Note: Descriptions are shown in the official language in which they were submitted.
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
METHODS AND COMPOSITIONS FOR TREATING FIBROTIC
DISEASES
BACKGROUND
[01] Fibrosis is the formation of excess fibrous connective tissue in an
organ or in
tissue. Connective tissue, such as extracellular matrix proteins, is produced
by
fibroblasts in response to pro-fibrotic factors including transforming growth
factor
beta (TGFI3), connective tissue growth factor (CTGF), platelet-derived growth
factor
(PDGF) and interleukin 4 (IL-4). Fibroblasts may differentiate into
myofibroblasts
through an epithelial to mesenchymal transition largely mediated by TGFP
(Midgley,
A. C. etal., "Transforming growth factor-131 (TGF-131)-stimulated fibroblast
to
myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated
epidermal
growth factor receptor (EGFR) and CD44 co-localization in lipid rafts",
Journal of
Biological Chemistry, Vol. 288, No. 21, p. 14824-14838 (2013)). Myofibroblasts
are
key mediators of profibrotic conditions and proliferate in response to TGFI3
signaling
(Harris, W. T. et al., "Myofibroblast differentiation and enhanced TGF-13
signaling in
cystic fibrosis lung disease", PLoS One, Vol. 8, No. 8, 8 pages (2013)).
[02] Fibrosis can result from specific trauma such as surgical
complications,
exposure to radiation, burns or physical injury. Some therapeutic treatments,
such
as the administration of chemotherapeutic drugs or exposure to ionizing
radiation
(radiotherapy) as used in treating cancer, are known to cause fibrosis as a
side
effect. Certain cancers, such as myelofibrosis (bone marrow cancer), can also
cause fibrosis.
[03] Fibrotic diseases can develop in many parts of the body in humans and
animals and may affect organs including the lungs (interstitial lung disease,
pulmonary fibrosis, acute lung injury), liver (liver fibrosis, cirrhosis),
kidneys (kidney
disease, nephrosclerosis), heart (cardiovascular disease) and eyes (macular
degeneration, vitreal retinopathy) (Wynn, T. A., "Fibrotic disease and the
TH1/TH2
paradigm", Nature Reviews Immunology, Vol. 4, No. 8, p. 583-594 (2004)).
Fibrotic
diseases that are characterized by excessive connective tissue accumulation
and
- 1 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
progressive tissue contraction are known as fibroproliferative disorders
(Bitternnan,
P.B. etal., "Fibroproliferative disorders", Chest, Vol. 99, No. 3, pp. 81S-84S
(1991)).
Examples of fibroproliferative disorders include scleroderma (systemic and
local),
hypertrophic scarring, keloid scarring, cirrhosis, nephrosis and restenosis.
[04] Pulmonary fibrosis (scarring of the lungs) is a respiratory disease
characterized by the development of fibrous tissue in the walls of the air
sacs of the
lungs. The formation of scar tissue impairs the ability of the lungs to
oxygenate
blood, which often presents as shortness of breath (dyspnea). Other symptoms
of
pulmonary fibrosis include a chronic dry or hacking cough, fatigue, weakness,
chest
pain or discomfort, loss of appetite, unexplained weight loss and clubbing of
the tips
of the fingers and toes. The scarring caused by pulmonary fibrosis is
permanent and
the loss of lung function cannot be restored.
[05] Pulmonary fibrosis may result from a variety of different sources.
Known
causes of pulmonary fibrosis include autoimmune diseases that produce
inflammation or scarring in the lungs, such as rheumatoid arthritis,
scleroderma and
autoimmune muscle diseases; occupational exposure to small inorganic
particles,
such as asbestos, beryllium, coal dust, silica and heavy metal dusts;
environmental
exposure to small organic particles, such as animal proteins, bacteria and
molds;
radiation exposure, including environmental radiation and cancer radiotherapy;
bacterial or viral infections; and exposure to medications, such as
nitrofurantoin,
sulfasalazine, amiodarone, propranolol, phenytoin, methotrexate, bleomycin and
oxaliplatin (Pulmonary Fibrosis Foundation, "About PF", available online at
www.pulmonaryfibrosis.org/life-with-pf/about-pf, accessed on July 18, 2017).
When
the specific cause of pulmonary fibrosis cannot be identified, the condition
is referred
to as idiopathic pulmonary fibrosis (IPF). Idiopathic pulmonary fibrosis may
result
from DNA abnormalities, and inheritable forms of idiopathic pulmonary fibrosis
include familial pulmonary fibrosis (FPF) and familial interstitial pneumonia
(FIP)
(j4.).
[06] There is no standard treatment for pulmonary fibrosis. Lifestyle
changes such
as not smoking and maintaining a healthy weight can improve symptoms of
- 2 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
pulmonary fibrosis. Pulmonary rehabilitation exercise programs can help to
preserve
lung function. Oxygen therapy may be used to improve oxygen delivery to the
body.
Various pharmacotherapies have been investigated for treating pulmonary
fibrosis
including corticosteroids (immunosuppressant/anti-inflammatory),
cyclophosphamide
(anti-inflammatory), azathioprine (immunosuppressant), mycophenolate mofetil
(immunosuppressant), N-acetylcysteine (antioxidant) and proton pump inhibitors
(gastric acid reducer). Nintedanib (OFEV8) and pirfenidone (ESBRIETe) are the
only U.S. Food and Drug Administration-approved medications for treating
idiopathic
pulmonary fibrosis. However, these medications slow the progression of
idiopathic
pulmonary fibrosis, but do not treat the disease or prevent its onset. Severe
cases of
pulmonary fibrosis may be treated with lung transplantation. There are no
phannacotherapies for treating or preventing the onset of pulmonary fibrosis.
[07] Liver fibrosis is the formation of fibrous tissue in the liver.
Cirrhosis is the
most well-known fibrotic liver disease and may be caused by alcoholism,
hepatitis B,
hepatitis C, non-alcoholic fatty liver disease, primary biliary cirrhosis,
primary
sclerosing cholangitis, autoimmune hepatitis, hereditary hemochromatosis,
Wilson's
disease, Indian childhood cirrhosis, alpha 1-antitrypsin deficiency (A1AD),
cardiac
cirrhosis, galactosemia, glycogen storage disease type IV, cystic fibrosis and
exposure to hepatoxic drugs or toxins. Cirrhosis may lead to complications
such as
ascites, esophageal variceal bleeding, hepatic encephalopathy, hepatorenal
syndrome, spontaneous bacterial peritonitis, portal hypertensive gastropathy,
infection and hepatocellular carcinoma.
[08] The possibility of developing cirrhosis may be reduced by vaccination
against
specific diseases that cause cirrhosis, such as hepatitis B or hepatitis C.
Similarly,
cirrhosis that develops as a side effect may be managed by treating the
underlying
disease, such as by administering interferon and corticosteroids to patients
experiencing cirrhosis due to hepatitis. Treatment options for cirrhosis are
limited.
Further liver damage may be prevented with lifestyle changes including
abstaining
from alcohol, avoiding acetaminophen and maintaining a healthy diet. Liver
transplantation may ultimately be necessary for patients who experience liver
failure
- 3 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
or cannot control the complications of cirrhosis. There are no
pharmacotherapies for
treating or preventing the onset of cirrhosis.
[09] Dermal fibrosis is the formation of fibrous tissue in the skin.
Fibrotic skin
disorders include keloid scarring, hypertrophic scarring, scleroderma,
nephrogenic
fibrosing dermopathy, mixed connective tissue disease, scleromyxedema,
scleredema and eosinophilic fasciitis. Dermal fibrosis may be a minor
aesthetic
inconvenience, or may result in severe health complications. For example,
scleroderma may affect major organs such as the lungs (loss of lung function,
severe
lung disease and lung tissue scarring), heart (scarring and weakness of the
heart,
swelling of the heart muscle and irregular heartbeat) and kidneys (high blood
pressure and kidney failure) ("Scleroderma Living With It", National Institute
of
Arthritis and Musculoskeletal and Skin Diseases, available online at
www.nianns.nih.gov/health-topics/scleroderma#tab-living-with (2016)).
(10] Many treatments for dermal fibrosis address only the local fibrous
tissue. For
example, keloid and hypertrophic scars may be treated with local steroid
injections,
pressure dressings, topical chemotherapy drugs, laser light therapy, pulsed-
dye
laser therapy, radiation therapy, surgical excision, silicone gel sheeting
application
and administration of hyaluronic acid (WO 2014/179262). Other treatments for
dermal fibrosis focus on the symptoms and complications of the specific
fibrotic
disorder. For example, scleroderma therapies include calcium channel blockers,
angiotensin II receptor antagonists, angiotensin converting enzyme (ACE)
inhibitors,
non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors,
acetaminophen,
corticosteroids, narcotics, antacids, histamine H2-receptor antagonists,
proton pump
inhibitors, prokinetic agents, somatostatin agonists, antibiotics,
prostaglandin
derivatives, endothelin receptor antagonists, IP receptor agonists,
phosphodiesterase type 5 (PDE5) inhibitors, anti-fibrotic agents, anti-
inflammatory
agents, tyrosine kinase inhibitors, immunosuppressants and alkylating agents
("Current treatments available for scleroderma patients", Scleroderma Research
Foundation, available online at www.srfcure.org/for-patients/current-
treatments
(2018)). These various treatments fail to address the underlying causes of
dermal
fibrosis.
- 4 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[1 1 ] The formation of fibrous tissue in the urinary system may occur
in the kidneys,
ureter, bladder or the urethra. Kidney fibrosis may be further classified
based on the
location of the affected tissue, such as nephrosclerosis (the nephron),
glomerulosclerosis (the glomerulus) or tubulointerstitial renal fibrosis (the
interstitium). Chronic kidney disease (CKD), a progressive disease, is
frequently
caused by kidney fibrosis. Two primary factors that promote CKD are diabetes
and
high blood pressure ("About chronic kidney disease: symptoms and causes",
National Kidney Foundation, available online at
www.kidney.orgiatoz/content/about-
chronic-kidney-disease (2020)).
[12] The damage caused by urinary fibrosis is permanent. Further damage
may
be reduced by managing underlying conditions that contribute to urinary
fibrosis,
especially diabetes and high blood pressure. Lifestyle changes such as not
smoking, limiting alcohol intake and maintaining a healthy weight can prevent
further
worsening of kidney fibrosis. Dietary changes such as adopting a low-salt and
low-
fat diet may also help. If untreated, urinary fibrosis may lead to loss of
kidney
function, end-stage renal disease or kidney failure. Individuals with kidney
failure
must undergo regular dialysis to cleanse the blood or receive a kidney
transplant, if
they are healthy enough for the procedure and are able to find a suitable
donor.
Pharmacotherapies for treating urinary fibrosis are limited and primarily
focus on
controlling symptoms, reducing complications and slowing the progression of
the
disease.
(13] Desmoplasia refers to growth of dense connective tissue or stoma,
and it
may occur as result of injury or neoplasia. The stromal reaction in cancer is
similar
to the stromal reaction induced by injury or wound repair, causing scar-like
tissue to
be built around the cancer. Thus, the surrounding stroma plays a very
important role
in the progression of cancer. It has been suggested that tumor cells cause the
proliferation of fibroblasts and subsequent secretion of collagen (El-Torky,
M., et al.,
Collagens in scar carcinoma of the lung, The American Journal of Pathology,
vol.
121, no. 2, pp. 322-326 (1985)). The newly secreted collagen is similar to
that of
collagen in scar formation, and it inhibits infiltration of cells to the site
of injury.
- 5 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[14] Senescent cells are cells that are partially-functional or non-
functional and are
in a state of proliferative arrest. Senescence is a distinct state of a cell,
and is
associated with biomarkers, such as activation of the biomarker p161"k4a, and
expression of p-galactosidase. Senescence begins with damage or stress (such
as
overstimulation by growth factors) of cells.
[15] Advanced glycation end-products (AGEs; also referred to as AGE-
modified
proteins or peptides, or glycation end-products) arise from a non-enzymatic
reaction
of sugars with protein side-chains (Ando, K. et al., Membrane Proteins of
Human
Erythrocytes Are Modified by Advanced Glycation End Products during Aging in
the
Circulation, Biochem Biophys Res Commun., Vol. 258, 123, 125 (1999)). This
process begins with a reversible reaction between the reducing sugar and the
amino
group to form a Schiff base, which proceeds to form a covalently-bonded
Amadori
rearrangement product. Once formed, the Amadori product undergoes further
rearrangement to produce AGEs. Hyperglycemia and oxidative stress promote this
post-translational modification of membrane proteins (Lindsey JB, et aL,
"Receptor
For Advanced Glycation End-Products (RAGE) and soluble RAGE (sRAGE):
Cardiovascular Implications," Diabetes Vascular Disease Research, Vol. 6(1), 7-
14,
(2009)). AGEs may also be formed from other processes. For example, the
advanced glycation end product, Ne-(carboxymethyl)lysine, is a product of both
lipid
peroxidation and glycoxidation reactions. AGEs have been associated with
several
pathological conditions including inflammation, atherosclerosis, stroke,
endothelial
cell dysfunction, and neurodegenerative disorders (Bierhaus A, "AGEs and their
interaction with AGE-receptors in vascular disease and diabetes mellitus. I.
The AGE
concept," Cardiovasc Res, Vol. 37(3), 586-600 (1998)).
[16] AGE-modified proteins are also a marker of senescent cells. This
association
between AGEs and senescence is well known in the art. See, for example,
Gruber,
L. (WO 2009/143411, 26 Nov. 2009), Ando, K. etal. (Membrane Proteins of Human
Erythrocytes Are Modified by Advanced Glycation End Products during Aging in
the
Circulation, Biochem Biophys Res Commun., Vol. 258, 123, 125 (1999)), Ahmed,
E.K. etal. ("Protein Modification and Replicative Senescence of WI-38 Human
Embryonic Fibroblasts" Aging Cells, vol. 9, 252, 260 (2010)), Vlassara, H.
etal.
- 6 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
(Advanced Glycosylation Endproducts on Erythrocyte Cell Surface Induce
Receptor-
Mediated Phagocytosis by Macrophages, J. Exp. Med., Vol. 166, 539, 545 (1987))
and Vlassara et al. ("High-affinity-receptor-mediated Uptake and Degradation
of
Glucose-modified Proteins: A Potential Mechanism for the Removal of Senescent
Macromolecules" Proc. Natl. Acad. Sc,. USA!, Vol. 82, 5588, 5591 (1985)).
Furthermore, Ahmed, E.K. et al. indicates that glycation end-products are "one
of the
major causes of spontaneous damage to cellular and extracellular proteins"
(Ahmed,
E.K. et al., see above, page 353). Accordingly, the accumulation of glycation
end-
products is associated with senescence and lack of function.
[17] The damage or stress that causes cellular senescence also negatively
impacts mitochondrial DNA in the cells to cause them to produce free radicals
which
react with sugars in the cell to form methyl glyoxal (MG). MG in turn reacts
with
proteins or lipids to generate advanced glycation end products. In the case of
the
protein component lysine, MG reacts to form carboxymethyllysine, which is an
AGE.
[18] Damage or stress to mitochondrial DNA also sets off a DNA damage
response which induces the cell to produce cell cycle blocking proteins. These
blocking proteins prevent the cell from dividing. Continued damage or stress
causes
mTOR production, which in turn activates protein synthesis and inactivates
protein
breakdown. Further stimulation of the cells leads to programmed cell death
(apoptosis).
[19] p16 is a protein involved in regulation of the cell cycle, by
inhibiting the S
phase (synthesis phase). It can be activated during ageing or in response to
various
stresses, such as DNA damage, oxidative stress or exposure to drugs. p16 is
typically considered a tumor suppressor protein, causing a cell to become
senescent
in response to DNA damage and irreversibly preventing the cell from entering a
hyperproliferative state. However, there has been some ambiguity in this
regard, as
some tumors show overexpression of p16, while others show downregulated
expression. Evidence suggests that overexpression of p16 is some tumors
results
from a defective retinoblastoma protein ("Rb"). p16 acts on Rb to inhibit the
S
phase, and Rb downregulates p16, creating negative feedback. Defective Rb
fails to
- 7 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
both inhibit the S phase and downregulate p16, thus resulting in
overexpression of
p16 in hyperproliferating cells (Romagosa, C. etal., p16In4a overexpression in
cancer: a tumor suppressor gene associated with senescence and high-grade
tumors, Onco gene, Vol. 30, 2087-2097 (2011)).
