Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/057960
PCT/1B2022/059574
SWEETENER CONCENTRATE FORMULATIONS
This application draws priority from US Patent Application No. 63/262,176,
filed
October 6, 2021, which application is incorporated by reference for all
purposes
as if fully set forth herein.
FIELD AND BACKGROUND OF THE INVENTION
The present invention primarily relates to edible formulations and edible
sweetener formulations therefor, and more particularly, to sweetener
concentrate
formulations containing one or more polysaccharides disposed in the sweetener
particles, and to edible or food formulations containing such sweetener
concentrate
formulations.
SUMMARY OF THE INVENTION
According to aspects of the invention there is provided a sweetener
formulation including: (a) sweetener particles containing a first sweetener;
and (b)
crystalline sugar particles; wherein a polysaccharide is disposed within the
sweetener
particles; and wherein a first weight ratio of the polysaccharide to the first
sweetener
is within a range of 1:100 to 95:5.
According to further aspects of the invention there is provided a food
formulation including: (a) a sweetener formulation; (b) a fat; and (c)
optionally, a
starch; wherein a total concentration of the first sweetener, the crystalline
sugar, the
fat, and the starch, within the food formulation, is at least 20%, on a weight
basis;
wherein the food formulation exhibits improved sweetness with respect to a
control
edible formulation that is identical to the food formulation, but devoid of
the
polysaccharide; and wherein, within the food formulation, at least 60% of the
total
amount of sweetener, by weight, is crystalline.
According to yet further aspects of the invention there is provided a
sweetener
formulation including: a first population of sweetener particles, the
sweetener
particles including: (a) crystalline sucrose; and (b) optionally, amorphous
sucrose;
wherein a total amount of sucrose within the sweetener particles includes the
crystalline sucrose and the amorphous sucrose; wherein a polysaccharide is
disposed
as at least one polysaccharide particle in each sweetener particle of the
sweetener
1
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
particles; and wherein, within the first population of sweetener particles:
(i) a first
weight ratio of the polysaccharide to the total amount of sucrose is within a
range of
1:100 to 95:5; and (ii) a second weight ratio of the amorphous sucrose to the
crystalline sucrose is at most 5:1.
According to yet further aspects of the invention there is provided a
formulation containing: a first population of sweetener particles, the
sweetener
particles including: (a) crystalline sucrose; and (b) optionally, amorphous
sucrose;
wherein a total amount of sucrose within the sweetener particles includes the
crystalline sucrose and the amorphous sucrose; wherein a polysaccharide is
disposed
as at least one polysaccharide particle in each sweetener particle of the
sweetener
particles; and wherein, within the first population of sweetener particles, a
first weight
ratio of the polysaccharide to the total amount of sucrose is within a range
of 6:100 to
95:5.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention is herein described, by way of example only, with reference to
the accompanying drawings. With specific reference now to the drawings in
detail, it
is stressed that the particulars shown are by way of example and for purposes
of
illustrative discussion of the preferred embodiments of the present invention
only.
In the drawings:
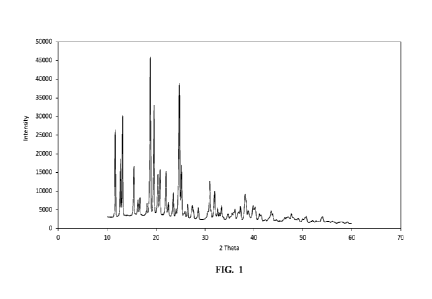
Figure 1 is an X-ray diffraction (XRD) plot of a solid sweetener concentrate
formulation containing 20% polysaccharide (pectin) and 80% sweetener
(sucrose),
according to an aspect of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present disclosure primarily describes sweetener concentrate formulations
containing one or more polysaccharides disposed in the sweetener particles,
and
edible formulations containing such sweetener concentrate formulations.
Such sweetener concentrate formulations may include one or more species of
polysaccharides that may exhibit any of various mucoadhesive properties.
The inventors have discovered that the location of the polysaccharides within
the food may be of cardinal importance, at least with respect to the sweetness
thereof
Specifically, the inventors have discovered when the polysaccharide is
incorporated
2
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
within the sweetener particles, the polysaccharide may not negatively impact
food
sweetness. In fact, the inventors have surprisingly discovered that under
certain
conditions (e.g., within a particular concentration range of the
polysaccharides), the
presence of such polysaccharides within the food may actually enhance food
sweetness.
Without wishing to be limited by theory, the inventors believe that
mucoadhesion of the polysaccharide to the mucosa or mucous membranes on the
tongue and within the oral cavity may contribute to the retention of sweetener
carbohydrates and sweetener polyols, resulting in an enhanced and extended
sensation
of sweetness. This
phenomenon occurs, or is greatly enhanced, when the
polysaccharide is incorporated within the sweetener particles, such that the
mucosal
adhesion between the mucin-containing mucosa and the polysaccharide in the
sweetener particle helps to fix the sweetener particle to the oral mucosa, or
to at least
increase the contact time between the sweetener particle to the oral mucosa.
This
translates into increased activation of the sweetness sensors/receptor sites
on the
tongue, by way of example.
The inventors have surprisingly discovered that within a particular, low range
of concentrations of polysaccharide disposed within the sweetener particles,
the
increased mucosal adhesion of these polysaccharides appears to more than
offset
various polysaccharide properties that deleteriously affect perceived
sweetness. These
deleterious properties include the increased viscosity of the food (inter alia
reducing
the solubility kinetics and hindering the transport of sweetener molecules to
the
sweetness sensors/receptor sites), covering and blocking oral sweetness
sensors/receptor sites, and the non-sweet taste of the polysaccharide itself.
By more
than offsetting these deleterious polysaccharide properties, the presence of
the
polysaccharide within the sweetener particles may impart appreciably enhanced
sweetness to the food.
The mucoadhesive agents for use in accordance with the formulations and
methods of the present invention may have various mucoadhesive properties. For
example, the mucoadhesive agents may have numerous hydrophilic groups, such as
hydroxyl and carboxyl groups, which aid attachment to mucus or cell membranes
through various interactions such as hydrogen bonding and hydrophobic or
electrostatic interactions.
3
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Mucoadhesion may generally refer to the attachment of particular
macromolecules to a mucin layer of a mucosal surface of a human tongue.
The mucoadhesive agent's affinity for attaching to a mucin layer of a mucosal
surface of a human tongue, may be characterized or quantified by at least one
of
several characterization methods, some of which are described hereinbelow.
Examples of such polysaccharides exhibiting mucoadhesive activity include,
but are not limited to, xanthan gum, guar gum, locust bean gum, tragacanth,
karaya
gum, gum Arabic, agar-agar, tara gum, sodium alginate, potassium alginate,
konjac
mannan, gellan and pectin, including both low methoxyl pectin (LMP) and high
methoxyl pectin (HMP).
As used herein in the specification and in the claims section that follows,
the
terms "mucoadhesion" and "mucosal adhesion" refer to the tendency of
particular
macromolecules such as polysaccharides to attach to a mucin layer of a mucosal
surface of a human tongue.
The inventors have yet further surprisingly discovered that within the
inventive ratio of polysaccharide to sweetener (w/w%) within the sweetener
particles,
the distribution of polysaccharide -- counterintuitively -- does not have to
be uniform.
In fact, high non-uniformity may actually enhance perceived sweetness.
Assume, by way of example, that a particular polysaccharide enhances
sweetness when disposed within sugar particles in a ratio of 0.3% (w/w%). The
inventors have discovered that a polysaccharide-sweetener concentrate (e.g.,
sweetener particles containing 50% polysaccharide and 50% sweetener) may be
diluted (e.g., with ordinary sugar, which does not contain polysaccharide) to
obtain
the desired average concentration of 0.3% (w/w%) polysaccharide with respect
to the
total amount of sugar. The inventors have found that such a diluted
concentrated
product containing an average of 0.3% may be no less effective -- and may
actually be
more effective -- in sweetness enhancement than the product having the even
distribution of 0.3% polysaccharide within the sweetener particles.
As used herein, the term "sweetener carbohydrate" refers to an edible
sweetener having at least one carbohydrate moiety, which carbohydrate is
processed
within the human body to produce energy. This definition is meant to include
sweetener carbohydrates having an energy value of at least 0.1 kcal/g, more
typically,
4
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
at least 0.2 kcal/g, more typically, at least 0.5 kcal/g, and yet more
typically, at least
1.0 kcal/g. This definition is specifically meant to include allulose.
The term "sweetener carbohydrate" is specifically meant to exclude high-
intensity sweeteners such as sucralose, aspartame, and acesulfame-K.
The term "sweetener", when used alone, is meant to include both sweetener
carbohydrates and sweetener polyols.
A sweetener carbohydrate produces a sweet taste when consumed by the
typical human consumer. If, on a normalized sweetness scale, on a weight
basis, in
which sucrose is taken as a standard of 1, maltose is about 031, and lactose
is about
0.22, the term "sweetener carbohydrate" would apply to lactose, and to any
sugar or
other nutritive, carbohydrate-containing sweetener having a sweetness within a
range
of 0.15 to 2.5 on this normalized sweetness scale. Alternatively, it may be
stated that
the minimum sweetness for the sugar or other nutritive, carbohydrate-
containing
sweetener would be that of raffinose (which has a sweetness of 0.15 on the
above-
mentioned scale). More typically, such a sweetener carbohydrate has a
sweetness
within a range of 0.25 to 2.5, 0.35 to 2.5, 0.45 to 2.5, 0.25 to 1.8, 0.45 to
1.7, 0.15 to
1.7, or 0.35 to 1.5 on this normalized sweetness scale.
It is noted that the relative sweetness of fructose reported in the literature
has
been reported to be as little as 0.91, and as much as about 1.7. For the
avoidance of
doubt, the term "sweetener carbohydrate" is meant to include fructose,
irrespective of
any of its reported relative sweetness values.
As used herein, the term "normalized sweetness scale", refers to a relative
sweetness scale, on a weight basis, in which sucrose is assigned a value of
1.00. More
specifically, the normalized sweetness scale is determined according to the
methods
disclosed in Moscowitz, H. "Ratio Scales of Sugar Sweetness"; Perception &
Psychophysics, 1970, Vol. 7 (5), in which the power function for the sugars
and
polyols/sugar alcohols has an exponent of 1.3 (n = 1.3), as disclosed therein
in Table
3, and as provided hereinbelow.
5
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
From "Ratio Scales of Sugar Sweetness- (Table 3)
Percent by Weight Basis
Relative
Rank
Sweetness
Sucrose 1 1.00
Fructose 2 0.91
Raftinose 15 0.15
Maltose 12 0.31
Lactose 14 0.22
Dulcitol 5 0.46
Glucose 4 0.45
Galactose 6 0.42
Sorbose 7 0.41
Sorbitol 9 0.37
Mannitol 11 0.33
Arabi nose 8 0.39
Rhamnose 10 0.35
Glycerol 3 0.50
Xylose 13 0.26
A sweetener carbohydrate may be a monosaccharide or a disaccharide.
Examples of sweetener carbohydrates include, but are not limited to, sucrose,
glucose,
maltose, fructose, lactose, or any combination of sweetener carbohydrates. One
or
more sweetener carbohydrate may be combined with one or more sweetener
polyols.
A sweetener carbohydrate may be naturally occurring or synthetically produced.
As used herein, the term "sweetener polyol" refers to a consumable polyol that
produces a sweet taste when consumed by the typical human consumer. Non-
limiting
examples of sweetener polyols include xylitol, maltitol, erythritol, sorbitol,
threitol,
arabitol, hydrogenated starch hydrolyzates (HSH), isomalt, lactitol, mannitol,
or
galactitol (dulcitol). In many instances, the polyol is a sugar alcohol. A
sugar alcohol
can be produced from a carbohydrate by any known method of reduction (via a
chemical or biological transformation) of an acid or aldehyde to an alcohol.
In other
cases, a sweetener polyol can be synthesized from a parent carbohydrate.
Alternatively, a sweetener polyol may be obtained from a biological source.
For the avoidance of doubt, the term "sweetener polyol" is meant to include
any polyol/sugar alcohol having a sweetness within a range of 0.15 to 2.5 on
the
above-described normalized sweetness scale. More typically, such a sweetener
polyol
6
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
has a sweetness within a range of 0.15 to 1.5, 0.15 to 1.0, 0.15 to 0.8, 0.15
to 0.7, 0.20
to 0.7, 0.15 to 0.6, or 0.25 to 0.6, on this normalized sweetness scale.
As used herein in the specification and in the claims section that follows,
the
term "polysaccharide" refers to a polymer comprising a plurality of
monosaccharide
building blocks or units, adjacent monosaccharide units being bound or linked
by a
glycosidic linkage. Such linkages may be effected using various enzymes. A
polysaccharide may be a homopolysaccharide, in which all of the monosaccharide
building blocks are identical (e.g., curdlan), or a heteropolysaccharide,
which contains
at least two monosaccharide building blocks (e.g., sodium alginate, tara gum).
