water permeation through the
composite material.
[0078] For certain examples having multilayer composites according to the
present disclosure,
several methods may be used to form these multilayer laminates. In a first
example, pre-
existing polymer sheets can be pressed at a suitable temperature and pressure
on one side or
each side of an aluminum sheet, with the use of one or more interdigitated or
interspersed
22
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
adhesive tie layers to enable or enhance the adhesion of the polymer sheet
with the aluminum
or metal foil. In a second example, the polymer layer and adhesive tie layers
can be directly
melt-extruded as thin films onto the surface of an aluminum or other metal
foil, using
multilayer film casting processes, known to one skilled in the art. Additional
metal foils or
films incorporating metals such as magnesium, titanium, iron, nickel, copper,
zinc, or alloys or
mixtures thereof may be used in accordance with the present disclosure.
[0079] In certain embodiments, other materials known to have water vapor
barrier properties
may be used. These materials must conform to the biodegradable and/or
compostable format
and include materials such as beeswax, plasticizers, and alternative
biodegradable polymer
composite films. In alternate embodiments wherein the water vapor barrier is
not a part of the
substrate of the electrochemical device, water vapor barriers may be used
having higher
temperature stability and resistance as compared to biodegradable materials,
polymers or
composites, having wider ranges of temperature resistance. Embodiments of
electrochemical
devices having biodegradable enclosures or water vapor barriers having
moisture barrier
properties may exhibit reduced water vapor transmission rates (WVTR) as
compared to
electrochemical devices without such barriers, layers, or enclosures,
exhibiting WVTR of from
about 0 % over 24 hour to about 5 % over 24 hour, from about 0.1 % over 24
hour to about 2
% over 24 hour, or from about 0.5 % over 24 hour to about 1 % over 24 hour.
WVTR may also
be expressed as a percentage of total weight of water lost as compared to a
total weight of the
electrochemical device including the enclosure or moisture barrier.
Embodiments of
electrochemical devices having biodegradable enclosures or water vapor
barriers having
moisture barrier properties in accordance with the present disclosure may
exhibit reduced water
vapor transmission rates (WVTR) as compared to electrochemical devices without
such barrier
layers or enclosures, the water vapor barrier exhibiting WVTR of from about
0.0 g/m2/24 hour
to about 10 g/m2/24 hour, from about 0.5 g/m2/24 hour to about 5 g/m2/24 hour,
or from about
1 g/m2/24 hour to about 2 g/m2/24 hour. Expressions of WVTR are provided
herein as weight
percent, or wt% of the total electrochemical device. Certain examples of
electrochemical
devices having biodegradable enclosures or water vapor barriers having
moisture barrier
properties may exhibit reduced water vapor transmission rates (WVTR) as
compared to
electrochemical devices without such barrier layers or enclosures, the water
vapor barriers of
the present disclosure exhibiting WVTR of from about 0 mg/cm2/24 hour to about
5.0
mg/cm2/24 hour, from about 0.1 mg/cm2/24 hour to about 1 mg/cm2/24 hour, or
from about 0.1
mg/cm2/24 hour to about 0.5 mg/cm2/24 hour.
23
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0080] In some embodiments, the electrochemical device may be arranged such
that a battery
or electrochemical device is contained in an enclosure or entirely within a
water vapor barrier
as described having improved water vapor barrier properties and oriented or
arranged such that
the cathode and anode are in a side-by-side or lateral X-Y plane geometry, as
illustrated in
Figure 1. In alternate embodiments, the electrochemical device may be arranged
such that a
battery or electrochemical device is contained in an enclosure as described
having improved
water vapor barrier properties and oriented or arranged such that the cathode
and anode are in
a stacked geometry, as illustrated in Figure 2.
[0081] Embodiments of the present disclosure may provide methods for
fabricating,
producing, or otherwise enclosing an electrochemical device having improved
moisture barrier
properties or water vapor barrier properties. The method may include orienting
a first metalized
PLA film having four edges and a second metalized PLA film having four edges
such that a
non-metalized side of the first metalized PLA film is facing a non-metalized
side of the second
metalized PLA film. One or more edges of the first and second metallized PLA
films may be
sealed together. A biodegradable or compostable electrochemical device may be
placed
between the first metalized PLA film and the second metalized PLA film,
followed by sealing
the edges of the first metalized PLA film and the edges of the second
metalized PLA film
together, such that one or more electrodes of the electrochemical device are
exposed through
at least one of four edges.
