Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/250833
PCT/US2022/026814
NEEDLE ASSEMBLY FOR VISUALIZATION OF FLUID MOVEMENT
BACKGROUND
[0001] Catheters are commonly used for a variety of infusion
therapies. For example, catheters
may be used for infusing fluids, such as normal saline solution, various
medicaments, and total
parenteral nutrition, into a patient. Catheters may also be used for
withdrawing blood from the
patient.
[0002] A common type of catheter is a peripheral intravenous catheter
("PIVC-) that is over-
the-needle. As its name implies, the PIVC that is over-the-needle may be
mounted over an
introducer needle having a sharp distal tip. The PIVC and the introducer
needle may be assembled
so that the distal tip of the introducer needle extends beyond a distal tip of
the PIVC with the bevel
of the needle facing up away from skin of the patient. The PIVC and the
introducer needle are
generally inserted at a shallow angle through the skin into vasculature of the
patient. The PIVC
may further be used with a guidewire for precise placement of the distal tip
of the PIVC at a desired
location with the patient vasculature. After successful placement of the
distal tip of the PIVC
within the vasculature, the guidewire may be withdrawn in conjunction with the
introducer needle.
[0003] In order to verify proper placement of the introducer needle
and/or the distal tip of the
PIVC in the vasculature, a user generally confirms that there is flashback of
blood within a catheter
system that includes the PIVC. The user visualizes the flashback of blood
within the catheter
system to determine the introducer needle is within the vasculature. Flashback
visualization in a
guidewire assisted PIVC is compromised by reduced flow rate of blood entering
the PIVC due to
placement of the guidewire in a fluid pathway of the catheter. Flashback
visualization in a
guidewire assisted PIVC may be achieved via a flashback chamber, however user
perception of
fluid advancement within the flashback chamber is difficult due to the large
volume of the
-1-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
flashback chamber and the slow flow rate of blood entering the flashback
chamber. Further, if the
catheter system includes the guidewire assisted PIVC system and is closed,
presence of the
guidewire in the PIVC and the closed nature of the catheter system may create
positive pressures
within the PIVC and the flashback chamber, thereby preventing the blood from
entering the
flashback chamber.
[0004] The subject matter claimed herein is not limited to
embodiments that solve any
disadvantages or that operate only in environments such as those described
above. Rather, this
background is only provided to illustrate one example technology area where
some
implementations described herein may be practiced.
SUMMARY
[0005] The present disclosure relates generally to vascular access
systems and related devices
and methods. In some embodiments, a catheter system may include a catheter
assembly and/or a
needle assembly. In some embodiments, the catheter assembly may include a
catheter adapter and
a catheter extending distally from a distal end of the catheter adapter. In
some embodiments, the
catheter may include a peripheral intravenous catheter (PIVC), a midline
catheter, or a peripherally
inserted central catheter. In some embodiments, a proximal end of the catheter
adapter may be
configured to receive or otherwise couple to a needle assembly.
[0006] In some embodiments, the needle assembly may include various
features to facilitate
use of a guidewire. In some embodiments, the needle assembly may include
various features to
facilitate flashback confirmation of proper intravenous catheterization. In
some embodiments, the
needle assembly may include various features to prevent buildup of positive
pressure within a
flashback chamber of the needle assembly.
-2-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
[0007] In some embodiments, the needle assembly may include a needle
hub, which may
include a proximal end, a distal end, and an interior interposed therebetween.
In some
embodiments, the proximal end of the needle hub may include a proximal
opening. In some
embodiments, the distal end of the needle hub may include a distal opening. In
some embodiments,
the needle assembly may include a plug and an introducer needle.
[0008] In some embodiments, the plug may include an outer surface,
which may include a first
portion in contact with an inner surface of the interior. In some embodiments,
the plug may include
a distal end, a proximal end, and an aperture interconnecting the distal end
of the plug and the
proximal end of the plug. In some embodiments, the first portion may include a
vent extending
between the distal end of the plug and the proximal end of the plug and
providing a fluid pathway
between the outer surface of the plug and the inner surface of the interior.
In some embodiments,
the fluid pathway may interconnect the interior and the proximal opening of
the needle hub.
