Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/269033
PCT/EP2022/067335
LINEAR FOURIER FIDUCIAL
CROSS-REFERENCE TO RELATED APPLICATION
[0001]
This application claims priority to and the benefit of U.S. Provisional
Application No. 63/215,152, entitled "LINEAR FOURIER FIDUCIAL", filed June 25,
2021 and U.S. Provisional Application No. 63/216,898, entitled "LINEAR FOURIER
FIDUCIAL", filed June 30, 2021, both which are herein incorporated by
reference in its
entirety.
BACKGROUND
100021
The present approach relates generally to image-based approaches for
detecting
deviations from a linear movement when scanning a surface. More particularly,
the
approach relates to the use of linear fiducials to detect, in real-time,
deviations from a linear
scan path during operation of a scanning imaging system.
[0003]
In a nucleic acid sequencing context, a sample holder, such as a flow cell
or
other sequencing substrate, for use in a sequencing instrument may provide a
number of
individual sites (e.g., sample wells or nanowells) at permanently or
transiently fixed
locations on a surface. Such sites may contain chemical groups or biological
molecules,
which can be identical or different among the many sites, and can interact
with other
materials of interest, such as a biological sample. Sites can be located
and/or analyzed by
taking an image of the substrate surface, such as by taking a planar image or
by line
scanning. The image data may be processed to locate and identify at least a
portion of the
sites and/or to obtain qualitative or quantitative measurements related to
samples being
analyzed. In such a context, where a chemical or biological interaction occurs
at a
particular site, the interaction may be detected at the site and correlated
with the location
and identity of the site, as well as the particular group or molecule present
at the site.
1
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0004] For sequencing instruments using a scanning imaging
system, any transverse
movement of the moving stage during scanning can cause deterioration of the
sequencing
data extracted from the acquired images. Straight line movements at a skew
angle can be
detected and compensations applied. However, typically there has not been a
suitable
method for detecting and compensating for deviations from straight line
movement. Such
deviations from linear movement can, in a sequencing context, impact the
quality of the
sequencing operation as such operations typically require that the location of
each sample
cluster is accurate to 0.1 to 0.2 pixels. On current systems, this equates to
approximately
70 nm. Further, it is anticipated that higher density flow cells under
development may
require accuracy of 40 nm or better. It is believed that deviations from
linear movement
of this magnitude may occur frequently using conventional readout approaches.
Hence,
detecting and compensating for deviations from linear motion is relevant in
such
sequencing contexts.
SUMMARY
[0005] The present invention provides an article of manufacture,
comprising a
substrate, on which a plurality of sites are disposed at fixed, physical
locations on the
surface of the substrate. An example of such an article may include a
patterned
arrangement of sites associated with a sequencing flow cell, where some or all
of the sites
may be configured to hold a material of interest.
[0006] In one embodiment, a substrate is provided that is
suitable for linear scanning.
In accordance with various implementations of such an embodiment, sample sites
or wells
(e.g., nanowells) may be arranged on non-fiducial regions of the substrate in
a periodic or
repeating pattern (e.g., a hexagonal or rectilinear pattern). Conversely, in
fiducial regions,
a fiducial (e.g., a linear fiducial) may be provided that is a combination of
sample sites and
"blank" regions or wells (e.g., locations where a well would normally be
formed (e.g.,
nano-imprinted) in accordance with the non-fiducial pattern but where no well
was formed
(or fully formed) during fabrication or where a well has been formed but which
contains
2
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
no sample. By way of example, in various embodiments discussed herein a
fiducial (e.g.,
a linear fiducial) may comprise a full row of sample sites between respective
rows of
"blank" sites or wells; a partial row of sample sites (e.g., alternating
sample wells and
"blanks") between respective rows of "blank" sites or wells; or multiple rows,
each row
comprising both sample sites and "blanks" but in which every row has at least
one sample
site (i.e., there are no "site-free- rows within the fiducial).
[0007]
With the preceding in mind, a respective embodiment a patterned flow cell
is
provided. In accordance with this embodiment, the patterned fl ow cell
comprises: a
substrate and a plurality of sample sites in a non-fiducial region of the
substrate. The
plurality of sample sites are arranged in a periodic pattern. The patterned
flow cell further
comprises a plurality of coarse-alignment fiducials formed on the substrate
separate from
the plurality of sample sites and a plurality of linear fiducials formed on
the substrate. Each
linear fiducial comprises sample sites and blanks arranged in accordance with
the periodic
pattern. Each blank corresponds to a location in the periodic pattern where a
sample site
should be located but is not or where an empty sample site is located.
[0008]
In a further embodiment a patterned flow cell is provided. In accordance
with
this embodiment, the patterned flow cell comprises: a substrate and a
plurality of sample
sites in a non-fiducial region of the substrate. The plurality of sample sites
are arranged in
a periodic pattern. The patterned flow cell further comprises a plurality of
coarse-
alignment fiducials formed on the substrate separate from the plurality of
sample sites and
a plurality of linear fiducials formed on the substrate. Each linear fiducial
comprises
elongated sample sites. Each elongated sample site spans the area associated
with two or
more sample sites.
[0009]
In another embodiment, a method is provided for correcting for deviations
from
a linear scan path in an imaging operation. In accordance with this method a
patterned
surface undergoing an imaging operation is advanced along a linear scan path.
The
patterned surface is imaged as it is advanced along the linear scan path. The
patterned
surface comprises a plurality of linear fiducials. Deviations from the linear
scan path are
3
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
detected using the plurality of linear fiducials. The deviations from the
linear scan path are
corrected for while the patterned surface is imaged.
[0010]
In a further embodiment, a sequencing instrument is provided. In
accordance
with this embodiment, the sequencing instrument comprises: a sample stage
configured to
support a sample container; an objective lens, a photodetector, and a light
source
configured to operate in combination to image the sample container when
present on the
sample stage; and a controller configured to perform operations comprising:
advancing the
sample container undergoing an imaging operation along a linear scan path;
imaging a
patterned surface of the sample container as it is advanced along the linear
scan path,
wherein the patterned surface comprises a plurality of linear fiducials;
detecting deviations
from the linear scan path using the plurality of linear fiducials; and
correcting for the
deviations from the linear scan path while the patterned surface is imaged by
the
sequencing instrument.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011]
These and other features, aspects, and advantages of the present invention
will
become better understood when the following detailed description is read with
reference
to the accompanying drawings, in which like characters represent like parts
throughout the
drawings, wherein:
[0012]
FIG. 1 illustrates a high-level overview of one example of an image
scanning
system, in accordance with aspects of the present disclosure;
[0013]
FIG. 2 is a block diagram illustration of an imaging and image processing
system, such as for biological samples, in accordance with aspects of the
present disclosure;
[0014]
FIG. 3 is a diagrammatical overview of functional components that may be
included in a data analysis system for use in a system of the type illustrated
in FIG. 2;
4
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0015]
FIG. 4 is a plan view of an example patterned surface, in accordance with
aspects of the present disclosure;
[0016]
FIG. 5 is an enlarged, cut-away view of a portion of the patterned surface
of
FIG. 4;
[0017]
FIG. 6 is a further cut-away diagram illustrating sites on an example
patterned
flow cell surface, in accordance with aspects of the present disclosure;
[0018]
FIG. 7 is an enlarged view of two example sites of a patterned flow cell
surface
illustrating pixilation in image data for the sites during processing;
[0019]
FIG. 8 depicts a process flow of steps for correcting deviations from a
linear
scan path using linear fiducials, in accordance with aspects of the present
disclosure;
[0020]
FIGS. 9A and 9B respectively depict examples of an image tile
incorporating
both conventional fiducials and linear fiducials, in accordance with aspects
of the present
disclosure;
[0021]
FTG. 10 depicts an example of a layout of a linear fiducial, in accordance
with
aspects of the present disclosure;
[0022]
FIG. 11 depicts an example of a layout of a vertically arranged linear
fiducial,
in accordance with aspects of the present disclosure;
[0023]
FIG. 12 depicts a further example of a layout of a linear fiducial, in
accordance
with aspects of the present disclosure;
[0024]
FIG. 13 depicts another example of a layout of a linear fiducial, in
accordance
with aspects of the present disclosure;
[0025]
FIG. 14 depicts an additional example of a layout of a linear fiducial, in
accordance with aspects of the present disclosure;
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0026]
FIG. 15 depicts a further example of a layout of a linear fiducial, in
accordance
with aspects of the present disclosure;
[0027]
FIG. 16 depicts an additional example of a layout of a linear fiducial, in
accordance with aspects of the present disclosure;
[0028]
FIG. 17 depicts another example of a layout of a linear fiducial, in
accordance
with aspects of the present disclosure;
[0029]
FIG. 18 depicts a further example of a layout of a linear fiducial, in
accordance
with aspects of the present disclosure;
[0030]
FIG. 19 depicts an additional example of a layout of a linear fiducial, in
accordance with aspects of the present disclosure; and
[0031]
FIG. 20 depicts another example of a layout of a linear fiducial, in
accordance
with aspects of the present disclosure.
DETAILED DESCRIPTION
[0032]
This disclosure provides methods and systems for processing, imaging, and
image data analysis that are useful for locating features of patterned
surfaces, such as sites
or wells of patterned flow cells, and for detecting deviations from linear
motion in real-
time during a scan operation. The systems and methods may be used to register
multiple
images or sub-images of such patterned surfaces. As discussed herein,
patterned surfaces
used in flow cells (the processing of which produces image data, or other
forms of detection
output, of sites on the surface) may be a type of analytical sample holder,
such as those
used for the analysis of biological samples. Such patterned surfaces may
contain repeating
patterns of features (e.g., sample sites, such as sample wells or nanowells)
that are to be
resolved at a suitable resolution (e.g., sub-micron resolution ranges) for
which the methods
and systems described herein are suited. In many applications, the material to
be imaged
and analyzed will be located on one or more surfaces of one or more supports,
such as a
6
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
glass material. Various chemical or structural features may be employed at
sites to bind or
anchor (or to otherwise localize) segments or fragments of material to be
processed (e.g.,
hybridized, combined with additional molecules, imaged, and analyzed).
Fiducial markers
or regions, or simply "fiducials" are located at known locations with respect
to the sites to
assist in locating the support in the system (e.g., for imaging), and for
locating the sites in
subsequent image data. As discussed herein, certain fiducials, namely linear
fiducials as
described herein, may be formed, at least in part, from sites used in the
processing of a
biological sample (i.e., sample sites) but which are arranged so as to be
optically discernible
from the non-fiducial pattern of sample sites, which are typically arranged in
a regular or
periodic pattern (e.g., a hexagonal or rectilinear pattern). As used herein,
such a regular or
periodic pattern is translationally periodic, repeating in one or more
directions.
[0033]
As discussed in greater detail below, sequencing instruments that employ a
scanning imaging system typically move the imaging optics relative to the
imaged substrate
during operation. Any transverse movement of the moving stage during scanning
can
cause deterioration of the sequencing data extracted from the acquired image.
While
straight line movements at a skew angle can be detected and compensated, there
has not
been a suitable approach for detecting and compensating for deviations from
straight line
movement. In a sequencing context, it is essential for base-calling quality
that the location
of each sample cluster is accurate to within 0.1 to 0.2 pixels. On current
sequencing
systems, this equates to approximately 70 nm. However, higher density flow
cells under
development may require accuracy of 40 nm or better. Unfortunately, analysis
of data from
current stages (which are configured to hold and move flow cells undergoing
imaging)
shows that deviations from linear movement of this magnitude may occur during
normal
operation at unacceptable rates.
[0034]
In order to compensate for such deviations from a linear movement,
deviations
may be detected in real time during operation of the scanning imaging system.
As
discussed herein, approaches and structures are described that allow such real-
time
detection of deviations from linear movement. By way of example, certain
embodiments
7
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
employ a specialized, linear fiducial for high-resolution, real-time detection
of transverse
movement of the scanning system, such as using one-dimensional Fourier
transforms of
pixel rows.
[0035]
It may be noted that as used herein, a "sequence flow cell", including
"patterned
flow cells" may be understood to be a sample holding and/or processing
structure or device.
