Note: Descriptions are shown in the official language in which they were submitted.
Ocular injection assembly, injection device and method of using the same
Technical field
The present invention relates to the field of ophthalmic therapeutics and
specifically to ocular
injection assemblies, injection devices and methods of use.
Background art
Eye is a very complex organ. As a visual organ, an eye consists of three
parts: eyeball, visual
pathway and accessory structures, with the eyeball and the visual pathway
accomplishing the
visual function. Eyeball is nearly spherical and consists of an eyeball wall
and contents therein,
which has a very complex structure. The wall of the eyeball contains three
layers, the outer layer
is the fibrous tunic, the middle layer is the uvea, and the inner layer is the
retina. Fibrous tunic is
mainly composed of fibrous tissue and forms the outer membrane of the eyeball,
mainly cornea
and sclera, of which the sclera accounted for 5/6. Sclera has tough texture
composed of densely
interwoven fibers. The sclera has different thickness in different parts of
the eyeball and is
individually specific among children, adults, and the elderly. The arrangement
of the collagen fiber
bundles within the sclera varies in different parts of the eye. The uvea
situated between the sclera
and the retina contains three contiguous parts from anterior to posterior: the
iris, the ciliary body,
and the choroid. The choroid consists of fibrous tissue, small blood vessels,
and capillaries, and is
a highly vascularized and pigmented tissue containing a meshwork of large
branching small
arterioles and arterioles as well as venous channels, the thickness of which
varies with changes in
vascular filling and decreases with age.
The eye, as an exposed organ, is susceptible to a variety of damages such as
conjunctival or
corneal injury caused by pathogens. The conjunctiva is a thin, transparent
mucous membrane rich
in blood vessels that covers the inner surface of the eyelid and the front of
the eyeball, helping to
prevent damage to the eye from foreign bodies and infections. However, the
conjunctiva itself is
not only very sensitive and prone to irritation by chemicals or allergic
substances, or infected by
viruses or bacteria to cause conjunctivitis, but also liable to guide a large
amount of medication
(>60%) into the system circulation through its rich blood vessels, causing
tissue toxicity and other
complications.
Currently, fundus diseases are one of the leading causes of irreparable visual
impairment or
loss. These fundus diseases include neovascular age-related macular
degeneration, diabetic
retinopathy, diabetic macular edema, central retinal vein occlusion and branch
retinal vein
occlusion. Ocular drug delivery devices play a critical role in the treatment
of ocular diseases
because tissue barriers (e.g., cornea, conjunctiva, blood-aqueous barrier, and
blood-retinal barrier)
limit drug delivery to the fundus. Conventional delivery methods such as
ocular surface
CA 03224603 2023- 12- 29 1
administration and intravitreal injection (IVT) are convenient but difficult
to deliver drugs to the
fundus efficiently and safely. Moreover, ocular injections that are performed
in an incorrect
position or at an incorrect angle may lead to complications, such as
intraocular hemorrhage, or
retinal damage, resulting in cataracts, retinal detachment, and so on.
Infection of other tissues in
the eye may be caused if leakage of the drug occurs.
Delivery of drugs to the eye has always been a challenge due to the unique
structure of the
eye. Delivery to the suprachoroidal space is particularly difficult due to the
structure of the
suprachoroidal space. The suprachoroidal space exists without a significant
gap in the absence of
fluid and/or tissue separation and is a potential space between the sclera and
choroid. When fluid
or other material accumulates between the choroid and the sclera, the
suprachoroidal space may
become visible in that region. Thus, by delivering, injecting and/or infusing
a drug concoction into
the suprachoroidal space, a fluid accumulation is intentionally created to
further create and/or
expand the separation of the choroid from the sclera, thereby creating a
suprachoroidal space.
Localized choroidal hemorrhage and retinal damage may occur during injection
of drugs
administered into the suprachoroidal space. Complications such as
endophthalmitis, scleral
dilatation, wound abscesses, and occasionally high intraocular pressure and
cataracts may also
occur as a result of injection.
In the prior art, devices for injecting drugs into the choroidal cavity are
disclosed, but all of
them produce side effects of damage to the eye. For example, CN112165923A
discloses a device
for injecting substance into the interlayer of a bodily tissue or an organ,
wherein the injection
needle comprises two layers of needles including an outer layer of a short 27G
stainless steel
needle, and a blunt separating needle inside thereof First, the 27G tunneling
needle obliquely
pierces into the sclera from the corneal rim, the direction of pierce being
kept parallel to the surface
of the eyeball; the control button on the syringe is pressed to stretch out
the separating needle to
strip the tissue and form an intra-scleral channel connecting to the
suprachoroidal space; and then
the obtuse separating needle is withdrawn by the control knob on the syringe;
and finally, the
medicinal solution inside the syringe is injected into the suprachoroidal
space to complete the
injection through the passageway punched out by the tunneling needle. The
device is more
damaging to the eye, prone to cause bleeding, and the piercing angle is very
critical and difficult
to precisely control to accurately separate the channel to the suprachoroidal
space, and prone to
puncture into the vitreous body or puncture the conjunctiva. A therapeutic
agent delivery device is
disclosed in CN107223042B. In performing an ocular injection, the sclera is
first incised behind
the corneal limbus. A cannula with a curved opening is inserted between the
sclera and the choroid
and advanced to the suprachoroidal chamber, and then an injection needle is
pushed into the
subretina to complete the injection. However, this delivery device requires
incision of the sclera
and pushing the cannula through the eye wall, which is damaging and causes
extensive scraping
CA 03224603 2023- 12- 29 2
of the choroid and sclera. It is also difficult to control the depth of the
needle, as it is easy to
penetrate the retina.
In order to solve the above problems, there is a need to provide a simple and
convenient
device that can effectively deliver the drug to the target tissues in the eye
and also solve the
problem of injection-induced eye injuries.
Summary of the invention
The present invention provides an ocular injection assembly which presses on
the
conjunctival tissue around the injection point through a clamping port to form
a bulge facing the
clamping port, which is capable of both effectively delivering a drug to a
target tissue in the eye
and solving the problem of injection-induced ocular injury.
In order to achieve the above purposes, the present invention comprises:
An ocular injection assembly comprising a sleeve and a needle, the needle
being capable of
being set within the sleeve, the sleeve having a clamping port at the end
thereof, the tip portion of
the needle being capable of passing through the gripping port. When the ocular
injection assembly
is used, the clamping port of the sleeve is contacted with and pressed against
ocular tissues at the
site of the injection, so as to cause the ocular tissues at the site of the
injection to protrude into the
sleeve.
Preferably, the clamping port has a minimum inner diameter of 1 mm to 3 mm.
The needle
is threaded into the injection point for injection, and the minimum inner
diameter of the clamping
port is the length of a line section passing through the injection point with
both ends thereof inside
the clamping port. The injection point is located in the middle of the line
section. The experimental
results showed that at an inner diameter of 1-3 mm, the area of ocular tissues
inside the clamping
port was moderate, and the ocular tissues were able to form a clear bump
inside the clamping port,
thereby facilitating the injection operation.
Preferably, the clamping port has an annular end face. The periphery of the
clamping port
forms an annular end surface capable of forming an annular face contact with
the ocular tissue
surrounding the injection site.
Preferably, the annular end face is planar and the needle is perpendicular to
the annular end
face. That is, the axial direction of the needle is perpendicular to the plane
in which the annular
end face is located.
Preferably, the clamping port is of round, oval or polygonal shape. Different
shapes of the
clamping port, such as round, oval, hexagonal, octagonal, square or irregular
shape, can realize the
pressure of the clamping port on the conjunctival tissue. When the clamping
port is round, the
annular end surface is circular, which is not only more conducive to avoid
damage to the ocular
tissues, but also more conducive to the recovery of the ocular tissues during
the injection process.
CA 03224603 2023- 12- 29 3
Preferably, the end of the sleeve is a shrinking section, and the clamping
port is located at
the end of the shrinking section. The size of the cross-section of the
shrinking section gradually
increases in a direction away from the clamping port, which facilitates
collecting and storing the
refluxed liquid in the shrinking section in the event of reflux.
Preferably, the side wall of the sleeve is provided with an observation window
made of
transparent material, or the sleeve is a transparent material member.
Providing an observation
window or adopting a sleeve made of transparent material facilitates observing
the return flow of
the drug solution and quickly assessing whether the injection is successful.
Preferably, the observation window or the transparent sleeve is provided with
a volume scale
line set in the axial direction along the sleeve which enables real-time
observation of the reflux of
the medicinal fluid, and when the warning limit is exceeded, can conveniently
remind the operator
of the injection failure and thus stop the injection.
Preferably, the length of the edge surface of the needle tip portion is less
than 1100
micrometers, preferably less than 900 micrometers, further preferably less
than 700 micrometers,
more preferably < 550 micrometers, and most preferably 250-550 micrometers.
Preferably, when the needle is mated with the sleeve, the length of the needle
tip portion
outside the clamping port portion is 500-2000 micrometers, preferably 700-1350
micrometers,
more preferably 700-1100 micrometers.
Preferably, in the case of a suprachoroidal space injection, the length of the
tip portion of the
needle outside the clamping portion is 500-1100 micrometers.
Preferably, the sleeve is provided with a canal and the needle is movably
coupled to the canal.
The needle is coupled to the sleeve via the canal, which facilitates the
needle to maintain a relative
position to the clamping port of the sleeve when piercing to reach the
injection site.
Preferably, a needle hub is attached to the needle at an end opposite to the
tip portion, and
an adjusting assembly is provided between the sleeve and the needle hub for
adjusting the size of
the length of the tip portion of the needle outside the clamping port portion,
facilitating the
adjustment of the length of the tip of the needle outside the clamping port,
and controlling the
depth of the puncture, so as to be applicable to different patients, or to
puncture different locations
of the eye, such as applying to the eye wall tissue for patients with abnormal
sclera thickness.
Preferably, the needle hub is coupled to the sleeve via the adjustment
assembly. Setting the
needle hub facilitates coupling with the syringe. Setting the adjustment
assembly between the
needle hub and the sleeve makes it less likely to be affected by other
components such as the
syringe when adjusting the length of the needle.
Preferably, the adjusting assembly comprises an externally threaded section
provided on the
needle hub, and an internally threaded section provided on a corresponding
sleeve, the externally
threaded section and internally threaded section being connectable to each
other. The needle hub
CA 03224603 2023- 12- 29 4
and the sleeve are rotatable relative to each other through the mating of the
externally threaded
section and the internally threaded section to realize the adjustment of the
length of the needle.
Preferably, the needle hub comprises a guide tube provided with the externally
threaded
section. The guide tube is connected to the front end of the needle hub, and
the guide tube set is
connected to the needle for reinforcing the strength of the needle and
avoiding bending or shaking
of the needle during puncture process.
Preferably, the thread pitch is 50-200 microns, more preferably 50-150
microns.
The adjustment assembly may also be as follows.
A needle hub is connected to the rear end of the sleeve and is provided with
an adjusting
assembly comprising a telescopic rod assembly and a driving mechanism. The
telescopic rod
assembly comprises an outer tube and an inner tube, one end of the outer tube
is connected to the
rear end of the needle hub and the other end is sheathed on the inner tube,
and the other end of the
inner tube is connected to the rear end of the needle. The outer tube, the
inner tube, and the needle
are connected in turn. Alternatively, the rear end of the needle passes
through the inner tube and
the outer tube in turn and extends outside the outer tube. A driving mechanism
is connected to the
inner tube for driving the inner tube to move axially relative to the outer
tube, and the inner tube
is capable of driving the needle.
The rear end of the needle passing sequentially through the inner tube and the
outer tube and
outside the outer tube means that the needle is fixed to the inner tube and
the rear end of the needle
extends outside the inner tube toward the outer tube and to the other end of
the outer tube.
Preferably, the needle is provided with a guide tube over the outer sleeve of
the needle, the
two ends of the needle are exposed from the guide tube, the front end of the
needle hub is set
outside the guide tube, and the guide tube and the needle hub are capable of
sliding relative to each
other. By setting the guide tube on the needle, the strength of the needle is
strengthened, and the
shaking and deformation of the needle during the puncturing of the ocular
tissues are reduced.
Preferably, the driving mechanism includes a driving housing, a rack guide, a
driving gear
and an operating lever. The drive housing is connected to the outer tube at
one end and slidingly
connected to the inner tube at the other end. The rack guide is connected to
the inner tube in an
axial direction. The drive gear is hingedly connected to the drive housing and
is engagedly
connected to the rack guide. One end of the operating lever is connected to
the drive gear, and the
other end passes through the through-hole of the drive housing and the through-
hole of the needle
hub to the outside of the needle hub. By rotating the operating lever in the
driving mechanism to
rotate the driving gear, the driving gear moves with the rack guide, and the
inner tube moves with
the rack guide, thereby driving the needle to move in the axial direction for
adjustment of the
position of the needle.
The adjustment assembly may also be used in conjunction with the release
assembly.