[20] Senescent cells are associated with secretion of many factors involved
in
intercellular signaling, including pro-inflammatory factors; secretion of
these factors
has been termed the senescence-associated secretory phenotype, or SASP
(Freund, A. "Inflammatory networks during cellular senescence: causes and
consequences" Trends Mol Med. 2010 May;16(5):238-46). Autoinnmune diseases,
such as Crohn's disease and rheumatoid arthritis, are associated with chronic
inflammation (Ferraccioli, G. et al. "Interleukin-113 and Interleukin-6 in
Arthritis Animal
Models: Roles in the Early Phase of Transition from Acute to Chronic
Inflammation
and Relevance for Human Rheumatoid Arthritis" Mol Med. 2010 Nov-Dec; 16(11-
12):
552-557). Chronic inflammation may be characterized by the presence of pro-
inflammatory factors at levels higher than baseline near the site of
pathology, but
lower than those found in acute inflammation. Examples of these factors
include
TNF, IL-la, IL-1p, IL-5, IL-6, IL-8, IL-12, IL-23, CD2, CD3, CD20, CD22, CD52,
CD80, C086, C5 complement protein, BAFF, APRIL, IgE, a4131 integrin and a407
integrin. Senescent cells also upregulate genes with roles in inflammation
including
IL-113, IL-8, ICAM1, TNFAP3, ESM1 and CCL2 (Burton, D.G.A. etal., "Microarray
analysis of senescent vascular smooth muscle cells: a link to atherosclerosis
and
vascular calcification", Experimental Gerontology, Vol. 44, No. 10, pp. 659-
665
(October 2009)).
[21] Senescent cells secrete reactive oxygen species ("ROS") as part of the
SASP. ROS are believed to play an important role in maintaining senescence of
cells. The secretion of ROS creates a bystander effect, where senescent cells
induce senescence in neighboring cells: ROS create the very cellular damage
known
to activate p16 expression, leading to senescence (Nelson, G., A senescent
cell
bystander effect: senescence-induced senescence, Aging Cell, Vo. 11, 345-349
(2012)). The p16/Rb pathway leads to the induction of ROS, which in turn
activates
the protein kinase C delta creating a positive feedback loop that further
enhance
- 8 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
ROS, helping maintain the irreversible cell cycle arrest; it has even been
suggested
that exposing cancer cells to ROS might be effective to treat cancer by
inducing cell
phase arrest in hyperproliferating cells (Rayess, H. etal., Cellular
senescence and
tumor suppressor gene p16, Int J Cancer, Vol. 130, 1715-1725 (2012)).
[22] Recent research demonstrates the therapeutic benefits of removing
senescent cells. In vivo animal studies at the Mayo Clinic in Rochester,
Minnesota,
found that elimination of senescent cells in transgenic mice carrying a
biomarker for
elimination delayed age-related disorders associated with cellular senescence.
Eliminating senescent cells in fat and muscle tissues substantially delayed
the onset
of sarcopenia and cataracts and reduced senescence indicators in skeletal
muscle
and the eye (Baker, D. J. etal., "Clearance of p16In4a-positive senescent
cells
delays ageing-associated disorders", Nature, Vol. 479, pp. 232-236, (2011)).
Mice
that were treated to induce senescent cell elimination were found to have
larger
diameters of muscle fibers as compared to untreated mice. Treadmill exercise
tests
indicated that treatment also preserved muscle function. Continuous treatment
of
transgenic mice for removal of senescent cells had no negative side effects
and
selectively delayed age-related phenotypes that depend on cells. This data
demonstrates that removal of senescent cells produces beneficial therapeutic
effects
and shows that these benefits may be achieved without adverse effects.
[23] Additional In vivo animal studies in mice found that removing
senescent cells
using senolytic agents treats aging-related disorders and atherosclerosis.
Short-
term treatment with senolytic drugs in chronologically aged or progeroid mice
alleviated several aging-related phenotypes (Zhu, Y. et al., "The Achilles'
heel of
senescent cells: from transcriptome to senolytic drugs", Aging Cell, vol. 14,
pp. 644-
658 (2015)). Long-term treatment with senolytic drugs improved vasomotor
function
in mice with established atherosclerosis and reduced intimal plaque
calcification
(Roos, C.M. et al., "Chronic senolytic treatment alleviates established
vasomotor
dysfunction in aged or atherosclerotic mice", Aging Cell (2016)). This data
further
demonstrates the benefits of removing senescent cells.
- 9 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[24] Vaccines have been widely used since their introduction by Edward
Jenner in
the 1770s to confer immunity against a wide range of diseases and afflictions.
Vaccine preparations contain a selected immunogenic agent capable of
stimulating
immunity to an antigen. Typically, antigens are used as the immunogenic agent
in
vaccines, such as, for example, viruses, either killed or attenuated, and
purified viral
components. Antigens used in the production of cancer vaccines include, for
example, tumor-associated carbohydrate antigens (TACAs), dendritic cells,
whole
cells and viral vectors. Different techniques are employed to produce the
desired
amount and type of antigen being sought. For example, pathogenic viruses are
grown either in eggs or cells. Recombinant DNA technology is often utilized to
generate attenuated viruses for vaccines.
[25] Vaccines may therefore be used to stimulate the production of
antibodies in
the body and provide immunity against antigens. When an antigen is introduced
to a
subject that has been vaccinated and developed immunity to that antigen, the
immune system may destroy or remove cells that express the antigen.
SUMMARY
[26] In a first aspect, the invention is a method of treating or preventing
the onset
of a fibrotic disease comprising administering to a subject a composition
comprising
an anti-AGE antibody.
[27] In a second aspect, the invention is a method of treating or
preventing the
onset of a fibrotic disease comprising administering to a subject a vaccine
comprising an AGE antigen.
[28] DEFINITIONS
[29] The term "fibrotic disease" means a disease or disorder characterized
by the
formation of fibrous tissue. Examples of fibrotic diseases include
interstitial lung
disease, pulmonary fibrosis, liver fibrosis, cirrhosis, urinary fibrosis,
kidney fibrosis,
nephrosclerosis, nephrosis, cardiovascular disease, macular degeneration,
vitreal
retinopathy, scleroderma (systemic and local), hypertrophic scarring, keloid
scarring,
- 10-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
restenosis, myelofibrosis, nephrogenic fibrosing dermopathy, mixed connective
tissue disease, scleromyxedema, scleredema, and eosinophilic fasciitis. As
used
herein, "fibrotic disease" does not include atherosclerosis.
[30] The term "pulmonary fibrosis" means a disease or disorder
characterized by
the formation of fibrous tissue in the lungs. Pulmonary fibrosis includes
idiopathic
pulmonary fibrosis (IPF), idiopathic nonspecific interstitial pneumonia
(NSIP),
cryptogenic organizing pneumonia (COP), sarcoidosis, familial pulmonary
fibrosis
(FPF), familial interstitial pneumonia (FIP), asbestosis, silicosis,
berylliosis,
hypersensitivity pneumonitis, atypical pneumonia, pneumocystis pneumonia,
tuberculosis, respiratory syncytial virus, acute interstitial
pneurnonitis/pneumonia
(also known as Hamman-Rich syndrome), chronic obstructive pulmonary disease
(COPD), emphysema and mesothelioma.
[31] The term "liver fibrosis" means a disease or disorder characterized by
the
formation of fibrous tissue in the liver. Liver fibrosis includes cirrhosis.
[32] The term "dermal fibrosis" means a disease or disorder characterized
by the
formation of fibrous tissue in the skin. Dermal fibrosis includes keloid
scarring,
hypertrophic scarring, scleroderma, nephrogenic fibrosing dermopathy, mixed
connective tissue disease, scleromyxedema, scleredema and eosinophilic
fasciitis.
[33] The term "urinary fibrosis" means a disease or disorder characterized
by the
formation of fibrous tissue in the kidneys, ureter, bladder or the urethra.
Urinary
fibrosis includes kidney fibrosis, nephrosclerosis, nephrosis,
glomerulosclerosis,
tubulointerstitial renal fibrosis, bladder fibrosis and urethral stricture.
Urinary fibrosis
may also be referred to as "renal fibrosis".
[34] The term "peptide" means a molecule composed of 2-50 amino acids.
[35] The term "protein" means a molecule composed of more than 50 amino
acids.
[36] The terms "advanced glycation end-product", "AGE", "AGE-modified
protein",
"AGE-modified peptide" and "glycation end-product" refer to modified proteins
or
peptides that are formed as the result of the reaction of sugars with protein
side
-11 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
chains that further rearrange and form irreversible cross-links. This process
begins
with a reversible reaction between a reducing sugar and an amino group to form
a
Schiff base, which proceeds to form a covalently-bonded Amadori rearrangement
product. Once formed, the Amadori product undergoes further rearrangement to
produce AGEs. AGE-modified proteins and antibodies to AGE-modified proteins
are
described in U.S. 5,702,704 to Bucala ("Bucala") and U.S. 6,380,165 to Al-Abed
et
al. ("Al-Abed"). Glycated proteins or peptides that have not undergone the
necessary rearrangement to form AGEs, such as N-deoxyfructosyllysine found on
glycated albumin, are not AGEs. AGEs may be identified by the presence of AGE
modifications (also referred to as AGE epitopes or AGE moieties) such as 2-(2-
furoy1)-4(5)-(2-furany1)-1H-imidazole ("FFI"); 5-hydroxymethy1-1-alkylpyrrole-
2-
carbaldehyde ("Pyrraline"); 1-alky1-2-formy1-3,4-diglycosyl pyrrole ("AFGP"),
a non-
fluorescent model AGE; carboxymethyllysine; carboxyethyllysine; and
pentosidine.
ALI, another AGE, is described in Al-Abed.
[37] The term "AGE antigen" means a substance that elicits an immune
response
against an AGE-modified protein or peptide of a cell. The immune response
against
an AGE-modified protein or peptide of a cell does not include the production
of
antibodies to the non-AGE-modified protein or peptide.
[38] "An antibody that binds to an AGE-modified protein on a cell", "anti-
AGE
antibody" or "AGE antibody" means an antibody, antibody fragment or other
protein
or peptide that binds to an AGE-modified protein or peptide which preferably
includes a constant region of an antibody, where the protein or peptide which
has
been AGE-modified is a protein or peptide normally found bound on the surface
of a
cell, preferably a mammalian cell, more preferably a human, cat, dog, horse,
camelid
(for example, camel or alpaca), cattle, sheep, pig, or goat cell. "An antibody
that
binds to an AGE-modified protein on a cell", "anti-AGE antibody" or "AGE
antibody"
does not include an antibody or other protein which binds with the same
specificity
and selectivity to both the AGE-modified protein or peptide, and the same non-
AGE-
modified protein or peptide (that is, the presence of the AGE modification
does not
increase binding). AGE-modified albumin is not an AGE-modified protein on a
cell,
because albumin is not a protein normally found bound on the surface of cells.
"An
- 12-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
antibody that binds to an AGE-modified protein on a cell", "anti-AGE antibody"
or
"AGE antibody" only includes those antibodies which lead to removal,
destruction, or
death of the cell. Also included are antibodies which are conjugated, for
example to
a toxin, drug, or other chemical or particle. Preferably, the antibodies are
monoclonal antibodies, but polyclonal antibodies are also possible.
[39] The term "senescent cell" means a cell which is in a state of
proliferative
arrest and expresses one or more biomarkers of senescence, such as activation
of
p16In4a or expression of senescence-associated 13-galactosidase. Also included
are
cells which express one or more biomarkers of senescence, do not proliferate
in
vivo, but may proliferate in vitro under certain conditions, such as some
satellite cells
found in the muscles of ALS patients.
[40] The term "senolytic agent" means a small molecule with a molecular
weight of
less than 900 daltons that destroys senescent cells. The term "senolytic
agent" does
not include antibodies, antibody conjugates, proteins, peptides or biologic
therapies.
[41] The term "variant" means a nucleotide, protein or amino acid sequence
different from the specifically identified sequences, wherein one or more
nucleotides,
proteins or amino acid residues is deleted, substituted or added. Variants may
be
naturally-occurring allelic variants, or non-naturally-occurring variants.
Variants of
the identified sequences may retain some or all of the functional
characteristics of
the identified sequences.
[42] The term "percent (%) sequence identity" is defined as the percentage
of
amino acid residues in a candidate sequence that are identical to the amino
acid
residues in a reference polypeptide sequence, after aligning the sequences and
introducing gaps, if necessary, to achieve the maximum percent sequence
identity,
and not considering any conservative substitutions as part of the sequence
identity.
Alignment for purposes of determining percent amino acid sequence identity can
be
achieved in various ways using publicly available computer software such as
BLAST,
BLAST-2, ALIGN or Megalign (DNASTAR) software. Preferably, % sequence
identity values are generated using the sequence comparison computer program
- 13-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
ALIGN-2. The ALIGN-2 sequence comparison computer program is publicly
available from Genentech, Inc. (South San Francisco, CA), or may be compiled
from
the source code, which has been filed with user documentation in the U.S.
Copyright
Office and is registered under U.S. Copyright Registration No. TXU510087. The
ALIGN-2 program should be compiled for use on a UNIX operating system,
including
digital UNIX V4.0D. All sequence comparison parameters are set by the ALIGN-2
program and do not vary.
[43] In situations where ALIGN-2 is employed for amino acid sequence
comparisons, the c/o sequence identity of a given amino acid sequence A to,
with, or
against a given amino acid sequence B (which can alternatively be phrased as a
given amino acid sequence A that has or comprises a certain % amino acid
sequence identity to, with, or against a given amino acid sequence B) is
calculated
as follows: 100 times the fraction X/Y where X is the number of amino acid
residues
scored as identical matches by the sequence alignment program ALIGN-2 in that
program's alignment of A and B, and where Y is the total number of amino acid
residues in B. Where the length of amino acid sequence A is not equal to the
length
of amino acid sequence B, the % amino acid sequence identity of A to B will
not
equal the % amino acid sequence identity of B to A. Unless specifically stated
otherwise, all % amino acid sequence identity values used herein are obtained
using
the ALIGN-2 computer program.
BRIEF DESCRIPTION OF THE DRAWINGS
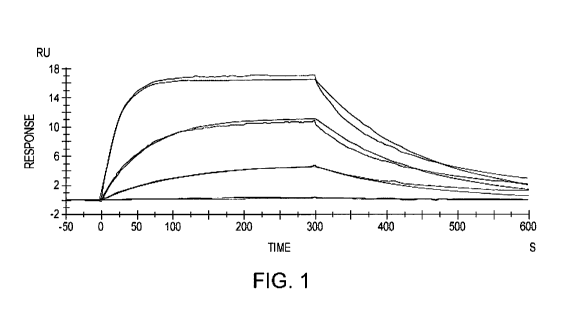
(44] FIG. 1 is a graph of the response versus time in an antibody
binding
experiment.
[45] FIG. 2 is a graph illustrating the effect of senescent cell clearance
on
peripheral capillary oxygen saturation (Sp02) in bleomycin exposed mice.
[46] FIG. 3A is a graph illustrating the effect of senescent cell clearance
with
ganciclovir on lung elastance in 3MR mice exposed to bleomycin.
-14-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[47] FIG. 3B is a graph illustrating the effect of senescent cell clearance
with
ganciclovir on dynamic lung compliance in 3MR mice exposed to bleomycin.
[48] FIG. 3C is a graph illustrating the effect of senescent cell clearance
with
ganciclovir on static lung compliance in 3MR mice exposed to bleonnycin.
[49] FIG. 4 is a graph illustrating the effect of senescent cell clearance
on
peripheral capillary oxygen saturation (Sp02) in mice after 2 months and 4
months of
cigarette smoke (CS) exposure. AP=AP20187; GAN=ganciclovir; Navi=Navitoclax
(ABT-263); and Nutlin=Nutlin 3A.
DETAILED DESCRIPTION
[50] Studies of fibrotic diseases have established that inflammation is
involved in
fibrosis. Pro-inflammatory factors, such as TGF13, PDGF, IL-13, IL-6, IL-10,
IL-13
and IFN-y, and reactive oxygen species have been recognized as mediators of
fibrosis (Bataller, R. etal., "Liver fibrosis", The Journal of Clinical
Investigation, Vol.
115, No. 2, p. 209-218 (2005)). Similarly, inflammatory cells and their
secreted
inflammatory factors have been recognized as the primary factors in activating
dermal fibroblasts to become fibrotic (Shaw, T. J. et al., "Wound-associated
skin
fibrosis: mechanisms and treatments based on modulating the inflammatory
response", Endocrine, Metabolic & Immune Disorders, Vol. 10, No. 4, pp. 320-
330
(2010)). These studies have led to the development of therapies that treat
fibrotic
diseases by targeting pro-inflammatory factors. Administration of resveratrol,
a
known anti-inflammatory agent, and an inhibitor of the inflammatory cytokine
monocyte chemoattractant protein-1 (MCP-1) was shown to inhibit fibroplasia in
wound healing and reduce or prevent scar formation (WO 2016/057831).