[0009] In some embodiments, the introducer needle may include a
distal end and a proximal
end. In some embodiments, the proximal end of the introducer needle may be
secured to the
proximal end of the needle hub via the aperture, and the distal end of the
introducer needle may
extend beyond the distal end of the needle hub. In some embodiments, a
proximal opening of the
introducer needle may be accessible at the proximal end of the needle hub such
that a guidewire
may be threaded through a lumen of the introducer needle via the proximal
opening of the
introducer needle and extend out through a distal opening of the introducer
needle. In some
embodiments, the introducer needle may include a notch forming a pathway
through a sidewall of
the introducer needle and in fluid communication with the lumen. In some
embodiments, the
flashback chamber may be in fluid communication with the notch and may define
a space between
the inner surface and a second portion of the outer surface.
-3 -
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
[0010] In some embodiments, the flashback chamber of the needle hub
may be positioned
between the distal end of the plug and the distal end of the needle hub. In
some embodiments, the
fluid pathway may include a cross-sectional area that permits the passage of
air but not of blood.
In some embodiments, the cross-sectional area may be between 0.0001 and 0.0003
inches2. In
some embodiments, the needle hub may include a fluid tight seal between the
distal end of the
plug and the inner surface of the interior.
[0011] In some embodiments, the distal end of the plug may include a
window, which may be
in fluid communication with the flashback chamber. In some embodiments, the
notch of the
introducer needle may be positioned within the window such that the notch is
in fluid
communication with the flashback chamber via the window. In some embodiments,
a fluid tight
seal may be provided between an outer surface of the introducer needle and the
aperture of the
plug such that blood is prevented from flowing therebetween.
[0012] In some embodiments, the second portion of the outer surface
of the plug may include
an outer diameter that is less than an inner diameter of the interior. In some
embodiments, the
notch of the introducer needle may be positioned within the flashback chamber
when the proximal
end of the introducer needle is aligned with the proximal end of the plug or
the proximal end of
the needle hub. In some embodiments, a distal end of the plug may be chamfered
inwardly from
the proximal end of the plug to form a chamfered surface. In some embodiments,
the chamfered
surface may assist in aligning and guiding the introducer needle through the
plug during assembly.
Various other proximal ends and/or distal ends of the components of the needle
assembly may be
similarly chamfered to assist alignment during assembly.
[0013] In some embodiments, a gap is provided between the proximal
end of the plug and the
inner surface of the interior in proximity to the proximal opening of the
needle hub. In some
-4-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
embodiments, the gap may permit passage of air after the air passes through
the vent of the plug.
In some embodiments, the gap may be interposed between the vent and the
proximal opening of
the needle hub.
[0014] It is to be understood that both the foregoing general
description and the following
detailed description are examples and explanatory and are not restrictive of
the invention, as
claimed. It should be understood that the various embodiments are not limited
to the arrangements
and instrumentality shown in the drawings. It should also be understood that
the embodiments may
be combined, or that other embodiments may be utilized and that structural
changes, unless so
claimed, may be made without departing from the scope of the various
embodiments of the present
invention. The following detailed description is, therefore, not to be taken
in a limiting sense.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0015] Example embodiments will be described and explained with
additional specificity and
detail through the use of the accompanying drawings in which:
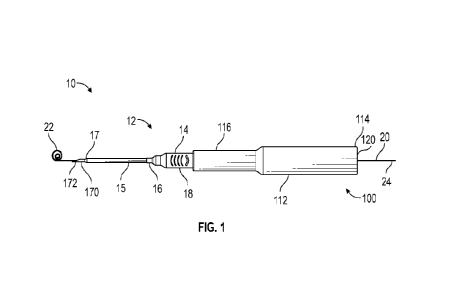
[0016] Figure 1 is an upper perspective view of an example catheter
system, illustrating an
example needle assembly, according to some embodiments;
[0017] Figure 2 is a cross-sectional view of the needle assembly,
according to some
embodiments;
[0018] Figure 3 is an exploded cross-sectional view of the needle
assembly, according to some
embodiments;
[0019] Figure 4 is an upper perspective view of an example plug,
according to some
embodiments;
[0020] Figure 5 is a cross-sectional view of a portion of the needle
assembly, according to some
embodiments;
-5-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
[0021] Figure 6 is a cross-sectional view of another example needle
assembly, according to
some embodiments;
[0022] Figure 7 is a partial cutaway view of the other needle
assembly, according to some
embodiments; and
[0023] Figure 8 is a cross-sectional view of the other needle
assembly, according to some
embodiments.
DESCRIPTION OF EMBODIMENTS
[0024] As used in the present disclosure, the term "proximal" refers
to the portion of a structure
closer to a user, and the term "distal" refers to the portion further from the
user. Referring now to
Figure 1, a catheter system 10 is illustrated, according to some embodiments.