[0082] The method may alternatively include steps to orient a first metalized
PLA film having
four edges on a top side of an electrochemical device such that a non-
metalized side is facing
the electrochemical device. A second metalized PLA film is oriented on a
bottom side of an
electrochemical device such that a non-metalized side is facing the
electrochemical device. All
four edges of the first metalized PLA film and the four edges of the second
metalized PLA film
may be sealed together, such that the one or more electrodes are exposed
through at least one
of four edges. Enclosures or water vapor barriers fabricated in this manner
from biodegradable
aluminized polymer barrier layers in combination with surface coatings and/or
polymer
additives may reduce or prevent water vapor loss from a biodegradable or
compostable
electrochemical device. Such devices may significantly extend the service life
of biodegradable
or compostable electrochemical devices by preventing the electrolyte solvent
from evaporating
over time.
24
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
EXAMPLES
[0083] The examples and other implementations described herein are exemplary
and not
intended to be limiting in describing the full scope of compositions and
methods of this
disclosure. Equivalent changes, modifications and variations of specific
implementations,
materials, compositions and methods may be made within the scope of the
present disclosure,
with substantially similar results.
Comparative Example 1
[0084] A surrogate test related to embodiments described herein of a
biodegradable battery
configuration having improved moisture retention is described in relation to
Comparative
Example 1. Figure 3 illustrates a cross-sectional view of a comparative
example of a fully
assembled surrogate for a biodegradable battery assembly having a water vapor
barrier,
according to one or more embodiments. This surrogate test was performed to
measure the water
loss through PLA-D biodegradable substrate within a similar context to a fully
assembled
biodegradable battery. Comparative Example 1 is a surrogate battery assembly
300 with film
enclosure 304 surrounding a patch of filter paper 302 as a surrogate for an
aqueous electrolyte
battery composition. Two sheets of 80 pm thick PLA-D bi-axially stretched
films were cut into
2cm x 3cm squares. Two of the cut films were stacked together with similar
orientation. An
MSK-130 heat sealer from MTI corporation set at 170 C for 5 seconds was used
to heat seal
three of the four edges of the film sheets together, resulting in a pouch or
enclosure with a
single, 3cm unsealed side. A lcm square patch of whatman 1825-150 filter paper
302 was
placed into the 2cm x 3cm pouch 304. The pouch with the filter paper 302
inside was then
weighed and tared. 3 drops of DI water were added to the paper in the pouch
and then the
remaining open edge of the pouch was sealed, forming a heat sealed edge 306
under the same
conditions as mentioned previously. The weight of the pouch was measured over
time to
determine the loss of water. The surrogate test includes the creation of a
full sized, sealed
substrate configuration, with known, measured amounts of water added to
reflect similar
amounts found in fully assembled biodegradable batteries. The water loss over
time is then
measured by periodically weighing the surrogate battery assembly 300. This
testing protocol
is then repeated with experimental Examples 1-3, to evaluate various
embodiments of film
enclosures to inhibit water transmission loss from aqueous based electrolyte
compositions
within biodegradable electrochemical devices.
Example 1
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0085] Figure 4 illustrates a cross-sectional view of the embodiment of a
fully assembled
surrogate for an electrochemical device assembly of Example 1, having a water
vapor barrier,
according to one or more embodiments. Two 4cm x 4cm square sheets of Enviromet
HS 75
pm thick Aluminized PLA manufactured by Celplast, Toronto Ontario, Canada,
were oriented
and stacked together such that the PLA side was facing inward to form an
enclosure 400 for an
electrochemical device. An MS K-130 heat sealer with a soft die set at 170 C
for 3 seconds was
used to seal three of the four edges of the sheet together to make an
enclosure pouch 400 with
one open side, resulting in an enclosure pouch 400 having an internal PLA
layer 410 and an
external aluminum layer 412. A water-soaked filter paper 402 and PLA-D
enclosure pouch 404
sealed at an edge similar to the embodiment described in regard to Comparative
Example 1
was then placed inside of the PLA-Al pouch 400 and the remaining edge 414 was
sealed using
the MSK-130. The weight of the pouch 400 was measured over time to determine
the loss of
water.