Similarly,
interference with TGFI3 signaling was found to influence liver fibrosis in
mouse
models (Weiler-Normann, C., et al., "Mouse models of liver fibrosis",
Zeitschrift fur
Gastroenterologie, Vol. 45, p. 43-50 (2007)). However, treatments that target
the
inflammatory cascade have been unsuccessful (Wynn). Recent research into the
pathogenesis of fibrotic diseases suggests that senescent cells, which secrete
pro-
- 15-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
inflammatory factors as part of the SASP, may be a more appropriate
therapeutic
target than pro-inflammatory factors.
[51] Senescent cells have been implicated in a number of fibrotic
diseases.
Senescent biomarkers such as p16, p21 and senescence-associated 13-
galactosidase (SA-13-gal) have been observed in fibroblasts and epithelial
cells in
human and mouse idiopathic lung fibrosis tissue (Schafer, M. J. et al.,
"Cellular
senescence mediates fibrotic pulmonary disease", Nature Communications, Vol.
8,
No. 14532, 11 pages (2017)). Pathogenic models of chronic obstructive
pulmonary
disease and idiopathic pulmonary fibrosis both involve premature cellular
senescence of progenitor cells, which results in stem cell exhaustion and
disease
progression (Chilosi, M. etal., "Premature lung aging and cellular senescence
in the
pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema",
Translational
Research, Vol. 162, p. 156-173 (2013)). Senescent cells in the lungs
contribute to
excess extracellular matrix deposition in an aged mouse model and in elderly
human
samples (Calhoun, C. et al., "Senescent cells contribute to the physiological
remodeling of aged lungs", Journals of Gerontology: Biological Sciences, Vol.
71,
No. 2, p. 153-160 (2016)). The cell surface protein vimentin has been
recognized as
a marker of cellular senescence in mice that have been immunized with
senescent
mouse lung fibroblasts, and the IgM antibody clone 9H4 was found to bind to
cell
surface vimentin on senescent cells (Frescas, D. etal., "Senescent cells
expose and
secrete an oxidized form of membrane-bound vimentin as revealed by a natural
polyreactive antibody", Proceedings of the National Academy of Sciences, p.
E1668-
E1677 (2017)). Cellular senescence and the SASP participate in the
pathological
process of CKD, and CKD accelerates the progression of cellular senescence and
the secretion of inflammatory factors through the SASP (Wang, W-J. et al.,
"Cellular
senescence, senescence-associated secretory phenotype, and chronic kidney
disease", Oncotarget, Vol. 8, No. 38, pp. 64520-64533 (2017)). Senescent cells
increase in the glomeruli in response to renal injury and aging, and increased
senescent markers have been detected in glomerulosclerosis (Valentijn, F.A.
etal.,
"Cellular senescence in the aging and diseased kidney", Journal of Cell
Communication and Signaling, Vol. 12, pp. 69-82 (2018)).
- 16-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[52] Advanced glycation end-products, known markers of senescent cells,
have
also been implicated in fibrotic diseases. RAGE, a receptor for advanced
glycation
end-products, is expressed by hepatic stellate cells and myofibroblasts, both
of
which are involved in the pathogenesis of liver fibrosis and cirrhosis
(Yagmur, E. et
al., "Elevation of NE-(carboxymethyplysine-modified advanced glycation end
products in chronic liver disease is an indicator of liver cirrhosis",
Clinical
Biochemistry, Vol. 39, p. 39-45 (2006)). In addition, serum levels of
carboxymethyllysine, the most well-studied advanced glycation end-product,
were
significantly affected by the stage of liver cirrhosis and were closely
associated with
liver function capacity (Yagmur et al.). AGE exposure followed by binding to
RAGE
results in tubulointerstitial fibrosis in the diabetic kidney (Oldfield, M.D.
et al.,
"Advanced glycation end products cause epithelial-myofibroblast
transdifferentiation
via the receptor for advanced glycation end products (RAGE)", The Journal of
Clinical Investigation, Vol. 108, No. 12, pp. 1853-1863 (2001)). CML and
pentosidine have also been identified in glomerulosclerosis, FSGS,
hypertensive
nephrosclerosis and lupus nephritis (Tanji, N. et al., "Expression of advanced
glycation end products and their cellular receptor RAGE in diabetic
nephropathy and
nondiabetic renal disease", Journal of the American Society of Nephrology,
Vol. 11,
pp. 1656-1666 (2000)).
[53] Desmoplasia forms around tumors, acting as a barrier to drugs and even
small molecules. The tumor cells are maintained by the cancer-associated
fibroblasts that form the desmoplasia, and the tumor cells contribute to the
growth of
the desmoplasia. Senescent fibroblasts are present in the desmoplasia. AGEs
have
been detected in higher staining of surrounding fibroblast foci in fibrotic
lungs of IPF
patients, compared to control patients (Machahua, C., et al., Increased AGE-
RAGE
ratio in idiopathic pulmonary fibrosis, Respiratory Research, vol. 17, no.
144, pp. 1-
11 (2016)). Eliminating or reducing the senescent fibroblasts would reduce the
size
of the desmoplasia, and it would enhance the permeability of the tumor to
antibodies,
natural killer cells (NK), immune effectors and other therapeutics, which
would
enhance the efficacy of the immune response or pharmaceuticals for treating
cancer.
- 17-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[54] Recent studies have found that elimination of senescent cells can be
used to
treat fibrotic diseases. Elimination of senescent fibroblasts in mice by
administration
of a combination of the senolytic agents dasatinib and quercetin improved lung
function (Schafer, M. J. et al.). Dasatinib alone attenuated bleomycin-induced
lung
fibrosis in vivo in mice, and suppressed TG93-induced endothelial mesenchymal
transition in vitro (Kato, M. et aL, "Dasatinib suppresses TFG13-induced
epithelial
mesenchymal transition and inhibits pulmonary fibrosis", European Respiratory
Journal, Vol. 44, No. Suppl. 58, p. 742 (2014); Yilmaz, 0. etal., "Dasatinib
attenuated bleonnycin-induced pulmonary fibrosis in mice", Growth Factors,
Vol. 33,
No. 5, p. 366-375 (2015)). Ablation of p19ARF-expressing cells in transgenic
mice
using a toxin receptor-mediated cell knockout system improved aging-associated
lung hypofunction (Hashimoto, M. etal., "Elimination of p191RF-expressing
cells
enhances pulmonary function in mice", Journal of Clinical Investigation
Insight, Vol.
1, No. 12, 15 pages (2016)). In addition, the elimination of p19ARF reversed
the
expression of other aging-associated genes. Administration of oral rapannycin
to
mice prevented cellular senescence in the vasculature and limited collagen
deposition in the lungs (Calhoun, C. et al.). Removing senescent cells by
administration of senolytic agents treated pulmonary fibrosis resulting from
ionizing
radiation (Pan, J. et al., "Inhibition of Bc1-2/xl with ABT-263 selectively
kills senescent
type II pneumocytes and reverses pulmonary fibrosis induced by ionizing
radiation in
mice", International Journal of Radiation Oncology Biology Physics, Vol. 99,
No. 2,
pp. 353-361 (2017)). Clearance of senescent cells by induction of apoptosis in
transgenic animal models attenuated glomerulosclerosis (Valentijn, F.A. et
al.).
These studies validate the selection of senescent cells as a target for
treating fibrotic
diseases.
[55] The therapeutic benefits of removing senescent cells has been
demonstrated
in vivo in an art-accepted model in treating age-related diseases such as
sarcopenia
(US 9,161,810) and treating metastatic cancer (WO 2017/143073). The
identification of a link between cellular senescence and fibrotic diseases
allows for
similar treatment possibilities. The present invention uses enhanced clearance
of
cells expressing AGE-modified proteins or peptides (AGE-modified cells) to
treat,
- 18-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
ameliorate or prevent the onset of fibrotic diseases by removing or killing
senescent
cells. This may be accomplished by administering anti-AGE antibodies to a
subject.
[56] Vaccination against AGE-modified proteins or peptides of a cell may
also be
used to control the presence of AGE-modified cells in a subject. The
continuous and
virtually ubiquitous surveillance exercised by the immune system in the body
in
response to a vaccination allows maintaining low levels of AGE-modified cells
in the
body. Vaccination against AGE-modified proteins or peptides of a cell removes
or
kills senescent cells. The process of senescent cell removal or destruction
allows
vaccination against AGE-modified proteins or peptides of a cell to be used to
treat or
prevent the onset of fibrotic diseases. Individuals may receive repeated
vaccinations
or boosters on a periodic basis to maintain their immunity.
[57] Anti-AGE antibodies are known in the art and are commercially
available.
Examples include those described in U.S. 5,702,704 (Bucala) and U.S. 6,380,165
(Al-Abed et al.). The antibody may bind to one or more AGE-modified proteins
or
peptides having an AGE modification such as FFI, pyrraline, AFGP, ALI,
carboxymethyllysine (CML), carboxyethyllysine (CEL) and pentosidine, and
mixtures
of such antibodies. Preferably, the antibody is non-immunogenic to the animal
in
which it will be used, such as non-immunogenic to humans; companion animals
including cats, dogs and horses; and commercially important animals, such
camels
(or alpaca), cattle (bovine), sheep, pig, and goats. More preferably, the
antibody has
the same species constant region as antibodies of the animal to reduce the
immune
response against the antibody, such as being humanized (for humans), felinized
(for
cats), caninized (for dogs), equuinized (for horses), camelized (for camels or
alpaca),
bovinized (for cattle), ovinized (for sheep), porcinized (for pigs), or
caperized (for
goats). Most preferably, the antibody is identical to that of the animal in
which it will
be used (except for the variable region), such as a human antibody, a cat
antibody, a
dog antibody, a horse antibody, a camel antibody, a bovine antibody, a sheep
antibody, a pig antibody, or a goat antibody. Details of the constant regions
and
other parts of antibodies for these animals are described below. The antibody
may
be monoclonal or polyclonal. Preferably, the antibody is a monoclonal
antibody.
- 19 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[58] Preferred anti-AGE antibodies include those which bind to proteins or
peptides that exhibit a carboxymethyllysine or carboxyethyllysine AGE
modification.
Carboxymethyllysine (also known as N(epsilon)-(carboxymethyl)lysine, N(6)-
carboxymethyllysine, or 2-Amino-6-(carboxymethylamino)hexanoic acid) and
carboxyethyllysine (also known as N-epsilon-(carboxyethyl)lysine) are found on
proteins or peptides and lipids as a result of oxidative stress and chemical
glycation.
CML- and CEL-modified proteins or peptides are recognized by the receptor RAGE
which is expressed on a variety of cells. CML and CEL have been well-studied
and
CML- and CEL-related products are commercially available. For example, Cell
Biolabs, Inc. sells CML-BSA antigens, CML polyclonal antibodies, CML
immunoblot
kits, and CML competitive ELISA kits (www.cellbiolabs.com/cml-assays) as well
as
CEL-BSA antigens and CEL competitive ELISA kits (www.cellbiolabs.com/cel-n-
epsilon-carboxyethyl-lysine-assays-and-reagents). A preferred antibody
includes the
variable region of the commercially available mouse anti-glycation end-product
antibody raised against carboxymethyl lysine conjugated with keyhole limpet
hemocyanin, the carboxymethyl lysine MAb (Clone 318003) available from R&D
Systems, Inc. (Minneapolis, MN; catalog no. MAB3247), modified to have a human
constant region (or the constant region of the animal into which it will be
administered). Commercially-available antibodies, such as the carboxymethyl
lysine
antibody corresponding to catalog no. MAB3247 from R&D Systems, Inc., may be
intended for diagnostic purposes and may contain material that is not suited
for use
in animals or humans. Preferably, commercially-available antibodies are
purified
and/or isolated prior to use in animals or humans to remove toxins or other
potentially-harmful material.
[59] The anti-AGE antibody preferably has a low rate of dissociation from
the
antibody-antigen complex, or kd (also referred to as kback or off-rate),
preferably at
most 9 x 10-3, 8 x 10-3, 7 x 10-3 or 6 x 10-3 (sec-1). The anti-AGE antibody
preferably
has a high affinity for the AGE-modified protein of a cell, which may be
expressed as
a low dissociation constant KD of at most 9 x 10-6, 8 x 10-6, 7 x 10-6, 6 x 10-
6, 5 x 10-6,
4 x 10-6 or 3 x 10-6 (M). Preferably, the binding properties of the anti-AGE
antibody
are similar to, the same as, or superior to the carboxymethyl lysine MAb
(Clone
-20 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
318003) available from R&D Systems, Inc. (Minneapolis, MN; catalog no.
MAB3247), illustrated in FIG. 1.
[60] The anti-AGE antibody may destroy AGE-modified cells through antibody-
dependent cell-mediated cytotoxicity (ADCC). ADCC is a mechanism of cell-
mediated immune defense in which an effector cell of the immune system
actively
lyses a target cell whose membrane-surface antigens have been bound by
specific
antibodies. ADCC may be mediated by natural killer (NK) cells, macrophages,
neutrophils or eosinophils. The effector cells bind to the Fc portion of the
bound
antibody. The anti-AGE antibody may also destroy AGE-modified cells through
complement-dependent cytotoxicity (CDC). In CDC, the complement cascade of the
immune system is triggered by an antibody binding to a target antigen.
[61] The anti-AGE antibody may be conjugated to an agent that causes the
destruction of AGE-modified cells. Such agents may be a toxin, a cytotoxic
agent,
magnetic nanoparticles, and magnetic spin-vortex discs.
[62] A toxin, such as pore-forming toxins (PFT) (Aroian R. et aL, "Pore-
Forming
Toxins and Cellular Non-Immune Defenses (CNIDs)," Current Opinion in
Microbiology, 10:57-61 (2007)), conjugated to an anti-AGE antibody may be
injected
into a patient to selectively target and remove AGE-modified cells. The anti-
AGE
antibody recognizes and binds to AGE-modified cells. Then, the toxin causes
pore
formation at the cell surface and subsequent cell removal through osmotic
lysis.
[63] Magnetic nanoparticles conjugated to the anti-AGE antibody may be
injected
into a patient to target and remove AGE-modified cells. The magnetic
nanoparticles
can be heated by applying a magnetic field in order to selectively remove the
AGE-
modified cells.
[64] As an alternative, magnetic spin-vortex discs, which are magnetized
only
when a magnetic field is applied to avoid self-aggregation that can block
blood
vessels, begin to spin when a magnetic field is applied, causing membrane
disruption of target cells. Magnetic spin-vortex discs, conjugated to anti-AGE
antibodies specifically target AGE-modified cell types, without removing other
cells.
-21-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[65] Antibodies are Y-shaped proteins composed of two heavy chains and two
light chains. The two arms of the Y shape form the fragment antigen-binding
(Fab)
region while the base or tail of the Y shape forms the fragment crystallizable
(Fc)
region of the antibody. Antigen binding occurs at the terminal portion of the
fragment
antigen-binding region (the tips of the arms of the Y shape) at a location
referred to
as the paratope, which is a set of complementarity determining regions (also
known
as CDRs or the hypervariable region). The complementarity determining regions
vary among different antibodies and gives a given antibody its specificity for
binding
to a given antigen. The fragment crystallizable region of the antibody
determines the
result of antigen binding and may interact with the immune system, such as by
triggering the complement cascade or initiating antibody-dependent cell-
mediated
cytotoxicity (ADCC). When antibodies are prepared recombinantly, it is also
possible
to have a single antibody with variable regions (or complementary determining
regions) that bind to two different antigens, with each tip of the Y shape
being
specific to one of the antigens; these are referred to as bi-specific
antibodies.
[66] A humanized anti-AGE antibody according to the present invention may
have
the human constant region sequence of amino acids shown in SEQ ID NO: 22. The
heavy chain complementarity determining regions of the humanized anti-AGE
antibody may have one or more of the protein sequences shown in SEQ ID NO: 23
(CDR1H), SEQ ID NO: 24 (CDR2H) and SEQ ID NO: 25 (CDR3H). The light chain
complementarity determining regions of the humanized anti-AGE antibody may
have
one or more of the protein sequences shown in SEQ ID NO: 26 (CDR1L), SEQ ID
NO: 27 (CDR2L) and SEQ ID NO: 28 (CDR3L).
[67] The heavy chain of a humanized anti-AGE antibody may have or may
include
the protein sequence of SEQ ID NO: 1. The variable domain of the heavy chain
may
have or may include the protein sequence of SEQ ID NO: 2. The complementarity
determining regions of the variable domain of the heavy chain (SEQ ID NO: 2)
are
shown in SEQ ID NO: 41, SEQ ID NO: 42 and SEQ ID NO: 43. The kappa light
chain of a humanized anti-AGE antibody may have or may include the protein
sequence of SEQ ID NO: 3. The variable domain of the kappa light chain may
have
or may include the protein sequence of SEQ ID NO: 4. Optionally, the arginine
(Arg
- 22 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
or R) residue at position 128 of SEQ ID NO: 4 may be omitted. The
complementarity
determining regions of the variable domain of the light chain (SEQ ID NO: 4)
are
shown in SEQ ID NO: 44, SEQ ID NO: 45 and SEQ ID NO: 46. The variable regions
may be codon-optimized, synthesized and cloned into expression vectors
containing
human immunoglobulin G1 constant regions. In addition, the variable regions
may
be used in the preparation of non-human anti-AGE antibodies.
