Note: Descriptions are shown in the official language in which they were submitted.
TITLE: PEROXYCARBOXYLIC ACID COMPOSITIONS CONTAINING A
STABILIZING AGENT AND MINERAL ACIDS FOR STABILIZATION
FIELD OF THE INVENTION
The invention relates to peroxycarboxylic acid compositions that are liable to
exothermic decomposition which are stabilized under highly acidic conditions
(e.g. high
mineral acid levels) to provide improved transport and/or storage according to
the invention.
The invention further relates to peroxycarboxylic acid sanitizing compositions
having
favorable foam profiles under various water conditions and/or mechanical
action during
applications of use. The invention still further relates to peroxycarboxylic
acid sanitizing
compositions containing a peroxycarboxylic acid stable fluorescent active
compound suitable
for monitoring by conductivity and/or optical sensors. Beneficially, the
sanitizing
peroxycarboxylic acid compositions are also low odor and low/no VOC dual
functioning acid
wash and sanitizing compositions. Still further, the stabilized, low foaming
compositions have
improved antimicrobial efficacy in comparison to conventional mixed
peroxycarboxylic acid
compositions for sanitizing applications.
BACKGROUND OF THE INVENTION
Improving Stabilization: Peroxycarboxylic acids (i.e. peracids, such as
peracetic acid)
fall into the chemical category of "organic peroxides" which in turn are
classified as self-
reactive, self-heating substances. Self-reactive substances are strictly
regulated by the US
Department of Transportation (DOT) following the guidance of the UN Committee
for the
Transport of Dangerous Goods (TDG). Like the US DOT most local and national
governments
strictly observe the UN TDG guidance thus making their "guidance" essentially
a world wide
requirement These guidances may be found in the UN document known as the
"orange book,"
titled Recommendations on the Transport of Dangerous Goods, 5th revised
edition, 2009.
The concern about self-heating substances is that most decomposition processes
accelerate as temperature rises and usually exponentially. A self-heating
process producing
heat faster than it can cool is the definition of a runaway reaction. In the
case of organic
Date Recue/Date Received 2023-12-12
peroxides the mnaway reaction is accompanied by the generation of large
volumes of gas and
therefore poses an extreme explosion risk. It is therefore an absolute
requirement for purposes
of safety, with the ancillary benefit of improving both shelf-life and
quality, that the heat
generating rate of the organic peroxide containing product not exceed the
cooling rate of the
package. In addition, since the cooling rates decrease with increased volume,
these self-
heating rates limit a commercial package size which in turn limits commercial
opportunities. If
for example a product falls into UN category 5.2 (D), as do some organic
peroxides, they may
not be sold in packages with a volume greater than 50 kg. For a customer
consuming hundreds
of kilograms of product per day such a limitation may be unacceptable.
In summary there are two aspects (and two sets of tests) of a prospective
"self-reactive
substance" to address, the first involves the characterization of the chemical
(5.2 A, B, C, D,
E, F or G) and the second set of tests assesses the chemistry in the proposed
package of
commerce. By testing the chemistry in the proposed commercial package the heat
loss
characteristics as well as the heat generating characteristics are assessed at
varirms "ambient"
temperatures. The minimum ambient temperature at which the chemistry self
heats to exceed
the ambient by at least 6 degrees Celsius is defined as the "Self Accelerating
Decomposition
Temperature" (SA TYT) Restrictions on shipping, storage (Le. refrigeration
requirements) come
therefore not just from the classification test but also the SADT. If for
example the package
has an SADT <45 degrees Celsius refrigeration is required. A requirement of
refrigeration,
like classification can severely restrict commercial opportunities.
Various factors impact the transportation and/or storage risk and therefore a
specific
product is required to be transported below its SADT. For example, the larger
a container, the
lower its surface-to-volume ratio will be, resulting in less transmittal of
heat to the
surroundings container when undergoing thermal decomposition and a reduction
in the SADT.
This increases the risk of storing and transporting peroxycarboxylic acid
compounds
susceptible to exothermic decomposition within large containers. This hazard
can be
minimized by storing and transporting such compositions in containers having
been diluted
with one or more liquids. The diluted peroxycarboxylic acids can also be
formulated into
suspensions, emulsions, or solutions. Aqueous emulsions or suspensions are
generally
2
Date Recue/Date Received 2023-12-12
considered safer formulations, because the active peroxide is dispersed in the
water phase (e.g.
suitable for removing heat of decomposing peroxide molecules, such as by
convection and/or
evaporation). Thus commercially-available peroxycarboxylic acids are usually
sold in an
equilibrium solution, containing the corresponding carboxylic acid to the
peroxycarboxylic
acid, hydrogen peroxide and water.
Storage and/or transportation containers may also be made of substances that
can
withstand the pressures resulting from the inevitable gaseous decay products
but they must
also be made of inert or semi-inert materials. For aqueous organic peroxides
the most common
containers are made of high density polyethylene or polypropylene fitted with
vented closures.
Corrosion sensitive steel for example is not used as it will contaminate the
product with
transition metal ions such as Fe3 which are catalytical decay accelerants for
most organic
peroxides. Packages range in size from several gram bottles to bulk storage
tanks depending
largely on their classification and their package-specific SADTs. Still
further,
pemxycarhoxylie acid compositions can he transported under refrigeration
In non-refrigeration transport and storage it becomes almost an absolute
necessity to
employ transition metal chelators or "stabilizers" to both elevate the SADTs
as well as to
maximize the shelf-life and quality of organic peroxides These stabilizers can
be used in
peroxycarboxylic acid compositions to stabilize the compositions. For example,
phosphonatc
based stabilizers, such as phosphoric acid and salts, pyrophosphoric acid and
salts and 1-
hydroxyethylidene-1,1-diphosphonic acid (1IEDP) and salts, are the most
commonly used
stabilizers in peroxycarboxylic acid compositions. When used individually at a
sufficient
concentration, these stabilizers can significantly improve the stability of
the peroxycarboxylic
acid compositions, and for the conventional (i.e. non-highly acidic)
peroxycarboxylic acid
compositions, the stability profile achieved with these stabilizers allows for
the commercial
transportation and use of these compositions. However, for peroxycarboxylic
acid
compositions with highly acidic formulations, including for example using
strong mineral
acids, these stabilizers' efficacy is greatly reduced, in many instances the
efficacy is
essentially non-existant.
3
Date Recue/Date Received 2023-12-12
Accordingly, it is an objective of the claimed invention to develop stabilized
peroxycarboxylic acid compositions having reduced storage and/or
transportation hazards.
In a particular aspect, the stabilized compositions which overcome the
challenges
associated with the SADT of conventional peroxycarboxylic acid compositions.
In addition
these stabilizer compositions may even affect the DOT classification,
providing in some cases
an exemption from the typical UN "5.2" class for organic peracids to the
reduced risk "5.1"
classification.
A further object of the invention is to provide a stabilized peroxycarboxylic
acid
composition suitable for storage and/or transport at temperatures of at least
50 C without
presenting SADT hazards.
A still further object of the invention is to provide a stabilized, highly
acidic, mixed
peroxycarboxylic acid composition utilizing a unique peracid stabilizing
agent.
Improved Foam Profiles: Peroxycarboxylic acids (i.e. peracids) are
commercially-
available in equilibrium solutions, containing the corresponding carboxylic
acid to the
peroxycarboxylic acid, hydrogen peroxide and water. Peroxycarboxylic acids are
known for
use as antimicrobials, sanitizing agents and/or bleaching agents. However,
formulations of
peroxycarboxylic acid compositions, including the selection of peracid
compatible low
foaming surfactants, presents formulation difficulties, including foam
profiles which can
interfere with various applications of use, including for example clean-in-
place uses.
Accordingly, it is an objective of the claimed invention to develop low
foaming, highly
acidic peroxycarboxylic acid compositions containing mineral acids.
A further object of the invention is to provide a highly acidic, mixed
peroxycarboxylic
acid composition utilizing a unique combination of a low foaming surfactant
and defoarning
agent to control foam under various water conditions, including for example
deionized or soft
water.
A still further object of the invention is to provide a defoaming agent having
synergistic biocidal efficacy with other peracid components.
Peroxycarboxylic acid Monitoring: Antimicrobial compositions are used in a
variety
of automated processing and cleaning applications to reduce microbial or viral
populations on
4
Date Recue/Date Received 2023-12-12
hard or soft surfaces or in a body or stream of water. Regardless of the
application, an
antimicrobial or "use" composition is a composition containing a defined
minimum
concentration of one or more active components which exhibit desired
antimicrobial
properties. The concentration of active components in the use composition is
chosen to
achieve the requisite level of antimicrobial activity. In use compositions in
which one or more
peracids are the active component, the concentration of hydrogen peroxide
tends to increase
over time while the concentration of peracid decreases. However, in order to
maintain the
requisite level of antimicrobial activity, the amount of peracid in the use
composition must be
maintained at a defined minimum concentration. In addition, as the amount of
hydrogen
peroxide in the use composition increases, the use composition may exceed a
defined
maximum concentration of hydrogen peroxide in the solution.
To ensure the amount of peracid is maintained at or above some minimum
concentration and to determine when the amount of hydrogen peroxide reaches or
exceeds a
maximum concentration, it is necessary to determine the concentration of
peracid(s) and
hydrogen peroxide in the use composition. In the past, to determine both the
peracid
concentration and the hydrogen peroxide concentration in a use composition has
required
multiple time consuming manual titrations, several different reagents and
relatively large
volumes of use composition. Moreover, past devices and methods for determining
both
peracid and hydrogen peroxide concentrations were effective over only a narrow
range of
concentrations.
Accordingly, it is an objective of the claimed invention to develop peracid
compositions having various beneficial aspects, including compatibility for
monitoring of
peracid concentration by conductivity and/or optical sensors.
A further object of the invention is to provide a highly acidic, mixed
peroxycarboxylic
acid composition having such compatibility for concentration monitoring that
is also low/no
VOC, low odor, low foaming and stabilized under such highly acidic conditions
(e.g. mineral
acids in formulation).
Other objects, advantages and features of the present invention will become
apparent
from the following specification taken in conjunction with the accompanying
drawings.
5
Date Recue/Date Received 2023-12-12
BRIEF SUMMARY OF THE INVENTION
The present invention relates to stable, low foaming and/or fluorescent active
peroxycarboxylic acid compositions and uses thereof. An advantage of the
invention is that
unconventionally acidic peroxycarboxylic acid compositions, including mixed
peracids, are
stabilized without impacting antimicrobial and/or sanitizing efficacy of the
compositions. A
further advantage of the invention is that the unconventionally acidic
peroxycarboxylic acid
compositions, including mixed peracids, are combined with at least one
surfactant and a
defoaming agent to provide broad applications of use which are not limited due
to the foaming
profile of the composition, without impacting antimicrobial and/or sanitizing
efficacy of the
compositions. A still further advantage of the invention is that the
peroxycarboxylic acids,
mineral acids, and fluorescent active compound provide long-term stability of
the traceable
component of the peroxycarboxylic acid composition concentrations. However,
the
fluorescent active compounds are also stable in alkaline environments
providing traceable
and/or measumble components. As a result, the compositions can be monitored by
optical
sensors and provided for broad applications of use, without impacting
antimicrobial and/or
sanitizing efficacy of the compositions
In an embodiment, the present invention is directed to a composition
comprising: a Cl-
C22 carboxylic acid; a Ci-C22percarboxylic acid; hydrogen peroxide; and a
stabilizing agent,
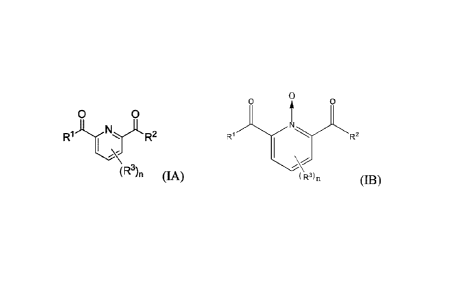
wherein the stabilizing agent is a picolinic acid or a compound having the
following Formula
(1A):
0 0
RljtyCTA R2
(R3), (IA)
wherein R1 is OH or NRiaRlb, wherein R1a and Rib are independently hydrogen or
(Ct -
C6)alkyl; R2 is OH or ¨NR2aR2b, wherein R2a and R21) are independently
hydrogen or (Ci -
C.6)alkyl; each R3 is independently (CI -C6)alkyl, (C2 -C6)alkenyl or (C2 -
C6)alkynyl; and n is a
number from zero to 3; or a salt thereof; or a compound having the following
Formula (IB):
6
Date Recue/Date Received 2023-12-12
0
0
R2
R3}n (IB)
wherein RI is OH or NRiaRib, wherein R1a and Rib are independently hydrogen or
(CI -
C6)alkyl; R2 is OH or ¨Wale% wherein R2a and R21' are independently hydrogen
or (Ci -
C6)alkyl; each R1 is independently (Ci -C6)alkyl, (C2 -C6)alkenyl or (C2 -
C6)allcynyl; and n is a
number from zero to 3; or a salt thereof; and wherein said composition has a
pH at about 3 or
less.
In a further embodiment, the present invention is directed to methods of
storing and/or
transporting a highly acidic, stabilized peroxycarboxylic acid composition
comprising: storing
the above composition, wherein said composition retains at least about 80% of
the C I-C22
percarboxylic acid activity after storage of about 30 days at about 50 C. Tn a
still further
aspect, the present invention is directed to a method for transporting the
highly acidic,
stabilized percarboxylic acid composition, preferably in bulk, wherein the
SADT of said
composition is elevated to at least above 45 C during transportation and/or
storage.
In a still further embodiment, the present invention is directed to methods of
using
highly acidic, stabilized peroxycarboxylic acid composition comprising:
providing the
peroxycarboxylic acid composition, contacting a surface or substrate with a
use solution of the
composition for sufficient time to reduce a microbial population, wherein said
use solution has
a pH below about 4, and wherein the composition retains at least about 80% of
the Ci-C22
peroxycarboxylic acid activity after storage of about 30 days at about 50 C.
In a further embodiment, the present invention is directed to low-foaming
equilibrium
peracid compositions comprising: a Ci-C22 peroxycarboxylic acid; a Ci-C22
carboxylic acid;
hydrogen peroxide; a mineral acid; an anionic surfactant; and a metal salt as
defoaming agent,
wherein said composition has a use solution pH below about 4. In further
aspects, the
composition include from about 1 wt-% to about 40 wt-% of the Ci.-C22
peroxycarboxylic acid,
7
Date Recue/Date Received 2023-12-12
from about 1 wt-% to about 80 wt-% of the CI-C22 carboxylic acid, from about 1
wt-% to
about 80 wt-% of the hydrogen peroxide, from about 1 wt-% to about 50 wt-% of
the mineral
acid, from about 0.01 wt-% to about 40 wt-% of the surfactant, and from about
0.001 wt-% to
about 10 wt-% of the defoaming agent. In still further aspects, the
composition includes at
least one additional agent selected from the group consisting of a hydrotrope,
a solvent, a
stabilizing agent and combinations thereof.
In a still further embodiment, the present invention is directed to methods of
reducing a
microbial population using a low-foaming equilibrium peroxycarboxylic acid
composition
comprising: providing the low-foaming peroxycarboxylic acid composition set
forth above;
and contacting a surface or substrate with a use solution of said composition
for sufficient time
to reduce a microbial population, wherein said use solution has a p11 below
about 4.
Tn a still further embodiment, the present invention is directed to an
equilibrium
peracid composition comprising: a Cl-C22 peroxycarboxylic acid; a Ci-Cn
carboxylic acid;
hydrogen peroxide; and a fluorescent active compound In a further aspect, the,
fluorescent
active compound is stable in the equilibrium peracid composition for
monitoring
peroxycarboxylic acid concentration by optical sensors. In a further aspect,
the composition
comprises from about 1 wt-% to about 40 wt- h of the CI-C22 peroxycarboxylic
acid, from
about 1 wt-% to about 80 wt-% of the Ci-C22 carboxylic acid, from about 1 wt-%
to about 80
wt-% of the hydrogen peroxide, and from about 0.001 wt-% to about 10 wt-% of
the
fluorescent active compound.
In a still further embodiment, the present invention is directed to methods
for
monitoring a concentration of peroxycarboxylic acid and/or hydrogen peroxide
in a sanitizing
composition and/or cleaning process includes providing an equilibrium
peroxycarboxylic acid
composition comprising a CI-C22 peroxycarboxylic acid, a CI-C22 carboxylic
acid, hydrogen
peroxide, and a fluorescent active compound, and wherein the fluorescent
active compound is
stable in the equilibrium peracid composition for monitoring peroxycarboxylic
acid
concentration, including for example by optical sensors. The method further
includes
measuring a fluorescence response with a fluorometer from the fluorescent
active compound
in the peroxycarboxylic acid composition or measuring an optical response from
the
8
Date Recue/Date Received 2023-12-12
fluorescent active compound in an optical cell, and determining a
concentration of the
peroxycarboxylic acid and/or the hydrogen peroxide.
While multiple embodiments are disclosed, still other embodiments of the
present
invention will become apparent to those skilled in the art from the following
detailed
description, which shows and describes illustrative embodiments of the
invention.
Accordingly, the drawings and detailed description are to be regarded as
illustrative in nature
and not restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 shows a graph of the comparison of the SADT study of a DPA-stabilized
peroxycarboxylic acid composition according to an embodiment of the invention
with a
phosphate based peroxycarboxylic acid composition.
FIG. 2 shows a graph of the comparison of the SADT study of a DPA-stabilized,
highly acidic peroxycarboxylic acid composition according to an embodiment of
the invention
with a phosphate based, highly acidic peroxycarboxylic acid composition.
FIGS. 3-4 show graphs of the comparison of the SADT study of a highly acidic
peracid
composition, where the TWA-stabilizing agent according to embodiments of the
invention
provided sufficient stabilization, such that the self-heating effects were not
sufficient to reach
the oven temperature within the 7 day period.
FIG. 5 shows a graph of the comparison of foam heights resulting from various
commercially-available peracid compositions in comparison to the low-foaming,
highly acidic
peroxycarboxylic acid composition according to embodiments of the invention.
FIG. 6 shows a graph of the additional biocidal efficacy provided according to
the low-
foaming, highly acidic peroxycarboxylic acid composition according to
embodiments of the
invention.
FIG. 7 shows a graph of the traceability of the peroxycarboxylic acid
concentration of
the highly acidic peroxycarboxylic acid compositions according to embodiments
of the
invention.
9
Date Recue/Date Received 2023-12-12
FIG. 8 shows a graph of the comparison of foam heights resulting from various
commercially-available peroxycarboxylic acid compositions in comparison to the
low-
foaming, highly acidic peroxycarboxylic acid composition according to
embodiments of the
invention.
FIG. 9 shows a graph of the emission (SU) of the highly acidic
peroxycarboxylic acid
composition according to embodiments of the invention in various types of
water.
FIG. 10 shows a graph of the conductivity (us/cm) of the highly acidic
peroxycarboxylic acid composition according to embodiments of the invention in
various
types of water.
Various embodiments of the present invention will be described in detail with
reference to the drawings, wherein like reference numerals represent like
parts throughout the
several views. Reference to various embodiments does not limit the scope of
the invention.
Figures represented herein are not limitations to the various embodiments
according to the
invention and are presented for exemplary illustration of the invention
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The embodiments of this invention are not limited to particular
peroxycarboxylic acid
compositions and methods of using the same, which can vary and arc understood
by skilled
artisans. It is further to be understood that all terminology used herein is
for the purpose of
describing particular embodiments only, and is not intended to be limiting in
any manner or
scope. For example, as used in this specification and the appended claims, the
singular forms
"a," "an" and "the" can include plural referents unless the content clearly
indicates otherwise.
Further, all units, prefixes, and symbols may be denoted in its SI accepted
form.
Numeric ranges recited within the specification are inclusive of the numbers
defining
.. the range and include each integer within the defined range. Throughout
this disclosure,
various aspects of this invention are presented in a range format. It should
be understood that
the description in range format is merely for convenience and brevity and
should not be
construed as an inflexible limitation on the scope of the invention.
Accordingly, the
description of a range should be considered to have specifically disclosed all
the possible sub-
Date Regue/Date Received 2023-12-12
ranges as well as individual numerical values within that range. For example,
description of a
range such as from 1 to 6 should be considered to have specifically disclosed
sub-ranges such
as from 1 to 3, from 1 to 4, from 1 to 5, from 2 to 4, from 2 to 6, from 3 to
6 etc., as well as
individual numbers within that range, for example, 1, 2, 3, 4, 5, and 6. This
applies regardless
of the breadth of the range.
So that the present invention may be more readily understood, certain terms
are first
defined. Unless defined otherwise, all technical and scientific terms used
herein have the same
meaning as commonly understood by one of ordinary skill in the art to which
embodiments of
the invention pertain. Many methods and materials similar, modified, or
equivalent to those
described herein can be used in the practice of the embodiments of the present
invention
without undue experimentation, the preferred materials and methods arc
described herein. In
describing and claiming the embodiments of the present invention, the
following terminology
will be used in accordance with the definitions set out below.
The i "about," as used herein, refers to variation in the
numerical quantity that can
.. occur, for example, through typical measuring and liquid handling
procedures used for making
concentrates or use solutions in the real world; through inadvertent error in
these procedures;
through differences in the manufacture, source, or purity of the ingredients
used to make the
compositions or carry out the methods; and the like. The term "about" also
encompasses
amounts that differ due to different equilibrium conditions for a composition
resulting from a
particular initial mixture. Whether or not modified by the term "about", the
claims include
equivalents to the quantities.
