Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/049079
PCT/US2022/044024
COMPOSITIONS AND METHODS FOR PROPOGATING INSULIN AND GLUCAGON
SECRETING CELLS FROM TYPE 1 DIABETIC PANCREATIC TISSUE
AND THERAPEUTIC USES THEREOF
CROSS REFERENCE TO RELATED APPLICATION
This application claims priority to U.S. provisional patent application No(s).
63/247,252, filed on September 22, 2021 and 63/337,137, filed on May 1,2022,
the
contents of which are herein incorporated by reference in their entirety.
BACKGROUND
Cell-based therapies offer the promise of treating and altering the course of
pancreatic disorders, such as Type 1 diabetes (T1D), which cannot be addressed
adequately by existing therapies, yet cell-based therapies present myriad
issues, mainly
related to safety and efficacy and scalability of manufacture. Many of the
problems
associated with cell-based therapies are described in Engineering the next
generation
of cell-based therapeutics by Bashor, C.J., et al., Nat Rev Drug Discov (2022)
(and
available online at https://doi.org/10.1038/s41573-022-00476-6).
SUMMARY OF THE DISCLOSURE
Disclosed herein are compositions and methods for generating compositions
comprising cell-based therapeutics useful for treating pancreatic disorders,
including
Type 1 diabetes. In one embodiment, a composition as disclosed herein
comprises an
insulin and glucagon secreting cell population generated from non-insulin
secreting
pancreatic cells collected via needle biopsy from a Type 1 diabetic donor
pancreas. In
another embodiment, a composition as disclosed herein comprises an insulin-and
glucagon secreting cell population generated from pancreatic cells collected
via needle
1
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
biopsy from a patient or donor suffering from chronic pancreatitis. In one
embodiment,
non-insulin secreting Type 1 diabetic pancreatic cells are treated, in vitro,
with an islet
cell culture medium comprising a base medium and an effective amount of a
polypeptide according to an amino acid sequence listed in SEQ ID 1 or 2,
wherein
treatment causes the treated cells to differentiate and propagate into a
population of
islet progenitor cells that secrete both insulin and glucagon in response to
stimuli and
are CD133 positive. The resulting insulin and glucagon secreting progenitor
cells can be
propagated to a desirable cell count for subsequent use in transplantation or
injection
and as a cell-based therapeutic for Type 1 diabetes or chronic pancreatitis.
The cellular
composition comprising an effective amount of an insulin and glucagon
secreting
progenitor cell population may be administered to a subject by infusion,
injection,
transplantation, intra portal delivery, or by other suitable delivery means
such as with a
medical device, as method for restoring secretion of insulin and glucagon in
response to
stimuli.
The compositions and methods disclosed herein have implications for producing
large volumes of insulin and glucagon secreting pancreatic cells useful for
cell-based
therapies and cellular transplantations, namely autologous or allogenic
transplantation
for the treatment of Type 1 diabetes or chronic pancreatitis.
Also disclosed herein is a method of treating a pancreatic disorder, such as
Type
1 diabetes or pancreatitis, comprising administering to a subject in need
thereof, a
therapeutically effective amount of a composition comprising an insulin and
glucagon
secreting pancreatic cell population, wherein the insulin and glucagon
secreting
pancreatic cell population is generated by treating pancreatic cells collected
from
2
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
diseased pancreatic tissue (for example, from a Type 1 diabetic subject or one
suffering
from chronic pancreatitis), such as via needle biopsy, with an islet cell
culture media
comprising a base medium and a peptide comprising an amino acid sequence
according to SEQ ID 1 or 2. The composition, when administered to a subject in
need
thereof, provides delivery of healthy pancreatic progenitor cells to a target
site in the
subject, wherein the healthy pancreatic progenitor cells are capable of
producing insulin
and glucagon in response to stimulation.
The composition comprising a therapeutically effective amount of insulin and
glucagon secreting progenitor cells generated by the methods disclosed herein
may be
used as an autologous or allogenic cell based therapeutic to supplement the
loss of
insulin production or replace insulin production in patients with Type 1
diabetes, or with
other diseases characterized by severe insulin deficiency, such as after total
or partial
pancreatectomy, with and without autologous or allogenic islet
transplantation.
In one embodiment, compositions may be prepared for transplantation by
supplementing the compositions with human serum albumin and/or human serum
from
the recipient prior to administration.
In another embodiment, an islet cell culture medium useful for stimulating
growth,
propagation and differentiation of insulin and glucagon secreting cells from
pancreatic
cells derived from Type 1 diabetic pancreatic tissue comprises a base medium
and an
effective amount of a polypeptide, wherein the polypeptide comprising an amino
acid
sequence according to one or more of SEQ ID NO. 1 ¨2 (listed in Table 1), or
active
fragment thereof. In one embodiment, the polypeptide comprises an amino acid
sequence having at least 50% sequence identity to the amino acid sequence set
forth in
3
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
SEQ ID NO. 01; in another embodiment, the polypeptide has at least 50%, 55%,
60%,
65%, 70%, 75%, 80%, 85%, 90%, 95%, 98% or 99% sequence identity to the amino
acid sequence set forth in SEQ ID NO. 1. Alternatively, the polypeptide
comprises an
amino acid sequence having at least 50% sequence identity to the amino acid
sequence set forth in SEQ ID NO. 2; in another embodiment, the polypeptide has
at
least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98% or 99% sequence
identity to the amino acid sequence set forth in SEQ ID NO. 2.
In yet another embodiment, a cellular composition comprises a population of
insulin and glucagon secreting cells generated by treatment of isolated Type 1
diabetic
pancreas tissue with an islet cell culture medium comprising a base medium and
an
effective amount of a polypeptide according to SEQ ID NO. 1 or 2 or active
fragment
thereof; further comprising measuring the response of the cells to glucose;
wherein the
cellular composition comprises a population of cells capable of secreting
insulin and
glucagon in response to appropriate stimuli.
In another embodiment, a method of manufacturing a cellular composition
comprises applying, in vitro, an islet cell culture medium comprising a base
medium and
an effective amount of a polypeptide according to SEQ ID NO. 1 or 2, or active
fragment
thereof, to human pancreatic tissue collected from a Type 1 diabetic;
incubating the
cells in the islet cell culture medium; screening the incubated cells for one
or more cell
markers selective for CD133 and insulin; and collecting the cells identified
by screening
as CD133 and insulin-positive from the cultured cell population; continuing to
grow the
cultured cells until a desired quantity of cells are propagated.
4
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
In yet another embodiment, cellular compositions comprising insulin and
glucagon secreting cells derived from T1D pancreatic tissue are packaged or
encapsulated for administration or implantation into a mammal for in vivo
therapy,
specifically to restore insulin production and secretion. The cellular
compositions may
be packaged as a delivery solution, or in a delivery vehicle, and administered
by
implantation, injection or infusion, whether administration is systemic,
localized or
directed to a target site.
