Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/076307
PCT/US2022/047771
STABILIZED HEMEPROTEIN COMPOSITIONS AND METHODS OF USE
THERE OF
CROSS REFERENCE TO RELATED APPLICATIONS
[001] The present application claims priority from the U.S. Provisional
Application No.
63/271,423, filed on October 25, 2021, the entire contents of which are hereby
incorporated by
reference.
FIELD
[002] Compositions for use in non-animal food products comprising one or
more purified
hemeproteins and one or more antioxidants, methods of making, and methods of
use thereof
BACKGROUND
[003] Consumer demand for alternatives to animal-based foods, such as meat,
eggs, and
milk, continues to rise because of growing environmental, health, and ethical
concerns
associated with the rearing and slaughter of livestock animals. In response to
these demands,
the food and biotechnology sectors have developed a number of innovative
approaches to
designing animal-free food products; however, many of these non-animal based
alternatives to
animal proteins do not fully emulate the sensory and functional properties of
animal proteins.
[004] Current difficulties with creating non-animal sourced food products
include
generating products having the appropriate color and taste profile. As an
example, there has
been great interest in finding non-animal alternatives to the hemeproteins
normally found in
meat, such as myoglobin, because these proteins provide a desirable red color
and meaty flavor
comparable to conventional animal-based foods. The characteristic red color
associated with
hemeproteins is determined by the protein's redox state, which depends on
environmental
conditions, such as pH, ionic strength, temperature, and oxygen levels. The
problem in isolating
hemeproteins from plant sources or producing them using cellular agriculture
approaches (e.g.,
fermentation), is that the resulting isolated hemeproteins are highly
susceptible to chemical
degradation during storage and utilization and the desired red color and taste
profile is lost soon
after production. As such, there is a need to develop non-animal based
hemeproteins with
increased stability, thereby extending their quality and shelf-life while
maintaining a color and
taste profile that emulates animal-based foods such as muscle-based meat
products.
1
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
SUMMARY OF THE INVENTION
[005] The present disclosure provides novel compositions suitable for use
in food
products wherein the compositions herein have improved color stability and
reduced protein
degradation (e.g., aggregation) over time.
[006] Embodiments of the present disclosure provide compositions for use in
a food
product comprising one or more purified hemeprotein compositions and one or
more
antioxidants, wherein the one or more purified hemeprotein compositions
comprise a
hemeprotein from a non-animal source.
[007] In certain embodiments, one or more purified hemeprotein compositions
herein
may comprise a globin. In some embodiments, one or more purified hemeproteins
compositions herein may comprise a leghemoglobin, non-symbiotic hemoglobin,
chlorocruorin, erythrocruorin, protoglobin, cytochrome, cyanoglobin,
flavohemoglobin,
myoglobin, phytoglobin, or any combination thereof
[008] In certain embodiments, one or more purified hemeproteins
compositions herein
may comprise a hemeprotein from a genetically modified non-animal source. In
some
embodiments, a genetically modified non-animal source herein may comprise a
genetically
modified plant, a genetically modified bacteria, a genetically modified yeast,
or any
combination thereof
[009] In some embodiments the current disclosure comprises hemeprotein
composition
for use in a food product comprising one or more purified hemeprotein and one
or more
antioxidants. In some embodiment, the one or more purified hemeprotein
comprises a globin.
In some aspects, the one or more purified hemeprotein comprise leghemoglobin,
non-symbiotic
hemoglobin, chlorocruorin, erythrocruorin, protoglobin, cyto chro me,
cyanoglobin,
av oh em ogl bin, my ogl obi n, phytogl obi n, or any combination thereof. In
some embodiments,
the hemeprotein compositions comprise a purified hemeprotein from a
genetically modified
source. In some aspects, the source comprises a genetically modified plant, a
genetically
modified bacteria, a genetically modified yeast, or any combination thereof.
In some
embodiments, the one or more purified hemeprotein compositions comprise a
polypeptide
expressed and/or secreted from a source, wherein the source comprises plants,
fungi, bacteria,
yeasts, algae, archaea, genetically modified plants, genetically modified
fungi, genetically
modified bacteria, genetically modified yeasts, genetically modified algae,
genetically
2
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
modified archaea_ or any combination thereof In some aspects, the
polynucleotide sequence
encoding the hemeprotein is derived from animals, plants, fungi, bacteria,
yeasts, algae,
archaea, or any combination thereof
10101
In certain embodiments, one or more antioxidants herein may comprise
antioxidant
vitamins, polyphenols, or any combination thereof. In some embodiments, one or
more
antioxidants herein may comprise vitamin C, vitamin E, any derivatives
thereof, or any
combination thereof In some embodiments, one or more antioxidants herein may
comprise
flavonoids or any derivatives thereof In some embodiments, one or more
antioxidants herein
may comprise isorhamnetin, kaempferol, myricetin, proanthocyanidins,
quercetin, rutin,
taxifolin, catechin, gall ocatechin, gall ocatechin gallate esters,
epicatechin, epigallocatechin,
epigallocatechin gallate esters, theaflavin, theaflavin gallate esters,
thearubigins, or any
combination thereof In some embodiments, one or more antioxidants herein may
have a
reduction potential less than about 500 mV.
[011] In certain embodiments, compositions herein may comprise a ratio of
the total
amount by weight of one or more purified hemeproteins to the total amount of
one or more
antioxidants as about 1:1 to about 30:1, for example about 1:1, about 2:1,
about 3:1, about 4:1,
about 5:1, about 6:1, about 7:1, about 8:1, about 9:1, about 10:1, about 11:1,
about 12:1, about
13:1, about 14:1, about 15:1, about 16:1, about 17:1, about 18:1, about 19:1,
about 20:1, about
21:1, about 22:1, about 23:1, about 24:1, about 25:1, about 26:1, about 27:1,
about 28:1, about
29:1, or about 30:1. In certain embodiments, hemeprotein compositions herein
may be stable
for at least 7 days at about 4 C.
[012] In certain embodiments, hemeprotein compositions herein may comprise
one or
more hemeproteins herein that may comprise a heme group bound to oxygen, a
heme group
bound to carbon monoxide, or a combination thereof for at least 7 days. In
certain
embodiments, hemeprotein composition results in an increase in peak height of
the UV-visible
absorption spectrum for the heme protein compositions at one or more of about
550 nm and
about 582 nm In some embodiments, the increase in peak height of the UV-
visible absorption
spectrum for the one or more heme protein compositions at one or more of about
550 nm and
about 582 nm is detectable for at least about 7 days after addition of the one
or more
antioxidants.
[013] In certain embodiments, the hemeprotein compositions herein may
comprise one or
more purified hemeproteins from a genetically modified non-animal source and
one or more
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
antioxidants herein that stabilizes the oxidation state of the one or more
purified hemeproteins.
In some embodiments, hemeprotein compositions herein may comprise one or more
purified
hemeproteins from a genetically modified non-animal source such as genetically
modified plant, a genetically modified bacteria, or a genetically modified
yeast, and one or
more antioxidants herein that stabilizes the oxidation state of the one or
more purified
hemeproteins.
[014] In certain embodiments, one or more purified hemeproteins herein may
be
recombinant hemeproteins produced from a genetically modified non-animal
source. In some
embodiments, recombinant hemeproteins herein may be encoded from a
polynucleotide,
wherein the polynucleotide may comprise an nucleic acid sequence from a plant,
animal,
fungus, or bacteria, encoding a hemeprotein. In some embodiments, the nucleic
acid sequence
encoding a hemeprotein herein may be derived from a legume. In some
embodiments, the
nucleic acid sequence encoding a hemeprotein herein may be derived from a
leopard, a bovine,
or a whale. In some embodiments, recombinant hemeproteins herein may be
recombinant
globin. In some embodiments, recombinant hemeproteins herein may be
recombinant
leghemoglobin, non-symbiotic hemoglobin, chlorocruorin, erythrocruorin,
protoglobin,
cytochrome, cyanoglobin, flavohemoglobin, myoglobin, phytoglobin, or any
combination
thereof
[015] In certain embodiments, one or more purified hemeproteins herein may
be
recombinant hemeproteins produced from a genetically modified yeast source,
wherein the
recombinant hemeproteins may be encoded from a polynucleotide, wherein the
polynucleotide
may comprise an endogenous nucleic acid sequence for a hemeprotein derived
from a legume,
an equine, a leopard, a bovine, a whale, or any combination thereof
[016] Other embodiments of the present disclosure provide for methods of
making any of
the hemeprotein compositions disclosed herein. In certain embodiments, methods
herein may
stabilize an oxidative state of one or more purified hemeproteins from a non-
animal source
herein by combining the one or more purified hemeproteins with one or more
antioxidants. In
some embodiments, methods herein may stabilize the visual appearance of the
purified
hemeproteins for at least 7 days. In some embodiments, methods herein may
stabilize the
purified hemeproteins from aggregation at pH ranging from about 5 to about 9.
In some
embodiments, methods herein may stabilize the oxidation state of the purified
hemeproteins
for at least 7 days. In certain embodiments, methods of making a hemeprotein
composition
4
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
herein for use in a food product may comprise obtaining one or more purified
hemeproteins
from a non-animal source and combining the one or more purified hemeproteins
with at least
one or more antioxidants herein. In some embodiments, methods herein may
comprise
recombinantly producing the one or more purified hemeproteins from a
genetically
modified non-animal source. In some embodiments, methods herein may comprise
combining
hemeproteins herein with about 0.01% to about 10% by weight of the composition
of the one
or more antioxidants. In some embodiments, methods herein may comprise
combining
antioxidants herein with about 1% to about 99% by weight of the composition of
the one or
more purified hemeproteins. In some embodiments, the method herein may
comprise
combining a comprise combining antioxidants herein with one or more purified
hemeproteins
wherein the hemeprotein composition results in an increase in peak height of
the UV-visible
absorption spectrum for the one or more hemeprotein compositions at one or
more of about
550 nm and about 582 nm, that is detectable for at least about 7 days after
addition of the one
or more antioxidants. In some embodiments, methods herein may further comprise
combining
hemeproteins and antioxidants herein with in a buffer solution, wherein the
buffer solution may
have a pH ranging from about 5 to about 9.
[017] In certain embodiments, the current disclosure also encompasses
composition
comprising one or more purified hemeprotein compositions and one or more
antioxidants,
wherein the one or more purified hemeprotein compositions comprise a
hemeprotein from a
genetically modified non-animal source selected from the group consisting of a
genetically
modified plant, a genetically modified bacteria, or a genetically modified
yeast; and wherein
the composition results in an increase in peak height of the UV-visible
absorption spectrum for
the one or more heme protein compositions at one or more of about 550 nm and
about 582 nm
that is detectable for at least about 7 days after addition of the one or more
antioxidants. In
certain embodiments, the one or more antioxidants has a reduction potential
ranging from about
280 my to about 500 mV. In certain embodiments, the one or more antioxidants
is selected
from ascorbic acid, quercetin, taxifolin, Trolox, and combinations thereof. In
certain
embodiments, the composition comprises a weight ratio of the one or more
purified
hemeprotein to the one or more antioxidants of about 1:1 to about 30:1 (for
example about 1:1,
about 2:1, about 3:1, about 4:1, about 5:1, about 6:1, about 7:1, about 8:1,
about 9:1, about
10:1, about 11:1, about 12:1, about 13:1, about 14:1, about 15:1, about 16:1,
about 17:1, about
18:1, about 19:1, about 20:1, about 21:1, about 22:1, about 23:1, about 24:1,
about 25:1, about
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
26:1, about 27:1, about 28:1, about 29:1, or about 30:1).
[018] In certain embodiments, the hemeprotein of the compositions comprising
the
hemeprotein composition are encoded from a polynucleotide, wherein the
polynucleotide
comprises an endogenous nucleic acid sequence for a hemeprotein derived from a
plant,
animal, fungus, or bacteria source. In certain embodiments the endogenous
nucleic acid
sequence for the hemeprotein is derived from a legume. In certain embodiments,
the
endogenous nucleic acid sequence for the hemeprotein is derived from an animal
source
selected from a group consisting of equine, leopard, bovine, or whale. In
certain embodiments,
the hemeprotein is a globin selected from a group consisting of leghemoglobin,
non-symbiotic
hemoglobin, chlorocruorin, erythrocruorin, protoglobin, cytochrome,
cyanoglobin,
flavohemoglobin, my oglobin, and phytoglobin. In certain embodiemnst, wherein
the one or
more purified hemeproteins are recombinant hemeproteins produced from a
genetically
modified yeast source, wherein the recombinant hemeproteins are encoded from a
polynucleotide, wherein the polynucleotide comprises an endogenous nucleic
acid sequence
for a hemeprotein derived from a legume, an equine, a leopard, a bovine, a
whale, or any
combination thereof. In certain embodiments, the hemeprotein composition
comprises a
myoglobin and wherein combining the one or more purified hemeprotein
compositions with
one or more antioxidants causes an increase in the relative amount of
oxymyoglobin and
carboxymyoglobin to metmyoglobin in the composition. In certain embodiments,
the increase
in relative amount of oxymyoglobin and carboxymyoglobin to metmyoglobin in the
composition is detectable for at least about 7 days after addition of the
antioxidant. In certain
embodiments, the increase in the relative amount of oxymyoglobin and
carboxymyoglobin to
metmyoglobin in the composition ranges from about 1.1-fold to about 5-fold.
10191 In certain embodiments the current disclosure encompasses a hemeprotein
composition
comprising one or more purified hemeprotein and one or more antioxidants,
wherein the one
or more purified hemeprotein compositions comprise a hemeprotein from a
genetically
modified source selected from the group consisting of a genetically modified
plant, a
genetically modified bacteria, a genetically modified fungi, or a genetically
modified yeast;
and wherein the composition results in an increase in peak height of the UV-
visible absorption
spectrum for the one or more heme protein compositions at one or more of about
550 nm and
about 582 nm that is detectable for at least about 7 days after addition of
the one or more
6
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
antioxidants.
