Note: Descriptions are shown in the official language in which they were submitted.
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
MISCARRIAGE IDENTIFICATION AND PREDICTION FROM WEARABLE-
BASED PHYSIOLOGICAL DATA
CROSS REFERENCE
[0001] The present Application for Patent claims the benefit of U.S. Non-
Provisional Patent Application No. 17/710,045 by Thigpen et al., entitled
"MISCARRIAGE IDENTIFICATION AND PREDICTION FROM WEARABLE-
BASED PHYSIOLOGICAL DATA," filed March 31, 2022, which claims the benefit of
U.S. Provisional Patent Application No. 63/169,314 by Aschbacher et al.,
entitled
"WOMEN'S HEALTH TRACKING," filed April 1, 2021, each of which is assigned to
the assignee hereof, and expressly incorporated by reference herein.
FIELD OF TECHNOLOGY
[0002] The following relates to wearable devices and data processing,
including
miscarriage identification and prediction from wearable-based physiological
data.
BACKGROUND
[0003] Some wearable devices may be configured to collect data from users
associated with body temperature and heart rate. For example, some wearable
devices
may be configured to detect cycles associated with reproductive health.
However,
conventional cycle detection techniques implemented by wearable devices are
deficient.
BRIEF DESCRIPTION OF THE DRAWINGS
[0004] FIG. 1 illustrates an example of a system that supports miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure.
[0005] FIG. 2 illustrates an example of a system that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure.
[0006] FIG. 3 illustrates an example of a system that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure.
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
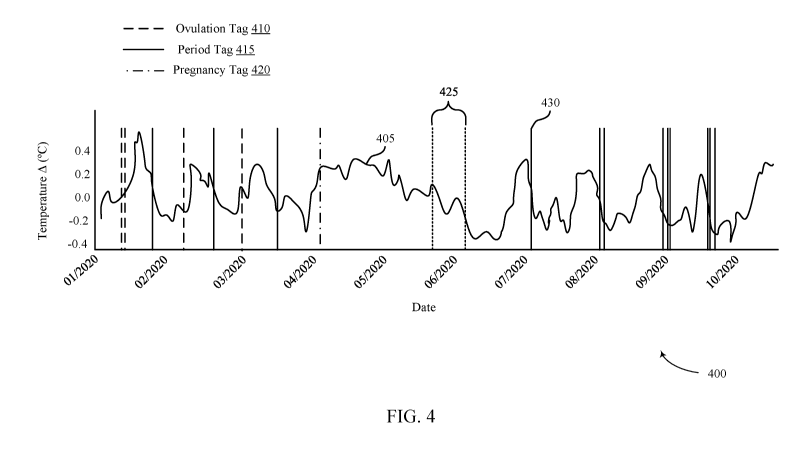
[0007] FIG. 4 illustrates an example of a timing diagram that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure.
[0008] FIG. 5 illustrates an example of a timing diagram that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure.
[0009] FIG. 6 illustrates an example of a graphical user interface (GUI)
that
supports miscarriage identification and prediction from wearable-based
physiological
data in accordance with aspects of the present disclosure.
[0010] FIG. 7 shows a block diagram of an apparatus that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure.
[0011] FIG. 8 shows a block diagram of a wearable application that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure.
[0012] FIG. 9 shows a diagram of a system including a device that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure.
[0013] FIGs. 10 through 12 show flowcharts illustrating methods that
support
.. miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure.
DETAILED DESCRIPTION
[0014] Some wearable devices may be configured to collect physiological
data from
users, including temperature data, heart rate data, and the like. Acquired
physiological
data may be used to analyze the user's movement and other activities, such as
sleeping
patterns. Many users have a desire for more insight regarding their physical
health,
including their sleeping patterns, activity, and overall physical well-being.
In particular,
many users may have a desire for more insight regarding women's health,
including
their menstrual cycle, ovulation, fertility patterns, and pregnancy. However,
typical
2
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
cycle tracking or women's health devices and applications lack the ability to
provide
robust prediction and insight for several reasons.
[0015] First, typical cycle prediction applications require users to
manually take
their temperature with a device at a discrete time each day. This single
temperature data
.. point may not provide sufficient context to accurately capture or predict
the true
temperature variations indicative of woman's health cycle patterns and
pregnancy
patterns, and may be difficult to accurately capture given the sensitivity of
the
measuring device to user movement or exertion. Second, even for devices that
are
wearable or that take a user's temperature more frequently throughout the day,
typical
devices and applications lack the ability to collect other physiological,
behavioral, or
contextual inputs from the user that can be combined with the measured
temperature to
more comprehensively understand the complete set of physiological contributors
to a
women's cycle and pregnancy.
[0016] Aspects of the present disclosure are directed to techniques for
identifying an
indication of an early pregnancy loss. In particular, computing devices of the
present
disclosure may receive physiological data including temperature data, from the
wearable device associated with the user and determine a time series of
temperature
values taken over a plurality of days. The physiological data may be
associated with a
user who is pregnant. For example, aspects of the present disclosure may
identify one or
more morphological features from a graphical representation of the time series
of
temperature values, such as deviations of the time series of temperature
values relative
to a pregnancy baseline of temperature values for the user. As such, aspects
of the
present disclosure detect an indication of an early pregnancy loss of the user
based on
identifying the morphological features (e.g., deviations). In such cases, an
indication of
an early pregnancy loss may be associated with temperature values that are
lower than
the pregnancy baseline of temperature values of the user. The indication of
early
pregnancy loss may be an example of detecting that the early pregnancy loss
has already
happened, is currently happening, and/or that the early pregnancy loss is
predicted to
happen in the future.
[0017] In some implementations, the system may analyze historical
temperature
data from a user and pregnancy baseline values of the user and identify an
indication of
the early pregnancy loss and may generate an indication that indicates the
user's early
3
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
pregnancy loss. The user may confirm whether the early pregnancy loss has
already
occurred as indicated by the system, and the system may incorporate this user
input into
a predictive function (e.g., a machine learning model for predicting a future
early
pregnancy loss). The system may also analyze temperature series data in real
time and
may predict an upcoming early pregnancy loss based on identifying one or more
morphological features in the time series of the temperature data and/or based
on the
user's input.
[0018] For the purposes of the present disclosure, the term "early
pregnancy loss"
may be used to refer to a spontaneous loss of a user's pregnancy before the
twentieth
week of pregnancy. An early pregnancy loss (e.g., miscarriage) begins with a
loss of
blood, fluid, or tissue and/or pain in the belly or lower back. For example, a
user may be
experiencing a miscarriage when the user's body discards the fetus from the
womb
before the fetus is able to survive independently.
[0019] Some aspects of the present disclosure are directed to the
detection of the
early pregnancy loss before the user experiences symptoms and effects of the
menstrual
cycle early pregnancy loss. However, techniques described herein may also be
used to
detect the early pregnancy loss in cases where the user does not become
symptomatic,
or does not become aware of their symptoms. In some implementations, the
computing
devices may identify an indication of the early pregnancy loss using a
temperature
sensor. In such cases, the computing devices may estimate the retrospective
dates of the
early pregnancy loss without the user tagging or labeling these events.
[0020] In conventional systems, an early pregnancy loss may be detected
by using a
fetal doppler and/or ultrasound after the early pregnancy loss has occurred.
In other
cases, an early pregnancy loss may be detected based on symptoms experienced
by the
user (e.g., cramping, bleeding, pain, etc.). In such cases, the early
pregnancy loss may
be detected after occurrence and/or confirmed at an appointment with the
clinician.
Techniques described herein may continuously collect the physiological data
from the
user based on measurements taken from a wearable that continuously measures a
user's
surface temperature and signals extracted from blood flow such as arterial
blood flow
(e.g., via PPG signal). In some implementations, the computing devices may
sample the
user's temperature continuously throughout the day and night. Sampling at a
sufficient
4
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
rate (e.g., one sample per minute) throughout the night may provide sufficient
temperature data for analysis described herein.
[0021] In some cases, continuous temperature measurement at the finger
may
capture temperature fluctuations (e.g., small or large fluctuations) that may
not be
evident in core temperature. For example, continuous temperature measurement
at the
finger may capture minute-to-minute or hour-to-hour temperature fluctuations
that
provide additional insight that may not be provided by other temperature
measurements
elsewhere in the body or if the user were manually taking their temperature
once per
day. As such, data collected by the computing devices may be used to identify
when the
user experiences an early pregnancy loss.
[0022] Techniques described herein may notify a user, clinician,
fertility specialist,
care-giver, or a combination thereof of the indication of the early pregnancy
loss in a
variety of ways. For example, a system may generate a message for display on a
graphical user interface (GUI) of a user device that indicates the indication
of the early
.. pregnancy loss. In such cases, the system may cause the GUI of a user
device to display
a message or other notification to notify the user, clinician, etc. of the
detected early
pregnancy loss, notify the user of an estimated likelihood of a future early
pregnancy
loss, make recommendations to the user, and the like. In some implementations,
the
system may make tag recommendations to a user. For example, the system may
.. recommend early pregnancy symptom tags (e.g., cramps, back pain) to users
in a
personalized manner.
[0023] The system may also include graphics or text that indicate the
data used to
make the detection/prediction of a likely pregnancy loss. For example, the GUI
may
display a notification of the likelihood of an early pregnancy loss based on
temperature
deviations from a pregnancy baseline of the user. In some cases, the GUI may
display a
notification of the likelihood of an early pregnancy loss based on heart rate
deviations
from a normal baseline, breath rate deviations from a normal baseline, heart
rate
variability (HRV) from a normal baseline, or a combination thereof Based on
the early
detection (e.g., before the user experiences symptoms), a user may take early
steps that
.. may help reduce the severity of upcoming symptoms associated with the early
pregnancy loss or limit the risk of having an early pregnancy loss altogether.
5
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0024] Aspects of the disclosure are initially described in the context
of systems
supporting physiological data collection from users via wearable devices.
Additional
aspects of the disclosure are described in the context of example timing
diagrams and
example GUIs. Aspects of the disclosure are further illustrated by and
described with
reference to apparatus diagrams, system diagrams, and flowcharts that relate
to
miscarriage identification and prediction from wearable-based physiological
data.
[0025] FIG. 1 illustrates an example of a system 100 that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure. The system 100 includes a plurality of
electronic
devices (e.g., wearable devices 104, user devices 106) that may be worn and/or
operated
by one or more users 102. The system 100 further includes a network 108 and
one or
more servers 110.
[0026] The electronic devices may include any electronic devices known
in the art,
including wearable devices 104 (e.g., ring wearable devices, watch wearable
devices,
etc.), user devices 106 (e.g., smartphones, laptops, tablets). The electronic
devices
associated with the respective users 102 may include one or more of the
following
functionalities: 1) measuring physiological data, 2) storing the measured
data, 3)
processing the data, 4) providing outputs (e.g., via GUIs) to a user 102 based
on the
processed data, and 5) communicating data with one another and/or other
computing
devices. Different electronic devices may perform one or more of the
functionalities.
[0027] Example wearable devices 104 may include wearable computing
devices,
such as a ring computing device (hereinafter "ring") configured to be worn on
a user's
102 finger, a wrist computing device (e.g., a smart watch, fitness band, or
bracelet)
configured to be worn on a user's 102 wrist, and/or a head mounted computing
device
(e.g., glasses/goggles). Wearable devices 104 may also include bands, straps
(e.g.,
flexible or inflexible bands or straps), stick-on sensors, and the like, that
may be
positioned in other locations, such as bands around the head (e.g., a forehead
headband),
arm (e.g., a forearm band and/or bicep band), and/or leg (e.g., a thigh or
calf band),
behind the ear, under the armpit, and the like. Wearable devices 104 may also
be
attached to, or included in, articles of clothing. For example, wearable
devices 104 may
be included in pockets and/or pouches on clothing. As another example,
wearable
device 104 may be clipped and/or pinned to clothing, or may otherwise be
maintained
6
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
within the vicinity of the user 102. Example articles of clothing may include,
but are not
limited to, hats, shirts, gloves, pants, socks, outerwear (e.g., jackets), and
undergarments. In some implementations, wearable devices 104 may be included
with
other types of devices such as training/sporting devices that are used during
physical
activity. For example, wearable devices 104 may be attached to, or included
in, a
bicycle, skis, a tennis racket, a golf club, and/or training weights.
[0028] Much of the present disclosure may be described in the context of
a ring
wearable device 104. Accordingly, the terms "ring 104," "wearable device 104,"
and
like terms, may be used interchangeably, unless noted otherwise herein.
However, the
.. use of the term "ring 104" is not to be regarded as limiting, as it is
contemplated herein
that aspects of the present disclosure may be performed using other wearable
devices
(e.g., watch wearable devices, necklace wearable device, bracelet wearable
devices,
earring wearable devices, anklet wearable devices, and the like).
[0029] In some aspects, user devices 106 may include handheld mobile
computing
devices, such as smartphones and tablet computing devices. User devices 106
may also
include personal computers, such as laptop and desktop computing devices.
Other
example user devices 106 may include server computing devices that may
communicate
with other electronic devices (e.g., via the Internet). In some
implementations,
computing devices may include medical devices, such as external wearable
computing
.. devices (e.g., Holter monitors). Medical devices may also include
implantable medical
devices, such as pacemakers and cardioverter defibrillators. Other example
user devices
106 may include home computing devices, such as internet of things (IoT)
devices (e.g.,
IoT devices), smart televisions, smart speakers, smart displays (e.g., video
call
displays), hubs (e.g., wireless communication hubs), security systems, smart
appliances
(e.g., thermostats and refrigerators), and fitness equipment.
[0030] Some electronic devices (e.g., wearable devices 104, user devices
106) may
measure physiological parameters of respective users 102, such as
photoplethysmography waveforms, continuous skin temperature, a pulse waveform,
respiration rate, heart rate, HRV, actigraphy, galvanic skin response, pulse
oximetry,
and/or other physiological parameters. Some electronic devices that measure
physiological parameters may also perform some/all of the calculations
described
herein. Some electronic devices may not measure physiological parameters, but
may
7
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
perform some/all of the calculations described herein. For example, a ring
(e.g.,
wearable device 104), mobile device application, or a server computing device
may
process received physiological data that was measured by other devices.
[0031] In some implementations, a user 102 may operate, or may be
associated
with, multiple electronic devices, some of which may measure physiological
parameters
and some of which may process the measured physiological parameters. In some
implementations, a user 102 may have a ring (e.g., wearable device 104) that
measures
physiological parameters. The user 102 may also have, or be associated with, a
user
device 106 (e.g., mobile device, smartphone), where the wearable device 104
and the
user device 106 are communicatively coupled to one another. In some cases, the
user
device 106 may receive data from the wearable device 104 and perform some/all
of the
calculations described herein. In some implementations, the user device 106
may also
measure physiological parameters described herein, such as motion/activity
parameters.
[0032] For example, as illustrated in FIG. 1, a first user 102-a (User
1) may operate,
or may be associated with, a wearable device 104-a (e.g., ring 104-a) and a
user device
106-a that may operate as described herein. In this example, the user device
106-a
associated with user 102-a may process/store physiological parameters measured
by the
ring 104-a. Comparatively, a second user 102-b (User 2) may be associated with
a ring
104-b, a watch wearable device 104-c (e.g., watch 104-c), and a user device
106-b,
where the user device 106-b associated with user 102-b may process/store
physiological
parameters measured by the ring 104-b and/or the watch 104-c. Moreover, an nth
user
102-n (User N) may be associated with an arrangement of electronic devices
described
herein (e.g., ring 104-n, user device 106-n). In some aspects, wearable
devices 104 (e.g.,
rings 104, watches 104) and other electronic devices may be communicatively
coupled
to the user devices 106 of the respective users 102 via Bluetooth, Wi-Fi, and
other
wireless protocols.
[0033] In some implementations, the rings 104 (e.g., wearable devices
104) of the
system 100 may be configured to collect physiological data from the respective
users
102 based on arterial blood flow within the user's finger. In particular, a
ring 104 may
.. utilize one or more LEDs (e.g., red LEDs, green LEDs) that emit light on
the palm-side
of a user's finger to collect physiological data based on arterial blood flow
within the
user's finger. In some implementations, the ring 104 may acquire the
physiological data
8
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
using a combination of both green and red LEDs. The physiological data may
include
any physiological data known in the art including, but not limited to,
temperature data,
accelerometer data (e.g., movement/motion data), heart rate data, HRV data,
blood
oxygen level data, or any combination thereof
[0034] The use of both green and red LEDs may provide several advantages
over
other solutions, as red and green LEDs have been found to have their own
distinct
advantages when acquiring physiological data under different conditions (e.g.,
light/dark, active/inactive) and via different parts of the body, and the
like. For example,
green LEDs have been found to exhibit better performance during exercise.
Moreover,
using multiple LEDs (e.g., green and red LEDs) distributed around the ring 104
has
been found to exhibit superior performance as compared to wearable devices
that utilize
LEDs that are positioned close to one another, such as within a watch wearable
device.
Furthermore, the blood vessels in the finger (e.g., arteries, capillaries) are
more
accessible via LEDs as compared to blood vessels in the wrist. In particular,
arteries in
the wrist are positioned on the bottom of the wrist (e.g., palm-side of the
wrist),
meaning only capillaries are accessible on the top of the wrist (e.g., back of
hand side of
the wrist), where wearable watch devices and similar devices are typically
worn. As
such, utilizing LEDs and other sensors within a ring 104 has been found to
exhibit
superior performance as compared to wearable devices worn on the wrist, as the
ring
.. 104 may have greater access to arteries (as compared to capillaries),
thereby resulting in
stronger signals and more valuable physiological data.
[0035] The electronic devices of the system 100 (e.g., user devices 106,
wearable
devices 104) may be communicatively coupled to one or more servers 110 via
wired or
wireless communication protocols. For example, as shown in FIG. 1, the
electronic
devices (e.g., user devices 106) may be communicatively coupled to one or more
servers 110 via a network 108. The network 108 may implement transfer control
protocol and internet protocol (TCP/IP), such as the Internet, or may
implement other
network 108 protocols. Network connections between the network 108 and the
respective electronic devices may facilitate transport of data via email, web,
text
messages, mail, or any other appropriate form of interaction within a computer
network
108. For example, in some implementations, the ring 104-a associated with the
first user
102-a may be communicatively coupled to the user device 106-a, where the user
device
9
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
106-a is communicatively coupled to the servers 110 via the network 108. In
additional
or alternative cases, wearable devices 104 (e.g., rings 104, watches 104) may
be directly
communicatively coupled to the network 108.