Such devices comprise sites (i.e., sample sites or binding sites) at which
analytes may be
located for processing and analysis.
[0036]
As discussed herein, in a nucleic acid sequencing technique, oligomeric or
polymeric chains of nucleic acids, which may be spatially separated and
localized on a
substrate, may be subjected to several cycles of biochemical processing and
imaging. In
some examples, each cycle can result in one of four different labels being
detected at each
feature, depending upon the nucleotide base that is processed biochemically in
that cycle.
In such examples, multiple (e.g., four) different images are obtained at a
given cycle and
each feature will be detected in the images. Sequencing includes multiple
cycles, and
alignment of features represented in image data from successive cycles is used
to determine
the sequence of nucleotides at each site based on the sequence of labels
detected at the
respective site. Improper registration of the images can adversely affect
sequence analysis,
including improper localization of a cluster at an imaged site due to
deviation from the
expected linear motion.
[0037]
As used herein, the term "fiducial" is intended to mean a distinguishable
region
(e.g., point or area) of reference in or on an object (such as a support or
substrate with sites
for molecular materials to be analyzed) as well as in image data acquired of
the object. The
fiducial can be, for example, a mark, an object, shape, edge, area,
irregularity, channel, pit,
post, or, as in many cases, a collection of features at known locations,
geometry, and/or
configuration that can be used as a reference. The fiducial can be detected in
an image of
the object or in another data set derived from detecting (e.g., imaging) the
object. The
fiducial can be characterized by an x- and/or y-coordinate in a plane of the
object (e.g., one
or more surfaces of the patterned flow cell). Alternatively or additionally,
the fiducial can
8
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
be specified by a z-coordinate that is orthogonal to the x, y plane, for
example, being
defined by the relative locations of the object and a detector. One or more
coordinates for
a fiducial can be specified relative to one or more other features of an
object or of an image
or other data set derived from the object.
[0038]
As used herein, certain fiducials (e.g., linear fiducials) may be
described or
otherwise characterized as constituting a grouping or arrangement of features
(e.g., sample
sites, such as sample wells or nanowells) as well as "blanks" or "blank
regions" where a
well may be expected (based on an underlying or implied pattern of sites) but
was not
formed or where a well is present, but empty of sample (i.e., a "dark" well))
which when
considered together or in the aggregate form a fiducial that is optically
discernible from a
pattern associated with non-fiducial regions. In certain embodiments discussed
herein, the
sample sites present in the linear fiducial may be elongated relative to non-
fiducial sample
sites, such as spanning two, three, or more site locations with respect to the
underlying and
shared pattern of sites (e.g., a hexagonal or rectilinear pattern). Thus, in
certain
embodiments discussed herein a fiducial may comprise a combination of sample
sites
(elongated or otherwise) and "blank" regions. By way of example, in various
embodiments
discussed herein a fiducial (e.g., a linear fiducial) may comprise a full row
of sample sites
between respective rows of "blank" sites or wells; a partial row of sample
sites (e.g.,
alternating sample wells and "blanks-) between respective rows of "blank"
sites or wells;
or multiple rows, each row comprising both sample sites and "blanks- but in
which every
row has at least one sample site (i.e., there are no "site-free" rows within
the fiducial).
Conversely, in other embodiments a fiducial (e.g., a linear fiducial) may
comprise
elongated sample sites with one or both of blanks or non-elongated sample
sites (e.g., as
found in the non-fiducial regions of the substrate).
[0039]
Several examples will be described herein with respect to fiducials, their
form,
their configuration, and their use in systems and methods of analysis. It will
be understood
that systems are also provided for carrying out the methods in an automated or
semi-
automated way, and such systems will include a processor; a data storage
device; and a
9
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
program for image analysis, the program including instructions for carrying
out one or
more methods provided for processing or leveraging fiducial data, such as
image
registration, distortion correction, and so forth. Accordingly, methods
discussed herein can
be carried out on a computer, for example, having components and executable
routines
needed for such purposes.
[0040]
The methods and systems described herein may be employed for analyzing any
of a variety of materials, such as biological samples and molecules, which may
be on or in
a variety of objects. Useful objects are solid supports or solid-phase
surfaces with attached
analytes. The methods and systems set forth may provide advantages when used
with
objects having a repeating pattern of features in an x, y plane, such as a
patterned flow cell
having an attached collection of molecules, such as DNA, RNA, biological
material from
viruses, proteins, antibodies, carbohydrates, small molecules (such as drug
candidates),
biologically active molecules, or any other analytes of interest.
[0041]
An increasing number of applications have been developed for substrates
with
patterned arrangements of features (e.g., sample wells or sites) having
biological
molecules, such as nucleic acids and polypeptides. Such patterned features may
include
DNA or RNA probes. These are specific for nucleotide sequences present in
plants,
animals (e.g., humans), and other organisms. In some applications, for
example, individual
DNA or RNA probes can be attached at individual features (e.g., sample wells
or sites) of
a surface of a patterned flow cell. A test sample, such as from a known or
unknown person
or organism, can be exposed to the sites, such that target nucleic acids
(e.g., gene fragments,
mRNA, or amplicons thereof) hybridize to complementary probes at respective
sites in the
pattern of sites. The probes can be labeled in a target specific process
(e.g., due to labels
present on the target nucleic acids or due to enzymatic labeling of the probes
or targets that
are present in hybridized form at the features). The patterned surface can
then be examined,
such as by scanning specific frequencies of light over the features to
identify which target
nucleic acids are present in the sample.
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0042]
Patterned flow cells may be used for genetic sequencing and similar
applications. In general, genetic sequencing includes determining the order of
nucleotides
in a length of target nucleic acid, such as a fragment of DNA or RNA.
Relatively short
sequences may be sequenced at each feature, and the resulting sequence
information may
be used in various bioinformatics methods to logically fit the sequence
fragments together,
so as to reliably determine the sequence of much more extensive lengths of
genetic material
from which the fragments are available. Automated, processor-executable
routines for
characterizing fragments may be employed, and have been used in endeavors such
as
genome mapping, identification of genes and their function, and so forth.
Patterned
arrangements of sample sites on a surface are useful for characterizing
genomic content
because a large number of variants may be present and this supplants the
alternative of
performing many experiments on individual probes and targets. Thus, the
patterned
surface (such as a patterned surface of a flow cell) may be a useful platform
for performing
such investigations in a practical manner.
[0043]
As noted above, any of a variety of patterned surface (e.g., patterned
flow cells)
having sample binding sites or wells can be used in a method or system set
forth herein.
Such patterned surface may contain features, each having an individual probe
or a
population of probes. In the latter case, the population of probes at each
feature may be
homogenous having a single species of probe. For example, in the case of a
nucleic acid
sequencing flow cell, each sample well or site can have multiple nucleic acid
molecules
each having a common sequence. However, in some other examples, the
populations at
each site or well of a patterned surface can be heterogeneous. Similarly,
protein based
patterned surfaces can have features with a single protein or a population of
proteins, which
may or may not have the same amino acid sequence_ The probes can be attached
to the
patterned surface, for example, via covalent linkage of the probes to the
surface or via non-
covalent interaction of the probes with the surface. In some examples, probes,
such as
nucleic acid molecules, can be attached to a surface via a gel layer as
described, for
example, in U.S. Pat. No. 9,012,022 and U.S. Pat. App. Pub. No. 2011/0059865
Al, each
of which is incorporated herein by reference in its entirety for all purposes.
11
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0044]
Patterned surfaces used for nucleic acid sequencing often have random
spatial
patterns of nucleic acid features. For example, HiSeqTM or MiSeqTM sequencing
platforms
available from Illumina Inc. utilize flow cells comprising supports (e.g.,
surfaces) upon
which nucleic acid(s) is/are disposed by random seeding followed by bridge
amplification.
However, patterned surfaces (upon which discrete reaction sites are formed in
a pattern on
the surface) can also be used for nucleic acid sequencing or other analytical
applications.
Example patterned surfaces, methods for their manufacture and methods for
their use are
set forth in U.S. Pat. Nos. 9,512,422; 8,895,249; and 9,012,022; and in U.S.
Pat. App. Pub.
Nos. 2013/0116153 Al; and 2012/0316086 Al, each of which is incorporated
herein by
reference in its entirety. The features (e.g., reaction or capture sites or
wells) of such
patterned surfaces can be used to capture a single nucleic acid template
molecule to seed
subsequent formation of a homogenous colony, for example, via bridge
amplification.
Such patterned surfaces are useful for nucleic acid sequencing applications.
[0045]
The size of features, such as reaction or sample binding sites (e.g.,
sample wells
or nanowells) on a patterned surface (or another object used in a method or
system herein),
can be selected to suit a desired application. In some non-limiting examples,
a feature of
a patterned surface can have a size that accommodates only a single nucleic
acid molecule.
A surface having a plurality of features in this size range is useful for
constructing a pattern
of molecules for detection at single molecule resolution. Features in this
size range are
also useful in patterned surfaces having features that each contain a colony
of nucleic acid
molecules. Thus, the features of a patterned surface can each have an area
that is no larger
than about 1 mm2, no larger than about 500 [im2, no larger than about 100
[im2, no larger
than about 10 um2, no larger than about 1 um2, no larger than about 500 nm2,
no larger
than about 100 nm2, no larger than about 10 nm2, no larger than about 5 nm2,
or no larger
than about 1 nm2. Alternatively or additionally, the features of a patterned
surface will be
no smaller than about 1 mm2, no smaller than about 500 am2, no smaller than
about 100
no smaller than about 10 mm2, no smaller than about 1 prn2, no smaller than
about 500
nm2, no smaller than about 100 nm2, no smaller than about 10 nm2, no smaller
than about
nm2, or no smaller than about 1 nm2. Indeed, a feature can have a size that is
in a range
12
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
between an upper and lower limit selected from those exemplified above.
Although several
size ranges for features of a surface have been exemplified with respect to
nucleic acids
and on the scale of nucleic acids, it will be understood that features in
these size ranges can
be used for applications that do not include nucleic acids. It will be further
understood that
the size of the features need not necessarily be confined to a scale used for
nucleic acid
applications.
[0046]
For examples that include an object (e.g., a flow cell surface) having a
plurality
of features, the features can be discrete, being separated with spaces between
each other.
A patterned surface useful in the present context can have features that are
separated by
edge to edge distance of at most about 100 gm, about 50 gm, about 10 gm, about
5 gm,
about 1 gm, about 0.5 gm, or less. Alternatively or additionally, a patterned
surface can
have features that are separated by an edge to edge distance of at least about
0.5 gm, about
1 gm, about 5 gm, about 10 gm, about 50 gm, about 100 gm, or more. These
example
ranges are provided by way of context, are non-limiting, and can apply to the
average edge
to edge spacing for features, as well as to the minimum or maximum spacing.
[0047]
The size of the features and/or pitch of the features can vary such that
the
features on a patterned surface can have a desired density. For example, the
average feature
pitch in a regular pattern can be at most about 100 gm, about 50 gm, about 10
gm, about
gm, about 1 gm, about 0.5 gm, or about 350 nm, or less. Alternatively or
additionally,
the average feature pitch in a regular pattern can be at least about 0.5 gm,
about 1 gm,
about 5 gm, about 10 gm, about 50 gm, or about 100 gm or more. These ranges
can apply
to the maximum or minimum pitch for a regular pattern as well. For example,
the
maximum feature pitch for a regular pattern can be at most about 100 [Lin,
about 50 [Lin,
about 10 gm, about 5 gm, about 1 gm, or about 0.5 gm or less; and/or the
minimum feature
pitch in a regular pattern can be at least about 0.5 gm, about 1 gm, about 5
gm, about 10
gm, about 50 gm, or about 100 gm or more.
13
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0048]
The density of features on a patterned surface can also be understood in
terms
of the number of features present per unit area. For example, the average
density of features
on a patterned surface can be at least about 1x103 features/mm2, about 1x104
features/mm2,
about 1x105 features/mm2 about 1x106 features/mm2, about 1x107 features/mm2,
about
lx108 features/mm2, or about lx109 features/mm2 or higher. Alternatively or
additionally,
the average density of features on a patterned surface can be at most about
1x109
features/mm2, about 1x108 features/mm2, about lx107 features/mm2, about 1x106
features/mm2, about 1x105 features/mm2, about 1x104 features/mm2, or about
1x103
features/mm2 or less.