Depending on which monosaccharides are connected, and which carbon atom
in the monosaccharides is involved in the linkage, polysaccharides may assume
a
variety of forms. A polysaccharide having solely a straight chain of
monosaccharides
is a "linear" polysaccharide; a polysaccharide having a branched backbone is a
"branched." polysaccharide.
As used herein in the specification and in the claims section that follows,
the
term "glycosidic linkage" refers to covalent bonding between adjacent building
blocks
or monosaccharide units within a polysaccharide by means of oxygen ("0-
glycosidic"
linkage), nitrogen ("N-glycosidic- linkage), or sulfur ("S-glycosidic-
linkage). Most
typically, the glycosidic linkage is an 0-glycosidic linkage.
As used herein in the specification and in the claims section that follows,
the
term "unsubstituted monosaccharide", with respect to building blocks within
the
polysaccharide, refers to a non-substituted cyclic monosaccharide such as a
cyclic
hexose sugar, cyclic pentose sugar, and cyclic heptose sugar.
As used herein in the specification and in the claims section that follows,
the
term "monosaccharide", with respect to building blocks within the
polysaccharide, is
meant to include unsubstituted monosaccharides and substituted
monosaccharides.
As used herein in the specification and in the claims section that follows,
the
term "substituted monosaccharide", with respect to building blocks within the
polysaccharide, refers to a cyclic monosaccharide having at least one moiety
other
than hydrogen (H-), hydrocarbon (e.g., alkyl), or hydroxyl (H0-). Typical
examples
of moieties in such substituted monosaccharides include acetyl (e.g., konjac
mannan,
locust bean gum), amino (e.g., chitosan), methoxy (e.g., pectin), sulfate
(e.g.,
7
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
carrageenan), pyruvate (e.g., carrageenan, xanthan gum), a carboxylate such as
acetate
(e.g., xanthan gum) and acyl (e.g., gellan gum) moieties.
In some embodiments, the carboxylate moiety is, or includes, a uronic acid.
Examples include pectin and sodium alginate.
In some embodiments, the polysaccharide is, or includes, an anionic
polysaccharide. Examples include gellan gum, xanthan gum, pectin, and sodium
alginate.
In some embodiments, the polysaccharide is, or includes, a non-ionic
polysaccharide. Examples include locust bean gum (LBG) and agar-agar.
The sweetener formulation or edible formulation is typically devoid of silicon-
containing species such as silica. In some embodiments, the concentration of
silicon
within the sweetener formulation or edible formulation is at most 1%, at most
0.5%, at
most 0.2%, at most 0.1%, at most 0.05%, at most 0.02%, at most 0.01%, at most
0.005%, or at most 0.003%. Typically, the concentration of silicon within the
sweetener formulation or edible formulation is at most 0.002%, at most 0.001%,
or
the formulation is devoid of silicon.
EXAMPLES
Reference is now made to the following examples, which together with the
above descriptions, illustrate the invention in a non-limiting fashion.
25
8
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EQUIPMENT
Measuring
Instruments Manufacturer Model Units Geometry
range
IKA T 25
High shear IK A ULTRA- 3000-25000 rpm
mixer TURRAX
SiIverson L5M-A 0-8000 rpm
Vacuum mixer-
dryer
Stephan UMC 5 300-3000 1/min
(cooking
mixer)
Vacuum pump Vacuubrand MZ 2C NT 50 Hz
Laboratory
MRC Ltd DFO-150 25-250 C
oven
Ultra
Retsch ZM200 50 Hz
centrifugal mill
Schmidt +
Refractometer VariRef A 0.00-100 Bx %
Haensch
A/MUC Muco-
Texture Stable Micro
TA.XTplus 0-5000 gr adhesion
Test
analyzer Systems
Rig
MCR 92 Bob-cup
Rheometer Anton Paar GmbH 0-1000 1/s
P/N:159000 cylinder
MATERIALS
Material Manufacturer Type
FMC Biopolymers
Manucol DH
Corporation
Ingredients Solutions, Inc. Nalgin MV-120
Sodium alginate
TIC gum TICA-al gin C 400
Powder
Qingdao Lanneret Lanneret
Biochemical Co., Ltd
CP Kelco IIMP: GENU pectin
type
D 100 buffered
Pectin
1-IMP: Pre-Hydrated
TIC gum
Pectin 1694 Powder
9
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
H&F CS538
LMP: Unipectine of
Cargill
100C LMP
TIC gum LMP: TIC Pretested
Apple Pectin LMA
Goodchem Technology
HMP: Citrus Pectin HM
Co., Limited
Rama Gum Ricol
Guar gum TIC gum Pre-Hydrated Guar
Gum 8/24 Powder
Lucid Colloids Ltd. Edicol FGDG 8
Cargill CX911
Pre-Hydrated
Xanthan Gum TIC gum Ticaxan Xanthan
EC
NGMO
CP Kelco KELTROL
Nnexira Instant gum BB
Gum Arabic TIC gum Pre-Hydrated Gum
Arabic SF Powder
Norevo GmbH Gum acacia
CP Kelco Kelcogel LT100
TIC gum Ticagel Gellan L-
6
Ticagel Gellan HS
Gellan Gum TIC gum
NGMO
CP Kelco Kelcogel JD HA B
Amstel Products BY Gellan gum
TIC gum 100
Agar-Agar Marine Hydrocolloids Agar Agar
Gracilaria
Norevo GmbH Agar Agar
Konj ac-Mannan TIC gum High viscosity
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Konjac Mannan Gel
Gfn-Selco
Powder
BOC Sciences Konjac glucomannan
TIC gum HV
TIC gum 100
Tara Gum
Ingredients UK Ltd Tara gum
Amstel Products BV Tara gum
TIC gum POR/A2
Locust Bean Gum (LBG) CP Kelco GENU GUM Refined
Locust Bean Gum
Amstel Products BV LBG
7LF
7MF
Na-CMC Blanose
7HOF
9H4F
Ca-CMC Maple Biotech Pvt. Ltd. E.G.C. 505
Beneo Orafti High
Soluble Inulin
Filler -- Inulin Cosucra Fibruline
Sensus Frutafit CLR
Filler -- Fructo-
Galam GofosTM
oligosaccharide
Various common materials (sugars, polyols, etc.) have not been included in
this list.
Properties of CMC Materials
Manufacturer ' type Viscosity Degree of
i25 C,mPa*S1
substitution
7MF 400-600 (1%) 0.65 -
0.90
Blanose (Na- 7HOF 1000-2800 (1%) 0.65 -
0.90
CMC) 9H4F 2500-4500 (1%) 0.80 -
0.90
Maple Biotech
E.G.C. 505 0.5 - 0.7
Pvt. Ltd.
11
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EXAMPLE 1: Production of a Polysaccharide-Sweetener Slurry
A sweetener syrup containing one or more carbohydrate sweeteners and/or one
or more polyol (typically sugar alcohol) sweeteners, is prepared prior to the
addition
of the polysaccharide. The temperature of the sweetener syrup is generally
maintained within a range of 25 C to as much as 80 C, in some cases. For
sucrose,
the default temperature is 60 C. Various polysaccharides may be temperature-
sensitive, and may dictate the maximum temperature for the preparation
procedure.
The concentration of sweetener, with respect to water, is typically within a
range of
lwt%-65we/0 (may depend on the ratio between the polysaccharide and the
sweetener) for most of the carbohydrate and polyol sweeteners. Some of the
lower
solubility sweeteners may require relatively high water concentrations and/or
temperatures in order to fully dissolve. The polysaccharide is then added
incrementally under constant mixing. Once the polysaccharide addition has been
completed, the mixing vessel continues to be stirred for at least 7 minutes
using a high
shear mixer, until the polysaccharide is fully dispersed within the sweetener
syrup.
For polysaccharides that are more difficult to disperse, the water fraction
may
be pre-heated.
EXAMPLE 2: Production of a Dry Crystalline Powder
Polysaccharide-sweetener concentrate syrup (e.g., produced according to
Example 1) is transferred to the heated double-jacketed vessel of the vacuum
dryer
(e.g., Stephan). The vessel is heated (typically to 60 C-70 C), maintained
under
vacuum, and mixed constantly, so as to evaporate the water slowly over time,
eventually producing a polysaccharide-sweetener concentrate powder that is
typically
fine and dry. To further improve the crystallinity of the product, the vessel
may be
seeded with fine sweetener crystals. Optionally, the powder may be transferred
to an
oven (typically operating at 65 C) for further drying for several hours or
overnight
EXAMPLE 2A: Size Reduction of the Polysaccharide-Sweetener Powder
The polysaccharide-sweetener concentrate, typically in powder form, may
optionally undergo size reduction. The polysaccharide-sweetener powder may be
milled to produce a fine powder having a D50 that is typically within the
range of 75 to
300 micrometers, depending on the particular polysaccharide(s) in the
concentrate.
12
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EXAMPLE 3: Dilution of the Polysaccharide-Sweetener Concentrate to Produce a
Sweetener Ingredient
The polysaccharide-sweetener concentrate, typically having a Dso within a
range of 75 to 300 micrometers (e.g., having undergone size reduction as in
Example
2A), is diluted with at least one ordinary carbohydrate sweetener and/or at
least one
polyol (typically a sugar alcohol) sweetener to yield the desired amount of
polysaccharide in the sweetener formulation. For example: in order to prepare
a
"diluted- polysaccharide-sweetener formulation or -regular-strength
polysaccharide-
sweetener" formulation containing an average of 0.3% polysaccharide, from a
polysaccharide-sweetener concentrate containing 50% polysaccharide; 0.6 grams
of
the polysaccharide-sweetener concentrate formulation is mixed with 99.4 grams
of the
ordinary carbohydrate sweeteners (e.g., sucrose) and/or polyol sweetener.
EXAMPLE 4: Utilizing the Sweetener Ingredient to Produce an Edible Formulation
The "diluted" or "regular-strength" polysaccharide-sweetener formulation
(e.g., as produced according to Example 3), which may be a mixture of
polysaccharide-sweetener concentrate and ordinary sweetener, is added as an
ingredient, along with other ingredients, and may be mixed and optionally
processed
further (e.g., baked) to produce an edible formulation (e.g., cake, muffins,
biscuits).
EXAMPLE 5
Another way to utilize the polysaccharide-sweetener concentrate formulation
is by adding -- as separate ingredients -- the requisite amount of the
polysaccharide-
sweetener concentrate along with the ordinary sweetener (carbohydrate
sweetener
and/or polyol sweetener) during the preparation of the edible formulation
(e.g.,
muffins). For example: to obtain, within the edible formulation, a sweetener
having an
average polysaccharide concentration of 0.3% from an ordinary sweetener and a
concentrated polysaccharide-containing sweetener containing 50%
polysaccharide,
0.6 grams of the polysaccharide-sweetener concentrate is added along with 99.4
grams of the ordinary sweetener. The polysaccharide-sweetener concentrate and
the
ordinary sweetener may thus be added as separate components, and not as a
mixture.
EXAMPLE 6
A dispersion (slurry) containing 50% pectin formulation (C5538, H&F, 89%
galacturonic acid) and 50% sucrose was prepared according to Example 1: 100
grams
of pectin formulation were added gradually to a sucrose syrup containing 100
grams
13
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
sucrose and 500 grams water. The syrup containing the pectin was then
transferred to
the heated double-jacketed vessel of the vacuum dryer, which was heated and
maintained under vacuum according to Example 2, to produce a polysaccharide-
sweetener concentrate as a fine dry powder.
EXAMPLE 7
A dispersion (slurry) containing 70% pectin formulation (C S538, H&F) and
30% sucrose was prepared according to Example 1: 100 grams of pectin
formulation
were added gradually to sucrose syrup containing 42.8 grams sucrose and 500
grams
water. The syrup containing the pectin was then transferred to the heated
double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 8
A dispersion (slurry) containing 10% pectin formulation (C S538, H&F) and
90% sucrose was prepared according to Example 1: 100 grams of pectin
formulation
were added gradually to sucrose syrup containing 900 grams sucrose and 500
grams
water. The syrup containing the pectin was then transferred to the heated
double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 9
A dispersion (slurry) containing 90% pectin formulation (C S538, H&F) and
10% sucrose was prepared according to Example 1: 100 grams of pectin
formulation
were added gradually to sucrose syrup containing 11.1 grams sucrose and 500
grams
water. The syrup containing the pectin was then transferred to the heated
double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 10
A dispersion (slurry) containing 30% pectin formulation (C S538, H&F) and
70% sucrose was prepared according to Example 1: 100 grams of pectin
formulation
were added gradually to sucrose syrup containing 233.3 grams sucrose and 500
grams
water. The syrup containing the pectin was then transferred to the heated
double-
14
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 11
A dispersion (slurry) containing 30% sodium alginate formulation (Manucol
DH) and 70% sucrose was prepared according to Example 1: 100 grams of sodium
alginate formulation were added gradually to sucrose syrup containing 233.3
grams
sucrose and 500 grams water. The syrup containing the sodium alginate was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 12
A dispersion (slurry) containing 70% sodium alginate formulation (Manucol
DH) and 30% sucrose was prepared according to Example 1: 100 grams of sodium
alginate formulation were added gradually to sucrose syrup containing 42.8
grams
sucrose and 500 grams water. The syrup containing the sodium alginate was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 13
A dispersion (slurry) containing 50% sodium alginate formulation (Manucol
DH) and 50% sucrose was prepared according to Example 1. 100 grams of sodium
alginate formulation were added gradually to sucrose syrup containing 100
grams
sucrose and 500 grams water. The syrup containing the sodium alginate was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 14
A dispersion containing 1% pectin formulation (C S538, H&F) was prepared
according to Example 1: a concentrated sweetener syrup containing 650 grams
sucrose was prepared prior to the addition of the pectin. 6.5 grams of pectin
were then
dispersed in the concentrated sweetener syrup. The syrup was transferred to
the heated
double-jacketed vessel of the vacuum dryer, which was heated and maintained
under
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate
as a fine dry powder.