As used herein, the term "cleaning" refers to a method used to facilitate or
aid in soil
removal, bleaching, microbial population reduction, and any combination
thereof. As used
herein, the term "microorganism" refers to any noncellular or unicellular
(including colonial)
organism. Microorganisms include all prokaryotes. Microorganisms include
bacteria
(including eyanobacteria), spores, lichens, fungi, protozoa, virinos, viroids,
viruses, phases,
and some algae. As used herein, the term "microbe" is synonymous with
microorganism.
As used herein, the term "disinfectant" refers to an agent that kills all
vegetative cells
including most recognized pathogenic microorganisms, using the procedure
described in
11
Date Recue/Date Received 2023-12-12
A.O.A.C. Use Dilution Methods, Official Methods of Analysis of the Association
of Official
Analytical Chemists, paragraph 955.14 and applicable sections, 15th Edition,
1990 (EPA
Guideline 91-2). As used herein, the term "high level disinfection" or "high
level
disinfectant" refers to a compound or composition that kills substantially all
organisms, except
high levels of bacterial spores, and is effected with a chemical germicide
cleared for marketing
as a sterilant by the Food and Drug Administration. As used herein, the term
"intermediate-
level disinfection" or "intermediate level disinfectant" refers to a compound
or composition
that kills mycobacteria, most viruses, and bacteria with a chemical germicide
registered as a
tuberculocide by the Environmental Protection Agency (EPA). As used herein,
the term "low-
level disinfection" or "low level disinfectant" refers to a compound or
composition that kills
some viruses and bacteria with a chemical germicide registered as a hospital
disinfectant by
the EPA.
The term "hard surface" refers to a solid, substantially non-flexible surface
such as a
counter top, tile, floor, wall, panel, window, plumbing fixture, kitchen and
bathroom furniture,
appliance, engine, circuit board, and dish. Hard surfaces may include for
example, health care
surfaces and food/plant/animal processing surfaces.
As used herein, the terms "mixed" or "mixture" when used relating to
"percarboxylic
acid composition," "percarboxylic acids," "peroxycarboxylic acid composition"
or
"peroxycarboxylic acids" refer to a composition or mixture including more than
one
percarboxylic acid or peroxycarboxylic acid.
For the purpose of this patent application, successful microbial reduction is
achieved
when the microbial populations are reduced by at least about 50%, or by
significantly more
than is achieved by a wash with water. Larger reductions in microbial
population provide
greater levels of protection. Differentiation of antimicrobial "-cidal" or "-
static" activity, the
definitions which describe the degree of efficacy, and the official laboratory
protocols for
measuring this efficacy are considerations for understanding the relevance of
antimicrobial
agents and compositions. Antimicrobial compositions can affect two kinds of
microbial cell
damage. The first is a lethal, irreversible action resulting in complete
microbial cell
destruction or incapacitation. The second type of cell damage is reversible,
such that if the
12
Date Recue/Date Received 2023-12-12
organism is rendered free of the agent, it can again multiply. The former is
termed
microbiocidal and the later, microbistatic. A sanitizer and a disinfectant
are, by definition,
agents which provide antimicrobial or microbiocidal activity. In contrast, a
preservative is
generally described as an inhibitor or microbistatic composition
As used herein, the term "sanitizer" refers to an agent that reduces the
number of
bacterial contaminants to safe levels as judged by public health requirements.
In an
embodiment, sanitizers for use in this invention will provide at least a
99_999% reduction (S-
log order reduction). These reductions can be evaluated using a procedure set
out in
Germicidal and Detergent Sanitizing Action ofDisinfectants, Official Methods
of Analysis of
the Association of Official Analytical Chemists, paragraph 960.09 and
applicable sections,
15th Edition, 1990 (EPA Guideline 91-2). According to this reference a
sanitizer should
provide a 99.999% reduction (5-log order reduction) within 30 seconds at room
temperature,
2512 C, against several test organisms.
As used herein, the ierill "Sllifoperoxyc:arboxylic acid," "sill inflated
peracid," or
"sulfonated peroxycarboxylic acid" refers to the peroxycarboxylic acid form of
a sulfonated
carboxylic acid. In some embodiments, the sulfonated peracids of the present
invention are
mid-chain sulfonated peracids As used herein, the term "mid-chain sulfonated
peracid" refers
to a peracid compound that includes a sulfonate group attached to a carbon
that is at least one
carbon (e.g., the three position or further) from the carbon of the
percarboxylic acid group in
the carbon backbone of the percarboxylic acid chain, wherein the at least one
carbon is not in
the terminal position. As used herein, the term "terminal position," refers to
the carbon on the
carbon backbone chain of a percarboxylic acid that is furthest from the
percarboxyl group.
The term "weight percent," "wt-%," "percent by weight," "% by weight," and
variations thereof, as used herein, refer to the concentration of a substance
as the weight of that
substance divided by the total weight of the composition and multiplied by
100. It is
understood that, as used here, "percent," "%," and the like are intended to be
synonymous with
"weight percent," "wt-%," etc.
The methods and compositions of the present invention may comprise, consist
essentially of, or consist of the components and ingredients of the present
invention as well as
13
Date Recue/Date Received 2023-12-12
other ingredients described herein. As used herein, "consisting essentially
of" means that the
methods and compositions may include additional steps, components or
ingredients, but only
if the additional steps, components or ingredients do not materially alter the
basic and novel
characteristics of the claimed methods and compositions.
Compositions
Stabilized Peroxycarboxylic Acids
While an understanding of the mechanism is not necessary to practice the
present
invention and while the present invention is not limited to any particular
mechanism of action,
it is contemplated that, in some embodiments, highly acidic peroxycarboxylic
acid
compositions are not sufficiently stabilized using conventional phosphate
stabilizers.
Phosphonate or other metal chelating stabilizers (e.g HEDP/Dequest 2010) are
either
incompatible and/or ineffective as stabilizers with the highly acidic peracid
compositions of
the present invention, which results in SADT that effectively limit the
transportation and/or
storage of these self-accelerating decomposition compotinds The present
invention provides
peroxycarboxylic acid stabilizing compounds suitable for use under highly
acidic, equilibrium
compositions. The present invention further provides peroxycarboxylic acid
stabilizing
compounds suitable for use in compositions having extreme ratios of peracid to
hydrogen
peroxide, wherein the concentration of the peroxyacids greatly exceed the
hydrogen peroxide.
In an embodiment, dipicolinic acid is provided as the peroxycarboxylic acid
stabilizer under
strong acidic conditions in place of conventional peracid stabilizers, such as
Dequest 2010
which is predominantly used in commercial peracid products. Beneficially, the
peroxycarboxylic acid stabilizer under strong acidic conditions elevates the
SADT of the
compositions providing transportation and/or storage benefits.
According to an embodiment of the invention the stabilized peroxycarboxylic
acid
compositions are suitable for storage and/or transport at ambient temperatures
that might
occasionally reach about 50 C. In an aspect, the stabilized composition
retains at least about
80% of the peroxycarboxylic acid activity after storage of about 30 days at
about 50 C.
Preferably, the peracid compositions retain at least about 85%, at least about
90% or greater
percentage of the peracid activity after storage of about 30 days at about
50"C. According to a
14
Date Recue/Date Received 2023-12-12
further embodiment, the stabilized compositions can be transported and/or
stored, preferably
in bulk, wherein the SADT of said composition is elevated to at least about 45
C during
transportation, or to at least about 50 C, or to at least about 60 C (for
moderate size packages)
during transportation.
In an aspect, the compositions include concentrated equilibrium compositions
comprising a stabilizing agent, peracid(s), hydrogen peroxide, carboxylic
acid(s), a solvent,
e.g., water, and optional additional functional ingredients (e.g. defoaming
agents, fluorescent
active compounds). In an aspect, the compositions include the exemplary ranges
shown in
Table 1 in weight percentage of the liquid concentrated equilibrium
compositions.
TARE .R 1 ¨ Stabilized Peracid Compositions
Material First Exemplary Second Third
Range wt-% Exemplary Exemplary
Range Range
wt-% wt-%
Solvent (e.g Water) 1-75 10-60 20-40
Pcroxycnrboxylic Acid 0.1-40 1-40 1-20
Carboxylic Acid 0.1-90 1-80 1-50
Hydrogen Peroxide 1-90 1-80 1-50
Mineral Acid 1-50 1-20 5-20
Stabilizing Agent 0.001-25 0.01-10 0.01-1
Additional Functional 0-25 0-20 0-10
Ingredients
(e.g. defoaming agent)
Low Foam Peroxycarboxylic Acids
While an understanding of the mechanism is not necessary to practice the
present
invention and while the present invention is not limited to any particular
mechanism of action,
Date Recue/Date Received 2023-12-12
it is contemplated that, in some embodiments, highly acidic peroxycarboxylic
acid
compositions are formulated to provide low foam profiles under various water
conditions
and/or under mechanical action as may be present in various applications of
use, including for
example, clean-in-place applications using high degrees of mechanical
action/force. The
present invention provides peroxycarboxylic acid compositions in highly
acidic, equilibrium
compositions. In an embodiment, suitable surfactants (e.g. some anionic
surfactants) are
combined with a defoaming agents resulting in critical coupling/wetting, which
result in
improved foaming profiles in comparison to known low foaming surfactants,
especially in
deionized or soft water. Beneficially, the defoaming agent is compatible with
the harsh acidic
.. peroxycarboxylic acid compositions containing mineral acids, and
unexpectedly results in
synergistic biocidal efficacy.
Due to the highly acidic equilibrium peracid compositions employed, as well as
the
intended application of the compositions for use under high degrees of
mechanical
action/force (e.g. CTP applications), conventional defoaming agents are either
not compatible
.. with the compositions or not effective. For example, such incompatible
and/or ineffective
defoaming agents for use with highly acidic equilibrium peracid compositions
include silica,
silicone or nonionic-based defoaming agents, aliphatic acids or esters; fatty
alcohols; fatty
amines or amides; halogenated compounds such as fluorochlorohydrocarbons;
vegetable oils,
waxes, mineral oils as well as their sulfonated or sulfated derivatives; fatty
acids; and
phosphates and phosphate esters such as alkyl and alkaline diphosphates, and
tributyl
phosphates among others; and mixtures thereof.
Similarly, use of various cations (e.g. calcium) to decrease the
hydrophilicity of the
surfactants may provide defoaming efficacy, however the cations are
incompatible with such
peracid compositions and result in the precipitation of the employed
surfactant. The various
cations which are known to be peracid compatible (e.g. Mg2' ) fail to provide
efficient
defoaming properties.
In an aspect, the compositions include concentrated equilibrium compositions
comprising peracid(s), hydrogen peroxide, carboxylic acid(s), a solvent, e.g.,
water, a
surfactant and/or defoaming agent, and other optional additional functional
ingredients (e.g.
16
Date Recue/Date Received 2023-12-12
stabilizing agent, fluorescent active compounds). In an aspect, the
compositions include the
exemplary ranges shown in Table 2 in weight percentage of the liquid
concentrated
equilibrium compositions.
TABLE 2
Material First Second Third
Exemplary Exemplary Exemplary
Range wt-% Range wt-% Range
wt-%
Solvent (e.g Water) 1-75 10-60 20-40
Peroxycarboxylic Acid 0.1-40 1-40 1-20
Carboxylic Acid 0.1-90 1-80 1-50
Hydrogen Peroxide 1-90 1-80 1-50
Mineral Acid 1-50 1-20 5-20
Defoaming Agent 0.001-10 0.1-5 0.1-1
Surfactant 0-40 0.1-25 1-20
Additional Functional 0-25 0-20 0-10
Ingredients (e.g. stabilizing
agent)
Fluorescent Active Peroxycarboxylic Acids
While an understanding of the mechanism is not necessary to practice the
present
invention and while the present invention is not limited to any particular
mechanism of action,
it is contemplated that, in some embodiments, equilibrium peroxycarboxylic
acid
compositions are formulated to preferably provide highly acidic compositions
with a stable
fluorescent active compound allowing for dose quantification, e.g. optical
measurement. The
formulation of stable fluorescent active compounds into highly acidic
equilibrium
peroxycarboxylic acid compositions allows quantification over extended periods
of time; e.g.
beyond the 48 hours of prior art peracid compositions that are combined with a
fluorescent
17
Date Recue/Date Received 2023-12-12
component at a point of use. Beneficially, this allows formulation of the
peroxycarboxylic
acid compositions to include the fluorescent active compound instead of such
point of use
dosing with a fluorescent compound as previously done in some applications of
use. In
addition, the present invention is a significant improvement over
peroxycarboxylic acid-
forming and/or containing compositions which merely contain a non-stable
fluorescent
compound that is only suitable for visual assessment of peracid under UV light
and in dry
conditions to confirm a disinfectant was applied. As a result, the
concentrated equilibrium
compositions containing the stable fluorescent active compounds arc suitable
for optical
measurement of peracid concentrations in various applications of use,
including for example,
clean-in-place, warewashing and other sanitizing applications, rather than
merely a visual
assessment of a treated surface.
Tn an aspect, the compositions include concentrated equilibrium compositions
comprising peracid(s), hydrogen peroxide, carboxylic acid(s), a solvent, e.g.,
water, a
fluorescent active compound, and other optional additicmal functional
ingredients (e.g.
.. stabilizing agent, a surfactant and/or defoaming agent). In an aspect, the
compositions include
the exemplary ranges shown in Table 3 in weight percentage of the liquid
concentrated
equilibrium compositions
TABLE 3
Material First Second Third
Exemplary Exemplary Exemplary
Range wt-% Range wt-% Range
wt-%
Solvent (e.g. Water) 1 75 10 60 20 40
Peroxycarboxylic Acid 0.1-40 1-40 1-20
Carboxylic Acid 0.1-90 1-80 1-50
Hydrogen Peroxide 1-90 1-80 1-50
Mineral Acid 0-50 0-20 0.1-20
18
Date Recue/Date Received 2023-12-12
Fluorescent Active 0.001-10 0.1-10 0.5-7.5
Compound
Surfactant 0-40 0.1-25 1-20
Additional Functional 0-25 0-20 0-10
Ingredients (e.g. stabilizing
agent, defoaming agent)
In yet other aspects, the compositions according to the invention may include
non-
equilibrium peracid compositions, such as where a peroxycarboxylic acid is
generated in situ
and/or on site through a process by one or more composition (e.g. one or more
part systems)
comprising individual reagents combined according to the invention. In an
exemplary aspect,
these reagents are described herein individually along and include at least
one ester of a
polyhydric alcohol and a Cl to C18 carboxylic acid, an oxidizing agent, a
source of alkalinity,
solvents, and other functional groups/agents. An acidulant is also described
herein as a reagent
to be added to the compositions after the formation of the percarboxylic
acid(s). Alternatively,
as described herein, there may be benefits to providing the reagents in
various premix
formulations to decrease the number of reagents and/or increase the simplicity
of the invention
for generating peracid compositions for a particular use. Premix formulations
suitable for use
according to the invention may comprise, consist of and/or consist essentially
of at least one
ester of a polyhydric alcohol and a Cl to C18 carboxylic acid, an oxidizing
agent, a solvent
and mixtures thereof. Premix formulations suitable for use according to the
invention may
also comprise, consist of and/or consist essentially of at least one ester, an
oxidizing agent,
water, solvents, dispersing agents, surfactants, defoamers and mixtures
thereof.
In some aspects the compositions, whether generated in situ or on site from
one or
more premix compositions or whether provided in a concentrated equilibrium
composition, in
a use solution has a pH at about 4 or less. Preferably, the compositions in a
use solution have a
pH at about 3 or less. In an aspect, the use solutions of the highly acidic,
stabilized
peroxycarboxylic acid compositions, when diluted pursuant to EPA sanitizer
suspension
preparations (e.g. dilute 1 oz. of the peracid composition to 8 Gallon with
500 ppm hard
19
Date Recue/Date Received 2023-12-12
water), such that the pH of the solution is less than about 3.0, preferably
between about 2.8.-
2.9.
Peracids
According to the invention, a peroxycarboxylic acid (i.e. peracid) is included
for
antimicrobial efficacy in the sanitizing compositions disclosed herein. As
used herein, the term
"peracid" may also be referred to as a "percarboxylic acid," "peroxycarboxylic
acid" or
"peroxyacid." Sulfoperoxycarboxylic acids, sulfonated peracids and sulfonated
peroxycarboxylic acids are also included within the terms "peroxycarboxylic
acid" and
"peracid" as used herein. The terms "sulfoperoxycarboxylic acid," "sulfonated
peracid," or
"sulfonated peroxycarboxylic acid" refers to the peroxycarboxylic acid form of
a sulfonated
carboxylic acid as disclosed in U.S. Patent No. 8,344,026, and U.S. Patent
Publication Nos.
2010/0048730 and 2012/0052134. As one of skill in the art appreciates, a
peracid refers to an
acid having the hydrogen of the hydroxyl group in carboxylic acid replaced by
a hydroxy
group Oxidi7ing peraeids may also he referred to herein as peroxycarboxylic
acids
A peracid includes any compound of the formula R--(C000H)n in which R can be
hydrogen, alkyl, alkenyl, alkyne, acylic, alicyclic group, aryl, heteroaryl,
or heterocyclic
group, and n is 1, 2, or 3, and named by prefixing the parent acid with
peroxy. Preferably R
includes hydrogen, alkyl, or alkenyl. The terms "alkyl," "alkcnyl," "alkyne,"
"acylic,"
"alicyclic group," "aryl," "heteroaryl," and "heterocyclic group" are as
defined herein.
As used herein, the term "alkyl" or "alkyl groups" refers to saturated
hydrocarbons
having one or more carbon atoms, including straight-chain alkyl groups (e.g.,
methyl, ethyl,
propyl, butyl, pentyl, hexyl, heptyl, octyl, nonyl, decyl, etc.), cyclic alkyl
groups (or
"cycloalkyl" or "alicyclic" or "carbocyclic" groups) (e.g., cyclopropyl,
cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, etc.), branched-chain alkyl groups (e.g.,
isopropyl, tert-
butyl, sec-butyl, isobutyl, etc.), and alkyl-substituted alkyl groups (e.g.,
alkyl-substituted
cycloalkyl groups and cycloalkyl-substituted alkyl groups). Preferably, a
straight or branched
saturated aliphatic hydrocarbon chain having from 1 to 22 carbon atoms, such
as, for example,
methyl, ethyl, propyl, isopropyl (1-methylethyl), butyl, tert-butyl (1,1-
dimethylethyl), and the
like.
Date Recue/Date Received 2023-12-12
Unless otherwise specified, the term "alkyl" includes both "unsubstituted
alkyls" and
"substituted alkyls." As used herein, the term "substituted alkyls" refers to
alkyl groups
having substituents replacing one or more hydrogens on one or more carbons of
the
hydrocarbon backbone. Such substituents may include, for example, alkenyl,
allcynyl,
halogeno, hydroxyl, alkylcarbonyloxy, arylcarbonyloxy, alkoxycarbonyloxy,
aryloxy,
aryloxycarbonyloxy, carboxylate, alkylearbonyl, arylcarbonyl, alkoxycaxbonyl,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl, alkylthiocarbonyl,
alkoxyl,
-phosphate, phosphonato, phosphinato, cyano, amino (including alkyl amino,
dialkylamino,
arylamino, diarylamino, and alkylarylamino), acylamino (including
aLkylcarbonylamino,
.. arylcarbonylamino, carbamoyl and ureido), imino, sulfhydryl, alkylthio,
arylthio,
thiocarboxylate, sulfates, alkylsulfinyl, sulfonates, sulfamoyl, sulfonamido,
nitro,
trifluoromethyl, cyano, azido, heterocyclic, alkylaryl, or aromatic (including
heteroaromatic)
groups.
The iPTM "alkenyl" includes an unsaturated aliphatic hydrocarbon chain having
from 2
to 12 carbon atoms, such as, for example, ethenyl, 1-propenyl, 2-propenyl, 1-
butenyl, 2-
methyl-I -propenyl, and the like. The alkyl or alkenyl can be terminally
substituted with a
heteroatom, such as, for example, a nitrogen, sulfur, or oxygen atom, forming
an aminoalkyl,
oxyalkyl, or thioalkyl, for example, aminomethyl, thioethyl, oxypropyl, and
the like. Similarly,
the above alkyl or alkenyl can be interrupted in the chain by a heteroatom
forming an
alkylaminoalkyl, alkylthioalkyl, or alkoxyalkyl, for example,
methylaminoethyl,
ethylthiopropyl, methoxymethyl, and the like.
Further, as used herein the term "alicyclic" includes any cyclic hydrocarbyl
containing
from 3 to 8 carbon atoms. Examples of suitable alicyclic groups include
cyclopropanyl,
cyclobutanyl, cyclopentanyl, etc. In some embodiments, substituted alkyls can
include a
heterocyclic group. As used herein, the term "heterocyclic group" includes
closed ring
structures analogous to carbocyclic groups in which one or more of the carbon
atoms in the
ring is an element other than carbon, for example, nitrogen, sulfur or oxygen.
Heterocyclic
groups may be saturated or unsaturated. Exemplary heterocyclic groups include,
but are not
limited to, aziridine, ethylene oxide (epoxides, oxiranes), thiirane
(episulfides), dioxirane,
21
Date Recue/Date Received 2023-12-12
azetidine, oxetane, thietane, dioxetane, dithietane, dithiete, azolidine,
pyrrolidine, pyrroline,
oxolane, dihydrofuran, and furan. Additional examples of suitable heterocyclic
groups include
groups derived from tetrahydrofurans, furans, thiophenes, pyrrolidines,
piperidines, pyridines,
pyrrols, picoline, coumaline, etc.