[0049]
The features provided on a patterned surface can have any of a variety of
shapes,
cross-sections, and layouts. For example, when observed in a two dimensional
plane, such
as on a surface, the features can have a perimeter that is rounded, circular,
oval, rectangular,
square, symmetric, asymmetric, triangular, polygonal, or the like. The
features can be
arranged in a regular repeating pattern including, for example, a hexagonal or
rectilinear
pattern. A pattern can be selected to achieve a desired level of packing. For
example, round
features are optimally packed in a hexagonal arrangement. Other packing
arrangements
can also be used for round features and vice versa.
[0050]
In general, a patterned surface might be characterized in terms of the
number of
features that are present in a subset that forms the smallest geometric unit
of the pattern.
The subset can include, for example, at least 2, 3, 4, 5, 6, 10 or more
features. Depending
upon the size and density of the features, the geometric unit can occupy an
area of less than
about 1 mm2, about 500 um2, about 100 [tm2, about 50 um2, about 10 [tm2, about
1 litm2,
about 500 nm2, about 100 nm2, about 50 nm2, or about 10 nm2 or less.
Alternatively or
additionally, the geometric unit can occupy an area of greater than about 10
nm2, about 50
nm2, about 100 nm2, about 500 nm2, about 1 p.m2, about 10 m2, about 50 tim2,
about 100
um2, about 500 um2, or about 1 mm2 or more. Characteristics of the features in
a geometric
unit, such as shape, size, pitch and the like, can be selected from those set
forth herein more
generally with regard to features provided on a patterned surface.
14
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0051]
A surface having a regular pattern of features can be ordered with respect
to the
relative locations of the features but random with respect to one or more
other characteristic
of each feature. For example, in the case of a nucleic acid sequencing
surface, the nucleic
acid features can be ordered with respect to their relative locations but
random with respect
to one's knowledge of the sequence for the nucleic acid species present at any
feature. As
a more specific example, nucleic acid sequencing surfaces formed by seeding a
repeating
pattern of features with template nucleic acids and amplifying the template at
each feature
to form copies of the template at the feature (e.g., via cluster amplification
or bridge
amplification) will have a regular pattern of nucleic acid features but will
be random with
regard to the distribution of sequences of the nucleic acids across the
pattern. Thus,
detection of the presence of nucleic acid material on the surface can yield a
repeating
pattern of features, whereas sequence specific detection can yield non-
repeating
distribution of signals across the surface.
[0052]
As may be appreciated, the description of patterns, order, randomness and
so
forth provided herein not only pertains to features on objects (e.g., a solid
substrate having
such features, such as features on solid-supports or surface), but also to
image data, or
images generated from such image data, that includes or depicts such an object
having
features as described herein. As such, patterns, order, randomness and so
forth can be
present in any of a variety of formats that are used to store, manipulate or
communicate
image data including, but not limited to, a computer readable medium or
computer
component such as a graphical user interface or other output device.
[0053]
As discussed above and throughout, patterned flow cells have a regular
pattern
of sample sites (e.g., wells or nanowells) imprinted in the surfaces of the
flow cell. This
pattern is normally hexagonal or square, and can have different orientations.
In practice, a
hexagonal pattern is conventionally used in current systems that employ a
linear scanning
imaging system. In such contexts, the hexagonal pattern may typically have one
axis
aligned at right angles to the scanning direction. This axis is typically
referred to as
"horizontal" due to how images are normally presented with the image vertical
axis being
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
aligned with the scanning direction. Location of the individual wells is
typically made
possible by using fiducials in known locations on the flow cell pattern.
[0054]
In conventional approaches, certain fiducials, which may be referred to
herein
as conventional or coarse-alignment fiducials, may be in the form of a
"bullseye" pattern
consisting of concentric dark and bright circles. Each image scanned from the
flow cells
(each image typically referred to as a "tile" or "image tile") may have from 4
to 8 bullseye
fiducials on current platforms. The image data acquired of such "bullseye"
fiducials is
used in the generation of geometric transforms, such as affine transforms,
that may be used
to perform image corrections, such as to compensate for shifts, skews, and
magnification
changes along both principal axes of the image. The image data acquired of
such
"bullseye" fiducials, however, does not provide sufficient information for non-
linear
corrections of the image geometry, i.e., to identify and correct for
deviations from linear
movement of the sample relative to the imaging optics.
[0055]
One method to detect transverse movements of the scanning mechanism (e.g.,
linear motion deviations) employees one-dimensional (1-D) Fourier transforms
of the well
pattern on the flow cell. This may be accomplished by detecting the phase of
the peaks in
the Fourier transform corresponding to the period of the well pattern. There
are, however,
two issues which can affect the utility of this approach. First, the spacing
(i.e., pitch) of
the well pattern of the flow cell may be below the Shannon-Nyquist sampling
limit for the
optical system. By way of example, there may be less than 2 pixels in the
image for each
well location (e.g., 1.9 pixels/well). As a result, the period of the well
pattern cannot be
directly represented in the 1-D Fourier transform. However, by utilizing
aliasing it is still
possible to reliably detect phase for slightly or moderately undersampled
data, thereby
allowing accurate estimates of transverse movement in the x-direction.
[0056]
However, to increase sample densities and, correspondingly, improve
throughput and efficiency, there is an incentive to reduce the pitch of flow
cells, such as to
a flow cell pitch of 1.8 pixels/pitch or less (e.g., 1.7 pixel/pitch). The
aliasing approach
16
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
used to address slight and moderate undersampled data is not adequate to
address this
degree of undersampling.
[0057]
A further issue relevant to hexagonal flow cell patterns is that alternate
rows are
offset by half a well distance relative to each other. As a result, some 1-D
Fourier
transforms lose signal where the pixel row spans two rows of sample wells on
the flow
cell.
[0058]
With these issues in mind, various fiducials, referred to herein as linear
fiducials, are described herein that may be used to address one or both of
these concerns.
In the linear fiducials discussed herein, multiple well locations may be
"blanked" out so as
to create breaks in the overall pattern of well sites. By way of example, a
fiducial pattern
may comprise three rows of wells over a central location in the flow cell
(e.g., centered
with respect to an x-axis or other axis), with wells in the two outer rows
blanked out. In
this manner, the issues arising from alternating well locations on adjacent
rows may be
addressed by the fiducial. In a second aspect of one such implementation,
alternating wells
in the center row of wells are also blanked out. In this manner, the effective
pitch in the
fiducial is increased such that the well pitch in this fiducial pattern will
not be affected the
Shannon-Nyquist limit, thereby allowing tighter pixel pitches to be used
overall within the
flow cell.
[0059]
While the preceding provides useful background and context with respect to
terminology and processes, the following provides an example of suitable
systems and
functional workflows that may utilize or process sample substrates having
fiducials as
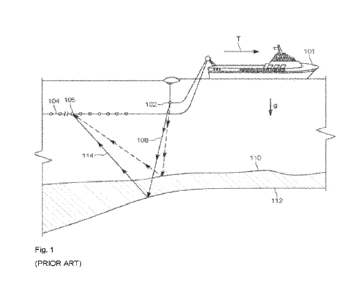
described herein. By way of example, FIG. 1 depicts an example of an optical
image
scanning system 10, such as a sequencing system, that may be used in
conjunction with the
disclosed fiducials and corresponding registration techniques to process
biological
samples. With respect to such an imaging system 10, it may be appreciated that
such
imaging systems typically include a sample stage or support that holds a
sample or other
object to be imaged (e.g., a flow cell or sequencing cartridge having a
patterned surface of
17
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
spaced apart sample sites, such as sample wells) and an optical stage that
includes the optics
used for the imaging operations.
[0060]
Turning to FIG. 1, the example imaging scanning system 10 may include a
device for obtaining or producing an image of a region, such as an image tile,
sub-tile, or
line of a flow cell. The example illustrated in FIG. 1 shows an example image
scanning
system configured in a backlit operational configuration. In the depicted
example, subject
samples are located on sample container 110 (such as a flow cell), which is
positioned on
a sample stage 170 under an objective lens 142. Light source 160 and
associated optics
direct a beam of light, such as laser light, to a chosen sample location on
the sample
container 110. The sample fluoresces and the resultant light is collected by
the objective
lens 142 and directed to a photodetector 140 to detect the florescence. Sample
stage 170
is moved relative to objective lens 142 to position the next sample location
on sample
container 110 at the focal point of the objective lens 142. Movement of sample
stage 170
relative to objective lens 142 can be achieved by moving the sample stage
itself, the
objective lens, the entire optical stage, or any combination of these
structures. Further
examples may also include moving the entire imaging system over a stationary
sample.
[0061]
A fluid delivery module or device 100, as discussed in greater detail
below,
directs a flow of reagents (e.g., fluorescent nucleotides, buffers, enzymes,
cleavage
reagents, etc.) to (and through) the sample container 110 and waste valve 120.
In some
applications, the sample container 110 can be implemented as a flow cell that
includes
clusters of nucleic acid sequences at a plurality of sample locations on the
sample container
110. The samples to be sequenced may be attached to the substrate of the flow
cell, along
with other optional components. In practice, the plurality of sample locations
provided on
a surface of the flow cell may be arranged as spaced apart sample sites (e.g.,
wells or
nanowells), which in turn may be subdivided into tile, sub-tile, and line
regions each
comprising a corresponding subset of the plurality of sample locations.
18
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0062]
The depicted example image scanning system 10 also comprises temperature
station actuator 130 and heater/cooler 135 that can optionally regulate the
temperature or
conditions of the fluids within the sample container 110. A camera system
(e.g.,
photodetector system 140) can be included to monitor and track the sequencing
of sample
container 110. The photodetector system 140 can be implemented, for example,
as a CCD
camera, which can interact with various filters within filter switching
assembly 145,
objective lens 142, and focusing laser assembly (e.g., focusing laser 150 and
focusing
detector 141). The photodetector system 140 is not limited to a CCD camera and
other
cameras and image sensor technologies can be used.
[0063]
Light source 160 (e.g., an excitation laser within an assembly optionally
comprising multiple lasers) or another light source can be included to
illuminate
fluorescent sequencing reactions within the samples via illumination through a
fiber optic
interface 161 (which can optionally comprise one or more re-imaging lenses, a
fiber optic
mounting, etc.). Low watt lamp 165 and reverse dichroic 185 are also presented
in the
example shown. In some applications focusing laser 150 may be turned off
during
imaging. In other applications, an alternative focus configuration can include
a second
focusing camera, which can be a quadrant detector, a position sensitive
detector, or similar
detector to measure the location of the scattered beam reflected from the
surface concurrent
with data collection.
[0064]
Although illustrated as a backlit device, other examples may include a
light
from a laser or other light source that is directed through the objective lens
142 onto the
samples on sample container 110 (i.e., a frontlit configuration). Sample
container 110 can
be mounted on a sample stage 170 to provide movement and alignment of the
sample
container 110 relative to the objective lens 142. The sample stage 170 can
have one or
more actuators to allow it to move in any of three directions. For example, in
terms of the
Cartesian coordinate system, actuators can be provided to allow the stage to
move in the
x-, y- and z-directions relative to the objective lens 142. This can allow one
or more sample
19
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
locations on sample container 110 to be positioned in optical alignment with
objective lens
142.
[0065]
A focus component 175 is shown in this example as being included to
control
positioning of the optical components relative to the sample container 110 in
the focus
direction (typically referred to as the z-axis, or z-direction). Focus
component 175 can
include one or more actuators physically coupled to the optical stage or the
sample stage,
or both, to move sample container 110 on sample stage 170 relative to the
optical
components (e.g., the objective lens 142) to provide proper focusing for the
imaging
operation. For example, the actuator may be physically coupled to the
respective stage
such as, for example, by mechanical, magnetic, fluidic or other attachment or
contact
directly or indirectly to or with the stage. The one or more actuators can be
configured to
move the stage in the z-direction while maintaining the sample stage in the
same plane
(e.g., maintaining a level or horizontal attitude, perpendicular to the
optical axis). The one
or more actuators can also be configured to tilt the stage. This can be done,
for example,
so that sample container 110 can be leveled dynamically to account for any
slope in its
surfaces.