Example 2
[0086] Figure 5 illustrates a cross-sectional view of the embodiment of a
fully assembled
surrogate for an electrochemical device assembly of Example 2, having a water
vapor barrier,
according to one or more embodiments. Eight 4cm x 4cm square sheets of
Enviromet 75 pm
thick Aluminized PLA were oriented and stacked together such that the PLA side
was facing
inward to form an enclosure pouch or water vapor barrier 500 having four
layers for an
electrochemical device. An MS K-130 heat sealer with a soft die set at 170 C
for 3 seconds was
used to seal three of the four edges of each sheet together to make an
enclosure pouch 500 with
one open side. In this embodiment, the enclosure pouch 500 walls consist of a
first layer having
a first PLA layer 508 and a first aluminum layer 510, a second layer having a
second PLA layer
512 and a second aluminum layer 514, a third layer having a third PLA layer
516 and a third
aluminum layer 518, and a fourth layer having a fourth PLA layer 520 and a
fourth aluminum
layer 522, resulting in four stacked sheets of Enviromet Aluminized PLA. A
water-soaked filter
paper 502 and PLA-D enclosure pouch 504 sealed at an edge 506 similar to the
embodiment
described in regard to Comparative Example 1 was then placed inside of the PLA-
Al pouch
500 and the remaining edge 522 was sealed using the MSK-130. The weight of the
enclosure
pouch 500 was measured over time to determine the loss of water.
Example 3
26
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
[0087] Figure 6 illustrates a cross-sectional view of the embodiment of a
fully assembled
surrogate for an electrochemical device assembly of Example 3, having a water
vapor barrier,
according to one or more embodiments. Two 4cm x 4cm square sheets of Enviromet
75 pm
thick Aluminized PLA were oriented and stacked together such that the PLA side
was facing
inward to form an enclosure pouch 600 having a single water vapor barrier
layer for an
electrochemical device. An MSK-130 heat sealer with a soft die set at 170C for
3 seconds was
used to seal 3 of the 4 edges together to make an enclosure pouch 600 with one
open side. In
this embodiment, the enclosure pouch 600 walls consists of a PLA layer 604 and
an aluminum
layer 606. A lcm square patch of Whatman 1825-150 filter paper 602 was placed
into the 4cm
x 4cm enclosure pouch 600. The pouch 600 with the paper patch 602 inside was
then weighed
and tared. 3 drops of DI water were added to the paper 602 in the pouch 600
and then last open
edge of the pouch was sealed as before with the MSK-130. The weight of the
pouch was
measured over time to determine the loss of water.
Examples 4, 5, 6, and 7
[0088] Figure 7 illustrates a cross-sectional view of an example of a
surrogate for an
electrochemical device assembly having a multilaminate enclosure structure, in
accordance
with the present disclosure. In the multilayer enclosure structure 700 a first
side and a second
side of a metal barrier layer 702 have a first adhesive tie layer 704 and a
second adhesive tie
layer 706 disposed onto the metal barrier layer 702. It should be noted that
in certain materials
configurations for a multilayer composite structures 700, no adhesive tie
layers are needed, but
in other examples, in instances where a polymer will not adhere to an aluminum
or other metal
surface without an adhesive, the presence of an adhesive tie layer can be
desirable. In certain
examples, the composition of the adhesive tie layers 704, 706 is
biodegradable, however, as
they are sufficiently thin compared to the metal and polymer layers, they may
be fabricated
partially or fully of non-biodegradable material. In such examples, the
relative weight
percentage of any non-biodegradable material would be less than 10% by weight
of the entire
enclosure material. In certain examples, the aluminum or metal barrier 702
surface may have
a surface treatment that can enhance the adhesion of one or more layers to a
surface of the
metal barrier layer 702, such as a plasma or corona treatment. It is expected
that both of the
external first biodegradable polymer layer 708 and a second biodegradable
polymer layer 710
will be constructed of the same material, however, in certain examples the
first biodegradable
polymer layer 708 and the second biodegradable polymer layer 710 may not be
constructed of
the same material and therefore the material composition of adhesive tie
layers 704, 706 could
27
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
differ as well, as would be required to optimize the adhesion with each
polymer type used in
the first biodegradable polymer layer 708 and the second biodegradable polymer
layer 710.