[020] In some embodiments, the hemeprotein is encoded by a polynucleotide
comprising a
nucleic acid sequence from a plant, animal, fungus, or bacteria. In some
embodiments, the
polynucleotide comprises a nucleic acid sequence derived from a legume,
wherein the nucleic
acid sequence encodes a hemeprotein. In some embodiments, the polynucleotide
sequence
comprises a nucleic acid sequence derived from a group consisting of an
equine, a feline, a
bovine, or a whale. In some embodiments, the hemeprotein is a globin selected
from a group
consisting of leghemoglobin, non-symbiotic hemoglobin, chlorocruorin,
erythrocruorin,
protoglobin, cytochrome, cyanoglobin, flavohemoglobin, myoglobin, and
phytoglobin. In
some embodiments, the one or more purified hemeproteins are recombinant
hemeproteins
produced from a genetically modified yeast source, wherein the recombinant
hemeproteins are
encoded from a polynucleotide, comprising a nucleic acid sequence derived from
a legume, an
equine, a leopard, a bovine, a whale and encoding a hemeprotein.
[020] In certain embodiments, the current disclosure also encompasses a
composition
comprising one or more purified hemeprotein compositions and one or more
antioxidants,
wherein the one or more purified hemeprotein compositions comprise
leghemoglobin, non-
symbiotic hemoglobin, chlorocruorin, erythrocruorin, protoglobin, cytochrome,
cyanoglobin,
flavohemoglobin, myoglobin, phytoglobin, or any combination thereof; wherein
the one or
more antioxidants is selected from the group consisting of ascorbic acid,
quercetin, taxifolin,
Trolox, and combinations thereof; wherein the composition comprises a weight
ratio of the one
or more purified hemeprotein to the one or more antioxidants of about 1:1 to
about 30:1 (for
example: about 1:1, about 2:1, about 3:1, about 4:1, about 5:1, about 6:1,
about 7:1, about 8:1,
about 9:1, about 10:1, about 11:1, about 12:1, about 13:1, about 14:1, about
15:1, about 16:1,
about 17:1, about 18:1, about 19:1, about 20:1, about 21:1, about 22:1, about
23:1, about 24:1,
about 25:1, about 26:1, about 27:1, about 28:1, about 29:1, or about 30:1),
and wherein the
composition results in an increase in peak height of the UV-visible absorption
spectrum for the
one or more hemeprotein compositions at one or more of about 550 nm and about
582 nm, that
is detectable for at least about 7 days after addition of the one or more
antioxidants.
[021] Other embodiments of the present disclosure include meat substitute
(e.g., meat replica)
compositions and methods of making thereof In some embodiments, methods of
preparing a
meat substitute comprise combining any of the compositions herein with a meat
replica matrix.
7
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
In some embodiments, methods that comprise combining any of the compositions
herein to a
meat replica matrix may result in the composition imparting a meat-like (e.g.,
a beef-like)
appearance to the meat substitutes herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[022]
The patent or application file contains at least one drawing executed in
color. Copies
of this patent or patent application publication with color drawing(s) will be
provided by the
Office upon request and payment of the necessary fee. The following drawings
form part of
the present specification and are included to further demonstrate certain
embodiments of the
present disclosure, which can be better understood by reference to the drawing
in combination
with the detailed description of specific embodiments presented herein.
Embodiments of the
present inventive concept are illustrated by way of example in which like
reference numerals
indicate similar elements and in which as follows.
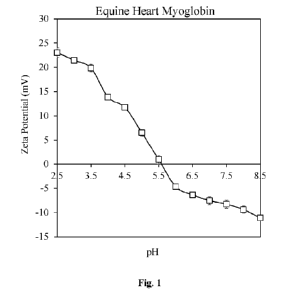
10231
Fig. 1 depicts a representative graph of zeta-potential versus pH profile
of equine
heart myoglobin (1 mg/mL) measured by electrophoresis.
[024]
Fig. 2 depicts a representative graph of protein content of equine heart
myoglobin
(1 mg/mL) at pH 2.5 to 8.5.
10251
Fig. 3A depicts a representative graph of absorption spectra and
representative
image of visual appearance of equine heart myoglobin solutions (-1 mg/mL)
stored at 4 C for
0 days.
[026]
Fig. 3B depicts a representative graph of absorption spectra and
representative
image of visual appearance of equine heart myoglobin solutions (-1 mg/mL)
stored at 4 C for
1 days.
[027]
Fig. 3C depicts a representative graph of absorption spectra and
representative
image of visual appearance of equine heart myoglobin solutions (-1 mg/mL)
stored at 4 C for
2 days.
[028]
Fig. 3D depicts a representative graph of absorption spectra and
representative
image of visual appearance of equine heart myoglobin solutions (-1 mg/mL)
stored at 4 C for
3 days.
[029]
Fig. 3E depicts a representative graph of absorption spectra and
representative
image of visual appearance of equine heart myoglobin solutions (-1 mg/mL)
stored at 4 C for
8
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
4 days.
[030]
Fig. 3F depicts a representative graph of absorption spectra and
representative
image of visual appearance of equine heart myoglobin solutions (-1 mg/mL)
stored at 4 C for
days.
[031] Fig. 4A depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions samples stored at 4 C in
combination with
(1 mM) Ascorbic Acid.
[032] Fig. 4B depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
samples stored at 4 C in combination with (1 mM) Ascorbic Acid.
[033] Fig. 4C are a series of representative images of visual appearance
over time (from
day 0 to day 27 as indicated) of equine heart myoglobin solutions samples
stored at 4 C in
combination with (1 mM) Ascorbic Acid.
[034] Fig. 4D depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions samples stored at 4 C in
combination with
(1 mM) Quercetin.
[035] Fig. 4E depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
samples stored at 4 C in combination with (1 mM) Quercetin.
10361
Fig. 4F are a series of representative images of visual appearance over
time (from
day 0 to day 27 as indicated) of equine heart myoglobin solutionssamples
stored at 4 C in
combination with (1 mM) Quercetin.
[037] Fig. 4G depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions (1 mM) samples stored at 4 C
in combination
with (1 mM) Epigallocatechin gallate (EGCG).
[038] Fig. 4H depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
samples stored at 4 C in combination with (1 mM) EGCG.
[039] Fig. 41 depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions samples stored at 4 C in
combination with
(1 mM) Taxifolin.
9
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[040] Fig. 4J depicts a representative graph of absorption spectra
intensity (at 544 nm and
582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
samples stored at 4 C in combination with (1 mM) Taxifolin.
[041] Fig. 4K depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions samples stored at 4 C in
combination with
(1 mM) 4-Methylcatechol.
[042] Fig. 4L depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
samples stored at 4 C in combination with (1 mM) 4-Methylcatechol.
[043] Fig. 4M are a series of representative images of visual appearance
over time (from
day () to day 27 as indicated) of equine heart myoglobin solutions ( I mM)
samples stored at
4 C in combination with antioxidants EGCG, Taxifolin and Methylcatechol
respectively.
[044] Fig. 4N depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions (1 mM) samples stored at 4 C
in combination
with Trolox.
[045] Fig. 40 depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
(1 mM) samples stored at 4 C in combination with Trolox.
[046] Fig. 4P depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions (1 mM) samples stored at 4 C
in combination
with Caffeic Acid.
[047] Fig. 4Q depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
(1 mM) samples stored at 4 C in combination with Caffeic Acid.
[048] Fig. 4R depicts a representative graph of absorption spectra (from
day 0 to day 27
as indicated) of equine heart myoglobin solutions (1 mM) samples stored at 4 C
in combination
with Gallic Acid.
[049] Fig. 4S depicts a representative graph of absorption spectra
intensity (at 544 nm
and 582 nm) over time (from day 0 to day 27 as indicated) of equine heart
myoglobin solution
(1 mM) samples stored at 4 C in combination with Gallic Acid.
[050] Fig. 4T are a series of representative images of visual appearance
over time (from
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
day 0 to day 27 as indicated) of equine heart myoglobin solutions (1 m1VI)
samples stored at
4 C in combination with antioxidants Trolox, Caffeic Acid and Gallic Acid
respectively.
[051] Fig. 5A depict representative graphs of zeta potential of recombinant
leopard,
bovine, whale, and soy hemeprotein samples in solutions having a pH of 2.5,
3.5, 4.5, 5.5, 6.5,
7.5, and 8.5.
[052] Fig. 5B are a series of representative images of visual appearance of
of
recombinant leopard, bovine, whale, and soy hemeprotein samples in solutions
having a pH of
2.5, 3.5, 4.5, 5.5, 6.5, 7.5, and 8.5.
[053] Fig. 6 depicts a representative graph showing the impact of pH on the
solubility of
my oglobin solutions (0.5 mg/mL) produced by cellular agriculture.
[054] Fig. 7A depicts representative graphs of absorption spectra of a 1
mg/mL solution
of recombinant hemeprotein from Panthera pardus (leopard) stored at 4 C for 27
days.
[055] Fig. 7B depicts representative graphs of absorption spectra of a
5mg/mL solution
of recombinant hemeprotein from Panthera pardus (leopard) stored at 4 C for 27
days.
[056] Fig. 7C are a series of representative images of visual appearance of
1 mg/mL and
5mg/mL solutions of recombinant hemeprotein from Panthera pardus (leopard)
stored at 4 C
for 27 days.
[057] Fig. 7D depicts representative graphs of absorption spectra of a 1
mg/mL solution
of recombinant hemeprotein from Bos taurus (bovine) stored at 4 C for 27 days.
[058] Fig. 7E depicts representative graphs of absorption spectra of a
5mg/mL solution
of recombinant hemeprotein from Bos taurus (bovine) stored at 4 C for 27 days.
[059] Fig. 7F are a series of representative images of visual appearance of
1 mg/mL and
5mg/mL solutions of recombinant hemeprotein from Bos taurus (bovine) stored at
4 C for 27
days.
[060] Fig. 7G depicts representative graphs of absorption spectra of a 1
mg/mL solution
of recombinant hemeprotein from Physeter rnacrocephalus (sperm whale) stored
at 4 C for 27
days.
[061] Fig. 7H depicts representative graphs of absorption spectra of a
5mg/mL solution
of recombinant hemeprotein from Physeter macrocephalus (sperm whale) stored at
4 C for 27
days.
[062] Fig. 71 are a series of representative images of visual appearance of
1 mg/mL and
11
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
5mg/mL solutions of recombinant hemeprotein from Physeter macrocephalus (sperm
whale)
stored at 4 C for 27 days.
[063] Fig. 7J depicts representative graphs of absorption spectra of a 1
mg/mL solution
of recombinant hemeprotein from soy leghemoglobin stored at 4 C for 27 days.
[064] Fig. 7K depicts representative graphs of absorption spectra of a
5mg/mL solution
of recombinant hemeprotein from soy leghemoglobin stored at 4 C for 27 days.
[065] Fig. 7L are a series of representative images of visual appearance of
1 mg/mL and
5mg/mL solutions of recombinant hemeprotein from soy leghemoglobin stored at 4
C for 27
days.
DETAILED DESCRIPTION
[066] The following detailed description references the accompanying
drawings that
illustrate various embodiments of the present inventive concept. The drawings
and description
are intended to describe embodiments and embodiments of the present inventive
concept in
sufficient detail to enable those skilled in the art to practice the present
inventive concept. Other
components can be utilized and changes can be made without departing from the
scope of the
present inventive concept. The following description is, therefore, not to be
taken in a limiting
sense. The scope of the present inventive concept is defined only by the
appended claims, along
with the full scope of equivalents to which such claims are entitled.
I. Terminology
[067] The phraseology and terminology employed herein are for the purpose
of
description and should not be regarded as limiting. For example, the use of a
singular term,
such as, "a" is not intended as limiting of the number of items. Also, the use
of relational terms
such as, but not limited to, "top," "bottom," "left," "right," "upper,"
"lower," "down," "up,"
and -side," are used in the description for clarity in specific reference to
the figures and are not
intended to limit the scope of the present inventive concept or the appended
claims.
[068] Further, as the present inventive concept is susceptible to
embodiments of many
different forms, it is intended that the present disclosure be considered as
an example of the
principles of the present inventive concept and not intended to limit the
present inventive
concept to the specific embodiments shown and described. Any one of the
features of the
present inventive concept may be used separately or in combination with any
other feature.
References to the terms -embodiment," -embodiments," and/or the like in the
description mean
12
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
that the feature and/or features being referred to are included in, at least,
one aspect of the
description. Separate references to the terms "embodiment," "embodiments,"
and/or the like in
the description do not necessarily refer to the same embodiment and are also
not mutually
exclusive unless so stated and/or except as will be readily apparent to those
skilled in the art
from the description. For example, a feature, structure, process, step,
action, or the like
described in one embodiment may also be included in other embodiments, but is
not necessarily
included. Thus, the present inventive concept may include a variety of
combinations and/or
integrations of the embodiments described herein. Additionally, all
embodiments of the present
disclosure, as described herein, are not essential for its practice. Likewise,
other systems,
methods, features, and advantages of the present inventive concept will be, or
become, apparent
to one with skill in the art upon examination of the figures and the
description. It is intended
that all such additional systems, methods, features, and advantages be
included within this
description, be within the scope of the present inventive concept, and be
encompassed by the
claims.
[069] As used herein, the term "about," can mean relative to the recited
value, e.g.,
amount, dose, temperature, time, percentage, etc., 10%, 9%, 8%, 7%, 6%,
5%, 4%,
3%, 2%, or 1%.
[070] The terms -comprising," -including," -encompassing" and -having" are
used
interchangeably in this disclosure. The terms "comprising," "including,"
"encompassing" and
-having" mean to include, but not necessarily be limited to the things so
described.