CA 03224603 2023- 12- 29 5
Preferably, a needle hub is connected to the rear end of the sleeve; a release
assembly
comprising a resilient member and a first stop member is provided between the
sleeve and the
needle hub; the sleeve is connected to the needle hub via the resilient
member; one end of the first
stop member is detachably connected to the sleeve and/or the other end of the
first stop member is
detachably connected to the needle hub. When the first stop member is
connected to the sleeve and
the needle hub, the resilient member is in stretching status and is capable of
providing an axial
tensile force to stretch the resilient member to increase the distance between
the needle hub and
the sleeve for inserting the first stop member into place to maintain the
distance between the needle
hub and the sleeve. At this point the resilient member is in the stretched
state and the front end of
the needle is retracted into the sleeve. During use, the clamping port is kept
in contact with the
ocular tissue, and after the first stop member is removed, the needle hub is
moved forward so that
the tip of the needle exceeds the clamping port and penetrates into the ocular
tissue.
The resilient member may be a reset spring, a compressed gas container, or a
container
comprising a propellant; and the first stop member may be a tab, a slot, a
ring, a slot, or a pawl.
Preferably, the adjustment assembly may be provided between the sleeve and the
needle hub,
the adjustment assembly comprising a telescopic rod assembly and a driving
mechanism. The
telescopic rod assembly comprises an outer tube and an inner tube, one end of
the outer tube being
connected to the front end of the needle hub, the other end being fit over the
inner tube, and the
other end of the inner tube being connected to the rear end of the needle. The
outer tube, the inner
tube and the needle are connected in turn, or the rear end of the needle
passes through the inner
tube and the outer tube in turn, and exceeds the outer tube. The driving
mechanism is connected
to the inner tube for driving the inner tube to move relative to the outer
tube in an axial direction,
and the inner tube is capable of driving the needle to move. In this
embodiment, the structure of
the adjusting assembly may be with the same as that in the previous
embodiment, or the adjusting
assembly may be provided outside of the needle hub and located in the middle
of the resilient
member.
Preferably, the driving mechanism comprises a driving housing, a rack guide, a
driving gear
and an operating lever; the driving housing is connected to the outer tube at
one end and is slidingly
connected to the inner tube at the other end; the rack guide is connected to
the inner tube in the
axial direction; the driving gear is hingedly attached to the driving housing
and is meshing with
the rack guide; one end of the operating lever is connected to the driving
gear, and the other end
passes through the through hole of the driving housing and the through hole of
the needle hub to
the outside of the needle hub. Turning the operating lever can rotate the
drive gear. The operating
lever and the drive gear are removably connected. When the operating lever is
separated from the
needle hub, the first stop member can be driven to be separated from the
sleeve and/or the needle
hub. By setting the operation lever outside the needle hub, the operation
lever can be removed by
CA 03224603 2023- 12- 29 6
pulling the operation lever out after adjusting the length of the needle using
the operation lever to
facilitate observation. Using the operation lever to drives the first stop
member to separate from
the sleeve and the needle hub facilitates one-handed operation by the
operator.
Preferably, when the clamping port of the sleeve contacts and presses on the
ocular tissue,
the clamping port is sealed and the ocular tissue forms a bulge towards the
inside of the sleeve.
Preferably, when the clamping port is sealed, the chamber within the sleeve
forms a
hermetically sealed chamber. In the event of reflux, the refluxed medicament
is stowed in the
sealed chamber, and when the clamping port is separated from the ocular
tissues, it is difficult for
the medicament to leak out of the sealed chamber, so as to avoid the
medicament dispersing to the
ocular tissues, which is advantageous for accurately determining the volume of
the refluxed liquid
and estimating the amount of liquid injected.
The present invention also provides an ocular injection device comprising a
syringe, and an
ocular injection assembly capable of being mounted on the syringe as described
above.
Preferably, the syringe comprises a medication container storing the
medication and fitting
with a needle, and a pushrod being slidable within the medication container.
Preferably, a thrust assembly is provided between the end of the pushrod and
the medication
container for generating a constant thrust force on the pushrod.
The thrust assembly may be a spring-loaded ball mechanism, a spring-loaded
pin, a cylinder,
or a container containing propellant.
Preferably, the constant thrust generated by the thrust assembly has a
threshold value less
than 6N. The constant thrust generated by the thrust assembly means that when
the thrust force
applied to the thrust assembly is less than the threshold value, the thrust
assembly cannot be pushed;
and when the thrust force is greater than the threshold value, the thrust
assembly can counteract
the thrust force exceeding threshold value so that the thrust force is
maintained at the threshold
value. For example, when the threshold value is 5N, the thrust assembly cannot
be pushed with a
force of 4N, and when the thrust force is 7N, the thrust assembly will
maintain a thrust force of
6N.
Preferably, the injection device further comprises a second stop member
provided between
the pushrod and the medication container for limiting the pushing stroke of
the pushrod.
The second stop member may be a tab, slot, ring, slot or pawl.
Preferably, the injection device further comprises a needle protection cap
cooperating with
the needle.
The present invention also provides a method of using the ocular injection
device described
above which uses the ocular injection device to contact and press the clamping
port of the sleeve
against the ocular tissue at the injection site to form a bulge into the
sleeve.
Preferably, after the clamping port is contacted and pressed against the
ocular tissue at the
CA 03224603 2023- 12- 29 7
injection site to form a bulge of the ocular tissue at the injection site into
the sleeve, the tip portion
of the needle is then made to puncture the ocular tissue at the injection site
so that the distal end
of the needle reaches the target ocular tissue at the injection site.
Preferably, while the clamping port contacts and presses the ocular tissue at
the injection site
to form a bulge of the ocular tissue at the injection site into the sleeve,
the tip portion of the needle
is made to puncture the ocular tissue at the injection site so that the distal
end of the needle reaches
the target ocular tissue at the injection site.
Preferably, the length of the tip portion of the needle that is exposed to the
clamping port is
adjusted using the adjustment assembly in the injection device described above
according to the
thickness of the ocular tissue at the injection site before the clamping port
contacts the ocular tissue
at the injection site of compression.
Preferably, one side of the clamping port is first brought into contact with
the ocular surface,
then is flipped with the contact part as a pivot point to allow the needle to
penetrate the ocular
tissue at the injection site, and continued to be flipped to allow the other
side of the clamping port
to contact the ocular surface, thus making ocular tissue form a bulge into the
sleeve. The ocular
surface may be an ocular surface, such as conjunctival tissue or other ocular
tissue.
Preferably, the tissue thickness of the eye at the injection site is measured
using one or more
selected from optical coherence tomography (OCT), OCT-enhanced deep imaging
(EDI-OCT),
swept frequency OCT (SS-OCT), or ultrasound biomicroscopy (UBM).
Preferably, at least a portion of the substance in the medication container is
delivered into
the target tissue of the eye via the needle by pushing the pushrod.
Preferably, a volume of the medicament reflux in the sleeve is observed using
an observation
window, or an injection device with a transparent sleeve while pushing the
pushrod to deliver at
least a portion of the substance in the medicament container to the target
tissue of the eye via the
distal end of the needle observation window.
Preferably, as the ocular injection assembly is pressed to puncture the ocular
tissue, the thrust
assembly, in conjunction with the syringe, applies a force to the clamping
port at the distal end of
the sleeve, causing the ocular tissue surrounding the injection site to bulge
into the sleeve.
Preferably, the thrust assembly exerts no more than 0.4N-6N, more preferably
1N-6N, more
preferably 1-3N, to the clamping port at the distal end of the sleeve with the
syringe cooperating
with the ocular injection assembly when the ocular injection assembly is
pressed to puncture the
ocular tissue.
Preferably, the injection pressure within the medication container does not
exceed 500 kPa
when pushing the pushrod to deliver at least a portion of the substance of the
medication container
to the target tissue of the eye via the distal end of the needle.
Preferably, the intraocular pressure rises no more than 30 mmHg, more
preferably no more
CA 03224603 2023- 12- 29 8
than 20 mmHg, more preferably no more than 10 mmHg, when the pushrod is pushed
to deliver
at least a portion of the substance of the medication container to the target
tissue of the eye via the
distal end of the needle.
Alternatively, the present invention also provides an ocular injection device
comprising a
syringe 1, a needle 2, and a sleeve 3, with a clamping port 31 provided at the
distal end of the
sleeve 3, wherein the clamping port 31 is tightly fitted to the ocular tissues
when injecting the eye
to enable the ocular tissues to form a bulge towards the sleeve 1.
The syringe 1 further comprises a medication container 11, a pushrod 12
coupled to the
medication container 11, and a needle 2 with its proximal end connected to the
medication
container 11, the distal end of the pushrod 12 being disposed within the
medication container 11,
the proximal end portion of the pushrod 12 being subjected to a force to move
the distal end of the
pushrod 12 within the medication container 11 for delivering at least a
portion of the substance
therein via the needle 2; a sleeve 3 being provided with a canal 32 and
movably coupled to the
proximal end of the needle 2, the distal end of the needle 2 being configured
to pierce the eye
tissue through the canal 32 of the sleeve 3 and the clamping port 31.
When the ocular injection device performs a clamping action, the sleeve 3
forms a bottom-
sealed chamber 33 with the clamped ocular tissue.
The shape of the clamping port 31 may be circular, hexagonal, octagonal,
square or irregular,
and preferably circular. The minimum inner diameter of the clamping port 31 is
0.5 mm- 10 mm,
preferably 1-6 mm, and more preferably 1 mm- 3 mm;
The length of the blade at the distal end of the needle 2 is less than 1100
micrometers,
preferably less than 900 micrometers, further preferably less than 700
micrometers, more
preferably < 550 micrometers, and most preferably 250-550 micrometers.
The penetration force at the distal end of the needle 2 is <0.7N, more
preferably <0.5N.
The force applied to the ocular tissue by the ocular injection device is
capable of inducing
an elastic deformation of the ocular tissue, preferably from 0.4N to 10N, more
preferably from 2N
to 6N, and most preferably from 3N to 5N.
The needle 2 of the ocular injection device comprises a needle hub 21 with its
proximal end
coupled to the distal end of the medication container 11 and the distal end
coupled to the proximal
end of the needle 2, and an adjusting assembly 4 is provided between the
proximal end and the
distal end of the needle hub 21, which cooperates with the sleeve 3 in
adjusting a length of the
needle 2 that extends outside the clamping port 31.
An adjustment release member 5 is provided between the needle hub 21 and the
sleeve
3adjustment release member configured for adjusting the length of the needle 2
extends outside
the clamping port 31 and for pushing the needle 2 piercing into the ocular
tissues via the distal
clamping port 31 during puncturing action by the ocular injection device.
CA 03224603 2023- 12- 29 9
The injection device may comprise a thrust assembly 6 configured to apply a
constant thrust
force on the proximal end portion of the push rod 12.
The injection device may further comprise a second stop member 7 configured to
selectively
restrict movement of the push rod 12 with respect to the medication container
11, as well as to
release the thrust assembly 6 when performing a drug injection.
The sleeve 3 is a transparent sleeve configured for observing the length of
the needle 2
extending outside the clamping port 31, or\and configured to observe the
amount of medicament
reflux to the chamber 33.
Alternatively, the sleeve 3 may be provided with an observation window 34
configured for
observing the length of the needle 2 outside the clamping port 31, or \and
configured for observing
the amount of medicament reflux in the chamber 33.
In summary, the following beneficial effects may be brought about by the above
technical
solutions:
1. By setting the clamping port as an annular end surface with an appropriate
area of ocular
tissues therein, a clear bulge can be formed inside the clamping port so that
the medical solution
can be delivered to the target site smoothly while effectively preventing
reflux and spreading of
the drug solution under the conjunctiva.
2. Provision of the adjusting assembly facilitates the adjustment of the
length of the tip of
the needle outside the clamping port and controlling the effective length of
the tip of the needle,
so that the effective dose of the drug is accurately delivered to the target
tissue via the needle. The
injection assemble of the present invention can be applied to different
patients and can pierce
different locations of the eye, such as being applied to the eyeball wall
tissue for patients with
abnormal scleral thickness.
3. The operation lever in the adjustment assembly is detachably connected, and
the first
stopper in the release assembly can be driven out of the release assembly,
which facilitates one-
handed operation by the operator and makes the operation more convenient.
4. The operation becomes more convenient by using the ocular injection
assembly in
conjunction with a syringe.
5. In the method of using the ocular injection device of the present
invention, different
injection assembly can be selected according to different patient conditions.
The needle puncture
is conducted after the peripheral tissues of the injection point form a bulge
inside the sleeve, which
facilitates the needle to reach the predetermined position and ensures the
injection effect.
6. The leakage of drug from the piercing site can be effectively prevented,
thereby improving
the success rate of one-time puncture at a predetermined site. Even if reflux
occurs due to operation
or other reasons, the refluxed medication will go back to the sleeve through
the clamping port,
effectively avoiding the diffusion of the refluxed medication on the ocular
surface as a result of
CA 03224603 2023- 12- 29 10
injection failure.
