Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/076990
PCT/US2022/078770
COMPOSITIONS, SYSTEMS AND METHODS
FOR TREATING A SUBSTRATE
CROSS-REFERENCE
[0001] This application claims the benefit of U.S. Provisional
Application Nos.
63/272,554. filed October 27, 2021, and 63/269,866, filed March 24, 2022, and
each entitled
"COMPOSITIONS, SYSTEMS AND METHODS FOR TREATING A SUBSTRATE", the
entire content of which is incorporated herein by reference.
FIELD
[0002] The present disclosure relates to compositions, systems
and methods for treating a
substrate.
BACKGROUND
[0003] It is known in the art of substrate protection to prevent
the oxidation and
degradation of the metal substrates by applying an inorganic protective
coating to the metal
surface.
SUMMARY
[0004] Disclosed herein are conversion compositions comprising: a
yield stress
component; and a corrosion inhibitor; wherein the conversion composition
comprises a yield
stress sufficient to overcome the effect of gravity when applied to a non-
horizontal surface.
[0005] Also disclosed herein are systems for treating a metal
substrate comprising: a
conversion composition comprising a yield stress component, and a corrosion
inhibitor, wherein
the conversion composition comprises a yield stress sufficient to overcome the
effect of gravity
when applied to a non-horizontal surface; and a least one of a cleaning
composition, a
deoxidizer, and a film-forming resin.
[0006] Also disclosed herein are methods of treating a metal
substrate, comprising:
contacting at least a portion of a surface of the substrate with a conversion
composition
comprising a yield stress component, and a corrosion inhibitor, wherein the
conversion
composition comprises a yield stress sufficient to overcome the effect of
gravity when applied to
a non-horizontal surface.
[0007] Also disclosed herein are substrates treated with the
conversion compositions,
systems or methods disclosed herein.
1
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0008] Also disclosed herein are uses of the disclosed conversion
compositions to
provide a composition comprising (i) a yield stress sufficient to overcome the
effect of gravity
when applied to a non-horizontal surface and (ii) a shear thinning rheology
profile.
[0009] Also disclosed herein arc uses of the disclosed conversion
compositions to
provide a film that overcomes the effect of gravity when applied to a non-
horizontal surface and
that provides corrosion protection to the surface such that the substrate
surface comprises less
than 1% corrosion of a 1.376 in2 area of the substrate surface following 24
hour exposure to a
neutral salt spray in a cabinet operated according to ASTM-B117 (2019) neutral
salt spray
testing and/or wherein the substrate passes corrosion testing according to
ASTM D610-08 (2019)
rating scale.
BRIEF DESCRIPTION OF THE FIGURES
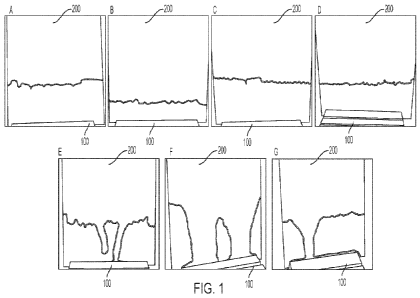
[0010] FIG. 1 shows schematics of photographs taken of panels
treated according to the
Example disclosed herein: FIG. lA (Sample A); FIG. 1B (Sample B); FIG. 1C
(Sample C); FIG.
1D (Sample D); FIG. 1E (Sample E, comparative); FIG. 1F (Sample F,
comparative); FIG. 1G
(Sample G, comparative).
[0011] FIG. 2A shows photographs of panels treated with Sample A
following exposure
to neutral salt spray according to the Example disclosed herein and FIG. 2B
shows photographs
of untreated panels (H) following exposure to neutral salt spray according to
the Example
disclosed herein.
DETAILED DESCRIPTION
[0012] For purposes of the following detailed description, it is
to be understood that the
disclosure may assume various alternative variations and step sequences,
except where expressly
specified to the contrary. Moreover, other than in any operating examples, or
where otherwise
indicated, all numbers such as those expressing values, amounts, percentages,
ranges, subranges
and fractions may be read as if prefaced by the word "about," even if the term
does not expressly
appear. Accordingly, unless indicated to the contrary, the numerical
parameters set forth in the
following specification and attached claims are approximations that may vary
depending upon
the desired properties to be obtained by the present disclosure. At the very
least, and not as an
attempt to limit the application of the doctrine of equivalents to the scope
of the claims, each
numerical parameter should at least be construed in light of the number of
reported significant
digits and by applying ordinary rounding techniques. Where a closed or open-
ended numerical
2
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
range is described herein, all numbers, values, amounts, percentages,
subranges and fractions
within or encompassed by the numerical range are to be considered as being
specifically
included in and belonging to the original disclosure of this application as if
these numbers,
values, amounts, percentages, subranges and fractions had been explicitly
written out in their
entirety.
[0013] Notwithstanding that the numerical ranges and parameters
setting forth the broad
scope of the disclosure are approximations, the numerical values set forth in
the specific
examples are reported as precisely as possible. Any numerical value, however,
inherently
contains certain errors necessarily resulting from the standard variation
found in their respective
testing measurements.
[0014] As used herein, unless indicated otherwise, a plural term
can encompass its
singular counterpart and vice versa, unless indicated otherwise. For example,
although reference
is made herein to -a" conversion composition, -a" cleaning composition, and -
a" a yield stress
component, a combination (i.e., a plurality) of these components can be used.
In addition, in this
application, the use of -or" means -and/or" unless specifically stated
otherwise, even though
"and/or" may be explicitly used in certain instances.
[0015] As used herein, "including," "containing" and like terms
are understood in the
context of this application to be synonymous with "comprising" and are
therefore open-ended
and do not exclude the presence of additional undescribed and/or unrecited
elements, materials,
ingredients and/or method steps.
[0016] As used herein, "consisting of' is understood in the
context of this application to
exclude the presence of any unspecified element, ingredient and/or method
step.
[0017] As used herein, "consisting essentially of' is understood
in the context of this
application to include the specified elements, materials, ingredients and/or
method steps "and
those that do not materially affect the basic and novel characteristic(s)" of
what is being
described.
[0018] As used herein, the terms "on," -onto," "applied on,"
"applied onto," "formed
on," "deposited on," "deposited onto," mean formed, overlaid, deposited,
and/or provided on but
not necessarily in contact with the surface. For example, a coating layer
"formed over" a
substrate does not preclude the presence of one or more other intervening
coating layers of the
same or different composition located between the formed coating layer and the
substrate.
3
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0019] Unless otherwise disclosed herein, the term "substantially
free," when used with
respect to the absence of a particular material, means that such material, if
present at all, in a
composition, in a bath containing the composition, and/or in layers formed
from and comprising
the composition, only is present in a trace amount of 5 ppm or less based on a
total weight of the
composition or layer(s), as the case may be, excluding any amount of such
material that may be
present or derived as a result of drag-in, substrate(s), and/or dissolution of
equipment. Unless
otherwise disclosed herein, the term "essentially free," when used with
respect to the absence of
a particular material, means that such material, if present at all, in a
composition, in a bath
containing the composition, and/or in layers formed from and comprising the
composition, only
is present in a trace amount of 1 ppm or less based on a total weight of the
composition or
layer(s), as the case may be. Unless otherwise disclosed herein, the term
"completely free,"
when used with respect to the absence of a particular material, means that
such material, if
present at all, in a composition, in a bath containing the composition, and/or
in layers formed
from and comprising the composition, is absent from the composition, the bath
containing the
composition, and/or layers formed from and comprising same (i.e., the
composition, bath
containing the composition, and/or layers formed from and comprising the
composition contain 0
ppm of such material).
[0020] As used herein, a "salt" refers to an ionic compound made
up of metal cations and
non-metallic anions and having an overall electrical charge of zero. Salts may
be hydrated or
anhydrous.
[0021] As used herein, "aqueous composition" refers to a solution
or dispersion in a
medium that comprises predominantly water. For example, the aqueous medium may
comprise
water in an amount of more than 50 wt.%, or more than 70 wt.% or more than 80
wt.% or more
than 90 wt.% or more than 95 wt.%, based on the total weight of the medium.
The aqueous
medium may for example consist substantially of water.
[0022] As used herein, "conversion composition" refers to a
composition that is capable
of reacting with and chemically altering the substrate surface and binding to
it to form a film that
affords corrosion protection.
[0023] As used herein, the term "transition metal" refers to an
element that is in any of
Groups IIIB to VIIIB, TB, and JIB of the CAS version of the Periodic Table of
Elements
excluding the lanthanide series elements and elements 89-103, as is shown, for
example, in the
4
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to
Groups 3 to 12 in the
actual IUPAC numbering.
[0024] As used herein, the term "transition metal compound"
refers to compounds that
include at least one clement that is a transition metal of the CAS version of
the Periodic Table of
Elements.
[0025] As used herein, the term "Group IA metal" refers to an
element that is in Group
IA of the CAS version of the Periodic Table of Elements as is shown, for
example, in the
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to Group
1 in the actual
IUPAC numbering.
[0026] As used herein, the term "Group IA metal compound- refers
to compounds that
include at least one element that is in Group IA of the CAS version of the
Periodic Table of
Elements.
[0027] As used herein, the term -Group IIA metal" refers to an
element that is in Group
IA of the CAS version of the Periodic Table of Elements as is shown, for
example, in the
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to Group
2 in the actual
IUPAC numbering.
[0028] As used herein, the term "Group IIA metal compound" refers
to compounds that
include at least one element that is in Group IIA of the CAS version of the
Periodic Table of
Elements.
[0029] As used herein, the term "Group IIIB metal" refers to
yttrium and scandium of the
CAS version of the Periodic Table of Elements as is shown, for example, in the
Handbook of
Chemistry and Physics, 63rd edition (1983), corresponding to Group 3 in the
actual IUPAC
numbering. For clarity, "Group MB metal" expressly excludes lanthanide series
elements.
[0030] As used herein, the term "Group IIIB metal compound"
refers to compounds that
include at least one element that is in group IIIB of the CAS version of the
Periodic Table of
Elements as defined above.
[0031] As used herein, the term "Group 1VB metal" refers to an
element that is in Group
IVB of the CAS version of the Periodic Table of Elements as is shown, for
example, in the
Handbook of Chemistry and Physics, 631d edition (1983), corresponding to Group
4 in the actual
IUPAC numbering.
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0032] As used herein, the term "Group IVB metal compound" refers
to compounds that
include at least one element that is in Group IVB of the CAS version of the
Periodic Table of
Elements.
[0033] As used herein, the term -Group VB metal" refers to an
element that is in Group
VB of the CAS version of the Periodic Table of Elements as is shown, for
example, in the
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to Group
5 in the actual
IUPAC numbering.
[0034] As used herein, the term "Group VB metal compound" refers
to compounds that
include at least one element that is in Group VB of the CAS version of the
Periodic Table of
Elements.