EXAMPLE 15
A dispersion containing 1.5% pectin formulation (C S538, H&F) was prepared
according to Example 1: a concentrated sweetener syrup containing 650 grams
sucrose was prepared prior to the addition of the pectin formulation. 9.75
grams of
pectin formulation were then dispersed in the concentrated sweetener syrup.
The
syrup was transferred to the heated double-jacketed vessel of the vacuum
dryer, which
was heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 15A
A dispersion containing 20% pectin formulation (C S538, H&F) was prepared
according to Example 1: a concentrated sweetener syrup containing 650 grams
sucrose was prepared prior to the addition of the pectin formulation. 130
grams of
pectin formulation were then dispersed in the concentrated sweetener syrup. No
seeding with sucrose crystals was conducted. The syrup was transferred to the
heated
double-jacketed vessel of the vacuum dryer, which was heated and maintained
under
vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate
as a fine dry powder.
The concentrate morphology was characterized by X-ray diffraction (XRD).
Figure 1 is an XRD plot of a solid sweetener concentrate formulation
containing 20%
polysaccharide (pectin) and 80% sweetener (sucrose), according to an aspect of
the
present invention. The sucrose is distinctly crystalline.
EXAMPLE 16
A dispersion containing 1% sodium alginate formulation (Manucol DH) was
prepared according to Example 1: a concentrated sweetener syrup containing 650
grams sucrose was prepared prior to the addition of the sodium alginate
formulation.
6.5 grams of sodium alginate formulation were then dispersed in the
concentrated
sweetener syrup. The syrup was transferred to the heated double-jacketed
vessel of the
vacuum dryer, which was heated and maintained under vacuum according to
Example
2, to produce a polysaccharide-sweetener concentrate as a fine dry powder.
16
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EXAMPLES 17-26
The formulations of Examples 6 to 15 were prepared, but using fructose
instead of sucrose.
EXAMPLE 27
A dispersion (slurry) containing 70% tara gum formulation (HV, TIC gum)
and 30% sucrose was prepared according to Example 1: 100 grams of tara gum
were
added gradually to sucrose syrup containing 42.8 grams sucrose and 500 grams
water.
The syrup containing the tara gum was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 28
A dispersion (slurry) containing 60% tara gum formulation (HV, TIC gum)
and 40% sucrose was prepared according to Example 1: 100 grams of tara gum
were
added gradually to sucrose syrup containing 66.6 grams sucrose and 500 grams
water.
The syrup containing the tara gum was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 29
A dispersion (slurry) containing 20% tara gum formulation (HV, TIC gum)
and 80% sucrose was prepared according to Example 1: 100 grams of tara gum
were
added gradually to sucrose syrup containing 400 grams sucrose and 500 grams
water.
The syrup containing the tara gum was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 30
A dispersion (slurry) containing 50% tara gum formulation (HV, TIC gum)
and 50% sucrose was prepared according to Example 1: 100 grams of tara gum
were
added gradually to sucrose syrup containing 100 grams sucrose and 500 grams
water.
The syrup containing the tara gum was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
17
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 31
A dispersion (slurry) containing 50% locust bean gum formulation (POR/A2,
TIC gum) and 50% sucrose was prepared according to Example 1: 100 grams of
locust bean gum formulation were added gradually to sucrose syrup containing
100
grams sucrose and 500 grams water. The syrup containing the locust bean gum
was
then transferred to the heated double-jacketed vessel of the vacuum dryer,
which was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 32
A dispersion (slurry) containing 70% locust bean gum formulation (POR/A2,
TIC gum) and 30% sucrose was prepared according to Example 1: 100 grams of
locust bean gum formulation were added gradually to sucrose syrup containing
42.86
grams sucrose and 500 grams water. The syrup containing the locust bean gum
was
then transferred to the heated double-jacketed vessel of the vacuum dryer,
which was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 33
A dispersion (slurry) containing 30% locust bean gum formulation (POR/A2,
TIC gum) and 70% sucrose was prepared according to Example 1: 100 grams of
locust bean gum formulation were added gradually to sucrose syrup containing
233.3
grams sucrose and 500 grams water. The syrup containing the locust bean gum
was
then transferred to the heated double-jacketed vessel of the vacuum dryer,
which was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 34
A dispersion (slurry) containing 90% locust bean gum formulation (POR/A2,
TIC gum) and 10% sucrose was prepared according to Example 1: 100 grams of
locust bean gum formulation were added gradually to sucrose syrup containing
11.1
grams sucrose and 500 grams water. The syrup containing the locust bean gum
was
then transferred to the heated double-jacketed vessel of the vacuum dryer,
which was
18
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 35
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 12 according to Example 1, and subsequently heating
under
vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate
as a fine dry powder. The powder was subjected to size reduction according to
Example 2A.
The milled polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.114 grams of the powder were mixed
with
79.885 grams of sucrose to yield 80 grams of the final sweetener formulation,
which
contained an average actual sodium alginate concentration of close to 0.1%.
EXAMPLE 36
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 32 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.114 grams of the powder were mixed
with
79.885 grams of sucrose to yield 80 grams of the final sweetener formulation,
which
contained an average actual locust bean gum concentration of close to 0.1%.
EXAMPLE 37
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 7 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.16 grams of the powder were mixed
with
99.84 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of about 0.1%.
19
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EXAMPLE 38
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 10 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.4 grams of the powder were mixed with
99.6 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of about 0.1%.
EXAMPLE 39
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 6 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.23 grams of the powder were mixed
with
99.77 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of about 0.1%.
EXAMPLE 40
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 6 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 1.12 grams of the powder were mixed
with
98.88 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of about 0.5%.
EXAMPLE 41
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 31 according to Example 1, and subsequently evaporating
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.2 grams of the powder were mixed with
99.8 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of close to 0.1%.
EXAMPLE 42
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 11 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.33 grams of the powder were mixed
with
99.67 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of close to 0.1%.
EXAMPLE 43
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 13 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 0.2 grams of the powder were mixed with
99.8 grams of sucrose to yield 100 grams of the final sweetener formulation,
which
contained an average actual polysaccharide concentration of close to 0.1%.
EXAMPLE 44
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 13 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
21
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
The polysaccharide-sweetener concentrate powder was then mixed with
ordinary sugar according to Example 3: 1.0 gram of the powder was mixed with
99
grams of sucrose to yield 100 grams of the final sweetener formulation, which
contained an average actual polysaccharide concentration of close to 0.5%.
EXAMPLE 45
A dispersion (slurry) containing 30% pectin formulation (CS538, H&F) and
70% allulose was prepared according to Example 1: 51.5 grams of pectin were
added
gradually to an allulose syrup containing 120 grams allulose and 480 grams
water.
The syrup containing the pectin was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 46
A dispersion (slurry) containing 50% locust bean gum formulation (POR/A2,
TIC gum) and 50% allulose was prepared according to Example 1: 100 grams of
locust bean gum were added gradually to an allulose syrup containing 100 grams
allulose and 500 grams water. The syrup containing the locust bean gum was
then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 47
A polysaccharide-sweetener concentrate was produced by processing the
formulation of Example 31 according to Example 1, and subsequently evaporating
under vacuum according to Example 2, to produce a polysaccharide-sweetener
concentrate as a fine dry powder. The powder was subjected to size reduction
according to Example 2A.
The polysaccharide-sweetener concentrate powder was then mixed with
allulose according to Example 3: 0.2 grams of the powder were mixed with 99.8
grams of allulose to yield 100 grams of the final sweetener formulation, which
contained an average actual polysaccharide concentration of close to 0.1%.
EXAMPLE 48
A dispersion (slurry) containing 30% guar gum (Ricol, Rama Gum) and 70%
sucrose was prepared according to Example 1: 100 grams of mung bean were added
22
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
gradually to a sucrose syrup containing 233.3 grams sucrose and 500 grams
water.
The syrup containing the mung bean was then transferred to the heated double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 49
A dispersion (slurry) containing 50% guar gum (Ricol, Rama Gum) and 50%
sucrose was prepared according to Example 1: 100 grams of guar gum were added
gradually to a sucrose syrup containing 100 grams sucrose and 500 grams water.
The
syrup containing the guar gum was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLE 50
A dispersion (slurry) containing 70% guar gum (Ricol, Rama Gum) and 30%
sucrose was prepared according to Example 1: 100 grams of guar gum were added
gradually to a sucrose syrup containing 42.86 grams sucrose and 500 grams
water.
The syrup containing the guar gum was then transferred to the heated double-
jacketed
vessel of the vacuum dryer, which was heated and maintained under vacuum
according to Example 2, to produce a polysaccharide-sweetener concentrate as a
fine
dry powder.
EXAMPLES 51-60
The formulations of Examples 6 to 15 were prepared, but using maltitol
instead of sucrose, and using 700 grams water.
EXAMPLE 61
A dispersion (slurry) containing 5% sodium alginate formulation (Manticol
DH) and 95% sucrose was prepared according to Example 1: 10 grams of sodium
alginate formulation were added gradually to sucrose syrup containing 190
grams
sucrose and 500 grams water. The syrup containing the polysaccharide was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
23
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EXAMPLE 61A
A polysaccharide-sweetener concentrate was produced: the powder of
Example 61 was subjected to size reduction according to Example 2A. The
polysaccharide-sweetener concentrate powder was then mixed with ordinary sugar
according to Example 3: 19 grams of the powder were mixed with 81 grams of
sucrose to yield 100 grams of the final sweetener formulation, which contained
an
average actual polysaccharide concentration of close to 0.95%.
EXAMPLE 62
A dispersion (slurry) containing 15% sodium alginate formulation (Manucol
DH) and 85% sucrose was prepared according to Example 1: 15 grams of sodium
alginate formulation were added gradually to a sucrose syrup containing 85
grams
sucrose and 500 grams water. The syrup containing the sodium alginate was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 62A
A polysaccharide-sweetener concentrate was produced: the powder of
Example 62 was subjected to size reduction according to Example 2A. The
polysaccharide-sweetener concentrate powder was then mixed with ordinary sugar
according to Example 3: 2 grams of the powder were mixed with 98 grams of
sucrose
to yield 100 grams of the final sweetener formulation, which contained an
average
actual polysaccharide concentration of close to 0.3%.
EXAMPLE 63
A dispersion (slurry) containing 95% sodium alginate formulation (Manucol
DH) and 5% sucrose was prepared according to Example 1: 95 grams of sodium
alginate formulation were added gradually to sucrose syrup containing 5 grams
sucrose and 500 grams water. The syrup containing the polysaccharide was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a
polysaccharide-sweetener concentrate as a fine dry powder.
EXAMPLE 63A
A polysaccharide-sweetener concentrate was produced: the powder of
Example 63 was subjected to size reduction according to Example 2A. The
24
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
polysaccharide-sweetener concentrate powder was then mixed with ordinary sugar
according to Example 3: 0.737 grams of the powder were mixed with 99.263 grams
of sucrose to yield 100 grams of the final sweetener formulation, which
contained an
average actual polysaccharide concentration of close to 0.7%.
EXAMPLES 64-67
The formulations of Examples 27 to 30 were prepared, but using sorbitol
instead of sucrose, and using 700 grams water.
EXAMPLE 68
A dispersion (slurry) containing 50% sodium carboxymethyl cellulose,
(Blanose 7MF) and 50% sucrose was prepared according to Example 1: 100 grams
of
the CMC formulation were added gradually to a sucrose syrup containing 100
grams
sucrose and 500 grams water. The syrup was then transferred to the heated
double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a CMC-sweetener concentrate as a fine dry
powder.
EXAMPLE 69
A dispersion (slurry) containing 50% sodium carboxymethyl cellulose
(Blanose 7LF) and 50% sucrose was prepared according to Example 1: 100 grams
of
the CMC formulation were added gradually to a sucrose syrup containing 100
grams
sucrose and 500 grams water. The syrup was then transferred to the heated
double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a CMC-sweetener concentrate as a fine dry
powder.