7. The chamber inside the sleeve forms a sealed chamber. In case of reflux,
the refluxed
liquid is collected and stored in the sealed chamber. When separating the
clamping port from the
ocular tissue, the liquid will not easily leak out of the sealed chamber,
preventing the liquid from
dispersing onto the ocular tissue. Combined with the use of a transparent
window and the setting
of a scale, it is more conducive to accurately judging the reflux situation,
as well as observing the
volume of the refluxed liquid and estimating the amount of liquid injected.
Brief description of drawings
FIG. 1 shows a cross-section of a human eye.
FIG. 2A is a schematic diagram of the subconjunctiva without fluid, and FIG.
2B is a
schematic diagram of the subconjunctiva with fluid.
FIG. 3A is a diagram showing the effect of no fluid in the suprachoroidal
space, and FIG. 3B
is a diagram showing the effect of no fluid in the suprachoroidal space.
FIGS. 4 to 18 are the accompanying drawings in Embodiments 1 to 9, in which:
FIG. 4 is a schematic diagram of the structure of an ocular injection device.
FIG. 5A is a front view of the sleeve shown in FIG. 4, FIG. 5B shows a top
view thereof, and
FIG. 5C shows an elevation view thereof
FIG. 6 is a schematic front view showing the ocular tissue structure in the
bulge formed by
the ocular syringe of FIG. 4 and the ocular tissue.
FIG. 7A is a front view of the blade at the distal end of the needle shown in
FIG. 4, and FIG.
7B shows a side view thereof
FIG. 8 is a schematic structural diagram of an ocular injection device
provided with a bottom
seal chamber and an observation window.
FIG. 9A is a schematic diagram of the sleeve shown in FIG. 8 with an
observation window
and a bottom sealing chamber, and FIG. 9B shows a schematic diagram of the
sleeve shown in
FIG. 8 with a volume scale.
FIG. 10 is a schematic diagram of the structure of an ocular injection device
comprising a
needle hub.
FIG. 11 is a schematic structural diagram of an ocular injection device
provided with a
threaded adjustment assembly.
FIG. 12A is a schematic diagram of a sleeve structure with internal threads,
FIG. 12B is a
schematic diagram of a needle hub structure provided with external threads,
and FIG. 12C is a
schematic diagram of a distal portion of a needle with a volume scale.
FIG. 13 is a schematic structural diagram of an ocular injection device
provided with a
telescoping rod adjustment assembly.
CA 03224603 2023- 12- 29 11
FIG. 14A is a schematic diagram of the structure of a needle provided with a
telescoping rod
adjustment assembly, FIG. 14B is a schematic diagram of the structure of the
telescoping rod
adjustment assembly, FIG. 14C is a schematic diagram of the internal structure
of the telescoping
rod adjusting mechanism, and FIG. 14D is a schematic diagram of the structure
of a needle hub
sleeve assembly with a volumetric scale.
FIG. 15 is a schematic structural diagram of an ocular injection device having
an adjustable
release assembly.
FIG. 16A is a schematic diagram of the structure of a needle having an
adjustable release
assembly, and FIG. 16B is a schematic diagram of the structure of a first stop
member.
FIG. 17 is a schematic diagram of the structure of an ocular injection device
comprising a
thrust assembly.
FIG. 18 is a schematic diagram of the structure of the second stop member.
Reference signs in FIGS. 1 to 18 are explained as follows:
el-eye e2-lens e3-cornea e4-sclera
e5-iris e6-anterior chamber e7-posterior chamber
e8-corneal rim e9-conjunctiva e 1 0-choroid
e11-retina e12-vitreous body e13-ciliary body
e14-suprachoroidal space e15-fluid layer;
1-syringe 11-medication container 12-pushrod 12
2-needle 21-needle hub 211-channel
212-flange 213-connecting portion 214-ribs
3-sleeve 31-clamping port 32-canal
33-chamber 34-observation window 35-volume scale
36-limit Hole 4-adjustment assembly 41-threads
42-telescoping rod assembly 421-outer tube
422-inner tube 423-guideway 424-drive mechanism
43-first operating lever 5-adjustment release
member
51-telescopic assembly 52-release assembly
53-second operating lever 54-first stop member
6-thrust assembly 7-second stop member 8-needle protection cap
FIG. 19 is a schematic diagram of the structure of the ocular injection
assembly of
Embodiment 10.
FIG. 20 is a schematic diagram showing assembling of the ocular injection
assembly of
Embodiment 10.
FIG. 21 is a schematic front view of the ocular injection assembly of
Embodiment 10.
CA 03224603 2023- 12- 29 12
FIG. 22 is a sectional view at A-A of FIG. 21.
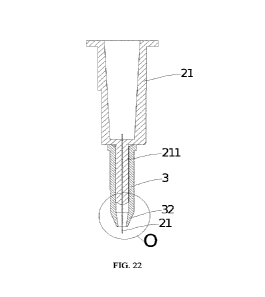
FIG. 23 is an enlarged schematic view at circle 0 of FIG. 22FIG., in which d
is the length of
the tip portion of the needle outside the portion of the clamping port.
FIG. 24 is a schematic diagram of a circular clamping port with an annular end
face.
FIG. 25 is a schematic view of an oval shaped clamping port with an annular
end face.
FIG. 26 is a schematic diagram of a polygonal clamping port with an annular
end face.
FIG. 27 is a schematic diagram of the clamping port in relation to the ocular
surface when
the ocular injection assembly is in use.
FIG. 28 is a schematic diagram of the clamping port in relation to the ocular
surface when
reflux occurs during use of the ocular injection assembly.
FIG. 29 is a schematic diagram of a sectional structure of the needle hub and
needle of the
injection assembly of Embodiment 11.
FIG. 30 is a schematic diagram of a sectional structure of the sleeve of the
injection assembly
of Embodiment 11.
FIG. 31 is a schematic diagram of a sectional structure of the sleeve of an
alternative
embodiment of the injection assembly of Embodiment 11.
FIG. 32 is a schematic diagram of a sectional structure of the injection
assembly of
Embodiment 12.
FIG. 33 is a schematic diagram of a sectional structure of another state of
the injection
assembly of Embodiment 12.
FIG. 34 is a schematic diagram of the structure of the adjustment assembly of
the injection
assembly of Embodiment 12.
FIG. 35 is a schematic diagram of a sectional structure of an adjustment
assembly of an
alternative embodiment of the injection assembly of Embodiment 12.
FIG. 36 is a schematic view of the sectional structure at B-B of FIG. 35.
FIG. 37 is a schematic diagram of a sectional structure of an alternative
embodiment of the
injection assembly of Embodiment 12.
FIG. 38 is a schematic diagram of a sectional structure of the injection
assembly of
Embodiment 13.
FIG. 39 is a schematic diagram of a sectional structure of another state of
the injection
assembly of Embodiment 13.
FIG. 40 is a schematic view of the structure of the first stop member of
Embodiment 13.
FIG. 41 is a schematic diagram of the structure of the injection device of
Embodiment 14.
FIG. 42 is an exploded view schematic of the injection device of Embodiment
14.
FIG. 43 is a schematic diagram of the structure of the injection device of
Embodiment 15.
FIG. 44 is a picture of a section of fundus tissue from Test Example 1.
CA 03224603 2023- 12- 29 13
FIG. 45 shows the ICGA contrast results and OCT scan results in Test Example
3.
FIG. 46 shows the results of the fundus fluorescence assay in Test Example 3.
FIG. 47 shows the results of the expression of the injected reagent in retinal
epithelial cells
and photoreceptor cells in Test Example 3.
FIG. 48 shows ICGA contrast results and OCT scan results in Test Example 4.
FIG. 49 shows ICGA contrast results and OCT scan results in Test Example 5.
FIG. 50 shows ICGA contrast results and OCT scan results in Test Example 6.
FIG. 51 shows the OCT scan results in Test Example 7.
FIG. 52 shows the results of the expression of the injected reagent in retinal
epithelial cells,
photoreceptor cells in Test example 7.
FIG. 53 shows the results of the OCT scan in Test example 8.
FIG. 54 shows the results of the expression of the injected reagent in retinal
epithelial cells,
photoreceptor cells in Test example 8.
FIG. 55 is a picture of a section of fundus tissue from Comparative example 2.
Reference signs in FIGS. 19 to 55 are explained as follows:
101-internally threaded section 102-externally
threaded section
200-bulge 2-needle 21-needle hub
211-guide tube 3-sleeve 31-clamping port
32-retracting section 33-annular end surface
34-chamber 35-observation window 351-volume graduations
4-adjustment assembly 41-drive mechanism 411-drive housing
412-drive gear 413-rack guide 414-operating
lever
42-retractable rod assembly
421-outer tube 422-inner tube
5-release assembly 51-resilient member 52-first stop member
6-thrust assembly 7-second stop member 8-needle
protection cap
Detailed description
Embodiments of the present invention are described in detail below in
conjunction with the
accompanying drawings.
The injection site, needle gauge, blade length, blade penetration force,
constant thrust and
piercing application force of the thrust assembly as well as the needle gauge
are described below:
1. Injection site
Injection sites described herein include points in any area of supranasal,
infranasal,
supratemporal, infratemporal, etc., of the conjunctiva, between the iris rim
and the corneal rim,
approximately 3-9 mm from the corneal rim, approximately 4-8 mm from the
corneal rim,
CA 03224603 2023- 12- 29 14
approximately 4-7 mm from the corneal rim, approximately 6-8 mm from the
corneal rim,
approximately 7-8 mm from the corneal rim, approximately 4-5 mm from the
corneal rim. A point
that is approximately 3 mm from the corneal rim, or approximately 4mm, or
approximately 5mm,
or approximately 6mm, or about 7mm, or about 8mm may be elected.
2. Needle specifications
The specification of needles can be selected from commercially available
conventional
injection needles such as 28G, 30G, 31G, 32G, 33G, 34G needle, or customized
using conventional
process of manufacturing the injection needle.
Effective length of needle
Also named as the effective piercing length of the needle, which is the length
of the needle
extending out of the clamping port and is about 1400 microns or less, about
1300 microns or less,
about 1200 microns or less, about 1100 microns or less, about 1000 microns or
less, about 900
microns or less, about 800 microns or less, about 850 microns or less, about
700 microns or less,
about 650 microns or less, about 500 microns or less, about 450 microns or
less. In some
embodiments, the needle effective length may be about 700 micrometers. In
other embodiments,
the effective length of needle may be about 750 microns, or about 800 microns,
or about 850
microns, or about 900 microns, or about 950 microns, or about 1000 microns, or
about 1100
microns, or about 1350 microns;
3. Blade length
A straight-line distance from the proximal inner edge of the needle wall at
the distal outlet
of the needle to the distal edge of the outer needle wall at the distal outlet
of the needle, about 800
microns or less, about 700 microns or less, about 650 microns or less, about
600 microns or less,
about 550 microns or less, about 500 microns or less, about 450 microns or
less, about 400 microns
or less, about 350 microns or less, about 300 microns or less, about 250
microns or less. In some
embodiments, the length of the distal edge of the needle is about 550 microns.
In other
embodiments, the length of the distal edge surface of the needle is about 700
micrometers, or about
650 micrometers, about 600 micrometers, about 500 micrometers, about 450
micrometers, about
300 micrometers, about 250 micrometers.
4. Blade piercing force
The blade surface piercing force of the distal outlet port of the needle is
about 0.7N or less,
about 0.65N or less, about 0.5N or less, about 0.4N or less, about 0.3N or
less, in order to facilitate
defining arrival at a desired location (e.g., the suprachoroidal space and/or
vitreous body) within
the target tissue and to form a medicament delivery channel. In some
embodiments, the blade
piercing force may be about 0.5N, and in other embodiments, the blade
penetration force or may
be about 0.7N, or about 0.65 N, about 0.4N, about 0.3N.
5. Needle specifications
CA 03224603 2023- 12- 29 15
Needle Gauge: The distal end of the needle is typically constructed to be
sharp, beveled
cutting, or other forms capable of piercing the ocular surface (e.g., sclera).
The needle employed
may be of any suitable gauge, e.g., about 25 G, about 26 G, about 27 G, about
28 G, about 29 G,
about 30 G, about 31 G, about 32 G, about 33 G, about 34 G, about 35 G, about
36 G. The needle
wall may be of any suitable thickness. For example, in addition to the regular
wall thickness (RW),
the wall of the needle may be designed as thin wall (TW), super/ultra thin
wall (XTW/UTW), or
super thin wall (XXTW), which are well known to those skilled in the art. For
example, the needle
may be a fine gauge cannula or needle. In some embodiments, the needle may
have a gauge
between about 25G and about 36G. In other embodiments, the catheter may have a
gauge between
about 27G and about 35G. In additional embodiments, the needle may have a
gauge between about
30G and about 33G.