[0035] As used herein, the term "Group VIB metal" refers to an
element that is in Group
VIB of the CAS version of the Periodic Table of Elements as is shown, for
example, in the
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to Group
6 in the actual
IUPAC numbering.
[0036] As used herein, the term -Group VIB metal compound" refers
to compounds that
include at least one element that is in Group V1B of the CAS version of the
Periodic Table of
Elements.
[0037] As used herein, the term "Group VIIB metal" refers to an
element that is in Group
VIIB of the CAS version of the Periodic Table of Elements as is shown, for
example, in the
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to Group
7 in the actual
IUPAC numbering.
[0038] As used herein, the term "Group VIIB metal compound"
refers to compounds that
include at least one element that is in Group VIIB of the CAS version of the
Periodic Table of
Elements.
[0039] As used herein, the term "Group JIB metal" refers to an
element that is in Group
11B of the CAS version of the Periodic Table of Elements as is shown, for
example. in the
Handbook of Chemistry and Physics, 63rd edition (1983), corresponding to Group
12 in the
actual IUPAC numbering.
[0040] As used herein, the term "Group JIB metal compound" refers
to compounds that
include at least one element that is in Group JIB of the CAS version of the
Periodic Table of
Elements.
6
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0041] As used herein, the term "lanthanide series elements"
refers to elements 57-71 of
the CAS version of the Periodic Table of Elements and includes elemental
versions of the
lanthanide series elements. According to the present disclosure, the
lanthanide series elements
may be those which have both common oxidation states of +3 and +4, referred to
hereinafter as
+3/+4 oxidation states.
[0042] As used herein, the term "lanthanide compound" refers to
compounds that include
at least one of elements 57-71 of the CAS version of the Periodic Table of
Elements.
[0043] As used herein, the term "halogen" refers to any of the
elements fluorine,
chlorine, bromine, iodine, and astatine of the CAS version of the Periodic
Table of Elements,
corresponding to Group VITA of the CAS version of the Periodic Table of
Elements.
[0044] As used herein, the term "halide" refers to compounds that
include at least one
halogen.
[0045] As used herein, the term -aluminum," when used in
reference to a substrate, refers
to substrates made of or comprising aluminum and/or aluminum alloy, and clad
aluminum
substrates.
[0046] "Pitting corrosion," as used herein, refers to the
localized formation of corrosion
by which cavities or holes are produced in a substrate. The term "pit," as
used herein, refers to
such cavities or holes resulting from pitting corrosion and is characterized
by (1) a rounded,
elongated or irregular appearance when viewed normal to the test panel
surface, (2) a "comet-
tail", a line, or a "halo" (i.e., a surface discoloration) emanating from the
pitting cavity, and/or
(3) the presence of corrosion byproduct (e.g., white, grayish or black
granular, powdery or
amorphous material) inside or immediately around the pit. Visual inspection
using a microscope
with 10X magnification may be used to determine the presence of corrosion
byproducts when
corrosion byproducts are not visible with the unaided eye.
[0047] Unless otherwise disclosed herein, as used herein, the
terms "total composition
weight", "total weight of a composition" or similar terms refer to the total
weight of all
ingredients being present in the respective composition including any carriers
and solvents.
[0048] As used herein, the term "yield stress" refers to the
stress corresponding to the
yield point above which a material begins to flow and below which the material
behaves as a
predominantly elastic solid.
7
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0049] As used herein, the term "yield stress component" refers
to a material that
behaves as a predominantly elastic solid below the yield stress (elastic
modulus greater than
viscous modulus) and that begins to flow above the yield stress, i.e., the
"yield stress
component" imparts yield stress to the conversion composition.
[0050] As used herein, the term "complex substrate" refers to a
substrate with portions
having a variety of orientations, such as horizontal, vertical, non-
horizontal, substantially vertical
and combinations thereof.
[0051] As used herein, the term "horizontal," when used with
respect to a surface, refers
to parallel to the plane of the horizon.
[0052] As used herein, the term "vertical,- when used with
respect to a surface, refers to
90 as measured from the horizontal plane.
[0053] As used herein, the term "non-horizontal" or "inclined,"
when used with respect
to a surface, refers to any angle greater than 0 as measured from the
horizontal plane.
[0054] As used herein, the term "substantially vertical," when
used with respect to a
surface, refers to 70 to 1100 as measured from the horizontal plane.
[0055] As used herein, the term "cling" refers to a yield stress
sufficient to overcome the
effect of gravity when a composition is applied to a non-horizontal surface.
Conversion Compositions
[0056] The present disclosure is directed to conversion
compositions. The conversion
composition may comprise, or may consist essentially of, or may consist of: a
yield stress
component; and a corrosion inhibitor; wherein the composition comprises a
yield stress sufficient
to overcome the effect of gravity when applied to a non-horizontal surface.
The conversion
composition may comprise a yield stress fluid that provides corrosion
protection to substrate
surfaces. The yield stress component may be dissolved or dispersed in a fluid
medium. The
fluid medium may comprise an aqueous medium.
[0057] The conversion composition may comprise a yield stress of
at least 0.6 Pa at a
frequency of 1 Hz and a temperature of 25 C when the composition is applied at
a thickness of
0.5 mil to 40 mil to a substantially vertical substrate surface, such as at
least 0.7 Pa, such as at
least 0.8 Pa, such as at least 0.9 Pa, such as at least 1.0 Pa, such as at
least 2.0 Pa, such as at least
3.0 Pa, such as at least 4.0 Pa, such as at least 5.0 Pa, such as at least 6.0
Pa, such as at least 7.0
Pa, such as at least 8.0 Pa, such as at least 9.0 Pa, such as at least 10.0
Pa.
8
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0058] The conversion composition may comprise a yield stress
greater than 10.0 Pa as
long as the composition maintains shear thinning behavior (i.e., as long as
the composition has a
low viscosity with a high shear rate) as described below, such as a yield
stress of no more than
50.0 Pa at a frequency of 1 Hz and a temperature of 25 C when the composition
is applied at a
thickness of 0.5 mil to 40 mil to a substantially vertical substrate surface,
such as no more than
40.0 Pa, such as no more than 30.0 Pa, such as no more than 20.0 Pa, such as
no more than 20.0
Pa.
[0059] The conversion composition may comprise a yield stress of
0.6 Pa to 50.0 Pa at a
frequency of 1 Hz and a temperature of 25 C when the composition is applied at
a thickness of
0.5 mil to 40 mil to a substantially vertical substrate surface, such as 0.7
Pa to 50.0 Pa, such as
0.8 Pa to 50.0 Pa, such as 0.9 Pa to 50.0, such as 1.0 Pa to 40.0 Pa, such as
2.0 Pa to 40.0 Pa,
such as 3.0 Pa to 30.0 Pa, such as 4.0 Pa to 20.0 Pa, such as 5.0 Pa to 20.0
Pa, such as 6.0 Pa to
20.0 Pa, such as 7.0 Pa to 20.0 Pa, such as 8.0 Pa to 20.0 Pa, such as 9.0 Pa
to 20.0 Pa, such as
10.0 Pa to 20.0 Pa.
[0060] Yield stress may be determined in water at 25 C using a
DHR-2 rheometer from
TA Instruments with a concentric cylinder geometry or equivalent instruments.
Elastic (G') and
viscous (G") moduli may be determined as a function of increasing stress
amplitude at a
frequency of 1 Hz and the crossover of G' and G" may be used to estimate the
yield stress. One
skilled in the art will understand that the yield stress required to support a
coating on a non-
horizontal surface will vary with the thickness of the coating and the angle
of incline.
[0061] The conversion composition may comprise a viscosity of
less than 700 mPa.s. at a
shear rate of 100 reciprocal seconds at a temperature of 25 C, such as a
viscosity of less than 600
mPa.s. at a shear rate of 100 reciprocal seconds at a temperature of 25 C,
such as a viscosity of
less than 500 mPa.s. at a shear rate of 100 reciprocal seconds at a
temperature of 25 C, such as a
viscosity of less than 400 mPa.s. at a shear rate of 100 reciprocal seconds at
a temperature of
25 C, such as a viscosity of less than 300 mPa.s. at a shear rate of 100
reciprocal seconds at a
temperature of 25 C, such as a viscosity of less than 200 mPa.s. at a shear
rate of 100 reciprocal
seconds at a temperature of 25 C, such as a viscosity of less than 100 mPa.s.
at a shear rate of
100 reciprocal seconds at a temperature of 25 C, such as a viscosity of less
than 75 mPa.s. at a
shear rate of 100 reciprocal seconds at a temperature of 25 C, such as a
viscosity of less than 70
mPa.s. at a shear rate of 100 reciprocal seconds at a temperature of 25 C,
such as a viscosity of
9
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
less than 60 mPa.s. at a shear rate of 100 reciprocal seconds at a temperature
of 25 C, such as a
viscosity of less than 55 mPa.s. at a shear rate of 100 reciprocal seconds at
a temperature of
25 C.
[0062] The conversion composition may comprise a viscosity of
less than 200 mPa.s. at a
shear rate of 1000 reciprocal seconds at a temperature of 25 C, such as a
viscosity of less than
175 mPa.s. at a shear rate of 1000 reciprocal seconds at a temperature of 25
C, such as a
viscosity of less than 150 mPa.s. at a shear rate of 1000 reciprocal seconds
at a temperature of
25 C, such as a viscosity of less than 125 mPa.s. at a shear rate of 1000
reciprocal seconds at a
temperature of 25 C, such as a viscosity of less than 100 mPa.s. at a shear
rate of 1000 reciprocal
seconds at a temperature of 25 C, such as a viscosity of less than 80 mPa.s.
at a shear rate of
1000 reciprocal seconds at a temperature of 25 C, such as a viscosity of less
than 60 mPa.s. at a
shear rate of 1000 reciprocal seconds at a temperature of 25 C, such as a
viscosity of less than 50
mPa.s. at a shear rate of 1000 reciprocal seconds at a temperature of 25 C,
such as a viscosity of
less than 40 mPa.s. at a shear rate of 1000 reciprocal seconds at a
temperature of 25 C, such as a
viscosity of less than 30 mPa.s. at a shear rate of 1000 reciprocal seconds at
a temperature of
25 C, such as a viscosity of less than 20 mPa.s. at a shear rate of 1000
reciprocal seconds at a
temperature of 25 C, such as a viscosity of less than 15 mPa.s. at a shear
rate of 1000 reciprocal
seconds at a temperature of 25 C.
[0063] Viscosity may be determined using a Discovery HR-2
Rheometer with concentric
cylinders from TA Instruments or equivalent instruments.
[0064] As mentioned above, the conversion composition may
comprise a yield stress
component. The yield stress component may comprise a crosslinked microgel
polymer, a
network-forming polymer or combinations thereof.