[0036] The system 100 may offer an on-demand database service between
the user
devices 106 and the one or more servers 110. In some cases, the servers 110
may
receive data from the user devices 106 via the network 108, and may store and
analyze
the data. Similarly, the servers 110 may provide data to the user devices 106
via the
network 108. In some cases, the servers 110 may be located at one or more data
centers.
The servers 110 may be used for data storage, management, and processing. In
some
implementations, the servers 110 may provide a web-based interface to the user
device
106 via web browsers.
[0037] In some aspects, the system 100 may detect periods of time during
which a
user 102 is asleep, and classify periods of time during which the user 102 is
asleep into
one or more sleep stages (e.g., sleep stage classification). For example, as
shown in
FIG. 1, User 102-a may be associated with a wearable device 104-a (e.g., ring
104-a)
and a user device 106-a. In this example, the ring 104-a may collect
physiological data
associated with the user 102-a, including temperature, heart rate, HRV,
respiratory rate,
and the like. In some aspects, data collected by the ring 104-a may be input
to a
machine learning classifier, where the machine learning classifier is
configured to
determine periods of time during which the user 102-a is (or was) asleep.
Moreover, the
machine learning classifier may be configured to classify periods of time into
different
sleep stages, including an awake sleep stage, a rapid eye movement (REM) sleep
stage,
a light sleep stage (non-REM (NREM)), and a deep sleep stage (NREM). In some
aspects, the classified sleep stages may be displayed to the user 102-a via a
GUI of the
user device 106-a. Sleep stage classification may be used to provide feedback
to a user
102-a regarding the user's sleeping patterns, such as recommended bedtimes,
recommended wake-up times, and the like. Moreover, in some implementations,
sleep
stage classification techniques described herein may be used to calculate
scores for the
respective user, such as Sleep Scores, Readiness Scores, and the like.
[0038] In some aspects, the system 100 may utilize circadian rhythm-derived
features to further improve physiological data collection, data processing
procedures,
and other techniques described herein. The term circadian rhythm may refer to
a natural,
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
internal process that regulates an individual's sleep-wake cycle, that repeats
approximately every 24 hours. In this regard, techniques described herein may
utilize
circadian rhythm adjustment models to improve physiological data collection,
analysis,
and data processing. For example, a circadian rhythm adjustment model may be
input
into a machine learning classifier along with physiological data collected
from the user
102-a via the wearable device 104-a. In this example, the circadian rhythm
adjustment
model may be configured to "weight," or adjust, physiological data collected
throughout
a user's natural, approximately 24-hour circadian rhythm. In some
implementations, the
system may initially start with a "baseline" circadian rhythm adjustment
model, and
may modify the baseline model using physiological data collected from each
user 102 to
generate tailored, individualized circadian rhythm adjustment models that are
specific to
each respective user 102.
[0039] In some aspects, the system 100 may utilize other biological
rhythms to
further improve physiological data collection, analysis, and processing by
phase of these
other rhythms. For example, if a weekly rhythm is detected within an
individual's
baseline data, then the model may be configured to adjust "weights" of data by
day of
the week. Biological rhythms that may require adjustment to the model by this
method
include: 1) ultradian (faster than a day rhythms, including sleep cycles in a
sleep state,
and oscillations from less than an hour to several hours periodicity in the
measured
physiological variables during wake state; 2) circadian rhythms; 3) non-
endogenous
daily rhythms shown to be imposed on top of circadian rhythms, as in work
schedules;
4) weekly rhythms, or other artificial time periodicities exogenously imposed
(e.g. in a
hypothetical culture with 12 day "weeks", 12 day rhythms could be used); 5)
multi-day
ovarian rhythms in women and spermatogenesis rhythms in men; 6) lunar rhythms
(relevant for individuals living with low or no artificial lights); and 7)
seasonal rhythms.
[0040] The biological rhythms are not always stationary rhythms. For
example,
many women experience variability in ovarian cycle length across cycles, and
ultradian
rhythms are not expected to occur at exactly the same time or periodicity
across days
even within a user. As such, signal processing techniques sufficient to
quantify the
frequency composition while preserving temporal resolution of these rhythms in
physiological data may be used to improve detection of these rhythms, to
assign phase
of each rhythm to each moment in time measured, and to thereby modify
adjustment
11
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
models and comparisons of time intervals. The biological rhythm-adjustment
models
and parameters can be added in linear or non-linear combinations as
appropriate to more
accurately capture the dynamic physiological baselines of an individual or
group of
individuals.
[0041] In some aspects, the respective devices of the system 100 may
support
techniques for miscarriage identification and prediction based on data
collected by a
wearable device 104. In particular, the system 100 illustrated in FIG. 1 may
support
techniques for detecting the indication of the early pregnancy loss of a user
102, and
causing a user device 106 corresponding to the user 102 to display the
indication of the
early pregnancy loss. The indication of early pregnancy loss may be an example
of
detecting that the early pregnancy loss has already happened, detecting that
the early
pregnancy loss is currently happening, and/or that the early pregnancy loss is
predicted
to occur in the future.
[0042] For example, as shown in FIG. 1, User 1 (user 102-a) may be
associated with
a wearable device 104-a (e.g., ring 104-a) and a user device 106-a. In this
example, the
ring 104-a may collect data associated with the user 102-a, including
temperature, heart
rate, HRV, respiratory rate, and the like. In some aspects, data collected by
the ring 104-
a may be used to detect the indication of the early pregnancy loss during
which User 1
experiences a miscarriage. Identifying and/or predicting the early pregnancy
loss may
be performed by any of the components of the system 100, including the ring
104-a, the
user device 106-a associated with User 1, the one or more servers 110, or any
combination thereof Upon identifying and/or predicting the early pregnancy
loss, the
system 100 may selectively cause the GUI of the user device 106 to display the
indication of the early pregnancy loss. In such cases, the user device 106 may
be
associated with User 1, User 2, User N, or a combination thereof where User 2
and User
N may be an example of a clinician, a caregiver, a user associated with User
1, or a
combination thereof
[0043] In some implementations, upon receiving physiological data (e.g.,
including
temperature data), the system 100 may determine a time series of temperature
values
taken over a plurality of days. The system 100 may identify that the
temperature values
are lower than a pregnancy baseline of temperature values for the user. As
described in
more detail herein, a pregnancy baseline may refer to a baseline or average
temperature,
12
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
or usual temperature variations for the user as measured throughout pregnancy
or
specific phases of pregnancy, which may differ from the user's normal or non-
pregnant
baselines. In such cases, the system 100 may detect the indication of the
early
pregnancy loss of the user based on identifying that the temperature values
are lower
than the pregnancy baseline of temperature values for the user.
[0044] In some implementations, the system 100 may generate alerts,
messages, or
recommendations for User 1, User, 2, and/or User N (e.g., via the ring 104-a,
user
device 106-a, or both) based on the detected indication of early pregnancy
loss, where
the messages may provide insights regarding the detected indication of early
pregnancy
.. loss, such as a timing of the early pregnancy loss. In some cases, the
messages may
provide insight regarding symptoms associated with the early pregnancy loss,
educational videos and/or text (e.g., content) associated with the early
pregnancy loss,
recommendations to improve symptoms associated with the early pregnancy loss,
or a
combination thereof
[0045] It should be appreciated by a person skilled in the art that one or
more
aspects of the disclosure may be implemented in a system 100 to additionally
or
alternatively solve other problems than those described above. Furthermore,
aspects of
the disclosure may provide technical improvements to "conventional" systems or
processes as described herein. However, the description and appended drawings
only
include example technical improvements resulting from implementing aspects of
the
disclosure, and accordingly do not represent all of the technical improvements
provided
within the scope of the claims.
[0046] FIG. 2 illustrates an example of a system 200 that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure. The system 200 may implement, or be
implemented
by, system 100. In particular, system 200 illustrates an example of a ring 104
(e.g.,
wearable device 104), a user device 106, and a server 110, as described with
reference
to FIG. 1.
[0047] In some aspects, the ring 104 may be configured to be worn around
a user's
finger, and may determine one or more user physiological parameters when worn
around the user's finger. Example measurements and determinations may include,
but
13
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
are not limited to, user skin temperature, pulse waveforms, respiratory rate,
heart rate,
HRV, blood oxygen levels, and the like.
[0048] System 200 further includes a user device 106 (e.g., a
smartphone) in
communication with the ring 104. For example, the ring 104 may be in wireless
and/or
wired communication with the user device 106. In some implementations, the
ring 104
may send measured and processed data (e.g., temperature data,
photoplethysmogram
(PPG) data, motion/accelerometer data, ring input data, and the like) to the
user device
106. The user device 106 may also send data to the ring 104, such as ring 104
firmware/configuration updates. The user device 106 may process data. In some
implementations, the user device 106 may transmit data to the server 110 for
processing
and/or storage.
[0049] The ring 104 may include a housing 205 that may include an inner
housing
205-a and an outer housing 205-b. In some aspects, the housing 205 of the ring
104 may
store or otherwise include various components of the ring including, but not
limited to,
device electronics, a power source (e.g., battery 210, and/or capacitor), one
or more
substrates (e.g., printable circuit boards) that interconnect the device
electronics and/or
power source, and the like. The device electronics may include device modules
(e.g.,
hardware/software), such as: a processing module 230-a, a memory 215, a
communication module 220-a, a power module 225, and the like. The device
electronics
may also include one or more sensors. Example sensors may include one or more
temperature sensors 240, a PPG sensor assembly (e.g., PPG system 235), and one
or
more motion sensors 245.
[0050] The sensors may include associated modules (not illustrated)
configured to
communicate with the respective components/modules of the ring 104, and
generate
signals associated with the respective sensors. In some aspects, each of the
components/modules of the ring 104 may be communicatively coupled to one
another
via wired or wireless connections. Moreover, the ring 104 may include
additional and/or
alternative sensors or other components that are configured to collect
physiological data
from the user, including light sensors (e.g., LEDs), oximeters, and the like.
[0051] The ring 104 shown and described with reference to FIG. 2 is
provided
solely for illustrative purposes. As such, the ring 104 may include additional
or
14
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
alternative components as those illustrated in FIG. 2. Other rings 104 that
provide
functionality described herein may be fabricated. For example, rings 104 with
fewer
components (e.g., sensors) may be fabricated. In a specific example, a ring
104 with a
single temperature sensor 240 (or other sensor), a power source, and device
electronics
configured to read the single temperature sensor 240 (or other sensor) may be
fabricated. In another specific example, a temperature sensor 240 (or other
sensor) may
be attached to a user's finger (e.g., using a clamps, spring loaded clamps,
etc.). In this
case, the sensor may be wired to another computing device, such as a wrist
worn
computing device that reads the temperature sensor 240 (or other sensor). In
other
examples, a ring 104 that includes additional sensors and processing
functionality may
be fabricated.
[0052] The housing 205 may include one or more housing 205 components.
The
housing 205 may include an outer housing 205-b component (e.g., a shell) and
an inner
housing 205-a component (e.g., a molding). The housing 205 may include
additional
components (e.g., additional layers) not explicitly illustrated in FIG. 2. For
example, in
some implementations, the ring 104 may include one or more insulating layers
that
electrically insulate the device electronics and other conductive materials
(e.g.,
electrical traces) from the outer housing 205-b (e.g., a metal outer housing
205-b). The
housing 205 may provide structural support for the device electronics, battery
210,
substrate(s), and other components. For example, the housing 205 may protect
the
device electronics, battery 210, and substrate(s) from mechanical forces, such
as
pressure and impacts. The housing 205 may also protect the device electronics,
battery
210, and substrate(s) from water and/or other chemicals.
[0053] The outer housing 205-b may be fabricated from one or more
materials. In
some implementations, the outer housing 205-b may include a metal, such as
titanium,
that may provide strength and abrasion resistance at a relatively light
weight. The outer
housing 205-b may also be fabricated from other materials, such polymers. In
some
implementations, the outer housing 205-b may be protective as well as
decorative.
[0054] The inner housing 205-a may be configured to interface with the
user's
finger. The inner housing 205-a may be formed from a polymer (e.g., a medical
grade
polymer) or other material. In some implementations, the inner housing 205-a
may be
transparent. For example, the inner housing 205-a may be transparent to light
emitted by
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
the PPG light emitting diodes (LEDs). In some implementations, the inner
housing
205-a component may be molded onto the outer housing 205-b. For example, the
inner
housing 205-a may include a polymer that is molded (e.g., injection molded) to
fit into
an outer housing 205-b metallic shell.
[0055] The ring 104 may include one or more substrates (not illustrated).
The
device electronics and battery 210 may be included on the one or more
substrates. For
example, the device electronics and battery 210 may be mounted on one or more
substrates. Example substrates may include one or more printed circuit boards
(PCBs),
such as flexible PCB (e.g., polyimide). In some implementations, the
electronics/battery
210 may include surface mounted devices (e.g., surface-mount technology (SMT)
devices) on a flexible PCB. In some implementations, the one or more
substrates (e.g.,
one or more flexible PCBs) may include electrical traces that provide
electrical
communication between device electronics. The electrical traces may also
connect the
battery 210 to the device electronics.
[0056] The device electronics, battery 210, and substrates may be arranged
in the
ring 104 in a variety of ways. In some implementations, one substrate that
includes
device electronics may be mounted along the bottom of the ring 104 (e.g., the
bottom
half), such that the sensors (e.g., PPG system 235, temperature sensors 240,
motion
sensors 245, and other sensors) interface with the underside of the user's
finger. In these
implementations, the battery 210 may be included along the top portion of the
ring 104
(e.g., on another substrate).
[0057] The various components/modules of the ring 104 represent
functionality
(e.g., circuits and other components) that may be included in the ring 104.
Modules may
include any discrete and/or integrated electronic circuit components that
implement
analog and/or digital circuits capable of producing the functions attributed
to the
modules herein. For example, the modules may include analog circuits (e.g.,
amplification circuits, filtering circuits, analog/digital conversion
circuits, and/or other
signal conditioning circuits). The modules may also include digital circuits
(e.g.,
combinational or sequential logic circuits, memory circuits etc.).
[0058] The memory 215 (memory module) of the ring 104 may include any
volatile,
non-volatile, magnetic, or electrical media, such as a random access memory
(RAM),
16
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
read-only memory (ROM), non-volatile RAM (NVRAM), electrically-erasable
programmable ROM (EEPROM), flash memory, or any other memory device. The
memory 215 may store any of the data described herein. For example, the memory
215
may be configured to store data (e.g., motion data, temperature data, PPG
data)
collected by the respective sensors and PPG system 235. Furthermore, memory
215 may
include instructions that, when executed by one or more processing circuits,
cause the
modules to perform various functions attributed to the modules herein. The
device
electronics of the ring 104 described herein are only example device
electronics. As
such, the types of electronic components used to implement the device
electronics may
.. vary based on design considerations.
[0059] The functions attributed to the modules of the ring 104 described
herein may
be embodied as one or more processors, hardware, firmware, software, or any
combination thereof Depiction of different features as modules is intended to
highlight
different functional aspects and does not necessarily imply that such modules
must be
realized by separate hardware/software components. Rather, functionality
associated
with one or more modules may be performed by separate hardware/software
components or integrated within common hardware/software components.
[0060] The processing module 230-a of the ring 104 may include one or
more
processors (e.g., processing units), microcontrollers, digital signal
processors, systems
on a chip (SOCs), and/or other processing devices. The processing module 230-a
communicates with the modules included in the ring 104. For example, the
processing
module 230-a may transmit/receive data to/from the modules and other
components of
the ring 104, such as the sensors. As described herein, the modules may be
implemented
by various circuit components. Accordingly, the modules may also be referred
to as
circuits (e.g., a communication circuit and power circuit).
[0061] The processing module 230-a may communicate with the memory 215.
The
memory 215 may include computer-readable instructions that, when executed by
the
processing module 230-a, cause the processing module 230-a to perform the
various
functions attributed to the processing module 230-a herein. In some
implementations,
the processing module 230-a (e.g., a microcontroller) may include additional
features
associated with other modules, such as communication functionality provided by
the
17
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
communication module 220-a (e.g., an integrated Bluetooth Low Energy
transceiver)
and/or additional onboard memory 215.
[0062] The communication module 220-a may include circuits that provide
wireless
and/or wired communication with the user device 106 (e.g., communication
module
220-b of the user device 106). In some implementations, the communication
modules
220-a, 220-b may include wireless communication circuits, such as Bluetooth
circuits
and/or Wi-Fi circuits. In some implementations, the communication modules 220-
a,
220-b can include wired communication circuits, such as Universal Serial Bus
(USB)
communication circuits. Using the communication module 220-a, the ring 104 and
the
user device 106 may be configured to communicate with each other. The
processing
module 230-a of the ring may be configured to transmit/receive data to/from
the user
device 106 via the communication module 220-a. Example data may include, but
is not
limited to, motion data, temperature data, pulse waveforms, heart rate data,
HRV data,
PPG data, and status updates (e.g., charging status, battery charge level,
and/or ring 104
configuration settings). The processing module 230-a of the ring may also be
configured
to receive updates (e.g., software/firmware updates) and data from the user
device 106.
[0063] The ring 104 may include a battery 210 (e.g., a rechargeable
battery 210).
An example battery 210 may include a Lithium-Ion or Lithium-Polymer type
battery
210, although a variety of battery 210 options are possible. The battery 210
may be
wirelessly charged. In some implementations, the ring 104 may include a power
source
other than the battery 210, such as a capacitor. The power source (e.g.,
battery 210 or
capacitor) may have a curved geometry that matches the curve of the ring 104.
In some
aspects, a charger or other power source may include additional sensors that
may be
used to collect data in addition to, or which supplements, data collected by
the ring 104
itself Moreover, a charger or other power source for the ring 104 may function
as a user
device 106, in which case the charger or other power source for the ring 104
may be
configured to receive data from the ring 104, store and/or process data
received from the
ring 104, and communicate data between the ring 104 and the servers 110.
[0064] In some aspects, the ring 104 includes a power module 225 that
may control
charging of the battery 210. For example, the power module 225 may interface
with an
external wireless charger that charges the battery 210 when interfaced with
the ring 104.