In some
embodiments, the catheter system 10 may be guidewire assisted or in other
words, configured for
use with a guidewire. In some embodiments, the catheter system 10 may include
a catheter
assembly 12, which may include a catheter adapter 14.
[0025] In some embodiments, the catheter adapter 14 may include a
distal end 16. In some
embodiments, the catheter assembly 12 may include a catheter 15, which may
extend distally from
distal end 16. In some embodiments, the catheter 15 may include a PIVC.
[0026] In some embodiments, the catheter adapter 14 may include a
proximal end 18, which
may be configured to selectively connect to a needle assembly 100 of the
catheter system 10. In
some embodiments, following catheterization of a patient, the catheter adapter
14 may be separated
from needle assembly 100, and needle assembly 100 may be disposed.
[0027] In some embodiments, the needle assembly 100 may include a
needle hub 112, which
may include a distal end 116 configured to selectively receive and retain the
proximal end 18 of
the catheter adapter 14 during catheterization. In some embodiments, the
catheter system 10 may
-6-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
further include additional known intravenous catheter assembly components and
features, such as,
for example, a needle shield, a securement platform, and various gripping
surfaces (not illustrated).
[0028] In some embodiments, the needle assembly 100 may include a
needle 170, which may
include an introducer needle. In some embodiments, the needle 170 may include
a proximal end
secured to the needle hub 112, and a sharpened distal end 172 that extends
distally beyond the
distal tip 17 of the catheter 15 when the needle assembly 100 is secured to
the catheter adapter 14.
[0029] In some embodiments, the catheter system 10 may include or may
be compatible for use
with a guidewire 20. In some embodiments, the guidewire 20 may be inserted
through a lumen of
the needle 170 such that a distal end 22 of the guidewire 20 may extend beyond
the sharpened
distal end 172 and such that a proximal end 24 of guidewire 20 extends
proximally from a proximal
opening 120 in a proximal end 114 of needle hub 112. As such, a user may
manipulate the distal
end 22 of guidewire 20 by directly contacting and controlling the proximal end
24 of the guidewire
20.
[0030] Referring now to Figures 2-5, the needle assembly 100 is
illustrated, according to some
embodiments. In some embodiments, the needle hub 112 may include a translucent
or optically
clear polymer material that is sufficiently rigid to withstand forces of
catheterization. As such, a
user may visualize interior surfaces, spaces, and components of the needle hub
112 during use. In
some embodiments, the needle hub 112 may include the proximal end 114 having
the proximal
opening 120, the distal end 116 having a distal opening 122, and an interior
118 interposed between
the proximal end 114 and the distal end 116.
[0031] In some embodiments, the interior 118 may include a distal
inner diameter 119, which
may be approximately equal to an outer diameter of needle 70 such that a fluid
tight interface or
seal is provided between needle 170 and the distal inner diameter 119. In some
embodiments, the
-7-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
interior 118 may include a proximal inner diameter 121 that is greater than
the outer diameter of
needle 70 such that a fluid (i.e., blood) may gather and flow between the
outer surface of needle
70 and the proximal inner diameter 121. In some embodiments, a portion of the
proximal inner
diameter 121 may include a flashback chamber 190 of needle hub 112.
[0032] In some embodiments, the needle 170 may include a distal
opening 177 and a notch 180
in fluid communication with distal opening 177 via a portion of a lumen 178 of
the needle 170. In
some embodiments, the notch 180 may be formed in and through a sidewall 182 of
the needle 170,
such that the notch 180 forms a pathway 181 through the sidewall 182.
Accordingly, the distal
opening 177 may be in fluid communication with the interior 118 via the notch
180.
[0033] In some embodiments, the flashback chamber 190 may include a
portion of the proximal
inner diameter 121 in fluid communication with the notch 180. In these and
other embodiments,
blood entering the lumen 178 via the distal opening 177 may flow through the
lumen 178, exit the
needle 170 via notch 180, and flow into flashback chamber 190. In some
embodiments, a small
spacing or fluid tight seal between the outer surface of the needle 170 and
the distal inner diameter
119 prevents blood from flowing therebetween.
