Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/002004
PCT/EP2022/070585
1
MULTIPARTICULATE PHARMACEUTICAL COMPOSITION
FIELD OF THE INVENTION
The present invention provides an oral solid pharmaceutical composition in the
form of
a multiparticulate composition comprising an immediate release core comprising
levetiracetam or a pharmaceutically acceptable salt thereof, coated with a
modified
release layer, a process for the preparation of the composition of the
invention, and its
use in therapy.
BACKGROUND OF THE INVENTION
Levetiracetam, having the IUPAC chemical name of (S)-2-(2-oxopyrrolidin-1-
yl)butanamide , Formula (1), is a medication developed by UCB, for the
treatment of
epilepsy either as a monotherapy or in combination, sold under the brand name
Keppra .
0
=-='-yi-LNE12
N 0
r
(1)
The exact mechanism of action is not properly understood, although it is
believed that
levetiracetam acts through the binding to the synaptic vesicle protein SV2A in
the brain.
Levetiracetam is rapidly and nearly completely absorbed upon oral
administration, with
peak plasma concentrations reached after approximately 1 hour, with minimal
binding
to plasma proteins.
Epilepsy is a chronic condition characterized by epileptic seizures, its
clinic
manifestation, which results from an abnormal, excessive electrical discharge
of
neurons in the brain. Epileptic seizures can be classified into three
categories, focal
seizures, generalized seizures and unclassified epileptic seizures, which can
present
convulsive or non-convulsive manifestations, and consciousness can be impaired
(totally or partially).
As a chronic condition, epilepsy requires in many patients medication for
life. In this
regard, a single-dose regimen is advantageous to increase patient compliance
as well
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
2
as keeping a steady level of medication in the body, thereby avoiding
undesired
fluctuations in medication levels.
The standard initial posology for levetiracetam is 500 mg twice daily, which
upon
tolerance and clinical response can be increased up to 1500 mg twice daily. In
Europe
it is available in the form of tablets (in different strengths), as a solution
for infusion,
and oral solution. An extended-release formulation of levetiracetam, is not
available in
Europe, despite being available in the USA as Keppra XR.
Keppra XR is available in 500 mg and 750 mg strengths, in the form of film-
coated
tablets, wherein the tablet core is a matrix tablet comprising a hydrophilic
rate
controlling polymer, such as high viscosity hypromellose. However, these
tablets do
not allow an easy fraction ing of the dose as they should not be broken or
crushed, and
their size may present an issue for patient's compliance, for instance in the
paediatric
population. Moreover, after oral administration of the tablets, the bitter
taste of
levetiracetam may be experienced due to the faint odor and bitter taste of
levetiracetam
active drug itself.
Document W02006/088864 Al discloses a controlled-release composition
cornprising
levetiracetam capable of producing a plasma profile similar to the plasma
profile
produced by administering two or more levetiracetam dosage forms sequentially.
However, no experimental results, or examples are provided in this document.
In W02009/069089 Al a levetiracetam controlled release formulation, in the
form of
coated tablets is disclosed. Examples of tablets according to the disclosure
are
provided, wherein can be seen that high dose tablets, for instance 1000 mg,
result in
large tablets, which can be a hindrance to patient compliance, especially in
the
paediatric population.
US2011/0217374 Al discloses compositions having, simultaneously, rapid and
long-
acting properties, by comprising a sustained-release part coated with a
hydrophobic
polymer comprising a first active ingredient, and an immediate release part
comprising
a second active ingredient.
In document US2010/0172979 Al, a controlled-release formulation is disclosed
wherein the core comprises an active ingredient, such as levetiracetam and a
wax
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
3
excipient, many of which require specific handling and/or process conditions
due to
their physical properties, i.e. melting point.
WO 2011/107855 A2 refers to a taste masked sustained release oral liquid
suspension
dosage form comprising a) inert pellets, surrounded by seal coating, b) drug
layer
surrounding the seal coated inert pellets comprising pharmaceutically active
ingredient
with one or more pharmaceutically acceptable excipient, and c) coating layer
surrounding the drug layer comprising rate controlling polymer, wherein the
sustained
release pellets are suspended with viscosity modifying agent or suspending
agent or
thickening agent or suspension stabilizers in addition to other
pharmaceutically
acceptable excipients in a suspending media at a suitable pH which is
maintained with
or without buffer.
International application WO 2014/025593 Al relates to a prolonged-release
levetiracetam pharmaceutical compositions to allow a once-a-day dosage regime.
The
disclosed compositions, rely on reservoir particulates having release
modifiers, which
can be further coated with a release-modifier polymer.
Notwithstanding the above, there is still a need for the development of
pharmaceutical
compositions comprising levetiracetam which can improve patient's compliance
with
the therapeutic regime, due to their facile administration and easy posology
adequation, together with an easy administration schedule (such as a once-a-
day
administration), while maintaining therapeutic levels of levetiracetam in the
patients.
BRIEF DESCRIPTION OF THE INVENTION
The present inventors have developed a multiparticulate composition comprising
immediate release cores comprising levetiracetam or a pharmaceutically
acceptable
salt thereof, wherein said cores are coated with a mixture comprising ethyl
cellulose
and hydroxypropylmethyl cellulose wherein hydroxypropylmethyl cellulose has a
viscosity comprised between about 1 mPa-s to about 1,000 mPa-s, which is
suitable
for a once-daily therapeutic regime.
In a first aspect of the present invention refers to a multiparticulate
pharmaceutical
composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
4
b) at least 50% of the immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPas to about 1,000 mPas.
A second aspect of the present invention provides a process for preparing the
solid
pharmaceutical composition according to the first aspect.
The third aspect of the present invention refers to the multiparticulate
composition
according to the first aspect for use in the treatment of epilepsy.
BRIEF DESCRIPTION OF THE DRAWINGS
- Fig. 1 is a graph showing mean plasma concentrations of a composition
according to
example 1 compared to reference treatment with Keppra .
- Fig. 2 is a graph showing mean plasma concentrations of a composition
according to
example 4 compared to reference treatment with Keppra .
- Fig. 3 is a graph showing mean plasma concentrations of a composition
according to
example 5 compared to reference treatment with Keppra .
- Fig. 4 is a graph showing mean plasma concentrations of a composition
according to
example 6 compared to reference treatment with Keppra .
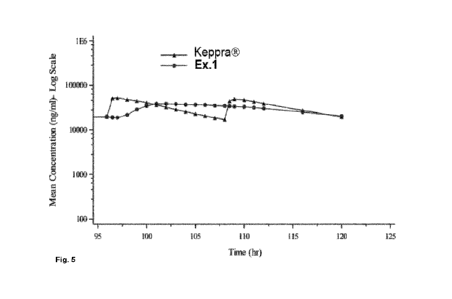
- Fig. 5 is a graph showing mean plasma concentrations of a composition
according to
example 1 compared to reference treatment with Keppra .
- Fig.6 is a photograph of the mini-tablets obtained as in examples 1 to 6.
DEFINITIONS
The term "about" as used herein refers to a statistically meaningful range of
a value,
typically within 10%. Such a range can lie within experimental error, typical
of standard
methods used for the measurement and/or determination of a given value or
range. In
one embodiment, the range is within 5% of the indicated value. In another
embodiment,
the range is within 1 % of the indicated value. In yet another embodiment, the
range is
within 0.5% of the indicated value.
The term "pharmaceutically acceptable" as used herein refers to those
compounds,
materials, compositions, and/or dosage forms which are, within the scope of
sound
medical judgment, suitable for contact with the tissues of animals, in
particular human
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
beings, without excessive toxicity, irritation, allergic response, or other
problematic
corn plications commensurate with a reasonable benefit/risk ratio.