Embodiments described herein relate to systems and devices for delivering a
fluid (e.g., drug)
into eye tissue. Additionally, the above embodiments relate to systems,
devices, and methods that
help the piercing of a delivery member (e.g., a needle) into the eye at a
predetermined point of
injection and/or help the effective metered injection of a drug into a target
ocular tissue. The above
embodiments also relate to systems, devices, and methods for avoiding the
formation of a
subconjunctival leakage channel around a delivery member (e.g., a needle) to
avoid conjunctival
and scleral gap diffusion during puncture to prevent diffusion of a substance
and/or ocular fluid
under the conjunctiva. The above embodiments also relate to systems, devices,
and methods for
forming a sealed chamber around a delivery member (e.g., a needle) during
puncture to prevent
diffusion of substances and/or ocular fluids around the ocular surface. The
above embodiments
also relate to systems, devices, and methods that are transparently structured
or provided with an
observation window around the sleeve to allow the operator to quickly and
visually observe the
needle length adjustment or the reflux of the substance and/or ocular fluid.
The "syringe" of the present invention is a conventional syringe assembly for
ophthalmic use,
comprising primarily a medication container 11, a pushrod 12 coupled to the
medication container
11, and a needle 2 with its proximal end connected to the medication container
11. The distal end
of pushrod 12 is placed within the medication container 11 and its proximal
end is subjected to a
force to move the distal end of the pushrod 12 within the medication container
11 to deliver at least
a portion of the substance in the medication container 11 via the needle 2.
Eye Structure
FIGS. 1-3 show a human eye for reference (of which FIGS. 2 and 3 are sectional
views).
Although the drawings of this specification relate to specific regions of the
eye, it is understood
by those skilled in the art that the drawings of specific regions of the eye
in the drawings of the
present application do not constitute the whole eye, but rather are intended
only as specific
embodiments applicable to the present invention so that those skilled in the
art may understand the
CA 03224603 2023- 12- 29 16
embodiments thereof Wherein eye el comprises an anterior section (the portion
of the eye anterior
to the lens e2 (and including the lens e2)) and a posterior section (the
portion of the eye posterior
to the lens e2). The anterior section is bounded by cornea e3 and lens e2, and
the posterior section
is bounded by sclera e4 and lens e2. The anterior section includes an anterior
chamber e6 between
iris e5 and cornea e3 and a posterior chamber e7 between lens e2 and iris e5.
Cornea e3 and sclera
e4 together form the corneal limbus at their junctional positions e8. the
exposed portion of sclera
e4 in the anterior section is the conjunctiva e9 to protect the eye. Below
sclera e4 are choroid el 0
and retina ell, collectively known as the retinochoroidal tissue. Vitreous
body el2 is between the
ciliary body el 3 and the retina el 1 . The loose connective tissue or
potential space between choroid
el 0 and sclera e4 is called suprachoroidal space e14. As shown in FIG. 2,
conjunctiva e9 is
specifically a soft, smooth, and elastic mucous membrane covering the inside
of the upper and
lower eyelids and in front of the eyeball, and is a transparent membrane
formed by a complex
columnar epithelium and a small amount of connective tissue, and a small
number of mucous
glands, which can secrete mucus and make the surface of the eyeball smoother.
Sclera e4 is divided
into three layers (surface layer, stroma, and brown-black plate layer). The
superficial layer is loose
connective tissue, and the stromal and brownish-black plate layers are
composed of dense
connective tissue and elastic fibers, rendering sclera e4 dense and tough.
During injection, if the
drug leaks, it tends to return to the subconjunctiva and diffuse to form the
fluid layer el5, as shown
in FIGS.FIGS. 2A and 2B.
As shown in FIG. 3A, there is not a distinct gap existing in suprachoroidal
space e14 in the
absence of fluid and/or tissue separation, which is a potential space between
sclera e4 and choroid
el O. As shown in FIG. 3B, in the presence of fluid e16 in the suprachoroidal
space e14, a distinct
gap appears with sclera e4 above and choroid el 0 below. Thus, suprachoroidal
space e14 can be
made visible in the region when fluid or other material accumulates between
choroid el0 and
sclera e4. Thus fluid accumulation is intentionally created by delivering,
injecting and/or
transfusing drug concoctions into the suprachoroidal space to further cause
and/or expand the
separation of the choroid from the sclera to form the suprachoroidal space.
The injection site location of either the ocular injection devices and/or
methods of the present
invention is in a region of 6-8 mm from the corneal rim, e.g., supranasal,
infranasal, supratemporal,
infratemporal, etc. An operator can confirm the injection site (7-8 mm from
the corneal limbus) by
measuring the distance using ophthalmic calipers. In this manner, the drug can
be introduced (e.g.,
via a needle) into the suprachoroidal space from the injection site and can be
pushed into the
suprachoroidal space away from the insertion site.
As shown in FIG. 7, the length of the distal edge of the needle in the present
invention is the
maximum distance between the opening in the inner wall of the needle and the
tip of the tip.
The term "distal" or "anterior" of the invention refers to the end near the
eye tissue, and
CA 03224603 2023- 12- 29 17
"proximal" or "posterior" refers to the end near the operator (e.g., physician
or nurse). "proximal"
or "posterior" refers to the end near the operator (e.g., a physician or
nurse).
Embodiments
Embodiment 1:
An ocular injection device of this embodiment is shown in FIGS. 4-6. The
ocular injection
device comprises a syringe 1, a needle 2, and a sleeve 3. the distal end of
the sleeve 3 is provided
with a clamping port 31. When injecting into the eye, the clamping port 31 is
tightly close-fitted
to the ocular tissue to enable the ocular tissue to form a bulge towards the
inside the sleeve 3.
The syringe 1 comprises a medication container 11 and a push rod 12. The
medication
container 11 is coupled to the proximal end of needle 2 coupled to push rod
12, and the distal end
of the push rod 12 is disposed within the medication container 11. A force is
applied on the
proximal end of push rod 12 to move the distal end of push rod 12 within the
medication container
11 to deliver at least a portion of the substance in the medication container
11 via the needle 2.
The sleeve 3 is provided with a canal 32 and is movably connected to the
proximal end of
needle 2. The distal end of needle 2 is configured to pierce the eye tissue
via clamping port 31
through canal 32.
The ocular injection method of the present embodiment comprises, inter alia:
A first step of measuring the distance with ophthalmic calipers to confirm the
injection site;
A second step of applying a force to a side of clamping port 31 of the sleeve
3 via the syringe
1 to form a pivot point on the conjunctival surface with the side of clamping
port 31;
A third step of pivoting the clamping port 31 around the pivot point toward
the other side of
clamping port 31 so that the distal end of needle 2 pierces the conjunctival
tissue at the injection
site;
A fourth step of continuously pivoting the clamping port 31 around the pivot
point to make
the other side of clamping port 31 attach the conjunctival surface, so that
clamping port 31 grips
the conjunctival tissue of the eye to form a bulge into sleeve 3, and the
distal end of needle 2 is
pressed into the target tissue of the eye at the injection site; and
A fifth step of administration to inject the drug to reach the site of
administration.
This embodiment also provides another method of ocular injection comprising:
A first step of measuring the distance with ophthalmic calipers to confirm the
injection site;
A second step of placing clamping port 31 in perpendicular to the ocular
surface of the
injection site;
A third step of piercing the distal end of needle 2 into the conjunctival
tissue at the injection
site and making clamping port 31 contact the ocular surface at the injection
site;
A fourth step of applying a force is applied to the ocular tissue at the
injection site via the
CA 03224603 2023- 12- 29 18
syringe 1 of the ocular injection device in conjunction with sleeve 3, so that
the conjunctival tissue
held by the clamping port 31 forms a bulge towards inside of sleeve 2, and the
distal end of the
needle 2 is pressed into the target ocular tissue at the injection site; and
A fifth step of administration to inject the drug to reach the site of
administration.
The needle 2 of this embodiment can either be selected from commercially
available
conventional ophthalmic needles such as 31G and 32G needles, or customized and
processed
through the conventional process of making injecting needles.
The needle and the sleeve can be produced in a matching fashion. Needle sleeve
assemblies
with different sizes can be customized according to the thickness between the
ocular surface of the
point of injection to the suprachoroidal space in which medication is to be
delivered. The different
sizes depend on the length of the portion of needle 2 outside the clamping
port 31, which may
range from 500 to 2,000 microns, such as 700 microns, 800 microns, 900
microns, 1,000 microns,
1,100 microns, 1,200 microns, 1,300 microns, and so forth, for selection by
the operator.
In order to avoid abrasion or laceration of the operator by the distal end of
needle 2, the ocular
injection device in this embodiment is further provided with a needle
protection cap 8 configured
to be movably connected to the sleeve 3 or the needle hub 21. Operator removes
the needle
protective cap 8 during use and install it on the needle after use for
recycling it together with other
components of the injection device.
A 700-micron needle sleeve assembly was selected to perform rabbit ocular
suprachoroidal
chamber injections according to the ocular injection method of the present
embodiment, and it was
found that the ocular injection device provided in the present embodiment not
only was successful
in a one-time puncture to deliver the drug to the target tissues of the eye,
but also significantly
reduced the subconjunctival leakage of the drug solution.
Through the study of the force applied to the ocular tissues by the ocular
injection device, it
is found that the force applied to the ocular tissues by the ocular injection
device according to the
embodiments should not be too large or too small. Too small a force cannot
cause elastic
deformation of the conjunctival tissue to form a bulge, and too large a force
may damage the
conjunctival tissue. A force applied between 0.4N and 1 ON can not only cause
the conjunctival
tissue to form a bulge in the sleeve 3, but also ensure that the bulge of the
conjunctival tissue is
automatically restored after completion of the injection, and significantly
reduce the leakage of the
liquid under the conjunctiva. When the applied force is controlled at a range
from 2N to 6N, there
is no leakage of drug under the conjunctiva.
Through the study of the shape of the clamping port 31, it is found that
various shapes of the
clamping port 31 of the ocular injection device, such as round, hexagonal,
octagonal, square or
irregular shapes, can realize the purpose of the present invention. Among
them, when the clamping
port 31 of the ocular injection device is round, it is not only more conducive
to avoiding damage
CA 03224603 2023- 12- 29 19
to the conjunctiva, but also more conducive to the recovery of ocular tissues
during the injection
process. When the inner diameter of the clamping port 31 is controlled at
0.5mm - 1 Omm, the
conjunctival tissue forms an arch-shaped raised structure, so that the needle
of the ocular injection
device is pierced through the raised top of the ophthalmic tissue to reach the
ophthalmic drug
delivery site, thus making the needle more fixed on the conjunctival tissue at
the injection site, and
making the injection at the identified injection site simpler. When the inner
diameter of the
clamping port 31 is limited at 1-6 mm, the arch-shaped bulge structure is
better, and the injection
effect is best when the inner diameter of the clamping port 31 is 1 mm - 3 mm.
Embodiment 2
The ocular injection device of this embodiment is detailed in FIGS. 7A-7B, and
the length of
the blade at the distal end of needle 2 is 700 micrometers.
The needle of this embodiment can be processed by the following process:
Step 1: Use seamless welding machine to wind the stainless-steel bar into a
tube by laser
welding, and then pull it thinly into 31G caliber stainless steel capillary
tube through wall reduction
machine, pipe drawing machine, straightening machine and other equipment;
Step 2: Cut the capillary to fixed length using a tube cutter;
Step 3: Fix the capillary by arranging it with a needle setting machine;
Step 4: A first sharpening at a set angle of 18 is carried out using a
sharpening machine,
followed by a second and a third sharpening at an angle of 35 . Stop
sharpening when the length
of the sharpened edge reaches 700 microns;
Step 5: Cleaning;
Step 6: Conduct needle assembly in a 100,000-class workshop.
The penetration force of the needle blade prepared by the above process was
less than 0.4N
according to the test by needle penetration experiments.
Embodiment 3
The ocular injection device of this embodiment is detailed in FIGS. 7A-7B, and
the length of
the blade at the distal end of the needle 2 is 550 micrometers.
The needle of this embodiment can be processed by the following process:
Step 1: Use seamless welding machine to wind the stainless-steel bar into a
tube by laser
welding, and then pull it thinly into 31G caliber stainless steel capillary
tube through wall reduction
machine, pipe drawing machine, straightening machine and other equipment;
Step 2: Cut the capillary to fixed length using a tube cutter;
Step 3: Fix the capillary by arranging it with a needle setting machine;
Step 4: A first sharpening at a set angle of 22 is carried out using a
sharpening machine,
CA 03224603 2023- 12- 29 20
followed by a second and a third sharpening at an angle of 35 . Stop
sharpening when the length
of the sharpened edge reaches 550 microns;
Step 5: Cleaning;
Step 6: Conduct needle assembly in a 100,000-class workshop.
The penetration force of the needle blade prepared by the above process was
less than 0.5N
according to the test by needle penetration experiments.
Embodiment 4
The ocular injection device of this embodiment is detailed in FIGS. 7A-7B, and
the length of
the blade at the distal end of the needle 2 is 250 micrometers.