[0066]
Focusing of the system generally refers to aligning the focal plane of the
objective lens 142 with the sample to be imaged at the chosen sample location.
However,
focusing can also refer to adjustments to the system to obtain or enhance a
desired
characteristic for a representation of the sample such as, for example, a
desired level of
sharpness or contrast for an image of a test sample. Because the usable depth
of field of
the focal plane of the objective lens 142 may be very small (sometimes on the
order of 1
[um or less), focus component 175 closely follows the surface being imaged.
Because the
sample container may not be perfectly flat as fixtured in the instrument,
focus component
175 may be set up to follow this profile while moving along in the scanning
direction
(typically referred to as the y-axis).
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0067]
The light emanating from a test sample at a sample location being imaged
can
be directed to one or more photodetectors 140. Photodetectors can include, for
example a
CCD camera. An aperture can be included and positioned to allow only light
emanating
from the focus area to pass to the photodetector(s). The aperture can be
included to
improve image quality by filtering out components of the light that emanate
from areas that
are outside of the focus area. Emission filters can be included in filter
switching assembly
145, which can be selected to record a determined emission wavelength and to
block any
stray laser light.
[0068]
In various examples, sample container 110 (e.g., a flow cell) can include
one or
more substrates upon which the samples are provided. For example, in the case
of a system
to analyze a large number of different nucleic acid sequences, sample
container 110 can
include one or more substrates on which nucleic acids to be sequenced are
bound, attached
or associated. In various examples, the substrate can include any inert
substrate or matrix
to which nucleic acids can be attached, such as for example glass surfaces,
plastic surfaces,
latex, dextran, polystyrene surfaces, polypropylene surfaces, polyacrylamide
gels, gold
surfaces, and silicon wafers. In some applications, the substrate is within a
channel or other
area at a plurality of locations formed in a matrix or pattern across the
sample container
110.
[0069]
One or more controllers 190 (e.g., processor or ASIC based controller(s))
can
be provided to control the operation of a scanning system, such as the example
image
scanning system 10 described with reference to FIG. 1. The controller 190 can
be
implemented to control aspects of system operation such as, for example,
focusing, stage
movement, and imaging operations. In various applications, the controller can
be
implemented using hardware, software, or a combination of the preceding. For
example,
in some implementations the controller can include one or more CPUs or
processors 192
with associated memory 194. As another example, the controller can comprise
hardware
or other circuitry to control the operation. For example, this circuitry can
include one or
more of the following: field programmable gate arrays (FPGA), application
specific
21
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
integrated circuits (ASIC), programmable logic devices (PLD), complex
programmable
logic devices (CPLD), a programmable logic array (PLA), programmable array
logic
(PAL), or other similar processing device or circuitry. As yet another
example, the
controller can comprise a combination of this circuitry with one or more
processors.
[0070]
Although acquisition and registration of image data of arrangements of
features
(e.g., sample sites, such as wells) for use as fiducials may be described and
discussed herein
in the context of this example system, this is only one example with which
these techniques
might be implemented. After reading this description, one of ordinary skill in
the art will
understand how the systems and methods described herein can be implemented
with this
and other scanners, microscopes and other imaging systems.
[0071]
While the preceding description covers aspects of an optical image
scanning
system 10, such as a sequencing system, FIGS. 2 and 3 discuss the use of such
a system 10
in the context of a functional work flow. This discussion is provided in order
to provide
useful, real-world context for the subsequent discussion of fiducials, such as
linear fiducials
used in the detection and correction of deviation from a linear scan path. In
this manner,
it is hoped that the use and significance of the fiducials and their use in
the approaches
subsequently described will be more fully appreciated.
[0072]
With this in mind, and turning to FIG. 2, a block diagram illustrating an
example
work flow in conjunction with system components is provided. In this example,
the work
flow and corresponding system components may be suitable for processing
patterned flow
cells (such as for biological applications), imaging the patterned flow cell
surface, and
analyzing data derived from the imaging.
[0073]
In the illustrated example, molecules (such as nucleotides,
oligonucleotides,
and other bioactive reagents) may be introduced into respective sample
container 110 that
may be prepared in advance. As noted herein, such sample containers 110 may
comprise
flow cells, sequencing cartridges, or other suitable structures having
substrates
encompassing sample sites for imaging. The depicted work flow with system
components
22
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
may be utilized for synthesizing biopolymers, such as DNA chains, or for
sequencing
biopolymers. However, it should be understood that the present technique is
not limited to
sequencing operations, gene expression operations, diagnostic applications,
and so forth,
but may be used more generally for analyzing collected image data for multiple
lines,
swaths or regions detected from imaging of a sample or sample holder, as
described below.
Other substrates containing reaction or capture sites for molecules or other
detectable
features can similarly be used with the techniques and systems disclosed.
[0074]
In the present context, example biopolymers may include, but are not
limited
to, nucleic acids, such as DNA, RNA, or analogs of DNA or RNA. Other example
biopolymers may include proteins (also referred to as polypeptides),
polysaccharides, or
analogs thereof Although any of a variety of biopolymers may be processed in
accordance
with the described techniques, to facilitate and simplify explanation the
systems and
methods used for processing and imaging in the example context will be
described with
regard to the processing of nucleic acids. In general, the described work flow
will process
sample containers 110, each of which may include a patterned surface of
reaction sites. As
used herein, a "patterned surface" refers to a surface of a support or
substrate having a
population of different discrete and spaced apart reaction sites, such that
different reaction
sites can be differentiated from each other according to their relative
location. A single
species of biopolymer may be attached to each individual reaction site.
However, multiple
copies of a species of biopolymer can be attached to a reaction site. The
pattern, taken as
a whole, may include a plurality of different biopolymers attached at a
plurality of different
sites. Reaction sites can be located at different addressable locations on the
same substrate.
Alternatively, a patterned surface can include separate substrates each
forming a different
reaction site_ The sites may include fragments of DNA attached at specific,
known
locations, or may be wells or nanowells in which a target product is to be
synthesized. In
some applications, the system may be designed for continuously synthesizing or
sequencing molecules, such as polymeric molecules based upon common
nucleotides.
23
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0075]
In the diagrammatical representation of FIG. 2, an analysis system may
include
a processing system 224 (e.g., a sequencing system or station) designed to
process samples
provided within sample containers 110 (such as may include biological
patterned surfaces),
and to generate image data representative of individual sites on the patterned
surface, as
well as spaces between sites, and representations of fiducials provided in or
on the
patterned surface. A data analysis system 226 receives the image data and
processes the
image data in accordance with the present disclosure to extract meaningful
values from the
imaging data as described herein. A downstream processing/storage system 228,
then, may
receive this information and store the information, along with imaging data,
where desired.
The downstream processing/storage system 228 may further analyze the image
data or
processed data derived from the image data, such as to diagnose physiological
conditions,
compile sequencing lists, analyze gene expression, and so forth.
[0076]
With respect to the data analysis system 226 and/or the downstream
process/storage system 228 as may be relevant to the present context, image
data may be
analyzed using a real-time analysis (RTA) protocol commercially available for
Illumina
sequencers. Fiducials may be formed and disposed as discussed below, such as
in or
partially within swaths of sites. Dark (non-signal producing regions or
pixels) and light
(signal producing regions or pixels) areas may be assigned an intensity level
of 0 and 255,
respectively, or any desired other level or levels between these. The data
indicating the
presence of a fiducial may be cross correlated at possible x- and y-offsets
and shifted to
maximize correlation. An area may be fit, for example to a two-dimensional
Gaussian to
determine a subpixel x- and y-shift that maximizes the cross correlation. This
process can
be repeated in different regions of the image where the fiducials are located.
The subpixel
x- and y-offsets determined in each region may be used to determine a
geometric transform
or set of geometric transforms describing how features on the designed
patterned surface
appear in the image data By way of example, an Affine transform or Projective
transform
may be derived in this manner. In particular embodiments discussed herein,
certain of the
fiducials, i.e., linear fiducials, may be used to determine if there is
deviation from linear
motion in real-time during a scanning operation and to allow for the
correction of such
24
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
detected deviation, either as one or both of a control-feedback loop to the
mechanisms
controlling motion of the sample stage and/or optics or as an image-based
correction factor.
[0077]
The processing system 224 may employ a biomolecule reagent delivery system
(shown as a nucleotide delivery system 230 in the example of FIG. 2) for
delivering various
reagents to a sample container 110 as processing progresses. The biomolecule
reagent
delivery system may correspond to the fluid delivery module or device 100 of
FIG. 1.
Processing system 224 may perform a plurality of operations through which
sample
container 110 and corresponding samples progress. This progression can be
achieved in a
number of ways including, for example, physical movement of the sample
container 110
to different stations, or loading of the sample container 110 (such as a flow
cell) in a system
in which the sample container 110 is moved or an optical system is moved, or
both, or the
delivery of fluids is performed via valve actuation. A system may be designed
for cyclic
operation in which reactions are promoted with single nucleotides or with
oligonucleotides,
followed by flushing, imaging, and de-blocking in preparation for a subsequent
cycle. In
a practical system, the sample containers 110 and corresponding samples are
disposed in
the processing system 224 and an automated or semi-automated sequence of
operations is
performed for reactions, flushing, imaging, de-blocking, and so forth, in a
number of
successive cycles before all useful information is extracted from the test
sample. Again, it
should be noted that the work flow illustrated in FIG. 2 is not limiting, and
the present
techniques may operate on image data acquired from any suitable system
employed for any
application. It should be noted that while reference is made in the present
disclosure to
"imaging" or "image data", in many practical systems this will entail actual
optical imaging
and extraction of data from electronic detection circuits (e.g., cameras or
imaging
electronic circuits or chips), although other detection techniques may also be
employed,
and the resulting electronic or digital detected data characterizing the
molecules of interest
should also be considered as "images" or "image data".
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0078]
In the example illustrated in FIG. 2, the nucleotide delivery system 230
provides
a process stream 232 to the sample containers 110. An effluent stream 234 from
the sample
containers 110 (e.g., a flow cell) may be recaptured and recirculated, for
example, in the
nucleotide delivery system 230. In the illustrated example, the patterned
surface of the
flow cell may be flushed at a flush station 236 (or in many cases by flushing
by actuation
of appropriate valving, such as waste valve 120 of FIG. 1) to remove
additional reagents
and to clarify the sample within the sample containers 110 for imaging. The
sample
containers 110 is then imaged, such as using line imaging or area imaging
techniques, by
an imaging system 10 (which may be within the same device). The image data
thereby
generated may be analyzed, for example, for determination of the sequence of a
progressively building nucleotide chain, such as based upon a template. In one
possible
embodiment, the imaging system 10 may employ confocal line scanning to produce
progressive pixilated image data that can be analyzed to locate individual
sites on the
patterned surface and to determine the type of nucleotide that was most
recently attached
or bound to each site. Other imaging techniques may also suitably be employed,
such as
techniques employing "step and shoot" or other area-based imaging approaches.
[0079]
As noted, the imaging components of the imaging system 10 may be more
generally considered a "detection apparatus", and any detection apparatus that
is capable
of high resolution imaging of surfaces may be employed. In some examples, the
detection
apparatus will have sufficient resolution to distinguish features at the
densities, pitches
and/or feature sizes set forth herein. Examples of the detection apparatus are
those that are
configured to maintain an object and detector in a static relationship while
obtaining a line
or area image. As noted, a line scanning apparatus can be used, as well as
systems that
obtain continuous or successive area images (e.g. "step and shoot" detectors).
Line
scanning detectors can be configured to scan a line along the y-dimension of
the surface of
an object, where the longest dimension of the line occurs along the x-
dimension. It will be
understood that the detection device, object, or both can be moved to achieve
scanning
detection. Detection apparatuses that are useful, for example in nucleic acid
sequencing
applications, are described in U.S. Pat. App. Pub. Nos. 2012/0270305 Ai;
2013/0023422
26
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
Al; and 2013/0260372 Al; and U.S. Pat. Nos. 5,528,050; 5,719,391; 8,158,926
and
8,241,573, all of which are incorporated herein by reference in their entirety
for all
purposes.