[0065] Suitable crosslinked microgel polymers comprise
polyelectrolyte microgel
polymers, crosslinked nonionic microgel polymers or combinations thereof. In
examples, the
crosslinked microgel polymer may be pH-activated and may comprise a pH-
responsive moiety.
The pH-responsive moiety may be either acidic or alkaline. In use, when the
crosslinked
microgel polymers are neutralized by the addition of an acid or a base
(resulting in ionization of
the acidic or basic groups), the polymers swell to form a randomly close-
packed network of
swollen crosslinked microgel particles that impart rheological features such
as yield stress. In
examples, the polymers may comprise vinyls.
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0066] The crosslinked microgel polymer may comprise a pKa of at
least 3, such as at
least 3.2, and may have a pKa of no more than 7, such as no more than 4, such
as no more than
3.7. The crosslinked microgel polymer may comprise a pKa of 3 to 7, such as a
pKa of 3 to 4,
such as a pKa of 3.2 to 3.7.
[0067] Suitable examples of crosslinked microgel polymers include
crosslinked
carboxylic acid polymers based on maleic acid, itaconic acid or (meth)acrylic
acid monomers.
Microgel polymers including polymers based on (meth)acrylic acid monomers are
commercially
available, for example, as Carbopol from The Lubrizol Corporation. As used
herein,
"(meth)acrylate" refers to either/or methacrylate or acrylate and
"(meth)acrylic" refers to
either/or methacrylic acid and acrylic acid.
[0068] Other suitable examples of crosslinked microgel polymers
include alkali
swellable polymers based on alkyl acrylic, (meth)acrylic acid, carboxylic
acid, non-acid vinyl
monomers and combinations thereof. The crosslinked alkali swellable polymer
may be
hydrophobically modified, may be amphiphilic and/or may be activated by a
surfactant. As used
herein, -amphiphilic" means molecules having a polar water-soluble group
attached to a water-
insoluble hydrocarbon chain.
[0069] As noted above, the yield stress component may comprise a
network-forming
polymer. The network-forming polymer may comprise a biopolymer. The biopolymer
may
comprise an ionic polymer such as an anionic polysaccharide. In other
examples, the biopolymer
may comprise a neutral polysaccharide. Suitable examples of biopolymers
include xanthan gum,
welan gum, diutan gum, scleroglucan or combinations thereof.
[0070] The biopolymers disclosed herein may comprise no more than
4 charged groups
per monosaccharide unit, such as no more than 3 charged groups per
monosaccharide unit, such
as no more than 2 charged groups per monosaccharide unit, such as no more than
I charged
group per monosaccharide unit.
[0071] The biopolymers disclosed herein may comprise at least 1
shielded charged
group. Charged groups in monosaccharide unit may be shielded by hydrophilic
side groups to
minimize interactions with monovalent and polyvalent cations that comprise the
conversion
coating composition.
[0072] The conversion composition may comprise the yield stress
component in an
amount of at least 0.2 percent by weight based on total weight of the
composition, such as at
11
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
least such as at least 0.3 percent by weight based on total weight of the
composition, such as at
least 0.4 percent by weight based on total weight of the composition, such as
at least 0.5 percent
by weight based on total weight of the composition, such as at least 0.6
percent by weight based
on total weight of the composition, such as at least 0.7 percent by weight
based on total weight of
the composition, such as at least 0.8 percent by weight based on total weight
of the composition,
such as at least 0.9 percent by weight based on total weight of the
composition, such as at least
1.0 percent by weight based on total weight of the composition, such as at
least 1.5 percent by
weight based on total weight of the composition, such as at least 2.0 percent
by weight based on
total weight of the composition.
[0073] The conversion composition may comprise the yield stress
component in any
amount such that the composition comprises yield stress and shear thinning
properties described
above while not negatively corrosion performance and coating behaviors such as
leveling, ease
of application and the like.
[0074] As mentioned above, the conversion composition may
comprise a trivalent
chromium. The trivalent chromium may be present in the conversion composition
in an amount
of at least 0.005 g/L based on total weight of the conversion composition,
such as at least 0.01
g/L, such as at least 0.5g/L, and in some instances, may be present in the
conversion composition
in an amount of no more than 2 g/L based on total weight of the conversion
composition, such as
no more than 1.5 g/L, such as no more than 1 g/L. The trivalent chromium may
be present in the
conversion composition in an amount of 0.005 g/L to 2 g/L based on total
weight of the
conversion composition, such as 0.01 g/L to 1.5 g/L, such as 0.5 g/L to 1 g/L.
[0075] The trivalent chromium may be present in the conversion
composition as a
compound, such as a trivalent chromium-containing salt. Thus, the composition
may further
comprise an anion that may be suitable for forming a salt with the trivalent
chromium, including
for example a halogen, a sulfate, a nitrate, an acetate, a carbonate, a
hydroxide, or combinations
thereof. Suitable examples of trivalent chromium salts include but are not
limited to basic
chromium sulphate, chromium (111) potassium sulfate. chromium (111) sulfate,
chromium (111)
halide (such as chromium (III) chloride) or combinations thereof. Trivalent
chromium salts may
be present in the conversion composition in their hydrated form or their
anhydrous form.
[0076] The anion suitable for forming the trivalent chromium salt
may be present in the
conversion composition in an amount of at least 0.01 g/L based on total weight
of the conversion
12
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
composition, such as at least 0.5 g/L, such as at least 1 g/L, and in some
instances, may be
present in an amount of no more than 4 g/L based on total weight of the
conversion composition,
such as no more than 3.5 g/L, such as no more than 2 g/L. The anion suitable
for forming the
trivalent chromium salt may be present in the conversion composition in an
amount of 0.01 g/L
to 4 g/L based on total weight of the conversion composition, such as 0.5 g/L
to 3.5 g/L, such as
1 g/L to 2 g/L.
[0077] Optionally, the conversion composition may further
comprise at least one
coinhibitor. In examples, the coinhibitor may comprise a Group IIA metal, a
transition metal, a
lanthanide series metal, a Group JIB metal, an azole, or combinations thereof.
In examples, the
Group IIA metal may comprise magnesium; the transition metal may comprise a
Group IIIB
metal such as yttrium, scandium, or combinations thereof, a Group IVB metal
such as zirconium,
titanium, hafnium, or combinations thereof, a Group VB metal such as vanadium,
a Group VIB
metal other than chromium such as molybdenum, and/or a Group VIIB metal such
as
manganese; the lanthanide series metal may comprise cerium, praseodymium,
terbium, or
combinations thereof; and the Group JIB metal may comprise zinc. Optionally,
the conversion
composition may be substantially free, or essentially free, or completely
free, of a Group 11B
metal.
[0078] The coinhibitor may be present in the conversion
composition as a compound
such as a salt. As such, the conversion composition may further comprise an
anion that may be
suitable for forming a salt with the metals of the coinhibitor(s), such as a
halogen, a nitrate, a
sulfate, a phosphate, a silicate (orthosilicates and metasilicates), a
carbonate, an acetate, a
hydroxide, a fluoride, and the like. Accordingly, the conversion composition
may comprise
sulfur-containing coinhibitors, phosphorous-containing coinhibitors, fluorine-
containing
coinhibitors, and the like.
[0079] The cation of the coinhibitor may be present in the
conversion composition in an
amount of at least 0.05 g/L based on total weight of the conversion
composition, such as at least
0.07 g/L, such as at least 0.5 g/L, and in some instances, may be present in
an amount of no more
than 5 g/L based on total weight of the conversion composition, such as no
more than 4 g/L, such
as no more than 1 g/L. The cation of the coinhibitor may be present in the
conversion
composition in an amount of 0.05 g/L to 5 g/L based on total weight of the
conversion
composition, such as 0.07 g/L to 4 g/L, such as 0.5 g/L to 1 g/L.
13
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[00801 The conversion composition may optionally further comprise
an indicator
compound, so named because it indicates, for example, the presence of a
chemical species, such
as a metal ion, the pH of a composition, and the like. An "indicator",
"indicator compound", and
like terms as used herein refer to a compound that changes color in response
to some external
stimulus, parameter, or condition, such as the presence of a metal ion, or in
response to a specific
pH or range of pHs.
[0081] The indicator compound may be any indicator known in the
art that indicates the
presence of a species, a particular pH, and the like. For example, a suitable
indicator may be one
that changes color after forming a metal ion complex with a particular metal
ion. The metal ion
indicator is generally a highly conjugated organic compound. A "conjugated
compound- as used
herein, and as will be understood by those skilled in the art, refers to a
compound having two
double bonds separated by a single bond, for example two carbon-carbon double
bonds with a
single carbon-carbon bond between them. Any conjugated compound can be used
according to
the present disclosure.
[0082] Similarly, the indicator compound can be one in which the
color changes upon
change of the pH; for example, the compound may be one color at an acidic or
neutral pH and
change color in an alkaline pH, or vice versa. Such indicators are well known
and widely
commercially available. An indicator that "changes color upon transition from
a first pH to a
second pH" (i.e., from a first pH to a second pH that is more or less acidic
or alkaline) therefore
has a first color (or is colorless) when exposed to a first pH and changes to
a second color (or
goes from colorless to colored) upon transition to a second pH (i.e., one that
is either more or
less acidic or alkaline than the first pH). For example, an indicator that
"changes color upon
transition to a more alkaline pH (or less acidic pH) goes from a first
color/colorless to a second
color/color when the pH transitions from acidic/neutral to alkaline. For
example, an indicator
that "changes color upon transition to a more acidic pH (or less alkaline pH)
goes from a first
color/colorless to a second color/color when the pH transitions from
alkaline/neutral to acidic.
[0083] Non-limiting examples of such indicator compounds include
methyl orange,
xylenol orange, catechol violet, bromophenol blue, green and purple,
eriochrome black T,
Celestine blue, hematoxylin, calmagite, gallocyanine, and combinations
thereof. Optionally, the
indicator compound may comprise an organic indicator compound that is a metal
ion indicator.
Nonlimiting examples of indicator compounds include those found in Table 1.
Fluorescent
14
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
indicators, which will emit light in certain conditions, can also be used
according to the present
disclosure, although the use of a fluorescent indicator also may be
specifically excluded. That is,
alternatively, conjugated compounds that exhibit fluorescence are specifically
excluded. As used
herein, "fluorescent indicator" and like terms refer to compounds, molecules,
pigments, and/or
dyes that will fluoresce or otherwise exhibit color upon exposure to
ultraviolet or visible light.
To "fluoresce" will be understood as emitting light following absorption of
shorter wavelength
light or other electromagnetic radiation. Examples of such indicators, often
referred to as "tags,"
include acridine, anthraquinone, coumarin, diphenylmethane,
diphenylnaphthlymethane,
quinoline, stilbene, triphenylmethane, anthracine and/or molecules containing
any of these
moieties and/or derivatives of any of these such as rhodamines,
phenanthridines, oxazines,
fluorones, cyanines and/or acridines.