EXAMPLE 70
A dispersion (slurry) containing 15% sodium carboxymethyl cellulose
(Blanose 9H4F) and 85% sucrose was prepared according to Example 1: 15 grams
of
the CMC formulation were added gradually to a sucrose syrup containing 85
grams
sucrose and 500 grams water. The syrup was then transferred to the heated
double-
jacketed vessel of the vacuum dryer, which was heated and maintained under
vacuum
according to Example 2, to produce a CMC-sweetener concentrate as a fine dry
powder.
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
EXAMPLE 71
A dispersion (slurry) containing 5% sodium carboxymethyl cellulose (Blanose
7H0F) and 95% sucrose was prepared according to Example 1: 10 grams of the CMC
formulation were added gradually to sucrose syrup containing 190 grams sucrose
and
500 grams water. The syrup was then transferred to the heated double-jacketed
vessel
of the vacuum dryer, which was heated and maintained under vacuum according to
Example 2, to produce a polysaccharide-sweetener concentrate as a fine dry
powder.
EXAMPLE 72
A dispersion (slurry) containing 15% calcium carboxymethyl cellulose
(E.G.C. 505, Maple Biotech Pvt. Ltd.) and 85% sucrose was prepared according
to
Example 1: 15 grams of the CMC formulation were added gradually to a sucrose
syrup containing 85 grams sucrose and 500 grams water. The syrup was then
transferred to the heated double-jacketed vessel of the vacuum dryer, which
was
heated and maintained under vacuum according to Example 2, to produce a CMC-
sweetener concentrate as a fine dry powder.
EXAMPLE 73: Preparation of Muffin Samples
Three types of muffin samples may be prepared. Type I is a "full sugar"
control muffin, which may be similar in composition to typical, commercially
available muffins. Type II is an inventive, reduced-sugar muffin containing
the
inventive polysaccharide-sweetener or polysaccharide-sweetener concentrate.
Type
III is a reduced sugar control muffin, having the identical composition as the
Type II
inventive, reduced-sugar muffin, but being devoid of the polysaccharide in the
sweetener particles.
The batter for each type of muffin contains sugar, 14.2% sunflower oil, 21.8%
wheat flour (containing approximately 68% starch), 24.5% eggs, baking powder
(1.1%), flavors or flavorants (0.1%), salt (0.1%), and about 16.4% water. The
batter
of the Type I muffin contains 21.8 wt.% sugar.
A fructooligosaccharide is used as a filler to make up for the reduced amount
of sugar in the Type II and Type III samples. Typically, GofosTM (typically
containing 2% sugar) is utilized.
The Type II muffin utilizes a sweetener formulation from various exemplary
formulations (many of which are described or exemplified hereinabove). Aside
from
26
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
the formulative differences, the preparation and baking process is identical
for the
inventive muffin and the control muffins.
EXAMPLE 73A
Typically, the Type II inventive, reduced-sugar muffin contains 39.1% less
sugar with respect to the Type I "full sugar" control muffin. For this
exemplary case,
the Type II and Type III muffins are formulated such that the batter contains
about
(100%-39.1%)=21.8% = 13.3 wt.% sugar. The fructooligosaccharide (GofosTM)
content of the muffin batter is about 8.5wt% (21.8% - 13.38%).
EXAMPLE 73B
In many cases, the Type II inventive, reduced-sugar muffin may contain
reduced sugar in an amount other than the typical reduction of 39.1%. By way
of
(non-exhaustive) example, the Type II muffin may contain 50% less sugar, 35%
less
sugar, 20% less sugar, or 10% less sugar. For an exemplary case of 20% less
sugar,
the Type II muffin is formulated such that the batter contains about (100%-
20%).21.8% = 17.44 wt.% sugar, and 4.36 wt.% GofosTM (21.8% - 17.44%). In any
event, strictly for comparative purposes, the Type II muffin contains at least
10% less
sugar with respect to the Type I "full sugar" control muffin.
EXAMPLE 74: Preparation of Butter Cookie Samples
Three types of butter cookie samples may be prepared. Type I is a "full
sugar'.
control butter cookie, which may be similar in composition to typical,
commercially
available butter cookies. Type II is an inventive, reduced-sugar butter cookie
containing the inventive polysaccharide-sweetener or polysaccharide-sweetener
concentrate. Type III is a reduced sugar control butter cookie, having the
identical
composition as the Type II inventive, reduced-sugar butter cookie, but being
devoid
of the polysaccharide in the sweetener particles.
The batter for each type of butter cookie contains sugar, 14.6% palm oil,
49.42% wheat flour (containing approximately 68% starch), corn starch (4.2%),
water
(5.7%), egg (3.6%), soy lecithin (0.19%), baking powder (0.3%), salt (0.2%),
1.2%
invert sugar (containing 5% water), 1.5% heavy cream (containing 37% fat and
3.5%
lactose), flavor or flavorants (0.1%), with water being the remainder. The
sugar
content of the Type I butter cookie is about 19.0%.
27
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Inulin is used as a filler to make up for the reduced amount of sugar in the
Type II and Type III samples. Typically, Orafti High Soluble Inulin (which
contains
10% sugar) is utilized.
The Type II butter cookie utilizes a sweetener formulation from various
exemplary formulations (many of which are described or exemplified
hereinabove).
Aside from the formulative differences, the preparation and baking process is
identical for the inventive butter cookie and the control butter cookies.
EXAMPLE 74A
Typically, the Type II inventive, reduced-sugar butter cookie contains about
40% less sugar with respect to the Type I "full sugar" control butter cookie.
For this
exemplary case, the Type II and Type III butter cookies are formulated such
that the
batter contains about (100%-40.45%)-19.0% = 11.3 wt.% sugar. The inulin
content
of the batter is about 7.7wt.% (19.0% - 11.3%).
Substantially as in the case of the muffin samples provided hereinabove, in
many cases, the Type II inventive, reduced-sugar butter cookie may contain
reduced
sugar in an amount other than the typical reduction of about 40%. By way of
(non-
exhaustive) example, the Type II butter cookie may contain 50% less sugar, 40%
less
sugar, 35% less sugar, 20% less sugar, or 10% less sugar. Strictly for
comparative
purposes, the Type II butter cookie contains at least 10% less sugar with
respect to the
Type I -full sugar" control butter cookie.
EXAMPLE 75: Preparation of Hazelnut Spread Samples
Three types of hazelnut spread samples may be prepared. Type I is a "full
sugar- control hazelnut spread, which may be similar in composition to
typical,
commercially available hazelnut spreads. Type II is an inventive, reduced-
sugar
hazelnut spread containing the inventive polysaccharide-sweetener or
polysaccharide-
sweetener concentrate. Type III is a reduced sugar control hazelnut spread,
having the
identical composition as the Type II inventive, reduced-sugar hazelnut spread,
but
being devoid of the polysaccharide in the sweetener particles.
Each type of hazelnut spread contains sugar, hazelnut paste (15%), palm oil
(21.7%), cocoa powder (7.4%) having 12% fat, skim milk powder (6.6%), rapeseed
lecithin (0.2%) and flavors or flavorants (0.1%). The sugar content of the
Type 1
hazelnut spread is 49%.
28
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
A fructooligosaccharide is used as a filler to make up for the reduced amount
of sugar in the Type II and Type III samples. Typically, GofosTM is utilized.
The Type II hazelnut spread utilizes a sweetener formulation from various
exemplary formulations (many of which are described or exemplified
hereinabove).
Aside from the formulative differences, the preparation process is identical
for the
inventive hazelnut spread and the control hazelnut spreads.
EXAMPLE 75A
Typically, the Type II inventive, reduced-sugar hazelnut spread contains about
41% less sugar with respect to the Type I "full sugar" control hazelnut
spread. For
this exemplary case, the Type II and Type III hazelnut spreads are formulated
to
contain about (100%-41.2%)=49% = 28.8 wt.% sugar. The inulin content of the
hazelnut spread is about 20.2 wt.% (49% - 29.4 %).
Substantially as in the case of the hazelnut spread samples provided
hereinabove, in many cases, the Type II inventive, reduced-sugar hazelnut
spread may
contain reduced sugar in an amount other than the typical reduction of 40%. By
way
of (non-exhaustive) example, the Type II hazelnut spread may contain 50% less
sugar,
35% less sugar, 20% less sugar, or 10% less sugar. Strictly for comparative
purposes,
the Type II hazelnut spread contains at least 10% less sugar with respect to
the Type
"full sugar" control hazelnut spread.
EXAMPLE 76
Sensory Evaluation
The exemplary sweetener or edible formulations (e.g., muffins, butter cookies
and hazelnut spreads) may be evaluated by trained sensory panelists using a
paired-
comparison test. The paired-comparison test is a two-product blind test, and
the
panelists' task is to choose/indicate the sweeter one of the two products or
samples
(Sensory Evaluation Practices, 4t11 Ed., Stone, Bleibaum, Thomas, eds.). The
results
are analyzed using binomial distribution tables, which allows the sensory
scientist to
determine whether perceived differences between the samples are statistically
significant.
A Comparative Sweetness Index may be calculated from the paired-
comparison test results, compiled from all the panelists. For example, if,
among 17
panelists, 10 chose the inventive product as being sweeter, while the other 7
panelists
29
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
chose the comparative or control product, the Comparative Sweetness Index
(CSI)
would be calculated as:
CSI = (10/17)=100 = 58.8 = 59 (rounded)
EXAMPLE 76A
Another sensory method used to evaluate samples is difference magnitude
estimation (DME). Here, each panelist tastes the two samples, choose the
sweetest,
and also chooses the difference in sweetness, from the following list:
= No difference at all
= Extremely small difference
= Small difference
= Moderate difference
= Large difference
= Extremely large difference
Each choice is given a numerical value of 0 to 5 (with "0" being "No
difference at
all"), and the average of the panel is calculated. When the inventive,
polysaccharide-
containing sample is indicated as sweeter, the values are taken as positive,
and vice
versa). Generally, a difference of up to 1.0 (i.e., within an absolute value
of 1), and
in some cases, up to 0.8 or up to 0.5, is considered to be insignificant
(i.e., the
sweetness of the samples is substantially the same). An insignificant
difference is
considered to be a good result for the inventive formulation vs. the control
formulation.
EXAMPLES 77-78
Various formulations exemplified hereinabove were used to prepare butter
cookies samples, according to Examples 74 and 74A.
Pair-comparison test results of the pair-comparison tests, performed and
evaluated according to Examples 76 and 76A, are listed below in Table 1.
TABLE 1
% poly-
Example No. % Poly-
saccharide
A Poly- within the
of Poly- saccharide Poly-
Example saccharide in sugar
Comparative
saccharide - in sactharide . MED
No. Concentrate particles in
Sweetness
sweetener Concentrate Type
(Nominal) (Actual) the total Index (CSI)
concentrate edible
formulation
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
77 10 30 Pectin 0.3 0.33
58
78 7 70 Pectin 0.3 -0.14
43
EXAMPLE 79
Tensile strength/Detachment Force-Texture Analysis
The mucoadhesion properties of sweetener formulations were evaluated by
performing detachment tests using the TA.XTplus Texture Analyzer. The effect
of
various mucoadhesive species of polysaccharide on the adhesiveness of the
sweetener
formulation was also investigated, at various concentrations.
Materials and methods
Before the detachment tests were executed, the following steps were
performed: tablet preparation from sugar samples, preparation of artificial
saliva
buffer solution and trimming of fresh pig tongues to pieces of 30 mm X 30 mm
with
thickness of around 20 mm. The tongue tissues were frozen at -20'C. Before the
test,
the tongue tissue was heated to 37 C for 5 minutes. In terms of artificial
saliva, the
solution was prepared according to the following composition (Table 2):
TABLE 2: Artificial Saliva Composition
Na HCO3 2.5 mM
KCI 10 m M
NaCI 7.4 mM
CaCl2 1.5 mM
NaH2PO4 5.8 mM
Tablet preparation
Tablets, made from various sweetener samples provided hereinabove, were
prepared for detachment test using the Tableting Minipress MIT machine. "Dry
Mix"
samples were ground and mixed with magnesium steal ate (as a lubricant) at 2
w/vv%
in a Tumble Mixer for 2 minutes. The mixture was introduced to the Minipress
and
pressed at an upper punch penetration of 11 mm, to produce flat tablets. The
31
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
sweetener samples, produced according to Example 3 and further processed
according
to Example 6 (including further drying overnight), were pressed at a lower
upper
punch penetration of 7.5 ¨ 9 mm. For all samples, the preparation rate was
around 40
tablets/minute, in automatic mode. The diameter of the tablet is 10 mm.