[0080]
In one example, an imaging system 10 that is used in a method or system
set
forth herein may scan along the y-dimension of a patterned surface, scanning
parallel
swaths of sites of the patterned surface in the process. The patterned surface
may include
coarse-alignment markers that distinguish the relative locations of the swaths
of sites along
the x-dimension. When used, the coarse-alignment markers can cooperate with
the
detection apparatus, such as to determine the location of at least one of the
swaths of sites.
Optionally, the relative position of the detection apparatus and/or the sample
container 110
having the patterned surface may be adjusted based on the location determined
for the
swaths. In some examples, the determining of the location of the swaths can be
performed
by an algorithm by a processor or computer, such as a computer used to perform
registration or feature identification. Thus, the system may function to
perform the
algorithm on the computer to determine locations for the features in the image
data, as well
as to characterize molecules at each site, referenced based on the fiducials.
[0081]
Following imaging (e.g., at imaging system 10), the sample container 110
may
progress to a deblock station 240 for de-blocking, during which a blocking
molecule or
protecting group is cleaved from the last added nucleotide, along with a
marking dye. If
the processing system 224 is used for sequencing, by way of example, image
data from the
imaging system 10 will be stored and forwarded to a data analysis system 226.
[0082]
The data analysis system 226 may include a general purpose or application-
specific programmed computer, which provides a user interface and automated or
semi-
automated analysis of the image data to determine which of the four common DNA
nucleotides may have been last added at each of the sites on a patterned
surface, as
described below. As will be appreciated by those skilled in the art, such
analysis may be
performed based upon the color of unique tagging dyes for each of the four
common DNA
nucleotides. This image data may be further analyzed by the downstream
processing/
27
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
storage system 228, which may store data derived from the image data as
described below,
as well as the image data itself, where appropriate. Again, the sequencing
application is
intended to be one example, and other operations, such as diagnostic
applications, clinical
applications, gene expression experiments, and so forth may be carried out
that will
generate similar imaging data operated on by the present techniques.
[0083]
As noted above, in some implementations, the sample container 110 (e.g., a
flow cell) having the patterned surface may remain in a fixed or substantially
fixed position,
and the "stations" referred to may include integrated subsystems that act on
the sample
container 110 as described (e.g., for introduction and reaction with desired
chemistries,
flushing, imaging, image data collection, and so forth). The data analysis may
be
performed contemporaneously with the other processing operations (i.e., in
"real time"),
or may be done post-processing by accessing the image data, or data derived
from the
image data, from an appropriate memory (in the same system, or elsewhere). In
many
applications, a patterned surface "container" will comprise a cartridge or
flow cell in which
the patterned surface exists and through which the desired chemistry is
circulated. In such
applications, imaging may be done through and via the flow cell. The flow cell
may be
appropriately located (e.g., in the x-y plane), and moved (e.g., in x-, y-,
and z-directions)
as needed for imaging. Connections for the desired chemistry may be made
directly to the
flow cell when it is mounted in the apparatus. Moreover, depending upon the
device design
and the imaging technique used, the patterned surface, encased in the flow
cell, may be
initially located in the x-y plane, and moved in this plane during imaging, or
imaging
components may be moved parallel to this plane during imaging. In general,
here again,
the "x-y plane" is the plane of the patterned surface that supports the sites,
or a plane
parallel to this. The flow cell, therefore, may be said to extend in the x-y
plane, with the
x-direction being the longer direction of the flow cell, and the y-direction
being the shorter
direction (the flow cells being rectangular). It is to be understood, however,
that this
orientation could be reversed. The flow cell and corresponding patterned
surface may also
be moved in the z-direction, which is the focus-direction, typically
orthogonal to both the
x- and y-directions. Such movements may be useful for securing the flow cell
into place,
28
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
for making fluid connections to the flow cell, and for imaging (e.g., focusing
the optic for
imaging sites at precise z-depths). In some applications, the optic may be
moved in the x-
direction for precise imaging.
[0084]
FIG. 3 illustrates an example data analysis system 226 and some of its
functional components that may be relevant to the present approach. As noted
above, the
data analysis system 226 may include one or more programmed computers, with
programming being stored on one or more machine readable media with code
executed to
carry out the processes described_ Alternatively or in addition, one or more
application
specific integrated circuits (ASICs) and/or field programmable gate arrays
(FPGAs) (or
other hardware based solutions) may be employed to perform some or all of the
functionality attributed to the data analysis system 226 as described herein.
In the
illustrated example, the data analysis system 226 includes an interface 260
designed to
permit networking of the data analysis system 226 to one or more imaging
systems 10
acquiring image data of patterned surfaces of reaction or capture sites (i.e.,
features, such
as wells) within a sample container 110. The interface 260 may receive and
condition data,
where appropriate. In general, however, the imaging system 10 will output
digital image
data representative of individual picture elements or pixels that, together,
form an image
of the patterned surface (or a portion (e.g., line or tile) of it). In the
depicted example, a
processor 262 processes the received image data in accordance with a plurality
of routines
defined by processing code. The processing code may be stored in various types
of
memory circuitry 264. As used in this disclosure, the term "machine readable"
means
detectable and interpretable by a machine, such as a computer, processor, or a
computer or
processor in cooperation with detection and signal interpretation devices or
circuits (e.g.,
computer memory and memory access components and circuits, imaging or other
detection
apparatus in cooperation with image or signal interpretation and processing
components
and circuits), and so forth.
29
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0085]
Computers and processors useful for the present techniques may include
specialized (e.g., application-specific) circuitry and/or general purpose
computing devices,
such as a processor that is part of a detection device, networked with a
detection device
used to obtain the data that is processed by the computer, or separate from
the detection
device. In some examples, information (e.g., image data) may be transmitted
between
components of a data analysis system 226 disclosed herein directly or via a
computer
network. A Local Area Network (LAN) or Wide Area Network (WAN) may be a
corporate
computing network, including access to the Internet, to which computers and
computing
devices comprising the data analysis system 226 are connected. In one example,
the LAN
conforms to the Transmission Control Protocol/Internet Protocol (TCP/IP)
industry
standard. In some instances, the information (e.g., image data) is input to a
data analysis
system 226 disclosed herein via an input device (e.g., disk drive, compact
disk player, USB
port, etc.). In some instances, the information is received by loading the
information, such
as from a storage device such as a disk or flash drive.
[0086]
As noted above, in some examples, the processing circuitry may process
image
data in real or near-real time while one or more sets of image data of the
support, sites,
molecules, etc. are being obtained. Such real time analysis is useful for
nucleic acid
sequencing applications where an imaged surface having attached of nucleic
acids is
subjected to repeated cycles of fluidic and detection operations. Further, as
discussed
herein, such real-time analysis is particularly beneficial to detect
deviations from linear
motion during image acquisition so as to allow appropriate corrective actions
to be
performed. As noted herein, for features that are sufficiently small in scale
(e.g., spanning
less than two or three pixels, such deviations from linearity may have
significant
downstream processing consequences. Analysis of the sequencing data can often
be
computationally intensive such that it can be beneficial to perform the
methods in real or
near-real time or in the background while other data acquisition or analysis
algorithms are
in process. Example real time analysis methods that can be used with the
present methods
are those used for the MiSeqTM and HiSeqTm sequencing devices commercially
available
from Illumina, Inc. and/or described in U.S. Pat. App. Pub. No. 2012/0020537
Al, which
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
is incorporated herein by reference in its entirety for all purposes. The
terms "real time"
and "near-real time", when used in conjunction with the processing of samples
and their
imaging are intended to convey that the processing occurs at least in part
during the time
the samples are being processed and imaged (i.e., processing occurs
simultaneously or
contemporaneously with data acquisition). In other examples, image data may be
obtained
and stored for subsequent analysis by similar algorithms. This may permit
other equipment
(e.g., powerful processing systems) to handle the processing tasks at the same
or a different
physical site from where imaging is performed. This may also allow for re-
processing,
quality verification, and so forth.
[0087]
In accordance with the presently contemplated examples, the processing
code
executed on the image data includes an image data analysis routine 270
designed to analyze
the image data. Image data analysis may be used to determine the locations of
individual
sites visible or encoded in the image data, as well as locations in which no
site is visible
(i.e., where there is no site, or where no meaningful radiation was detected
from an existing
site). Image data analysis may also be used to determine locations of
fiducials that aid in
locating the sites.
[0088]
As will be appreciated by those skilled in the art, in a biological
patterned
surface imaging context, respective sites of the patterned surface will appear
brighter than
non-site locations due to the presence of fluorescing dyes attached to the
imaged molecules.
It will be understood that the sites need not appear brighter than their
surrounding area, for
example, when a target for the probe at the site is not present in a sample
being detected.
The color at which individual sites appear may be a function of the dye
employed, as well
as of the wavelength range of the light used by the imaging system 28 for
imaging purposes
(e.g., the excitation wavelength range of light). Sites to which targets are
not bound or that
are otherwise devoid of a label can be identified according to other
characteristics, such as
their expected location on the patterned surface. Any fiducial markers may
appear on one
or more of the images, depending upon the design and function of the markers.
31
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0089]
Once the image data analysis routine 270 has located individual sites in
the
image data, a value assignment may be carried out at step 272, often as a
function of, or by
reference to any fiducial markers provided. In general, the value assignment
step 272 will
assign a digital value to each site based upon characteristics of the image
data represented
by pixels at the corresponding location. That is, for example, the value
assignment routine
272 may be designed to recognize that a specific color range or wavelength
range of light
was detected at a specific location within a threshold time after excitation,
as indicated by
a group or cluster of pixels at the location. The value assignment carried out
at step 272 in
such a context will assign the corresponding value to the entire site,
alleviating the need to
further process the image data itself, which will be much more voluminous
(e.g., many
pixels may correspond to each site) and of significantly larger numerical
values (i.e., much
larger number of bits to encode each pixel).
[0090]
By way of further example, the present compositions, devices, and methods
suitably can be used so as to generate luminescent images in sequencing-by-
synthesis
(SBS) techniques and devices. In such SBS approaches, a flow cell or other
microfluidic
device may include a sample and sample capture sites as described herein and
one or more
analytes may be flowed over the sites as part of a sequencing operation. A
suitable number
of luminophores may be employed that can be excited in sequence using any
suitable
number of excitation wavelengths. By way of example, four distinct excitation
sources at
four resonant wavelengths (ki, X.2, X. 3, and k 4) may be employed in a 4-
channel SBS
chemistry scheme, or two excitation wavelengths (X, 1 and X.2) may be employed
in a 2-
channel SBS chemistry scheme, or one excitation wavelength () 1) may be
employed in a
1-channel SBS chemistry scheme. Examples of 4-channel, 3-channel, 2-channel or
1-
channel SBS schemes are described, for example, in US Pat. App. Pub. No.
2013/0079232
Al, which is hereby incorporated herein by reference in its entirety, and can
be modified
for use with the apparatus and methods set forth herein. As will be
appreciated, in one
such SBS approach for use in sequencing DNA using luminescent imaging, a first
luminophore can be coupled to A, a second luminophore can be coupled to G, a
third
luminophore can be coupled to C, and a fourth luminophore can be coupled to T.
As
32
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
another example, in techniques for use in sequencing RNA using luminescent
imaging, a
first luminophore can be coupled to A, a second luminophore can be coupled to
G, a third
luminophore can be coupled to C, and a fourth luminophore can be coupled to U.
[0091]
In practice, in a multi-channel system (e.g., a four-channel system) each
respective sequencing-by-synthesis (SBS) cycle has an associated separate
excitation and
readout operation for each channel and each channel is separately read out
each cycle. That
is, for each SBS cycle in a four-channel system, there are four excitation and
readout
operations, each corresponding to a different channel. In a DNA imaging
application, for
example, the four common nucleotides may be represented by separate and
distinguishable
colors (or more generally, wavelengths or wavelength ranges of light), each
color
corresponding to a separate channel that is separately readout out during each
SBS cycle.
