Note: Descriptions are shown in the official language in which they were submitted.
CA 03225436 2023-12-22
1
METHOD OF REGULATING GENE EXPRESSION
PRIORITY
This patent application is a Canadian national phase application of
PCT/US2022/034706, which claims priority from Provisional United States Patent
Application Number 63/214,239, filed June 23, 2021, entitled, "FLUID
MANAGEMENT
SYSTEM," and naming Gianna N. Riccardi, Marcie Glicksman, Anthony DePasqua,
Kevin
Kalish, Rajan Patel, and Alan Watson as inventors.
CROSS REFERENCE TO RELATED APPLICATIONS
The subject matter of this patent may be related to the subject matter of U.S.
Patent Application No. 17/489,620 filed on September 29, 2021, and entitled,
"SUBARACHNOID FLUID MANAGEMENT METHOD AND SYSTEM WITH VARYING
RATES," which claims the benefit of, and priority to, United States
provisional patent
application number 63/084,996, filed September 29, 2020, and United States
provisional
patent application number 63/117,975, filed November 24, 2020.
The subject matter of this patent may be related to the subject matter of U.S.
Patent Application No. 17/495,682, filed on October 6, 2020, and entitled,
"SYSTEM
AND METHOD FOR CONTROLLING CSF FLOW AND MANAGING INTRACRANIAL
PRESSURE," which claims the benefit of, and priority to, United States
provisional patent
application number 63/088,401, filed October 6, 2020.
The subject matter of this patent may be related to the subject matter of U.S.
Patent Application No. 17/669,883 filed on February 11, 2022, and entitled
"METHODS
OF AMELIORATION OF CEREBROSPINAL FLUID AND DEVICES AND SYSTEMS
THEREFOR," which is a continuation of U.S. Patent Application Number
17/062,440, filed
on October 2, 2020, which is a continuation of PCT Patent Application Number
PCT/US20/27683, filed on April 10, 2020, which claims priority to and the
benefit of U.S.
Date Recue/Date Received 2023-12-22
CA 03225436 2023-12-22
2
Provisional Patent Application Number 62/832,486, filed on April 11, 2019, and
U.S.
Provisional Patent Application Number 62/960,861, filed on January 14, 2020.
GOVERNMENT RIGHTS
None
FIELD
Illustrative embodiments generally relate to medical devices and methods and,
more particularly, illustrative embodiments relate to devices and methods for
managing
subarachnoid fluid, such as cerebrospinal fluid ("CSF"), and/or drug delivery
that may be
used to treat neurodegenerative disorders.
BACKGROUND
When delivering a drug intrathecally, it is difficult to ensure that the
delivered
dosage reaches the target anatomy (e.g., part of the brain correlating to a
specific
disease, such as the cortical versus subcortical). It also is difficult to
verify the actual
dosage delivered to the target anatomy, as well as control, in real time, the
concentration of the drug in the fluid surrounding the target anatomy.
Date Recue/Date Received 2023-12-22
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
3
SUMMARY OF VARIOUS EMBODIMENTS
In accordance with one embodiment of the invention, a method of
regulating gene expression of a patient couples a fluid channel between the
lumbar region of the patient and the ventricle of the patient's brain. The
fluid
channel fluidly connects the lumbar region and the ventricle, and the fluid
channel has an access port and a pump. The pump is energized to cause CSF
to flow between the lumbar and ventricle of the patient. Antisense
oligonucleotide material (ASO) is added to the access port before, during,
and/or after energizing the pump.
The method may further include de-energizing the pump to cause the
CSF to flow under natural flow processes. The CSF may flow in one direction
only between energizing and de-energizing the pump.
The method may further de-energize the pump to cause the CSF to
flow under natural flow processes. The CSF may flow in two or more
directions between energizing and de-energizing the pump.
The method may further reverse the direction of the pump output to
cause the ASO to pass by the same point in the ventricle or lumbar regions in
two directions. The fluid channel may further include a catheter having a
lumen configured to transport ASO mixed with CSF of the patient. Adding
antisense oligonucleotide material may include adding a bolus of antisense
oligonucleotide material to the fluid channel through the access port.
The method may further include controlling, via a controller, the pump
output rate as a function of a parameter of the patient. The fluid channel may
form a closed loop configured to circulate CSF and ASO mixture through the
ventricle, the body chambers through which CSF flows, the lumbar, and fluid
channel in one or two directions. The fluid channel may include a catheter.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
4
Coupling a fluid channel between the lumbar region of the patient and
the ventricle may include locating a lumbar catheter in-vivo to the patient
and
extending from the patient's lumbar, locating a ventricle catheter in-vivo to
the patient and extending from the patient's ventricle, mechanically coupling
the catheter to the lumbar catheter, mechanically coupling the catheter to the
ventricle catheter, and mechanically coupling the to the lumbar and ventricle
catheters fluidly coupling the lumbar and ventricle of the patient via the
fluid
channel.
In accordance with another embodiment, a kit configured to assist with
regulating gene expression includes an antisense oligonucleotide material
(ASO) and a fluid management circuit. The fluid management circuit includes
a pump, a catheter having an internal lumen, an access port configured to
facilitate addition of ASO to the internal lumen of the catheter, and
mechanical couplings configured to couple the catheter between and with at
least two spaced apart internal catheters. The pump may be configured to
selectively flow in either of two directions. The kit may further include a
controller configured for controlling the pump output.
In accordance with another embodiment, a method of regulating gene
expression of a patient includes providing fluid management system
comprising a pump, a catheter, and an access port that is either part of the
catheter or separate from the catheter. The method includes securing
together the pump, catheter and access port to form a CSF fluid channel,
coupling the catheter to an internal lumbar catheter extending from and
embedded within the patient's the lumbar region, coupling another end of the
catheter to an internal ventricle catheter extending from and embedded
within the patient's the ventricle region, energizing the pump to cause CSF to
flow in one or two opposite directions between the lumbar region and
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
ventricle region of the patient; and adding antisense oligonucleotide material
(ASO) to the catheter through the access port before, during, and/or after
energizing the pump.
The method may further include de-energizing the pump to cause the
5 CSF to flow under natural flow processes. The CSF may flow in one
direction
only between energizing and de-energizing the pump.
The method may further include de-energizing the pump to cause the
CSF to flow under natural flow processes. The CSF may flow in two or more
directions between energizing and de-energizing the pump.
The method may further include reversing the direction of the pump
output to cause the ASO to pass by the same point within the ventricle or
lumbar region in two directions. The catheter may form a closed loop
configured to circulate CSF and ASO mixture through the ventricle, the body
chambers through which CSF flows, and the lumbar in one or two directions.
Adding antisense oligonucleotide material may include adding a bolus
of antisense oligonucleotide material to the catheter through the access port.
Adding antisense oligonucleotide material may include adding a
gradual flow of antisense oligonucleotide material to the catheter through the
access port.
The method may further include controlling, via a controller, the pump
to oscillate CSF fluid flow.