The needle of this embodiment can be processed by the following process:
Step 1: Use seamless welding machine to wind the stainless-steel bar into a
tube by laser
welding, and then pull it thinly into 32G caliber stainless steel capillary
tube through wall reduction
machine, pipe drawing machine, straightening machine and other equipment;
Step 2: Cut the capillary to fixed length using a tube cutter;
Step 3: Fix the capillary by arranging it with a needle setting machine;
Step 4: A first sharpening at a set angle of 30 is carried out using a
sharpening machine,
followed by a second and a third sharpening at an angle of 35 . Stop
sharpening when the length
of the sharpened edge reaches 250 microns;
Step 5: Cleaning;
Step 6: Conduct needle assembly in a 100,000-class workshop.
The penetration force of the needle blade prepared by the above process was
less than 0.7N
according to the test by needle penetration experiments.
When the ocular injection devices provided in embodiments 2-4 of the present
invention are
used for suprachoroidal space injection in accordance with the injection
method of Embodiment
1, it was found that not only reflux of the medicament did not occur, but also
less puncture force
is needed to be exerted by the operator, and it is more convenient to operate,
while reducing the
safety risk of the patient's ocular tissues due to puncture.
The edge surface of the distal end of needle 2 of the present invention can be
machined into
the distal end of needle 2 of Embodiments 2-4 by employing a single or
multiple cuts, in addition
to being machined into a three-sided structure by the three-knife cutting
process provided in the
above embodiments.
Embodiment 5
The ocular injection device of this embodiment is shown in FIGS. 8-9, wherein
the ocular
CA 03224603 2023- 12- 29 21
injection device performs a clamping action, wherein sleeve 3 forms a bottom-
sealed chamber 33
with the clamped ocular tissue.
Sleeve 3 is a transparent structure, or an observation window 34 is provided
on the wall of
sleeve 3, and the operator observes the reverse osmosis of the medicament
through the transparent
sleeve 3 or the observation window 34.
Injecting in accordance with the suprachoroidal chamber injection method of
Embodiment 1,
the refluxed medicament produced by the ocular injection device of the present
embodiment is
automatically refluxed into the bottom sealed chamber 33 formed by the sleeve
3 and the clamped
ocular tissues via the injection port formed at the injection site. The
operator can observe the
refluxing medicament through the sleeve 3 or the observation window 34 on the
sleeve 3, quickly
assess whether the injection is successful or not, and quickly take remedial
measures accordingly.
For example, the operator, without removing the needle 2, increases the
penetration force to make
the distal end of the needle 2 penetrate the sclera; the operator, without
removing needle 14, can
adjust the length of needle 2 outside the clamping port 31 at the distal end
of sleeve 3 through the
adjustment assembly 4 of the present invention, and drive the distal end of
the needle 2 to penetrate
the sclera.
Meanwhile, this embodiment of the ocular injection device collects the reflux
liquid centrally
in the chamber 33, avoiding the reflux liquid from spreading around on the
ocular surface, and
greatly reducing the difficulty of cleaning up the ocular surface during the
injection process or
after the injection is completed.
In order to facilitate the operator in determining the amount of medicament
reflux so as to
assess whether this injection is successful, the embodiment provides a
medicament reflux volume
scale 35 in the observation window 34 or the transparent sleeve 3, and
provides a warning limit in
the scale, so as to remind the operator that the injection has failed and that
the injection should be
stopped.
Embodiment 6
An ocular injection device is shown in FIG. 10, which comprises a needle hub
21 with a
proximal end coupled to a distal end of a medication container 11 and a distal
end coupled to a
proximal end of a needle 2. The needle hub 21 is provided with a channel 211
to deliver at least a
portion of substance from the medication container 11 to needle 2 via channel
211. The proximal
and distal ends of the needle hub 21 are provided with a flange 212 configured
to be coupled to
sleeve 3.
The distal end of needle hub 21 is provided with a connecting portion 313
fixedly connected
to the proximal end of needle 2. This embodiment allows the proximal end of
needle 2 to be
inserted through connecting portion 213 and to be fixed by sealing between the
connecting portion
CA 03224603 2023- 12- 29 22
213 and the proximal end of the 2 by means of dispensing glue.
Flange 212 is provided with ribs 214 to allow an operator to easily grip
needle hub 21 when
installing the same.
The ocular injection device of this embodiment not only secures the needle in
the connecting
portion 213 to avoid the distal end of needle 2 from wobbling during puncture
caused by bending
of needle 2, but also facilitates the installation by the operator and makes
it easier to use adjusting
assembly 4 of the present invention to adjust the length of the distal end of
needle 2 outside the
clamping port 31 of the sleeve 3.
Embodiment 7
The ocular injection device of this embodiment is shown in FIGS. 11-14, with
an adjustment
assembly 4 provided between the proximal and distal ends of needle hub 31,
which cooperates
with sleeve 3 to adjust the length of needle 2 outside the sleeve 3 clamping
port 31.
The adjustment assembly 4 is provided with threads 41 in the outer wall of the
distal portion
of needle hub 21 and the inner wall of sleeve 3 respectively, and the length
of the distal end of
needle 2 outside the clamping port 31 is adjusted by rotating sleeve 3 through
threads 41.
The adjustment assembly 4 is provided with a telescopic rod assembly 42 and a
first operating
lever 43. The telescopic rod assembly 42 includes an outer tube 421, an inner
tube 422, a guide
rail 423, and a drive mechanism 424. The outer tube 42 is connected to the
needle hub 21, and the
inner tube 422 is connected to a connecting portion 313. The guide rail 423 is
mounted in the outer
tube 421, and the inner tube 422 moves axially on the guide rail 42 driven by
the drive mechanism
434.
One end of the first operating lever 43 is movably connected to the driving
mechanism 434,
and the other end is fixed on the outer side of the sleeve 3. By acting on the
outer side of the sleeve
3, the first operating lever 43 drives the driving mechanism 434 to drive the
inner tube 422 to move
axially on the guide rail 423, so as to realize the free adjustment of the
length of the needle 2
outside the clamping mouth 31.
The drive mechanism 434 of the telescoping rod assembly 42 is a linear drive
mechanism,
which can be driven by the first operating lever 43 through a conventional
screw, a rack and pinion,
a ball screw, or a pneumatic cylinder to adjust the length of needle 2 outside
clamping port 31.
The operator can measure the length of needle 2 outside clamping port 31
adjusted by the
adjusting assembly 4 by using ophthalmic calipers. This embodiment of the
adjustment assembly
4 has the advantage of a flexible and adjustable length of the exposed distal
end of needle 2, and
may be suitable for patients with abnormalities in the thickness of ocular
wall tissue, such as sclera.
Meanwhile, in order to facilitate the operator to more accurately observe and
adjust the length
of the needle 2, this embodiment is also provided with a volume scale line of
different variables
CA 03224603 2023- 12- 29 23
on the outer wall of transparent sleeve 3 or observation window 34. When
manufacturing
adjustment assembly 4 of this embodiment, a starting line for length
adjustment of needle 2 can
be provided on the outer thread of connecting portion 313, aligned with the
starting line of the
scale set on the outer wall of the transparent sleeve or the observation
window, and the distal end
of needle 2 can be aligned with the clamping port of the sleeve. The operator
actuates the
adjustment assembly to make an axial movement of the distal end of the needle
towards the sleeve
gripping port, so as to realize a precise adjustment of the length of the
portion of needle 2 outside
clamping port 31 without using ophthalmic calipers for measurement.
This embodiment also provides volume scale lines of different variables at the
distal end of
needle 2. The volume scale lines may be labeled by laser marking. When the
volume scale line at
the distal end of needle 2 is aligned with the clamping port, the length of
the distal end of needle
2 outside clamping port 31 can be measured without using ophthalmic calipers.
Embodiment 8
The ocular injection device of this embodiment is shown in FIGS. 15-16. An
adjustment
release member 5 is provided between the needle hub 21 and the sleeve 3. The
adjustment release
member 5 is configured to adjust the length of the needle 2 outside the distal
clamping port 31 of
the sleeve 3, and to push the distal end of the needle 2 to pierce into the
target tissue of eye via the
distal clamping port 31.
The adjustment release member 5 includes a telescoping assembly 51, a release
assembly 52,
and a second operating lever 53. the telescoping assembly 51 in this
embodiment is the same as
the telescoping rod assembly of Embodiment 8, and the second operating lever
53 is the same as
the first operating lever of Embodiment 8.
Release assembly 52 is connected to flange 312 of needle hub 21 at one end and
to the
proximal end of sleeve 2 at the other end. The other end of second operating
lever 53 connected to
the sleeve is provided with a first stop 54, which is stuck between needle hub
21 and sleeve 3 via
a limit hole 36 provided in sleeve 3. Release assembly 52 may be released by
removing second
operating lever 53 and its first stop to push the distal end of needle 2 into
the ocular tissue via
clamping port 31.
Release assembly 52 may be any of a reset spring, a compressed gas container,
or a container
containing propellant.
In manufacturing the adjustment release member 5 of this embodiment, the first
stop 54 can
be made as tab, slot, ring, or pawl, which can restrict the release assembly
52 from releasing the
pull of the sleeve toward the proximal end of the needle hub to drive the
distal end of the needle 2
toward the clamping port 31 for piercing into the ocular tissues. To release
the tension of the release
assembly 52, the operator removes the first stop 54 by pulling out the second
operating lever 53.
CA 03224603 2023- 12- 29 24
The operator can adjust the length of the portion of needle 2 outside clamping
port 31 by
operating the second operating lever 53, and make the release assembly 52 push
the distal end
portion of needle 2 through clamping port 31 to pierce the target tissue of
the eye by removing the
second operating lever 53 with the first stop 54.
This embodiment also provides another method of injecting ocular tissue,
comprising the
following steps.
Step 1, the length of needle 2 outside the clamping port 31 at the distal end
of the sleeve 3 is
adjusted by the adjusting release member 5 of the ocular injection device of
this embodiment;
Step 2, measure the distance with ophthalmic calipers to confirm the injection
site;
Step 3, clamping port 31 of the ocular injection device is placed
perpendicular to the
conjunctiva of the injection site;
Step 4, force is applied to the conjunctival tissue at the injection site by
syringe 1 of the ocular
injection device and sleeve 3 cooperatively, so that clamping port 31 is
tightly attached to and
clamps the conjunctival tissue to make the clamped conjunctival tissue
projects toward sleeve 3;
Step 5, first stop 54 is removed using second operating lever 53 to release
the release assembly
52, actuating the distal end portion of needle 2 to be inserted into the
ocular target tissue from the
raised conjunctival tissue through clamping port 31.
Step 6, drug is administered by injection to reach the administration site.
As shown by the results of the suprachoroidal space injection performed in
accordance with
the ocular injection device of the present embodiment and the ocular injection
method thereof, the
distal end of needle 2 of the ocular injection device of the present
embodiment forms an arched
structure with the projection formed by the ocular tissues, so that needle 2
of the ocular injection
device can pierce the top of the projection of the ocular tissues to reach the
target tissues of the
ocular region, which makes it simpler to perform the injection at the
identified injection site.
In industrial manufacture of the ocular injection device of this embodiment,
release assembly
52 is adjusted for a stroke length that is the distance from the distal end of
needle 2 to clamping
port 31 when first stop 54 restricts the release thrust of release assembly
52.
Embodiment 9
The ocular injection device of this embodiment is shown in FIGS. 17-18 and
includes a thrust
assembly 6. The thrust assembly 6 is configured to generate a constant thrust
force on a proximal
portion of the pushrod 12 to push the distal end of the pushrod 12 within the
drug chamber 11
toward the needle 2, causing at least a portion of the drug within the drug
chamber 11 to be injected
into the target tissue of the eye via the injection site to reach the site of
administration.
In the manufacture of the thrust assembly 6 of the ocular injection device of
this embodiment,
any of conventional mechanism, such as spring-loaded ball mechanisms, spring-
loaded pins,
CA 03224603 2023- 12- 29 25
cylinders, or container containing propellant may be used.
The operator may, when starting injection after completing the puncture, use
thrust assembly
6 to generate a constant thrust force on the proximal portion of pushrod 12 to
inject the drug into
the target tissue of eye at a uniform rate, thereby preventing the operator
from applying too large
force and pushing the pushrod 12 too fast which may result in the destruction
of the eye tissue of
the site of administration due to the excessive pressure of the local
injection.
The present embodiment of the injection device further comprises a second stop
member 7
configured to selectively limit the movement of push rod 12 relative to the
medication container.
The second stop member 7 in the manufacture of the ocular injection device of
this embodiment
may utilize any mechanical structure commonly used in the prior art, such as
snap rings, slots,
pawls, and the like, that can limit the movement of pushrod 12 relative to the
medication container.
The operator may simply pull off the second stop member 7 to release thrust
assembly 6 when
performing the drug injection.