[68] The antibody heavy chain may be encoded by the DNA sequence of SEQ ID
NO: 12, a murine anti-AGE immunoglobulin G2b heavy chain. The protein sequence
of the murine anti-AGE immunoglobulin G2b heavy chain encoded by SEQ ID NO:
12 is shown in SEQ ID NO: 16. The variable region of the murine antibody is
shown
in SEQ ID NO: 20, which corresponds to positions 25-142 of SEQ ID NO: 16. The
antibody heavy chain may alternatively be encoded by the DNA sequence of SEQ
ID
NO: 13, a chimeric anti-AGE human immunoglobulin G1 heavy chain. The protein
sequence of the chimeric anti-AGE human immunoglobulin G1 heavy chain encoded
by SEQ ID NO: 13 is shown in SEQ ID NO: 17. The chimeric anti-AGE human
immunoglobulin includes the murine variable region of SEQ ID NO: 20 in
positions
25-142. The antibody light chain may be encoded by the DNA sequence of SEQ ID
NO: 14, a murine anti-AGE kappa light chain. The protein sequence of the
murine
anti-AGE kappa light chain encoded by SEQ ID NO: 14 is shown in SEQ ID NO: 18.
The variable region of the murine antibody is shown in SEQ ID NO: 21, which
corresponds to positions 21-132 of SEQ ID NO: 18. The antibody light chain may
alternatively be encoded by the DNA sequence of SEQ ID NO: 15, a chimeric anti-
AGE human kappa light chain. The protein sequence of the chimeric anti-AGE
human kappa light chain encoded by SEQ ID NO: 15 is shown in SEQ ID NO: 19.
The chimeric anti-AGE human immunoglobulin includes the murine variable region
of
SEQ ID NO: 21 in positions 21-132.
[69] A humanized anti-AGE antibody according to the present invention may
have
or may include one or more humanized heavy chains or humanized light chains. A
humanized heavy chain may be encoded by the DNA sequence of SEQ ID NO: 30,
32 or 34. The protein sequences of the humanized heavy chains encoded by SEQ
ID NOs: 30, 32 and 34 are shown in SEQ ID NOs: 29, 31 and 33, respectively. A
- 23 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
humanized light chain may be encoded by the DNA sequence of SEQ ID NO: 36, 38
or 40. The protein sequences of the humanized light chains encoded by SEQ ID
NOs: 36, 38 and 40 are shown in SEQ ID NOs: 35, 37 and 39, respectively.
Preferably, the humanized anti-AGE antibody maximizes the amount of human
sequence while retaining the original antibody specificity. A complete
humanized
antibody may be constructed that contains a heavy chain having a protein
sequence
chosen from SEQ ID NOs: 29, 31 and 33 and a light chain having a protein
sequence chosen from SEQ ID NOs: 35, 37 and 39.
[70] Particularly preferred anti-AGE antibodies may be obtained by
humanizing
murine monoclonal anti-AGE antibodies. Murine monoclonal anti-AGE antibodies
have the heavy chain protein sequence shown in SEQ ID NO: 47 (the protein
sequence of the variable domain is shown in SEQ ID NO: 52) and the light chain
protein sequence shown in SEQ ID NO: 57 (the protein sequence of the variable
domain is shown in SEQ ID NO: 62). A preferred humanized heavy chain may have
the protein sequence shown in SEQ ID NO: 48, SEQ ID NO: 49, SEQ ID NO: 50 or
SEQ ID NO: 51 (the protein sequences of the variable domains of the humanized
heavy chains are shown in SEQ ID NO: 53, SEQ ID NO: 54, SEQ ID NO: 55 and
SEQ ID NO: 56, respectively). A preferred humanized light chain may have the
protein sequence shown in SEQ ID NO: 58, SEQ ID NO: 59, SEQ ID NO: 60 or SEQ
ID NO: 61 (the protein sequences of the variable domains of the humanized
light
chains are shown in SEQ ID NO: 63, SEQ ID NO: 64, SEQ ID NO: 65 and SEQ ID
NO: 66, respectively). Preferably, a humanized anti-AGE monoclonal antibody is
composed a heavy chain having a protein sequence selected from the group
consisting of SEQ ID NO: 48, SEQ ID NO: 49, SEQ ID NO: 50 and SEQ ID NO: 51
and a light chain having a protein sequence selected from the group consisting
of
SEQ ID NO: 58, SEQ ID NO: 59, SEQ ID NO: 60 and SEQ ID NO: 61. Humanized
monoclonal anti-AGE antibodies composed of these protein sequences may have
better binding and/or improved activation of the immune system, resulting in
greater
efficacy.
iti l The protein sequence of an antibody from a non-human species may
be
modified to include the variable domain of the heavy chain having the sequence
-24 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
shown in SEQ ID NO: 2 or the kappa light chain having the sequence shown in
SEQ
ID NO: 4. The non-human species may be a companion animal, such as the
domestic cat or domestic dog, or livestock, such as cattle, the horse or the
camel.
Preferably, the non-human species is not the mouse. The heavy chain of the
horse
(Equus cabal/us) antibody immunoglobulin gamma 4 may have or may include the
protein sequence of SEQ ID NO: 5 (EMBUGenBank accession number AY445518).
The heavy chain of the horse (Equus cabal/us) antibody immunoglobulin delta
may
have or may include the protein sequence of SEQ ID NO: 6 (EMBUGenBank
accession number AY631942). The heavy chain of the dog (Canis familiaris)
antibody immunoglobulin A may have or may include the protein sequence of SEQ
ID NO: 7 (GenBank accession number L36871). The heavy chain of the dog (Canis
familiaris) antibody immunoglobulin E may have or may include the protein
sequence
of SEQ ID NO: 8 (GenBank accession number L36872). The heavy chain of the cat
(Fells catus) antibody immunoglobulin G2 may have or may include the protein
sequence of SEQ ID NO: 9 (DDBJ/EMBUGenBank accession number KF811175).
[72] Animals of the camelid family, such as camels (Came/us dromedarius
and
Came/us bactrianus), llamas (Lama glama, Lama pacos and Lama vicugna), alpacas
(Vicugna pacos) and guanacos (Lama guanicoe), have a unique antibody that is
not
found in other mammals. In addition to conventional immunoglobulin G
antibodies
composed of heavy and light chain tetramers, camelids also have heavy chain
immunoglobulin G antibodies that do not contain light chains and exist as
heavy
chain dimers. These antibodies are known as heavy chain antibodies, HCAbs,
single-domain antibodies or sdAbs, and the variable domain of a camelid heavy
chain antibody is known as the VHH. The camelid heavy chain antibodies lack
the
heavy chain CHI domain and have a hinge region that is not found in other
species.
The variable region of the Arabian camel (Came/us dromedarius) single-domain
antibody may have or may include the protein sequence of SEQ ID NO: 10
(GenBank accession number AJ245148). The variable region of the heavy chain of
the Arabian camel (Came/us dromedarius) tetrameric immunoglobulin may have or
may include the protein sequence of SEQ ID NO: 11 (GenBank accession number
AJ245184).
- 25 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[73] In addition to camelids, heavy chain antibodies are also found in
cartilaginous
fishes, such as sharks, skates and rays. This type of antibody is known as an
immunoglobulin new antigen receptor or IgNAR, and the variable domain of an
IgNAR is known as the VNAR. The IgNAR exists as two identical heavy chain
dimers composed of one variable domain and five constant domains each. Like
camelids, there is no light chain.
[74] The protein sequences of additional non-human species may be readily
found
in online databases, such as the International ImMunoGeneTics Information
System
(www.imgt.org), the European Bioinformatics Institute (www.ebi.ac.uk), the DNA
Databank of Japan (ddbj.nig.ac.jp/arsa) or the National Center for
Biotechnology
Information (www.ncbi.nlm.nih.gov).
[75] An anti-AGE antibody or a variant thereof may include a heavy chain
having
at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 100% sequence
identity to the amino acid sequence of SEQ ID NO: 1, SEQ ID NO: 16, SEQ ID NO:
17, SEQ ID NO: 29, SEQ ID NO: 31, SEQ ID NO: 33, SEQ ID NO: 47, SEQ ID NO:
48, SEQ ID NO: 49, SEQ ID NO: 50 or SEQ ID NO: 51, including post-
translational
modifications thereof. A heavy chain having at least 90%, 91%, 92%, 93%, 94%,
95%, 96%, 97%, 98%, or 99% sequence identity may contain substitutions (e.g.,
conservative substitutions), insertions, or deletions relative to the
reference
sequence, but an anti-AGE antibody including that sequence retains the ability
to
bind to AGE.
[76] An anti-AGE antibody or a variant thereof may include a heavy chain
variable
region having at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or
100% sequence identity to the amino acid sequence of SEQ ID NO: 2, SEQ ID NO:
20, SEQ ID NO: 23, SEQ ID NO: 24, SEQ ID NO: 25, SEQ ID NO: 41, SEQ ID NO:
42, SEQ ID NO: 43, SEQ ID NO: 52, SEQ ID NO: 53, SEQ ID NO: 54, SEQ ID NO:
55, or SEQ ID NO: 56, including post-translational modifications thereof. A
variable
region having at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
sequence identity may contain substitutions (e.g., conservative
substitutions),
insertions, or deletions relative to the reference sequence, but an anti-AGE
antibody
- 26 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
including that sequence retains the ability to bind to AGE. The substitutions,
insertions, or deletions may occur in regions outside the variable region.
[77] An anti-AGE antibody or a variant thereof may include a light chain
having at
least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 100% sequence
identity to the amino acid sequence of SEQ ID NO: 3, SEQ ID NO: 18, SEQ ID NO:
19, SEQ ID NO: 35, SEQ ID NO: 37, SEQ ID NO: 39, SEQ ID NO: 57, SEQ ID NO:
58, SEQ ID NO: 59, SEQ ID NO: 60 or SEQ ID NO: 61, including post-
translational
modifications thereof. A light chain having at least 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, 98%, or 99% sequence identity may contain substitutions (e.g.,
conservative substitutions), insertions, or deletions relative to the
reference
sequence, but an anti-AGE antibody including that sequence retains the ability
to
bind to AGE. The substitutions, insertions, or deletions may occur in regions
outside
the variable region.
[78] An anti-AGE antibody or a variant thereof may include a light chain
variable
region having at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or
100% sequence identity to the amino acid sequence of SEQ ID NO: 4, SEQ ID NO:
21, SEQ ID NO: 26, SEQ ID NO: 27, SEQ ID NO: 28, SEQ ID NO: 44, SEQ ID NO:
45, SEQ ID NO: 46, SEQ ID NO: 62, SEQ ID NO: 63, SEQ ID NO: 64, SEQ ID NO:
65 or SEQ ID NO: 66, including post-translational modifications thereof. A
variable
region having at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
sequence identity may contain substitutions (e.g., conservative
substitutions),
insertions, or deletions relative to the reference sequence, but an anti-AGE
antibody
including that sequence retains the ability to bind to AGE. The substitutions,
insertions, or deletions may occur in regions outside the variable region.
[79] Alternatively, the antibody may have the connplementarity determining
regions
of commercially available mouse anti-glycation end-product antibody raised
against
carboxymethyl lysine conjugated with keyhole limpet hemocyanin (CML-KLH), the
carboxymethyl lysine MAb (Clone 318003) available from R&D Systems, Inc.
(Minneapolis, MN; catalog no. MAB3247).
- 27 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[80] The antibody may have or may include constant regions which permit
destruction of targeted cells by a subject's immune system.
[81] Mixtures of antibodies that bind to more than one type AGE of AGE-
modified
proteins may also be used.
[82] Bi-specific antibodies, which are anti-AGE antibodies directed to two
different
epitopes, may also be used. Such antibodies will have a variable region (or
complementary determining region) from those of one anti-AGE antibody, and a
variable region (or complementary determining region) from a different
antibody.
[83] Antibody fragments may be used in place of whole antibodies. For
example,
immunoglobulin G may be broken down into smaller fragments by digestion with
enzymes. Papain digestion cleaves the N-terminal side of inter-heavy chain
disulfide
bridges to produce Fab fragments. Fab fragments include the light chain and
one of
the two N-terminal domains of the heavy chain (also known as the Fd fragment).
Pepsin digestion cleaves the C-terminal side of the inter-heavy chain
disulfide
bridges to produce F(ate)2 fragments. F(ab)2 fragments include both light
chains
and the two N-terminal domains linked by disulfide bridges. Pepsin digestion
may
also form the Fv (fragment variable) and Fc (fragment crystallizable)
fragments. The
Fv fragment contains the two N-terminal variable domains. The Fc fragment
contains the domains which interact with immunoglobulin receptors on cells and
with
the initial elements of the complement cascade. Pepsin may also cleave
immunoglobulin G before the third constant domain of the heavy chain (CH3) to
produce a large fragment F(abc) and a small fragment pFc'. Antibody fragments
may alternatively be produced recombinantly. Preferably, such antibody
fragments
are conjugated to an agent that causes the destruction of AGE-modified cells.
[84] If additional antibodies are desired, they can be produced using well-
known
methods. For example, polyclonal antibodies (pAbs) can be raised in a
mammalian
host by one or more injections of an immunogen, and if desired, an adjuvant.
Typically, the immunogen (and adjuvant) is injected in a mammal by a
subcutaneous
or intraperitoneal injection. The immunogen may be an AGE-modified protein of
a
- 28 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
cell, such as AGE-antithrombin III, AGE-calmodulin, AGE-insulin, AGE-
ceruloplasmin, AGE-collagen, AGE-cathepsin B, AGE-albumin such as AGE-bovine
serum albumin (AGE-BSA), AGE-human serum albumin and ovalbunnin, AGE-
crystallin, AGE-plasminogen activator, AGE-endothelial plasma membrane
protein,
AGE-aldehyde red uctase, AGE-transferrin, AGE-fibrin, AGE-copper/zinc SOD, AGE-
apo B, AGE-fibronectin, AGE-pancreatic ribose, AGE-apo A-I and II, AGE-
hemoglobin, AGE-Na+/K+-ATPase, AGE-plasminogen, AGE-myelin, AGE-lysozyme,
AGE-immunoglobulin, AGE-red cell Glu transport protein, AGE-6-N-acetyl
hexokinase, AGE-apo E, AGE-red cell membrane protein, AGE-aldose reductase,
AGE-ferritin, AGE-red cell spectrin, AGE-alcohol dehydrogenase, AGE-
haptoglobin,
AGE-tubulin, AGE-thyroid hormone, AGE-fibrinogen, AGE-62-microglobulin, AGE-
sorbitol dehydrogenase, AGE-m-antitrypsin, AGE-carbonate dehydratase, AGE-
RNAse, AGE-hexokinase, AGE-apo C-I, AGE-hemoglobin such as AGE-human
hemoglobin, AGE-low density lipoprotein (AGE-LDL) and AGE-collagen IV. AGE-
modified cells, such as AGE-modified erythrocytes, whole, lysed, or partially
digested, may also be used as AGE antigens. Examples of adjuvants include
Freund 's complete, monophosphoryl Lipid A synthetic-trehalose
dicorynomycolate,
aluminum hydroxide (alum), heat shock proteins HSP 70 or HSP96, squalene
emulsion containing monophosphoryl lipid A, a2-macroglobulin and surface
active
substances, including oil emulsions, pleuronic polyols, polyanions and
dinitrophenol.
To improve the immune response, an innmunogen may be conjugated to a
polypeptide that is immunogenic in the host, such as keyhole limpet hemocyanin
(KLH), serum albumin, bovine thyroglobulin, cholera toxin, labile enterotoxin,
silica
particles or soybean trypsin inhibitor. A preferred immunogen conjugate is AGE-
KLH. Alternatively, pAbs may be made in chickens, producing IgY molecules.
[85] Monoclonal antibodies (mAbs) may also be made by immunizing a host
or
lymphocytes from a host, harvesting the mAb-secreting (or potentially
secreting)
lymphocytes, fusing those lymphocytes to immortalized cells (for example,
myeloma
cells), and selecting those cells that secrete the desired mAb. Other
techniques may
be used, such as the EBV-hybridoma technique. Non-human antibodies may be
made less immunogenic to humans by engineering the antibodies to contain a
- 29 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
combination of non-human and human antibody components. A chimeric antibody
may be produced by combining the variable region of a non-human antibody with
a
human constant region. A humanized antibody may be produced by replacing the
complementarity determining regions (CDRs) of a human antibody with those of a
non-human antibody. Similarly, antibodies may be made less immunogenic to
other
species by being substantially "ized" to a given animal, such as cat, dog,
horse,
camel or alpaca, cattle, sheep, pig, or goat, at the amino acid level. If
desired, the
mAbs may be purified from the culture medium or ascites fluid by conventional
procedures, such as protein A-sepharose, hydroxyapatite chromatography, gel
electrophoresis, dialysis, ammonium sulfate precipitation or affinity
chromatography.