Illustrative embodiments of the invention are implemented as a
computer program product having a computer usable medium with computer
readable program code thereon. The computer readable code may be read
and utilized by a computer system in accordance with conventional processes.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
6
BRIEF DESCRIPTION OF THE DRAWINGS
Those skilled in the art should more fully appreciate advantages of
various embodiments of the invention from the following "Description of
Illustrative Embodiments," discussed with reference to the drawings
summarized immediately below.
Figure 1A schematically shows a cerebrospinal fluid circuit that may be
used with illustrative embodiments of the invention.
Figure 1B schematically shows an external catheter configured in
accordance with illustrative embodiments.
Figure 1C shows a high level surgical flow process in accordance with
illustrative embodiments of the invention.
Figure 2 schematically shows a two pump circuit with drug fed into
pump through separate fluid line in accordance with illustrative embodiments.
Figure 3 schematically shows a two pump circuit with drug introduced
directly into fluid line
Figure 4A schematically shows a flow control valve circuit that may be
used with illustrative embodiments.
Figure 4B schematically shows a syringe pump dosing circuit with a
drug introduced directly into the fluid line configured and usable with
illustrative embodiments.
Figure 5 schematically shows a two-pump circuit with a mixing
chamber in accordance with illustrative embodiments.
Figure 6 schematically shows a flow control valve with a mixing
chamber in accordance with other embodiments.
Figures 7 and 8 schematically show two different user interfaces in
accordance with illustrative embodiments.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
7
Figure 9 shows a process of localizing drug delivery to a target area of
the brain in accordance with illustrative embodiments.
Figure 10 schematically shows directing flow from lumbar to ventricle in
accordance with illustrative embodiments.
Figure 11 schematically shows directing flow from ventricle to lumbar in
accordance with illustrative embodiments.
Figure 12 schematically shows directing flow from lumbar to ventricle
with a pulsatile pattern in accordance with illustrative embodiments.
Figures 13A and 13B schematically show bidirectional pump circuits
that enable flow in two opposite directions (Figure 13B between right and left
ventricles in the brain) in accordance with illustrative embodiments.
Figure 14 schematically shows another system interface in accordance
with illustrative embodiments.
Figure 15 shows a process of manually programming drug delivery in
accordance with illustrative embodiments.
Figures 16A, 16B, and 16C detail an example of illustrative
embodiments of the invention.
Figures 17A, 17B, and 17C detail another example of illustrative
embodiments of the invention.
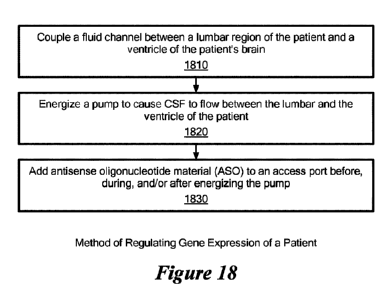
Figure 18 shows a method of regulating gene expression of a patient in
accordance with illustrative embodiments.
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
Illustrative embodiments regulate patient gene expression by
controllably circulating antisense oligonucleotide material (ASO) through a
closed fluid circuit formed between the patient's ventricle and lumbar
regions.
To that end, after coupling a fluid channel between those regions, such
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
8
embodiments add ASO to the fluid channel (preferably after the channel is
primed with CSF) and energize a pump to controllably flow the CSF and the
ASO mixed with the CSF. CSF/ASO fluid flow may be managed to localize
treatment (e.g., providing deep brain distribution) while minimizing toxicity
potentially caused by the ASO to certain nerves (e.g., the peripheral nerve).
Details of various embodiments are discussed below.
In additional illustrative embodiments, a system controllably applies a
therapeutic material, such as a drug (e.g., methotrexate, a chemotherapy,
small interfering RNA (siRNA), microRNA (miRNA), plasmid DNA, messenger
RNA (mRNA), small activating RNA (saRNA), splicing-modulatory AS0s, and
CRISPR (clustered regularly interspaced short palindromic repeats)/Cas
(CRISPR-associated protein, adenoviral vectors (AAV), and immunosuppressive
drug) to a specific anatomical location within the subarachnoid space or other
area. The therapeutic material, which also may be referred to herein as a
"drug," may be applied in a single large volume as a bolus, or dosed gradually
over a longer time. To that end, the system has a controller or control system
that manages distribution of the therapeutic material within a CSF circuit
through which cerebrospinal fluid ("CSF") flows. Specifically, among other
things, the controller (or "control system") manages pumps, valves, catheters,
and/or other structure(s) to control fluid flow, flow direction, and
frequencies
of certain periodic flows of bodily fluids (e.g., CSF), to provide a more
localized
and efficient therapeutic application to a patient.
Preferred embodiments enable the therapeutic material to penetrate
the blood-brain barrier by either selecting appropriate CSF and therapeutic
material flow rates, and/or controlling CSF flow to maintain a bolus of the
therapeutic material within CSF at/near a desired location in the CSF circuit.
Consequently, using various embodiments, medical practitioners can be more
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
9
comfortable applying the appropriate application of the therapeutic in the
patient, while reducing toxicity and, in some cases, reducing the need for
larger volumes of the therapeutic. Details of illustrative embodiments are
discussed below.
Many neurodegenerative diseases have been tied to the accumulation
of biomolecules (e.g., toxic proteins) contained in cerebrospinal fluid (CSF)
or
other fluids (e.g., interstitial fluid) within the subarachnoid space (SAS) of
a
mammalian subject. Problematically, these (e.g., toxic) biomolecules may be
secreted and then transported by the CSF to other cells in the body, which
process may occur over the span of years. For example, dipeptide repeat
proteins (DPRs) and/or TDP-43 have been implicated in neuronal death in the
pathology of amyotrophic lateral sclerosis (ALS, or Lou Gehrig's disease),
Alzheimer disease (AD), frontotemporal degeneration (FTD), Parkinson's
disease (PD), Huntington's disease (HD), and progressive supranuclear palsy
(PSP), to name just a few. Hence, research has focused primarily on the
removal of harmful DPRs. Techniques for removing DPRs and/or TDP-43 have
included: shunting CSF from the CSF space, diluting the CSF (e.g., with an
artificial fluid), administering a drug into the CSF, conditioning the CSF,
and/or
manipulating CSF flow.
Recent breakthrough techniques for handling this problem include
ameliorating the CSF, and treating a neurological disorder by removing or
degrading a specific (toxic) protein.
Amelioration, as used in various embodiments, involves systems and
methods for ameliorating a fluid in the subarachnoid space (SAS) (e.g., a
cerebrospinal fluid (CSF), an interstitial fluid (ISF), blood, and the like)
of a
mammalian subject, unless otherwise particularly distinguished (e.g., referred
to as solely CSF). Representative systems may be completely or partially
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
implanted within the body of the mammalian subject (discussed below).
Within the body, the systems and/or components thereof may also be
completely or partially implanted within the SAS and exposed to the exterior
via a port 16 (e.g., a medical valve that provides selective access to the
interior
5 system components). These systems execute processes that may occur
entirely in-vivo, or some steps that occur extracorporeally. Illustrative
embodiments ameliorate with a CSF circuit, discussed below.