TABLE 1
Compound Structure
CAS Reg. No.
Catechol Violet 0 115-41-3
Synonyms: OH
OH
Catecholsulfonphthalein; U=S=0
Pyrocatecholsulfonephthalein; HO
Pyrocatechol Violet HO
Xylenol Orange 3618-43-
7
Synonym:
0
3,3 '-Bis[N,N-
bis(carboxymethyl)aminomethyl]- 4
o-cresolsulfonephthalein tetrasodium OH
z 9
salt tis
r OH
[0084] According to the present disclosure, the conjugated
compound useful as indicator
may for example comprise catechol violet, as shown in Table 1. Catechol violet
(CV) is a
sulfone phthalein dye made from condensing two moles of pyrocatechol with one
mole of o-
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
sulfobenzoic acid anhydride. It has been found that CV has indicator
properties and when
incorporated into compositions having metal ions, it forms complexes, making
it useful as a
complexiometric reagent. As the composition containing the CV chelates metal
ions coming
from the metal substrate (i.e., those having hi- or higher valence), a
generally blue to blue-violet
color is observed.
[0085] Xylenol orange, as shown in Table 1, may likewise be
employed in the
compositions according to the present disclosure. It has been found that
xylenol orange has
metal ion (i.e., those having bi- or higher valence) indicator properties and
when incorporated
into compositions having metal ions, it forms complexes, making it useful as a
complexiometric
reagent. As the composition containing the xylenol orange chelates metal ions,
a solution of
xylenol orange turns from red to a generally blue color.
[0086] According to the present disclosure, the indicator
compound may be present in the
conversion composition in an amount of at least 0.01 g/1000 g conversion
composition, such as
at least 0.05 g/1000 g conversion composition, and in some instances, no more
than 3 g/1000 g
conversion composition, such as no more than 0.3g/1000 g conversion
composition. According
to the present disclosure, the indicator compound may be present in the
conversion composition
in an amount of 0.01 g/1000 g conversion composition to 3 g/1000 g conversion
composition,
such as 0.05 g/1000 g conversion composition to 0.3 g/1000 g conversion
composition.
[0087] The indicator compound changing color in response to a
certain external stimulus
provides a benefit when using the conversion composition in that it can serve,
for example, as a
visual indication that a substrate has been treated with the composition. For
example, a
conversion composition comprising an indicator that changes color when exposed
to a metal ion
that is present in the substrate will change color upon complexing with metal
ions in that
substrate; this allows the user to see that the substrate has been contacted
with the composition.
Similar benefits can be realized by depositing an alkaline or acid layer on a
substrate and
contacting the substrate with a composition of the present disclosure that
changes color when
exposed to an alkaline or acidic pH.
[0088] The pH of the conversion composition may, in some
instances, be less than 7,
such as less than 5, such as 1.5 to 6.9, such as 2.0 to 6.0, such as 2.5 to
4.5, such as 2.8 to 4.5.
The pH may be adjusted using, for example, any acid and/or base as is
necessary. Thus, the pH
of the conversion composition may be maintained through the inclusion of an
acidic material,
16
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
including water soluble and/or water dispersible acids, such as nitric acid,
sulfuric acid, and/or
phosphoric acid. Additionally, the pH of the composition may be maintained
through the
inclusion of a basic material, including water soluble and/or water
dispersible bases, such as
sodium hydroxide, sodium carbonate, potassium carbonate, potassium hydroxide,
ammonium
hydroxide, ammonia, and/or amines such as triethylamine, methylethyl amine, or
mixtures
thereof.
[0089] The conversion composition may exclude hexavalent chromium
or compounds
that include hexavalent chromium. Non-limiting examples of such materials
include chromic
acid, chromium trioxide, chromic acid anhydride, dichromate salts, such as
ammonium
dichromate, sodium dichromate, potassium dichromate, and calcium, barium,
magnesium, zinc,
cadmium, and strontium dichromate. When a composition and/or a coating or a
layer formed
from the composition is substantially free, essentially free, or completely
free of hexavalent
chromium, this includes hexavalent chromium in any form, such as, but not
limited to, the
hexavalent chromium-containing compounds listed above.
[0090] Thus, optionally, the conversion composition and/or
coatings or layers,
respectively, deposited from the same may be substantially free, may be
essentially free, and/or
may be completely free of one or more of any of the elements or compounds
listed in the
preceding paragraph. A composition and/or coating or layer, respectively,
formed from the same
that is substantially free of hexavalent chromium or derivatives thereof means
that hexavalent
chromium or derivatives thereof are not intentionally added, but may be
present in trace
amounts, such as because of impurities or unavoidable contamination from the
environment. In
other words, the amount of material is so small that it does not affect the
properties of the
composition; in the case of hexavalent chromium, this may further include that
the element or
compounds thereof are not present in the compositions and/or coatings or
layers, respectively,
formed from the same at such a level that it causes a burden on the
environment. The term
"substantially free" means that the composition and/or coating or layers,
respectively, formed
from the same contain less than 10 ppm of any or all of the elements or
compounds listed in the
preceding paragraph, based on total weight of the composition or the layer,
respectively, if any at
all. The term "essentially free" means that the composition and/or coatings or
layers,
respectively, formed from the same contain less than 1 ppm of any or all of
the elements or
compounds listed in the preceding paragraph, if any at all. The term
"completely free" means
17
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
that the composition and/or coatings or layers, respectively, formed from the
same contain less
than 1 ppb of any or all of the elements or compounds listed in the preceding
paragraph, if any at
all.
[0091] The conversion composition may comprise an aqueous medium
and may
optionally contain other materials such as nonionic surfactants and
auxiliaries conventionally
used in the art of conversion compositions. In the aqueous medium, water
dispersible organic
solvents, for example, alcohols with up to about 8 carbon atoms such as
methanol, isopropanol,
and the like, may be present; or glycol ethers such as the monoalkyl ethers of
ethylene glycol,
diethylene glycol, or propylene glycol, and the like. When present, water
dispersible organic
solvents are typically used in amounts up to about 10% by volume, based on the
total volume of
aqueous medium. Additionally, in the aqueous medium, thickeners such as
cellulosic, silicated,
or acrylic thickeners may be present. When present, such thickeners are
typically used in
amounts of at least 0.00001 wt.%, such as at least 0.5 wt.%, and in some
instances, no more than
wt.%, such as no more than 1 wt.%. When present, such thickeners are typically
used in
amounts of 0.00001 wt.% to 5 wt.%, such as 0.5 wt.% to 1 wt/%, based on total
weight of the
composition. As used herein, "thickener" refers to materials that are
substantially free of
crosslinking. and "substantially free of crosslinking" refers to materials
that have a weight
average molecular weight, as determined by gel permeation chromatography, of
less than
100,000.
[0092] Other optional materials that may be included in the
conversion composition
include surfactants that function as defoamers or substrate wetting agents.
Anionic, cationic,
amphoteric, and/or nonionic surfactants may be used. Defoaming surfactants may
optionally be
present at levels up to 1 wt.%, such as up to 0.1 wt.%, and wetting agents are
typically present at
levels up to 2 wt.%, such as up to 0.5 wt.%, based on the total weight of the
conversion
composition.
[0093] As mentioned above, the conversion composition may
comprise a carrier, often an
aqueous medium, so that the conversion composition is in the form of a
solution or dispersion of
the yield stress component, the trivalent chromium compound and optionally
other metal
compounds and/or coinhibitors in the carrier.
[0094] It has been surprisingly discovered that the conversion
compositions disclosed
herein (i) comprise a yield stress sufficient for the conversion composition
to cling to a non-
18
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
horizontal substrate, such as a substantially vertical substrate, (ii)
comprise a shear thinning
rheology profile (a decrease in viscosity with increasing shear rate) with
sufficiently low
viscosity at the higher shear rates to enable easy application to a substrate
surface, and (iii)
provide corrosion protection to substrates exposed to neutral salt spray
testing for 24 hours.
Systems
[0095] Also disclosed herein are systems for treating a
substrate. The system may
comprise, or consist essentially of, or consist of, any of the conversion
compositions described
herein above and at least one of a cleaning composition, a deoxidizer, a film-
forming resin or
combinations thereof.
[0096] As used herein, "cleaning compositions- included in the
systems and methods of
the present disclosure may have deoxidizing functionality in addition to
degreasing
characteristics and/or may eliminate the need for application of separate
treatment compositions
that deoxidize the substrate surface.
[0097] As mentioned above, the cleaning composition may be
alkaline and may have a
pH greater than 7, such as greater than 9, such as greater than 11. The pH of
the cleaning
composition may be 7 to 13, such as 9 to 12.7. In other instances, the
cleaning composition may
be acidic and may have a p1-1 less than 7, such as less than 6, such as less
than 5.5. The p1-1 of the
cleaning composition may be 0.5 to 6, such as 1.5 to 4.5.
[0098] The cleaning composition may include commercially
available alkaline cleaners,
including ChemkleenTM 163, 177, 611L. 490MX, 2010LP, and 181ALP, Ultrax 32,
Ultrax 97,
and Ultrax 94D, each of which are commercially available from PPG Industries,
Inc. (Cleveland,
OH), and any of the DFM Series, RECC 1001, and 88X1002 cleaners commercially
available
from PRC-DeSoto International (Sylmar, CA), and Turco 4215-NCLT and Ridolene
commercially available from Henkel Technologies (Madison Heights, MI), and any
of the
SOCOCLEAN series of cleaners commercially available from Soconaore.
Optionally, the
cleaner may be substantially free, or essentially free, or completely free of
borate.
[0099] The cleaning composition may comprise a hydroxide-
containing and/or a
phosphate-containing compound and/or a metasilicate. The hydroxide ion of the
hydroxide-
containing compound, if present at all, may be present in the cleaning
composition in an amount
of 0.05 to 25 g/1000 g solution, for example 18 to 20 g/1000 g solution based
on total weight of
the cleaning composition. In cleaning compositions having a phosphate-
containing compound,
19
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
the phosphate may comprise phosphate (PO4)3-, di-hydrogen phosphate (1-12PO4)-
, and/or
pyrophosphate (P207)4-, for example, phosphate (PO4)3- and/or pyrophosphate
(P207)4-. The
phosphate may be present in the composition in an amount of 50 g/1000 g
solution to 10 g/1000
g solution, for example 70 g/1000 g solution to 90 g/1000 g solution based on
total weight of the
cleaning composition. Other nonlimiting examples of suitable phosphate-
containing compounds
include organ phosphates, such as DequestO obtainable from Monsanto (St.
Louis, MO).
[0100] The cleaning composition may comprise hydrogen and/or
minerals such as iron,
potassium, etc. For example, the cleaning composition may comprise phosphoric
acid, acetic
acid, nitric acid, sulfuric acid, hydrofluoric acid, hydrochloric acid, and/or
iron sulfate.