[071] The terms "or" and "and/or," as used herein, are to be interpreted as
inclusive or
meaning any one or any combination. Therefore, "A, B or C" or "A, B and/or C"
mean any of
the following: "A," "B- or -C"; "A and B"; "A and C"; "B and C";
B and C." An exception
to this definition will occur only when a combination of elements, functions,
steps or acts are
in some way inherently mutually exclusive.
[072] The term "nucleic acid" or "polynucleotide- refers to
deoxyribonucleic acids
(DNA) or ribonucleic acids (RNA) and polymers thereof in either single- or
double-stranded
form. Unless specifically limited, the term encompasses nucleic acids
containing known
analogues of natural nucleotides that have similar binding properties as the
reference nucleic
acid and are metabolized in a manner similar to naturally occurring
nucleotides. Unless
otherwise indicated, a particular nucleic acid sequence also implicitly
encompasses
conservatively modified variants thereof (e.g., degenerate codon
substitutions), alleles,
13
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
orthologs, SNPs, and complementary sequences as well as the sequence
explicitly indicated.
Specifically, degenerate codon substitutions may be achieved by generating
sequences in
which the third position of one or more selected (or all) codons is
substituted with mixed-base
and/or deoxyinosine residues (Batzer et al., Nucleic Acid Res. 19:5081(1991);
Ohtsuka et al.,
I. Biol. Chem. 260:2605-2608 (1985); and Rossolini et al., Mol. Cell. Probes
8:91-98 (1994)).
[073] The terms -peptide," -polypeptide," and "protein" are used
interchangeably, and
refer to a compound comprised of amino acid residues covalently linked by
peptide bonds. A
protein or peptide must contain at least two amino acids, and no limitation is
placed on the
maximum number of amino acids that can comprise a protein's or peptide's
sequence.
Polypepti des include any peptide or protein comprising two or more amino
acids joined to each
other by peptide bonds. As used herein, the term refers to both short chains,
which also
commonly are referred to in the art as peptides, oligopeptides and oligomers,
for example, and
to longer chains, which generally are referred to in the art as proteins, of
which there are many
types. "Polypeptides" include, for example, biologically active fragments,
substantially
homologous polypeptides, oligopeptides, homodimers, heterodimers, variants of
polypeptides,
modified polypeptides, derivatives, analogs, fusion proteins, among others. A
polypeptide
includes a natural peptide, a recombinant peptide, or a combination thereof
[074] As used herein, -recombinant" refers to a cell, nucleic acid,
protein, or vector,
which has been modified due to the introduction of an exogenous nucleic acid
or the alteration
of a native nucleic acid. The nucleic acid can be of genomic, cDNA,
semisynthetic, and/or
synthetic origin, which, by virtue of its origin or manipulation, is not
associated with all or a
portion of the polynucleotide with which it is associated in nature.
[075] "Transformation" refers to the transfer of a nucleic acid fragment
into a host
organism or the genome of a host organism, resulting in genetically stable
inheritance. Host
organisms containing the transformed nucleic acid fragments are referred to as
"recombinant",
"transgenic" or "transformed" organisms. Thus, isolated polynucleotides of the
present
invention can be incorporated into recombinant constructs, typically DNA
constructs, capable
of introduction into and replication in a host cell. Such a construct can be a
vector that includes
a replication system and sequences that are capable of transcription and
translation of a
polypeptide-encoding sequence in a given host cell. Typically, expression
vectors include, for
example, one or more cloned genes under the transcriptional control of 5' and
3' regulatory
sequences and a selectable marker. Such vectors also can contain a promoter
regulatory region
14
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
(e.g., a regulatory region controlling inducible or constitutive,
environmentally- or
developmentally-regulated, or location-specific expression), a transcription
initiation start site,
a ribosome binding site, a transcription termination site, and/or a
polyadenylation signal.
10761
"Stabilization" or "stabilized" in the context of hemeprotein compositions
provided
herein, refers to a composition wherein the combination of hemeprotein with an
antioxidant
causes a lasting increase in the levels of oxygenated hemeprotein or carboxy
hemeprotein, as
indicated by a change in visual appearance to a more red composition, a change
in the oxidation
state (from Met state to oxygen or Carbon monooxide bound state), or a change
in UV-Visible
spectra such that the peak height at 550 nm (corresponding to CarboxyMb),
and/or at 582nm
(corresponding to OxyMb) increases in comparison to a composition without
antioxidant. In
some aspects, the increase in the peak height can be detected within 1-48
hours of combining
the antioxidant with the hemeprotein. This period between adding the
antioxidant and a
detectible increase in the peak height is referred to as the lag-time or lag-
phase. In some aspects,
depending on the antioxidant used and the storage conditions, the increase in
peak height can
last or is "stably" maintained for about 0.5 days to about 90 days, though in
some embodiments,
the absolute peak height may decrease through this stable period.
[077] "Cellular Agriculture- is a method of producing animal products from
cell culture,
rather than animals using a combination of biotechnology, tissue engineering,
molecular
biology, and synthetic biology to create and design new methods of producing
proteins, fats,
and tissues that would otherwise come from traditional agriculture. In the
context of the current
application, in some aspects, hemeproteins can be sourced and purified from
cellular
agriculture.
[078] As used herein, a polynucleotide encoding a hemeprotein "derived
from" or which
is a -derivative of' an endogenous polynucleotide refers to a polynucleotide
related to the
endogenous polynucleotide by sequence. In some aspects the polynucleotide may
be a variant
or comprise a fragment of the endogenous polynucleotide and may comprise
mutations,
insertions, deletions, truncations, modifications, or combinations thereof
compared to an
endogenous polynucleotide. In some aspects, the polynucleotide may comprise a
nucleic acid
sequence at least about 60% identical to an endogenous polynucleotide or a
fragment thereof,
encoding a hemeprotein.
[079] As used herein, the term "source" refers to an organism that
comprises a
polynucleotide sequence encoding an endogenous or a recombinant hemeprotein
which can be
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
purified for use in the compositions and methods disclosed herein. In some
aspects, a source
can comprise a polynucleotide sequence that encodes an endogenous hemeprotein,
for example
Glycine max (soybean) comprises a polynucleotide sequence encoding soy
leghemoglobin
which can be purified and used in the compositions and methods disclosed
herein. In some
aspects, the source may be a genetically modified source. As used herein, the
term "genetically
modified source" refers to a recombinant organism for example a genetically
modified plant,
genetically modified fungi, genetically modified bacteria, genetically
modified yeast,
genetically modified algae, genetically modified archaea comprising a
polynucleotide
sequence encoding a hemeprotein and from which the hemeprotein can be purified
for use in
the compositions and methods of the current disclosure. In some aspects, the
recombinant
organism comprises a polynucleotide sequence that is at least about 60%
identical to an
endogenous polynucleotide or a fragment thereof from a plant or at least about
60% identical
to an endogenous polynucleotide or a fragment thereof from a bovine, equine,
feline or whale.
[080] It should also be understood that, unless clearly indicated to the
contrary, in any
methods claimed herein that include more than one step or act, the order of
the steps or acts of
the method is not necessarily limited to the order in which the steps or acts
of the method are
recited.
II. Compositions
[081] The present disclosure provides for hemeprotein compositions suitable
for use in
food products having improved color stability and reduced protein degradation
over time. In
some embodiments, the current disclosure results from the surprising result
that addition of
certain antioxidants to these compositions greatly increases the desirable
characteristics of the
hemeproteins. In some embodiments, compositions herein may have one or more
purified
hemeproteins and one or more antioxidants.
A. Hemeproteins
[082] In certain embodiments, hemeprotein compositions suitable for use in
food products
herein may have one or more purified hemeproteins. As used herein, the term -
hemeprotein"
includes any polypeptide that can covalently or noncovalently bind to a heme
moiety. In some
embodiments, hemeproteins herein may be a monomer (i.e., a single polypeptide
chain), a
dimer, a trimer, tetramer, a higher order oligomer, or any combination thereof
[083] In some embodiments, hemeproteins herein may be a globin. Non-
limiting
16
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
examples of globins that can covalently or noncovalently bind to a heme moiety
for use herein
can include an androglobin, a cytoglobin, a globin E, a globin X, a globin Y,
a hemoglobin, a
myoglobin, an erythrocruorin, a beta hemoglobin, an alpha hemoglobin, a
protoglobin, a
cyanoglobin, a histoglobin, a neuroglobin, a chlorocruorin, a truncated
hemoglobin (e.g., HbN,
HbO, a truncated 2/2 globin, a hemoglobin 3 (e.g., Glb3)), a cytochrome, or a
peroxidase. In
accordance with certain embodiments herein, globins may have a globin fold
having a series
of about seven to about nine alpha helices. In accordance with certain
embodiments herein,
globins may be of any class (e.g., class I, class II, or class III). In
accordance with certain
embodiments herein, globins may transport and/or store oxygen.
[084] In some embodiments, hemeproteins herein may have an oxygenated Fe +
state
similar to that of globin (e.g., myoglobin). In some embodiments, hemeproteins
herein may
have an oxygenated Fe (iron) state higher than globin (e.g., myoglobin). In
some
embodiments, hemeproteins herein may have an oxygenated Fe + state about 10%,
20%, 30%,
40%, 50%, 100% or higher than globin (e.g., myoglobin). In some embodiments,
hemeproteins
herein may be similar to oxymyoglobin. As used herein "oxymyoglobin" refers to
the
oxygenated form of myoglobin which is a single chain globular protein.
[085] In some embodiments, hemeproteins herein may be a non-symbiotic
hemoglobin, a
leghemoglobin, a chlorocruorin, an erythrocruorin, a protoglobin, a
cytochrome, a
cyanoglobin, a flavohemoglobin, a myoglobin, a phytoglobin, or any combination
thereof.
[086] In some embodiments, hemeproteins herein may be derived from non-
animal
sources. Non-limiting examples of non-animal sources include plants, fungi,
bacteria, yeasts,
algae, archaea, genetically modified organisms such as genetically modified
bacteria, plants,
or yeast, chemical or in vitro synthesis. In some embodiments, hemeproteins
herein may be a
polypeptide derived from non-animal sources. In some embodiments, hemeproteins
herein may
be a polypeptide expressed and/or secreted from a non-animal source. In some
embodiments,
hemeproteins herein may be a polypeptide expressed and/or secreted from a non-
animal source
wherein the polypeptide may be encoded from a polynucleotide derived from
animals, plants,
fungi, bacteria, yeasts, algae, archaea, or any combination thereof.
10871
In some embodiments, hemeproteins herein may be isolated from or may be
encoded from a polynucleotide derived from a -wild-type" source. A wild-type
source of
hemeproteins herein may be mammals, fish, birds, plants, algae, fungi (e.g.,
yeast or
filamentous fungi), ciliates, bacteria, or any combination thereof
17
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[088]
In some embodiments, hemeproteins herein may be isolated from or may be
encoded from a polynucleotide derived from a mammal belonging to any of the 27
orders of
mammalian species, the orders including: Afrosoricida; Carnivora;
Cetartiodactyla;
Chiroptera; Cingulata; Dasyuromorphia; Dermoptera; Didelphimorphia;
Diprotodontia;
Eulipotyphla; Hyracoidea; Lagomorpha; Macroscelidea; Microbiotheria;
Monotremata;
Notoryctemorphia; Paucituberculata; Peramelemorphia; Perissodactyla;
Pholidota; Pilosa;
Primates; Proboscidea; Rodentia; Scandentia; Sirenia; and Tubulidentata.
10891
In some embodiments, hemeproteins herein may be isolated from or may be
encoded from a polynucleotide derived from human, non-human primate (e.g.,
gibbon, rhesus
macaque, bonobo, chimpanzee, gorilla, orangutan, lemur, loris, tarsier),
bovinae (e.g., cow,
zebu, bison, water buffalo, African buffalo, antelopes), ovine, caprine,
camelid, canine (e.g.,
domestic dog, wolves, coyotes, jackals, foxes), cetacean (e.g., whales,
dolphins, porpoises),
feline (e.g., domestic cat, tiger, lion, cheetah, leopard, jaguar, bobcat,
caracal, margay, oncilla,
cougar, serval, ocelot, lynx, puma), equine (e.g., horses, donkeys, mules,
zebras), marsupial,
or from any other mammal of interest. In some embodiments, hemeproteins herein
may be
isolated from or may be encoded from a polynucleotide derived from a mammal
such as a cow,
goat, sheep, horse, pig, ox, mule, rabbit, yak, llama, camel, deer, cat, dog,
bear, or any
combination thereof
[090] In some embodiments, hemeproteins herein may be isolated from or may
be
encoded from a polynucleotide derived from a bird. In some embodiments,
hemeproteins
herein may be isolated from or may be encoded from a polynucleotide derived
from
Anseriformes (e.g., ducks, swans, geese), Falconiformes (e.g., falcons,
eagles, hawks)
Galliformes (e.g. chickens, turkeys, pheasants), Struthioniformes (e.g., emus,
ostriches, kiwis),
Passeriformes (e.g., perching birds and songbirds such as sparrows, larks,
crows, swallows,
and the like), Sphenisciformes (e.g., penguins), Pelecaniformes (e.g., Ibis,
herons, pelicans),
Strigiformes (e.g., owls), Gaviiformes (e.g., loons), Gruiformes (e.g.,
terrestrial, marsh birds),
or any combination thereof.
[091] In some embodiments, hemeproteins herein may be isolated from or may
be
encoded from a polynucleotide derived from a fish. In some embodiments,
hemeproteins herein
may be isolated from or may be encoded from a polynucleotide derived from a
Scombridae
(e.g., tuna), Salmonidae (e.g., salmon), Gadidae (e.g., cod, haddock),
Clupeidae (e.g., herrings,
shads, sardines, hilsa, menhadens), Engraulidae (e.g., anchovies), and the
like. In some
18
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
embodiments, hemeproteins herein may be isolated from or may be encoded from a
polynucleotide derived from shrimp, oysters, clams, mussels, and the like.