The term "treating", as used herein, unless otherwise indicated, includes the
5 amelioration, cure, and/or maintenance of a cure (i.e., the prevention or
delay of
relapse) of a disease or disorder. Treatment after a disorder has started aims
to reduce,
alleviate, ameliorate or altogether eliminate the disorder, and/or its
associated
symptoms, to prevent it from becoming worse, to slow the rate of progression,
or to
prevent the disorder from re-occurring once it has been initially eliminated
(i.e., to
prevent a relapse).
The term, "extended release" as used herein refers to a dosage form that is
deliberately
modified to protract the release rate of the drug substance compared to that
observed
for an immediate-release dosage form. The release pattern in an extended-
release
dosage may begin with a burst effect that mimics an immediate release,
followed by a
slower release of the remaining drug substance in the dosage form.
The term "immediate release" as used herein refers to a pharmaceutical
formulation
which, in water at 25 C using the paddle test (50 rpm), releases at least 80%
of the
active pharmaceutical ingredient within 30 minutes.
The term "multiparticulate pharmaceutical composition" as used herein refers
to a
pharmaceutical composition in the form of multiple solid units, such as,
pellets, mini-
tablets, granules, and/or mixtures thereof.
The term "pellet" or "pellets" as used herein refers to free-flowing,
substantially
spherical particulates having a size from 90 micrometres to 2000 micrometres
and
which are, preferably manufactured by the agglomeration of fine powders or
granules.
In an embodiment of the present invention the terms "mini-tablet" and "mini-
tablets"
refers to a solid pharmaceutical dosage form with a diameter of typically 3
mm,
preferably a diameter of less or equal to 3 mm, more preferably a diameter of
less or
equal to 2.5 mm, even more preferably a diameter between about 2.0 to at least
1.0
mm, manufactured by compression, which can be manufactured in a conventional
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
6
tableting machine adapted to small size tablet pressing. The mini-tablets may
be
compressed in any common tablet shape selected from flat round, biconvex
round,
oval convex and cylindrical, preferably in a biconvex round shape. The shape
of said
mini-tablets is depicted in Figure 6.
In another embodiment of the present invention the terms "mini-tablet" and
"mini-
tablets" refers to a solid form manufactured by compression which has a
surface area
of less than 20 mm2, preferably from 16 to 18 mm2, more preferably from 12 to
15 mm2
and has a volume of less than 10 mm3, preferably from 7 to 10 mm3, more
preferably
from 4 to 6 mm3.
The terms "granule" and "granules" as used herein refers to a solid
pharmaceutical
dose form, of irregular shape, comprising powder particles that have been
aggregated
to form larger particles sufficiently robust to withstand handling.
The term "coating" as used herein refers to adherence, and/or adsorption,
preferable
uniformly, of at least one solution, dispersion or suspension coating material
onto a
substrate. The coating material on the substrate may be of any thickness.
Preferably
the coating material is a thin and uniform film applied onto the substrate. A
thin and
uniform film can be of the type of a "film coating" or/and an "isolating film
coating"
or/and an "external film coating".
The term "curing" as used herein refers to the process of physical or chemical
hardening by any method, such as cooling, and/or drying. In particular, the
physical
hardening of the coating mixture (film coating) applied to the immediate
release cores
of the multiparticulate pharmaceutical composition of the invention.
The term "pharmaceutically acceptable salt" as used herein refers to the
relatively non-
toxic, inorganic and organic acid or base addition salts of a compound. These
salts can
be prepared in situ during the final isolation and purification of the
compounds or by
separately reacting the purified compound in its free base/acid form with a
suitable
organic or inorganic acid/base and isolating the salt thus formed.
The term "bioequivalence" as used herein refers to a pharmaceutical product
which is
pharmaceutically equivalent or pharmaceutical alternative to another
pharmaceutical
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
7
product(s), and their bioavailabilities, in terms of rate (Cmax and tmax) and
extent of
absorption (area under the curve), after administration of the same molar dose
under
the same conditions, are similar to such a degree that their effects can be
expected to
be essentially the same. This is considered to be demonstrated if the 90%
confidence
intervals of the ratios between the AUCo_t and Cmax, between the products
being
compared is in the range of 80-125%.
DETAILED DESCRIPTION OF THE INVENTION
The inventors of the present invention have surprisingly found that coating
immediate
release cores comprising levetiracetam or a pharmaceutically acceptable salt
thereof
with a mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose
having a
viscosity comprised between about 1 mPa-s to about 1,000 mPas allows the
preparation of an oral solid pharmaceutical composition having a modified
release
suitable for a once-daily dose regimen.
Notably, the compositions according to the invention allow the maintenance of
a
plasmatic concentration of levetiracetam in a once-daily dose regime identical
to that
achieved with the currently recommended twice-daily dosage for levetiracetam
with
KeppraG. Furthermore, the compositions of the invention show resistance to
dose
dumping when exposed to ethanol.
Thus, the first aspect of the present invention refers to a multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least about 50% of the immediate release cores a) are coated with a
mixture
comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa-s.
In an embodiment of the present invention the immediate release core is solid.
In an embodiment of the present invention the pharmaceutical compositions of
the
present invention do not comprise wax excipients. More specifically, they do
not
comprise any excipient selected from the group consisting of camauba wax,
vegetable
wax, fruit wax, microcrystalline wax ("petroleum wax"), bees wax (white or
bleached,
and yellow), hydrocarbon wax, paraffin wax, cetyl esters wax, non-ionic
emulsifying
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
8
wax, anionic emulsifying wax, candelilla wax, fatty alcohols (such as lauryl,
myristyl,
stearyl, cetyl or cetostearyl alcohol), hydrogenated vegetable oil,
hydrogenated castor
oil, fatty acids such as stearic acid, fatty acid esters including fatty acid
glycerides
(mono-, di-, and tri-glycerides), polyethylene glycol (PEG) having a molecular
weight
of greater than about 3000 number average molecular weight, Mn (e.g., PEG
3350,
PEG 4000, PEG 4600, PEG 6000, and PEG 8000), or a combination comprising at
least one of the foregoing wax excipients.
In an embodiment of the present invention the hydroxypropylmethyl cellulose
has a
viscosity comprised between 80 mPa-s to 120 mPa-s, preferably about 100 mPa.s.
In an embodiment of the present invention the ethyl cellulose has a viscosity
comprised
between 400 mPa=s and 1500 mPa=s.
In an embodiment of the present invention the coating mixture is used in an
amount
comprised between 9 and 14 g of coating solution (expressed as dry matter) per
100 g
of immediate release core.
In an embodiment of the present invention the immediate release cores comprise
levetiracetam or a pharmaceutically acceptable salt, solvate, hydrate,
crystalline form,
or non-crystalline form thereof, preferably the immediate release cores
comprise
crystalline levetiracetam The final dosage form prepared for administration to
a patient
comprises the multiparticulate composition of the invention which is composed
of
various particles, preferably mini-tablets, comprising levetiracetam which may
be
present in the final dosage form in an amount of between 250 mg to about 3000
mg.
For example, the levetiracetam may be present in the final dosage form in an
amount
of any of about 250, 275, 300, 325, 350, 375, 400, 425, 450, 475, 500, 525,
550, 575,
600, 625, 650, 675, 700, 725, 750, 775, 800, 825, 850, 875, 900, 925, 950,
975, 1000,
1025, 1050,1075, 1100,1125, 1150, 1175,1200, 1225,1250, 1275, 1300,1325, 1350,
1375, 1400, 1425, 1450, 1475, 1500, 1525, 1550, 1575, 1600, 1625, 1650, 1675,
1700,
1725, 1750,1775, 1800,1825, 1850, 1875, 1900, 1925,1950, 1975, 2000, 2025,
2050,
2075, or 3000 mg. In one embodiment, the levetiracetam may be present in the
final
dosage form in an amount of about 1000 mg. In one embodiment, the
levetiracetam
may be present in the final dosage form in an amount of about 1500 mg. In one
embodiment, the levetiracetam may be present in the final dosage form in an
amount
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
9
of about 2000 mg. In one embodiment, the levetiracetam may be present in the
final
dosage form in an amount of about 3000 mg.