[0092]
An indexing assignment routine 274 associates each of the assigned values
with
a location in an image index or map, which may be made by reference to known
or detected
locations of fiducial markers, or to any data encoded by such markers. As
described more
fully below, the map will correspond to the known or determined locations of
individual
sites within the sample container 110. Data analysis routines (shown as data
stitching step
276 in FIG. 3), which may be provided in the same or a different physical
device, allows
for identification or characterization of the molecules of the sample present
within the
sample container 110, as well as for logical analysis of the molecular data,
where desired.
For sequencing, for example, the data analysis routines may permit
characterization of the
molecules at each site by reference to the emission spectrum (that is, whether
the site is
detectable in an image, indicating that a tag or other mechanism produced a
detectable
signal when excited by a wavelength of light). The molecules at the sites, and
subsequent
molecules detected at the same sites may then be assembled logically into
sequences.
These short sequences may then be further analyzed by the data analysis
routines 276 to
determine probable longer sequences in which they may occur in the sample
donor subject.
33
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[0093]
It may be noted that as in the illustration of FIG. 3, an operator (OP)
interface
280 may be provided, which may consist of a device-specific interface, or in
some
applications, to a conventional computer monitor, keyboard, mouse, and so
forth to interact
with the routines executed by the processor 262. The operator interface 280
may be used
to control, visualize or otherwise interact with the routines as imaging data
is processed,
analyzed and resulting values are indexed and processed.
[0094]
FIG. 4 illustrates an example of a patterned surface 288 that may be
present as
part of or within a sample container 110. As shown in FIG 4, a plurality of
grids or swaths
290 (here depicted as vertical swaths) may be provided such that each includes
a multitude
of individual tiles 294 to be imaged. Each image tile 294 in turn comprises
multitudes of
sample sites (e.g., capture or reaction sites) which may display activity of
interest at
different cycles of a processing operation (e.g., a sequencing operation). As
noted herein,
a wide range of layouts for patterned surfaces 288 are possible, and the
present techniques
are not intended to be limited to any desired or particular layout. In a
progressive scanning
context, as imaging progresses, the sample container 110 (or patterned surface
288 therein)
will undergo relative motion in an indexed direction so that each of the
swaths 290 can be
imaged. Coarse alignment fiducials (e.g., "auto-centering" fiducials) may be
formed in or
on the support, such as to allow for properly locating the grids or swaths 290
with respect
to the imager, or for locating the patterned features in a processing system
224 or imaging
system 10. It should be noted that in the view of FIG. 4, the surrounding flow
cell in which
the patterned surface 288 may be located is not shown.
[0095]
FIG. 5 is an enlargement of one of the swaths 290 of the patterned surface
288
of FIG. 4. As shown in FIG. 5, depending upon the imaging technique employed,
the swath
290 may be scanned by the imaging system 10 in parallel scan lines 310 that
progressively
move along the swath 290. Moreover, in many systems the patterned surface will
be moved
slowly in one direction, as indicated by arrow 312, while the imaging optic
will remain
stationary. The parallel scan lines 310 will then result from the progressive
movement of
the sample. Each swath 290 may include regions designated as fiducial markers
that can
34
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
be similarly imaged and identified in resulting image data. Although not
shown, area scans
may also be used in which an area of the surface, as opposed to a series of
lines, may be
scanned each pass or acquisition.
[0096]
In the illustrated example, the grid or swath 290 of the patterned surface
has a
width 316 which may be wider than the length 318 of the scan lines 310 of
which the
imaging system 10 is capable of generating or imaging in each pass. That is,
the entire
width 316 may not be scanned or imaged in a single pass. This may be due to
the inherent
limitation of the line length 318 due to the imaging optics, limitations
relating to focusing
or movement of components, such as mirrors or other optical components used to
generate
the scan lines, limitations in digital detectors, and so forth. The swath 290
may be scanned
in multiple passes, and values for each of the sites may be extracted from the
image data.
[0097]
In FIG. 5, for example, the overall width 316 of the swath 290 can be
accommodated in two overlapping areas 320 and 322. The width of each area 320
and
322, as indicated by reference numerals 324 and 326, respectively, may be
slightly less
than the length 318 of the scan lines 310. In such implementations, this will
permit
detection of a feature used to integrate the values derived from the image
data, such as by
reference to an edge or other feature. It may be noted that a common area or
overlap 328
exists that may be imaged in both passes.
[0098]
FIG. 6 illustrates, in somewhat greater detail, scan lines 310 over a
plurality of
sample sites 340 (e.g., wells or nanowells) in a swath 290. By way of example,
in the
context of a flow cell the sites 340 may be gel-filled wells, each well
occupied by a nucleic
acid (e.g., DNA) colony. As noted above, in some implementations, the sites
340 may be
laid out in any suitable grid pattern, or even randomly. In the illustrated
example, the sites
340 are laid out in a hexagonal pattern, although rectangular patterns (e.g.,
rectilinear
patterns), and other patterns may be employed. The location of each site 340
will be known
with reference to one or more fiducial or reference features, such as an edge
342 of the grid
or portion of the patterned surface. In the case of random site locations,
these may be
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
located and mapped by an initial imaging sequence designed to detect the
location of all
sites of interest.
[0099]
FIG. 7 represents a portion of an example image of a type that may be
generated
based upon image data collected by progressive scanning of a region of
interest of a
patterned surface. The actual image 350 is composed of a large number of
pixels 352 each
of which is assigned a digital value by the imaging system 10. In a
contemplated context
the pixel data, which represents the image 350, may encode values
corresponding to bright
pixels 354 and darker pixels 356. By way of example, dark (i.e., non-signal
producing
regions or pixels) and light (i.e., signal producing regions or pixels) may be
assigned an
intensity level of 0 and 255, respectively, or any desired other level or
levels between these.
In practice, various grey levels or even color encoding can be employed such
that the
individual sites 340 can be identified by detecting contrast or color value
differences
between the pixels as indicated by their individual digital values.
[00100] It may be noted that, for the purpose of illustration and explanation,
FIG. 7
illustrates each site 340 (e.g., well) as spanning a multitude of pixels 352.
In practice
however, as well sizes continue to decrease in order to increase throughput
and efficiency,
each sample site 340 may effectively be imaged by a small number of pixels
(e.g., 1, 2, 3,
or 4 pixels). As a result, and as discussed herein, the reduced pixel coverage
for each
sample site 340 substantially increases the consequence of deviations from the
linear
motion associated with the scanning operation. By way of example, due to
deviations in
the expected linear motion, the primary pixels 354 associated with a given
well site 340
may be misconstrued so that useful signal is missed or is applied incorrectly
(e.g., to a
different well). Present approaches discussed herein may be used to address
such
deviations.
[00101] Before discussing some presently contemplated forms, types, and uses
of
fiducials, a brief discussion is provided of example processing for the use,
data encoding
and decoding, and registration of site and image data based on the fiducial
techniques
disclosed. Registration of fiducials as described herein, and thereby of sites
340 detectible
36
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
in image data of sequential imaging operations, can be carried out by lining
up (e.g.,
locating and overlaying or otherwise aligning) fiducials, determining the two
dimensional
cross-correlation (or other measure of the similarity of fit), for example,
based on the
number of bright pixels 354 from the image data, and determining the offset
between the
fiducials in one or more dimensions (e.g., in the x- and y-dimensions). The
offset can be
determined, for example, via an iterative process whereby the following
operations are
repeated: one of the fiducials is shifted relative to the other, the change in
level of
correlation of fit is determined (e.g., an increase in correlation being
indicated by an
increase in the number of bright pixels 354 of fiducials that overlap), and a
determined
location of one or more of the fiducials is shifted in a direction that
increases the correlation
of fit. Iterations can proceed until an offset that produces an optimal
correlation, a specified
threshold correlation, or otherwise desired correlation is determined. A
transform can be
determined based on the offset and the transform can be applied to the rest of
the features
(e.g., sites 340) in the target image. Thus, the locations for the features in
a target image
can be determined by shifting the relative scale and/or orientation between
the image data,
using a transform based on an offset determined between fiducials in the image
data when
overlaid.
[00102] Any of a variety of transform models can be used. Global transforms
are useful
including, for example, linear transforms, geometric transforms, projective
transforms, or
affine transforms. The transformations can include, for example, one or more
of rotation,
translation, scaling, shear, and so forth. An elastic or non-rigid transform
can also be
useful, for example, to adjust for distortions in the target detection data or
reference data.
Distortions can arise when using a detection apparatus that scans a line along
they
dimension of an object, where the longest dimension of the line occurs along
the x-
dimension. For example, stretching distortions can occur along the x-dimension
(and
sometimes only along the x-dimension). Distortions can arise for other
detectors including,
for example, spreading distortions in both the x- and y-dimensions in the
context of an area
detector. An elastic or non-rigid transform can be used to correct for
distortions, such as
linear distortions present in image data obtained from line scanning
instruments, or
37
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
spreading distortions present in image data obtained from area detectors.
Alternatively or
additionally, a correction factor can be applied to the reference data, target
data and/or the
transform to correct distortions introduced (or expected to be introduced) by
a detection
apparatus. For examples where patterned features are imaged, a non-linear
correction can
be applied to feature locations as a function of position in the x-dimension.
For example,
the non-linear correction that is applied can be a third order polynomial to
account for
distortion arising from the optical system that was used for detection of the
features.
[00103]
As discussed herein, conventional fiducials that are in current use, such
as
"bullseye" fiducials, are two-dimensional (2-D) and registration utilizes a 2-
D cross-
correlation with a template image for each fiducial. This requires 2-D Fast
Fourier
Transforms (FFT) for each fiducial. While FFTs are highly optimized, 1-D FFTs
are
significantly less computationally intensive and may be more suitable for real-
time
processing and corrections. While the conventional fiducials are useful for
correctly
applying the affine transform, increasing the number of conventional fiducials
may not be
the optimal choice for increasing the precision of registration along the x-
axis.
[00104] As discussed herein and developed in greater detail below, using a
method
based on 1-D FFTs (i.e., linear FFTs) along the x-axis provides high
resolution in the x-
dimension within a limited distance range. By way of example, a linear FFT can
be
performed for each pixel row within an image tile. In one embodiment the
linear FFT has
a length of 1,024 pixels and is centered in the image tile. The linear FFT
allows the well
pattern within the respective pixel row to be directly resolved. That is, by
performing a
linear FFT along the x-axis over the central 1,024 pixels of each pixel row,
the complex
phase can be extracted from the bin at the position of the peak for each pixel
row of the
image tile. In this manner, the conventional fiducials (as described above)
may be used for
absolute coordinate determination along both the x- and y-axes and to assist
in locating the
linear fiducials, described below), and the linear FFTs can provide detailed
information for
intermediate locations based on the linear fiducials.
38
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[00105]
With this in mind, and as discussed below, linear features (e.g., linear
fiducials)
can be incorporated onto a patterned surface. One possibility is using a
number of vertical
lines as linear fiducials. While this may be workable, this approach may not
fully utilize
the possible advantages of linear FFTs. As discussed herein, other possible
embodiments
of a linear fiducial design may be optimized for linear FFT-based
registration. By way of
example, the accuracy of an FFT-based registration method is based on a long
sequence of
a regular pattern. In order to avoid reliance on aliasing effects, the pitch
of the pattern
should be larger than the Shannon-Nyquist limit for the system, however, it
should be as
dense as possible within that limit in order to achieve maximum precision.