In yet another embodiment, a method of treating a pancreatic disorder, wherein
the pancreatic disorder is characterized by an insufficient production of
insulin, in a
mammal, comprises: culturing, in vitro, a population of insulin and glucagon
secreting
cells from pancreatic tissue collected from a Type 1 diabetic donor pancreas,
in an islet
cell culture medium comprising a base medium and an effective amount of a
polypeptide according to SEQ ID NO. 1 or 2 or active fragment thereof, whereby
a
population of CD133 positive, insulin and glucagon secreting cells are
produced; further
comprising isolating and expanding the population to generate a predominantly
(at least
60% or greater) insulin and glucagon secreting cell population; and further
comprising
collecting the insulin and glucagon secreting cells and suspending the
collected cells in
a physiologic buffer, such as phosphate buffered saline (PBS) or Hanks
Balanced Salt
Solution (HBSS), and implanting or injecting into a mammal a cellular
composition
comprising the insulin and glucagon secreting cells in suspension with
physiologic
buffer. In one embodiment, the composition may be delivered as an aqueous
solution, a
suspension, an encapsulation, a microencapsulation, and/or an encapsulated, or
semi-
solid formulation; wherein the composition may be delivered to the mammal via
one or
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
more of an injection, infusion, omental or peritoneal pouch, surgical
implantation, or via
packaging the composition as part of a device to a target site in the mammal.
In another embodiment, a cellular composition comprises an insulin and
glucagon secreting cell population further comprising one or more of a buffer,
a
pharmaceutically acceptable carrier, a pharmaceutically acceptable additive,
an
antibiotic or other pharmaceutical agent.
DETAILED DESCRIPTION OF THE DRAWINGS
The compositions and methods disclosed herein are further described by the
accompanying figures. The term "IPCs" are used in the figures to refer to the
insulin-
producing cells (I PCs) described and claimed herein.
FIG. 1 shows that T1D-derived insulin and glucagon secreting cells propagated
according to methods herein are greater than 50% triple positive for CD133,
insulin and
glucagon.
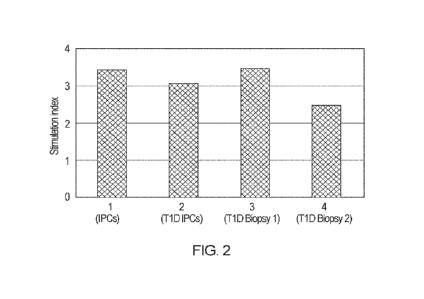
FIG. 2 shows T1D pancreatic tissue cultured with islet cell culture medium
comprising a
peptide according to SEQ ID NO. 1 or 2 produce cells that secrete insulin in
response to
glucose stimulation, as shown by the stimulation index, which is the ratio
between
insulin secretion under high glucose conditions vs basal release under
unstimulated
conditions. A value above 2 represents glucose responsiveness in the cells.
Sample 1 is
a cell population propagated from normal pancreatic tissue according to
methods
herein; Samples 2 ¨ 4 are cell populations propagated from T1D pancreatic
tissue
according to methods herein.
FIG. 3 shows the down regulation and up regulation of gene (families)
associated with
pancreatic function in a single Ti D biopsy-derived cell preparation compared
to native
6
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
pancreatic tissue. Each family of genes includes 5 to 13 genes. As the figure
shows, cell
populations comprising insulin and glucagon secreting cells generated
according to the
methods herein exhibit upregulation of gene families essential for mature
islet cells,
beta cell maturation, GSIS, insulin granules and cell cycle.
FIG. 4 shows serum insulin levels following transplantation in streptozotocin
(STZ, a 13-
cell-specific toxin that induces irreversible damage to pancreatic islets and
induces
diabetes) treated mice of cellular compositions comprising insulin and
glucagon
propagated according to methods herein. The cellular compositions were shown
to
promote the secretion of human insulin in vivo, which was present in the serum
for up to
100 days of STZ mice treated with cellular compositions disclosed herein.
FIG. 5 shows that insulin and glucagon secreting cells generated from Type 1
diabetic
(Ti D) cells propagated according to methods herein can normalize blood
glucose levels
upon injection in a STZ diabetic mouse model. M1 ¨ 4 refer to STZ mouse sample
numbers, ie, Mouse-1, Mouse-2, Mouse-3, Mouse-4.
DETAILED DESCRIPTION OF THE DISCLOSURE
The following terms are used in this disclosure to describe different
embodiments. These terms are used for explanation purposes only and are not
intended to limit the scope for any aspect of the subject matter claimed
herein.
As used herein "SEQ ID NO 1 or 2" refers to a protein, polypeptide, peptide
fragment, or analogue thereof, and including any modification thereto, having
an amino
acid sequence having at least 85%, 90%, 95%, 98% or 99% sequence identity to
the
amino acid sequence according to SEQ ID NO 1 or 2 (See Table 1). Also
contemplated
is a peptide fragment, or analogue thereof, and including any modification
thereto,
7
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
having an amino acid sequence having at least 85%, 90%, 95%, 98% or 99%
sequence
identity to the amino acid sequence according to SEQ ID NO(s): 1 and 2. It has
been
demonstrated by in vitro studies with polypeptides according to SEQ ID NO 1
and SEQ
ID NO 2 that treatment of cells (keratinocytes, enterocytes, islet,
endothelial and
pneumocyte cells) with a polypeptide according to SEQ ID NO 1 or SEQ ID NO 2
added
to cell culture medium causes stimulation and increase in cell growth,
resulting in viable
progenitor cells, as measured by the percent of CD133 positive cells in
culture and MTT
cell proliferation assays (Sigma Aldrich Cell Proliferation Kit). These
progenitor cells can
be regenerated and propagated into the billions.
As used herein, the term "insulin and glucagon secreting cells" or "insulin
and
glucagon secreting islet cells" or "insulin and glucagon secreting progenitor
cells" are
used interchangeably herein to refer to the cellular composition comprising
insulin and
glucagon secreting cells and/or cell population(s), which are generated from
non-insulin
secreting T1D pancreatic cells according to the methods described herein, and
which
are positive for the cell markers: CD133, insulin, glucagon, and that produce
insulin and
glucagon in response to stimuli, and are further characterized by cell markers
PDX-1,
SST, IIAP, Pax4, Pax6, Nkx2, Nkx6, NeuroD1, MafA, MafB.
The term "propagation" refers to an increase in the number of cells present in
a
culture as a result of cell division.
As used herein "culture", "cultured" or "culturing" refers to removal or
isolation of
cells from an environment (such as in a host mammal) and their subsequent
growth in a
favorable artificial environment in vitro. "Cultured cells" is intended to
include sub-
8
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
cultured (i.e., passaged) by transferring the cells to a new vessel with fresh
growth
medium to provide more room for continued growth, differentiation and/or
propagation.
Reference to "pancreatic cells" include those cells normally found in the
pancreas of a
mammal, and include pancreatic islet cells, e.g., glucagon-synthesizing alpha
cells,
insulin-producing beta cells, and any combination thereof.
The term "target site" as used herein refers to a region in the recipient host
(a
mammal, preferably human) that requires treatment or supplementation. The
target site
can be a single region within a specific organ or can be multiple regions in
the host. In
some embodiments, the supplementation or replacement results in the same
physiological response as normal tissue, such as pancreatic tissue, whether or
not
targeting the pancreas.
As used herein, the terms "treat," "treating" or "treatment," and other
grammatical
equivalents as used herein, include alleviating, abating or ameliorating a
disease or
condition symptoms, preventing additional symptoms, ameliorating or preventing
the
underlying metabolic causes of symptoms, inhibiting the disease or condition,
e.g.,
arresting the development of the disease or condition, relieving the disease
or condition,
causing regression of the disease or condition, relieving a condition caused
by the
disease or condition, or stopping the symptoms of the disease or condition,
and
prophylaxis. The terms further include achieving a therapeutic benefit and/or
a
prophylactic benefit. By therapeutic benefit is meant eradication or
amelioration of the
underlying disorder being treated. Also, a therapeutic benefit is achieved
with the
eradication or amelioration of one or more of the physiological symptoms
associated
9
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
with the underlying disorder such that an improvement is observed in the
patient,
notwithstanding that the patient may still be afflicted with the underlying
disorder.