Advantageously, the plurality of individual immediate release cores comprising
levetiracetam or a pharmaceutically acceptable salt thereof of the present
invention,
preferably in the form of mini-tablets, show good chemical and mechanical
properties
such as good uniformity, friability, hardness (resistant to crushing), and
disintegration
time suitable for successful coating application.
Additionally, the dosage forms comprise the multiparticulate composition of
the
invention, preferably in the form of mini-tablets, so that said dosage forms
comprise
several mini-tablets and the total amount of levetiracetam in the dosage from
will be of
about 1000 mg, 1500 mg, 2000 mg, or 3000 mg of levetiracetam. When the
levetiracetam is in the form of dosage forms comprising several coated mini-
tablets it
is possible to dose high amounts of levetiracetam such as of about 1000 mg,
1500 mg,
2000 mg, or 3000 mg by oral administration to a patient without the patient
noticing the
bitter taste, undesirable taste of levetiracetam and, avoiding the need to add
sweeteners or flavoring agents to the composition to mask the bitter or
unpleasant taste
of the drug.
In an embodiment of the first aspect, at least about 55% of the immediate
release cores
are coated with a mixture comprising ethyl cellulose and hydroxypropylmethyl
cellulose
having a viscosity comprised between about 1 mPa-s to about 1,000 mPa-s,
preferably
about at least 60%, more preferably about at least 65%, more preferably about
at least
70%, more preferably about at least, more preferably about at least 75%, more
preferably about at least 80%, more preferably about at least 85%, more
preferably
about at least 90%, more preferably about at least 95%, more preferably about
at least
99%, even more preferably all (100%) immediate release cores are coated.
Typically,
the coating layer represents from about 5% to about 20% weight of the total
weight of
the composition, preferably from about 7% to about 15%, more preferably from
about
8% to about 13%, more preferably from about 9% to about 12%, most preferably
about
11.5% by weight of the total weight of the composition. In an embodiment of
the first
aspect, the weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose
having a
viscosity comprised between about 1 mPa-s to about 1,000 mPa-s in the coating
mixture is comprised between about 4:1 to about 20:1, preferably from about
5:1 to
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
about 18:1, more preferably from about 8:1 to about 15:1, even more preferably
from
about 1 1: 1 to about 15: 1 , and even more preferably about 12.5:1.
The skilled person recognizes that ethyl cellulose is available in various
forms, and
under different brand names, such as AqualonTM, AquacoatO, EthocelTm,
Surelease .
5 Preferably, the ethyl cellulose for the compositions of the invention is
an aqueous
dispersion of ethyl cellulose, such as Surelease E-7-19029, Surelease E-7-
19030,
Surelease0E-7-19040, preferably Surelease0E-7-19040 (which is an 25% w/w
aqueous dispersion of ethyl cellulose, comprising medium chain triglycerides,
oleic
acid, and ammonium hydroxide). These preferred Surelease ethyl celluloses
allow
10 obtaining a pH independent drug release.
In the context of the present invention, hydroxypropylmethyl cellulose has a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa-s. Preferably, the
hydroxypropylmethyl cellulose has a viscosity comprised between about 5 mPa-s
to
about 400 mPa-s, more preferably comprised between about 50 mPa-s to about 250
mPa-s, even more preferably comprised between about 70 mPa-s to about 200 mPa-
s,
most preferably comprised between about 80 mPa-s to about 120 mPa-s. The
viscosity
values shown correspond to the measured viscosity of a 2% w/w aqueous solution
of
hydroxypropylmethyl cellulose at 20 C, measured according to USP method. The
preferred hydroxypropylmethyl cellulose are selected from the group consisting
of
cellulose ethers graded as E5LV, E15LV, E5OLV, and K100LV, preferably K100 LV,
commercially available as MethocelTM (from Dow Chemicals), or BenecelTM (from
Ashland).
In an embodiment of the first aspect the hydroxypropylmethyl cellulose has a
viscosity
comprised between about 80 mPa-s to about 120 mPa-s in a 2% w/w aqueous
solution
at 20 C (measured according to USP method).
In an embodiment of the first aspect, the coating mixture comprising ethyl
cellulose and
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPa.s to
about 1,000 mPas further comprises I) plasticizers selected from the group
comprising
medium chain triglycerides, oleic acid, ethylene glycol, glycol, 1,2-butylene
glycol, 2,3-
butylene glycol, styrene glycol, diethylene glycol, triethylene glycol,
tetraethylene glycol
20, monopropylene glycol monoisopropyl ether, propylene glycol monoethyl
ether,
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
11
ethylene glycol monoethyl ether, diethylene glycol monoethyl ether, sorbitol
lactate,
ethyl lactate, butyl lactate, ethyl glycolate, dibutyl sebacate, acetyl
tributyl citrate,
triethyl citrate, acetyl triethyl citrate, tributyl citrate and allyl
glycolate, and/or mixtures
thereof, preferably selected from medium chain triglycerides, oleic acid,
and/or
mixtures thereof; and II) stabilizers selected from the group comprising
ammonium
hydroxide, sodium hydroxide, lithium hydroxide, potassium hydroxide, caesium
hydroxide, and/or mixtures thereof, preferably ammonium hydroxide.
The term "medium chain triglyceride" or "MCI" refers to triesters of glycerol
and C6-C12
fatty acids, examples of said fatty acids being caproic acid (C6), caprylic
acid (Cs),
capric acid (C10) and lauric acid (C12). The three fatty acid residues of the
MCT can be
the same or different, preferably there are two different fatty acid residues.
Preferred
medium chain triglycerides are caprylic/capric acid triglyceride (marketed as
Stelliesterse MCT 65/35, Estasan , Crodamol GTC/C, Miglyol 812 or 810, and
Neobee M5).
In an embodiment of the first aspect, the coating mixture does not comprise
sodium
lauryl sulfate.
The multiparticulate composition of the invention can be in the form of mini-
tablets,
pellets, granules, and/or mixtures thereof, preferably mini-tablets. The mini-
tablets,
pellets, granules, and/or mixtures thereof can be used to fill capsules or
sachets and
stick-packs, preferably sachets, more suitable for packaging relatively large
numbers
of mini-tablets since have larger fill volumes compared to stick-packs.
In an embodiment of the first aspect, the immediate release cores of the
multiparticulate
pharmaceutical composition are solid immediate release cores, preferably in
the form
of mini-tablets. The multiparticulate composition of the present invention may
comprise
further pharmaceutically acceptable excipients. Suitable excipients include,
but are not
limited to, binders, diluents, disintegrants, lubricants, sweetening agent,
colouring
agent, flavouring agent, or plasticizers_
Preferably, the multiparticulate composition does neither contain lactose nor
gluten.ln
a further embodiment of the first aspect, the percentage in weight of
levetiracetam or a
pharmaceutically acceptable salt thereof in the composition of the invention
is from
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
12
about 60% to about 90% by weight, preferably from about 70% to about 80% by
weight,
more preferably about 73% to 78% by weight of the total weight of the
composition.