According to the invention, alkyl, alkenyl, alicyclic groups, and heterocyclic
groups
can be =substituted or substituted by, for example, aryl, heteroaryl, C14
alkyl, C1-4 alkenyl,
C1-4 alkoxy, amino, carboxy, halo, nitro, cyano, --S03H, phosphono, or
hydroxy. When alkyl,
alkenyl, alicyclic group, or heterocyclic group is substituted, preferably the
substitution is C1-4
alkyl, halo, nitro, amido, hydroxy, carboxy, sulpho, or phosphono. In one
embodiment, R
includes alkyl substituted with hydroxy. The term "aryl" includes aromatic
hydrocarbyl,
including fused aromatic rings, such as, for example, phenyl and naphthyl. The
term
"heteroaryl" includes heterocyclic aromatic derivatives having at least one
heteroatom such as,
for example, nitrogen, oxygen, phosphorus, or sulfur, and includes, for
example, fury!,
pyrmlyl, ihienyl, oxazolyl, pyridyl, irnitiazolA thiazolyl, isoxazolyl,
pyraznlyl, isothia7nlyl,
etc. The term wheteroaryl" also includes fused rings in which at least one
ring is aromatic, such
as, for example, indolyl, purinyl, benzofuryl, etc.
According to the invention, aryl and heteroaryl groups can he unsubstituted or
substituted on the ring by, for example, aryl, heteroaryl, alkyl, alkenyl,
alkoxy, amino,
carboxy, halo, nitro, cyan , --S0311, phosphono, or hydroxy. When aryl,
aralkyl, or heteroaryl
is substituted, preferably the substitution is C1-4 alkyl, halo, nitro, amido,
hydroxy, carboxy,
sulpho, or phosphono. In one embodiment, R includes aryl substituted with C1-4
alkyl.
Peracids suitable for use include any peroxycarboxylic acids, including
varying lengths
of peroxycarboxylic acids (e.g. C1-22) that can be prepared from the acid-
catalyzed
equilibrium reaction between a carboxylic acid described above and hydrogen
peroxide. A
peroxycarboxylic acid can also be prepared by the auto-oxidation of aldehydes
or by the
reaction of hydrogen peroxide with an acid chloride, acid anhydride,
carboxylic acid
anhydride, sodium alcoholate or alkyl and aryl esters. Alternatively, peracids
can be prepared
through non-equilibrium reactions, which may be generated for use in situ,
such as the
methods disclosed in U.S. Patent Publication Nos. 2012/0172440 and
2012/0172441 each
22
Date Regue/Date Received 2023-12-12
titled "In Situ Generation of Peroxycarboxylic Acids at Alkaline pH, and
Methods of Use
Thereof,". Preferably a composition of the invention includes peroxyacetic
acid,
peroxyoctanoic acid, peroxypropionic acid, peroxylactic acid, peroxyheptanoic
acid,
peroxyoctanoic acid and/or peroxynonanoic acid.
In some embodiments, a peroxycarboxylic acid includes at least one water-
soluble
peroxycarboxylic acid in which R includes alkyl of 1-22 carbon atoms. For
example, in one
embodiment, a peroxycarboxylic acid includes peroxyacetic acid. In another
embodiment, a
peroxycarboxylic acid has R that is an alkyl of 1-22 carbon atoms substituted
with a hydroxyl
group or other polar substituent such that the substituent improves the water
solubility..
Methods of preparing peroxyacetic acid are known to those of skill in the art
including those
disclosed in U.S. Pat No. 2,833,813.
Tn another embodiment, a sulfoperoxycarboxylic acid has the following formula:
R1¨CH¨ R2 ¨ C000 H
S 03-Xf
wherein RI is hydrogen, or a substituted or unsubstituted alkyl group; R2 is a
substituted or unsubstituted alkylene group; X is hydrogen, a cationic group,
or an ester
forming moiety; or salts or esters thereof. In some embodiments, Ri is a
substituted or
unsubstituted Cm alkyl group; X is hydrogen a cationic group, or an ester
forming moiety; R2
is a substituted or unsubstituted C. alkyl group; m=1 to 10; n=1 to 10; and
m+n is less than 18,
or salts, esters or mixtures thereof.
In some embodiments, Ri is hydrogen. In other embodiments, RI is a substituted
or
unsubstituted alkyl group. In some embodiments, R1 is a substituted or
unsubstituted alkyl
group that does not include a cyclic alkyl group. In some embodiments, Ri is a
substituted
alkyl group. In some embodiments, RI is an unsubstituted Ci-C9 alkyl group. In
some
embodiments, RI is an unsubstituted C7 OT C8 alkyl. In other embodiments, Ri
is a substituted
Cs-Cio alkylene group. In some embodiments, RI is a substituted Cs-Cto alkyl
group is
substituted with at least 1, or at least 2 hydroxyl groups. In still yet other
embodiments, RI is a
substituted Ci-C9 alkyl group. In some embodiments, RI is a substituted Cl-C9
substituted alkyl
23
Date Recue/Date Received 2023-12-12
group is substituted with at least 1 S031-1 group. In other embodiments, Ri is
a C9-Cio
substituted alkyl group. In some embodiments, Ri is a substituted C9-CIO alkyl
group wherein
at least two of the carbons on the carbon backbone form a heterocyclic group.
In some
embodiments, the heterocyclic group is an epoxide group.
In some embodiments, R2 is a substituted CI-Cm alkylene group. In some
embodiments,R2 is a substituted C8-Cioalkylene. In some embodiments, R2is an
unsubstituted C6-C9 alkylene. In other embodiments, R2 is a Cs-Cio alkylene
group substituted
with at least one hydroxyl group. In some embodiments. R2 is a Cio alkylene
group substituted
with at least two hydroxyl groups_ In other embodiments, R2 is a Cs alkylene
group substituted
with at least one S0311 group. In some embodiments, P.2 is a substituted C9
group, wherein at
least two of the carbons on the carbon backbone form a heterocyclic group. In
some
embodiments, the heterocyclic group is an epoxide group. In some embodiments,
RI is a Cs-C9
substituted or unsubstituted alkyl, and R2 is a C7-C8 substituted or
unsubstituted alkylene.
These and other suitable sill foperoxycarboxylic acid compounds for use in the
stabilized peroxycarboxylic acid compositions of the invention are further
disclosed in U.S.
Patent No. 8,344,026 and U.S. Patent Publication Nos. 2010/0048730 and
2012/0052134.
In additional embodiments a sulfoperoxycarboxylic acid is combined with a
single or
mixed peroxycarboxylic acid composition, such as a sulfoperoxycarboxylic acid
with
peroxyacetic acid and peroxyoctanoic acid (PSOAJPOOA/POAA). In other
embodiments, a
mixed peracid is employed, such as a peroxycarboxylic acid including at least
one
peroxycarboxylic acid of limited water solubility in which R includes alkyl of
5-22 carbon
atoms and at least one water-soluble peroxycarboxylic acid in which R includes
alkyl of 1-4
carbon atoms For example, in one embodiment, a peroxycarboxylic acid includes
peroxyacetic acid and at least one other peroxycarboxylic acid such as those
named above.
Preferably a composition of the invention includes peroxyacetic acid and
peroxyoetanoic acid,
such as disclosed in U.S. Patent No. 5,314,687 In an aspect, the peracid
mixture is a
hydrophilic peracetic acid and a hydrophobic peroctanoic acid, providing
antimicrobial
synergy. In an aspect, the synergy of a mixed peracid system allows the use of
lower dosages
of the peracids_
24
Date Regue/Date Received 2023-12-12
In another embodiment, a tertiary peracid mixture composition, such as
peroxysulfonated oleic acid, peracetic acid and peroctanoic acid are employed,
such as
disclosed in U.S. Patent No. 8,344,026. Advantageously, a combination of
peroxycarboxylic
acids provides a composition with desirable antimicrobial activity in the
presence of high
organic soil loads. The mixed peroxycarboxylic acid compositions often provide
synergistic
micro efficacy. Accordingly, compositions of the invention can include a
peroxycarboxylic
acid, or mixtures thereof_
Various commercial formulations of peracids are available, including for
example
peracetic acid (approximately 15%) available as EnviroSan (Ecolab, Inc., St.
Paul MN). Most
commercial peracid solutions state a specific percarboxylic acid concentration
without
reference to the other chemical components in a use solution. However, it
should be
understood that commercial products, such as peracetic acid, will also contain
the
corresponding carboxylic acid (e.g. acetic acid), hydrogen peroxide and water.
In an aspect, any suitable CI-C22perca1boxylic acid can he used in the present
compositions. In some embodiments, the CI-C22 percarboxylic acid is a C2-C2o
percarboxylic
acid. In other embodiments, the CI-C22 percarboxylic is a CI, C2, C3, C5,
C6, C7, Cs, C9,
CIO, Cii, C12, C13, C14, C15, C16, C17, CU, C19, C20, C21, or C22 carboxylic
acid In still other
embodiments, the C1-C22 percarboxylic acid comprises peroxyacctic acid,
peroxyoctanoic acid
and/or peroxysulfonated oleic acid.
In an aspect of the invention, a peracid may be selected from a concentrated
composition having a ratio of hydrogen peroxide to peracid from about 0:10 to
about 10:0,
preferably from about 0.5:10 to about 10:0.5, preferably from about 1:8 to
8:1. Various
concentrated peracid compositions having the hydrogen peroxide to peracid
ratios of about
0.5:10 to about 10:0.5, preferably from about 1:8 to 8:1, may be employed to
produce a use
solution for treatment according to the methods of the invention. In a further
aspect of the
invention, a peracid may have a ratio of hydrogen peroxide to peracid as low
as from about
0.01 part hydrogen peroxide to about 1 part peracid. Without limiting the
scope of invention,
the numeric ranges are inclusive of the numbers defining the range and include
each integer
within the defined range.
Date Recue/Date Received 2023-12-12
Obtaining the preferred hydrogen peroxide to peroxycarboxylic acid ratios in a
peracid
composition may be obtained by a variety of methods suitable for producing a
very low
hydrogen peroxide to peracid ratio. In an aspect, equilibrium peracid
compositions may be
distilled to recover a very low hydrogen peroxide peracid mixture. In yet
another aspect,
catalysts for hydrogen peroxide decomposition may be combined with a peracid
composition,
including for example, peroxide-reducing agents and/or other biomimetic
complexes. In yet
another aspect, perhydrolysis of peracid precursors, such as esters (e.g.
triacetin) and amides
may be employed to obtain peracids with very low hydrogen peroxide. These and
other
methods of reducing hydrogen peroxide ratios in a peracid composition are
disclosed in U.S.
Patent Publication Nos. 2013/0259743 titled "Use of Peracetic Acid/Hydrogen
Peroxide and
Catalase for Treatment of Drilling Fluids, Frac Fluids, Flowback Water and
Disposal Water,
and 2013/0264293 and 2013/0264059 titled "Use of Peracetic Acid/Hydrogen
Peroxide and
Peroxide Reducing Agents for Treatment of Drilling Fluids, Frac Fluids,
Flowback Water and
Disposal Water,"
In a preferred aspect, the C1-C22 percarboxylic acid can be used at any
suitable
concentration. In some embodiments, the Ci-C22 percarboxylic acid has a
concentration from
about 0.1 wt-% to about 40 wt-% in a concentrated equilibrium composition In
other
embodiments, the C1-C22 percarboxylic acid has a concentration from about 1 wt-
% to about
40 wt-%, or from about 1 wt-% to about 20 wt-%. In still other embodiments,
the Ci-C22
percarboxylic acid has a concentration at about 1 wt-%, 2 wt-%, 3 wt-%, 4 wt-
%, 5 wt-%, 6
wt-%, 7 wt-%, 8 wt-%, 9 wt-%, 10 wt-%, 11 wt-%, 12 wt-%, 13 wt-%, 14 wt-%, 15
wt-%, 16
wt-%, 17 wt-%, 18 wt-%, 19 wt-%, 20 wt-%, 25 wt-%, 30 wt-%, 35 wt-%, or 40 wt-
%.
Without limiting the scope of invention, the numeric ranges are inclusive of
the numbers
defining the range and include each integer within the defined range.
Carboxylic Acid
The present invention includes a carboxylic acid with the peracid composition
and
hydrogen peroxide. A carboxylic acid includes any compound of the formula R--
(COOH). in
which R can be hydrogen, alkyl, alkenyl, alkyne, acylic, alicyclic group,
aryl, heteroaryl, or
heterocylic group, and n is 1, 2, or 3. Preferably R includes hydrogen, alkyl,
or alkenyl. The
26
Date Recue/Date Received 2023-12-12
terms "alkyl," "alkenyl," "alkyne," "acylic," "alicyclic group," "aryl,"
"heteroaryl," and
"heterocyclic group" are as defined above with respect to pemcids.
Examples of suitable carboxylic acids according to the equilibrium systems of
peracids
according to the invention include a variety monocarboxylic acids,
dicarboxylic acids, and
tricarboxylic acids. Monocarboxylic acids include, for example, formic acid,
acetic acid,
propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid,
octanoic acid,
nonanoic acid, decanoic acid, undecanoic acid, dodecanoic acid, glycolic acid,
lactic acid,
salicylic acid, acetylsalicylic acid, mandelic acid, etc. Dicarboxylic acids
include, for example,
adipic acid, fumaric acid, glutaric acid, maleic acid, succinic acid, malic
acid, tartaric acid, etc.
Tricarboxylic acids include, for example, citric acid, trimellitic acid,
isocitric acid, agaicic
acid. etc.
Tn an aspect of the invention, a particularly well suited carboxylic acid is
water soluble
such as formic acid, acetic acid, propionic acid, butanoic acid, lactic acid,
glycolic acid, citric
acid, mandelic acid, glntaric acid, maleic acid, malic acid, adipic acid,
succinic acid, tartaric
acid, etc. Preferably a composition of the invention includes acetic acid,
octanoic acid, or
propionic acid, lactic acid, heptanoic acid, octanoic acid, or nonanoic acid.
Additional
examples of suitable carboxylic acids are employed in sulfoperoxycarboxylic
acid or
sulfonatcd peracid systems, which arc disclosed in U.S. Patent No. 8,344,026,
and U.S. Patent
Publication Nos. 2010/0048730 and 2012/0052134.
Any suitable CI-Cm carboxylic acid can be used in the present compositions. In
some
embodiments, the Ci-C22 carboxylic acid is a C2-C2o carboxylic acid. In other
embodiments,
the Ci-C22 carboxylic acid is a CI, C2, C3, C4, C5, Co, C7, C8, C9, C10, Cll,
C12, C13, C14, C15,
C16, C17, Clg, C19, C20, C21, Or C22 carboxylic acid. In still other
embodiments, the C1-C22
carboxylic acid comprises acetic acid, octanoic acid and/or sulfonated oleic
acid.
The Ci-C22 carboxylic acid can be used at any suitable concentration. In some
embodiments, the Ci-C22 carboxylic acid has a concentration in an equilibrium
composition
from about 0.1 wt-% to about 90 wt-%. In other embodiments, the Ci-C22
carboxylic acid has
a concentration from about I wt-% to about 80 wt-%. In still other
embodiments, the C1-C22
carboxylic acid has a concentration at about I wt-% to about SO wt-%. Without
limiting the
27
Date Recue/Date Received 2023-12-12
scope of invention, the numeric ranges are inclusive of the numbers defining
the range and
include each integer within the defined range.
Hydrogen Peroxide
The present invention includes hydrogen peroxide. Hydrogen peroxide, H202,
provides
the advantages of having a high ratio of active oxygen because of its low
molecular weight
(34.014 g/mole) and being compatible with numerous substances that can be
treated by
methods of the invention because it is a weakly acidic, clear, and colorless
liquid. Another
advantage of hydrogen peroxide is that it decomposes into water and oxygen. It
is
advantageous to have these decomposition products because they are generally
compatible
with substances being treated. For example, the decomposition products are
generally
compatible with metallic substance (e.g., substantially noncorrosive) and arc
generally
innocuous to incidental contact and are environmentally friendly.
In one aspect of the invention, hydrogen peroxide is initially in an
antimicrobial
peracid composition in an amount effective for maintaining an equilibrium
between a
carboxylic acid, hydrogen peroxide, and a peracid. The amount of hydrogen
peroxide should
not exceed an amount that would adversely affect the antimicrobial activity of
a composition
of the invention. In further aspects of the invention, hydrogen peroxide
concentration can he
significantly reduced within an antimicrobial peracid composition. In some
aspects, an
advantage of minimizing the concentration of hydrogen peroxide is that
antimicrobial activity
of a composition of the invention is improved as compared to conventional
equilibrium
peracid compositions.
The hydrogen peroxide can be used at any suitable concentration. In some
embodiments, a concentrated equilibrium composition has a concentration of
hydrogen
peroxide from about 0.5 wt-% to about 90 wt-%, or from about 1 wt-% to about
90 wt-%. In
still other embodiments, the hydrogen peroxide has a concentration from about
1 wt-% to
about 80 wt-%, from about 1 wt-% to about SO wt-%. Without limiting the scope
of invention,
the numeric ranges are inclusive of the numbers defining the range and include
each integer
within the defined range.
28
Date Regue/Date Received 2023-12-12
Beneficially, the compositions and methods of the invention in providing
stabilized
equilibrium peracid compositions, are not reliant and/or limited according to
any particular
ratio of hydrogen peroxide to peracid for such enhanced stability. Instead, it
is unexpected
that the stabilizing agent (e.g. DPA) is suitable for providing peracid
stability under high
acidity / mineral acid conditions, while constraining the peracid SADT. This
represents a
significant improvement over the prior art, wherein DPA is an optional peracid
stabilizing
agent for low hydrogen peroxide containing peracid compositions. See e.g. U.S.
Publication
No. 2010/021558.
Peracid Stabilizing Agent
A peracid stabilizing agent or agents are included in compositions according
to the
invention. Beneficially, the peracid stabilizing agent or agents prevent the
decomposition of
peracid in an equilibrium peracid composition. In addition, peracid
stabilizing agent(s)
prevent an equilibrium peracid composition from reaching their self-
accelerating
decomposition temperatures. (SA DT) The use of the peracid stabilizing agent
beneficially
stabilizes highly acidic equilibrium peracids including mixed peracid
compositions, as well as
extreme chemistries with problematic high peracid to hydrogen peroxide ratios.
By elevating
the SADTs of the compositions the stabilizers contribute significant safety
benefits for
transportation and storage of the compositions. In some aspects, the
stabilizing agents delay or
prevent the composition from meeting its native SADT.
In an aspect of the invention, the stabilizing agent is a pyridine carboxylic
acid
compound. Pyridine carboxylic acids include dipicolinic acids, including for
example, 2,6-
pyridinedicarboxylic acid (DPA). In a further aspect, the stabilizing agent is
a picolinic acid,
or a salt thereof.
In an aspect of the invention, the stabilizing agent is a picolinic acid or a
compound
having the following Formula (IA):
0 0
(IA)
29
Date Regue/Date Received 2023-12-12
laR lb,
wherein Ri is OH or ¨NR wherein Ria and Rib are independently
hydrogen or (Ci
-C6)alkyl; R2 is OH or ¨NR2aR2b, wherein R' and R21' are independently
hydrogen or (Ci -
C6)alkyl; each R3 is independently (Ci -C6)alkyl, (C2 -C6)alkenyl or (C2 -
C6)alkynyl; and n is a
number from zero to 3; or a salt thereof.
In a further aspect of the invention, the peracid stabilizing agent is a
compound having
the following Formula (IB):
0 0
rR2
(R3). (113)
wherein W is OH or ¨NRiaR 1 b, wherein Ria and Rib are independently hydrogen
or (Ci
-C6)alkyl; R2 is OH or ¨NR2aR21', wherein R' and R21' are independently
hydrogen or (Ci -
C6)alkyl; each R3 is independently (Ci -C6)alkyl, (C2 -C6)alkenyl or (C2 -
C6)alkynyl; and n is a
number from zero to 3; or a salt thereof.
In a preferred aspect, the peracid stabilizing agent is dipicolinic acid
(picolinic acid,
2,6-Pyridinedicarboxylic acid) and provides stabilization for high mineral
content peracids,
wherein the resulting peracid composition has an elevated SADT.
Dipicolinic acid has been used as a stabilizer for peracid compositions, such
as
disclosed in WO 91/07375 and U.S. Patent No. 2,609,391. However, use of such
DPA
stabilizer for peracid compositions has not previously been disclosed and/or
exploited for its
SADT- elevating properties.
In a further aspect, the stabilizing agent may be combined with additional
conventional
stabilizing agents, e.g. a phosphonate based stabilizer, to beneficially
provide further increase
in stability of the composition, and in some aspects provide synergistic
increase in SADT and
peracid stability according to embodiments of the invention.
Stabilizing agents may be present in amounts sufficient to provide the
intended
stabilizing benefits, namely achieving the desired shelf life, and elevating
the SADT of the
Date Recue/Date Received 2023-12-12
highly acidic peroxycarboxylic acid compositions having a use solution pH of
below at least 4,
preferably below at least 3. As the property of the composition will vary
depending upon the
acidity of the particular peracid composition according to the invention, such
peracid
stabilizing agents may be present in a concentrated equilibrium peracid
composition in
amounts from about 0.001 wt-% to about 25 wt-%, 0.01 wt-% to about 10 wt-%,
and more
preferably from about 0.01 wt-% to about 1 wt-%. Without limiting the scope of
invention,
the numeric ranges are inclusive of the numbers defining the range and include
each integer
within the defined range.