The ocular injection device and method of use provided herein can be widely
used for ocular
diseases and their associated conditions, including but are not limited to
uveitis, glaucoma, diabetic
macular edema or retinopathy, macular degeneration, retinal germ cell tumors,
and genetic
disorders, and are particularly suitable for delivery of drugs to localized
areas in the posterior
portion of the eye, such as the retinal choroidal tissue, the macula, and the
optic nerve in the
posterior section of the eye. The ocular injection device and method of use
provided herein can
also be used in gene-based therapy applications to deliver a medicament
containing a therapeutic
gene fragment into the suprachoroidal space to the target ocular tissue in any
one or more carriers
selected from DNA, RNA, or oligonucleotides.
The medication container 11 of the present invention contains a pharmaceutical
solution of
one or two or more pharmaceutically active substances selected from
antibodies, antiviral agents,
chemotherapeutic agents, analgesics, anesthetics, aptamers, antihistamines,
anti-inflammatory
agents, and antitumor agents.
The various embodiments of the present invention are described in a
progressive manner,
with each embodiment focusing on the differences from other embodiments, and
the same or
similar parts between the embodiments may be cross-referenced. Since the
device disclosed in the
embodiments corresponds to the method disclosed in the embodiments, it is only
briefly described,
and the relevant contents can be found in paragraphs illustrating the methods.
Embodiment 10
The ocular injection assembly, as shown in FIGS. 19 to 21, comprises a sleeve
3 and a needle
2 disposed within the sleeve 3. As shown in FIG. 22 and FIG. 23, the sleeve 3
is provided with a
clamping port 31 at the end of the sleeve 3. A tip portion of the needle 2 may
pass through the
CA 03224603 2023- 12- 29 26
clamping port 31 in use.
Referring to FIG. 23, the clamping port 31 has an annular end face 33, i.e.,
the end face of
the sleeve 3 is annular in shape and includes an inner ring curve and an outer
ring curve which are
smooth curves and spaced. The space surrounding the inner ring curve is the
clamping port. The
portion between the inner ring curve and the outer ring curve is the sleeve
wall. The clamping port
31 is circular in this embodiment. The annular end face 33 is shown as an
annular end face in FIG.
24. In other embodiments, an elliptical annular end face as shown in FIG. 25
or a polygonal annular
end face as shown in FIG. 26 may be used. Different shapes of clamping port
such as round, oval,
hexagonal, octagonal, square, or irregular can be used to enable the clamping
port to press against
the ocular tissues. The circular end surface 33 is not only more conducive to
avoiding damage to
the conjunctiva, but also more conducive to recovery of the ocular tissues
during the injection
process.
The minimum inner diameter of the clamping port 31 is 0.5mm-1 Omm, preferably
1 mm-
6mm, more preferably lmm-3mm.
Returning to FIG. 23, the sleeve 3 ends in a constriction section 32. A
clamping port 31 is
located at the end of the constriction section 32. The cross-section of the
constriction section 33
gradually increases in a direction away from the clamping port 31.
The length of the edge surface of the tip portion of the needle 2 is less than
1100 micrometers,
preferably less than 900 micrometers, further preferably less than 700
micrometers, more
preferably < 550 micrometers, and most preferably 250-550 micrometers. The
needle structure is
referred to FIGS. 7A and 7B.
When needle 2 is mated with sleeve 3, the distance d labeled in FIG. 23 is a
length of 500-
2000 micrometers of the tip portion of needle 2 outside the clamping port 21.
The distance from
the tip of needle 2 to the annular end surface 33 is the length of the tip
portion of the needle 2
outside the clamping port 31. In this embodiment annular end face 33 is planar
and needle 2 is
perpendicular to annular end face 33. That is, the axial direction of needle 2
is perpendicular to the
plane where annular end face 33 is located.
When clamping port 31 of sleeve 3 contacts and presses on the eye tissue,
clamping port 31
is sealed. As shown in FIG. 27, the ocular tissue forms a bulge 200 towards
the inside of the sleeve.
As shown in FIG. 28, when clamping port 31 is sealed, chamber 34 within sleeve
3 forms a
hermetically sealed chamber 34. In the event of reflux, the refluxed
medicament is stowed into the
hermetically sealed chamber 34. When separating clamping port 31 from the
ocular tissue, the
medicament is less likely to leak out of the hermetically sealed chamber 34,
preventing the
medicament from dispersing to the ocular tissue.
Sleeve 3 is provided with a canal, and needle 2 is movably connected to the
canal. Needle 2
is communicated to sleeve 3 through the canal, which facilitates the needle to
maintain a position
CA 03224603 2023- 12- 29 27
relative to the clamping port of the sleeve when the needle pierces to reach
the injection point.
Returning to FIG. 22, when needle 2 is connected to sleeve 3, needle 2 has a
needle hub 21
attached to needle 2 at an opposite end located at the tip portion. Needle hub
21 includes a guide
tube 211 at the front end. Needle 2 is secured within guide tube 211 and
extends into needle hub
21. Sleeve 3 is assembled to guide tube 211 so that sleeve 3 is fixed with
respect to needle 2.
Embodiment 11
Based on embodiment 10, in this embodiment an adjustment assembly is provided
between
sleeve 3 and needle 2 for adjusting the size of the length of the tip portion
of the needle 2 outside
the portion 31 of the clamping port. A needle hub 21 is attached to the needle
2 at an opposite end
located at the tip portion, coupled to the sleeve 3 by the adjustment
assembly. The adjustment
assembly comprises an externally threaded section 102 provided on the needle
hub 21 and an
internally threaded section 101 provided on the corresponding sleeve 3. The
externally threaded
section 102 and the internally threaded section 101 are matingly coupled to
each other. Through
the mating of the externally threaded section 102 and the internally threaded
section 101, the
needle hub 21 and the sleeve 3 are rotated relative to each other to realize
the adjustment of the
length of the needle. As shown in FIG. 29, the needle hub 21 includes a guide
tube 211 provided
with the externally threaded section 102. The guide tube 211 is connected to
the front end of the
needle hub 21 and is connected to the needle for enhancing the strength of the
needle to avoid
bending or shaking thereof during the puncturing process. As shown in FIG. 30,
sleeve 3 is
provided with an internally threaded section 101. An adjustment assembly is
provided between the
needle hub 21 and the sleeve 3 such that adjustment of the length of needle 2
is less likely to be
affected by other components such as the syringe. The thread pitch is 50-200
micrometers, more
preferably 50-150 micrometers.
Another option is shown in FIG. 31. The side wall of sleeve 3 is provided with
an observation
window 35 of transparent material, or the sleeve is a transparent material
member. The provision
of the observation window 35 or the use of the transparent material sleeve
facilitates the
observation of the medicament reflux to quickly determine whether the
injection is successful. The
observation window 35 or the transparent sleeve 3 is provided with a volume
scale line 351 along
the axial direction of the sleeve 3 for real-time observation of the
medicament reflux. When the
warning limit is exceeded, it is convenient to remind the operator of the
injection failure and thus
stop the injection.
The adjustment assembly is provided to facilitate the adjustment of the length
of the tip of
needle 2 outside clamping port 31 and to control the depth of puncture, which
is applicable to
different patients, or to puncture different locations of the eye. For
example, when a patient has
abnormal sclera thickness, it may be applied to the eye wall tissue.
CA 03224603 2023- 12- 29 28
Embodiment 12
This embodiment adds an adjustment assembly to embodiment 10.
As shown in FIG. 32, a needle hub 21 is connected to the rear end of the
sleeve 3, and an
adjustment assembly is provided therein. The adjustment assembly includes a
telescoping rod
assembly 42 and a drive mechanism 41. the telescoping rod assembly 42 includes
an outer tube
421 and an inner tube 422. one end of the outer tube 421 is connected to the
rear end of the needle
hub 21 via a connecting block 423, and the other end is sheathed on the inner
tube 422. The other
end of the inner tube 422 is connected to the rear end of the needle 2. The
outer tube 421, the inner
tube 422 and the needle 2 are connected in turn. A drive mechanism 41 is
connected to the inner
tube 422 to drive the inner tube 422 to move axially relative to the outer
tube 421 and to be able
to move the needle 2. A guide tube 211 is provided outside the needle 2, and
both ends of the
needle 2 extend outside the guide tube 211. A front end of the needle hub 21
is disposed outside
the guide tube 211. The guide tube 211 and the needle hub 21 are capable of
sliding relative to
each other. FIG. 33 shows that the front end of the needle 2 is incorporated
into the sleeve 3 by
means of an adjustment assembly.
As shown in FIG. 34, the drive mechanism 41 includes a drive housing 411, a
drive gear 412,
a rack guide 413, and an operating lever 414. one end of the drive housing 411
is connected to the
outer tube 422, and the other end is slidably connected with the inner tube
422. The rack guide
413 is connected to the inner tube 422 and is provided in the axial direction.
In this embodiment,
a concave section is set in the middle of the inner tube 422, in which the
rack guide 413 is directly
provided. A drive gear 412 is hingedly connected to the drive housing 411 and
is engaged with the
rack guide 413. One end of the operating lever 414 is connected to the drive
gear 412, and the
other end passes through the through-hole of the drive housing 411 and the
through-hole of the
needle hub 21 to the outside of the needle hub 21. Turning the operating lever
412 will rotate the
drive gear 412. By rotating the operating lever 412 in the rotary drive
mechanism to turn the drive
gear 414 which then moves the rack guide 413. The inner tube 422 moves with
the rack guide 413
and drives the needle 2 in the axial direction, realizing the adjustment of
the position of the needle
2.
Another embodiment is shown in FIGS. 35 and 36. The inner tube 422 includes
upper and
lower sections, wherein the upper section is socketed within the outer tube
421 and the lower
section is connected to the guide tube 211. The upper section and the lower
section are connected
to each other by a rack guide 413 to facilitate setting the drive gear 412 in
the middle of the drive
housing 411.
A further embodiment is shown in FIG. 37. The rear end of the needle 2 passes
through the
inner tube 422 and the outer tube 421 in turn and extends outside the outer
tube 421. The needle 2
CA 03224603 2023- 12- 29 29
is fixedly connected to the inner tube 422 by a guide tube 211, and the rear
end of the needle 2
extends outside the inner tube 422 toward the outer tube 421 to the other end
of the outer tube 421.
The rear end of the needle 2 extends into the syringe in use with a syringe.
Embodiment 13
This embodiment adds a release assembly to embodiment 10.
As shown in FIG. 38, a needle hub 21 is attached to the rear end of the sleeve
3. A release
assembly 5, including a resilient member 51 and a first stop member 52, is
provided between the
sleeve 3 and the needle hub 2. The sleeve 3 is connected to the needle hub 21
via the resilient
member 51. One end of the first stop member 52 is removably connected to the
sleeve 3 and/or
the other end is removably connected to the needle hub 21. When the first stop
member 32 is
connected to the sleeve 3 and the needle hub 21, the resilient member 51 is in
a stretched state,
providing tension along the axial direction. The resilient member 51 may be a
reset spring, a
compressed gas container or a container containing propellant. The first stop
member 52 may be
a tab, a slot, a ring, a slot or a pawl, etc. In this embodiment the resilient
member 51 is a reset
spring and the first stop member 52 is a snap ring, as shown in FIG. 40.
As shown in FIG. 38, the resilient member 51 is stretched to increase the
distance between
the needle hub 21 and the sleeve 3. The first stop member 52 is snapped
between the needle hub
21 and the sleeve 3 to maintain the distance between the needle hub 21 and the
sleeve 3. At this
point the resilient member is in a stretched state and the front end of the
needle 2 is recovered into
the sleeve 3. The clamping port 31 is kept in contact with the eye tissue
during use. After removing
the first stop member 52 the needle hub 21 is moved forwardly so that the
front end of the needle
2 is outside the clamping port 31, as shown in FIG. 39, and pierces into the
eye tissue.
Similarly, an adjustment assembly is provided between the sleeve 3 and the
needle hub 21,
including a telescoping rod assembly 42 and a drive mechanism 41. The
telescoping rod assembly
42 includes an outer tube 421 and an inner tube 422. One end of the outer tube
421 is connected
to the front end of the needle hub 21, and the other end is sheathed on the
inner tube 422. The other
end of the inner tube 422 is connected to the rear end of the needle hub 21.
The outer tube 421, the
inner tube 422 and the needle 21 are connected in turn, or the rear end of the
needle 2 passes
through the inner tube 422 and the outer tube 421 in turn and extends outside
the outer tube 421.
the drive mechanism 41 is connected to the inner tube 421 to drive the inner
tube 422 to move in
an axial direction with respect to the outer tube 421 and drive the needle 21.
As shown in FIG. 34, the drive mechanism 41 includes a drive housing 411, a
drive gear 412,
a rack guide 413, and an operating lever 414. One end of the drive housing 411
is connected to the
outer tube 421, and the other end is slidingly connected to the inner tube
422. The rack guide 413
is connected to the inner tube 421 and is provided in an axial direction. A
drive gear 412 is hingedly
CA 03224603 2023- 12- 29 30
connected within the drive housing 411 and is engaged with the rack guide 413.