As used herein, an "effective amount" refers to an amount that is sufficient
to
achieve the stated effect. A therapeutically effective amount to treat a
condition is an
amount capable of achieving a clinically relevant end-point in a patient or
patient
population. As non-limiting examples, administration of an effective amount of
a
composition comprising insulin and glucagon secreting cells is an amount of
approximately 1.2 to about 2.5 x 106 cells/kg, or greater than 200 x 106
cells, in order to
produce sufficient insulin to cause a reduction in blood glucose levels to
approximately
100 to 125 mg/di (5.6 to 6.9 mmol/L), or to under 250 mg/d1. Other ranges
include
approximately 3 x 106 cells to about 25 x 106 cells per kg of body mass; or
approximately 5 x 106 to about 10 x 106 millions cells/kilo. The appropriate
dose of the
composition may depend on the route of administration, such as injection or
infusion or
transplantation, and may depend on the subject being treated as well as the
severity of
the condition to be treated. Using scaling methods, such as allometric
scaling, it is
possible to predict suitable and exemplary dosage ranges for the
administration of
compositions, as disclosed herein, to adult humans. Dose scaling is an
empirical
approach, is well characterized and understood in the art. This approach
assumes that
there are some unique characteristics on anatomical, physiological, and
biochemical
process among species, and the possible difference in
pharmacokinetics/physiological
time is, as such, accounted for by scaling. As one example, not intending to
be limiting,
the human pancreas, based on the literature, has between 6 x 10 to about 2 x
106
islets; hence there are approximately 600 x 106 islet cells in the normal
human
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
pancreas, with half of them being beta cells. Considering that an islet mass
of 30% is
sufficient to maintain normoglycemia, a dose of 1.2 x 106 of the insulin and
glucagon
secreting cells as disclosed herein would be expected to sufficiently replace
the insulin
producing capacity of a non-diseased human pancreas.
As used herein, the term "sequence identity" refers to the identity between
two or
more amino acid sequences expressed in terms of the identity or similarity
between the
sequences. Sequence identity can be measured in terms of percentage identity;
the
higher the percentage, the more identical the sequences are. The percentage
identity is
calculated over the entire length of the sequence. Homologs or orthologs of
amino acid
sequences possess a relatively high degree of sequence identity when aligned
using
standard methods. This homology is more significant when the orthologous
proteins are
derived from species which are more closely related (e.g., human and mouse
sequences), compared to species more distantly related (e.g., human and C.
elegans
sequences). Methods of alignment of sequences for comparison are well known in
the
art. Various programs and alignment algorithms are described in: Smith &
Waterman;
Needleman & Wunsch, J. Mol. Biol. 48:443, 1970; Pearson & Lipman, Proc. Nat.
Acad
Sci. USA 85:2444, 1988; Higgins & Sharp, Gene, 73:23744, 1988; Higgins &
Sharp,
CABIOS 5:151-3, 1989; Carpet et al., Nuc. Acids Res. 16:10881-90, 1988; Huang
et al.
Computer Appls. in the Biosciences 8, 155-65, 1992; and Pearson et al., Meth
Mol. Bio.
24:307-31, 1994. Altschul et al., J. Mol. Biol. 215:403-10, 1990, presents a
detailed
consideration of sequence alignment methods and homology calculations. The
level of
sequence identity may be determined using the NCB! Basic Local Alignment
Search
Tool (BLAST) (Altschul et al., J. Mol. Biol. 215:403-10, 1990), which is
available from
11
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
several sources, including the National Center for Biological Information
(NCBI, National
Library of Medicine, Building 38A, Room 8N805, Bethesda, Md. 20894, US) and on
the
Internet.
It will be understood that a numerical value may be associated with a certain
amount of experimental error. Thus, recitation of the qualifier "about" (or
"approximately") prior to a numerical error is meant to embody the
experimental error
that may be associated with the recited numerical value. To the extent that a
numerical
value obtained experimentally is not preceded by the expression "about" (or
"approximately") does not mean that the numerical value is not associated with
a certain
amount of experimental error.
Representative cultures of the insulin and glucagon secreting cells
characterized
herein have been deposited with ATCC on September 7, 2022 [Accession
Number ____________________________________________________________ ] under
the terms of the Budapest Treaty. Cultured cells, propagated
cells, isolated cells, and the like may be protected from external mutagenic
stimuli, such
as, for example UV radiation.
Utilizing the methods disclosed herein, it has been determined that 30 days
post
isolation of T1D pancreatic tissue, a single pancreas can product 77 billion
insulin and
glucagon secreting (islet) cells; 2 trillion cells by day 60, which is enough
cells to infuse
100 ¨ 150 patients in need of treatment (depending on the severity of illness
or dosage
used), or bank (freeze/store) cells for future expansion and re-infusion.
Table I: SEQ ID NO(s)
SEQ ID NO. 01 MADDAGAAGGPGGPGGPGMGNRGGFRGGFGSGIRGRGRGRGRGRGRGRGARGG
(293 aa) KAEDKEWMPVTKLGRLVKDMKIKSLEEIYLFSLPIKESEIIDFFLGASLKDEVLKIMPVQK
QTRAGQRTRFKAFVAIGDYNGHVGLGVKCSKEVATAI RGAIILAKLSIVPVRRGYWGNK
12
CA 03231258 2024- 3-7
WO 2023/049079
PCT/1152022/044024
IG KPHTVPCKVTGRCGSVLVRL I PAPRGTG IVSAPVPKKLLM MAGIDDCYTSARGCTAT
LGNFAKATFDAISKTYSYLTPDLWKETVFTKSPYQEFTDHLVKTHTRVSVORTQAPAVATT
SEQ ID NO. 2: GHVGLGVKCSKEVATAIRGAI I LAKLSIVPVRRGYWG NKIGKPHTVPCKVTGRCGSVLVR
(159 aa) L I PAPRGTG IVSAPVPKKLLMMAG I DDCYTSARGCTATLGN
FAKATFDAISKTYSYLTPD
LVVKETVFTKSPYQEFTDHLVKTHTRVSVQRTQAPAVATT
Amino acid residues of the active agents may be post-translationally modified
or
conjugated with other functional or non-functional molecular groups. See,
e.g., Guo et
al. Mol. Biosyst. 7(7): 2286-2295, 2011, describing generally antagonistic
citrullination
and methylation of human ribosomal protein S2 (e.g., SEQ ID NO. 1). Naturally,
such
modified amino acid residues are included in the amino acid sequences and
within the
scope of the active agents described herein.
The polypeptides and/or polypeptide fragments according to, for example, SEQ
ID NO(s): 1 and 2 may be produced under conditions known in the art for
protein
production, such as production in bacteria, yeast or by synthetic means, or as
described
in United States Patent Application No.15/811,060.
In one embodiment, the cellular compositions may be packaged as a delivery
solution, or in a delivery vehicle comprising a medical device, and may be
administered
by implantation, injection, or infusion, whether administration is parenteral,
systemic,
localized, or directed to a target site. In one embodiment, encapsulation of
in vitro-
generated insulin and glucagon secreting islet cells and implantation into a
mammal
have been previously characterized in the art (see, for example, Altman, et
al., 1984,
Trans. Am. Soc. Art. Organs 30:382-386, and U.S. Pat. No. 6,703,017 B1, herein
incorporated by reference)¨and would be suitable for the insulin and glucagon
secreting cells generated according to the methods disclosed herein.