In an embodiment of the first aspect, the percentage in weight of
levetiracetam in the
composition of the invention is from about 60% to about 90% by weight,
preferably from
about 70% to about 80% by weight, more preferably about 73% to 78% by weight
of
the total weight of the composition.
In an embodiment of the first aspect, the immediate release cores comprising
levetiracetam, further comprise one or more excipients selected from the group
consisting of diluents, binders, glidants, and lubricants.
In an embodiment of the first aspect, the immediate release cores further
comprise a
diluent selected from the group consisting of cellulose derivatives, such as
cellulose
powder, microcrystalline cellulose, silicified microcrystalline cellulose,
hydroxypropyl
cellulose; natural starches, such as maize starch and potato starch;
pregelatinized
starch and mixtures thereof, preferably, the diluent is a cellulose derivate
selected from
methyl cellulose, microcrystalline cellulose, silicified microcrystalline
cellulose,
hydroxypropyl cellulose; pregelatinized starch and/or mixtures thereof; more
preferably, the diluent is microcrystalline cellulose. Typically, a diluent
can be present
in an amount from about 4% to about 15% by weight, preferably from about 7% to
about 12% by weight, more preferably from about 8% to about 10% by weight of
the
total weight of the composition.
In an embodiment of the first aspect, the immediate release cores further
comprise a
binder selected from the group consisting of povidone, copovidone,
polyethylene
glycol, gelatin, polyethylene oxide, alginic acid, modified corn starch,
and/or mixtures
thereof, preferably the binder is selected from povidone, copovidone and/or
mixtures
thereof; more preferably the binder is copovidone. Typically, a binder can be
present
in an amount from about 0.5% to about 5% by weight, preferably from about 1%
to
about 3% by weight, even more preferably from about 1.5% to about 2% by weight
of
the total weight of the composition.
In an embodiment of the first aspect, the immediate release cores further
comprise a
glidant selected from the group consisting of calcium silicate, magnesium
silicate, corn
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
13
starch, colloidal silicon dioxide, silicon hydrogel, talc, colloidal silicon
dioxide, sodium
stearyl fumarate, sodium lauryl sulfate, mineral oil, and/or mixtures thereof,
preferably
talc. Typically, a glidant can be present in an amount from about 0.5% to
about 5% by
weight, preferably from about 1% to 3% by weight, more preferably about 2% by
weight
of the total weight of the composition.
In an embodiment of the first aspect, the immediate release cores further
comprise a
lubricant selected from the group consisting of magnesium stearate, calcium
stearate,
zinc stearate glyceryl behenate, mineral oil, stearic acid, and/or mixtures
thereof;
preferably selected from magnesium stearate, calcium stearate, zinc stearate
and/or
mixtures thereof; more preferably magnesium stearate. Typically, a lubricant
can be
present in an amount from about 0.1% to about 2% by weight, preferably from
about
0.3% to about 1.5% by weight, more preferably from about 0.5% to about 1.0% by
weight, even more preferably about 0.5% by weight of the total weight of the
composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least about 50%, preferably about at least about 60%, more preferably
about at
least 65%, more preferably about at least 70%, more preferably about at least
75%,
more preferably about at least 80%, more preferably about at least about 85%,
more
preferably about at least about 90%, more preferably about at least about 95%,
more
preferably about at least about 99%, even more preferably all (100%) of the
individual
immediate release cores a) are coated with a mixture comprising ethyl
cellulose and
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPa.s to
about 1,000 mPa-s, further wherein the weight ratio of ethyl cellulose to
hydroxypropylmethyl cellulose having a viscosity comprised between about 1 mPa-
s to
about 1,000 mPa-s in the coating mixture is comprised between about 4:1 to
about
20:1, preferably from about 5:1 to about 18:1, more preferably from about 8:1
to about
15:1, more preferably from about 11:1 to about 15:1, and even more preferably
about
12.5:1.
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
14
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least about 50%, preferably about at least about 60%, more preferably
about at
least 65%, more preferably about at least 70%, more preferably about at least
75%,
more preferably about at least 80%, more preferably about at least about 85%,
more
preferably about at least about 90%, more preferably about at least about 95%,
more
preferably about at least about 99%, even more preferably all (100%) of the
individual
immediate release cores a) are coated with a mixture comprising ethyl
cellulose and
hydroxypropylmethyl cellulose having a viscosity comprised between about 1 mPa-
s to
about 1,000 mPa-s, further wherein the weight ratio of ethyl cellulose to
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPas to
about 1,000 nnPa-s in the coating mixture is comprised from about 8:1 to about
15:1,
preferably from about 11:1 to about 15:1.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least about 50% preferably about at least about 60%, more preferably
about at
least 65%, more preferably about at least 70%, more preferably about at least
75%,
more preferably about at least 80%, more preferably about at least 85%, more
preferably about at least 90%, more preferably about at least 95%, more
preferably
about at least 99%, even more preferably all (100%) of the individual
immediate release
cores a) are coated with a mixture comprising ethyl cellulose and
hydroxypropylmethyl
cellulose having a viscosity comprised between about 1 mPa-s to about 1,000
mPa-s,
further wherein the weight ratio of ethyl cellulose to hydroxypropylnnethyl
cellulose
having a viscosity comprised between about 1 mPa-s to about 1,000 mPa-s in the
coating mixture is about 12.5:1.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least about 90% of the individual immediate release cores a) are coated
with a
mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
5 viscosity comprised between about 1 mPa-s to about 1,000 mPa-s, further
wherein the
weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is
comprised between about 4:1 to about 20:1, preferably from about 5:1 to about
18:1,
more preferably from about 8:1 to about 15:1, more preferably from about 11:1
to about
10 15:1, and even more preferably about 12.5:1.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
15 b) at least about 90% of the individual immediate release cores a) are
coated with a
mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity comprised between about 1 mPa-s to about 1,000 mPa-s, further
wherein the
weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is
about
12.5:1.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa.s in the coating mixture is comprised between about
4:1
to about 20:1, preferably from about 5:1 to about 18:1, more preferably from
about 8:1
to about 15:1, more preferably from about 11:1 to about 15:1, and even more
preferably
about 12.5:1.
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
16
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa-s in the coating mixture is about 12.5:1.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least 50%, preferably about at least 60%, more preferably about at least
65%,
more preferably about at least 70%, more preferably about at least, more
preferably
about at least 75%, more preferably about at least 80%, more preferably about
at least
85%, more preferably about at least 90%, more preferably about at least 95%,
more
preferably about at least 99%, even more preferably all (100%) of the
individual
immediate release cores a) are coated with a mixture comprising ethyl
cellulose and
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPa.s to
about 1,000 mPa-s, further wherein the weight ratio of ethyl cellulose to
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPa.s to
about 1,000 mPa-s in the coating mixture is comprised between about 8:1 to
about
15:1, more preferably from about 11:1 to about 15:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides oleic acid, and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least 50%, preferably about at least 60%, more preferably about at least
65%,
more preferably about at least 70%, more preferably about at least, more
preferably
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
17
about at least 75%, more preferably about at least 80%, more preferably about
at least
85%, more preferably about at least 90%, more preferably about at least 95%,
more
preferably about at least 99%, even more preferably all (100%) of the
individual
immediate release cores a) are coated with a mixture comprising ethyl
cellulose and
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPa.s to
about 1,000 mPa-s, further wherein the weight ratio of ethyl cellulose to
hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPa.s to
about 1,000 mPa-s in the coating mixture is about 12.5:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid, and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least 90% of the individual immediate release cores a) are coated with a
mixture
comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa.s, further wherein the
weight
ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a viscosity
comprised
between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is comprised
between about 4:1 to about 20:1, preferably from about 5:1 to about 18:1, more
preferably from about 8:1 to about 15:1, more preferably from about 11:1 to
about 15:1,
even more preferably about 12.5:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid, and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
18
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa.s in the coating mixture is comprised between about
4:1
to about 20:1, preferably from about 5:1 to about 18:1, more preferably from
about 8:1
to about 15:1, more preferably from about 11:1 to about 15:1, even more
preferably
about 12.5:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least 90% of the individual immediate release cores a) are coated with a
mixture
comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa-s, further wherein the
weight
ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a viscosity
comprised
between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is comprised
between about 8:1 to about 15:1, more preferably from about 11:1 to about
15:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) at least 90% of the immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
19
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa-s in the coating mixture is about 12.5:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa.s in the coating mixture is comprised between about
8:1
to about 15:1, more preferably from about 11:1 to about 15:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof; wherein
b) all the immediate release cores a) are coated with a mixture comprising
ethyl
cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between
about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio of ethyl
cellulose
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
to hydroxypropylmethyl cellulose having a viscosity comprised between about 1
mPas
to about 1,000 mPas in the coating mixture is about 12.5:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
5 ammonium hydroxide;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) at least about 90% of the individual immediate release cores a) are coated
with a
mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity comprised between about 1 mPas to about 1,000 mPa-s, further wherein
the
weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPas in the coating mixture is
comprised between about 8:1 to about 15:1, more preferably from about 11:1 to
about
15:1.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) at least about 90% of the individual immediate release cores a) are coated
with a
mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity comprised between about 1 mPas to about 1,000 mPa-s, further wherein
the
weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPas to about 1,000 mPas in the coating mixture is
about
12.5.