[092] In some embodiments, hemeproteins herein may be isolated from or may
be
encoded from a polynucleotide derived from a plant. In some embodiments,
hemeproteins
herein may be isolated from or may be encoded from a polynucleo tide derived
from Nicotianu
tabacum or Nicotiana sylvestris (tobacco); Zea mays (corn), Arabidopsis
thaliana, a legume
such as Glycine max (soybean), Cicer arietinum (garbanzo or chick pea), Pisum
sativum (pea)
varieties such as garden peas or sugar snap peas, Phase lus vulgaris varieties
of common beans
such as green beans, black beans, navy beans, northern beans, or pinto beans,
Vigna
unguiculata varieties (cow peas), Vigna radiate (Mung beans), Lupinus albus
(lupin), or
Medicago scItivct (alfalfa); Brass/ca nctpus (canola); Triticum sps. (wheat,
including wheat
berries, and spelt); Gossypium hirsutum (cotton); Oryza sativa (rice); Zizania
sps. (wild rice);
Helianthus annuus (sunflower); Beta vulgaris (sugarbeet); Pennisetum glaucum
(pearl millet);
Chenopodium sp. (quinoa); Sesamum sp. (sesame); Linum usitatissimum (flax); or
Hordeum
vulgare (barley).
[093] In some embodiments, hemeproteins herein may be isolated from or may
be
encoded from a polynucleotide derived from fungi. In some embodiments,
hemeproteins herein
may be isolated from or may be encoded from a polynucleotide derived from
Saccharomyces
cerevisiae, Pichia pastoris, Magnaporthe oryzae, Fusarium graminearum, or
Fusarium
oxysporum.
[094] In some embodiments, hemeproteins herein may be isolated from or may
be
encoded from a polynucleotide derived from bacteria. In some embodiments,
hemeproteins
herein may be isolated from or may be encoded from a polynucleotide derived
from
Escherichia coli, Bacillus subtilis, Synechocistis sp., Aquifex aeolicus,
Methylacidiphilum
infernorum, or thermophilic bacteria such as Thermophilus.
[095] In some embodiments, hemeproteins herein may be isolated from or may
be
encoded from a polynucleotide derived from non-symbiotic hemoglobin. In some
embodiments, hemeproteins herein may be non-symbiotic hemoglobins isolated
from or
encoded from a polynucleotide derived from soybean, sprouted soybean, alfalfa,
golden flax,
black bean, black eyed pea, northern, garbanzo, moong bean, cowpeas, pinto
beans, pod peas,
quinoa, sesame, sunflower, wheat berries, spelt, barley, wild rice, rice, or
any combination
thereof
19
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[096] In some embodiments, hemeproteins described herein may have an amino
acid
sequence corresponding to a wild-type hemeprotein, fragments, truncations,
variants or fusions
thereof that contain a heme-binding motif One of skill in the art will
appreciate that the amino
acid sequences of any of the wild-type hemeproteins contemplated herein can be
found in
sequence databases such as, but not limited to, the UniProtKB/Swiss-Prot
database and the
Heme Protein Database. In some embodiments, hemeproteins described herein may
have an
amino acid sequence with at least 60% (e.g., at least 65%, 70%, 75%, 80%, 85%,
90%, 95%,
97%, 98%, 99%, or 100%) sequence identity to the amino acid sequence
corresponding to a
wild-type hemeprotein, fragments, truncations, variants or fusions thereof
that contain a heme-
binding motif. One of skill in the art will appreciate how to determine the
percent identity
between two amino acid sequences using methods known in the art. Such methods
include, but
are not limited to, use of a BLAST 2 Sequences (B12seq) program provided by
the National
Center for Biotechnology Information (www.ncbi.nlm.nih.gov) and the like.
[097] In certain embodiments, hemeproteins herein may be from a genetically
modified non-animal source. In accordance with certain embodiments herein, a
genetically
modified non-animal source may be a genetically modified plant, a genetically
modified
bacteria, a genetically modified yeast, or any combination thereof In some
embodiments,
hemeproteins herein may be recombinant hemeproteins. As used herein -
recombinant
hemeproteins" refers to hemeproteins recombinantly produced using polypeptide
expression
techniques (e.g., heterologous expression techniques using bacterial cells,
insect cells, fungal
cells such as yeast, plant cells such as tobacco, soybean, or Arabidopsis, or
mammalian cells).
In some embodiments, recombinant hemeproteins herein may be a polypeptide
encoded from
a polynucleotide, wherein the polynucleotide may have an endogenous (i.e.,
wild-type) nucleic
acid sequence for a hemeprotein derived from a plant, animal, fish, bird,
fungus, or bacteria
source as described herein. In accordance with certain embodiments herein, an
endogenous
nucleic acid sequence for the hemeprotein may be derived from a plant. In
accordance with
certain embodiments herein, an endogenous nucleic acid sequence for the
hemeprotein may be
derived from a legume. In accordance with certain embodiments herein, an
endogenous nucleic
acid sequence for the hemeprotein may be derived from a mammal. In accordance
with certain
embodiments herein, an endogenous nucleic acid sequence for the hemeprotein
may be derived
from an equine, feline (e.g_, leopard), bovine, or cetacean (e.g., whale).
[098] In some embodiments, standard polypeptide synthesis techniques (e.g.,
liquid-phase
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
polypeptide synthesis techniques or solid-phase polypeptide synthesis
techniques) may be used
to produce any of the recombinant hemeproteins herein. In some embodiments, in
vitro
transcription-translation techniques may be used to produce any of the
recombinant
hemeproteins herein
[099]
In some embodiments, recombinant hemeproteins may be expressed by a
microbial
expression system. In some embodiments, microbial expression systems for use
herein may
comprise at least one expression vector. Microbial expression systems and
expression vectors
containing regulatory sequences that direct high level expression of
recombinant proteins are
well known to those skilled in the art, any of which may be used to produce
the any one of the
gene products (e.g., recombinant hemeproteins) of the polynucleotides
disclosed herein.
Vectors or cassettes useful for the transformation of suitable host cells are
well known in the
art. -Expression vector" or -expression construct" or -plasmid" or
"recombinant DNA
construct- refers to a vehicle for introducing a nucleic acid into a host
cell. A nucleic acid for
use herein can be one that has been generated via human intervention,
including by
recombinant means or direct chemical synthesis, with a series of specified
nucleic acid
elements that permit transcription and/or translation of a particular nucleic
acid. In some
embodiments, expression vectors for use herein can be part of a plasmid,
virus, or nucleic acid
fragment, or other suitable vehicle. In some embodiments, expression vectors
for use herein
may further include a nucleic acid to be transcribed operably linked to a
promoter.
[0100] In accordance with certain embodiments herein, vectors comprising
polynucleotides disclosed herein can be introduced into appropriate
microorganisms (i.e., host
cells) via transformation techniques to provide high-level expression of the
recombinant
hemeproteins for use herein. Expression of a polypeptide (e.g., recombinant
hemeprotein) of
the disclosure may include transient expression and/or constitutive expression
(e.g., developing
of a stable cell line) in a suitable host cell. Host cells herein may be
transformed by any suitable
technique including, e.g., biolistics, electroporation, glass bead
transformation and silicon
carbide whisker transformation. Any convenient technique for introducing a
transgene into a
microorganism can be employed in the present invention. Transformation can be
achieved by,
for example, the method of D. M. Morrison (Methods in Enzymology 68, 326
(1979)), the
method by increasing permeability of recipient cells for DNA with calcium
chloride (Mandel.
M. and Higa, A., J. Mol. Biol., 53, 159 (1970)), or the like.
[0101]
In some embodiments, a suitable host cell for production of recombinant
21
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
hemeproteins herein may be from a genetically modified organism. In certain
embodiments, a
genetically modified organism herein may be a bacterium, a yeast, a fungus, an
algae, a
mammalian cell, an insect cell, or any combination thereof In some
embodiments, a suitable
host cell for production of recombinant hemeproteins herein may be from a
genetically
modified plant, a genetically modified bacteria, and/or a genetically modified
yeast. In certain
embodiments, a genetically engineered organism suitable for production of
recombinant
hemeproteins herein may be Acetobacter, Acinetobacter calcoaceticus.
Alcaligenes eutropha,
Arxtila adeninivorans, Aspergillus nidulans, Aspergillus niger, Aspergillus
orzyae, Asper gillus
terreus, Aurantiochytriurn spp., Bacillus licheniforms, Bacillus methanolicus,
Bacillus
stearothermophilus, Bacillus subtilis, Candida utilis, Chlamydomonas
reinharchii, Clostridium
acetobutylicum, Clostridium thermocellum, Corynebacterium glutcunicum,
Escherichict coli,
Hansenula polymorpha, Isochrysts spp., Kluyveromyces lactis, Kluyveromyces
marnanus,
Lactococcus lactis, Micrococcus lysodeikticus, Nannochloropsis spp., Ogatctea,
Paracoccus
denitrifi cans, Pavlova spp., Penicillium chrysogenum, Pichia guilliermondii,
Pichia pastoris,
Pichia stipitis, Pseudomonas putida, Rhizopus spp., Rhodoporidium spp.,
Rhodotorula spp.,
Saccharomyces cerevisiae, Schizosaccharomyces pombe, Streptococcus lactis,
Streptomyces,
Synechococcus elongatus, Tetraselmis spp.,
Thermoanaerobcicter spp.,
Ihermoanaerobacterium spp., Trichoderma reesei, Xanthaomonas campestris,
and/or
Yarrawia lipolytica.
[0102]
In some embodiments, following introduction of a polynucleotide comprising
the
coding sequence for a hemeprotein of the disclosure, a host cell may be
cultured in conventional
nutrient media modified as appropriate for activating promoters, selecting
transformants,
and/or amplifying expression of a polypeptide-encoding polynucleotide. In some
embodiments, following the introduction of a polynucleotide comprising the
coding sequence
for a hemeprotein of the disclosure, a host cell may be cultured by
fermentation. Culturing may
be accomplished in a growth medium having one or more supplements to aid in
culture growth
including, but not limited to, aqueous mineral salts medium, organic growth
factors, carbon
and/or energy source material, molecular oxygen, and the like. In some
embodiments,
polypeptides (e.g., hemeproteins) may be recovered from the culture (e.g., by
centrifugation,
purification, etc.), and purified as described herein.
[0103]
In certain embodiments, hemeproteins for use herein may be purified
hemeproteins.
As used herein, the term -purified" refers to a polypeptide or protein (e.g.,
a hemeprotein) that
22
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
has been separated from other components of the source material (e.g., other
animal, fish, plant,
fungal, algal, bacterial, genetically modified plant, genetically modified
bacteria, or genetically
modified yeast proteins). In some embodiments, purified hemeproteins herein
may be free of
least about 2% to about 100% (e.g., about 5%, 10%, 20%, 25%, 30%, 35%, 40%,
45%, 50%,
55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 99%, 100%) of the other
components of
the source material. Hemeproteins herein can be purified using methods of
protein separation
known in the art, including but not limited to, size exclusion chromatography,
affinity
chromatography, anion exchange chromatography, cation exchange chromatography,
ultrafiltration through membranes, or density centrifugation, isoelectric
precipitation,
ammonium sulfate precipitation, i s o el ectri c precipitation, surfactants,
detergents, and solvent
extraction.
B. Antioxidants
[0104]
In certain embodiments, compositions suitable for use in food products
herein may
have one or more purified hemeproteins and one or more antioxidants. As used
herein,
"antioxidants" are agents that inhibit oxidation and thus can be used to
prevent the deterioration
of preparations by the oxidative process. In some embodiments, antioxidants
suitable for use
herein may be an antioxidant vitamin, a polyphenol, or any combination thereof
[0105]
In some embodiments, an antioxidant herein may be a naturally occurring or
a
synthetic form of a vitamin having antioxidant properties. In some
embodiments, an
antioxidant vitamin may be vitamin C, a derivative thereof, and/or an analogue
thereof In
accordance with certain embodiments herein, an antioxidant vitamin may be
ascorbic acid, L-
ascorbic acid, ethylated L-ascorbic acid, vitamin C, or of the erythorbic acid
isomer thereof, or
salts or esters thereof In some embodiments, an antioxidant vitamin may be
vitamin E, a
derivative thereof, and/or an analogue thereof Vitamin E is a group of eight
fat soluble
compounds that include four tocopherols and four tocotrienols. In accordance
with certain
embodiments herein, an antioxidant vitamin may be alpha-Tocopherol, beta-
Tocopherol,
gamm a-To coph erol , d el ta-Tocoph erol , Tocopheryl acetate, RRR-al ph a-to
coph erol , S SR-
alpha-tocopherol, alpha-tocotrienol, vitamin E, or salts or esters thereof.
101061
In some embodiments, an antioxidant herein may be a naturally occurring or
a
synthetic form of a polyphenol having antioxidant properties. Polyphenols are
common
constituents of foods of plant origin and contribute the major antioxidants
found in diets_ The
main dietary sources of polyphenols include, but are not limited to, fruits,
vegetables, and
23
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
beverages (e.g., coffee). Non-limiting examples of antioxidants are
polyphenolic compounds,
chlorogenic acids, flavonoids, tocopherols, di- or tri-carboxylic acids (such
as citric acid),
EDTA (ethylenediaminetetraacetic acid), ascorbic acid (vitamin C),
anthocyanins, catechins,
quercetin, resveratrol, rosmarinic acid, camosol, Maillard reaction products,
enzymes such as
superoxide dismutase, certain proteins, amino acids, and protein hydrolyzates,
etc. Several
thousand different polyphenols have been identified in foods. In some
embodiments,
antioxidant polyphenols herein may be a naturally occurring or a synthetic
form of a flavonoid
having antioxidant properties. Non-limiting examples flavonoids include
quercetin (found in
onion, tea, apple), catechin (tea, fruit), hesperidin (citrus fruits), and
cyanidin (red fruits). In
some embodiments, antioxidant flavonoids herein may be isorhamnetin,
kaempferol,
myricetin, proanthocyanidins, quercetin, rutin, taxifolin, catechin,
gallocatechin, gallocatechin
gallate esters, epicatechin, epigallocatechin, epigallocatechin gallate
esters, theaflavin,
theaflavin gallate esters, thearubigins, or any combination thereof In some
embodiments,
antioxidant polyphenols herein may be a naturally occurring or a synthetic
form of a phenolic
acids having antioxidant properties. A non-limiting example of a phenolic acid
includes caffeic
acid which is present in many fruits and vegetables. Caffeic acid, most often
esterified with
quinic acid as in chlorogenic acid, is the major phenolic compound in coffee.