The charger may include a datum structure that mates with a ring 104 datum
structure to
18
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
create a specified orientation with the ring 104 during 104 charging. The
power module
225 may also regulate voltage(s) of the device electronics, regulate power
output to the
device electronics, and monitor the state of charge of the battery 210. In
some
implementations, the battery 210 may include a protection circuit module (PCM)
that
protects the battery 210 from high current discharge, over voltage during 104
charging,
and under voltage during 104 discharge. The power module 225 may also include
electro-static discharge (ESD) protection.
[0065] The one or more temperature sensors 240 may be electrically
coupled to the
processing module 230-a. The temperature sensor 240 may be configured to
generate a
temperature signal (e.g., temperature data) that indicates a temperature read
or sensed
by the temperature sensor 240. The processing module 230-a may determine a
temperature of the user in the location of the temperature sensor 240. For
example, in
the ring 104, temperature data generated by the temperature sensor 240 may
indicate a
temperature of a user at the user's finger (e.g., skin temperature). In some
implementations, the temperature sensor 240 may contact the user's skin. In
other
implementations, a portion of the housing 205 (e.g., the inner housing 205-a)
may form
a barrier (e.g., a thin, thermally conductive barrier) between the temperature
sensor 240
and the user's skin. In some implementations, portions of the ring 104
configured to
contact the user's finger may have thermally conductive portions and thermally
insulative portions. The thermally conductive portions may conduct heat from
the user's
finger to the temperature sensors 240. The thermally insulative portions may
insulate
portions of the ring 104 (e.g., the temperature sensor 240) from ambient
temperature.
[0066] In some implementations, the temperature sensor 240 may generate
a digital
signal (e.g., temperature data) that the processing module 230-a may use to
determine
the temperature. As another example, in cases where the temperature sensor 240
includes a passive sensor, the processing module 230-a (or a temperature
sensor 240
module) may measure a current/voltage generated by the temperature sensor 240
and
determine the temperature based on the measured current/voltage. Example
temperature
sensors 240 may include a thermistor, such as a negative temperature
coefficient (NTC)
thermistor, or other types of sensors including resistors, transistors,
diodes, and/or other
electrical/electronic components.
19
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0067] The processing module 230-a may sample the user's temperature
over time.
For example, the processing module 230-a may sample the user's temperature
according
to a sampling rate. An example sampling rate may include one sample per
second,
although the processing module 230-a may be configured to sample the
temperature
signal at other sampling rates that are higher or lower than one sample per
second. In
some implementations, the processing module 230-a may sample the user's
temperature
continuously throughout the day and night. Sampling at a sufficient rate
(e.g., one
sample per second, one sample per minute, and the like) throughout the day may
provide sufficient temperature data for analysis described herein.
[0068] The processing module 230-a may store the sampled temperature data
in
memory 215. In some implementations, the processing module 230-a may process
the
sampled temperature data. For example, the processing module 230-a may
determine
average temperature values over a period of time. In one example, the
processing
module 230-a may determine an average temperature value each minute by summing
all
temperature values collected over the minute and dividing by the number of
samples
over the minute. In a specific example where the temperature is sampled at one
sample
per second, the average temperature may be a sum of all sampled temperatures
for one
minute divided by sixty seconds. The memory 215 may store the average
temperature
values over time. In some implementations, the memory 215 may store average
temperatures (e.g., one per minute) instead of sampled temperatures in order
to conserve
memory 215.
[0069] The sampling rate, which may be stored in memory 215, may be
configurable. In some implementations, the sampling rate may be the same
throughout
the day and night. In other implementations, the sampling rate may be changed
throughout the day/night. In some implementations, the ring 104 may
filter/reject
temperature readings, such as large spikes in temperature that are not
indicative of
physiological changes (e.g., a temperature spike from a hot shower). In some
implementations, the ring 104 may filter/reject temperature readings that may
not be
reliable due to other factors, such as excessive motion during 104 exercise
(e.g., as
indicated by a motion sensor 245).
[0070] The ring 104 (e.g., communication module) may transmit the
sampled and/or
average temperature data to the user device 106 for storage and/or further
processing.
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
The user device 106 may transfer the sampled and/or average temperature data
to the
server 110 for storage and/or further processing.
[0071] Although the ring 104 is illustrated as including a single
temperature sensor
240, the ring 104 may include multiple temperature sensors 240 in one or more
locations, such as arranged along the inner housing 205-a near the user's
finger. In some
implementations, the temperature sensors 240 may be stand-alone temperature
sensors
240. Additionally, or alternatively, one or more temperature sensors 240 may
be
included with other components (e.g., packaged with other components), such as
with
the accelerometer and/or processor.
[0072] The processing module 230-a may acquire and process data from
multiple
temperature sensors 240 in a similar manner described with respect to a single
temperature sensor 240. For example, the processing module 230 may
individually
sample, average, and store temperature data from each of the multiple
temperature
sensors 240. In other examples, the processing module 230-a may sample the
sensors at
.. different rates and average/store different values for the different
sensors. In some
implementations, the processing module 230-a may be configured to determine a
single
temperature based on the average of two or more temperatures determined by two
or
more temperature sensors 240 in different locations on the finger.
[0073] The temperature sensors 240 on the ring 104 may acquire distal
temperatures
.. at the user's finger (e.g., any finger). For example, one or more
temperature sensors 240
on the ring 104 may acquire a user's temperature from the underside of a
finger or at a
different location on the finger. In some implementations, the ring 104 may
continuously acquire distal temperature (e.g., at a sampling rate). Although
distal
temperature measured by a ring 104 at the finger is described herein, other
devices may
.. measure temperature at the same/different locations. In some cases, the
distal
temperature measured at a user's finger may differ from the temperature
measured at a
user's wrist or other external body location. Additionally, the distal
temperature
measured at a user's finger (e.g., a "shell" temperature) may differ from the
user's core
temperature. As such, the ring 104 may provide a useful temperature signal
that may not
.. be acquired at other internal/external locations of the body. In some
cases, continuous
temperature measurement at the finger may capture temperature fluctuations
(e.g., small
or large fluctuations) that may not be evident in core temperature. For
example,
21
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
continuous temperature measurement at the finger may capture minute-to-minute
or
hour-to-hour temperature fluctuations that provide additional insight that may
not be
provided by other temperature measurements elsewhere in the body.
[0074] The ring 104 may include a PPG system 235. The PPG system 235 may
include one or more optical transmitters that transmit light. The PPG system
235 may
also include one or more optical receivers that receive light transmitted by
the one or
more optical transmitters. An optical receiver may generate a signal
(hereinafter "PPG"
signal) that indicates an amount of light received by the optical receiver.
The optical
transmitters may illuminate a region of the user's finger. The PPG signal
generated by
the PPG system 235 may indicate the perfusion of blood in the illuminated
region. For
example, the PPG signal may indicate blood volume changes in the illuminated
region
caused by a user's pulse pressure. The processing module 230-a may sample the
PPG
signal and determine a user's pulse waveform based on the PPG signal. The
processing
module 230-a may determine a variety of physiological parameters based on the
user's
pulse waveform, such as a user's respiratory rate, heart rate, HRV, oxygen
saturation,
and other circulatory parameters.
[0075] In some implementations, the PPG system 235 may be configured as
a
reflective PPG system 235 in which the optical receiver(s) receive transmitted
light that
is reflected through the region of the user's finger. In some implementations,
the PPG
system 235 may be configured as a transmissive PPG system 235 in which the
optical
transmitter(s) and optical receiver(s) are arranged opposite to one another,
such that
light is transmitted directly through a portion of the user's finger to the
optical
receiver(s).
[0076] The number and ratio of transmitters and receivers included in
the PPG
system 235 may vary. Example optical transmitters may include light-emitting
diodes
(LEDs). The optical transmitters may transmit light in the infrared spectrum
and/or
other spectrums. Example optical receivers may include, but are not limited
to,
photosensors, phototransistors, and photodiodes. The optical receivers may be
configured to generate PPG signals in response to the wavelengths received
from the
optical transmitters. The location of the transmitters and receivers may vary.
Additionally, a single device may include reflective and/or transmissive PPG
systems
235.
22
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0077] The PPG system 235 illustrated in FIG. 2 may include a reflective
PPG
system 235 in some implementations. In these implementations, the PPG system
235
may include a centrally located optical receiver (e.g., at the bottom of the
ring 104) and
two optical transmitters located on each side of the optical receiver. In this
implementation, the PPG system 235 (e.g., optical receiver) may generate the
PPG
signal based on light received from one or both of the optical transmitters.
In other
implementations, other placements, combinations, and/or configurations of one
or more
optical transmitters and/or optical receivers are contemplated.
[0078] The processing module 230-a may control one or both of the
optical
transmitters to transmit light while sampling the PPG signal generated by the
optical
receiver. In some implementations, the processing module 230-a may cause the
optical
transmitter with the stronger received signal to transmit light while sampling
the PPG
signal generated by the optical receiver. For example, the selected optical
transmitter
may continuously emit light while the PPG signal is sampled at a sampling rate
(e.g.,
250 Hz).
[0079] Sampling the PPG signal generated by the PPG system 235 may
result in a
pulse waveform that may be referred to as a "PPG." The pulse waveform may
indicate
blood pressure vs time for multiple cardiac cycles. The pulse waveform may
include
peaks that indicate cardiac cycles. Additionally, the pulse waveform may
include
respiratory induced variations that may be used to determine respiration rate.
The
processing module 230-a may store the pulse waveform in memory 215 in some
implementations. The processing module 230-a may process the pulse waveform as
it is
generated and/or from memory 215 to determine user physiological parameters
described herein.
[0080] The processing module 230-a may determine the user's heart rate
based on
the pulse waveform. For example, the processing module 230-a may determine
heart
rate (e.g., in beats per minute) based on the time between peaks in the pulse
waveform.
The time between peaks may be referred to as an interbeat interval (IBI). The
processing module 230-a may store the determined heart rate values and IBI
values in
memory 215.
23
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0081] The processing module 230-a may determine HRV over time. For
example,
the processing module 230-a may determine HRV based on the variation in the
IBls.
The processing module 230-a may store the HRV values over time in the memory
215.
Moreover, the processing module 230-a may determine the user's respiratory
rate over
time. For example, the processing module 230-a may determine respiratory rate
based
on frequency modulation, amplitude modulation, or baseline modulation of the
user's
IBI values over a period of time. Respiratory rate may be calculated in
breaths per
minute or as another breathing rate (e.g., breaths per 30 seconds). The
processing
module 230-a may store user respiratory rate values over time in the memory
215.
[0082] The ring 104 may include one or more motion sensors 245, such as one
or
more accelerometers (e.g., 6-D accelerometers) and/or one or more gyroscopes
(gyros).
The motion sensors 245 may generate motion signals that indicate motion of the
sensors. For example, the ring 104 may include one or more accelerometers that
generate acceleration signals that indicate acceleration of the
accelerometers. As another
example, the ring 104 may include one or more gyro sensors that generate gyro
signals
that indicate angular motion (e.g., angular velocity) and/or changes in
orientation. The
motion sensors 245 may be included in one or more sensor packages. An example
accelerometer/gyro sensor is a Bosch BM1160 inertial micro electro-mechanical
system
(MEMS) sensor that may measure angular rates and accelerations in three
perpendicular
axes.
[0083] The processing module 230-a may sample the motion signals at a
sampling
rate (e.g., 50Hz) and determine the motion of the ring 104 based on the
sampled motion
signals. For example, the processing module 230-a may sample acceleration
signals to
determine acceleration of the ring 104. As another example, the processing
module
230-a may sample a gyro signal to determine angular motion. In some
implementations,
the processing module 230-a may store motion data in memory 215. Motion data
may
include sampled motion data as well as motion data that is calculated based on
the
sampled motion signals (e.g., acceleration and angular values).
[0084] The ring 104 may store a variety of data described herein. For
example, the
ring 104 may store temperature data, such as raw sampled temperature data and
calculated temperature data (e.g., average temperatures). As another example,
the ring
104 may store PPG signal data, such as pulse waveforms and data calculated
based on
24
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
the pulse waveforms (e.g., heart rate values, IBI values, HRV values, and
respiratory
rate values). The ring 104 may also store motion data, such as sampled motion
data that
indicates linear and angular motion.
[0085] The ring 104, or other computing device, may calculate and store
additional
values based on the sampled/calculated physiological data. For example, the
processing
module 230 may calculate and store various metrics, such as sleep metrics
(e.g., a Sleep
Score), activity metrics, and readiness metrics. In some implementations,
additional
values/metrics may be referred to as "derived values." The ring 104, or other
computing/wearable device, may calculate a variety of values/metrics with
respect to
motion. Example derived values for motion data may include, but are not
limited to,
motion count values, regularity values, intensity values, metabolic
equivalence of task
values (METs), and orientation values. Motion counts, regularity values,
intensity
values, and METs may indicate an amount of user motion (e.g.,
velocity/acceleration)
over time. Orientation values may indicate how the ring 104 is oriented on the
user's
finger and if the ring 104 is worn on the left hand or right hand.
[0086] In some implementations, motion counts and regularity values may
be
determined by counting a number of acceleration peaks within one or more
periods of
time (e.g., one or more 30 second to 1 minute periods). Intensity values may
indicate a
number of movements and the associated intensity (e.g., acceleration values)
of the
movements. The intensity values may be categorized as low, medium, and high,
depending on associated threshold acceleration values. METs may be determined
based
on the intensity of movements during a period of time (e.g., 30 seconds), the
regularity/irregularity of the movements, and the number of movements
associated with
the different intensities.
[0087] In some implementations, the processing module 230-a may compress
the
data stored in memory 215. For example, the processing module 230-a may delete
sampled data after making calculations based on the sampled data. As another
example,
the processing module 230-a may average data over longer periods of time in
order to
reduce the number of stored values. In a specific example, if average
temperatures for a
user over one minute are stored in memory 215, the processing module 230-a may
calculate average temperatures over a five minute time period for storage, and
then
subsequently erase the one minute average temperature data. The processing
module
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
230-a may compress data based on a variety of factors, such as the total
amount of
used/available memory 215 and/or an elapsed time since the ring 104 last
transmitted
the data to the user device 106.
[0088] Although a user's physiological parameters may be measured by
sensors
included on a ring 104, other devices may measure a user's physiological
parameters.
For example, although a user's temperature may be measured by a temperature
sensor
240 included in a ring 104, other devices may measure a user's temperature. In
some
examples, other wearable devices (e.g., wrist devices) may include sensors
that measure
user physiological parameters. Additionally, medical devices, such as external
medical
devices (e.g., wearable medical devices) and/or implantable medical devices,
may
measure a user's physiological parameters. One or more sensors on any type of
computing device may be used to implement the techniques described herein.
[0089] The physiological measurements may be taken continuously
throughout the
day and/or night. In some implementations, the physiological measurements may
be
.. taken during 104 portions of the day and/or portions of the night. In some
implementations, the physiological measurements may be taken in response to
determining that the user is in a specific state, such as an active state,
resting state,
and/or a sleeping state. For example, the ring 104 can make physiological
measurements
in a resting/sleep state in order to acquire cleaner physiological signals. In
one example,
.. the ring 104 or other device/system may detect when a user is resting
and/or sleeping
and acquire physiological parameters (e.g., temperature) for that detected
state. The
devices/systems may use the resting/sleep physiological data and/or other data
when the
user is in other states in order to implement the techniques of the present
disclosure.
[0090] In some implementations, as described previously herein, the ring
104 may
be configured to collect, store, and/or process data, and may transfer any of
the data
described herein to the user device 106 for storage and/or processing. In some
aspects,
the user device 106 includes a wearable application 250, an operating system
(OS), a
web browser application (e.g., web browser 280), one or more additional
applications,
and a GUI 275. The user device 106 may further include other modules and
components, including sensors, audio devices, haptic feedback devices, and the
like.
The wearable application 250 may include an example of an application (e.g.,
"app")
that may be installed on the user device 106. The wearable application 250 may
be
26
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
configured to acquire data from the ring 104, store the acquired data, and
process the
acquired data as described herein. For example, the wearable application 250
may
include a user interface (UI) module 255, an acquisition module 260, a
processing
module 230-b, a communication module 220-b, and a storage module (e.g.,
database
265) configured to store application data.
[0091] The various data processing operations described herein may be
performed
by the ring 104, the user device 106, the servers 110, or any combination
thereof For
example, in some cases, data collected by the ring 104 may be pre-processed
and
transmitted to the user device 106. In this example, the user device 106 may
perform
some data processing operations on the received data, may transmit the data to
the
servers 110 for data processing, or both. For instance, in some cases, the
user device
106 may perform processing operations that require relatively low processing
power
and/or operations that require a relatively low latency, whereas the user
device 106 may
transmit the data to the servers 110 for processing operations that require
relatively high
processing power and/or operations that may allow relatively higher latency.
[0092] In some aspects, the ring 104, user device 106, and server 110 of
the system
200 may be configured to evaluate sleep patterns for a user. In particular,
the respective
components of the system 200 may be used to collect data from a user via the
ring 104,
and generate one or more scores (e.g., Sleep Score, Readiness Score) for the
user based
on the collected data. For example, as noted previously herein, the ring 104
of the
system 200 may be worn by a user to collect data from the user, including
temperature,
heart rate, HRV, and the like. Data collected by the ring 104 may be used to
determine
when the user is asleep in order to evaluate the user's sleep for a given
"sleep day." In
some aspects, scores may be calculated for the user for each respective sleep
day, such
that a first sleep day is associated with a first set of scores, and a second
sleep day is
associated with a second set of scores. Scores may be calculated for each
respective
sleep day based on data collected by the ring 104 during the respective sleep
day. Scores
may include, but are not limited to, Sleep Scores, Readiness Scores, and the
like.
[0093] In some cases, "sleep days" may align with the traditional
calendar days,
such that a given sleep day runs from midnight to midnight of the respective
calendar
day. In other cases, sleep days may be offset relative to calendar days. For
example,
sleep days may run from 6:00 pm (18:00) of a calendar day until 6:00 pm
(18:00) of the
27
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
subsequent calendar day. In this example, 6:00 pm may serve as a "cut-off
time," where
data collected from the user before 6:00 pm is counted for the current sleep
day, and
data collected from the user after 6:00 pm is counted for the subsequent sleep
day. Due
to the fact that most individuals sleep the most at night, offsetting sleep
days relative to
calendar days may enable the system 200 to evaluate sleep patterns for users
in such a
manner that is consistent with their sleep schedules. In some cases, users may
be able to
selectively adjust (e.g., via the GUI) a timing of sleep days relative to
calendar days so
that the sleep days are aligned with the duration of time in which the
respective users
typically sleep.