[0034] In some embodiments, the needle assembly 100 may include a
plug 150, which may be
fixedly seated within proximal opening 120. In some embodiments, the plug 150
may include an
aperture 156 for receiving the proximal end 174 of needle 170. In some
embodiments, the proximal
opening 176 of the lumen 178 may be accessible at the proximal end 154, and
the small spacing
or fluid tight seal is provided between aperture 156 and the outer surface of
needle 170 to prevent
blood within flashback chamber 190 from flowing between aperture 156 and the
outer surface of
needle 170. In some embodiments, the plug 150 may include an outer surface,
which may include
a first portion 158 in contact with a proximal inner surface 123 of interior
118. In some
-8-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
embodiments, the plug 150 may include a second portion 159 defining or forming
at least one
surface of flashback chamber 190. In some embodiments, the second portion 159
may define or
form a proximal end surface of the flashback chamber 190. In some embodiments,
the second
portion 159 may define or form an inner diameter of the flashback chamber 190.
[0035] In a closed system, blood flowing through an introducer needle
and into a flashback
chamber generally produces a positive pressure within the lumen of the needle
and within the
flashback chamber, thereby reducing or preventing the flow of blood into the
flashback chamber.
This effect is enhanced when the volume of a lumen of the introducer needle is
further reduced by
a presence of a guidewire within the lumen. This buildup of positive pressure
prevents the user
from visualizing advancement of the blood within the flashback chamber,
thereby reducing the
benefits of using a flashback chamber to confirm proper placement of the
distal tip of the catheter
during catheterization. Thus, the first portion 158 of the plug 150 may
include one or more vents
160, which may be configured to enable airflow through or around the plug 150
to relieve positive
pressure within the flashback chamber 190. In some embodiments, the vents 160
may permit air,
but not blood, to exit therethrough. As such, the catheter system 10 may
provide a facilitate
observable flashback, which may facilitate accurate placement of the catheter
15 and reduce a risk
of transfixing a vein.
[0036] In some embodiments, the first portion 158 of the plug 150 may
be modified to include
the vents 160. In some embodiments, the vents 160 may include channels
interposed between the
outer surface of the plug 150 and the proximal inner surface 123 of the
interior 118. In some
embodiments, the vents 160 may provide a fluid pathway 161 between the plug
150 and the
proximal inner surface 123 of the interior 118. In some embodiments, the vents
160 may be
configured to prevent or relieve positive pressure within the flashback
chamber 190 by providing
-9-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
an access for air to bypass the plug 150 into the exterior environment via a
gap 163 provided
between a proximal outer surface 157 of the plug 150 and a proximal inner
surface 125 of the
needle hub 112. In some embodiments, the gap 163 may be annular.
[0037] In some embodiments, the gap 163 may be in fluid communication
with the fluid
pathway 161. In some embodiments, the vents 160 may be constructed by removing
material from
the first portion 158, which may result in multiple parallel grooves or
channels. A close-up
perspective view of the plug 150 is provided in Figure 4, according to some
embodiments. In other
embodiments, the vents 160 may be formed as channels through the plug 150
rather than on the
outer surface of plug 150.
[0038] In some embodiments, a rate at which air flows through the
fluid pathway 161 of the
vents 160 in the plug 150 may be adjusted by manufacturing the plug 150 to
include a greater or
lesser number of vents 160 or by changing the cross-sectioned area of the
vents 160. Thus, in some
embodiments the rate at which air and/or fluid flows through the lumen 178 and
the flashback
chamber 190 may be increased by manufacturing the plug 150 to have either an
increased number
of the vents 160 or with vents 160 having a greater cross-sectioned area.
Conversely, in other
embodiments, the rate at which air flows through the lumen 178 and the
flashback chamber 190
may be decreased by manufacturing the plug 150 with either a decreased number
of the vents 160
or with the vents 160 having a lesser cross-sectioned area.
[0039] One having skill in the art will appreciate that the blood
pressure of the patient may be
largely responsible for the rate at which blood flows through lumen 178 and
flashback chamber
190, and therefore also may be largely responsible for the rate at which air
within the lumen 178
and the flashback chamber 190 exits through the vents 160. As such, in some
embodiments, the
flow rate through the catheter system 10 may be affected by a combined
effective hydraulic
-10-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
diameter of all flow paths. Thus, in some embodiments, the hydraulic diameter
of the vents 160
may be modified to increase or decrease the rate of flow through the lumen 178
and the flashback
chamber 190. In other embodiments, the hydraulic diameter of the vents 160 may
be decreased
thereby resulting in substantially reduced or stopped flow therethrough. The
governing equation
for controlling the flow rate through the vents 160 is given in Equation 1,
where BP is the blood
pressure, A is the surface area of vents 160, G is the surface tension of the
blood, and P is the
perimeter of the vents 160.