[0101] The cleaning composition may optionally comprise a
corrosion inhibitor
comprising a metal compound and/or an azole compound. The metal of the metal
compound in
the corrosion inhibitor (when included) may comprise various metals which have
corrosion
inhibiting characteristics. For example, the metal may comprise a lanthanide
series element, a
Group IA metal, a Group IIA metal, and/or a transition metal, such as any of
those described
above.
[0102] The cleaning composition may comprise a corrosion
inhibitor comprising a metal
at a concentration of at least 0.01 g/L, such as at least 0.05 g/L, such as at
least 0.1 g/L, such as at
least 1 g/L, and in some instances may be present in the cleaning composition
at a concentration
of no more than 25 g/L, such as no more than 16 g/L, such as no more than 10
g/L, such as no
more than 5 g/L. The metal can be present in the cleaning composition at a
concentration of 0.01
g/L of composition to 25 g/L of composition, such as 0.05 g/L to 16 g/L, such
as 0.1 g/L to 10
g/L, such as 1 g/L to 5 g/L based on total weight of the cleaning composition.
[0103] The corrosion-inhibiting metal may be provided in the
cleaning composition in
the form of a salt having an anion and the metal as the cation of the salt.
The anion of the salt
may be any suitable anion capable of forming a salt with the lanthanide series
element, Group IA
metal, Group 11A metal, and/or transition metal. Nonlimiting examples of such
anions include a
carbonate, a hydroxide, a nitrate, a halogen, a sulfate, a phosphate and/or a
silicate (e.g.,
orthosilicates and metasilicates). Optionally, the cleaning composition may
include at least two
metal salts, and the at least two metal salts may comprise different anions
and/or cations from
each other. For example, the at least two metal salts may comprise different
anions but the same
cations or may comprise different cations but the same anions.
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0104] As mentioned above, the cleaning composition may comprise
a halogen. The
halogen may be provided in the composition in the form of a salt with the
metals described
above. The halogen may be present in the cleaning composition in an amount of
at least 0.2 g/L
based on total weight of the cleaning composition, and in some instances may
he present in an
amount of no more than 1.5 g/L based on total weight of the cleaning
composition. The halogen
may be present in the cleaning composition in an amount of 0.2 g/L to 1.5 g/L
based on total
weight of the cleaning composition. In other examples, the cleaning
composition may be
substantially free, or essentially free, or completely free, of halogen.
[0105] Optionally, the cleaning composition may further comprise
a nitrogen-containing
heterocyclic compound. The nitrogen-containing heterocyclic compound may
include cyclic
compounds having 1 nitrogen atom, such as pyrroles, and azole compounds having
2 or more
nitrogen atoms, such as pyrazoles, imidazoles, triazoles, tetrazoles and
pentazoles, 1 nitrogen
atom and 1 oxygen atom, such as oxazoles and isoxazoles, or 1 nitrogen atom
and 1 sulfur atom,
such as thiazoles and isothiazoles. Nonlimiting examples of suitable azole
compounds include
2,5-dimercapto-1,3.4-thiadiazole (CAS:1072-71-5),11-1-benzotriazole (CAS: 95-
14-7), 1H-1,2,3-
triazole (CAS: 288-36-8), 2-amino-5-mercapto-1,3,4-thiadiazole (CAS: 2349-67-
9), also named
5-amino-1,3,4-thiadiazc-)1e-2-thiol, and 2-amino-1,3,4-thiadiazole (CAS: 4005-
51-0). In some
embodiments, for example, the azole compound comprises 2,5-dimercapto-1,3,4-
thiadiazole.
Additionally, the nitrogen-containing heterocyclic compound may be in the form
of a salt, such
as a sodium salt.
[0106] The nitrogen-containing heterocyclic compound may be
present in the cleaning
composition in an amount of at least 0.5 g/L based on total weight of the
cleaning composition,
such as at least 1 g/L based on total weight of the cleaning composition, such
as at least 5 g/L
based on total weight of the cleaning composition, and in some instances may
be present in an
amount of no more than 15 g/L based on total weight of the cleaning
composition, such as no
more than 12 g/L based on total weight of the cleaning composition, such as no
more than 10 g/L
based on total weight of the cleaning. The nitrogen-containing heterocyclic
compound may be
present in the cleaning composition in an effective corrosion inhibiting
amount, for example, 0.5
g/L to 15 g/L based on total weight of the cleaning composition, such as 1 g/L
to 12 g/L based on
total weight of the cleaning composition, such as 5 g/L to 10 g/L based on
total weight of the
cleaning composition.
21
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0107] The cleaning composition may contain additives such as,
but not limited to,
carbonates, surfactants, chelators, thickeners, allantoin,
polyvinylpyrrolidone, 2,5-dimercapto-
1,3,4-thiadiazole, halides, adhesion promotors, such as adhesion promoting
silanes (e.g., silanes
having an amine and/or hydroxyl functionality; or a zirconium alkoxide and/or
a si lanc coupling
agent) and alcohols (collectively, "additives"). Surfactants suitable for use
in the present
disclosure include Dynol 604 and CarhowetTM DC01 surfactant both commercially
available
from Air Products, having offices in Allentown, PA. and Triton X-100 available
from The Dow
Chemical Company (Midland MI). Such additives, if present at all, may be
present in the
cleaning composition in an amount of at least 0.01 g/L based on total weight
of the cleaning
composition, such as at least 0.5 g/L, such as at least 1 g/L, such as at
least 10 g/L, such as at
least 20 g/L, and may be present in the cleaning composition in an amount of
no more than 60
g/L based on total weight of the cleaning composition, such as no more than 50
g/L, such as no
more than 40 g/L, such as no more than 30 g/L, such as no more than 10 g/L,
such as no more
than 5 g/L, such as no more than 3 g/L. Such additives, if present at all, may
be present in the
cleaning composition in an amount of 0.01 g/L to 60 g/L based on total weight
of the cleaning
composition, such as 0.5 g/L to 50 g/L, such as 1 g/L to 40 g/L, such as 10
g/L to 30 g/L, such as
g/L to 20 g/L, such as 0.01 g/L to 5 g/L, such as 0.05 g/L to 3 g/L.
[0108] The cleaning composition of the present disclosure may
comprise a carrier such as
water such that the cleaning composition is in the form of a solution or
dispersion.
[0109] The system may further comprise a deoxidizer. As used
herein, the term
"deoxidizer" refers to a material or a substance that is capable of removing
an oxide layer from a
surface of a substrate. Deoxidizers may comprise physical deoxidizers and/or
chemical
deoxidizers. Suitable physical deoxidizer may uniformly roughen the substrate
surface, such as
by using a scouring or cleaning pad. Suitable chemical deoxidizers include,
for example, acid-
based deoxidizers such as phosphoric acid, nitric acid, fluoroboric acid,
sulfuric acid, chromic
acid, hydrofluoric acid, and ammonium bifluoride, or Amchem 7/17 deoxidizers
available from
Henkel Technologies (Madison Heights, MI), OAK1TE DEOXID1ZER LNC commercially
available from Chemetall, TURCO DEOXIDIZER 6 commercially available from
Henkel,
Socosurf deoxiders commercially available from Socomore) or combinations
thereof. Often, the
chemical deoxidizer comprises a carrier, often an aqueous medium, so that the
deoxidizer may be
in the form of a solution or dispersion in the carrier.
22
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0110] The system of the present disclosure may further comprise
a thermosetting film-
forming resin or a thermoplastic film-forming resin. As used herein, the term
"film-forming
resin" refers to resins that can form a self-supporting continuous film on at
least a horizontal
surface of a substrate upon removal of any diluents or carriers present in the
composition or upon
curing at ambient or elevated temperatures. Conventional film-forming resins
that may be used
include, without limitation, those typically used in automotive OEM coating
compositions,
automotive refinish coating compositions, industrial coating compositions,
architectural coating
compositions, coil coating compositions, and aerospace coating compositions,
among others. As
used herein, the term "thermosetting" refers to resins that "set" irreversibly
upon curing or
crosslinking. wherein the polymer chains of the polymeric components are
joined together by
covalent bonds. This property is usually associated with a cross-linking
reaction of the
composition constituents often induced, for example, by heat or radiation.
Curing or
cros slinking reactions also may be carried out under ambient conditions. Once
cured or
crosslinked, a thermosetting resin will not melt upon the application of heat
and is insoluble in
solvents. As used herein, the term -thermoplastic" refers to resins that
comprise polymeric
components that are not joined by covalent bonds and thereby can undergo
liquid flow upon
heating and are soluble in solvents.
[0111] In general, the colorant can be present in the coating
composition in any amount
sufficient to impart the desired visual and/or color effect. The colorant may
comprise from 1
wt.% to 65 wt.%, such as from 3 wt.% to 40 wt.% or 5 wt.% to 35 wt.%, with
weight percent
based on the total weight of the composition.
[0112] In an example, the film-forming resin may be an
electrodepositable coating
composition comprising a water dispersible, ionic salt group-containing film-
forming resin that
may be deposited onto a surface of the substrate by electrodeposition.
[0113] The ionic salt group-containing film-forming polymer may
comprise a cationic
salt group-containing film-forming polymer for use in a cationic
electrodepositable coating
composition. As used herein, the term "cationic salt group-containing film-
forming polymer"
refers to polymers that include at least partially neutralized cationic
groups, such as sulfonium
groups and ammonium groups, that impart a positive charge. The cationic salt
group-containing
film-forming polymer may comprise active hydrogen functional groups,
including, for example,
hydroxyl groups, primary or secondary amine groups, and thiol groups. Cationic
salt group-
23
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
containing film-forming polymers that comprise active hydrogen functional
groups may be
referred to as active hydrogen-containing, cationic salt group-containing film-
forming polymers.
Examples of polymers that are suitable for use as the cationic salt group-
containing film-forming
polymer include, hut arc not limited to, alkyd polymers, acrylics,
polyepoxides, polyamides,
polyurethanes, polyureas, polyethers, and polyesters, among others.
[0114] The cationic salt group-containing film-forming polymer
may be present in the
cationic electrodepositable coating composition in an amount of 40 wt.% to 90
wt.%, such as 50
wt.% to 80 wt.%, such as 60 wt.% to 75 wt.%, based on the total weight of the
resin solids of the
electrodepositable coating composition. As used herein, the "resin solids"
include the ionic salt
group-containing film-forming polymer, curing agent, and any additional water
dispersible non-
pigmented component(s) present in the electrodepositable coating composition.