Preferably, the
13
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
encapsulant is hypoallergenic, is easily and stably situated in a target
tissue, and
provides added protection to the implanted cellular composition, to protect
and prevent
from the destruction of the implanted cells.
The appropriate implantation dosage in humans can be determined from existing
information relating to ex vivo islet transplantation in humans, further in
vitro and animal
experiments, and from human clinical trials. From data relating to
transplantation of ex
vivo islets in humans, it is expected that about 8,000-12,000 islets per
patient kg may be
required. Assuming long-term survival of the implants following
transplantation, less
than the number of naturally occurring islets (about 2 million in a normal
human adult
pancreas), or possibly even less than the amount used in ex vivo islet
transplantation
may be necessary.
In one embodiment, the cellular compositions are of therapeutic benefit for
treating a pancreatic disorder, wherein the pancreatic disorder is
hyperglycemia, Type 1
diabetes, or chronic pancreatitis, in a mammal, which comprises: administering
a
therapeutically effective amount of the insulin and glucagon secreting cell
population,
thereby providing a treatment for the pancreatic disorder.
In one embodiment, the composition comprising a therapeutically effective
amount of an insulin and glucagon secreting cell population may be formulated
as an
aqueous solution, a suspension, an encapsulation, a microencapsulation, and/or
an
encapsulated, or semi-solid formulation; wherein the composition may be
delivered to
the patient in need via one or more of an injection, infusion, omental or
peritoneal
pouch, port, surgical implantation, or via packaging the composition as part
of a device
to a target site in the mammal.
14
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
In one embodiment, a composition comprises an insulin and glucagon secreting
progenitor cell population further comprising one or more of a
pharmaceutically
acceptable excipients, and/or one or more of a pharmaceutically acceptable
additive
and/or one or more a pharmaceutical agent. Suitable excipients and additives
include,
but are not limited to buffering agents such as PBS, or HBSS, amino acids,
stabilizer or
bulking agents, surfactants, antimicrobial/preservatives, antifungal agents,
metal
ions/chelators, polymers, polyanions, salts, sugars, cyclodextrin based
excipients,
lyoprotectants, solubilizing agents, antioxidants, complexing agents, anti-
adhesive
agents, dispersing agents, serum additives.
In another embodiment, the disclosure provides a method for treating a mammal,
preferably a human, suffering from, or at risk of developing Type 1 diabetes
or severe
pancreatitis, which comprises: removing pancreatic tissue from the mammal;
culturing
the excised pancreatic tissue in vitro to propagate a population of insulin
and glucagon
secreting islet cells; and implanting, transplanting, infusing, injecting, or
otherwise
inserting the population of insulin and glucagon secreting islet cells, alone
or together
with a medical or delivery device, into the mammal.
EXAMPLES
The following Examples are offered by way of illustration and not by way of
limitation with respect to subject matter claimed herein.
Cell cultures performed under the Examples were incubated at 37 C under
standard CO (5%) conditions; culturing (plating, splitting of cells) was
performed using
standard aseptic techniques and conditions in a vertical laminar flow hood.
Unless
stated otherwise, cells (including controls) were cultured in the islet cell
culture media
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
described in Table 2 and 3. Cells were split when they reached confluency of
approximately 70 - 80% in culture.
In one embodiment, the splitting technique involved removal of supernatant
from
the culture plates (supernatant was preserved). Plates were then washed with 2
- 5 ml
PBS (wash was preserved). Cells were detached using approximately 3 - 5 ml of
trypsin
(such as available from Sigma-Aldrich) by incubating the cells in the presence
of trypsin
at 37 C for approximately 3 - 5 minutes until cells detached. Plates were then
washed a
second time with PBS. The trypsinized cells, as well as the preserved PBS
washes and
collected cell culture supernatant, were then centrifuged at 300 g for 7
minutes at 4 C.
In one embodiment, the resulting supernatant was decanted, and the pellet
resuspended in 2 nil PBS, and recentrifuged. The supernatant was then removed,
and
the pellet resuspended in culture medium containing SEQ ID 1 or 2 and re-
plated at a
cell density of ¨ 1000 cells/cm2. While the Examples may refer to cell culture
plates, it
will be understood that cell culture flasks are an acceptable alternative to
plates.
TABLE 2 Islet Cell Culture Medium TABLE 3 CONTROL Medium
MEDIUM COMPONENT AMOUNT MEDIUM COMPONENT AMOUNT
CMRL medium (or other 500 mL CMRL medium (or other 500 mL
similar equivalent nutrient similar equivalent
media which could be nutrient media which
prepared and utilized by could be prepared and
those skilled in the art) utilized by those skilled
in the art)
L-glutamine 2 mmol
Ciprofloxacin 2mg/L
Ciprofloxacin 2mg/L
Amphotericin B 0.1mg/L
Amphotericin B 0.1mg/L
Penicillin 100,000
units/L
Penicillin 100,000 units/L
16
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
Streptomycin 100,000 Streptomycin 100,000
micrograms/L
micrograms/L
SEQ ID 1 or 2 3 -20 pg/mL Fetal calf serum (FCS) 10%
(or horse or bovine
Fetal calf serum (FCS) (or 10% serum)
horse, or bovine serum)
Human Serum 10%
Human Serum 10%
Example 1
Method of generating an insulin and glucagon secreting pancreatic cell
population from non-insulin-producing pancreatic tissue collected by needle
biopsy from
a Type 1 Diabetic donor.
Human pancreatic tissue was collected via needle biopsy from a Type 1 diabetic
(T1D) donor patient (58-year-old female; 53 years diabetic). Biopsies of
1x1mm3 were
obtained from the donor pancreas (procured from a center for organ recovery
and
education) preserved on ice in a commercially available solution (sold under
names
such as Viaspan, Belzer UW, Bel-Gen or StoreProtec).
The collected T1D tissue was then cultured in an islet cell culture medium
(See
Table 2) comprising CMRL (such as Mediatech #99-663-CV Transplant Medium (CMRL
1066) without phenol red) supplemented with L-glutamine (2mmo1), Ciprofloxacin
(2mg/L), Amphotericin B (0.1mg/L) Penicillin (100,000 units/L) and
Streptomycin
(100,000 micrograms/L), and a polypeptide according to SEQ ID NO. 1 or 2 (in a
range
of 3 - 20 pg/ml, and specifically 10 pg/ml), with fetal calf serum (FCS) (10%)
and human
serum (10%). Control culture medium is one which comprises CMRL supplemented
with
L-glutamine (2mm01), Ciprofloxacin (2mg/L), Amphotericin B (0.1mg/L)
Penicillin
(100,000 units/L) and Streptomycin (100,000 micrograms/L) and fetal calf serum
(FCS)
17
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
(10%) and human serum (10%) (without the addition of a polypeptide according
to SEQ
ID NO. 1 or 2). Standard tissue/cell culture conditions (37 C, 5% CO2) were
used.
Tissue is cultured on plates or in flasks coated with an attachment factor
mixture
(AFM) comprising Collagen Type I (Collagen from rat tail, Sigma-Aldrich C3867)
and
Endothelial Cell Attachment Factor (ECAF, Sigma-Aldrich E9765). Various ratios
of
ECAF and collagen may be used, including but not limited to a 50/50 ratio of
collagen to
ECAF. Briefly, plates (or flasks) were prepared by applying a thin layer of
AFM
(between 3¨ 10 ml) to the plates, and after setting for 30 minutes the excess
AFM was
removed. The plates were allowed to dry for 45 minutes in a hood. Prior to use
the
plates were washed with PBS to remove any potential contaminants. The
collected
tissue was incubated on the AFM-treated plates in islet cell culture medium
supplemented with a polypeptide according to SEQ ID NO. 1 or 2 until cells
began to
mobilize and proliferate (approximately 10 to 20 days).