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
21
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) at least about 90% of the individual immediate release cores a) are coated
with a
mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity comprised between about 1 mPa-s to about 1,000 mPa-s, further
wherein the
weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPas in the coating mixture is
comprised between about 8:1 to about 15:1, more preferably from about 11:1 to
about
15:1;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) at least about 90% of the individual immediate release cores a) are coated
with a
mixture comprising ethyl cellulose and hydroxypropylmethyl cellulose having a
viscosity comprised between about 1 mPa-s to about 1,000 mPa-s, further
wherein the
weight ratio of ethyl cellulose to hydroxypropylmethyl cellulose having a
viscosity
comprised between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is
about
12.5:1;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
22
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa.s in the coating mixture is comprised between about
8:1
to about 15:1, more preferably from about 11:1 to about 15:1;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa-s in the coating mixture is about 12.5:1;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition_
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
23
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa-s in the coating mixture is comprised between about
8:1
to about 15:1, more preferably from about 11:1 to about 15:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and optionally one or more
excipients
selected from diluents, binders, glidants, lubricants; wherein
b) all the individual immediate release cores a) are coated with a mixture
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio
of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about
1 mPa-s to about 1,000 mPa-s in the coating mixture is about 12.5:1;
wherein the coating mixture further comprises I) plasticizers selected from
medium
chain triglycerides, oleic acid and/or mixtures thereof; and II) stabilizers
selected from
ammonium hydroxide;
wherein the coating layer represents from about 5% to about 20% weight of the
total
weight of the composition, preferably from about 7% to about 15%, more
preferably
from about 8% to about 13%, more preferably from about 9% to about 12%, most
preferably about 11.5% by weight of the total weight of the composition.
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
24
In an embodiment of the first aspect, the optional one or more excipients are
present,
and the diluent is microcrystalline cellulose, the binder is copovidone, the
glidant is talc,
and the lubricant is magnesium stearate.
In an embodiment of the first aspect, the present invention refers to a
multiparticulate
pharmaceutical composition comprising:
a) a plurality of individual immediate release cores comprising levetiracetam
or a
pharmaceutically acceptable salt thereof, and one or more excipients selected
from
diluents, binders, glidants, and lubricants; wherein
b) at least about 90% of the individual immediate release cores, preferably
all (100%)
of the individual immediate release cores a) are coated with a mixture
comprising ethyl
cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between
about 1 mPa-s to about 1,000 mPa-s, further wherein the weight ratio of ethyl
cellulose
to hydroxypropylnnethyl cellulose in the coating mixture is comprised between
about
4:1 to about 20:1, preferably from about 5:1 to about 18:1, more preferably
from about
8:1 to about 15:1, more preferably from about 11:1 to about 15:1, and even
more
preferably about 12.5:1,
wherein the multiparticulate pharmaceutical composition is in the form of mini-
tablets,
pellets and/or granules, preferably mini-tablets.
In a second aspect, the present invention relates to a process for the
preparation of the
multiparticulate composition of the first aspect, comprising:
i) providing levetiracetam or a pharmaceutically
acceptable salt thereof
ii) optionally, mixing levetiracetam or a pharmaceutically acceptable salt
thereof with a diluent from about 1 min to about 10 min, preferably from about
2 min to about 7 min, more preferably for about 5 min;
iii) optionally, granulating the mixture resulting from step ii) with a
solution
comprising a binder;
iv) optionally, drying the product resulting from step i) or from steps ii)
and iii)
when these steps are present, preferably at a temperature from about 55 C
to 70 C, preferably from about 60 C to about 65 C, more preferably about
60 C;
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
v) optionally, mixing the product resulting from step i) or from steps ii),
iii) and
iv) when these steps are present with a glidant, preferably from about 5 min
to about 30 min, more preferably from about 10 min to about 20 min, even
more preferably for about 15 min;
5 vi) optionally, mixing the product resulting from step i) or from
steps ii), iii), iv)
or v) when these steps are present, with a lubricant from about 1 min to about
10 min, preferably from about 2 min to about 7 min, more preferably for about
5 min;
vii) optionally, compressing the product resulting from step i) or from steps
ii),
10 iii), iv), V) and vi) when these steps are present;
viii) coating the product resulting from step i) or from steps ii), iii), iv),
v), vi) and
vii) when these steps are present, with a mixture comprising ethyl cellulose
and hydroxypropylmethyl cellulose having a viscosity comprised between
about 1 mPa-s to about 1,000 mPa-s;
15 ix) curing the coated product of step viii) at a temperature from
about 15 C to
about 30 C, preferably from about 20 C to about 25 C.
In an embodiment of the second aspect the process comprises:
i) providing levetiracetam or a pharmaceutically acceptable salt thereof
ii) optionally, mixing levetiracetam or a pharmaceutically acceptable salt
20 thereof with a diluent for about 5 min;
iii) optionally, granulating the mixture resulting from step ii) with a
solution
comprising a binder;
iv) optionally, drying the product resulting from step i) or from steps ii)
and iii)
when these steps are present, at a temperature from about 60 C to about
25 65 C;
v) optionally, mixing the product resulting from step i) or from steps ii),
iii) and
iv) when these steps are present, with a glidant, from about 10 min to about
20 min;
vi) optionally, mixing the product resulting from step i) or from steps ii),
iii), iv)
or v) when these steps are present, with a lubricant for about 5 min;
vii) optionally, compressing the product resulting from step i) or from steps
ii),
iii), iv), v) and vi) when these steps are present;
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
26
viii) coating the product resulting from step i) or from steps ii), iii), iv),
v), vi) and
vii) when these steps are present, with a mixture comprising ethyl cellulose
and hydroxypropylmethyl cellulose having a viscosity comprised between
about 1 mPa-s to about 1,000 mPa-s;
ix) curing the coated product of step viii) at a temperature from about 20 C
to
about 25 C.