Detachment Tests
The trimmed pig tongue piece was pressure-fixed between a plastic platform
and a lid, by means of four screws. A hole (13 mm in diameter), disposed in
the
middle of the lid, enables tablet-tongue contact. The plastic platform and pig
tongue
arrangement was maintained in the artificial saliva solution under constant
temperature of 37 C. A sweetener tablet was attached to the Texture Analyzer
(TA)
probe (cylinder) by means of a double-sided adhesive tape. The measurement was
performed using the following procedure: the probe, together with the tablet,
was
lowered at constant speed until a pre-determined applied force was exerted,
for a fixed
contact time, with the tongue tissue. Once finished, the probe and tablet were
lifted,
and the (maximum) detachment force (Fmax) and detachment work (area between
the
curve and X-axis, also termed "total work of adhesion") were recorded for each
of the
sweetener tablets. The whole process was controlled by the TA adhesion test
rig,
utilizing the settings provided in Table 3.
TABLE 3: Measurement conditions for the detachment tests
Pre-test speed 0.5 mim/s
Test speed 0.5 mim/s
Post-test speed 0.1 mm/s
Applied force 200 gr
Return distance 5.0 mm
Contact time 40 sec
Trigger force 5.0 gr
Saliva buffer amount 100 1.1L
32
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
As used herein, the above-described detachment test procedure is referred to
as a "standard detachment test".
Tablets of various sweetener samples were evaluated to determine the
maximum detachment force and the work of detachment, using the equipment and
procedures disclosed in Example 79.
In some embodiments, the mucosal adhesion of the sweetener formulation, as
characterized by the maximum detachment force, is greater than that of the
control
composition, (i.e., a formulation being devoid of the polysaccharide, but
being
otherwise identical to the sweetener formulation in both composition and
preparation
method). Typically, the mucosal adhesion of the sweetener formulation, as
characterized by the maximum detachment force (or by the maximum force of
detachment determination (FD_D), defined hereinbelow), is greater than that of
the
control composition by at least 1%, at least 1.5%, at least 2%, at least 3%,
or at least
4%, and in some cases, at least 5%, at least 7%, at least 10%, at least 12%,
or at least
15%.
The inventors have further discovered that at relatively high levels of
mucosal
adhesion (e.g., as characterized by at least one of the maximum detachment
force and
the work of detachment), the presence of the polysaccharide may actually be
detrimental to the sweetness of the food or formulation, as perceived by taste-
testing.
Thus, in some embodiments, the mucosal adhesion of the sweetener
formulation, as characterized by the maximum detachment force (or by FD_D), is
greater than that of the control composition by at most 200%, at most 150%, at
most
100%, at most 80%, and more typically, at most 60%, at most 50%, at most 40%,
at
most 35%, or at most 30%.
In some embodiments, the mucosal adhesion of the sweetener formulation, as
characterized by the maximum detachment force (or by FD_D), is greater than
that of
the control composition by a value within a range of 1% to 200%, 1% to 120%,
1% to
80%, 1% to 60%, 1% to 40%, 1% to 30%, 1% to 25%, 1% to 20%, 1.5% to 60%,
1.5% to 40%, 1.5% to 30%, 1.5% to 25%, 1.5% to 20%, 2% to 200%, 2% to 120%,
2% to 80%, 2% to 60%, 2% to 50%, 2% to 40%, 2% to 30%, 2% to 25%, 2% to 20%,
3% to 80%, 3% to 60%, 3% to 40%, 3% to 30%, 3% to 25%, 3% to 20%, 4% to 60%,
4% to 40%, 4% to 30%, 4% to 25%, 4% to 20%, 5% to 60%, 5% to 40%, 5% to 30%,
5% to 25%, 5% to 20%, 6% to 60%, 6% to 40%, 6% to 30%, 6% to 25%, 6% to 20%,
33
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
8% to 50%, 8% to 30%, 8% to 25%, 8% to 20%, 10% to 50%, 10% to 30%, 10% to
25%, or 10% to 20%.
In some embodiments, the mucosal adhesion of the sweetener formulation, as
characterized by the work of detachment (or by the detachment work (WD),
defined
hereinbelow), is greater than that of the control composition, (i.e., as
above, a
formulation being devoid of the polysaccharide, but being otherwise identical
to the
sweetener formulation in both composition and preparation method). Typically,
the
mucosal adhesion of the sweetener formulation, as characterized by the work of
detachment, is greater than that of the control composition by at least 1%, at
least
1.5%, at least 2%, at least 3%, at least 5%, at least 7%, at least 10%, at
least 20%, at
least 30%, at least 40%, or at least 45%.
In some embodiments, the mucosal adhesion of the sweetener formulation, as
characterized by the work of detachment (or by WD), is greater than that of
the control
composition by at most 200%, at most 150%, at most 125%, at most 110%, at most
100%, at most 90%, at most 80%, at most 70%, at most 60%, or at most 50%.
In some embodiments, the mucosal adhesion of the sweetener formulation, as
characterized by the work of detachment (or by WD), is greater than that of
the control
composition by a value within a range of 10% to 150%, 10% to 125%, 10% to
100%,
10% to 80%, 20% to 150%, 20% to 125%, 20% to 100%, 20% to 80%, 30% to 150%,
30% to 125%, 30% to 100%, 30% to 80%, 40% to 150%, 40% to 125%, 40% to
100%, 40% to 80%, 50% to 150%, 50% to 125%, 50% to 100%, or 50% to 90%.
As used herein in the specification and in the claims section that follows,
the
term "maximum detachment force" (Farnax) refers to the maximum detachment
force
as measured by the standard detachment test.
As used herein in the specification and in the claims section that follows,
the
term "detachment work" (WD) refers to the work of detachment as measured by
the
standard detachment test.
As used herein in the specification and in the claims section that follows,
the
term "work of detachment determination" (WD_D) for a sweetener formulation
containing a particular species of polysaccharide within the sweetener
particles
thereof, refers to the work of detachment for the identical vegetable-protein-
containing sweetener formulation, but having a concentration of 1% of that
particular
34
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
species of polysaccharide with respect to the sweetener, and prepared and
measured
according to the standard procedure of Example 79, the obtained detachment
work
(We) then being linearly applied using a coefficient Kconc based on the actual
concentration (Cactual), in %, of that particular polysaccharide disposed
within the
sweetener particles of the formulation. Similarly, as used herein in the
specification
and in the claims section that follows, the term -maximum force of detachment
determination" (FD_D) for a sweetener formulation containing a particular
species of
polysaccharide within the sweetener particles thereof, refers to the maximum
detachment force (FDinax) for the identical vegetable-protein-containing
sweetener
formulation, but having a concentration of 1% of that particular species of
polysaccharide with respect to the sweetener, and prepared and measured
according to
the standard procedure of Example 79, the obtained maximum detachment force
(FD.) then being linearly applied using a coefficient Kconc based on the
actual
concentration (Cactual), in %, of that particular species of polysaccharide
disposed
within the sweetener particles of the formulation. Thus:
Kconc = Cactual / 1% (A)
FD-D = Kconc = EDmax (B)
WD-D =Kconc = WD (C)
As used herein in the specification and in the claims section that follows,
the
term "mucosal adhesion" and the like, with respect to a formulation, is meant
to refer
to mucosal adhesion as exhibited by at least one of maximum detachment force
(FDmax), maximum force of detachment determination (FD_D), detachment work
(WD),
and work of detachment determination (WD-D).
EXAMPLE 80: Rheological Characterization of Mucoadhesivity
The mucoadhesive properties of various species of polysaccharide were
characterized using rheological measurements. It is known that the rheological
behavior of the mixture containing the mucoadhesive polysaccharide and mucin
may
be appreciably influenced by chemical interactions, conformational changes and
chain
interlocking between the two species. Rheological techniques are used to study
the
deformation of material and their flow behavior under shear. Such measurement
allows monitoring the interactions between polymers (Hassan and Gallo, 1990).
Interactions between the mucoadhesive polysaccharides and the mucin are
manifested
by viscosity enhancement, such that the viscosity of the mixture exceeds the
sum of
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
the individual viscosities of the mucin and the polysaccharide. Thus, by
measuring
the individual viscosities, along with the viscosity of the mucin ¨ vegetable-
protein
mixture, the mucoadhesive force between the mucin and the polysaccharide may
be
characterized, according to the following equation:
Ilt=11m+11p+Ilb
where it is the total (measured) viscosity of the system (mixture), ib is the
viscosity
component of bioadhesion (viscosity enhancement), im and ip are the
individually-
measured viscosities of mucin and polysaccharide single-component dispersions,
respectively.
Various polysaccharide dispersions of 2 wt% in distilled water were prepared
according to the manufacturer instructions and were gently mixed for 3 hours.
Dried
mucin was hydrated with distilled water (sufficient to make a lOwt%
dispersion) by
gentle stirring for 1 hour at room temperature followed by sonication of 10
minutes
(at room temperature). The mucin solution was then gently stirred for 2 hours
to yield
the 1 Owt% mucin dispersion. Equal amounts of each polysaccharide dispersion
and
the lOwt% mucin dispersion were mixed to yield a final concentration of lwt%
polysaccharide and 5wt% mucin for each mixed dispersion. All mixture systems
were maintained at 37 C for 1 hour to equilibrate prior to analysis.
All measurements were performed using the Anton Paar MRC92 rheometer
having a Peltier temperature chamber: C-PTD 180/air, rotating bob (CC27
concentric
cylinder) and a fixed cup (C-CC27/SS/AIR) having a diameter of 28.992mm. Prior
to
the measurement, each sample formulation was allowed to rest for another 2
minutes.
The measurements were performed at 37 C at a shear rate ranging between 0.1-
350 s-1
(logarithmic ramp).
Measurements for each polysaccharide (1 wt%) dispersion and for a 5 wt%
mucin dispersion were performed in order to yield the individual viscosities
(rip,
The enhanced viscosity (bioadhesion) was then calculated for each vegetable-
protein -
mucin, according to the above-provided equation
The mucoadhesive properties of various samples of were characterized using
the rheologi cal equipment and methodology provided in Example 80
It was found that a particular species of polysaccharide can be considered to
be
mucoadhesive, or to be a mucoadhesive agent, if the bioadhesion viscosity
36
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
component (rib), as measured according to the standard procedure of Example
80, at a
polysaccharide concentration of 1%, is at least 3 mPa=s. More typically, rib
is at least
mPa-s, at least 7 mPa-s, or at least 10 mPa=s. As used herein in the
specification
and in the claims section that follows, this determination of mucoadhesivity
(i.e.,
5
whether the polysaccharide is considered to be mucoadhesive, or to be a
mucoadhesive agent) is referred to as a "standard rheological determination-.
Typically, this bioadhesion viscosity component (rib) is within a range of 2-
400 mPa-s, 2.5-400 mPa-s, 2-350 mPa=s, 2.5-350 mPa-s, 3-400 mPa-s, 3-350
mPa=s,
3-300 mPa-s, 3-250 mPa-s, 3-200 mPa-s, 3-150 mPa-s, 4-400 mPa-s, 4-350 mPa-s,
4-
300 mPa=s, 4-250 mPa=s, 5-400 mPa=s, 5-350 mPa=s, 5-300 mPa=s, 5-250 mPa=s, 5-
200 mPa=s, 5-150 mPa=s, 6-400 mPa=s, 6-350 mPa=s, 6-300 mPa=s, 6-200 mPa=s, 6-
150 mPa-s, 7-200 mPa-s, 7-150 mPa-s, 8-200 mPa-s, 8-150 mPa-s, 10-200 mPa-s,
10-
150 mPa=s, 10-100 mPa=s, 12-200 mPa=s, 12-150 mPa=s, 15-200 mPa=s, 15-150
mPa=s, 20-200 mPa=s, 20-150 mPa=s, or 20-100 mPa=s.
As used herein in the specification and in the claims section that follows,
the
term "bioadhesive concentration of polysaccharide" and the like refers to a
particular
concentration of at least one species of polysaccharide disposed within the
sweetener
particles of a formulation, the particular concentration of the at least one
species of
polysaccharide being sufficient to attain a value of at least 3 mPa=s for a
bioadhesion
viscosity component (rib), as measured according to the standard procedure of
Example 80, but at that particular concentration.
As used herein in the specification and in the claims section that follows,
the
term "bioadhesive content of polysaccharide- and the like, with respect to a
vegetable-protein -containing formulation, refers to an actual concentration
(Cactual) of
at least one species of polysaccharide disposed within the sweetener particles
of the
formulation, the actual concentration being sufficient to attain a bioadhesion
viscosity
increase (Arips)
of at least 1.0 mPa=s, wherein the bioadhesion viscosity
component (rib) is measured according to the standard procedure of Example 80
at a
concentration of 1% polysaccharide, and then linearly applied to obtain Arips
using a
coefficient Kconc based on the actual concentration (Cactual), in %, of the at
least one
species of polysaccharide disposed within the sweetener particles of the
formulation:
Kconc = Cactuai / 1% (I)
37
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
bioadhesion viscosity increase (/.rips) = Kcon, = lib (II)
Thus, when the bioadhesion viscosity increase (Arips) is at least 1.0 mPa-s
for Cactual,
the formulation is deemed to have a bioadhesive content of polysaccharide.