After 12-15 days in culture the biopsy-derived cells were mobilized and began
to
adhere to the dish and to proliferate. During a subsequent 4 ¨ 6-week period,
the
adherent cells continued to proliferate, became confluent and continue to
multiply,
doubling every 3 days. The biopsy-derived cells in culture exhibited
morphological
similarities to cellular compositions comprising insulin secreting cells
generated from
non-diabetic donors, previously characterized in US 2021/0205371, published
July 8,
2021. Similarly, the cells formed islet-like cell clusters with size
consistent with that of
islets of Langerhans and were shown to secrete insulin in response to
stimulation with
glucose.
18
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
The resulting cell cultures derived from T1D pancreatic biopsy tissue were
also
assayed for the expression of CD133, as well as intracellular insulin and
glucagon
expression, by fluorescence activated cell sorting (FACS) using a flow
cytometry
instrument (Becton Dickinson FACS Aria cell sorter). The cultured cells were
initially
labeled for CD133 expression and then fixed and pernneabilized with FOXP3
Fixation/Permeabilization Buffer, as per the manufacturer's instructions, and
stained
with conjugated fluorometric antibodies for glucagon and intra-cellular
insulin,
respectively. FACS analysis followed FOXP3 Fixation Permeabilization and
staining
with conjugated fluorometric antibodies. T1D pancreas biopsy-derived cells
cultured in
islet cell culture medium comprising a peptide according to SEQ ID NO. 1 or 2
were
found to be positive for CD133, glucagon and insulin (referred to herein as
"triple
positive"), specifically, 48¨ 73% triple positive for insulin, glucagon and
0D133, 26 ¨
42% double positive for glucagon and CD133, 14 ¨ 18% triple negative for
insulin,
glucagon and CD133, 9¨ 23% single positive for glucagon, and 0 ¨ 7% single
positive
CD133, and negative for insulin and glucagon; negative for insulin and CD133;
and
negative for insulin. It was determined that, overall, the cultured cell
population was
greater than 65% positive for insulin, CD133 and glucagon (triple positive).
(See FIG. 1)
Example 2
An insulin and glucagon secreting pancreatic cell population generated from
non-
insulin producing pancreatic tissue collected by needle biopsy from a Type 1
Diabetic
donor secretes insulin in response to glucose stimulation in vitro.
To test glucose responsiveness of the insulin and glucagon secreting cells
propagated from T1D biopsy tissue (See Example 1), the insulin and glucagon
secreting
19
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
cells were subjected to a glucose stimulation insulin secretion assay.
Approximately 1 x
106 cells/well were plated in a 6-well dish and underwent 2 stimulus
conditions to
assess insulin secretion. The cells were incubated for 30 minutes with either:
(1) islet
cell culture medium (See Table 2); or (2) islet cell culture medium
supplemented with a
higher glucose concentration (final concentration 16.7 mM, as a stimulus for
insulin
secretion). Following incubation, the supernatant was stored at -20 C until
undergoing a
standard ELISA assay for insulin quantification. The cells cultured in the
islet cell culture
medium supplemented with a higher glucose concentration were shown to secrete
higher insulin amounts than those cells treated with standard islet cell
culture medium
(unstimulated controls). There was a relative increase in insulin secretion
following
glucose stimulation compared to the unstimulated controls, more specifically,
higher
insulin amounts (95 +/- 11 pMol/L) were seen in the stimulated cultures
compared to
those cells treated with standard islet cell culture medium (32 +/- 7 pMol/L
the
"unstimulated control"). See FIG 2, which shows the stimulation index (the
measure of
the ratio between insulin secretion with high glucose versus basal release) of
the cells
treated with islet cell culture medium supplemented with high glucose,
compared to
unstimulated controls.
Example 3
Characterization and mRNA analysis of insulin and glucagon secreting cells
derived from Ti D donor tissue.
Using RNA sequencing methods, characteristics of several cell populations were
determined, including pancreatic tissue from a deceased donor, insulin and
glucagon
secreting cells generated by methods described herein, and denovo-pseudo-
islets from
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
the same deceased donor. Characteristics of the various cell types were
assessed
using an IIlumina NovaSeq TM platform. Markers assessed were: insulin,
glucagon,
PDX-1, SST, IIAP, Pax4, Pax-6, NKx2, Nkx6, NeuroD1, MafA, and MafB. It was
also
determined that cellular compositions comprising insulin and glucagon
secreting cells
generated from T1D-derived pancreatic tissue exhibited a substantial decrease
in
markers of exocrine function (AMY and CTRC), which are expressed in the native
pancreas. Concurrently, markers of proliferation PCNA and CCND1 (cyclin
family)
increased in expression in the cellular compositions, potentially signifying
de-
differentiation to motile, proliferating cells, consistent with the
observation of cell
expansion in vitro. Following longer periods of culture (-20 days or more),
the insulin
and glucagon secreting cells generated from pancreatic tissue collected by
needle
biopsy from a T1D donor underwent morphological re-arrangement and
spontaneously
generated de novo-pseudo-islets. These islet-like structures were
characterized by a
significant increase in expression of an endocrine progenitor and islet
signature markers
that included insulin, glucagon, PDX-1, SST, IIAP, Pax4, Pax-6, NKx2, Nkx6,
NeuroD1,
MafA, and MafB, while exhibiting downregulation of the cell proliferation
pathways.
Moreover, IGFBP1 a marker of 13-cell regeneration, was found expressed at
higher
levels in pseudo-islets when compared to the cellular compositions comprising
insulin
and glucagon secreting cells, whereas the stern cell markers LY6E and PROM1
were
more highly expressed in the cellular compositions, suggesting the maturation
to a more
differentiated endocrine progenitor cell population committed to generate
alpha, beta
and delta cells.
21
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
Microarray mRNA profile of the cells confirmed a gene profile compatible with
a
pancreatic endocrine islet cell population, with expression of insulin, and
glucagon.
Furthermore, these T1D-derived cell populations also expressed the pancreatic
transcription factors PDX1, Nkx6, ngn3, NeuroD, and MafA and MafB, as well as
the
islet neogenesis factor nestin, the glucose transporter Glut-2, the secretory
product of 13-
cells IAPP, and somatostatin which, is secreted by islet 6-cells. These
findings suggest
that the T1D-derived cell populations carry all factors necessary for islet
neogenesis.
See FIG. 3, which shows an overview of the upregulation and downregulation of
gene
families in the insulin and glucagon secreting cells derived from T1D
pancreatic tissue.
Example 4
Method of increasing insulin secretion in vivo via transplantation into a host
animal of cellular compositions comprising insulin and glucagon secreting
cells.
To test the effectiveness of the T1D-derived insulin and glucagon secreting
cells
as both a therapeutic and a method for transplantation, four STZ-treated mice
(NOD-
SC ID, 5-6 weeks old; Jackson Laboratory, Bar Harbor, ME) were injected with
approximately 2.5 x 106 T1D-derived biopsy-derived insulin and glucagon
secreting
cells (cells were counted using a Neubauer Chamber) twice, one week apart
(total of 2
doses). Follow up was 30 days. Blood samples were obtained via tail vein after
14 days
post first dose, and at the end of the follow up. Serum was obtained and
stored at -20 C
until used to measure human insulin and human C-peptide concentrations by
ELISA
(Abcam and Alpco, respectively).