Additionally, human monoclonal antibodies can be generated by immunization of
transgenic mice containing a third copy IgG human trans-loci and silenced
endogenous mouse Ig loci or using human-transgenic mice. Production of
humanized monoclonal antibodies and fragments thereof can also be generated
through phage display technologies.
[86] A "pharmaceutically acceptable carrier" includes any and all solvents,
dispersion media, coatings, antibacterial and antifungal agents, isotonic and
absorption delaying agents, and the like, compatible with pharmaceutical
administration. Preferred examples of such carriers or diluents include water,
saline,
Ringer's solutions and dextrose solution. Supplementary active compounds can
also
be incorporated into the compositions. Solutions and suspensions used for
parenteral administration can include a sterile diluent, such as water for
injection,
saline solution, polyethylene glycols, glycerin, propylene glycol or other
synthetic
solvents; antibacterial agents such as benzyl alcohol or methyl parabens;
antioxidants such as ascorbic acid or sodium bisulfite; buffers such as
acetates,
citrates or phosphates, and agents for the adjustment of tonicity such as
sodium
chloride or dextrose. The pH can be adjusted with acids or bases, such as
hydrochloric acid or sodium hydroxide. The parenteral preparation can be
enclosed
in ampoules, disposable syringes or multiple dose vials made of glass or
plastic.
[87] The antibodies may be administered systemically, such as by
intravenous
injection or infusion. Alternatively, the antibodies may be administered
locally with a
-30-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
pharmaceutically acceptable carrier suitable for the administration site, such
as by
percutaneous injection into an affected organ, topical administration at the
site of
dermal fibrosis or administration via nebulizer for pulmonary fibrosis.
Pharmaceutical compositions suitable for injection include sterile aqueous
solutions
or dispersions for the extemporaneous preparation of sterile injectable
solutions or
dispersion. Various excipients may be included in pharmaceutical compositions
of
antibodies suitable for injection. Suitable carriers include physiological
saline,
bacteriostatic water, CREMOPHOR EL S (BASF; Parsippany, NJ) or phosphate
buffered saline (PBS). In all cases, the composition must be sterile and
should be
fluid so as to be administered using a syringe. Such compositions should be
stable
during manufacture and storage and must be preserved against contamination
from
microorganisms such as bacteria and fungi. Various antibacterial and anti-
fungal
agents, for example, parabens, chlorobutanol, phenol, ascorbic acid, and
thimerosal,
can contain microorganism contamination. Isotonic agents such as sugars,
polyalcohols, such as mannitol, sorbitol, and sodium chloride can be included
in the
composition. Compositions that can delay absorption include agents such as
aluminum monostearate and gelatin. Sterile injectable solutions can be
prepared by
incorporating antibodies, and optionally other therapeutic components, in the
required amount in an appropriate solvent with one or a combination of
ingredients
as required, followed by sterilization. Methods of preparation of sterile
solids for the
preparation of sterile injectable solutions include vacuum drying and freeze-
drying to
yield a solid.
[88] For administration by inhalation, the antibodies may be delivered as
an
aerosol spray from a nebulizer or a pressurized container that contains a
suitable
propellant, for example, a gas such as carbon dioxide. Antibodies may also be
delivered via inhalation as a dry powder, for example using the ISPERSETM
inhaled
drug delivery platform (PULMATR1X, Lexington, Mass.). The use of anti-AGE
antibodies which are chicken antibodies (IgY) may be non-immunogenic in a
variety
of animals, including humans, when administered by inhalation.
[89] An appropriate dosage level of each type of antibody will generally be
about
0.01 to 500 mg per kg patient body weight. Preferably, the dosage level will
be
- 31 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
about 0.1 to about 250 mg/kg; more preferably about 0.5 to about 100 ring/kg.
A
suitable dosage level may be about 0.01 to 250 mg/kg, about 0.05 to 100 mg/kg,
or
about 0.1 to 50 mg/kg. Within this range the dosage may be 0.05 to 0.5, 0.5 to
5 or
to 50 mg/kg. Although each type of antibody may be administered on a regimen
of
1 to 4 times per day, such as once or twice per day, antibodies typically have
a long
half-life in vivo. Accordingly, each type of antibody may be administered once
a day,
once a week, once every two or three weeks, once a month, or once every 60 to
90
days.
[90] A subject that receives administration of an anti-AGE antibody may be
tested
to determine if the administration has been effective to treat fibrotic
diseases. A
subject may be considered to have received an effective antibody treatment if
he or
she demonstrates an improvement in symptoms between subsequent
measurements or over time. The efficacy of treatment may be determined with a
diagnostic test that is suitable for a given fibrotic disease. For example,
liver fibrosis
may be monitored with a liver biopsy and pulmonary fibrosis may be monitored
with
high-resolution computed tomography, pulmonary function tests, bronchoscopy or
bronchoalveolar lavage. Alternatively, the concentration and/or number of
senescent
cells may be measured over time. Administration of antibody and subsequent
testing may be repeated until the desired therapeutic result is achieved.
[91] Unit dosage forms can be created to facilitate administration and
dosage
uniformity. Unit dosage form refers to physically discrete units suited as
single
dosages for the subject to be treated, containing a therapeutically effective
quantity
of one or more types of antibodies in association with the required
pharmaceutical
carrier. Preferably, the unit dosage form is in a sealed container and is
sterile.
[92] Vaccines against AGE-modified proteins or peptides contain an AGE
antigen,
an adjuvant, optional preservatives and optional excipients. Examples of AGE
antigens include AGE-modified proteins or peptides such as AGE-antithrombin
Ill,
AGE-calmodulin, AGE-insulin, AGE-ceruloplasmin, AGE-collagen, AGE-cathepsin B,
AGE-albumin such as AGE-bovine serum albumin (AGE-BSA), AGE-human serum
albumin and ovalbumin, AGE-crystallin, AGE-plasminogen activator, AGE-
- 32 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
endothelial plasma membrane protein, AGE-aldehyde reductase, AGE-transferrin,
AGE-fibrin, AGE-copper/zinc SOD, AGE-apo B, AGE-fibronectin, AGE-pancreatic
ribose, AGE-apo A-I and II, AGE-hemoglobin, AGE-Na+/K+-ATPase, AGE-
plasminogen, AGE-myelin, AGE-lysozyme, AGE-immunoglobulin, AGE-red cell Glu
transport protein, AGE-13-N-acetyl hexokinase, AGE-apo E, AGE-red cell
membrane
protein, AGE-aldose reductase, AGE-ferritin, AGE-red cell spectrin, AGE-
alcohol
dehydrogenase, AGE-haptoglobin, AGE-tubulin, AGE-thyroid hormone, AGE-
fibrinogen, AGE-132-microglobulin, AGE-sorbitol dehydrogenase, AGE-ai-
antitrypsin,
AGE-carbonate dehydratase, AGE-RNAse, AGE-hexokinase, AGE-apo C-I, AGE-
hemoglobin such as AGE-human hemoglobin, AGE-low density lipoprotein (AGE-
LDL) and AGE-collagen IV. AGE-modified cells, such as AGE-modified
erythrocytes, whole, lysed, or partially digested, may also be used as AGE
antigens.
Suitable AGE antigens also include proteins or peptides that exhibit AGE
modifications (also referred to as AGE epitopes or AGE moieties) such as
carboxymethyllysine (CML), carboxyethyllysine (CEL), pentosidine, pyrraline,
FFI,
AFGP and ALI. The AGE antigen may be an AGE-protein conjugate, such as AGE
conjugated to keyhole limpet hemocyanin (AGE-KLH). Further details of some of
these AGE-modified proteins or peptides and their preparation are described in
Bucala.
[93] Particularly preferred AGE antigens include proteins or peptides
that exhibit a
carboxymethyllysine or carboxyethyllysine AGE modification.
Carboxymethyllysine
(also known as N(epsilon)-(carboxymethyl)lysine, N(6)-carboxymethyllysine, or
2-
Amino-6-(carboxymethylamino)hexanoic acid) and carboxyethyllysine (also known
as N-epsilon-(carboxyethyl)lysine) are found on proteins or peptides and
lipids as a
result of oxidative stress and chemical glycation, and have been correlated
with
juvenile genetic disorders. CML- and CEL-modified proteins or peptides are
recognized by the receptor RAGE which is expressed on a variety of cells. CML
and
CEL have been well-studied and CML- and CEL-related products are commercially
available. For example, Cell Biolabs, Inc. sells CML-BSA antigens, CML
polyclonal
antibodies, CML immunoblot kits, and CML competitive ELISA kits
(www.cellbiolabs.com/cml-assays) as well as CEL-BSA antigens and CEL
- 33 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
competitive ELISA kits (www.cellbiolabs.connicel-n-epsilon-carboxyethyl-lysine-
assays-and-reagents).
[94] AGE antigens may be conjugated to carrier proteins to enhance antibody
production in a subject. Antigens that are not sufficiently immunogenic alone
may
require a suitable carrier protein to stimulate a response from the immune
system.
Examples of suitable carrier proteins include keyhole limpet hemocyanin (KLH),
serum albumin, bovine thyroglobulin, cholera toxin, labile enterotoxin, silica
particles
and soybean trypsin inhibitor. Preferably, the carrier protein is KLH (AGE-
KLH).
KLH has been extensively studied and has been identified as an effective
carrier
protein in experimental cancer vaccines. Preferred AGE antigen-carrier protein
conjugates include CML-KLH and CEL-KLH.
[95] The administration of an AGE antigen allows the immune system to
develop
immunity to the antigen. Immunity is a long-term immune response, either
cellular or
humoral. A cellular immune response is activated when an antigen is presented,
preferably with a co-stimulator to a T-cell which causes it to differentiate
and produce
cytokines. The cells involved in the generation of the cellular immune
response are
two classes of T-helper (Th) cells, Th1 and Th2. Th1 cells stimulate B cells
to
produce predominantly antibodies of the IgG2A isotype, which activates the
complement cascade and binds the Fc receptors of macrophages, while Th2 cells
stimulate B cells to produce IgG1 isotype antibodies in mice, IgG4 isotype
antibodies
in humans, and IgE isotype antibodies. The human body also contains
"professional" antigen-presenting cells such as dendritic cells, macrophages,
and B
cells.
[96] A humoral immune response is triggered when a B cell selectively binds
to an
antigen and begins to proliferate, leading to the production of a clonal
population of
cells that produce antibodies that specifically recognize that antigen and
which may
differentiate into antibody-secreting cells, referred to as plasma-cells or
memory-B
cells. Antibodies are molecules produced by B-cells that bind a specific
antigen.
The antigen-antibody complex triggers several responses, either cell-mediated,
for
example by natural killers (NK) or macrophages, or serum-mediated, for example
by
- 34-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
activating the complement system, a complex of several serum proteins that act
sequentially in a cascade that result in the lysis of the target cell.
[97] Immunological adjuvants (also referred to simply as "adjuvants") are
the
component(s) of a vaccine which augment the immune response to the
immunogenic agent. Adjuvants function by attracting macrophages to the
immunogenic agent and then presenting the agent to the regional lymph nodes to
initiate an effective antigenic response. Adjuvants may also act as carriers
themselves for the immunogenic agent. Adjuvants may induce an inflammatory
response, which may play an important role in initiating the immune response.
[98] Adjuvants include mineral compounds such as aluminum salts, oil
emulsions,
bacterial products, liposomes, immunostimulating complexes and squalene.
Aluminum compounds are the most widely used adjuvants in human and veterinary
vaccines. These aluminum compounds include aluminum salts such as aluminum
phosphate (AIP04) and aluminum hydroxide (Al(OH)3) compounds, typically in the
form of gels, and are generically referred to in the field of vaccine
immunological
adjuvants as "alum." Aluminum hydroxide is a poorly crystalline aluminum
oxyhydroxide having the structure of the mineral boehmite. Aluminum phosphate
is
an amorphous aluminum hydroxyphosphate. Negatively charged species (for
example, negatively charged antigens) can absorb onto aluminum hydroxide gels
at
neutral pH, whereas positively charged species (for example, positively
charged
antigens) can absorb onto aluminum phosphate gels at neutral pH. It is
believed that
these aluminum compounds provide a depot of antigen at the site of
administration,
thereby providing a gradual and continuous release of antigen to stimulate
antibody
production. Aluminum compounds tend to more effectively stimulate a cellular
response mediated by Th2, rather than Thl cells.
[99] Emulsion adjuvants include water-in-oil emulsions (for example,
Freund's
adjuvants, such as killed mycobacteria in oil emulsion) and oil-in-water
emulsions
(for example, MF-59). Emulsion adjuvants include an immunogenic component, for
example squalene (MF-59) or mannide oleate (Incomplete Freund's Adjuvants),
- 35 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
which can induce an elevated humoral response, increased T cell proliferation,
cytotoxic lymphocytes and cell-mediated immunity.
[100] Liposomal or vesicular adjuvants (including paucilannellar lipid
vesicles) have
lipophilic bilayer domains and an aqueous milieu which can be used to
encapsulate
and transport a variety of materials, for example an antigen. Paucilamellar
vesicles
(for example, those described in U.S. Pat. No. 6,387,373) can be prepared by
mixing, under high pressure or shear conditions, a lipid phase comprising a
non-
phospholipid material (for example, an amphiphile surfactant; see U.S. Pat.
Nos.
4,217,344; 4,917,951; and 4,911,928), optionally a sterol, and any water-
immiscible
oily material to be encapsulated in the vesicles (for example, an oil such as
squalene
oil and an oil-soluble or oil-suspended antigen); and an aqueous phase such as
water, saline, buffer or any other aqueous solution used to hydrate the
lipids.
Liposomal or vesicular adjuvants are believed to promote contact of the
antigen with
immune cells, for example by fusion of the vesicle to the immune cell
membrane,
and preferentially stimulate the Th1 sub-population of T-helper cells.
[101] Other types of adjuvants include Mycobacterium bovis bacillus
Calmette¨
Guerin (BCG), quill-saponin and unmethylated CpG dinucleotides (CpG motifs).
Additional adjuvants are described in U.S. Patent Application Publication Pub.
No.
US 2010/0226932 (September 9, 2010) and Jiang, Z-H. etal. "Synthetic vaccines:
the role of adjuvants in immune targeting", Current Medicinal Chemistry, Vol.
10(15),
pp. 1423-39 (2003). Preferable adjuvants include Freund's complete adjuvant
and
Freund's incomplete adjuvant.
[102] The vaccine may optionally include one or more preservatives, such as
antioxidants, antibacterial and antimicrobial agents, as well as combinations
thereof.
Examples include benzethonium chloride, ethylenediamine-tetraacetic acid
sodium
(EDTA), thimerosal, phenol, 2-phenoxyethanol, formaldehyde and formalin;
antibacterial agents such as amphotericin B, chlortetracycline, gentamicin,
neomycin, polymyxin B and streptomycin; antimicrobial surfactants such as
polyoxyethylene-9, 10-nonyl phenol (Triton N-101, octoxyno1-9), sodium
deoxycholate and polyoxyethylated octyl phenol (Triton X-100). The production
and
- 36 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
packaging of the vaccine may eliminate the need for a preservative. For
example, a
vaccine that has been sterilized and stored in a sealed container may not
require a
preservative.
[103] Other components of vaccines include pharmaceutically acceptable
excipients, such as stabilizers, thickening agents, toxin detoxifiers,
diluents, pH
adjusters, tonicity adjustors, surfactants, antifoaming agents, protein
stabilizers, dyes
and solvents. Examples of such excipients include hydrochloric acid, phosphate
buffers, sodium acetate, sodium bicarbonate, sodium borate, sodium citrate,
sodium
hydroxide, potassium chloride, potassium chloride, sodium chloride,
polydimethylsilozone, brilliant green, phenol red (phenolsulfon-phthalein),
glycine,
glycerin, sorbitol, histidine, monosodium glutamate, potassium glutamate,
sucrose,
urea, lactose, gelatin, sorbitol, polysorbate 20, polysorbate 80 and
glutaraldehyde. A
variety of these components of vaccines, as well as adjuvants, are described
in
www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/B/excipient-table-
2.pdf
and Vogel, F. R. et al., "A compendium of vaccine adjuvants and excipients",
Pharmaceutical Biotechnology, Vol. 6, pp. 141-228 (1995).