Mineral Acid
In some embodiments, the present composition is a strongly acidic peracid as a
result
of inclusion of a strong acid. In some aspects the peracid composition has a
use solution pH of
4 or less, and preferably has a use solution pH of 3 or less. In some
embodiments, the present
composition includes an inorganic acid. In preferred embodiments, the present
composition
includes a mineral acid
Particularly suitable mineral acids include sulfuric acid (1H2SO4), sodium
hydrogen
sulfate, nitric acid, sulfamic acid and sulfonic acids both alkyl and aryl, in
particular methane
sulfonic acid and dodecyltien7ene, toluene, xylene, naphthalene and cumene
sulfonic acid,
and/or phosphoric acid (II3PO4). Additional phosphonic acids which may be used
according to
the invention include, for example, aminotrimethylene phosphonic acid,
ethylene diamin
tetramethylene phosphonic acid, hexamethylene diamin tetramethylene phosphonic
acid,
diethylene triamin tetramethylene phosphonic acid, and 1- hydroxyethylidene-
1,1-
diphosphonic acid (HEDP).
In a further aspect, the acids suitable for use include are not limited to
mineral acids.
Instead, acids suitable for use include strong acids, which are defined as
those with a pKa near
or below the lower pKas of HEDP which may cause significant protonation of the
HEDP and
other phosphate and phosphonate stabilizers and thus diminish their ability to
stabilize the
peracid chemistries. Additional description of mineral acids for use in
peracid compositions is
disclosed in WO 91/07375.
31
Date Recue/Date Received 2023-12-12
In an aspect of the invention, the mineral acid providing the strong acidity
of the
peracid compositions can be used at any suitable concentration. In some
embodiments, a
concentrated equilibrium composition has a concentration of the mineral acid
from about 0.5
wt-% to about 50 wt-%, or from about 1 wt-% to about 50 wt-%. hi still other
embodiments,
the mineral acid has a concentration from about 1 wt-% to about 20 wt-%, or
more preferably
from about 5 wt-% to about 20 wt-%. Without limiting the scope of invention,
the numeric
ranges are inclusive of the numbers defining the range and include each
integer within the
defined range.
Defoaming Agent
The present invention includes a defoaming agent. Defoaming agents suitable
for use
in the peroxycarboxylic acid compositions according to the invention are
compatible with the
highly acidic peracid compositions and anionic and/or nonionic surfactants
which may be
employed in the peracid compositions. The defoaming agents suitable for use in
the
pemxycarhoxylic, acid compositions according in the inventinn, maintain a low
foam profile
under various water conditions, preferably under deionized or soft water
conditions, and/or
under mechanical action. In a still further aspect, the defoaming agents are
compatible with
surfactants, preferably anionic surfactants, to achieve critical performance
such as
coupling/wetting, improved material compatibility and enhanced biocidal
efficacy. In
preferred aspects, the defoaming agent provides a synergistic biocidal
efficacy.
In an aspect of the invention, the defoaming agent is a metal salt, including
for
example, aluminum, magnesium, calcium, zinc and/or other rare earth metal
salts. In a
preferred aspect, the defoaming agent is a cation with high charge density,
such as Fe', AP'
and La3. In a preferred aspect, the defoaming agent is aluminum sulfate.
In an aspect, the defoaming agent is not a transition metal compound, which
are
incompatible with the highly acidic equilibrium peracid compositions according
to the
invention.
In some embodiments, the compositions of the present invention can include
antifoaming agents or defoamers which are of food grade quality given the
application of the
method of the invention.
32
Date Recue/Date Received 2023-12-12
In a further embodiment, the compositions of the present invention can include
defoaming agents which are stable in acid environments (e.g. the peracid
compositions
containing a mineral acid and having a use solution pH of about 4 or less)
and/or are
oxidatively stable.
In an aspect of the invention, the defoaming agent can be used at any suitable
concentration to provide defoaming with the surfactants according to the
invention and to
provide synergistic biocidal efficacy. In some embodiments, a concentrated
equilibrium
composition has a concentration of the a defoaming agent from about 0.001 wt-%
to about 10
wt-%, or from about 0.1 wt-% to about 5 wt-%. In still other embodiments, the
defoaming
agent has a concentration from about 0.1 wt-% to about 1 wt-%. Without
limiting the scope of
invention, the numeric ranges are inclusive of the numbers defining the range
and include each
integer within the defined range.
Surfactants
In some embodiments, the compositions of the present invention include a
surfactant
Surfactants suitable for use with the compositions of the present invention
include, but are not
limited to nonionic surfactants and/or anionic surfactants. Preferably, a low
foaming anionic
surfactant is included in the peroxycarboxylic acid compositions Beneficially,
according to
embodiments of the invention, the use of the defoaming agent (e.g. aluminum
sulfate) in
combination with the surfactant overcomes the foaming issues that are known to
result from
the use of conventional low-foaming surfactants in peroxycarboxylic acid
compositions,
especially in deionized or soft water.
In some embodiments, the compositions of the present invention include about 0
wt-%
to about 40 wt-% of a surfactant. In other embodiments the compositions of the
present
invention include about 0.1 wt-% to about 40 wt-% of a surfactant, preferably
from about 0.1
wt-% to about 25 wt-% of a surfactant, and more preferably from about 1 wt-%
to about 20
wt-% of a surfactant.
Anionic surfactants
Preferably, surface active substances which are categorized as anionics
because the
charge on the hydrophobe is negative are utilized according to the present
invention; or
33
Date Recue/Date Received 2023-12-12
surfactants in which the hydrophobic section of the molecule carries no charge
unless the pH
is elevated to neutrality or above (e.g. carboxylic acids). Carboxylate,
sulfonate, sulfate and
phosphate are the polar (hydrophilic) solubilizing groups found in anionic
surfactants. Of the
cations (counter ions) associated with these polar groups, sodium, lithium and
potassium
impart water solubility; ammonium and substituted ammonium ions provide both
water and oil
solubility; and, calcium, barium, and magnesium promote oil solubility. As
those skilled in the
art understand, anionics are excellent detersive surfactants and are therefore
favored additions
to heavy duty detergent compositions.
Anionic sulfate surfactants suitable for use in the present compositions
include alkyl
ether sulfates, alkyl sulfates, the linear and branched primary and secondary
alkyl sulfates,
alkyl ethoxysulfates, fatty olcyl glycerol sulfates, alkyl phenol ethylene
oxide ether sulfates,
the Cs -C17 acyl-N-(Ci -C4 alkyl) and -N-(Ci -C2 hydroxyalkyl) glucamine
sulfates, and
sulfates of alkylpolysaccharides such as the sulfates of alkylpolyglucoside,
and the like. Also
included are the alkyl sulfates, alkyl poly(ethylenenxy) ether sulfates and
aromatic
poly(ethyleneoxy) sulfates such as the sulfates or condensation products of
ethylene oxide and
nonyl phenol (usually having I to 6 oxyethylene groups per molecule).
Anionic sulfonate surfactants suitable for use in the present compositions
also include
alkyl sulfonates, the linear and branched primary and secondary alkyl
sulfonates, and the
aromatic sulfonates with or without substituents.
Anionic carboxylate surfactants suitable for use in the present compositions
include
carboxylic acids (and salts), such as alkanoic acids (and alkanoates), ester
carboxylic acids
(e.g. alkyl succinates), ether carboxylic acids, sulfonated fatty acids, such
as sulfonated oleic
acid, and the like. Such carboxylates include alkyl ethoxy carboxylates, alkyl
aryl ethoxy
carboxylates, alkyl polyethoxy polycarboxylate surfactants and soaps (e.g.
alkyl carboxyls).
Secondary carboxylates useful in the present compositions include those which
contain a
carboxyl unit connected to a secondary carbon. The secondary carbon can be in
a ring
structure, e.g. as in p-octyl benzoic acid, or as in alkyl-substituted
cyclohexyl carboxylates.
The secondary carboxylate surfactants typically contain no ether linkages, no
ester linkages
and no hydroxyl groups. Further, they typically lack nitrogen atoms in the
head-group
34
Date Recue/Date Received 2023-12-12
(amphiphilic portion). Suitable secondary soap surfactants typically contain
11-13 total
carbon atoms, although more carbons atoms (e.g., up to 16) can be present.
Suitable
carboxylates also include acylamino acids (and salts), such as acylgluamates,
acyl peptides,
sarcosinates (e.g. N-acyl sarcosinates), taurates (e.g. N-acyl taurates and
fatty acid amides of
methyl tauride), and the like.
Suitable anionic surfactants include alkyl or alkylaryl ethoxy carboxylates of
the
following formula:
R - 0 - (C112C1120).(C112)m - CO2X (3)
it, Km
in which R is a CR to C22 alkyl group or
, in which 121 is a C4-C16 alkyl
group; n is an integer of 1-20; in is an integer of 1-3; and X is a counter
ion, such as hydrogen,
sodium, potassium, lithium, ammonium, or an amine salt such as
monoethanolamine,
diethanolamine or briethanolamine. In some embodiments, n is an integer of 4
to 10 and m is
1 In some embodiments, R is a Cs-C16 alkyl group. In some embodiments, R is a
C12-C14
alkyl group, n is 4, and m is l
RI 411
In other embodiments, R is and RI is
a C6-C12 alkyl group. In still yet
other embodiments, R1 is a C9 alkyl group, n is 10 and in is 1.
Such alkyl and alkylaryl elhoxy earboxylatec are commercially available.
These ethoxy carboxylates are typically available as the acid forms, which can
be readily
converted to the anionic or salt form. Commercially available carboxylates
include, Neodox
23-4, a C17-11 alkyl polyethoxy (4) carboxylic acid (Shell Chemical), and
Emcol CNP-l10, a
C9 alkylaryl polyethoxy (10) carboxylic acid (Witco Chemical). Carboxylates
are also
available from Clan i ant, e.g. the product Sandopan DTC, a C13 alkyl
polyethoxy (7)
carboxylic acid.
Nonionic Swfaclants
Useful nonionic surfactants are generally characterized by the presence of an
organic hydrophobic group and an organic hydrophilic group and are typically
produced by
Date Regue/Date Received 2023-12-12
the condensation of an organic aliphatic, alkyl aromatic or polyoxyalkylene
hydrophobic
compound with a hydrophilic alkaline oxide moiety which in common practice is
ethylene
oxide or a polyhydration product thereof, polyethylene glycol. Practically any
hydrophobic
compound having a hydroxyl, carboxyl, amino, or amido group with a reactive
hydrogen atom
can be condensed with ethylene oxide, or its polyhydration adducts, or its
mixtures with
alkoxylenes such as propylene oxide to form a nonionic surface-active agent.
The length of the
hydrophilic polyoxyalkylene moiety which is condensed with any particular
hydrophobic
compound can be readily adjusted to yield a water dispersible or water soluble
compound
having the desired degree of balance between hydrophilic and hydrophobic
properties. Useful
nonionic surfactants include:
Block polyoxypropylene-polyoxyethylene polymeric compounds based upon
propylene glycol, ethylene glycol, glycerol, trimethylolpropane, and
ethylenediamine as the
initiator reactive hydrogen compound. Examples of polymeric compounds made
from a
sequential prnpoxylation and ethoxylation of initiator are commercially
available under the
trade names Pluronicg and Tetronic manufactured by BASF Corp. Pluronic
compounds are
difunetional (two reactive hydrogens) compounds formed by condensing ethylene
oxide with a
hydrophobic base formed by the addition of propylene oxide to the two hydroxyl
groups of
propylene glycol. This hydrophobic portion of the molecule weighs from about
1,000 to about
4,000. Ethylene oxide is then added to sandwich this hydrophobe between
hydrophilic groups,
controlled by length to constitute from about 100/4) by weight to about 80% by
weight of the
final molecule. Tetronic compounds are tetra-flinctional block copolymers
derived from the
sequential addition of propylene oxide and ethylene oxide to ethylenediamine.
The molecular
weight of the propylene oxide hydrotype ranges from about 500 to about 7,000;
and, the
hydrophile, ethylene oxide, is added to constitute from about 10% by weight to
about 80% by
weight of the molecule.
2. Condensation products of one mole of alkyl phenol wherein the
alkyl chain, of
straight chain or branched chain configuration, or of single or dual alkyl
constituent, contains
from about 8 to about 18 carbon atoms with from about 3 to about 50 moles of
ethylene oxide.
The alkyl group can, for example, be represented by diisobutylene, di-amyl,
polymerized
36
Date Recue/Date Received 2023-12-12
propylene, iso-octyl, nonyl, and di-nonyl. These surfactants can be
polyethylene,
polypropylene, and polybutylene oxide condensates of alkyl phenols. Examples
of commercial
compounds of this chemistry are available on the market under the trade names
Igepal
manufactured by Rhone-Poulenc and Triton manufactured by Union Carbide.
3. Condensation products of one mole of a saturated or unsaturated,
straight or
branched chain alcohol having from about 6 to about 24 carbon atoms with from
about 3 to
about 50 moles of ethylene oxide. The alcohol moiety can consist of mixtures
of alcohols in
the above delineated carbon range or it can consist of an alcohol having a
specific number of
carbon atoms within this range. Examples of like commercial surfactant are
available under
the trade names Neodol'I'm manufactured by Shell Chemical Co. and Alfoniem
manufactured
by Vista Chemical Co_
4. Condensation products of one mole of saturated or unsaturated, straight
or
branched chain carboxylic acid having from about 8 to about 18 carbon atoms
with from about
6 to about 50 moles of ethylene oxide The acid -moiety can consist of -
mixtures of acids in the
above defined carbon atoms range or it can consist of an acid having a
specific number of
carbon atoms within the range. Examples of commercial compounds of this
chemistry are
available on the market under the trade names Napoleon manufactured by Henkel
Corporation and Lipopee manufactured by Lipo Chemicals, Inc.
In addition to ethoxylated carboxylic acids, commonly called polyethylene
glycol
esters, other alkanoic acid esters formed by reaction with glycerides,
glycerin, and polyhydric
(saccharide or sorbitan/sorbitol) alcohols have application in this invention
for specialized
embodiments, particularly indirect food additive applications. All of these
ester moieties have
one or more reactive hydrogen sites on their molecule which can undergo
further acylation or
ethylene oxide (alkoxide) addition to control the hydrophilicity of these
substances. Care must
be exercised when adding these fatty ester or acylated carbohydrates to
compositions of the
present invention containing amylase and/or lipase enzymes because of
potential
incompatibility.
Examples of nonionic low foaming surfactants include:
37
Date Recue/Date Received 2023-12-12
5. Compounds from (1) which are modified, essentially reversed, by adding
ethylene oxide to ethylene glycol to provide a hydrophile of designated
molecular weight; and,
then adding propylene oxide to obtain hydrophobic blocks on the outside (ends)
of the
molecule. The hydrophobic portion of the molecule weighs from about 1,000 to
about 3,100
with the central hydrophile including 10% by weight to about 80% by weight of
the final
molecule. These reverse Pluronics' are manufactured by BASF Corporation under
the trade
name Pluronic R surfactants. Likewise, the Tetronic' R surfactants are
produced by BASF
Corporation by the sequential addition of ethylene oxide and propylene oxide
to
ethylenediamine. The hydrophobic portion of the molecule weighs from about
2,100 to about
6,700 with the central hydrophile including 10% by weight to 80% by weight of
the final
molecule.
6. Compounds from groups (1), (2), (3) and (4) which are modified by
"capping"
or "end blocking" the terminal hydroxy group or groups (of multi-functional
moieties) to
reduce foaming by reaction with a small hydrophobic molecule such as propylene
oxide,
butylene oxide, benzyl chloride; and, short chain fatty acids, alcohols or
alkyl halides
containing from 1 to about 5 carbon atoms; and mixtures thereof. Also included
are reactants
such as tbionyl chloride which convert terminal hydroxy groups to a chloride
group Such
modifications to the terminal hydroxy group may lead to all-block, block-
heteric, heteric-block
or all-heteric nonionics.
Additional examples of effective low foaming nonionics include:
7. The alkylphenoxypolyethoxyalkanols of U.S. Pat. No. 2,903,486 issued
Sep. 8,
1959 to Brown et al. and represented by the formula
R
i0A11 in which R is an alkyl group of 8 to 9 carbon atoms, A is an alkylene
chain of 3 to 4
carbon atoms, n is an integer of 7 to 16, and m is an integer of 1 to 10.
The polyalkylene glycol condensates of U.S. Pat. No. 3,048,548 issued Aug. 7,
1962 to Martin et al. having alternating hydrophilic oxyethylene chains and
hydrophobic
38
Date Regue/Date Received 2023-12-12
oxypropylene chains where the weight of the terminal hydrophobic chains, the
weight of the
middle hydrophobic unit and the weight of the linking hydrophilic units each
represent about
one-third of the condensate.
The defoaming nonionic surfactants disclosed in U.S. Pat. No. 3,382,178 issued
May 7,
1968 to Lissant et al. having the general formula Zr(OR)n01117 wherein Z is
alkoxylatable
material, R is a radical derived from an alkaline oxide which can be ethylene
and propylene
and n is an integer from, for example, 10 to 2,000 or more and z is an integer
determined by
the number of reactive oxyalkylatable groups.
The conjugated polyoxyalkylene compounds described in U.S. Pat. No. 2,677,700,
issued May 4, 1954 to Jackson et al. corresponding to the formula Y(C3H60)n
(C2H40)mH
wherein V is the residue of organic compound having from about] to 6 carbon
atoms and one
reactive hydrogen atom, n has an average value of at least about 6.4, as
determined by
hydroxyl number and m has a value such that the oxyethylene portion
constitutes about 10% to
about 90% by weight of the molecule
The conjugated polyoxyalkylene compounds described in U.S. Pat. No. 2,674,619,
issued Apr. 6, 1954 to Lundsted et al. having the formula YI (C3H6On (C2H40),H
ix wherein Y
is the residue of an organic compound having from about 2 to 6 carbon atoms
and containing x
reactive hydrogen atoms in which x has a value of at least about 2, n has a
value such that the
molecular weight of the polyoxypropylene hydrophobic base is at least about
900 and m has
value such that the oxyethylene content of the molecule is from about 10% to
about 90% by
weight. Compounds falling within the scope of the definition for Y include,
for example,
propylene glycol, glycerine, pentaerythritol, trimethylolpropane,
ethylenediamine and the like.
The oxypropylene chains optionally, but advantageously, contain small amounts
of ethylene
oxide and the oxyethylene chains also optionally, but advantageously, contain
small amounts
of propylene oxide.
Additional conjugated polyoxyalkylene surface-active agents which are
advantageously used in the compositions of this invention correspond to the
formula:
P[(C3H60)n (C2H40)infl]x wherein P is the residue of an organic compound
having from about
8 to 18 carbon atoms and containing x reactive hydrogen atoms in which x has a
value of 1 or
39
Date Recue/Date Received 2023-12-12
2, n has a value such that the molecular weight of the polyoxyethylene portion
is at least about
44 and m has a value such that the oxypropylene content of the molecule is
from about 10% to
about 90% by weight. In either case the oxypropylene chains may contain
optionally, but
advantageously, small amounts of ethylene oxide and the oxyethylene chains may
contain also
optionally, but advantageously, small amounts of propylene oxide.
8. Polyhydroxy fatty acid amide surfactants suitable for use in the present
compositions include those having the structural formula R2CONRIZ in which: R1
is H, CI-C4
hydrocarbyl, 2-hydroxy ethyl, 2-hydroxy propyl, ethoxy, propoxy group, or a
mixture thereof;
R2 is a C5-C31 hydrocarbyl, which can be straight-chain; and Z is a
polyhydroxyhydrocarbyl
having a linear hydrocarbyl chain with at least 3 hydroxyls directly connected
to the chain, or
an alkoxylated derivative (preferably ethoxylated or propoxylatcd) thereof Z
can be derived
from a reducing sugar in a reductive amination reaction; such as a glycityl
moiety.
9. The alkyl ethoxylate condensation products of aliphatic alcohols with
from
ahout 0 to about 25 moles of ethylene oxide are suitable for use in the
pre.sent compositions
The alkyl chain of the aliphatic alcohol can either be straight or branched,
primary or
secondary, and generally contains from 6 to 22 carbon atoms.
10_ The ethoxylated C6-Cift fatty alcohols and CG-Cis mixed
ethoxylated and
propoxylated fatty alcohols are suitable surfactants for use in the present
compositions,
particularly those that are water soluble. Suitable ethoxylated fatty alcohols
include the C6-Cis
ethoxylated fatty alcohols with a degree of ethoxylation of from 3 to 50.
11. Suitable nonionic allcylpolysaccharide surfactants,
particularly for use in the
present compositions include those disclosed in U.S. Pat. No. 4,565,647,
Llenado, issued Jan.
21, 1986. These surfactants include a hydrophobic group containing from about
6 to about 30
carbon atoms and a polysaccharide, e.g., a polyglycoside, hydrophilic group
containing from
about 1.3 to about 10 saccharide units. Any reducing saccharide containing 5
or 6 carbon
atoms can be used, e.g., glucose, galactose and galactosyl moieties can be
substituted for the
glucosyl moieties. (Optionally the hydrophobic group is attached at the 2-, 3-
, 4-, etc. positions
thus giving a glucose or galactose as opposed to a glucoside or galactoside.)
The
Date Recue/Date Received 2023-12-12
intersaccharide bonds can be, e.g., between the one position of the additional
saccharide units
and the 2-, 3-, 4-, and/or 6-positions on the preceding saccharide units.
12. Fatty acid amide surfactants suitable for use the present
compositions include
those having the formula: R6CON(11.7)2 in which R6 is an alkyl group
containing from 7 to 21
carbon atoms and each R7 is independently hydrogen, Ci- C4 alkyl, Ci- C4
hydroxyalkyl, or --(
C2H40)xH, where x is in the range of from 1 to 3.