[0094] In some implementations, each overall score for a user for each
respective
day (e.g., Sleep Score, Readiness Score) may be determined/calculated based on
one or
more "contributors," "factors," or "contributing factors." For example, a
user's overall
Sleep Score may be calculated based on a set of contributors, including: total
sleep,
efficiency, restfulness, REM sleep, deep sleep, latency, timing, or any
combination
thereof The Sleep Score may include any quantity of contributors. The "total
sleep"
contributor may refer to the sum of all sleep periods of the sleep day. The
"efficiency"
contributor may reflect the percentage of time spent asleep compared to time
spent
awake while in bed, and may be calculated using the efficiency average of long
sleep
periods (e.g., primary sleep period) of the sleep day, weighted by a duration
of each
sleep period. The "restfulness" contributor may indicate how restful the
user's sleep is,
and may be calculated using the average of all sleep periods of the sleep day,
weighted
by a duration of each period. The restfulness contributor may be based on a
"wake up
count" (e.g., sum of all the wake-ups (when user wakes up) detected during
different
sleep periods), excessive movement, and a "got up count" (e.g., sum of all the
got-ups
(when user gets out of bed) detected during the different sleep periods).
[0095] The "REM sleep" contributor may refer to a sum total of REM sleep
durations across all sleep periods of the sleep day including REM sleep.
Similarly, the
"deep sleep" contributor may refer to a sum total of deep sleep durations
across all sleep
periods of the sleep day including deep sleep. The "latency" contributor may
signify
how long (e.g., average, median, longest) the user takes to go to sleep, and
may be
calculated using the average of long sleep periods throughout the sleep day,
weighted by
a duration of each period and the number of such periods (e.g., consolidation
of a given
28
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
sleep stage or sleep stages may be its own contributor or weight other
contributors).
Lastly, the "timing" contributor may refer to a relative timing of sleep
periods within
the sleep day and/or calendar day, and may be calculated using the average of
all sleep
periods of the sleep day, weighted by a duration of each period.
[0096] By way of another example, a user's overall Readiness Score may be
calculated based on a set of contributors, including: sleep, sleep balance,
heart rate,
HRV balance, recovery index, temperature, activity, activity balance, or any
combination thereof The Readiness Score may include any quantity of
contributors.
The "sleep" contributor may refer to the combined Sleep Score of all sleep
periods
within the sleep day. The "sleep balance" contributor may refer to a
cumulative duration
of all sleep periods within the sleep day. In particular, sleep balance may
indicate to a
user whether the sleep that the user has been getting over some duration of
time (e.g.,
the past two weeks) is in balance with the user's needs. Typically, adults
need 7-9 hours
of sleep a night to stay healthy, alert, and to perform at their best both
mentally and
physically. However, it is normal to have an occasional night of bad sleep, so
the sleep
balance contributor takes into account long-term sleep patterns to determine
whether
each user's sleep needs are being met. The "resting heart rate" contributor
may indicate
a lowest heart rate from the longest sleep period of the sleep day (e.g.,
primary sleep
period) and/or the lowest heart rate from naps occurring after the primary
sleep period.
[0097] Continuing with reference to the "contributors" (e.g., factors,
contributing
factors) of the Readiness Score, the "HRV balance" contributor may indicate a
highest
HRV average from the primary sleep period and the naps happening after the
primary
sleep period. The HRV balance contributor may help users keep track of their
recovery
status by comparing their HRV trend over a first time period (e.g., two weeks)
to an
average HRV over some second, longer time period (e.g., three months). The
"recovery
index" contributor may be calculated based on the longest sleep period.
Recovery index
measures how long it takes for a user's resting heart rate to stabilize during
the night. A
sign of a very good recovery is that the user's resting heart rate stabilizes
during the first
half of the night, at least six hours before the user wakes up, leaving the
body time to
recover for the next day. The "body temperature" contributor may be calculated
based
on the longest sleep period (e.g., primary sleep period) or based on a nap
happening
after the longest sleep period if the user's highest temperature during the
nap is at least
29
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
0.5 C higher than the highest temperature during the longest period. In some
aspects,
the ring may measure a user's body temperature while the user is asleep, and
the system
200 may display the user's average temperature relative to the user's baseline
temperature. If a user's body temperature is outside of their normal range
(e.g., clearly
above or below 0.0), the body temperature contributor may be highlighted
(e.g., go to a
"Pay attention" state) or otherwise generate an alert for the user.
[0098] In some aspects, the system 200 may support techniques for
miscarriage
identification and prediction. In particular, the respective components of the
system 200
may be used to detect the indication of the early pregnancy loss based on
identifying
that the temperature values in a time series representing the user's
temperature over
time are lower than a pregnancy baseline of temperature values for the user.
The
indication of the early pregnancy loss for the user may be identified and/or
predicted by
leveraging temperature sensors on the ring 104 of the system 200. In some
cases, the
indication of the early pregnancy loss may be estimated by identifying one or
more
morphological features such as deviations in the time series representing the
user's
temperature over time relative to the pregnancy baseline of temperature values
and
detecting the indication of early pregnancy loss that corresponds to the
deviations of the
time series. The indication of early pregnancy loss may be an example of
identifying
that the early pregnancy loss has already occurred, is currently occurring,
and/or that the
.. early pregnancy loss is predicted to occur in the future.
[0099] For example, as noted previously herein, the ring 104 of the
system 200 may
be worn by a user to collect data from the user, including temperature, heart
rate, HRV,
respiratory data, and the like. The ring 104 of the system 200 may collect the
physiological data from the user based on temperature sensors and measurements
extracted from arterial blood flow (e.g., using PPG signals). The
physiological data may
be collected continuously. In some implementations, the processing module 230-
a may
sample the user's temperature continuously throughout the day and night.
Sampling at a
sufficient rate (e.g., one sample per minute) throughout the day and/or night
may
provide sufficient temperature data for analysis described herein. In some
implementations, the ring 104 may continuously acquire temperature data (e.g.,
at a
sampling rate). In some examples, even though temperature is collected
continuously,
the system 200 may leverage other information about the user that it has
collected or
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
otherwise derived (e.g., sleep stage, activity levels, illness onset, etc.) to
select a
representative temperature for a particular day that is an accurate
representation of the
underlying physiological phenomenon.
[0100] In contrast, systems that require a user to manually take their
temperature
each day and/or systems that measure temperature continuously but lack any
other
contextual information about the user may select inaccurate or inconsistent
temperature
values for their menstrual cycle predictions and/or pregnancy tracking,
leading to
inaccurate predictions and decreased user experience. In contrast, data
collected by the
ring 104 may be used to accurately detect the indication of the early
pregnancy loss of
the user. Early pregnancy loss identification and prediction and related
techniques are
further shown and described with reference to FIG. 3.
[0101] FIG. 3 illustrates an example of a system 300 that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure. The system 300 may implement, or be
implemented
by, system 100, system 200, or both. In particular, system 300 illustrates an
example of
a ring 104 (e.g., wearable device 104), a user device 106, and a server 110,
as described
with reference to FIG. 1.
[0102] The ring 305 may acquire temperature data 320, heart rate data
325,
respiratory rate data 330, HRV data 335, and sleep data 340, among other forms
of
physiological data as described herein. In such cases, the ring 305 may
transmit
temperature data 320, heart rate data 325, respiratory rate data 330, HRV data
335, and
sleep data 340 to the user device 310. The temperature data 320 may include
continuous
nighttime temperature data. The respiratory rate data 330 may include
continuous
nighttime breath rate data. In some cases, multiple devices may acquire
physiological
.. data. For example, a first computing device (e.g., user device 310) and a
second
computing device (e.g., the ring 305) may acquire temperature data 320, heart
rate data
325, respiratory rate data 330, HRV data 335, sleep data 340, or a combination
thereof
[0103] For example, the ring 305 may acquire user physiological data,
such as user
temperature data 320, respiratory rate data 330, heart rate data 325, HRV data
335, and
.. sleep data 340, galvanic skin response, blood oxygen saturation,
actigraphy, and/or
other user physiological data. For example, the ring 305 may acquire raw data
and
31
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
convert the raw data to features with daily granularity. In some
implementations,
different granularity input data may be used. The ring 305 may send the data
to another
computing device, such as a mobile device (e.g., user device 310) for further
processing.
[0104] For example, the user device 310 may identify and/or predict the
indication
of the early pregnancy loss based on the received data. In some cases, the
system 300
may identify and/or predict the indication of the early pregnancy loss based
on
temperature data 320, respiratory rate data 330, heart rate data 325, HRV data
335, sleep
data 340, galvanic skin response, blood oxygen saturation, activity, sleep
architecture,
or a combination thereof In some cases, the system 300 may determine which
features
are useful predictors for early pregnancy losses. Although the system may be
implemented by a ring 305 and a user device 310, any combination of computing
devices described herein may implement the features attributed to the system
300.
[0105] The user device 310-a may include a ring application 345. The
ring
application 345 may include at least modules 350 and application data 355. In
some
cases, the application data 355 may include historical temperature patterns
for the user
and other data. The other data may include temperature data 320, heart rate
data 325,
respiratory rate data 330, HRV data 335, sleep data 340, or a combination
thereof
[0106] The ring application 345 may present a predicted and/or detected
early
pregnancy loss to the user. The ring application 345 may include an
application data
.. processing module that may perform data processing. For example, the
application data
processing module may include modules 350 that provide functions attributed to
the
system 300. Example modules 350 may include a daily temperature determination
module, a time series processing module, a miscarriage identification module,
and
miscarriage prediction module.
[0107] The daily temperature determination module may determine daily
temperature values (e.g., by selecting a representative temperature value for
that day
from a series of temperature values that were collected continuously
throughout the
night). The time series processing module may process time series data to
identify that
the plurality of temperature values are lower than a pregnancy baseline of
temperature
values. The miscarriage identification module may identify the indication of
the early
pregnancy loss of the user based on the processed time series data. The
miscarriage
32
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
prediction module may predict the indication of the early pregnancy loss of
the user
based on the processed time series data. In such cases, the system 300 may
receive user
physiological data (e.g., from a ring 305) and output daily classification of
whether
pregnancy loss is identified or predicted. The ring application 345 may store
application
data 355, such as acquired temperature data, other physiological data,
pregnancy
tracking data (e.g., event data), and miscarriage tracking data.
[0108] In some cases, the system 300 may generate pregnancy and/or
miscarriage
tracking data based on user physiological data (e.g., temperature data 320).
The
pregnancy and/or miscarriage tracking data may include a detected indication
of the
early pregnancy loss for the user, which may be determined based on acquired
user
temperature data (e.g., daily temperature data 320) over an analysis time
period (e.g., a
period of weeks/months). For example, the system 300 may receive physiological
data
associated with a user from a wearable device (e.g., ring 305). The
physiological data
may include at least temperature data 320, heart rate data 325, respiratory
rate data 330,
HRV data 335, sleep data 340, or a combination thereof For example, the system
300
acquires user physiological data over an analysis time period (e.g., a
plurality of days).
In such cases, the system 300 may acquire and process user physiological data
over an
analysis time period to generate one or more time series of user physiological
data.
[0109] In some cases, the system 300 may acquire daily user temperature
data 320
over an analysis time period. For example, the system 300 may calculate a
single
temperature value for each day. The system 300 may acquire a plurality of
temperature
values during the night and process the acquired temperature values to
determine the
single daily temperature value. In some implementations, the system 300 may
determine
a time series of a plurality of temperature values taken over a plurality of
days based on
the received temperature data 320. The system 300 may detect the indication of
the
early pregnancy loss in the time series of the temperature values based on
identifying
that the plurality of temperature values are lower than a pregnancy baseline
of
temperature values for the user, as further shown and described with reference
to FIG.
5.
[0110] In some implementations, the system 300 may determine that the
received
heart rate data 325 exceeds a pregnancy baseline heart rate for the user for
at least a
portion of the plurality of days. In such cases, the system 300 may detect the
indication
33
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
of the early pregnancy loss based on determining that the received heart rate
data 325
exceeds the pregnancy baseline heart rate for the user. In some examples, the
system
300 may determine that the received respiratory rate data 330 exceeds a
pregnancy
baseline respiratory rate for the user for at least a portion of the plurality
of days. In
.. such cases, the system 300 may detect the indication of the early pregnancy
loss based
on determining that the received respiratory rate data 330 exceeds the
pregnancy
baseline respiratory rate for the user.
[0111] In some implementations, the system 300 may determine that the
received
HRV data 335 is less than a pregnancy baseline HRV for the user for at least a
portion
.. of the plurality of days. In such cases, the system 300 may detect the
indication of the
early pregnancy loss based on determining that the received HRV data 335 is
less than a
pregnancy baseline HRV for the user. In some implementations, the system 300
may
determine that a quantity of detected sleep disturbances from the received
sleep data
340 exceeds a pregnancy baseline sleep disturbance threshold for the user for
at least a
portion of the plurality of days. In such cases, the system 300 may detect the
indication
of the early pregnancy loss based on determining that the quantity of detected
sleep
disturbances from the received sleep data 340 exceeds a pregnancy baseline
sleep
disturbance threshold for the user. In such cases, the pregnancy baselines
(e.g.,
temperature, heart rate, respiratory rate, HRV, sleep data, and the like) may
be tailored-
specific to the user based on historical data 360 acquired by the system 300.
For
example, these pregnancy baselines may represent baseline or average values of
physiological parameters or typical trends of physiological values throughout
a user's
pregnancy, which may differ from the user's normal or non-pregnant baselines.
In some
cases, the pregnancy baselines may differ throughout the user's pregnancy
(e.g., based
on the different stages of pregnancy) for each physiological parameter. In
some cases,
the pregnancy baselines may be based on known standards, averages among users,
demographic-specific, and/or based on a user's prior pregnancies.
[0112] In some cases, one or more physiological measurements may be
combined to
detect the indication of the early pregnancy loss. In such cases, identifying
the
indication of the pregnancy loss may be based on one physiological measurement
or a
combination of physiological measurements (e.g., temperature data 320, heart
rate data
325, respiratory rate data 330, HRV data 335, sleep data 340). For example,
the user's
34
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
heart rate data 325 in combination with the user's temperature data 320 may be
an
indicator that may characterize an early pregnancy loss. In some cases, the
user's heart
rate data 325 may confirm (e.g., provide a definitive indication of or better
prediction
of) the indication of the early pregnancy loss in light of the user's
temperature data 320.
For example, if the system 300 determines that the received heart rate data
325 exceeds
the pregnancy baseline heart rate for the user and that the received
temperature data 320
is greater than the pregnancy baseline temperature for the user, the system
300 may
validate or detect the indication of the early pregnancy loss with greater
accuracy and
precision than if one of the heart rate data 325 or temperature data 320
deviates from the
pregnancy baseline.
[0113] In some examples, one or more physiological measurements may be
combined to disprove or reduce the likelihood of a detected indication of the
early
pregnancy loss. In such cases, the system 300 may identify a false positive
for
identifying the indication of the early pregnancy loss based on one
physiological
measurement or a combination of physiological measurements. For example, if
the
system 300 determines that the received temperature data 320 is greater than
the
pregnancy baseline temperature for the user but the received respiratory rate
data 330
still aligns with the pregnancy baseline respiratory rate for the user, the
system 300 may
determine that the detected indication of the early pregnancy loss is invalid
or at least
less likely than if both the temperate and respiratory rate deviated from
their pregnancy
baselines. In such cases, the system 300 may determine that the user may be
experiencing an illness, hormonal shift in the menstrual cycle, and the like.
[0114] In some cases, the user's logged symptoms (e.g., tags) in
combination with
the user's physiological data (e.g., temperature data 320, heart rate data
325, respiratory
rate data 330, HRV data 335, sleep data 340, or a combination thereof) may be
an
indicator that may characterize an indication of the early pregnancy loss. In
such cases,
the user's logged symptoms may confirm (e.g., provide a definitive indication
of or
better prediction of) the indication of the early pregnancy loss in light of
the user's
physiological data. For example, if the system 300 determines that the
received
temperature data 320 is greater than the pregnancy baseline temperature for
the user and
the system receives user input associated with a miscarriage (e.g., bleeding,
pain, etc.),
the system may validate or detect the indication of the early pregnancy loss
with greater
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
accuracy and precision than if one of the temperature data 320 deviates from
the
pregnancy baseline or the user logs early pregnancy loss symptoms.
[0115] In some examples, the system 300 may identify a false positive
for
identifying the indication of the early pregnancy loss based on the user
input, one
physiological measurement, a combination of physiological measurements, or a
combination thereof For example, if the system 300 determines that the
received heart
rate data 325 is greater than the pregnancy baseline heart rate for the user
but the user
input indicates a symptom associated with stress, illness, anxiety, a change
in
medication, and the like, the system 300 may determine that the detected
indication of
early pregnancy loss is invalid (e.g., a false positive). In such cases, the
system 300 may
determine that the user may be experiencing an illness, stress, hormonal shift
in the
menstrual cycle, and the like based on receiving the user input.
[0116] In some implementations, the system may identify personalized
lifestyle
factors to improve fertility treatment success. For example, the system may
track
behavioral metrics related to sleep, activity, and nutrition to help users
improve fertility
and guide fertility treatment. In some cases, there may be a relationship
between sleep
disturbance and reproductive health (e.g., whether the user experiences a
miscarriage
during pregnancy). Sleep disturbance may be detected in users such as users
under
stress and/or shift workers that experience sleep disruption and circadian
misalignment
impacting reproductive health outcomes including: menstrual irregularities,
dysmenorrhea, reduced rates of conception, increased miscarriages, and lower
birth
weights. By monitoring sleep data for the user and aggregate users undergoing
fertility
treatments, the system 300 may provide valuable insights into the relationship
between
various dimensions and metrics of sleep and fertility in order to increase
successful
pregnancy rates.