Amelioration, for the purpose of illustration, may include changing the
physical parameters of the fluid, as well as digestion, removal,
immobilization,
10 reduction, and/or alteration, to become more acceptable and/or
inactivation
of certain entities, including: target molecules, proteins, agglomerations,
viruses, bacteria, cells, couples, enzymes, antibodies, substances, and/or any
combination thereof. For example, in some embodiments and applications,
amelioration may refer to removing toxic proteins from or conditioning one or
more of the blood, interstitial fluid, or glymph contained therein, or other
fluid, as well as the impact that this removal has on treating diseases or
conditions that affect various bodily functions, (i.e., improving the clinical
condition of the patient). Moreover, amelioration may be performed by any
one of: digestion, enzymatic digestion, filtration, size filtration,
tangential flow
filtering, countercurrent cascade ultrafiltration, centrifugation, separation,
magnetic separation (including with nanoparticles and the like),
electrophysical separation (performed by means of one or more of enzymes,
antibodies, nanobodies, molecular imprinted polymers, ligand-receptor
complexes, and other charge and/or bioaffinity interactions), photonic
methods (including fluorescence-activated cell sorting (FACS), ultraviolet
(UV)
sterilization, and/or optical tweezers), photo-acoustical interactions,
chemical
treatments, thermal methods, and combinations thereof. Advantageously,
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
11
various embodiments or implementations of the present invention may
reduce levels of toxicity and, after reduced, facilitate maintaining the
reduced
levels over time.
The extent of amelioration, as reflected by the concentration of the
target biomolecules, may be detected through a variety of means. These
include optical techniques (e.g., Raman, coherent Stokes, and anti-Stokes
Raman spectroscopy; surface enhanced Raman spectroscopy; diamond
nitrogen vacancy magnetometry; fluorescence correlation spectroscopy;
dynamic light scattering; and the like) and use of nanostructures such as
carbon nanotubes, enzyme linked immunosorbent assays, surface plasmon
resonance, liquid chromatography, mass spectrometry, circular proximity
ligation assays, and the like.
Amelioration may include the use of a treatment system (e.g., UV
radiation, IR radiation), as well as a substance, whose properties make it
suitable for amelioration. Amelioration of CSF or ameliorated CSF ¨ which
terms may be used interchangeably herein ¨ refers to a treated volume of CSF
in which one or more target compounds have been partially, mostly, or
entirely removed. It will be appreciated that the term removed, as used
herein, can refer not only to spatially separating, as in taking away, but
also
effectively removing by sequestering, immobilizing, or transforming the
molecule (e.g., by shape change, denaturing, digestion, isomerization, or post-
translational modification) to make it less toxic, non-toxic or irrelevant.
The term, "ameliorating agent" generally refers to a material or process
capable of ameliorating a fluid, including enzymes, antibodies, or antibody
fragments, nucleic acids, receptors, anti-bacterial, anti-viral, anti-DNA/RNA,
protein/amino acid, carbohydrate, enzymes, isomerases, compounds with
high-low biospecific binding affinity, aptamers, exosomes, ultraviolet light,
CA 03225436 2023-12-22
12
temperature change, electric field, molecular imprinted polymers, living
cells, and the
like. Additional details of amelioration are taught by PCT Application No.
PCT/US20/27683 (Publication No. WO 2020210634), filed on April 10, 2020. In a
similar
manner, details for further treatments are taught by PCT Application No.
PCT/US19/042880 (Publication No. WO 2020/023418), filed July 22, 2019.
To control CSF flow within the body (e.g., through the ventricle),
illustrative
embodiments form a CSF circuit/channel (identified by reference number "10")
that
manages fluid flow in a closed loop. Figure 1A, for example, shows one
embodiment of
such a CSF circuit 10. In this example, internal catheters 12 positioned in-
vivo/interior to
the body fluidly couple together via the subarachnoid space. To that end, a
first internal
catheter 12 fluidly couples a prescribed region of the brain (e.g., the
ventricle) to a first
port 16, which itself is configured and positioned to be accessible by
external
components. In a corresponding manner, a second catheter couples the lumbar
region
or the lower abdomen of the subarachnoid space with a second port 16 that,
like the
first port 16, also is configured to be positioned and accessible by external
components.
The first and second ports 16 may be those conventionally used for such
purposes, such
as a valved Luer-lock or removable needle. The first and second internal
catheters 12
thus may be considered to form a fluid channel extending from the first port
16, to the
ventricle, down the spine/subarachnoid space to the lumbar, and then to the
second
port 16. These internal components, which may be referred to as "internal CSF
circuit
components," are typically surgically implanted by skilled professionals in a
hospital
setting.
Date Recue/Date Received 2023-12-22
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
13
The CSF circuit 10 also has external components (referred to as
"external CSF circuit components). To that end, the external CSF circuit
components include at least two fluid conduits 14. Specifically, the external
CSF circuit components include a first external fluid conduit 14, that couples
with the first port 16 for access to the ventricle. The other end of the first
external conduit 14 is coupled with a management system 19, which includes
one or more CSF pumps (all pumps are generically identified in the figures as
reference number "18"), one or more user interface/displays 20, one or more
drug pumps 18, and a control system/controller 22. The fluid external fluid
conduit 14 may be implemented as a catheter and thus, that term may be
used interchangeably with the term "conduit" and be identified by the same
reference number 14.
Illustratively, this management system 19 is supported by a
conventional support structure (e.g., a hospital pole 24 in Figure 1A). To
close
the CSF circuit 10, a second external catheter 14 extends from that same CSF
management system 19 and couples with the second port 16 and the
management system 19. This management system 19 and external catheters
14 therefore form the exterior part of a closed CSF circuit 10 for circulating
the
CSF and therapeutic material.
It should be noted that the CSF circuit 10 may have one or more
components between the first and second ports 16 and the respective
removable connections of the first and second external catheters 14. For
example, the first port 16 may have an adapter that couples with the first
external catheter 14, or another catheter with a flow sensor may couple
between such external catheter 14 and port 16. As such, this still may be
considered a removable connection, albeit an indirect fluid connection. There
may be corresponding arrangements with the other end of the first external
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
14
catheter 14, as well as corresponding ends of the second external catheter 14.
Accordingly, the connection can be a direct connection or an indirect
connection.
The first and second external catheters 12 and 14 preferably are
configured to have removable connections/couplings with the management
system 19, as well as their respective ports 16. Examples of removable
couplings may include a screw-on fit, an interference fit, a snap-fit, or
other
known removable couplings known in the art. Accordingly, a removable
coupling or removable connection does not necessarily require that one
forcibly break, cut, or otherwise permanently break the ports 16 for such a
connection or disconnection. Some embodiments, however, may enable a
disconnection form the first and/or second ports 16 via breaking or otherwise,
but the first and/or second ports 16 should remain in-tact to receive another
external catheter 14 (e.g., at the end of life of the removed external
catheter
14).