[0107]
In some embodiments, an antioxidant herein may be ascorbic acid, ascorbyl
palmitate, butylated hydroxyanisole, butylated hydroxytoluene, hypophosphorous
acid,
monothioglycerol, propyl gallate, sodium ascorbate, sodium bisulfite, sodium
formaldehyde
sulfoxylate and sodium metabisulfite and other materials known to one of
ordinary skill in the
art.
[0108]
In some preferred embodiments, an antioxidant herein may be ascorbic acid,
quercetin, epigallocatechin gallate, EGCG, trolox, taxifolin, 4-methycatechol,
caffeic acid,
gallic acid, or any combination thereof.
[0109]
In some embodiments, antioxidants for use herein may have one or more
characteristics that impart a desirable property to the compositions described
herein. In some
embodiments, antioxidants for use herein may have a strong reducing potential.
Standard
reduction potential describes the ability of a compound to accept electrons.
In some
embodiments, antioxidants for use herein may have reduction potential less
than about 500 mV
(e.g., about 0.5, 1, 10, 25, 50, 75, 100, 125, 150, 175, 200, 225, 250, 275,
300, 325_ 350, 375,
400, 425, 450, 475, 500 mV). In some embodiments, antioxidants for use herein
may have
24
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
reduction potential less than about 200, or 250, or 300, or 350, or 400, or
450, or 500 mV.
C. Hemeprotein Compositions
[OHO]
In some embodiments, hemeprotein compositions as described herein may
include
one or more purified hemeproteins. In some embodiments, the compositions
described herein
may comprise about 1% to about 99% (e.g., about 1%, about 5%, about 10%, about
20%, about
30%, about 40%, about 50%, about 60%, about 70%, about 80%, about 90%, about
95%, about
99%) by weight of the composition of one or more purified hemeprotein
compositions, as
disclosed herein. As used herein, a "hemeprotein composition" includes the
hemeprotein
hydrated or in solution. In certain embodiments, the hemeprotein content can
be calculated on
a dry basis, meaning the hemeprotein content and concentration is determined
with the liquid
from the hemeprotein composition removed. In such embodiments, the
compositions described
herein may comprise about 1% to about 99% (e.g., about 1%, about 5%, about
10%, about
20%, about 30%, about 40%, about 50%, about 60%, about 70%, about 80%, about
90%, about
95%, about 99%) by weight of the composition of one or more purified
hemeproteins on a dry
weight basis.
101111
In some embodiments, the compositions described herein may comprise about
0.01% to about 10% (e.g., about 0.01%, about 0.1%, about 0.5%, about 1%, about
2%, about
3%, about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, about 10%) by
weight of
the composition of one or more antioxidants disclosed herein. In some
embodiments,
compositions herein may comprise about 1% to about 99% (e.g., about 5%, 10%,
20%, 30%,
40%, 50%, 60%, 70%, 80%, 90%, 95%, 99%) by weight of the composition of one or
more
purified hemeproteins disclosed herein and about 0.01% to about 10% (e.g.,
about 0.01%,
0.1%, 0.5%, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%) by weight of the
composition of
one or more antioxidants disclosed herein. In some embodiments, compositions
herein may
comprise a ratio of total hemeprotein amount to total antioxidant amount. In
some
embodiments, compositions herein may have a weight ratio of total hemeprotein
composition
content to antioxidant content ranging from about 1:1 to about 30:1. In
certain embodiments,
the eight ratio of total hemeprotein content to total antioxidant content is
about 1:1 to about
30:1, for example about 1:1, about 2:1, about 3:1, about 4:1, about 5:1, about
6:1, about 7:1,
about 8:1, about 9:1, about 10:1, about 11:1, about 12:1, about 13:1, about
14:1, about 15:1,
about 16:1, about 17:1, about 18:1, about 19:1, about 20:1, about 21:1, about
22:1, about 23:1,
about 24:1, about 25:1, about 26:1, about 27:1, about 28:1, about 29:1, or
about 30:1..
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[0112]
In certain embodiments, the composition comprises a leghemoglobin and one
or
more antioxidants. In some embodiments, the composition comprises a soy
leghemoglobin
and one or more antioxidant selected from any one of Quercetin. Ascorbic acid,
EGCG, Trolox
or Taxifolin. In some embodiments, the compositions herein may have a weight
ratio of total
soy leghemoglobin content to total antioxidant content of about 1:1, about
2:1, about 3:1, about
4:1, about 5:1, about 6:1, about 7:1, about 8:1, about 9:1, about 10:1, about
11:1, about 12:1,
about 13:1, about 14:1, about 15:1, about 16:1, about 17:1, about 18:1, about
19:1, or about
20:1.. In some additional embodiments, the composition comprises a myoglobin
and one or
more antioxidants. In some embodiments, the composition comprises one or more
myoglobin
selected from an equine, bovine, feline (for example leopard) or whale (for
example, sperm
whale) and one or more antioxidant selected from any one of Quercetin,
Ascorbic acid, EGCG,
Trolox or Taxifolin. In some embodiments, the compositions herein may have a
weight ratio
of total soy leghemoglobin content to total antioxidant content of about 1:1,
about 2:1, about
3:1, about 4:1, about 5:1, about 6:1, about 7:1, about 8:1, about 9:1, about
10:1, about 11:1,
about 12:1, about 13:1, about 14:1, about 15:1, about 16:1, about 17:1, about
18:1, about 19:1,
or about 20:1.
[0113]
In some embodiments, hemeprotein compositions herein may have one or more
purified hemeproteins as disclosed herein and one or more antioxidants as
disclosed herein in
a buffer solution. A buffer solution for use herein may be any solution
suitable for use in a food
product. In some embodiments, hemeprotein compositions herein may also include
a buffer
solution having or more buffering agents wherein "buffering agents" are
compounds used to
resist change in pH upon dilution or addition of acid or alkali. Buffering
agents for use herein
can include, by way of example and without limitation, potassium
metaphosphate, potassium
phosphate, monobasic sodium acetate and sodium citrate anhydrous and dihydrate
and other
materials known to one of ordinary skill in the art. In some embodiments, any
food-grade
organic or inorganic buffer can be used. In some embodiments, compositions
disclosed herein
may comprise at least 5%, at least 10%, at least 20%, at least 25%, at least
30%, at least 35%,
at least 40%, at least 45%, at least 50% total amount of one or more buffering
agents by total
weight of the composition. In some embodiments, the amount of one or more
buffering agents
may depend on the desired pH level of compositions herein. In some
embodiments, buffer
solutions herein may have a pH ranging from about 5 to about 9 (e.g., about 5,
5.5, 6, 6.5, 7,
7.5, 8, 8.4, 8.5, 9). In some embodiments, compositions disclosed herein may
have a pH
26
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
ranging from about 4 to about 9 (e.g., about 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5,
8, 8.5, 9).
[0114]
In some embodiments, the hemeprotein compositions herein may comprise
additional components for example, binding agents, flavor enhancers,
oligosaccharides,
stabilizing agents, pH regulators, preservatives, non-heme proteins, dietary
fibers, gelling
agents, surfactants, water, fats, oils emulsifiers, starches, coloring agents
and combinations
thereof In some embodiments, the additional components may include for
example, one or
more of, glucose, fructose, ribose, arabinose, glucose-6-phosphate, fructose 6-
phosphate,
fructose 1,6-diphosphate, inositol, maltose, sucrose, maltodextrin, glycogen,
nucleotide-bound
sugars, molasses, a phospholipid, a lecithin, inosine, inosine monophosphate
(IMP), guanosine
monophosphate (GMP), pyrazine, adenosine monophosphate (AMP), lactic acid,
succinic acid,
glycolic acid, thiamine, creatine, pyrophosphate, vegetable oil, algal oil,
corn oil, soybean oil,
palm fruit oil, palm kernel oil, safflower oil, flaxseed oil, rice bran oil,
cottonseed oil, sunflower
oil, canola oil, olive oil, a free fatty acid, cysteine, methionine,
isoleucine, leucine, lysine,
phenylalanine, threonine, tryptophan, valine, arginine, histidine, alanine,
asparagine, aspartate,
glutamate, glutamine, glycine, proline, serine, tyrosine, glutathione, an
amino acid derivative,
a protein hydrolysate, a malt extract, a yeast extract, vitamins, dietary
fibers like vegetable
fibers from carrots, bamboo, peas, broccoli, potatoes, sweet potatoes, corn,
whole grains,
alfalfa, collard greens, celery, celery root, parsley, cabbage, squash, green
beans, common
beans, black beans, red beans, white beans, beets, cauliflower, nuts, apple
peels, oats, wheat or
plantain, or mixtures thereof, onion flavor, garlic flavor, or herb flavors,
basil, celery leaves,
chervil, chives, cilantro, parsley, oregano, tarragon, thyme, spice extracts,
spice oils, natural
smoke solutions, natural smoke extracts, yeast extract, and shiitake extract.
[0115]
In some embodiments, the hemeprotein compositions herein comprising one or
more of the antioxidants described herein and one or more of the hemeproteins
described herein
can be formulated into for example, liquids, gels, pastes, sauces, powder or
cubes, ingredients
of flavor packets, seasoning packets or shakers. In some embodiments, the
compositions herein
may be formulated into or added to, for example, soup or stew bases, bouillon
or broths.
D. Characteristics of Hemeprotein Compositions
101161
The present disclosure provides for hemeprotein compositions suitable for
use in
food products having improved color stability and reduced protein degradation
over time.
[0117]
In certain embodiments, hemeprotein compositions herein may comprise
antioxidants for stabilization of the oxidation state of the one or more
purified hemeproteins.
27
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
The iron atom in the heme group of a hemeprotein can be in the ferrous (Fe2+)
oxidation state
to support oxygen and other gases' binding and transport Hemoglobin in normal
red blood
cells is protected by a reduction system to stabilize these states. Initial
oxidation to the ferric
(Fe3+) state without oxygen converts hemoglobin into "methemoglobin" which
cannot bind
oxygen. For example, the hemeprotein myoglobin can exist in an oxygen bound
ferrous (Fe')
state referred herein as Oxymyoglobin (OxyMb), in a deoxygenated state
referred herein as
Deoxymyoglobin (DeoxyMb), a state bound to carbon monoxide referred herein as
Carboxymyoglobin (CarboxyMb) or in the ferric state referred to as
Metmyoglobin (MetMb).
Changes in the oxidative states of hemeprotein can be determined from the UV-
visible
absorption spectra, for example of a solution comprising the composition
disclosed herein. For
example, the absorption spectra may comprise peaks consistent with CarboxyMb
(550 nm),
OxyMb (582 nm), and MetMb (503 nm and 632 nm).
[0118]
In some embodiments, change in the oxidative states can also result in
change in
the appearance of the solution comprising the composition disclosed herein.
For example, a
solution comprising higher amounts of CarboxyMb or OxyMb is characterized by a
brighter
red color, while MetMb results in a more undesirable brown coloration. In some
embodiments
as provided herein, presence of antioxidant results in change and/or
stabilization of the
oxidative state of the hemeprotein.
[0119]
In some embodiments, hemeprotein compositions herein may comprise
antioxidants to act as a reduction system for changing and/or stabilization of
the oxidation state
of the one or more purified hemeproteins. "Stabilization- or "stabilized- in
context of
hemeprotein compositions provided herein, refers to a composition wherein the
combination
of hemeprotein with an antioxidant causes a lasting increase in the levels of
oxygenated
hemeprotein or carboxy hemeprotein, as indicated by a change in appearance to
a more red
composition, or a change in UV-Visible spectra such that the peak height at
550 nm
(corresponding to CarboxyMb), and/or at 582nm (corresponding to OxyMb)
increases in
comparison to a composition without antioxidant. In some aspects, the increase
in the peak
height can be detected within 1-48 hours of combining the antioxidant with the
hemeprotein.
This period between adding the antioxidant and a detectible increase in the
peak height is
refered to as the lag-time or lag-phase. In some aspects, depending on the
antioxidant used and
the storage conditions, the increase in peak height can last or is "stably"
maintained for about
0.5 days to about 90 days, though in some embodiments, the absolute peak
height may decrease
28
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
through this stable period. A guidance for some exemplary antioxidants and
their impact on
peak heights is provided in the Examples included herein.
[0120]
In some embodiments, the current disclosure encompasses hemeprotein
compositions comprising a hemeprotein and antioxidant, wherein the antioxidant
causes an
increase in relative amount of oxygenated or carboxygenated hemeprotein
compared to the
oxidized Met state in the composition. In certain embodiments of the
compositions disclosed
herein, the hemeprotein is a myoglobin and the antioxidant causes an increase
in relative
amount of oxymyoglobin to metmyoglobin in the composition as measured by the
change in
the UV-visible spectrum of a solution of the composition. In some embodiments,
the increase
in relative amount of oxymyoglobin to metmyoglobin in the composition is
detectable at about
0.5 to about 90 days, or at about 0.5 to 1 day, or about 1-10 days or about 10-
20 days, or about
20-30 days, or about 30-40 days, or about 40-50 days after addition of the
antioxidant and
stored at refrigeration temperatures (e.g., about 2 C to about 8 C). In some
embodiments, the
increase in the relative amount of oxymyoglobin to metmyoglobin in the
composition is at least
about 1.1 to about 5-fold, or about 1.1, about 1.2, about 1.3, about 1.4,
about 1.5, about 1.6,
about 1.7, about 1.8, about 1.9, about 2, about 3, about 4 or about 5-fold. In
some embodiments
the antioxidant causes a change in the UV-visible absorption spectrum with an
increase in peak
height at about 550 nm and about 582 nm, at about 4 hours to about 50 days, or
about 0.5 to
about 1 day, or about 1-10 days or about 10-20 days, or about 20-30 days, or
about 30-40 days,
or about 40-50 days after addition of the antioxidant and stored in
refrigeration (e.g., about 2 C
to about 8 C).