[0117] In some implementations, the system 300 may ask a user to
indicate whether
they are shift workers (e.g., during a sign-up process) or experience other
sleep
disturbances. In such cases, metrics (e.g., self-reported and signal-derived
metrics of
circadian disruption) may be incorporated as features in a machine learning
model to
.. predict risk for subfertility, infertility, fertility treatment success,
fertility complications,
the likelihood of miscarriage, or a combination thereof The system 300 that
considers
the user's sleep history and quantifies the magnitude of circadian disruption
may surface
36
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
tags that may provide input on overall health as well as fertility status
(e.g., late night
meal, back pain, chest pain). In some implementations, these factors may be
used to
drive the selection and personalization of insights displayed to the users.
The system
300 may provide feedback on fertility odds while considering menstrual cycle
irregularities. For example, the user may be provided with information related
to
circadian rhythms and sleep hygiene. The user may be presented the option to
connect
with a human-in-the-loop live sleep coach to set goals and an action plan that
may lead
to application supported behavioral changes under the user's personal
constraints and
limitations (e.g., time, nutrition). In some implementations, the system 300
may allow
users to tag melatonin and see related changes in sleep and fertility, as
melatonin levels
and supplementation may influence fertility outcomes (e.g., via biological
mechanisms
such as reduction of oxidative stress-mediated effects on reproductive
tissues).
[0118] The system 300 may cause a GUI of the user devices 310-a, 310-b
to display
the indication of the early pregnancy loss. In some cases, the system 300 may
cause the
GUI to display the time series. The system 300 may generate a tracking GUI
that
includes physiological data (e.g., at least temperature data 320), tagged
events, and/or
other GUI elements described herein with reference to FIG. 4. In such cases,
the system
300 may render ovulations, periods, pregnancy, a miscarriage, and the like in
a tracking
GUI.
[0119] The system 300 may generate a message 370 for display on a GUI on a
user
device 310-a or 310-b that indicates the indication of the early pregnancy
loss. For
example, the system 300 (e.g., user device 310-a or server 315) may transmit
the
message 370 that indicates the predicted and/or identified early pregnancy
loss to the
user device 310-b. In such cases, the user device 310-b maybe associated with
a
clinician, a fertility specialist, a care-taker, a partner, or a combination
thereof The
detection of a probable pregnancy loss may trigger a personalized message 370
to a user
highlighting the pattern detected in the temperature data and providing an
educational
link about pregnancy loss.
[0120] In some implementations, the ring application 345 may notify the
user of
indication of early pregnancy loss and/or prompt the user to perform a variety
of tasks
in the activity GUI. The notifications and prompts may include text, graphics,
and/or
other user interface elements. The notifications and prompts may be included
in the ring
37
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
application 345 such as when there is identified and/or predicted pregnancy
loss, the
ring application 345 may display notifications and prompts. The user device
310 may
display notifications and prompts in a separate window on the home screen
and/or
overlaid onto other screens (e.g., at the very top of the home screen). In
some cases, the
user device 310 may display the notifications and prompts on a mobile device,
a user's
watch device, or both.
[0121] In some examples, the system 300 provide mitigation advice. In
such cases,
the system 300 may provide recommendations on steps to take to confirm or
disprove
the indication of the early pregnancy loss. For example, if the system 300
determines
that the user's respiratory rate is elevated above the pregnancy baseline
heart rate, the
system 300 may prompt the user to perform a meditation and/or breathing
exercise a
few times a day for a couple of days and the re-evaluate the respiratory rate
data 330. In
such cases, the system 300 may receive the physiological data after the user
performs
the mitigation advice to determine whether the user is experiencing a
miscarriage, if the
user is experiencing a period of anxiety, or both.
[0122] In some implementations, the user device 310 may store historical
user data.
In some cases, the historical user data may include historical data 360. The
historical
data 360 may include historical temperature patterns of the user, historical
heart rate
patterns of the user, historical respiratory rate patterns of the user,
historical HRV
patterns of the user, historical sleep patterns of the user, historical
menstrual cycle onset
events (e.g., cycle length, cycle start date, etc.) of the user, or a
combination thereof
The historical data 360 may be selected from the last few months. The
historical data
360 may be used (e.g., by the user device 310 or server 315) to determine a
threshold
(e.g., pregnancy baseline) for the user, determine temperature values of the
user, predict
an early pregnancy loss, identify an early pregnancy loss, or a combination
thereof The
historical data 360 may be used by the server 315. Using the historical data
360 may
allow the user device 310 and/or server 315 to personalize the GUI by taking
into
consideration user's historical data 360.
[0123] In such cases, the user device 310 may transmit historical data
360 to the
server 315. In some cases, the transmitted historical data 360 may be the same
historical
data stored in the ring application 345. In other examples, the historical
data 360 may be
different than the historical data stored in the ring application 345. The
server 315 may
38
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
receive the historical data 360. The server 315 may store the historical data
360 in
server data 365.
[0124] In some implementations, the user device 310 and/or server 315
may also
store other data which may be an example of user information. The user
information
may include, but is not limited to, user age, weight, height, and gender. In
some
implementations, the user information may be used as features for predicting
or
identifying early pregnancy loss. The server data 365 may include the other
data such as
user information.
[0125] In some implementations, the system 300 may include one or more
user
devices 310 for different users. For example, the system 300 may include user
device
310-a for a primary user and user device 310-b for a second user 302
associated with the
primary user (e.g., partner). The user devices 310 may measure physiological
parameters of the different users, provide GUIs for the different users, and
receive user
input from the different users. In some implementations, the different user
devices 310
may acquire physiological information and provide output related to a woman's
health,
such as menstrual cycles, ovarian cycles, illness, fertility, and/or
pregnancy. In some
implementations, the user device 310-b may acquire physiological information
related
to the second user 302, such as male illness and fertility.
[0126] In some implementations, the system 300 may provide GUIs that
inform the
second user 302 of relevant information. For example, the first user and the
second user
302 may share their information with one another via one or more user devices
310,
such as via a server device, mobile device, or other device. In some
implementations,
the second user 302 may share one or more of their accounts (e.g., usernames,
login
information, etc.) and/or associated data with one another (e.g., the first
user). By
sharing information between users, the system 300 may assist second users 302
in
making health decisions related to pregnancy. In some implementations, the
users may
be prompted (e.g., in a GUI) to share specific information. For example, the
user may
use a GUI to opt into sharing her pregnancy information with the second user
302. In
such cases, the user and the second user 302 may receive notifications on
their
respective user devices 310. In other examples, a second user 302 may make
their
information (e.g., illness, pregnancy data, etc.) available to the user via a
notification or
39
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
other sharing arrangement. In such cases, the second user 302 may be an
example of a
clinician, a fertility specialist, a care-taker, a partner, or a combination
thereof
[0127] FIG. 4 illustrates an example of a timing diagram 400 that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure. The timing diagrams 400 may
implement, or be implemented by, aspects of the system 100, system 200, system
300,
or a combination thereof For example, in some implementations, the timing
diagram
400 may be displayed to a user via the GUI 275 of the user device 106, as
shown in
FIG. 2.
[0128] As described in further detail herein, the system may be configured
to
identify and predict a miscarriage. In some cases, the user's body temperature
pattern
throughout the day and night may be an indicator that may characterize a
miscarriage.
For example, skin temperature during the day and night may identify and/or
predict a
miscarriage (e.g., early pregnancy loss). As such, the timing diagram 400
illustrates a
relationship between a user's temperature data and a time (e.g., over a
plurality of
months). In this regard, the solid curved line illustrated in the timing
diagram 400 may
be understood to refer to the "temperature values 405." The dashed vertical
line
illustrated in the timing diagram 400 may be understood to refer to the
"ovulation tag
410." The dashed-dotted vertical line illustrated in the timing diagram 400
may be
understood to refer to the "pregnancy tag 420." The solid vertical bars
illustrated in the
timing diagram 400 may be understood to refer to the "period tag 415." The
user's
temperature values 405 may be relative to a baseline temperature.
[0129] In some cases, the system (e.g., ring 104, user device 106,
server 110) may
receive physiological data associated with a user from a wearable device. The
physiological data may include at least temperature data. The system may
determine a
time series of temperature values 405 taken over a plurality of days based on
the
received temperature data. With reference to timing diagram 400, the plurality
of days
may be an example of ten months. The system may process original time series
temperature data (e.g., temperature values 405) to detect the indication of
the early
pregnancy loss 425. In some cases, the time series may include a plurality of
events
tagged by the user in the system. For example, the time series may include
ovulation
tags 410, period tags 415, and a pregnancy tag 420. In some cases, the
ovulation tags
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
410, period tags 415, and a pregnancy tag 420 may be determined by the system
based
on physiological data continuously collected by the system.
[0130] The temperature values 405 may be continuously collected by the
wearable
device. The physiological measurements may be taken continuously throughout
the day
and/or night. For example, in some implementations, the ring may be configured
to
acquire physiological data (e.g., temperature data, sleep data, heart rate,
HRV data,
respiratory rate data, sleep data, MET data, and the like) continuously in
accordance
with one or more measurement periodicities throughout the entirety of each
day/sleep
day. In other words, the ring may continuously acquire physiological data from
the user
without regard to "trigger conditions" for performing such measurements. In
some
cases, continuous temperature measurement at the finger may capture
temperature
fluctuations (e.g., small or large fluctuations) that may not be evident in
core
temperature. For example, continuous temperature measurement at the finger may
capture minute-to-minute or hour-to-hour temperature fluctuations that provide
additional insight that may not be provided by other temperature measurements
elsewhere in the body or if the user were manually taking their temperature
once per
day.
[0131] In some implementations, the system may detect the indication of
the early
pregnancy loss 425 by observing a user's relative body temperature for many
days and
marking the decrease in temperature relative to a pregnancy baseline, which
may
indicate a miscarriage (e.g., early pregnancy loss). The indication of the
early pregnancy
loss 425 may include a duration of time (e.g., time span) including at least a
day, a
plurality of days, a week, a plurality of weeks, or a month. In such cases,
the indication
of the early pregnancy loss 425 may include a start date and an end date. The
indication
of the early pregnancy loss 425 may be an example of a detected miscarriage
that
previously occurred or currently occurs and/or a predicted miscarriage that
likely occurs
in the future.
[0132] The system may detect the indication of the early pregnancy loss
425 in the
time series of the temperature values 405 based on identifying that the
temperature
values 405 are lower than a pregnancy baseline of temperature values for the
user. For
example, the system may identify the temperature values 405 after determining
the time
series and identify the pregnancy baseline of temperature values. The system
may detect
41
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
the indication of the early pregnancy loss 425 of the user in response to
identifying that
the temperature values 405 are lower than the pregnancy baseline of
temperature values
for the user.
[0133] As described in further detail herein, the system may be
configured to track
menstrual cycles, ovulation, pregnancy, miscarriage, and the like. In some
cases, the
user's body temperature pattern throughout the night may be an indicator that
may
characterize miscarriage. For example, skin temperature during the night may
identify
the indication of the early pregnancy loss 425. As such, the timing diagram
400
illustrates a relationship between a user's temperature data and a time (e.g.,
over a
plurality of months).
[0134] The timing diagram 400 may illustrate a user with three periods
(e.g., period
tags 415), an indication of pregnancy (e.g., pregnancy tag 420), the
indication of the
early pregnancy loss 425, followed by at least four more menstrual cycles
(e.g. period
tags 415). For example, the timing diagram 400 may indicate that the
miscarriage
occurred after 2 months (e.g., eight weeks) from the pregnancy tag 420. The
timing
diagram 400 may indicate a user who had several periods (e.g., period tags
415) that
may have been identified automatically and/or by user tags in the application.
For
example, timing diagram 400 illustrates that the user became pregnant (e.g.,
indicated
via pregnancy tag 420) and then returned to having periods (e.g.,
automatically detected
or tagged via period tags 415) within less than 9 months after becoming
pregnant,
thereby indicating the user likely had a miscarriage. The user's temperature
trajectory
around the time of the pregnancy tag 420 may be not much higher than the
temperature
peak at the time of the period tags 415. In such cases, the system may
determine that the
temperature trajectory of the user may peak (e.g., thereby indicating a
period) within
less than 9 months after becoming pregnant such that the system may determine
that the
user is likely experiencing a miscarriage.
[0135] In some cases, the system may identify a presence of a menstrual
cycle 430
within a time period after pregnancy onset (e.g., pregnancy tag 420) based on
determining the time series. In such cases, the indication of the early
pregnancy loss 425
.. may be detected based on identifying the presence of the menstrual cycle
430. For
example, if the system identifies the menstrual cycle 430 within 20 weeks of
the
pregnancy tag 420, the system may detect the indication of the early pregnancy
loss
42
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
425. The menstrual cycle 430 may be identified based on the user's
continuously
collected physiological data, a received confirmation, or both. For example,
the system
may receive a confirmation of a menstrual cycle 430 within a time period after
the
pregnancy tag 420. In some cases, the system may receive a confirmation of a
.. pregnancy loss. In such cases, the system may detect the indication of the
early
pregnancy loss 425 in response to receiving the confirmation of the menstrual
cycle
430, the pregnancy loss, or both.
[0136] FIG. 5 illustrates an example of a timing diagram 500 that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure. The timing diagram 500 may
implement, or be implemented by, aspects of the system 100, system 200, system
300,
or a combination thereof For example, in some implementations, the timing
diagram
500 may be displayed to a user via the GUI 275 of the user device 106, as
shown in
FIG. 2.
[0137] As described in further detail herein, the system may be configured
to
identify and predict a miscarriage based on deviations relative to a pregnancy
baseline.
In some cases, the user's body temperature pattern throughout the night may be
an
indicator that may characterize a miscarriage. For example, skin temperature
during the
day and/or night may identify and/or predict a miscarriage (e.g., early
pregnancy loss).
As such, the timing diagram 500 illustrates a relationship between a user's
temperature
data and a time (e.g., over a plurality of days relative to pregnancy). In
this regard, the
dashed curved line illustrated in the timing diagram 500 may be understood to
refer to
the "temperature values 505." In this regard, the solid curved line
illustrated in the
timing diagram 500 may be understood to refer to the "pregnancy baseline of
temperature values 510." The user's temperature values 505 and pregnancy
baseline of
temperature values 510 may be relative to a baseline temperature.
[0138] In some cases, the system (e.g., ring 104, user device 106,
server 110) may
receive physiological data associated with a user from a wearable device. The
physiological data may include at least temperature values 505. The system may
determine a time series of the temperature values 505 taken over a plurality
of days
based on the received temperature data. With reference to timing diagram 500,
the
plurality of days may be an example of at least three months (e.g., one month
prior to
43
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
pregnancy onset and two months after pregnancy onset). The system may process
original time series temperature data (e.g., temperature values 505) to detect
the
indication of an early pregnancy loss 520. In some cases, the time series may
include a
plurality of events tagged by the user in the system. For example, the time
series may
include an indication of pregnancy 515. In some cases, indication of pregnancy
515 may
be determined by the system based on physiological data continuously collected
by the
system, based on a user input, or both.
[0139] The temperature values 505 may be continuously collected by the
wearable
device. The physiological measurements may be taken continuously throughout
the
.. night. For example, in some implementations, the ring may be configured to
acquire
physiological data (e.g., temperature data, sleep data, heart rate, HRV data,
respiratory
rate data, MET data, sleep data, and the like) continuously in accordance with
one or
more measurement periodicities throughout the entirety of each day/sleep day.
In other
words, the ring may continuously acquire physiological data from the user
without
.. regard to "trigger conditions" for performing such measurements. In some
cases,
continuous temperature measurement at the finger may capture temperature
fluctuations
(e.g., small or large fluctuations) that may not be evident in core
temperature. For
example, continuous temperature measurement at the finger may capture minute-
to-
minute or hour-to-hour temperature fluctuations that provide additional
insight that may
.. not be provided by other temperature measurements elsewhere in the body or
if the user
were manually taking their temperature once per day.
[0140] In some implementations, the system may identify and/or predict
the
indication of the early pregnancy loss 520 by observing a user's relative body
temperature for many days and marking the decrease in temperature relative to
a
pregnancy baseline (e.g., pregnancy baseline of temperature values 510), which
may
indicate a miscarriage. The indication of the early pregnancy loss 520 may
include a
duration of time (e.g., time span) including at least a day, a plurality of
days, a week, or
a plurality of weeks. In such cases, the indication of the early pregnancy
loss 520 may
include a start date and an end date. The indication of the early pregnancy
loss 520 may
be an example of a detected miscarriage that previously occurred or currently
occurs
and/or a predicted miscarriage that likely occurs in the future.
44
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0141] The system may identify and/or predict the indication of the
early pregnancy
loss 520 in the time series of the temperature values 505 based on identifying
that the
temperature values 505 are lower than the pregnancy baseline of temperature
values 510
for the user. For example, the system may identify the temperature values 505
after
determining the time series and identify the pregnancy baseline of temperature
values
510. The system may detect the indication of the early pregnancy loss 520 of
the user in
response to identifying that the temperature values 505 are lower than the
pregnancy
baseline of temperature values 510 for the user.
[0142] In some cases, the temperature values 505 may deviate from the
pregnancy
baseline of temperature values 510 for the user after the indication of
pregnancy 515.
For example, after identifying the indication of pregnancy 515, the system may
determine that the temperature values 505 deviation from (e.g., are less than)
the
pregnancy baseline of temperature values 510. The system may compute a
deviation in
the time series of the temperature values 505 relative to the pregnancy
baseline of
temperature values 510 for the user in response to determining the time
series. The
deviation may include a decrease in the temperature values 505 from the
pregnancy
baseline of temperature values 510 for the user. In such cases, identifying
that the
temperature values 505 are lower than the pregnancy baseline of temperature
values 510
is in response to computing the deviation.
[0143] In some cases, the system may determine, or estimate, the
temperature
maximum and/or minimum for a user after determining the time series of the
temperature values 505 for the user collected via the ring. The system may
identify the
one or more positive slopes 530 of the time series of the temperature values
505 based
on determining the maximum and/or minimum. In some cases, calculating the
difference between the maximum and minimum may determine the positive slope
530.
In other examples, identifying the one or more positive slopes 530 of the time
series of
the temperature values 505 may be in response to computing a derivative of the
original
time series temperature data (e.g., temperature values 505).
[0144] The system may identify the one or more positive slopes 525 of
the time
series of the pregnancy baseline of temperature values 510 based on
determining the
maximum and/or minimum. In some cases, calculating the difference between the
maximum and minimum may determine the positive slope 525. In other examples,
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
identifying the one or more positive slopes 525 of the time series of the
pregnancy
baseline of temperature values 510 may be in response to computing a
derivative of the
pregnancy baseline of temperature values 510.