Figure 1 B schematically shows more details of the first and/or second
external conduits/catheters 14. This figure shows an example of an external
catheter 14 operating with other parts of the system. As shown, the system
receives a drug reservoir 17 (e.g., a single-use syringe) configured to
deliver a
dose of therapeutic material (e.g., a drug) that fluidly couples with the
catheter
14 via a check valve 28 and T-port 19 on the catheter 14. In addition, the
catheter 14 is coupled with a mechanical pump 18 and also preferably
includes a sample port 23 with flow diverters 25 for diverting flow toward or
away from a sample port 23. The sample port 23 preferably has sample port
flow sensors 23A to track samples.
Some embodiments may be implemented as a simple catheter having a
body forming a fluid-flow bore with removably couplable ends (or only one
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
removably couplable end). Illustrative embodiments, however, add intelligence
to make one or both of these external catheters 14 "smart" catheters,
effectively creating a more intelligent flow system. For example, either one
or
both of the external catheters 14 can have a processor, ASIC, memory,
5 EEPROM (discussed below), FPGAs, RFID, NFC, or other logic (generally
identified as reference number "27") configured to collect, manage, control
the device, and store information for the purposes of security, patient
monitoring, catheter usage, or communicating with the management system
19 to actively control fluid dynamics of the CSF circuit 10. Among other
10 things, the management system 19 may be configured to coordinate with an
EEPROM 27 to control CSF fluid flow as a function of the therapeutic material
infusion flow added to the CSF circuit 10 (discussed below) via the check
valve
28 at the output of the drug reservoir 17.
As shown in Figure 1B, one embodiment of the external catheter 14 has
15 .. the noted electrically erasable programmable read-only memory, EEPROM
27,
(or other logic/electronics) that can be implemented to accomplish a variety
of
functions. Among others, the EEPROM 27 can ensure that the CSF circuit 10
and its operation is customized/individualized to a patient, a treatment type,
a
specific disease, and/or a therapeutic material. For example, in response to
reading information stored in the EEPROM 27, the control system 22 may be
configured to control fluid flow as a function of the therapeutic material.
Importantly, as a disposable device, the EEPROM 27 or other logic of
the external catheter 14 can be configured to provide alerts, and/or produce
or cause production of some indicia (e.g., a message, visual indication, audio
indication, etc.) indicating that the external catheter 14 has reached an end
of
its lifecycle, or indicating how much of its lifecycle remains. For example,
an
external surface of the catheter 14 may have a tag that turns red when the
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
16
EEPROM 27 and/or other logic 27 determines that the external catheter 14 has
reached its full lifetime use. For example, the external catheter 14 may be
considered to have a usage meter, implemented as some logic or EEPROM 27,
configured to track use of the CSF fluid conduit 14 to help ensure it is not
used beyond its rated lifetime. Moreover, the logic or EEPROM 27 can register
with the control system 22 to start use timers to reduce tampering or use
beyond a lifetime.
Some embodiments have a printable circuit board (PCB) equipped with
a wireless interface (e.g., Bluetooth antenna) or a hardware connection
configured to communicate the pump 18 and/or control system 22. The
external catheter 14 can be configured to time out after a certain period,
capture data, and communicate back and forth with the control system 22 or
other off-catheter or on-catheter apparatus to share system specifications and
parameters. The intelligent flow catheter 14 can be designed with proprietary
connections such that design of knockoffs or cartridges 26 (discussed below)
can be prevented to ensure safety and efficacy of the CSF circuit 10 and
accompanying processes.
In addition to the management logic, the external catheter(s) 14 also
may have a set of one or more flow sensors and/or a set of one or more
pressure sensors. Both of those flow sensors are shown generically at
reference number 29, and may be located upstream or downstream from their
locations in Figure 1B. For example, the left sensor(s) 29 generically shown
in
Figure 1B can be a flow sensor, pressure, or both a flow sensor and pressure.
The same can be said for the right sensor(s) 29 generically shown in Figure
1B.
They preferably are positioned between the ports 16 on the body and the
remaining components as shown.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
17
Of course, the flow sensor(s) 29 may be configured to detect flow
through the bore of the catheter body, while the pressure sensor(s) 29 may be
configured to detect pressure within the bore of the body. Among other
functions, the flow sensor(s) 29 may monitor flow rate of fluid through the
conduit bore and/or total flow volume through the conduit bore.
The catheter 14 preferably is configured to have different hardness
values at different locations. Specifically, illustrative embodiments may use
a
mechanical pump 18, as shown and noted above. The pump 18 may
periodically urge a compressive force along that portion of the catheter 14 it
contacts at its interface 18A with the catheter 14. The outlet of the pump 18
in
this case may be the portion of the catheter 14 that is receiving the output
of
a neighboring compressed catheter portion (e.g., a portion that is adjacent to
the compressed catheter portion(s). To operate efficiently, illustrative
embodiments form the catheter 14 to have a specially configured hardness at
that location (e.g., 25-35 Shore A). Diameter also is important for flow and
thus, one skilled in the art should determine appropriate diameters as a
function of performance and durometer/hardness. Preferably, the catheter
portion that contacts the pump 18 is softer than the remainder of the catheter
14, although both could have the same hardness. Accordingly, the catheter
preferably has a variable hardness along its length and may even have a
variable diameter.
Alternative embodiments may provide an open-loop CSF fluid circuit
10. For example, the CSF fluid circuit 10 may have an open bath (not shown)
to which fluid is added and then removed. The inventors expect the closed-
loop embodiment to deliver better results, however, than those of the open-
loop CSF fluid circuit 10.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
18
Illustrative embodiments are distributed to healthcare facilities and/or
hospitals as one or more kits. For example, one more inclusive kit may include
the internal and external catheters 12 and 14. Another exemplary kit may
include just the internal catheters 12 and the ports 16 (e.g., for a
hospital),
while a second kit may have the external catheters 14 and/or a single-use
syringe. Other exemplary kits may include the external catheters 14 and other
components, such as the management system 19 and/or a CSF treatment
cartridge 26. See below for various embodiments of the CSF circuit 10 and
exterior components that also may be part of this kit.
In some embodiments, the kit may include a number of other or similar
items. For example, the kit may include an antisense oligonucleotide material
(ASO) and a fluid management circuit with a pump, catheter, access port, and
mechanical couplings. Some of those features, such as the access port, may be
integrated into the other components. For example, the access port may be
.. part of the catheter, or a separate component. As another example, the
mechanical couplings may be integral with the catheter or separate.
Accordingly, when coupled, these pumps 18, valves (discussed below
and all valves generally identified by reference number 28), internal and
external catheters 14, and other components may be considered to form a
fluid conduit/channel that directs CSF to the desired locations in the body.
It
should be noted that although specific locations and CSF containing
compartments are discussed, those skilled in the art should recognize that
other compartments can be managed (e.g., the lateral ventricles, the lumbar
thecal sac, the third ventricle, the fourth ventricle, and/or the cisterna
magna).