11 A useful class of non-ionic surfactants include the class
defined as alkoxylated
amines or, most particularly, alcohol alkoxylated/aminated/alkoxylated
surfactants. These non-
ionic surfactants may be at least in part represented by the general formulae:
R20--(PO)sN--
(EO) tH, R20--(P0)814--(E0)1H(E0)tH, and R20--N(E0)tH; in which R2 is an
alkyl, alkenyl or
other aliphatic group, or an alkyl-aryl group of from 8 to 20, preferably 12
to 14 carbon atoms,
EO is oxyethylene, PO is oxypropylene, s is 1 to 20, preferably 2-5, t is 1-
10, preferably 2-5,
and u is 1-10, preferably 2-5. Other variations on the scope of these
compounds may be
represented by the alternative formula. R20--(P0)v--NI(F.0) w1-111(F.0) MI in
which R2 is aft
defined above, v is 1 to 20 (e.g., 1, 2, 3, or 4 (preferably 2)), and w and z
are independently 1-
10, preferably 2-5. These compounds are represented commercially by a line of
products sold
by Huntsman Chemicals as nonionic surfactants A preferred chemical of this
class includes
SurfonicTm PEA 25 Amine Alkoxylate. Preferred nonionic surfactants for the
compositions of
the invention include alcohol alkoxylates, EO/P0 block copolymers, alkylphenol
alkoxylates,
and the like.
The treatise Nonionic Surfactants, edited by Schick, M. J., Vol. 1 of the
Surfactant
Science Series, Marcel Dekker, Inc., New York, 1983 is an excellent reference
on the wide
variety of nonionic compounds generally employed in the practice of the
present invention. A
typical listing of nonionic classes, and species of these surfactants, is
given in U.S. Pat. No.
3,929,678 issued to Laughlin and Heuring on Dec. 30, 1975. Further examples
are given in
"Surface Active Agents and detergents" (Vol. 1 and II by Schwartz, Perry and
Berch).
Semi-Polar Nonionic Surfactants
The semi-polar type of nonionic surface active agents are another class of
nonionic
surfactant useful in compositions of the present invention. Generally, semi-
polar nonionies are
41
Date Regue/Date Received 2023-12-12
high foamers and foam stabilizers, which can limit their application in CIP
systems. However,
within compositional embodiments of this invention designed for high foam
cleaning
methodology, semi-polar nonionics would have immediate utility. The semi-polar
nonionic
surfactants include the amine oxides, phosphine oxides, sulfoxides and their
alkoxylated
derivatives.
14. Amine oxides are tertiary amine oxides corresponding to the
general formula:
wherein the arrow is a conventional representation of a semi-polar bond; and,
R', R2,
and R3 may be aliphatic, aromatic, heterocyclic, alicyclic, or combinations
thereof. Generally,
for amine oxides of detergent interest, R' is an alkyl radical of from about 8
to about 24 carbon
atoms; R2 and R3 are alkyl or hydroxyalkyl of 1-3 carbon atoms or a mixture
thereof; R2 and
R3 can be attached to each other, e.g. through an oxygen or nitrogen atom, to
form a ring
structure; le is an alkaline or a hydroxyalkylene group containing 2 to 3
carbon atoms; and n
ranges from 0 to about 20.
Useful water soluble amine oxide surfactants are selected from the coconut or
tallow
alkyl di-(lower alkyl) amine oxides, specific examples of which are
dodecyldimethylamine
oxide, tridecyldimethylamine oxide, etradecyldimethylamine oxide,
pentadecyldimethylamine
oxide, hexadecyldimethylamine oxide, heptadecyldimethylamMe oxide,
octadecyldimethylaine oxide, dodecyldipropylamine oxide,
tetradecyldipropylamine oxide,
hexadecyldipropylamine oxide, tetradecyldibutylamine oxide,
octadecyldibutylamine oxide,
bis(2-hydroxyethyl)dodecylfunine oxide, bis(2-hydroxyethyl)-3-dodecoxy-1-
hydroxypropylamine oxide, dimethyl-(2-hydroxydodecypamine oxide, 3,6,9-
trioctadecyldimethylamine oxide and 3-dodecoxy-2-hydroxypropyldi-(2-
hydroxyethyl)amine
oxide.
Useful semi-polar nonionic surfactants also include the water soluble
phosphine oxides
42
Date Recue/Date Received 2023-12-12
having the following structure:
RJ1.4o.()
/3
wherein the arrow is a conventional representation of a semi-polar bond; and,
121 is an
alkyl, alkenyl or hydroxyalkyl moiety ranging from 10 to about 24 carbon atoms
in chain
length; and, R.' and R3 are each alkyl moieties separately selected from alkyl
or hydroxyalkyl
groups containing 1 to 3 carbon atoms.
Examples of useful phosphine oxides include dimethyldecylphosphine oxide,
dimethyltetradecylphosphine oxide, methylethyltetradecylphosphone oxide,
dimethylhexadecylphosphine oxide, di ethyl-2-hydroxyoctyldecylphosphine oxide,
bis(2-
hydroxyethyl)dodecylphosphine oxide, and bis(hydroxymethyptetradecylphosphine
oxide.
Semi-polar nonionic surfactants useful herein also include the water soluble
sulfoxide
compounds which have the structure:
11./
wherein the arrow is a conventional representation of a semi-polar bond; and,
R' is an
alkyl or hydroxyalkyl moiety of about 8 to about 28 carbon atoms, from 0 to
about 5 ether
linkages and from 0 to about 2 hydroxyl substituents; and R2 is an alkyl
moiety consisting of
alkyl and hydroxyalkyl groups having 1 to 3 carbon atoms.
Useful examples of these sulfoxides include dodecyl methyl sulfoxide; 3-
hydroxy
tridecyl methyl sulfoxide; 3-methoxy tridecyl methyl sulfoxide; and 3-hydroxy-
4-
dodecoxybutyl methyl sulfoxide.
Semi-polar nonionic surfactants for the compositions of the invention include
dimethyl
amine oxides, such as lauryl dimethyl amine oxide, myristyl dimethyl amine
oxide, cetyl
dimethyl amine oxide, combinations thereof, and the like. Useful water soluble
amine oxide
surfactants are selected from the octyl, decyl, dodecyl, isododecyl, coconut,
or tallow alkyl di-
43
Date Recue/Date Received 2023-12-12
(lower alkyl) amine oxides, specific examples of which are octyldimethylamine
oxide,
nonyldimethylamine oxide, decyldimethylamine oxide, undecyldimethylamine
oxide,
dodecyldimethylamine oxide, iso-dodecyldimethyl amine oxide,
tridecyldimethylamine oxide,
tetradecyldimethylamine oxide, pentadecyldimethylamine oxide,
hexadecyldimethylamine
oxide, heptadecyldimethylamine oxide, octadecyldimethylaine oxide,
dodecyldipropylamine
oxide, tetradecyldipropylamine oxide, hexadecyldipropylamine oxide,
tetradecyldibutylamine
oxide, octadecyldibutylamine oxide, bis(2-hydroxyethyl)dodecylamine oxide,
bis(2-
hydroxyethyl)-3-dodecoxy-1-hydroxypropylamine oxide, dimethyl-(2-
hydroxydodecyl)amine
oxide, 3,6,9-trioctadecyldimethylamine oxide and 3-dodecoxy-2-hydroxypropyldi-
(2-
hydroxyethyl)amine oxide.
Suitable nonionic surfactants suitable for use with the compositions of the
present
invention include alkoxylated surfactants. Suitable alkoxylated surfactants
include ED/PO
copolymers, capped EO/PO copolymers, alcohol alkoxylates, capped alcohol
alkoxylates,
mixtures thereof, or the like Suitable alkoxylated surfactants for use as
solvents include
EO/PO block copolymers, such as the Pluronic and reverse Pluronic surfactants;
alcohol
alkoxylates, such as Dehypon LS-54 (R-(E0)5(P0)4) and Dehypon LS-36 (R-
(E0)3(P0)6); and
capped alcohol alkoxylates, such as Plurafac 1F221 and Tegoten FE 1 1;
mixtures thereof, or
the like.
Additional Functional Ingredients
In some embodiments, the present composition can further comprise additional
functional ingredients. In some embodiments, the highly acidic peracid
composition including
the defoaming agent and/or surfactant, mineral acid, peroxycarboxylic acid,
carboxylic acid,
hydrogen peroxide and water make up a large amount, or even substantially all
of the total
weight of the peracid compositions. For example, in some embodiments few or no
additional
.. functional ingredients are disposed therein.
In other embodiments, additional functional ingredients may be included in the
compositions. The functional ingredients provide desired properties and
functionalities to the
compositions. For the purpose of this application, the term "functional
ingredient" includes a
material that when dispersed or dissolved in a use andJor concentrate
solution, such as an
44
Date Recue/Date Received 2023-12-12
aqueous solution, provides a beneficial property in a particular use. Some
particular examples
of functional materials are discussed in more detail below, although the
particular materials
discussed are given by way of example only, and that a broad variety of other
functional
ingredients may be used. In some aspects, the compositions may include
stabilizing agents,
additional surfactants, additional antimicrobial agents, anti-redeposition
agents, bleaching
agents, solubility modifiers, dispersants, rinse aids, metal protecting
agents, stabilizing agents,
corrosion inhibitors, fragrances and/or dyes, rheology modifiers or
thickeners, hydrotropes or
couplers, buffers, solvents and the like.
In preferred embodiments, the compositions further include a peracid
stabilizing agent.
In additional preferred embodiments, the compositions do not include
phosphonic acid based
stabilizers (e.g. pyrophosphoric acids and/or salts thereof, IIEDP, (I
In42PnO3n, I)).
In preferred embodiments, the compositions further include substances that aid
in the
solubilization of the stabilizing agent(s), including for example, hydrotropes
such as sodium
xylene sulfonate (SXS), sodium cumene sulfonates (SCS), surfactants, such as
anionic
.. surfactants and nonionic surfactants, and a defoaming agent. In further
aspects, the
composition may utilize alternative hydrotropes for solubilization of the
stabilizing agent,
including for example, n-octanesulfonate, a xylene sulfonate, a naphthalene
sulfonate,
ethylhexyl sulfate, lauryl sulfate, an amine oxide, etc.
Peracid Stabilizing Agent
A peracid stabilizing agent or agents are preferably included in compositions
according
to the invention. Beneficially, the peracid stabilizing agent(s) prevents the
decomposition of
peracid in an equilibrium peracid composition. In addition, the use of the
peracid stabilizing
agent also help to elevate the SADT of the compositions providing significant
benefits for
transportation and storage of the compositions. In some aspects, the
stabilizing agents delay or
prevent the composition from meeting its native SADT.
In an aspect of the invention, the stabilizing agent is a pyridine carboxylic
acid
compound. Pyridine carboxylic acids include dipicolinic acids, including for
example, 2,6-
pyridinedicarboxylic acid (DPA). In a further aspect, the stabilizing agent is
a picolinic acid,
or a salt thereof.
Date Recue/Date Received 2023-12-12
In an aspect of the invention, the stabilizing agent is a picolinic acid or a
compound
having the following Formula (IA):
0 0
==e"R2
(R3)õ .. (IA)
wherein R' is OH or ¨NRIalt lb, wherein Rand R11) are independently hydrogen
or (Ci
-C6)alkyl; R2 is OH or ¨NeR21', wherein R2a and R21' are independently
hydrogen or (CI -
C6)alkyl; each R3 is independently (CI -C6)alkyl, (C2 -C6)alkenyl or (C2 -
C6)alkynyl; and n is a
number from zero to 3; or a salt thereof.
Tn a further aspect of the invention, the peracid stabilizing agent is a
compound having
the following Formula (IB):
R I R2
R3h, (IB)
wherein R1 is OH or ¨NR1'11.1b, wherein Ria and Rib are independently hydrogen
or (Ci
-C6)alkyl; R2 is 011 or ¨NR2alt 2b, wherein lea and R2b are independently
hydrogen or (Ci -
C6)alkyl; each IV is independently (Ci -C6)alkyl, (C2 -C6)alkenyl or (C2 -
C6)alkynyl; and n is a
number from zero to 3; or a salt thereof.
In a preferred aspect, the peracid stabilizing agent is dipicolinic acid
(picolinic acid,
2,6-Pyridinedicarboxylic acid) and provides stabilization for high mineral
content peracids,
wherein the resulting peracid composition has an elevated SADT.
Dipicolinic acid has been used as a stabilizer for peracid compositions, such
as
disclosed in WO 91/07375 and U.S. Patent No. 2,609,391. However, use of such
DPA
stabilizer for peracid compositions has not previously been disclosed and/or
exploited for its
46
Date Recue/Date Received 2023-12-12
SADT elevating properties. In a further aspect, the stabilizing agent may be
combined with
additional conventional stabilizing agents, e.g. a phosphonate based
stabilizer.
Stabilizing agents may be present in amounts sufficient to provide the
intended
stabilizing benefits, namely achieving the desired shelf life, and elevating
the SADT of the
highly acidic peroxycarboxylic acid compositions having a use solution pH of
below at least 4,
preferably below at least 3. As the property of the composition will vary
depending upon the
acidity of the particular peracid composition according to the invention, such
peracid
stabilizing agents may be present in a concentrated equilibrium peracid
composition in
amounts from about 0.001 wt-% to about 25 wt-%, 0.01 wt-% to about 10 wt-%,
and more
preferably from about 0.01 wt-% to about 1 wt-%. Without limiting the scope of
invention,
the numeric ranges are inclusive of the numbers defining the range and include
each integer
within the defined range.
Fluorescent Active Compound
In an aspect, the present composition is a strongly acidic equilibrium peracid
containing a fluorescent active compound that is stable in the peracid
compositions according
to the invention. In an aspect, the fluorescent active compound is formulated
directly into the
equilibrium peracid composition, instead of contained in a two or more part
system (e.g.
peracid precursors or preformed peracids with a fluorescent active compound
added prior to
use and having short stability). In additional aspects, the fluorescent active
compound is
further stable in and suitable for use in other peracid compositions,
including compositions in
concentrate and/or use solutions at both acidic and alkaline plls. For
example, in some aspects,
the fluorescent active compound is further stable in highly acidic
compositions (e.g. cleaning
and sanitizing compositions) and caustic compositions (e.g. laundry
compositions). In further
aspects, the fluorescent active compound is further stable in strongly oxidant
systems (often
employed for sanitizing compositions), such as chlorine.
In some aspects, the fluorescent active compound may be an inert component of
the
composition (e.g. sanitizing compositions). In other aspects, the fluorescent
active compound
is an active component of the composition (e.g. cleaning compositions).
47
Date Recue/Date Received 2023-12-12
In an aspect, the fluorescent active compound is an aryl sulfonate. In other
aspects, the
fluorescent active compound is an alkyl aryl sulfonate. In further aspects,
the fluorescent
active compound is an aromatic ring with a hydrophilic group (e.g. sulfonate,
carboxylic).
Without being limited to a particular theory or mechanism of the invention,
the inclusion of
the hydrophilic group of the aromatic ring beneficially results in the
compatibility of the
fluorescent active compound with the peracid composition.
Exemplary suitable alkyl aryl sulfonates that can be used in the compositions
as
fluorescent active compounds can have an alkyl group that contains 0 to 16
carbon atoms and
the aryl group can be at least one of benzene, diphenyl oxide, and/or
naphthalene. A suitable
alkyl aryl sulfonate includes linear alkyl benzene sulfonate. A suitable
linear alkyl benzene
sulfonate includes linear dodecyl benzyl sulfonate that can be provided as an
acid that is
neutralized to form the sulfonate. Additional suitable alkyl aryl sulfonates
include benzene
sulfonate, toluene sulfonate, xylene sulfonate, cumene sulfonate, diphenyl
oxide
disulfonate, naphthalene sulfonate and naphthalene di sulfonates.
Additional exemplary suitable aromatic rings having a hydrophilic group are
shown in
the following formulas:
503m R3 03M
SO3M
SO3M
1 2 41 0
1 R2 Rl
M=H+, or Na R2
M=H , or Na Ri= H, or Alkyl M=H ,or Na
+
RI= H, or Alkyl R2= H, or Alkyl R1= H. or Alkyl
R2= H, or Alkyl R3=S03M, H, or Alkyl R2= H, or Alkyl
In an aspect, the fluorescent active compound is sodium xylene sulfonate
(SXS), such
as is commercially available from the Stepan Company, and/or sodium cumene
sulfonate
(SCS), such as is commercially available from AkzoNobel. In an aspect, the
fluorescent active
compound is sodium alkyl diphenyl disulfonate, such as commercially available
from the Dow
Company as Dowfax, such as Dowfax 2A1. In an aspect, the fluorescent active
compound is
48
Date Recue/Date Received 2023-12-12
sodium naphthalene sulfonate and/or disodium naphthalene disulfonate, and or
alkyl
naphthalene sulfonate, such as commercially available from AlczoNobel as
PetroLBA.
In an aspect, the fluorescent active compound is suitable for indirect food
use. In a
further aspect, the fluorescent active compound is suitable for more than only
visual
assessment of peracid concentrations (e.g. UV light source to confirm on a dry
substrate a
disinfectant was applied). Instead, the fluorescent active compounds are
suitable for dose
quantification by optical measurement.
Additional fluorescent tracers that may have applications of use according to
the
invention are commercially available under the trade name TRASAR (Nalco
Company
(Naperville, Ill.)) and/or may be synthesized using techniques known to
persons of ordinary
skill in the art of organic chemistry.
In an aspect of the invention, fluorescent active compound can be used at any
suitable
concentration. In some embodiments, a concentrated equilibrium composition has
a
concentration of the fluorescent active compound from about 0.001 wt-% to
about 10 wt-%, or
from about 0.1 wt-% to about 10 wt-%. In still other embodiments, the
fluorescent active
compound has a concentration from about 0.5 wt-% to about 7.5 wt-%, or more
preferably
from about 1 wt-% to about 5 wt-%. Without limiting the scope of invention,
the numeric
ranges are inclusive of the numbers defining the range and include each
integer within the
defined range.
Additional Functional Ingredients
In some embodiments, the present composition can further comprise additional
functional ingredients. In some embodiments few or no additional functional
ingredients are
disposed therein.
In other embodiments, additional functional ingredients may be included in the
compositions. The functional ingredients provide desired properties and
functionalities to the
compositions. For the purpose of this application, the term "functional
ingredient" includes a
material that when dispersed or dissolved in a use and/or concentrate
solution, such as an
aqueous solution, provides a beneficial property in a particular use. Some
particular examples
of functional materials are discussed in more detail below, although the
particular materials
49
Date Recue/Date Received 2023-12-12
discussed are given by way of example only, and that a broad variety of other
functional
ingredients may be used. In some aspects, the compositions may include
defoaming agents,
surfactants, additional antimicrobial agents, anti-redeposition agents,
bleaching agents,
solubility modifiers, dispersants, rinse aids, metal protecting agents,
stabilizing agents,
corrosion inhibitors, fragrances and/or dyes, rheology modifiers or
thickeners, hydrotropes or
couplers, buffers, solvents and the like.
In preferred embodiments, the compositions further include substances that aid
in the
solubilization of the stabilizing agent(s), including for example, hydrotropes
such as sodium
xylene sulfonate (SXS), sodium cumene sulfonates (SCS), surfactants, such as
anionic
surfactants and noinionic surfactants, and a dcfoaming agent. In further
aspects, the
composition may utilize alternative hydrotropes for solubilization of the
stabilizing agent,
including for example, n-oetanesulfonate, a xylene sulfonate, a naphthalene
sulfonate,
ethylhexyl sulfate, lauryl sulfate, an amine oxide, etc.
In preferred enrthodiments, the compositions do nni include phosphonic acid
based
stabilizers (e.g. pyrophosphoric acids and/or salts thereof, HEDP, (1-
1.+2PnO3ni
Methods' of Delivery and Methods of Use
Tn an aspect, the present invention is directed to a method for storing and/or
transporting a peroxycarboxylic acid composition, including storing the
compositions, wherein
at least about 80% of the peroxycarboxylic acid activity is retained after
storage for any
suitable time under any suitable conditions, e.g, retaining at least about 80%
of the
peroxycarboxylic acid activity after storage of about 30 days at about 50 C.
Preferably, the
methods include retaining at least about 85%, at least about 90%, or at least
about 95% or
higher of the peroxycarboxylic acid activity after storage of about 30 days at
about 50 C.
In another aspect, the present invention is directed to a method for
transporting a
peroxycarboxylic acid containing composition, which method comprises
transporting the
compositions under ambient conditions wherein the SADT of the composition is
at least about
45 C during transportation. Preferably, the SADT of the composition is higher
than at least
about 50 C, about 55 C, about 60 C, about 65 C or about 70 C. In a further
aspect, the
Date Recue/Date Received 2023-12-12
transporting of the peroxycarboxylic acid containing composition is in bulk
e.g., 1,000 gallons
and above.
In still another aspect, the present invention includes use of the
compositions for
sanitizing surfaces and/or products. In another aspect, the compositions of
the invention are
particularly suitable for use as a hard surface sanitizer and/or disinfectant,
a CIP sanitizer, food
and/or tissue treatment sanitizer (including direct or indirect contact
sanitizer), an
environmental disinfectant, a laundry bleach and disinfectant, and/or an
indirect food contact
sanitizer. The present methods can be used in the methods, processes or
procedures described
and/or claimed in U.S. Patent Nos. 5,200,189, 5,314,687, 5,718,910, 6,165,483,
6,238,685,
8,017,409 and 8,236,573.