As used herein in the specification and in the claims section that follows,
the
terms "bioadhesive formulation", "bioadhesive sweet formulation" and the like
refer
to a formulation containing at least one of a bioadhesive concentration of
polysaccharide and a bioadhesive content of polysaccharide.
EXAMPLE 81: Exemplary Starch Content Calculation
A cookie is made from fat (palm oil, 17%), white wheat flour (61%), sucrose
(11%), a polysaccharide-sweetener concentrate of Example 8 (1%), and a fructan
(inulin, 10%). The only starch-containing ingredient is the white wheat flour,
which
contains about 68% starch. Thus, the starch content of the cookie is 68% of
61%, or
about 41.5%.
EXAMPLE 82: Exemplary Fat Content Calculation
A hazelnut spread is made from fat (palm oil, 24%), sucrose (28%), a
polysaccharide-sweetener concentrate of Example 11 (2%), pure hazelnut paste
(13%,
having a 61% fat content), non-fat milk powder (6%), cocoa powder (7% having a
12% fat content) and a fructan (inulin, 20%). The total fat content of the
hazelnut
spread is 24% + (61% of 13%) + (12% of 7%), or about 32.8%.
Additional Embodiments
Additional Embodiments (Clauses) 1 to 171 are provided hereinbelow.
Embodiment 1. A sweet formulation comprising:
(a) sweetener particles containing a first sweetener; and
(b) crystalline sugar particles;
wherein a polysaccharide is disposed within the sweetener particles;
and wherein a first weight ratio of the polysaccharide to the first sweetener
is within a
range of 1:100 to 95:5.
Embodiment 1A. A sweet formulation comprising:
(a) sucrose particles; and
(b) crystalline sugar particles;
38
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
wherein a polysaccharide is disposed within the sucrose particles;
and wherein a first weight ratio of the polysaccharide to the sucrose in the
sucrose
particles is within a range of 1:100 to 95:5.
Embodiment 1B. The formulation of Embodiment 1 or 1A, wherein
the sugar of
the crystalline sugar particles is sucrose.
Embodiment 1C. The formulation of Embodiment 1B, wherein the
crystalline
sugar particles is table sugar.
Embodiment 2. The formulation of any one of Embodiments 1 to
1C, wherein
at least 20% of the total amount of sweetener within the sweet formulation, by
weight,
is crystalline.
Embodiment 3. The formulation of any one of the preceding
Embodiments,
wherein at least 50% of the total amount of sweetener within the sweet
formulation,
by weight, is crystalline.
Embodiment 4. The sweet formulation of any one of the
preceding
Embodiments, wherein a polysaccharide-sweetener concentrate consisting of the
sweetener particles, including the polysaccharide, when provided within a
standard
reduced sugar edible formulation, is less sweet with respect to a standard
reduced
sugar control edible formulation that is identical to the standard reduced
sugar edible
formulation, but devoid of the polysaccharide.
Embodiment 5. The sweet formulation of Embodiment 4, wherein, when the
entire sweet formulation is provided within the standard reduced sugar edible
formulation, the standard reduced sugar formulation exhibits improved
sweetness
with respect to the standard reduced sugar edible formulation.
Embodiment 6. The sweet formulation of any one of Embodiments
1 to 3,
wherein a polysaccharide-sweetener concentrate consisting of the sweetener
particles,
including the polysaccharide, is less sweet with respect to a first control
sweetener
that is identical to the polysaccharide-sweetener concentrate, but devoid of
the
polysaccharide.
Embodiment 7. The sweet formulation of Embodiment 6, wherein
the sweet
formulation exhibits improved sweetness with respect to a second control
sweetener
that is identical to the sweet formulation, but devoid of the polysaccharide
39
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 8. The sweet formulation of any one of the
preceding
Embodiments, wherein a second weight ratio of a total polysaccharide weight
(PS
total)
of the polysaccharide in the sweetener particles and any polysaccharide
disposed in
the crystalline sugar particles, to the total weight of the first sweetener
and the
crystalline sugar particles, is within a range of 0.02% to 50%.
Embodiment 9. The sweet formulation of Embodiment 8, wherein
the second
weight ratio is within a range of 0.02% to 20%.
Embodiment 10. The sweet formulation of Embodiment 8, wherein
the second
weight ratio is within a range of 0.02% to 10%.
Embodiment 11. The sweet formulation of Embodiment 8, wherein the second
weight ratio is within a range of 0.02% to 3%.
Embodiment 12. The sweet formulation of Embodiment 8, wherein
the second
weight ratio is at most 1%, at most 0.6%, or at most 0.3%.
Embodiment 13. The sweet formulation of any one of the
preceding
Embodiments, wherein, within the sweetener particles, a weight ratio R is
defined by
R = Wsweetener-a / Wsweetener-c,
wherein:
Wsweetener-a is the weight of any amorphous sucrose; and
Wsweetener-c is the weight of the crystalline sucrose;
and wherein R is at most 5:1.
Embodiment 14. The sweet formulation of Embodiment 13, wherein
the
sweetener includes, or predominantly includes sucrose.
Embodiment 15. The sweet formulation of Embodiment 13, wherein
the
sweetener is sucrose.
Embodiment 16. The sweet formulation of any one of Embodiments 13 to 15,
wherein R is at most at most 3.3:1.
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 17. The sweet formulation of Embodiment 16, wherein
R is at most
at most 0.8:1.
Embodiment 18. A food formulation comprising:
(a) the sweet formulation of any one of the preceding
Embodiments;
(b) a fat;
(c) optionally, a starch; and
(d) optionally, an edible filler;
wherein a total concentration of the first sweetener, the crystalline sugar,
the fat, and
the starch, within the food formulation, is at least 20%, on a weight basis;
wherein the food formulation exhibits improved sweetness with respect to a
control
edible formulation that is identical to the food formulation, but devoid of
the
polysaccharide;
and wherein, within the food formulation, at least 60% of the total amount of
sweetener, by weight, is crystalline.
Embodiment 18A A food formulation comprising:
(al) sweetener particles containing a first sweetener;
(a2) crystalline sugar particles;
wherein a polysaccharide is disposed within the sweetener particles;
and wherein a first weight ratio of the polysaccharide to the first sweetener
is within a
range of 1:100 to 95:5;
(b) a fat;
(c) optionally, a starch; and
(d) optionally, an edible filler;
wherein a total concentration of the first sweetener, the crystalline sugar,
the fat, and
the starch, within the food formulation, is at least 20%, on a weight basis;
wherein the food formulation exhibits improved sweetness with respect to a
control
edible formulation that is identical to the food formulation, but devoid of
the
polysaccharide;
41
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 18B. The food formulation of Embodiment 18 or 18A,
wherein,
within the food formulation, at least 60% of the total amount of sweetener, by
weight,
is crystalline.
Embodiment 19. The food formulation of any one of Embodiments
18 to 18B,
wherein at least 95% of the total amount of sweetener, by weight, within the
food
formulation, is crystalline.
Embodiment 20. The food formulation of any one of Embodiments
18 to 19,
wherein a total weight content of sweeteners within the food formulation is
within a
range of 10% to 80%.
Embodiment 21. The food formulation of any one of Embodiments 18 to 20, the
food formulation containing at least 5% of the fat.
Embodiment 22. The food formulation of Embodiment 21, the food
formulation
containing at least 5% of the starch.
Embodiment 23. The food formulation of any one Embodiments 18
to 22,
containing at least 2% of the edible filler.
Embodiment 24. The food formulation of Embodiment 23,
containing at least
5% of the edible filler.
Embodiment 25. The food formulation of Embodiment 23,
containing at least
10% of the edible filler.
Embodiment 26. The food formulation of any one of Embodiments 18 to 25,
wherein a total concentration of the first sweetener, the crystalline sugar,
the fat, the
starch, and the edible filler, within the food formulation, is at least 50%,
on a weight
basis.
Embodiment 27. The food formulation of any one of Embodiments
18 to 25,
wherein a total concentration of the first sweetener, the crystalline sugar,
the fat, the
starch, and the and the edible filler, within the food formulation, is at
least 70%, on a
weight basis.
Embodiment 28. The food formulation of any one of Embodiments
18 to 27,
wherein the edible filler is a dietary fiber.
Embodiment 29. The food formulation of any one of Embodiments 18 to 28,
wherein the control edible formulation is a standard reduced sugar control
edible
formulation.
42
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 29A. The food formulation of any one of Embodiments
18 to 29,
wherein the food formulation is a flour confection.
Embodiment 30. The food formulation of any one of Embodiments
18 to 29,
wherein the food formulation is a sugar confection.
Embodiment 30A. An edible formulation comprising:
a first population of sweetener particles, the sweetener particles including:
(a) crystalline sucrose; and
(b) optionally, amorphous sucrose;
wherein a total amount of sucrose within the sweetener particles includes the
crystalline sucrose and the amorphous sucrose;
wherein a polysaccharide is disposed as at least one polysaccharide particle
in each
sweetener particle of the sweetener particles;
and wherein, within the first population of sweetener particles:
(i) a first weight ratio of the polysaccharide to the total amount of sucrose
is within a range of 1:100 to 95:5; and
(ii) a second weight ratio of the amorphous sucrose to the crystalline
sucrose is at most 5:1.
Embodiment 31. An edible formulation comprising:
a first population of sweetener particles, the sweetener particles including:
(a) crystalline sucrose; and
(b) optionally, amorphous sucrose;
wherein a total amount of sucrose within the sweetener particles includes the
crystalline sucrose and the amorphous sucrose;
wherein a polysaccharide is disposed as at least one polysaccharide particle
in each
sweetener particle of the sweetener particles;
and wherein, within the first population of sweetener particles, a first
weight ratio of
the polysaccharide to the total amount of sucrose is within a range of 6:100
to 95:5.
Embodiment 32. The formulation of any one of Embodiments 1 to
31, wherein
the first sweetener and the at least one polysaccharide make up at least 30%
of the
formulation.
43
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 33. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 40%
of the
formulation.
Embodiment 34. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 50%
of the
formulation.
Embodiment 35. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 60%
of the
formulation.
Embodiment 36. The formulation of any one of Embodiments 1 to 32, wherein
the first sweetener and the at least one polysaccharide make up at least 70%
of the
formulation.
Embodiment 37. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 80%
of the
formulation.
Embodiment 38. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 85%
of the
formulation.
Embodiment 39. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 90%
of the
formulation.
Embodiment 40. The formulation of any one of Embodiments 1 to
32, wherein
the first sweetener and the at least one polysaccharide make up at least 95%
of the
formulation.
Embodiment 41. The formulation of any one of the preceding Embodiments,
wherein the first sweetener includes allulose.
Embodiment 42. The formulation of any one of the preceding
Embodiments,
wherein the sweetener carbohydrate includes sucrose.
Embodiment 43. The formulation of any one of the preceding
Embodiments,
wherein the sweetener carbohydrate is predominantly sucrose.
Embodiment 44. The formulation of any one of the preceding
Embodiments,
wherein the sweetener carbohydrate includes glucose.
44
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 45. The formulation of any one of the preceding
Embodiments,
wherein the sweetener carbohydrate includes fructose.
Embodiment 46. The formulation of any one of the preceding
Embodiments,
wherein the sweetener polyol is selected from at least one of the group
consisting of
xylitol, maltitol, erythritol, sorbitol, threitol, arabitol, hydrogenated
starch
hydrolysates (HSH), isomalt, lactitol, mannitol, and galactitol (dulcitol).
Embodiment 47. The formulation of any one of the preceding
Embodiments,
wherein the sweetener formulation is in the form of a particulate solid such
as a free-
flowing powder.
Embodiment 48. The formulation of Embodiment 47, wherein the particulate
solid is a powder.
Embodiment 49. The sweetener formulation of any one of the
preceding
Embodiments, wherein the concentration of silicon within the sweetener
formulation
is at most 0.2%, at most 0.1%, or at most 0.05%.
Embodiment 50. The sweetener formulation of any one of the preceding
Embodiments, wherein the concentration of silicon within the sweetener
formulation
is at most 0.01%, at most 0.005%, or at most 0.003%.
Embodiment 51. The formulation of any one of the preceding
Embodiments,
wherein glycosidic linkages within the at least one polysaccharide are 0-
glycosidic
linkages [oxygenic linkages (-0-)];
Embodiment 52. The formulation of any one of the preceding
Embodiments,
wherein an average molecular weight of the at least one polysaccharide
disposed
within the sweetener particles, in Daltons, is within a range of 8,000 to
2,000,000.
Embodiment 53. The formulation of any one of the preceding
Embodiments,
wherein an average degree of polymerization of the at least one polysaccharide
disposed within the sweetener particles is within a range of 50 to 40,000
monosaccharide building blocks.
Embodiment 54. The edible formulation of any one of the
preceding
Embodiments, wherein the at least one polysaccharide is a mucoadhesive agent.
Embodiment 55. The edible formulation of any one of the preceding
Embodiments, wherein a or the mucosa] adhesion of the edible formulation is
greater
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
than that of a control formulation, the control formulation being devoid of
the at least
one polysaccharide, but being otherwise identical to the edible formulation.