Rather than accessing the ventricle and the lumbar thecal sac, both lateral
ventricles could be accessed with the kit. With both internal catheters 12
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
19
implanted, CSF may be circulated between the two lateral ventricles, or a drug
could be delivered to both ventricles simultaneously.
In illustrative embodiments, the CSF management system 19 generally
manages fluid flow to target anatomy through the CSF circuit 10. To that end,
that management system 19 has at least one pump 18 that directs flow of the
CSF, and at least one pump 18 that directs flow of a therapeutic material
(e.g.,
a drug) though the CSF circuit 10 to desired anatomy. Alternative
embodiments may have more pumps 18 for these functions, or combine
pumps 18 for these functions. The management system 19 also has a plurality
of valves 28 to control flow, and the control system 22, as noted, is
configured
to control the pumps 18 to selectively apply the drug-carrying CSF to desired
local anatomy. Figure 1A also shows a user interface 20 that enables a
clinician to control drug and fluid parameters in the CSF circuit 10
(discussed
below) via the control system 22.
Some embodiments may use a monitoring process, such as real-time
spectroscopy, to monitor drug concentrations in the CSF. In some of these
embodiments, a spectrophotometric sensor may be placed in the CSF circuit
10 to measure the localized concentration of a substance based on its
absorption at various wavelengths. For example, some embodiments may use
a sensor constructed to measure a single wavelength or multiple wavelengths.
The reading taken by the sensor may be relayed to the control system 22,
where it would then be stored or processed for various purposes. This signal
could be processed for a number of purposes, such as to trigger the control
system 22 to alter the fluid flow, flow direction, and/or frequencies of
certain
periodic flows of bodily fluids (e.g., CSF) to provide a more localized and
efficient therapeutic application to a patient in real-time. It will be
appreciated
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
that the signal could also be stored or displayed such that the changes to
flow, direction or frequencies of period flows could be adjust manually.
Figure 1C shows a high level surgical flow process that may incorporate
the CSF circuit 10 of Figure 1A in accordance with illustrative embodiments of
5 the invention. It should be noted that this process is substantially
simplified
from a longer process that normally would be used to complete the surgical
flow. Accordingly, this process may have many additional steps that those
skilled in the art likely would use. In addition, some of the steps may be
performed in a different order than that shown, or at the same time. Those
10 skilled in the art therefore can modify the process as appropriate.
Moreover,
as noted above and below, many of the materials, devices, and structures
noted are but one of a wide variety of different materials and structures that
may be used. Those skilled in the art can select the appropriate materials and
structures depending upon the application and other constraints.
15 Accordingly, discussion of specific materials, devices, and structures
is not
intended to limit all embodiments.
The process begins at step 100 by setting up the internal catheters 12
inside the patient. To that end, step 100 accesses the ventricles and thecal
sacs
using standard catheters and techniques, thus providing access to the CSF.
20 Step 102 then connects access catheters 12 to peritoneal catheters 12,
which
are tunneled subcutaneously to the lower abdomen. The tunneled catheters
12 then are connected at step 104 to the ports 16 implanted in the abdomen.
At this point, the process sets up an extracorporeal circulation set (i.e.,
the external catheters 14, or the "smart catheters" in some embodiments). To
that end, step 106 may prime and connect the extracorporeal circulation set
14 to the subcutaneous access ports 16. Preferably, this step uses an
extracorporeal circulation set, such as one provided by Endear Therapies, Inc.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
21
of Newburyport, MA, and/or the external catheters 14 discussed above. The
process continues to step 110, which connects an infusion line or other
external catheter 14 to the management system 19, and then sets the target
flow rate and time. At this point, setup is complete and treatment may begin
(step 112).
The process then removes endogenous CSF from the ventricle. This CSF
may then be passed through a digestion region (e.g., through a cartridge 26
having a specific digesting material), where certain target proteins in the
CSF
are digested. For example, the cartridge 26 may have an inner plenum space
1830 of the cartridge 26 filled with a plurality of (e.g., porous,
chromatography
resin) beads that have been compression packed. To prevent constituents
from entering or escaping from the cartridge 26, a filter membrane may be
disposed at the first end of the cartridge 26 and a second filter membrane
may be disposed at the second end of the cartridge 26. In some applications,
the ameliorating agent may be decorated on the beads 1835.
In some applications, the cartridge 26 may be compression packed with
a chromatography resin (e.g., agarose, epoxy methacrylate, amino resin, and
the like) that has a protease covalently bonded (i.e. immobilized) to the
three-
dimensional resin matrix. The selected protease may be configured to
degrade and/or removing target toxic biomolecules by way of proteolytic
degradation. The resin may be a porous structure having a particle size
commonly ranging between 75-300 micrometers and, depending on the
specific grade, a pore size commonly ranging between 300-1800 A. Thus, at a
high level, the cartridge 26 has ameliorating agent that removes and/or
substantially mitigates the presence of toxic proteins from the CSF.
This and similar embodiments may consider this to be an input for the
digesting enzyme. Any location providing access to the drug may be
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
22
considered to be an input for the drug. At step 116, the treated CSF exits the
digestion region and is returned via the CSF circuit 10 to the lumbar thecal
sac. The process concludes at step 118, which stops the pump 18 when
treatment is complete. The management system 19 then may be disconnected
and the ports 16 flushed.
In accordance with illustrative embodiments, the CSF circuit 10 is
configured to improve the likelihood of the drug passing through the blood-
brain barrier. To that end, the management system 19 enables the user or
logic to independently set both the flow rate of CSF circulation (e.g.,
between
.05 ml/min and 2.0 ml/min, such as 0.5 mVmin) and the dosing rate of the
drug (e.g., between .01 ml/min to 2.0 ml/min, such as .02 ml/min). Preferably,
these rates are different, although they can be the same. In illustrative
embodiments, the CSF circulation rate is controlled to be different from the
natural CSF flow rate. Note that the natural CSF flow rate is the rate of CSF
flow without intervention by outside equipment, such as the pumps 18 and
other CSF circuit components¨even if it is within a range of typical non-
interventionally controlled CSF flow rates. Thus, unless the context dictates
otherwise, the non-natural CSF flow rate is the flow rate with such
intervention. In other embodiments, the CSF flow rate is simply changed from
its truly natural flow rate¨i.e., the rate at which the CSF flows without
intervention.
Depending on a number of factors, the CSF flow rate may be greater
than the rate of drug infusion, while in other embodiments, the CSF flow rate
is less than rate of the drug infusion rate. Other embodiments may set them
to be equal. Those skilled in the art can select the appropriate flow rate
based
on a variety of factors, including the drug being delivered, the illness,
patient
profile, rated pressure of the CSF circuit 10, etc.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
23
The inventors recognized that varying the two rates in a coordinated
manner enables more control of the drug dose as well as more control of the
drug treatment time. Stated another way, these two independent flow rates
enable setting of the dosing rate, which allows the user to optimize drug
.. concentration. At the same time, having the ability to set the flow rate
allows
the user to control the rate of delivery (as opposed to relying upon natural
CSF flow).