[0121]
In some embodiments, hemeprotein compositions herein may comprise
antioxidants for stabilization of the oxidation state of the one or more
purified hemeproteins
for about 1 to about 120 days, about 2 to about 100 days, about 3 to about 80
days, about 4 to
about 60 days, or about 7 to about 30 days. In some embodiments, compositions
herein may
comprise antioxidants for stabilization of the oxidation state of the one or
more purified
hemeproteins for at least about 7 days (e.g., about 0.5, 1, 2, 3, 4õ5 ,6, 7
days).
[0122]
In some embodiments, hemeprotein compositions herein may comprise
antioxidants for stabilization of the oxidation state of the one or more
purified hemeproteins
for about 1 to about 120 days, about 2 to about 100 days, about 3 to about 80
days, about 4 to
about 60 days, or about 7 to about 30 days when the composition is stored at
temperatures
below freezing (e.g., below 0 C). In some embodiments, hemeprotein
compositions herein may
29
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
comprise antioxidants for stabilization of the oxidation state of the one or
more purified
hemeproteins for about 1 to about 120 days, about 2 to about 100 days, about 3
to about 80
days, about 4 to about 60 days, or about 7 to about 30 days when the
composition is stored in
refrigeration (e.g., about 2 C to about 8 C). In some embodiments, hemeprotein
compositions
herein may comprise antioxidants for stabilization of the oxidation state of
the one or more
purified hemeproteins for about 1 to about 120 days, about 2 to about 100
days, about 3 to
about 80 days, about 4 to about 60 days, or about 7 to about 30 days when the
composition is
stored at room temperature (e.g., about 22 C to about 27 C). In some
embodiments,
compositions herein may comprise antioxidants for stabilization of the
oxidation state of the
one or more purified hemeproteins for about 1 to about 120 days, about 2 to
about 100 days,
about 3 to about 80 days, about 4 to about 60 days, or about 7 to about 30
days when the
composition is stored at about -30 C to about 30 C (e.g., about -30 C, about -
20 C, about -
C, about 0 C, about 10 C, about 20 C, about 30 C, about 40 C). In some
embodiments,
compositions herein may comprise antioxidants for stabilization of the
oxidation state of the
one or more purified hemeproteins for about 1 to about 120 days, about 2 to
about 100 days,
about 3 to about 80 days, about 4 to about 60 days, or about 7 to about 30
days when the
composition is stored at about 4 C. In some embodiments, hemeprotein
compositions herein
may comprise antioxidants for stabilization of the oxidation state of the one
or more purified
hemeproteins for about 7 days when the hemeprotein composition is stored at
about 4 C. In
some embodiments, compositions herein may comprise antioxidants for
stabilization of the
oxidation state of the one or more purified hemeproteins for about 1 to about
120 days, about
2 to about 100 days, about 3 to about 80 days, about 4 to about 60 days, or
about 7 to about 30
days when the composition is stored sequentially at two or more temperatures
ranging from
about -30 C, to about 40 C.
101231
In some embodiments, hemeprotein compositions herein may comprise
antioxidants and hemeproteins wherein the heme group of the hemeprotein is
bound to oxygen,
carbon monoxide, or a combination thereof. In some embodiments, hemeprotein
compositions
herein may comprise antioxidants and hemeproteins wherein the heme group of
the
hemeprotein is bound to oxygen, carbon monoxide, or a combination thereof for
about 1 to
about 120 days, about 2 to about 100 days, about 3 to about 80 days, about 4
to about 60 days,
or about 7 to about 30 days. In some embodiments, hemeprotein compositions
herein may
comprise antioxidants and hemeproteins wherein the heme group of a higher
fraction of
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
hemeprotein is bound to oxygen_ carbon monoxide, or a combination thereof for
at least about
0.5 days, at least about 1 day, at least about 2 days, at least about 3 days,
at least about 4 days,
at least about 5 days, at least about 6 days, or at least about 7 days or at
least about 8, or at least
about 9, or at least about 10, or at least about 11, at least about 12, or at
least about 13, or at
least about 14, or at least about 15, or at least about 16, or at least about
17, or at least about
18, or at least about 19, or at least about 20 or more days. In some
embodiments, compositions
herein may comprise antioxidants and hemeproteins wherein the heme group of a
higher
fraction of the hemeprotein is bound to oxygen, carbon monoxide, or a
combination thereof
for about 1 to about 120 days, about 2 to about 100 days, about 3 to about 80
days, about 4 to
about 60 days, or about 7 to about 30 days when the composition is stored at
about -30 C to
about 40 C (e.g., about -30 C, -20 C, -10 C, 0 C, 10 C, 20 C, 30 C, 40 C).
101241
In some embodiments, hemeprotein compositions herein may comprise
antioxidants for stabilization of the visual appearance of the one or more
purified hemeproteins.
As used herein, a stabilized visual appearance of a hemeprotein herein refers
to the appearance
of a red composition in visible light. As used herein, a destabilized visual
appearance of a
hemeprotein herein refers to the appearance of a brown composition in visible
light. In some
embodiments, hemeprotein compositions herein may comprise antioxidants for
stabilization of
the visual appearance of the one or more purified hemeproteins for about 1 to
about 120 days,
about 2 to about 100 days, about 3 to about 80 days, about 4 to about 60 days,
or about 7 to
about 30 days. In some embodiments, hemeprotein compositions herein may
comprise
antioxidants for stabilization of the visual appearance of the one or more
purified hemeproteins
for at least about 0.5 days, at least about 1 day, at least about 2 days, at
least about 3 days, at
least about 4 days, at least about 5 days, at least about 6 days, or at least
about 7 days, or at
least about 8, or at least about 9, or at least about 10, or at least about
11, at least about 12, or
at least about 13, or at least about 14, or at least about 15, or at least
about 16, or at least about
17, or at least about 18, or at least about 19, or at least about 20, or at
least about 21, or at least
about 22, or at least about 23, or at least about 24, or at least about 25, or
at least about 26, or
at least about 27, or at least about 28, or at least about 29, or at least
about 30 or more days. In
some embodiments, hemeprotein compositions herein may comprise antioxidants
for
stabilization ofthe visual appearance of the one or more purified hemeproteins
for at least about
0.5 days, at least about 1 day, at least about 2 days, at least about 3 days,
at least about 4 days,
at least about 5 days, at least about 6 days, or at least about 7 days, or at
least about 8, or at
31
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
least about 9, or at least about 10, or at least about 11, at least about 12_
or at least about 11 or
at least about 14, or at least about 15, or at least about 16, or at least
about 17, or at least about
18, or at least about 19, or at least about 20, or at least about 21, or at
least about 22, or at least
about 23, or at least about 24, or at least about 25, or at least about 26, or
at least about 27, or
at least about 28, or at least about 29, or at least about 30 or more days,
when the hemeprotein
composition is stored at about -30 C to about 40 C (e.g., about -30 C, about -
20 C, about -
C, about 0 C, about 10 C, about 20 C, about 30 C, about 40 C).
101251
In some embodiments, hemeprotein compositions herein may comprise
antioxidants for stabilization of the visual appearance of the one or more
purified hemeproteins,
wherein intensity of the red color of the composition decreases slowly over
time compared to
compositions only comprising hemeproteins. In some embodiments, hemeprotein
compositions herein may comprise antioxidants for stabilization of the visual
appearance of
the one or more purified hemeproteins, wherein intensity of the red color of
the composition
decreases by about 0.5% to about 70% after about 30 days (e.g., about 0.5,
about 1, about 5,
about 10, about 15, about 20, about 25, about 30 days). In some embodiments,
hemeprotein
compositions herein may comprise antioxidants for stabilization of the visual
appearance of
the one or more purified hemeproteins, wherein intensity of the red color of
the composition
decreases in a linear manner per day after making the compositions according
to the methods
herein. In some embodiments, hemeprotein compositions herein may comprise
antioxidants for
stabilization of the visual appearance of the one or more purified
hemeproteins, wherein
intensity of the red color of the composition decreases by about 0.001% to
about 0.5% per day
after making the compositions according to the methods herein.
[0126]
In some embodiments, hemeprotein compositions herein comprising one or more
antioxidants herein and one or more hemeproteins herein may have almost no
protein
degradation of the hemeprotein. In some embodiments, hemeprotein compositions
herein
comprising one or more antioxidants herein and one or more hemeproteins herein
may have
about 0.01% to about 15% (e.g., about 0.01%, about 0.1%, about 0.5%, about 1%,
about 2%,
about 3%, about 4%, about 5%, about 6%, about 7%, about 8%, about 9%, about
10%, about
11%, about 12%, about 13%, about 14%, about 15%) protein degradation of the
hemeprotein.
In some embodiments, hemeprotein compositions herein comprising one or more
antioxidants
herein and one or more hemeproteins herein may have about 0.01% to about 15%
(e.g., about
0.01%, about 0.1%, about 0.5%, about 1%, about 2%, about 3%, about 4%, about
5%, about
32
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
6%, about 7%, about 8%, about 9%, about 10%, about 11%, about 12%, about 13%,
about
14%, about 15%) protein degradation of the hemeprotein after about 1 to about
120 days, about
2 to about 100 days, about 3 to about 80 days, about 4 to about 60 days, or
about 7 to about 30
days. In some embodiments, hemeprotein compositions herein comprising one or
more
antioxidants herein and one or more hemeproteins herein may have about 0.01%
to about 15%
(e.g., (e.g., about 0.01%, about 0.1%, about 0.5%, about 1%, about 2%, about
3%, about 4%,
about 5%, about 6%, about 7%, about 8%, about 9%, about 10%, about 11%, about
12%, about
13%, about 14%, about 15%)) protein degradation of the hemeprotein after about
1 to about
120 days, about 2 to about 100 days, about 3 to about 80 days, about 4 to
about 60 days, or
about 7 to about 30 days when the composition is stored at about -30 C to
about 40 C (e.g.,
about -30 C, about -20 C, about -10 C, about 0 C, about 10 C, about 20 C,
about 30 C, about
40 C).
III. Methods of Use
[0127]
The present disclosure provides for food products and methods of making
such food
products using the hemeprotein compositions herein. In certain embodiments,
hemeprotein
compositions herein can be used for producing meat substitute food products
("meat replicas"
or -meat analogs"). As used herein, the term -meat replica" or -meat analog"
has the same
meaning as commonly understood by one of ordinary skill in the art and
includes but is not
restricted to plant-derived meat, vegan meat, meat substitute, mock meat, meat
alternative,
imitation meat, vegetarian meat, fake meat or faux meat. The terms "meat
replica" or "meat
analog" refer to a food product aiming to have a realistic meat-like
appearance without
containing an animal-based component. In some embodiments, compositions herein
can be
used as a materials in and in methods of making meat replicas, including, but
not limited to
ground meat replicas (e.g., ground beef, ground chicken, ground turkey, ground
lamb, or
ground pork), as well as replicas of cuts of meat and fish.
[0128]
In some embodiments, methods of making food products (e.g., meat replicas)
may
include combining the hemeprotein compositions herein with non-animal-based
fat, non-
animal-based matrixes, non-animal-based edible fibrous components, or any
combination
thereof In some embodiments, methods of making food products (e.g., meat
replicas) may
include combining the compositions herein with non-animal-based fat, non-
animal-based
matrixes, non-animal-based edible fibrous components, or any combination
thereof wherein
33
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
the addition of the composition herein provides a meat-like appearance to the
meat substitute.
In some embodiments, methods of making food products (e.g., meat replicas) may
include
combining the compositions herein with non-animal-based fat, non-animal-based
matrices,
non-animal-based edible fibrous components, or any combination thereof wherein
the addition
of the composition herein provides a meat-like taste to the meat substitute.
In some
embodiments, methods of making food products (e.g., meat replicas) may include
combining
the compositions herein with non-animal-based fat, non-animal-based matrixes,
non-animal-
based edible fibrous components, or any combination thereof wherein the
addition of the
composition herein provides a meat-like smell to the meat substitute. In
accordance with some
embodiments herein, a meat-like appearance, taste, or smell may be a beef-like
appearance,
taste, or smell, a poultry-like appearance, taste, or smell, a seafood-like
appearance, taste, or
smell, a game-like appearance, taste, or smell, a pork-like appearance, taste,
or smell, a lamb-
like appearance, taste, or smell, or any combination thereof
[0129]
In some embodiments, the methods of making food-products may include
combining the compositions herein with non-animal-based meat-like, poultry-
like, or seafood-
like base dough, that can be used in meat replicas sold in a form such as -
ground meat",
burgers/patties, or other forms, for example comparable to Impossible Burger
(from
Impossiblem Foods), Beyond Burger (from Beyond Meat ), Veggie Chik Patty
(from
Morningstar Farms ), and Plant-Based Patties from Good & GatherTM. Other
examples of
poultry, meat and seafood analog products that may include compositions
provided herein
include products like Veggie Meal Starters from Morningstar Farms , such as
Veggie
CHIK'N Nugget, Veggie Popcorn CHIK'N, Veggie CHIK'N Strips, Veggie Grillers ,
Veggie
Buffalo, beef analogue products made by Beyond Meat products such as Beyond
Beef
Crumbles, Beyond Beef(?) Ground Beef, and Beyond Beef(?) Sausage, or fish
analog products
made by Good Catch like salmon burgers, fish sticks, fish fillets, crab cakes,
fish burgers and
fish cakes.