[104] The vaccine may contain from 1 pg to 100 mg of at least one AGE
antigen,
including 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 400, 800 or 1000 pg,
or 2, 3, 4,
5, 6, 7, 8, 9, 10, 20, 30, 40, 50, 60, 70, 80 or 90 mg. The amount used for a
single
injection corresponds to a unit dosage.
[105] The vaccine may be provided in unit dosage form or in multidosage
form,
such as 2-100 or 2-10 doses. The unit dosages may be provided in a vial with a
septum, or in a syringe with or without a needle. The vaccine may be
administered
intravenously, subdermally or intraperitoneally. Preferably, the vaccine is
sterile.
[106] The vaccine may be administered one or more times, such as Ito 10
times,
including 2, 3, 4, 5, 6, 7, 8 or 9 times, and may be administered over a
period of time
ranging from 1 week to 1 year, 2-10 weeks or 2-10 months. Furthermore, booster
vaccinations may be desirable, over the course of 1 year to 20 years,
including 2, 5,
and 15 years.
- 37 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[107] A subject that receives a vaccine for AGE-modified proteins or
peptides of a
cell may be tested to determine if he or she has developed an immunity to the
AGE-
modified proteins or peptides. Suitable tests may include blood tests for
detecting
the presence of an antibody, such as immunoassays or antibody titers. An
immunity
to AGE-modified proteins or peptides may also be determined by monitoring the
concentration and/or number of senescent cells over time. In addition to
testing for
the development of an immunity to AGE-modified proteins or peptides, a subject
may
also be tested to determine if the vaccination has been effective to treat
fibrotic
diseases. A subject may be considered to have received an effective
vaccination if
he or she demonstrates an improvement in symptoms between subsequent
measurements or over time, or by measuring the concentration and/or number of
senescent cells. Vaccination and subsequent testing may be repeated until the
desired therapeutic result is achieved.
[108] The vaccination process may be designed to provide immunity against
multiple AGE moieties. A single AGE antigen may induce the production of AGE
antibodies which are capable of binding to multiple AGE moieties.
Alternatively, the
vaccine may contain multiple AGE antigens. In addition, a subject may receive
multiple vaccines, where each vaccine contains a different AGE antigen.
[109] Any mammal that could develop fibrotic diseases may be treated by the
methods herein described. Humans are a preferred mammal for treatment. Other
mammals that may be treated include mice, rats, goats, sheep, pigs, cows,
horses
and companion animals, such as dogs or cats. Alternatively, any of the mammals
or
subjects identified above may be excluded from the patient population in need
of
treatment for fibrotic diseases.
[110] A subject may be identified as in need of treatment based on a
diagnosis with
at least one fibrotic disease, or with a disease that is known to contribute
to fibrosis.
Fibrotic diseases include interstitial lung disease, pulmonary fibrosis, liver
fibrosis,
cirrhosis, urinary fibrosis, kidney fibrosis, kidney disease, chronic kidney
disease
(CKD), nephrosclerosis, nephrosis, glomerulosclerosis, bladder fibrosis,
urethral
stricture, cardiovascular disease, macular degeneration, vitreal retinopathy,
- 38 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
scleroderma (systemic and local), restenosis, myelofibrosis (bone marrow
cancer),
keloid scarring, hypertrophic scarring, nephrogenic fibrosing dermopathy,
mixed
connective tissue disease, scleromyxedema, scleredema and eosinophilic
fasciitis.
Fibrotic diseases include desnnoplasia, as fibrotic tissue in the surrounding
stroma
plays a very important role in the progression of cancer. Diagnosis may
involve any
suitable diagnostic test or procedure for a given disease or disorder. A
subject may
also be identified as in need of treatment after experiencing physical trauma
that is
known to result in fibrosis, such as surgical complications, administration of
chemotherapeutic drugs, exposure to radiation, burns or physical injury.
[111] Subjects may also be identified as in need of treatment based on
detection of
advanced glycation end products in a sample obtained from the subject.
Suitable
samples include blood, skin, serum, saliva and urine. The diagnostic use of
anti-
AGE antibodies is discussed in more detail in International Publication No. WO
2018/204679.
[112] The Present Application includes 66 nucleotide and amino acid
sequences in
the Sequence Listing filed herewith. Variants of the nucleotide and amino acid
sequences are possible. Known variants include substitutions, deletions and
additions to the sequences shown in SEQ ID NO: 4, 16 and 20. In SEQ ID NO: 4,
the arginine (Arg or R) residue at position 128 may optionally be omitted. In
SEQ ID
NO: 16, the alanine residue at position 123 may optionally be replaced with a
serine
residue, and/or the tyrosine residue at position 124 may optionally be
replaced with a
phenylalanine residue. SEQ ID NO: 20 may optionally include the same
substitutions as SEQ ID NO: 16 at positions 123 and 124. In addition, SEQ ID
NO:
20 may optionally contain one additional lysine residue after the terminal
valine
residue.
[113] EXAMPLES
[114] Example 1: in vivo study of the administration of anti-glycation end-
product
antibody
- 39 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[115] To examine the effects of an anti-glycation end-product antibody,
the antibody
was administered to the aged CD1(ICR) mouse (Charles River Laboratories),
twice
daily by intravenous injection, once a week, for three weeks (Days 1, 8 and
15),
followed by a 10 week treatment-free period. The test antibody was a
commercially
available mouse anti-glycation end-product antibody raised against
carboxymethyl
lysine conjugated with keyhole limpet hemocyanin, the carboxymethyl lysine MAb
(Clone 318003) available from R&D Systems, Inc. (Minneapolis, MN; catalog no.
MAB3247). A control reference of physiological saline was used in the control
animals.
[116] Mice referred to as "young" were 8 weeks old, while mice referred
to as "old"
were 88 weeks ( 2 days) old. No adverse events were noted from the
administration
of the antibody. The different groups of animals used in the study are shown
in
Table 1.
[117] Table 1: The
different groups of animals used in the study
Number of Animals
Main Study Treatment-
Grou Test Dose Level
Free
p No. Material Mice (pg/gm/BID/ week) Females
Females
1 Saline young 0 20
2 Saline old 0 20 20
3 Antibody old 2.5 20 20
4 None old 0 20 pre
Antibody old 5.0 20 20
- = Not Applicable, Pre = Subset of animals euthanized prior to treatment
start for collection
of adipose tissue.
,
[118] F1 6INK4a mRNA, a marker for senescent cells, was quantified in
adipose tissue
of the groups by Real Time-qPCR. The results are shown in Table 2. In the
table
AACt = ACt mean control Group (2) ¨ ACt mean experimental Group (1 or 3 or 5);
Fold Expression= 2 - tmc .
[119] Table 2: P161"ma mRNA quantified in adipose tissue
Calculation (unadjusted Group 2 vs Group 1 Group 2 vs Group 3 Group 2 vs Group
5
-40-
CA 03229602 2024-02-19
WO 2023/023654 PCT/US2022/075226
to Group 4: 5.59) Group 2 Group 2 Group 1 Group 1
Group 1
Group 5
1 3 2
Mean ACt 5.79 7.14 5.79 6.09 5.79 7.39
AACt -1.35 -0.30 -1.60
Fold Expression 2.55 1.23 3.03
[120] The table above indicates that untreated old mice (Control Group 2)
express
2.55-fold more p161"4a mRNA than the untreated young mice (Control Group 1),
as
expected. This was observed when comparing Group 2 untreated old mice
euthanized at end of recovery Day 85 to Group 1 untreated young mice
euthanized
at end of treatment Day 22. When results from Group 2 untreated old mice were
compared to results from Group 3 treated old mice euthanized Day 85, it was
observed that p161"k4a mRNA was 1.23-fold higher in Group 2 than in Group 3.
Therefore, the level of p161"k4a mRNA expression was lower when the old mice
were
treated with 2.5 pg/gram/BID/week of antibody.
[121] When results from Group 2 (Control) untreated old mice were compared
to
results from Group 5 (5 pg/gram) treated old mice euthanized Day 22, it was
observed that p161"k4a mRNA was 3.03-fold higher in Group 2 (controls) than in
Group 5 (5 pg/gram). This comparison indicated that the Group 5 animals had
lower
levels of p161"k4a mRNA expression when they were treated with 5.0
pg/gram/BID/week, providing p161"k4a mRNA expression levels comparable to that
of
the young untreated mice (i.e. Group 1). Unlike Group 3 (2.5 pg/gram) mice
that
were euthanized at end of recovery Day 85, Group 5 mice were euthanized at end
of
treatment Day 22.
[122] These results indicate the antibody administration resulted in the
killing of
senescent cells.
[123] The mass of the gastrocnemius muscle was also measured, to determine
the
effect of antibody administration on sarcopenia. The results are provided in
Table 3.
The results indicate that administration of the antibody increased muscle mass
as
compared to controls, but only at the higher dosage of 5.0 pg/gm/BID/ week.
- 41 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[124] Table 3: Effect of antibody administration on mass of the
gastrocnemius
muscle
Summary Absolute weight of Weight relative to body
mass of
Group Information Gastrocnemius Muscle (g) Gastrocnemius Muscle (%)
1 Mean 0.3291 1.1037
SD 0.0412 0.1473
20 20
2 Mean 0.3304 0.7671
SD 0.0371 0.1246
20 20
3 Mean 0.3410 0.7706
SD 0.0439 0.0971
19 19
Mean 0.4074 0.9480
SD 0.0508 0.2049
9 9
[125] These results demonstrate that administration of antibodies that
bind to AGEs
of a cell resulted in a reduction of cells expressing p16Ink4a, a biomarker of
senescence. The data show that reducing senescent cells leads directly to an
increase in muscle mass in aged mice. These results indicate that the loss of
muscle mass, a classic sign of sarcopenia, can be treated by administration of
antibodies that bind to AGEs of a cell. The results suggest that
administration of the
antibodies would be effective in treating or preventing the onset of fibrotic
diseases
by removing senescent cells.
[126] Example 2: Affinity and kinetics of test antibody
[127] The affinity and kinetics of the test antibody used in Example 1
were analyzed
using Na,Na-bis(carboxymethyl)-L-lysine trifluoroacetate salt (Sigma-Aldrich,
St.
Louis, MO) as a model substrate for an AGE-modified protein of a cell. Label-
free
-42 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
interaction analysis was carried out on a BIACORETM T200 (GE Healthcare,
Pittsburgh, PA), using a Series S sensor chip CM5 (GE Healthcare, Pittsburgh,
PA),
with Fc1 set as blank, and Fc2 immobilized with the test antibody (molecular
weigh
of 150,000 Da). The running buffer was an HBS-EP buffer (10 mM HEPES, 150 mM
NaCI, 3 mM EDTA and 0.05% P-20, pH of 7.4), at a temperature of 25 C.
Software
was BIACORETM T200 evaluation software, version 2Ø A double reference (Fc2-1
and only buffer injection), was used in the analysis, and the data was fitted
to a
Langmuir 1:1 binding model.
[128] Table 4: Experimental set-up of affinity and kinetics analysis
Association and dissociation
Flow path Fc1 and Fc2
Flow rate (hl/mm.) 30
Association time (s) 300
Dissociation time (s) 300
Sample concentration (pM) 20 ¨ 5¨ 1.25 (x2) ¨ 0.3125 ¨ 0.078 - 0
_
[129] A graph of the response versus time is illustrated in FIG. 1. The
following
values were determined from the analysis: ka (1/Ms) = 1.857 x 103; kd (1/s) =
6.781 x
10-3; !(o (M) = 3.651 x 10-6; Rmax (RU) = 19.52; and Chi2 = 0.114. Because the
Chi2
value of the fitting is less than 10% of Rmax, the fit is reliable.
[130] Example 3: Construction and production of murine anti-AGE IgG2b
antibody
and chimeric anti-AGE IgG1 antibody
[131] Murine and chimeric human anti-AGE antibodies were prepared. The DNA
sequence of nnurine anti-AGE antibody IgG2b heavy chain is shown in SEQ ID NO:
12. The DNA sequence of chimeric human anti-AGE antibody IgG1 heavy chain is
-43 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
shown in SEQ ID NO: 13. The DNA sequence of murine anti-AGE antibody kappa
light chain is shown in SEQ ID NO: 14. The DNA sequence of chimeric human anti-
AGE antibody kappa light chain is shown in SEQ ID NO: 15. The gene sequences
were synthesized and cloned into high expression mammalian vectors. The
sequences were codon optimized. Completed constructs were sequence confirmed
before proceeding to transfection.
[132] HEK293 cells were seeded in a shake flask one day before
transfection, and
were grown using serum-free chemically defined media. The DNA expression
constructs were transiently transfected into 0.03 liters of suspension HEK293
cells.
After 20 hours, cells were sampled to obtain the viabilities and viable cell
counts, and
titers were measured (Octet QKe, ForteBio). Additional readings were taken
throughout the transient transfection production runs. The cultures were
harvested
on day 5, and an additional sample for each was measured for cell density,
viability
and titer.
[133] The conditioned media for murine and chimeric anti-AGE antibodies
were
harvested and clarified from the transient transfection production runs by
centrifugation and filtration. The supernatants were run over a Protein A
column and
eluted with a low pH buffer. Filtration using a 0.2 pm membrane filter was
performed
before aliquoting. After purification and filtration, the protein
concentrations were
calculated from the 0D280 and the extinction coefficient. A summary of yields
and
aliquots is shown in Table 5:
[134] Table 5: Yields and aliquots
Protein Concentration Volume No. of vials
Total Yield (mg)
(mg/mL) (mL)
Murine anti-AGE 0.08 1.00 3 0.24
Chimeric anti-AGE 0.23 1.00 3 0.69
- 44 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[135] Antibody purity was evaluated by capillary electrophoresis sodium-
dodecyl
sulfate (CE-SDS) analysis using LabChip GXII, (PerkinElmer).
[136] Example 4: Binding of murine (parental) and chimeric anti-AGE
antibodies
[137] The binding of the murine (parental) and chimeric anti-AGE antibodies
described in Example 3 was investigated by a direct binding ELISA. An anti-
carboxymethyl lysine (CML) antibody (R&D Systems, MAB3247) was used as a
control. CML was conjugated to KLH (CML-KLH) and both CML and CML-KLH were
coated overnight onto an ELISA plate. HRP-goat anti-mouse Fc was used to
detect
the control and murine (parental) anti-AGE antibodies. HRP-goat anti-human Fc
was used to detect the chimeric anti-AGE antibody.
[138] The antigens were diluted to 1 pg/mL in 1x phosphate buffer at pH
6.5. A 96-
well microtiter ELISA plate was coated with 100 p1./well of the diluted
antigen and let
sit at 4 C overnight. The plate was blocked with lx PBS, 2.5% BSA and allowed
to
sit for 1-2 hours the next morning at room temperature. The antibody samples
were
prepared in serial dilutions with lx PBS, 1% BSA with the starting
concentration of
50 pg/mL. Secondary antibodies were diluted 1:5,000. 100 pL of the antibody
dilutions was applied to each well. The plate was incubated at room
temperature for
0.5-1 hour on a microplate shaker. The plate was washed 3 times with lx PBS.
100
pL/well diluted HRP-conjugated goat anti-human Fc secondary antibody was
applied
to the wells. The plate was incubated for 1 hour on a microplate shaker. The
plate
was then washed 3 times with lx PBS. 100 pL HRP substrate TMB was added to
each well to develop the plate. After 3-5 minutes elapsed, the reaction was
terminated by adding 100 pL of IN HCl. A second direct binding ELISA was
performed with only CML coating. The absorbance at 0D450 was read using a
microplate reader.
[139] The 0D450 absorbance raw data for the CML and CML-KLH ELISA is shown
in the plate map below. 48 of the 96 wells in the well plate were used. Blank
wells in
the plate map indicate unused wells.
[140] Plate map of CML and CML-KLH ELISA:
- 45 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
Conc.
(pg/mL) 1 2 3 4 5 6 7
50 0.462 0.092 0.42 1.199 0.142
1.852
16.67 0.312 0.067 0.185 0.31 0.13
0.383
5.56 0.165 0.063 0.123 0.19 0.115
0.425
1.85 0.092 0.063 0.088 0.146 0.099
0.414
0.62 0.083 0.072 0.066 0.108 0.085
0.248
0.21 0.075 0.066 0.09 0.096 0.096
0.12
0.07 0.086 0.086 0.082 0.098 0.096
0.098
0 0.09 0.085 0.12 0.111 0.083
0.582
R&D Parental Chimeric R&D Parental Chimeric
Positive Anti- Anti- Positive Anti- Anti-
Control AGE AGE Control AGE AGE
CML-KLH Coat CML
Coat
[141] The 0D450 absorbance raw data for the CML-only ELISA is shown in the
plate map below. 24 of the 96 wells in the well plate were used. Blank wells
in the
plate map indicate unused wells.
[142] Plate map of CML-only ELISA:
Conc.