T1D-derived Insulin and glucagon secreting cells were generated using methods
described herein, for example by culturing T1D pancreatic tissue in a culture
medium
22
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
comprising a polypeptide according to SEQ ID NO. 1 or 2 at a concentration
ranging
from 3 to 20 pg/ml. One example of islet cell culture medium is described as
follows
(and shown in Table 2 and Example 1): CMRL supplemented with L-glutamine
(2mm01),
Ciprofloxacin (2mg/L), Amphotericin B (0.1mg/L) Penicillin (100,000 units/L)
and
Streptomycin (100,000 micrograms/L), and a polypeptide according to SEQ ID NO.
1 or
2 ( at 10 pg/m1,) and Fetal calf serum (FCS) (10%) and human serum (10%).
Control
culture medium is described in Table 3.
Prior to transplantation, cells were cultured for approximately 50 ¨ 60 days.
At
the time of transplant, cells were detached from the bottom of the plate/flask
using
trypsin (available from Gibco). Following filtration via a 40pm sterile mesh,
single cells
were washed in a phosphate buffer solution (without Calcium and Magnesium),
Hanks
Balanced Salt Solution may also be used (both available from Sigma Aldrich) by
spinning (from 180 g ¨300 g, for approximately 10 minutes) counted and
resuspended
in approximately 200 pl of sterile PBS (or HBSS) at a dose of 1.25 x 106/100p1
and
injected into anesthetized mice via the tail vein. Injection was carried out
over the
course of one minute.
Human insulin was detected in all mice on day 14 and day 30 (the concentration
range was measured as 12.5 to 33 pmol/L) post first injection. Human C-peptide
confirmed positive results when measured on day 30 (at a level up to 10 pmol).
As an alternative to intra venous injection, the cells can be resuspended in
PBS
at a more concentrated volume of 20 ¨ 50 pl and inserted in the sub capsular
space
(occupying an area of approximately 1cm2) of the kidney by using a PE-50
tubing
connected to a syringe (method described by Bertera et al, Journal of
Transplantation
23
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
Volume 2012, Article ID 856386, 9 pages doi:10.1155/2012/856386). Using this
approach, larger doses of cells can be administered at once (for example, from
about 5
¨ 10 x 106 cells), in contrast to intravenous injection of human cells, of
which doses
higher than 2.5 x 106 cells may not be as well tolerated.
Considering that normal range of insulin in the human serum of non-diabetic
individuals is approximately 35.9 to 143.5 pmo1/1, and considering the
limitations derived
from the difference in clearance of human insulin in mice compared to humans,
it is
reasonable to expect that a dose of 3.0 to 25 x 106 cells/kg of body weight in
human
recipients would supply insulin in quantities that have the potential to
affect glucose
regulation. Administration may be once, or multiple administrations, repeated
every 3 to
6 months, or on an annual basis, as needed. The dose will depend on many
factors,
such as severity of illness, gender, weight, and age.
Example 5
Method of increasing insulin secretion in vivo via transplantation into a host
animal of cellular compositions comprising insulin and glucagon secreting
cells.
It has been determined that cellular compositions comprising insulin and
glucagon secreting cells propagated from murine islets, using the islet cell
culture
medium described herein, can be safely injected and/or transplanted via the
kidney
capsule into animals, such as mice.
In one example, human insulin secretion following implantation of insulin and
glucagon secreting cells under the kidney capsule in mice was detected for a
duration of
at least 100 days (See FIG. 4).
24
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
The insulin and glucagon secreting cells propagated were collected, then
suspended in a solution, such as one comprising Hanks Balanced Salt Solution
(HBSS)
or Phosphate Buffered Saline (PBS) (available from Sigma Aldrich) to form a
cellular
composition. In this example, the cellular composition comprising insulin and
glucagon
secreting cells suspended in Hanks Balanced Salt Solution was transplanted
under the
kidney capsule of streptozotocin-diabetic (STZ) nude mice (5-6 weeks old;
Jackson
Laboratory, Bar Harbor, ME) using known methods, such as an approach described
in
Bertera et al 2012.
Briefly, prior to transplantation, mice were injected with streptozotocin
(240mg/kg
IP) and hyperglycemia (non-fasting blood glucose levels > 350mg/dI on 2
consecutive
readings) was confirmed.
On the day of transplantation, a cellular composition is made by detaching the
insulin and glucagon secreting cells from culture, such as with trypsin;
centrifuging (at
300 g) and counting the detached cells. Approximately 4 x 106 cells; were
suspended in
a solution comprising HBSS; and loaded into a tubing or catheter, such as PE50
tubing.
The cellular composition is then transplanted into an STZ mouse by placing the
catheter
or tubing containing the cellular composition under the kidney capsule of a
fully
anesthetized STZ mouse, via a small incision of the left flank, and following
exposure of
the kidney.
On days 14, 56 and 100 post-transplantation, blood was obtained from the tail
vein of the STZ mice receiving the cellular compositions, and plasma was
separated
and stored. Insulin levels were measured using an ELISA kit specific for human
insulin
(ALPCO Diagnostics, Salem, NH, USA). See FIG. 4, showing insulin levels in the
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
streptozotocin-diabetic mice treated, via transplantation, with a composition
comprising
insulin and glucagon secreting cells.
Example 6
Method of treating hyperglycemia and diabetes via transplantation into a host
animal of cellular compositions comprising insulin and glucagon secreting
cells.
Cellular compositions comprising insulin and glucagon secreting cells
generated
by treating non-type 1 diabetic pancreatic tissue (human and murine in origin)
with an
islet cell culture medium comprising a base medium and a polypeptide according
to SQ
ID NO: 1 or 2 have been shown to secrete insulin following glucose stimulation
at
sufficient levels to lower blood glucose. These cellular compositions
comprising
approximately 4 x 106 cells, when injected intravenously, not only secrete
insulin and
glucagon but home and engraft in the pancreas. Cellular compositions
comprising
insulin and glucagon secreting cells may be delivered via cell transplantation
for the
treatment of pancreatic disorders, including diabetes and hyperglycemia.
The expression of the stem cell marker CD133 correlates with the capacity of
cells to engraft long-term, and these cells have the innate capability to
migrate and
home to injury sites. It was determined that cellular compositions as
disclosed herein,
when injected into an STZ-diabetic mouse, migrate to the pancreas and are
shown to
normalize hyperglycemia by a corresponding lowering of blood glucose levels
following
treatment. For example, six (6) four STZ-treated mice were injected with a
cellular
composition comprising (approximately) 20 x 106 cells isolated from murine
pancreatic
tissue and treated with islet cell culture medium comprising a polypeptide
according to
SEQ ID NO. 1 01 2. Results showed that by day 10 all mice had lower blood
glucose
26
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
levels; by day 22 post injection two of the four animals exhibited a fasting
blood glucose
lower than 200 mg/d1. All recipient animals had a decrease in blood glucose
levels, two
of the animals-maintained blood glucose levels close to 250 mg/di, with one
animal
having levels as low as 180 mg/di on day 85 of the experiment. See FIG. 5.