[0115] Alternatively, the ionic salt group-containing film-
forming polymer may comprise
an anionic salt group-containing film-forming polymer for use in an anionic
electrodepositable
coating composition. As used herein, the term "anionic salt group-containing
film-forming
polymer" refers to an anionic polymer comprising at least partially
neutralized anionic functional
groups, such as carboxylic acid and phosphoric acid groups that impart a
negative charge. The
anionic salt group-containing film-forming polymer may comprise active
hydrogen functional
groups. Anionic salt group-containing film-forming polymers that comprise
active hydrogen
functional groups may be referred to as active hydrogen-containing, anionic
salt group-
containing film-forming polymers.
[0116] The anionic salt group-containing film-forming polymer may
comprise base-
solubilized, carboxylic acid group-containing film-forming polymers such as
the reaction
product or adduct of a drying oil or semi-drying fatty acid ester with a
dicarboxylic acid or
anhydride; and the reaction product of a fatty acid ester, unsaturated acid or
anhydride and any
additional unsaturated modifying materials which are further reacted with
polyol. Also suitable
are the at least partially neutralized interpolymers of hydroxy-alkyl esters
of unsaturated
carboxylic acids, unsaturated carboxylic acid and at least one other
ethylenically unsaturated
monomer. Still another suitable anionic electrodepositable resin comprises an
alkyd-aminoplast
vehicle, i.e., a vehicle containing an alkyd resin and an amine-aldehyde
resin. Another suitable
anionic electrodepositable resin composition comprises mixed esters of a
resinous polyol. Other
acid functional polymers may also be used such as phosphatized polyepoxide or
phosphatized
24
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
acrylic polymers. Exemplary phosphatized polyepoxides are disclosed in U.S.
Patent
Application Publication No. 2009-0045071 at [0004]-[0015] and U.S. Patent
Application Serial
No. 13/232,093 at [0014]-[0040], the cited portions of which being
incorporated herein by
reference.
[0117] The anionic salt group-containing film-forming polymer may
be present in the
anionic electrodepositable coating composition in an amount 50% to 90%, such
as 55% to 80%,
such as 60% to 75%, based on the total weight of the resin solids of the
electrodepositable
coating composition.
[0118] The electrodepositable coating composition may further
comprise a curing agent.
The curing agent may react with the reactive groups, such as active hydrogen
groups, of the ionic
salt group-containing film-forming polymer to effectuate cure of the coating
composition to form
a coating. Non-limiting examples of suitable curing agents are at least
partially blocked
polyisocyanates, aminoplast resins and phenoplast resins, such as
phenolformaldehyde
condensates including allyl ether derivatives thereof.
[0119] The curing agent may be present in the cationic
electrodepositable coating
composition in an amount of 10 wt.% to 60 wt.%, such as 20 wt.% to 50 wt.%,
such as 25 wt.%
to 40 wt.%, based on the total weight of the resin solids of the
electrodepositable coating
composition. Alternatively, the curing agent may be present in the anionic
electrodepositable
coating composition in an amount of 10 wt.% to 50 wt.%, such as 20 wt.% to 45
wt.%, such as
25 wt.% to 40 wt.%, based on the total weight of the resin solids of the
electrodepositable
coating composition.
[0120] The electrodepositable coating composition may further
comprise other optional
ingredients, such as a pigment composition and, if desired, various additives
such as fillers,
plasticizers, antioxidants, biocides, UV light absorbers and stabilizers,
hindered amine light
stabilizers, defoamers, fungicides, dispersing aids, flow control agents,
surfactants, wetting
agents, or combinations thereof.
[0121] The electrodepositable coating composition may comprise
water and/or one or
more organic solvent(s). Water can, for example, be present in amounts of 40
wt.% to 90 wt.%,
such as 50 wt.% to 75 wt.%, based on total weight of the electrodepositable
coating composition.
If used, the organic solvents may typically be present in an amount of less
than 10 wt.%, such as
less than 5 wt.%, based on total weight of the electrodepositable coating
composition. The
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
electrodepositable coating composition may in particular be provided in the
form of an aqueous
dispersion. The total solids content of the electrodepositable coating
composition may be from 1
wt.% to 50 wt.%, such as 5 wt.% to 40 wt.%, such as 5 wt.% to 20 wt.%, based
on the total
weight of the electrodepositable coating composition. As used herein, -total
solids" refers to the
non-volatile content of the electrodepositable coating composition, i.e.,
materials which will not
volatilize when heated to 110 C for 15 minutes.
[0122]
The cationic electrodepositable coating composition may be deposited upon
an
electrically conductive substrate by placing the composition in contact with
an electrically
conductive cathode and an electrically conductive anode, with the surface to
be coated being the
cathode. Alternatively, the anionic electrodepositable coating composition may
be deposited
upon an electrically conductive substrate by placing the composition in
contact with an
electrically conductive cathode and an electrically conductive anode, with the
surface to be
coated being the anode. An adherent film of the electrodepositable coating
composition may be
deposited in a substantially continuous manner on the cathode or anode when a
sufficient voltage
is impressed between the electrodes. The applied voltage may be varied and can
be, for
example, as low as one volt to as high as several thousand volts, such as
between 50 and 500
volts. Current density is usually between 1.0 ampere and 15 amperes per square
foot (10.8 to
161.5 amperes per square meter) and tends to decrease quickly during the
electrodeposition
process, indicating formation of a continuous self-insulating film.
[0123]
The film-forming resin may comprise a powder coating composition. As used
herein, "powder coating composition refers to a coating composition which is
completely free
of water and/or solvent. Accordingly, the powder coating composition disclosed
herein is not
synonymous to waterborne and/or solvent-borne coating compositions known in
the art. The
powder coating composition may comprise: (a) a film-forming polymer having a
reactive
functional group; and (b) a curing agent that is reactive with the functional
group. Examples of
powder coating compositions that may be used in the present disclosure include
the polyester-
based ENV1ROCRON line of powder coating compositions commercially available
from PPG
Industries, Inc. or epoxy-polyester hybrid powder coating compositions.
Alternative examples of
powder coating compositions that may be used in the present disclosure include
low temperature
cure thermosetting powder coating compositions comprising (a) at least one
tertiary aminourea
compound, at least one tertiary aminourethane compound, or mixtures thereof,
and (b) at least
26
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
one film-forming epoxy-containing resin and/or at least one siloxane-
containing resin (such as
those described in U.S. Patent No. 7,470,752, assigned to PPG Industries Ohio,
Inc. and
incorporated herein by reference); curable powder coating compositions
generally comprising (a)
at least one tertiary aminourea compound, at least one tertiary aminourethane
compound, or
mixtures thereof, and (b) at least one film-forming epoxy-containing resin
and/or at least one
siloxane-containing resin (such as those described in U.S. Patent No.
7,432,333, assigned to PPG
Industries Ohio, Inc. and incorporated herein by reference); and those
comprising a solid
particulate mixture of a reactive group-containing polymer having a Tg of at
least 30 C (such as
those described in U.S. Patent No. 6,797,387, assigned to PPG Industries Ohio,
Inc. and
incorporated herein by reference).
[0124] The film-forming resin may comprise a liquid coating
composition. As used
herein, "liquid coating composition" refers to a coating composition which
contains a portion of
water and/or solvent. Accordingly, the liquid coating composition disclosed
herein is
synonymous with waterborne and/or solvent-borne coating compositions known in
the art. The
liquid coating composition may comprise, for example, (a) a film-forming
polymer having a
reactive functional group and (b) a curing agent that is reactive with the
functional group. In
other examples, the liquid coating may contain a film-forming polymer that may
react with
oxygen in the air or coalesce into a film with the evaporation of water and/or
solvents. These
film-forming mechanisms may require or be accelerated by the application of
heat or some type
of radiation such as Ultraviolet or Infrared. Examples of liquid coating
compositions that may be
used in the present disclosure include the SPECTRACRONO line of solvent-based
coating
compositions, the AQUACRONO line of water-based coating compositions, and the
RAYCRONO line of UV cured coatings all commercially available from PPG
Industries, Inc.
[0125] Suitable film-forming polymers that may be used in the
liquid coating
composition of the present disclosure may comprise a (poly)ester, an alkyd, a
(poly)urethane, an
isocyanurate, a (poly)urea. a (poly)epoxy, an anhydride, an acrylic, a
(poly)ether, a (poly)sulfide,
a (poly)amine, a (poly)amide, (poly)vinyl chloride, (poly)olefin,
(poly)vinylidene fluoride,
(poly)siloxane, or combinations thereof.
[0126] The film-forming polymer composition may comprise a primer
composition. The
primer compositions may comprise, for example, chromate-based primer
compositions such as
those available from PPG Industries, Inc. (product code 44GN072), or a chrome-
free primer
27
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
composition such as those available from PPG (DESOPRIME CA7502, DESOPRIME
CA7521,
Deft 02GN083, Deft 02GN084) or such as those described in U.S. Patent
Application Serial No.
10/758,973, titled "Corrosion Resistant Coatings Containing Carbon", and U.S.
Patent
Application Serial Nos. 10/758,972 and 10/758,972, both titled -Corrosion
Resistant Coatings",
all of which are incorporated herein by reference. In examples, the primer
composition may be
one which can pass the military requirement of MIL-PRF-85582 Class N or MIL-
PRF-23377
Class N.
[0127] The film-forming polymer composition may comprise a
topcoat composition. As
used herein, the term "topcoat composition" refers to a mixture of binder(s)
(i.e., organic or
inorganic based polymer(s)) and at least one pigment, which can optionally
contain at least one
solvent and/or at least one curing agent. Topcoat compositions form a topcoat
on a substrate,
which is typically the coating layer in a single or multi-layer coating system
whose outer surface
is exposed to the atmosphere or environment, and whose inner surface is in
contact with another
coating layer or polymeric substrate. Examples of suitable topcoat
compositions include those
conforming to MIL-PRF-85285D, such as those available from PPG (Deft 03W127A
and Deft
03GY292). Other suitable topcoat compositions include advanced performance
topcoat
compositions such as those available from PPG (Defthanee ELT.TM. 99GY001 and
99W009).
However, other topcoat compositions and advanced performance topcoat
compositions can be
used in the present disclosure as will be understood by those of skill in the
art with reference to
this disclosure.
[0128] The film-forming polymer composition may comprise a self-
priming topcoat or
an enhanced self-priming topcoat. Examples of suitable self-priming topcoat
compositions
include those that conform to TT-P-2756A. Examples of self-priming topcoat
compositions
include those available from PPG (03W169 and 03GY369), and examples of
enhanced self-
priming topcoat compositions include Defthanee ELTIm/ESPT (product code
97GY121),
available from PPG. However, other self-priming topcoats and enhanced self-
priming topcoats
can be used in the coating system according to the present disclosure as will
be understood by
those of skill in the art with reference to this disclosure.
[0129] In addition to the components described above, such film-
forming resins may
further comprise colorants, surfactants, wetting agents and/or catalysts. As
used herein, the term
"colorant" means any substance that is capable of imparting color, opacity
and/or other visual
28
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
effect to the composition. Example colorants include pigments, dyes and tints,
such as those
used in the paint industry and/or listed in the Dry Color Manufacturers
Association (DCMA).