Metal layers or metal barrier layers as described herein provide multilayer
enclosure structures
of the present disclosure with a moisture impermeable layer, or moisture
impenetrable layer.
In certain examples of such multilayer enclosure structures, the metal layer
or metal barrier
layer can allow for no water, moisture, or solvent to pass through the
enclosure.
[0089] Example 4: production of a half-structure multilayer composite using a
commercial tri-
layer PLA thin film as a tie layer. A PLA sheet of approximately 100 um thick
was obtained
by melt-extruding a formulation of a commercial extrusion grade PLA, obtained
from Corbion,
blended with a crystallization agent (agent D, Luminy D070 by Corbion). A
multilayer stack
was prepared using a 40 um thick aluminum foil, obtained from All Foils Inc.,
Ohio, USA, a
sheet of the extruded PLA-D polymer and as an in-between adhesive tie layer, a
tri-layer PLA
thin film, Evlon EV-HS1The multilayer stack was pressed at 120 C under 5000
PSI for 20
minutes. The resulting multilayer film can be considered as half the
multilayer structure
detailed in Figure 7. However, since the aluminum layer is acting as a water
vapor barrier layer,
this half-structure is sufficient to demonstrate the barrier properties in
surrogate evaluation in
accordance with the present disclosure. The complete structure may only be
needed in certain
examples to protect the aluminum layer from being mechanically damaged by
adding a
polymer scratch-resistant layer. The complete structure can be fabricated
using the same
method as the half structure.
[0090] Example 5: production of a half-structure multilayer composite using a
non-
biodegradable polyamide-based adhesive as a tie layer. A 40 um thick aluminum
foil was
coated with a thin layer of polyamide-based adhesive powder (Evonik Vestamelt
Hylink) using
an electrostatic spray gun, then placed in an oven at 140 C for 10 minutes. A
PLA-D sheet
obtained as detailed in Example 4 was then applied to the adhesive tie layer
side of the
aluminum foil and pressed at 120 C under 5000 PSI for 20 minutes. The adhesive
tie layer
weights 2 mg/cm2 whereas the half structure weights 32.4 mg/cm2, making the
adhesive tie
layer weight approximately 6 wt% based on a total weight of the half
structure. It can be
calculated that the adhesive tie layer weight of a full symmetric
multilaminate structure would
be 4 mg/cm2 out of 54.4 mg/cm2 or 7.4 wt% of non-biodegradable adhesive tie
layer.
[0091] Example 6: production of a half-structure multilayer composite using a
thin film of
polycaprolactone (PCL) as an adhesive tie layer. A thin film of a PCL adhesive
tie layer was
obtained by pressing CAPA6500 PCL pellets, obtained from Ingevity, at 100 C
under 5000
PSI for 20 min. A multilayer stack was prepared using a 40 um thick aluminum
foil, a sheet of
28
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
extruded PLA-D, as used in Example 4 and in accordance with the present
disclosure, and PCL
thin film as an adhesive tie-layer. The multilayer stack was pressed at 120 C
under 5000 PSI
for 20 minutes.
[0092] Example 7: production of a half-structure multilayer composite using a
thin film of an
amorphous grade of PLA as an adhesive tie layer. A thin film of amorphous PLA
layer was
produced by pressing PLA pellets at 200 C under 5000 PSI for 20 mm. A
multilayer stack was
prepared using a 40 um thick aluminum foil, a sheet of extruded PLA-D (as in
Example 4) and
as an interdigitated amorphous PLA thin film as an adhesive tie layer. The
multilayer stack was
pressed at 120 C under 5000 PSI for 20 minutes.
[0093] In certain embodiments, the multilayer composites from Examples 6 and 7
can be
produced in a single-step by directly melt-extruding a dual layer of adhesive
tie layer and PLA
film on to of an aluminum roll, as commonly performed by the multilayer
packaging
manufacturing industry.