As cells from normal pancreatic tissue display very similar characteristics to
the
cells generated from T1D pancreas samples, ie: triple positive for CD133,
glucagon and
insulin, it is reasonable to expect that the cellular compositions comprising
insulin and
glucagon secreting cells generated from a T1D pancreatic tissue sample treated
with
islet cell culture medium comprising a polypeptide according to SEQ NO: 1 or 2
will
provide a measurable reduction in blood glucose levels in vivo. Moreover,
implantations
of cellular compositions comprising insulin and glucagon secreting cells have
been
shown to be non-oncogenic, making them a desirable option for treatment of
pancreatic
disorders by transplantation (by one of injection, infusion, engraftment).
Example 7
Method of cultivation, viability testing and cryopreservation of insulin and
glucagon secreting islet cells.
In one embodiment, an islet cell culture medium comprises CMRL-1066
(Mediatech, #99-663-CV; Transplant Medium (CMRL 1066) without phenol red)
supplemented with: 10% Heat Inactivated Fetal Calf Serum (Gibco, #16140071),
10%
Human Serum (Gemini, #100512),L-Glutamine (2mM, Gibco, #25030081),
Ciprofloxacin
(2m1/L, Bioworld, #403100313), Amphotericin-B (0.1mg/L, Gibco,
#15290026),Penicillin-
Streptomycin (100,000 U/L-100,000 pg/L, Gibco, #10378016), and a peptide
according
27
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
to SEQ ID 1 or 2 (range of 3 - 20 pg/ml, such as, 3 pg/ml, 5 pg/ml, or 10.0
pg/ml). In
another embodiment, a cell culture medium for cryopreservation comprises a
cryoprotectant medium (Gibco #12648010). In another embodiment, viability
testing was
carried out using methods comprising utilizing fluoroscein diacetate (FDA)
(Sigma
Aldrich #F7378) and propidium Iodide (PI) (Sigma Aldrich #P4170)
In one embodiment, human pancreatic tissue was collected via needle biopsy
from human donors affected by Type 1 diabetes or severe pancreatitis and
cultured in
vitro in islet cell culture medium comprising CMRL 1066 supplemented with 10%
fetal
calf serum, 10% human serum, 2 mmol/L-glutamine, antibiotics, and a peptide
according to SEQ ID NO 1 or 2 at a concentration range of 3 pg/mL to 20 pg/ml,
such
as 10.0 pg/mL, on plates (or flasks) coated with an attachment factor mixture
(AFM)
comprising Collagen Type I and Endothelial Cell Attachment Factor (ECAF).
Various
ratios of ECAF and collagen were used, including a 50/50 ratio of collagen to
ECAF. A
thin layer of AFM (between 3 ¨ 10 ml) was applied and after setting for 30
minutes the
excess AFM was removed; the plates were dried for 45 minutes in a hood. Plates
are
then washed with Phosphate Buffer Solution (PBS) to remove any potential
contaminants. The collected pancreatic tissue was incubated on AFM-treated
flasks/plates in islet cell culture medium for 10 to 20 days until cells began
to mobilize
and proliferate. Cultured cells are split when they reached confluency of
approximately
70 - 80% in culture using standard aseptic techniques in a vertical laminar
flow hood.
The splitting technique involved removal of supernatant from the culture
plates
(supernatant was preserved). Plates are then washed with 2 ¨ 5 ml PBS (wash
was
preserved). The cultured cells were detached using approximately 3 ¨ 5 ml of
trypsin
28
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
(25% solution) by incubating the cells in the presence of trypsin at 37 C for
approximately 3 ¨ 5 minutes until cells detached. The plate was then washed a
second
time with PBS. The trypsinized cells, as well as the preserved PBS washes and
collected cell culture supernatant, were then centrifuged at 1000 rpm for 7
minutes at
4 C. The resulting supernatant was decanted and the pellet resuspended in 2 ml
PBS,
and centrifuged. The supernatant was then removed, and the pellet resuspended
in islet
cell culture medium comprising SEQ ID 1 01 2, and re-plated at a cell density
of ¨ 1000
cells/cm2. Cultured cells that were not used for replating were resuspended at
the
maximum concentration of 1x106/m1 in a cryopreservation medium. Cultured cells
were
centrifuged (1000 rpm for 7 minutes) at 4 C. The supernatant was aspirated and
replaced by fresh cryopreservation medium and the cells were transferred into
a
freezing container Mr. Frosty (Thermo-Fisher #5100-0036) overnight and then
transferred and stored in vapor phase liquid nitrogen.
Cells were subject to a viability assay prior to cryopreservation. The minimal
viability
accepted for cryopreservation = 95% (thus 95% of all cells analyzed were
stained with
FDA, viable fluorescence dye).
Two aliquots of approximately 200 cells were transferred in approximately 50
pl
volume in a solution of 400 pl containing FDA and PI at the concentration of
0.46 pM for
FDA and 14.34 pM for PI, in an Eppendorf tube. Cells were centrifuged (1000
rpm for 7
minutes) and approximately 95% of the Supernatant was aspirated and cells were
transferred in the residual fluid in a microscope slide using a micropipette.
Cells were
analyzed under a fluorescence microscope (Olympus model CKX3) using a
green/red
filter set. Viable cells stain in green (FDA) and dead cells in red (P1).
Percent of viable
29
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
cells over total (expressed in percentage) was determined by two operators
independently.
Although the foregoing invention has been described in some detail by way of
illustration and example for purposes of clarity of understanding, it will be
obvious that
certain changes and modifications may be practiced within the scope of the
appended
claims. It will be clear to a person skilled in the art that features
described in relation to
any of the aspects and various embodiments described above can be applicable
interchangeably between the different embodiments.
ASPECTS
Aspect 1. A composition comprising an insulin and glucagon secreting cell
population generated from non-insulin secreting pancreatic cells collected via
needle
biopsy from a Type 1 diabetic donor pancreas, a pancreatitis donor pancreas,
or a
combination thereof.
Aspect 2. The composition of Aspect 1, wherein at least about 50% of the
insulin
and glucagon secreting cell population expresses CD133, glucagon, and insulin.
Aspect 3. The composition of any one of Aspects 1 ¨ 2, wherein about 50% to
about 100% of insulin and glucagon secreting cell population expresses CD133,
glucagon, and insulin, including all values in between, such as, for example,
about 55%,
about 60%, about 65%, about 70%, about 75%, about 80%, about 85%, about 90%,
and about 95%; specify percent as a total of medium cell population).
Aspect 4. The composition of any one of Aspects 1 ¨3, wherein the insulin and
glucagon secreting cell population comprises from about 3 x 106 cells to about
25 x 106
cells per kg of body weight in a human recipient, including all values in
between, such
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
as, for example about 4 x 106 cells, about 5 x 106 cells, about 6 x 106 cells,
about 7 x
106 cells, about 8 x 106 cells, about 9 x 106 cells, about 10 x 106 cells,
about 11 x 106
cells, about 12 x 106 cells, about 13 x 106 cells, about 14 x 106 cells, about
15 x 106
cells, about 16 x 106 cells, about 17 x 106 cells, about 18 x 106 cells, about
19 x 106
cells, about 20 x 106 cells, about 21 x 106 cells, about 22 x 106 cells, about
23 x 106
cells, and about 24 x 106 cells; where the mass (in kg) for typical human
recipient
depends on, for example, age height, and may range from about 4 kg to about
225 kg,
including all values in between, such as about 10 kg, about 20 kg, about 30
kg, 4 about
0 kg, about 50 kg, about 60 kg, about 70 kg, about 80 kg, about 90 kg, about
100 kg,
about 110 kg, about 120 kg, about 130 kg, about 140 kg, about 150 kg, about
160 kg,
about 170 kg, about 180 kg, about 190 kg, about 200 kg, about 210 kg, and
about 220
kg.