In an embodiment of the second aspect, the process comprises:
I) providing levetiracetam or a pharmaceutically
acceptable salt thereof;
ii) mixing levetiracetam or a pharmaceutically acceptable salt thereof and a
diluent for about 5 min;
iii) granulating the product resulting from step i) with a solution comprising
a
binder;
iv) drying the product resulting from step ii) at a temperature of about 60
C;
v) mixing the product resulting from step iii) with a glidant for about 15
min;
vi) mixing the product resulting from step iv), with a lubricant for about 5
min;
vii) compressing the product resulting from step v);
viii) coating the product from step vi) with a mixture comprising ethyl
cellulose
and hydroxypropylmethyl cellulose having a viscosity comprised between
about 1 mPa-s to about 1,000 mPa-s;
ix) curing the product of step vii) at a temperature from about 20 C to about
25
C.
In an embodiment of the second aspect, the coating layer represents from about
5%
to about 20% by weight of the total weight of the composition, preferably from
about
7% to about 15%, more preferably from about 8% to about 13%, more preferably
from
about 9% to about 12%, most preferably about 11.5% by weight of the total
weight of
the composition_
In an embodiment of the second aspect, the process comprises:
i) providing levetiracetam or a pharmaceutically
acceptable salt thereof;
ii) optionally, mixing levetiracetam or a pharmaceutically acceptable salt
thereof with a diluent from about 2 min to about 7 min;
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
27
iii) optionally, granulating the mixture resulting from step ii) with a
solution
comprising a binder;
iv) optionally, drying the product resulting from step i) or from steps ii)
and iii)
when these steps are present, at a temperature from about 60 C to about
65 C;
v) optionally, mixing the product resulting from step i) or from steps ii),
iii) and
iv) when these steps are present with a glidant, from about 10 min to about
20 min;
vi) optionally, mixing the product resulting from step i) or from steps ii),
iii), iv)
or v) when these steps are present, with a lubricant from about 2 min to about
7 min;
vii) optionally, compressing the product resulting from step i) or from steps
ii),
iii), iv), v) and vi) when these steps are present;
viii) coating the product resulting from step i) or from steps ii), iii), iv),
v), vi) and
Vii) when these steps are present, with a mixture comprising ethyl cellulose
and hydroxypropylmethyl cellulose having a viscosity comprised between
about 1 mPas to about 1,000 mPa-s wherein the weight ratio of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is
comprised between about 8:1 to about 15:1, preferably from about 11:1 to
about 15:1, more preferably about 12.5:1;
ix) curing the coated product of step viii) at a temperature from about 20 C
to
about 25 C.
In an embodiment of the second aspect, the process comprises:
i) providing levetiracetam or a pharmaceutically acceptable salt thereof
ii) optionally, mixing levetiracetam or a pharmaceutically acceptable salt
thereof with a diluent for about 5min;
iii) optionally, granulating the mixture resulting from step ii) with a
solution
comprising a binder;
iv) optionally, drying the product resulting from step i) or from steps ii)
and iii)
when these steps are present, at a temperature of about 60 C;
v) optionally, mixing the product resulting from step i) or from steps ii),
iii) and
iv) when these steps are present with a glidant, for about 15 min;
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
28
vi) optionally, mixing the product resulting from step i) or from steps ii),
iii), iv)
or v) when these steps are present, with a lubricant for about 5 min;
vii) optionally, compressing the product resulting from step i) or from steps
ii),
iii), iv), v) and vi) when these steps are present;
viii) coating the product resulting from step i) or from steps ii), iii), iv),
v), vi) and
vii) when these steps are present, with a mixture comprising ethyl cellulose
and hydroxypropylmethyl cellulose having a viscosity comprised between
about 1 mPa-s to about 1,000 mPa-s wherein the weight ratio of ethyl
cellulose to hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s in the coating mixture is
comprised between about 8:1 to about 15:1, preferably from about 11:1 to
about 15:1, more preferably about 12.5:1;
ix) curing the coated product of step viii) at a temperature from about 20 C
to
about 25 C.
In an embodiment of the second aspect, if uncoated immediate release cores are
desired, steps viii) and ix) are not carried out.
In an embodiment of the second aspect, steps ii), iii), iv), v), vi) and vii)
are carried Out
(i.e. are present).
In an embodiment of the second aspect, steps ii) to ix) are carried out (i.e.
are present).
In an embodiment of the second aspect, the diluent of step ii) is selected
from cellulose
derivatives, such as cellulose powder, microcrystalline cellulose, silicified
microcrystalline cellulose, hydroxypropyl cellulose; natural starches, such as
maize
starch and potato starch; pregelatinized starch and mixtures thereof,
preferably, the
diluent is a cellulose derivate selected from methyl cellulose,
microcrystalline cellulose,
silicified microcrystalline cellulose, hydroxypropyl cellulose; pregelatinized
starch
and/or mixtures thereof; preferably microcrystalline cellulose.
In an embodiment of the second aspect, the binder of step iii) is selected
from
povidone, copovidone, polyethylene glycol, gelatin, polyethylene oxide,
alginic acid,
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
29
modified corn starch, and/or mixtures thereof, preferably povidone, copovidone
and/or
mixtures thereof; preferably copovidone.
In an embodiment of the second aspect, the glidant of step v) is selected from
calcium
silicate, magnesium silicate, corn starch, colloidal silicon dioxide, silicon
hydrogel, talc,
colloidal silicon dioxide, sodium stearyl fumarate, sodium lauryl sulfate,
mineral oil,
and/or mixtures thereof, preferably talc.
In an embodiment of the second aspect, the lubricant of step vi) is selected
from
magnesium stearate, calcium stearate, zinc stearate glyceryl behenate, mineral
oil,
stearic acid, and/or mixtures thereof; preferably selected from magnesium
stearate,
calcium stearate, zinc stearate and/or mixtures thereof; more preferably
magnesium
stearate.
In an embodiment of the second aspect, the binder is copovidone, the diluent
is
microcrystalline cellulose, the glidant is talc, and the lubricant is
magnesium stearate.
In an embodiment of the second aspect the hydroxypropylmethyl cellulose has a
viscosity comprised between about 80 mPa-s to about 120 mPa-s in a 2% w/w
aqueous
solution at 20 C (measured according to USP method).
In an embodiment of the second aspect, the coating mixture of step vii)
comprising
ethyl cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between about 1 mPa-s to about 1,000 mPa-s, further comprises I) plasticizers
selected
from the group comprising medium chain triglycerides, oleic acid ethylene
glycol,
glycol, 1,2-butylene glycol, 2,3-butylene glycol, styrene glycol, diethylene
glycol,
triethylene glycol, tetraethylene glycol 20, monopropylene glycol
monoisopropyl ether,
propylene glycol monoethyl ether, ethylene glycol monoethyl ether, diethylene
glycol
monoethyl ether, sorbitol lactate, ethyl lactate, butyl lactate, ethyl
glycolate,
dibutylsebacate, acetyltributylcitrate, triethyl citrate, acetyl triethyl
citrate, tributyl citrate
and allyl glycolate, and/or mixtures thereof, preferably selected from medium
chain
triglycerides, oleic acid, and/or mixtures thereof; and II) stabilizers
selected from the
group comprising ammonium hydroxide sodium hydroxide, lithium hydroxide,
potassium hydroxide, caesium hydroxide, and/or mixtures thereof, preferably
ammonium hydroxide, and/or mixtures thereof.
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
In an embodiment of the second aspect the coating mixture of step vii)
comprising ethyl
cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between
about 1 mPa-s to about 1,000 mPa-s, further comprises I) plasticizers selected
from
the group comprising medium chain triglycerides, oleic acid, and or mixtures
thereof;
5 and II) stabilizers selected from ammonium hydroxide.
In an embodiment, the coating mixture of step viii) may be applied by a
perforated pan-
coating process or a fluid-bed coating process, preferably by a fluid-bed
coating
process commonly used technique for coating of small particles.