(pg/mL) 1 2 3 4 5 6 7
50 1.913 0.165 0.992
-46 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
16.66667 1.113 0.226 0.541
5.555556 0.549 0.166 0.356
1.851852 0.199 0.078 0.248
0.617284 0.128 0.103 0.159
0.205761 0.116 0.056 0.097
0.068587 0.073 0.055 0.071
0 0.053 0.057 0.06
R&D Parental Chimeric
Positive Anti- Anti-
Control AGE AGE
[143] The control and chimeric anti-AGE antibodies showed binding to both
CML
and CML-KLH. The murine (parental) anti-AGE antibody showed very weak to no
binding to either CML or CML-KLH. Data from repeated ELISA confirms binding of
the control and chimeric anti-AGE to CML. All buffer control showed negative
signal.
[144] Example 5: Humanized antibodies
[145] Humanized antibodies were designed by creating multiple hybrid
sequences
that fuse select parts of the parental (mouse) antibody sequence with the
human
framework sequences. Acceptor frameworks were identified based on the overall
sequence identity across the framework, matching interface position, similarly
classed CDR canonical positions, and presence of N-glycosylation sites that
would
have to be removed. Three humanized light chains and three humanized heavy
chains were designed based on two different heavy and light chain human
acceptor
frameworks. The amino acid sequences of the heavy chains are shown in SEQ ID
NO: 29, 31 and 33, which are encoded by the DNA sequences shown in SEQ ID NO:
-47 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
30, 32 and 34, respectively. The amino acid sequences of the light chains are
shown in SEQ ID NO: 35, 37 and 39, which are encoded by the DNA sequences
shown in SEQ ID NO: 36, 38 and 40, respectively. The humanized sequences were
methodically analyzed by eye and computer modeling to isolate the sequences
that
would most likely retain antigen binding. The goal was to maximize the amount
of
human sequence in the final humanized antibodies while retaining the original
antibody specificity. The light and heavy humanized chains could be combined
to
create nine variant fully humanized antibodies.
[146] The three heavy chains and three light chains were analyzed to
determine
their humanness. Antibody humanness scores were calculated according to the
method described in Gao, S. H., etal., "Monoclonal antibody humanness score
and its applications", BMC Biotechnology, 13:55 (July 5, 2013). The humanness
score represents how human-like an antibody variable region sequence looks.
For heavy chains a score of 79 or above is indicative of looking human-like;
for
light chains a score of 86 or above is indicative of looking human-like. The
humanness of the three heavy chains, three light chains, a parental (mouse)
heavy chain and a parental (mouse) light chain are shown below in Table 6:
[147] Table 6: Antibody humanness
Antibody Humanness (Framework + CDR)
Parental (mouse) heavy chain 63.60
Heavy chain 1 (SEQ ID NO: 29) 82.20
Heavy chain 2 (SEQ ID NO: 31) 80.76
Heavy chain 3 (SEQ ID NO: 33) 81.10
Parental (mouse) light chain 77.87
Light chain 1 (SEQ ID NO: 35) 86.74
-48 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
Light chain 2 (SEQ ID NO: 37) 86.04
Light chain 3 (SEQ IN NO: 39) 83.57
[148] Full-length antibody genes were constructed by first synthesizing the
variable
region sequences. The sequences were optimized for expression in mammalian
cells. These variable region sequences were then cloned into expression
vectors
that already contain human Fc domains; for the heavy chain, the IgG1 was used.
[149] Small scale production of humanized antibodies was carried out by
transfecting plasmids for the heavy and light chains into suspension HEK293
cells
using chemically defined media in the absence of serum. Whole antibodies in
the
conditioned media were purified using MabSelect SuRe Protein A medium (GE
Healthcare).
[150] Nine humanized antibodies were produced from each combination of the
three heavy chains having the amino acid sequences shown in SEQ ID NO: 29, 31
and 33 and three light chains having the amino acid sequences shown in SEQ ID
NO: 35, 37 and 39. A comparative chimeric parental antibody was also prepared.
The antibodies and their respective titers are shown below in Table 7:
[151] Table 7: Antibody titers
Antibody Titer (rng/L)
Chimeric parental 23.00
SEQ ID NO: 29+ SEQ ID NO: 35 24.67
SEQ ID NO: 29+ SEQ ID NO: 37 41.67
SEQ ID NO: 29+ SEQ ID NO: 39 29.67
SEQ ID NO: 31 + SEQ ID NO: 35 26.00
-49 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
SEQ ID NO: 31 + SEQ ID NO: 37 27.33
SEQ ID NO: 31 + SEQ ID NO: 39 35.33
SEQ ID NO: 33 + SEQ ID NO: 35 44.00
SEQ ID NO: 33 + SEQ ID NO: 37 30.33
SEQ ID NO: 33 + SEQ ID NO: 39 37.33
[152] The binding of the humanized antibodies may be evaluated, for
example, by
dose-dependent binding ELISA or cell-based binding assay.
[153] Example 6 (Prophetic): An AGE-RNAse containing vaccine in a human
subject.
[154] AGE-RNAse is prepared by incubating RNAse in a phosphate buffer
solution
containing 0.1-3 M glucose, glucose-6-phosphate, fructose or ribose for 10-100
days. The AGE-RNAse solution is dialyzed and the protein content is measured.
Aluminum hydroxide or aluminum phosphate, as an adjuvant, is added to 100 pg
of
the AGE-RNAse. Formaldehyde or formalin is added as a preservative to the
preparation. Ascorbic acid is added as an antioxidant. The vaccine also
includes
phosphate buffer to adjust the pH and glycine as a protein stabilizer. The
composition is injected intravenously into a subject with idiopathic pulmonary
fibrosis.
[155] Example 7 (Prophetic): Injection regimen for an AGE-RNAse containing
vaccine in a human subject.
[156] The same vaccine as described in Example 6 is injected
intraperitoneally into
a subject with liver cirrhosis. The titer of antibodies to AGE-RNAse is
determined by
ELISA after two weeks. Additional injections are performed after three weeks
and
six weeks, respectively. Further titer determination is performed two weeks
after
each injection.
- 50 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[157] Example 8 (Prophetic): An AGE-hemoglobin containing vaccine in a
human
subject.
[158] AGE-hemoglobin is prepared by incubating human hemoglobin in a
phosphate buffer solution containing 0.1-3 M glucose, glucose-6-phosphate,
fructose
or ribose for 10-100 days. The AGE-hemoglobin solution is dialyzed and the
protein
content is measured. All vaccine components are the same as in Example 6,
except
AGE-hemoglobin is substituted for AGE-RNAse. Administration is carried out as
in
Example 6, or as in Example 7.
[159] Example 9 (Prophetic): An AGE-human serum albumin containing vaccine
in
a human subject.
[160] AGE-human serum albumin is prepared by incubating human serum albumin
in a phosphate buffer solution containing 0.1-3 M glucose, glucose-6-
phosphate,
fructose or ribose for 10-100 days. The AGE-human serum albumin solution is
dialyzed and the protein content is measured. All vaccine components are the
same
as in Example 6, except AGE-human serum albumin is substituted for AGE-RNAse.
Administration is carried out as in Example 6, or as in Example 7.
[161] Example 10 (Prophetic): Carboxymethyllysine-modified protein vaccine
for a
human subject
[162] A vaccine is prepared by combining a carboxymethyllysine-modified
protein
as an AGE antigen, aluminum hydroxide as an adjuvant, formaldehyde as a
preservative, ascorbic acid as an antioxidant, a phosphate buffer to adjust
the pH of
the vaccine and glycine as a protein stabilizer. The vaccine is injected
subcutaneously into a subject with myelofibrosis.
[163] Example 11 (Prophetic): Carboxyethyllysine-modified peptide vaccine
for a
human subject
[164] A vaccine is prepared by combining a carboxyethyllysine-modified
peptide
conjugated to KLH as an AGE antigen, aluminum hydroxide as an adjuvant,
formaldehyde as a preservative, ascorbic acid as an antioxidant, a phosphate
buffer
- 51 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
to adjust the pH of the vaccine and glycine as a protein stabilizer. The
vaccine is
injected subcutaneously into a subject with chronic kidney disease.
[165] Example 12: In vivo study of the administration of a carboxymethyl
lysine
monoclonal antibody
[166] The effect of a carboxymethyl lysine antibody on tumor growth,
metastatic
potential and cachexia was investigated. In vivo studies were carried out in
mice
using a murine breast cancer tumor model. Female BALB/c mice (BALB/cAnNCrl,
Charles River) were eleven weeks old on Day 1 of the study.
[167] 4T1 murine breast tumor cells (ATCC CRL-2539) were cultured in RPM!
1640
medium containing 10% fetal bovine serum, 2 nnM glutamine, 25 pg/mL
gentamicin,
100 units/mL penicillin G Na and 100 pg/mL streptomycin sulfate. Tumor cells
were
maintained in tissue culture flasks in a humidified incubator at 37 C in an
atmosphere of 5% CO2 and 95% air.
[168] The cultured breast cancer cells were then implanted in the mice. 4T1
cells
were harvested during log phase growth and re-suspended in phosphate buffered
saline (PBS) at a concentration of 1 x 106 cells/mL on the day of implant.
Tumors
were initiated by subcutaneously implanting 1 x 105 4T1 cells (0.1 mL
suspension)
into the right flank of each test animal. Tumors were monitored as their
volumes
approached a target range of 80-120 mm3. Tumor volume was determined using
the formula: tumor volume = (tumor width)2(tumor length)/2. Tumor weight was
approximated using the assumption that 1 mm3 of tumor volume has a weight of 1
mg. Thirteen days after implantation, designated as Day 1 of the study, mice
were
sorted into four groups (n=15/group) with individual tumor volumes ranging
from 108
to 126 mm3 and a group mean tumor volume of 112 mm3. The four treatment groups
are shown in Table 8 below:
[169] Table 8: Treatment groups
Group Description Agent Dosing
- 52 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
r (pg/g)
1 Control phosphate buffered saline (PBS) N/A
2 Low-dose carboxymethyl lysine monoclonal 5
antibody
3 High-dose carboxymethyl lysine monoclonal 10
antibody
4 Observation None N/A
only
[170] An anti-carboxymethyl lysine monoclonal antibody was used as a
therapeutic
agent. 250 mg of carboxymethyl lysine monoclonal antibody was obtained from
R&D Systems (Minneapolis, MN). Dosing solutions of the carboxymethyl lysine
monoclonal antibody were prepared at 1 and 0.5 mg/mL in a vehicle (PBS) to
provide the active dosages of 10 and 5 pg/g, respectively, in a dosing volume
of 10
mL/kg. Dosing solutions were stored at 4 C protected from light.
[171] All treatments were administered intravenously (i.v.) twice daily for
21 days,
except on Day 1 of the study where the mice were administered one dose. On Day
19 of the study, i.v. dosing was changed to intraperitoneal (i.p.) dosing for
those
animals that could not be dosed i.v. due to tail vein degradation. The dosing
volume
was 0.200 mL per 20 grams of body weight (10 mL/kg), and was scaled to the
body
weight of each individual animal.
[172] The study continued for 23 days. Tumors were measured using calipers
twice
per week. Animals were weighed daily on Days 1-5, then twice per week until
the
completion of the study. Mice were also observed for any side effects.
Acceptable
toxicity was defined as a group mean body weight loss of less than 20% during
the
study and not more than 10% treatment-related deaths. Treatment efficacy was
determined using data from the final day of the study (Day 23).
- 53 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[173] The ability of the anti-carboxymethyl lysine antibody to inhibit
tumor growth
was determined by comparing the median tumor volume (MTV) for Groups 1-3.
Tumor volume was measured as described above. Percent tumor growth inhibition
(%TGI) was defined as the difference between the MW of the control group
(Group
1) and the MTV of the drug-treated group, expressed as a percentage of the MTV
of
the control group. %TGI may be calculated according to the formula: %TGI = (1-
MTVtreated/MTVcontrol) X 100.
[174] The ability of the anti-carboxymethyl lysine antibody to inhibit
cancer
metastasis was determined by comparing lung cancer foci for Groups 1-3.
Percent
inhibition (%Inhibition) was defined as the difference between the mean count
of
metastatic foci of the control group and the mean count of metastatic foci of
a drug-
treated group, expressed as a percentage of the mean count of metastatic foci
of the
control group. %Inhibition may be calculated according to the following
formula:
%Inhibition = (1-Mean Count of Focit reated. ¨ /mean Count of FOCicontrol) X
100.
[175] The ability of the anti-carboxymethyl lysine antibody to inhibit
cachexia was
determined by comparing the weights of the lungs and gastrocnemius muscles for
Groups 1-3. Tissue weights were also normalized to 100 g body weight.
[176] Treatment efficacy was also evaluated by the incidence and magnitude
of
regression responses observed during the study. Treatment may cause partial
regression (PR) or complete regression (CR) of the tumor in an animal. In a PR
response, the tumor volume was 50% or less of its Day 1 volume for three
consecutive measurements during the course of the study, and equal to or
greater
than 13.5 mm3 for one or more of these three measurements. In a CR response,
the
tumor volume was less than 13.5 mm3 for three consecutive measurements during
the course of the study.
[177] Statistical analysis was carried out using Prism (GraphPad) for
Windows 6.07.
Statistical analyses of the differences between Day 23 mean tumor volumes
(MTVs)
of two groups were accomplished using the Mann-Whitney U test. Comparisons of
metastatic foci were assessed by ANOVA-Dunnett. Normalized tissue weights were
- 54 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
compared by ANOVA. Two-tailed statistical analyses were conducted at
significance
level P = 0.05. Results were classified as statistically significant or not
statistically
significant.
[178] The results of the study are shown below in Table 9:
[179] Table 9: Results
Group MTV cATGI Lung %Inhibition PR CR Gastroc. Lung
weight/
(mm3)
foci weight/
normalized
normalized (mg)
(mg)
1 1800 N/A 70.4 N/A
0 0 353.4/19.68 2799.4/292.98
2 1568 13% 60.3 14%
0 0 330.4/21.62 2388.9/179.75
3 1688 6% 49.0 30%
0 0 398.6/24.91 2191.6/214.90
[180] All treatment regimens were acceptably tolerated with no
treatment-related
deaths. The only animal deaths were non-treatment-related deaths due to
metastasis. The %TGI trended towards significance (P > 0.05, Mann-Whitney) for
the 5 pg/g (Group 2) or 10 pg/g treatment group (Group 3). The %Inhibition
trended
towards significance (P > 0.05, ANOVA-Dunnett) for the 5 pg/g treatment group.
The %Inhibition was statistically significant (P < 0.01, ANOVA-Dunnett) for
the 10
pg/g treatment group. The ability of the carboxymethyl lysine antibody to
treat
cachexia trended towards significance (P > 0.05, ANOVA) based on a comparison
of
the organ weights of the lung and gastrocnemius between treatment groups and
the
control group. The results indicate that administration of an anti-
carboxymethyl
lysine monoclonal antibody is able to reduce cancer metastases. This data
provides
additional evidence that in vivo administration of anti-AGE antibodies can
provide
therapeutic benefits safely and effectively.
- 55 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[181] Example 13: Treatment of fibrotic lung diseases by removal of
senescent cells
[182] The following example was obtained from International Publication no.
WO
2015/116740 and describes research carried out by parties other than the
inventor
and applicant of the Present Application regarding therapeutic removal of
senescent
cells in vivo by administration of senolytic agents in a mouse model, as well
as
potential future studies that could be carried out.
[183] One animal model study assessed the effect of clearance of senescence
cells
in the transgenic mouse strain 3MR that has bleomycin induced lung injury. In
the
bleomycin injury model for idiopathic pulmonary fibrosis, mice develop lung
fibrosis
within 7-14 days after bleomycin treatment (see, e.g., Limjunyawong et al.,
2014,
Physiological Reports 2:e00249; Daniels et al, 2004, J. Clin. Invest. 114:
1308-
1316). Bleomycin was administered to anesthetized 6-8 week old 3MR mice by
intratracheal aspiration (2.5U/kg of bleomycin in 50 pi' PBS) using a
microsprayer
syringe (Penn- Century, Inc.) as described in Daniels et al. (2004, J. Clin.
Invest.
114: 1308-1316). Control mice were administered saline. The day following
bleomycin treatment, ganciclovir (GCV) (25mg/kg in PBS) was administered. 3MR
mice were treated via intraperitoneal injection with ganciclovir for 5
consecutive
days, followed by 5 days of rest, followed by a second treatment cycle of 5
consecutive days. Untreated mice received an equal volume of vehicle. At 7,
14, and
21 days post-bleomycin treatment, lung function was assessed by monitoring
oxygen
saturation using the MouseSTAT PhysioSuite pulse oximeter (Kent Scientific).
Animals were anesthetized with isoflurane (1.5%) and a toe clip was applied.