Methods
[0130] Also disclosed herein arc methods for treating a
substrate. The method may
comprise, or consist essentially of, or consist of, contacting at least a
portion of a surface of a
substrate with one of the conversion compositions described herein above.
[0131] The solution or dispersion of the conversion composition
may be brought into
contact with the substrate by any of a variety of known techniques, such as
dipping or
immersion, spraying, intermittent spraying, dipping followed by spraying,
spraying followed by
dipping, brushing, or roll-coating. The solution or dispersion, when applied
to the metal
substrate, may be at a temperature ranging from 40 F to 160 F, such as 60 F to
110 F, such as
70 F to 90 F. For example, the process may be carried out at ambient or room
temperature. The
contact time is often from 1 second to 30 minutes, such as 30 seconds to 15
minutes, such as 4
minutes to 10 minutes.
[0132] Following the contacting with the conversion composition,
the substrate
optionally may be air dried at room temperature or may be dried with hot air,
for example, by
using an air knife, by flashing off the water by brief exposure of the
substrate to a high
temperature, such as by drying the substrate in an oven at 15 C to 100 C, such
as 20 C to 90 C,
or in a heater assembly using, for example, infrared heat, such as for 10
minutes at 70 C, or by
passing the substrate between squeegee rolls. Alternatively, following the
contacting with the
conversion composition, the substrate optionally may be rinsed with tap water,
deionized water,
reverse osmosis (RO) water and/or an aqueous solution of rinsing agents in
order to remove any
residue and then optionally may be dried, for example air dried or dried with
hot air as described
in the preceding sentence.
[0133] The metal substrate optionally may be prepared by first
solvent treating the metal
substrate prior to contacting the metal substrate with a cleaning composition,
a deoxidizing
composition, or one of the conversion compositions described herein. As used
herein, the term
"solvent treating" refers to rinsing, wiping, spraying, or immersing the
substrate in a solvent that
assists in the removal of inks, oils, etc., that may be on the metal surface.
Nonlimiting examples
of suitable solvents include methyl ethyl ketone (MEK), methyl propyl ketone
(MPK), acetone,
and the like. Alternately, the metal substrate may be prepared by degreasing
the metal substrate
29
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
using conventional degreasing methods prior to contacting the metal substrate
with the cleaning
composition.
[0134] Additional optional procedures for preparing the metal
substrate include the use
of a surface brightener, such as an acid pickle or light acid etch, or a smut
remover.
[0135] Optionally, at least a portion of the substrate surface
may be cleaned and/or
deoxidized prior to contacting at least a portion of the substrate surface
with conversion
compositions described above, in order to remove grease, dirt, and/or other
extraneous matter,
using any of the cleaners and/or deoxidizers described above. Such cleaners
and/or deoxidizers
are often preceded or followed by a water rinse, such as with tap water,
distilled water, RO
water, or combinations thereof. For example, the methods of the present
disclosure may include
cleaning compositions and deoxidizing compositions which are applied to the
substrate surface
in sequential steps, optionally with a rinse step(s) intervening.
[0136] Optionally, as mentioned above, optionally, at least a
portion of the cleaned
substrate surface may be deoxidized, mechanically and/or chemically. Often,
the chemical
deoxidizer comprises a carrier, often an aqueous medium, so that the
deoxidizer may be in the
form of a solution or dispersion in the carrier, in which case the solution or
dispersion may be
brought into contact with the substrate by any of a variety of known
techniques, such as dipping
or immersion, spraying, intermittent spraying, dipping followed by spraying,
spraying followed
by dipping, brushing, or roll-coating. The skilled artisan will select a
temperature range of the
solution or dispersion, when applied to the metal substrate, based on etch
rates, for example, at a
temperature ranging from 50 F to 150 F (10 C to 66 C), such as from 70 F to
130 F (21 C to
54 C), such as from 80 F to 120 F (27 C to 49 C). The contact time may be from
30 seconds to
20 minutes, such as 1 minute to 15 minutes, such as 90 seconds to 12 minutes,
such as 3 minutes
to 9 minutes.
[0137] The cleaning and deoxidizing compositions may be brought
into contact with the
substrate surface by any of a variety of techniques. including, but not
limited to, dip immersion,
spraying, swabbing, or spreading using a brush, roller, or the like. With
regard to application via
spraying, conventional (automatic or manual) spray techniques and equipment
used for air
spraying may be used. The dwell time in which the cleaning composition remains
in contact
with the metal substrate may vary from a few seconds to several hours, for
example less than 30
minutes or 3 minutes or less.
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0138] After contacting the metal substrate with the cleaning
composition, the metal
substrate may optionally be air dried, and then rinsed with tap water, RO
water, and/or
distilled/de-ionized water. Alternately, after contacting the metal substrate
with the composition,
the metal substrate may be rinsed with tap water, RU water, and/or
distilled/de-ionized water,
and then subsequently air dried (if desired). However, the substrate need not
be dried; and in
some instances, drying is omitted. Additionally, as noted above, the substrate
need not be rinsed,
and the metal substrate may then be further coated with conversion coatings,
primers and/or
topcoats to achieve a substrate with a finished coating. Accordingly, in some
instances, this
subsequent rinse may be omitted. For example, a solvent (e.g., alcohol) may be
used to rinse the
substrate, which allows the omission of a drying step.
[0139] After the substrate is contacted with the conversion
composition, a coating
composition comprising a film-forming resin may be deposited onto at least a
portion of the
surface of the substrate that has been contacted with the conversion
composition. Any suitable
technique may be used to deposit such a coating composition onto the
substrate, including, for
example, brushing, dipping, flow coating, spraying and the like. In some
instances, however, as
described in more detail below, such depositing of a coating composition may
comprise an
electrocoating step wherein an electrodepositable composition is deposited
onto a metal substrate
by electrodeposition. In certain other instances, as described in more detail
below, such
depositing of a coating composition comprises a powder coating step. In still
other instances, the
coating composition may be a liquid coating composition.
[0140] Once the cationic or anionic electrodepositable coating
composition is
electrodeposited over at least a portion of the electroconductive substrate,
the coated substrate
may be heated to a temperature and for a time sufficient to cure the
electrodeposited coating on
the substrate. For cationic electrodeposition, the coated substrate may be
heated to a temperature
ranging from 250 F to 450 F (121.1 C to 232.2 C), such as from 275 F to 400 F
(135 C to
204.4 C), such as from 300 F to 360 F (149 C to 180 C). For anionic
electrodeposition, the
coated substrate may be heated to a temperature ranging from 200 F to 450 F
(93 C to 232.2 C),
such as from 275 F to 400 F (135 C to 204.4 C), such as from 300 F to 360 F
(149 C to
180 C), such as 200 F to 210.2 F (93 C to 99 C). The curing time may be
dependent upon the
curing temperature as well as other variables, for example, the film thickness
of the
electrodeposited coating, level and type of catalyst present in the
composition and the like. For
31
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
example, the curing time can range from 10 to 60 minutes, such as 20 to 40
minutes. The
thickness of the resultant cured electrodeposited coating may range from 2 to
50 microns.
[0141] After deposition of the powder coating composition, the
coating is often heated to
cure the deposited composition. The heating or curing operation is often
carried out at a
temperature in the range of from 150 C to 200 C, such as from 170 C to 190 C,
for a period of
time ranging from 10 to 20 minutes. The thickness of the resultant film is
from 50 to 125
microns.
[0142] The self-priming topcoat and enhanced self-priming topcoat
may be applied
directly to the substrate treated with the conversion composition. The self-
priming topcoat and
enhanced self-priming topcoat can optionally be applied to an organic or
inorganic polymeric
coating, such as a primer or paint film. The self-priming topcoat layer and
enhanced self-
priming topcoat is typically the coating layer in a single or multi-layer
coating system where the
outer surface of the coating is exposed to the atmosphere or environment, and
the inner surface
of the coating is typically in contact with the substrate or optional polymer
coating or primer.
[0143] The topcoat, self-priming topcoat, and enhanced self-
priming topcoat can be
applied to the treated substrate, in either a wet or "not fully cured"
condition that dries or cures
over time, that is, solvent evaporates and/or there is a chemical reaction.
The coatings can dry or
cure either naturally or by accelerated means, for example, an ultraviolet
light cured system to
form a film or "cured" paint. The coatings can also be applied in a semi or
fully cured state, such
as an adhesive.
Substrates
[0144] Disclosed herein are substrates treated with the
conversion compositions
described above. Also disclosed herein are substrates treated with the systems
and methods
described above.
[0145] Suitable substrates that may be used in the present
disclosure include metal
substrates, metal alloy substrates, and/or substrates that have been
metallized, such as nickel-
plated plastic. The metal or metal alloy can comprise or be steel, aluminum,
zinc, nickel, and/or
magnesium. For example, the steel substrate could be cold rolled steel, hot
rolled steel,
electrogalvanized steel, and/or hot dipped galvanized steel. Aluminum alloys
of the 1XXX,
2XXX, 3XXX, 4XXX, 5XXX, 6XXX, or 7XXX series as well as clad aluminum alloys
also may
be used as the substrate. Aluminum alloys may comprise 0.01 wt.% copper to 10
wt.% copper.
32
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
Aluminum alloys which are treated may also include castings, such as 1XX.X,
2XX.X, 3XX.X,
4XX.X, 5XX.X, 6XX.X, 7XX.X. 8XX.X, or 9XX.X (e.g., A356.0). Magnesium alloys
of the
AZ31B, AZ91C, AM60B, or EV31A series also may be used as the substrate. The
substrate
used in the present disclosure may also comprise titanium and/or titanium
alloys, zinc and/or
zinc alloys, and/or nickel and/or nickel alloys. The substrate also may
comprise assemblies or
multi-metal substrates. The substrate may comprise a portion of a vehicle such
as a vehicular
body (e.g., without limitation, door, body panel, trunk deck lid, roof panel,
hood, roof and/or
stringers, rivets, landing gear components, and/or skins used on an aircraft)
and/or a vehicular
frame. As used herein. "vehicle" or variations thereof include, but is not
limited to, civilian,
commercial, and military aircraft, and/or land vehicles such as cars,
motorcycles, and/or trucks.
[0146] In examples, the substrate may be a complex part and/or
may have a non-
horizontal surface. The surface may be a substantially vertical surface.
[0147] Disclosed herein are treated substrates. The treated
substrates may comprise, or
consist essentially of, or consist of, a film formed from one of the
conversion compositions
disclosed herein. The film may be formed from a conversion composition
comprising, or
consisting essentially of, or consisting of, a yield stress component and a
corrosion inhibitor,
wherein the conversion composition comprises a yield stress sufficient to
overcome the effect of
gravity when applied to a non-horizontal surface.