The methods of use are suitable for treating a variety of surfaces, products
and/or
target For example, these may include a food item or a plant item and/or at
least a portion of
a medium, a container, an equipment, a system or a facility for growing,
holding, processing,
packaging, storing, transporting, preparing, cooking or serving the fond item
or the plant item
The present methods can be used for treating any suitable plant item. In some
embodiments,
the plant item is a grain, fruit, vegetable or flower plant item, a living
plant item or a harvested
plant item In addition, the present methods can be used for treating any
suitable food item,
e.g., an animal product, an animal carcass or an egg, a fruit item, a
vegetable item, or a grain
item. In still other embodiments, the food item may include a fruit, grain
and/or vegetable
item.
The present methods can be used for treating a target that is at least a
portion of a
container, an equipment, a system or a facility for holding, processing,
packaging, storing,
transporting, preparing, cooking or serving the food item or the plant item.
In some
embodiments, the target is at least a portion of a container, an equipment, a
system or a facility
for holding, processing, packaging, storing, transporting, preparing, cooking
or serving a meat
item, a fruit item, a vegetable item, or a grain item. In other embodiments,
the target is at least
a portion of a container, an equipment, a system or a facility for holding,
processing,
packaging, storing, or transporting an animal carcass. In still other
embodiments, the target is
at least a portion of a container, an equipment, a system or a facility used
in food processing,
51
Date Recue/Date Received 2023-12-12
food service or health care industry. In yet other embodiments, the target is
at least a portion
of a fixed in-place process facility. An exemplary fixed in-place process
facility can comprise
a milk line dairy, a continuous brewing system, a pumpable food system or a
beverage
processing line.
The present methods can be used for treating a target that is at least a
portion of a
container, an equipment, a system or a facility for holding, processing,
packaging, storing,
transporting, preparing, cooking or serving the food item or the plant item.
In some
embodiments, the target is at least a portion of a container, an equipment, a
system or a facility
for holding, processing, packaging, storing, transporting, preparing, cooking
or serving a meat
item, a fruit item, a vegetable item, or a grain item. In other embodiments,
the target is at least
a portion of a container, an equipment, a system or a facility for holding,
processing,
packaging, storing, or transporting an animal carcass. In still other
embodiments, the target is
at least a portion of a container, an equipment, a system or a facility used
in food processing,
food service or health care industry In yet other embodiments, the target is
at least a portion
of a fixed in-place process facility. An exemplary fixed in-place process
facility can comprise
a milk line dairy, a continuous brewing system, a pumpable food system or a
beverage
processing line
The present methods can be used for treating a target that is at least a
portion of a solid
surface or liquid media. In some embodiments, the solid surface is an
inanimate solid surface.
The inanimate solid surface can be contaminated by a biological fluid, eg., a
biological fluid
comprising blood, other hanrdous body fluid, or a mixture thereof. In other
embodiments, the
solid surface can be a contaminated surface. An exemplary contaminated surface
can
comprise the surface of food service wares or equipment, or the surface of a
fabric.
The various methods of treatment can include the use of any suitable level of
the
peroxycarboxylic acid. In some embodiments, the treated target composition
comprises from
about 10 ppm to about 1000 ppm of the peroxycarboxylic acid, including any of
the
peroxycarboxylic acid compositions according to the invention.
In still another aspect, the present invention includes water treatment
methods and
other industrial processes uses of the compositions for sanitizing surfaces
and/or products. In
52
Date Recue/Date Received 2023-12-12
some aspects, the invention includes methods of using the peroxycarboxylic
acid compositions
to prevent biological fouling in various industrial processes and industries,
including oil and
gas operations, to control microorganism growth, eliminate microbial
contamination, limit or
prevent biological fouling in liquid systems, process waters or on the
surfaces of equipment
that come in contact with such liquid systems. As referred to herein,
microbial contamination
can occur in various industrial liquid systems including, but not limited to,
air-borne
contamination, water make-up, process leaks and improperly cleaned equipment.
In another
aspect, the peroxycarboxylic acid compositions arc used to control the growth
of
microorganisms in water used in various oil and gas operations. In a further
aspect, the
compositions are suitable for incorporating into fracturing fluids to control
or eliminate
microorgan isms.
For the various industrial processes disclosed herein, "liquid system" refers
to flood
waters or an environment within at least one artificial artifact, containing a
substantial amount
of liquid that is capable of undergoing biological fouling, it includes btu is
not limited to
industrial liquid systems, industrial water systems, liquid process streams,
industrial liquid
process streams, industrial process water systems, process water applications,
process waters,
utility waters, water used in manufacturing, water used in industrial
services, aqueous liquid
streams, liquid streams containing two or more liquid phases, and any
combination thereof.
In at least one embodiment this technology would be applicable to any process
or
utility liquid system where microorganisms are known to grow and are an issue,
and biocides
are added. Examples of some industrial process water systems where the method
of this
invention could be applied are in process water applications (fluine water,
shower water,
washers, thermal processing waters, brewing, fermentation, OP (clean in
place), hard surface
sanitization, etc.), Ethanol/Bio-fuels process waters, pretreatment and
utility waters
.. (membrane systems, ion-exchange beds), water used in the
process/manufacture of paper,
ceiling tiles, fiber board, microelectronics, E-coat or electro deposition
applications, process
cleaning, oil exploration and energy services (completion and work over
fluids, drilling
additive fluids, fracturing fluids, flood waters, etc.; oil fields - oil and
gas wells/flow line,
53
Date Regue/Date Received 2023-12-12
water systems, gas systems, etc.), and in particular water systems where the
installed process
equipment exhibits lowered compatibility to halogenated biocides.
The methods by which the peroxycarboxylic acid compositions are introduced
into the
aqueous fluids or liquid systems are not critical. Introduction of the peracid
compositions may
be carried out in a continuous or intermittent mariner and will depend on the
type of water
and/or liquid being treated. In some embodiments, the peracid compositions are
introduced
into an aqueous fluid according to the methods disclosed in U.S. Patent
Application Serial No.
13/645,671, titled "New Method and Arrangement for Feeding Chemicals into a
Hydrofracturing Process and Oil and Gas Applications".
Those skilled in the art will recognize, or be able to ascertain using no more
than
routine experimentation, numerous equivalents to the specific procedures,
embodiments,
claims, and examples described herein. Such equivalents are considered to be
within the
scope of this invention and covered by the claims appended hereto. All
publications and
patent applications in this specification are indicative of the level of
ordinary skill in the art to
which this invention pertains. The invention is further illustrated by the
following examples,
which should not be construed as further limiting.
The various applications of use described herein provide the peroxycarhoxylic
acid
compositions to a surface, liquid and/or product in need of antimicrobial
and/or sanitizing
treatment. Beneficially, the compositions of the invention are fast-acting.
However, the
present methods require a certain minimal contact time of the compositions
with the surface,
liquid and/or product in need of treatment for occurrence of sufficient
antimicrobial effect.
The contact time can vary with concentration of the use compositions, method
of applying the
use compositions, temperature of the use compositions, pll of the use
compositions, amount of
the surface, liquid and/or product to be treated, amount of soil or substrates
on/in the surface,
liquid and/or product to be treated, or the like. The contact or exposure time
can be at least
about 15 seconds. In some embodiments, the exposure time is about 1 to 5
minutes. In other
embodiments, the exposure time is at least about 10 minutes, 30 minutes, or 60
minutes. In
other embodiments, the exposure time is a few minutes to hours. In other
embodiments, the
54
Date Recue/Date Received 2023-12-12
exposure time is a few hours to days. The contact time will further vary based
upon the
concentration of peracid in a use solution.
The present methods can be conducted at any suitable temperature. In some
embodiments, the present methods are conducted at a temperature ranging from
about 0 C to
about 70 C, e.g., from about 0 C to about 4 C or 5 C, from about 5 C to about
10 C, from
about 1 1 C to about 20 C, from about 21 C to about 30 C, from about 31 C to
about 40 C,
including at about 37 C, from about 41 C to about 50 C, from about 51 C to
about 60 C, or
from about 61 C to about 70 C.
The compositions are suitable for antimicrobial efficacy against a broad
spectrum of
microorganisms, providing broad spectrum bactericidal and fungistatic
activity. For example,
the peracid biocides of this invention provide broad spectrum activity against
wide range of
different types of microorganisms (including both aerobic and anaerobic
microorganisms),
including bacteria, yeasts, molds, fungi, algae, and other problematic
microorganisms.
The present methods can he used to acliieve any suitable reduction of the
microbial
population in and/or on the target or the treated target composition. In some
embodiments, the
present methods can be used to reduce the microbial population in and/or on
the target or the
treated target composition by at least one login In other embodiments, the
present methods
can be used to reduce the microbial population in and/or on the target or the
treated target
composition by at least two login. In still other embodiments, the present
methods can be used
to reduce the microbial population in and/or on the target or the treated
target composition by
at least three login.
The peroxycarboxylic acid compositions may include concentrate compositions or
may
be diluted to form use compositions. In general, a concentrate refers to a
composition that is
intended to be diluted with water to provide a use solution that contacts a
surface, liquid and/or
product in need of treatment to provide the desired cleaning, sanitizing or
the like. The
peroxycarboxylic acid composition that contacts the surface, liquid and/or
product in need of
treatment can be referred to as a concentrate or a use composition (or use
solution) dependent
upon the formulation employed in methods according to the invention. It should
be understood
Date Recue/Date Received 2023-12-12
that the concentration of the peroxycarboxylic acid in the composition will
vary depending on
whether the composition is provided as a concentrate or as a use solution.
A use solution may be prepared from the concentrate by diluting the
concentrate with
water at a dilution ratio that provides a use solution having desired
sanitizing and/or other
antimicrobial properties. The water that is used to dilute the concentrate to
form the use
composition can be referred to as water of dilution or a diluent, and can vary
from one location
to another. The typical dilution factor is between approximately 1 and
approximately 10,000
but will depend on factors including water hardness, the amount of soil to be
removed and the
like_ In an embodiment, the concentrate is diluted at a ratio of between about
1:10 and about
1:10,000 concentrate to water. Particularly, the concentrate is diluted at a
ratio of between
about 1:100 and about 1:5,000 concentrate to water. More particularly, the
concentrate is
diluted at a ratio of between about 1:250 and about 1:2,000 concentrate to
water.
Methods of Monitoring and/or Determining Peracid Concentration
The -methods of monitoring and/or detecting the concentrations of peracid
and/or
hydrogen peroxide in a use composition employing the peracid compositions of
the invention
provide a significant benefit of traceability, monitoring and/or measurement
of such
concentrations within various peroxycarboxylic acid compositions, including
equilibrium
peracid compositions, as well as other strong oxidizing compositions (e.g.
sanitizing
compositions). Previously, various inert fluorescent tracers have been used
(e.g. 1,3,6,8-
pyrenetetrasulfonic acid, tetrasodium salt, 2-anthracenesulfonic acid sodium
salt, etc.) and are
disclosed for example in U.S. Patent No. 7,910,371; however, these fluorescent
compounds
are not approved for non-rinse food contact application, and are not stable in
the highly
concentrated equilibrium peracid compositions and/or strongly oxidizing
compositions
according to the present invention.
In an aspect, the methods of the invention are suitable for use in monitoring
and/or
detecting the concentration of the peroxycarboxylic acid compositions that are
delivered to a
system and/or cleaning application. In another aspect, the methods of the
invention are suitable
for use in monitoring and/or detecting the concentration of the
peroxycarboxylic acid
compositions that are circulated within a system and/or within a cleaning
application (e.g.
56
Date Recue/Date Received 2023-12-12
prior to and/or during an application of use). In a still further aspect, the
methods of the
invention are suitable for use in monitoring and/or detecting the
concentration of the
peroxycarboxylic acid compositions that are stored and/or housed prior to an
application of
use.
In an aspect of the invention, the methods of monitoring and/or detecting the
concentration of the peroxycarboxylic acid compositions are suitable for
extended use due to
the stability of the fluorescent active compound within the various
compositions, including the
equilibrium peroxycarboxylic acid compositions. Beneficially, the fluorescent
active
compounds are stable and can be monitoring and/or detecting according to the
methods
.. disclosed herein for at least about 180 days, preferably at least about 12
months, or greater.
The compositions according to the invention are suitable for monitoring the
concentration of the peroxycarboxylic acid compositions using light absorbance
or
fluorescence. As one skilled in the art appreciates, the fluorescence can be
measured using a
variety of different and suitable techniques For exa-mple, fluorescence
emission spectroscopy
(e.g. fluorometrically monitoring) on a substantially continuous basis, at
least over a given
time period, is one of the preferred analytical techniques according to an
embodiment of this
invention One method for the continuous on-stream measuring of chemical
tracers by
fluorescence emission spectroscopy and other analysis methods is described in
U.S. Pat. No.
4,992,380.
Examples of fluorometers that may be used in the practice of this invention
include the
Xe II and TRASAR 8000 fluorometer (available from Nalco Company); the Hitachi
F-4500
fluorometer (available from Hitachi through Hitachi Instruments Inc.); the
JOBIN YVON
FluoroMax 3 "SPEX" fluorometer (available from JORIN YVON Inc.); and the
Gilford
Fluoro-IV spectrophotometer or the SFM 25 (available from Bio-tech Kontron
through
Research Instruments International). It should be appreciated that the
foregoing list is not
comprehensive and is intended only to show examples of representative
fluorometers. Other
commercially available fluorometers and modifications thereof can also be used
in this
invention.
57
Date Recue/Date Received 2023-12-12
In another aspect, a variety of other suitable analytical techniques may be
utilized to
measure the amount of fluorescent active compounds. Examples of such
techniques include
combined HPLC-fluorescence analysis, colorimetry analysis, ion selective
electrode analysis,
transition metal analysis, chemiluminescence, pulsed fluorescence
measurements, and the like.
In an aspect, this allows for precise control of the peroxycarboxylic acid
composition
dosage. For example, the fluorescent signal of the fluorescent active compound
may be used
to determine the concentration of the peroxycarboxylic acid and/or hydrogen
peroxide in a
cleaning and/or sanitizing system. In an aspect, the fluorescent signal of the
fluorescent active
compound is then used to determine whether the desired amount of the
peroxycarboxylic acid
and/or hydrogen peroxide is present in a concentrate and/or use solution. As a
result, the feed
of the concentrate and/or use solution of the compositions according to the
invention can then
be adjusted.
As one skilled in the art ascertains, the fluorescent active compound is used
to detect
fluorescence at one or more locations within a storage container, cleaning
system, water
system, piping, or the like that is containing/housing the peroxycarboxylic
acid compositions
(or use solutions thereof) according to the invention. In an aspect, the
fluorescence is
correlated with the concentration of the peroxycarhoxylic acid and/or hydrogen
peroxide
concentration of the tested solution. Optionally, corrective action may be
taken.
In an aspect, the methods of the invention include providing one or more
fluorometers
and locating said fluorometer(s) in position to sample a use solution of the
concentrated
peroxycarboxylic acid compositions according to the invention. In an aspect,
the fluorometer
is placed in or along a feed line delivering the peroxycarboxylic acid
compositions to a
cleaning application, such as for example a ware wash machine and/or OP
application.
The compositions according to the invention are also suitable for use in
monitoring by
conductivity and/or an optical sensor (may also be referred to as an optical
cell and/or an
optical detector), such as is disclosed, for example, in the methods and/or
apparatuses in U.S.
Patent Publication Nos. 2012/014912, 2012/0085931, and 2011/0260079, and U.S.
Patent Nos.
8,229,204, 8,187,540, 8,143,070, 8,119,412, 8,076,155, 8,076,154, 8,071,390
and 7,169,236.
The apparatuses, sensors and/or cells suitable for measuring or monitoring
peroxycarboxylic
58
Date Regue/Date Received 2023-12-12
acid and/or hydrogen peroxide content within a use solution are not limited
according to the
invention. Beneficially, any such apparatuses, sensors and/or cells which are
compatible with
the highly acidic peroxycarboxylic acid compositions according to the
invention may be
employed.
In an aspect, the methods of the invention include providing one or more
optical
sensors/cells and locating said optical sensors/cells in position to measure a
sample of a use
solution of the concentrated peroxycarboxylic acid compositions according to
the invention. In
an aspect, the optical sensor/cell is placed in or along a feed line
delivering the
peroxycarboxylic acid compositions to a cleaning application, such as for
example a ware
wash machine and/or CIP application.
In an aspect, the methods of the invention include determining the
concentrations of
peracid and/or hydrogen peroxide in a use composition as a result of the
compositions
formulated to include a fluorescent active compound. In an aspect of the
invention, the
monitoring includes determining whether a concentration of peracid satisfies
at least a
minimum threshold concentration. In another aspect of the invention, the
monitoring includes
determining when the concentration of hydrogen peroxide exceeds a maximum
threshold
concentration Various types of apparatus and methods for determining the
concentration of
peracid and/or hydrogen peroxide in a use composition may employ the
compositions
according to the invention.
In an aspect, a detector is used to determine the concentrations of peracid
and/or
hydrogen peroxide in a use composition. In an aspect of the invention, a
detector measures at
least one characteristic of the sample mixture indicative of the
concentrations of peracid and/or
hydrogen peroxide in the use composition, such as the fluorescent active
compound. The
measurements obtained by detector may be referred to herein as "response
data." In an aspect,
a processor determines the concentration of peracid and/or hydrogen peroxide
in a use
composition based on the response data. In one embodiment, the detector is an
optical
sensor/cell/detector that measures the transmittance and/or the absorbance of
the sample. In
that embodiment, the response data may be the optical transmittance data or
optical
absorbance data of the fluorescent active compound as a function of time. In
other
59
Date Recue/Date Received 2023-12-12
embodiments, other characteristics indicative of the concentrations of peracid
and/or hydrogen
peroxide in the sample may be measure, such as pH, oxidation-reduction
potential,
conductivity, mass spectra and/or combinations thereof. In such embodiments,
the response
data is the corresponding measured characteristic at the appropriate points in
time.
Suitable exemplary detectors include photometric detectors, fluorometers,
and/or
optical cells/sensors/detectors. In an aspect a detector operates in the
visible, ultraviolet or
infrared wavelength range, although other luminescence detection techniques
may also be
used without departing from the scope of the present invention. Any suitable
optical detector
may be used without departing from the scope of the present invention, and
that the invention
is not limited in this respect.
In an aspect, a detector receives the sample mixture, and a processor collects
the
response data from detector. In the case of an optical detector, the response
data is the
measured change in the optical response of the detector over time. In some
embodiments,
detector measures response data by measuring the color change (e.g.,
ahcorbanc.e or
transmittance) of the sample solution within detector as a function of time.
In other words, the
voltage response of detector as a function of time corresponds to the amount
of light
transmitted through the sample mixture and hence the color the of the sample
mixture as the
chemical reaction progresses. The response data is indicative of the
concentrations of peracid
and hydrogen peroxide in the use composition.
In an aspect, suitable carriers or solvents for forming a use solution may
include
various types of water. in an aspect, deionized water, soft water and/or low
grain (e.g. 5 grain)
water is preferred for the use of certain detecting devices, namely for
measuring conductivity.
Beneficially, however, the type of water or solvent employed does not impact
the optical
measurements obtained from a composition according to the invention. However,
it shall be
understood that other suitable reagents and carriers may also be used without
departing from
the scope of the present invention, and that the invention is not limited in
this respect.
The concentrations of peracid and/or hydrogen peroxide determined according to
the
methods of the invention may be used, for example, as feedback to a controller
to maintain the
peracid concentration in the use composition within a predefined range and/or
to cause the
Date Recue/Date Received 2023-12-12
emptying of a use composition vessel and production of a new use composition
when the
hydrogen peroxide concentration exceeds the maximum peroxide threshold
concentration.
As an exemplary application of use, the methods for determining the
concentrations of
peracid and/or hydrogen peroxide according to the methods of the invention,
may be used to
ensure a use composition has a predefined range of such concentrations. For
example,
application specific concentrations may include: Aseptic bottle rinse
generally requiring
between about 1000-5000 ppm peracid and/or between about 5000-40,000 ppm
hydrogen
peroxide; or Central Sanitizing generally requiring between about 100-1000 ppm
peracid and
100-5000 ppm hydrogen peroxide. If, for example, the concentration of peracid
in the use
composition decreases below a predetermined level, the use composition may be
replenished
by adding the concentrated equilibrium peracid composition according to the
invention to the
use composition.
As another example, if the concentration of hydrogen peroxide in the use
composition
exceeds a predetermined level, the use composition may be replenished by
emptying the use
composition vessel of the spent use composition and generating a new use
composition using
the concentrated equilibrium peracid composition according to the invention.
The various methods for detecting peracid and/or hydrogen peroxide
concentration of a
use solution according to the invention is based upon the correlation of the
amount of the
fluorescent active compound and the equilibrium peroxycarboxylic acid
composition (e.g. the
concentration of peroxycarboxylic acid that is required for the various
cleaning and/or
sanitizing applications of use of the invention). In an exemplary embodiment,
the amount of
fluorescent active compound added being proportional to the amount of the
peroxycarboxylic
acid. By using a fluorometer or other optical means to measure the fluorescent
signal of the
fluorescent active compound, the amount of the fluorescent active compound can
be
determined by using a calibration curve to relate the amount of fluorescent
signal detected to
the amount of the inert fluorescent active compound present. Because the inert
fluorescent
active compound and the peroxycarboxylic acid are formulated in known
proportions, by
knowing the amount of fluorescent active compound present then the amount of
peroxycarboxylic acid present can be calculated.