Embodiment 56. The edible formulation of any one of the
preceding
Embodiments, wherein a or the mucosal adhesion of the edible formulation is
greater
than that of a control formulation by a value of at least 10%, and optionally,
at least
20%, at least 30%, at least 40%, at least 50%, at least 75%, or at least 100%,
the
control formulation being devoid of the at least one polysaccharide, but being
otherwise identical to the edible formulation.
Embodiment 57. The edible formulation of any one of Embodiments
1 to 56,
wherein a or the mucosal adhesion of the edible formulation is greater than
that of a
control formulation by a value of 5% to 200%, the control formulation being
devoid
of the at least one polysaccharide, but being otherwise identical to the
edible
formulation.
Embodiment 58. The edible formulation of any one of Embodiments
55 to 57,
wherein the mucosal adhesion of the edible formulation is greater than that of
the
control formulation by a value of 10% to 90%.
Embodiment 59. The edible formulation of any one of Embodiments
55 to 57,
wherein the mucosal adhesion of the edible formulation is greater than that of
the
control formulation by a value of 10% to 50%, 15% to 90%, 15% to 80%, 15% to
70%, 15% to 50%, 20% to 90%, 20% to 70%, 25% to 90%, or 25% to 70%.
Embodiment 60. The edible formulation of any one of Embodiments
55 to 57,
wherein the mucosal adhesion of the edible formulation is greater than that of
the
control formulation by a value of 10% to 70%.
Embodiment 61. The edible formulation of any one of the
preceding
Embodiments, wherein the a value of the mucosal adhesion of the edible
formulation
is determined by a standard maximum detachment force determination.
Embodiment 62. The edible formulation of any one of the
preceding
Embodiments, wherein a or the mucosal adhesion of the edible formulation is
determined by a standard work of detachment determination.
Embodiment 63. The formulation of any one of the preceding Embodiments,
wherein an average molecular weight of the polysaccharide disposed within the
sweetener particles, in Daltons, is within a range of 10,000 to 2,000,000.
46
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 64.
The formulation of any one of the preceding Embodiments,
wherein an average degree of polymerization of the polysaccharide disposed
within
the sweetener particles is within a range of 50 to 40,000 monosaccharide
building
blocks.
Embodiment 65. The
formulation of any one of the preceding Embodiments,
wherein the formulation is a bioadhesive formulation.
Embodiment 66.
The formulation of Embodiment 73, wherein the bioadhesive
formulation contains a bioadhesive concentration of polysaccharide.
Embodiment 67.
The formulation of Embodiment 73, wherein the bioadhesive
formulation contains a bioadhesive content of polysaccharide.
Embodiment 68.
The formulation of any one of the preceding Embodiments,
wherein the average molecular weight of the polysaccharide disposed within the
sweetener particles, in Daltons, is within a range of 15,000 to 2,000,000;
35,000 to
2,000,000; 50,000 to 2,000,000; 75,000 to 2,000,000; 100,000 to 2,000,000;
100,000
to 1,500,000; 100,000 to 1,000,000; 150,000 to 2,000,000; 200,000 to
2,000,000;
200,000 to 1,500,000; 200,000 to 1,200,000; 200,000 to 1,000,000; 300,000 to
2,000,000; 300,000 to 1,500,000; 300,000 to 1,200,000; 300,000 to 1,000,000;
300,000 to 800,000; 150,000 to 400,000; 100,000 to 800,000; 100,000 to
650,000;
100,000 to 500,000; or 100,000 to 400,000.
Embodiment 69. The
formulation of any one of the preceding Embodiments,
wherein the average molecular weight of the polysaccharide disposed within the
sweetener particles, in Daltons, is within a range of 15,000 to 2,000,000.
Embodiment 70.
The formulation of Embodiment 69, wherein the average
molecular weight of the polysaccharide disposed within the sweetener
particles, in
Daltons, is within a range of 35,000 to 1,200,000.
Embodiment 71.
The formulation of Embodiment 69, wherein the average
molecular weight of the polysaccharide disposed within the sweetener
particles, in
Daltons, is within a range of 50,000 to 1,000,000.
Embodiment 72.
The formulation of Embodiment 69, wherein the average
molecular weight of the polysaccharide disposed within the sweetener
particles, in
Daltons, is within a range of 15,000 to 400,000.
47
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 73.
The formulation of any one of the preceding Embodiments,
wherein the average degree of polymerization of the polysaccharide disposed
within
the sweetener particles is within a range of 50 to 10,000 monosaccharide
building
blocks.
Embodiment 74. The
formulation of Embodiment 73, wherein the average
degree of polymerization of the polysaccharide disposed within the sweetener
particles is within a range of 50 to 1,500 monosaccharide building blocks.
Embodiment 75.
The formulation of Embodiment 73, wherein the average
degree of polymerization of the polysaccharide disposed within the sweetener
particles is at least 120 monosaccharide building blocks.
Embodiment 76.
The formulation of any one of the preceding Embodiments,
wherein the average degree of polymerization of the polysaccharide disposed
within
the sweetener particles is at least 400 monosaccharide building blocks.
Embodiment 77.
The formulation of any one of the preceding Embodiments,
wherein the average degree of polymerization of the polysaccharide disposed
within
the sweetener particles is at most 700 monosaccharide building blocks.
Embodiment 78.
The formulation of any one of the preceding Embodiments,
wherein the substituted monosaccharides contain an acetate moiety.
Embodiment 79.
The formulation of any one of the preceding Embodiments,
wherein the substituted monosaccharides contain a methoxy moiety.
Embodiment 80.
The formulation of any one of the preceding Embodiments,
wherein the substituted monosaccharides contain a pyruvate moiety.
Embodiment 81.
The formulation of any one of the preceding Embodiments,
wherein the substituted monosaccharides contain a sulfate moiety.
Embodiment 82. The
formulation of any one of the preceding Embodiments,
wherein the polysaccharide is a homopolysaccharide.
Embodiment 83.
The formulation of any one of the preceding Embodiments,
wherein the polysaccharide is a heteropolysaccharide.
Embodiment 84.
The formulation of any one of the preceding Embodiments,
wherein the polysaccharide is a linear polysaccharide.
Embodiment 85.
The formulation of any one of the preceding Embodiments,
wherein the polysaccharide is a branched polysaccharide
48
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 86. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide is an anionic polysaccharide.
Embodiment 87. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide is a non-ionic polysaccharide.
Embodiment 88. The formulation of any one of the preceding Embodiments,
wherein the monosaccharide building blocks are cyclic monosaccharides.
Embodiment 89. The formulation of any one of the preceding
Embodiments,
wherein the monosaccharide building blocks are, or include, unsubstituted
monosaccharides
Embodiment 90. The formulation of Embodiment 89, wherein the unsubstituted
monosaccharides include hexose sugars.
Embodiment 91. The formulation of Embodiment 89, wherein the
unsubstituted
monosaccharides include pentose sugars.
Embodiment 92. The formulation of Embodiment 89, wherein the
unsubstituted
monosaccharides include heptose sugars.
Embodiment 93. The formulation of any one of the preceding
Embodiments,
wherein the monosaccharide building blocks are, or include, substituted
monosaccharides.
Embodiment 94. The formulation of Embodiment 93, wherein the
substituted
monosaccharides contain an amine moiety.
Embodiment 95. The formulation of Embodiment 93, wherein the
substituted
monosaccharides contain an acetyl moiety.
Embodiment 96. The formulation of Embodiment 93, wherein the
substituted
monosaccharides contain a carboxylate moiety.
Embodiment 97. The formulation of Embodiment 93, wherein the substituted
monosaccharides are, or include, a uronic acid.
Embodiment 98. The formulation of any one of the preceding
Embodiments,
wherein the unsubstituted monosaccharides include glucose.
Embodiment 99. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes xylose.
49
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 100. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes arabinose.
Embodiment 101. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes rhamnose.
Embodiment 102. The formulation of any one of the preceding Embodiments,
wherein the polysaccharide includes mannuronate.
Embodiment 103. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes galactose.
Embodiment 104. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes mannose.
Embodiment 105. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes glucuronate.
Embodiment 106. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes galactopyranose.
Embodiment 107. The formulation of any one of the preceding Embodiments,
wherein the polysaccharide includes galacturonic acid.
Embodiment 108. The formulation of any one of the preceding
Embodiments,
wherein the heteropolysaccharide includes mannose and glucose.
Embodiment 109. The formulation of any one of the preceding
Embodiments,
wherein the heteropolysaccharide includes mannose and galactose.
Embodiment 110. The formulation of any one of the preceding
Embodiments,
wherein a molar ratio of the mannose to the galactose is between 1:1 and 6:1.
Embodiment 111. The formulation of any one of the preceding
Embodiments,
wherein the heteropolysaccharide includes mannuronate and glucuronate.
Embodiment 112. The formulation of any one of the preceding Embodiments,
wherein the heteropolysaccharide includes mannuronate and glucuronate disposed
in a
block polymer structure.
Embodiment 113. The formulation of any one of the preceding
Embodiments,
wherein the heteropolysaccharide includes mannuronate and glucuronate disposed
in
an alternating structure.
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 114. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes an arabinogalactan proteoglycan.
Embodiment 115. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes 13-D-mannopyranosyl units.
Embodiment 116. The formulation of any one of the preceding Embodiments,
wherein the polysaccharide has a P-D-Glucose backbone having mannose and
glucuronic acid side chains.
Embodiment 117. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes xanthan gum
Embodiment 118. The formulation of any one of the preceding Embodiments,
wherein the polysaccharide includes agar-agar.
Embodiment 119. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes gum Arabic.
Embodiment 120. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes Konjac Mannan.
Embodiment 121. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes an alkali alginate optionally selected
from the
group of sodium alginate and potassium alginate.
Embodiment 122. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes pectin.
Embodiment 123. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes guar gum.
Embodiment 124. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes gellan gum.
Embodiment 125. The formulation of any one of the preceding Embodiments,
wherein the polysaccharide includes locust bean gum.
Embodiment 126. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes tara gum.
Embodiment 127. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes Karaya gum.
51
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 128. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes curdlan.
Embodiment 129. The formulation of any one of the preceding
Embodiments,
wherein the polysaccharide includes tragacanth.
Embodiment 130. The formulation of any one of the preceding Embodiments,
wherein the at least one polysaccharide includes a carboxymethyl cellulose.
Embodiment 131. The formulation of any one of the preceding
Embodiments,
wherein the at least one polysaccharide includes a sodium carboxymethyl
cellulose.
Embodiment 132. The formulation of any one of the preceding
Embodiments,
wherein the at least one polysaccharide includes a calcium carboxymethyl
cellulose.
Embodiment 133. The formulation of any one of the preceding
Embodiments,
wherein an or the alkali alginate has an average molecular weight above
10,000.
Embodiment 134. The formulation of Embodiment 133, wherein the
alkali
alginate has an average molecular weight above 50,000.
Embodiment 135. The formulation of any one of the preceding Embodiments,
wherein an or the alkali alginate has an average molecular weight of at most
1,000,000.
Embodiment 136. The formulation of Embodiment 135, wherein the
alkali
alginate has an average molecular weight of at most 600,000.
Embodiment 137. The formulation of Embodiment 135, wherein the alkali
alginate has an average molecular weight of at most 300,000.
Embodiment 138. The formulation of Embodiment 135, wherein the
alkali
alginate has an average molecular weight of at most 125,000.
Embodiment 139. The formulation of Embodiment 135, wherein the
alkali
alginate has an average molecular weight within a range of 10,000 to
1,000,000.
Embodiment 140. The formulation of Embodiment 135, wherein the
alkali
alginate has an average molecular weight within a range of 10,000 to 250,000.
Embodiment 141. The formulation of Embodiment 135, wherein the
alkali
alginate has an average molecular weight within a range of 10,000 to 120,000.
Embodiment 142. The formulation of Embodiment 135, wherein the alkali
alginate has an average molecular weight within a range of 20,000 to 350,000.
52
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 143. The formulation of any one of the preceding
Embodiments,
wherein a molar ratio of the alkali alginate to silicon within the sweetener
particles is
at least 3:1, and optionally, at least 5:1, at least 10:1, or at least 50:1.
Embodiment 144. The formulation of any one of the preceding
Embodiments,
wherein the alkali alginate includes sodium alginate.
Embodiment 145. The formulation of any one of the preceding
Embodiments,
wherein the alkali alginate includes potassium alginate.
Embodiment 146. An edible formulation containing the formulation
of any one of
Embodiments 1 to 145.
Embodiment 147. An edible formulation comprising:
(a) a first population of sweetener particles containing a first sweetener
selected from the group consisting of a first sweetener carbohydrate
and a first sweetener polyol;
(b) a second population of sweetener particles containing a second
sweetener selected from the group consisting of a second sweetener
carbohydrate and a second sweetener polyol;
(c) at least one polysaccharide disposed within the first population of
sweetener particles;
(d) at least one fat; and
(e) optionally, at least one starch;
wherein a second weight-to-weight ratio of total polysaccharide content to the
second
sweetener within the second population of sweetener particles is at most 0.1%;
and wherein a total weight-to-weight ratio of total polysaccharide content to
the first
and second sweeteners within the first and second populations is within a
range of
0.02% to 0.99%.