Mice
were monitored for 30 seconds and the average peripheral capillary oxygen
saturation (Sp02) measurement over this duration was calculated. As shown in
Figure 2, bleomycin administration significantly reduced Sp02 levels in
vehicle
treated mice, and removal of senescent cells resulted in higher Sp02 levels,
which
approached normal levels at 21 days post bleomycin administration. At 21 days
post-
bleomycin treatment, airway hyper-reactivity (AHR) of mice was examined. AHR
of
mice was measured by methacholine challenge while other parameters of lung
function (airway mechanics, lung volume and lung compliance) were determined
using a SCIREQ flexiVent ventilator. While under ketamine/xylazine anesthesia
and
- 56 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
subjected to cannulation of the trachea via a tracheostomy (19Fr blunt Luer
cannula), airway resistance (elastance) and compliance of mice were assessed
at
baseline and in response to increasing concentrations of nnethacholine (0 to
50
mg/ml in PBS) delivered via nebulization (AeroNeb) as described in Aravannudan
et
al. (Am. J. Physiol. Lung Cell. Mol. Physiol. (2012) 303:L669-L681). Animals
were
maintained at 37 C, and while under muscle paralysis (pancuronium); airway
function was measured by using the FlexiVentTM ventilator and lung mechanics
system (SCIREQ, Montreal, Quebec, Canada), which was housed on Stabile 8. As
shown in Figure 3A, in vehicle treated mice, bleomycin administration
increased lung
elastance, whereas ganciclovir treatment reduced lung elastance. As shown in
Figures 3B-C, bleomycin administration reduced static compliance and (dynamic)
compliance in vehicle treated mice. Clearance of senescent cells with
ganciclovir in
bleomycin exposed mice improved compliance values significantly (Figures 3B-
C).
Although not statistically significant because the animal group size was too
small,
data suggested that clearance of senescent cells with a senolytic agent
(Nutlin-3A)
also reduced lung elastance and increased lung compliance in a bleomycin
exposed
mouse. Mice were euthanized by i.p injection of pentobarbital.
[1 8 4] Bronchoalveolar lavage (BAL) fluids and lungs is obtained and
analyzed.
Hydroxyproline content of lungs is measured as described in Christensen et al.
(1999, Am . J. Pathol. 155: 1773-1779), and quantitative histopathology is
performed. RNA is extracted from lung tissue to measure senescence cell
markers
by qRT-PCR in treated and control mice. The effect of clearance of senescence
cells in the bleomycin induced lung injury model of IPF may also be studied in
INK-
KIT AC transgenic mice in the study design described above. INK- AU AC
(p161"ma
apoptosis through targeted activation of caspase) transgenic mice have an
FK506-
binding protein (FKBP)-caspase 8 (Casp8) fusion polypeptide under the control
of
the p16Ink" promoter (see, e.g., Baker et al, Nature, supra; Intl Patent
Application
Publication No. WO/2012/177927). In the presence of AP20187, a synthetic drug
that induces dimerization of a membrane bound myristoylated FKBP-Casp8 fusion
protein, senescent cells specifically expressing the FKBP-Casp8 fusion protein
via
- 57 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
the pl6In4a promoter undergo programmed cell death (apoptosis) (see, e.g.,
Baker,
Nature, supra, Figure 1 therein).
[185] A second study also assesses the effect of clearance of senescence
cells
using a senolytic agent in C57BL6/J mice that have bleomycin induced lung
injury.
Bleomycin is administered to 6 week old C57BL6/J mice as described above. A
senolytic agent is administered during the first and third week post-bleomycin
treatment. Control mice are treated with vehicle. At 21 days post-bleomycin
treatment, clearance of senescent cells and lung function/histopathology is
assessed.
[186] In a second animal model for pulmonary diseases (e.g., COPD), mice
were
exposed to cigarette smoke. The effect of a senolytic agent on the mice
exposed to
smoke is assessed by senescent cell clearance, lung function, and
histopathology.
[187] Six week-old 3 MR (n=35) or INK-ATTAC (n=35) mice were chronically
exposed to cigarette smoke generated from a Teague TE-10 system, an
automatically-controlled cigarette smoking machine that produces a combination
of
side-stream and mainstream cigarette smoke in a chamber, which is transported
to a
collecting and mixing chamber where varying amounts of air is mixed with the
smoke
mixture. The COPD protocol was adapted from the COPD core facility at Johns
Hopkins University (at Internet site web
.jhu.edu/Biswal/exposure_core/copd.html#Cigarette_Snnoke) (Rangasamy et al,
2004, J. Clin. Invest. 114: 1248-1259; Yao et al, 2012, J. Clin. Invest.
122:2032-
2045). Mice received a total of 6 hours of cigarette smoke exposure per day, 5
days
a week for 6 months. Each lighted cigarette (3R4F research cigarettes
containing
10.9 mg of total particulate matter (TPM), 9.4 mg of tar, and 0.726 mg of
nicotine,
and 11.9 mg carbon monoxide per cigarette [University of Kentucky, Lexington,
KY])
was puffed for 2 seconds and once every minute for a total of 8 puffs, with
the flow
rate of 1.05 Unnin, to provide a standard puff of 35 cm3. The smoke machine
was
adjusted to produce a mixture of side stream smoke (89%) and mainstream smoke
(11%) by smoldering 2 cigarettes at one time. The smoke chamber atmosphere was
monitored for total suspended particulates (80-120 mg/m3) and carbon monoxide
- 58 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
(350 ppm). Beginning at day 7, (10) INK-ATTAC and (10) 3 MR mice were treated
with AP20187 (3x per week) or ganciclovir (5 consecutive days of treatment
followed
by 16 days off drug, repeated until the end of the experiment), respectively.
An equal
number of mice received the corresponding vehicle. The remaining 30 mice (15
INK-
ATTAC and 15 3 MR) were evenly split with 5 of each genetically modified
strain
placed into three different treatment groups. One group (n=10) received Nutlin-
3A
(25 mg/kg dissolved in 10% DMSO/3%) Tween-20 in PBS, treated 14 days
consecutively followed by 14 days off drug, repeated until the end of the
experiment).
One group (n=10) received ABT-263 (Navitoclax) (100 mg/kg dissolved in 15%
DMSO/5% Tween-20, treated 7 days consecutively followed by 14 days off drug,
repeated until the end of the experiment), and the last group (n=10) received
only the
vehicle used for ABT-263 (15% DMSO/5% Tween-20), following the same treatment
regimen as ABT-263. An additional 70 animals that did not receive exposure to
cigarette smoke were used as controls for the experiment.
[188] After two months of cigarette smoke exposure, lung function was
assessed by
monitoring oxygen saturation using the MouseSTAT PhysioSuite pulse oximeter
(Kent Scientific). Animals were anesthetized with isoflurane (1.5%) and the
toe clip
was applied. Mice were monitored for 30 seconds and the average peripheral
capillary oxygen saturation (Sp02) measurement over this duration was
calculated.
As shown in Figure 4, clearance of senescent cells via AP20187, ganciclovir,
ABT-
263 (Navi), or Nutlin-3A resulted in statistically significant increases in
Sp02 levels in
mice after 2 months of cigarette smoke exposure compared to untreated
controls.
[189] At the end of the experimental period, airway hyper-reactivity (AHR)
of mice to
methacholine challenge using a SCIREQ flexiVent ventilator and lung mechanics
system is examined as described above. After AHR measurement, mice are killed
by
i.p. injection of pentobarbital for in-depth analysis of lung histopathology
as
previously described (Rangasamy et al, 2004, J. Clin. Invest. 114: 1248-1259).
Briefly, lungs are inflated with 0.5% low-melting agarose at a constant
pressure of 25
cm. Part of the lung tissue is collected for RNA extraction and qRT-PCR
analysis of
senescent markers. Other parts of lungs are fixed in 10% buffered formalin and
embedded in paraffin. Sections (5 um) are stained with hematoxylin and eosin.
Mean
- 59 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
=
alveolar diameter, alveolar length, and mean linear intercepts are determined
by
computer- assisted morphometry with Image Pro Plus software (Media
Cybernetics).
[190] The potential therapeutic effect of clearance of senescent cells
after COPD is
fully developed may be assessed in 3MR or INK-ATTAC mice. Six week-old 3MR or
INK-ATTAC mice are chronically exposed to cigarette smoke for 6 months as
described above. At 6 months following the start of smoke exposure, 3MR or INK-
ATTAC mice are treated with ganciclovir (5 consecutive days of treatment
followed
by 16 days off drug) or AP20187 (3x/week), respectively, until 9 months
following the
start of smoke exposure, when assessment of senescent cell clearance, lung
function, and histopathology is performed.
[191] These studies demonstrate that fibrotic lung diseases may be treated
by the
removal of senescent cells. In addition, these studies confirm that senescent
cells
are appropriate targets for treating fibrotic diseases.
[192] Example 14 (Prophetic): Treatment of idiopathic pulmonary fibrosis
[193] A patient presents with idiopathic pulmonary fibrosis. She is
administered an
anti-AGE antibody by inhalation using a nebulizer. The anti-AGE antibody will
specifically bind to cells expressing cell-surface AGEs, such as senescent
myofibroblasts, and allow her immune system to destroy those cells. Killing
and
removing senescent cells will treat her idiopathic pulmonary fibrosis and
prevent the
disease from worsening.
[194] Example 15 (Prophetic): Treatment of scleroderma
[195] A patient presents with scleroderma on his right arm. He is
administered a
topical cream containing an anti-AGE antibody. The anti-AGE antibody will
specifically bind to cells expressing cell-surface AGEs, such as senescent
myofibroblasts, and allow his immune system to destroy those cells. Killing
and
removing senescent cells will treat his scleroderma and prevent further
progression
of the disease.
- 60 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
REFERENCES
[196] 1. "About PF", Pulmonary Fibrosis Foundation, available online at
www.pulmonaryfibrosis.org/life-with-pf/about-pf, accessed on July 18, 2017.
[197] 2. "Idiopathic pulmonary fibrosis", available online at
en.wikipedia.org/wiki/Idiopathic_pulmonary_fibrosis (April 18, 2017).
[198] 3. "Pulmonary fibrosis" available online at
en.wikipedia.org/wiki/Pulmonary_fibrosis (July 12, 2017).
[199] 4. "Epithelial-mesenchymal transition", available online at
en.wikipedia.org/wiki/Epithelial¨mesenchyrnal_transition (July 15, 2017).
[200] 5. Medline Plus, "Pulmonary fibrosis", National Institute of
Health,
available online at medlineplus.gov/pulrnonaryfibrosis.html (April 21, 2017).
[201] 6. Schafer, M. J. et al., "Cellular senescence mediates fibrotic
pulmonary
disease", Nature Communications, Vol. 8, No. 14532, 11 pages (2017).
[202] 7. Yilmaz, 0. et aL, "Dasatinib attenuated bleomycin-induced
pulmonary
fibrosis in mice", Growth Factors, Vol. 33, No. 5, p. 366-375 (2015).
[203] 8. Kato, M. etal., "Dasatinib suppresses TFG13-induced epithelial
mesenchymal transition and inhibits pulmonary fibrosis", European Respiratory
Journal, Vol. 44, No. Suppl. 58, p. 742 (2014).
[204] 9. Yagmur, E. at al., "Elevation of NE-(carboxymethyl)lysine-
modified
advanced glycation end products in chronic liver disease is an indicator of
liver
cirrhosis", Clinical Biochemistry, Vol. 39, p. 39-45 (2006).
[205] 10. Chilosi, M. etal., "Premature lung aging and cellular
senescence in the
pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema",
Translational
Research, Vol. 162, p. 156-173 (2013).
-61-
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[206] 11. Calhoun, C. et al., "Senescent cells contribute to the
physiological
remodeling of aged lungs", Journals of Gerontology: Biological Sciences, Vol.
71,
No. 2, p. 153-160 (2016).
[207] 12. Duffield, J., "Cellular and molecular mechanisms in kidney
fibrosis",
The Journal of Clinical Investigation, Vol. 124, No. 6, p. 2299-2306 (2014).
[208] 13. Weiler-Normann, C., etal., "Mouse models of liver fibrosis",
Zeitschrift
Mr Gastroenterologie, Vol. 45, p. 43-50 (2007).
[209] 14. Harris, W. T. etal., "Myofibroblast differentiation and
enhanced TGF-I3
signaling in cystic fibrosis lung disease", PLoS One, Vol. 8, No. 8, 8 pages
(2013).
[210] 15. Hashimoto, M. etal., "Elimination of p19ARF-expressing cells
enhances
pulmonary function in mice", Journal of Clinical Investigation Insight, Vol.
1, No. 12,
15 pages (2016).
[211] 16. Childs, B. G. etal., "Senescent foam cells are deleterious at
all stages
of atherosclerosis", Science, Vol. 354, No. 6311, p. 472-477 (2016).
[212] 17. Frescas, D. et a/., "Senescent cells expose and secrete an
oxidized
form of membrane-bound vimentin as revealed by a natural polyreactive
antibody",
Proceedings of the National Academy of Sciences, p. E1668-E1677 (2017).
[213] 18. "Targeting pro-inflammatory cells in idiopathic pulmonary
fibrosis: a
human trial (IPF)", available online at
clinicaltrials.gov/ct2/show/study/NCT02874989
(accessed on February 6, 2017).
[214] 19. "Safety evaluation of dasatinib in subjects with scleroderma
pulmonary
fibrosis", available online at clinicaltrials.govict2/show/study/NCT00764309
(accessed on February 6, 2017).
[215] 20. "Beneficial effects of quercetin in chronic obstructive
pulmonary
disease (COPD) (quercetin)", available online at
clinicaltrials.govict2/show/study/NCT01708278 (accessed on February 6, 2017).
- 62 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[216] 21. Wynn, T. A., "Fibrotic disease and the TH1fTH2 paradigm",
Nature
Reviews Immunology, Vol. 4, No. 8, P. 583-594 (2004).
[217] 22. BataIler, R. et aL, "Liver fibrosis", The Journal of Clinical
Investigation,
Vol. 115, No. 2, p. 209-218 (2005).
[218] 23. "Myelofibrosis", Mayo Clinic, available online at
www.mayoclinic.org/diseases-conditions/myelofibrosis/home/ovc-20261141
(accessed on February 14, 2017).
[219] 24. Midgley, A. C. etal., "Transforming growth factor-I31 (TGF-
131)-
stimulated fibroblast to myofibroblast differentiation is mediated by
hyaluronan (HA)-
facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization
in lipid
rafts", Journal of Biological Chemistry, Vol. 288, No. 21, P. 14824-14838
(2013).
[220] 25. Shaw, T. J. etal., "Wound-associated skin fibrosis: mechanisms
and
treatments based on modulating the inflammatory response", Endocrine,
Metabolic &
Immune Disorders, Vol. 10, No. 4, pp. 320-330 (2010).
[221] 26. "Scleroderma Living With It", National Institute of Arthritis
and
Musculoskeletal and Skin Diseases, available online at
www.niams.nih.gov/health-
topics/scleroderma#tab-living-with (2016).
[222] 27. "Current treatments available for scleroderma patients",
Scleroderma
Research Foundation, available online at www.srIcure.org/for-patientsicurrent-
treatments (2018).
[223] 28. W02014/179262.
[224] 29. Pan, J. et al., "Inhibition of Bc1-2/xl with ABT-263
selectively kills
senescent type II pneumocytes and reverses pulmonary fibrosis induced by
ionizing
radiation in mice", International Journal of Radiation Oncology Biology
Physics, Vol.
99, No. 2, pp. 353-361 (2017).
[225] 30. W02015/116740.
- 63 -
CA 03229602 2024-02-19
WO 2023/023654
PCT/US2022/075226
[226] 31. Wang, W-J. et aL, "Cellular senescence, senescence-associated
secretory phenotype, and chronic kidney disease", Oncotarget, Vol. 8, No. 38,
pp.
64520-64533 (2017).
[227] 32. Valentijn, F.A. etal., "Cellular senescence in the aging and
diseased
kidney", Journal of Cell Communication and Signaling, Vol. 12, pp. 69-82
(2018).
[228] 33. Oldfield, M.D. etal., "Advanced glycation end products cause
epithelial-myofibroblast transdifferentiation via the receptor for advanced
glycation
end products (RAGE)", The Journal of Clinical Investigation, Vol. 108, No. 12,
pp.
1853-1863 (2001).
[229] 34. Tanji, N. et al., "Expression of advanced glycation end
products and
their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal
disease",
Journal of the American Society of Nephrology, Vol. 11, pp. 1656-1666 (2000).
[230] 35. Machahua, C., et al., Increased AGE-RAGE ratio in idiopathic
pulmonary fibrosis, Respiratory Research, vol. 17, no. 144, pp. 1-11 (2016).
[231] 36. El-Torky, M., et al., Collagens in scar carcinoma of the lung,
The
American Journal of Pathology, vol. 121, no. 2, pp. 322-326 (1985)
-64 -