[00106] With the preceding in mind, certain embodiments discussed herein
utilize a
linear fiducial oriented (e.g., centered) along the x-axis of an image tile at
appropriate
intervals, including between each pair of conventional fiducials, but also at
intervals
between them. Such linear fiducials may include both sample sites (e.g.,
wells) and
"blanks" or "blank regions", where a well may be expected (based on an
underlying or
implied pattern of sites 340) but was not formed or where a well is present,
but empty of
sample (i.e., a "dark" well)). When the sites 340 and blanks forming a linear
fiducial (e.g.,
a linear fiducial region) are considered together or in the aggregate, they
form a linear
fiducial that is optically discernible from a pattern associated with non-
fiducial regions. In
addition, as discussed herein coarse-alignment markers, when present, can be
used to
roughly align (step 360, FIG. 8) a detection device with the patterned
surface, such as prior
to assessing linear motion using linear fiducials as discussed herein. For
example, in
contexts where the detector is an optical scanning device, the flow cell
surface can include
one or more coarse-alignment markers that are used to roughly align the
imaging optics
with a location of the patterned surface, such as a location to initiate
sequential area or line
imaging. In this case, the coarse-alignment markers can be positioned near the
proximal
edge of the patterned surface, the proximal edge being at or near the
initiation location for
scanning of the sites 340. Coarse-alignment markers are useful when a
patterned surface
is scanned in multiple swaths. In certain implementations, each swath of the
patterned
surface will include one or more linear fiducials as described herein, which
may be used
39
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
for detecting (step 364) and/or correcting (step 368) deviations from linear
motion during
scanning (step 362) in real-time. In this way, both coarse-alignment markers
and fiducials
(including linear fiducials) within, among, or between swaths can be used by a
detection
system to locate features (e.g., sites 340) on the patterned surface. In
certain embodiments
coarse alignment markers may be absent from the flow cell and instead the
corresponding
alignment functions are performed using other fiducials present on the
patterned surface.
[00107] With the preceding background and context in mind, FIGS. 9A and 9B
respectively depict differing examples of a layout of an image tile 294. As
previously
noted, in conventional approaches, certain fiducials 380 (e.g., conventional
or coarse
alignment fiducials) may be in the form of a "bullseye" pattern consisting of
concentric
and alternating dark and bright circles. Each image tile 294 scanned from the
flow cells
may have from 4 to 8 (e.g., 4, 6, or 8) conventional fiducials 380 and the
image data
acquired of such fiducials 380 may be used for absolute positioning and in the
generation
of geometric transforms, such as affine transforms, that may be used to
perform image
corrections, such as to compensate for shifts, skews, and magnification
changes along both
principal axes of the image. The image data acquired of such conventional
fiducials 380
(such as bullseye fiducials), however, does not provide sufficient information
for non-
linear corrections of the image geometry, i.e., to identify and correct for
deviations from
linear movement of the sample relative to the imaging optics.
[00108] In addition to the fiducials 380 used for conventional registration
and site
positioning, the image tile 294 of FIGS. 9A and 9B is shown as including
linear fiducials
384 that may be used for determining deviations from linear motion during a
scan
operation. By way of example, such linear fiducials 384 may be used to perform
high
resolution localization within the x-dimension and, in the example shown in
FIG. 9A, may
be positioned on the image tile 294 between pairs of aligned conventional
fiducials 380 as
well as at intervals between the paired conventional fiducials 380. In a
differing layout,
and as shown in FIG. 9B, conventional fiducials 380 may be spaced apart (i.e.,
offset) in
the depicted y-dimension instead of being aligned. Due to the known offset,
the
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
conventional fiducials 380 (e.g., coarse alignment or "bullseye" fiducials)
may still be used
for coarse alignment functions as well as for localizing linear fiducials 384
as discussed
herein.
[00109] In an example implementation of a linear fiducial 384, and turning to
FIG. 10,
each linear fiducial in the depicted example comprises three rows based on the
normal well
pattern present in non-fiducial regions 388. In the depicted example, the
three rows
corresponding to the linear fiducial 384 are flanked on the top and bottom
with sample sites
340 in the normal, periodic well pattern present in non-fiducial regions 388.
The linear
fiducial 384 in this example comprises two outer rows 392 of "blank" wells or
sites 396 or
are otherwise "blank" (i.e., do not have wells formed at the positions that
would correspond
to the pattern present in non-fiducial regions 388 or have empty, dark wells).
In this
example, the "blank" rows 392 address issues that may arise related to
alternating well
locations on adjacent rows. In addition, the adjacent rows of bordering non-
fiducial regions
388 will have the same sample site (e.g., well) alignment as the center row
400 of the linear
fiducial 384. In one sample embodiment, in order to minimize or otherwise
limit the effects
of optical distortion, the length of the linear fiducial 384 is at least 1,024
pixels and it is
centered in the respective swath such that the non-fiducial well pattern may
be present on
the sides of the linear fiducial 384 as well as above and below the linear
fiducial 384.
[00110] In the depicted example, the center row of the linear fiducial 384
includes well
sites 340, which may be capable of holding sample and thus may be used to
acquire and
collect sample data. As described herein, if the spacing (i.e., pitch) of the
well pattern is
below the Shannon-Nyquist sampling limit for the optical system the period of
the well
pattern cannot be directly represented in the 1-D Fourier transform. With this
in mind, the
depicted example alternates sample sites 340 with blanks 396 so as to bring
the effective
fiducial pitch within the center row 400 above the limit. As a result, a
linear fiducial as
shown in FIG. 10 will have significantly fewer lost well site locations
compared to the
conventional fiducials, and can thus be positioned at smaller intervals. The
linear fiducial
design illustrated has higher resolution compared to a conventional fiducial,
but with a
41
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
limited total range. Correspondingly, the illustrated linear fiducial 384 is
better suited than
a conventional fiducial (e.g., bullseye fiducial) for detecting relative
displacement along
the x-axis (e.g., deviation from a linear motion associated with a scan path).
Conversely,
however, the linear fiducial 384 has little to no sensitivity in the y-
dimension.
[00111] In an alternative embodiment to that illustrated in FIG. 10, and as
shown in FIG.
11 and as noted above, the linear fiducials 384 that may be used to correct
for x-dimension
deviations or shifts may instead involve "vertical" fiducials (i.e., linear
fiducials running
length-wise in the y-dimension. By way of example, such "vertical" linear
fiducials 384
may be formed as a trench or sharp line formed on the substrate, such as
running between
the conventional or coarse alignment fiducials 380.
[00112] Turning to FIG. 12, a further example of an implementation of a linear
fiducial
384 is depicted. In this example, each linear fiducial 384 comprises three
rows based
generally on the normal well pattern present in the non-fiducial regions 388.
In the depicted
example, the three rows corresponding to the linear fiducial 384 are flanked
on the top and
bottom with sample sites 340 in the normal, periodic well pattern present in
the non-fiducial
regions 388. The linear fiducial 384 in this example comprises two outer rows
392 of
"blank- wells or sites 396 or are otherwise "blank- (i.e., do not have wells
formed at the
positions that would correspond to the pattern 388 or have empty, dark wells).
As in
preceding examples, the "blank" rows 392 address matters that may arise
related to the
tight pitch density associated with alternating well locations on adjacent
rows in a high-
density context for the sample wells 340.
[00113] In addition, in the depicted example of FIG. 12 the center row of the
linear
fiducial 384 includes horizontally-oriented (with the direction of line scan
corresponding
to a vertical dimension) elongated sample well sites 344 (also referred to
herein as linear
features 344) alternated with blank sites 396. The linear features344 in the
depicted
embodiment span two normally spaced sites 340 plus the intervening pitch
distance in the
depicted example, though other geometries and/or dimensions are also
contemplated. As
described herein, if the spacing (i.e., pitch) of the well pattern is below
the Shannon-
42
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
Nyquist sampling limit for the optical system the period of the well pattern
cannot be
directly represented in the 1 -D Fourier transform. With this in mind, the
depicted example
alternates linear features344 with blanks 396 so as to bring the effective
fiducial pitch
within the center row 400 above the limit.
[00114] In further examples illustrated in FIGS. 13-15 the linear fiducials
384 are
depicted as comprising more than three rows of sample sites 340 and blanks
396. By way
of example, in the depicted embodiments the linear fiducials 384 comprise five
rows of
sample sites 340 and blanks 396. As noted herein, in order to minimize or
otherwise limit
the effects of optical distortion, the length of the linear fiducial 384 may
be at least 1,024
pixels and the linear fiducial 384 may be centered (with respect to the x-
dimension) in the
respective swath.
[00115] For example, turning to FIG. 13, the depicted linear fiducial 384
comprises five
rows. The top, center, and bottom rows of the linear fiducial 384 comprise a
periodic,
alternating arrangement of blank sites 396 and sample sites 340. In this
example, the
periodic arrangement is: one blank site 396, followed by two sample sites 340,
followed
by a blank site 396, and so forth. The linear fiducial 384 of FIG. 13 includes
two non-
blank rows 404 of sample sites 340 with no intervening or spacing blank sites
396 within
the row. One non-blank row 404 is positioned between the top and center rows
of the
linear fiducial 384 while the other non-blank row 404 is positioned between
the center and
bottom rows of the linear fiducial 384. As in preceding examples, the breaks
in the overall
pattern provided by the blank sites 396 within the linear fiducial 384 may
provide useful
regions where the pitch or spacing between sample sites 340 within the linear
fiducial 384
exceeds the Shannon-Nyquist sampling limit and, further, allows deviations in
linear
motion to be detected.
[00116] With further reference to FIG. 13, in certain embodiments a linear
fiducial 384
comprises two or more non-blank sites 340 (e.g., sample wells) generally
aligned in a
column substantially parallel to the scan direction, such as the scan
direction of a line scan.
As shown in FIG. 13, three non-blank sites 340A, 340B, 340C are aligned in a
column
43
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
substantially parallel to the scan direction. In certain aspects, two or more
non-blank
sample sites 340 aligned in a column substantially parallel provide a larger
"positive"
signal upon Fourier transform since their values are additive.
[00117] Further, in certain embodiments, a linear fiducial 384 comprises two
or more
blank sites 396 generally aligned in a column substantially parallel to the
scan direction,
such as the scan direction of a line scan. As shown in FIG. 13, three blank
sites 396A,
396B, 396C are aligned in a column substantially parallel to the scan
direction. In certain
aspects, two or more blank sites 396 aligned in a column substantially
parallel provide a
larger "negative" signal upon Fourier transform since their values are
additive.
[00118] As further illustrated, in certain embodiments a linear fiducial 384
comprises
two or more non-blank wells 340 generally aligned in a column substantially
parallel to the
scan direction and comprises two or more blank sites 396 generally aligned in
a column
substantially parallel to the scan direction. For example, as shown in FIG.
13, the linear
fiducial 384 comprises five columns (denoted by filled lines 1-5) of two or
more non-blank
sample sites 340 between columns (one of which is denoted by the non-filled
line 1) of two
or more blank sites 396.
[00119] In certain aspects, the arrangement of non-blank sample sites 340
(e.g., sample
wells) and blank sites 396 provide increased detectability of the linear
fiducial 384 while
also allowing the non-blank wells to be used as sample/analyte regions.
[00120] Turning to FIG. 14, a further example of a linear
fiducial 384 is illustrated.
Unlike the example linear fiducial 384 of FIG. 13, where rows of alternating
blank sites
396 and sample sites 340 are alternated with full rows of sample sites 340,
the example
linear fiducial 384 of FIG. 14 utilizes rows of blank sites 396 and sample
sites 340 having
different periodic, repeating patterns. In this example, rows 420 of the
linear fiducial 384
(here constituting the bottom, center, and top rows of the linear fiducial
384) comprise
sample sites 340 and blank sites 396 in a one to two alternating pattern
(e.g., one blank site
396, two sample sites 340, one blank site 396, and so forth). Positioned
between the rows
44
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
420 (i.e., between the top and center rows and between the center and bottom
rows) are
rows 424 of the linear fiducial 384, in which the sample sites 340 and blank
sites 396 are
in a one to one alternating pattern (e.g., one blank site 396, one sample site
340, one blank
site 396, and so forth). As in preceding examples, the breaks in the overall
pattern provided
by the blank sites 396 within the linear fiducial 384 may provide useful
regions where the
pitch or spacing between sample sites 340 within the linear fiducial 384
exceeds the
Shannon-Nyquist sampling limit and, further, allows deviations in linear
motion to be
detected.
[00121] With further reference to FIG. 14, in certain embodiments a linear
fiducial 384
comprises two or more non-blank sample sites 340 (e.g., sample wells)
generally aligned
in a column substantially parallel to the scan direction and comprises two or
more blank
sites 396 generally aligned in a column substantially parallel to the scan
direction. For
example, as shown in FIG. 14, the linear fiducial 384 comprises three columns
of two or
more non-blank sample sites 340 (denoted by filled lines 1-3) between sets
(e.g., pairs) of
two columns of two or more blank sites 396 (one pair of which is denoted by
the non-filled
lines 1 and 2).