[0040] Equation 1: BP(A) = G(P)
100411 Thus, according to Equation 1, when the perimeter of the vents
160 is small, the vents
160 will allow air venting, but will prevent blood flow due to the relatively
high surface tension
(a) of blood. Therefore, by adjusting the variables of Equation 1, a desired
flow rate may be
achieved. Thus, based on the size and/or number of the vents 160 around the
plug 150, blood flow
may provide customized, controlled, and predictable air flow around the plug
150, thereby
providing a desired observable flashback while preventing blood from exiting
the proximal
opening 120 of the needle hub 112. Accordingly, in some embodiments the vents
160 may be
designed to allow the flow of air and stop the flow of blood.
[0042] In some embodiments, a number of the vents 160 may be between
1 and 40. In other
embodiments, the number of the vents 160 may be between 1 and 20. In some
embodiments, the
number of the vents 160 may be six or more. In some embodiments, the number of
the vents 160
may be five or fewer vents 160. In some embodiments, the vents 160 may have a
cross-sectional
area between about 0.000007 to 0.00004 inches2. In other embodiments, the
vents 160 may have
a cross-sectional area between about 0.00001 to 0.00003 inches2. In other
embodiments, the vents
160 may have a cross-sectional area of about 0.00002 inches2. For instance, in
some embodiments,
-11 -
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
the vents 160 may have a height of about 0.001 to 0.003 inches and a width of
about 0.010 inches.
In other embodiments, the vents 160 may have a height of about 0.002 to 0.003
inches and a width
of about 0.005 inches.
[0043] In some embodiments, the vents 160 may include a cross-
sectional area to permit blood
flow through the lumen 178 or through the flashback chamber 190 at a rate
between about 10 to
200 ml/hr. In some embodiments, the cross-sectional area of the vents 160 can
permit blood to
flow through the lumen 178 or the flashback chamber 190 at a rate between
about 15 to 150 ml/hr.
In some embodiments, the cross-sectional area of vents 160 can permit blood to
flow through
lumen 178 or flashback chamber 190 at a rate between at a rate between about
50 to 100 ml/hr. At
these rates, the rate of blood flow into the flashback chamber 190 can be
paced to provide a
clinician with adequate time to correctly locate the catheter within a
patient's blood vessel.
Accordingly, in some embodiments, vents 160 have a cross-sectional area
greater than 0.00003
inches2, a cross-sectional area greater than 0.00004 inches2, a cross-
sectional area of about 0.0001
inches2, or a cross-sectional area of about 0.001 inches2.
[0044] In some embodiments, the flow rate of blood through lumen 178
is affected by the
presence of a guidewire inserted through lumen 178. Accordingly, in some
embodiments, a gauge
of the guidewire may be selected to provide a desire rate of blood flow
through lumen 178. In
some embodiments a gauge of the needle 170 may be selected to provide a cross-
sectioned area
between the inner surface of the needle 170 and the outer surface of the
guidewire to provide a
desired rate of blood flow through lumen 178. In some embodiments, an inner
diameter of a distal
portion of the lumen 178 of the needle 170 may be greater than an inner
diameter of a proximal
portion of the lumen 178 of the needle 170.
-12-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
[0045] In some embodiments, the notch 180 may separate the distal and
proximal portions of
lumen 178. In some embodiments, the inner diameter of the distal portion of
the lumen 178 may
permit blood flow between the distal portion of the lumen and the outer
surface of the guidewire
located within the distal portion of the lumen. In some embodiments, the inner
diameter of the
proximal portion of the lumen 178 may prevent blood flow between the proximal
portion of the
lumen 178 and the outer surface of the guidewire located within the proximal
portion of the lumen
178.
[0046] In some embodiments, the guidewire may include a distal
portion, which may include a
smaller outer diameter. In some embodiments, the guidewire may include a
proximal portion,
which may include a larger outer diameter compared to the distal portion of
the guidewire. In some
embodiments, the notch 180 may be disposed proximate and/or distal to the
proximal portion of
the guidewire, such that blood is permitted to flow between distal portion of
the guidewire and the
lumen 178 of the needle 170, and blood is prevented from flowing between the
proximal portion
of the guidewire and the lumen 178 of the needle 170. In some embodiments, an
outer diameter of
the guidewire may be increased by placing a portion of the guidewire in a
sleeve. In some
embodiments, an inner diameter of lumen 178 may be decreased by lining a
portion of lumen 178
with a sleeve.