As noted in the example below, the inventors were surprised to
discover that varying the rates in this manner enabled penetration of the drug
across the otherwise difficult to penetrate blood-brain barrier. The selected
CSF flow rate may be constant or variable. For example, the CSF flow rate may
be set to a first rate for a first period of time, a second rate for a second
period of time, and a third rate for a third period of time. As such, various
embodiments enable flow of the CSF within the CSF circuit 10 at two or more
flow rates at two or more different times. The drug delivery rate may be
constant or variable in a similar manner, but coordinated with the CSF flow
rate to deliver preferred results.
The inventors recognized that a wide variety of different CSF circuit
configurations can accomplish the desired goals. Figure 2 schematically shows
a two pump CSF circuit 10 with the drug fed into the pump 18 through a
separate fluid line/catheter 12/14 in accordance with illustrative
embodiments.
In a corresponding manner, Figure 3 schematically shows a two pump CSF
circuit 10 with drug introduced directly into fluid line.
In one embodiment, the CSF circuit 10 has two pumps 18, Pump 1 and
Pump 2, to enable a user to set flow rate and dosing rate independently. To
that end, Pump 1 may be programmed to control the rate of CSF circulation,
while Pump 2 may be programmed to control the dosing rate of the drug to
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
24
be delivered. Both pumps 18 could be programmed to achieve a desired
delivery profile. Check valves 28 or other flow control devices prevent
backflow into either pump 18.
As show, the drug may be fed into the pump 18 through a separate
fluid line/catheter (Figure 2) and input to mix with the patient's CSF in the
internal catheter/tubing set 12 before being reintroduced to the body.
Alternatively, the drug may also be pre-loaded into a cartridge 26 or other
type of drug reservoir and connected directly into the fluid line/catheter 14
(Figure 3). In this latter embodiment, the CSF mixes with the drug as it flows
through the cartridge 26 and tubing set 12/14. Figure 4A schematically shows
another embodiment in which a flow control valve 28 is used in place of Pump
2. In this embodiment, that flow control valve 28 preferably is programmed to
control the dosing rate (i.e., the rate of adding the drug to the CSF circuit
10
carrying the CSF.
Figure 5 schematically shows a two-pump circuit 10 with a mixing
chamber 30 in accordance with illustrative embodiments. In particular, to
ensure a homogeneous mixture of CSF and the drug being delivered, the
noted mixing chamber 30 is added to both the two-pump circuit 10 (Figure 5)
and the flow control valve 28 circuit (Figure 6). The mixing chamber 30 can
contain a sensor that provides a readout of a drug's concentration in the CSF,
or the management system 19 could simply be programmed to produce a
specific drug concentration in the CSF.
In Figure 5, the CSF-circulating pump 18 and the dosing pump 18 (via
an input) feed into the mixing chamber 30 at independent programmable
rates. Upon entering the chamber, a small turbine mixes the fluids and the
homogeneous mixture is expelled and returned to the patient anatomy. The
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
same concept applies to Figure 6, but some in-line mixing occurs before the
fluids reach the mixing chamber 30.
Whether controlling dosing rate by a second pump 18 or by a flow
control valve 28, CSF delivery may be manually programmed on an
5 interface/display 20 similar to Figures 7 and 8. Specifically, Figures 7
and 8
schematically show two different user interfaces 20 in accordance with
illustrative embodiments. Rather than requiring the user to input a dosing
rate, however, the user may specify a drug concentration and the
management system 19 responsively may adjust the dosing rate accordingly
10 to achieve that concentration. The user can also input a maximum dosage.
After this dosage was reached, the management system 19 would
automatically stop treatment. Figure 8 shows one such interface 20 (e.g., a
graphical user interface or a manual interface).
It should be noted that the during actual processing, the CSF flow rate
15 may differ at different parts of the CSF circuit 10¨total CSF flow rate
in the
CSF circuit 10 is not necessarily homogenous. For example, some parts of the
CSF circuit 10 may be wider (e.g., certain human geographies) and thus, may
be slower than the average CSF circuit flow rate, while other portions may be
narrower, causing a nozzle effect and increasing the CSF flow rate at that
20 point. Near the pump 18 (e.g., at the pump outlet), however, the CSF
flow rate
can be controlled to provide a desired rate across the entire CSF circuit 10,
even if that rate may deviate in local parts of the CSF circuit.
The discussion above relates to delivering a therapeutic material, such
as a drug, over a longer infusion period (e.g., 5 minutes, 10 minutes, 30
25 minutes, 1-6 hours, days, etc.). Figure 9 shows another embodiment that
localizes drug delivery at a target area of the brain using a bolus drug
infusion. Specifically as known by those in the art, a dose of drug can be
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
26
delivered in a short time period (e.g., 10 seconds, 20 seconds, 60 seconds),
or
over a longer period (i.e., gradual drug administration, as noted above). The
shorter drug delivery is known in the art as a "bolus" drug delivery.
Specifically, to optimize delivery, Figure 9 alternates the flow direction
of the pump 18. The pump 18 thus has programmable controls, via the control
system 22, for flow rate and frequency of these alternations. The flow rate
and
frequency preferably are programmed to achieve a desired delivery profile.
In a manner similar to the other process discussed above, the process
of Figure 9 is substantially simplified from a longer process that normally
would be used to complete the localize drug delivery. Accordingly, this
process may have many additional steps that those skilled in the art likely
would use. In addition, some of the steps may be performed in a different
order than that shown, or at the same time. Those skilled in the art therefore
can modify the process as appropriate. Moreover, as noted above and below,
many of the materials, devices, and structures noted are but one of a wide
variety of different materials and structures that may be used. Those skilled
in
the art can select the appropriate materials and structures depending upon
the application and other constraints. Accordingly, discussion of specific
materials, devices, and structures is not intended to limit all embodiments.
Figure 9 therefore delivers a drug intrathecally using positive
displacement at a desired flow rate. It may incorporate the components
discussed above, as well as principles discussed for other embodiments, such
as that discussed above with regard to Figure 2. The process of Figure 9
begins at step 900 by adding the drug in a bolus dose to the CSF circuit 10,
and/or administering a tag for imaging to the drug. In the latter example, its
position can then be tracked using standard imaging techniques to determine
when the drug has reached the target anatomy. Alternative embodiments add
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
27
the drug to the CSF without administering a tag. Such steps may use other
techniques to ensure the drug is localized at the desired target anatomy.
Step 902 sets the desired flow rate, direction, timing, and other
parameters for the CSF circuit 10 to accomplish the bolus application. For
example, specific computer program code on a tangible medium within the
control system 22 may cooperate with other components of the CSF circuit 10
to control addition of the therapeutic material, localize the therapeutic
material, or both. Next, after step 904 verifies the position of the drug at a
target anatomy, step 906 controls the pump 18 to maintain the drug at that
target location. Among other ways, step 906 may control the pump(s) 18 to
oscillate at a desired flow rate and frequency to contain the drug at that
prescribed or desired anatomical location for a pre-set period of time.