61
Date Recue/Date Received 2023-12-12
The frequency at which the peracid and/or hydrogen peroxide concentration of a
use
solution is monitored according to the invention (e.g. monitoring frequency)
will vary
according to the desired applications of use. For example, a monitoring device
may be
programmed to monitor the concentrations of peracid and hydrogen peroxide in
the use
composition every 15 minutes, every 30 minutes, every hour, every two hours,
every day or
other appropriate time. The monitoring frequency/interval may vary depending
on, among
other things, the particular application to which the use composition is
directed and the
corresponding threshold concentrations of peracid and hydrogen peroxide.
Beneficially,
according to the invention, the fluorescent active compound is stable in the
highly acidic,
.. equilibrium peracid compositions according to the invention and allows
measurement/detecting over such extended periods of time.
In an aspect, methods for determining the concentrations of one or more use
compositions may apply to compositions undergoing kinetic reactions (e.g.
obtaining response
data from a peracid composition according to the invention over time) A use
solution of the
peracid composition of the present invention may be provided for measuring the
concentration
of peracid and/or hydrogen peroxide to obtain a desired measurement location
(e.g. a location
along a mixing profile) In such embodiments, the measurement location is to he
selected to
provide appropriate response data.
All publications and patent applications in this specification are indicative
of the level
of ordinary skill in the art to which this invention pertains.
EXAMPLES
Embodiments of the present invention are further defined in the following non-
limiting
Examples. It should be understood that these Examples, while indicating
certain embodiments
of the invention, are given by way of illustration only. From the above
discussion and these
Examples, one skilled in the art can ascertain the essential characteristics
of this invention, and
without departing from the spirit and scope thereof, can make various changes
and
modifications of the embodiments of the invention to adapt it to various
usages and conditions.
Thus, various modifications of the embodiments of the invention, in addition
to those shown
62
Date Recue/Date Received 2023-12-12
and described herein, will be apparent to those skilled in the art from the
foregoing
description. Such modifications are also intended to fall within the scope of
the appended
claims.
EXAMPLE 1
Self-Accelerating Decomposition Test. As used herein, SADT refers to the
lowest
temperature at which self-accelerating decomposition may occur with the
peracid composition.
In some embodiments, SADT refers to the lowest temperature at which self-
accelerating
.. decomposition may occur under the commercial packaging, storage,
transportation and/or use
condition(s). SADT can be estimated, calculated, predicted and/or measured by
any suitable
methods. For example, SADT can be estimated, or measured directly by one of 3
methods
(HI, H2 and H4) recommended by the UN Committee for the Transportation of
Dangerous
Goods in "Recommendations on the Transport of Dangerous Goods, Model
Regulations"
(Rev.17) ST/SG/AC.10/1/Rev.17. For example, the methodology disclosed in Malow
and
Wehrstedt, J. Hazard Mater., 120(1-3):21-4 (2005) can be used.
The full test protocol used in this Example is available at "Recommendations
on the
Transport of Dangerous Goods," Manual of Tests and Criteria, 5th revised
edition: (United
Nations): Classification procedures, test methods and criteria relating to
self-reactive
substances of Division 4.1 and organic peroxides of Division 5.2: Test H.4
Heat accumulation
stomp test (28.4.4).
Since peroxycarboxylic acids fall into the organic peroxides classification
and
therefore are self-reactive, self-heating products, testing was conducted to
demonstrate if
cooling is required for a given package of a peroxycarboxylic acid product.
One of the four
published recommended methods of the UN Committee for the Transport of
Dangerous Goods
allows the modeling of a large volume package with Dewar flasks. Again, this
method is
utilized to model large commercial packages which if tested directly (i.e.
with the Ill method)
might pose a significant hazard as well as inconvenience due to size. In this
example the
product was intended to be sold in 1000 L plastic tanks known as totes and it
was necessary to
determine if the SADT was greater or less than 45 C. If it was found to be
less than 45 C
63
Date Recue/Date Received 2023-12-12
refrigeration would be required both in shipping and in storage and use thus
severely limiting
the products potential for a wide application in the marketplace. Additionally
if the product
has an SADT equal to or less than 50 C the product is not permitted to be
shipped stored or
used in conatainers as large as 1000 L but is essentially limited to 200 L
drums or smaller,
again severely limiting the products applicationsA 1 L cylindrical Dewar flask
is fitted with a
closure that causes it to cool at the same rate as the 1000 L polyethylene
tank. The Dewar flask
is filled to 80% of full volume with the product, fitted with the specific
closure and a recording
thermometer, and is placed in an oven set at 45 C. Once the internal package
temperature
warms to 43 C temperature, time recording is begun. If the temperature exceeds
the oven temp
of 45 C by a magnitude of 6 C (51 C ) before 7 days have elapsed the SADT for
the product
in that 1000 L package is defined as < 45 C and the product is deemed to
require cooling. As
such a requirement can severely limit use of a product in many industries,
this SADT is
considered an unfavorable property. If the temperature doesn't exceed a 6 C
rise over the oven
temperature the SADT is deemed > 45 C and may be considered for shipping and
storage in
that 1000L package without refrigeration.
The H4 test methodology was employed at 50 C. The tested peroxycarboxylic acid
compositions are shown in Table 4.
TABLE 4
Composition Wt%
DPA Formula HEDP Formula
DPA 0.05 0.0
HEDP (60%) 0.0 1.5
Sulfonated oleic acid (70%) 1-10 1-10
Octanoic acid 1-10 1-10
Acetic acid 2-20 2-20
Sodium xylene sulfonate (40%) 3-20 3-20
64
Date Recue/Date Received 2023-12-12
Sodium cumene sulfonate 1-5 1-5
(96%)
H202(35%) 10-50 10-50
Al2(SO4)3.1. 81-120 1-5 1-5
14.2SO4 (96%) 12.5 12. 5
Deionized Water 20-50 20-50
Total 100.0 100.0
The resulting SA-DT from the evaluated formulations is shown in FIG. 1. While
the
formulae were identical aside from their stabilizers, the DPA-stabilized
peracid composition
showed a much more gradual increase in temperature (below the SADT of the
peracid)
demonstrating suitability for stabilization for transportation and/or storag.
In contrast, the
phosphate / HEDP-stabilized peracid composition showed excessive increase in
temperature in
the first day, necessitating the termination of the experiment to avoid
explosion of the
composition/container. The results illustrate an advantage in DPA
stabilization in a high acid
vi romn era,
EXAMPLE 2
The methods of Example 1 were further employed to analyze the SADT of a fatty
peracid, a peroxyoctanoic acid compositions having an even further increased
acidity. While
the formulae were identical aside from their stabilizers, the results shown in
Table 5 again
illustrate an advantage in DPA stabilization in a high acid environment that
extends to fatty
peracids such as peroxy octanoic acid.
TABLE 5-
Composition Wt%
I IE DP Formula DP A Formula
Date Recue/Date Received 2023-12-12
DPA 0.0 0.05
HEDP (60%) 2.54 0.0
Octanoic acid 1-10 1-10
Sodium octane SI,11 fonate (40%) 10-30 10-30
14202 (35%) 10-50 10-50
.H2SO4 (96%) 14.14 14.14
Delonized Water 20-50 20-50
Total 100.0
1 100.0
The resulting SADT from the evaluated formulations is shown in FIG.2. The DPA-
stabilized peracid composition, even under increased acidity of the peracid
composition,
showed a much more gradual increase in temperature (below the SADT of the
peracid) in
a comparison to the phosphaie HEDP-stabilized petacid composition. The DPA-
stabilized
peracid composition would likely meet DOT standards for transportation since
its temperature
projected out to 7 days since reaching 48 C would likely not exceed 56 C.
EXAMPLE 3
As set forth in Table 6 (and shown in FIG. 3) a strong acid containing a
reduced
hydrogen peroxide concentration was evaluated by the H4-SADT protocol in a
Dewar flask
modeling a 300 gallon IBC or plastic tote.
.. TABU 6
Composition Wt%
D.PA 0.05
11LDP (60%) 0.0
Sulfonated oleic acid 1-10
(70%)
66
Date Recue/Date Received 2023-12-12
Octanoic acid 1-10
Acetic acid 2-20
Sodium xylene sulfonate 1-5
(40%)
Sodium cumene sulfonate 1-5
(96%)
H202 (35%) 35
Al2(SO4) 3.181420 0.05 2.0
112SO4 (96%) 18
Deionized Water 20-50
Total 100.0
The evaluation of the stability of the composition was conducted and despite
the
presence of the -17% sulfuric acid, the composition containing the DPA
stabilizing agent was
stabilized. The stabilization of the composition, as shown in FIG. 3,
illustrates the stabilization
of the solution temperature only exceeding the 50 C ambient temperature by 4.7
C by day 7.
Beneficially, these results qualify the highly acidic peracid composition for
shipping and
storage in a 300 gallon tote without refrigeration, demonstrating a
significant improvement
over the instability of HEDP-containing equivalent peracid compositions.
EXAMPLE 4
The methods of Example I were further employed to analyze the SADT of a fatty
peracid, a peroxyoctanoic acid compositions with an HEDP stabilizer, in
comparison to an
equivalent peracid composition with a DPA stabilizer, as shown in Table 7.
TABLE 7
Composition Wt%
Formula HEDP stabilizer Formula with DPA stabilizer
67
Date Recue/Date Received 2023-12-12
DPA 0.0 0.05
HEDP (60%) 2.54 0.0
Octanoic acid 1-10 1-10
Sodium octane 10-30 10-30
sulfonate (40%)
H202 (35%) 23.00 23.00
H2SO4 (96%) 14.14 14.14
Deionized Water 20-50 20-50
Total 100.0 100.0
The evaluation of the stability of the composition was conducted and despite
the
presence of the ¨14% sulfuric acid, the composition containing the DPA
stabilizing agent
according to embodiments of the invention was stabilized whereas the formula
containing only
an HEDP stabilizer failed prior to the 7th day. The stabilization of the
composition, as shown
in FIG. 4, illustrates the stabilization of the solution temperature only
exceeding the 50 C
ambient temperature by 2.8uC by day 7. Again, these results qualify the highly
acidic peracid
composition stabilized by the DPA stabilizing agent for shipping and storage
in a 300 gallon
tote without refrigeration. This demonstrates a significant improvement over
the instability of
HEDP-containing equivalent peracid compositions.
EXAMPLE 5
A study was performed to determine the foam properties of selected
compositions of
the present invention, compared to peracid compositions including commercially
available
surfactants and/or defoaming agents. The following peracid compositions were
prepared:
Test solution A: 2262 ppm composition A in soft water at pH 2.9 with
sulfonated oleic
acid and sodium octane sulfonate surfactants.
68
Date Recue/Date Received 2023-12-12
Test solution A (plus aluminum sulfate): 2262 ppm composition A in soft water
at pH
2.9 with anionic surfactants, plus the aluminum sulfate defoaming agent.
Test solution B: 2262 ppm composition B in soft water at pH 2.9 with an
anionic
surfactant (commercially-available formulation).
Test solution C: 2262 ppm composition C in soft water at pH 2.9 (commercially-
available formulation).
Test solution D: 2262 composition D in soft water pH 2.9 (commercially-
available
formulation).
A Glewwe Foam meter provides a dynamic foam test rather than a static test (as
in the
case of the Ross-Miles foam test). A dynamic foam meter is considered more
appropriate for
simulation of industrial conditions, e.g. the conditions in a flume. The
equipment and general
procedure for the Glewwe form test is described in U.S. Patent No. 3,899,387,
column 12, line
45 et seq. The foam meter itself consists of a thermostated reservoir and a
pump to recirculate
The aqueous medium with foaming tendencies The foam developed by the action of
the
aqueous stream impinging on the surface in the reservoir causes foam
formation.
The foam heights of the tested compositions were determined using the
following
method First 3000 mi. of each formula was prepared and gently poured into
Glewwe cylinder.
The foam height is measured after various time intervals and provides a
relative measure of
the effectiveness of the defoamer. The reservoir of this foam meter consists
of a stainless steel
laboratory beaker of 3,000 mL capacity. Sealed to this beaker by means of a
silicone sealant is
a clear Plexiglass tubing which snugly fits into the inner walls of the
beaker. This enables the
operator to measure the foam height above the liquor level. The beaker
measures about 19 cm
high by about 17 to 18 cm in diameter and the Plexiglass tube extends about 30
to 35 cm
above the lip of this beaker. Further detail regarding the Glewwe foam test is
shown in, U.S.
Patent No. 5,447,648.
A ruler was attached to the side of the cylinder, and the solution was level
with the
bottom of the ruler. The pump was turned on. Foam height was estimated by
reading the
average level of foaming according to the ruler. Foam height readings were
taken versus time
69
Date Recue/Date Received 2023-12-12
with a stopwatch or timer. The pump was turned off and height of the foam was
recorded at
various times. The results are shown in FIG. 5.
As shown in FIG. 5, the aluminum sulfate defoaming agent incorporated into the
highly acidic peracid composition according to the invention, resulted in
significant
improvement in foam profile, as evidenced by the significantly lower foam
height at all time
points. The chart in FIG. 5 measured the impact of aluminum sulfate on the
foam height of a
peracid compositions in soft water, illustrating that the formulation
containing the aluminum
sulfate (test solution of Composition A plus aluminum sulfate) yielded
significantly decreased
foam height in comparison to several other commercial peracid compositions.
Beneficially, the reduced foam height of the compositions of the present
invention is
useful when using the compositions in applications where the production of
foam is
detrimental to the application, for example, in a clean in place cleaning
and/or sanitizing
application.
EXAMPLE 6
As a result of the superior defoaming performance of the aluminum sulfate
defoaming
agent as set forth in Example 5, further evaluation of the low-foaming peracid
composition
(tt solution of Composition A (plus aluminum sulfate)) of Example 5 was
conducted to
determine whether any effects on biocidal efficacy resulted from the
formations containing the
defoaming agent
A test solution of Composition A was diluted 1 oz. to 7 Gallon with 500 ppm
hard
water, and the target pH of the solution was less than about 3Ø The log
reduction of S'. Aureus
achieved by the evaluated composition was measured at varying concentrations
at 15 second
and 30 second intervals.
As shown in FIG. 6, the aluminum sulfate defoaming agent incorporated into the
highly acidic peracid composition according to the invention, further resulted
in not only
compatibility with the peracid compositions, it further showed the synergistic
biocidal efficacy
of the defoaming agent with the anionic surfactant. Beneficially, the
defoaming agent and
surfactant combination in the highly acidic peracid compositions according to
the invention
Date Recue/Date Received 2023-12-12
provide improved biocidal. activity. Without being limited to a particular
theory of the
invention, the defoaming agent may result in an increase in the hydrophobicity
of the peracid
composition resulting in such improved biocidal activity.
EXAMPLE 7
The biocidal efficacy of various equilibrium peroxvearboxylic acid
compositions
shown in 'fable 8 according to the invention were evaluated under various
conditions as set
forth in 'fables 9-11.
TABLE 8
Material Formula 1 Formula 2
wt-% wt-%
õ.
Water 20-40 20-40
Sulfonated oleic acid (70%) 1-20 1-20
Octanoic acid 1-25 1-25
Acolic acid I -75 1-25
Hydrogen Peroxide (35%) 1-50 1-50
Sulfuric acid (96%) 5-20 5-20
SCS (93%) 0.5-7.5 0
SXS (40%) 0 0.5-7.5
Additional :Functional 0-25 0-25
mgedi cuts (e.g. surfac tat t,
stabilizing agent, defoarning
agent)
71
Date Recue/Date Received 2023-12-12
AOAC Official Method 960.09 (Germicidal and Detergent Sanitizing Action of
'Disinfectants) was employed to test the compositions for food contact
sanitizing, as shown in
Table 9.
$ TABLE 9
Food Contact Sanitizing Results
Formula Exposure Log
Concentration pH Test System
Time Reduction
=
1 oz. 6 gallons 5 li. co/i 6.82
30 seconds 3 S. aureus
6.49
E. coil
>6.99
1 oz. / 7 gallons
S. aureus
>7.113
E. coil
2.60
Oz. / 6 gallons 5, E. coil 6.64
oz. / 7 gallons 30 seconds 3 aureus
7.13
E. coil
>6.99
S. aureus >7.13
E. coil
2.93
European Standard EN 1276 (November 2001) (Chemical Disinfectants and
Antiseptics ¨ Quantitative Suspension Test for the Evaluation of Bactericidal
Activity of
Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic, and
Institutional.
Areas) was employed to test the compositions against dirty soil conditions at
20 C, as shown
in Table 10.
TABLE 10
EN 1276 Results
Formula Exposure Log
Concentration pH Test System
Time Reduction
72
Date Recue/Date Received 2023-12-12
minutes S. aureus 4.20
2.5 E. hirae
3.70
1. 1 oz. / 6 gallons E. coil >6.36
P.
>6.26
aeruginosa
5 minutes S. aureus
5.66
2.5 E. hirae
5.40
2 1 oz. / 6 gallons E. cob >6.34
>6.31
aeruginosa
AOAC Official Method 964.02 (Testing Disinfectants ¨ Use Dilution Methods) was
employed to test the compositions at a more concentrated use solution, as
shown in Table11.
5 TABLE 11
UDT Results
Formula # Negative
Exposure
Concentration pH Test System Carriers / #
Time
Test Carriers
S. aureus 60 / 60,
7
1 oz. / 4 gallons 10 minutes 2.P.
60 /60
aeruginosa
S. aureus 60 / 60
2.7
P. 58 / 60
2 1 oz. / 4 gallons 10 minutes
aeruginosa
EXAMPLE 8
73
Date Recue/Date Received 2023-12-12
The formula stability of Formula 1 of Example 7 was evaluated under
accelerated
conditions, namely 100 F over 4 weeks. The total peracid and hydrogen peroxide
concentration was measured, as shown in FIG. 7. The results indicate that
under the
accelerated conditions, the highly acidic equilibrium peroxycarboxylic acid
composition are
.. stable for at least 4 weeks, which is equal to about a year under ambient
conditions..
EXAMPLE 9
A study was performed to determine the foam properties of selected
compositions of
.. the present invention, compared to peracid compositions including
commercially available
surfactants and/or defoaming agents. The following peracid compositions were
prepared:
Test solution A: 2262 ppm composition A in soft water at pH 2.9 with
sulfonated oleic
acid and sodium octane sulfonate surfactants.
Test solution A (plus aluminum sulfate): 2262 ppm composition A in soft water
at pH
2.9 with anionic surfactants, plus the aluminum sulfate defoaming agent.
Test solution B: 2262 ppm composition B in soft water at pH 2.9 with an
anionic
surfactant (commercially-available formulation).
Test solution C: 2262 ppm composition C in soft water at pH 2.9 (commercially-
available formulation).
Test solution D: 2262 composition D in soft water pI1 2.9 (commercially-
available
formulation).
A Glewwe Foam meter provides a dynamic foam test rather than a static test (as
in the
case of the Ross-Miles foam test) A dynamic foam meter is considered more
appropriate for
simulation of industrial conditions, e.g. the conditions in a flume. The
equipment and general
procedure for the Glewwe form test is described in U.S. Patent No. 3,899,387,
column 12, line
45 et seq. The foam meter itself consists of a thermostated reservoir and a
pump to recirculate
the aqueous medium with foaming tendencies. The foam developed by the action
of the
aqueous stream impinging on the surface in the reservoir causes foam
formation.
74
Date Recue/Date Received 2023-12-12
The foam heights of the tested compositions were determined using the
following
method. First 3000 mL of each formula was prepared and gently poured into
Glewwe cylinder.
The foam height is measured after various time intervals and provides a
relative measure of
the effectiveness of the defoamer. The reservoir of this foam meter consists
of a stainless steel
laboratory beaker of 3,000 mL capacity. Sealed to this beaker by means of a
silicone sealant is
a clear Plexiglass tubing which snugly fits into the inner walls of the
beaker. This enables the
operator to measure the foam height above the liquor level. The beaker
measures about 19 cm
high by about 17 to 18 cm in diameter and the Plexiglass tube extends about 30
to 35 cm
above the lip of this beaker. Further detail regarding the Glewwe foam test is
shown in, U.S.
Patent No. 5,447,648.
A ruler was attached to the side of the cylinder, and the solution was level
with the
bottom of the ruler. The pump was turned on. Foam height was estimated by
reading the
average level of foaming according to the ruler. Foam height readings were
taken versus time
with a stopwatc.h or timer The pump was tamed off and height of the foam Was
recorded at
various times. The results are shown in FIG. 8.
As shown in FIG. 8, the aluminum sulfate defoaming agent incorporated into the
highly acidic peracid composition according to the invention, resulted in
significant
improvement in foam profile, as evidenced by the significantly lower foam
height at all time
points. The chart in FIG.8 measured the impact of aluminum sulfate on the foam
height of a
peracid compositions in soft water, illustrating that the formulation
containing the aluminum
sulfate (test solution of Composition A plus aluminum sulfate) yielded
significantly decreased
foam height in comparison to several other commercial peracid compositions.
Beneficially, the reduced foam height of the compositions of the present
invention is
useful when using the compositions in applications where the production of
foam is
detrimental to the application, for example, in a clean in place cleaning
and/or sanitizing
application.
Date Recue/Date Received 2023-12-12
EXAMPLE 10
A use solution of the concentrated equilibrium peroxycarboxylic acid
composition
according to the invention, as shown in Example 7 (equilibrium composition),
was monitored
by both conductivity and fluorescence.
Conductivity was measured by a conductivity meters; and the fluorescent
intensity was
measured by a hand hold fluorescent meter (made by Ecolab) with excitation
wavelength at
275 nm. The emission of fluorescence was measured in SU and conductivity was
measured in
us/cm.