[0145] In some implementations, the system may identify that one or more
positive
slopes 530 of the temperature values 505 are lower than a positive slope 525
for a
pregnancy baseline of temperature values 510 for the user in response to
determining
the time series. For example, the slope 530 of the temperature values 505 may
be below
the slope 525 of the pregnancy baseline of temperature values 510 for the
user. In some
cases, the degree (e.g., angle of slope) of the slope 530 of the temperature
values 505
.. may be less than the degree of the slope 525 of the pregnancy baseline of
temperature
values 510 for the user. In such cases, identifying that the temperature
values 505 are
lower than the pregnancy baseline of temperature values 510 for the user is in
response
to identifying that the one or more positive slopes 530 are lower than the
positive slope
525 for the pregnancy baseline of temperature values 510 for the user. For
example, the
temperature rise is lower and the temperature decrease occurs earlier for the
temperature
values 505 as compared to the pregnancy baseline of temperature values 510 for
the
user. In such cases, the temperature patterns for a user experiencing or
predicted to
experience a miscarriage is different than a temperature pattern for a user
experiencing a
full-term pregnancy.
[0146] As described in further detail herein, the system may be configured
to track
menstrual cycles, ovulation, pregnancy, and the like. In some cases, the
user's body
temperature pattern throughout the night may be an indicator that may
characterize
miscarriage. For example, skin temperature during the night may identify the
indication
of the early pregnancy loss 520. As such, the timing diagram 500 illustrates a
relationship between a user's temperature data and a time (e.g., over a
plurality of
months).
[0147] In some cases, the system may estimate a likelihood of future
early
pregnancy loss 520, a likelihood that the user will experience the early
pregnancy loss
520, or both, in response to identifying that the temperature values 505 are
lower than
the pregnancy baseline of temperature values 510 for the user. In such cases,
the system
may predict the indication of the early pregnancy loss 520, detect the
indication of the
early pregnancy loss 520, or both.
46
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0148] FIG. 6 illustrates an example of a GUI 600 that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure. The GUI 600 may implement, or be
implemented by,
aspects of the system 100, system 200, system 300, timing diagram 400, timing
diagram
500, or any combination thereof For example, the GUI 600 may be an example of
a
GUI 275 of a user device 106 (e.g., user device 106-a, 106-b, 106-c)
corresponding to a
user 102.
[0149] In some examples, the GUI 600 illustrates a series of application
pages 605
which may be displayed to a user via the GUI 600 (e.g., GUI 275 illustrated in
FIG. 2).
The server of the system may cause the GUI 600 of the user device (e.g.,
mobile device)
to display inquiries of whether the user activates the pregnancy mode and
wants to track
their pregnancy (e.g., via application page 605). In such cases, the system
may generate
a personalized tracking experience on the GUI 600 of the user device to
predict a risk
for pregnancy loss or detect when the pregnancy is no longer viable based on
the
contextual tags and user questions.
[0150] Continuing with the examples above, prior to detecting the
indication of the
early pregnancy loss of the user, the user may be presented with an
application page
upon opening the wearable application. The application page 605 may display a
request
to activate the pregnancy mode and enable the system to track the pregnancy.
In such
cases, the application page 605 may display an invitation card where the users
are
invited to enroll in the pregnancy tracking applications. The application page
605 may
display a prompt to the user to verify whether the pregnancy may be tracked or
dismiss
the message if the pregnancy is not tracked. The system may receive an
indication of
whether the user selects to opt-in to tracking the pregnancy or opt-out to
tracking the
pregnancy.
[0151] The user may be presented with an application page 605 upon
selecting
"yes" to tracking the pregnancy. The application page 605 may display a prompt
to the
user to verify the main reason to track pregnancy. In such cases, the
application page
605 may prompt the user to confirm the intent of tracking the pregnancy. For
example,
the system may receive, via the user device, a confirmation of the intended
use of the
pregnancy tracking system.
47
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0152] In some cases, the user may be presented with an application page
605 upon
confirming the intent. The application page 605 may display a prompt to the
user to
verify the day of conception, the due date, and the like. For example, the
system may
receive, via the user device, a confirmation of the due date. In some cases,
the
application page 605 may display a prompt to the user to indicate whether due
date may
not be determined.
[0153] In some cases, the user may be presented with an application page
605 upon
confirming the due date. The application page may display a prompt to the user
to verify
whether the user experience any pregnancy-related complications, any pre-
existing
medical conditions, any fertility treatments used to achieve pregnancy, any
sleep
disturbances of the use (e.g., whether the user is a shift worker), and the
like. For
example, the system may receive, via the user device, a confirmation of
whether the
user experience any pregnancy-related complications, any pre-existing medical
conditions, any fertility treatments used to achieve pregnancy, any sleep
disturbances of
.. the use (e.g., whether the user is a shift worker), and the like. Upon
receiving the
confirmations, the user may be presented with a GUI 600 that may be further
shown and
described with reference to application page 605.
[0154] The server of the system may generate a message for display on
the GUI 600
on a user device that indicates the indication of the early pregnancy loss.
For example,
the server of system may cause the GUI 600 of the user device (e.g., mobile
device) to
display a message 620 associated with the indication of the early pregnancy
loss (e.g.,
via application page 605). In such cases, the system may output the indication
of the
early pregnancy loss on the GUI 600 of the user device to indicate that the
pregnancy is
no longer viable, that the user is experiencing a risk of pregnancy loss,
and/or a
pregnancy loss is predicted for the future.
[0155] Continuing with the example above, upon detecting the indication
of the
early pregnancy loss of the user, the user may be presented with the
application page
605 upon opening the wearable application. As shown in FIG. 6, the application
page
605 may display the indication that the early pregnancy loss is predicted
and/or
identified via message 620. In such cases, the application page 605 may
include the
message 620 on the home page. In cases where a user's early pregnancy loss is
predicted and/or identified, as described herein, the server may transmit a
message 620
48
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
to the user, where the message 620 is associated with the predicted and/or
identified
early pregnancy loss. In some cases, the server may transmit a message 620 to
a
clinician, a fertility specialist, a care-taker, a partner of the user, or a
combination
thereof In such cases, the system may present application page 605 on the user
device
associated with the clinician, the fertility specialists, the care-taker, the
partner, or a
combination thereof
[0156] For example, the user may receive message 620, which may indicate
a time
interval during which the early pregnancy loss occurred, a time interval
during which
the early pregnancy loss is predicted to occur, a request to input symptoms
associated
with the early pregnancy loss, educational content associated with the early
pregnancy
loss, an adjusted set of sleep targets, an adjusted set of activity targets,
recommendations to improve symptoms associated with the early pregnancy loss,
and
the like. For example, the message 620 may indicate a risk for pregnancy loss.
The
messages 620 may be configurable/customizable, such that the user may receive
different messages 620 based on the prediction and identification of the early
pregnancy
loss, as described previously herein.
[0157] As shown in FIG. 6, the application page 605 may display the
indication of
the early pregnancy loss via alert 610. The user may receive alert 610, which
may
prompt the user to verify whether the early pregnancy loss has occurred or
dismiss the
alert 610 if the early pregnancy loss has not occurred. In such cases, the
application
page 605 may prompt the user to confirm or dismiss the early pregnancy loss
(e.g.,
confirm/deny whether the system correctly detected the indication of the early
pregnancy loss and/or confirm/deny whether the pregnancy loss has been
confirmed via
a clinician). For example, the system may receive, via the user device and in
response to
detecting the indication of the early pregnancy loss, a confirmation of the
pregnancy
loss.
[0158] Additionally, in some implementations, the application page 605
may
display one or more scores (e.g., Sleep Score, Readiness Score, etc.) for the
user for the
respective day. Moreover, in some cases, the predicted and/or identified
pregnancy loss
may be used to update (e.g., modify) one or more scores associated with the
user (e.g.,
Sleep Score, Readiness Score, etc.). That is, data associated with the
predicted and/or
identified pregnancy loss may be used to update the scores for the user for
the following
49
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
calendar days. In such cases, the system may notify the user of the score
update via alert
610.
[0159] In some cases, the Readiness Score may be updated based on the
detected
indication of the early pregnancy loss. In such cases, the Readiness Score may
indicate
.. to the user to "pay attention" based on the predicted and/or identified
early pregnancy
loss. If the Readiness Score changes for the user, the system may implement a
recovery
mode for users whose symptoms may be severe and may benefit from adjusted
activity
and readiness guidance for a couple of days. In other examples, the Readiness
Score
may be updated based on the Sleep Score. However, the system may determine
that the
user is experiencing a miscarriage or predicted to experience a miscarriage
and may
adjust the Readiness Score, Sleep Score, and/or Activity Score to offset the
effects of
the miscarriage.
[0160] In some cases, the messages 620 displayed to the user via the GUI
600 of the
user device may indicate how the predicted and/or identified early pregnancy
loss
affected the overall scores (e.g., overall Readiness Score) and/or the
individual
contributing factors. For example, a message may indicate "It looks like your
body is
under strain right now, but if you're feeling ok, doing a light or medium
intensity
exercise can help your body battle the symptoms" or "From your recovery
metrics it
looks like your body is still doing ok, so some light activity can help
relieve the
.. symptoms. Hope you'll feel better tomorrow!" In cases where the early
pregnancy loss
is predicted and/or identified, the messages 620 may provide suggestions for
the user in
order to improve their general health based on user history of the user, a
group of users,
general knowledge, or a combination thereof For example, the message may
indicate
"If you feel really low on energy, why not switch to rest mode for today," or
"Since you
.. have cramps and pain, devote today for rest." In such cases, the messages
620 displayed
to the user may provide targeted insights to help the user adjust their
lifestyle.
[0161] The application page 605 may indicate one or more parameters,
including a
temperature, heart rate, HRV, respiratory rate, sleep data, and the like
experienced by
the user during the pregnancy loss via the graphical representation 615. The
graphical
representation 615 may be an example of the timing diagram 400, as described
with
reference to FIG. 4. In such cases, the system may cause the GUI 600 of a user
device to
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
display a message 620, alert 610, or graphical representation 615 associated
with the
detected indication of pregnancy loss.
[0162] In some cases, the user may log symptoms via user input 625. For
example,
the system may receive user input (e.g., tags) to log symptoms associated with
the
pregnancy loss, or the like (e.g., flow, cramps, headaches, migraine, pain,
etc.). The
system may recommend tags to the user based on user history for the user, a
group of
users, the predicted and/or identified early pregnancy loss, or a combination
thereof In
some cases, the system may cause the GUI 600 of the user device to display
symptom
tags based on a correlation between prior user symptom tags and a timing of
the early
pregnancy loss.
[0163] Application page 605 may also include message 620 that includes
insights,
recommendations, and the like associated with the predicted and/or identified
early
pregnancy loss. The server of system may cause the GUI 600 of the user device
to
display a message 620 associated with the predicted and/or identified early
pregnancy
loss. The user device may display recommendations and/or information
associated with
the predicted and/or identified early pregnancy loss via message 620. As noted
previously herein, an accurately predicted and/or identified early pregnancy
loss may be
beneficial to a user's overall health and recovery process.
[0164] In some implementations, the system may provide additional
insight
regarding the user's predicted and/or identified pregnancy loss. For example,
the
application pages 605 may indicate one or more physiological parameters (e.g.,
contributing factors) which resulted in the user's predicted and/or identified
early
pregnancy loss, such as decreased temperature relative to a pregnancy
baseline, and the
like. In other words, the system may be configured to provide some information
or other
.. insights regarding the predicted and/or identified pregnancy loss.
Personalized insights
may indicate aspects of collected physiological data (e.g., contributing
factors within the
physiological data) which were used to generate the predicted and/or
identified
pregnancy loss.
[0165] In some implementations, the system may be configured to receive
user
inputs regarding the identified and/or predicted early pregnancy loss in order
to train
classifiers (e.g., supervised learning for a machine learning classifier) and
improve
51
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
pregnancy loss determination and/or prediction techniques. For example, the
user device
may receive user inputs 625, and these user inputs 625 may then be input into
the
classifier to train the classifier. In some examples, the system may use the
detected
indication of pregnancy loss to train the system to more accurately and/or
precisely
predict future pregnancy loss for the user and/or other users. For example, if
the user
tags a pregnancy loss that the system did not initially predict, the system
may detect a
pattern indicative of pregnancy loss and apply the pattern to future
pregnancies of the
user or a group of users.
[0166] Upon predicting and/or identifying the early pregnancy loss on
application
.. page 605, the GUI 600 may display a calendar view that may indicate a
current date that
the user is viewing application page 605, a date range including the day when
the early
pregnancy loss is predicted and/or identified, and a date range including the
day when
the early pregnancy loss is predicted and/or identified. For example, the date
range may
encircle the calendar days using a dashed line configuration, the current date
may
.. encircle the calendar day, and the day when early pregnancy loss is
predicted may be
encircled. The calendar view may also include a message including the current
calendar
day and indication of the day of the user's pregnancy (e.g., that the user is
8 weeks
pregnant).
[0167] FIG. 7 shows a block diagram 700 of a device 705 that supports
miscarriage
identification and prediction from wearable-based physiological data in
accordance with
aspects of the present disclosure. The device 705 may include an input module
710, an
output module 715, and a wearable application 720. The device 705 may also
include a
processor. Each of these components may be in communication with one another
(e.g.,
via one or more buses).
[0168] The input module 710 may provide a means for receiving information
such
as packets, user data, control information, or any combination thereof
associated with
various information channels (e.g., control channels, data channels,
information
channels related to illness detection techniques). Information may be passed
on to other
components of the device 705. The input module 710 may utilize a single
antenna or a
set of multiple antennas.
52
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0169] The output module 715 may provide a means for transmitting
signals
generated by other components of the device 705. For example, the output
module 715
may transmit information such as packets, user data, control information, or
any
combination thereof associated with various information channels (e.g.,
control
channels, data channels, information channels related to illness detection
techniques). In
some examples, the output module 715 may be co-located with the input module
710 in
a transceiver module. The output module 715 may utilize a single antenna or a
set of
multiple antennas.
[0170] For example, the wearable application 720 may include a data
acquisition
component 725, a temperature data component 730, a calculation component 735,
a
miscarriage component 740, a user interface component 745, or any combination
thereof In some examples, the wearable application 720, or various components
thereof, may be configured to perform various operations (e.g., receiving,
monitoring,
transmitting) using or otherwise in cooperation with the input module 710, the
output
module 715, or both. For example, the wearable application 720 may receive
information from the input module 710, send information to the output module
715, or
be integrated in combination with the input module 710, the output module 715,
or both
to receive information, transmit information, or perform various other
operations as
described herein.
[0171] The data acquisition component 725 may be configured as or otherwise
support a means for receiving, from a wearable device, physiological data
associated
with a user that is pregnant, the physiological data comprising at least
temperature data.
The temperature data component 730 may be configured as or otherwise support a
means for determining a time series of a plurality of temperature values taken
over a
plurality of days based at least in part on the received temperature data. The
calculation
component 735 may be configured as or otherwise support a means for
identifying that
the plurality of temperature values are lower than a pregnancy baseline of
temperature
values for the user based at least in part on determining the time series. The
miscarriage
component 740 may be configured as or otherwise support a means for detecting
an
indication of an early pregnancy loss of the user based at least in part on
identifying that
the plurality of temperature values are lower than the pregnancy baseline of
temperature
values for the user. The user interface component 745 may be configured as or
53
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
otherwise support a means for generating a message for display on a graphical
user
interface on a user device that indicates the indication of the early
pregnancy loss.
[0172] FIG. 8 shows a block diagram 800 of a wearable application 820
that
supports miscarriage identification and prediction from wearable-based
physiological
data in accordance with aspects of the present disclosure. The wearable
application 820
may be an example of aspects of a wearable application or a wearable
application 720,
or both, as described herein. The wearable application 820, or various
components
thereof, may be an example of means for performing various aspects of
miscarriage
identification and prediction from wearable-based physiological data as
described
herein. For example, the wearable application 820 may include a data
acquisition
component 825, a temperature data component 830, a calculation component 835,
a
miscarriage component 840, a user interface component 845, or any combination
thereof Each of these components may communicate, directly or indirectly, with
one
another (e.g., via one or more buses).
[0173] The data acquisition component 825 may be configured as or otherwise
support a means for receiving, from a wearable device, physiological data
associated
with a user that is pregnant, the physiological data comprising at least
temperature data.
The temperature data component 830 may be configured as or otherwise support a
means for determining a time series of a plurality of temperature values taken
over a
plurality of days based at least in part on the received temperature data. The
calculation
component 835 may be configured as or otherwise support a means for
identifying that
the plurality of temperature values are lower than a pregnancy baseline of
temperature
values for the user based at least in part on determining the time series. The
miscarriage
component 840 may be configured as or otherwise support a means for detecting
an
indication of an early pregnancy loss of the user based at least in part on
identifying that
the plurality of temperature values are lower than the pregnancy baseline of
temperature
values for the user. The user interface component 845 may be configured as or
otherwise support a means for generating a message for display on a graphical
user
interface on a user device that indicates the indication of the early
pregnancy loss.
[0174] In some examples, the calculation component 835 may be configured as
or
otherwise support a means for computing a deviation in the time series of the
plurality
of temperature values relative to the pregnancy baseline of temperature values
for the
54
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
user based at least in part on determining the time series, wherein the
deviation
comprises a decrease in the plurality of temperature values from the pregnancy
baseline
of temperature values for the user, wherein identifying that the plurality of
temperature
values are lower than the pregnancy baseline of temperature values is based at
least in
part on computing the deviation.
[0175] In some examples, the calculation component 835 may be configured
as or
otherwise support a means for identifying that one or more positive slopes of
the
plurality of temperature values are lower than a positive slope for a
pregnancy baseline
of temperature values for the user based at least in part on determining the
time series,
wherein identifying that the plurality of temperature values are lower than
the
pregnancy baseline of temperature values for the user is based at least in
part on
identifying that the one or more positive slopes are lower than the positive
slope for the
pregnancy baseline of temperature values for the user.
[0176] In some examples, the physiological data further comprises heart
rate data,
and the data acquisition component 825 may be configured as or otherwise
support a
means for determining that the received heart rate data exceeds a pregnancy
baseline
heart rate for the user for at least a portion of the plurality of days,
wherein detecting the
indication of the early pregnancy loss is based at least in part on
determining that the
received heart rate data exceeds the pregnancy baseline heart rate for the
user.