[0047] Referring now to Figures 6-8, a needle assembly 200 is
illustrated, according to some
embodiments. In some embodiments, the needle assembly 200 may include a plug
250, which may
include a first portion 258 in contact with the proximal inner surface 123 of
the interior 118. In
some embodiments, the plug 250 may include a second portion 259, which may
define at least one
surface of the flashback chamber 190. In some embodiments, the first portion
258 may include
one or more vents 260, which may be configured to enable airflow through or
around the plug 250
-13 -
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
to relieve positive pressure within the flashback chamber 190. In some
embodiments, the vents
260 may permit air, but not blood, to exit therethrough.
[0048] In some embodiments, the needle assembly 200 may be similar or
identical to the needle
assembly 100 in terms of one or more features and/or operation. In further
detail, in some
embodiments, the plug 250 may be similar or identical to the plug 150 in terms
of one or more
features and/or operation. As another example, in some embodiments, the vents
260 may be similar
or identical to the vents 160 in terms of one or more features and/or
operation.
[0049] In some embodiments, the second portion 259 may include an
outer diameter 221 that
is less than the proximal inner diameter 121 of the interior 118. In some
embodiments, the
flashback chamber 190 may include the space between the second portion 259 and
the proximal
inner surface 123 of the interior 118. In some embodiments, placement of the
plug 250 within the
interior 118 may reduce the volume of the flashback chamber 190, thereby
reducing the volume
of blood needed to visualize the advancement of the blood within the flashback
chamber 190. In
some embodiments, the second portion 259 may be elongated to entirely span the
proximal inner
surface 123 of the interior 118, such that the distal end 252 of the plug 250
forms a fluid tight seal
with the distal inner diameter 119 of needle hub 112, thereby forming the
distal end of the
flashback chamber 190.
[0050] In some embodiments, the second portion 259 may include a
contrasting color
configured to enhance the user's visualization of blood with flashback chamber
190. For example,
in some embodiments, the second portion 259 may include an opaque hue that
contrasts the red
hue of blood. In further detail, the second portion 259 may include a white,
yellow, green or blue
hue. In some embodiments, the second portion 259 may include two or more hues
that contrast
with the red hue of blood. In some embodiments, the second portion 259 may
include two
-14-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
alternating hues configured to provide a scale by which the user may gauge the
speed of the
advancement of the blood within the flash chamber 190. In some embodiments,
the second portion
259 may include a color pattern by which a user may gauge the speed of the
advancement of the
blood within chamber 190.
[0051] In some embodiments, the plug 250 may include a window 253,
which may form a fluid
pathway between the aperture 256 of the plug 250 and the flashback chamber
190, such that the
window 253 is in fluid communication with the flashback chamber 190. In some
embodiments,
the window 253 may be positioned in proximity to the distal end 252 of the
plug 250 such that the
notch 180 of the needle 170 may be positioned within the window 253 when the
proximal end 174
of the needle 170 is aligned with the proximal end 254 of the plug 250. Thus,
a pathway 181 is
provided between the notch 180 and the flashback chamber 190 via the window
253. In some
embodiments, a fluid tight seal may be provided between remaining outer
surfaces of the needle
170 and the aperture 256 to prevent blood from leaking therebetween. In some
embodiments, the
plug 250 may include no more than one window 253. In some embodiments, the
plug 250 may
include two or more windows 253.
[0052] In some embodiments, when a guidewire is inserted through the
proximal opening 176
of the lumen 178 of the needle 170, blood may be permitted to flow between the
inner surface of
the lumen 178 and the outer surface of the guidewire along the distal portion
of the lumen 178
such that the blood exits the notch 180 and flows through the window 253,
however blood may be
substantially prevented from flowing through the portions of the lumen 178 and
the aperture 256
between the window 253 and the proximal ends 174 and 254, respectively.
Accordingly, in some
embodiments, the catheter system 10 is configured for use with the guidewire,
and flashback
confirmation is provided via a vented flashback chamber.
-15-
CA 03217740 2023- 11- 2
WO 2022/250833
PCT/US2022/026814
[0053] All examples and conditional language recited herein are
intended for pedagogical
objects to aid the reader in understanding the invention and the concepts
contributed by the
inventor to furthering the art and are to be construed as being without
limitation to such specifically
recited examples and conditions. Although embodiments of the present
inventions have been
described in detail, it should be understood that the various changes,
substitutions, and alterations
could be made hereto without departing from the spirit and scope of the
invention.
-16-
CA 03217740 2023- 11- 2