Embodiment 148. An edible formulation comprising:
(a) a first population of sweetener particles containing a first sweetener
including a first sweetener carbohydrate,
(b) at least one polysaccharide disposed within the sweetener particles;
(c) at least one fat; and
(d) optionally, at least one starch;
53
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
wherein a total concentration of the first sweetener, the at least one fat,
and the at least
one starch, within the edible formulation, is at least 30%, on a weight basis.
Embodiment 149. The edible formulation of any one of Embodiments
18 to 30
and 147 to 148, wherein a total concentration of the first sweetener, a or the
second
sweetener, the at least one fat, and the at least one starch, within the
edible
formulation, is at least 32%, on a weight basis.
Embodiment 150. The edible formulation of any one of Embodiments
18 to 30
and 147 to 149, wherein a weight content of the first sweetener and a or the
second
sweetener, within the edible formulation is at least 8%.
Embodiment 151. The edible formulation of any one of Embodiments 18 to 30
and 147 to 150, the edible formulation containing a total of at least 5% of
the first
sweetener and a or the second sweetener, and at least 5% of the at least one
fat.
Embodiment 152. The edible formulation of any one of Embodiments
18 to 30
and 147 to 151, the edible formulation containing a total of at least 5% of
the first
sweetener and a or the second sweetener, and at least 5% of the at least one
starch.
Embodiment 153. The edible formulation of any one of Embodiments
18 to 30
and 147 to 152, wherein a weight concentration of all sweetener particles
within the
edible formulation is within a range of 10% to 80%.
Embodiment 154. The edible formulation of any one of the
preceding
Embodiments, the edible formulation containing at least 5% of the first
sweetener and
a or the second sweetener, at least 5% of a or the at least one fat, and at
least 5% of a
or the at least one starch.
Embodiment 155. The edible formulation of any one of the
preceding
Embodiments, the edible formulation containing at least 2%, at least 5%, or at
least
10% of an edible filler.
Embodiment 156. The edible formulation of any one of the
preceding
Embodiments, the edible formulation containing at least one edible filler.
Embodiment 157. The edible formulation of Embodiment 156, the at
least one
edible filler including a dietary fiber.
Embodiment 158. The edible formulation of Embodiment 156 or Embodiment
157, the at least one edible filler including a soluble fiber.
54
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Embodiment 159. The edible formulation of Embodiment 157 or 158,
the at least
one edible filler including a polysaccharide filler.
Embodiment 160. The edible formulation of Embodiment 159, the
polysaccharide
filler including a fructan.
Embodiment 161. The edible formulation of Embodiment 160, the
polysaccharide
filler including inulin.
Embodiment 162. The edible formulation of any one of the
preceding
Embodiments, the edible formulation containing at least one edible filler
including an
oligosaccharide.
Embodiment 163. The edible formulation of Embodiment 162, the
oligosaccharide including a fructooligosacchari de.
Embodiment 164. The edible formulation of any one of the
preceding
Embodiments, the edible formulation containing at least one edible filler
including a
soluble fiber, the soluble fiber including resistant maltodextrin.
Embodiment 165 The edible formulation of any one of the preceding
Embodiments, the edible formulation containing at least one edible filler
including a
soluble fiber, the soluble fiber including polydextrose.
Embodiment 166. The edible formulation of any one of the
preceding
Embodiments, containing at least 10% of the first sweetener and a or the
second
sweetener, at least 10% of a or the at least one fat, and at least 10% of a or
the at least
one starch.
Embodiment 167. The edible formulation of any one of the
preceding
Embodiments, wherein the first reduced sugar edible formulation is a standard
reduced sugar edible formulation.
Embodiment 168. The edible formulation of any one of the preceding
Embodiments, wherein the polysaccharide includes a carboxymethyl cellulose.
Embodiment 169. The edible formulation of Embodiment 168,
wherein the
carboxymethyl cellulose includes a sodium carboxymethyl cellulose.
Embodiment 170. The edible formulation of Embodiment 168,
wherein the
carboxymethyl cellulose includes a calcium carboxymethyl cellulose
Embodiment 171. The edible formulation of Embodiments 147 to
148, further
containing any of the limitations of Embodiments 1 to 146.
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
As opposed to small molecules, which may have a unique molecular weight
readily derived from their chemical formula, generally provided in grams/mole,
polymers and other macromolecules typically exist as a diverse population of
distinct
molecules, which are therefore characterized by an average molecular weight
often
expressed in Daltons.
The molecular weight or average molecular weight of such materials is
generally provided by the manufacturer or supplier thereof. In addition, the
molecular
weight or average molecular weight of such materials may be independently
determined by known analytical methods, including, by way of example, gel
permeation chromatography, high pressure liquid chromatography (HPLC), or
matrix-
assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-
TOF
MS).
As used herein in the specification and in the claims section that follows,
the
term "starch" is meant to include edible starches that are used or may be used
in
foodstuffs. Typically, such starches include at least one of amylose and
amylopectin,
and more typically, both amylose and amylopectin. It will be appreciated that
various
modifications of starch may be made, in order to impart to a particular
foodstuff, or to
the starch therein, specific chemical and/or physical properties, including,
by way of
example, the prevention of gelling at cold temperatures, withstanding low pH,
or
resistance to high shear or to high temperatures.
Often, starch is present in an ingredient, e.g., flour. In white wheat flour,
the
starch content is typically about 68%. In oats, the starch content is
typically about
58%.
In addition to including fats that are solid at room temperature (25 C), e.g.,
beef fat, shortening, palm oil, and butter, as used herein in the
specification and in the
claims section that follows, the term "fat" is meant to include edible oils,
including
those that are liquid at room temperature, e.g., cooking oils. Specific
examples of
edible oils are olive oil, walnut oil, corn oil, and cottonseed oil.
Fats may be a separate ingredient, or may be an ingredient within a food
ingredient. For example, hazelnut paste and cocoa powder both contain fat.
56
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
Average particle size (D50) may be based on the number of particles in the
population ("DN50") or may be based on the volume of particles (Dv50). These
measurements may be obtained by various known methods including static light
scattering (SLS), dynamic light scattering (DLS), sieving, and various methods
of
microscopy. Some methods may be preferred for larger ranges of particles,
others
may be preferred for smaller ranges of particles.
As used herein in the specification and in the claims section that follows,
the
term "percent", or "%", refers to percent by weight, unless specifically
indicated
otherwise. However, with specific regard to formulations containing at least
one
polysaccharide and at least one sweetener, the weight-percent of the
polysaccharide is
with respect to the sweetener. By way of example, in such a formulation
containing
1.95 grams polysaccharide dispersed in a syrup containing 650 grams sucrose
and 350
grams water, the weight-percent of polysaccharide is 1.95/650 = 0.3%.
As used herein in the specification and in the claims section that follows,
the
term "concentration" refers to concentration on a weight basis, unless
specifically
indicated otherwise.
As used herein in the specification and in the claims section that follows,
the
term "polysaccharide-sweetener concentrate- refers to a population of
sweetener
particles containing a sweetener selected from the group consisting of a
sweetener
carbohydrate and a sweetener polyol; and at least one polysaccharide disposed
within
the population of sweetener particles; wherein a weight-to-weight ratio of the
at least
one polysaccharide to the sweetener within the population of sweetener
particles is at
least 0.01:1, at least 0.02:1, at least 0.03:1, or at least 0.05:1, and more
typically, at
least 0.06:1, at least 0.08:1, at least 0.1:1, at least 0.15:1, or at least
0.20:1. Typically,
this weight ratio is at most 20:1, and more typically at most 4:1 or at most
2:1.
As used herein in the specification and in the claims section that follows,
the
term "reduced sugar", "less sugar" and the like, refers to a lower relative
amount of
sugar. Thus, if a Type II reduced-sugar muffin contains 40% less sugar with
respect
to a Type I "full sugar" control muffin, and the Type I muffin contains 21.8%
sugar,
the Type II reduced-sugar muffin contains 60% (100% - 40%) of the sugar
contained
in the Type I muffin, i.e., 0.60 = 21.8% = 13.08 wt.% sugar.
As used herein in the specification and in the claims section that follows,
the
term "less sweet", typically used with respect to a polysaccharide-sweetener
57
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
concentrate vs. a control sweetener, refers to a lower sweetness result as
exhibited by
the Comparative Sweetness Index calculated from paired-comparison test
results, as
described in Examples 76 and 76A.
As used herein in the specification and in the claims section that follows,
the
term -reduced sugar edible formulation-, -first reduced sugar edible
formulation-, or
the like, refers to any one of the "Type II" reduced sugar products as
formulated
according to any one of Examples 73B, 74B, and 75B.
As used herein in the specification and in the claims section that follows,
the
term "reduced sugar control edible formulation- refers to any one of the
reduced sugar
control products as described and formulated according to any one of Examples
73,
74, and 75.
As used herein in the specification and in the claims section that follows,
the
term "standard reduced sugar edible formulation- refers to any one of the Type
II
reduced sugar products as formulated according to any pair of Examples 73-73A,
74-
74A, and 75-75A.
As used herein in the specification and in the claims section that follows,
the
term "standard reduced sugar control edible formulation- refers to any one of
the
"Type III" reduced sugar control products as formulated according to any pair
of
Examples 73-73A, 74-74A, and 75-75A.
As used herein in the specification and in the claims section that follows,
the
term "exhibits improved sweetness" and the like, typically with reference to a
first
edible formulation (e.g., a reduced sugar edible formulation) containing a
polysaccharide-sweetener concentrate relative to a control edible formulation
(e.g., a
reduced sugar control edible formulation) that is identical to the edible
formulation,
but devoid of the polysaccharide contained in that polysaccharide-sweetener
concentrate, refers to a higher sweetness result as exhibited by the
Comparative
Sweetness Index calculated from paired-comparison test results, as described
in
Example 76 and/or the difference magnitude estimation (DME) as described in
Example 76A. For evaluation purposes, the concentration of polysaccharide from
the
polysaccharide-sweetener concentrate distributed within the first edible
formulation is
0.1%, 0.3%, or 0.5%.
58
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
As used herein in the specification and in the claims section that follows,
the
term "a first sweetener" refers to at least one sweetener selected from the
group
consisting of a first sweetener carbohydrate and a first sweetener polyol.
As used herein in the specification and in the claims section that follows,
the
term -a second sweetener- refers to at least one sweetener selected from the
group
consisting of a first sweetener carbohydrate and a first sweetener polyol,
wherein the
chemical identity of the second sweetener may be identical to the "first
sweetener",
unless otherwise indicated.
As used herein in the specification and in the claims section that follows,
the
term "majority", with respect to the number of particles of a formulation
component,
refers to at least 50%, by number.
As used herein in the specification and in the claims section that follows,
the
term "majority-, with respect to the concentration of a formulation component,
refers
to at least 50%, by weight.
As used herein in the specification and in the claims section that follows,
the
term "predominantly-, with respect to the concentration of a formulation
component,
refers to at least 65%, by weight.
The term "ratio", as used herein in the specification and in the claims
section
that follows, refers to a weight ratio, unless specifically indicated
otherwise.
The modifier "about" and "substantially" used in connection with a quantity is
inclusive of the stated value and has the meaning dictated by the context (for
example,
it includes at least the degree of error associated with the measurement of
the
particular quantity). When used with a specific value, it should also be
considered as
disclosing that value.
In the context of the present application and claims, the phrase "at least one
of
A and B" is equivalent to an inclusive "or", and includes any one of "only A",
"only
B", or "A and B". Similarly, the phrase "at least one of A, B, and C" is
equivalent to
an inclusive "or", and includes any one of "only A", "only B", "only C", "A
and B",
"A and C", "B and C", or "A and B and C".
It will be appreciated that certain features of the invention, which are, for
clarity, described in the context of separate embodiments, may also be
provided in
combination in a single embodiment. Conversely, various features of the
invention,
59
CA 03233710 2024- 4- 2
WO 2023/057960
PCT/IB2022/059574
which are, for brevity, described in the context of a single embodiment, may
also be
provided separately or in any suitable sub-combination.
Although the invention has been described in conjunction with specific
embodiments thereof, it is evident that many alternatives, modifications and
variations
will be apparent to those skilled in the art. Accordingly, it is intended to
embrace all
such alternatives, modifications and variations that fall within the spirit
and broad
scope of the appended claims. All publications, patents and patent
applications
mentioned in this specification are herein incorporated in their entirety by
reference
into the specification, to the same extent as if each individual publication,
patent or
patent application was specifically and individually indicated to be
incorporated
herein by reference. In addition, citation or identification of any reference
in this
application shall not be construed as an admission that such reference is
available as
prior art to the present invention.
CA 03233710 2024- 4- 2