In an embodiment of the first and/or second aspect of the invention, the
multiparticulate
10 composition is in the form of mini-tablets.The third aspect of the
present invention refers
to the multiparticulate composition according to the first aspect for use in
the treatment
of epilepsy.
In an embodiment of the third aspect, the composition according to the first
aspect is
for use in the treatment of epilepsy in a once-a-day administration regime. In
an
15 embodiment, the multiparticulate composition of the present invention
comprises
dosage strengths of levetiracetam of about 1000 mg, 1500 mg, 2000 mg, or 3000
mg
for once-a-day administration to treat epilepsy.
EXPERIMENTAL
General methods
20 Multimedia Dissolution test
Dissolution tests were carried out according to the basket method described in
the
Pharmacopea Europea, as follows:
= Apparatus Agilent Technologies 708-DS integrated with an 850-DS sampling
station;
25 = Stirring speed: 100 rpm
= Temperature: 37 C 0.5 C
The sample is placed in a basket, and then stirred at 100 rpm for 2 hours in
750 mL of
HCI 0.1N, followed by 2 hours in 900 mL of pH 4.5 acetate buffer, and finally
for 8 hours
30 in 900 mL pH 6.8, or pH 6.0 phosphate buffer. Alternatively, the last
stage can be
carried out until complete dissolution.
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
31
Phosphate buffer pH 6.0 preparation: Dissolve 6.8 g of potassium dihydrogen
phosphate and 0.2 g of sodium hydroxide in 1000 mL of deionized water. If
necessary,
adjust with 1 N sodium hydroxide to a pH 6Ø
Phosphate buffer p/-i 6.8 preparation: Dissolve 6.8 g of potassium dihydrogen
phosphate, and 0.896 g of sodium hydroxide in 900 mL of deionized water. If
necessary, adjust to pH 6.8 with phosphoric acid, or sodium hydroxide.
Alcohol dose-dumping assay: The sample is placed in a basket, and then stirred
at
100 rpm in 750 mL of HCI 0.1N or 900 mL phosphate buffer pH 6.0, in the
presence of
the corresponding ethanol % (v/v), and samples are taken every 15 minutes.
HPLC method
The amount of active ingredient released in the dissolution test is determined
by High
Performance Liquid Chromatography (HPLC), as follows:
HPLC system: Agilent 1260 Infinity II, equipped with pump, a diode-array
detector, and
an auto-sampler.
Column: Kromasil C18 5 pnn, 150 x4.6 mm
Oven temperature: 20 C
Wavelength: 230 nm
Eluent: 85% buffer solution pH 5.5: 15% acetonitrile
Flow: 1.0 mL/min
Injection volume: 5 pL
Buffer solution pH 5.5: Dissolve 0.26 g of potassium dihydrogen phosphate in
1000 mL
of water. Adjust with 0.1 M potassium hydroxide to a pH 5.5. Filter through
0.45 pm
filter.
Standard solution: Prepare a levetiracetam solution in water containing a
concentration
exactly calculated of about 0.15 ring/mL.
Test solution: Take a minimum of 5 sachets, weight and calculate the average
weight.
Grind the mini-tablets to a fine and homogeneous powder. Prepare a solution of
the
powder in solvent at a concentration of about 0.15 mg/mL of levetiracetann.
Stir 30
minutes. Centrifuge.
Examples
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
32
Examples 1 to 6.
Table 1. Immediate release cores composition for examples 1 to 6.
COMPONENT AMOUNT (MG)
Levetiracetam 1000
Microcrystalline cellulose 120
Copovidone 23.53
Purified waterl q.s
Talc 25.88
Magnesium stearate 7.06
Table 2. Physical parameters of mechanical properties of immediate release
cores
composition for examples 1 to 6. The parameters are determined by standard
methods
defined in the European Pharmacopoeia.
PARAMETERS VALUES
Diameter (mm) 2.00-2.06
Thickness (mm) 1.64-1.70
Weight (mg) 4.95-5.05
Hardness (N) 5.30-8.10
Friability (%) 0.10-0.52
Disintegration time (min) 4-9 min
Water content by KF ((Yip) 0.30-0.50
Bulk density (g/ml) 0.7
Volume of mini-tablets (ml/g) 1.42
Content Uniformity (%) 98-102
AV Values 2.5
Table 3. Coating layer composition for examples 1 to 6
EX. 1 EX. 2 EX. 3 EX. 4
EX. 5 EX. 6
COMPONENT
COATING COMPOSITION (AMOUNTS IN MG)
Ethyl cellulose 2 105.37 81.49 81.49
94.68 62.42 85.21
Medium chain triglycerides2 18.37 14.21 14.21
16.50 10.87 .. 14.85
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
33
Oleic Acid2 10.36 8.01 8.01 9.31
6.14 8.38
Ammonium hydroxide2 10.42 8.06 8.06 9.36
6.17 8.43
Hydroxypropylmethyl
8.41 5.88 11.14 7.43 10.03
cellulose3
Guar Gum 5.88
Purified water1'2 q.s q.s q.s. q.s.
q.s q.s
Ethyl cellulose:
Hydroxypropylmethyl
12.5:1 13.85:1 13.85:1 8:5:1 8.4:1 8.5:1
cellulose (or guar gum)
weight ratio
1. Evaporated during drying of the composition.
2. Ethyl cellulose, medium chain triglycerides, oleic acid, ammonium hydroxide
and water are
added in the form of the commercial product Surelease 8 E-7-19040 which is an
aqueous
dispersion of ethyl cellulose 25% w/w (solids content), comprising medium
chain triglycerides,
oleic acid and ammonium hydroxide.
3. MethocelTm K100LV was used having a viscosity of 80 mPa.s to about 120
mPa.s in a 2%
w/w aqueous solution at 20 C (measured according to USP method).
All examples were prepared in the form of mini-tablets wherein the uncoated
cores,
weigh 5 mg. For Ex. 6, 10% of the mini-tablet cores, were left uncoated,
thereby being
a combination of 90% coated mini-tablets and 10% uncoated mini-tablets. The
resulting
mini-tablets have a biconvex round shape.
The mini-tablets were prepared as follows:
i) microcrystalline cellulose PH 101 and levetiracetam were sifted through
sieve 0.8
mm;
ii) microcrystalline cellulose PH 101 and levetiracetam were mixed in high
shear
granulator for 5 min;
iii) copovidone was dissolved in purified water while stirring with propeller
stirrer;
iv) the mixture resulting from step ii) was granulated in a high shear
granulator using
copovidone solution resulting from step iii);
v) the granules resulting from step iv) were dried in fluid bed dryer at a
temperature of
about 60 C ¨ 65 C;
vi) the dried granules resulting from step v) were sifted using screening mill
through a
stainless-steel sieve of 0.8 mm;
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
34
vii) talc was added to the granules resulting from step vi) in container mixer
for 15
minutes;
viii) magnesium stearate as added to the mixture resulting from step vii) in
container
mixer for 5 minutes;
ix) the mixture resulting from step viii) was compressed in a tablet
compression
machine using 2 mm multiunit punches obtaining 5 mg weight of each mini-
tablet;
x) the mini-tablets resulting from step ix) were coated with a water
suspension of ethyl
cellulose and hydroxypropylmethyl cellulose having a viscosity comprised
between
about 1 mPa-s to about 1,000 mPa-s, or guar gum in example 2;
Xi) the mini-tablets resulting from step x) were cured at room temperature.
The white or off-white round biconvex functional coated mini-tablets of 2 mm
of
diameter obtained as in examples Ito 6 showed a bulk density of 0.71-0.75 g/ml
and
an apparent volume of 1.33 to 1.41 ml/g. The apparent volume was calculated
considering the bulk density of mini-tablets (i.e. the unit in ml per 1 g of
mini-tablets).