Aspect 5. The composition of any one of Aspects 1 ¨ 4, wherein the non-insulin
secreting pancreatic cells are collected via needle biopsy are obtained from a
Type 1
diabetic donor pancreas.
Aspect 6. The composition of any one of Aspects 1 ¨ 5, wherein the non-insulin
secreting pancreatic cells collected via needle biopsy are obtained from a
pancreatitis
donor pancreas.
Aspect 7. The composition of any one of Aspects1 ¨ 6, wherein the non-insulin
secreting pancreatic cells are isogenic, allogenic, or a combination thereof.
Aspect 8. A method of treating a pancreatic disorder, comprising administering
to
a subject in need thereof a therapeutically effective amount of the
composition of any
31
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
one of Aspects1 ¨ 7, wherein the pancreatic disorder comprises Type 1
diabetes,
pancreatitis, or a combination thereof.
Aspect 9. The method of Aspect 8, wherein said administering comprises
delivering to the subject the therapeutically effective amount of the
composition of any
one of Aspects1 ¨7 via one or more of an injection, infusion, omental or
peritoneal
pouch, surgical implantation, or via packaging the composition as part of a
device to a
target site in the subject.
Aspect 10. A method for preparing a composition comprising an insulin and
glucagon secreting cell population, which comprises: treating in vitro a
population of
non-insulin secreting Type 1 diabetic pancreatic cells with an islet cell
culture medium
comprising a base medium and an effective amount of a polypeptide comprising
SEQ
ID NO. 1, an active fragment of SEQ ID No. 1, SEQ ID NO. 2, an active fragment
of
SEQ ID No. 2, or a combination thereof.
Aspect 11. The method of Aspect 10, wherein the treating further comprises
differentiating the population of non-insulin secreting Type 1 diabetic
pancreatic cells
and propagating the insulin and glucagon secreting cell population.
Aspect 12. The method of Aspect 10, wherein the treating further comprises
differentiating the population of non-insulin secreting Type 1 diabetic
pancreatic cells
and propagating the insulin and glucagon secreting cell population, wherein
the insulin
and glucagon secreting cell population at least about 50% of insulin and
glucagon
secreting cell population expresses CD133, glucagon, and insulin.
Aspect 13. The method of Aspect 10, wherein the treating further comprises
differentiating the population of non-insulin secreting Type 1 diabetic
pancreatic cells
32
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
and propagating the insulin and glucagon secreting cell population, wherein
the insulin
and glucagon secreting cell population about 50% to about 100% of insulin and
glucagon secreting cell population expresses C0133, glucagon, and insulin.
Aspect 14. The method of Aspect 10, wherein the treating further comprises
differentiating the population of non-insulin secreting Type 1 diabetic
pancreatic cells
and propagating the insulin and glucagon secreting cell population, wherein
the insulin
and glucagon secreting cell population comprises from about 3 x 106 cells to
about 25 x
106 cells per kg of body weight in a human recipient.
Aspect 15. The method of any one of Aspects 10 ¨ 14 further comprising
extracting the population of non-insulin secreting Type 1 diabetic pancreatic
cells from a
donor.
Aspect 16. The method of any one of Aspects 10 ¨ 14 further comprising
extracting the population of non-insulin secreting Type 1 diabetic pancreatic
cells from a
donor via needle biopsy.
Aspect 17. The method of any one of Aspects 10 ¨ 16 further comprising
extracting the population of non-insulin secreting Type 1 diabetic pancreatic
cells from
an isogenic donor, and allogenic donor, or a combination thereof.
Aspect 18. The method of any one of Aspects 10¨ 17, wherein the islet cell
culture medium comprises the polypeptide in amount that ranges from about 3
pg/mL to
about 20 pg/mL and all values in between, such as, for example, about 4 pg/mL,
about
pg/mL, about 6 pg/mL, about 7 pg/mL, about 8 pg/mL, about 9 pg/mL, about 10
pg/mL, about 11 pg/mL, about 12 pg/mL, about 13 pg/mL, about 14 pg/mL, about
15
pg/mL, about 16 pg/mL, about 17 pg/mL, about 18 pg/mL, and about 19 pg/mL.
33
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
Aspect 19. The method of any one of Aspects 10¨ 19, wherein the treating
further comprises differentiating the population of non-insulin secreting Type
1 diabetic
pancreatic cells and propagating the insulin and glucagon secreting cell
population to
obtain a therapeutically amount of the insulin and glucagon secreting cell
population
and the method further comprises administering to a subject in need thereof
the
therapeutically effective amount of the insulin and glucagon secreting cell
population.
Aspect 20. A composition (e.g., any one of Aspects 1 ¨7) comprising an insulin
and glucagon secreting cell population generated from non-insulin secreting
pancreatic
cells collected via needle biopsy from a Type 1 diabetic donor pancreas for
use in the
treatment of Type 1 diabetes, pancreatitis, or a combination thereof.
Although the foregoing information highlights aspects disclosed herein by way
of
illustration and example for purposes of clarity of understanding, it will be
obvious that
certain changes and modifications may be practiced within the scope of the
subject
matter claimed herein. It will be clear to a person skilled in the art that
features
described in relation to any of the aspects and various embodiments described
above
can be applicable interchangeably between the different embodiments.
The aspects and embodiments described above are examples to illustrate
various features of the subject matter claimed herein. All publications and
patent
applications disclosed herein are indicative of the level of those skilled in
the art to
which this disclosure and the subject matter of the claims pertains.
Throughout the description and claims of this specification, the words
"comprise"
and "contain" and variations of them mean "including but not limited to", and
they are
not intended to (and do not) exclude other moieties, additives, components, or
steps.
34
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
Throughout the description and claims of this specification, the singular
encompasses
the plural unless the context otherwise requires. In particular, where the
indefinite article
is used, the specification is to be understood as contemplating plurality as
well as
singularity, unless the context requires otherwise.
Features, characteristics, compounds, chemical moieties, or groups described
in
conjunction with a particular aspect, embodiment, or example are to be
understood to
be applicable to any other aspect, embodiment or example described herein
unless
incompatible therewith. All of the features disclosed in this specification
(including any
accompanying claims, abstract and drawings), and/or all of the steps of any
method or
process so disclosed, may be combined in any combination, except combinations
where at least some of such features and/or steps are mutually exclusive. The
subject
matter claimed herein is not restricted to the details of any foregoing
embodiments. The
subject matter claimed herein extends to any novel one, or any novel
combination, of
the features disclosed in this specification (including any accompanying
claims, abstract
and drawings), or to any novel one, or any novel combination, of the steps of
any
method or process so disclosed.
All publications and patent applications are herein incorporated by reference
to
the same extent as if each individual publication or patent application was
specifically
and individually to be incorporated by reference. Specific patent applications
incorporated by reference include, for example, United States Patent
Application No.
15/811,060, filed on November 13, 2017 (and published as US 2018/0133280 Al);
and
International Patent Application No. PCT/U52019/038305 filed on June 20, 2019
(and
published as WO 2020/005721 Al). To the extent that terms and/or expressions
CA 03231258 2024- 3-7
WO 2023/049079
PCT/US2022/044024
incorporated herein conflict with the terms and/or expression disclosed
herein, the
information disclosed herein controls.
36
CA 03231258 2024- 3-7