[0148] Also disclosed herein are substrates treated with any of
the systems disclosed
hereinabove. The substrate may be treated with a system comprising, or
consisting essentially
of, or consisting of: one of the conversion compositions disclosed
hereinabove; and at least one
of a cleaning composition, a deoxidizer, a film-forming resin or combinations
thereof.
[0149] Also disclosed herein are substrates treated with any of
the methods disclosed
hereinabove. The substrate may be treated with a method comprising, or
consisting essentially
of, or consisting of: contacting at least a portion of a surface of the
substrate with one of the
conversion composition disclosed hereinabove; and optionally contacting at
least a portion of the
surface with a cleaning composition and/or a deoxidizer disclosed hereinabove.
[0150] It has been surprisingly discovered that the conversion
compositions disclosed
herein provide substrates with corrosion protection when exposed to neutral
salt spray testing for
24 hours. For example, the substrate surface may comprise less than 1%
corrosion over a 1.376
in2 area of the substrate surface following 24-hour exposure to a 5% sodium
chloride salt fog
33
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
environment (salt spray cabinet operated according to ASTM B117 (2019)), such
as less than
0.75% corrosion, such as less than 0.5% corrosion, such as less than 0.25%
corrosion, such as
less than 0.2% corrosion. For example, the treated substrate may pass
corrosion testing
according to ASTM D610-08 (2019) rating scale, such as a rating of at least
7P, such as at least
8P, such as at least 9P.
Uses
[0151] Also disclosed are uses of the compositions disclosed
herein to provide a
conversion composition comprising (i) a yield stress sufficient to overcome
the effect of gravity
when applied to a non-horizontal surface and (ii) a shear thinning rheology
profile.
[0152] Also disclosed are uses of a film formed on a substrate
surface from the
conversion compositions disclosed herein to provide a film that overcomes the
effect of gravity
when applied to a non-horizontal surface and that provides corrosion
protection to the surface
such that the substrate surface comprises less than 1% corrosion of a 1.376
in2 area of the
substrate surface following 24 hour exposure to a 5% sodium chloride salt fog
environment (salt
spray cabinet operated according to ASTM B117 (2019)) and/or wherein the
substrate passes
corrosion testing according to ASTM D610-08 (2019) rating scale. The substrate
may comprise
a substantially vertical surface. The substrate may comprise a complex
substrate.
[0153] The films formed by the conversion compositions, systems
and methods may be
used to repair a surface of a substrate.
Methods of Making the Conversion Compositions
[0154] The conversion compositions disclosed herein may be made
by first optionally
adjusting a pH of the yield stress component to a pH of less than 7; and then
mixing the yield
stress component with the corrosion inhibitor. Optionally, the pH of the
conversion composition
may be adjusted to a pH less than 7, such as a pH of 2.8 to 4.5.
[0155] Optionally, the yield stress fluid may be made using
conventional free-radical
polymerization techniques including emulsion, dispersion or solution
processes.
[0156] Illustrating the disclosed subject matter are the
following examples that are not to
be considered as limiting the disclosure to their details. All parts and
percentages in the
examples, as well as throughout the specification, are by weight unless
otherwise indicated.
34
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
EXAMPLES
Preparation of corrosion inhibitor compositions
[0157] A 1.0 wt% solution of diutan gum in deionized water was
prepared by adding
5.0g of diutan gum to 495g of warmed, deionized water and stirring for 18
hours to obtain a
homogeneous solution.
[0158] A concentrated solution of corrosion inhibitor was
prepared in the following
manner. Potassium hexafluorozirconate (1.5g) was added to 500g of deionized
distilled water
and stirred until completely dissolved. Chromium(III) chloride hexahydrate
(2.2g) was then
added and the mixture stirred to obtain a homogeneous solution.
[0159] The concentrated solution of corrosion inhibitor was then
combined with the
solution of diutan gum and diluted to the final concentrations shown in Table
2 to form the
compositions of Samples A to E, with Sample E being a comparative example.
[0160] Additional comparative Samples F and G were prepared using
MethocelT" A4M
as shown in Table 2. The concentrated solution of corrosion inhibitor was
combined with
deionized water and dry McthocclTM A4M powder, then stirred at high speed for
six hours, to
obtain a homogenous solution.
Table 2. Corrosion inhibitor compositions
Sample Diutan MethocelTM A4M Chromium(III)
Potassium
gum (wt%) (wt%) chloride
hexafluorozirconate
hexahydrate (g/L)
(g/L)
A 0.20 0.0 2.2 1.5
0.30 0.0 2.2 1.5
0.40 0.0 2.2 1.5
0.50 0.0 2.2 1.5
0.10 0.0 2.2 1.5
(comparative)
0.0 0.20 2.2 1.5
(comparative)
0.0 2.0 2.2 1.5
(comparative)
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
Rheology measurements
[0161] Dynamic and steady state rheology measurements on samples
A to G were
performed at 25 C on a Discovery HR-2 rheometer from TA Instruments with
concentric
cylinder geometry.
[0162] The elastic (G') and viscous (G") moduli were determined
as a function of
increasing stress amplitude at a frequency of 1 Hz and the crossover of G' and
G" was used to
estimate the yield stress. Data are reported in Table 3 below.
Table 3. Yield Stress
Sample Yield Stress (Pa)
A 1.62
3.07
5.96
10.1
E (comparative) 0.536
F (comparative) Not detected
G (comparative) Not detected
[0163] Steady shear viscosities were measured in the shear rate
range of 1 to 1000 s-1.
Data are reported in Table 4 below.
Table 4. Viscosity Measurements
Shear Rate (s-1) Viscosity sample Viscosity sample Viscosity
Sample
A (mPa.$) E (naPa.$) G (naPa.$)
1 2.57x103 625 2.20x103
395 114 1.62x103
100 51.2 19.5 771
1000 10.6 8.10 230
[0164] Both samples A and E exhibited shear thinning (decrease in
viscosity with
increasing shear rate) with sufficiently low viscosity at the higher shear
rates to enable easy
application but sample A exhibited much stronger shear thinning and a higher
yield stress.
36
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
Sample G showed shear thinning that was much weaker than sample A resulting in
a relatively
high viscosity at high shear rates that is not desirable for application.
Sample Application and 24 Hours Corrosion Testing
[0165] FIG. 1 shows schematics of photographs taken of panels 100
treated as follows.
Panels 100 are shown in holders 200 which held the panels at a substantially
vertical orientation.
[0166] Samples A to G were applied on 2024-T3 bare aluminum
panels (3 inches wide,
inches long and 0.032 inches thick) using the following procedure. The panels
were first
subjected to a tap water abrade that consisted of wetting the substrate with
tap water, scrubbing
the surface with a Scotch-Brite 7447 pad, spray rinsing with tap water, wiping
down the rinsed
surface with a cheesecloth to remove residual smut followed by a final tap
rinse. The panels
were then dried in air prior to application of the conversion coatings. The
panels were secured
on a rack in a substantially vertical orientation at an inclination of 70-90
degrees above the
horizontal. One of the conversion compositions was applied by brushing once in
an upward
direction, starting at the bottom of the panel, to wet the surface and
allowing the formula to dwell
in place for a total of 5 minutes. A wet gauze wipe was performed on the
treated panels by
wiping three times with a clean cheesecloth that had been saturated with
deionized water.
[0167] As shown in FIG. 1E, 1F and 1G, Samples E, F and G
(comparatives) did not
have sufficient yield stress for the composition to cling to the substrate
without dripping when
the substrate was oriented substantially vertically during application.
However, as shown in FIG.
1A, 1B, 1C, and 1D, Samples A, B, C and D had sufficient yield stress to
counteract the effect of
gravity on the wet coated film (i.e., to cling to the substrate surface) and
little to no dripping was
observed in these Samples.
[0168] Five panels prepared with Sample A were then dried in air
again and exposed to
neutral salt spray (5% sodium chloride salt fog environment) for 24 hours.
Five untreated panels
H were prepared the same way as those treated with Sample A, but without any
conversion
coating (i.e., subjected to a tap water abrade, scrubbing, rinsing and wiping
as described above
but not treated with any of Samples A to G) were run as control H.
[0169] After salt spray exposure, panels were rinsed and dried.
FIG. 2A shows
photographs of panels treated with Sample A following exposure to neutral salt
spray according
to the Example disclosed herein and FIG. 2B shows photographs of untreated
panels (H)
following exposure to neutral salt spray. As shown, panels treated with Sample
A had virtually
37
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
no corrosion on the substrate surface (FIG. 2A), while the substrate surface
of the untreated
panel H was significantly corroded as shown by the significant number of pits
(see arrow as an
example) and corrosion products formed on the substrate surface (FIG. 2B).
[0170] Panels were analyzed for corrosion with a VHX-2000 super
resolution digital
microscope from Keyence equipped with a VH-ZOOR lens. A magnified image of a
1.376 in2
region in the center of the panel was taken using the auto area measurement
tool in the
"mesr/draw" menu. Extraction parameters were set so that the area of the panel
that had
corroded was highlighted, and the total highlighted area measured as a
fraction of the total
analysis area and reported below as a percent value. Three sections of each
panel were measured
and then averaged. Data are presented in Table 5 below. Corrosion on panels
also was rated
according to "Table 1 Scale and Description of Rust Ratings" from the
specification ASTM
D610-08 (2019), and the ratings are shown in Table 5 below.
Table 5. Corrosion data following Exposure to ASTM B117 (2019) Neutral Salt
Spray
Corrosion Results After 24 Hours Neutral Salt Spray
Sample A CONTROL H
Panel Set Corrosion ASTM D610 Corrosion
ASTM D610
Area (%) Rating Area (%)
Rating
1 0.139 7P 69.116
0
2 0.049 8P 75.548
0
3 0.186 7P 72.853
0
4 0.132 7P 69.128
0
0.123 7P 71.212 0
Average 0.126 7P 71.571
0
[0171] Sample A provided significant improvement in corrosion
protection. The control
panels without the conversion coating averaged >70% corroded area per panel
and failed testing
according to ASTM D610 rating scale, whereas panels with Sample A had no more
than 0.2%
corroded area and passed according to the ASTM D610 rating scale.
[0172] Collectively, the data presented herein demonstrate that
Samples containing more
than 0.10% diutan gum and trivalent chromium not only provide corrosion
protection to treated
substrates, but also combines the advantages of low viscosity at high shear
rates for easy brush
application with a high enough yield stress to prevent the wet coating from
dripping when
applied to vertically oriented surfaces.
38
CA 03233870 2024- 4- 3
WO 2023/076990
PCT/US2022/078770
[0173] Whereas particular features of the present disclosure have
been described above
for purposes of illustration, it will be evident to those skilled in the art
that numerous variations
of the details of the coating composition, coating, and methods disclosed
herein may be made
without departing from the scope in the appended claims.
39
CA 03233870 2024- 4- 3