As shown in FIG. 9, the increase of the concentration of peroxycarboxylic acid
is
proportional to the increase of the emission of the fluorescent active
compound in all type of
water tested, demonstrating the suitability of using the fluorescent active
compounds to
measure the concentration of peroxycarboxylic acid compositions according to
the invention.
As shown in FIG. 10, in deionized and 5 grain water, the emission intensity of
the
fluorescent active compound has linear relationship with the concentration of
peroxycarboxylic acid, however such linear relationship does not hold in well
water. This
demonstrates the superiority of fluorescent according to this invention vs.
conductivity
approach in measuring the concentration of peroxycarboxylic acids.
EMBODIMENTS
The following are non-limiting embodiments according to the disclosure herein.
Emnodiment 1. A stabilized equilibrium peracid composition comprising:
a C1-C22 peroxycarboxylic acid;
a CI-C22 carboxylic acid;
hydrogen peroxide;
a mineral acid; and
a peroxycarboxylic acid stabilizing agent, wherein the stabilizing agent is a
picolinic acid
76
Date Recue/Date Received 2023-12-12
or a compound having the following Formula (IA):
0 0
R. , JL R-
,
1
(R3)n (IA)
wherein le is OH or NRialeb, wherein Rla and Rib are independently hydrogen or
(CI -C6)alkyl; wherein R2 is OH or ¨NR2ali=''213, wherein R2a and R2b are
independently
hydrogen or (Ci -C6)a1kyl; wherein each le is independently (Ci -C6)alkyl, (C2
-
C(,)alkenyl or (C2 -C6)alkynyl; and n is a number from zero to 3; or a salt
thereof;
or a compound having the following Formula (113):
1,o R
Ri 2
oon (113)
wherein R1 is OH or NRIaRlb, wherein Rla and Rlb are independently hydrogen or
(Ci -C6)alkyl; wherein R2 is OH or ¨NR2aR2b, wherein R2aand R2b are
independently hydrogen or (Ci -C6)alkyl; wherein each R3 is independently (CI
C6)alkyl, (C2 -C6)alkenyl or (C2 -C6)alkynyl; and n is a number from zero to
3; or a
salt thereof;
wherein said composition has a use solution pH below about 4, and wherein the
stabilizing
agent delays or prevents the peroxycarboxylic acid from exceeding its self-
accelerating
decomposition temperature (SADT).
Embodiment 2. The composition of Embodiment 1, wherein the stabilizing
agent is a
picolinic acid, or a salt thereof.
77
Date Recue/Date Received 2023-12-12
Embodiment 3. The composition of Embodiment 2, wherein the stabilizing
agent is 2,6-
pyridinedicarboxylic acid, or a salt thereof.
Embodiment 4. The composition of any Embodiment 1, wherein the additional
agent is
a hydrotrope that aids solubilization of the stabilizing agent.
Embodiment 5. The composition of Embodiment 1, wherein at least about
80% of the
CI-C22 peroxyearboxylic acid activity is retained after storage of at least 30
days at about
50 C.
Embodiment 6. A low-foaming equilibrium peracid composition
comprising:
a C1-C22 peroxycarboxylic acid;
CA-C22 carboxylic acid;
hydrogen peroxide;
a mineral acid;
an anionic surfactant; and
a metal salt defoaming agent,
wherein said composition has a use solution pH below about 4.
Embodiment 7. The composition of Embodiment 6, wherein the defoaming
agent is an
aluminum salt, a magnesium salt, a calcium salt, a zinc salt and/or a salt of
a rare earth metal.
Embodiment 8. The composition of Embodiment 7, wherein the defoaming
agent is
aluminum sulfate.
Embodiment 9. The composition of Embodiment 6, wherein the
of the use solution
is below about 3.
78
Date Recue/Date Received 2023-12-12
Embodiment 10. The composition of Embodiment 6, wherein the defoaming
agent is
aluminum sulfate and the anionic surfactant is sodium octane sulfonate and
sulfonated oleic
acid.
Embodiment 11. The composition of Embodiment 6, further comprising a
peroxycarboxylic acid stabilizing agent, wherein the stabilizing agent is a
picolinic acid or a
compound having the following Formula (IA):
0 0
RiA"--rN.,-)L R2
(R3), (IA)
wherein R1 is OH or ¨NR1921b, wherein RI' and Rib are independently hydrogen
or (Ci
-C6)alkyl; wherein R2 is OH or ¨NR2aR2b, wherein R" and R2U are independently
hydrogen or
(CI -C6)alkyl; wherein each R3 is independently (CI -C6)alkyl, (C2 -C6)alkenyl
or (C2 -
C6)alkynyl; and n is a number from zero to 3; or a salt thereof;
or a compound having the following Formula (TB):
0
R Ri 2
(R3). (113)
wherein R1 is OH or wherein R1a and Rib are independently
hydrogen or (CI
-C6)alkyl; wherein R2 is OH or ¨NR'R wherein R2a and R211 are independently
hydrogen or
(CI -C6)alkyl; wherein each R3 is independently (Ci -C6)alkyl, (C2 -C6)alkenyl
or (C2 -
C6)alkynyl; and n is a number from zero to 3; or a salt thereof, wherein the
stabilizing agent
79
Date Recue/Date Received 2023-12-12
delays or prevents the peroxycarboxylic acid from exceeding its self-
accelerating
decomposition temperature (SADT).
Embodiment 12. An equilibrium peracid composition comprising:
a Ci-C22 peroxycarboxylic acid;
a C1-C22 carboxylic acid;
hydrogen peroxide; and
an aryl sulfonate fluorescent active compound, wherein the fluorescent active
compound is
stable in the equilibrium peracid composition for monitoring peroxycarboxylic
acid
concentration by an optical sensor.
Embodiment 13. The composition of Embodiment 12, wherein the
fluorescent active
compound is sodium xylene sulfonate, toluene sulfonate, alkyl
benzenesulfonate, alkyl
cliphenyl oxide clisulfonate, naphthalene sulfonate, alkyl naphthalene
sulfonate, naphthalene
disulfonate or sodium cumene sulfonate.
Embodiment 14. The composition of Embodiment 12, wherein the pH of the
use solution
is below about 4.
Embodiment 15. The composition of Embodiment 12, further comprising an
alkaline
metal defoaming agent, an anionic surfactant, and a peroxycarboxylic acid
stabilizing agent,
wherein the stabilizing agent is a picolinic acid or a compound having the
following Formula
(14
0 0
R R2
(R3)1, (IA)
Date Recue/Date Received 2023-12-12
laR lb,
wherein R1 is OH or ¨NR wherein R1a and Rib are independently
hydrogen or (CI
-C6)alkyl; wherein R2 is OH or ¨NR2aR2b, wherein R2a and R21' are
independently hydrogen or
(Ci -C6)alkyl; wherein each R3 is independently (Ci -C6)alkyl, (C2 -C6)alkenyl
or (C2 -
G)alkynyl; and n is a number from zero to 3; or a salt thereof;
or a compound having the following Formula (TB):
Rijr
(R3) (TB)
wherein R1 is OH or ¨NRlar. lb,
K wherein Rand Rib are independently
hydrogen or (CI
-C6)alkyl; wherein R2 is OH or _NR2a,K-.2b,
wherein R2a and R21' are independently hydrogen or
(CI -C6)alkyl; wherein each R.3 is independently (CI -C6)alkyl, (C2 -
C6)alkenyl or (C2 -
C6)alkynyl; and n is a number from zero to 3; or a salt thereof, wherein the
stabilizing agent
delays or prevents the peroxycarboxylic acid from exceeding its self-
accelerating
decomposition temperature (SADT).
Embodiment 16. The composition of Embodiment 1, 6 or 12, wherein the CI-
C22
peroxycarboxylic acid comprises from about 1 wt-% to about 40 wt-%, the CI-C22
carboxylic
acid comprises from about 1 wt-% to about 80 wt-%, the hydrogen peroxide
comprises from
about 1 wt-% to about 80 wt-%, the mineral acid comprises from about 5 wt-% to
about 50
wt-%, and the stabilizing agent comprises from about 0.01 wt-% to about 10 wt-
%.
Embodiment 17. The composition of Embodiment 1, 6 or 12, further
comprising at least
one additional agent selected from the group consisting of an anionic
surfactant, a hydrotrope,
a defoaming agent, a solvent, a fluorescent active agent and combinations
thereof.
81
Date Recue/Date Received 2023-12-12
Embodiment 18. The composition of Embodiment 1,6 or 12, wherein the Ci-
C22
peroxycarboxylic acid
is a C2-C2o peroxycarboxylic acid and wherein the CI-C22 carboxylic acid is a
C2-C2o
carboxylic acid.
Embodiment 19. The composition of Embodiment 1, 6 or 12, wherein the
peroxycarboxylic acid is peroxyacetic acid, peroxyoctanoic acid and/or
peroxysulfonated oleic
acid, and wherein the carboxylic acid is acetic acid, octanoic acid,
sulfonated oleic acid or a
combinations of the same.
Embodiment 20. A low-foaming, stabilized equilibrium peracid
composition comprising:
from about 1 wt-% to about 40 wt-% of a CI-C22 peroxycarboxylic acid;
from about 1 wt-% to about 80 wt-% of a CI-C22 carboxylic acid;
from about I wt-% to about Re wt-% of hydrogen peroxide;
from about 1 wt-% to about 50 wt-% of a mineral acid;
from about 0.01 wt-% to about 40 wt-% of an anionic surfactant;
from about 0.001 wt-% to about 10 wt-% of a metal salt defoaming agent; and
from about 0.01 wt-% to about 10 wt-% of a peroxycarboxylic acid stabilizing
agent,
wherein the stabilizing agent is a picolinic acid or a compound having the
following
Formula (IA):
0
R2
(R3)11 (IA)
wherein RI is OH or ¨NRiaR lb, wherein Rla and Rib are independently hydrogen
or (CI
-C6)alkyl; wherein R2 is OH or ¨NR2amK2b,
wherein R2 and R2b are independently
hydrogen or (CI -C6)alkyl; wherein each R3 is independently (CI -C6)alkyl, (C2
-
C(,)alkenyl or (C2 -C6)alkynyl; and n is a number from zero to 3; or a salt
thereof;
82
Date Recue/Date Received 2023-12-12
or a compound having the following Formula (IB):
===`...õ7\R2
(R3). (TB)
wherein R.' is OH or ¨NRIclb, wherein Riaand Rib are independently hydrogen or
(CI
-C6)alkyl; wherein R2 is OH or ¨NR2aR213, wherein leu and leb are
independently
hydrogen or (CI -C6)alkyl; wherein each le is independently (CI -C6)alkyl, (C2
-
C6)alkenyl or (C2 -C6)alkynyl; and n is a number from zero to 3; or a salt
thereof;
wherein said composition has a use solution pH below about 4, and wherein the
defoaming agent and anionic surfactant provide enhanced biocidal efficacy for
the
composition.
Embodiment 21. The composition of Embodiment 20, wherein the CI-C22
peroxycarboxylic acid comprises from about 1 wt-% to about 20 wt-%, the CI-C22
carboxylic
acid comprises from about 1 wt-% to about 50 wt-%, the hydrogen peroxide
comprises from
about 1 wt-% to about 50 wt-%, the mineral acid comprises from about 1 wt-% to
about 20
wt-%, the surfactant comprises from about 0. 1 wt-% to about 25 wt-%, the
defoaming agent
comprises from about 0.1 wt-% to about 5 wt-%, and the stabilizing agent
comprises from
about 0.01 wt-% to about lwt-%.
Embodiment 22. The composition of Embodiment 20, further comprising a
hydrotrope, a
solvent or combinations thereof, wherein the CI-C22 peroxycarboxylic acid is a
C2-C20
peroxycarboxylic acid and wherein the Cl-C22 carboxylic acid is a C2-C2o
carboxylic acid.
Embodiment 23. The composition of Embodiment 22, wherein the
peroxycarboxylic acid
is peroxyacetic acid, peroxyoctanoic acid and/or peroxysulfonated oleic acid,
wherein the
83
Date Recue/Date Received 2023-12-12
carboxylic acid is acetic acid, octanoic acid, sulfonated oleic acid or a
combinations of the
same, and wherein the defoaming agent is aluminum sulfate.
Embodiment 24. A method for storing and/or transporting a stabilized
equilibrium
peroxycarboxylic acid composition comprising:
storing the peroxycarboxylic acid composition of Embodiment I wherein said
composition
retains at least about 80% of the CI-C22 peroxycarboxylic acid activity after
storage
of about 30 days at about 50 C.
Embodiment 25. The method of Embodiment 24, wherein the SADT of said
composition
is elevated to at least 45 C during transportation.
Embodiment 26. The method of Embodiment 24, wherein the C1-C22
peroxycarboxylic
acid comprises from about I wt-% to about 40 wi-%, the C1-1722 carboxylic acid
comprises
from about 1 wt-% to about 80 wt-%, the hydrogen peroxide comprises from about
1 wt-% to
about 80 wt-%, the mineral acid comprises from about 5 wt-% to about 50 wt-%,
and the
stabilizing agent comprises from about 001 wt-% to about 10 wt-%.
Embodiment 27. The method of Embodiment 24, further comprising at least
one
additional agent selected from the group consisting of an anionic surfactant,
a hydrotrope, a
defoaming agent, a solvent, a fluorescent active agent and combinations
thereof.
Embodiment 28. The method of Embodiment 24, wherein the CI-C22
peroxycarboxylic
acid is a C2-C2o peroxycarboxylic acid selected from the group consisting of
peroxyacetic
.. acid, peroxyoctanoic acid peroxysulfonated oleic acid and combinations
thereof, wherein the
Ct-C22 carboxylic acid is a C2-C2o carboxylic acid selected from the group
consisting of acetic
acid, octanoic acid, sulfonated oleic acid and combinations thereof, and
wherein the
stabilizing agent is 2,6-pyridineclicarboxylic acid, or a salt thereof.
84
Date Recue/Date Received 2023-12-12
Embodiment 29. A method for detecting a concentration of
peroxycarboxylic acid and/or
hydrogen peroxide in a sanitizing composition and/or cleaning process
comprising:
providing an equilibrium peroxycarboxylic acid composition comprising a CI-C22
peroxycarboxylic acid, a CI-C22 carboxylic acid, hydrogen peroxide, and an
aryl
sulfonate fluorescent active compound, wherein the fluorescent active compound
is
stable in the equilibrium peracid composition for monitoring peroxycarboxylic
acid
concentration by an optical sensor;
measuring a fluorescence response with an optical cell, wherein the
fluorescence response
measures the intensity of fluorescent emission; and
determining a concentration of the peroxycarboxylic acid and/or the hydrogen
peroxide.
Embodiment 10. The method of Embodiment 29, further comprising diluting
the
equilibrium peroxycarboxylic acid composition into a use solution.
Embodiment 31. The method of Embodiment 29, wherein the use solution of the
equilibrium peroxycarboxylic acid composition is directed to an optical cell.
Embodiment 32. The method of Embodiment 30, wherein the use solution of
the
equilibrium peroxycarboxylic acid composition is measured for a fluorescence
response from
the fluorescent active compound.
Embodiment 33. The method of Embodiment 32, wherein the fluorescence
response is a
fluorescent signal detected by an optical cell during a circulating, soaking,
cleaning and/or
rinsing step.
Embodiment 34. The method of Embodiment 29, further comprising placing
an optical
cell in or along a feed line delivering the peroxycarboxylic acid compositions
to a cleaning
application.
Date Recue/Date Received 2023-12-12
Embodiment 35. The method of Embodiment 29, further comprising
adjusting the
concentration of the peroxycarboxylic acid and/or the hydrogen peroxide of the
composition
based on the measured fluorescence response.
Embodiment 36. The method of Embodiment 29, further comprising removal of
at least a
portion of the peroxycarboxylic acid composition to provide a new equilibrium
peroxycarboxylic acid composition for dilution and use in a cleaning and/or
sanitizing
application.
Embodiment 37. The method of Embodiment 29, wherein the cleaning process is
performed on-line or is conducted off-line using a clean-in-place system.
Embodiment 38. The method of Embodiment 29, wherein the Cl-C22
peroxycarboxylic
acid is a C2-C7.0 peroxycarhoxylic acid selected from the group consisting of
peroxyacetic
acid, peroxyoctanoic acid, peroxysulfonated oleic acid and combinations
thereof, wherein the
Cl -C22 carboxylic acid is a C2-C20 carboxylic acid selected from the group
consisting of
acetic acid, octanoic acid, sulfonated oleic acid and combinations thereof,
and wherein the
fluorescent active compound is sodium xylene sulfonate, toluene sulfonate,
alkyl
benzenesulfonate, alkyl diphenyl oxide disulfonate, naphthalene sulfonate,
alkyl naphthalene
sulfonate, naphthalene disulfonate or sodium cumene sulfonate.
Embodiment 39. The method of Embodiment 29, wherein the C1-C22
peroxycarboxylic
acid comprises from about 1 wt-% to about 40 wt-%, the Cl-C22 carboxylic acid
comprises
from about 1 wt-% to about 80 wt-%, the hydrogen peroxide comprises from about
1 wt-% to
about 80 wt-%, and the fluorescent active compound comprises from about 0.001
wt-% to
about 10 wt-%, and wherein the equilibrium peroxycarboxylic acid composition
further
comprises at least one additional agent selected from the group consisting of
a hydrotrope, a
solvent, a stabilizing agent, an anionic surfactant, a metal salt defoaming
agent, and
combinations thereof.
86
Date Recue/Date Received 2023-12-12
Embodiment 40. The method of Embodiment 29, wherein the fluorescent
active
compound is sodium xylene sulfonate, toluene sulfonate, alkyl
benzenesulfonate, alkyl
diphenyl oxide disulfonate, naphthalene sulfonate, alkyl naphthalene
sulfonate, naphthalene
disulfonate or sodium cumene sulfonate, and wherein the equilibrium
peroxycarboxylic acid
composition further comprises an metal salt defoaming agent, an anionic
surfactant, and a
peroxycarboxylic acid stabilizing agent, wherein the stabilizing agent is a
picolinic acid or a
compound having the following Formula (1A):
0 0
R1 JC R2
(R3)11 (IA)
wherein R1 is OH or ¨NitlaR11), wherein Rh and Rib are independently hydrogen
or (CI
-C6)alkyl; wherein R2 is OH or ¨NR2aR2b, wherein le and R21' are independently
hydrogen or
(CA -C6)alkyl; wherein each R3 is independently (Ci -C6)alkyl, (C2 -C6)alkenyl
or (C2 -
C6)alkynyl; and n is a number from zero to 3; or a salt thereof;
or a compound having the following Formula (IB):
0
Ri R2
R3)n (IB)
wherein Ri is OH or ¨Nit"Rii), wherein Rand Rib are independently hydrogen or
(Ci
-C6)alkyl; wherein le is OH or ¨N1k2aR2b, wherein
and R21' are independently hydrogen or
(CI -C6)alkyl; wherein each R3 is independently (CI -C6)alkyl, (C2 -C6)alkenyl
or (C2 -
C6)alkynyl; and n is a number from zero to 3; or a salt thereof, wherein the
stabilizing agent
87
Date Recue/Date Received 2023-12-12
delays or prevents the peroxycarboxylic acid from exceeding its self-
accelerating
decomposition temperature (SADT).
Embodiment 41. A method for reducing a microbial population using a
stabilized
equilibrium peroxycarboxylic acid composition comprising:
providing the peroxycarboxylic acid composition of Embodiment 1, 6, 12 or 20;
and
contacting a surface or substrate with a use solution of said composition for
sufficient time
to reduce a microbial population, wherein said use solution has a pll below
about 4,
and wherein said composition retains at least about 80% of the Ci-C22
peroxycarboxylic acid activity after storage of about 30 days at about 50 C.
Embodiment 42. The method of Embodiment 41, wherein the Cl-C22
peroxycarboxylic
acid is a C2-C20 peroxycarboxylic acid selected from the group consisting of
peroxyacetic
acid, peroxynctanoic acid pemxystilfonated oleic acid and combinations
thereof, wherein the
C1-C22 carboxylic acid is a C2-C20 carboxylic acid selected from the group
consisting of
acetic acid, octanoic acid, sulfonated oleic acid and combinations thereof.
Embodiment 43. The method of Embodiment 41, wherein the stabilizing
agent is 2,6-
pyridinedicarboxylic acid, or a salt thereof.
Embodiment 44. The method of Embodiment 41, wherein the defoaming agent
is an
aluminum salt, a magnesium salt, a calcium salt, a zinc salt and/or a salt of
a rare earth metal.
Embodiment 45. The method of Embodiment 41, wherein the surface or
substrate is a
food item, plant item, animal item, a container, an equipment, a system or a
facility for
growing, holding, processing, packaging, storing, transporting, preparing,
cooking or serving
the food item, plant item or the animal item, an instrument, a hard surface, a
liquid media,
equipment, a fabric surface (e.g. laundry), a fouled water or industrial
processing liquid
source, liquid system, or process water used in oil, gas and/or industrial
processing operations.
88
Date Recue/Date Received 2023-12-12
Embodiment 46. The method of Embodiment 41, wherein the contacting step
lasts for at
least 10 seconds.
Embodiment 47. The method of any Embodiment 41, wherein the microbial
population
in and/or on the surface or substrate is reduced by at least two logio.
The inventions being thus described, it will be obvious that the same may be
varied in
many ways. Such variations are not to be regarded as a departure from the
spirit and scope of
the inventions and all such modifications are intended to be included within
the scope of the
following claims. The above specification provides a description of the
manufacture and use
of the disclosed compositions and methods. Since many embodiments can be made
without
departing from the spirit and scope of the invention, the invention resides in
the claims.
89
Date Recue/Date Received 2023-12-12