Example 6. Dissolution profiles
Table 4.
Time (hours) Ex. 1 Ex.2 Ex.3
0 0.0 0.0 0.0
pH 0.5 1.0 6.0 1.0
1.2 1 3.0 32.0 5.0
2 5.0 64.0 15.0
pH 3 9.0 87.0 30.0
4.5 4 15.0 95.0 44.0
5 25.0 101.0 54.0
6 34.0 64.0
pH
8 48.0 71.0
6.8
10 59.0 79.0
12 70.0 88.0
As can be seen Ex.2, containing guar gum instead of hydroxypropylmethyl
cellulose
having a viscosity comprised between about 1 mPa-s to about 1,000 mPa-s shows
a
pH-dependent release of levetiracetam, whereas Ex.1 and 3 show a non-pH
CA 03226799 2024- 1-23
WO 2023/002004 PC T/EP2022/070585
dependent liberation profile, in line with the dissolution profile observed
for Keppra
XRO, the reference product on the market.
Example 7. Stability experiments
5 Stability data of composition according to Ex. 1, upon conditions at time
of preparation
(day 0), and after 3 months and 6 months at 40 C/75% Relative Humidity (RH)
and
after 12 months at 25 C/60% RH, is shown below.
Dissolution test according to general methods above (multimedia dissolution
test: 2
10 hours at HCl 0.1N, then 2 hours at pH 4.5 acetate buffer, followed by pH
6.8 phosphate
buffer until complete dissolution).
Table 5.
RESULTS
Time ________________________________________________________________________
(hours) Day 0 3M 6M 12M
C/75%RH 40 C/75%RH 25 C/60%RH
0 0.0 0.0 0.0 0.0
1 1.0 1.0 2.0 2.0
2 5.0 6.0 7.0 6.0
4 15.0 26.0 27.0 23.0
8 48.0 56.0 59.0 55.0
12 70.0 74.0 79.0 75.0
15 81.0 84.0 87.0 85.0
The composition according to Ex. 1, was further evaluated for levetiracetam
content,
15 and impurities or degradation products that could have occurred during
the storage
conditions. HPLC analysis (according to the general method) of levetiracetam
content,
and related impurities, after stability test_
Table 6.
RESULTS
Sample Levetiracetam Impurity A Any unspecified
Total Impurities
impurity
T=0 100.2 <0.1% 50.05
<0.1%
6M -
25 C/60%RH 100.3 <0.1% 50.05 <0.1%
6M -
40 C/75%RH 100.3 <0.1% 50.05 <0.1%
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
36
As can be seen, from table 5, the tablets according to the present invention
show a
good stability even after 6 months storage under accelerated storage
conditions, since
the dissolution profile is nearly identical to day 0. Additionally, the
composition showed
an excellent ability in preventing the degradation of levetiracetam as shown
in table 6.
Example 8. Alcohol-induced dose dumping
The compositions according to the invention were tested to determine their
resistance
to an unintended, rapid release in a short period of time of the entire amount
or a
significant fraction of levetiracetam. The test was performed in the presence
of varying
percentages of ethanol, and carried out at different pH.
Table 7.
Ex. 1
Time (min)
0% Et0H 5% Et0H 10% Et0H 20%
Et0H
0 0 0 0 0
15 0 0 0 0
30 0 0 0 3
pH 1.2 45 0 0 1
8
60 0 1 3 16
75 1 2 5 26
90 2 3 7 37
0 0 0 0 0
15 0 0 0 0
30 0 0 1 3
pH 6.0 45 0 1 1
3
60 1 3 6 15
75 3 4 11 25
90 5 7 16 35
As can be seen in table 7, the composition of Ex.1 is able to withstand
increasing
alcohol concentrations up to 20%, without significantly releasing
levetiracetam to the
media (below 50% liberation of the active ingredient).
Example 9. In vivo Pharmacokinetics Assay
CA 03226799 2024- 1-23
WO 2023/002004 PC
T/EP2022/070585
37
An in vivo study was performed in healthy human volunteers to assess the
plasma
concentrations of levetiracetam formulated according to Ex. 1, 4, 5 and 6,
compared
with the reference treatment with immediate release levetiracetam tablets,
Keppra0.
The study was designed as an open label, four-period, four-way crossover,
block
randomized single dose bioequivalence on healthy human, male and female,
volunteers, with oral administration under fasting conditions. The
participants were
given 1000 mg of levetiracetam, orally. For immediate release tablets, Keppra0
tablets
were provided, following the recommended posology of 500 mg twice-daily, one
in the
morning and one in the evening, at 12 hours after the morning administration.
The
extended-release formulations according to Ex.1, 4, 5 and 6 were administered
in the
morning as a single dose.
Mean plasma levels of levetiracetam were determined using an HPLC validated
method, with MS/MS detection, from blood samples drawn from each participant.
The results are shown in Fig. 1, 2, 3 and 4. As can be seen in the graphs, the
average
mean plasmatic concentrations of levetiracetam obtained with a single dose of
the
formulations according to Ex.1, 4, 5 and 6, respectively, maintain and/or
achieve
levetiracetam levels in plasma of the subjects comparable to the ones achieved
with
twice-daily intake with Keppra0.
This clearly shows that the compositions according to the invention are
suitable for a
single-dose posology regime for levetiracetam.
Example 10. In vivo Multiple Dose Bioequivalence Study
An in Vivo study was performed in healthy human volunteers to demonstrate the
steady
state bioequivalence between the extended release levetiracetam formulated
according to Ex. 1 (test=Levetiracetam 1000 mg prolonged-release mini-tablets
in
sachets, taken as 3000 mg dose in the morning, in fasting conditions) and a
corresponding dose of the reference medicinal product (Keppra0 500 mg
immediate
release film-coated tablets, administered twice daily on each treatment day,
in fasting
conditions: the first dose in the morning ¨3 tablets of Keppra0 500 mg - and
the second
one in the evening 12 hours after the morning dose ¨ 3 tablets of Keppra0 500
mg).
The study was designed as open label, two-period, two-way crossover, block
CA 03226799 2024- 1-23
WO 2023/002004
PCT/EP2022/070585
38
randomized, multiple dose bioequivalence pivotal study on healthy volunteers
with
administration under fasting conditions for 5 consecutive days. The
bioequivalence
assessment was based on plasma drug levels of levetiracetam determined using
an
HPLC validated method, with MS/MS detection, from blood samples drawn from
each
participant.
The results are shown in Table 8 and Fig. 5. As can be seen, a dose of 3000 mg
of
Levetiracetam formulation according to Ex.1 and a corresponding dose of the
reference
formulation are bioequivalent with respect to Levetiracetam extent of
absorption
(AUCO-tau) after multiple dosing in fasting conditions.
Table 8. Bioequivalence comparison (based on primary PK parameters)
Test Parameter Geo Mean 90% Confidence Acceptance
Infra-
name Ratio Interval range
subject
(Test!
CV (%)
Reference)
Lower Upper Lower Upper
90% CL 90% CL limit limit
(`)/0) (%)
Classic AUCO-tau 89.669 86.099 93.387 80.00 125.00
10.808
90% Cl
Classic Cmax.ss 69.259 65.320 73.434 80.00 125.00
15.623
90% Cl
Classic Cmin,ss 98.505 90.005 107.809 80.00 125.00
24.287
90% Cl
If the lower and upper CL (Confidence Interval limits) lie within accepted
regulatory criteria, then
we may conclude for bioequivalence.
CA 03226799 2024- 1-23