[0177] In some examples, the physiological data further comprises heart
rate
variability data, and the data acquisition component 825 may be configured as
or
otherwise support a means for determining that the received heart rate
variability data is
less than a pregnancy baseline heart rate variability for the user for at
least a portion of
the plurality of days, wherein detecting the indication of the early pregnancy
loss is
based at least in part on determining that the received heart rate variability
data satisfies
the threshold.
[0178] In some examples, the physiological data further comprises
respiratory rate
data, and the data acquisition component 825 may be configured as or otherwise
support
a means for determining that the received respiratory rate data exceeds a
pregnancy
baseline respiratory rate for the user for at least a portion of the plurality
of days,
wherein detecting the indication of the early pregnancy loss is based at least
in part on
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
determining that the received respiratory rate data exceeds the pregnancy
baseline
respiratory rate for the user.
[0179] In some examples, the physiological data further comprises sleep
data, and
the data acquisition component 825 may be configured as or otherwise support a
means
for determining that a quantity of detected sleep disturbances from the
received sleep
data exceeds a pregnancy baseline sleep disturbance threshold for the user for
at least a
portion of the plurality of days, wherein detecting the indication of the
early pregnancy
loss is based at least in part on determining that the quantity of detected
sleep
disturbances exceeds the pregnancy baseline sleep disturbance threshold for
the user.
[0180] In some examples, the miscarriage component 840 may be configured as
or
otherwise support a means for identifying a presence of a menstrual cycle
within a time
period after pregnancy based at least in part on determining the time series,
wherein
detecting the indication of the early pregnancy loss is based at least in part
on
identifying the presence of the menstrual cycle.
[0181] In some examples, the user interface component 845 may be configured
as
or otherwise support a means for receiving a confirmation of a menstrual cycle
within a
time period after pregnancy, a confirmation of a pregnancy loss, or both,
wherein
detecting the indication of the early pregnancy loss is based at least in part
on receiving
the confirmation.
[0182] In some examples, the temperature data component 830 may be
configured
as or otherwise support a means for determining each temperature value of the
plurality
of temperature values based at least in part on receiving the temperature
data, wherein
the temperature data comprises continuous nighttime temperature data.
[0183] In some examples, the miscarriage component 840 may be configured
as or
otherwise support a means for estimating a likelihood of future early
pregnancy loss, a
likelihood that the user will experience the early pregnancy loss, or both,
based at least
in part on identifying that the plurality of temperature values are lower than
the
pregnancy baseline of temperature values for the user, wherein detecting the
indication
of the early pregnancy loss is based at least in part on the estimation.
56
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0184] In some examples, the calculation component 835 may be configured
as or
otherwise support a means for updating a readiness score associated with the
user, an
activity score associated with the user, a sleep score associated with the
user, or a
combination thereof, based at least in part on detecting the indication of the
early
.. pregnancy loss.
[0185] In some examples, the user interface component 845 may be
configured as
or otherwise support a means for transmitting the message that indicates the
indication
of the early pregnancy loss to the user device, wherein the user device is
associated with
a clinician, the user, or both.
[0186] In some examples, the user interface component 845 may be configured
as
or otherwise support a means for causing a graphical user interface of a user
device
associated with the user to display early pregnancy loss symptom tags based at
least in
part on detecting the indication of the early pregnancy loss.
[0187] In some examples, the user interface component 845 may be
configured as
or otherwise support a means for causing a graphical user interface of a user
device
associated with the user to display a message associated with the indication
of the early
pregnancy loss.
[0188] In some examples, the message further comprises a time interval
during
which the early pregnancy loss occurred, a time interval during which the
early
pregnancy loss is predicted to occur, a request to input symptoms associated
with the
early pregnancy loss, educational content associated with the early pregnancy
loss, an
adjusted set of sleep targets, an adjusted set of activity targets,
recommendations to
improve symptoms associated with the early pregnancy loss, or a combination
thereof
[0189] In some examples, the calculation component 835 may be configured
as or
otherwise support a means for inputting the physiological data into a machine
learning
classifier, wherein detecting the indication of the early pregnancy loss is
based at least
in part on inputting the physiological data into the machine learning
classifier.
[0190] In some examples, the wearable device comprises a wearable ring
device.
[0191] In some examples, the wearable device collects the physiological
data from
the user based on arterial blood flow.
57
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0192] FIG. 9 shows a diagram of a system 900 including a device 905
that
supports miscarriage identification and prediction from wearable-based
physiological
data in accordance with aspects of the present disclosure. The device 905 may
be an
example of or include the components of a device 705 as described herein. The
device
905 may include an example of a user device 106, as described previously
herein. The
device 905 may include components for bi-directional communications including
components for transmitting and receiving communications with a wearable
device 104
and a server 110, such as a wearable application 920, a communication module
910, an
antenna 915, a user interface component 925, a database (application data)
930, a
memory 935, and a processor 940. These components may be in electronic
communication or otherwise coupled (e.g., operatively, communicatively,
functionally,
electronically, electrically) via one or more buses (e.g., a bus 945).
[0193] The communication module 910 may manage input and output signals
for
the device 905 via the antenna 915. The communication module 910 may include
an
example of the communication module 220-b of the user device 106 shown and
described in FIG. 2. In this regard, the communication module 910 may manage
communications with the ring 104 and the server 110, as illustrated in FIG. 2.
The
communication module 910 may also manage peripherals not integrated into the
device
905. In some cases, the communication module 910 may represent a physical
connection or port to an external peripheral. In some cases, the communication
module
910 may utilize an operating system such as i0S0, ANDROID , MS-DOS , MS-
WINDOWS , OS/20, UNIX , LINUX , or another known operating system. In other
cases, the communication module 910 may represent or interact with a wearable
device
(e.g., ring 104), modem, a keyboard, a mouse, a touchscreen, or a similar
device. In
.. some cases, the communication module 910 may be implemented as part of the
processor 940. In some examples, a user may interact with the device 905 via
the
communication module 910, user interface component 925, or via hardware
components
controlled by the communication module 910.
[0194] In some cases, the device 905 may include a single antenna 915.
However, in
some other cases, the device 905 may have more than one antenna 915, which may
be
capable of concurrently transmitting or receiving multiple wireless
transmissions. The
communication module 910 may communicate bi-directionally, via the one or more
58
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
antennas 915, wired, or wireless links as described herein. For example, the
communication module 910 may represent a wireless transceiver and may
communicate
bi-directionally with another wireless transceiver. The communication module
910 may
also include a modem to modulate the packets, to provide the modulated packets
to one
or more antennas 915 for transmission, and to demodulate packets received from
the
one or more antennas 915.
[0195] The user interface component 925 may manage data storage and
processing
in a database 930. In some cases, a user may interact with the user interface
component
925. In other cases, the user interface component 925 may operate
automatically
without user interaction. The database 930 may be an example of a single
database, a
distributed database, multiple distributed databases, a data store, a data
lake, or an
emergency backup database.
[0196] The memory 935 may include RAM and ROM. The memory 935 may store
computer-readable, computer-executable software including instructions that,
when
executed, cause the processor 940 to perform various functions described
herein. In
some cases, the memory 935 may contain, among other things, a BIOS which may
control basic hardware or software operation such as the interaction with
peripheral
components or devices.
[0197] The processor 940 may include an intelligent hardware device,
(e.g., a
general-purpose processor, a DSP, a CPU, a microcontroller, an ASIC, an FPGA,
a
programmable logic device, a discrete gate or transistor logic component, a
discrete
hardware component, or any combination thereof). In some cases, the processor
940
may be configured to operate a memory array using a memory controller. In
other cases,
a memory controller may be integrated into the processor 940. The processor
940 may
be configured to execute computer-readable instructions stored in a memory 935
to
perform various functions (e.g., functions or tasks supporting a method and
system for
sleep staging algorithms).
[0198] For example, the wearable application 920 may be configured as or
otherwise support a means for receiving, from a wearable device, physiological
data
.. associated with a user that is pregnant, the physiological data comprising
at least
temperature data. The wearable application 920 may be configured as or
otherwise
59
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
support a means for determining a time series of a plurality of temperature
values taken
over a plurality of days based at least in part on the received temperature
data. The
wearable application 920 may be configured as or otherwise support a means for
identifying that the plurality of temperature values are lower than a
pregnancy baseline
of temperature values for the user based at least in part on determining the
time series.
The wearable application 920 may be configured as or otherwise support a means
for
detecting an indication of an early pregnancy loss of the user based at least
in part on
identifying that the plurality of temperature values are lower than the
pregnancy
baseline of temperature values for the user. The wearable application 920 may
be
configured as or otherwise support a means for generating a message for
display on a
graphical user interface on a user device that indicates the indication of the
early
pregnancy loss.
[0199] By including or configuring the wearable application 920 in
accordance with
examples as described herein, the device 905 may support techniques for
improved
communication reliability, reduced latency, improved user experience related
to reduced
processing, reduced power consumption, more efficient utilization of
communication
resources, improved coordination between devices, longer battery life,
improved
utilization of processing capability.
[0200] The wearable application 920 may include an application (e.g.,
"app"),
program, software, or other component which is configured to facilitate
communications with a ring 104, server 110, other user devices 106, and the
like. For
example, the wearable application 920 may include an application executable on
a user
device 106 which is configured to receive data (e.g., physiological data) from
a ring
104, perform processing operations on the received data, transmit and receive
data with
the servers 110, and cause presentation of data to a user 102.
[0201] FIG. 10 shows a flowchart illustrating a method 1000 that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure. The operations of the
method 1000
may be implemented by a user device or its components as described herein. For
example, the operations of the method 1000 may be performed by a user device
as
described with reference to FIGs. 1 through 9. In some examples, a user device
may
execute a set of instructions to control the functional elements of the user
device to
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
perform the described functions. Additionally, or alternatively, the user
device may
perform aspects of the described functions using special-purpose hardware.
[0202] At 1005, the method may include receiving, from a wearable
device,
physiological data associated with a user that is pregnant, the physiological
data
comprising at least temperature data. The operations of 1005 may be performed
in
accordance with examples as disclosed herein. In some examples, aspects of the
operations of 1005 may be performed by a data acquisition component 825 as
described
with reference to FIG. 8.
[0203] At 1010, the method may include determining a time series of a
plurality of
temperature values taken over a plurality of days based at least in part on
the received
temperature data. The operations of 1010 may be performed in accordance with
examples as disclosed herein. In some examples, aspects of the operations of
1010 may
be performed by a temperature data component 830 as described with reference
to
FIG. 8.
[0204] At 1015, the method may include identifying that the plurality of
temperature values are lower than a pregnancy baseline of temperature values
for the
user based at least in part on determining the time series. The operations of
1015 may be
performed in accordance with examples as disclosed herein. In some examples,
aspects
of the operations of 1015 may be performed by a calculation component 835 as
described with reference to FIG. 8.
[0205] At 1020, the method may include detecting an indication of an
early
pregnancy loss of the user based at least in part on identifying that the
plurality of
temperature values are lower than the pregnancy baseline of temperature values
for the
user. The operations of 1020 may be performed in accordance with examples as
disclosed herein. In some examples, aspects of the operations of 1020 may be
performed by a miscarriage component 840 as described with reference to FIG.
8.
[0206] At 1025, the method may include generating a message for display
on a
graphical user interface on a user device that indicates the indication of the
early
pregnancy loss. The operations of 1025 may be performed in accordance with
examples
as disclosed herein. In some examples, aspects of the operations of 1025 may
be
performed by a user interface component 845 as described with reference to
FIG. 8.
61
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0207] FIG. 11 shows a flowchart illustrating a method 1100 that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure. The operations of the
method 1100
may be implemented by a user device or its components as described herein. For
example, the operations of the method 1100 may be performed by a user device
as
described with reference to FIGs. 1 through 9. In some examples, a user device
may
execute a set of instructions to control the functional elements of the user
device to
perform the described functions. Additionally, or alternatively, the user
device may
perform aspects of the described functions using special-purpose hardware.
[0208] At 1105, the method may include receiving, from a wearable device,
physiological data associated with a user that is pregnant, the physiological
data
comprising at least temperature data. The operations of 1105 may be performed
in
accordance with examples as disclosed herein. In some examples, aspects of the
operations of 1105 may be performed by a data acquisition component 825 as
described
with reference to FIG. 8.
[0209] At 1110, the method may include determining a time series of a
plurality of
temperature values taken over a plurality of days based at least in part on
the received
temperature data. The operations of 1110 may be performed in accordance with
examples as disclosed herein. In some examples, aspects of the operations of
1110 may
be performed by a temperature data component 830 as described with reference
to
FIG. 8.
[0210] At 1115, the method may include computing a deviation in the time
series of
the plurality of temperature values relative to the pregnancy baseline of
temperature
values for the user based at least in part on determining the time series,
wherein the
.. deviation comprises a decrease in the plurality of temperature values from
the
pregnancy baseline of temperature values for the user, wherein identifying
that the
plurality of temperature values are lower than the pregnancy baseline of
temperature
values is based at least in part on computing the deviation. The operations of
1115 may
be performed in accordance with examples as disclosed herein. In some
examples,
aspects of the operations of 1115 may be performed by a calculation component
835 as
described with reference to FIG. 8.
62
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0211] At 1120, the method may include identifying that the plurality of
temperature values are lower than a pregnancy baseline of temperature values
for the
user based at least in part on determining the time series. The operations of
1120 may be
performed in accordance with examples as disclosed herein. In some examples,
aspects
of the operations of 1120 may be performed by a calculation component 835 as
described with reference to FIG. 8.
[0212] At 1125, the method may include detecting an indication of an
early
pregnancy loss of the user based at least in part on identifying that the
plurality of
temperature values are lower than the pregnancy baseline of temperature values
for the
user. The operations of 1125 may be performed in accordance with examples as
disclosed herein. In some examples, aspects of the operations of 1125 may be
performed by a miscarriage component 840 as described with reference to FIG.
8.
[0213] At 1130, the method may include generating a message for display
on a
graphical user interface on a user device that indicates the indication of the
early
pregnancy loss. The operations of 1130 may be performed in accordance with
examples
as disclosed herein. In some examples, aspects of the operations of 1130 may
be
performed by a user interface component 845 as described with reference to
FIG. 8.
[0214] FIG. 12 shows a flowchart illustrating a method 1200 that
supports
miscarriage identification and prediction from wearable-based physiological
data in
accordance with aspects of the present disclosure. The operations of the
method 1200
may be implemented by a user device or its components as described herein. For
example, the operations of the method 1200 may be performed by a user device
as
described with reference to FIGs. 1 through 9. In some examples, a user device
may
execute a set of instructions to control the functional elements of the user
device to
perform the described functions. Additionally, or alternatively, the user
device may
perform aspects of the described functions using special-purpose hardware.
[0215] At 1205, the method may include receiving, from a wearable
device,
physiological data associated with a user that is pregnant, the physiological
data
comprising at least temperature data. The operations of 1205 may be performed
in
accordance with examples as disclosed herein. In some examples, aspects of the
63
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
operations of 1205 may be performed by a data acquisition component 825 as
described
with reference to FIG. 8.
[0216] At 1210, the method may include determining a time series of a
plurality of
temperature values taken over a plurality of days based at least in part on
the received
temperature data. The operations of 1210 may be performed in accordance with
examples as disclosed herein. In some examples, aspects of the operations of
1210 may
be performed by a temperature data component 830 as described with reference
to
FIG. 8.
[0217] At 1215, the method may include identifying that one or more
positive
slopes of the plurality of temperature values are lower than a positive slope
for a
pregnancy baseline of temperature values for the user based at least in part
on
determining the time series, wherein identifying that the plurality of
temperature values
are lower than the pregnancy baseline of temperature values for the user is
based at least
in part on identifying that the one or more positive slopes are lower than the
positive
slope for the pregnancy baseline of temperature values for the user. The
operations of
1215 may be performed in accordance with examples as disclosed herein. In some
examples, aspects of the operations of 1215 may be performed by a calculation
component 835 as described with reference to FIG. 8.
[0218] At 1220, the method may include identifying that the plurality of
temperature values are lower than a pregnancy baseline of temperature values
for the
user based at least in part on determining the time series. The operations of
1220 may be
performed in accordance with examples as disclosed herein. In some examples,
aspects
of the operations of 1220 may be performed by a calculation component 835 as
described with reference to FIG. 8.
[0219] At 1225, the method may include detecting an indication of an early
pregnancy loss of the user based at least in part on identifying that the
plurality of
temperature values are lower than the pregnancy baseline of temperature values
for the
user. The operations of 1225 may be performed in accordance with examples as
disclosed herein. In some examples, aspects of the operations of 1225 may be
performed by a miscarriage component 840 as described with reference to FIG.
8.
64
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0220] At 1230, the method may include generating a message for display
on a
graphical user interface on a user device that indicates the indication of the
early
pregnancy loss. The operations of 1230 may be performed in accordance with
examples
as disclosed herein. In some examples, aspects of the operations of 1230 may
be
performed by a user interface component 845 as described with reference to
FIG. 8.
[0221] It should be noted that the methods described above describe
possible
implementations, and that the operations and the steps may be rearranged or
otherwise
modified and that other implementations are possible. Furthermore, aspects
from two or
more of the methods may be combined.
[0222] A method is described. The method may include receiving, from a
wearable
device, physiological data associated with a user that is pregnant, the
physiological data
comprising at least temperature data, determining a time series of a plurality
of
temperature values taken over a plurality of days based at least in part on
the received
temperature data, identifying that the plurality of temperature values are
lower than a
pregnancy baseline of temperature values for the user based at least in part
on
determining the time series, detecting an indication of an early pregnancy
loss of the
user based at least in part on identifying that the plurality of temperature
values are
lower than the pregnancy baseline of temperature values for the user, and
generating a
message for display on a graphical user interface on a user device that
indicates the
indication of the early pregnancy loss.