[0130]
In some embodiments, the methods of making food-products may include
combining the liquids, gels, pastes, sauces, powder or cubes, ingredients of
flavor packets,
seasoning packets or shakers, soup or stew bases, bouillon or broths into a
food product before,
during, or after cooking of the consumable food product. In some embodiments,
the
compositions herein can be used to modulate the flavor and/or aroma profile
for a variety of
consumable food products.
34
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[0131]
In some embodiments, food products herein (e.g., meat replicas) may
comprise
about 1% to about 99% (e.g., about 1%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%,
80%,
90%, 95%, 99%) of any of the hemeprotein compositions herein by weight. In
some
embodiments, food products herein (e.g., meat replicas) may comprise about
0.01% to about
99% (e.g., about 1%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%,
99%) of
any of the antioxidants herein by weight. In some embodiments, food products
herein (e.g.,
meat replicas) may comprise about 1% to about 99% (e.g., about 1%, 5%, 10%,
20%, 30%,
40%, 50%, 60%, 70%, 80%, 90%, 95%, 99%) of any of the hemeproteins herein by
weight.
EXAMPLES
[0132]
The following examples are included to demonstrate preferred embodiments of
the
disclosure. It should be appreciated by those of skill in the art that the
techniques disclosed in
the examples that follow represent techniques discovered by the inventor to
function well in
the practice of the present disclosure, and thus can be considered to
constitute preferred modes
for its practice. However, those of skill in the art should, in light of the
present disclosure,
appreciate that many changes can be made in the specific embodiments which are
disclosed
and still obtain a like or similar result without departing from the spirit
and scope of the present
disclosure.
[0133]
The Examples 1-4 below were conducted using the exemplary techniques
provided
herein for guidance, though equivalent techniques known to those skilled in
the art can be used.
Exemplary Techniques
[0134]
In exemplary methods as disclosed in Examples 1-4 below, the following
techniques were performed as detailed below.
[0135]
Preparation of equine heart myoglobin solution. 50 mg/ml of equine heart
myoglobin solution was prepared by dispersing a weighed mass of the protein
powder into a
buffer solution (2.5 mM sodium phosphate/2.5 mM histidine, pH 9.0). The
resulting myoglobin
solution was stirred continually for at least 1 hour to ensure the protein was
fully dissolved.
The conversion of myoglobin to metmyoglobin was carried out according to the
method
described by Tang et al., Journal of Food Science, 69(9) (2004) with some
slight modifications.
In brief, potassium ferricyanide (5 mg/mL) was added to the myoglobin solution
and then the
mixture was stirred for 1 hour in an ice bath (4 C). Any residual ferricyanide
was then removed
from the mixture using a desalting column (Sephadex G-25 PD-10).
[0136]
The metmyoglobin (MetMb) was then reduced to oxymyoglobin (OxyMb) by
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
adding either sodium hydrosulfite or antioxidants: ascorbic acid, caffeic
acid, EGCG_ gallic
acid, quercetin, taxifolin, Trolox, and 4-methylcatechol. The desalted MetMb
solution was
diluted to 2 mg Mb/mL with buffer solution (2.5 mM sodium phosphate/2.5
histidine, pH
9.0) and then reduced by adding either sodium hydrosulfite (0.5 mg/mL) or
antioxidant (1 mM)
solutions. This was achieved by adding a weighed mass of the reducing agent to
the OxyMb
solution and mixing well. Any excess sodium hydrosulfite was subsequently
removed using a
desalting column (Sephadex G-25 PD-10). For addition of the antioxidants to
the myoglobin
solution (¨ 2.0 mg Mb/mL ¨ desalted), non-water-soluble antioxidants (caffeic
acid, quercetin,
or taxifolin) were first added to ethanol (5 to 10% total volume) prior to
adding to the MetMb
solutions. The reduction potential and solubility of antioxidants used are
listed in Table 1. For
all systems, the pH of the myoglobin solutions was measured after incubation
for at least 2
hours. All samples were stored at 4 C in screw-capped glass vials or 1.5 mL
plastic cuvettes
with a lid for up to 52 days.
[0137]
Preparation of celhilar agriculture hemeprotein solutions. Powdered
hemeprotein
samples obtained using a cellular agriculture approach (e.g., fermentation)
were used to prepare
the protein solutions: bovine, leopard, and sperm whale myoglobin and soy
leghemoglobin.
Weighed masses of each myoglobin powder were dissolved in aqueous buffer
solutions (2.5
m1\4 sodium phosphate/2.5 mM histidine, pH 9.0) to obtain a range of protein
concentrations
(0.5, 1.0, and 5 mg Mb/mL). The resulting mixtures were then stirred
continuously for at least
1 hour to fully dissolve the protein. All solutions were prepared fresh daily
or stored at 4 C for
a maximum of 1 day, prior to being analyzed.
101381
Appearance The appearance of the myoglobin samples was captured using a
digital
camera.
[0139]
Electrical characteristics. The zeta-potential (t-potential) of the protein
solutions
(1.0 or 5.0 mg/mL) was measured using a particle electrophoresis instrument
(Zetasizer Pro,
Malvern Instruments Ltd., Worcestershire, UK). The -potential was measured
from pH 8.5 to
2.5 by titrating the initial solutions with either acid (0.1 to 0.25 M HC1) or
alkaline (0.25 M
NaOH) solutions with continuous stirring.
[0140]
Solubility. The pH-dependence of the solubility of the proteins in the
myoglobin
solutions was determined by measuring the soluble and total protein
concentrations over a
range of pH values. The myoglobin solutions (0.5 mg/mL) were stored overnight
(4 C) and
then the protein concentrations were measured using a Bradford assay kit. The
total protein
36
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
concentration (ctotat) was determined by directly analyzing the myoglobin
solutions, whereas
the soluble protein concentration (csolubie) was determined by centrifuging
them at 13,000 rpm
for 15 minutes and then analyzing the supernatant. The protein concentration
measurements
were performed according to the instructions provided with the Bradford assay
kit. In brief, 5
1.1L protein samples (centrifuged or non-centrifuged) were pipetted into the
wells of a 96-well
micro plate (Costar, Corning Inc., Corning, NY, USA) and then 250 1.1L of
Bradford reagent
was added to each well, and the solutions were mixed well. The mixtures were
then incubated
at room temperature for 5 minutes before being analyzed. The absorbance of the
samples was
then measured at 595 nm using a UV-visible spectrophotometer (SpectraMax M2e,
Sunnyvale,
CA, USA). A standard curve was prepared by measuring the absorbance of a
series of bovine
serum albumin (BSA) solutions with different protein concentrations (0.125
mg/mL to 1
mg/mL), which led to a linear calibration curve with R2= 0.995. The myoglobin
solubility was
calculated using the following expression: %Solubility = 100xcsoltib1e/ctotai.
[0141]
Absorption spectra measurement. The absorption spectra of myoglobin
solutions
(1 and 5 mg/mL) were measured from 450 to 750 nm using a UV-visible
spectrophotometer
(Cary 100, Agilent Technologies, Santa Clara, CA, USA) (Tang et al., 2004).
All samples were
contained in cuvettes (volume = 1.5 mL; path length = 1 cm) that were stored
at 4 C throughout
the storage study. The myoglobin redox state proportions (deoxygenated
myoglobin (DeoMb),
oxygenated myoglobin (OxyMb), and metmyoglobin (MetMb)) in the myoglobin
systems
containing antioxidant were calculated according to the modified Krzywicki
equations by
similar to that described in Tang et al., Journal of Food Science, 69(9)
(2004).
[0142]
Computational Modeling. Some insights into the nature of the molecular
interactions involved between the antioxidants and the proteins were obtained
by carrying out
molecular docking analysis for equine heart myoglobin and selected
antioxidants. Molecular
docking was carried out using Glide software (Version 2019-3, Schrodinger,
LLC, New York)
to obtain some additional insights into the nature of the binding interactions
between the
antioxidants and myoglobin. The myoglobin crystal structure (PDB ID 1MNH) was
used as a
starting configuration and was processed and optimized using the Protein
Preparation Wizard,
setting PROPKA at pH = 8Ø The 3D structures of four ligands (ascorbic acid,
quercetin, gallic
acidõ and 4-methylcatechol) were built using Maestro 3D Builder. All the
ligand structures
were then prepared with LigPrep with OPLS3 force field. Ligand chirality was
maintained, and
possible states at the target pH 8.0 1.0 were generated. A docking grid was
created and
37
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
centered on the heme group, which enabled the entire myoglobin to be covered
by adjusting
the size of ligand diameter midpoint box, and no other constraint was applied.
Docking
experiments were then performed using ligand docking with extra precision (XP)
mode. The
number of poses to be reported was not limited, but post-docking minimization
was applied
with 20 poses per ligand included with strain correction terms. Then, all the
reported results
were ranked by docking scores, and poses with a docking score above zero were
excluded.
Only the specific configurations (binding site and binding pose) with the
highest Glide docking
scores (most negative) in docking experiments are discussed and illustrated in
the Results
section.
[0143]
Statistical analysis. For each myoglobin system, replicate analyses were
performed
and at least triplicate measurements were carried out for each analytical
test. The mean and
standard deviations of the combined data were calculated from the 6 or more
experimental data
points using Microsoft Excel 2016.
Example 1. Effect of pH on properties of equine heart myoglobin
[0144]
In an exemplary method, an initial characterization of the physical
properties,
including electrical charge, solubility and stability of an exemplary
myoglobin (equine heart
myoglobin) was conducted at different pH.
Electrical charge:
[0145]
The electrical characteristics of equine heart myoglobin were determined by
measuring the pH-dependence of their -potential values. In particular, the
isoelectric point (pI)
of each protein was determined from the point where their net charge was zero.
The isoelectric
point of the equine heart myoglobin (1 mg/mL) was around pH 5.5 (Fig. 1).
Visual observation
indicated that a thin sediment layer formed at the bottom of the samples
around and below pH
5.5 (data not shown), which can be attributed to aggregation of the protein.
Protein solubility
[0146]
The effect of pH on solubility of the equine heart myoglobin was determined
by
measuring the soluble and total protein contents of the solutions using the
Bradford assay. The
protein was highly soluble across the entire pH range studied, with the
solubility always being
greater than 90% (Fig. 2).
Example 2. Impact of antioxidant addition on oxymyoglobin stability
[0147]
In another exemplary method, the impact of various natural and synthetic
antioxidants to convert metmyoglobin to oxymyoglobin in the form of solutions
instead of meat
38
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
muscle, and thereby improve the color characteristics of myoglobin solutions
for application
in food product development was examined. The oxygenated form of myoglobin
(oxymyoglobin: OxyMb) plays a critical role in determining the desirable
bright red color of
many meat products. However, it is highly susceptible to oxidation to
metmyoglobin (MetMb),
which causes a change in color from red to brown.
[0148]
The UV-visible absorption spectra of equine heart myoglobin (OxyMb and
MetMb)
solutions without antioxidant are shown in Fig. 3A. The oxymyoglobin spectrum
had two
distinct absorption peaks around 544 and 582 nm, which are responsible for the
bright red color
of these solutions. Conversely, the metmyoglobin only had relatively small
absorption peaks
around 503 and 632 nm, which are responsible for its brown color. The
oxymyoglobin solutions
only remained bright red for about 1 day before turning brown and exhibiting a
UV-visible
spectrum similar to that of metmyoglobin. Figs. 3B-3F shows the concentration
of oxygenated
myoglobin decreased gradually over time and metmyoglobin concentration
increased to about
100% on day 5.
[0149]
Antioxidants with different reduction potential values (E ) were examined
for their
potential to convert metmyoglobin to oxymyoglobin, thereby creating a
desirable red color in
the system. The exemplary antioxidants used herein are provided in Table 1.
TABLE 1
Reduction Potential Solubility in
Water
Type LogP / xLogP3
(E , mV)* (mg/mL or mM)
Ascorbic Acid 282 -1.6 to -2.15 100 mg/mL
Soluble in Ethanol;
Quercetin 330 0.9 to 1.5
Water: <1 mg/mL
Epigallocatechin 430 1 . 2 to 2 . 984 Soluble in
Ethanol;
gallate; EGCG Water: <10 mM
Soluble in Ethanol
Trolox 480 1.272
Water: 0.38 mg/mL
Soluble in Ethanol;
Taxifolin 500 0.803
Water: <1 mg/mL
4-methycatechol 520 1.37 38 mg/mL
Caffeic Acid 534 0.89 to 1.2 Soluble in
Ethanol, DMS0
Water: <1 mg/mL
Gallic Acid 560 0.7 to 0.96 11.5 mg/mL or
70 mM
39
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[0150]
It was found that addition of 1 mM ascorbic acid to the metmyoglobin
solutions (-
2 mg/mL) converted the MetMb to OxyMb, as seen in the UV-visible absorption
and color
measurements (Figs. 4A-4C). After forming OxyMb and reaching peak
concentration (99.9%)
at Day 1, the OxyMb portion decreased gradually over 13 days in the presence
of the ascorbic
acid, compared to only 2 to 3 days in its absence (Figs. 4A-4C).
Interestingly, there was a "lag
time" that ranged from about 4 to 24 hours for the OxyMb concentration to
reach maximum
and the color of the solution became bright red (Figs. 4A-4C). The origin of
this effect may
have been due to the delay in the ability of the ascorbic acid to reach the
heme group.
Consequently, the observed lag-time may have been due to the time taken for
the ascorbic acid
molecules to move from the aqueous solution to the active site where they
could reduce the
heme group. Without wishing to be bound by theory, the longer lag-time
observed in the
samples herein may have been because they were stored at 4 C, which could have
slowed down
the reaction kinetics. Moreover, the lag-time time observed the myoglobin
solutions herein
may be related to the "bloom" time observed in freshly cut meat, i.e., the
time for the interior
of the meat to turn from brown to red due to oxygenation of the myoglobin.