[0094] To demonstrate the barrier properties of the multilayer composites
produced in
Examples 4, 5, 6 and 7, hermetically sealed pouches of each multilayer
laminate (half
structures) were prepared by cutting sheets of similar dimensions and
thermally sealing their
edges by pressing them between the jaws of a hand-held thermal sealer set at
200 C for 5
seconds. Each pouch contained a paper tissue soaked with water. The weight of
each pouch
was then measured each day as a means to monitor the water permeation through
the multilayer
composites and subsequent evaporation.
[0095] Figure 8 illustrates a plot of water loss in milligrams per square
centimeter vs. time in
days for the Comparative Example of Figure 3 compared to Examples 1 to 7 of
Figures 4, 5, 6,
and 7, respectively. The cumulative water loss in milligrams per square
centimeter was
measured by weight every 24 hrs for each example, normalized for surface area,
and then
plotted. Comparative Example 1, having only PLA-D substrate as a barrier layer
shows a high
cumulative water loss of ¨15 mg/cm2, exhibiting total loss of water by day 9.
Example 1 with
an extra aluminized PLA barrier layer shows a significantly reduced cumulative
water loss of
about 1.34 mg/cm2 by day 9. Example 2 with four combined aluminized PLA
barrier layers
shows a significant reduction in cumulative water loss at 0.66 mg/cm2 by day
9. Example 3
with only aluminized PLA as a barrier layer shows a moderately improved
reduction in
cumulative water loss of 2.84 mg/cm2 on day 9 as compared to the total water
loss of
Comparative Example 1 at a similar time. The multilaminate samples of Examples
4, 5, 6, and
7 showed virtually no or negligible water loss by day 9. Full results are
reported in Tables 1
and 2.
29
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
Time (days) COMPARATIVEEXAMPLE 1 EXAMPLE 2 EXAMPLE 3
EXAMPLE 1
Water Loss -> mg/cm2 mg/cm2 mg/cm2 mg/cm2
0 0 0 0 0
1 1.58 0.16 0.08 0.38
2 4.08 0.31 0.16 0.69
3 5.67 0.44 0.23 0.97
4 7.67 0.59 0.31 1.34
6
7 12.08 1.06 0.52 2.22
8 13.58 1.25 0.59 2.53
9 14.8 1.34 0.66 2.84
15 1.56 0.73 3.09
11 15 1.72 0.81 3.38
12
13
14 15 2.25 1.03 3.81
15 2.44 1.11 3.81
16 15 2.56 1.19 3.81
22 15 3.38 1.66 3.81
26 15 3.44 1.97 3.81
30 15 3.44 2.28 3.81
Table 1
CA 03213871 2023-09-15
WO 2022/212454 PCT/US2022/022475
Time (days) EXAMPLE 4 EXAMPLE 5 EXAMPLE 6 EXAMPLE 7
Water Loss 4 mg/cm2 mg/cm2 mg/cm2 mg/cm2
0 0 0 0 0
1 0.04 0
2 0
3
4 0.16 0
0.12 0.16 0
6 0.14 0 0.16
7 0.17 0
8 0.17 0
9
11 0.16 0
12 0.24 0.16 0
13 0.24 0
14 0.26 0
16
19 0.33
0.34
Table 2
Continuity Testing
[0096] The utilization of embodiments of biodegradable batteries having
barrier layers as
described herein presents the possible issue of impacting the continuity of
the battery tabs,
particularly with the use of heat seal across the tabs. This was evaluated by
assembling several
full sized batteries using aluminized PLA as an external barrier layer,
similar to Example 2. A
lower temperature of 135 C for the MS K sealer was used to prevent unnecessary
heat exposure
and potential damage to the battery tab while still providing a strong seal.
The MSK sealer was
set to the lower temperature of 135 C in soft mode for a longer time of 6
seconds. The device
was found to be fully functional with no loss of continuity across the tabs
after the heat sealing.
[0097] The present disclosure has been described with reference to exemplary
implementations. Although a limited number of implementations have been shown
and
described, it will be appreciated by those skilled in the art that changes may
be made in these
implementations without departing from the principles and spirit of the
preceding detailed
description. It is intended that the present disclosure be construed as
including all such
31
CA 03213871 2023-09-15
WO 2022/212454
PCT/US2022/022475
modifications and alterations insofar as they come within the scope of the
appended claims or
the equivalents thereof.
32