After the bolus reaches the target anatomy, the pump 18, which can be
programmable and/or have logic, can reverse CSF flow; specifically, the pump
18 can alternate quickly between pushing and pulling flow of the CSF so that
the bolus of drug is localized to the target anatomy in the brain (or another
target anatomy). In other words, the higher concentration of drug in a portion
of the CSF can moved back and forth over the target region. Other
embodiments can simply slow down the CSF flow rate to ensure a longer drug
application to the target. Either way, these embodiments preferably "soak" the
target with the drug, providing a higher quality drug administration. As a
result, despite using less of the drug than would be administered by prior art
systems, this embodiment still administers a desired amount of the drug to
the target by this localizing technique, consequently minimizing toxicity and
drug costs (step 908).
It should be noted that "reaching" the target anatomy may be defined
by the user or other entity within the control system 22. For example, the
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
28
portion of CSF in the CSF circuit 10 having the higher concentration of drug
(from the bolus) may be considered to have reached the target anatomy when
some identifiable portion of it (e.g., the highest concentration, or an
interior
point within the spread of the drug in the CSF) may be within a prescribed
distance upstream of the target, or a prescribed distance downstream of the
target. Some embodiments may require the defined portion of CSF with the
high drug concentration to actually be at or in contact with that target
region.
Other embodiments may consider the drug to have "reached" the target
simply by calculating the time it should take to reach that area, using
artificial
intelligence/machine learning, and/or through empirical studies.
Illustrative embodiments can be implemented in a number of different
manners with catheters 12/14, pumps 18, valves 28, etc. similar to those
discussed above (including the noted external catheters 14). Figures 10-14
show several exemplary implementations. In the embodiment shown in Figure
10, the CSF circuit 10 has four pinch valves 28 on tubing (i.e., external
catheters 14), enabling fluid oscillation between opposing flow directions. To
flow from lumbar to ventricle (Figure 10), pinch valves 1 and 2 are opened
while pinch valves 3 and 4 are closed. Conversely, to switch flow direction
from ventricle to lumbar (Figure 11), pinch valves 1 and 2 are closed while
pinch valves 3 and 4 are opened. Controlling the pinch valves 28 in this
manner enables flow direction oscillation. The frequency at which the pinch
valves 28 switched between open and closed may be set by the user as could
the flow rate of the pump 18 (e.g., via the control system 22). Alternative
embodiments may pre-program such parameters into the management
system 19.
In fact, the same pinch valve 28 configuration (Figure 12) may be used
to create a pulsatile flow pattern. For example, when flowing from lumbar to
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
29
ventricle, pinch valves 3 and 4 remain closed, while pinch valves 1 and 2 are
pulsed (i.e., periodically switched between open and closed) at a frequency
set
by the user.
The ability to set the frequency at which the pinch valves 28 open and
close enables a range of pulsatile effects to be implemented. For example,
rather than rapidly switching between open and closed pinch valves 28, the
valves 28 can remain closed long enough to build up a set pressure in the
fluid line. Shortly after opening the pinch valves 28, a bolus of the drug can
be
released as a result of the pressure build-up.
Flow direction oscillation and a pulsatile flow pattern could also be
produced using a bidirectional pump 18 instead of using pinch valves 28 (e.g.,
Figure 13A and Figure 13B). The pump 18 can be programmed to switch flow
directions at a frequency set by the user. While flowing in one direction, the
pump 18 can be programmed to pulse by starting and stopping at a
frequency also set by the user. Those skilled in the art may use other
techniques to provide bidirectional flow.
In addition to those noted above, some embodiments may set the
frequency, flow rate, and other parameters as a function of the requirements
and structure of the anatomy and devices used in the treatment (e.g., in the
CSF circuit 10). Among others, those requirements may include the diameter
of the catheters in the CSF circuit 10, physical properties of the drug, the
interaction of the drug at the localized region, the properties of the
localized
region, and other requirements and parameters relevant to the treatment.
Those skilled in the art may select appropriate parameters as a function of
the
requisite properties.
Figure 14 schematically shows another system interface 20 configured
in accordance with illustrative embodiments. Specifically, whether controlling
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
delivery parameters by pinch valve, a bidirectional pump 18, or other means,
the delivery profile can be controlled manually with an interface 20, such as
the interface 20 shown in Figure 14, and/or a delivery profile loaded onto the
management system 19. As with the other interfaces, this interface 20 may be
5 a fixed control panel, or a graphical user interface on a display device.
Figure 15 shows a process of manually programming drug delivery of a
bolus in accordance with illustrative embodiments. In a manner similar to the
other process discussed above, this process is substantially simplified from a
longer process that normally would be used to complete the localize drug
10 delivery. Accordingly, this process may have many additional steps that
those
skilled in the art likely would use. In addition, some of the steps may be
performed in a different order than that shown, or at the same time. Those
skilled in the art therefore can modify the process as appropriate. Moreover,
as noted above and below, many of the materials, devices, and structures
15 noted are but one of a wide variety of different materials and
structures that
may be used. Those skilled in the art can select the appropriate materials and
structures depending upon the application and other constraints.
Accordingly, discussion of specific materials, devices, and structures is not
intended to limit all embodiments.
20 The process begins at step 1500, which sets the flow direction. Three
options include lumbar to ventricle (1502A), ventricle to lumbar (step 1502B),
or oscillating between flow directions (step 1502C). Next, the process sets
the
flow rate at steps 1504A or 1504B, and sets the frequency of the pulse (step
1506A) or oscillation frequency (step 15068). Alternative embodiments can be
25 programmed using artificial intelligence algorithms or other program
logic.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
31
Example 1: Administration of Methotrexate to a sheep using illustrative
embodiments
The inventors administered methotrexate to sheep using illustrative
embodiments. An outline of the study is depicted by Figures 16A-16C. A
sheep used for this experiment received the CSF circuit 10 of illustrative
embodiments. Circulation was started at the same time that methotrexate was
infused. Methotrexate was infused at a gradual rate of 2 mLs over 2 minutes
and then recirculation from lumbar to ventricle was started at a rate of 0.2
mLs/min. Circulation continued after drug infusion was stopped for four more
hours. At zero to three hours, the circulation rate was at 0.1 mLimin and from
four to six hours, circulation was at 0.3 mL/min. The dose of methotrexate
infused was 12 mg. Drug concentration was measured with LC/MS (liquid
chromatography with the mass analysis capabilities of mass spectrometry) in
the CSF, spinal cord, and multiple brain regions.
Figure 16B schematically shows the results. CSF levels of methotrexate
were analyzed over time. Drug levels were found to be very high in the lumbar
region where the drug was infused and a decline over time was measured
except for an increase at 5 hours and then a subsequent decline. CSF levels of
methotrexate were very low in the ventricular samples initially, but with
time,
increased at 4 and 5 hours, before declining to a similar level as the lumbar
samples.