Fig. 4A also shows
that deoxygenated myoglobin (DeoMb) concentration was increasing at day 10 and
onward.
This occurrence could be due to depletion of oxygen around the myoglobin
molecules and
formation of a low partial oxygen pressure environment, as ascorbic acid is an
oxygen
scavenger, and hence the DeoMb conversion from MetMb.
[0151]
Quercetin, a natural hydrophobic antioxidant, was also studied for its
potential in
stabilizing the oxymyoglobin. 1 mM quercetin (originally dissolved in ethanol)
reduced the
metmyoglobin to oxymyoglobin, with 70% oxymyoglobin formed at day 1 and then
decreased
gradually over 21 days (Figs. 4D-4F). The lag-time for the red color to
stabilize was around 2
to 10 hours (data not shown). The quercetin was observed to have a shorter lag-
time.
[0152]
Epigallocatechin gallate (EGCG), which is soluble in both water and organic
solvents, was also examined. The addition of EGCG (1 mM) was also effective at
reducing the
metmyoglobin to oxymyoglobin (around 40%) and turning the protein solution to
slightly red
color (Figs. 4G, 4H and 4M). The 40% oxymyoglobin formed was not very stable
and reverted
back to metmyoglobin by 10% after 24 hours, causing the solution to turn brown
quickly.
[0153]
The addition of taxifolin (1 mM), a hydrophobic antioxidant, to the
solution
converted the MetMb to OxyMb and the red color remained relatively stable over
27 days
(Figs. 41, 4J and 4M). There was around a 24-hour lag-time for the
oxymyoglobin to fully
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
stabilize and reach a peak concentration of about 65% (Fig. 41) and form a
bright red color in
the system. The characteristic absorption peaks and red color associated with
the OxyMb
slowly decreased in intensity over 27 days, with OxyMb concentration
decreasing gradually to
22% and the MetMb state reaching 72% concentration.
[0154]
The addition of 1 mM 4-methylcatechol to myoglobin did not yield the
typical
characteristic double-peak spectra of oxymyoglobin, with OxyMb state reaching
10 to 20%
only (Figs. 4K-4M). This suggested that 4-methylcatechol at 1 mM was not an
effective
reducing agent or antioxidant. Instead, there was a large increase in the
measured absorbance
at the lower wavelengths (Fig. 4K), which suggested that some pigmented
molecules or
colloidal particles were formed that absorbed or scattered light,
respectively. A "blood red"
color was observed in these samples, which may have applications in some food
systems.
[0155]
The addition of 1 mM Trolox, a water-soluble analog of vitamin E, to the
protein
solution was also able to reduce the MetMb to OxyMb (52%), which then remained
stable for
up to 26 days (Figs. 4N, 40 and 4T). This system had a 24-hour lag-time before
the
characteristic absorption spectra, OxyMb maximum concentration and red color
associated
with OxyMb was first observed (Fig. 4T). The OxyMb concentration decreased
gradually from
52% to 30% over 26 days with the MetMb portion reaching 61%. The deoxygenated
myoglobin
concentration increased almost linearly on day 7 onward, which could be due to
oxygen being
depleted (Fig. 40).
[0156]
The addition of 1 mM caffeic acid, a hydrophobic antioxidant, was able to
reduce
the metmyoglobin solution to only about 25% oxymyoglobin after 24 hours. Only
slight red
color was observed in the solution on day 1 and it then turned brown (Figs.
4P, 4Q and 4T).
The 24 hour lag-time with only slight red color forming may have been because
of the relatively
low reducing potential power of caffeic acid. Interestingly, the metmyoglobin
concentration
also decreased over time after reaching a peak at 78% on day 4 while the DeoMb
portion
increased linearly from day 0 over 15 days (Fig. 4P). Again, the DeoMb
conversion from
MetMb could be due to a low oxygen partial pressure formed in the system as a
result of the
radical scavenging properties of caffeic acid.
101571
The addition of 1 mM gallic acid (water-soluble) was able to reduce
metmyoglobin
and form about 35% oxymyoglobin (Figs. 4R, 4S and 4T). The slightly reddish
myoglobin
solution observed on Day 0 turned dark brown after 1 day of storage and the
OxyMb
concentration decreased to 14% only after 24 hours (Day I). The low conversion
of MetMb to
41
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
OxyMb may have been due to the relatively high reduction potential of gallic
acid among the
other antioxidants used (Table 1). Around 20% DeoMb was measured in the system
throughout
the storage span (Fig. 4R).
101581
Overall, the results of these exemplary methods showed that different
natural and
synthetic antioxidants had different abilities to convert MetMb to OxyMb and
then stabilize
the oxymyoglobin form. In particular, ascorbic acid and quercetin, which both
have relatively
low reduction potentials (Table 1), were effective at stabilizing the color of
the oxymyoglobin.
These insights are important for the application of myoglobin in food products
that are expected
to have a long shelf life so that the color is maintained for prolonged
periods.
Example 3. Molecular docking experiments
[0159]
In another exemplary method, molecular docking experiments were performed
for
selected antioxidants using Glide (Schrodinger Suite) to obtain some insight
into key molecular
interactions involved when antioxidants bind to myoglobin. The docking results
showed that
there was a similar ligand binding site close to the heme group for ascorbic
acid, quercetin, and
gallic acid and the distance of these antioxidant ligands to the heme group
were around 10 to
12 angstroms.
[0160]
Ascorbic acid: For ascorbic acid, three lysines (1(42, 1(47, and 1(98) and
an aspartic
acid (D44) on the myoglobin were involved in the binding interaction. In terms
of the nature
of the bonds, a salt-bridge and five hydrogen bonds were formed between
hydroxyl/carbonyl
groups on the ascorbic acid and these amino acids on the protein.
[0161]
Quercetin: For quercetin, two lysines (1(96 and 1(98), an aspartic acid
(D44), and a
glutamic acid (E41) participated in the binding interactions. In this case,
several hydrogen
bonds were formed between the hydroxyl/carbonyl groups on the quercetin and
the amino acids
on the protein.
101621
Gallic acid: For gallic acid, three lysines (K42, K96, and K98) and an
aspartic acid
(D44) participated in the binding interaction. In this case, two salt-bridges
were formed
between the carboxylate on the gallic acid and the K42/K98 side chains. In
addition, three
hydrogen bonds were formed between the hydroxyl groups on the gallic acid and
the K47/D44
side chains and 1(96 carbonyl on the protein.
[0163]
4-methylcatechol: Also performed was a molecular docking experiment for 4-
methylcatechol, since this compound did not yield a typical oxymyoglobin
spectra when added
to the myoglobin solution (Figs. 4K and 4L). Indeed, this compound was bound
to a different
42
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
site on the protein molecule, near to the K34 and E52 residues, which is far
away from the
heme group. There were only two hydrogen bonds formed between the hydroxyl
groups on the
4-methylcatechol and the K34/E52 side chains on the protein. The different
binding site for the
4- methylcatechol may be the reason for the different absorption spectra
measured in the mixed
system.
Example 4. Characteristics of myoglobin produced by cellular agriculture
[0164]
In another exemplary method, four different myoglobin samples (leopard,
bovine,
sperm whale, and soy legume) generated using a cellular agriculture
(fermentation) approach
were characterized. In particular, the electrical charge, color, solubility,
and redox stability of
the proteins was measured using similar methods as for the equine heart
myoglobin discussed
in Examples 1 and 2 and described in detail herein.
[0165]
Electrical charge. The -potential versus pH profiles of the four different
myoglobin samples was measured to determine their isoelectric points (pi) and
stability to
aggregation under different solution conditions (Fig. 5A). The leopard
myoglobin, bovine
myoglobin, and soy leghemoglobin all had similar isoelectric points (pH 4.5),
while the sperm
whale myoglobin had a much higher isoelectric point (pH 6.5) and the equine
myoglobin had
isoelectric point in between these four samples, i.e., at pH 5.5. When the pH
was reduced from
8.5 to 2.5, the c-potential changed from about -18 to +14 mV for leopard
myoglobin, -30 to
+17 mV for bovine myoglobin and soy leghemoglobin, and -18 to +28 mV for sperm
whale
myoglobin.
[0166]
Appearance. All four myoglobin solutions turned from red to brown when the
pH
was reduced from 8.5 to 2.5, with this effect being most pronounced for the
animal-origin
myoglobin samples (Fig. 5B), including the equine heart myoglobin. This change
in color can
be attributed to protein unfolding and exposure of the heme group at low pH
values, which
leads to the loss of oxygen molecules bound to the iron heme. Visually, the
soy and leopard
myoglobin solutions appeared to maintain their strong red color longer than
the other samples,
which may be an advantage for food applications.
[0167]
Protein solubility. Visually, some protein aggregation was observed around
and
below the isoelectric point of the myoglobin solutions, which can be
attributed to the reduction
in electrostatic repulsion and protein unfolding effects. In general, however,
the four myoglobin
samples had a relatively high solubility (>78%) across the entire pH range
studied (Figs_ 7A-
7D). This suggested that there was either a strong repulsion (electrostatic or
steric) or weak
43
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
attraction (van der Waals or hydrophobic) between the myoglobin molecules. For
the sake of
comparison, the average solubilities of the proteins across the whole pH range
was calculated:
soy (101%) > leopard (97%) > sperm whale (94%) > bovine (91%). Overall, these
results
suggested that all the hemeproteins had a high water-solubility across the
whole pH range but
that there were some differences between them. These differences may have
arisen due to
differences in the primary structure (amino acid sequence) of the proteins,
which led to
differences in their conformations, surface charges, and hydrophobicity.
101681
Oxidative stability. The absorption spectra of the freshly prepared
myoglobin
solutions (1 and 5 mg/mL) were measured to determine their initial redox
state. The leopard
myoglobin solutions appeared red with the intensity of the color increasing
with protein
concentration. These solutions had distinct absorption peaks at 550 and 582 nm
(Fig. 6);
however, the spectrum differed somewhat from that expected for oxymyoglobin
(Fig. 4A). In
particular, the peak at 550 nm was strongest for the leopard myoglobin, while
the peak at 582
nm was strongest for the reduced equine heart myoglobin. When myoglobin binds
carbon
monoxide it produces a double-peak spectrum that closely resembles that of
OxyMb but with
a more prominent peak around 541 nm. Both OxyMb and CarboxyMb also have a
fairly similar
bright cherry red color. It was therefore possible that mixtures of OxyMb and
CarboxyMb, as
well as some MetMb (small peak around 635 nm) were formed in the initial
leopard myoglobin
system. This may have occurred because some carbon monoxide attached to the
myoglobin
during the production, isolation, and dehydration of the protein. Over time,
the OxyMb and
CarboxyMb were gradually oxidized to MetMb, as shown by the change in
absorption spectra,
i.e., the formation of peaks around 503 and 632 nm (Figs. 7A-7C). A higher
absorbance
intensity and a longer redox stability were observed for the more concentrated
myoglobin
solution (5 mg/mL), which would be expected because there is more active
compound present.
101691
The absorption spectra of the bovine myoglobin solution also suggested that
it
contained a mixture of CarboxyMb, OxyMb, and MetMb, with peaks being observed
at 554
and 630 nm (Fig. 711-7F). The bovine myoglobin had slightly better oxidative
stability than
the leopard myoglobin, with the characteristic double-peak spectra still being
observed at day
21. The bovine myoglobin solutions did not, however, display a strong red
color even on Day
0, and over time the solutions turned brown. This change in color was
consistent with the
gradual change of the absorption spectra from CarboxyMb/OxyMb to MetMb, with a
prominent peak gradually forming at 632 nm over time (Figs. 7D-7F).
44
CA 03233394 2024- 3- 27
WO 2023/076307
PCT/US2022/047771
[0170]
Fig. 7G show that the initial sperm whale myoglobin had a slightly
different redox
state than the other proteins examined herein. The absorption spectrum
contained peaks
consistent with CarboxyMb (550 nm). OxyMb (582 nm), and Metmyoglobin (503 nm
and 632
nm). Over about 5 days storage, however, the absorption spectrum gradually
became more
similar to that of pure MetMb, with the solution turning from red to brown
(Figs. 7G-7I). This
study suggested that the sperm whale sample used in this exemplary study may
have undergone
some autoxidation.
101711
Soy leghemoglobin produced solutions herein with a much stronger bright
cherry
red color than the three animal myoglobin solutions. Initially, the absorption
spectrum of this
sample had peaks that were consistent with the presence of CarboNyMb and ONyMb
(Fig. 7J-
.1L). A double-peak absorption spectrum was still observed in the soy
leghemoglobin at Day
52, suggesting that this hemeprotein was highly resistant to oxidation.
Consequently, the soy
leghemoglobin used in this study may have applications in food products that
have a long shelf
life.
[0172]
In comparison to the equine heart myoglobin without antioxidant addition,
the
cellular agriculture-produced myoglobin had longer oxidative stability
(compare Fig. 3 and
Figs. 7A-7L). This may be contributed to the method used to yield these
myoglobins.
Nonetheless, all these systems had typical oxymyoglobin/carboxymyoglobin
absorption
spectra.
*****************************
[0173]
The foregoing discussion of the disclosure has been presented for purposes
of
illustration and description. The foregoing is not intended to limit the
disclosure to the form or
forms disclosed herein. Although the description of the disclosure has
included description of
one or more embodiments and certain variations and modifications, other
variations and
modifications are within the scope of the disclosure, e.g., as can be within
the skill and
knowledge of those in the art, after understanding the present disclosure. It
is intended to obtain
rights which include alternative embodiments to the extent permitted,
including alternate,
interchangeable and/or equivalent structures, functions, ranges or steps to
those claimed,
whether or not such alternate, interchangeable and/or equivalent structures,
functions, ranges
or steps are disclosed herein, and without intending to publicly dedicate any
patentable subject
matter_
CA 03233394 2024- 3- 27