One end of the
operating lever 414 is connected to the drive gear 412, and the other end
passes through the
through-hole of the drive housing 411 and the through-hole of the needle hub
21 to the outside of
the needle hub 21. Turning the operating lever 414 will rotate the drive gear
412.
In yet another embodiment, the operating lever 414 is detachably connected to
the drive gear
412. The first stop 52 may be driven when the operating lever 414 is separated
from the needle
hub 21 to separate the first stop member 52 from the sleeve 3 and/or the
needle hub 21. By
providing the operating lever 414 outside the needle hub 21, after completion
of adjusting the
length of the needle 2 using the operating lever 414, the operating lever 414
can be removed by
pulling out it for easy observation. Moreover, the operation lever 414 drives
the first stop member
52 to separate from the sleeve 3 and the needle hub 21, which facilitates one-
handed operation by
the operator. In this embodiment, the structure of the adjustment assembly 4
may be the same as
the structure of the adjustment assembly in the previous embodiment, or the
adjustment assembly
may be provided outside the needle hub and in the middle of the resilient
member 5.
Embodiment 14
The ocular injection device is shown in FIG. 41 and includes a syringe 1 and
an ocular
injection assembly of any of embodiments 10-13 mounted on the syringe.
As shown in FIG. 42, the syringe 1 includes a medication container 11, a
pushrod 12. the
pushrod 12 is slidable within the medication container 11. The medication
container 11 is used to
store the medication and is matingly connected to the injection assembly 2.
The injection assembly
also includes a needle protection cap 8 that fits over the needle 2.
Embodiment 15
This embodiment is based on embodiment 14, and a thrust assembly 6 is provided
between
the end of the pushrod 12 and the medication container 11 for generating a
constant thrust force
on the pushrod.
The thrust assembly 6 is a spring-loaded ball mechanism, a spring-loaded pin,
a cylinder, or
a container storing a propellant, which generates a constant thrust with a
threshold value of no
more than 6 N. The thrust assembly 6 generates a constant thrust in such a way
that it is not able
to propel the thrust assembly when the thrust applied to the thrust assembly
is less than a threshold
value, and the thrust assembly is capable of counteracting the thrust to
maintain the thrust at the
threshold when the thrust is greater than the threshold value. For example,
when the threshold
value is 5N, the thrust assembly cannot be pushed using a force of 4N, and
when the thrust force
is 7N, the thrust assembly is able to maintain a thrust force of 6N.
The injection device further comprises a second stop member 7 provided between
the
CA 03224603 2023- 12- 29 31
pushrod 12 and the medication container 11 for limiting the size of the
pushing stroke of the
pushrod. The second stop member 7 may be a tab, a slot, a ring, a slot or a
pawl.
Embodiment 16
A method of using an ocular injection device, using the ocular injection
device of
embodiment 14 or 15, contacting and pressing the clamping port 31 of the
sleeve 3 against the
ocular tissue at the injection site so that the ocular tissue at the injection
site forms a bulge 200
into the sleeve 3.
After the sleeve clamping port 31 is contacted and pressed against the ocular
tissue at the
injection site to form a bulge 200 of the ocular tissue at the injection site
into the sleeve, the tip
portion of the needle 2 is then made to puncture the conjunctival tissue and
scleral tissue at the
injection site to allow the distal end of the needle to reach the target
tissue of the eye at the injection
site.
The length of the tip portion of the needle exposed to the clamping port is
adjusted according
to the thickness of the ocular tissue at the injection site before the sleeve
clamping port 31 contacts
the ocular tissue at the press injection site. The adjustment is performed
using the adjustment
assembly in the injection assembly described above.
Alternatively, one side of the sleeve clamping port 31 is first brought into
contact with the
ocular surface, then the sleeve clamping port 31 is pivoted using that contact
part as a pivot point
to allow the needle to pierce the ocular tissue at the injection site, and is
further pivoted so that the
other side of the clamping port 31 is in contact with the ocular surface, the
ocular tissue forms a
bulge into the sleeve 3. The ocular surface may be a conjunctival surface or
other ocular tissue.
Ocular tissue thickness at the injection site was obtained by testing with one
or more of
optical coherence tomography (OCT), enhanced deep imaging with OCT (EDI-OCT),
swept-
frequency OCT (SS-OCT), or ultrasound biomicroscopy (UBM).
After the distal end of the needle 2 reaches the target tissue of the eye at
the injection site,
the pushrod 12 is pushed to inject at least a portion of the substance in the
medication container
11 into the target tissue of the eye via the needle 2.
While pushing the pushrod 12 to deliver at least a portion of the substance in
the medication
container 11 to the target tissue of the eye via the distal end of the needle
2, a return volume of the
refluxed drug solution in the sleeve 3 is observed. The observation can be
made using an
observation window or a transparent sleeve in the injection device, and the
volume of the refluxed
medicament is recorded with reference to a volume scale.
Test example 1
CA 03224603 2023- 12- 29 32
Experiment No. 1-1. The ocular injection assembly and syringe of embodiment 10
were used
to form an injection device, and a socket needle with an effective length of
700 m and a blade
length of 500 50 m was set according to the conventional thickness of the New
Zealand rabbit
scleral tissues and choroid, and the socket clamping port 31 was round with an
inner diameter of
1.5mm.
The effective length, i.e., the length of the tip portion of needle 2 outside
clamping port 31,
is 700p.m. That is, the distance from the tip portion of needle 2 to the
annular end surface is 700 m.
Needle 2 is substantially perpendicular to the annular end surface.
Experimental method: after pentobarbital anesthesia, ocular surface anesthesia
was
performed using proparacaine hydrochloride eye drops, and waited for 1-2
minutes after the eye
drops, then suprachoroidal space injection was performed using a homemade
socket needle.
Test animals: Healthy New Zealand rabbits, 1.8-2.2 kg;
Injection frequency: single-site injection in the right eye;
Injection reagent: 0.2% ICG, 100 1;
The method of suprachoroidal space injection comprises the following steps.
A first step is to measure the distance with ophthalmic calipers to confirm
the injection site.
In the second step, the injection reagent is drawn and a force is applied to
the side of the
clamping port 31 via the syringe, so that the side of the clamping port 31 is
tightly pressed against
the ocular tissues at the injection site and forms a fulcrum on the ocular
surface of the injection
site.
In the third step, the injection device is flipped around the pivot point to
the other side of the
clamping port 31 so that the distal end of the needle 2 pierces the ocular
tissue at the injection site.
In the fourth step, the other side of the clamping port 31 is attached closely
to the ocular
surface of the injection site by continuing to flip around the pivot point, so
that the clamping port
31 grips the ocular tissue to form a bulge 200 into the sleeve 3. The distal
end of the needle 2 is
pressed vertically into the sclera to reach the target tissue of the eye at
the injection site.
In the fifth step, the drug is injected to reach the site of administration.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Rabbits were
executed, and the eyeballs were dissected to prepare flat samples of fundus
tissue. Photographs
were taken to record the distribution of ICG solution in the suprachoroidal
space.
Test result: It is observed that there was no reflux and no subconjunctival
residue caused by
ICG injections, and the results of fundus tissue sections showed that all the
fundus tissues were
transfected with ICG, and the stained fundus tissues were light green in
color. The fundus tissue
sections are shown in FIG. 44.
CA 03224603 2023- 12- 29 33
Test example 2
On the basis of Test example 1, other conditions were kept constant, and the
inner diameter
of the sleeve clamping port was adjusted, thereby investigating the effect of
the inner diameter of
sleeve 3 on the reflux of the drug solution. The inner diameters of sleeve 3
were 0.25 mm
(Experiment No. 1-2), 0.5 mm (Experiment No. 1-3), 1.0 mm (Experiment No. 1-
4), 2.0 mm
(Experiment No. 1-5), 2.5 mm (Experiment No. 1-6), 3.0 mm (Experiment No. 1-
7), 5.0 mm
(Experiment No. 1-8) and 10.0 mm (Experiment No. 1-9), respectively.
Test example 1 and Test example 2 are summarized below:
Table 1 Effect of sleeves with different inner diameters on return flow
test Sleeve inner Experimental Phenomena Experimental
Phenomena
serial diameter (right eye) (Tissue
slices)
number (mm)
1-1 1.5 No reflux, and no ocular surface All
transfected with ICG, light
and subconjunctival spreading green
1-2 0.25 Significant reflux, significant Severe
reflux, failed injections,
ocular surface and rabbits not
executed for
subconjunctival spreading preparing fundus
sections
1-3 0.5 Significant reflux, significant Severe
reflux, failed injections,
ocular surface and rabbits not
executed for
subconjunctival spreading preparing fundus
sections
1-4 1.0 No significant reflux, and no About 3/4 of
the area stained,
ocular surface and green
subconjunctival spreading
1-5 2.0 No significant reflux, no ocular Over 3/4
of the area stained,
surface spreading and trace dark green
residue in the subconjunctiva but
not spreading
1-6 0.5 No significant reflux, no ocular About 2/3
of the area stained,
surface spreading and trace dark green
residue in the subconjunctiva but
not spreading
1-7 3.0 No significant reflux, no ocular About 3/5
of the area stained,
surface spreading and small dark green color
subconjunctival residue, slight
bulging but not spreading
CA 03224603 2023- 12- 29 34
1-8 5.0 No reflux, no ocular surface Fundus tissue
unstained,
spread and no visible vitreous stained
subconjunctival residue
1-9 10.0 No reflux, no ocular surface Fundus tissue
unstained,
spread and no visible vitreous stained
subconjunctival residue
The test results showed no significant reflux or ocular surface and
subconjunctival spreading
at an internal diameter of 1-3 mm. Tissue section conditions showed a stained
area between 3/5
and 3/4 of the area, and the injection was successful.
Test example 3
Unlike Test example 1, the injection reagent was AAV8-EGFP recombinant adeno-
associated
virus, in which the target gene was EGFP, dosage: 1.02E+11vg/eye, single-site
injection in the left
eye.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Immediately after
injection, OCT was performed on the injected eye to observe whether the
injection site was the
suprachoroidal space, whether it caused damage to the surrounding tissues, and
to assess the extent
of drug diffusion in the suprachoroidal space. After injection, the animals
were continued to be fed
for observation and were examined by autofluorescence at 28 days. Eyes were
isolated after
euthanizing the animals, and frozen sections and staining were performed to
examine AAV-EGFP
transduction.
Test results: It is observed that there was no reflux or ocular surface
spreading caused by the
drug injection, and there was no residue, hemorrhage, or congestion in the
conjunctiva. The OCT
scan results are shown in FIG. 45, with no scleral puncture openings. The
choroidal detachment
was obvious on OCT, and there was no retinal detachment, bulge, or
lacrimation, which indicated
that the injection depth was appropriate without penetrating the choroid or
the retina.
Autofluorescence detection was shown in FIG. 46, with fluorescent dots
distributed throughout
the fundus on the upper and lower left and right sides, and the optic disc was
obvious on the upper
and lower sides. Frozen retinal tissue sections are shown in FIG. 47, and the
injected reagent was
clearly expressed in retinal epithelial cells and photoreceptor cells. The
above test results showed
that the injection was successful.
Note: RGC, ganglion cells; INL, inner nuclear layer of the retina; ONL, outer
nuclear layer
of the retina; RPE, retinal pigment epithelial cells.
Test example 4
CA 03224603 2023- 12- 29 35
The ocular injection assembly and syringe of Embodiment 1 were used to form an
injection
device, and a socket needle with an effective length of 700 m and a blade
length of 500 50m
was customized according to the conventional thickness of the scleral tissues
and choroid of rhesus
monkeys, with a sleeve 3 having an inner diameter of 1.5 mm.
The effective length, i.e., the length of the tip portion of the needle 2
outside the portion of
the clamping port is 700 m. That is, the distance from the tip portion of the
needle 2 to the annular
end surface is 700 m; and the needle 2 is substantially perpendicular to the
annular end surface.
Experimental method: After pentobarbital anesthesia, ocular surface anesthesia
was
performed using proparacaine hydrochloride eye drops, and waited for 1-2
minutes after the drops,
suprachoroidal space injection was performed using a homemade socket needle.
Test animals: rhesus monkeys;
Frequency of injections: single-site injections in both eyes;
Injection reagent: 0.2% ICG, 100 1;
Injection method: same as in Test example 1;
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Immediately after
injection, ICGA contrast and OCT were performed on the injected eye to observe
whether the
injection site was the suprachoroidal space and whether damage is caused to
the surrounding
tissues, and to assess the extent of drug diffusion in the suprachoroidal
space.
Test results: It is observed from the injection results that there was a small
amount of reflux
in the right and left eyes, the reflux fluid was adsorbed by the sleeve, no
ocular surface diffusion
was seen, and no subconjunctival residue, diffusion, hemorrhage, or congestion
was seen. ICGA
contrast results as shown in Fig. 48 showed that striated hyper-fluorescence
was seen in the right
and left eyes, localized obvious fluorescence at the injection point was
visible, no intravascular
hyper-fluorescence was seen, no diffuse lamellar fluorescence was seen, and
fluorescence
diffusion was seen up to supratemporal level. OCT scanning results were shown
in FIG. 48, with
extra choroidal dark areas visible in both the right and left eyes, shallow
choroidal detachment,
and no retinal detachment, bulge, or lacunae. The above test results showed
that the injection was
successful.