Figure 16C shows how samples from twelve regions of the brain were
homogenized and analyzed for methotrexate levels and measured as ug/mL/g
of tissue. The x-axis of this graph is drug levels. All areas of the brain and
spinal cord had good levels of methotrexate. It will be appreciated that
methotrexate that is administered typically subdural in the thigh typically
does
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
32
not cross the blood-brain barrier and would not be found in appreciable levels
in brain and spinal cord as a result.
Example 2: Administration of Antisense Oligonucleotide to a sheep using
illustrative embodiments
The inventors administered an antisense oligonucleotide (ASO) for
Huntington's disease to sheep using illustrative embodiments. An outline of
the study is depicted in Figures 17A-C. Each sheep used for this experiment
received the CSF circuit 10 of illustrative embodiments. Circulation was
started
at the same time that ASO was infused. ASO was infused at a rate of 2 mLs
over 2 min. The dose of ASO infused was 30 mg. Circulation continued after
drug infusion was stopped for four more hours. At zero to four hours, the
circulation rate was at 0.2 mL/min. For unidirectional flow, the direction of
the
flow was lumbar to ventricle me for the entire 4h. For the bidirectional flow,
the first lh of flow was lumbar to ventricle, then the direction of flow was
reversed to ventricle to lumbar for 10 min, then switched repeatedly for 10
min intervals for the remainder of the 4h. All sheep in the study were
necropsied after 48h and tissues were collected to assay drug levels.
The assay for detection of the ASO is depicted in Figure 17B. Sandwich
hybridization ELISA (Enzyme-Linked-immuno-absorption-Assay) quantification
used to measure the concentration of CAG repeats in tissue, CSF, and blood
samples. Probes comprised of a sequence complementary to the analyte.
Capture DNA probe conjugated to biotin label and applied to a streptavidin-
coated microtiter plate. Detection DNA probe with digoxigenin label was used.
To detect digoxigenin label, anti-digoxigenin (anti-Dig) peroxidase (POD) is
reacted with substrate TMB for the signal measurement by an absorption
change with a plate reader.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
33
Figure 17C schematically shows the results. Samples from seventeen
regions of the brain were homogenized and analyzed for ASO levels and
measured as ug/g of tissue. The x-axis of this graph is drug levels. Most
areas
of the brain and spinal cord had good levels of ASO. It will be appreciated
that the ASO if administered orally, subdurally, or intravenously typically
does
not cross the blood-brain barrier and would not be found in appreciable levels
in brain and spinal cord as a result.
Figure 18 shows a method of regulating gene expression of a patient in
accordance with illustrative embodiments. It should be noted that this
process is substantially simplified from a longer process that normally would
be used to regulate gene expression. Accordingly, the process may have
additional steps that those skilled in the art likely would use. In addition,
some of the steps may be performed in a different order than that shown, or
at the same time. Those skilled in the art therefore can modify the process as
appropriate. Moreover, as noted above and below, many of the materials and
structures noted are but one of a wide variety of different materials and
structures that may be used. Those skilled in the art can select the
appropriate materials and structures depending upon the application and
other constraints. Accordingly, discussion of specific materials and
structures
is not intended to limit all embodiments.
The method begins with step 1810, which couples a fluid channel
between the lumbar region of the patient and the ventricle of the patient's
brain. To that end, a physician or other caregiver first may locate an in-vivo
lumbar catheter extending from the patient's lumbar, and an in-vivo ventricle
catheter extending from the patient's ventricle. After locating those
catheters,
the ventricle catheter may be mechanically coupled to the lumbar catheter via
an intermediate catheter, such as those discussed above, to produce the fluid
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
34
channel Accordingly, this fluid channel fluidly couples the lumbar and
ventricle
of the patient. In preferred embodiments, the catheter has a lumen
configured to transport ASO mixed with CSF of the patient, and an access port
to receive an ASO or other therapeutic.
Step 1820 energizes a pump 18 to cause CSF to flow between the
lumbar and the ventricle of the patient.
Next, the physician or other caregiver (e.g., a nurse) adds antisense
oligonucleotide material (ASO) to the access port. Indeed, as noted above,
this
step can be performed before, during, and/or after step 1802¨energizing the
pump 18. Among other ways, the ASO may be added via a bolus to the fluid
channel through the access port. In other embodiments, the ASO may be
added more gradually without a bolus as discussed above. Preferably, the fluid
channel forms a closed loop configured to circulate CSF and ASO mixture
through the ventricle, the body chambers through which CSF flows, the
lumbar, and fluid channel in one or two directions. As such, a controller
20/22
or other apparatus may manage the pump 18 to cause the ASO/CF mixture to
flow at prescribed, non-natural rates in one direction or in two different
directions. As suggested above, some embodiments may oscillate the mixture
so that a prescribed region of the brain receives a more concentrated/focused
dose of the ASO.
Of course, those skilled in the art should recognize that the above
examples are two of many examples that may be used with illustrative
embodiments.
Accordingly, illustrative embodiments enable a clinician to more
effectively treat various diseases by targeting drug delivery via CSF in the
subarachnoid space.
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
Various embodiments of the invention may be implemented at least in
part in any conventional computer programming language. For example,
some embodiments may be implemented in a procedural programming
language (e.g., "C"), or in an object oriented programming language (e.g.,
5 '1C++"). Other embodiments of the invention may be implemented as a pre-
configured, stand-along hardware element and/or as preprogrammed
hardware elements (e.g., application specific integrated circuits, FPGAs, and
digital signal processors), or other related components.
In an alternative embodiment, the disclosed apparatus and methods
10 (e.g., see the various flow charts described above) may be implemented
as a
computer program product for use with a computer system. Such
implementation may include a series of computer instructions fixed either on
a tangible, non-transitory medium, such as a computer readable medium (e.g.,
a diskette, CD-ROM, ROM, or fixed disk). The series of computer instructions
15 can embody all or part of the functionality previously described herein
with
respect to the system.
Those skilled in the art should appreciate that such computer
instructions can be written in a number of programming languages for use
with many computer architectures or operating systems. Furthermore, such
20 instructions may be stored in any memory device, such as semiconductor,
magnetic, optical or other memory devices, and may be transmitted using any
communications technology, such as optical, infrared, microwave, or other
transmission technologies.
Among other ways, such a computer program product may be
25 distributed as a removable medium with accompanying printed or
electronic
documentation (e.g., shrink wrapped software), preloaded with a computer
system (e.g., on system ROM or fixed disk), or distributed from a server or
CA 03225436 2023-12-22
WO 2022/271938
PCT/US2022/034706
36
electronic bulletin board over the network (e.g., the Internet or World Wide
Web). In fact, some embodiments may be implemented in a software-as-a-
service model ("SAAS") or cloud computing model. Of course, some
embodiments of the invention may be implemented as a combination of both
software (e.g., a computer program product) and hardware. Still other
embodiments of the invention are implemented as entirely hardware, or
entirely software.
The embodiments of the invention described above are intended to be
merely exemplary; numerous variations and modifications will be apparent to
those skilled in the art. Such variations and modifications are intended to be
within the scope of the present invention as defined by any of the appended
claims.