[00122] Turning to FIG. 15, a further example is illustrated in which the rows
of the
linear fiducial 384 comprise blank sites 396 and sample sites 340 in a
repeating pattern that
is offset from row to row. In this example, there are four rows in the linear
fiducial 384.
Unlike the example of FIG. 14, the rows 428 of the linear fiducial 384 of FIG.
15 each have
the same pattern of alternation (e.g., two blank sites 396, one sample site
340, two blank
sites 396, and so forth), but are offset from one another in adjacent rows. As
a result, in
the two interior rows of the linear fiducial 384 each sample site 340 has no
other sample
sites 340 adjacent, and instead is surrounded by adjacent blank sites 396. As
in preceding
examples, the breaks in the overall pattern provided by the blank sites 396
within the linear
fiducial 384 may provide useful regions where the pitch or spacing between
sample sites
340 within the linear fiducial 384 exceeds the Shannon-Nyquist sampling limit
and, further,
allows deviations in linear motion to be detected.
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[00123] With further reference to FIG. 15, in certain embodiments a linear
fiducial 384
comprises two or more non-blank sample sites 340 (e.g., sample wells)
generally aligned
in a column substantially parallel to the scan direction and comprises two or
more blank
sites 396 generally aligned in a column substantially parallel to the scan
direction. For
example, as shown in FIG. 15, the linear fiducial 384 comprises one column of
two or more
non-blank sample sites 340 (denoted by filled line 1) between sets (e.g.,
pairs) of two
columns of two or more blank sites 396 (one pair of which is denoted by the
non-filled
lines 1 and 2).
[00124] Turning to FIGS. 16 through 20, five additional embodiments of a
linear
fiducial are depicted and described. As with the embodiment illustrated in
FIG. 12,
elongated sample well sites 344 (also referred to herein as linear features
344) are
incorporated into the embodiments depicted in FIGS. 16 through 20. However,
unlike the
embodiment of FIG. 12, in which the linear features 344 are disposed
horizontally along
the x-dimension of the substrate (i.e., each linear feature 344 is within a
row of the linear
fiducial 384), in the depicted example embodiments of FIGS. 16 through 20, the
linear
features 344 are instead disposed vertically along the y-dimension of the
substrate (i.e., in
the direction of line scanning), thereby spanning multiple rows (e.g., two,
three, four, etc.)
of the linear fiducial 384 while, within each row, each linear feature 344
only occupies the
space associated with a single sample site 340 within the respective row. As
previously
noted, in some embodiments the elongated sample sites (e.g., linear features
344), as with
other sample sites 340, may be used as sample/analyte regions and thus may
provide sites
for meaningful data collection or generation in contrast to techniques in
which the regions
or spots associated with a fiducial are associated with fixed or pre-
determined markers.
[00125] By way of example, and turning to FIG. 16, one embodiment is
illustrated. In
this example, each linear fiducial 384 comprises three rows based generally on
the normal
well pattern present in the non-fiducial regions 388. In the depicted example,
the three
rows corresponding to the linear fiducial 384 include vertically-oriented
linear features 344
that span, in the depicted example, all three rows corresponding to the linear
fiducial 384.
46
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
That is, the vertically-oriented linear features 344 in the depicted
embodiment span three
normally spaced sample sites 340 plus the intervening pitch distance, though
other
geometries and/or dimensions are also contemplated. As will be appreciated, in
other
embodiments the vertically-oriented linear features 344 may span less than all
rows of the
linear fiducial 384. In the depicted example the linear fiducial 384 is
flanked on the top
and bottom with sample sites 340 in the normal, periodic well pattern present
in the non-
fiducial regions 388. As the linear fiducial may have a limited extent in the
x-dimension
(e.g., 1,024 pixels), it may also be flanked on the sides by the normal,
periodic well pattern
present in the non-fiducial regions 388.
[00126] In the depicted example the embodiment of a linear fiducial 384 does
not
include blank sites 396, as discussed and described elsewhere herein. Instead,
both the
depicted sample sites 340 and linear features 344 are configured to hold
sample and/or
analyte undergoing analysis, and thus no substrate surface space is lost to
non-sample
holding blank sites 396.
[00127] In this example, the topmost and bottommost rows of the linear
fiducial 384
comprise sample sites 340 and portions of linear features 344 in a one to two
alternating
pattern (e.g., one linear feature 344, two sample sites 340, one linear
feature 344, and so
forth). The center row of the linear fiducial 384 in the depicted example
(i.e., between the
top and bottom rows) instead exhibits a one-to-one alternating pattern of
linear features
344 and sample sites 340 (e.g., one linear feature 344, one sample site 340,
one linear
feature 344, and so forth). In this example, the changes in pitch between the
linear features
344 and sample sites 340 may provide useful regions where the pitch or spacing
between
sample sites 340 and/or between sample sites 340 and linear features 344
within the linear
fiducial 384 exceeds the Shannon-Nyquist sampling limit and, further, allows
deviations
in linear motion to be detected. Further, as noted above, this may be
facilitated by the
vertically-oriented linear features 344 providing a larger -positive" signal
upon Fourier
transform.
47
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
[00128] In a further example, and turning to FIG. 17, in this example, each
linear
fiducial 384 corresponds in extent to three rows of the normal well pattern
present in the
non-fiducial regions 388. In the depicted example, the three rows
corresponding to the
linear fiducial 384 include vertically-oriented linear features 344 that span,
in the depicted
example, all three rows corresponding to the linear fiducial 384. That is, the
linear features
344 in the depicted embodiment span the height of the linear fiducial 384
corresponding to
the direction of line scan and, in this example corresponding to the extent
associated with
three normally spaced sample sites 340 plus the intervening pitch distance,
though other
geometries and/or dimensions are also contemplated. As will be appreciated, in
other
embodiments the vertically-oriented linear features 344 may span less than all
rows of the
linear fiducial 384 and in such cases may be vertically aligned or may be
alternately offset
in the vertical dimension (i.e., the direction of line scan illustrated). In
the depicted
example the linear fiducial 384 is flanked on the top and bottom with sample
sites 340 in
the normal, periodic well pattern present in the non-fiducial regions 388. As
the linear
fiducial may have a limited extent in the x-dimension (e.g., 1,024 pixels), it
may also be
flanked on the sides by the normal, periodic well pattern present in the non-
fiducial regions
388.
[00129] In the depicted example, within the linear fiducial 384 the vertically-
oriented
linear features 344 may be offset or spaced apart horizontally, such as via a
spacing or
blank region 408 that may correspond to blanks or blanks spaces or regions
(i.e., blank
wells 396 or regions where no wells, blank or otherwise, are formed), as
discussed herein.
For the purpose of illustration and clarification, in the depicted example the
corresponding
spacing of the blank or unformed wells is not shown so as to better illustrate
the extent of
the blank regions 408 in a real-world context, though at the expense of the
underlying
pattern being less evident. As in preceding examples, the blank spaces or
regions 408 help
address matters that may arise related to the pitch density associated with
alternating well
locations on adjacent rows in a high-density context for the sample wells. In
this manner,
issues arising from alternating well locations on adjacent rows that are too
closely spaced
may be addressed by the linear fiducial 384. Further, the effective pitch in
the linear
48
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
fiducial 384 at certain regions having blanks regions 408 or no wells is
increased so as not
to be below the Shannon-Nyquist sampling limit for the optical system, thus
facilitating
representation and/or determination of the period of the well pattern using 1-
D Fourier
transform. Indeed, in certain aspects the vertically-oriented linear features
344 provide a
larger "positive" signal upon Fourier transform. With this in mind, the
depicted example
alternates vertically-oriented linear features 344 with blanks regions 408 so
as to achieve
regions within the linear fiducial 384 in which the effective fiducial pitch
is above the
Shannon-Nyquist sampling limit for the optical system.
[00130] As previously noted, in the preceding and other examples described
herein, the
horizontal spacing between features comprising a linear fiducial may be
something other
than an integer multiple of the well spacing associated with the well pattern
(i.e., the
spacing between adjacent wells in the non-fiducial hexagonal well pattern). By
way of
reference, the preceding example illustrated in FIG. 17 has a spacing between
linear
features 344 of the linear fiducial 384 that in an integer multiple (here, 2x)
of the well
spacing. However, it should be appreciated that the spacing within a linear
fiducial 384
between such linear features 344 need not be an integer multiple of well
spacing, and may
instead be a non-integer multiple of well spacing. Further, and with the
preceding in mind,
in various implementations of linear fiducials 384 spacing patterns (e.g.,
alternating
spacing patterns) between linear features 344 of the linear fiducial 384 may
be employed.
By way of example, where a "unit- is a length unit that may or may not be
related to the
underlying pattern of wells, a linear feature spacing pattern (in units) of
2:3:2:3... ;
1:2:1:2... ; 1:3:1:3... ; and so forth may be employed within a linear
fiducial 384.
[00131] With the preceding in mind, and turning to the examples illustrated in
FIGS.
18-20, various aspects of such variations are illustrated. Turning to FIG. 18,
in this
example an arrangement of linear features 344 similar to what is seen in the
embodiment
of FIG. 17 is depicted. However, in contrast to the prior example, the spacing
between
linear features 344 (illustrated as corresponding to blank regions 408) is a
non-integer
multiple of the well spacing corresponding to wells 340 of the non-fiducial
regions 388.
49
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
For the purpose of illustration, in this example, the linear features 344 are
spaced apart in
the linear fiducial 384 by a distance corresponding to 1.5x the well spacing.
As will be
appreciated however, and suitable non-integer multiple of well spacing (e.g.,
1.25x, 1.33x,
1.5x, 1.66x 1.75x, and so forth) may be employed.
[00132] Turning to FIG. 19, in this example, the spacing between linear
features 344 of
the linear fiducial 384 is also a non-integer multiple of well spacing
corresponding to wells
340 of the non-fiducial regions 388. In addition, the linear features 344 of
the linear
fiducial 384 are shown as having a lesser extent (e.g., length) than in the
prior examples.
In particular, in the depicted example, the vertically-oriented linear
features 344 two rows
of the underlying hexagonal pattern of the non-fiducial regions 388 (i.e., the
area of two
normally spaced sample sites 340 plus the intervening pitch distance. In this
manner, the
linear fiducial 384 may be designed of configured to occupy a lesser vertical
extent of the
substrate, thereby allowing a greater area to be allowed for conventional
sample wells 340.
[00133] In a further example, and turning to FIG. 20, in certain embodiments
the spacing
between linear features 344 of the linear fiducial 384 may incorporate an
alternating pattern
of spacing between linear features 344. That is, the blank regions 408 between
linear
features may be of two different, alternated widths or extents. An example of
this is
illustrated in FIG. 20. In the depicted example, each linear feature 344 is
separated from
the nearest linear feature on a first side by a blank region 408A having a
first width or
extent and is separated from the nearest linear feature on a second side
(e.g., the opposite
or opposing side) by a blank region 408B having a second width or extent
different than
the first width or extent. In this manner a pattern of linear features 344 may
be formed
which itself provides information as to position in the x-dimension. As will
be appreciated
the spacing between linear features with respect to both the first and second
blank regions
408A and 408B may or may not be related to the underlying pattern of wells.
That is one
or both spacing intervals may or may not be based on an integer multiple of
the well spacing
present int eh hexagonal pattern observed in the non-fiducial regions. As in
other examples
described herein, the alternated linear features 344 with blanks regions 408A
and 408B
CA 03224034 2023- 12-22
WO 2022/269033
PCT/EP2022/067335
provide regions within the linear fiducial 384 in which the effective fiducial
pitch is above
the Shannon-Nyquist sampling limit for the optical system.
[00134] This written description uses examples to disclose the invention,
including the
best mode, and also to enable any person skilled in the art to practice the
invention,
including making and using any devices or systems and performing any
incorporated
methods. The patentable scope of the invention is defined by the claims, and
may include
other examples that occur to those skilled in the art. Such other examples are
intended to
be within the scope of the claims if they have structural elements that do not
differ from
the literal language of the claims, or if they include equivalent structural
elements with
insubstantial differences from the literal languages of the claims
51
CA 03224034 2023- 12-22