Test example 5
The difference between this Test example and Test example 4 is that the socket
needle with
an effective length of 800 m and a blade length of 500 50 m was customized
according to the
conventional thickness of the scleral tissue and choroid of rhesus monkeys,
and the inner diameter
of the sleeve 3 was 1.5 mm.
CA 03224603 2023- 12- 29 36
Test results: it is observed from the injection results that a small amount of
reflux present in
the left eye and a trace amount of reflux in the right eye, but the reflux
fluid in both eyes was
adsorbed by the sleeve, and no ocular surface diffusion, subconjunctival
residue, diffusion,
hemorrhage or congestion was seen in either eye. The results of trans-ICGA
contrast are shown in
FIG. 49, with streaked hyper-fluorescence in both the right and left eyes,
visible localized obvious
fluorescence at the injection site, no intravascular hyper-fluorescence, no
diffuse flakiness
fluorescence, and fluorescence can be seen at the macula or supratemporal. The
results of the OCT
scans are shown in FIG. 49, with extra-choroidal dark areas and superficial
choroidal detachment
in both the right and left eyes; the retina of the left eye was slightly
elevated, and the right eye did
not have retinal detachments, elevations, or lacunae. The test results showed
that the injection was
successful.
Test example 6
The difference between this Test example and Test example 4 is that the
scleral thickness was
examined. Injection device used is a socket needle with a blade length of 500
50 m and including
a threaded adjusting member to adjust the effective length at 2,000 m, and a
single-site injection
in the right eye was performed.
The injection method is as follows:
In a first step, the operator detected the thicknesses of retinal, choroidal,
and scleral in the
injection region of the right eye of rhesus monkeys using any one of optical
coherence tomography,
OCT-enhanced deep imaging, swept-frequency OCT, or ultrasound biomicroscopy,
and used the
adjusting component 4 of the ocular injection device to adjust the length of
the needle 2 outside
the clamping port 31 of the sleeve 3 in accordance with the detected
thicknesses, wherein the
adjusted length of the right eye was 650 m.
In a second step, measure the distance with ophthalmic calipers to confirm the
injection site.
In a third step, clamping port 31 of the ocular injection device is placed
perpendicular to the
conjunctiva of the injection site.
In a fourth step, a force is applied to the conjunctival tissue at the
injection site through the
syringe 1 of the ocular injection device and the sleeve 3 in conjunction, so
that the clamping port
31 was close-fitting and gripped on the conjunctival tissue, so that the
gripped conjunctival tissue
is projected toward the sleeve 3. The distal end of the needle 2 is vertically
pierced into the scleral
tissue to reach the target tissue of the eye at the injection site.
In a fifth step, the drug is administered by injection so that the drug
reaches the site of
administration.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Immediately after
CA 03224603 2023- 12- 29 37
injection, ICGA contrast and OCT were performed on the injected eye to observe
whether the
injection site was the suprachoroidal space and whether damage is caused to
the surrounding
tissues, and to assess the extent of drug diffusion in the suprachoroidal
space.
Test results: it is observed from the injection result that no reflux, ocular
surface diffusion,
and subconjunctival residual, diffuse hemorrhage or congestion was seen. ICGA
contrast was
shown in FIG. 50, in which streaky hyper-fluorescence was visible, localized
significant
fluorescence at the injection site, no intravascular hyper-fluorescence, and
no diffuse lamellar
fluorescence, which was fluorescent up to the macular area or supratemporal
level. OCT scans, as
shown in Fig. 50, did not show any scleral puncture openings, and the OCT
visualized choroidal
detachment was evident, and no retinal detachment, bulge, or fissure was seen.
The test results
showed that the injection was successful.
Test example 7
The difference between this Test example and Test example 4 is that the
scleral thickness was
examined, and the injection device used is a socket needle with a needle 2
having a blade length
of 400 50 m and including a threaded adjustment member to adjust the effective
length at
2000 m, and that a single-site injection in the left eye was performed.
The injection method was the same as in Test example 6, in which the left eye
was adjusted
to a length of 750 p.m.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Immediately after
injection, OCT was performed on the injected eye to observe whether the
injection site was the
suprachoroidal space and whether damage is caused to the surrounding tissues,
and to assess the
extent of drug diffusion in the suprachoroidal space. The animals were
continued to be kept for
observation for 28 days after injection. After euthanizing the animals, the
eyeballs were separated,
and frozen sections and staining were performed to examine AAV-EGFP
transduction.
Test results: it is observed from the injection results that no reflux, ocular
surface diffusion as
well as subconjunctival residual, diffuse hemorrhage or congestion is visible.
OCT scanning
results were shown in FIG. 51, and no scleral puncture opening was seen. OCT
showed obvious
choroidal detachment, no retinal detachment, bulge or lacunae, indicating that
the injection depth
was appropriate and did not penetrate the choroid and retina. Frozen retinal
tissue sections are
shown in FIG. 52, which shows that after injection through the suprachoroidal
space, AAV-EGFP
mainly transduced RPE cells and photoreceptor cells. The test results showed
that the injection
was successful.
CA 03224603 2023- 12- 29 38
In the figure, RPE is retinal pigment epithelium, IS/OS is retinal
photoreceptor inner and
outer ganglion connections, INL is inner nuclear layer of the retina, ONL is
outer nuclear layer of
the retina, and RGC is ganglion cells.
Test example 8
This Test example differs from Test example 4 in that the scleral thickness
was examined,
and using an injection device which is a socket needle containing a threaded
adjusting member for
adjusting the effective length of a needle blade length of 300 50 m, with an
adjustable effective
length of 2000 m, wherein the injection device was provided with a thrust
assembly and a single-
point injection for the right eye.
The injection method is as follows:
In a first step, the operator detected the thickness of the conjunctiva to the
choroid in the
injection area of the rhesus monkey using optical coherence tomography, OCT-
enhanced deep
imaging, swept OCT, or ultrasound biomicroscopy, and adjusted the length of
the needle exposing
the clamping port of the sleeve according to the detected conjunctiva to
choroid thickness via the
adjustment component of the ocular injection device, in which the adjustment
length was 850 m
for the right eye.
In a second step, measure the distance with ophthalmic calipers to confirm the
injection site.
In a third step, the clamping port 31 of the ocular injection device is placed
perpendicular to
the ocular surface at the injection site.
In a fourth step, the operator applied a force to the ocular tissue at the
injection site via the
thrust assembly 6 via the syringe of the ocular injection device and the
sleeve 3 in conjunction,
such that the clamping port 31 abuts and grips the ocular tissue to cause the
ocular tissue gripped
to form a project 200 towards the sleeve 3, so that the distal end of the
needle 2 is pressed vertically
from the projecting ocular surface tissue into the sclera through the clamping
port of the sleeve 3
to reach the target tissue of the eye at the injection site.
In a fifth step, the operator opens the second stop member 7, presses the
distal end of the
pushrod 12, and injects the medication through the constant thrust created by
the thrust assembly
on the proximal portion of the pushrod 12, so that the medication reaches the
site of administration.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Immediately after
injection, OCT was performed on the injected eyes to observe whether the
injection site was the
suprachoroidal space and whether damage is caused to the surrounding tissues,
and to assess the
extent of drug diffusion in the suprachoroidal space. After injection, the
animals were continued
to be kept for observation and were examined by autofluorescence at 28 days.
The eyes were
CA 03224603 2023- 12- 29 39
isolated after euthanizing the animals, and frozen sections and staining were
performed to examine
AAV-EGFP transduction.
Test results: Observation of the injection results showed no reflux, ocular
surface diffusion,
and subconjunctival residual, diffuse hemorrhage or congestion. OCT scan
results were shown in
FIG. 53, and no choroidal or retinal detachment, bulge, or lacunae was seen,
suggesting that the
injection depth was appropriate, and that it did not penetrate the choroid or
the retina. Frozen
retinal tissue sections are shown in FIG. 54, after injection through the
suprachoroidal space, AAV-
EGFP mainly transduced RPE cells and photoreceptor cells. The test results
showed that the
injection was successful.
Test example 9
This Test example differs from Test example 4 in that the injection dose was
100 microliters
per dose per eye.
Monocular injections were performed according to the injection method of Test
example 4,
in which three monkeys were injected with ICG, three monkeys were injected
with buffer, and the
remaining nine monkeys were injected with AAV8-containing drugs.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
Test results: no reflux, ocular surface spread, or subconjunctival residual,
spreading
hemorrhage or congestion was seen in any of the 36 eyes during the injection.
The test results
showed that the injection was successful.
Comparative example 1
Open conjunctival injection method for suprachoroidal space injection
The Comparative example differs from Test example 1 in that a common syringe
was used
for injection.
Syringes: BD syringe, 34G needle; WPI 34G blunt needle
Test method: After pentobarbital anesthesia, proparacaine hydrochloride eye
drops were used
for ocular surface anesthesia, and waited for 1 min-2 min after the eye drops,
the suprachoroidal
space was injected using the open conjunctival injection method. Specific
procedures: the
conjunctiva was first cut open using conjunctival scissors to completely
expose the sclera at the
injection site, then the sclera was punctured using a BD syringe needle 34G,
and finally the
suprachoroidal space was injected using a WPI 34G blunt needle.
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
After execution of
CA 03224603 2023- 12- 29 40
the rabbits, their eyeballs were dissected and flat samples of the fundus
tissue were prepared, and
photographs were taken to record the distribution of the ICG solution in the
suprachoroidal space.
Test results: it is observed that severe reflux and subconjunctival residue of
the injected ICG,
severe ocular surface and subconjunctival spreading, and conjunctival
hemorrhage occurred.
Fundus tissue section results showed no staining of the fundus tissue. The
test results showed that
the injection was unsuccessful.
Comparative example 2
Hub body microneedle was used for suprachoroidal space injection (without
sleeve)
Hub body microneedle used was the one with an effective length for
suprachoroidal space
being set as 700 gm and a blade length being set as 450 gm based on the
scleral tissue and the
regular thickness of the choroid in New Zealand rabbits.
Test methods: after pentobarbital anesthesia, ocular surface anesthesia was
performed using
proparacaine hydrochloride eye drops, waited for 1-2 minutes after the eye
drops, and the sclera
was punctured vertically using a homemade hub-body microneedle and
suprachoroidal space
injection was performed.
Test animals: healthy New Zealand rabbits, 1.8-2.2 kg
Injection frequency: single-site injection in the right eye
Injection reagent: 0.2% ICG, 100 gl
The injection conditions such as reflux, ocular surface spread, and
subconjunctival residue,
spread, hemorrhage, or congestion were recorded during the injection process.
After execution of
the rabbits, the eyeballs were dissected and flat samples of the fundus tissue
were prepared, and
photographs were taken to record the distribution of the ICG solution in the
suprachoroidal space.
Test results: it is observed that ICG injection reflux and subconjunctival
residue were obvious,
ocular surface and subconjunctival diffusion were obvious, and a small amount
of congestion
existed in the subconjunctiva. As shown in FIG. 55, the results of the fundus
tissue section showed
that the ICG-stained area of the fundus tissue was less than 2/3, and the
stained fundus tissue was
light green. The test results showed that the injection was unsuccessful.
The various embodiments of the present invention are described in a
progressive manner,
with each embodiment focusing on the differences from other embodiments, and
the same or
similar parts between the embodiments may be cross-referenced. Since the
device disclosed in the
embodiments corresponds to the method disclosed in the embodiments, it is only
briefly described,
and the relevant contents can be found in paragraphs illustrating the methods.
Although the devices and methods of the present invention are described for
delivering a drug
in the suprachoroidal space, in other embodiments, the devices and methods of
the disclosure may
be adapted to deliver any suitable therapeutic substance to any portion of the
eye, such as the
CA 03224603 2023- 12- 29 41
cornea, the conjunctiva, the retinal region, or the vitreous. In other
embodiments, either of the
disclosed devices and methods may be suitable for delivering any suitable
therapeutic substance
to any desired target tissue of the eye.
Specific components arranged in particular orientations or positions as
indicated above in
conjunction with the schematic drawings and/or embodiments may be adapted.
Similarly, the order
of particular methods and/or steps of the disclosure may be adapted. Although
embodiments have
been specifically shown and described, it should be understood that various
changes in form and
detail may be made.
An ocular injection assembly, an injection device and a method of use provided
by the present
invention are described in detail above. Specific embodiments have been
applied herein to
illustrate the principles and embodiments of the present invention. The above
illustrations of the
embodiments are only for helping understanding of the method of the present
invention and its
core ideas. It should be noted that, a number of improvements and
modifications can be made to
the present invention for a person of ordinary skilled in the art without
departing from the
principles of the present invention, and these improvements and modifications
also fall within the
scope of protection of the claims of the present invention.
CA 03224603 2023- 12- 29 42