[0223] An apparatus is described. The apparatus may include a processor,
memory
coupled with the processor, and instructions stored in the memory. The
instructions may
be executable by the processor to cause the apparatus to receive, from a
wearable
device, physiological data associated with a user that is pregnant, the
physiological data
comprising at least temperature data, determine a time series of a plurality
of
temperature values taken over a plurality of days based at least in part on
the received
temperature data, identify that the plurality of temperature values are lower
than a
pregnancy baseline of temperature values for the user based at least in part
on
determining the time series, detect an indication of an early pregnancy loss
of the user
based at least in part on identifying that the plurality of temperature values
are lower
than the pregnancy baseline of temperature values for the user, and generate a
message
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
for display on a graphical user interface on a user device that indicates the
indication of
the early pregnancy loss.
[0224] Another apparatus is described. The apparatus may include means
for
receiving, from a wearable device, physiological data associated with a user
that is
.. pregnant, the physiological data comprising at least temperature data,
means for
determining a time series of a plurality of temperature values taken over a
plurality of
days based at least in part on the received temperature data, means for
identifying that
the plurality of temperature values are lower than a pregnancy baseline of
temperature
values for the user based at least in part on determining the time series,
means for
.. detecting an indication of an early pregnancy loss of the user based at
least in part on
identifying that the plurality of temperature values are lower than the
pregnancy
baseline of temperature values for the user, and means for generating a
message for
display on a graphical user interface on a user device that indicates the
indication of the
early pregnancy loss.
[0225] A non-transitory computer-readable medium storing code is described.
The
code may include instructions executable by a processor to receive, from a
wearable
device, physiological data associated with a user that is pregnant, the
physiological data
comprising at least temperature data, determine a time series of a plurality
of
temperature values taken over a plurality of days based at least in part on
the received
temperature data, identify that the plurality of temperature values are lower
than a
pregnancy baseline of temperature values for the user based at least in part
on
determining the time series, detect an indication of an early pregnancy loss
of the user
based at least in part on identifying that the plurality of temperature values
are lower
than the pregnancy baseline of temperature values for the user, and generate a
message
for display on a graphical user interface on a user device that indicates the
indication of
the early pregnancy loss.
[0226] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for computing a deviation in the time series of the plurality of
temperature
values relative to the pregnancy baseline of temperature values for the user
based at
least in part on determining the time series, wherein the deviation comprises
a decrease
in the plurality of temperature values from the pregnancy baseline of
temperature values
66
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
for the user, wherein identifying that the plurality of temperature values may
be lower
than the pregnancy baseline of temperature values may be based at least in
part on
computing the deviation.
[0227] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for identifying that one or more positive slopes of the plurality
of
temperature values may be lower than a positive slope for a pregnancy baseline
of
temperature values for the user based at least in part on determining the time
series,
wherein identifying that the plurality of temperature values may be lower than
the
pregnancy baseline of temperature values for the user may be based at least in
part on
identifying that the one or more positive slopes may be lower than the
positive slope for
the pregnancy baseline of temperature values for the user.
[0228] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the physiological data further comprises
heart rate
data and the method, apparatuses, and non-transitory computer-readable medium
may
include further operations, features, means, or instructions for determining
that the
received heart rate data exceeds a pregnancy baseline heart rate for the user
for at least a
portion of the plurality of days, wherein detecting the indication of the
early pregnancy
loss may be based at least in part on determining that the received heart rate
data
exceeds the pregnancy baseline heart rate for the user.
[0229] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the physiological data further comprises
heart rate
variability data and the method, apparatuses, and non-transitory computer-
readable
medium may include further operations, features, means, or instructions for
determining
that the received heart rate variability data may be less than a pregnancy
baseline heart
rate variability for the user for at least a portion of the plurality of days,
wherein
detecting the indication of the early pregnancy loss may be based at least in
part on
determining that the received heart rate variability data satisfies the
threshold.
[0230] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the physiological data further comprises
respiratory
rate data and the method, apparatuses, and non-transitory computer-readable
medium
67
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
may include further operations, features, means, or instructions for
determining that the
received respiratory rate data exceeds a pregnancy baseline respiratory rate
for the user
for at least a portion of the plurality of days, wherein detecting the
indication of the
early pregnancy loss may be based at least in part on determining that the
received
respiratory rate data exceeds the pregnancy baseline respiratory rate for the
user.
[0231] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the physiological data further comprises
sleep data
and the method, apparatuses, and non-transitory computer-readable medium may
include further operations, features, means, or instructions for determining
that a
quantity of detected sleep disturbances from the received sleep data exceeds a
pregnancy baseline sleep disturbance threshold for the user for at least a
portion of the
plurality of days, wherein detecting the indication of the early pregnancy
loss may be
based at least in part on determining that the quantity of detected sleep
disturbances
exceeds the pregnancy baseline sleep disturbance threshold for the user.
[0232] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for identifying a presence of a menstrual cycle within a time
period after
pregnancy based at least in part on determining the time series, wherein
detecting the
indication of the early pregnancy loss may be based at least in part on
identifying the
presence of the menstrual cycle.
[0233] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for receiving a confirmation of a menstrual cycle within a time
period after
pregnancy, a confirmation of a pregnancy loss, or both, wherein detecting the
indication
of the early pregnancy loss may be based at least in part on receiving the
confirmation.
[0234] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for determining each temperature value of the plurality of
temperature
values based at least in part on receiving the temperature data, wherein the
temperature
data comprises continuous nighttime temperature data.
68
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0235] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for estimating a likelihood of future early pregnancy loss, a
likelihood that
the user will experience the early pregnancy loss, or both, based at least in
part on
identifying that the plurality of temperature values may be lower than the
pregnancy
baseline of temperature values for the user, wherein detecting the indication
of the early
pregnancy loss may be based at least in part on the estimation.
[0236] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for updating a readiness score associated with the user, an
activity score
associated with the user, a sleep score associated with the user, or a
combination
thereof, based at least in part on detecting the indication of the early
pregnancy loss.
[0237] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for transmitting the message that indicates the indication of the
early
pregnancy loss to the user device, wherein the user device may be associated
with a
clinician, the user, or both.
[0238] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for causing a graphical user interface of a user device
associated with the
user to display early pregnancy loss symptom tags based at least in part on
detecting the
indication of the early pregnancy loss.
[0239] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for causing a graphical user interface of a user device
associated with the
user to display a message associated with the indication of the early
pregnancy loss.
[0240] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the message further comprises a time
interval during
which the early pregnancy loss occurred, a time interval during which the
early
pregnancy loss may be predicted to occur, a request to input symptoms
associated with
the early pregnancy loss, educational content associated with the early
pregnancy loss,
69
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
an adjusted set of sleep targets, an adjusted set of activity targets,
recommendations to
improve symptoms associated with the early pregnancy loss, or a combination
thereof
[0241] Some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein may further include operations, features,
means, or
instructions for inputting the physiological data into a machine learning
classifier,
wherein detecting the indication of the early pregnancy loss may be based at
least in
part on inputting the physiological data into the machine learning classifier.
[0242] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the wearable device comprises a wearable
ring
device.
[0243] In some examples of the method, apparatuses, and non-transitory
computer-
readable medium described herein, the wearable device collects the
physiological data
from the user based on arterial blood flow.
[0244] The following provides an overview of aspects of the present
disclosure:
[0245] Aspect 1: A method comprising: receiving, from a wearable device,
physiological data associated with a user that is pregnant, the physiological
data
comprising at least temperature data; determining a time series of a plurality
of
temperature values taken over a plurality of days based at least in part on
the received
temperature data; identifying that the plurality of temperature values are
lower than a
pregnancy baseline of temperature values for the user based at least in part
on
determining the time series; detecting an indication of an early pregnancy
loss of the
user based at least in part on identifying that the plurality of temperature
values are
lower than the pregnancy baseline of temperature values for the user; and
generating a
message for display on a graphical user interface on a user device that
indicates the
indication of the early pregnancy loss.
[0246] Aspect 2: The method of aspect 1, further comprising: computing a
deviation
in the time series of the plurality of temperature values relative to the
pregnancy
baseline of temperature values for the user based at least in part on
determining the time
series, wherein the deviation comprises a decrease in the plurality of
temperature values
from the pregnancy baseline of temperature values for the user, wherein
identifying that
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
the plurality of temperature values are lower than the pregnancy baseline of
temperature
values is based at least in part on computing the deviation.
[0247] Aspect 3: The method of any of aspects 1 through 2, further
comprising:
identifying that one or more positive slopes of the plurality of temperature
values are
lower than a positive slope for a pregnancy baseline of temperature values for
the user
based at least in part on determining the time series, wherein identifying
that the
plurality of temperature values are lower than the pregnancy baseline of
temperature
values for the user is based at least in part on identifying that the one or
more positive
slopes are lower than the positive slope for the pregnancy baseline of
temperature
values for the user.
[0248] Aspect 4: The method of any of aspects 1 through 3, wherein the
physiological data further comprises heart rate data, the method further
comprising:
determining that the received heart rate data exceeds a pregnancy baseline
heart rate for
the user for at least a portion of the plurality of days, wherein detecting
the indication of
the early pregnancy loss is based at least in part on determining that the
received heart
rate data exceeds the pregnancy baseline heart rate for the user.
[0249] Aspect 5: The method of any of aspects 1 through 4, wherein the
physiological data further comprises heart rate variability data, the method
further
comprising: determining that the received heart rate variability data is less
than a
.. pregnancy baseline heart rate variability for the user for at least a
portion of the plurality
of days, wherein detecting the indication of the early pregnancy loss is based
at least in
part on determining that the received heart rate variability data satisfies
the threshold.
[0250] Aspect 6: The method of any of aspects 1 through 5, wherein the
physiological data further comprises respiratory rate data, the method further
comprising: determining that the received respiratory rate data exceeds a
pregnancy
baseline respiratory rate for the user for at least a portion of the plurality
of days,
wherein detecting the indication of the early pregnancy loss is based at least
in part on
determining that the received respiratory rate data exceeds the pregnancy
baseline
respiratory rate for the user.
[0251] Aspect 7: The method of any of aspects 1 through 6, wherein the
physiological data further comprises sleep data, the method further
comprising:
71
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
determining that a quantity of detected sleep disturbances from the received
sleep data
exceeds a pregnancy baseline sleep disturbance threshold for the user for at
least a
portion of the plurality of days, wherein detecting the indication of the
early pregnancy
loss is based at least in part on determining that the quantity of detected
sleep
disturbances exceeds the pregnancy baseline sleep disturbance threshold for
the user.
[0252] Aspect 8: The method of any of aspects 1 through 7, further
comprising:
identifying a presence of a menstrual cycle within a time period after
pregnancy based
at least in part on determining the time series, wherein detecting the
indication of the
early pregnancy loss is based at least in part on identifying the presence of
the menstrual
cycle.
[0253] Aspect 9: The method of any of aspects 1 through 8, further
comprising:
receiving a confirmation of a menstrual cycle within a time period after
pregnancy, a
confirmation of a pregnancy loss, or both, wherein detecting the indication of
the early
pregnancy loss is based at least in part on receiving the confirmation.
[0254] Aspect 10: The method of any of aspects 1 through 9, further
comprising:
determining each temperature value of the plurality of temperature values
based at least
in part on receiving the temperature data, wherein the temperature data
comprises
continuous nighttime temperature data.
[0255] Aspect 11: The method of any of aspects 1 through 10, further
comprising:
estimating a likelihood of future early pregnancy loss, a likelihood that the
user will
experience the early pregnancy loss, or both, based at least in part on
identifying that the
plurality of temperature values are lower than the pregnancy baseline of
temperature
values for the user, wherein detecting the indication of the early pregnancy
loss is based
at least in part on the estimation.
[0256] Aspect 12: The method of any of aspects 1 through 11, further
comprising:
updating a readiness score associated with the user, an activity score
associated with the
user, a sleep score associated with the user, or a combination thereof, based
at least in
part on detecting the indication of the early pregnancy loss.
72
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0257] Aspect 13: The method of any of aspects 1 through 12, further
comprising:
transmitting the message that indicates the indication of the early pregnancy
loss to the
user device, wherein the user device is associated with a clinician, the user,
or both.
[0258] Aspect 14: The method of any of aspects 1 through 13, further
comprising:
causing a graphical user interface of a user device associated with the user
to display
early pregnancy loss symptom tags based at least in part on detecting the
indication of
the early pregnancy loss.
[0259] Aspect 15: The method of any of aspects 1 through 14, further
comprising:
causing a graphical user interface of a user device associated with the user
to display a
message associated with the indication of the early pregnancy loss.
[0260] Aspect 16: The method of aspect 15, wherein the message further
comprises
a time interval during which the early pregnancy loss occurred, a time
interval during
which the early pregnancy loss is predicted to occur, a request to input
symptoms
associated with the early pregnancy loss, educational content associated with
the early
pregnancy loss, an adjusted set of sleep targets, an adjusted set of activity
targets,
recommendations to improve symptoms associated with the early pregnancy loss,
or a
combination thereof
[0261] Aspect 17: The method of any of aspects 1 through 16, further
comprising:
inputting the physiological data into a machine learning classifier, wherein
detecting the
indication of the early pregnancy loss is based at least in part on inputting
the
physiological data into the machine learning classifier.
[0262] Aspect 18: The method of any of aspects 1 through 17, wherein the
wearable
device comprises a wearable ring device.
[0263] Aspect 19: The method of any of aspects 1 through 18, wherein the
wearable
device collects the physiological data from the user based on arterial blood
flow.
[0264] Aspect 20: An apparatus comprising a processor; memory coupled
with the
processor; and instructions stored in the memory and executable by the
processor to
cause the apparatus to perform a method of any of aspects 1 through 19.
[0265] Aspect 21: An apparatus comprising at least one means for
performing a
method of any of aspects 1 through 19.
73
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
[0266] Aspect 22: A non-transitory computer-readable medium storing code
the
code comprising instructions executable by a processor to perform a method of
any of
aspects 1 through 19.
[0267] The description set forth herein, in connection with the appended
drawings,
describes example configurations and does not represent all the examples that
may be
implemented or that are within the scope of the claims. The term "exemplary"
used
herein means "serving as an example, instance, or illustration," and not
"preferred" or
"advantageous over other examples." The detailed description includes specific
details
for the purpose of providing an understanding of the described techniques.
These
techniques, however, may be practiced without these specific details. In some
instances,
well-known structures and devices are shown in block diagram form in order to
avoid
obscuring the concepts of the described examples.
[0268] In the appended figures, similar components or features may have
the same
reference label. Further, various components of the same type may be
distinguished by
following the reference label by a dash and a second label that distinguishes
among the
similar components. If just the first reference label is used in the
specification, the
description is applicable to any one of the similar components having the same
first
reference label irrespective of the second reference label.
[0269] Information and signals described herein may be represented using
any of a
.. variety of different technologies and techniques. For example, data,
instructions,
commands, information, signals, bits, symbols, and chips that may be
referenced
throughout the above description may be represented by voltages, currents,
electromagnetic waves, magnetic fields or particles, optical fields or
particles, or any
combination thereof
[0270] The various illustrative blocks and modules described in connection
with the
disclosure herein may be implemented or performed with a general-purpose
processor, a
DSP, an ASIC, an FPGA or other programmable logic device, discrete gate or
transistor
logic, discrete hardware components, or any combination thereof designed to
perform
the functions described herein. A general-purpose processor may be a
microprocessor,
but in the alternative, the processor may be any conventional processor,
controller,
microcontroller, or state machine. A processor may also be implemented as a
74
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
combination of computing devices (e.g., a combination of a DSP and a
microprocessor,
multiple microprocessors, one or more microprocessors in conjunction with a
DSP core,
or any other such configuration).
[0271] The functions described herein may be implemented in hardware,
software
.. executed by a processor, firmware, or any combination thereof If
implemented in
software executed by a processor, the functions may be stored on or
transmitted over as
one or more instructions or code on a computer-readable medium. Other examples
and
implementations are within the scope of the disclosure and appended claims.
For
example, due to the nature of software, functions described above can be
implemented
using software executed by a processor, hardware, firmware, hardwiring, or
combinations of any of these. Features implementing functions may also be
physically
located at various positions, including being distributed such that portions
of functions
are implemented at different physical locations. Also, as used herein,
including in the
claims, "or" as used in a list of items (for example, a list of items prefaced
by a phrase
such as "at least one of" or "one or more of") indicates an inclusive list
such that, for
example, a list of at least one of A, B, or C means A or B or C or AB or AC or
BC or
ABC (i.e., A and B and C). Also, as used herein, the phrase "based on" shall
not be
construed as a reference to a closed set of conditions. For example, an
exemplary step
that is described as "based on condition A" may be based on both a condition A
and a
condition B without departing from the scope of the present disclosure. In
other words,
as used herein, the phrase "based on" shall be construed in the same manner as
the
phrase "based at least in part on."
[0272] Computer-readable media includes both non-transitory computer
storage
media and communication media including any medium that facilitates transfer
of a
computer program from one place to another. A non-transitory storage medium
may be
any available medium that can be accessed by a general purpose or special
purpose
computer. By way of example, and not limitation, non-transitory computer-
readable
media can comprise RAM, ROM, electrically erasable programmable ROM
(EEPROM), compact disk (CD) ROM or other optical disk storage, magnetic disk
storage or other magnetic storage devices, or any other non-transitory medium
that can
be used to carry or store desired program code means in the form of
instructions or data
structures and that can be accessed by a general-purpose or special-purpose
computer,
CA 03215868 2023-09-29
WO 2022/212755
PCT/US2022/022905
or a general-purpose or special-purpose processor. Also, any connection is
properly
termed a computer-readable medium. For example, if the software is transmitted
from a
website, server, or other remote source using a coaxial cable, fiber optic
cable, twisted
pair, digital subscriber line (DSL), or wireless technologies such as
infrared, radio, and
microwave, then the coaxial cable, fiber optic cable, twisted pair, DSL, or
wireless
technologies such as infrared, radio, and microwave are included in the
definition of
medium. Disk and disc, as used herein, include CD, laser disc, optical disc,
digital
versatile disc (DVD), floppy disk and Blu-ray disc where disks usually
reproduce data
magnetically, while discs reproduce data optically with lasers. Combinations
of the
above are also included within the scope of computer-readable media.
[0273] The description herein is provided to enable a person skilled in
the art to
make or use the disclosure. Various modifications to the disclosure will be
readily
apparent to those skilled in the art, and the generic principles defined
herein may be
applied to other variations without departing from the scope of the
disclosure. Thus, the
disclosure is not limited to the examples and designs described herein, but is
to be
accorded the broadest scope consistent with the principles and novel features
disclosed
herein.
76